User login
Mitchel is a reporter for MDedge based in the Philadelphia area. He started with the company in 1992, when it was International Medical News Group (IMNG), and has since covered a range of medical specialties. Mitchel trained as a virologist at Roswell Park Memorial Institute in Buffalo, and then worked briefly as a researcher at Boston Children's Hospital before pivoting to journalism as a AAAS Mass Media Fellow in 1980. His first reporting job was with Science Digest magazine, and from the mid-1980s to early-1990s he was a reporter with Medical World News. @mitchelzoler
Lipidologists welcome bempedoic acid as new lipid-lowering option
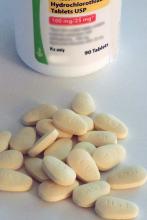
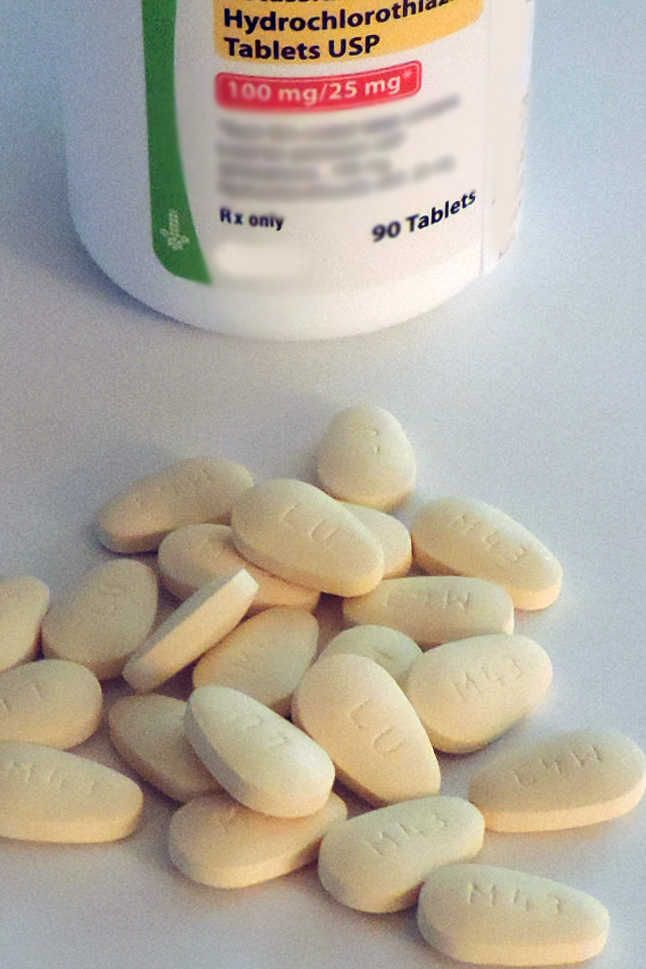
Bempedoic acid, the first agent in a new class of drugs that reduce LDL cholesterol, received Food and Drug Administration approval on Feb. 21 for treating selected hypercholesterolemic patients and is a welcome addition to the medicine cabinet, say lipid experts.
However, it is a tertiary option at least until results from a 14,000 patient clinical-outcome trial of bempedoic acid come out, likely in 2022, they agreed.
“I’m excited to have a new tool in the toolkit for treating high-risk patients, but I will always reach first for the drugs proven to reduce clinical outcomes,” said Erin D. Michos, MD, director of Women’s Cardiovascular Health and associate director of Preventive Cardiology at Johns Hopkins Medicine in Baltimore. That sentiment, shared by other experts, should for the time being relegate bempedoic acid (Nexletol) to a backup role behind statins, ezetimibe, and the PCSK9 inhibitor antibodies that are all now on the U.S. market and all buttressed with evidence of their ability to cut cardiovascular disease death and other CVD outcomes from large outcome studies.
The existing evidence base for bempedoic acid rests primarily two multicenter, randomized, placebo-controlled clinical trials of bempedoic acid in patients with LDL levels above 70 mg/dL while on maximally tolerated lipid-lowering therapy. In CLEAR Harmony, results showed that treatment with bempedoic acid cut LDL-cholesterol levels by an average of 18% more compared with placebo (N Engl J Med 2019;380:1022-32). In CLEAR Wisdom, bempedoic acid reduced LDL cholesterol levels by 17% (JAMA. 2019;322[18]:1780-8).
While those two trials proved the drug’s ability to lower levels of LDL cholesterol, they lacked the power to address whether this effect cut the incidence of CVD events, a question that the CLEAR Outcomes trial aims to answer.
“I believe in the lipid hypothesis, but the main thing we need to see is whether bempedoic acid leads to a meaningful reduction in CVD events. The window for bempedoic acid will remain narrow until we see the outcomes results,” Dr. Michos said in an interview.
Bempedoic acid is a prodrug that’s activated in liver and targets the same cholesterol synthesis pathway as statins by inhibition of ATP-citrate lyase, an enzyme that’s upstream of HMG-CoA reductase, thereby enhancing LDL cholesterol clearance via up-regulation of LDL receptors
.
“,” said Jennifer G. Robinson, MD, professor of epidemiology and medicine and director of the Prevention Intervention Center of the University of Iowa in Iowa City. That would be just a portion of the newly labeled target population. The FDA’s approved label for bempedoic acid cites the drug as an “adjunct to diet and maximally tolerated statin therapy for the treatment of adults with heterozygous familial hypercholesterolemia (HeFH) or established atherosclerotic cardiovascular disease (ASCVD) who require additional lowering of LDL-C.”
The current lack of outcomes evidence for bempedoic acid was not an issue for Robert H. Eckel, MD, an endocrinologist and lipid management specialist at the University of Colorado at Denver in Aurora. Having results from CLEAR Outcomes “may be helpful, but LDL cholesterol lowering in the range where the FDA has indicated using bempedoic acid seems all we need for now,” he said in an interview. Viewing bempedoic acid as potentially useful for both HeFH and ASCVD patients, Dr. Eckel particularly cited the possibility of using the new drug in combination with ezetimibe, another oral, once-daily agent with a moderate but additive effect for cutting LDL cholesterol.
Combined treatment with bempedoic acid and ezetimibe “may be successful in avoiding [using] a PCSK9 inhibitor in some patients, and in particular patients with HeFH or those who are statin intolerant.” But like his colleagues, Dr. Eckel agreed that, for the moment, ezetimibe has an edge over bempedoic acid because of its more extensive evidence base. “If the combination of bempedoic acid and ezetimibe is not needed, the decision [of which one of these to use] needs to depend on the outcome trial results for ezetimibe,” he said. Other factors clinicians could apply if faced with choosing between these two agents include the significant reduction in high-sensitivity C-reactive protein that bempedoic acid produces; the downside that bempedoic acid can cause in some patients an early and persistent rise in serum uric acid levels that can trigger gout flares in patients with a history of gout or at risk for gout; and cost, he said.
Cost is the room-dwelling elephant that colors many decisions about which lipid-lowering drug to use for patients, with options running the price gamut from the generic and uniformly affordable statins and ezetimibe, to the notoriously pricey PCSK9 inhibitors that remain for many patients either prohibitively expensive or hard to get covered by some insurers. Bempedoic acid seems on track to fall somewhere between these two poles, although staff members from Esperion, the company that developed and will market bempedoic acid as Nexletol starting on March 30, declared in a conference call on Feb. 24 that “cost will not be an issue,” for indicated patients prescribed the drug. Company representatives cited a program of coupons, discounts, and rebates they have planned that they anticipate will allow patients who meet the labeled indications to have an out-of-pocket cost for bempedoic acid of “as low as” $10 for a 90-pill supply. They also noted their goal of getting bempedoic acid onto the lowest tier of the Medicare formulary.
How these steps actually play out in the fun house of U.S. prescription drug pricing and preauthorizations remains to be seen. “Out-of-pocket costs are not the real drivers” of drug access, noted Dr. Robinson. “Insurers will likely start with restricted access and prior authorization requirements, just as they did with ezetimibe when it was on patent and prior to having the results from a CVD outcomes trial.” For the time being, bempedoic acid can generally be seen as “expensive ezetimibe,” summed up Dr. Robinson.
Despite that somewhat dismissive characterization, experts are intrigued by the possibility of combining two moderately potent, oral, and safe lipid-lowering drugs in selected patients as a potential alternative to the still financially challenging PCSK9 inhibitors. Combining bempedoic acid and ezetimibe “has a lot of appeal,” said Dr. Michos. “Even though preauthorization has gotten better, it’s still a challenge to get a PCSK9 inhibitor approved.”
Much of her enthusiasm stems from a study reported last year that randomized 301 patients to treatment with bempedoic acid, ezetimibe, or both. The results showed that combined treatment has a similar safety profile to treatment with either drug alone, and produced a cut in LDL cholesterol that was roughly additive for the reductions produced by each drug by itself: Ezetimibe alone cut LDL by about 23%, bempedoic acid alone by about 17%, and the two dosed together once daily resulted in an average 36% drop (Eur J Prev Cardiol. 2019 Jul 29. doi: 10.1177/2047487319864671). The results showed that, “in patients requiring intensive LDL cholesterol lowering, who cannot afford PCSK9 inhibitors, or have statin intolerance, bempedoic acid and ezetimibe are stronger together and can serve as an alternative approach for lipid management in ASCVD prevention,” wrote Dr. Michos and a coauthor in a commentary that appeared with the study results (Eur J Prev Cardiol. 2019 Jul 29. doi: 10.1177/2047487319864672).
The concept of combined bempedoic acid and ezetimibe treatment is so appealing that the bempedoic acid manufacturer, Esperion, has already developed a single-pill formulation of the two drugs that received FDA marketing approval on February 26. A company statement said that marketing of this combined formulation, Nexlizet, will start in July 2020.
Although interest in bempedoic acid seems running high for patients included in the new FDA indication, Dr. Michos and others see possibly greater potential for what would now be off-label use for primary prevention in high-risk patients without HeFH, patients who generally don’t qualify for insurance coverage of a PCSK9 inhibitor.
“Use in primary prevention in [non-HeFH] patients with insufficient lowering of LDL cholesterol wouldn’t surprise me,” but a big concern will be out-of-pocket cost when off-label use precludes insurance coverage or discount eligibility, noted Dr. Eckel. An Esperion spokesperson said that the undiscounted, wholesale acquisition cost for bempedoic acid is expected to be roughly $10/pill, or about $300 for a 30-day supply, positioning it more or less midway between generic statins and ezetimibe and the list price for a PCSk9 inhibitor of roughly $500/month.
“I’m most excited about bempedoic acid in the off-label space, for patients who can’t get approved for a PCSK9 inhibitor, for treating patients with subclinical ASCVD, or really high-risk patients with multiple risk factors including diabetes,” especially when these patients are intolerant of a high-intensity statin regimen, said Dr. Michos. “I have a clinic full of patients” who can’t take their full, indicated dosage of a high-intensity statin, and when those patients also can’t get on treatment with a PCSK9 inhibitor then bempedoic acid will be an important part of their alternative regimen, she explained.
Dr. Michos had no disclosures. Dr. Robinson has received research funding from Esperion and from several other companies, and she has been a consultant to Amgen, Merck, Novartis, Novo Nordisk, Pfizer, Regeneron, and Sanofi. Dr. Eckel has received honoraria from Kowa, Merck, Novo Nordisk, and Sanofi/Regeneron.
This article was updated 2/27/20.
Bempedoic acid, the first agent in a new class of drugs that reduce LDL cholesterol, received Food and Drug Administration approval on Feb. 21 for treating selected hypercholesterolemic patients and is a welcome addition to the medicine cabinet, say lipid experts.
However, it is a tertiary option at least until results from a 14,000 patient clinical-outcome trial of bempedoic acid come out, likely in 2022, they agreed.
“I’m excited to have a new tool in the toolkit for treating high-risk patients, but I will always reach first for the drugs proven to reduce clinical outcomes,” said Erin D. Michos, MD, director of Women’s Cardiovascular Health and associate director of Preventive Cardiology at Johns Hopkins Medicine in Baltimore. That sentiment, shared by other experts, should for the time being relegate bempedoic acid (Nexletol) to a backup role behind statins, ezetimibe, and the PCSK9 inhibitor antibodies that are all now on the U.S. market and all buttressed with evidence of their ability to cut cardiovascular disease death and other CVD outcomes from large outcome studies.
The existing evidence base for bempedoic acid rests primarily two multicenter, randomized, placebo-controlled clinical trials of bempedoic acid in patients with LDL levels above 70 mg/dL while on maximally tolerated lipid-lowering therapy. In CLEAR Harmony, results showed that treatment with bempedoic acid cut LDL-cholesterol levels by an average of 18% more compared with placebo (N Engl J Med 2019;380:1022-32). In CLEAR Wisdom, bempedoic acid reduced LDL cholesterol levels by 17% (JAMA. 2019;322[18]:1780-8).
While those two trials proved the drug’s ability to lower levels of LDL cholesterol, they lacked the power to address whether this effect cut the incidence of CVD events, a question that the CLEAR Outcomes trial aims to answer.
“I believe in the lipid hypothesis, but the main thing we need to see is whether bempedoic acid leads to a meaningful reduction in CVD events. The window for bempedoic acid will remain narrow until we see the outcomes results,” Dr. Michos said in an interview.
Bempedoic acid is a prodrug that’s activated in liver and targets the same cholesterol synthesis pathway as statins by inhibition of ATP-citrate lyase, an enzyme that’s upstream of HMG-CoA reductase, thereby enhancing LDL cholesterol clearance via up-regulation of LDL receptors
.
“,” said Jennifer G. Robinson, MD, professor of epidemiology and medicine and director of the Prevention Intervention Center of the University of Iowa in Iowa City. That would be just a portion of the newly labeled target population. The FDA’s approved label for bempedoic acid cites the drug as an “adjunct to diet and maximally tolerated statin therapy for the treatment of adults with heterozygous familial hypercholesterolemia (HeFH) or established atherosclerotic cardiovascular disease (ASCVD) who require additional lowering of LDL-C.”
The current lack of outcomes evidence for bempedoic acid was not an issue for Robert H. Eckel, MD, an endocrinologist and lipid management specialist at the University of Colorado at Denver in Aurora. Having results from CLEAR Outcomes “may be helpful, but LDL cholesterol lowering in the range where the FDA has indicated using bempedoic acid seems all we need for now,” he said in an interview. Viewing bempedoic acid as potentially useful for both HeFH and ASCVD patients, Dr. Eckel particularly cited the possibility of using the new drug in combination with ezetimibe, another oral, once-daily agent with a moderate but additive effect for cutting LDL cholesterol.
Combined treatment with bempedoic acid and ezetimibe “may be successful in avoiding [using] a PCSK9 inhibitor in some patients, and in particular patients with HeFH or those who are statin intolerant.” But like his colleagues, Dr. Eckel agreed that, for the moment, ezetimibe has an edge over bempedoic acid because of its more extensive evidence base. “If the combination of bempedoic acid and ezetimibe is not needed, the decision [of which one of these to use] needs to depend on the outcome trial results for ezetimibe,” he said. Other factors clinicians could apply if faced with choosing between these two agents include the significant reduction in high-sensitivity C-reactive protein that bempedoic acid produces; the downside that bempedoic acid can cause in some patients an early and persistent rise in serum uric acid levels that can trigger gout flares in patients with a history of gout or at risk for gout; and cost, he said.
Cost is the room-dwelling elephant that colors many decisions about which lipid-lowering drug to use for patients, with options running the price gamut from the generic and uniformly affordable statins and ezetimibe, to the notoriously pricey PCSK9 inhibitors that remain for many patients either prohibitively expensive or hard to get covered by some insurers. Bempedoic acid seems on track to fall somewhere between these two poles, although staff members from Esperion, the company that developed and will market bempedoic acid as Nexletol starting on March 30, declared in a conference call on Feb. 24 that “cost will not be an issue,” for indicated patients prescribed the drug. Company representatives cited a program of coupons, discounts, and rebates they have planned that they anticipate will allow patients who meet the labeled indications to have an out-of-pocket cost for bempedoic acid of “as low as” $10 for a 90-pill supply. They also noted their goal of getting bempedoic acid onto the lowest tier of the Medicare formulary.
How these steps actually play out in the fun house of U.S. prescription drug pricing and preauthorizations remains to be seen. “Out-of-pocket costs are not the real drivers” of drug access, noted Dr. Robinson. “Insurers will likely start with restricted access and prior authorization requirements, just as they did with ezetimibe when it was on patent and prior to having the results from a CVD outcomes trial.” For the time being, bempedoic acid can generally be seen as “expensive ezetimibe,” summed up Dr. Robinson.
Despite that somewhat dismissive characterization, experts are intrigued by the possibility of combining two moderately potent, oral, and safe lipid-lowering drugs in selected patients as a potential alternative to the still financially challenging PCSK9 inhibitors. Combining bempedoic acid and ezetimibe “has a lot of appeal,” said Dr. Michos. “Even though preauthorization has gotten better, it’s still a challenge to get a PCSK9 inhibitor approved.”
Much of her enthusiasm stems from a study reported last year that randomized 301 patients to treatment with bempedoic acid, ezetimibe, or both. The results showed that combined treatment has a similar safety profile to treatment with either drug alone, and produced a cut in LDL cholesterol that was roughly additive for the reductions produced by each drug by itself: Ezetimibe alone cut LDL by about 23%, bempedoic acid alone by about 17%, and the two dosed together once daily resulted in an average 36% drop (Eur J Prev Cardiol. 2019 Jul 29. doi: 10.1177/2047487319864671). The results showed that, “in patients requiring intensive LDL cholesterol lowering, who cannot afford PCSK9 inhibitors, or have statin intolerance, bempedoic acid and ezetimibe are stronger together and can serve as an alternative approach for lipid management in ASCVD prevention,” wrote Dr. Michos and a coauthor in a commentary that appeared with the study results (Eur J Prev Cardiol. 2019 Jul 29. doi: 10.1177/2047487319864672).
The concept of combined bempedoic acid and ezetimibe treatment is so appealing that the bempedoic acid manufacturer, Esperion, has already developed a single-pill formulation of the two drugs that received FDA marketing approval on February 26. A company statement said that marketing of this combined formulation, Nexlizet, will start in July 2020.
Although interest in bempedoic acid seems running high for patients included in the new FDA indication, Dr. Michos and others see possibly greater potential for what would now be off-label use for primary prevention in high-risk patients without HeFH, patients who generally don’t qualify for insurance coverage of a PCSK9 inhibitor.
“Use in primary prevention in [non-HeFH] patients with insufficient lowering of LDL cholesterol wouldn’t surprise me,” but a big concern will be out-of-pocket cost when off-label use precludes insurance coverage or discount eligibility, noted Dr. Eckel. An Esperion spokesperson said that the undiscounted, wholesale acquisition cost for bempedoic acid is expected to be roughly $10/pill, or about $300 for a 30-day supply, positioning it more or less midway between generic statins and ezetimibe and the list price for a PCSk9 inhibitor of roughly $500/month.
“I’m most excited about bempedoic acid in the off-label space, for patients who can’t get approved for a PCSK9 inhibitor, for treating patients with subclinical ASCVD, or really high-risk patients with multiple risk factors including diabetes,” especially when these patients are intolerant of a high-intensity statin regimen, said Dr. Michos. “I have a clinic full of patients” who can’t take their full, indicated dosage of a high-intensity statin, and when those patients also can’t get on treatment with a PCSK9 inhibitor then bempedoic acid will be an important part of their alternative regimen, she explained.
