User login
GM-CSF did not increase survival in high-risk melanoma
Neither adjuvant granulocyte macrophage colony-stimulating factor (GM-CSF) nor a peptide vaccination (PV) significantly improved relapse-free or overall survival in patients with high-risk stage III and IV melanoma who had already undergone surgical resection, according to a study published online Sept. 8 in the Journal of Clinical Oncology.
Findings from early clinical trials support the possible benefit of GM-CSF as adjuvant therapy for melanoma, said the authors, led by Dr. David H. Lawson of Winship Cancer Institute, Emory University, Atlanta. Some studies have also shown that combining cytokines, including GM-CSF, with melanoma vaccines yields augmented immunologic responses and clinically significant tumor responses.
However, the improvement in survival in the current study did not reach statistical significance. The median survival for patients who received GM-CSF was 69.6 months, compared to 59.3 months for the patients who received placebo. This represented a 17.4% improvement in patients who received GM-CSF, but it was less than the projected absolute increase of 13.3 months and the relative improvement of 33% required for significance (HR, 0.94; 95% repeated CI, 0.77 to 1.15; stratified log-rank P =.528).
The results for relapse-free survival also did not reach statistical significance. For patients treated with GM-CSF it was 11.4 months (95% CI,9.4 to 14.8 months) compared to 8.8 month in the placebo group (95% CI, 7.5 to 11.2 months), which was an increase of 2.6 months, or 30% (HR, 0.88; 95% CI,0.74 to 1.04; stratified log-rank P = .131).
Just over half of the cohort (53.3%) were HLA-A2–positive patients. This group was randomized to receive PV alone or with GM-CSF, or placebo. The median overall survival was 68.6 months (95% CI, 47.0 to 92.3 months) in patients who received PV and 63.3 months (95% CI,49.2 to 105.0 months) for those who received placebo (HR, 0.93; 95% CI0.71 to1.21; P = .598)
Also in this subgroup, the median relapse-free survival was only 1.7 months longer in patients who received PV than for those who got placebo (11.5 v 9.8 months, for a 17.3% improvement). This was also lower than what was expected (3 months; 33% improvement), and also did not reach statistical significance.
Even though this study did not support their hypotheses, “trials that test GM-CSF in patients with resected visceral melanoma metastases are worthy of consideration,” wrote the authors. “GM-CSF may find its greatest use in melanoma in combination with other agents” (J Clin Oncol. 2015 Sep 8. doi:10.1200/JCO.2015.62.0500).
The study was supported by grants from the Public Health Service, National Institutes of Health, and the National Cancer Institute. Several of the coauthors report financial relationships with industry.
Neither adjuvant granulocyte macrophage colony-stimulating factor (GM-CSF) nor a peptide vaccination (PV) significantly improved relapse-free or overall survival in patients with high-risk stage III and IV melanoma who had already undergone surgical resection, according to a study published online Sept. 8 in the Journal of Clinical Oncology.
Findings from early clinical trials support the possible benefit of GM-CSF as adjuvant therapy for melanoma, said the authors, led by Dr. David H. Lawson of Winship Cancer Institute, Emory University, Atlanta. Some studies have also shown that combining cytokines, including GM-CSF, with melanoma vaccines yields augmented immunologic responses and clinically significant tumor responses.
However, the improvement in survival in the current study did not reach statistical significance. The median survival for patients who received GM-CSF was 69.6 months, compared to 59.3 months for the patients who received placebo. This represented a 17.4% improvement in patients who received GM-CSF, but it was less than the projected absolute increase of 13.3 months and the relative improvement of 33% required for significance (HR, 0.94; 95% repeated CI, 0.77 to 1.15; stratified log-rank P =.528).
The results for relapse-free survival also did not reach statistical significance. For patients treated with GM-CSF it was 11.4 months (95% CI,9.4 to 14.8 months) compared to 8.8 month in the placebo group (95% CI, 7.5 to 11.2 months), which was an increase of 2.6 months, or 30% (HR, 0.88; 95% CI,0.74 to 1.04; stratified log-rank P = .131).
Just over half of the cohort (53.3%) were HLA-A2–positive patients. This group was randomized to receive PV alone or with GM-CSF, or placebo. The median overall survival was 68.6 months (95% CI, 47.0 to 92.3 months) in patients who received PV and 63.3 months (95% CI,49.2 to 105.0 months) for those who received placebo (HR, 0.93; 95% CI0.71 to1.21; P = .598)
Also in this subgroup, the median relapse-free survival was only 1.7 months longer in patients who received PV than for those who got placebo (11.5 v 9.8 months, for a 17.3% improvement). This was also lower than what was expected (3 months; 33% improvement), and also did not reach statistical significance.
Even though this study did not support their hypotheses, “trials that test GM-CSF in patients with resected visceral melanoma metastases are worthy of consideration,” wrote the authors. “GM-CSF may find its greatest use in melanoma in combination with other agents” (J Clin Oncol. 2015 Sep 8. doi:10.1200/JCO.2015.62.0500).
The study was supported by grants from the Public Health Service, National Institutes of Health, and the National Cancer Institute. Several of the coauthors report financial relationships with industry.
Neither adjuvant granulocyte macrophage colony-stimulating factor (GM-CSF) nor a peptide vaccination (PV) significantly improved relapse-free or overall survival in patients with high-risk stage III and IV melanoma who had already undergone surgical resection, according to a study published online Sept. 8 in the Journal of Clinical Oncology.
Findings from early clinical trials support the possible benefit of GM-CSF as adjuvant therapy for melanoma, said the authors, led by Dr. David H. Lawson of Winship Cancer Institute, Emory University, Atlanta. Some studies have also shown that combining cytokines, including GM-CSF, with melanoma vaccines yields augmented immunologic responses and clinically significant tumor responses.
However, the improvement in survival in the current study did not reach statistical significance. The median survival for patients who received GM-CSF was 69.6 months, compared to 59.3 months for the patients who received placebo. This represented a 17.4% improvement in patients who received GM-CSF, but it was less than the projected absolute increase of 13.3 months and the relative improvement of 33% required for significance (HR, 0.94; 95% repeated CI, 0.77 to 1.15; stratified log-rank P =.528).
The results for relapse-free survival also did not reach statistical significance. For patients treated with GM-CSF it was 11.4 months (95% CI,9.4 to 14.8 months) compared to 8.8 month in the placebo group (95% CI, 7.5 to 11.2 months), which was an increase of 2.6 months, or 30% (HR, 0.88; 95% CI,0.74 to 1.04; stratified log-rank P = .131).
Just over half of the cohort (53.3%) were HLA-A2–positive patients. This group was randomized to receive PV alone or with GM-CSF, or placebo. The median overall survival was 68.6 months (95% CI, 47.0 to 92.3 months) in patients who received PV and 63.3 months (95% CI,49.2 to 105.0 months) for those who received placebo (HR, 0.93; 95% CI0.71 to1.21; P = .598)
Also in this subgroup, the median relapse-free survival was only 1.7 months longer in patients who received PV than for those who got placebo (11.5 v 9.8 months, for a 17.3% improvement). This was also lower than what was expected (3 months; 33% improvement), and also did not reach statistical significance.
Even though this study did not support their hypotheses, “trials that test GM-CSF in patients with resected visceral melanoma metastases are worthy of consideration,” wrote the authors. “GM-CSF may find its greatest use in melanoma in combination with other agents” (J Clin Oncol. 2015 Sep 8. doi:10.1200/JCO.2015.62.0500).
The study was supported by grants from the Public Health Service, National Institutes of Health, and the National Cancer Institute. Several of the coauthors report financial relationships with industry.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Adjuvant granulocyte macrophage colony-stimulating factor and peptide vaccination did not significantly improve relapse-free or overall survival in patients with high-risk resected melanoma.
Major finding: The median overall survival with GM-CSF versus placebo treatments was 69.6 months versus 59.3 months, and relapse-free survival was 11.4 months versus 8.8 months.
Data source: A study of 815 patients with high-risk resected melanoma who were randomly assigned to receive GM-CSF, PV, both, or placebo.
Disclosures: The study was supported by grants from the Public Health Service, National Institutes of Health, and the National Cancer Institute. Several of the coauthors report financial relationships with industry.
Early results encouraging for nivolumab in ovarian cancer patients
Nivolumab demonstrated encouraging clinical efficacy and tolerability in patients with platinum-resistant ovarian cancer in a small phase II trial, according to a study published online Sept. 7 in the Journal of Clinical Oncology.
The safety and antitumor efficacy of nivolumab, a fully human immunoglobulin G4 anti–PD-1 receptor–blocking monoclonal antibody, was evaluated in 20 patients with platinum-resistant, recurrent, or advanced ovarian cancer, reported Dr. Junzo Hamanishi of Kyoto of University Graduate School of Medicine, Kyoto, Japan, and his colleagues. They noted that to their knowledge, this is the first investigator-initiated, phase II clinical trial to be done in this setting.
The patients were treated with an intravenous infusion of nivolumab every 2 weeks at a dose of either 1 or 3 mg/kg (two 10-patient cohorts), and the primary end point of this study was best overall response.
Patients received up to six cycles (four doses per cycle) of nivolumab treatment or remained on therapy until their disease progressed.
The best overall response rate across both groups was 15% (3 patients; 95% CI, 3.2% to 37.9%) and the disease control rate was 45% (9 patients; 95%CI, 23.1% to 68.5%). Among patients who received 1 mg/kg, there was one partial response and four had stable disease. The objective response rate was 10% (95% CI, 0.3% to 44.5%), while disease control rate was 50% (95% CI, 18.7% to 81.3%).
In the 3-mg/kg cohort, two patients achieved a complete response, and two had stable disease, with a total objective response rate of 20% (95% CI, 2.5% to 55.6%), and a disease control rate was 40% (95% CI, 12.2% to 73.8%).
For both groups, the median progression-free survival was 3.5 months and median overall survival was 20.0 months.
The most common adverse events were increased serum AST, hypothyroidism, lymphocytopenia, decreased serum albumin, fever, increased serum ALT, maculopapular rash, arthralgia, arrhythmia, fatigue, and anemia. Grade 3 or 4 treatment-related adverse events occurred in eight (40%) patients, although the majority of the cohort was able to continue on the regimen (n = 19, 95%). The authors pointed out that the frequencies of arrhythmias and elevated AST were relatively higher in this study than in larger previous studies that assessed nivolumab in other types of solid tumors, but all of these events were grade 1 or 2 and manageable.
“This study indicates the merit of a large-scale investigation to evaluate the priority of nivolumab for platinum-resistant ovarian cancer,” the researchers wrote, adding that they plan to conduct such a study in the future (J Clin Oncol. 2015 Sep 7. doi:10.1200/JCO.2015.62.3397).
Nivolumab demonstrated encouraging clinical efficacy and tolerability in patients with platinum-resistant ovarian cancer in a small phase II trial, according to a study published online Sept. 7 in the Journal of Clinical Oncology.
The safety and antitumor efficacy of nivolumab, a fully human immunoglobulin G4 anti–PD-1 receptor–blocking monoclonal antibody, was evaluated in 20 patients with platinum-resistant, recurrent, or advanced ovarian cancer, reported Dr. Junzo Hamanishi of Kyoto of University Graduate School of Medicine, Kyoto, Japan, and his colleagues. They noted that to their knowledge, this is the first investigator-initiated, phase II clinical trial to be done in this setting.
The patients were treated with an intravenous infusion of nivolumab every 2 weeks at a dose of either 1 or 3 mg/kg (two 10-patient cohorts), and the primary end point of this study was best overall response.
