User login
Another risk to US travelers—malaria
› Assess the need for nonpharmacologic, behavioral interventions and for chemoprophylaxis based on a destination’s relative risk to travelers, planned and potential activities, and patient comorbidities. B
› Choose an antimalarial medication based on knowledge of area-specific drug effectiveness or resistance patterns, trip duration, drug cost, tolerance for adverse effects, and comorbidities. C
› Presume a diagnosis of malaria until proven otherwise in any traveler who is febrile after returning from a malaria-endemic region. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Although malaria was eradicated as an endemic disease in the United States in the early 1950s,1 it still returns yearly in approximately 1500 individuals who travel to foreign countries2—most of whom neglected to use prophylactic measures or use them properly.3 In more than 60 documented cases, these infected individuals have been the source of local transmission in their communities.2 To reduce the individual and public health risks associated with malaria, this article focuses on steps that international travelers can take to limit their risk of the disease.
What travelers need to know
In 2013, more than 61.5 million residents of the United States traveled abroad, approximately 30% of whom visited malaria-endemic regions: Mexico and equatorial nations in Central and South America; Africa; the Middle East; and South, East, and Southeast Asia.4 Counseling on appropriate preventive measures fits the Medical Home concept of comprehensive, preventive, patient-centered care, and pre-travel consultation—including a review of health data and itineraries, and patient education—can be a team-based effort.5
Begin your planning for malaria prophylaxis by assessing your patient’s individual risk. Key variables are a patient’s detailed itinerary, a credible and current source of information on location-specific malaria prevalence, personal risk factors, and risk tolerance. Shared decision-making is vital and enhances adherence to the prescribed regimen.
Endemicity varies regionally. Without chemoprophylaxis, risk of infection ranges from more than 20% in Papua New Guinea to 0.01% in Central America, with wide exposure risk variations likely, even within regions.6 Travel to areas of high endemicity requires more aggressive malaria prevention strategies than travel to low-endemicity regions.
Risk of exposure is lower with short visits,7 business-only travel, urban-only stays in some countries, day trips to endemic areas,7 and travel during seasons with lower mosquito burden. Likewise, travelers staying in a hotel with sealed windows will face lower nighttime Anopheles mosquito exposure. In these cases, nonpharmacologic measures alone may be appropriate.
Those at particularly high risk for complicated or lethal malarial infection are children, pregnant women, elderly individuals, and immunocompromised patients.7 In addition to counseling high-risk patients about prophylactic measures, consider advising against travel in certain circumstances. Among those at highest risk for acquiring malaria are immigrants and refugees traveling to their ancestral homelands to visit friends and relatives (VFR).2 Many VFR travelers fail to take appropriate prophylactic measures when “going home.”8 A significant number of cases of travel-acquired malaria occurs in VFR children.9
Individualizing prevention directives
The mainstays of malaria prevention include nonpharmacologic and behavioral interventions, as well as chemoprophylaxis. Most cases of malaria in travelers returning to the United States result from the improper implementation of prophylactic measures.3 Discussing individual risk with travelers is an easy way to bolster adherence to malaria prevention measures, and some evidence suggests it is effective10 (strength of recommendation [SOR]: C). Other limited studies have also shown that malaria education can improve knowledge about malaria transmission and increase the likelihood that preventive measures will be used.11,12
Recommend nonpharmacologic measures even for those using chemoprophylaxis
Nonpharmacologic interventions such as sleeping under permethrin-treated bednets, wearing long sleeves and full-length pants, treating clothes with permethrin, and applying DEET (N,N-diethyl-meta-toluamide) to exposed skin are effective and have the added benefit of preventing non-malarial arthropod-borne diseases4 (SOR: B). Studies have shown that, compared with sleeping without nets, the use of insecticide treated-nets can reduce child mortality by 17% and the incidence of uncomplicated malarial episodes by 50%.13 In areas with malaria transmission, 10% to 30% DEET—used alone or in combination with permethrin-treated clothing— can reduce bite load, although the American Academy of Pediatrics recommends against using DEET in children younger than 2 months of age.14,15
Using these measures in combination from dusk to dawn, when Anopheles mosquitoes are active, has been shown to be effective, although randomized, controlled studies are lacking.16 Remaining indoors during these peak biting periods is also advisable. In certain areas, and with the right itinerary, the traveler may only need to employ nonpharmacologic methods of preventing malarial infection. Recommend them to all patients traveling to malarial regions, even to individuals using pharmacologic prophylaxis.
Factors determining the need for, and selection of, chemoprophylaxis
When used properly, chemoprophylactic drugs are effective in preventing malaria (SOR: A). Atovaquone-proguanil achieves efficacy of 95% to 100%,17 while doxycycline, primaquine, and mefloquine are slightly less effective.18-20 Chloroquine is effective in 6 regions of the tropics and subtropics where Plasmodium falciparum resistance has not developed. Select a drug based on your assessment of an individual’s level of risk according to the personal itinerary, trip duration and accommodations, cost of medication, tolerance for adverse effects, and other factors (eg, comorbidities, concurrent drug usage, pregnancy).
Location matters. The risk of malaria transmission can vary considerably not only between countries, but also regionally within countries and even between a city and its immediate surroundings. Therefore, select a chemoprophylactic agent based on the specific itinerary, planned activities, the potential for unforeseen additional excursions, and local Plasmodium resistance patterns. For example, chloroquine is effective only in the Caribbean, Central America, and some countries in the Middle East.21 Mefloquine resistance has been reported in parts of Cambodia, Thailand, Vietnam, Burma, China, and Laos.21
On its Travelers’ Health Web site (www.cdc.gov/travel), the Centers for Disease Control and Prevention (CDC) reports for each country 1) the risk of malaria transmission, 2) areas within the country that pose a risk, 3) evidence of Plasmodium drug resistance, 4) which Plasmodium species are active, and 5) which chemoprophylactic medications are recommended.22 Additional Web sites, either free or subscription-based, allow users to view this same information on maps, advise on where insect precautions alone are sufficiently protective, and provide information about the traveler’s risk of contracting other diseases (TABLE 1).
TABLE 1
| Web resources on infectious diseases of concern to international travelers | |
| Resource | Notes |
Centers for Disease Control and Prevention | Free Site Go to Yellow Book » Contents » Chapter 3 » “Travel Vaccines & Malaria Information, by Country” for country-specific information about the risk of malaria transmission |
VHI Healthcare | Free Site Destination-specific information about travel alerts and vaccine recommendations Does not report malaria transmission data |
Gideon | Subscription only Online application that helps with diagnosing infectious diseases and keeping up to date with global health literature |
Travax | Subscription only Information about recommended vaccines and country-specific risk of malaria transmission |
Tropimed | Subscription only Information about recommended vaccines and country-specific risk of malaria transmission |
Comparative adverse effects of antimalarial agents. A Cochrane Review on the tolerability of chemoprophylactic agents concluded that atovaquone-proguanil and doxycycline were better tolerated than mefloquine (SOR: B). Compared with mefloquine, atovaquone-proguanil led to fewer reports of any adverse effects (relative risk [RR]=0.72), gastrointestinal adverse effects (RR=0.54), and neuropsychiatric adverse events (RR= 0.49-0.86, depending on the studies).23 Doxycycline users have reported fewer neuropsychiatric events (RR=0.84) than mefloquine users.23 These are relatively small differences, and the authors point out that these figures are based on low-quality evidence. Additional research is likely to have an impact on the confidence in the estimate of effect and to ultimately change the estimate.
Mefloquine is contraindicated in travelers with seizures, active or recent history of depression, generalized anxiety disorder, psychosis, schizophrenia, or other psychiatric disorders. Compared with mefloquine, atovaquone-proguanil and doxycycline cause fewer neuropsychiatric adverse effects (such as vivid dreams, dizziness, anxiety, depression, visual disturbance, or seizures).24 Caution is advised when prescribing chloroquine for patients with epilepsy because the medication has the potential to lower the seizure threshold.25
Use caution when prescribing mefloquine for patients with cardiac conduction disturbances. Electrocardiogram alterations such as sinus bradycardia, first-degree AV block, prolongation of QTc intervals, and abnormal T wave changes have been reported.26 Chloroquine can also prolong QTc intervals.26
Safety in pregnancy and breastfeeding. Malaria in pregnancy is associated with increased rates of anemia, low birth weight, prematurity, intrauterine growth restriction, and infant mortality.27 Chloroquine and mefloquine are considered safe during pregnancy and breastfeeding. Doxycycline has been associated with increased risk of harm to the fetus. Atovaquone-proguanil can be used in breastfeeding women if the child is ≥5 kg (≥11 lbs). Chemoprophylaxis taken by the mother while breastfeeding does not protect the infant from infection.
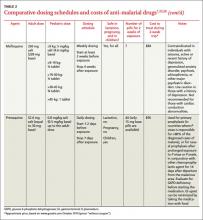
Dosing considerations. Mefloquine and chloroquine are dosed weekly; doxycycline and atovaquone-proguanil are taken daily. Travelers staying in a malaria-endemic region for longer periods (months rather than weeks) often prefer the weekly rather than daily medications; however, this may not be possible due to the adverse-effect profile of mefloquine or to traveling in an area with known chloroquine resistance. Some individuals prefer the routine of taking a medication daily, since remembering to take a single dose on the same day each week can be challenging. Others may not want to carry a large number of pills and therefore prefer weekly dosing. Have patients take medications before the trip, to assess tolerability and to ensure adequate blood concentrations before exposure.
Because mefloquine, doxycycline, and chloroquine target only the blood stages of Plasmodium, patients must continue these medications for 4 weeks following the exposure period to ensure adequate coverage as parasites are released from the liver. Because doxycycline is taken daily and has to be continued for 4 weeks following the exposure period, the total number of pills taken is higher for this regimen. Atovaquone-proguanil is active against hepatic and blood stages and can be discontinued a week following the exposure period.
With children, base dosing on body weight and do not exceed the recommended adult dose. When fractions of tablets are used (such as with mefloquine and atovaquone-proguanil dosing), pharmacists can crush tablets and place divided doses in capsules, to be sprinkled as needed into food such as applesauce or jelly. Mefloquine and chloroquine can be given to children of all ages and weights. Although atovaquone-proguanil is approved only for children ≥11 kg (24 lbs), dosing schedules have been calculated for children who weigh ≥5 kg.21 Doxycycline is recommended only for children who are at least 8 years of age.
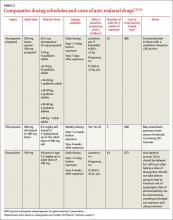
Cost. For a 2-week exposure period, chloroquine is the least expensive medication (although regions in which it is recommended are limited due to resistance) (TABLE 27,25,26).
Ask about accommodations
Since Anopheles mosquitoes feed between dusk and dawn, inquiring about accommodations can further clarify a patient’s malaria risk. Staying in air-conditioned housing (implying that the interior can be sealed) or that has screened windows can reduce exposure to mosquitoes, although data are lacking regarding whether the latter practice reduces the incidence of malaria transmission28 (SOR: C).
Share decision making
After considering the key factors determining a patient’s level of risk, you may decide to recommend no specific interventions, to advise insect avoidance measures only, to combine insect avoidance with chemoprophylaxis, or to caution against traveling to a malaria-endemic region. The patient’s contribution to the final decision includes personal preferences, values, and risk tolerance—particularly when comorbidities are involved.
When preventive measures fail
Approximately 0.2% of travelers to malaria-endemic regions will become infected, despite proper pre-travel counseling and prophylaxis.29 In the United States, malaria is often misdiagnosed or improperly treated.30 The time from initial presentation to correct diagnosis of malaria has been reported as an astonishingly high 4 to 8.5 days, depending on the population.31,32
A high index of suspicion is needed and will ensure timely care when any febrile traveler returns from a malaria-endemic area.33 Be sure to advise patients to seek medical attention if they are feverish upon returning home.
Once suspected, the diagnosis of malaria can be readily confirmed through the use of antibody-, nucleic acid-, or microscopy-based techniques (the latter to directly visualize Plasmodium species in blood smears).
Although malaria chemoprophylaxis is relatively straightforward, malaria treatment—especially in cases of chemoprophylaxis failures—may not be, and the topic is beyond the scope of this article. For guidance on treating malaria, consult a knowledgeable physician or contact the CDC at www.cdc.gov/malaria/, or at (855) 856-4713 (weekdays, 9 am to 5 pm EST) or (770) 488-7100 (weekends or after normal business hours; ask for the Malaria Branch clinician on call).
CORRESPONDENCE
Mark K. Huntington, MD, PhD, Center for Family Medicine, 1115 East 20th Street, Sioux Falls, SD 57105; [email protected]
1. Mali S, Steele S, Slutsker L, et al; Centers for Disease Control and Prevention (CDC). Malaria surveillance - United States, 2008. MMWR Surveill Summ. 2010;59:1-15.
2. Centers for Disease Control and Prevention. Malaria facts. Centers for Disease Control and Prevention Web site. Available at: www.cdc.gov/malaria/about/facts.html. Accessed September 29, 2014.
3. Huntington MK. Healthy people, malaria and South Dakota. S D Med. 2012;65:297-300.
4. Office of Travel and Tourism Industries. U.S. citizen travel to international regions, 2013. Office of Travel and Tourism Industries Web site. Available at: http://travel.trade.gov/view/m-2013-O-001/index.html. Accessed September 29, 2014.
5. Bazemore AW, Huntington M. The pretravel consultation. Am Fam Physician. 2009;80:583-590.
6. Bradley DJ, Warhurst DC, Blaze M, et al. Malaria imported into the United Kingdom in 1996. Euro Surveill. 1998;3:40-42.
7. Arguin PM, Tan KR, et al; Centers for Disease Control and Prevention. Infectious diseases related to travel. Centers for Disease Control and Prevention Web site. Available at: http://wwwnc.cdc.gov/travel/yellowbook/2014/chapter-3-infectious-diseases-related-to-travel/malaria. Accessed October 15, 2014.
8. Pavli A, Maltezou HC. Malaria and travellers visiting friends and relatives. Travel Med Infect Dis. 2010;8:161-168.
9. Stäger K, Legros F, Krause G, et al. Imported malaria in children in industrialized countries, 1992-2002. Emerg Infect Dis. 2009;15:185-191.
10. Hartjes LB, Baumann LC, Henriques JB. Travel health risk perceptions and prevention behaviors of US study abroad students. J Travel Med. 2009;16:338-343.
11. Kishore J, Gupta VK, Singh SV, et al. Impact of health education intervention on knowledge and community action for malaria control in Delhi. J Commun Dis. 2008;40:183-192.
12. Chirdan OO, Zoakah AI, Ejembi CL. Impact of health education on home treatment and prevention of malaria in Jengre, North Central Nigeria. Ann Afr Med. 2008;7:112-119.
13. Lengeler C. Insecticide-treated bed nets and curtains for preventing malaria. Cochrane Database Syst Rev. 2004;(2): CD000363.
14. Centers for Disease Control and Prevention. Fight the bite for protection from malaria: Guidelines for DEET insect repellent use. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/malaria/toolkit/DEET.pdf. Accessed September 29, 2014.
15. American Academy of Pediatrics. Safety & prevention. Healthychildren.org Web site. Available at: http://www.healthychildren. org/English/safety-prevention/at-play/Pages/Insect-Repellents. aspx. Accessed September 29, 2014.
16. Croft AM, Baker D, von Bertele MJ. An evidence-based vector control strategy for military deployments: the British Army experience. Med Trop (Mars). 2001;61:91-98.
17. Boggild AK, Parise ME, Lewis LS, et al. Atovaquone-proguanil: report from the CDC expert meeting on malaria chemoprophylaxis (II). Am J Trop Med Hyg. 2007;76:208-223.
18. Tan KR, Magill AJ, Parise ME, et al; Centers for Disease Control and Prevention. Doxycycline for malaria chemoprophylaxis and treatment: report from the CDC expert meeting on malaria chemoprophylaxis. Am J Trop Med Hyg. 2011;84:517-531.
19. Hill DR, Baird JK, Parise ME, et al. Primaquine: report from CDC expert meeting on malaria chemoprophylaxis I. Am J Trop Med Hyg. 2006;75:402-415.
20. Steffen R, Fuchs E, Schildknecht J, et al. Mefloquine compared with other malaria chemoprophylactic regimens in tourists visiting east Africa. Lancet. 1993;341:1299-1303.
21. Centers for Disease Control and Prevention. CDC Health Information for International Travel 2014. New York, NY: Oxford University Press; 2014.
22. Gershman MD, Jentes ES, Johnson KJ, et al; Centers for Disease Control and Prevention. Infectious diseases related to travel. Centers for Disease Control and Prevention Web site. Available at: http://wwwnc.cdc.gov/travel/yellowbook/2012/chapter-3- infectious-diseases-related-to-travel/yellow-fever-and-malaria- information-by-country.htm. Accessed September 29, 2014.
23. Jacquerioz FA, Croft AM. Drugs for preventing malaria in travellers. Cochrane Database Syst Rev. 2009;(4):CD006491.
24. Schlagenhauf P, Tschopp A, Johnson R, et al. Tolerability of malaria chemoprophylaxis in non-immune travellers to sub-Saharan Africa: multicentre, randomised, double blind, four arm study. BMJ. 2003;327:1078.
25. Chloroquine phosphate [package insert]. Eatontown, NJ: Westward Pharmaceutical Corp; 2010.
26. Lariam [package insert]. Roche Laboratories, Inc: Nutley, NJ; 2004.
27. Steketee RW, Nahlen BL, Parise ME, et al. The burden of malaria in pregnancy in malaria-endemic areas. Am J Trop Med Hyg. 2001;64(1-2 suppl):28-35.
28. Kirby MJ, Ameh D, Bottomley C, et al. Effect of two different house screening interventions on exposure to malaria vectors and on anaemia in children in The Gambia: a randomised controlled trial. Lancet. 2009;374:998-1009.
29. Steffen R, Amitirigala I, Mutsch M. Health risks among travelers--need for regular updates. J Travel Med. 2008;15:145-146.
30. Dorsey G, Gandhi M, Oyugi JH, et al. Difficulties in the prevention, diagnosis, and treatment of imported malaria. Arch Intern Med. 2000;160:2505-2510.
31. Newman RD, Parise ME, Barber AM, et al. Malaria-related deaths among U.S. travelers, 1963-2001. Ann Intern Med. 2004;141: 547-555.
32. Lesko CR, Arguin PM, Newman RD. Congenital malaria in the United States: a review of cases from 1966 to 2005. Arch Pediatr Adolesc Med. 2007;161:1062-1067.
33. Blair JE. Evaluation of fever in the international traveler. Unwanted ‘souvenir’ can have many causes. Postgrad Med. 2004;116: 13-20,29.
› Assess the need for nonpharmacologic, behavioral interventions and for chemoprophylaxis based on a destination’s relative risk to travelers, planned and potential activities, and patient comorbidities. B
› Choose an antimalarial medication based on knowledge of area-specific drug effectiveness or resistance patterns, trip duration, drug cost, tolerance for adverse effects, and comorbidities. C
› Presume a diagnosis of malaria until proven otherwise in any traveler who is febrile after returning from a malaria-endemic region. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Although malaria was eradicated as an endemic disease in the United States in the early 1950s,1 it still returns yearly in approximately 1500 individuals who travel to foreign countries2—most of whom neglected to use prophylactic measures or use them properly.3 In more than 60 documented cases, these infected individuals have been the source of local transmission in their communities.2 To reduce the individual and public health risks associated with malaria, this article focuses on steps that international travelers can take to limit their risk of the disease.
What travelers need to know
In 2013, more than 61.5 million residents of the United States traveled abroad, approximately 30% of whom visited malaria-endemic regions: Mexico and equatorial nations in Central and South America; Africa; the Middle East; and South, East, and Southeast Asia.4 Counseling on appropriate preventive measures fits the Medical Home concept of comprehensive, preventive, patient-centered care, and pre-travel consultation—including a review of health data and itineraries, and patient education—can be a team-based effort.5
Begin your planning for malaria prophylaxis by assessing your patient’s individual risk. Key variables are a patient’s detailed itinerary, a credible and current source of information on location-specific malaria prevalence, personal risk factors, and risk tolerance. Shared decision-making is vital and enhances adherence to the prescribed regimen.
Endemicity varies regionally. Without chemoprophylaxis, risk of infection ranges from more than 20% in Papua New Guinea to 0.01% in Central America, with wide exposure risk variations likely, even within regions.6 Travel to areas of high endemicity requires more aggressive malaria prevention strategies than travel to low-endemicity regions.
Risk of exposure is lower with short visits,7 business-only travel, urban-only stays in some countries, day trips to endemic areas,7 and travel during seasons with lower mosquito burden. Likewise, travelers staying in a hotel with sealed windows will face lower nighttime Anopheles mosquito exposure. In these cases, nonpharmacologic measures alone may be appropriate.
Those at particularly high risk for complicated or lethal malarial infection are children, pregnant women, elderly individuals, and immunocompromised patients.7 In addition to counseling high-risk patients about prophylactic measures, consider advising against travel in certain circumstances. Among those at highest risk for acquiring malaria are immigrants and refugees traveling to their ancestral homelands to visit friends and relatives (VFR).2 Many VFR travelers fail to take appropriate prophylactic measures when “going home.”8 A significant number of cases of travel-acquired malaria occurs in VFR children.9
Individualizing prevention directives
The mainstays of malaria prevention include nonpharmacologic and behavioral interventions, as well as chemoprophylaxis. Most cases of malaria in travelers returning to the United States result from the improper implementation of prophylactic measures.3 Discussing individual risk with travelers is an easy way to bolster adherence to malaria prevention measures, and some evidence suggests it is effective10 (strength of recommendation [SOR]: C). Other limited studies have also shown that malaria education can improve knowledge about malaria transmission and increase the likelihood that preventive measures will be used.11,12
Recommend nonpharmacologic measures even for those using chemoprophylaxis
Nonpharmacologic interventions such as sleeping under permethrin-treated bednets, wearing long sleeves and full-length pants, treating clothes with permethrin, and applying DEET (N,N-diethyl-meta-toluamide) to exposed skin are effective and have the added benefit of preventing non-malarial arthropod-borne diseases4 (SOR: B). Studies have shown that, compared with sleeping without nets, the use of insecticide treated-nets can reduce child mortality by 17% and the incidence of uncomplicated malarial episodes by 50%.13 In areas with malaria transmission, 10% to 30% DEET—used alone or in combination with permethrin-treated clothing— can reduce bite load, although the American Academy of Pediatrics recommends against using DEET in children younger than 2 months of age.14,15
Using these measures in combination from dusk to dawn, when Anopheles mosquitoes are active, has been shown to be effective, although randomized, controlled studies are lacking.16 Remaining indoors during these peak biting periods is also advisable. In certain areas, and with the right itinerary, the traveler may only need to employ nonpharmacologic methods of preventing malarial infection. Recommend them to all patients traveling to malarial regions, even to individuals using pharmacologic prophylaxis.
Factors determining the need for, and selection of, chemoprophylaxis
When used properly, chemoprophylactic drugs are effective in preventing malaria (SOR: A). Atovaquone-proguanil achieves efficacy of 95% to 100%,17 while doxycycline, primaquine, and mefloquine are slightly less effective.18-20 Chloroquine is effective in 6 regions of the tropics and subtropics where Plasmodium falciparum resistance has not developed. Select a drug based on your assessment of an individual’s level of risk according to the personal itinerary, trip duration and accommodations, cost of medication, tolerance for adverse effects, and other factors (eg, comorbidities, concurrent drug usage, pregnancy).
Location matters. The risk of malaria transmission can vary considerably not only between countries, but also regionally within countries and even between a city and its immediate surroundings. Therefore, select a chemoprophylactic agent based on the specific itinerary, planned activities, the potential for unforeseen additional excursions, and local Plasmodium resistance patterns. For example, chloroquine is effective only in the Caribbean, Central America, and some countries in the Middle East.21 Mefloquine resistance has been reported in parts of Cambodia, Thailand, Vietnam, Burma, China, and Laos.21
On its Travelers’ Health Web site (www.cdc.gov/travel), the Centers for Disease Control and Prevention (CDC) reports for each country 1) the risk of malaria transmission, 2) areas within the country that pose a risk, 3) evidence of Plasmodium drug resistance, 4) which Plasmodium species are active, and 5) which chemoprophylactic medications are recommended.22 Additional Web sites, either free or subscription-based, allow users to view this same information on maps, advise on where insect precautions alone are sufficiently protective, and provide information about the traveler’s risk of contracting other diseases (TABLE 1).
TABLE 1
| Web resources on infectious diseases of concern to international travelers | |
| Resource | Notes |
Centers for Disease Control and Prevention | Free Site Go to Yellow Book » Contents » Chapter 3 » “Travel Vaccines & Malaria Information, by Country” for country-specific information about the risk of malaria transmission |
VHI Healthcare | Free Site Destination-specific information about travel alerts and vaccine recommendations Does not report malaria transmission data |
Gideon | Subscription only Online application that helps with diagnosing infectious diseases and keeping up to date with global health literature |
Travax | Subscription only Information about recommended vaccines and country-specific risk of malaria transmission |
Tropimed | Subscription only Information about recommended vaccines and country-specific risk of malaria transmission |
Comparative adverse effects of antimalarial agents. A Cochrane Review on the tolerability of chemoprophylactic agents concluded that atovaquone-proguanil and doxycycline were better tolerated than mefloquine (SOR: B). Compared with mefloquine, atovaquone-proguanil led to fewer reports of any adverse effects (relative risk [RR]=0.72), gastrointestinal adverse effects (RR=0.54), and neuropsychiatric adverse events (RR= 0.49-0.86, depending on the studies).23 Doxycycline users have reported fewer neuropsychiatric events (RR=0.84) than mefloquine users.23 These are relatively small differences, and the authors point out that these figures are based on low-quality evidence. Additional research is likely to have an impact on the confidence in the estimate of effect and to ultimately change the estimate.
