User login
No reduction in oral mucositis with folinic acid post transplant
ORLANDO – Folinic acid does not prevent oral mucositis in patients who are receiving a calcineurin inhibitor and methotrexate for graft-vs.-host disease prophylaxis, results of a multicenter study from Israel suggest.
A randomized clinical trial to determine whether folinic acid rescue 24 hours after a methotrexate dose protects patients against severe oral mucositis was halted for futility after an interim analysis showed no advantage to adding folinic acid, reported Moshe Yeshrun, MD, of Rabin Medical Center at Tel Aviv University, Israel.
“Regarding the primary and secondary endpoints, we observed identical rates and duration of severe oral mucositis, as well as identical rates of oral mucositis of any grade in the folinic acid and placebo groups,” he said at the annual Transplantation and Cellular Therapy Meetings.
There were also no significant differences between the folinic acid and placebo control groups in time to neutrophil and platelet engraftment, rates of febrile neutropenia and bloodstream infections, veno-occlusive disease, need for opiates or total parenteral nutrition (TPN), or time from transplant to discharge, Dr. Yeshrun added at the meeting, held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
Folinic acid therapy did not, however, appear to abrogate or interfere with the effects of methotrexate on prevention of either acute or chronic graft-vs-host disease (GVHD).
Severe adverse event
Oral mucositis can be a serious complication of therapy, associated with increased morbidity and mortality; significant pain; difficulty with eating or speaking; difficulty swallowing water, food, and medications; prolonged hospitalizations; and increased costs of care, Dr. Yeshrun noted.
The presence of oral mucositis sometimes leads clinicians to reduce or even skip methotrexate doses, thereby increasing risk for GVHD.
There are limited data from nonrandomized studies indicating that folinic acid (also called leucovorin) may reduce methotrexate-associated toxicities, and both the European Society for Blood and Marrow Transplantation and European LeukemiaNet working group recommend the use of folinic-acid rescue 24 hours following each methotrexate dose, Dr. Yeshrun said.
To see whether folinic acid rescue actually reduces the rate of methotrexate-induced toxicity and affects outcomes for patients who receive methotrexate post transplant for GVHD prophylaxis, Dr. Yeshrun and colleagues in three Israeli medical centers conducted a randomized, placebo-controlled trial.
The eligible study population included patients 18 and older with hematological malignancies in complete remission or minimal residual disease who underwent myeloablative conditioning and allogeneic transplant from HLA-matched or 1-antigen mismatched siblings or unrelated donors.
The patients were stratified by treatment center and intensity of the conditioning regimen, and then randomized on a 1:1 basis to receive folinic acid or placebo beginning 24 hours after each methotrexate dose, with the assigned medication given at a dose of 15 mg three times daily on the first day, and once daily on days 3 and 6.
Patients who received a transplant from an unrelated donor were also given anti-thymocyte globulin at a total dose of 15 mg/kg.
Supportive care included filgrastim (Neupogen) 5 mcg/kg from day 7 until neutrophil engraftment, infection prophylaxis, and ursodeoxycholic acid for prevention of veno-occlusive disease.
Trial stopped
Although the study was designed to enroll 116 patients to have a power of 80% to detect a 50% reduction in the rate of severe oral mucositis from an anticipated 50% in the placebo arm, a planned interim analysis conducted after approximately half of the target events occurred showed no difference in the rates of severe oral mucositis, and the trial was halted.
A total of 28 patients in the folinic acid group and 24 in the placebo group were available for the analysis.
The rate of grade 3 or 4 mucositis, the primary endpoint, was 46.6% in the folinic acid group, and 45.8% in the placebo group.
Respectively, the median duration of severe oral mucositis was 4 days in each group, days to neutrophil engraftment were a median of 12 and to platelet engraftment were a median of 13 days in each group, rates of febrile neutropenia were 57.1% and 58.3%, rates of bloodstream infections were 10.7% and 16.6%, rates of veno-occlusive disease were 7% and 12.5%, need for TPN occurred in 14.2% vs. 25%, need for opiates occurred in 78.5% vs. 66.6%, and median time to discharge was 18 and 19 days. As noted, none of the differences were statistically significant.
“These unequivocal interim results led to our decision to discontinue the study,” Dr. Yeshrun said.
Arnon Nagler, MD, MSc, from Sheba Medical Center Tel HaShomer at Tel Aviv University in Israel, who was not involved in the study, said in an interview “I think this is a very practical and important study, because we need evidence-based data such as this.”
Dr. Nagler, who comoderated the session where the data were presented, noted that the study was limited by the small number of patients and the lack of a subgroup analysis, but emphasized that the findings were important nonetheless.
His comoderator, Maria Gilleece, MD, director of the Yorkshire (England) Blood and Marrow Transplant Program, noted in an interview that folinic acid is frequently used in the United Kingdom for patients receiving high-dose methotrexate, ”and certainly, this study suggests that may be inappropriate.”
Rabin Medical Center sponsored the trial. Dr. Yeshrun, Dr. Nagler, and Dr. Gilleece reported no relevant conflicts of interest.
SOURCE: Yeshrun M. et al. TCT 2020. Abstract 61.
ORLANDO – Folinic acid does not prevent oral mucositis in patients who are receiving a calcineurin inhibitor and methotrexate for graft-vs.-host disease prophylaxis, results of a multicenter study from Israel suggest.
A randomized clinical trial to determine whether folinic acid rescue 24 hours after a methotrexate dose protects patients against severe oral mucositis was halted for futility after an interim analysis showed no advantage to adding folinic acid, reported Moshe Yeshrun, MD, of Rabin Medical Center at Tel Aviv University, Israel.
“Regarding the primary and secondary endpoints, we observed identical rates and duration of severe oral mucositis, as well as identical rates of oral mucositis of any grade in the folinic acid and placebo groups,” he said at the annual Transplantation and Cellular Therapy Meetings.
There were also no significant differences between the folinic acid and placebo control groups in time to neutrophil and platelet engraftment, rates of febrile neutropenia and bloodstream infections, veno-occlusive disease, need for opiates or total parenteral nutrition (TPN), or time from transplant to discharge, Dr. Yeshrun added at the meeting, held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
Folinic acid therapy did not, however, appear to abrogate or interfere with the effects of methotrexate on prevention of either acute or chronic graft-vs-host disease (GVHD).
Severe adverse event
Oral mucositis can be a serious complication of therapy, associated with increased morbidity and mortality; significant pain; difficulty with eating or speaking; difficulty swallowing water, food, and medications; prolonged hospitalizations; and increased costs of care, Dr. Yeshrun noted.
The presence of oral mucositis sometimes leads clinicians to reduce or even skip methotrexate doses, thereby increasing risk for GVHD.
There are limited data from nonrandomized studies indicating that folinic acid (also called leucovorin) may reduce methotrexate-associated toxicities, and both the European Society for Blood and Marrow Transplantation and European LeukemiaNet working group recommend the use of folinic-acid rescue 24 hours following each methotrexate dose, Dr. Yeshrun said.
To see whether folinic acid rescue actually reduces the rate of methotrexate-induced toxicity and affects outcomes for patients who receive methotrexate post transplant for GVHD prophylaxis, Dr. Yeshrun and colleagues in three Israeli medical centers conducted a randomized, placebo-controlled trial.
The eligible study population included patients 18 and older with hematological malignancies in complete remission or minimal residual disease who underwent myeloablative conditioning and allogeneic transplant from HLA-matched or 1-antigen mismatched siblings or unrelated donors.
The patients were stratified by treatment center and intensity of the conditioning regimen, and then randomized on a 1:1 basis to receive folinic acid or placebo beginning 24 hours after each methotrexate dose, with the assigned medication given at a dose of 15 mg three times daily on the first day, and once daily on days 3 and 6.
Patients who received a transplant from an unrelated donor were also given anti-thymocyte globulin at a total dose of 15 mg/kg.
Supportive care included filgrastim (Neupogen) 5 mcg/kg from day 7 until neutrophil engraftment, infection prophylaxis, and ursodeoxycholic acid for prevention of veno-occlusive disease.
Trial stopped
Although the study was designed to enroll 116 patients to have a power of 80% to detect a 50% reduction in the rate of severe oral mucositis from an anticipated 50% in the placebo arm, a planned interim analysis conducted after approximately half of the target events occurred showed no difference in the rates of severe oral mucositis, and the trial was halted.
A total of 28 patients in the folinic acid group and 24 in the placebo group were available for the analysis.
The rate of grade 3 or 4 mucositis, the primary endpoint, was 46.6% in the folinic acid group, and 45.8% in the placebo group.
Respectively, the median duration of severe oral mucositis was 4 days in each group, days to neutrophil engraftment were a median of 12 and to platelet engraftment were a median of 13 days in each group, rates of febrile neutropenia were 57.1% and 58.3%, rates of bloodstream infections were 10.7% and 16.6%, rates of veno-occlusive disease were 7% and 12.5%, need for TPN occurred in 14.2% vs. 25%, need for opiates occurred in 78.5% vs. 66.6%, and median time to discharge was 18 and 19 days. As noted, none of the differences were statistically significant.
“These unequivocal interim results led to our decision to discontinue the study,” Dr. Yeshrun said.
Arnon Nagler, MD, MSc, from Sheba Medical Center Tel HaShomer at Tel Aviv University in Israel, who was not involved in the study, said in an interview “I think this is a very practical and important study, because we need evidence-based data such as this.”
Dr. Nagler, who comoderated the session where the data were presented, noted that the study was limited by the small number of patients and the lack of a subgroup analysis, but emphasized that the findings were important nonetheless.
His comoderator, Maria Gilleece, MD, director of the Yorkshire (England) Blood and Marrow Transplant Program, noted in an interview that folinic acid is frequently used in the United Kingdom for patients receiving high-dose methotrexate, ”and certainly, this study suggests that may be inappropriate.”
Rabin Medical Center sponsored the trial. Dr. Yeshrun, Dr. Nagler, and Dr. Gilleece reported no relevant conflicts of interest.
SOURCE: Yeshrun M. et al. TCT 2020. Abstract 61.
ORLANDO – Folinic acid does not prevent oral mucositis in patients who are receiving a calcineurin inhibitor and methotrexate for graft-vs.-host disease prophylaxis, results of a multicenter study from Israel suggest.
A randomized clinical trial to determine whether folinic acid rescue 24 hours after a methotrexate dose protects patients against severe oral mucositis was halted for futility after an interim analysis showed no advantage to adding folinic acid, reported Moshe Yeshrun, MD, of Rabin Medical Center at Tel Aviv University, Israel.
“Regarding the primary and secondary endpoints, we observed identical rates and duration of severe oral mucositis, as well as identical rates of oral mucositis of any grade in the folinic acid and placebo groups,” he said at the annual Transplantation and Cellular Therapy Meetings.
There were also no significant differences between the folinic acid and placebo control groups in time to neutrophil and platelet engraftment, rates of febrile neutropenia and bloodstream infections, veno-occlusive disease, need for opiates or total parenteral nutrition (TPN), or time from transplant to discharge, Dr. Yeshrun added at the meeting, held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
Folinic acid therapy did not, however, appear to abrogate or interfere with the effects of methotrexate on prevention of either acute or chronic graft-vs-host disease (GVHD).
Severe adverse event
Oral mucositis can be a serious complication of therapy, associated with increased morbidity and mortality; significant pain; difficulty with eating or speaking; difficulty swallowing water, food, and medications; prolonged hospitalizations; and increased costs of care, Dr. Yeshrun noted.
The presence of oral mucositis sometimes leads clinicians to reduce or even skip methotrexate doses, thereby increasing risk for GVHD.
There are limited data from nonrandomized studies indicating that folinic acid (also called leucovorin) may reduce methotrexate-associated toxicities, and both the European Society for Blood and Marrow Transplantation and European LeukemiaNet working group recommend the use of folinic-acid rescue 24 hours following each methotrexate dose, Dr. Yeshrun said.
To see whether folinic acid rescue actually reduces the rate of methotrexate-induced toxicity and affects outcomes for patients who receive methotrexate post transplant for GVHD prophylaxis, Dr. Yeshrun and colleagues in three Israeli medical centers conducted a randomized, placebo-controlled trial.
The eligible study population included patients 18 and older with hematological malignancies in complete remission or minimal residual disease who underwent myeloablative conditioning and allogeneic transplant from HLA-matched or 1-antigen mismatched siblings or unrelated donors.
The patients were stratified by treatment center and intensity of the conditioning regimen, and then randomized on a 1:1 basis to receive folinic acid or placebo beginning 24 hours after each methotrexate dose, with the assigned medication given at a dose of 15 mg three times daily on the first day, and once daily on days 3 and 6.
Patients who received a transplant from an unrelated donor were also given anti-thymocyte globulin at a total dose of 15 mg/kg.
Supportive care included filgrastim (Neupogen) 5 mcg/kg from day 7 until neutrophil engraftment, infection prophylaxis, and ursodeoxycholic acid for prevention of veno-occlusive disease.
Trial stopped
Although the study was designed to enroll 116 patients to have a power of 80% to detect a 50% reduction in the rate of severe oral mucositis from an anticipated 50% in the placebo arm, a planned interim analysis conducted after approximately half of the target events occurred showed no difference in the rates of severe oral mucositis, and the trial was halted.
A total of 28 patients in the folinic acid group and 24 in the placebo group were available for the analysis.
The rate of grade 3 or 4 mucositis, the primary endpoint, was 46.6% in the folinic acid group, and 45.8% in the placebo group.
Respectively, the median duration of severe oral mucositis was 4 days in each group, days to neutrophil engraftment were a median of 12 and to platelet engraftment were a median of 13 days in each group, rates of febrile neutropenia were 57.1% and 58.3%, rates of bloodstream infections were 10.7% and 16.6%, rates of veno-occlusive disease were 7% and 12.5%, need for TPN occurred in 14.2% vs. 25%, need for opiates occurred in 78.5% vs. 66.6%, and median time to discharge was 18 and 19 days. As noted, none of the differences were statistically significant.
“These unequivocal interim results led to our decision to discontinue the study,” Dr. Yeshrun said.
Arnon Nagler, MD, MSc, from Sheba Medical Center Tel HaShomer at Tel Aviv University in Israel, who was not involved in the study, said in an interview “I think this is a very practical and important study, because we need evidence-based data such as this.”
Dr. Nagler, who comoderated the session where the data were presented, noted that the study was limited by the small number of patients and the lack of a subgroup analysis, but emphasized that the findings were important nonetheless.
His comoderator, Maria Gilleece, MD, director of the Yorkshire (England) Blood and Marrow Transplant Program, noted in an interview that folinic acid is frequently used in the United Kingdom for patients receiving high-dose methotrexate, ”and certainly, this study suggests that may be inappropriate.”
Rabin Medical Center sponsored the trial. Dr. Yeshrun, Dr. Nagler, and Dr. Gilleece reported no relevant conflicts of interest.
SOURCE: Yeshrun M. et al. TCT 2020. Abstract 61.
REPORTING FROM TCT 2020
Safer CAR uses modified NK cells for advanced CLL, NHL
A chimeric antigen receptor (CAR) construct using transduced natural killer cells instead of T cells was associated with a high complete remission rate without the cytokine release syndrome frequently seen with CAR T cell therapy, early clinical trial results show.
The construct, consisting of natural killer (NK) cells derived from umbilical cord blood that have been transduced to target CD19-expressing cells combined with interleukin 15 and equipped with an “off” switch, offers the prospect of an off-the-shelf CAR product, reported Enli Liu, MD, and colleagues at the University of Texas MD Anderson Cancer Center in Houston.
“We found that allogeneic CAR-NK cells can be delivered in adoptive transfer without the serious cytokine release syndrome and neurologic toxic effects that have been associated with CAR T-cell therapy,” they wrote in The New England Journal of Medicine.
The modified NK cells were delivered to 9 of 11 patients with only partial human leukocyte antigen (HLA) matching, and in 2 patients with no matching, yet there were no cases of graft-versus host disease (GvHD), and no patients had symptoms of cytokine release syndrome (CRS), neurotoxicity, or hemophagocytic lymphohistiocytosis.
CAR T cell production “is a cumbersome process that requires coordination and collection of the cells and there’s several weeks of manufacturing, during which time patients often can have their lymphoma worsen, and so at times it’s a little bit of a race against the clock to get those cells manufactured,” Brian Hill, MD, PhD, director of the lymphoid malignancies program at Taussig Cancer Institute at Cleveland Clinic, said in an interview.
Dr. Hill, who was not involved in the study, said that the proof-of-principle study shows promising early results and offers the prospect of an effective and safe off-the-shelf therapeutic option for patients with lymphoid malignancies.
