User login
CLINICAL GUIDELINES: Primary care bronchiolitis guidelines
Bronchiolitis is the most common cause of hospitalization among infants during the first 12 months of life. Approximately 100,000 bronchiolitis admissions occur annually in children in the United States, at an estimated cost of $1.73 billion. The American Academy of Pediatrics recently published new guidelines for the diagnosis, management, and prevention of bronchiolitis in children younger than 2 years.
Diagnosis
Diagnosis is based on patient history and physical examination. The course and severity of bronchiolitis vary, ranging from mild disease with simple runny nose and cough, to transient apneic events, and on to progressive respiratory distress secondary to airway obstruction. Management of bronchiolitis must be determined in the context of increased risk factors for severe disease, including age less than 12 weeks, history of prematurity, underlying cardiopulmonary disease, and immunodeficiency. Current evidence does not support routine labs or diagnostic imaging as helping with risk assessment. Abnormalities on chest x-ray, which are common in children with bronchiolitis, do reliably predict severity of disease, so chest x-rays are only indicated when another etiology of respiratory distress such as pneumothorax or pneumonia is a concern. Routine virologic testing is not recommended, as it does not appear to aid in guiding the treatment of the child with bronchiolitis.
Management
Randomized trials have not shown any benefit from alpha- or beta-adrenergic agonist administration. Bronchodilators can lessen symptoms scores, but their use does not speed disease resolution or decrease the length of stay or need for hospitalization. A Cochrane analysis concluded that there was no benefit to giving bronchodilators to infants with bronchiolitis. Adverse effects included tachycardia, tremors, and cost, all of which outweigh potential benefits. While previous versions of the AAP guidelines recommended bronchodilators as an option, the 2014 guidelines state, “Clinicians should not administer albuterol (or salbutamol) to infants and children with a diagnosis of bronchiolitis (Evidence Quality: B; Recommendation Strength: Strong).” It is noted that there may be some children who have reversible airway obstruction, but it is impossible to tell ahead of time who they are; and due to the variability of the disease, it is even hard to tell in whom the medication is effective. It is acknowledged that children with severe disease were usually excluded from the studies of bronchodilators. Epinephrine should also not be used except potentially as a rescue agent in severe disease.
Nebulized hypertonic saline appears to increase mucociliary clearance. Nebulized 3% saline is safe and effective in improving symptoms of mild to moderate bronchiolitis when measured after 24 hours of use, and it possibly decreases the length of hospital stay in studies where the length of stay exceeded 3 days. The guidelines conclude that hypertonic saline may be helpful to infants who are hospitalized with bronchiolitis, but probably is of very little benefit when administered in an emergency department setting.
Although there is strong evidence of benefit of systemic corticosteroids in asthma and croup, there is no evidence that systemic corticosteroids provide benefit in bronchiolitis. In addition, there is some evidence that corticosteroids may prolong viral shedding. For these reasons, the 2014 guidelines state, “Clinicians should not administer systemic corticosteroids to infants with a diagnosis of bronchiolitis in any setting (Evidence Quality: A; Recommendation Strength: Strong Recommendation).”
Physicians may choose not to give supplemental oxygen if oxyhemoglobin saturation is more than 90%, and also not to use continuous pulse oximetry given that it is prone to errors of measurement. Chest physiotherapy is not recommended. Antibiotics use is not recommended unless there is a concomitant bacterial infection or strong suspicion of one.
Prevention
The guidelines advise that palivizumab (Synagis) should not be given to otherwise healthy infants with a gestational age of 29 weeks or greater. Palivizumab should be given in the first year of life to infants with hemodynamically significant heart disease or chronic lung disease of prematurity (defined as infants of less than 32 weeks’ gestation who required more than 21% oxygen for at least the first 28 days of life). Infants who qualify for palivizumab at the start of respiratory syncytial virus season should receive a maximum of five monthly doses (15 mg/kg per dose) of palivizumab or until the end of RSV season, whichever comes first. Because of the low risk of RSV hospitalization in the second year of life, palivizumab prophylaxis is not recommended for children in the second year of life, unless the child meets the criteria for chronic lung disease and continues to require supplemental oxygen or is on chronic corticosteroids or diuretic therapy within 6 months of the onset of the second RSV season.
Reference
Ralston S.L. "Clinical Practice Guideline: The Diagnosis, Management, and Prevention of Bronchiolitis." Pediatrics 2014;134:e1474-502).
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital and professor of family and community medicine at Temple University in Philadelphia. Dr. Rastogi is a third-year resident in the family medicine residency program at Abington Memorial Hospital.
Ralston S.L. “Clinical Practice Guideline: The Diagnosis, Management, and Prevention of Bronchiolitis.” Pediatrics 2014;134:e1474-502).
Bronchiolitis is the most common cause of hospitalization among infants during the first 12 months of life. Approximately 100,000 bronchiolitis admissions occur annually in children in the United States, at an estimated cost of $1.73 billion. The American Academy of Pediatrics recently published new guidelines for the diagnosis, management, and prevention of bronchiolitis in children younger than 2 years.
Diagnosis
Diagnosis is based on patient history and physical examination. The course and severity of bronchiolitis vary, ranging from mild disease with simple runny nose and cough, to transient apneic events, and on to progressive respiratory distress secondary to airway obstruction. Management of bronchiolitis must be determined in the context of increased risk factors for severe disease, including age less than 12 weeks, history of prematurity, underlying cardiopulmonary disease, and immunodeficiency. Current evidence does not support routine labs or diagnostic imaging as helping with risk assessment. Abnormalities on chest x-ray, which are common in children with bronchiolitis, do reliably predict severity of disease, so chest x-rays are only indicated when another etiology of respiratory distress such as pneumothorax or pneumonia is a concern. Routine virologic testing is not recommended, as it does not appear to aid in guiding the treatment of the child with bronchiolitis.
Management
Randomized trials have not shown any benefit from alpha- or beta-adrenergic agonist administration. Bronchodilators can lessen symptoms scores, but their use does not speed disease resolution or decrease the length of stay or need for hospitalization. A Cochrane analysis concluded that there was no benefit to giving bronchodilators to infants with bronchiolitis. Adverse effects included tachycardia, tremors, and cost, all of which outweigh potential benefits. While previous versions of the AAP guidelines recommended bronchodilators as an option, the 2014 guidelines state, “Clinicians should not administer albuterol (or salbutamol) to infants and children with a diagnosis of bronchiolitis (Evidence Quality: B; Recommendation Strength: Strong).” It is noted that there may be some children who have reversible airway obstruction, but it is impossible to tell ahead of time who they are; and due to the variability of the disease, it is even hard to tell in whom the medication is effective. It is acknowledged that children with severe disease were usually excluded from the studies of bronchodilators. Epinephrine should also not be used except potentially as a rescue agent in severe disease.
Nebulized hypertonic saline appears to increase mucociliary clearance. Nebulized 3% saline is safe and effective in improving symptoms of mild to moderate bronchiolitis when measured after 24 hours of use, and it possibly decreases the length of hospital stay in studies where the length of stay exceeded 3 days. The guidelines conclude that hypertonic saline may be helpful to infants who are hospitalized with bronchiolitis, but probably is of very little benefit when administered in an emergency department setting.
Although there is strong evidence of benefit of systemic corticosteroids in asthma and croup, there is no evidence that systemic corticosteroids provide benefit in bronchiolitis. In addition, there is some evidence that corticosteroids may prolong viral shedding. For these reasons, the 2014 guidelines state, “Clinicians should not administer systemic corticosteroids to infants with a diagnosis of bronchiolitis in any setting (Evidence Quality: A; Recommendation Strength: Strong Recommendation).”
Physicians may choose not to give supplemental oxygen if oxyhemoglobin saturation is more than 90%, and also not to use continuous pulse oximetry given that it is prone to errors of measurement. Chest physiotherapy is not recommended. Antibiotics use is not recommended unless there is a concomitant bacterial infection or strong suspicion of one.
Prevention
The guidelines advise that palivizumab (Synagis) should not be given to otherwise healthy infants with a gestational age of 29 weeks or greater. Palivizumab should be given in the first year of life to infants with hemodynamically significant heart disease or chronic lung disease of prematurity (defined as infants of less than 32 weeks’ gestation who required more than 21% oxygen for at least the first 28 days of life). Infants who qualify for palivizumab at the start of respiratory syncytial virus season should receive a maximum of five monthly doses (15 mg/kg per dose) of palivizumab or until the end of RSV season, whichever comes first. Because of the low risk of RSV hospitalization in the second year of life, palivizumab prophylaxis is not recommended for children in the second year of life, unless the child meets the criteria for chronic lung disease and continues to require supplemental oxygen or is on chronic corticosteroids or diuretic therapy within 6 months of the onset of the second RSV season.
Reference
Ralston S.L. "Clinical Practice Guideline: The Diagnosis, Management, and Prevention of Bronchiolitis." Pediatrics 2014;134:e1474-502).
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital and professor of family and community medicine at Temple University in Philadelphia. Dr. Rastogi is a third-year resident in the family medicine residency program at Abington Memorial Hospital.
Bronchiolitis is the most common cause of hospitalization among infants during the first 12 months of life. Approximately 100,000 bronchiolitis admissions occur annually in children in the United States, at an estimated cost of $1.73 billion. The American Academy of Pediatrics recently published new guidelines for the diagnosis, management, and prevention of bronchiolitis in children younger than 2 years.
Diagnosis
Diagnosis is based on patient history and physical examination. The course and severity of bronchiolitis vary, ranging from mild disease with simple runny nose and cough, to transient apneic events, and on to progressive respiratory distress secondary to airway obstruction. Management of bronchiolitis must be determined in the context of increased risk factors for severe disease, including age less than 12 weeks, history of prematurity, underlying cardiopulmonary disease, and immunodeficiency. Current evidence does not support routine labs or diagnostic imaging as helping with risk assessment. Abnormalities on chest x-ray, which are common in children with bronchiolitis, do reliably predict severity of disease, so chest x-rays are only indicated when another etiology of respiratory distress such as pneumothorax or pneumonia is a concern. Routine virologic testing is not recommended, as it does not appear to aid in guiding the treatment of the child with bronchiolitis.
Management
Randomized trials have not shown any benefit from alpha- or beta-adrenergic agonist administration. Bronchodilators can lessen symptoms scores, but their use does not speed disease resolution or decrease the length of stay or need for hospitalization. A Cochrane analysis concluded that there was no benefit to giving bronchodilators to infants with bronchiolitis. Adverse effects included tachycardia, tremors, and cost, all of which outweigh potential benefits. While previous versions of the AAP guidelines recommended bronchodilators as an option, the 2014 guidelines state, “Clinicians should not administer albuterol (or salbutamol) to infants and children with a diagnosis of bronchiolitis (Evidence Quality: B; Recommendation Strength: Strong).” It is noted that there may be some children who have reversible airway obstruction, but it is impossible to tell ahead of time who they are; and due to the variability of the disease, it is even hard to tell in whom the medication is effective. It is acknowledged that children with severe disease were usually excluded from the studies of bronchodilators. Epinephrine should also not be used except potentially as a rescue agent in severe disease.
Nebulized hypertonic saline appears to increase mucociliary clearance. Nebulized 3% saline is safe and effective in improving symptoms of mild to moderate bronchiolitis when measured after 24 hours of use, and it possibly decreases the length of hospital stay in studies where the length of stay exceeded 3 days. The guidelines conclude that hypertonic saline may be helpful to infants who are hospitalized with bronchiolitis, but probably is of very little benefit when administered in an emergency department setting.
Although there is strong evidence of benefit of systemic corticosteroids in asthma and croup, there is no evidence that systemic corticosteroids provide benefit in bronchiolitis. In addition, there is some evidence that corticosteroids may prolong viral shedding. For these reasons, the 2014 guidelines state, “Clinicians should not administer systemic corticosteroids to infants with a diagnosis of bronchiolitis in any setting (Evidence Quality: A; Recommendation Strength: Strong Recommendation).”
Physicians may choose not to give supplemental oxygen if oxyhemoglobin saturation is more than 90%, and also not to use continuous pulse oximetry given that it is prone to errors of measurement. Chest physiotherapy is not recommended. Antibiotics use is not recommended unless there is a concomitant bacterial infection or strong suspicion of one.
Prevention
The guidelines advise that palivizumab (Synagis) should not be given to otherwise healthy infants with a gestational age of 29 weeks or greater. Palivizumab should be given in the first year of life to infants with hemodynamically significant heart disease or chronic lung disease of prematurity (defined as infants of less than 32 weeks’ gestation who required more than 21% oxygen for at least the first 28 days of life). Infants who qualify for palivizumab at the start of respiratory syncytial virus season should receive a maximum of five monthly doses (15 mg/kg per dose) of palivizumab or until the end of RSV season, whichever comes first. Because of the low risk of RSV hospitalization in the second year of life, palivizumab prophylaxis is not recommended for children in the second year of life, unless the child meets the criteria for chronic lung disease and continues to require supplemental oxygen or is on chronic corticosteroids or diuretic therapy within 6 months of the onset of the second RSV season.
Reference
Ralston S.L. "Clinical Practice Guideline: The Diagnosis, Management, and Prevention of Bronchiolitis." Pediatrics 2014;134:e1474-502).
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital and professor of family and community medicine at Temple University in Philadelphia. Dr. Rastogi is a third-year resident in the family medicine residency program at Abington Memorial Hospital.
Ralston S.L. “Clinical Practice Guideline: The Diagnosis, Management, and Prevention of Bronchiolitis.” Pediatrics 2014;134:e1474-502).
Ralston S.L. “Clinical Practice Guideline: The Diagnosis, Management, and Prevention of Bronchiolitis.” Pediatrics 2014;134:e1474-502).
Sharing is caring: A primer on EHR interoperability
The debate over the future of meaningful use seems to have found its bellwether issue: interoperability. For the uninitiated, this is the concept of sharing patient information across systems with the promise of improving the ease and quality of care. As you might expect, it is full of challenges, not the least of which is standardization. Competing vendors of electronic health record (EHR) software and technological hurdles have made the goal of true interoperability quite elusive, and there is no clear path to victory. Meaningful use and other incentive programs have set requirements for widespread rapid adoption of data sharing. Unfortunately, instead of encouraging innovation, they seem only to have created more stumbling blocks for physicians. Now providers are facing penalties for noncompliance, and national physician advocacy groups are taking notice.
On Oct. 15 of this year, a letter from key stakeholders including the American Medical Association (AMA), American Academy of Family Physicians (AAFP), and Medical Group Management Association (MGMA) to the Department of Health and Human Services laid out a “blueprint” for revamping the meaningful use program. The center point of this communication was a call for more emphasis on interoperability, as well as flexibility for both vendors and physicians. We tend to agree with these ideas, but wonder on a global scale what this interoperability should look like. In this column, we’ll address the essential pieces to making this a reality, and how physicians and patients can benefit from enhanced information exchange.
Information should be standardized
One of the fundamental challenges standing in the way of true interoperability is standardization: allowing data to be shared and viewed anywhere, independent of hardware or software. This is an idea that has allowed the World Wide Web to flourish; websites are readable by any computer or mobile device, using any operating system or browser. As of now, very little standardization exists in the world of medical data, in part because EHRs have been developed and promoted by private corporations, all competing for market share.
This evolution is quite unlike the history of the Internet, which was developed by government and educational institutions, with the express intent of connecting disparate computer systems. EHRs have essentially been developed in isolation, with much more emphasis placed on keeping information private than on making it shareable. Now meaningful use is forcing vendors to share data across systems, and – not surprisingly – each vendor is attempting to create their own method of doing so.
Some argue that EHR companies would prefer not to share, as this might threaten their hold on the market. In fact, Epic Health Systems, the world’s largest EHR vendor, has recently faced accusations of limiting interoperability to encourage physicians to use its software exclusively. Epic has fired back with statistics pointing to its accomplishments in data exchange. Both sides clearly disagree on what true interoperability should look like. This underscores a critical point: The concept of interoperability and what the standards should look like may mean different things to different parties.
One attempt at standardization that is commonly referenced is the continuity of care document (CCD), a key requirement for data exchange outlined in Stage 2 of meaningful use. This document, endorsed by the U.S. Healthcare Information Technology Standards Panel, has gained popularity as it can contain large amounts of data in one file. Unfortunately, it too is still limited and often isn’t user friendly at the point of care. It is in many ways merely a jumping-off point that will hopefully facilitate improved data accessibility and ease of sharing.
To improve usability and confidence in data exchange, many practices and health systems have joined together to create Regional Health Information Organizations and provide some governance structure to the process of data exchange. We strongly recommend getting involved in such an organization and engaging in the process of standardization. Regardless of your position on the usefulness or practicality of sharing patient records, a few notions are indisputable: Interoperability is coming, and point-of-care data availability – if accurate, secure, and useful – can ultimately usher in the promise of better patient care.
Information should be secure
In the process of seeking easier data exchange, we cannot lose sight of the importance of data security. Health care entities need to feel confident the information they are sending electronically will stay private until it reaches its ultimate destination. An attempt to address this issue led to the development of the “direct” encryption standard in 2010. Also known as Direct Exchange and Direct Secure Messaging, it specifies a secure method for the exchange of Protected Health Information. Providers can take advantage of the security offered through Direct by developing their own infrastructure or engaging the services of a Health Information Service Provider. These are private, HIPAA-compliant data exchange services that serve a health care community and facilitate direct messaging between health care settings. Ultimately, the goal is to create a robust Nationwide Health Information Network and achieve true widespread health information exchange. But before we actually achieve this, there is one more element essential to interoperability and improving patient care.
The information should be useful
In the preliminary stages of EHR interoperability, attempts at meaningful information exchange have led to only modest success. Outside of private health systems that have developed their own proprietary interfaces, data extraction and sharing between disparate electronic platforms have yet to have a meaningful impact on patient care. In part, this is because the information is not provided to clinicians in a useful format. Even the CCD described above is often confusing and replete with extraneous information – filtering through it during a patient encounter can be tedious and frustrating. Also, ensuring data integrity can be a real challenge, not only technically, but also practically. Questions occur regularly, such as “Did the data come through in the correct fields?” or “Did the medical resident remember to include all of the medications or allergies associated with the patient?” Ultimately, physicians need to decide whether or not to trust the information they receive before making it a permanent part of a patient’s health record.
Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. Dr. Skolnik is associate director of the family medicine residency program at Abington Memorial Hospital and professor of family and community medicine at Temple University in Philadelphia.
The debate over the future of meaningful use seems to have found its bellwether issue: interoperability. For the uninitiated, this is the concept of sharing patient information across systems with the promise of improving the ease and quality of care. As you might expect, it is full of challenges, not the least of which is standardization. Competing vendors of electronic health record (EHR) software and technological hurdles have made the goal of true interoperability quite elusive, and there is no clear path to victory. Meaningful use and other incentive programs have set requirements for widespread rapid adoption of data sharing. Unfortunately, instead of encouraging innovation, they seem only to have created more stumbling blocks for physicians. Now providers are facing penalties for noncompliance, and national physician advocacy groups are taking notice.
On Oct. 15 of this year, a letter from key stakeholders including the American Medical Association (AMA), American Academy of Family Physicians (AAFP), and Medical Group Management Association (MGMA) to the Department of Health and Human Services laid out a “blueprint” for revamping the meaningful use program. The center point of this communication was a call for more emphasis on interoperability, as well as flexibility for both vendors and physicians. We tend to agree with these ideas, but wonder on a global scale what this interoperability should look like. In this column, we’ll address the essential pieces to making this a reality, and how physicians and patients can benefit from enhanced information exchange.
Information should be standardized
One of the fundamental challenges standing in the way of true interoperability is standardization: allowing data to be shared and viewed anywhere, independent of hardware or software. This is an idea that has allowed the World Wide Web to flourish; websites are readable by any computer or mobile device, using any operating system or browser. As of now, very little standardization exists in the world of medical data, in part because EHRs have been developed and promoted by private corporations, all competing for market share.
This evolution is quite unlike the history of the Internet, which was developed by government and educational institutions, with the express intent of connecting disparate computer systems. EHRs have essentially been developed in isolation, with much more emphasis placed on keeping information private than on making it shareable. Now meaningful use is forcing vendors to share data across systems, and – not surprisingly – each vendor is attempting to create their own method of doing so.
Some argue that EHR companies would prefer not to share, as this might threaten their hold on the market. In fact, Epic Health Systems, the world’s largest EHR vendor, has recently faced accusations of limiting interoperability to encourage physicians to use its software exclusively. Epic has fired back with statistics pointing to its accomplishments in data exchange. Both sides clearly disagree on what true interoperability should look like. This underscores a critical point: The concept of interoperability and what the standards should look like may mean different things to different parties.
One attempt at standardization that is commonly referenced is the continuity of care document (CCD), a key requirement for data exchange outlined in Stage 2 of meaningful use. This document, endorsed by the U.S. Healthcare Information Technology Standards Panel, has gained popularity as it can contain large amounts of data in one file. Unfortunately, it too is still limited and often isn’t user friendly at the point of care. It is in many ways merely a jumping-off point that will hopefully facilitate improved data accessibility and ease of sharing.
To improve usability and confidence in data exchange, many practices and health systems have joined together to create Regional Health Information Organizations and provide some governance structure to the process of data exchange. We strongly recommend getting involved in such an organization and engaging in the process of standardization. Regardless of your position on the usefulness or practicality of sharing patient records, a few notions are indisputable: Interoperability is coming, and point-of-care data availability – if accurate, secure, and useful – can ultimately usher in the promise of better patient care.
Information should be secure
In the process of seeking easier data exchange, we cannot lose sight of the importance of data security. Health care entities need to feel confident the information they are sending electronically will stay private until it reaches its ultimate destination. An attempt to address this issue led to the development of the “direct” encryption standard in 2010. Also known as Direct Exchange and Direct Secure Messaging, it specifies a secure method for the exchange of Protected Health Information. Providers can take advantage of the security offered through Direct by developing their own infrastructure or engaging the services of a Health Information Service Provider. These are private, HIPAA-compliant data exchange services that serve a health care community and facilitate direct messaging between health care settings. Ultimately, the goal is to create a robust Nationwide Health Information Network and achieve true widespread health information exchange. But before we actually achieve this, there is one more element essential to interoperability and improving patient care.
The information should be useful
In the preliminary stages of EHR interoperability, attempts at meaningful information exchange have led to only modest success. Outside of private health systems that have developed their own proprietary interfaces, data extraction and sharing between disparate electronic platforms have yet to have a meaningful impact on patient care. In part, this is because the information is not provided to clinicians in a useful format. Even the CCD described above is often confusing and replete with extraneous information – filtering through it during a patient encounter can be tedious and frustrating. Also, ensuring data integrity can be a real challenge, not only technically, but also practically. Questions occur regularly, such as “Did the data come through in the correct fields?” or “Did the medical resident remember to include all of the medications or allergies associated with the patient?” Ultimately, physicians need to decide whether or not to trust the information they receive before making it a permanent part of a patient’s health record.
Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. Dr. Skolnik is associate director of the family medicine residency program at Abington Memorial Hospital and professor of family and community medicine at Temple University in Philadelphia.
The debate over the future of meaningful use seems to have found its bellwether issue: interoperability. For the uninitiated, this is the concept of sharing patient information across systems with the promise of improving the ease and quality of care. As you might expect, it is full of challenges, not the least of which is standardization. Competing vendors of electronic health record (EHR) software and technological hurdles have made the goal of true interoperability quite elusive, and there is no clear path to victory. Meaningful use and other incentive programs have set requirements for widespread rapid adoption of data sharing. Unfortunately, instead of encouraging innovation, they seem only to have created more stumbling blocks for physicians. Now providers are facing penalties for noncompliance, and national physician advocacy groups are taking notice.
On Oct. 15 of this year, a letter from key stakeholders including the American Medical Association (AMA), American Academy of Family Physicians (AAFP), and Medical Group Management Association (MGMA) to the Department of Health and Human Services laid out a “blueprint” for revamping the meaningful use program. The center point of this communication was a call for more emphasis on interoperability, as well as flexibility for both vendors and physicians. We tend to agree with these ideas, but wonder on a global scale what this interoperability should look like. In this column, we’ll address the essential pieces to making this a reality, and how physicians and patients can benefit from enhanced information exchange.
Information should be standardized
One of the fundamental challenges standing in the way of true interoperability is standardization: allowing data to be shared and viewed anywhere, independent of hardware or software. This is an idea that has allowed the World Wide Web to flourish; websites are readable by any computer or mobile device, using any operating system or browser. As of now, very little standardization exists in the world of medical data, in part because EHRs have been developed and promoted by private corporations, all competing for market share.
This evolution is quite unlike the history of the Internet, which was developed by government and educational institutions, with the express intent of connecting disparate computer systems. EHRs have essentially been developed in isolation, with much more emphasis placed on keeping information private than on making it shareable. Now meaningful use is forcing vendors to share data across systems, and – not surprisingly – each vendor is attempting to create their own method of doing so.
Some argue that EHR companies would prefer not to share, as this might threaten their hold on the market. In fact, Epic Health Systems, the world’s largest EHR vendor, has recently faced accusations of limiting interoperability to encourage physicians to use its software exclusively. Epic has fired back with statistics pointing to its accomplishments in data exchange. Both sides clearly disagree on what true interoperability should look like. This underscores a critical point: The concept of interoperability and what the standards should look like may mean different things to different parties.
One attempt at standardization that is commonly referenced is the continuity of care document (CCD), a key requirement for data exchange outlined in Stage 2 of meaningful use. This document, endorsed by the U.S. Healthcare Information Technology Standards Panel, has gained popularity as it can contain large amounts of data in one file. Unfortunately, it too is still limited and often isn’t user friendly at the point of care. It is in many ways merely a jumping-off point that will hopefully facilitate improved data accessibility and ease of sharing.
To improve usability and confidence in data exchange, many practices and health systems have joined together to create Regional Health Information Organizations and provide some governance structure to the process of data exchange. We strongly recommend getting involved in such an organization and engaging in the process of standardization. Regardless of your position on the usefulness or practicality of sharing patient records, a few notions are indisputable: Interoperability is coming, and point-of-care data availability – if accurate, secure, and useful – can ultimately usher in the promise of better patient care.
Information should be secure
In the process of seeking easier data exchange, we cannot lose sight of the importance of data security. Health care entities need to feel confident the information they are sending electronically will stay private until it reaches its ultimate destination. An attempt to address this issue led to the development of the “direct” encryption standard in 2010. Also known as Direct Exchange and Direct Secure Messaging, it specifies a secure method for the exchange of Protected Health Information. Providers can take advantage of the security offered through Direct by developing their own infrastructure or engaging the services of a Health Information Service Provider. These are private, HIPAA-compliant data exchange services that serve a health care community and facilitate direct messaging between health care settings. Ultimately, the goal is to create a robust Nationwide Health Information Network and achieve true widespread health information exchange. But before we actually achieve this, there is one more element essential to interoperability and improving patient care.
The information should be useful
In the preliminary stages of EHR interoperability, attempts at meaningful information exchange have led to only modest success. Outside of private health systems that have developed their own proprietary interfaces, data extraction and sharing between disparate electronic platforms have yet to have a meaningful impact on patient care. In part, this is because the information is not provided to clinicians in a useful format. Even the CCD described above is often confusing and replete with extraneous information – filtering through it during a patient encounter can be tedious and frustrating. Also, ensuring data integrity can be a real challenge, not only technically, but also practically. Questions occur regularly, such as “Did the data come through in the correct fields?” or “Did the medical resident remember to include all of the medications or allergies associated with the patient?” Ultimately, physicians need to decide whether or not to trust the information they receive before making it a permanent part of a patient’s health record.
Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. Dr. Skolnik is associate director of the family medicine residency program at Abington Memorial Hospital and professor of family and community medicine at Temple University in Philadelphia.
Contraception for adolescents
Approximately 750,000 adolescents become pregnant each year in the United States and nearly half of high school students report having had sexual intercourse. More than 80% of teenage pregnancies are unplanned and result in substantial health care costs, reduced earning potential, and increased health risks to both the mother and newborn. The American Academy of Pediatrics has released an updated policy statement that endorses long-acting reversible contraception (LARC) as a first-line consideration for adolescent contraception.
Sexual history taking and counseling
The American Academy of Pediatrics (AAP) encourages the 5 P’s of sexual history taking: partners, prevention of pregnancy, protection from sexually transmitted infections (STIs), sexual practices, past history of STIs, and pregnancy. Confidentiality is advised for issues revolving around sexuality and sexually transmitted infections. Most states have legislation regarding minor consent for contraception, details of which can be found at the Guttmacher Institute. The adolescent should be encouraged to delay onset of sexual activity until they are ready, but abstinence should not be the only focus of contraception counseling, and adolescents should be supported in choosing, and adhering to, a method of contraception if they choose to be sexually active.
Methods of contraception
The updated AAP policy statement advises that physicians offer methods of contraception by discussing methods that are more effective preventing pregnancy as preferred over methods that are less effective. The effectiveness of all contraceptive methods is described by typical user rates and average user rates, expressed as the percent of women who become pregnant using the method for 1 year. The gap between perfect and typical user rates is explained by the amount of effort that is needed to reliably use the method. Typical user rates should guide decisions about contraceptive efficacy for adolescents. Long-acting reversible contraception (LARC), specifically implants and intrauterine devices, are the most effective methods and should be encouraged for adolescents who desire birth control.
Progestin implants: Implanon and Nexplanon (Merck) are both implants containing the active metabolite of desogestrel, a progestin. Failure rates are less than 1% for the 3-year duration of the implant. Unpredictable bleeding or spotting is common, but implants are ideal for patients who desire an extended length of pregnancy prevention, without any schedule of adherence.
IUDs: These LARCs include two levonorgestrel-releasing IUDs (Mirena, 52 mg levonorgestrel and Skyla, 13.5 mg levonorgestrel, Bayer HealthCare Pharmaceuticals) and a copper-containing IUD (ParaGard, Teva ). All remain in place for 3-10 years, depending on brand, and have less than 1% failure rates. Known to be safe for use in nulliparous adolescents and patients with a previous episode of pelvic inflammatory disease (PID), STI screening in the asymptomatic patient can be performed on the day of IUD insertion. Infection can be treated while the IUD remains in place. Contraindications to an IUD are current, symptomatic PID or current, purulent cervicitis.
Progestin-only injectable contraception: Depot medroxyprogesterone acetate (DMPA or Depo-Provera, Pfizer) is a long-acting progestin given as a single intramuscular injection every 13 weeks. DMPA has a typical use failure rate of 6% in the first year and can be initiated on the same day of the visit if the patient is not pregnant. Bone density reduction seems to recover once DMPA is discontinued and bone density does not need to be measured repeatedly. However, individual risk for osteoporosis must be assessed, and all patients need to be counseled on adequate calcium and vitamin D intake.
Combined oral contraceptives (COCs): COCs all contain an estrogen and a progestin. A follow-up visit is advised in 1-3 months after starting COCs, and no gynecologic examination is necessary prior to COC use. COC can be started on the same day as the office visit in nonpregnant adolescents. The typical use failure rates are 9%, and adolescents should be educated on what to do when pills are missed. A serious adverse event associated with COC is up to 4/10,000 risk of thromboembolism, but the risk is up to 20/10,000 during pregnancy. Rifampin and antiviral and antiepileptic medications can decrease efficacy. Contraindications to COCs should be reviewed prior to prescribing.
Contraceptive vaginal ring: The vaginal ring (NuvaRing, Merck) has similar efficacy and side effects as the COCs, since it releases a combination of estrogen and progestin. Inserted and left in place for 3 weeks, it is then removed for 1 week to induce withdrawal bleeding.
Transdermal contraceptive patch: The patch has similar a similar profile as COCs and the vaginal ring. It is placed on the upper arm, torso, or abdomen, left in place for 3 weeks, and removed for 1 week. Users have estrogen exposure of 1.6 times that of COC users, so there is a potential for increased thromboembolism with patch use. Obese patients have a higher risk of pregnancy with perfect use.
Progestin-only pills: Progestin only pills work by thickening cervical mucus. Failure rates are elevated, due to the need for strict, timed dosing schedule. They provide an option for the patient who has concerns with estrogen use.
Male condom use: Condoms remain a cheap, easily accessible form of contraception, used by 53% of female and 75% of male adolescents studied. With an 18% failure rate with typical use when used alone, condom use should be additional to another effective hormonal or long-acting contraceptive.
Emergency contraception: Various hormonal options can be used up to 5 days after unprotected intercourse. Plan B One-Step is a nonprescription form available for all women of childbearing potential.
Withdrawal: 57% of female adolescents report using this method. A 22% failure rate and lack of STI protection is important to relay to the patient and more effective contraception methods should be encouraged.
The Bottom Line
The IUD and implant should be considered safe, first-line contraceptive choices for adolescents. Physicians should counsel adolescent patients on all available methods of contraception in a developmentally appropriate, confidential manner that falls within the limits of state and federal law. Condoms should always be encouraged for STI protection.
Reference: Contraception for Adolescents. Pediatrics 2014;134:e1244-e56
Dr. Skolnik is associate director of the family medicine residency program at Abington Memorial Hospital and professor of family and community medicine at Temple University in Philadelphia. Dr. Roesing is an assistant director in the Family Medicine Residency Program at Abington Memorial Hospital.
Approximately 750,000 adolescents become pregnant each year in the United States and nearly half of high school students report having had sexual intercourse. More than 80% of teenage pregnancies are unplanned and result in substantial health care costs, reduced earning potential, and increased health risks to both the mother and newborn. The American Academy of Pediatrics has released an updated policy statement that endorses long-acting reversible contraception (LARC) as a first-line consideration for adolescent contraception.
Sexual history taking and counseling
The American Academy of Pediatrics (AAP) encourages the 5 P’s of sexual history taking: partners, prevention of pregnancy, protection from sexually transmitted infections (STIs), sexual practices, past history of STIs, and pregnancy. Confidentiality is advised for issues revolving around sexuality and sexually transmitted infections. Most states have legislation regarding minor consent for contraception, details of which can be found at the Guttmacher Institute. The adolescent should be encouraged to delay onset of sexual activity until they are ready, but abstinence should not be the only focus of contraception counseling, and adolescents should be supported in choosing, and adhering to, a method of contraception if they choose to be sexually active.
Methods of contraception
The updated AAP policy statement advises that physicians offer methods of contraception by discussing methods that are more effective preventing pregnancy as preferred over methods that are less effective. The effectiveness of all contraceptive methods is described by typical user rates and average user rates, expressed as the percent of women who become pregnant using the method for 1 year. The gap between perfect and typical user rates is explained by the amount of effort that is needed to reliably use the method. Typical user rates should guide decisions about contraceptive efficacy for adolescents. Long-acting reversible contraception (LARC), specifically implants and intrauterine devices, are the most effective methods and should be encouraged for adolescents who desire birth control.
Progestin implants: Implanon and Nexplanon (Merck) are both implants containing the active metabolite of desogestrel, a progestin. Failure rates are less than 1% for the 3-year duration of the implant. Unpredictable bleeding or spotting is common, but implants are ideal for patients who desire an extended length of pregnancy prevention, without any schedule of adherence.
IUDs: These LARCs include two levonorgestrel-releasing IUDs (Mirena, 52 mg levonorgestrel and Skyla, 13.5 mg levonorgestrel, Bayer HealthCare Pharmaceuticals) and a copper-containing IUD (ParaGard, Teva ). All remain in place for 3-10 years, depending on brand, and have less than 1% failure rates. Known to be safe for use in nulliparous adolescents and patients with a previous episode of pelvic inflammatory disease (PID), STI screening in the asymptomatic patient can be performed on the day of IUD insertion. Infection can be treated while the IUD remains in place. Contraindications to an IUD are current, symptomatic PID or current, purulent cervicitis.
Progestin-only injectable contraception: Depot medroxyprogesterone acetate (DMPA or Depo-Provera, Pfizer) is a long-acting progestin given as a single intramuscular injection every 13 weeks. DMPA has a typical use failure rate of 6% in the first year and can be initiated on the same day of the visit if the patient is not pregnant. Bone density reduction seems to recover once DMPA is discontinued and bone density does not need to be measured repeatedly. However, individual risk for osteoporosis must be assessed, and all patients need to be counseled on adequate calcium and vitamin D intake.