Dr. Michos had no disclosures. Dr. Robinson has received research funding from Esperion and from several other companies, and she has been a consultant to Amgen, Merck, Novartis, Novo Nordisk, Pfizer, Regeneron, and Sanofi. Dr. Eckel has received honoraria from Kowa, Merck, Novo Nordisk, and Sanofi/Regeneron.
This article was updated 2/27/20.
Bempedoic acid, the first agent in a new class of drugs that reduce LDL cholesterol, received Food and Drug Administration approval on Feb. 21 for treating selected hypercholesterolemic patients and is a welcome addition to the medicine cabinet, say lipid experts.
However, it is a tertiary option at least until results from a 14,000 patient clinical-outcome trial of bempedoic acid come out, likely in 2022, they agreed.
“I’m excited to have a new tool in the toolkit for treating high-risk patients, but I will always reach first for the drugs proven to reduce clinical outcomes,” said Erin D. Michos, MD, director of Women’s Cardiovascular Health and associate director of Preventive Cardiology at Johns Hopkins Medicine in Baltimore. That sentiment, shared by other experts, should for the time being relegate bempedoic acid (Nexletol) to a backup role behind statins, ezetimibe, and the PCSK9 inhibitor antibodies that are all now on the U.S. market and all buttressed with evidence of their ability to cut cardiovascular disease death and other CVD outcomes from large outcome studies.
The existing evidence base for bempedoic acid rests primarily two multicenter, randomized, placebo-controlled clinical trials of bempedoic acid in patients with LDL levels above 70 mg/dL while on maximally tolerated lipid-lowering therapy. In CLEAR Harmony, results showed that treatment with bempedoic acid cut LDL-cholesterol levels by an average of 18% more compared with placebo (N Engl J Med 2019;380:1022-32). In CLEAR Wisdom, bempedoic acid reduced LDL cholesterol levels by 17% (JAMA. 2019;322[18]:1780-8).
While those two trials proved the drug’s ability to lower levels of LDL cholesterol, they lacked the power to address whether this effect cut the incidence of CVD events, a question that the CLEAR Outcomes trial aims to answer.
“I believe in the lipid hypothesis, but the main thing we need to see is whether bempedoic acid leads to a meaningful reduction in CVD events. The window for bempedoic acid will remain narrow until we see the outcomes results,” Dr. Michos said in an interview.
Bempedoic acid is a prodrug that’s activated in liver and targets the same cholesterol synthesis pathway as statins by inhibition of ATP-citrate lyase, an enzyme that’s upstream of HMG-CoA reductase, thereby enhancing LDL cholesterol clearance via up-regulation of LDL receptors
.
“,” said Jennifer G. Robinson, MD, professor of epidemiology and medicine and director of the Prevention Intervention Center of the University of Iowa in Iowa City. That would be just a portion of the newly labeled target population. The FDA’s approved label for bempedoic acid cites the drug as an “adjunct to diet and maximally tolerated statin therapy for the treatment of adults with heterozygous familial hypercholesterolemia (HeFH) or established atherosclerotic cardiovascular disease (ASCVD) who require additional lowering of LDL-C.”
The current lack of outcomes evidence for bempedoic acid was not an issue for Robert H. Eckel, MD, an endocrinologist and lipid management specialist at the University of Colorado at Denver in Aurora. Having results from CLEAR Outcomes “may be helpful, but LDL cholesterol lowering in the range where the FDA has indicated using bempedoic acid seems all we need for now,” he said in an interview. Viewing bempedoic acid as potentially useful for both HeFH and ASCVD patients, Dr. Eckel particularly cited the possibility of using the new drug in combination with ezetimibe, another oral, once-daily agent with a moderate but additive effect for cutting LDL cholesterol.
Combined treatment with bempedoic acid and ezetimibe “may be successful in avoiding [using] a PCSK9 inhibitor in some patients, and in particular patients with HeFH or those who are statin intolerant.” But like his colleagues, Dr. Eckel agreed that, for the moment, ezetimibe has an edge over bempedoic acid because of its more extensive evidence base. “If the combination of bempedoic acid and ezetimibe is not needed, the decision [of which one of these to use] needs to depend on the outcome trial results for ezetimibe,” he said. Other factors clinicians could apply if faced with choosing between these two agents include the significant reduction in high-sensitivity C-reactive protein that bempedoic acid produces; the downside that bempedoic acid can cause in some patients an early and persistent rise in serum uric acid levels that can trigger gout flares in patients with a history of gout or at risk for gout; and cost, he said.
Cost is the room-dwelling elephant that colors many decisions about which lipid-lowering drug to use for patients, with options running the price gamut from the generic and uniformly affordable statins and ezetimibe, to the notoriously pricey PCSK9 inhibitors that remain for many patients either prohibitively expensive or hard to get covered by some insurers. Bempedoic acid seems on track to fall somewhere between these two poles, although staff members from Esperion, the company that developed and will market bempedoic acid as Nexletol starting on March 30, declared in a conference call on Feb. 24 that “cost will not be an issue,” for indicated patients prescribed the drug. Company representatives cited a program of coupons, discounts, and rebates they have planned that they anticipate will allow patients who meet the labeled indications to have an out-of-pocket cost for bempedoic acid of “as low as” $10 for a 90-pill supply. They also noted their goal of getting bempedoic acid onto the lowest tier of the Medicare formulary.
How these steps actually play out in the fun house of U.S. prescription drug pricing and preauthorizations remains to be seen. “Out-of-pocket costs are not the real drivers” of drug access, noted Dr. Robinson. “Insurers will likely start with restricted access and prior authorization requirements, just as they did with ezetimibe when it was on patent and prior to having the results from a CVD outcomes trial.” For the time being, bempedoic acid can generally be seen as “expensive ezetimibe,” summed up Dr. Robinson.
Despite that somewhat dismissive characterization, experts are intrigued by the possibility of combining two moderately potent, oral, and safe lipid-lowering drugs in selected patients as a potential alternative to the still financially challenging PCSK9 inhibitors. Combining bempedoic acid and ezetimibe “has a lot of appeal,” said Dr. Michos. “Even though preauthorization has gotten better, it’s still a challenge to get a PCSK9 inhibitor approved.”
Much of her enthusiasm stems from a study reported last year that randomized 301 patients to treatment with bempedoic acid, ezetimibe, or both. The results showed that combined treatment has a similar safety profile to treatment with either drug alone, and produced a cut in LDL cholesterol that was roughly additive for the reductions produced by each drug by itself: Ezetimibe alone cut LDL by about 23%, bempedoic acid alone by about 17%, and the two dosed together once daily resulted in an average 36% drop (Eur J Prev Cardiol. 2019 Jul 29. doi: 10.1177/2047487319864671). The results showed that, “in patients requiring intensive LDL cholesterol lowering, who cannot afford PCSK9 inhibitors, or have statin intolerance, bempedoic acid and ezetimibe are stronger together and can serve as an alternative approach for lipid management in ASCVD prevention,” wrote Dr. Michos and a coauthor in a commentary that appeared with the study results (Eur J Prev Cardiol. 2019 Jul 29. doi: 10.1177/2047487319864672).
The concept of combined bempedoic acid and ezetimibe treatment is so appealing that the bempedoic acid manufacturer, Esperion, has already developed a single-pill formulation of the two drugs that received FDA marketing approval on February 26. A company statement said that marketing of this combined formulation, Nexlizet, will start in July 2020.
Although interest in bempedoic acid seems running high for patients included in the new FDA indication, Dr. Michos and others see possibly greater potential for what would now be off-label use for primary prevention in high-risk patients without HeFH, patients who generally don’t qualify for insurance coverage of a PCSK9 inhibitor.
“Use in primary prevention in [non-HeFH] patients with insufficient lowering of LDL cholesterol wouldn’t surprise me,” but a big concern will be out-of-pocket cost when off-label use precludes insurance coverage or discount eligibility, noted Dr. Eckel. An Esperion spokesperson said that the undiscounted, wholesale acquisition cost for bempedoic acid is expected to be roughly $10/pill, or about $300 for a 30-day supply, positioning it more or less midway between generic statins and ezetimibe and the list price for a PCSk9 inhibitor of roughly $500/month.
“I’m most excited about bempedoic acid in the off-label space, for patients who can’t get approved for a PCSK9 inhibitor, for treating patients with subclinical ASCVD, or really high-risk patients with multiple risk factors including diabetes,” especially when these patients are intolerant of a high-intensity statin regimen, said Dr. Michos. “I have a clinic full of patients” who can’t take their full, indicated dosage of a high-intensity statin, and when those patients also can’t get on treatment with a PCSK9 inhibitor then bempedoic acid will be an important part of their alternative regimen, she explained.
Dr. Michos had no disclosures. Dr. Robinson has received research funding from Esperion and from several other companies, and she has been a consultant to Amgen, Merck, Novartis, Novo Nordisk, Pfizer, Regeneron, and Sanofi. Dr. Eckel has received honoraria from Kowa, Merck, Novo Nordisk, and Sanofi/Regeneron.
This article was updated 2/27/20.
Higher endovascular thrombectomy volumes yield better stroke outcomes
LOS ANGELES – Higher case volumes matter for getting better outcomes in acute ischemic stroke patients treated with endovascular thrombectomy, according to data from more than 13,000 Medicare patients treated during 2016 and 2017.
That’s hardly surprising, given that it’s consistent with what’s already been reported for several other types of endovascular and transcatheter procedures: The more cases a center or individual proceduralist performs, the better their patients do. Routine use of endovascular thrombectomy to treat selected acute ischemic stroke patients is a new-enough paradigm that until now few reports have come out that looked at this issue (Stroke. 2019 May;50[5]:1178-83).
The new analysis of Medicare data “is one of the first contemporary studies of the volume-outcome relationship in endovascular thrombectomy,” Laura K. Stein, MD, said at the International Stroke Conference sponsored by the American Heart Association. The analysis showed that, when the researchers adjusted the Medicare data to better reflect overall case volumes (Medicare patients represent just 59% of all endovascular thrombectomies performed on U.S. acute ischemic stroke patients), the minimum case number for a stroke center to have statistically better in-hospital survival than lower volume centers was 24 cases/year, and 29 cases/year to have a statistically significant higher rate of “good” outcomes than lower-volume centers, reported Dr. Stein, a stroke neurologist with the Mount Sinai Health System in New York. For individual proceduralists, the minimum, adjusted case number to have statistically better acute patient survival was 4 cases/year, and 19 cases/year to have a statistically better rate of good outcomes.
For this analysis, good outcomes were defined as cases when patients left the hospital following their acute care and returned home with either self care or a home health care service, and also patients discharged to rehabilitation. “Bad” outcomes for this analysis were discharges to a skilled nursing facility or hospice, as well as patients who died during their acute hospitalization.
The analyses also showed no plateau to the volume effect for any of the four parameters examined: in-hospital mortality by center and by proceduralist, and the rates of good outcomes by center and by proceduralist. For each of these measures, as case volume increased above the minimum number needed to produce statistically better outcomes, the rate of good outcomes continued to steadily rise and acute mortality continued to steadily fall.
The study run by Dr. Stein and associates used data collected by the Center for Medicare & Medicaid Services on 13,311 Medicare patients who underwent endovascular thrombectomy for acute ischemic stroke at any of 641 U.S. hospitals and received treatment from any of 2,754 thrombectomy proceduralists. Outcomes rated as good occurred in 56% of the patients. The statistical adjustments that the researchers applied to calculate the incremental effect of increasing case volume took into account the variables of patient age, sex, and comorbidities measured by the Charlson Comorbidity Index.
The analysis also showed that, during this 2-year period, the average number of endovascular thrombectomy cases among Medicare patients was just under 21 cases per center, with a range of 1-160 cases; for individual proceduralists, the average was just under 5 cases, with a range of 1-82 cases.
The 19 case/year volume minimum that the analysis identified for an individual proceduralist to have a statistically significant higher rate of good outcomes, compared with lower-volume proceduralists, came close to the 15 cases/year minimum set by the Joint Commission in 2019 for individual operators at centers seeking accreditation from the Joint Commission as either a Thrombectomy-Capable Stroke Center or a Comprehensive Stroke Center. The CMS has not yet set thrombectomy case-load requirements for centers or operators to qualify for Medicare reimbursements, although CMS has set such standards for other endovascular procedures, such as transcatheter aortic valve replacement. When setting such standards, CMS has cited its need to balance the better outcomes produced by higher-volume centers against a societal interest in facilitating access to vital medical services, a balance that Dr. Stein also highlighted in her talk.
“We want to optimize access as well as outcomes for every patient,” she said. “These data support certification volume standards,” but they are “in no way an argument for limiting access based on volume.”
Dr. Stein had no disclosures.
SOURCE: Stein LK et al. ISC 2020, Abstract LB11.
The results reported by Dr. Stein raise issues about balancing the access to certain therapies with the outcomes of those therapies. Having procedures like endovascular thrombectomy for acute ischemic stroke done primarily at high-volume centers might improve procedural outcomes, but having more centers offering this treatment across wider geographical areas would make this treatment more broadly available to more people.
For endovascular thrombectomy, center volume and experience may be much more important than proceduralist volume because having a smoothly functioning system in place is so important for rapid stroke assessment and treatment. It’s also important for programs to provide experienced and comprehensive postthrombectomy care. Success in endovascular thrombectomy involves much more than just taking a clot out. It means quickly and smoothly moving patients through the steps that precede thrombectomy and then following the intervention with a range of services that optimize recovery.
Ashutosh P. Jadhav, MD, PhD , is director of the comprehensive stroke center at the University of Pittsburgh. He had no relevant disclosures. He made these comments in an interview.
The results reported by Dr. Stein raise issues about balancing the access to certain therapies with the outcomes of those therapies. Having procedures like endovascular thrombectomy for acute ischemic stroke done primarily at high-volume centers might improve procedural outcomes, but having more centers offering this treatment across wider geographical areas would make this treatment more broadly available to more people.
For endovascular thrombectomy, center volume and experience may be much more important than proceduralist volume because having a smoothly functioning system in place is so important for rapid stroke assessment and treatment. It’s also important for programs to provide experienced and comprehensive postthrombectomy care. Success in endovascular thrombectomy involves much more than just taking a clot out. It means quickly and smoothly moving patients through the steps that precede thrombectomy and then following the intervention with a range of services that optimize recovery.
Ashutosh P. Jadhav, MD, PhD , is director of the comprehensive stroke center at the University of Pittsburgh. He had no relevant disclosures. He made these comments in an interview.
The results reported by Dr. Stein raise issues about balancing the access to certain therapies with the outcomes of those therapies. Having procedures like endovascular thrombectomy for acute ischemic stroke done primarily at high-volume centers might improve procedural outcomes, but having more centers offering this treatment across wider geographical areas would make this treatment more broadly available to more people.
For endovascular thrombectomy, center volume and experience may be much more important than proceduralist volume because having a smoothly functioning system in place is so important for rapid stroke assessment and treatment. It’s also important for programs to provide experienced and comprehensive postthrombectomy care. Success in endovascular thrombectomy involves much more than just taking a clot out. It means quickly and smoothly moving patients through the steps that precede thrombectomy and then following the intervention with a range of services that optimize recovery.
Ashutosh P. Jadhav, MD, PhD , is director of the comprehensive stroke center at the University of Pittsburgh. He had no relevant disclosures. He made these comments in an interview.
LOS ANGELES – Higher case volumes matter for getting better outcomes in acute ischemic stroke patients treated with endovascular thrombectomy, according to data from more than 13,000 Medicare patients treated during 2016 and 2017.
That’s hardly surprising, given that it’s consistent with what’s already been reported for several other types of endovascular and transcatheter procedures: The more cases a center or individual proceduralist performs, the better their patients do. Routine use of endovascular thrombectomy to treat selected acute ischemic stroke patients is a new-enough paradigm that until now few reports have come out that looked at this issue (Stroke. 2019 May;50[5]:1178-83).
The new analysis of Medicare data “is one of the first contemporary studies of the volume-outcome relationship in endovascular thrombectomy,” Laura K. Stein, MD, said at the International Stroke Conference sponsored by the American Heart Association. The analysis showed that, when the researchers adjusted the Medicare data to better reflect overall case volumes (Medicare patients represent just 59% of all endovascular thrombectomies performed on U.S. acute ischemic stroke patients), the minimum case number for a stroke center to have statistically better in-hospital survival than lower volume centers was 24 cases/year, and 29 cases/year to have a statistically significant higher rate of “good” outcomes than lower-volume centers, reported Dr. Stein, a stroke neurologist with the Mount Sinai Health System in New York. For individual proceduralists, the minimum, adjusted case number to have statistically better acute patient survival was 4 cases/year, and 19 cases/year to have a statistically better rate of good outcomes.
For this analysis, good outcomes were defined as cases when patients left the hospital following their acute care and returned home with either self care or a home health care service, and also patients discharged to rehabilitation. “Bad” outcomes for this analysis were discharges to a skilled nursing facility or hospice, as well as patients who died during their acute hospitalization.
The analyses also showed no plateau to the volume effect for any of the four parameters examined: in-hospital mortality by center and by proceduralist, and the rates of good outcomes by center and by proceduralist. For each of these measures, as case volume increased above the minimum number needed to produce statistically better outcomes, the rate of good outcomes continued to steadily rise and acute mortality continued to steadily fall.
The study run by Dr. Stein and associates used data collected by the Center for Medicare & Medicaid Services on 13,311 Medicare patients who underwent endovascular thrombectomy for acute ischemic stroke at any of 641 U.S. hospitals and received treatment from any of 2,754 thrombectomy proceduralists. Outcomes rated as good occurred in 56% of the patients. The statistical adjustments that the researchers applied to calculate the incremental effect of increasing case volume took into account the variables of patient age, sex, and comorbidities measured by the Charlson Comorbidity Index.
The analysis also showed that, during this 2-year period, the average number of endovascular thrombectomy cases among Medicare patients was just under 21 cases per center, with a range of 1-160 cases; for individual proceduralists, the average was just under 5 cases, with a range of 1-82 cases.
The 19 case/year volume minimum that the analysis identified for an individual proceduralist to have a statistically significant higher rate of good outcomes, compared with lower-volume proceduralists, came close to the 15 cases/year minimum set by the Joint Commission in 2019 for individual operators at centers seeking accreditation from the Joint Commission as either a Thrombectomy-Capable Stroke Center or a Comprehensive Stroke Center. The CMS has not yet set thrombectomy case-load requirements for centers or operators to qualify for Medicare reimbursements, although CMS has set such standards for other endovascular procedures, such as transcatheter aortic valve replacement. When setting such standards, CMS has cited its need to balance the better outcomes produced by higher-volume centers against a societal interest in facilitating access to vital medical services, a balance that Dr. Stein also highlighted in her talk.
“We want to optimize access as well as outcomes for every patient,” she said. “These data support certification volume standards,” but they are “in no way an argument for limiting access based on volume.”
Dr. Stein had no disclosures.
SOURCE: Stein LK et al. ISC 2020, Abstract LB11.
LOS ANGELES – Higher case volumes matter for getting better outcomes in acute ischemic stroke patients treated with endovascular thrombectomy, according to data from more than 13,000 Medicare patients treated during 2016 and 2017.