Patients received up to six cycles (four doses per cycle) of nivolumab treatment or remained on therapy until their disease progressed.
The best overall response rate across both groups was 15% (3 patients; 95% CI, 3.2% to 37.9%) and the disease control rate was 45% (9 patients; 95%CI, 23.1% to 68.5%). Among patients who received 1 mg/kg, there was one partial response and four had stable disease. The objective response rate was 10% (95% CI, 0.3% to 44.5%), while disease control rate was 50% (95% CI, 18.7% to 81.3%).
In the 3-mg/kg cohort, two patients achieved a complete response, and two had stable disease, with a total objective response rate of 20% (95% CI, 2.5% to 55.6%), and a disease control rate was 40% (95% CI, 12.2% to 73.8%).
For both groups, the median progression-free survival was 3.5 months and median overall survival was 20.0 months.
The most common adverse events were increased serum AST, hypothyroidism, lymphocytopenia, decreased serum albumin, fever, increased serum ALT, maculopapular rash, arthralgia, arrhythmia, fatigue, and anemia. Grade 3 or 4 treatment-related adverse events occurred in eight (40%) patients, although the majority of the cohort was able to continue on the regimen (n = 19, 95%). The authors pointed out that the frequencies of arrhythmias and elevated AST were relatively higher in this study than in larger previous studies that assessed nivolumab in other types of solid tumors, but all of these events were grade 1 or 2 and manageable.
“This study indicates the merit of a large-scale investigation to evaluate the priority of nivolumab for platinum-resistant ovarian cancer,” the researchers wrote, adding that they plan to conduct such a study in the future (J Clin Oncol. 2015 Sep 7. doi:10.1200/JCO.2015.62.3397).
Nivolumab demonstrated encouraging clinical efficacy and tolerability in patients with platinum-resistant ovarian cancer in a small phase II trial, according to a study published online Sept. 7 in the Journal of Clinical Oncology.
The safety and antitumor efficacy of nivolumab, a fully human immunoglobulin G4 anti–PD-1 receptor–blocking monoclonal antibody, was evaluated in 20 patients with platinum-resistant, recurrent, or advanced ovarian cancer, reported Dr. Junzo Hamanishi of Kyoto of University Graduate School of Medicine, Kyoto, Japan, and his colleagues. They noted that to their knowledge, this is the first investigator-initiated, phase II clinical trial to be done in this setting.
The patients were treated with an intravenous infusion of nivolumab every 2 weeks at a dose of either 1 or 3 mg/kg (two 10-patient cohorts), and the primary end point of this study was best overall response.
Patients received up to six cycles (four doses per cycle) of nivolumab treatment or remained on therapy until their disease progressed.
The best overall response rate across both groups was 15% (3 patients; 95% CI, 3.2% to 37.9%) and the disease control rate was 45% (9 patients; 95%CI, 23.1% to 68.5%). Among patients who received 1 mg/kg, there was one partial response and four had stable disease. The objective response rate was 10% (95% CI, 0.3% to 44.5%), while disease control rate was 50% (95% CI, 18.7% to 81.3%).
In the 3-mg/kg cohort, two patients achieved a complete response, and two had stable disease, with a total objective response rate of 20% (95% CI, 2.5% to 55.6%), and a disease control rate was 40% (95% CI, 12.2% to 73.8%).
For both groups, the median progression-free survival was 3.5 months and median overall survival was 20.0 months.
The most common adverse events were increased serum AST, hypothyroidism, lymphocytopenia, decreased serum albumin, fever, increased serum ALT, maculopapular rash, arthralgia, arrhythmia, fatigue, and anemia. Grade 3 or 4 treatment-related adverse events occurred in eight (40%) patients, although the majority of the cohort was able to continue on the regimen (n = 19, 95%). The authors pointed out that the frequencies of arrhythmias and elevated AST were relatively higher in this study than in larger previous studies that assessed nivolumab in other types of solid tumors, but all of these events were grade 1 or 2 and manageable.
“This study indicates the merit of a large-scale investigation to evaluate the priority of nivolumab for platinum-resistant ovarian cancer,” the researchers wrote, adding that they plan to conduct such a study in the future (J Clin Oncol. 2015 Sep 7. doi:10.1200/JCO.2015.62.3397).
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Nivolumab demonstrated encouraging clinical efficacy and tolerability in patients with platinum-resistant ovarian cancer.
Major finding: The best overall response was 15%, including 2 patients with a durable complete response, and the disease control rate in all 20 patients was 45%.
Data source: A phase II trial of 20 patients with platinum-resistant ovarian cancer who received an intravenous infusion of nivolumab every 2 weeks at a dose of 1 or 3 mg/kg.
Disclosures: The study was supported by a Health and Labour Sciences Research grant, by a grant from the Translational Research Network Program of the Ministry of Education, Culture, Sports, Science and Technology of Japan, and a grant from Kyoto University. Several of the coauthors reported financial relationships with industry.
Obinutuzumab trends better than rituxumab in relapsed indolent lymphoma
Patients with relapsed follicular lymphoma who were treated with obinutuzumab experienced higher response rates than did patients given rituximab with an acceptable safety profile, according to new findings.
However, the difference did not translate into an improvement in progression-free survival, so the clinical value of obinutuzumab in this patient population is still unclear.
The quality of remissions was better with obinutuzumab, with an almost twofold higher complete response/unconfirmed complete response rate (41.9% vs. 22.7%; P = .006),” wrote Dr. Laurie Sehn from the Centre for Lymphoid Cancer, British Columbia Cancer Agency and the University of British Columbia, Vancouver, and her colleagues (J Clin Oncol. 2015 Aug 17. doi:10.1200/JCO.2014.59.2139).
On the basis of an independent review, the best overall response was better in the obinutuzumab arm (P = .04), but the complete response/unconfirmed response rate was not different for the two groups.
The study was published online Aug. 17 in the Journal of Clinical Oncology.
A total of 175 patients with relapsed CD20+ indolent lymphoma were randomized 1:1 to four once-per-week infusions of either obinutuzumab (1,000 mg) or rituximab (375 mg/m2). Those without any evidence of disease progression after completing induction therapy received obinutuzumab or rituximab maintenance therapy every 2 months for up to 2 years.
At the end of induction, the investigator assessed overall response rate was 44.6% in the obinutuzumab arm and 33.3% in the rituximab arm (P = .08); nine patients receiving obinutuzumab (12.2%) and four given rituximab (5.3%) achieved complete response or unconfirmed complete response, but the difference was not significant (P = .07).
Independent review also found the overall response rate to be higher with obinutuzumab vs. rituximab (44.6% vs. 26.7%; P = .01), but with no difference in complete response/unconfirmed complete response rate (5.4 vs. 4.0; P = .34).
Adverse events were similar in each group, and most episodes were grade 1 to 2. Higher rates of infusion-related reactions (74% vs. 51%) and cough (24% vs. 9%) were observed in the obinutuzumab vs. the rituximab arm.
Dr. Sehn receives research funding and honoraria from, and serves in a consulting or advisory role to, Roche/Genentech, the maker of obinutuzumab (Gyzyva) and rituximab (Rituxan). She also receives honoraria from and serves in a consulting or advisory role to Amgen, Janssen, Seattle Genetics, Lundbeck, and Celgene.
Patients with relapsed follicular lymphoma who were treated with obinutuzumab experienced higher response rates than did patients given rituximab with an acceptable safety profile, according to new findings.
However, the difference did not translate into an improvement in progression-free survival, so the clinical value of obinutuzumab in this patient population is still unclear.
The quality of remissions was better with obinutuzumab, with an almost twofold higher complete response/unconfirmed complete response rate (41.9% vs. 22.7%; P = .006),” wrote Dr. Laurie Sehn from the Centre for Lymphoid Cancer, British Columbia Cancer Agency and the University of British Columbia, Vancouver, and her colleagues (J Clin Oncol. 2015 Aug 17. doi:10.1200/JCO.2014.59.2139).
On the basis of an independent review, the best overall response was better in the obinutuzumab arm (P = .04), but the complete response/unconfirmed response rate was not different for the two groups.
The study was published online Aug. 17 in the Journal of Clinical Oncology.
A total of 175 patients with relapsed CD20+ indolent lymphoma were randomized 1:1 to four once-per-week infusions of either obinutuzumab (1,000 mg) or rituximab (375 mg/m2). Those without any evidence of disease progression after completing induction therapy received obinutuzumab or rituximab maintenance therapy every 2 months for up to 2 years.
At the end of induction, the investigator assessed overall response rate was 44.6% in the obinutuzumab arm and 33.3% in the rituximab arm (P = .08); nine patients receiving obinutuzumab (12.2%) and four given rituximab (5.3%) achieved complete response or unconfirmed complete response, but the difference was not significant (P = .07).
Independent review also found the overall response rate to be higher with obinutuzumab vs. rituximab (44.6% vs. 26.7%; P = .01), but with no difference in complete response/unconfirmed complete response rate (5.4 vs. 4.0; P = .34).
Adverse events were similar in each group, and most episodes were grade 1 to 2. Higher rates of infusion-related reactions (74% vs. 51%) and cough (24% vs. 9%) were observed in the obinutuzumab vs. the rituximab arm.
Dr. Sehn receives research funding and honoraria from, and serves in a consulting or advisory role to, Roche/Genentech, the maker of obinutuzumab (Gyzyva) and rituximab (Rituxan). She also receives honoraria from and serves in a consulting or advisory role to Amgen, Janssen, Seattle Genetics, Lundbeck, and Celgene.
Patients with relapsed follicular lymphoma who were treated with obinutuzumab experienced higher response rates than did patients given rituximab with an acceptable safety profile, according to new findings.
However, the difference did not translate into an improvement in progression-free survival, so the clinical value of obinutuzumab in this patient population is still unclear.
The quality of remissions was better with obinutuzumab, with an almost twofold higher complete response/unconfirmed complete response rate (41.9% vs. 22.7%; P = .006),” wrote Dr. Laurie Sehn from the Centre for Lymphoid Cancer, British Columbia Cancer Agency and the University of British Columbia, Vancouver, and her colleagues (J Clin Oncol. 2015 Aug 17. doi:10.1200/JCO.2014.59.2139).
On the basis of an independent review, the best overall response was better in the obinutuzumab arm (P = .04), but the complete response/unconfirmed response rate was not different for the two groups.
The study was published online Aug. 17 in the Journal of Clinical Oncology.
A total of 175 patients with relapsed CD20+ indolent lymphoma were randomized 1:1 to four once-per-week infusions of either obinutuzumab (1,000 mg) or rituximab (375 mg/m2). Those without any evidence of disease progression after completing induction therapy received obinutuzumab or rituximab maintenance therapy every 2 months for up to 2 years.
At the end of induction, the investigator assessed overall response rate was 44.6% in the obinutuzumab arm and 33.3% in the rituximab arm (P = .08); nine patients receiving obinutuzumab (12.2%) and four given rituximab (5.3%) achieved complete response or unconfirmed complete response, but the difference was not significant (P = .07).
Independent review also found the overall response rate to be higher with obinutuzumab vs. rituximab (44.6% vs. 26.7%; P = .01), but with no difference in complete response/unconfirmed complete response rate (5.4 vs. 4.0; P = .34).
Adverse events were similar in each group, and most episodes were grade 1 to 2. Higher rates of infusion-related reactions (74% vs. 51%) and cough (24% vs. 9%) were observed in the obinutuzumab vs. the rituximab arm.
