User login
RECOVERY trial of COVID-19 treatments stops colchicine arm
On the advice of its independent data monitoring committee (DMC), the RECOVERY trial has stopped recruitment to the colchicine arm for lack of efficacy in patients hospitalized with COVID-19.
“The DMC saw no convincing evidence that further recruitment would provide conclusive proof of worthwhile mortality benefit either overall or in any prespecified subgroup,” the British investigators announced on March 5.
“The RECOVERY trial has already identified two anti-inflammatory drugs – dexamethasone and tocilizumab – that improve the chances of survival for patients with severe COVID-19. So, it is disappointing that colchicine, which is widely used to treat gout and other inflammatory conditions, has no effect in these patients,” cochief investigator Martin Landray, MBChB, PhD, said in a statement.
“We do large, randomized trials to establish whether a drug that seems promising in theory has real benefits for patients in practice. Unfortunately, colchicine is not one of those,” said Dr. Landry, University of Oxford (England).
The RECOVERY trial is evaluating a range of potential treatments for COVID-19 at 180 hospitals in the United Kingdom, Indonesia, and Nepal, and was designed with the expectation that drugs would be added or dropped as the evidence changes. Since November 2020, the trial has included an arm comparing colchicine with usual care alone.
As part of a routine meeting March 4, the DMC reviewed data from a preliminary analysis based on 2,178 deaths among 11,162 patients, 94% of whom were being treated with a corticosteroid such as dexamethasone.
The results showed no significant difference in the primary endpoint of 28-day mortality in patients randomized to colchicine versus usual care alone (20% vs. 19%; risk ratio, 1.02; 95% confidence interval, 0.94-1.11; P = .63).
Follow-up is ongoing and final results will be published as soon as possible, the investigators said. Thus far, there has been no convincing evidence of an effect of colchicine on clinical outcomes in hospitalized COVID-19 patients.
Recruitment will continue to all other treatment arms – aspirin, baricitinib, Regeneron’s antibody cocktail, and, in select hospitals, dimethyl fumarate – the investigators said.
Cochief investigator Peter Hornby, MD, PhD, also from the University of Oxford, noted that this has been the largest trial ever of colchicine. “Whilst we are disappointed that the overall result is negative, it is still important information for the future care of patients in the U.K. and worldwide.”
A version of this article first appeared on Medscape.com.
On the advice of its independent data monitoring committee (DMC), the RECOVERY trial has stopped recruitment to the colchicine arm for lack of efficacy in patients hospitalized with COVID-19.
“The DMC saw no convincing evidence that further recruitment would provide conclusive proof of worthwhile mortality benefit either overall or in any prespecified subgroup,” the British investigators announced on March 5.
“The RECOVERY trial has already identified two anti-inflammatory drugs – dexamethasone and tocilizumab – that improve the chances of survival for patients with severe COVID-19. So, it is disappointing that colchicine, which is widely used to treat gout and other inflammatory conditions, has no effect in these patients,” cochief investigator Martin Landray, MBChB, PhD, said in a statement.
“We do large, randomized trials to establish whether a drug that seems promising in theory has real benefits for patients in practice. Unfortunately, colchicine is not one of those,” said Dr. Landry, University of Oxford (England).
The RECOVERY trial is evaluating a range of potential treatments for COVID-19 at 180 hospitals in the United Kingdom, Indonesia, and Nepal, and was designed with the expectation that drugs would be added or dropped as the evidence changes. Since November 2020, the trial has included an arm comparing colchicine with usual care alone.
As part of a routine meeting March 4, the DMC reviewed data from a preliminary analysis based on 2,178 deaths among 11,162 patients, 94% of whom were being treated with a corticosteroid such as dexamethasone.
The results showed no significant difference in the primary endpoint of 28-day mortality in patients randomized to colchicine versus usual care alone (20% vs. 19%; risk ratio, 1.02; 95% confidence interval, 0.94-1.11; P = .63).
Follow-up is ongoing and final results will be published as soon as possible, the investigators said. Thus far, there has been no convincing evidence of an effect of colchicine on clinical outcomes in hospitalized COVID-19 patients.
Recruitment will continue to all other treatment arms – aspirin, baricitinib, Regeneron’s antibody cocktail, and, in select hospitals, dimethyl fumarate – the investigators said.
Cochief investigator Peter Hornby, MD, PhD, also from the University of Oxford, noted that this has been the largest trial ever of colchicine. “Whilst we are disappointed that the overall result is negative, it is still important information for the future care of patients in the U.K. and worldwide.”
A version of this article first appeared on Medscape.com.
On the advice of its independent data monitoring committee (DMC), the RECOVERY trial has stopped recruitment to the colchicine arm for lack of efficacy in patients hospitalized with COVID-19.
“The DMC saw no convincing evidence that further recruitment would provide conclusive proof of worthwhile mortality benefit either overall or in any prespecified subgroup,” the British investigators announced on March 5.
“The RECOVERY trial has already identified two anti-inflammatory drugs – dexamethasone and tocilizumab – that improve the chances of survival for patients with severe COVID-19. So, it is disappointing that colchicine, which is widely used to treat gout and other inflammatory conditions, has no effect in these patients,” cochief investigator Martin Landray, MBChB, PhD, said in a statement.
“We do large, randomized trials to establish whether a drug that seems promising in theory has real benefits for patients in practice. Unfortunately, colchicine is not one of those,” said Dr. Landry, University of Oxford (England).
The RECOVERY trial is evaluating a range of potential treatments for COVID-19 at 180 hospitals in the United Kingdom, Indonesia, and Nepal, and was designed with the expectation that drugs would be added or dropped as the evidence changes. Since November 2020, the trial has included an arm comparing colchicine with usual care alone.
As part of a routine meeting March 4, the DMC reviewed data from a preliminary analysis based on 2,178 deaths among 11,162 patients, 94% of whom were being treated with a corticosteroid such as dexamethasone.
The results showed no significant difference in the primary endpoint of 28-day mortality in patients randomized to colchicine versus usual care alone (20% vs. 19%; risk ratio, 1.02; 95% confidence interval, 0.94-1.11; P = .63).
Follow-up is ongoing and final results will be published as soon as possible, the investigators said. Thus far, there has been no convincing evidence of an effect of colchicine on clinical outcomes in hospitalized COVID-19 patients.
Recruitment will continue to all other treatment arms – aspirin, baricitinib, Regeneron’s antibody cocktail, and, in select hospitals, dimethyl fumarate – the investigators said.
Cochief investigator Peter Hornby, MD, PhD, also from the University of Oxford, noted that this has been the largest trial ever of colchicine. “Whilst we are disappointed that the overall result is negative, it is still important information for the future care of patients in the U.K. and worldwide.”
A version of this article first appeared on Medscape.com.
No vascular benefit of testosterone over exercise in aging men
Exercise training – but not testosterone therapy – improved vascular health in aging men with widening midsections and low to normal testosterone, new research suggests.
“Previous studies have suggested that men with higher levels of testosterone, who were more physically active, might have better health outcomes,” Bu Beng Yeap, MBBS, PhD, University of Western Australia, Perth, said in an interview. “We formulated the hypothesis that the combination of testosterone treatment and exercise training would improve the health of arteries more than either alone.”
To test this hypothesis, the investigators randomly assigned 80 men, aged 50-70 years, to 12 weeks of 5% testosterone cream 2 mL applied daily or placebo plus a supervised exercise program that included machine-based resistance and aerobic (cycling) exercises two to three times a week or no additional exercise.
The men (mean age, 59 years) had low-normal testosterone (6-14 nmol/L), a waist circumference of at least 95 cm (37.4 inches), and no known cardiovascular disease (CVD), type 1 diabetes, or other clinically significant illnesses. Current smokers and men on testosterone or medications that would alter testosterone levels were also excluded.
High-resolution ultrasound of the brachial artery was used to assess flow-mediated dilation (FMD) and sublingual glyceryl trinitrate (GTN) responses. FMD has been shown to be predictive of CVD risk, with a 1% increase in FMD associated with a 9%-13% decrease in future CVD events.
Based on participants’ daily dairies, testosterone adherence was 97.6%. Exercise adherence was 96.5% for twice-weekly attendance and 80.0% for thrice-weekly attendance, with no between-group differences.
As reported Feb. 22, 2021, in Hypertension, testosterone levels increased, on average, 3.0 nmol/L in both testosterone groups by week 12 (P = .003). In all, 62% of these men had levels of the hormone exceeding 14 nmol/L, compared with 29% of those receiving placebo.
Testosterone levels improved with exercise training plus placebo by 0.9 nmol/L, but fell with no exercise and placebo by 0.9 nmol/L.
In terms of vascular function, exercise training increased FMD when expressed as both the delta change (mm; P = .004) and relative rise from baseline diameter (%; P = .033).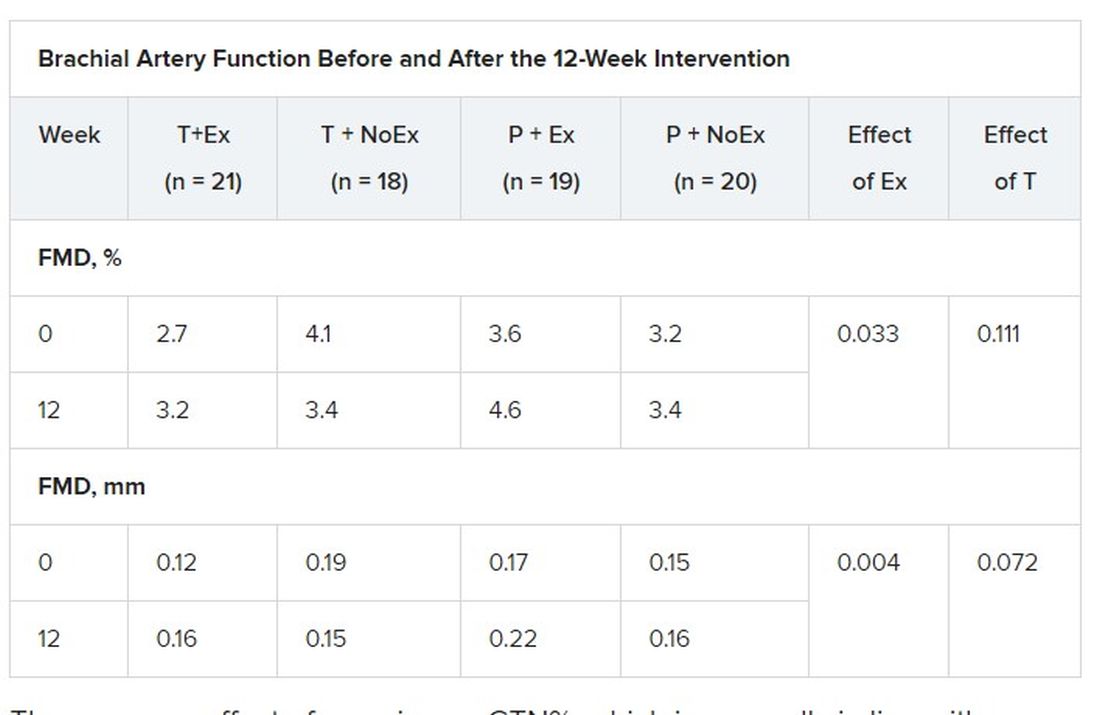
There was no effect of exercise on GTN%, which is generally in line with exercise literature indicating that shear-mediated adaptations in response to episodic exercise occur largely in endothelial cells, the authors noted.
Testosterone did not affect any measures of FMD nor was there an effect on GTN response, despite previous evidence that lower testosterone doses might enhance smooth muscle function.
“Our main finding was that testosterone – at this dose over this duration of treatment – did not have a beneficial effect on artery health, nor did it enhance the effect of exercise,” said Dr. Yeap, who is also president of the Endocrine Society of Australia. “For middle-aged and older men wanting to improve the health of their arteries, exercise is better than testosterone!”
Shalender Bhasin, MBBS, director of research programs in men’s health, aging, and metabolism at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School, both in Boston, said the study is interesting from a mechanistic perspective and adds to the overall body of evidence on how testosterone affects performance, but was narrowly focused.
“They looked at very specific markers and what they’re showing is that this is not the mechanism by which testosterone improves performance,” he said. “That may be so, but it doesn’t negate the finding that testosterone improves endurance and has other vascular effects: it increases capillarity, increases blood flow to the tissues, and improves myocardial function.”
Although well done, the study doesn’t get at the larger question of whether testosterone increases cardiovascular risk, observed Dr. Bhasin. “None of the randomized studies have been large enough or long enough to determine the effect on cardiovascular events rates. There’s a lot of argument on both sides but we need some data to address that.”
The 6,000-patient TRAVERSE trial is specifically looking at long-term major cardiovascular events with topical testosterone, compared with placebo, in hypogonadal men aged 45-80 years age who have evidence of or are at increased risk for CVD. The study, which is set to be completed in April 2022, should also provide information on fracture risk in these men, said Dr. Bhasin, one of the trial’s principal investigators and lead author of the Endocrine Society’s 2018 clinical practice guideline on testosterone therapy for hypogonadism in men.
William Evans, MD, adjunct professor of human nutrition, University of California, Berkley, said in an interview that the positive effects of testosterone occur at much lower doses in men and women who are hypogonadal but, in this particular population, exercise is the key and the major recommendation.
“Testosterone has been overprescribed and overadvertised for essentially a lifetime of sedentary living, and it’s advertised as a way to get all that back without having to work for it,” he said. “Exercise has a profound and positive effect on control of blood pressure, function, and strength, and testosterone may only affect in people who are sick, people who have really low levels.”
The study was funded by the Heart Foundation of Australia. Lawley Pharmaceuticals provided the study medication and placebo. Dr. Yeap has received speaker honoraria and conference support from Bayer, Eli Lilly, and Besins Healthcare; research support from Bayer, Lily, and Lawley; and served as an adviser for Lily, Besins Healthcare, Ferring, and Lawley. Dr. Shalender reports consultation or advisement for GTx, Pfizer, and TAP; grant or other research support from Solvay and GlaxoSmithKline; and honoraria from Solvay and Auxilium. Dr. Evans reported having no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Exercise training – but not testosterone therapy – improved vascular health in aging men with widening midsections and low to normal testosterone, new research suggests.
“Previous studies have suggested that men with higher levels of testosterone, who were more physically active, might have better health outcomes,” Bu Beng Yeap, MBBS, PhD, University of Western Australia, Perth, said in an interview. “We formulated the hypothesis that the combination of testosterone treatment and exercise training would improve the health of arteries more than either alone.”
To test this hypothesis, the investigators randomly assigned 80 men, aged 50-70 years, to 12 weeks of 5% testosterone cream 2 mL applied daily or placebo plus a supervised exercise program that included machine-based resistance and aerobic (cycling) exercises two to three times a week or no additional exercise.
The men (mean age, 59 years) had low-normal testosterone (6-14 nmol/L), a waist circumference of at least 95 cm (37.4 inches), and no known cardiovascular disease (CVD), type 1 diabetes, or other clinically significant illnesses. Current smokers and men on testosterone or medications that would alter testosterone levels were also excluded.
High-resolution ultrasound of the brachial artery was used to assess flow-mediated dilation (FMD) and sublingual glyceryl trinitrate (GTN) responses. FMD has been shown to be predictive of CVD risk, with a 1% increase in FMD associated with a 9%-13% decrease in future CVD events.
Based on participants’ daily dairies, testosterone adherence was 97.6%. Exercise adherence was 96.5% for twice-weekly attendance and 80.0% for thrice-weekly attendance, with no between-group differences.
As reported Feb. 22, 2021, in Hypertension, testosterone levels increased, on average, 3.0 nmol/L in both testosterone groups by week 12 (P = .003). In all, 62% of these men had levels of the hormone exceeding 14 nmol/L, compared with 29% of those receiving placebo.
Testosterone levels improved with exercise training plus placebo by 0.9 nmol/L, but fell with no exercise and placebo by 0.9 nmol/L.
In terms of vascular function, exercise training increased FMD when expressed as both the delta change (mm; P = .004) and relative rise from baseline diameter (%; P = .033).
There was no effect of exercise on GTN%, which is generally in line with exercise literature indicating that shear-mediated adaptations in response to episodic exercise occur largely in endothelial cells, the authors noted.
Testosterone did not affect any measures of FMD nor was there an effect on GTN response, despite previous evidence that lower testosterone doses might enhance smooth muscle function.
“Our main finding was that testosterone – at this dose over this duration of treatment – did not have a beneficial effect on artery health, nor did it enhance the effect of exercise,” said Dr. Yeap, who is also president of the Endocrine Society of Australia. “For middle-aged and older men wanting to improve the health of their arteries, exercise is better than testosterone!”
Shalender Bhasin, MBBS, director of research programs in men’s health, aging, and metabolism at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School, both in Boston, said the study is interesting from a mechanistic perspective and adds to the overall body of evidence on how testosterone affects performance, but was narrowly focused.
“They looked at very specific markers and what they’re showing is that this is not the mechanism by which testosterone improves performance,” he said. “That may be so, but it doesn’t negate the finding that testosterone improves endurance and has other vascular effects: it increases capillarity, increases blood flow to the tissues, and improves myocardial function.”
