User login
PACT-HF: Transitional care derives no overall benefit
Women respond more to intervention
PHILADELPHIA – A clinical trial of a program that transitions heart failure patients after they’re discharged from the hospital didn’t result in any appreciable improvement in all-cause death, readmissions or emergency department visits after 6 months overall, but it did show that women responded more favorably than men.
Harriette G.C. Van Spall, MD, MPH, reported 6-month results of the Patient-Centered Transitional Care Services in Heart Failure (PACT-HF) trial of 2,494 HF patients at 10 hospitals in Ontario during February 2015 to March 2016. They were randomized to the care-transition program or usual care. The findings, she said at the American Heart Association scientific sessions, “highlight the gap between efficacy that’s often demonstrated in mechanistic clinical trials and effectiveness when we aim to implement these results in real-world settings.” Three-month PACT-HF results were reported previously (JAMA. 2019 Feb 26;321:753-61).
The transitional-care model consisted of a comprehensive needs assessment by a nurse who also provided self-care education, a patient-centered discharge summary, and follow-up with a family physician within 7 days of discharge, which Dr. Van Spall noted “is not current practice in our health care system.”
Patients deemed high risk for readmission or death also received nurse home visits and scheduled visits to a multidisciplinary heart function clinic within 2-4 weeks of discharge and continuing as long as clinically suitable, said Dr. Van Spall, a principal investigator at the Population Health Research Institute, Hamilton, Ont., and assistant professor in cardiology at McMaster University in Hamilton.
The trial found no difference between the intervention and usual-care groups in the two composite endpoints at 6 months, Dr. Van Spall said: all-cause death, readmissions, or ED visits (63.1% and 64.5%, respectively; P = .50); or all-cause readmissions or ED visits (60.8% and 62.4%; P = .36).
“Despite the mutual overall clinical outcomes, we noted specific differences in response to treatment,” she said. With regard to the composite endpoint that included all-cause death, “Men had an attenuated response to the treatment with a hazard ratio of 1.05 (95% confidence interval, 0.87-1.26), whereas women had a hazard ratio of 0.85 (95% CI, 0.71-1.03), demonstrating that women have more of a treatment response to this health care service,” she said.
In men, rates for the first primary composite outcome were 66.3% and 64.1% in the intervention and usual-care groups, whereas in women those rates were 59.9% and 64.8% (P = .04 for sex interaction).
In the second composite endpoint, all-cause readmission or ED visit, “again, men had an attenuated response” with a HR of 1.03, whereas women had a HR of 0.83. Results were similar to those for the first primary composite outcome: 63.4% and 61.7% for intervention and usual care in men and 57.7% and 63% in women (P = .03 for sex interaction).
In putting the findings into context, Dr. Van Spall said tailoring services to risk in HF patients may be fraught with pitfalls. “We delivered intensive services to those patients at high risk of readmission or death, but it is quite possible they are the least likely to derive benefit by virtue of their advanced heart failure,” she said. “It may be that more benefit would have been derived had we chosen low- or moderate-risk patients to receive the intervention.”
She also said the sex-specific outcomes must be interpreted with caution. “But they do give us pause to consider that services could be titrated more effectively if delivered to patients who are more likely to derive benefit,” Dr. Van Spall said. The finding that women derived more of a benefit is in line with other prospective and observational studies that have found that women have a higher sense of self-care, self-efficacy, and confidence in managing their own health care needs than men.
Dr. Van Spall has no financial relationships to disclose.
Women respond more to intervention
Women respond more to intervention
PHILADELPHIA – A clinical trial of a program that transitions heart failure patients after they’re discharged from the hospital didn’t result in any appreciable improvement in all-cause death, readmissions or emergency department visits after 6 months overall, but it did show that women responded more favorably than men.
Harriette G.C. Van Spall, MD, MPH, reported 6-month results of the Patient-Centered Transitional Care Services in Heart Failure (PACT-HF) trial of 2,494 HF patients at 10 hospitals in Ontario during February 2015 to March 2016. They were randomized to the care-transition program or usual care. The findings, she said at the American Heart Association scientific sessions, “highlight the gap between efficacy that’s often demonstrated in mechanistic clinical trials and effectiveness when we aim to implement these results in real-world settings.” Three-month PACT-HF results were reported previously (JAMA. 2019 Feb 26;321:753-61).
The transitional-care model consisted of a comprehensive needs assessment by a nurse who also provided self-care education, a patient-centered discharge summary, and follow-up with a family physician within 7 days of discharge, which Dr. Van Spall noted “is not current practice in our health care system.”
Patients deemed high risk for readmission or death also received nurse home visits and scheduled visits to a multidisciplinary heart function clinic within 2-4 weeks of discharge and continuing as long as clinically suitable, said Dr. Van Spall, a principal investigator at the Population Health Research Institute, Hamilton, Ont., and assistant professor in cardiology at McMaster University in Hamilton.
The trial found no difference between the intervention and usual-care groups in the two composite endpoints at 6 months, Dr. Van Spall said: all-cause death, readmissions, or ED visits (63.1% and 64.5%, respectively; P = .50); or all-cause readmissions or ED visits (60.8% and 62.4%; P = .36).
“Despite the mutual overall clinical outcomes, we noted specific differences in response to treatment,” she said. With regard to the composite endpoint that included all-cause death, “Men had an attenuated response to the treatment with a hazard ratio of 1.05 (95% confidence interval, 0.87-1.26), whereas women had a hazard ratio of 0.85 (95% CI, 0.71-1.03), demonstrating that women have more of a treatment response to this health care service,” she said.
In men, rates for the first primary composite outcome were 66.3% and 64.1% in the intervention and usual-care groups, whereas in women those rates were 59.9% and 64.8% (P = .04 for sex interaction).
In the second composite endpoint, all-cause readmission or ED visit, “again, men had an attenuated response” with a HR of 1.03, whereas women had a HR of 0.83. Results were similar to those for the first primary composite outcome: 63.4% and 61.7% for intervention and usual care in men and 57.7% and 63% in women (P = .03 for sex interaction).
In putting the findings into context, Dr. Van Spall said tailoring services to risk in HF patients may be fraught with pitfalls. “We delivered intensive services to those patients at high risk of readmission or death, but it is quite possible they are the least likely to derive benefit by virtue of their advanced heart failure,” she said. “It may be that more benefit would have been derived had we chosen low- or moderate-risk patients to receive the intervention.”
She also said the sex-specific outcomes must be interpreted with caution. “But they do give us pause to consider that services could be titrated more effectively if delivered to patients who are more likely to derive benefit,” Dr. Van Spall said. The finding that women derived more of a benefit is in line with other prospective and observational studies that have found that women have a higher sense of self-care, self-efficacy, and confidence in managing their own health care needs than men.
Dr. Van Spall has no financial relationships to disclose.
PHILADELPHIA – A clinical trial of a program that transitions heart failure patients after they’re discharged from the hospital didn’t result in any appreciable improvement in all-cause death, readmissions or emergency department visits after 6 months overall, but it did show that women responded more favorably than men.
Harriette G.C. Van Spall, MD, MPH, reported 6-month results of the Patient-Centered Transitional Care Services in Heart Failure (PACT-HF) trial of 2,494 HF patients at 10 hospitals in Ontario during February 2015 to March 2016. They were randomized to the care-transition program or usual care. The findings, she said at the American Heart Association scientific sessions, “highlight the gap between efficacy that’s often demonstrated in mechanistic clinical trials and effectiveness when we aim to implement these results in real-world settings.” Three-month PACT-HF results were reported previously (JAMA. 2019 Feb 26;321:753-61).
The transitional-care model consisted of a comprehensive needs assessment by a nurse who also provided self-care education, a patient-centered discharge summary, and follow-up with a family physician within 7 days of discharge, which Dr. Van Spall noted “is not current practice in our health care system.”
Patients deemed high risk for readmission or death also received nurse home visits and scheduled visits to a multidisciplinary heart function clinic within 2-4 weeks of discharge and continuing as long as clinically suitable, said Dr. Van Spall, a principal investigator at the Population Health Research Institute, Hamilton, Ont., and assistant professor in cardiology at McMaster University in Hamilton.
The trial found no difference between the intervention and usual-care groups in the two composite endpoints at 6 months, Dr. Van Spall said: all-cause death, readmissions, or ED visits (63.1% and 64.5%, respectively; P = .50); or all-cause readmissions or ED visits (60.8% and 62.4%; P = .36).
“Despite the mutual overall clinical outcomes, we noted specific differences in response to treatment,” she said. With regard to the composite endpoint that included all-cause death, “Men had an attenuated response to the treatment with a hazard ratio of 1.05 (95% confidence interval, 0.87-1.26), whereas women had a hazard ratio of 0.85 (95% CI, 0.71-1.03), demonstrating that women have more of a treatment response to this health care service,” she said.
In men, rates for the first primary composite outcome were 66.3% and 64.1% in the intervention and usual-care groups, whereas in women those rates were 59.9% and 64.8% (P = .04 for sex interaction).
In the second composite endpoint, all-cause readmission or ED visit, “again, men had an attenuated response” with a HR of 1.03, whereas women had a HR of 0.83. Results were similar to those for the first primary composite outcome: 63.4% and 61.7% for intervention and usual care in men and 57.7% and 63% in women (P = .03 for sex interaction).
In putting the findings into context, Dr. Van Spall said tailoring services to risk in HF patients may be fraught with pitfalls. “We delivered intensive services to those patients at high risk of readmission or death, but it is quite possible they are the least likely to derive benefit by virtue of their advanced heart failure,” she said. “It may be that more benefit would have been derived had we chosen low- or moderate-risk patients to receive the intervention.”
She also said the sex-specific outcomes must be interpreted with caution. “But they do give us pause to consider that services could be titrated more effectively if delivered to patients who are more likely to derive benefit,” Dr. Van Spall said. The finding that women derived more of a benefit is in line with other prospective and observational studies that have found that women have a higher sense of self-care, self-efficacy, and confidence in managing their own health care needs than men.
Dr. Van Spall has no financial relationships to disclose.
REPORTING FROM AHA 2019
Analyses clarify who benefits from ARNI-ARB combination
PHILADELPHIA – Two clinical trials of the combination therapy of the neprilysin inhibitor sacubitril and the angiotensin II receptor blocker valsartan in patients with heart failure and reduced ejection fraction found that it lowered rates of all-cause death, compared to a renin-angiotensin-system inhibitor alone.
Furthermore, the treatment produced a more beneficial effect in women, who are more prone to heart failure and preserved ejection fraction (HFpEF), lead investigators reported at the American Heart Association scientific sessions.
A prespecified subgroup analysis of 4,796 patients in the PARAGON-HF trial found that the sacubitril/valsartan, or sac/val, combination had a significantly more beneficial risk reduction of first and recurrent hospitalizations for heart failure, as well as cardiovascular death, in women than men. A prespecified pooled analysis of 13,195 patients in the PARAGON-HF and the PARADIGM-HF trials also found women derived a greater benefit from the combination therapy than men, but also concluded that patients with heart failure and even mildly reduced ejection fraction had better outcomes. The results of both studies were published simultaneously with the presentations on Nov. 17 in Circulation (doi: 10.1161/circulationaha.119.044491; doi: 10.1161/circulationaha.119.044586).
The findings underscore the effectiveness of sac/val combination in patients with HF and EF in the lower ranges, defined as 40% or less, commented discussant Lynne Warner Stevenson, MD, of Vanderbilt Heart and Vascular Institute in Nashville, Tenn. “We all agree now that the use of sacubitril/valsartan is very appropriate to improve outcomes in those patients, even if they’ve never been hospitalized,” she said in an interview.
PARAGON-HF subanalysis
John J.V. McMurray, MD, of the University of Glasgow presented the PARAGON-HF subgroup analysis. He said it initially focused on 12 subgroups, but that only two baseline variables showed a modified effect of sac/val: sex and left-ventricle ejection fraction (LVEF). The findings, he said, “stood up in a very robust, multivariable analysis.”
The women in the subgroup analysis were older, had higher baseline New York Heart Association class status, and worse quality of life as measured by Kansas City Cardiomyopathy Questionnaire clinical summary score. At baseline, women also had higher average LVEF (59% vs. 56%), lower N-terminal prohormone brain natriuretic peptide levels, and higher rates of renal dysfunction and chronic kidney disease, but lower incidence of a previous MI and coronary artery disease. Prestudy treatments were similar between the sexes.
In terms of the primary outcome – a composite of total hospitalizations for heart failure and cardiovascular death – “there was an apparent 27% relative risk reduction in women and no overall effect in men,” Dr. McMurray said of the treatment group. “The difference was driven completely by hospitalizations.” Rates of CV death were similar between the valsartan-only and sac/val groups in both men and women, he said.
In the analysis of LVEF, women in the treatment group seemed to cross over to a heightened risk of hospitalization and CV death at an LVEF in the 60%-65% range, Dr. McMurray said, whereas men made that cross over in the 50%-55% range. “It looks as though women might be getting more benefit from this treatment up to a higher EF than in men,” he said.
However, the differences between men and women did not hold up in the analysis of secondary outcomes. At 8 months, women in the sac/val group had a 0.6-point greater decline than did the valsartan-only patients in KCCQ-CSS score, whereas men on sac/val had a 2.8-point lesser decline than did those on valsartan only. Similar differences were seen between the treatment and valsartan-only groups within the sexes, with women showing a noticeable improvement surpassing the men.
Posttreatment hypotension rates in both sexes were higher in the sac/val groups, and the risk of renal dysfunction was a bit less in both treatment groups. Women in the treatment group had significantly higher rates of angioedema than did the valsartan-only group and men in either group.
“Compared to valsartan, it’s important to say that sacubitril/valsartan seemed to reduce the risk of heart failure and hospitalization more in women than men, but we didn’t find a similar differential for other endpoints,” Dr. McMurray said. “Therefore, we’re not sure this is a real effect or a chance finding. It’s very statistically robust, but it could still be a chance finding.”
A possible explanation could be than men may not be responding to sac/val, or that valsartan alone may be more effective in men than women, he said. “This possible effect modification of sac/val vs. valsartan by sex deserves further investigation,” he said.
PARAGON-HF and PARADIGM-HF pooled analysis
Likewise, the prespecified pooled analysis of the PARADIGM-HF and PARAGON-HF trials found a greater benefit of sac/val in women, according to results presented by Scott D. Solomon, MD, of Brigham and Women’s Hospital in Boston. Where PARAGON-HF compared combination therapy with valsartan 160 mg twice daily alone, PARADIGM-HF used enalapril 10 mg twice daily alone as the comparator renin-angiotensin-system (RAS) inhibitor.
“These data suggest that the therapeutic effect of sacubitril/valsartan vs. RAS inhibition alone appear to extend to patients with heart failure and mildly reduced EF, with therapeutic benefits that extend to a higher left-ventricle EF range in women compared to men,” Dr. Solomon said.
The pooled analysis divided patients into six different EF groups: up to 22.5%, then in 10-point increments from 22.5% to 62.5%, and 62.5% or greater. PARADIGM-HF enrolled patients age 18 years and older, whereas PARAGON-HF involved those aged 50 years and older.
The analysis showed that, as LVEF rates increased across the EF groups, the rates of the primary composite outcome – HF hospitalizations, CV death, and all-cause mortality – decreased, but the decline was greatest for CV death and less so for HF hospitalization. And while rates of all-cause mortality decreased as EF increased, rates of non-CV death increased substantially with increasing LVEF.
“For each of these endpoints, there are significant benefits to sacubitril/valsartan in the pooled analysis, and this includes HF hospitalization, CV death, either total or first events, and all-cause mortality, which was reduced overall by 12% in the combination group,” Dr. Solomon said. That benefit was seen in the first five categories of EF, but all but disappeared in the highest category (at least 62.5%), he said.
At the lower end of the EF spectrum, the effect of sac/val is more pronounced and similar for men and women, Dr. Solomon said. “But as EF goes up, we see an attenuation of that effect in both men and women, but it occurs at a different point,” he said. “Women seem to derive a benefit to a higher ejection fraction than men.” As in Dr. McMurray’s research, the benefit seems to extend to LVEF of 55%-60% in men and 65%-70% in women.
“These findings were driven by an observed benefit in patients with chronic heart failure and LVEF below the normal range,” he said. “The benefit in the EF range above the ranking ‘reduced’ but below normal was driven primarily by reduction in HF hospitalization.”
Dr. Stevenson said that these findings indicate that a previous hospitalization for HF with preserved EF may be a telling marker for the effectiveness of sac/val. “As opposed to the patient who has exertional dyspnea but has never decompensated to the level needing hospitalization, if they have pEF, our current analyses would suggest sac/val may not offer them much benefit,” she said.
In real-world practice, cost would be an issue, Dr. Stevenson said. “This drug is very expensive; the majority of patients pay more than $100 a month in out-of-pocket costs, and we have to recognize this is not a therapy that everyone can afford,” she said in an interview. “In many areas, and particularly in the disadvantaged populations, this is not going to be a therapy that we’re going to be able to offer everyone, and that gives me great concern as we move toward trying to treat the whole disease that we’re developing therapies that will be limited by finance rather than by physiology. That’s a major call to action for all of us.”
Novartis sponsored the studies. Dr. McMurray has no disclosures. Dr. Solomon disclosed financial relationships with trial sponsor Novartis along with numerous pharmaceutical companies and the National Heart, Lung, and Blood Institute.
SOURCE: McMurray JJ and Solomon SD. AHA 2019, Late Breaking Science Session 5.
PHILADELPHIA – Two clinical trials of the combination therapy of the neprilysin inhibitor sacubitril and the angiotensin II receptor blocker valsartan in patients with heart failure and reduced ejection fraction found that it lowered rates of all-cause death, compared to a renin-angiotensin-system inhibitor alone.
Furthermore, the treatment produced a more beneficial effect in women, who are more prone to heart failure and preserved ejection fraction (HFpEF), lead investigators reported at the American Heart Association scientific sessions.
A prespecified subgroup analysis of 4,796 patients in the PARAGON-HF trial found that the sacubitril/valsartan, or sac/val, combination had a significantly more beneficial risk reduction of first and recurrent hospitalizations for heart failure, as well as cardiovascular death, in women than men. A prespecified pooled analysis of 13,195 patients in the PARAGON-HF and the PARADIGM-HF trials also found women derived a greater benefit from the combination therapy than men, but also concluded that patients with heart failure and even mildly reduced ejection fraction had better outcomes. The results of both studies were published simultaneously with the presentations on Nov. 17 in Circulation (doi: 10.1161/circulationaha.119.044491; doi: 10.1161/circulationaha.119.044586).
The findings underscore the effectiveness of sac/val combination in patients with HF and EF in the lower ranges, defined as 40% or less, commented discussant Lynne Warner Stevenson, MD, of Vanderbilt Heart and Vascular Institute in Nashville, Tenn. “We all agree now that the use of sacubitril/valsartan is very appropriate to improve outcomes in those patients, even if they’ve never been hospitalized,” she said in an interview.
