User login
A Multipronged Approach to Decrease the Risk of Clostridium difficile Infection at a Community Hospital and Long-Term Care Facility
From Sharp HealthCare, San Diego, CA.
Abstract
- Objective: To examine the relationship between the rate of Clostridium difficile infections (CDI) and implementation of 3 interventions aimed at preserving the fecal microbiome: (1) reduction of antimicrobial pressure; (2) reduction in intensity of gastrointestinal prophylaxis with proton-pump inhibitors (PPIs); and (3) expansion of probiotic therapy.
- Methods: We conducted a retrospective analysis of all inpatients with CDI between January 2009 and December 2013 receiving care at our community hospital and associated long-term care (LTC) facility. We used interrupted time series analysis to assess CDI rates during the implementation phase (2008–2010) and the postimplementation phase (2011–2013).
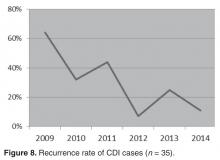
- Results: A reduction in the rate of health care facility–associated CDIs was seen. The mean number of cases per 10,000 patient days fell from 11.9 to 3.6 in acute care and 6.1 to 1.1 in LTC. Recurrence rates decreased from 64% in 2009 to 16% by 2014. The likelihood of CDI recurring was 3 times higher in those exposed to PPI and 0.35 times less likely in those who received probiotics with their initial CDI therapy.
- Conclusion: The risk of CDI incidence and recurrence was significantly reduced in our inpatients, with recurrent CDI associated with PPI use, multiple antibiotic courses, and lack of probiotics. We attribute our success to the combined effect of intensified antibiotic stewardship, reduced PPI use, and expanded probiotic use.
Clostridium difficile is classified as an urgent public health threat by the Centers for Disease Control and Prevention [1]. A recent study by the CDC found that it caused more than 400,000 infections in the United States in 2011, leading to over 29,000 deaths [2]. The costs of treating CDI are substantial and recurrences are common. While rates for many health care–associated infections are declining, C. difficile infection (CDI) rates remain at historically high levels [1] with the elderly at greatest risk for infection and mortality from the illness [3].
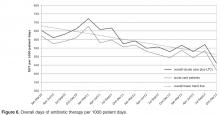
CDIs can be prevented. A principal recommendation for preventing CDIs is improving antibiotic use. Antibiotic use increases the risk for developing CDI by disrupting the colonic microbiome. Hospitalized and long-term care (LTC) patients are frequently prescribed antibiotics, but studies indicate that much of this use is inappropriate [4]. Antimicrobial stewardship has been shown to be effective in reducing CDI rates. Other infection prevention measures commonly employed to decrease the risk of hospital-onset CDI include monitoring of hand hygiene compliance using soap and water, terminal cleaning with bleach products of rooms occupied by patients with CDI, and daily cleaning of highly touched areas. At our institution, patients identified with CDI are placed on contact precautions until they have been adequately treated and have had resolution of diarrhea for 48 hours.
In addition to preventing CDI transmission through antimicrobial stewardship, attention is being paid to the possibility that restricting PPI use may help in preventing CDI. The increasing utilization of proton-pump inhibitors (PPIs) in recent years has coincided with the trend of increasing CDI rates. Although C. difficile spores are acid-resistant, vegetative forms are easily affected by acidity. Several studies have shown the association of acid suppression and greater susceptibility of acquiring CDI or recurrences [5–7]. Elevated gastric pH by PPIs facilitates the growth of potentially pathogenic upper and lower gastrointestinal (GI) tract flora, including the conversion of C. difficile from spore to vegetative form in the upper GI tract [5,8].
A growing body of evidence indicates that probiotics are both safe and effective for preventing CDIs [9]. Probiotics may counteract disturbances in intestinal flora, thereby reducing the risk for colonization by pathogenic bacteria. Probiotics can inhibit pathogen adhesion, colonization, and invasion of the gastrointestinal mucosa [10].
We hypothesized that preservation and/or restoration of the diversity of the fecal microbiome would prevent CDI and disease recurrence in our facility. Prior to 2009, we had strict infection prevention measures in place to prevent disease transmission, similar to many other institutions. In 2009, we implemented 3 additional interventions to reduce the rising incidence of CDI: (1) an antibiotic stewardship program, (2) lowering the intensity of acid suppression, and (3) expanding the use of probiotic therapy. The 3 interventions were initiated over the 19-month period January 2009 through July 2010. This study addresses the effects of these interventions.
Methods
Patients and Data Collection
The study was conducted at a community hospital (59 beds) that has an associated LTC facility (122 beds). We conducted a retrospective analysis of hospital and LTC data from all documented cases of CDI between January 2009 and December 2013. Study subjects included all patients with stools positive for C. difficile antigen and toxin with associated symptoms of infection (n = 123). Institutional review board approval was obtained prior to data collection.
The following information was collected: admission diagnosis, number of days from admission until confirmed CDI, residence prior to admission, duration and type of antibiotics received prior to or during symptoms of CDI, type of GI prophylaxis received within 14 days prior to and during CDI treatment, probiotic received and duration, and the type and duration of antibiotic treatment given for the CDI. The data collected was used to determine the likely origin of each C. difficile case, dates of recurrences, and the possible effects of the interventions. Antibiotic use was categorized as: (1) recent antibiotic course (antibiotics received within the preceding 4 weeks), (2) antibiotic courses greater than 10 days, and (3) multiple antibiotic courses (more than 1 antibiotic course received sequentially or concurrently).
Positive C. difficile infections were detected using a 2-step algorithm, starting in 2009. The samples were first screened with a rapid membrane enzyme immunoassay for glutamate dehydrogenase (GDH) antigen and toxin A and B in stool (C. Diff Quik Chek Complete, Techlab, Blacksburg, VA). Discrepant samples (GDH positive and toxin A and B negative) were reflexed to DNA-based PCR testing. The PCR assay was changed to the Verigene C. difficile test (Nanosphere, Northbrook, IL) in 2012. Up to 30 days after discharge from our facility, positive results were considered as acquired from our facility and positive results within 2 days of admission with symptoms of CDI were considered positive on admission and were not attributed to our facility. A primary episode of CDI was defined to be the first identified episode or event in each patient. Recurrent CDI was defined as a repeated case of CDI within 180 days of the original CDI event.
Interventions to Reduce CDI
Reduction of Antibiotic Pressure
Other actions taken to improve antimicrobial prescribing as part of the stewardship program included medication usage evaluations (MUEs) for levofloxacin and carbapenems, implementing an automatic dosing/duration protocol for levofloxacin, and carbapenem restriction to prevent inappropriate use. Nursing and pharmacy staffs were educated on vancomycin appropriateness, benefits of MRSA screening for de-escalation, procalcitonin, and treatment of sepsis. Emergency department staff was educated on (1) empiric antimicrobial treatment recommendations for urinary and skin and soft tissue infections based on outpatient antibiogram data, (2) renal adjustment of antimicrobials, (3) fluoroquinolones: resistance formation, higher CDI risk and higher dosing recommendations, (4) GI prophylaxis recommendations, and (5) probiotics.
Reduction in the Intensity of Acid Suppression for GI Prophylaxis
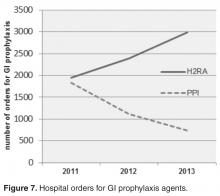
PPIs were substituted with histamine-2 receptor antagonists (H2RA) whenever acid suppression for GI prophylaxis was warranted. If GI symptoms persisted, sucralfate was added. In May 2010, all eligible LTC patients were converted from PPIs to H2RA.