That’s hardly surprising, given that it’s consistent with what’s already been reported for several other types of endovascular and transcatheter procedures: The more cases a center or individual proceduralist performs, the better their patients do. Routine use of endovascular thrombectomy to treat selected acute ischemic stroke patients is a new-enough paradigm that until now few reports have come out that looked at this issue (Stroke. 2019 May;50[5]:1178-83).
The new analysis of Medicare data “is one of the first contemporary studies of the volume-outcome relationship in endovascular thrombectomy,” Laura K. Stein, MD, said at the International Stroke Conference sponsored by the American Heart Association. The analysis showed that, when the researchers adjusted the Medicare data to better reflect overall case volumes (Medicare patients represent just 59% of all endovascular thrombectomies performed on U.S. acute ischemic stroke patients), the minimum case number for a stroke center to have statistically better in-hospital survival than lower volume centers was 24 cases/year, and 29 cases/year to have a statistically significant higher rate of “good” outcomes than lower-volume centers, reported Dr. Stein, a stroke neurologist with the Mount Sinai Health System in New York. For individual proceduralists, the minimum, adjusted case number to have statistically better acute patient survival was 4 cases/year, and 19 cases/year to have a statistically better rate of good outcomes.
For this analysis, good outcomes were defined as cases when patients left the hospital following their acute care and returned home with either self care or a home health care service, and also patients discharged to rehabilitation. “Bad” outcomes for this analysis were discharges to a skilled nursing facility or hospice, as well as patients who died during their acute hospitalization.
The analyses also showed no plateau to the volume effect for any of the four parameters examined: in-hospital mortality by center and by proceduralist, and the rates of good outcomes by center and by proceduralist. For each of these measures, as case volume increased above the minimum number needed to produce statistically better outcomes, the rate of good outcomes continued to steadily rise and acute mortality continued to steadily fall.
The study run by Dr. Stein and associates used data collected by the Center for Medicare & Medicaid Services on 13,311 Medicare patients who underwent endovascular thrombectomy for acute ischemic stroke at any of 641 U.S. hospitals and received treatment from any of 2,754 thrombectomy proceduralists. Outcomes rated as good occurred in 56% of the patients. The statistical adjustments that the researchers applied to calculate the incremental effect of increasing case volume took into account the variables of patient age, sex, and comorbidities measured by the Charlson Comorbidity Index.
The analysis also showed that, during this 2-year period, the average number of endovascular thrombectomy cases among Medicare patients was just under 21 cases per center, with a range of 1-160 cases; for individual proceduralists, the average was just under 5 cases, with a range of 1-82 cases.
The 19 case/year volume minimum that the analysis identified for an individual proceduralist to have a statistically significant higher rate of good outcomes, compared with lower-volume proceduralists, came close to the 15 cases/year minimum set by the Joint Commission in 2019 for individual operators at centers seeking accreditation from the Joint Commission as either a Thrombectomy-Capable Stroke Center or a Comprehensive Stroke Center. The CMS has not yet set thrombectomy case-load requirements for centers or operators to qualify for Medicare reimbursements, although CMS has set such standards for other endovascular procedures, such as transcatheter aortic valve replacement. When setting such standards, CMS has cited its need to balance the better outcomes produced by higher-volume centers against a societal interest in facilitating access to vital medical services, a balance that Dr. Stein also highlighted in her talk.
“We want to optimize access as well as outcomes for every patient,” she said. “These data support certification volume standards,” but they are “in no way an argument for limiting access based on volume.”
Dr. Stein had no disclosures.
SOURCE: Stein LK et al. ISC 2020, Abstract LB11.
REPORTING FROM ISC 2020
Carotid endarterectomy surpasses stenting in elderly, asymptomatic patients
LOS ANGELES – Carotid artery stenting in older, asymptomatic patients with severe carotid artery stenosis is, in general, as bad an idea as it has already proven to be in symptomatic patients, with a multifold increase in adverse short- and mid-term outcomes, compared with similar older, asymptomatic patients who underwent endarterectomy, according to a combined-study analysis with more than 2,500 patients.
The risk for poor outcomes in patients with severe but asymptomatic carotid artery disease who underwent carotid artery stenting (CAS), compared with patients who instead underwent carotid endarterectomy (CEA) “abruptly increased around age 75,” in an analysis that combined data from the two major, published, randomized trials that compared these two interventions in this patient population, Jenifer H. Voeks, PhD said at the International Stroke Conference sponsored by the American Heart Association.
These results “largely mirror” the findings from a similar combined analysis of data from four major, randomized trials that compared CEA and CAS in patients with symptomatic carotid disease, she noted (Lancet. 2016 Mar 26;387[10025]:1305-11). The new findings in an expanded population of asymptomatic patients derived from two separate studies showed that, in patients aged 70 years or less, “CAS appears to be a reasonable alternative to CEA, but above age 70, and certainly above age 75, age-related risk factors such as cerebrovascular anatomy and underlying cerebral pathology should be carefully considered before selecting patients for CAS,” said Dr. Voeks, a neurology researcher at the Medical University of South Carolina, Charleston. Many experts also believe that, for asymptomatic patients, intensive medical management may have returned as an alternative to either of these invasive approaches for treating severe carotid stenosis and has achieved a level of equipoise that led to the launch of CREST 2 (Carotid Revascularization and Medical Management for Asymptomatic Carotid Stenosis Trial). CREST 2 is comparing CEA and CAS with medical management, and is scheduled to report results in 2021.
The data for this analysis in asymptomatic patients came from the first CREST (Carotid Revascularization Endarterectomy Versus Stenting Trial; N Engl J Med. 2010 Jul 1;363[1]:11-23), which included 1,181 asymptomatic patients (nearly half the total enrollment, with symptomatic patients making up the balance) and had no age ceiling, as well as all 1,453 patients from the ACT 1 trial, which enrolled exclusively asymptomatic patients and limited enrollment to patients aged 79 years or less (N Engl J Med. 2016 Mar 17;374[11]: 1011-20). Because the maximum age of patients in ACT 1 was 79 years, for this analysis Dr. Voeks and associates only included the 1,091 asymptomatic CREST patients who also were within the same age ceiling. The resulting cohort of 2,544 included 1,637 patients who underwent CAS and 907 who underwent CEA (because of a 3:1 randomization ratio in ACT 1), creating the largest data set to compare CAS and CEA by age in asymptomatic patients, Dr. Voeks noted. When subdivided by age, 30% of the cohort was younger that 65 years, 54% were 65-74, and 16% were 75-79.
The primary outcome the researchers used for their analysis was the combined incidence of periprocedural stroke, MI, or death, plus the incidence of ipsilateral stroke during 4 years of follow-up post procedure. Among patients who underwent CAS, this outcome occurred in roughly 9% of patients aged 75-79 years and in about 3% of those younger than 65 years, a hazard ratio of 2.9 that was statistically significant. In contrast, the incidence of the primary outcome among patients aged 65-74 years was just 30% higher, compared with patients aged less than 65 years, a difference that was not statistically significant.
Patients who underwent CEA showed no similar relationship between age and outcome. The incidence of the primary outcome among the CEA patients was roughly the same, about 3.5%, regardless of their age.
A second analysis that considered age as a continuous variable showed a sharply spiked increase in the risk for CAS patients, compared with CEA patients once they reached about age 73-75 years. Until about age 72, the rate of the primary outcome was nearly the same regardless of whether patients underwent CAS or CEA, but the risk for adverse outcomes rose “steeply” starting at about age 75 so that by age 79 the rate of the primary outcome approached 300% higher among the CAS patients compared with CEA patients, Dr. Voeks said.
She cautioned that the analysis included just 115 total primary-outcome events, which makes the incidence rate estimates somewhat imprecise, and that the data reflect outcomes in patients who were treated more than a decade ago, but these data remain the only reported results from large randomized trials that compared CAS and CEA in asymptomatic patients.
Dr. Voeks reported no disclosures.
SOURCE: Voeks JH al. Stroke. 2020 Feb 12;51[suppl 1], Abstract 70.
The role for carotid intervention in asymptomatic patients with severe carotid stenosis, usually defined as a stenosis that obstructs at least 70% of the carotid lumen, is controversial right now because intensive medical management has not been compared with invasive treatments, such as carotid endarterectomy and carotid stenting, for well over a decade. New drugs and new regimens have become treatment options for patients with advanced atherosclerotic carotid artery disease, and this has returned us to a state of equipoise for medical versus interventional management. That’s the premise behind CREST 2 (Carotid Revascularization and Medical Management for Asymptomatic Carotid Stenosis Trial), which is comparing medical treatment against endarterectomy and against carotid stenting in a randomized study. The results may be available in 2021.
The new findings are very important for helping patients and their families make informed decisions. CAS is often perceived as the safer option for older patients because it is less traumatic and invasive than CEA. The data that Dr. Voeks reported show once again that this intuitive impression about CAS in the elderly is belied by the evidence. But the findings also require cautious interpretation because they came from a post hoc, subgroup analysis.
Mai N. Nguyen-Huynh, MD , is a vascular neurologist with Kaiser Permanente Northern California in Oakland. She had no relevant disclosures. She made these comments in an interview.
The role for carotid intervention in asymptomatic patients with severe carotid stenosis, usually defined as a stenosis that obstructs at least 70% of the carotid lumen, is controversial right now because intensive medical management has not been compared with invasive treatments, such as carotid endarterectomy and carotid stenting, for well over a decade. New drugs and new regimens have become treatment options for patients with advanced atherosclerotic carotid artery disease, and this has returned us to a state of equipoise for medical versus interventional management. That’s the premise behind CREST 2 (Carotid Revascularization and Medical Management for Asymptomatic Carotid Stenosis Trial), which is comparing medical treatment against endarterectomy and against carotid stenting in a randomized study. The results may be available in 2021.
The new findings are very important for helping patients and their families make informed decisions. CAS is often perceived as the safer option for older patients because it is less traumatic and invasive than CEA. The data that Dr. Voeks reported show once again that this intuitive impression about CAS in the elderly is belied by the evidence. But the findings also require cautious interpretation because they came from a post hoc, subgroup analysis.
Mai N. Nguyen-Huynh, MD , is a vascular neurologist with Kaiser Permanente Northern California in Oakland. She had no relevant disclosures. She made these comments in an interview.
The role for carotid intervention in asymptomatic patients with severe carotid stenosis, usually defined as a stenosis that obstructs at least 70% of the carotid lumen, is controversial right now because intensive medical management has not been compared with invasive treatments, such as carotid endarterectomy and carotid stenting, for well over a decade. New drugs and new regimens have become treatment options for patients with advanced atherosclerotic carotid artery disease, and this has returned us to a state of equipoise for medical versus interventional management. That’s the premise behind CREST 2 (Carotid Revascularization and Medical Management for Asymptomatic Carotid Stenosis Trial), which is comparing medical treatment against endarterectomy and against carotid stenting in a randomized study. The results may be available in 2021.
The new findings are very important for helping patients and their families make informed decisions. CAS is often perceived as the safer option for older patients because it is less traumatic and invasive than CEA. The data that Dr. Voeks reported show once again that this intuitive impression about CAS in the elderly is belied by the evidence. But the findings also require cautious interpretation because they came from a post hoc, subgroup analysis.
Mai N. Nguyen-Huynh, MD , is a vascular neurologist with Kaiser Permanente Northern California in Oakland. She had no relevant disclosures. She made these comments in an interview.
LOS ANGELES – Carotid artery stenting in older, asymptomatic patients with severe carotid artery stenosis is, in general, as bad an idea as it has already proven to be in symptomatic patients, with a multifold increase in adverse short- and mid-term outcomes, compared with similar older, asymptomatic patients who underwent endarterectomy, according to a combined-study analysis with more than 2,500 patients.
The risk for poor outcomes in patients with severe but asymptomatic carotid artery disease who underwent carotid artery stenting (CAS), compared with patients who instead underwent carotid endarterectomy (CEA) “abruptly increased around age 75,” in an analysis that combined data from the two major, published, randomized trials that compared these two interventions in this patient population, Jenifer H. Voeks, PhD said at the International Stroke Conference sponsored by the American Heart Association.
These results “largely mirror” the findings from a similar combined analysis of data from four major, randomized trials that compared CEA and CAS in patients with symptomatic carotid disease, she noted (Lancet. 2016 Mar 26;387[10025]:1305-11). The new findings in an expanded population of asymptomatic patients derived from two separate studies showed that, in patients aged 70 years or less, “CAS appears to be a reasonable alternative to CEA, but above age 70, and certainly above age 75, age-related risk factors such as cerebrovascular anatomy and underlying cerebral pathology should be carefully considered before selecting patients for CAS,” said Dr. Voeks, a neurology researcher at the Medical University of South Carolina, Charleston. Many experts also believe that, for asymptomatic patients, intensive medical management may have returned as an alternative to either of these invasive approaches for treating severe carotid stenosis and has achieved a level of equipoise that led to the launch of CREST 2 (Carotid Revascularization and Medical Management for Asymptomatic Carotid Stenosis Trial). CREST 2 is comparing CEA and CAS with medical management, and is scheduled to report results in 2021.
The data for this analysis in asymptomatic patients came from the first CREST (Carotid Revascularization Endarterectomy Versus Stenting Trial; N Engl J Med. 2010 Jul 1;363[1]:11-23), which included 1,181 asymptomatic patients (nearly half the total enrollment, with symptomatic patients making up the balance) and had no age ceiling, as well as all 1,453 patients from the ACT 1 trial, which enrolled exclusively asymptomatic patients and limited enrollment to patients aged 79 years or less (N Engl J Med. 2016 Mar 17;374[11]: 1011-20). Because the maximum age of patients in ACT 1 was 79 years, for this analysis Dr. Voeks and associates only included the 1,091 asymptomatic CREST patients who also were within the same age ceiling. The resulting cohort of 2,544 included 1,637 patients who underwent CAS and 907 who underwent CEA (because of a 3:1 randomization ratio in ACT 1), creating the largest data set to compare CAS and CEA by age in asymptomatic patients, Dr. Voeks noted. When subdivided by age, 30% of the cohort was younger that 65 years, 54% were 65-74, and 16% were 75-79.
The primary outcome the researchers used for their analysis was the combined incidence of periprocedural stroke, MI, or death, plus the incidence of ipsilateral stroke during 4 years of follow-up post procedure. Among patients who underwent CAS, this outcome occurred in roughly 9% of patients aged 75-79 years and in about 3% of those younger than 65 years, a hazard ratio of 2.9 that was statistically significant. In contrast, the incidence of the primary outcome among patients aged 65-74 years was just 30% higher, compared with patients aged less than 65 years, a difference that was not statistically significant.
Patients who underwent CEA showed no similar relationship between age and outcome. The incidence of the primary outcome among the CEA patients was roughly the same, about 3.5%, regardless of their age.
A second analysis that considered age as a continuous variable showed a sharply spiked increase in the risk for CAS patients, compared with CEA patients once they reached about age 73-75 years. Until about age 72, the rate of the primary outcome was nearly the same regardless of whether patients underwent CAS or CEA, but the risk for adverse outcomes rose “steeply” starting at about age 75 so that by age 79 the rate of the primary outcome approached 300% higher among the CAS patients compared with CEA patients, Dr. Voeks said.
She cautioned that the analysis included just 115 total primary-outcome events, which makes the incidence rate estimates somewhat imprecise, and that the data reflect outcomes in patients who were treated more than a decade ago, but these data remain the only reported results from large randomized trials that compared CAS and CEA in asymptomatic patients.
Dr. Voeks reported no disclosures.
SOURCE: Voeks JH al. Stroke. 2020 Feb 12;51[suppl 1], Abstract 70.
LOS ANGELES – Carotid artery stenting in older, asymptomatic patients with severe carotid artery stenosis is, in general, as bad an idea as it has already proven to be in symptomatic patients, with a multifold increase in adverse short- and mid-term outcomes, compared with similar older, asymptomatic patients who underwent endarterectomy, according to a combined-study analysis with more than 2,500 patients.
The risk for poor outcomes in patients with severe but asymptomatic carotid artery disease who underwent carotid artery stenting (CAS), compared with patients who instead underwent carotid endarterectomy (CEA) “abruptly increased around age 75,” in an analysis that combined data from the two major, published, randomized trials that compared these two interventions in this patient population, Jenifer H. Voeks, PhD said at the International Stroke Conference sponsored by the American Heart Association.
These results “largely mirror” the findings from a similar combined analysis of data from four major, randomized trials that compared CEA and CAS in patients with symptomatic carotid disease, she noted (Lancet. 2016 Mar 26;387[10025]:1305-11). The new findings in an expanded population of asymptomatic patients derived from two separate studies showed that, in patients aged 70 years or less, “CAS appears to be a reasonable alternative to CEA, but above age 70, and certainly above age 75, age-related risk factors such as cerebrovascular anatomy and underlying cerebral pathology should be carefully considered before selecting patients for CAS,” said Dr. Voeks, a neurology researcher at the Medical University of South Carolina, Charleston. Many experts also believe that, for asymptomatic patients, intensive medical management may have returned as an alternative to either of these invasive approaches for treating severe carotid stenosis and has achieved a level of equipoise that led to the launch of CREST 2 (Carotid Revascularization and Medical Management for Asymptomatic Carotid Stenosis Trial). CREST 2 is comparing CEA and CAS with medical management, and is scheduled to report results in 2021.
The data for this analysis in asymptomatic patients came from the first CREST (Carotid Revascularization Endarterectomy Versus Stenting Trial; N Engl J Med. 2010 Jul 1;363[1]:11-23), which included 1,181 asymptomatic patients (nearly half the total enrollment, with symptomatic patients making up the balance) and had no age ceiling, as well as all 1,453 patients from the ACT 1 trial, which enrolled exclusively asymptomatic patients and limited enrollment to patients aged 79 years or less (N Engl J Med. 2016 Mar 17;374[11]: 1011-20). Because the maximum age of patients in ACT 1 was 79 years, for this analysis Dr. Voeks and associates only included the 1,091 asymptomatic CREST patients who also were within the same age ceiling. The resulting cohort of 2,544 included 1,637 patients who underwent CAS and 907 who underwent CEA (because of a 3:1 randomization ratio in ACT 1), creating the largest data set to compare CAS and CEA by age in asymptomatic patients, Dr. Voeks noted. When subdivided by age, 30% of the cohort was younger that 65 years, 54% were 65-74, and 16% were 75-79.
The primary outcome the researchers used for their analysis was the combined incidence of periprocedural stroke, MI, or death, plus the incidence of ipsilateral stroke during 4 years of follow-up post procedure. Among patients who underwent CAS, this outcome occurred in roughly 9% of patients aged 75-79 years and in about 3% of those younger than 65 years, a hazard ratio of 2.9 that was statistically significant. In contrast, the incidence of the primary outcome among patients aged 65-74 years was just 30% higher, compared with patients aged less than 65 years, a difference that was not statistically significant.
Patients who underwent CEA showed no similar relationship between age and outcome. The incidence of the primary outcome among the CEA patients was roughly the same, about 3.5%, regardless of their age.
A second analysis that considered age as a continuous variable showed a sharply spiked increase in the risk for CAS patients, compared with CEA patients once they reached about age 73-75 years. Until about age 72, the rate of the primary outcome was nearly the same regardless of whether patients underwent CAS or CEA, but the risk for adverse outcomes rose “steeply” starting at about age 75 so that by age 79 the rate of the primary outcome approached 300% higher among the CAS patients compared with CEA patients, Dr. Voeks said.