Although well done, the study doesn’t get at the larger question of whether testosterone increases cardiovascular risk, observed Dr. Bhasin. “None of the randomized studies have been large enough or long enough to determine the effect on cardiovascular events rates. There’s a lot of argument on both sides but we need some data to address that.”
The 6,000-patient TRAVERSE trial is specifically looking at long-term major cardiovascular events with topical testosterone, compared with placebo, in hypogonadal men aged 45-80 years age who have evidence of or are at increased risk for CVD. The study, which is set to be completed in April 2022, should also provide information on fracture risk in these men, said Dr. Bhasin, one of the trial’s principal investigators and lead author of the Endocrine Society’s 2018 clinical practice guideline on testosterone therapy for hypogonadism in men.
William Evans, MD, adjunct professor of human nutrition, University of California, Berkley, said in an interview that the positive effects of testosterone occur at much lower doses in men and women who are hypogonadal but, in this particular population, exercise is the key and the major recommendation.
“Testosterone has been overprescribed and overadvertised for essentially a lifetime of sedentary living, and it’s advertised as a way to get all that back without having to work for it,” he said. “Exercise has a profound and positive effect on control of blood pressure, function, and strength, and testosterone may only affect in people who are sick, people who have really low levels.”
The study was funded by the Heart Foundation of Australia. Lawley Pharmaceuticals provided the study medication and placebo. Dr. Yeap has received speaker honoraria and conference support from Bayer, Eli Lilly, and Besins Healthcare; research support from Bayer, Lily, and Lawley; and served as an adviser for Lily, Besins Healthcare, Ferring, and Lawley. Dr. Shalender reports consultation or advisement for GTx, Pfizer, and TAP; grant or other research support from Solvay and GlaxoSmithKline; and honoraria from Solvay and Auxilium. Dr. Evans reported having no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Exercise training – but not testosterone therapy – improved vascular health in aging men with widening midsections and low to normal testosterone, new research suggests.
“Previous studies have suggested that men with higher levels of testosterone, who were more physically active, might have better health outcomes,” Bu Beng Yeap, MBBS, PhD, University of Western Australia, Perth, said in an interview. “We formulated the hypothesis that the combination of testosterone treatment and exercise training would improve the health of arteries more than either alone.”
To test this hypothesis, the investigators randomly assigned 80 men, aged 50-70 years, to 12 weeks of 5% testosterone cream 2 mL applied daily or placebo plus a supervised exercise program that included machine-based resistance and aerobic (cycling) exercises two to three times a week or no additional exercise.
The men (mean age, 59 years) had low-normal testosterone (6-14 nmol/L), a waist circumference of at least 95 cm (37.4 inches), and no known cardiovascular disease (CVD), type 1 diabetes, or other clinically significant illnesses. Current smokers and men on testosterone or medications that would alter testosterone levels were also excluded.
High-resolution ultrasound of the brachial artery was used to assess flow-mediated dilation (FMD) and sublingual glyceryl trinitrate (GTN) responses. FMD has been shown to be predictive of CVD risk, with a 1% increase in FMD associated with a 9%-13% decrease in future CVD events.
Based on participants’ daily dairies, testosterone adherence was 97.6%. Exercise adherence was 96.5% for twice-weekly attendance and 80.0% for thrice-weekly attendance, with no between-group differences.
As reported Feb. 22, 2021, in Hypertension, testosterone levels increased, on average, 3.0 nmol/L in both testosterone groups by week 12 (P = .003). In all, 62% of these men had levels of the hormone exceeding 14 nmol/L, compared with 29% of those receiving placebo.
Testosterone levels improved with exercise training plus placebo by 0.9 nmol/L, but fell with no exercise and placebo by 0.9 nmol/L.
In terms of vascular function, exercise training increased FMD when expressed as both the delta change (mm; P = .004) and relative rise from baseline diameter (%; P = .033).
There was no effect of exercise on GTN%, which is generally in line with exercise literature indicating that shear-mediated adaptations in response to episodic exercise occur largely in endothelial cells, the authors noted.
Testosterone did not affect any measures of FMD nor was there an effect on GTN response, despite previous evidence that lower testosterone doses might enhance smooth muscle function.
“Our main finding was that testosterone – at this dose over this duration of treatment – did not have a beneficial effect on artery health, nor did it enhance the effect of exercise,” said Dr. Yeap, who is also president of the Endocrine Society of Australia. “For middle-aged and older men wanting to improve the health of their arteries, exercise is better than testosterone!”
Shalender Bhasin, MBBS, director of research programs in men’s health, aging, and metabolism at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School, both in Boston, said the study is interesting from a mechanistic perspective and adds to the overall body of evidence on how testosterone affects performance, but was narrowly focused.
“They looked at very specific markers and what they’re showing is that this is not the mechanism by which testosterone improves performance,” he said. “That may be so, but it doesn’t negate the finding that testosterone improves endurance and has other vascular effects: it increases capillarity, increases blood flow to the tissues, and improves myocardial function.”
Although well done, the study doesn’t get at the larger question of whether testosterone increases cardiovascular risk, observed Dr. Bhasin. “None of the randomized studies have been large enough or long enough to determine the effect on cardiovascular events rates. There’s a lot of argument on both sides but we need some data to address that.”
The 6,000-patient TRAVERSE trial is specifically looking at long-term major cardiovascular events with topical testosterone, compared with placebo, in hypogonadal men aged 45-80 years age who have evidence of or are at increased risk for CVD. The study, which is set to be completed in April 2022, should also provide information on fracture risk in these men, said Dr. Bhasin, one of the trial’s principal investigators and lead author of the Endocrine Society’s 2018 clinical practice guideline on testosterone therapy for hypogonadism in men.
William Evans, MD, adjunct professor of human nutrition, University of California, Berkley, said in an interview that the positive effects of testosterone occur at much lower doses in men and women who are hypogonadal but, in this particular population, exercise is the key and the major recommendation.
“Testosterone has been overprescribed and overadvertised for essentially a lifetime of sedentary living, and it’s advertised as a way to get all that back without having to work for it,” he said. “Exercise has a profound and positive effect on control of blood pressure, function, and strength, and testosterone may only affect in people who are sick, people who have really low levels.”
The study was funded by the Heart Foundation of Australia. Lawley Pharmaceuticals provided the study medication and placebo. Dr. Yeap has received speaker honoraria and conference support from Bayer, Eli Lilly, and Besins Healthcare; research support from Bayer, Lily, and Lawley; and served as an adviser for Lily, Besins Healthcare, Ferring, and Lawley. Dr. Shalender reports consultation or advisement for GTx, Pfizer, and TAP; grant or other research support from Solvay and GlaxoSmithKline; and honoraria from Solvay and Auxilium. Dr. Evans reported having no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Ivabradine knocks down heart rate, symptoms in POTS
The heart failure drug ivabradine (Corlanor) can provide relief from the elevated heart rate and often debilitating symptoms associated with postural orthostatic tachycardia syndrome (POTS), a new study suggests.
Ivabradine significantly lowered standing heart rate, compared with placebo (77.9 vs. 94.2 beats/min; P < .001). The typical surge in heart rate that occurs upon standing in these patients was also blunted, compared with baseline (13.0 vs. 21.4 beats/min; P = .001).
“There are really not a lot of great options for patients with POTS and, mechanistically, ivabradine just make sense because it’s a drug that lowers heart rate very selectively and doesn’t lower blood pressure,” lead study author Pam R. Taub, MD, told this news organization.
Surprisingly, the reduction in heart rate translated into improved physical (P = .008) and social (P = .021) functioning after just 1 month of ivabradine, without any other background POTS medications or a change in nonpharmacologic therapies, she said. “What’s really nice to see is when you tackle a really significant part of the disease, which is the elevated heart rate, just how much better they feel.”
POTS patients are mostly healthy, active young women, who after some inciting event – such as viral infection, trauma, or surgery – experience an increase in heart rate of at least 30 beats/min upon standing accompanied by a range of symptoms, including dizziness, palpitations, brain fog, and fatigue.
A COVID connection?
The study enrolled patients with hyperadrenergic POTS as the predominant subtype, but another group to keep in mind that might benefit is the post-COVID POTS patient, said Dr. Taub, from the University of California, San Diego.
“We’re seeing an incredible number of patients post COVID that meet the criteria for POTS, and a lot of these patients also have COVID fatigue,” she said. “So clinically, myself and many other cardiologists who understand ivabradine have been using it off-label for the COVID patients, as long as they meet the criteria. You don’t want to use it in every COVID patient, but if someone’s predominant complaint is that their heart rate is going up when they’re standing and they’re debilitated by it, this is a drug to consider.”
Anecdotal findings in patients with long-hauler COVID need to be translated into rigorous research protocols, but mechanistically, whether it’s POTS from COVID or from another type of infection – like Lyme disease or some other viral syndrome – it should work the same, Dr. Taub said. “POTS is POTS.”
There are no first-line drugs for POTS, and current class IIb recommendations include midodrine, which increases blood pressure and can make people feel awful, and fludrocortisone, which can cause a lot of weight gain and fluid retention, she observed. Other agents that lower heart rate, like beta-blockers, also lower blood pressure and can aggravate depression and fatigue.
Ivabradine regulates heart rate by specifically blocking the Ifunny channel of the sinoatrial node. It was approved in 2015 in the United States to reduce hospitalizations in patients with systolic heart failure, and it also has a second class IIb recommendation for inappropriate sinus tachycardia.
The present study, reported in the Feb. 23 issue of the Journal of the American College of Cardiology, is the first randomized clinical trial using ivabradine to treat POTS.
A total of 26 patients with POTS were started on ivabradine 5 mg or placebo twice daily for 1 month, then were crossed over to the other treatment for 1 month after a 1-week washout period. Six patients were started on a 2.5-mg twice-daily dose. Doses were adjusted during the study based on the patient’s heart rate response and tolerance. Patients had seven clinic visits in which norepinephrine (NE) levels were measured and head-up tilt testing conducted.
Four patients in the ivabradine arm withdrew because of adverse effects, and one withdrew during crossover.
Among the 22 patients who completed the study, exploratory analyses showed a strong trend for greater reduction in plasma NE upon standing with ivabradine (P = .056). The effect was also more profound in patients with very high baseline standing NE levels (at least 1,000 pg/mL) than in those with lower NE levels (600 to 1,000 pg/mL).
“It makes sense because that means their sympathetic nervous system is more overactive; they have a higher heart rate,” Dr. Taub said. “So it’s a potential clinical tool that people can use in their practice to determine, ‘okay, is this a patient I should be considering ivabradine on?’ ”
Although the present study had only 22 patients, “it should definitely be looked at as a step forward, both in terms of ivabradine specifically and in terms of setting the standard for the types of studies we want to see in our patients,” Satish R. Raj, MD, MSCI, University of Calgary (Alta.), said in an interview.
In a related editorial, however, Dr. Raj and coauthor Robert S. Sheldon, MD, PhD, also from the University of Calgary, point out that the standing heart rate in the placebo phase was only 94 beats/min, “suggesting that these patients may be affected only mildly by their POTS.”
Asked about the point, Dr. Taub said: “I don’t know if I agree with that.” She noted that the diagnosis of POTS was confirmed by tilt-table testing and NE levels and that patients’ symptoms vary from day to day. “The standard deviation was plus or minus 16.8, so there’s variability.”
Both Dr. Raj and Dr. Taub said they expect the results will be included in the next scientific statement for POTS, but in the meantime, it may be a struggle to get the drug covered by insurance.
“The challenge is that this is a very off-label use for this medication, and the medication’s not cheap,” Dr. Raj observed. The price for 60 tablets, which is about a 1-month supply, is $485 on GoodRx.
Another question going forward, he said, is whether ivabradine is superior to beta-blockers, which will be studied in a 20-patient crossover trial sponsored by the University of Calgary that is about to launch. The primary completion date is set for 2024.
The study was supported by a grant from Amgen. Dr. Taub has served as a consultant for Amgen, Bayer, Esperion, Boehringer Ingelheim, Novo Nordisk, and Sanofi; is a shareholder in Epirium Bio; and has received research grants from the National Institutes of Health, the American Heart Association, and the Department of Homeland Security/FEMA. Dr. Raj has received a research grant from the Canadian Institutes of Health Research and research grants from Dysautonomia International to address the pathophysiology of POTS. Dr. Sheldon has received a research grant from Dysautonomia International for a clinical trial assessing ivabradine and propranolol for the treatment of POTS.
A version of this article first appeared on Medscape.com.
The heart failure drug ivabradine (Corlanor) can provide relief from the elevated heart rate and often debilitating symptoms associated with postural orthostatic tachycardia syndrome (POTS), a new study suggests.
Ivabradine significantly lowered standing heart rate, compared with placebo (77.9 vs. 94.2 beats/min; P < .001). The typical surge in heart rate that occurs upon standing in these patients was also blunted, compared with baseline (13.0 vs. 21.4 beats/min; P = .001).
“There are really not a lot of great options for patients with POTS and, mechanistically, ivabradine just make sense because it’s a drug that lowers heart rate very selectively and doesn’t lower blood pressure,” lead study author Pam R. Taub, MD, told this news organization.
Surprisingly, the reduction in heart rate translated into improved physical (P = .008) and social (P = .021) functioning after just 1 month of ivabradine, without any other background POTS medications or a change in nonpharmacologic therapies, she said. “What’s really nice to see is when you tackle a really significant part of the disease, which is the elevated heart rate, just how much better they feel.”
POTS patients are mostly healthy, active young women, who after some inciting event – such as viral infection, trauma, or surgery – experience an increase in heart rate of at least 30 beats/min upon standing accompanied by a range of symptoms, including dizziness, palpitations, brain fog, and fatigue.
A COVID connection?
The study enrolled patients with hyperadrenergic POTS as the predominant subtype, but another group to keep in mind that might benefit is the post-COVID POTS patient, said Dr. Taub, from the University of California, San Diego.
“We’re seeing an incredible number of patients post COVID that meet the criteria for POTS, and a lot of these patients also have COVID fatigue,” she said. “So clinically, myself and many other cardiologists who understand ivabradine have been using it off-label for the COVID patients, as long as they meet the criteria. You don’t want to use it in every COVID patient, but if someone’s predominant complaint is that their heart rate is going up when they’re standing and they’re debilitated by it, this is a drug to consider.”
Anecdotal findings in patients with long-hauler COVID need to be translated into rigorous research protocols, but mechanistically, whether it’s POTS from COVID or from another type of infection – like Lyme disease or some other viral syndrome – it should work the same, Dr. Taub said. “POTS is POTS.”
There are no first-line drugs for POTS, and current class IIb recommendations include midodrine, which increases blood pressure and can make people feel awful, and fludrocortisone, which can cause a lot of weight gain and fluid retention, she observed. Other agents that lower heart rate, like beta-blockers, also lower blood pressure and can aggravate depression and fatigue.
Ivabradine regulates heart rate by specifically blocking the Ifunny channel of the sinoatrial node. It was approved in 2015 in the United States to reduce hospitalizations in patients with systolic heart failure, and it also has a second class IIb recommendation for inappropriate sinus tachycardia.
The present study, reported in the Feb. 23 issue of the Journal of the American College of Cardiology, is the first randomized clinical trial using ivabradine to treat POTS.
A total of 26 patients with POTS were started on ivabradine 5 mg or placebo twice daily for 1 month, then were crossed over to the other treatment for 1 month after a 1-week washout period. Six patients were started on a 2.5-mg twice-daily dose. Doses were adjusted during the study based on the patient’s heart rate response and tolerance. Patients had seven clinic visits in which norepinephrine (NE) levels were measured and head-up tilt testing conducted.
Four patients in the ivabradine arm withdrew because of adverse effects, and one withdrew during crossover.
Among the 22 patients who completed the study, exploratory analyses showed a strong trend for greater reduction in plasma NE upon standing with ivabradine (P = .056). The effect was also more profound in patients with very high baseline standing NE levels (at least 1,000 pg/mL) than in those with lower NE levels (600 to 1,000 pg/mL).
“It makes sense because that means their sympathetic nervous system is more overactive; they have a higher heart rate,” Dr. Taub said. “So it’s a potential clinical tool that people can use in their practice to determine, ‘okay, is this a patient I should be considering ivabradine on?’ ”
Although the present study had only 22 patients, “it should definitely be looked at as a step forward, both in terms of ivabradine specifically and in terms of setting the standard for the types of studies we want to see in our patients,” Satish R. Raj, MD, MSCI, University of Calgary (Alta.), said in an interview.
In a related editorial, however, Dr. Raj and coauthor Robert S. Sheldon, MD, PhD, also from the University of Calgary, point out that the standing heart rate in the placebo phase was only 94 beats/min, “suggesting that these patients may be affected only mildly by their POTS.”
Asked about the point, Dr. Taub said: “I don’t know if I agree with that.” She noted that the diagnosis of POTS was confirmed by tilt-table testing and NE levels and that patients’ symptoms vary from day to day. “The standard deviation was plus or minus 16.8, so there’s variability.”
Both Dr. Raj and Dr. Taub said they expect the results will be included in the next scientific statement for POTS, but in the meantime, it may be a struggle to get the drug covered by insurance.
