User login
Abnormal growth of the amygdala in infants tied to autism
A new study suggests that overgrowth of the amygdala in infants during the first 6-12 months of life is tied to a later diagnosis of autism spectrum disorder (ASD).
“The faster the amygdala grew in infancy, the more social difficulties the child showed when diagnosed with autism a year later,” first author Mark Shen, PhD, assistant professor of psychiatry and neuroscience, University of North Carolina, Chapel Hill, told this news organization.
The study was published online in the American Journal of Psychiatry.
Unique to autism
The amygdala plays a key role in processing memory, emotional responses, and decisionmaking.
It’s long been known that the amygdala is abnormally large in school-aged children with ASD, but until now, it was not known precisely when aberrant amygdala growth happens, what the clinical consequences may be, and whether amygdala overgrowth is unique to autism.
To investigate, Dr. Shen and colleagues evaluated 1,099 longitudinal MRI scans obtained during natural sleep at 6, 12, and 24 months of age in 408 infants in the Infant Brain Imaging Study (IBIS) Network.
The cohort included 58 infants at high likelihood of developing ASD who were later diagnosed with the disorder, 212 infants at high likelihood of ASD who did not develop ASD, 109 typically-developing control infants, and 29 infants with fragile X syndrome.
At 6 months, infants who developed ASD had typically sized amygdala volumes but showed significantly faster amygdala growth between 6 and 24 months, such that by 12 months the ASD group had significantly larger amygdala volume (Cohen’s d = 0.56), compared with all other groups.
Amygdala growth rate between 6 and 12 months was significantly associated with greater social deficits at 24 months when the children were diagnosed with ASD.
“We found that the amygdala grows too rapidly between 6 and 12 months of age, during a presymptomatic period in autism, prior to when the diagnostic symptoms of autism (social difficulties and repetitive behaviors) are evident and lead to the later diagnosis of autism,” Dr. Shen said in an interview.
This brain growth pattern appears to be unique to autism, as babies with the genetic disorder fragile X syndrome – another neurodevelopmental condition – showed a markedly different brain growth pattern: no differences in amygdala growth but enlargement of a different brain structure, the caudate, which was linked to increased repetitive behaviors, the investigators found.
Earlier intervention
Prior research has shown that children who are later diagnosed with ASD often display problems in infancy with how they attend to visual stimuli in their surroundings.
These early problems with processing visual and sensory information may put increased stress on the amygdala, potentially leading to amygdala hyperactivity, deficits in pruning dendritic connections, and overgrowth, Dr. Shen and colleagues hypothesize.
Amygdala overgrowth has also been linked to chronic stress in studies of other psychiatric conditions, such as depression and anxiety, and may provide a clue to understanding this observation in infants who later develop autism.
“This research suggests that an optimal time to begin supports for children who are at the highest likelihood of developing autism may be during the first year of life: to improve early precursors to social development, such as sensory processing, in babies even before social difficulties arise,” Dr. Shen said.
Cyrus A. Raji, MD, PhD, assistant professor of radiology and neurology, Washington University, St. Louis, said, “What makes this study important is the finding of abnormally increased amygdala growth rate in autism using a longitudinal design that focuses on earlier development.”
“While we are typically used to understanding brain structure as abnormally decreasing over time in certain disorders like Alzheimer’s disease, this study challenges us to understand that too much brain volume growth can also be abnormal in specific conditions,” Dr. Raji added.
This research was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institute of Environmental Health Sciences, and National Institute of Mental Health, along with Autism Speaks and the Simons Foundation. Dr. Shen and Dr. Raji have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new study suggests that overgrowth of the amygdala in infants during the first 6-12 months of life is tied to a later diagnosis of autism spectrum disorder (ASD).
“The faster the amygdala grew in infancy, the more social difficulties the child showed when diagnosed with autism a year later,” first author Mark Shen, PhD, assistant professor of psychiatry and neuroscience, University of North Carolina, Chapel Hill, told this news organization.
The study was published online in the American Journal of Psychiatry.
Unique to autism
The amygdala plays a key role in processing memory, emotional responses, and decisionmaking.
It’s long been known that the amygdala is abnormally large in school-aged children with ASD, but until now, it was not known precisely when aberrant amygdala growth happens, what the clinical consequences may be, and whether amygdala overgrowth is unique to autism.
To investigate, Dr. Shen and colleagues evaluated 1,099 longitudinal MRI scans obtained during natural sleep at 6, 12, and 24 months of age in 408 infants in the Infant Brain Imaging Study (IBIS) Network.
The cohort included 58 infants at high likelihood of developing ASD who were later diagnosed with the disorder, 212 infants at high likelihood of ASD who did not develop ASD, 109 typically-developing control infants, and 29 infants with fragile X syndrome.
At 6 months, infants who developed ASD had typically sized amygdala volumes but showed significantly faster amygdala growth between 6 and 24 months, such that by 12 months the ASD group had significantly larger amygdala volume (Cohen’s d = 0.56), compared with all other groups.
Amygdala growth rate between 6 and 12 months was significantly associated with greater social deficits at 24 months when the children were diagnosed with ASD.
“We found that the amygdala grows too rapidly between 6 and 12 months of age, during a presymptomatic period in autism, prior to when the diagnostic symptoms of autism (social difficulties and repetitive behaviors) are evident and lead to the later diagnosis of autism,” Dr. Shen said in an interview.
This brain growth pattern appears to be unique to autism, as babies with the genetic disorder fragile X syndrome – another neurodevelopmental condition – showed a markedly different brain growth pattern: no differences in amygdala growth but enlargement of a different brain structure, the caudate, which was linked to increased repetitive behaviors, the investigators found.
Earlier intervention
Prior research has shown that children who are later diagnosed with ASD often display problems in infancy with how they attend to visual stimuli in their surroundings.
These early problems with processing visual and sensory information may put increased stress on the amygdala, potentially leading to amygdala hyperactivity, deficits in pruning dendritic connections, and overgrowth, Dr. Shen and colleagues hypothesize.
Amygdala overgrowth has also been linked to chronic stress in studies of other psychiatric conditions, such as depression and anxiety, and may provide a clue to understanding this observation in infants who later develop autism.
“This research suggests that an optimal time to begin supports for children who are at the highest likelihood of developing autism may be during the first year of life: to improve early precursors to social development, such as sensory processing, in babies even before social difficulties arise,” Dr. Shen said.
Cyrus A. Raji, MD, PhD, assistant professor of radiology and neurology, Washington University, St. Louis, said, “What makes this study important is the finding of abnormally increased amygdala growth rate in autism using a longitudinal design that focuses on earlier development.”
“While we are typically used to understanding brain structure as abnormally decreasing over time in certain disorders like Alzheimer’s disease, this study challenges us to understand that too much brain volume growth can also be abnormal in specific conditions,” Dr. Raji added.
This research was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institute of Environmental Health Sciences, and National Institute of Mental Health, along with Autism Speaks and the Simons Foundation. Dr. Shen and Dr. Raji have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new study suggests that overgrowth of the amygdala in infants during the first 6-12 months of life is tied to a later diagnosis of autism spectrum disorder (ASD).
“The faster the amygdala grew in infancy, the more social difficulties the child showed when diagnosed with autism a year later,” first author Mark Shen, PhD, assistant professor of psychiatry and neuroscience, University of North Carolina, Chapel Hill, told this news organization.
The study was published online in the American Journal of Psychiatry.
Unique to autism
The amygdala plays a key role in processing memory, emotional responses, and decisionmaking.
It’s long been known that the amygdala is abnormally large in school-aged children with ASD, but until now, it was not known precisely when aberrant amygdala growth happens, what the clinical consequences may be, and whether amygdala overgrowth is unique to autism.
To investigate, Dr. Shen and colleagues evaluated 1,099 longitudinal MRI scans obtained during natural sleep at 6, 12, and 24 months of age in 408 infants in the Infant Brain Imaging Study (IBIS) Network.
The cohort included 58 infants at high likelihood of developing ASD who were later diagnosed with the disorder, 212 infants at high likelihood of ASD who did not develop ASD, 109 typically-developing control infants, and 29 infants with fragile X syndrome.
At 6 months, infants who developed ASD had typically sized amygdala volumes but showed significantly faster amygdala growth between 6 and 24 months, such that by 12 months the ASD group had significantly larger amygdala volume (Cohen’s d = 0.56), compared with all other groups.
Amygdala growth rate between 6 and 12 months was significantly associated with greater social deficits at 24 months when the children were diagnosed with ASD.
“We found that the amygdala grows too rapidly between 6 and 12 months of age, during a presymptomatic period in autism, prior to when the diagnostic symptoms of autism (social difficulties and repetitive behaviors) are evident and lead to the later diagnosis of autism,” Dr. Shen said in an interview.
This brain growth pattern appears to be unique to autism, as babies with the genetic disorder fragile X syndrome – another neurodevelopmental condition – showed a markedly different brain growth pattern: no differences in amygdala growth but enlargement of a different brain structure, the caudate, which was linked to increased repetitive behaviors, the investigators found.
Earlier intervention
Prior research has shown that children who are later diagnosed with ASD often display problems in infancy with how they attend to visual stimuli in their surroundings.
These early problems with processing visual and sensory information may put increased stress on the amygdala, potentially leading to amygdala hyperactivity, deficits in pruning dendritic connections, and overgrowth, Dr. Shen and colleagues hypothesize.
Amygdala overgrowth has also been linked to chronic stress in studies of other psychiatric conditions, such as depression and anxiety, and may provide a clue to understanding this observation in infants who later develop autism.
“This research suggests that an optimal time to begin supports for children who are at the highest likelihood of developing autism may be during the first year of life: to improve early precursors to social development, such as sensory processing, in babies even before social difficulties arise,” Dr. Shen said.
Cyrus A. Raji, MD, PhD, assistant professor of radiology and neurology, Washington University, St. Louis, said, “What makes this study important is the finding of abnormally increased amygdala growth rate in autism using a longitudinal design that focuses on earlier development.”
“While we are typically used to understanding brain structure as abnormally decreasing over time in certain disorders like Alzheimer’s disease, this study challenges us to understand that too much brain volume growth can also be abnormal in specific conditions,” Dr. Raji added.
This research was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institute of Environmental Health Sciences, and National Institute of Mental Health, along with Autism Speaks and the Simons Foundation. Dr. Shen and Dr. Raji have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Physical fitness tied to lower risk of Alzheimer’s disease
, new findings suggest. “One exciting finding of this study is that as people’s fitness improved, their risk of Alzheimer’s disease decreased – it was not an all-or-nothing proposition,” study investigator Edward Zamrini, MD, of the Washington DC VA Medical Center, said in a news release.
The findings suggest that people can work toward making incremental changes and improvements in their physical fitness, which may help decrease their risk of dementia, Dr. Zamrini added.
The findings were presented at the 2022 annual meeting of the American Academy of Neurology.
Effective prevention strategy
Using the Veterans Health Administration database, Dr. Zamrini and colleagues identified 649,605 veterans (mean age, 61 years) free of Alzheimer’s disease and related disorders (ADRD) when they completed standardized exercise treadmill tests between 2000 and 2017.
They divided participants into five age-specific fitness groups, from least fit to most fit, based on peak metabolic equivalents (METs) achieved during the treadmill test: lowest-fit (METs, ±3.8), low-fit (METs, ±5.8), moderate-fit (METs, ±7.5), fit (METs, ±9.2), and highest-fit (METs, ±11.7).
In unadjusted analysis, veterans with the lowest cardiorespiratory fitness developed ADRD at a rate of 9.5 cases per 1,000 person-years, compared with a rate of 6.4 cases per 1,000 person-years for the most fit group (P < .001).
After adjusting for factors that could affect risk of ADRD, compared with the lowest-fit group, the highest-fit and fit groups were 33% and 26% less likely to develop ADRD, respectively, while the moderate-fit and low-fit groups were 20% and 13% less likely to develop the disease, respectively.
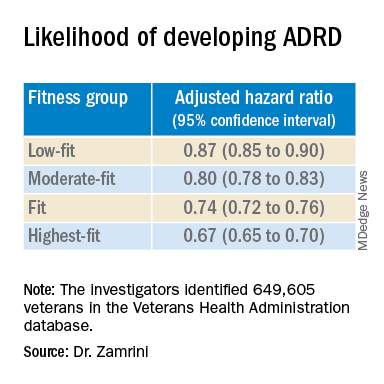
The findings suggest that the association between cardiorespiratory fitness and ADRD risk is “inverse, independent, and graded,” the researchers said in their conference abstract.
“The idea that you can reduce your risk for Alzheimer’s disease by simply increasing your activity is very promising, especially since there are no adequate treatments to prevent or stop the progression of the disease,” Dr. Zamrini added in the news release.
“We hope to develop a simple scale that can be individualized so people can see the benefits that even incremental improvements in fitness can deliver,” he said.
The next vital sign?
Commenting on the study, Shaheen E. Lakhan, MD, PhD, a neurologist in Boston, noted that “for decades and with increasing body of support from studies like this, we have known that preventing dementia is based on healthy behaviors for the brain including a proper diet (NASH and/or Mediterranean), exercise regimen (aerobic/cardio more than anaerobic/weight-lifting), sleep hygiene, and social and intellectual engagements.”