Advanced B-cell cancers
The investigators conducted a phase 1/2 trial in patients with B-cell lymphoid malignancies, including five patients with chronic lymphocytic leukemia (CLL), one patient with Richter’s transformation and one with accelerated CLL, three with transformed follicular lymphoma, two with diffuse large B-cell lymphoma (DLBCL), and one with follicular lymphoma (focally grade 3B).
The patients were all heavily pretreated, with 3 to as many as 11 prior lines of therapy.
The patients received cord blood-derived NK cells that had been transduced with a retroviral vector expressing genes that encode anti-CD19 CAR, interleukin-15, and inducible caspase 9 as a safety switch.
The cells were expanded in the lab and after the patients underwent lymphodepleting chemotherapy, they received the cells in a single infusion at one of three doses, either 1×105, 1×106, or 1×107 CAR-NK cells per kilogram of body weight.
As noted before, there were no cases of CRS, neurotoxicity, or GvHD and no increase over baseline in inflammatory cytokines, including interleukin-6, a key factor in the development and severity of CRS. The maximum tolerated dose was not reached.
Early efficacy
Of the 11 patients, 8 had a clinical response, and 7 had a complete remission, including 4 patients with lymphomas and 3 with CLL.
The patient with CLL with Richter’s transformation had a remission of the Richter’s component, but not of the CLL itself.
“This is particularly remarkable, because these patients are notoriously very difficult to treat, and the efficacy of autologous CAR T cell therapy in CLL and Richter’s patients has been hampered by lack of fitness of the patient’s own T cells when manufacturing the CAR T cell product, so this approach may obviate the need for autologous T cells in these patients,” Dr. Hill said.
The responses were rapid and occurred within 30 days of infusion at all dose levels. In addition, there was evidence of expansion and persistence of the modified NK cells at low levels for at least 1 year, despite the HLA mismatches between the NK cells and the recipients. The investigators speculated that the inclusion of interleukin-15 in the NL construct may at least partially account for the persistence of the cells and their antitumor activity.
Of the eight patients with a response, five had postremission therapy, including two patients with CLL who had minimal residual disease (MRD), one patient with follicular lymphoma and one with transformed follicular lymphoma who underwent hematopoietic stem-cell transplantation while in complete remission without evidence of MRD, and the patient with CLLL with Richter’s transformation with remission of the lymphoma component, who received a course of venetoclax.
The authors acknowledged that it may be difficult to assess the durability of response after CAR NK therapy in this study because of the allowed consolidation therapy for patients in remission.
They noted that although the patients in the current study each had a fresh CAR NK product manufactured for them, “we have shown that it is possible to produce more than 100 doses of CAR-NK cells from a single cord-blood unit. This capability, together with the apparently minimal HLA-matching requirements between the donor of CAR-NK cells and the patient, may pave the way for a truly off-the-shelf product that could increase treatment accessibility for many more patients.”
The National Institutes of Health supported the study. Dr. Liu disclosed a pending patent for methods of production of CAR-NK cells, and a patent held by MD Anderson for methods of treatment with NK cells. Dr. Hill is a member of the Hematology News editorial advisory board.
SOURCE: Liu E et al. N Engl J Med. 2020 Feb 6;382:545-53.
A chimeric antigen receptor (CAR) construct using transduced natural killer cells instead of T cells was associated with a high complete remission rate without the cytokine release syndrome frequently seen with CAR T cell therapy, early clinical trial results show.
The construct, consisting of natural killer (NK) cells derived from umbilical cord blood that have been transduced to target CD19-expressing cells combined with interleukin 15 and equipped with an “off” switch, offers the prospect of an off-the-shelf CAR product, reported Enli Liu, MD, and colleagues at the University of Texas MD Anderson Cancer Center in Houston.
“We found that allogeneic CAR-NK cells can be delivered in adoptive transfer without the serious cytokine release syndrome and neurologic toxic effects that have been associated with CAR T-cell therapy,” they wrote in The New England Journal of Medicine.
The modified NK cells were delivered to 9 of 11 patients with only partial human leukocyte antigen (HLA) matching, and in 2 patients with no matching, yet there were no cases of graft-versus host disease (GvHD), and no patients had symptoms of cytokine release syndrome (CRS), neurotoxicity, or hemophagocytic lymphohistiocytosis.
CAR T cell production “is a cumbersome process that requires coordination and collection of the cells and there’s several weeks of manufacturing, during which time patients often can have their lymphoma worsen, and so at times it’s a little bit of a race against the clock to get those cells manufactured,” Brian Hill, MD, PhD, director of the lymphoid malignancies program at Taussig Cancer Institute at Cleveland Clinic, said in an interview.
Dr. Hill, who was not involved in the study, said that the proof-of-principle study shows promising early results and offers the prospect of an effective and safe off-the-shelf therapeutic option for patients with lymphoid malignancies.
Advanced B-cell cancers
The investigators conducted a phase 1/2 trial in patients with B-cell lymphoid malignancies, including five patients with chronic lymphocytic leukemia (CLL), one patient with Richter’s transformation and one with accelerated CLL, three with transformed follicular lymphoma, two with diffuse large B-cell lymphoma (DLBCL), and one with follicular lymphoma (focally grade 3B).
The patients were all heavily pretreated, with 3 to as many as 11 prior lines of therapy.
The patients received cord blood-derived NK cells that had been transduced with a retroviral vector expressing genes that encode anti-CD19 CAR, interleukin-15, and inducible caspase 9 as a safety switch.
The cells were expanded in the lab and after the patients underwent lymphodepleting chemotherapy, they received the cells in a single infusion at one of three doses, either 1×105, 1×106, or 1×107 CAR-NK cells per kilogram of body weight.
As noted before, there were no cases of CRS, neurotoxicity, or GvHD and no increase over baseline in inflammatory cytokines, including interleukin-6, a key factor in the development and severity of CRS. The maximum tolerated dose was not reached.
Early efficacy
Of the 11 patients, 8 had a clinical response, and 7 had a complete remission, including 4 patients with lymphomas and 3 with CLL.
The patient with CLL with Richter’s transformation had a remission of the Richter’s component, but not of the CLL itself.
“This is particularly remarkable, because these patients are notoriously very difficult to treat, and the efficacy of autologous CAR T cell therapy in CLL and Richter’s patients has been hampered by lack of fitness of the patient’s own T cells when manufacturing the CAR T cell product, so this approach may obviate the need for autologous T cells in these patients,” Dr. Hill said.
The responses were rapid and occurred within 30 days of infusion at all dose levels. In addition, there was evidence of expansion and persistence of the modified NK cells at low levels for at least 1 year, despite the HLA mismatches between the NK cells and the recipients. The investigators speculated that the inclusion of interleukin-15 in the NL construct may at least partially account for the persistence of the cells and their antitumor activity.
Of the eight patients with a response, five had postremission therapy, including two patients with CLL who had minimal residual disease (MRD), one patient with follicular lymphoma and one with transformed follicular lymphoma who underwent hematopoietic stem-cell transplantation while in complete remission without evidence of MRD, and the patient with CLLL with Richter’s transformation with remission of the lymphoma component, who received a course of venetoclax.
The authors acknowledged that it may be difficult to assess the durability of response after CAR NK therapy in this study because of the allowed consolidation therapy for patients in remission.
They noted that although the patients in the current study each had a fresh CAR NK product manufactured for them, “we have shown that it is possible to produce more than 100 doses of CAR-NK cells from a single cord-blood unit. This capability, together with the apparently minimal HLA-matching requirements between the donor of CAR-NK cells and the patient, may pave the way for a truly off-the-shelf product that could increase treatment accessibility for many more patients.”
The National Institutes of Health supported the study. Dr. Liu disclosed a pending patent for methods of production of CAR-NK cells, and a patent held by MD Anderson for methods of treatment with NK cells. Dr. Hill is a member of the Hematology News editorial advisory board.
SOURCE: Liu E et al. N Engl J Med. 2020 Feb 6;382:545-53.
A chimeric antigen receptor (CAR) construct using transduced natural killer cells instead of T cells was associated with a high complete remission rate without the cytokine release syndrome frequently seen with CAR T cell therapy, early clinical trial results show.
The construct, consisting of natural killer (NK) cells derived from umbilical cord blood that have been transduced to target CD19-expressing cells combined with interleukin 15 and equipped with an “off” switch, offers the prospect of an off-the-shelf CAR product, reported Enli Liu, MD, and colleagues at the University of Texas MD Anderson Cancer Center in Houston.
“We found that allogeneic CAR-NK cells can be delivered in adoptive transfer without the serious cytokine release syndrome and neurologic toxic effects that have been associated with CAR T-cell therapy,” they wrote in The New England Journal of Medicine.
The modified NK cells were delivered to 9 of 11 patients with only partial human leukocyte antigen (HLA) matching, and in 2 patients with no matching, yet there were no cases of graft-versus host disease (GvHD), and no patients had symptoms of cytokine release syndrome (CRS), neurotoxicity, or hemophagocytic lymphohistiocytosis.
CAR T cell production “is a cumbersome process that requires coordination and collection of the cells and there’s several weeks of manufacturing, during which time patients often can have their lymphoma worsen, and so at times it’s a little bit of a race against the clock to get those cells manufactured,” Brian Hill, MD, PhD, director of the lymphoid malignancies program at Taussig Cancer Institute at Cleveland Clinic, said in an interview.
Dr. Hill, who was not involved in the study, said that the proof-of-principle study shows promising early results and offers the prospect of an effective and safe off-the-shelf therapeutic option for patients with lymphoid malignancies.
Advanced B-cell cancers
The investigators conducted a phase 1/2 trial in patients with B-cell lymphoid malignancies, including five patients with chronic lymphocytic leukemia (CLL), one patient with Richter’s transformation and one with accelerated CLL, three with transformed follicular lymphoma, two with diffuse large B-cell lymphoma (DLBCL), and one with follicular lymphoma (focally grade 3B).
The patients were all heavily pretreated, with 3 to as many as 11 prior lines of therapy.
The patients received cord blood-derived NK cells that had been transduced with a retroviral vector expressing genes that encode anti-CD19 CAR, interleukin-15, and inducible caspase 9 as a safety switch.
The cells were expanded in the lab and after the patients underwent lymphodepleting chemotherapy, they received the cells in a single infusion at one of three doses, either 1×105, 1×106, or 1×107 CAR-NK cells per kilogram of body weight.
As noted before, there were no cases of CRS, neurotoxicity, or GvHD and no increase over baseline in inflammatory cytokines, including interleukin-6, a key factor in the development and severity of CRS. The maximum tolerated dose was not reached.
Early efficacy
Of the 11 patients, 8 had a clinical response, and 7 had a complete remission, including 4 patients with lymphomas and 3 with CLL.
The patient with CLL with Richter’s transformation had a remission of the Richter’s component, but not of the CLL itself.
“This is particularly remarkable, because these patients are notoriously very difficult to treat, and the efficacy of autologous CAR T cell therapy in CLL and Richter’s patients has been hampered by lack of fitness of the patient’s own T cells when manufacturing the CAR T cell product, so this approach may obviate the need for autologous T cells in these patients,” Dr. Hill said.
The responses were rapid and occurred within 30 days of infusion at all dose levels. In addition, there was evidence of expansion and persistence of the modified NK cells at low levels for at least 1 year, despite the HLA mismatches between the NK cells and the recipients. The investigators speculated that the inclusion of interleukin-15 in the NL construct may at least partially account for the persistence of the cells and their antitumor activity.
Of the eight patients with a response, five had postremission therapy, including two patients with CLL who had minimal residual disease (MRD), one patient with follicular lymphoma and one with transformed follicular lymphoma who underwent hematopoietic stem-cell transplantation while in complete remission without evidence of MRD, and the patient with CLLL with Richter’s transformation with remission of the lymphoma component, who received a course of venetoclax.
The authors acknowledged that it may be difficult to assess the durability of response after CAR NK therapy in this study because of the allowed consolidation therapy for patients in remission.
They noted that although the patients in the current study each had a fresh CAR NK product manufactured for them, “we have shown that it is possible to produce more than 100 doses of CAR-NK cells from a single cord-blood unit. This capability, together with the apparently minimal HLA-matching requirements between the donor of CAR-NK cells and the patient, may pave the way for a truly off-the-shelf product that could increase treatment accessibility for many more patients.”
The National Institutes of Health supported the study. Dr. Liu disclosed a pending patent for methods of production of CAR-NK cells, and a patent held by MD Anderson for methods of treatment with NK cells. Dr. Hill is a member of the Hematology News editorial advisory board.
SOURCE: Liu E et al. N Engl J Med. 2020 Feb 6;382:545-53.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Adrenal-permissive genotype linked to poor prognosis in prostate cancer
Men with low-volume, advanced prostate cancer who carry one or more adrenal-permissive alleles of the HSD3B1 gene have worse prognosis than do men with adrenal-restrictive alleles of HSD3B1, a retrospective analysis suggests.
Among 475 white men in the CHAARTED trial, those with the adrenal-permissive allele, HSD3B1(1245C), had a nearly twofold greater risk for developing early castration-resistant prostate cancer (CRPC) and for lower overall survival, compared with men who had the adrenal-restrictive allele, HSD3B1(1245A).
“Our findings suggest that the HSD3B1 genotype can be used to risk stratify white men with low-volume, metastatic prostate cancer,” Jason W.D. Hearn, MD, of the University of Michigan in Ann Arbor and colleagues wrote in JAMA Oncology.
HSD3B1 encodes for an enzyme involved in regulating the rate of metabolic conversion of the precursor hormone dehydroepiandrosterone into testosterone and dihydrotestosterone in prostate tissues. A common missense-encoding germline mutation in the gene leads to variant metabolic phenotypes, including the adrenal-permissive type that allows for ramped-up conversion of the precursor hormone into dihydrotestosterone and the adrenal-restrictive type that results in more rapid enzyme degradation, limiting the conversion from the precursor into the active hormone.
“The population frequency of the adrenal-permissive HSD3B1(1245C) allele varies tremendously by race and is disproportionally high in white men (e.g., carried in approximately 70% of Italian and Spanish men and only about 9% of Yoruba Nigerian men),” Dr. Hearn and colleagues wrote.
Previous studies have suggested that men with both metastatic and nonmetastatic prostate cancer who inherit the adrenal permissive HSD3B1(1245C) allele have worse clinical outcomes, compared with men without that allele.
To validate these findings, Dr. Hearn and colleagues retrospectively determined the germline genotype of HSD3B1 for 475 white men enrolled in CHAARTED. In that trial, men with metastatic, hormone-sensitive prostate cancer were randomized to either castration alone or castration plus docetaxel. The current analysis showed that 270 of those patients carried the adrenal-permissive allele.
Among patients with low-volume disease, the adrenal-permissive genotype was associated with significantly lower freedom from CRPC at 2 years, compared with the adrenal-restrictive genotype: 51% and 70.5%, respectively (hazard ratio, 1.89; P = .02). The 5-year overall survival rate was significantly lower for adrenal-permissive allele carriers than adrenal-restrictive allele carriers: 57.5% and 70.8%, respectively (HR, 1.74; P = .045).
Among men with high-volume disease, there was no association between HSD3B1 genotypes and outcomes, and there was no interaction between genotype and benefit from docetaxel. High-volume disease was defined as the presence of visceral metastases or four or more bone metastases with one or more lesions beyond the pelvis and vertebral bodies.
“Regardless of genotype, men with high-volume disease achieved better outcomes when docetaxel was added to ADT [androgen deprivation therapy], whereas outcomes in men with low-volume disease varied only by genotype, with no clear benefit from docetaxel,” the investigators wrote.
The trial was funded by the National Cancer Institute. The authors disclosed relationships with several pharmaceutical companies. One author is a coinventor on a patent for a treatment method based on HSD3B1.
SOURCE: Hearn JWD et al. JAMA Oncol. 2020 Feb 13. doi: 10.1001/jamaoncol.2019.6496.
Men with low-volume, advanced prostate cancer who carry one or more adrenal-permissive alleles of the HSD3B1 gene have worse prognosis than do men with adrenal-restrictive alleles of HSD3B1, a retrospective analysis suggests.
Among 475 white men in the CHAARTED trial, those with the adrenal-permissive allele, HSD3B1(1245C), had a nearly twofold greater risk for developing early castration-resistant prostate cancer (CRPC) and for lower overall survival, compared with men who had the adrenal-restrictive allele, HSD3B1(1245A).
“Our findings suggest that the HSD3B1 genotype can be used to risk stratify white men with low-volume, metastatic prostate cancer,” Jason W.D. Hearn, MD, of the University of Michigan in Ann Arbor and colleagues wrote in JAMA Oncology.
