User login
Ticagrelor not superior to clopidogrel in peripheral artery disease
Ticagrelor was found to be “not superior” to clopidogrel at preventing cardiovascular events in the largest clinical trial to date involving patients with symptomatic peripheral artery disease (PAD), Manesh Patel, MD, reported at the American Heart Association scientific sessions.*
Ticagrelor also was no better than clopidogrel at preventing acute limb ischemia in this study of 13,885 patients.
Regarding safety issues, the two drugs had identical rates of major bleeding adverse events, but ticagrelor was discontinued significantly more often than clopidogrel was, because of its other well-known adverse effects, said Dr. Patel of Duke University, Durham, N.C.
The findings of the EUCLID (Examining the Use of Ticagrelor in PAD) study were presented at the meeting and simultaneously published online Nov. 13 in the New England Journal of Medicine (2016. doi: 10.1056/NEJMoa1611688).
In addition, the results of a substudy of the EUCLID trial involving the 7,875 participants who had previously undergone lower-limb revascularization were reported at the meeting and simultaneously published online in Circulation (2016 Nov 13; doi: 10.1161/CIRCULATIONAHA.116.025880).
Until now, there have been no large studies comparing antiplatelet therapies in patients with PAD. Clopidogrel is considered superior to aspirin in this patient population based on limited evidence, often extrapolated from studies of acute coronary syndromes or coronary artery disease. Ticagrelor also proved beneficial in these contexts, so researchers performed the EUCLID study, comparing the two medications head to head in patients with PAD.
The manufacturer-funded double-blind trial was conducted at 811 medical centers in eight countries. Patients aged 50 years or older (median age, 66 years) were randomly assigned to receive either oral ticagrelor, 90 mg twice daily (6,930 patients), or oral clopidogrel, 75 mg once daily (6,955 patients), and were followed for a median of 30 months.
The primary efficacy endpoint – the first occurrence of any event in the composite of cardiovascular death, myocardial infarction, or ischemic stroke – occurred in 10.8% of the ticagrelor group and 10.6% of the clopidogrel group, for a hazard ratio of 1.02.
When the components of this composite endpoint were considered individually, only the rate of ischemic stroke was significantly different between the two study groups, occurring in 1.9% of patients taking ticagrelor and 2.4% of those taking clopidogrel (HR, 0.78).
Other important secondary and composite efficacy endpoints, including acute limb ischemia and revascularization, were similar between the two study groups, Dr. Patel said.
The primary safety endpoint – the rate of major bleeding events – occurred in the same percentage of both study groups (1.6%), and individual rates of fatal bleeding, intracranial bleeding, and minor bleeding were similar.
“There were numerically fewer fatal bleeding events in the ticagrelor group than in the clopidogrel group (10 vs. 20), but there were significantly more bleeding events leading to drug discontinuation with ticagrelor than with clopidogrel (168 vs. 112),” he noted.
Ticagrelor was discontinued more often than clopidogrel during the study (30.1% of patients vs. 25.9%; HR, 1.21). Discontinuation was driven mainly by the occurrence of dyspnea (4.8% vs. 0.8%) and minor bleeding, both of which are well-described adverse effects of ticagrelor, Dr. Patel said.
“Our findings show the hazards of extrapolating evidence from patients with coronary artery disease to those with peripheral artery disease,” he added.
In a separate report in Circulation, the results were similar in the substudy of EUCLID participants who had already undergone lower-extremity revascularization procedures before enrollment, reported W. Schuyler Jones, MD, also of of Duke University.
The primary efficacy endpoint occurred in 11.4% of the ticagrelor group and 11.3% of the clopidogrel group, a nonsignificant difference (HR, 1.01). “Other key secondary and composite endpoints, including repeat revascularization, also were not different between the two study groups,” Dr. Jones said.
Regarding safety endpoints, the rates of major bleeding, fatal bleeding, intracranial bleeding, and minor bleeding all were similar between the two study groups.
“These findings suggest that patients with prior revascularization have a substantial residual rate of cardiovascular and acute limb events, despite high adherence to antiplatelet and statin medications, and require further study,” Dr. Jones noted.
*Correction 11/14/16: An earlier version of this article misstated the name of the investigator who presented the study at the meeting.
Ticagrelor was found to be “not superior” to clopidogrel at preventing cardiovascular events in the largest clinical trial to date involving patients with symptomatic peripheral artery disease (PAD), Manesh Patel, MD, reported at the American Heart Association scientific sessions.*
Ticagrelor also was no better than clopidogrel at preventing acute limb ischemia in this study of 13,885 patients.
Regarding safety issues, the two drugs had identical rates of major bleeding adverse events, but ticagrelor was discontinued significantly more often than clopidogrel was, because of its other well-known adverse effects, said Dr. Patel of Duke University, Durham, N.C.
The findings of the EUCLID (Examining the Use of Ticagrelor in PAD) study were presented at the meeting and simultaneously published online Nov. 13 in the New England Journal of Medicine (2016. doi: 10.1056/NEJMoa1611688).
In addition, the results of a substudy of the EUCLID trial involving the 7,875 participants who had previously undergone lower-limb revascularization were reported at the meeting and simultaneously published online in Circulation (2016 Nov 13; doi: 10.1161/CIRCULATIONAHA.116.025880).
Until now, there have been no large studies comparing antiplatelet therapies in patients with PAD. Clopidogrel is considered superior to aspirin in this patient population based on limited evidence, often extrapolated from studies of acute coronary syndromes or coronary artery disease. Ticagrelor also proved beneficial in these contexts, so researchers performed the EUCLID study, comparing the two medications head to head in patients with PAD.
The manufacturer-funded double-blind trial was conducted at 811 medical centers in eight countries. Patients aged 50 years or older (median age, 66 years) were randomly assigned to receive either oral ticagrelor, 90 mg twice daily (6,930 patients), or oral clopidogrel, 75 mg once daily (6,955 patients), and were followed for a median of 30 months.
The primary efficacy endpoint – the first occurrence of any event in the composite of cardiovascular death, myocardial infarction, or ischemic stroke – occurred in 10.8% of the ticagrelor group and 10.6% of the clopidogrel group, for a hazard ratio of 1.02.
When the components of this composite endpoint were considered individually, only the rate of ischemic stroke was significantly different between the two study groups, occurring in 1.9% of patients taking ticagrelor and 2.4% of those taking clopidogrel (HR, 0.78).
Other important secondary and composite efficacy endpoints, including acute limb ischemia and revascularization, were similar between the two study groups, Dr. Patel said.
The primary safety endpoint – the rate of major bleeding events – occurred in the same percentage of both study groups (1.6%), and individual rates of fatal bleeding, intracranial bleeding, and minor bleeding were similar.
“There were numerically fewer fatal bleeding events in the ticagrelor group than in the clopidogrel group (10 vs. 20), but there were significantly more bleeding events leading to drug discontinuation with ticagrelor than with clopidogrel (168 vs. 112),” he noted.
Ticagrelor was discontinued more often than clopidogrel during the study (30.1% of patients vs. 25.9%; HR, 1.21). Discontinuation was driven mainly by the occurrence of dyspnea (4.8% vs. 0.8%) and minor bleeding, both of which are well-described adverse effects of ticagrelor, Dr. Patel said.
“Our findings show the hazards of extrapolating evidence from patients with coronary artery disease to those with peripheral artery disease,” he added.
In a separate report in Circulation, the results were similar in the substudy of EUCLID participants who had already undergone lower-extremity revascularization procedures before enrollment, reported W. Schuyler Jones, MD, also of of Duke University.
The primary efficacy endpoint occurred in 11.4% of the ticagrelor group and 11.3% of the clopidogrel group, a nonsignificant difference (HR, 1.01). “Other key secondary and composite endpoints, including repeat revascularization, also were not different between the two study groups,” Dr. Jones said.
Regarding safety endpoints, the rates of major bleeding, fatal bleeding, intracranial bleeding, and minor bleeding all were similar between the two study groups.
“These findings suggest that patients with prior revascularization have a substantial residual rate of cardiovascular and acute limb events, despite high adherence to antiplatelet and statin medications, and require further study,” Dr. Jones noted.
*Correction 11/14/16: An earlier version of this article misstated the name of the investigator who presented the study at the meeting.
Ticagrelor was found to be “not superior” to clopidogrel at preventing cardiovascular events in the largest clinical trial to date involving patients with symptomatic peripheral artery disease (PAD), Manesh Patel, MD, reported at the American Heart Association scientific sessions.*
Ticagrelor also was no better than clopidogrel at preventing acute limb ischemia in this study of 13,885 patients.
Regarding safety issues, the two drugs had identical rates of major bleeding adverse events, but ticagrelor was discontinued significantly more often than clopidogrel was, because of its other well-known adverse effects, said Dr. Patel of Duke University, Durham, N.C.
The findings of the EUCLID (Examining the Use of Ticagrelor in PAD) study were presented at the meeting and simultaneously published online Nov. 13 in the New England Journal of Medicine (2016. doi: 10.1056/NEJMoa1611688).
In addition, the results of a substudy of the EUCLID trial involving the 7,875 participants who had previously undergone lower-limb revascularization were reported at the meeting and simultaneously published online in Circulation (2016 Nov 13; doi: 10.1161/CIRCULATIONAHA.116.025880).
Until now, there have been no large studies comparing antiplatelet therapies in patients with PAD. Clopidogrel is considered superior to aspirin in this patient population based on limited evidence, often extrapolated from studies of acute coronary syndromes or coronary artery disease. Ticagrelor also proved beneficial in these contexts, so researchers performed the EUCLID study, comparing the two medications head to head in patients with PAD.
The manufacturer-funded double-blind trial was conducted at 811 medical centers in eight countries. Patients aged 50 years or older (median age, 66 years) were randomly assigned to receive either oral ticagrelor, 90 mg twice daily (6,930 patients), or oral clopidogrel, 75 mg once daily (6,955 patients), and were followed for a median of 30 months.
The primary efficacy endpoint – the first occurrence of any event in the composite of cardiovascular death, myocardial infarction, or ischemic stroke – occurred in 10.8% of the ticagrelor group and 10.6% of the clopidogrel group, for a hazard ratio of 1.02.
When the components of this composite endpoint were considered individually, only the rate of ischemic stroke was significantly different between the two study groups, occurring in 1.9% of patients taking ticagrelor and 2.4% of those taking clopidogrel (HR, 0.78).
Other important secondary and composite efficacy endpoints, including acute limb ischemia and revascularization, were similar between the two study groups, Dr. Patel said.
The primary safety endpoint – the rate of major bleeding events – occurred in the same percentage of both study groups (1.6%), and individual rates of fatal bleeding, intracranial bleeding, and minor bleeding were similar.
“There were numerically fewer fatal bleeding events in the ticagrelor group than in the clopidogrel group (10 vs. 20), but there were significantly more bleeding events leading to drug discontinuation with ticagrelor than with clopidogrel (168 vs. 112),” he noted.
Ticagrelor was discontinued more often than clopidogrel during the study (30.1% of patients vs. 25.9%; HR, 1.21). Discontinuation was driven mainly by the occurrence of dyspnea (4.8% vs. 0.8%) and minor bleeding, both of which are well-described adverse effects of ticagrelor, Dr. Patel said.
“Our findings show the hazards of extrapolating evidence from patients with coronary artery disease to those with peripheral artery disease,” he added.
In a separate report in Circulation, the results were similar in the substudy of EUCLID participants who had already undergone lower-extremity revascularization procedures before enrollment, reported W. Schuyler Jones, MD, also of of Duke University.
The primary efficacy endpoint occurred in 11.4% of the ticagrelor group and 11.3% of the clopidogrel group, a nonsignificant difference (HR, 1.01). “Other key secondary and composite endpoints, including repeat revascularization, also were not different between the two study groups,” Dr. Jones said.
Regarding safety endpoints, the rates of major bleeding, fatal bleeding, intracranial bleeding, and minor bleeding all were similar between the two study groups.
“These findings suggest that patients with prior revascularization have a substantial residual rate of cardiovascular and acute limb events, despite high adherence to antiplatelet and statin medications, and require further study,” Dr. Jones noted.
*Correction 11/14/16: An earlier version of this article misstated the name of the investigator who presented the study at the meeting.
FROM THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: The primary efficacy end point – the first occurrence of any event in the composite of cardiovascular death, myocardial infarction, or ischemic stroke – occurred in 10.8% of the ticagrelor group and 10.6% of the clopidogrel group (HR, 1.02).
Data source: An international double-blind, randomized trial involving 13,885 patients followed for a median of 30 months.
Disclosures: The study was funded by AstraZeneca, maker of ticagrelor. Dr. Patel reported receiving funding from AstraZeneca and several pharmaceutical companies, and his associates reported ties to numerous industry sources.
Dystrophic epidermolysis bullosa: 104 mutations found in single gene
according to a report published online in the Journal of Investigative Dermatology.
Identifying which specific mutations in the COL7A1 gene a given patient is carrying should allow clinicians to render a more accurate prognosis and may even dictate management strategies to counteract manifestations of the disease. Dystrophic epidermolysis bullosa (EB) can have an extremely variable progression that depends, in part, on the underlying molecular defects in many different genes expressed in the dermal-epidermal junction, according to study investigators Hassan Vahidnezhad, MD, of the department of dermatology and cutaneous biology, Thomas Jefferson University, Philadelphia, and his associates.
The study cohort included many consanguineous marriages, which are customary in Iran as in many parts of the Middle East, North Africa, and sub-Saharan Africa. In addition, many migrant communities from these regions now reside in Western countries and continue to practice these marriage customs, most commonly marriages between first cousins. “It is estimated that globally at least 20% of the human population live in communities with a preference for consanguineous marriage,” the researchers wrote (J Investig Dermatol. 2016. doi: 10.1016/j.jid.2016.10.023).