She cautioned that the analysis included just 115 total primary-outcome events, which makes the incidence rate estimates somewhat imprecise, and that the data reflect outcomes in patients who were treated more than a decade ago, but these data remain the only reported results from large randomized trials that compared CAS and CEA in asymptomatic patients.
Dr. Voeks reported no disclosures.
SOURCE: Voeks JH al. Stroke. 2020 Feb 12;51[suppl 1], Abstract 70.
REPORTING FROM ISC 2020
Pulsed field catheter ablation shows huge clinical promise for AFib
NATIONAL HARBOR, MD. – Cardiac electrophysiologists have reported using pulsed field ablation, a new power source for catheter ablation of atrial fibrillation, on fewer than 150 patients worldwide in initial clinical studies, but its performance so far and the promise it carries for substantially improving the safety and efficacy of catheter ablation has convinced many experts that it represents the future for this intervention.
“I’m very excited about PFA [pulsed field ablation]. It may make everything else obsolete,” Andrea Natale, MD, said at the annual International AF Symposium. “We need to see more efficacy data, but just for safety alone there is no reason to use anything else,” commented Dr. Natale, executive medical director of the Texas Cardiac Arrhythmia Institute at St. David’s Medical Center in Austin,Tex.
“The main issue is safety, and if PFA lives up to its promise, then [using it preferentially] is not a difficult decision,” commented Francis E. Marchlinski, MD, professor of medicine and director of electrophysiology at the University of Pennsylvania.
“The only question is whether it has good long-term efficacy” because so far no patients have been followed for longer than about a year after PFA treatment, noted Moussa Mansour, MD, director of the cardiac electrophysiology laboratory at Massachusetts General Hospital in Boston. “If that piece turns out to be true, then I think it will be a winner.”
Vivek Y. Reddy, MD, one of the few investigators to have already collaborated on clinical studies that used PFA to catheter ablate both in patients with paroxysmal and, more recently, persistent atrial fibrillation (AFib), put it this way: “I’m 99% sure” PFA will be the energy of choice in the near future for AFib catheter ablation. The 1% of uncertainty “is only because of what might be unknown, something we’re not expecting,” said Dr. Reddy, professor of medicine and director of the cardiac arrhythmia service at Mount Sinai Medical Center in New York.
He and his associates at a center in Prague and at a second site in Bordeaux, France, reported their collective experience in 2019 regarding use of PFA on 81 patients with symptomatic, paroxysmal AFib who had not responded to at least one antiarrhythmic drug (J Am Coll Cardiol. 2019 Jul;74[3]:315-26). During a session on PFA at the symposium, Pierre Jaïs, MD, a cardiac electrophysiologist and professor of cardiology at the University of Bordeaux, updated this experience to now include 113 patients treated by the end of 2019 at the same two centers plus now an added third site, an experience accumulated by a total of five operators. Fifty-one patients have now been followed for at least a year, with no “unexpected” safety events, said Dr. Jaïs, The most recent 88 patients underwent PFA without general anesthesia. The ablation technique has undergone several refinements during this experience, and with use of the most recent, biphasic protocol that’s so far treated 26 patients, 24 (92%) of the treated patients had no reconnected AFib circuits in their atrial tissue when they underwent remapping 3 months after their procedure.
Magnetic resonance imaging of the left atria of these patients after pulmonary vein isolation with PFA showed a uniquely homogeneous and continuous lesion that functionally isolated each vein from surrounding atrial tissue and denoted a more uniform and complete ablation, Dr. Jaïs noted. “I have never seen [an ablation] as homogeneous.” The Magnetic resonance pictures also showed that the esophagus in each treated patient remained completely undamaged. “Esophageal sparing is systematically observed,” along with phrenic nerve sparing that’s in notable contrast with what’s seen with conventional energy sources, he said. The images also indicated that edema was substantially reduced compared with both radiofrequency and cryoablation, while mechanical function of treated left atria has consistently been “well preserved.”
“For the first time, we can use extra power to ensure durable lesions without compromising safety,” Dr. Jaïs concluded. PFA appears to put AFib ablation “on the verge of a totally new era.”
The less extensive and briefer experience in patients with persistent AFib has been completely consistent. This included 25 patients who had not responded to at least one antiarrhythmic drug treated by either of two operators, one in Prague and the other in Split, Croatia. All 25 patients who underwent pulmonary vein isolation had the procedure successfully completed as assessed with acute mapping of arrhythmia circuits after ablation, and the 24 of these patients who also underwent posterior wall ablation with the PFA device all had a successful acute result according to mapping, Dr. Reddy reported. No patient had an adverse event. PFA treatments were relatively fast, with an average procedure time in this series of 132 minutes. Repeat mapping 3 months after treatment is still pending.
At the heart of PFA’s safety is its “myocardial selectivity” which has so far kept PFA from causing any esophageal or phrenic nerve injuries, two potential complications of conventional AFib catheter ablation with use of either radiofrequency or cryo energy. Dr. Reddy was quick to highlight that there is no absolute selectivity for myocardium. “If you create a big enough field, it will electroporate everything, but the margin [between safety and damage] seems wide enough to take advantage” of focally damaging myocardial tissue in the left atrium to disrupt arrhythmia circuits while sparing adjacent tissue. Irreversible electroporation is the means by which PFA destroys targets cells while leaving other tissue unscathed, and a precisely adjusted PFA signal can focus its lethal effect exclusively on myocardial cells, a feature of PFA that Dr. Reddy called “lucky.”
The pulsed field ablation studies have been sponsored by Farapulse, the company developing this device, which in May 2019 received breakthrough designation for priority review from the Food and Drug Administration.
Dr. Reddy and Dr. Jaïs are both consultants to and shareholders in Farapulse. Dr. Natale has received honoraria from or has been a consultant to Biotronik, Janssen, Medtronic, and St. Jude. Dr. Marchlinski has been a consultant to or has received honoraria from Abbott EP/St. Jude, Biotronik, and Medtronic. Dr. Mansour has been a consultant for Abbott and Medtronic, has an equity interest or stock options in NewPace and EPD Solutions, and has received research grants from Abbott, Boehringer Ingelheim, Pfizer, and Sentre Heart. In addition, all sources have received consulting fees, honoraria, and/or research grants from Biosense Webster and Boston Scientific.
NATIONAL HARBOR, MD. – Cardiac electrophysiologists have reported using pulsed field ablation, a new power source for catheter ablation of atrial fibrillation, on fewer than 150 patients worldwide in initial clinical studies, but its performance so far and the promise it carries for substantially improving the safety and efficacy of catheter ablation has convinced many experts that it represents the future for this intervention.
“I’m very excited about PFA [pulsed field ablation]. It may make everything else obsolete,” Andrea Natale, MD, said at the annual International AF Symposium. “We need to see more efficacy data, but just for safety alone there is no reason to use anything else,” commented Dr. Natale, executive medical director of the Texas Cardiac Arrhythmia Institute at St. David’s Medical Center in Austin,Tex.
“The main issue is safety, and if PFA lives up to its promise, then [using it preferentially] is not a difficult decision,” commented Francis E. Marchlinski, MD, professor of medicine and director of electrophysiology at the University of Pennsylvania.
“The only question is whether it has good long-term efficacy” because so far no patients have been followed for longer than about a year after PFA treatment, noted Moussa Mansour, MD, director of the cardiac electrophysiology laboratory at Massachusetts General Hospital in Boston. “If that piece turns out to be true, then I think it will be a winner.”
Vivek Y. Reddy, MD, one of the few investigators to have already collaborated on clinical studies that used PFA to catheter ablate both in patients with paroxysmal and, more recently, persistent atrial fibrillation (AFib), put it this way: “I’m 99% sure” PFA will be the energy of choice in the near future for AFib catheter ablation. The 1% of uncertainty “is only because of what might be unknown, something we’re not expecting,” said Dr. Reddy, professor of medicine and director of the cardiac arrhythmia service at Mount Sinai Medical Center in New York.
He and his associates at a center in Prague and at a second site in Bordeaux, France, reported their collective experience in 2019 regarding use of PFA on 81 patients with symptomatic, paroxysmal AFib who had not responded to at least one antiarrhythmic drug (J Am Coll Cardiol. 2019 Jul;74[3]:315-26). During a session on PFA at the symposium, Pierre Jaïs, MD, a cardiac electrophysiologist and professor of cardiology at the University of Bordeaux, updated this experience to now include 113 patients treated by the end of 2019 at the same two centers plus now an added third site, an experience accumulated by a total of five operators. Fifty-one patients have now been followed for at least a year, with no “unexpected” safety events, said Dr. Jaïs, The most recent 88 patients underwent PFA without general anesthesia. The ablation technique has undergone several refinements during this experience, and with use of the most recent, biphasic protocol that’s so far treated 26 patients, 24 (92%) of the treated patients had no reconnected AFib circuits in their atrial tissue when they underwent remapping 3 months after their procedure.
Magnetic resonance imaging of the left atria of these patients after pulmonary vein isolation with PFA showed a uniquely homogeneous and continuous lesion that functionally isolated each vein from surrounding atrial tissue and denoted a more uniform and complete ablation, Dr. Jaïs noted. “I have never seen [an ablation] as homogeneous.” The Magnetic resonance pictures also showed that the esophagus in each treated patient remained completely undamaged. “Esophageal sparing is systematically observed,” along with phrenic nerve sparing that’s in notable contrast with what’s seen with conventional energy sources, he said. The images also indicated that edema was substantially reduced compared with both radiofrequency and cryoablation, while mechanical function of treated left atria has consistently been “well preserved.”
“For the first time, we can use extra power to ensure durable lesions without compromising safety,” Dr. Jaïs concluded. PFA appears to put AFib ablation “on the verge of a totally new era.”
The less extensive and briefer experience in patients with persistent AFib has been completely consistent. This included 25 patients who had not responded to at least one antiarrhythmic drug treated by either of two operators, one in Prague and the other in Split, Croatia. All 25 patients who underwent pulmonary vein isolation had the procedure successfully completed as assessed with acute mapping of arrhythmia circuits after ablation, and the 24 of these patients who also underwent posterior wall ablation with the PFA device all had a successful acute result according to mapping, Dr. Reddy reported. No patient had an adverse event. PFA treatments were relatively fast, with an average procedure time in this series of 132 minutes. Repeat mapping 3 months after treatment is still pending.
At the heart of PFA’s safety is its “myocardial selectivity” which has so far kept PFA from causing any esophageal or phrenic nerve injuries, two potential complications of conventional AFib catheter ablation with use of either radiofrequency or cryo energy. Dr. Reddy was quick to highlight that there is no absolute selectivity for myocardium. “If you create a big enough field, it will electroporate everything, but the margin [between safety and damage] seems wide enough to take advantage” of focally damaging myocardial tissue in the left atrium to disrupt arrhythmia circuits while sparing adjacent tissue. Irreversible electroporation is the means by which PFA destroys targets cells while leaving other tissue unscathed, and a precisely adjusted PFA signal can focus its lethal effect exclusively on myocardial cells, a feature of PFA that Dr. Reddy called “lucky.”
The pulsed field ablation studies have been sponsored by Farapulse, the company developing this device, which in May 2019 received breakthrough designation for priority review from the Food and Drug Administration.
Dr. Reddy and Dr. Jaïs are both consultants to and shareholders in Farapulse. Dr. Natale has received honoraria from or has been a consultant to Biotronik, Janssen, Medtronic, and St. Jude. Dr. Marchlinski has been a consultant to or has received honoraria from Abbott EP/St. Jude, Biotronik, and Medtronic. Dr. Mansour has been a consultant for Abbott and Medtronic, has an equity interest or stock options in NewPace and EPD Solutions, and has received research grants from Abbott, Boehringer Ingelheim, Pfizer, and Sentre Heart. In addition, all sources have received consulting fees, honoraria, and/or research grants from Biosense Webster and Boston Scientific.
NATIONAL HARBOR, MD. – Cardiac electrophysiologists have reported using pulsed field ablation, a new power source for catheter ablation of atrial fibrillation, on fewer than 150 patients worldwide in initial clinical studies, but its performance so far and the promise it carries for substantially improving the safety and efficacy of catheter ablation has convinced many experts that it represents the future for this intervention.
“I’m very excited about PFA [pulsed field ablation]. It may make everything else obsolete,” Andrea Natale, MD, said at the annual International AF Symposium. “We need to see more efficacy data, but just for safety alone there is no reason to use anything else,” commented Dr. Natale, executive medical director of the Texas Cardiac Arrhythmia Institute at St. David’s Medical Center in Austin,Tex.
“The main issue is safety, and if PFA lives up to its promise, then [using it preferentially] is not a difficult decision,” commented Francis E. Marchlinski, MD, professor of medicine and director of electrophysiology at the University of Pennsylvania.
“The only question is whether it has good long-term efficacy” because so far no patients have been followed for longer than about a year after PFA treatment, noted Moussa Mansour, MD, director of the cardiac electrophysiology laboratory at Massachusetts General Hospital in Boston. “If that piece turns out to be true, then I think it will be a winner.”
Vivek Y. Reddy, MD, one of the few investigators to have already collaborated on clinical studies that used PFA to catheter ablate both in patients with paroxysmal and, more recently, persistent atrial fibrillation (AFib), put it this way: “I’m 99% sure” PFA will be the energy of choice in the near future for AFib catheter ablation. The 1% of uncertainty “is only because of what might be unknown, something we’re not expecting,” said Dr. Reddy, professor of medicine and director of the cardiac arrhythmia service at Mount Sinai Medical Center in New York.
He and his associates at a center in Prague and at a second site in Bordeaux, France, reported their collective experience in 2019 regarding use of PFA on 81 patients with symptomatic, paroxysmal AFib who had not responded to at least one antiarrhythmic drug (J Am Coll Cardiol. 2019 Jul;74[3]:315-26). During a session on PFA at the symposium, Pierre Jaïs, MD, a cardiac electrophysiologist and professor of cardiology at the University of Bordeaux, updated this experience to now include 113 patients treated by the end of 2019 at the same two centers plus now an added third site, an experience accumulated by a total of five operators. Fifty-one patients have now been followed for at least a year, with no “unexpected” safety events, said Dr. Jaïs, The most recent 88 patients underwent PFA without general anesthesia. The ablation technique has undergone several refinements during this experience, and with use of the most recent, biphasic protocol that’s so far treated 26 patients, 24 (92%) of the treated patients had no reconnected AFib circuits in their atrial tissue when they underwent remapping 3 months after their procedure.
Magnetic resonance imaging of the left atria of these patients after pulmonary vein isolation with PFA showed a uniquely homogeneous and continuous lesion that functionally isolated each vein from surrounding atrial tissue and denoted a more uniform and complete ablation, Dr. Jaïs noted. “I have never seen [an ablation] as homogeneous.” The Magnetic resonance pictures also showed that the esophagus in each treated patient remained completely undamaged. “Esophageal sparing is systematically observed,” along with phrenic nerve sparing that’s in notable contrast with what’s seen with conventional energy sources, he said. The images also indicated that edema was substantially reduced compared with both radiofrequency and cryoablation, while mechanical function of treated left atria has consistently been “well preserved.”
“For the first time, we can use extra power to ensure durable lesions without compromising safety,” Dr. Jaïs concluded. PFA appears to put AFib ablation “on the verge of a totally new era.”
The less extensive and briefer experience in patients with persistent AFib has been completely consistent. This included 25 patients who had not responded to at least one antiarrhythmic drug treated by either of two operators, one in Prague and the other in Split, Croatia. All 25 patients who underwent pulmonary vein isolation had the procedure successfully completed as assessed with acute mapping of arrhythmia circuits after ablation, and the 24 of these patients who also underwent posterior wall ablation with the PFA device all had a successful acute result according to mapping, Dr. Reddy reported. No patient had an adverse event. PFA treatments were relatively fast, with an average procedure time in this series of 132 minutes. Repeat mapping 3 months after treatment is still pending.
At the heart of PFA’s safety is its “myocardial selectivity” which has so far kept PFA from causing any esophageal or phrenic nerve injuries, two potential complications of conventional AFib catheter ablation with use of either radiofrequency or cryo energy. Dr. Reddy was quick to highlight that there is no absolute selectivity for myocardium. “If you create a big enough field, it will electroporate everything, but the margin [between safety and damage] seems wide enough to take advantage” of focally damaging myocardial tissue in the left atrium to disrupt arrhythmia circuits while sparing adjacent tissue. Irreversible electroporation is the means by which PFA destroys targets cells while leaving other tissue unscathed, and a precisely adjusted PFA signal can focus its lethal effect exclusively on myocardial cells, a feature of PFA that Dr. Reddy called “lucky.”
The pulsed field ablation studies have been sponsored by Farapulse, the company developing this device, which in May 2019 received breakthrough designation for priority review from the Food and Drug Administration.
Dr. Reddy and Dr. Jaïs are both consultants to and shareholders in Farapulse. Dr. Natale has received honoraria from or has been a consultant to Biotronik, Janssen, Medtronic, and St. Jude. Dr. Marchlinski has been a consultant to or has received honoraria from Abbott EP/St. Jude, Biotronik, and Medtronic. Dr. Mansour has been a consultant for Abbott and Medtronic, has an equity interest or stock options in NewPace and EPD Solutions, and has received research grants from Abbott, Boehringer Ingelheim, Pfizer, and Sentre Heart. In addition, all sources have received consulting fees, honoraria, and/or research grants from Biosense Webster and Boston Scientific.
EXPERT ANALYSIS FROM THE AF SYMPOSIUM 2020
Risk factors found for respiratory AEs in children following OSA surgery
Underlying cardiac disease, airway anomalies, and younger age each independently boosted the risk of severe perioperative respiratory adverse events (PRAE) in children undergoing adenotonsillectomy to treat obstructive sleep apnea, in a review of 374 patients treated at a single Canadian tertiary-referral center.
In contrast, the analysis failed to show independent, significant effects from any assessed polysomnography or oximetry parameters on the rate of postoperative respiratory complications. The utility of preoperative polysomnography or oximetry for risk stratification is questionable for pediatric patients scheduled to adenotonsillectomy to treat obstructive sleep apnea, wrote Sherri L. Katz, MD, of the University of Ottawa, and associates in a recent report published in the Journal of Clinical Sleep Medicine, although they also added that making these assessments may be “unavoidable” because of their need for diagnosing obstructive sleep apnea and determining the need for surgery.
Despite this caveat, “overall our study results highlight the need to better define the complex interaction between comorbidities, age, nocturnal respiratory events, and gas exchange abnormalities in predicting risk for PRAE” after adenotonsillectomy, the researchers wrote. These findings “are consistent with existing clinical care guidelines,” and “cardiac and craniofacial conditions have been associated with risk of postoperative complications in other studies.”
The analysis used data collected from all children aged 0-18 years who underwent polysomnography assessment followed by adenotonsillectomy at one Canadian tertiary-referral center, Children’s Hospital of Eastern Ontario in Ottawa, during 2010-2016. Their median age was just over 6 years, and 39 patients (10%) were younger than 3 years at the time of their surgery. More than three-quarters of the patients, 286, had at least one identified comorbidity, and nearly half had at least two comorbidities. Polysomnography identified sleep-disordered breathing in 344 of the children (92%), and diagnosed obstructive sleep apnea in 256 (68%), including 148 (43% of the full cohort) with a severe apnea-hypopnea index.