“The challenge is that this is a very off-label use for this medication, and the medication’s not cheap,” Dr. Raj observed. The price for 60 tablets, which is about a 1-month supply, is $485 on GoodRx.
Another question going forward, he said, is whether ivabradine is superior to beta-blockers, which will be studied in a 20-patient crossover trial sponsored by the University of Calgary that is about to launch. The primary completion date is set for 2024.
The study was supported by a grant from Amgen. Dr. Taub has served as a consultant for Amgen, Bayer, Esperion, Boehringer Ingelheim, Novo Nordisk, and Sanofi; is a shareholder in Epirium Bio; and has received research grants from the National Institutes of Health, the American Heart Association, and the Department of Homeland Security/FEMA. Dr. Raj has received a research grant from the Canadian Institutes of Health Research and research grants from Dysautonomia International to address the pathophysiology of POTS. Dr. Sheldon has received a research grant from Dysautonomia International for a clinical trial assessing ivabradine and propranolol for the treatment of POTS.
A version of this article first appeared on Medscape.com.
The heart failure drug ivabradine (Corlanor) can provide relief from the elevated heart rate and often debilitating symptoms associated with postural orthostatic tachycardia syndrome (POTS), a new study suggests.
Ivabradine significantly lowered standing heart rate, compared with placebo (77.9 vs. 94.2 beats/min; P < .001). The typical surge in heart rate that occurs upon standing in these patients was also blunted, compared with baseline (13.0 vs. 21.4 beats/min; P = .001).
“There are really not a lot of great options for patients with POTS and, mechanistically, ivabradine just make sense because it’s a drug that lowers heart rate very selectively and doesn’t lower blood pressure,” lead study author Pam R. Taub, MD, told this news organization.
Surprisingly, the reduction in heart rate translated into improved physical (P = .008) and social (P = .021) functioning after just 1 month of ivabradine, without any other background POTS medications or a change in nonpharmacologic therapies, she said. “What’s really nice to see is when you tackle a really significant part of the disease, which is the elevated heart rate, just how much better they feel.”
POTS patients are mostly healthy, active young women, who after some inciting event – such as viral infection, trauma, or surgery – experience an increase in heart rate of at least 30 beats/min upon standing accompanied by a range of symptoms, including dizziness, palpitations, brain fog, and fatigue.
A COVID connection?
The study enrolled patients with hyperadrenergic POTS as the predominant subtype, but another group to keep in mind that might benefit is the post-COVID POTS patient, said Dr. Taub, from the University of California, San Diego.
“We’re seeing an incredible number of patients post COVID that meet the criteria for POTS, and a lot of these patients also have COVID fatigue,” she said. “So clinically, myself and many other cardiologists who understand ivabradine have been using it off-label for the COVID patients, as long as they meet the criteria. You don’t want to use it in every COVID patient, but if someone’s predominant complaint is that their heart rate is going up when they’re standing and they’re debilitated by it, this is a drug to consider.”
Anecdotal findings in patients with long-hauler COVID need to be translated into rigorous research protocols, but mechanistically, whether it’s POTS from COVID or from another type of infection – like Lyme disease or some other viral syndrome – it should work the same, Dr. Taub said. “POTS is POTS.”
There are no first-line drugs for POTS, and current class IIb recommendations include midodrine, which increases blood pressure and can make people feel awful, and fludrocortisone, which can cause a lot of weight gain and fluid retention, she observed. Other agents that lower heart rate, like beta-blockers, also lower blood pressure and can aggravate depression and fatigue.
Ivabradine regulates heart rate by specifically blocking the Ifunny channel of the sinoatrial node. It was approved in 2015 in the United States to reduce hospitalizations in patients with systolic heart failure, and it also has a second class IIb recommendation for inappropriate sinus tachycardia.
The present study, reported in the Feb. 23 issue of the Journal of the American College of Cardiology, is the first randomized clinical trial using ivabradine to treat POTS.
A total of 26 patients with POTS were started on ivabradine 5 mg or placebo twice daily for 1 month, then were crossed over to the other treatment for 1 month after a 1-week washout period. Six patients were started on a 2.5-mg twice-daily dose. Doses were adjusted during the study based on the patient’s heart rate response and tolerance. Patients had seven clinic visits in which norepinephrine (NE) levels were measured and head-up tilt testing conducted.
Four patients in the ivabradine arm withdrew because of adverse effects, and one withdrew during crossover.
Among the 22 patients who completed the study, exploratory analyses showed a strong trend for greater reduction in plasma NE upon standing with ivabradine (P = .056). The effect was also more profound in patients with very high baseline standing NE levels (at least 1,000 pg/mL) than in those with lower NE levels (600 to 1,000 pg/mL).
“It makes sense because that means their sympathetic nervous system is more overactive; they have a higher heart rate,” Dr. Taub said. “So it’s a potential clinical tool that people can use in their practice to determine, ‘okay, is this a patient I should be considering ivabradine on?’ ”
Although the present study had only 22 patients, “it should definitely be looked at as a step forward, both in terms of ivabradine specifically and in terms of setting the standard for the types of studies we want to see in our patients,” Satish R. Raj, MD, MSCI, University of Calgary (Alta.), said in an interview.
In a related editorial, however, Dr. Raj and coauthor Robert S. Sheldon, MD, PhD, also from the University of Calgary, point out that the standing heart rate in the placebo phase was only 94 beats/min, “suggesting that these patients may be affected only mildly by their POTS.”
Asked about the point, Dr. Taub said: “I don’t know if I agree with that.” She noted that the diagnosis of POTS was confirmed by tilt-table testing and NE levels and that patients’ symptoms vary from day to day. “The standard deviation was plus or minus 16.8, so there’s variability.”
Both Dr. Raj and Dr. Taub said they expect the results will be included in the next scientific statement for POTS, but in the meantime, it may be a struggle to get the drug covered by insurance.
“The challenge is that this is a very off-label use for this medication, and the medication’s not cheap,” Dr. Raj observed. The price for 60 tablets, which is about a 1-month supply, is $485 on GoodRx.
Another question going forward, he said, is whether ivabradine is superior to beta-blockers, which will be studied in a 20-patient crossover trial sponsored by the University of Calgary that is about to launch. The primary completion date is set for 2024.
The study was supported by a grant from Amgen. Dr. Taub has served as a consultant for Amgen, Bayer, Esperion, Boehringer Ingelheim, Novo Nordisk, and Sanofi; is a shareholder in Epirium Bio; and has received research grants from the National Institutes of Health, the American Heart Association, and the Department of Homeland Security/FEMA. Dr. Raj has received a research grant from the Canadian Institutes of Health Research and research grants from Dysautonomia International to address the pathophysiology of POTS. Dr. Sheldon has received a research grant from Dysautonomia International for a clinical trial assessing ivabradine and propranolol for the treatment of POTS.
A version of this article first appeared on Medscape.com.
More Americans hospitalized, readmitted for heart failure
Overall primary HF hospitalization rates per 1,000 adults declined from 4.4 in 2010 to 4.1 in 2013, and then increased from 4.2 in 2014 to 4.9 in 2017.
Rates of unique patient visits for HF were also on the way down – falling from 3.4 in 2010 to 3.2 in 2013 and 2014 – before climbing to 3.8 in 2017.
Similar trends were observed for rates of postdischarge HF readmissions (from 1.0 in 2010 to 0.9 in 2014 to 1.1 in 2017) and all-cause 30-day readmissions (from 0.8 in 2010 to 0.7 in 2014 to 0.9 in 2017).
“We should be emphasizing the things we know work to reduce heart failure hospitalization, which is, No. 1, prevention,” senior author Boback Ziaeian, MD, PhD, said in an interview.
Comorbidities that can lead to heart failure crept up over the study period, such that by 2017, hypertension was present in 91.4% of patients, diabetes in 48.9%, and lipid disorders in 53.1%, up from 76.5%, 44.9%, and 40.4%, respectively, in 2010. Half of all patients had coronary artery disease at both time points. Renal disease shot up from 45.9% to 60.6% by 2017.
“If we did a better job of controlling our known risk factors, we would really cut down on the incidence of heart failure being developed and then, among those estimated 6.6 million heart failure patients, we need to get them on our cornerstone therapies,” said Dr. Ziaeian, of the Veterans Affairts Greater Los Angeles Healthcare System and the University of California, Los Angeles.
Sodium-glucose cotransporter 2 (SGLT2) inhibitors, which have shown clear efficacy and safety in trials like DAPA-HF and EMPEROR-Reduced, provide a “huge opportunity” to add on to standard therapies, he noted. Competition for VA contracts has brought the price down to about $50 a month for veterans, compared with a cash price of about $500-$600 a month.
Yet in routine practice, only 8% of veterans with HF at his center are on an SGLT2 inhibitor, compared with 80% on ACE inhibitors or beta blockers, observed Dr. Ziaeian. “This medication has been indicated for the last year and a half and we’re only at 8% in a system where we have pretty easy access to medications.”
As reported online Feb. 10 in JAMA Cardiology, notable sex differences were found in hospitalization, with higher rates per 1,000 persons among men.
In contrast, a 2020 report on HF trends in the VA system showed a 2% decrease in unadjusted 30-day readmissions from 2007 to 2017 and a decline in the adjusted 30-day readmission risk.
The present study did not risk-adjust readmission risk and included a population that was 51% male, compared with about 98% male in the VA, the investigators noted.
“The increasing hospitalization rate in our study may represent an actual increase in HF hospitalizations or shifts in administrative coding practices, increased use of HF biomarkers, or lower thresholds for diagnosis of HF with preserved ejection fraction,” they wrote.
The analysis was based on data from the Nationwide Readmission Database, which included 35,197,725 hospitalizations with a primary or secondary diagnosis of HF and 8,273,270 primary HF hospitalizations from January 2010 to December 2017.
A single primary HF admission occurred in 5,092,626 unique patients and 1,269,109 had two or more HF hospitalizations. The mean age was 72.1 years.
The administrative database did not include clinical data, so it wasn’t possible to differentiate between HF with preserved or reduced ejection fraction, the authors noted. Patient race and ethnicity data also were not available.
“Future studies are needed to verify our findings to better develop and improve individualized strategies for HF prevention, management, and surveillance for men and women,” the investigators concluded.
One coauthor reporting receiving personal fees from Abbott, Amgen, AstraZeneca, Bayer, CHF Solutions, Edwards Lifesciences, Janssen Pharmaceuticals, Medtronic, Merck, and Novartis. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
Overall primary HF hospitalization rates per 1,000 adults declined from 4.4 in 2010 to 4.1 in 2013, and then increased from 4.2 in 2014 to 4.9 in 2017.
Rates of unique patient visits for HF were also on the way down – falling from 3.4 in 2010 to 3.2 in 2013 and 2014 – before climbing to 3.8 in 2017.
Similar trends were observed for rates of postdischarge HF readmissions (from 1.0 in 2010 to 0.9 in 2014 to 1.1 in 2017) and all-cause 30-day readmissions (from 0.8 in 2010 to 0.7 in 2014 to 0.9 in 2017).
“We should be emphasizing the things we know work to reduce heart failure hospitalization, which is, No. 1, prevention,” senior author Boback Ziaeian, MD, PhD, said in an interview.
Comorbidities that can lead to heart failure crept up over the study period, such that by 2017, hypertension was present in 91.4% of patients, diabetes in 48.9%, and lipid disorders in 53.1%, up from 76.5%, 44.9%, and 40.4%, respectively, in 2010. Half of all patients had coronary artery disease at both time points. Renal disease shot up from 45.9% to 60.6% by 2017.
“If we did a better job of controlling our known risk factors, we would really cut down on the incidence of heart failure being developed and then, among those estimated 6.6 million heart failure patients, we need to get them on our cornerstone therapies,” said Dr. Ziaeian, of the Veterans Affairts Greater Los Angeles Healthcare System and the University of California, Los Angeles.
Sodium-glucose cotransporter 2 (SGLT2) inhibitors, which have shown clear efficacy and safety in trials like DAPA-HF and EMPEROR-Reduced, provide a “huge opportunity” to add on to standard therapies, he noted. Competition for VA contracts has brought the price down to about $50 a month for veterans, compared with a cash price of about $500-$600 a month.
Yet in routine practice, only 8% of veterans with HF at his center are on an SGLT2 inhibitor, compared with 80% on ACE inhibitors or beta blockers, observed Dr. Ziaeian. “This medication has been indicated for the last year and a half and we’re only at 8% in a system where we have pretty easy access to medications.”
As reported online Feb. 10 in JAMA Cardiology, notable sex differences were found in hospitalization, with higher rates per 1,000 persons among men.
In contrast, a 2020 report on HF trends in the VA system showed a 2% decrease in unadjusted 30-day readmissions from 2007 to 2017 and a decline in the adjusted 30-day readmission risk.
The present study did not risk-adjust readmission risk and included a population that was 51% male, compared with about 98% male in the VA, the investigators noted.
“The increasing hospitalization rate in our study may represent an actual increase in HF hospitalizations or shifts in administrative coding practices, increased use of HF biomarkers, or lower thresholds for diagnosis of HF with preserved ejection fraction,” they wrote.
The analysis was based on data from the Nationwide Readmission Database, which included 35,197,725 hospitalizations with a primary or secondary diagnosis of HF and 8,273,270 primary HF hospitalizations from January 2010 to December 2017.
A single primary HF admission occurred in 5,092,626 unique patients and 1,269,109 had two or more HF hospitalizations. The mean age was 72.1 years.
The administrative database did not include clinical data, so it wasn’t possible to differentiate between HF with preserved or reduced ejection fraction, the authors noted. Patient race and ethnicity data also were not available.
“Future studies are needed to verify our findings to better develop and improve individualized strategies for HF prevention, management, and surveillance for men and women,” the investigators concluded.
One coauthor reporting receiving personal fees from Abbott, Amgen, AstraZeneca, Bayer, CHF Solutions, Edwards Lifesciences, Janssen Pharmaceuticals, Medtronic, Merck, and Novartis. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
Overall primary HF hospitalization rates per 1,000 adults declined from 4.4 in 2010 to 4.1 in 2013, and then increased from 4.2 in 2014 to 4.9 in 2017.
Rates of unique patient visits for HF were also on the way down – falling from 3.4 in 2010 to 3.2 in 2013 and 2014 – before climbing to 3.8 in 2017.
Similar trends were observed for rates of postdischarge HF readmissions (from 1.0 in 2010 to 0.9 in 2014 to 1.1 in 2017) and all-cause 30-day readmissions (from 0.8 in 2010 to 0.7 in 2014 to 0.9 in 2017).
“We should be emphasizing the things we know work to reduce heart failure hospitalization, which is, No. 1, prevention,” senior author Boback Ziaeian, MD, PhD, said in an interview.
Comorbidities that can lead to heart failure crept up over the study period, such that by 2017, hypertension was present in 91.4% of patients, diabetes in 48.9%, and lipid disorders in 53.1%, up from 76.5%, 44.9%, and 40.4%, respectively, in 2010. Half of all patients had coronary artery disease at both time points. Renal disease shot up from 45.9% to 60.6% by 2017.
“If we did a better job of controlling our known risk factors, we would really cut down on the incidence of heart failure being developed and then, among those estimated 6.6 million heart failure patients, we need to get them on our cornerstone therapies,” said Dr. Ziaeian, of the Veterans Affairts Greater Los Angeles Healthcare System and the University of California, Los Angeles.
Sodium-glucose cotransporter 2 (SGLT2) inhibitors, which have shown clear efficacy and safety in trials like DAPA-HF and EMPEROR-Reduced, provide a “huge opportunity” to add on to standard therapies, he noted. Competition for VA contracts has brought the price down to about $50 a month for veterans, compared with a cash price of about $500-$600 a month.
Yet in routine practice, only 8% of veterans with HF at his center are on an SGLT2 inhibitor, compared with 80% on ACE inhibitors or beta blockers, observed Dr. Ziaeian. “This medication has been indicated for the last year and a half and we’re only at 8% in a system where we have pretty easy access to medications.”
As reported online Feb. 10 in JAMA Cardiology, notable sex differences were found in hospitalization, with higher rates per 1,000 persons among men.
In contrast, a 2020 report on HF trends in the VA system showed a 2% decrease in unadjusted 30-day readmissions from 2007 to 2017 and a decline in the adjusted 30-day readmission risk.
The present study did not risk-adjust readmission risk and included a population that was 51% male, compared with about 98% male in the VA, the investigators noted.
“The increasing hospitalization rate in our study may represent an actual increase in HF hospitalizations or shifts in administrative coding practices, increased use of HF biomarkers, or lower thresholds for diagnosis of HF with preserved ejection fraction,” they wrote.
The analysis was based on data from the Nationwide Readmission Database, which included 35,197,725 hospitalizations with a primary or secondary diagnosis of HF and 8,273,270 primary HF hospitalizations from January 2010 to December 2017.
A single primary HF admission occurred in 5,092,626 unique patients and 1,269,109 had two or more HF hospitalizations. The mean age was 72.1 years.
The administrative database did not include clinical data, so it wasn’t possible to differentiate between HF with preserved or reduced ejection fraction, the authors noted. Patient race and ethnicity data also were not available.