PARAGON-HF subanalysis
John J.V. McMurray, MD, of the University of Glasgow presented the PARAGON-HF subgroup analysis. He said it initially focused on 12 subgroups, but that only two baseline variables showed a modified effect of sac/val: sex and left-ventricle ejection fraction (LVEF). The findings, he said, “stood up in a very robust, multivariable analysis.”
The women in the subgroup analysis were older, had higher baseline New York Heart Association class status, and worse quality of life as measured by Kansas City Cardiomyopathy Questionnaire clinical summary score. At baseline, women also had higher average LVEF (59% vs. 56%), lower N-terminal prohormone brain natriuretic peptide levels, and higher rates of renal dysfunction and chronic kidney disease, but lower incidence of a previous MI and coronary artery disease. Prestudy treatments were similar between the sexes.
In terms of the primary outcome – a composite of total hospitalizations for heart failure and cardiovascular death – “there was an apparent 27% relative risk reduction in women and no overall effect in men,” Dr. McMurray said of the treatment group. “The difference was driven completely by hospitalizations.” Rates of CV death were similar between the valsartan-only and sac/val groups in both men and women, he said.
In the analysis of LVEF, women in the treatment group seemed to cross over to a heightened risk of hospitalization and CV death at an LVEF in the 60%-65% range, Dr. McMurray said, whereas men made that cross over in the 50%-55% range. “It looks as though women might be getting more benefit from this treatment up to a higher EF than in men,” he said.
However, the differences between men and women did not hold up in the analysis of secondary outcomes. At 8 months, women in the sac/val group had a 0.6-point greater decline than did the valsartan-only patients in KCCQ-CSS score, whereas men on sac/val had a 2.8-point lesser decline than did those on valsartan only. Similar differences were seen between the treatment and valsartan-only groups within the sexes, with women showing a noticeable improvement surpassing the men.
Posttreatment hypotension rates in both sexes were higher in the sac/val groups, and the risk of renal dysfunction was a bit less in both treatment groups. Women in the treatment group had significantly higher rates of angioedema than did the valsartan-only group and men in either group.
“Compared to valsartan, it’s important to say that sacubitril/valsartan seemed to reduce the risk of heart failure and hospitalization more in women than men, but we didn’t find a similar differential for other endpoints,” Dr. McMurray said. “Therefore, we’re not sure this is a real effect or a chance finding. It’s very statistically robust, but it could still be a chance finding.”
A possible explanation could be than men may not be responding to sac/val, or that valsartan alone may be more effective in men than women, he said. “This possible effect modification of sac/val vs. valsartan by sex deserves further investigation,” he said.
PARAGON-HF and PARADIGM-HF pooled analysis
Likewise, the prespecified pooled analysis of the PARADIGM-HF and PARAGON-HF trials found a greater benefit of sac/val in women, according to results presented by Scott D. Solomon, MD, of Brigham and Women’s Hospital in Boston. Where PARAGON-HF compared combination therapy with valsartan 160 mg twice daily alone, PARADIGM-HF used enalapril 10 mg twice daily alone as the comparator renin-angiotensin-system (RAS) inhibitor.
“These data suggest that the therapeutic effect of sacubitril/valsartan vs. RAS inhibition alone appear to extend to patients with heart failure and mildly reduced EF, with therapeutic benefits that extend to a higher left-ventricle EF range in women compared to men,” Dr. Solomon said.
The pooled analysis divided patients into six different EF groups: up to 22.5%, then in 10-point increments from 22.5% to 62.5%, and 62.5% or greater. PARADIGM-HF enrolled patients age 18 years and older, whereas PARAGON-HF involved those aged 50 years and older.
The analysis showed that, as LVEF rates increased across the EF groups, the rates of the primary composite outcome – HF hospitalizations, CV death, and all-cause mortality – decreased, but the decline was greatest for CV death and less so for HF hospitalization. And while rates of all-cause mortality decreased as EF increased, rates of non-CV death increased substantially with increasing LVEF.
“For each of these endpoints, there are significant benefits to sacubitril/valsartan in the pooled analysis, and this includes HF hospitalization, CV death, either total or first events, and all-cause mortality, which was reduced overall by 12% in the combination group,” Dr. Solomon said. That benefit was seen in the first five categories of EF, but all but disappeared in the highest category (at least 62.5%), he said.
At the lower end of the EF spectrum, the effect of sac/val is more pronounced and similar for men and women, Dr. Solomon said. “But as EF goes up, we see an attenuation of that effect in both men and women, but it occurs at a different point,” he said. “Women seem to derive a benefit to a higher ejection fraction than men.” As in Dr. McMurray’s research, the benefit seems to extend to LVEF of 55%-60% in men and 65%-70% in women.
“These findings were driven by an observed benefit in patients with chronic heart failure and LVEF below the normal range,” he said. “The benefit in the EF range above the ranking ‘reduced’ but below normal was driven primarily by reduction in HF hospitalization.”
Dr. Stevenson said that these findings indicate that a previous hospitalization for HF with preserved EF may be a telling marker for the effectiveness of sac/val. “As opposed to the patient who has exertional dyspnea but has never decompensated to the level needing hospitalization, if they have pEF, our current analyses would suggest sac/val may not offer them much benefit,” she said.
In real-world practice, cost would be an issue, Dr. Stevenson said. “This drug is very expensive; the majority of patients pay more than $100 a month in out-of-pocket costs, and we have to recognize this is not a therapy that everyone can afford,” she said in an interview. “In many areas, and particularly in the disadvantaged populations, this is not going to be a therapy that we’re going to be able to offer everyone, and that gives me great concern as we move toward trying to treat the whole disease that we’re developing therapies that will be limited by finance rather than by physiology. That’s a major call to action for all of us.”
Novartis sponsored the studies. Dr. McMurray has no disclosures. Dr. Solomon disclosed financial relationships with trial sponsor Novartis along with numerous pharmaceutical companies and the National Heart, Lung, and Blood Institute.
SOURCE: McMurray JJ and Solomon SD. AHA 2019, Late Breaking Science Session 5.
PHILADELPHIA – Two clinical trials of the combination therapy of the neprilysin inhibitor sacubitril and the angiotensin II receptor blocker valsartan in patients with heart failure and reduced ejection fraction found that it lowered rates of all-cause death, compared to a renin-angiotensin-system inhibitor alone.
Furthermore, the treatment produced a more beneficial effect in women, who are more prone to heart failure and preserved ejection fraction (HFpEF), lead investigators reported at the American Heart Association scientific sessions.
A prespecified subgroup analysis of 4,796 patients in the PARAGON-HF trial found that the sacubitril/valsartan, or sac/val, combination had a significantly more beneficial risk reduction of first and recurrent hospitalizations for heart failure, as well as cardiovascular death, in women than men. A prespecified pooled analysis of 13,195 patients in the PARAGON-HF and the PARADIGM-HF trials also found women derived a greater benefit from the combination therapy than men, but also concluded that patients with heart failure and even mildly reduced ejection fraction had better outcomes. The results of both studies were published simultaneously with the presentations on Nov. 17 in Circulation (doi: 10.1161/circulationaha.119.044491; doi: 10.1161/circulationaha.119.044586).
The findings underscore the effectiveness of sac/val combination in patients with HF and EF in the lower ranges, defined as 40% or less, commented discussant Lynne Warner Stevenson, MD, of Vanderbilt Heart and Vascular Institute in Nashville, Tenn. “We all agree now that the use of sacubitril/valsartan is very appropriate to improve outcomes in those patients, even if they’ve never been hospitalized,” she said in an interview.
PARAGON-HF subanalysis
John J.V. McMurray, MD, of the University of Glasgow presented the PARAGON-HF subgroup analysis. He said it initially focused on 12 subgroups, but that only two baseline variables showed a modified effect of sac/val: sex and left-ventricle ejection fraction (LVEF). The findings, he said, “stood up in a very robust, multivariable analysis.”
The women in the subgroup analysis were older, had higher baseline New York Heart Association class status, and worse quality of life as measured by Kansas City Cardiomyopathy Questionnaire clinical summary score. At baseline, women also had higher average LVEF (59% vs. 56%), lower N-terminal prohormone brain natriuretic peptide levels, and higher rates of renal dysfunction and chronic kidney disease, but lower incidence of a previous MI and coronary artery disease. Prestudy treatments were similar between the sexes.
In terms of the primary outcome – a composite of total hospitalizations for heart failure and cardiovascular death – “there was an apparent 27% relative risk reduction in women and no overall effect in men,” Dr. McMurray said of the treatment group. “The difference was driven completely by hospitalizations.” Rates of CV death were similar between the valsartan-only and sac/val groups in both men and women, he said.
In the analysis of LVEF, women in the treatment group seemed to cross over to a heightened risk of hospitalization and CV death at an LVEF in the 60%-65% range, Dr. McMurray said, whereas men made that cross over in the 50%-55% range. “It looks as though women might be getting more benefit from this treatment up to a higher EF than in men,” he said.
However, the differences between men and women did not hold up in the analysis of secondary outcomes. At 8 months, women in the sac/val group had a 0.6-point greater decline than did the valsartan-only patients in KCCQ-CSS score, whereas men on sac/val had a 2.8-point lesser decline than did those on valsartan only. Similar differences were seen between the treatment and valsartan-only groups within the sexes, with women showing a noticeable improvement surpassing the men.
Posttreatment hypotension rates in both sexes were higher in the sac/val groups, and the risk of renal dysfunction was a bit less in both treatment groups. Women in the treatment group had significantly higher rates of angioedema than did the valsartan-only group and men in either group.
“Compared to valsartan, it’s important to say that sacubitril/valsartan seemed to reduce the risk of heart failure and hospitalization more in women than men, but we didn’t find a similar differential for other endpoints,” Dr. McMurray said. “Therefore, we’re not sure this is a real effect or a chance finding. It’s very statistically robust, but it could still be a chance finding.”
A possible explanation could be than men may not be responding to sac/val, or that valsartan alone may be more effective in men than women, he said. “This possible effect modification of sac/val vs. valsartan by sex deserves further investigation,” he said.
PARAGON-HF and PARADIGM-HF pooled analysis
Likewise, the prespecified pooled analysis of the PARADIGM-HF and PARAGON-HF trials found a greater benefit of sac/val in women, according to results presented by Scott D. Solomon, MD, of Brigham and Women’s Hospital in Boston. Where PARAGON-HF compared combination therapy with valsartan 160 mg twice daily alone, PARADIGM-HF used enalapril 10 mg twice daily alone as the comparator renin-angiotensin-system (RAS) inhibitor.
“These data suggest that the therapeutic effect of sacubitril/valsartan vs. RAS inhibition alone appear to extend to patients with heart failure and mildly reduced EF, with therapeutic benefits that extend to a higher left-ventricle EF range in women compared to men,” Dr. Solomon said.
The pooled analysis divided patients into six different EF groups: up to 22.5%, then in 10-point increments from 22.5% to 62.5%, and 62.5% or greater. PARADIGM-HF enrolled patients age 18 years and older, whereas PARAGON-HF involved those aged 50 years and older.
The analysis showed that, as LVEF rates increased across the EF groups, the rates of the primary composite outcome – HF hospitalizations, CV death, and all-cause mortality – decreased, but the decline was greatest for CV death and less so for HF hospitalization. And while rates of all-cause mortality decreased as EF increased, rates of non-CV death increased substantially with increasing LVEF.
“For each of these endpoints, there are significant benefits to sacubitril/valsartan in the pooled analysis, and this includes HF hospitalization, CV death, either total or first events, and all-cause mortality, which was reduced overall by 12% in the combination group,” Dr. Solomon said. That benefit was seen in the first five categories of EF, but all but disappeared in the highest category (at least 62.5%), he said.
At the lower end of the EF spectrum, the effect of sac/val is more pronounced and similar for men and women, Dr. Solomon said. “But as EF goes up, we see an attenuation of that effect in both men and women, but it occurs at a different point,” he said. “Women seem to derive a benefit to a higher ejection fraction than men.” As in Dr. McMurray’s research, the benefit seems to extend to LVEF of 55%-60% in men and 65%-70% in women.
“These findings were driven by an observed benefit in patients with chronic heart failure and LVEF below the normal range,” he said. “The benefit in the EF range above the ranking ‘reduced’ but below normal was driven primarily by reduction in HF hospitalization.”
Dr. Stevenson said that these findings indicate that a previous hospitalization for HF with preserved EF may be a telling marker for the effectiveness of sac/val. “As opposed to the patient who has exertional dyspnea but has never decompensated to the level needing hospitalization, if they have pEF, our current analyses would suggest sac/val may not offer them much benefit,” she said.
In real-world practice, cost would be an issue, Dr. Stevenson said. “This drug is very expensive; the majority of patients pay more than $100 a month in out-of-pocket costs, and we have to recognize this is not a therapy that everyone can afford,” she said in an interview. “In many areas, and particularly in the disadvantaged populations, this is not going to be a therapy that we’re going to be able to offer everyone, and that gives me great concern as we move toward trying to treat the whole disease that we’re developing therapies that will be limited by finance rather than by physiology. That’s a major call to action for all of us.”
Novartis sponsored the studies. Dr. McMurray has no disclosures. Dr. Solomon disclosed financial relationships with trial sponsor Novartis along with numerous pharmaceutical companies and the National Heart, Lung, and Blood Institute.
SOURCE: McMurray JJ and Solomon SD. AHA 2019, Late Breaking Science Session 5.
REPORTING FROM THE AHA SCIENTIFIC SESSIONS
Navigators improve medication adherence in HFrEF
PHILADELPHIA – Treatment guidelines are clear about optimal treatment of heart failure in patients with reduced ejection fraction (HFrEF), but adherence breakdowns often occur.
So, Brigham and Women’s Hospital in Boston implemented a navigator-administered patient outreach program that led to improved medication adherence over usual care, according to study results reported at the American Heart Association scientific sessions.
Although the study was done at a major academic center, the findings have implications for community practitioners, lead study author Akshay S. Desai, MD, MPH, said in an interview. “The impact of the intervention is clearly greater in those practitioners who manage heart failure and have the least support around them,” he said.
“Our sense is that the kind of population where this intervention would have the greater impact would be a community-dwelling heart failure population managed by community cardiologists, where the infrastructure to provide longitudinal heart failure care is less robust than may be in an academic center,” Dr. Desai said.
The study evaluated adherence in guideline-directed medical therapy (GDMT) at 3 months. “The navigator-led remote medication optimization strategy improved utilization and dosing of all categories of GDMP and was associated with a lower rate of adverse events,” Dr. Desai said. “The impact was more pronounced in patients followed by general practitioners than by a HF specialist.” In the outreach, health navigators contacted patients by phone and managed medications based on remote surveillance of labs, blood pressure, and symptoms under supervision of a pharmacist, nurse practitioner, and heart failure specialist.
The study included 1,028 patients with chronic HFrEF who’d visited a cardiologist at Brigham and Women’s in the year prior to the study: 197 patients and their providers consented to participate in the program with the remainder serving as the reference usual-care group. Most HF specialists at Brigham and Women’s declined to participate in the navigator-led program, Dr. Desai said.
Treating providers were approached for consent to adjust medical therapy according to a sequential, stepped titration algorithm modeled on the current American College of Cardiology/American Heart Association HF Guidelines. The study population did not include patients with end-stage HF, those with a severe noncardiac illness with a life expectancy of less than a year, and patients with a pattern of nonadherence. Baseline characteristics of the two groups were well balanced, Dr. Desai said.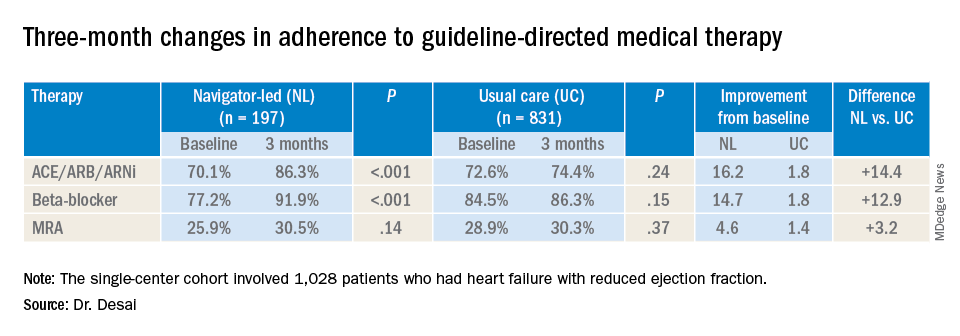
At baseline, 74% (759) participants were treated with ACE inhibitors/angiotensin receptor blockers/angiotensin-receptor neprilysin inhibitors (ACE/ARB/ARNi), 73% (746) with guideline-directed beta-blockers, and 29% (303) with mineralocorticoid receptor antagonists (MRAs), with 10% (107) and 11% (117) treated with target doses of ACE/ARB/ARNi and beta-blockers, respectively.
In the navigator-led group, beta-blocker adherence improved from 77.2% at baseline to 91.9% at 3 months (P less than 0.001) compared with an increase from 84.5% to 86.3% in the usual-care patients (P = 0.15), Dr. Desai said. ACE/ARB/ARNi adherence increased 16.2 percentage points to 86.3% (P less than 0.001) in the navigator-group versus 1.8 percentage points to 74.4% (P = 0.24) for usual care. In the MRA subgroup, 3-month adherence to GDMT was almost identical: 30.5% (P = 0.14) and 30.3% (P = 0.37) for the two treatment groups, respectively, although the navigator-led patients averaged a larger increase of 4.6 versus 1.4 percentage points from baseline.
Adverse event rates were similar in both groups, although the navigator group had “slightly higher rates” of hypotension and hyperkalemia but no serious events, Dr. Desai said. This group also had similarly higher rates of worsening renal function, but most were asymptomatic change in creatinine that was addressed with medication changes, he said. There were no hospitalizations for adverse events.
He said the navigator-led optimization has potential in a community setting because the referral nature of Brigham and Women’s HF population “reflects potentially a worst-case scenario for such a program.” The greatest impact was seen in patients managed by general cardiologists, he said. “If we were to move this forward, which we hope to do with scale, the impact might be greater in a community population where there are fewer specialists and less severe illnesses present.”
This study represents a proof of concept, Dr. Desai said in an interview. “What we would like to do is demonstrate that this can be done on a larger scale,” he said. “That might involve partnership with a payer or health care system to see if we can replicate these findings across a broader range of providers.”
Dr. Desai disclosed financial relationships with Novartis, AstraZeneca, Abbott, Boehringer-Ingelheim, Coston Scientific, Biofourmis, DalCor, Relypsa, Regeneron, and Alnylam. Novartis provided an unrestricted grant for the investigator-initiated trial.
SOURCE: Desai AS. AHA 2019 Featured Science session AOS.07.
PHILADELPHIA – Treatment guidelines are clear about optimal treatment of heart failure in patients with reduced ejection fraction (HFrEF), but adherence breakdowns often occur.