Combined oral contraceptives (COCs): COCs all contain an estrogen and a progestin. A follow-up visit is advised in 1-3 months after starting COCs, and no gynecologic examination is necessary prior to COC use. COC can be started on the same day as the office visit in nonpregnant adolescents. The typical use failure rates are 9%, and adolescents should be educated on what to do when pills are missed. A serious adverse event associated with COC is up to 4/10,000 risk of thromboembolism, but the risk is up to 20/10,000 during pregnancy. Rifampin and antiviral and antiepileptic medications can decrease efficacy. Contraindications to COCs should be reviewed prior to prescribing.
Contraceptive vaginal ring: The vaginal ring (NuvaRing, Merck) has similar efficacy and side effects as the COCs, since it releases a combination of estrogen and progestin. Inserted and left in place for 3 weeks, it is then removed for 1 week to induce withdrawal bleeding.
Transdermal contraceptive patch: The patch has similar a similar profile as COCs and the vaginal ring. It is placed on the upper arm, torso, or abdomen, left in place for 3 weeks, and removed for 1 week. Users have estrogen exposure of 1.6 times that of COC users, so there is a potential for increased thromboembolism with patch use. Obese patients have a higher risk of pregnancy with perfect use.
Progestin-only pills: Progestin only pills work by thickening cervical mucus. Failure rates are elevated, due to the need for strict, timed dosing schedule. They provide an option for the patient who has concerns with estrogen use.
Male condom use: Condoms remain a cheap, easily accessible form of contraception, used by 53% of female and 75% of male adolescents studied. With an 18% failure rate with typical use when used alone, condom use should be additional to another effective hormonal or long-acting contraceptive.
Emergency contraception: Various hormonal options can be used up to 5 days after unprotected intercourse. Plan B One-Step is a nonprescription form available for all women of childbearing potential.
Withdrawal: 57% of female adolescents report using this method. A 22% failure rate and lack of STI protection is important to relay to the patient and more effective contraception methods should be encouraged.
The Bottom Line
The IUD and implant should be considered safe, first-line contraceptive choices for adolescents. Physicians should counsel adolescent patients on all available methods of contraception in a developmentally appropriate, confidential manner that falls within the limits of state and federal law. Condoms should always be encouraged for STI protection.
Reference: Contraception for Adolescents. Pediatrics 2014;134:e1244-e56
Dr. Skolnik is associate director of the family medicine residency program at Abington Memorial Hospital and professor of family and community medicine at Temple University in Philadelphia. Dr. Roesing is an assistant director in the Family Medicine Residency Program at Abington Memorial Hospital.
Approximately 750,000 adolescents become pregnant each year in the United States and nearly half of high school students report having had sexual intercourse. More than 80% of teenage pregnancies are unplanned and result in substantial health care costs, reduced earning potential, and increased health risks to both the mother and newborn. The American Academy of Pediatrics has released an updated policy statement that endorses long-acting reversible contraception (LARC) as a first-line consideration for adolescent contraception.
Sexual history taking and counseling
The American Academy of Pediatrics (AAP) encourages the 5 P’s of sexual history taking: partners, prevention of pregnancy, protection from sexually transmitted infections (STIs), sexual practices, past history of STIs, and pregnancy. Confidentiality is advised for issues revolving around sexuality and sexually transmitted infections. Most states have legislation regarding minor consent for contraception, details of which can be found at the Guttmacher Institute. The adolescent should be encouraged to delay onset of sexual activity until they are ready, but abstinence should not be the only focus of contraception counseling, and adolescents should be supported in choosing, and adhering to, a method of contraception if they choose to be sexually active.
Methods of contraception
The updated AAP policy statement advises that physicians offer methods of contraception by discussing methods that are more effective preventing pregnancy as preferred over methods that are less effective. The effectiveness of all contraceptive methods is described by typical user rates and average user rates, expressed as the percent of women who become pregnant using the method for 1 year. The gap between perfect and typical user rates is explained by the amount of effort that is needed to reliably use the method. Typical user rates should guide decisions about contraceptive efficacy for adolescents. Long-acting reversible contraception (LARC), specifically implants and intrauterine devices, are the most effective methods and should be encouraged for adolescents who desire birth control.
Progestin implants: Implanon and Nexplanon (Merck) are both implants containing the active metabolite of desogestrel, a progestin. Failure rates are less than 1% for the 3-year duration of the implant. Unpredictable bleeding or spotting is common, but implants are ideal for patients who desire an extended length of pregnancy prevention, without any schedule of adherence.
IUDs: These LARCs include two levonorgestrel-releasing IUDs (Mirena, 52 mg levonorgestrel and Skyla, 13.5 mg levonorgestrel, Bayer HealthCare Pharmaceuticals) and a copper-containing IUD (ParaGard, Teva ). All remain in place for 3-10 years, depending on brand, and have less than 1% failure rates. Known to be safe for use in nulliparous adolescents and patients with a previous episode of pelvic inflammatory disease (PID), STI screening in the asymptomatic patient can be performed on the day of IUD insertion. Infection can be treated while the IUD remains in place. Contraindications to an IUD are current, symptomatic PID or current, purulent cervicitis.
Progestin-only injectable contraception: Depot medroxyprogesterone acetate (DMPA or Depo-Provera, Pfizer) is a long-acting progestin given as a single intramuscular injection every 13 weeks. DMPA has a typical use failure rate of 6% in the first year and can be initiated on the same day of the visit if the patient is not pregnant. Bone density reduction seems to recover once DMPA is discontinued and bone density does not need to be measured repeatedly. However, individual risk for osteoporosis must be assessed, and all patients need to be counseled on adequate calcium and vitamin D intake.
Combined oral contraceptives (COCs): COCs all contain an estrogen and a progestin. A follow-up visit is advised in 1-3 months after starting COCs, and no gynecologic examination is necessary prior to COC use. COC can be started on the same day as the office visit in nonpregnant adolescents. The typical use failure rates are 9%, and adolescents should be educated on what to do when pills are missed. A serious adverse event associated with COC is up to 4/10,000 risk of thromboembolism, but the risk is up to 20/10,000 during pregnancy. Rifampin and antiviral and antiepileptic medications can decrease efficacy. Contraindications to COCs should be reviewed prior to prescribing.
Contraceptive vaginal ring: The vaginal ring (NuvaRing, Merck) has similar efficacy and side effects as the COCs, since it releases a combination of estrogen and progestin. Inserted and left in place for 3 weeks, it is then removed for 1 week to induce withdrawal bleeding.
Transdermal contraceptive patch: The patch has similar a similar profile as COCs and the vaginal ring. It is placed on the upper arm, torso, or abdomen, left in place for 3 weeks, and removed for 1 week. Users have estrogen exposure of 1.6 times that of COC users, so there is a potential for increased thromboembolism with patch use. Obese patients have a higher risk of pregnancy with perfect use.
Progestin-only pills: Progestin only pills work by thickening cervical mucus. Failure rates are elevated, due to the need for strict, timed dosing schedule. They provide an option for the patient who has concerns with estrogen use.
Male condom use: Condoms remain a cheap, easily accessible form of contraception, used by 53% of female and 75% of male adolescents studied. With an 18% failure rate with typical use when used alone, condom use should be additional to another effective hormonal or long-acting contraceptive.
Emergency contraception: Various hormonal options can be used up to 5 days after unprotected intercourse. Plan B One-Step is a nonprescription form available for all women of childbearing potential.
Withdrawal: 57% of female adolescents report using this method. A 22% failure rate and lack of STI protection is important to relay to the patient and more effective contraception methods should be encouraged.
The Bottom Line
The IUD and implant should be considered safe, first-line contraceptive choices for adolescents. Physicians should counsel adolescent patients on all available methods of contraception in a developmentally appropriate, confidential manner that falls within the limits of state and federal law. Condoms should always be encouraged for STI protection.
Reference: Contraception for Adolescents. Pediatrics 2014;134:e1244-e56
Dr. Skolnik is associate director of the family medicine residency program at Abington Memorial Hospital and professor of family and community medicine at Temple University in Philadelphia. Dr. Roesing is an assistant director in the Family Medicine Residency Program at Abington Memorial Hospital.
Clinical Guidelines: Obstructive sleep apnea
The Greek word apnea literally translates to “without breath.” More than 18 million American adults experience several moments “without breath” every night, according to the National Sleep Foundation. These pauses in breathing can last from a few seconds to minutes and can occur up to 30 times per hour or more. There are three types of apnea: obstructive, central, and mixed. Of the three, obstructive sleep apnea (OSA) is the most common. If left untreated, sleep apnea can have serious or life-threatening consequences, such as high blood pressure, heart disease, and day-time sleepiness that can lead to car accidents, depression, and headaches.
The significance of this condition has led the American College of Physicians (ACP) to publish guidelines regarding the management of OSA in adults. These guidelines are intended to provide clinicians with evidence-based recommendations that will have positive, long-term effects on patients’ cardiovascular risk, as well overall health and quality of life.
Recommendation 1: Encourage weight loss in all overweight and obese patients diagnosed with OSA.
There is strong evidence that shows how weight loss interventions can reduce the apnea/hypopnea index (AHI) and improve symptoms. The apnea/hypopnea index is a measure of the number of apnea and hypopnea episodes per hour of monitored sleep. According to the American Academy of Sleep Medicine, an OSA diagnosis is defined by ≥ 15 events/hr (with or without OSA symptoms) or ≥ 5 events/hr with OSA symptoms. Severity of OSA is classified as:
• Mild: 5-14 events/hr
• Moderate: 15-30 events/hr
• Severe: >30 events/hr
In patients with mild OSA, weight loss alone can be sufficient to normalize the apnea/hypopnea index, thereby reducing the need for continuous positive airway pressure (CPAP). In those with persistent OSA, weight loss can reduce the amount of PAP pressure required, which can increase tolerance of and adherence to CPAP. However, weight loss alone may not be sufficient to reduce OSA in all patients. In obese patients, weight loss must be encouraged with another primary treatment. In a meta-analysis of 342 patients in 12 published trials, weight loss produced substantial reductions in apnea/hypopnea index; however, the majority of patients continued to have persistent OSA after significant weight reduction. So, while weight loss should always be encouraged in patients with OSA, it should not be assumed that this intervention alone will be sufficient; patients need to be reassessed to determine whether OSA persists and if so, CPAP should be continued.
Recommendation 2: Prescribe CPAP as the initial therapy for patients diagnosed with OSA.
CPAP is as critical as it is effective not only in reducing the AHI but also in improving sleep continuity and architecture and decreasing the sleep hypoxia that is associated with OSA. Because of the lack of adherence to CPAP, many new features have been added including heated humidification, broad pressure adjustments, and expiratory pressure relief; while many of these features mildly improve patients’ preferences, there is no evidence to suggest that these changes improve efficacy or adherence. Again, it is critical that each patient’s needs be taken into consideration. Some patients, particularly those with mild forms of OSA, may not need CPAP and instead, may benefit from positional therapy or weight loss. Other therapy such as surgery or mandibular advancement devices (MADs) may be better suited for some patients despite their lessor effectiveness when compared with CPAP.
Recommendation 3: Consider MAD as an alternate therapy to CPAP for patients diagnosed with OSA who prefer MAD or for those with adverse effects associated with CPAP.
MADs have been recommended for patients with moderate to severe OSA, those with apnea/hypopnea index values between 18-40 events/hour, and for individuals who experienced adverse events on CPAP or who can’t tolerate using it. While CPAP still is considered to be primary therapy, for those who must use a mandibular advancement device, it can often be very effective. Patients may find it easier to be compliant with MAD than with CPAP. One group of researchers observed that MADs were able to provide appropriate decrease in obstructive events in 70% of patients with mild OSA, 48% of those with moderate OSA, and 42% of those with severe OSA (Chest 2011:139:1331-9). The ACP concluded that, without sufficient evidence, it is unclear which patients will benefit from MADs. Data from several studies show that patients who are younger and thinner, with less severe OSA, may benefit more from the use of MADs. Although the ACP does not recommend surgery or pharmaceutical therapy for OSA, the ACP does acknowledge that it may work for certain patients. However, the documented efficacy rate ranges from 20%-100%, thereby making it challenging to determine its true effect.
Bottom Line
The ACP recommends weight loss for all overweight patients with OSA. CPAP is first-line therapy, particularly if weight loss cannot be sustained or does not sufficiently lessen symptoms. For selected patients who are younger and thinner, with less severe OSA, or for those patients who are unable to tolerate CPAP, MADs can be used as an alternative first-line therapy with moderate success.
Reference
Qaseem A., Holty J.C., Owens D. et al. Diagnosis of obstructive sleep apnea in adults: a Clinical Practice Guideline from the American College of Physicians. Ann. Intern. Med. 2014;161:210-20.
Dr. Grover is a second-year resident in the family medicine residency program at Abington Memorial Hospital. Dr. Skolnik is associate director of the family medicine residency program at Abington Memorial Hospital and professor of family and community medicine at Temple University in Philadelphia.
The Greek word apnea literally translates to “without breath.” More than 18 million American adults experience several moments “without breath” every night, according to the National Sleep Foundation. These pauses in breathing can last from a few seconds to minutes and can occur up to 30 times per hour or more. There are three types of apnea: obstructive, central, and mixed. Of the three, obstructive sleep apnea (OSA) is the most common. If left untreated, sleep apnea can have serious or life-threatening consequences, such as high blood pressure, heart disease, and day-time sleepiness that can lead to car accidents, depression, and headaches.
The significance of this condition has led the American College of Physicians (ACP) to publish guidelines regarding the management of OSA in adults. These guidelines are intended to provide clinicians with evidence-based recommendations that will have positive, long-term effects on patients’ cardiovascular risk, as well overall health and quality of life.
Recommendation 1: Encourage weight loss in all overweight and obese patients diagnosed with OSA.
There is strong evidence that shows how weight loss interventions can reduce the apnea/hypopnea index (AHI) and improve symptoms. The apnea/hypopnea index is a measure of the number of apnea and hypopnea episodes per hour of monitored sleep. According to the American Academy of Sleep Medicine, an OSA diagnosis is defined by ≥ 15 events/hr (with or without OSA symptoms) or ≥ 5 events/hr with OSA symptoms. Severity of OSA is classified as:
• Mild: 5-14 events/hr
• Moderate: 15-30 events/hr
• Severe: >30 events/hr
In patients with mild OSA, weight loss alone can be sufficient to normalize the apnea/hypopnea index, thereby reducing the need for continuous positive airway pressure (CPAP). In those with persistent OSA, weight loss can reduce the amount of PAP pressure required, which can increase tolerance of and adherence to CPAP. However, weight loss alone may not be sufficient to reduce OSA in all patients. In obese patients, weight loss must be encouraged with another primary treatment. In a meta-analysis of 342 patients in 12 published trials, weight loss produced substantial reductions in apnea/hypopnea index; however, the majority of patients continued to have persistent OSA after significant weight reduction. So, while weight loss should always be encouraged in patients with OSA, it should not be assumed that this intervention alone will be sufficient; patients need to be reassessed to determine whether OSA persists and if so, CPAP should be continued.
Recommendation 2: Prescribe CPAP as the initial therapy for patients diagnosed with OSA.
CPAP is as critical as it is effective not only in reducing the AHI but also in improving sleep continuity and architecture and decreasing the sleep hypoxia that is associated with OSA. Because of the lack of adherence to CPAP, many new features have been added including heated humidification, broad pressure adjustments, and expiratory pressure relief; while many of these features mildly improve patients’ preferences, there is no evidence to suggest that these changes improve efficacy or adherence. Again, it is critical that each patient’s needs be taken into consideration. Some patients, particularly those with mild forms of OSA, may not need CPAP and instead, may benefit from positional therapy or weight loss. Other therapy such as surgery or mandibular advancement devices (MADs) may be better suited for some patients despite their lessor effectiveness when compared with CPAP.
Recommendation 3: Consider MAD as an alternate therapy to CPAP for patients diagnosed with OSA who prefer MAD or for those with adverse effects associated with CPAP.
MADs have been recommended for patients with moderate to severe OSA, those with apnea/hypopnea index values between 18-40 events/hour, and for individuals who experienced adverse events on CPAP or who can’t tolerate using it. While CPAP still is considered to be primary therapy, for those who must use a mandibular advancement device, it can often be very effective. Patients may find it easier to be compliant with MAD than with CPAP. One group of researchers observed that MADs were able to provide appropriate decrease in obstructive events in 70% of patients with mild OSA, 48% of those with moderate OSA, and 42% of those with severe OSA (Chest 2011:139:1331-9). The ACP concluded that, without sufficient evidence, it is unclear which patients will benefit from MADs. Data from several studies show that patients who are younger and thinner, with less severe OSA, may benefit more from the use of MADs. Although the ACP does not recommend surgery or pharmaceutical therapy for OSA, the ACP does acknowledge that it may work for certain patients. However, the documented efficacy rate ranges from 20%-100%, thereby making it challenging to determine its true effect.
Bottom Line
The ACP recommends weight loss for all overweight patients with OSA. CPAP is first-line therapy, particularly if weight loss cannot be sustained or does not sufficiently lessen symptoms. For selected patients who are younger and thinner, with less severe OSA, or for those patients who are unable to tolerate CPAP, MADs can be used as an alternative first-line therapy with moderate success.
Reference
Qaseem A., Holty J.C., Owens D. et al. Diagnosis of obstructive sleep apnea in adults: a Clinical Practice Guideline from the American College of Physicians. Ann. Intern. Med. 2014;161:210-20.
Dr. Grover is a second-year resident in the family medicine residency program at Abington Memorial Hospital. Dr. Skolnik is associate director of the family medicine residency program at Abington Memorial Hospital and professor of family and community medicine at Temple University in Philadelphia.