“Future studies are needed to verify our findings to better develop and improve individualized strategies for HF prevention, management, and surveillance for men and women,” the investigators concluded.
One coauthor reporting receiving personal fees from Abbott, Amgen, AstraZeneca, Bayer, CHF Solutions, Edwards Lifesciences, Janssen Pharmaceuticals, Medtronic, Merck, and Novartis. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
ColCORONA: More questions than answers for colchicine in COVID-19
Science by press release and preprint has cooled clinician enthusiasm for the use of colchicine in nonhospitalized patients with COVID-19, despite a pressing need for early treatments.
As previously reported by this news organization, a Jan. 22 press release announced that the massive ColCORONA study missed its primary endpoint of hospitalization or death among 4,488 newly diagnosed patients at increased risk for hospitalization.
But it also touted that use of the anti-inflammatory drug significantly reduced the primary endpoint in 4,159 of those patients with polymerase chain reaction–confirmed COVID and led to reductions of 25%, 50%, and 44%, respectively, for hospitalizations, ventilations, and death.
Lead investigator Jean-Claude Tardif, MD, director of the Montreal Heart Institute Research Centre, deemed the findings a “medical breakthrough.”
When the preprint released a few days later, however, newly revealed confidence intervals showed colchicine did not meaningfully reduce the need for mechanical ventilation (odds ratio, 0.50; 95% confidence interval, 0.23-1.07) or death alone (OR, 0.56; 95% CI, 0.19-1.66).
Further, the significant benefit on the primary outcome came at the cost of a fivefold increase in pulmonary embolism (11 vs. 2; P = .01), which was not mentioned in the press release.
“Whether this represents a real phenomenon or simply the play of chance is not known,” Dr. Tardif and colleagues noted later in the preprint.
“I read the preprint on colchicine and I have so many questions,” Aaron E. Glatt, MD, spokesperson for the Infectious Diseases Society of America and chief of infectious diseases, Mount Sinai South Nassau, Hewlett, N.Y., said in an interview. “I’ve been burned too many times with COVID and prefer to see better data.
“People sometimes say if you wait for perfect data, people are going to die,” he said. “Yeah, but we have no idea if people are going to die from getting this drug more than not getting it. That’s what concerns me. How many pulmonary emboli are going to be fatal versus the slight benefit that the study showed?”
The pushback to the non–peer-reviewed data on social media and via emails was so strong that Dr. Tardif posted a nearly 2,000-word letter responding to the many questions at play.
Chief among them was why the trial, originally planned for 6,000 patients, was stopped early by the investigators without consultation with the data safety monitoring board (DSMB).
The explanation in the letter that logistical issues like running the study call center, budget constraints, and a perceived need to quickly communicate the results left some calling foul that the study wasn’t allowed to finish and come to a more definitive conclusion.
“I can be a little bit sympathetic to their cause but at the same time the DSMB should have said no,” said David Boulware, MD, MPH, who led a recent hydroxychloroquine trial in COVID-19. “The problem is we’re sort of left in limbo, where some people kind of believe it and some say it’s not really a thing. So it’s not really moving the needle, as far as guidelines go.”
Indeed, a Twitter poll by cardiologist James Januzzi Jr., MD, captured the uncertainty, with 28% of respondents saying the trial was “neutral,” 58% saying “maybe but meh,” and 14% saying “colchicine for all.”
Another poll cheekily asked whether ColCORONA was the Gamestop/Reddit equivalent of COVID.
“The press release really didn’t help things because it very much oversold the effect. That, I think, poisoned the well,” said Dr. Boulware, professor of medicine in infectious diseases at the University of Minnesota, Minneapolis.
“The question I’m left with is not whether colchicine works, but who does it work in,” he said. “That’s really the fundamental question because it does seem that there are probably high-risk groups in their trial and others where they benefit, whereas other groups don’t benefit. In the subgroup analysis, there was absolutely no beneficial effect in women.”
According to the authors, the number needed to treat to prevent one death or hospitalization was 71 overall, but 29 for patients with diabetes, 31 for those aged 70 years and older, 53 for patients with respiratory disease, and 25 for those with coronary disease or heart failure.
Men are at higher risk overall for poor outcomes. But “the authors didn’t present a multivariable analysis, so it is unclear if another factor, such as a differential prevalence of smoking or cardiovascular risk factors, contributed to the differential benefit,” Rachel Bender Ignacio, MD, MPH, infectious disease specialist, University of Washington, Seattle, said in an interview.
Importantly, in this pragmatic study, duration and severity of symptoms were not reported, observed Dr. Bender Ignacio, who is also a STOP-COVID-2 investigator. “We don’t yet have data as to whether colchicine shortens duration or severity of symptoms or prevents long COVID, so we need more data on that.”
The overall risk for serious adverse events was lower in the colchicine group, but the difference in pulmonary embolism (PE) was striking, she said. This could be caused by a real biologic effect, or it’s possible that persons with shortness of breath and hypoxia, without evident viral pneumonia on chest x-ray after a positive COVID-19 test, were more likely to receive a CT-PE study.
The press release also failed to include information, later noted in the preprint, that the MHI has submitted two patents related to colchicine: “Methods of treating a coronavirus infection using colchicine” and “Early administration of low-dose colchicine after myocardial infarction.”
Reached for clarification, MHI communications adviser Camille Turbide said in an interview that the first patent “simply refers to the novel concept of preventing complications of COVID-19, such as admission to the hospital, with colchicine as tested in the ColCORONA study.”
The second patent, she said, refers to the “novel concept that administering colchicine early after a major adverse cardiovascular event is better than waiting several days,” as supported by the COLCOT study, which Dr. Tardif also led.
The patents are being reviewed by authorities and “Dr. Tardif has waived his rights in these patents and does not stand to benefit financially at all if colchicine becomes used as a treatment for COVID-19,” Ms. Turbide said.
Dr. Tardif did not respond to interview requests for this story. Dr. Glatt said conflicts of interest must be assessed and are “something that is of great concern in any scientific study.”
Cardiologist Steve Nissen, MD, of the Cleveland Clinic said in an interview that, “despite the negative results, the study does suggest that colchicine might have a benefit and should be studied in future trials. These findings are not sufficient evidence to suggest use of the drug in patients infected with COVID-19.”
He noted that adverse effects like diarrhea were expected but that the excess PE was unexpected and needs greater clarification.
“Stopping the trial for administrative reasons is puzzling and undermined the ability of the trial to give a reliable answer,” Dr. Nissen said. “This is a reasonable pilot study that should be viewed as hypothesis generating but inconclusive.”
Several sources said a new trial is unlikely, particularly given the cost and 28 trials already evaluating colchicine. Among these are RECOVERY and COLCOVID, testing whether colchicine can reduce the duration of hospitalization or death in hospitalized patients with COVID-19.
Because there are so many trials ongoing right now, including for antivirals and other immunomodulators, it’s important that, if colchicine comes to routine clinical use, it provides access to treatment for those not able or willing to access clinical trials, rather than impeding clinical trial enrollment, Dr. Bender Ignacio suggested.
“We have already learned the lesson in the pandemic that early adoption of potentially promising therapies can negatively impact our ability to study and develop other promising treatments,” she said.
The trial was coordinated by the Montreal Heart Institute and funded by the government of Quebec; the National Heart, Lung, and Blood Institute of the National Institutes of Health; Montreal philanthropist Sophie Desmarais, and the COVID-19 Therapeutics Accelerator launched by the Bill & Melinda Gates Foundation, Wellcome, and Mastercard. CGI, Dacima, and Pharmascience of Montreal were also collaborators. Dr. Glatt reported no conflicts of interest. Dr. Boulware reported receiving $18 in food and beverages from Gilead Sciences in 2018.
A version of this article first appeared on Medscape.com.
Science by press release and preprint has cooled clinician enthusiasm for the use of colchicine in nonhospitalized patients with COVID-19, despite a pressing need for early treatments.
As previously reported by this news organization, a Jan. 22 press release announced that the massive ColCORONA study missed its primary endpoint of hospitalization or death among 4,488 newly diagnosed patients at increased risk for hospitalization.
But it also touted that use of the anti-inflammatory drug significantly reduced the primary endpoint in 4,159 of those patients with polymerase chain reaction–confirmed COVID and led to reductions of 25%, 50%, and 44%, respectively, for hospitalizations, ventilations, and death.
Lead investigator Jean-Claude Tardif, MD, director of the Montreal Heart Institute Research Centre, deemed the findings a “medical breakthrough.”
When the preprint released a few days later, however, newly revealed confidence intervals showed colchicine did not meaningfully reduce the need for mechanical ventilation (odds ratio, 0.50; 95% confidence interval, 0.23-1.07) or death alone (OR, 0.56; 95% CI, 0.19-1.66).
Further, the significant benefit on the primary outcome came at the cost of a fivefold increase in pulmonary embolism (11 vs. 2; P = .01), which was not mentioned in the press release.
“Whether this represents a real phenomenon or simply the play of chance is not known,” Dr. Tardif and colleagues noted later in the preprint.
“I read the preprint on colchicine and I have so many questions,” Aaron E. Glatt, MD, spokesperson for the Infectious Diseases Society of America and chief of infectious diseases, Mount Sinai South Nassau, Hewlett, N.Y., said in an interview. “I’ve been burned too many times with COVID and prefer to see better data.
“People sometimes say if you wait for perfect data, people are going to die,” he said. “Yeah, but we have no idea if people are going to die from getting this drug more than not getting it. That’s what concerns me. How many pulmonary emboli are going to be fatal versus the slight benefit that the study showed?”
The pushback to the non–peer-reviewed data on social media and via emails was so strong that Dr. Tardif posted a nearly 2,000-word letter responding to the many questions at play.
Chief among them was why the trial, originally planned for 6,000 patients, was stopped early by the investigators without consultation with the data safety monitoring board (DSMB).
The explanation in the letter that logistical issues like running the study call center, budget constraints, and a perceived need to quickly communicate the results left some calling foul that the study wasn’t allowed to finish and come to a more definitive conclusion.
“I can be a little bit sympathetic to their cause but at the same time the DSMB should have said no,” said David Boulware, MD, MPH, who led a recent hydroxychloroquine trial in COVID-19. “The problem is we’re sort of left in limbo, where some people kind of believe it and some say it’s not really a thing. So it’s not really moving the needle, as far as guidelines go.”
Indeed, a Twitter poll by cardiologist James Januzzi Jr., MD, captured the uncertainty, with 28% of respondents saying the trial was “neutral,” 58% saying “maybe but meh,” and 14% saying “colchicine for all.”
Another poll cheekily asked whether ColCORONA was the Gamestop/Reddit equivalent of COVID.
“The press release really didn’t help things because it very much oversold the effect. That, I think, poisoned the well,” said Dr. Boulware, professor of medicine in infectious diseases at the University of Minnesota, Minneapolis.
“The question I’m left with is not whether colchicine works, but who does it work in,” he said. “That’s really the fundamental question because it does seem that there are probably high-risk groups in their trial and others where they benefit, whereas other groups don’t benefit. In the subgroup analysis, there was absolutely no beneficial effect in women.”
According to the authors, the number needed to treat to prevent one death or hospitalization was 71 overall, but 29 for patients with diabetes, 31 for those aged 70 years and older, 53 for patients with respiratory disease, and 25 for those with coronary disease or heart failure.
Men are at higher risk overall for poor outcomes. But “the authors didn’t present a multivariable analysis, so it is unclear if another factor, such as a differential prevalence of smoking or cardiovascular risk factors, contributed to the differential benefit,” Rachel Bender Ignacio, MD, MPH, infectious disease specialist, University of Washington, Seattle, said in an interview.
Importantly, in this pragmatic study, duration and severity of symptoms were not reported, observed Dr. Bender Ignacio, who is also a STOP-COVID-2 investigator. “We don’t yet have data as to whether colchicine shortens duration or severity of symptoms or prevents long COVID, so we need more data on that.”
The overall risk for serious adverse events was lower in the colchicine group, but the difference in pulmonary embolism (PE) was striking, she said. This could be caused by a real biologic effect, or it’s possible that persons with shortness of breath and hypoxia, without evident viral pneumonia on chest x-ray after a positive COVID-19 test, were more likely to receive a CT-PE study.
The press release also failed to include information, later noted in the preprint, that the MHI has submitted two patents related to colchicine: “Methods of treating a coronavirus infection using colchicine” and “Early administration of low-dose colchicine after myocardial infarction.”
Reached for clarification, MHI communications adviser Camille Turbide said in an interview that the first patent “simply refers to the novel concept of preventing complications of COVID-19, such as admission to the hospital, with colchicine as tested in the ColCORONA study.”
The second patent, she said, refers to the “novel concept that administering colchicine early after a major adverse cardiovascular event is better than waiting several days,” as supported by the COLCOT study, which Dr. Tardif also led.
The patents are being reviewed by authorities and “Dr. Tardif has waived his rights in these patents and does not stand to benefit financially at all if colchicine becomes used as a treatment for COVID-19,” Ms. Turbide said.
Dr. Tardif did not respond to interview requests for this story. Dr. Glatt said conflicts of interest must be assessed and are “something that is of great concern in any scientific study.”
Cardiologist Steve Nissen, MD, of the Cleveland Clinic said in an interview that, “despite the negative results, the study does suggest that colchicine might have a benefit and should be studied in future trials. These findings are not sufficient evidence to suggest use of the drug in patients infected with COVID-19.”
He noted that adverse effects like diarrhea were expected but that the excess PE was unexpected and needs greater clarification.
“Stopping the trial for administrative reasons is puzzling and undermined the ability of the trial to give a reliable answer,” Dr. Nissen said. “This is a reasonable pilot study that should be viewed as hypothesis generating but inconclusive.”
Several sources said a new trial is unlikely, particularly given the cost and 28 trials already evaluating colchicine. Among these are RECOVERY and COLCOVID, testing whether colchicine can reduce the duration of hospitalization or death in hospitalized patients with COVID-19.
Because there are so many trials ongoing right now, including for antivirals and other immunomodulators, it’s important that, if colchicine comes to routine clinical use, it provides access to treatment for those not able or willing to access clinical trials, rather than impeding clinical trial enrollment, Dr. Bender Ignacio suggested.
“We have already learned the lesson in the pandemic that early adoption of potentially promising therapies can negatively impact our ability to study and develop other promising treatments,” she said.
The trial was coordinated by the Montreal Heart Institute and funded by the government of Quebec; the National Heart, Lung, and Blood Institute of the National Institutes of Health; Montreal philanthropist Sophie Desmarais, and the COVID-19 Therapeutics Accelerator launched by the Bill & Melinda Gates Foundation, Wellcome, and Mastercard. CGI, Dacima, and Pharmascience of Montreal were also collaborators. Dr. Glatt reported no conflicts of interest. Dr. Boulware reported receiving $18 in food and beverages from Gilead Sciences in 2018.
A version of this article first appeared on Medscape.com.
Science by press release and preprint has cooled clinician enthusiasm for the use of colchicine in nonhospitalized patients with COVID-19, despite a pressing need for early treatments.
As previously reported by this news organization, a Jan. 22 press release announced that the massive ColCORONA study missed its primary endpoint of hospitalization or death among 4,488 newly diagnosed patients at increased risk for hospitalization.
But it also touted that use of the anti-inflammatory drug significantly reduced the primary endpoint in 4,159 of those patients with polymerase chain reaction–confirmed COVID and led to reductions of 25%, 50%, and 44%, respectively, for hospitalizations, ventilations, and death.
Lead investigator Jean-Claude Tardif, MD, director of the Montreal Heart Institute Research Centre, deemed the findings a “medical breakthrough.”
When the preprint released a few days later, however, newly revealed confidence intervals showed colchicine did not meaningfully reduce the need for mechanical ventilation (odds ratio, 0.50; 95% confidence interval, 0.23-1.07) or death alone (OR, 0.56; 95% CI, 0.19-1.66).
Further, the significant benefit on the primary outcome came at the cost of a fivefold increase in pulmonary embolism (11 vs. 2; P = .01), which was not mentioned in the press release.
“Whether this represents a real phenomenon or simply the play of chance is not known,” Dr. Tardif and colleagues noted later in the preprint.
“I read the preprint on colchicine and I have so many questions,” Aaron E. Glatt, MD, spokesperson for the Infectious Diseases Society of America and chief of infectious diseases, Mount Sinai South Nassau, Hewlett, N.Y., said in an interview. “I’ve been burned too many times with COVID and prefer to see better data.
“People sometimes say if you wait for perfect data, people are going to die,” he said. “Yeah, but we have no idea if people are going to die from getting this drug more than not getting it. That’s what concerns me. How many pulmonary emboli are going to be fatal versus the slight benefit that the study showed?”
The pushback to the non–peer-reviewed data on social media and via emails was so strong that Dr. Tardif posted a nearly 2,000-word letter responding to the many questions at play.
Chief among them was why the trial, originally planned for 6,000 patients, was stopped early by the investigators without consultation with the data safety monitoring board (DSMB).
The explanation in the letter that logistical issues like running the study call center, budget constraints, and a perceived need to quickly communicate the results left some calling foul that the study wasn’t allowed to finish and come to a more definitive conclusion.