Mefloquine is contraindicated in travelers with seizures, active or recent history of depression, generalized anxiety disorder, psychosis, schizophrenia, or other psychiatric disorders. Compared with mefloquine, atovaquone-proguanil and doxycycline cause fewer neuropsychiatric adverse effects (such as vivid dreams, dizziness, anxiety, depression, visual disturbance, or seizures).24 Caution is advised when prescribing chloroquine for patients with epilepsy because the medication has the potential to lower the seizure threshold.25
Use caution when prescribing mefloquine for patients with cardiac conduction disturbances. Electrocardiogram alterations such as sinus bradycardia, first-degree AV block, prolongation of QTc intervals, and abnormal T wave changes have been reported.26 Chloroquine can also prolong QTc intervals.26
Safety in pregnancy and breastfeeding. Malaria in pregnancy is associated with increased rates of anemia, low birth weight, prematurity, intrauterine growth restriction, and infant mortality.27 Chloroquine and mefloquine are considered safe during pregnancy and breastfeeding. Doxycycline has been associated with increased risk of harm to the fetus. Atovaquone-proguanil can be used in breastfeeding women if the child is ≥5 kg (≥11 lbs). Chemoprophylaxis taken by the mother while breastfeeding does not protect the infant from infection.
Dosing considerations. Mefloquine and chloroquine are dosed weekly; doxycycline and atovaquone-proguanil are taken daily. Travelers staying in a malaria-endemic region for longer periods (months rather than weeks) often prefer the weekly rather than daily medications; however, this may not be possible due to the adverse-effect profile of mefloquine or to traveling in an area with known chloroquine resistance. Some individuals prefer the routine of taking a medication daily, since remembering to take a single dose on the same day each week can be challenging. Others may not want to carry a large number of pills and therefore prefer weekly dosing. Have patients take medications before the trip, to assess tolerability and to ensure adequate blood concentrations before exposure.
Because mefloquine, doxycycline, and chloroquine target only the blood stages of Plasmodium, patients must continue these medications for 4 weeks following the exposure period to ensure adequate coverage as parasites are released from the liver. Because doxycycline is taken daily and has to be continued for 4 weeks following the exposure period, the total number of pills taken is higher for this regimen. Atovaquone-proguanil is active against hepatic and blood stages and can be discontinued a week following the exposure period.
With children, base dosing on body weight and do not exceed the recommended adult dose. When fractions of tablets are used (such as with mefloquine and atovaquone-proguanil dosing), pharmacists can crush tablets and place divided doses in capsules, to be sprinkled as needed into food such as applesauce or jelly. Mefloquine and chloroquine can be given to children of all ages and weights. Although atovaquone-proguanil is approved only for children ≥11 kg (24 lbs), dosing schedules have been calculated for children who weigh ≥5 kg.21 Doxycycline is recommended only for children who are at least 8 years of age.
Cost. For a 2-week exposure period, chloroquine is the least expensive medication (although regions in which it is recommended are limited due to resistance) (TABLE 27,25,26).
Ask about accommodations
Since Anopheles mosquitoes feed between dusk and dawn, inquiring about accommodations can further clarify a patient’s malaria risk. Staying in air-conditioned housing (implying that the interior can be sealed) or that has screened windows can reduce exposure to mosquitoes, although data are lacking regarding whether the latter practice reduces the incidence of malaria transmission28 (SOR: C).
Share decision making
After considering the key factors determining a patient’s level of risk, you may decide to recommend no specific interventions, to advise insect avoidance measures only, to combine insect avoidance with chemoprophylaxis, or to caution against traveling to a malaria-endemic region. The patient’s contribution to the final decision includes personal preferences, values, and risk tolerance—particularly when comorbidities are involved.
When preventive measures fail
Approximately 0.2% of travelers to malaria-endemic regions will become infected, despite proper pre-travel counseling and prophylaxis.29 In the United States, malaria is often misdiagnosed or improperly treated.30 The time from initial presentation to correct diagnosis of malaria has been reported as an astonishingly high 4 to 8.5 days, depending on the population.31,32
A high index of suspicion is needed and will ensure timely care when any febrile traveler returns from a malaria-endemic area.33 Be sure to advise patients to seek medical attention if they are feverish upon returning home.
Once suspected, the diagnosis of malaria can be readily confirmed through the use of antibody-, nucleic acid-, or microscopy-based techniques (the latter to directly visualize Plasmodium species in blood smears).
Although malaria chemoprophylaxis is relatively straightforward, malaria treatment—especially in cases of chemoprophylaxis failures—may not be, and the topic is beyond the scope of this article. For guidance on treating malaria, consult a knowledgeable physician or contact the CDC at www.cdc.gov/malaria/, or at (855) 856-4713 (weekdays, 9 am to 5 pm EST) or (770) 488-7100 (weekends or after normal business hours; ask for the Malaria Branch clinician on call).
CORRESPONDENCE
Mark K. Huntington, MD, PhD, Center for Family Medicine, 1115 East 20th Street, Sioux Falls, SD 57105; [email protected]
› Assess the need for nonpharmacologic, behavioral interventions and for chemoprophylaxis based on a destination’s relative risk to travelers, planned and potential activities, and patient comorbidities. B
› Choose an antimalarial medication based on knowledge of area-specific drug effectiveness or resistance patterns, trip duration, drug cost, tolerance for adverse effects, and comorbidities. C
› Presume a diagnosis of malaria until proven otherwise in any traveler who is febrile after returning from a malaria-endemic region. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Although malaria was eradicated as an endemic disease in the United States in the early 1950s,1 it still returns yearly in approximately 1500 individuals who travel to foreign countries2—most of whom neglected to use prophylactic measures or use them properly.3 In more than 60 documented cases, these infected individuals have been the source of local transmission in their communities.2 To reduce the individual and public health risks associated with malaria, this article focuses on steps that international travelers can take to limit their risk of the disease.
What travelers need to know
In 2013, more than 61.5 million residents of the United States traveled abroad, approximately 30% of whom visited malaria-endemic regions: Mexico and equatorial nations in Central and South America; Africa; the Middle East; and South, East, and Southeast Asia.4 Counseling on appropriate preventive measures fits the Medical Home concept of comprehensive, preventive, patient-centered care, and pre-travel consultation—including a review of health data and itineraries, and patient education—can be a team-based effort.5
Begin your planning for malaria prophylaxis by assessing your patient’s individual risk. Key variables are a patient’s detailed itinerary, a credible and current source of information on location-specific malaria prevalence, personal risk factors, and risk tolerance. Shared decision-making is vital and enhances adherence to the prescribed regimen.
Endemicity varies regionally. Without chemoprophylaxis, risk of infection ranges from more than 20% in Papua New Guinea to 0.01% in Central America, with wide exposure risk variations likely, even within regions.6 Travel to areas of high endemicity requires more aggressive malaria prevention strategies than travel to low-endemicity regions.
Risk of exposure is lower with short visits,7 business-only travel, urban-only stays in some countries, day trips to endemic areas,7 and travel during seasons with lower mosquito burden. Likewise, travelers staying in a hotel with sealed windows will face lower nighttime Anopheles mosquito exposure. In these cases, nonpharmacologic measures alone may be appropriate.
Those at particularly high risk for complicated or lethal malarial infection are children, pregnant women, elderly individuals, and immunocompromised patients.7 In addition to counseling high-risk patients about prophylactic measures, consider advising against travel in certain circumstances. Among those at highest risk for acquiring malaria are immigrants and refugees traveling to their ancestral homelands to visit friends and relatives (VFR).2 Many VFR travelers fail to take appropriate prophylactic measures when “going home.”8 A significant number of cases of travel-acquired malaria occurs in VFR children.9
Individualizing prevention directives
The mainstays of malaria prevention include nonpharmacologic and behavioral interventions, as well as chemoprophylaxis. Most cases of malaria in travelers returning to the United States result from the improper implementation of prophylactic measures.3 Discussing individual risk with travelers is an easy way to bolster adherence to malaria prevention measures, and some evidence suggests it is effective10 (strength of recommendation [SOR]: C). Other limited studies have also shown that malaria education can improve knowledge about malaria transmission and increase the likelihood that preventive measures will be used.11,12
Recommend nonpharmacologic measures even for those using chemoprophylaxis
Nonpharmacologic interventions such as sleeping under permethrin-treated bednets, wearing long sleeves and full-length pants, treating clothes with permethrin, and applying DEET (N,N-diethyl-meta-toluamide) to exposed skin are effective and have the added benefit of preventing non-malarial arthropod-borne diseases4 (SOR: B). Studies have shown that, compared with sleeping without nets, the use of insecticide treated-nets can reduce child mortality by 17% and the incidence of uncomplicated malarial episodes by 50%.13 In areas with malaria transmission, 10% to 30% DEET—used alone or in combination with permethrin-treated clothing— can reduce bite load, although the American Academy of Pediatrics recommends against using DEET in children younger than 2 months of age.14,15
Using these measures in combination from dusk to dawn, when Anopheles mosquitoes are active, has been shown to be effective, although randomized, controlled studies are lacking.16 Remaining indoors during these peak biting periods is also advisable. In certain areas, and with the right itinerary, the traveler may only need to employ nonpharmacologic methods of preventing malarial infection. Recommend them to all patients traveling to malarial regions, even to individuals using pharmacologic prophylaxis.
Factors determining the need for, and selection of, chemoprophylaxis
When used properly, chemoprophylactic drugs are effective in preventing malaria (SOR: A). Atovaquone-proguanil achieves efficacy of 95% to 100%,17 while doxycycline, primaquine, and mefloquine are slightly less effective.18-20 Chloroquine is effective in 6 regions of the tropics and subtropics where Plasmodium falciparum resistance has not developed. Select a drug based on your assessment of an individual’s level of risk according to the personal itinerary, trip duration and accommodations, cost of medication, tolerance for adverse effects, and other factors (eg, comorbidities, concurrent drug usage, pregnancy).
Location matters. The risk of malaria transmission can vary considerably not only between countries, but also regionally within countries and even between a city and its immediate surroundings. Therefore, select a chemoprophylactic agent based on the specific itinerary, planned activities, the potential for unforeseen additional excursions, and local Plasmodium resistance patterns. For example, chloroquine is effective only in the Caribbean, Central America, and some countries in the Middle East.21 Mefloquine resistance has been reported in parts of Cambodia, Thailand, Vietnam, Burma, China, and Laos.21
On its Travelers’ Health Web site (www.cdc.gov/travel), the Centers for Disease Control and Prevention (CDC) reports for each country 1) the risk of malaria transmission, 2) areas within the country that pose a risk, 3) evidence of Plasmodium drug resistance, 4) which Plasmodium species are active, and 5) which chemoprophylactic medications are recommended.22 Additional Web sites, either free or subscription-based, allow users to view this same information on maps, advise on where insect precautions alone are sufficiently protective, and provide information about the traveler’s risk of contracting other diseases (TABLE 1).
TABLE 1
| Web resources on infectious diseases of concern to international travelers | |
| Resource | Notes |
Centers for Disease Control and Prevention | Free Site Go to Yellow Book » Contents » Chapter 3 » “Travel Vaccines & Malaria Information, by Country” for country-specific information about the risk of malaria transmission |
VHI Healthcare | Free Site Destination-specific information about travel alerts and vaccine recommendations Does not report malaria transmission data |
Gideon | Subscription only Online application that helps with diagnosing infectious diseases and keeping up to date with global health literature |
Travax | Subscription only Information about recommended vaccines and country-specific risk of malaria transmission |
Tropimed | Subscription only Information about recommended vaccines and country-specific risk of malaria transmission |
Comparative adverse effects of antimalarial agents. A Cochrane Review on the tolerability of chemoprophylactic agents concluded that atovaquone-proguanil and doxycycline were better tolerated than mefloquine (SOR: B). Compared with mefloquine, atovaquone-proguanil led to fewer reports of any adverse effects (relative risk [RR]=0.72), gastrointestinal adverse effects (RR=0.54), and neuropsychiatric adverse events (RR= 0.49-0.86, depending on the studies).23 Doxycycline users have reported fewer neuropsychiatric events (RR=0.84) than mefloquine users.23 These are relatively small differences, and the authors point out that these figures are based on low-quality evidence. Additional research is likely to have an impact on the confidence in the estimate of effect and to ultimately change the estimate.
Mefloquine is contraindicated in travelers with seizures, active or recent history of depression, generalized anxiety disorder, psychosis, schizophrenia, or other psychiatric disorders. Compared with mefloquine, atovaquone-proguanil and doxycycline cause fewer neuropsychiatric adverse effects (such as vivid dreams, dizziness, anxiety, depression, visual disturbance, or seizures).24 Caution is advised when prescribing chloroquine for patients with epilepsy because the medication has the potential to lower the seizure threshold.25
Use caution when prescribing mefloquine for patients with cardiac conduction disturbances. Electrocardiogram alterations such as sinus bradycardia, first-degree AV block, prolongation of QTc intervals, and abnormal T wave changes have been reported.26 Chloroquine can also prolong QTc intervals.26
Safety in pregnancy and breastfeeding. Malaria in pregnancy is associated with increased rates of anemia, low birth weight, prematurity, intrauterine growth restriction, and infant mortality.27 Chloroquine and mefloquine are considered safe during pregnancy and breastfeeding. Doxycycline has been associated with increased risk of harm to the fetus. Atovaquone-proguanil can be used in breastfeeding women if the child is ≥5 kg (≥11 lbs). Chemoprophylaxis taken by the mother while breastfeeding does not protect the infant from infection.
Dosing considerations. Mefloquine and chloroquine are dosed weekly; doxycycline and atovaquone-proguanil are taken daily. Travelers staying in a malaria-endemic region for longer periods (months rather than weeks) often prefer the weekly rather than daily medications; however, this may not be possible due to the adverse-effect profile of mefloquine or to traveling in an area with known chloroquine resistance. Some individuals prefer the routine of taking a medication daily, since remembering to take a single dose on the same day each week can be challenging. Others may not want to carry a large number of pills and therefore prefer weekly dosing. Have patients take medications before the trip, to assess tolerability and to ensure adequate blood concentrations before exposure.
Because mefloquine, doxycycline, and chloroquine target only the blood stages of Plasmodium, patients must continue these medications for 4 weeks following the exposure period to ensure adequate coverage as parasites are released from the liver. Because doxycycline is taken daily and has to be continued for 4 weeks following the exposure period, the total number of pills taken is higher for this regimen. Atovaquone-proguanil is active against hepatic and blood stages and can be discontinued a week following the exposure period.
With children, base dosing on body weight and do not exceed the recommended adult dose. When fractions of tablets are used (such as with mefloquine and atovaquone-proguanil dosing), pharmacists can crush tablets and place divided doses in capsules, to be sprinkled as needed into food such as applesauce or jelly. Mefloquine and chloroquine can be given to children of all ages and weights. Although atovaquone-proguanil is approved only for children ≥11 kg (24 lbs), dosing schedules have been calculated for children who weigh ≥5 kg.21 Doxycycline is recommended only for children who are at least 8 years of age.
Cost. For a 2-week exposure period, chloroquine is the least expensive medication (although regions in which it is recommended are limited due to resistance) (TABLE 27,25,26).
Ask about accommodations
Since Anopheles mosquitoes feed between dusk and dawn, inquiring about accommodations can further clarify a patient’s malaria risk. Staying in air-conditioned housing (implying that the interior can be sealed) or that has screened windows can reduce exposure to mosquitoes, although data are lacking regarding whether the latter practice reduces the incidence of malaria transmission28 (SOR: C).
Share decision making
After considering the key factors determining a patient’s level of risk, you may decide to recommend no specific interventions, to advise insect avoidance measures only, to combine insect avoidance with chemoprophylaxis, or to caution against traveling to a malaria-endemic region. The patient’s contribution to the final decision includes personal preferences, values, and risk tolerance—particularly when comorbidities are involved.
When preventive measures fail
Approximately 0.2% of travelers to malaria-endemic regions will become infected, despite proper pre-travel counseling and prophylaxis.29 In the United States, malaria is often misdiagnosed or improperly treated.30 The time from initial presentation to correct diagnosis of malaria has been reported as an astonishingly high 4 to 8.5 days, depending on the population.31,32
A high index of suspicion is needed and will ensure timely care when any febrile traveler returns from a malaria-endemic area.33 Be sure to advise patients to seek medical attention if they are feverish upon returning home.
Once suspected, the diagnosis of malaria can be readily confirmed through the use of antibody-, nucleic acid-, or microscopy-based techniques (the latter to directly visualize Plasmodium species in blood smears).
Although malaria chemoprophylaxis is relatively straightforward, malaria treatment—especially in cases of chemoprophylaxis failures—may not be, and the topic is beyond the scope of this article. For guidance on treating malaria, consult a knowledgeable physician or contact the CDC at www.cdc.gov/malaria/, or at (855) 856-4713 (weekdays, 9 am to 5 pm EST) or (770) 488-7100 (weekends or after normal business hours; ask for the Malaria Branch clinician on call).
CORRESPONDENCE
Mark K. Huntington, MD, PhD, Center for Family Medicine, 1115 East 20th Street, Sioux Falls, SD 57105; [email protected]
1. Mali S, Steele S, Slutsker L, et al; Centers for Disease Control and Prevention (CDC). Malaria surveillance - United States, 2008. MMWR Surveill Summ. 2010;59:1-15.
2. Centers for Disease Control and Prevention. Malaria facts. Centers for Disease Control and Prevention Web site. Available at: www.cdc.gov/malaria/about/facts.html. Accessed September 29, 2014.
3. Huntington MK. Healthy people, malaria and South Dakota. S D Med. 2012;65:297-300.
4. Office of Travel and Tourism Industries. U.S. citizen travel to international regions, 2013. Office of Travel and Tourism Industries Web site. Available at: http://travel.trade.gov/view/m-2013-O-001/index.html. Accessed September 29, 2014.
5. Bazemore AW, Huntington M. The pretravel consultation. Am Fam Physician. 2009;80:583-590.
6. Bradley DJ, Warhurst DC, Blaze M, et al. Malaria imported into the United Kingdom in 1996. Euro Surveill. 1998;3:40-42.
7. Arguin PM, Tan KR, et al; Centers for Disease Control and Prevention. Infectious diseases related to travel. Centers for Disease Control and Prevention Web site. Available at: http://wwwnc.cdc.gov/travel/yellowbook/2014/chapter-3-infectious-diseases-related-to-travel/malaria. Accessed October 15, 2014.
8. Pavli A, Maltezou HC. Malaria and travellers visiting friends and relatives. Travel Med Infect Dis. 2010;8:161-168.
9. Stäger K, Legros F, Krause G, et al. Imported malaria in children in industrialized countries, 1992-2002. Emerg Infect Dis. 2009;15:185-191.
10. Hartjes LB, Baumann LC, Henriques JB. Travel health risk perceptions and prevention behaviors of US study abroad students. J Travel Med. 2009;16:338-343.
11. Kishore J, Gupta VK, Singh SV, et al. Impact of health education intervention on knowledge and community action for malaria control in Delhi. J Commun Dis. 2008;40:183-192.
12. Chirdan OO, Zoakah AI, Ejembi CL. Impact of health education on home treatment and prevention of malaria in Jengre, North Central Nigeria. Ann Afr Med. 2008;7:112-119.
13. Lengeler C. Insecticide-treated bed nets and curtains for preventing malaria. Cochrane Database Syst Rev. 2004;(2): CD000363.
14. Centers for Disease Control and Prevention. Fight the bite for protection from malaria: Guidelines for DEET insect repellent use. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/malaria/toolkit/DEET.pdf. Accessed September 29, 2014.
15. American Academy of Pediatrics. Safety & prevention. Healthychildren.org Web site. Available at: http://www.healthychildren. org/English/safety-prevention/at-play/Pages/Insect-Repellents. aspx. Accessed September 29, 2014.
16. Croft AM, Baker D, von Bertele MJ. An evidence-based vector control strategy for military deployments: the British Army experience. Med Trop (Mars). 2001;61:91-98.
17. Boggild AK, Parise ME, Lewis LS, et al. Atovaquone-proguanil: report from the CDC expert meeting on malaria chemoprophylaxis (II). Am J Trop Med Hyg. 2007;76:208-223.
18. Tan KR, Magill AJ, Parise ME, et al; Centers for Disease Control and Prevention. Doxycycline for malaria chemoprophylaxis and treatment: report from the CDC expert meeting on malaria chemoprophylaxis. Am J Trop Med Hyg. 2011;84:517-531.
19. Hill DR, Baird JK, Parise ME, et al. Primaquine: report from CDC expert meeting on malaria chemoprophylaxis I. Am J Trop Med Hyg. 2006;75:402-415.
20. Steffen R, Fuchs E, Schildknecht J, et al. Mefloquine compared with other malaria chemoprophylactic regimens in tourists visiting east Africa. Lancet. 1993;341:1299-1303.
21. Centers for Disease Control and Prevention. CDC Health Information for International Travel 2014. New York, NY: Oxford University Press; 2014.
22. Gershman MD, Jentes ES, Johnson KJ, et al; Centers for Disease Control and Prevention. Infectious diseases related to travel. Centers for Disease Control and Prevention Web site. Available at: http://wwwnc.cdc.gov/travel/yellowbook/2012/chapter-3- infectious-diseases-related-to-travel/yellow-fever-and-malaria- information-by-country.htm. Accessed September 29, 2014.
23. Jacquerioz FA, Croft AM. Drugs for preventing malaria in travellers. Cochrane Database Syst Rev. 2009;(4):CD006491.
24. Schlagenhauf P, Tschopp A, Johnson R, et al. Tolerability of malaria chemoprophylaxis in non-immune travellers to sub-Saharan Africa: multicentre, randomised, double blind, four arm study. BMJ. 2003;327:1078.
25. Chloroquine phosphate [package insert]. Eatontown, NJ: Westward Pharmaceutical Corp; 2010.
26. Lariam [package insert]. Roche Laboratories, Inc: Nutley, NJ; 2004.
27. Steketee RW, Nahlen BL, Parise ME, et al. The burden of malaria in pregnancy in malaria-endemic areas. Am J Trop Med Hyg. 2001;64(1-2 suppl):28-35.
28. Kirby MJ, Ameh D, Bottomley C, et al. Effect of two different house screening interventions on exposure to malaria vectors and on anaemia in children in The Gambia: a randomised controlled trial. Lancet. 2009;374:998-1009.
29. Steffen R, Amitirigala I, Mutsch M. Health risks among travelers--need for regular updates. J Travel Med. 2008;15:145-146.
30. Dorsey G, Gandhi M, Oyugi JH, et al. Difficulties in the prevention, diagnosis, and treatment of imported malaria. Arch Intern Med. 2000;160:2505-2510.
31. Newman RD, Parise ME, Barber AM, et al. Malaria-related deaths among U.S. travelers, 1963-2001. Ann Intern Med. 2004;141: 547-555.
32. Lesko CR, Arguin PM, Newman RD. Congenital malaria in the United States: a review of cases from 1966 to 2005. Arch Pediatr Adolesc Med. 2007;161:1062-1067.
33. Blair JE. Evaluation of fever in the international traveler. Unwanted ‘souvenir’ can have many causes. Postgrad Med. 2004;116: 13-20,29.
1. Mali S, Steele S, Slutsker L, et al; Centers for Disease Control and Prevention (CDC). Malaria surveillance - United States, 2008. MMWR Surveill Summ. 2010;59:1-15.
2. Centers for Disease Control and Prevention. Malaria facts. Centers for Disease Control and Prevention Web site. Available at: www.cdc.gov/malaria/about/facts.html. Accessed September 29, 2014.
3. Huntington MK. Healthy people, malaria and South Dakota. S D Med. 2012;65:297-300.
4. Office of Travel and Tourism Industries. U.S. citizen travel to international regions, 2013. Office of Travel and Tourism Industries Web site. Available at: http://travel.trade.gov/view/m-2013-O-001/index.html. Accessed September 29, 2014.
5. Bazemore AW, Huntington M. The pretravel consultation. Am Fam Physician. 2009;80:583-590.
6. Bradley DJ, Warhurst DC, Blaze M, et al. Malaria imported into the United Kingdom in 1996. Euro Surveill. 1998;3:40-42.
7. Arguin PM, Tan KR, et al; Centers for Disease Control and Prevention. Infectious diseases related to travel. Centers for Disease Control and Prevention Web site. Available at: http://wwwnc.cdc.gov/travel/yellowbook/2014/chapter-3-infectious-diseases-related-to-travel/malaria. Accessed October 15, 2014.