Expanding the Use of Probiotics
We expanded the use of probiotics as an adjunctive treatment for CDI with metronidazole ± vancomycin oral therapies. Probiotics were included concurrently with any broad-spectrum antibiotic administration, longer antibiotic courses (≥ 7 days), and/or multiple courses of antibiotics. The combination of Saccromyces boulardii plus Lactobacillus acidophilus and L. bulgaricus was given with twice daily dosing until the end of 2011. In January 2012, our facility switched over to daily administration of a probiotic with the active ingredients of Lactobacillus acidophilus and Lactobacillus casei, 50 billion colony-forming units. Probiotics were given during the antibiotic course plus for 1 additional week after course completion. Probiotics were not administered to selected groups of patients: (1) immunocompromised patients, (2) patients who were NPO, or (3) patients excluded by their physicians.
There was no change or enhanced targeting of infection prevention or environmental hygiene strategies during the study period.
Data Analysis and Statistical Methods
All data were collected on data collection sheets and transcribed into Microsoft Office Excel 2007 Service Pack 3. No data were excluded from analysis. Continuous variables, eg, number of cases of CDI, are reported as mean ± standard deviation. Categorical variables, eg, number of recurrent CDI cases, are reported as the count and percentage. Comparison of populations was done with the Wilcoxon rank sum test. Segments of the interrupted time series were assessed using linear regression. Associations were tested using χ2. Statistical tests were deemed significant when the α probability was < 0.05. No adjustments were made for multiplicity. Data descriptive statistics (including frequency histograms for visual examination of distributions) and statistical analyses were performed using Stata 11.1 (StataCorp, College Station, TX).
Results
CDIs
Within the population of patients having a CDI or recurrence, we found that those patients in the later time period (2011–2013) were significantly less likely to have a recurrence than those in the earlier time period (pre- Jan 2011) (chi square = 5.975, df = 1, P = 0.015). The odds ratio (OR) was 0.35 (95% CI 0.15 to 0.83).
Patients in the earlier (2009–2010) vs. the later post-intervention group (2011–2013) had more likely received multiple antibiotic courses (chi square = 5.32, df = 1, P = 0.021, OR 2.56), a PPI (chi square = 8.86, df = 1, P = 0.003, OR 3.38), and had a health care facility–associated infection originating from our institution as opposed to outside facility transfers or community-acquired cases (chi square = 7.09, df = 1, P = 0.008, OR 2.94).
Antibiotic Pressure
Acid Suppression
In evaluating the effects of limiting the use of PPIs, patients who received an H2RA or no antacid prophylaxis were significantly less likely to have a recurrence of CDI than those who received a PPI (chi square = 6.35, df = 1, P = 0.012). The OR for recurrence with PPIs was 3.05 (95% CI 1.25 to 7.44). Of patients exposed to PPIs, those exposed in the later time period (2011 through 2013) were significantly less likely to have a recurrence than those exposed in the early time period (third quarter 2008 through 2010; chi square = 15.14, df = 1, P < 0.001). The OR was 0.23 (95% CI, 0.11 to 0.49).
Probiotics
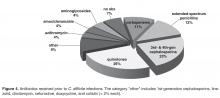
During 2009–2011, only 15% of the CDI patients had received probiotics with an antibiotic course. Probiotic therapy as part of CDI treatment increased from 60% in 2009 to 91% in 2011. Among patients that contracted CDI in 2012–2013, only 2 patients received probiotics with their antibiotic courses.
Recurrences
With regard to the effect of probiotics within this population, those who received
One patient with significant initial antibiotic pressure was continued on her PPI during CDI treatment and continued to have recurrences, despite probiotic use. After her fourth recurrence, her PPI was changed to an H2RA, and she had no further recurrences. She continues off PPI therapy and is CDI-free 2 years later. Another patient who remained on his PPI had 3 recurrences, until finally a probiotic was added and the recurrences abated.
Discussion
CDI is common in hospitalized patients, and its incidence has increased due to multiple factors, which include the widespread use of broad-spectrum antimicrobials and increased use of PPIs. Our observational study showed a statistically significant reduction in the number of health care–associated CDI cases during our implementation period (mid–2008 through 2010). From 2011 on, all initiatives were maintained. As the lower rates of CDI continued, physician confidence in antimicrobial stewardship recommendations increased. During this latter portion of the study period, hospitalists uniformly switched patients to H2RA for GI prophylaxis, added prophylactic probiotics to antibiotic courses as well as CDI therapy, and were more receptive to streamlining and limiting durations of antibiotic therapy. Although the study was completed in 2013, follow-up data have shown that the low CDI incidence has continued through 2014.
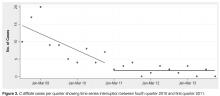
The average age of the patients in our study was 69 years. In 2009, there were 41 C. difficile cases originating from our institution; however, by the end of 2011, only 9 cases had been reported, a 75% reduction. The majority of our cases of C. difficile in 2009–2010 originated from our facility’s LTC units (Figure 2). Risk factors in the LTC population included older age (72% are > 65 years) with multiple comorbidities, exposure to frequent multiple courses of broad-spectrum antibiotics, and use of PPIs as the standard for GI prophylaxis therapy. Multiple antibiotic courses had a strong association with PPI administration in the patients who contracted CDI, while recent antibiotics and antibiotics greater than 10 days did not. Implications may include an increased risk of CDI in patients requiring multiple antibiotic courses concurrent with PPI exposure.
Infection prevention strategies were promulgated among the health care team during the study period but were not specifically targeted for quality improvement efforts. Therefore, in contrast to other studies where infection prevention measures and environmental hygiene were prominent components of a CDI prevention “bundle,” our focus was on antimicrobial stewardship and PPI and probiotic use, not enhancement of standard infection prevention and environmental hygiene measures.
The antibiotics used prior to the development of CDI in our study were similar to findings from other studies that have associated broad-spectrum antibiotics with increased susceptibility to CDI [11]. Antimicrobials disrupt the normal GI flora, which is essential for eradicating many C. difficile spores [12]. The utilization of high-risk antibiotics and prolonged antimicrobial therapy were reduced with implementation of our antimicrobial stewardship program. In 2012, the antimicrobial stewardship program developed a LTC fever protocol, providing education to LTC nurses, physicians, and pharmacists using the modified McGeer criteria [13] for infection in LTC units and empiric antibiotic recommendations from our epidemiologist. A formal recommendation for a LTC 7-day stop date for urinary, respiratory, and skin and soft tissue infections was initiated, which included are-assessment at day 6–7 for resolution of symptoms.
With regard to PPI therapy, our study revealed that patients who had received a PPI at some point were 3.05 times more likely to have a recurrence of CDI than those who had not. These findings are consistent with the literature. Linsky et al [5] found a 42% increased risk of CDI recurrence in patients receiving PPIs concurrent with CDI treatment while considering covariates that may influence the risk of recurrent CDI or exposure to PPIs. A meta-analysis of 16 observational studies involving more than 1.2 million hospitalized patients by Janarthanan et al [14] explored the association between CDI and PPIs and showed a 65% increase in the incidence of CDI among PPI users. Those receiving PPI for GI prophylaxis in the earlier time period (before 2011) were 77% more likely to have a recurrence than those who received PPI in the later period. This finding might be associated with the more appropriate antimicrobial use and the more consistent use of consistent prophylactic probiotics in the later study period.