So, Brigham and Women’s Hospital in Boston implemented a navigator-administered patient outreach program that led to improved medication adherence over usual care, according to study results reported at the American Heart Association scientific sessions.
Although the study was done at a major academic center, the findings have implications for community practitioners, lead study author Akshay S. Desai, MD, MPH, said in an interview. “The impact of the intervention is clearly greater in those practitioners who manage heart failure and have the least support around them,” he said.
“Our sense is that the kind of population where this intervention would have the greater impact would be a community-dwelling heart failure population managed by community cardiologists, where the infrastructure to provide longitudinal heart failure care is less robust than may be in an academic center,” Dr. Desai said.
The study evaluated adherence in guideline-directed medical therapy (GDMT) at 3 months. “The navigator-led remote medication optimization strategy improved utilization and dosing of all categories of GDMP and was associated with a lower rate of adverse events,” Dr. Desai said. “The impact was more pronounced in patients followed by general practitioners than by a HF specialist.” In the outreach, health navigators contacted patients by phone and managed medications based on remote surveillance of labs, blood pressure, and symptoms under supervision of a pharmacist, nurse practitioner, and heart failure specialist.
The study included 1,028 patients with chronic HFrEF who’d visited a cardiologist at Brigham and Women’s in the year prior to the study: 197 patients and their providers consented to participate in the program with the remainder serving as the reference usual-care group. Most HF specialists at Brigham and Women’s declined to participate in the navigator-led program, Dr. Desai said.
Treating providers were approached for consent to adjust medical therapy according to a sequential, stepped titration algorithm modeled on the current American College of Cardiology/American Heart Association HF Guidelines. The study population did not include patients with end-stage HF, those with a severe noncardiac illness with a life expectancy of less than a year, and patients with a pattern of nonadherence. Baseline characteristics of the two groups were well balanced, Dr. Desai said.
At baseline, 74% (759) participants were treated with ACE inhibitors/angiotensin receptor blockers/angiotensin-receptor neprilysin inhibitors (ACE/ARB/ARNi), 73% (746) with guideline-directed beta-blockers, and 29% (303) with mineralocorticoid receptor antagonists (MRAs), with 10% (107) and 11% (117) treated with target doses of ACE/ARB/ARNi and beta-blockers, respectively.
In the navigator-led group, beta-blocker adherence improved from 77.2% at baseline to 91.9% at 3 months (P less than 0.001) compared with an increase from 84.5% to 86.3% in the usual-care patients (P = 0.15), Dr. Desai said. ACE/ARB/ARNi adherence increased 16.2 percentage points to 86.3% (P less than 0.001) in the navigator-group versus 1.8 percentage points to 74.4% (P = 0.24) for usual care. In the MRA subgroup, 3-month adherence to GDMT was almost identical: 30.5% (P = 0.14) and 30.3% (P = 0.37) for the two treatment groups, respectively, although the navigator-led patients averaged a larger increase of 4.6 versus 1.4 percentage points from baseline.
Adverse event rates were similar in both groups, although the navigator group had “slightly higher rates” of hypotension and hyperkalemia but no serious events, Dr. Desai said. This group also had similarly higher rates of worsening renal function, but most were asymptomatic change in creatinine that was addressed with medication changes, he said. There were no hospitalizations for adverse events.
He said the navigator-led optimization has potential in a community setting because the referral nature of Brigham and Women’s HF population “reflects potentially a worst-case scenario for such a program.” The greatest impact was seen in patients managed by general cardiologists, he said. “If we were to move this forward, which we hope to do with scale, the impact might be greater in a community population where there are fewer specialists and less severe illnesses present.”
This study represents a proof of concept, Dr. Desai said in an interview. “What we would like to do is demonstrate that this can be done on a larger scale,” he said. “That might involve partnership with a payer or health care system to see if we can replicate these findings across a broader range of providers.”
Dr. Desai disclosed financial relationships with Novartis, AstraZeneca, Abbott, Boehringer-Ingelheim, Coston Scientific, Biofourmis, DalCor, Relypsa, Regeneron, and Alnylam. Novartis provided an unrestricted grant for the investigator-initiated trial.
SOURCE: Desai AS. AHA 2019 Featured Science session AOS.07.
PHILADELPHIA – Treatment guidelines are clear about optimal treatment of heart failure in patients with reduced ejection fraction (HFrEF), but adherence breakdowns often occur.
So, Brigham and Women’s Hospital in Boston implemented a navigator-administered patient outreach program that led to improved medication adherence over usual care, according to study results reported at the American Heart Association scientific sessions.
Although the study was done at a major academic center, the findings have implications for community practitioners, lead study author Akshay S. Desai, MD, MPH, said in an interview. “The impact of the intervention is clearly greater in those practitioners who manage heart failure and have the least support around them,” he said.
“Our sense is that the kind of population where this intervention would have the greater impact would be a community-dwelling heart failure population managed by community cardiologists, where the infrastructure to provide longitudinal heart failure care is less robust than may be in an academic center,” Dr. Desai said.
The study evaluated adherence in guideline-directed medical therapy (GDMT) at 3 months. “The navigator-led remote medication optimization strategy improved utilization and dosing of all categories of GDMP and was associated with a lower rate of adverse events,” Dr. Desai said. “The impact was more pronounced in patients followed by general practitioners than by a HF specialist.” In the outreach, health navigators contacted patients by phone and managed medications based on remote surveillance of labs, blood pressure, and symptoms under supervision of a pharmacist, nurse practitioner, and heart failure specialist.
The study included 1,028 patients with chronic HFrEF who’d visited a cardiologist at Brigham and Women’s in the year prior to the study: 197 patients and their providers consented to participate in the program with the remainder serving as the reference usual-care group. Most HF specialists at Brigham and Women’s declined to participate in the navigator-led program, Dr. Desai said.
Treating providers were approached for consent to adjust medical therapy according to a sequential, stepped titration algorithm modeled on the current American College of Cardiology/American Heart Association HF Guidelines. The study population did not include patients with end-stage HF, those with a severe noncardiac illness with a life expectancy of less than a year, and patients with a pattern of nonadherence. Baseline characteristics of the two groups were well balanced, Dr. Desai said.
At baseline, 74% (759) participants were treated with ACE inhibitors/angiotensin receptor blockers/angiotensin-receptor neprilysin inhibitors (ACE/ARB/ARNi), 73% (746) with guideline-directed beta-blockers, and 29% (303) with mineralocorticoid receptor antagonists (MRAs), with 10% (107) and 11% (117) treated with target doses of ACE/ARB/ARNi and beta-blockers, respectively.
In the navigator-led group, beta-blocker adherence improved from 77.2% at baseline to 91.9% at 3 months (P less than 0.001) compared with an increase from 84.5% to 86.3% in the usual-care patients (P = 0.15), Dr. Desai said. ACE/ARB/ARNi adherence increased 16.2 percentage points to 86.3% (P less than 0.001) in the navigator-group versus 1.8 percentage points to 74.4% (P = 0.24) for usual care. In the MRA subgroup, 3-month adherence to GDMT was almost identical: 30.5% (P = 0.14) and 30.3% (P = 0.37) for the two treatment groups, respectively, although the navigator-led patients averaged a larger increase of 4.6 versus 1.4 percentage points from baseline.
Adverse event rates were similar in both groups, although the navigator group had “slightly higher rates” of hypotension and hyperkalemia but no serious events, Dr. Desai said. This group also had similarly higher rates of worsening renal function, but most were asymptomatic change in creatinine that was addressed with medication changes, he said. There were no hospitalizations for adverse events.
He said the navigator-led optimization has potential in a community setting because the referral nature of Brigham and Women’s HF population “reflects potentially a worst-case scenario for such a program.” The greatest impact was seen in patients managed by general cardiologists, he said. “If we were to move this forward, which we hope to do with scale, the impact might be greater in a community population where there are fewer specialists and less severe illnesses present.”
This study represents a proof of concept, Dr. Desai said in an interview. “What we would like to do is demonstrate that this can be done on a larger scale,” he said. “That might involve partnership with a payer or health care system to see if we can replicate these findings across a broader range of providers.”
Dr. Desai disclosed financial relationships with Novartis, AstraZeneca, Abbott, Boehringer-Ingelheim, Coston Scientific, Biofourmis, DalCor, Relypsa, Regeneron, and Alnylam. Novartis provided an unrestricted grant for the investigator-initiated trial.
SOURCE: Desai AS. AHA 2019 Featured Science session AOS.07.
REPORTING FROM AHA 2019
Mechanical circulatory support in PCI needs clearer guidance
PHILADELPHIA – Use of the Impella ventricular-assist device in patients with cardiogenic shock having percutaneous coronary interventions (PCI) has increased rapidly since its approval in 2008, but two studies comparing it with intra-aortic balloon pumps in PCI patients have raised questions about the safety, effectiveness, and cost of the ventricular-assist device, according to results of two studies presented at the American Heart Association scientific sessions.
The results of an observational analysis of 48,306 patients and a national real-world study of 28,304 patients may not be telling the complete story of the utility of ventricular assist in patients requiring mechanical circulatory support (MCS), one interventional cardiologist said in an interview. “It’s concerning; it’s sobering,” said Ranya N. Sweis, MD, of Northwestern University, Chicago. However, the data didn’t parse out patients who would have been routed to palliative care and otherwise wouldn’t have been candidates for PCI without MCS.
“What I take from it is that we need to get more randomized data,” she said. “Who are the patients that were doing worse? Who are the patients who really needed the Impella support for the PCI after cardiogenic shock?”
In the observational study, Amit P. Amin, MD, of Washington University, St. Louis, said that the use of MCS devices increased steadily to 32% of all PCI patients receiving MCS from 2008 to 2016 while use of intra-aortic balloon pump (IABP) declined, but that Impella was less likely to be used in critically ill patients. The study analyzed patients in the Premier Healthcare Database who had PCI with MCS at 432 hospitals from 2004 to 2016.
Outcomes in what Dr. Amin called “the Impella era,” showed significantly higher risks for death, acute kidney injury, and stroke, with odds ratios of 1.17, 1.91 and 3.34, respectively (P less than .001 for all). In the patient-level comparison of Impella versus IABP, Impella had a 24% higher risk of death (P less than .0001), 10% for bleeding (P = .0445), 8% for acute kidney injury (P = .0521) and 34% for stroke (P less than .0001). The findings were published simultaneously with the presentation (Circulation. 2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044007)
“The total length of stay, as well as the ICU length of stay, were actually lower with Impella use, by approximately a half day to 1 day,” Dr. Amin said. “Despite that, the total costs were approximately $15,000.”
Yet, the study found wide variation in the use of Impella among hospitals, some doing no cases with the device and others all of them, Dr. Amin said. The risk analysis also found wide variations in outcomes across hospitals using Impella. “We saw a 2.5-fold variation in bleeding across hospitals and a 1.5-fold variation in acute kidney injury, stroke and death,” he noted. The study found less variation in hospital stays and total cost of Impella, “perhaps related to the uniformly high device acquisition costs.”
“These data underscore the need for defining the appropriate use of mechanical circulatory support in patients undergoing PCI,” Dr. Amin said.
Dr. Sweis wasn’t surprised by the cost findings. “New technology is going to cost more,” she said in an interview. “I’m actually surprised that the cost wasn’t more significantly different just knowing the cost of some of these devices.
Patients who require MCS represent a small portion of PCI cases: 2%, according to Dr. Sweis. “It’s not like all PCI has increased because of MCS, and there’s a potential improvement in the length of stay so there are going to be cost savings that way.”
The national real-world study that Sanket S. Dhruva, MD, MHS, of the University of California San Francisco, reported on focused on Impella and IABP in PCI patients with acute MI complicated by cardiogenic shock (CS). The study used outcomes of patients with AMI-CS who had PCI from October 2015 to December 2017 in the National Cardiovascular Data Registry’s CathPCI and Chest Pain–MI registries. An estimated 4%-12% of AMIs present with CS.
Most patients in the study population had medical therapy only, but this study focused on the 1,768 who had Impella only and the 8,471 who had IABP only. The rates of in-hospital death and bleeding were 34.1% 16% in the IABP group, and 45% and 31.3% in the Impella group, Dr. Dhruva said. In this study population, the rate of Impella use increased from 3.5% in 2015 to 8.7% by the end of 2017 (P less than .001).
Dr. Dhruva acknowledged a number of limitations to the study findings, including residual confounding. However, the “robust propensity match” of 95% of the Impella-only patients and the results were consistent across multiple sensitivity analyses. “There may have been questions about the clinical severity of AMI-CS patients in the NCDR Registry,” he said. “However, the registry definition is similar to that used in the trials.”
The trial also failed to distinguish between the different types of Impella devices, but the results mostly pertain to the Impella 2.5 and CP because the 5.0 device requires a surgical cutdown, and the study excluded patients who received multiple devices.
“Better evidence and guidance are needed regarding the optimal management of patients with AMI-CS as well as the role of mechanical circulatory support devices in general and Impella in particular,” he said, adding that Impella has been on the U.S. market since 2008, but with limited randomized clinical trial evidence in cardiogenic shock.
The study population of patient’s with CS is “only a piece of the puzzle,” Dr. Sweis said. “We know that there are sick hearts that aren’t in shock right now, but you’re going to do triple-vessel intervention and use atherectomy. Those patients would not do very well during the procedure itself and it may not even be offered to them if there weren’t support.”
Impella is not going away, Dr. Sweis said. “It provides an option that a patient wouldn’t otherwise have. This is really stressing to me that we need to get rid of that variability in the safety related to these devices.”
Dr. Amin disclosed financial relationships with Terumo and GE Healthcare. Dr. Dhruva had no financial relationships to disclose. The study was supported in part by a Center of Excellence in Regulatory Science and Innovation grant from the Food and Drug Administration and the American College of Cardiology’s National Cardiovascular Data Registry.
PHILADELPHIA – Use of the Impella ventricular-assist device in patients with cardiogenic shock having percutaneous coronary interventions (PCI) has increased rapidly since its approval in 2008, but two studies comparing it with intra-aortic balloon pumps in PCI patients have raised questions about the safety, effectiveness, and cost of the ventricular-assist device, according to results of two studies presented at the American Heart Association scientific sessions.
The results of an observational analysis of 48,306 patients and a national real-world study of 28,304 patients may not be telling the complete story of the utility of ventricular assist in patients requiring mechanical circulatory support (MCS), one interventional cardiologist said in an interview. “It’s concerning; it’s sobering,” said Ranya N. Sweis, MD, of Northwestern University, Chicago. However, the data didn’t parse out patients who would have been routed to palliative care and otherwise wouldn’t have been candidates for PCI without MCS.
“What I take from it is that we need to get more randomized data,” she said. “Who are the patients that were doing worse? Who are the patients who really needed the Impella support for the PCI after cardiogenic shock?”
In the observational study, Amit P. Amin, MD, of Washington University, St. Louis, said that the use of MCS devices increased steadily to 32% of all PCI patients receiving MCS from 2008 to 2016 while use of intra-aortic balloon pump (IABP) declined, but that Impella was less likely to be used in critically ill patients. The study analyzed patients in the Premier Healthcare Database who had PCI with MCS at 432 hospitals from 2004 to 2016.
Outcomes in what Dr. Amin called “the Impella era,” showed significantly higher risks for death, acute kidney injury, and stroke, with odds ratios of 1.17, 1.91 and 3.34, respectively (P less than .001 for all). In the patient-level comparison of Impella versus IABP, Impella had a 24% higher risk of death (P less than .0001), 10% for bleeding (P = .0445), 8% for acute kidney injury (P = .0521) and 34% for stroke (P less than .0001). The findings were published simultaneously with the presentation (Circulation. 2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044007)
“The total length of stay, as well as the ICU length of stay, were actually lower with Impella use, by approximately a half day to 1 day,” Dr. Amin said. “Despite that, the total costs were approximately $15,000.”
Yet, the study found wide variation in the use of Impella among hospitals, some doing no cases with the device and others all of them, Dr. Amin said. The risk analysis also found wide variations in outcomes across hospitals using Impella. “We saw a 2.5-fold variation in bleeding across hospitals and a 1.5-fold variation in acute kidney injury, stroke and death,” he noted. The study found less variation in hospital stays and total cost of Impella, “perhaps related to the uniformly high device acquisition costs.”
“These data underscore the need for defining the appropriate use of mechanical circulatory support in patients undergoing PCI,” Dr. Amin said.
Dr. Sweis wasn’t surprised by the cost findings. “New technology is going to cost more,” she said in an interview. “I’m actually surprised that the cost wasn’t more significantly different just knowing the cost of some of these devices.
Patients who require MCS represent a small portion of PCI cases: 2%, according to Dr. Sweis. “It’s not like all PCI has increased because of MCS, and there’s a potential improvement in the length of stay so there are going to be cost savings that way.”
The national real-world study that Sanket S. Dhruva, MD, MHS, of the University of California San Francisco, reported on focused on Impella and IABP in PCI patients with acute MI complicated by cardiogenic shock (CS). The study used outcomes of patients with AMI-CS who had PCI from October 2015 to December 2017 in the National Cardiovascular Data Registry’s CathPCI and Chest Pain–MI registries. An estimated 4%-12% of AMIs present with CS.
Most patients in the study population had medical therapy only, but this study focused on the 1,768 who had Impella only and the 8,471 who had IABP only. The rates of in-hospital death and bleeding were 34.1% 16% in the IABP group, and 45% and 31.3% in the Impella group, Dr. Dhruva said. In this study population, the rate of Impella use increased from 3.5% in 2015 to 8.7% by the end of 2017 (P less than .001).
Dr. Dhruva acknowledged a number of limitations to the study findings, including residual confounding. However, the “robust propensity match” of 95% of the Impella-only patients and the results were consistent across multiple sensitivity analyses. “There may have been questions about the clinical severity of AMI-CS patients in the NCDR Registry,” he said. “However, the registry definition is similar to that used in the trials.”
The trial also failed to distinguish between the different types of Impella devices, but the results mostly pertain to the Impella 2.5 and CP because the 5.0 device requires a surgical cutdown, and the study excluded patients who received multiple devices.
“Better evidence and guidance are needed regarding the optimal management of patients with AMI-CS as well as the role of mechanical circulatory support devices in general and Impella in particular,” he said, adding that Impella has been on the U.S. market since 2008, but with limited randomized clinical trial evidence in cardiogenic shock.
The study population of patient’s with CS is “only a piece of the puzzle,” Dr. Sweis said. “We know that there are sick hearts that aren’t in shock right now, but you’re going to do triple-vessel intervention and use atherectomy. Those patients would not do very well during the procedure itself and it may not even be offered to them if there weren’t support.”
Impella is not going away, Dr. Sweis said. “It provides an option that a patient wouldn’t otherwise have. This is really stressing to me that we need to get rid of that variability in the safety related to these devices.”