8. Pavli A, Maltezou HC. Malaria and travellers visiting friends and relatives. Travel Med Infect Dis. 2010;8:161-168.
9. Stäger K, Legros F, Krause G, et al. Imported malaria in children in industrialized countries, 1992-2002. Emerg Infect Dis. 2009;15:185-191.
10. Hartjes LB, Baumann LC, Henriques JB. Travel health risk perceptions and prevention behaviors of US study abroad students. J Travel Med. 2009;16:338-343.
11. Kishore J, Gupta VK, Singh SV, et al. Impact of health education intervention on knowledge and community action for malaria control in Delhi. J Commun Dis. 2008;40:183-192.
12. Chirdan OO, Zoakah AI, Ejembi CL. Impact of health education on home treatment and prevention of malaria in Jengre, North Central Nigeria. Ann Afr Med. 2008;7:112-119.
13. Lengeler C. Insecticide-treated bed nets and curtains for preventing malaria. Cochrane Database Syst Rev. 2004;(2): CD000363.
14. Centers for Disease Control and Prevention. Fight the bite for protection from malaria: Guidelines for DEET insect repellent use. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/malaria/toolkit/DEET.pdf. Accessed September 29, 2014.
15. American Academy of Pediatrics. Safety & prevention. Healthychildren.org Web site. Available at: http://www.healthychildren. org/English/safety-prevention/at-play/Pages/Insect-Repellents. aspx. Accessed September 29, 2014.
16. Croft AM, Baker D, von Bertele MJ. An evidence-based vector control strategy for military deployments: the British Army experience. Med Trop (Mars). 2001;61:91-98.
17. Boggild AK, Parise ME, Lewis LS, et al. Atovaquone-proguanil: report from the CDC expert meeting on malaria chemoprophylaxis (II). Am J Trop Med Hyg. 2007;76:208-223.
18. Tan KR, Magill AJ, Parise ME, et al; Centers for Disease Control and Prevention. Doxycycline for malaria chemoprophylaxis and treatment: report from the CDC expert meeting on malaria chemoprophylaxis. Am J Trop Med Hyg. 2011;84:517-531.
19. Hill DR, Baird JK, Parise ME, et al. Primaquine: report from CDC expert meeting on malaria chemoprophylaxis I. Am J Trop Med Hyg. 2006;75:402-415.
20. Steffen R, Fuchs E, Schildknecht J, et al. Mefloquine compared with other malaria chemoprophylactic regimens in tourists visiting east Africa. Lancet. 1993;341:1299-1303.
21. Centers for Disease Control and Prevention. CDC Health Information for International Travel 2014. New York, NY: Oxford University Press; 2014.
22. Gershman MD, Jentes ES, Johnson KJ, et al; Centers for Disease Control and Prevention. Infectious diseases related to travel. Centers for Disease Control and Prevention Web site. Available at: http://wwwnc.cdc.gov/travel/yellowbook/2012/chapter-3- infectious-diseases-related-to-travel/yellow-fever-and-malaria- information-by-country.htm. Accessed September 29, 2014.
23. Jacquerioz FA, Croft AM. Drugs for preventing malaria in travellers. Cochrane Database Syst Rev. 2009;(4):CD006491.
24. Schlagenhauf P, Tschopp A, Johnson R, et al. Tolerability of malaria chemoprophylaxis in non-immune travellers to sub-Saharan Africa: multicentre, randomised, double blind, four arm study. BMJ. 2003;327:1078.
25. Chloroquine phosphate [package insert]. Eatontown, NJ: Westward Pharmaceutical Corp; 2010.
26. Lariam [package insert]. Roche Laboratories, Inc: Nutley, NJ; 2004.
27. Steketee RW, Nahlen BL, Parise ME, et al. The burden of malaria in pregnancy in malaria-endemic areas. Am J Trop Med Hyg. 2001;64(1-2 suppl):28-35.
28. Kirby MJ, Ameh D, Bottomley C, et al. Effect of two different house screening interventions on exposure to malaria vectors and on anaemia in children in The Gambia: a randomised controlled trial. Lancet. 2009;374:998-1009.
29. Steffen R, Amitirigala I, Mutsch M. Health risks among travelers--need for regular updates. J Travel Med. 2008;15:145-146.
30. Dorsey G, Gandhi M, Oyugi JH, et al. Difficulties in the prevention, diagnosis, and treatment of imported malaria. Arch Intern Med. 2000;160:2505-2510.
31. Newman RD, Parise ME, Barber AM, et al. Malaria-related deaths among U.S. travelers, 1963-2001. Ann Intern Med. 2004;141: 547-555.
32. Lesko CR, Arguin PM, Newman RD. Congenital malaria in the United States: a review of cases from 1966 to 2005. Arch Pediatr Adolesc Med. 2007;161:1062-1067.
33. Blair JE. Evaluation of fever in the international traveler. Unwanted ‘souvenir’ can have many causes. Postgrad Med. 2004;116: 13-20,29.
Treatment in the face of uncertainty following traumatic anhydrous ammonia exposure
JD is a previously healthy 33-year-old white man, married and father of 2 children, who, while working as an agronomist, was inadvertently exposed to anhydrous ammonia. His only recollection of the event was a "puff of smoke” and the smell of ammonia. He lost consciousness almost immediately and awoke several days later in the intensive care unit. He was blind. Over the days that followed, he regained central vision; however, the loss of peripheral vision in all fields persisted. He said that upon first waking in the morning, he could "only see shadows.” For the rest of the day, he had “tunnel vision.” Ophthalmology and neurology evaluations uncovered no obvious reasons for the persistent vision loss.
The patient also complained of mild headache and discomfort behind his eyes for which he was taking aspirin. The discomfort behind his left eye was worse than on the right. He remained on disability following his work injury, and began to feel increasingly distressed and hopeless. His wife noted he was uncharacteristically irritable with her and the children, and that he had vivid nightmares and said he could smell ammonia. He also had trouble keeping up his yard because of the agitation and anxiety he experienced in approaching his workshed and equipment on the property.
A month later, an ophthalmologist reexamined JD and, again, found no cause for the ocular abnormality and suggested artificial tears for dry eyes. Two months later, he saw an optometrist, who documented constricted visual fields and referred JD to a second ophthalmologist. This consultant suggested possible brain injury and doubted it was psychosomatic in nature. He referred the patient to a neurologist. The neurologist found no organic explanation for his vision loss. He suspected a somatoform disorder and told JD his vision should recover. JD and his wife initially declined the neurologist’s idea of a neuroophthalmology consultation, but eventually agreed. The neuro-ophthalmologist also suspected a functional disturbance as the cause for visual impairment; and he required the patient to stop driving a motor vehicle until his vision improved.
The patient was subsequently referred for psychological evaluation. When initially seen by a psychologist and a family medicine resident, JD was working as a farmhand to make ends meet.
Effects of ammonia exposure
Ammonia is a water-soluble, colorless gas—an alkaloid with a unique odor. In the past, most exposures were related to its use as a fertilizer, as was the case with JD. In recent years, it has also been used to illegally manufacture methamphetamine, which has led to ammonia accidents and increased exposures.1-3
Systems commonly injured are the respiratory tract, ocular system, skin, and gastrointestinal tract (only if ingested).2
Ammonia destroys the mucosal barrier of the respiratory tract, causing loss of cilia, edema, and smooth muscle contraction.3,4 Long-term effects include chronic cough or hoarseness, obstructive or restrictive airway disease, reactive airway disease, or bronchiectasis.1,3
The extent of ocular injury is related to the degree of ammonia exposure. In mild cases, there is eye irritation, increased tear production, a sensation of stinging or burning, and perhaps conjunctivitis or spasmodic winking. The patient may also experience photophobia.1,3,4 In more severe cases, there may be corneal ulcerations, iritis, anterior or posterior synechia, opacification of the cornea, cataracts, glaucoma, atrophy of the retina, or severe pain.1,3 Blindness may occur, temporarily or permanently.4 This complete or partial vision loss is secondary to physical damage that can be seen during an ophthalmologic examination.1,4
Skin injuries can range from a mild erythematous rash to a full thickness burn with bullae and even denudation.1 Long-term effects include scarring or dermatitis.3
Our patient had respiratory and skin symptoms that fit with classic ammonia exposure (respiratory distress requiring intubation, rash). His initial blindness was consistent with ammonia exposure; however, his subsequent peripheral loss was inconsistent with known reaction to ammonia.
Causes of acute visual loss
Vision loss can be caused by injury to the media of the eye (cornea, lens, etc), the retina, or the neural visual pathways. It may also have a psychogenic component.5 Media-related causes of acute vision loss include keratitis or uveitis, edema of the cornea, blood in the anterior chamber (hyphema), disturbance of the lens, or hemorrhage into the vitreous.5,6 Retinal causes include occlusion of the central retinal artery or vein, detachment of the retina, or acute maculopathy.5-8 Neurologic causes include injury to the optic nerve itself (normally monocular) or defects in the chiasmal or retrochiasmal regions (causing partial loss in both eyes).5,9 If all of the above possibilities have been ruled out, consider psychogenic contribution to visual loss.5 Often this diagnosis is called “functional vision loss,” which can include feigning visual loss for secondary gain or subjective blindness as is seen with a somatoform disorder (eg, conversion disorder).
JD had bilateral peripheral vision loss of both the medial and lateral visual fields with macular sparing bilaterally. But he had an otherwise normal physical examination. At this point, the neurologist suspected conversion disorder, while one ophthalmologist thought a neuropathic disorder was responsible.
The neurologist had, early on, recognized that JD was significantly distressed by the accident and encouraged a psychological consultation. With the absence of identifiable ophthalmologic pathology, the patient reluctantly accepted this referral.
The psychologist, aided by family medicine residents, entertained the diagnoses of Post-Traumatic Stress Disorder (PTSD) and somatoform disorders, particularly conversion. PTSD is unique among psychiatric diagnoses in that a patient must meet all 6 DSM V criteria:10
• exposure to a traumatic event involving actual or threatened death or serious injury
• recollections, dreams, or hallucinations in which the trauma is re-experienced
• avoidance of stimuli associated with the trauma
• persistent symptoms of increased arousal (eg, irritability, agitation)
• symptoms and behavior that last for longer than one month
• distress that is clinically significant.
He met the criteria for a PTSD diagnosis and likely would benefit from treatment for it. However, sensory loss related to PTSD alone would be unusual, perhaps as unusual as peripheral vision loss secondary to ammonia exposure. Other factors needed to be explored.
Conversion disorders consist of disorders of movement, such as seizures or paralysis, or disorders of sensations, such as numbness or blindness. These may be episodic or sustained and have acute or chronic onset.11
Psychological factors are judged to be associated with the symptom or deficit because conflicts or other stressors precede the initiation or exacerbation of the symptom or deficit. This was possible in JD, but a degree of uncertainty lingered because he did not exhibit behavior typically seen with factitious disorder, and performance anxiety could conceivably account for the outcome on his vision tests.
In general, he could meet the criteria for conversion disorder, but questions remained. The biggest question is whether the accident resulting in PTSD is the cause of the psychological stress, or is the peripheral vision loss the source of the psychological stress, which would mean it is not a conversion disorder?
Treatment of visual defects
As is the case in many disorders, a definitive diagnosis of the cause of vision loss is not necessary to begin treatment. When you suspect a somatoform disorder including conversion, start therapy and treat the symptoms as “real.”12 Tell the patient that no specific treatment will completely resolve the symptoms, but that it can help.13 Whether the primary cause is neurologic or conversion based, there is often some spontaneous recovery of vision that occurs between 2 weeks and 3 or more months.14 Peripheral field defects have a guarded prognosis, although an extensive rehabilitation program may improve the vision fields somewhat.15-18
Conversion disorders effectively respond to cognitive behavioral therapy (CBT) including gradual exposure to anxiety triggers.19 Rehabilitation for neurologic damage based on remodeling of pathways responds to a similar gradual exercise or exposure to the lost function. Since these interventions are similar processes, a definitive diagnosis was unnecessary in JD’s case. A proprietary visual rehabilitation therapy program is available17 that exposes the patient to visual field activity that requires a cognitive reaction.15 This treatment facilitates recovery even into the sixth month of therapy.16 However, the cost of the software is approximately $6000 and is not yet covered by insurance.15
JD could not afford the commercially available programmed therapy. Therefore, we introduced an alternative treatment plan to challenge the transitional zone. With this plan, JD would play video games for 30 minutes at least twice a week, and preferably daily. He sat close enough to the television so that the transitional zone was approximately 1 to 2 inches from the peripheral portion of the television screen. The game was an action-packed video in which the peripheral portion of the screen was important (such as in first-person shooter games). He was to continue staring at the center of the screen during play in order to truly exercise the peripheral vision. Every day, he reassessed where the transitional zone was located and adjusted his seating accordingly. JD practiced this at least once a day and found that he had to sit closer and closer to the television screen to allow the transitional zone to remain in the screen’s periphery.
The patient, being very motivated, also developed treatments that worked well. He would stare at a blank white wall approximately 2 feet away, focusing on one location. One of his family members would take a laser pointer and start very far away, then slowly move the light closer to the patient’s center of vision. JD would tell his family member when he could see the light and they would move on to a different portion of his visual field.
After 3 months, we retested JD’s vision, which showed great improvement. JD felt he had significant improvement in his vision. The ophthalmologist retested JD about 2 months later and he passed a visual test well enough to obtain a modified driving license so he could return to his work as an agronomist.
Treatment of PTSD
Therapy for PTSD is complex and best approached with a long-term, multifaceted plan.20 Both pharmacotherapy and psychotherapy can be considered for initial treatment; however, no placebo-controlled randomized trials comparing the 2 modalities have been conducted. Combination therapy can also be employed.
Drug therapy, particularly selective serotonin reuptake inhibitors (SSRIs), has been shown to be generally effective in ameliorating positive symptoms associated with PTSD, such as nightmares and flashbacks. But they are less effective at treating negative symptoms such as withdrawal and avoidance.21,22
There is no clinical evidence to support the use of anxiolytics such as benzodiazepines in treating PTSD-specific symptoms. One small study did find a significant reduction in anxiety with alprazolam compared with placebo; however, the response was modest, and specific PTSD symptoms were unchanged.2 Given the high prevalence of comorbid substance abuse in PTSD, benzodiazepines are best avoided since evidence for their effectiveness is lacking.23
Both CBT and eye movement desensitization and reprocessing (EMDR) can be effective therapy for PTSD.24 Both modalities center on desensitization through exposure to traumatic recollections and symptom triggers.
The CBT approach we used focused on JD’s phobic reaction to ammonia that prevented his return to work. First, he listened to relaxation CDs to practice deep breathing and relaxation techniques. Once he was familiar with the techniques, he practiced them in the presence of the shed that contained ammonia products, which was a trigger for his anxiety. At first he was only able to approach the shed while using the breathing exercises to calm his anxiety. Over several weeks, he became more comfortable moving closer to the shed, and he eventually stepped into the shed and began staying for longer periods of time. The course of therapy took several months, but by the end of the sessions he was able to perform necessary tasks in the he was able to perform necessary tasks in the shed with only mild anxiety.
He also suffered from persistent troubling nightmares that significantly affected his sleep and led to physical symptoms of headache and vomiting. These, too, were overcome with the CBT approach.25 We instructed him to immediately write down as much as he could recall of a nightmare upon waking from it. During the following day, he re-read the dream and attempted to re-experience it while using the relaxation techniques to temper anxiety. Over several months of therapy, his nightmares lessened and eventually stopped.
On the last day of therapy, JD reported he had 3 job offers and 2 more interviews lined up, and that he was excited about his opportunities. We congratulated him on his visual recovery and applauded him for his hard work.
Discussion
While it is possible that JD spontaneously recovered his vision loss, it’s more likely that treatment can be credited, given that he did not improve in the 6 months prior to treatment and that his condition resolved over the 3-month rehabilitation period.
Research that guides practice must necessarily limit variables, but real-life patients often have multiple variables complicating both diagnosis and treatment. Our patient is a graphic example. He was exposed to anhydrous ammonia with its multiple physiologic sequelae and it was a traumatic event leading to additional sequelae. Furthermore, his inability to perform his job and fulfill social obligations contributed to his impairment.
JD’s referral to a neural-ophthalmologist did not provide a definitive diagnosis. He then followed up on a referral to the local residency clinic, where the family physician/psychologist team treats patients from a biopsychosocial perspective. Although physicians feel most comfortable when they arrive at a specific diagnosis with a specific evidence-based treatment that predicts a good outcome, this case yielded no definitive diagnosis. Instead, the psychologist and family physician relied on general research findings showing that in the context of traumatic injury or illness followed by debilitating anxiety symptoms, desensitization and rehabilitation provide the best chance of improvement. This shift in treatment approach very likely was responsible for the patient’s improvement.
This approach had the added benefit of helping the patient feel empowered. When JD a and family physician, he was frustrated that no explanation had been found for his problem. As a result, he feared that nothing could be done. When told that a rehabilitation and gradual exposure approach would likely help him, even if we were uncertain of the absolute cause, he became an eager participant in his treatment. He also embraced the idea that it wasn’t up to the doctors alone to improve his condition, but that he could be an active participant. In our view, his enthusiastic efforts contributed to the ultimate treatment outcome.
Often family physicians think they should refer these “complicated” patients to other specialists. However, for patients with combined psychological and physiological pathologies, we believe there are no better experts than family physicians. Properly trained family physicians could have treated this patient without the aid of a psychologist. Other patients who can benefit from this type of integrated biopsychosocial rehabilitation include those with chronic fatigue syndrome, chronic low back pain, and debilitating epilepsy.26-28
1. Lessenger JE. Anhydrous ammonia injuries. J Agromedicine. 1996;3:191-203.
2. Souther L, Small-Johnson J, Messing R. A description of agricultural releases of anhydrous ammonia in Minnesota. Chem Health Safety. 2000;7:16-22.
3. Welch A. Exposing the dangers of anhydrous ammonia. Nurse Pract. 2006;31:40-45.
4. Makarovsky I, Markel G, Dushnitsky T, et al. Ammonia—when something smells wrong. Isr Med Assoc J. 2008;10:537-543.
5. Leveque T. Approach to the adult with acute persistent visual loss. In: UpToDate, Trobe J (ed), UpToDate, Waltham, MA, 2013.
6. Morgan A, Hemphill RR. Acute visual change. Emerg Med Clin North Am. 1998;16:825-843,vii.
7. Beran DI, Murphy-Lavoie H. Acute, painless vision loss. J La State Med Soc. 2009;161:214-216, 218-223.
8. Vortmann M, Schneider JI. Acute monocular vision loss. Emerg Med Clin North Am. 2008;26:73-96.
9. Chamberlain MC, Chalmers L. Acute binocular blindness. Can- cer. 2007;109:1851-1854.
10. American Psychiatric Association. Diagnostic and Statistical Manual. 5th ed. Arlington, VA: American Psychiatric Publishing, Inc.; 2013.
11. Aybek S, Kanaan RA, David AS. The neuropsychiatry of conversion disorder. Curr Opin Psychiatry. 2008;21:275-280.
12. Stone J, Vuilleumier P, Friedman JH. Conversion disorder: separating “how” from “why.” Neurology. 2010;74:190-191.
13. Tocchio SL. Treatment of conversion disorder. A clinical and holistic approach. J Psychosoc Nurs Ment Health Serv. 2009; 47:42-49.
14. Mueller I, Gall C, Kasten E, et al. Long-term learning of visual functions in patients after brain damage. Behav Brain Res. 2008;191:32-42.
15. Glisson CC. Capturing the benefit of vision restoration therapy. Curr Opin Ophthalmol. 2006;17:504-508.
16. Marshall RS, Chmayssani M, O’Brien KA, et al. Visual field expansion after visual restoration therapy. Clin Rehabil. 2010;24: 1027-1035.
17. Mueller I, Mast H, Sabel BA. Recovery of visual field defects: a large clinical observational study using vision restoration therapy. Restor Neurol Neurosci. 2007;25:563-572.
18. Romano JG, Schulz P, Kenkel S, et al. Visual field changes after a rehabilitation intervention: vision restoration therapy. J Neurol Sci. 2008;273:70-74.
19. Allen LA, Woolfolk RL. Cognitive behavioral therapy for somatoform disorders. Psychiatr Clin North Am. 2010;33:579-593.
20. Cukor J, Olden M, Lee F, et al. Evidence-based treatments for PTSD, new directions, and special challenges. Ann N Y Acad Sci. 2010;1208:82-89.
21. Meltzer-Brody S, Connor K, Churchill E, et al. Symptom-specific effects of fluoxetine in PTSD. Intl Clin Psychopharmacol. 2000;15:227-231.
22. Stein DJ, Ipser JC, Seedat S. Pharmacotherapy for post traumatic stress disorder. Cochrane Database Syst Rev. 2006(1):CD002795.
23. Braun P, Greenberg D, Dasberg H, et al. Core symptoms of post-traumatic stress disorder unimproved by alprazolam treatment. J Clin Psychiatry. 1990;51:236-238.
24. Bisson J, Andrew M. Psychological treatment of post-traumatic stress disorder. Cochrane Database Syst Rev. 2007(3): CD003388.
25. Aurora RN, Zak RS, Auerbach SH, et al. Best practice guide for the treatment of nightmare disorder in adults. J Clin Sleep Med. 2010;6:389-401.
26. Reid SF, Chalder T, Cleare A, et al. Chronic fatigue syndrome. Clin Evid (Online). 2008 Aug 28;2008:1101.
27. Hall H, McIntosh G. Low back pain (chronic) Clin Evid (Online). 2008 Oct 1;2008:1116.
28. Prevedini A,Presti G,Ragitti E, et al.Acceptance and commitment Therapy (ACT): the foundation of the therapeutic model and an overview of its contribution to the treatment of patients with chronic physical diseases. G Ital Med Lav Ergon. 2011;33(1suppl A):A53-63.
JD is a previously healthy 33-year-old white man, married and father of 2 children, who, while working as an agronomist, was inadvertently exposed to anhydrous ammonia. His only recollection of the event was a "puff of smoke” and the smell of ammonia. He lost consciousness almost immediately and awoke several days later in the intensive care unit. He was blind. Over the days that followed, he regained central vision; however, the loss of peripheral vision in all fields persisted. He said that upon first waking in the morning, he could "only see shadows.” For the rest of the day, he had “tunnel vision.” Ophthalmology and neurology evaluations uncovered no obvious reasons for the persistent vision loss.
The patient also complained of mild headache and discomfort behind his eyes for which he was taking aspirin. The discomfort behind his left eye was worse than on the right. He remained on disability following his work injury, and began to feel increasingly distressed and hopeless. His wife noted he was uncharacteristically irritable with her and the children, and that he had vivid nightmares and said he could smell ammonia. He also had trouble keeping up his yard because of the agitation and anxiety he experienced in approaching his workshed and equipment on the property.
A month later, an ophthalmologist reexamined JD and, again, found no cause for the ocular abnormality and suggested artificial tears for dry eyes. Two months later, he saw an optometrist, who documented constricted visual fields and referred JD to a second ophthalmologist. This consultant suggested possible brain injury and doubted it was psychosomatic in nature. He referred the patient to a neurologist. The neurologist found no organic explanation for his vision loss. He suspected a somatoform disorder and told JD his vision should recover. JD and his wife initially declined the neurologist’s idea of a neuroophthalmology consultation, but eventually agreed. The neuro-ophthalmologist also suspected a functional disturbance as the cause for visual impairment; and he required the patient to stop driving a motor vehicle until his vision improved.
The patient was subsequently referred for psychological evaluation. When initially seen by a psychologist and a family medicine resident, JD was working as a farmhand to make ends meet.
Effects of ammonia exposure
Ammonia is a water-soluble, colorless gas—an alkaloid with a unique odor. In the past, most exposures were related to its use as a fertilizer, as was the case with JD. In recent years, it has also been used to illegally manufacture methamphetamine, which has led to ammonia accidents and increased exposures.1-3
Systems commonly injured are the respiratory tract, ocular system, skin, and gastrointestinal tract (only if ingested).2
Ammonia destroys the mucosal barrier of the respiratory tract, causing loss of cilia, edema, and smooth muscle contraction.3,4 Long-term effects include chronic cough or hoarseness, obstructive or restrictive airway disease, reactive airway disease, or bronchiectasis.1,3
The extent of ocular injury is related to the degree of ammonia exposure. In mild cases, there is eye irritation, increased tear production, a sensation of stinging or burning, and perhaps conjunctivitis or spasmodic winking. The patient may also experience photophobia.1,3,4 In more severe cases, there may be corneal ulcerations, iritis, anterior or posterior synechia, opacification of the cornea, cataracts, glaucoma, atrophy of the retina, or severe pain.1,3 Blindness may occur, temporarily or permanently.4 This complete or partial vision loss is secondary to physical damage that can be seen during an ophthalmologic examination.1,4
Skin injuries can range from a mild erythematous rash to a full thickness burn with bullae and even denudation.1 Long-term effects include scarring or dermatitis.3
Our patient had respiratory and skin symptoms that fit with classic ammonia exposure (respiratory distress requiring intubation, rash). His initial blindness was consistent with ammonia exposure; however, his subsequent peripheral loss was inconsistent with known reaction to ammonia.