Dr. Sehn receives research funding and honoraria from, and serves in a consulting or advisory role to, Roche/Genentech, the maker of obinutuzumab (Gyzyva) and rituximab (Rituxan). She also receives honoraria from and serves in a consulting or advisory role to Amgen, Janssen, Seattle Genetics, Lundbeck, and Celgene.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Obinutuzumab was associated with a higher overall response rate as compared with rituximab, but obinutuzumab’s clinical benefit in non–Hodgkin lymphoma is still unclear.
Major finding: Among patients with follicular lymphoma (n = 149), overall response rate trended higher for obinutuzumab, compared with rituximab (44.6% vs. 33.3%; P = .08).
Data source: An open-label, multicenter, randomized, phase II study of 175 patients with relapsed CD20+ indolent lymphoma that compared induction with obinutuzumab vs. rituximab.
Disclosures: Dr. Sehn receives research funding and honoraria from, and serves in a consulting or advisory role to Roche/Genentech, the maker of obinutuzumab (Gyzyva) and rituximab (Rituxan). She also receives honoraria from and serves in a consulting or advisory role to Amgen, Janssen, Seattle Genetics, Lundbeck, and Celgene.
CLL patients achieve remission with CAR-modified T-cells
Treatment with chimeric antigen receptor (CAR)-modified T cells targeting CD19 achieved a response in 8 of 14 patients (57%) with advanced chronic lymphocytic leukemia (CLL), of whom 4 experienced a complete remission without relapse, based on the mature results of a small pilot study.
Of these four patients, two have remained free of their disease for up to 4 years after they received treatment. An analysis of blood samples also showed that these modified T cells can multiply and persist in the body for a period of years, the researchers report in a study published Sept. 2 in Science Translational Medicine
“Both patients remain alive and cancer free and just passed the 5-year anniversary of their treatment this summer,” said Dr. David L. Porter, the Jodi Fisher Horowitz Professor in Leukemia Care Excellence and director of blood and marrow transplantation at the University of Pennsylvania’s Abramson Cancer Center in Philadelphia. “A third patient in remission just passed the 3-year anniversary with no signs of leukemia” (Sci Transl Med. 2015;7:303ra139).
The current study indicates the mature results from this trial, which began in the summer of 2010. In 2011, preliminary findings from the first three patients to enroll in the study were published and showed that two of them had experienced a complete response. Their disease currently remains in remission more than 4 years after beginning treatment. The first patient to receive the therapy has been cancer free for 5 years.
In the current trial, 14 patients with relapsed or refractory CLL received at least one infusion of autologous T cells transduced with a CD19-directed CAR (CTL019) lentiviral vector. All of the patients had active disease at the time they received the experimental treatment, and had received a median of 5 previous therapies (range, 1-11). One participant had undergone two previous autologous stem cell transplants and one had progressed on ibrutinib therapy.
In addition to those who achieved a complete remission, four other patients (29%) had partial responses to the therapy with responses that persisted for a median of 7 months. Two died of disease progression at 10 and 27 months after receiving CTL019, and one died from a pulmonary embolism; the remaining patient remains alive after CLL progressed at 13 months, and is receiving other therapies.
Overall, the CTL019 infusions were well tolerated, with grade less than 2 toxicities that included primarily low-grade fevers and chills. The most frequent related events were associated with complications of neutropenia and delayed cytokine release syndrome, which correlated with in vivo CTL019 expansion. There were two cases of tumor lysis syndrome, and one patient died in remission 21 months after T cell infusion, after developing ecthyma gangrenosum after pseudomonas infection at a skin biopsy site.
Six subjects (43%) had no response and all six progressed within 1-9 months (median, 4 months) of CTL019 therapy. “We are working hard to determine why this therapy may be appropriate for some patients and not others, and trying to optimize either treatment conditions or patient-specific factors so that this might be more effective for more patients,” Dr. Porter wrote.
Minimal residual disease was not detectable in patients who achieved a complete response, suggesting that disease eradication may be possible in some patients with advanced CLL. The activity of CTLO19 seemed to be on par with results achieved with allogeneic stem cell transplantation, suggesting that this therapy could possibly cure CLL. But Dr. Porter pointed out that this study was conducted with a small number of patients and for CLL, a relatively short follow-up.
“However, these patients all had heavily pretreated resistant disease,” he said. “Though we do not know if patients are indeed cured, it is certainly our goal to find a cure for CLL and without the toxicities and limitations of allogeneic stem cell transplantation. Indeed, longer follow-up will be needed but we are quite excited about the results to date.”
Dr. Porter said he and his team have ongoing trials in CLL in progress, where they are working on trying to identify the optimal dose of T cells for this approach. Also, “this research has led to expansion of this approach to other B cell malignancies such as acute lymphocytic leukemia.”
Novartis, the Leukemia and Lymphoma Society (Specialized Center of Research Award), and the National Institutes of Health funded the study. The University of Pennsylvania has licensed technologies involved in this trial to Novartis. Some scientists involved in these trials, including Dr. Porter, are inventors of these technologies. As a result of the licensing relationship with Novartis, the University of Pennsylvania receives significant financial benefit, and these inventors have benefited financially and/or may benefit financially in the future.
Treatment with chimeric antigen receptor (CAR)-modified T cells targeting CD19 achieved a response in 8 of 14 patients (57%) with advanced chronic lymphocytic leukemia (CLL), of whom 4 experienced a complete remission without relapse, based on the mature results of a small pilot study.
Of these four patients, two have remained free of their disease for up to 4 years after they received treatment. An analysis of blood samples also showed that these modified T cells can multiply and persist in the body for a period of years, the researchers report in a study published Sept. 2 in Science Translational Medicine
“Both patients remain alive and cancer free and just passed the 5-year anniversary of their treatment this summer,” said Dr. David L. Porter, the Jodi Fisher Horowitz Professor in Leukemia Care Excellence and director of blood and marrow transplantation at the University of Pennsylvania’s Abramson Cancer Center in Philadelphia. “A third patient in remission just passed the 3-year anniversary with no signs of leukemia” (Sci Transl Med. 2015;7:303ra139).
The current study indicates the mature results from this trial, which began in the summer of 2010. In 2011, preliminary findings from the first three patients to enroll in the study were published and showed that two of them had experienced a complete response. Their disease currently remains in remission more than 4 years after beginning treatment. The first patient to receive the therapy has been cancer free for 5 years.
In the current trial, 14 patients with relapsed or refractory CLL received at least one infusion of autologous T cells transduced with a CD19-directed CAR (CTL019) lentiviral vector. All of the patients had active disease at the time they received the experimental treatment, and had received a median of 5 previous therapies (range, 1-11). One participant had undergone two previous autologous stem cell transplants and one had progressed on ibrutinib therapy.
In addition to those who achieved a complete remission, four other patients (29%) had partial responses to the therapy with responses that persisted for a median of 7 months. Two died of disease progression at 10 and 27 months after receiving CTL019, and one died from a pulmonary embolism; the remaining patient remains alive after CLL progressed at 13 months, and is receiving other therapies.
Overall, the CTL019 infusions were well tolerated, with grade less than 2 toxicities that included primarily low-grade fevers and chills. The most frequent related events were associated with complications of neutropenia and delayed cytokine release syndrome, which correlated with in vivo CTL019 expansion. There were two cases of tumor lysis syndrome, and one patient died in remission 21 months after T cell infusion, after developing ecthyma gangrenosum after pseudomonas infection at a skin biopsy site.
Six subjects (43%) had no response and all six progressed within 1-9 months (median, 4 months) of CTL019 therapy. “We are working hard to determine why this therapy may be appropriate for some patients and not others, and trying to optimize either treatment conditions or patient-specific factors so that this might be more effective for more patients,” Dr. Porter wrote.
Minimal residual disease was not detectable in patients who achieved a complete response, suggesting that disease eradication may be possible in some patients with advanced CLL. The activity of CTLO19 seemed to be on par with results achieved with allogeneic stem cell transplantation, suggesting that this therapy could possibly cure CLL. But Dr. Porter pointed out that this study was conducted with a small number of patients and for CLL, a relatively short follow-up.
“However, these patients all had heavily pretreated resistant disease,” he said. “Though we do not know if patients are indeed cured, it is certainly our goal to find a cure for CLL and without the toxicities and limitations of allogeneic stem cell transplantation. Indeed, longer follow-up will be needed but we are quite excited about the results to date.”
Dr. Porter said he and his team have ongoing trials in CLL in progress, where they are working on trying to identify the optimal dose of T cells for this approach. Also, “this research has led to expansion of this approach to other B cell malignancies such as acute lymphocytic leukemia.”
Novartis, the Leukemia and Lymphoma Society (Specialized Center of Research Award), and the National Institutes of Health funded the study. The University of Pennsylvania has licensed technologies involved in this trial to Novartis. Some scientists involved in these trials, including Dr. Porter, are inventors of these technologies. As a result of the licensing relationship with Novartis, the University of Pennsylvania receives significant financial benefit, and these inventors have benefited financially and/or may benefit financially in the future.
Treatment with chimeric antigen receptor (CAR)-modified T cells targeting CD19 achieved a response in 8 of 14 patients (57%) with advanced chronic lymphocytic leukemia (CLL), of whom 4 experienced a complete remission without relapse, based on the mature results of a small pilot study.
Of these four patients, two have remained free of their disease for up to 4 years after they received treatment. An analysis of blood samples also showed that these modified T cells can multiply and persist in the body for a period of years, the researchers report in a study published Sept. 2 in Science Translational Medicine
“Both patients remain alive and cancer free and just passed the 5-year anniversary of their treatment this summer,” said Dr. David L. Porter, the Jodi Fisher Horowitz Professor in Leukemia Care Excellence and director of blood and marrow transplantation at the University of Pennsylvania’s Abramson Cancer Center in Philadelphia. “A third patient in remission just passed the 3-year anniversary with no signs of leukemia” (Sci Transl Med. 2015;7:303ra139).
The current study indicates the mature results from this trial, which began in the summer of 2010. In 2011, preliminary findings from the first three patients to enroll in the study were published and showed that two of them had experienced a complete response. Their disease currently remains in remission more than 4 years after beginning treatment. The first patient to receive the therapy has been cancer free for 5 years.
In the current trial, 14 patients with relapsed or refractory CLL received at least one infusion of autologous T cells transduced with a CD19-directed CAR (CTL019) lentiviral vector. All of the patients had active disease at the time they received the experimental treatment, and had received a median of 5 previous therapies (range, 1-11). One participant had undergone two previous autologous stem cell transplants and one had progressed on ibrutinib therapy.
In addition to those who achieved a complete remission, four other patients (29%) had partial responses to the therapy with responses that persisted for a median of 7 months. Two died of disease progression at 10 and 27 months after receiving CTL019, and one died from a pulmonary embolism; the remaining patient remains alive after CLL progressed at 13 months, and is receiving other therapies.
Overall, the CTL019 infusions were well tolerated, with grade less than 2 toxicities that included primarily low-grade fevers and chills. The most frequent related events were associated with complications of neutropenia and delayed cytokine release syndrome, which correlated with in vivo CTL019 expansion. There were two cases of tumor lysis syndrome, and one patient died in remission 21 months after T cell infusion, after developing ecthyma gangrenosum after pseudomonas infection at a skin biopsy site.
Six subjects (43%) had no response and all six progressed within 1-9 months (median, 4 months) of CTL019 therapy. “We are working hard to determine why this therapy may be appropriate for some patients and not others, and trying to optimize either treatment conditions or patient-specific factors so that this might be more effective for more patients,” Dr. Porter wrote.