HSD3B1 encodes for an enzyme involved in regulating the rate of metabolic conversion of the precursor hormone dehydroepiandrosterone into testosterone and dihydrotestosterone in prostate tissues. A common missense-encoding germline mutation in the gene leads to variant metabolic phenotypes, including the adrenal-permissive type that allows for ramped-up conversion of the precursor hormone into dihydrotestosterone and the adrenal-restrictive type that results in more rapid enzyme degradation, limiting the conversion from the precursor into the active hormone.
“The population frequency of the adrenal-permissive HSD3B1(1245C) allele varies tremendously by race and is disproportionally high in white men (e.g., carried in approximately 70% of Italian and Spanish men and only about 9% of Yoruba Nigerian men),” Dr. Hearn and colleagues wrote.
Previous studies have suggested that men with both metastatic and nonmetastatic prostate cancer who inherit the adrenal permissive HSD3B1(1245C) allele have worse clinical outcomes, compared with men without that allele.
To validate these findings, Dr. Hearn and colleagues retrospectively determined the germline genotype of HSD3B1 for 475 white men enrolled in CHAARTED. In that trial, men with metastatic, hormone-sensitive prostate cancer were randomized to either castration alone or castration plus docetaxel. The current analysis showed that 270 of those patients carried the adrenal-permissive allele.
Among patients with low-volume disease, the adrenal-permissive genotype was associated with significantly lower freedom from CRPC at 2 years, compared with the adrenal-restrictive genotype: 51% and 70.5%, respectively (hazard ratio, 1.89; P = .02). The 5-year overall survival rate was significantly lower for adrenal-permissive allele carriers than adrenal-restrictive allele carriers: 57.5% and 70.8%, respectively (HR, 1.74; P = .045).
Among men with high-volume disease, there was no association between HSD3B1 genotypes and outcomes, and there was no interaction between genotype and benefit from docetaxel. High-volume disease was defined as the presence of visceral metastases or four or more bone metastases with one or more lesions beyond the pelvis and vertebral bodies.
“Regardless of genotype, men with high-volume disease achieved better outcomes when docetaxel was added to ADT [androgen deprivation therapy], whereas outcomes in men with low-volume disease varied only by genotype, with no clear benefit from docetaxel,” the investigators wrote.
The trial was funded by the National Cancer Institute. The authors disclosed relationships with several pharmaceutical companies. One author is a coinventor on a patent for a treatment method based on HSD3B1.
SOURCE: Hearn JWD et al. JAMA Oncol. 2020 Feb 13. doi: 10.1001/jamaoncol.2019.6496.
Men with low-volume, advanced prostate cancer who carry one or more adrenal-permissive alleles of the HSD3B1 gene have worse prognosis than do men with adrenal-restrictive alleles of HSD3B1, a retrospective analysis suggests.
Among 475 white men in the CHAARTED trial, those with the adrenal-permissive allele, HSD3B1(1245C), had a nearly twofold greater risk for developing early castration-resistant prostate cancer (CRPC) and for lower overall survival, compared with men who had the adrenal-restrictive allele, HSD3B1(1245A).
“Our findings suggest that the HSD3B1 genotype can be used to risk stratify white men with low-volume, metastatic prostate cancer,” Jason W.D. Hearn, MD, of the University of Michigan in Ann Arbor and colleagues wrote in JAMA Oncology.
HSD3B1 encodes for an enzyme involved in regulating the rate of metabolic conversion of the precursor hormone dehydroepiandrosterone into testosterone and dihydrotestosterone in prostate tissues. A common missense-encoding germline mutation in the gene leads to variant metabolic phenotypes, including the adrenal-permissive type that allows for ramped-up conversion of the precursor hormone into dihydrotestosterone and the adrenal-restrictive type that results in more rapid enzyme degradation, limiting the conversion from the precursor into the active hormone.
“The population frequency of the adrenal-permissive HSD3B1(1245C) allele varies tremendously by race and is disproportionally high in white men (e.g., carried in approximately 70% of Italian and Spanish men and only about 9% of Yoruba Nigerian men),” Dr. Hearn and colleagues wrote.
Previous studies have suggested that men with both metastatic and nonmetastatic prostate cancer who inherit the adrenal permissive HSD3B1(1245C) allele have worse clinical outcomes, compared with men without that allele.
To validate these findings, Dr. Hearn and colleagues retrospectively determined the germline genotype of HSD3B1 for 475 white men enrolled in CHAARTED. In that trial, men with metastatic, hormone-sensitive prostate cancer were randomized to either castration alone or castration plus docetaxel. The current analysis showed that 270 of those patients carried the adrenal-permissive allele.
Among patients with low-volume disease, the adrenal-permissive genotype was associated with significantly lower freedom from CRPC at 2 years, compared with the adrenal-restrictive genotype: 51% and 70.5%, respectively (hazard ratio, 1.89; P = .02). The 5-year overall survival rate was significantly lower for adrenal-permissive allele carriers than adrenal-restrictive allele carriers: 57.5% and 70.8%, respectively (HR, 1.74; P = .045).
Among men with high-volume disease, there was no association between HSD3B1 genotypes and outcomes, and there was no interaction between genotype and benefit from docetaxel. High-volume disease was defined as the presence of visceral metastases or four or more bone metastases with one or more lesions beyond the pelvis and vertebral bodies.
“Regardless of genotype, men with high-volume disease achieved better outcomes when docetaxel was added to ADT [androgen deprivation therapy], whereas outcomes in men with low-volume disease varied only by genotype, with no clear benefit from docetaxel,” the investigators wrote.
The trial was funded by the National Cancer Institute. The authors disclosed relationships with several pharmaceutical companies. One author is a coinventor on a patent for a treatment method based on HSD3B1.
SOURCE: Hearn JWD et al. JAMA Oncol. 2020 Feb 13. doi: 10.1001/jamaoncol.2019.6496.
FROM JAMA ONCOLOGY
Survival for older AML patients better with HSCT from unrelated donors
For adults aged 50 and older in first or second remission after induction therapy for acute myeloid leukemia, hematopoietic stem cell transplants (HSCT) from young matched unrelated donors was associated with better overall survival and lower risk for relapse than transplants from haploidentical donors, a retrospective study suggests,
Among 823 patients from the aged 50 to 75 with acute myeloid leukemia (AML) in a transplant registry, hazard ratios for both mortality and relapse were significantly higher for patients who received transplants from haploidentical siblings or offspring, compared with patients who received transplants from HLA-matched unrelated donors aged 40 or younger, reported Miguel-Angel Perales, MD, who is affiliated with Memorial Sloan Kettering Cancer Center in New York City, and colleagues.
“Our findings lend support to our hypothesis that a young [matched unrelated donor] should be the donor of choice when available. Furthermore, the data presented here suggest comparable times to transplantation in both treatment groups, confirming timely access to unrelated donors is no longer a barrier,” they wrote in Haematologica.Allogeneic transplants from matched unrelated donors have been performed for more than 30 years for treatment of patients with advanced myeloid and lymphoid malignancies. More recently, T-cell-replete bone marrow or peripheral blood transplants from haploidentical relatives, with post-transplant cyclophosphamide, tacrolimus, and mycophenolate mofetil to lower risk for graft-versus-host disease (GvHD) have become commonplace worldwide, and are established treatment options for patients with myeloid and lymphoid malignancies. There are conflicting studies suggesting that outcomes with haploidentical transplants are equivalent or superior to those seen with matched unrelated donors, the authors noted, but pointed to a 2018 study from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplant and the Center for International Blood and Marrow Transplant Research (CIBMTR). Those study results found that, among transplant recipients aged 55 through 76, graft failure, nonrelapse mortality, and overall mortality were higher when the donors were haploidentical offspring rather than HLA-matched siblings.
To see whether patients aged 50 and older with AML might benefit more with transplants from hapolidentical relatives or matched unrelated donors, the investigators used CIBMTR data to review outcomes for 823 adults with AML who received a transplant in first or second remission at one of 90 U.S. centers from 2008 through 2015.
Of this cohort, 192 patients received grafts from haploidentical donors (25% from siblings and 75% from offspring), and 631 received grafts from matched unrelated donors ranging from 18 to 40 years of age.
Although the two groups were generally similar in demographic and disease characteristics, patients in the matched unrelated donor group had significantly higher frequency of poor-risk cytogenetics (P = .03) and were significantly more likely to have received a myeloablative condition regimen than a reduced-intensity regimen (P less than .001).
In the haploidentical group, 76% of patients were in first complete remission, and the remaining 24% were in second complete remission. In the HLA-matched group the respective proportions were 83% and 17%.
The median follow-up was 42 months in the haploidentical group and 47 months in the HLA-matched group. Five-year overall survival rates were 32% and 42%, respectively.
In multivariable models controlling for donor and recipient age, sex, performance score, hematopoietic cell transplant comorbidity score, cytomegalovirus serostatus, disease status, cytogenetic risk, transplant conditioning regimen intensity and transplant period, the hazard ratio (HR) for the primary endpoint of overall mortality was 1.27 for haploidentical vs. HLA-matched grafts (P = .04). The HR for relapse risk with haploidentical transplants was 1.32 (P =.04). No significant differences in risk of nonrelapse mortality were found between the two study arms.
Bone marrow grafts from matched unrelated donors were associated with significantly higher risk for chronic GvHD than haploidentical grafts (HR, 3.12; P less than .001), but there was no difference in chronic graft-versus-host disease (GvHD) incidence between peripheral blood grafts from matched unrelated donors and haploidentical grafts.
“These data support the view that matched unrelated donor transplant with donors younger than 40 years is to be preferred,” the investigators wrote.
But in an interview, coauthor
“Even though there appears to be that clinical benefit for this older AML patient population, that benefit is not huge, and when you’re also accounting for the process of finding a donor and just getting someone into transplant, a lot of us weren’t sure if this was really going to be practice changing as the field does move into haploidentical transplants being more common,” he said.
He noted that the better outcomes among patients who received transplants from matched unrelated donors may be at least in part explained by the higher proportion of patients with unrelated donors who received myeloablative conditioning regimens. In this study, 65% of patients with haploidentical donors underwent reduced-intensity conditioning with total body irradiation, cyclophosphamide, and fludarabine.“If we do a comparison of equal conditioning regimens, are we really going to see the same outcomes in this setting? This might actually argue that, if you’re going to do a haploidentical transplant, you might start thinking about those newer, more ablative conditioning regimens,” he said.Dr. Tomlinson added that the data are reassuring, because of the modest size of the benefit, and because “many, many of our studies are showing that haploidentical transplants do almost as well as the matched ones. The big question mark will be what are the long-term outcomes? What happens after 3 years from those transplants? And that is going to take a lot more high quality, mature data.”In an editorial accompanying the study, Richard E. Champlin, MD, of the University of Texas MD Anderson Cancer Center in Houston, noted that the more frequent use of reduced-intensity conditioning used for most patients in the haploidentical group has been associated in other studies with higher relapse rates, compared with other, more intense reduced-intensity regimens.
While he agreed that the study by Dr. Perales and colleagues “should give pause for thought, however, for those considering jumping to haploidentical transplants as a preferred approach in general,” he also noted that the study’s conclusion might not apply to cases where time-to-transplant is critical, or when other conditioning and GvHD prophylaxis regimens are used.
“The ideal study would compare optimized versions of both haploidentical and unrelated donor transplants, and use “intention-to-treat” analysis, including all patients for whom a transplant is intended from the time of initial HLA typing,” he wrote.
The study was funded by grants from the National Institutes of Health and the Office of Naval Research. Dr. Tomlinson reported no relevant disclosures. Dr. Champlin did not report disclosures.
SOURCE: Perales M-A et al. Haematologica. 2020 Jan 31;105(2):407-13.
For adults aged 50 and older in first or second remission after induction therapy for acute myeloid leukemia, hematopoietic stem cell transplants (HSCT) from young matched unrelated donors was associated with better overall survival and lower risk for relapse than transplants from haploidentical donors, a retrospective study suggests,
Among 823 patients from the aged 50 to 75 with acute myeloid leukemia (AML) in a transplant registry, hazard ratios for both mortality and relapse were significantly higher for patients who received transplants from haploidentical siblings or offspring, compared with patients who received transplants from HLA-matched unrelated donors aged 40 or younger, reported Miguel-Angel Perales, MD, who is affiliated with Memorial Sloan Kettering Cancer Center in New York City, and colleagues.
“Our findings lend support to our hypothesis that a young [matched unrelated donor] should be the donor of choice when available. Furthermore, the data presented here suggest comparable times to transplantation in both treatment groups, confirming timely access to unrelated donors is no longer a barrier,” they wrote in Haematologica.Allogeneic transplants from matched unrelated donors have been performed for more than 30 years for treatment of patients with advanced myeloid and lymphoid malignancies. More recently, T-cell-replete bone marrow or peripheral blood transplants from haploidentical relatives, with post-transplant cyclophosphamide, tacrolimus, and mycophenolate mofetil to lower risk for graft-versus-host disease (GvHD) have become commonplace worldwide, and are established treatment options for patients with myeloid and lymphoid malignancies. There are conflicting studies suggesting that outcomes with haploidentical transplants are equivalent or superior to those seen with matched unrelated donors, the authors noted, but pointed to a 2018 study from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplant and the Center for International Blood and Marrow Transplant Research (CIBMTR). Those study results found that, among transplant recipients aged 55 through 76, graft failure, nonrelapse mortality, and overall mortality were higher when the donors were haploidentical offspring rather than HLA-matched siblings.
To see whether patients aged 50 and older with AML might benefit more with transplants from hapolidentical relatives or matched unrelated donors, the investigators used CIBMTR data to review outcomes for 823 adults with AML who received a transplant in first or second remission at one of 90 U.S. centers from 2008 through 2015.
Of this cohort, 192 patients received grafts from haploidentical donors (25% from siblings and 75% from offspring), and 631 received grafts from matched unrelated donors ranging from 18 to 40 years of age.
Although the two groups were generally similar in demographic and disease characteristics, patients in the matched unrelated donor group had significantly higher frequency of poor-risk cytogenetics (P = .03) and were significantly more likely to have received a myeloablative condition regimen than a reduced-intensity regimen (P less than .001).
In the haploidentical group, 76% of patients were in first complete remission, and the remaining 24% were in second complete remission. In the HLA-matched group the respective proportions were 83% and 17%.
The median follow-up was 42 months in the haploidentical group and 47 months in the HLA-matched group. Five-year overall survival rates were 32% and 42%, respectively.
In multivariable models controlling for donor and recipient age, sex, performance score, hematopoietic cell transplant comorbidity score, cytomegalovirus serostatus, disease status, cytogenetic risk, transplant conditioning regimen intensity and transplant period, the hazard ratio (HR) for the primary endpoint of overall mortality was 1.27 for haploidentical vs. HLA-matched grafts (P = .04). The HR for relapse risk with haploidentical transplants was 1.32 (P =.04). No significant differences in risk of nonrelapse mortality were found between the two study arms.
Bone marrow grafts from matched unrelated donors were associated with significantly higher risk for chronic GvHD than haploidentical grafts (HR, 3.12; P less than .001), but there was no difference in chronic graft-versus-host disease (GvHD) incidence between peripheral blood grafts from matched unrelated donors and haploidentical grafts.
“These data support the view that matched unrelated donor transplant with donors younger than 40 years is to be preferred,” the investigators wrote.
But in an interview, coauthor
“Even though there appears to be that clinical benefit for this older AML patient population, that benefit is not huge, and when you’re also accounting for the process of finding a donor and just getting someone into transplant, a lot of us weren’t sure if this was really going to be practice changing as the field does move into haploidentical transplants being more common,” he said.
He noted that the better outcomes among patients who received transplants from matched unrelated donors may be at least in part explained by the higher proportion of patients with unrelated donors who received myeloablative conditioning regimens. In this study, 65% of patients with haploidentical donors underwent reduced-intensity conditioning with total body irradiation, cyclophosphamide, and fludarabine.“If we do a comparison of equal conditioning regimens, are we really going to see the same outcomes in this setting? This might actually argue that, if you’re going to do a haploidentical transplant, you might start thinking about those newer, more ablative conditioning regimens,” he said.Dr. Tomlinson added that the data are reassuring, because of the modest size of the benefit, and because “many, many of our studies are showing that haploidentical transplants do almost as well as the matched ones. The big question mark will be what are the long-term outcomes? What happens after 3 years from those transplants? And that is going to take a lot more high quality, mature data.”In an editorial accompanying the study, Richard E. Champlin, MD, of the University of Texas MD Anderson Cancer Center in Houston, noted that the more frequent use of reduced-intensity conditioning used for most patients in the haploidentical group has been associated in other studies with higher relapse rates, compared with other, more intense reduced-intensity regimens.
