User login
Mitchel is a reporter for MDedge based in the Philadelphia area. He started with the company in 1992, when it was International Medical News Group (IMNG), and has since covered a range of medical specialties. Mitchel trained as a virologist at Roswell Park Memorial Institute in Buffalo, and then worked briefly as a researcher at Boston Children's Hospital before pivoting to journalism as a AAAS Mass Media Fellow in 1980. His first reporting job was with Science Digest magazine, and from the mid-1980s to early-1990s he was a reporter with Medical World News. @mitchelzoler
Fear of blindness hobbles hydroxychloroquine treatment of SLE
CHICAGO – The dosage of hydroxychloroquine (HCQ) for treating patients with systemic lupus erythematosus (SLE) is often overly influenced by fear that the drug could cause blindness, Michelle A. Petri, MD, said at the annual meeting of the American College of Rheumatology.
HCQ “is the most important drug we have to treat lupus. It’s the only treatment shown to improve survival of lupus patients. There is no reason to make patients afraid of this very important drug. I am very concerned that fear of blindness is causing our patients to be less adherent” or making them receive an inadequate dosage, said Dr. Petri, a professor of medicine and the director of the Lupus Center at Johns Hopkins University in Baltimore. “I have had no patients who went blind on HCQ. A few patients developed retinopathy, but none went blind.”
Dr. Petri spoke about experiences with some of her SLE patients who were frightened by what an ophthalmologist told them about the retinal effects of HCQ and had their dosage of the drug unilaterally cut by the ophthalmologist.
“The message we should give patients is that retinopathy is a real complication that can happen, but usually not until after 16 years of treatment with HCQ, and we will work together to make sure you are regularly screened so that, if retinopathy developed, we would pick it up early and you’ll remain asymptomatic,” she said. ”We need to put the risk into perspective for our patients.”
Reports differ on the incidence of retinopathy in SLE patients on long-term HCQ treatment. During the session in which Dr. Petri spoke, James T. Rosenbaum, MD, cited a seminal report from 2014 that tracked 2,361 U.S. patients treated with HCQ daily for at least 5 years with regular retinal follow-up. The results showed a steady, cumulative increase in patients who developed retinopathy, an increase that was also dose dependent. For example, patients who received daily dosage of 5-5.9 mg/kg and took the drug for 20 or more years had a cumulative retinopathy incidence of 30% (JAMA Opthalmol. 2014 Dec;132[12]:1453-60). For patients on higher dosages, the cumulative risk at 20 years or longer jumped above 50%.
The findings from this study led directly to the most recent recommendations from the American Academy of Ophthalmology for retinopathy screening for patients on chronic HCQ treatment, said Dr. Rosenbaum, a professor of medicine and opthalmology at Oregon Health & Science University in Portland. The recommendations called for a dosage cap of less than 5 mg/kg real weight, a baseline retinal examination, and then at least annual follow-up examinations starting after 5 years of daily HCQ use, ideally using both automated visual fields and spectral-domain optical coherence tomography (Ophthalmology. 2016 June;123[6]:1386-94). The recommendations also noted that the presence of retinopathy risk factors, including renal disease or concomitant tamoxifen use, warrant starting screening at 5 years or sooner on HCQ.
Clinicians have often exceeded recommended dosage guidelines. Dr. Rosenbaum cited a 2017 report from one U.S. health care system that reviewed the treatment of 554 patients on HCQ and found that roughly half were overdosed in their starting regimen based on prevailing treatment recommendations (Opthalmology. 2017 May;124[5]:604-8).
But Dr. Petri contended that concerns about excessive HCQ dosages are overblown. At the meeting, she reported data showing a different perspective on the retinopathy risk of patients on chronic HCQ treatment. In a prospective series of 477 SLE patients on chronic HCQ treatment and followed at Johns Hopkins, the incidence of retinopathy was 10% among patients on treatment for 16 or more years, she reported in a talk at the meeting (Arthritis Rheumatol. 2018;70[Suppl 10]: Abstract 2897).
A 10% retinopathy rate after 16 or more years on treatment “sounds a lot safer to patients” than rates as high as 30% or 50% that were reported in the JAMA Opthalmology report from 2014, Dr. Petri said. “There is a danger relying on one retrospective study,” she warned. In addition, the 10% risk for retinopathy is “manageable” when patients receive regular screening that produces early detection of retinal damage.
“There has been acceptance of suboptimal dosing of HCQ when discussion has only been about safety, not about efficacy. We need to put the risks into perspective and stop scaring patients.”
Dr. Petri presented her recommendations for treating SLE patients with HCQ: Treat with dosages as high as 6.5 mg/kg but without exceeding 400 mg/day, and cut the dosage for patients with renal insufficiency or failure, those with liver disease, or the elderly. In addition, Dr. Petri endorsed monitoring blood levels of HCQ. Data she reported at the meeting showed a roughly fourfold higher rate of retinopathy among patients who had a maximum HCQ blood level of 1,733 ng/mL or higher when compared with patients whose maximum level remained at 1,194 ng/mL or lower. Clinicians could use blood levels of HCQ to better focus screening and its intensity, she said. “We should embrace monitoring HCQ blood levels.”
Dr. Petri has been a consultant to Amgen, Exagen, GlaxoSmithKline, Inova Diagnostics, Janssen, Lilly, Merck, Novartis, Quintiles, and EMD Serono, and she has received research funding from AstraZeneca and Exagen. Dr. Rosenbaum has been a consultant to AbbVie, Eyevensys, Gilead, Janssen, Novartis, Regeneron, and Roche, and has received research funding from Pfizer.
CHICAGO – The dosage of hydroxychloroquine (HCQ) for treating patients with systemic lupus erythematosus (SLE) is often overly influenced by fear that the drug could cause blindness, Michelle A. Petri, MD, said at the annual meeting of the American College of Rheumatology.
HCQ “is the most important drug we have to treat lupus. It’s the only treatment shown to improve survival of lupus patients. There is no reason to make patients afraid of this very important drug. I am very concerned that fear of blindness is causing our patients to be less adherent” or making them receive an inadequate dosage, said Dr. Petri, a professor of medicine and the director of the Lupus Center at Johns Hopkins University in Baltimore. “I have had no patients who went blind on HCQ. A few patients developed retinopathy, but none went blind.”
Dr. Petri spoke about experiences with some of her SLE patients who were frightened by what an ophthalmologist told them about the retinal effects of HCQ and had their dosage of the drug unilaterally cut by the ophthalmologist.
“The message we should give patients is that retinopathy is a real complication that can happen, but usually not until after 16 years of treatment with HCQ, and we will work together to make sure you are regularly screened so that, if retinopathy developed, we would pick it up early and you’ll remain asymptomatic,” she said. ”We need to put the risk into perspective for our patients.”
Reports differ on the incidence of retinopathy in SLE patients on long-term HCQ treatment. During the session in which Dr. Petri spoke, James T. Rosenbaum, MD, cited a seminal report from 2014 that tracked 2,361 U.S. patients treated with HCQ daily for at least 5 years with regular retinal follow-up. The results showed a steady, cumulative increase in patients who developed retinopathy, an increase that was also dose dependent. For example, patients who received daily dosage of 5-5.9 mg/kg and took the drug for 20 or more years had a cumulative retinopathy incidence of 30% (JAMA Opthalmol. 2014 Dec;132[12]:1453-60). For patients on higher dosages, the cumulative risk at 20 years or longer jumped above 50%.
The findings from this study led directly to the most recent recommendations from the American Academy of Ophthalmology for retinopathy screening for patients on chronic HCQ treatment, said Dr. Rosenbaum, a professor of medicine and opthalmology at Oregon Health & Science University in Portland. The recommendations called for a dosage cap of less than 5 mg/kg real weight, a baseline retinal examination, and then at least annual follow-up examinations starting after 5 years of daily HCQ use, ideally using both automated visual fields and spectral-domain optical coherence tomography (Ophthalmology. 2016 June;123[6]:1386-94). The recommendations also noted that the presence of retinopathy risk factors, including renal disease or concomitant tamoxifen use, warrant starting screening at 5 years or sooner on HCQ.
Clinicians have often exceeded recommended dosage guidelines. Dr. Rosenbaum cited a 2017 report from one U.S. health care system that reviewed the treatment of 554 patients on HCQ and found that roughly half were overdosed in their starting regimen based on prevailing treatment recommendations (Opthalmology. 2017 May;124[5]:604-8).
But Dr. Petri contended that concerns about excessive HCQ dosages are overblown. At the meeting, she reported data showing a different perspective on the retinopathy risk of patients on chronic HCQ treatment. In a prospective series of 477 SLE patients on chronic HCQ treatment and followed at Johns Hopkins, the incidence of retinopathy was 10% among patients on treatment for 16 or more years, she reported in a talk at the meeting (Arthritis Rheumatol. 2018;70[Suppl 10]: Abstract 2897).
A 10% retinopathy rate after 16 or more years on treatment “sounds a lot safer to patients” than rates as high as 30% or 50% that were reported in the JAMA Opthalmology report from 2014, Dr. Petri said. “There is a danger relying on one retrospective study,” she warned. In addition, the 10% risk for retinopathy is “manageable” when patients receive regular screening that produces early detection of retinal damage.
“There has been acceptance of suboptimal dosing of HCQ when discussion has only been about safety, not about efficacy. We need to put the risks into perspective and stop scaring patients.”
Dr. Petri presented her recommendations for treating SLE patients with HCQ: Treat with dosages as high as 6.5 mg/kg but without exceeding 400 mg/day, and cut the dosage for patients with renal insufficiency or failure, those with liver disease, or the elderly. In addition, Dr. Petri endorsed monitoring blood levels of HCQ. Data she reported at the meeting showed a roughly fourfold higher rate of retinopathy among patients who had a maximum HCQ blood level of 1,733 ng/mL or higher when compared with patients whose maximum level remained at 1,194 ng/mL or lower. Clinicians could use blood levels of HCQ to better focus screening and its intensity, she said. “We should embrace monitoring HCQ blood levels.”
Dr. Petri has been a consultant to Amgen, Exagen, GlaxoSmithKline, Inova Diagnostics, Janssen, Lilly, Merck, Novartis, Quintiles, and EMD Serono, and she has received research funding from AstraZeneca and Exagen. Dr. Rosenbaum has been a consultant to AbbVie, Eyevensys, Gilead, Janssen, Novartis, Regeneron, and Roche, and has received research funding from Pfizer.
CHICAGO – The dosage of hydroxychloroquine (HCQ) for treating patients with systemic lupus erythematosus (SLE) is often overly influenced by fear that the drug could cause blindness, Michelle A. Petri, MD, said at the annual meeting of the American College of Rheumatology.
HCQ “is the most important drug we have to treat lupus. It’s the only treatment shown to improve survival of lupus patients. There is no reason to make patients afraid of this very important drug. I am very concerned that fear of blindness is causing our patients to be less adherent” or making them receive an inadequate dosage, said Dr. Petri, a professor of medicine and the director of the Lupus Center at Johns Hopkins University in Baltimore. “I have had no patients who went blind on HCQ. A few patients developed retinopathy, but none went blind.”
Dr. Petri spoke about experiences with some of her SLE patients who were frightened by what an ophthalmologist told them about the retinal effects of HCQ and had their dosage of the drug unilaterally cut by the ophthalmologist.
“The message we should give patients is that retinopathy is a real complication that can happen, but usually not until after 16 years of treatment with HCQ, and we will work together to make sure you are regularly screened so that, if retinopathy developed, we would pick it up early and you’ll remain asymptomatic,” she said. ”We need to put the risk into perspective for our patients.”
Reports differ on the incidence of retinopathy in SLE patients on long-term HCQ treatment. During the session in which Dr. Petri spoke, James T. Rosenbaum, MD, cited a seminal report from 2014 that tracked 2,361 U.S. patients treated with HCQ daily for at least 5 years with regular retinal follow-up. The results showed a steady, cumulative increase in patients who developed retinopathy, an increase that was also dose dependent. For example, patients who received daily dosage of 5-5.9 mg/kg and took the drug for 20 or more years had a cumulative retinopathy incidence of 30% (JAMA Opthalmol. 2014 Dec;132[12]:1453-60). For patients on higher dosages, the cumulative risk at 20 years or longer jumped above 50%.
The findings from this study led directly to the most recent recommendations from the American Academy of Ophthalmology for retinopathy screening for patients on chronic HCQ treatment, said Dr. Rosenbaum, a professor of medicine and opthalmology at Oregon Health & Science University in Portland. The recommendations called for a dosage cap of less than 5 mg/kg real weight, a baseline retinal examination, and then at least annual follow-up examinations starting after 5 years of daily HCQ use, ideally using both automated visual fields and spectral-domain optical coherence tomography (Ophthalmology. 2016 June;123[6]:1386-94). The recommendations also noted that the presence of retinopathy risk factors, including renal disease or concomitant tamoxifen use, warrant starting screening at 5 years or sooner on HCQ.
Clinicians have often exceeded recommended dosage guidelines. Dr. Rosenbaum cited a 2017 report from one U.S. health care system that reviewed the treatment of 554 patients on HCQ and found that roughly half were overdosed in their starting regimen based on prevailing treatment recommendations (Opthalmology. 2017 May;124[5]:604-8).
But Dr. Petri contended that concerns about excessive HCQ dosages are overblown. At the meeting, she reported data showing a different perspective on the retinopathy risk of patients on chronic HCQ treatment. In a prospective series of 477 SLE patients on chronic HCQ treatment and followed at Johns Hopkins, the incidence of retinopathy was 10% among patients on treatment for 16 or more years, she reported in a talk at the meeting (Arthritis Rheumatol. 2018;70[Suppl 10]: Abstract 2897).
A 10% retinopathy rate after 16 or more years on treatment “sounds a lot safer to patients” than rates as high as 30% or 50% that were reported in the JAMA Opthalmology report from 2014, Dr. Petri said. “There is a danger relying on one retrospective study,” she warned. In addition, the 10% risk for retinopathy is “manageable” when patients receive regular screening that produces early detection of retinal damage.
“There has been acceptance of suboptimal dosing of HCQ when discussion has only been about safety, not about efficacy. We need to put the risks into perspective and stop scaring patients.”
Dr. Petri presented her recommendations for treating SLE patients with HCQ: Treat with dosages as high as 6.5 mg/kg but without exceeding 400 mg/day, and cut the dosage for patients with renal insufficiency or failure, those with liver disease, or the elderly. In addition, Dr. Petri endorsed monitoring blood levels of HCQ. Data she reported at the meeting showed a roughly fourfold higher rate of retinopathy among patients who had a maximum HCQ blood level of 1,733 ng/mL or higher when compared with patients whose maximum level remained at 1,194 ng/mL or lower. Clinicians could use blood levels of HCQ to better focus screening and its intensity, she said. “We should embrace monitoring HCQ blood levels.”
Dr. Petri has been a consultant to Amgen, Exagen, GlaxoSmithKline, Inova Diagnostics, Janssen, Lilly, Merck, Novartis, Quintiles, and EMD Serono, and she has received research funding from AstraZeneca and Exagen. Dr. Rosenbaum has been a consultant to AbbVie, Eyevensys, Gilead, Janssen, Novartis, Regeneron, and Roche, and has received research funding from Pfizer.
REPORTING FROM THE ACR ANNUAL MEETING
Ganglion stimulation boosts cerebral blood flow, improves stroke outcomes
MONTREAL – Stimulation of the sphenopalatine ganglion (SPG) using a small, implanted electrode for 5 days in patients who had just had an acute ischemic stroke led to statistically significant and clinically meaningful improvements in the subset of patients with confirmed cortical involvement in a pivotal, sham-controlled trial.
