User login
Sharon Worcester is an award-winning medical journalist for MDedge News. She has been with the company since 1996, first as the Southeast Bureau Chief (1996-2009) when the company was known as International Medical News Group, then as a freelance writer (2010-2015) before returning as a reporter in 2015. She previously worked as a daily newspaper reporter covering health and local government. Sharon currently reports primarily on oncology and hematology. She has a BA from Eckerd College and an MA in Mass Communication/Print Journalism from the University of Florida. Connect with her via LinkedIn and follow her on twitter @SW_MedReporter.
Diet appears to play an important role in response to anti-PD-1 cancer immunotherapy
Diet plays an important role in patient response to anti-programmed death-1 (PD-1) cancer immunotherapy, preliminary findings from a gut microbiome study involving 146 melanoma patients suggest.
Specifically, a high-fiber diet was associated with a more diverse gut microbiome and with improved response to anti-PD-1 therapy, whereas a diet high in sugar and processed meat was associated with fewer of the gut bacteria known to be associated with improved response, Christine Spencer, PhD, reported during a press conference highlighting data to be presented at the upcoming American Association for Cancer Research (AACR) annual meeting in Atlanta.
“We found that patients who reported eating high-fiber diets were about five times as likely to respond to anti-PD-1 checkpoint blockade immunotherapy (odds ratio vs. low-fiber diet, 5.3),” said Dr. Spencer, a research scientist at the Parker Institute for Cancer Immunotherapy.
Notably, more than 40% of patients reported taking probiotics, and those, surprisingly, were also associated with reduced gut microbiome diversity, she said.
For this study, Dr. Spencer and her colleagues at the University of Texas MD Anderson Cancer Center, Houston, analyzed prospectively collected fecal samples from 146 melanoma patients, and collected baseline diet information via the National Cancer Institute dietary screener questionnaire, as well as information about probiotic and antibiotic use, in a subset of 113 who were initiating therapy at MD Anderson. Those patients were then followed to assess therapy response.
“Our early data suggest that different foods and supplements may impact response to cancer immunotherapy in patients, and we think this is likely mediated by the gut microbiome,” she said.
Since only 20%-30% of cancer patients respond to immunotherapy, the findings hint at potential approaches for improving gut microbiome diversity, and thus response to anti-PD-1 cancer immunotherapy.
“Eat your high-fiber foods: fruits, vegetables, and whole grains – lots of different kinds and lots of them,” she said. “High-fiber diets have been linked to health benefits in several other contexts, and this study, although preliminary, shows fiber is linked to more favorable gut microbiome in patients, and better response to cancer immunotherapy.”
Conversations about the use of probiotic supplements also are important, she said.
“A lot of people have the perception that probiotics will provide health benefits, but that might not be the case in cancer patients. We’re not saying all of them are bad, but the message is that these factors have never before been studied in patients on immunotherapy, and our data suggest for the first time that they could matter,” she said, noting that future directions include validation of the findings in larger cohorts.
Some of that work has already been done, and updated results will be reported at the AACR meeting.
AACR president and press conference comoderator Elizabeth M. Jaffee, MD, said the findings highlight an “exciting area that’s emerging in cancer research right now.”
Although microbiome research is in its infancy, the MD Anderson group and others are “really making headway,” said Dr. Jaffee, professor of oncology and deputy director of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, Baltimore.
“It’s exciting ... to learn from your study that patients and healthy individuals can also be empowered through diet to control cancer development, and also how they can be empowered to influence favorable response to our therapies,” she said, cautioning, however, that “this is early and certainly we need more research in this area.”
Also of note, the findings show that gut bacteria affect cancers that aren’t necessarily deriving from the gut.
“So the microbiome has importance in, probably, many different cancers and their response to therapy – and possibly in the development of those cancers, so those are areas of research that we need to prioritize in future work, as well,” she said.
This study was sponsored by the Melanoma Research Alliance, the MD Anderson Melanoma Moonshot, the Miriam and Jim Mulva Fund for Melanoma Research, and the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation. Dr. Spencer disclosed that she is a contributor to U.S. patent application (PCT/US17/53.717) submitted by MD Anderson Cancer Center that covers methods to enhance immune checkpoint blockade responses by modulating the microbiome.
SOURCE: Spencer C et al. AACR 2019, Abstract preview.
Diet plays an important role in patient response to anti-programmed death-1 (PD-1) cancer immunotherapy, preliminary findings from a gut microbiome study involving 146 melanoma patients suggest.
Specifically, a high-fiber diet was associated with a more diverse gut microbiome and with improved response to anti-PD-1 therapy, whereas a diet high in sugar and processed meat was associated with fewer of the gut bacteria known to be associated with improved response, Christine Spencer, PhD, reported during a press conference highlighting data to be presented at the upcoming American Association for Cancer Research (AACR) annual meeting in Atlanta.
“We found that patients who reported eating high-fiber diets were about five times as likely to respond to anti-PD-1 checkpoint blockade immunotherapy (odds ratio vs. low-fiber diet, 5.3),” said Dr. Spencer, a research scientist at the Parker Institute for Cancer Immunotherapy.
Notably, more than 40% of patients reported taking probiotics, and those, surprisingly, were also associated with reduced gut microbiome diversity, she said.
For this study, Dr. Spencer and her colleagues at the University of Texas MD Anderson Cancer Center, Houston, analyzed prospectively collected fecal samples from 146 melanoma patients, and collected baseline diet information via the National Cancer Institute dietary screener questionnaire, as well as information about probiotic and antibiotic use, in a subset of 113 who were initiating therapy at MD Anderson. Those patients were then followed to assess therapy response.
“Our early data suggest that different foods and supplements may impact response to cancer immunotherapy in patients, and we think this is likely mediated by the gut microbiome,” she said.
Since only 20%-30% of cancer patients respond to immunotherapy, the findings hint at potential approaches for improving gut microbiome diversity, and thus response to anti-PD-1 cancer immunotherapy.
“Eat your high-fiber foods: fruits, vegetables, and whole grains – lots of different kinds and lots of them,” she said. “High-fiber diets have been linked to health benefits in several other contexts, and this study, although preliminary, shows fiber is linked to more favorable gut microbiome in patients, and better response to cancer immunotherapy.”
Conversations about the use of probiotic supplements also are important, she said.
“A lot of people have the perception that probiotics will provide health benefits, but that might not be the case in cancer patients. We’re not saying all of them are bad, but the message is that these factors have never before been studied in patients on immunotherapy, and our data suggest for the first time that they could matter,” she said, noting that future directions include validation of the findings in larger cohorts.
Some of that work has already been done, and updated results will be reported at the AACR meeting.
AACR president and press conference comoderator Elizabeth M. Jaffee, MD, said the findings highlight an “exciting area that’s emerging in cancer research right now.”
Although microbiome research is in its infancy, the MD Anderson group and others are “really making headway,” said Dr. Jaffee, professor of oncology and deputy director of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, Baltimore.
“It’s exciting ... to learn from your study that patients and healthy individuals can also be empowered through diet to control cancer development, and also how they can be empowered to influence favorable response to our therapies,” she said, cautioning, however, that “this is early and certainly we need more research in this area.”
Also of note, the findings show that gut bacteria affect cancers that aren’t necessarily deriving from the gut.
“So the microbiome has importance in, probably, many different cancers and their response to therapy – and possibly in the development of those cancers, so those are areas of research that we need to prioritize in future work, as well,” she said.
This study was sponsored by the Melanoma Research Alliance, the MD Anderson Melanoma Moonshot, the Miriam and Jim Mulva Fund for Melanoma Research, and the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation. Dr. Spencer disclosed that she is a contributor to U.S. patent application (PCT/US17/53.717) submitted by MD Anderson Cancer Center that covers methods to enhance immune checkpoint blockade responses by modulating the microbiome.
SOURCE: Spencer C et al. AACR 2019, Abstract preview.
Diet plays an important role in patient response to anti-programmed death-1 (PD-1) cancer immunotherapy, preliminary findings from a gut microbiome study involving 146 melanoma patients suggest.
Specifically, a high-fiber diet was associated with a more diverse gut microbiome and with improved response to anti-PD-1 therapy, whereas a diet high in sugar and processed meat was associated with fewer of the gut bacteria known to be associated with improved response, Christine Spencer, PhD, reported during a press conference highlighting data to be presented at the upcoming American Association for Cancer Research (AACR) annual meeting in Atlanta.
“We found that patients who reported eating high-fiber diets were about five times as likely to respond to anti-PD-1 checkpoint blockade immunotherapy (odds ratio vs. low-fiber diet, 5.3),” said Dr. Spencer, a research scientist at the Parker Institute for Cancer Immunotherapy.
Notably, more than 40% of patients reported taking probiotics, and those, surprisingly, were also associated with reduced gut microbiome diversity, she said.
For this study, Dr. Spencer and her colleagues at the University of Texas MD Anderson Cancer Center, Houston, analyzed prospectively collected fecal samples from 146 melanoma patients, and collected baseline diet information via the National Cancer Institute dietary screener questionnaire, as well as information about probiotic and antibiotic use, in a subset of 113 who were initiating therapy at MD Anderson. Those patients were then followed to assess therapy response.
“Our early data suggest that different foods and supplements may impact response to cancer immunotherapy in patients, and we think this is likely mediated by the gut microbiome,” she said.
Since only 20%-30% of cancer patients respond to immunotherapy, the findings hint at potential approaches for improving gut microbiome diversity, and thus response to anti-PD-1 cancer immunotherapy.
“Eat your high-fiber foods: fruits, vegetables, and whole grains – lots of different kinds and lots of them,” she said. “High-fiber diets have been linked to health benefits in several other contexts, and this study, although preliminary, shows fiber is linked to more favorable gut microbiome in patients, and better response to cancer immunotherapy.”
Conversations about the use of probiotic supplements also are important, she said.
“A lot of people have the perception that probiotics will provide health benefits, but that might not be the case in cancer patients. We’re not saying all of them are bad, but the message is that these factors have never before been studied in patients on immunotherapy, and our data suggest for the first time that they could matter,” she said, noting that future directions include validation of the findings in larger cohorts.
Some of that work has already been done, and updated results will be reported at the AACR meeting.
AACR president and press conference comoderator Elizabeth M. Jaffee, MD, said the findings highlight an “exciting area that’s emerging in cancer research right now.”
Although microbiome research is in its infancy, the MD Anderson group and others are “really making headway,” said Dr. Jaffee, professor of oncology and deputy director of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, Baltimore.
“It’s exciting ... to learn from your study that patients and healthy individuals can also be empowered through diet to control cancer development, and also how they can be empowered to influence favorable response to our therapies,” she said, cautioning, however, that “this is early and certainly we need more research in this area.”
Also of note, the findings show that gut bacteria affect cancers that aren’t necessarily deriving from the gut.
“So the microbiome has importance in, probably, many different cancers and their response to therapy – and possibly in the development of those cancers, so those are areas of research that we need to prioritize in future work, as well,” she said.
This study was sponsored by the Melanoma Research Alliance, the MD Anderson Melanoma Moonshot, the Miriam and Jim Mulva Fund for Melanoma Research, and the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation. Dr. Spencer disclosed that she is a contributor to U.S. patent application (PCT/US17/53.717) submitted by MD Anderson Cancer Center that covers methods to enhance immune checkpoint blockade responses by modulating the microbiome.
SOURCE: Spencer C et al. AACR 2019, Abstract preview.
Myositis mimics: Clues for making the right diagnosis
A number of conditions can mimic myositis, but clues that can point to the correct diagnosis are often present in cases involving the mimics, according to Lisa Christopher-Stine, MD.
For example, elevated levels of certain muscle enzymes are an important source of diagnostic information, Dr. Christopher-Stine, director of the Johns Hopkins Myositis Center, Baltimore, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
Isolated elevations in aldolase can be seen in connective tissue–associated interstitial lung disease or in patients with fascial edema, and aspartate aminotransferase, alanine aminotransferase, lactate dehydrogenase (LDH), and creatine kinase (CK) levels can also be helpful, she explained.
The latter can also be elevated in the absence of muscle disease, for example, in healthy individuals following exercise. CK peaks at 24 hours after exercise before returning to baseline by 72 hours. In an experimental setting, a threefold increase in CK levels has been seen at 8-24 hours after exercise, Dr. Christopher-Stine said.
“HyperCKemia”
Trauma from causes such as intramuscular injection, electromyography (EMG), major surgery, or biopsy can also lead to increased CK levels. Motor neuron disease can also cause such increases. In one study, 75% of patients with amyotrophic lateral sclerosis had a mean twofold increase in CK levels, she said.
Asymptomatic CK elevations may also represent presymptomatic myopathies, type 1 or 2 macro-CK, manual labor occupations, or they may be idiopathic.
Race can play a role in CK levels as well. Black people tend to have higher CK levels than white people, she said, noting that one study of more than 10,000 adults showed that black race was strongly associated with CK, and that body composition largely explained differences in CK by age, but not by race/ethnicity (Medicine. Aug 2016;95[33]:e4344).
“So elevated CK may not herald any discernible illness,” she said.
Dr. Christopher-Stine described a case involving an otherwise healthy 30-year-old man with a CK level of 695 IU/L that was found incidentally. He had a desk job, no recent travel, and denied weakness, myalgias, joint pain, dysphagia, shortness of breath, and fevers. In this case, the elevated CK was felt to be secondary to his African American race given that other causes were ruled out.
Another case involved a 72-year-old man with left-arm pain. A cardiac event was ruled out, and CK was found to be about 4,500 IU/L. He reported “flare-ups” of diffuse swelling of the hands and feet. X-rays showed concerning signs of erosions. His transaminases and electromyogram were normal; he reported no weakness or myalgia; and an MRI showed no muscle edema. He was diagnosed with macro-CK, which refers to CK with an increased molecular weight. A clue to this diagnosis is a normal liver function test. In some cases, muscle/brain CK levels (CK-MB) are elevated and higher than total CK, she noted.
She presented an algorithm for the diagnostic work-up of patients presenting with elevated CK of unclear significance. Her recommended approach involves repeat CK assessment and a closer look at family history, medication, drug/toxin history, examination for weakness and neurologic abnormalities, and additional lab assessments in those whose levels remain elevated. In those in whom a diagnosis is not identified, the algorithm calls for observation every 3 months – including physical examination and labs – in asymptomatic patients with levels at less than five times the upper limit of normal, and further evaluation, including EMG with nerve-conduction velocity testing, muscle biopsy, and MRI in those with (or who later develop) marked elevation greater than five times the upper limit of normal and/or symptoms.
Patient assessment
The physical examination should involve localization and quantification of weakness, and assessment for fever, rash, atrophy/wasting/scooping of forearms, fasciculations, cranial nerve involvement, Raynaud’s phenomenon, nailfold capillary changes, arthritis, calcinosis, “mechanic’s hands,” signs of other autoimmune diseases, and lung crackles. Initial laboratory testing should include HIV and hepatitis B and C testing; measurement of CK, AST, ALT, aldolase, thyroid-stimulating hormone, and magnesium levels; a comprehensive metabolic panel and complete blood count; and measurement of erythrocyte sedimentation rate and C-reactive protein.
“Weakness may be secondary to a neuropathy, myopathy, or a problem at the neuropathic junction. Many causes of weakness can be readily identified by careful history taking, focused physical examination, and directed laboratory evaluation,” she said.
Features pointing toward a diagnosis of myositis include characteristic rashes, gradual symptom onset, proximal limb and truncal weakness, other connective tissue disease features such as Raynaud’s and arthritis, and the presence of lung disease, including interstitial lung disease or unexplained infiltrates, she said.
Features pointing away from a diagnosis of myositis include a family history of a similar illness, weakness that is associated with eating or fasting, neurologic signs, cranial nerve involvement, fasciculations, severe muscle cramping, early atrophy, and creatine phosphokinase levels that are either less than 2 times or more than 100 times the upper limit of normal.
Among the conditions to consider in the presence of the features that point away from a myositis diagnosis are muscular dystrophies, metabolic myopathies, and toxic (drug-induced) myopathies, to name a few, Dr. Christopher-Stine said.
She described a number of other cases to illustrate the need for – and to help develop – a differential diagnosis in patients presenting with apparent myositis.
Muscular dystrophies
A 38-year-old woman with limited scleroderma and anti-PM/Scl autoantibodies developed proximal weakness over 9 months and was eventually unable to walk up a flight of stairs. She had heliotrope rash and Gottron’s sign, her serum CK was 723 IU/L, and EMG showed an irritable myopathy.
Muscle biopsy showed inflammation, and she was treated with prednisone, but this led to worsening weakness. She complained of prominent fatigue and double vision at the end of the day, and these symptoms did not improve with steroids.
Anti-AChR and anti-MuSK antibodies were negative, but she had a decrement on repetitive nerve stimulation testing.
She was treated with pyridostigmine and experienced near-complete resolution of her proximal weakness and double vision. A chest CT scan showed thymic hyperplasia; thymectomy was recommended.
In another case, a 19-year-old woman who complained of leg pain after exercise was found to have intact strength but asymmetric calf hypertrophy. Her CK level was 5,000 IU/L, and she was referred to rule out acute myositis.
A quadriceps biopsy was performed and showed abnormal dystrophin immunostaining but no inflammation. A molecular genetic analysis showed deletions in Xp21 and she was diagnosed as a manifesting carrier of Duchenne muscular dystrophy. It was recommended that she be evaluated for cardiomyopathy and receive genetic counseling.
A number of other cases presented by Dr. Christopher-Stine highlighted other muscular dystrophies that can mimic myositis, such as:
- Myotonic dystrophies. These are more often type 2 than type 1. Myotonia may be subtle, cataracts are seen early in all patients, and cardiac arrhythmias are common.
- Limb girdle muscular dystrophy type 2 B (dysferlinopathy). In the legs, this often affects the gastrocnemius muscle, and this will be visible on MRI. In the arms, it most often affects the biceps, sparing the deltoids. CKs are typically very high.
- Facioscapulohumeral muscular dystrophy (FSHD). This involves facial weakness, especially obicularis oris, in 95% of cases, as well as scapular weakness and winging, inflammation on muscle biopsy in 75% of cases, and typically is endomysial or perivascular.
Metabolic myopathies
Among metabolic myopathies that can mimic myositis are disorders of carbohydrate metabolism such as McArdle’s disease, 6-phosphofructokinase deficiency, and Pompe’s disease (adult acid maltase deficiency); disorders of lipid metabolism such as carnitine deficiency and carnitine palmitoyltransferase 2 (CPT2) deficiency; and disorders of purine metabolism, such as myoadenylate deaminase deficiency.
A 27-year-old patient who complained of weakness with activity was referred for possible myositis and was found to have a CK of 3,650 IU/L that never normalized. Physical examination showed intact strength and no muscle atrophy or fasciculations, and an enzyme stain for myophosphorylase showed a normal staining pattern and complete absence of the enzyme on quadricep biopsy. A 22-year-old man with similar symptoms plus recent onset of brown/black urine after physical activity had CK of 110,000 IU/L when symptomatic, and also underwent biopsy after being referred for possible myopathy. Both patients were ultimately diagnosed with CPT2 deficiency, which is associated with risk of rhabdomyolysis triggered by prolonged exercise, diets low in carbohydrates and high in fat, or by fasting.
Myalgias are common, and CK levels are normal or only mildly elevated between episodes in CPT2 deficiency, Dr. Christopher-Stine noted.
Toxic myopathies
Drug-induced myopathies are among the most common etiologies of myopathy and can range from mild myalgia to massive rhabdomyolysis. They can cause mild to severe weakness and may be chronic. The mechanism of toxic injury is direct via myotoxins such as ethyl alcohol, glucocorticoids, lipid-lowering drugs, cocaine, antimalarial drugs, antipsychotic drugs, colchicine, and Ipecac syrup.
One case described by Dr. Christopher-Stine involved “statin myopathy.”
A 55-year-old man on atorvastatin complained of myalgias and brown urine, but had no definitive weakness. He had intact strength and diffuse myalgias that weren’t reproducible. His CK was 45,000 IU/L.
Statin myopathy, as seen in this patient, is usually self-limited and is not associated with autoimmunity or with anti-HMGCR autoantibody positivity.
The mechanism is unknown, but statin myopathy has an incidence of 1.2 per 10,000 patient-years. Myalgias, myositis, rhabdomyolysis, and asymptomatic hyperCKemia are commonly seen. This is in contrast to the immune-mediated necrotizing myelitis that can be secondary to statins and is responsive to immunosuppression, she noted.