While he agreed that the study by Dr. Perales and colleagues “should give pause for thought, however, for those considering jumping to haploidentical transplants as a preferred approach in general,” he also noted that the study’s conclusion might not apply to cases where time-to-transplant is critical, or when other conditioning and GvHD prophylaxis regimens are used.
“The ideal study would compare optimized versions of both haploidentical and unrelated donor transplants, and use “intention-to-treat” analysis, including all patients for whom a transplant is intended from the time of initial HLA typing,” he wrote.
The study was funded by grants from the National Institutes of Health and the Office of Naval Research. Dr. Tomlinson reported no relevant disclosures. Dr. Champlin did not report disclosures.
SOURCE: Perales M-A et al. Haematologica. 2020 Jan 31;105(2):407-13.
For adults aged 50 and older in first or second remission after induction therapy for acute myeloid leukemia, hematopoietic stem cell transplants (HSCT) from young matched unrelated donors was associated with better overall survival and lower risk for relapse than transplants from haploidentical donors, a retrospective study suggests,
Among 823 patients from the aged 50 to 75 with acute myeloid leukemia (AML) in a transplant registry, hazard ratios for both mortality and relapse were significantly higher for patients who received transplants from haploidentical siblings or offspring, compared with patients who received transplants from HLA-matched unrelated donors aged 40 or younger, reported Miguel-Angel Perales, MD, who is affiliated with Memorial Sloan Kettering Cancer Center in New York City, and colleagues.
“Our findings lend support to our hypothesis that a young [matched unrelated donor] should be the donor of choice when available. Furthermore, the data presented here suggest comparable times to transplantation in both treatment groups, confirming timely access to unrelated donors is no longer a barrier,” they wrote in Haematologica.Allogeneic transplants from matched unrelated donors have been performed for more than 30 years for treatment of patients with advanced myeloid and lymphoid malignancies. More recently, T-cell-replete bone marrow or peripheral blood transplants from haploidentical relatives, with post-transplant cyclophosphamide, tacrolimus, and mycophenolate mofetil to lower risk for graft-versus-host disease (GvHD) have become commonplace worldwide, and are established treatment options for patients with myeloid and lymphoid malignancies. There are conflicting studies suggesting that outcomes with haploidentical transplants are equivalent or superior to those seen with matched unrelated donors, the authors noted, but pointed to a 2018 study from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplant and the Center for International Blood and Marrow Transplant Research (CIBMTR). Those study results found that, among transplant recipients aged 55 through 76, graft failure, nonrelapse mortality, and overall mortality were higher when the donors were haploidentical offspring rather than HLA-matched siblings.
To see whether patients aged 50 and older with AML might benefit more with transplants from hapolidentical relatives or matched unrelated donors, the investigators used CIBMTR data to review outcomes for 823 adults with AML who received a transplant in first or second remission at one of 90 U.S. centers from 2008 through 2015.
Of this cohort, 192 patients received grafts from haploidentical donors (25% from siblings and 75% from offspring), and 631 received grafts from matched unrelated donors ranging from 18 to 40 years of age.
Although the two groups were generally similar in demographic and disease characteristics, patients in the matched unrelated donor group had significantly higher frequency of poor-risk cytogenetics (P = .03) and were significantly more likely to have received a myeloablative condition regimen than a reduced-intensity regimen (P less than .001).
In the haploidentical group, 76% of patients were in first complete remission, and the remaining 24% were in second complete remission. In the HLA-matched group the respective proportions were 83% and 17%.
The median follow-up was 42 months in the haploidentical group and 47 months in the HLA-matched group. Five-year overall survival rates were 32% and 42%, respectively.
In multivariable models controlling for donor and recipient age, sex, performance score, hematopoietic cell transplant comorbidity score, cytomegalovirus serostatus, disease status, cytogenetic risk, transplant conditioning regimen intensity and transplant period, the hazard ratio (HR) for the primary endpoint of overall mortality was 1.27 for haploidentical vs. HLA-matched grafts (P = .04). The HR for relapse risk with haploidentical transplants was 1.32 (P =.04). No significant differences in risk of nonrelapse mortality were found between the two study arms.
Bone marrow grafts from matched unrelated donors were associated with significantly higher risk for chronic GvHD than haploidentical grafts (HR, 3.12; P less than .001), but there was no difference in chronic graft-versus-host disease (GvHD) incidence between peripheral blood grafts from matched unrelated donors and haploidentical grafts.
“These data support the view that matched unrelated donor transplant with donors younger than 40 years is to be preferred,” the investigators wrote.
But in an interview, coauthor
“Even though there appears to be that clinical benefit for this older AML patient population, that benefit is not huge, and when you’re also accounting for the process of finding a donor and just getting someone into transplant, a lot of us weren’t sure if this was really going to be practice changing as the field does move into haploidentical transplants being more common,” he said.
He noted that the better outcomes among patients who received transplants from matched unrelated donors may be at least in part explained by the higher proportion of patients with unrelated donors who received myeloablative conditioning regimens. In this study, 65% of patients with haploidentical donors underwent reduced-intensity conditioning with total body irradiation, cyclophosphamide, and fludarabine.“If we do a comparison of equal conditioning regimens, are we really going to see the same outcomes in this setting? This might actually argue that, if you’re going to do a haploidentical transplant, you might start thinking about those newer, more ablative conditioning regimens,” he said.Dr. Tomlinson added that the data are reassuring, because of the modest size of the benefit, and because “many, many of our studies are showing that haploidentical transplants do almost as well as the matched ones. The big question mark will be what are the long-term outcomes? What happens after 3 years from those transplants? And that is going to take a lot more high quality, mature data.”In an editorial accompanying the study, Richard E. Champlin, MD, of the University of Texas MD Anderson Cancer Center in Houston, noted that the more frequent use of reduced-intensity conditioning used for most patients in the haploidentical group has been associated in other studies with higher relapse rates, compared with other, more intense reduced-intensity regimens.
While he agreed that the study by Dr. Perales and colleagues “should give pause for thought, however, for those considering jumping to haploidentical transplants as a preferred approach in general,” he also noted that the study’s conclusion might not apply to cases where time-to-transplant is critical, or when other conditioning and GvHD prophylaxis regimens are used.
“The ideal study would compare optimized versions of both haploidentical and unrelated donor transplants, and use “intention-to-treat” analysis, including all patients for whom a transplant is intended from the time of initial HLA typing,” he wrote.
The study was funded by grants from the National Institutes of Health and the Office of Naval Research. Dr. Tomlinson reported no relevant disclosures. Dr. Champlin did not report disclosures.
SOURCE: Perales M-A et al. Haematologica. 2020 Jan 31;105(2):407-13.
FROM HAEMATOLOGICA
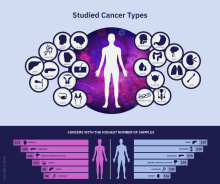
Global project reveals cancer’s genomic playbook
A massive collaborative project spanning four continents and 744 research centers has revealed driver mutations in both protein-coding and noncoding regions of 38 cancer types.
The Pan-Cancer Analysis of Whole Genomes (PCAWG) is an integrative analysis of the whole-genome sequences from 2,658 donors across 38 common tumor types. The findings are expected to add exponentially to what’s currently known about the complex genetics of cancer, and they point to possible strategies for improving cancer prevention, diagnosis, and care.
Six articles summarizing the findings are presented in a series of papers in Nature, and 16 more appear in affiliated publications.
“It’s humbling that it was only 14 years ago that the genomics community sequenced its very first cancer exome, and it was able to identify mutations within the roughly 20,000 protein-coding genes in the human cell,” investigator Lincoln Stein, MD, PhD, of the Ontario Institute for Cancer Research in Toronto, said in a telephone briefing.
Exome sequencing, however, covers only protein-coding genomic regions, which constitute only about 1% of the entire genome, “so assembling an accurate portrait of the cancer genome using just the exome data is like trying to put together a 100,000-piece jigsaw puzzle when you’re missing 99% of the pieces and there’s no puzzle box with a completed picture to guide you,” Dr. Stein said.
Members of the PCAWG from centers in North America, Europe, Asia, and Australia screened 2,658 whole-cancer genomes and matched samples of noncancerous tissues from the same individuals, along with 1,188 transcriptomes cataloging the sequences and expression of RNA transcripts in a given tumor. The 6-year project netted more than 800 terabytes of genomic data, roughly equivalent to the digital holdings of the U.S. Library of Congress multiplied by 11.
The findings are summarized in papers focusing on cancer drivers, noncoding changes, mutational signatures, structural variants, cancer evolution over time, and RNA alterations.
Driver mutations
Investigators found that the average cancer genome contains four or five driver mutations located in both coding and noncoding regions. They also found, however, that in approximately 5% of cases no driver mutations could be identified.
A substantial proportion of tumors displayed “hallmarks of genomic catastrophes.” About 22% of tumors exhibited chromothripsis, a mutational process marked by hundreds or even thousands of clustered chromosomal rearrangements. About 18% showed chromoplexy, which is characterized by scattering and rearrangement of multiple strands of DNA from one or more chromosomes.
Analyzing driver point mutations and structural variants in noncoding regions, the investigators found the usual suspects – previously reported culprits – as well as novel candidates.
For example, they identified point mutations in the five prime region of the tumor suppressor gene TP53 and the three prime untranslated regions of NFKBIZ (a nuclear factor kappa B inhibitor) and TOB1 (an antiproliferative protein), focal deletion in BRD4 (a transcriptional and epigenetic regulator), and rearrangements in chromosomal loci in members of the AKR1C family of enzymes thought to play a role in disease progression.
In addition, investigators identified mutations in noncoding regions of TERT, a telomerase gene. These mutations result in ramped-up expression of telomerase, which in turn promotes uncontrollable division of tumor cells.
Mutational signatures
In a related line of research, PCAWG investigators identified new DNA mutational signatures ranging from single nucleotide polymorphisms to insertions and deletions, as well as to structural variants – rearrangements of large sections of the genome.
“The substantial size of our dataset, compared with previous analyses, enabled the discovery of new signatures, the separation of overlapping signatures, and the decomposition of signatures into components that may represent associated – but distinct – DNA damage, repair, and/or replication mechanisms. By estimating the contribution of each signature to the mutational catalogs of individual cancer genomes, we revealed associations of signatures to exogenous or endogenous exposures, as well as to defective DNA maintenance processes,” the investigators wrote.
They also acknowledged, however, that “many signatures are of unknown cause.”
Cancer evolution
One of the six main studies focused on the evolution of cancer over time. Instead of providing a “snapshot” of the genome as captured by sequencing tissue from a single biopsy, consortium investigators created full-length features of the “life history and evolution of mutational processes and driver mutation sequences.”
They found that early cancer development was marked by relatively few mutations in driver genes and by identifiable copy-number gains, including trisomy 7 in glioblastoma, and an abnormal mirroring of the arms (isochromosome) of chromosome 17 in medulloblastoma.
In 40% of the samples, however, there were significant changes in the mutational spectrum as the cancers grew, leading to a near quadrupling of driver genes and increased genomic instability in later-stage tumors.
“Copy-number alterations often occur in mitotic crises and lead to simultaneous gains of chromosomal segments,” the investigators wrote. “Timing analyses suggest that driver mutations often precede diagnosis by many years, if not decades. Together, these results determine the evolutionary trajectories of cancer and highlight opportunities for early cancer detection.”
Implications for cancer care
“When I used to treat patients with cancer, I was always completely amazed and puzzled by how two patients could have what looked like the same tumor. It would look the same under the microscope, have the same size, and the two patients would receive exactly the same treatment, but the two patients would have completely opposite outcomes; one would survive, and one would die. What this analysis … has done is really laid bare the reasons for that unpredictability in clinical outcomes,” Peter Campbell, MD, PhD, of the Wellcome Sanger Institute in Hinxton, England, said during the telebriefing.
“The most striking finding out of all of the suite of papers is just how different one person’s cancer genome is from another person’s. We see thousands of different combinations of mutations that can cause the cancer, and more than 80 different underlying processes generating the mutations in a cancer, and that leads to very different shapes and patterns in the genome that result,” he added.
On a positive note, the research shows that one or more driver mutations can be identified in about 95% of all cancer patients, and it elucidates the sequence of events leading to oncogenesis and tumor evolution, providing opportunities for earlier identification and potential interventions to prevent cancer, Dr. Campbell said.
The PCAWG was a collaborative multinational effort with multiple funding sources and many investigators.
SOURCE: Nature. 2020 Feb 5. https://www.nature.com/collections/pcawg/
A massive collaborative project spanning four continents and 744 research centers has revealed driver mutations in both protein-coding and noncoding regions of 38 cancer types.
The Pan-Cancer Analysis of Whole Genomes (PCAWG) is an integrative analysis of the whole-genome sequences from 2,658 donors across 38 common tumor types. The findings are expected to add exponentially to what’s currently known about the complex genetics of cancer, and they point to possible strategies for improving cancer prevention, diagnosis, and care.
Six articles summarizing the findings are presented in a series of papers in Nature, and 16 more appear in affiliated publications.
“It’s humbling that it was only 14 years ago that the genomics community sequenced its very first cancer exome, and it was able to identify mutations within the roughly 20,000 protein-coding genes in the human cell,” investigator Lincoln Stein, MD, PhD, of the Ontario Institute for Cancer Research in Toronto, said in a telephone briefing.
Exome sequencing, however, covers only protein-coding genomic regions, which constitute only about 1% of the entire genome, “so assembling an accurate portrait of the cancer genome using just the exome data is like trying to put together a 100,000-piece jigsaw puzzle when you’re missing 99% of the pieces and there’s no puzzle box with a completed picture to guide you,” Dr. Stein said.
Members of the PCAWG from centers in North America, Europe, Asia, and Australia screened 2,658 whole-cancer genomes and matched samples of noncancerous tissues from the same individuals, along with 1,188 transcriptomes cataloging the sequences and expression of RNA transcripts in a given tumor. The 6-year project netted more than 800 terabytes of genomic data, roughly equivalent to the digital holdings of the U.S. Library of Congress multiplied by 11.
The findings are summarized in papers focusing on cancer drivers, noncoding changes, mutational signatures, structural variants, cancer evolution over time, and RNA alterations.
Driver mutations
Investigators found that the average cancer genome contains four or five driver mutations located in both coding and noncoding regions. They also found, however, that in approximately 5% of cases no driver mutations could be identified.
A substantial proportion of tumors displayed “hallmarks of genomic catastrophes.” About 22% of tumors exhibited chromothripsis, a mutational process marked by hundreds or even thousands of clustered chromosomal rearrangements. About 18% showed chromoplexy, which is characterized by scattering and rearrangement of multiple strands of DNA from one or more chromosomes.
Analyzing driver point mutations and structural variants in noncoding regions, the investigators found the usual suspects – previously reported culprits – as well as novel candidates.
For example, they identified point mutations in the five prime region of the tumor suppressor gene TP53 and the three prime untranslated regions of NFKBIZ (a nuclear factor kappa B inhibitor) and TOB1 (an antiproliferative protein), focal deletion in BRD4 (a transcriptional and epigenetic regulator), and rearrangements in chromosomal loci in members of the AKR1C family of enzymes thought to play a role in disease progression.
In addition, investigators identified mutations in noncoding regions of TERT, a telomerase gene. These mutations result in ramped-up expression of telomerase, which in turn promotes uncontrollable division of tumor cells.
Mutational signatures
In a related line of research, PCAWG investigators identified new DNA mutational signatures ranging from single nucleotide polymorphisms to insertions and deletions, as well as to structural variants – rearrangements of large sections of the genome.
“The substantial size of our dataset, compared with previous analyses, enabled the discovery of new signatures, the separation of overlapping signatures, and the decomposition of signatures into components that may represent associated – but distinct – DNA damage, repair, and/or replication mechanisms. By estimating the contribution of each signature to the mutational catalogs of individual cancer genomes, we revealed associations of signatures to exogenous or endogenous exposures, as well as to defective DNA maintenance processes,” the investigators wrote.
They also acknowledged, however, that “many signatures are of unknown cause.”
Cancer evolution
One of the six main studies focused on the evolution of cancer over time. Instead of providing a “snapshot” of the genome as captured by sequencing tissue from a single biopsy, consortium investigators created full-length features of the “life history and evolution of mutational processes and driver mutation sequences.”