SPG stimulation started within 24 hours of an acute ischemic stroke “reduced poststroke disability over the entire outcome range and increased the proportion of patients who were alive and independent 3 months after their stroke” in the subgroup with a confirmed cortical infarction (CCI), Jeffrey L. Saver, MD, said at the World Stroke Congress. Five days of SPG stimulation, done once daily starting within 24 hours of stroke onset, “enhances ipsilateral collateral blood flow” and may also stabilize the blood brain barrier, explained Dr. Saver, professor of neurology and director of the stroke center at the University of California, Los Angeles. The study included a prespecified primary endpoint analysis that focused exclusively on the CCI subgroup, 52% of the total enrolled population.
If the reported data result in Food and Drug Administration marketing approval for the system, Dr. Saver said that he anticipated “substantial uptake” of the strategy, which he tested in patients who had not undergone thrombectomy or thrombolysis treatment. In current U.S. practice, there is “a large group of patients with a missed opportunity for recanalization” who would be candidates for treatment with SPG stimulation, a treatment that appeared to provide benefits beyond current standard care, he said in an interview.
Ongoing studies are also testing whether SPG stimulation can benefit acute ischemic stroke patients who have already undergone treatment with thrombectomy or thrombolysis, he added. The same SPG stimulation device is additionally undergoing U.S. testing as a treatment for headache and has regulatory approval in the European Union for treating headache and migraine.
The ImpACT-24B (Implant for Augmentation of Cerebral Blood Flow Trial, Effectiveness and Safety in a 24-Hour Window) trial involved 1,000 patients at 73 centers in 18 countries, including the United States. The investigators enrolled acute ischemic stroke patients 8-24 hours after stroke onset who had a National Institutes of Health Stroke Scale (NIHSS) score of 7-18.
Each patient received an implant of a short, thin metal electrode placed through the soft palate at the rear roof of the mouth, near the SPG. Neurologists primarily performed the implants in a procedure that had a “skin to skin” time of less than 5 minutes. Patients received either electrical stimulation or a sham stimulation through the electrode immediately after placement and then daily for the next 4 days. The investigators titrated the strength of the treatment stimulation in each patient to maximize its strength while maintaining patient comfort. Subsequent analysis of the results showed that the stronger the tolerated stimulation, the bigger the treatment effect in a clear dose-response pattern, Dr. Saver reported.
The study’s primary endpoint was improvement in the modified Rankin scale (mRS) score at 90 days after the index stroke when measured against historical expectations. By this measure, the overall study cohort showed a small, statistically insignificant improvement in actively treated patients, compared with sham-treated patients. However, in the prespecified, coprimary endpoint cohort of patients with a CCI, active treatment resulted in 50% of patients having a better-than-expected 90-day outcome, compared with 40% of controls, a 48% relative improvement in this measure that met the prespecified definition of statistical significance. The results also showed about a 50% relative improvement in each of three secondary outcomes in the CCI cohort: the percentage of patients with a mRS score of 0-2 after 90 days, the percentage with a mRS score of 0-3 after 90 days, and average stroke-related quality of life at 90 days.
Dr. Saver also reported results of a meta-analysis that combined the results he reported from 520 patients with CCI with results from 87 CCI patients enrolled in the preceding pilot study of this treatment strategy, ImpACT-1. The pilot findings were completely consistent and when combined with the current results strengthened the statistical significance of the primary and secondary endpoints.
“There is a compelling story” of efficacy based on the study results, the dose-response relationship, and the meta-analysis results, Dr. Saver said. “I think it’s a very strong case.”
He also reported “no safety concerns” raised in the new study, with no serious adverse effects seen in or experienced by the patients on active treatment.
“The data are compelling” for safety and efficacy, for this novel approach for treating acute ischemic stroke, commented Pooja Khatri, MD, professor of neurology and director of the acute stroke program at the University of Cincinnati.
The study was sponsored by BrainsGate, the company developing the tested device. Dr. Saver has been a consultant to BrainsGate. Dr. Khatri has been a consultant to Biogen, Greenwich, Lumosa, and PTC Therapeutics.
SOURCE: Saver J et al. Int J. Stroke. 2018 Oct;13(2S):28, Abstract 104.
MONTREAL – Stimulation of the sphenopalatine ganglion (SPG) using a small, implanted electrode for 5 days in patients who had just had an acute ischemic stroke led to statistically significant and clinically meaningful improvements in the subset of patients with confirmed cortical involvement in a pivotal, sham-controlled trial.
SPG stimulation started within 24 hours of an acute ischemic stroke “reduced poststroke disability over the entire outcome range and increased the proportion of patients who were alive and independent 3 months after their stroke” in the subgroup with a confirmed cortical infarction (CCI), Jeffrey L. Saver, MD, said at the World Stroke Congress. Five days of SPG stimulation, done once daily starting within 24 hours of stroke onset, “enhances ipsilateral collateral blood flow” and may also stabilize the blood brain barrier, explained Dr. Saver, professor of neurology and director of the stroke center at the University of California, Los Angeles. The study included a prespecified primary endpoint analysis that focused exclusively on the CCI subgroup, 52% of the total enrolled population.
If the reported data result in Food and Drug Administration marketing approval for the system, Dr. Saver said that he anticipated “substantial uptake” of the strategy, which he tested in patients who had not undergone thrombectomy or thrombolysis treatment. In current U.S. practice, there is “a large group of patients with a missed opportunity for recanalization” who would be candidates for treatment with SPG stimulation, a treatment that appeared to provide benefits beyond current standard care, he said in an interview.
Ongoing studies are also testing whether SPG stimulation can benefit acute ischemic stroke patients who have already undergone treatment with thrombectomy or thrombolysis, he added. The same SPG stimulation device is additionally undergoing U.S. testing as a treatment for headache and has regulatory approval in the European Union for treating headache and migraine.
The ImpACT-24B (Implant for Augmentation of Cerebral Blood Flow Trial, Effectiveness and Safety in a 24-Hour Window) trial involved 1,000 patients at 73 centers in 18 countries, including the United States. The investigators enrolled acute ischemic stroke patients 8-24 hours after stroke onset who had a National Institutes of Health Stroke Scale (NIHSS) score of 7-18.
Each patient received an implant of a short, thin metal electrode placed through the soft palate at the rear roof of the mouth, near the SPG. Neurologists primarily performed the implants in a procedure that had a “skin to skin” time of less than 5 minutes. Patients received either electrical stimulation or a sham stimulation through the electrode immediately after placement and then daily for the next 4 days. The investigators titrated the strength of the treatment stimulation in each patient to maximize its strength while maintaining patient comfort. Subsequent analysis of the results showed that the stronger the tolerated stimulation, the bigger the treatment effect in a clear dose-response pattern, Dr. Saver reported.
The study’s primary endpoint was improvement in the modified Rankin scale (mRS) score at 90 days after the index stroke when measured against historical expectations. By this measure, the overall study cohort showed a small, statistically insignificant improvement in actively treated patients, compared with sham-treated patients. However, in the prespecified, coprimary endpoint cohort of patients with a CCI, active treatment resulted in 50% of patients having a better-than-expected 90-day outcome, compared with 40% of controls, a 48% relative improvement in this measure that met the prespecified definition of statistical significance. The results also showed about a 50% relative improvement in each of three secondary outcomes in the CCI cohort: the percentage of patients with a mRS score of 0-2 after 90 days, the percentage with a mRS score of 0-3 after 90 days, and average stroke-related quality of life at 90 days.
Dr. Saver also reported results of a meta-analysis that combined the results he reported from 520 patients with CCI with results from 87 CCI patients enrolled in the preceding pilot study of this treatment strategy, ImpACT-1. The pilot findings were completely consistent and when combined with the current results strengthened the statistical significance of the primary and secondary endpoints.
“There is a compelling story” of efficacy based on the study results, the dose-response relationship, and the meta-analysis results, Dr. Saver said. “I think it’s a very strong case.”
He also reported “no safety concerns” raised in the new study, with no serious adverse effects seen in or experienced by the patients on active treatment.
“The data are compelling” for safety and efficacy, for this novel approach for treating acute ischemic stroke, commented Pooja Khatri, MD, professor of neurology and director of the acute stroke program at the University of Cincinnati.
The study was sponsored by BrainsGate, the company developing the tested device. Dr. Saver has been a consultant to BrainsGate. Dr. Khatri has been a consultant to Biogen, Greenwich, Lumosa, and PTC Therapeutics.
SOURCE: Saver J et al. Int J. Stroke. 2018 Oct;13(2S):28, Abstract 104.
MONTREAL – Stimulation of the sphenopalatine ganglion (SPG) using a small, implanted electrode for 5 days in patients who had just had an acute ischemic stroke led to statistically significant and clinically meaningful improvements in the subset of patients with confirmed cortical involvement in a pivotal, sham-controlled trial.
SPG stimulation started within 24 hours of an acute ischemic stroke “reduced poststroke disability over the entire outcome range and increased the proportion of patients who were alive and independent 3 months after their stroke” in the subgroup with a confirmed cortical infarction (CCI), Jeffrey L. Saver, MD, said at the World Stroke Congress. Five days of SPG stimulation, done once daily starting within 24 hours of stroke onset, “enhances ipsilateral collateral blood flow” and may also stabilize the blood brain barrier, explained Dr. Saver, professor of neurology and director of the stroke center at the University of California, Los Angeles. The study included a prespecified primary endpoint analysis that focused exclusively on the CCI subgroup, 52% of the total enrolled population.
If the reported data result in Food and Drug Administration marketing approval for the system, Dr. Saver said that he anticipated “substantial uptake” of the strategy, which he tested in patients who had not undergone thrombectomy or thrombolysis treatment. In current U.S. practice, there is “a large group of patients with a missed opportunity for recanalization” who would be candidates for treatment with SPG stimulation, a treatment that appeared to provide benefits beyond current standard care, he said in an interview.
Ongoing studies are also testing whether SPG stimulation can benefit acute ischemic stroke patients who have already undergone treatment with thrombectomy or thrombolysis, he added. The same SPG stimulation device is additionally undergoing U.S. testing as a treatment for headache and has regulatory approval in the European Union for treating headache and migraine.
The ImpACT-24B (Implant for Augmentation of Cerebral Blood Flow Trial, Effectiveness and Safety in a 24-Hour Window) trial involved 1,000 patients at 73 centers in 18 countries, including the United States. The investigators enrolled acute ischemic stroke patients 8-24 hours after stroke onset who had a National Institutes of Health Stroke Scale (NIHSS) score of 7-18.
Each patient received an implant of a short, thin metal electrode placed through the soft palate at the rear roof of the mouth, near the SPG. Neurologists primarily performed the implants in a procedure that had a “skin to skin” time of less than 5 minutes. Patients received either electrical stimulation or a sham stimulation through the electrode immediately after placement and then daily for the next 4 days. The investigators titrated the strength of the treatment stimulation in each patient to maximize its strength while maintaining patient comfort. Subsequent analysis of the results showed that the stronger the tolerated stimulation, the bigger the treatment effect in a clear dose-response pattern, Dr. Saver reported.
The study’s primary endpoint was improvement in the modified Rankin scale (mRS) score at 90 days after the index stroke when measured against historical expectations. By this measure, the overall study cohort showed a small, statistically insignificant improvement in actively treated patients, compared with sham-treated patients. However, in the prespecified, coprimary endpoint cohort of patients with a CCI, active treatment resulted in 50% of patients having a better-than-expected 90-day outcome, compared with 40% of controls, a 48% relative improvement in this measure that met the prespecified definition of statistical significance. The results also showed about a 50% relative improvement in each of three secondary outcomes in the CCI cohort: the percentage of patients with a mRS score of 0-2 after 90 days, the percentage with a mRS score of 0-3 after 90 days, and average stroke-related quality of life at 90 days.
Dr. Saver also reported results of a meta-analysis that combined the results he reported from 520 patients with CCI with results from 87 CCI patients enrolled in the preceding pilot study of this treatment strategy, ImpACT-1. The pilot findings were completely consistent and when combined with the current results strengthened the statistical significance of the primary and secondary endpoints.
“There is a compelling story” of efficacy based on the study results, the dose-response relationship, and the meta-analysis results, Dr. Saver said. “I think it’s a very strong case.”
He also reported “no safety concerns” raised in the new study, with no serious adverse effects seen in or experienced by the patients on active treatment.
“The data are compelling” for safety and efficacy, for this novel approach for treating acute ischemic stroke, commented Pooja Khatri, MD, professor of neurology and director of the acute stroke program at the University of Cincinnati.
The study was sponsored by BrainsGate, the company developing the tested device. Dr. Saver has been a consultant to BrainsGate. Dr. Khatri has been a consultant to Biogen, Greenwich, Lumosa, and PTC Therapeutics.
SOURCE: Saver J et al. Int J. Stroke. 2018 Oct;13(2S):28, Abstract 104.
REPORTING FROM THE WORLD STROKE CONGRESS
Key clinical point: Sphenopalatine ganglion stimulation of acute ischemic stroke patients boosted cerebral blood flow and improved 90-day outcomes in patients with confirmed cortical infarctions.
Major finding: For confirmed cortical infarctions ganglion stimulation led to a 48% higher rate of better-than-expected outcomes, compared with controls.
Study details: ImpACT-24B, a multicenter pivotal trial with 1,000 acute ischemic stroke patients.
Disclosures: The study was sponsored by BrainsGate, the company developing the tested device. Dr. Saver has been a consultant to BrainsGate. Dr. Khatri has been a consultant to Biogen, Greenwich, Lumosa, and PTC Therapeutics.
Source: Saver J et al. Int J. Stroke. 2018 Oct;13(2S):28, Abstract 104.
Endoscopic vein-graft harvest equals open harvest at 3 years
CHICAGO – Patients who underwent coronary artery bypass grafting using saphenous veins harvested endoscopically had similar clinical outcomes after nearly 3 years of follow-up as those seen with patients who received vein grafts taken by open harvesting in a multicenter, randomized trial in the United States with 1,150 patients.
As expected, follow-up also showed that endoscopic vein-graft harvesting (EVH) resulted in about half the number of wound infections as did open vein-graft harvesting (OVH). This combination of similar clinical outcomes after a median 2.8 years of follow-up, as well as fewer leg-wound adverse events, makes EVH “the preferred vein-harvesting modality,” Marco A. Zenati, MD, said at the American Heart Association scientific sessions.
Although patients far and away prefer EVH because of the reduced pain and faster healing, questions about its clinical efficacy when compared with that of OVH have lingered. That’s because observational data published almost a decade ago taken from the PREVENT IV (Project of Ex-Vivo Vein Graft Engineering via Transfection IV) trial suggested that patients who underwent coronary artery bypass grafting (CABG) using vein grafts collected by EVH had more vein-graft failures after 12-18 months and a higher rate of death, MI, or need for revascularization after 3 years, compared with patients treated using OVH (N Engl J Med. 2009 July 16;361[3]:235-44).
The results from the prospective, randomized trial reported by Dr. Zenati “take the cloud away from endovascular vein-graft harvesting that PREVENT IV had made,” commented Timothy J. Gardner, MD, a cardiac surgeon who chaired the session.
“I think this answers the question,” commented Marc Ruel, MD, a professor of surgery and the chief of cardiac surgery at the University of Ottawa. “The results show that endoscopic harvesting of vein grafts is as good as open harvesting for preventing major adverse cardiac events, which is the goal of CABG. This is a definitive trial, with no trend toward more events with endoscopic harvested vein grafts,” said Dr. Ruel, the designated discussant for Dr. Zenati’s report.