Other myositis mimics
In addition to these common myositis mimics, certain other neurologic diseases (such as ALS and cervical myelopathy), endocrinopathies (such as hypothyroidism), and infections (like toxoplasmosis) can also be mistaken for myositis, Dr. Christopher-Stine said, noting that cases illustrating these mimics underscore the need for careful consideration of possible alternate diagnoses.
“While most noninflammatory myopathies are self-limited or have no therapies available, knowing the diagnosis can be helpful for genetic counseling of the patient and family, for mitigating risk factors, and for precluding the use of unwarranted immunosuppressive agents,” she said.
Dr. Christopher-Stine reported having intellectual property interest in a novel Inova Diagnostics autoantibody assay detection for anti-HMGCR. She was also the safety officer for the JBT-101 Trial sponsored by Corbus and funded by the National Institutes of Health.
A number of conditions can mimic myositis, but clues that can point to the correct diagnosis are often present in cases involving the mimics, according to Lisa Christopher-Stine, MD.
For example, elevated levels of certain muscle enzymes are an important source of diagnostic information, Dr. Christopher-Stine, director of the Johns Hopkins Myositis Center, Baltimore, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
Isolated elevations in aldolase can be seen in connective tissue–associated interstitial lung disease or in patients with fascial edema, and aspartate aminotransferase, alanine aminotransferase, lactate dehydrogenase (LDH), and creatine kinase (CK) levels can also be helpful, she explained.
The latter can also be elevated in the absence of muscle disease, for example, in healthy individuals following exercise. CK peaks at 24 hours after exercise before returning to baseline by 72 hours. In an experimental setting, a threefold increase in CK levels has been seen at 8-24 hours after exercise, Dr. Christopher-Stine said.
“HyperCKemia”
Trauma from causes such as intramuscular injection, electromyography (EMG), major surgery, or biopsy can also lead to increased CK levels. Motor neuron disease can also cause such increases. In one study, 75% of patients with amyotrophic lateral sclerosis had a mean twofold increase in CK levels, she said.
Asymptomatic CK elevations may also represent presymptomatic myopathies, type 1 or 2 macro-CK, manual labor occupations, or they may be idiopathic.
Race can play a role in CK levels as well. Black people tend to have higher CK levels than white people, she said, noting that one study of more than 10,000 adults showed that black race was strongly associated with CK, and that body composition largely explained differences in CK by age, but not by race/ethnicity (Medicine. Aug 2016;95[33]:e4344).
“So elevated CK may not herald any discernible illness,” she said.
Dr. Christopher-Stine described a case involving an otherwise healthy 30-year-old man with a CK level of 695 IU/L that was found incidentally. He had a desk job, no recent travel, and denied weakness, myalgias, joint pain, dysphagia, shortness of breath, and fevers. In this case, the elevated CK was felt to be secondary to his African American race given that other causes were ruled out.
Another case involved a 72-year-old man with left-arm pain. A cardiac event was ruled out, and CK was found to be about 4,500 IU/L. He reported “flare-ups” of diffuse swelling of the hands and feet. X-rays showed concerning signs of erosions. His transaminases and electromyogram were normal; he reported no weakness or myalgia; and an MRI showed no muscle edema. He was diagnosed with macro-CK, which refers to CK with an increased molecular weight. A clue to this diagnosis is a normal liver function test. In some cases, muscle/brain CK levels (CK-MB) are elevated and higher than total CK, she noted.
She presented an algorithm for the diagnostic work-up of patients presenting with elevated CK of unclear significance. Her recommended approach involves repeat CK assessment and a closer look at family history, medication, drug/toxin history, examination for weakness and neurologic abnormalities, and additional lab assessments in those whose levels remain elevated. In those in whom a diagnosis is not identified, the algorithm calls for observation every 3 months – including physical examination and labs – in asymptomatic patients with levels at less than five times the upper limit of normal, and further evaluation, including EMG with nerve-conduction velocity testing, muscle biopsy, and MRI in those with (or who later develop) marked elevation greater than five times the upper limit of normal and/or symptoms.
Patient assessment
The physical examination should involve localization and quantification of weakness, and assessment for fever, rash, atrophy/wasting/scooping of forearms, fasciculations, cranial nerve involvement, Raynaud’s phenomenon, nailfold capillary changes, arthritis, calcinosis, “mechanic’s hands,” signs of other autoimmune diseases, and lung crackles. Initial laboratory testing should include HIV and hepatitis B and C testing; measurement of CK, AST, ALT, aldolase, thyroid-stimulating hormone, and magnesium levels; a comprehensive metabolic panel and complete blood count; and measurement of erythrocyte sedimentation rate and C-reactive protein.
“Weakness may be secondary to a neuropathy, myopathy, or a problem at the neuropathic junction. Many causes of weakness can be readily identified by careful history taking, focused physical examination, and directed laboratory evaluation,” she said.
Features pointing toward a diagnosis of myositis include characteristic rashes, gradual symptom onset, proximal limb and truncal weakness, other connective tissue disease features such as Raynaud’s and arthritis, and the presence of lung disease, including interstitial lung disease or unexplained infiltrates, she said.
Features pointing away from a diagnosis of myositis include a family history of a similar illness, weakness that is associated with eating or fasting, neurologic signs, cranial nerve involvement, fasciculations, severe muscle cramping, early atrophy, and creatine phosphokinase levels that are either less than 2 times or more than 100 times the upper limit of normal.
Among the conditions to consider in the presence of the features that point away from a myositis diagnosis are muscular dystrophies, metabolic myopathies, and toxic (drug-induced) myopathies, to name a few, Dr. Christopher-Stine said.
She described a number of other cases to illustrate the need for – and to help develop – a differential diagnosis in patients presenting with apparent myositis.
Muscular dystrophies
A 38-year-old woman with limited scleroderma and anti-PM/Scl autoantibodies developed proximal weakness over 9 months and was eventually unable to walk up a flight of stairs. She had heliotrope rash and Gottron’s sign, her serum CK was 723 IU/L, and EMG showed an irritable myopathy.
Muscle biopsy showed inflammation, and she was treated with prednisone, but this led to worsening weakness. She complained of prominent fatigue and double vision at the end of the day, and these symptoms did not improve with steroids.
Anti-AChR and anti-MuSK antibodies were negative, but she had a decrement on repetitive nerve stimulation testing.
She was treated with pyridostigmine and experienced near-complete resolution of her proximal weakness and double vision. A chest CT scan showed thymic hyperplasia; thymectomy was recommended.
In another case, a 19-year-old woman who complained of leg pain after exercise was found to have intact strength but asymmetric calf hypertrophy. Her CK level was 5,000 IU/L, and she was referred to rule out acute myositis.
A quadriceps biopsy was performed and showed abnormal dystrophin immunostaining but no inflammation. A molecular genetic analysis showed deletions in Xp21 and she was diagnosed as a manifesting carrier of Duchenne muscular dystrophy. It was recommended that she be evaluated for cardiomyopathy and receive genetic counseling.
A number of other cases presented by Dr. Christopher-Stine highlighted other muscular dystrophies that can mimic myositis, such as:
- Myotonic dystrophies. These are more often type 2 than type 1. Myotonia may be subtle, cataracts are seen early in all patients, and cardiac arrhythmias are common.
- Limb girdle muscular dystrophy type 2 B (dysferlinopathy). In the legs, this often affects the gastrocnemius muscle, and this will be visible on MRI. In the arms, it most often affects the biceps, sparing the deltoids. CKs are typically very high.
- Facioscapulohumeral muscular dystrophy (FSHD). This involves facial weakness, especially obicularis oris, in 95% of cases, as well as scapular weakness and winging, inflammation on muscle biopsy in 75% of cases, and typically is endomysial or perivascular.
Metabolic myopathies
Among metabolic myopathies that can mimic myositis are disorders of carbohydrate metabolism such as McArdle’s disease, 6-phosphofructokinase deficiency, and Pompe’s disease (adult acid maltase deficiency); disorders of lipid metabolism such as carnitine deficiency and carnitine palmitoyltransferase 2 (CPT2) deficiency; and disorders of purine metabolism, such as myoadenylate deaminase deficiency.
A 27-year-old patient who complained of weakness with activity was referred for possible myositis and was found to have a CK of 3,650 IU/L that never normalized. Physical examination showed intact strength and no muscle atrophy or fasciculations, and an enzyme stain for myophosphorylase showed a normal staining pattern and complete absence of the enzyme on quadricep biopsy. A 22-year-old man with similar symptoms plus recent onset of brown/black urine after physical activity had CK of 110,000 IU/L when symptomatic, and also underwent biopsy after being referred for possible myopathy. Both patients were ultimately diagnosed with CPT2 deficiency, which is associated with risk of rhabdomyolysis triggered by prolonged exercise, diets low in carbohydrates and high in fat, or by fasting.
Myalgias are common, and CK levels are normal or only mildly elevated between episodes in CPT2 deficiency, Dr. Christopher-Stine noted.
Toxic myopathies
Drug-induced myopathies are among the most common etiologies of myopathy and can range from mild myalgia to massive rhabdomyolysis. They can cause mild to severe weakness and may be chronic. The mechanism of toxic injury is direct via myotoxins such as ethyl alcohol, glucocorticoids, lipid-lowering drugs, cocaine, antimalarial drugs, antipsychotic drugs, colchicine, and Ipecac syrup.
One case described by Dr. Christopher-Stine involved “statin myopathy.”
A 55-year-old man on atorvastatin complained of myalgias and brown urine, but had no definitive weakness. He had intact strength and diffuse myalgias that weren’t reproducible. His CK was 45,000 IU/L.
Statin myopathy, as seen in this patient, is usually self-limited and is not associated with autoimmunity or with anti-HMGCR autoantibody positivity.
The mechanism is unknown, but statin myopathy has an incidence of 1.2 per 10,000 patient-years. Myalgias, myositis, rhabdomyolysis, and asymptomatic hyperCKemia are commonly seen. This is in contrast to the immune-mediated necrotizing myelitis that can be secondary to statins and is responsive to immunosuppression, she noted.
Other myositis mimics
In addition to these common myositis mimics, certain other neurologic diseases (such as ALS and cervical myelopathy), endocrinopathies (such as hypothyroidism), and infections (like toxoplasmosis) can also be mistaken for myositis, Dr. Christopher-Stine said, noting that cases illustrating these mimics underscore the need for careful consideration of possible alternate diagnoses.
“While most noninflammatory myopathies are self-limited or have no therapies available, knowing the diagnosis can be helpful for genetic counseling of the patient and family, for mitigating risk factors, and for precluding the use of unwarranted immunosuppressive agents,” she said.
Dr. Christopher-Stine reported having intellectual property interest in a novel Inova Diagnostics autoantibody assay detection for anti-HMGCR. She was also the safety officer for the JBT-101 Trial sponsored by Corbus and funded by the National Institutes of Health.
A number of conditions can mimic myositis, but clues that can point to the correct diagnosis are often present in cases involving the mimics, according to Lisa Christopher-Stine, MD.
For example, elevated levels of certain muscle enzymes are an important source of diagnostic information, Dr. Christopher-Stine, director of the Johns Hopkins Myositis Center, Baltimore, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
Isolated elevations in aldolase can be seen in connective tissue–associated interstitial lung disease or in patients with fascial edema, and aspartate aminotransferase, alanine aminotransferase, lactate dehydrogenase (LDH), and creatine kinase (CK) levels can also be helpful, she explained.
The latter can also be elevated in the absence of muscle disease, for example, in healthy individuals following exercise. CK peaks at 24 hours after exercise before returning to baseline by 72 hours. In an experimental setting, a threefold increase in CK levels has been seen at 8-24 hours after exercise, Dr. Christopher-Stine said.
“HyperCKemia”
Trauma from causes such as intramuscular injection, electromyography (EMG), major surgery, or biopsy can also lead to increased CK levels. Motor neuron disease can also cause such increases. In one study, 75% of patients with amyotrophic lateral sclerosis had a mean twofold increase in CK levels, she said.
Asymptomatic CK elevations may also represent presymptomatic myopathies, type 1 or 2 macro-CK, manual labor occupations, or they may be idiopathic.
Race can play a role in CK levels as well. Black people tend to have higher CK levels than white people, she said, noting that one study of more than 10,000 adults showed that black race was strongly associated with CK, and that body composition largely explained differences in CK by age, but not by race/ethnicity (Medicine. Aug 2016;95[33]:e4344).
“So elevated CK may not herald any discernible illness,” she said.
Dr. Christopher-Stine described a case involving an otherwise healthy 30-year-old man with a CK level of 695 IU/L that was found incidentally. He had a desk job, no recent travel, and denied weakness, myalgias, joint pain, dysphagia, shortness of breath, and fevers. In this case, the elevated CK was felt to be secondary to his African American race given that other causes were ruled out.
Another case involved a 72-year-old man with left-arm pain. A cardiac event was ruled out, and CK was found to be about 4,500 IU/L. He reported “flare-ups” of diffuse swelling of the hands and feet. X-rays showed concerning signs of erosions. His transaminases and electromyogram were normal; he reported no weakness or myalgia; and an MRI showed no muscle edema. He was diagnosed with macro-CK, which refers to CK with an increased molecular weight. A clue to this diagnosis is a normal liver function test. In some cases, muscle/brain CK levels (CK-MB) are elevated and higher than total CK, she noted.
She presented an algorithm for the diagnostic work-up of patients presenting with elevated CK of unclear significance. Her recommended approach involves repeat CK assessment and a closer look at family history, medication, drug/toxin history, examination for weakness and neurologic abnormalities, and additional lab assessments in those whose levels remain elevated. In those in whom a diagnosis is not identified, the algorithm calls for observation every 3 months – including physical examination and labs – in asymptomatic patients with levels at less than five times the upper limit of normal, and further evaluation, including EMG with nerve-conduction velocity testing, muscle biopsy, and MRI in those with (or who later develop) marked elevation greater than five times the upper limit of normal and/or symptoms.
Patient assessment
The physical examination should involve localization and quantification of weakness, and assessment for fever, rash, atrophy/wasting/scooping of forearms, fasciculations, cranial nerve involvement, Raynaud’s phenomenon, nailfold capillary changes, arthritis, calcinosis, “mechanic’s hands,” signs of other autoimmune diseases, and lung crackles. Initial laboratory testing should include HIV and hepatitis B and C testing; measurement of CK, AST, ALT, aldolase, thyroid-stimulating hormone, and magnesium levels; a comprehensive metabolic panel and complete blood count; and measurement of erythrocyte sedimentation rate and C-reactive protein.
“Weakness may be secondary to a neuropathy, myopathy, or a problem at the neuropathic junction. Many causes of weakness can be readily identified by careful history taking, focused physical examination, and directed laboratory evaluation,” she said.
Features pointing toward a diagnosis of myositis include characteristic rashes, gradual symptom onset, proximal limb and truncal weakness, other connective tissue disease features such as Raynaud’s and arthritis, and the presence of lung disease, including interstitial lung disease or unexplained infiltrates, she said.
Features pointing away from a diagnosis of myositis include a family history of a similar illness, weakness that is associated with eating or fasting, neurologic signs, cranial nerve involvement, fasciculations, severe muscle cramping, early atrophy, and creatine phosphokinase levels that are either less than 2 times or more than 100 times the upper limit of normal.
Among the conditions to consider in the presence of the features that point away from a myositis diagnosis are muscular dystrophies, metabolic myopathies, and toxic (drug-induced) myopathies, to name a few, Dr. Christopher-Stine said.
She described a number of other cases to illustrate the need for – and to help develop – a differential diagnosis in patients presenting with apparent myositis.
Muscular dystrophies
A 38-year-old woman with limited scleroderma and anti-PM/Scl autoantibodies developed proximal weakness over 9 months and was eventually unable to walk up a flight of stairs. She had heliotrope rash and Gottron’s sign, her serum CK was 723 IU/L, and EMG showed an irritable myopathy.
Muscle biopsy showed inflammation, and she was treated with prednisone, but this led to worsening weakness. She complained of prominent fatigue and double vision at the end of the day, and these symptoms did not improve with steroids.
Anti-AChR and anti-MuSK antibodies were negative, but she had a decrement on repetitive nerve stimulation testing.
She was treated with pyridostigmine and experienced near-complete resolution of her proximal weakness and double vision. A chest CT scan showed thymic hyperplasia; thymectomy was recommended.
In another case, a 19-year-old woman who complained of leg pain after exercise was found to have intact strength but asymmetric calf hypertrophy. Her CK level was 5,000 IU/L, and she was referred to rule out acute myositis.
A quadriceps biopsy was performed and showed abnormal dystrophin immunostaining but no inflammation. A molecular genetic analysis showed deletions in Xp21 and she was diagnosed as a manifesting carrier of Duchenne muscular dystrophy. It was recommended that she be evaluated for cardiomyopathy and receive genetic counseling.
A number of other cases presented by Dr. Christopher-Stine highlighted other muscular dystrophies that can mimic myositis, such as:
- Myotonic dystrophies. These are more often type 2 than type 1. Myotonia may be subtle, cataracts are seen early in all patients, and cardiac arrhythmias are common.
- Limb girdle muscular dystrophy type 2 B (dysferlinopathy). In the legs, this often affects the gastrocnemius muscle, and this will be visible on MRI. In the arms, it most often affects the biceps, sparing the deltoids. CKs are typically very high.
- Facioscapulohumeral muscular dystrophy (FSHD). This involves facial weakness, especially obicularis oris, in 95% of cases, as well as scapular weakness and winging, inflammation on muscle biopsy in 75% of cases, and typically is endomysial or perivascular.
Metabolic myopathies
Among metabolic myopathies that can mimic myositis are disorders of carbohydrate metabolism such as McArdle’s disease, 6-phosphofructokinase deficiency, and Pompe’s disease (adult acid maltase deficiency); disorders of lipid metabolism such as carnitine deficiency and carnitine palmitoyltransferase 2 (CPT2) deficiency; and disorders of purine metabolism, such as myoadenylate deaminase deficiency.
A 27-year-old patient who complained of weakness with activity was referred for possible myositis and was found to have a CK of 3,650 IU/L that never normalized. Physical examination showed intact strength and no muscle atrophy or fasciculations, and an enzyme stain for myophosphorylase showed a normal staining pattern and complete absence of the enzyme on quadricep biopsy. A 22-year-old man with similar symptoms plus recent onset of brown/black urine after physical activity had CK of 110,000 IU/L when symptomatic, and also underwent biopsy after being referred for possible myopathy. Both patients were ultimately diagnosed with CPT2 deficiency, which is associated with risk of rhabdomyolysis triggered by prolonged exercise, diets low in carbohydrates and high in fat, or by fasting.
Myalgias are common, and CK levels are normal or only mildly elevated between episodes in CPT2 deficiency, Dr. Christopher-Stine noted.
Toxic myopathies
Drug-induced myopathies are among the most common etiologies of myopathy and can range from mild myalgia to massive rhabdomyolysis. They can cause mild to severe weakness and may be chronic. The mechanism of toxic injury is direct via myotoxins such as ethyl alcohol, glucocorticoids, lipid-lowering drugs, cocaine, antimalarial drugs, antipsychotic drugs, colchicine, and Ipecac syrup.
One case described by Dr. Christopher-Stine involved “statin myopathy.”
A 55-year-old man on atorvastatin complained of myalgias and brown urine, but had no definitive weakness. He had intact strength and diffuse myalgias that weren’t reproducible. His CK was 45,000 IU/L.
Statin myopathy, as seen in this patient, is usually self-limited and is not associated with autoimmunity or with anti-HMGCR autoantibody positivity.
The mechanism is unknown, but statin myopathy has an incidence of 1.2 per 10,000 patient-years. Myalgias, myositis, rhabdomyolysis, and asymptomatic hyperCKemia are commonly seen. This is in contrast to the immune-mediated necrotizing myelitis that can be secondary to statins and is responsive to immunosuppression, she noted.
Other myositis mimics
In addition to these common myositis mimics, certain other neurologic diseases (such as ALS and cervical myelopathy), endocrinopathies (such as hypothyroidism), and infections (like toxoplasmosis) can also be mistaken for myositis, Dr. Christopher-Stine said, noting that cases illustrating these mimics underscore the need for careful consideration of possible alternate diagnoses.
“While most noninflammatory myopathies are self-limited or have no therapies available, knowing the diagnosis can be helpful for genetic counseling of the patient and family, for mitigating risk factors, and for precluding the use of unwarranted immunosuppressive agents,” she said.