Minimal residual disease was not detectable in patients who achieved a complete response, suggesting that disease eradication may be possible in some patients with advanced CLL. The activity of CTLO19 seemed to be on par with results achieved with allogeneic stem cell transplantation, suggesting that this therapy could possibly cure CLL. But Dr. Porter pointed out that this study was conducted with a small number of patients and for CLL, a relatively short follow-up.
“However, these patients all had heavily pretreated resistant disease,” he said. “Though we do not know if patients are indeed cured, it is certainly our goal to find a cure for CLL and without the toxicities and limitations of allogeneic stem cell transplantation. Indeed, longer follow-up will be needed but we are quite excited about the results to date.”
Dr. Porter said he and his team have ongoing trials in CLL in progress, where they are working on trying to identify the optimal dose of T cells for this approach. Also, “this research has led to expansion of this approach to other B cell malignancies such as acute lymphocytic leukemia.”
Novartis, the Leukemia and Lymphoma Society (Specialized Center of Research Award), and the National Institutes of Health funded the study. The University of Pennsylvania has licensed technologies involved in this trial to Novartis. Some scientists involved in these trials, including Dr. Porter, are inventors of these technologies. As a result of the licensing relationship with Novartis, the University of Pennsylvania receives significant financial benefit, and these inventors have benefited financially and/or may benefit financially in the future.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point: CAR-modified T cell therapy lacks the toxicities and limitations of allogeneic stem cell transplantation and may be an effective treatment for chronic lymphocytic leukemia.
Major finding: CAR-modified T cell therapy elicited a response in 8 of 14 patients (57%) with relapsed and refractory chronic lymphocytic leukemia, and 4 patients (29%) achieved a complete remission.
Data source: Mature results from a pilot clinical trial.
Disclosures: Novartis, the Leukemia and Lymphoma Society (Specialized Center of Research Award), and the National Institutes of Health funded the study. The University of Pennsylvania has licensed technologies involved in this trial to Novartis. Some scientists involved in these trials, including Dr. Porter, are inventors of these technologies. As a result of the licensing relationship with Novartis, the University of Pennsylvania receives significant financial benefit, and these inventors have benefited financially and/or may benefit financially in the future.
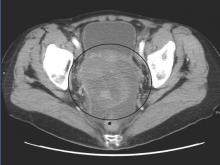
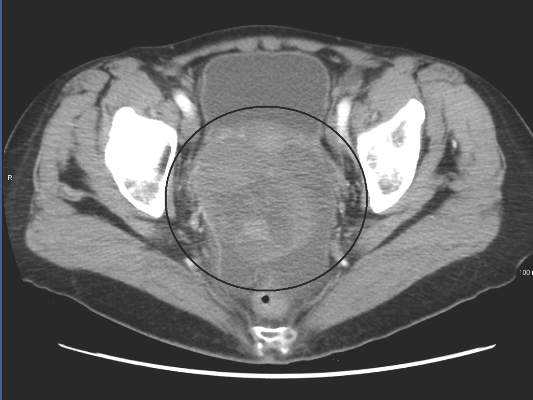
Low-risk prostate cancer: Replace immediate intervention with immediate contemplation
Men with favorable-risk prostate cancer have a very low risk of disease progression to a lethal phenotype, and they should be encouraged to consider surveillance rather than curative intervention, investigators said online in the Journal of Clinical Oncology.
Cancer-specific survival rates were 99.9% at 10 years and again at 15 years, in a single-arm prospective study of 1,298 men with favorable-risk disease offered active surveillance beginning in 1995, Dr. Jeffrey J. Tosoian and his colleagues from Johns Hopkins University School of Medicine and Hospitals in Baltimore reported. The study enrolled 926 men (71%) who met all criteria for very-low-risk cancer and 372 (29%) who met criteria for low-risk disease, they said (Journ Clin Onc 2015 Aug 31 [doi: 10.1200/JCO.2015.62.5764]).
Curative intervention was recommended for disease reclassification when patients no longer met the inclusion criteria following biopsy results.
A total of 38 (3%) men died from other causes before any reclassification or treatment, while 9 succumbed to other causes after receiving prostate cancer treatment. The 47 men who died from causes other than prostate cancer had been receiving active surveillance for an average of 7 years (range, 1-18 years).
There were two deaths (0.15%) from prostate cancer, both in patients with very-low-risk disease. In addition, there were two cases of lymph node metastases and one distant metastasis.
The overall cancer-specific and metastasis-free survival rates were 93%, 99.9%, and 99.4%, respectively, at 10 years and 69%, 99.9%, and 99.4%, respectively, at 15 years.
Disease was reclassified during biopsy in 467 men at a median of 2 years (range, 0.3-16 years) after enrollment. Of this group, 233 involved reclassification of the tumor grade and 234 involved volume reclassification. At 5, 10, and 15 years after active surveillance was initiated, the cumulative incidence of any biopsy reclassification was 35%, 49%, and 56%, respectively, while the cumulative incidence of grade reclassification was 17%, 26%, and 31%, respectively.
This study was begun at a time when there was substantial resistance to monitoring men with prostate cancer, the authors noted, and therefore, “our intents were to demonstrate the safety of this approach for carefully selected men and to identify markers of a lethal phenotype that might lead to wider inclusion in active surveillance.”
“The expansion of active surveillance during the past decade and the sharing of institutional data sets will likely help delineate factors associated with varying outcomes and will allow patients to play a greater role in selecting the strategy that best suits their individual preferences,” wrote Dr. Tosoian and coauthors.
“Our data suggest that, for men with favorable-risk prostate cancer, the paradigm of immediate intervention must be replaced by one of immediate contemplation – a thoughtful assessment of prognostic risk, life expectancy, and the relative risks and benefits of available management options considered in the context of personal preferences,” the authors concluded.
There were no outside funding sources reported. Some coauthors reported consulting or advisory roles with Metamark Genetics, MDxHealth, Dianon Systems, DAKO, Trock, SonaCare Medical, Myriad Genetics, Rochon Genova, Rothwell Figg, and Roche.
In this study, the authors suggest that men with favorable-risk prostate cancer should be informed of the low likelihood of harm from their diagnosis and should be encouraged to consider active surveillance rather than curative intervention. There is no doubt that, for some men diagnosed today with very-low-risk or low-risk prostate cancer this statement is valid; however, to whom specifically, on an individual basis, this statement should apply remains unanswered.
The authors have also provided helpful information about patient- and cancer-related parameters associated with upgrading to Gleason score 7 or greater at surveillance biopsy. These parameters included increasing age, PSA density, and the number of positive cores. However, information about patient factors, including comorbidity, ethnicity, and family history, should also be considered in decisions about when to use active surveillance and in which patients active surveillance will not lead to missing occult high-grade prostate cancer that can that can progress to metastasis during their remaining life expectancy.
Attention should shift away from establishing that long-term rates of metastasis and death as a result of prostate cancer are low for a population of men diagnosed with favorable-risk prostate cancer who are observed on an active surveillance protocol or who are randomly assigned to active surveillance versus intervention. Rather, the focus should be on defining a validated risk-assessment scheme that is based on a panel of cancer and patient factors capable of determining whether an individual man of a given age, health, and ethnicity, and with specific tumor characteristics, family history, and perhaps multiparametric MRI imaging characteristics, is best served by being observed on an active surveillance protocol.
Dr. Anthony V. D’Amico is from Brigham and Women’s Hospital and Dana Farber Cancer Institute, Boston, and has no disclosures. These remarks were taken from the editorial accompanying Dr. Tosoian’s report (Journ Clin Onc 2015 Aug 31. doi: 10.1200/JCO.2015.63.6118)
In this study, the authors suggest that men with favorable-risk prostate cancer should be informed of the low likelihood of harm from their diagnosis and should be encouraged to consider active surveillance rather than curative intervention. There is no doubt that, for some men diagnosed today with very-low-risk or low-risk prostate cancer this statement is valid; however, to whom specifically, on an individual basis, this statement should apply remains unanswered.
The authors have also provided helpful information about patient- and cancer-related parameters associated with upgrading to Gleason score 7 or greater at surveillance biopsy. These parameters included increasing age, PSA density, and the number of positive cores. However, information about patient factors, including comorbidity, ethnicity, and family history, should also be considered in decisions about when to use active surveillance and in which patients active surveillance will not lead to missing occult high-grade prostate cancer that can that can progress to metastasis during their remaining life expectancy.
Attention should shift away from establishing that long-term rates of metastasis and death as a result of prostate cancer are low for a population of men diagnosed with favorable-risk prostate cancer who are observed on an active surveillance protocol or who are randomly assigned to active surveillance versus intervention. Rather, the focus should be on defining a validated risk-assessment scheme that is based on a panel of cancer and patient factors capable of determining whether an individual man of a given age, health, and ethnicity, and with specific tumor characteristics, family history, and perhaps multiparametric MRI imaging characteristics, is best served by being observed on an active surveillance protocol.
Dr. Anthony V. D’Amico is from Brigham and Women’s Hospital and Dana Farber Cancer Institute, Boston, and has no disclosures. These remarks were taken from the editorial accompanying Dr. Tosoian’s report (Journ Clin Onc 2015 Aug 31. doi: 10.1200/JCO.2015.63.6118)
In this study, the authors suggest that men with favorable-risk prostate cancer should be informed of the low likelihood of harm from their diagnosis and should be encouraged to consider active surveillance rather than curative intervention. There is no doubt that, for some men diagnosed today with very-low-risk or low-risk prostate cancer this statement is valid; however, to whom specifically, on an individual basis, this statement should apply remains unanswered.
The authors have also provided helpful information about patient- and cancer-related parameters associated with upgrading to Gleason score 7 or greater at surveillance biopsy. These parameters included increasing age, PSA density, and the number of positive cores. However, information about patient factors, including comorbidity, ethnicity, and family history, should also be considered in decisions about when to use active surveillance and in which patients active surveillance will not lead to missing occult high-grade prostate cancer that can that can progress to metastasis during their remaining life expectancy.
Attention should shift away from establishing that long-term rates of metastasis and death as a result of prostate cancer are low for a population of men diagnosed with favorable-risk prostate cancer who are observed on an active surveillance protocol or who are randomly assigned to active surveillance versus intervention. Rather, the focus should be on defining a validated risk-assessment scheme that is based on a panel of cancer and patient factors capable of determining whether an individual man of a given age, health, and ethnicity, and with specific tumor characteristics, family history, and perhaps multiparametric MRI imaging characteristics, is best served by being observed on an active surveillance protocol.
Dr. Anthony V. D’Amico is from Brigham and Women’s Hospital and Dana Farber Cancer Institute, Boston, and has no disclosures. These remarks were taken from the editorial accompanying Dr. Tosoian’s report (Journ Clin Onc 2015 Aug 31. doi: 10.1200/JCO.2015.63.6118)
Men with favorable-risk prostate cancer have a very low risk of disease progression to a lethal phenotype, and they should be encouraged to consider surveillance rather than curative intervention, investigators said online in the Journal of Clinical Oncology.
Cancer-specific survival rates were 99.9% at 10 years and again at 15 years, in a single-arm prospective study of 1,298 men with favorable-risk disease offered active surveillance beginning in 1995, Dr. Jeffrey J. Tosoian and his colleagues from Johns Hopkins University School of Medicine and Hospitals in Baltimore reported. The study enrolled 926 men (71%) who met all criteria for very-low-risk cancer and 372 (29%) who met criteria for low-risk disease, they said (Journ Clin Onc 2015 Aug 31 [doi: 10.1200/JCO.2015.62.5764]).
Curative intervention was recommended for disease reclassification when patients no longer met the inclusion criteria following biopsy results.