Causes of acute visual loss
Vision loss can be caused by injury to the media of the eye (cornea, lens, etc), the retina, or the neural visual pathways. It may also have a psychogenic component.5 Media-related causes of acute vision loss include keratitis or uveitis, edema of the cornea, blood in the anterior chamber (hyphema), disturbance of the lens, or hemorrhage into the vitreous.5,6 Retinal causes include occlusion of the central retinal artery or vein, detachment of the retina, or acute maculopathy.5-8 Neurologic causes include injury to the optic nerve itself (normally monocular) or defects in the chiasmal or retrochiasmal regions (causing partial loss in both eyes).5,9 If all of the above possibilities have been ruled out, consider psychogenic contribution to visual loss.5 Often this diagnosis is called “functional vision loss,” which can include feigning visual loss for secondary gain or subjective blindness as is seen with a somatoform disorder (eg, conversion disorder).
JD had bilateral peripheral vision loss of both the medial and lateral visual fields with macular sparing bilaterally. But he had an otherwise normal physical examination. At this point, the neurologist suspected conversion disorder, while one ophthalmologist thought a neuropathic disorder was responsible.
The neurologist had, early on, recognized that JD was significantly distressed by the accident and encouraged a psychological consultation. With the absence of identifiable ophthalmologic pathology, the patient reluctantly accepted this referral.
The psychologist, aided by family medicine residents, entertained the diagnoses of Post-Traumatic Stress Disorder (PTSD) and somatoform disorders, particularly conversion. PTSD is unique among psychiatric diagnoses in that a patient must meet all 6 DSM V criteria:10
• exposure to a traumatic event involving actual or threatened death or serious injury
• recollections, dreams, or hallucinations in which the trauma is re-experienced
• avoidance of stimuli associated with the trauma
• persistent symptoms of increased arousal (eg, irritability, agitation)
• symptoms and behavior that last for longer than one month
• distress that is clinically significant.
He met the criteria for a PTSD diagnosis and likely would benefit from treatment for it. However, sensory loss related to PTSD alone would be unusual, perhaps as unusual as peripheral vision loss secondary to ammonia exposure. Other factors needed to be explored.
Conversion disorders consist of disorders of movement, such as seizures or paralysis, or disorders of sensations, such as numbness or blindness. These may be episodic or sustained and have acute or chronic onset.11
Psychological factors are judged to be associated with the symptom or deficit because conflicts or other stressors precede the initiation or exacerbation of the symptom or deficit. This was possible in JD, but a degree of uncertainty lingered because he did not exhibit behavior typically seen with factitious disorder, and performance anxiety could conceivably account for the outcome on his vision tests.
In general, he could meet the criteria for conversion disorder, but questions remained. The biggest question is whether the accident resulting in PTSD is the cause of the psychological stress, or is the peripheral vision loss the source of the psychological stress, which would mean it is not a conversion disorder?
Treatment of visual defects
As is the case in many disorders, a definitive diagnosis of the cause of vision loss is not necessary to begin treatment. When you suspect a somatoform disorder including conversion, start therapy and treat the symptoms as “real.”12 Tell the patient that no specific treatment will completely resolve the symptoms, but that it can help.13 Whether the primary cause is neurologic or conversion based, there is often some spontaneous recovery of vision that occurs between 2 weeks and 3 or more months.14 Peripheral field defects have a guarded prognosis, although an extensive rehabilitation program may improve the vision fields somewhat.15-18
Conversion disorders effectively respond to cognitive behavioral therapy (CBT) including gradual exposure to anxiety triggers.19 Rehabilitation for neurologic damage based on remodeling of pathways responds to a similar gradual exercise or exposure to the lost function. Since these interventions are similar processes, a definitive diagnosis was unnecessary in JD’s case. A proprietary visual rehabilitation therapy program is available17 that exposes the patient to visual field activity that requires a cognitive reaction.15 This treatment facilitates recovery even into the sixth month of therapy.16 However, the cost of the software is approximately $6000 and is not yet covered by insurance.15
JD could not afford the commercially available programmed therapy. Therefore, we introduced an alternative treatment plan to challenge the transitional zone. With this plan, JD would play video games for 30 minutes at least twice a week, and preferably daily. He sat close enough to the television so that the transitional zone was approximately 1 to 2 inches from the peripheral portion of the television screen. The game was an action-packed video in which the peripheral portion of the screen was important (such as in first-person shooter games). He was to continue staring at the center of the screen during play in order to truly exercise the peripheral vision. Every day, he reassessed where the transitional zone was located and adjusted his seating accordingly. JD practiced this at least once a day and found that he had to sit closer and closer to the television screen to allow the transitional zone to remain in the screen’s periphery.
The patient, being very motivated, also developed treatments that worked well. He would stare at a blank white wall approximately 2 feet away, focusing on one location. One of his family members would take a laser pointer and start very far away, then slowly move the light closer to the patient’s center of vision. JD would tell his family member when he could see the light and they would move on to a different portion of his visual field.
After 3 months, we retested JD’s vision, which showed great improvement. JD felt he had significant improvement in his vision. The ophthalmologist retested JD about 2 months later and he passed a visual test well enough to obtain a modified driving license so he could return to his work as an agronomist.
Treatment of PTSD
Therapy for PTSD is complex and best approached with a long-term, multifaceted plan.20 Both pharmacotherapy and psychotherapy can be considered for initial treatment; however, no placebo-controlled randomized trials comparing the 2 modalities have been conducted. Combination therapy can also be employed.
Drug therapy, particularly selective serotonin reuptake inhibitors (SSRIs), has been shown to be generally effective in ameliorating positive symptoms associated with PTSD, such as nightmares and flashbacks. But they are less effective at treating negative symptoms such as withdrawal and avoidance.21,22
There is no clinical evidence to support the use of anxiolytics such as benzodiazepines in treating PTSD-specific symptoms. One small study did find a significant reduction in anxiety with alprazolam compared with placebo; however, the response was modest, and specific PTSD symptoms were unchanged.2 Given the high prevalence of comorbid substance abuse in PTSD, benzodiazepines are best avoided since evidence for their effectiveness is lacking.23
Both CBT and eye movement desensitization and reprocessing (EMDR) can be effective therapy for PTSD.24 Both modalities center on desensitization through exposure to traumatic recollections and symptom triggers.
The CBT approach we used focused on JD’s phobic reaction to ammonia that prevented his return to work. First, he listened to relaxation CDs to practice deep breathing and relaxation techniques. Once he was familiar with the techniques, he practiced them in the presence of the shed that contained ammonia products, which was a trigger for his anxiety. At first he was only able to approach the shed while using the breathing exercises to calm his anxiety. Over several weeks, he became more comfortable moving closer to the shed, and he eventually stepped into the shed and began staying for longer periods of time. The course of therapy took several months, but by the end of the sessions he was able to perform necessary tasks in the he was able to perform necessary tasks in the shed with only mild anxiety.
He also suffered from persistent troubling nightmares that significantly affected his sleep and led to physical symptoms of headache and vomiting. These, too, were overcome with the CBT approach.25 We instructed him to immediately write down as much as he could recall of a nightmare upon waking from it. During the following day, he re-read the dream and attempted to re-experience it while using the relaxation techniques to temper anxiety. Over several months of therapy, his nightmares lessened and eventually stopped.
On the last day of therapy, JD reported he had 3 job offers and 2 more interviews lined up, and that he was excited about his opportunities. We congratulated him on his visual recovery and applauded him for his hard work.
Discussion
While it is possible that JD spontaneously recovered his vision loss, it’s more likely that treatment can be credited, given that he did not improve in the 6 months prior to treatment and that his condition resolved over the 3-month rehabilitation period.
Research that guides practice must necessarily limit variables, but real-life patients often have multiple variables complicating both diagnosis and treatment. Our patient is a graphic example. He was exposed to anhydrous ammonia with its multiple physiologic sequelae and it was a traumatic event leading to additional sequelae. Furthermore, his inability to perform his job and fulfill social obligations contributed to his impairment.
JD’s referral to a neural-ophthalmologist did not provide a definitive diagnosis. He then followed up on a referral to the local residency clinic, where the family physician/psychologist team treats patients from a biopsychosocial perspective. Although physicians feel most comfortable when they arrive at a specific diagnosis with a specific evidence-based treatment that predicts a good outcome, this case yielded no definitive diagnosis. Instead, the psychologist and family physician relied on general research findings showing that in the context of traumatic injury or illness followed by debilitating anxiety symptoms, desensitization and rehabilitation provide the best chance of improvement. This shift in treatment approach very likely was responsible for the patient’s improvement.
This approach had the added benefit of helping the patient feel empowered. When JD a and family physician, he was frustrated that no explanation had been found for his problem. As a result, he feared that nothing could be done. When told that a rehabilitation and gradual exposure approach would likely help him, even if we were uncertain of the absolute cause, he became an eager participant in his treatment. He also embraced the idea that it wasn’t up to the doctors alone to improve his condition, but that he could be an active participant. In our view, his enthusiastic efforts contributed to the ultimate treatment outcome.
Often family physicians think they should refer these “complicated” patients to other specialists. However, for patients with combined psychological and physiological pathologies, we believe there are no better experts than family physicians. Properly trained family physicians could have treated this patient without the aid of a psychologist. Other patients who can benefit from this type of integrated biopsychosocial rehabilitation include those with chronic fatigue syndrome, chronic low back pain, and debilitating epilepsy.26-28
JD is a previously healthy 33-year-old white man, married and father of 2 children, who, while working as an agronomist, was inadvertently exposed to anhydrous ammonia. His only recollection of the event was a "puff of smoke” and the smell of ammonia. He lost consciousness almost immediately and awoke several days later in the intensive care unit. He was blind. Over the days that followed, he regained central vision; however, the loss of peripheral vision in all fields persisted. He said that upon first waking in the morning, he could "only see shadows.” For the rest of the day, he had “tunnel vision.” Ophthalmology and neurology evaluations uncovered no obvious reasons for the persistent vision loss.
The patient also complained of mild headache and discomfort behind his eyes for which he was taking aspirin. The discomfort behind his left eye was worse than on the right. He remained on disability following his work injury, and began to feel increasingly distressed and hopeless. His wife noted he was uncharacteristically irritable with her and the children, and that he had vivid nightmares and said he could smell ammonia. He also had trouble keeping up his yard because of the agitation and anxiety he experienced in approaching his workshed and equipment on the property.
A month later, an ophthalmologist reexamined JD and, again, found no cause for the ocular abnormality and suggested artificial tears for dry eyes. Two months later, he saw an optometrist, who documented constricted visual fields and referred JD to a second ophthalmologist. This consultant suggested possible brain injury and doubted it was psychosomatic in nature. He referred the patient to a neurologist. The neurologist found no organic explanation for his vision loss. He suspected a somatoform disorder and told JD his vision should recover. JD and his wife initially declined the neurologist’s idea of a neuroophthalmology consultation, but eventually agreed. The neuro-ophthalmologist also suspected a functional disturbance as the cause for visual impairment; and he required the patient to stop driving a motor vehicle until his vision improved.
The patient was subsequently referred for psychological evaluation. When initially seen by a psychologist and a family medicine resident, JD was working as a farmhand to make ends meet.
Effects of ammonia exposure
Ammonia is a water-soluble, colorless gas—an alkaloid with a unique odor. In the past, most exposures were related to its use as a fertilizer, as was the case with JD. In recent years, it has also been used to illegally manufacture methamphetamine, which has led to ammonia accidents and increased exposures.1-3
Systems commonly injured are the respiratory tract, ocular system, skin, and gastrointestinal tract (only if ingested).2
Ammonia destroys the mucosal barrier of the respiratory tract, causing loss of cilia, edema, and smooth muscle contraction.3,4 Long-term effects include chronic cough or hoarseness, obstructive or restrictive airway disease, reactive airway disease, or bronchiectasis.1,3
The extent of ocular injury is related to the degree of ammonia exposure. In mild cases, there is eye irritation, increased tear production, a sensation of stinging or burning, and perhaps conjunctivitis or spasmodic winking. The patient may also experience photophobia.1,3,4 In more severe cases, there may be corneal ulcerations, iritis, anterior or posterior synechia, opacification of the cornea, cataracts, glaucoma, atrophy of the retina, or severe pain.1,3 Blindness may occur, temporarily or permanently.4 This complete or partial vision loss is secondary to physical damage that can be seen during an ophthalmologic examination.1,4
Skin injuries can range from a mild erythematous rash to a full thickness burn with bullae and even denudation.1 Long-term effects include scarring or dermatitis.3
Our patient had respiratory and skin symptoms that fit with classic ammonia exposure (respiratory distress requiring intubation, rash). His initial blindness was consistent with ammonia exposure; however, his subsequent peripheral loss was inconsistent with known reaction to ammonia.
Causes of acute visual loss
Vision loss can be caused by injury to the media of the eye (cornea, lens, etc), the retina, or the neural visual pathways. It may also have a psychogenic component.5 Media-related causes of acute vision loss include keratitis or uveitis, edema of the cornea, blood in the anterior chamber (hyphema), disturbance of the lens, or hemorrhage into the vitreous.5,6 Retinal causes include occlusion of the central retinal artery or vein, detachment of the retina, or acute maculopathy.5-8 Neurologic causes include injury to the optic nerve itself (normally monocular) or defects in the chiasmal or retrochiasmal regions (causing partial loss in both eyes).5,9 If all of the above possibilities have been ruled out, consider psychogenic contribution to visual loss.5 Often this diagnosis is called “functional vision loss,” which can include feigning visual loss for secondary gain or subjective blindness as is seen with a somatoform disorder (eg, conversion disorder).
JD had bilateral peripheral vision loss of both the medial and lateral visual fields with macular sparing bilaterally. But he had an otherwise normal physical examination. At this point, the neurologist suspected conversion disorder, while one ophthalmologist thought a neuropathic disorder was responsible.
The neurologist had, early on, recognized that JD was significantly distressed by the accident and encouraged a psychological consultation. With the absence of identifiable ophthalmologic pathology, the patient reluctantly accepted this referral.
The psychologist, aided by family medicine residents, entertained the diagnoses of Post-Traumatic Stress Disorder (PTSD) and somatoform disorders, particularly conversion. PTSD is unique among psychiatric diagnoses in that a patient must meet all 6 DSM V criteria:10
• exposure to a traumatic event involving actual or threatened death or serious injury
• recollections, dreams, or hallucinations in which the trauma is re-experienced
• avoidance of stimuli associated with the trauma
• persistent symptoms of increased arousal (eg, irritability, agitation)
• symptoms and behavior that last for longer than one month
• distress that is clinically significant.
He met the criteria for a PTSD diagnosis and likely would benefit from treatment for it. However, sensory loss related to PTSD alone would be unusual, perhaps as unusual as peripheral vision loss secondary to ammonia exposure. Other factors needed to be explored.
Conversion disorders consist of disorders of movement, such as seizures or paralysis, or disorders of sensations, such as numbness or blindness. These may be episodic or sustained and have acute or chronic onset.11
Psychological factors are judged to be associated with the symptom or deficit because conflicts or other stressors precede the initiation or exacerbation of the symptom or deficit. This was possible in JD, but a degree of uncertainty lingered because he did not exhibit behavior typically seen with factitious disorder, and performance anxiety could conceivably account for the outcome on his vision tests.
In general, he could meet the criteria for conversion disorder, but questions remained. The biggest question is whether the accident resulting in PTSD is the cause of the psychological stress, or is the peripheral vision loss the source of the psychological stress, which would mean it is not a conversion disorder?
Treatment of visual defects
As is the case in many disorders, a definitive diagnosis of the cause of vision loss is not necessary to begin treatment. When you suspect a somatoform disorder including conversion, start therapy and treat the symptoms as “real.”12 Tell the patient that no specific treatment will completely resolve the symptoms, but that it can help.13 Whether the primary cause is neurologic or conversion based, there is often some spontaneous recovery of vision that occurs between 2 weeks and 3 or more months.14 Peripheral field defects have a guarded prognosis, although an extensive rehabilitation program may improve the vision fields somewhat.15-18
Conversion disorders effectively respond to cognitive behavioral therapy (CBT) including gradual exposure to anxiety triggers.19 Rehabilitation for neurologic damage based on remodeling of pathways responds to a similar gradual exercise or exposure to the lost function. Since these interventions are similar processes, a definitive diagnosis was unnecessary in JD’s case. A proprietary visual rehabilitation therapy program is available17 that exposes the patient to visual field activity that requires a cognitive reaction.15 This treatment facilitates recovery even into the sixth month of therapy.16 However, the cost of the software is approximately $6000 and is not yet covered by insurance.15
JD could not afford the commercially available programmed therapy. Therefore, we introduced an alternative treatment plan to challenge the transitional zone. With this plan, JD would play video games for 30 minutes at least twice a week, and preferably daily. He sat close enough to the television so that the transitional zone was approximately 1 to 2 inches from the peripheral portion of the television screen. The game was an action-packed video in which the peripheral portion of the screen was important (such as in first-person shooter games). He was to continue staring at the center of the screen during play in order to truly exercise the peripheral vision. Every day, he reassessed where the transitional zone was located and adjusted his seating accordingly. JD practiced this at least once a day and found that he had to sit closer and closer to the television screen to allow the transitional zone to remain in the screen’s periphery.
The patient, being very motivated, also developed treatments that worked well. He would stare at a blank white wall approximately 2 feet away, focusing on one location. One of his family members would take a laser pointer and start very far away, then slowly move the light closer to the patient’s center of vision. JD would tell his family member when he could see the light and they would move on to a different portion of his visual field.
After 3 months, we retested JD’s vision, which showed great improvement. JD felt he had significant improvement in his vision. The ophthalmologist retested JD about 2 months later and he passed a visual test well enough to obtain a modified driving license so he could return to his work as an agronomist.
Treatment of PTSD
Therapy for PTSD is complex and best approached with a long-term, multifaceted plan.20 Both pharmacotherapy and psychotherapy can be considered for initial treatment; however, no placebo-controlled randomized trials comparing the 2 modalities have been conducted. Combination therapy can also be employed.
Drug therapy, particularly selective serotonin reuptake inhibitors (SSRIs), has been shown to be generally effective in ameliorating positive symptoms associated with PTSD, such as nightmares and flashbacks. But they are less effective at treating negative symptoms such as withdrawal and avoidance.21,22
There is no clinical evidence to support the use of anxiolytics such as benzodiazepines in treating PTSD-specific symptoms. One small study did find a significant reduction in anxiety with alprazolam compared with placebo; however, the response was modest, and specific PTSD symptoms were unchanged.2 Given the high prevalence of comorbid substance abuse in PTSD, benzodiazepines are best avoided since evidence for their effectiveness is lacking.23
Both CBT and eye movement desensitization and reprocessing (EMDR) can be effective therapy for PTSD.24 Both modalities center on desensitization through exposure to traumatic recollections and symptom triggers.
The CBT approach we used focused on JD’s phobic reaction to ammonia that prevented his return to work. First, he listened to relaxation CDs to practice deep breathing and relaxation techniques. Once he was familiar with the techniques, he practiced them in the presence of the shed that contained ammonia products, which was a trigger for his anxiety. At first he was only able to approach the shed while using the breathing exercises to calm his anxiety. Over several weeks, he became more comfortable moving closer to the shed, and he eventually stepped into the shed and began staying for longer periods of time. The course of therapy took several months, but by the end of the sessions he was able to perform necessary tasks in the he was able to perform necessary tasks in the shed with only mild anxiety.
He also suffered from persistent troubling nightmares that significantly affected his sleep and led to physical symptoms of headache and vomiting. These, too, were overcome with the CBT approach.25 We instructed him to immediately write down as much as he could recall of a nightmare upon waking from it. During the following day, he re-read the dream and attempted to re-experience it while using the relaxation techniques to temper anxiety. Over several months of therapy, his nightmares lessened and eventually stopped.
On the last day of therapy, JD reported he had 3 job offers and 2 more interviews lined up, and that he was excited about his opportunities. We congratulated him on his visual recovery and applauded him for his hard work.
Discussion
While it is possible that JD spontaneously recovered his vision loss, it’s more likely that treatment can be credited, given that he did not improve in the 6 months prior to treatment and that his condition resolved over the 3-month rehabilitation period.
Research that guides practice must necessarily limit variables, but real-life patients often have multiple variables complicating both diagnosis and treatment. Our patient is a graphic example. He was exposed to anhydrous ammonia with its multiple physiologic sequelae and it was a traumatic event leading to additional sequelae. Furthermore, his inability to perform his job and fulfill social obligations contributed to his impairment.
JD’s referral to a neural-ophthalmologist did not provide a definitive diagnosis. He then followed up on a referral to the local residency clinic, where the family physician/psychologist team treats patients from a biopsychosocial perspective. Although physicians feel most comfortable when they arrive at a specific diagnosis with a specific evidence-based treatment that predicts a good outcome, this case yielded no definitive diagnosis. Instead, the psychologist and family physician relied on general research findings showing that in the context of traumatic injury or illness followed by debilitating anxiety symptoms, desensitization and rehabilitation provide the best chance of improvement. This shift in treatment approach very likely was responsible for the patient’s improvement.
This approach had the added benefit of helping the patient feel empowered. When JD a and family physician, he was frustrated that no explanation had been found for his problem. As a result, he feared that nothing could be done. When told that a rehabilitation and gradual exposure approach would likely help him, even if we were uncertain of the absolute cause, he became an eager participant in his treatment. He also embraced the idea that it wasn’t up to the doctors alone to improve his condition, but that he could be an active participant. In our view, his enthusiastic efforts contributed to the ultimate treatment outcome.
Often family physicians think they should refer these “complicated” patients to other specialists. However, for patients with combined psychological and physiological pathologies, we believe there are no better experts than family physicians. Properly trained family physicians could have treated this patient without the aid of a psychologist. Other patients who can benefit from this type of integrated biopsychosocial rehabilitation include those with chronic fatigue syndrome, chronic low back pain, and debilitating epilepsy.26-28
1. Lessenger JE. Anhydrous ammonia injuries. J Agromedicine. 1996;3:191-203.
2. Souther L, Small-Johnson J, Messing R. A description of agricultural releases of anhydrous ammonia in Minnesota. Chem Health Safety. 2000;7:16-22.
3. Welch A. Exposing the dangers of anhydrous ammonia. Nurse Pract. 2006;31:40-45.
4. Makarovsky I, Markel G, Dushnitsky T, et al. Ammonia—when something smells wrong. Isr Med Assoc J. 2008;10:537-543.
5. Leveque T. Approach to the adult with acute persistent visual loss. In: UpToDate, Trobe J (ed), UpToDate, Waltham, MA, 2013.
6. Morgan A, Hemphill RR. Acute visual change. Emerg Med Clin North Am. 1998;16:825-843,vii.
7. Beran DI, Murphy-Lavoie H. Acute, painless vision loss. J La State Med Soc. 2009;161:214-216, 218-223.
8. Vortmann M, Schneider JI. Acute monocular vision loss. Emerg Med Clin North Am. 2008;26:73-96.
9. Chamberlain MC, Chalmers L. Acute binocular blindness. Can- cer. 2007;109:1851-1854.
10. American Psychiatric Association. Diagnostic and Statistical Manual. 5th ed. Arlington, VA: American Psychiatric Publishing, Inc.; 2013.
11. Aybek S, Kanaan RA, David AS. The neuropsychiatry of conversion disorder. Curr Opin Psychiatry. 2008;21:275-280.
12. Stone J, Vuilleumier P, Friedman JH. Conversion disorder: separating “how” from “why.” Neurology. 2010;74:190-191.
13. Tocchio SL. Treatment of conversion disorder. A clinical and holistic approach. J Psychosoc Nurs Ment Health Serv. 2009; 47:42-49.
14. Mueller I, Gall C, Kasten E, et al. Long-term learning of visual functions in patients after brain damage. Behav Brain Res. 2008;191:32-42.
15. Glisson CC. Capturing the benefit of vision restoration therapy. Curr Opin Ophthalmol. 2006;17:504-508.
16. Marshall RS, Chmayssani M, O’Brien KA, et al. Visual field expansion after visual restoration therapy. Clin Rehabil. 2010;24: 1027-1035.
17. Mueller I, Mast H, Sabel BA. Recovery of visual field defects: a large clinical observational study using vision restoration therapy. Restor Neurol Neurosci. 2007;25:563-572.
18. Romano JG, Schulz P, Kenkel S, et al. Visual field changes after a rehabilitation intervention: vision restoration therapy. J Neurol Sci. 2008;273:70-74.
19. Allen LA, Woolfolk RL. Cognitive behavioral therapy for somatoform disorders. Psychiatr Clin North Am. 2010;33:579-593.
20. Cukor J, Olden M, Lee F, et al. Evidence-based treatments for PTSD, new directions, and special challenges. Ann N Y Acad Sci. 2010;1208:82-89.
21. Meltzer-Brody S, Connor K, Churchill E, et al. Symptom-specific effects of fluoxetine in PTSD. Intl Clin Psychopharmacol. 2000;15:227-231.
22. Stein DJ, Ipser JC, Seedat S. Pharmacotherapy for post traumatic stress disorder. Cochrane Database Syst Rev. 2006(1):CD002795.
23. Braun P, Greenberg D, Dasberg H, et al. Core symptoms of post-traumatic stress disorder unimproved by alprazolam treatment. J Clin Psychiatry. 1990;51:236-238.