Dr. Christopher-Stine reported having intellectual property interest in a novel Inova Diagnostics autoantibody assay detection for anti-HMGCR. She was also the safety officer for the JBT-101 Trial sponsored by Corbus and funded by the National Institutes of Health.
EXPERT ANALYSIS FROM THE WINTER RHEUMATOLOGY SYMPOSIUM
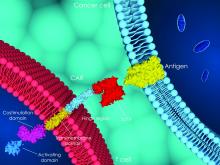
Barriers to CAR T use in the spotlight at first European meeting
outcomes data suggest.
For that reason, and because bone marrow units are profit centers and CAR T-cell therapy reimbursement remains problematic, CAR T in the United States is “effectively being used as a bridge to transplant” – at a cost of more than $1 million per dose, economist Duane Schulthess told attendees at a recent, first-of-its-kind joint European CAR T-cell meeting in Paris, which was cosponsored by the European Hematology Association (EHA) and the European Society for Blood and Marrow Transplantation (EBMT).
“This is the way clinical practice is evolving right now; the price is not allowing enough experimentation for CAR T to flow up and be used in the less-diseased population,” said Mr. Schulthess, managing director of Vital Transformation, a consulting company based in Wezembeek-Oppem, Belgium.
In Europe, there is a slightly different problem in that health technology assessment bodies (HTAs) “have to figure out what they want to do” given the 2018 approvals of the first CAR T therapies there, he said, explaining that the data he presented was from a study commissioned by the Dutch government to help determine “what [CAR T] looks like from an effectiveness standpoint while they’re trying to figure out how much it’s worth and what they should pay.”
“Increasingly these are the big issues,” Mr. Schulthess said.
In August, the European Commission approved tisagenlecleucel (Kymriah) and axicabtagene ciloleucel (Yescarta) on the recommendation of the European Medicines Agency. Kymriah was approved for pediatric and young adult patients up to age 25 years with refractory B-cell acute lymphoblastic leukemia in relapse after transplant or in second or later relapse, as well as for adults patients with relapsed/refractory diffuse large B-cell lymphoma after failing at least two lines of systemic therapy, and Yescarta was approved for the latter and for the treatment of primary refractory mediastinal large B-cell lymphoma after at least two lines of systemic therapy.
The approvals have researchers and clinicians there clamoring for information about the therapy, which is revolutionizing the field of hematologic malignancies, according to Christian Chabannon, MD, PhD, chair of the EBMT Cellular Therapy & Immunobiology Working Party and vice-chair of the EBMT Scientific Council.
“An increasing number of European institutions are starting to administer this new category of medicinal products and increasingly contribute to ongoing clinical protocols and preclinical studies,” Dr. Chabannon said in an interview, explaining the urgency in planning the 1st European CAR T Cell Meeting just 6 months after the CAR T approvals in Europe.
EHA and EBMT brought together patient advocates, young investigators, and experts from across the globe to present the latest relevant information and data on topics ranging from current trials and experience, CAR T implementation and management, the preclinical and clinical pipelines, various CAR T applications, industry perspectives, and relevant economic issues, he said.
The latter is where Mr. Schulthess came in.
His research involved patient-level treatment pathway data from a database of more than 3 million patients treated with either allogeneic hematopoietic stem cell transplant (allo-HCT) or CAR T therapy across 5 years of experience. The data showed up to 85% response rates for each in the first-line setting. He and his colleagues then looked at therapy choices for those who failed to respond to second-line therapies and at how decisions were made regarding transplant and CAR T therapy – and specifically whether CAR T can be a substitute for transplant.
Ultimately, they looked at 29 allo-HCT recipients and 14 CAR T therapy recipients for a head-to-head comparison of the two treatments and performed an in-depth cost-efficacy analysis using a novel “visual pathology” methodology to account for limitations in the data.
The 3-year relapse-free survival probability was nearly 68% in the transplant recipients and 46% with CAR T.
“Now why is that? [Because] ... these populations are not the same; the CAR T population has a much higher disease burden,” Mr. Schulthess said. “So what we’re seeing [among] actual clinical doctors doing this for real – they are defaulting to bone marrow transplants, except in those cases where they do not have enough time or the patient does not respond. Then and only then are they giving CAR T.”
And that comes back to the fact that bone marrow units make money, he said.
CAR T is costly, and reimbursement can be problematic; these are disincentives for doctors to use CAR T therapy, at least in the United States, and while this is currently “being worked out,” the choice more often is “giving bone marrow transplant first and seeing what happens,” Mr. Schulthess said.
In Europe, that creates “a tough choice” for the HTAs, he said, noting that, in the absence of evidence of CAR T being curative in the subpopulation of patients with high disease burden who fail transplant and given the high cost, there is a push to determine at what point it begins to make sense economically.
“We think that you gain efficiency at ... roughly $277,000 [per dose] because [at that cost] you can do more CAR Ts than you can do bone marrow transplants. [CAR T] is less invasive, it’s lighter touch, it’s more efficient,” he said. “So if we were to see an efficiency cost of between $222,000 and $277,000, we think that works.”
Another recent study came to similar conclusions based on quality assessments, he said (J Clin Oncol. 2018 Sep 13. doi: 10.1200/JCO.2018.79.0642).
“We think that’s where this is going to end up, so we think that, if someone starts producing this for a couple hundred thousand bucks, then – certainly in Europe – it will make sense for this to start drifting up and being used as a substitute [to transplant],” he added.
Mr. Schulthess was one of scores of experts and investigators who presented at the EHA/EBMT joint meeting, which included numerous U.S. pioneers in the field and young European investigators, among others, Dr. Chabannon said.
Attesting to the enthusiasm in Europe regarding CAR T, Dr. Chabannon said that there were “more requests for registration than the venue could safely accommodate, a long waiting list, and a high number of individuals on the waiting list who registered for the live streaming” of the event.
“The field of CAR T cells is growing at a fast pace since the first clinical successes reported in the early 2010s, and one can wonder whether the expectations are not in excess of what reality will deliver,” he said. “Nevertheless, CAR T cells represent an essential innovation, not an incremental progress in biomedical sciences. They combine new mechanisms of action, clinical activity in advanced malignancies (and possibly beyond the field of cancer), transfer of manufacturing of human cell-based therapeutics to the industry, and potentially the first commercial success for a gene therapy.”
Surveys conducted by various professional associations, including EBMT, have clearly identified the potential for clinical successes that CAR T cells represent and the tremendous challenges raised by these innovations, he said, noting that “these include fulfilling specific educational needs.”
Therefore, EBMT and EHA have already announced that a second edition of the meeting is planned for Jan. 30 – Feb. 1, 2020, he noted.
Mr. Schulthess reported that his research was funded by the Dutch government.
outcomes data suggest.
For that reason, and because bone marrow units are profit centers and CAR T-cell therapy reimbursement remains problematic, CAR T in the United States is “effectively being used as a bridge to transplant” – at a cost of more than $1 million per dose, economist Duane Schulthess told attendees at a recent, first-of-its-kind joint European CAR T-cell meeting in Paris, which was cosponsored by the European Hematology Association (EHA) and the European Society for Blood and Marrow Transplantation (EBMT).
“This is the way clinical practice is evolving right now; the price is not allowing enough experimentation for CAR T to flow up and be used in the less-diseased population,” said Mr. Schulthess, managing director of Vital Transformation, a consulting company based in Wezembeek-Oppem, Belgium.
In Europe, there is a slightly different problem in that health technology assessment bodies (HTAs) “have to figure out what they want to do” given the 2018 approvals of the first CAR T therapies there, he said, explaining that the data he presented was from a study commissioned by the Dutch government to help determine “what [CAR T] looks like from an effectiveness standpoint while they’re trying to figure out how much it’s worth and what they should pay.”
“Increasingly these are the big issues,” Mr. Schulthess said.
In August, the European Commission approved tisagenlecleucel (Kymriah) and axicabtagene ciloleucel (Yescarta) on the recommendation of the European Medicines Agency. Kymriah was approved for pediatric and young adult patients up to age 25 years with refractory B-cell acute lymphoblastic leukemia in relapse after transplant or in second or later relapse, as well as for adults patients with relapsed/refractory diffuse large B-cell lymphoma after failing at least two lines of systemic therapy, and Yescarta was approved for the latter and for the treatment of primary refractory mediastinal large B-cell lymphoma after at least two lines of systemic therapy.
The approvals have researchers and clinicians there clamoring for information about the therapy, which is revolutionizing the field of hematologic malignancies, according to Christian Chabannon, MD, PhD, chair of the EBMT Cellular Therapy & Immunobiology Working Party and vice-chair of the EBMT Scientific Council.
“An increasing number of European institutions are starting to administer this new category of medicinal products and increasingly contribute to ongoing clinical protocols and preclinical studies,” Dr. Chabannon said in an interview, explaining the urgency in planning the 1st European CAR T Cell Meeting just 6 months after the CAR T approvals in Europe.
EHA and EBMT brought together patient advocates, young investigators, and experts from across the globe to present the latest relevant information and data on topics ranging from current trials and experience, CAR T implementation and management, the preclinical and clinical pipelines, various CAR T applications, industry perspectives, and relevant economic issues, he said.
The latter is where Mr. Schulthess came in.
His research involved patient-level treatment pathway data from a database of more than 3 million patients treated with either allogeneic hematopoietic stem cell transplant (allo-HCT) or CAR T therapy across 5 years of experience. The data showed up to 85% response rates for each in the first-line setting. He and his colleagues then looked at therapy choices for those who failed to respond to second-line therapies and at how decisions were made regarding transplant and CAR T therapy – and specifically whether CAR T can be a substitute for transplant.
Ultimately, they looked at 29 allo-HCT recipients and 14 CAR T therapy recipients for a head-to-head comparison of the two treatments and performed an in-depth cost-efficacy analysis using a novel “visual pathology” methodology to account for limitations in the data.
The 3-year relapse-free survival probability was nearly 68% in the transplant recipients and 46% with CAR T.
“Now why is that? [Because] ... these populations are not the same; the CAR T population has a much higher disease burden,” Mr. Schulthess said. “So what we’re seeing [among] actual clinical doctors doing this for real – they are defaulting to bone marrow transplants, except in those cases where they do not have enough time or the patient does not respond. Then and only then are they giving CAR T.”
And that comes back to the fact that bone marrow units make money, he said.
CAR T is costly, and reimbursement can be problematic; these are disincentives for doctors to use CAR T therapy, at least in the United States, and while this is currently “being worked out,” the choice more often is “giving bone marrow transplant first and seeing what happens,” Mr. Schulthess said.
In Europe, that creates “a tough choice” for the HTAs, he said, noting that, in the absence of evidence of CAR T being curative in the subpopulation of patients with high disease burden who fail transplant and given the high cost, there is a push to determine at what point it begins to make sense economically.
“We think that you gain efficiency at ... roughly $277,000 [per dose] because [at that cost] you can do more CAR Ts than you can do bone marrow transplants. [CAR T] is less invasive, it’s lighter touch, it’s more efficient,” he said. “So if we were to see an efficiency cost of between $222,000 and $277,000, we think that works.”
Another recent study came to similar conclusions based on quality assessments, he said (J Clin Oncol. 2018 Sep 13. doi: 10.1200/JCO.2018.79.0642).
“We think that’s where this is going to end up, so we think that, if someone starts producing this for a couple hundred thousand bucks, then – certainly in Europe – it will make sense for this to start drifting up and being used as a substitute [to transplant],” he added.
Mr. Schulthess was one of scores of experts and investigators who presented at the EHA/EBMT joint meeting, which included numerous U.S. pioneers in the field and young European investigators, among others, Dr. Chabannon said.
Attesting to the enthusiasm in Europe regarding CAR T, Dr. Chabannon said that there were “more requests for registration than the venue could safely accommodate, a long waiting list, and a high number of individuals on the waiting list who registered for the live streaming” of the event.
“The field of CAR T cells is growing at a fast pace since the first clinical successes reported in the early 2010s, and one can wonder whether the expectations are not in excess of what reality will deliver,” he said. “Nevertheless, CAR T cells represent an essential innovation, not an incremental progress in biomedical sciences. They combine new mechanisms of action, clinical activity in advanced malignancies (and possibly beyond the field of cancer), transfer of manufacturing of human cell-based therapeutics to the industry, and potentially the first commercial success for a gene therapy.”
Surveys conducted by various professional associations, including EBMT, have clearly identified the potential for clinical successes that CAR T cells represent and the tremendous challenges raised by these innovations, he said, noting that “these include fulfilling specific educational needs.”
Therefore, EBMT and EHA have already announced that a second edition of the meeting is planned for Jan. 30 – Feb. 1, 2020, he noted.
Mr. Schulthess reported that his research was funded by the Dutch government.
outcomes data suggest.
For that reason, and because bone marrow units are profit centers and CAR T-cell therapy reimbursement remains problematic, CAR T in the United States is “effectively being used as a bridge to transplant” – at a cost of more than $1 million per dose, economist Duane Schulthess told attendees at a recent, first-of-its-kind joint European CAR T-cell meeting in Paris, which was cosponsored by the European Hematology Association (EHA) and the European Society for Blood and Marrow Transplantation (EBMT).
“This is the way clinical practice is evolving right now; the price is not allowing enough experimentation for CAR T to flow up and be used in the less-diseased population,” said Mr. Schulthess, managing director of Vital Transformation, a consulting company based in Wezembeek-Oppem, Belgium.
In Europe, there is a slightly different problem in that health technology assessment bodies (HTAs) “have to figure out what they want to do” given the 2018 approvals of the first CAR T therapies there, he said, explaining that the data he presented was from a study commissioned by the Dutch government to help determine “what [CAR T] looks like from an effectiveness standpoint while they’re trying to figure out how much it’s worth and what they should pay.”
“Increasingly these are the big issues,” Mr. Schulthess said.
In August, the European Commission approved tisagenlecleucel (Kymriah) and axicabtagene ciloleucel (Yescarta) on the recommendation of the European Medicines Agency. Kymriah was approved for pediatric and young adult patients up to age 25 years with refractory B-cell acute lymphoblastic leukemia in relapse after transplant or in second or later relapse, as well as for adults patients with relapsed/refractory diffuse large B-cell lymphoma after failing at least two lines of systemic therapy, and Yescarta was approved for the latter and for the treatment of primary refractory mediastinal large B-cell lymphoma after at least two lines of systemic therapy.
The approvals have researchers and clinicians there clamoring for information about the therapy, which is revolutionizing the field of hematologic malignancies, according to Christian Chabannon, MD, PhD, chair of the EBMT Cellular Therapy & Immunobiology Working Party and vice-chair of the EBMT Scientific Council.
“An increasing number of European institutions are starting to administer this new category of medicinal products and increasingly contribute to ongoing clinical protocols and preclinical studies,” Dr. Chabannon said in an interview, explaining the urgency in planning the 1st European CAR T Cell Meeting just 6 months after the CAR T approvals in Europe.
EHA and EBMT brought together patient advocates, young investigators, and experts from across the globe to present the latest relevant information and data on topics ranging from current trials and experience, CAR T implementation and management, the preclinical and clinical pipelines, various CAR T applications, industry perspectives, and relevant economic issues, he said.
The latter is where Mr. Schulthess came in.
His research involved patient-level treatment pathway data from a database of more than 3 million patients treated with either allogeneic hematopoietic stem cell transplant (allo-HCT) or CAR T therapy across 5 years of experience. The data showed up to 85% response rates for each in the first-line setting. He and his colleagues then looked at therapy choices for those who failed to respond to second-line therapies and at how decisions were made regarding transplant and CAR T therapy – and specifically whether CAR T can be a substitute for transplant.
Ultimately, they looked at 29 allo-HCT recipients and 14 CAR T therapy recipients for a head-to-head comparison of the two treatments and performed an in-depth cost-efficacy analysis using a novel “visual pathology” methodology to account for limitations in the data.
The 3-year relapse-free survival probability was nearly 68% in the transplant recipients and 46% with CAR T.
“Now why is that? [Because] ... these populations are not the same; the CAR T population has a much higher disease burden,” Mr. Schulthess said. “So what we’re seeing [among] actual clinical doctors doing this for real – they are defaulting to bone marrow transplants, except in those cases where they do not have enough time or the patient does not respond. Then and only then are they giving CAR T.”
And that comes back to the fact that bone marrow units make money, he said.
CAR T is costly, and reimbursement can be problematic; these are disincentives for doctors to use CAR T therapy, at least in the United States, and while this is currently “being worked out,” the choice more often is “giving bone marrow transplant first and seeing what happens,” Mr. Schulthess said.
In Europe, that creates “a tough choice” for the HTAs, he said, noting that, in the absence of evidence of CAR T being curative in the subpopulation of patients with high disease burden who fail transplant and given the high cost, there is a push to determine at what point it begins to make sense economically.
“We think that you gain efficiency at ... roughly $277,000 [per dose] because [at that cost] you can do more CAR Ts than you can do bone marrow transplants. [CAR T] is less invasive, it’s lighter touch, it’s more efficient,” he said. “So if we were to see an efficiency cost of between $222,000 and $277,000, we think that works.”
Another recent study came to similar conclusions based on quality assessments, he said (J Clin Oncol. 2018 Sep 13. doi: 10.1200/JCO.2018.79.0642).
“We think that’s where this is going to end up, so we think that, if someone starts producing this for a couple hundred thousand bucks, then – certainly in Europe – it will make sense for this to start drifting up and being used as a substitute [to transplant],” he added.
Mr. Schulthess was one of scores of experts and investigators who presented at the EHA/EBMT joint meeting, which included numerous U.S. pioneers in the field and young European investigators, among others, Dr. Chabannon said.
Attesting to the enthusiasm in Europe regarding CAR T, Dr. Chabannon said that there were “more requests for registration than the venue could safely accommodate, a long waiting list, and a high number of individuals on the waiting list who registered for the live streaming” of the event.
“The field of CAR T cells is growing at a fast pace since the first clinical successes reported in the early 2010s, and one can wonder whether the expectations are not in excess of what reality will deliver,” he said. “Nevertheless, CAR T cells represent an essential innovation, not an incremental progress in biomedical sciences. They combine new mechanisms of action, clinical activity in advanced malignancies (and possibly beyond the field of cancer), transfer of manufacturing of human cell-based therapeutics to the industry, and potentially the first commercial success for a gene therapy.”
Surveys conducted by various professional associations, including EBMT, have clearly identified the potential for clinical successes that CAR T cells represent and the tremendous challenges raised by these innovations, he said, noting that “these include fulfilling specific educational needs.”
Therefore, EBMT and EHA have already announced that a second edition of the meeting is planned for Jan. 30 – Feb. 1, 2020, he noted.
Mr. Schulthess reported that his research was funded by the Dutch government.
NILE: Liquid biopsy bests tissue testing for targetable mutations in NSCLC
A cell-free DNA (cfDNA) test, or “liquid biopsy,” identifies more biomarkers and does so more quickly than tissue-based genotyping for guiding treatment in newly diagnosed advanced non–small cell lung cancer (NSCLC), according to a finding from a prospective study.
In 282 patients with newly diagnosed advanced NSCLC who were enrolled in the multicenter Noninvasive versus Invasive Lung Evaluation (NILE) study between July 2016 and April 2018, the “well-validated, comprehensive, and highly sensitive test” – Guardant360 – detected at least one guideline-recommended biomarker mutation in significantly more cases than did tissue-based tests alone (77 vs. 60 patients), Vassiliki A. Papadimitrakopoulou, MD, reported during a press conference highlighting data to be presented at the upcoming American Association for Cancer Research annual meeting in Atlanta.
“Additionally, the cfDNA results were delivered significantly faster than the standard-of-care tissue results [median, 9 vs. 15 days],” said Dr. Papadimitrakopoulou, chief of the section of thoracic medical oncology and the Jay and Lori Eisenberg Distinguished Professor in the department of thoracic/head and neck medical oncology at the University of Texas MD Anderson Cancer Center, Houston.
Guardant360 assesses for all guideline-recommended genomic biomarkers, Dr. Papadimitrakopoulou said, noting that nine such biomarkers have been identified. All biomarkers identified using the liquid biopsy were also detected in tissue every time.
“Plasma cfDNA testing therefore had 100% positive predictive value,” she said.
This is important, because “we know that about 30% of patients with newly diagnosed advanced non–small lung cancer have therapeutically targetable genomic alterations that make them eligible for targeted therapies,” she said.