Our results showed that those who received probiotics with the initial CDI treatment were significantly less likely to have a recurrence than those who did not. Patients receiving probiotics in the later period (2011–2013) were 74% less likely to have a recurrence than patients in the earlier group (2009–2010). Despite the standard use of probiotics for primary CDI prevention at our institution, we could not show direct significance to the lack of probiotic use found in the identified CDI patients with this observational study design. The higher benefit in more recent years could possibly be attributed to the fact that these patients were much less likely to have received a PPI, that most had likely received probiotics concurrently plus 1 week after their antibiotic courses, and their antibiotic therapy was likely more focused and streamlined to prevent C. difficile infection. A meta-analysis of probiotic efficacy in primary CDI prevention suggested that probiotics can lead to a 64% reduction in the incidence of CDI, in addition to reducing GI-associated symptoms related to infection or antibiotic use [9]. A dose-response study of the efficacy of a probiotic formula showed a lower incidence of CDI, 1.2% for higher dose vs. 9.4% for lower dose vs. 23.8% for placebo [15]. Maziade et al [16] added prophylactic probiotics to a bundle of standard preventative measures for C. difficile infections, and were able to show an enhanced and sustained decrease in CDI rates (73%) and recurrences (39%). However, many of the probiotic studies which have studied the relationship to CDI have been criticized for reporting abnormally high rates of infection [9,16] missing data, a lack of controls or excessive patient exclusion criteria [17,18] The more recent PLACIDE study by Allen et al [19] was a large multicenter randomized controlled trial that did not show any benefit to CDI prevention with probiotics; however, with 83% of screened patients excluded, the patients were low risk, with the resulting CDI incidence (0.99%) too low to show a benefit. Acid suppression was also not revealed in the specific CDI cases, and others have found this to be a significant risk factor [5–7].
Limitations of this study include the study design (an observational, retrospective analysis), the small size of our facility, and the difficulty in obtaining probiotic history prior to admission in some cases. Due to a change in computer systems, hospital orders for GI prophylaxis agents could not be obtained for 2009–2010. Due to the fact that we instituted our interventions somewhat concurrently, it is difficult to analyze their individual impact. Randomized controlled trials evaluating the combined role of probiotics, GI prophylaxis, and antibiotic pressure in CDI are needed to further define the importance of this approach.
Corresponding author: Bridget Olson, RPh, Sharp Coronado Hospital & Villa Coronado Long-Term Care Facility, 250
Prospect Pl., Coronado CA 92118, [email protected].
Financial disclosures: None.
Author contributions: conception and design, BO, TH, KW, RO; analysis and interpretation of data, RAF; drafting of article, BO, RAF; critical revision of the article, RAF, JH, TH; provision of study materials or patients, BO; statistical expertise, RAF; administrative or technical support, KW, RO; collection and assembly of data, BO.
1. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. http://www.cdc.gov/drugresistance/threat-report-2013/index.html.
2. Lessa FC, Mu Y, Bamberg WM, Beldavs ZG, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med 2015;372:825–34.
3. Pepin J, Valiquette L, Cossette B. Mortality attributable to nosocomial Clostridium difficile-associated disease during an epidemic caused by a hypervirulent strain in Quebec. CMAJ 2005;173:1037–42.
4. Warren JW, Palumbo FB, Fitterman L, Speedie SM. Incidence and characteristics of antibiotic use in aged nursing home patients. J Am Geriatr Soc 1991;39:963–72.
5. Linsky A, Gupta K, Lawler E, et al. Proton pump inhibitors and risk for recurrent Clostridium difficile infection. Arch Intern Med 2010;170:772–8.
6. Dial S, Delaney JA, Barkun AN, Sulssa S. Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile-associated disease. JAMA 2005;294:2989–95.
7. Howell M, Novack V, Grgurich P, et.al. Iatrogenic gastric acid suppression and the risk if nosocomial Clostridium difficile infection. Arch Intern Med 2010;170:784–90.
8. Radulovic Z, Petrovic T, Bulajic S. Antibiotic susceptibility of probiotic bacteria. In Pana M, editor. Antibiotic resistant bacteria: a continuous challenge in the new millennium. Rijeka, Croatia: InTech; 2012.
9. Goldenberg JZ, Ma SS, Saxton JD, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev 2013;5:CD006095.
10. Johnston BC, Ma SY, Goldenberg JZ, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea. Ann Intern Med 2012;157:878–88.
11. Blondeau JM. What have we learned about antimicrobial use and the risks for Clostridium difficile-associated diarrhoea? J Antimicrob Chemother 2009;63:203–37.
12. Elliott B, Chang BJ, Golledge CL et al. Clostridium difficile-associated diarrhoea. Intern Med J 2007;37:561–8.
13. Stone, ND, Ashraf, MS et al. Surveillance definitions of infections in long-term care facilities: revisiting the McGeer criteria. Infect Control Hosp Epidemiol 2012;33:965–77.
14. Janarthanan S, Ditah I, Adler DG, Ehrinpreis MN. Clostridium difficile-associated diarrhea and proton pump inhibitor therapy: a meta-analysis. Am J Gastroenterol 2012;107:1001–10.
15. Gao XW, Mubasher M, Fang CY, et al. Dose-response efficacy of a proprietary probiotic formula of Lactobacillus acidophilus CL1285 and Lactobacillus casei LBC80R for antibiotic-associated diarrhea and Clostridium difficile-associated diarrhea prophylaxis in adult patients. Am J Gastroenterol 2010;105:1636-41.
16. Maziade PJ, Andriessen JA, Pereira P, et.al. Impact of adding prophylactic probiotics to a bundle of standard preventative measures for Clostridium difficile infections: enhanced and sustained decrease in the incidence and severity of infection at a community hospital. Curr Med Res Opin 2013;29:1341–7.
17. Islam, J, Cohen J, Rajkumar C, Llewelyn M. Probiotics for the prevention and treatment of Clostridium difficile in older patients. Age Ageing 2012;41:706–11.
18. Hickson M, D’Souza AL, Muthu N, et al. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: randomised double blind placebo controlled trial. BMJ 2007;335:80.
19. Allen S J, Wareham K, Wang, D, et.al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomized, double-blind, placebo-controlled, multi-centre trial. Lancet 2013;382:1249–57.
From Sharp HealthCare, San Diego, CA.
Abstract
- Objective: To examine the relationship between the rate of Clostridium difficile infections (CDI) and implementation of 3 interventions aimed at preserving the fecal microbiome: (1) reduction of antimicrobial pressure; (2) reduction in intensity of gastrointestinal prophylaxis with proton-pump inhibitors (PPIs); and (3) expansion of probiotic therapy.
- Methods: We conducted a retrospective analysis of all inpatients with CDI between January 2009 and December 2013 receiving care at our community hospital and associated long-term care (LTC) facility. We used interrupted time series analysis to assess CDI rates during the implementation phase (2008–2010) and the postimplementation phase (2011–2013).
- Results: A reduction in the rate of health care facility–associated CDIs was seen. The mean number of cases per 10,000 patient days fell from 11.9 to 3.6 in acute care and 6.1 to 1.1 in LTC. Recurrence rates decreased from 64% in 2009 to 16% by 2014. The likelihood of CDI recurring was 3 times higher in those exposed to PPI and 0.35 times less likely in those who received probiotics with their initial CDI therapy.
- Conclusion: The risk of CDI incidence and recurrence was significantly reduced in our inpatients, with recurrent CDI associated with PPI use, multiple antibiotic courses, and lack of probiotics. We attribute our success to the combined effect of intensified antibiotic stewardship, reduced PPI use, and expanded probiotic use.
Clostridium difficile is classified as an urgent public health threat by the Centers for Disease Control and Prevention [1]. A recent study by the CDC found that it caused more than 400,000 infections in the United States in 2011, leading to over 29,000 deaths [2]. The costs of treating CDI are substantial and recurrences are common. While rates for many health care–associated infections are declining, C. difficile infection (CDI) rates remain at historically high levels [1] with the elderly at greatest risk for infection and mortality from the illness [3].