“I can be a little bit sympathetic to their cause but at the same time the DSMB should have said no,” said David Boulware, MD, MPH, who led a recent hydroxychloroquine trial in COVID-19. “The problem is we’re sort of left in limbo, where some people kind of believe it and some say it’s not really a thing. So it’s not really moving the needle, as far as guidelines go.”
Indeed, a Twitter poll by cardiologist James Januzzi Jr., MD, captured the uncertainty, with 28% of respondents saying the trial was “neutral,” 58% saying “maybe but meh,” and 14% saying “colchicine for all.”
Another poll cheekily asked whether ColCORONA was the Gamestop/Reddit equivalent of COVID.
“The press release really didn’t help things because it very much oversold the effect. That, I think, poisoned the well,” said Dr. Boulware, professor of medicine in infectious diseases at the University of Minnesota, Minneapolis.
“The question I’m left with is not whether colchicine works, but who does it work in,” he said. “That’s really the fundamental question because it does seem that there are probably high-risk groups in their trial and others where they benefit, whereas other groups don’t benefit. In the subgroup analysis, there was absolutely no beneficial effect in women.”
According to the authors, the number needed to treat to prevent one death or hospitalization was 71 overall, but 29 for patients with diabetes, 31 for those aged 70 years and older, 53 for patients with respiratory disease, and 25 for those with coronary disease or heart failure.
Men are at higher risk overall for poor outcomes. But “the authors didn’t present a multivariable analysis, so it is unclear if another factor, such as a differential prevalence of smoking or cardiovascular risk factors, contributed to the differential benefit,” Rachel Bender Ignacio, MD, MPH, infectious disease specialist, University of Washington, Seattle, said in an interview.
Importantly, in this pragmatic study, duration and severity of symptoms were not reported, observed Dr. Bender Ignacio, who is also a STOP-COVID-2 investigator. “We don’t yet have data as to whether colchicine shortens duration or severity of symptoms or prevents long COVID, so we need more data on that.”
The overall risk for serious adverse events was lower in the colchicine group, but the difference in pulmonary embolism (PE) was striking, she said. This could be caused by a real biologic effect, or it’s possible that persons with shortness of breath and hypoxia, without evident viral pneumonia on chest x-ray after a positive COVID-19 test, were more likely to receive a CT-PE study.
The press release also failed to include information, later noted in the preprint, that the MHI has submitted two patents related to colchicine: “Methods of treating a coronavirus infection using colchicine” and “Early administration of low-dose colchicine after myocardial infarction.”
Reached for clarification, MHI communications adviser Camille Turbide said in an interview that the first patent “simply refers to the novel concept of preventing complications of COVID-19, such as admission to the hospital, with colchicine as tested in the ColCORONA study.”
The second patent, she said, refers to the “novel concept that administering colchicine early after a major adverse cardiovascular event is better than waiting several days,” as supported by the COLCOT study, which Dr. Tardif also led.
The patents are being reviewed by authorities and “Dr. Tardif has waived his rights in these patents and does not stand to benefit financially at all if colchicine becomes used as a treatment for COVID-19,” Ms. Turbide said.
Dr. Tardif did not respond to interview requests for this story. Dr. Glatt said conflicts of interest must be assessed and are “something that is of great concern in any scientific study.”
Cardiologist Steve Nissen, MD, of the Cleveland Clinic said in an interview that, “despite the negative results, the study does suggest that colchicine might have a benefit and should be studied in future trials. These findings are not sufficient evidence to suggest use of the drug in patients infected with COVID-19.”
He noted that adverse effects like diarrhea were expected but that the excess PE was unexpected and needs greater clarification.
“Stopping the trial for administrative reasons is puzzling and undermined the ability of the trial to give a reliable answer,” Dr. Nissen said. “This is a reasonable pilot study that should be viewed as hypothesis generating but inconclusive.”
Several sources said a new trial is unlikely, particularly given the cost and 28 trials already evaluating colchicine. Among these are RECOVERY and COLCOVID, testing whether colchicine can reduce the duration of hospitalization or death in hospitalized patients with COVID-19.
Because there are so many trials ongoing right now, including for antivirals and other immunomodulators, it’s important that, if colchicine comes to routine clinical use, it provides access to treatment for those not able or willing to access clinical trials, rather than impeding clinical trial enrollment, Dr. Bender Ignacio suggested.
“We have already learned the lesson in the pandemic that early adoption of potentially promising therapies can negatively impact our ability to study and develop other promising treatments,” she said.
The trial was coordinated by the Montreal Heart Institute and funded by the government of Quebec; the National Heart, Lung, and Blood Institute of the National Institutes of Health; Montreal philanthropist Sophie Desmarais, and the COVID-19 Therapeutics Accelerator launched by the Bill & Melinda Gates Foundation, Wellcome, and Mastercard. CGI, Dacima, and Pharmascience of Montreal were also collaborators. Dr. Glatt reported no conflicts of interest. Dr. Boulware reported receiving $18 in food and beverages from Gilead Sciences in 2018.
A version of this article first appeared on Medscape.com.
Cardiac activity not uncommon after lifesaving measures stop
Among critically ill patients pulseless after planned withdrawal of life-sustaining therapies, cardiac activity restarted in 14% of cases, research shows.
Reassuringly, most resumption of heart activity happened in the first 1-2 minutes and most lasted 1 or 2 seconds.
“The reason we wanted to look at death determination specifically is we know that the stories persist about people coming back to life following death, and that’s not just in the public, it’s in the medical community as well,” lead author Sonny Dhanani, MD, of Children’s Hospital of Eastern Ontario, Ottawa, said in an interview.
“We thought that if we provided scientific evidence of whether this happened or not, we might dispel some myths and misunderstanding, which would hopefully promote organ donation.”
About 70% of organ donations occur after brain death, but an increasing number follow circulatory determination of death, he noted. Most protocols recommend 5 minutes of apnea and pulselessness by arterial catheter monitor before declaring death. But practices vary from 10 minutes in some European countries to 75 seconds in infant heart donors at one Colorado hospital.
Reports of patients recovering 10 minutes after pulselessness have raised concerns about the Lazarus phenomenon, or autoresuscitation, but are based in patients after cardiopulmonary resuscitation was terminated.
The present study, known as Death Prediction and Physiology after Removal of Therapy (DePParRT), enrolled patients at 20 intensive care sites in Canada, the Czech Republic, and the Netherlands, only if surrogate decision-makers agreed on withdrawal of life-sustaining measures without CPR and imminent death was anticipated.
As reported Jan. 28 in the New England Journal of Medicine, physicians observed resumption of circulation or cardiac activity prospectively in 1% of 631 patients based on bedside ECG, arterial pressure catheter monitors, palpated arterial pulse, breaths, or physical movements.
A retrospective review of data from 480 patients with complete ECG and arterial waveforms and at least 5 minutes of continuous waveform monitoring after pulselessness showed resumption of cardiac activity in 14% of patients.
The longest period of pulselessness before the heart showed signs of activity again was 4 minutes and 20 seconds. “So that was a reassuring number, because that’s within our 5-minute window that we currently use,” Dr. Dhanani said.
Importantly, “nobody woke up, nobody ended up being resuscitated, and all of these individuals died. And I think that’s going to be very helpful in this context,” he added.
In all, there were 77 cessations and resumptions in 67 of the 480 patients. The median duration of resumed cardiac activity was 3.9 seconds but, notably, ranged from 1 second to 13 minutes and 14 seconds.
“Though surprising, I think maybe not unreasonable,” observed Dr. Dhanani. “The heart is a very robust organ, and we maybe should anticipate these things happening, where at the end of life the heart may restart for minutes.”
In this situation, it’s important to wait the 13 minutes for the heart to stop again and then “wait another 5 minutes to make sure it doesn’t restart before determining death,” he said. “I think that’s where this study is going to now inform policy makers and guidelines, especially in the context of donations.”
The findings will be taken as strong support for the 5-minute window, said Robert Truog, MD, director of the Harvard Medical School Center for Bioethics and the Frances Glessner Lee Professor of Medical Ethics, Anaesthesia, and Pediatrics, Boston.
“I think it’s a safe point, I think people will refer to it, and it will be used to support the 5-minute window, and that’s probably reasonable,” he told this news organization. “Certainly, if it’s read in Europe it will cut the time from 10 minutes to 5 minutes, and that’s a good thing because 10 minutes is a very long time to wait.”
He noted that the 5-minute window provides reasonable assurance to the public and, with new technologies, permits most organs to be usable for donation after cardiac death. That said, there’s nothing magical about the number.
“In some ways I see this paper as providing interesting data but not actually providing an answer, because from the patient’s perspective and from the recipient’s perspective, waiting until the heart has made its last squeeze may not be the most relevant ethical question,” Dr. Truog said. “It may be, once we know this patient is not going to have return of cardiorespiratory function, is not going to wake up, that’s the point at which we ought to focus on organ preservation and organ retrieval, and that can be much sooner than 5 minutes.”
Dr. Dhanani and colleagues note that the generalizability of the results might be limited because patients without arterial pressure catheters were excluded, and 24% of enrolled patients could not be included in the retrospective waveform analysis owing to incomplete data.
“Our study definition of cardiac activity used an arbitrary threshold of pulse pressure (less than 5 mm Hg) that does not imply meaningful circulation,” they add. “This conservative consensus definition may have been partially responsible for the ostensibly high incidence (14%) of transient resumptions of cardiac activity identified through waveform adjudication.”
The study was supported by the Canadian Institutes for Health Research as part of the Canadian Donation and Transplantation Research Program, CHEO Research Institute, and Karel Pavlík Foundation. Dr. Dhanani has consulted for Canadian Blood Services. Dr. Truog reports no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Among critically ill patients pulseless after planned withdrawal of life-sustaining therapies, cardiac activity restarted in 14% of cases, research shows.
Reassuringly, most resumption of heart activity happened in the first 1-2 minutes and most lasted 1 or 2 seconds.
“The reason we wanted to look at death determination specifically is we know that the stories persist about people coming back to life following death, and that’s not just in the public, it’s in the medical community as well,” lead author Sonny Dhanani, MD, of Children’s Hospital of Eastern Ontario, Ottawa, said in an interview.
“We thought that if we provided scientific evidence of whether this happened or not, we might dispel some myths and misunderstanding, which would hopefully promote organ donation.”
About 70% of organ donations occur after brain death, but an increasing number follow circulatory determination of death, he noted. Most protocols recommend 5 minutes of apnea and pulselessness by arterial catheter monitor before declaring death. But practices vary from 10 minutes in some European countries to 75 seconds in infant heart donors at one Colorado hospital.
Reports of patients recovering 10 minutes after pulselessness have raised concerns about the Lazarus phenomenon, or autoresuscitation, but are based in patients after cardiopulmonary resuscitation was terminated.
The present study, known as Death Prediction and Physiology after Removal of Therapy (DePParRT), enrolled patients at 20 intensive care sites in Canada, the Czech Republic, and the Netherlands, only if surrogate decision-makers agreed on withdrawal of life-sustaining measures without CPR and imminent death was anticipated.
As reported Jan. 28 in the New England Journal of Medicine, physicians observed resumption of circulation or cardiac activity prospectively in 1% of 631 patients based on bedside ECG, arterial pressure catheter monitors, palpated arterial pulse, breaths, or physical movements.
A retrospective review of data from 480 patients with complete ECG and arterial waveforms and at least 5 minutes of continuous waveform monitoring after pulselessness showed resumption of cardiac activity in 14% of patients.
The longest period of pulselessness before the heart showed signs of activity again was 4 minutes and 20 seconds. “So that was a reassuring number, because that’s within our 5-minute window that we currently use,” Dr. Dhanani said.
Importantly, “nobody woke up, nobody ended up being resuscitated, and all of these individuals died. And I think that’s going to be very helpful in this context,” he added.
In all, there were 77 cessations and resumptions in 67 of the 480 patients. The median duration of resumed cardiac activity was 3.9 seconds but, notably, ranged from 1 second to 13 minutes and 14 seconds.
“Though surprising, I think maybe not unreasonable,” observed Dr. Dhanani. “The heart is a very robust organ, and we maybe should anticipate these things happening, where at the end of life the heart may restart for minutes.”
In this situation, it’s important to wait the 13 minutes for the heart to stop again and then “wait another 5 minutes to make sure it doesn’t restart before determining death,” he said. “I think that’s where this study is going to now inform policy makers and guidelines, especially in the context of donations.”
The findings will be taken as strong support for the 5-minute window, said Robert Truog, MD, director of the Harvard Medical School Center for Bioethics and the Frances Glessner Lee Professor of Medical Ethics, Anaesthesia, and Pediatrics, Boston.
“I think it’s a safe point, I think people will refer to it, and it will be used to support the 5-minute window, and that’s probably reasonable,” he told this news organization. “Certainly, if it’s read in Europe it will cut the time from 10 minutes to 5 minutes, and that’s a good thing because 10 minutes is a very long time to wait.”
He noted that the 5-minute window provides reasonable assurance to the public and, with new technologies, permits most organs to be usable for donation after cardiac death. That said, there’s nothing magical about the number.
“In some ways I see this paper as providing interesting data but not actually providing an answer, because from the patient’s perspective and from the recipient’s perspective, waiting until the heart has made its last squeeze may not be the most relevant ethical question,” Dr. Truog said. “It may be, once we know this patient is not going to have return of cardiorespiratory function, is not going to wake up, that’s the point at which we ought to focus on organ preservation and organ retrieval, and that can be much sooner than 5 minutes.”
Dr. Dhanani and colleagues note that the generalizability of the results might be limited because patients without arterial pressure catheters were excluded, and 24% of enrolled patients could not be included in the retrospective waveform analysis owing to incomplete data.
“Our study definition of cardiac activity used an arbitrary threshold of pulse pressure (less than 5 mm Hg) that does not imply meaningful circulation,” they add. “This conservative consensus definition may have been partially responsible for the ostensibly high incidence (14%) of transient resumptions of cardiac activity identified through waveform adjudication.”
The study was supported by the Canadian Institutes for Health Research as part of the Canadian Donation and Transplantation Research Program, CHEO Research Institute, and Karel Pavlík Foundation. Dr. Dhanani has consulted for Canadian Blood Services. Dr. Truog reports no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Among critically ill patients pulseless after planned withdrawal of life-sustaining therapies, cardiac activity restarted in 14% of cases, research shows.
Reassuringly, most resumption of heart activity happened in the first 1-2 minutes and most lasted 1 or 2 seconds.
“The reason we wanted to look at death determination specifically is we know that the stories persist about people coming back to life following death, and that’s not just in the public, it’s in the medical community as well,” lead author Sonny Dhanani, MD, of Children’s Hospital of Eastern Ontario, Ottawa, said in an interview.
“We thought that if we provided scientific evidence of whether this happened or not, we might dispel some myths and misunderstanding, which would hopefully promote organ donation.”
About 70% of organ donations occur after brain death, but an increasing number follow circulatory determination of death, he noted. Most protocols recommend 5 minutes of apnea and pulselessness by arterial catheter monitor before declaring death. But practices vary from 10 minutes in some European countries to 75 seconds in infant heart donors at one Colorado hospital.
Reports of patients recovering 10 minutes after pulselessness have raised concerns about the Lazarus phenomenon, or autoresuscitation, but are based in patients after cardiopulmonary resuscitation was terminated.
The present study, known as Death Prediction and Physiology after Removal of Therapy (DePParRT), enrolled patients at 20 intensive care sites in Canada, the Czech Republic, and the Netherlands, only if surrogate decision-makers agreed on withdrawal of life-sustaining measures without CPR and imminent death was anticipated.
As reported Jan. 28 in the New England Journal of Medicine, physicians observed resumption of circulation or cardiac activity prospectively in 1% of 631 patients based on bedside ECG, arterial pressure catheter monitors, palpated arterial pulse, breaths, or physical movements.
A retrospective review of data from 480 patients with complete ECG and arterial waveforms and at least 5 minutes of continuous waveform monitoring after pulselessness showed resumption of cardiac activity in 14% of patients.
The longest period of pulselessness before the heart showed signs of activity again was 4 minutes and 20 seconds. “So that was a reassuring number, because that’s within our 5-minute window that we currently use,” Dr. Dhanani said.
Importantly, “nobody woke up, nobody ended up being resuscitated, and all of these individuals died. And I think that’s going to be very helpful in this context,” he added.
In all, there were 77 cessations and resumptions in 67 of the 480 patients. The median duration of resumed cardiac activity was 3.9 seconds but, notably, ranged from 1 second to 13 minutes and 14 seconds.
“Though surprising, I think maybe not unreasonable,” observed Dr. Dhanani. “The heart is a very robust organ, and we maybe should anticipate these things happening, where at the end of life the heart may restart for minutes.”
In this situation, it’s important to wait the 13 minutes for the heart to stop again and then “wait another 5 minutes to make sure it doesn’t restart before determining death,” he said. “I think that’s where this study is going to now inform policy makers and guidelines, especially in the context of donations.”
The findings will be taken as strong support for the 5-minute window, said Robert Truog, MD, director of the Harvard Medical School Center for Bioethics and the Frances Glessner Lee Professor of Medical Ethics, Anaesthesia, and Pediatrics, Boston.