“Identifying these patients is important, as the response rate to the properly identified targeted therapy is higher than response rates to first-line chemotherapy or immune checkpoint inhibitor therapy,” she added, explaining that tissue-based assessment has long been the standard of care option for identifying genomic biomarkers, but is limited by the risks associated with the biopsy procedure, the inability to test for all relevant mutations, and the time it takes – up to 30 days – to obtain results.
“[The NILE] results have very exciting implications for clinical practice, especially in light of the expanding list of genomic biomarkers to be assessed,” she said, concluding that the findings from NILE – the largest study of newly diagnosed advanced NSCLC – demonstrate that the clinical utility of this well-validated, comprehensive, sensitive cfDNA test “is cardinal in identification of patients with guideline-recommended biomarker-positive tumors, and it is an alternative to SOC [standard of care] tissue testing in the first-line testing.”
Clinical follow-up of patients is ongoing, she noted.
This study was funded by Guardant Health. Dr. Papadimitrakopoulou serves on the advisory boards of several pharmaceutical companies. She reported receiving CME speaker fees from F. Hoffmann–La Roche, and has received research support from Eli Lilly, Novartis, Merck, AstraZeneca, F. Hoffmann–La Roche, Nektar Therapeutics, Janssen, Bristol-Myers Squibb, Checkmate, Incyte and Guardant Health.
SOURCE: Papadimitrakopoulou VA et al. AACR 2019, Abstract 4460..
A cell-free DNA (cfDNA) test, or “liquid biopsy,” identifies more biomarkers and does so more quickly than tissue-based genotyping for guiding treatment in newly diagnosed advanced non–small cell lung cancer (NSCLC), according to a finding from a prospective study.
In 282 patients with newly diagnosed advanced NSCLC who were enrolled in the multicenter Noninvasive versus Invasive Lung Evaluation (NILE) study between July 2016 and April 2018, the “well-validated, comprehensive, and highly sensitive test” – Guardant360 – detected at least one guideline-recommended biomarker mutation in significantly more cases than did tissue-based tests alone (77 vs. 60 patients), Vassiliki A. Papadimitrakopoulou, MD, reported during a press conference highlighting data to be presented at the upcoming American Association for Cancer Research annual meeting in Atlanta.
“Additionally, the cfDNA results were delivered significantly faster than the standard-of-care tissue results [median, 9 vs. 15 days],” said Dr. Papadimitrakopoulou, chief of the section of thoracic medical oncology and the Jay and Lori Eisenberg Distinguished Professor in the department of thoracic/head and neck medical oncology at the University of Texas MD Anderson Cancer Center, Houston.
Guardant360 assesses for all guideline-recommended genomic biomarkers, Dr. Papadimitrakopoulou said, noting that nine such biomarkers have been identified. All biomarkers identified using the liquid biopsy were also detected in tissue every time.
“Plasma cfDNA testing therefore had 100% positive predictive value,” she said.
This is important, because “we know that about 30% of patients with newly diagnosed advanced non–small lung cancer have therapeutically targetable genomic alterations that make them eligible for targeted therapies,” she said.
“Identifying these patients is important, as the response rate to the properly identified targeted therapy is higher than response rates to first-line chemotherapy or immune checkpoint inhibitor therapy,” she added, explaining that tissue-based assessment has long been the standard of care option for identifying genomic biomarkers, but is limited by the risks associated with the biopsy procedure, the inability to test for all relevant mutations, and the time it takes – up to 30 days – to obtain results.
“[The NILE] results have very exciting implications for clinical practice, especially in light of the expanding list of genomic biomarkers to be assessed,” she said, concluding that the findings from NILE – the largest study of newly diagnosed advanced NSCLC – demonstrate that the clinical utility of this well-validated, comprehensive, sensitive cfDNA test “is cardinal in identification of patients with guideline-recommended biomarker-positive tumors, and it is an alternative to SOC [standard of care] tissue testing in the first-line testing.”
Clinical follow-up of patients is ongoing, she noted.
This study was funded by Guardant Health. Dr. Papadimitrakopoulou serves on the advisory boards of several pharmaceutical companies. She reported receiving CME speaker fees from F. Hoffmann–La Roche, and has received research support from Eli Lilly, Novartis, Merck, AstraZeneca, F. Hoffmann–La Roche, Nektar Therapeutics, Janssen, Bristol-Myers Squibb, Checkmate, Incyte and Guardant Health.
SOURCE: Papadimitrakopoulou VA et al. AACR 2019, Abstract 4460..
A cell-free DNA (cfDNA) test, or “liquid biopsy,” identifies more biomarkers and does so more quickly than tissue-based genotyping for guiding treatment in newly diagnosed advanced non–small cell lung cancer (NSCLC), according to a finding from a prospective study.
In 282 patients with newly diagnosed advanced NSCLC who were enrolled in the multicenter Noninvasive versus Invasive Lung Evaluation (NILE) study between July 2016 and April 2018, the “well-validated, comprehensive, and highly sensitive test” – Guardant360 – detected at least one guideline-recommended biomarker mutation in significantly more cases than did tissue-based tests alone (77 vs. 60 patients), Vassiliki A. Papadimitrakopoulou, MD, reported during a press conference highlighting data to be presented at the upcoming American Association for Cancer Research annual meeting in Atlanta.
“Additionally, the cfDNA results were delivered significantly faster than the standard-of-care tissue results [median, 9 vs. 15 days],” said Dr. Papadimitrakopoulou, chief of the section of thoracic medical oncology and the Jay and Lori Eisenberg Distinguished Professor in the department of thoracic/head and neck medical oncology at the University of Texas MD Anderson Cancer Center, Houston.
Guardant360 assesses for all guideline-recommended genomic biomarkers, Dr. Papadimitrakopoulou said, noting that nine such biomarkers have been identified. All biomarkers identified using the liquid biopsy were also detected in tissue every time.
“Plasma cfDNA testing therefore had 100% positive predictive value,” she said.
This is important, because “we know that about 30% of patients with newly diagnosed advanced non–small lung cancer have therapeutically targetable genomic alterations that make them eligible for targeted therapies,” she said.
“Identifying these patients is important, as the response rate to the properly identified targeted therapy is higher than response rates to first-line chemotherapy or immune checkpoint inhibitor therapy,” she added, explaining that tissue-based assessment has long been the standard of care option for identifying genomic biomarkers, but is limited by the risks associated with the biopsy procedure, the inability to test for all relevant mutations, and the time it takes – up to 30 days – to obtain results.
“[The NILE] results have very exciting implications for clinical practice, especially in light of the expanding list of genomic biomarkers to be assessed,” she said, concluding that the findings from NILE – the largest study of newly diagnosed advanced NSCLC – demonstrate that the clinical utility of this well-validated, comprehensive, sensitive cfDNA test “is cardinal in identification of patients with guideline-recommended biomarker-positive tumors, and it is an alternative to SOC [standard of care] tissue testing in the first-line testing.”
Clinical follow-up of patients is ongoing, she noted.
This study was funded by Guardant Health. Dr. Papadimitrakopoulou serves on the advisory boards of several pharmaceutical companies. She reported receiving CME speaker fees from F. Hoffmann–La Roche, and has received research support from Eli Lilly, Novartis, Merck, AstraZeneca, F. Hoffmann–La Roche, Nektar Therapeutics, Janssen, Bristol-Myers Squibb, Checkmate, Incyte and Guardant Health.
SOURCE: Papadimitrakopoulou VA et al. AACR 2019, Abstract 4460..
Much still unknown about inflammation’s role in RA patients’ CVD risk
A variety of trials, some recent and some a decade old, have highlighted the role of inflammation on cardiovascular disease risk in both patients with and without rheumatoid arthritis, spurring greater interest in alleviating inflammation across a wide range of patients, Jon T. Giles, MD, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
However, questions remain about the unique contributions of inflammation to CVD risk in RA patients and the effect of RA treatments on that risk, which future studies hope to answer.
Hints of inflammation’s effects in non-RA patients
The JUPITER trial published more than a decade ago, for example, tested the effects of statins in nearly 18,000 older adults without rheumatoid arthritis (RA) who had elevated levels of inflammation, defined as a C-reactive protein (CRP) level of greater than 2 mg/L and low-density lipoprotein (LDL) cholesterol less than 130 mg/dL. Such patients would otherwise be considered low risk and not eligible for statin therapy, said Dr. Giles, a rheumatologist, epidemiologist, and clinical researcher in the division of rheumatology at Columbia University, New York.
A marked decrease in the incidence of cardiovascular disease (CVD) events was seen in those treated with statins, compared with those who received placebo, and all patient subgroups benefited; the number needed to treat to prevent one event was 32 at 5 years (N Engl J Med. 2008;359:2195-207).
The trial was remarkable in that it was stopped early for efficacy, he noted.
“So the question is: Should we be thinking about systemic inflammation as the real target here? And should RA patients who have elevated persistent levels of CRP really be the people that we’re thinking about?” he asked. “Obviously this needs to be tested; we don’t know.”
The more recent CANTOS trial looking at secondary CVD prevention in more than 10,000 non-RA patients with a prior myocardial infarction also highlighted the role of inflammation and provided “some support that decreasing inflammatory cytokines may be important for reducing [CVD] events,” he said (N Engl J Med. 2017;377:1119-31).
Participants were treated with the interleukin-1 inhibitor canakinumab (Ilaris) or placebo, and canakinumab was associated with about a 15% reduction in CVD events, providing “more proof of concept to look at the inflammatory innate immune contribution to CVD risk,” Dr. Giles said.
Treated patients had more infections, but they also had less gout, less arthritis, and less cancer than did those who received placebo, he noted.
Effect of RA treatments on CVD risk
The effects of existing treatments for RA also highlight the importance of inflammation in CVD risk in RA patients, he said, noting that data support a role for immunomodulators for risk reduction.
“There’s a lot of observational epidemiology in this space – mostly for methotrexate and [tumor necrosis factor (TNF)] inhibitors,” he said.
One analysis showed that across 8 cohort studies involving methotrexate, the disease-modifying antirheumatic agent reduced the risk of CVD events by 28%, and that across 16 cohort studies, TNF inhibitors reduced the risk by 30% (Ann Rheum Dis. 2015 Mar;74[3]:480-9).
All of the methotrexate studies showed a reduction, and almost all of the TNF inhibitor trials showed a reduction, Dr. Giles noted.
With respect to other non-TNF biologics, claims data suggest that abatacept (Orencia) is similar to the TNF inhibitor etanercept (Enbrel) with respect to CVD risk, and in a head-to-head, randomized clinical trial of more than 3,000 RA patients presented as a late-breaking abstract at the ACR annual meeting in 2016, Dr. Giles and his colleagues found similar cardiovascular safety between the anti-IL-6 receptor blocker tocilizumab (Actemra) and etanercept.
“I think we’ll know more about this in the near future,” he said.
As for the mechanisms of these agents, early data and animal models suggest that abatacept may play “a special role” in atherosclerosis reduction related to its effects on T cell CTLA-4 over-expression, and methotrexate also seems to have a number of “potential mechanistic benefits” that render it atheroprotective, he said.
The disappointing findings from the recently reported CIRT trial, which showed no benefit of methotrexate for secondary CVD prevention in non-RA patients (N Engl J Med. 2019;380:752-62), has dampened enthusiasm regarding methotrexate’s role here, but it is important to note that patients enrolled in CIRT, unlike those in JUPITER and CANTOS, were not enrolled based on elevated levels of CRP, Dr. Giles said.
Various studies of TNF inhibitors have shown atheroprotective effects through reductions in macrophage-derived inflammatory cytokines, downregulation of adhesion molecules on endothelial cells, improving the function of high-density lipoprotein, stabilizing atherosclerotic plaque remodeling, and reducing procoagulant states.
The TARGET trial
In a recent study of 17 patients with RA, Dr. Giles and his colleagues showed that TNF inhibitor therapy with either adalimumab (Humira) or etanercept significantly reduced aortic inflammation as measured by baseline and 8-week fluorodeoxyglucose (FDG) PET-CT.
“Is this proof that this helps? It’s not proof; there’s no control group, we don’t know that this is not the natural progression of vascular inflammation in these patients,” he said.
However, the findings were suggestive enough to prompt the launch of the TARGET trial, which is now enrolling patients at centers in the United States and Canada, Dr. Giles said.
The TARGET trial is a project involving his team at Columbia University along with researchers from Brigham and Women’s Hospital, Boston. They plan to enroll 200 RA patients without CVD who have an inadequate response to methotrexate. Participants will be randomized to receive an added TNF inhibitor or added triple therapy, and the primary outcome will be changes in inflammation in the aortic and carotid arteries on FDG PET-CT at 6 months versus baseline.
“So stay tuned and hopefully we’ll have some good information about the effect of two different types of treatments for rheumatoid arthritis on vascular inflammation,” Dr. Giles said.
A final question he addressed is whether the RA-CVD risk link is really a problem that has already been solved – one that “we’re just learning about after the fact.”
“The answer is partially yes and partially no,” he said.
The most up-to-date estimate of whether RA patients have a problem with CVD comes from a Swedish population-based study of more than 15,700 RA patients and nearly 70,900 comparators, which was published in 2018 and showed across-the-board declines in CVD rates over time.
RA and non-RA patients experienced an overall 40% reduction in acute coronary syndromes between 1997 and 2014, but the relative difference in event rates between the groups persisted (Ann Rheum Dis. 2017;76:1642-7).
“There is still a gap ... so we haven’t answered this question yet,” he said, adding that “the rates have been reduced, but we want those rates to be equal or maybe even less.
“Why can’t RA patients have less cardiovascular disease if we’re using drugs that are so effective for treating the inflammatory component of atherogenesis?” he asked.
The authors of the study noted that most RA patients in Sweden are in low disease activity by 3-6 months, so they were “a little confounded by why there is no equalization of these rates as of yet,” he said.
“I think we still have more to learn about this problem, and it is still a problem in our patients,” Dr. Giles said.
Dr. Giles is a consultant for Genentech, Lilly, Horizon, Bristol-Myers Squibb, and UCB, and he has received grant support from Pfizer.
A variety of trials, some recent and some a decade old, have highlighted the role of inflammation on cardiovascular disease risk in both patients with and without rheumatoid arthritis, spurring greater interest in alleviating inflammation across a wide range of patients, Jon T. Giles, MD, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
However, questions remain about the unique contributions of inflammation to CVD risk in RA patients and the effect of RA treatments on that risk, which future studies hope to answer.
Hints of inflammation’s effects in non-RA patients
The JUPITER trial published more than a decade ago, for example, tested the effects of statins in nearly 18,000 older adults without rheumatoid arthritis (RA) who had elevated levels of inflammation, defined as a C-reactive protein (CRP) level of greater than 2 mg/L and low-density lipoprotein (LDL) cholesterol less than 130 mg/dL. Such patients would otherwise be considered low risk and not eligible for statin therapy, said Dr. Giles, a rheumatologist, epidemiologist, and clinical researcher in the division of rheumatology at Columbia University, New York.
A marked decrease in the incidence of cardiovascular disease (CVD) events was seen in those treated with statins, compared with those who received placebo, and all patient subgroups benefited; the number needed to treat to prevent one event was 32 at 5 years (N Engl J Med. 2008;359:2195-207).
The trial was remarkable in that it was stopped early for efficacy, he noted.
“So the question is: Should we be thinking about systemic inflammation as the real target here? And should RA patients who have elevated persistent levels of CRP really be the people that we’re thinking about?” he asked. “Obviously this needs to be tested; we don’t know.”
The more recent CANTOS trial looking at secondary CVD prevention in more than 10,000 non-RA patients with a prior myocardial infarction also highlighted the role of inflammation and provided “some support that decreasing inflammatory cytokines may be important for reducing [CVD] events,” he said (N Engl J Med. 2017;377:1119-31).
Participants were treated with the interleukin-1 inhibitor canakinumab (Ilaris) or placebo, and canakinumab was associated with about a 15% reduction in CVD events, providing “more proof of concept to look at the inflammatory innate immune contribution to CVD risk,” Dr. Giles said.
Treated patients had more infections, but they also had less gout, less arthritis, and less cancer than did those who received placebo, he noted.
Effect of RA treatments on CVD risk
The effects of existing treatments for RA also highlight the importance of inflammation in CVD risk in RA patients, he said, noting that data support a role for immunomodulators for risk reduction.
“There’s a lot of observational epidemiology in this space – mostly for methotrexate and [tumor necrosis factor (TNF)] inhibitors,” he said.
One analysis showed that across 8 cohort studies involving methotrexate, the disease-modifying antirheumatic agent reduced the risk of CVD events by 28%, and that across 16 cohort studies, TNF inhibitors reduced the risk by 30% (Ann Rheum Dis. 2015 Mar;74[3]:480-9).
All of the methotrexate studies showed a reduction, and almost all of the TNF inhibitor trials showed a reduction, Dr. Giles noted.
With respect to other non-TNF biologics, claims data suggest that abatacept (Orencia) is similar to the TNF inhibitor etanercept (Enbrel) with respect to CVD risk, and in a head-to-head, randomized clinical trial of more than 3,000 RA patients presented as a late-breaking abstract at the ACR annual meeting in 2016, Dr. Giles and his colleagues found similar cardiovascular safety between the anti-IL-6 receptor blocker tocilizumab (Actemra) and etanercept.
“I think we’ll know more about this in the near future,” he said.
As for the mechanisms of these agents, early data and animal models suggest that abatacept may play “a special role” in atherosclerosis reduction related to its effects on T cell CTLA-4 over-expression, and methotrexate also seems to have a number of “potential mechanistic benefits” that render it atheroprotective, he said.
The disappointing findings from the recently reported CIRT trial, which showed no benefit of methotrexate for secondary CVD prevention in non-RA patients (N Engl J Med. 2019;380:752-62), has dampened enthusiasm regarding methotrexate’s role here, but it is important to note that patients enrolled in CIRT, unlike those in JUPITER and CANTOS, were not enrolled based on elevated levels of CRP, Dr. Giles said.
Various studies of TNF inhibitors have shown atheroprotective effects through reductions in macrophage-derived inflammatory cytokines, downregulation of adhesion molecules on endothelial cells, improving the function of high-density lipoprotein, stabilizing atherosclerotic plaque remodeling, and reducing procoagulant states.
The TARGET trial
In a recent study of 17 patients with RA, Dr. Giles and his colleagues showed that TNF inhibitor therapy with either adalimumab (Humira) or etanercept significantly reduced aortic inflammation as measured by baseline and 8-week fluorodeoxyglucose (FDG) PET-CT.
“Is this proof that this helps? It’s not proof; there’s no control group, we don’t know that this is not the natural progression of vascular inflammation in these patients,” he said.
However, the findings were suggestive enough to prompt the launch of the TARGET trial, which is now enrolling patients at centers in the United States and Canada, Dr. Giles said.
The TARGET trial is a project involving his team at Columbia University along with researchers from Brigham and Women’s Hospital, Boston. They plan to enroll 200 RA patients without CVD who have an inadequate response to methotrexate. Participants will be randomized to receive an added TNF inhibitor or added triple therapy, and the primary outcome will be changes in inflammation in the aortic and carotid arteries on FDG PET-CT at 6 months versus baseline.
“So stay tuned and hopefully we’ll have some good information about the effect of two different types of treatments for rheumatoid arthritis on vascular inflammation,” Dr. Giles said.
A final question he addressed is whether the RA-CVD risk link is really a problem that has already been solved – one that “we’re just learning about after the fact.”
“The answer is partially yes and partially no,” he said.
The most up-to-date estimate of whether RA patients have a problem with CVD comes from a Swedish population-based study of more than 15,700 RA patients and nearly 70,900 comparators, which was published in 2018 and showed across-the-board declines in CVD rates over time.
RA and non-RA patients experienced an overall 40% reduction in acute coronary syndromes between 1997 and 2014, but the relative difference in event rates between the groups persisted (Ann Rheum Dis. 2017;76:1642-7).
“There is still a gap ... so we haven’t answered this question yet,” he said, adding that “the rates have been reduced, but we want those rates to be equal or maybe even less.
“Why can’t RA patients have less cardiovascular disease if we’re using drugs that are so effective for treating the inflammatory component of atherogenesis?” he asked.
The authors of the study noted that most RA patients in Sweden are in low disease activity by 3-6 months, so they were “a little confounded by why there is no equalization of these rates as of yet,” he said.
“I think we still have more to learn about this problem, and it is still a problem in our patients,” Dr. Giles said.
Dr. Giles is a consultant for Genentech, Lilly, Horizon, Bristol-Myers Squibb, and UCB, and he has received grant support from Pfizer.