The Greek word apnea literally translates to “without breath.” More than 18 million American adults experience several moments “without breath” every night, according to the National Sleep Foundation. These pauses in breathing can last from a few seconds to minutes and can occur up to 30 times per hour or more. There are three types of apnea: obstructive, central, and mixed. Of the three, obstructive sleep apnea (OSA) is the most common. If left untreated, sleep apnea can have serious or life-threatening consequences, such as high blood pressure, heart disease, and day-time sleepiness that can lead to car accidents, depression, and headaches.
The significance of this condition has led the American College of Physicians (ACP) to publish guidelines regarding the management of OSA in adults. These guidelines are intended to provide clinicians with evidence-based recommendations that will have positive, long-term effects on patients’ cardiovascular risk, as well overall health and quality of life.
Recommendation 1: Encourage weight loss in all overweight and obese patients diagnosed with OSA.
There is strong evidence that shows how weight loss interventions can reduce the apnea/hypopnea index (AHI) and improve symptoms. The apnea/hypopnea index is a measure of the number of apnea and hypopnea episodes per hour of monitored sleep. According to the American Academy of Sleep Medicine, an OSA diagnosis is defined by ≥ 15 events/hr (with or without OSA symptoms) or ≥ 5 events/hr with OSA symptoms. Severity of OSA is classified as:
• Mild: 5-14 events/hr
• Moderate: 15-30 events/hr
• Severe: >30 events/hr
In patients with mild OSA, weight loss alone can be sufficient to normalize the apnea/hypopnea index, thereby reducing the need for continuous positive airway pressure (CPAP). In those with persistent OSA, weight loss can reduce the amount of PAP pressure required, which can increase tolerance of and adherence to CPAP. However, weight loss alone may not be sufficient to reduce OSA in all patients. In obese patients, weight loss must be encouraged with another primary treatment. In a meta-analysis of 342 patients in 12 published trials, weight loss produced substantial reductions in apnea/hypopnea index; however, the majority of patients continued to have persistent OSA after significant weight reduction. So, while weight loss should always be encouraged in patients with OSA, it should not be assumed that this intervention alone will be sufficient; patients need to be reassessed to determine whether OSA persists and if so, CPAP should be continued.
Recommendation 2: Prescribe CPAP as the initial therapy for patients diagnosed with OSA.
CPAP is as critical as it is effective not only in reducing the AHI but also in improving sleep continuity and architecture and decreasing the sleep hypoxia that is associated with OSA. Because of the lack of adherence to CPAP, many new features have been added including heated humidification, broad pressure adjustments, and expiratory pressure relief; while many of these features mildly improve patients’ preferences, there is no evidence to suggest that these changes improve efficacy or adherence. Again, it is critical that each patient’s needs be taken into consideration. Some patients, particularly those with mild forms of OSA, may not need CPAP and instead, may benefit from positional therapy or weight loss. Other therapy such as surgery or mandibular advancement devices (MADs) may be better suited for some patients despite their lessor effectiveness when compared with CPAP.
Recommendation 3: Consider MAD as an alternate therapy to CPAP for patients diagnosed with OSA who prefer MAD or for those with adverse effects associated with CPAP.
MADs have been recommended for patients with moderate to severe OSA, those with apnea/hypopnea index values between 18-40 events/hour, and for individuals who experienced adverse events on CPAP or who can’t tolerate using it. While CPAP still is considered to be primary therapy, for those who must use a mandibular advancement device, it can often be very effective. Patients may find it easier to be compliant with MAD than with CPAP. One group of researchers observed that MADs were able to provide appropriate decrease in obstructive events in 70% of patients with mild OSA, 48% of those with moderate OSA, and 42% of those with severe OSA (Chest 2011:139:1331-9). The ACP concluded that, without sufficient evidence, it is unclear which patients will benefit from MADs. Data from several studies show that patients who are younger and thinner, with less severe OSA, may benefit more from the use of MADs. Although the ACP does not recommend surgery or pharmaceutical therapy for OSA, the ACP does acknowledge that it may work for certain patients. However, the documented efficacy rate ranges from 20%-100%, thereby making it challenging to determine its true effect.
Bottom Line
The ACP recommends weight loss for all overweight patients with OSA. CPAP is first-line therapy, particularly if weight loss cannot be sustained or does not sufficiently lessen symptoms. For selected patients who are younger and thinner, with less severe OSA, or for those patients who are unable to tolerate CPAP, MADs can be used as an alternative first-line therapy with moderate success.
Reference
Qaseem A., Holty J.C., Owens D. et al. Diagnosis of obstructive sleep apnea in adults: a Clinical Practice Guideline from the American College of Physicians. Ann. Intern. Med. 2014;161:210-20.
Dr. Grover is a second-year resident in the family medicine residency program at Abington Memorial Hospital. Dr. Skolnik is associate director of the family medicine residency program at Abington Memorial Hospital and professor of family and community medicine at Temple University in Philadelphia.
Meaningful use – Stage 2 (Part 1 of 2)
The words "meaningful use" have been making providers cringe for more than 2 years now. Those clinicians who worked hard to demonstrate meaningful use under the stage 1 requirements now must go on to demonstrate meaningful use under the stage 2 requirements. We recently heard one of our colleagues describe stage 2 of meaningful use as reminiscent of the 1978 movie "Jaws 2," the ads for which ran with the tagline: "Just when you thought it was safe to go back in the water..."
As you may be aware, on August 29th the Department of Health and Human Services published a final rule allowing certain eligible providers the flexibility to continue using the Stage 1 criteria for the 2014 attestation year, even if they were due to start Stage 2. This only applies to those who have been unable to obtain the 2014-certified software in time due to vendor delays. Unfortunately, this flexibility does not extend to those who can’t meet Stage 2 due to measure difficulty or procrastination in purchasing software or adopting new workflows (we recommend speaking with a meaningful use expert or consultant before attempting to take advantage of this flexibility). Regardless of stage or year, everyone is on a 90-day reporting period for 2014, but remember that 2015 will require a full year of reporting (January through December). So even if you qualify for the flexibility and opt to stick with the Stage 1 measures, you’ll need to be ready to hit the ground running with Stage 2 as soon as the ball drops on January 1st, 2015.
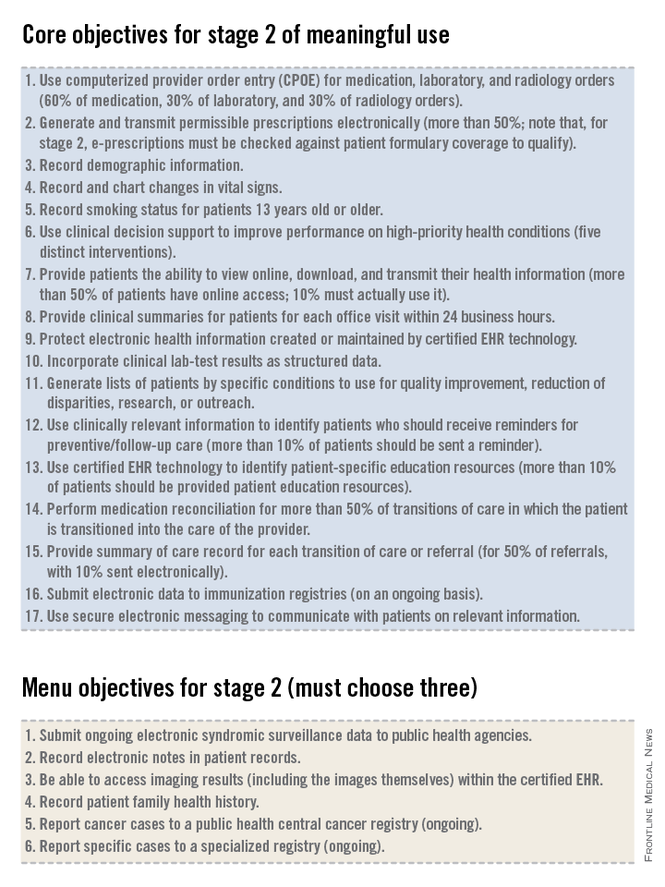
The government’s intent with the EHR incentive program is to ensure that practitioners use an EHR to do more than what could otherwise be done on a paper note. As we review the criteria that must be met for stage 2 of meaningful use, we will see the inclusion of menu items and quality measures that are aimed at enhancing actionable decision support to improve the quality of medical care, population management (even for patients who might not come in to the office), and physician-patient communication. By articulating these goals we can see that they are very different from what most practitioners perceive to be the main outcome of the meaningful use rules: the creation of a lot of unnecessary busywork in the office that yields very little benefit for practitioners or patients.
The EHR incentive program consists of three stages.
• Stage 1, which many practitioners have already accomplished and received incentive dollars for completing, focused on basic data capture.
• Stage 2, which focuses on more advanced processes including additional requirements for e-prescribing, incorporating lab results into the record, electronic transmission of patient summaries across systems, and increased patient engagement.
• Stage 3, which will utilize the processes put in place in the first two stages and focus on improved patient outcomes.
For stage 2 of meaningful use, clinicians must meet or exceed the thresholds for the 17 core objectives and 3 menu objectives, as well as report on defined Clinical Quality Measures (CQMs). Many of the objectives in stage 2 are the same as those from stage 1. Some objectives that were in the set of choices in stage 1 are now part of the mandatory core set for stage 2, required for all providers. Some objectives that were in the core set in stage 1 now have higher thresholds or percentages of patients that must meet the criteria in order to qualify for meaningful use in stage 2. The data reported to the Centers for Medicare & Medicaid Services for CQMs must originate from an EHR that has been certified for 2014 standards. This rule requires that clinicians upgrade their EHR to current technology standards, a rule that is good for EHR vendors, makes sense when we look at the system as a whole, but may be very expensive for many practitioners.
In addition to the 17 core objectives, and 3 out of 6 menu objectives, clinicians will need to report on nine CQMs. We will review the details of reporting on CQMs in next month in part 2 of our overview of Meaningful Use Stage 2.
Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. Dr. Skolnik is associate director of the family medicine residency program at Abington Memorial Hospital and professor of family and community medicine at Temple University in Philadelphia.
The words "meaningful use" have been making providers cringe for more than 2 years now. Those clinicians who worked hard to demonstrate meaningful use under the stage 1 requirements now must go on to demonstrate meaningful use under the stage 2 requirements. We recently heard one of our colleagues describe stage 2 of meaningful use as reminiscent of the 1978 movie "Jaws 2," the ads for which ran with the tagline: "Just when you thought it was safe to go back in the water..."

As you may be aware, on August 29th the Department of Health and Human Services published a final rule allowing certain eligible providers the flexibility to continue using the Stage 1 criteria for the 2014 attestation year, even if they were due to start Stage 2. This only applies to those who have been unable to obtain the 2014-certified software in time due to vendor delays. Unfortunately, this flexibility does not extend to those who can’t meet Stage 2 due to measure difficulty or procrastination in purchasing software or adopting new workflows (we recommend speaking with a meaningful use expert or consultant before attempting to take advantage of this flexibility). Regardless of stage or year, everyone is on a 90-day reporting period for 2014, but remember that 2015 will require a full year of reporting (January through December). So even if you qualify for the flexibility and opt to stick with the Stage 1 measures, you’ll need to be ready to hit the ground running with Stage 2 as soon as the ball drops on January 1st, 2015.
The government’s intent with the EHR incentive program is to ensure that practitioners use an EHR to do more than what could otherwise be done on a paper note. As we review the criteria that must be met for stage 2 of meaningful use, we will see the inclusion of menu items and quality measures that are aimed at enhancing actionable decision support to improve the quality of medical care, population management (even for patients who might not come in to the office), and physician-patient communication. By articulating these goals we can see that they are very different from what most practitioners perceive to be the main outcome of the meaningful use rules: the creation of a lot of unnecessary busywork in the office that yields very little benefit for practitioners or patients.
The EHR incentive program consists of three stages.
• Stage 1, which many practitioners have already accomplished and received incentive dollars for completing, focused on basic data capture.
• Stage 2, which focuses on more advanced processes including additional requirements for e-prescribing, incorporating lab results into the record, electronic transmission of patient summaries across systems, and increased patient engagement.
• Stage 3, which will utilize the processes put in place in the first two stages and focus on improved patient outcomes.
For stage 2 of meaningful use, clinicians must meet or exceed the thresholds for the 17 core objectives and 3 menu objectives, as well as report on defined Clinical Quality Measures (CQMs). Many of the objectives in stage 2 are the same as those from stage 1. Some objectives that were in the set of choices in stage 1 are now part of the mandatory core set for stage 2, required for all providers. Some objectives that were in the core set in stage 1 now have higher thresholds or percentages of patients that must meet the criteria in order to qualify for meaningful use in stage 2. The data reported to the Centers for Medicare & Medicaid Services for CQMs must originate from an EHR that has been certified for 2014 standards. This rule requires that clinicians upgrade their EHR to current technology standards, a rule that is good for EHR vendors, makes sense when we look at the system as a whole, but may be very expensive for many practitioners.
In addition to the 17 core objectives, and 3 out of 6 menu objectives, clinicians will need to report on nine CQMs. We will review the details of reporting on CQMs in next month in part 2 of our overview of Meaningful Use Stage 2.
Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. Dr. Skolnik is associate director of the family medicine residency program at Abington Memorial Hospital and professor of family and community medicine at Temple University in Philadelphia.
The words "meaningful use" have been making providers cringe for more than 2 years now. Those clinicians who worked hard to demonstrate meaningful use under the stage 1 requirements now must go on to demonstrate meaningful use under the stage 2 requirements. We recently heard one of our colleagues describe stage 2 of meaningful use as reminiscent of the 1978 movie "Jaws 2," the ads for which ran with the tagline: "Just when you thought it was safe to go back in the water..."

As you may be aware, on August 29th the Department of Health and Human Services published a final rule allowing certain eligible providers the flexibility to continue using the Stage 1 criteria for the 2014 attestation year, even if they were due to start Stage 2. This only applies to those who have been unable to obtain the 2014-certified software in time due to vendor delays. Unfortunately, this flexibility does not extend to those who can’t meet Stage 2 due to measure difficulty or procrastination in purchasing software or adopting new workflows (we recommend speaking with a meaningful use expert or consultant before attempting to take advantage of this flexibility). Regardless of stage or year, everyone is on a 90-day reporting period for 2014, but remember that 2015 will require a full year of reporting (January through December). So even if you qualify for the flexibility and opt to stick with the Stage 1 measures, you’ll need to be ready to hit the ground running with Stage 2 as soon as the ball drops on January 1st, 2015.
The government’s intent with the EHR incentive program is to ensure that practitioners use an EHR to do more than what could otherwise be done on a paper note. As we review the criteria that must be met for stage 2 of meaningful use, we will see the inclusion of menu items and quality measures that are aimed at enhancing actionable decision support to improve the quality of medical care, population management (even for patients who might not come in to the office), and physician-patient communication. By articulating these goals we can see that they are very different from what most practitioners perceive to be the main outcome of the meaningful use rules: the creation of a lot of unnecessary busywork in the office that yields very little benefit for practitioners or patients.
The EHR incentive program consists of three stages.
• Stage 1, which many practitioners have already accomplished and received incentive dollars for completing, focused on basic data capture.
• Stage 2, which focuses on more advanced processes including additional requirements for e-prescribing, incorporating lab results into the record, electronic transmission of patient summaries across systems, and increased patient engagement.
• Stage 3, which will utilize the processes put in place in the first two stages and focus on improved patient outcomes.
For stage 2 of meaningful use, clinicians must meet or exceed the thresholds for the 17 core objectives and 3 menu objectives, as well as report on defined Clinical Quality Measures (CQMs). Many of the objectives in stage 2 are the same as those from stage 1. Some objectives that were in the set of choices in stage 1 are now part of the mandatory core set for stage 2, required for all providers. Some objectives that were in the core set in stage 1 now have higher thresholds or percentages of patients that must meet the criteria in order to qualify for meaningful use in stage 2. The data reported to the Centers for Medicare & Medicaid Services for CQMs must originate from an EHR that has been certified for 2014 standards. This rule requires that clinicians upgrade their EHR to current technology standards, a rule that is good for EHR vendors, makes sense when we look at the system as a whole, but may be very expensive for many practitioners.
In addition to the 17 core objectives, and 3 out of 6 menu objectives, clinicians will need to report on nine CQMs. We will review the details of reporting on CQMs in next month in part 2 of our overview of Meaningful Use Stage 2.
Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. Dr. Skolnik is associate director of the family medicine residency program at Abington Memorial Hospital and professor of family and community medicine at Temple University in Philadelphia.
Management of atrial fibrillation
Atrial fibrillation is a common disorder with a lifetime prevalence of approximately 10%-15%. Risk factors include advancing age, diabetes, myocardial infarction, valvular disease, smoking, hypertension, obstructive sleep apnea, hyperthyroidism, alcohol abuse, illicit drug use, and obesity.
The diagnosis of AF should be based on the patient’s history and physical exam, including an ECG and lab work to evaluate thyroid, renal, and hepatic function, as well as a blood count. Further testing should be individualized and may include exercise testing, Holter monitoring, transesophageal echocardiogram (TEE), electrophysiology study, sleep study, or chest x-ray.
An important consideration in the approach to AF is prevention of thromboembolic events; multiple scoring systems have been developed to help stratify risk. The guideline recommends using the newer CHA2DS2-VASc scoring system, rather than the older CHADS2 scoring system, for patients with nonvalvular AF. Points are awarded as follows: congestive heart failure, 1; hypertension, 1; age of 75 years or older, 2; diabetes mellitus, 1; stroke/transient ischemic attack, 2; vascular disease (myocardial infarction, peripheral arterial disease, or aortic plaque), 1; age 65-74 years, 1; sex category (female), 1; for a maximum of 9 points. The CHA2DS2-VASc system reflects the increased risk attributed to age of 75 years or older, vascular disease, and female sex not accounted for in the CHADS2 system, and it is better than CHADS2 at discriminating among low-risk individuals. The annual risk of stroke ranges from less than 1% for a score of 0, to greater than 15% with a score of 9.