However, the study did have some significant limitations, Dr. Ruel added. The new, randomized trial, run at 16 U.S. VA cardiac surgery centers, exclusively used surgeons who were experts in endovascular vein harvesting, which could have meant that they and their surgical teams were not as expert in open vein harvesting, he said. Also, in the broader context of CABG and conduit selection, new evidence suggests the superiority of pedicled vein grafts (Ann Thoracic Surg. 2017 Oct;104[4]1313-17), and “we could also do better by using the radial artery” rather than a saphenous vein graft, Dr. Ruel said. He cited a meta-analysis published in 2018 that showed the superiority of CABG when it combined an internal thoracic artery graft with a radial artery graft rather than with a vein graft (N Engl J Med. 2018 May 31;378[22]:2069-77).
“The operation of the future is not necessarily what you saw” in Dr. Zenati’s study, Dr. Ruel cautioned.
The results Dr. Zenati reported came from the REGROUP (Randomized End-Vein Graft Prospective) trial, which enrolled patients who underwent CABG during 2014-2017. All patients received an internal thoracic artery graft and were randomized to receive additional saphenous vein grafts with the conduits collected either by the EVH or OVH method. The study’s primary endpoint of all-cause death, nonfatal MI, or need for repeat revascularization after a median follow-up of 2.8 years occurred in 14% of the patients who received vein grafts with EVH and in 16% of the patients who received grafts with OVH, a difference that was not statistically significant, reported Dr. Zenati, a professor of surgery at Harvard Medical School in Boston and the chief of cardiothoracic surgery for the VA Boston Health System. The incidence of wound infection was 3.1% in the OVH patients and 1.4% in the EVH patients, a difference that came close to but did not reach statistical significance. Concurrently with Dr. Zenati’s report, an article with the results appeared online (N Engl J Med. 2018 Nov 11. doi: 0.1056/NEJMoa1812390).
The REGROUP trial did not collect data on vein-graft patency following CABG. The investigators were concerned about having enough patients return for follow-up angiography to produce a meaningful result for this endpoint, and they believed that the clinical endpoint they used sufficed for demonstrating equivalence of the two harvesting methods, Dr. Zenati said during his talk.
“The more arterial conduit used in CABG, the better the durability of the grafts, but often surgeons use vein grafts because there is not enough arterial conduit,” commented Donald M. Lloyd-Jones, MD, professor and chair of preventive medicine at Northwestern University in Chicago.* “The recovery from endoscopic vein-graft harvesting is very different from open harvesting. Endoscopic harvesting produces much less pain and infection, and recovery is much easier for patients, so it’s reassuring to see that the quality of the vein is not affected by endoscopic harvesting when done by experts,” he said.
Dr. Zenati, Dr. Gardner, Dr. Ruel, and Dr. Lloyd-Jones had no disclosures.
SOURCE: Zenati M et al. AHA 2018, Abstract 19055.
*Correction, 11/12/18: An earlier version of this article misstated the name of Dr. Donald M. Lloyd-Jones.
This article was updated 11/14/18.
The results from the REGROUP trial are interesting and open the field for additional comparisons of endoscopic and open saphenous vein-graft harvesting, but this trial is not the definitive answer regarding whether these two harvesting approaches produce similar results. Greater reassurance of equivalence would come from studies that included more patients and a more diverse patient population; REGROUP largely enrolled male veterans and patients with multiple comorbidities. Longer follow-up is also needed. A median follow-up of 3 years is too brief for complete reassurance that long-term patency is the same with both approaches. It would also help to have follow-up data on graft patency. Many factors besides patency can lead to differences in clinical outcomes following coronary bypass surgery.
Endoscopic vein harvesting is preferred by patients, and it results in fewer wound infections, as was confirmed in REGROUP. Because of these advantages for endoscopic harvesting, it would be great if we could definitively document that these vein grafts functioned as well as those taken with open harvesting.
Evidence now suggests that the more arterial conduits used during coronary bypass, the better. If I were having triple-vessel bypass surgery, I’d want to get two thoracic-artery bypass grafts and a radial artery graft. But studies like REGROUP are important because a majority of heart surgeons use vein grafts for several reasons including convenience. Surgeons will likely continue to use vein grafts for the foreseeable future, so we need to know whether endoscopic harvesting is an acceptable approach.
Jennifer S. Lawton, MD , is a professor of surgery and chief of cardiac surgery at Johns Hopkins Medicine in Baltimore. She had no disclosures. She made these comments in an interview.
The results from the REGROUP trial are interesting and open the field for additional comparisons of endoscopic and open saphenous vein-graft harvesting, but this trial is not the definitive answer regarding whether these two harvesting approaches produce similar results. Greater reassurance of equivalence would come from studies that included more patients and a more diverse patient population; REGROUP largely enrolled male veterans and patients with multiple comorbidities. Longer follow-up is also needed. A median follow-up of 3 years is too brief for complete reassurance that long-term patency is the same with both approaches. It would also help to have follow-up data on graft patency. Many factors besides patency can lead to differences in clinical outcomes following coronary bypass surgery.
Endoscopic vein harvesting is preferred by patients, and it results in fewer wound infections, as was confirmed in REGROUP. Because of these advantages for endoscopic harvesting, it would be great if we could definitively document that these vein grafts functioned as well as those taken with open harvesting.
Evidence now suggests that the more arterial conduits used during coronary bypass, the better. If I were having triple-vessel bypass surgery, I’d want to get two thoracic-artery bypass grafts and a radial artery graft. But studies like REGROUP are important because a majority of heart surgeons use vein grafts for several reasons including convenience. Surgeons will likely continue to use vein grafts for the foreseeable future, so we need to know whether endoscopic harvesting is an acceptable approach.
Jennifer S. Lawton, MD , is a professor of surgery and chief of cardiac surgery at Johns Hopkins Medicine in Baltimore. She had no disclosures. She made these comments in an interview.
The results from the REGROUP trial are interesting and open the field for additional comparisons of endoscopic and open saphenous vein-graft harvesting, but this trial is not the definitive answer regarding whether these two harvesting approaches produce similar results. Greater reassurance of equivalence would come from studies that included more patients and a more diverse patient population; REGROUP largely enrolled male veterans and patients with multiple comorbidities. Longer follow-up is also needed. A median follow-up of 3 years is too brief for complete reassurance that long-term patency is the same with both approaches. It would also help to have follow-up data on graft patency. Many factors besides patency can lead to differences in clinical outcomes following coronary bypass surgery.
Endoscopic vein harvesting is preferred by patients, and it results in fewer wound infections, as was confirmed in REGROUP. Because of these advantages for endoscopic harvesting, it would be great if we could definitively document that these vein grafts functioned as well as those taken with open harvesting.
Evidence now suggests that the more arterial conduits used during coronary bypass, the better. If I were having triple-vessel bypass surgery, I’d want to get two thoracic-artery bypass grafts and a radial artery graft. But studies like REGROUP are important because a majority of heart surgeons use vein grafts for several reasons including convenience. Surgeons will likely continue to use vein grafts for the foreseeable future, so we need to know whether endoscopic harvesting is an acceptable approach.
Jennifer S. Lawton, MD , is a professor of surgery and chief of cardiac surgery at Johns Hopkins Medicine in Baltimore. She had no disclosures. She made these comments in an interview.
CHICAGO – Patients who underwent coronary artery bypass grafting using saphenous veins harvested endoscopically had similar clinical outcomes after nearly 3 years of follow-up as those seen with patients who received vein grafts taken by open harvesting in a multicenter, randomized trial in the United States with 1,150 patients.
As expected, follow-up also showed that endoscopic vein-graft harvesting (EVH) resulted in about half the number of wound infections as did open vein-graft harvesting (OVH). This combination of similar clinical outcomes after a median 2.8 years of follow-up, as well as fewer leg-wound adverse events, makes EVH “the preferred vein-harvesting modality,” Marco A. Zenati, MD, said at the American Heart Association scientific sessions.
Although patients far and away prefer EVH because of the reduced pain and faster healing, questions about its clinical efficacy when compared with that of OVH have lingered. That’s because observational data published almost a decade ago taken from the PREVENT IV (Project of Ex-Vivo Vein Graft Engineering via Transfection IV) trial suggested that patients who underwent coronary artery bypass grafting (CABG) using vein grafts collected by EVH had more vein-graft failures after 12-18 months and a higher rate of death, MI, or need for revascularization after 3 years, compared with patients treated using OVH (N Engl J Med. 2009 July 16;361[3]:235-44).
The results from the prospective, randomized trial reported by Dr. Zenati “take the cloud away from endovascular vein-graft harvesting that PREVENT IV had made,” commented Timothy J. Gardner, MD, a cardiac surgeon who chaired the session.
“I think this answers the question,” commented Marc Ruel, MD, a professor of surgery and the chief of cardiac surgery at the University of Ottawa. “The results show that endoscopic harvesting of vein grafts is as good as open harvesting for preventing major adverse cardiac events, which is the goal of CABG. This is a definitive trial, with no trend toward more events with endoscopic harvested vein grafts,” said Dr. Ruel, the designated discussant for Dr. Zenati’s report.
However, the study did have some significant limitations, Dr. Ruel added. The new, randomized trial, run at 16 U.S. VA cardiac surgery centers, exclusively used surgeons who were experts in endovascular vein harvesting, which could have meant that they and their surgical teams were not as expert in open vein harvesting, he said. Also, in the broader context of CABG and conduit selection, new evidence suggests the superiority of pedicled vein grafts (Ann Thoracic Surg. 2017 Oct;104[4]1313-17), and “we could also do better by using the radial artery” rather than a saphenous vein graft, Dr. Ruel said. He cited a meta-analysis published in 2018 that showed the superiority of CABG when it combined an internal thoracic artery graft with a radial artery graft rather than with a vein graft (N Engl J Med. 2018 May 31;378[22]:2069-77).
“The operation of the future is not necessarily what you saw” in Dr. Zenati’s study, Dr. Ruel cautioned.
The results Dr. Zenati reported came from the REGROUP (Randomized End-Vein Graft Prospective) trial, which enrolled patients who underwent CABG during 2014-2017. All patients received an internal thoracic artery graft and were randomized to receive additional saphenous vein grafts with the conduits collected either by the EVH or OVH method. The study’s primary endpoint of all-cause death, nonfatal MI, or need for repeat revascularization after a median follow-up of 2.8 years occurred in 14% of the patients who received vein grafts with EVH and in 16% of the patients who received grafts with OVH, a difference that was not statistically significant, reported Dr. Zenati, a professor of surgery at Harvard Medical School in Boston and the chief of cardiothoracic surgery for the VA Boston Health System. The incidence of wound infection was 3.1% in the OVH patients and 1.4% in the EVH patients, a difference that came close to but did not reach statistical significance. Concurrently with Dr. Zenati’s report, an article with the results appeared online (N Engl J Med. 2018 Nov 11. doi: 0.1056/NEJMoa1812390).
The REGROUP trial did not collect data on vein-graft patency following CABG. The investigators were concerned about having enough patients return for follow-up angiography to produce a meaningful result for this endpoint, and they believed that the clinical endpoint they used sufficed for demonstrating equivalence of the two harvesting methods, Dr. Zenati said during his talk.
“The more arterial conduit used in CABG, the better the durability of the grafts, but often surgeons use vein grafts because there is not enough arterial conduit,” commented Donald M. Lloyd-Jones, MD, professor and chair of preventive medicine at Northwestern University in Chicago.* “The recovery from endoscopic vein-graft harvesting is very different from open harvesting. Endoscopic harvesting produces much less pain and infection, and recovery is much easier for patients, so it’s reassuring to see that the quality of the vein is not affected by endoscopic harvesting when done by experts,” he said.
Dr. Zenati, Dr. Gardner, Dr. Ruel, and Dr. Lloyd-Jones had no disclosures.
SOURCE: Zenati M et al. AHA 2018, Abstract 19055.
*Correction, 11/12/18: An earlier version of this article misstated the name of Dr. Donald M. Lloyd-Jones.
This article was updated 11/14/18.
CHICAGO – Patients who underwent coronary artery bypass grafting using saphenous veins harvested endoscopically had similar clinical outcomes after nearly 3 years of follow-up as those seen with patients who received vein grafts taken by open harvesting in a multicenter, randomized trial in the United States with 1,150 patients.
As expected, follow-up also showed that endoscopic vein-graft harvesting (EVH) resulted in about half the number of wound infections as did open vein-graft harvesting (OVH). This combination of similar clinical outcomes after a median 2.8 years of follow-up, as well as fewer leg-wound adverse events, makes EVH “the preferred vein-harvesting modality,” Marco A. Zenati, MD, said at the American Heart Association scientific sessions.
Although patients far and away prefer EVH because of the reduced pain and faster healing, questions about its clinical efficacy when compared with that of OVH have lingered. That’s because observational data published almost a decade ago taken from the PREVENT IV (Project of Ex-Vivo Vein Graft Engineering via Transfection IV) trial suggested that patients who underwent coronary artery bypass grafting (CABG) using vein grafts collected by EVH had more vein-graft failures after 12-18 months and a higher rate of death, MI, or need for revascularization after 3 years, compared with patients treated using OVH (N Engl J Med. 2009 July 16;361[3]:235-44).
The results from the prospective, randomized trial reported by Dr. Zenati “take the cloud away from endovascular vein-graft harvesting that PREVENT IV had made,” commented Timothy J. Gardner, MD, a cardiac surgeon who chaired the session.
“I think this answers the question,” commented Marc Ruel, MD, a professor of surgery and the chief of cardiac surgery at the University of Ottawa. “The results show that endoscopic harvesting of vein grafts is as good as open harvesting for preventing major adverse cardiac events, which is the goal of CABG. This is a definitive trial, with no trend toward more events with endoscopic harvested vein grafts,” said Dr. Ruel, the designated discussant for Dr. Zenati’s report.
However, the study did have some significant limitations, Dr. Ruel added. The new, randomized trial, run at 16 U.S. VA cardiac surgery centers, exclusively used surgeons who were experts in endovascular vein harvesting, which could have meant that they and their surgical teams were not as expert in open vein harvesting, he said. Also, in the broader context of CABG and conduit selection, new evidence suggests the superiority of pedicled vein grafts (Ann Thoracic Surg. 2017 Oct;104[4]1313-17), and “we could also do better by using the radial artery” rather than a saphenous vein graft, Dr. Ruel said. He cited a meta-analysis published in 2018 that showed the superiority of CABG when it combined an internal thoracic artery graft with a radial artery graft rather than with a vein graft (N Engl J Med. 2018 May 31;378[22]:2069-77).
“The operation of the future is not necessarily what you saw” in Dr. Zenati’s study, Dr. Ruel cautioned.
The results Dr. Zenati reported came from the REGROUP (Randomized End-Vein Graft Prospective) trial, which enrolled patients who underwent CABG during 2014-2017. All patients received an internal thoracic artery graft and were randomized to receive additional saphenous vein grafts with the conduits collected either by the EVH or OVH method. The study’s primary endpoint of all-cause death, nonfatal MI, or need for repeat revascularization after a median follow-up of 2.8 years occurred in 14% of the patients who received vein grafts with EVH and in 16% of the patients who received grafts with OVH, a difference that was not statistically significant, reported Dr. Zenati, a professor of surgery at Harvard Medical School in Boston and the chief of cardiothoracic surgery for the VA Boston Health System. The incidence of wound infection was 3.1% in the OVH patients and 1.4% in the EVH patients, a difference that came close to but did not reach statistical significance. Concurrently with Dr. Zenati’s report, an article with the results appeared online (N Engl J Med. 2018 Nov 11. doi: 0.1056/NEJMoa1812390).
The REGROUP trial did not collect data on vein-graft patency following CABG. The investigators were concerned about having enough patients return for follow-up angiography to produce a meaningful result for this endpoint, and they believed that the clinical endpoint they used sufficed for demonstrating equivalence of the two harvesting methods, Dr. Zenati said during his talk.