A variety of trials, some recent and some a decade old, have highlighted the role of inflammation on cardiovascular disease risk in both patients with and without rheumatoid arthritis, spurring greater interest in alleviating inflammation across a wide range of patients, Jon T. Giles, MD, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
However, questions remain about the unique contributions of inflammation to CVD risk in RA patients and the effect of RA treatments on that risk, which future studies hope to answer.
Hints of inflammation’s effects in non-RA patients
The JUPITER trial published more than a decade ago, for example, tested the effects of statins in nearly 18,000 older adults without rheumatoid arthritis (RA) who had elevated levels of inflammation, defined as a C-reactive protein (CRP) level of greater than 2 mg/L and low-density lipoprotein (LDL) cholesterol less than 130 mg/dL. Such patients would otherwise be considered low risk and not eligible for statin therapy, said Dr. Giles, a rheumatologist, epidemiologist, and clinical researcher in the division of rheumatology at Columbia University, New York.
A marked decrease in the incidence of cardiovascular disease (CVD) events was seen in those treated with statins, compared with those who received placebo, and all patient subgroups benefited; the number needed to treat to prevent one event was 32 at 5 years (N Engl J Med. 2008;359:2195-207).
The trial was remarkable in that it was stopped early for efficacy, he noted.
“So the question is: Should we be thinking about systemic inflammation as the real target here? And should RA patients who have elevated persistent levels of CRP really be the people that we’re thinking about?” he asked. “Obviously this needs to be tested; we don’t know.”
The more recent CANTOS trial looking at secondary CVD prevention in more than 10,000 non-RA patients with a prior myocardial infarction also highlighted the role of inflammation and provided “some support that decreasing inflammatory cytokines may be important for reducing [CVD] events,” he said (N Engl J Med. 2017;377:1119-31).
Participants were treated with the interleukin-1 inhibitor canakinumab (Ilaris) or placebo, and canakinumab was associated with about a 15% reduction in CVD events, providing “more proof of concept to look at the inflammatory innate immune contribution to CVD risk,” Dr. Giles said.
Treated patients had more infections, but they also had less gout, less arthritis, and less cancer than did those who received placebo, he noted.
Effect of RA treatments on CVD risk
The effects of existing treatments for RA also highlight the importance of inflammation in CVD risk in RA patients, he said, noting that data support a role for immunomodulators for risk reduction.
“There’s a lot of observational epidemiology in this space – mostly for methotrexate and [tumor necrosis factor (TNF)] inhibitors,” he said.
One analysis showed that across 8 cohort studies involving methotrexate, the disease-modifying antirheumatic agent reduced the risk of CVD events by 28%, and that across 16 cohort studies, TNF inhibitors reduced the risk by 30% (Ann Rheum Dis. 2015 Mar;74[3]:480-9).
All of the methotrexate studies showed a reduction, and almost all of the TNF inhibitor trials showed a reduction, Dr. Giles noted.
With respect to other non-TNF biologics, claims data suggest that abatacept (Orencia) is similar to the TNF inhibitor etanercept (Enbrel) with respect to CVD risk, and in a head-to-head, randomized clinical trial of more than 3,000 RA patients presented as a late-breaking abstract at the ACR annual meeting in 2016, Dr. Giles and his colleagues found similar cardiovascular safety between the anti-IL-6 receptor blocker tocilizumab (Actemra) and etanercept.
“I think we’ll know more about this in the near future,” he said.
As for the mechanisms of these agents, early data and animal models suggest that abatacept may play “a special role” in atherosclerosis reduction related to its effects on T cell CTLA-4 over-expression, and methotrexate also seems to have a number of “potential mechanistic benefits” that render it atheroprotective, he said.
The disappointing findings from the recently reported CIRT trial, which showed no benefit of methotrexate for secondary CVD prevention in non-RA patients (N Engl J Med. 2019;380:752-62), has dampened enthusiasm regarding methotrexate’s role here, but it is important to note that patients enrolled in CIRT, unlike those in JUPITER and CANTOS, were not enrolled based on elevated levels of CRP, Dr. Giles said.
Various studies of TNF inhibitors have shown atheroprotective effects through reductions in macrophage-derived inflammatory cytokines, downregulation of adhesion molecules on endothelial cells, improving the function of high-density lipoprotein, stabilizing atherosclerotic plaque remodeling, and reducing procoagulant states.
The TARGET trial
In a recent study of 17 patients with RA, Dr. Giles and his colleagues showed that TNF inhibitor therapy with either adalimumab (Humira) or etanercept significantly reduced aortic inflammation as measured by baseline and 8-week fluorodeoxyglucose (FDG) PET-CT.
“Is this proof that this helps? It’s not proof; there’s no control group, we don’t know that this is not the natural progression of vascular inflammation in these patients,” he said.
However, the findings were suggestive enough to prompt the launch of the TARGET trial, which is now enrolling patients at centers in the United States and Canada, Dr. Giles said.
The TARGET trial is a project involving his team at Columbia University along with researchers from Brigham and Women’s Hospital, Boston. They plan to enroll 200 RA patients without CVD who have an inadequate response to methotrexate. Participants will be randomized to receive an added TNF inhibitor or added triple therapy, and the primary outcome will be changes in inflammation in the aortic and carotid arteries on FDG PET-CT at 6 months versus baseline.
“So stay tuned and hopefully we’ll have some good information about the effect of two different types of treatments for rheumatoid arthritis on vascular inflammation,” Dr. Giles said.
A final question he addressed is whether the RA-CVD risk link is really a problem that has already been solved – one that “we’re just learning about after the fact.”
“The answer is partially yes and partially no,” he said.
The most up-to-date estimate of whether RA patients have a problem with CVD comes from a Swedish population-based study of more than 15,700 RA patients and nearly 70,900 comparators, which was published in 2018 and showed across-the-board declines in CVD rates over time.
RA and non-RA patients experienced an overall 40% reduction in acute coronary syndromes between 1997 and 2014, but the relative difference in event rates between the groups persisted (Ann Rheum Dis. 2017;76:1642-7).
“There is still a gap ... so we haven’t answered this question yet,” he said, adding that “the rates have been reduced, but we want those rates to be equal or maybe even less.
“Why can’t RA patients have less cardiovascular disease if we’re using drugs that are so effective for treating the inflammatory component of atherogenesis?” he asked.
The authors of the study noted that most RA patients in Sweden are in low disease activity by 3-6 months, so they were “a little confounded by why there is no equalization of these rates as of yet,” he said.
“I think we still have more to learn about this problem, and it is still a problem in our patients,” Dr. Giles said.
Dr. Giles is a consultant for Genentech, Lilly, Horizon, Bristol-Myers Squibb, and UCB, and he has received grant support from Pfizer.
EXPERT ANALYSIS FROM THE WINTER RHEUMATOLOGY SYMPOSIUM
Midostaurin maintenance may reduce relapse risk in FLT3-ITD+ AML
HOUSTON – Midostaurin maintenance therapy along with standard-of-care treatment after allogeneic stem cell transplant (alloSCT) in patients with acute myeloid leukemia (AML) appears to reduce the risk of relapse, according to findings from the randomized, phase 2 RADIUS trial.
Notably, the effect of midostaurin in this open-label, exploratory trial was most pronounced in patients with high levels of phosphorylated FLT3 (pFLT3) inhibition as assessed by plasma inhibitor activity assay, Richard T. Maziarz, MD, reported at the Transplantation & Cellular Therapy Meetings.
“The median [pFLT3 reduction] was less than 70% ... those patients who had the deepest level inhibition maintained the highest likelihood of staying free of disease,” Dr. Maziarz, a professor of medicine at Oregon Health & Science University, Portland, said at the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research. At its meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: American Society for Transplantation and Cellular Therapy (ASTCT).
Midostaurin is a multitargeted tyrosine kinase inhibitor (TKI) that was shown in the pivotal RATIFY trial to significantly improve event-free and overall survival versus placebo when interspersed with induction and consolidation chemotherapy and also when used for maintenance in adults with newly diagnosed FLT3-mutated AML, Dr. Maziarz explained. He noted that patients in the RATIFY study who underwent alloSCT did not receive midostaurin maintenance (N Engl J Med. 2017; 377:454-64).
Although alloSCT provides the greatest likelihood of sustained remission in AML, relapse rates remain high at 30%-59%, he said, adding that, “in the setting of transplantation, FLT3 expression, or FLT3-ITD [internal tandem duplication] ... is a poor risk feature.”
Studies are increasingly suggesting that posttransplant maintenance therapy may improve this outcome. For example, the small, randomized, phase 2 SORMAIN study presented at the 2018 annual meeting of the American Society of Hematology showed a signal for benefit with posttransplant maintenance with the TKI sorafenib. Data regarding midostaurin in this setting are limited, Dr. Maziarz noted.
The RADIUS trial was a small study designed to look for a similar signal with midostaurin and thus was not adequately powered to detect a statistical difference between the arms, he explained.
RADIUS included 60 AML patients aged 18-70 years who underwent myeloablative alloSCT and were in their first complete remission. The primary endpoint was relapse-free survival (RFS) at 18 months after transplant. Results were presented at ASH 2018.
RFS was 89% in 16 of 30 patients who were randomized to receive 50 mg of midostaurin twice daily along with standard-of-care (SOC) treatment and completed 12 4-week cycles. This compared with an RFS rate of 76% in 14 of 30 patients who received SOC only and completed 12 cycles (hazard ratio, 0.46).
The predicted relative reduction in the risk of relapse with the addition of midostaurin was 54%, and at 24 months, both RFS and overall survival were 85% in the midostaurin group and 76% in the SOC-only group, Dr. Maziarz reported.
The median duration of exposure to midostaurin was 10.5 months and the median dose intensity was 93 mg/day, indicating that full-dose therapy was achievable in most patients who stayed on the study.
Treatment was generally well tolerated; there was a comparable number of early discontinuations in the midostaurin and SOC-only arms. The discontinuations were caused mainly by adverse events (typically gastrointestinal toxicities) in the midostaurin arm and by consent withdrawal in the SOC-only arm, he said, adding that there were no significant differences between the groups with respect to serious adverse events or acute or chronic graft-versus-host disease.
Following the presentation of the primary RADIUS results at ASH 2018, an exploratory analysis was conducted to assess midostaurin’s inhibitory effects on FLT3 in plasma.
FLT3 plasma inhibitor activity, assessed by coculturing plasma samples taken on the first day of the treatment cycles with the FLT3-positive AML to look for a reduction in pFLT3, was evaluable in 28 patients in each arm.
“What we see is when you start there are high levels of FLT3, but the pFLT3 drops significantly with exposure to the plasma,” he said, noting that the effect was most prominent during the first two cycles of therapy.
The patients with the highest levels of inhibition had the greatest likelihood of RFS, whereas RFS in those with suboptimal pFLT3 inhibition was similar to that seen in the SOC-only arm, Dr. Maziarz said. Two patients in the midostaurin group who relapsed did so after 12 months – when midostaurin had been discontinued, he noted.
“Our conclusion is that maintenance midostaurin may contribute to a reduction in relapse risk at 18 months post transplant ... and can be safely administered in the posttransplant setting,” Dr. Maziarz said. “pFLT3 inhibition to less than 70% of baseline, at least in this study, was associated with improved relapse-free survival and overall survival, and it was achieved in more than 50% of patients on the midostaurin.”
It is likely that a more definitive answer will be provided by the Blood and Marrow Transplant Clinical Trials Network Protocol 1506, a large, multinational, placebo-controlled trial now recruiting to look at this question of whether maintenance therapy in the posttransplant setting will improve outcomes.
However, it is important to note that no patient in the RADIUS trial received pretransplant midostaurin, as RADIUS was conducted at the same time as the RATIFY trial.
“Patients today who will go to transplant with FLT3-ITD, the vast majority will have been treated during induction ... and we may have a totally different biology going forward,” he said.
Dr. Maziarz reported financial relationships with Incyte, Novartis, Celgene/Juno, Kite/Gilead, Juno Therapeutics, Kite Therapeutics, and Athersys.
HOUSTON – Midostaurin maintenance therapy along with standard-of-care treatment after allogeneic stem cell transplant (alloSCT) in patients with acute myeloid leukemia (AML) appears to reduce the risk of relapse, according to findings from the randomized, phase 2 RADIUS trial.
Notably, the effect of midostaurin in this open-label, exploratory trial was most pronounced in patients with high levels of phosphorylated FLT3 (pFLT3) inhibition as assessed by plasma inhibitor activity assay, Richard T. Maziarz, MD, reported at the Transplantation & Cellular Therapy Meetings.
“The median [pFLT3 reduction] was less than 70% ... those patients who had the deepest level inhibition maintained the highest likelihood of staying free of disease,” Dr. Maziarz, a professor of medicine at Oregon Health & Science University, Portland, said at the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research. At its meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: American Society for Transplantation and Cellular Therapy (ASTCT).
Midostaurin is a multitargeted tyrosine kinase inhibitor (TKI) that was shown in the pivotal RATIFY trial to significantly improve event-free and overall survival versus placebo when interspersed with induction and consolidation chemotherapy and also when used for maintenance in adults with newly diagnosed FLT3-mutated AML, Dr. Maziarz explained. He noted that patients in the RATIFY study who underwent alloSCT did not receive midostaurin maintenance (N Engl J Med. 2017; 377:454-64).
Although alloSCT provides the greatest likelihood of sustained remission in AML, relapse rates remain high at 30%-59%, he said, adding that, “in the setting of transplantation, FLT3 expression, or FLT3-ITD [internal tandem duplication] ... is a poor risk feature.”
Studies are increasingly suggesting that posttransplant maintenance therapy may improve this outcome. For example, the small, randomized, phase 2 SORMAIN study presented at the 2018 annual meeting of the American Society of Hematology showed a signal for benefit with posttransplant maintenance with the TKI sorafenib. Data regarding midostaurin in this setting are limited, Dr. Maziarz noted.
The RADIUS trial was a small study designed to look for a similar signal with midostaurin and thus was not adequately powered to detect a statistical difference between the arms, he explained.
RADIUS included 60 AML patients aged 18-70 years who underwent myeloablative alloSCT and were in their first complete remission. The primary endpoint was relapse-free survival (RFS) at 18 months after transplant. Results were presented at ASH 2018.
RFS was 89% in 16 of 30 patients who were randomized to receive 50 mg of midostaurin twice daily along with standard-of-care (SOC) treatment and completed 12 4-week cycles. This compared with an RFS rate of 76% in 14 of 30 patients who received SOC only and completed 12 cycles (hazard ratio, 0.46).
The predicted relative reduction in the risk of relapse with the addition of midostaurin was 54%, and at 24 months, both RFS and overall survival were 85% in the midostaurin group and 76% in the SOC-only group, Dr. Maziarz reported.
The median duration of exposure to midostaurin was 10.5 months and the median dose intensity was 93 mg/day, indicating that full-dose therapy was achievable in most patients who stayed on the study.
Treatment was generally well tolerated; there was a comparable number of early discontinuations in the midostaurin and SOC-only arms. The discontinuations were caused mainly by adverse events (typically gastrointestinal toxicities) in the midostaurin arm and by consent withdrawal in the SOC-only arm, he said, adding that there were no significant differences between the groups with respect to serious adverse events or acute or chronic graft-versus-host disease.
Following the presentation of the primary RADIUS results at ASH 2018, an exploratory analysis was conducted to assess midostaurin’s inhibitory effects on FLT3 in plasma.
FLT3 plasma inhibitor activity, assessed by coculturing plasma samples taken on the first day of the treatment cycles with the FLT3-positive AML to look for a reduction in pFLT3, was evaluable in 28 patients in each arm.
“What we see is when you start there are high levels of FLT3, but the pFLT3 drops significantly with exposure to the plasma,” he said, noting that the effect was most prominent during the first two cycles of therapy.
The patients with the highest levels of inhibition had the greatest likelihood of RFS, whereas RFS in those with suboptimal pFLT3 inhibition was similar to that seen in the SOC-only arm, Dr. Maziarz said. Two patients in the midostaurin group who relapsed did so after 12 months – when midostaurin had been discontinued, he noted.
“Our conclusion is that maintenance midostaurin may contribute to a reduction in relapse risk at 18 months post transplant ... and can be safely administered in the posttransplant setting,” Dr. Maziarz said. “pFLT3 inhibition to less than 70% of baseline, at least in this study, was associated with improved relapse-free survival and overall survival, and it was achieved in more than 50% of patients on the midostaurin.”
It is likely that a more definitive answer will be provided by the Blood and Marrow Transplant Clinical Trials Network Protocol 1506, a large, multinational, placebo-controlled trial now recruiting to look at this question of whether maintenance therapy in the posttransplant setting will improve outcomes.
However, it is important to note that no patient in the RADIUS trial received pretransplant midostaurin, as RADIUS was conducted at the same time as the RATIFY trial.
“Patients today who will go to transplant with FLT3-ITD, the vast majority will have been treated during induction ... and we may have a totally different biology going forward,” he said.
Dr. Maziarz reported financial relationships with Incyte, Novartis, Celgene/Juno, Kite/Gilead, Juno Therapeutics, Kite Therapeutics, and Athersys.
HOUSTON – Midostaurin maintenance therapy along with standard-of-care treatment after allogeneic stem cell transplant (alloSCT) in patients with acute myeloid leukemia (AML) appears to reduce the risk of relapse, according to findings from the randomized, phase 2 RADIUS trial.
Notably, the effect of midostaurin in this open-label, exploratory trial was most pronounced in patients with high levels of phosphorylated FLT3 (pFLT3) inhibition as assessed by plasma inhibitor activity assay, Richard T. Maziarz, MD, reported at the Transplantation & Cellular Therapy Meetings.
“The median [pFLT3 reduction] was less than 70% ... those patients who had the deepest level inhibition maintained the highest likelihood of staying free of disease,” Dr. Maziarz, a professor of medicine at Oregon Health & Science University, Portland, said at the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research. At its meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: American Society for Transplantation and Cellular Therapy (ASTCT).
Midostaurin is a multitargeted tyrosine kinase inhibitor (TKI) that was shown in the pivotal RATIFY trial to significantly improve event-free and overall survival versus placebo when interspersed with induction and consolidation chemotherapy and also when used for maintenance in adults with newly diagnosed FLT3-mutated AML, Dr. Maziarz explained. He noted that patients in the RATIFY study who underwent alloSCT did not receive midostaurin maintenance (N Engl J Med. 2017; 377:454-64).
Although alloSCT provides the greatest likelihood of sustained remission in AML, relapse rates remain high at 30%-59%, he said, adding that, “in the setting of transplantation, FLT3 expression, or FLT3-ITD [internal tandem duplication] ... is a poor risk feature.”
Studies are increasingly suggesting that posttransplant maintenance therapy may improve this outcome. For example, the small, randomized, phase 2 SORMAIN study presented at the 2018 annual meeting of the American Society of Hematology showed a signal for benefit with posttransplant maintenance with the TKI sorafenib. Data regarding midostaurin in this setting are limited, Dr. Maziarz noted.
The RADIUS trial was a small study designed to look for a similar signal with midostaurin and thus was not adequately powered to detect a statistical difference between the arms, he explained.
RADIUS included 60 AML patients aged 18-70 years who underwent myeloablative alloSCT and were in their first complete remission. The primary endpoint was relapse-free survival (RFS) at 18 months after transplant. Results were presented at ASH 2018.
RFS was 89% in 16 of 30 patients who were randomized to receive 50 mg of midostaurin twice daily along with standard-of-care (SOC) treatment and completed 12 4-week cycles. This compared with an RFS rate of 76% in 14 of 30 patients who received SOC only and completed 12 cycles (hazard ratio, 0.46).
The predicted relative reduction in the risk of relapse with the addition of midostaurin was 54%, and at 24 months, both RFS and overall survival were 85% in the midostaurin group and 76% in the SOC-only group, Dr. Maziarz reported.
The median duration of exposure to midostaurin was 10.5 months and the median dose intensity was 93 mg/day, indicating that full-dose therapy was achievable in most patients who stayed on the study.
Treatment was generally well tolerated; there was a comparable number of early discontinuations in the midostaurin and SOC-only arms. The discontinuations were caused mainly by adverse events (typically gastrointestinal toxicities) in the midostaurin arm and by consent withdrawal in the SOC-only arm, he said, adding that there were no significant differences between the groups with respect to serious adverse events or acute or chronic graft-versus-host disease.
Following the presentation of the primary RADIUS results at ASH 2018, an exploratory analysis was conducted to assess midostaurin’s inhibitory effects on FLT3 in plasma.