“Frankly, what’s good for the body is good for the brain,” said Dr. Lakhan.
“It should be noted that the measure studied here is cardiorespiratory fitness, which has been associated with heart disease and resulting death, death from any cause, and now brain health,” Dr. Lakhan said.
“This powerful predictor may in fact be the next vital sign, after your heart rate and blood pressure, from which your primary care provider can make a personalized treatment plan,” he added.
“Accelerating this process, the ability to measure cardiorespiratory fitness traditionally from huge stationary machines down to wearables like a watch or ring, or even your iPhone or Android, is just on the horizon,” Dr. Lakhan said.
“Instead of tracking just your weight, shape, and BMI, personal fitness may be tailored to optimizing this indicator and further empowering individuals to take charge of their health,” he said.
The study was supported by the National Institute on Aging, the National Institutes of Health, the U.S. Department of Veterans Affairs, the Washington DC VA Medical Center, and George Washington University. Dr. Zamrini and Dr. Lakhan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new findings suggest. “One exciting finding of this study is that as people’s fitness improved, their risk of Alzheimer’s disease decreased – it was not an all-or-nothing proposition,” study investigator Edward Zamrini, MD, of the Washington DC VA Medical Center, said in a news release.
The findings suggest that people can work toward making incremental changes and improvements in their physical fitness, which may help decrease their risk of dementia, Dr. Zamrini added.
The findings were presented at the 2022 annual meeting of the American Academy of Neurology.
Effective prevention strategy
Using the Veterans Health Administration database, Dr. Zamrini and colleagues identified 649,605 veterans (mean age, 61 years) free of Alzheimer’s disease and related disorders (ADRD) when they completed standardized exercise treadmill tests between 2000 and 2017.
They divided participants into five age-specific fitness groups, from least fit to most fit, based on peak metabolic equivalents (METs) achieved during the treadmill test: lowest-fit (METs, ±3.8), low-fit (METs, ±5.8), moderate-fit (METs, ±7.5), fit (METs, ±9.2), and highest-fit (METs, ±11.7).
In unadjusted analysis, veterans with the lowest cardiorespiratory fitness developed ADRD at a rate of 9.5 cases per 1,000 person-years, compared with a rate of 6.4 cases per 1,000 person-years for the most fit group (P < .001).
After adjusting for factors that could affect risk of ADRD, compared with the lowest-fit group, the highest-fit and fit groups were 33% and 26% less likely to develop ADRD, respectively, while the moderate-fit and low-fit groups were 20% and 13% less likely to develop the disease, respectively.

The findings suggest that the association between cardiorespiratory fitness and ADRD risk is “inverse, independent, and graded,” the researchers said in their conference abstract.
“The idea that you can reduce your risk for Alzheimer’s disease by simply increasing your activity is very promising, especially since there are no adequate treatments to prevent or stop the progression of the disease,” Dr. Zamrini added in the news release.
“We hope to develop a simple scale that can be individualized so people can see the benefits that even incremental improvements in fitness can deliver,” he said.
The next vital sign?
Commenting on the study, Shaheen E. Lakhan, MD, PhD, a neurologist in Boston, noted that “for decades and with increasing body of support from studies like this, we have known that preventing dementia is based on healthy behaviors for the brain including a proper diet (NASH and/or Mediterranean), exercise regimen (aerobic/cardio more than anaerobic/weight-lifting), sleep hygiene, and social and intellectual engagements.”
“Frankly, what’s good for the body is good for the brain,” said Dr. Lakhan.
“It should be noted that the measure studied here is cardiorespiratory fitness, which has been associated with heart disease and resulting death, death from any cause, and now brain health,” Dr. Lakhan said.
“This powerful predictor may in fact be the next vital sign, after your heart rate and blood pressure, from which your primary care provider can make a personalized treatment plan,” he added.
“Accelerating this process, the ability to measure cardiorespiratory fitness traditionally from huge stationary machines down to wearables like a watch or ring, or even your iPhone or Android, is just on the horizon,” Dr. Lakhan said.
“Instead of tracking just your weight, shape, and BMI, personal fitness may be tailored to optimizing this indicator and further empowering individuals to take charge of their health,” he said.
The study was supported by the National Institute on Aging, the National Institutes of Health, the U.S. Department of Veterans Affairs, the Washington DC VA Medical Center, and George Washington University. Dr. Zamrini and Dr. Lakhan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new findings suggest. “One exciting finding of this study is that as people’s fitness improved, their risk of Alzheimer’s disease decreased – it was not an all-or-nothing proposition,” study investigator Edward Zamrini, MD, of the Washington DC VA Medical Center, said in a news release.
The findings suggest that people can work toward making incremental changes and improvements in their physical fitness, which may help decrease their risk of dementia, Dr. Zamrini added.
The findings were presented at the 2022 annual meeting of the American Academy of Neurology.
Effective prevention strategy
Using the Veterans Health Administration database, Dr. Zamrini and colleagues identified 649,605 veterans (mean age, 61 years) free of Alzheimer’s disease and related disorders (ADRD) when they completed standardized exercise treadmill tests between 2000 and 2017.
They divided participants into five age-specific fitness groups, from least fit to most fit, based on peak metabolic equivalents (METs) achieved during the treadmill test: lowest-fit (METs, ±3.8), low-fit (METs, ±5.8), moderate-fit (METs, ±7.5), fit (METs, ±9.2), and highest-fit (METs, ±11.7).
In unadjusted analysis, veterans with the lowest cardiorespiratory fitness developed ADRD at a rate of 9.5 cases per 1,000 person-years, compared with a rate of 6.4 cases per 1,000 person-years for the most fit group (P < .001).
After adjusting for factors that could affect risk of ADRD, compared with the lowest-fit group, the highest-fit and fit groups were 33% and 26% less likely to develop ADRD, respectively, while the moderate-fit and low-fit groups were 20% and 13% less likely to develop the disease, respectively.

The findings suggest that the association between cardiorespiratory fitness and ADRD risk is “inverse, independent, and graded,” the researchers said in their conference abstract.
“The idea that you can reduce your risk for Alzheimer’s disease by simply increasing your activity is very promising, especially since there are no adequate treatments to prevent or stop the progression of the disease,” Dr. Zamrini added in the news release.
“We hope to develop a simple scale that can be individualized so people can see the benefits that even incremental improvements in fitness can deliver,” he said.
The next vital sign?
Commenting on the study, Shaheen E. Lakhan, MD, PhD, a neurologist in Boston, noted that “for decades and with increasing body of support from studies like this, we have known that preventing dementia is based on healthy behaviors for the brain including a proper diet (NASH and/or Mediterranean), exercise regimen (aerobic/cardio more than anaerobic/weight-lifting), sleep hygiene, and social and intellectual engagements.”
“Frankly, what’s good for the body is good for the brain,” said Dr. Lakhan.
“It should be noted that the measure studied here is cardiorespiratory fitness, which has been associated with heart disease and resulting death, death from any cause, and now brain health,” Dr. Lakhan said.
“This powerful predictor may in fact be the next vital sign, after your heart rate and blood pressure, from which your primary care provider can make a personalized treatment plan,” he added.
“Accelerating this process, the ability to measure cardiorespiratory fitness traditionally from huge stationary machines down to wearables like a watch or ring, or even your iPhone or Android, is just on the horizon,” Dr. Lakhan said.
“Instead of tracking just your weight, shape, and BMI, personal fitness may be tailored to optimizing this indicator and further empowering individuals to take charge of their health,” he said.
The study was supported by the National Institute on Aging, the National Institutes of Health, the U.S. Department of Veterans Affairs, the Washington DC VA Medical Center, and George Washington University. Dr. Zamrini and Dr. Lakhan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AAN 2022
First comprehensive guidelines for managing anorexia in pregnancy
The first comprehensive guidelines to manage pregnant women with anorexia nervosa (AN) have been released.
Pregnant women with AN are at greater risk of poor outcomes, including stillbirth, underweight infant, or pre-term birth, yet there are no clear guidelines on the management of the condition.
“Anorexia in pregnancy has been an overlooked area of clinical care, as many believed only women in remission become pregnant, and it is clear that is not the case,” lead author Megan Galbally, MBBS, PhD, professor and director, Centre of Women’s and Children’s Mental Health at Monash University School of Clinical Sciences, Melbourne, told this news organization.
“There are great opportunities to support women in their mental health and give them and their babies a healthier start to parenthood and life,” said Dr. Galbally.
“For instance, reducing the likelihood of prematurity or low birth weight at birth that can be associated with anorexia in pregnancy has extraordinary benefits for that child for lifelong health and well-being,” she added.
The guidelines were published online in Lancet Psychiatry.
Spike in cases
Dr. Galbally noted that during her 20 years of working in perinatal mental health within tertiary maternity services, she only ever saw an occasional pregnant woman with current AN.
In contrast, over the last 3 to 4 years, there has been a “steep increase in women presenting in pregnancy with very low body mass index (BMI) and current anorexia nervosa requiring treatment in pregnancy,” Dr. Galbally said.
Despite the complexity of managing AN in pregnancy, few studies are available to guide care. In a systematic literature review, the researchers identified only eight studies that addressed the management of AN in pregnancy. These studies were case studies or case reports examining narrow aspects of management.
Digging deeper, the researchers conducted a state-of-the-art research review in relevant disciplines and areas of expertise for managing anorexia nervosa in pregnancy. They synthesized their findings into “recommendations and principles” for multidisciplinary care of pregnant women with AN.
The researchers note that AN in pregnancy is associated with increased risks of pregnancy complications and poorer outcomes for infants, and measures such as BMI are less accurate in pregnancy for assessing severity or change in anorexia nervosa.
Anorexia affects pregnancy and neonatal outcomes through low calorie intake, nutritional and vitamin deficiencies, stress, fasting, low body mass, and poor placentation and uteroplacental function.
The authors note that managing AN in pregnancy requires multidisciplinary care that considers the substantial physiological changes for women and requirements for monitoring fetal growth and development.
At a minimum, they recommend monitoring the following:
- Sodium, potassium, magnesium, phosphate, and chloride concentration
- Iron status, vitamin D and bone mineral density, blood sugar concentration (fasting or random), and A1c
- Liver function (including bilirubin, aspartate transaminase, alanine aminotransferase, and gamma-glutamyl transferase) and bone marrow function (including full blood examination, white cell count, neutrophil count, platelets, and hemoglobin)
- Inflammatory markers (C-reactive protein and erythrocyte sedimentation rate)
- Cardiac function (electrocardiogram and echocardiogram)
- Blood pressure and heart rate (lying and standing) and body temperature
“There are considerable risks for women and their unborn child in managing moderate to severe AN in pregnancy,” said Dr. Galbally.
“While we have provided some recommendations, it still requires considerable adaptation to individual presentations and circumstances, and this is best done with a maternity service that manages other high-risk pregnancies such as through maternal-fetal medicine teams,” she said.
“While this area of clinical care can be new to high-risk pregnancy teams, it is clearly important that high-risk pregnancy services and mental health work together to improve care for women with anorexia in pregnancy,” Dr. Galbally added.
A nightmare, a dream come true
Reached for comment, Kamryn T. Eddy, PhD, co-director, Eating Disorders Clinical and Research Program, Massachusetts General Hospital, said, “for many with anorexia nervosa, pregnancy realizes their greatest nightmare and dream come true, both at once.”
“The physical demands of pregnancy can be taxing, and for those with anorexia nervosa, closer clinical management makes sense and may help to support patients who are at risk for return to or worsening of symptoms with the increased nutritional needs and weight gain that occur in pregnancy,” Dr. Eddy, associate professor, department of psychiatry, Harvard Medical School, Boston, told this news organization.
“At the same time, the desire to have a child can be a strong motivator for patients to make the changes needed to recover, and for some, the transition to mother can also help in recovery by broadening the range of things that influence their self-worth,” Dr. Eddy added.
This research had no specific funding. Dr. Galbally and Dr. Eddy report no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
The first comprehensive guidelines to manage pregnant women with anorexia nervosa (AN) have been released.
Pregnant women with AN are at greater risk of poor outcomes, including stillbirth, underweight infant, or pre-term birth, yet there are no clear guidelines on the management of the condition.
“Anorexia in pregnancy has been an overlooked area of clinical care, as many believed only women in remission become pregnant, and it is clear that is not the case,” lead author Megan Galbally, MBBS, PhD, professor and director, Centre of Women’s and Children’s Mental Health at Monash University School of Clinical Sciences, Melbourne, told this news organization.
“There are great opportunities to support women in their mental health and give them and their babies a healthier start to parenthood and life,” said Dr. Galbally.
“For instance, reducing the likelihood of prematurity or low birth weight at birth that can be associated with anorexia in pregnancy has extraordinary benefits for that child for lifelong health and well-being,” she added.
The guidelines were published online in Lancet Psychiatry.
Spike in cases
Dr. Galbally noted that during her 20 years of working in perinatal mental health within tertiary maternity services, she only ever saw an occasional pregnant woman with current AN.