“The more arterial conduit used in CABG, the better the durability of the grafts, but often surgeons use vein grafts because there is not enough arterial conduit,” commented Donald M. Lloyd-Jones, MD, professor and chair of preventive medicine at Northwestern University in Chicago.* “The recovery from endoscopic vein-graft harvesting is very different from open harvesting. Endoscopic harvesting produces much less pain and infection, and recovery is much easier for patients, so it’s reassuring to see that the quality of the vein is not affected by endoscopic harvesting when done by experts,” he said.
Dr. Zenati, Dr. Gardner, Dr. Ruel, and Dr. Lloyd-Jones had no disclosures.
SOURCE: Zenati M et al. AHA 2018, Abstract 19055.
*Correction, 11/12/18: An earlier version of this article misstated the name of Dr. Donald M. Lloyd-Jones.
This article was updated 11/14/18.
REPORTING FROM THE AHA SCIENTIFIC SESSION
Key clinical point:
Major finding: Clinical events occurred in 16% of open-harvest vein-graft patients and in 14% who received endoscopically harvested veins.
Study details: REGROUP, a multicenter, randomized trial with 1,150 patients.
Disclosures: Dr. Zenati, Dr. Gardner, Dr. Ruel, and Dr. Lloyd-Jones had no disclosures.
Source: Zenati M et al. AHA 2018, Abstract 19055.
Revised U.S. cholesterol guidelines promote personalized risk assessment
CHICAGO – The latest cholesterol management guideline for U.S. practice has a core treatment principal that propels the field from its long-held focus on “know your cholesterol number,” that then became “know your risk” with the 2013 guideline, to what is now “personalize your risk.”
“The new guideline put special focus not just on risk, but on risk assessment that uses ‘enhancing factors’ to help patients understand their risk in a personal way and decide whether statin treatment is right for them” Neil J. Stone, MD, said at the American Heart Association scientific sessions.
Other novel features of the 2018 edition of the cholesterol management guideline included: specification of the role for two types of drugs other than statins – ezetimibe and PCSK9 inhibitors (including mention of the cost-value consideration when prescribing an expensive PCSK9 inhibitor); inclusion of coronary artery calcium (CAC) score assessment for patients with intermediate risk who are unsure whether statin treatment is right for them; and acknowledgment that nonfasting measurement of blood cholesterol levels is fine for most screening circumstances.
“Nonfasting is okay for many situations,” said Dr. Stone, a professor of medicine at Northwestern University here, and vice-chair of the writing panel for the guidelines, released by the American College of Cardiology, the American Heart Association, and 10 additional endorsing societies (J Am Coll Cardiol. 2018;doi:10.1016/j.jacc.2018.11.003)
But among the changes in the 2018 guideline that distinguish it from the preceding, 2013 version (Circulation. 2014 June 24;129[25, suppl 2]:S1-S45), the expanded approach to risk assessment in the primary-prevention setting stood out as the biggest shift.
“In 2013 we said calculate a person’s risk” for atherosclerotic cardiovascular disease. “Now that is much more fleshed out,” said Donald M. Lloyd-Jones, MD, a member of the guideline-writing group who helped develop the risk assessment tools used by the guideline.*
“The risk equations now are the same as we introduced in 2013,” he noted, and research done by Dr. Lloyd-Jones and others since that introduction showed that the “pooled cohort equations” are “well calibrated” for estimating a person’s 10-year risk for a cardiovascular event, especially at a risk level around 7.5%, which serves as the threshold for identifying a person with enough risk to warrant statin treatment. “But there are subgroups where the risk calculator clearly over- or under-estimates risk,” and that’s why the new guideline introduced the concept of risk enhancers--additional features not included in the basic risk calculation that enhance risk: family history; metabolic syndrome; chronic kidney disease; chronic inflammatory diseases such as psoriasis, rheumatoid arthritis, or HIV infection; a history of premature menopause or preeclampsia, certain ethnicity, or high levels of Lp(a) or apolipoprotein B.
“We didn’t need new risk scores; we needed to understand how to use the scores better, and the new guideline goes a long way toward helping clinicians do that,” Dr. Lloyd-Jones said in an interview.
Another aspect of this new, more nuanced approach to individualized risk assessment is the introduction of the CAC score as a possible tie breaker when a person who is otherwise a candidate for statin treatment for primary prevention is unsure about committing to possibly decades of daily statin treatment.
The guideline does not endorse obtaining a person’s CAC score for everyone as screening, stressed Dr. Stone, but this score, obtained by noncontrast CT with a radiation dose of about 1 mSv – comparable to a mammography exam, received a IIa rating” – is reasonable” for helping patients decide. Dr. Stone and others cited the importance of a CAC score of zero for patients on the fence for statin treatment as a strong indicator for many people that they can safely defer treatment.
As a result of this new endorsement for selectively obtaining CAC scores, “I think the number of tests will increase, probably fairly substantially,” said Dr. Lloyd-Jones, professor and chair of preventive medicine at Northwestern University here. He also expressed hope that this acknowledgment of an evidenced-based role for selected CAC score imaging may prompt health insurers to start coving this expense, something they don’t now do. Patients generally pay out-of-pocket from $50 to $300 for CAC score imaging. “I hope they will start paying for this,” Dr. Lloyd-Jones said.
The guidelines also deal, at least in passing, with another financial issue that has loomed large for cholesterol treatment, the role of the notoriously expensive PCSK9 inhibitors, alirocumab (Praluent) and evolocumab (Repatha). For secondary prevention patients or for patients with familial hypercholesterolemia who do not reach their LDL cholesterol goal on statin treatment alone, the guideline recommended treatment first with generic ezetimibe. If the goal remains elusive, the next step is prescribing a PCSK9 inhibitor. The guideline also noted the poor cost-benefit ratio for the PCSK9 inhibitors at the U.S. list prices that existed in mid-2018, about $14,000 a year.
The guideline writers noted that this is one of the first times that cost considerations found their way into cardiology guidelines. Clinicians “need to be attuned to prescribing PCSK9 inhibitors only in those settings when it provides good value to patients,” explained Mark A. Hlatky, MD, a professor of medicine, cardiologist, and health policy specialist at Stanford (Calif.) University. The guideline “focuses on patient selection for PCSK9 inhibitors, limiting it to patients who get the most benefit,” said Dr. Hlatky, another member of the writing panel.
Although 10 medical groups joined the American College of Cardiology and American Heart Association in endorsing the guideline, conspicuously absent were the two largest U.S. societies representing primary care physicians, the American College of Physicians and American Academy of Family Practitioners. The guideline’s organizers invited both these societies to participate in the process and they declined, said Sidney C. Smith, Jr., MD, a member of the guideline committee.
Dr. Stone and Dr. Lloyd-Jones had no financial disclosures. The writing committee members’ disclosures can be found at jaccjacc.acc.org/Clinical_Document/Cholesterol_GL_Au_Comp_RWI.pdf.
*Correction, 11/13/18: An earlier version of this article misstated the name of Dr. Donald M. Lloyd-Jones.
SOURCE: AHA 2018 and Grundy S et al. J Am Coll Cardiol. 2018;doi:10.1016/j.jacc.2018.11.003.
CHICAGO – The latest cholesterol management guideline for U.S. practice has a core treatment principal that propels the field from its long-held focus on “know your cholesterol number,” that then became “know your risk” with the 2013 guideline, to what is now “personalize your risk.”
“The new guideline put special focus not just on risk, but on risk assessment that uses ‘enhancing factors’ to help patients understand their risk in a personal way and decide whether statin treatment is right for them” Neil J. Stone, MD, said at the American Heart Association scientific sessions.
Other novel features of the 2018 edition of the cholesterol management guideline included: specification of the role for two types of drugs other than statins – ezetimibe and PCSK9 inhibitors (including mention of the cost-value consideration when prescribing an expensive PCSK9 inhibitor); inclusion of coronary artery calcium (CAC) score assessment for patients with intermediate risk who are unsure whether statin treatment is right for them; and acknowledgment that nonfasting measurement of blood cholesterol levels is fine for most screening circumstances.
“Nonfasting is okay for many situations,” said Dr. Stone, a professor of medicine at Northwestern University here, and vice-chair of the writing panel for the guidelines, released by the American College of Cardiology, the American Heart Association, and 10 additional endorsing societies (J Am Coll Cardiol. 2018;doi:10.1016/j.jacc.2018.11.003)
But among the changes in the 2018 guideline that distinguish it from the preceding, 2013 version (Circulation. 2014 June 24;129[25, suppl 2]:S1-S45), the expanded approach to risk assessment in the primary-prevention setting stood out as the biggest shift.
“In 2013 we said calculate a person’s risk” for atherosclerotic cardiovascular disease. “Now that is much more fleshed out,” said Donald M. Lloyd-Jones, MD, a member of the guideline-writing group who helped develop the risk assessment tools used by the guideline.*
“The risk equations now are the same as we introduced in 2013,” he noted, and research done by Dr. Lloyd-Jones and others since that introduction showed that the “pooled cohort equations” are “well calibrated” for estimating a person’s 10-year risk for a cardiovascular event, especially at a risk level around 7.5%, which serves as the threshold for identifying a person with enough risk to warrant statin treatment. “But there are subgroups where the risk calculator clearly over- or under-estimates risk,” and that’s why the new guideline introduced the concept of risk enhancers--additional features not included in the basic risk calculation that enhance risk: family history; metabolic syndrome; chronic kidney disease; chronic inflammatory diseases such as psoriasis, rheumatoid arthritis, or HIV infection; a history of premature menopause or preeclampsia, certain ethnicity, or high levels of Lp(a) or apolipoprotein B.
“We didn’t need new risk scores; we needed to understand how to use the scores better, and the new guideline goes a long way toward helping clinicians do that,” Dr. Lloyd-Jones said in an interview.
Another aspect of this new, more nuanced approach to individualized risk assessment is the introduction of the CAC score as a possible tie breaker when a person who is otherwise a candidate for statin treatment for primary prevention is unsure about committing to possibly decades of daily statin treatment.
The guideline does not endorse obtaining a person’s CAC score for everyone as screening, stressed Dr. Stone, but this score, obtained by noncontrast CT with a radiation dose of about 1 mSv – comparable to a mammography exam, received a IIa rating” – is reasonable” for helping patients decide. Dr. Stone and others cited the importance of a CAC score of zero for patients on the fence for statin treatment as a strong indicator for many people that they can safely defer treatment.
As a result of this new endorsement for selectively obtaining CAC scores, “I think the number of tests will increase, probably fairly substantially,” said Dr. Lloyd-Jones, professor and chair of preventive medicine at Northwestern University here. He also expressed hope that this acknowledgment of an evidenced-based role for selected CAC score imaging may prompt health insurers to start coving this expense, something they don’t now do. Patients generally pay out-of-pocket from $50 to $300 for CAC score imaging. “I hope they will start paying for this,” Dr. Lloyd-Jones said.
The guidelines also deal, at least in passing, with another financial issue that has loomed large for cholesterol treatment, the role of the notoriously expensive PCSK9 inhibitors, alirocumab (Praluent) and evolocumab (Repatha). For secondary prevention patients or for patients with familial hypercholesterolemia who do not reach their LDL cholesterol goal on statin treatment alone, the guideline recommended treatment first with generic ezetimibe. If the goal remains elusive, the next step is prescribing a PCSK9 inhibitor. The guideline also noted the poor cost-benefit ratio for the PCSK9 inhibitors at the U.S. list prices that existed in mid-2018, about $14,000 a year.
The guideline writers noted that this is one of the first times that cost considerations found their way into cardiology guidelines. Clinicians “need to be attuned to prescribing PCSK9 inhibitors only in those settings when it provides good value to patients,” explained Mark A. Hlatky, MD, a professor of medicine, cardiologist, and health policy specialist at Stanford (Calif.) University. The guideline “focuses on patient selection for PCSK9 inhibitors, limiting it to patients who get the most benefit,” said Dr. Hlatky, another member of the writing panel.
Although 10 medical groups joined the American College of Cardiology and American Heart Association in endorsing the guideline, conspicuously absent were the two largest U.S. societies representing primary care physicians, the American College of Physicians and American Academy of Family Practitioners. The guideline’s organizers invited both these societies to participate in the process and they declined, said Sidney C. Smith, Jr., MD, a member of the guideline committee.
Dr. Stone and Dr. Lloyd-Jones had no financial disclosures. The writing committee members’ disclosures can be found at jaccjacc.acc.org/Clinical_Document/Cholesterol_GL_Au_Comp_RWI.pdf.
*Correction, 11/13/18: An earlier version of this article misstated the name of Dr. Donald M. Lloyd-Jones.
SOURCE: AHA 2018 and Grundy S et al. J Am Coll Cardiol. 2018;doi:10.1016/j.jacc.2018.11.003.
CHICAGO – The latest cholesterol management guideline for U.S. practice has a core treatment principal that propels the field from its long-held focus on “know your cholesterol number,” that then became “know your risk” with the 2013 guideline, to what is now “personalize your risk.”
“The new guideline put special focus not just on risk, but on risk assessment that uses ‘enhancing factors’ to help patients understand their risk in a personal way and decide whether statin treatment is right for them” Neil J. Stone, MD, said at the American Heart Association scientific sessions.
Other novel features of the 2018 edition of the cholesterol management guideline included: specification of the role for two types of drugs other than statins – ezetimibe and PCSK9 inhibitors (including mention of the cost-value consideration when prescribing an expensive PCSK9 inhibitor); inclusion of coronary artery calcium (CAC) score assessment for patients with intermediate risk who are unsure whether statin treatment is right for them; and acknowledgment that nonfasting measurement of blood cholesterol levels is fine for most screening circumstances.
“Nonfasting is okay for many situations,” said Dr. Stone, a professor of medicine at Northwestern University here, and vice-chair of the writing panel for the guidelines, released by the American College of Cardiology, the American Heart Association, and 10 additional endorsing societies (J Am Coll Cardiol. 2018;doi:10.1016/j.jacc.2018.11.003)
But among the changes in the 2018 guideline that distinguish it from the preceding, 2013 version (Circulation. 2014 June 24;129[25, suppl 2]:S1-S45), the expanded approach to risk assessment in the primary-prevention setting stood out as the biggest shift.
“In 2013 we said calculate a person’s risk” for atherosclerotic cardiovascular disease. “Now that is much more fleshed out,” said Donald M. Lloyd-Jones, MD, a member of the guideline-writing group who helped develop the risk assessment tools used by the guideline.*
“The risk equations now are the same as we introduced in 2013,” he noted, and research done by Dr. Lloyd-Jones and others since that introduction showed that the “pooled cohort equations” are “well calibrated” for estimating a person’s 10-year risk for a cardiovascular event, especially at a risk level around 7.5%, which serves as the threshold for identifying a person with enough risk to warrant statin treatment. “But there are subgroups where the risk calculator clearly over- or under-estimates risk,” and that’s why the new guideline introduced the concept of risk enhancers--additional features not included in the basic risk calculation that enhance risk: family history; metabolic syndrome; chronic kidney disease; chronic inflammatory diseases such as psoriasis, rheumatoid arthritis, or HIV infection; a history of premature menopause or preeclampsia, certain ethnicity, or high levels of Lp(a) or apolipoprotein B.
“We didn’t need new risk scores; we needed to understand how to use the scores better, and the new guideline goes a long way toward helping clinicians do that,” Dr. Lloyd-Jones said in an interview.
Another aspect of this new, more nuanced approach to individualized risk assessment is the introduction of the CAC score as a possible tie breaker when a person who is otherwise a candidate for statin treatment for primary prevention is unsure about committing to possibly decades of daily statin treatment.
The guideline does not endorse obtaining a person’s CAC score for everyone as screening, stressed Dr. Stone, but this score, obtained by noncontrast CT with a radiation dose of about 1 mSv – comparable to a mammography exam, received a IIa rating” – is reasonable” for helping patients decide. Dr. Stone and others cited the importance of a CAC score of zero for patients on the fence for statin treatment as a strong indicator for many people that they can safely defer treatment.