In patients with nonvalvular AF with a CHA2DS2-VASc score of 0, it is reasonable to forego antithrombotic therapy altogether. For patients with nonvalvular AF and a CHA2DS2-VASc score of 1, it is reasonable to choose either no anticoagulation therapy or use of daily aspirin or an oral anticoagulant. For a CHA2DS2-VASc score of 2, using an oral anticoagulant is recommended, either warfarin or a newer agent – dabigatran, rivaroxaban, or apixaban. For patients who have mechanical heart valves, warfarin is the only recommended choice for anticoagulation, with dabigatran specifically noted as causing harm.
Prevention of thromboembolism is just one of the treatment goals in AF. Affected patients generally require either rate- or rhythm-control therapy. Choosing which treatment to pursue depends on multiple individualized factors. The guideline points out that over time, attempting to maintain sinus rhythm results in more hospitalizations than does therapy focused on rate control, with no decrease in mortality. The guidelines state: "Routine use of a rhythm-control strategy is not warranted for some patients." Persistent symptoms are the most important indication for a rhythm-control strategy.
If a rate-control approach is chosen, the guideline recommends choosing a beta-blocker or nondihydropyridine calcium channel blocker as first-line therapy unless contraindicated, regardless of whether the AF is paroxysmal, persistent, or permanent. The dose of these medications should be titrated according to resting heart rate. Previously a target of fewer than 80 bpm was recommended; however, findings from the RACE-II (Rate Control Efficacy in Permanent Atrial Fibrillation) trial demonstrated no higher rates of cardiovascular death, hospitalization for heart failure, stroke, embolism, bleeding, or life-threatening arrhythmic events in patients with permanent AF who were allowed a target heart rate of fewer than 110 bpm.
While it is not clear if the conclusions of the RACE-II trial are applicable to all patients, the guideline recommends that for patients with permanent AF, it is reasonable to use a goal heart rate of fewer than 110 bpm if the patient is still asymptomatic and maintains normal left ventricular function. If rate control cannot be achieved using beta-blockers or calcium channel blockers, amiodarone may be a reasonable choice.
It may be reasonable to pursue restoration of sinus rhythm in patients with persistent, bothersome symptoms, a first episode of AF, or an acute illness or event leading to the onset of AF. Other situations where a rhythm-control approach may be reasonable include young patient age, inadequate rate control despite appropriate therapy, tachycardia-mediated cardiomyopathy, or patient preference.
If considering cardioversion, the length of time the patient has been in AF is critical. If the onset of AF can be reliably pinpointed to fewer than 48 hours ago, the guideline states that cardioversion is reasonable at that point, and preprocedure anticoagulation is not necessary if the patient is at low risk for thromboembolic events. If the duration of AF is 48 hours or unknown, anticoagulation with warfarin or a newer agent for 3 weeks prior to and 4 weeks after cardioversion is recommended. Alternatively, the preprocedure period of anticoagulation can be eliminated if anticoagulation can be established and a TEE performed that shows no thrombus in the left atrium (LA) or left atrium appendage (LAA).
For patients who achieve sinus rhythm, there are several choices of antiarrhythmic agent to be considered for a rhythm-control strategy, including amiodarone, dofetilide, dronedarone, flecainide, propafenone, and sotalol. It is important to note that these medications are reasonable to continue even if the patient experiences episodes of AF, if the medication decreases the number of episodes or the symptoms associated with them. These medications should be stopped, however, if AF becomes permanent.
The bottom line
Atrial fibrillation is a common rhythm disorder that requires ongoing management of thromboembolic risk as well as adherence to either a rate-control or rhythm-control strategy. The choice for anticoagulation should be made jointly between the patient and physician, and should be based on the CHA2DS2-VASc score.
Reference
2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society (Circulation 2014 March 28 [doi: 10.1161/CIR.0000000000000040]).
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital and professor of family and community medicine at Temple University, Philadelphia. Dr. George-Reichley is a third-year resident in the family medicine residency program at Abington Memorial Hospital.
Atrial fibrillation is a common disorder with a lifetime prevalence of approximately 10%-15%. Risk factors include advancing age, diabetes, myocardial infarction, valvular disease, smoking, hypertension, obstructive sleep apnea, hyperthyroidism, alcohol abuse, illicit drug use, and obesity.
The diagnosis of AF should be based on the patient’s history and physical exam, including an ECG and lab work to evaluate thyroid, renal, and hepatic function, as well as a blood count. Further testing should be individualized and may include exercise testing, Holter monitoring, transesophageal echocardiogram (TEE), electrophysiology study, sleep study, or chest x-ray.
An important consideration in the approach to AF is prevention of thromboembolic events; multiple scoring systems have been developed to help stratify risk. The guideline recommends using the newer CHA2DS2-VASc scoring system, rather than the older CHADS2 scoring system, for patients with nonvalvular AF. Points are awarded as follows: congestive heart failure, 1; hypertension, 1; age of 75 years or older, 2; diabetes mellitus, 1; stroke/transient ischemic attack, 2; vascular disease (myocardial infarction, peripheral arterial disease, or aortic plaque), 1; age 65-74 years, 1; sex category (female), 1; for a maximum of 9 points. The CHA2DS2-VASc system reflects the increased risk attributed to age of 75 years or older, vascular disease, and female sex not accounted for in the CHADS2 system, and it is better than CHADS2 at discriminating among low-risk individuals. The annual risk of stroke ranges from less than 1% for a score of 0, to greater than 15% with a score of 9.
In patients with nonvalvular AF with a CHA2DS2-VASc score of 0, it is reasonable to forego antithrombotic therapy altogether. For patients with nonvalvular AF and a CHA2DS2-VASc score of 1, it is reasonable to choose either no anticoagulation therapy or use of daily aspirin or an oral anticoagulant. For a CHA2DS2-VASc score of 2, using an oral anticoagulant is recommended, either warfarin or a newer agent – dabigatran, rivaroxaban, or apixaban. For patients who have mechanical heart valves, warfarin is the only recommended choice for anticoagulation, with dabigatran specifically noted as causing harm.
Prevention of thromboembolism is just one of the treatment goals in AF. Affected patients generally require either rate- or rhythm-control therapy. Choosing which treatment to pursue depends on multiple individualized factors. The guideline points out that over time, attempting to maintain sinus rhythm results in more hospitalizations than does therapy focused on rate control, with no decrease in mortality. The guidelines state: "Routine use of a rhythm-control strategy is not warranted for some patients." Persistent symptoms are the most important indication for a rhythm-control strategy.
If a rate-control approach is chosen, the guideline recommends choosing a beta-blocker or nondihydropyridine calcium channel blocker as first-line therapy unless contraindicated, regardless of whether the AF is paroxysmal, persistent, or permanent. The dose of these medications should be titrated according to resting heart rate. Previously a target of fewer than 80 bpm was recommended; however, findings from the RACE-II (Rate Control Efficacy in Permanent Atrial Fibrillation) trial demonstrated no higher rates of cardiovascular death, hospitalization for heart failure, stroke, embolism, bleeding, or life-threatening arrhythmic events in patients with permanent AF who were allowed a target heart rate of fewer than 110 bpm.
While it is not clear if the conclusions of the RACE-II trial are applicable to all patients, the guideline recommends that for patients with permanent AF, it is reasonable to use a goal heart rate of fewer than 110 bpm if the patient is still asymptomatic and maintains normal left ventricular function. If rate control cannot be achieved using beta-blockers or calcium channel blockers, amiodarone may be a reasonable choice.
It may be reasonable to pursue restoration of sinus rhythm in patients with persistent, bothersome symptoms, a first episode of AF, or an acute illness or event leading to the onset of AF. Other situations where a rhythm-control approach may be reasonable include young patient age, inadequate rate control despite appropriate therapy, tachycardia-mediated cardiomyopathy, or patient preference.
If considering cardioversion, the length of time the patient has been in AF is critical. If the onset of AF can be reliably pinpointed to fewer than 48 hours ago, the guideline states that cardioversion is reasonable at that point, and preprocedure anticoagulation is not necessary if the patient is at low risk for thromboembolic events. If the duration of AF is 48 hours or unknown, anticoagulation with warfarin or a newer agent for 3 weeks prior to and 4 weeks after cardioversion is recommended. Alternatively, the preprocedure period of anticoagulation can be eliminated if anticoagulation can be established and a TEE performed that shows no thrombus in the left atrium (LA) or left atrium appendage (LAA).
For patients who achieve sinus rhythm, there are several choices of antiarrhythmic agent to be considered for a rhythm-control strategy, including amiodarone, dofetilide, dronedarone, flecainide, propafenone, and sotalol. It is important to note that these medications are reasonable to continue even if the patient experiences episodes of AF, if the medication decreases the number of episodes or the symptoms associated with them. These medications should be stopped, however, if AF becomes permanent.
The bottom line
Atrial fibrillation is a common rhythm disorder that requires ongoing management of thromboembolic risk as well as adherence to either a rate-control or rhythm-control strategy. The choice for anticoagulation should be made jointly between the patient and physician, and should be based on the CHA2DS2-VASc score.
Reference
2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society (Circulation 2014 March 28 [doi: 10.1161/CIR.0000000000000040]).
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital and professor of family and community medicine at Temple University, Philadelphia. Dr. George-Reichley is a third-year resident in the family medicine residency program at Abington Memorial Hospital.
Atrial fibrillation is a common disorder with a lifetime prevalence of approximately 10%-15%. Risk factors include advancing age, diabetes, myocardial infarction, valvular disease, smoking, hypertension, obstructive sleep apnea, hyperthyroidism, alcohol abuse, illicit drug use, and obesity.
The diagnosis of AF should be based on the patient’s history and physical exam, including an ECG and lab work to evaluate thyroid, renal, and hepatic function, as well as a blood count. Further testing should be individualized and may include exercise testing, Holter monitoring, transesophageal echocardiogram (TEE), electrophysiology study, sleep study, or chest x-ray.
An important consideration in the approach to AF is prevention of thromboembolic events; multiple scoring systems have been developed to help stratify risk. The guideline recommends using the newer CHA2DS2-VASc scoring system, rather than the older CHADS2 scoring system, for patients with nonvalvular AF. Points are awarded as follows: congestive heart failure, 1; hypertension, 1; age of 75 years or older, 2; diabetes mellitus, 1; stroke/transient ischemic attack, 2; vascular disease (myocardial infarction, peripheral arterial disease, or aortic plaque), 1; age 65-74 years, 1; sex category (female), 1; for a maximum of 9 points. The CHA2DS2-VASc system reflects the increased risk attributed to age of 75 years or older, vascular disease, and female sex not accounted for in the CHADS2 system, and it is better than CHADS2 at discriminating among low-risk individuals. The annual risk of stroke ranges from less than 1% for a score of 0, to greater than 15% with a score of 9.
In patients with nonvalvular AF with a CHA2DS2-VASc score of 0, it is reasonable to forego antithrombotic therapy altogether. For patients with nonvalvular AF and a CHA2DS2-VASc score of 1, it is reasonable to choose either no anticoagulation therapy or use of daily aspirin or an oral anticoagulant. For a CHA2DS2-VASc score of 2, using an oral anticoagulant is recommended, either warfarin or a newer agent – dabigatran, rivaroxaban, or apixaban. For patients who have mechanical heart valves, warfarin is the only recommended choice for anticoagulation, with dabigatran specifically noted as causing harm.
Prevention of thromboembolism is just one of the treatment goals in AF. Affected patients generally require either rate- or rhythm-control therapy. Choosing which treatment to pursue depends on multiple individualized factors. The guideline points out that over time, attempting to maintain sinus rhythm results in more hospitalizations than does therapy focused on rate control, with no decrease in mortality. The guidelines state: "Routine use of a rhythm-control strategy is not warranted for some patients." Persistent symptoms are the most important indication for a rhythm-control strategy.
If a rate-control approach is chosen, the guideline recommends choosing a beta-blocker or nondihydropyridine calcium channel blocker as first-line therapy unless contraindicated, regardless of whether the AF is paroxysmal, persistent, or permanent. The dose of these medications should be titrated according to resting heart rate. Previously a target of fewer than 80 bpm was recommended; however, findings from the RACE-II (Rate Control Efficacy in Permanent Atrial Fibrillation) trial demonstrated no higher rates of cardiovascular death, hospitalization for heart failure, stroke, embolism, bleeding, or life-threatening arrhythmic events in patients with permanent AF who were allowed a target heart rate of fewer than 110 bpm.
While it is not clear if the conclusions of the RACE-II trial are applicable to all patients, the guideline recommends that for patients with permanent AF, it is reasonable to use a goal heart rate of fewer than 110 bpm if the patient is still asymptomatic and maintains normal left ventricular function. If rate control cannot be achieved using beta-blockers or calcium channel blockers, amiodarone may be a reasonable choice.
It may be reasonable to pursue restoration of sinus rhythm in patients with persistent, bothersome symptoms, a first episode of AF, or an acute illness or event leading to the onset of AF. Other situations where a rhythm-control approach may be reasonable include young patient age, inadequate rate control despite appropriate therapy, tachycardia-mediated cardiomyopathy, or patient preference.
If considering cardioversion, the length of time the patient has been in AF is critical. If the onset of AF can be reliably pinpointed to fewer than 48 hours ago, the guideline states that cardioversion is reasonable at that point, and preprocedure anticoagulation is not necessary if the patient is at low risk for thromboembolic events. If the duration of AF is 48 hours or unknown, anticoagulation with warfarin or a newer agent for 3 weeks prior to and 4 weeks after cardioversion is recommended. Alternatively, the preprocedure period of anticoagulation can be eliminated if anticoagulation can be established and a TEE performed that shows no thrombus in the left atrium (LA) or left atrium appendage (LAA).
For patients who achieve sinus rhythm, there are several choices of antiarrhythmic agent to be considered for a rhythm-control strategy, including amiodarone, dofetilide, dronedarone, flecainide, propafenone, and sotalol. It is important to note that these medications are reasonable to continue even if the patient experiences episodes of AF, if the medication decreases the number of episodes or the symptoms associated with them. These medications should be stopped, however, if AF becomes permanent.
The bottom line
Atrial fibrillation is a common rhythm disorder that requires ongoing management of thromboembolic risk as well as adherence to either a rate-control or rhythm-control strategy. The choice for anticoagulation should be made jointly between the patient and physician, and should be based on the CHA2DS2-VASc score.
Reference
2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society (Circulation 2014 March 28 [doi: 10.1161/CIR.0000000000000040]).
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital and professor of family and community medicine at Temple University, Philadelphia. Dr. George-Reichley is a third-year resident in the family medicine residency program at Abington Memorial Hospital.
Is it time for telemedicine?
In the constantly advancing world of health information technology, the buzzwords are always changing. In the 90s, HIPAA dominated the literature. In the past few years, meaningful use took over. Most recently, a great deal of attention has been paid to telemedicine. For the uninitiated, telemedicine, a.k.a. telehealth, is not simply returning patient phone calls after hours. Telemedicine is the idea of substituting in-person patient encounters with virtual ones, typically using a secure video interface. In this column, we will explore a general overview of telemedicine and consider how this new medium fits into the ever-changing landscape of patient care.
The patient will ‘see’ you now
As evidenced by the feedback we have received on previous columns, many of our readers are still reeling from the arduous task of implementing an EHR and shudder at the thought of introducing even more technology into their office workflow. Others who enjoy being on the cutting edge of medicine are eager to embrace these virtual visits. We’ve spoken to several early adopters and have learned that it can be a rewarding way to handle certain patient interactions, but telemedicine obviously isn’t right for every patient or condition. Some feel that the lack of physical presence is not conducive to handling personal conversations or conveying sensitive information. Others have met with resistance from patients who just can’t get used to the idea of hands-free care. As with anything else, there will be patients who remain skeptical, but there will also be those who prefer it.
One patient we engaged saw his physician’s choice to offer virtual visits as a huge benefit, saying that "the idea of saving the 30 minutes of driving in each direction to visit the doctor, along with avoiding the hassle of taking time off work, makes [telehealth] a perfect way to handle my blood pressure control." In this case, as with others, patients use information from devices such as home blood pressure monitors or glucometers to supplement the visit and provide objective data. As home technology advances, connected devices are becoming more sophisticated and allowing more complicated diagnostics to be done remotely. Smartphone-connected otoscopes and ECG machines are just two examples of what’s to come, and we plan to highlight some of these in an upcoming column.
Getting started: Is it safe?
There are several things to consider when getting started in telemedicine in your practice. First is hardware. In general, this is fairly straightforward as most practices are now set up with broadband Internet and computers or tablets. Obviously, a good quality webcam is essential to ensure quality communication with patients. Beyond this, it’s critical to think about the security of the video interaction.
Standard consumer video-conferencing software such as Skype and Apple’s FaceTime are not HIPAA-compliant and can’t be used for patient communications. An encrypted video portal is essential to maintain patient privacy and make sure the patient interaction remains secure. The good news is that several easy-to-use software packages exist to allow this, if the functionality is not already built in to your EHR. A quick web search for "HIPAA-compliant video conferencing" returned several low-cost examples – some that are even free for a few interactions per month. This may be a good way to see if there is interest from patients prior to a large outlay of money. But what about the question of liability?
Unfortunately, physicians in the 21st century are wired to always be concerned about the threat of litigation, and there is no question that telemedicine offers a new opportunity for scrutiny of our clinical decisions. In addition, many rightfully fear the idea of diagnosis and treatment without the laying on of hands. As of now, there is little-to-no legal precedent for the malpractice implications of telehealth visits, but Teledoc, the country’s largest purveyor of telemedicine, advertises 7.5 million members and zero malpractice claims. Whether or not this is enough to convince the skeptics remains to be seen, but so far telemedicine has seemingly managed to fly below the radar of the courts. Following the pattern of all areas of medicine, this is likely to change, but it may be prevented if physicians limit the kinds of services offered through virtual visits. Many providers, such as Teledoc, for example, do not prescribe Drug Enforcement Administration–controlled or so-called nontherapeutic medications. (If you’re wondering, Viagra and Cialis are both on this list.) In this way, they are able to avoid becoming confused with illegitimate drug fulfillment warehouses and limit encounters that might raise questions of legality.
Can I get paid for this?