They found that early cancer development was marked by relatively few mutations in driver genes and by identifiable copy-number gains, including trisomy 7 in glioblastoma, and an abnormal mirroring of the arms (isochromosome) of chromosome 17 in medulloblastoma.
In 40% of the samples, however, there were significant changes in the mutational spectrum as the cancers grew, leading to a near quadrupling of driver genes and increased genomic instability in later-stage tumors.
“Copy-number alterations often occur in mitotic crises and lead to simultaneous gains of chromosomal segments,” the investigators wrote. “Timing analyses suggest that driver mutations often precede diagnosis by many years, if not decades. Together, these results determine the evolutionary trajectories of cancer and highlight opportunities for early cancer detection.”
Implications for cancer care
“When I used to treat patients with cancer, I was always completely amazed and puzzled by how two patients could have what looked like the same tumor. It would look the same under the microscope, have the same size, and the two patients would receive exactly the same treatment, but the two patients would have completely opposite outcomes; one would survive, and one would die. What this analysis … has done is really laid bare the reasons for that unpredictability in clinical outcomes,” Peter Campbell, MD, PhD, of the Wellcome Sanger Institute in Hinxton, England, said during the telebriefing.
“The most striking finding out of all of the suite of papers is just how different one person’s cancer genome is from another person’s. We see thousands of different combinations of mutations that can cause the cancer, and more than 80 different underlying processes generating the mutations in a cancer, and that leads to very different shapes and patterns in the genome that result,” he added.
On a positive note, the research shows that one or more driver mutations can be identified in about 95% of all cancer patients, and it elucidates the sequence of events leading to oncogenesis and tumor evolution, providing opportunities for earlier identification and potential interventions to prevent cancer, Dr. Campbell said.
The PCAWG was a collaborative multinational effort with multiple funding sources and many investigators.
SOURCE: Nature. 2020 Feb 5. https://www.nature.com/collections/pcawg/
A massive collaborative project spanning four continents and 744 research centers has revealed driver mutations in both protein-coding and noncoding regions of 38 cancer types.
The Pan-Cancer Analysis of Whole Genomes (PCAWG) is an integrative analysis of the whole-genome sequences from 2,658 donors across 38 common tumor types. The findings are expected to add exponentially to what’s currently known about the complex genetics of cancer, and they point to possible strategies for improving cancer prevention, diagnosis, and care.
Six articles summarizing the findings are presented in a series of papers in Nature, and 16 more appear in affiliated publications.
“It’s humbling that it was only 14 years ago that the genomics community sequenced its very first cancer exome, and it was able to identify mutations within the roughly 20,000 protein-coding genes in the human cell,” investigator Lincoln Stein, MD, PhD, of the Ontario Institute for Cancer Research in Toronto, said in a telephone briefing.
Exome sequencing, however, covers only protein-coding genomic regions, which constitute only about 1% of the entire genome, “so assembling an accurate portrait of the cancer genome using just the exome data is like trying to put together a 100,000-piece jigsaw puzzle when you’re missing 99% of the pieces and there’s no puzzle box with a completed picture to guide you,” Dr. Stein said.
Members of the PCAWG from centers in North America, Europe, Asia, and Australia screened 2,658 whole-cancer genomes and matched samples of noncancerous tissues from the same individuals, along with 1,188 transcriptomes cataloging the sequences and expression of RNA transcripts in a given tumor. The 6-year project netted more than 800 terabytes of genomic data, roughly equivalent to the digital holdings of the U.S. Library of Congress multiplied by 11.
The findings are summarized in papers focusing on cancer drivers, noncoding changes, mutational signatures, structural variants, cancer evolution over time, and RNA alterations.
Driver mutations
Investigators found that the average cancer genome contains four or five driver mutations located in both coding and noncoding regions. They also found, however, that in approximately 5% of cases no driver mutations could be identified.
A substantial proportion of tumors displayed “hallmarks of genomic catastrophes.” About 22% of tumors exhibited chromothripsis, a mutational process marked by hundreds or even thousands of clustered chromosomal rearrangements. About 18% showed chromoplexy, which is characterized by scattering and rearrangement of multiple strands of DNA from one or more chromosomes.
Analyzing driver point mutations and structural variants in noncoding regions, the investigators found the usual suspects – previously reported culprits – as well as novel candidates.
For example, they identified point mutations in the five prime region of the tumor suppressor gene TP53 and the three prime untranslated regions of NFKBIZ (a nuclear factor kappa B inhibitor) and TOB1 (an antiproliferative protein), focal deletion in BRD4 (a transcriptional and epigenetic regulator), and rearrangements in chromosomal loci in members of the AKR1C family of enzymes thought to play a role in disease progression.
In addition, investigators identified mutations in noncoding regions of TERT, a telomerase gene. These mutations result in ramped-up expression of telomerase, which in turn promotes uncontrollable division of tumor cells.
Mutational signatures
In a related line of research, PCAWG investigators identified new DNA mutational signatures ranging from single nucleotide polymorphisms to insertions and deletions, as well as to structural variants – rearrangements of large sections of the genome.
“The substantial size of our dataset, compared with previous analyses, enabled the discovery of new signatures, the separation of overlapping signatures, and the decomposition of signatures into components that may represent associated – but distinct – DNA damage, repair, and/or replication mechanisms. By estimating the contribution of each signature to the mutational catalogs of individual cancer genomes, we revealed associations of signatures to exogenous or endogenous exposures, as well as to defective DNA maintenance processes,” the investigators wrote.
They also acknowledged, however, that “many signatures are of unknown cause.”
Cancer evolution
One of the six main studies focused on the evolution of cancer over time. Instead of providing a “snapshot” of the genome as captured by sequencing tissue from a single biopsy, consortium investigators created full-length features of the “life history and evolution of mutational processes and driver mutation sequences.”
They found that early cancer development was marked by relatively few mutations in driver genes and by identifiable copy-number gains, including trisomy 7 in glioblastoma, and an abnormal mirroring of the arms (isochromosome) of chromosome 17 in medulloblastoma.
In 40% of the samples, however, there were significant changes in the mutational spectrum as the cancers grew, leading to a near quadrupling of driver genes and increased genomic instability in later-stage tumors.
“Copy-number alterations often occur in mitotic crises and lead to simultaneous gains of chromosomal segments,” the investigators wrote. “Timing analyses suggest that driver mutations often precede diagnosis by many years, if not decades. Together, these results determine the evolutionary trajectories of cancer and highlight opportunities for early cancer detection.”
Implications for cancer care
“When I used to treat patients with cancer, I was always completely amazed and puzzled by how two patients could have what looked like the same tumor. It would look the same under the microscope, have the same size, and the two patients would receive exactly the same treatment, but the two patients would have completely opposite outcomes; one would survive, and one would die. What this analysis … has done is really laid bare the reasons for that unpredictability in clinical outcomes,” Peter Campbell, MD, PhD, of the Wellcome Sanger Institute in Hinxton, England, said during the telebriefing.
“The most striking finding out of all of the suite of papers is just how different one person’s cancer genome is from another person’s. We see thousands of different combinations of mutations that can cause the cancer, and more than 80 different underlying processes generating the mutations in a cancer, and that leads to very different shapes and patterns in the genome that result,” he added.
On a positive note, the research shows that one or more driver mutations can be identified in about 95% of all cancer patients, and it elucidates the sequence of events leading to oncogenesis and tumor evolution, providing opportunities for earlier identification and potential interventions to prevent cancer, Dr. Campbell said.
The PCAWG was a collaborative multinational effort with multiple funding sources and many investigators.
SOURCE: Nature. 2020 Feb 5. https://www.nature.com/collections/pcawg/
FROM NATURE
Lenvatinib/pembrolizumab has good activity in advanced RCC, other solid tumors
A combination of the tyrosine kinase inhibitor lenvatinib (Lenvima) and the immune checkpoint inhibitor pembrolizumab (Keytruda) was safe and showed promising activity against advanced renal cell carcinoma and other solid tumors in a phase 1b/2 study.
Overall response rates (ORR) at 24 weeks ranged from 63% for patients with advanced renal cell carcinomas (RCC) to 25% for patients with urothelial cancers, reported Matthew H. Taylor, MD, of Knight Cancer Institute at Oregon Health & Science University in Portland, and colleagues.
The findings from this study sparked additional clinical trials for patients with gastric cancer, gastroesophageal cancer, and differentiated thyroid cancer, and set the stage for larger phase 3 trials in patients with advanced RCC, endometrial cancer, malignant melanoma, and non–small cell lung cancer (NSCLC).
“In the future, we also plan to study lenvatinib plus pembrolizumab in patients with RCC who have had disease progression after treatment with immune checkpoint inhibitors,” they wrote. The report was published in Journal of Clinical Oncology.
Lenvatinib is a multitargeted tyrosine kinase inhibitor (TKI) with action against vascular endothelial growth factor (VEGF) receptors 1-3, fibroblast growth factor (FGF) receptors 1-4, platelet-derived growth factor receptors alpha and the RET and KIT kinases.
“Preclinical and clinical studies suggest that modulation of VEGF-mediated immune suppression via angiogenesis inhibition could potentially augment the immunotherapeutic activity of immune checkpoint inhibitors,” the investigators wrote.
They reported results from the dose finding (1b) phase including 13 patients and initial phase 2 expansion cohorts with a total of 124 patients.
The maximum tolerated dose of lenvatinib in combination with pembrolizumab was established as 20 mg/day.
At 24 weeks of follow-up, the ORR for 30 patients with RCC was 63%; two additional patients had responses after week 24, for a total ORR at study cutoff in this cohort of 70%. The median duration of response for these patients was 20 months, and the median progression-free survival (PFS) was 19.8 months. At the time of data cutoff for this analysis, 9 of the 30 patients with RCC were still on treatment.
For 23 patients with endometrial cancer, the 24-week and overall ORR were 52%, with a median duration of response not reached, and a median PFS of 9.7 months. Seven patients were still on treatment at data cutoff.
For 21 patients with melanoma, the 24-week and overall ORR were 48%, median duration of response was 12.5 months, and median PFS was 5.5 months. Two of the patients were still on treatment at data cutoff.
For the 22 patients with squamous cell cancer of the head and neck, the 24-week ORR was 36%, with two patients having a response after week 24 for a total ORR at data cutoff of 46%. The median duration of response was 8.2 months and the median PFS was 4.7 months. Three patients remained on treatment at data cutoff.
For 21 patients with NSCLC, the 24-week and overall ORR were 33%, the median duration of response was 10.9 months, and median PFS was 5.9 months. Six of the patients were still receiving treatment at data cutoff.
For 20 patients with urothelial cancer, the 24-week and overall ORR were 25%, with a median duration of response not reached, and a median PFS of 5.4 months. Three patients were still receiving the combination at the time of data cutoff.
Treatment related adverse events (TRAEs) occurred in 133 of all 137 patients enrolled in the two study phases. The adverse events were similar across all cohorts, with any grade of events including fatigue in 58%, diarrhea in 52%, hypertension in 47%, hypothyroidism in 42%, and decreased appetite in 39%.
The most frequent grade 3 or 4 TRAEs were hypertension in 20%, fatigue in 12%, diarrhea in 9%, proteinuria in 8%, and increased lipase levels in 7%.
In all, 85% of patients had a TRAE leading to lenvatinib dose reduction and/or interruption, and 13% required lenvatinib discontinuation.
Events leading to pembrolizumab dose interruption occurred in 45% of patients, and pembrolizumab discontinuation in 15%.
The study was sponsored by Eisai with collaboration from Merck Sharp & Dohme. Dr. Taylor disclosed a consulting or advisory role for Bristol-Myers Squibb, Eisai, Array BioPharma, Loxo, Bayer, ArQule, Blueprint Medicines, Novartis, and Sanofi/Genzyme, and speakers bureau activities for BMS and Eisai.
SOURCE: Taylor MH et al. J Clin Oncol. 2020 Jan. 21 doi: 10.1200/JCO.19.01598.
A combination of the tyrosine kinase inhibitor lenvatinib (Lenvima) and the immune checkpoint inhibitor pembrolizumab (Keytruda) was safe and showed promising activity against advanced renal cell carcinoma and other solid tumors in a phase 1b/2 study.
Overall response rates (ORR) at 24 weeks ranged from 63% for patients with advanced renal cell carcinomas (RCC) to 25% for patients with urothelial cancers, reported Matthew H. Taylor, MD, of Knight Cancer Institute at Oregon Health & Science University in Portland, and colleagues.
The findings from this study sparked additional clinical trials for patients with gastric cancer, gastroesophageal cancer, and differentiated thyroid cancer, and set the stage for larger phase 3 trials in patients with advanced RCC, endometrial cancer, malignant melanoma, and non–small cell lung cancer (NSCLC).
“In the future, we also plan to study lenvatinib plus pembrolizumab in patients with RCC who have had disease progression after treatment with immune checkpoint inhibitors,” they wrote. The report was published in Journal of Clinical Oncology.
Lenvatinib is a multitargeted tyrosine kinase inhibitor (TKI) with action against vascular endothelial growth factor (VEGF) receptors 1-3, fibroblast growth factor (FGF) receptors 1-4, platelet-derived growth factor receptors alpha and the RET and KIT kinases.
“Preclinical and clinical studies suggest that modulation of VEGF-mediated immune suppression via angiogenesis inhibition could potentially augment the immunotherapeutic activity of immune checkpoint inhibitors,” the investigators wrote.
They reported results from the dose finding (1b) phase including 13 patients and initial phase 2 expansion cohorts with a total of 124 patients.
The maximum tolerated dose of lenvatinib in combination with pembrolizumab was established as 20 mg/day.
At 24 weeks of follow-up, the ORR for 30 patients with RCC was 63%; two additional patients had responses after week 24, for a total ORR at study cutoff in this cohort of 70%. The median duration of response for these patients was 20 months, and the median progression-free survival (PFS) was 19.8 months. At the time of data cutoff for this analysis, 9 of the 30 patients with RCC were still on treatment.
For 23 patients with endometrial cancer, the 24-week and overall ORR were 52%, with a median duration of response not reached, and a median PFS of 9.7 months. Seven patients were still on treatment at data cutoff.
For 21 patients with melanoma, the 24-week and overall ORR were 48%, median duration of response was 12.5 months, and median PFS was 5.5 months. Two of the patients were still on treatment at data cutoff.
For the 22 patients with squamous cell cancer of the head and neck, the 24-week ORR was 36%, with two patients having a response after week 24 for a total ORR at data cutoff of 46%. The median duration of response was 8.2 months and the median PFS was 4.7 months. Three patients remained on treatment at data cutoff.
For 21 patients with NSCLC, the 24-week and overall ORR were 33%, the median duration of response was 10.9 months, and median PFS was 5.9 months. Six of the patients were still receiving treatment at data cutoff.
For 20 patients with urothelial cancer, the 24-week and overall ORR were 25%, with a median duration of response not reached, and a median PFS of 5.4 months. Three patients were still receiving the combination at the time of data cutoff.
Treatment related adverse events (TRAEs) occurred in 133 of all 137 patients enrolled in the two study phases. The adverse events were similar across all cohorts, with any grade of events including fatigue in 58%, diarrhea in 52%, hypertension in 47%, hypothyroidism in 42%, and decreased appetite in 39%.
The most frequent grade 3 or 4 TRAEs were hypertension in 20%, fatigue in 12%, diarrhea in 9%, proteinuria in 8%, and increased lipase levels in 7%.
In all, 85% of patients had a TRAE leading to lenvatinib dose reduction and/or interruption, and 13% required lenvatinib discontinuation.
Events leading to pembrolizumab dose interruption occurred in 45% of patients, and pembrolizumab discontinuation in 15%.
The study was sponsored by Eisai with collaboration from Merck Sharp & Dohme. Dr. Taylor disclosed a consulting or advisory role for Bristol-Myers Squibb, Eisai, Array BioPharma, Loxo, Bayer, ArQule, Blueprint Medicines, Novartis, and Sanofi/Genzyme, and speakers bureau activities for BMS and Eisai.
SOURCE: Taylor MH et al. J Clin Oncol. 2020 Jan. 21 doi: 10.1200/JCO.19.01598.
A combination of the tyrosine kinase inhibitor lenvatinib (Lenvima) and the immune checkpoint inhibitor pembrolizumab (Keytruda) was safe and showed promising activity against advanced renal cell carcinoma and other solid tumors in a phase 1b/2 study.
Overall response rates (ORR) at 24 weeks ranged from 63% for patients with advanced renal cell carcinomas (RCC) to 25% for patients with urothelial cancers, reported Matthew H. Taylor, MD, of Knight Cancer Institute at Oregon Health & Science University in Portland, and colleagues.