Sixty-six of the children (18%) had at least one severe PRAE that required intervention. Specifically these were either oxygen desaturations requiring intervention or need for airway or ventilatory support with interventions such as jaw thrust, oral or nasal airway placement, bag and mask ventilation, or endotracheal intubation.
A multivariate regression analysis of the measured comorbidity, polysomnography, and oximetry parameters, as well as age, identified three factors that independently linked with a statistically significant increase in the rate of severe PRAE: airway anomaly, underlying cardiac disease, and young age. Patients with an airway anomaly had a 219% increased rate of PRAE, compared with those with no anomaly; patients with underlying cardiac disease had a 109% increased rate, compared with those without cardiac disease; and patients aged younger than 3 years had a 310% higher rate of PRAE, compared with the children aged 6 years or older, while children aged 3-5 years had a 121% higher rate of PRAE, compared with older children.
The study received no commercial funding. Dr. Katz has received honoraria for speaking from Biogen that had no relevance to the study.
SOURCE: Katz SL et al. J Clin Sleep Med. 2020 Jan 15;16(1):41-8.
This well-conducted, retrospective, chart-review study adds important information to the published literature about risk stratification for children in a tertiary-referral population undergoing adenotonsillectomy. Their findings indicate that younger children remain at higher risk as well as those children with complex comorbid medical disease. They also show that children with severe sleep apnea or significant oxyhemoglobin desaturation are likewise at higher risk of postoperative respiratory compromise – emphasizing the need for preoperative polysomnography – particularly in a tertiary setting where many patients have medical comorbidities.
Despite the strengths of this study in assessing perioperative risk for respiratory compromise in a referral population with highly prevalent medical comorbidities, this study does not provide significant insight into the management of otherwise healthy children in a community setting who are undergoing adenotonsillectomy. This is important because a large number of adenotonsillectomies are performed outside of a tertiary-referral center and many of these children may not have undergone preoperative polysomnography to stratify risk. The utility of preoperative polysomnography in the evaluation of all children undergoing adenotonsillectomy remains controversial, with diverging recommendations from two major U.S. medical groups.
This study does not address the utility of polysomnography in community-based populations of otherwise healthy children. It is imperative to accurately ascertain risk so perioperative planning can ensure the safety of children at higher risk following adenotonsillectomy; however, there remains a paucity of studies assessing the cost-effectiveness as well as the positive and negative predictive value of polysomnographic findings. This study highlights the need for community-based studies of otherwise healthy children undergoing adenotonsillectomy to ensure that children at risk receive appropriate monitoring in an inpatient setting whereas those at lesser risk are not unnecessarily hospitalized postoperatively.
Heidi V. Connolly, MD, and Laura E. Tomaselli, MD, are pediatric sleep medicine physicians, and Margo K. McKenna Benoit, MD, is an otolaryngologist at the University of Rochester (N.Y.). They made these comments in a commentary that accompanied the published report ( J Clin Sleep Med. 2020 Jan 15;16[1]:3-4 ). They had no disclosures.
This well-conducted, retrospective, chart-review study adds important information to the published literature about risk stratification for children in a tertiary-referral population undergoing adenotonsillectomy. Their findings indicate that younger children remain at higher risk as well as those children with complex comorbid medical disease. They also show that children with severe sleep apnea or significant oxyhemoglobin desaturation are likewise at higher risk of postoperative respiratory compromise – emphasizing the need for preoperative polysomnography – particularly in a tertiary setting where many patients have medical comorbidities.
Despite the strengths of this study in assessing perioperative risk for respiratory compromise in a referral population with highly prevalent medical comorbidities, this study does not provide significant insight into the management of otherwise healthy children in a community setting who are undergoing adenotonsillectomy. This is important because a large number of adenotonsillectomies are performed outside of a tertiary-referral center and many of these children may not have undergone preoperative polysomnography to stratify risk. The utility of preoperative polysomnography in the evaluation of all children undergoing adenotonsillectomy remains controversial, with diverging recommendations from two major U.S. medical groups.
This study does not address the utility of polysomnography in community-based populations of otherwise healthy children. It is imperative to accurately ascertain risk so perioperative planning can ensure the safety of children at higher risk following adenotonsillectomy; however, there remains a paucity of studies assessing the cost-effectiveness as well as the positive and negative predictive value of polysomnographic findings. This study highlights the need for community-based studies of otherwise healthy children undergoing adenotonsillectomy to ensure that children at risk receive appropriate monitoring in an inpatient setting whereas those at lesser risk are not unnecessarily hospitalized postoperatively.
Heidi V. Connolly, MD, and Laura E. Tomaselli, MD, are pediatric sleep medicine physicians, and Margo K. McKenna Benoit, MD, is an otolaryngologist at the University of Rochester (N.Y.). They made these comments in a commentary that accompanied the published report ( J Clin Sleep Med. 2020 Jan 15;16[1]:3-4 ). They had no disclosures.
This well-conducted, retrospective, chart-review study adds important information to the published literature about risk stratification for children in a tertiary-referral population undergoing adenotonsillectomy. Their findings indicate that younger children remain at higher risk as well as those children with complex comorbid medical disease. They also show that children with severe sleep apnea or significant oxyhemoglobin desaturation are likewise at higher risk of postoperative respiratory compromise – emphasizing the need for preoperative polysomnography – particularly in a tertiary setting where many patients have medical comorbidities.
Despite the strengths of this study in assessing perioperative risk for respiratory compromise in a referral population with highly prevalent medical comorbidities, this study does not provide significant insight into the management of otherwise healthy children in a community setting who are undergoing adenotonsillectomy. This is important because a large number of adenotonsillectomies are performed outside of a tertiary-referral center and many of these children may not have undergone preoperative polysomnography to stratify risk. The utility of preoperative polysomnography in the evaluation of all children undergoing adenotonsillectomy remains controversial, with diverging recommendations from two major U.S. medical groups.
This study does not address the utility of polysomnography in community-based populations of otherwise healthy children. It is imperative to accurately ascertain risk so perioperative planning can ensure the safety of children at higher risk following adenotonsillectomy; however, there remains a paucity of studies assessing the cost-effectiveness as well as the positive and negative predictive value of polysomnographic findings. This study highlights the need for community-based studies of otherwise healthy children undergoing adenotonsillectomy to ensure that children at risk receive appropriate monitoring in an inpatient setting whereas those at lesser risk are not unnecessarily hospitalized postoperatively.
Heidi V. Connolly, MD, and Laura E. Tomaselli, MD, are pediatric sleep medicine physicians, and Margo K. McKenna Benoit, MD, is an otolaryngologist at the University of Rochester (N.Y.). They made these comments in a commentary that accompanied the published report ( J Clin Sleep Med. 2020 Jan 15;16[1]:3-4 ). They had no disclosures.
Underlying cardiac disease, airway anomalies, and younger age each independently boosted the risk of severe perioperative respiratory adverse events (PRAE) in children undergoing adenotonsillectomy to treat obstructive sleep apnea, in a review of 374 patients treated at a single Canadian tertiary-referral center.
In contrast, the analysis failed to show independent, significant effects from any assessed polysomnography or oximetry parameters on the rate of postoperative respiratory complications. The utility of preoperative polysomnography or oximetry for risk stratification is questionable for pediatric patients scheduled to adenotonsillectomy to treat obstructive sleep apnea, wrote Sherri L. Katz, MD, of the University of Ottawa, and associates in a recent report published in the Journal of Clinical Sleep Medicine, although they also added that making these assessments may be “unavoidable” because of their need for diagnosing obstructive sleep apnea and determining the need for surgery.
Despite this caveat, “overall our study results highlight the need to better define the complex interaction between comorbidities, age, nocturnal respiratory events, and gas exchange abnormalities in predicting risk for PRAE” after adenotonsillectomy, the researchers wrote. These findings “are consistent with existing clinical care guidelines,” and “cardiac and craniofacial conditions have been associated with risk of postoperative complications in other studies.”
The analysis used data collected from all children aged 0-18 years who underwent polysomnography assessment followed by adenotonsillectomy at one Canadian tertiary-referral center, Children’s Hospital of Eastern Ontario in Ottawa, during 2010-2016. Their median age was just over 6 years, and 39 patients (10%) were younger than 3 years at the time of their surgery. More than three-quarters of the patients, 286, had at least one identified comorbidity, and nearly half had at least two comorbidities. Polysomnography identified sleep-disordered breathing in 344 of the children (92%), and diagnosed obstructive sleep apnea in 256 (68%), including 148 (43% of the full cohort) with a severe apnea-hypopnea index.
Sixty-six of the children (18%) had at least one severe PRAE that required intervention. Specifically these were either oxygen desaturations requiring intervention or need for airway or ventilatory support with interventions such as jaw thrust, oral or nasal airway placement, bag and mask ventilation, or endotracheal intubation.
A multivariate regression analysis of the measured comorbidity, polysomnography, and oximetry parameters, as well as age, identified three factors that independently linked with a statistically significant increase in the rate of severe PRAE: airway anomaly, underlying cardiac disease, and young age. Patients with an airway anomaly had a 219% increased rate of PRAE, compared with those with no anomaly; patients with underlying cardiac disease had a 109% increased rate, compared with those without cardiac disease; and patients aged younger than 3 years had a 310% higher rate of PRAE, compared with the children aged 6 years or older, while children aged 3-5 years had a 121% higher rate of PRAE, compared with older children.
The study received no commercial funding. Dr. Katz has received honoraria for speaking from Biogen that had no relevance to the study.
SOURCE: Katz SL et al. J Clin Sleep Med. 2020 Jan 15;16(1):41-8.
Underlying cardiac disease, airway anomalies, and younger age each independently boosted the risk of severe perioperative respiratory adverse events (PRAE) in children undergoing adenotonsillectomy to treat obstructive sleep apnea, in a review of 374 patients treated at a single Canadian tertiary-referral center.
In contrast, the analysis failed to show independent, significant effects from any assessed polysomnography or oximetry parameters on the rate of postoperative respiratory complications. The utility of preoperative polysomnography or oximetry for risk stratification is questionable for pediatric patients scheduled to adenotonsillectomy to treat obstructive sleep apnea, wrote Sherri L. Katz, MD, of the University of Ottawa, and associates in a recent report published in the Journal of Clinical Sleep Medicine, although they also added that making these assessments may be “unavoidable” because of their need for diagnosing obstructive sleep apnea and determining the need for surgery.
Despite this caveat, “overall our study results highlight the need to better define the complex interaction between comorbidities, age, nocturnal respiratory events, and gas exchange abnormalities in predicting risk for PRAE” after adenotonsillectomy, the researchers wrote. These findings “are consistent with existing clinical care guidelines,” and “cardiac and craniofacial conditions have been associated with risk of postoperative complications in other studies.”
The analysis used data collected from all children aged 0-18 years who underwent polysomnography assessment followed by adenotonsillectomy at one Canadian tertiary-referral center, Children’s Hospital of Eastern Ontario in Ottawa, during 2010-2016. Their median age was just over 6 years, and 39 patients (10%) were younger than 3 years at the time of their surgery. More than three-quarters of the patients, 286, had at least one identified comorbidity, and nearly half had at least two comorbidities. Polysomnography identified sleep-disordered breathing in 344 of the children (92%), and diagnosed obstructive sleep apnea in 256 (68%), including 148 (43% of the full cohort) with a severe apnea-hypopnea index.
Sixty-six of the children (18%) had at least one severe PRAE that required intervention. Specifically these were either oxygen desaturations requiring intervention or need for airway or ventilatory support with interventions such as jaw thrust, oral or nasal airway placement, bag and mask ventilation, or endotracheal intubation.
A multivariate regression analysis of the measured comorbidity, polysomnography, and oximetry parameters, as well as age, identified three factors that independently linked with a statistically significant increase in the rate of severe PRAE: airway anomaly, underlying cardiac disease, and young age. Patients with an airway anomaly had a 219% increased rate of PRAE, compared with those with no anomaly; patients with underlying cardiac disease had a 109% increased rate, compared with those without cardiac disease; and patients aged younger than 3 years had a 310% higher rate of PRAE, compared with the children aged 6 years or older, while children aged 3-5 years had a 121% higher rate of PRAE, compared with older children.
The study received no commercial funding. Dr. Katz has received honoraria for speaking from Biogen that had no relevance to the study.
SOURCE: Katz SL et al. J Clin Sleep Med. 2020 Jan 15;16(1):41-8.
FROM THE JOURNAL OF CLINICAL SLEEP MEDICINE
Thrombectomy access lags for U.S. stroke patients
In 2017, roughly 3 years after evidence from several studies made endovascular thrombectomy first-line treatment for selected acute ischemic stroke patients, the treatment was available at barely more than one-third of all U.S. stroke centers, available within 30-minute access to just over 30% of Americans, and available within 15-minute access to one-fifth of U.S. residents, based on information in a comprehensive U.S. database.
These numbers showed that “current direct EVT [endovascular thrombectomy] access in the United States is suboptimal under predominate EMS routing protocols,” Amrou Sarraj, MD, and his associates wrote in an article published online in Stroke on Feb. 12. “Only in eight states did the coverage exceed 25% of the population, and nine states had coverage for less than 10% of the population. These results reflect limited access to an effective treatment modality that would improve clinical outcomes in patients with large strokes and prevent potentially devastating disability,” wrote Dr. Sarraj, chief of the general neurology service at Memorial-Hermann Hospital in Houston and coauthors.
Their analysis of data collected in 2017 by the Medicare Provider Analysis and Review (MEDPAR) database, maintained by the Centers for Medicare & Medicaid Services, identified two apparently effective ways to improve EVT access for acute ischemic stroke patients: First, systematically divert patients to a nearby center that offers EVT even when it means bypassing a closer stroke center that does not perform EVT when the added travel time is less than 15 minutes. Second, convert selected stroke centers that currently do not perform EVT into centers that do. Between these two approaches, the strategy of having ambulances bypass stroke centers that do not perform EVT and continuing to ones that do generally has the greater potential to boost access, the authors found. They based their analysis exclusively on their calculations of expected consequences rather than actual experience.
The calculations showed that bypassing non-EVT centers when the added bypass time computed to less than 15 minutes linked with an anticipated overall U.S. gain in access of about 17%, or 52 million people, extending the ability of acute ischemic stroke patients able to quickly reach an EVT center to about 37% of the American public. The second approach to boost access, converting the top 10% of stroke centers based on case volume that currently do not provide EVT to centers that do offer it, would result in expanded access for about 23 million additional Americans, raising the total with access to about 27% of the public, the new report said.
As part of this analysis, the MEDPAR data identified 1,941 U.S. centers providing stroke services during 2017, of which 713 (37%) had performed at least one EVT procedure. By comparison, 2015 MEDPAR data showed 577 U.S. stroke centers performing EVT, indicating that during the 2-3 years following several reports in early 2015 on the net benefits of EVT for acute ischemic stroke patients, the number of U.S. stroke centers offering this treatment had grown by a relative 24%. Based on the locations of the stroke centers that made EVT available in 2017, Dr. Sarraj and coauthors calculated that the 713 EVT-capable stroke centers provided emergency access within a 15-minute ground-ambulance trip for 61 million Americans (20% of the U.S. population), and within a 30-minute ground-transport trip to 95 million residents (31%).
Boosting these numbers by implementing a systematic bypass of stroke patients past non-EVT stroke centers to nearby centers that are EVT capable “has the benefit of ease of implementation and requires less time and resources,” the authors said. However, they also noted the heterogeneity of circumstances based on variables like population density and stroke center distribution, which means that in some locations the most effective way to boost access would be by increasing the number of stroke centers that provide EVT.
In 2018, Dr. Sarraj and associates reported results from a similar analysis of MEDPAR data that used 30-minute and 60-minute ground-transport times as the criteria for their calculations.
The study received no commercial funding. Dr. Sarraj reported receiving research funding from Stryker Neurovascular outside of this work. One coauthor reported serving in roles for the University of Texas Health System for which the institution has been funded via various industry and government grants, and another coauthor reported receiving research funding from the Patient-Centered Outcomes Research Institute, the National Institutes of Health, Genentech, and CSL Behring, as well as consulting fees from Frazer Ltd.
SOURCE: Sarraj A et al. Stroke. 2020 Feb 12. doi: 10.1161/STROKEAHA.120.028850.
In 2017, roughly 3 years after evidence from several studies made endovascular thrombectomy first-line treatment for selected acute ischemic stroke patients, the treatment was available at barely more than one-third of all U.S. stroke centers, available within 30-minute access to just over 30% of Americans, and available within 15-minute access to one-fifth of U.S. residents, based on information in a comprehensive U.S. database.
These numbers showed that “current direct EVT [endovascular thrombectomy] access in the United States is suboptimal under predominate EMS routing protocols,” Amrou Sarraj, MD, and his associates wrote in an article published online in Stroke on Feb. 12. “Only in eight states did the coverage exceed 25% of the population, and nine states had coverage for less than 10% of the population. These results reflect limited access to an effective treatment modality that would improve clinical outcomes in patients with large strokes and prevent potentially devastating disability,” wrote Dr. Sarraj, chief of the general neurology service at Memorial-Hermann Hospital in Houston and coauthors.
Their analysis of data collected in 2017 by the Medicare Provider Analysis and Review (MEDPAR) database, maintained by the Centers for Medicare & Medicaid Services, identified two apparently effective ways to improve EVT access for acute ischemic stroke patients: First, systematically divert patients to a nearby center that offers EVT even when it means bypassing a closer stroke center that does not perform EVT when the added travel time is less than 15 minutes. Second, convert selected stroke centers that currently do not perform EVT into centers that do. Between these two approaches, the strategy of having ambulances bypass stroke centers that do not perform EVT and continuing to ones that do generally has the greater potential to boost access, the authors found. They based their analysis exclusively on their calculations of expected consequences rather than actual experience.
The calculations showed that bypassing non-EVT centers when the added bypass time computed to less than 15 minutes linked with an anticipated overall U.S. gain in access of about 17%, or 52 million people, extending the ability of acute ischemic stroke patients able to quickly reach an EVT center to about 37% of the American public. The second approach to boost access, converting the top 10% of stroke centers based on case volume that currently do not provide EVT to centers that do offer it, would result in expanded access for about 23 million additional Americans, raising the total with access to about 27% of the public, the new report said.
As part of this analysis, the MEDPAR data identified 1,941 U.S. centers providing stroke services during 2017, of which 713 (37%) had performed at least one EVT procedure. By comparison, 2015 MEDPAR data showed 577 U.S. stroke centers performing EVT, indicating that during the 2-3 years following several reports in early 2015 on the net benefits of EVT for acute ischemic stroke patients, the number of U.S. stroke centers offering this treatment had grown by a relative 24%. Based on the locations of the stroke centers that made EVT available in 2017, Dr. Sarraj and coauthors calculated that the 713 EVT-capable stroke centers provided emergency access within a 15-minute ground-ambulance trip for 61 million Americans (20% of the U.S. population), and within a 30-minute ground-transport trip to 95 million residents (31%).
Boosting these numbers by implementing a systematic bypass of stroke patients past non-EVT stroke centers to nearby centers that are EVT capable “has the benefit of ease of implementation and requires less time and resources,” the authors said. However, they also noted the heterogeneity of circumstances based on variables like population density and stroke center distribution, which means that in some locations the most effective way to boost access would be by increasing the number of stroke centers that provide EVT.