Dr. Amin disclosed financial relationships with Terumo and GE Healthcare. Dr. Dhruva had no financial relationships to disclose. The study was supported in part by a Center of Excellence in Regulatory Science and Innovation grant from the Food and Drug Administration and the American College of Cardiology’s National Cardiovascular Data Registry.
PHILADELPHIA – Use of the Impella ventricular-assist device in patients with cardiogenic shock having percutaneous coronary interventions (PCI) has increased rapidly since its approval in 2008, but two studies comparing it with intra-aortic balloon pumps in PCI patients have raised questions about the safety, effectiveness, and cost of the ventricular-assist device, according to results of two studies presented at the American Heart Association scientific sessions.
The results of an observational analysis of 48,306 patients and a national real-world study of 28,304 patients may not be telling the complete story of the utility of ventricular assist in patients requiring mechanical circulatory support (MCS), one interventional cardiologist said in an interview. “It’s concerning; it’s sobering,” said Ranya N. Sweis, MD, of Northwestern University, Chicago. However, the data didn’t parse out patients who would have been routed to palliative care and otherwise wouldn’t have been candidates for PCI without MCS.
“What I take from it is that we need to get more randomized data,” she said. “Who are the patients that were doing worse? Who are the patients who really needed the Impella support for the PCI after cardiogenic shock?”
In the observational study, Amit P. Amin, MD, of Washington University, St. Louis, said that the use of MCS devices increased steadily to 32% of all PCI patients receiving MCS from 2008 to 2016 while use of intra-aortic balloon pump (IABP) declined, but that Impella was less likely to be used in critically ill patients. The study analyzed patients in the Premier Healthcare Database who had PCI with MCS at 432 hospitals from 2004 to 2016.
Outcomes in what Dr. Amin called “the Impella era,” showed significantly higher risks for death, acute kidney injury, and stroke, with odds ratios of 1.17, 1.91 and 3.34, respectively (P less than .001 for all). In the patient-level comparison of Impella versus IABP, Impella had a 24% higher risk of death (P less than .0001), 10% for bleeding (P = .0445), 8% for acute kidney injury (P = .0521) and 34% for stroke (P less than .0001). The findings were published simultaneously with the presentation (Circulation. 2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044007)
“The total length of stay, as well as the ICU length of stay, were actually lower with Impella use, by approximately a half day to 1 day,” Dr. Amin said. “Despite that, the total costs were approximately $15,000.”
Yet, the study found wide variation in the use of Impella among hospitals, some doing no cases with the device and others all of them, Dr. Amin said. The risk analysis also found wide variations in outcomes across hospitals using Impella. “We saw a 2.5-fold variation in bleeding across hospitals and a 1.5-fold variation in acute kidney injury, stroke and death,” he noted. The study found less variation in hospital stays and total cost of Impella, “perhaps related to the uniformly high device acquisition costs.”
“These data underscore the need for defining the appropriate use of mechanical circulatory support in patients undergoing PCI,” Dr. Amin said.
Dr. Sweis wasn’t surprised by the cost findings. “New technology is going to cost more,” she said in an interview. “I’m actually surprised that the cost wasn’t more significantly different just knowing the cost of some of these devices.
Patients who require MCS represent a small portion of PCI cases: 2%, according to Dr. Sweis. “It’s not like all PCI has increased because of MCS, and there’s a potential improvement in the length of stay so there are going to be cost savings that way.”
The national real-world study that Sanket S. Dhruva, MD, MHS, of the University of California San Francisco, reported on focused on Impella and IABP in PCI patients with acute MI complicated by cardiogenic shock (CS). The study used outcomes of patients with AMI-CS who had PCI from October 2015 to December 2017 in the National Cardiovascular Data Registry’s CathPCI and Chest Pain–MI registries. An estimated 4%-12% of AMIs present with CS.
Most patients in the study population had medical therapy only, but this study focused on the 1,768 who had Impella only and the 8,471 who had IABP only. The rates of in-hospital death and bleeding were 34.1% 16% in the IABP group, and 45% and 31.3% in the Impella group, Dr. Dhruva said. In this study population, the rate of Impella use increased from 3.5% in 2015 to 8.7% by the end of 2017 (P less than .001).
Dr. Dhruva acknowledged a number of limitations to the study findings, including residual confounding. However, the “robust propensity match” of 95% of the Impella-only patients and the results were consistent across multiple sensitivity analyses. “There may have been questions about the clinical severity of AMI-CS patients in the NCDR Registry,” he said. “However, the registry definition is similar to that used in the trials.”
The trial also failed to distinguish between the different types of Impella devices, but the results mostly pertain to the Impella 2.5 and CP because the 5.0 device requires a surgical cutdown, and the study excluded patients who received multiple devices.
“Better evidence and guidance are needed regarding the optimal management of patients with AMI-CS as well as the role of mechanical circulatory support devices in general and Impella in particular,” he said, adding that Impella has been on the U.S. market since 2008, but with limited randomized clinical trial evidence in cardiogenic shock.
The study population of patient’s with CS is “only a piece of the puzzle,” Dr. Sweis said. “We know that there are sick hearts that aren’t in shock right now, but you’re going to do triple-vessel intervention and use atherectomy. Those patients would not do very well during the procedure itself and it may not even be offered to them if there weren’t support.”
Impella is not going away, Dr. Sweis said. “It provides an option that a patient wouldn’t otherwise have. This is really stressing to me that we need to get rid of that variability in the safety related to these devices.”
Dr. Amin disclosed financial relationships with Terumo and GE Healthcare. Dr. Dhruva had no financial relationships to disclose. The study was supported in part by a Center of Excellence in Regulatory Science and Innovation grant from the Food and Drug Administration and the American College of Cardiology’s National Cardiovascular Data Registry.
REPORTING FROM AHA 2019
Colchicine pre-PCI improves biomarkers, not injury risk
PHILADELPHIA – Giving patients a single shot of colchicine before percutaneous coronary intervention was found to favorably impact inflammatory biomarkers linked to vascular injury, but not to lower the risk of procedure-related myocardial injury, according to results of the COLCHICINE-PCI randomized trial reported at the American Heart Association scientific sessions.
This is the first study to evaluate pre-PCI colchicine versus placebo on markers of myocardial injury and inflammation, said Binita Shah, MD, of Veterans Affairs New York Harbor Healthcare System and New York University.
“More work is needed to determine the optimal dosing and timing regimen of colchicine administration in patients undergoing PCI,” Dr. Shah said in an interview. “In this study, we saw inflammatory markers decrease around the 24-hour time point post PCI, so an earlier start to preprocedural colchicine regimen warrants further investigation.” The study found that pre-PCI colchicine attenuated increases in interleukin-6 and high-sensitivity C-reactive protein (CRP) concentrations at 24 hours post PCI, Dr. Shah said.
The results followed by a day the presentation of results from the COLCOT trial (Colchicine Cardiovascular Outcomes Trial) that showed a 23% reduction in cardiovascular events in patients with coronary disease on colchicine 0.5 mg daily vs. placebo (N Engl J Med. 2019 Nov 16. doi: 10.1056/NEJMoa1912388), as Subodh Verma, MD, PhD, of the University of Toronto noted in his discussant comments. COLCHICINE-PCI “has important implications, since patients with acute coronary syndrome often have variable biomarker responses, as biomarkers often function as acute-phase reactants in that setting.”
The COLCHICINE-PCI study of 400 patients investigated oral 1.8 mg colchicine given 1-2 hours before the patient went to the cath lab. The drug was given in a 1.2-mg dose followed an hour later by an 0.6-mg dose. Patients received placebo at the same intervals. An inflammatory biomarker substudy of 280 patients evaluated differences in plasma interleukin-6 levels at baseline and 1 hour post PCI, as well as other key biomarkers at longer intervals.
The primary outcome of PCI-related myocardial injury showed no statistically significant difference between the two groups, Dr. Shah said: 57.3% for colchicine and 64.2% for placebo (P = 0.19). The same was true of 30-day major adverse cardiovascular events, she said: 11.7% and 12.9% for the treatment and placebo groups, respectively (P = 0.82). Rates of PCI-related MI were also similar between the two groups.
However, the biomarker substudy told a different story. IL-6 levels in the treatment group were stable at 1 and 6-8 hours post PCI. “However, at 22-24 hours we see a significant attenuation in the rise of IL-6 with colchicine,” she said.
While IL-beta levels showed no deviation after PCI, the colchicine group showed a noticeable attenuation in the rise of high-sensitivity CRP levels at 22-24 hours.
“This is the first study to demonstrate that an oral load of colchicine prevents a rise of inflammatory markers in an acute-injury setting,” Dr. Shah said.
While results of the COLCOT trial affirmed a “resounding yes” for the use of colchicine in patients who’ve had a recent MI, Dr. Verma said the COLCHICINE-PCI results did not give as clear an answer.
“What about pre- or peri-PCI?” he said. “I don’t think we’re there yet, but I do think that more studies are needed that target residual inflammatory risk and potentially couple an acute loading dose with a chronic, ongoing treatment.” Results from higher-risk primary prevention studies, such as the CLEAR SYNERGY (OASIS 9) of a colchicine-spironolactone combination in patients with STEMI having PCI, are needed, he said.
Dr. Shah disclosed financial relationships with Phillips Volcano and Radux. The VA Office of Research and Development and AHA provided grant funding and Takeda Pharmaceuticals provided the drug.
SOURCE: Shah B. AHA 2019, Late Breaking Science session IV.
PHILADELPHIA – Giving patients a single shot of colchicine before percutaneous coronary intervention was found to favorably impact inflammatory biomarkers linked to vascular injury, but not to lower the risk of procedure-related myocardial injury, according to results of the COLCHICINE-PCI randomized trial reported at the American Heart Association scientific sessions.
This is the first study to evaluate pre-PCI colchicine versus placebo on markers of myocardial injury and inflammation, said Binita Shah, MD, of Veterans Affairs New York Harbor Healthcare System and New York University.
“More work is needed to determine the optimal dosing and timing regimen of colchicine administration in patients undergoing PCI,” Dr. Shah said in an interview. “In this study, we saw inflammatory markers decrease around the 24-hour time point post PCI, so an earlier start to preprocedural colchicine regimen warrants further investigation.” The study found that pre-PCI colchicine attenuated increases in interleukin-6 and high-sensitivity C-reactive protein (CRP) concentrations at 24 hours post PCI, Dr. Shah said.
The results followed by a day the presentation of results from the COLCOT trial (Colchicine Cardiovascular Outcomes Trial) that showed a 23% reduction in cardiovascular events in patients with coronary disease on colchicine 0.5 mg daily vs. placebo (N Engl J Med. 2019 Nov 16. doi: 10.1056/NEJMoa1912388), as Subodh Verma, MD, PhD, of the University of Toronto noted in his discussant comments. COLCHICINE-PCI “has important implications, since patients with acute coronary syndrome often have variable biomarker responses, as biomarkers often function as acute-phase reactants in that setting.”
The COLCHICINE-PCI study of 400 patients investigated oral 1.8 mg colchicine given 1-2 hours before the patient went to the cath lab. The drug was given in a 1.2-mg dose followed an hour later by an 0.6-mg dose. Patients received placebo at the same intervals. An inflammatory biomarker substudy of 280 patients evaluated differences in plasma interleukin-6 levels at baseline and 1 hour post PCI, as well as other key biomarkers at longer intervals.
The primary outcome of PCI-related myocardial injury showed no statistically significant difference between the two groups, Dr. Shah said: 57.3% for colchicine and 64.2% for placebo (P = 0.19). The same was true of 30-day major adverse cardiovascular events, she said: 11.7% and 12.9% for the treatment and placebo groups, respectively (P = 0.82). Rates of PCI-related MI were also similar between the two groups.
However, the biomarker substudy told a different story. IL-6 levels in the treatment group were stable at 1 and 6-8 hours post PCI. “However, at 22-24 hours we see a significant attenuation in the rise of IL-6 with colchicine,” she said.
While IL-beta levels showed no deviation after PCI, the colchicine group showed a noticeable attenuation in the rise of high-sensitivity CRP levels at 22-24 hours.
“This is the first study to demonstrate that an oral load of colchicine prevents a rise of inflammatory markers in an acute-injury setting,” Dr. Shah said.
While results of the COLCOT trial affirmed a “resounding yes” for the use of colchicine in patients who’ve had a recent MI, Dr. Verma said the COLCHICINE-PCI results did not give as clear an answer.
“What about pre- or peri-PCI?” he said. “I don’t think we’re there yet, but I do think that more studies are needed that target residual inflammatory risk and potentially couple an acute loading dose with a chronic, ongoing treatment.” Results from higher-risk primary prevention studies, such as the CLEAR SYNERGY (OASIS 9) of a colchicine-spironolactone combination in patients with STEMI having PCI, are needed, he said.
Dr. Shah disclosed financial relationships with Phillips Volcano and Radux. The VA Office of Research and Development and AHA provided grant funding and Takeda Pharmaceuticals provided the drug.
SOURCE: Shah B. AHA 2019, Late Breaking Science session IV.
PHILADELPHIA – Giving patients a single shot of colchicine before percutaneous coronary intervention was found to favorably impact inflammatory biomarkers linked to vascular injury, but not to lower the risk of procedure-related myocardial injury, according to results of the COLCHICINE-PCI randomized trial reported at the American Heart Association scientific sessions.
This is the first study to evaluate pre-PCI colchicine versus placebo on markers of myocardial injury and inflammation, said Binita Shah, MD, of Veterans Affairs New York Harbor Healthcare System and New York University.
“More work is needed to determine the optimal dosing and timing regimen of colchicine administration in patients undergoing PCI,” Dr. Shah said in an interview. “In this study, we saw inflammatory markers decrease around the 24-hour time point post PCI, so an earlier start to preprocedural colchicine regimen warrants further investigation.” The study found that pre-PCI colchicine attenuated increases in interleukin-6 and high-sensitivity C-reactive protein (CRP) concentrations at 24 hours post PCI, Dr. Shah said.
The results followed by a day the presentation of results from the COLCOT trial (Colchicine Cardiovascular Outcomes Trial) that showed a 23% reduction in cardiovascular events in patients with coronary disease on colchicine 0.5 mg daily vs. placebo (N Engl J Med. 2019 Nov 16. doi: 10.1056/NEJMoa1912388), as Subodh Verma, MD, PhD, of the University of Toronto noted in his discussant comments. COLCHICINE-PCI “has important implications, since patients with acute coronary syndrome often have variable biomarker responses, as biomarkers often function as acute-phase reactants in that setting.”
The COLCHICINE-PCI study of 400 patients investigated oral 1.8 mg colchicine given 1-2 hours before the patient went to the cath lab. The drug was given in a 1.2-mg dose followed an hour later by an 0.6-mg dose. Patients received placebo at the same intervals. An inflammatory biomarker substudy of 280 patients evaluated differences in plasma interleukin-6 levels at baseline and 1 hour post PCI, as well as other key biomarkers at longer intervals.
The primary outcome of PCI-related myocardial injury showed no statistically significant difference between the two groups, Dr. Shah said: 57.3% for colchicine and 64.2% for placebo (P = 0.19). The same was true of 30-day major adverse cardiovascular events, she said: 11.7% and 12.9% for the treatment and placebo groups, respectively (P = 0.82). Rates of PCI-related MI were also similar between the two groups.
However, the biomarker substudy told a different story. IL-6 levels in the treatment group were stable at 1 and 6-8 hours post PCI. “However, at 22-24 hours we see a significant attenuation in the rise of IL-6 with colchicine,” she said.
While IL-beta levels showed no deviation after PCI, the colchicine group showed a noticeable attenuation in the rise of high-sensitivity CRP levels at 22-24 hours.
“This is the first study to demonstrate that an oral load of colchicine prevents a rise of inflammatory markers in an acute-injury setting,” Dr. Shah said.
While results of the COLCOT trial affirmed a “resounding yes” for the use of colchicine in patients who’ve had a recent MI, Dr. Verma said the COLCHICINE-PCI results did not give as clear an answer.
“What about pre- or peri-PCI?” he said. “I don’t think we’re there yet, but I do think that more studies are needed that target residual inflammatory risk and potentially couple an acute loading dose with a chronic, ongoing treatment.” Results from higher-risk primary prevention studies, such as the CLEAR SYNERGY (OASIS 9) of a colchicine-spironolactone combination in patients with STEMI having PCI, are needed, he said.
Dr. Shah disclosed financial relationships with Phillips Volcano and Radux. The VA Office of Research and Development and AHA provided grant funding and Takeda Pharmaceuticals provided the drug.
SOURCE: Shah B. AHA 2019, Late Breaking Science session IV.
REPORTING FROM AHA 2019
Cardiac arrests peak with pollution in Japan
PHILADELPHIA – Out-of-hospital cardiac arrests spike with daily counts of emissions-related particulate matter – a key contributor to urban smog – and particularly affect men and people older than age 75, according to results of a nationwide Japanese study presented at the American Heart Association scientific sessions.
“Short-term exposure to particulate pollutants is a potential trigger for cardiac-origin, out-of-hospital cardiac arrest [OHCA] onset in Japan,” said Sunao Kojima, MD, a professor at Kawasaki Medical School in Kurashiki, Japan.
The study used the All-Japan Utstein Registry of OHCA throughout all 47 prefectures in Japan. The analysis then applied prefecture-specific estimates of PM2.5 – particulate matter that measures 2.5 mcm in average diameter – using a time-stratified, case-crossover design. By comparison, PM2.5 is about 1/40th the diameter of human hair (approximately 100 mcm) and about 1/12th that of cedar pollen (30 mcm).
“Increased OHCAs incidence correlated with the average increase in PM2.5 concentrations over those observed 1 day before cardiac arrest,” Dr. Kojima said.
What’s noteworthy about the Utstein registry, Dr. Kojima said, is that emergency medical service personnel in Japan are not authorized to terminate resuscitation efforts, so most OHCA patients are transported to the nearest hospital and are thus counted in the registry.
From a total count of 1.4 million EMS-assessed OHCAs from 2005 through 2016, the study focused on 103,189 bystander-witnessed events from April 2011 through 2016. The analysis further divided that population into three groups: those presenting with initial ventricular fibrillation/pulseless ventricular tachycardia (20,848); those without initial VF/pulseless VT (80,110); and those with initial cardiac rhythm of unknown origin (2,231).
“The pathways linking PM2.5 exposure with OHCA remain unknown, but several mechanisms have been suspected,” Dr. Kojima said. “A major mechanism is thought to be associated with oxidative stress and systemic inflammation.”
The average daily concentration for PM2.5 was 13.9/m3 across all of Japan, Dr. Kojima said, with the highest concentrations in western Japan (16.3/m3). A 10-mcg/m3 increase in the average PM2.5 concentrations on the day of OHCA from the previous day (lag 0-1) was associated with a 1.6% increase in OHCAs (95% confidence interval, 0.1-3.1%), he said.
“Increased PM2.5 concentrations were closely associated with OHCA incidence, even when adjusted for other pollutants, such as ozone, nitrogen dioxide, sulfur dioxide, and lag 0-1,” Dr. Kojima said.