The findings from this study sparked additional clinical trials for patients with gastric cancer, gastroesophageal cancer, and differentiated thyroid cancer, and set the stage for larger phase 3 trials in patients with advanced RCC, endometrial cancer, malignant melanoma, and non–small cell lung cancer (NSCLC).
“In the future, we also plan to study lenvatinib plus pembrolizumab in patients with RCC who have had disease progression after treatment with immune checkpoint inhibitors,” they wrote. The report was published in Journal of Clinical Oncology.
Lenvatinib is a multitargeted tyrosine kinase inhibitor (TKI) with action against vascular endothelial growth factor (VEGF) receptors 1-3, fibroblast growth factor (FGF) receptors 1-4, platelet-derived growth factor receptors alpha and the RET and KIT kinases.
“Preclinical and clinical studies suggest that modulation of VEGF-mediated immune suppression via angiogenesis inhibition could potentially augment the immunotherapeutic activity of immune checkpoint inhibitors,” the investigators wrote.
They reported results from the dose finding (1b) phase including 13 patients and initial phase 2 expansion cohorts with a total of 124 patients.
The maximum tolerated dose of lenvatinib in combination with pembrolizumab was established as 20 mg/day.
At 24 weeks of follow-up, the ORR for 30 patients with RCC was 63%; two additional patients had responses after week 24, for a total ORR at study cutoff in this cohort of 70%. The median duration of response for these patients was 20 months, and the median progression-free survival (PFS) was 19.8 months. At the time of data cutoff for this analysis, 9 of the 30 patients with RCC were still on treatment.
For 23 patients with endometrial cancer, the 24-week and overall ORR were 52%, with a median duration of response not reached, and a median PFS of 9.7 months. Seven patients were still on treatment at data cutoff.
For 21 patients with melanoma, the 24-week and overall ORR were 48%, median duration of response was 12.5 months, and median PFS was 5.5 months. Two of the patients were still on treatment at data cutoff.
For the 22 patients with squamous cell cancer of the head and neck, the 24-week ORR was 36%, with two patients having a response after week 24 for a total ORR at data cutoff of 46%. The median duration of response was 8.2 months and the median PFS was 4.7 months. Three patients remained on treatment at data cutoff.
For 21 patients with NSCLC, the 24-week and overall ORR were 33%, the median duration of response was 10.9 months, and median PFS was 5.9 months. Six of the patients were still receiving treatment at data cutoff.
For 20 patients with urothelial cancer, the 24-week and overall ORR were 25%, with a median duration of response not reached, and a median PFS of 5.4 months. Three patients were still receiving the combination at the time of data cutoff.
Treatment related adverse events (TRAEs) occurred in 133 of all 137 patients enrolled in the two study phases. The adverse events were similar across all cohorts, with any grade of events including fatigue in 58%, diarrhea in 52%, hypertension in 47%, hypothyroidism in 42%, and decreased appetite in 39%.
The most frequent grade 3 or 4 TRAEs were hypertension in 20%, fatigue in 12%, diarrhea in 9%, proteinuria in 8%, and increased lipase levels in 7%.
In all, 85% of patients had a TRAE leading to lenvatinib dose reduction and/or interruption, and 13% required lenvatinib discontinuation.
Events leading to pembrolizumab dose interruption occurred in 45% of patients, and pembrolizumab discontinuation in 15%.
The study was sponsored by Eisai with collaboration from Merck Sharp & Dohme. Dr. Taylor disclosed a consulting or advisory role for Bristol-Myers Squibb, Eisai, Array BioPharma, Loxo, Bayer, ArQule, Blueprint Medicines, Novartis, and Sanofi/Genzyme, and speakers bureau activities for BMS and Eisai.
SOURCE: Taylor MH et al. J Clin Oncol. 2020 Jan. 21 doi: 10.1200/JCO.19.01598.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
High-dose chemo offers survival benefit only for highest-risk breast cancer
High-dose chemotherapy in the adjuvant setting offers a long-term survival advantage for women with very-high-risk stage III breast cancer, but does not improve survival odds for women with lower-risk cancers, an analysis of 20 years of follow-up data shows.
Among 885 women younger than 56 years at the time of treatment who had 4 or more involved axilliary lymph nodes, there was no overall survival difference over 2 decades between the total population of women randomized to receive adjuvant high-dose chemotherapy (HDCT) and those assigned to receive conventional-dose chemotherapy (CDCT).
However, women with 10 or more involved axilliary nodes and those with triple-negative breast cancer had an approximately 15% absolute improvement in 20-year overall survival with high-dose chemotherapy, although the difference for triple-negative disease fell just short of statistical significance, reported Tessa G. Steenbruggen, MD, from the Netherlands Cancer Institute in Amsterdam and colleagues.
“Our analysis confirms earlier results that HDCT has no significant overall survival benefit compared with CDCT for unselected patients with stage III [breast cancer]. However, we found a 14.6%improvement in 20-year OS estimates with HDCT in the predefined subgroup of patients with 10 or more involved [axilliary lymph nodes],” they wrote in JAMA Oncology.
And although other studies of chemotherapy regimens containing high doses of alkylating agents have shown increases in risk of late second malignancies and major cardiovascular events, there were no significant increases of either adverse event with HDCT in this study, the authors noted.
They reported 20-year follow-up results for 885 women who were enrolled in a 10-center randomized clinical trial conducted in the Netherlands from August 1, 1993, through July 31, 1999.
The participants were younger than age 56 years with breast cancer involving at least 4 axillary lymph nodes. All patients underwent surgery with complete axillary clearance and were then randomized to receive either conventional chemotherapy, which consisted of five cycles of fluorouracil 500mg/m2, epirubicin 90 mg/m2, and cyclophosphamide 500mg/m2 (FEC), or high-dose chemotherapy, with the first 4 cycles identical to conventional-dose chemotherapy but the fifth cycle consisting of cyclophosphamide 6000 mg/m2, thiotepa 480 mg/m2, and carboplatin 1600 mg/m2, supported with autologous hematopoietic stem cell transplant.
In addition, all patients received radiotherapy according to the local standard and 2 years of adjuvant tamoxifen.
After a median follow-up of 20.4 years, the 20-year overall survival (OS) rates were 45.3% for patients who had received high-dose chemotherapy and 41.5% for those who had received the conventional dose. This translated into a nonsignificant hazard ratio of 0.89.
However, for patients with 10 or more involved axillary nodes, the 20-year OS rates were 44.5% with HDCT and 29.9% with CDCT, translating into an absolute OS advantage for high-dose chemotherapy of 14.6% and an HR of 0.72 (P = .02).
Respective 20-year OS rates for women with triple-negative breast cancer were 52.9% and 37.5%, an absolute difference of 15.4% and a HR of 0.67, which fell just short of statistical significance, possibly because of the small number of patients with triple-negative breast cancer (140).
“In our 20-year follow-up analysis, there was no increase in cumulative risk for a second malignant neoplasm or for incidence of major cardiovascular events after HDCT,” the investigators wrote.
They noted that women randomized to high-dose chemotherapy had more frequent dysrhythmias, hypertension, and hypercholesterolemia, adding that the latter two adverse events may be partly attributable to a higher incidence of menopause induction among women who received HDCT.
The study was sponsored by University Medical Center Groningen and the The Netherlands Cancer Institute. Dr Steenbruggen reported receiving grants from the Dutch Health Insurance Council during the conduct of the study.
SOURCE: Steenbruggen TG et al. JAMA Oncology. 2020 Jan 30. doi: 10.1001/jamaoncol.2019.6276.
High-dose chemotherapy in the adjuvant setting offers a long-term survival advantage for women with very-high-risk stage III breast cancer, but does not improve survival odds for women with lower-risk cancers, an analysis of 20 years of follow-up data shows.
Among 885 women younger than 56 years at the time of treatment who had 4 or more involved axilliary lymph nodes, there was no overall survival difference over 2 decades between the total population of women randomized to receive adjuvant high-dose chemotherapy (HDCT) and those assigned to receive conventional-dose chemotherapy (CDCT).
However, women with 10 or more involved axilliary nodes and those with triple-negative breast cancer had an approximately 15% absolute improvement in 20-year overall survival with high-dose chemotherapy, although the difference for triple-negative disease fell just short of statistical significance, reported Tessa G. Steenbruggen, MD, from the Netherlands Cancer Institute in Amsterdam and colleagues.
“Our analysis confirms earlier results that HDCT has no significant overall survival benefit compared with CDCT for unselected patients with stage III [breast cancer]. However, we found a 14.6%improvement in 20-year OS estimates with HDCT in the predefined subgroup of patients with 10 or more involved [axilliary lymph nodes],” they wrote in JAMA Oncology.
And although other studies of chemotherapy regimens containing high doses of alkylating agents have shown increases in risk of late second malignancies and major cardiovascular events, there were no significant increases of either adverse event with HDCT in this study, the authors noted.
They reported 20-year follow-up results for 885 women who were enrolled in a 10-center randomized clinical trial conducted in the Netherlands from August 1, 1993, through July 31, 1999.
The participants were younger than age 56 years with breast cancer involving at least 4 axillary lymph nodes. All patients underwent surgery with complete axillary clearance and were then randomized to receive either conventional chemotherapy, which consisted of five cycles of fluorouracil 500mg/m2, epirubicin 90 mg/m2, and cyclophosphamide 500mg/m2 (FEC), or high-dose chemotherapy, with the first 4 cycles identical to conventional-dose chemotherapy but the fifth cycle consisting of cyclophosphamide 6000 mg/m2, thiotepa 480 mg/m2, and carboplatin 1600 mg/m2, supported with autologous hematopoietic stem cell transplant.
In addition, all patients received radiotherapy according to the local standard and 2 years of adjuvant tamoxifen.
After a median follow-up of 20.4 years, the 20-year overall survival (OS) rates were 45.3% for patients who had received high-dose chemotherapy and 41.5% for those who had received the conventional dose. This translated into a nonsignificant hazard ratio of 0.89.
However, for patients with 10 or more involved axillary nodes, the 20-year OS rates were 44.5% with HDCT and 29.9% with CDCT, translating into an absolute OS advantage for high-dose chemotherapy of 14.6% and an HR of 0.72 (P = .02).
Respective 20-year OS rates for women with triple-negative breast cancer were 52.9% and 37.5%, an absolute difference of 15.4% and a HR of 0.67, which fell just short of statistical significance, possibly because of the small number of patients with triple-negative breast cancer (140).
“In our 20-year follow-up analysis, there was no increase in cumulative risk for a second malignant neoplasm or for incidence of major cardiovascular events after HDCT,” the investigators wrote.
They noted that women randomized to high-dose chemotherapy had more frequent dysrhythmias, hypertension, and hypercholesterolemia, adding that the latter two adverse events may be partly attributable to a higher incidence of menopause induction among women who received HDCT.
The study was sponsored by University Medical Center Groningen and the The Netherlands Cancer Institute. Dr Steenbruggen reported receiving grants from the Dutch Health Insurance Council during the conduct of the study.
SOURCE: Steenbruggen TG et al. JAMA Oncology. 2020 Jan 30. doi: 10.1001/jamaoncol.2019.6276.
High-dose chemotherapy in the adjuvant setting offers a long-term survival advantage for women with very-high-risk stage III breast cancer, but does not improve survival odds for women with lower-risk cancers, an analysis of 20 years of follow-up data shows.
Among 885 women younger than 56 years at the time of treatment who had 4 or more involved axilliary lymph nodes, there was no overall survival difference over 2 decades between the total population of women randomized to receive adjuvant high-dose chemotherapy (HDCT) and those assigned to receive conventional-dose chemotherapy (CDCT).
However, women with 10 or more involved axilliary nodes and those with triple-negative breast cancer had an approximately 15% absolute improvement in 20-year overall survival with high-dose chemotherapy, although the difference for triple-negative disease fell just short of statistical significance, reported Tessa G. Steenbruggen, MD, from the Netherlands Cancer Institute in Amsterdam and colleagues.
“Our analysis confirms earlier results that HDCT has no significant overall survival benefit compared with CDCT for unselected patients with stage III [breast cancer]. However, we found a 14.6%improvement in 20-year OS estimates with HDCT in the predefined subgroup of patients with 10 or more involved [axilliary lymph nodes],” they wrote in JAMA Oncology.
And although other studies of chemotherapy regimens containing high doses of alkylating agents have shown increases in risk of late second malignancies and major cardiovascular events, there were no significant increases of either adverse event with HDCT in this study, the authors noted.
They reported 20-year follow-up results for 885 women who were enrolled in a 10-center randomized clinical trial conducted in the Netherlands from August 1, 1993, through July 31, 1999.
The participants were younger than age 56 years with breast cancer involving at least 4 axillary lymph nodes. All patients underwent surgery with complete axillary clearance and were then randomized to receive either conventional chemotherapy, which consisted of five cycles of fluorouracil 500mg/m2, epirubicin 90 mg/m2, and cyclophosphamide 500mg/m2 (FEC), or high-dose chemotherapy, with the first 4 cycles identical to conventional-dose chemotherapy but the fifth cycle consisting of cyclophosphamide 6000 mg/m2, thiotepa 480 mg/m2, and carboplatin 1600 mg/m2, supported with autologous hematopoietic stem cell transplant.
In addition, all patients received radiotherapy according to the local standard and 2 years of adjuvant tamoxifen.
After a median follow-up of 20.4 years, the 20-year overall survival (OS) rates were 45.3% for patients who had received high-dose chemotherapy and 41.5% for those who had received the conventional dose. This translated into a nonsignificant hazard ratio of 0.89.
However, for patients with 10 or more involved axillary nodes, the 20-year OS rates were 44.5% with HDCT and 29.9% with CDCT, translating into an absolute OS advantage for high-dose chemotherapy of 14.6% and an HR of 0.72 (P = .02).
Respective 20-year OS rates for women with triple-negative breast cancer were 52.9% and 37.5%, an absolute difference of 15.4% and a HR of 0.67, which fell just short of statistical significance, possibly because of the small number of patients with triple-negative breast cancer (140).
“In our 20-year follow-up analysis, there was no increase in cumulative risk for a second malignant neoplasm or for incidence of major cardiovascular events after HDCT,” the investigators wrote.
They noted that women randomized to high-dose chemotherapy had more frequent dysrhythmias, hypertension, and hypercholesterolemia, adding that the latter two adverse events may be partly attributable to a higher incidence of menopause induction among women who received HDCT.
The study was sponsored by University Medical Center Groningen and the The Netherlands Cancer Institute. Dr Steenbruggen reported receiving grants from the Dutch Health Insurance Council during the conduct of the study.
SOURCE: Steenbruggen TG et al. JAMA Oncology. 2020 Jan 30. doi: 10.1001/jamaoncol.2019.6276.
FROM JAMA ONCOLOGY
Key clinical point: High-dose chemotherapy offers a long-term breast cancer survival advantage only for women with very-high-risk disease.
Major finding: The absolute 20-year overall survival benefit for women with 10 or more involved lymph nodes was 14.6%.
Study details: Long-term, follow-up study of 885 women under age 56 years with stage III breast cancer treated with adjuvant high- or conventional-dose chemotherapy.
Disclosures: The study was sponsored by University Medical Center Groningen and the The Netherlands Cancer Institute. Dr. Steenbruggen reported receiving grants from the Dutch Health Insurance Council during the conduct of the study.
Source: Steenbruggen TG et al. JAMA Oncology. 2020 Jan 30. doi: 10.1001/jamaoncol.2019.6276.
Echoes of SARS mark 2019 novel coronavirus outbreak
The current outbreak of severe respiratory infections caused by the 2019 novel coronarvirus (2019-nCoV) has a clinical presentation resembling the Severe Acute Respiratory Syndrome coronavirus (SARS-CoV) outbreak that began in 2002, Chinese investigators caution.

By Jan. 2, 2020, 41 patients with confirmed 2019-nCoV had been admitted to a designated hospital in the city of Wuhan, Hubei Province, in central China. Thirteen required ICU admission and six died, reported Chaolin Huang, MD, from Jin Yin-tan Hospital in Wuhan, and colleagues.
“2019-nCoV still needs to be studied deeply in case it becomes a global health threat. Reliable quick pathogen tests and feasible differential diagnosis based on clinical description are crucial for clinicians in their first contact with suspected patients. Because of the pandemic potential of 2019-nCoV, careful surveillance is essential to monitor its future host adaption, viral evolution, infectivity, transmissibility, and pathogenicity,” they wrote in a review published online by The Lancet.