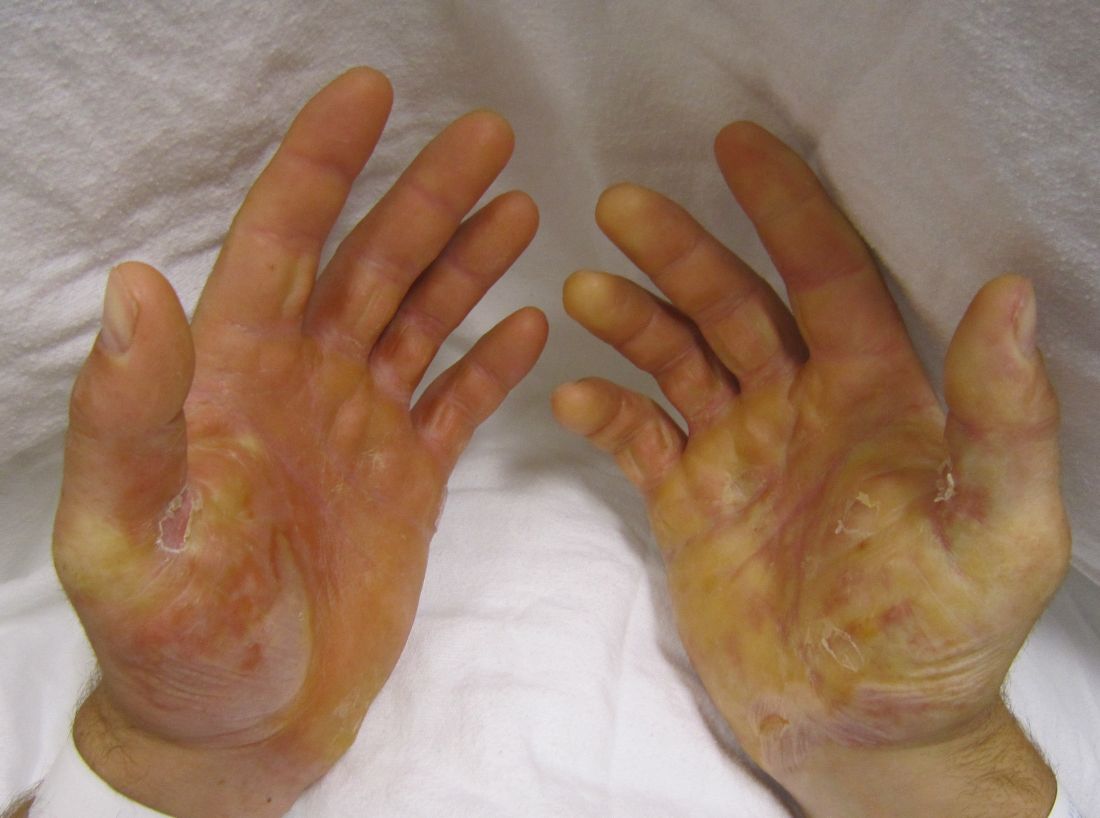
Dystrophic epidermolysis bullosa is characterized by blistering of the sublamina densa that leads to erosions; chronic ulcers; and extensive scarring, especially at sites of trauma on the hands and feet. Blistering can also develop in corneal and gastrointestinal epithelium, which adversely affects vision and feeding, and patients often develop aggressive squamous cell carcinoma with its attendant premature mortality.
Dystrophic EB has long been associated with mutations in COL7A1 as well as other genes. COL7A1 encodes type VII collagen, “a major protein component of the anchoring fibrils, which play a critical role in securing the attachment of the dermal-epidermal basement membrane to the underlying dermis.” Many patients with dystrophic EB show marked reductions in, or a total absence of, anchoring fibrils.
The investigators explored COL7A1 mutations in a multiethnic database comprising 238 patients in 152 extended families from different parts of the country. (The approximately 80 million people in Iran include several ethnic groups with distinct ancestries, languages, cultures, and geographic areas of residence.) A total of 139 of these families were consanguineous.
A total of 104 distinct COL7A1 mutations were detected in 149 of the 152 families, for a detection rate of 98%. Many mutations had been reported previously, but 56 had never been reported before. Approximately 90% of the families in the study cohort showed homozygous recessive mutations, which appears to reflect the consanguinity of this population, Dr. Vahidnezhad and his associates said.
They concluded that “the overwhelming majority” of patients with dystrophic EB carry COL7A1 genes harboring mutations.
This study was supported by DEBRA International and the Sidney Kimmel Cancer Center at Thomas Jefferson University. Dr. Vahidnezhad and his associates reported having no relevant financial disclosures.
according to a report published online in the Journal of Investigative Dermatology.
Identifying which specific mutations in the COL7A1 gene a given patient is carrying should allow clinicians to render a more accurate prognosis and may even dictate management strategies to counteract manifestations of the disease. Dystrophic epidermolysis bullosa (EB) can have an extremely variable progression that depends, in part, on the underlying molecular defects in many different genes expressed in the dermal-epidermal junction, according to study investigators Hassan Vahidnezhad, MD, of the department of dermatology and cutaneous biology, Thomas Jefferson University, Philadelphia, and his associates.
The study cohort included many consanguineous marriages, which are customary in Iran as in many parts of the Middle East, North Africa, and sub-Saharan Africa. In addition, many migrant communities from these regions now reside in Western countries and continue to practice these marriage customs, most commonly marriages between first cousins. “It is estimated that globally at least 20% of the human population live in communities with a preference for consanguineous marriage,” the researchers wrote (J Investig Dermatol. 2016. doi: 10.1016/j.jid.2016.10.023).
Dystrophic epidermolysis bullosa is characterized by blistering of the sublamina densa that leads to erosions; chronic ulcers; and extensive scarring, especially at sites of trauma on the hands and feet. Blistering can also develop in corneal and gastrointestinal epithelium, which adversely affects vision and feeding, and patients often develop aggressive squamous cell carcinoma with its attendant premature mortality.
Dystrophic EB has long been associated with mutations in COL7A1 as well as other genes. COL7A1 encodes type VII collagen, “a major protein component of the anchoring fibrils, which play a critical role in securing the attachment of the dermal-epidermal basement membrane to the underlying dermis.” Many patients with dystrophic EB show marked reductions in, or a total absence of, anchoring fibrils.
The investigators explored COL7A1 mutations in a multiethnic database comprising 238 patients in 152 extended families from different parts of the country. (The approximately 80 million people in Iran include several ethnic groups with distinct ancestries, languages, cultures, and geographic areas of residence.) A total of 139 of these families were consanguineous.
A total of 104 distinct COL7A1 mutations were detected in 149 of the 152 families, for a detection rate of 98%. Many mutations had been reported previously, but 56 had never been reported before. Approximately 90% of the families in the study cohort showed homozygous recessive mutations, which appears to reflect the consanguinity of this population, Dr. Vahidnezhad and his associates said.
They concluded that “the overwhelming majority” of patients with dystrophic EB carry COL7A1 genes harboring mutations.
This study was supported by DEBRA International and the Sidney Kimmel Cancer Center at Thomas Jefferson University. Dr. Vahidnezhad and his associates reported having no relevant financial disclosures.
according to a report published online in the Journal of Investigative Dermatology.
Identifying which specific mutations in the COL7A1 gene a given patient is carrying should allow clinicians to render a more accurate prognosis and may even dictate management strategies to counteract manifestations of the disease. Dystrophic epidermolysis bullosa (EB) can have an extremely variable progression that depends, in part, on the underlying molecular defects in many different genes expressed in the dermal-epidermal junction, according to study investigators Hassan Vahidnezhad, MD, of the department of dermatology and cutaneous biology, Thomas Jefferson University, Philadelphia, and his associates.
The study cohort included many consanguineous marriages, which are customary in Iran as in many parts of the Middle East, North Africa, and sub-Saharan Africa. In addition, many migrant communities from these regions now reside in Western countries and continue to practice these marriage customs, most commonly marriages between first cousins. “It is estimated that globally at least 20% of the human population live in communities with a preference for consanguineous marriage,” the researchers wrote (J Investig Dermatol. 2016. doi: 10.1016/j.jid.2016.10.023).
Dystrophic epidermolysis bullosa is characterized by blistering of the sublamina densa that leads to erosions; chronic ulcers; and extensive scarring, especially at sites of trauma on the hands and feet. Blistering can also develop in corneal and gastrointestinal epithelium, which adversely affects vision and feeding, and patients often develop aggressive squamous cell carcinoma with its attendant premature mortality.
Dystrophic EB has long been associated with mutations in COL7A1 as well as other genes. COL7A1 encodes type VII collagen, “a major protein component of the anchoring fibrils, which play a critical role in securing the attachment of the dermal-epidermal basement membrane to the underlying dermis.” Many patients with dystrophic EB show marked reductions in, or a total absence of, anchoring fibrils.
The investigators explored COL7A1 mutations in a multiethnic database comprising 238 patients in 152 extended families from different parts of the country. (The approximately 80 million people in Iran include several ethnic groups with distinct ancestries, languages, cultures, and geographic areas of residence.) A total of 139 of these families were consanguineous.
A total of 104 distinct COL7A1 mutations were detected in 149 of the 152 families, for a detection rate of 98%. Many mutations had been reported previously, but 56 had never been reported before. Approximately 90% of the families in the study cohort showed homozygous recessive mutations, which appears to reflect the consanguinity of this population, Dr. Vahidnezhad and his associates said.
They concluded that “the overwhelming majority” of patients with dystrophic EB carry COL7A1 genes harboring mutations.
This study was supported by DEBRA International and the Sidney Kimmel Cancer Center at Thomas Jefferson University. Dr. Vahidnezhad and his associates reported having no relevant financial disclosures.
FROM THE JOURNAL OF INVESTIGATIVE DERMATOLOGY
Key clinical point: 104 distinct mutations in a single gene, COL7A1, were discovered in a multiethnic cohort of 152 Iranian families with dystrophic epidermolysis bullosa.
Major finding: 104 distinct COL7A1 mutations were detected in 149 of the 152 families in the database, for a detection rate of 98%.
Data source: Analyses of mutations in the COL741 gene in 152 extended Iranian families with a high frequency of dystrophic epidermolysis bullosa.
Disclosures: This study was supported by DEBRA International and the Sidney Kimmel Cancer Center at Thomas Jefferson University. Dr. Vahidnezhad and his associates reported having no relevant financial disclosures.
Study finds 19% of Merkel cell carcinomas are virus negative
Nineteen percent of Merkel cell carcinomas are not driven by the Merkel cell polyomavirus and are substantially more aggressive than those that are virus positive, according to a report published online in the Journal of Investigative Dermatology.
This and other findings from a retrospective analysis of samples from 282 Merkel cell carcinomas in a Seattle repository “suggest that it may be clinically indicated to determine tumor viral status at the time of diagnosis, as the results may affect prognosis as well as optimal clinical management,” wrote Ata Moshiri, MD, who was with the University of Washington, Seattle, at the time of the study, and his associates.
Given that virus-negative Merkel cell carcinomas carry a markedly higher risk of recurrence, progression, and patient mortality, “clinicians may consider larger initial surgical margins, larger radiotherapy fields, and the use of regional nodal therapy even in the absence of documented nodal metastasis. Closer clinical follow-up and more frequent radiologic surveillance may be justified for patients with virus-negative tumors because ... serologic monitoring is not feasible for this patient population,” the investigators noted.
The incidence of Merkel cell carcinoma, a rare and aggressive neuroendocrine skin cancer with an overall disease-related mortality of 40%, has quadrupled during the last 20 years. This is likely because of the increasing prevalence of risk factors for the cancer, including advanced age, increased cumulative exposure to ultraviolet light, and systemic immune suppression.
Data concerning the presence of Merkel cell polyomavirus in these cancers are conflicting, with estimates of virus positivity ranging from 20% all the way to 100% in some studies. Part of the reason for this wide range of estimates is that there is no accepted preferred method for measuring the viral status of these tumors. Moreover, the prognostic significance of that viral status is also debated. Thus, most Merkel cell cancers are not routinely analyzed for the presence of Merkel cell polyomavirus.
To pin down the prevalence of virus positivity and establish whether it impacts clinical outcomes, Dr. Moshiri and his associates analyzed 282 Merkel cell specimens collected since 1980 and stored in a Seattle repository, along with clinical data. They tested each specimen using an immunohistochemical assay to detect one antibody (CM2B4), a different immunohistochemical assay to detect another antibody (Ab3), and a quantitative PCR assay for polyomavirus DNA. To be considered virus positive, each specimen had to show the presence of the virus on at least two of these tests.
By these criteria, 53 tumors (18.8%) were found to be virus negative and 229 (81.2%) to be virus positive.
Virus-negative tumors tended to be smaller than virus-positive tumors at presentation. Despite their smaller size, virus-negative tumors tended to be more advanced at presentation: 66.7% had nodal or distant metastases, compared with 48.3% of virus-positive tumors.
A total of 66.7% of virus-negative carcinomas progressed, compared with only 43.6% of virus-positive carcinomas. The median time to progression was 1.2 years for virus-negative cancers, but was not reached for virus-positive cancers. In a univariate analysis, virus-negative tumors had a nearly twofold higher risk of progression. In a multivariate analysis that adjusted for differences in disease stage at presentation, the HR fell slightly to 1.55.
Cancer-specific mortality was 45.3% for virus-negative tumors, compared with 26.3% for virus-positive tumors. Median time to death from Merkel cell carcinoma was 3.7 years for virus-negative tumors but was not reached for virus-positive tumors. In a univariate analysis, virus-negative tumors carried a nearly twofold higher risk of death from Merkel cell carcinoma. In a multivariate analysis that adjusted for differences in disease stage at presentation, the HR fell somewhat to 1.50.