24. Bisson J, Andrew M. Psychological treatment of post-traumatic stress disorder. Cochrane Database Syst Rev. 2007(3): CD003388.
25. Aurora RN, Zak RS, Auerbach SH, et al. Best practice guide for the treatment of nightmare disorder in adults. J Clin Sleep Med. 2010;6:389-401.
26. Reid SF, Chalder T, Cleare A, et al. Chronic fatigue syndrome. Clin Evid (Online). 2008 Aug 28;2008:1101.
27. Hall H, McIntosh G. Low back pain (chronic) Clin Evid (Online). 2008 Oct 1;2008:1116.
28. Prevedini A,Presti G,Ragitti E, et al.Acceptance and commitment Therapy (ACT): the foundation of the therapeutic model and an overview of its contribution to the treatment of patients with chronic physical diseases. G Ital Med Lav Ergon. 2011;33(1suppl A):A53-63.
1. Lessenger JE. Anhydrous ammonia injuries. J Agromedicine. 1996;3:191-203.
2. Souther L, Small-Johnson J, Messing R. A description of agricultural releases of anhydrous ammonia in Minnesota. Chem Health Safety. 2000;7:16-22.
3. Welch A. Exposing the dangers of anhydrous ammonia. Nurse Pract. 2006;31:40-45.
4. Makarovsky I, Markel G, Dushnitsky T, et al. Ammonia—when something smells wrong. Isr Med Assoc J. 2008;10:537-543.
5. Leveque T. Approach to the adult with acute persistent visual loss. In: UpToDate, Trobe J (ed), UpToDate, Waltham, MA, 2013.
6. Morgan A, Hemphill RR. Acute visual change. Emerg Med Clin North Am. 1998;16:825-843,vii.
7. Beran DI, Murphy-Lavoie H. Acute, painless vision loss. J La State Med Soc. 2009;161:214-216, 218-223.
8. Vortmann M, Schneider JI. Acute monocular vision loss. Emerg Med Clin North Am. 2008;26:73-96.
9. Chamberlain MC, Chalmers L. Acute binocular blindness. Can- cer. 2007;109:1851-1854.
10. American Psychiatric Association. Diagnostic and Statistical Manual. 5th ed. Arlington, VA: American Psychiatric Publishing, Inc.; 2013.
11. Aybek S, Kanaan RA, David AS. The neuropsychiatry of conversion disorder. Curr Opin Psychiatry. 2008;21:275-280.
12. Stone J, Vuilleumier P, Friedman JH. Conversion disorder: separating “how” from “why.” Neurology. 2010;74:190-191.
13. Tocchio SL. Treatment of conversion disorder. A clinical and holistic approach. J Psychosoc Nurs Ment Health Serv. 2009; 47:42-49.
14. Mueller I, Gall C, Kasten E, et al. Long-term learning of visual functions in patients after brain damage. Behav Brain Res. 2008;191:32-42.
15. Glisson CC. Capturing the benefit of vision restoration therapy. Curr Opin Ophthalmol. 2006;17:504-508.
16. Marshall RS, Chmayssani M, O’Brien KA, et al. Visual field expansion after visual restoration therapy. Clin Rehabil. 2010;24: 1027-1035.
17. Mueller I, Mast H, Sabel BA. Recovery of visual field defects: a large clinical observational study using vision restoration therapy. Restor Neurol Neurosci. 2007;25:563-572.
18. Romano JG, Schulz P, Kenkel S, et al. Visual field changes after a rehabilitation intervention: vision restoration therapy. J Neurol Sci. 2008;273:70-74.
19. Allen LA, Woolfolk RL. Cognitive behavioral therapy for somatoform disorders. Psychiatr Clin North Am. 2010;33:579-593.
20. Cukor J, Olden M, Lee F, et al. Evidence-based treatments for PTSD, new directions, and special challenges. Ann N Y Acad Sci. 2010;1208:82-89.
21. Meltzer-Brody S, Connor K, Churchill E, et al. Symptom-specific effects of fluoxetine in PTSD. Intl Clin Psychopharmacol. 2000;15:227-231.
22. Stein DJ, Ipser JC, Seedat S. Pharmacotherapy for post traumatic stress disorder. Cochrane Database Syst Rev. 2006(1):CD002795.
23. Braun P, Greenberg D, Dasberg H, et al. Core symptoms of post-traumatic stress disorder unimproved by alprazolam treatment. J Clin Psychiatry. 1990;51:236-238.
24. Bisson J, Andrew M. Psychological treatment of post-traumatic stress disorder. Cochrane Database Syst Rev. 2007(3): CD003388.
25. Aurora RN, Zak RS, Auerbach SH, et al. Best practice guide for the treatment of nightmare disorder in adults. J Clin Sleep Med. 2010;6:389-401.
26. Reid SF, Chalder T, Cleare A, et al. Chronic fatigue syndrome. Clin Evid (Online). 2008 Aug 28;2008:1101.
27. Hall H, McIntosh G. Low back pain (chronic) Clin Evid (Online). 2008 Oct 1;2008:1116.
28. Prevedini A,Presti G,Ragitti E, et al.Acceptance and commitment Therapy (ACT): the foundation of the therapeutic model and an overview of its contribution to the treatment of patients with chronic physical diseases. G Ital Med Lav Ergon. 2011;33(1suppl A):A53-63.
When bed bugs bite
• Provide symptomatic relief for bed bug bites with antihistamines or corticosteroids. C
• Advise patients experiencing an infestation to consider the CDC’s recommended integrated pest management program (eg, heat treatment, vacuuming, nonchemical pesticides) to increase the likelihood of successful extermination. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Bed bugs, Cimex spp, are a re-emerging public health problem in the United States. First recorded by the ancient Greeks,1 bed bugs have plagued societies for centuries. In the United States, infestations peaked in intensity in the 1920s and 1930s, then were largely eliminated as a significant concern after World War II, thanks to synthetic, residual pesticides.2 By the mid-1990s, bed bugs were so uncommon that specimens could not be obtained for medical education purposes.3
That has all changed.
Although commonly perceived as disproportionately affecting the underprivileged,4 bed bugs are equal-opportunity pests, infesting the most posh hotels, retailers, and theaters.5,6 According to one news report summarizing data from a national pest control firm, US cities with the highest infestation rates are, in descending order: Cincinnati, Columbus, Chicago, Denver, Detroit, Washington DC, New York, Philadelphia, Dayton, and Baltimore.7 Although bed bugs are not known to transmit infectious diseases, they are responsible for significant dermatitis, allergic reactions, and psychological distress.
Bed bug biology and behavior
The bed bug life cycle has 7 stages. All but the egg stage require blood meals before the arthropod can molt to the next stage. Bed bugs are attracted to their hosts by body heat and exhaled carbon dioxide, and they feed only through the skin. This makes baiting and trapping challenging, although it’s a common extermination strategy for other domestic pests. Also, unlike cockroaches, flies, or other pests, bed bug infestations are not associated with hygienic deficiencies. Improved housekeeping does not significantly affect their populations; bed bugs feed on household inhabitants, not their spilled or improperly stored food. However, clutter does increase their chances of finding refuge.
Interestingly, researchers recently discovered that bed bugs are themselves hosts to the endosymbiotic bacterium, Wolbachia.8,9 This genus is found in many invertebrates and appears to be essential for normal bed bug fertility and reproduction. Targeting the bacteria may inhibit the ability of bed bugs to breed—something we’ll discuss a bit later.
Clinical assessment
Patients with bed bug bites complain about intensely pruritic lesions. These are typically erythematous and indurated and may be hemorrhagic. The pattern of bites is often linear, and 3 bites in a row are common, sometimes referred to as “breakfast, lunch, and dinner.” Patients typically have no recollection of being bitten, as bed bugs feed on sleeping hosts, and their bite is usually painless.
Clues to bed bugs as the source. Scabies mites also cause linear pruritic lesions, but bed bug lesions differ in appearance and distribution. Scabies lesions are subtle, appearing as burrows and excoriations, in contrast to the more prominent erythematous papule seen with bed bugs and other arthropod bites. Scabies tend to occur in skin folds, finger webbing, genitals, and areas where clothing is tight, such as beltlines. In contrast, bed bugs tend to attack easily accessible, exposed areas. Areas covered with loose clothing are less affected, and areas covered by tight clothing are essentially spared. Multiple members of the household are often affected.
Flea bite? Bed bug bites may be virtually indistinguishable from those of other arthropods such as fleas, spiders, or mosquitoes. While the linear 3-bite pattern may suggest bed bug exposure, it is not pathognomonic. Capturing the arthropod or finding evidence of infestation (discussed in a bit) is needed to confirm a bed bug as the source of the bite.
The etiology of pruritic papules is broad. Besides arthropod bites, include conditions such as papular eczema, papular pruritic eruption, and eosinophilic folliculitis in the differential diagnosis.
Potential for complications
As with any break in the skin, secondary infection is a risk, although it is rarely a complication of the bite. If infection occurs, it is more likely due to scratching. Bed bug bites are allergenic, and they have also been implicated in asthma exacerbations and even anaphylaxis.10,11 In severe infestations, anemia from the extensive blood-meals can occur.12
Experimental studies have found that >45 human pathogens—ranging from viruses to methicillin-resistant Staphylococcus aureus to helminths—can survive ingestion by bed bugs, but none have shown pathogens to be transmitted to humans by bed bugs.11 Fortunately, bed bugs do not appear to be competent as vectors, although prospective studies are ongoing.13 In addition to allergic manifestations, bed bug bites have been associated with significant, even incapacitating, psychiatric problems such as anxiety, obsession, and depression to the point of suicide.14
Treating symptoms and cause
Management of bed bugs consists of symptomatic treatment of the bites and elimination of the infestation—treating both patients and their environments.15
Treating patients
Treating bed bug bites mainly involves providing symptomatic relief with antipruritic agents (antihistamines, topical or oral corticosteroids, over-the-counter topical anesthetics).16 When, rarely, a bite becomes infected, antibiotics may be indicated. Address psychological distress associated with an infestation. Counseling with cognitive behavioral therapy is effective most of the time, although some cases may warrant short-term psychopharmacotherapy.
Symptomatic relief will be short-lived, however, without remediation of the underlying infestation. If the bugs remain, the biting will continue.
Treating the environment
Every object and location in which bed bugs may have taken refuge must be treated. The first step in eradicating an infestation is to find it. In light infestations, evidence may be limited. However, they are dirty bugs. Significant amounts of litter, including molted exoskeletons, dark feces, and eggs, are found wherever there is an infestation. These signs of infestation may be found on mattresses or box springs, or in the bottom of bureau drawers and the corners of rooms. Anywhere just out of reach of the vacuum cleaner can harbor their detritus. Some success has been reported using bed bug detectors/monitors.17 Bed bug-sniffing dogs have been trained and employed in both identifying infestations and monitoring the efficacy of eradication interventions.
A number of extermination methods have been used. The most commonly used chemicals are permethrins, the same agents that have proven effective in antimalarial bed net programs. This agent is applied to the environment, not to the patient. Generally, at least 2 applications are required. Although as recently as 1990 no bed bug resistance to permethrin had been reported,18 there is now widespread resistance.19 Efforts at developing new agents are progressing.
Besides resistance, toxicity to humans is a concern. The Centers for Disease Control and Prevention (CDC) has reported both morbidity and mortality from chemical pesticides used in bed bug extermination efforts.20,21
Physical methods have also been applied.
Thermal treatment (heating or steaming to >48°C [120°F] for one hour or freezing to -20°C [-4°F] for one hour) has proven effective.22 Books, clothing, and other small items may be placed in an oven or freezer (as long as specified temperatures are met); steamers are useful for treating furniture and baseboards. If an oven is used, diligent attention must be paid to avoid too high a temperature, which could create a fire hazard. Let patients know that, even at 120°F, some book bindings and slipcovers could be damaged.
Desiccant dusts such as silica gel and diatomaceous earth, applied along the baseboards and the back of bookshelves, have also demonstrated efficacy.23 As with chemical pesticides, it is important to follow directions when using desiccant dusts to minimize potential health hazards.
The CDC recommends a comprehensive, integrated pest management program to control bed bugs. This program includes a number of methods, such as removing clutter and sealing cracks and crevices where bed bugs take refuge, applying heat treatment, vacuuming, using nonchemical pesticides, and cautiously applying effective chemical pesticides.17 An approach such as this is labor- and time-intensive, and can be costly.
Given the inadequacies of current strategies in controlling infestations, new approaches are needed. One such approach may be xenointoxication, in which patients take an oral arthropodicidal agent, making the blood meal toxic to the parasite and decimating the population. Although there are no literature reports of its application to bed bugs, the technique, using ivermectin, has been successfully applied to other ectoparasites, including scabies,24,25 lice,25,26 and the medically important arthropod vectors Triatoma27 and Anopheles.28 The TABLE shows dosing recommendations for 3 of these indications.
In vitro studies demonstrate that Cimex is susceptible to this same class of agents,29 so there is reason for optimism. Future studies will reveal the viability of this approach. Another potential approach to bed bug control is targeting the Wolbachia endosymbionts. Elimination of these bacteria has been associated with a significant decrease in parasite reproduction9; this strategy has also been efficacious in treating human filarial infections.30
TABLE
Xenointoxication with ivermectin has proven effective against several ectoparasite infestations24-28
| Ectoparasite | Condition | Ivermectin dosing |
|---|---|---|
| Sarcoptes scabiei | Scabies | 0.2 mg/kg, single dose |
| Pediculus capitis | Head lice | 0.2 mg/kg every 10 days x 2 doses |
| Pediculus corpora | Body lice | 0.2 mg/kg every 7 days x 3 doses |
In vitro research has shown that bed bugs are also susceptible to this class of antiparasitic drugs.
Preventing infestation
Bed bugs depend largely on humans for their dissemination. They take refuge in or near their host’s bed during the day, and when the bed or other object in which they are hiding is moved, they are transplanted to a new location. They also migrate directly to adjacent apartments, hotel rooms, etc, along plumbing and wiring or through cracks.16 Bed bugs are effective at hiding, and can survive for up to a year without feeding.31 This contributes to the frequent failure of elimination efforts and the presence of bed bugs in hotels, furnished apartments, theaters, shopping centers, airplanes, newly purchased houses, and other places.
Avoiding bites while in an infested facility is difficult, if not impossible. But people can take steps to decrease the likelihood of bringing them home. Although there are no strong evidence-based guidelines on preventing infestation, pest control experts make a number of recommendations,32 which you can pass on to your patients.
Protect luggage when traveling. When staying in hotels, for instance, patients should keep suitcases tightly closed when not in use. Protection is further enhanced by placing suitcases in a sealed plastic bag; “contractor” trash bags available at hardware stores are large and durable. Keeping suitcases in the bathroom rather than the sleeping quarters also decreases the possibility of stowaways, as bed bugs typically shelter within a few feet of their host’s sleeping place.
Immediate laundering of clothes upon returning home from a trip, and storing suitcases outside the living quarters can decrease risk, too.33 There are commercially available suitcase heaters that raise the temperature of the suitcase and its contents to insecticidal levels, but they are fairly cost-prohibitive.
Screen items brought into the home. Used items, especially furniture, may harbor bed bugs. Fumigating used furniture was once common; it is still a good idea before second-hand items are brought into the house. Cardboard boxes in which used items are commonly stored or transported can shelter bed bugs, too.33
Deprive bed bugs of hiding places. Decluttering one’s sleeping quarters decreases the number of places bed bugs can hide. This tactic diminishes the likelihood of an infestation becoming firmly established before being discovered. Intervention early in the course of infestation, when it is limited to a single room, increases the likelihood of successful elimination.33
Mattress and box-spring encasements can prevent bed bug infestations by blocking movement of the bugs into and out of their shelters. If encasements are placed during an infestation, it is important to keep them in place for an extended period, given that bed bugs can survive for up to a year without feeding. Also effective is caulking and sealing molding, joints, and cracks wider than the thickness of a credit card in the room and in furniture.33
Vacuuming is part of the CDC’s recommendation for household pest control. But vacuum cleaners can also transfer bed bugs from infested to uninfested rooms. During an infestation, it’s important to empty vacuum bags immediately. And sharing vacuum cleaners between dwellings is best avoided.
A need for better solutions
Although bed bugs are not competent as vectors for the transmission of infectious diseases, they are responsible for significant dermatitis, allergic reactions, and psychological distress. Treatment of symptoms is effective in the short-term, but current methods of eliminating infestation are cumbersome, toxic, and are seldom completely successful. New strategies are desperately needed. The CDC Web page (http://www.cdc.gov/nceh/ehs/topics/bedbugs.htm) is regularly updated, and is a good source of information as new approaches are developed.
CORRESPONDENCE Mark K. Huntington, MD, PhD, Center for Family Medicine, 1115 East Twentieth Street, Sioux Falls, SD 57105; [email protected]
1. Usinger RI. Monograph of the Cimicidae (Hemiptera-Heteroptera). Vol VII. College Park, Md: Entomological Society of America; 1966.
2. Berg R. Bed bugs: the pesticide dilemma. J Environ Health. 2010;72:32-35.
3. Snetsinger R. Bed bugs & other bugs. In: Hedges S, ed. Mallis’ Handbook of Pest Control. 8th ed. Cleveland, Ohio: GIE Media; 1997:392–424.
4. Eddy C, Jones SC. Bed bugs, public health, and social justice: part 1, a call to action. J Environ Health. 2011;73:8-14.
5. Hurst S, Humphreys M. Bedbugs: not back by popular demand. Dimens Crit Care Nurs. 2011;30:94-96.
6. Anderson A. The decade of bedbugs and fear. Environ Health Insights. 2011;5:53-54.
7. America’s 10 most infested cities. The Daily Beast; August 24, 2010. Available at: http://www.thedailybeast.com/articles/2010/08/24/bedbug-outbreak-which-cities-are-most-infested.html. Accessed August 28, 2011.
8. Hosokawa T, Koga R, Kikuchi Y, et al. Wolbachia as a bacteriocyte-associated nutritional mutualist. Proc Natl Acad Sci U S A. 2010;107:769-774.
9. Sakamoto JM, Rasgon JL. Geographic distribution of Wolbachia infections in Cimex lectularius (Heteroptera: Cimicidae). J Med Entomol. 2006;43:696-700.
10. Abou Gamra EM, el Shayed FA, Morsy TA, et al. The relation between Cimex lectularius antigen and bronchial asthma in Egypt. J Egypt Soc Parasitol. 1991;21:735-746.
11. Delaunay P, Blanc V, Del Giudice P, et al. Bedbugs and infectious diseases. Clin Infect Dis. 2011;52:200-210.
12. Pritchard MJ, Hwang SW. Cases: severe anemia from bedbugs. CMAJ. 2009;181:287-288.
13. Delaunay P. Cimex lectularius or bed bug: vector of infectious agents and pathogenic role. Available at: http://www.clinicaltrials.gov. Identifier: NCT01089465. Accessed November 29, 2011.
14. Rieder E, Hamalian G, Ying P. Psychiatric implications of bedbugs. Presented at: 164th Annual Meeting of the American Psychiatric Association; May 14-18, 2011; Honolulu, Hawaii. Abstract NR01-51.
15. Roos TC, Alam M, Roos S, et al. Pharmacotherapy of ectoparasitic infections. Drugs. 2001;61:1067-1088.
16. Goddard J, deShazo R. Bed bugs (Cimex lectularius) and clinical consequences of their bites. JAMA. 2009;301:1358-1366.
17. Centers for Disease Control and Prevention and Environmental Protection Agency. Joint statement on bed bug control in the United States from the U.S. Centers for Disease Control and Prevention (CDC) and the U.S. Environmental Protection Agency (EPA). Atlanta, Ga: US Department of Health and Human Services; 2010. Available at: http://www.cdc.gov/nceh/ehs/Publications/Bed_Bugs_CDC-EPA_Statement.htm. Accessed June 15, 2012.
18. Axtell RC, Arends JJ. Ecology and management of arthropod pests of poultry. Annu Rev Entomol. 1990;35:101-126.
19. Moore DJ, Miller DM. Field evaluations of insecticide treatment regimens for control of the common bed bug, Cimex lectularius (L.). Pest Manag Sci. 2009;65:332-338.
20. CDC. Acute illnesses associated with insecticides used to control bed bugs—seven states, 2003–2010. MMWR Morb Mortal Wkly Rep. 2011;60:1269-1274.
21. Tawatsin A, Thavara U, Chompoosri J, et al. Insecticide resistance in bedbugs in Thailand and laboratory evaluation of insecticides for the control of Cimex hemipterus and Cimex lectularius (Hemiptera: Cimicidae). J Med Entomol. 2011;48:1023-1030.
22. Benoit JB, Lopez-Martinez G, Teets NM, et al. Responses of the bed bug, Cimex lectularius, to temperature extremes and dehydration: levels of tolerance, rapid cold hardening and expression of heat shock proteins. Med Vet Entomol. 2009;23:418-425.
23. Benoit JB, Phillips SA, Croxall TJ, et al. Addition of alarm pheromone components improves the effectiveness of desiccant dusts against Cimex lectularius. J Med Entomol. 2009;46:572-579.
24. Heukelbach J, Feldmeier H. Scabies. Lancet. 2006;367:1767-1774.
25. Fox LM. Ivermectin: uses and impact 20 years on. Curr Opin Infect Dis. 2006;19:588-593.
26. Foucault C, Ranque S, Badiaga S, et al. Oral ivermectin in the treatment of body lice. J Infect Dis. 2006;193:474-476.
27. Dias JC, Schofield CJ, Machado EM, et al. Ticks, ivermectin, and experimental Chagas disease. Mem Inst Oswaldo Cruz. 2005;100:829-832.
28. Kobylinski KC, Sylla M, Chapman PL, et al. Ivermectin mass drug administration to humans disrupts malaria parasite transmission in Senegalese villages. Am J Trop Med Hyg. 2011;85:3-5.
29. Ostlind DA, Cifelli S, Conroy JA, etal. A novel Cimex lectularius– rodent assay for the detection of systemic ectoparasiticide activity. Southwest Entomol. 2001;26:181-186.
30. Tamarozzi F, Halliday A, Gentil K, et al. Onchocerciasis: the role of Wolbachia bacterial endosymbionts in parasite biology, disease pathogenesis, and treatment. Clin Microbiol Rev. 2011;24:459-468.
31. Kells SA, Hahn J. Prevention and control of bed bugs in residences. Available at: http://www.extension.umn.edu/distribution/housingandclothing/dk1022.html. Accessed November 29, 2011.
32. Got bedbugs? Your hotel might! Available at: http://www.smartertravel.com/travel-advice/avoiding-bedbugs-every-traveler-nightmare.html?id=4726511. Published April 25, 2010. Accessed November 29, 2011.
33. US Environmental Protection Agency. Bed bug information. Available at: http://www.epa.gov/bedbugs/index.html. Accessed November 29, 2011.
• Provide symptomatic relief for bed bug bites with antihistamines or corticosteroids. C
• Advise patients experiencing an infestation to consider the CDC’s recommended integrated pest management program (eg, heat treatment, vacuuming, nonchemical pesticides) to increase the likelihood of successful extermination. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Bed bugs, Cimex spp, are a re-emerging public health problem in the United States. First recorded by the ancient Greeks,1 bed bugs have plagued societies for centuries. In the United States, infestations peaked in intensity in the 1920s and 1930s, then were largely eliminated as a significant concern after World War II, thanks to synthetic, residual pesticides.2 By the mid-1990s, bed bugs were so uncommon that specimens could not be obtained for medical education purposes.3
That has all changed.
Although commonly perceived as disproportionately affecting the underprivileged,4 bed bugs are equal-opportunity pests, infesting the most posh hotels, retailers, and theaters.5,6 According to one news report summarizing data from a national pest control firm, US cities with the highest infestation rates are, in descending order: Cincinnati, Columbus, Chicago, Denver, Detroit, Washington DC, New York, Philadelphia, Dayton, and Baltimore.7 Although bed bugs are not known to transmit infectious diseases, they are responsible for significant dermatitis, allergic reactions, and psychological distress.
Bed bug biology and behavior
The bed bug life cycle has 7 stages. All but the egg stage require blood meals before the arthropod can molt to the next stage. Bed bugs are attracted to their hosts by body heat and exhaled carbon dioxide, and they feed only through the skin. This makes baiting and trapping challenging, although it’s a common extermination strategy for other domestic pests. Also, unlike cockroaches, flies, or other pests, bed bug infestations are not associated with hygienic deficiencies. Improved housekeeping does not significantly affect their populations; bed bugs feed on household inhabitants, not their spilled or improperly stored food. However, clutter does increase their chances of finding refuge.
Interestingly, researchers recently discovered that bed bugs are themselves hosts to the endosymbiotic bacterium, Wolbachia.8,9 This genus is found in many invertebrates and appears to be essential for normal bed bug fertility and reproduction. Targeting the bacteria may inhibit the ability of bed bugs to breed—something we’ll discuss a bit later.
Clinical assessment
Patients with bed bug bites complain about intensely pruritic lesions. These are typically erythematous and indurated and may be hemorrhagic. The pattern of bites is often linear, and 3 bites in a row are common, sometimes referred to as “breakfast, lunch, and dinner.” Patients typically have no recollection of being bitten, as bed bugs feed on sleeping hosts, and their bite is usually painless.