In 2018, Dr. Sarraj and associates reported results from a similar analysis of MEDPAR data that used 30-minute and 60-minute ground-transport times as the criteria for their calculations.
The study received no commercial funding. Dr. Sarraj reported receiving research funding from Stryker Neurovascular outside of this work. One coauthor reported serving in roles for the University of Texas Health System for which the institution has been funded via various industry and government grants, and another coauthor reported receiving research funding from the Patient-Centered Outcomes Research Institute, the National Institutes of Health, Genentech, and CSL Behring, as well as consulting fees from Frazer Ltd.
SOURCE: Sarraj A et al. Stroke. 2020 Feb 12. doi: 10.1161/STROKEAHA.120.028850.
In 2017, roughly 3 years after evidence from several studies made endovascular thrombectomy first-line treatment for selected acute ischemic stroke patients, the treatment was available at barely more than one-third of all U.S. stroke centers, available within 30-minute access to just over 30% of Americans, and available within 15-minute access to one-fifth of U.S. residents, based on information in a comprehensive U.S. database.
These numbers showed that “current direct EVT [endovascular thrombectomy] access in the United States is suboptimal under predominate EMS routing protocols,” Amrou Sarraj, MD, and his associates wrote in an article published online in Stroke on Feb. 12. “Only in eight states did the coverage exceed 25% of the population, and nine states had coverage for less than 10% of the population. These results reflect limited access to an effective treatment modality that would improve clinical outcomes in patients with large strokes and prevent potentially devastating disability,” wrote Dr. Sarraj, chief of the general neurology service at Memorial-Hermann Hospital in Houston and coauthors.
Their analysis of data collected in 2017 by the Medicare Provider Analysis and Review (MEDPAR) database, maintained by the Centers for Medicare & Medicaid Services, identified two apparently effective ways to improve EVT access for acute ischemic stroke patients: First, systematically divert patients to a nearby center that offers EVT even when it means bypassing a closer stroke center that does not perform EVT when the added travel time is less than 15 minutes. Second, convert selected stroke centers that currently do not perform EVT into centers that do. Between these two approaches, the strategy of having ambulances bypass stroke centers that do not perform EVT and continuing to ones that do generally has the greater potential to boost access, the authors found. They based their analysis exclusively on their calculations of expected consequences rather than actual experience.
The calculations showed that bypassing non-EVT centers when the added bypass time computed to less than 15 minutes linked with an anticipated overall U.S. gain in access of about 17%, or 52 million people, extending the ability of acute ischemic stroke patients able to quickly reach an EVT center to about 37% of the American public. The second approach to boost access, converting the top 10% of stroke centers based on case volume that currently do not provide EVT to centers that do offer it, would result in expanded access for about 23 million additional Americans, raising the total with access to about 27% of the public, the new report said.
As part of this analysis, the MEDPAR data identified 1,941 U.S. centers providing stroke services during 2017, of which 713 (37%) had performed at least one EVT procedure. By comparison, 2015 MEDPAR data showed 577 U.S. stroke centers performing EVT, indicating that during the 2-3 years following several reports in early 2015 on the net benefits of EVT for acute ischemic stroke patients, the number of U.S. stroke centers offering this treatment had grown by a relative 24%. Based on the locations of the stroke centers that made EVT available in 2017, Dr. Sarraj and coauthors calculated that the 713 EVT-capable stroke centers provided emergency access within a 15-minute ground-ambulance trip for 61 million Americans (20% of the U.S. population), and within a 30-minute ground-transport trip to 95 million residents (31%).
Boosting these numbers by implementing a systematic bypass of stroke patients past non-EVT stroke centers to nearby centers that are EVT capable “has the benefit of ease of implementation and requires less time and resources,” the authors said. However, they also noted the heterogeneity of circumstances based on variables like population density and stroke center distribution, which means that in some locations the most effective way to boost access would be by increasing the number of stroke centers that provide EVT.
In 2018, Dr. Sarraj and associates reported results from a similar analysis of MEDPAR data that used 30-minute and 60-minute ground-transport times as the criteria for their calculations.
The study received no commercial funding. Dr. Sarraj reported receiving research funding from Stryker Neurovascular outside of this work. One coauthor reported serving in roles for the University of Texas Health System for which the institution has been funded via various industry and government grants, and another coauthor reported receiving research funding from the Patient-Centered Outcomes Research Institute, the National Institutes of Health, Genentech, and CSL Behring, as well as consulting fees from Frazer Ltd.
SOURCE: Sarraj A et al. Stroke. 2020 Feb 12. doi: 10.1161/STROKEAHA.120.028850.
FROM STROKE
Statin, antihypertensive treatment don’t guarantee healthier lifestyles
When people learn they have enough cardiovascular disease risk to start treatment with a statin or antihypertensive drug, the impact on their healthy-lifestyle choices seems to often be a wash, based on findings from more than 40,000 Finland residents followed for at least 4 years after starting their primary-prevention regimen.
“Patients’ awareness of their risk factors alone seems not to be effective in improving health behaviors,” wrote Maarit J. Korhonen, PhD, and associates in a report published in the Journal of the American Heart Association.
“Initiation of antihypertensive or statin therapy appears to be associated with lifestyle changes, some positive and others negative,” wrote Dr. Korhonen, a pharmacoepidemiologist at the University of Turku (Finland), and associates. This was the first reported study to assess a large-scale and prospectively followed cohort to look for associations between the use of medicines that prevent cardiovascular disease (CVD) and lifestyle changes. Most previous studies of these associations “have been cross sectional and provide no information on potential lifestyle changes during the time window around the initiation of medication use,” they added.
The new study specifically found that, on average, people who began treatment with at least one CVD-prevention medication for the first time were more likely to gain weight and more likely to become less active during the years following their treatment onset. But at the same time, these patients were also more likely to either quit or cut down on their smoking and alcohol consumption, the researchers found.
Their analysis used data from 41,225 people enrolled in the Finnish Public Sector Study, which prospectively began collecting data on a large number of Finland residents in the 1990s. They specifically focused on 81,772 completed questionnaires – collected at 4-year intervals – from people who completed at least two consecutive rounds of the survey during 2000-2013, and who were also at least 40 years old and free of prevalent CVD at the time of their first survey. The participants averaged nearly 53 years of age at their first survey, and 84% were women.
The researchers subdivided the survey responses into 8,837 (11%) people who began a statin, antihypertensive drug, or both during their participation; 26,914 (33%) already on a statin or antihypertensive drug when they completed their first questionnaire; and 46,021 response sets (56%) from people who never began treatment with either drug class. People who initiated a relevant drug began a median of 1.7 years following completion of their first survey, and a median of 2.4 years before their next survey. During follow-up, about 2% of all participants became newly diagnosed with some form of CVD.
The results showed that, after full adjustment for possible confounders, the mean increase in body mass index was larger among those who initiated a CVD-prevention drug, compared with those who did not. Among participants who were obese at entry, those who started a CVD drug had a statistically significant 37% increased rate of remaining obese, compared with those not starting these drugs. Among those who were not obese at baseline, those who began a CVD prevention drug had a statistically significant 82%% higher rate of becoming obese, compared with those not on a CVD-prevention drug. In addition, average daily energy expenditure, a measure of physical activity, showed a statistically significant decline among those who started a CVD drug, compared with those who did not. In contrast, CVD drug initiators had an average 1.85 gram/week decline in alcohol intake, compared with noninitiators, and those who were current smokers at the first survey and then started a CVD drug had a 26% relative drop in their smoking prevalence, compared with those who did not start a CVD drug, both statistically significant differences.
The findings suggest that “patients’ awareness of their risk factors alone seems not to be effective in improving health behaviors,” the authors concluded. “This means that expansion of pharmacologic interventions toward populations at low CVD risk may not necessarily lead to expected benefits at the population level.”
The study received no commercial funding. Dr. Korhonen had no disclosures.
SOURCE: Korhonen MJ et al. J Am Heart Assoc. 2020 Feb 5. doi: 10.1161/JAHA.119.014.168.
When people learn they have enough cardiovascular disease risk to start treatment with a statin or antihypertensive drug, the impact on their healthy-lifestyle choices seems to often be a wash, based on findings from more than 40,000 Finland residents followed for at least 4 years after starting their primary-prevention regimen.
“Patients’ awareness of their risk factors alone seems not to be effective in improving health behaviors,” wrote Maarit J. Korhonen, PhD, and associates in a report published in the Journal of the American Heart Association.
“Initiation of antihypertensive or statin therapy appears to be associated with lifestyle changes, some positive and others negative,” wrote Dr. Korhonen, a pharmacoepidemiologist at the University of Turku (Finland), and associates. This was the first reported study to assess a large-scale and prospectively followed cohort to look for associations between the use of medicines that prevent cardiovascular disease (CVD) and lifestyle changes. Most previous studies of these associations “have been cross sectional and provide no information on potential lifestyle changes during the time window around the initiation of medication use,” they added.
The new study specifically found that, on average, people who began treatment with at least one CVD-prevention medication for the first time were more likely to gain weight and more likely to become less active during the years following their treatment onset. But at the same time, these patients were also more likely to either quit or cut down on their smoking and alcohol consumption, the researchers found.
Their analysis used data from 41,225 people enrolled in the Finnish Public Sector Study, which prospectively began collecting data on a large number of Finland residents in the 1990s. They specifically focused on 81,772 completed questionnaires – collected at 4-year intervals – from people who completed at least two consecutive rounds of the survey during 2000-2013, and who were also at least 40 years old and free of prevalent CVD at the time of their first survey. The participants averaged nearly 53 years of age at their first survey, and 84% were women.
The researchers subdivided the survey responses into 8,837 (11%) people who began a statin, antihypertensive drug, or both during their participation; 26,914 (33%) already on a statin or antihypertensive drug when they completed their first questionnaire; and 46,021 response sets (56%) from people who never began treatment with either drug class. People who initiated a relevant drug began a median of 1.7 years following completion of their first survey, and a median of 2.4 years before their next survey. During follow-up, about 2% of all participants became newly diagnosed with some form of CVD.
The results showed that, after full adjustment for possible confounders, the mean increase in body mass index was larger among those who initiated a CVD-prevention drug, compared with those who did not. Among participants who were obese at entry, those who started a CVD drug had a statistically significant 37% increased rate of remaining obese, compared with those not starting these drugs. Among those who were not obese at baseline, those who began a CVD prevention drug had a statistically significant 82%% higher rate of becoming obese, compared with those not on a CVD-prevention drug. In addition, average daily energy expenditure, a measure of physical activity, showed a statistically significant decline among those who started a CVD drug, compared with those who did not. In contrast, CVD drug initiators had an average 1.85 gram/week decline in alcohol intake, compared with noninitiators, and those who were current smokers at the first survey and then started a CVD drug had a 26% relative drop in their smoking prevalence, compared with those who did not start a CVD drug, both statistically significant differences.
The findings suggest that “patients’ awareness of their risk factors alone seems not to be effective in improving health behaviors,” the authors concluded. “This means that expansion of pharmacologic interventions toward populations at low CVD risk may not necessarily lead to expected benefits at the population level.”
The study received no commercial funding. Dr. Korhonen had no disclosures.
SOURCE: Korhonen MJ et al. J Am Heart Assoc. 2020 Feb 5. doi: 10.1161/JAHA.119.014.168.
When people learn they have enough cardiovascular disease risk to start treatment with a statin or antihypertensive drug, the impact on their healthy-lifestyle choices seems to often be a wash, based on findings from more than 40,000 Finland residents followed for at least 4 years after starting their primary-prevention regimen.
“Patients’ awareness of their risk factors alone seems not to be effective in improving health behaviors,” wrote Maarit J. Korhonen, PhD, and associates in a report published in the Journal of the American Heart Association.
“Initiation of antihypertensive or statin therapy appears to be associated with lifestyle changes, some positive and others negative,” wrote Dr. Korhonen, a pharmacoepidemiologist at the University of Turku (Finland), and associates. This was the first reported study to assess a large-scale and prospectively followed cohort to look for associations between the use of medicines that prevent cardiovascular disease (CVD) and lifestyle changes. Most previous studies of these associations “have been cross sectional and provide no information on potential lifestyle changes during the time window around the initiation of medication use,” they added.
The new study specifically found that, on average, people who began treatment with at least one CVD-prevention medication for the first time were more likely to gain weight and more likely to become less active during the years following their treatment onset. But at the same time, these patients were also more likely to either quit or cut down on their smoking and alcohol consumption, the researchers found.
Their analysis used data from 41,225 people enrolled in the Finnish Public Sector Study, which prospectively began collecting data on a large number of Finland residents in the 1990s. They specifically focused on 81,772 completed questionnaires – collected at 4-year intervals – from people who completed at least two consecutive rounds of the survey during 2000-2013, and who were also at least 40 years old and free of prevalent CVD at the time of their first survey. The participants averaged nearly 53 years of age at their first survey, and 84% were women.
The researchers subdivided the survey responses into 8,837 (11%) people who began a statin, antihypertensive drug, or both during their participation; 26,914 (33%) already on a statin or antihypertensive drug when they completed their first questionnaire; and 46,021 response sets (56%) from people who never began treatment with either drug class. People who initiated a relevant drug began a median of 1.7 years following completion of their first survey, and a median of 2.4 years before their next survey. During follow-up, about 2% of all participants became newly diagnosed with some form of CVD.
The results showed that, after full adjustment for possible confounders, the mean increase in body mass index was larger among those who initiated a CVD-prevention drug, compared with those who did not. Among participants who were obese at entry, those who started a CVD drug had a statistically significant 37% increased rate of remaining obese, compared with those not starting these drugs. Among those who were not obese at baseline, those who began a CVD prevention drug had a statistically significant 82%% higher rate of becoming obese, compared with those not on a CVD-prevention drug. In addition, average daily energy expenditure, a measure of physical activity, showed a statistically significant decline among those who started a CVD drug, compared with those who did not. In contrast, CVD drug initiators had an average 1.85 gram/week decline in alcohol intake, compared with noninitiators, and those who were current smokers at the first survey and then started a CVD drug had a 26% relative drop in their smoking prevalence, compared with those who did not start a CVD drug, both statistically significant differences.
The findings suggest that “patients’ awareness of their risk factors alone seems not to be effective in improving health behaviors,” the authors concluded. “This means that expansion of pharmacologic interventions toward populations at low CVD risk may not necessarily lead to expected benefits at the population level.”
The study received no commercial funding. Dr. Korhonen had no disclosures.
SOURCE: Korhonen MJ et al. J Am Heart Assoc. 2020 Feb 5. doi: 10.1161/JAHA.119.014.168.
FROM THE JOURNAL OF THE AMERICAN HEART ASSOCIATION
Heart rhythm data from wearables confounds EP practice
NATIONAL HARBOR, MD. – or other warnings that flagged a possible cardiac arrhythmia.
While the clinical community has yet to reach an evidence-based consensus on how to deal with this information, or even asymptomatic arrhythmias identified by more standard means, the American public is voting with their wrists. People seem to like collecting and reviewing readouts on their heart rhythm and other vital data, and then they often take their numbers to a physician, especially when their device suggests a possible problem.
“The whole paradigm of ordering a test only if you intend to act on the result has been flipped. People now get what they want directly, and our job is to guide them” after the fact. “You need to teach people what’s actionable and what’s not,” Mintu P. Turakhia, MD, said at the annual International AF Symposium.
“We’re in a situation where the ability of a sensor to detect things is separate from access to the health care system. They are no longer coupled; they are disjointed. People can create their own ICU in their house just by shopping online, but what do we do with this information, whether it’s an irregular heart rhythm or their whole genome?” asked Dr. Turakhia, executive director of the Center for Digital Health at Stanford (Calif.) University and director of cardiac electrophysiology at the VA Palo Alto (Calif.) Health Care System.
“The main challenge is people without a diagnosis who get a notification. How much monitoring should you do until you can say it was a false positive? We don’t know what to do, so we monitor them. People are trying to figure this out.” Dr. Turakhia said. Some people who seek out electrophysiologists this way “may not even have a primary care physician,” he noted.
The potential implications of widespread monitoring for heartbeat irregularities in the general public began to surface in a study that Dr. Turakhia helped run that collected wearable data from nearly 420,000 Americans. Results from the Apple Heart Study showed that, during a median 117 days of monitoring with a smart watch, 2,161 people (0.5%) received a report of an irregular pulse, which led to further investigations that eventually diagnosed atrial fibrillation (AFib) in 153 people of the 450 who underwent follow-up assessment (N Engl J Med. 2019 Nov 14;381[20]:1909-17). These results “raise questions” about the large number of people who underwent follow-up testing who did not have arrhythmia, Dr. Turakhia noted.
“The dissemination of wearables has been quite dramatic, and electrophysiologists end up owning this,” commented Jeremy N. Ruskin, MD, professor of medicine at Harvard Medical School and director emeritus of the cardiac arrhythmia service at Massachusetts General Hospital, both in Boston. “I get a ton of calls from people whom I wish never bought a smart watch, and they say ‘I have atrial fibrillation. What do I do?’ ”
To document the growth of this trend, Dr. Turakhia cited results from a survey he collaborated on, run by Stanford and Rock Health, that found a 33% ownership rate among American adults of a wearable device capable of collecting health data, and a 42% rate of people who tracked their health data with a device, app, journal, or log. Both of these rates more than doubled what a similar survey found in 2015.
The cardiac electrophysiology community took a first step toward addressing one aspect of this evolving situation. In early 2020, the Heart Rhythm Society and the Consumer Technology Association jointly issued a guidance document targeted at consumers that walks them through the kinds of information their wearable devices might collect and how to approach this information. The main message: If you have questions or concerns about your data, consult a clinician. What remains in short supply is guidance to clinicians on what to do when they see these patients.
“Until recently, device-detected AFib was the sole purview of electrophysiologists using implanted rhythm-monitoring devices. Now mobile and other devices raise issues [of asymptomatic AFib] for a much broader population,” noted Daniel E. Singer, MD, professor of medicine and epidemiology at Harvard and Massachusetts General Hospital. “The world of device-detected AFib now includes watches.”
Researchers have tried for years to better understand the stroke risk faced by patients with asymptomatic or subclinical AFib that’s detected by an implanted device, as in the ASSERT study of nearly 2,600 patients followed for a median of 2.5 years that found an increased stroke risk when the duration of individual, subclinical AFib episodes surpassed 24 hours (Eur Heart J. 2017 May 1;38[17]:1339-44). More recently, a study of AFib duration collected by implanted cardiac devices in nearly 22,000 Americans showed a relationship between stroke risk and both the duration of AFib episodes and the underlying risk of a person for stroke as measured by their CHA2DS2-VAScscore (Circulation. 2019 Nov 12;140[20]:1639-46). Just under 30% of patients in the study were diagnosed with AFib at entry.
The implications of asymptomatic episodes of AFib have so far been largely studied in people with an implanted cardiac device, which may have limited applicability to wearable users. In addition, the field has not yet fully sorted out the relationships between the duration of individual AFib episodes and overall AFib burden, and a person’s stroke risk and the window of time when the potential stroke-preventing benefits of anticoagulation outweigh its bleeding risk.
The results of trials now in progress that are examining the safety and efficacy of medical interventions designed to avert strokes in patients with asymptomatic AFib “will bear on the use of anticoagulants in a large group of patients,” including people with AFib detected by a wearable, Dr. Singer predicted.