Median overall survival was 3.3 years for patients with virus-negative tumors, compared with 4.6 years for patients with virus-positive tumors.
These findings indicate that a more advanced cancer stage at diagnosis accounts for some but not all of the poorer clinical outcomes seen with virus-negative tumors, the investigators said.
This study could not assess why virus-negative Merkel cell carcinomas are more aggressive and lethal than virus-positive ones, but previous studies have proposed some plausible biological mechanisms. Virus-negative tumors carry a greater number of chromosomal aberrations, a greater burden of nucleotide mutations, and a greater number of mutations in known oncogenic pathways. They also may be more immunogenic “due to their constitutive expression of oncoproteins that may serve as targets for cytotoxic tumor-infiltrating lymphocytes,” Dr. Moshiri and his associates said.
They added that in this study, the immunohistochemical assay for CM2B4 antibodies was the test that most accurately identified tumors that had worse outcomes. “We believe that the CM2B4 antibody test may be well-suited for routine clinical use” because of its sensitivity and specificity in this application, its commercial availability, “and the ease with which it could be included in the work flow of clinical laboratories accustomed to immunohistochemistry.”
Dr. Moshiri is currently at the University of Pennsylvania, Philadelphia.
The National Institutes of Health, the Colin Johnston Fund, and the Janet Canning Fund supported the study. Dr. Moshiri reported having no relevant financial disclosures; one of his associates reported that her institute received research funding from Valeant and Pfizer unrelated to this work.
Nineteen percent of Merkel cell carcinomas are not driven by the Merkel cell polyomavirus and are substantially more aggressive than those that are virus positive, according to a report published online in the Journal of Investigative Dermatology.
This and other findings from a retrospective analysis of samples from 282 Merkel cell carcinomas in a Seattle repository “suggest that it may be clinically indicated to determine tumor viral status at the time of diagnosis, as the results may affect prognosis as well as optimal clinical management,” wrote Ata Moshiri, MD, who was with the University of Washington, Seattle, at the time of the study, and his associates.
Given that virus-negative Merkel cell carcinomas carry a markedly higher risk of recurrence, progression, and patient mortality, “clinicians may consider larger initial surgical margins, larger radiotherapy fields, and the use of regional nodal therapy even in the absence of documented nodal metastasis. Closer clinical follow-up and more frequent radiologic surveillance may be justified for patients with virus-negative tumors because ... serologic monitoring is not feasible for this patient population,” the investigators noted.
The incidence of Merkel cell carcinoma, a rare and aggressive neuroendocrine skin cancer with an overall disease-related mortality of 40%, has quadrupled during the last 20 years. This is likely because of the increasing prevalence of risk factors for the cancer, including advanced age, increased cumulative exposure to ultraviolet light, and systemic immune suppression.
Data concerning the presence of Merkel cell polyomavirus in these cancers are conflicting, with estimates of virus positivity ranging from 20% all the way to 100% in some studies. Part of the reason for this wide range of estimates is that there is no accepted preferred method for measuring the viral status of these tumors. Moreover, the prognostic significance of that viral status is also debated. Thus, most Merkel cell cancers are not routinely analyzed for the presence of Merkel cell polyomavirus.
To pin down the prevalence of virus positivity and establish whether it impacts clinical outcomes, Dr. Moshiri and his associates analyzed 282 Merkel cell specimens collected since 1980 and stored in a Seattle repository, along with clinical data. They tested each specimen using an immunohistochemical assay to detect one antibody (CM2B4), a different immunohistochemical assay to detect another antibody (Ab3), and a quantitative PCR assay for polyomavirus DNA. To be considered virus positive, each specimen had to show the presence of the virus on at least two of these tests.
By these criteria, 53 tumors (18.8%) were found to be virus negative and 229 (81.2%) to be virus positive.
Virus-negative tumors tended to be smaller than virus-positive tumors at presentation. Despite their smaller size, virus-negative tumors tended to be more advanced at presentation: 66.7% had nodal or distant metastases, compared with 48.3% of virus-positive tumors.
A total of 66.7% of virus-negative carcinomas progressed, compared with only 43.6% of virus-positive carcinomas. The median time to progression was 1.2 years for virus-negative cancers, but was not reached for virus-positive cancers. In a univariate analysis, virus-negative tumors had a nearly twofold higher risk of progression. In a multivariate analysis that adjusted for differences in disease stage at presentation, the HR fell slightly to 1.55.
Cancer-specific mortality was 45.3% for virus-negative tumors, compared with 26.3% for virus-positive tumors. Median time to death from Merkel cell carcinoma was 3.7 years for virus-negative tumors but was not reached for virus-positive tumors. In a univariate analysis, virus-negative tumors carried a nearly twofold higher risk of death from Merkel cell carcinoma. In a multivariate analysis that adjusted for differences in disease stage at presentation, the HR fell somewhat to 1.50.
Median overall survival was 3.3 years for patients with virus-negative tumors, compared with 4.6 years for patients with virus-positive tumors.
These findings indicate that a more advanced cancer stage at diagnosis accounts for some but not all of the poorer clinical outcomes seen with virus-negative tumors, the investigators said.
This study could not assess why virus-negative Merkel cell carcinomas are more aggressive and lethal than virus-positive ones, but previous studies have proposed some plausible biological mechanisms. Virus-negative tumors carry a greater number of chromosomal aberrations, a greater burden of nucleotide mutations, and a greater number of mutations in known oncogenic pathways. They also may be more immunogenic “due to their constitutive expression of oncoproteins that may serve as targets for cytotoxic tumor-infiltrating lymphocytes,” Dr. Moshiri and his associates said.
They added that in this study, the immunohistochemical assay for CM2B4 antibodies was the test that most accurately identified tumors that had worse outcomes. “We believe that the CM2B4 antibody test may be well-suited for routine clinical use” because of its sensitivity and specificity in this application, its commercial availability, “and the ease with which it could be included in the work flow of clinical laboratories accustomed to immunohistochemistry.”
Dr. Moshiri is currently at the University of Pennsylvania, Philadelphia.
The National Institutes of Health, the Colin Johnston Fund, and the Janet Canning Fund supported the study. Dr. Moshiri reported having no relevant financial disclosures; one of his associates reported that her institute received research funding from Valeant and Pfizer unrelated to this work.
Nineteen percent of Merkel cell carcinomas are not driven by the Merkel cell polyomavirus and are substantially more aggressive than those that are virus positive, according to a report published online in the Journal of Investigative Dermatology.
This and other findings from a retrospective analysis of samples from 282 Merkel cell carcinomas in a Seattle repository “suggest that it may be clinically indicated to determine tumor viral status at the time of diagnosis, as the results may affect prognosis as well as optimal clinical management,” wrote Ata Moshiri, MD, who was with the University of Washington, Seattle, at the time of the study, and his associates.
Given that virus-negative Merkel cell carcinomas carry a markedly higher risk of recurrence, progression, and patient mortality, “clinicians may consider larger initial surgical margins, larger radiotherapy fields, and the use of regional nodal therapy even in the absence of documented nodal metastasis. Closer clinical follow-up and more frequent radiologic surveillance may be justified for patients with virus-negative tumors because ... serologic monitoring is not feasible for this patient population,” the investigators noted.
The incidence of Merkel cell carcinoma, a rare and aggressive neuroendocrine skin cancer with an overall disease-related mortality of 40%, has quadrupled during the last 20 years. This is likely because of the increasing prevalence of risk factors for the cancer, including advanced age, increased cumulative exposure to ultraviolet light, and systemic immune suppression.
Data concerning the presence of Merkel cell polyomavirus in these cancers are conflicting, with estimates of virus positivity ranging from 20% all the way to 100% in some studies. Part of the reason for this wide range of estimates is that there is no accepted preferred method for measuring the viral status of these tumors. Moreover, the prognostic significance of that viral status is also debated. Thus, most Merkel cell cancers are not routinely analyzed for the presence of Merkel cell polyomavirus.
To pin down the prevalence of virus positivity and establish whether it impacts clinical outcomes, Dr. Moshiri and his associates analyzed 282 Merkel cell specimens collected since 1980 and stored in a Seattle repository, along with clinical data. They tested each specimen using an immunohistochemical assay to detect one antibody (CM2B4), a different immunohistochemical assay to detect another antibody (Ab3), and a quantitative PCR assay for polyomavirus DNA. To be considered virus positive, each specimen had to show the presence of the virus on at least two of these tests.
By these criteria, 53 tumors (18.8%) were found to be virus negative and 229 (81.2%) to be virus positive.
Virus-negative tumors tended to be smaller than virus-positive tumors at presentation. Despite their smaller size, virus-negative tumors tended to be more advanced at presentation: 66.7% had nodal or distant metastases, compared with 48.3% of virus-positive tumors.
A total of 66.7% of virus-negative carcinomas progressed, compared with only 43.6% of virus-positive carcinomas. The median time to progression was 1.2 years for virus-negative cancers, but was not reached for virus-positive cancers. In a univariate analysis, virus-negative tumors had a nearly twofold higher risk of progression. In a multivariate analysis that adjusted for differences in disease stage at presentation, the HR fell slightly to 1.55.
Cancer-specific mortality was 45.3% for virus-negative tumors, compared with 26.3% for virus-positive tumors. Median time to death from Merkel cell carcinoma was 3.7 years for virus-negative tumors but was not reached for virus-positive tumors. In a univariate analysis, virus-negative tumors carried a nearly twofold higher risk of death from Merkel cell carcinoma. In a multivariate analysis that adjusted for differences in disease stage at presentation, the HR fell somewhat to 1.50.
Median overall survival was 3.3 years for patients with virus-negative tumors, compared with 4.6 years for patients with virus-positive tumors.
These findings indicate that a more advanced cancer stage at diagnosis accounts for some but not all of the poorer clinical outcomes seen with virus-negative tumors, the investigators said.
This study could not assess why virus-negative Merkel cell carcinomas are more aggressive and lethal than virus-positive ones, but previous studies have proposed some plausible biological mechanisms. Virus-negative tumors carry a greater number of chromosomal aberrations, a greater burden of nucleotide mutations, and a greater number of mutations in known oncogenic pathways. They also may be more immunogenic “due to their constitutive expression of oncoproteins that may serve as targets for cytotoxic tumor-infiltrating lymphocytes,” Dr. Moshiri and his associates said.
They added that in this study, the immunohistochemical assay for CM2B4 antibodies was the test that most accurately identified tumors that had worse outcomes. “We believe that the CM2B4 antibody test may be well-suited for routine clinical use” because of its sensitivity and specificity in this application, its commercial availability, “and the ease with which it could be included in the work flow of clinical laboratories accustomed to immunohistochemistry.”
Dr. Moshiri is currently at the University of Pennsylvania, Philadelphia.
The National Institutes of Health, the Colin Johnston Fund, and the Janet Canning Fund supported the study. Dr. Moshiri reported having no relevant financial disclosures; one of his associates reported that her institute received research funding from Valeant and Pfizer unrelated to this work.
FROM THE JOURNAL OF INVESTIGATIVE DERMATOLOGY
Key clinical point: Nineteen percent of Merkel cell carcinomas are not driven by the Merkel cell polyomavirus and are substantially more aggressive than those that are virus positive.
Major finding: The 53 virus-negative tumors carried a cancer-specific mortality of 45.3%, while the 229 virus-positive tumors carried a cancer-specific mortality of 26.2%.
Data source: A retrospective molecular analysis of samples from 282 Merkel cell carcinomas in a Seattle repository for the presence of Merkel cell polyomavirus.
Disclosures: The National Institutes of Health, the Colin Johnston Fund, and the Janet Canning Fund supported the study. Dr. Moshiri reported having no relevant financial disclosures; one of his associates reported that her institute received research funding from Valeant and Pfizer unrelated to this work.
Genetically corrected skin grafts explored in dystrophic EB
Genetically corrected autologous skin grafts produced wound healing in a phase I trial involving four men with recessive dystrophic epidermolysis bullosa in what the investigators described as the first human trial of cutaneous gene therapy for this indication, according to a report published in JAMA.
The wound healing varied according to graft site and across the four patients, and generally declined over the course of 1 year of follow-up. Given that this study focused on safety outcomes and that the treatment’s safety profile was deemed “acceptable,” the Food and Drug Administration has permitted a phase IIA trial (NCT01263379) that is currently enrolling adolescents with the disease and will focus on clinical outcomes. Longer-term follow-up of these four patients will continue, and “controlled trials are needed with a broader range of patients to better understand the potential long-term efficacy of genetically corrected autologous epidermal grafts,” said Zurab Siprashvili, Ph.D., of Stanford (Calif.) University and associates.
The investigators assessed whether grafting of type VII collagen gene-corrected autologous keratinocytes onto existing wounds would promote their healing. They first harvested and cultured keratinocytes from biopsies of the patients’ unwounded, unscarred skin and transduced these samples with COL7A1-containing retrovirus, producing eight gene-corrected grafts for each patient. All the biopsy sites healed completely, without complications.
The wound beds were prepared for engraftment by cauterization to minimize any retained epidermal stem cells. Then grafts were applied using dissolvable sutures to six wound sites on each patient; the sites had been selected for their accessibility, their potential to enhance the patients’ quality of life, and their ability to ease the period of immobilization after grafting.
All 24 grafts were well tolerated, and no serious adverse events occurred during 1 year of follow-up, which represented approximately 10 epidermal turnover cycles. The most common adverse events – pruritus (three patients) and drainage (two patients) at the graft site – were mild. None of the patients showed systemic autoimmune symptoms or increased blistering outside of the grafted areas, and no clinical signs of malignancy developed.