Clues to bed bugs as the source. Scabies mites also cause linear pruritic lesions, but bed bug lesions differ in appearance and distribution. Scabies lesions are subtle, appearing as burrows and excoriations, in contrast to the more prominent erythematous papule seen with bed bugs and other arthropod bites. Scabies tend to occur in skin folds, finger webbing, genitals, and areas where clothing is tight, such as beltlines. In contrast, bed bugs tend to attack easily accessible, exposed areas. Areas covered with loose clothing are less affected, and areas covered by tight clothing are essentially spared. Multiple members of the household are often affected.
Flea bite? Bed bug bites may be virtually indistinguishable from those of other arthropods such as fleas, spiders, or mosquitoes. While the linear 3-bite pattern may suggest bed bug exposure, it is not pathognomonic. Capturing the arthropod or finding evidence of infestation (discussed in a bit) is needed to confirm a bed bug as the source of the bite.
The etiology of pruritic papules is broad. Besides arthropod bites, include conditions such as papular eczema, papular pruritic eruption, and eosinophilic folliculitis in the differential diagnosis.
Potential for complications
As with any break in the skin, secondary infection is a risk, although it is rarely a complication of the bite. If infection occurs, it is more likely due to scratching. Bed bug bites are allergenic, and they have also been implicated in asthma exacerbations and even anaphylaxis.10,11 In severe infestations, anemia from the extensive blood-meals can occur.12
Experimental studies have found that >45 human pathogens—ranging from viruses to methicillin-resistant Staphylococcus aureus to helminths—can survive ingestion by bed bugs, but none have shown pathogens to be transmitted to humans by bed bugs.11 Fortunately, bed bugs do not appear to be competent as vectors, although prospective studies are ongoing.13 In addition to allergic manifestations, bed bug bites have been associated with significant, even incapacitating, psychiatric problems such as anxiety, obsession, and depression to the point of suicide.14
Treating symptoms and cause
Management of bed bugs consists of symptomatic treatment of the bites and elimination of the infestation—treating both patients and their environments.15
Treating patients
Treating bed bug bites mainly involves providing symptomatic relief with antipruritic agents (antihistamines, topical or oral corticosteroids, over-the-counter topical anesthetics).16 When, rarely, a bite becomes infected, antibiotics may be indicated. Address psychological distress associated with an infestation. Counseling with cognitive behavioral therapy is effective most of the time, although some cases may warrant short-term psychopharmacotherapy.
Symptomatic relief will be short-lived, however, without remediation of the underlying infestation. If the bugs remain, the biting will continue.
Treating the environment
Every object and location in which bed bugs may have taken refuge must be treated. The first step in eradicating an infestation is to find it. In light infestations, evidence may be limited. However, they are dirty bugs. Significant amounts of litter, including molted exoskeletons, dark feces, and eggs, are found wherever there is an infestation. These signs of infestation may be found on mattresses or box springs, or in the bottom of bureau drawers and the corners of rooms. Anywhere just out of reach of the vacuum cleaner can harbor their detritus. Some success has been reported using bed bug detectors/monitors.17 Bed bug-sniffing dogs have been trained and employed in both identifying infestations and monitoring the efficacy of eradication interventions.
A number of extermination methods have been used. The most commonly used chemicals are permethrins, the same agents that have proven effective in antimalarial bed net programs. This agent is applied to the environment, not to the patient. Generally, at least 2 applications are required. Although as recently as 1990 no bed bug resistance to permethrin had been reported,18 there is now widespread resistance.19 Efforts at developing new agents are progressing.
Besides resistance, toxicity to humans is a concern. The Centers for Disease Control and Prevention (CDC) has reported both morbidity and mortality from chemical pesticides used in bed bug extermination efforts.20,21
Physical methods have also been applied.
Thermal treatment (heating or steaming to >48°C [120°F] for one hour or freezing to -20°C [-4°F] for one hour) has proven effective.22 Books, clothing, and other small items may be placed in an oven or freezer (as long as specified temperatures are met); steamers are useful for treating furniture and baseboards. If an oven is used, diligent attention must be paid to avoid too high a temperature, which could create a fire hazard. Let patients know that, even at 120°F, some book bindings and slipcovers could be damaged.
Desiccant dusts such as silica gel and diatomaceous earth, applied along the baseboards and the back of bookshelves, have also demonstrated efficacy.23 As with chemical pesticides, it is important to follow directions when using desiccant dusts to minimize potential health hazards.
The CDC recommends a comprehensive, integrated pest management program to control bed bugs. This program includes a number of methods, such as removing clutter and sealing cracks and crevices where bed bugs take refuge, applying heat treatment, vacuuming, using nonchemical pesticides, and cautiously applying effective chemical pesticides.17 An approach such as this is labor- and time-intensive, and can be costly.
Given the inadequacies of current strategies in controlling infestations, new approaches are needed. One such approach may be xenointoxication, in which patients take an oral arthropodicidal agent, making the blood meal toxic to the parasite and decimating the population. Although there are no literature reports of its application to bed bugs, the technique, using ivermectin, has been successfully applied to other ectoparasites, including scabies,24,25 lice,25,26 and the medically important arthropod vectors Triatoma27 and Anopheles.28 The TABLE shows dosing recommendations for 3 of these indications.
In vitro studies demonstrate that Cimex is susceptible to this same class of agents,29 so there is reason for optimism. Future studies will reveal the viability of this approach. Another potential approach to bed bug control is targeting the Wolbachia endosymbionts. Elimination of these bacteria has been associated with a significant decrease in parasite reproduction9; this strategy has also been efficacious in treating human filarial infections.30
TABLE
Xenointoxication with ivermectin has proven effective against several ectoparasite infestations24-28
| Ectoparasite | Condition | Ivermectin dosing |
|---|---|---|
| Sarcoptes scabiei | Scabies | 0.2 mg/kg, single dose |
| Pediculus capitis | Head lice | 0.2 mg/kg every 10 days x 2 doses |
| Pediculus corpora | Body lice | 0.2 mg/kg every 7 days x 3 doses |
In vitro research has shown that bed bugs are also susceptible to this class of antiparasitic drugs.
Preventing infestation
Bed bugs depend largely on humans for their dissemination. They take refuge in or near their host’s bed during the day, and when the bed or other object in which they are hiding is moved, they are transplanted to a new location. They also migrate directly to adjacent apartments, hotel rooms, etc, along plumbing and wiring or through cracks.16 Bed bugs are effective at hiding, and can survive for up to a year without feeding.31 This contributes to the frequent failure of elimination efforts and the presence of bed bugs in hotels, furnished apartments, theaters, shopping centers, airplanes, newly purchased houses, and other places.
Avoiding bites while in an infested facility is difficult, if not impossible. But people can take steps to decrease the likelihood of bringing them home. Although there are no strong evidence-based guidelines on preventing infestation, pest control experts make a number of recommendations,32 which you can pass on to your patients.
Protect luggage when traveling. When staying in hotels, for instance, patients should keep suitcases tightly closed when not in use. Protection is further enhanced by placing suitcases in a sealed plastic bag; “contractor” trash bags available at hardware stores are large and durable. Keeping suitcases in the bathroom rather than the sleeping quarters also decreases the possibility of stowaways, as bed bugs typically shelter within a few feet of their host’s sleeping place.
Immediate laundering of clothes upon returning home from a trip, and storing suitcases outside the living quarters can decrease risk, too.33 There are commercially available suitcase heaters that raise the temperature of the suitcase and its contents to insecticidal levels, but they are fairly cost-prohibitive.
Screen items brought into the home. Used items, especially furniture, may harbor bed bugs. Fumigating used furniture was once common; it is still a good idea before second-hand items are brought into the house. Cardboard boxes in which used items are commonly stored or transported can shelter bed bugs, too.33
Deprive bed bugs of hiding places. Decluttering one’s sleeping quarters decreases the number of places bed bugs can hide. This tactic diminishes the likelihood of an infestation becoming firmly established before being discovered. Intervention early in the course of infestation, when it is limited to a single room, increases the likelihood of successful elimination.33
Mattress and box-spring encasements can prevent bed bug infestations by blocking movement of the bugs into and out of their shelters. If encasements are placed during an infestation, it is important to keep them in place for an extended period, given that bed bugs can survive for up to a year without feeding. Also effective is caulking and sealing molding, joints, and cracks wider than the thickness of a credit card in the room and in furniture.33
Vacuuming is part of the CDC’s recommendation for household pest control. But vacuum cleaners can also transfer bed bugs from infested to uninfested rooms. During an infestation, it’s important to empty vacuum bags immediately. And sharing vacuum cleaners between dwellings is best avoided.
A need for better solutions
Although bed bugs are not competent as vectors for the transmission of infectious diseases, they are responsible for significant dermatitis, allergic reactions, and psychological distress. Treatment of symptoms is effective in the short-term, but current methods of eliminating infestation are cumbersome, toxic, and are seldom completely successful. New strategies are desperately needed. The CDC Web page (http://www.cdc.gov/nceh/ehs/topics/bedbugs.htm) is regularly updated, and is a good source of information as new approaches are developed.
CORRESPONDENCE Mark K. Huntington, MD, PhD, Center for Family Medicine, 1115 East Twentieth Street, Sioux Falls, SD 57105; [email protected]
• Provide symptomatic relief for bed bug bites with antihistamines or corticosteroids. C
• Advise patients experiencing an infestation to consider the CDC’s recommended integrated pest management program (eg, heat treatment, vacuuming, nonchemical pesticides) to increase the likelihood of successful extermination. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Bed bugs, Cimex spp, are a re-emerging public health problem in the United States. First recorded by the ancient Greeks,1 bed bugs have plagued societies for centuries. In the United States, infestations peaked in intensity in the 1920s and 1930s, then were largely eliminated as a significant concern after World War II, thanks to synthetic, residual pesticides.2 By the mid-1990s, bed bugs were so uncommon that specimens could not be obtained for medical education purposes.3
That has all changed.
Although commonly perceived as disproportionately affecting the underprivileged,4 bed bugs are equal-opportunity pests, infesting the most posh hotels, retailers, and theaters.5,6 According to one news report summarizing data from a national pest control firm, US cities with the highest infestation rates are, in descending order: Cincinnati, Columbus, Chicago, Denver, Detroit, Washington DC, New York, Philadelphia, Dayton, and Baltimore.7 Although bed bugs are not known to transmit infectious diseases, they are responsible for significant dermatitis, allergic reactions, and psychological distress.
Bed bug biology and behavior
The bed bug life cycle has 7 stages. All but the egg stage require blood meals before the arthropod can molt to the next stage. Bed bugs are attracted to their hosts by body heat and exhaled carbon dioxide, and they feed only through the skin. This makes baiting and trapping challenging, although it’s a common extermination strategy for other domestic pests. Also, unlike cockroaches, flies, or other pests, bed bug infestations are not associated with hygienic deficiencies. Improved housekeeping does not significantly affect their populations; bed bugs feed on household inhabitants, not their spilled or improperly stored food. However, clutter does increase their chances of finding refuge.
Interestingly, researchers recently discovered that bed bugs are themselves hosts to the endosymbiotic bacterium, Wolbachia.8,9 This genus is found in many invertebrates and appears to be essential for normal bed bug fertility and reproduction. Targeting the bacteria may inhibit the ability of bed bugs to breed—something we’ll discuss a bit later.
Clinical assessment
Patients with bed bug bites complain about intensely pruritic lesions. These are typically erythematous and indurated and may be hemorrhagic. The pattern of bites is often linear, and 3 bites in a row are common, sometimes referred to as “breakfast, lunch, and dinner.” Patients typically have no recollection of being bitten, as bed bugs feed on sleeping hosts, and their bite is usually painless.
Clues to bed bugs as the source. Scabies mites also cause linear pruritic lesions, but bed bug lesions differ in appearance and distribution. Scabies lesions are subtle, appearing as burrows and excoriations, in contrast to the more prominent erythematous papule seen with bed bugs and other arthropod bites. Scabies tend to occur in skin folds, finger webbing, genitals, and areas where clothing is tight, such as beltlines. In contrast, bed bugs tend to attack easily accessible, exposed areas. Areas covered with loose clothing are less affected, and areas covered by tight clothing are essentially spared. Multiple members of the household are often affected.
Flea bite? Bed bug bites may be virtually indistinguishable from those of other arthropods such as fleas, spiders, or mosquitoes. While the linear 3-bite pattern may suggest bed bug exposure, it is not pathognomonic. Capturing the arthropod or finding evidence of infestation (discussed in a bit) is needed to confirm a bed bug as the source of the bite.
The etiology of pruritic papules is broad. Besides arthropod bites, include conditions such as papular eczema, papular pruritic eruption, and eosinophilic folliculitis in the differential diagnosis.
Potential for complications
As with any break in the skin, secondary infection is a risk, although it is rarely a complication of the bite. If infection occurs, it is more likely due to scratching. Bed bug bites are allergenic, and they have also been implicated in asthma exacerbations and even anaphylaxis.10,11 In severe infestations, anemia from the extensive blood-meals can occur.12
Experimental studies have found that >45 human pathogens—ranging from viruses to methicillin-resistant Staphylococcus aureus to helminths—can survive ingestion by bed bugs, but none have shown pathogens to be transmitted to humans by bed bugs.11 Fortunately, bed bugs do not appear to be competent as vectors, although prospective studies are ongoing.13 In addition to allergic manifestations, bed bug bites have been associated with significant, even incapacitating, psychiatric problems such as anxiety, obsession, and depression to the point of suicide.14
Treating symptoms and cause
Management of bed bugs consists of symptomatic treatment of the bites and elimination of the infestation—treating both patients and their environments.15
Treating patients
Treating bed bug bites mainly involves providing symptomatic relief with antipruritic agents (antihistamines, topical or oral corticosteroids, over-the-counter topical anesthetics).16 When, rarely, a bite becomes infected, antibiotics may be indicated. Address psychological distress associated with an infestation. Counseling with cognitive behavioral therapy is effective most of the time, although some cases may warrant short-term psychopharmacotherapy.
Symptomatic relief will be short-lived, however, without remediation of the underlying infestation. If the bugs remain, the biting will continue.
Treating the environment
Every object and location in which bed bugs may have taken refuge must be treated. The first step in eradicating an infestation is to find it. In light infestations, evidence may be limited. However, they are dirty bugs. Significant amounts of litter, including molted exoskeletons, dark feces, and eggs, are found wherever there is an infestation. These signs of infestation may be found on mattresses or box springs, or in the bottom of bureau drawers and the corners of rooms. Anywhere just out of reach of the vacuum cleaner can harbor their detritus. Some success has been reported using bed bug detectors/monitors.17 Bed bug-sniffing dogs have been trained and employed in both identifying infestations and monitoring the efficacy of eradication interventions.
A number of extermination methods have been used. The most commonly used chemicals are permethrins, the same agents that have proven effective in antimalarial bed net programs. This agent is applied to the environment, not to the patient. Generally, at least 2 applications are required. Although as recently as 1990 no bed bug resistance to permethrin had been reported,18 there is now widespread resistance.19 Efforts at developing new agents are progressing.
Besides resistance, toxicity to humans is a concern. The Centers for Disease Control and Prevention (CDC) has reported both morbidity and mortality from chemical pesticides used in bed bug extermination efforts.20,21
Physical methods have also been applied.
Thermal treatment (heating or steaming to >48°C [120°F] for one hour or freezing to -20°C [-4°F] for one hour) has proven effective.22 Books, clothing, and other small items may be placed in an oven or freezer (as long as specified temperatures are met); steamers are useful for treating furniture and baseboards. If an oven is used, diligent attention must be paid to avoid too high a temperature, which could create a fire hazard. Let patients know that, even at 120°F, some book bindings and slipcovers could be damaged.
Desiccant dusts such as silica gel and diatomaceous earth, applied along the baseboards and the back of bookshelves, have also demonstrated efficacy.23 As with chemical pesticides, it is important to follow directions when using desiccant dusts to minimize potential health hazards.
The CDC recommends a comprehensive, integrated pest management program to control bed bugs. This program includes a number of methods, such as removing clutter and sealing cracks and crevices where bed bugs take refuge, applying heat treatment, vacuuming, using nonchemical pesticides, and cautiously applying effective chemical pesticides.17 An approach such as this is labor- and time-intensive, and can be costly.
Given the inadequacies of current strategies in controlling infestations, new approaches are needed. One such approach may be xenointoxication, in which patients take an oral arthropodicidal agent, making the blood meal toxic to the parasite and decimating the population. Although there are no literature reports of its application to bed bugs, the technique, using ivermectin, has been successfully applied to other ectoparasites, including scabies,24,25 lice,25,26 and the medically important arthropod vectors Triatoma27 and Anopheles.28 The TABLE shows dosing recommendations for 3 of these indications.
In vitro studies demonstrate that Cimex is susceptible to this same class of agents,29 so there is reason for optimism. Future studies will reveal the viability of this approach. Another potential approach to bed bug control is targeting the Wolbachia endosymbionts. Elimination of these bacteria has been associated with a significant decrease in parasite reproduction9; this strategy has also been efficacious in treating human filarial infections.30
TABLE
Xenointoxication with ivermectin has proven effective against several ectoparasite infestations24-28
| Ectoparasite | Condition | Ivermectin dosing |
|---|---|---|
| Sarcoptes scabiei | Scabies | 0.2 mg/kg, single dose |
| Pediculus capitis | Head lice | 0.2 mg/kg every 10 days x 2 doses |
| Pediculus corpora | Body lice | 0.2 mg/kg every 7 days x 3 doses |
In vitro research has shown that bed bugs are also susceptible to this class of antiparasitic drugs.
Preventing infestation
Bed bugs depend largely on humans for their dissemination. They take refuge in or near their host’s bed during the day, and when the bed or other object in which they are hiding is moved, they are transplanted to a new location. They also migrate directly to adjacent apartments, hotel rooms, etc, along plumbing and wiring or through cracks.16 Bed bugs are effective at hiding, and can survive for up to a year without feeding.31 This contributes to the frequent failure of elimination efforts and the presence of bed bugs in hotels, furnished apartments, theaters, shopping centers, airplanes, newly purchased houses, and other places.
Avoiding bites while in an infested facility is difficult, if not impossible. But people can take steps to decrease the likelihood of bringing them home. Although there are no strong evidence-based guidelines on preventing infestation, pest control experts make a number of recommendations,32 which you can pass on to your patients.
Protect luggage when traveling. When staying in hotels, for instance, patients should keep suitcases tightly closed when not in use. Protection is further enhanced by placing suitcases in a sealed plastic bag; “contractor” trash bags available at hardware stores are large and durable. Keeping suitcases in the bathroom rather than the sleeping quarters also decreases the possibility of stowaways, as bed bugs typically shelter within a few feet of their host’s sleeping place.
Immediate laundering of clothes upon returning home from a trip, and storing suitcases outside the living quarters can decrease risk, too.33 There are commercially available suitcase heaters that raise the temperature of the suitcase and its contents to insecticidal levels, but they are fairly cost-prohibitive.
Screen items brought into the home. Used items, especially furniture, may harbor bed bugs. Fumigating used furniture was once common; it is still a good idea before second-hand items are brought into the house. Cardboard boxes in which used items are commonly stored or transported can shelter bed bugs, too.33
Deprive bed bugs of hiding places. Decluttering one’s sleeping quarters decreases the number of places bed bugs can hide. This tactic diminishes the likelihood of an infestation becoming firmly established before being discovered. Intervention early in the course of infestation, when it is limited to a single room, increases the likelihood of successful elimination.33
Mattress and box-spring encasements can prevent bed bug infestations by blocking movement of the bugs into and out of their shelters. If encasements are placed during an infestation, it is important to keep them in place for an extended period, given that bed bugs can survive for up to a year without feeding. Also effective is caulking and sealing molding, joints, and cracks wider than the thickness of a credit card in the room and in furniture.33
Vacuuming is part of the CDC’s recommendation for household pest control. But vacuum cleaners can also transfer bed bugs from infested to uninfested rooms. During an infestation, it’s important to empty vacuum bags immediately. And sharing vacuum cleaners between dwellings is best avoided.
A need for better solutions
Although bed bugs are not competent as vectors for the transmission of infectious diseases, they are responsible for significant dermatitis, allergic reactions, and psychological distress. Treatment of symptoms is effective in the short-term, but current methods of eliminating infestation are cumbersome, toxic, and are seldom completely successful. New strategies are desperately needed. The CDC Web page (http://www.cdc.gov/nceh/ehs/topics/bedbugs.htm) is regularly updated, and is a good source of information as new approaches are developed.
CORRESPONDENCE Mark K. Huntington, MD, PhD, Center for Family Medicine, 1115 East Twentieth Street, Sioux Falls, SD 57105; [email protected]
1. Usinger RI. Monograph of the Cimicidae (Hemiptera-Heteroptera). Vol VII. College Park, Md: Entomological Society of America; 1966.
2. Berg R. Bed bugs: the pesticide dilemma. J Environ Health. 2010;72:32-35.
3. Snetsinger R. Bed bugs & other bugs. In: Hedges S, ed. Mallis’ Handbook of Pest Control. 8th ed. Cleveland, Ohio: GIE Media; 1997:392–424.
4. Eddy C, Jones SC. Bed bugs, public health, and social justice: part 1, a call to action. J Environ Health. 2011;73:8-14.
5. Hurst S, Humphreys M. Bedbugs: not back by popular demand. Dimens Crit Care Nurs. 2011;30:94-96.
6. Anderson A. The decade of bedbugs and fear. Environ Health Insights. 2011;5:53-54.
7. America’s 10 most infested cities. The Daily Beast; August 24, 2010. Available at: http://www.thedailybeast.com/articles/2010/08/24/bedbug-outbreak-which-cities-are-most-infested.html. Accessed August 28, 2011.
8. Hosokawa T, Koga R, Kikuchi Y, et al. Wolbachia as a bacteriocyte-associated nutritional mutualist. Proc Natl Acad Sci U S A. 2010;107:769-774.
9. Sakamoto JM, Rasgon JL. Geographic distribution of Wolbachia infections in Cimex lectularius (Heteroptera: Cimicidae). J Med Entomol. 2006;43:696-700.
10. Abou Gamra EM, el Shayed FA, Morsy TA, et al. The relation between Cimex lectularius antigen and bronchial asthma in Egypt. J Egypt Soc Parasitol. 1991;21:735-746.
11. Delaunay P, Blanc V, Del Giudice P, et al. Bedbugs and infectious diseases. Clin Infect Dis. 2011;52:200-210.
12. Pritchard MJ, Hwang SW. Cases: severe anemia from bedbugs. CMAJ. 2009;181:287-288.
13. Delaunay P. Cimex lectularius or bed bug: vector of infectious agents and pathogenic role. Available at: http://www.clinicaltrials.gov. Identifier: NCT01089465. Accessed November 29, 2011.
14. Rieder E, Hamalian G, Ying P. Psychiatric implications of bedbugs. Presented at: 164th Annual Meeting of the American Psychiatric Association; May 14-18, 2011; Honolulu, Hawaii. Abstract NR01-51.
15. Roos TC, Alam M, Roos S, et al. Pharmacotherapy of ectoparasitic infections. Drugs. 2001;61:1067-1088.
16. Goddard J, deShazo R. Bed bugs (Cimex lectularius) and clinical consequences of their bites. JAMA. 2009;301:1358-1366.
17. Centers for Disease Control and Prevention and Environmental Protection Agency. Joint statement on bed bug control in the United States from the U.S. Centers for Disease Control and Prevention (CDC) and the U.S. Environmental Protection Agency (EPA). Atlanta, Ga: US Department of Health and Human Services; 2010. Available at: http://www.cdc.gov/nceh/ehs/Publications/Bed_Bugs_CDC-EPA_Statement.htm. Accessed June 15, 2012.
18. Axtell RC, Arends JJ. Ecology and management of arthropod pests of poultry. Annu Rev Entomol. 1990;35:101-126.
19. Moore DJ, Miller DM. Field evaluations of insecticide treatment regimens for control of the common bed bug, Cimex lectularius (L.). Pest Manag Sci. 2009;65:332-338.
20. CDC. Acute illnesses associated with insecticides used to control bed bugs—seven states, 2003–2010. MMWR Morb Mortal Wkly Rep. 2011;60:1269-1274.
21. Tawatsin A, Thavara U, Chompoosri J, et al. Insecticide resistance in bedbugs in Thailand and laboratory evaluation of insecticides for the control of Cimex hemipterus and Cimex lectularius (Hemiptera: Cimicidae). J Med Entomol. 2011;48:1023-1030.
22. Benoit JB, Lopez-Martinez G, Teets NM, et al. Responses of the bed bug, Cimex lectularius, to temperature extremes and dehydration: levels of tolerance, rapid cold hardening and expression of heat shock proteins. Med Vet Entomol. 2009;23:418-425.
23. Benoit JB, Phillips SA, Croxall TJ, et al. Addition of alarm pheromone components improves the effectiveness of desiccant dusts against Cimex lectularius. J Med Entomol. 2009;46:572-579.
24. Heukelbach J, Feldmeier H. Scabies. Lancet. 2006;367:1767-1774.
25. Fox LM. Ivermectin: uses and impact 20 years on. Curr Opin Infect Dis. 2006;19:588-593.
26. Foucault C, Ranque S, Badiaga S, et al. Oral ivermectin in the treatment of body lice. J Infect Dis. 2006;193:474-476.