CDIs can be prevented. A principal recommendation for preventing CDIs is improving antibiotic use. Antibiotic use increases the risk for developing CDI by disrupting the colonic microbiome. Hospitalized and long-term care (LTC) patients are frequently prescribed antibiotics, but studies indicate that much of this use is inappropriate [4]. Antimicrobial stewardship has been shown to be effective in reducing CDI rates. Other infection prevention measures commonly employed to decrease the risk of hospital-onset CDI include monitoring of hand hygiene compliance using soap and water, terminal cleaning with bleach products of rooms occupied by patients with CDI, and daily cleaning of highly touched areas. At our institution, patients identified with CDI are placed on contact precautions until they have been adequately treated and have had resolution of diarrhea for 48 hours.
In addition to preventing CDI transmission through antimicrobial stewardship, attention is being paid to the possibility that restricting PPI use may help in preventing CDI. The increasing utilization of proton-pump inhibitors (PPIs) in recent years has coincided with the trend of increasing CDI rates. Although C. difficile spores are acid-resistant, vegetative forms are easily affected by acidity. Several studies have shown the association of acid suppression and greater susceptibility of acquiring CDI or recurrences [5–7]. Elevated gastric pH by PPIs facilitates the growth of potentially pathogenic upper and lower gastrointestinal (GI) tract flora, including the conversion of C. difficile from spore to vegetative form in the upper GI tract [5,8].
A growing body of evidence indicates that probiotics are both safe and effective for preventing CDIs [9]. Probiotics may counteract disturbances in intestinal flora, thereby reducing the risk for colonization by pathogenic bacteria. Probiotics can inhibit pathogen adhesion, colonization, and invasion of the gastrointestinal mucosa [10].
We hypothesized that preservation and/or restoration of the diversity of the fecal microbiome would prevent CDI and disease recurrence in our facility. Prior to 2009, we had strict infection prevention measures in place to prevent disease transmission, similar to many other institutions. In 2009, we implemented 3 additional interventions to reduce the rising incidence of CDI: (1) an antibiotic stewardship program, (2) lowering the intensity of acid suppression, and (3) expanding the use of probiotic therapy. The 3 interventions were initiated over the 19-month period January 2009 through July 2010. This study addresses the effects of these interventions.
Methods
Patients and Data Collection
The study was conducted at a community hospital (59 beds) that has an associated LTC facility (122 beds). We conducted a retrospective analysis of hospital and LTC data from all documented cases of CDI between January 2009 and December 2013. Study subjects included all patients with stools positive for C. difficile antigen and toxin with associated symptoms of infection (n = 123). Institutional review board approval was obtained prior to data collection.
The following information was collected: admission diagnosis, number of days from admission until confirmed CDI, residence prior to admission, duration and type of antibiotics received prior to or during symptoms of CDI, type of GI prophylaxis received within 14 days prior to and during CDI treatment, probiotic received and duration, and the type and duration of antibiotic treatment given for the CDI. The data collected was used to determine the likely origin of each C. difficile case, dates of recurrences, and the possible effects of the interventions. Antibiotic use was categorized as: (1) recent antibiotic course (antibiotics received within the preceding 4 weeks), (2) antibiotic courses greater than 10 days, and (3) multiple antibiotic courses (more than 1 antibiotic course received sequentially or concurrently).
Positive C. difficile infections were detected using a 2-step algorithm, starting in 2009. The samples were first screened with a rapid membrane enzyme immunoassay for glutamate dehydrogenase (GDH) antigen and toxin A and B in stool (C. Diff Quik Chek Complete, Techlab, Blacksburg, VA). Discrepant samples (GDH positive and toxin A and B negative) were reflexed to DNA-based PCR testing. The PCR assay was changed to the Verigene C. difficile test (Nanosphere, Northbrook, IL) in 2012. Up to 30 days after discharge from our facility, positive results were considered as acquired from our facility and positive results within 2 days of admission with symptoms of CDI were considered positive on admission and were not attributed to our facility. A primary episode of CDI was defined to be the first identified episode or event in each patient. Recurrent CDI was defined as a repeated case of CDI within 180 days of the original CDI event.
Interventions to Reduce CDI
Reduction of Antibiotic Pressure
Other actions taken to improve antimicrobial prescribing as part of the stewardship program included medication usage evaluations (MUEs) for levofloxacin and carbapenems, implementing an automatic dosing/duration protocol for levofloxacin, and carbapenem restriction to prevent inappropriate use. Nursing and pharmacy staffs were educated on vancomycin appropriateness, benefits of MRSA screening for de-escalation, procalcitonin, and treatment of sepsis. Emergency department staff was educated on (1) empiric antimicrobial treatment recommendations for urinary and skin and soft tissue infections based on outpatient antibiogram data, (2) renal adjustment of antimicrobials, (3) fluoroquinolones: resistance formation, higher CDI risk and higher dosing recommendations, (4) GI prophylaxis recommendations, and (5) probiotics.
Reduction in the Intensity of Acid Suppression for GI Prophylaxis
PPIs were substituted with histamine-2 receptor antagonists (H2RA) whenever acid suppression for GI prophylaxis was warranted. If GI symptoms persisted, sucralfate was added. In May 2010, all eligible LTC patients were converted from PPIs to H2RA.
Expanding the Use of Probiotics
We expanded the use of probiotics as an adjunctive treatment for CDI with metronidazole ± vancomycin oral therapies. Probiotics were included concurrently with any broad-spectrum antibiotic administration, longer antibiotic courses (≥ 7 days), and/or multiple courses of antibiotics. The combination of Saccromyces boulardii plus Lactobacillus acidophilus and L. bulgaricus was given with twice daily dosing until the end of 2011. In January 2012, our facility switched over to daily administration of a probiotic with the active ingredients of Lactobacillus acidophilus and Lactobacillus casei, 50 billion colony-forming units. Probiotics were given during the antibiotic course plus for 1 additional week after course completion. Probiotics were not administered to selected groups of patients: (1) immunocompromised patients, (2) patients who were NPO, or (3) patients excluded by their physicians.
There was no change or enhanced targeting of infection prevention or environmental hygiene strategies during the study period.
Data Analysis and Statistical Methods
All data were collected on data collection sheets and transcribed into Microsoft Office Excel 2007 Service Pack 3. No data were excluded from analysis. Continuous variables, eg, number of cases of CDI, are reported as mean ± standard deviation. Categorical variables, eg, number of recurrent CDI cases, are reported as the count and percentage. Comparison of populations was done with the Wilcoxon rank sum test. Segments of the interrupted time series were assessed using linear regression. Associations were tested using χ2. Statistical tests were deemed significant when the α probability was < 0.05. No adjustments were made for multiplicity. Data descriptive statistics (including frequency histograms for visual examination of distributions) and statistical analyses were performed using Stata 11.1 (StataCorp, College Station, TX).
Results
CDIs
Within the population of patients having a CDI or recurrence, we found that those patients in the later time period (2011–2013) were significantly less likely to have a recurrence than those in the earlier time period (pre- Jan 2011) (chi square = 5.975, df = 1, P = 0.015). The odds ratio (OR) was 0.35 (95% CI 0.15 to 0.83).
Patients in the earlier (2009–2010) vs. the later post-intervention group (2011–2013) had more likely received multiple antibiotic courses (chi square = 5.32, df = 1, P = 0.021, OR 2.56), a PPI (chi square = 8.86, df = 1, P = 0.003, OR 3.38), and had a health care facility–associated infection originating from our institution as opposed to outside facility transfers or community-acquired cases (chi square = 7.09, df = 1, P = 0.008, OR 2.94).
Antibiotic Pressure
Acid Suppression
In evaluating the effects of limiting the use of PPIs, patients who received an H2RA or no antacid prophylaxis were significantly less likely to have a recurrence of CDI than those who received a PPI (chi square = 6.35, df = 1, P = 0.012). The OR for recurrence with PPIs was 3.05 (95% CI 1.25 to 7.44). Of patients exposed to PPIs, those exposed in the later time period (2011 through 2013) were significantly less likely to have a recurrence than those exposed in the early time period (third quarter 2008 through 2010; chi square = 15.14, df = 1, P < 0.001). The OR was 0.23 (95% CI, 0.11 to 0.49).