The gene-corrected graft sites showed type VII collagen localization to anchoring fibrils at the dermal-epidermal junction, in contrast to control sites that showed no such localization or fibril formation. “Gene-corrected graft sites showed fully differentiated epidermis with spinous and granular layers, which were positive for epidermal markers keratin 14, keratin 1, and loricirin resembling normal skin,” Dr. Siprashvili and his associates wrote.
At 1-month follow-up, 20 of the 24 grafts showed 75% or greater wound healing, compared with baseline, and the other 4 grafts showed 50%-74% wound healing. At 3 months, 21 of the 24 graft sites showed 75% or greater wound healing while the remaining 3 grafts showed 50%-74% wound healing. At 6 months, 16 of 24 graft sites showed 75% or greater wound healing and 5 graft sites showed 50%-74% wound healing, but 3 graft sites showed extensive blisters or erosions and were considered graft failures. At 12 months, only 12 of the 24 graft sites showed 75% or greater wound healing.
This general decline in efficacy might be related to the relatively small number of stem cells available for transplantation in these patients, who had only small areas of unscarred skin for harvesting. Or it may be that uncorrected cells in the wound beds began competing with corrected cells within the graft and eventually overcame them, the researchers said.
This study was supported by the National Institutes of Health, the Epidermolysis Bullosa Medical Research Foundation, the Epidermolysis Bullosa Research Partnership, and the Palo Alto VA Medical Center. Dr. Siprashvili and some associates reported having U.S. patents pending; two associates also reported ties to Scioderm and Fibrocell.
Genetically corrected autologous skin grafts produced wound healing in a phase I trial involving four men with recessive dystrophic epidermolysis bullosa in what the investigators described as the first human trial of cutaneous gene therapy for this indication, according to a report published in JAMA.
The wound healing varied according to graft site and across the four patients, and generally declined over the course of 1 year of follow-up. Given that this study focused on safety outcomes and that the treatment’s safety profile was deemed “acceptable,” the Food and Drug Administration has permitted a phase IIA trial (NCT01263379) that is currently enrolling adolescents with the disease and will focus on clinical outcomes. Longer-term follow-up of these four patients will continue, and “controlled trials are needed with a broader range of patients to better understand the potential long-term efficacy of genetically corrected autologous epidermal grafts,” said Zurab Siprashvili, Ph.D., of Stanford (Calif.) University and associates.
The investigators assessed whether grafting of type VII collagen gene-corrected autologous keratinocytes onto existing wounds would promote their healing. They first harvested and cultured keratinocytes from biopsies of the patients’ unwounded, unscarred skin and transduced these samples with COL7A1-containing retrovirus, producing eight gene-corrected grafts for each patient. All the biopsy sites healed completely, without complications.
The wound beds were prepared for engraftment by cauterization to minimize any retained epidermal stem cells. Then grafts were applied using dissolvable sutures to six wound sites on each patient; the sites had been selected for their accessibility, their potential to enhance the patients’ quality of life, and their ability to ease the period of immobilization after grafting.
All 24 grafts were well tolerated, and no serious adverse events occurred during 1 year of follow-up, which represented approximately 10 epidermal turnover cycles. The most common adverse events – pruritus (three patients) and drainage (two patients) at the graft site – were mild. None of the patients showed systemic autoimmune symptoms or increased blistering outside of the grafted areas, and no clinical signs of malignancy developed.
The gene-corrected graft sites showed type VII collagen localization to anchoring fibrils at the dermal-epidermal junction, in contrast to control sites that showed no such localization or fibril formation. “Gene-corrected graft sites showed fully differentiated epidermis with spinous and granular layers, which were positive for epidermal markers keratin 14, keratin 1, and loricirin resembling normal skin,” Dr. Siprashvili and his associates wrote.
At 1-month follow-up, 20 of the 24 grafts showed 75% or greater wound healing, compared with baseline, and the other 4 grafts showed 50%-74% wound healing. At 3 months, 21 of the 24 graft sites showed 75% or greater wound healing while the remaining 3 grafts showed 50%-74% wound healing. At 6 months, 16 of 24 graft sites showed 75% or greater wound healing and 5 graft sites showed 50%-74% wound healing, but 3 graft sites showed extensive blisters or erosions and were considered graft failures. At 12 months, only 12 of the 24 graft sites showed 75% or greater wound healing.
This general decline in efficacy might be related to the relatively small number of stem cells available for transplantation in these patients, who had only small areas of unscarred skin for harvesting. Or it may be that uncorrected cells in the wound beds began competing with corrected cells within the graft and eventually overcame them, the researchers said.
This study was supported by the National Institutes of Health, the Epidermolysis Bullosa Medical Research Foundation, the Epidermolysis Bullosa Research Partnership, and the Palo Alto VA Medical Center. Dr. Siprashvili and some associates reported having U.S. patents pending; two associates also reported ties to Scioderm and Fibrocell.
Genetically corrected autologous skin grafts produced wound healing in a phase I trial involving four men with recessive dystrophic epidermolysis bullosa in what the investigators described as the first human trial of cutaneous gene therapy for this indication, according to a report published in JAMA.
The wound healing varied according to graft site and across the four patients, and generally declined over the course of 1 year of follow-up. Given that this study focused on safety outcomes and that the treatment’s safety profile was deemed “acceptable,” the Food and Drug Administration has permitted a phase IIA trial (NCT01263379) that is currently enrolling adolescents with the disease and will focus on clinical outcomes. Longer-term follow-up of these four patients will continue, and “controlled trials are needed with a broader range of patients to better understand the potential long-term efficacy of genetically corrected autologous epidermal grafts,” said Zurab Siprashvili, Ph.D., of Stanford (Calif.) University and associates.
The investigators assessed whether grafting of type VII collagen gene-corrected autologous keratinocytes onto existing wounds would promote their healing. They first harvested and cultured keratinocytes from biopsies of the patients’ unwounded, unscarred skin and transduced these samples with COL7A1-containing retrovirus, producing eight gene-corrected grafts for each patient. All the biopsy sites healed completely, without complications.
The wound beds were prepared for engraftment by cauterization to minimize any retained epidermal stem cells. Then grafts were applied using dissolvable sutures to six wound sites on each patient; the sites had been selected for their accessibility, their potential to enhance the patients’ quality of life, and their ability to ease the period of immobilization after grafting.
All 24 grafts were well tolerated, and no serious adverse events occurred during 1 year of follow-up, which represented approximately 10 epidermal turnover cycles. The most common adverse events – pruritus (three patients) and drainage (two patients) at the graft site – were mild. None of the patients showed systemic autoimmune symptoms or increased blistering outside of the grafted areas, and no clinical signs of malignancy developed.
The gene-corrected graft sites showed type VII collagen localization to anchoring fibrils at the dermal-epidermal junction, in contrast to control sites that showed no such localization or fibril formation. “Gene-corrected graft sites showed fully differentiated epidermis with spinous and granular layers, which were positive for epidermal markers keratin 14, keratin 1, and loricirin resembling normal skin,” Dr. Siprashvili and his associates wrote.
At 1-month follow-up, 20 of the 24 grafts showed 75% or greater wound healing, compared with baseline, and the other 4 grafts showed 50%-74% wound healing. At 3 months, 21 of the 24 graft sites showed 75% or greater wound healing while the remaining 3 grafts showed 50%-74% wound healing. At 6 months, 16 of 24 graft sites showed 75% or greater wound healing and 5 graft sites showed 50%-74% wound healing, but 3 graft sites showed extensive blisters or erosions and were considered graft failures. At 12 months, only 12 of the 24 graft sites showed 75% or greater wound healing.
This general decline in efficacy might be related to the relatively small number of stem cells available for transplantation in these patients, who had only small areas of unscarred skin for harvesting. Or it may be that uncorrected cells in the wound beds began competing with corrected cells within the graft and eventually overcame them, the researchers said.
This study was supported by the National Institutes of Health, the Epidermolysis Bullosa Medical Research Foundation, the Epidermolysis Bullosa Research Partnership, and the Palo Alto VA Medical Center. Dr. Siprashvili and some associates reported having U.S. patents pending; two associates also reported ties to Scioderm and Fibrocell.
FROM JAMA
Key clinical point: Genetically corrected autologous skin grafts produced wound healing in a phase I study of four patients with recessive dystrophic epidermolysis bullosa.
Major finding: All 24 grafts were well tolerated, and no serious adverse events occurred during 1 year of follow-up; none of the patients showed systemic autoimmune symptoms or increased blistering outside of the grafted areas, and no clinical signs of malignancy developed.
Data source: A single-center open-label phase I study involving four men followed for 1 year.
Disclosures: This study was supported by the National Institutes of Health, the Epidermolysis Bullosa Medical Research Foundation, the Epidermolysis Bullosa Research Partnership, and the Palo Alto VA Medical Center. Dr. Siprashvili and some associates reported having U.S. patents pending; two associates also reported ties to Scioderm and Fibrocell.
Giving women HIV self-tests increases male partner testing
Providing Kenyan women attending prenatal or postpartum health care visits with HIV self-testing kits raised the rates of partner and couples testing to more than 90%, according to a report published online in PLOS Medicine.
HIV testing remains underused in many parts of sub-Saharan Africa, particularly among men, for reasons including social stigma, fear of poor prognosis, lack of awareness of HIV risk, fear that their results would be disclosed, inconvenience, and transportation costs. To assess one strategy for improving male testing rates, researchers performed a study at a hospital and two clinics in urban and suburban Kisumu, Kenya, where the HIV prevalence is approximately 20% among adult residents.
The trial involved 600 women aged 18-39 years (mean age, 24 years) who were seeking either prenatal or postpartum health care and agreed to participate. They were randomly assigned to receive either a few HIV self-test kits plus counseling regarding HIV testing (intervention group) or counseling alone (control group) and followed for 3 months. A total of 95% of the women – 284 in the intervention group and 286 in the control group – completed the study, said Harsha Thirumurthy, PhD, of the department of health policy and management, Gillings School of Global Public Health, University of North Carolina, Chapel Hill, and his associates.
At follow-up, 258 (90.8%) of the women in the intervention group reported that their partners had been tested for HIV, compared with 148 (51.7%) of the control group. This significant difference persisted across all subgroups of patients, regardless of study site and whether or not partners said they had been tested during the preceding year. “This result is encouraging since it suggests that the strategy of giving multiple self-tests to women can effectively increase access to HIV testing in hard-to-reach populations such as men who do not test regularly,” the investigators said (PLOS Med. 2016 Nov 8. doi: 10.1371/journal.pmed.1002166).
In three-fourths of the cases where male partners were tested for HIV, both members of the couple were tested together. This is beneficial because it helps women learn their partners’ HIV status, and because couples who test together are “more likely to adopt a range of HIV prevention and care behaviors,” Dr. Thirumurthy and his associates wrote.
Approximately one-third of the women who were eligible for this study declined to participate, often because they feared that their partners would become violent if offered an HIV self-test. Even women who did participate reported a high rate (27%) of partner violence at baseline. It is encouraging that none of the study participants reported any such incidents in response to the HIV testing, the investigators added.
The International Initiative for Impact Evaluation funded the study. Dr. Thirumurthy and his associates reported having no relevant financial disclosures.
Providing Kenyan women attending prenatal or postpartum health care visits with HIV self-testing kits raised the rates of partner and couples testing to more than 90%, according to a report published online in PLOS Medicine.
HIV testing remains underused in many parts of sub-Saharan Africa, particularly among men, for reasons including social stigma, fear of poor prognosis, lack of awareness of HIV risk, fear that their results would be disclosed, inconvenience, and transportation costs. To assess one strategy for improving male testing rates, researchers performed a study at a hospital and two clinics in urban and suburban Kisumu, Kenya, where the HIV prevalence is approximately 20% among adult residents.
The trial involved 600 women aged 18-39 years (mean age, 24 years) who were seeking either prenatal or postpartum health care and agreed to participate. They were randomly assigned to receive either a few HIV self-test kits plus counseling regarding HIV testing (intervention group) or counseling alone (control group) and followed for 3 months. A total of 95% of the women – 284 in the intervention group and 286 in the control group – completed the study, said Harsha Thirumurthy, PhD, of the department of health policy and management, Gillings School of Global Public Health, University of North Carolina, Chapel Hill, and his associates.
At follow-up, 258 (90.8%) of the women in the intervention group reported that their partners had been tested for HIV, compared with 148 (51.7%) of the control group. This significant difference persisted across all subgroups of patients, regardless of study site and whether or not partners said they had been tested during the preceding year. “This result is encouraging since it suggests that the strategy of giving multiple self-tests to women can effectively increase access to HIV testing in hard-to-reach populations such as men who do not test regularly,” the investigators said (PLOS Med. 2016 Nov 8. doi: 10.1371/journal.pmed.1002166).
In three-fourths of the cases where male partners were tested for HIV, both members of the couple were tested together. This is beneficial because it helps women learn their partners’ HIV status, and because couples who test together are “more likely to adopt a range of HIV prevention and care behaviors,” Dr. Thirumurthy and his associates wrote.
Approximately one-third of the women who were eligible for this study declined to participate, often because they feared that their partners would become violent if offered an HIV self-test. Even women who did participate reported a high rate (27%) of partner violence at baseline. It is encouraging that none of the study participants reported any such incidents in response to the HIV testing, the investigators added.
The International Initiative for Impact Evaluation funded the study. Dr. Thirumurthy and his associates reported having no relevant financial disclosures.
Providing Kenyan women attending prenatal or postpartum health care visits with HIV self-testing kits raised the rates of partner and couples testing to more than 90%, according to a report published online in PLOS Medicine.
HIV testing remains underused in many parts of sub-Saharan Africa, particularly among men, for reasons including social stigma, fear of poor prognosis, lack of awareness of HIV risk, fear that their results would be disclosed, inconvenience, and transportation costs. To assess one strategy for improving male testing rates, researchers performed a study at a hospital and two clinics in urban and suburban Kisumu, Kenya, where the HIV prevalence is approximately 20% among adult residents.