The incidence for PM2.5-related OHCA was higher for people age 75 and older and for men, during the warm season and in the central region. In the central region, the incidence increased around 6% for every 10-mcg/m3 day-to-day increase in the average PM2.5 compared to less than 1% increases in the eastern and western regions, Dr. Kojima said.
PM2.5 levels also seemed to influence outcomes depending on the origin of the OHCA, he said. Patients with VF/pulseless VT and pulseless electrical activity had better outcomes than did those with asystole. Increased PM2.5 levels were linked with lower rates of restoration of spontaneous circulation, 1-month survival, and 1-month survival with minimal neurological impairment, he said. Patients who had chest-compression-only CPR seemed to do significantly better than did those who had chest compression with rescue breathing, he said.
“There may be room for further discussion regarding the impact of performing rescue breathing in CPR and the consequent effects of short-term PM2.5 exposure on patients with cardiac origin,” he said.
Dr. Kojima has no financial relationships to disclose. The study received funding from the Japan Ministry of the Environment, Japan Society for the Promotion of Science, and Foundation for Total Health Promotion, Japan.
SOURCE: Kojima S. AHA 2019, Session FS.AOS.F1.
PHILADELPHIA – Out-of-hospital cardiac arrests spike with daily counts of emissions-related particulate matter – a key contributor to urban smog – and particularly affect men and people older than age 75, according to results of a nationwide Japanese study presented at the American Heart Association scientific sessions.
“Short-term exposure to particulate pollutants is a potential trigger for cardiac-origin, out-of-hospital cardiac arrest [OHCA] onset in Japan,” said Sunao Kojima, MD, a professor at Kawasaki Medical School in Kurashiki, Japan.
The study used the All-Japan Utstein Registry of OHCA throughout all 47 prefectures in Japan. The analysis then applied prefecture-specific estimates of PM2.5 – particulate matter that measures 2.5 mcm in average diameter – using a time-stratified, case-crossover design. By comparison, PM2.5 is about 1/40th the diameter of human hair (approximately 100 mcm) and about 1/12th that of cedar pollen (30 mcm).
“Increased OHCAs incidence correlated with the average increase in PM2.5 concentrations over those observed 1 day before cardiac arrest,” Dr. Kojima said.
What’s noteworthy about the Utstein registry, Dr. Kojima said, is that emergency medical service personnel in Japan are not authorized to terminate resuscitation efforts, so most OHCA patients are transported to the nearest hospital and are thus counted in the registry.
From a total count of 1.4 million EMS-assessed OHCAs from 2005 through 2016, the study focused on 103,189 bystander-witnessed events from April 2011 through 2016. The analysis further divided that population into three groups: those presenting with initial ventricular fibrillation/pulseless ventricular tachycardia (20,848); those without initial VF/pulseless VT (80,110); and those with initial cardiac rhythm of unknown origin (2,231).
“The pathways linking PM2.5 exposure with OHCA remain unknown, but several mechanisms have been suspected,” Dr. Kojima said. “A major mechanism is thought to be associated with oxidative stress and systemic inflammation.”
The average daily concentration for PM2.5 was 13.9/m3 across all of Japan, Dr. Kojima said, with the highest concentrations in western Japan (16.3/m3). A 10-mcg/m3 increase in the average PM2.5 concentrations on the day of OHCA from the previous day (lag 0-1) was associated with a 1.6% increase in OHCAs (95% confidence interval, 0.1-3.1%), he said.
“Increased PM2.5 concentrations were closely associated with OHCA incidence, even when adjusted for other pollutants, such as ozone, nitrogen dioxide, sulfur dioxide, and lag 0-1,” Dr. Kojima said.
The incidence for PM2.5-related OHCA was higher for people age 75 and older and for men, during the warm season and in the central region. In the central region, the incidence increased around 6% for every 10-mcg/m3 day-to-day increase in the average PM2.5 compared to less than 1% increases in the eastern and western regions, Dr. Kojima said.
PM2.5 levels also seemed to influence outcomes depending on the origin of the OHCA, he said. Patients with VF/pulseless VT and pulseless electrical activity had better outcomes than did those with asystole. Increased PM2.5 levels were linked with lower rates of restoration of spontaneous circulation, 1-month survival, and 1-month survival with minimal neurological impairment, he said. Patients who had chest-compression-only CPR seemed to do significantly better than did those who had chest compression with rescue breathing, he said.
“There may be room for further discussion regarding the impact of performing rescue breathing in CPR and the consequent effects of short-term PM2.5 exposure on patients with cardiac origin,” he said.
Dr. Kojima has no financial relationships to disclose. The study received funding from the Japan Ministry of the Environment, Japan Society for the Promotion of Science, and Foundation for Total Health Promotion, Japan.
SOURCE: Kojima S. AHA 2019, Session FS.AOS.F1.
PHILADELPHIA – Out-of-hospital cardiac arrests spike with daily counts of emissions-related particulate matter – a key contributor to urban smog – and particularly affect men and people older than age 75, according to results of a nationwide Japanese study presented at the American Heart Association scientific sessions.
“Short-term exposure to particulate pollutants is a potential trigger for cardiac-origin, out-of-hospital cardiac arrest [OHCA] onset in Japan,” said Sunao Kojima, MD, a professor at Kawasaki Medical School in Kurashiki, Japan.
The study used the All-Japan Utstein Registry of OHCA throughout all 47 prefectures in Japan. The analysis then applied prefecture-specific estimates of PM2.5 – particulate matter that measures 2.5 mcm in average diameter – using a time-stratified, case-crossover design. By comparison, PM2.5 is about 1/40th the diameter of human hair (approximately 100 mcm) and about 1/12th that of cedar pollen (30 mcm).
“Increased OHCAs incidence correlated with the average increase in PM2.5 concentrations over those observed 1 day before cardiac arrest,” Dr. Kojima said.
What’s noteworthy about the Utstein registry, Dr. Kojima said, is that emergency medical service personnel in Japan are not authorized to terminate resuscitation efforts, so most OHCA patients are transported to the nearest hospital and are thus counted in the registry.
From a total count of 1.4 million EMS-assessed OHCAs from 2005 through 2016, the study focused on 103,189 bystander-witnessed events from April 2011 through 2016. The analysis further divided that population into three groups: those presenting with initial ventricular fibrillation/pulseless ventricular tachycardia (20,848); those without initial VF/pulseless VT (80,110); and those with initial cardiac rhythm of unknown origin (2,231).
“The pathways linking PM2.5 exposure with OHCA remain unknown, but several mechanisms have been suspected,” Dr. Kojima said. “A major mechanism is thought to be associated with oxidative stress and systemic inflammation.”
The average daily concentration for PM2.5 was 13.9/m3 across all of Japan, Dr. Kojima said, with the highest concentrations in western Japan (16.3/m3). A 10-mcg/m3 increase in the average PM2.5 concentrations on the day of OHCA from the previous day (lag 0-1) was associated with a 1.6% increase in OHCAs (95% confidence interval, 0.1-3.1%), he said.
“Increased PM2.5 concentrations were closely associated with OHCA incidence, even when adjusted for other pollutants, such as ozone, nitrogen dioxide, sulfur dioxide, and lag 0-1,” Dr. Kojima said.
The incidence for PM2.5-related OHCA was higher for people age 75 and older and for men, during the warm season and in the central region. In the central region, the incidence increased around 6% for every 10-mcg/m3 day-to-day increase in the average PM2.5 compared to less than 1% increases in the eastern and western regions, Dr. Kojima said.
PM2.5 levels also seemed to influence outcomes depending on the origin of the OHCA, he said. Patients with VF/pulseless VT and pulseless electrical activity had better outcomes than did those with asystole. Increased PM2.5 levels were linked with lower rates of restoration of spontaneous circulation, 1-month survival, and 1-month survival with minimal neurological impairment, he said. Patients who had chest-compression-only CPR seemed to do significantly better than did those who had chest compression with rescue breathing, he said.
“There may be room for further discussion regarding the impact of performing rescue breathing in CPR and the consequent effects of short-term PM2.5 exposure on patients with cardiac origin,” he said.
Dr. Kojima has no financial relationships to disclose. The study received funding from the Japan Ministry of the Environment, Japan Society for the Promotion of Science, and Foundation for Total Health Promotion, Japan.
SOURCE: Kojima S. AHA 2019, Session FS.AOS.F1.
REPORTING FROM AHA 2019
Icosapent ethyl cost effective in REDUCE-IT analysis
PHILADELPHIA – The overall costs of icosapent ethyl were less than placebo, and the medication reduced cardiovascular events by 30% at a cost that fits well within acceptable quality-adjusted life-year (QALY) parameters, according to a cost-effectiveness analysis of the REDUCE-IT trial.
Days before the presentation of the analysis at the American Heart Association scientific sessions, a Food and Drug Administration advisory panel unanimously recommended approval of icosapent ethyl (Vascepa) for a new indication for reducing CV event risk. Icosapent ethyl, a highly purified form of the ethyl ester of eicosapentaenoic acid derived from fish oil, received FDA approval in 2012 for treatment of triglyceride levels of at least 500 mg/dL.
“What we found here is that icosapent ethyl is a dominant strategy,” said William S. Weintraub, MD, director of outcomes research at MedStar Heart & Vascular Institute in Washington, in reporting preliminary cost-analysis findings from REDUCE-IT (Reduction of Cardiovascular Events With Icosapent Ethyl – Intervention Trial). “It’s offering better outcomes at a lower cost.”
The dominant strategy was demonstrated by cost savings in 70% of simulations the cost-effectiveness analysis ran, Dr. Weintraub said.
“These are very impressive results,” said session moderator Seth S. Martin, MD, an internist and cardiologist at Johns Hopkins University, Baltimore. “We don’t often see dominant strategies for new drugs. This is very exciting.”
“Almost never,” Dr. Weintraub responded.
REDUCE-IT randomized 8,179 patients with a diagnosis of CVD or with diabetes and other risk factors who had been on statins and had triglycerides of 135-499 mg/dL to either 4 g of icosapent ethyl daily or placebo (N Engl J Med. 2019;380:11-22). Trial results showed the treatment group had an absolute risk reduction of 4.8% and a relative risk reduction of 25% of first CV events and a 30% relative risk reduction for total events, Dr. Weintraub said.
The analysis determined that the QALYs for icosapent ethyl versus those for placebo were 3.34 and 3.27, respectively, during the trial period and 11.61 and 11.35 over a lifetime. The mean costs for the two treatments were $27,576 and $28,205 during the trial period and $235,352 and $236,636 lifetime, respectively, Dr. Weintraub said.
An analysis of cost effectiveness showed that almost all of the estimates fell below the willingness-to-pay (WTP) threshold of $50,000 per QALY gained, Dr. Weintraub said. “In fact, some 70% plus are in what’s called quadrant two; that is, decreased cost and increased efficacy.”
The analysis also calculated the value of icosapent ethyl at three different WTP thresholds: up to $6 a day at a WTP of $50,000, up to $12 a day at $100,000, and up to $18 a day at $150,000. The analysis used the actual net pricing of $4.16 a day, Dr. Weintraub said. “That’s why we showed we have the dominant strategy,” he said.
Further cost-effectiveness analyses of the REDUCE-IT data will focus on subgroups, such as U.S. and non–U.S. patients and people with diabetes. He also emphasized the data he reported were preliminary. “We have a lot more work to do,” Dr. Weintraub said.
Dr. Weintraub reported having financial relationships with Amarin Pharma, which markets Vascepa, and AstraZeneca.
SOURCE: Weintraub WS. AHA 2019, Session FS.AOS.F1.
PHILADELPHIA – The overall costs of icosapent ethyl were less than placebo, and the medication reduced cardiovascular events by 30% at a cost that fits well within acceptable quality-adjusted life-year (QALY) parameters, according to a cost-effectiveness analysis of the REDUCE-IT trial.
Days before the presentation of the analysis at the American Heart Association scientific sessions, a Food and Drug Administration advisory panel unanimously recommended approval of icosapent ethyl (Vascepa) for a new indication for reducing CV event risk. Icosapent ethyl, a highly purified form of the ethyl ester of eicosapentaenoic acid derived from fish oil, received FDA approval in 2012 for treatment of triglyceride levels of at least 500 mg/dL.
“What we found here is that icosapent ethyl is a dominant strategy,” said William S. Weintraub, MD, director of outcomes research at MedStar Heart & Vascular Institute in Washington, in reporting preliminary cost-analysis findings from REDUCE-IT (Reduction of Cardiovascular Events With Icosapent Ethyl – Intervention Trial). “It’s offering better outcomes at a lower cost.”
The dominant strategy was demonstrated by cost savings in 70% of simulations the cost-effectiveness analysis ran, Dr. Weintraub said.
“These are very impressive results,” said session moderator Seth S. Martin, MD, an internist and cardiologist at Johns Hopkins University, Baltimore. “We don’t often see dominant strategies for new drugs. This is very exciting.”
“Almost never,” Dr. Weintraub responded.
REDUCE-IT randomized 8,179 patients with a diagnosis of CVD or with diabetes and other risk factors who had been on statins and had triglycerides of 135-499 mg/dL to either 4 g of icosapent ethyl daily or placebo (N Engl J Med. 2019;380:11-22). Trial results showed the treatment group had an absolute risk reduction of 4.8% and a relative risk reduction of 25% of first CV events and a 30% relative risk reduction for total events, Dr. Weintraub said.
The analysis determined that the QALYs for icosapent ethyl versus those for placebo were 3.34 and 3.27, respectively, during the trial period and 11.61 and 11.35 over a lifetime. The mean costs for the two treatments were $27,576 and $28,205 during the trial period and $235,352 and $236,636 lifetime, respectively, Dr. Weintraub said.
An analysis of cost effectiveness showed that almost all of the estimates fell below the willingness-to-pay (WTP) threshold of $50,000 per QALY gained, Dr. Weintraub said. “In fact, some 70% plus are in what’s called quadrant two; that is, decreased cost and increased efficacy.”
The analysis also calculated the value of icosapent ethyl at three different WTP thresholds: up to $6 a day at a WTP of $50,000, up to $12 a day at $100,000, and up to $18 a day at $150,000. The analysis used the actual net pricing of $4.16 a day, Dr. Weintraub said. “That’s why we showed we have the dominant strategy,” he said.
Further cost-effectiveness analyses of the REDUCE-IT data will focus on subgroups, such as U.S. and non–U.S. patients and people with diabetes. He also emphasized the data he reported were preliminary. “We have a lot more work to do,” Dr. Weintraub said.
Dr. Weintraub reported having financial relationships with Amarin Pharma, which markets Vascepa, and AstraZeneca.
SOURCE: Weintraub WS. AHA 2019, Session FS.AOS.F1.
PHILADELPHIA – The overall costs of icosapent ethyl were less than placebo, and the medication reduced cardiovascular events by 30% at a cost that fits well within acceptable quality-adjusted life-year (QALY) parameters, according to a cost-effectiveness analysis of the REDUCE-IT trial.
Days before the presentation of the analysis at the American Heart Association scientific sessions, a Food and Drug Administration advisory panel unanimously recommended approval of icosapent ethyl (Vascepa) for a new indication for reducing CV event risk. Icosapent ethyl, a highly purified form of the ethyl ester of eicosapentaenoic acid derived from fish oil, received FDA approval in 2012 for treatment of triglyceride levels of at least 500 mg/dL.
“What we found here is that icosapent ethyl is a dominant strategy,” said William S. Weintraub, MD, director of outcomes research at MedStar Heart & Vascular Institute in Washington, in reporting preliminary cost-analysis findings from REDUCE-IT (Reduction of Cardiovascular Events With Icosapent Ethyl – Intervention Trial). “It’s offering better outcomes at a lower cost.”
The dominant strategy was demonstrated by cost savings in 70% of simulations the cost-effectiveness analysis ran, Dr. Weintraub said.
“These are very impressive results,” said session moderator Seth S. Martin, MD, an internist and cardiologist at Johns Hopkins University, Baltimore. “We don’t often see dominant strategies for new drugs. This is very exciting.”
“Almost never,” Dr. Weintraub responded.
REDUCE-IT randomized 8,179 patients with a diagnosis of CVD or with diabetes and other risk factors who had been on statins and had triglycerides of 135-499 mg/dL to either 4 g of icosapent ethyl daily or placebo (N Engl J Med. 2019;380:11-22). Trial results showed the treatment group had an absolute risk reduction of 4.8% and a relative risk reduction of 25% of first CV events and a 30% relative risk reduction for total events, Dr. Weintraub said.
The analysis determined that the QALYs for icosapent ethyl versus those for placebo were 3.34 and 3.27, respectively, during the trial period and 11.61 and 11.35 over a lifetime. The mean costs for the two treatments were $27,576 and $28,205 during the trial period and $235,352 and $236,636 lifetime, respectively, Dr. Weintraub said.
An analysis of cost effectiveness showed that almost all of the estimates fell below the willingness-to-pay (WTP) threshold of $50,000 per QALY gained, Dr. Weintraub said. “In fact, some 70% plus are in what’s called quadrant two; that is, decreased cost and increased efficacy.”
The analysis also calculated the value of icosapent ethyl at three different WTP thresholds: up to $6 a day at a WTP of $50,000, up to $12 a day at $100,000, and up to $18 a day at $150,000. The analysis used the actual net pricing of $4.16 a day, Dr. Weintraub said. “That’s why we showed we have the dominant strategy,” he said.
Further cost-effectiveness analyses of the REDUCE-IT data will focus on subgroups, such as U.S. and non–U.S. patients and people with diabetes. He also emphasized the data he reported were preliminary. “We have a lot more work to do,” Dr. Weintraub said.
Dr. Weintraub reported having financial relationships with Amarin Pharma, which markets Vascepa, and AstraZeneca.
SOURCE: Weintraub WS. AHA 2019, Session FS.AOS.F1.
REPORTING FROM AHA 2019
GALILEO, GALILEO 4D: Mixed results in post-TAVR anticoagulation
PHILADELPHIA – The results of the first randomized prospective trial of an anticoagulation strategy versus standard dual antiplatelet (DAPT) therapy for patients undergoing transcatheter aortic valve replacement (TAVR) show that routine anticoagulation is not suitable for all comers in a high-risk population.
In the main GALILEO trial of elderly patients after TAVR, those who received an investigational anticoagulation strategy with the direct factor Xa inhibitor rivaroxaban (Xarelto; Bayer/Janssen) had worse survival and more thromboembolic and bleeding events than patients who received standard DAPT.
However, in the GALILEO 4D substudy of patients who underwent four-dimensional computed tomography (4DCT) randomized to the two therapies, those in the rivaroxaban arm were less likely to show subclinical leaflet motion abnormalities and leaflet thickening.
Preliminary results from GALILEO were disclosed in an October 3, 2018, “Dear Healthcare Professional” letter from Bayer, and the trial was stopped after a median of 17 months due to safety concerns.