Although there are many conceivable benefits to telemedicine, such as access to patients who are homebound or live in isolated rural areas, it is hard to imagine setting off down the virtual path without the promise of reimbursement. According to the American Telemedicine Association, 21 states and Washington, D.C., require coverage of telemedicine services with reimbursement at a rate on par with in-person visits, while providers in the others are left wanting. Currently, 120 members of the U.S. Congress support various bills to expand the reach and acceptance of telemedicine, according to the telemedicine association, but it’s clear there is a long way to go before telemedicine receives the full support of insurers. In the meantime, its only financial benefit may be as a marketing advantage for practices. Those who leverage this may be able to draw new business from patients seeking more options in the delivery of their care.
Are you ready?
As with all burgeoning areas of health IT, telemedicine in its early stages appears at best like science fiction and at worst like just another headache. Unlike the other buzzwords of the past few years, adoption of telemedicine seems to be less of a requirement and more of an option. It may or may not work in your practice or for your patient population. Most importantly, though, it represents one point on the continuum of care that will take on an increasingly prominent role in the consumer-driven health care market, and it may represent a boon to both patients and providers alike.
Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. Dr. Skolnik is associate director of the family medicine residency program at Abington Memorial Hospital and professor of family and community medicine at Temple University in Philadelphia.
In the constantly advancing world of health information technology, the buzzwords are always changing. In the 90s, HIPAA dominated the literature. In the past few years, meaningful use took over. Most recently, a great deal of attention has been paid to telemedicine. For the uninitiated, telemedicine, a.k.a. telehealth, is not simply returning patient phone calls after hours. Telemedicine is the idea of substituting in-person patient encounters with virtual ones, typically using a secure video interface. In this column, we will explore a general overview of telemedicine and consider how this new medium fits into the ever-changing landscape of patient care.
The patient will ‘see’ you now
As evidenced by the feedback we have received on previous columns, many of our readers are still reeling from the arduous task of implementing an EHR and shudder at the thought of introducing even more technology into their office workflow. Others who enjoy being on the cutting edge of medicine are eager to embrace these virtual visits. We’ve spoken to several early adopters and have learned that it can be a rewarding way to handle certain patient interactions, but telemedicine obviously isn’t right for every patient or condition. Some feel that the lack of physical presence is not conducive to handling personal conversations or conveying sensitive information. Others have met with resistance from patients who just can’t get used to the idea of hands-free care. As with anything else, there will be patients who remain skeptical, but there will also be those who prefer it.
One patient we engaged saw his physician’s choice to offer virtual visits as a huge benefit, saying that "the idea of saving the 30 minutes of driving in each direction to visit the doctor, along with avoiding the hassle of taking time off work, makes [telehealth] a perfect way to handle my blood pressure control." In this case, as with others, patients use information from devices such as home blood pressure monitors or glucometers to supplement the visit and provide objective data. As home technology advances, connected devices are becoming more sophisticated and allowing more complicated diagnostics to be done remotely. Smartphone-connected otoscopes and ECG machines are just two examples of what’s to come, and we plan to highlight some of these in an upcoming column.
Getting started: Is it safe?
There are several things to consider when getting started in telemedicine in your practice. First is hardware. In general, this is fairly straightforward as most practices are now set up with broadband Internet and computers or tablets. Obviously, a good quality webcam is essential to ensure quality communication with patients. Beyond this, it’s critical to think about the security of the video interaction.
Standard consumer video-conferencing software such as Skype and Apple’s FaceTime are not HIPAA-compliant and can’t be used for patient communications. An encrypted video portal is essential to maintain patient privacy and make sure the patient interaction remains secure. The good news is that several easy-to-use software packages exist to allow this, if the functionality is not already built in to your EHR. A quick web search for "HIPAA-compliant video conferencing" returned several low-cost examples – some that are even free for a few interactions per month. This may be a good way to see if there is interest from patients prior to a large outlay of money. But what about the question of liability?
Unfortunately, physicians in the 21st century are wired to always be concerned about the threat of litigation, and there is no question that telemedicine offers a new opportunity for scrutiny of our clinical decisions. In addition, many rightfully fear the idea of diagnosis and treatment without the laying on of hands. As of now, there is little-to-no legal precedent for the malpractice implications of telehealth visits, but Teledoc, the country’s largest purveyor of telemedicine, advertises 7.5 million members and zero malpractice claims. Whether or not this is enough to convince the skeptics remains to be seen, but so far telemedicine has seemingly managed to fly below the radar of the courts. Following the pattern of all areas of medicine, this is likely to change, but it may be prevented if physicians limit the kinds of services offered through virtual visits. Many providers, such as Teledoc, for example, do not prescribe Drug Enforcement Administration–controlled or so-called nontherapeutic medications. (If you’re wondering, Viagra and Cialis are both on this list.) In this way, they are able to avoid becoming confused with illegitimate drug fulfillment warehouses and limit encounters that might raise questions of legality.
Can I get paid for this?
Although there are many conceivable benefits to telemedicine, such as access to patients who are homebound or live in isolated rural areas, it is hard to imagine setting off down the virtual path without the promise of reimbursement. According to the American Telemedicine Association, 21 states and Washington, D.C., require coverage of telemedicine services with reimbursement at a rate on par with in-person visits, while providers in the others are left wanting. Currently, 120 members of the U.S. Congress support various bills to expand the reach and acceptance of telemedicine, according to the telemedicine association, but it’s clear there is a long way to go before telemedicine receives the full support of insurers. In the meantime, its only financial benefit may be as a marketing advantage for practices. Those who leverage this may be able to draw new business from patients seeking more options in the delivery of their care.
Are you ready?
As with all burgeoning areas of health IT, telemedicine in its early stages appears at best like science fiction and at worst like just another headache. Unlike the other buzzwords of the past few years, adoption of telemedicine seems to be less of a requirement and more of an option. It may or may not work in your practice or for your patient population. Most importantly, though, it represents one point on the continuum of care that will take on an increasingly prominent role in the consumer-driven health care market, and it may represent a boon to both patients and providers alike.
Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. Dr. Skolnik is associate director of the family medicine residency program at Abington Memorial Hospital and professor of family and community medicine at Temple University in Philadelphia.
In the constantly advancing world of health information technology, the buzzwords are always changing. In the 90s, HIPAA dominated the literature. In the past few years, meaningful use took over. Most recently, a great deal of attention has been paid to telemedicine. For the uninitiated, telemedicine, a.k.a. telehealth, is not simply returning patient phone calls after hours. Telemedicine is the idea of substituting in-person patient encounters with virtual ones, typically using a secure video interface. In this column, we will explore a general overview of telemedicine and consider how this new medium fits into the ever-changing landscape of patient care.
The patient will ‘see’ you now
As evidenced by the feedback we have received on previous columns, many of our readers are still reeling from the arduous task of implementing an EHR and shudder at the thought of introducing even more technology into their office workflow. Others who enjoy being on the cutting edge of medicine are eager to embrace these virtual visits. We’ve spoken to several early adopters and have learned that it can be a rewarding way to handle certain patient interactions, but telemedicine obviously isn’t right for every patient or condition. Some feel that the lack of physical presence is not conducive to handling personal conversations or conveying sensitive information. Others have met with resistance from patients who just can’t get used to the idea of hands-free care. As with anything else, there will be patients who remain skeptical, but there will also be those who prefer it.
One patient we engaged saw his physician’s choice to offer virtual visits as a huge benefit, saying that "the idea of saving the 30 minutes of driving in each direction to visit the doctor, along with avoiding the hassle of taking time off work, makes [telehealth] a perfect way to handle my blood pressure control." In this case, as with others, patients use information from devices such as home blood pressure monitors or glucometers to supplement the visit and provide objective data. As home technology advances, connected devices are becoming more sophisticated and allowing more complicated diagnostics to be done remotely. Smartphone-connected otoscopes and ECG machines are just two examples of what’s to come, and we plan to highlight some of these in an upcoming column.
Getting started: Is it safe?
There are several things to consider when getting started in telemedicine in your practice. First is hardware. In general, this is fairly straightforward as most practices are now set up with broadband Internet and computers or tablets. Obviously, a good quality webcam is essential to ensure quality communication with patients. Beyond this, it’s critical to think about the security of the video interaction.
Standard consumer video-conferencing software such as Skype and Apple’s FaceTime are not HIPAA-compliant and can’t be used for patient communications. An encrypted video portal is essential to maintain patient privacy and make sure the patient interaction remains secure. The good news is that several easy-to-use software packages exist to allow this, if the functionality is not already built in to your EHR. A quick web search for "HIPAA-compliant video conferencing" returned several low-cost examples – some that are even free for a few interactions per month. This may be a good way to see if there is interest from patients prior to a large outlay of money. But what about the question of liability?
Unfortunately, physicians in the 21st century are wired to always be concerned about the threat of litigation, and there is no question that telemedicine offers a new opportunity for scrutiny of our clinical decisions. In addition, many rightfully fear the idea of diagnosis and treatment without the laying on of hands. As of now, there is little-to-no legal precedent for the malpractice implications of telehealth visits, but Teledoc, the country’s largest purveyor of telemedicine, advertises 7.5 million members and zero malpractice claims. Whether or not this is enough to convince the skeptics remains to be seen, but so far telemedicine has seemingly managed to fly below the radar of the courts. Following the pattern of all areas of medicine, this is likely to change, but it may be prevented if physicians limit the kinds of services offered through virtual visits. Many providers, such as Teledoc, for example, do not prescribe Drug Enforcement Administration–controlled or so-called nontherapeutic medications. (If you’re wondering, Viagra and Cialis are both on this list.) In this way, they are able to avoid becoming confused with illegitimate drug fulfillment warehouses and limit encounters that might raise questions of legality.
Can I get paid for this?
Although there are many conceivable benefits to telemedicine, such as access to patients who are homebound or live in isolated rural areas, it is hard to imagine setting off down the virtual path without the promise of reimbursement. According to the American Telemedicine Association, 21 states and Washington, D.C., require coverage of telemedicine services with reimbursement at a rate on par with in-person visits, while providers in the others are left wanting. Currently, 120 members of the U.S. Congress support various bills to expand the reach and acceptance of telemedicine, according to the telemedicine association, but it’s clear there is a long way to go before telemedicine receives the full support of insurers. In the meantime, its only financial benefit may be as a marketing advantage for practices. Those who leverage this may be able to draw new business from patients seeking more options in the delivery of their care.
Are you ready?
As with all burgeoning areas of health IT, telemedicine in its early stages appears at best like science fiction and at worst like just another headache. Unlike the other buzzwords of the past few years, adoption of telemedicine seems to be less of a requirement and more of an option. It may or may not work in your practice or for your patient population. Most importantly, though, it represents one point on the continuum of care that will take on an increasingly prominent role in the consumer-driven health care market, and it may represent a boon to both patients and providers alike.
Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. Dr. Skolnik is associate director of the family medicine residency program at Abington Memorial Hospital and professor of family and community medicine at Temple University in Philadelphia.
EHR Report: Across the ages
Eighty percent of physicians are now using electronic health records in their offices. We have been impressed that the younger physicians to whom we have spoken often view their experience with EHRs very differently from older physicians. Is such a difference inevitable, perhaps, not just because change is more difficult for many people as they get older but also because expectations are influenced by experience. Noticing these different thoughts and feelings, we’ve asked two physicians more than 55 years old and two younger physicians to share some thoughts on their experiences with electronic records.
Mathew Clark (family physician)
I’ve been in practice for 31 years and using an EHR system for just under 5. I’m not thrilled with it, but I accept that it’s an unavoidable part of my practice now, and so I don’t waste energy being upset about it. I’ve learned to function efficiently with an EHR, doing the best I can. I remember physicians, before the days of SOAP notes, who would write pithy, useful notes such as "probable strep, Pen VK 500 bid for 10 days" on 3x5 index cards. Such notes lacked detail, and it’s not hard to imagine the problems this lack of detail might create, but they were readable at a glance, and told you what you needed to know. On the other hand, the massively detailed, bloated notes we see with our EHRs, obscured by "copy-forward" text and fictional (in other words, never really asked or examined) information, present very significant practical and legal issues of their own, and take hours of physician time to complete. Given a choice, I’d probably go for the index cards.
Natalie McGann (family physician)
I have been a family physician in practice for 4 years since graduating from residency. The advent of the EHR hasn’t been an overwhelming transition for those of us in the early stages of our careers. Much of our schooling to date has included laptops and other electronic devices that for many prove an easier means of communication. Despite that fact that EHRs require a host of extraneous clicks and check boxes, it is still less cumbersome than documenting encounters on paper. For the generation of young physicians accustomed to having answers at their fingertips, the idea of flipping through paper charts to collate a patient’s medical record seems far more complicated than clicking a few tabs without ever leaving your chair. I, and most colleagues in my peer group to whom I’ve spoken, agree that we would not be likely to a join a practice that doesn’t utilize an EHR or have a current plan to adopt one. Anything less would feel like a step back at this point.
Danielle Carcia (intern, family medicine residency)
Overall, I enjoy using electronic medical records. I feel that it places all pertinent information about the patient in an easy-to-follow and concise manner. The ability to read through past providers and even at times specialists visits with a patient can be very helpful when navigating an appointment with a new patient. As a young physician, electronics have been an extension of myself for my entire adult life, so a computer in front of me during an office visit is comforting. I do not feel it distracts from my interaction with patients, or takes away from their experience at all, just the opposite, it allows me to more confidently care for them with up to date, and organized information at my fingertips.
Dave Depietro (family physician)
I have been a family physician for 25 years and feel that the EHRs have affected my office in a number of ways. It has definitely improved the efficacy of office tasks such as doing prescription refills, interoffice communication, and scheduling. Also before EHRs, the turnaround time for a dictated note was about a week, and now most notes are completed by the end of the day. This makes it easier if I am taking care of one of my partner’s patients or dealing with a patient I recently saw. Also in this day of pay for performance we can now gather data much easier. This would be almost impossible to do if we still had paper charts.
EHRs unfortunately also have their downsides. The main problem I see is that they add a significant amount of time for providers to complete tasks. When I dictated a note, I could have completed a note within 1-2 minutes where now with EHRs, it can take maybe 3-5 minutes/patient. Also to approve labs, x-rays, etc. it just takes longer. I feel that EHRs have added about 1½ hr to my day. I feel most of my colleagues have the same complaint. They routinely take work home at night and spend 1-2 hours at home completing notes. Many of my peers seem stressed and frustrated. Even though EHRs make the office more efficient, I feel that the provider pays the price. My other complaint is the cost of IT support to keep the EHRs running smoothly. The promise of EHRs is that they would save physicians’ money and reduce staffing, however I have not seen that happen.
I ask myself, at the end of the day, would I go back to paper charts? The answer is no. Despite their downsides, I feel that the positives of EHRs outweigh the negatives. Older doctors just need to adapt to this new way of practicing medicine.
The Bottom Line
Clearly there is a range of opinion about the effect of electronic health records on our practices and our lives, with those opinions at least partly segregated by age. We are interested in your thoughts and plan to publish some of those thoughts in future columns, so please let us know at [email protected]. Thanks.
Dr. Notte is a family physician and clinical informaticist for Abington Memorial Hospital. He is a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital and professor of family and community medicine at Temple University, Philadelphia. He is editor in chief of Redi-Reference Inc., a software company that creates mobile apps.
Eighty percent of physicians are now using electronic health records in their offices. We have been impressed that the younger physicians to whom we have spoken often view their experience with EHRs very differently from older physicians. Is such a difference inevitable, perhaps, not just because change is more difficult for many people as they get older but also because expectations are influenced by experience. Noticing these different thoughts and feelings, we’ve asked two physicians more than 55 years old and two younger physicians to share some thoughts on their experiences with electronic records.
Mathew Clark (family physician)
I’ve been in practice for 31 years and using an EHR system for just under 5. I’m not thrilled with it, but I accept that it’s an unavoidable part of my practice now, and so I don’t waste energy being upset about it. I’ve learned to function efficiently with an EHR, doing the best I can. I remember physicians, before the days of SOAP notes, who would write pithy, useful notes such as "probable strep, Pen VK 500 bid for 10 days" on 3x5 index cards. Such notes lacked detail, and it’s not hard to imagine the problems this lack of detail might create, but they were readable at a glance, and told you what you needed to know. On the other hand, the massively detailed, bloated notes we see with our EHRs, obscured by "copy-forward" text and fictional (in other words, never really asked or examined) information, present very significant practical and legal issues of their own, and take hours of physician time to complete. Given a choice, I’d probably go for the index cards.
Natalie McGann (family physician)
I have been a family physician in practice for 4 years since graduating from residency. The advent of the EHR hasn’t been an overwhelming transition for those of us in the early stages of our careers. Much of our schooling to date has included laptops and other electronic devices that for many prove an easier means of communication. Despite that fact that EHRs require a host of extraneous clicks and check boxes, it is still less cumbersome than documenting encounters on paper. For the generation of young physicians accustomed to having answers at their fingertips, the idea of flipping through paper charts to collate a patient’s medical record seems far more complicated than clicking a few tabs without ever leaving your chair. I, and most colleagues in my peer group to whom I’ve spoken, agree that we would not be likely to a join a practice that doesn’t utilize an EHR or have a current plan to adopt one. Anything less would feel like a step back at this point.
Danielle Carcia (intern, family medicine residency)
Overall, I enjoy using electronic medical records. I feel that it places all pertinent information about the patient in an easy-to-follow and concise manner. The ability to read through past providers and even at times specialists visits with a patient can be very helpful when navigating an appointment with a new patient. As a young physician, electronics have been an extension of myself for my entire adult life, so a computer in front of me during an office visit is comforting. I do not feel it distracts from my interaction with patients, or takes away from their experience at all, just the opposite, it allows me to more confidently care for them with up to date, and organized information at my fingertips.
Dave Depietro (family physician)
I have been a family physician for 25 years and feel that the EHRs have affected my office in a number of ways. It has definitely improved the efficacy of office tasks such as doing prescription refills, interoffice communication, and scheduling. Also before EHRs, the turnaround time for a dictated note was about a week, and now most notes are completed by the end of the day. This makes it easier if I am taking care of one of my partner’s patients or dealing with a patient I recently saw. Also in this day of pay for performance we can now gather data much easier. This would be almost impossible to do if we still had paper charts.