FLT3 plasma inhibitor activity, assessed by coculturing plasma samples taken on the first day of the treatment cycles with the FLT3-positive AML to look for a reduction in pFLT3, was evaluable in 28 patients in each arm.
“What we see is when you start there are high levels of FLT3, but the pFLT3 drops significantly with exposure to the plasma,” he said, noting that the effect was most prominent during the first two cycles of therapy.
The patients with the highest levels of inhibition had the greatest likelihood of RFS, whereas RFS in those with suboptimal pFLT3 inhibition was similar to that seen in the SOC-only arm, Dr. Maziarz said. Two patients in the midostaurin group who relapsed did so after 12 months – when midostaurin had been discontinued, he noted.
“Our conclusion is that maintenance midostaurin may contribute to a reduction in relapse risk at 18 months post transplant ... and can be safely administered in the posttransplant setting,” Dr. Maziarz said. “pFLT3 inhibition to less than 70% of baseline, at least in this study, was associated with improved relapse-free survival and overall survival, and it was achieved in more than 50% of patients on the midostaurin.”
It is likely that a more definitive answer will be provided by the Blood and Marrow Transplant Clinical Trials Network Protocol 1506, a large, multinational, placebo-controlled trial now recruiting to look at this question of whether maintenance therapy in the posttransplant setting will improve outcomes.
However, it is important to note that no patient in the RADIUS trial received pretransplant midostaurin, as RADIUS was conducted at the same time as the RATIFY trial.
“Patients today who will go to transplant with FLT3-ITD, the vast majority will have been treated during induction ... and we may have a totally different biology going forward,” he said.
Dr. Maziarz reported financial relationships with Incyte, Novartis, Celgene/Juno, Kite/Gilead, Juno Therapeutics, Kite Therapeutics, and Athersys.
REPORTING FROM TCT 2019
AlloBMT should be option for older adults with hematologic malignancies
HOUSTON – Allogeneic blood and marrow transplantation (alloBMT) with posttransplant cyclophosphamide (PTCy) is relatively safe and feasible in septuagenarians with hematologic malignancies and should be considered in this population, findings from a review of 108 cases suggest.
The main difference in outcomes in older versus younger patients is a higher – but still low – rate of nonrelapse mortality (NRM) that appears to be due in part to age-related causes, Philip Hollingsworth Imus, MD, of Johns Hopkins University and the Sidney Kimmel Comprehensive Cancer Center, Baltimore, reported at the Transplantation & Cellular Therapy Meetings.
Overall survival (OS) among the 108 consecutive patients over age 70 years who underwent alloBMT at Johns Hopkins from Jan. 1, 2009, through March 31, 2018, was 64% at 1 year and 43% at 3 years, and progression-free survival (PFS) was 50% at 1 year and 32% at 3 years, Dr. Imus said at the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research. At its meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: American Society for Transplantation and Cellular Therapy (ASTCT).
NRM, especially later NRM, however, seemed to be increased, he said, noting that 6-month NRM was acceptable at 14%, but at 1 and 3 years it was 20% and 29%, respectively.
“In contrast to younger patients, [hematopoietic cell transplantation–specific comorbidity index] did not seem to predict NRM in our cohort,” he said.
Early causes of NRM were “in keeping with what we typically see,” and included pneumonia, sepsis, and a few cases of cytokine release syndrome, but later causes of NRM included some that are commonly seen in older patients without hematologic malignancies, such as secondary malignancies, dementia, falls, and cerebrovascular accidents, he said.
Based on frailty research suggesting that weight loss and gain may contribute to outcomes in older patients, Dr. Imus and his colleagues also performed a landmark analysis looking at weight change at 6 months versus pretreatment weight, and found that OS was 31 months in those with greater than the median loss of 4.4 kg, compared with 79 months in those who maintained or regained weight and who therefore had less than the median weight loss at 6 months.
The patients in this series had a median age of 72 years, and the refined disease risk index was low in 9% of patients, intermediate in 77%, and high or very high in 13%. All received nonmyeloablative (NMA) conditioning, PTCy and mycophenolate mofetil prophylaxis from day 5 to day 35, and either tacrolimus and sirolimus from day 5 to day 60-180.
The graft source was bone marrow in 75% of patients.
Engraftment in this population was acceptable and similar to that seen in younger patients; there were seven graft failures, with most occurring in patients who received bone marrow grafts, Dr. Imus said.
The incidence of severe, acute, and chronic graft-versus-host disease was about 10% for each, and was also similar to what is seen in younger patients, he noted.
The findings are of interest because many hematologic malignancies in septuagenarians are associated with very poor survival in the absence of alloBMT. It has only been in recent years that advances in NMA conditioning and haploidentical donor use have made alloBMT more available to older patients, he explained.
With increasing numbers of patients over age 70 years being offered the therapy – about 10% of adult alloBMT recipients at Johns Hopkins are over age 70 now – it was of interest to look at these outcomes, he said, adding that the findings demonstrate that hematologic malignancies in older patients are curable with alloBMT.
“Patients should not be denied therapy based on age alone,” Dr. Imus said, noting that in an effort to address the finding of increased graft failures in those receiving bone marrow grafts at Johns Hopkins, peripheral blood is now being used in certain cases.
“Nonrelapse mortality continues to be a major challenge in this group. It rivals relapse for poor outcomes, especially for late nonrelapse mortality,” he said, concluding that prospective studies looking at NRM are warranted.
Dr. Imus reported having no financial disclosures.
SOURCE: Imus P et al. TCT 2019, Abstract 42.
HOUSTON – Allogeneic blood and marrow transplantation (alloBMT) with posttransplant cyclophosphamide (PTCy) is relatively safe and feasible in septuagenarians with hematologic malignancies and should be considered in this population, findings from a review of 108 cases suggest.
The main difference in outcomes in older versus younger patients is a higher – but still low – rate of nonrelapse mortality (NRM) that appears to be due in part to age-related causes, Philip Hollingsworth Imus, MD, of Johns Hopkins University and the Sidney Kimmel Comprehensive Cancer Center, Baltimore, reported at the Transplantation & Cellular Therapy Meetings.
Overall survival (OS) among the 108 consecutive patients over age 70 years who underwent alloBMT at Johns Hopkins from Jan. 1, 2009, through March 31, 2018, was 64% at 1 year and 43% at 3 years, and progression-free survival (PFS) was 50% at 1 year and 32% at 3 years, Dr. Imus said at the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research. At its meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: American Society for Transplantation and Cellular Therapy (ASTCT).
NRM, especially later NRM, however, seemed to be increased, he said, noting that 6-month NRM was acceptable at 14%, but at 1 and 3 years it was 20% and 29%, respectively.
“In contrast to younger patients, [hematopoietic cell transplantation–specific comorbidity index] did not seem to predict NRM in our cohort,” he said.
Early causes of NRM were “in keeping with what we typically see,” and included pneumonia, sepsis, and a few cases of cytokine release syndrome, but later causes of NRM included some that are commonly seen in older patients without hematologic malignancies, such as secondary malignancies, dementia, falls, and cerebrovascular accidents, he said.
Based on frailty research suggesting that weight loss and gain may contribute to outcomes in older patients, Dr. Imus and his colleagues also performed a landmark analysis looking at weight change at 6 months versus pretreatment weight, and found that OS was 31 months in those with greater than the median loss of 4.4 kg, compared with 79 months in those who maintained or regained weight and who therefore had less than the median weight loss at 6 months.
The patients in this series had a median age of 72 years, and the refined disease risk index was low in 9% of patients, intermediate in 77%, and high or very high in 13%. All received nonmyeloablative (NMA) conditioning, PTCy and mycophenolate mofetil prophylaxis from day 5 to day 35, and either tacrolimus and sirolimus from day 5 to day 60-180.
The graft source was bone marrow in 75% of patients.
Engraftment in this population was acceptable and similar to that seen in younger patients; there were seven graft failures, with most occurring in patients who received bone marrow grafts, Dr. Imus said.
The incidence of severe, acute, and chronic graft-versus-host disease was about 10% for each, and was also similar to what is seen in younger patients, he noted.
The findings are of interest because many hematologic malignancies in septuagenarians are associated with very poor survival in the absence of alloBMT. It has only been in recent years that advances in NMA conditioning and haploidentical donor use have made alloBMT more available to older patients, he explained.
With increasing numbers of patients over age 70 years being offered the therapy – about 10% of adult alloBMT recipients at Johns Hopkins are over age 70 now – it was of interest to look at these outcomes, he said, adding that the findings demonstrate that hematologic malignancies in older patients are curable with alloBMT.
“Patients should not be denied therapy based on age alone,” Dr. Imus said, noting that in an effort to address the finding of increased graft failures in those receiving bone marrow grafts at Johns Hopkins, peripheral blood is now being used in certain cases.
“Nonrelapse mortality continues to be a major challenge in this group. It rivals relapse for poor outcomes, especially for late nonrelapse mortality,” he said, concluding that prospective studies looking at NRM are warranted.
Dr. Imus reported having no financial disclosures.
SOURCE: Imus P et al. TCT 2019, Abstract 42.
HOUSTON – Allogeneic blood and marrow transplantation (alloBMT) with posttransplant cyclophosphamide (PTCy) is relatively safe and feasible in septuagenarians with hematologic malignancies and should be considered in this population, findings from a review of 108 cases suggest.
The main difference in outcomes in older versus younger patients is a higher – but still low – rate of nonrelapse mortality (NRM) that appears to be due in part to age-related causes, Philip Hollingsworth Imus, MD, of Johns Hopkins University and the Sidney Kimmel Comprehensive Cancer Center, Baltimore, reported at the Transplantation & Cellular Therapy Meetings.
Overall survival (OS) among the 108 consecutive patients over age 70 years who underwent alloBMT at Johns Hopkins from Jan. 1, 2009, through March 31, 2018, was 64% at 1 year and 43% at 3 years, and progression-free survival (PFS) was 50% at 1 year and 32% at 3 years, Dr. Imus said at the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research. At its meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: American Society for Transplantation and Cellular Therapy (ASTCT).
NRM, especially later NRM, however, seemed to be increased, he said, noting that 6-month NRM was acceptable at 14%, but at 1 and 3 years it was 20% and 29%, respectively.
“In contrast to younger patients, [hematopoietic cell transplantation–specific comorbidity index] did not seem to predict NRM in our cohort,” he said.
Early causes of NRM were “in keeping with what we typically see,” and included pneumonia, sepsis, and a few cases of cytokine release syndrome, but later causes of NRM included some that are commonly seen in older patients without hematologic malignancies, such as secondary malignancies, dementia, falls, and cerebrovascular accidents, he said.
Based on frailty research suggesting that weight loss and gain may contribute to outcomes in older patients, Dr. Imus and his colleagues also performed a landmark analysis looking at weight change at 6 months versus pretreatment weight, and found that OS was 31 months in those with greater than the median loss of 4.4 kg, compared with 79 months in those who maintained or regained weight and who therefore had less than the median weight loss at 6 months.
The patients in this series had a median age of 72 years, and the refined disease risk index was low in 9% of patients, intermediate in 77%, and high or very high in 13%. All received nonmyeloablative (NMA) conditioning, PTCy and mycophenolate mofetil prophylaxis from day 5 to day 35, and either tacrolimus and sirolimus from day 5 to day 60-180.
The graft source was bone marrow in 75% of patients.
Engraftment in this population was acceptable and similar to that seen in younger patients; there were seven graft failures, with most occurring in patients who received bone marrow grafts, Dr. Imus said.
The incidence of severe, acute, and chronic graft-versus-host disease was about 10% for each, and was also similar to what is seen in younger patients, he noted.
The findings are of interest because many hematologic malignancies in septuagenarians are associated with very poor survival in the absence of alloBMT. It has only been in recent years that advances in NMA conditioning and haploidentical donor use have made alloBMT more available to older patients, he explained.
With increasing numbers of patients over age 70 years being offered the therapy – about 10% of adult alloBMT recipients at Johns Hopkins are over age 70 now – it was of interest to look at these outcomes, he said, adding that the findings demonstrate that hematologic malignancies in older patients are curable with alloBMT.
“Patients should not be denied therapy based on age alone,” Dr. Imus said, noting that in an effort to address the finding of increased graft failures in those receiving bone marrow grafts at Johns Hopkins, peripheral blood is now being used in certain cases.
“Nonrelapse mortality continues to be a major challenge in this group. It rivals relapse for poor outcomes, especially for late nonrelapse mortality,” he said, concluding that prospective studies looking at NRM are warranted.
Dr. Imus reported having no financial disclosures.
SOURCE: Imus P et al. TCT 2019, Abstract 42.
REPORTING FROM TCT 2019
Dr. Lisa Christopher-Stine: Polymyositis? It’s more likely something else
“When someone refers you [a patient with suspected] polymyositis, I want you to do a checklist in your head and say, ‘Have I thought about these five things?’ ” Dr. Christopher-Stine, director of the Johns Hopkins Myositis Center, Baltimore, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
The five most common diagnoses in patients labeled as having polymyositis are immune-mediated necrotizing myopathy (IMNM), overlap with other rheumatologic conditions, antisynthetase syndrome, inclusion body myositis (IBM), and muscular dystrophy, she explained.
“You may say, ‘look, it’s all what you call it,’ but I think we need to be a little bit more careful in what we call it,” she said.
IMNM
Patients with IMNM present with clinical symptoms similar to those seen in polymyositis and dermatomyositis – mainly proximal muscle weakness.
However, there are some important differences, both clinically and histologically, Dr. Christopher-Stine said.
“Look for higher [creatine kinase (CK)] levels,” she said. “In the thousands, usually multiple thousands ... like 5,000, 10,000, 2,000 ... that’s when you’re thinking about a necrotizing phenotype before you even look at the biopsy.”
CK levels will usually be under 30,000 U/L in IMNM, she noted, adding that data increasingly suggest that the extensive muscle necrosis in IMNM explains the elevated CK levels versus those seen in other myopathies.
Myalgias also tend to be more prominent in IMNM than in polymyositis.
“These folks hurt,” she said, noting that IMNM patients tend to have more extensive muscle atrophy and functional disability. “Many will be wheelchair bound within 9 months of diagnosis; it’s not subtle.”
The most important tool for making an IMNM diagnosis is muscle biopsy; look for prominent myocyte necrosis and a relative paucity of lymphocytes, she advised.
Overlap
Sometimes patients with polymyositis also have other rheumatologic conditions that shouldn’t be overlooked, therefore “overlap is its own category,” she said.
“In our experience, the most common overlap is scleroderma,” she noted, adding that the scleroderma is often, but not always, subtle, and that there may be overlapping autoantibodies.
Overt sclerodactyly is rarely seen, although a small amount may be present, but significant Raynaud’s phenomenon is common in these patients, and tiny telangiectasias across the neck are a tell-tale sign.
“Why does that matter? It’s not an esoteric argument; those are the folks that go on to have pulmonary hypertension,” she said. “They can have the same [interstitial lung disease] and all of the other internal scleroderma manifestations.”
Think about overlap and “look close phenotypically and with antibodies,” she advised.
There is also “the typical RA seropositive overlap,” she said, but lupus only rarely overlaps with myositis.
“However, the next diagnosis on the list – antisynthetase syndrome – can be a forme fruste where you first see a seronegative RA-like picture, and it’s important to think about that as well,” she said.
Antisynthetase syndrome
In patients referred for polymyositis, it’s also important to evaluate for antisynthetase syndrome, Dr. Christopher-Stine said.
The arthritis seen in the extramuscular phenotype of the syndrome is rarely deforming, but despite what many physicians were taught, “it absolutely can be erosive,” she said.
In fact, 40% of people with this syndrome present with an isolated forme fruste seronegative rheumatoid arthritis, she said.
Roughening and desquamation of the skin on the radial surface of fingers or palms – a sign known as mechanic’s hands – that doesn’t have another identifiable cause suggests this diagnosis in patients with this type of arthritis, as does interstitial lung disease and Raynaud’s phenomenon.
The Raynaud’s can be “fairly significant in the sense that it is bothersome,” but it usually doesn’t lead to ulceration or digital necrosis.
This is different from what is seen with the scleroderma phenotype, she said, adding that “if you’re starting to see gangrene and digital loss, think of something else.”
IBM
IBM is “probably the No. 1 most-missed diagnosis” among patients referred for what is initially believed to be polymyositis, Dr. Christopher-Stine said.
“I used to think that this was missed at entry, that everybody [with IBM] had all of these criteria and that rheumatologists really didn’t understand this phenotype ... but some people morph into this,” she said, explaining that they often start out looking like they have polymyositis with proximal muscle weakness.
“They may even initially respond to steroids. And then they get this phenotype,” she said.
Older men are more likely to present with the phenotype from the beginning; women, in her experience, tend to present with what appears to be polymyositis, and then develop the phenotype over time, she noted.
An IBM diagnosis requires age over 30 years, but most patients are over 50, she said.
“This is the only one of the myopathies that is preferential to men,” she added, noting that it affects men twice as often as it does women.
The syndrome is characterized by proximal strength loss and muscle atrophy. Also, a finding that a patient’s knee extensors are weaker than their hip flexors is “a fantastic bedside sign” differentiating IBM from polymyositis, she said.
That’s not to say IBM patients don’t have hip flexor weakness, but their knee extensors usually are “considerably weaker by a grade strength or more” versus their hip flexors, she explained.
“It’s a very easy bedside test. In typical other myopathies we have this, but the knee extensors aren’t that weak in general, or they’re not as weak as the hip flexors,” she added.
Another sign is distal strength loss, particularly in the forearm and finger flexors.
“I was taught to have them make a fist; don’t have them make a fist,” she said, explaining that this recruits intrinsic muscles which basically allows cheating that may mask weakness.
Instead, ask them to flex just their distal interphalangeal joints by making a claw and using the fingers to pull against your fingers, she suggested.
Mixed myopathic and neuropathic features on electromyography also indicate IBM, she said.
Muscle biopsy may be helpful, but inclusions are seen in less than one-third of IBM patients.
“At times, we have had to biopsy three times to see them at all, and some people never show them, so you have to rely on your clinical acumen if you don’t see them,” she said.
Also, keep in mind that these patients are often labeled as having treatment-resistant polymyositis.
“Please, when somebody refers to you somebody that’s treatment resistant, that may be the case, but I want you to think maybe they’re treatment resistant because they don’t have that disease.”
Muscular dystrophy
Some cases of myositis mimic certain types of muscular dystrophy, Dr. Christopher-Stine said, providing a checklist of muscular dystrophies that can look “clinically completely indistinguishable from a typical inflammatory myopathy,” and should therefore be considered in these patients.
The checklist includes Duchenne’s manifesting carrier, limb girdle muscular dystrophy type 2b, myotonic dystrophy (usually type 2), and facioscapulohumeral muscular dystrophy.
Dr. Christopher-Stine reported having intellectual property interest in a novel Inova Diagnostics autoantibody assay detection for anti-HMGCR. She was also the safety officer for the JBT-101 Trial sponsored by Corbus and funded by the National Institutes of Health.
“When someone refers you [a patient with suspected] polymyositis, I want you to do a checklist in your head and say, ‘Have I thought about these five things?’ ” Dr. Christopher-Stine, director of the Johns Hopkins Myositis Center, Baltimore, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
The five most common diagnoses in patients labeled as having polymyositis are immune-mediated necrotizing myopathy (IMNM), overlap with other rheumatologic conditions, antisynthetase syndrome, inclusion body myositis (IBM), and muscular dystrophy, she explained.
“You may say, ‘look, it’s all what you call it,’ but I think we need to be a little bit more careful in what we call it,” she said.
IMNM
Patients with IMNM present with clinical symptoms similar to those seen in polymyositis and dermatomyositis – mainly proximal muscle weakness.
However, there are some important differences, both clinically and histologically, Dr. Christopher-Stine said.
“Look for higher [creatine kinase (CK)] levels,” she said. “In the thousands, usually multiple thousands ... like 5,000, 10,000, 2,000 ... that’s when you’re thinking about a necrotizing phenotype before you even look at the biopsy.”
CK levels will usually be under 30,000 U/L in IMNM, she noted, adding that data increasingly suggest that the extensive muscle necrosis in IMNM explains the elevated CK levels versus those seen in other myopathies.
Myalgias also tend to be more prominent in IMNM than in polymyositis.
“These folks hurt,” she said, noting that IMNM patients tend to have more extensive muscle atrophy and functional disability. “Many will be wheelchair bound within 9 months of diagnosis; it’s not subtle.”