In contrast, over the last 3 to 4 years, there has been a “steep increase in women presenting in pregnancy with very low body mass index (BMI) and current anorexia nervosa requiring treatment in pregnancy,” Dr. Galbally said.
Despite the complexity of managing AN in pregnancy, few studies are available to guide care. In a systematic literature review, the researchers identified only eight studies that addressed the management of AN in pregnancy. These studies were case studies or case reports examining narrow aspects of management.
Digging deeper, the researchers conducted a state-of-the-art research review in relevant disciplines and areas of expertise for managing anorexia nervosa in pregnancy. They synthesized their findings into “recommendations and principles” for multidisciplinary care of pregnant women with AN.
The researchers note that AN in pregnancy is associated with increased risks of pregnancy complications and poorer outcomes for infants, and measures such as BMI are less accurate in pregnancy for assessing severity or change in anorexia nervosa.
Anorexia affects pregnancy and neonatal outcomes through low calorie intake, nutritional and vitamin deficiencies, stress, fasting, low body mass, and poor placentation and uteroplacental function.
The authors note that managing AN in pregnancy requires multidisciplinary care that considers the substantial physiological changes for women and requirements for monitoring fetal growth and development.
At a minimum, they recommend monitoring the following:
- Sodium, potassium, magnesium, phosphate, and chloride concentration
- Iron status, vitamin D and bone mineral density, blood sugar concentration (fasting or random), and A1c
- Liver function (including bilirubin, aspartate transaminase, alanine aminotransferase, and gamma-glutamyl transferase) and bone marrow function (including full blood examination, white cell count, neutrophil count, platelets, and hemoglobin)
- Inflammatory markers (C-reactive protein and erythrocyte sedimentation rate)
- Cardiac function (electrocardiogram and echocardiogram)
- Blood pressure and heart rate (lying and standing) and body temperature
“There are considerable risks for women and their unborn child in managing moderate to severe AN in pregnancy,” said Dr. Galbally.
“While we have provided some recommendations, it still requires considerable adaptation to individual presentations and circumstances, and this is best done with a maternity service that manages other high-risk pregnancies such as through maternal-fetal medicine teams,” she said.
“While this area of clinical care can be new to high-risk pregnancy teams, it is clearly important that high-risk pregnancy services and mental health work together to improve care for women with anorexia in pregnancy,” Dr. Galbally added.
A nightmare, a dream come true
Reached for comment, Kamryn T. Eddy, PhD, co-director, Eating Disorders Clinical and Research Program, Massachusetts General Hospital, said, “for many with anorexia nervosa, pregnancy realizes their greatest nightmare and dream come true, both at once.”
“The physical demands of pregnancy can be taxing, and for those with anorexia nervosa, closer clinical management makes sense and may help to support patients who are at risk for return to or worsening of symptoms with the increased nutritional needs and weight gain that occur in pregnancy,” Dr. Eddy, associate professor, department of psychiatry, Harvard Medical School, Boston, told this news organization.
“At the same time, the desire to have a child can be a strong motivator for patients to make the changes needed to recover, and for some, the transition to mother can also help in recovery by broadening the range of things that influence their self-worth,” Dr. Eddy added.
This research had no specific funding. Dr. Galbally and Dr. Eddy report no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
The first comprehensive guidelines to manage pregnant women with anorexia nervosa (AN) have been released.
Pregnant women with AN are at greater risk of poor outcomes, including stillbirth, underweight infant, or pre-term birth, yet there are no clear guidelines on the management of the condition.
“Anorexia in pregnancy has been an overlooked area of clinical care, as many believed only women in remission become pregnant, and it is clear that is not the case,” lead author Megan Galbally, MBBS, PhD, professor and director, Centre of Women’s and Children’s Mental Health at Monash University School of Clinical Sciences, Melbourne, told this news organization.
“There are great opportunities to support women in their mental health and give them and their babies a healthier start to parenthood and life,” said Dr. Galbally.
“For instance, reducing the likelihood of prematurity or low birth weight at birth that can be associated with anorexia in pregnancy has extraordinary benefits for that child for lifelong health and well-being,” she added.
The guidelines were published online in Lancet Psychiatry.
Spike in cases
Dr. Galbally noted that during her 20 years of working in perinatal mental health within tertiary maternity services, she only ever saw an occasional pregnant woman with current AN.
In contrast, over the last 3 to 4 years, there has been a “steep increase in women presenting in pregnancy with very low body mass index (BMI) and current anorexia nervosa requiring treatment in pregnancy,” Dr. Galbally said.
Despite the complexity of managing AN in pregnancy, few studies are available to guide care. In a systematic literature review, the researchers identified only eight studies that addressed the management of AN in pregnancy. These studies were case studies or case reports examining narrow aspects of management.
Digging deeper, the researchers conducted a state-of-the-art research review in relevant disciplines and areas of expertise for managing anorexia nervosa in pregnancy. They synthesized their findings into “recommendations and principles” for multidisciplinary care of pregnant women with AN.
The researchers note that AN in pregnancy is associated with increased risks of pregnancy complications and poorer outcomes for infants, and measures such as BMI are less accurate in pregnancy for assessing severity or change in anorexia nervosa.
Anorexia affects pregnancy and neonatal outcomes through low calorie intake, nutritional and vitamin deficiencies, stress, fasting, low body mass, and poor placentation and uteroplacental function.
The authors note that managing AN in pregnancy requires multidisciplinary care that considers the substantial physiological changes for women and requirements for monitoring fetal growth and development.
At a minimum, they recommend monitoring the following:
- Sodium, potassium, magnesium, phosphate, and chloride concentration
- Iron status, vitamin D and bone mineral density, blood sugar concentration (fasting or random), and A1c
- Liver function (including bilirubin, aspartate transaminase, alanine aminotransferase, and gamma-glutamyl transferase) and bone marrow function (including full blood examination, white cell count, neutrophil count, platelets, and hemoglobin)
- Inflammatory markers (C-reactive protein and erythrocyte sedimentation rate)
- Cardiac function (electrocardiogram and echocardiogram)
- Blood pressure and heart rate (lying and standing) and body temperature
“There are considerable risks for women and their unborn child in managing moderate to severe AN in pregnancy,” said Dr. Galbally.
“While we have provided some recommendations, it still requires considerable adaptation to individual presentations and circumstances, and this is best done with a maternity service that manages other high-risk pregnancies such as through maternal-fetal medicine teams,” she said.
“While this area of clinical care can be new to high-risk pregnancy teams, it is clearly important that high-risk pregnancy services and mental health work together to improve care for women with anorexia in pregnancy,” Dr. Galbally added.
A nightmare, a dream come true
Reached for comment, Kamryn T. Eddy, PhD, co-director, Eating Disorders Clinical and Research Program, Massachusetts General Hospital, said, “for many with anorexia nervosa, pregnancy realizes their greatest nightmare and dream come true, both at once.”
“The physical demands of pregnancy can be taxing, and for those with anorexia nervosa, closer clinical management makes sense and may help to support patients who are at risk for return to or worsening of symptoms with the increased nutritional needs and weight gain that occur in pregnancy,” Dr. Eddy, associate professor, department of psychiatry, Harvard Medical School, Boston, told this news organization.
“At the same time, the desire to have a child can be a strong motivator for patients to make the changes needed to recover, and for some, the transition to mother can also help in recovery by broadening the range of things that influence their self-worth,” Dr. Eddy added.
This research had no specific funding. Dr. Galbally and Dr. Eddy report no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Keto diet in MS tied to less disability, better quality of life
, new research suggests.
High-fat, low-carbohydrate ketogenic diets mimic a fasting state and promote a more efficient use of energy – and have previously been shown to affect immune regulation. The diet helps lower blood sugar in individuals with type 2 diabetes and has been used for years to improve seizure control in patients with epilepsy, researchers note.
However, “there is a paucity of literature on the ketogenic diet in MS currently,” said principal investigator J. Nicholas Brenton, MD, University of Virginia, Charlottesville.
“The current study demonstrates the safety, tolerability, and potential clinical benefits of a ketogenic diet over 6 months in patients with relapsing MS,” Dr. Brenton said.
The were presented at the 2022 annual meeting of the American Academy of Neurology.
Palatable, beneficial
The open-label, uncontrolled study included 65 patients with relapsing MS who followed a ketogenic diet for 6 months. Investigators monitored adherence by daily urine ketone testing.
Patient-reported fatigue, depression, and quality-of-life scores were obtained at baseline, in addition to fasting adipokines and pertinent MS-related clinical outcome metrics. Baseline study metrics were repeated at 3 and/or 6 months while on the ketogenic diet.
Of the patient group, 83% adhered to the ketogenic diet for the full 6-month study period.
The ketogenic diet was associated with reductions in fat mass from baseline to 6 months (41.3 vs. 32.0 kg; P < .001) and a significant decline in fatigue and depression scores, the investigators reported.
MS quality-of-life physical and mental composite scores also improved while on the ketogenic diet (P < .001 for both).
A significant decrease from baseline to 6 months in Expanded Disability Status Scale scores, signifying improvement, was observed (2.3 vs. 1.9; P < .001).
Improvements were also shown on the 6-minute walk (1,631 vs. 1,733 feet; P < .001) and the nine-hole peg test (21.5 vs. 20.3 seconds; P < .001).
At 6 months on the diet, fasting serum leptin was significantly lower (25.5 vs. 14 ng/mL; P <.001), and adiponectin was higher (11.4 vs. 13.5 μg/mL, P = .002).
Justifies further research
The current study builds on an earlier one that Dr. Brenton and colleagues conducted in 2019 that showed that the ketogenic diet was feasible in patients with MS. “Our data justify the need for future studies of ketogenic diets as a complementary therapeutic approach to the treatment of MS,” Dr. Brenton said.
He noted that there may be multiple mechanisms of benefit when considering the ketogenic diet. “One avenue is via reduction in total body fat. This is an important aspect as we continue to learn more about the role of obesity and fat-derived inflammation in MS,” Dr. Brenton said.
“Ketogenic diets also have immunomodulatory properties,” such as the capacity to reduce oxidative damage from metabolic stress, increase mitochondrial biogenesis, and reduce systemic inflammation, he added. “These intrinsic properties of the ketogenic diet make it appealing to study in immune-mediated diseases, such as MS.”
Dr. Brenton cautioned that the data demonstrate the diet’s safety over 6 months but that the study was not designed to assess its long-term implications in MS. “Thus, while our results support the rationale for a larger-scale study of ketogenic diets as a complementary treatment for MS, our data does not support its widespread adoption outside of a clinical trial,” he said.
Remarkable adherence
Commenting on the study, Shaheen E. Lakhan, MD, PhD, a neurologist in Boston, noted that “variations of the ketogenic diet have been popularized in the general population for weight loss and further studied for other medical conditions [that are] largely immune-related, including MS.”
He noted that it was “remarkable” that the vast majority of study participants with MS adhered to the very regimented ketogenic diet for 6 months.
Seeing this translate into the real world “will be the next milestone, in addition to its impact on relapses and brain lesions as seen on MRI,” which are the classic markers of MS, said Dr. Lakhan, who was not involved with the research.
He cautioned that “even if one can follow the ketogenic diet, certain conditions can be made worse. This includes kidney stones, liver disease, reflux, constipation, and other metabolic disorders.”
The study was funded by the National Center for Advancing Translational Sciences of the National Institutes of Health and by the ZiMS Foundation. Dr. Brenton and Dr. Lakhan have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
High-fat, low-carbohydrate ketogenic diets mimic a fasting state and promote a more efficient use of energy – and have previously been shown to affect immune regulation. The diet helps lower blood sugar in individuals with type 2 diabetes and has been used for years to improve seizure control in patients with epilepsy, researchers note.
However, “there is a paucity of literature on the ketogenic diet in MS currently,” said principal investigator J. Nicholas Brenton, MD, University of Virginia, Charlottesville.
“The current study demonstrates the safety, tolerability, and potential clinical benefits of a ketogenic diet over 6 months in patients with relapsing MS,” Dr. Brenton said.
The were presented at the 2022 annual meeting of the American Academy of Neurology.
Palatable, beneficial
The open-label, uncontrolled study included 65 patients with relapsing MS who followed a ketogenic diet for 6 months. Investigators monitored adherence by daily urine ketone testing.
Patient-reported fatigue, depression, and quality-of-life scores were obtained at baseline, in addition to fasting adipokines and pertinent MS-related clinical outcome metrics. Baseline study metrics were repeated at 3 and/or 6 months while on the ketogenic diet.
Of the patient group, 83% adhered to the ketogenic diet for the full 6-month study period.
The ketogenic diet was associated with reductions in fat mass from baseline to 6 months (41.3 vs. 32.0 kg; P < .001) and a significant decline in fatigue and depression scores, the investigators reported.
MS quality-of-life physical and mental composite scores also improved while on the ketogenic diet (P < .001 for both).
A significant decrease from baseline to 6 months in Expanded Disability Status Scale scores, signifying improvement, was observed (2.3 vs. 1.9; P < .001).
Improvements were also shown on the 6-minute walk (1,631 vs. 1,733 feet; P < .001) and the nine-hole peg test (21.5 vs. 20.3 seconds; P < .001).
