User login
Nivolumab demonstrated encouraging clinical efficacy and tolerability in patients with platinum-resistant ovarian cancer in a small phase II trial, according to a study published online Sept. 7 in the Journal of Clinical Oncology.
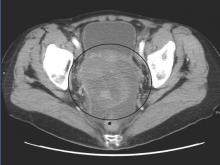
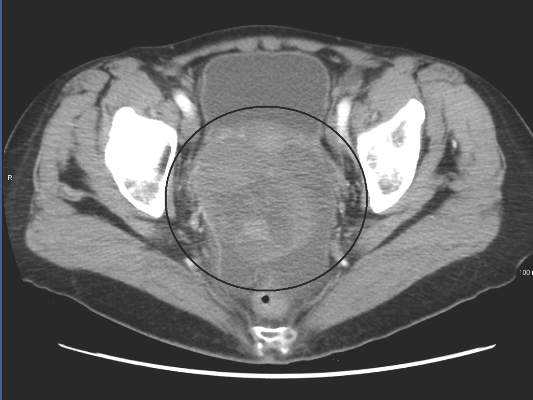
The safety and antitumor efficacy of nivolumab, a fully human immunoglobulin G4 anti–PD-1 receptor–blocking monoclonal antibody, was evaluated in 20 patients with platinum-resistant, recurrent, or advanced ovarian cancer, reported Dr. Junzo Hamanishi of Kyoto of University Graduate School of Medicine, Kyoto, Japan, and his colleagues. They noted that to their knowledge, this is the first investigator-initiated, phase II clinical trial to be done in this setting.
The patients were treated with an intravenous infusion of nivolumab every 2 weeks at a dose of either 1 or 3 mg/kg (two 10-patient cohorts), and the primary end point of this study was best overall response.
Patients received up to six cycles (four doses per cycle) of nivolumab treatment or remained on therapy until their disease progressed.
The best overall response rate across both groups was 15% (3 patients; 95% CI, 3.2% to 37.9%) and the disease control rate was 45% (9 patients; 95%CI, 23.1% to 68.5%). Among patients who received 1 mg/kg, there was one partial response and four had stable disease. The objective response rate was 10% (95% CI, 0.3% to 44.5%), while disease control rate was 50% (95% CI, 18.7% to 81.3%).
In the 3-mg/kg cohort, two patients achieved a complete response, and two had stable disease, with a total objective response rate of 20% (95% CI, 2.5% to 55.6%), and a disease control rate was 40% (95% CI, 12.2% to 73.8%).
For both groups, the median progression-free survival was 3.5 months and median overall survival was 20.0 months.
The most common adverse events were increased serum AST, hypothyroidism, lymphocytopenia, decreased serum albumin, fever, increased serum ALT, maculopapular rash, arthralgia, arrhythmia, fatigue, and anemia. Grade 3 or 4 treatment-related adverse events occurred in eight (40%) patients, although the majority of the cohort was able to continue on the regimen (n = 19, 95%). The authors pointed out that the frequencies of arrhythmias and elevated AST were relatively higher in this study than in larger previous studies that assessed nivolumab in other types of solid tumors, but all of these events were grade 1 or 2 and manageable.
“This study indicates the merit of a large-scale investigation to evaluate the priority of nivolumab for platinum-resistant ovarian cancer,” the researchers wrote, adding that they plan to conduct such a study in the future (J Clin Oncol. 2015 Sep 7. doi:10.1200/JCO.2015.62.3397).
Nivolumab demonstrated encouraging clinical efficacy and tolerability in patients with platinum-resistant ovarian cancer in a small phase II trial, according to a study published online Sept. 7 in the Journal of Clinical Oncology.
The safety and antitumor efficacy of nivolumab, a fully human immunoglobulin G4 anti–PD-1 receptor–blocking monoclonal antibody, was evaluated in 20 patients with platinum-resistant, recurrent, or advanced ovarian cancer, reported Dr. Junzo Hamanishi of Kyoto of University Graduate School of Medicine, Kyoto, Japan, and his colleagues. They noted that to their knowledge, this is the first investigator-initiated, phase II clinical trial to be done in this setting.
The patients were treated with an intravenous infusion of nivolumab every 2 weeks at a dose of either 1 or 3 mg/kg (two 10-patient cohorts), and the primary end point of this study was best overall response.
Patients received up to six cycles (four doses per cycle) of nivolumab treatment or remained on therapy until their disease progressed.