According to the U.S. Centers for Disease Control and Prevention, as of Jan. 28, 2020, the total number of 2019-nCoV cases reported in the United States stood at five, but further cases of the infection – which Chinese health officials have confirmed can be transmitted person-to-person – are expected.
Dr. Huang and colleagues note that although most human coronavirus infections are mild, SARS-CoV and the Middle East respiratory syndrome coronavirus (MERS-CoV) were responsible for more than 10,000 infections, with mortality rates ranging from 10% with SARS to 37% with MERS. To date, 2019-nCoV has “caused clusters of fatal pneumonia greatly resembling SARS-CoV,” they write.
The authors studied the epidemiological, clinical, laboratory, and radiological characteristics as well as treatments and clinical outcomes of 41 patients admitted or transferred to the Jin Yin-tan Hospital with laboratory-confirmed 2019-nCoV infections.
The median patient age was 49 years. Thirty of the 41 patients (73%) were male. Comorbid conditions included diabetes in 13 of the 41 patients (32%), hypertension in 6 (15%), and cardiovascular disease in 6.
In all 27 of the 41 patients had been exposed to the Huanan seafood market in Wuhan, the suspected epicenter of the outbreak that was shut down by health authorities on Jan. 1 of this year.
The most common symptoms at the onset of the illness were fever in all but one of the 41 patients, cough in 31, and myalgia or fatigue in 18. Other, less frequent symptoms included sputum production in 11, headache in three, hemoptysis in two, and diarrhea in one.
“In this cohort, most patients presented with fever, dry cough, dyspnoea, and bilateral ground-glass opacities on chest CT scans. These features of 2019-nCoV infection bear some resemblance to SARS-CoV and MERS-CoV infections. However, few patients with 2019-nCoV infection had prominent upper respiratory tract signs and symptoms (e.g., rhinorrhoea, sneezing, or sore throat), indicating that the target cells might be located in the lower airway. Furthermore, 2019-nCoV patients rarely developed intestinal signs and symptoms (e.g., diarrhoea), whereas about 20%-25% of patients with MERS-CoV or SARS-CoV infection had diarrhoea.”
In all, 22 patients developed dyspnea, with a median time from illness onset to dyspnea of 8 days. The median time from illness onset to admission was 7 days, median time to shortness of breath was 8 days, median time to acute respiratory distress syndrome (ARDS) was 9 days, and median time to both mechanical ventilation and ICU admission was 10.5 days.
All of the patients developed pneumonia with abnormal findings on chest CT scan. In addition, 12 patients developed ARDS, six had RNAaemia, five developed acute cardiac injury, and four developed a secondary infection. As noted before, 13 of the 14 patients were admitted to an ICU, and six died. RNAaemia is a positive result for real-time polymerase chain reaction in plasma samples. Patients admitted to the ICU had higher initial concentrations of multiple inflammatory cytokines than patients who did not need ICU care, “suggesting that the cytokine storm was associated with disease severity.”
All of the patients received empirical antibiotics, 38 were treated with oseltamivir (Tamiflu), and 9 received systemic corticosteroids.
The investigators have initiated a randomized controlled trial of the antiviral agents lopinavir and ritonavir for patients hospitalized with 2019-nCoV infection.
The study was funded by the Chinese Ministry of Science and Technology, Chinese Academy of Medical Sciences, National Natural Science Foundation of China, and Beijing Municipal Science and Technology Commission. All authors declared having no competing interests.
SOURCE: Huang C et al. Lancet. 2020 Jan 24. doi: 10.1016/S0140-6736(20)30183-5.
The current outbreak of severe respiratory infections caused by the 2019 novel coronarvirus (2019-nCoV) has a clinical presentation resembling the Severe Acute Respiratory Syndrome coronavirus (SARS-CoV) outbreak that began in 2002, Chinese investigators caution.

By Jan. 2, 2020, 41 patients with confirmed 2019-nCoV had been admitted to a designated hospital in the city of Wuhan, Hubei Province, in central China. Thirteen required ICU admission and six died, reported Chaolin Huang, MD, from Jin Yin-tan Hospital in Wuhan, and colleagues.
“2019-nCoV still needs to be studied deeply in case it becomes a global health threat. Reliable quick pathogen tests and feasible differential diagnosis based on clinical description are crucial for clinicians in their first contact with suspected patients. Because of the pandemic potential of 2019-nCoV, careful surveillance is essential to monitor its future host adaption, viral evolution, infectivity, transmissibility, and pathogenicity,” they wrote in a review published online by The Lancet.
According to the U.S. Centers for Disease Control and Prevention, as of Jan. 28, 2020, the total number of 2019-nCoV cases reported in the United States stood at five, but further cases of the infection – which Chinese health officials have confirmed can be transmitted person-to-person – are expected.
Dr. Huang and colleagues note that although most human coronavirus infections are mild, SARS-CoV and the Middle East respiratory syndrome coronavirus (MERS-CoV) were responsible for more than 10,000 infections, with mortality rates ranging from 10% with SARS to 37% with MERS. To date, 2019-nCoV has “caused clusters of fatal pneumonia greatly resembling SARS-CoV,” they write.
The authors studied the epidemiological, clinical, laboratory, and radiological characteristics as well as treatments and clinical outcomes of 41 patients admitted or transferred to the Jin Yin-tan Hospital with laboratory-confirmed 2019-nCoV infections.
The median patient age was 49 years. Thirty of the 41 patients (73%) were male. Comorbid conditions included diabetes in 13 of the 41 patients (32%), hypertension in 6 (15%), and cardiovascular disease in 6.
In all 27 of the 41 patients had been exposed to the Huanan seafood market in Wuhan, the suspected epicenter of the outbreak that was shut down by health authorities on Jan. 1 of this year.
The most common symptoms at the onset of the illness were fever in all but one of the 41 patients, cough in 31, and myalgia or fatigue in 18. Other, less frequent symptoms included sputum production in 11, headache in three, hemoptysis in two, and diarrhea in one.
“In this cohort, most patients presented with fever, dry cough, dyspnoea, and bilateral ground-glass opacities on chest CT scans. These features of 2019-nCoV infection bear some resemblance to SARS-CoV and MERS-CoV infections. However, few patients with 2019-nCoV infection had prominent upper respiratory tract signs and symptoms (e.g., rhinorrhoea, sneezing, or sore throat), indicating that the target cells might be located in the lower airway. Furthermore, 2019-nCoV patients rarely developed intestinal signs and symptoms (e.g., diarrhoea), whereas about 20%-25% of patients with MERS-CoV or SARS-CoV infection had diarrhoea.”
In all, 22 patients developed dyspnea, with a median time from illness onset to dyspnea of 8 days. The median time from illness onset to admission was 7 days, median time to shortness of breath was 8 days, median time to acute respiratory distress syndrome (ARDS) was 9 days, and median time to both mechanical ventilation and ICU admission was 10.5 days.
All of the patients developed pneumonia with abnormal findings on chest CT scan. In addition, 12 patients developed ARDS, six had RNAaemia, five developed acute cardiac injury, and four developed a secondary infection. As noted before, 13 of the 14 patients were admitted to an ICU, and six died. RNAaemia is a positive result for real-time polymerase chain reaction in plasma samples. Patients admitted to the ICU had higher initial concentrations of multiple inflammatory cytokines than patients who did not need ICU care, “suggesting that the cytokine storm was associated with disease severity.”
All of the patients received empirical antibiotics, 38 were treated with oseltamivir (Tamiflu), and 9 received systemic corticosteroids.
The investigators have initiated a randomized controlled trial of the antiviral agents lopinavir and ritonavir for patients hospitalized with 2019-nCoV infection.
The study was funded by the Chinese Ministry of Science and Technology, Chinese Academy of Medical Sciences, National Natural Science Foundation of China, and Beijing Municipal Science and Technology Commission. All authors declared having no competing interests.
SOURCE: Huang C et al. Lancet. 2020 Jan 24. doi: 10.1016/S0140-6736(20)30183-5.
The current outbreak of severe respiratory infections caused by the 2019 novel coronarvirus (2019-nCoV) has a clinical presentation resembling the Severe Acute Respiratory Syndrome coronavirus (SARS-CoV) outbreak that began in 2002, Chinese investigators caution.

By Jan. 2, 2020, 41 patients with confirmed 2019-nCoV had been admitted to a designated hospital in the city of Wuhan, Hubei Province, in central China. Thirteen required ICU admission and six died, reported Chaolin Huang, MD, from Jin Yin-tan Hospital in Wuhan, and colleagues.
“2019-nCoV still needs to be studied deeply in case it becomes a global health threat. Reliable quick pathogen tests and feasible differential diagnosis based on clinical description are crucial for clinicians in their first contact with suspected patients. Because of the pandemic potential of 2019-nCoV, careful surveillance is essential to monitor its future host adaption, viral evolution, infectivity, transmissibility, and pathogenicity,” they wrote in a review published online by The Lancet.
According to the U.S. Centers for Disease Control and Prevention, as of Jan. 28, 2020, the total number of 2019-nCoV cases reported in the United States stood at five, but further cases of the infection – which Chinese health officials have confirmed can be transmitted person-to-person – are expected.
Dr. Huang and colleagues note that although most human coronavirus infections are mild, SARS-CoV and the Middle East respiratory syndrome coronavirus (MERS-CoV) were responsible for more than 10,000 infections, with mortality rates ranging from 10% with SARS to 37% with MERS. To date, 2019-nCoV has “caused clusters of fatal pneumonia greatly resembling SARS-CoV,” they write.
The authors studied the epidemiological, clinical, laboratory, and radiological characteristics as well as treatments and clinical outcomes of 41 patients admitted or transferred to the Jin Yin-tan Hospital with laboratory-confirmed 2019-nCoV infections.
The median patient age was 49 years. Thirty of the 41 patients (73%) were male. Comorbid conditions included diabetes in 13 of the 41 patients (32%), hypertension in 6 (15%), and cardiovascular disease in 6.
In all 27 of the 41 patients had been exposed to the Huanan seafood market in Wuhan, the suspected epicenter of the outbreak that was shut down by health authorities on Jan. 1 of this year.
The most common symptoms at the onset of the illness were fever in all but one of the 41 patients, cough in 31, and myalgia or fatigue in 18. Other, less frequent symptoms included sputum production in 11, headache in three, hemoptysis in two, and diarrhea in one.
“In this cohort, most patients presented with fever, dry cough, dyspnoea, and bilateral ground-glass opacities on chest CT scans. These features of 2019-nCoV infection bear some resemblance to SARS-CoV and MERS-CoV infections. However, few patients with 2019-nCoV infection had prominent upper respiratory tract signs and symptoms (e.g., rhinorrhoea, sneezing, or sore throat), indicating that the target cells might be located in the lower airway. Furthermore, 2019-nCoV patients rarely developed intestinal signs and symptoms (e.g., diarrhoea), whereas about 20%-25% of patients with MERS-CoV or SARS-CoV infection had diarrhoea.”
In all, 22 patients developed dyspnea, with a median time from illness onset to dyspnea of 8 days. The median time from illness onset to admission was 7 days, median time to shortness of breath was 8 days, median time to acute respiratory distress syndrome (ARDS) was 9 days, and median time to both mechanical ventilation and ICU admission was 10.5 days.
All of the patients developed pneumonia with abnormal findings on chest CT scan. In addition, 12 patients developed ARDS, six had RNAaemia, five developed acute cardiac injury, and four developed a secondary infection. As noted before, 13 of the 14 patients were admitted to an ICU, and six died. RNAaemia is a positive result for real-time polymerase chain reaction in plasma samples. Patients admitted to the ICU had higher initial concentrations of multiple inflammatory cytokines than patients who did not need ICU care, “suggesting that the cytokine storm was associated with disease severity.”
All of the patients received empirical antibiotics, 38 were treated with oseltamivir (Tamiflu), and 9 received systemic corticosteroids.
The investigators have initiated a randomized controlled trial of the antiviral agents lopinavir and ritonavir for patients hospitalized with 2019-nCoV infection.
The study was funded by the Chinese Ministry of Science and Technology, Chinese Academy of Medical Sciences, National Natural Science Foundation of China, and Beijing Municipal Science and Technology Commission. All authors declared having no competing interests.
SOURCE: Huang C et al. Lancet. 2020 Jan 24. doi: 10.1016/S0140-6736(20)30183-5.
FROM THE LANCET
Adding cannabinoids to opioids doesn’t improve cancer pain control
Adding cannabinoids to opioids does not appear to improve control of cancer-related pain in adults with late-stage disease, authors of a systematic review and meta-analysis contend.
Among 1,442 participants in five randomized controlled trials of cannabinoids compared with placebo, there was no significant difference in the primary outcome of pain intensity scores, reported Elaine G. Boland, MD, PhD, of Hull University Teaching Hospitals NHS Trust in Cottingham, England, and colleagues.
“For a medication to be useful, there needs to be a net overall benefit, with the positive effects (analgesia) outweighing adverse effects. None of the included phase III studies show benefit of cannabinoids,” they wrote. Their report is in BMJ Supportive & Palliative Care.
According to NORML, the National Organization for the Reform of Marijuana Laws, 33 U.S. states currently have legalized medical use of marijuana or cannabinoids, and Dr. Boland and coauthors report that medical marijuana is legal in some 40 nations worldwide.
Survey data and a randomized sample of urine tests from a cancer center in Washington State, were marijuana is legal, show that cannabis or cannabinoid use is common among cancer patients. Despite its widespread use, good quality evidence of the efficacy of cannabis for control of cancer pain is sparse, the investigators said.-
They designed a systematic review and meta-analysis to identify randomized controlled trials with a low risk for bias, eventually settling on five with a total of 1,442 patients. Four of the studies evaluated nabiximols (Sativex), an oromucosal formulation of delta-9-tetrahydrocannabinol:cannabidiol (THC:CBD), and one tested THC:CBD or THC abstract vs. placebo.
To bolster confidence in their results, the investigators contacted the authors of the included studies to obtain additional findings and information about each study’s design.
They found that in the pooled data there was no significant difference between cannabinoids and placebo for the difference in average pain on a Numeric Rating Scale (NRS). The mean difference was –0.21 (P = .14) and did not reach significance when the analysis was restricted to phase 3 trials (mean difference –.02, P = .80).
For the secondary outcomes of adverse events and dropouts, they found that cannabinoids were associated with significantly higher risk for somnolence (odds ratio [OR] 2.69, P less than .001) and dizziness (OR 1.58, P = .05), and that dropouts due to adverse events were more frequent in the cannabinoid arms.
The investigators acknowledged that the study was limited by its reliance on the NRS pain score “as this simple instrument does not capture the complexity of pain especially when it has been [a] long-standing problem,” and by the possibility that vagaries in the use of the oromucosal spray might affect the absorption and efficacy of the cannabinoids.
The authors did not report a funding source. No conflicts of interest were reported.
SOURCE: Boland EG et al. BMJ Supportive & Palliative Care 2020 Jan 20. doi: 10.1136/bmjspcare-2019-002032.
Adding cannabinoids to opioids does not appear to improve control of cancer-related pain in adults with late-stage disease, authors of a systematic review and meta-analysis contend.
Among 1,442 participants in five randomized controlled trials of cannabinoids compared with placebo, there was no significant difference in the primary outcome of pain intensity scores, reported Elaine G. Boland, MD, PhD, of Hull University Teaching Hospitals NHS Trust in Cottingham, England, and colleagues.
“For a medication to be useful, there needs to be a net overall benefit, with the positive effects (analgesia) outweighing adverse effects. None of the included phase III studies show benefit of cannabinoids,” they wrote. Their report is in BMJ Supportive & Palliative Care.
According to NORML, the National Organization for the Reform of Marijuana Laws, 33 U.S. states currently have legalized medical use of marijuana or cannabinoids, and Dr. Boland and coauthors report that medical marijuana is legal in some 40 nations worldwide.
Survey data and a randomized sample of urine tests from a cancer center in Washington State, were marijuana is legal, show that cannabis or cannabinoid use is common among cancer patients. Despite its widespread use, good quality evidence of the efficacy of cannabis for control of cancer pain is sparse, the investigators said.-
They designed a systematic review and meta-analysis to identify randomized controlled trials with a low risk for bias, eventually settling on five with a total of 1,442 patients. Four of the studies evaluated nabiximols (Sativex), an oromucosal formulation of delta-9-tetrahydrocannabinol:cannabidiol (THC:CBD), and one tested THC:CBD or THC abstract vs. placebo.
To bolster confidence in their results, the investigators contacted the authors of the included studies to obtain additional findings and information about each study’s design.