At 6 months on the diet, fasting serum leptin was significantly lower (25.5 vs. 14 ng/mL; P <.001), and adiponectin was higher (11.4 vs. 13.5 μg/mL, P = .002).
Justifies further research
The current study builds on an earlier one that Dr. Brenton and colleagues conducted in 2019 that showed that the ketogenic diet was feasible in patients with MS. “Our data justify the need for future studies of ketogenic diets as a complementary therapeutic approach to the treatment of MS,” Dr. Brenton said.
He noted that there may be multiple mechanisms of benefit when considering the ketogenic diet. “One avenue is via reduction in total body fat. This is an important aspect as we continue to learn more about the role of obesity and fat-derived inflammation in MS,” Dr. Brenton said.
“Ketogenic diets also have immunomodulatory properties,” such as the capacity to reduce oxidative damage from metabolic stress, increase mitochondrial biogenesis, and reduce systemic inflammation, he added. “These intrinsic properties of the ketogenic diet make it appealing to study in immune-mediated diseases, such as MS.”
Dr. Brenton cautioned that the data demonstrate the diet’s safety over 6 months but that the study was not designed to assess its long-term implications in MS. “Thus, while our results support the rationale for a larger-scale study of ketogenic diets as a complementary treatment for MS, our data does not support its widespread adoption outside of a clinical trial,” he said.
Remarkable adherence
Commenting on the study, Shaheen E. Lakhan, MD, PhD, a neurologist in Boston, noted that “variations of the ketogenic diet have been popularized in the general population for weight loss and further studied for other medical conditions [that are] largely immune-related, including MS.”
He noted that it was “remarkable” that the vast majority of study participants with MS adhered to the very regimented ketogenic diet for 6 months.
Seeing this translate into the real world “will be the next milestone, in addition to its impact on relapses and brain lesions as seen on MRI,” which are the classic markers of MS, said Dr. Lakhan, who was not involved with the research.
He cautioned that “even if one can follow the ketogenic diet, certain conditions can be made worse. This includes kidney stones, liver disease, reflux, constipation, and other metabolic disorders.”
The study was funded by the National Center for Advancing Translational Sciences of the National Institutes of Health and by the ZiMS Foundation. Dr. Brenton and Dr. Lakhan have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
High-fat, low-carbohydrate ketogenic diets mimic a fasting state and promote a more efficient use of energy – and have previously been shown to affect immune regulation. The diet helps lower blood sugar in individuals with type 2 diabetes and has been used for years to improve seizure control in patients with epilepsy, researchers note.
However, “there is a paucity of literature on the ketogenic diet in MS currently,” said principal investigator J. Nicholas Brenton, MD, University of Virginia, Charlottesville.
“The current study demonstrates the safety, tolerability, and potential clinical benefits of a ketogenic diet over 6 months in patients with relapsing MS,” Dr. Brenton said.
The were presented at the 2022 annual meeting of the American Academy of Neurology.
Palatable, beneficial
The open-label, uncontrolled study included 65 patients with relapsing MS who followed a ketogenic diet for 6 months. Investigators monitored adherence by daily urine ketone testing.
Patient-reported fatigue, depression, and quality-of-life scores were obtained at baseline, in addition to fasting adipokines and pertinent MS-related clinical outcome metrics. Baseline study metrics were repeated at 3 and/or 6 months while on the ketogenic diet.
Of the patient group, 83% adhered to the ketogenic diet for the full 6-month study period.
The ketogenic diet was associated with reductions in fat mass from baseline to 6 months (41.3 vs. 32.0 kg; P < .001) and a significant decline in fatigue and depression scores, the investigators reported.
MS quality-of-life physical and mental composite scores also improved while on the ketogenic diet (P < .001 for both).
A significant decrease from baseline to 6 months in Expanded Disability Status Scale scores, signifying improvement, was observed (2.3 vs. 1.9; P < .001).
Improvements were also shown on the 6-minute walk (1,631 vs. 1,733 feet; P < .001) and the nine-hole peg test (21.5 vs. 20.3 seconds; P < .001).
At 6 months on the diet, fasting serum leptin was significantly lower (25.5 vs. 14 ng/mL; P <.001), and adiponectin was higher (11.4 vs. 13.5 μg/mL, P = .002).
Justifies further research
The current study builds on an earlier one that Dr. Brenton and colleagues conducted in 2019 that showed that the ketogenic diet was feasible in patients with MS. “Our data justify the need for future studies of ketogenic diets as a complementary therapeutic approach to the treatment of MS,” Dr. Brenton said.
He noted that there may be multiple mechanisms of benefit when considering the ketogenic diet. “One avenue is via reduction in total body fat. This is an important aspect as we continue to learn more about the role of obesity and fat-derived inflammation in MS,” Dr. Brenton said.
“Ketogenic diets also have immunomodulatory properties,” such as the capacity to reduce oxidative damage from metabolic stress, increase mitochondrial biogenesis, and reduce systemic inflammation, he added. “These intrinsic properties of the ketogenic diet make it appealing to study in immune-mediated diseases, such as MS.”
Dr. Brenton cautioned that the data demonstrate the diet’s safety over 6 months but that the study was not designed to assess its long-term implications in MS. “Thus, while our results support the rationale for a larger-scale study of ketogenic diets as a complementary treatment for MS, our data does not support its widespread adoption outside of a clinical trial,” he said.
Remarkable adherence
Commenting on the study, Shaheen E. Lakhan, MD, PhD, a neurologist in Boston, noted that “variations of the ketogenic diet have been popularized in the general population for weight loss and further studied for other medical conditions [that are] largely immune-related, including MS.”
He noted that it was “remarkable” that the vast majority of study participants with MS adhered to the very regimented ketogenic diet for 6 months.
Seeing this translate into the real world “will be the next milestone, in addition to its impact on relapses and brain lesions as seen on MRI,” which are the classic markers of MS, said Dr. Lakhan, who was not involved with the research.
He cautioned that “even if one can follow the ketogenic diet, certain conditions can be made worse. This includes kidney stones, liver disease, reflux, constipation, and other metabolic disorders.”
The study was funded by the National Center for Advancing Translational Sciences of the National Institutes of Health and by the ZiMS Foundation. Dr. Brenton and Dr. Lakhan have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AAN 2022
Psychotropic med use tied to ‘striking’ post-COVID dementia risk
, new research suggests.
Results from a large study of more than 1,700 patients who had been hospitalized with COVID showed a greater than twofold increased risk for post-COVID dementia in those taking antipsychotics and mood stabilizers/anticonvulsants – medications often used to treat schizophrenia, psychosis, bipolar disorder, and seizures.
“We know that pre-existing psychiatric illness is associated with poor COVID-19 outcomes, but our study is the first to show an association with certain psychiatric medications and dementia,” co-investigator Liron Sinvani, MD, the Feinstein Institutes for Medical Research, Manhasset, New York, said in an interview.
“Our study highlights the potential interaction between baseline neuropsychiatric disease, psychotropic medications, COVID-19, and dementia,” Dr. Sinvani added.
The findings were published online March 18 in Frontiers in Medicine.
‘Striking’ dementia rate
Using electronic health records, the researchers evaluated pre-COVID psychotropic medication use and post-COVID dementia onset in 1,755 adults aged 65 and older. All were hospitalized with COVID-19 at Northwell Health between March 1 and April 20, 2020.
A “striking” 13% of the participants (n = 223) developed dementia within 1-year of follow-up, the investigators report.
Among the 438 patients (25%) exposed to at least one psychotropic medication before COVID-19, 105 (24%) developed dementia in the year following COVID versus 118 of 1,317 (9%) patients with no pre-COVID exposure to psychotropic medication (odds ratio, 3.2; 95% confidence interval, 2.37-4.32).
Both pre-COVID psychotropic medication use (OR, 2.7; 95% CI, 1.8-4.0, P < .001) and delirium (OR, 3.0; 95% CI, 1.9-4.6, P < .001) were significantly associated with post-COVID dementia at 1 year.
In a sensitivity analysis in the subset of 423 patients with at least one documented neurologic or psychiatric diagnosis at the time of COVID admission, and after adjusting for confounding factors, pre-COVID psychotropic medication use remained significantly linked to post-COVID dementia onset (OR, 3.09; 95% CI, 1.5-6.6, P = .002).
Drug classes most strongly associated with 1-year post-COVID dementia onset were antipsychotics (OR, 2.8, 95% CI, 1.7-4.4, P < .001) and mood stabilizers/anticonvulsants (OR, 2.4, 95% CI, 1.39-4.02, P = .001).
In a further exploratory analysis, the psychotropics valproic acid (multiple brands) and haloperidol (Haldol) had the largest association with post-COVID dementia.
Antidepressants as a class were not associated with post-COVID dementia, but the potential effects of two commonly prescribed antidepressants in older adults, mirtazapine (Remeron) and escitalopram (Lexapro), “warrant further investigation,” the researchers note.
Predictive risk marker?
“This research shows that psychotropic medications can be considered a predictive risk marker for post-COVID dementia. In patients taking psychotropic medications, COVID-19 could have accelerated progression of dementia after hospitalization,” lead author Yun Freudenberg-Hua, MD, the Feinstein Institutes, said in a news release.
It is unclear why psychotropic medications may raise the risk for dementia onset after COVID, the investigators note.
“It is intuitive that psychotropic medications indicate pre-existing neuropsychiatric conditions in which COVID-19 occurs. It is possible that psychotropic medications may potentiate the neurostructural changes that have been found in the brain of those who have recovered from COVID-19,” they write.
The sensitivity analysis in patients with documented neurologic and psychiatric diagnoses supports this interpretation.
COVID-19 may also accelerate the underlying brain disorders for which psychotropic medications were prescribed, leading to the greater incidence of post-COVID dementia, the researchers write.
“It is important to note that this study is in no way recommending people should stop taking antipsychotics but simply that clinicians need to factor in a patient’s medication history while considering post-COVID aftereffects,” Dr. Freudenberg-Hua said.
“Given that the number of patients with dementia is projected to triple in the next 30 years, these findings have significant public health implications,” Dr. Sinvani added.
She noted that “care partners and health care professionals” should look for early signs of dementia, such as forgetfulness and depressive symptoms, in their patients.
“Future studies must continue to evaluate these associations, which are key for potential future interventions to prevent dementia,” Dr. Sinvani said.
The study was funded by the National Institutes of Health. Dr. Freudenberg-Hua co-owns stock and stock options from Regeneron Pharmaceuticals. Dr. Sinvani has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
Results from a large study of more than 1,700 patients who had been hospitalized with COVID showed a greater than twofold increased risk for post-COVID dementia in those taking antipsychotics and mood stabilizers/anticonvulsants – medications often used to treat schizophrenia, psychosis, bipolar disorder, and seizures.
“We know that pre-existing psychiatric illness is associated with poor COVID-19 outcomes, but our study is the first to show an association with certain psychiatric medications and dementia,” co-investigator Liron Sinvani, MD, the Feinstein Institutes for Medical Research, Manhasset, New York, said in an interview.
“Our study highlights the potential interaction between baseline neuropsychiatric disease, psychotropic medications, COVID-19, and dementia,” Dr. Sinvani added.
The findings were published online March 18 in Frontiers in Medicine.
‘Striking’ dementia rate
Using electronic health records, the researchers evaluated pre-COVID psychotropic medication use and post-COVID dementia onset in 1,755 adults aged 65 and older. All were hospitalized with COVID-19 at Northwell Health between March 1 and April 20, 2020.
A “striking” 13% of the participants (n = 223) developed dementia within 1-year of follow-up, the investigators report.
Among the 438 patients (25%) exposed to at least one psychotropic medication before COVID-19, 105 (24%) developed dementia in the year following COVID versus 118 of 1,317 (9%) patients with no pre-COVID exposure to psychotropic medication (odds ratio, 3.2; 95% confidence interval, 2.37-4.32).
Both pre-COVID psychotropic medication use (OR, 2.7; 95% CI, 1.8-4.0, P < .001) and delirium (OR, 3.0; 95% CI, 1.9-4.6, P < .001) were significantly associated with post-COVID dementia at 1 year.
In a sensitivity analysis in the subset of 423 patients with at least one documented neurologic or psychiatric diagnosis at the time of COVID admission, and after adjusting for confounding factors, pre-COVID psychotropic medication use remained significantly linked to post-COVID dementia onset (OR, 3.09; 95% CI, 1.5-6.6, P = .002).
Drug classes most strongly associated with 1-year post-COVID dementia onset were antipsychotics (OR, 2.8, 95% CI, 1.7-4.4, P < .001) and mood stabilizers/anticonvulsants (OR, 2.4, 95% CI, 1.39-4.02, P = .001).
In a further exploratory analysis, the psychotropics valproic acid (multiple brands) and haloperidol (Haldol) had the largest association with post-COVID dementia.
Antidepressants as a class were not associated with post-COVID dementia, but the potential effects of two commonly prescribed antidepressants in older adults, mirtazapine (Remeron) and escitalopram (Lexapro), “warrant further investigation,” the researchers note.
Predictive risk marker?