The most important tool for making an IMNM diagnosis is muscle biopsy; look for prominent myocyte necrosis and a relative paucity of lymphocytes, she advised.
Overlap
Sometimes patients with polymyositis also have other rheumatologic conditions that shouldn’t be overlooked, therefore “overlap is its own category,” she said.
“In our experience, the most common overlap is scleroderma,” she noted, adding that the scleroderma is often, but not always, subtle, and that there may be overlapping autoantibodies.
Overt sclerodactyly is rarely seen, although a small amount may be present, but significant Raynaud’s phenomenon is common in these patients, and tiny telangiectasias across the neck are a tell-tale sign.
“Why does that matter? It’s not an esoteric argument; those are the folks that go on to have pulmonary hypertension,” she said. “They can have the same [interstitial lung disease] and all of the other internal scleroderma manifestations.”
Think about overlap and “look close phenotypically and with antibodies,” she advised.
There is also “the typical RA seropositive overlap,” she said, but lupus only rarely overlaps with myositis.
“However, the next diagnosis on the list – antisynthetase syndrome – can be a forme fruste where you first see a seronegative RA-like picture, and it’s important to think about that as well,” she said.
Antisynthetase syndrome
In patients referred for polymyositis, it’s also important to evaluate for antisynthetase syndrome, Dr. Christopher-Stine said.
The arthritis seen in the extramuscular phenotype of the syndrome is rarely deforming, but despite what many physicians were taught, “it absolutely can be erosive,” she said.
In fact, 40% of people with this syndrome present with an isolated forme fruste seronegative rheumatoid arthritis, she said.
Roughening and desquamation of the skin on the radial surface of fingers or palms – a sign known as mechanic’s hands – that doesn’t have another identifiable cause suggests this diagnosis in patients with this type of arthritis, as does interstitial lung disease and Raynaud’s phenomenon.
The Raynaud’s can be “fairly significant in the sense that it is bothersome,” but it usually doesn’t lead to ulceration or digital necrosis.
This is different from what is seen with the scleroderma phenotype, she said, adding that “if you’re starting to see gangrene and digital loss, think of something else.”
IBM
IBM is “probably the No. 1 most-missed diagnosis” among patients referred for what is initially believed to be polymyositis, Dr. Christopher-Stine said.
“I used to think that this was missed at entry, that everybody [with IBM] had all of these criteria and that rheumatologists really didn’t understand this phenotype ... but some people morph into this,” she said, explaining that they often start out looking like they have polymyositis with proximal muscle weakness.
“They may even initially respond to steroids. And then they get this phenotype,” she said.
Older men are more likely to present with the phenotype from the beginning; women, in her experience, tend to present with what appears to be polymyositis, and then develop the phenotype over time, she noted.
An IBM diagnosis requires age over 30 years, but most patients are over 50, she said.
“This is the only one of the myopathies that is preferential to men,” she added, noting that it affects men twice as often as it does women.
The syndrome is characterized by proximal strength loss and muscle atrophy. Also, a finding that a patient’s knee extensors are weaker than their hip flexors is “a fantastic bedside sign” differentiating IBM from polymyositis, she said.
That’s not to say IBM patients don’t have hip flexor weakness, but their knee extensors usually are “considerably weaker by a grade strength or more” versus their hip flexors, she explained.
“It’s a very easy bedside test. In typical other myopathies we have this, but the knee extensors aren’t that weak in general, or they’re not as weak as the hip flexors,” she added.
Another sign is distal strength loss, particularly in the forearm and finger flexors.
“I was taught to have them make a fist; don’t have them make a fist,” she said, explaining that this recruits intrinsic muscles which basically allows cheating that may mask weakness.
Instead, ask them to flex just their distal interphalangeal joints by making a claw and using the fingers to pull against your fingers, she suggested.
Mixed myopathic and neuropathic features on electromyography also indicate IBM, she said.
Muscle biopsy may be helpful, but inclusions are seen in less than one-third of IBM patients.
“At times, we have had to biopsy three times to see them at all, and some people never show them, so you have to rely on your clinical acumen if you don’t see them,” she said.
Also, keep in mind that these patients are often labeled as having treatment-resistant polymyositis.
“Please, when somebody refers to you somebody that’s treatment resistant, that may be the case, but I want you to think maybe they’re treatment resistant because they don’t have that disease.”
Muscular dystrophy
Some cases of myositis mimic certain types of muscular dystrophy, Dr. Christopher-Stine said, providing a checklist of muscular dystrophies that can look “clinically completely indistinguishable from a typical inflammatory myopathy,” and should therefore be considered in these patients.
The checklist includes Duchenne’s manifesting carrier, limb girdle muscular dystrophy type 2b, myotonic dystrophy (usually type 2), and facioscapulohumeral muscular dystrophy.
Dr. Christopher-Stine reported having intellectual property interest in a novel Inova Diagnostics autoantibody assay detection for anti-HMGCR. She was also the safety officer for the JBT-101 Trial sponsored by Corbus and funded by the National Institutes of Health.
“When someone refers you [a patient with suspected] polymyositis, I want you to do a checklist in your head and say, ‘Have I thought about these five things?’ ” Dr. Christopher-Stine, director of the Johns Hopkins Myositis Center, Baltimore, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
The five most common diagnoses in patients labeled as having polymyositis are immune-mediated necrotizing myopathy (IMNM), overlap with other rheumatologic conditions, antisynthetase syndrome, inclusion body myositis (IBM), and muscular dystrophy, she explained.
“You may say, ‘look, it’s all what you call it,’ but I think we need to be a little bit more careful in what we call it,” she said.
IMNM
Patients with IMNM present with clinical symptoms similar to those seen in polymyositis and dermatomyositis – mainly proximal muscle weakness.
However, there are some important differences, both clinically and histologically, Dr. Christopher-Stine said.
“Look for higher [creatine kinase (CK)] levels,” she said. “In the thousands, usually multiple thousands ... like 5,000, 10,000, 2,000 ... that’s when you’re thinking about a necrotizing phenotype before you even look at the biopsy.”
CK levels will usually be under 30,000 U/L in IMNM, she noted, adding that data increasingly suggest that the extensive muscle necrosis in IMNM explains the elevated CK levels versus those seen in other myopathies.
Myalgias also tend to be more prominent in IMNM than in polymyositis.
“These folks hurt,” she said, noting that IMNM patients tend to have more extensive muscle atrophy and functional disability. “Many will be wheelchair bound within 9 months of diagnosis; it’s not subtle.”
The most important tool for making an IMNM diagnosis is muscle biopsy; look for prominent myocyte necrosis and a relative paucity of lymphocytes, she advised.
Overlap
Sometimes patients with polymyositis also have other rheumatologic conditions that shouldn’t be overlooked, therefore “overlap is its own category,” she said.
“In our experience, the most common overlap is scleroderma,” she noted, adding that the scleroderma is often, but not always, subtle, and that there may be overlapping autoantibodies.
Overt sclerodactyly is rarely seen, although a small amount may be present, but significant Raynaud’s phenomenon is common in these patients, and tiny telangiectasias across the neck are a tell-tale sign.
“Why does that matter? It’s not an esoteric argument; those are the folks that go on to have pulmonary hypertension,” she said. “They can have the same [interstitial lung disease] and all of the other internal scleroderma manifestations.”
Think about overlap and “look close phenotypically and with antibodies,” she advised.
There is also “the typical RA seropositive overlap,” she said, but lupus only rarely overlaps with myositis.
“However, the next diagnosis on the list – antisynthetase syndrome – can be a forme fruste where you first see a seronegative RA-like picture, and it’s important to think about that as well,” she said.
Antisynthetase syndrome
In patients referred for polymyositis, it’s also important to evaluate for antisynthetase syndrome, Dr. Christopher-Stine said.
The arthritis seen in the extramuscular phenotype of the syndrome is rarely deforming, but despite what many physicians were taught, “it absolutely can be erosive,” she said.
In fact, 40% of people with this syndrome present with an isolated forme fruste seronegative rheumatoid arthritis, she said.
Roughening and desquamation of the skin on the radial surface of fingers or palms – a sign known as mechanic’s hands – that doesn’t have another identifiable cause suggests this diagnosis in patients with this type of arthritis, as does interstitial lung disease and Raynaud’s phenomenon.
The Raynaud’s can be “fairly significant in the sense that it is bothersome,” but it usually doesn’t lead to ulceration or digital necrosis.
This is different from what is seen with the scleroderma phenotype, she said, adding that “if you’re starting to see gangrene and digital loss, think of something else.”
IBM
IBM is “probably the No. 1 most-missed diagnosis” among patients referred for what is initially believed to be polymyositis, Dr. Christopher-Stine said.
“I used to think that this was missed at entry, that everybody [with IBM] had all of these criteria and that rheumatologists really didn’t understand this phenotype ... but some people morph into this,” she said, explaining that they often start out looking like they have polymyositis with proximal muscle weakness.
“They may even initially respond to steroids. And then they get this phenotype,” she said.
Older men are more likely to present with the phenotype from the beginning; women, in her experience, tend to present with what appears to be polymyositis, and then develop the phenotype over time, she noted.
An IBM diagnosis requires age over 30 years, but most patients are over 50, she said.
“This is the only one of the myopathies that is preferential to men,” she added, noting that it affects men twice as often as it does women.
The syndrome is characterized by proximal strength loss and muscle atrophy. Also, a finding that a patient’s knee extensors are weaker than their hip flexors is “a fantastic bedside sign” differentiating IBM from polymyositis, she said.
That’s not to say IBM patients don’t have hip flexor weakness, but their knee extensors usually are “considerably weaker by a grade strength or more” versus their hip flexors, she explained.
“It’s a very easy bedside test. In typical other myopathies we have this, but the knee extensors aren’t that weak in general, or they’re not as weak as the hip flexors,” she added.
Another sign is distal strength loss, particularly in the forearm and finger flexors.
“I was taught to have them make a fist; don’t have them make a fist,” she said, explaining that this recruits intrinsic muscles which basically allows cheating that may mask weakness.
Instead, ask them to flex just their distal interphalangeal joints by making a claw and using the fingers to pull against your fingers, she suggested.
Mixed myopathic and neuropathic features on electromyography also indicate IBM, she said.
Muscle biopsy may be helpful, but inclusions are seen in less than one-third of IBM patients.
“At times, we have had to biopsy three times to see them at all, and some people never show them, so you have to rely on your clinical acumen if you don’t see them,” she said.
Also, keep in mind that these patients are often labeled as having treatment-resistant polymyositis.
“Please, when somebody refers to you somebody that’s treatment resistant, that may be the case, but I want you to think maybe they’re treatment resistant because they don’t have that disease.”
Muscular dystrophy
Some cases of myositis mimic certain types of muscular dystrophy, Dr. Christopher-Stine said, providing a checklist of muscular dystrophies that can look “clinically completely indistinguishable from a typical inflammatory myopathy,” and should therefore be considered in these patients.
The checklist includes Duchenne’s manifesting carrier, limb girdle muscular dystrophy type 2b, myotonic dystrophy (usually type 2), and facioscapulohumeral muscular dystrophy.
Dr. Christopher-Stine reported having intellectual property interest in a novel Inova Diagnostics autoantibody assay detection for anti-HMGCR. She was also the safety officer for the JBT-101 Trial sponsored by Corbus and funded by the National Institutes of Health.
EXPERT ANALYSIS FROM THE WINTER RHEUMATOLOGY SYMPOSIUM
Take action to mitigate CVD risk in RA patients
Emerging understanding of the increased incidence of cardiovascular disease in patients with rheumatoid arthritis is providing greater insight regarding mitigation of risk, according to Jon T. Giles, MD.
The mechanisms of increased cardiovascular disease (CVD) risk in rheumatoid arthritis (RA) are multifactorial; in addition to traditional risk factors for CVD, chronically elevated levels of systemic inflammatory cytokines likely play a major role in atherogenesis and myocardial dysfunction in RA patients, and the interactions between those elevated cytokine levels and traditional risk factors also play a likely role, Dr. Giles, a rheumatologist, epidemiologist, and clinical researcher in the division of rheumatology at Columbia University, New York, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
In addition, the relationship between traditional fasting lipids and atherosclerosis is different in RA patients from that in non-RA patients, he said, noting that this has important implications for CVD screening and risk management. The phenotype of CVD in RA based on the current literature is also one involving more coronary atherosclerosis in which the atherosclerotic plaques are more inflamed.
“There’s more myocardial dysfunction,” Dr. Giles said, noting that this dysfunction may be partly mediated by more myocardial fibrosis and possibly active subclinical low-grade myocarditis.
It is possible that traditional CVD risk factors such as smoking and hypertension have a greater impact in RA patients, but it’s likely that most of the differences are related to RA-specific factors such as autoimmunity, inflammation, and genetics, as well as some nontraditional CVD risk factors such as stress, anxiety, and depression that may be increased in RA patients, he noted.
With respect to traditional CVD risk factors, a recent study of more than 5,600 RA patients without CVD who were followed for an average of almost 6 years at 13 centers in Europe and the United States showed that 389 experienced CVD events, and the most common CVD risk factors in those patients were smoking – particularly in men – and hypertension.
“But also, RA characteristics played a big role,” he said.
Disease activity was one of the major risk factors for CVD events in RA patients, and the traditional CVD risk factor of hyperlipidemia, and particularly elevated low-density lipoprotein (LDL) levels, had less influence in RA patients than in the general population. In men it was “a negative predictor” of CVD events, he said (Ann Rheum Dis. 2018;77:48-54).
Prior studies have also shown a “strange relationship” between lipids and CVD risk in RA patients. For example, RA patients with very low LDL cholesterol have been shown to have higher CVD event rates – a phenomenon known as the “lipid paradox” – and it may be related to inflammation, but the mechanism is unclear,” he said.
To further assess whether RA patients with abnormally low LDL cholesterol levels without the use of statin therapy had more atherosclerotic burden, Dr. Giles and his colleagues looked at more than 600 RA patients and more than 1,000 non-RA patients (Arthritis Rheumatol. 2015;67[suppl 10]:Abstract 2127). They found that RA patients did, indeed, have a greater atherosclerotic burden, which was “quite shocking,” he said.
The burden rivaled that seen in patients with LDL levels greater than 160 mg/dL, he noted.
“Interestingly, these patients did not have higher levels of inflammatory markers, and they did not have higher disease activity scores. They looked exactly the same as the rest of the RA patients,” he said, noting that investigation into why the LDL levels in these patients are so low is ongoing.
As for how atherogenic lipoproteins allow for atherosclerosis and atherogenesis to occur in the setting of low LDL in RA patients, it turns out it’s not just the amount, but also the characteristics of the lipoproteins, such as the size and oxidization of the LDL, which change in the context of systemic inflammation, he explained.
Further, during an acute-phase reaction, high-density lipoprotein (HDL) composition changes rapidly from antiatherogenic to proinflammatory.
Extensive evidence shows that endothelial function is diminished in RA, and that RA patients have these and other proatherogenic mechanisms, as well as other elements of immunity that are associated with atherogenesis, including aspects of both innate and adaptive immune function, he said.
Given the emerging understanding of CVD risk in RA, mitigation of that risk is an important consideration. In fact, the European League Against Rheumatism updated its CVD management guidelines in 2015/2016, including a statement that rheumatologists are responsible for CVD risk management in RA patients (Ann Rheum Dis. 2017;76:17-28).
The guidelines are intended for all inflammatory arthritis, and the recommendation with the strongest level of evidence relates to optimization of disease activity. Also recommended are:
- CVD screening every 5 years.
- Use of a 1.5 multiplier for risk scores.
- Secondary screening with imaging for select patients.
- Management of traditional risk factors according to local guidelines.
- Minimization of the use of NSAIDs and corticosteroids.
- Emphasis on lifestyle management.
Importantly, research has suggested that screening for hyperlipidemia is substandard in RA, and that standard risk stratification tools underperform in the setting of RA, he said.
“So my approach, and this is not evidence based yet ... comes down to what [a patient’s] apparent risk is. So if an RA patient is high risk based on your apparent risk prediction, then they are likely high risk and maybe even higher than estimated,” Dr. Giles said. These patients need optimization of their traditional risk factors and their inflammatory factors and should therefore receive a high-intensity statin regardless of lipid levels, he said.
That means atorvastatin at a dose of at least 40 mg or rosuvastatin at a dose of at least 20 mg, he said, adding that some studies have suggested that statins work as well in RA patients as in non-RA patients, and that RA patients with CVD risk do better with a statin than without.
He considers patients who have intermediate risk based on the risk calculation to actually be high risk in most cases, and they, too, need maximal optimization of traditional CVD risk factors and inflammatory factors.
“Consider one-time secondary imaging for all of these patients,” he advised, noting that a coronary calcium score from a chest CT scan is a good option that has low radiation, can be quantified, and is increasingly covered by insurance.
A coronary calcium score of 0 on chest CT is highly reassuring, and a score that is greater than what is expected for age, gender, and/or race can help define the intensity of intervention, he said.
For example, if a patient’s score is 300 but should be 50, that patient should be treated as if he or she has coronary artery disease. Patients with high scores in general – particularly those with scores over 300 – should also be maximally managed, he said.
Patients at low risk based on risk calculations usually are low risk, but some can be high risk, so again, maximal optimization of risk factors is recommended.
Secondary imaging can be considered in some of these patients, and while it’s not entirely clear which are at greatest risk, Dr. Giles said he recommends screening those with treatment-resistant active disease, those with high disease severity, and those with abnormally low LDL.
Dr. Giles is a consultant for Genentech, Lilly, Horizon, Bristol-Myers Squibb, and UCB, and he has received grant support from Pfizer.
Emerging understanding of the increased incidence of cardiovascular disease in patients with rheumatoid arthritis is providing greater insight regarding mitigation of risk, according to Jon T. Giles, MD.
The mechanisms of increased cardiovascular disease (CVD) risk in rheumatoid arthritis (RA) are multifactorial; in addition to traditional risk factors for CVD, chronically elevated levels of systemic inflammatory cytokines likely play a major role in atherogenesis and myocardial dysfunction in RA patients, and the interactions between those elevated cytokine levels and traditional risk factors also play a likely role, Dr. Giles, a rheumatologist, epidemiologist, and clinical researcher in the division of rheumatology at Columbia University, New York, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
In addition, the relationship between traditional fasting lipids and atherosclerosis is different in RA patients from that in non-RA patients, he said, noting that this has important implications for CVD screening and risk management. The phenotype of CVD in RA based on the current literature is also one involving more coronary atherosclerosis in which the atherosclerotic plaques are more inflamed.
“There’s more myocardial dysfunction,” Dr. Giles said, noting that this dysfunction may be partly mediated by more myocardial fibrosis and possibly active subclinical low-grade myocarditis.
It is possible that traditional CVD risk factors such as smoking and hypertension have a greater impact in RA patients, but it’s likely that most of the differences are related to RA-specific factors such as autoimmunity, inflammation, and genetics, as well as some nontraditional CVD risk factors such as stress, anxiety, and depression that may be increased in RA patients, he noted.
With respect to traditional CVD risk factors, a recent study of more than 5,600 RA patients without CVD who were followed for an average of almost 6 years at 13 centers in Europe and the United States showed that 389 experienced CVD events, and the most common CVD risk factors in those patients were smoking – particularly in men – and hypertension.
“But also, RA characteristics played a big role,” he said.
Disease activity was one of the major risk factors for CVD events in RA patients, and the traditional CVD risk factor of hyperlipidemia, and particularly elevated low-density lipoprotein (LDL) levels, had less influence in RA patients than in the general population. In men it was “a negative predictor” of CVD events, he said (Ann Rheum Dis. 2018;77:48-54).
Prior studies have also shown a “strange relationship” between lipids and CVD risk in RA patients. For example, RA patients with very low LDL cholesterol have been shown to have higher CVD event rates – a phenomenon known as the “lipid paradox” – and it may be related to inflammation, but the mechanism is unclear,” he said.
To further assess whether RA patients with abnormally low LDL cholesterol levels without the use of statin therapy had more atherosclerotic burden, Dr. Giles and his colleagues looked at more than 600 RA patients and more than 1,000 non-RA patients (Arthritis Rheumatol. 2015;67[suppl 10]:Abstract 2127). They found that RA patients did, indeed, have a greater atherosclerotic burden, which was “quite shocking,” he said.
The burden rivaled that seen in patients with LDL levels greater than 160 mg/dL, he noted.