The trial involved 600 women aged 18-39 years (mean age, 24 years) who were seeking either prenatal or postpartum health care and agreed to participate. They were randomly assigned to receive either a few HIV self-test kits plus counseling regarding HIV testing (intervention group) or counseling alone (control group) and followed for 3 months. A total of 95% of the women – 284 in the intervention group and 286 in the control group – completed the study, said Harsha Thirumurthy, PhD, of the department of health policy and management, Gillings School of Global Public Health, University of North Carolina, Chapel Hill, and his associates.
At follow-up, 258 (90.8%) of the women in the intervention group reported that their partners had been tested for HIV, compared with 148 (51.7%) of the control group. This significant difference persisted across all subgroups of patients, regardless of study site and whether or not partners said they had been tested during the preceding year. “This result is encouraging since it suggests that the strategy of giving multiple self-tests to women can effectively increase access to HIV testing in hard-to-reach populations such as men who do not test regularly,” the investigators said (PLOS Med. 2016 Nov 8. doi: 10.1371/journal.pmed.1002166).
In three-fourths of the cases where male partners were tested for HIV, both members of the couple were tested together. This is beneficial because it helps women learn their partners’ HIV status, and because couples who test together are “more likely to adopt a range of HIV prevention and care behaviors,” Dr. Thirumurthy and his associates wrote.
Approximately one-third of the women who were eligible for this study declined to participate, often because they feared that their partners would become violent if offered an HIV self-test. Even women who did participate reported a high rate (27%) of partner violence at baseline. It is encouraging that none of the study participants reported any such incidents in response to the HIV testing, the investigators added.
The International Initiative for Impact Evaluation funded the study. Dr. Thirumurthy and his associates reported having no relevant financial disclosures.
FROM PLOS MEDICINE
Key clinical point: Providing Kenyan women attending prenatal or postpartum health care visits with HIV self-testing kits raised the rates of partner and couples testing to more than 90%.
Major finding: After 3 months, 258 women (90.8%) in the intervention group reported that their partners had been tested for HIV, compared with 148 (51.7%) of the control group.
Data source: A multicenter randomized trial involving 600 women in Kenya receiving prenatal or postpartum care, and their sexual partners.
Disclosures: The International Initiative for Impact Evaluation funded the study. Dr. Thirumurthy and his associates reported having no relevant financial disclosures.
Abatacept may benefit ACPA-negative undifferentiated arthritis
Abatacept may benefit ACPA-negative undifferentiated arthritis, which generally has a poor prognosis and for which there is no proven therapy currently available, according to a report published in Rheumatology.
A 1-year course of abatacept (Orencia) reduced disease activity and an ultrasound measure of synovial inflammation in a manufacturer-funded, open-label, proof-of-concept study. However, it did not achieve the primary composite endpoint of remission on the DAS44 (44-joint Disease Activity Score), a maximum of one swollen joint for at least 3 consecutive months, and no radiographic progression after 6 months of treatment.
They assessed 20 adults with undifferentiated arthritis that had lasted 12 weeks to 18 months who showed definite, active synovitis in at least 1 of 20 scanned joints and had no previous disease-modifying antirheumatic drug (DMARD) therapy; these study subjects were considered likely to progress to rheumatoid arthritis. They received 14 doses of IV abatacept for 1 year.
Two patients withdrew from the study at 6 months and 12 months because of adverse events, and another three were lost to follow-up. Only 2 of the 20 patients (10%) achieved the composite primary endpoint. The majority of patients met two of the individual components – 15 patients showed no radiographic progression and 12 had a maximum of one swollen joint – but only 6 achieved DAS44 remission, the investigators said (Rheumatology [Oxford]. 2016 Oct 22. doi: 10.1093/rheumatology/kew357).
The treatment appeared to suppress C-reactive protein levels immediately, from a median of 9 mg/L at baseline to 0 mg/L at 3 and 6 months. This implies a clear biologic effect. The median number of swollen joints also decreased from a median of 2 at baseline to 0 at 6 and 12 months. There were small reductions in median scores on a disability index and a small decrease in patient-reported visual analog scale disease activity from a median of 52 at baseline to 28 at 6 months and 24 at 12 months. Median ultrasound scores of synovitis decreased from 10 at baseline to 3 at both 6 and 12 months.
Most of these benefits persisted for 1 year after abatacept therapy was stopped, but approximately half of patients required a synthetic DMARD to maintain the benefits. “Overall, these data suggest that in the vast majority of patients (18 of 20), abatacept therapy prevented further progression of disease, but on cessation, additional therapy was indicated to maintain this,” Dr. Buch and her associates said.
There were no serious adverse events during treatment, including no infections and no abnormal liver function tests. A total of 131 nonserious adverse events were reported.
This study was supported by a research grant from Bristol-Myers Squibb and by institution-level grants from Arthritis UK and the U.K. National Institute for Health Research. Dr. Buch reported ties to Bristol-Myers Squibb, AbbVie, AstraZeneca, Pfizer, and Roche-Chugai; her associates reported ties to numerous industry sources.
Abatacept may benefit ACPA-negative undifferentiated arthritis, which generally has a poor prognosis and for which there is no proven therapy currently available, according to a report published in Rheumatology.
A 1-year course of abatacept (Orencia) reduced disease activity and an ultrasound measure of synovial inflammation in a manufacturer-funded, open-label, proof-of-concept study. However, it did not achieve the primary composite endpoint of remission on the DAS44 (44-joint Disease Activity Score), a maximum of one swollen joint for at least 3 consecutive months, and no radiographic progression after 6 months of treatment.
They assessed 20 adults with undifferentiated arthritis that had lasted 12 weeks to 18 months who showed definite, active synovitis in at least 1 of 20 scanned joints and had no previous disease-modifying antirheumatic drug (DMARD) therapy; these study subjects were considered likely to progress to rheumatoid arthritis. They received 14 doses of IV abatacept for 1 year.
Two patients withdrew from the study at 6 months and 12 months because of adverse events, and another three were lost to follow-up. Only 2 of the 20 patients (10%) achieved the composite primary endpoint. The majority of patients met two of the individual components – 15 patients showed no radiographic progression and 12 had a maximum of one swollen joint – but only 6 achieved DAS44 remission, the investigators said (Rheumatology [Oxford]. 2016 Oct 22. doi: 10.1093/rheumatology/kew357).
The treatment appeared to suppress C-reactive protein levels immediately, from a median of 9 mg/L at baseline to 0 mg/L at 3 and 6 months. This implies a clear biologic effect. The median number of swollen joints also decreased from a median of 2 at baseline to 0 at 6 and 12 months. There were small reductions in median scores on a disability index and a small decrease in patient-reported visual analog scale disease activity from a median of 52 at baseline to 28 at 6 months and 24 at 12 months. Median ultrasound scores of synovitis decreased from 10 at baseline to 3 at both 6 and 12 months.
Most of these benefits persisted for 1 year after abatacept therapy was stopped, but approximately half of patients required a synthetic DMARD to maintain the benefits. “Overall, these data suggest that in the vast majority of patients (18 of 20), abatacept therapy prevented further progression of disease, but on cessation, additional therapy was indicated to maintain this,” Dr. Buch and her associates said.
There were no serious adverse events during treatment, including no infections and no abnormal liver function tests. A total of 131 nonserious adverse events were reported.
This study was supported by a research grant from Bristol-Myers Squibb and by institution-level grants from Arthritis UK and the U.K. National Institute for Health Research. Dr. Buch reported ties to Bristol-Myers Squibb, AbbVie, AstraZeneca, Pfizer, and Roche-Chugai; her associates reported ties to numerous industry sources.
Abatacept may benefit ACPA-negative undifferentiated arthritis, which generally has a poor prognosis and for which there is no proven therapy currently available, according to a report published in Rheumatology.
A 1-year course of abatacept (Orencia) reduced disease activity and an ultrasound measure of synovial inflammation in a manufacturer-funded, open-label, proof-of-concept study. However, it did not achieve the primary composite endpoint of remission on the DAS44 (44-joint Disease Activity Score), a maximum of one swollen joint for at least 3 consecutive months, and no radiographic progression after 6 months of treatment.
They assessed 20 adults with undifferentiated arthritis that had lasted 12 weeks to 18 months who showed definite, active synovitis in at least 1 of 20 scanned joints and had no previous disease-modifying antirheumatic drug (DMARD) therapy; these study subjects were considered likely to progress to rheumatoid arthritis. They received 14 doses of IV abatacept for 1 year.
Two patients withdrew from the study at 6 months and 12 months because of adverse events, and another three were lost to follow-up. Only 2 of the 20 patients (10%) achieved the composite primary endpoint. The majority of patients met two of the individual components – 15 patients showed no radiographic progression and 12 had a maximum of one swollen joint – but only 6 achieved DAS44 remission, the investigators said (Rheumatology [Oxford]. 2016 Oct 22. doi: 10.1093/rheumatology/kew357).
The treatment appeared to suppress C-reactive protein levels immediately, from a median of 9 mg/L at baseline to 0 mg/L at 3 and 6 months. This implies a clear biologic effect. The median number of swollen joints also decreased from a median of 2 at baseline to 0 at 6 and 12 months. There were small reductions in median scores on a disability index and a small decrease in patient-reported visual analog scale disease activity from a median of 52 at baseline to 28 at 6 months and 24 at 12 months. Median ultrasound scores of synovitis decreased from 10 at baseline to 3 at both 6 and 12 months.
Most of these benefits persisted for 1 year after abatacept therapy was stopped, but approximately half of patients required a synthetic DMARD to maintain the benefits. “Overall, these data suggest that in the vast majority of patients (18 of 20), abatacept therapy prevented further progression of disease, but on cessation, additional therapy was indicated to maintain this,” Dr. Buch and her associates said.
There were no serious adverse events during treatment, including no infections and no abnormal liver function tests. A total of 131 nonserious adverse events were reported.
This study was supported by a research grant from Bristol-Myers Squibb and by institution-level grants from Arthritis UK and the U.K. National Institute for Health Research. Dr. Buch reported ties to Bristol-Myers Squibb, AbbVie, AstraZeneca, Pfizer, and Roche-Chugai; her associates reported ties to numerous industry sources.
Key clinical point:
Major finding: Only 2 of the 20 patients (10%) achieved the composite primary endpoint of DAS44 remission, a maximum of 1 swollen joint for at least 3 consecutive months, and no radiographic progression at 6-month follow-up.
Data source: A manufacturer-supported, open-label, proof-of-concept study involving 20 adults treated for 1 year and followed for 1 further year.
Disclosures: This study was supported by a research grant from Bristol-Myers Squibb and by institution-level grants from Arthritis UK and the U.K. National Institute for Health Research. Dr. Buch reported ties to Bristol-Myers Squibb, AbbVie, AstraZeneca, Pfizer, and Roche-Chugai; her associates reported ties to numerous industry sources.
Nivolumab + ipilimumab induced fulminant, fatal myocarditis
Two patients taking the immune checkpoint inhibitors nivolumab and ipilimumab for metastatic melanoma developed fulminant, fatal myocarditis, investigators reported in the New England Journal of Medicine.
Even though this adverse effect is rare, “clinicians should be vigilant for immune-mediated myocarditis, particularly because of its early onset, nonspecific symptomatology, and fulminant progression,” said Douglas B. Johnson, MD, of Vanderbilt University Medical Center, Nashville, and his associates.
The first case involved a 65-year-old woman with no cardiac risk factors who was admitted to the hospital with chest pain, dyspnea, and fatigue 12 days after she received her first dose of the combination therapy. She was found to have myocarditis and myositis with rhabdomylysis. Despite treatment with high-dose glucocorticoids, she developed intraventricular conduction delay within 24 hours, followed by complete heart block. She died from multisystem organ failure and refractory ventricular tachycardia.
The second case involved a 63-year-old man with no cardiac risk factors who was admitted with fatigue and myalgias 15 days after he received his first dose of the combination therapy. He showed profound ST-segment depression, an intraventricular conduction delay, myocarditis, and myositis. He also was treated with high-dose glucocorticoids but developed complete heart block and died from cardiac arrest.
Both patients had “strikingly elevated troponin levels and refractory conduction-system abnormalities with preserved cardiac function,” the investigators noted. Postmortem assessments showed intense lymphocytic infiltrates only in striated cardiac and skeletal muscle and in metastases; adjacent smooth muscle and other tissues were unaffected. Pathology results “were reminiscent of those observed in patients with acute allograft rejection after cardiac transplantation,” Dr. Johnson and his associates said (N Engl J Med. 2016 Nov 3. doi: 10.1056/NEJMoa1609214).
To assess the frequency of myocarditis and myositis in patients receiving immune checkpoint inhibitors for many different cancers, the investigators searched Bristol-Myers Squibb safety databases. They found 18 drug-related cases of severe myocarditis among 20,594 patients, for a frequency of 0.09%. Patients who received combined nivolumab and ipilimumab had more frequent and more severe myocarditis than those who took either agent alone.
“There are no known data regarding what monitoring strategy may be of value; in our practice, we are performing baseline ECG and weekly testing of troponin levels during weeks 1-3 for patients receiving combination immunotherapy,” the researchers noted.
This work was supported by the Bready Family Foundation, the National Cancer Institute, Vanderbilt-Ingram Cancer Center Ambassadors, the Breast Cancer Specialized Program of Research Excellence, the National Comprehensive Cancer Network, the National Institutes of Health, the Howard Hughes Medical Institute, and Gilead Life Sciences. Dr. Johnson reported receiving personal fees from Genoptix and Bristol-Myers Squibb, and his associates reported ties to numerous industry sources.
Two patients taking the immune checkpoint inhibitors nivolumab and ipilimumab for metastatic melanoma developed fulminant, fatal myocarditis, investigators reported in the New England Journal of Medicine.