Probiotics
During 2009–2011, only 15% of the CDI patients had received probiotics with an antibiotic course. Probiotic therapy as part of CDI treatment increased from 60% in 2009 to 91% in 2011. Among patients that contracted CDI in 2012–2013, only 2 patients received probiotics with their antibiotic courses.
Recurrences
With regard to the effect of probiotics within this population, those who received
One patient with significant initial antibiotic pressure was continued on her PPI during CDI treatment and continued to have recurrences, despite probiotic use. After her fourth recurrence, her PPI was changed to an H2RA, and she had no further recurrences. She continues off PPI therapy and is CDI-free 2 years later. Another patient who remained on his PPI had 3 recurrences, until finally a probiotic was added and the recurrences abated.
Discussion
CDI is common in hospitalized patients, and its incidence has increased due to multiple factors, which include the widespread use of broad-spectrum antimicrobials and increased use of PPIs. Our observational study showed a statistically significant reduction in the number of health care–associated CDI cases during our implementation period (mid–2008 through 2010). From 2011 on, all initiatives were maintained. As the lower rates of CDI continued, physician confidence in antimicrobial stewardship recommendations increased. During this latter portion of the study period, hospitalists uniformly switched patients to H2RA for GI prophylaxis, added prophylactic probiotics to antibiotic courses as well as CDI therapy, and were more receptive to streamlining and limiting durations of antibiotic therapy. Although the study was completed in 2013, follow-up data have shown that the low CDI incidence has continued through 2014.
The average age of the patients in our study was 69 years. In 2009, there were 41 C. difficile cases originating from our institution; however, by the end of 2011, only 9 cases had been reported, a 75% reduction. The majority of our cases of C. difficile in 2009–2010 originated from our facility’s LTC units (Figure 2). Risk factors in the LTC population included older age (72% are > 65 years) with multiple comorbidities, exposure to frequent multiple courses of broad-spectrum antibiotics, and use of PPIs as the standard for GI prophylaxis therapy. Multiple antibiotic courses had a strong association with PPI administration in the patients who contracted CDI, while recent antibiotics and antibiotics greater than 10 days did not. Implications may include an increased risk of CDI in patients requiring multiple antibiotic courses concurrent with PPI exposure.
Infection prevention strategies were promulgated among the health care team during the study period but were not specifically targeted for quality improvement efforts. Therefore, in contrast to other studies where infection prevention measures and environmental hygiene were prominent components of a CDI prevention “bundle,” our focus was on antimicrobial stewardship and PPI and probiotic use, not enhancement of standard infection prevention and environmental hygiene measures.
The antibiotics used prior to the development of CDI in our study were similar to findings from other studies that have associated broad-spectrum antibiotics with increased susceptibility to CDI [11]. Antimicrobials disrupt the normal GI flora, which is essential for eradicating many C. difficile spores [12]. The utilization of high-risk antibiotics and prolonged antimicrobial therapy were reduced with implementation of our antimicrobial stewardship program. In 2012, the antimicrobial stewardship program developed a LTC fever protocol, providing education to LTC nurses, physicians, and pharmacists using the modified McGeer criteria [13] for infection in LTC units and empiric antibiotic recommendations from our epidemiologist. A formal recommendation for a LTC 7-day stop date for urinary, respiratory, and skin and soft tissue infections was initiated, which included are-assessment at day 6–7 for resolution of symptoms.
With regard to PPI therapy, our study revealed that patients who had received a PPI at some point were 3.05 times more likely to have a recurrence of CDI than those who had not. These findings are consistent with the literature. Linsky et al [5] found a 42% increased risk of CDI recurrence in patients receiving PPIs concurrent with CDI treatment while considering covariates that may influence the risk of recurrent CDI or exposure to PPIs. A meta-analysis of 16 observational studies involving more than 1.2 million hospitalized patients by Janarthanan et al [14] explored the association between CDI and PPIs and showed a 65% increase in the incidence of CDI among PPI users. Those receiving PPI for GI prophylaxis in the earlier time period (before 2011) were 77% more likely to have a recurrence than those who received PPI in the later period. This finding might be associated with the more appropriate antimicrobial use and the more consistent use of consistent prophylactic probiotics in the later study period.
Our results showed that those who received probiotics with the initial CDI treatment were significantly less likely to have a recurrence than those who did not. Patients receiving probiotics in the later period (2011–2013) were 74% less likely to have a recurrence than patients in the earlier group (2009–2010). Despite the standard use of probiotics for primary CDI prevention at our institution, we could not show direct significance to the lack of probiotic use found in the identified CDI patients with this observational study design. The higher benefit in more recent years could possibly be attributed to the fact that these patients were much less likely to have received a PPI, that most had likely received probiotics concurrently plus 1 week after their antibiotic courses, and their antibiotic therapy was likely more focused and streamlined to prevent C. difficile infection. A meta-analysis of probiotic efficacy in primary CDI prevention suggested that probiotics can lead to a 64% reduction in the incidence of CDI, in addition to reducing GI-associated symptoms related to infection or antibiotic use [9]. A dose-response study of the efficacy of a probiotic formula showed a lower incidence of CDI, 1.2% for higher dose vs. 9.4% for lower dose vs. 23.8% for placebo [15]. Maziade et al [16] added prophylactic probiotics to a bundle of standard preventative measures for C. difficile infections, and were able to show an enhanced and sustained decrease in CDI rates (73%) and recurrences (39%). However, many of the probiotic studies which have studied the relationship to CDI have been criticized for reporting abnormally high rates of infection [9,16] missing data, a lack of controls or excessive patient exclusion criteria [17,18] The more recent PLACIDE study by Allen et al [19] was a large multicenter randomized controlled trial that did not show any benefit to CDI prevention with probiotics; however, with 83% of screened patients excluded, the patients were low risk, with the resulting CDI incidence (0.99%) too low to show a benefit. Acid suppression was also not revealed in the specific CDI cases, and others have found this to be a significant risk factor [5–7].
Limitations of this study include the study design (an observational, retrospective analysis), the small size of our facility, and the difficulty in obtaining probiotic history prior to admission in some cases. Due to a change in computer systems, hospital orders for GI prophylaxis agents could not be obtained for 2009–2010. Due to the fact that we instituted our interventions somewhat concurrently, it is difficult to analyze their individual impact. Randomized controlled trials evaluating the combined role of probiotics, GI prophylaxis, and antibiotic pressure in CDI are needed to further define the importance of this approach.
Corresponding author: Bridget Olson, RPh, Sharp Coronado Hospital & Villa Coronado Long-Term Care Facility, 250
Prospect Pl., Coronado CA 92118, [email protected].
Financial disclosures: None.
Author contributions: conception and design, BO, TH, KW, RO; analysis and interpretation of data, RAF; drafting of article, BO, RAF; critical revision of the article, RAF, JH, TH; provision of study materials or patients, BO; statistical expertise, RAF; administrative or technical support, KW, RO; collection and assembly of data, BO.
From Sharp HealthCare, San Diego, CA.
Abstract
- Objective: To examine the relationship between the rate of Clostridium difficile infections (CDI) and implementation of 3 interventions aimed at preserving the fecal microbiome: (1) reduction of antimicrobial pressure; (2) reduction in intensity of gastrointestinal prophylaxis with proton-pump inhibitors (PPIs); and (3) expansion of probiotic therapy.
- Methods: We conducted a retrospective analysis of all inpatients with CDI between January 2009 and December 2013 receiving care at our community hospital and associated long-term care (LTC) facility. We used interrupted time series analysis to assess CDI rates during the implementation phase (2008–2010) and the postimplementation phase (2011–2013).