The Apple Heart Study was sponsored Apple. Dr. Turakhia has received funding from Apple, and he has received honoraria or research funding from several other companies. Dr. Ruskin has been a consultant or adviser to several companies, is a steering committee member for Pfizer, and holds equity or options in Portola, Element Science, NewPace, Gilead, and InfoBionic. Dr. Singer has been a consultant and adviser to Boehringer Ingelheim, Bristol-Myers Squibb, Johnson & Johnson, Merck, and Pfizer, and he has received research grants from Bristol-Myers Squibb.
NATIONAL HARBOR, MD. – or other warnings that flagged a possible cardiac arrhythmia.
While the clinical community has yet to reach an evidence-based consensus on how to deal with this information, or even asymptomatic arrhythmias identified by more standard means, the American public is voting with their wrists. People seem to like collecting and reviewing readouts on their heart rhythm and other vital data, and then they often take their numbers to a physician, especially when their device suggests a possible problem.
“The whole paradigm of ordering a test only if you intend to act on the result has been flipped. People now get what they want directly, and our job is to guide them” after the fact. “You need to teach people what’s actionable and what’s not,” Mintu P. Turakhia, MD, said at the annual International AF Symposium.
“We’re in a situation where the ability of a sensor to detect things is separate from access to the health care system. They are no longer coupled; they are disjointed. People can create their own ICU in their house just by shopping online, but what do we do with this information, whether it’s an irregular heart rhythm or their whole genome?” asked Dr. Turakhia, executive director of the Center for Digital Health at Stanford (Calif.) University and director of cardiac electrophysiology at the VA Palo Alto (Calif.) Health Care System.
“The main challenge is people without a diagnosis who get a notification. How much monitoring should you do until you can say it was a false positive? We don’t know what to do, so we monitor them. People are trying to figure this out.” Dr. Turakhia said. Some people who seek out electrophysiologists this way “may not even have a primary care physician,” he noted.
The potential implications of widespread monitoring for heartbeat irregularities in the general public began to surface in a study that Dr. Turakhia helped run that collected wearable data from nearly 420,000 Americans. Results from the Apple Heart Study showed that, during a median 117 days of monitoring with a smart watch, 2,161 people (0.5%) received a report of an irregular pulse, which led to further investigations that eventually diagnosed atrial fibrillation (AFib) in 153 people of the 450 who underwent follow-up assessment (N Engl J Med. 2019 Nov 14;381[20]:1909-17). These results “raise questions” about the large number of people who underwent follow-up testing who did not have arrhythmia, Dr. Turakhia noted.
“The dissemination of wearables has been quite dramatic, and electrophysiologists end up owning this,” commented Jeremy N. Ruskin, MD, professor of medicine at Harvard Medical School and director emeritus of the cardiac arrhythmia service at Massachusetts General Hospital, both in Boston. “I get a ton of calls from people whom I wish never bought a smart watch, and they say ‘I have atrial fibrillation. What do I do?’ ”
To document the growth of this trend, Dr. Turakhia cited results from a survey he collaborated on, run by Stanford and Rock Health, that found a 33% ownership rate among American adults of a wearable device capable of collecting health data, and a 42% rate of people who tracked their health data with a device, app, journal, or log. Both of these rates more than doubled what a similar survey found in 2015.
The cardiac electrophysiology community took a first step toward addressing one aspect of this evolving situation. In early 2020, the Heart Rhythm Society and the Consumer Technology Association jointly issued a guidance document targeted at consumers that walks them through the kinds of information their wearable devices might collect and how to approach this information. The main message: If you have questions or concerns about your data, consult a clinician. What remains in short supply is guidance to clinicians on what to do when they see these patients.
“Until recently, device-detected AFib was the sole purview of electrophysiologists using implanted rhythm-monitoring devices. Now mobile and other devices raise issues [of asymptomatic AFib] for a much broader population,” noted Daniel E. Singer, MD, professor of medicine and epidemiology at Harvard and Massachusetts General Hospital. “The world of device-detected AFib now includes watches.”
Researchers have tried for years to better understand the stroke risk faced by patients with asymptomatic or subclinical AFib that’s detected by an implanted device, as in the ASSERT study of nearly 2,600 patients followed for a median of 2.5 years that found an increased stroke risk when the duration of individual, subclinical AFib episodes surpassed 24 hours (Eur Heart J. 2017 May 1;38[17]:1339-44). More recently, a study of AFib duration collected by implanted cardiac devices in nearly 22,000 Americans showed a relationship between stroke risk and both the duration of AFib episodes and the underlying risk of a person for stroke as measured by their CHA2DS2-VAScscore (Circulation. 2019 Nov 12;140[20]:1639-46). Just under 30% of patients in the study were diagnosed with AFib at entry.
The implications of asymptomatic episodes of AFib have so far been largely studied in people with an implanted cardiac device, which may have limited applicability to wearable users. In addition, the field has not yet fully sorted out the relationships between the duration of individual AFib episodes and overall AFib burden, and a person’s stroke risk and the window of time when the potential stroke-preventing benefits of anticoagulation outweigh its bleeding risk.
The results of trials now in progress that are examining the safety and efficacy of medical interventions designed to avert strokes in patients with asymptomatic AFib “will bear on the use of anticoagulants in a large group of patients,” including people with AFib detected by a wearable, Dr. Singer predicted.
The Apple Heart Study was sponsored Apple. Dr. Turakhia has received funding from Apple, and he has received honoraria or research funding from several other companies. Dr. Ruskin has been a consultant or adviser to several companies, is a steering committee member for Pfizer, and holds equity or options in Portola, Element Science, NewPace, Gilead, and InfoBionic. Dr. Singer has been a consultant and adviser to Boehringer Ingelheim, Bristol-Myers Squibb, Johnson & Johnson, Merck, and Pfizer, and he has received research grants from Bristol-Myers Squibb.
NATIONAL HARBOR, MD. – or other warnings that flagged a possible cardiac arrhythmia.
While the clinical community has yet to reach an evidence-based consensus on how to deal with this information, or even asymptomatic arrhythmias identified by more standard means, the American public is voting with their wrists. People seem to like collecting and reviewing readouts on their heart rhythm and other vital data, and then they often take their numbers to a physician, especially when their device suggests a possible problem.
“The whole paradigm of ordering a test only if you intend to act on the result has been flipped. People now get what they want directly, and our job is to guide them” after the fact. “You need to teach people what’s actionable and what’s not,” Mintu P. Turakhia, MD, said at the annual International AF Symposium.
“We’re in a situation where the ability of a sensor to detect things is separate from access to the health care system. They are no longer coupled; they are disjointed. People can create their own ICU in their house just by shopping online, but what do we do with this information, whether it’s an irregular heart rhythm or their whole genome?” asked Dr. Turakhia, executive director of the Center for Digital Health at Stanford (Calif.) University and director of cardiac electrophysiology at the VA Palo Alto (Calif.) Health Care System.
“The main challenge is people without a diagnosis who get a notification. How much monitoring should you do until you can say it was a false positive? We don’t know what to do, so we monitor them. People are trying to figure this out.” Dr. Turakhia said. Some people who seek out electrophysiologists this way “may not even have a primary care physician,” he noted.
The potential implications of widespread monitoring for heartbeat irregularities in the general public began to surface in a study that Dr. Turakhia helped run that collected wearable data from nearly 420,000 Americans. Results from the Apple Heart Study showed that, during a median 117 days of monitoring with a smart watch, 2,161 people (0.5%) received a report of an irregular pulse, which led to further investigations that eventually diagnosed atrial fibrillation (AFib) in 153 people of the 450 who underwent follow-up assessment (N Engl J Med. 2019 Nov 14;381[20]:1909-17). These results “raise questions” about the large number of people who underwent follow-up testing who did not have arrhythmia, Dr. Turakhia noted.
“The dissemination of wearables has been quite dramatic, and electrophysiologists end up owning this,” commented Jeremy N. Ruskin, MD, professor of medicine at Harvard Medical School and director emeritus of the cardiac arrhythmia service at Massachusetts General Hospital, both in Boston. “I get a ton of calls from people whom I wish never bought a smart watch, and they say ‘I have atrial fibrillation. What do I do?’ ”
To document the growth of this trend, Dr. Turakhia cited results from a survey he collaborated on, run by Stanford and Rock Health, that found a 33% ownership rate among American adults of a wearable device capable of collecting health data, and a 42% rate of people who tracked their health data with a device, app, journal, or log. Both of these rates more than doubled what a similar survey found in 2015.
The cardiac electrophysiology community took a first step toward addressing one aspect of this evolving situation. In early 2020, the Heart Rhythm Society and the Consumer Technology Association jointly issued a guidance document targeted at consumers that walks them through the kinds of information their wearable devices might collect and how to approach this information. The main message: If you have questions or concerns about your data, consult a clinician. What remains in short supply is guidance to clinicians on what to do when they see these patients.
“Until recently, device-detected AFib was the sole purview of electrophysiologists using implanted rhythm-monitoring devices. Now mobile and other devices raise issues [of asymptomatic AFib] for a much broader population,” noted Daniel E. Singer, MD, professor of medicine and epidemiology at Harvard and Massachusetts General Hospital. “The world of device-detected AFib now includes watches.”
Researchers have tried for years to better understand the stroke risk faced by patients with asymptomatic or subclinical AFib that’s detected by an implanted device, as in the ASSERT study of nearly 2,600 patients followed for a median of 2.5 years that found an increased stroke risk when the duration of individual, subclinical AFib episodes surpassed 24 hours (Eur Heart J. 2017 May 1;38[17]:1339-44). More recently, a study of AFib duration collected by implanted cardiac devices in nearly 22,000 Americans showed a relationship between stroke risk and both the duration of AFib episodes and the underlying risk of a person for stroke as measured by their CHA2DS2-VAScscore (Circulation. 2019 Nov 12;140[20]:1639-46). Just under 30% of patients in the study were diagnosed with AFib at entry.
The implications of asymptomatic episodes of AFib have so far been largely studied in people with an implanted cardiac device, which may have limited applicability to wearable users. In addition, the field has not yet fully sorted out the relationships between the duration of individual AFib episodes and overall AFib burden, and a person’s stroke risk and the window of time when the potential stroke-preventing benefits of anticoagulation outweigh its bleeding risk.
The results of trials now in progress that are examining the safety and efficacy of medical interventions designed to avert strokes in patients with asymptomatic AFib “will bear on the use of anticoagulants in a large group of patients,” including people with AFib detected by a wearable, Dr. Singer predicted.
The Apple Heart Study was sponsored Apple. Dr. Turakhia has received funding from Apple, and he has received honoraria or research funding from several other companies. Dr. Ruskin has been a consultant or adviser to several companies, is a steering committee member for Pfizer, and holds equity or options in Portola, Element Science, NewPace, Gilead, and InfoBionic. Dr. Singer has been a consultant and adviser to Boehringer Ingelheim, Bristol-Myers Squibb, Johnson & Johnson, Merck, and Pfizer, and he has received research grants from Bristol-Myers Squibb.
REPORTING FROM THE AF SYMPOSIUM 2020
Expanded indication for leadless pacemaker triples eligible patients
The U.S. Food and Drug Administration’s approval of an expanded indication for a leadless pacemaker for patients “who may benefit from maintenance of atrioventricular synchrony” will make this technology potentially available to nearly half of the Americans who need a pacemaker, roughly triple the number of patients who have been candidates for a leadless pacemaker up to now.
“This approval was huge. The complication rate with leadless pacemakers has been 63% less than the rate using pacemakers with transvenous leads,” said Larry A. Chinitz, MD, a cardiac electrophysiologist and a coinvestigator on some of the studies that led to the new indication. By expanding the types of patients suitable for leadless pacing “we’ll achieve AV [atrioventricular] synchrony in more patients with fewer complications,” said Dr. Chinitz, professor of medicine and director of the Cardiac Electrophysiology and Heart Rhythm Center at NYU Langone Health in New York.
Because the device is both leadless and requires no pocket owing to its small size and placement in a patient’s right ventricle, it has implications for potentially broadening the population that could benefit from the device, he said in an interview. “When we started with this pacemaker, it was limited to elderly patients with persistent atrial fibrillation who needed only ventricular pacing, a very small group,” just under 15% of the universe of patients who need pacemakers. The broadened indication, for patients with high-grade AV block who also have atrial function, makes it possible to think of using this safer and easier-to-place device in patients who need infrequent pacing, and in patients with multiple comorbidities that give them an increased complication risk, he said. The new indication means “you’re treating a much broader patient population, doing it more safely, and creating the foundation for expanding this technology.”
The Micra AV pacemaker uses the same basic design as the previously approved Micra Transcatheter Pacing System, which came onto the U.S. market in 2016 and provides single-chamber pacing. An accelerometer on the device allows it to detect atrial motion and thereby synchronize ventricular and atrial contractions, which led to the new indication. Although the Micra AV device looks similar to the original single-chamber model, it has an entirely new circuitry that prolongs battery life during dual-chamber pacing as well as new software that incorporates the accelerometer data, explained Robert Kowal, MD, a cardiac electrophysiologist, and vice president of medical affairs and chief medical officer of cardiac rhythm and heart failure at Medtronic in Minneapolis. The battery of the Micra AV is designed to last about 15 years, Dr. Chinitz noted.
Results from two studies that Dr. Chinitz helped run established the safety and efficacy of the device for dual-chamber pacing. The MARVEL (Micra Atrial Tracking Using a Ventricular Accelerometer) study included 64 patients who completed the study at 12 worldwide centers, which produced an average 80% AV synchrony in 33 patients with high-degree AV block (The other patients in the study had predominantly intrinsic AV conduction; Heart Rhythm. 2018 Sep;15[9]:1363-71). The MARVEL 2 study included 75 patients with either second- or third-degree AV block at 12 worldwide centers and showed that AV synchrony increased from an average of 27% without two-chamber pacing to 89% with the dual-chamber function turned on, and with 95% of patients achieving at least 70% AV synchrony (JACC Clin Electrophysiol. 2020 Jan;6[1]:94-106).
The 2016 indication for single-chamber pacing included patients with “high-grade” AV bloc with or without atrial fibrillation, typically patients for whom dual-chamber pacemaker was not a great option because of the risks for complication but with the downside of limited AV synchrony, a limitation now mitigated by the option of mechanical synchronization, Dr. Kowal said. The AV device remains intended for patients with high-grade AV node block, which means patients with second- or third-degree block, he added in an interview. The estimated prevalence of third-degree AV block among U.S. adults is about 0.02%, which translates into about 50,000 people; the estimated prevalence of second-degree AV block is much less, about 10% of the third-degree prevalence.
Despite the substantial cut in complications by a leadless and pocketless pacemaker, “some patients may still benefit from a traditional dual-chamber pacemaker,” specifically active patients who might sometimes get their heart rates up with exercise to levels of about 150 beats/min or higher, Dr. Kowal said. That’s because currently the programing algorithms used to synchronize the ventricle and atrium become less reliable at heart rates above 105 beats/min, he explained. However, the ability for mechanical synchronization to keep up at higher heart rates should improve as additional data are collected that can refine the algorithms. It’s also unusual for most patients who are pacemaker candidates to reach heart rates this high, he said.
The MARVEL and MARVEL 2 studies were sponsored by Medtronic, the company that markets Micra pacemakers. Dr. Chinitz has received fees and fellowship support from Medtronic, and has also received fees from Abbott, Biosense Webster, Biotronik, and Pfizer, and he has also received fellowship support from Biotronik and Boston Scientific. Dr. Kowal is a Medtronic employee.
The U.S. Food and Drug Administration’s approval of an expanded indication for a leadless pacemaker for patients “who may benefit from maintenance of atrioventricular synchrony” will make this technology potentially available to nearly half of the Americans who need a pacemaker, roughly triple the number of patients who have been candidates for a leadless pacemaker up to now.
“This approval was huge. The complication rate with leadless pacemakers has been 63% less than the rate using pacemakers with transvenous leads,” said Larry A. Chinitz, MD, a cardiac electrophysiologist and a coinvestigator on some of the studies that led to the new indication. By expanding the types of patients suitable for leadless pacing “we’ll achieve AV [atrioventricular] synchrony in more patients with fewer complications,” said Dr. Chinitz, professor of medicine and director of the Cardiac Electrophysiology and Heart Rhythm Center at NYU Langone Health in New York.
Because the device is both leadless and requires no pocket owing to its small size and placement in a patient’s right ventricle, it has implications for potentially broadening the population that could benefit from the device, he said in an interview. “When we started with this pacemaker, it was limited to elderly patients with persistent atrial fibrillation who needed only ventricular pacing, a very small group,” just under 15% of the universe of patients who need pacemakers. The broadened indication, for patients with high-grade AV block who also have atrial function, makes it possible to think of using this safer and easier-to-place device in patients who need infrequent pacing, and in patients with multiple comorbidities that give them an increased complication risk, he said. The new indication means “you’re treating a much broader patient population, doing it more safely, and creating the foundation for expanding this technology.”
The Micra AV pacemaker uses the same basic design as the previously approved Micra Transcatheter Pacing System, which came onto the U.S. market in 2016 and provides single-chamber pacing. An accelerometer on the device allows it to detect atrial motion and thereby synchronize ventricular and atrial contractions, which led to the new indication. Although the Micra AV device looks similar to the original single-chamber model, it has an entirely new circuitry that prolongs battery life during dual-chamber pacing as well as new software that incorporates the accelerometer data, explained Robert Kowal, MD, a cardiac electrophysiologist, and vice president of medical affairs and chief medical officer of cardiac rhythm and heart failure at Medtronic in Minneapolis. The battery of the Micra AV is designed to last about 15 years, Dr. Chinitz noted.
Results from two studies that Dr. Chinitz helped run established the safety and efficacy of the device for dual-chamber pacing. The MARVEL (Micra Atrial Tracking Using a Ventricular Accelerometer) study included 64 patients who completed the study at 12 worldwide centers, which produced an average 80% AV synchrony in 33 patients with high-degree AV block (The other patients in the study had predominantly intrinsic AV conduction; Heart Rhythm. 2018 Sep;15[9]:1363-71). The MARVEL 2 study included 75 patients with either second- or third-degree AV block at 12 worldwide centers and showed that AV synchrony increased from an average of 27% without two-chamber pacing to 89% with the dual-chamber function turned on, and with 95% of patients achieving at least 70% AV synchrony (JACC Clin Electrophysiol. 2020 Jan;6[1]:94-106).
The 2016 indication for single-chamber pacing included patients with “high-grade” AV bloc with or without atrial fibrillation, typically patients for whom dual-chamber pacemaker was not a great option because of the risks for complication but with the downside of limited AV synchrony, a limitation now mitigated by the option of mechanical synchronization, Dr. Kowal said. The AV device remains intended for patients with high-grade AV node block, which means patients with second- or third-degree block, he added in an interview. The estimated prevalence of third-degree AV block among U.S. adults is about 0.02%, which translates into about 50,000 people; the estimated prevalence of second-degree AV block is much less, about 10% of the third-degree prevalence.
Despite the substantial cut in complications by a leadless and pocketless pacemaker, “some patients may still benefit from a traditional dual-chamber pacemaker,” specifically active patients who might sometimes get their heart rates up with exercise to levels of about 150 beats/min or higher, Dr. Kowal said. That’s because currently the programing algorithms used to synchronize the ventricle and atrium become less reliable at heart rates above 105 beats/min, he explained. However, the ability for mechanical synchronization to keep up at higher heart rates should improve as additional data are collected that can refine the algorithms. It’s also unusual for most patients who are pacemaker candidates to reach heart rates this high, he said.