27. Dias JC, Schofield CJ, Machado EM, et al. Ticks, ivermectin, and experimental Chagas disease. Mem Inst Oswaldo Cruz. 2005;100:829-832.
28. Kobylinski KC, Sylla M, Chapman PL, et al. Ivermectin mass drug administration to humans disrupts malaria parasite transmission in Senegalese villages. Am J Trop Med Hyg. 2011;85:3-5.
29. Ostlind DA, Cifelli S, Conroy JA, etal. A novel Cimex lectularius– rodent assay for the detection of systemic ectoparasiticide activity. Southwest Entomol. 2001;26:181-186.
30. Tamarozzi F, Halliday A, Gentil K, et al. Onchocerciasis: the role of Wolbachia bacterial endosymbionts in parasite biology, disease pathogenesis, and treatment. Clin Microbiol Rev. 2011;24:459-468.
31. Kells SA, Hahn J. Prevention and control of bed bugs in residences. Available at: http://www.extension.umn.edu/distribution/housingandclothing/dk1022.html. Accessed November 29, 2011.
32. Got bedbugs? Your hotel might! Available at: http://www.smartertravel.com/travel-advice/avoiding-bedbugs-every-traveler-nightmare.html?id=4726511. Published April 25, 2010. Accessed November 29, 2011.
33. US Environmental Protection Agency. Bed bug information. Available at: http://www.epa.gov/bedbugs/index.html. Accessed November 29, 2011.
1. Usinger RI. Monograph of the Cimicidae (Hemiptera-Heteroptera). Vol VII. College Park, Md: Entomological Society of America; 1966.
2. Berg R. Bed bugs: the pesticide dilemma. J Environ Health. 2010;72:32-35.
3. Snetsinger R. Bed bugs & other bugs. In: Hedges S, ed. Mallis’ Handbook of Pest Control. 8th ed. Cleveland, Ohio: GIE Media; 1997:392–424.
4. Eddy C, Jones SC. Bed bugs, public health, and social justice: part 1, a call to action. J Environ Health. 2011;73:8-14.
5. Hurst S, Humphreys M. Bedbugs: not back by popular demand. Dimens Crit Care Nurs. 2011;30:94-96.
6. Anderson A. The decade of bedbugs and fear. Environ Health Insights. 2011;5:53-54.
7. America’s 10 most infested cities. The Daily Beast; August 24, 2010. Available at: http://www.thedailybeast.com/articles/2010/08/24/bedbug-outbreak-which-cities-are-most-infested.html. Accessed August 28, 2011.
8. Hosokawa T, Koga R, Kikuchi Y, et al. Wolbachia as a bacteriocyte-associated nutritional mutualist. Proc Natl Acad Sci U S A. 2010;107:769-774.
9. Sakamoto JM, Rasgon JL. Geographic distribution of Wolbachia infections in Cimex lectularius (Heteroptera: Cimicidae). J Med Entomol. 2006;43:696-700.
10. Abou Gamra EM, el Shayed FA, Morsy TA, et al. The relation between Cimex lectularius antigen and bronchial asthma in Egypt. J Egypt Soc Parasitol. 1991;21:735-746.
11. Delaunay P, Blanc V, Del Giudice P, et al. Bedbugs and infectious diseases. Clin Infect Dis. 2011;52:200-210.
12. Pritchard MJ, Hwang SW. Cases: severe anemia from bedbugs. CMAJ. 2009;181:287-288.
13. Delaunay P. Cimex lectularius or bed bug: vector of infectious agents and pathogenic role. Available at: http://www.clinicaltrials.gov. Identifier: NCT01089465. Accessed November 29, 2011.
14. Rieder E, Hamalian G, Ying P. Psychiatric implications of bedbugs. Presented at: 164th Annual Meeting of the American Psychiatric Association; May 14-18, 2011; Honolulu, Hawaii. Abstract NR01-51.
15. Roos TC, Alam M, Roos S, et al. Pharmacotherapy of ectoparasitic infections. Drugs. 2001;61:1067-1088.
16. Goddard J, deShazo R. Bed bugs (Cimex lectularius) and clinical consequences of their bites. JAMA. 2009;301:1358-1366.
17. Centers for Disease Control and Prevention and Environmental Protection Agency. Joint statement on bed bug control in the United States from the U.S. Centers for Disease Control and Prevention (CDC) and the U.S. Environmental Protection Agency (EPA). Atlanta, Ga: US Department of Health and Human Services; 2010. Available at: http://www.cdc.gov/nceh/ehs/Publications/Bed_Bugs_CDC-EPA_Statement.htm. Accessed June 15, 2012.
18. Axtell RC, Arends JJ. Ecology and management of arthropod pests of poultry. Annu Rev Entomol. 1990;35:101-126.
19. Moore DJ, Miller DM. Field evaluations of insecticide treatment regimens for control of the common bed bug, Cimex lectularius (L.). Pest Manag Sci. 2009;65:332-338.
20. CDC. Acute illnesses associated with insecticides used to control bed bugs—seven states, 2003–2010. MMWR Morb Mortal Wkly Rep. 2011;60:1269-1274.
21. Tawatsin A, Thavara U, Chompoosri J, et al. Insecticide resistance in bedbugs in Thailand and laboratory evaluation of insecticides for the control of Cimex hemipterus and Cimex lectularius (Hemiptera: Cimicidae). J Med Entomol. 2011;48:1023-1030.
22. Benoit JB, Lopez-Martinez G, Teets NM, et al. Responses of the bed bug, Cimex lectularius, to temperature extremes and dehydration: levels of tolerance, rapid cold hardening and expression of heat shock proteins. Med Vet Entomol. 2009;23:418-425.
23. Benoit JB, Phillips SA, Croxall TJ, et al. Addition of alarm pheromone components improves the effectiveness of desiccant dusts against Cimex lectularius. J Med Entomol. 2009;46:572-579.
24. Heukelbach J, Feldmeier H. Scabies. Lancet. 2006;367:1767-1774.
25. Fox LM. Ivermectin: uses and impact 20 years on. Curr Opin Infect Dis. 2006;19:588-593.
26. Foucault C, Ranque S, Badiaga S, et al. Oral ivermectin in the treatment of body lice. J Infect Dis. 2006;193:474-476.
27. Dias JC, Schofield CJ, Machado EM, et al. Ticks, ivermectin, and experimental Chagas disease. Mem Inst Oswaldo Cruz. 2005;100:829-832.
28. Kobylinski KC, Sylla M, Chapman PL, et al. Ivermectin mass drug administration to humans disrupts malaria parasite transmission in Senegalese villages. Am J Trop Med Hyg. 2011;85:3-5.
29. Ostlind DA, Cifelli S, Conroy JA, etal. A novel Cimex lectularius– rodent assay for the detection of systemic ectoparasiticide activity. Southwest Entomol. 2001;26:181-186.
30. Tamarozzi F, Halliday A, Gentil K, et al. Onchocerciasis: the role of Wolbachia bacterial endosymbionts in parasite biology, disease pathogenesis, and treatment. Clin Microbiol Rev. 2011;24:459-468.
31. Kells SA, Hahn J. Prevention and control of bed bugs in residences. Available at: http://www.extension.umn.edu/distribution/housingandclothing/dk1022.html. Accessed November 29, 2011.
32. Got bedbugs? Your hotel might! Available at: http://www.smartertravel.com/travel-advice/avoiding-bedbugs-every-traveler-nightmare.html?id=4726511. Published April 25, 2010. Accessed November 29, 2011.
33. US Environmental Protection Agency. Bed bug information. Available at: http://www.epa.gov/bedbugs/index.html. Accessed November 29, 2011.
Infertility: Help for couples starts with you
• Evaluate the fallopian tubes and their patency when menstruation is normal. Also, consider arranging for a hysterosalpingogram. B
• Suspect polycystic ovarian syndrome when adiposity, acne, and hirsutism with menstrual irregularity are factors. B
• Suspect androgen deficiency when a man’s arm span is >2 cm longer than his height, or when he has experienced a loss of pubic, axillary, or facial hair. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
During an annual visit, a patient confides in you that she and her husband have been trying to get pregnant for a year, but haven’t had any success. She tells you that she’s starting to get worried.
How do you advise her? What are your next steps?
The approach to evaluating infertility complaints is usually straightforward and can lead to a positive outcome. Not surprisingly, the dialogue often begins with you, the family physician. Your attention to clues in each partner’s history can do much to get to the heart of the problem. And even if a couple requires a specialty referral, it’s best to be familiar with the more extensive evaluation and management options they’ll encounter to help them anticipate likely discussions in their consultations. In this article, we review the best evidence for the care of your patients who want to conceive.
Who’s affected?
Infertility difficulties may be attributable to one or both partners, may be multifactorial, or may be unexplained (TABLE).
In women, infertility is the inability to conceive after 1 year of unprotected regular intercourse in those younger than 35 years, and after 6 months in those 35 years and older.1 Fecundability is the probability of achieving pregnancy in 1 menstrual cycle. Normal fecundability with a single menstrual cycle is ~20%, peaking between the ages of 20 and 24 years.2 Fecundability decreases slightly at age 32 and declines progressively and more rapidly after age 40. Spontaneous miscarriage is a factor; its rate in younger women is ~10% and in women >40 years is ~40%.2 Overall, approximately 13% of women between the ages of 15 and 44 have fecundity impairment, with more than 6 million women in the United States affected.2
About 24% of all cases of infertility are due to male factors—seminiferous dysfunction, including problems with motility, morphology, and volume of sperm; primary hypogonadism; posttesticular defects; and hypothalamic pituitary disease.3 Recent observational trends show declines in fertility among men older than 40, and among men from different areas in the country, thus raising the issue of the role that environmental pollutants or toxins may play. Supposed increases in urogenital abnormalities and testicular cancers may also contribute to declining fertility rates.4,5
TABLE
Consider these factors in cases of suspected infertility3,6,21,23,40,41
| Major causes of infertility | Infertility risk factors | |
|---|---|---|
| Female | ||
| Cause | Contribution | |
| Endocrine factors | 45%-55% |
|
| PCOS Thyroid Diabetes mellitus Prolactinemia | 21%-28% 10%-20% 10%-20% 7% | |
| Tubal and peritoneal pathology | 30%-40% | |
| Ovulatory dysfunction* | 15% | |
| Cervical and uterine factors | <5% | |
| Male | ||
| Cause | Contribution | |
| Seminiferous tubule dysfunction | 60%-80% |
|
| Posttesticular defects | 10%-20% | |
| Primary hypogonadism | 10%-15% | |
| Hypothalamic pituitary disease | 1%-2% | |
| *Assuming appropriate ovarian reserve, indicated by follicle-stimulating hormone (FSH) level <10 mIU/mL, FSH-to-luteinizing hormone ratio <2, and estradiol level <50 pg/mL. GU, genitourinary; PCOS, polycystic ovarian syndrome; STIs, sexually transmitted infections | ||
Zero in on these areas of the history
As with any diagnostic work-up, the most important aspect of an infertility evaluation is the history. Document menstrual cycle length and regularity and the timing of intercourse. (Ideally, this would be done when a couple first decides to conceive.) It’s important to know how long the couple has been trying to become pregnant. More time may be all they need to achieve pregnancy. Educate them on reproductive cycles and optimal timing to achieve pregnancy. Some women experience lower abdominal pain (mittelschmerz) signifying release of an egg from the ovary, which can help identify the time of ovulation.
Remember, too, the role that a couple’s psychological state can play; worries over suspected infertility may cause anxiety, anger, depression, and marital troubles.
Is it her?
Regular menstrual cycles—menses occurring every 21 to 35 days—carry an ovulation probability of 95% with each cycle.6 With normal menses, ovulatory dysfunction is an unlikely cause of infertility. If menstrual cycles are irregular, ovulatory function is not normal and cyclical. Explore the woman’s medical, surgical, and gynecologic histories, looking particularly for thyroid disease, galactorrhea, hirsutism, pelvic or abdominal pain, dysmenorrhea, dyspareunia, pelvic inflammatory disease (PID), and abdominal or pelvic surgery.
The fallopian tubes. When menstruation is normal, evaluate the fallopian tubes and their patency; 30% to 40% of infertility cases can be related to peritoneal pathology.3,7 Inability to conceive in a previous relationship, history of PID, or prior tubal surgery all correlate to infertility. Ten percent of patients with a history of one PID episode and 54% to 75% of patients with 3 episodes will have patency issues.7
Consider arranging for a hysterosalpingogram (HSG) in all patients as part of an initial work-up for infertility.8 HSG is useful in evaluating tubal patency and the uterine cavity, and it can be therapeutic. HSG is not useful in detecting peritubal adhesions or endometriosis; patients in whom you suspect these conditions should undergo diagnostic laparoscopy. If abnormalities are found on HSG, refer patients to a reproductive endocrinologist to evaluate treatment options.
Chlamydia trachomatis IgG antibody testing can predict the presence of tubal disease. For women with low risk of tubal disease, it may be more cost effective to test for the Chlamydia antibody and proceed with HSG if the result is positive. Antibody testing is also useful for women with an allergy to contrast dye who cannot undergo an HSG. If the antibody test result is positive, consider arranging for a sonohysterogram to evaluate for the presence of fluid in the cul-de-sac, or an intrauterine infusion of saline to evaluate the patency of at least one tube.9
Ovulatory function. To assess ovulatory function, measure a midluteal-phase serum progesterone level, drawn 1 week before the expected day of menses (Day 21 of a 28-day cycle). A level >3 ng/mL is evidence of ovulation. Over-the-counter ovulation kits detect the luteinizing hormone (LH) surge but have false-positive and false-negative rates of 5% to 10%, respectively.10 Recording basal body temperature is a noninvasive and inexpensive means of evaluating ovulation. The patient must record temperatures at exactly the same time each day. Have her log the temperatures and watch for a spike that occurs 1 to 2 days after the LH surge. The average woman’s temperature rises above 98ºF in progressing from the follicular to the luteal phase. Since the spike occurs 1 to 2 days after ovulation, this method is best used for many months so the woman can predict her cycle.11
Once timing of ovulation has been established, you can check lab results at Day 3 of the woman’s cycle for follicle-stimulating hormone (FSH), LH, estradiol, thyroid-stimulating hormone (TSH), prolactin, and 2-hour fasting glucose tolerance. In addition to polycystic ovarian syndrome (PCOS), patients may have ovulatory dysfunction secondary to glucose intolerance.
A clomiphene (Clomid) challenge can help in assessing ovarian reserve. Administer 100 mg clomiphene on Days 5 through 9 of the patient’s cycle, and check FSH and estradiol levels on Day 10. With diminished ovarian reserve, FSH will increase to >12 mIU/mL and estradiol to >300 pg/mL.12 If this occurs, consider referring for an ultrasound measurement of antral follicle count. The presence of 4 to 10 follicles measuring 2 to 10 mm in diameter suggests adequate reserve.13
Although not widely available in the United States, the test for antimüllerian hormone (AMH) levels may be useful in reflecting the size of the primordial follicle pool. At menopause, the level is undetectable. A level above 0.5 ng/mL correlates with good ovarian reserve; levels <0.15 ng/mL suggest poor response to in vitro fertilization (IVF).14
Endocrine factors account for 45% to 55% of female infertility and include thyroid disease, PCOS, diabetes mellitus, prolactinemia, and luteal phase defects. Subclinical hypothyroidism, often evidenced only by high levels of TSH, decreases the chance of a successful pregnancy. This can occur even if the dysfunction is not severe enough to affect cycle regularity.15 Clinical hypo- or hyperthyroidism can affect ovulation by interfering with normal hormonal feedback loops, and correcting thyroid disease can improve fertility.
Galactorrhea discovered during the history and physical may be caused by elevated prolactin levels, which also inhibit normal ovulatory function. Chronically elevated prolactin levels in patients with PCOS can be attributed to elevated estrogen levels. Adiposity, acne, and hirsutism with menstrual irregularity can indicate PCOS as the primary cause, and your work-up should focus on a hyperandrogenic state.16 Low or normal FSH levels are common in patients with PCOS. Also test for 17a-hydroxyprogestrone and serum testosterone levels.17
Endometriosis. How endometriosis affects fertility is controversial. One hypothesis is that it is associated with overproduction of prostaglandins, metalloproteinases, cytokines, and chemokines. The inflammatory process impairs ovarian, peritoneal, tubal, and endometrial function.18
Discourage the use of the postcoital test. Patients may inquire about this test, in which the cervical mucus is obtained after intercourse to assess stretch ability and sperm motility. This test has been used for more than a century, but has poor predictive value and is not recommended.19
Or is it him?
Inquire about sexual development and medical history, including mumps orchitis or other infections, sinopulmonary symptoms suggesting cystic fibrosis, sexually transmitted infections (STIs) and genitourinary infections, and surgical procedures of the inguinal and scrotal areas. Also ask about prescription and illicit drug use, environmental exposures, and sexual history.
Physical exam. Look for signs of androgen deficiency, such as an arm span >2 cm longer than height (eunuchoidal proportions), or loss of pubic, axillary, or facial hair.20 Examine the external genitalia to evaluate for complete sexual development (Tanner stage of 5). The scrotum can provide clues to disorders that can affect sperm maturation and transport. Examination may reveal absence of the vas deferens, epididymal thickening, varicocele, or hernia.21 Testicular volume, if <15 mL with testicular length <3.6 cm, can point to a decreased number of seminiferous tubules.21
Semen analysis. If the physical examination is normal, analyze semen for volume and pH; microscopic debris and agglutination; sperm concentration, motility, and morphology; leukocyte count; and immature germ cells. Have the man abstain from sex for 2 to 7 days before semen collection. If collection is not possible to do in the office, the patient can drop it off at a lab within an hour of collection. Analyze 2 samples at least 2 weeks apart.22
More detailed semen analysis can be done, especially if evaluation of the female partner does not reveal a cause of infertility. Tests include sperm autoantibodies, sperm biochemistry, semen culture, sperm function tests, and sperm-cervical mucus interaction. Typically, these tests and further evaluation of the male partner after an abnormal semen analysis are best done by a urologist specializing in reproduction.
Oligospermia or azoospermia point toward hypogonadism. Elevated morning FSH and low total testosterone correlate with primary hypogonadism, whereas low levels of both hormones correlate with secondary hypogonadism. Hyperprolactinemia is a cause of secondary hypogonadism.3 Low volume of semen can be further evaluated by testing a postejaculatory urinalysis and transrectal ultrasonography to rule out retrograde ejaculation and ejaculatory duct obstruction.23
Fixing the problem
Focus initial counseling for couples on lifestyle modifications. Advise patients to quit smoking, reduce excessive caffeine and alcohol consumption, and engage in intercourse every day or every other day around ovulation. Patients should also avoid lubricants and douching as they can interfere with sperm deposition.
Managing female infertility
Tubal, pelvic, and uterine infertility. Patients with bilateral tubal obstruction may wish to undergo tubal reconstruction, especially if IVF treatments are not readily available to them. Counsel them that surgery for proximal tubal occlusion is not effective and the risk of ectopic pregnancy in the future is high, at approximately 20%.24 Because of the low efficacy of surgery and high ectopic rate, most patients with tubal disease favor IVF. Patients with endometriosis sometimes benefit from laser ablation or surgical resection, but often do well with intrauterine insemination (IUI) or IVF in conjunction with ovulation induction.25 Uterine abnormalities including submucous fibroid, endometrial polyp, septate uterus, or uterine synechiae frequently benefit from surgical correction.26 Patients with irreparable defects may want to consider a surrogate.
Ovulatory dysfunction. Anovulation can be hypogonadotropic hypogonadal (secondary to functional factors such as exercise and weight), normogonadotropic normoestrogenic with PCOS, or hypergonadotropic hypoestrogenic infertility (premature ovarian failure).
A body mass index >17 and <27 kg/m2 is optimal to achieve fertility and to sustain a healthy pregnancy.27 Individuals who are obese or very thin or who overexercise and do not respond to behavioral modification are known to benefit from pulsatile gonadotropin-releasing hormone therapy. This treatment, however, is not available in the United States.28
Dopaminergic agents can restore normal ovulation in patients with hyperprolactinemia,29 but they should receive ovulation induction first. Patients who have glucose intolerance may benefit from an insulin-sensitizing agent such as metformin. It is particularly useful if patients also have PCOS; however, it is not an FDA-approved indication for the medication. Clomiphene has recently been shown to result in a higher rate of ovulation, but not pregnancy, than metformin.30
Most patients with ovulatory dysfunction are best treated with clomiphene.31 Give 50 mg of the drug on cycle Days 3 through 7; ovulation occurs between Days 10 through 15.12 If, after the first cycle, pregnancy has not occurred, increase the dose by 50 mg with each cycle, to a maximum of 150 mg daily.32 Higher doses are not FDA approved, nor are they more effective. Clomiphene is most effective in the first 6 cycles, and the American Congress of Obstetricians and Gynecologists recommends limiting its use to fewer than 12 cycles due to the risk of ovarian neoplasm.33 Clomiphene yields an ovulation rate of 73% and a pregnancy rate of 36% per cycle. Multiple births, primarily twinning, occur at a rate of 8% to 13%.33 If clomiphene is unsuccessful, refer patients to a reproductive endocrinologist for evaluation for IVF and injectable ovulation-inducing agents.
Managing male infertility
Men who have hyperprolactinemic infertility can often be treated with dopaminergic agents such as bromocriptine. Inform them that normal spermatogenesis can take 3 to 6 months. Gonadotropin therapy may be effective for patients with hypothalamic or pituitary diseases. Surgery may correct obstruction, but may not actually increase pregnancy rates. Repairing a varicocele, for instance, increases sperm counts but not conception rates.34 Other obstructive problems may need sperm extraction followed by IUI or IVF, with or without intracytoplasmic sperm injection, where the sperm is injected into the ovum in the lab before implantation.34
Managing unexplained infertility
Fifteen percent of infertility is unexplained.35 Assisting these patients is challenging. Performing IUI with or without clomiphene, or giving clomiphene alone is often attempted. Pregnancy rates are 2% for expectant management, 5% for IUI alone, 9.5% for clomiphene alone, and 19% for combined IUI with clomiphene.36 Gonadotropins are no more effective in achieving conception than clomiphene, but gonadotropin injection and IUI together are more effective than no treatment.37 IVF, if successful, leads to pregnancy in the shortest amount of time. But it is the most costly intervention and the most likely to result in multiple births. In randomized controlled trials, however, IVF has not proved beneficial for unexplained infertility.38
Trends likely to affect fertility treatment
Currently in the United States, there is little regulation to guide reproductive technologies. But there is a trend, varying by state, toward legislation similar to child protection laws and adoption services, under which couples are evaluated for suitability as parents for the potential child’s safety.39 Other countries have acts regulating reproductive technologies and infertility services. England focuses on the child’s welfare; Australia restricts access by eligibility requirements.39 In the United States we may see similar policies, especially as controversy grows regarding multiple births. Cost is a factor in the treatment of infertility. Education and household income correlate with the amount of money spent on fertility care.
CORRESPONDENCE Heather Bell, MD, Center for Family Medicine, 1115 East 20th Street, Sioux Falls, SD 57105; [email protected]
1. Practice Committee of the American Society for Reproductive Medicine. Definitions of infertility and recurrent pregnancy loss. Fertil Steril. 2008;90(suppl):S60.-
2. Boivin J, Bunting L, Collins JA, et al. International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum Reprod. 2007;22:1506-1512.
3. Hull MGR, Glazener CMJ, Kelly NJ, et al. Population studies of causes, treatment, and outcome of infertility. BMJ. 1985;291:1693-1697.
4. Carlsen E, Giwercman A, Keiding N, et al. Evidence for decreasing quality of semen during past 50 years. BMJ. 1992;305:609-613.
5. de La Rochebrochard E, Thonneau P. Paternal age >or=40 years: an important risk factor for infertility. Am J Obstet Gynecol. 2003;189:901-905.
6. Behre HM, Kuhlage J, Gassner C, et al. Prediction of ovulation by urinary hormone measurements with the home use ClearPlan Fertility Monitor: comparison with transvaginal ultrasound scans and serum hormone measurements. Hum Reprod. 2000;15:2478-2482.
7. Brassard M, AinMelk Y, Baillargeon J. Basic infertility including polycystic ovary syndrome. Med Clin North Am. 2008;92:1163-1192.
8. Papaioannou S, Bourdrez P, Varma R, et al. Tubal evaluation in the investigation of subfertility: a structured comparison of tests. BJOG. 2004;111:1313-1321.
9. Mol BW, Collins JA, Van Der Veen F, et al. Cost-effectiveness of hysterosalpingography, laparoscopy, and Chlamydia antibody testing in subfertile couples. Fertil Steril. 2001;75:571-580.
10. Corson SL. Self-prediction of ovulation using a urinary luteinizing hormone test. J Reprod Med. 1986;31(8 suppl):760-763.
11. Kambic R, Gray RH. Interobserver variation in estimation of day of conception intercourse using selected natural family planning charts. Fertil Steril. 1989;51:430-434.
12. Wu CH, Winkel CA. The effect of therapy initiation day on clomiphene citrate therapy. Fertil Steril. 1989;52:564-568.