A total of 38 (3%) men died from other causes before any reclassification or treatment, while 9 succumbed to other causes after receiving prostate cancer treatment. The 47 men who died from causes other than prostate cancer had been receiving active surveillance for an average of 7 years (range, 1-18 years).
There were two deaths (0.15%) from prostate cancer, both in patients with very-low-risk disease. In addition, there were two cases of lymph node metastases and one distant metastasis.
The overall cancer-specific and metastasis-free survival rates were 93%, 99.9%, and 99.4%, respectively, at 10 years and 69%, 99.9%, and 99.4%, respectively, at 15 years.
Disease was reclassified during biopsy in 467 men at a median of 2 years (range, 0.3-16 years) after enrollment. Of this group, 233 involved reclassification of the tumor grade and 234 involved volume reclassification. At 5, 10, and 15 years after active surveillance was initiated, the cumulative incidence of any biopsy reclassification was 35%, 49%, and 56%, respectively, while the cumulative incidence of grade reclassification was 17%, 26%, and 31%, respectively.
This study was begun at a time when there was substantial resistance to monitoring men with prostate cancer, the authors noted, and therefore, “our intents were to demonstrate the safety of this approach for carefully selected men and to identify markers of a lethal phenotype that might lead to wider inclusion in active surveillance.”
“The expansion of active surveillance during the past decade and the sharing of institutional data sets will likely help delineate factors associated with varying outcomes and will allow patients to play a greater role in selecting the strategy that best suits their individual preferences,” wrote Dr. Tosoian and coauthors.
“Our data suggest that, for men with favorable-risk prostate cancer, the paradigm of immediate intervention must be replaced by one of immediate contemplation – a thoughtful assessment of prognostic risk, life expectancy, and the relative risks and benefits of available management options considered in the context of personal preferences,” the authors concluded.
There were no outside funding sources reported. Some coauthors reported consulting or advisory roles with Metamark Genetics, MDxHealth, Dianon Systems, DAKO, Trock, SonaCare Medical, Myriad Genetics, Rochon Genova, Rothwell Figg, and Roche.
Men with favorable-risk prostate cancer have a very low risk of disease progression to a lethal phenotype, and they should be encouraged to consider surveillance rather than curative intervention, investigators said online in the Journal of Clinical Oncology.
Cancer-specific survival rates were 99.9% at 10 years and again at 15 years, in a single-arm prospective study of 1,298 men with favorable-risk disease offered active surveillance beginning in 1995, Dr. Jeffrey J. Tosoian and his colleagues from Johns Hopkins University School of Medicine and Hospitals in Baltimore reported. The study enrolled 926 men (71%) who met all criteria for very-low-risk cancer and 372 (29%) who met criteria for low-risk disease, they said (Journ Clin Onc 2015 Aug 31 [doi: 10.1200/JCO.2015.62.5764]).
Curative intervention was recommended for disease reclassification when patients no longer met the inclusion criteria following biopsy results.
A total of 38 (3%) men died from other causes before any reclassification or treatment, while 9 succumbed to other causes after receiving prostate cancer treatment. The 47 men who died from causes other than prostate cancer had been receiving active surveillance for an average of 7 years (range, 1-18 years).
There were two deaths (0.15%) from prostate cancer, both in patients with very-low-risk disease. In addition, there were two cases of lymph node metastases and one distant metastasis.
The overall cancer-specific and metastasis-free survival rates were 93%, 99.9%, and 99.4%, respectively, at 10 years and 69%, 99.9%, and 99.4%, respectively, at 15 years.
Disease was reclassified during biopsy in 467 men at a median of 2 years (range, 0.3-16 years) after enrollment. Of this group, 233 involved reclassification of the tumor grade and 234 involved volume reclassification. At 5, 10, and 15 years after active surveillance was initiated, the cumulative incidence of any biopsy reclassification was 35%, 49%, and 56%, respectively, while the cumulative incidence of grade reclassification was 17%, 26%, and 31%, respectively.
This study was begun at a time when there was substantial resistance to monitoring men with prostate cancer, the authors noted, and therefore, “our intents were to demonstrate the safety of this approach for carefully selected men and to identify markers of a lethal phenotype that might lead to wider inclusion in active surveillance.”
“The expansion of active surveillance during the past decade and the sharing of institutional data sets will likely help delineate factors associated with varying outcomes and will allow patients to play a greater role in selecting the strategy that best suits their individual preferences,” wrote Dr. Tosoian and coauthors.
“Our data suggest that, for men with favorable-risk prostate cancer, the paradigm of immediate intervention must be replaced by one of immediate contemplation – a thoughtful assessment of prognostic risk, life expectancy, and the relative risks and benefits of available management options considered in the context of personal preferences,” the authors concluded.
There were no outside funding sources reported. Some coauthors reported consulting or advisory roles with Metamark Genetics, MDxHealth, Dianon Systems, DAKO, Trock, SonaCare Medical, Myriad Genetics, Rochon Genova, Rothwell Figg, and Roche.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Men with favorable-risk prostate cancer have a low risk of progression to a lethal phenotype and should consider active surveillance.
Major finding: Of 1,298 men with favorable-risk prostate cancer who were enrolled in an active surveillance program overall, cancer-specific, and metastasis-free survival rates were 69%, 99.9%, and 99.4%, respectively, at 15 years.
Data source: A follow-up of a cohort of men with favorable-risk prostate cancer receiving active surveillance at a single institution that used a clearly defined protocol for enrollment, monitoring, and intervention.
Disclosures: There were no outside funding sources reported. Some coauthors reported consulting or advisory roles with Metamark Genetics, MDxHealth, Dianon Systems, DAKO, Trock, SonaCare Medical, Myriad Genetics, Rochon Genova, Rothwell Figg, and Roche.
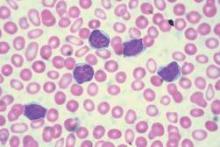
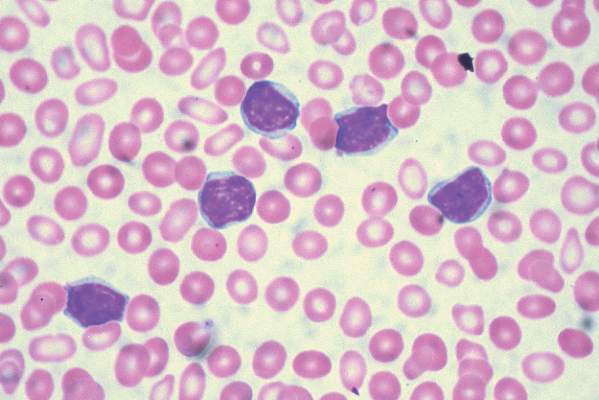
Persistent mutations linked to poorer outcomes in AML
Persistent leukemia-associated mutations that can be detected in at least 5% of bone marrow cells at 30 days after remission were associated with a significantly increased risk of relapse and reduced overall survival in patients with acute myeloid leukemia (AML), in a study published Aug. 25 in JAMA.
About 20% of adult patients with AML fail to achieve remission following standard initial induction chemotherapy, and approximately half of them will subsequently experience a relapse after achieving complete remission. Currently, tests that predict outcomes for these patients are imprecise, especially for those with intermediate-risk disease.
“The data presented in this report begin to define a genomic method for the risk stratification of patients with AML that places greater emphasis on the clearance of somatic mutations after chemotherapy than the identification of specific mutations at the time of presentation,” wrote Dr. Jeffery M. Klco, Washington University, St Louis, and his colleagues. (JAMA. 2015;314[8]:811-22).
Whole-genome or exome sequencing was performed on samples that were obtained at disease presentation from 71 patients with AML who were treated with standard induction chemotherapy in March 2002, with follow-up through January 2015. A subsequent re-analysis was conducted in a cohort of 50 patients, who had available samples from both presentation and documented remission.
Of this group, 24 (48%) had persistent leukemia-associated mutations in at least 5% of bone marrow cells at remission, while 26 patients had cleared all mutations.
The investigators noted that patients with at least one persistent mutation on day 30 had significantly reduced event-free survival compared with those who had cleared all mutations (median, 6.0 months [95% CI, 3.7-9.6] vs 17.9 months [95% CI, 11.3-40.4], hazard ratio [HR], 3.67 [95%CI, 1.93-7.11], P less than .001).
Findings were similar for overall survival. Median survival was 10.5 months [95% CI, 7.5-22.2] for those with persistent mutations vs 42.2 months [95% CI, 20.6-not estimable] for those without them (HR, 2.86 [95% CI, 1.39-5.88], P = .004).
The results were similar for the 32 patients with intermediate-risk AML, in that persistent mutations were associated with reduced event-free survival as well as overall survival.
As well as providing critical insights into the role of molecular monitoring in AML and the dynamics of genetic mutations during AML treatment, the findings of this study suggest that the clearance of all leukemia-associated mutations was associated with favorable overall survival. Thus, clearance of all leukemia cells and of preleukemic cells with founder mutations is necessary to achieve a cure in this disease.
But to cure patients with AML, it may be important to direct therapy after remission toward the eradication of disease-initiating mutations, including epigenetic modifiers, given these mutations are often present at clinical remission and can initiate relapse through the acquisition of additional mutations.
Since this was a small, single-institution cohort, high-quality studies in larger AML cohorts are needed, to ascertain if whole-genome or whole-exome sequencing or other state-of-the-art genomic approaches used at the time of diagnosis can better predict prognosis than currently used methodologies.
Although subsequent studies will be needed to validate these findings and to credential clinical-grade assays for dynamic molecular studies, these data illustrate that the depth of remission after initial therapy represents an important parameter that is not sufficiently interrogated in the clinical context.
Dr. Friederike Pastore, of the human oncology and pathogenesis program, Memorial Sloan Kettering Cancer Center, New York, is receiving a grant from the German Research Foundation. Dr. Ross L Levine, of the leukemia service, department of medicine, Memorial Sloan Kettering Cancer Center, New York, has no disclosures. These remarks were taken from their editorial accompanying Dr. KLco’s report (JAMA. 2015;314[8]:778-80.).
As well as providing critical insights into the role of molecular monitoring in AML and the dynamics of genetic mutations during AML treatment, the findings of this study suggest that the clearance of all leukemia-associated mutations was associated with favorable overall survival. Thus, clearance of all leukemia cells and of preleukemic cells with founder mutations is necessary to achieve a cure in this disease.
But to cure patients with AML, it may be important to direct therapy after remission toward the eradication of disease-initiating mutations, including epigenetic modifiers, given these mutations are often present at clinical remission and can initiate relapse through the acquisition of additional mutations.
Since this was a small, single-institution cohort, high-quality studies in larger AML cohorts are needed, to ascertain if whole-genome or whole-exome sequencing or other state-of-the-art genomic approaches used at the time of diagnosis can better predict prognosis than currently used methodologies.
Although subsequent studies will be needed to validate these findings and to credential clinical-grade assays for dynamic molecular studies, these data illustrate that the depth of remission after initial therapy represents an important parameter that is not sufficiently interrogated in the clinical context.
Dr. Friederike Pastore, of the human oncology and pathogenesis program, Memorial Sloan Kettering Cancer Center, New York, is receiving a grant from the German Research Foundation. Dr. Ross L Levine, of the leukemia service, department of medicine, Memorial Sloan Kettering Cancer Center, New York, has no disclosures. These remarks were taken from their editorial accompanying Dr. KLco’s report (JAMA. 2015;314[8]:778-80.).
As well as providing critical insights into the role of molecular monitoring in AML and the dynamics of genetic mutations during AML treatment, the findings of this study suggest that the clearance of all leukemia-associated mutations was associated with favorable overall survival. Thus, clearance of all leukemia cells and of preleukemic cells with founder mutations is necessary to achieve a cure in this disease.