“This research shows that psychotropic medications can be considered a predictive risk marker for post-COVID dementia. In patients taking psychotropic medications, COVID-19 could have accelerated progression of dementia after hospitalization,” lead author Yun Freudenberg-Hua, MD, the Feinstein Institutes, said in a news release.
It is unclear why psychotropic medications may raise the risk for dementia onset after COVID, the investigators note.
“It is intuitive that psychotropic medications indicate pre-existing neuropsychiatric conditions in which COVID-19 occurs. It is possible that psychotropic medications may potentiate the neurostructural changes that have been found in the brain of those who have recovered from COVID-19,” they write.
The sensitivity analysis in patients with documented neurologic and psychiatric diagnoses supports this interpretation.
COVID-19 may also accelerate the underlying brain disorders for which psychotropic medications were prescribed, leading to the greater incidence of post-COVID dementia, the researchers write.
“It is important to note that this study is in no way recommending people should stop taking antipsychotics but simply that clinicians need to factor in a patient’s medication history while considering post-COVID aftereffects,” Dr. Freudenberg-Hua said.
“Given that the number of patients with dementia is projected to triple in the next 30 years, these findings have significant public health implications,” Dr. Sinvani added.
She noted that “care partners and health care professionals” should look for early signs of dementia, such as forgetfulness and depressive symptoms, in their patients.
“Future studies must continue to evaluate these associations, which are key for potential future interventions to prevent dementia,” Dr. Sinvani said.
The study was funded by the National Institutes of Health. Dr. Freudenberg-Hua co-owns stock and stock options from Regeneron Pharmaceuticals. Dr. Sinvani has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
Results from a large study of more than 1,700 patients who had been hospitalized with COVID showed a greater than twofold increased risk for post-COVID dementia in those taking antipsychotics and mood stabilizers/anticonvulsants – medications often used to treat schizophrenia, psychosis, bipolar disorder, and seizures.
“We know that pre-existing psychiatric illness is associated with poor COVID-19 outcomes, but our study is the first to show an association with certain psychiatric medications and dementia,” co-investigator Liron Sinvani, MD, the Feinstein Institutes for Medical Research, Manhasset, New York, said in an interview.
“Our study highlights the potential interaction between baseline neuropsychiatric disease, psychotropic medications, COVID-19, and dementia,” Dr. Sinvani added.
The findings were published online March 18 in Frontiers in Medicine.
‘Striking’ dementia rate
Using electronic health records, the researchers evaluated pre-COVID psychotropic medication use and post-COVID dementia onset in 1,755 adults aged 65 and older. All were hospitalized with COVID-19 at Northwell Health between March 1 and April 20, 2020.
A “striking” 13% of the participants (n = 223) developed dementia within 1-year of follow-up, the investigators report.
Among the 438 patients (25%) exposed to at least one psychotropic medication before COVID-19, 105 (24%) developed dementia in the year following COVID versus 118 of 1,317 (9%) patients with no pre-COVID exposure to psychotropic medication (odds ratio, 3.2; 95% confidence interval, 2.37-4.32).
Both pre-COVID psychotropic medication use (OR, 2.7; 95% CI, 1.8-4.0, P < .001) and delirium (OR, 3.0; 95% CI, 1.9-4.6, P < .001) were significantly associated with post-COVID dementia at 1 year.
In a sensitivity analysis in the subset of 423 patients with at least one documented neurologic or psychiatric diagnosis at the time of COVID admission, and after adjusting for confounding factors, pre-COVID psychotropic medication use remained significantly linked to post-COVID dementia onset (OR, 3.09; 95% CI, 1.5-6.6, P = .002).
Drug classes most strongly associated with 1-year post-COVID dementia onset were antipsychotics (OR, 2.8, 95% CI, 1.7-4.4, P < .001) and mood stabilizers/anticonvulsants (OR, 2.4, 95% CI, 1.39-4.02, P = .001).
In a further exploratory analysis, the psychotropics valproic acid (multiple brands) and haloperidol (Haldol) had the largest association with post-COVID dementia.
Antidepressants as a class were not associated with post-COVID dementia, but the potential effects of two commonly prescribed antidepressants in older adults, mirtazapine (Remeron) and escitalopram (Lexapro), “warrant further investigation,” the researchers note.
Predictive risk marker?
“This research shows that psychotropic medications can be considered a predictive risk marker for post-COVID dementia. In patients taking psychotropic medications, COVID-19 could have accelerated progression of dementia after hospitalization,” lead author Yun Freudenberg-Hua, MD, the Feinstein Institutes, said in a news release.
It is unclear why psychotropic medications may raise the risk for dementia onset after COVID, the investigators note.
“It is intuitive that psychotropic medications indicate pre-existing neuropsychiatric conditions in which COVID-19 occurs. It is possible that psychotropic medications may potentiate the neurostructural changes that have been found in the brain of those who have recovered from COVID-19,” they write.
The sensitivity analysis in patients with documented neurologic and psychiatric diagnoses supports this interpretation.
COVID-19 may also accelerate the underlying brain disorders for which psychotropic medications were prescribed, leading to the greater incidence of post-COVID dementia, the researchers write.
“It is important to note that this study is in no way recommending people should stop taking antipsychotics but simply that clinicians need to factor in a patient’s medication history while considering post-COVID aftereffects,” Dr. Freudenberg-Hua said.
“Given that the number of patients with dementia is projected to triple in the next 30 years, these findings have significant public health implications,” Dr. Sinvani added.
She noted that “care partners and health care professionals” should look for early signs of dementia, such as forgetfulness and depressive symptoms, in their patients.
“Future studies must continue to evaluate these associations, which are key for potential future interventions to prevent dementia,” Dr. Sinvani said.
The study was funded by the National Institutes of Health. Dr. Freudenberg-Hua co-owns stock and stock options from Regeneron Pharmaceuticals. Dr. Sinvani has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM FRONTIERS IN MEDICINE
Surgery in CJD patients a potential risk factor for transmission
About one in six patients with Creutzfeldt-Jakob disease (CJD) undergo surgery, raising the risk of iatrogenic transmission of this rare but universally fatal prion disease.
In a retrospective analysis, researchers found that 26 of 121 (21%) patients with probable or definite CJD at four U.S. academic medical centers underwent a total of 55 procedures.
These included high-risk procedures for two patients with neuropathologically proven CJD. One underwent ophthalmic artery aneurysm clipping for unruptured aneurysm, and the other underwent diagnostic brain biopsy.
“The findings were definitely surprising to me and my team – particularly the high frequency with which patients with an irreversible and particularly transmissible neurologic disease underwent invasive medical procedures either just before or shortly after the emergence of symptoms later attributed to CJD,” study investigator Gregory Day, MD, with the Mayo Clinic, Jacksonville, Fla., said in an interview.
The study was published online March 9, 2022, in JAMA Network Open.
Poor infection control
The investigators noted that the majority of CJD cases are sporadic or are inherited, but research shows that prion transmission can occur via contaminated tissues or reusable medical equipment.
While the risk of iatrogenic transmission is highest following procedures involving the central nervous system, where prion burden is highest, experimental models suggest CJD transmission can occur after contact with other tissues, including nasal mucosa, lung, lymph nodes, and spleen, the researchers noted.
“If these models are accurate, surgical procedures involving these tissues may pose a risk to patients,” the investigators wrote.
To determine the potential scope of this problem, the researchers examined the frequency of invasive procedures performed in patients with CJD at four tertiary care centers.
“In several cases, these procedures were done with clear indications [such as] fixation or joint replacement following a fracture. In several others, however, the procedures were unlikely to help the patient. For instance, a hip replacement for walking difficulties that were actually due to changes in the brain due to CJD,” Dr. Day said.
“Even more surprising was the low frequency with which appropriate surgical precautions/infection control procedures were used in patients with established diagnoses of CJD,” he noted.
Only one procedure was performed with sterilization techniques adequate to prevent CJD.
Dr. Day said the findings aren’t necessarily cause for immediate alarm, but they do highlight an area for potential improvement, including better screening of patients who have new and unexplained symptoms before proceeding with surgery, especially surgery of the central nervous system, where prion burden is high.
Another potential solution is to develop and support program surveillance and to work with public health organizations such the Centers for Disease Control and Prevention and the National Prion Disease Pathology Surveillance Center to elicit a surgical history in patients diagnosed with prion disease.
“Active nationwide surveillance is needed to determine the true scope of this potential problem and to develop strategies to mitigate the potential risk of iatrogenic prion transmission to future patients,” Dr. Day said.
True prevalence unknown
The authors of an invited commentary noted that, while most CJD infections occur sporadically, iatrogenic transmission is possible. Approximately 500 such cases have been reported worldwide to date.
“Yet, reported transmission from surgical procedures remains rare, with fewer than 10 confirmed CJD cases described in the literature, although the true prevalence is difficult to quantify as confirmed diagnosis requires autopsy,” wrote Beatrice Sun, MD, and Joseph Forrester, MD, with the department of surgery, Stanford (Calif.) University.
They noted that, over a 15-year period, 19 suspected iatrogenic CJD exposures were reported to the CDC – two from ophthalmology procedures, and 17 from neurosurgical procedures.
In all 19 cases, the diagnosis of CJD was unknown before the intervention, and all surgical instruments underwent normal decontamination protocols, which are inadequate to eradicate prion disease.
For patients with suspected or confirmed CJD, the World Health Organization has published infection control guidelines to prevent transmission of spongiform encephalopathies.
The guidelines recommend proper communication with all staff involved in the surgical procedure and the sterilization of supplies to be aware of potential exposure; minimizing the number of staff in the operating room; using single-use equipment whenever possible and disposing of it by incineration; using protective coverings for all nondisposable equipment; and scheduling such procedures at the end of the day to allow adequate time for decontamination.
Funding for the study was provided by the National Institutes of Health. Dr. Day owns stock in ANI Pharmaceuticals; serves as a consultant for Parabon Nanolabs, as a topic editor (dementia) for DynaMed, and as the clinical director of the Anti-NMDA Receptor Encephalitis Foundation (uncompensated). Dr. Forrester reported receiving unrestricted research funding from Varian and has received grant funding from the Surgical Infections Society.
A version of this article first appeared on Medscape.com.
About one in six patients with Creutzfeldt-Jakob disease (CJD) undergo surgery, raising the risk of iatrogenic transmission of this rare but universally fatal prion disease.
In a retrospective analysis, researchers found that 26 of 121 (21%) patients with probable or definite CJD at four U.S. academic medical centers underwent a total of 55 procedures.
These included high-risk procedures for two patients with neuropathologically proven CJD. One underwent ophthalmic artery aneurysm clipping for unruptured aneurysm, and the other underwent diagnostic brain biopsy.
“The findings were definitely surprising to me and my team – particularly the high frequency with which patients with an irreversible and particularly transmissible neurologic disease underwent invasive medical procedures either just before or shortly after the emergence of symptoms later attributed to CJD,” study investigator Gregory Day, MD, with the Mayo Clinic, Jacksonville, Fla., said in an interview.
The study was published online March 9, 2022, in JAMA Network Open.
Poor infection control
The investigators noted that the majority of CJD cases are sporadic or are inherited, but research shows that prion transmission can occur via contaminated tissues or reusable medical equipment.
While the risk of iatrogenic transmission is highest following procedures involving the central nervous system, where prion burden is highest, experimental models suggest CJD transmission can occur after contact with other tissues, including nasal mucosa, lung, lymph nodes, and spleen, the researchers noted.
“If these models are accurate, surgical procedures involving these tissues may pose a risk to patients,” the investigators wrote.
To determine the potential scope of this problem, the researchers examined the frequency of invasive procedures performed in patients with CJD at four tertiary care centers.
“In several cases, these procedures were done with clear indications [such as] fixation or joint replacement following a fracture. In several others, however, the procedures were unlikely to help the patient. For instance, a hip replacement for walking difficulties that were actually due to changes in the brain due to CJD,” Dr. Day said.
“Even more surprising was the low frequency with which appropriate surgical precautions/infection control procedures were used in patients with established diagnoses of CJD,” he noted.
Only one procedure was performed with sterilization techniques adequate to prevent CJD.
Dr. Day said the findings aren’t necessarily cause for immediate alarm, but they do highlight an area for potential improvement, including better screening of patients who have new and unexplained symptoms before proceeding with surgery, especially surgery of the central nervous system, where prion burden is high.
Another potential solution is to develop and support program surveillance and to work with public health organizations such the Centers for Disease Control and Prevention and the National Prion Disease Pathology Surveillance Center to elicit a surgical history in patients diagnosed with prion disease.
“Active nationwide surveillance is needed to determine the true scope of this potential problem and to develop strategies to mitigate the potential risk of iatrogenic prion transmission to future patients,” Dr. Day said.
True prevalence unknown
The authors of an invited commentary noted that, while most CJD infections occur sporadically, iatrogenic transmission is possible. Approximately 500 such cases have been reported worldwide to date.
“Yet, reported transmission from surgical procedures remains rare, with fewer than 10 confirmed CJD cases described in the literature, although the true prevalence is difficult to quantify as confirmed diagnosis requires autopsy,” wrote Beatrice Sun, MD, and Joseph Forrester, MD, with the department of surgery, Stanford (Calif.) University.