As a result of this new endorsement for selectively obtaining CAC scores, “I think the number of tests will increase, probably fairly substantially,” said Dr. Lloyd-Jones, professor and chair of preventive medicine at Northwestern University here. He also expressed hope that this acknowledgment of an evidenced-based role for selected CAC score imaging may prompt health insurers to start coving this expense, something they don’t now do. Patients generally pay out-of-pocket from $50 to $300 for CAC score imaging. “I hope they will start paying for this,” Dr. Lloyd-Jones said.
The guidelines also deal, at least in passing, with another financial issue that has loomed large for cholesterol treatment, the role of the notoriously expensive PCSK9 inhibitors, alirocumab (Praluent) and evolocumab (Repatha). For secondary prevention patients or for patients with familial hypercholesterolemia who do not reach their LDL cholesterol goal on statin treatment alone, the guideline recommended treatment first with generic ezetimibe. If the goal remains elusive, the next step is prescribing a PCSK9 inhibitor. The guideline also noted the poor cost-benefit ratio for the PCSK9 inhibitors at the U.S. list prices that existed in mid-2018, about $14,000 a year.
The guideline writers noted that this is one of the first times that cost considerations found their way into cardiology guidelines. Clinicians “need to be attuned to prescribing PCSK9 inhibitors only in those settings when it provides good value to patients,” explained Mark A. Hlatky, MD, a professor of medicine, cardiologist, and health policy specialist at Stanford (Calif.) University. The guideline “focuses on patient selection for PCSK9 inhibitors, limiting it to patients who get the most benefit,” said Dr. Hlatky, another member of the writing panel.
Although 10 medical groups joined the American College of Cardiology and American Heart Association in endorsing the guideline, conspicuously absent were the two largest U.S. societies representing primary care physicians, the American College of Physicians and American Academy of Family Practitioners. The guideline’s organizers invited both these societies to participate in the process and they declined, said Sidney C. Smith, Jr., MD, a member of the guideline committee.
Dr. Stone and Dr. Lloyd-Jones had no financial disclosures. The writing committee members’ disclosures can be found at jaccjacc.acc.org/Clinical_Document/Cholesterol_GL_Au_Comp_RWI.pdf.
*Correction, 11/13/18: An earlier version of this article misstated the name of Dr. Donald M. Lloyd-Jones.
SOURCE: AHA 2018 and Grundy S et al. J Am Coll Cardiol. 2018;doi:10.1016/j.jacc.2018.11.003.
REPORTING FROM THE AHA SCIENTIFIC SESSIONS
New cholesterol guidelines expand options for primary care
CHICAGO – New U.S. cholesterol guidelines spell out the role for ezetimibe and PCSK9 inhibitors, expand the scope of individualized risk assessment, and cite the potential value of a coronary artery calcium score as an additional risk determinant.
Neil J. Stone MD, vice chair of the of the 2018 Cholesterol Guidelines Committee, sat down for an interview and detailed the research behind the guidelines and how new features can help guide treatment decisions for patients at risk for a cardiovascular event.
CHICAGO – New U.S. cholesterol guidelines spell out the role for ezetimibe and PCSK9 inhibitors, expand the scope of individualized risk assessment, and cite the potential value of a coronary artery calcium score as an additional risk determinant.
Neil J. Stone MD, vice chair of the of the 2018 Cholesterol Guidelines Committee, sat down for an interview and detailed the research behind the guidelines and how new features can help guide treatment decisions for patients at risk for a cardiovascular event.
CHICAGO – New U.S. cholesterol guidelines spell out the role for ezetimibe and PCSK9 inhibitors, expand the scope of individualized risk assessment, and cite the potential value of a coronary artery calcium score as an additional risk determinant.
Neil J. Stone MD, vice chair of the of the 2018 Cholesterol Guidelines Committee, sat down for an interview and detailed the research behind the guidelines and how new features can help guide treatment decisions for patients at risk for a cardiovascular event.
REPORTING FROM THE AHA SCIENTIFIC SESSIONS
Draft JIA recommendations from ACR seek inactive disease
CHICAGO – New draft guidelines for treating juvenile idiopathic arthritis (JIA) written by experts assembled by the American College of Rheumatology “formalize inactive disease as the goal of treatment,” Timothy G. Beukelman, MD, said at the annual meeting of the American College of Rheumatology.
“We defined low disease activity as patients with a single active joint, and the goal is to have zero active joints. Low disease activity should not be tolerated” in patients with JIA, said Dr. Beukelman, a pediatric rheumatologist at the University of Alabama at Birmingham and a member of the guideline-writing committee. “Until now, treating these patients to zero active joints has not been recommended. But clinically inactive disease is a realistic target for a majority of JIA patients,” he said in an interview.
Despite this shift in the recommended treatment goal, the writing panel was forced to rely largely on their expertise rather than reported evidence. The recommendation by the committee to escalate therapy in patients with low disease activity was “conditional,” with a level of evidence deemed “very low,” Dr. Beukelman said during a talk in which he cited selected highlights from the committee’s full list of 39 recommendations.
The paucity of evidence reflected the status of many of the recommendations: 31 of the 39 recommendations were conditional, which means that the desirable effects from treatment “probably” outweigh the undesirable effects, and they may not apply to some patients. The writing panel pegged 22 of their recommendations as having a very low evidence backing and another 13 recommendations had low evidence. The JIA committee believed that none of its recommendations had strong evidence to back them up.
Dr. Beukelman defended writing recommendations despite this absence of evidence. “We should continue to write conditional recommendations when we don’t have the evidence. These recommendations had approval from at least 70% of the writing group, a diverse committee of experts. They should not be taken lightly just because they are conditional.”
Dr. Beukelman also stressed that he was presenting draft recommendations that still awaited approval from the ACR and the Arthritis Foundation, which collaborated with the ACR on this project. Once adopted, the new document would revise the existing management recommendations that the ACR approved in 2011 (Arthritis Rheum. 2011 Apr;63[4]:465-82).
The recommendations Dr. Beukelman outlined focused on treatment of polyarthritis, sacroiliitis, and enthesitis, and Dr. Beukelman devoted the most time to detailing some of the statements on polyarthritis. The panel conditionally recommended methotrexate over leflunomide (Arava) or sulfasalazine with moderate or very low evidence and said that subcutaneous methotrexate was conditionally preferred over oral dosing for reasons of both better efficacy and tolerability. Combination of a biologic agent with a nonbiologic received a conditional recommendation over biologic monotherapy, with moderate to very low evidence, but with a strong recommendation for this combined approach when using infliximab (Remicade), based on moderate evidence.
Another strong recommendation was to avoid treating patients with a chronic course of a low-dose, systemic glucocorticoid, based on a very low level of evidence. A brief course, less than 3 months, of an oral glucocorticoid received conditional recommendation for patients with moderate or high disease activity, based on very low evidence, but also received a conditional negative recommendation for patients with low disease activity, also based on very low evidence.
Initial therapy with a disease-modifying antirheumatic drug (DMARD) instead of monotherapy with an NSAID received a strong recommendation, based on a moderate level of evidence, while initial therapy with a DMARD received conditional support over initial therapy with a biologic agent, based on low evidence.
The panel gave a conditional endorsement to the idea of switching from a tumor necrosis factor inhibitor (TNFi) to a different biologic drug class when patients remained with moderate or high disease activity, based on a very low level of evidence.
Regarding sacroiliitis, the panel strongly recommended starting a TNFi in patients with active sacroiliitis despite NSAID treatment, based on low evidence, and the committee strongly recommended against starting methotrexate treatment in these patients, based on very low evidence. For treating active enthesitis despite NSAID treatment, the panel conditionally recommended adding a TNFi over treatment with methotrexate or sulfasalazine, based on a low level of evidence. Dr. Beukelman highlighted that the new recommendations placed increased emphasis on treating sacroiliitis, compared with the 2011 statement, and that the new recommendations dealt with treating enthesitis for the first time.
Dr. Beukelman has been a consultant to Bristol-Myers Squibb, Novartis, Sobi, and UCB.
CHICAGO – New draft guidelines for treating juvenile idiopathic arthritis (JIA) written by experts assembled by the American College of Rheumatology “formalize inactive disease as the goal of treatment,” Timothy G. Beukelman, MD, said at the annual meeting of the American College of Rheumatology.
“We defined low disease activity as patients with a single active joint, and the goal is to have zero active joints. Low disease activity should not be tolerated” in patients with JIA, said Dr. Beukelman, a pediatric rheumatologist at the University of Alabama at Birmingham and a member of the guideline-writing committee. “Until now, treating these patients to zero active joints has not been recommended. But clinically inactive disease is a realistic target for a majority of JIA patients,” he said in an interview.
Despite this shift in the recommended treatment goal, the writing panel was forced to rely largely on their expertise rather than reported evidence. The recommendation by the committee to escalate therapy in patients with low disease activity was “conditional,” with a level of evidence deemed “very low,” Dr. Beukelman said during a talk in which he cited selected highlights from the committee’s full list of 39 recommendations.
The paucity of evidence reflected the status of many of the recommendations: 31 of the 39 recommendations were conditional, which means that the desirable effects from treatment “probably” outweigh the undesirable effects, and they may not apply to some patients. The writing panel pegged 22 of their recommendations as having a very low evidence backing and another 13 recommendations had low evidence. The JIA committee believed that none of its recommendations had strong evidence to back them up.
Dr. Beukelman defended writing recommendations despite this absence of evidence. “We should continue to write conditional recommendations when we don’t have the evidence. These recommendations had approval from at least 70% of the writing group, a diverse committee of experts. They should not be taken lightly just because they are conditional.”
Dr. Beukelman also stressed that he was presenting draft recommendations that still awaited approval from the ACR and the Arthritis Foundation, which collaborated with the ACR on this project. Once adopted, the new document would revise the existing management recommendations that the ACR approved in 2011 (Arthritis Rheum. 2011 Apr;63[4]:465-82).
The recommendations Dr. Beukelman outlined focused on treatment of polyarthritis, sacroiliitis, and enthesitis, and Dr. Beukelman devoted the most time to detailing some of the statements on polyarthritis. The panel conditionally recommended methotrexate over leflunomide (Arava) or sulfasalazine with moderate or very low evidence and said that subcutaneous methotrexate was conditionally preferred over oral dosing for reasons of both better efficacy and tolerability. Combination of a biologic agent with a nonbiologic received a conditional recommendation over biologic monotherapy, with moderate to very low evidence, but with a strong recommendation for this combined approach when using infliximab (Remicade), based on moderate evidence.
Another strong recommendation was to avoid treating patients with a chronic course of a low-dose, systemic glucocorticoid, based on a very low level of evidence. A brief course, less than 3 months, of an oral glucocorticoid received conditional recommendation for patients with moderate or high disease activity, based on very low evidence, but also received a conditional negative recommendation for patients with low disease activity, also based on very low evidence.
Initial therapy with a disease-modifying antirheumatic drug (DMARD) instead of monotherapy with an NSAID received a strong recommendation, based on a moderate level of evidence, while initial therapy with a DMARD received conditional support over initial therapy with a biologic agent, based on low evidence.
The panel gave a conditional endorsement to the idea of switching from a tumor necrosis factor inhibitor (TNFi) to a different biologic drug class when patients remained with moderate or high disease activity, based on a very low level of evidence.
Regarding sacroiliitis, the panel strongly recommended starting a TNFi in patients with active sacroiliitis despite NSAID treatment, based on low evidence, and the committee strongly recommended against starting methotrexate treatment in these patients, based on very low evidence. For treating active enthesitis despite NSAID treatment, the panel conditionally recommended adding a TNFi over treatment with methotrexate or sulfasalazine, based on a low level of evidence. Dr. Beukelman highlighted that the new recommendations placed increased emphasis on treating sacroiliitis, compared with the 2011 statement, and that the new recommendations dealt with treating enthesitis for the first time.
Dr. Beukelman has been a consultant to Bristol-Myers Squibb, Novartis, Sobi, and UCB.
CHICAGO – New draft guidelines for treating juvenile idiopathic arthritis (JIA) written by experts assembled by the American College of Rheumatology “formalize inactive disease as the goal of treatment,” Timothy G. Beukelman, MD, said at the annual meeting of the American College of Rheumatology.
“We defined low disease activity as patients with a single active joint, and the goal is to have zero active joints. Low disease activity should not be tolerated” in patients with JIA, said Dr. Beukelman, a pediatric rheumatologist at the University of Alabama at Birmingham and a member of the guideline-writing committee. “Until now, treating these patients to zero active joints has not been recommended. But clinically inactive disease is a realistic target for a majority of JIA patients,” he said in an interview.
Despite this shift in the recommended treatment goal, the writing panel was forced to rely largely on their expertise rather than reported evidence. The recommendation by the committee to escalate therapy in patients with low disease activity was “conditional,” with a level of evidence deemed “very low,” Dr. Beukelman said during a talk in which he cited selected highlights from the committee’s full list of 39 recommendations.
The paucity of evidence reflected the status of many of the recommendations: 31 of the 39 recommendations were conditional, which means that the desirable effects from treatment “probably” outweigh the undesirable effects, and they may not apply to some patients. The writing panel pegged 22 of their recommendations as having a very low evidence backing and another 13 recommendations had low evidence. The JIA committee believed that none of its recommendations had strong evidence to back them up.
Dr. Beukelman defended writing recommendations despite this absence of evidence. “We should continue to write conditional recommendations when we don’t have the evidence. These recommendations had approval from at least 70% of the writing group, a diverse committee of experts. They should not be taken lightly just because they are conditional.”
Dr. Beukelman also stressed that he was presenting draft recommendations that still awaited approval from the ACR and the Arthritis Foundation, which collaborated with the ACR on this project. Once adopted, the new document would revise the existing management recommendations that the ACR approved in 2011 (Arthritis Rheum. 2011 Apr;63[4]:465-82).
The recommendations Dr. Beukelman outlined focused on treatment of polyarthritis, sacroiliitis, and enthesitis, and Dr. Beukelman devoted the most time to detailing some of the statements on polyarthritis. The panel conditionally recommended methotrexate over leflunomide (Arava) or sulfasalazine with moderate or very low evidence and said that subcutaneous methotrexate was conditionally preferred over oral dosing for reasons of both better efficacy and tolerability. Combination of a biologic agent with a nonbiologic received a conditional recommendation over biologic monotherapy, with moderate to very low evidence, but with a strong recommendation for this combined approach when using infliximab (Remicade), based on moderate evidence.
Another strong recommendation was to avoid treating patients with a chronic course of a low-dose, systemic glucocorticoid, based on a very low level of evidence. A brief course, less than 3 months, of an oral glucocorticoid received conditional recommendation for patients with moderate or high disease activity, based on very low evidence, but also received a conditional negative recommendation for patients with low disease activity, also based on very low evidence.
Initial therapy with a disease-modifying antirheumatic drug (DMARD) instead of monotherapy with an NSAID received a strong recommendation, based on a moderate level of evidence, while initial therapy with a DMARD received conditional support over initial therapy with a biologic agent, based on low evidence.
The panel gave a conditional endorsement to the idea of switching from a tumor necrosis factor inhibitor (TNFi) to a different biologic drug class when patients remained with moderate or high disease activity, based on a very low level of evidence.
Regarding sacroiliitis, the panel strongly recommended starting a TNFi in patients with active sacroiliitis despite NSAID treatment, based on low evidence, and the committee strongly recommended against starting methotrexate treatment in these patients, based on very low evidence. For treating active enthesitis despite NSAID treatment, the panel conditionally recommended adding a TNFi over treatment with methotrexate or sulfasalazine, based on a low level of evidence. Dr. Beukelman highlighted that the new recommendations placed increased emphasis on treating sacroiliitis, compared with the 2011 statement, and that the new recommendations dealt with treating enthesitis for the first time.