But to cure patients with AML, it may be important to direct therapy after remission toward the eradication of disease-initiating mutations, including epigenetic modifiers, given these mutations are often present at clinical remission and can initiate relapse through the acquisition of additional mutations.
Since this was a small, single-institution cohort, high-quality studies in larger AML cohorts are needed, to ascertain if whole-genome or whole-exome sequencing or other state-of-the-art genomic approaches used at the time of diagnosis can better predict prognosis than currently used methodologies.
Although subsequent studies will be needed to validate these findings and to credential clinical-grade assays for dynamic molecular studies, these data illustrate that the depth of remission after initial therapy represents an important parameter that is not sufficiently interrogated in the clinical context.
Dr. Friederike Pastore, of the human oncology and pathogenesis program, Memorial Sloan Kettering Cancer Center, New York, is receiving a grant from the German Research Foundation. Dr. Ross L Levine, of the leukemia service, department of medicine, Memorial Sloan Kettering Cancer Center, New York, has no disclosures. These remarks were taken from their editorial accompanying Dr. KLco’s report (JAMA. 2015;314[8]:778-80.).
Persistent leukemia-associated mutations that can be detected in at least 5% of bone marrow cells at 30 days after remission were associated with a significantly increased risk of relapse and reduced overall survival in patients with acute myeloid leukemia (AML), in a study published Aug. 25 in JAMA.
About 20% of adult patients with AML fail to achieve remission following standard initial induction chemotherapy, and approximately half of them will subsequently experience a relapse after achieving complete remission. Currently, tests that predict outcomes for these patients are imprecise, especially for those with intermediate-risk disease.
“The data presented in this report begin to define a genomic method for the risk stratification of patients with AML that places greater emphasis on the clearance of somatic mutations after chemotherapy than the identification of specific mutations at the time of presentation,” wrote Dr. Jeffery M. Klco, Washington University, St Louis, and his colleagues. (JAMA. 2015;314[8]:811-22).
Whole-genome or exome sequencing was performed on samples that were obtained at disease presentation from 71 patients with AML who were treated with standard induction chemotherapy in March 2002, with follow-up through January 2015. A subsequent re-analysis was conducted in a cohort of 50 patients, who had available samples from both presentation and documented remission.
Of this group, 24 (48%) had persistent leukemia-associated mutations in at least 5% of bone marrow cells at remission, while 26 patients had cleared all mutations.
The investigators noted that patients with at least one persistent mutation on day 30 had significantly reduced event-free survival compared with those who had cleared all mutations (median, 6.0 months [95% CI, 3.7-9.6] vs 17.9 months [95% CI, 11.3-40.4], hazard ratio [HR], 3.67 [95%CI, 1.93-7.11], P less than .001).
Findings were similar for overall survival. Median survival was 10.5 months [95% CI, 7.5-22.2] for those with persistent mutations vs 42.2 months [95% CI, 20.6-not estimable] for those without them (HR, 2.86 [95% CI, 1.39-5.88], P = .004).
The results were similar for the 32 patients with intermediate-risk AML, in that persistent mutations were associated with reduced event-free survival as well as overall survival.
Persistent leukemia-associated mutations that can be detected in at least 5% of bone marrow cells at 30 days after remission were associated with a significantly increased risk of relapse and reduced overall survival in patients with acute myeloid leukemia (AML), in a study published Aug. 25 in JAMA.
About 20% of adult patients with AML fail to achieve remission following standard initial induction chemotherapy, and approximately half of them will subsequently experience a relapse after achieving complete remission. Currently, tests that predict outcomes for these patients are imprecise, especially for those with intermediate-risk disease.
“The data presented in this report begin to define a genomic method for the risk stratification of patients with AML that places greater emphasis on the clearance of somatic mutations after chemotherapy than the identification of specific mutations at the time of presentation,” wrote Dr. Jeffery M. Klco, Washington University, St Louis, and his colleagues. (JAMA. 2015;314[8]:811-22).
Whole-genome or exome sequencing was performed on samples that were obtained at disease presentation from 71 patients with AML who were treated with standard induction chemotherapy in March 2002, with follow-up through January 2015. A subsequent re-analysis was conducted in a cohort of 50 patients, who had available samples from both presentation and documented remission.
Of this group, 24 (48%) had persistent leukemia-associated mutations in at least 5% of bone marrow cells at remission, while 26 patients had cleared all mutations.
The investigators noted that patients with at least one persistent mutation on day 30 had significantly reduced event-free survival compared with those who had cleared all mutations (median, 6.0 months [95% CI, 3.7-9.6] vs 17.9 months [95% CI, 11.3-40.4], hazard ratio [HR], 3.67 [95%CI, 1.93-7.11], P less than .001).
Findings were similar for overall survival. Median survival was 10.5 months [95% CI, 7.5-22.2] for those with persistent mutations vs 42.2 months [95% CI, 20.6-not estimable] for those without them (HR, 2.86 [95% CI, 1.39-5.88], P = .004).
The results were similar for the 32 patients with intermediate-risk AML, in that persistent mutations were associated with reduced event-free survival as well as overall survival.
FROM JAMA
Key clinical point: Leukemia-associated mutations that persisted 30 days after chemotherapy initiation were associated with a significantly increased risk of relapse and reduced overall survival in patients with AML.
Major finding: Patients with one persistent mutation at day 30 had an overall median survival of 10.5 months compared to 42.2 months for those who cleared all mutations (P = .003; HR, 2.86 [95% CI, 1.39-5.88]).
Data source: Whole-genome or exome sequencing was performed on specimens from 71 AML patients treated at a single center with standard induction chemotherapy.
Disclosures: The study was supported by grants from the National Institutes of Health and from the Barnes–Jewish Hospital Foundation. Dr. Spencer reports receiving personal fees from Cofactor Genomics, Dr Duncavage reports receiving personal fees from Cofactor Genomics and nonfinancial support from Agilent Technologies, and Dr Ozenberger reports receiving grant funding from the National Cancer Institute. There were no other disclosures.
Lenalidomide + rituximab combo effective in recurrent follicular lymphoma
The combination of lenalidomide and rituximab was more active in patients with recurrent follicular lymphoma, compared with lenalidomide alone, and significantly increased the overall response rate, according to new data published online Aug. 24 in the Journal of Clinical Oncology.
Although both lenalidomide and rituximab are active agents in follicular lymphoma, their combined use in recurrent follicular lymphoma has not been previously evaluated in randomized clinical trials, said Dr. John P. Leonard of Cornell University, New York, and his colleagues.
The overall response rate of patients receiving the combination regimen was significantly higher than that of patients who received lenalidomide alone (P = .029). In the cohort receiving lenalidomide alone, 24 patients (53%) achieved an objective response (9 complete responses [20%]), while 35 patients (76%) in the lenalidomide/rituximab group were responders (18 complete responses [39%]).
At a median follow-up of 2.5 years (range, 0.1-4.8 years), the addition of rituximab to lenalidomide in this population also significantly increased the median time to progression: 1.1 year for lenalidomide alone versus 2 years for the combined therapy (P = .002).
Overall survival was 4.5 years for lenalidomide alone and has not yet been reached for the combination arm (P = .149.
This trial helps to establish the safety profile of single-agent lenalidomide in follicular lymphoma, while its “randomized nature also allows a direct assessment of potential toxicity resulting from the addition of rituximab to lenalidomide,” wrote Dr. Leonard and his associates (J Clin Oncol. 2015 Aug 24. doi: 10.1200/JCO.2014.59.9258).
“There was no evidence of increased toxicity from the lenalidomide/rituximab combination compared with lenalidomide alone,” they pointed out.
Both lenalidomide alone and lenalidomide/rituximab were well tolerated, with grade 3-4 adverse events occurring in 58% and 53% of patients, respectively, with 9% and 11% of patients experiencing grade 4 toxicity, respectively. The most common grade 3-4 adverse events included neutropenia (16% vs. 20%), fatigue (9% vs. 13%), and rash (4% vs. 4%).
The study was supported in part by grants from the National Cancer Institute to the Alliance for Clinical Trials in Oncology. Dr. Leonard reported financial relationships with Celgene and Genentech, and several coauthors also reported relationships with industry.
The combination of lenalidomide and rituximab was more active in patients with recurrent follicular lymphoma, compared with lenalidomide alone, and significantly increased the overall response rate, according to new data published online Aug. 24 in the Journal of Clinical Oncology.
Although both lenalidomide and rituximab are active agents in follicular lymphoma, their combined use in recurrent follicular lymphoma has not been previously evaluated in randomized clinical trials, said Dr. John P. Leonard of Cornell University, New York, and his colleagues.
The overall response rate of patients receiving the combination regimen was significantly higher than that of patients who received lenalidomide alone (P = .029). In the cohort receiving lenalidomide alone, 24 patients (53%) achieved an objective response (9 complete responses [20%]), while 35 patients (76%) in the lenalidomide/rituximab group were responders (18 complete responses [39%]).
At a median follow-up of 2.5 years (range, 0.1-4.8 years), the addition of rituximab to lenalidomide in this population also significantly increased the median time to progression: 1.1 year for lenalidomide alone versus 2 years for the combined therapy (P = .002).
Overall survival was 4.5 years for lenalidomide alone and has not yet been reached for the combination arm (P = .149.
This trial helps to establish the safety profile of single-agent lenalidomide in follicular lymphoma, while its “randomized nature also allows a direct assessment of potential toxicity resulting from the addition of rituximab to lenalidomide,” wrote Dr. Leonard and his associates (J Clin Oncol. 2015 Aug 24. doi: 10.1200/JCO.2014.59.9258).
“There was no evidence of increased toxicity from the lenalidomide/rituximab combination compared with lenalidomide alone,” they pointed out.
Both lenalidomide alone and lenalidomide/rituximab were well tolerated, with grade 3-4 adverse events occurring in 58% and 53% of patients, respectively, with 9% and 11% of patients experiencing grade 4 toxicity, respectively. The most common grade 3-4 adverse events included neutropenia (16% vs. 20%), fatigue (9% vs. 13%), and rash (4% vs. 4%).
The study was supported in part by grants from the National Cancer Institute to the Alliance for Clinical Trials in Oncology. Dr. Leonard reported financial relationships with Celgene and Genentech, and several coauthors also reported relationships with industry.
The combination of lenalidomide and rituximab was more active in patients with recurrent follicular lymphoma, compared with lenalidomide alone, and significantly increased the overall response rate, according to new data published online Aug. 24 in the Journal of Clinical Oncology.
Although both lenalidomide and rituximab are active agents in follicular lymphoma, their combined use in recurrent follicular lymphoma has not been previously evaluated in randomized clinical trials, said Dr. John P. Leonard of Cornell University, New York, and his colleagues.
The overall response rate of patients receiving the combination regimen was significantly higher than that of patients who received lenalidomide alone (P = .029). In the cohort receiving lenalidomide alone, 24 patients (53%) achieved an objective response (9 complete responses [20%]), while 35 patients (76%) in the lenalidomide/rituximab group were responders (18 complete responses [39%]).
At a median follow-up of 2.5 years (range, 0.1-4.8 years), the addition of rituximab to lenalidomide in this population also significantly increased the median time to progression: 1.1 year for lenalidomide alone versus 2 years for the combined therapy (P = .002).
Overall survival was 4.5 years for lenalidomide alone and has not yet been reached for the combination arm (P = .149.