Even though this adverse effect is rare, “clinicians should be vigilant for immune-mediated myocarditis, particularly because of its early onset, nonspecific symptomatology, and fulminant progression,” said Douglas B. Johnson, MD, of Vanderbilt University Medical Center, Nashville, and his associates.
The first case involved a 65-year-old woman with no cardiac risk factors who was admitted to the hospital with chest pain, dyspnea, and fatigue 12 days after she received her first dose of the combination therapy. She was found to have myocarditis and myositis with rhabdomylysis. Despite treatment with high-dose glucocorticoids, she developed intraventricular conduction delay within 24 hours, followed by complete heart block. She died from multisystem organ failure and refractory ventricular tachycardia.
The second case involved a 63-year-old man with no cardiac risk factors who was admitted with fatigue and myalgias 15 days after he received his first dose of the combination therapy. He showed profound ST-segment depression, an intraventricular conduction delay, myocarditis, and myositis. He also was treated with high-dose glucocorticoids but developed complete heart block and died from cardiac arrest.
Both patients had “strikingly elevated troponin levels and refractory conduction-system abnormalities with preserved cardiac function,” the investigators noted. Postmortem assessments showed intense lymphocytic infiltrates only in striated cardiac and skeletal muscle and in metastases; adjacent smooth muscle and other tissues were unaffected. Pathology results “were reminiscent of those observed in patients with acute allograft rejection after cardiac transplantation,” Dr. Johnson and his associates said (N Engl J Med. 2016 Nov 3. doi: 10.1056/NEJMoa1609214).
To assess the frequency of myocarditis and myositis in patients receiving immune checkpoint inhibitors for many different cancers, the investigators searched Bristol-Myers Squibb safety databases. They found 18 drug-related cases of severe myocarditis among 20,594 patients, for a frequency of 0.09%. Patients who received combined nivolumab and ipilimumab had more frequent and more severe myocarditis than those who took either agent alone.
“There are no known data regarding what monitoring strategy may be of value; in our practice, we are performing baseline ECG and weekly testing of troponin levels during weeks 1-3 for patients receiving combination immunotherapy,” the researchers noted.
This work was supported by the Bready Family Foundation, the National Cancer Institute, Vanderbilt-Ingram Cancer Center Ambassadors, the Breast Cancer Specialized Program of Research Excellence, the National Comprehensive Cancer Network, the National Institutes of Health, the Howard Hughes Medical Institute, and Gilead Life Sciences. Dr. Johnson reported receiving personal fees from Genoptix and Bristol-Myers Squibb, and his associates reported ties to numerous industry sources.
Two patients taking the immune checkpoint inhibitors nivolumab and ipilimumab for metastatic melanoma developed fulminant, fatal myocarditis, investigators reported in the New England Journal of Medicine.
Even though this adverse effect is rare, “clinicians should be vigilant for immune-mediated myocarditis, particularly because of its early onset, nonspecific symptomatology, and fulminant progression,” said Douglas B. Johnson, MD, of Vanderbilt University Medical Center, Nashville, and his associates.
The first case involved a 65-year-old woman with no cardiac risk factors who was admitted to the hospital with chest pain, dyspnea, and fatigue 12 days after she received her first dose of the combination therapy. She was found to have myocarditis and myositis with rhabdomylysis. Despite treatment with high-dose glucocorticoids, she developed intraventricular conduction delay within 24 hours, followed by complete heart block. She died from multisystem organ failure and refractory ventricular tachycardia.
The second case involved a 63-year-old man with no cardiac risk factors who was admitted with fatigue and myalgias 15 days after he received his first dose of the combination therapy. He showed profound ST-segment depression, an intraventricular conduction delay, myocarditis, and myositis. He also was treated with high-dose glucocorticoids but developed complete heart block and died from cardiac arrest.
Both patients had “strikingly elevated troponin levels and refractory conduction-system abnormalities with preserved cardiac function,” the investigators noted. Postmortem assessments showed intense lymphocytic infiltrates only in striated cardiac and skeletal muscle and in metastases; adjacent smooth muscle and other tissues were unaffected. Pathology results “were reminiscent of those observed in patients with acute allograft rejection after cardiac transplantation,” Dr. Johnson and his associates said (N Engl J Med. 2016 Nov 3. doi: 10.1056/NEJMoa1609214).
To assess the frequency of myocarditis and myositis in patients receiving immune checkpoint inhibitors for many different cancers, the investigators searched Bristol-Myers Squibb safety databases. They found 18 drug-related cases of severe myocarditis among 20,594 patients, for a frequency of 0.09%. Patients who received combined nivolumab and ipilimumab had more frequent and more severe myocarditis than those who took either agent alone.
“There are no known data regarding what monitoring strategy may be of value; in our practice, we are performing baseline ECG and weekly testing of troponin levels during weeks 1-3 for patients receiving combination immunotherapy,” the researchers noted.
This work was supported by the Bready Family Foundation, the National Cancer Institute, Vanderbilt-Ingram Cancer Center Ambassadors, the Breast Cancer Specialized Program of Research Excellence, the National Comprehensive Cancer Network, the National Institutes of Health, the Howard Hughes Medical Institute, and Gilead Life Sciences. Dr. Johnson reported receiving personal fees from Genoptix and Bristol-Myers Squibb, and his associates reported ties to numerous industry sources.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Two patients taking the immune checkpoint inhibitors nivolumab and ipilimumab for metastatic melanoma developed fulminant, fatal myocarditis.
Major finding: A search of Bristol-Myers Squibb safety databases found 18 drug-related cases of severe myocarditis among 20,594 patients, for a frequency of 0.09%.
Data source: Two case reports of a rare adverse effect of treatment with immune checkpoint inhibitors.
Disclosures: This work was supported by the Bready Family Foundation, the National Cancer Institute, Vanderbilt-Ingram Cancer Center Ambassadors, the Breast Cancer Specialized Program of Research Excellence, the National Comprehensive Cancer Network, the National Institutes of Health, the Howard Hughes Medical Institute, and Gilead Life Sciences. Dr. Johnson reported receiving personal fees from Genoptix and Bristol-Myers Squibb, and his associates reported ties to numerous industry sources.
‘Excellent’ real-world experience with LAA closure device
A device that closes the left atrial appendage to prevent stroke in patients with nonvalvular atrial fibrillation showed a 95.6% procedural success rate in a study of real-world experience since it was approved by the FDA in 2015, according to a report presented at the Transcatheter Cardiovascular Therapeutics annual meeting and published simultaneously in the Journal of the American College of Cardiology.
The study was based on data collected by Boston Scientific, the manufacturer of the Watchman device, regarding 3,822 consecutive patients who underwent the implantation during a 14-month period. The “excellent” procedural success rate, together with low short-term complication rates, are especially “remarkable” because 71% of the interventional cardiologists and electrophysiologists who performed these procedures had no experience with the device prior to FDA approval, said Vivek Y. Reddy, MD, of Mount Sinai Medical Center, New York.
For this study, the implantations were done by 382 physicians at 169 U.S. medical centers. A total of 3,653 procedures were successful. The median duration of the implantation was “an acceptable” 50 minutes (range, 10-210 minutes), and an average of 1.38 devices (range, 1-6) were required per patient. In 23% of cases, a “partial recapture” of a device was necessary to reposition it (J Am Coll Cardiol. 2016 Nov. doi: 10.1016/j.jacc.2016.10.010).
The rates of major complications within 1 week – pericardial tamponade (<1%), procedure-related stroke (0.08%), and mortality (0.08%) – were characterized as “favorable.”
The most common complication was pericardial effusion requiring intervention, which developed in 39 patients (1.02%). The effusions were drained percutaneously in most (24) of these patients. Another 11 patients (0.29%) developed mild pericardial effusions requiring only conservative management.
Three strokes, two ischemic and one hemorrhagic, were deemed related to the procedure, though the hemorrhagic bleed may have resulted chiefly from anticoagulation medications. Three deaths were judged to be related to the procedure: All were secondary to pericardial tamponade associated with perforation by the device.
“It is worth comparing [this] cardiac tamponade rate with [that of] another left atrial cardiovascular procedure, catheter ablation of atrial fibrillation,” Dr. Reddy noted.
A worldwide survey of more than 20,000 catheter ablations reported a pericardial tamponade rate of 1.31%, and another study of more than 93,000 ablation procedures performed during a 1-year period reported a rate of 1.52%, he said at the meeting, which was sponsored by the Cardiovascular Research Foundation.
Other short-term complications in this study included nine cases of device embolization (0.24%). Six of these required surgical removal of the device, while three were retrieved percutaneously.
No sponsor was cited for this study. Boston Scientific, maker of the Watchman left atrial appendage closure device, collected the data on all implantations of the device in the United States following FDA approval. Dr. Reddy and his associates reported ties to Boston Scientific, Coherex, SentreHeart, Abbott Vascular, and St. Jude Medical.
The results reported by Dr. Reddy and his colleagues are remarkably favorable for the earliest phase of widespread dissemination of this technology, especially in the context of the much higher rates of medication-related adverse events that occur with long-term oral anticoagulation. These findings should reassure us that left atrial appendage device closure has been safely introduced into clinical practice in the United States.
The study design, however, raises some concerns, and clinicians should be aware that complications may have been underreported. The manufacturer’s employees collected the data regarding complications in a somewhat informal manner, so this was not an objective study meeting the rigorous standards of clinical trials or postmarketing registries. These “clinical specialists” only included complications that developed within 1 week of the procedure, which fails to address possible delayed complications such as device-related thrombus. And they also didn’t track some important complications such as vascular events and bleeding events.
Jacqueline Saw, MD, of Vancouver General Hospital, and Matthew J. Price, MD, of Scripps Clinic in La Jolla, Calif., made these remarks in an editorial (J Am Coll Cardiol. 2016 Nov. doi: 10.1016/j.jacc.2016.10.019) accompanying Dr. Reddy’s report. Dr. Saw reported ties to Boston Scientific, AstraZeneca, Abbott Vascular, St. Jude Medical, Servier, Bayer, and Sunovion. Dr. Price reported ties to Boston Scientific, St. Jude Medical, W.L. Gore, Medtronic, AstraZeneca, Abbott Vascular, and Terumo.
The results reported by Dr. Reddy and his colleagues are remarkably favorable for the earliest phase of widespread dissemination of this technology, especially in the context of the much higher rates of medication-related adverse events that occur with long-term oral anticoagulation. These findings should reassure us that left atrial appendage device closure has been safely introduced into clinical practice in the United States.
The study design, however, raises some concerns, and clinicians should be aware that complications may have been underreported. The manufacturer’s employees collected the data regarding complications in a somewhat informal manner, so this was not an objective study meeting the rigorous standards of clinical trials or postmarketing registries. These “clinical specialists” only included complications that developed within 1 week of the procedure, which fails to address possible delayed complications such as device-related thrombus. And they also didn’t track some important complications such as vascular events and bleeding events.
Jacqueline Saw, MD, of Vancouver General Hospital, and Matthew J. Price, MD, of Scripps Clinic in La Jolla, Calif., made these remarks in an editorial (J Am Coll Cardiol. 2016 Nov. doi: 10.1016/j.jacc.2016.10.019) accompanying Dr. Reddy’s report. Dr. Saw reported ties to Boston Scientific, AstraZeneca, Abbott Vascular, St. Jude Medical, Servier, Bayer, and Sunovion. Dr. Price reported ties to Boston Scientific, St. Jude Medical, W.L. Gore, Medtronic, AstraZeneca, Abbott Vascular, and Terumo.
The results reported by Dr. Reddy and his colleagues are remarkably favorable for the earliest phase of widespread dissemination of this technology, especially in the context of the much higher rates of medication-related adverse events that occur with long-term oral anticoagulation. These findings should reassure us that left atrial appendage device closure has been safely introduced into clinical practice in the United States.
The study design, however, raises some concerns, and clinicians should be aware that complications may have been underreported. The manufacturer’s employees collected the data regarding complications in a somewhat informal manner, so this was not an objective study meeting the rigorous standards of clinical trials or postmarketing registries. These “clinical specialists” only included complications that developed within 1 week of the procedure, which fails to address possible delayed complications such as device-related thrombus. And they also didn’t track some important complications such as vascular events and bleeding events.
Jacqueline Saw, MD, of Vancouver General Hospital, and Matthew J. Price, MD, of Scripps Clinic in La Jolla, Calif., made these remarks in an editorial (J Am Coll Cardiol. 2016 Nov. doi: 10.1016/j.jacc.2016.10.019) accompanying Dr. Reddy’s report. Dr. Saw reported ties to Boston Scientific, AstraZeneca, Abbott Vascular, St. Jude Medical, Servier, Bayer, and Sunovion. Dr. Price reported ties to Boston Scientific, St. Jude Medical, W.L. Gore, Medtronic, AstraZeneca, Abbott Vascular, and Terumo.
A device that closes the left atrial appendage to prevent stroke in patients with nonvalvular atrial fibrillation showed a 95.6% procedural success rate in a study of real-world experience since it was approved by the FDA in 2015, according to a report presented at the Transcatheter Cardiovascular Therapeutics annual meeting and published simultaneously in the Journal of the American College of Cardiology.
The study was based on data collected by Boston Scientific, the manufacturer of the Watchman device, regarding 3,822 consecutive patients who underwent the implantation during a 14-month period. The “excellent” procedural success rate, together with low short-term complication rates, are especially “remarkable” because 71% of the interventional cardiologists and electrophysiologists who performed these procedures had no experience with the device prior to FDA approval, said Vivek Y. Reddy, MD, of Mount Sinai Medical Center, New York.