“I think it’s a safe point, I think people will refer to it, and it will be used to support the 5-minute window, and that’s probably reasonable,” he told this news organization. “Certainly, if it’s read in Europe it will cut the time from 10 minutes to 5 minutes, and that’s a good thing because 10 minutes is a very long time to wait.”
He noted that the 5-minute window provides reasonable assurance to the public and, with new technologies, permits most organs to be usable for donation after cardiac death. That said, there’s nothing magical about the number.
“In some ways I see this paper as providing interesting data but not actually providing an answer, because from the patient’s perspective and from the recipient’s perspective, waiting until the heart has made its last squeeze may not be the most relevant ethical question,” Dr. Truog said. “It may be, once we know this patient is not going to have return of cardiorespiratory function, is not going to wake up, that’s the point at which we ought to focus on organ preservation and organ retrieval, and that can be much sooner than 5 minutes.”
Dr. Dhanani and colleagues note that the generalizability of the results might be limited because patients without arterial pressure catheters were excluded, and 24% of enrolled patients could not be included in the retrospective waveform analysis owing to incomplete data.
“Our study definition of cardiac activity used an arbitrary threshold of pulse pressure (less than 5 mm Hg) that does not imply meaningful circulation,” they add. “This conservative consensus definition may have been partially responsible for the ostensibly high incidence (14%) of transient resumptions of cardiac activity identified through waveform adjudication.”
The study was supported by the Canadian Institutes for Health Research as part of the Canadian Donation and Transplantation Research Program, CHEO Research Institute, and Karel Pavlík Foundation. Dr. Dhanani has consulted for Canadian Blood Services. Dr. Truog reports no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Study flags cardiovascular disease in men with breast cancer
.
Among 24 male breast cancer patients evaluated over a decade in the Washington area, 88% were obese or overweight, 58% had hypertension, and 54% had hyperlipidemia.
Tachyarrhythmia existed in 8% of the men before cancer treatment and developed in 13% during treatment.
Two patients had preexisting heart failure, two patients developed the disease after treatment, and another two patients experienced a decline in left ventricular ejection fraction during the course of their cancer treatment.
“Our hope is that treating male breast cancer patients becomes a multidisciplinary approach where oncologists recruit their cardio-oncologist counterparts to mitigate cardiovascular risk factors, so patients live a long and healthy life after cancer treatment,” said Michael Ibrahim, one of the study authors and a 4th-year medical student at Georgetown University in Washington.
The data were presented Jan. 25 as part of the American College of Cardiology’s Advancing the Cardiovascular Care of the Oncology Patient virtual course, which is hosting live sessions Feb. 5-6.
Although the association between cardiovascular disease and breast cancer is well documented in female breast cancer patients, there is little evidence in their male counterparts, especially African Americans, Mr. Ibrahim noted.
To provide some context, Mr. Ibrahim highlighted a 2018 report in nearly 3,500 female breast cancer patients, ages 40-79, in whom 52% were obese/overweight, 35% had hypertension, and 28% had hyperlipidemia.
Diabetes was present in 7.5% of the women, which was roughly equivalent to the 8% found among the men, Mr. Ibrahim said. The men were of similar age (38-79 years), with 42% being African American, 29% White, 4% Hispanic, and 25% another ethnicity.
Importantly, half of the men had a family history of breast cancer, and two were positive for a mutation in the BRCA gene.
A 2017 in-depth review of male breast cancer cites advancing age, hormonal imbalance, radiation exposure, and family history of breast cancer as key risk factors for the development of the disease, but the “most relevant risk factor” is a mutation in the BRCA2 gene.
Male breast cancer accounts for less than 1% of all breast cancers, but the incidence is rising and, in some patient groups, reaching 15% over their lifetimes, the paper notes. Additionally, these patients are at special risk for developing a second cancer.
Remarkably, 25% of men in the D.C. cohort were diagnosed with a second primary malignancy, 13% a third primary cancer, and 4% a fourth primary cancer, Mr. Ibrahim reported. “This goes to show that male breast cancer patients should routinely undergo cancer screening,” he said.
The initial diagnosis was invasive ductal carcinoma in 79% of the men, with the remaining ductal carcinoma in situ. All patients underwent mastectomy, 17% had anthracycline chemotherapy, 8% received HER2-targeted therapy, 16% had radiation, and 71% received hormone therapy.
In terms of cardiovascular management, statins were the most prescribed medication (46%), followed by antiplatelet therapy (42%) and angiotensin-converting enzyme inhibitors/angiotensin-receptor blockers (38%).
An implantable cardioverter defibrillator/pacemaker was the most common intervention (16%), followed by bypass surgery in 8% and coronary angioplasty in 4%.
Mr. Ibrahim noted that the study was limited by the small sample size and that further research is needed to understand the risk of preexisting cardiovascular disease on long-term outcomes as well as the cardiotoxic effects of chemoradiation in male breast cancer patients.
In a statement, Mr. Ibrahim reiterated the need for a multidisciplinary cancer care team to evaluate patients’ cardiovascular risk prior to and through cancer treatment.
“On a more personal level, cancer patients are already surprised by their cancer diagnosis,” he added. “Similar to the pretreatment consultation with radiation oncology, breast surgery, and medical oncology, an upfront cardiovascular risk assessment provides greater comfort and further minimizes psychological surprise with cardiovascular complications going into cancer treatment.”
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
.
Among 24 male breast cancer patients evaluated over a decade in the Washington area, 88% were obese or overweight, 58% had hypertension, and 54% had hyperlipidemia.
Tachyarrhythmia existed in 8% of the men before cancer treatment and developed in 13% during treatment.
Two patients had preexisting heart failure, two patients developed the disease after treatment, and another two patients experienced a decline in left ventricular ejection fraction during the course of their cancer treatment.
“Our hope is that treating male breast cancer patients becomes a multidisciplinary approach where oncologists recruit their cardio-oncologist counterparts to mitigate cardiovascular risk factors, so patients live a long and healthy life after cancer treatment,” said Michael Ibrahim, one of the study authors and a 4th-year medical student at Georgetown University in Washington.
The data were presented Jan. 25 as part of the American College of Cardiology’s Advancing the Cardiovascular Care of the Oncology Patient virtual course, which is hosting live sessions Feb. 5-6.
Although the association between cardiovascular disease and breast cancer is well documented in female breast cancer patients, there is little evidence in their male counterparts, especially African Americans, Mr. Ibrahim noted.
To provide some context, Mr. Ibrahim highlighted a 2018 report in nearly 3,500 female breast cancer patients, ages 40-79, in whom 52% were obese/overweight, 35% had hypertension, and 28% had hyperlipidemia.
Diabetes was present in 7.5% of the women, which was roughly equivalent to the 8% found among the men, Mr. Ibrahim said. The men were of similar age (38-79 years), with 42% being African American, 29% White, 4% Hispanic, and 25% another ethnicity.
Importantly, half of the men had a family history of breast cancer, and two were positive for a mutation in the BRCA gene.
A 2017 in-depth review of male breast cancer cites advancing age, hormonal imbalance, radiation exposure, and family history of breast cancer as key risk factors for the development of the disease, but the “most relevant risk factor” is a mutation in the BRCA2 gene.
Male breast cancer accounts for less than 1% of all breast cancers, but the incidence is rising and, in some patient groups, reaching 15% over their lifetimes, the paper notes. Additionally, these patients are at special risk for developing a second cancer.
Remarkably, 25% of men in the D.C. cohort were diagnosed with a second primary malignancy, 13% a third primary cancer, and 4% a fourth primary cancer, Mr. Ibrahim reported. “This goes to show that male breast cancer patients should routinely undergo cancer screening,” he said.
The initial diagnosis was invasive ductal carcinoma in 79% of the men, with the remaining ductal carcinoma in situ. All patients underwent mastectomy, 17% had anthracycline chemotherapy, 8% received HER2-targeted therapy, 16% had radiation, and 71% received hormone therapy.
In terms of cardiovascular management, statins were the most prescribed medication (46%), followed by antiplatelet therapy (42%) and angiotensin-converting enzyme inhibitors/angiotensin-receptor blockers (38%).
An implantable cardioverter defibrillator/pacemaker was the most common intervention (16%), followed by bypass surgery in 8% and coronary angioplasty in 4%.
Mr. Ibrahim noted that the study was limited by the small sample size and that further research is needed to understand the risk of preexisting cardiovascular disease on long-term outcomes as well as the cardiotoxic effects of chemoradiation in male breast cancer patients.
In a statement, Mr. Ibrahim reiterated the need for a multidisciplinary cancer care team to evaluate patients’ cardiovascular risk prior to and through cancer treatment.
“On a more personal level, cancer patients are already surprised by their cancer diagnosis,” he added. “Similar to the pretreatment consultation with radiation oncology, breast surgery, and medical oncology, an upfront cardiovascular risk assessment provides greater comfort and further minimizes psychological surprise with cardiovascular complications going into cancer treatment.”
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
.
Among 24 male breast cancer patients evaluated over a decade in the Washington area, 88% were obese or overweight, 58% had hypertension, and 54% had hyperlipidemia.
Tachyarrhythmia existed in 8% of the men before cancer treatment and developed in 13% during treatment.
Two patients had preexisting heart failure, two patients developed the disease after treatment, and another two patients experienced a decline in left ventricular ejection fraction during the course of their cancer treatment.
“Our hope is that treating male breast cancer patients becomes a multidisciplinary approach where oncologists recruit their cardio-oncologist counterparts to mitigate cardiovascular risk factors, so patients live a long and healthy life after cancer treatment,” said Michael Ibrahim, one of the study authors and a 4th-year medical student at Georgetown University in Washington.
The data were presented Jan. 25 as part of the American College of Cardiology’s Advancing the Cardiovascular Care of the Oncology Patient virtual course, which is hosting live sessions Feb. 5-6.
Although the association between cardiovascular disease and breast cancer is well documented in female breast cancer patients, there is little evidence in their male counterparts, especially African Americans, Mr. Ibrahim noted.
To provide some context, Mr. Ibrahim highlighted a 2018 report in nearly 3,500 female breast cancer patients, ages 40-79, in whom 52% were obese/overweight, 35% had hypertension, and 28% had hyperlipidemia.
Diabetes was present in 7.5% of the women, which was roughly equivalent to the 8% found among the men, Mr. Ibrahim said. The men were of similar age (38-79 years), with 42% being African American, 29% White, 4% Hispanic, and 25% another ethnicity.
Importantly, half of the men had a family history of breast cancer, and two were positive for a mutation in the BRCA gene.
A 2017 in-depth review of male breast cancer cites advancing age, hormonal imbalance, radiation exposure, and family history of breast cancer as key risk factors for the development of the disease, but the “most relevant risk factor” is a mutation in the BRCA2 gene.
Male breast cancer accounts for less than 1% of all breast cancers, but the incidence is rising and, in some patient groups, reaching 15% over their lifetimes, the paper notes. Additionally, these patients are at special risk for developing a second cancer.
Remarkably, 25% of men in the D.C. cohort were diagnosed with a second primary malignancy, 13% a third primary cancer, and 4% a fourth primary cancer, Mr. Ibrahim reported. “This goes to show that male breast cancer patients should routinely undergo cancer screening,” he said.
The initial diagnosis was invasive ductal carcinoma in 79% of the men, with the remaining ductal carcinoma in situ. All patients underwent mastectomy, 17% had anthracycline chemotherapy, 8% received HER2-targeted therapy, 16% had radiation, and 71% received hormone therapy.
In terms of cardiovascular management, statins were the most prescribed medication (46%), followed by antiplatelet therapy (42%) and angiotensin-converting enzyme inhibitors/angiotensin-receptor blockers (38%).
An implantable cardioverter defibrillator/pacemaker was the most common intervention (16%), followed by bypass surgery in 8% and coronary angioplasty in 4%.
Mr. Ibrahim noted that the study was limited by the small sample size and that further research is needed to understand the risk of preexisting cardiovascular disease on long-term outcomes as well as the cardiotoxic effects of chemoradiation in male breast cancer patients.
In a statement, Mr. Ibrahim reiterated the need for a multidisciplinary cancer care team to evaluate patients’ cardiovascular risk prior to and through cancer treatment.
“On a more personal level, cancer patients are already surprised by their cancer diagnosis,” he added. “Similar to the pretreatment consultation with radiation oncology, breast surgery, and medical oncology, an upfront cardiovascular risk assessment provides greater comfort and further minimizes psychological surprise with cardiovascular complications going into cancer treatment.”
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
ColCORONA: Colchicine reduces complications in outpatient COVID-19
The oral, anti-inflammatory drug colchicine can prevent complications and hospitalizations in nonhospitalized patients newly diagnosed with COVID-19, according to a press release from the ColCORONA trial investigators.
After 1 month of therapy, there was a 21% risk reduction in the primary composite endpoint of death or hospitalizations that missed statistical significance, compared with placebo among 4,488 outpatients enrolled in the global, phase 3 trial.
After excluding 329 patients without a confirmatory polymerase chain reaction test, however, the use of colchicine was reported to significantly reduce hospitalizations by 25%, the need for mechanical ventilation by 50%, and deaths by 44%.
“We believe that this is a medical breakthrough. There’s no approved therapy to prevent complications of COVID-19 in outpatients, to prevent them from reaching the hospital,” lead investigator Jean-Claude Tardif, MD, from the Montreal Heart Institute, said in an interview.
“I know that several countries will be reviewing the data very rapidly and that Greece approved it today,” he said. “So this is providing hope for patients.”
Having been burned by hydroxychloroquine and other treatments brought forth without peer review, the response to the announcement was tempered by a desire for more details.
Asked for comment, Steven E. Nissen, MD, of the Cleveland Clinic Foundation, was cautious. “The press release about the trial is vague and lacks details such as hazard ratios, confidence intervals, and P values,” he said in an interview.
“It is impossible to evaluate the results of this trial without these details. It is also uncertain how rigorously data were collected,” he added. “We’ll need to see the manuscript to adequately interpret the results.”
The evidence in the press release is hard to interpret, but early intervention with anti-inflammatory therapy has considerable biologic appeal in COVID, said Paul Ridker, MD, MPH, who led the pivotal CANTOS trial of the anti-inflammatory drug canakinumab in the post-MI setting, and is also chair of the ACTIV-4B trial currently investigating anticoagulants and antithrombotics in outpatient COVID-19.
“Colchicine is both inexpensive and generally well tolerated, and the apparent benefits so far reported are substantial,” Dr. Ridker, from Brigham and Women’s Hospital in Boston, said in an interview. “We are eager to see the full data as rapidly as possible.”
The commonly used gout and rheumatic disease agent costs about 26 cents in Canada and between $4 and $6 in the United States. As previously reported, it reduced the time to clinical deterioration and hospital stay but not mortality in the 105-patient Greek Study in the Effects of Colchicine in COVID-19 Complications Prevention (GRECCO-19) study.
Dr. Tardif said he’s looking forward to having the data in the public domain and that they acted swiftly because the evidence was “clinically persuasive” and “the health system is congested now.”
“We received the results Friday, Jan. 22 at 5 p.m., an hour later we were in meetings with our data safety monitoring board [DSMB], 2 hours later we issued a press release, and a day later we’re submitting a full manuscript to a major scientific journal, so I don’t know if anyone has done this at this speed,” he said. “So we are actually very proud of what we did.”
ColCORONA was designed to enroll 6,000 outpatients, at least 40 years of age, who were diagnosed with COVID-19 infection within the previous 24 hours, and had a least one high-risk criterion, including age at least 70 years, body mass index of at least 30 kg/m2, diabetes mellitus, uncontrolled hypertension, known respiratory disease, heart failure or coronary disease, fever of at least 38.4° C within the last 48 hours, dyspnea at presentation, bicytopenia, pancytopenia, or the combination of high neutrophil count and low lymphocyte count.
Participants were randomly assigned to receive either placebo or colchicine 0.5 mg twice daily for 3 days and then once daily for another 27 days.
The number needed to prevent one COVID-19 complication is about 60 patients, Dr. Tardif said.
Colchicine was well tolerated and resulted in fewer serious adverse events than with placebo, he said. Diarrhea occurred more often with colchicine, but there was no increase in pneumonia. Caution should be used, however, in treating patients with severe renal disease.
Dr. Tardif said he would not prescribe colchicine to an 18-year-old COVID outpatient who doesn’t have any concomitant diseases, but would for those meeting the study protocol.
“As long as a patient appears to me to be at risk of a complication, I would prescribe it, without a doubt,” he said. “I can tell you that when we held the meeting with the DSMB Friday evening, I actually put each member on the spot and asked them: ‘If it were you – not even treating a patient, but if you had COVID today, would you take it based on the data you’ve seen?’ and all of the DSMB members said they would.
“So we’ll have that debate in the public domain when the paper is out, but I believe most physicians will use it to treat their patients.”
The trial was coordinated by the Montreal Heart Institute and funded by the government of Quebec; the U.S. National Heart, Lung, and Blood Institute; Montreal philanthropist Sophie Desmarais; and the COVID-19 Therapeutics Accelerator launched by the Bill & Melinda Gates Foundation, Wellcome, and Mastercard. CGI, Dacima, and Pharmascience of Montreal were also collaborators.