This trial helps to establish the safety profile of single-agent lenalidomide in follicular lymphoma, while its “randomized nature also allows a direct assessment of potential toxicity resulting from the addition of rituximab to lenalidomide,” wrote Dr. Leonard and his associates (J Clin Oncol. 2015 Aug 24. doi: 10.1200/JCO.2014.59.9258).
“There was no evidence of increased toxicity from the lenalidomide/rituximab combination compared with lenalidomide alone,” they pointed out.
Both lenalidomide alone and lenalidomide/rituximab were well tolerated, with grade 3-4 adverse events occurring in 58% and 53% of patients, respectively, with 9% and 11% of patients experiencing grade 4 toxicity, respectively. The most common grade 3-4 adverse events included neutropenia (16% vs. 20%), fatigue (9% vs. 13%), and rash (4% vs. 4%).
The study was supported in part by grants from the National Cancer Institute to the Alliance for Clinical Trials in Oncology. Dr. Leonard reported financial relationships with Celgene and Genentech, and several coauthors also reported relationships with industry.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Lenalidomide combined with rituximab is more active in recurrent follicular lymphoma, compared with lenalidomide monotherapy.
Major finding: Overall response rate was 53% (20% complete response) for lenalidomide alone versus 76% (39% complete response) for lenalidomide and rituximab (P = .029).
Data source: Randomized phase II clinical trial of 91 patients with follicular lymphoma who were assigned to receive lenalidomide alone or lenalidomide combined with rituximab.
Disclosures: The study was supported in part by grants from the National Cancer Institute to the Alliance for Clinical Trials in Oncology. Dr. Leonard reported financial relationships with Celgene and Genentech, and several coauthors also reported relationships with industry.
Continuous therapy improved outcomes in multiple myeloma
Compared with fixed duration therapy for patients with multiple myeloma, continuous therapy significantly prolonged median progression by approximately 1 year and improved overall survival by about 10%, investigators reported online Aug. 17 in the Journal of Clinical Oncology.
The goal of continuous therapy is to maintain the results of first-line therapy by keeping the patient free of symptoms and preventing or delaying disease progression. There has been some concern, however, that patients who progress while on continuous therapy may become resistant to at least that particular regimen.
In the current study, Dr. Antonio Palumbo of the University of Torino (Italy) and his colleagues pooled the results of three randomized phase III trials to evaluate the impact of continuous therapy, compared with fixed duration of therapy on time-to-event endpoints. In addition to overall survival, they evaluated progression-free survival (PFS) at two different time points in the course of therapy.
PFS1 was defined as the time from random assignment until the first disease progression or death, while PFS2 was defined as the time randomization until the second disease progression or death. Using both of these endpoints more accurately estimated the impact of both first- and second-line therapies on patient outcomes, the investigators said.
The pooled analysis of the three trials included 604 patients who were randomly assigned to continuous therapy and 614 who were randomized to fixed duration therapy. The median follow-up was 52 months. In the intent-to-treat population, continuous therapy (n = 417), compared with fixed duration (n = 410) significantly improved PFS1 (median, 32 vs. 16 months, respectively; hazard ratio, 0.47; 95% confidence interval, 0.40-0.56; P less than .001), as well as PFS2 (median, 55 vs. 40 months, respectively; HR, 0.61; 95% CI, 0.50-0.75; P less than .001). Overall survival also was improved in the continuous therapy group (4-year overall survival, 69% vs. 60%, respectively; HR, 0.69; 95% CI, 0.54-0.88; P = .003) (J Clin Oncol. 2015 Aug. 17 doi: 10.1200/JCO.2014.60.2466).
“Our findings suggest that most of the PFS1 advantage associated with CT [continuous therapy] up front is maintained after first relapse and that CT does not induce a significant chemotherapy resistance,” Dr. Palumbo and his associates wrote. “The improvement in PFS2 suggests that most of the benefit observed during the first remission is not affected by a short second remission.”
The GIMEMA-MM-03-05 study was sponsored by Fondazione Neoplasie Sangue Onlus and supported by Janssen-Cilag and Celgene. The RV-MM-PI-209 study was sponsored by Fondazione Neoplasie Sangue Onlus and supported by Celgene. The CC-5013-MM-015 study was sponsored and supported by Celgene. Several of the coauthors reported financial relationships with industry, including Celgene and Janssen-Citag.
Compared with fixed duration therapy for patients with multiple myeloma, continuous therapy significantly prolonged median progression by approximately 1 year and improved overall survival by about 10%, investigators reported online Aug. 17 in the Journal of Clinical Oncology.
The goal of continuous therapy is to maintain the results of first-line therapy by keeping the patient free of symptoms and preventing or delaying disease progression. There has been some concern, however, that patients who progress while on continuous therapy may become resistant to at least that particular regimen.
In the current study, Dr. Antonio Palumbo of the University of Torino (Italy) and his colleagues pooled the results of three randomized phase III trials to evaluate the impact of continuous therapy, compared with fixed duration of therapy on time-to-event endpoints. In addition to overall survival, they evaluated progression-free survival (PFS) at two different time points in the course of therapy.
PFS1 was defined as the time from random assignment until the first disease progression or death, while PFS2 was defined as the time randomization until the second disease progression or death. Using both of these endpoints more accurately estimated the impact of both first- and second-line therapies on patient outcomes, the investigators said.
The pooled analysis of the three trials included 604 patients who were randomly assigned to continuous therapy and 614 who were randomized to fixed duration therapy. The median follow-up was 52 months. In the intent-to-treat population, continuous therapy (n = 417), compared with fixed duration (n = 410) significantly improved PFS1 (median, 32 vs. 16 months, respectively; hazard ratio, 0.47; 95% confidence interval, 0.40-0.56; P less than .001), as well as PFS2 (median, 55 vs. 40 months, respectively; HR, 0.61; 95% CI, 0.50-0.75; P less than .001). Overall survival also was improved in the continuous therapy group (4-year overall survival, 69% vs. 60%, respectively; HR, 0.69; 95% CI, 0.54-0.88; P = .003) (J Clin Oncol. 2015 Aug. 17 doi: 10.1200/JCO.2014.60.2466).
“Our findings suggest that most of the PFS1 advantage associated with CT [continuous therapy] up front is maintained after first relapse and that CT does not induce a significant chemotherapy resistance,” Dr. Palumbo and his associates wrote. “The improvement in PFS2 suggests that most of the benefit observed during the first remission is not affected by a short second remission.”
The GIMEMA-MM-03-05 study was sponsored by Fondazione Neoplasie Sangue Onlus and supported by Janssen-Cilag and Celgene. The RV-MM-PI-209 study was sponsored by Fondazione Neoplasie Sangue Onlus and supported by Celgene. The CC-5013-MM-015 study was sponsored and supported by Celgene. Several of the coauthors reported financial relationships with industry, including Celgene and Janssen-Citag.
Compared with fixed duration therapy for patients with multiple myeloma, continuous therapy significantly prolonged median progression by approximately 1 year and improved overall survival by about 10%, investigators reported online Aug. 17 in the Journal of Clinical Oncology.
The goal of continuous therapy is to maintain the results of first-line therapy by keeping the patient free of symptoms and preventing or delaying disease progression. There has been some concern, however, that patients who progress while on continuous therapy may become resistant to at least that particular regimen.
In the current study, Dr. Antonio Palumbo of the University of Torino (Italy) and his colleagues pooled the results of three randomized phase III trials to evaluate the impact of continuous therapy, compared with fixed duration of therapy on time-to-event endpoints. In addition to overall survival, they evaluated progression-free survival (PFS) at two different time points in the course of therapy.
PFS1 was defined as the time from random assignment until the first disease progression or death, while PFS2 was defined as the time randomization until the second disease progression or death. Using both of these endpoints more accurately estimated the impact of both first- and second-line therapies on patient outcomes, the investigators said.
The pooled analysis of the three trials included 604 patients who were randomly assigned to continuous therapy and 614 who were randomized to fixed duration therapy. The median follow-up was 52 months. In the intent-to-treat population, continuous therapy (n = 417), compared with fixed duration (n = 410) significantly improved PFS1 (median, 32 vs. 16 months, respectively; hazard ratio, 0.47; 95% confidence interval, 0.40-0.56; P less than .001), as well as PFS2 (median, 55 vs. 40 months, respectively; HR, 0.61; 95% CI, 0.50-0.75; P less than .001). Overall survival also was improved in the continuous therapy group (4-year overall survival, 69% vs. 60%, respectively; HR, 0.69; 95% CI, 0.54-0.88; P = .003) (J Clin Oncol. 2015 Aug. 17 doi: 10.1200/JCO.2014.60.2466).
“Our findings suggest that most of the PFS1 advantage associated with CT [continuous therapy] up front is maintained after first relapse and that CT does not induce a significant chemotherapy resistance,” Dr. Palumbo and his associates wrote. “The improvement in PFS2 suggests that most of the benefit observed during the first remission is not affected by a short second remission.”
The GIMEMA-MM-03-05 study was sponsored by Fondazione Neoplasie Sangue Onlus and supported by Janssen-Cilag and Celgene. The RV-MM-PI-209 study was sponsored by Fondazione Neoplasie Sangue Onlus and supported by Celgene. The CC-5013-MM-015 study was sponsored and supported by Celgene. Several of the coauthors reported financial relationships with industry, including Celgene and Janssen-Citag.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Continuous therapy improved outcomes in multiple myeloma, compared with fixed duration therapy.
Major finding: Overall survival was improved (4-year OS, 69% v 60%, respectively; HR, 0.69; P = .003) as well as progression-free survival.
Data source: Pooled analysis of the three trials that included 604 patients randomly assigned to continuous therapy and 614 to fixed duration therapy.
Disclosures: The GIMEMA-MM-03-05 study was sponsored by Fondazione Neoplasie Sangue Onlus and supported by Janssen-Cilag and Celgene. The RV-MM-PI-209 study was sponsored by Fondazione Neoplasie Sangue Onlus and supported by Celgene. The CC-5013-MM-015 study was sponsored and supported by Celgene. Several of the coauthors reported financial relationships with industry, including Celgene and Janssen-Citag.
Weight program effective for breast cancer survivors
A group-based behavioral weight loss program supplemented with personal contact and support was effective in helping overweight or obese breast cancer survivors lose a clinically meaningful amount of weight, investigators reported online Aug. 17 in the Journal of Clinical Oncology.
“Women who have been diagnosed and treated for breast cancer often have special issues and problems, such as treatment-related adverse effects, fatigue, and depression, that can complicate weight management efforts,” wrote Cheryl L. Rock, Ph.D., of the University of California, San Diego, Moores Cancer Center in La Jolla, and her colleagues.
The results from this trial demonstrate that weight loss, even though it was modest, and increased physical activity can be achieved in this population and suggest that these issues can be overcome, the authors noted.
The multicenter trial included 692 overweight or obese breast cancer survivors who were randomly assigned to either the intervention group – which included a cognitive-behavioral weight loss program with telephone counseling and tailored newsletters to support initial weight loss and subsequent maintenance – or a control group with a less intensive intervention (J Clin Oncol. 2015 Aug. 17 doi: 10.1200/JCO.2015.61.1095).
At 6 months, the mean weight loss in the intervention group was 5.9%; at 12 months, the weight loss held steady and was maintained at 6%. In the control group, weight loss was 1.3% at 6 months and 1.5% at 12 months.
At 18 months, the women in the intervention group were 4.7% below their baseline weight, and at 24 months they weighed 3.7% less than at entry into the study. Those in the control group were 1.3% below baseline weight at 18 months and 1.1% below at 24 months.