EHRs unfortunately also have their downsides. The main problem I see is that they add a significant amount of time for providers to complete tasks. When I dictated a note, I could have completed a note within 1-2 minutes where now with EHRs, it can take maybe 3-5 minutes/patient. Also to approve labs, x-rays, etc. it just takes longer. I feel that EHRs have added about 1½ hr to my day. I feel most of my colleagues have the same complaint. They routinely take work home at night and spend 1-2 hours at home completing notes. Many of my peers seem stressed and frustrated. Even though EHRs make the office more efficient, I feel that the provider pays the price. My other complaint is the cost of IT support to keep the EHRs running smoothly. The promise of EHRs is that they would save physicians’ money and reduce staffing, however I have not seen that happen.
I ask myself, at the end of the day, would I go back to paper charts? The answer is no. Despite their downsides, I feel that the positives of EHRs outweigh the negatives. Older doctors just need to adapt to this new way of practicing medicine.
The Bottom Line
Clearly there is a range of opinion about the effect of electronic health records on our practices and our lives, with those opinions at least partly segregated by age. We are interested in your thoughts and plan to publish some of those thoughts in future columns, so please let us know at [email protected]. Thanks.
Dr. Notte is a family physician and clinical informaticist for Abington Memorial Hospital. He is a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital and professor of family and community medicine at Temple University, Philadelphia. He is editor in chief of Redi-Reference Inc., a software company that creates mobile apps.
Eighty percent of physicians are now using electronic health records in their offices. We have been impressed that the younger physicians to whom we have spoken often view their experience with EHRs very differently from older physicians. Is such a difference inevitable, perhaps, not just because change is more difficult for many people as they get older but also because expectations are influenced by experience. Noticing these different thoughts and feelings, we’ve asked two physicians more than 55 years old and two younger physicians to share some thoughts on their experiences with electronic records.
Mathew Clark (family physician)
I’ve been in practice for 31 years and using an EHR system for just under 5. I’m not thrilled with it, but I accept that it’s an unavoidable part of my practice now, and so I don’t waste energy being upset about it. I’ve learned to function efficiently with an EHR, doing the best I can. I remember physicians, before the days of SOAP notes, who would write pithy, useful notes such as "probable strep, Pen VK 500 bid for 10 days" on 3x5 index cards. Such notes lacked detail, and it’s not hard to imagine the problems this lack of detail might create, but they were readable at a glance, and told you what you needed to know. On the other hand, the massively detailed, bloated notes we see with our EHRs, obscured by "copy-forward" text and fictional (in other words, never really asked or examined) information, present very significant practical and legal issues of their own, and take hours of physician time to complete. Given a choice, I’d probably go for the index cards.
Natalie McGann (family physician)
I have been a family physician in practice for 4 years since graduating from residency. The advent of the EHR hasn’t been an overwhelming transition for those of us in the early stages of our careers. Much of our schooling to date has included laptops and other electronic devices that for many prove an easier means of communication. Despite that fact that EHRs require a host of extraneous clicks and check boxes, it is still less cumbersome than documenting encounters on paper. For the generation of young physicians accustomed to having answers at their fingertips, the idea of flipping through paper charts to collate a patient’s medical record seems far more complicated than clicking a few tabs without ever leaving your chair. I, and most colleagues in my peer group to whom I’ve spoken, agree that we would not be likely to a join a practice that doesn’t utilize an EHR or have a current plan to adopt one. Anything less would feel like a step back at this point.
Danielle Carcia (intern, family medicine residency)
Overall, I enjoy using electronic medical records. I feel that it places all pertinent information about the patient in an easy-to-follow and concise manner. The ability to read through past providers and even at times specialists visits with a patient can be very helpful when navigating an appointment with a new patient. As a young physician, electronics have been an extension of myself for my entire adult life, so a computer in front of me during an office visit is comforting. I do not feel it distracts from my interaction with patients, or takes away from their experience at all, just the opposite, it allows me to more confidently care for them with up to date, and organized information at my fingertips.
Dave Depietro (family physician)
I have been a family physician for 25 years and feel that the EHRs have affected my office in a number of ways. It has definitely improved the efficacy of office tasks such as doing prescription refills, interoffice communication, and scheduling. Also before EHRs, the turnaround time for a dictated note was about a week, and now most notes are completed by the end of the day. This makes it easier if I am taking care of one of my partner’s patients or dealing with a patient I recently saw. Also in this day of pay for performance we can now gather data much easier. This would be almost impossible to do if we still had paper charts.
EHRs unfortunately also have their downsides. The main problem I see is that they add a significant amount of time for providers to complete tasks. When I dictated a note, I could have completed a note within 1-2 minutes where now with EHRs, it can take maybe 3-5 minutes/patient. Also to approve labs, x-rays, etc. it just takes longer. I feel that EHRs have added about 1½ hr to my day. I feel most of my colleagues have the same complaint. They routinely take work home at night and spend 1-2 hours at home completing notes. Many of my peers seem stressed and frustrated. Even though EHRs make the office more efficient, I feel that the provider pays the price. My other complaint is the cost of IT support to keep the EHRs running smoothly. The promise of EHRs is that they would save physicians’ money and reduce staffing, however I have not seen that happen.
I ask myself, at the end of the day, would I go back to paper charts? The answer is no. Despite their downsides, I feel that the positives of EHRs outweigh the negatives. Older doctors just need to adapt to this new way of practicing medicine.
The Bottom Line
Clearly there is a range of opinion about the effect of electronic health records on our practices and our lives, with those opinions at least partly segregated by age. We are interested in your thoughts and plan to publish some of those thoughts in future columns, so please let us know at [email protected]. Thanks.
Dr. Notte is a family physician and clinical informaticist for Abington Memorial Hospital. He is a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital and professor of family and community medicine at Temple University, Philadelphia. He is editor in chief of Redi-Reference Inc., a software company that creates mobile apps.
Primary care management of persons infected with HIV
According to the U.S. Preventive Services Task Force, 1.2 million Americans are living with human immunodeficiency virus infection, and there are 50,000 new cases per year. In 20%-25% of those new cases, patients are unaware of having HIV infection. The recommendation for HIV screening by the USPSTF in 2013 is that all patients between 15 and 65 years of age should receive screening. The HIV Medicine Association of the Infectious Diseases Society of America released updated evidence-based guidelines for primary care management of persons infected with HIV.
Initial assessment
The new guidelines emphasize that all new patients who present to the office with an existing HIV infection undergo a comprehensive assessment, including a complete history, physical, and specific testing. The history must include the date of HIV diagnosis and approximate date of infection. Review the patient’s highest viral load, lowest CD4 count, and past and current treatment regimens; and document any history of opportunistic infections or HIV-related comorbidities. Note any chronic medical conditions, such as cardiovascular disease, diabetes mellitus, and/or renal or liver dysfunction that may impact future treatment regimens for HIV. A general discussion of the person’s understanding of their HIV infection may uncover any mental illness, economic burdens, and lack of social support that may present barriers to treatment adherence.
Testing
The guidelines recommend specific testing that should be performed on initial visit and subsequent further monitoring. It is suggested that the CD4 count be documented and the HIV viral load be quantified in patients who already have the diagnosis of HIV infection. In newly diagnosed patients with HIV infection, it is recommended that you get a CD4 cell count, viral load, and HIV genotype/resistance testing to help choose future antiretroviral therapies. The initial assessment should include testing for possible coexisting conditions, including other sexually transmitted infections: gonorrhea, chlamydia, and syphilis. The patient should be screened for hepatitis A, B, and C; toxoplasmosis; CMV; and tuberculosis. TB screening can be done with either the tuberculin skin test or interferon-gamma release assay.
It is also important to establish a baseline fasting comprehensive metabolic panel, complete blood count, urinalysis, creatinine clearance, and fasting lipid panel because HIV infection and certain antiretroviral therapy (ART) can have a negative effect on a range of organ systems.
Immunization and preventive health screening
Immunization should be routine for all patients with HIV infection. In addition to standard recommendations for all adults, the recommendations for patients with HIV include:
• Hepatitis A and hepatitis B vaccines series. Note that for hepatitis B vaccine, the dose is the 40-mcg dose that is used for immunocompromised patients. Patients should be tested for hepatitis A total, or IgG antibody, 1-2 months after the second dose of the HAV vaccine and HBsAb response 1-2 months after the third dose of the HBV vaccine.
• Pneumococcal vaccine. Patients should receive a dose of PCV13 (Prevnar 13), followed by a dose of PPV23 (Pneumovax) at least 8 weeks later. If a patient was previously vaccinated with PPV23, give PCV13 at least 1 year after PPV23.
• Influenza. Annual flu shot should be given.
• Varicella. Administer to HIV-infected persons with a CD4 count greater than 200 cells/mcL who do not have evidence of immunity to varicella.
• HPV. Indicated for females aged 9-26 years and males aged 9-26 years.
Ongoing monitoring
The main tests to follow in a patient with HIV infection to determine if treatment is successful are viral loads and CD4 counts. The viral load should be checked every 3-4 months after initial diagnosis or more frequently when beginning a new regimen. After 2-3 years of being treated and having their viral load successfully suppressed, a patient is considered stable, and the interval of time to test can be extended to every 6 months.
CD4 counts are a useful tool to assess for the need to start ART urgently as well as the effectiveness of ART. If the count drops below certain numbers, the CD4 count can determine the need for prophylactic therapy against opportunistic infections. For CD4 counts in a stable patient who has a suppressed viral load and an adequate CD4 response, the interval of time between monitoring can be extended to every 6-12 months. There are certain metabolic concerns that need frequent monitoring that are associated with the HIV infection itself and the different side effects of specific treatments. Renal function, complete blood count, lipid panel, fasting glucose, hemoglobin A1c, and liver function studies all require frequent monitoring.
Cervical cancer screening is important, and HIV patients are an exception to the newer lengthened screening interval recommendations. Women with HIV infection should have a pap smear at the time of HIV infection diagnosis, repeated at 6 months, then annually thereafter.
The bottom line
The prognosis of people living with HIV infection continues to improve because of new classes of treatment therapies. HIV infection has become a chronic illness, with which patients can live a long life if they take an active role in their treatment. For this reason, the primary care provider has a vital role in the management of HIV-infected persons. At each visit, the primary care provider should discuss the person’s HIV infection and emphasize the importance of adherence to ART, follow-up visits, and overall participation in their care.
References
Aberg J.A., et al. Primary Care Guidelines for the Management of Persons Infected With HIV: 2013 Update by the HIV Medicine Association of the Infectious Diseases Society of America. IDSA Guidelines 2013, 1-30.
Moyer, V., et al. Screening for HIV: U.S. Preventive Services Task Force Recommendation Statement. Ann. Int. Med., April 30, 2013, 1-10.
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital and professor of family and community medicine at Temple University, Philadelphia. Dr. Baranck is a second-year resident and will be one of next year’s chief residents in the Family Medicine Residency Program at Abington Memorial Hospital.
According to the U.S. Preventive Services Task Force, 1.2 million Americans are living with human immunodeficiency virus infection, and there are 50,000 new cases per year. In 20%-25% of those new cases, patients are unaware of having HIV infection. The recommendation for HIV screening by the USPSTF in 2013 is that all patients between 15 and 65 years of age should receive screening. The HIV Medicine Association of the Infectious Diseases Society of America released updated evidence-based guidelines for primary care management of persons infected with HIV.
Initial assessment
The new guidelines emphasize that all new patients who present to the office with an existing HIV infection undergo a comprehensive assessment, including a complete history, physical, and specific testing. The history must include the date of HIV diagnosis and approximate date of infection. Review the patient’s highest viral load, lowest CD4 count, and past and current treatment regimens; and document any history of opportunistic infections or HIV-related comorbidities. Note any chronic medical conditions, such as cardiovascular disease, diabetes mellitus, and/or renal or liver dysfunction that may impact future treatment regimens for HIV. A general discussion of the person’s understanding of their HIV infection may uncover any mental illness, economic burdens, and lack of social support that may present barriers to treatment adherence.
Testing
The guidelines recommend specific testing that should be performed on initial visit and subsequent further monitoring. It is suggested that the CD4 count be documented and the HIV viral load be quantified in patients who already have the diagnosis of HIV infection. In newly diagnosed patients with HIV infection, it is recommended that you get a CD4 cell count, viral load, and HIV genotype/resistance testing to help choose future antiretroviral therapies. The initial assessment should include testing for possible coexisting conditions, including other sexually transmitted infections: gonorrhea, chlamydia, and syphilis. The patient should be screened for hepatitis A, B, and C; toxoplasmosis; CMV; and tuberculosis. TB screening can be done with either the tuberculin skin test or interferon-gamma release assay.
It is also important to establish a baseline fasting comprehensive metabolic panel, complete blood count, urinalysis, creatinine clearance, and fasting lipid panel because HIV infection and certain antiretroviral therapy (ART) can have a negative effect on a range of organ systems.
Immunization and preventive health screening
Immunization should be routine for all patients with HIV infection. In addition to standard recommendations for all adults, the recommendations for patients with HIV include:
• Hepatitis A and hepatitis B vaccines series. Note that for hepatitis B vaccine, the dose is the 40-mcg dose that is used for immunocompromised patients. Patients should be tested for hepatitis A total, or IgG antibody, 1-2 months after the second dose of the HAV vaccine and HBsAb response 1-2 months after the third dose of the HBV vaccine.
• Pneumococcal vaccine. Patients should receive a dose of PCV13 (Prevnar 13), followed by a dose of PPV23 (Pneumovax) at least 8 weeks later. If a patient was previously vaccinated with PPV23, give PCV13 at least 1 year after PPV23.
• Influenza. Annual flu shot should be given.
• Varicella. Administer to HIV-infected persons with a CD4 count greater than 200 cells/mcL who do not have evidence of immunity to varicella.
• HPV. Indicated for females aged 9-26 years and males aged 9-26 years.
Ongoing monitoring
The main tests to follow in a patient with HIV infection to determine if treatment is successful are viral loads and CD4 counts. The viral load should be checked every 3-4 months after initial diagnosis or more frequently when beginning a new regimen. After 2-3 years of being treated and having their viral load successfully suppressed, a patient is considered stable, and the interval of time to test can be extended to every 6 months.
CD4 counts are a useful tool to assess for the need to start ART urgently as well as the effectiveness of ART. If the count drops below certain numbers, the CD4 count can determine the need for prophylactic therapy against opportunistic infections. For CD4 counts in a stable patient who has a suppressed viral load and an adequate CD4 response, the interval of time between monitoring can be extended to every 6-12 months. There are certain metabolic concerns that need frequent monitoring that are associated with the HIV infection itself and the different side effects of specific treatments. Renal function, complete blood count, lipid panel, fasting glucose, hemoglobin A1c, and liver function studies all require frequent monitoring.
Cervical cancer screening is important, and HIV patients are an exception to the newer lengthened screening interval recommendations. Women with HIV infection should have a pap smear at the time of HIV infection diagnosis, repeated at 6 months, then annually thereafter.
The bottom line
The prognosis of people living with HIV infection continues to improve because of new classes of treatment therapies. HIV infection has become a chronic illness, with which patients can live a long life if they take an active role in their treatment. For this reason, the primary care provider has a vital role in the management of HIV-infected persons. At each visit, the primary care provider should discuss the person’s HIV infection and emphasize the importance of adherence to ART, follow-up visits, and overall participation in their care.
References
Aberg J.A., et al. Primary Care Guidelines for the Management of Persons Infected With HIV: 2013 Update by the HIV Medicine Association of the Infectious Diseases Society of America. IDSA Guidelines 2013, 1-30.
Moyer, V., et al. Screening for HIV: U.S. Preventive Services Task Force Recommendation Statement. Ann. Int. Med., April 30, 2013, 1-10.
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital and professor of family and community medicine at Temple University, Philadelphia. Dr. Baranck is a second-year resident and will be one of next year’s chief residents in the Family Medicine Residency Program at Abington Memorial Hospital.
According to the U.S. Preventive Services Task Force, 1.2 million Americans are living with human immunodeficiency virus infection, and there are 50,000 new cases per year. In 20%-25% of those new cases, patients are unaware of having HIV infection. The recommendation for HIV screening by the USPSTF in 2013 is that all patients between 15 and 65 years of age should receive screening. The HIV Medicine Association of the Infectious Diseases Society of America released updated evidence-based guidelines for primary care management of persons infected with HIV.
Initial assessment
The new guidelines emphasize that all new patients who present to the office with an existing HIV infection undergo a comprehensive assessment, including a complete history, physical, and specific testing. The history must include the date of HIV diagnosis and approximate date of infection. Review the patient’s highest viral load, lowest CD4 count, and past and current treatment regimens; and document any history of opportunistic infections or HIV-related comorbidities. Note any chronic medical conditions, such as cardiovascular disease, diabetes mellitus, and/or renal or liver dysfunction that may impact future treatment regimens for HIV. A general discussion of the person’s understanding of their HIV infection may uncover any mental illness, economic burdens, and lack of social support that may present barriers to treatment adherence.
Testing
The guidelines recommend specific testing that should be performed on initial visit and subsequent further monitoring. It is suggested that the CD4 count be documented and the HIV viral load be quantified in patients who already have the diagnosis of HIV infection. In newly diagnosed patients with HIV infection, it is recommended that you get a CD4 cell count, viral load, and HIV genotype/resistance testing to help choose future antiretroviral therapies. The initial assessment should include testing for possible coexisting conditions, including other sexually transmitted infections: gonorrhea, chlamydia, and syphilis. The patient should be screened for hepatitis A, B, and C; toxoplasmosis; CMV; and tuberculosis. TB screening can be done with either the tuberculin skin test or interferon-gamma release assay.
It is also important to establish a baseline fasting comprehensive metabolic panel, complete blood count, urinalysis, creatinine clearance, and fasting lipid panel because HIV infection and certain antiretroviral therapy (ART) can have a negative effect on a range of organ systems.
Immunization and preventive health screening
Immunization should be routine for all patients with HIV infection. In addition to standard recommendations for all adults, the recommendations for patients with HIV include:
• Hepatitis A and hepatitis B vaccines series. Note that for hepatitis B vaccine, the dose is the 40-mcg dose that is used for immunocompromised patients. Patients should be tested for hepatitis A total, or IgG antibody, 1-2 months after the second dose of the HAV vaccine and HBsAb response 1-2 months after the third dose of the HBV vaccine.