For this study, the implantations were done by 382 physicians at 169 U.S. medical centers. A total of 3,653 procedures were successful. The median duration of the implantation was “an acceptable” 50 minutes (range, 10-210 minutes), and an average of 1.38 devices (range, 1-6) were required per patient. In 23% of cases, a “partial recapture” of a device was necessary to reposition it (J Am Coll Cardiol. 2016 Nov. doi: 10.1016/j.jacc.2016.10.010).
The rates of major complications within 1 week – pericardial tamponade (<1%), procedure-related stroke (0.08%), and mortality (0.08%) – were characterized as “favorable.”
The most common complication was pericardial effusion requiring intervention, which developed in 39 patients (1.02%). The effusions were drained percutaneously in most (24) of these patients. Another 11 patients (0.29%) developed mild pericardial effusions requiring only conservative management.
Three strokes, two ischemic and one hemorrhagic, were deemed related to the procedure, though the hemorrhagic bleed may have resulted chiefly from anticoagulation medications. Three deaths were judged to be related to the procedure: All were secondary to pericardial tamponade associated with perforation by the device.
“It is worth comparing [this] cardiac tamponade rate with [that of] another left atrial cardiovascular procedure, catheter ablation of atrial fibrillation,” Dr. Reddy noted.
A worldwide survey of more than 20,000 catheter ablations reported a pericardial tamponade rate of 1.31%, and another study of more than 93,000 ablation procedures performed during a 1-year period reported a rate of 1.52%, he said at the meeting, which was sponsored by the Cardiovascular Research Foundation.
Other short-term complications in this study included nine cases of device embolization (0.24%). Six of these required surgical removal of the device, while three were retrieved percutaneously.
No sponsor was cited for this study. Boston Scientific, maker of the Watchman left atrial appendage closure device, collected the data on all implantations of the device in the United States following FDA approval. Dr. Reddy and his associates reported ties to Boston Scientific, Coherex, SentreHeart, Abbott Vascular, and St. Jude Medical.
A device that closes the left atrial appendage to prevent stroke in patients with nonvalvular atrial fibrillation showed a 95.6% procedural success rate in a study of real-world experience since it was approved by the FDA in 2015, according to a report presented at the Transcatheter Cardiovascular Therapeutics annual meeting and published simultaneously in the Journal of the American College of Cardiology.
The study was based on data collected by Boston Scientific, the manufacturer of the Watchman device, regarding 3,822 consecutive patients who underwent the implantation during a 14-month period. The “excellent” procedural success rate, together with low short-term complication rates, are especially “remarkable” because 71% of the interventional cardiologists and electrophysiologists who performed these procedures had no experience with the device prior to FDA approval, said Vivek Y. Reddy, MD, of Mount Sinai Medical Center, New York.
For this study, the implantations were done by 382 physicians at 169 U.S. medical centers. A total of 3,653 procedures were successful. The median duration of the implantation was “an acceptable” 50 minutes (range, 10-210 minutes), and an average of 1.38 devices (range, 1-6) were required per patient. In 23% of cases, a “partial recapture” of a device was necessary to reposition it (J Am Coll Cardiol. 2016 Nov. doi: 10.1016/j.jacc.2016.10.010).
The rates of major complications within 1 week – pericardial tamponade (<1%), procedure-related stroke (0.08%), and mortality (0.08%) – were characterized as “favorable.”
The most common complication was pericardial effusion requiring intervention, which developed in 39 patients (1.02%). The effusions were drained percutaneously in most (24) of these patients. Another 11 patients (0.29%) developed mild pericardial effusions requiring only conservative management.
Three strokes, two ischemic and one hemorrhagic, were deemed related to the procedure, though the hemorrhagic bleed may have resulted chiefly from anticoagulation medications. Three deaths were judged to be related to the procedure: All were secondary to pericardial tamponade associated with perforation by the device.
“It is worth comparing [this] cardiac tamponade rate with [that of] another left atrial cardiovascular procedure, catheter ablation of atrial fibrillation,” Dr. Reddy noted.
A worldwide survey of more than 20,000 catheter ablations reported a pericardial tamponade rate of 1.31%, and another study of more than 93,000 ablation procedures performed during a 1-year period reported a rate of 1.52%, he said at the meeting, which was sponsored by the Cardiovascular Research Foundation.
Other short-term complications in this study included nine cases of device embolization (0.24%). Six of these required surgical removal of the device, while three were retrieved percutaneously.
No sponsor was cited for this study. Boston Scientific, maker of the Watchman left atrial appendage closure device, collected the data on all implantations of the device in the United States following FDA approval. Dr. Reddy and his associates reported ties to Boston Scientific, Coherex, SentreHeart, Abbott Vascular, and St. Jude Medical.
FROM TCT 2016
Key clinical point: A left atrial appendage closure device to prevent stroke in patients with nonvalvular atrial fibrillation showed a 95.6% procedural success rate.
Major finding: Of 3,822 implantations, 3,653 (95.6%) were successful, and 1-week complication rates were low, with <1% pericardial tamponade, 0.08% procedure-related stroke, and 0.08% mortality.
Data source: An analysis of manufacturer-collected data on all 3,822 consecutive device implantations at 169 medical centers from March 2015 to May 2016.
Disclosures: No sponsor was cited for this study. Boston Scientific, maker of the Watchman left atrial appendage closure device, collected the data on all implantations of the device in the United States following FDA approval. Dr. Reddy and his associates reported ties to Boston Scientific, Coherex, SentreHeart, Abbott Vascular, and St. Jude Medical.
Results puzzling for embolic protection during TAVR
The largest randomized clinical trial to assess the safety and efficacy of cerebral embolic protection systems during transcatheter aortic valve replacement yielded puzzling and somewhat contradictory results, according to a report presented at the Transcatheter Cardiovascular Therapeutics annual meeting and published simultaneously in the Journal of the American College of Cardiology.
Virtually every device in this industry-sponsored study involving 363 elderly patients (mean age, 83.4 years) with severe aortic stenosis trapped particulate debris as intended, the mean volume of new lesions in the protected areas of the brain was reduced by 42%, and the number and volume of new lesions correlated with neurocognitive outcomes at 30 days.
However, the reduction in lesion volume did not achieve statistical significance, and the improvement in neurocognitive function also did not reach statistical significance.
In addition, “the sample size was clearly too low to assess clinical outcomes, and in retrospect, was also too low to evaluate follow-up MRI findings or neurocognitive outcomes.” Nevertheless, the trial “provides reassuring evidence of device safety,” said Samir R. Kapadia, MD, of the Cleveland Clinic (J Am Coll Cardiol. 2016 Nov 1. doi: 10.1016/j.jacc.2016.10.023).
In this prospective study, the investigators assessed patients at 17 medical centers in the United States and 2 in Germany. In addition to being elderly, the study patients were at high risk because of frequent comorbidities, including atrial fibrillation (31.7%) and prior stroke (5.8%).
The remaining 123 patients underwent TAVR but not MRI in a safety arm of the trial.
The protection devices were placed “without safety concerns” in most patients. The rate of major adverse events with the device was 7.3%, markedly less than the 18.3% prespecified performance goal for this outcome. Total procedure time was lengthened by only 13 minutes when the device was used, and total fluoroscopy time was increased by only 3 minutes. These findings demonstrate the overall safety of using the device, Dr. Kapadia said.
Debris including thrombus with tissue elements, artery wall particles, calcifications, valve tissue, and foreign materials was retrieved from the filters in 99% of patients.
The mean volume of new cerebral lesions in areas of the brain protected by the device was reduced by 42%, compared with that in patients who underwent TAVR without the protection device. However, this reduction was not statistically significant, so the primary efficacy endpoint of the study was not met.
Similarly, neurocognitive testing at 30 days showed that the volume of new lesions correlated with poorer outcomes. However, the difference in neurocognitive function between the intervention group and the control group did not reach statistical significance.
Several limitations likely contributed to this lack of statistical significance, Dr. Kapadia said.
First, the 5-day “window” for MRI assessment was too long. Both the number and the volume of new lesions rapidly changed over time, which led to marked variance in MRI findings depending on when the images were taken.
In addition, only one TAVR device was available at the time the trial was designed, so the study wasn’t stratified by type of valve device. But several new devices became available during the study, and the study investigators were permitted to use any of them. Both pre- and postimplantation techniques differ among these TAVR devices, but these differences could not be accounted for, given the study design.
Also, certain risk factors for stroke, especially certain findings on baseline MRI, were not understood when the trial was designed, and those factors also were not accounted for, Dr. Kapadia said.
Claret Medical funded the study. Dr. Kapadia reported having no relevant financial disclosures; his associates reported numerous ties to industry sources. The meeting was sponsored by the Cardiovascular Research Foundation.
From a logical standpoint, a device that collects cerebral embolic material in 99% of cases should prevent ischemic brain injury, yet the findings from this randomized trial don’t appear to support the routine use of such devices. But it would be inappropriate and unfair to close the book on cerebral protection after this chapter.
The authors acknowledge that an MRI “window” of 5 days creates too much heterogeneity in the data, that multiple TAVR devices requiring different implantation techniques further muddy the picture, and that in retrospect the sample size was inadequate and the study was underpowered. In addition, rigorous neurocognitive assessment can be challenging in elderly, recovering patients, and results can depend on the time of day and the patient’s alertness.
Despite the negative findings regarding both primary and secondary endpoints, the data do show the overall safety of embolic protection devices. We are dealing with a potential benefit that cannot be ignored as TAVR shifts to younger and lower-risk patients.
Azeem Latib, MD, is in the interventional cardiology unit at San Raffaele Scientific Institute in Milan. Matteo Pagnesi, MD, is in the interventional cardiology unit at EMO-GVM Centro Cuore Columbus in Milan. San Raffaele Scientific Institute has been involved in clinical studies of embolic protection devices made by Claret Medical, Innovative Cardiovascular Solutions, and Keystone Heart. Dr. Latib and Dr. Pagnesi reported having no other relevant financial disclosures. They made these remarks in an editorial accompanying Dr. Kapadia’s report (J Am Coll Cardiol. 2016 Nov 1. doi: 10.1016/j.jacc.2016.10.036).
From a logical standpoint, a device that collects cerebral embolic material in 99% of cases should prevent ischemic brain injury, yet the findings from this randomized trial don’t appear to support the routine use of such devices. But it would be inappropriate and unfair to close the book on cerebral protection after this chapter.
The authors acknowledge that an MRI “window” of 5 days creates too much heterogeneity in the data, that multiple TAVR devices requiring different implantation techniques further muddy the picture, and that in retrospect the sample size was inadequate and the study was underpowered. In addition, rigorous neurocognitive assessment can be challenging in elderly, recovering patients, and results can depend on the time of day and the patient’s alertness.
Despite the negative findings regarding both primary and secondary endpoints, the data do show the overall safety of embolic protection devices. We are dealing with a potential benefit that cannot be ignored as TAVR shifts to younger and lower-risk patients.
Azeem Latib, MD, is in the interventional cardiology unit at San Raffaele Scientific Institute in Milan. Matteo Pagnesi, MD, is in the interventional cardiology unit at EMO-GVM Centro Cuore Columbus in Milan. San Raffaele Scientific Institute has been involved in clinical studies of embolic protection devices made by Claret Medical, Innovative Cardiovascular Solutions, and Keystone Heart. Dr. Latib and Dr. Pagnesi reported having no other relevant financial disclosures. They made these remarks in an editorial accompanying Dr. Kapadia’s report (J Am Coll Cardiol. 2016 Nov 1. doi: 10.1016/j.jacc.2016.10.036).
From a logical standpoint, a device that collects cerebral embolic material in 99% of cases should prevent ischemic brain injury, yet the findings from this randomized trial don’t appear to support the routine use of such devices. But it would be inappropriate and unfair to close the book on cerebral protection after this chapter.
The authors acknowledge that an MRI “window” of 5 days creates too much heterogeneity in the data, that multiple TAVR devices requiring different implantation techniques further muddy the picture, and that in retrospect the sample size was inadequate and the study was underpowered. In addition, rigorous neurocognitive assessment can be challenging in elderly, recovering patients, and results can depend on the time of day and the patient’s alertness.
Despite the negative findings regarding both primary and secondary endpoints, the data do show the overall safety of embolic protection devices. We are dealing with a potential benefit that cannot be ignored as TAVR shifts to younger and lower-risk patients.
Azeem Latib, MD, is in the interventional cardiology unit at San Raffaele Scientific Institute in Milan. Matteo Pagnesi, MD, is in the interventional cardiology unit at EMO-GVM Centro Cuore Columbus in Milan. San Raffaele Scientific Institute has been involved in clinical studies of embolic protection devices made by Claret Medical, Innovative Cardiovascular Solutions, and Keystone Heart. Dr. Latib and Dr. Pagnesi reported having no other relevant financial disclosures. They made these remarks in an editorial accompanying Dr. Kapadia’s report (J Am Coll Cardiol. 2016 Nov 1. doi: 10.1016/j.jacc.2016.10.036).
The largest randomized clinical trial to assess the safety and efficacy of cerebral embolic protection systems during transcatheter aortic valve replacement yielded puzzling and somewhat contradictory results, according to a report presented at the Transcatheter Cardiovascular Therapeutics annual meeting and published simultaneously in the Journal of the American College of Cardiology.
Virtually every device in this industry-sponsored study involving 363 elderly patients (mean age, 83.4 years) with severe aortic stenosis trapped particulate debris as intended, the mean volume of new lesions in the protected areas of the brain was reduced by 42%, and the number and volume of new lesions correlated with neurocognitive outcomes at 30 days.
However, the reduction in lesion volume did not achieve statistical significance, and the improvement in neurocognitive function also did not reach statistical significance.
In addition, “the sample size was clearly too low to assess clinical outcomes, and in retrospect, was also too low to evaluate follow-up MRI findings or neurocognitive outcomes.” Nevertheless, the trial “provides reassuring evidence of device safety,” said Samir R. Kapadia, MD, of the Cleveland Clinic (J Am Coll Cardiol. 2016 Nov 1. doi: 10.1016/j.jacc.2016.10.023).