They noted that, over a 15-year period, 19 suspected iatrogenic CJD exposures were reported to the CDC – two from ophthalmology procedures, and 17 from neurosurgical procedures.
In all 19 cases, the diagnosis of CJD was unknown before the intervention, and all surgical instruments underwent normal decontamination protocols, which are inadequate to eradicate prion disease.
For patients with suspected or confirmed CJD, the World Health Organization has published infection control guidelines to prevent transmission of spongiform encephalopathies.
The guidelines recommend proper communication with all staff involved in the surgical procedure and the sterilization of supplies to be aware of potential exposure; minimizing the number of staff in the operating room; using single-use equipment whenever possible and disposing of it by incineration; using protective coverings for all nondisposable equipment; and scheduling such procedures at the end of the day to allow adequate time for decontamination.
Funding for the study was provided by the National Institutes of Health. Dr. Day owns stock in ANI Pharmaceuticals; serves as a consultant for Parabon Nanolabs, as a topic editor (dementia) for DynaMed, and as the clinical director of the Anti-NMDA Receptor Encephalitis Foundation (uncompensated). Dr. Forrester reported receiving unrestricted research funding from Varian and has received grant funding from the Surgical Infections Society.
A version of this article first appeared on Medscape.com.
About one in six patients with Creutzfeldt-Jakob disease (CJD) undergo surgery, raising the risk of iatrogenic transmission of this rare but universally fatal prion disease.
In a retrospective analysis, researchers found that 26 of 121 (21%) patients with probable or definite CJD at four U.S. academic medical centers underwent a total of 55 procedures.
These included high-risk procedures for two patients with neuropathologically proven CJD. One underwent ophthalmic artery aneurysm clipping for unruptured aneurysm, and the other underwent diagnostic brain biopsy.
“The findings were definitely surprising to me and my team – particularly the high frequency with which patients with an irreversible and particularly transmissible neurologic disease underwent invasive medical procedures either just before or shortly after the emergence of symptoms later attributed to CJD,” study investigator Gregory Day, MD, with the Mayo Clinic, Jacksonville, Fla., said in an interview.
The study was published online March 9, 2022, in JAMA Network Open.
Poor infection control
The investigators noted that the majority of CJD cases are sporadic or are inherited, but research shows that prion transmission can occur via contaminated tissues or reusable medical equipment.
While the risk of iatrogenic transmission is highest following procedures involving the central nervous system, where prion burden is highest, experimental models suggest CJD transmission can occur after contact with other tissues, including nasal mucosa, lung, lymph nodes, and spleen, the researchers noted.
“If these models are accurate, surgical procedures involving these tissues may pose a risk to patients,” the investigators wrote.
To determine the potential scope of this problem, the researchers examined the frequency of invasive procedures performed in patients with CJD at four tertiary care centers.
“In several cases, these procedures were done with clear indications [such as] fixation or joint replacement following a fracture. In several others, however, the procedures were unlikely to help the patient. For instance, a hip replacement for walking difficulties that were actually due to changes in the brain due to CJD,” Dr. Day said.
“Even more surprising was the low frequency with which appropriate surgical precautions/infection control procedures were used in patients with established diagnoses of CJD,” he noted.
Only one procedure was performed with sterilization techniques adequate to prevent CJD.
Dr. Day said the findings aren’t necessarily cause for immediate alarm, but they do highlight an area for potential improvement, including better screening of patients who have new and unexplained symptoms before proceeding with surgery, especially surgery of the central nervous system, where prion burden is high.
Another potential solution is to develop and support program surveillance and to work with public health organizations such the Centers for Disease Control and Prevention and the National Prion Disease Pathology Surveillance Center to elicit a surgical history in patients diagnosed with prion disease.
“Active nationwide surveillance is needed to determine the true scope of this potential problem and to develop strategies to mitigate the potential risk of iatrogenic prion transmission to future patients,” Dr. Day said.
True prevalence unknown
The authors of an invited commentary noted that, while most CJD infections occur sporadically, iatrogenic transmission is possible. Approximately 500 such cases have been reported worldwide to date.
“Yet, reported transmission from surgical procedures remains rare, with fewer than 10 confirmed CJD cases described in the literature, although the true prevalence is difficult to quantify as confirmed diagnosis requires autopsy,” wrote Beatrice Sun, MD, and Joseph Forrester, MD, with the department of surgery, Stanford (Calif.) University.
They noted that, over a 15-year period, 19 suspected iatrogenic CJD exposures were reported to the CDC – two from ophthalmology procedures, and 17 from neurosurgical procedures.
In all 19 cases, the diagnosis of CJD was unknown before the intervention, and all surgical instruments underwent normal decontamination protocols, which are inadequate to eradicate prion disease.
For patients with suspected or confirmed CJD, the World Health Organization has published infection control guidelines to prevent transmission of spongiform encephalopathies.
The guidelines recommend proper communication with all staff involved in the surgical procedure and the sterilization of supplies to be aware of potential exposure; minimizing the number of staff in the operating room; using single-use equipment whenever possible and disposing of it by incineration; using protective coverings for all nondisposable equipment; and scheduling such procedures at the end of the day to allow adequate time for decontamination.
Funding for the study was provided by the National Institutes of Health. Dr. Day owns stock in ANI Pharmaceuticals; serves as a consultant for Parabon Nanolabs, as a topic editor (dementia) for DynaMed, and as the clinical director of the Anti-NMDA Receptor Encephalitis Foundation (uncompensated). Dr. Forrester reported receiving unrestricted research funding from Varian and has received grant funding from the Surgical Infections Society.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Psychedelics’ interaction with psych meds: More questions than answers
“Despite prolific psychedelic research and public interest, I was surprised to see little clinical research on how psilocybin and common psychiatric treatments interact,” study investigator Aryan Sarparast, MD, department of psychiatry, Oregon Health and Science University, Portland, told this news organization.
The review was published online March 7, 2022, in Psychopharmacology.
Need for RCTs
The Food and Drug Administration recently granted breakthrough therapy designation to psilocybin-assisted psychotherapy for major depression and treatment-resistant depression and to MDMA-assisted psychotherapy for PTSD.
The investigators assessed the volume of available research on interactions between psychedelics and traditional psychiatric medications, such as antidepressants.
They found 40 studies dating back to 1958, including 26 randomized controlled trials, 11 case reports, and 3 epidemiologic studies.
Only one randomized clinical trial evaluated the interaction between psilocybin and the most common psychiatric treatment, SSRIs, said Dr. Sarparast.
However, this study is “reassuring and overlaps with our hypothesis that there is a low risk of psilocybin and most psychiatric drugs causing harm when combined,” he noted.
Yet all of the clinical trials exclusively included young healthy adults, who were often recruited from university campuses. “We don’t have data on what happens when a depressed person on an SSRI takes psilocybin,” said Dr. Sarparast.
He added that he is concerned that the lack of evidence on drug-drug interactions will lead some providers to require patients to be tapered off existing traditional psychiatric medications before initiation of psilocybin therapy.
This may force vulnerable patients to choose between their existing therapy and psilocybin.
In addition, patients who opt for the “DIY method” of tapering risk mental health relapse and medication withdrawal effects. “That’s a very, very tough place to be,” Dr. Sarparast said.
Ideally, Dr. Sarparast would like to see a study in which depressed patients who have been receiving long-term antidepressant treatment are randomly assigned to received low, medium, and high doses of psilocybin. “This would clarify a lot of question marks.”
Evidence gap
In a comment, Roger McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, said: “The point in this article is very well taken. Indeed, more research is needed” on potential interactions between psychedelics and traditional psychiatric medications.
“Before we embark on completing research and development for psychedelics – or, for that matter, any psychoactive substance – we should endeavor to identify what the potential safety and toxicity concerns are when they are coprescribed with other prescribed medications, over-the-counter medications, and other substances (e.g., marijuana) that people take,” Dr. McIntyre said.
A case in point – a 2017 study conducted by McIntyre and colleagues revealed “significant drug-drug interactions with cannabis, which never receives that much attention.”
Also weighing in, Albert Garcia-Romeu, PhD, assistant professor of psychiatry and behavioral sciences, Johns Hopkins University, Baltimore, confirmed that there is “an evidence gap” on psilocybin’s and other psychedelic drugs’ interactions with other medications.
“This has not been formally studied for a number of reasons, but mainly because psilocybin has primarily been considered a drug of abuse. Psilocybin has only recently started to be looked at as a potential medication, and as such, research on drug-drug interactions is still limited, but growing,” Dr. Garcia-Romeu told this news organization.
He noted that studies are underway to better understand potential interactions between psilocybin and other medications.
“This will allow us to better understand how psilocybin should be used medically and what types of interactions could occur with other drugs or medications,” Dr. Garcia-Romeu added.
The study had no specific funding. Dr. Sarparast, Dr. McIntyre, and Dr. Garcia-Romeu reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“Despite prolific psychedelic research and public interest, I was surprised to see little clinical research on how psilocybin and common psychiatric treatments interact,” study investigator Aryan Sarparast, MD, department of psychiatry, Oregon Health and Science University, Portland, told this news organization.
The review was published online March 7, 2022, in Psychopharmacology.
Need for RCTs
The Food and Drug Administration recently granted breakthrough therapy designation to psilocybin-assisted psychotherapy for major depression and treatment-resistant depression and to MDMA-assisted psychotherapy for PTSD.
The investigators assessed the volume of available research on interactions between psychedelics and traditional psychiatric medications, such as antidepressants.
They found 40 studies dating back to 1958, including 26 randomized controlled trials, 11 case reports, and 3 epidemiologic studies.
Only one randomized clinical trial evaluated the interaction between psilocybin and the most common psychiatric treatment, SSRIs, said Dr. Sarparast.
However, this study is “reassuring and overlaps with our hypothesis that there is a low risk of psilocybin and most psychiatric drugs causing harm when combined,” he noted.
Yet all of the clinical trials exclusively included young healthy adults, who were often recruited from university campuses. “We don’t have data on what happens when a depressed person on an SSRI takes psilocybin,” said Dr. Sarparast.
He added that he is concerned that the lack of evidence on drug-drug interactions will lead some providers to require patients to be tapered off existing traditional psychiatric medications before initiation of psilocybin therapy.
This may force vulnerable patients to choose between their existing therapy and psilocybin.
In addition, patients who opt for the “DIY method” of tapering risk mental health relapse and medication withdrawal effects. “That’s a very, very tough place to be,” Dr. Sarparast said.
Ideally, Dr. Sarparast would like to see a study in which depressed patients who have been receiving long-term antidepressant treatment are randomly assigned to received low, medium, and high doses of psilocybin. “This would clarify a lot of question marks.”
Evidence gap
In a comment, Roger McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, said: “The point in this article is very well taken. Indeed, more research is needed” on potential interactions between psychedelics and traditional psychiatric medications.
“Before we embark on completing research and development for psychedelics – or, for that matter, any psychoactive substance – we should endeavor to identify what the potential safety and toxicity concerns are when they are coprescribed with other prescribed medications, over-the-counter medications, and other substances (e.g., marijuana) that people take,” Dr. McIntyre said.
A case in point – a 2017 study conducted by McIntyre and colleagues revealed “significant drug-drug interactions with cannabis, which never receives that much attention.”
Also weighing in, Albert Garcia-Romeu, PhD, assistant professor of psychiatry and behavioral sciences, Johns Hopkins University, Baltimore, confirmed that there is “an evidence gap” on psilocybin’s and other psychedelic drugs’ interactions with other medications.
“This has not been formally studied for a number of reasons, but mainly because psilocybin has primarily been considered a drug of abuse. Psilocybin has only recently started to be looked at as a potential medication, and as such, research on drug-drug interactions is still limited, but growing,” Dr. Garcia-Romeu told this news organization.
He noted that studies are underway to better understand potential interactions between psilocybin and other medications.
“This will allow us to better understand how psilocybin should be used medically and what types of interactions could occur with other drugs or medications,” Dr. Garcia-Romeu added.
The study had no specific funding. Dr. Sarparast, Dr. McIntyre, and Dr. Garcia-Romeu reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“Despite prolific psychedelic research and public interest, I was surprised to see little clinical research on how psilocybin and common psychiatric treatments interact,” study investigator Aryan Sarparast, MD, department of psychiatry, Oregon Health and Science University, Portland, told this news organization.
The review was published online March 7, 2022, in Psychopharmacology.
Need for RCTs
The Food and Drug Administration recently granted breakthrough therapy designation to psilocybin-assisted psychotherapy for major depression and treatment-resistant depression and to MDMA-assisted psychotherapy for PTSD.
The investigators assessed the volume of available research on interactions between psychedelics and traditional psychiatric medications, such as antidepressants.
They found 40 studies dating back to 1958, including 26 randomized controlled trials, 11 case reports, and 3 epidemiologic studies.
Only one randomized clinical trial evaluated the interaction between psilocybin and the most common psychiatric treatment, SSRIs, said Dr. Sarparast.