• Pneumococcal vaccine. Patients should receive a dose of PCV13 (Prevnar 13), followed by a dose of PPV23 (Pneumovax) at least 8 weeks later. If a patient was previously vaccinated with PPV23, give PCV13 at least 1 year after PPV23.
• Influenza. Annual flu shot should be given.
• Varicella. Administer to HIV-infected persons with a CD4 count greater than 200 cells/mcL who do not have evidence of immunity to varicella.
• HPV. Indicated for females aged 9-26 years and males aged 9-26 years.
Ongoing monitoring
The main tests to follow in a patient with HIV infection to determine if treatment is successful are viral loads and CD4 counts. The viral load should be checked every 3-4 months after initial diagnosis or more frequently when beginning a new regimen. After 2-3 years of being treated and having their viral load successfully suppressed, a patient is considered stable, and the interval of time to test can be extended to every 6 months.
CD4 counts are a useful tool to assess for the need to start ART urgently as well as the effectiveness of ART. If the count drops below certain numbers, the CD4 count can determine the need for prophylactic therapy against opportunistic infections. For CD4 counts in a stable patient who has a suppressed viral load and an adequate CD4 response, the interval of time between monitoring can be extended to every 6-12 months. There are certain metabolic concerns that need frequent monitoring that are associated with the HIV infection itself and the different side effects of specific treatments. Renal function, complete blood count, lipid panel, fasting glucose, hemoglobin A1c, and liver function studies all require frequent monitoring.
Cervical cancer screening is important, and HIV patients are an exception to the newer lengthened screening interval recommendations. Women with HIV infection should have a pap smear at the time of HIV infection diagnosis, repeated at 6 months, then annually thereafter.
The bottom line
The prognosis of people living with HIV infection continues to improve because of new classes of treatment therapies. HIV infection has become a chronic illness, with which patients can live a long life if they take an active role in their treatment. For this reason, the primary care provider has a vital role in the management of HIV-infected persons. At each visit, the primary care provider should discuss the person’s HIV infection and emphasize the importance of adherence to ART, follow-up visits, and overall participation in their care.
References
Aberg J.A., et al. Primary Care Guidelines for the Management of Persons Infected With HIV: 2013 Update by the HIV Medicine Association of the Infectious Diseases Society of America. IDSA Guidelines 2013, 1-30.
Moyer, V., et al. Screening for HIV: U.S. Preventive Services Task Force Recommendation Statement. Ann. Int. Med., April 30, 2013, 1-10.
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital and professor of family and community medicine at Temple University, Philadelphia. Dr. Baranck is a second-year resident and will be one of next year’s chief residents in the Family Medicine Residency Program at Abington Memorial Hospital.
Big Data and the art of medicine
Life is short, and the Art long to learn.
–Chaucer
If you are anything like us, you still hold firmly to the notion that medicine, at its core, is an art. Sure, medicine is informed by cutting-edge sciences, such as biochemistry and molecular biology. But one can’t capture the nuances of the patient interaction by studying the Krebs cycle, nor define the motivating forces driving physicians by looking through a microscope. On the contrary, physicians are artists, much like musicians, sculptors, or dancers. And, like any of these artists, it would seem that a physician’s craft should improve through "practice," not by studying data or using a computer. So how do we reconcile this in the era of "Big Data"? How can the "ones and zeros" living deep in the "guts" of our electronic health records (EHRs) promise to revolutionize an art that has relied solely on the judgment – or gut sense – of physicians for centuries? Here we will attempt to answer these questions and ponder whether or not we really can improve the art of medicine with the help of data.
It’s more than the ‘ones and zeros’
With the right information and the proper tools to analyze it, it is possible to conceive of an improvement in our ability to care for patients. Take, for example, the prevention of malignancies. Currently, early detection of cancer relies heavily on a physician’s knowledge of – and compliance with – current cancer screening guidelines. Success also depends on a patient’s willingness to come in for annual visits to receive the instruction. If the patient doesn’t show up for a physical, or if the provider neglects to mention the need for a colonoscopy when the patient does appear, the test may go unordered (much to the patient’s relief, perhaps!). But the right tools and analytics won’t let that happen. Instead, the technology will identify the highest-risk populations with ever-improving accuracy and notify both physician and patient of the need for action. With enough data, we may even be able to make observations in trends of cancer inheritance never before possible and predict cancer long before it might be detected by conventional screening protocols.
It pays to care about the data
Physicians may not realize it, but data can have a significant financial advantage, by improving reimbursement and decreasing the overall cost of care. This can be achieved in two ways. The first way is by using the data to paint a more accurate picture of patient complexity. Medicare assigns a risk-adjusted score to patient cohorts based on the severity of their diagnoses and reimburses Medicare Advantage plans based on that score: the higher the score, the better the reimbursement. Occasionally, those additional dollars are passed along to the treating physicians. But all too often physicians do not use ICD-9 codes properly on their claims, making their patients appear less complicated and thereby receiving lower reimbursement. Through emerging data collection tools, improper coding can be identified and corrected, and missed opportunities can be discovered early enough to capture additional funds.
The second way these tools can be used leads to direct benefit to the health care system in general, through improved medical cost management. By interfacing with insurers and analyzing claims, the software can identify patients who are high utilizers and can show trends in medical costs across a community or health system. This allows providers to target certain patients or disease states around which to build cost-containment strategies and create win-win scenarios that decrease hospital readmissions, limit cost, and improve patient quality of life.
We recently learned of a great example of this. Using a population management data tool, a community health system was able to identify a geographic area in their region with a large uninsured population. This group had a disproportionately high utilization of emergency medical services and very low care quality markers (such as diabetes control, vaccination rates, etc.). Through targeted outreach based on these data, the system was able to direct individuals into low-cost, high-quality primary care sites and reduce emergency service utilization to levels below the surrounding neighborhoods. Simultaneously, the health of the community improved through better disease management and care coordination. Finally, data analytics tools uncovered additional opportunities for savings by identifying expensive brand-name drug prescriptions that could be replaced with generic drug alternatives.
A reluctant revolution
As we have lamented on previous occasions, the adoption of health care information technology is often driven by artificial external forces, such as stimulus programs or regulatory requirements. The government has routinely used incentive payments and reimbursement adjustments in order to spur widespread acceptance of EHRs. Most infamously, the Meaningful Use Regulations program has become the poster child for government involvement in direct patient care. Through the use of annual payments over a 5-year period (combined with the threat of penalties for lack of compliance), Meaningful Use has almost single-handedly enabled the Big Data revolution in health care by requiring physicians to purchase electronic health records systems and use them for population management. While seemingly a good thing, most physicians would hardly regard these systems as "meaningful." In fact, many question if there is any value in having an electronic record at all.
Whether or not their detractors admit it, EHRs do form the backbone of a new and very powerful information network – one which many believe has the power to revolutionize health care. While we certainly do not view the "data revolution" as the panacea others have claimed it to be, we do recognize that the right tools are emerging to enable physicians to learn from data and implement new, novel, and "disruptive" strategies to improve patient care.
Art is not static; the canvas, the paints, and the viewpoints change over time as experience evolves. Leonardo da Vinci furthered the world’s understanding of perspective. Pablo Picasso led a revolution in modern art. Each was different from his predecessors, and each expressed a human need to understand and portray the world in a manner consistent with his age. The same is true of our age and the art of medicine. The science has changed, as has the viewpoint and perspective from which we provide care. Our ability to record, retrieve, and understand health and disease will never be the same. But the attention to the patient is ever present. The necessity of interpreting the shifting world of health and disease to provide an empathic understanding of each patient’s individual and unique place in the world will never go away. Therein lies the Art.
Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. Dr. Skolnik is associate director of the family medicine residency program at Abington Memorial Hospital and professor of family and community medicine at Temple University, Philadelphia. He is editor in chief of Redi-Reference Inc., a software company that creates mobile apps.
Life is short, and the Art long to learn.
–Chaucer
If you are anything like us, you still hold firmly to the notion that medicine, at its core, is an art. Sure, medicine is informed by cutting-edge sciences, such as biochemistry and molecular biology. But one can’t capture the nuances of the patient interaction by studying the Krebs cycle, nor define the motivating forces driving physicians by looking through a microscope. On the contrary, physicians are artists, much like musicians, sculptors, or dancers. And, like any of these artists, it would seem that a physician’s craft should improve through "practice," not by studying data or using a computer. So how do we reconcile this in the era of "Big Data"? How can the "ones and zeros" living deep in the "guts" of our electronic health records (EHRs) promise to revolutionize an art that has relied solely on the judgment – or gut sense – of physicians for centuries? Here we will attempt to answer these questions and ponder whether or not we really can improve the art of medicine with the help of data.
It’s more than the ‘ones and zeros’
With the right information and the proper tools to analyze it, it is possible to conceive of an improvement in our ability to care for patients. Take, for example, the prevention of malignancies. Currently, early detection of cancer relies heavily on a physician’s knowledge of – and compliance with – current cancer screening guidelines. Success also depends on a patient’s willingness to come in for annual visits to receive the instruction. If the patient doesn’t show up for a physical, or if the provider neglects to mention the need for a colonoscopy when the patient does appear, the test may go unordered (much to the patient’s relief, perhaps!). But the right tools and analytics won’t let that happen. Instead, the technology will identify the highest-risk populations with ever-improving accuracy and notify both physician and patient of the need for action. With enough data, we may even be able to make observations in trends of cancer inheritance never before possible and predict cancer long before it might be detected by conventional screening protocols.
It pays to care about the data
Physicians may not realize it, but data can have a significant financial advantage, by improving reimbursement and decreasing the overall cost of care. This can be achieved in two ways. The first way is by using the data to paint a more accurate picture of patient complexity. Medicare assigns a risk-adjusted score to patient cohorts based on the severity of their diagnoses and reimburses Medicare Advantage plans based on that score: the higher the score, the better the reimbursement. Occasionally, those additional dollars are passed along to the treating physicians. But all too often physicians do not use ICD-9 codes properly on their claims, making their patients appear less complicated and thereby receiving lower reimbursement. Through emerging data collection tools, improper coding can be identified and corrected, and missed opportunities can be discovered early enough to capture additional funds.
The second way these tools can be used leads to direct benefit to the health care system in general, through improved medical cost management. By interfacing with insurers and analyzing claims, the software can identify patients who are high utilizers and can show trends in medical costs across a community or health system. This allows providers to target certain patients or disease states around which to build cost-containment strategies and create win-win scenarios that decrease hospital readmissions, limit cost, and improve patient quality of life.
We recently learned of a great example of this. Using a population management data tool, a community health system was able to identify a geographic area in their region with a large uninsured population. This group had a disproportionately high utilization of emergency medical services and very low care quality markers (such as diabetes control, vaccination rates, etc.). Through targeted outreach based on these data, the system was able to direct individuals into low-cost, high-quality primary care sites and reduce emergency service utilization to levels below the surrounding neighborhoods. Simultaneously, the health of the community improved through better disease management and care coordination. Finally, data analytics tools uncovered additional opportunities for savings by identifying expensive brand-name drug prescriptions that could be replaced with generic drug alternatives.
A reluctant revolution
As we have lamented on previous occasions, the adoption of health care information technology is often driven by artificial external forces, such as stimulus programs or regulatory requirements. The government has routinely used incentive payments and reimbursement adjustments in order to spur widespread acceptance of EHRs. Most infamously, the Meaningful Use Regulations program has become the poster child for government involvement in direct patient care. Through the use of annual payments over a 5-year period (combined with the threat of penalties for lack of compliance), Meaningful Use has almost single-handedly enabled the Big Data revolution in health care by requiring physicians to purchase electronic health records systems and use them for population management. While seemingly a good thing, most physicians would hardly regard these systems as "meaningful." In fact, many question if there is any value in having an electronic record at all.
Whether or not their detractors admit it, EHRs do form the backbone of a new and very powerful information network – one which many believe has the power to revolutionize health care. While we certainly do not view the "data revolution" as the panacea others have claimed it to be, we do recognize that the right tools are emerging to enable physicians to learn from data and implement new, novel, and "disruptive" strategies to improve patient care.
Art is not static; the canvas, the paints, and the viewpoints change over time as experience evolves. Leonardo da Vinci furthered the world’s understanding of perspective. Pablo Picasso led a revolution in modern art. Each was different from his predecessors, and each expressed a human need to understand and portray the world in a manner consistent with his age. The same is true of our age and the art of medicine. The science has changed, as has the viewpoint and perspective from which we provide care. Our ability to record, retrieve, and understand health and disease will never be the same. But the attention to the patient is ever present. The necessity of interpreting the shifting world of health and disease to provide an empathic understanding of each patient’s individual and unique place in the world will never go away. Therein lies the Art.
Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. Dr. Skolnik is associate director of the family medicine residency program at Abington Memorial Hospital and professor of family and community medicine at Temple University, Philadelphia. He is editor in chief of Redi-Reference Inc., a software company that creates mobile apps.
Life is short, and the Art long to learn.
–Chaucer
If you are anything like us, you still hold firmly to the notion that medicine, at its core, is an art. Sure, medicine is informed by cutting-edge sciences, such as biochemistry and molecular biology. But one can’t capture the nuances of the patient interaction by studying the Krebs cycle, nor define the motivating forces driving physicians by looking through a microscope. On the contrary, physicians are artists, much like musicians, sculptors, or dancers. And, like any of these artists, it would seem that a physician’s craft should improve through "practice," not by studying data or using a computer. So how do we reconcile this in the era of "Big Data"? How can the "ones and zeros" living deep in the "guts" of our electronic health records (EHRs) promise to revolutionize an art that has relied solely on the judgment – or gut sense – of physicians for centuries? Here we will attempt to answer these questions and ponder whether or not we really can improve the art of medicine with the help of data.
It’s more than the ‘ones and zeros’
With the right information and the proper tools to analyze it, it is possible to conceive of an improvement in our ability to care for patients. Take, for example, the prevention of malignancies. Currently, early detection of cancer relies heavily on a physician’s knowledge of – and compliance with – current cancer screening guidelines. Success also depends on a patient’s willingness to come in for annual visits to receive the instruction. If the patient doesn’t show up for a physical, or if the provider neglects to mention the need for a colonoscopy when the patient does appear, the test may go unordered (much to the patient’s relief, perhaps!). But the right tools and analytics won’t let that happen. Instead, the technology will identify the highest-risk populations with ever-improving accuracy and notify both physician and patient of the need for action. With enough data, we may even be able to make observations in trends of cancer inheritance never before possible and predict cancer long before it might be detected by conventional screening protocols.
It pays to care about the data
Physicians may not realize it, but data can have a significant financial advantage, by improving reimbursement and decreasing the overall cost of care. This can be achieved in two ways. The first way is by using the data to paint a more accurate picture of patient complexity. Medicare assigns a risk-adjusted score to patient cohorts based on the severity of their diagnoses and reimburses Medicare Advantage plans based on that score: the higher the score, the better the reimbursement. Occasionally, those additional dollars are passed along to the treating physicians. But all too often physicians do not use ICD-9 codes properly on their claims, making their patients appear less complicated and thereby receiving lower reimbursement. Through emerging data collection tools, improper coding can be identified and corrected, and missed opportunities can be discovered early enough to capture additional funds.
The second way these tools can be used leads to direct benefit to the health care system in general, through improved medical cost management. By interfacing with insurers and analyzing claims, the software can identify patients who are high utilizers and can show trends in medical costs across a community or health system. This allows providers to target certain patients or disease states around which to build cost-containment strategies and create win-win scenarios that decrease hospital readmissions, limit cost, and improve patient quality of life.
We recently learned of a great example of this. Using a population management data tool, a community health system was able to identify a geographic area in their region with a large uninsured population. This group had a disproportionately high utilization of emergency medical services and very low care quality markers (such as diabetes control, vaccination rates, etc.). Through targeted outreach based on these data, the system was able to direct individuals into low-cost, high-quality primary care sites and reduce emergency service utilization to levels below the surrounding neighborhoods. Simultaneously, the health of the community improved through better disease management and care coordination. Finally, data analytics tools uncovered additional opportunities for savings by identifying expensive brand-name drug prescriptions that could be replaced with generic drug alternatives.
A reluctant revolution
As we have lamented on previous occasions, the adoption of health care information technology is often driven by artificial external forces, such as stimulus programs or regulatory requirements. The government has routinely used incentive payments and reimbursement adjustments in order to spur widespread acceptance of EHRs. Most infamously, the Meaningful Use Regulations program has become the poster child for government involvement in direct patient care. Through the use of annual payments over a 5-year period (combined with the threat of penalties for lack of compliance), Meaningful Use has almost single-handedly enabled the Big Data revolution in health care by requiring physicians to purchase electronic health records systems and use them for population management. While seemingly a good thing, most physicians would hardly regard these systems as "meaningful." In fact, many question if there is any value in having an electronic record at all.
Whether or not their detractors admit it, EHRs do form the backbone of a new and very powerful information network – one which many believe has the power to revolutionize health care. While we certainly do not view the "data revolution" as the panacea others have claimed it to be, we do recognize that the right tools are emerging to enable physicians to learn from data and implement new, novel, and "disruptive" strategies to improve patient care.
Art is not static; the canvas, the paints, and the viewpoints change over time as experience evolves. Leonardo da Vinci furthered the world’s understanding of perspective. Pablo Picasso led a revolution in modern art. Each was different from his predecessors, and each expressed a human need to understand and portray the world in a manner consistent with his age. The same is true of our age and the art of medicine. The science has changed, as has the viewpoint and perspective from which we provide care. Our ability to record, retrieve, and understand health and disease will never be the same. But the attention to the patient is ever present. The necessity of interpreting the shifting world of health and disease to provide an empathic understanding of each patient’s individual and unique place in the world will never go away. Therein lies the Art.
Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. Dr. Skolnik is associate director of the family medicine residency program at Abington Memorial Hospital and professor of family and community medicine at Temple University, Philadelphia. He is editor in chief of Redi-Reference Inc., a software company that creates mobile apps.