The full data analysis from GALILEO as well as the results from GALILEO 4D were presented at the American Heart Association scientific sessions to coincide with their publication on Nov. 16, 2019, in the New England Journal of Medicine.
The takeaway message is that, despite the positive imaging finding in GALILEO 4D, “there is no reason to give 10 mg rivaroxaban-based treatment routinely after TAVR in patients who don’t need anticoagulation anyhow,” lead author in the main GALILEO trial, George D. Dangas, MD, PhD, Mount Sinai Hospital, New York, said in an interview.
However, because rivaroxaban had an effect in reducing the clots on leaflets, he said, further investigation is required to determine the optimal therapeutic strategy after TAVR.
Similarly, the assigned discussant for GALILEO, Elaine Hylek, MD, of Boston University said in an interview that “we just don’t know right now what the overall added benefit of an oral anticoagulant would be in this high-risk patient population after having a TAVR.”
Ole De Backer, MD, PhD, of Rigshospitalet University Hospital, Copenhagen, lead author of the GALILEO 4D substudy, concluded that, although the rivaroxaban-based strategy was associated with fewer valve abnormalities in this analysis, those positive outcomes need to be taken in context with worse clinical outcomes in the main GALILEO trial.
GALILEO
Guidelines recommend DAPT after TAVR, but this advice is based on expert consensus or small studies, the GALILEO study authors noted. Several years ago, there were random case reports and then case series of patients who had undergone TAVR or surgical aortic valve replacement (SAVR) and developed clots around the valve, Dr. Dangas explained.
These developments coincided with the first available high-quality CT angiography images that captured valve abnormalities that had not been seen before.
In parallel, there were rare reports of stroke and transient ischemic attack (TIA) that may have been associated with TAVR or SAVR. This triggered a series of studies to investigate an anticoagulation strategy after TAVR.
From December 2015 to May 2018, GALILEO enrolled 1,644 patients at 136 sites in 16 countries who had undergone successful TAVR, and had no indication for an anticoagulant (e.g., no atrial fibrillation).
The patients had a mean age of 80.6 years (plus or minus 6.6 years) and 49.5% were female. The median time from TAVR to randomization was 2 days (range, 0-8 days).
Half were randomized to receive an antithrombotic strategy, rivaroxaban 10 mg once daily plus aspirin 75-100 mg once daily for the first 90 days followed by rivaroxaban alone. The other half received an antiplatelet-based strategy, aspirin 75-100 mg once daily plus clopidogrel 75 mg once daily for the first 90 days followed by aspirin alone.
In the intention-to-treat analysis, death or first thromboembolic event, the primary efficacy outcome, occurred in 105 patients in the rivaroxaban group and 78 patients in the antiplatelet group (hazard ratio, 1.35; 95% CI, 1.01-1.81; P = .04).
Major, disabling, or life-threatening bleeding, the primary safety outcome, occurred in 46 and 31 patients, respectively (HR, 1.50; P = .08).
A total of 64 deaths occurred in the rivaroxaban group and 38 in the antiplatelet group (HR, 1.69; 95% CI, 1.13-2.53).
The individuals who were enrolled in this study were 80 and older, Dr. Hylek pointed out. “The age in and of itself is an uncontested risk factor for everything, whether it be bleeding, embolic event, or obviously mortality.”
Although the dose was half that used to prevent stroke in patients with atrial fibrillation, perhaps a “twice-daily lower dose” might be the way to go, moving forward, she said.
Patients who did not have atrial fibrillation may have developed atrial fibrillation in the interim, and “you would have to change the dose of the rivaroxaban.”
Also, patients who may have been taking aspirin for 5 or 10 years and “survived” aspirin, who were then newly exposed to an anticoagulant, would be more likely to experience bleeding.
“I certainly wouldn’t close the door on novel anticoagulants,” she concluded. “There are still other drug trials that are out there with this TAVR population. We’ll wait for that,” and see if the results corroborate these findings.
The high-risk patients may turn out to be a potential niche group for drugs being developed to inhibit factor XIa, she speculated.
GALILEO 4D
However, despite the negative results of the overall GALILEO study, results from the substudy that used 4DCT to evaluate function of the bioprosthetic aortic valves suggested rivaroxaban may have potentially beneficial effects on valve function.
The results showed that patients on the rivaroxaban and aspirin regimen had lower rates of subclinical reduced leaflet motion and leaflet thickening than patients on the antiplatelet strategy, said Dr. De Backer, reporting on behalf of the GALILEO-4D investigators.
The substudy evaluated 205 patients who had 4DCT 90 days after TAVR. The primary substudy endpoint was at least one prosthetic valve leaflet with a grade 3 or higher motion reduction, which 2 of 97 patients in the rivaroxaban group had (2.1%) versus 11 of 101 in the antiplatelet group (10.9%, P = .01).
“This indicated an 80% greater reduction of the primary endpoint in the rivaroxaban arm,” Dr. De Backer said. The chief secondary endpoint, the proportion of patients with at least one thickened leaflet, was met by 12.4% of the rivaroxaban group and 32.4% of the antiplatelet arm, “a 60% significant reduction by rivaroxaban,” Dr. De Backer said.
However, when the 10 patients in each group who didn’t adhere to the study drug regimen were excluded, he said, “then we see no single patient had reduced leaflet motion of grade 3 or more in the rivaroxaban arm.”
Another takeaway from the substudy is the ineffectiveness of transthoracic echocardiography as opposed to 4DCT in TAVR patients. Echocardiography (ECG) failed to show any significant differences in the mean valve gradient between the treatment groups, Dr. De Backer said.
Eleven patients who didn’t have leaflet thickening (7.3%) and 7 patients who did (15.9%) showed an increase of 5 mm Hg or more in the mean valve gradient on echo. ECG also showed a similar increase in the mean valve gradient in 14 patients who had no to moderate reduced leaflet motion (grade 3 or lower, 7.7%) and in four patients (30.8) who had grade 3 or higher reduced leaflet motion.
“This basically confirms results from observational studies that transthoracic echocardiography is often not good enough to detect these phenomena,” Dr. De Backer said.
The percentages of substudy patients who had major clinical events – major bleeding, thromboembolic events, or death at 90 days – were each less than 3%, he said. “There were too few clinical events to permit any assessment of the impact of leaflet thickening or reduced leaflet motion on clinical outcomes,” he said.
That lack of clarity with regard to clinical events is one of the questions the study leaves unanswered, said discussant Victoria Delgado, MD, PhD, of Leiden University Medical Center in the Netherlands.
“With stroke or TIA, there are too few events to draw any conclusions,” she said of the substudy. “We don’t know when we need to use CT, when we need to evaluate these patients, or maybe when we should go for more advanced imaging techniques where we can see the biology of those changes in the leaflets.” Hopefully, she said, future studies provide those insights.
“CT can be more sensitive than ECG to see these subclinical changes,” she said, “but the open questions that we have are to see if there is a correlation between thrombosis rate on imaging versus the stroke rate.”
The substudy’s conclusion on ECG, however, has been borne out by previous retrospective studies, Dr. Delgado added.
Robert A. Harrington, MD, of Stanford Medicine, tried to put the seemingly conflicting findings of the main GALILEO study and the 4D substudy into context.
“There you have the disconnect between the mechanism and the clinical observation and those are sometimes difficult to reconcile because the assumption is that the mechanism leads to the clinical outcome.”
While the main study shows that routine anticoagulation after TAVR is not indicated, the findings raise questions about the risk of clots forming on bioprosthetic valves. “Yes, maybe there are clots forming on these valves, but maybe that’s not causing the bad clinical outcomes,” Dr. Harrington said.
The findings also raise questions about the use of newer anticoagulants to prevent stroke post TAVR, he said. “It appears that warfarin is better than the newer anticoagulants for reasons that aren’t entirely clear.”
Dr. Dangas, lead author of the main GALILEO trial, said the substudy results could help design future trials of even-lower doses of anticoagulation in a more selective group of TAVR patients.
“In order to decrease the clots, first of all you don’t need the full dose of anticoagulation; even a low dose may do the trick,” he said. Further investigations can evaluate the clinical significance of having a blood clot in the valve as an indication for anticoagulation versus antiplatelet therapy.
“Even though this obviously doesn’t mean you’re going to have a stroke in a year or two,” Dr. Dangas said, “could it perhaps mean that the valve is not going to have such a good durability later on?”
Perhaps future studies of anticoagulation in TAVR should concentrate on patients who actually have clotting in the valve, he said.
The trial was supported by Bayer and Janssen. Dr. Dangas reported receiving grants from Bayer during the conduct of the study, personal fees from Bayer and Janssen, grants and personal fees from Daiichi-Sankyo, and “other” funding from Medtronic outside the submitted work. Dr. De Backer reported receiving grants from Bayer during the conduct of the study and personal fees from Abbott and Boston Scientific outside the submitted work.
SOURCE: Dangas GD and De Backer O. AHA 19, Late-Breaking Science 3 session.
This article also appears on Medscape.com.
PHILADELPHIA – The results of the first randomized prospective trial of an anticoagulation strategy versus standard dual antiplatelet (DAPT) therapy for patients undergoing transcatheter aortic valve replacement (TAVR) show that routine anticoagulation is not suitable for all comers in a high-risk population.
In the main GALILEO trial of elderly patients after TAVR, those who received an investigational anticoagulation strategy with the direct factor Xa inhibitor rivaroxaban (Xarelto; Bayer/Janssen) had worse survival and more thromboembolic and bleeding events than patients who received standard DAPT.
However, in the GALILEO 4D substudy of patients who underwent four-dimensional computed tomography (4DCT) randomized to the two therapies, those in the rivaroxaban arm were less likely to show subclinical leaflet motion abnormalities and leaflet thickening.
Preliminary results from GALILEO were disclosed in an October 3, 2018, “Dear Healthcare Professional” letter from Bayer, and the trial was stopped after a median of 17 months due to safety concerns.
The full data analysis from GALILEO as well as the results from GALILEO 4D were presented at the American Heart Association scientific sessions to coincide with their publication on Nov. 16, 2019, in the New England Journal of Medicine.
The takeaway message is that, despite the positive imaging finding in GALILEO 4D, “there is no reason to give 10 mg rivaroxaban-based treatment routinely after TAVR in patients who don’t need anticoagulation anyhow,” lead author in the main GALILEO trial, George D. Dangas, MD, PhD, Mount Sinai Hospital, New York, said in an interview.
However, because rivaroxaban had an effect in reducing the clots on leaflets, he said, further investigation is required to determine the optimal therapeutic strategy after TAVR.
Similarly, the assigned discussant for GALILEO, Elaine Hylek, MD, of Boston University said in an interview that “we just don’t know right now what the overall added benefit of an oral anticoagulant would be in this high-risk patient population after having a TAVR.”
Ole De Backer, MD, PhD, of Rigshospitalet University Hospital, Copenhagen, lead author of the GALILEO 4D substudy, concluded that, although the rivaroxaban-based strategy was associated with fewer valve abnormalities in this analysis, those positive outcomes need to be taken in context with worse clinical outcomes in the main GALILEO trial.
GALILEO
Guidelines recommend DAPT after TAVR, but this advice is based on expert consensus or small studies, the GALILEO study authors noted. Several years ago, there were random case reports and then case series of patients who had undergone TAVR or surgical aortic valve replacement (SAVR) and developed clots around the valve, Dr. Dangas explained.
These developments coincided with the first available high-quality CT angiography images that captured valve abnormalities that had not been seen before.
In parallel, there were rare reports of stroke and transient ischemic attack (TIA) that may have been associated with TAVR or SAVR. This triggered a series of studies to investigate an anticoagulation strategy after TAVR.
From December 2015 to May 2018, GALILEO enrolled 1,644 patients at 136 sites in 16 countries who had undergone successful TAVR, and had no indication for an anticoagulant (e.g., no atrial fibrillation).
The patients had a mean age of 80.6 years (plus or minus 6.6 years) and 49.5% were female. The median time from TAVR to randomization was 2 days (range, 0-8 days).
Half were randomized to receive an antithrombotic strategy, rivaroxaban 10 mg once daily plus aspirin 75-100 mg once daily for the first 90 days followed by rivaroxaban alone. The other half received an antiplatelet-based strategy, aspirin 75-100 mg once daily plus clopidogrel 75 mg once daily for the first 90 days followed by aspirin alone.
In the intention-to-treat analysis, death or first thromboembolic event, the primary efficacy outcome, occurred in 105 patients in the rivaroxaban group and 78 patients in the antiplatelet group (hazard ratio, 1.35; 95% CI, 1.01-1.81; P = .04).
Major, disabling, or life-threatening bleeding, the primary safety outcome, occurred in 46 and 31 patients, respectively (HR, 1.50; P = .08).
A total of 64 deaths occurred in the rivaroxaban group and 38 in the antiplatelet group (HR, 1.69; 95% CI, 1.13-2.53).
The individuals who were enrolled in this study were 80 and older, Dr. Hylek pointed out. “The age in and of itself is an uncontested risk factor for everything, whether it be bleeding, embolic event, or obviously mortality.”
Although the dose was half that used to prevent stroke in patients with atrial fibrillation, perhaps a “twice-daily lower dose” might be the way to go, moving forward, she said.
Patients who did not have atrial fibrillation may have developed atrial fibrillation in the interim, and “you would have to change the dose of the rivaroxaban.”
Also, patients who may have been taking aspirin for 5 or 10 years and “survived” aspirin, who were then newly exposed to an anticoagulant, would be more likely to experience bleeding.
“I certainly wouldn’t close the door on novel anticoagulants,” she concluded. “There are still other drug trials that are out there with this TAVR population. We’ll wait for that,” and see if the results corroborate these findings.
The high-risk patients may turn out to be a potential niche group for drugs being developed to inhibit factor XIa, she speculated.
GALILEO 4D
However, despite the negative results of the overall GALILEO study, results from the substudy that used 4DCT to evaluate function of the bioprosthetic aortic valves suggested rivaroxaban may have potentially beneficial effects on valve function.
The results showed that patients on the rivaroxaban and aspirin regimen had lower rates of subclinical reduced leaflet motion and leaflet thickening than patients on the antiplatelet strategy, said Dr. De Backer, reporting on behalf of the GALILEO-4D investigators.
The substudy evaluated 205 patients who had 4DCT 90 days after TAVR. The primary substudy endpoint was at least one prosthetic valve leaflet with a grade 3 or higher motion reduction, which 2 of 97 patients in the rivaroxaban group had (2.1%) versus 11 of 101 in the antiplatelet group (10.9%, P = .01).
“This indicated an 80% greater reduction of the primary endpoint in the rivaroxaban arm,” Dr. De Backer said. The chief secondary endpoint, the proportion of patients with at least one thickened leaflet, was met by 12.4% of the rivaroxaban group and 32.4% of the antiplatelet arm, “a 60% significant reduction by rivaroxaban,” Dr. De Backer said.
However, when the 10 patients in each group who didn’t adhere to the study drug regimen were excluded, he said, “then we see no single patient had reduced leaflet motion of grade 3 or more in the rivaroxaban arm.”
Another takeaway from the substudy is the ineffectiveness of transthoracic echocardiography as opposed to 4DCT in TAVR patients. Echocardiography (ECG) failed to show any significant differences in the mean valve gradient between the treatment groups, Dr. De Backer said.
Eleven patients who didn’t have leaflet thickening (7.3%) and 7 patients who did (15.9%) showed an increase of 5 mm Hg or more in the mean valve gradient on echo. ECG also showed a similar increase in the mean valve gradient in 14 patients who had no to moderate reduced leaflet motion (grade 3 or lower, 7.7%) and in four patients (30.8) who had grade 3 or higher reduced leaflet motion.
“This basically confirms results from observational studies that transthoracic echocardiography is often not good enough to detect these phenomena,” Dr. De Backer said.
The percentages of substudy patients who had major clinical events – major bleeding, thromboembolic events, or death at 90 days – were each less than 3%, he said. “There were too few clinical events to permit any assessment of the impact of leaflet thickening or reduced leaflet motion on clinical outcomes,” he said.
That lack of clarity with regard to clinical events is one of the questions the study leaves unanswered, said discussant Victoria Delgado, MD, PhD, of Leiden University Medical Center in the Netherlands.
“With stroke or TIA, there are too few events to draw any conclusions,” she said of the substudy. “We don’t know when we need to use CT, when we need to evaluate these patients, or maybe when we should go for more advanced imaging techniques where we can see the biology of those changes in the leaflets.” Hopefully, she said, future studies provide those insights.
“CT can be more sensitive than ECG to see these subclinical changes,” she said, “but the open questions that we have are to see if there is a correlation between thrombosis rate on imaging versus the stroke rate.”
The substudy’s conclusion on ECG, however, has been borne out by previous retrospective studies, Dr. Delgado added.
Robert A. Harrington, MD, of Stanford Medicine, tried to put the seemingly conflicting findings of the main GALILEO study and the 4D substudy into context.
“There you have the disconnect between the mechanism and the clinical observation and those are sometimes difficult to reconcile because the assumption is that the mechanism leads to the clinical outcome.”
While the main study shows that routine anticoagulation after TAVR is not indicated, the findings raise questions about the risk of clots forming on bioprosthetic valves. “Yes, maybe there are clots forming on these valves, but maybe that’s not causing the bad clinical outcomes,” Dr. Harrington said.
The findings also raise questions about the use of newer anticoagulants to prevent stroke post TAVR, he said. “It appears that warfarin is better than the newer anticoagulants for reasons that aren’t entirely clear.”
Dr. Dangas, lead author of the main GALILEO trial, said the substudy results could help design future trials of even-lower doses of anticoagulation in a more selective group of TAVR patients.
“In order to decrease the clots, first of all you don’t need the full dose of anticoagulation; even a low dose may do the trick,” he said. Further investigations can evaluate the clinical significance of having a blood clot in the valve as an indication for anticoagulation versus antiplatelet therapy.
“Even though this obviously doesn’t mean you’re going to have a stroke in a year or two,” Dr. Dangas said, “could it perhaps mean that the valve is not going to have such a good durability later on?”
Perhaps future studies of anticoagulation in TAVR should concentrate on patients who actually have clotting in the valve, he said.
The trial was supported by Bayer and Janssen. Dr. Dangas reported receiving grants from Bayer during the conduct of the study, personal fees from Bayer and Janssen, grants and personal fees from Daiichi-Sankyo, and “other” funding from Medtronic outside the submitted work. Dr. De Backer reported receiving grants from Bayer during the conduct of the study and personal fees from Abbott and Boston Scientific outside the submitted work.
SOURCE: Dangas GD and De Backer O. AHA 19, Late-Breaking Science 3 session.
This article also appears on Medscape.com.
PHILADELPHIA – The results of the first randomized prospective trial of an anticoagulation strategy versus standard dual antiplatelet (DAPT) therapy for patients undergoing transcatheter aortic valve replacement (TAVR) show that routine anticoagulation is not suitable for all comers in a high-risk population.