A version of this article first appeared on Medscape.com.
The oral, anti-inflammatory drug colchicine can prevent complications and hospitalizations in nonhospitalized patients newly diagnosed with COVID-19, according to a press release from the ColCORONA trial investigators.
After 1 month of therapy, there was a 21% risk reduction in the primary composite endpoint of death or hospitalizations that missed statistical significance, compared with placebo among 4,488 outpatients enrolled in the global, phase 3 trial.
After excluding 329 patients without a confirmatory polymerase chain reaction test, however, the use of colchicine was reported to significantly reduce hospitalizations by 25%, the need for mechanical ventilation by 50%, and deaths by 44%.
“We believe that this is a medical breakthrough. There’s no approved therapy to prevent complications of COVID-19 in outpatients, to prevent them from reaching the hospital,” lead investigator Jean-Claude Tardif, MD, from the Montreal Heart Institute, said in an interview.
“I know that several countries will be reviewing the data very rapidly and that Greece approved it today,” he said. “So this is providing hope for patients.”
Having been burned by hydroxychloroquine and other treatments brought forth without peer review, the response to the announcement was tempered by a desire for more details.
Asked for comment, Steven E. Nissen, MD, of the Cleveland Clinic Foundation, was cautious. “The press release about the trial is vague and lacks details such as hazard ratios, confidence intervals, and P values,” he said in an interview.
“It is impossible to evaluate the results of this trial without these details. It is also uncertain how rigorously data were collected,” he added. “We’ll need to see the manuscript to adequately interpret the results.”
The evidence in the press release is hard to interpret, but early intervention with anti-inflammatory therapy has considerable biologic appeal in COVID, said Paul Ridker, MD, MPH, who led the pivotal CANTOS trial of the anti-inflammatory drug canakinumab in the post-MI setting, and is also chair of the ACTIV-4B trial currently investigating anticoagulants and antithrombotics in outpatient COVID-19.
“Colchicine is both inexpensive and generally well tolerated, and the apparent benefits so far reported are substantial,” Dr. Ridker, from Brigham and Women’s Hospital in Boston, said in an interview. “We are eager to see the full data as rapidly as possible.”
The commonly used gout and rheumatic disease agent costs about 26 cents in Canada and between $4 and $6 in the United States. As previously reported, it reduced the time to clinical deterioration and hospital stay but not mortality in the 105-patient Greek Study in the Effects of Colchicine in COVID-19 Complications Prevention (GRECCO-19) study.
Dr. Tardif said he’s looking forward to having the data in the public domain and that they acted swiftly because the evidence was “clinically persuasive” and “the health system is congested now.”
“We received the results Friday, Jan. 22 at 5 p.m., an hour later we were in meetings with our data safety monitoring board [DSMB], 2 hours later we issued a press release, and a day later we’re submitting a full manuscript to a major scientific journal, so I don’t know if anyone has done this at this speed,” he said. “So we are actually very proud of what we did.”
ColCORONA was designed to enroll 6,000 outpatients, at least 40 years of age, who were diagnosed with COVID-19 infection within the previous 24 hours, and had a least one high-risk criterion, including age at least 70 years, body mass index of at least 30 kg/m2, diabetes mellitus, uncontrolled hypertension, known respiratory disease, heart failure or coronary disease, fever of at least 38.4° C within the last 48 hours, dyspnea at presentation, bicytopenia, pancytopenia, or the combination of high neutrophil count and low lymphocyte count.
Participants were randomly assigned to receive either placebo or colchicine 0.5 mg twice daily for 3 days and then once daily for another 27 days.
The number needed to prevent one COVID-19 complication is about 60 patients, Dr. Tardif said.
Colchicine was well tolerated and resulted in fewer serious adverse events than with placebo, he said. Diarrhea occurred more often with colchicine, but there was no increase in pneumonia. Caution should be used, however, in treating patients with severe renal disease.
Dr. Tardif said he would not prescribe colchicine to an 18-year-old COVID outpatient who doesn’t have any concomitant diseases, but would for those meeting the study protocol.
“As long as a patient appears to me to be at risk of a complication, I would prescribe it, without a doubt,” he said. “I can tell you that when we held the meeting with the DSMB Friday evening, I actually put each member on the spot and asked them: ‘If it were you – not even treating a patient, but if you had COVID today, would you take it based on the data you’ve seen?’ and all of the DSMB members said they would.
“So we’ll have that debate in the public domain when the paper is out, but I believe most physicians will use it to treat their patients.”
The trial was coordinated by the Montreal Heart Institute and funded by the government of Quebec; the U.S. National Heart, Lung, and Blood Institute; Montreal philanthropist Sophie Desmarais; and the COVID-19 Therapeutics Accelerator launched by the Bill & Melinda Gates Foundation, Wellcome, and Mastercard. CGI, Dacima, and Pharmascience of Montreal were also collaborators.
A version of this article first appeared on Medscape.com.
The oral, anti-inflammatory drug colchicine can prevent complications and hospitalizations in nonhospitalized patients newly diagnosed with COVID-19, according to a press release from the ColCORONA trial investigators.
After 1 month of therapy, there was a 21% risk reduction in the primary composite endpoint of death or hospitalizations that missed statistical significance, compared with placebo among 4,488 outpatients enrolled in the global, phase 3 trial.
After excluding 329 patients without a confirmatory polymerase chain reaction test, however, the use of colchicine was reported to significantly reduce hospitalizations by 25%, the need for mechanical ventilation by 50%, and deaths by 44%.
“We believe that this is a medical breakthrough. There’s no approved therapy to prevent complications of COVID-19 in outpatients, to prevent them from reaching the hospital,” lead investigator Jean-Claude Tardif, MD, from the Montreal Heart Institute, said in an interview.
“I know that several countries will be reviewing the data very rapidly and that Greece approved it today,” he said. “So this is providing hope for patients.”
Having been burned by hydroxychloroquine and other treatments brought forth without peer review, the response to the announcement was tempered by a desire for more details.
Asked for comment, Steven E. Nissen, MD, of the Cleveland Clinic Foundation, was cautious. “The press release about the trial is vague and lacks details such as hazard ratios, confidence intervals, and P values,” he said in an interview.
“It is impossible to evaluate the results of this trial without these details. It is also uncertain how rigorously data were collected,” he added. “We’ll need to see the manuscript to adequately interpret the results.”
The evidence in the press release is hard to interpret, but early intervention with anti-inflammatory therapy has considerable biologic appeal in COVID, said Paul Ridker, MD, MPH, who led the pivotal CANTOS trial of the anti-inflammatory drug canakinumab in the post-MI setting, and is also chair of the ACTIV-4B trial currently investigating anticoagulants and antithrombotics in outpatient COVID-19.
“Colchicine is both inexpensive and generally well tolerated, and the apparent benefits so far reported are substantial,” Dr. Ridker, from Brigham and Women’s Hospital in Boston, said in an interview. “We are eager to see the full data as rapidly as possible.”
The commonly used gout and rheumatic disease agent costs about 26 cents in Canada and between $4 and $6 in the United States. As previously reported, it reduced the time to clinical deterioration and hospital stay but not mortality in the 105-patient Greek Study in the Effects of Colchicine in COVID-19 Complications Prevention (GRECCO-19) study.
Dr. Tardif said he’s looking forward to having the data in the public domain and that they acted swiftly because the evidence was “clinically persuasive” and “the health system is congested now.”
“We received the results Friday, Jan. 22 at 5 p.m., an hour later we were in meetings with our data safety monitoring board [DSMB], 2 hours later we issued a press release, and a day later we’re submitting a full manuscript to a major scientific journal, so I don’t know if anyone has done this at this speed,” he said. “So we are actually very proud of what we did.”
ColCORONA was designed to enroll 6,000 outpatients, at least 40 years of age, who were diagnosed with COVID-19 infection within the previous 24 hours, and had a least one high-risk criterion, including age at least 70 years, body mass index of at least 30 kg/m2, diabetes mellitus, uncontrolled hypertension, known respiratory disease, heart failure or coronary disease, fever of at least 38.4° C within the last 48 hours, dyspnea at presentation, bicytopenia, pancytopenia, or the combination of high neutrophil count and low lymphocyte count.
Participants were randomly assigned to receive either placebo or colchicine 0.5 mg twice daily for 3 days and then once daily for another 27 days.
The number needed to prevent one COVID-19 complication is about 60 patients, Dr. Tardif said.
Colchicine was well tolerated and resulted in fewer serious adverse events than with placebo, he said. Diarrhea occurred more often with colchicine, but there was no increase in pneumonia. Caution should be used, however, in treating patients with severe renal disease.
Dr. Tardif said he would not prescribe colchicine to an 18-year-old COVID outpatient who doesn’t have any concomitant diseases, but would for those meeting the study protocol.
“As long as a patient appears to me to be at risk of a complication, I would prescribe it, without a doubt,” he said. “I can tell you that when we held the meeting with the DSMB Friday evening, I actually put each member on the spot and asked them: ‘If it were you – not even treating a patient, but if you had COVID today, would you take it based on the data you’ve seen?’ and all of the DSMB members said they would.
“So we’ll have that debate in the public domain when the paper is out, but I believe most physicians will use it to treat their patients.”
The trial was coordinated by the Montreal Heart Institute and funded by the government of Quebec; the U.S. National Heart, Lung, and Blood Institute; Montreal philanthropist Sophie Desmarais; and the COVID-19 Therapeutics Accelerator launched by the Bill & Melinda Gates Foundation, Wellcome, and Mastercard. CGI, Dacima, and Pharmascience of Montreal were also collaborators.
A version of this article first appeared on Medscape.com.
Full-dose anticoagulation reduces need for life support in COVID-19
Full-dose anticoagulation was superior to low, prophylactic doses in reducing the need for vital organ support such as ventilation in moderately ill patients hospitalized for COVID-19, according to a report released Jan. 22 by the National Institutes of Health (NIH).
“This is a major advance for patients hospitalized with COVID. Full dose of anticoagulation in these non-ICU patients improved outcomes and there’s a trend toward a reduction in mortality,” Judith Hochman, MD, director of the Cardiovascular Clinical Research Center at NYU Langone Medical Center, New York, said in an interview.
“We have treatments that are improving outcomes but not as many that reduce mortality, so we’re hopeful when the full dataset comes in that will be confirmed,” she said.
The observation of increased rates of blood clots and inflammation among COVID-19 patients, which can lead to complications such as lung failure, heart attack, and stroke, has given rise to various anticoagulant treatment protocols and a need for randomized data on routinely administering increased doses of anticoagulation to hospitalized patients.
Today’s top-line findings come from three linked clinical trials – REMAP-CAP, ACTIV-4, and ATTACC – examining the safety and efficacy of full-dose anticoagulation to treat moderately ill or critically ill adults hospitalized with COVID-19 compared with a lower dose typically used to prevent blood clots in hospitalized patients.
In December 2020, all three trials paused enrollment of the critically ill subgroup after results showed that full-dose anticoagulation started in the intensive care unit (ICU) was not beneficial and may have been harmful in some patients.
Moderately ill patients with COVID-19, defined as those who did not require ICU care or organ support, made up 80% of participants at enrollment in the three trials, Dr. Hochman said.
Among more than 1,000 moderately ill patients reviewed as of the data cut with the data safety monitoring board, full doses of low molecular weight or unfractionated heparin were superior to low prophylactic doses for the primary endpoint of need for ventilation or other organ supportive interventions at 21 days after randomization.
This met the predefined threshold for 99% probability of superiority and recruitment was stopped, Dr. Hochman reported. “Obviously safety figured into this decision. The risk/benefit ratio was very clear.”
The results do not pertain to patients with a previous indication for anticoagulation, who were excluded from the trials.
Data from an additional 1,000 patients will be reviewed and the data published sometime in the next 2-3 months, she said.
With large numbers of COVID-19 patients requiring hospitalization, the outcomes could help reduce the overload on intensive care units around the world, the NIH noted.
The results also highlight the critical role of timing in the course of COVID-19.
“We believe that full anticoagulation is effective early in the disease course,” Dr. Hochman said. “Based on the results so far from these three platform trials, those that were very, very sick at the time of enrollment really didn’t benefit and we needed to have caught them at an earlier stage.
“It’s possible that the people in the ICU are just different and the minute they get sick they need the ICU; so we haven’t clearly demonstrated this time course and when to intervene, but that’s the implication of the findings.”
The question of even earlier treatment is being examined in the partner ACTIV-4B trial, which is enrolling patients with COVID-19 illness not requiring hospitalization and randomizing them to the direct oral anticoagulant apixaban or aspirin or placebo.
“It’s a very important trial and we really want to get the message out that patients should volunteer for it,” said Dr. Hochman, principal investigator of the ACTIV-4 trial.
In the United States, the ACTIV-4 trial is being led by a collaborative effort involving a number of universities, including the University of Pittsburgh and New York University.
The REMAP-CAP, ACTIV-4, and ATTACC study platforms span five continents in more than 300 hospitals and are supported by multiple international funding organizations including the National Institutes of Health, Canadian Institutes of Health Research, the National Institute for Health Research (United Kingdom), the National Health and Medical Research Council (Australia), and the PREPARE and RECOVER consortia (European Union).
A version of this article first appeared on Medscape.com.
Full-dose anticoagulation was superior to low, prophylactic doses in reducing the need for vital organ support such as ventilation in moderately ill patients hospitalized for COVID-19, according to a report released Jan. 22 by the National Institutes of Health (NIH).
“This is a major advance for patients hospitalized with COVID. Full dose of anticoagulation in these non-ICU patients improved outcomes and there’s a trend toward a reduction in mortality,” Judith Hochman, MD, director of the Cardiovascular Clinical Research Center at NYU Langone Medical Center, New York, said in an interview.
“We have treatments that are improving outcomes but not as many that reduce mortality, so we’re hopeful when the full dataset comes in that will be confirmed,” she said.
The observation of increased rates of blood clots and inflammation among COVID-19 patients, which can lead to complications such as lung failure, heart attack, and stroke, has given rise to various anticoagulant treatment protocols and a need for randomized data on routinely administering increased doses of anticoagulation to hospitalized patients.
Today’s top-line findings come from three linked clinical trials – REMAP-CAP, ACTIV-4, and ATTACC – examining the safety and efficacy of full-dose anticoagulation to treat moderately ill or critically ill adults hospitalized with COVID-19 compared with a lower dose typically used to prevent blood clots in hospitalized patients.
In December 2020, all three trials paused enrollment of the critically ill subgroup after results showed that full-dose anticoagulation started in the intensive care unit (ICU) was not beneficial and may have been harmful in some patients.
Moderately ill patients with COVID-19, defined as those who did not require ICU care or organ support, made up 80% of participants at enrollment in the three trials, Dr. Hochman said.
Among more than 1,000 moderately ill patients reviewed as of the data cut with the data safety monitoring board, full doses of low molecular weight or unfractionated heparin were superior to low prophylactic doses for the primary endpoint of need for ventilation or other organ supportive interventions at 21 days after randomization.
This met the predefined threshold for 99% probability of superiority and recruitment was stopped, Dr. Hochman reported. “Obviously safety figured into this decision. The risk/benefit ratio was very clear.”
The results do not pertain to patients with a previous indication for anticoagulation, who were excluded from the trials.
Data from an additional 1,000 patients will be reviewed and the data published sometime in the next 2-3 months, she said.
With large numbers of COVID-19 patients requiring hospitalization, the outcomes could help reduce the overload on intensive care units around the world, the NIH noted.
The results also highlight the critical role of timing in the course of COVID-19.
“We believe that full anticoagulation is effective early in the disease course,” Dr. Hochman said. “Based on the results so far from these three platform trials, those that were very, very sick at the time of enrollment really didn’t benefit and we needed to have caught them at an earlier stage.
“It’s possible that the people in the ICU are just different and the minute they get sick they need the ICU; so we haven’t clearly demonstrated this time course and when to intervene, but that’s the implication of the findings.”
The question of even earlier treatment is being examined in the partner ACTIV-4B trial, which is enrolling patients with COVID-19 illness not requiring hospitalization and randomizing them to the direct oral anticoagulant apixaban or aspirin or placebo.
“It’s a very important trial and we really want to get the message out that patients should volunteer for it,” said Dr. Hochman, principal investigator of the ACTIV-4 trial.
In the United States, the ACTIV-4 trial is being led by a collaborative effort involving a number of universities, including the University of Pittsburgh and New York University.
The REMAP-CAP, ACTIV-4, and ATTACC study platforms span five continents in more than 300 hospitals and are supported by multiple international funding organizations including the National Institutes of Health, Canadian Institutes of Health Research, the National Institute for Health Research (United Kingdom), the National Health and Medical Research Council (Australia), and the PREPARE and RECOVER consortia (European Union).
A version of this article first appeared on Medscape.com.
Full-dose anticoagulation was superior to low, prophylactic doses in reducing the need for vital organ support such as ventilation in moderately ill patients hospitalized for COVID-19, according to a report released Jan. 22 by the National Institutes of Health (NIH).