- Results: A reduction in the rate of health care facility–associated CDIs was seen. The mean number of cases per 10,000 patient days fell from 11.9 to 3.6 in acute care and 6.1 to 1.1 in LTC. Recurrence rates decreased from 64% in 2009 to 16% by 2014. The likelihood of CDI recurring was 3 times higher in those exposed to PPI and 0.35 times less likely in those who received probiotics with their initial CDI therapy.
- Conclusion: The risk of CDI incidence and recurrence was significantly reduced in our inpatients, with recurrent CDI associated with PPI use, multiple antibiotic courses, and lack of probiotics. We attribute our success to the combined effect of intensified antibiotic stewardship, reduced PPI use, and expanded probiotic use.
Clostridium difficile is classified as an urgent public health threat by the Centers for Disease Control and Prevention [1]. A recent study by the CDC found that it caused more than 400,000 infections in the United States in 2011, leading to over 29,000 deaths [2]. The costs of treating CDI are substantial and recurrences are common. While rates for many health care–associated infections are declining, C. difficile infection (CDI) rates remain at historically high levels [1] with the elderly at greatest risk for infection and mortality from the illness [3].
CDIs can be prevented. A principal recommendation for preventing CDIs is improving antibiotic use. Antibiotic use increases the risk for developing CDI by disrupting the colonic microbiome. Hospitalized and long-term care (LTC) patients are frequently prescribed antibiotics, but studies indicate that much of this use is inappropriate [4]. Antimicrobial stewardship has been shown to be effective in reducing CDI rates. Other infection prevention measures commonly employed to decrease the risk of hospital-onset CDI include monitoring of hand hygiene compliance using soap and water, terminal cleaning with bleach products of rooms occupied by patients with CDI, and daily cleaning of highly touched areas. At our institution, patients identified with CDI are placed on contact precautions until they have been adequately treated and have had resolution of diarrhea for 48 hours.
In addition to preventing CDI transmission through antimicrobial stewardship, attention is being paid to the possibility that restricting PPI use may help in preventing CDI. The increasing utilization of proton-pump inhibitors (PPIs) in recent years has coincided with the trend of increasing CDI rates. Although C. difficile spores are acid-resistant, vegetative forms are easily affected by acidity. Several studies have shown the association of acid suppression and greater susceptibility of acquiring CDI or recurrences [5–7]. Elevated gastric pH by PPIs facilitates the growth of potentially pathogenic upper and lower gastrointestinal (GI) tract flora, including the conversion of C. difficile from spore to vegetative form in the upper GI tract [5,8].
A growing body of evidence indicates that probiotics are both safe and effective for preventing CDIs [9]. Probiotics may counteract disturbances in intestinal flora, thereby reducing the risk for colonization by pathogenic bacteria. Probiotics can inhibit pathogen adhesion, colonization, and invasion of the gastrointestinal mucosa [10].
We hypothesized that preservation and/or restoration of the diversity of the fecal microbiome would prevent CDI and disease recurrence in our facility. Prior to 2009, we had strict infection prevention measures in place to prevent disease transmission, similar to many other institutions. In 2009, we implemented 3 additional interventions to reduce the rising incidence of CDI: (1) an antibiotic stewardship program, (2) lowering the intensity of acid suppression, and (3) expanding the use of probiotic therapy. The 3 interventions were initiated over the 19-month period January 2009 through July 2010. This study addresses the effects of these interventions.
Methods
Patients and Data Collection
The study was conducted at a community hospital (59 beds) that has an associated LTC facility (122 beds). We conducted a retrospective analysis of hospital and LTC data from all documented cases of CDI between January 2009 and December 2013. Study subjects included all patients with stools positive for C. difficile antigen and toxin with associated symptoms of infection (n = 123). Institutional review board approval was obtained prior to data collection.
The following information was collected: admission diagnosis, number of days from admission until confirmed CDI, residence prior to admission, duration and type of antibiotics received prior to or during symptoms of CDI, type of GI prophylaxis received within 14 days prior to and during CDI treatment, probiotic received and duration, and the type and duration of antibiotic treatment given for the CDI. The data collected was used to determine the likely origin of each C. difficile case, dates of recurrences, and the possible effects of the interventions. Antibiotic use was categorized as: (1) recent antibiotic course (antibiotics received within the preceding 4 weeks), (2) antibiotic courses greater than 10 days, and (3) multiple antibiotic courses (more than 1 antibiotic course received sequentially or concurrently).
Positive C. difficile infections were detected using a 2-step algorithm, starting in 2009. The samples were first screened with a rapid membrane enzyme immunoassay for glutamate dehydrogenase (GDH) antigen and toxin A and B in stool (C. Diff Quik Chek Complete, Techlab, Blacksburg, VA). Discrepant samples (GDH positive and toxin A and B negative) were reflexed to DNA-based PCR testing. The PCR assay was changed to the Verigene C. difficile test (Nanosphere, Northbrook, IL) in 2012. Up to 30 days after discharge from our facility, positive results were considered as acquired from our facility and positive results within 2 days of admission with symptoms of CDI were considered positive on admission and were not attributed to our facility. A primary episode of CDI was defined to be the first identified episode or event in each patient. Recurrent CDI was defined as a repeated case of CDI within 180 days of the original CDI event.
Interventions to Reduce CDI
Reduction of Antibiotic Pressure
Other actions taken to improve antimicrobial prescribing as part of the stewardship program included medication usage evaluations (MUEs) for levofloxacin and carbapenems, implementing an automatic dosing/duration protocol for levofloxacin, and carbapenem restriction to prevent inappropriate use. Nursing and pharmacy staffs were educated on vancomycin appropriateness, benefits of MRSA screening for de-escalation, procalcitonin, and treatment of sepsis. Emergency department staff was educated on (1) empiric antimicrobial treatment recommendations for urinary and skin and soft tissue infections based on outpatient antibiogram data, (2) renal adjustment of antimicrobials, (3) fluoroquinolones: resistance formation, higher CDI risk and higher dosing recommendations, (4) GI prophylaxis recommendations, and (5) probiotics.
Reduction in the Intensity of Acid Suppression for GI Prophylaxis
PPIs were substituted with histamine-2 receptor antagonists (H2RA) whenever acid suppression for GI prophylaxis was warranted. If GI symptoms persisted, sucralfate was added. In May 2010, all eligible LTC patients were converted from PPIs to H2RA.
Expanding the Use of Probiotics
We expanded the use of probiotics as an adjunctive treatment for CDI with metronidazole ± vancomycin oral therapies. Probiotics were included concurrently with any broad-spectrum antibiotic administration, longer antibiotic courses (≥ 7 days), and/or multiple courses of antibiotics. The combination of Saccromyces boulardii plus Lactobacillus acidophilus and L. bulgaricus was given with twice daily dosing until the end of 2011. In January 2012, our facility switched over to daily administration of a probiotic with the active ingredients of Lactobacillus acidophilus and Lactobacillus casei, 50 billion colony-forming units. Probiotics were given during the antibiotic course plus for 1 additional week after course completion. Probiotics were not administered to selected groups of patients: (1) immunocompromised patients, (2) patients who were NPO, or (3) patients excluded by their physicians.
There was no change or enhanced targeting of infection prevention or environmental hygiene strategies during the study period.
Data Analysis and Statistical Methods
All data were collected on data collection sheets and transcribed into Microsoft Office Excel 2007 Service Pack 3. No data were excluded from analysis. Continuous variables, eg, number of cases of CDI, are reported as mean ± standard deviation. Categorical variables, eg, number of recurrent CDI cases, are reported as the count and percentage. Comparison of populations was done with the Wilcoxon rank sum test. Segments of the interrupted time series were assessed using linear regression. Associations were tested using χ2. Statistical tests were deemed significant when the α probability was < 0.05. No adjustments were made for multiplicity. Data descriptive statistics (including frequency histograms for visual examination of distributions) and statistical analyses were performed using Stata 11.1 (StataCorp, College Station, TX).