In the main GALILEO trial of elderly patients after TAVR, those who received an investigational anticoagulation strategy with the direct factor Xa inhibitor rivaroxaban (Xarelto; Bayer/Janssen) had worse survival and more thromboembolic and bleeding events than patients who received standard DAPT.
However, in the GALILEO 4D substudy of patients who underwent four-dimensional computed tomography (4DCT) randomized to the two therapies, those in the rivaroxaban arm were less likely to show subclinical leaflet motion abnormalities and leaflet thickening.
Preliminary results from GALILEO were disclosed in an October 3, 2018, “Dear Healthcare Professional” letter from Bayer, and the trial was stopped after a median of 17 months due to safety concerns.
The full data analysis from GALILEO as well as the results from GALILEO 4D were presented at the American Heart Association scientific sessions to coincide with their publication on Nov. 16, 2019, in the New England Journal of Medicine.
The takeaway message is that, despite the positive imaging finding in GALILEO 4D, “there is no reason to give 10 mg rivaroxaban-based treatment routinely after TAVR in patients who don’t need anticoagulation anyhow,” lead author in the main GALILEO trial, George D. Dangas, MD, PhD, Mount Sinai Hospital, New York, said in an interview.
However, because rivaroxaban had an effect in reducing the clots on leaflets, he said, further investigation is required to determine the optimal therapeutic strategy after TAVR.
Similarly, the assigned discussant for GALILEO, Elaine Hylek, MD, of Boston University said in an interview that “we just don’t know right now what the overall added benefit of an oral anticoagulant would be in this high-risk patient population after having a TAVR.”
Ole De Backer, MD, PhD, of Rigshospitalet University Hospital, Copenhagen, lead author of the GALILEO 4D substudy, concluded that, although the rivaroxaban-based strategy was associated with fewer valve abnormalities in this analysis, those positive outcomes need to be taken in context with worse clinical outcomes in the main GALILEO trial.
GALILEO
Guidelines recommend DAPT after TAVR, but this advice is based on expert consensus or small studies, the GALILEO study authors noted. Several years ago, there were random case reports and then case series of patients who had undergone TAVR or surgical aortic valve replacement (SAVR) and developed clots around the valve, Dr. Dangas explained.
These developments coincided with the first available high-quality CT angiography images that captured valve abnormalities that had not been seen before.
In parallel, there were rare reports of stroke and transient ischemic attack (TIA) that may have been associated with TAVR or SAVR. This triggered a series of studies to investigate an anticoagulation strategy after TAVR.
From December 2015 to May 2018, GALILEO enrolled 1,644 patients at 136 sites in 16 countries who had undergone successful TAVR, and had no indication for an anticoagulant (e.g., no atrial fibrillation).
The patients had a mean age of 80.6 years (plus or minus 6.6 years) and 49.5% were female. The median time from TAVR to randomization was 2 days (range, 0-8 days).
Half were randomized to receive an antithrombotic strategy, rivaroxaban 10 mg once daily plus aspirin 75-100 mg once daily for the first 90 days followed by rivaroxaban alone. The other half received an antiplatelet-based strategy, aspirin 75-100 mg once daily plus clopidogrel 75 mg once daily for the first 90 days followed by aspirin alone.
In the intention-to-treat analysis, death or first thromboembolic event, the primary efficacy outcome, occurred in 105 patients in the rivaroxaban group and 78 patients in the antiplatelet group (hazard ratio, 1.35; 95% CI, 1.01-1.81; P = .04).
Major, disabling, or life-threatening bleeding, the primary safety outcome, occurred in 46 and 31 patients, respectively (HR, 1.50; P = .08).
A total of 64 deaths occurred in the rivaroxaban group and 38 in the antiplatelet group (HR, 1.69; 95% CI, 1.13-2.53).
The individuals who were enrolled in this study were 80 and older, Dr. Hylek pointed out. “The age in and of itself is an uncontested risk factor for everything, whether it be bleeding, embolic event, or obviously mortality.”
Although the dose was half that used to prevent stroke in patients with atrial fibrillation, perhaps a “twice-daily lower dose” might be the way to go, moving forward, she said.
Patients who did not have atrial fibrillation may have developed atrial fibrillation in the interim, and “you would have to change the dose of the rivaroxaban.”
Also, patients who may have been taking aspirin for 5 or 10 years and “survived” aspirin, who were then newly exposed to an anticoagulant, would be more likely to experience bleeding.
“I certainly wouldn’t close the door on novel anticoagulants,” she concluded. “There are still other drug trials that are out there with this TAVR population. We’ll wait for that,” and see if the results corroborate these findings.
The high-risk patients may turn out to be a potential niche group for drugs being developed to inhibit factor XIa, she speculated.
GALILEO 4D
However, despite the negative results of the overall GALILEO study, results from the substudy that used 4DCT to evaluate function of the bioprosthetic aortic valves suggested rivaroxaban may have potentially beneficial effects on valve function.
The results showed that patients on the rivaroxaban and aspirin regimen had lower rates of subclinical reduced leaflet motion and leaflet thickening than patients on the antiplatelet strategy, said Dr. De Backer, reporting on behalf of the GALILEO-4D investigators.
The substudy evaluated 205 patients who had 4DCT 90 days after TAVR. The primary substudy endpoint was at least one prosthetic valve leaflet with a grade 3 or higher motion reduction, which 2 of 97 patients in the rivaroxaban group had (2.1%) versus 11 of 101 in the antiplatelet group (10.9%, P = .01).
“This indicated an 80% greater reduction of the primary endpoint in the rivaroxaban arm,” Dr. De Backer said. The chief secondary endpoint, the proportion of patients with at least one thickened leaflet, was met by 12.4% of the rivaroxaban group and 32.4% of the antiplatelet arm, “a 60% significant reduction by rivaroxaban,” Dr. De Backer said.
However, when the 10 patients in each group who didn’t adhere to the study drug regimen were excluded, he said, “then we see no single patient had reduced leaflet motion of grade 3 or more in the rivaroxaban arm.”
Another takeaway from the substudy is the ineffectiveness of transthoracic echocardiography as opposed to 4DCT in TAVR patients. Echocardiography (ECG) failed to show any significant differences in the mean valve gradient between the treatment groups, Dr. De Backer said.
Eleven patients who didn’t have leaflet thickening (7.3%) and 7 patients who did (15.9%) showed an increase of 5 mm Hg or more in the mean valve gradient on echo. ECG also showed a similar increase in the mean valve gradient in 14 patients who had no to moderate reduced leaflet motion (grade 3 or lower, 7.7%) and in four patients (30.8) who had grade 3 or higher reduced leaflet motion.
“This basically confirms results from observational studies that transthoracic echocardiography is often not good enough to detect these phenomena,” Dr. De Backer said.
The percentages of substudy patients who had major clinical events – major bleeding, thromboembolic events, or death at 90 days – were each less than 3%, he said. “There were too few clinical events to permit any assessment of the impact of leaflet thickening or reduced leaflet motion on clinical outcomes,” he said.
That lack of clarity with regard to clinical events is one of the questions the study leaves unanswered, said discussant Victoria Delgado, MD, PhD, of Leiden University Medical Center in the Netherlands.
“With stroke or TIA, there are too few events to draw any conclusions,” she said of the substudy. “We don’t know when we need to use CT, when we need to evaluate these patients, or maybe when we should go for more advanced imaging techniques where we can see the biology of those changes in the leaflets.” Hopefully, she said, future studies provide those insights.
“CT can be more sensitive than ECG to see these subclinical changes,” she said, “but the open questions that we have are to see if there is a correlation between thrombosis rate on imaging versus the stroke rate.”
The substudy’s conclusion on ECG, however, has been borne out by previous retrospective studies, Dr. Delgado added.
Robert A. Harrington, MD, of Stanford Medicine, tried to put the seemingly conflicting findings of the main GALILEO study and the 4D substudy into context.
“There you have the disconnect between the mechanism and the clinical observation and those are sometimes difficult to reconcile because the assumption is that the mechanism leads to the clinical outcome.”
While the main study shows that routine anticoagulation after TAVR is not indicated, the findings raise questions about the risk of clots forming on bioprosthetic valves. “Yes, maybe there are clots forming on these valves, but maybe that’s not causing the bad clinical outcomes,” Dr. Harrington said.
The findings also raise questions about the use of newer anticoagulants to prevent stroke post TAVR, he said. “It appears that warfarin is better than the newer anticoagulants for reasons that aren’t entirely clear.”
Dr. Dangas, lead author of the main GALILEO trial, said the substudy results could help design future trials of even-lower doses of anticoagulation in a more selective group of TAVR patients.
“In order to decrease the clots, first of all you don’t need the full dose of anticoagulation; even a low dose may do the trick,” he said. Further investigations can evaluate the clinical significance of having a blood clot in the valve as an indication for anticoagulation versus antiplatelet therapy.
“Even though this obviously doesn’t mean you’re going to have a stroke in a year or two,” Dr. Dangas said, “could it perhaps mean that the valve is not going to have such a good durability later on?”
Perhaps future studies of anticoagulation in TAVR should concentrate on patients who actually have clotting in the valve, he said.
The trial was supported by Bayer and Janssen. Dr. Dangas reported receiving grants from Bayer during the conduct of the study, personal fees from Bayer and Janssen, grants and personal fees from Daiichi-Sankyo, and “other” funding from Medtronic outside the submitted work. Dr. De Backer reported receiving grants from Bayer during the conduct of the study and personal fees from Abbott and Boston Scientific outside the submitted work.
SOURCE: Dangas GD and De Backer O. AHA 19, Late-Breaking Science 3 session.
This article also appears on Medscape.com.
REPORTING FROM AHA 2019
Weaknesses exposed in valsartan recall
ED visits for hypertension in month after the 2018 recall spiked 55%
PHILADELPHIA – The 2018 recall of generic forms of the antihypertensive valsartan exposed weaknesses in the recall systems for generic drugs in both the United States and Canada that caused many patients on the drug to fall through the cracks, according to a study of prescribing patterns in Ontario before and after the recall reported at the American Heart Association scientific sessions.
The results also have been published online in the journal Circulation (2019 Nov 11. doi: 10.1161/CIRCULATIONAHA.119.044494).
Cynthia Jackevicius, PharmD, of the Western University of Health Sciences in Pomona, Calif., reported that 90% of patients on recalled generic valsartan products switched to another antihypertension drug, but called the 10% for whom the study had no data “concerning.” She also said that ED visits for hypertension (HTN) in the month after the recall spiked 55%, from a rate of 0.11% to 0.17% (P = .02). While small, that increase was statistically significant, she said.
The Food and Drug Administration and Health Canada issued voluntary recalls of generic forms of valsartan in July 2018 following reports of N-nitrosodimethylamine (NDMA), a suspected carcinogen, being found in the products. Eventually, the recalls expanded to include valsartan products containing the contaminants N-nitrosodiethylamine (NDEA) and N-nitroso-N-methyl-4-aminobutyric acid (NMBA), as well as losartan and irbesartan products.
The Ontario study evaluated prescribing patterns and health system utilization in four different provincewide health databases and involved 55,461 patients, all of whom were on recalled generic valsartan when Health Canada issued the recall. The study also computed monthly rates of ED visits and hospitalizations for HTN, congestive heart failure, stroke/transit ischemic attack, and MI as primary diagnoses for 18 months before and 6 months after the recall. Rates of utilization for CHF and MI remained relatively flat through the study period, Dr. Jackevicius said, but rates of ED visits for stroke/TIA showed “a very small relative increase: 6% and 8% in ED visits and hospitalizations, respectively.” Respective P values were .020 and .037.
As for the nature of the ED visits after the recall, Dr. Jackevicius said the study did not tease that out. Many visits could have been for uncontrolled HTN or to get expired prescriptions refilled.
“But either way, even if it is just getting a new prescription, this isn’t the best response,” she said. We need to have a better system where patients can more easily or with less burden deal with a recall.”
Session moderator Seth S. Martin, MD, MHS, of Johns Hopkins University in Baltimore, echoed Dr. Jackevicius’s concerns about the handling of drug recalls. “Recalls are increasing,” he said. “Is this just the tip of the iceberg on the quality of generics and we’re going to see these floodgates open? Is this going to be chaos or is this more isolated to this class of medication, the ARBs? This is becoming a little concerning.”
Dr. Jackevicius made note of the recalls that followed the original valsartan recall.
“This really opened a lot of questions in terms of the quality of generic products,” she said. Drug manufacturers are putting safeguards into place to detect these potential contaminants, she said, “but a lot more work needs to be done to ensure the supply. All of these recalls and the prominence of this will be increased.”
The response to the recalls also must undergo revision, she said, citing the experiences of the United States and Canada. “There isn’t really a good system or strategy for recalls in either country,” Dr. Jackevicius said, noting that regulatory bodies notify prescribers and physicians, but “they don’t know which patients are on it.”
A better strategy would be to involve pharmacies more in the process. “The pharmacies have the lot numbers, and they will know what patients are on the recalled drug,” she said. “The pharmacists are the ones who are making the changes in the drugs, and giving them the responsibility so patients don’t have to go into the ED is important. If it’s a basic interchange of a drug, the pharmacists can do that to help raise compliance.”
Dr. Jackevicius had no relevant relationships to disclose.
SOURCE: Jackevicius J. AHA 2019. Session FS.AOS.F1.
ED visits for hypertension in month after the 2018 recall spiked 55%
ED visits for hypertension in month after the 2018 recall spiked 55%
PHILADELPHIA – The 2018 recall of generic forms of the antihypertensive valsartan exposed weaknesses in the recall systems for generic drugs in both the United States and Canada that caused many patients on the drug to fall through the cracks, according to a study of prescribing patterns in Ontario before and after the recall reported at the American Heart Association scientific sessions.
The results also have been published online in the journal Circulation (2019 Nov 11. doi: 10.1161/CIRCULATIONAHA.119.044494).
Cynthia Jackevicius, PharmD, of the Western University of Health Sciences in Pomona, Calif., reported that 90% of patients on recalled generic valsartan products switched to another antihypertension drug, but called the 10% for whom the study had no data “concerning.” She also said that ED visits for hypertension (HTN) in the month after the recall spiked 55%, from a rate of 0.11% to 0.17% (P = .02). While small, that increase was statistically significant, she said.
The Food and Drug Administration and Health Canada issued voluntary recalls of generic forms of valsartan in July 2018 following reports of N-nitrosodimethylamine (NDMA), a suspected carcinogen, being found in the products. Eventually, the recalls expanded to include valsartan products containing the contaminants N-nitrosodiethylamine (NDEA) and N-nitroso-N-methyl-4-aminobutyric acid (NMBA), as well as losartan and irbesartan products.
The Ontario study evaluated prescribing patterns and health system utilization in four different provincewide health databases and involved 55,461 patients, all of whom were on recalled generic valsartan when Health Canada issued the recall. The study also computed monthly rates of ED visits and hospitalizations for HTN, congestive heart failure, stroke/transit ischemic attack, and MI as primary diagnoses for 18 months before and 6 months after the recall. Rates of utilization for CHF and MI remained relatively flat through the study period, Dr. Jackevicius said, but rates of ED visits for stroke/TIA showed “a very small relative increase: 6% and 8% in ED visits and hospitalizations, respectively.” Respective P values were .020 and .037.
As for the nature of the ED visits after the recall, Dr. Jackevicius said the study did not tease that out. Many visits could have been for uncontrolled HTN or to get expired prescriptions refilled.
“But either way, even if it is just getting a new prescription, this isn’t the best response,” she said. We need to have a better system where patients can more easily or with less burden deal with a recall.”
Session moderator Seth S. Martin, MD, MHS, of Johns Hopkins University in Baltimore, echoed Dr. Jackevicius’s concerns about the handling of drug recalls. “Recalls are increasing,” he said. “Is this just the tip of the iceberg on the quality of generics and we’re going to see these floodgates open? Is this going to be chaos or is this more isolated to this class of medication, the ARBs? This is becoming a little concerning.”
Dr. Jackevicius made note of the recalls that followed the original valsartan recall.
“This really opened a lot of questions in terms of the quality of generic products,” she said. Drug manufacturers are putting safeguards into place to detect these potential contaminants, she said, “but a lot more work needs to be done to ensure the supply. All of these recalls and the prominence of this will be increased.”
The response to the recalls also must undergo revision, she said, citing the experiences of the United States and Canada. “There isn’t really a good system or strategy for recalls in either country,” Dr. Jackevicius said, noting that regulatory bodies notify prescribers and physicians, but “they don’t know which patients are on it.”
A better strategy would be to involve pharmacies more in the process. “The pharmacies have the lot numbers, and they will know what patients are on the recalled drug,” she said. “The pharmacists are the ones who are making the changes in the drugs, and giving them the responsibility so patients don’t have to go into the ED is important. If it’s a basic interchange of a drug, the pharmacists can do that to help raise compliance.”
Dr. Jackevicius had no relevant relationships to disclose.
SOURCE: Jackevicius J. AHA 2019. Session FS.AOS.F1.
PHILADELPHIA – The 2018 recall of generic forms of the antihypertensive valsartan exposed weaknesses in the recall systems for generic drugs in both the United States and Canada that caused many patients on the drug to fall through the cracks, according to a study of prescribing patterns in Ontario before and after the recall reported at the American Heart Association scientific sessions.
The results also have been published online in the journal Circulation (2019 Nov 11. doi: 10.1161/CIRCULATIONAHA.119.044494).
Cynthia Jackevicius, PharmD, of the Western University of Health Sciences in Pomona, Calif., reported that 90% of patients on recalled generic valsartan products switched to another antihypertension drug, but called the 10% for whom the study had no data “concerning.” She also said that ED visits for hypertension (HTN) in the month after the recall spiked 55%, from a rate of 0.11% to 0.17% (P = .02). While small, that increase was statistically significant, she said.
The Food and Drug Administration and Health Canada issued voluntary recalls of generic forms of valsartan in July 2018 following reports of N-nitrosodimethylamine (NDMA), a suspected carcinogen, being found in the products. Eventually, the recalls expanded to include valsartan products containing the contaminants N-nitrosodiethylamine (NDEA) and N-nitroso-N-methyl-4-aminobutyric acid (NMBA), as well as losartan and irbesartan products.
The Ontario study evaluated prescribing patterns and health system utilization in four different provincewide health databases and involved 55,461 patients, all of whom were on recalled generic valsartan when Health Canada issued the recall. The study also computed monthly rates of ED visits and hospitalizations for HTN, congestive heart failure, stroke/transit ischemic attack, and MI as primary diagnoses for 18 months before and 6 months after the recall. Rates of utilization for CHF and MI remained relatively flat through the study period, Dr. Jackevicius said, but rates of ED visits for stroke/TIA showed “a very small relative increase: 6% and 8% in ED visits and hospitalizations, respectively.” Respective P values were .020 and .037.
As for the nature of the ED visits after the recall, Dr. Jackevicius said the study did not tease that out. Many visits could have been for uncontrolled HTN or to get expired prescriptions refilled.