“Interestingly, these patients did not have higher levels of inflammatory markers, and they did not have higher disease activity scores. They looked exactly the same as the rest of the RA patients,” he said, noting that investigation into why the LDL levels in these patients are so low is ongoing.
As for how atherogenic lipoproteins allow for atherosclerosis and atherogenesis to occur in the setting of low LDL in RA patients, it turns out it’s not just the amount, but also the characteristics of the lipoproteins, such as the size and oxidization of the LDL, which change in the context of systemic inflammation, he explained.
Further, during an acute-phase reaction, high-density lipoprotein (HDL) composition changes rapidly from antiatherogenic to proinflammatory.
Extensive evidence shows that endothelial function is diminished in RA, and that RA patients have these and other proatherogenic mechanisms, as well as other elements of immunity that are associated with atherogenesis, including aspects of both innate and adaptive immune function, he said.
Given the emerging understanding of CVD risk in RA, mitigation of that risk is an important consideration. In fact, the European League Against Rheumatism updated its CVD management guidelines in 2015/2016, including a statement that rheumatologists are responsible for CVD risk management in RA patients (Ann Rheum Dis. 2017;76:17-28).
The guidelines are intended for all inflammatory arthritis, and the recommendation with the strongest level of evidence relates to optimization of disease activity. Also recommended are:
- CVD screening every 5 years.
- Use of a 1.5 multiplier for risk scores.
- Secondary screening with imaging for select patients.
- Management of traditional risk factors according to local guidelines.
- Minimization of the use of NSAIDs and corticosteroids.
- Emphasis on lifestyle management.
Importantly, research has suggested that screening for hyperlipidemia is substandard in RA, and that standard risk stratification tools underperform in the setting of RA, he said.
“So my approach, and this is not evidence based yet ... comes down to what [a patient’s] apparent risk is. So if an RA patient is high risk based on your apparent risk prediction, then they are likely high risk and maybe even higher than estimated,” Dr. Giles said. These patients need optimization of their traditional risk factors and their inflammatory factors and should therefore receive a high-intensity statin regardless of lipid levels, he said.
That means atorvastatin at a dose of at least 40 mg or rosuvastatin at a dose of at least 20 mg, he said, adding that some studies have suggested that statins work as well in RA patients as in non-RA patients, and that RA patients with CVD risk do better with a statin than without.
He considers patients who have intermediate risk based on the risk calculation to actually be high risk in most cases, and they, too, need maximal optimization of traditional CVD risk factors and inflammatory factors.
“Consider one-time secondary imaging for all of these patients,” he advised, noting that a coronary calcium score from a chest CT scan is a good option that has low radiation, can be quantified, and is increasingly covered by insurance.
A coronary calcium score of 0 on chest CT is highly reassuring, and a score that is greater than what is expected for age, gender, and/or race can help define the intensity of intervention, he said.
For example, if a patient’s score is 300 but should be 50, that patient should be treated as if he or she has coronary artery disease. Patients with high scores in general – particularly those with scores over 300 – should also be maximally managed, he said.
Patients at low risk based on risk calculations usually are low risk, but some can be high risk, so again, maximal optimization of risk factors is recommended.
Secondary imaging can be considered in some of these patients, and while it’s not entirely clear which are at greatest risk, Dr. Giles said he recommends screening those with treatment-resistant active disease, those with high disease severity, and those with abnormally low LDL.
Dr. Giles is a consultant for Genentech, Lilly, Horizon, Bristol-Myers Squibb, and UCB, and he has received grant support from Pfizer.
Emerging understanding of the increased incidence of cardiovascular disease in patients with rheumatoid arthritis is providing greater insight regarding mitigation of risk, according to Jon T. Giles, MD.
The mechanisms of increased cardiovascular disease (CVD) risk in rheumatoid arthritis (RA) are multifactorial; in addition to traditional risk factors for CVD, chronically elevated levels of systemic inflammatory cytokines likely play a major role in atherogenesis and myocardial dysfunction in RA patients, and the interactions between those elevated cytokine levels and traditional risk factors also play a likely role, Dr. Giles, a rheumatologist, epidemiologist, and clinical researcher in the division of rheumatology at Columbia University, New York, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
In addition, the relationship between traditional fasting lipids and atherosclerosis is different in RA patients from that in non-RA patients, he said, noting that this has important implications for CVD screening and risk management. The phenotype of CVD in RA based on the current literature is also one involving more coronary atherosclerosis in which the atherosclerotic plaques are more inflamed.
“There’s more myocardial dysfunction,” Dr. Giles said, noting that this dysfunction may be partly mediated by more myocardial fibrosis and possibly active subclinical low-grade myocarditis.
It is possible that traditional CVD risk factors such as smoking and hypertension have a greater impact in RA patients, but it’s likely that most of the differences are related to RA-specific factors such as autoimmunity, inflammation, and genetics, as well as some nontraditional CVD risk factors such as stress, anxiety, and depression that may be increased in RA patients, he noted.
With respect to traditional CVD risk factors, a recent study of more than 5,600 RA patients without CVD who were followed for an average of almost 6 years at 13 centers in Europe and the United States showed that 389 experienced CVD events, and the most common CVD risk factors in those patients were smoking – particularly in men – and hypertension.
“But also, RA characteristics played a big role,” he said.
Disease activity was one of the major risk factors for CVD events in RA patients, and the traditional CVD risk factor of hyperlipidemia, and particularly elevated low-density lipoprotein (LDL) levels, had less influence in RA patients than in the general population. In men it was “a negative predictor” of CVD events, he said (Ann Rheum Dis. 2018;77:48-54).
Prior studies have also shown a “strange relationship” between lipids and CVD risk in RA patients. For example, RA patients with very low LDL cholesterol have been shown to have higher CVD event rates – a phenomenon known as the “lipid paradox” – and it may be related to inflammation, but the mechanism is unclear,” he said.
To further assess whether RA patients with abnormally low LDL cholesterol levels without the use of statin therapy had more atherosclerotic burden, Dr. Giles and his colleagues looked at more than 600 RA patients and more than 1,000 non-RA patients (Arthritis Rheumatol. 2015;67[suppl 10]:Abstract 2127). They found that RA patients did, indeed, have a greater atherosclerotic burden, which was “quite shocking,” he said.
The burden rivaled that seen in patients with LDL levels greater than 160 mg/dL, he noted.
“Interestingly, these patients did not have higher levels of inflammatory markers, and they did not have higher disease activity scores. They looked exactly the same as the rest of the RA patients,” he said, noting that investigation into why the LDL levels in these patients are so low is ongoing.
As for how atherogenic lipoproteins allow for atherosclerosis and atherogenesis to occur in the setting of low LDL in RA patients, it turns out it’s not just the amount, but also the characteristics of the lipoproteins, such as the size and oxidization of the LDL, which change in the context of systemic inflammation, he explained.
Further, during an acute-phase reaction, high-density lipoprotein (HDL) composition changes rapidly from antiatherogenic to proinflammatory.
Extensive evidence shows that endothelial function is diminished in RA, and that RA patients have these and other proatherogenic mechanisms, as well as other elements of immunity that are associated with atherogenesis, including aspects of both innate and adaptive immune function, he said.
Given the emerging understanding of CVD risk in RA, mitigation of that risk is an important consideration. In fact, the European League Against Rheumatism updated its CVD management guidelines in 2015/2016, including a statement that rheumatologists are responsible for CVD risk management in RA patients (Ann Rheum Dis. 2017;76:17-28).
The guidelines are intended for all inflammatory arthritis, and the recommendation with the strongest level of evidence relates to optimization of disease activity. Also recommended are:
- CVD screening every 5 years.
- Use of a 1.5 multiplier for risk scores.
- Secondary screening with imaging for select patients.
- Management of traditional risk factors according to local guidelines.
- Minimization of the use of NSAIDs and corticosteroids.
- Emphasis on lifestyle management.
Importantly, research has suggested that screening for hyperlipidemia is substandard in RA, and that standard risk stratification tools underperform in the setting of RA, he said.
“So my approach, and this is not evidence based yet ... comes down to what [a patient’s] apparent risk is. So if an RA patient is high risk based on your apparent risk prediction, then they are likely high risk and maybe even higher than estimated,” Dr. Giles said. These patients need optimization of their traditional risk factors and their inflammatory factors and should therefore receive a high-intensity statin regardless of lipid levels, he said.
That means atorvastatin at a dose of at least 40 mg or rosuvastatin at a dose of at least 20 mg, he said, adding that some studies have suggested that statins work as well in RA patients as in non-RA patients, and that RA patients with CVD risk do better with a statin than without.
He considers patients who have intermediate risk based on the risk calculation to actually be high risk in most cases, and they, too, need maximal optimization of traditional CVD risk factors and inflammatory factors.
“Consider one-time secondary imaging for all of these patients,” he advised, noting that a coronary calcium score from a chest CT scan is a good option that has low radiation, can be quantified, and is increasingly covered by insurance.
A coronary calcium score of 0 on chest CT is highly reassuring, and a score that is greater than what is expected for age, gender, and/or race can help define the intensity of intervention, he said.
For example, if a patient’s score is 300 but should be 50, that patient should be treated as if he or she has coronary artery disease. Patients with high scores in general – particularly those with scores over 300 – should also be maximally managed, he said.
Patients at low risk based on risk calculations usually are low risk, but some can be high risk, so again, maximal optimization of risk factors is recommended.
Secondary imaging can be considered in some of these patients, and while it’s not entirely clear which are at greatest risk, Dr. Giles said he recommends screening those with treatment-resistant active disease, those with high disease severity, and those with abnormally low LDL.
Dr. Giles is a consultant for Genentech, Lilly, Horizon, Bristol-Myers Squibb, and UCB, and he has received grant support from Pfizer.
EXPERT ANALYSIS FROM THE WINTER RHEUMATOLOGY SYMPOSIUM
PD-1 blockade plus CD19 CAR T boosts CAR T-cell persistence
SAN DIEGO – Checkpoint inhibition can be used safely and effectively with CD19-directed chimeric antigen receptor (CAR) T-cell therapy in children with relapsed B-cell acute lymphoblastic leukemia (ALL), and it may bolster CAR T-cell effects and persistence, suggest the findings in a series of 14 patients at the Children’s Hospital of Philadelphia.
Combined programmed death-1 (PD-1) blockade and CAR T-cell therapy appeared to have particular benefit in patients with early B-cell recovery and in those with bulky extramedullary disease, Shannon Maude, MD, PhD, reported during a press conference at the annual meeting of the American Society of Hematology.
The patients, aged 4-17 years with heavily pretreated relapsed B-ALL (13 patients) or B lymphoblastic lymphoma (1 patient), were treated with CD19-directed CAR T-cell therapy, including CTL019 in 4 patients and CTL119 in 10 patients, followed by pembrolizumab (in 13 patients) or nivolumab (in 1 patient).
Six patients received the combination therapy because of early B-cell recovery after initial CAR T-cell infusion, four patients had relapsed or refractory (R/R) bulky extramedullary disease, and four patients had failed to respond or relapsed after initial CAR T-cell therapy.
Three of the six with poor persistence of response reestablished B-cell aplasia (a reflection of CAR T-cell function) after reinfusion of the CAR T-cell product followed by infusion with PD-1 blockade, and they have “sustained CR [complete response] with B-cell aplasia, showing continued persistence of their CAR T cells,” said Dr. Maude, an attending physician in the Cancer Center at Children’s Hospital of Philadelphia.
Of the four patients with R/R bulky extramedullary disease, two patients had a partial response and two patients had CR, she said, explaining that it was hypothesized that the “PD-1 checkpoint pathway may be activated through the microenvironment in that extramedullary situation.”
However, all four patients who had partial or no response to initial CAR T-cell therapy progressed after PD-1 administration, she said, noting that “in one patient, this progression was marked by reduced CD19 expression, which was probably the mode of escape from CD19 CAR T cells.”
Prior studies have shown that patients who respond to CAR T-cell therapy have persistence of CD19 CAR T cells, whereas those with loss of CD19 CAR T cells within 6 months of infusion have a higher rate of relapse, Dr. Maude explained.
“Our hypothesis was that T cells, upon activation, may become exhausted through activation of immune checkpoint pathways, that one such pathway – PD-1 – may be involved in early loss of CD19 CAR T cells and therefore that the combination [of CD19 CAR T-cell therapy] with PD-1 checkpoint blockade may improve the function of the CAR T cells and their persistence,” she said.
The combined approach was well tolerated in this study, she said, noting that mild cytokine release syndrome symptoms and fever typical of CAR T-cell proliferative responses were observed in three patients within 2 days of starting pembrolizumab.
Other adverse effects associated with PD-1 inhibition, including acute pancreatitis, hypothyroidism, arthralgias, and urticaria, occurred in one patient each. There were four cases of grade 3-4 cytopenias that were deemed tolerable or reversible upon discontinuation.
“We show that PD-1 checkpoint inhibitors can be safely combined with CD19 CAR T-cell therapy and that this mechanism may be useful to improve CAR T-cell persistence,” Dr. Maude said.
These findings, which showed particular benefit in patients with poor persistence marked by early B-cell recovery and in those with R/R bulky extramedullary disease, should help inform future use of checkpoint inhibitors after CAR T-cell therapy, she added.
Dr. Maude reported financial ties to Novartis.
SOURCE: Li AM et al. ASH 2018, Abstract 556.
SAN DIEGO – Checkpoint inhibition can be used safely and effectively with CD19-directed chimeric antigen receptor (CAR) T-cell therapy in children with relapsed B-cell acute lymphoblastic leukemia (ALL), and it may bolster CAR T-cell effects and persistence, suggest the findings in a series of 14 patients at the Children’s Hospital of Philadelphia.
Combined programmed death-1 (PD-1) blockade and CAR T-cell therapy appeared to have particular benefit in patients with early B-cell recovery and in those with bulky extramedullary disease, Shannon Maude, MD, PhD, reported during a press conference at the annual meeting of the American Society of Hematology.
The patients, aged 4-17 years with heavily pretreated relapsed B-ALL (13 patients) or B lymphoblastic lymphoma (1 patient), were treated with CD19-directed CAR T-cell therapy, including CTL019 in 4 patients and CTL119 in 10 patients, followed by pembrolizumab (in 13 patients) or nivolumab (in 1 patient).
Six patients received the combination therapy because of early B-cell recovery after initial CAR T-cell infusion, four patients had relapsed or refractory (R/R) bulky extramedullary disease, and four patients had failed to respond or relapsed after initial CAR T-cell therapy.
Three of the six with poor persistence of response reestablished B-cell aplasia (a reflection of CAR T-cell function) after reinfusion of the CAR T-cell product followed by infusion with PD-1 blockade, and they have “sustained CR [complete response] with B-cell aplasia, showing continued persistence of their CAR T cells,” said Dr. Maude, an attending physician in the Cancer Center at Children’s Hospital of Philadelphia.
Of the four patients with R/R bulky extramedullary disease, two patients had a partial response and two patients had CR, she said, explaining that it was hypothesized that the “PD-1 checkpoint pathway may be activated through the microenvironment in that extramedullary situation.”
However, all four patients who had partial or no response to initial CAR T-cell therapy progressed after PD-1 administration, she said, noting that “in one patient, this progression was marked by reduced CD19 expression, which was probably the mode of escape from CD19 CAR T cells.”
Prior studies have shown that patients who respond to CAR T-cell therapy have persistence of CD19 CAR T cells, whereas those with loss of CD19 CAR T cells within 6 months of infusion have a higher rate of relapse, Dr. Maude explained.
“Our hypothesis was that T cells, upon activation, may become exhausted through activation of immune checkpoint pathways, that one such pathway – PD-1 – may be involved in early loss of CD19 CAR T cells and therefore that the combination [of CD19 CAR T-cell therapy] with PD-1 checkpoint blockade may improve the function of the CAR T cells and their persistence,” she said.
The combined approach was well tolerated in this study, she said, noting that mild cytokine release syndrome symptoms and fever typical of CAR T-cell proliferative responses were observed in three patients within 2 days of starting pembrolizumab.
Other adverse effects associated with PD-1 inhibition, including acute pancreatitis, hypothyroidism, arthralgias, and urticaria, occurred in one patient each. There were four cases of grade 3-4 cytopenias that were deemed tolerable or reversible upon discontinuation.
“We show that PD-1 checkpoint inhibitors can be safely combined with CD19 CAR T-cell therapy and that this mechanism may be useful to improve CAR T-cell persistence,” Dr. Maude said.
These findings, which showed particular benefit in patients with poor persistence marked by early B-cell recovery and in those with R/R bulky extramedullary disease, should help inform future use of checkpoint inhibitors after CAR T-cell therapy, she added.
Dr. Maude reported financial ties to Novartis.
SOURCE: Li AM et al. ASH 2018, Abstract 556.
SAN DIEGO – Checkpoint inhibition can be used safely and effectively with CD19-directed chimeric antigen receptor (CAR) T-cell therapy in children with relapsed B-cell acute lymphoblastic leukemia (ALL), and it may bolster CAR T-cell effects and persistence, suggest the findings in a series of 14 patients at the Children’s Hospital of Philadelphia.
Combined programmed death-1 (PD-1) blockade and CAR T-cell therapy appeared to have particular benefit in patients with early B-cell recovery and in those with bulky extramedullary disease, Shannon Maude, MD, PhD, reported during a press conference at the annual meeting of the American Society of Hematology.
The patients, aged 4-17 years with heavily pretreated relapsed B-ALL (13 patients) or B lymphoblastic lymphoma (1 patient), were treated with CD19-directed CAR T-cell therapy, including CTL019 in 4 patients and CTL119 in 10 patients, followed by pembrolizumab (in 13 patients) or nivolumab (in 1 patient).
Six patients received the combination therapy because of early B-cell recovery after initial CAR T-cell infusion, four patients had relapsed or refractory (R/R) bulky extramedullary disease, and four patients had failed to respond or relapsed after initial CAR T-cell therapy.
Three of the six with poor persistence of response reestablished B-cell aplasia (a reflection of CAR T-cell function) after reinfusion of the CAR T-cell product followed by infusion with PD-1 blockade, and they have “sustained CR [complete response] with B-cell aplasia, showing continued persistence of their CAR T cells,” said Dr. Maude, an attending physician in the Cancer Center at Children’s Hospital of Philadelphia.
Of the four patients with R/R bulky extramedullary disease, two patients had a partial response and two patients had CR, she said, explaining that it was hypothesized that the “PD-1 checkpoint pathway may be activated through the microenvironment in that extramedullary situation.”
However, all four patients who had partial or no response to initial CAR T-cell therapy progressed after PD-1 administration, she said, noting that “in one patient, this progression was marked by reduced CD19 expression, which was probably the mode of escape from CD19 CAR T cells.”
Prior studies have shown that patients who respond to CAR T-cell therapy have persistence of CD19 CAR T cells, whereas those with loss of CD19 CAR T cells within 6 months of infusion have a higher rate of relapse, Dr. Maude explained.
“Our hypothesis was that T cells, upon activation, may become exhausted through activation of immune checkpoint pathways, that one such pathway – PD-1 – may be involved in early loss of CD19 CAR T cells and therefore that the combination [of CD19 CAR T-cell therapy] with PD-1 checkpoint blockade may improve the function of the CAR T cells and their persistence,” she said.
The combined approach was well tolerated in this study, she said, noting that mild cytokine release syndrome symptoms and fever typical of CAR T-cell proliferative responses were observed in three patients within 2 days of starting pembrolizumab.
Other adverse effects associated with PD-1 inhibition, including acute pancreatitis, hypothyroidism, arthralgias, and urticaria, occurred in one patient each. There were four cases of grade 3-4 cytopenias that were deemed tolerable or reversible upon discontinuation.
“We show that PD-1 checkpoint inhibitors can be safely combined with CD19 CAR T-cell therapy and that this mechanism may be useful to improve CAR T-cell persistence,” Dr. Maude said.
These findings, which showed particular benefit in patients with poor persistence marked by early B-cell recovery and in those with R/R bulky extramedullary disease, should help inform future use of checkpoint inhibitors after CAR T-cell therapy, she added.
Dr. Maude reported financial ties to Novartis.
SOURCE: Li AM et al. ASH 2018, Abstract 556.
REPORTING FROM ASH 2018
Key clinical point: Major finding: Three of six patients with poor CAR T-cell persistence had a complete response after treatment with the combination therapy. Study details: A clinical study of 14 patients with relapsed B-ALL.
Disclosures: Dr. Maude reported financial relationships with Novartis.
Source: Li AM et al. ASH 2018, Abstract 556.