However, this study is “reassuring and overlaps with our hypothesis that there is a low risk of psilocybin and most psychiatric drugs causing harm when combined,” he noted.
Yet all of the clinical trials exclusively included young healthy adults, who were often recruited from university campuses. “We don’t have data on what happens when a depressed person on an SSRI takes psilocybin,” said Dr. Sarparast.
He added that he is concerned that the lack of evidence on drug-drug interactions will lead some providers to require patients to be tapered off existing traditional psychiatric medications before initiation of psilocybin therapy.
This may force vulnerable patients to choose between their existing therapy and psilocybin.
In addition, patients who opt for the “DIY method” of tapering risk mental health relapse and medication withdrawal effects. “That’s a very, very tough place to be,” Dr. Sarparast said.
Ideally, Dr. Sarparast would like to see a study in which depressed patients who have been receiving long-term antidepressant treatment are randomly assigned to received low, medium, and high doses of psilocybin. “This would clarify a lot of question marks.”
Evidence gap
In a comment, Roger McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, said: “The point in this article is very well taken. Indeed, more research is needed” on potential interactions between psychedelics and traditional psychiatric medications.
“Before we embark on completing research and development for psychedelics – or, for that matter, any psychoactive substance – we should endeavor to identify what the potential safety and toxicity concerns are when they are coprescribed with other prescribed medications, over-the-counter medications, and other substances (e.g., marijuana) that people take,” Dr. McIntyre said.
A case in point – a 2017 study conducted by McIntyre and colleagues revealed “significant drug-drug interactions with cannabis, which never receives that much attention.”
Also weighing in, Albert Garcia-Romeu, PhD, assistant professor of psychiatry and behavioral sciences, Johns Hopkins University, Baltimore, confirmed that there is “an evidence gap” on psilocybin’s and other psychedelic drugs’ interactions with other medications.
“This has not been formally studied for a number of reasons, but mainly because psilocybin has primarily been considered a drug of abuse. Psilocybin has only recently started to be looked at as a potential medication, and as such, research on drug-drug interactions is still limited, but growing,” Dr. Garcia-Romeu told this news organization.
He noted that studies are underway to better understand potential interactions between psilocybin and other medications.
“This will allow us to better understand how psilocybin should be used medically and what types of interactions could occur with other drugs or medications,” Dr. Garcia-Romeu added.
The study had no specific funding. Dr. Sarparast, Dr. McIntyre, and Dr. Garcia-Romeu reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM PSYCHOPHARMACOLOGY
Cancer survivors: Move more, sit less for a longer life, study says
Being physically active, on the other hand, lowers the risk of early death, new research shows.
What’s “alarming” is that so many cancer survivors have a sedentary lifestyle, Chao Cao and Lin Yang, PhD, with Alberta Health Services in Calgary, who worked on the study, said in an interview.
The American Cancer Society recommends that cancer survivors follow the same physical activity guidance as the general population. The target is 150-300 minutes of moderate activity or 75-150 minutes of vigorous activity each week (or a combination of these).
“Getting to or exceeding the upper limit of 300 minutes is ideal,” Mr. Cao and Dr. Yang say.
Yet in their study of more than 1,500 cancer survivors, more than half (57%) were inactive, reporting no weekly leisure-time physical activity in the past week.
About 16% were “insufficiently” active, or getting less than 150 minutes per week. Meanwhile, 28% were active, achieving more than 150 minutes of weekly physical activity.
Digging deeper, the researchers found that more than one-third of cancer survivors reported sitting for 6-8 hours each day, and one-quarter reported sitting for more than 8 hours per day.
Over the course of up to 9 years, 293 of the cancer survivors died – 114 from cancer, 41 from heart diseases, and 138 from other causes.
After accounting for things that might influence the results, the risk of dying from any cause or cancer was about 65% lower in cancer survivors who were physically active, relative to their inactive peers.
Sitting for long periods was especially risky, according to the study in JAMA Oncology.
Compared with cancer survivors who sat for less than 4 hours each day, cancer survivors who reported sitting for more than 8 hours a day had nearly twice the risk of dying from any cause and more than twice the risk of dying from cancer.
Cancer survivors who sat for more than 8 hours a day, and were inactive or not active enough, had as much as five times the risk of death from any cause or cancer.
“Be active and sit less, move more, and move frequently,” advise Mr. Cao and Dr. Yang. “Avoiding prolonged sitting is essential for most cancer survivors to reduce excess mortality risks.”
A version of this article first appeared on WebMD.com.
Being physically active, on the other hand, lowers the risk of early death, new research shows.
What’s “alarming” is that so many cancer survivors have a sedentary lifestyle, Chao Cao and Lin Yang, PhD, with Alberta Health Services in Calgary, who worked on the study, said in an interview.
The American Cancer Society recommends that cancer survivors follow the same physical activity guidance as the general population. The target is 150-300 minutes of moderate activity or 75-150 minutes of vigorous activity each week (or a combination of these).
“Getting to or exceeding the upper limit of 300 minutes is ideal,” Mr. Cao and Dr. Yang say.
Yet in their study of more than 1,500 cancer survivors, more than half (57%) were inactive, reporting no weekly leisure-time physical activity in the past week.
About 16% were “insufficiently” active, or getting less than 150 minutes per week. Meanwhile, 28% were active, achieving more than 150 minutes of weekly physical activity.
Digging deeper, the researchers found that more than one-third of cancer survivors reported sitting for 6-8 hours each day, and one-quarter reported sitting for more than 8 hours per day.
Over the course of up to 9 years, 293 of the cancer survivors died – 114 from cancer, 41 from heart diseases, and 138 from other causes.
After accounting for things that might influence the results, the risk of dying from any cause or cancer was about 65% lower in cancer survivors who were physically active, relative to their inactive peers.
Sitting for long periods was especially risky, according to the study in JAMA Oncology.
Compared with cancer survivors who sat for less than 4 hours each day, cancer survivors who reported sitting for more than 8 hours a day had nearly twice the risk of dying from any cause and more than twice the risk of dying from cancer.
Cancer survivors who sat for more than 8 hours a day, and were inactive or not active enough, had as much as five times the risk of death from any cause or cancer.
“Be active and sit less, move more, and move frequently,” advise Mr. Cao and Dr. Yang. “Avoiding prolonged sitting is essential for most cancer survivors to reduce excess mortality risks.”
A version of this article first appeared on WebMD.com.
Being physically active, on the other hand, lowers the risk of early death, new research shows.
What’s “alarming” is that so many cancer survivors have a sedentary lifestyle, Chao Cao and Lin Yang, PhD, with Alberta Health Services in Calgary, who worked on the study, said in an interview.
The American Cancer Society recommends that cancer survivors follow the same physical activity guidance as the general population. The target is 150-300 minutes of moderate activity or 75-150 minutes of vigorous activity each week (or a combination of these).
“Getting to or exceeding the upper limit of 300 minutes is ideal,” Mr. Cao and Dr. Yang say.
Yet in their study of more than 1,500 cancer survivors, more than half (57%) were inactive, reporting no weekly leisure-time physical activity in the past week.
About 16% were “insufficiently” active, or getting less than 150 minutes per week. Meanwhile, 28% were active, achieving more than 150 minutes of weekly physical activity.
Digging deeper, the researchers found that more than one-third of cancer survivors reported sitting for 6-8 hours each day, and one-quarter reported sitting for more than 8 hours per day.
Over the course of up to 9 years, 293 of the cancer survivors died – 114 from cancer, 41 from heart diseases, and 138 from other causes.
After accounting for things that might influence the results, the risk of dying from any cause or cancer was about 65% lower in cancer survivors who were physically active, relative to their inactive peers.
Sitting for long periods was especially risky, according to the study in JAMA Oncology.
Compared with cancer survivors who sat for less than 4 hours each day, cancer survivors who reported sitting for more than 8 hours a day had nearly twice the risk of dying from any cause and more than twice the risk of dying from cancer.
Cancer survivors who sat for more than 8 hours a day, and were inactive or not active enough, had as much as five times the risk of death from any cause or cancer.
“Be active and sit less, move more, and move frequently,” advise Mr. Cao and Dr. Yang. “Avoiding prolonged sitting is essential for most cancer survivors to reduce excess mortality risks.”
A version of this article first appeared on WebMD.com.
FROM JAMA ONCOLOGY
Few new cancer drugs replace current standards of care
, a new analysis shows.
Of more than 200 agents evaluated, most (42%) received approval as second-, third-, or later-line therapies.
“While there is justified enthusiasm for the high volume of new cancer drug approvals in oncology and malignant hematology, these approvals must be evaluated in the context of their use,” the authors note in a report published online March 15 in JAMA Network Open. Later-line drugs may, for instance, “benefit patients with few alternatives but also add to cost of care and further delay palliative and comfort services” compared to first-line therapies, which may alter “the treatment paradigm for a certain indication.”
The U.S. Food and Drug Administration approves several new cancer drugs each month, but it’s not clear how many transform the treatment landscape.
To investigate, David Benjamin, MD, with the Division of Hematology and Oncology, University of California, Irvine, and colleagues evaluated all 207 cancer drugs approved in the U.S. between May 1, 2016 and May 31, 2021.
The researchers found that only 28 drugs (14%) displaced the prior first-line standard of care for an indication.
Examples of these cancer drugs include alectinib for anaplastic lymphoma kinase rearrangement–positive metastatic non–small cell lung cancer (NSCLC), osimertinib for epidermal growth factor receptor exon 19 deletion or exon 21 L858R substitution NSCLC, atezolizumab plus bevacizumab for unresectable or metastatic hepatocellular carcinoma, and cabozantinib for advanced kidney cancer.
A total of 32 drugs (15%) were approved as first-line alternatives or new drugs. These drugs were approved for use in the first-line setting but did not necessarily replace the standard of care at the time of approval or were first-of-their-class therapies.
Examples of these drug approvals include apalutamide for nonmetastatic castrate-resistant prostate cancer, tepotinib for metastatic MET exon 14-skipping NSCLC, and avapritinib for unresectable or metastatic gastrointestinal stromal tumor with platelet-derived growth factor receptor alpha exon 18 variant, including D842V variant.
A total of 61 drugs (29%) were approved as add-on therapies for use in combination with a previously approved therapy or in the adjuvant or maintenance settings. These drugs “can only increase the cost of care,” the study team says.
Most new approvals (n = 86) were for use in second-, third- or later-line settings, often for patients for whom other treatment options had been exhausted.
The authors highlight disparities among approvals based on tumor type. Lung-related tumors received the most approvals (n = 37), followed by genitourinary tumors (n = 28), leukemia (n = 25), lymphoma (n = 22), breast cancer (n = 19), and gastrointestinal cancers (n = 14).
The authors note that cancer drugs considered new standards of care or approved as first-line setting alternatives could “provide market competition and work to lower cancer drug prices.”
The study was funded by a grant from Arnold Ventures.
A version of this article first appeared on Medscape.com.
, a new analysis shows.
Of more than 200 agents evaluated, most (42%) received approval as second-, third-, or later-line therapies.
“While there is justified enthusiasm for the high volume of new cancer drug approvals in oncology and malignant hematology, these approvals must be evaluated in the context of their use,” the authors note in a report published online March 15 in JAMA Network Open. Later-line drugs may, for instance, “benefit patients with few alternatives but also add to cost of care and further delay palliative and comfort services” compared to first-line therapies, which may alter “the treatment paradigm for a certain indication.”
The U.S. Food and Drug Administration approves several new cancer drugs each month, but it’s not clear how many transform the treatment landscape.
To investigate, David Benjamin, MD, with the Division of Hematology and Oncology, University of California, Irvine, and colleagues evaluated all 207 cancer drugs approved in the U.S. between May 1, 2016 and May 31, 2021.
The researchers found that only 28 drugs (14%) displaced the prior first-line standard of care for an indication.
Examples of these cancer drugs include alectinib for anaplastic lymphoma kinase rearrangement–positive metastatic non–small cell lung cancer (NSCLC), osimertinib for epidermal growth factor receptor exon 19 deletion or exon 21 L858R substitution NSCLC, atezolizumab plus bevacizumab for unresectable or metastatic hepatocellular carcinoma, and cabozantinib for advanced kidney cancer.
A total of 32 drugs (15%) were approved as first-line alternatives or new drugs. These drugs were approved for use in the first-line setting but did not necessarily replace the standard of care at the time of approval or were first-of-their-class therapies.
Examples of these drug approvals include apalutamide for nonmetastatic castrate-resistant prostate cancer, tepotinib for metastatic MET exon 14-skipping NSCLC, and avapritinib for unresectable or metastatic gastrointestinal stromal tumor with platelet-derived growth factor receptor alpha exon 18 variant, including D842V variant.
A total of 61 drugs (29%) were approved as add-on therapies for use in combination with a previously approved therapy or in the adjuvant or maintenance settings. These drugs “can only increase the cost of care,” the study team says.
Most new approvals (n = 86) were for use in second-, third- or later-line settings, often for patients for whom other treatment options had been exhausted.
The authors highlight disparities among approvals based on tumor type. Lung-related tumors received the most approvals (n = 37), followed by genitourinary tumors (n = 28), leukemia (n = 25), lymphoma (n = 22), breast cancer (n = 19), and gastrointestinal cancers (n = 14).