They found that in the pooled data there was no significant difference between cannabinoids and placebo for the difference in average pain on a Numeric Rating Scale (NRS). The mean difference was –0.21 (P = .14) and did not reach significance when the analysis was restricted to phase 3 trials (mean difference –.02, P = .80).
For the secondary outcomes of adverse events and dropouts, they found that cannabinoids were associated with significantly higher risk for somnolence (odds ratio [OR] 2.69, P less than .001) and dizziness (OR 1.58, P = .05), and that dropouts due to adverse events were more frequent in the cannabinoid arms.
The investigators acknowledged that the study was limited by its reliance on the NRS pain score “as this simple instrument does not capture the complexity of pain especially when it has been [a] long-standing problem,” and by the possibility that vagaries in the use of the oromucosal spray might affect the absorption and efficacy of the cannabinoids.
The authors did not report a funding source. No conflicts of interest were reported.
SOURCE: Boland EG et al. BMJ Supportive & Palliative Care 2020 Jan 20. doi: 10.1136/bmjspcare-2019-002032.
Adding cannabinoids to opioids does not appear to improve control of cancer-related pain in adults with late-stage disease, authors of a systematic review and meta-analysis contend.
Among 1,442 participants in five randomized controlled trials of cannabinoids compared with placebo, there was no significant difference in the primary outcome of pain intensity scores, reported Elaine G. Boland, MD, PhD, of Hull University Teaching Hospitals NHS Trust in Cottingham, England, and colleagues.
“For a medication to be useful, there needs to be a net overall benefit, with the positive effects (analgesia) outweighing adverse effects. None of the included phase III studies show benefit of cannabinoids,” they wrote. Their report is in BMJ Supportive & Palliative Care.
According to NORML, the National Organization for the Reform of Marijuana Laws, 33 U.S. states currently have legalized medical use of marijuana or cannabinoids, and Dr. Boland and coauthors report that medical marijuana is legal in some 40 nations worldwide.
Survey data and a randomized sample of urine tests from a cancer center in Washington State, were marijuana is legal, show that cannabis or cannabinoid use is common among cancer patients. Despite its widespread use, good quality evidence of the efficacy of cannabis for control of cancer pain is sparse, the investigators said.-
They designed a systematic review and meta-analysis to identify randomized controlled trials with a low risk for bias, eventually settling on five with a total of 1,442 patients. Four of the studies evaluated nabiximols (Sativex), an oromucosal formulation of delta-9-tetrahydrocannabinol:cannabidiol (THC:CBD), and one tested THC:CBD or THC abstract vs. placebo.
To bolster confidence in their results, the investigators contacted the authors of the included studies to obtain additional findings and information about each study’s design.
They found that in the pooled data there was no significant difference between cannabinoids and placebo for the difference in average pain on a Numeric Rating Scale (NRS). The mean difference was –0.21 (P = .14) and did not reach significance when the analysis was restricted to phase 3 trials (mean difference –.02, P = .80).
For the secondary outcomes of adverse events and dropouts, they found that cannabinoids were associated with significantly higher risk for somnolence (odds ratio [OR] 2.69, P less than .001) and dizziness (OR 1.58, P = .05), and that dropouts due to adverse events were more frequent in the cannabinoid arms.
The investigators acknowledged that the study was limited by its reliance on the NRS pain score “as this simple instrument does not capture the complexity of pain especially when it has been [a] long-standing problem,” and by the possibility that vagaries in the use of the oromucosal spray might affect the absorption and efficacy of the cannabinoids.
The authors did not report a funding source. No conflicts of interest were reported.
SOURCE: Boland EG et al. BMJ Supportive & Palliative Care 2020 Jan 20. doi: 10.1136/bmjspcare-2019-002032.
FROM BMJ SUPPORTIVE & PALLIATIVE CARE
Promising early efficacy of venetoclax/navitoclax in r/r acute lymphoblastic leukemia
ORLANDO – A combination of venetoclax (Venclexta) and the experimental BCL-2 inhibitor navitoclax showed good activity and acceptable safety in both children and adults with relapsed or refractory acute lymphoblastic leukemia or lymphoblastic lymphoma, investigators in a phase 1 trial reported.
The overall rate of combined complete response (CR), CR with incomplete marrow recovery (CRi) or incomplete platelet recovery (CRp) was 49% among 45 patients: 24 with acute lymphoblastic leukemia of B-cell lineage (B-ALL), 18 with T-cell lineage ALL (T-ALL), and 3 with lymphoblastic lymphoma, reported Norman J. Lacayo, MD, from Stanford (Calif.) University.
“Venetoclax, navitoclax, and chemotherapy is well tolerated with few discontinuations for dose reductions due to adverse events in patients with relapsed/refractory ALL or lymphoblastic lymphoma. The preliminary efficacy was promising in this heavily pretreated population of patients, including pediatric patients and patients with prior stem cell transplantation of CAR [chimeric antigen receptor] T-cell therapy,” he said at the annual meeting of the American Society of Hematology.
Venetoclax is a highly selective inhibitor of the B-cell lymphoma 2 (BCL-2) pathway. Navitoclax inhibits BCL-2, the BCL–extra large (BCL-XL) transmembrane molecule, and the apoptotic protein BCL-W, but was associated with dose-limiting toxicities when used at standard doses in monotherapy, he noted.
To see whether adding venetoclax to low-dose navitoclax could be safe and have synergistic activity against BCL-2, Dr. Lacayo and colleagues are conducting a phase 1, open-label, dose-escalation study of patients aged 4 years and older with relapsed/refractory ALL or lymphoblastic lymphoma.
Patients receive the weight-adjusted equivalent of 200 mg venetoclax on day 1, and 400 mg equivalent daily thereafter. Beginning on day 3, patients receive oral navitoclax daily at doses of 25, 50, or 100 mg for patients weighing 45 kg or more, or 25 or 50 mg for patients weighing from 20 to less than 45 kg.
Patients can also receive two cycles of chemotherapy with asparaginase, vincristine, and dexamethasone, with additional cycles allowed at the investigators’ discretion.
The patients reported on at ASH 2019 had received a median of 4 prior lines of therapy (range, 1-10).
After a median time on study of 8 months, preliminary efficacy – a secondary endpoint of this phase 1 trial – was promising, Dr. Lacayo said. The CR rate was 25% among patients with B-ALL, 11% in patients with T-ALL, and 67% (two of three patients) with lymphoblastic lymphoma. The respective CRi rates were 13%, 17%, and 0%, and respective CRp rates were 17%, 11%, and 0%.
In addition, 3 of 24 patients (13%) with B-ALL had a partial response, as did 1 patient with lymphoblastic lymphoma.
The median time to first response was about 1.1 months. The median duration of response was 9.1 months for patients with B-ALL, 4.2 months for patients with T-ALL, and had not been reached among patients with lymphoblastic leukemia.
The median overall survival was 9.7 months for patients with B-ALL, 6.8 months for those with T-ALL, and not reached for those with lymphoblastic leukemia.
In all, 6 of 24 patients with B-ALL, 3 of 18 with T-ALL, and 2 of 3 with lymphoblastic leukemia survived long enough to proceed to stem cell transplantation or CAR T-cell therapy.
Analysis of the combination’s safety, the primary endpoint, showed that 58% of patients had grade 3-4 adverse events (AEs) related to venetoclax, and 42% had grade 3-4 AEs related to navitoclax. Four patients had to discontinue the combination because of treatment-related AEs.
Dose-limiting toxicities, which occurred in seven patients, included delayed count recovery, drug-induced liver injury, intestinal ischemia, and increase in serum bilirubin.
One patient died from an intestinal ischemic event deemed to be related to the combination, and seven other patients had fatal adverse events considered not related to the study drugs. The causes of death included sepsis, septic shock, cardiac arrest, and neurotoxicity.
The investigators are enrolling an expansion cohort to see whether a 21-day dosing schedule of venetoclax with 50 mg navitoclax, or 25 mg for patients under 45 kg, could improve count recovery time.
The study was funded by AbbVie. Dr. Lacayo reported having no conflict of interests to disclose. Several coauthors are AbbVie employees.
SOURCE: Lacayo NJ et al. ASH 2019, Abstract 285.
ORLANDO – A combination of venetoclax (Venclexta) and the experimental BCL-2 inhibitor navitoclax showed good activity and acceptable safety in both children and adults with relapsed or refractory acute lymphoblastic leukemia or lymphoblastic lymphoma, investigators in a phase 1 trial reported.
The overall rate of combined complete response (CR), CR with incomplete marrow recovery (CRi) or incomplete platelet recovery (CRp) was 49% among 45 patients: 24 with acute lymphoblastic leukemia of B-cell lineage (B-ALL), 18 with T-cell lineage ALL (T-ALL), and 3 with lymphoblastic lymphoma, reported Norman J. Lacayo, MD, from Stanford (Calif.) University.
“Venetoclax, navitoclax, and chemotherapy is well tolerated with few discontinuations for dose reductions due to adverse events in patients with relapsed/refractory ALL or lymphoblastic lymphoma. The preliminary efficacy was promising in this heavily pretreated population of patients, including pediatric patients and patients with prior stem cell transplantation of CAR [chimeric antigen receptor] T-cell therapy,” he said at the annual meeting of the American Society of Hematology.
Venetoclax is a highly selective inhibitor of the B-cell lymphoma 2 (BCL-2) pathway. Navitoclax inhibits BCL-2, the BCL–extra large (BCL-XL) transmembrane molecule, and the apoptotic protein BCL-W, but was associated with dose-limiting toxicities when used at standard doses in monotherapy, he noted.
To see whether adding venetoclax to low-dose navitoclax could be safe and have synergistic activity against BCL-2, Dr. Lacayo and colleagues are conducting a phase 1, open-label, dose-escalation study of patients aged 4 years and older with relapsed/refractory ALL or lymphoblastic lymphoma.
Patients receive the weight-adjusted equivalent of 200 mg venetoclax on day 1, and 400 mg equivalent daily thereafter. Beginning on day 3, patients receive oral navitoclax daily at doses of 25, 50, or 100 mg for patients weighing 45 kg or more, or 25 or 50 mg for patients weighing from 20 to less than 45 kg.
Patients can also receive two cycles of chemotherapy with asparaginase, vincristine, and dexamethasone, with additional cycles allowed at the investigators’ discretion.
The patients reported on at ASH 2019 had received a median of 4 prior lines of therapy (range, 1-10).
After a median time on study of 8 months, preliminary efficacy – a secondary endpoint of this phase 1 trial – was promising, Dr. Lacayo said. The CR rate was 25% among patients with B-ALL, 11% in patients with T-ALL, and 67% (two of three patients) with lymphoblastic lymphoma. The respective CRi rates were 13%, 17%, and 0%, and respective CRp rates were 17%, 11%, and 0%.
In addition, 3 of 24 patients (13%) with B-ALL had a partial response, as did 1 patient with lymphoblastic lymphoma.
The median time to first response was about 1.1 months. The median duration of response was 9.1 months for patients with B-ALL, 4.2 months for patients with T-ALL, and had not been reached among patients with lymphoblastic leukemia.
The median overall survival was 9.7 months for patients with B-ALL, 6.8 months for those with T-ALL, and not reached for those with lymphoblastic leukemia.
In all, 6 of 24 patients with B-ALL, 3 of 18 with T-ALL, and 2 of 3 with lymphoblastic leukemia survived long enough to proceed to stem cell transplantation or CAR T-cell therapy.
Analysis of the combination’s safety, the primary endpoint, showed that 58% of patients had grade 3-4 adverse events (AEs) related to venetoclax, and 42% had grade 3-4 AEs related to navitoclax. Four patients had to discontinue the combination because of treatment-related AEs.
Dose-limiting toxicities, which occurred in seven patients, included delayed count recovery, drug-induced liver injury, intestinal ischemia, and increase in serum bilirubin.
One patient died from an intestinal ischemic event deemed to be related to the combination, and seven other patients had fatal adverse events considered not related to the study drugs. The causes of death included sepsis, septic shock, cardiac arrest, and neurotoxicity.
The investigators are enrolling an expansion cohort to see whether a 21-day dosing schedule of venetoclax with 50 mg navitoclax, or 25 mg for patients under 45 kg, could improve count recovery time.
The study was funded by AbbVie. Dr. Lacayo reported having no conflict of interests to disclose. Several coauthors are AbbVie employees.
SOURCE: Lacayo NJ et al. ASH 2019, Abstract 285.
ORLANDO – A combination of venetoclax (Venclexta) and the experimental BCL-2 inhibitor navitoclax showed good activity and acceptable safety in both children and adults with relapsed or refractory acute lymphoblastic leukemia or lymphoblastic lymphoma, investigators in a phase 1 trial reported.
The overall rate of combined complete response (CR), CR with incomplete marrow recovery (CRi) or incomplete platelet recovery (CRp) was 49% among 45 patients: 24 with acute lymphoblastic leukemia of B-cell lineage (B-ALL), 18 with T-cell lineage ALL (T-ALL), and 3 with lymphoblastic lymphoma, reported Norman J. Lacayo, MD, from Stanford (Calif.) University.
“Venetoclax, navitoclax, and chemotherapy is well tolerated with few discontinuations for dose reductions due to adverse events in patients with relapsed/refractory ALL or lymphoblastic lymphoma. The preliminary efficacy was promising in this heavily pretreated population of patients, including pediatric patients and patients with prior stem cell transplantation of CAR [chimeric antigen receptor] T-cell therapy,” he said at the annual meeting of the American Society of Hematology.
Venetoclax is a highly selective inhibitor of the B-cell lymphoma 2 (BCL-2) pathway. Navitoclax inhibits BCL-2, the BCL–extra large (BCL-XL) transmembrane molecule, and the apoptotic protein BCL-W, but was associated with dose-limiting toxicities when used at standard doses in monotherapy, he noted.
To see whether adding venetoclax to low-dose navitoclax could be safe and have synergistic activity against BCL-2, Dr. Lacayo and colleagues are conducting a phase 1, open-label, dose-escalation study of patients aged 4 years and older with relapsed/refractory ALL or lymphoblastic lymphoma.
Patients receive the weight-adjusted equivalent of 200 mg venetoclax on day 1, and 400 mg equivalent daily thereafter. Beginning on day 3, patients receive oral navitoclax daily at doses of 25, 50, or 100 mg for patients weighing 45 kg or more, or 25 or 50 mg for patients weighing from 20 to less than 45 kg.
Patients can also receive two cycles of chemotherapy with asparaginase, vincristine, and dexamethasone, with additional cycles allowed at the investigators’ discretion.
The patients reported on at ASH 2019 had received a median of 4 prior lines of therapy (range, 1-10).
After a median time on study of 8 months, preliminary efficacy – a secondary endpoint of this phase 1 trial – was promising, Dr. Lacayo said. The CR rate was 25% among patients with B-ALL, 11% in patients with T-ALL, and 67% (two of three patients) with lymphoblastic lymphoma. The respective CRi rates were 13%, 17%, and 0%, and respective CRp rates were 17%, 11%, and 0%.
In addition, 3 of 24 patients (13%) with B-ALL had a partial response, as did 1 patient with lymphoblastic lymphoma.
The median time to first response was about 1.1 months. The median duration of response was 9.1 months for patients with B-ALL, 4.2 months for patients with T-ALL, and had not been reached among patients with lymphoblastic leukemia.
The median overall survival was 9.7 months for patients with B-ALL, 6.8 months for those with T-ALL, and not reached for those with lymphoblastic leukemia.
In all, 6 of 24 patients with B-ALL, 3 of 18 with T-ALL, and 2 of 3 with lymphoblastic leukemia survived long enough to proceed to stem cell transplantation or CAR T-cell therapy.
Analysis of the combination’s safety, the primary endpoint, showed that 58% of patients had grade 3-4 adverse events (AEs) related to venetoclax, and 42% had grade 3-4 AEs related to navitoclax. Four patients had to discontinue the combination because of treatment-related AEs.
Dose-limiting toxicities, which occurred in seven patients, included delayed count recovery, drug-induced liver injury, intestinal ischemia, and increase in serum bilirubin.
One patient died from an intestinal ischemic event deemed to be related to the combination, and seven other patients had fatal adverse events considered not related to the study drugs. The causes of death included sepsis, septic shock, cardiac arrest, and neurotoxicity.
The investigators are enrolling an expansion cohort to see whether a 21-day dosing schedule of venetoclax with 50 mg navitoclax, or 25 mg for patients under 45 kg, could improve count recovery time.
The study was funded by AbbVie. Dr. Lacayo reported having no conflict of interests to disclose. Several coauthors are AbbVie employees.
SOURCE: Lacayo NJ et al. ASH 2019, Abstract 285.
REPORTING FROM ASH 2019