The best overall response rate across both groups was 15% (3 patients; 95% CI, 3.2% to 37.9%) and the disease control rate was 45% (9 patients; 95%CI, 23.1% to 68.5%). Among patients who received 1 mg/kg, there was one partial response and four had stable disease. The objective response rate was 10% (95% CI, 0.3% to 44.5%), while disease control rate was 50% (95% CI, 18.7% to 81.3%).
In the 3-mg/kg cohort, two patients achieved a complete response, and two had stable disease, with a total objective response rate of 20% (95% CI, 2.5% to 55.6%), and a disease control rate was 40% (95% CI, 12.2% to 73.8%).
For both groups, the median progression-free survival was 3.5 months and median overall survival was 20.0 months.
The most common adverse events were increased serum AST, hypothyroidism, lymphocytopenia, decreased serum albumin, fever, increased serum ALT, maculopapular rash, arthralgia, arrhythmia, fatigue, and anemia. Grade 3 or 4 treatment-related adverse events occurred in eight (40%) patients, although the majority of the cohort was able to continue on the regimen (n = 19, 95%). The authors pointed out that the frequencies of arrhythmias and elevated AST were relatively higher in this study than in larger previous studies that assessed nivolumab in other types of solid tumors, but all of these events were grade 1 or 2 and manageable.
“This study indicates the merit of a large-scale investigation to evaluate the priority of nivolumab for platinum-resistant ovarian cancer,” the researchers wrote, adding that they plan to conduct such a study in the future (J Clin Oncol. 2015 Sep 7. doi:10.1200/JCO.2015.62.3397).
Nivolumab demonstrated encouraging clinical efficacy and tolerability in patients with platinum-resistant ovarian cancer in a small phase II trial, according to a study published online Sept. 7 in the Journal of Clinical Oncology.
The safety and antitumor efficacy of nivolumab, a fully human immunoglobulin G4 anti–PD-1 receptor–blocking monoclonal antibody, was evaluated in 20 patients with platinum-resistant, recurrent, or advanced ovarian cancer, reported Dr. Junzo Hamanishi of Kyoto of University Graduate School of Medicine, Kyoto, Japan, and his colleagues. They noted that to their knowledge, this is the first investigator-initiated, phase II clinical trial to be done in this setting.
The patients were treated with an intravenous infusion of nivolumab every 2 weeks at a dose of either 1 or 3 mg/kg (two 10-patient cohorts), and the primary end point of this study was best overall response.
Patients received up to six cycles (four doses per cycle) of nivolumab treatment or remained on therapy until their disease progressed.
The best overall response rate across both groups was 15% (3 patients; 95% CI, 3.2% to 37.9%) and the disease control rate was 45% (9 patients; 95%CI, 23.1% to 68.5%). Among patients who received 1 mg/kg, there was one partial response and four had stable disease. The objective response rate was 10% (95% CI, 0.3% to 44.5%), while disease control rate was 50% (95% CI, 18.7% to 81.3%).
In the 3-mg/kg cohort, two patients achieved a complete response, and two had stable disease, with a total objective response rate of 20% (95% CI, 2.5% to 55.6%), and a disease control rate was 40% (95% CI, 12.2% to 73.8%).
For both groups, the median progression-free survival was 3.5 months and median overall survival was 20.0 months.
The most common adverse events were increased serum AST, hypothyroidism, lymphocytopenia, decreased serum albumin, fever, increased serum ALT, maculopapular rash, arthralgia, arrhythmia, fatigue, and anemia. Grade 3 or 4 treatment-related adverse events occurred in eight (40%) patients, although the majority of the cohort was able to continue on the regimen (n = 19, 95%). The authors pointed out that the frequencies of arrhythmias and elevated AST were relatively higher in this study than in larger previous studies that assessed nivolumab in other types of solid tumors, but all of these events were grade 1 or 2 and manageable.
“This study indicates the merit of a large-scale investigation to evaluate the priority of nivolumab for platinum-resistant ovarian cancer,” the researchers wrote, adding that they plan to conduct such a study in the future (J Clin Oncol. 2015 Sep 7. doi:10.1200/JCO.2015.62.3397).
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Nivolumab demonstrated encouraging clinical efficacy and tolerability in patients with platinum-resistant ovarian cancer.
Major finding: The best overall response was 15%, including 2 patients with a durable complete response, and the disease control rate in all 20 patients was 45%.
Data source: A phase II trial of 20 patients with platinum-resistant ovarian cancer who received an intravenous infusion of nivolumab every 2 weeks at a dose of 1 or 3 mg/kg.
Disclosures: The study was supported by a Health and Labour Sciences Research grant, by a grant from the Translational Research Network Program of the Ministry of Education, Culture, Sports, Science and Technology of Japan, and a grant from Kyoto University. Several of the coauthors reported financial relationships with industry.