The MARVEL and MARVEL 2 studies were sponsored by Medtronic, the company that markets Micra pacemakers. Dr. Chinitz has received fees and fellowship support from Medtronic, and has also received fees from Abbott, Biosense Webster, Biotronik, and Pfizer, and he has also received fellowship support from Biotronik and Boston Scientific. Dr. Kowal is a Medtronic employee.
The U.S. Food and Drug Administration’s approval of an expanded indication for a leadless pacemaker for patients “who may benefit from maintenance of atrioventricular synchrony” will make this technology potentially available to nearly half of the Americans who need a pacemaker, roughly triple the number of patients who have been candidates for a leadless pacemaker up to now.
“This approval was huge. The complication rate with leadless pacemakers has been 63% less than the rate using pacemakers with transvenous leads,” said Larry A. Chinitz, MD, a cardiac electrophysiologist and a coinvestigator on some of the studies that led to the new indication. By expanding the types of patients suitable for leadless pacing “we’ll achieve AV [atrioventricular] synchrony in more patients with fewer complications,” said Dr. Chinitz, professor of medicine and director of the Cardiac Electrophysiology and Heart Rhythm Center at NYU Langone Health in New York.
Because the device is both leadless and requires no pocket owing to its small size and placement in a patient’s right ventricle, it has implications for potentially broadening the population that could benefit from the device, he said in an interview. “When we started with this pacemaker, it was limited to elderly patients with persistent atrial fibrillation who needed only ventricular pacing, a very small group,” just under 15% of the universe of patients who need pacemakers. The broadened indication, for patients with high-grade AV block who also have atrial function, makes it possible to think of using this safer and easier-to-place device in patients who need infrequent pacing, and in patients with multiple comorbidities that give them an increased complication risk, he said. The new indication means “you’re treating a much broader patient population, doing it more safely, and creating the foundation for expanding this technology.”
The Micra AV pacemaker uses the same basic design as the previously approved Micra Transcatheter Pacing System, which came onto the U.S. market in 2016 and provides single-chamber pacing. An accelerometer on the device allows it to detect atrial motion and thereby synchronize ventricular and atrial contractions, which led to the new indication. Although the Micra AV device looks similar to the original single-chamber model, it has an entirely new circuitry that prolongs battery life during dual-chamber pacing as well as new software that incorporates the accelerometer data, explained Robert Kowal, MD, a cardiac electrophysiologist, and vice president of medical affairs and chief medical officer of cardiac rhythm and heart failure at Medtronic in Minneapolis. The battery of the Micra AV is designed to last about 15 years, Dr. Chinitz noted.
Results from two studies that Dr. Chinitz helped run established the safety and efficacy of the device for dual-chamber pacing. The MARVEL (Micra Atrial Tracking Using a Ventricular Accelerometer) study included 64 patients who completed the study at 12 worldwide centers, which produced an average 80% AV synchrony in 33 patients with high-degree AV block (The other patients in the study had predominantly intrinsic AV conduction; Heart Rhythm. 2018 Sep;15[9]:1363-71). The MARVEL 2 study included 75 patients with either second- or third-degree AV block at 12 worldwide centers and showed that AV synchrony increased from an average of 27% without two-chamber pacing to 89% with the dual-chamber function turned on, and with 95% of patients achieving at least 70% AV synchrony (JACC Clin Electrophysiol. 2020 Jan;6[1]:94-106).
The 2016 indication for single-chamber pacing included patients with “high-grade” AV bloc with or without atrial fibrillation, typically patients for whom dual-chamber pacemaker was not a great option because of the risks for complication but with the downside of limited AV synchrony, a limitation now mitigated by the option of mechanical synchronization, Dr. Kowal said. The AV device remains intended for patients with high-grade AV node block, which means patients with second- or third-degree block, he added in an interview. The estimated prevalence of third-degree AV block among U.S. adults is about 0.02%, which translates into about 50,000 people; the estimated prevalence of second-degree AV block is much less, about 10% of the third-degree prevalence.
Despite the substantial cut in complications by a leadless and pocketless pacemaker, “some patients may still benefit from a traditional dual-chamber pacemaker,” specifically active patients who might sometimes get their heart rates up with exercise to levels of about 150 beats/min or higher, Dr. Kowal said. That’s because currently the programing algorithms used to synchronize the ventricle and atrium become less reliable at heart rates above 105 beats/min, he explained. However, the ability for mechanical synchronization to keep up at higher heart rates should improve as additional data are collected that can refine the algorithms. It’s also unusual for most patients who are pacemaker candidates to reach heart rates this high, he said.
The MARVEL and MARVEL 2 studies were sponsored by Medtronic, the company that markets Micra pacemakers. Dr. Chinitz has received fees and fellowship support from Medtronic, and has also received fees from Abbott, Biosense Webster, Biotronik, and Pfizer, and he has also received fellowship support from Biotronik and Boston Scientific. Dr. Kowal is a Medtronic employee.
Vigilance safely keeps AFib patients off anticoagulants post ablation
NATIONAL HARBOR, MD. – A pilot program of daily arrhythmia self-vigilance has allowed selected patients with no atrial fibrillation following a catheter ablation procedure to safely come off a regimen of daily oral anticoagulation despite having residual risk factors for ischemic stroke.
This program, which started several years ago at the University of Pennsylvania in Philadelphia, has now managed 190 patients and followed them for a median of just over 3 years, and during 576 patient-years of follow-up, just a single patient had an ischemic cerebrovascular event that occurred with no atrial fibrillation (AFib) recurrence and appeared to be caused by an atherosclerotic embolism, Francis E. Marchlinski, MD, said at the annual International AF Symposium.
Although this strategy has not yet been tested in a prospective, randomized trial, this anecdotal, single-center experience suggests that the approach is “safe and effective” for selected patients who are eager to come off of their anticoagulation regimen when they remain arrhythmia free following catheter ablation of their AFib, said Dr. Marchlinski, professor of medicine and director of electrophysiology at the University of Pennsylvania. He and his associates developed this strategy as a way to more safely allow these patients to stop taking a daily oral anticoagulant because he found that many patients were stopping on their own, with no safety strategy in place.
“Patients tell me they don’t want to be on an oral anticoagulant because a parent had a hemorrhagic stroke, and they say they’re willing to accept the risk” of having an ischemic stroke by coming off anticoagulation. “This is a way for them to do it safely,” Dr. Marchlinski said in an interview. He stressed that he only allows his patients to go this route if they understand the risk and accept their shared responsibility for vigilant, twice-daily pulse monitoring to detect resumption of an irregular heart beat.
Since 2011, Dr. Marchlinski’s program ablated 1,216 patients with AFib who then remained arrhythmia free during 3 weeks of continuous ECG monitoring following their procedure. Among these patients, 443 had a CHA2DS2-VAScscore of either 0 (men) or 1 (women) that indicated no ongoing need for oral anticoagulation according to current guidelines. Of the remaining 773 patients with a CHA2DS2-VASc score of at least 1 in men and 2 in women, the clinicians determined 583 to be ineligible for the program because of their unwillingness to accept the risk, unwillingness to comply with daily pulse checks, a history of asymptomatic AFib, a CHA2DS2-VASc score greater than 4, or a resting pulse above 90 beats per minute, leaving 190 patients eligible to participate. Among these patients, 105 (55%) had a CHA2DS2-VASc score of 2-4, which should prompt anticoagulation according to current guidelines.Participating patients committed to check their resting pulse by palpation at least twice daily and to contacting the program immediately if their resting rate spiked by more than 20 beats per minutes or in another way seemed irregular. Patients were also instructed to restart their oral anticoagulation immediately if they experienced AFib symptoms that persisted for more than 5 minutes. Many patients in the program also use a wearable device (usually a watch) to monitor their resting pulse and to generate a 30-second ECG recording that they can send as an electronic file to the University of Pennsylvania staff. “We embrace wearables,” Dr. Marchlinski said. Those without a wearable can undergo transtelephonic EEG monitoring to document a suspected arrhythmia recurrence, and all patients undergo annual monitoring by continuous ECG for at least 2 weeks.During follow-up, in addition to the 1 patient free from recurrent AFib who had an atherosclerotic embolism, 34 patients resumed anticoagulant treatment because of AFib recurrence; 12 withdrew from the program because of noncompliance or preference, or because an exclusion appeared; 29 resumed oral anticoagulation transiently but then discontinued the drug a second time when their AFib recurrence resolved; and 114 patients (60% of the starting cohort of 190) remained completely off anticoagulation during a median of 37 months. These data updated a published report from Dr. Marchlinski and his associates on their first 99 patients followed for a median of 30 months (J Cardiovasc Electrophysiol. 2019 May;30[5]:631-8).
This experience underscored the need for ongoing rhythm monitoring even in the absence of AFib symptoms, as six patients developed asymptomatic AFib detected by monitoring, including one patient whose recurrence occurred 30 months after the ablation procedure.
Dr. Marchlinski stressed the stringent selection process he applies to limit this approach to patients who are willing to faithfully monitor their pulse and symptoms daily, and who accept the risk that this approach may pose and their responsibility to stay in contact with the clinical team. The program calls patients at the 6-month mark between annual monitoring to remind them of their need for daily attention.
“Being off anticoagulants is very important to these patients,” he explained, and he highlighted the added workload this strategy places on his staff. “I think this has legs” for adoption by other cardiac arrhythmia programs, “but it depends on the time the staff is willing to spend” monitoring and following these patients, some of whom regularly send in ECG traces from their wearable devices for assessment. “It takes a village” to make this program work, he said.
Dr. Marchlinski has been a consultant to or has received honoraria from Abbott EP/St. Jude, Biosense Webster, Biotronik, Boston Scientific, and Medtronic.
NATIONAL HARBOR, MD. – A pilot program of daily arrhythmia self-vigilance has allowed selected patients with no atrial fibrillation following a catheter ablation procedure to safely come off a regimen of daily oral anticoagulation despite having residual risk factors for ischemic stroke.
This program, which started several years ago at the University of Pennsylvania in Philadelphia, has now managed 190 patients and followed them for a median of just over 3 years, and during 576 patient-years of follow-up, just a single patient had an ischemic cerebrovascular event that occurred with no atrial fibrillation (AFib) recurrence and appeared to be caused by an atherosclerotic embolism, Francis E. Marchlinski, MD, said at the annual International AF Symposium.
Although this strategy has not yet been tested in a prospective, randomized trial, this anecdotal, single-center experience suggests that the approach is “safe and effective” for selected patients who are eager to come off of their anticoagulation regimen when they remain arrhythmia free following catheter ablation of their AFib, said Dr. Marchlinski, professor of medicine and director of electrophysiology at the University of Pennsylvania. He and his associates developed this strategy as a way to more safely allow these patients to stop taking a daily oral anticoagulant because he found that many patients were stopping on their own, with no safety strategy in place.
“Patients tell me they don’t want to be on an oral anticoagulant because a parent had a hemorrhagic stroke, and they say they’re willing to accept the risk” of having an ischemic stroke by coming off anticoagulation. “This is a way for them to do it safely,” Dr. Marchlinski said in an interview. He stressed that he only allows his patients to go this route if they understand the risk and accept their shared responsibility for vigilant, twice-daily pulse monitoring to detect resumption of an irregular heart beat.
Since 2011, Dr. Marchlinski’s program ablated 1,216 patients with AFib who then remained arrhythmia free during 3 weeks of continuous ECG monitoring following their procedure. Among these patients, 443 had a CHA2DS2-VAScscore of either 0 (men) or 1 (women) that indicated no ongoing need for oral anticoagulation according to current guidelines. Of the remaining 773 patients with a CHA2DS2-VASc score of at least 1 in men and 2 in women, the clinicians determined 583 to be ineligible for the program because of their unwillingness to accept the risk, unwillingness to comply with daily pulse checks, a history of asymptomatic AFib, a CHA2DS2-VASc score greater than 4, or a resting pulse above 90 beats per minute, leaving 190 patients eligible to participate. Among these patients, 105 (55%) had a CHA2DS2-VASc score of 2-4, which should prompt anticoagulation according to current guidelines.Participating patients committed to check their resting pulse by palpation at least twice daily and to contacting the program immediately if their resting rate spiked by more than 20 beats per minutes or in another way seemed irregular. Patients were also instructed to restart their oral anticoagulation immediately if they experienced AFib symptoms that persisted for more than 5 minutes. Many patients in the program also use a wearable device (usually a watch) to monitor their resting pulse and to generate a 30-second ECG recording that they can send as an electronic file to the University of Pennsylvania staff. “We embrace wearables,” Dr. Marchlinski said. Those without a wearable can undergo transtelephonic EEG monitoring to document a suspected arrhythmia recurrence, and all patients undergo annual monitoring by continuous ECG for at least 2 weeks.During follow-up, in addition to the 1 patient free from recurrent AFib who had an atherosclerotic embolism, 34 patients resumed anticoagulant treatment because of AFib recurrence; 12 withdrew from the program because of noncompliance or preference, or because an exclusion appeared; 29 resumed oral anticoagulation transiently but then discontinued the drug a second time when their AFib recurrence resolved; and 114 patients (60% of the starting cohort of 190) remained completely off anticoagulation during a median of 37 months. These data updated a published report from Dr. Marchlinski and his associates on their first 99 patients followed for a median of 30 months (J Cardiovasc Electrophysiol. 2019 May;30[5]:631-8).
This experience underscored the need for ongoing rhythm monitoring even in the absence of AFib symptoms, as six patients developed asymptomatic AFib detected by monitoring, including one patient whose recurrence occurred 30 months after the ablation procedure.
Dr. Marchlinski stressed the stringent selection process he applies to limit this approach to patients who are willing to faithfully monitor their pulse and symptoms daily, and who accept the risk that this approach may pose and their responsibility to stay in contact with the clinical team. The program calls patients at the 6-month mark between annual monitoring to remind them of their need for daily attention.
“Being off anticoagulants is very important to these patients,” he explained, and he highlighted the added workload this strategy places on his staff. “I think this has legs” for adoption by other cardiac arrhythmia programs, “but it depends on the time the staff is willing to spend” monitoring and following these patients, some of whom regularly send in ECG traces from their wearable devices for assessment. “It takes a village” to make this program work, he said.
Dr. Marchlinski has been a consultant to or has received honoraria from Abbott EP/St. Jude, Biosense Webster, Biotronik, Boston Scientific, and Medtronic.
NATIONAL HARBOR, MD. – A pilot program of daily arrhythmia self-vigilance has allowed selected patients with no atrial fibrillation following a catheter ablation procedure to safely come off a regimen of daily oral anticoagulation despite having residual risk factors for ischemic stroke.
This program, which started several years ago at the University of Pennsylvania in Philadelphia, has now managed 190 patients and followed them for a median of just over 3 years, and during 576 patient-years of follow-up, just a single patient had an ischemic cerebrovascular event that occurred with no atrial fibrillation (AFib) recurrence and appeared to be caused by an atherosclerotic embolism, Francis E. Marchlinski, MD, said at the annual International AF Symposium.
Although this strategy has not yet been tested in a prospective, randomized trial, this anecdotal, single-center experience suggests that the approach is “safe and effective” for selected patients who are eager to come off of their anticoagulation regimen when they remain arrhythmia free following catheter ablation of their AFib, said Dr. Marchlinski, professor of medicine and director of electrophysiology at the University of Pennsylvania. He and his associates developed this strategy as a way to more safely allow these patients to stop taking a daily oral anticoagulant because he found that many patients were stopping on their own, with no safety strategy in place.
“Patients tell me they don’t want to be on an oral anticoagulant because a parent had a hemorrhagic stroke, and they say they’re willing to accept the risk” of having an ischemic stroke by coming off anticoagulation. “This is a way for them to do it safely,” Dr. Marchlinski said in an interview. He stressed that he only allows his patients to go this route if they understand the risk and accept their shared responsibility for vigilant, twice-daily pulse monitoring to detect resumption of an irregular heart beat.
Since 2011, Dr. Marchlinski’s program ablated 1,216 patients with AFib who then remained arrhythmia free during 3 weeks of continuous ECG monitoring following their procedure. Among these patients, 443 had a CHA2DS2-VAScscore of either 0 (men) or 1 (women) that indicated no ongoing need for oral anticoagulation according to current guidelines. Of the remaining 773 patients with a CHA2DS2-VASc score of at least 1 in men and 2 in women, the clinicians determined 583 to be ineligible for the program because of their unwillingness to accept the risk, unwillingness to comply with daily pulse checks, a history of asymptomatic AFib, a CHA2DS2-VASc score greater than 4, or a resting pulse above 90 beats per minute, leaving 190 patients eligible to participate. Among these patients, 105 (55%) had a CHA2DS2-VASc score of 2-4, which should prompt anticoagulation according to current guidelines.Participating patients committed to check their resting pulse by palpation at least twice daily and to contacting the program immediately if their resting rate spiked by more than 20 beats per minutes or in another way seemed irregular. Patients were also instructed to restart their oral anticoagulation immediately if they experienced AFib symptoms that persisted for more than 5 minutes. Many patients in the program also use a wearable device (usually a watch) to monitor their resting pulse and to generate a 30-second ECG recording that they can send as an electronic file to the University of Pennsylvania staff. “We embrace wearables,” Dr. Marchlinski said. Those without a wearable can undergo transtelephonic EEG monitoring to document a suspected arrhythmia recurrence, and all patients undergo annual monitoring by continuous ECG for at least 2 weeks.During follow-up, in addition to the 1 patient free from recurrent AFib who had an atherosclerotic embolism, 34 patients resumed anticoagulant treatment because of AFib recurrence; 12 withdrew from the program because of noncompliance or preference, or because an exclusion appeared; 29 resumed oral anticoagulation transiently but then discontinued the drug a second time when their AFib recurrence resolved; and 114 patients (60% of the starting cohort of 190) remained completely off anticoagulation during a median of 37 months. These data updated a published report from Dr. Marchlinski and his associates on their first 99 patients followed for a median of 30 months (J Cardiovasc Electrophysiol. 2019 May;30[5]:631-8).
This experience underscored the need for ongoing rhythm monitoring even in the absence of AFib symptoms, as six patients developed asymptomatic AFib detected by monitoring, including one patient whose recurrence occurred 30 months after the ablation procedure.
Dr. Marchlinski stressed the stringent selection process he applies to limit this approach to patients who are willing to faithfully monitor their pulse and symptoms daily, and who accept the risk that this approach may pose and their responsibility to stay in contact with the clinical team. The program calls patients at the 6-month mark between annual monitoring to remind them of their need for daily attention.
“Being off anticoagulants is very important to these patients,” he explained, and he highlighted the added workload this strategy places on his staff. “I think this has legs” for adoption by other cardiac arrhythmia programs, “but it depends on the time the staff is willing to spend” monitoring and following these patients, some of whom regularly send in ECG traces from their wearable devices for assessment. “It takes a village” to make this program work, he said.
Dr. Marchlinski has been a consultant to or has received honoraria from Abbott EP/St. Jude, Biosense Webster, Biotronik, Boston Scientific, and Medtronic.
REPORTING FROM THE AF SYMPOSIUM 2020