The weight loss intervention was more effective among women older than 55 years than in younger women, who may need counseling and resources beyond that offered in this study, to achieve sufficient weight loss, Dr. Rock and her associates added.
The study was supported by the National Cancer Institute and by a grant from the National Center for Research Resources, a component of the NIH, and NIH Roadmap for Medical Research. Dr. Rock reported research funding from Jenny Craig and Nestle USA, and several of the coauthors reported financial relationships with various corporations.
A group-based behavioral weight loss program supplemented with personal contact and support was effective in helping overweight or obese breast cancer survivors lose a clinically meaningful amount of weight, investigators reported online Aug. 17 in the Journal of Clinical Oncology.
“Women who have been diagnosed and treated for breast cancer often have special issues and problems, such as treatment-related adverse effects, fatigue, and depression, that can complicate weight management efforts,” wrote Cheryl L. Rock, Ph.D., of the University of California, San Diego, Moores Cancer Center in La Jolla, and her colleagues.
The results from this trial demonstrate that weight loss, even though it was modest, and increased physical activity can be achieved in this population and suggest that these issues can be overcome, the authors noted.
The multicenter trial included 692 overweight or obese breast cancer survivors who were randomly assigned to either the intervention group – which included a cognitive-behavioral weight loss program with telephone counseling and tailored newsletters to support initial weight loss and subsequent maintenance – or a control group with a less intensive intervention (J Clin Oncol. 2015 Aug. 17 doi: 10.1200/JCO.2015.61.1095).
At 6 months, the mean weight loss in the intervention group was 5.9%; at 12 months, the weight loss held steady and was maintained at 6%. In the control group, weight loss was 1.3% at 6 months and 1.5% at 12 months.
At 18 months, the women in the intervention group were 4.7% below their baseline weight, and at 24 months they weighed 3.7% less than at entry into the study. Those in the control group were 1.3% below baseline weight at 18 months and 1.1% below at 24 months.
The weight loss intervention was more effective among women older than 55 years than in younger women, who may need counseling and resources beyond that offered in this study, to achieve sufficient weight loss, Dr. Rock and her associates added.
The study was supported by the National Cancer Institute and by a grant from the National Center for Research Resources, a component of the NIH, and NIH Roadmap for Medical Research. Dr. Rock reported research funding from Jenny Craig and Nestle USA, and several of the coauthors reported financial relationships with various corporations.
A group-based behavioral weight loss program supplemented with personal contact and support was effective in helping overweight or obese breast cancer survivors lose a clinically meaningful amount of weight, investigators reported online Aug. 17 in the Journal of Clinical Oncology.
“Women who have been diagnosed and treated for breast cancer often have special issues and problems, such as treatment-related adverse effects, fatigue, and depression, that can complicate weight management efforts,” wrote Cheryl L. Rock, Ph.D., of the University of California, San Diego, Moores Cancer Center in La Jolla, and her colleagues.
The results from this trial demonstrate that weight loss, even though it was modest, and increased physical activity can be achieved in this population and suggest that these issues can be overcome, the authors noted.
The multicenter trial included 692 overweight or obese breast cancer survivors who were randomly assigned to either the intervention group – which included a cognitive-behavioral weight loss program with telephone counseling and tailored newsletters to support initial weight loss and subsequent maintenance – or a control group with a less intensive intervention (J Clin Oncol. 2015 Aug. 17 doi: 10.1200/JCO.2015.61.1095).
At 6 months, the mean weight loss in the intervention group was 5.9%; at 12 months, the weight loss held steady and was maintained at 6%. In the control group, weight loss was 1.3% at 6 months and 1.5% at 12 months.
At 18 months, the women in the intervention group were 4.7% below their baseline weight, and at 24 months they weighed 3.7% less than at entry into the study. Those in the control group were 1.3% below baseline weight at 18 months and 1.1% below at 24 months.
The weight loss intervention was more effective among women older than 55 years than in younger women, who may need counseling and resources beyond that offered in this study, to achieve sufficient weight loss, Dr. Rock and her associates added.
The study was supported by the National Cancer Institute and by a grant from the National Center for Research Resources, a component of the NIH, and NIH Roadmap for Medical Research. Dr. Rock reported research funding from Jenny Craig and Nestle USA, and several of the coauthors reported financial relationships with various corporations.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: A group-based behavioral weight loss intervention can lead to clinically meaningful weight loss in overweight/obese breast cancer survivors
Major finding: At 24 months, mean weight loss was more than double in the intervention group, compared with controls (3.7% vs 1.3%).
Data source: Multicenter randomized trial involving 692 overweight/obese women who were about 2 years post treatment for early-stage breast cancer.
Disclosures: The study was supported by the National Cancer Institute and by a grant from the National Center for Research Resources, a component of the NIH, and NIH Roadmap for Medical Research. Dr. Rock reported research funding from Jenny Craig and Nestle USA, and several of the coauthors reported financial relationships with various corporations.
Novel triple therapy in ITP provides enduring responses
For patients with chronic primary immune thrombocytopenia, a three-drug regimen was associated with a high response rate and relapse-free survival, according to the results of a single-arm, phase IIb trial published online in Blood.
Twenty patients with primary immune thrombocytopenia (ITP) received an investigative triple therapy of oral dexamethasone 40 mg (days 1-4), oral cyclosporine 2.5-3.0 mg/kg daily (days 1-28), and intravenous rituximab 100 mg (day 7, 14, 21, and 28), and of this group, 12 patients responded. The median time to response was 7.4 days, and all patients maintained their response for at least 7 months, Dr. Philip Young-Ill Choi, of St. George Clinical School, University of New South Wales, Kogarah, Australia, and his colleagues reported (Blood 2015;126[4]:500-3).
Complete response was 30% at 6 months, and only two patients relapsed during a median follow-up period of 17.5 months (range, 7-47 months). Among patients who responded, relapse-free survival at 12 and 24 months was 92% and 76%, respectively (95% confidence intervals, 53%-98% and 30%-93%, respectively).
Peripheral T cells declined for all patients, irrespective of response, but responders had lower CD4+ T cells than did nonresponders for 6 months after treatment (median, 0.62 vs. 0.91 x 109/L; P less than .0001). Peripheral CD19+ B cells became undetectable for all patients by day 28, but recovery was earlier for patients younger than 50 years (median, 6.5 months vs. not reached; P = .0105).
The regimen was generally well tolerated, without any deaths, treatment-related serious adverse events, serum sickness, interruptions, or delays caused by toxicity.
A major advantage of this regimen is its short duration of therapy, and yet 12 of 20 patients enjoyed a prolonged remission of 7 months or longer without needing further treatment. However, interpretation of the data was limited by the small sample size, the investigators noted.
“Although our study shows encouraging results, the incremental benefit of cyclosporine to rituximab and dexamethasone remains unresolved, and randomized controlled trials are required,” they wrote.
No funding source for the study was given. One author reported receiving speaker’s fees from Roche, and another is on the speakers bureau and receives research funding from GlaxoSmithKline and Amgen. The remaining authors declared no competing financial interests.
For patients with chronic primary immune thrombocytopenia, a three-drug regimen was associated with a high response rate and relapse-free survival, according to the results of a single-arm, phase IIb trial published online in Blood.
Twenty patients with primary immune thrombocytopenia (ITP) received an investigative triple therapy of oral dexamethasone 40 mg (days 1-4), oral cyclosporine 2.5-3.0 mg/kg daily (days 1-28), and intravenous rituximab 100 mg (day 7, 14, 21, and 28), and of this group, 12 patients responded. The median time to response was 7.4 days, and all patients maintained their response for at least 7 months, Dr. Philip Young-Ill Choi, of St. George Clinical School, University of New South Wales, Kogarah, Australia, and his colleagues reported (Blood 2015;126[4]:500-3).
Complete response was 30% at 6 months, and only two patients relapsed during a median follow-up period of 17.5 months (range, 7-47 months). Among patients who responded, relapse-free survival at 12 and 24 months was 92% and 76%, respectively (95% confidence intervals, 53%-98% and 30%-93%, respectively).
Peripheral T cells declined for all patients, irrespective of response, but responders had lower CD4+ T cells than did nonresponders for 6 months after treatment (median, 0.62 vs. 0.91 x 109/L; P less than .0001). Peripheral CD19+ B cells became undetectable for all patients by day 28, but recovery was earlier for patients younger than 50 years (median, 6.5 months vs. not reached; P = .0105).
The regimen was generally well tolerated, without any deaths, treatment-related serious adverse events, serum sickness, interruptions, or delays caused by toxicity.
A major advantage of this regimen is its short duration of therapy, and yet 12 of 20 patients enjoyed a prolonged remission of 7 months or longer without needing further treatment. However, interpretation of the data was limited by the small sample size, the investigators noted.
“Although our study shows encouraging results, the incremental benefit of cyclosporine to rituximab and dexamethasone remains unresolved, and randomized controlled trials are required,” they wrote.
No funding source for the study was given. One author reported receiving speaker’s fees from Roche, and another is on the speakers bureau and receives research funding from GlaxoSmithKline and Amgen. The remaining authors declared no competing financial interests.
For patients with chronic primary immune thrombocytopenia, a three-drug regimen was associated with a high response rate and relapse-free survival, according to the results of a single-arm, phase IIb trial published online in Blood.
Twenty patients with primary immune thrombocytopenia (ITP) received an investigative triple therapy of oral dexamethasone 40 mg (days 1-4), oral cyclosporine 2.5-3.0 mg/kg daily (days 1-28), and intravenous rituximab 100 mg (day 7, 14, 21, and 28), and of this group, 12 patients responded. The median time to response was 7.4 days, and all patients maintained their response for at least 7 months, Dr. Philip Young-Ill Choi, of St. George Clinical School, University of New South Wales, Kogarah, Australia, and his colleagues reported (Blood 2015;126[4]:500-3).
Complete response was 30% at 6 months, and only two patients relapsed during a median follow-up period of 17.5 months (range, 7-47 months). Among patients who responded, relapse-free survival at 12 and 24 months was 92% and 76%, respectively (95% confidence intervals, 53%-98% and 30%-93%, respectively).
Peripheral T cells declined for all patients, irrespective of response, but responders had lower CD4+ T cells than did nonresponders for 6 months after treatment (median, 0.62 vs. 0.91 x 109/L; P less than .0001). Peripheral CD19+ B cells became undetectable for all patients by day 28, but recovery was earlier for patients younger than 50 years (median, 6.5 months vs. not reached; P = .0105).
The regimen was generally well tolerated, without any deaths, treatment-related serious adverse events, serum sickness, interruptions, or delays caused by toxicity.
A major advantage of this regimen is its short duration of therapy, and yet 12 of 20 patients enjoyed a prolonged remission of 7 months or longer without needing further treatment. However, interpretation of the data was limited by the small sample size, the investigators noted.
“Although our study shows encouraging results, the incremental benefit of cyclosporine to rituximab and dexamethasone remains unresolved, and randomized controlled trials are required,” they wrote.
No funding source for the study was given. One author reported receiving speaker’s fees from Roche, and another is on the speakers bureau and receives research funding from GlaxoSmithKline and Amgen. The remaining authors declared no competing financial interests.
FROM BLOOD
Key clinical point: Patients with primary immune thrombocytopenia can achieve an enduring response with a novel triple drug regimen.
Major finding: Relapse-free survival was 92% at 12 months for responders and 76% at 24 months
Data source: Prospective, single-arm, phase IIb study involving 20 patients.
Disclosures: No funding source for the study was given. One author reported receiving speaker’s fees from Roche, and another is on the speakers bureau and receives research funding from GlaxoSmithKline and Amgen. The remaining authors declared no competing financial interests.