“But either way, even if it is just getting a new prescription, this isn’t the best response,” she said. We need to have a better system where patients can more easily or with less burden deal with a recall.”
Session moderator Seth S. Martin, MD, MHS, of Johns Hopkins University in Baltimore, echoed Dr. Jackevicius’s concerns about the handling of drug recalls. “Recalls are increasing,” he said. “Is this just the tip of the iceberg on the quality of generics and we’re going to see these floodgates open? Is this going to be chaos or is this more isolated to this class of medication, the ARBs? This is becoming a little concerning.”
Dr. Jackevicius made note of the recalls that followed the original valsartan recall.
“This really opened a lot of questions in terms of the quality of generic products,” she said. Drug manufacturers are putting safeguards into place to detect these potential contaminants, she said, “but a lot more work needs to be done to ensure the supply. All of these recalls and the prominence of this will be increased.”
The response to the recalls also must undergo revision, she said, citing the experiences of the United States and Canada. “There isn’t really a good system or strategy for recalls in either country,” Dr. Jackevicius said, noting that regulatory bodies notify prescribers and physicians, but “they don’t know which patients are on it.”
A better strategy would be to involve pharmacies more in the process. “The pharmacies have the lot numbers, and they will know what patients are on the recalled drug,” she said. “The pharmacists are the ones who are making the changes in the drugs, and giving them the responsibility so patients don’t have to go into the ED is important. If it’s a basic interchange of a drug, the pharmacists can do that to help raise compliance.”
Dr. Jackevicius had no relevant relationships to disclose.
SOURCE: Jackevicius J. AHA 2019. Session FS.AOS.F1.
REPORTING FROM AHA 2019
Key clinical point: Neither Canada nor the United States has a good system or strategy for recalling generic drugs.
Major finding: One in 10 patients may have discontinued therapy after the recall.
Study details: Population study of prescribing patterns and health utilization rates of 55,461 patients on valsartan before and after the July 2018 recall.
Disclosures: Dr. Jackevicius has no relevant financial relationships to report.
Source: Jackevicius C. AHA 2019. Session FS.AOS.F1.
What repair is best for juxtarenal aneurysm?
CHICAGO – Outcomes with fenestrated endografts and endograft anchors to repair abdominal aortic aneurysms (AAAs) in the region of the renal artery have improved as the techniques have gained popularity in recent years, but open repair may still achieve better overall results, vascular surgeons on opposite sides of the controversy contended during a debate at the annual meeting of the Midwestern Vascular Surgery Society.
Fenestrated endovascular aortic repair (FEVAR) “is as safe as open surgery to treat complex aneurysm,” said Carlos Bechara, MD, of Loyola University Medical Center in Chicago. “EndoAnchors [Medtronic] do provide an excellent off-the-shelf solution to treat short, hostile necks with promising short-term results.”
Arguing for open repair was Paul DiMusto, MD, of the University of Wisconsin–Madison. “Open repair has an equal perioperative mortality to FEVAR,” Dr. DiMusto said, adding that the open approach also has a higher long-term branch patency rate, lower secondary-intervention rate, a lower incidence of long-term renal failure, and higher long-term survival. “So putting that all together, open repair is best,” he said.
They staked out their positions by citing a host of published trials.
“The presence of a short neck can create a challenging clinical scenario for an endovascular repair of abdominal aortic aneurysm,” Dr. Bechara said. However, he noted he was discussing complex aneurysm in which the aortic clamp is placed above the renal arteries, differentiating it from infrarenal AAA in which the clamp is below the renal arteries with no renal ischemia time. He noted a 2011 study that determined a short neck was a predictor of Type 1A endoleak after AAA repair, but that compliance with best practices at the time was poor; more than 44% of EVARs did not follow the manufacturer’s instruction (Circulation. 2011;123:2848-55).
But FEVAR was approved by the Food and Drug Administration in 2012, with an indication for an infrarenal neck length of 4-14 mm, Dr. Bechara noted. Since then, several studies have reported excellent outcomes with the technique. An early small study of 67 patients reported a 100% technical success rate with one patient having a Type 1 endoleak at 3 years (J Vasc Surg. 2014;60:1420-8).
This year, a larger study evaluated 6,825 patients in the American College of Surgeons National Surgical Quality Improvement Program who had FEVAR, open AAA repair or standard infrarenal endovascular repair during 2012-2016. “Actually, the fenestrated approach had fewer complications than open repair and the outcomes were comparable to standard EVAR,” Dr. Bechara noted. The trial reported FEVAR had lower rates of perioperative mortality (1.8% vs. 8.8%; P = .001), postoperative renal dysfunction (1.4% vs. 7.7%; P = .002), and overall complications (11% vs. 33%; P less than.001) than did open repair (J Vasc Surg. 2019;69:1670-78).
In regard to the use of endograft anchors for treatment of endoleaks, migrating grafts, and high-risk seal zones, Dr. Bechara noted they are a good “off-the-shelf” choice for complex AAA repair. He cited current results of a cohort of 70 patients with short-neck AAA (J Vasc Surg. 2019;70:732-40). “This study showed a procedural success rate at 97% and a technical success rate at 88.6%,” he said. “They had no stent migration, no increase in sac size or AAA rupture or open conversion.”
He also pointed to just-published results from a randomized trial of 881 patients with up to 14 years of follow-up that found comparable rates of death/secondary procedures, as well as durability, between patients who had endovascular and open repairs (77.7% and 75.5%, respectively, N Engl J Med. 2019;380:2126-35). Also, he noted that hospital volume is an important predictor of success with open repair, with high-volume centers reporting lower mortality (3.9%) than low-volume centers (9%; Ann Surg. 2018 Nov 29. doi: 10.1097/SLA.0000000000002873). “So not many centers are doing high-volume open aortic surgery,” he said.
To make his case that open surgery for juxtarenal AAAs is superior, Dr. DiMusto cited a number of recent studies, including a three-center trial of 200 patients who had open and FEVAR procedures (J Endovasc Ther. 2019;26:105-12). “There was no difference in perioperative mortality [2.2% for FEVAR, 1.9% in open repair], ” Dr. DiMusto said “There was a higher freedom from reintervention in the open group [96% vs. 78%], and there was higher long-term vessel patency in the open group” (97.5% having target patency for open vs. 93.3% for FEVAR).
He also pointed to a meta-analysis of 2,326 patients that found similar outcomes for mortality and postoperative renal insufficiency between FEVAR and open repair, around 4.1%, but showed significantly higher rates of renal failure in FEVAR, at 19.7% versus 7.7% (J Vasc Surg. 2015;61:242-55). This study also reported significantly more secondary interventions with FEVAR, 12.7% vs. 4.9%, Dr. DiMusto said.
Another study of 3,253 complex AAA repairs, including 887 FEVAR and 2,125 open procedures, showed that FEVAR had a technical success rate of 97%, with no appreciable difference in perioperative mortality between the two procedures (Ann Surg. 2019 Feb 1. doi: 10.1097/SLA.0000000000003094).
However, Dr. DiMusto said, adjusted 3-year mortality in this study was higher with FEVAR, and further analysis yielded outcomes that favored open repair. “After excluding perioperative deaths, differences remained, with 9% mortality for FEVAR and 5% for open repair [P = .02],” he said. “This corresponded to a 66% higher risk for overall mortality following FEVAR.”
What’s more, Dr. DiMusto said, draft guidelines from the National Institute for Health and Care Excellence in the United Kingdom advise against offering complex EVAR to people with an unruptured AAA under two scenarios: if open surgery is an option; and even if they’re unable to have surgery because of anesthetic or medical issues. The final guidelines have yet to be released.
Dr. Bechara disclosed financial relationships with Gore Medical and Cook Medical and equity interest in MOKITA Medical. Dr. DiMusto has no relevant financial disclosures.
CHICAGO – Outcomes with fenestrated endografts and endograft anchors to repair abdominal aortic aneurysms (AAAs) in the region of the renal artery have improved as the techniques have gained popularity in recent years, but open repair may still achieve better overall results, vascular surgeons on opposite sides of the controversy contended during a debate at the annual meeting of the Midwestern Vascular Surgery Society.
Fenestrated endovascular aortic repair (FEVAR) “is as safe as open surgery to treat complex aneurysm,” said Carlos Bechara, MD, of Loyola University Medical Center in Chicago. “EndoAnchors [Medtronic] do provide an excellent off-the-shelf solution to treat short, hostile necks with promising short-term results.”
Arguing for open repair was Paul DiMusto, MD, of the University of Wisconsin–Madison. “Open repair has an equal perioperative mortality to FEVAR,” Dr. DiMusto said, adding that the open approach also has a higher long-term branch patency rate, lower secondary-intervention rate, a lower incidence of long-term renal failure, and higher long-term survival. “So putting that all together, open repair is best,” he said.
They staked out their positions by citing a host of published trials.
“The presence of a short neck can create a challenging clinical scenario for an endovascular repair of abdominal aortic aneurysm,” Dr. Bechara said. However, he noted he was discussing complex aneurysm in which the aortic clamp is placed above the renal arteries, differentiating it from infrarenal AAA in which the clamp is below the renal arteries with no renal ischemia time. He noted a 2011 study that determined a short neck was a predictor of Type 1A endoleak after AAA repair, but that compliance with best practices at the time was poor; more than 44% of EVARs did not follow the manufacturer’s instruction (Circulation. 2011;123:2848-55).
But FEVAR was approved by the Food and Drug Administration in 2012, with an indication for an infrarenal neck length of 4-14 mm, Dr. Bechara noted. Since then, several studies have reported excellent outcomes with the technique. An early small study of 67 patients reported a 100% technical success rate with one patient having a Type 1 endoleak at 3 years (J Vasc Surg. 2014;60:1420-8).
This year, a larger study evaluated 6,825 patients in the American College of Surgeons National Surgical Quality Improvement Program who had FEVAR, open AAA repair or standard infrarenal endovascular repair during 2012-2016. “Actually, the fenestrated approach had fewer complications than open repair and the outcomes were comparable to standard EVAR,” Dr. Bechara noted. The trial reported FEVAR had lower rates of perioperative mortality (1.8% vs. 8.8%; P = .001), postoperative renal dysfunction (1.4% vs. 7.7%; P = .002), and overall complications (11% vs. 33%; P less than.001) than did open repair (J Vasc Surg. 2019;69:1670-78).
In regard to the use of endograft anchors for treatment of endoleaks, migrating grafts, and high-risk seal zones, Dr. Bechara noted they are a good “off-the-shelf” choice for complex AAA repair. He cited current results of a cohort of 70 patients with short-neck AAA (J Vasc Surg. 2019;70:732-40). “This study showed a procedural success rate at 97% and a technical success rate at 88.6%,” he said. “They had no stent migration, no increase in sac size or AAA rupture or open conversion.”
He also pointed to just-published results from a randomized trial of 881 patients with up to 14 years of follow-up that found comparable rates of death/secondary procedures, as well as durability, between patients who had endovascular and open repairs (77.7% and 75.5%, respectively, N Engl J Med. 2019;380:2126-35). Also, he noted that hospital volume is an important predictor of success with open repair, with high-volume centers reporting lower mortality (3.9%) than low-volume centers (9%; Ann Surg. 2018 Nov 29. doi: 10.1097/SLA.0000000000002873). “So not many centers are doing high-volume open aortic surgery,” he said.
To make his case that open surgery for juxtarenal AAAs is superior, Dr. DiMusto cited a number of recent studies, including a three-center trial of 200 patients who had open and FEVAR procedures (J Endovasc Ther. 2019;26:105-12). “There was no difference in perioperative mortality [2.2% for FEVAR, 1.9% in open repair], ” Dr. DiMusto said “There was a higher freedom from reintervention in the open group [96% vs. 78%], and there was higher long-term vessel patency in the open group” (97.5% having target patency for open vs. 93.3% for FEVAR).
He also pointed to a meta-analysis of 2,326 patients that found similar outcomes for mortality and postoperative renal insufficiency between FEVAR and open repair, around 4.1%, but showed significantly higher rates of renal failure in FEVAR, at 19.7% versus 7.7% (J Vasc Surg. 2015;61:242-55). This study also reported significantly more secondary interventions with FEVAR, 12.7% vs. 4.9%, Dr. DiMusto said.
Another study of 3,253 complex AAA repairs, including 887 FEVAR and 2,125 open procedures, showed that FEVAR had a technical success rate of 97%, with no appreciable difference in perioperative mortality between the two procedures (Ann Surg. 2019 Feb 1. doi: 10.1097/SLA.0000000000003094).
However, Dr. DiMusto said, adjusted 3-year mortality in this study was higher with FEVAR, and further analysis yielded outcomes that favored open repair. “After excluding perioperative deaths, differences remained, with 9% mortality for FEVAR and 5% for open repair [P = .02],” he said. “This corresponded to a 66% higher risk for overall mortality following FEVAR.”
What’s more, Dr. DiMusto said, draft guidelines from the National Institute for Health and Care Excellence in the United Kingdom advise against offering complex EVAR to people with an unruptured AAA under two scenarios: if open surgery is an option; and even if they’re unable to have surgery because of anesthetic or medical issues. The final guidelines have yet to be released.
Dr. Bechara disclosed financial relationships with Gore Medical and Cook Medical and equity interest in MOKITA Medical. Dr. DiMusto has no relevant financial disclosures.
CHICAGO – Outcomes with fenestrated endografts and endograft anchors to repair abdominal aortic aneurysms (AAAs) in the region of the renal artery have improved as the techniques have gained popularity in recent years, but open repair may still achieve better overall results, vascular surgeons on opposite sides of the controversy contended during a debate at the annual meeting of the Midwestern Vascular Surgery Society.
Fenestrated endovascular aortic repair (FEVAR) “is as safe as open surgery to treat complex aneurysm,” said Carlos Bechara, MD, of Loyola University Medical Center in Chicago. “EndoAnchors [Medtronic] do provide an excellent off-the-shelf solution to treat short, hostile necks with promising short-term results.”
Arguing for open repair was Paul DiMusto, MD, of the University of Wisconsin–Madison. “Open repair has an equal perioperative mortality to FEVAR,” Dr. DiMusto said, adding that the open approach also has a higher long-term branch patency rate, lower secondary-intervention rate, a lower incidence of long-term renal failure, and higher long-term survival. “So putting that all together, open repair is best,” he said.
They staked out their positions by citing a host of published trials.
“The presence of a short neck can create a challenging clinical scenario for an endovascular repair of abdominal aortic aneurysm,” Dr. Bechara said. However, he noted he was discussing complex aneurysm in which the aortic clamp is placed above the renal arteries, differentiating it from infrarenal AAA in which the clamp is below the renal arteries with no renal ischemia time. He noted a 2011 study that determined a short neck was a predictor of Type 1A endoleak after AAA repair, but that compliance with best practices at the time was poor; more than 44% of EVARs did not follow the manufacturer’s instruction (Circulation. 2011;123:2848-55).
But FEVAR was approved by the Food and Drug Administration in 2012, with an indication for an infrarenal neck length of 4-14 mm, Dr. Bechara noted. Since then, several studies have reported excellent outcomes with the technique. An early small study of 67 patients reported a 100% technical success rate with one patient having a Type 1 endoleak at 3 years (J Vasc Surg. 2014;60:1420-8).
This year, a larger study evaluated 6,825 patients in the American College of Surgeons National Surgical Quality Improvement Program who had FEVAR, open AAA repair or standard infrarenal endovascular repair during 2012-2016. “Actually, the fenestrated approach had fewer complications than open repair and the outcomes were comparable to standard EVAR,” Dr. Bechara noted. The trial reported FEVAR had lower rates of perioperative mortality (1.8% vs. 8.8%; P = .001), postoperative renal dysfunction (1.4% vs. 7.7%; P = .002), and overall complications (11% vs. 33%; P less than.001) than did open repair (J Vasc Surg. 2019;69:1670-78).
In regard to the use of endograft anchors for treatment of endoleaks, migrating grafts, and high-risk seal zones, Dr. Bechara noted they are a good “off-the-shelf” choice for complex AAA repair. He cited current results of a cohort of 70 patients with short-neck AAA (J Vasc Surg. 2019;70:732-40). “This study showed a procedural success rate at 97% and a technical success rate at 88.6%,” he said. “They had no stent migration, no increase in sac size or AAA rupture or open conversion.”
He also pointed to just-published results from a randomized trial of 881 patients with up to 14 years of follow-up that found comparable rates of death/secondary procedures, as well as durability, between patients who had endovascular and open repairs (77.7% and 75.5%, respectively, N Engl J Med. 2019;380:2126-35). Also, he noted that hospital volume is an important predictor of success with open repair, with high-volume centers reporting lower mortality (3.9%) than low-volume centers (9%; Ann Surg. 2018 Nov 29. doi: 10.1097/SLA.0000000000002873). “So not many centers are doing high-volume open aortic surgery,” he said.
To make his case that open surgery for juxtarenal AAAs is superior, Dr. DiMusto cited a number of recent studies, including a three-center trial of 200 patients who had open and FEVAR procedures (J Endovasc Ther. 2019;26:105-12). “There was no difference in perioperative mortality [2.2% for FEVAR, 1.9% in open repair], ” Dr. DiMusto said “There was a higher freedom from reintervention in the open group [96% vs. 78%], and there was higher long-term vessel patency in the open group” (97.5% having target patency for open vs. 93.3% for FEVAR).
He also pointed to a meta-analysis of 2,326 patients that found similar outcomes for mortality and postoperative renal insufficiency between FEVAR and open repair, around 4.1%, but showed significantly higher rates of renal failure in FEVAR, at 19.7% versus 7.7% (J Vasc Surg. 2015;61:242-55). This study also reported significantly more secondary interventions with FEVAR, 12.7% vs. 4.9%, Dr. DiMusto said.
Another study of 3,253 complex AAA repairs, including 887 FEVAR and 2,125 open procedures, showed that FEVAR had a technical success rate of 97%, with no appreciable difference in perioperative mortality between the two procedures (Ann Surg. 2019 Feb 1. doi: 10.1097/SLA.0000000000003094).
However, Dr. DiMusto said, adjusted 3-year mortality in this study was higher with FEVAR, and further analysis yielded outcomes that favored open repair. “After excluding perioperative deaths, differences remained, with 9% mortality for FEVAR and 5% for open repair [P = .02],” he said. “This corresponded to a 66% higher risk for overall mortality following FEVAR.”
What’s more, Dr. DiMusto said, draft guidelines from the National Institute for Health and Care Excellence in the United Kingdom advise against offering complex EVAR to people with an unruptured AAA under two scenarios: if open surgery is an option; and even if they’re unable to have surgery because of anesthetic or medical issues. The final guidelines have yet to be released.
Dr. Bechara disclosed financial relationships with Gore Medical and Cook Medical and equity interest in MOKITA Medical. Dr. DiMusto has no relevant financial disclosures.
EXPERT ANALYSIS FROM MIDWESTERN VASCULAR 2019