Results
CDIs
Within the population of patients having a CDI or recurrence, we found that those patients in the later time period (2011–2013) were significantly less likely to have a recurrence than those in the earlier time period (pre- Jan 2011) (chi square = 5.975, df = 1, P = 0.015). The odds ratio (OR) was 0.35 (95% CI 0.15 to 0.83).
Patients in the earlier (2009–2010) vs. the later post-intervention group (2011–2013) had more likely received multiple antibiotic courses (chi square = 5.32, df = 1, P = 0.021, OR 2.56), a PPI (chi square = 8.86, df = 1, P = 0.003, OR 3.38), and had a health care facility–associated infection originating from our institution as opposed to outside facility transfers or community-acquired cases (chi square = 7.09, df = 1, P = 0.008, OR 2.94).
Antibiotic Pressure
Acid Suppression
In evaluating the effects of limiting the use of PPIs, patients who received an H2RA or no antacid prophylaxis were significantly less likely to have a recurrence of CDI than those who received a PPI (chi square = 6.35, df = 1, P = 0.012). The OR for recurrence with PPIs was 3.05 (95% CI 1.25 to 7.44). Of patients exposed to PPIs, those exposed in the later time period (2011 through 2013) were significantly less likely to have a recurrence than those exposed in the early time period (third quarter 2008 through 2010; chi square = 15.14, df = 1, P < 0.001). The OR was 0.23 (95% CI, 0.11 to 0.49).
Probiotics
During 2009–2011, only 15% of the CDI patients had received probiotics with an antibiotic course. Probiotic therapy as part of CDI treatment increased from 60% in 2009 to 91% in 2011. Among patients that contracted CDI in 2012–2013, only 2 patients received probiotics with their antibiotic courses.
Recurrences
With regard to the effect of probiotics within this population, those who received
One patient with significant initial antibiotic pressure was continued on her PPI during CDI treatment and continued to have recurrences, despite probiotic use. After her fourth recurrence, her PPI was changed to an H2RA, and she had no further recurrences. She continues off PPI therapy and is CDI-free 2 years later. Another patient who remained on his PPI had 3 recurrences, until finally a probiotic was added and the recurrences abated.
Discussion
CDI is common in hospitalized patients, and its incidence has increased due to multiple factors, which include the widespread use of broad-spectrum antimicrobials and increased use of PPIs. Our observational study showed a statistically significant reduction in the number of health care–associated CDI cases during our implementation period (mid–2008 through 2010). From 2011 on, all initiatives were maintained. As the lower rates of CDI continued, physician confidence in antimicrobial stewardship recommendations increased. During this latter portion of the study period, hospitalists uniformly switched patients to H2RA for GI prophylaxis, added prophylactic probiotics to antibiotic courses as well as CDI therapy, and were more receptive to streamlining and limiting durations of antibiotic therapy. Although the study was completed in 2013, follow-up data have shown that the low CDI incidence has continued through 2014.
The average age of the patients in our study was 69 years. In 2009, there were 41 C. difficile cases originating from our institution; however, by the end of 2011, only 9 cases had been reported, a 75% reduction. The majority of our cases of C. difficile in 2009–2010 originated from our facility’s LTC units (Figure 2). Risk factors in the LTC population included older age (72% are > 65 years) with multiple comorbidities, exposure to frequent multiple courses of broad-spectrum antibiotics, and use of PPIs as the standard for GI prophylaxis therapy. Multiple antibiotic courses had a strong association with PPI administration in the patients who contracted CDI, while recent antibiotics and antibiotics greater than 10 days did not. Implications may include an increased risk of CDI in patients requiring multiple antibiotic courses concurrent with PPI exposure.
Infection prevention strategies were promulgated among the health care team during the study period but were not specifically targeted for quality improvement efforts. Therefore, in contrast to other studies where infection prevention measures and environmental hygiene were prominent components of a CDI prevention “bundle,” our focus was on antimicrobial stewardship and PPI and probiotic use, not enhancement of standard infection prevention and environmental hygiene measures.
The antibiotics used prior to the development of CDI in our study were similar to findings from other studies that have associated broad-spectrum antibiotics with increased susceptibility to CDI [11]. Antimicrobials disrupt the normal GI flora, which is essential for eradicating many C. difficile spores [12]. The utilization of high-risk antibiotics and prolonged antimicrobial therapy were reduced with implementation of our antimicrobial stewardship program. In 2012, the antimicrobial stewardship program developed a LTC fever protocol, providing education to LTC nurses, physicians, and pharmacists using the modified McGeer criteria [13] for infection in LTC units and empiric antibiotic recommendations from our epidemiologist. A formal recommendation for a LTC 7-day stop date for urinary, respiratory, and skin and soft tissue infections was initiated, which included are-assessment at day 6–7 for resolution of symptoms.
With regard to PPI therapy, our study revealed that patients who had received a PPI at some point were 3.05 times more likely to have a recurrence of CDI than those who had not. These findings are consistent with the literature. Linsky et al [5] found a 42% increased risk of CDI recurrence in patients receiving PPIs concurrent with CDI treatment while considering covariates that may influence the risk of recurrent CDI or exposure to PPIs. A meta-analysis of 16 observational studies involving more than 1.2 million hospitalized patients by Janarthanan et al [14] explored the association between CDI and PPIs and showed a 65% increase in the incidence of CDI among PPI users. Those receiving PPI for GI prophylaxis in the earlier time period (before 2011) were 77% more likely to have a recurrence than those who received PPI in the later period. This finding might be associated with the more appropriate antimicrobial use and the more consistent use of consistent prophylactic probiotics in the later study period.
Our results showed that those who received probiotics with the initial CDI treatment were significantly less likely to have a recurrence than those who did not. Patients receiving probiotics in the later period (2011–2013) were 74% less likely to have a recurrence than patients in the earlier group (2009–2010). Despite the standard use of probiotics for primary CDI prevention at our institution, we could not show direct significance to the lack of probiotic use found in the identified CDI patients with this observational study design. The higher benefit in more recent years could possibly be attributed to the fact that these patients were much less likely to have received a PPI, that most had likely received probiotics concurrently plus 1 week after their antibiotic courses, and their antibiotic therapy was likely more focused and streamlined to prevent C. difficile infection. A meta-analysis of probiotic efficacy in primary CDI prevention suggested that probiotics can lead to a 64% reduction in the incidence of CDI, in addition to reducing GI-associated symptoms related to infection or antibiotic use [9]. A dose-response study of the efficacy of a probiotic formula showed a lower incidence of CDI, 1.2% for higher dose vs. 9.4% for lower dose vs. 23.8% for placebo [15]. Maziade et al [16] added prophylactic probiotics to a bundle of standard preventative measures for C. difficile infections, and were able to show an enhanced and sustained decrease in CDI rates (73%) and recurrences (39%). However, many of the probiotic studies which have studied the relationship to CDI have been criticized for reporting abnormally high rates of infection [9,16] missing data, a lack of controls or excessive patient exclusion criteria [17,18] The more recent PLACIDE study by Allen et al [19] was a large multicenter randomized controlled trial that did not show any benefit to CDI prevention with probiotics; however, with 83% of screened patients excluded, the patients were low risk, with the resulting CDI incidence (0.99%) too low to show a benefit. Acid suppression was also not revealed in the specific CDI cases, and others have found this to be a significant risk factor [5–7].