“This is a major advance for patients hospitalized with COVID. Full dose of anticoagulation in these non-ICU patients improved outcomes and there’s a trend toward a reduction in mortality,” Judith Hochman, MD, director of the Cardiovascular Clinical Research Center at NYU Langone Medical Center, New York, said in an interview.
“We have treatments that are improving outcomes but not as many that reduce mortality, so we’re hopeful when the full dataset comes in that will be confirmed,” she said.
The observation of increased rates of blood clots and inflammation among COVID-19 patients, which can lead to complications such as lung failure, heart attack, and stroke, has given rise to various anticoagulant treatment protocols and a need for randomized data on routinely administering increased doses of anticoagulation to hospitalized patients.
Today’s top-line findings come from three linked clinical trials – REMAP-CAP, ACTIV-4, and ATTACC – examining the safety and efficacy of full-dose anticoagulation to treat moderately ill or critically ill adults hospitalized with COVID-19 compared with a lower dose typically used to prevent blood clots in hospitalized patients.
In December 2020, all three trials paused enrollment of the critically ill subgroup after results showed that full-dose anticoagulation started in the intensive care unit (ICU) was not beneficial and may have been harmful in some patients.
Moderately ill patients with COVID-19, defined as those who did not require ICU care or organ support, made up 80% of participants at enrollment in the three trials, Dr. Hochman said.
Among more than 1,000 moderately ill patients reviewed as of the data cut with the data safety monitoring board, full doses of low molecular weight or unfractionated heparin were superior to low prophylactic doses for the primary endpoint of need for ventilation or other organ supportive interventions at 21 days after randomization.
This met the predefined threshold for 99% probability of superiority and recruitment was stopped, Dr. Hochman reported. “Obviously safety figured into this decision. The risk/benefit ratio was very clear.”
The results do not pertain to patients with a previous indication for anticoagulation, who were excluded from the trials.
Data from an additional 1,000 patients will be reviewed and the data published sometime in the next 2-3 months, she said.
With large numbers of COVID-19 patients requiring hospitalization, the outcomes could help reduce the overload on intensive care units around the world, the NIH noted.
The results also highlight the critical role of timing in the course of COVID-19.
“We believe that full anticoagulation is effective early in the disease course,” Dr. Hochman said. “Based on the results so far from these three platform trials, those that were very, very sick at the time of enrollment really didn’t benefit and we needed to have caught them at an earlier stage.
“It’s possible that the people in the ICU are just different and the minute they get sick they need the ICU; so we haven’t clearly demonstrated this time course and when to intervene, but that’s the implication of the findings.”
The question of even earlier treatment is being examined in the partner ACTIV-4B trial, which is enrolling patients with COVID-19 illness not requiring hospitalization and randomizing them to the direct oral anticoagulant apixaban or aspirin or placebo.
“It’s a very important trial and we really want to get the message out that patients should volunteer for it,” said Dr. Hochman, principal investigator of the ACTIV-4 trial.
In the United States, the ACTIV-4 trial is being led by a collaborative effort involving a number of universities, including the University of Pittsburgh and New York University.
The REMAP-CAP, ACTIV-4, and ATTACC study platforms span five continents in more than 300 hospitals and are supported by multiple international funding organizations including the National Institutes of Health, Canadian Institutes of Health Research, the National Institute for Health Research (United Kingdom), the National Health and Medical Research Council (Australia), and the PREPARE and RECOVER consortia (European Union).
A version of this article first appeared on Medscape.com.
Ticking all the right boxes for same-day discharge PCI
The American College of Cardiology has released a new expert consensus decision pathway to provide practical guidance on same-day discharge after percutaneous coronary intervention (PCI).
“There’s been a lot of interest in people wanting to start these programs, so we thought this is an ideal topic for a consensus pathway that will help programs that want to implement these things – give them kind of a road map for how to do that,” writing committee chair Sunil Rao, MD, Duke University Medical Center, Durham, N.C., said.
Although the document reviews the evidence supporting same-day discharge much like a guideline, the focus is on implementation, he said in an interview. It features a checklist of patient- and systems-specific considerations along with key definitions and a series of clinical scenarios showing the rationale for same-day discharge or overnight monitoring.
The checklist can be used for anyone presenting for an elective PCI or for ad hoc cases that flow directly from the diagnostic cath lab and make up about 80% of procedures. It is not applicable for those presenting with ST-elevation myocardial infarction (STEMI) or non-STEMI, but can be used for staged procedures performed after their index PCI, according to the report, published online Jan. 7 in the Journal of the American College of Cardiology.
When establishing a new same-day discharge program, the basic approach can be distilled down to the “three Ps”– the patient, the procedure, and the program – Dr. Rao explained. The patient has to be the right patient, be willing to go home that night, and have some kind of support structure at home in case they run into trouble. The procedure itself should be without complications and the recovery unremarkable, with a stable access site and a return to baseline mental status and ambulation. Finally, “this all has to take place in the context of a program with buy-in from the different stakeholders,” he said.
The report points out that the need for administrative buy-in “should not be underestimated” and recommends physician-champions meet with staff administrators to present the data on PCI utility and safety and to communicate the need for staff to complete the checklist.
Implementing the checklist also requires buy-in from nurses and other team members who may be tasked with educating patients on issues like access site complications and ensuring they receive relevant discharge information, a loading dose of a P2Y12 inhibitor, and appropriate prescriptions.
“If you’re only going to observe the patient for 6 hours, you’ve got to make sure that they’re on all the secondary prevention medications and the referral to cardiac rehabilitation takes place,” Dr. Rao said. “So I think that, in a funny way, the implementation of same-day discharge allows us to actually focus a little bit more on these kinds of postprocedure aspects that I think we were taking for granted a little bit when patients were being observed overnight.”
The checklist is detailed but was designed so it can be tailored to the needs of individual institutions, writing committee member Connie N. Hess, MD, MHS, University of Colorado at Denver, Aurora, pointed out.
“At every level there is a lot of variance in institutional resources or even a patient’s resources,” she said. “So we didn’t want to seem too prescriptive.”
Some institutions, for example, may feel strongly that accessibility to a caregiver means someone staying in the house who can monitor the patient’s access site and call 911 if need be, whereas others may define it as having a neighbor who’s easy to reach by phone, Dr. Hess noted in an interview.
Exactly when the last patient can be eligible for same-day discharge may also vary between urban and rural settings where patients may drive hours for their care. The built-in flexibility also allows institutions to incorporate their own preexisting documents into the checklist.
“I don’t think the hospital buy-in is necessarily the hard part because there is a clear monetary benefit as long as you can show that it’s done safely and you’re not harming patients, which I think has been done,” Dr. Hess said. “I think then the next level down, you have the provider buy-in and that may be where there might be a little bit more work depending on the preexisting culture.”
Part of the hesitancy may reflect a generational gap, whereby younger interventionalists who trained in programs with same-day discharge may be more willing to support the checklist.
“This actually parallels radial artery access where data exists on its benefits but it’s not used,” Dr. Hess said. “And I think a lot of this has to do with provider comfort levels with sending patients home and just not necessarily knowing how to implement a program at their institution.”
Both Dr. Rao and Dr. Hess pointed out that uptake of same-day discharge PCI is low in the United States, compared with other part of the world, including the United Kingdom, with estimates at about 16%-20% of PCIs.
That said, the timing of the new expert consensus document is “fortuitous,” Dr. Rao noted. Since work on the document began 2 years ago, the Centers for Medicare & Medicaid Services’ greenlit reimbursement for PCI performed in an ambulatory surgical center and the pandemic walloped U.S. hospitals. “I think those two things really do highlight the importance of a document like this.”
“A potential advantage of the same-day discharge program is that you won’t be exposing patients to the hospital setting where COVID is a problem, and you’ll keep your beds open for the COVID patients that really do need it,” he said.
The ability to go home without an overnight stay may also encourage some patients to seek care. “Patients with cardiovascular disease really need to understand that you may be stable at one point but then obviously can become unstable, and we don’t want people to stay away from the hospital because they are worried about being admitted,” Dr. Rao said.
Dr. Rao and Dr. Hess report no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
The American College of Cardiology has released a new expert consensus decision pathway to provide practical guidance on same-day discharge after percutaneous coronary intervention (PCI).
“There’s been a lot of interest in people wanting to start these programs, so we thought this is an ideal topic for a consensus pathway that will help programs that want to implement these things – give them kind of a road map for how to do that,” writing committee chair Sunil Rao, MD, Duke University Medical Center, Durham, N.C., said.
Although the document reviews the evidence supporting same-day discharge much like a guideline, the focus is on implementation, he said in an interview. It features a checklist of patient- and systems-specific considerations along with key definitions and a series of clinical scenarios showing the rationale for same-day discharge or overnight monitoring.
The checklist can be used for anyone presenting for an elective PCI or for ad hoc cases that flow directly from the diagnostic cath lab and make up about 80% of procedures. It is not applicable for those presenting with ST-elevation myocardial infarction (STEMI) or non-STEMI, but can be used for staged procedures performed after their index PCI, according to the report, published online Jan. 7 in the Journal of the American College of Cardiology.
When establishing a new same-day discharge program, the basic approach can be distilled down to the “three Ps”– the patient, the procedure, and the program – Dr. Rao explained. The patient has to be the right patient, be willing to go home that night, and have some kind of support structure at home in case they run into trouble. The procedure itself should be without complications and the recovery unremarkable, with a stable access site and a return to baseline mental status and ambulation. Finally, “this all has to take place in the context of a program with buy-in from the different stakeholders,” he said.
The report points out that the need for administrative buy-in “should not be underestimated” and recommends physician-champions meet with staff administrators to present the data on PCI utility and safety and to communicate the need for staff to complete the checklist.
Implementing the checklist also requires buy-in from nurses and other team members who may be tasked with educating patients on issues like access site complications and ensuring they receive relevant discharge information, a loading dose of a P2Y12 inhibitor, and appropriate prescriptions.
“If you’re only going to observe the patient for 6 hours, you’ve got to make sure that they’re on all the secondary prevention medications and the referral to cardiac rehabilitation takes place,” Dr. Rao said. “So I think that, in a funny way, the implementation of same-day discharge allows us to actually focus a little bit more on these kinds of postprocedure aspects that I think we were taking for granted a little bit when patients were being observed overnight.”
The checklist is detailed but was designed so it can be tailored to the needs of individual institutions, writing committee member Connie N. Hess, MD, MHS, University of Colorado at Denver, Aurora, pointed out.
“At every level there is a lot of variance in institutional resources or even a patient’s resources,” she said. “So we didn’t want to seem too prescriptive.”
Some institutions, for example, may feel strongly that accessibility to a caregiver means someone staying in the house who can monitor the patient’s access site and call 911 if need be, whereas others may define it as having a neighbor who’s easy to reach by phone, Dr. Hess noted in an interview.
Exactly when the last patient can be eligible for same-day discharge may also vary between urban and rural settings where patients may drive hours for their care. The built-in flexibility also allows institutions to incorporate their own preexisting documents into the checklist.
“I don’t think the hospital buy-in is necessarily the hard part because there is a clear monetary benefit as long as you can show that it’s done safely and you’re not harming patients, which I think has been done,” Dr. Hess said. “I think then the next level down, you have the provider buy-in and that may be where there might be a little bit more work depending on the preexisting culture.”
Part of the hesitancy may reflect a generational gap, whereby younger interventionalists who trained in programs with same-day discharge may be more willing to support the checklist.
“This actually parallels radial artery access where data exists on its benefits but it’s not used,” Dr. Hess said. “And I think a lot of this has to do with provider comfort levels with sending patients home and just not necessarily knowing how to implement a program at their institution.”
Both Dr. Rao and Dr. Hess pointed out that uptake of same-day discharge PCI is low in the United States, compared with other part of the world, including the United Kingdom, with estimates at about 16%-20% of PCIs.
That said, the timing of the new expert consensus document is “fortuitous,” Dr. Rao noted. Since work on the document began 2 years ago, the Centers for Medicare & Medicaid Services’ greenlit reimbursement for PCI performed in an ambulatory surgical center and the pandemic walloped U.S. hospitals. “I think those two things really do highlight the importance of a document like this.”
“A potential advantage of the same-day discharge program is that you won’t be exposing patients to the hospital setting where COVID is a problem, and you’ll keep your beds open for the COVID patients that really do need it,” he said.
The ability to go home without an overnight stay may also encourage some patients to seek care. “Patients with cardiovascular disease really need to understand that you may be stable at one point but then obviously can become unstable, and we don’t want people to stay away from the hospital because they are worried about being admitted,” Dr. Rao said.
Dr. Rao and Dr. Hess report no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
The American College of Cardiology has released a new expert consensus decision pathway to provide practical guidance on same-day discharge after percutaneous coronary intervention (PCI).
“There’s been a lot of interest in people wanting to start these programs, so we thought this is an ideal topic for a consensus pathway that will help programs that want to implement these things – give them kind of a road map for how to do that,” writing committee chair Sunil Rao, MD, Duke University Medical Center, Durham, N.C., said.
Although the document reviews the evidence supporting same-day discharge much like a guideline, the focus is on implementation, he said in an interview. It features a checklist of patient- and systems-specific considerations along with key definitions and a series of clinical scenarios showing the rationale for same-day discharge or overnight monitoring.
The checklist can be used for anyone presenting for an elective PCI or for ad hoc cases that flow directly from the diagnostic cath lab and make up about 80% of procedures. It is not applicable for those presenting with ST-elevation myocardial infarction (STEMI) or non-STEMI, but can be used for staged procedures performed after their index PCI, according to the report, published online Jan. 7 in the Journal of the American College of Cardiology.
When establishing a new same-day discharge program, the basic approach can be distilled down to the “three Ps”– the patient, the procedure, and the program – Dr. Rao explained. The patient has to be the right patient, be willing to go home that night, and have some kind of support structure at home in case they run into trouble. The procedure itself should be without complications and the recovery unremarkable, with a stable access site and a return to baseline mental status and ambulation. Finally, “this all has to take place in the context of a program with buy-in from the different stakeholders,” he said.
The report points out that the need for administrative buy-in “should not be underestimated” and recommends physician-champions meet with staff administrators to present the data on PCI utility and safety and to communicate the need for staff to complete the checklist.
Implementing the checklist also requires buy-in from nurses and other team members who may be tasked with educating patients on issues like access site complications and ensuring they receive relevant discharge information, a loading dose of a P2Y12 inhibitor, and appropriate prescriptions.
“If you’re only going to observe the patient for 6 hours, you’ve got to make sure that they’re on all the secondary prevention medications and the referral to cardiac rehabilitation takes place,” Dr. Rao said. “So I think that, in a funny way, the implementation of same-day discharge allows us to actually focus a little bit more on these kinds of postprocedure aspects that I think we were taking for granted a little bit when patients were being observed overnight.”
The checklist is detailed but was designed so it can be tailored to the needs of individual institutions, writing committee member Connie N. Hess, MD, MHS, University of Colorado at Denver, Aurora, pointed out.
“At every level there is a lot of variance in institutional resources or even a patient’s resources,” she said. “So we didn’t want to seem too prescriptive.”
Some institutions, for example, may feel strongly that accessibility to a caregiver means someone staying in the house who can monitor the patient’s access site and call 911 if need be, whereas others may define it as having a neighbor who’s easy to reach by phone, Dr. Hess noted in an interview.
Exactly when the last patient can be eligible for same-day discharge may also vary between urban and rural settings where patients may drive hours for their care. The built-in flexibility also allows institutions to incorporate their own preexisting documents into the checklist.
“I don’t think the hospital buy-in is necessarily the hard part because there is a clear monetary benefit as long as you can show that it’s done safely and you’re not harming patients, which I think has been done,” Dr. Hess said. “I think then the next level down, you have the provider buy-in and that may be where there might be a little bit more work depending on the preexisting culture.”
Part of the hesitancy may reflect a generational gap, whereby younger interventionalists who trained in programs with same-day discharge may be more willing to support the checklist.
“This actually parallels radial artery access where data exists on its benefits but it’s not used,” Dr. Hess said. “And I think a lot of this has to do with provider comfort levels with sending patients home and just not necessarily knowing how to implement a program at their institution.”
Both Dr. Rao and Dr. Hess pointed out that uptake of same-day discharge PCI is low in the United States, compared with other part of the world, including the United Kingdom, with estimates at about 16%-20% of PCIs.
That said, the timing of the new expert consensus document is “fortuitous,” Dr. Rao noted. Since work on the document began 2 years ago, the Centers for Medicare & Medicaid Services’ greenlit reimbursement for PCI performed in an ambulatory surgical center and the pandemic walloped U.S. hospitals. “I think those two things really do highlight the importance of a document like this.”
“A potential advantage of the same-day discharge program is that you won’t be exposing patients to the hospital setting where COVID is a problem, and you’ll keep your beds open for the COVID patients that really do need it,” he said.
The ability to go home without an overnight stay may also encourage some patients to seek care. “Patients with cardiovascular disease really need to understand that you may be stable at one point but then obviously can become unstable, and we don’t want people to stay away from the hospital because they are worried about being admitted,” Dr. Rao said.
Dr. Rao and Dr. Hess report no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.