The authors note that cancer drugs considered new standards of care or approved as first-line setting alternatives could “provide market competition and work to lower cancer drug prices.”
The study was funded by a grant from Arnold Ventures.
A version of this article first appeared on Medscape.com.
, a new analysis shows.
Of more than 200 agents evaluated, most (42%) received approval as second-, third-, or later-line therapies.
“While there is justified enthusiasm for the high volume of new cancer drug approvals in oncology and malignant hematology, these approvals must be evaluated in the context of their use,” the authors note in a report published online March 15 in JAMA Network Open. Later-line drugs may, for instance, “benefit patients with few alternatives but also add to cost of care and further delay palliative and comfort services” compared to first-line therapies, which may alter “the treatment paradigm for a certain indication.”
The U.S. Food and Drug Administration approves several new cancer drugs each month, but it’s not clear how many transform the treatment landscape.
To investigate, David Benjamin, MD, with the Division of Hematology and Oncology, University of California, Irvine, and colleagues evaluated all 207 cancer drugs approved in the U.S. between May 1, 2016 and May 31, 2021.
The researchers found that only 28 drugs (14%) displaced the prior first-line standard of care for an indication.
Examples of these cancer drugs include alectinib for anaplastic lymphoma kinase rearrangement–positive metastatic non–small cell lung cancer (NSCLC), osimertinib for epidermal growth factor receptor exon 19 deletion or exon 21 L858R substitution NSCLC, atezolizumab plus bevacizumab for unresectable or metastatic hepatocellular carcinoma, and cabozantinib for advanced kidney cancer.
A total of 32 drugs (15%) were approved as first-line alternatives or new drugs. These drugs were approved for use in the first-line setting but did not necessarily replace the standard of care at the time of approval or were first-of-their-class therapies.
Examples of these drug approvals include apalutamide for nonmetastatic castrate-resistant prostate cancer, tepotinib for metastatic MET exon 14-skipping NSCLC, and avapritinib for unresectable or metastatic gastrointestinal stromal tumor with platelet-derived growth factor receptor alpha exon 18 variant, including D842V variant.
A total of 61 drugs (29%) were approved as add-on therapies for use in combination with a previously approved therapy or in the adjuvant or maintenance settings. These drugs “can only increase the cost of care,” the study team says.
Most new approvals (n = 86) were for use in second-, third- or later-line settings, often for patients for whom other treatment options had been exhausted.
The authors highlight disparities among approvals based on tumor type. Lung-related tumors received the most approvals (n = 37), followed by genitourinary tumors (n = 28), leukemia (n = 25), lymphoma (n = 22), breast cancer (n = 19), and gastrointestinal cancers (n = 14).
The authors note that cancer drugs considered new standards of care or approved as first-line setting alternatives could “provide market competition and work to lower cancer drug prices.”
The study was funded by a grant from Arnold Ventures.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Jury is out on universal screening for eating disorders
Eating disorders (binge eating disorder, bulimia nervosa, and anorexia nervosa) can cause “serious harms to physical and psychosocial health and take a tremendous toll on individuals and families,” task force member Lori Pbert, PhD, told this news organization.
“Screening for eating disorders has the potential to improve health by leading to early detection and effective treatment,” said Dr. Pbert, with the department of population and quantitative health sciences, University of Massachusetts, Worcester.
However, a “deep dive” into the available literature failed to turn up adequate evidence to recommend for or against routine screening for eating disorders for children and adolescents aged 10 years and older and for adults who have no signs or symptoms of an eating disorder or concerns about their eating and who have not previously been diagnosed with an eating disorder, Dr. Pbert said.
The task force, therefore, issued an “I” statement (insufficient evidence), meaning it cannot at this time recommend for or against screening for eating disorders.
An “I” statement is “fundamentally a call for more research,” Dr. Pbert noted.
Adolescents and adults who have signs and symptoms of an eating disorder – which include rapid weight loss; weight gain or pronounced deviation from growth trajectory; pubertal delay; bradycardia; oligomenorrhea; and amenorrhea – are not included in this recommendation.
The USPSTF recommendation statement and accompanying evidence report were published online March 15 in JAMA.
Clinical judgment key
In the absence of evidence, clinicians should use their judgment when determining whether or not to screen an individual patient for an eating disorder, Dr. Pbert advised.
One thing to consider is whether the patient is in a group at higher risk for eating disorders, such as athletes, females, young adults aged 18-29, and transgender individuals.
Another is whether the patient reports engaging in unhealthy weight control behaviors, such as fasting or skipping meals, Dr. Pbert said.
Importantly, any patient who has signs or symptoms of an eating disorder or is expressing concerns about their eating should be assessed and referred for appropriate care, Dr. Pbert said.
“The good news is that eating disorders can be treated,” she said.
Several organizations currently recommend screening in the context of monitoring changes in weight and other vital signs or signs and symptoms to determine whether a patient might have an eating disorder.
Dr. Pbert said it’s important to recognize that the USPSTF statement “doesn’t really conflict” with the recommendations of other organizations. “We all agree that patients who present with signs or symptoms of an eating disorder should be assessed further.”
Evidence gaps
The authors of an invited commentary in JAMA) say the task force has identified several “notable deficiencies” in the available data on screening for eating disorders.
“Directing attention to rigorous research to close this evidence gap will be important to find optimal approaches to identify patients with these complex disorders and improve their health outcomes,” write Evelyn Attia, MD, with Weill Cornell Medicine in New York, and Angela Guarda, MD, with Johns Hopkins University, Baltimore.
This “I” statement, they say, “highlights the need to prioritize research aimed at closing the evidence gap identified by USPSTF in a timely manner and underscores the need for new studies that address screening for eating disorders, treatment trials that enroll screen-detected populations from primary care settings, and screening in specific populations.
“Research on screening in primary care also should be paired with development and assessment of early brief intervention strategies for those individuals who screen positive, especially adolescents,” Dr. Attia and Dr. Guarda say.
Members of the USPSTF have disclosed no relevant financial relationships. Dr. Attia has received research support from the National Institute of Mental Health and the Hilda & Preston David Foundation; royalties from UpToDate; and has served as a clinical advisor to Equip Health. Dr. Guarda has received support from the Stephen and Jean Robinson Fund and research funding from the Klarman Family Foundation.
A version of this article first appeared on Medscape.com.
Eating disorders (binge eating disorder, bulimia nervosa, and anorexia nervosa) can cause “serious harms to physical and psychosocial health and take a tremendous toll on individuals and families,” task force member Lori Pbert, PhD, told this news organization.
“Screening for eating disorders has the potential to improve health by leading to early detection and effective treatment,” said Dr. Pbert, with the department of population and quantitative health sciences, University of Massachusetts, Worcester.
However, a “deep dive” into the available literature failed to turn up adequate evidence to recommend for or against routine screening for eating disorders for children and adolescents aged 10 years and older and for adults who have no signs or symptoms of an eating disorder or concerns about their eating and who have not previously been diagnosed with an eating disorder, Dr. Pbert said.
The task force, therefore, issued an “I” statement (insufficient evidence), meaning it cannot at this time recommend for or against screening for eating disorders.
An “I” statement is “fundamentally a call for more research,” Dr. Pbert noted.
Adolescents and adults who have signs and symptoms of an eating disorder – which include rapid weight loss; weight gain or pronounced deviation from growth trajectory; pubertal delay; bradycardia; oligomenorrhea; and amenorrhea – are not included in this recommendation.
The USPSTF recommendation statement and accompanying evidence report were published online March 15 in JAMA.
Clinical judgment key
In the absence of evidence, clinicians should use their judgment when determining whether or not to screen an individual patient for an eating disorder, Dr. Pbert advised.
One thing to consider is whether the patient is in a group at higher risk for eating disorders, such as athletes, females, young adults aged 18-29, and transgender individuals.
Another is whether the patient reports engaging in unhealthy weight control behaviors, such as fasting or skipping meals, Dr. Pbert said.
Importantly, any patient who has signs or symptoms of an eating disorder or is expressing concerns about their eating should be assessed and referred for appropriate care, Dr. Pbert said.
“The good news is that eating disorders can be treated,” she said.
Several organizations currently recommend screening in the context of monitoring changes in weight and other vital signs or signs and symptoms to determine whether a patient might have an eating disorder.
Dr. Pbert said it’s important to recognize that the USPSTF statement “doesn’t really conflict” with the recommendations of other organizations. “We all agree that patients who present with signs or symptoms of an eating disorder should be assessed further.”
Evidence gaps
The authors of an invited commentary in JAMA) say the task force has identified several “notable deficiencies” in the available data on screening for eating disorders.
“Directing attention to rigorous research to close this evidence gap will be important to find optimal approaches to identify patients with these complex disorders and improve their health outcomes,” write Evelyn Attia, MD, with Weill Cornell Medicine in New York, and Angela Guarda, MD, with Johns Hopkins University, Baltimore.
This “I” statement, they say, “highlights the need to prioritize research aimed at closing the evidence gap identified by USPSTF in a timely manner and underscores the need for new studies that address screening for eating disorders, treatment trials that enroll screen-detected populations from primary care settings, and screening in specific populations.
“Research on screening in primary care also should be paired with development and assessment of early brief intervention strategies for those individuals who screen positive, especially adolescents,” Dr. Attia and Dr. Guarda say.
Members of the USPSTF have disclosed no relevant financial relationships. Dr. Attia has received research support from the National Institute of Mental Health and the Hilda & Preston David Foundation; royalties from UpToDate; and has served as a clinical advisor to Equip Health. Dr. Guarda has received support from the Stephen and Jean Robinson Fund and research funding from the Klarman Family Foundation.
A version of this article first appeared on Medscape.com.
Eating disorders (binge eating disorder, bulimia nervosa, and anorexia nervosa) can cause “serious harms to physical and psychosocial health and take a tremendous toll on individuals and families,” task force member Lori Pbert, PhD, told this news organization.
“Screening for eating disorders has the potential to improve health by leading to early detection and effective treatment,” said Dr. Pbert, with the department of population and quantitative health sciences, University of Massachusetts, Worcester.
However, a “deep dive” into the available literature failed to turn up adequate evidence to recommend for or against routine screening for eating disorders for children and adolescents aged 10 years and older and for adults who have no signs or symptoms of an eating disorder or concerns about their eating and who have not previously been diagnosed with an eating disorder, Dr. Pbert said.
The task force, therefore, issued an “I” statement (insufficient evidence), meaning it cannot at this time recommend for or against screening for eating disorders.
An “I” statement is “fundamentally a call for more research,” Dr. Pbert noted.
Adolescents and adults who have signs and symptoms of an eating disorder – which include rapid weight loss; weight gain or pronounced deviation from growth trajectory; pubertal delay; bradycardia; oligomenorrhea; and amenorrhea – are not included in this recommendation.
The USPSTF recommendation statement and accompanying evidence report were published online March 15 in JAMA.
Clinical judgment key
In the absence of evidence, clinicians should use their judgment when determining whether or not to screen an individual patient for an eating disorder, Dr. Pbert advised.
One thing to consider is whether the patient is in a group at higher risk for eating disorders, such as athletes, females, young adults aged 18-29, and transgender individuals.
Another is whether the patient reports engaging in unhealthy weight control behaviors, such as fasting or skipping meals, Dr. Pbert said.
Importantly, any patient who has signs or symptoms of an eating disorder or is expressing concerns about their eating should be assessed and referred for appropriate care, Dr. Pbert said.
“The good news is that eating disorders can be treated,” she said.
Several organizations currently recommend screening in the context of monitoring changes in weight and other vital signs or signs and symptoms to determine whether a patient might have an eating disorder.
Dr. Pbert said it’s important to recognize that the USPSTF statement “doesn’t really conflict” with the recommendations of other organizations. “We all agree that patients who present with signs or symptoms of an eating disorder should be assessed further.”
Evidence gaps
The authors of an invited commentary in JAMA) say the task force has identified several “notable deficiencies” in the available data on screening for eating disorders.
“Directing attention to rigorous research to close this evidence gap will be important to find optimal approaches to identify patients with these complex disorders and improve their health outcomes,” write Evelyn Attia, MD, with Weill Cornell Medicine in New York, and Angela Guarda, MD, with Johns Hopkins University, Baltimore.
This “I” statement, they say, “highlights the need to prioritize research aimed at closing the evidence gap identified by USPSTF in a timely manner and underscores the need for new studies that address screening for eating disorders, treatment trials that enroll screen-detected populations from primary care settings, and screening in specific populations.
“Research on screening in primary care also should be paired with development and assessment of early brief intervention strategies for those individuals who screen positive, especially adolescents,” Dr. Attia and Dr. Guarda say.
Members of the USPSTF have disclosed no relevant financial relationships. Dr. Attia has received research support from the National Institute of Mental Health and the Hilda & Preston David Foundation; royalties from UpToDate; and has served as a clinical advisor to Equip Health. Dr. Guarda has received support from the Stephen and Jean Robinson Fund and research funding from the Klarman Family Foundation.
A version of this article first appeared on Medscape.com.