Limitations of this study include the study design (an observational, retrospective analysis), the small size of our facility, and the difficulty in obtaining probiotic history prior to admission in some cases. Due to a change in computer systems, hospital orders for GI prophylaxis agents could not be obtained for 2009–2010. Due to the fact that we instituted our interventions somewhat concurrently, it is difficult to analyze their individual impact. Randomized controlled trials evaluating the combined role of probiotics, GI prophylaxis, and antibiotic pressure in CDI are needed to further define the importance of this approach.
Corresponding author: Bridget Olson, RPh, Sharp Coronado Hospital & Villa Coronado Long-Term Care Facility, 250
Prospect Pl., Coronado CA 92118, [email protected].
Financial disclosures: None.
Author contributions: conception and design, BO, TH, KW, RO; analysis and interpretation of data, RAF; drafting of article, BO, RAF; critical revision of the article, RAF, JH, TH; provision of study materials or patients, BO; statistical expertise, RAF; administrative or technical support, KW, RO; collection and assembly of data, BO.
1. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. http://www.cdc.gov/drugresistance/threat-report-2013/index.html.
2. Lessa FC, Mu Y, Bamberg WM, Beldavs ZG, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med 2015;372:825–34.
3. Pepin J, Valiquette L, Cossette B. Mortality attributable to nosocomial Clostridium difficile-associated disease during an epidemic caused by a hypervirulent strain in Quebec. CMAJ 2005;173:1037–42.
4. Warren JW, Palumbo FB, Fitterman L, Speedie SM. Incidence and characteristics of antibiotic use in aged nursing home patients. J Am Geriatr Soc 1991;39:963–72.
5. Linsky A, Gupta K, Lawler E, et al. Proton pump inhibitors and risk for recurrent Clostridium difficile infection. Arch Intern Med 2010;170:772–8.
6. Dial S, Delaney JA, Barkun AN, Sulssa S. Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile-associated disease. JAMA 2005;294:2989–95.
7. Howell M, Novack V, Grgurich P, et.al. Iatrogenic gastric acid suppression and the risk if nosocomial Clostridium difficile infection. Arch Intern Med 2010;170:784–90.
8. Radulovic Z, Petrovic T, Bulajic S. Antibiotic susceptibility of probiotic bacteria. In Pana M, editor. Antibiotic resistant bacteria: a continuous challenge in the new millennium. Rijeka, Croatia: InTech; 2012.
9. Goldenberg JZ, Ma SS, Saxton JD, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev 2013;5:CD006095.
10. Johnston BC, Ma SY, Goldenberg JZ, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea. Ann Intern Med 2012;157:878–88.
11. Blondeau JM. What have we learned about antimicrobial use and the risks for Clostridium difficile-associated diarrhoea? J Antimicrob Chemother 2009;63:203–37.
12. Elliott B, Chang BJ, Golledge CL et al. Clostridium difficile-associated diarrhoea. Intern Med J 2007;37:561–8.
13. Stone, ND, Ashraf, MS et al. Surveillance definitions of infections in long-term care facilities: revisiting the McGeer criteria. Infect Control Hosp Epidemiol 2012;33:965–77.
14. Janarthanan S, Ditah I, Adler DG, Ehrinpreis MN. Clostridium difficile-associated diarrhea and proton pump inhibitor therapy: a meta-analysis. Am J Gastroenterol 2012;107:1001–10.
15. Gao XW, Mubasher M, Fang CY, et al. Dose-response efficacy of a proprietary probiotic formula of Lactobacillus acidophilus CL1285 and Lactobacillus casei LBC80R for antibiotic-associated diarrhea and Clostridium difficile-associated diarrhea prophylaxis in adult patients. Am J Gastroenterol 2010;105:1636-41.
16. Maziade PJ, Andriessen JA, Pereira P, et.al. Impact of adding prophylactic probiotics to a bundle of standard preventative measures for Clostridium difficile infections: enhanced and sustained decrease in the incidence and severity of infection at a community hospital. Curr Med Res Opin 2013;29:1341–7.
17. Islam, J, Cohen J, Rajkumar C, Llewelyn M. Probiotics for the prevention and treatment of Clostridium difficile in older patients. Age Ageing 2012;41:706–11.
18. Hickson M, D’Souza AL, Muthu N, et al. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: randomised double blind placebo controlled trial. BMJ 2007;335:80.
19. Allen S J, Wareham K, Wang, D, et.al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomized, double-blind, placebo-controlled, multi-centre trial. Lancet 2013;382:1249–57.
1. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. http://www.cdc.gov/drugresistance/threat-report-2013/index.html.
2. Lessa FC, Mu Y, Bamberg WM, Beldavs ZG, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med 2015;372:825–34.
3. Pepin J, Valiquette L, Cossette B. Mortality attributable to nosocomial Clostridium difficile-associated disease during an epidemic caused by a hypervirulent strain in Quebec. CMAJ 2005;173:1037–42.
4. Warren JW, Palumbo FB, Fitterman L, Speedie SM. Incidence and characteristics of antibiotic use in aged nursing home patients. J Am Geriatr Soc 1991;39:963–72.
5. Linsky A, Gupta K, Lawler E, et al. Proton pump inhibitors and risk for recurrent Clostridium difficile infection. Arch Intern Med 2010;170:772–8.
6. Dial S, Delaney JA, Barkun AN, Sulssa S. Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile-associated disease. JAMA 2005;294:2989–95.
7. Howell M, Novack V, Grgurich P, et.al. Iatrogenic gastric acid suppression and the risk if nosocomial Clostridium difficile infection. Arch Intern Med 2010;170:784–90.
8. Radulovic Z, Petrovic T, Bulajic S. Antibiotic susceptibility of probiotic bacteria. In Pana M, editor. Antibiotic resistant bacteria: a continuous challenge in the new millennium. Rijeka, Croatia: InTech; 2012.
9. Goldenberg JZ, Ma SS, Saxton JD, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev 2013;5:CD006095.
10. Johnston BC, Ma SY, Goldenberg JZ, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea. Ann Intern Med 2012;157:878–88.
11. Blondeau JM. What have we learned about antimicrobial use and the risks for Clostridium difficile-associated diarrhoea? J Antimicrob Chemother 2009;63:203–37.
12. Elliott B, Chang BJ, Golledge CL et al. Clostridium difficile-associated diarrhoea. Intern Med J 2007;37:561–8.
13. Stone, ND, Ashraf, MS et al. Surveillance definitions of infections in long-term care facilities: revisiting the McGeer criteria. Infect Control Hosp Epidemiol 2012;33:965–77.
14. Janarthanan S, Ditah I, Adler DG, Ehrinpreis MN. Clostridium difficile-associated diarrhea and proton pump inhibitor therapy: a meta-analysis. Am J Gastroenterol 2012;107:1001–10.
15. Gao XW, Mubasher M, Fang CY, et al. Dose-response efficacy of a proprietary probiotic formula of Lactobacillus acidophilus CL1285 and Lactobacillus casei LBC80R for antibiotic-associated diarrhea and Clostridium difficile-associated diarrhea prophylaxis in adult patients. Am J Gastroenterol 2010;105:1636-41.
16. Maziade PJ, Andriessen JA, Pereira P, et.al. Impact of adding prophylactic probiotics to a bundle of standard preventative measures for Clostridium difficile infections: enhanced and sustained decrease in the incidence and severity of infection at a community hospital. Curr Med Res Opin 2013;29:1341–7.
17. Islam, J, Cohen J, Rajkumar C, Llewelyn M. Probiotics for the prevention and treatment of Clostridium difficile in older patients. Age Ageing 2012;41:706–11.
18. Hickson M, D’Souza AL, Muthu N, et al. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: randomised double blind placebo controlled trial. BMJ 2007;335:80.
19. Allen S J, Wareham K, Wang, D, et.al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomized, double-blind, placebo-controlled, multi-centre trial. Lancet 2013;382:1249–57.