Dr. Beukelman has been a consultant to Bristol-Myers Squibb, Novartis, Sobi, and UCB.
EXPERT ANALYSIS FROM THE ACR ANNUAL MEETING
Genetic profile flags scleroderma patients with best HSCT responses
CHICAGO – Subgroup categorization of patients with severe scleroderma by their gene-expression profile correlated with responses to a newly proven treatment for the disease that involves myeloablation and autologous hematopoietic stem cell transplantation (HSCT).
Patients who fell into the “fibroproliferative” scleroderma subgroup, roughly one-third of patients enrolled in the treatment study, showed a high level of benefit from myeloablation and autologous HSCT, Michael L. Whitfield, PhD, said at the annual meeting of the American College of Rheumatology.
In contrast, the roughly one-third of patients in the study with a gene-expression profile that placed them into the “normal-like” subgroup had outcomes that closely matched the normal-like patients in the control group, who were treated with cyclophosphamide, which suggests that the normal-like patients are probably not good candidates for HSCT, said Dr. Whitfield, a professor of molecular and systems biology at the Geisel School of Medicine at Dartmouth in Hanover, N.H.
This study starts to get at the question of “How do you do personalized medicine in a disease like scleroderma?” he explained. “HSCT may be a game changer for patients with the fibroproliferative type of scleroderma,” who did relatively poorly in the control group of the trial when they received cyclophosphamide. Categorization of patients by their gene-expression profiles is a way to find order among scleroderma patients in what is otherwise “a very heterogeneous disease, where some patients improve on a treatment and others do not,” Dr Whitfield said.
The study used data collected in the SCOT (Scleroderma: Cyclophosphamide or Transplantation) trial, which enrolled 75 patients with severe scleroderma at any of 26 sites in the United States and Canada. SCOT compared the safety and efficacy of myeloablation followed by autologous HSCT with that of treatment with cyclophosphamide, and it followed patients for a median of 54 months. The results showed that overall HSCT was superior for the primary endpoint and several secondary endpoints, including event-free survival, which was 79% after HSCT and 50% in control patients by the end of follow-up (N Engl J Med. 2018 Jan 4;378[1]:35-47).
Dr. Whitfield’s group took peripheral blood cells from 30 of the patients treated by HSCT and from 33 of the patients treated with cyclophosphamide per protocol and analyzed the gene-expression profiles of the cells to categorize patients into the gene-expression subtypes of scleroderma that had been previously defined by Dr. Whitfield and his associates: fibroproliferative, inflammatory, limited, or normal-like (PLOS One. 2008 Jul 18;3[7]:e2696). The gene-expression analysis, which now looks at the activity of about 1,300 genes, showed that the 33 cyclophosphamide-treated patients included 12 with an inflammatory profile, 12 with a normal-like profile, and 9 with a fibroproliferative profile. Among the 30 patients treated with HSCT, 11 were in the fibroproliferative group, 11 were normal-like, and 8 had an inflammatory pattern.
Analysis of event-free survival out to 6 years following enrollment showed that, in the fibroproliferative subgroup, roughly 90% of patients treated with HSCT remained alive and event free, compared with about 35% of the cyclophosphamide patients, a highly statistically significant difference. In the inflammatory subgroup, event-free survival persisted in about 90% in the HSCT recipients, compared with about 50% of those in the control arm, a difference that did not reach statistical significance. Among patients with normal-like gene expression, the event-free survival rate was about the same regardless of treatment, about 60% in each treatment arm. The results suggest that patients with normal-like disease “are probably not good candidates for a treatment as intensive as HSCT,” Dr. Whitfield said in an interview.
Although the HSCT and cyclophosphamide treatment groups included relatively small numbers of patients, when the researchers subdivided the trial cohort into three different scleroderma types, the analysis remained “powered well enough to see a difference; the difference was very clearly statistically significant,” Dr. Whitfield declared.
“Now that we have a treatment [HSCT] to tie to the [gene-expression analysis], we can think about using this in routine practice,” he concluded.
Dr. Whitfield is a cofounder of Celdara, and he has been a consultant to Bristol-Myers Squibb, Corbus, UCB, and Third Rock Ventures.
SOURCE: Franks J et al. Arthritis Rheumatol. 2018;70(suppl 10): Abstract 1876.
CHICAGO – Subgroup categorization of patients with severe scleroderma by their gene-expression profile correlated with responses to a newly proven treatment for the disease that involves myeloablation and autologous hematopoietic stem cell transplantation (HSCT).
Patients who fell into the “fibroproliferative” scleroderma subgroup, roughly one-third of patients enrolled in the treatment study, showed a high level of benefit from myeloablation and autologous HSCT, Michael L. Whitfield, PhD, said at the annual meeting of the American College of Rheumatology.
In contrast, the roughly one-third of patients in the study with a gene-expression profile that placed them into the “normal-like” subgroup had outcomes that closely matched the normal-like patients in the control group, who were treated with cyclophosphamide, which suggests that the normal-like patients are probably not good candidates for HSCT, said Dr. Whitfield, a professor of molecular and systems biology at the Geisel School of Medicine at Dartmouth in Hanover, N.H.
This study starts to get at the question of “How do you do personalized medicine in a disease like scleroderma?” he explained. “HSCT may be a game changer for patients with the fibroproliferative type of scleroderma,” who did relatively poorly in the control group of the trial when they received cyclophosphamide. Categorization of patients by their gene-expression profiles is a way to find order among scleroderma patients in what is otherwise “a very heterogeneous disease, where some patients improve on a treatment and others do not,” Dr Whitfield said.
The study used data collected in the SCOT (Scleroderma: Cyclophosphamide or Transplantation) trial, which enrolled 75 patients with severe scleroderma at any of 26 sites in the United States and Canada. SCOT compared the safety and efficacy of myeloablation followed by autologous HSCT with that of treatment with cyclophosphamide, and it followed patients for a median of 54 months. The results showed that overall HSCT was superior for the primary endpoint and several secondary endpoints, including event-free survival, which was 79% after HSCT and 50% in control patients by the end of follow-up (N Engl J Med. 2018 Jan 4;378[1]:35-47).
Dr. Whitfield’s group took peripheral blood cells from 30 of the patients treated by HSCT and from 33 of the patients treated with cyclophosphamide per protocol and analyzed the gene-expression profiles of the cells to categorize patients into the gene-expression subtypes of scleroderma that had been previously defined by Dr. Whitfield and his associates: fibroproliferative, inflammatory, limited, or normal-like (PLOS One. 2008 Jul 18;3[7]:e2696). The gene-expression analysis, which now looks at the activity of about 1,300 genes, showed that the 33 cyclophosphamide-treated patients included 12 with an inflammatory profile, 12 with a normal-like profile, and 9 with a fibroproliferative profile. Among the 30 patients treated with HSCT, 11 were in the fibroproliferative group, 11 were normal-like, and 8 had an inflammatory pattern.
Analysis of event-free survival out to 6 years following enrollment showed that, in the fibroproliferative subgroup, roughly 90% of patients treated with HSCT remained alive and event free, compared with about 35% of the cyclophosphamide patients, a highly statistically significant difference. In the inflammatory subgroup, event-free survival persisted in about 90% in the HSCT recipients, compared with about 50% of those in the control arm, a difference that did not reach statistical significance. Among patients with normal-like gene expression, the event-free survival rate was about the same regardless of treatment, about 60% in each treatment arm. The results suggest that patients with normal-like disease “are probably not good candidates for a treatment as intensive as HSCT,” Dr. Whitfield said in an interview.
Although the HSCT and cyclophosphamide treatment groups included relatively small numbers of patients, when the researchers subdivided the trial cohort into three different scleroderma types, the analysis remained “powered well enough to see a difference; the difference was very clearly statistically significant,” Dr. Whitfield declared.
“Now that we have a treatment [HSCT] to tie to the [gene-expression analysis], we can think about using this in routine practice,” he concluded.
Dr. Whitfield is a cofounder of Celdara, and he has been a consultant to Bristol-Myers Squibb, Corbus, UCB, and Third Rock Ventures.
SOURCE: Franks J et al. Arthritis Rheumatol. 2018;70(suppl 10): Abstract 1876.
CHICAGO – Subgroup categorization of patients with severe scleroderma by their gene-expression profile correlated with responses to a newly proven treatment for the disease that involves myeloablation and autologous hematopoietic stem cell transplantation (HSCT).
Patients who fell into the “fibroproliferative” scleroderma subgroup, roughly one-third of patients enrolled in the treatment study, showed a high level of benefit from myeloablation and autologous HSCT, Michael L. Whitfield, PhD, said at the annual meeting of the American College of Rheumatology.
In contrast, the roughly one-third of patients in the study with a gene-expression profile that placed them into the “normal-like” subgroup had outcomes that closely matched the normal-like patients in the control group, who were treated with cyclophosphamide, which suggests that the normal-like patients are probably not good candidates for HSCT, said Dr. Whitfield, a professor of molecular and systems biology at the Geisel School of Medicine at Dartmouth in Hanover, N.H.
This study starts to get at the question of “How do you do personalized medicine in a disease like scleroderma?” he explained. “HSCT may be a game changer for patients with the fibroproliferative type of scleroderma,” who did relatively poorly in the control group of the trial when they received cyclophosphamide. Categorization of patients by their gene-expression profiles is a way to find order among scleroderma patients in what is otherwise “a very heterogeneous disease, where some patients improve on a treatment and others do not,” Dr Whitfield said.
The study used data collected in the SCOT (Scleroderma: Cyclophosphamide or Transplantation) trial, which enrolled 75 patients with severe scleroderma at any of 26 sites in the United States and Canada. SCOT compared the safety and efficacy of myeloablation followed by autologous HSCT with that of treatment with cyclophosphamide, and it followed patients for a median of 54 months. The results showed that overall HSCT was superior for the primary endpoint and several secondary endpoints, including event-free survival, which was 79% after HSCT and 50% in control patients by the end of follow-up (N Engl J Med. 2018 Jan 4;378[1]:35-47).
Dr. Whitfield’s group took peripheral blood cells from 30 of the patients treated by HSCT and from 33 of the patients treated with cyclophosphamide per protocol and analyzed the gene-expression profiles of the cells to categorize patients into the gene-expression subtypes of scleroderma that had been previously defined by Dr. Whitfield and his associates: fibroproliferative, inflammatory, limited, or normal-like (PLOS One. 2008 Jul 18;3[7]:e2696). The gene-expression analysis, which now looks at the activity of about 1,300 genes, showed that the 33 cyclophosphamide-treated patients included 12 with an inflammatory profile, 12 with a normal-like profile, and 9 with a fibroproliferative profile. Among the 30 patients treated with HSCT, 11 were in the fibroproliferative group, 11 were normal-like, and 8 had an inflammatory pattern.
Analysis of event-free survival out to 6 years following enrollment showed that, in the fibroproliferative subgroup, roughly 90% of patients treated with HSCT remained alive and event free, compared with about 35% of the cyclophosphamide patients, a highly statistically significant difference. In the inflammatory subgroup, event-free survival persisted in about 90% in the HSCT recipients, compared with about 50% of those in the control arm, a difference that did not reach statistical significance. Among patients with normal-like gene expression, the event-free survival rate was about the same regardless of treatment, about 60% in each treatment arm. The results suggest that patients with normal-like disease “are probably not good candidates for a treatment as intensive as HSCT,” Dr. Whitfield said in an interview.
Although the HSCT and cyclophosphamide treatment groups included relatively small numbers of patients, when the researchers subdivided the trial cohort into three different scleroderma types, the analysis remained “powered well enough to see a difference; the difference was very clearly statistically significant,” Dr. Whitfield declared.
“Now that we have a treatment [HSCT] to tie to the [gene-expression analysis], we can think about using this in routine practice,” he concluded.
Dr. Whitfield is a cofounder of Celdara, and he has been a consultant to Bristol-Myers Squibb, Corbus, UCB, and Third Rock Ventures.
SOURCE: Franks J et al. Arthritis Rheumatol. 2018;70(suppl 10): Abstract 1876.
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point:
Major finding: About 90% of fibroproliferative scleroderma patients had prolonged event-free survival after stem cell transplant, compared with about 35% of controls.
Study details: The study used data collected in the SCOT trial of 75 patients with severe scleroderma.
Disclosures: Dr. Whitfield is a cofounder of Celdara, and he has been a consultant to Bristol-Myers Squibb, Corbus, UCB, and Third Rock Ventures.
Source: Franks J et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 1876.
ACR and EULAR draft classification criteria for IgG4-related disease
CHICAGO – A joint American College of Rheumatology and European League Against Rheumatism panel has written the first-ever classification criteria for immunoglobulin G4-related disease (IgG4-RD), and the draft version of the criteria identified the disorder with 99.2% specificity and 85.5% sensitivity when compared with expert case opinions.
“We’ve come a long way” to write these criteria 17 years after the first case report, and about a decade after IgG4-RD first became part of routine rheumatology practice, John H. Stone, MD, said at the annual meeting of the American College of Rheumatology. He cited one estimate that about 185,000 Americans currently have IgG4-RD.
Approval of the draft criteria by both the ACR and EULAR remains pending.
The working group assembled by the American College of Rheumatology and the European League Against Rheumatism to write the classification criteria included 89 members, and the draft document they produced combined inclusion and exclusion criteria, “the first ACR and EULAR classification criteria to include specific exclusions, to my knowledge,” said Dr. Stone professor of medicine at Harvard Medical School and director of clinical rheumatology at Massachusetts General Hospital in Boston. The exclusions reflect the many other disorders that can mimic IgG4-RD, including cancers and several rheumatologic diseases, especially granulomatosis with polyangiitis and Sjögren’s syndrome.
The writing panel used 487 case reports from 272 patients diagnosed with IgG4-RD and 215 patients diagnosed with a different, mimic disease to derive the classification criteria, and then used 908 case reports – 493 from IgG4-RD patients and 415 reports from mimic cases – to test and validate the criteria.
The first step in classifying a patient with IgG4-RD is to identify involvement of at least one organ from the list the panel compiled of 10 organs where involvement typifies the disease: pancreas, bile ducts, orbits, lacrimal glands, major salivary glands, retroperitoneum, kidney, aorta, pachymeninges, and thyroid gland (Riedel’s thyroiditis, but not Hashimoto’s disease). Patients who do not have disease involvement in at least one of these organs don’t qualify as having IgG4-RD.