In this prospective study, the investigators assessed patients at 17 medical centers in the United States and 2 in Germany. In addition to being elderly, the study patients were at high risk because of frequent comorbidities, including atrial fibrillation (31.7%) and prior stroke (5.8%).
The remaining 123 patients underwent TAVR but not MRI in a safety arm of the trial.
The protection devices were placed “without safety concerns” in most patients. The rate of major adverse events with the device was 7.3%, markedly less than the 18.3% prespecified performance goal for this outcome. Total procedure time was lengthened by only 13 minutes when the device was used, and total fluoroscopy time was increased by only 3 minutes. These findings demonstrate the overall safety of using the device, Dr. Kapadia said.
Debris including thrombus with tissue elements, artery wall particles, calcifications, valve tissue, and foreign materials was retrieved from the filters in 99% of patients.
The mean volume of new cerebral lesions in areas of the brain protected by the device was reduced by 42%, compared with that in patients who underwent TAVR without the protection device. However, this reduction was not statistically significant, so the primary efficacy endpoint of the study was not met.
Similarly, neurocognitive testing at 30 days showed that the volume of new lesions correlated with poorer outcomes. However, the difference in neurocognitive function between the intervention group and the control group did not reach statistical significance.
Several limitations likely contributed to this lack of statistical significance, Dr. Kapadia said.
First, the 5-day “window” for MRI assessment was too long. Both the number and the volume of new lesions rapidly changed over time, which led to marked variance in MRI findings depending on when the images were taken.
In addition, only one TAVR device was available at the time the trial was designed, so the study wasn’t stratified by type of valve device. But several new devices became available during the study, and the study investigators were permitted to use any of them. Both pre- and postimplantation techniques differ among these TAVR devices, but these differences could not be accounted for, given the study design.
Also, certain risk factors for stroke, especially certain findings on baseline MRI, were not understood when the trial was designed, and those factors also were not accounted for, Dr. Kapadia said.
Claret Medical funded the study. Dr. Kapadia reported having no relevant financial disclosures; his associates reported numerous ties to industry sources. The meeting was sponsored by the Cardiovascular Research Foundation.
The largest randomized clinical trial to assess the safety and efficacy of cerebral embolic protection systems during transcatheter aortic valve replacement yielded puzzling and somewhat contradictory results, according to a report presented at the Transcatheter Cardiovascular Therapeutics annual meeting and published simultaneously in the Journal of the American College of Cardiology.
Virtually every device in this industry-sponsored study involving 363 elderly patients (mean age, 83.4 years) with severe aortic stenosis trapped particulate debris as intended, the mean volume of new lesions in the protected areas of the brain was reduced by 42%, and the number and volume of new lesions correlated with neurocognitive outcomes at 30 days.
However, the reduction in lesion volume did not achieve statistical significance, and the improvement in neurocognitive function also did not reach statistical significance.
In addition, “the sample size was clearly too low to assess clinical outcomes, and in retrospect, was also too low to evaluate follow-up MRI findings or neurocognitive outcomes.” Nevertheless, the trial “provides reassuring evidence of device safety,” said Samir R. Kapadia, MD, of the Cleveland Clinic (J Am Coll Cardiol. 2016 Nov 1. doi: 10.1016/j.jacc.2016.10.023).
In this prospective study, the investigators assessed patients at 17 medical centers in the United States and 2 in Germany. In addition to being elderly, the study patients were at high risk because of frequent comorbidities, including atrial fibrillation (31.7%) and prior stroke (5.8%).
The remaining 123 patients underwent TAVR but not MRI in a safety arm of the trial.
The protection devices were placed “without safety concerns” in most patients. The rate of major adverse events with the device was 7.3%, markedly less than the 18.3% prespecified performance goal for this outcome. Total procedure time was lengthened by only 13 minutes when the device was used, and total fluoroscopy time was increased by only 3 minutes. These findings demonstrate the overall safety of using the device, Dr. Kapadia said.
Debris including thrombus with tissue elements, artery wall particles, calcifications, valve tissue, and foreign materials was retrieved from the filters in 99% of patients.
The mean volume of new cerebral lesions in areas of the brain protected by the device was reduced by 42%, compared with that in patients who underwent TAVR without the protection device. However, this reduction was not statistically significant, so the primary efficacy endpoint of the study was not met.
Similarly, neurocognitive testing at 30 days showed that the volume of new lesions correlated with poorer outcomes. However, the difference in neurocognitive function between the intervention group and the control group did not reach statistical significance.
Several limitations likely contributed to this lack of statistical significance, Dr. Kapadia said.
First, the 5-day “window” for MRI assessment was too long. Both the number and the volume of new lesions rapidly changed over time, which led to marked variance in MRI findings depending on when the images were taken.
In addition, only one TAVR device was available at the time the trial was designed, so the study wasn’t stratified by type of valve device. But several new devices became available during the study, and the study investigators were permitted to use any of them. Both pre- and postimplantation techniques differ among these TAVR devices, but these differences could not be accounted for, given the study design.
Also, certain risk factors for stroke, especially certain findings on baseline MRI, were not understood when the trial was designed, and those factors also were not accounted for, Dr. Kapadia said.
Claret Medical funded the study. Dr. Kapadia reported having no relevant financial disclosures; his associates reported numerous ties to industry sources. The meeting was sponsored by the Cardiovascular Research Foundation.
Key clinical point: The largest randomized clinical trial to assess the safety and efficacy of cerebral embolic protection systems during TAVR yielded puzzling and contradictory results.
Major finding: Debris including thrombus with tissue elements, artery wall particles, calcifications, valve tissue, and foreign materials was retrieved from the cerebral protection filters in 99% of patients.
Data source: A prospective, international, randomized trial involving 363 elderly patients undergoing TAVR for severe aortic stenosis.
Disclosures: Claret Medical funded the study. Dr. Kapadia reported having no relevant financial disclosures; his associates reported numerous ties to industry sources.
Resorbable scaffold appears safe, effective in diabetes patients
An everolimus-eluting resorbable scaffold appeared to be safe and effective for percutaneous coronary intervention (PCI) in patients with diabetes and noncomplex coronary lesions, according to a study presented at the Transcatheter Cardiovascular Therapeutics annual meeting and published simultaneously in the Journal of the American College of Cardiology: Cardiovascular Interventions.
Patients with diabetes constitute an important and increasingly prevalent subgroup of PCI patients, who are at high risk of adverse clinical and angiographic outcomes such as MI, stent thrombosis, restenosis, and death. This is thought to be due to diabetic patients’ greater level of vascular inflammation and tendency toward a prothrombotic state and more complex angiographic features, said Dean J. Kereiakes, MD, of the Christ Hospital Heart and Vascular Center, Lindner Research Center, Cincinnati.
The substudy participants all received at least one resorbable scaffold in at least one target lesion. A total of 27.3% were insulin dependent and nearly 60% had HbA1c levels exceeding 7.0%. Notably, 18% of all the treated lesions in this analysis were less than 2.25 mm in diameter as assessed by quantitative coronary angiography, and approximately 60% had moderately to severely complex morphology.
The primary endpoint – the rate of target-lesion failure at 1-year follow-up – was 8.3%, which was well below the prespecified performance goal of 12.7%. This rate ranged from 4.4% to 10.9% across the different trials. A sensitivity analysis confirmed that the 1-year rate of target-lesion failure was significantly lower than the prespecified performance goal.
The rates of target-lesion failure, target-vessel MI, ischemia-driven target-lesion revascularization, and scaffold thrombosis were significantly higher in diabetic patients who required insulin than in those who did not. Older patient age, insulin dependency, and small target-vessel diameter all were independent predictors of target-lesion failure at 1 year.
The overall 1-year rate of scaffold thrombosis in this study was 2.3%, which is not surprising given the study population’s risk factors. For diabetic patients with appropriately sized vessels of greater than 2.25 mm diameter, the scaffold thrombosis rate was lower (1.3%).
In addition to being underpowered to assess rare adverse events, this study was limited in that it reported outcomes at 1 year, before resorption of the device was complete. It also reflects the first-time clinical experience with a resorbable scaffold for most of the participating investigators, “and one would expect that as with all new medical procedures, results will improve over time with increased operator experience,” the coauthors wrote.
Dr. Kereiakes reported being a consultant to Abbott Vascular, and his associates also reported ties to the company and to other industry sources.
An everolimus-eluting resorbable scaffold appeared to be safe and effective for percutaneous coronary intervention (PCI) in patients with diabetes and noncomplex coronary lesions, according to a study presented at the Transcatheter Cardiovascular Therapeutics annual meeting and published simultaneously in the Journal of the American College of Cardiology: Cardiovascular Interventions.
Patients with diabetes constitute an important and increasingly prevalent subgroup of PCI patients, who are at high risk of adverse clinical and angiographic outcomes such as MI, stent thrombosis, restenosis, and death. This is thought to be due to diabetic patients’ greater level of vascular inflammation and tendency toward a prothrombotic state and more complex angiographic features, said Dean J. Kereiakes, MD, of the Christ Hospital Heart and Vascular Center, Lindner Research Center, Cincinnati.
The substudy participants all received at least one resorbable scaffold in at least one target lesion. A total of 27.3% were insulin dependent and nearly 60% had HbA1c levels exceeding 7.0%. Notably, 18% of all the treated lesions in this analysis were less than 2.25 mm in diameter as assessed by quantitative coronary angiography, and approximately 60% had moderately to severely complex morphology.
The primary endpoint – the rate of target-lesion failure at 1-year follow-up – was 8.3%, which was well below the prespecified performance goal of 12.7%. This rate ranged from 4.4% to 10.9% across the different trials. A sensitivity analysis confirmed that the 1-year rate of target-lesion failure was significantly lower than the prespecified performance goal.
The rates of target-lesion failure, target-vessel MI, ischemia-driven target-lesion revascularization, and scaffold thrombosis were significantly higher in diabetic patients who required insulin than in those who did not. Older patient age, insulin dependency, and small target-vessel diameter all were independent predictors of target-lesion failure at 1 year.
The overall 1-year rate of scaffold thrombosis in this study was 2.3%, which is not surprising given the study population’s risk factors. For diabetic patients with appropriately sized vessels of greater than 2.25 mm diameter, the scaffold thrombosis rate was lower (1.3%).
In addition to being underpowered to assess rare adverse events, this study was limited in that it reported outcomes at 1 year, before resorption of the device was complete. It also reflects the first-time clinical experience with a resorbable scaffold for most of the participating investigators, “and one would expect that as with all new medical procedures, results will improve over time with increased operator experience,” the coauthors wrote.
Dr. Kereiakes reported being a consultant to Abbott Vascular, and his associates also reported ties to the company and to other industry sources.
An everolimus-eluting resorbable scaffold appeared to be safe and effective for percutaneous coronary intervention (PCI) in patients with diabetes and noncomplex coronary lesions, according to a study presented at the Transcatheter Cardiovascular Therapeutics annual meeting and published simultaneously in the Journal of the American College of Cardiology: Cardiovascular Interventions.
Patients with diabetes constitute an important and increasingly prevalent subgroup of PCI patients, who are at high risk of adverse clinical and angiographic outcomes such as MI, stent thrombosis, restenosis, and death. This is thought to be due to diabetic patients’ greater level of vascular inflammation and tendency toward a prothrombotic state and more complex angiographic features, said Dean J. Kereiakes, MD, of the Christ Hospital Heart and Vascular Center, Lindner Research Center, Cincinnati.
The substudy participants all received at least one resorbable scaffold in at least one target lesion. A total of 27.3% were insulin dependent and nearly 60% had HbA1c levels exceeding 7.0%. Notably, 18% of all the treated lesions in this analysis were less than 2.25 mm in diameter as assessed by quantitative coronary angiography, and approximately 60% had moderately to severely complex morphology.
The primary endpoint – the rate of target-lesion failure at 1-year follow-up – was 8.3%, which was well below the prespecified performance goal of 12.7%. This rate ranged from 4.4% to 10.9% across the different trials. A sensitivity analysis confirmed that the 1-year rate of target-lesion failure was significantly lower than the prespecified performance goal.
The rates of target-lesion failure, target-vessel MI, ischemia-driven target-lesion revascularization, and scaffold thrombosis were significantly higher in diabetic patients who required insulin than in those who did not. Older patient age, insulin dependency, and small target-vessel diameter all were independent predictors of target-lesion failure at 1 year.
The overall 1-year rate of scaffold thrombosis in this study was 2.3%, which is not surprising given the study population’s risk factors. For diabetic patients with appropriately sized vessels of greater than 2.25 mm diameter, the scaffold thrombosis rate was lower (1.3%).
In addition to being underpowered to assess rare adverse events, this study was limited in that it reported outcomes at 1 year, before resorption of the device was complete. It also reflects the first-time clinical experience with a resorbable scaffold for most of the participating investigators, “and one would expect that as with all new medical procedures, results will improve over time with increased operator experience,” the coauthors wrote.
Dr. Kereiakes reported being a consultant to Abbott Vascular, and his associates also reported ties to the company and to other industry sources.
Key clinical point:
Major finding: The primary endpoint – the rate of target-lesion failure at 1 year follow-up – was 8.3%, which was well below the prespecified performance goal of 12.7%.
Data source: A prespecified formal substudy of 754 patients with diabetes who participated in three clinical trials and one device registry, assessing 1-year outcomes after PCI.
Disclosures: This pooled analysis, plus all the contributing trials and the device registry, were funded by Abbott Vascular, maker of the resorbable scaffold. Dr. Kereiakes reported being a consultant to Abbott Vascular, and his associates also reported ties to the company and to other industry sources.