The next step is to rule out patients who have at least one exclusion criterion from a list of 21 exclusions the panel cited, divided into four categories based on the test that finds each exclusion: clinical examination, serology, radiology, or pathology.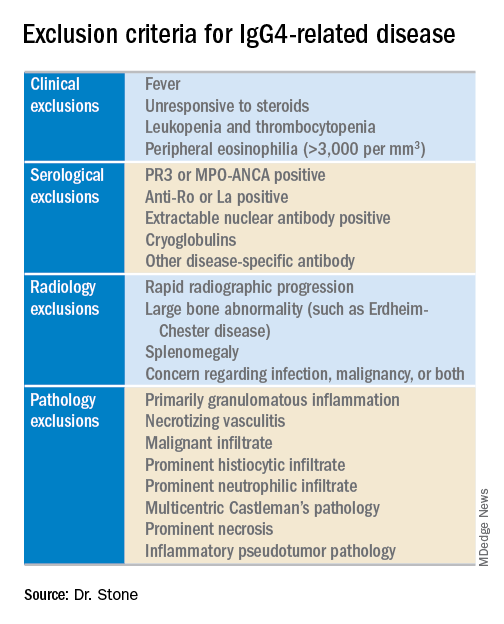
The last step is to identify enough individual classification hallmarks in the patient so that collectively they definitively identify IgG4-RD. The writing panel endorsed seven inclusion-criteria domains that each contain at least two different disease manifestations that confer points if fulfilled. To qualify for IgG4-RD classification, a patient needs to have enough manifestations to tally at least 19 points.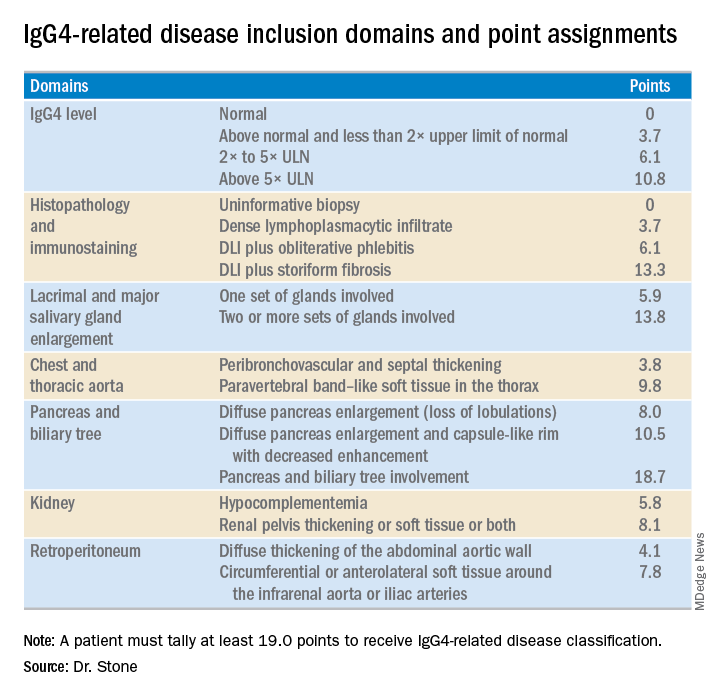
Fulfilling the inclusion criteria is the key step in classification, but the exclusion criteria also play a role in helping to rule the disease in or out, Dr. Stone noted. Without the exclusion criteria, the remaining classification criteria identified the 1,395 total cases and mimics studied with an increased sensitivity of 90% (compared with 85.5% when the exclusion criteria also apply), but with reduced specificity of 88.5% (compared with 99.2%). High specificity is a key aim. The criteria are supposed to give greater uniformity to patient selection for studies and ensure that enrolled patients actually have IgG4-RD. “Our goal was criteria that would prevent enrollment of patients without IgG4-RD,” he said.
Although IgG4 level is one of the seven inclusion domains and can give a patient as many as 10.8 points toward classification when the level exceeds five times the upper limit of normal, the criteria solidify the notion that “we have greatly overemphasized IgG4” in past considerations of the disease, said Dr. Stone. Elevation of IgG4 is one of several disease markers in most patients, but it’s not essential to classification and is missing in nearly a third of patients. While the cause of IgG4-RD remains unknown, it appears to involve an abnormal interaction between B cells and a CD4+ cytotoxic T lymphocyte, an understanding that has led to testing investigational therapies that target B cells including rituximab (Rituxan) and an agent called XmAb5871. “Rituximab works very well,” Dr. Stone said. The absence of a known cause is a reason why classification is so complex.
Dr. Stone also reminded his audience that IgG4-RD is an indolent disease that can produce symptoms for months or years before getting diagnosed. It often is accompanied by significant weight loss of 20 or more pounds, but without fever, and often features a dissociation between a high erythrocyte sedimentation rate but a relatively low level of C-reactive protein. “It’s astonishing how much weight patients lose,” he said.
Though barely more than a decade on the scene, awareness of IgG4-RD among rheumatologists has become widespread, though awareness has probably lagged among many primary care physicians, Dr. Stone said in an interview. The estimated prevalence of about 185,000 U.S. residents with IgG4-RD is probably an underestimate, he added. His group at Massachusetts General Hospital in Boston averages 3-5 patients evaluated each week as possibly having IgG4-RD, and this one group is now following more than 350 patients who have been diagnosed with the disease. “It’s probably more common than a lot of other conditions that rheumatologists treat, more common than scleroderma or ANCA-associated vasculitis,” Dr. Stone said. “The new criteria will help further raise awareness.”
Dr. Stone has been a consultant to and has received research funding from Genentech, Roche, and Xencor.
CHICAGO – A joint American College of Rheumatology and European League Against Rheumatism panel has written the first-ever classification criteria for immunoglobulin G4-related disease (IgG4-RD), and the draft version of the criteria identified the disorder with 99.2% specificity and 85.5% sensitivity when compared with expert case opinions.
“We’ve come a long way” to write these criteria 17 years after the first case report, and about a decade after IgG4-RD first became part of routine rheumatology practice, John H. Stone, MD, said at the annual meeting of the American College of Rheumatology. He cited one estimate that about 185,000 Americans currently have IgG4-RD.
Approval of the draft criteria by both the ACR and EULAR remains pending.
The working group assembled by the American College of Rheumatology and the European League Against Rheumatism to write the classification criteria included 89 members, and the draft document they produced combined inclusion and exclusion criteria, “the first ACR and EULAR classification criteria to include specific exclusions, to my knowledge,” said Dr. Stone professor of medicine at Harvard Medical School and director of clinical rheumatology at Massachusetts General Hospital in Boston. The exclusions reflect the many other disorders that can mimic IgG4-RD, including cancers and several rheumatologic diseases, especially granulomatosis with polyangiitis and Sjögren’s syndrome.
The writing panel used 487 case reports from 272 patients diagnosed with IgG4-RD and 215 patients diagnosed with a different, mimic disease to derive the classification criteria, and then used 908 case reports – 493 from IgG4-RD patients and 415 reports from mimic cases – to test and validate the criteria.
The first step in classifying a patient with IgG4-RD is to identify involvement of at least one organ from the list the panel compiled of 10 organs where involvement typifies the disease: pancreas, bile ducts, orbits, lacrimal glands, major salivary glands, retroperitoneum, kidney, aorta, pachymeninges, and thyroid gland (Riedel’s thyroiditis, but not Hashimoto’s disease). Patients who do not have disease involvement in at least one of these organs don’t qualify as having IgG4-RD.
The next step is to rule out patients who have at least one exclusion criterion from a list of 21 exclusions the panel cited, divided into four categories based on the test that finds each exclusion: clinical examination, serology, radiology, or pathology.
The last step is to identify enough individual classification hallmarks in the patient so that collectively they definitively identify IgG4-RD. The writing panel endorsed seven inclusion-criteria domains that each contain at least two different disease manifestations that confer points if fulfilled. To qualify for IgG4-RD classification, a patient needs to have enough manifestations to tally at least 19 points.
Fulfilling the inclusion criteria is the key step in classification, but the exclusion criteria also play a role in helping to rule the disease in or out, Dr. Stone noted. Without the exclusion criteria, the remaining classification criteria identified the 1,395 total cases and mimics studied with an increased sensitivity of 90% (compared with 85.5% when the exclusion criteria also apply), but with reduced specificity of 88.5% (compared with 99.2%). High specificity is a key aim. The criteria are supposed to give greater uniformity to patient selection for studies and ensure that enrolled patients actually have IgG4-RD. “Our goal was criteria that would prevent enrollment of patients without IgG4-RD,” he said.
Although IgG4 level is one of the seven inclusion domains and can give a patient as many as 10.8 points toward classification when the level exceeds five times the upper limit of normal, the criteria solidify the notion that “we have greatly overemphasized IgG4” in past considerations of the disease, said Dr. Stone. Elevation of IgG4 is one of several disease markers in most patients, but it’s not essential to classification and is missing in nearly a third of patients. While the cause of IgG4-RD remains unknown, it appears to involve an abnormal interaction between B cells and a CD4+ cytotoxic T lymphocyte, an understanding that has led to testing investigational therapies that target B cells including rituximab (Rituxan) and an agent called XmAb5871. “Rituximab works very well,” Dr. Stone said. The absence of a known cause is a reason why classification is so complex.
Dr. Stone also reminded his audience that IgG4-RD is an indolent disease that can produce symptoms for months or years before getting diagnosed. It often is accompanied by significant weight loss of 20 or more pounds, but without fever, and often features a dissociation between a high erythrocyte sedimentation rate but a relatively low level of C-reactive protein. “It’s astonishing how much weight patients lose,” he said.
Though barely more than a decade on the scene, awareness of IgG4-RD among rheumatologists has become widespread, though awareness has probably lagged among many primary care physicians, Dr. Stone said in an interview. The estimated prevalence of about 185,000 U.S. residents with IgG4-RD is probably an underestimate, he added. His group at Massachusetts General Hospital in Boston averages 3-5 patients evaluated each week as possibly having IgG4-RD, and this one group is now following more than 350 patients who have been diagnosed with the disease. “It’s probably more common than a lot of other conditions that rheumatologists treat, more common than scleroderma or ANCA-associated vasculitis,” Dr. Stone said. “The new criteria will help further raise awareness.”
Dr. Stone has been a consultant to and has received research funding from Genentech, Roche, and Xencor.
CHICAGO – A joint American College of Rheumatology and European League Against Rheumatism panel has written the first-ever classification criteria for immunoglobulin G4-related disease (IgG4-RD), and the draft version of the criteria identified the disorder with 99.2% specificity and 85.5% sensitivity when compared with expert case opinions.
“We’ve come a long way” to write these criteria 17 years after the first case report, and about a decade after IgG4-RD first became part of routine rheumatology practice, John H. Stone, MD, said at the annual meeting of the American College of Rheumatology. He cited one estimate that about 185,000 Americans currently have IgG4-RD.
Approval of the draft criteria by both the ACR and EULAR remains pending.
The working group assembled by the American College of Rheumatology and the European League Against Rheumatism to write the classification criteria included 89 members, and the draft document they produced combined inclusion and exclusion criteria, “the first ACR and EULAR classification criteria to include specific exclusions, to my knowledge,” said Dr. Stone professor of medicine at Harvard Medical School and director of clinical rheumatology at Massachusetts General Hospital in Boston. The exclusions reflect the many other disorders that can mimic IgG4-RD, including cancers and several rheumatologic diseases, especially granulomatosis with polyangiitis and Sjögren’s syndrome.
The writing panel used 487 case reports from 272 patients diagnosed with IgG4-RD and 215 patients diagnosed with a different, mimic disease to derive the classification criteria, and then used 908 case reports – 493 from IgG4-RD patients and 415 reports from mimic cases – to test and validate the criteria.
The first step in classifying a patient with IgG4-RD is to identify involvement of at least one organ from the list the panel compiled of 10 organs where involvement typifies the disease: pancreas, bile ducts, orbits, lacrimal glands, major salivary glands, retroperitoneum, kidney, aorta, pachymeninges, and thyroid gland (Riedel’s thyroiditis, but not Hashimoto’s disease). Patients who do not have disease involvement in at least one of these organs don’t qualify as having IgG4-RD.
The next step is to rule out patients who have at least one exclusion criterion from a list of 21 exclusions the panel cited, divided into four categories based on the test that finds each exclusion: clinical examination, serology, radiology, or pathology.
The last step is to identify enough individual classification hallmarks in the patient so that collectively they definitively identify IgG4-RD. The writing panel endorsed seven inclusion-criteria domains that each contain at least two different disease manifestations that confer points if fulfilled. To qualify for IgG4-RD classification, a patient needs to have enough manifestations to tally at least 19 points.
Fulfilling the inclusion criteria is the key step in classification, but the exclusion criteria also play a role in helping to rule the disease in or out, Dr. Stone noted. Without the exclusion criteria, the remaining classification criteria identified the 1,395 total cases and mimics studied with an increased sensitivity of 90% (compared with 85.5% when the exclusion criteria also apply), but with reduced specificity of 88.5% (compared with 99.2%). High specificity is a key aim. The criteria are supposed to give greater uniformity to patient selection for studies and ensure that enrolled patients actually have IgG4-RD. “Our goal was criteria that would prevent enrollment of patients without IgG4-RD,” he said.
Although IgG4 level is one of the seven inclusion domains and can give a patient as many as 10.8 points toward classification when the level exceeds five times the upper limit of normal, the criteria solidify the notion that “we have greatly overemphasized IgG4” in past considerations of the disease, said Dr. Stone. Elevation of IgG4 is one of several disease markers in most patients, but it’s not essential to classification and is missing in nearly a third of patients. While the cause of IgG4-RD remains unknown, it appears to involve an abnormal interaction between B cells and a CD4+ cytotoxic T lymphocyte, an understanding that has led to testing investigational therapies that target B cells including rituximab (Rituxan) and an agent called XmAb5871. “Rituximab works very well,” Dr. Stone said. The absence of a known cause is a reason why classification is so complex.
Dr. Stone also reminded his audience that IgG4-RD is an indolent disease that can produce symptoms for months or years before getting diagnosed. It often is accompanied by significant weight loss of 20 or more pounds, but without fever, and often features a dissociation between a high erythrocyte sedimentation rate but a relatively low level of C-reactive protein. “It’s astonishing how much weight patients lose,” he said.
Though barely more than a decade on the scene, awareness of IgG4-RD among rheumatologists has become widespread, though awareness has probably lagged among many primary care physicians, Dr. Stone said in an interview. The estimated prevalence of about 185,000 U.S. residents with IgG4-RD is probably an underestimate, he added. His group at Massachusetts General Hospital in Boston averages 3-5 patients evaluated each week as possibly having IgG4-RD, and this one group is now following more than 350 patients who have been diagnosed with the disease. “It’s probably more common than a lot of other conditions that rheumatologists treat, more common than scleroderma or ANCA-associated vasculitis,” Dr. Stone said. “The new criteria will help further raise awareness.”
Dr. Stone has been a consultant to and has received research funding from Genentech, Roche, and Xencor.
REPORTING FROM THE ACR ANNUAL MEETING
Complications cluster in inflammatory arthritis patients after total knee replacement
CHICAGO – Patients with an inflammatory arthritis had significantly higher rates of infections, transfusions, and readmissions following total knee replacement than did patients without inflammatory arthritis in a study of more than 137,000 Americans who underwent this surgery.
A sampling of U.S. patients who underwent total knee arthroplasty (TKA) during 2007-2016 showed that among the small percentage of these patients who had an inflammatory arthritis (IA), the rate of periprosthetic joint or wound infection while hospitalized or out to 30 days after surgery was a statistically significant 64% higher relative to patients without inflammatory arthritis, after adjustment for several demographic and clinical confounders, including recent glucocorticoid treatment, Susan M. Goodman, MD, said at the annual meeting of the American College of Rheumatology. The analysis also showed a statistically significant 46% higher relative rate of hospital readmission for any cause during the 90 days after surgery, and a significant 39% relative increase in blood transfusions during the 30 days after TKA in the IA patients.
“These results have important implications for evolving bundled payment models” for TKA, said Dr. Goodman, a rheumatologist at the Hospital for Special Surgery in New York. “Hospitals should receive commensurate resources to maintain access to total TKA for patients with IA.”
For this analysis, Dr. Goodman and her associates classified IA as a patient with a recorded diagnosis of rheumatoid arthritis, spondyloarthritis, or systemic lupus erythematosus if the patient had also received treatment during the year before surgery with a disease-modifying antirheumatic drug, a biologic agent, or a drug that treats systemic lupus erythematosus.
Complications following TKA became a particular concern to hospitals starting in 2013 when the Centers for Medicare & Medicaid Services began a program that penalized hospitals for outcomes such as excessive readmissions following selected types of hospitalizations and also with recent steps to bundle TKA reimbursement with related 90-day outcomes.
“My concern is to ensure that patients with IA aren’t penalized and can maintain access” to TKA despite recent policy moves by the CMS. Faced with potential disincentives to treat patients with an IA, “hospitals might cherry pick patients,” Dr. Goodman said in an interview. The new findings “are a reason for administrators to argue for patients with IA to come out of the cost bundle.”
Dr. Goodman expressed hope that future policies will better reflect the higher levels of risk faced by patients with an IA undergoing TKA. CMS “is pretty responsive,” she said.
The study used data collected by Humana for about 25 million American health insurance beneficiaries during 2007-2016, which included 137,550 people who underwent a TKA. Of these, 3,067 (2%) met the study’s definition for IA, and 134,483 did not. Most of those who did not meet the definition likely had osteoarthritis, Dr. Goodman said. This low percentage of U.S. TKA patients with IA was consistent with numbers in prior reports.
The researchers calculated the relative risk of the IA patients, compared with all the others, for nine potential complications, including acute MI, pneumonia, sepsis, pulmonary embolism, and death. The complications with significantly higher rates among the IA patients after confounder adjustment were 30-day infections, 30-day transfusions, and 90-day readmissions.
Dr. Goodman had no relevant disclosures.
SOURCE: Richardson S et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 1932.
CHICAGO – Patients with an inflammatory arthritis had significantly higher rates of infections, transfusions, and readmissions following total knee replacement than did patients without inflammatory arthritis in a study of more than 137,000 Americans who underwent this surgery.
A sampling of U.S. patients who underwent total knee arthroplasty (TKA) during 2007-2016 showed that among the small percentage of these patients who had an inflammatory arthritis (IA), the rate of periprosthetic joint or wound infection while hospitalized or out to 30 days after surgery was a statistically significant 64% higher relative to patients without inflammatory arthritis, after adjustment for several demographic and clinical confounders, including recent glucocorticoid treatment, Susan M. Goodman, MD, said at the annual meeting of the American College of Rheumatology. The analysis also showed a statistically significant 46% higher relative rate of hospital readmission for any cause during the 90 days after surgery, and a significant 39% relative increase in blood transfusions during the 30 days after TKA in the IA patients.
“These results have important implications for evolving bundled payment models” for TKA, said Dr. Goodman, a rheumatologist at the Hospital for Special Surgery in New York. “Hospitals should receive commensurate resources to maintain access to total TKA for patients with IA.”
For this analysis, Dr. Goodman and her associates classified IA as a patient with a recorded diagnosis of rheumatoid arthritis, spondyloarthritis, or systemic lupus erythematosus if the patient had also received treatment during the year before surgery with a disease-modifying antirheumatic drug, a biologic agent, or a drug that treats systemic lupus erythematosus.
Complications following TKA became a particular concern to hospitals starting in 2013 when the Centers for Medicare & Medicaid Services began a program that penalized hospitals for outcomes such as excessive readmissions following selected types of hospitalizations and also with recent steps to bundle TKA reimbursement with related 90-day outcomes.
“My concern is to ensure that patients with IA aren’t penalized and can maintain access” to TKA despite recent policy moves by the CMS. Faced with potential disincentives to treat patients with an IA, “hospitals might cherry pick patients,” Dr. Goodman said in an interview. The new findings “are a reason for administrators to argue for patients with IA to come out of the cost bundle.”
Dr. Goodman expressed hope that future policies will better reflect the higher levels of risk faced by patients with an IA undergoing TKA. CMS “is pretty responsive,” she said.
The study used data collected by Humana for about 25 million American health insurance beneficiaries during 2007-2016, which included 137,550 people who underwent a TKA. Of these, 3,067 (2%) met the study’s definition for IA, and 134,483 did not. Most of those who did not meet the definition likely had osteoarthritis, Dr. Goodman said. This low percentage of U.S. TKA patients with IA was consistent with numbers in prior reports.
The researchers calculated the relative risk of the IA patients, compared with all the others, for nine potential complications, including acute MI, pneumonia, sepsis, pulmonary embolism, and death. The complications with significantly higher rates among the IA patients after confounder adjustment were 30-day infections, 30-day transfusions, and 90-day readmissions.
Dr. Goodman had no relevant disclosures.
SOURCE: Richardson S et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 1932.
CHICAGO – Patients with an inflammatory arthritis had significantly higher rates of infections, transfusions, and readmissions following total knee replacement than did patients without inflammatory arthritis in a study of more than 137,000 Americans who underwent this surgery.
A sampling of U.S. patients who underwent total knee arthroplasty (TKA) during 2007-2016 showed that among the small percentage of these patients who had an inflammatory arthritis (IA), the rate of periprosthetic joint or wound infection while hospitalized or out to 30 days after surgery was a statistically significant 64% higher relative to patients without inflammatory arthritis, after adjustment for several demographic and clinical confounders, including recent glucocorticoid treatment, Susan M. Goodman, MD, said at the annual meeting of the American College of Rheumatology. The analysis also showed a statistically significant 46% higher relative rate of hospital readmission for any cause during the 90 days after surgery, and a significant 39% relative increase in blood transfusions during the 30 days after TKA in the IA patients.
“These results have important implications for evolving bundled payment models” for TKA, said Dr. Goodman, a rheumatologist at the Hospital for Special Surgery in New York. “Hospitals should receive commensurate resources to maintain access to total TKA for patients with IA.”
For this analysis, Dr. Goodman and her associates classified IA as a patient with a recorded diagnosis of rheumatoid arthritis, spondyloarthritis, or systemic lupus erythematosus if the patient had also received treatment during the year before surgery with a disease-modifying antirheumatic drug, a biologic agent, or a drug that treats systemic lupus erythematosus.
Complications following TKA became a particular concern to hospitals starting in 2013 when the Centers for Medicare & Medicaid Services began a program that penalized hospitals for outcomes such as excessive readmissions following selected types of hospitalizations and also with recent steps to bundle TKA reimbursement with related 90-day outcomes.
“My concern is to ensure that patients with IA aren’t penalized and can maintain access” to TKA despite recent policy moves by the CMS. Faced with potential disincentives to treat patients with an IA, “hospitals might cherry pick patients,” Dr. Goodman said in an interview. The new findings “are a reason for administrators to argue for patients with IA to come out of the cost bundle.”
Dr. Goodman expressed hope that future policies will better reflect the higher levels of risk faced by patients with an IA undergoing TKA. CMS “is pretty responsive,” she said.
The study used data collected by Humana for about 25 million American health insurance beneficiaries during 2007-2016, which included 137,550 people who underwent a TKA. Of these, 3,067 (2%) met the study’s definition for IA, and 134,483 did not. Most of those who did not meet the definition likely had osteoarthritis, Dr. Goodman said. This low percentage of U.S. TKA patients with IA was consistent with numbers in prior reports.
The researchers calculated the relative risk of the IA patients, compared with all the others, for nine potential complications, including acute MI, pneumonia, sepsis, pulmonary embolism, and death. The complications with significantly higher rates among the IA patients after confounder adjustment were 30-day infections, 30-day transfusions, and 90-day readmissions.
Dr. Goodman had no relevant disclosures.
SOURCE: Richardson S et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 1932.
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point: Complications were more common after total knee arthroplasty in patients with an inflammatory arthritis.
Major finding: Inflammatory arthritis patients had a 64% higher rate of infections after total knee arthroplasty, compared with patients without inflammatory arthritis.
Study details: Data analysis for 137,550 Americans who underwent total knee arthroplasty during 2007-2016.
Disclosures: Dr. Goodman had no relevant disclosures.
Source: Richardson S et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 1932.
Childhood abuse linked with tripled adult SLE incidence
CHICAGO – , in a study of more than 67,000 American nurses.
The results also suggested that development of depression and post-traumatic stress disorder (PTSD) may have been intermediary steps between episodes of childhood abuse and later development of systemic lupus erythematosus (SLE), Candace H. Feldman, MD, said at the annual meeting of the American College of Rheumatology.
These findings suggest the “importance of screening for childhood abuse exposures as well as for depression and PTSD in routine practice,” although Dr. Feldman acknowledged that interventions aimed at treating depression and PTSD have as of now no proven role for mitigating SLE.
The analysis Dr. Feldman and her associates ran on data collected in the Nurses Health Study II also documented a “striking” number of the enrolled women who completed the survey in 2001 and reported a history of abuse when they were 11 years old or younger: 30% of the 67,516 respondents reported a moderate level of abuse, and 24% reported a high level of abuse. An additional 22% reported either no or a very low level of abuse. These numbers suggest that abuse of girls “is very common and probably underreported,” she said in a video interview.
The Nurses Health Study II enrolled more than 116,429 U.S. women in 1989 who were 25-42 years old and had no history of SLE. Recording of incident SLE cases began in 1991 and for this analysis continued for 24 years, through 2015, during which time 94 women developed SLE that was confirmed in a review by two rheumatologists applying the 1997 SLE classification criteria (Arthritis Rheum. 1997 Sept;40[9]:1725. The incidence of SLE was 2.57-fold more common among women who reported a high level of abuse, compared with those who had no or very low abuse, after adjustment for several demographic and clinical confounders, reported Dr. Feldman, a rheumatologist at Brigham and Women’s Hospital in Boston.
“To our knowledge this is the first study to prospectively look at exposure to different forms of childhood abuse and SLE incidence in a general population of women,” she said.
To make the analysis more prospective the researchers also ran a calculation that considered only SLE cases that appeared after completion of the 2001 abuse survey. Using this criterion the incidence was 3.11-fold higher among women who reported a high level of childhood abuse. Further analyses showed that statistically a diagnosis of PTSD accounted for about 23% of the risk for developing SLE, and depression appeared responsible for about 17% of the risk. The analysis also showed no statistically significant link between sexual abuse in childhood or as a teenager and later onset of SLE.
The findings are consistent with prior reports that linked stress to development of various autoimmune diseases, Dr. Feldman noted. She speculated that high childhood stress could cause changes in inflammation, immune function, epigenetics, the autonomic nervous system, and endocrine pathways that could play a role in triggering depression or PTSD, and eventually SLE.
[email protected]
On Twitter @mitchelzoler
SOURCE:Feldman C et al. Arthritis Rheumatol. 2018;70(suppl 10) Abstract 2807.
CHICAGO – , in a study of more than 67,000 American nurses.
The results also suggested that development of depression and post-traumatic stress disorder (PTSD) may have been intermediary steps between episodes of childhood abuse and later development of systemic lupus erythematosus (SLE), Candace H. Feldman, MD, said at the annual meeting of the American College of Rheumatology.
These findings suggest the “importance of screening for childhood abuse exposures as well as for depression and PTSD in routine practice,” although Dr. Feldman acknowledged that interventions aimed at treating depression and PTSD have as of now no proven role for mitigating SLE.
The analysis Dr. Feldman and her associates ran on data collected in the Nurses Health Study II also documented a “striking” number of the enrolled women who completed the survey in 2001 and reported a history of abuse when they were 11 years old or younger: 30% of the 67,516 respondents reported a moderate level of abuse, and 24% reported a high level of abuse. An additional 22% reported either no or a very low level of abuse. These numbers suggest that abuse of girls “is very common and probably underreported,” she said in a video interview.
The Nurses Health Study II enrolled more than 116,429 U.S. women in 1989 who were 25-42 years old and had no history of SLE. Recording of incident SLE cases began in 1991 and for this analysis continued for 24 years, through 2015, during which time 94 women developed SLE that was confirmed in a review by two rheumatologists applying the 1997 SLE classification criteria (Arthritis Rheum. 1997 Sept;40[9]:1725. The incidence of SLE was 2.57-fold more common among women who reported a high level of abuse, compared with those who had no or very low abuse, after adjustment for several demographic and clinical confounders, reported Dr. Feldman, a rheumatologist at Brigham and Women’s Hospital in Boston.
“To our knowledge this is the first study to prospectively look at exposure to different forms of childhood abuse and SLE incidence in a general population of women,” she said.
To make the analysis more prospective the researchers also ran a calculation that considered only SLE cases that appeared after completion of the 2001 abuse survey. Using this criterion the incidence was 3.11-fold higher among women who reported a high level of childhood abuse. Further analyses showed that statistically a diagnosis of PTSD accounted for about 23% of the risk for developing SLE, and depression appeared responsible for about 17% of the risk. The analysis also showed no statistically significant link between sexual abuse in childhood or as a teenager and later onset of SLE.
The findings are consistent with prior reports that linked stress to development of various autoimmune diseases, Dr. Feldman noted. She speculated that high childhood stress could cause changes in inflammation, immune function, epigenetics, the autonomic nervous system, and endocrine pathways that could play a role in triggering depression or PTSD, and eventually SLE.
[email protected]
On Twitter @mitchelzoler
SOURCE:Feldman C et al. Arthritis Rheumatol. 2018;70(suppl 10) Abstract 2807.
CHICAGO – , in a study of more than 67,000 American nurses.
The results also suggested that development of depression and post-traumatic stress disorder (PTSD) may have been intermediary steps between episodes of childhood abuse and later development of systemic lupus erythematosus (SLE), Candace H. Feldman, MD, said at the annual meeting of the American College of Rheumatology.
These findings suggest the “importance of screening for childhood abuse exposures as well as for depression and PTSD in routine practice,” although Dr. Feldman acknowledged that interventions aimed at treating depression and PTSD have as of now no proven role for mitigating SLE.
The analysis Dr. Feldman and her associates ran on data collected in the Nurses Health Study II also documented a “striking” number of the enrolled women who completed the survey in 2001 and reported a history of abuse when they were 11 years old or younger: 30% of the 67,516 respondents reported a moderate level of abuse, and 24% reported a high level of abuse. An additional 22% reported either no or a very low level of abuse. These numbers suggest that abuse of girls “is very common and probably underreported,” she said in a video interview.
The Nurses Health Study II enrolled more than 116,429 U.S. women in 1989 who were 25-42 years old and had no history of SLE. Recording of incident SLE cases began in 1991 and for this analysis continued for 24 years, through 2015, during which time 94 women developed SLE that was confirmed in a review by two rheumatologists applying the 1997 SLE classification criteria (Arthritis Rheum. 1997 Sept;40[9]:1725. The incidence of SLE was 2.57-fold more common among women who reported a high level of abuse, compared with those who had no or very low abuse, after adjustment for several demographic and clinical confounders, reported Dr. Feldman, a rheumatologist at Brigham and Women’s Hospital in Boston.
“To our knowledge this is the first study to prospectively look at exposure to different forms of childhood abuse and SLE incidence in a general population of women,” she said.
To make the analysis more prospective the researchers also ran a calculation that considered only SLE cases that appeared after completion of the 2001 abuse survey. Using this criterion the incidence was 3.11-fold higher among women who reported a high level of childhood abuse. Further analyses showed that statistically a diagnosis of PTSD accounted for about 23% of the risk for developing SLE, and depression appeared responsible for about 17% of the risk. The analysis also showed no statistically significant link between sexual abuse in childhood or as a teenager and later onset of SLE.
The findings are consistent with prior reports that linked stress to development of various autoimmune diseases, Dr. Feldman noted. She speculated that high childhood stress could cause changes in inflammation, immune function, epigenetics, the autonomic nervous system, and endocrine pathways that could play a role in triggering depression or PTSD, and eventually SLE.
[email protected]
On Twitter @mitchelzoler
SOURCE:Feldman C et al. Arthritis Rheumatol. 2018;70(suppl 10) Abstract 2807.
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point: A history of high childhood abuse linked with a nearly three-fold higher incidence of systemic lupus erythematosus during adulthood.
Major finding: The incidence of systemic lupus erythematosus was 2.57-fold higher among women with high childhood abuse compared with unabused women.
Study details: Data from 67,516 women enrolled in the Nurses Health Study II.
Disclosures: Dr. Feldman had no disclosures.
Source: Feldman C et al. Arthritis Rheumatol. 2018;70(suppl 10) Abstract 2807.





