13. Chang MY, Chiang CH, Hsieh TT, et al. Use of the antral follicle count to predict the outcome of assisted reproductive technologies. Fertil Steril. 1998;69:505-510.
14. de Vet A, Laven JS, de Jong FH, et al. Antimüllerian hormone serum levels: a putative marker for ovarian aging. Fertil Steril. 2002;77:357-362.
15. De Sutter P. Rational diagnosis and treatment in infertility. Best Pract Res Clin Obstet Gynaecol. 2006;20:647-664.
16. Ehrmann DA. Polycystic ovary syndrome. N Engl J Med. 2005;352:1223-1236.
17. Azziz R, Zacur HA. 21-Hydroxylase deficiency in female hyperandrogenism, screening and diagnosis. J Clin Endocrinol Metab. 1989;69:577-584.
18. Bulun SE. Endometriosis. N Engl J Med. 2009;360:268-279.
19. van der Steeg JW, Steures P, Eijkemans MJ, et al. Should the post-coital test (PCT) be part of the routine fertility work-up? Hum Reprod. 2004;19:1373-1379.
20. Themmen APN, Huhtaniemi IT. Mutations of gonadotropins and gonadotropin receptors: elucidating the physiology and pathophysiology of pituitary-gonadal function. Endocr Rev. 2000;21:551-583.
21. Rowe PJ. WHO Manual for the Standardization Investigation and Diagnosis of the Infertile Couple. New York, NY: Cambridge University Press; 1993.
22. World Health Organization Department of Reproductive Health and Research World Health Organization Laboratory Manual for the Examination and Processing of Human Semen. 5th ed. Geneva, Switzerland: World Health Organization; 2010.
23. Male Infertility Best Practice Policy Committee of the American Urological Association; Practical Committee of the American Society for Reproductive Medicine. Report on optimal evaluation of the infertile male. Fertil Steril. 2004;82(suppl 1):S123-S130.
24. Honoré GM, Holden AE, Schenken RS. Pathophysiology and management of proximal tubal blockage. Fertil Steril. 1999;71:785-795.
25. Tummon IS, Asher LF, Martin JS, et al. Randomized controlled trial of superovulation and insemination for infertility associated with minimal or mild endometriosis. Fertil Steril. 1997;68:8-12.
26. Heinonen PK, Saarikoski S, Pystynen P. Reproductive performance of women with uterine anomalies. An evaluation of 182 cases. Acta Obstet Gynecol Scand. 1982;61:157-162.
27. Frisch RE. The right weight: body fat, menarche and ovulation. Baillieres Clin Obstet Gynaecol. 1990;4:419-439.
28. Abraham S, Mira M, Llewellyn-Jones D. Should ovulation be induced in women recovering from an eating disorder or who are compulsive exercisers? Fertil Steril. 1990;53:566-568.
29. Crosignan PG. Management of hyperprolactinemia in infertility. J Reprod Med. 1999;44(12 suppl):1116-1120.
30. Baran S, Api M, Godsedef BP, et al. Comparison of metformin and clomiphene citrate therapy for induction in the polycystic ovary syndrome. Arch Gynecol Obstet. 2010;282:439-443.
31. Kerin JF, Liu JH, Phillipou G, et al. Evidence for a hypothalamic site of action of clomiphene citrate in women. J Clin Endocrinol Metab. 1985;61:265-268.
32. Huang KE. The primary treatment of luteal phase inadequacy: progesterone versus clomiphene citrate. Am J Obstet Gynecol. 1986;155:824-828.
33. Homburg R. Clomiphene citrate—end of an era? A mini-review. Hum Reprod. 2005;20:2043-2051.
34. Hirsh A. Male subfertility. BMJ. 2003;327:669-672.
35. Collins JA, Crosignani PG. Unexplained infertility: a review of diagnosis, prognosis, treatment efficacy and management. Int J Gynaecol Obstet. 1992;39:267-275.
36. Fisch P, Casper RF, Brown SE, et al. Unexplained infertility: evaluation of treatment with clomiphene citrate and human chorionic gonadotropin. Fertil Steril. 1989;51:828-833.
37. Verhulst SM, Cohlen BJ, Hughes E, et al. Intra-uterine insemination for unexplained subfertility. Cochrane Database Syst Rev. 2006;(4):CD001838.-
38. Pandian Z, Bhattacharya S, Vale L, et al. In vitro fertilization for unexplained subfertility. Cochrane Database Syst Rev. 2005;(2):CD003357.-
39. Liu C. Restricting access to infertility services: what is a justified limitation on reproductive freedom? Minn J Law Sci Technol. 2009;10:291. Available at: http://mjlst.umn.edu/uploads/QJ/WD/QJWDUZ1D-pV28sHvArqp6A/101_liu.pdf. Accessed May 4, 2011.
40. Practice Committee of the American Society for Reproductive Medicine. Optimal evaluation of the infertile female. Fertil Steril. 2006;86(suppl 1):S264-S267.
41. Practice Committee of the American Society for Reproductive Medicine. Current evaluation of amenorrhea. Fertil Steril. 2004;82(suppl 1):S33-S39.
• Evaluate the fallopian tubes and their patency when menstruation is normal. Also, consider arranging for a hysterosalpingogram. B
• Suspect polycystic ovarian syndrome when adiposity, acne, and hirsutism with menstrual irregularity are factors. B
• Suspect androgen deficiency when a man’s arm span is >2 cm longer than his height, or when he has experienced a loss of pubic, axillary, or facial hair. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
During an annual visit, a patient confides in you that she and her husband have been trying to get pregnant for a year, but haven’t had any success. She tells you that she’s starting to get worried.
How do you advise her? What are your next steps?
The approach to evaluating infertility complaints is usually straightforward and can lead to a positive outcome. Not surprisingly, the dialogue often begins with you, the family physician. Your attention to clues in each partner’s history can do much to get to the heart of the problem. And even if a couple requires a specialty referral, it’s best to be familiar with the more extensive evaluation and management options they’ll encounter to help them anticipate likely discussions in their consultations. In this article, we review the best evidence for the care of your patients who want to conceive.
Who’s affected?
Infertility difficulties may be attributable to one or both partners, may be multifactorial, or may be unexplained (TABLE).
In women, infertility is the inability to conceive after 1 year of unprotected regular intercourse in those younger than 35 years, and after 6 months in those 35 years and older.1 Fecundability is the probability of achieving pregnancy in 1 menstrual cycle. Normal fecundability with a single menstrual cycle is ~20%, peaking between the ages of 20 and 24 years.2 Fecundability decreases slightly at age 32 and declines progressively and more rapidly after age 40. Spontaneous miscarriage is a factor; its rate in younger women is ~10% and in women >40 years is ~40%.2 Overall, approximately 13% of women between the ages of 15 and 44 have fecundity impairment, with more than 6 million women in the United States affected.2
About 24% of all cases of infertility are due to male factors—seminiferous dysfunction, including problems with motility, morphology, and volume of sperm; primary hypogonadism; posttesticular defects; and hypothalamic pituitary disease.3 Recent observational trends show declines in fertility among men older than 40, and among men from different areas in the country, thus raising the issue of the role that environmental pollutants or toxins may play. Supposed increases in urogenital abnormalities and testicular cancers may also contribute to declining fertility rates.4,5
TABLE
Consider these factors in cases of suspected infertility3,6,21,23,40,41
| Major causes of infertility | Infertility risk factors | |
|---|---|---|
| Female | ||
| Cause | Contribution | |
| Endocrine factors | 45%-55% |
|
| PCOS Thyroid Diabetes mellitus Prolactinemia | 21%-28% 10%-20% 10%-20% 7% | |
| Tubal and peritoneal pathology | 30%-40% | |
| Ovulatory dysfunction* | 15% | |
| Cervical and uterine factors | <5% | |
| Male | ||
| Cause | Contribution | |
| Seminiferous tubule dysfunction | 60%-80% |
|
| Posttesticular defects | 10%-20% | |
| Primary hypogonadism | 10%-15% | |
| Hypothalamic pituitary disease | 1%-2% | |
| *Assuming appropriate ovarian reserve, indicated by follicle-stimulating hormone (FSH) level <10 mIU/mL, FSH-to-luteinizing hormone ratio <2, and estradiol level <50 pg/mL. GU, genitourinary; PCOS, polycystic ovarian syndrome; STIs, sexually transmitted infections | ||
Zero in on these areas of the history
As with any diagnostic work-up, the most important aspect of an infertility evaluation is the history. Document menstrual cycle length and regularity and the timing of intercourse. (Ideally, this would be done when a couple first decides to conceive.) It’s important to know how long the couple has been trying to become pregnant. More time may be all they need to achieve pregnancy. Educate them on reproductive cycles and optimal timing to achieve pregnancy. Some women experience lower abdominal pain (mittelschmerz) signifying release of an egg from the ovary, which can help identify the time of ovulation.
Remember, too, the role that a couple’s psychological state can play; worries over suspected infertility may cause anxiety, anger, depression, and marital troubles.
Is it her?
Regular menstrual cycles—menses occurring every 21 to 35 days—carry an ovulation probability of 95% with each cycle.6 With normal menses, ovulatory dysfunction is an unlikely cause of infertility. If menstrual cycles are irregular, ovulatory function is not normal and cyclical. Explore the woman’s medical, surgical, and gynecologic histories, looking particularly for thyroid disease, galactorrhea, hirsutism, pelvic or abdominal pain, dysmenorrhea, dyspareunia, pelvic inflammatory disease (PID), and abdominal or pelvic surgery.
The fallopian tubes. When menstruation is normal, evaluate the fallopian tubes and their patency; 30% to 40% of infertility cases can be related to peritoneal pathology.3,7 Inability to conceive in a previous relationship, history of PID, or prior tubal surgery all correlate to infertility. Ten percent of patients with a history of one PID episode and 54% to 75% of patients with 3 episodes will have patency issues.7
Consider arranging for a hysterosalpingogram (HSG) in all patients as part of an initial work-up for infertility.8 HSG is useful in evaluating tubal patency and the uterine cavity, and it can be therapeutic. HSG is not useful in detecting peritubal adhesions or endometriosis; patients in whom you suspect these conditions should undergo diagnostic laparoscopy. If abnormalities are found on HSG, refer patients to a reproductive endocrinologist to evaluate treatment options.
Chlamydia trachomatis IgG antibody testing can predict the presence of tubal disease. For women with low risk of tubal disease, it may be more cost effective to test for the Chlamydia antibody and proceed with HSG if the result is positive. Antibody testing is also useful for women with an allergy to contrast dye who cannot undergo an HSG. If the antibody test result is positive, consider arranging for a sonohysterogram to evaluate for the presence of fluid in the cul-de-sac, or an intrauterine infusion of saline to evaluate the patency of at least one tube.9
Ovulatory function. To assess ovulatory function, measure a midluteal-phase serum progesterone level, drawn 1 week before the expected day of menses (Day 21 of a 28-day cycle). A level >3 ng/mL is evidence of ovulation. Over-the-counter ovulation kits detect the luteinizing hormone (LH) surge but have false-positive and false-negative rates of 5% to 10%, respectively.10 Recording basal body temperature is a noninvasive and inexpensive means of evaluating ovulation. The patient must record temperatures at exactly the same time each day. Have her log the temperatures and watch for a spike that occurs 1 to 2 days after the LH surge. The average woman’s temperature rises above 98ºF in progressing from the follicular to the luteal phase. Since the spike occurs 1 to 2 days after ovulation, this method is best used for many months so the woman can predict her cycle.11
Once timing of ovulation has been established, you can check lab results at Day 3 of the woman’s cycle for follicle-stimulating hormone (FSH), LH, estradiol, thyroid-stimulating hormone (TSH), prolactin, and 2-hour fasting glucose tolerance. In addition to polycystic ovarian syndrome (PCOS), patients may have ovulatory dysfunction secondary to glucose intolerance.
A clomiphene (Clomid) challenge can help in assessing ovarian reserve. Administer 100 mg clomiphene on Days 5 through 9 of the patient’s cycle, and check FSH and estradiol levels on Day 10. With diminished ovarian reserve, FSH will increase to >12 mIU/mL and estradiol to >300 pg/mL.12 If this occurs, consider referring for an ultrasound measurement of antral follicle count. The presence of 4 to 10 follicles measuring 2 to 10 mm in diameter suggests adequate reserve.13
Although not widely available in the United States, the test for antimüllerian hormone (AMH) levels may be useful in reflecting the size of the primordial follicle pool. At menopause, the level is undetectable. A level above 0.5 ng/mL correlates with good ovarian reserve; levels <0.15 ng/mL suggest poor response to in vitro fertilization (IVF).14
Endocrine factors account for 45% to 55% of female infertility and include thyroid disease, PCOS, diabetes mellitus, prolactinemia, and luteal phase defects. Subclinical hypothyroidism, often evidenced only by high levels of TSH, decreases the chance of a successful pregnancy. This can occur even if the dysfunction is not severe enough to affect cycle regularity.15 Clinical hypo- or hyperthyroidism can affect ovulation by interfering with normal hormonal feedback loops, and correcting thyroid disease can improve fertility.
Galactorrhea discovered during the history and physical may be caused by elevated prolactin levels, which also inhibit normal ovulatory function. Chronically elevated prolactin levels in patients with PCOS can be attributed to elevated estrogen levels. Adiposity, acne, and hirsutism with menstrual irregularity can indicate PCOS as the primary cause, and your work-up should focus on a hyperandrogenic state.16 Low or normal FSH levels are common in patients with PCOS. Also test for 17a-hydroxyprogestrone and serum testosterone levels.17
Endometriosis. How endometriosis affects fertility is controversial. One hypothesis is that it is associated with overproduction of prostaglandins, metalloproteinases, cytokines, and chemokines. The inflammatory process impairs ovarian, peritoneal, tubal, and endometrial function.18
Discourage the use of the postcoital test. Patients may inquire about this test, in which the cervical mucus is obtained after intercourse to assess stretch ability and sperm motility. This test has been used for more than a century, but has poor predictive value and is not recommended.19
Or is it him?
Inquire about sexual development and medical history, including mumps orchitis or other infections, sinopulmonary symptoms suggesting cystic fibrosis, sexually transmitted infections (STIs) and genitourinary infections, and surgical procedures of the inguinal and scrotal areas. Also ask about prescription and illicit drug use, environmental exposures, and sexual history.
Physical exam. Look for signs of androgen deficiency, such as an arm span >2 cm longer than height (eunuchoidal proportions), or loss of pubic, axillary, or facial hair.20 Examine the external genitalia to evaluate for complete sexual development (Tanner stage of 5). The scrotum can provide clues to disorders that can affect sperm maturation and transport. Examination may reveal absence of the vas deferens, epididymal thickening, varicocele, or hernia.21 Testicular volume, if <15 mL with testicular length <3.6 cm, can point to a decreased number of seminiferous tubules.21
Semen analysis. If the physical examination is normal, analyze semen for volume and pH; microscopic debris and agglutination; sperm concentration, motility, and morphology; leukocyte count; and immature germ cells. Have the man abstain from sex for 2 to 7 days before semen collection. If collection is not possible to do in the office, the patient can drop it off at a lab within an hour of collection. Analyze 2 samples at least 2 weeks apart.22
More detailed semen analysis can be done, especially if evaluation of the female partner does not reveal a cause of infertility. Tests include sperm autoantibodies, sperm biochemistry, semen culture, sperm function tests, and sperm-cervical mucus interaction. Typically, these tests and further evaluation of the male partner after an abnormal semen analysis are best done by a urologist specializing in reproduction.
Oligospermia or azoospermia point toward hypogonadism. Elevated morning FSH and low total testosterone correlate with primary hypogonadism, whereas low levels of both hormones correlate with secondary hypogonadism. Hyperprolactinemia is a cause of secondary hypogonadism.3 Low volume of semen can be further evaluated by testing a postejaculatory urinalysis and transrectal ultrasonography to rule out retrograde ejaculation and ejaculatory duct obstruction.23
Fixing the problem
Focus initial counseling for couples on lifestyle modifications. Advise patients to quit smoking, reduce excessive caffeine and alcohol consumption, and engage in intercourse every day or every other day around ovulation. Patients should also avoid lubricants and douching as they can interfere with sperm deposition.
Managing female infertility
Tubal, pelvic, and uterine infertility. Patients with bilateral tubal obstruction may wish to undergo tubal reconstruction, especially if IVF treatments are not readily available to them. Counsel them that surgery for proximal tubal occlusion is not effective and the risk of ectopic pregnancy in the future is high, at approximately 20%.24 Because of the low efficacy of surgery and high ectopic rate, most patients with tubal disease favor IVF. Patients with endometriosis sometimes benefit from laser ablation or surgical resection, but often do well with intrauterine insemination (IUI) or IVF in conjunction with ovulation induction.25 Uterine abnormalities including submucous fibroid, endometrial polyp, septate uterus, or uterine synechiae frequently benefit from surgical correction.26 Patients with irreparable defects may want to consider a surrogate.
Ovulatory dysfunction. Anovulation can be hypogonadotropic hypogonadal (secondary to functional factors such as exercise and weight), normogonadotropic normoestrogenic with PCOS, or hypergonadotropic hypoestrogenic infertility (premature ovarian failure).
A body mass index >17 and <27 kg/m2 is optimal to achieve fertility and to sustain a healthy pregnancy.27 Individuals who are obese or very thin or who overexercise and do not respond to behavioral modification are known to benefit from pulsatile gonadotropin-releasing hormone therapy. This treatment, however, is not available in the United States.28
Dopaminergic agents can restore normal ovulation in patients with hyperprolactinemia,29 but they should receive ovulation induction first. Patients who have glucose intolerance may benefit from an insulin-sensitizing agent such as metformin. It is particularly useful if patients also have PCOS; however, it is not an FDA-approved indication for the medication. Clomiphene has recently been shown to result in a higher rate of ovulation, but not pregnancy, than metformin.30
Most patients with ovulatory dysfunction are best treated with clomiphene.31 Give 50 mg of the drug on cycle Days 3 through 7; ovulation occurs between Days 10 through 15.12 If, after the first cycle, pregnancy has not occurred, increase the dose by 50 mg with each cycle, to a maximum of 150 mg daily.32 Higher doses are not FDA approved, nor are they more effective. Clomiphene is most effective in the first 6 cycles, and the American Congress of Obstetricians and Gynecologists recommends limiting its use to fewer than 12 cycles due to the risk of ovarian neoplasm.33 Clomiphene yields an ovulation rate of 73% and a pregnancy rate of 36% per cycle. Multiple births, primarily twinning, occur at a rate of 8% to 13%.33 If clomiphene is unsuccessful, refer patients to a reproductive endocrinologist for evaluation for IVF and injectable ovulation-inducing agents.
Managing male infertility
Men who have hyperprolactinemic infertility can often be treated with dopaminergic agents such as bromocriptine. Inform them that normal spermatogenesis can take 3 to 6 months. Gonadotropin therapy may be effective for patients with hypothalamic or pituitary diseases. Surgery may correct obstruction, but may not actually increase pregnancy rates. Repairing a varicocele, for instance, increases sperm counts but not conception rates.34 Other obstructive problems may need sperm extraction followed by IUI or IVF, with or without intracytoplasmic sperm injection, where the sperm is injected into the ovum in the lab before implantation.34
Managing unexplained infertility
Fifteen percent of infertility is unexplained.35 Assisting these patients is challenging. Performing IUI with or without clomiphene, or giving clomiphene alone is often attempted. Pregnancy rates are 2% for expectant management, 5% for IUI alone, 9.5% for clomiphene alone, and 19% for combined IUI with clomiphene.36 Gonadotropins are no more effective in achieving conception than clomiphene, but gonadotropin injection and IUI together are more effective than no treatment.37 IVF, if successful, leads to pregnancy in the shortest amount of time. But it is the most costly intervention and the most likely to result in multiple births. In randomized controlled trials, however, IVF has not proved beneficial for unexplained infertility.38
Trends likely to affect fertility treatment
Currently in the United States, there is little regulation to guide reproductive technologies. But there is a trend, varying by state, toward legislation similar to child protection laws and adoption services, under which couples are evaluated for suitability as parents for the potential child’s safety.39 Other countries have acts regulating reproductive technologies and infertility services. England focuses on the child’s welfare; Australia restricts access by eligibility requirements.39 In the United States we may see similar policies, especially as controversy grows regarding multiple births. Cost is a factor in the treatment of infertility. Education and household income correlate with the amount of money spent on fertility care.
CORRESPONDENCE Heather Bell, MD, Center for Family Medicine, 1115 East 20th Street, Sioux Falls, SD 57105; [email protected]
• Evaluate the fallopian tubes and their patency when menstruation is normal. Also, consider arranging for a hysterosalpingogram. B
• Suspect polycystic ovarian syndrome when adiposity, acne, and hirsutism with menstrual irregularity are factors. B
• Suspect androgen deficiency when a man’s arm span is >2 cm longer than his height, or when he has experienced a loss of pubic, axillary, or facial hair. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
During an annual visit, a patient confides in you that she and her husband have been trying to get pregnant for a year, but haven’t had any success. She tells you that she’s starting to get worried.
How do you advise her? What are your next steps?
The approach to evaluating infertility complaints is usually straightforward and can lead to a positive outcome. Not surprisingly, the dialogue often begins with you, the family physician. Your attention to clues in each partner’s history can do much to get to the heart of the problem. And even if a couple requires a specialty referral, it’s best to be familiar with the more extensive evaluation and management options they’ll encounter to help them anticipate likely discussions in their consultations. In this article, we review the best evidence for the care of your patients who want to conceive.
Who’s affected?
Infertility difficulties may be attributable to one or both partners, may be multifactorial, or may be unexplained (TABLE).
In women, infertility is the inability to conceive after 1 year of unprotected regular intercourse in those younger than 35 years, and after 6 months in those 35 years and older.1 Fecundability is the probability of achieving pregnancy in 1 menstrual cycle. Normal fecundability with a single menstrual cycle is ~20%, peaking between the ages of 20 and 24 years.2 Fecundability decreases slightly at age 32 and declines progressively and more rapidly after age 40. Spontaneous miscarriage is a factor; its rate in younger women is ~10% and in women >40 years is ~40%.2 Overall, approximately 13% of women between the ages of 15 and 44 have fecundity impairment, with more than 6 million women in the United States affected.2
About 24% of all cases of infertility are due to male factors—seminiferous dysfunction, including problems with motility, morphology, and volume of sperm; primary hypogonadism; posttesticular defects; and hypothalamic pituitary disease.3 Recent observational trends show declines in fertility among men older than 40, and among men from different areas in the country, thus raising the issue of the role that environmental pollutants or toxins may play. Supposed increases in urogenital abnormalities and testicular cancers may also contribute to declining fertility rates.4,5
TABLE
Consider these factors in cases of suspected infertility3,6,21,23,40,41
| Major causes of infertility | Infertility risk factors | |
|---|---|---|
| Female | ||
| Cause | Contribution | |
| Endocrine factors | 45%-55% |
|
| PCOS Thyroid Diabetes mellitus Prolactinemia | 21%-28% 10%-20% 10%-20% 7% | |
| Tubal and peritoneal pathology | 30%-40% | |
| Ovulatory dysfunction* | 15% | |
| Cervical and uterine factors | <5% | |
| Male | ||
| Cause | Contribution | |
| Seminiferous tubule dysfunction | 60%-80% |
|
| Posttesticular defects | 10%-20% | |
| Primary hypogonadism | 10%-15% | |
| Hypothalamic pituitary disease | 1%-2% | |
| *Assuming appropriate ovarian reserve, indicated by follicle-stimulating hormone (FSH) level <10 mIU/mL, FSH-to-luteinizing hormone ratio <2, and estradiol level <50 pg/mL. GU, genitourinary; PCOS, polycystic ovarian syndrome; STIs, sexually transmitted infections | ||
Zero in on these areas of the history
As with any diagnostic work-up, the most important aspect of an infertility evaluation is the history. Document menstrual cycle length and regularity and the timing of intercourse. (Ideally, this would be done when a couple first decides to conceive.) It’s important to know how long the couple has been trying to become pregnant. More time may be all they need to achieve pregnancy. Educate them on reproductive cycles and optimal timing to achieve pregnancy. Some women experience lower abdominal pain (mittelschmerz) signifying release of an egg from the ovary, which can help identify the time of ovulation.
Remember, too, the role that a couple’s psychological state can play; worries over suspected infertility may cause anxiety, anger, depression, and marital troubles.
Is it her?
Regular menstrual cycles—menses occurring every 21 to 35 days—carry an ovulation probability of 95% with each cycle.6 With normal menses, ovulatory dysfunction is an unlikely cause of infertility. If menstrual cycles are irregular, ovulatory function is not normal and cyclical. Explore the woman’s medical, surgical, and gynecologic histories, looking particularly for thyroid disease, galactorrhea, hirsutism, pelvic or abdominal pain, dysmenorrhea, dyspareunia, pelvic inflammatory disease (PID), and abdominal or pelvic surgery.
The fallopian tubes. When menstruation is normal, evaluate the fallopian tubes and their patency; 30% to 40% of infertility cases can be related to peritoneal pathology.3,7 Inability to conceive in a previous relationship, history of PID, or prior tubal surgery all correlate to infertility. Ten percent of patients with a history of one PID episode and 54% to 75% of patients with 3 episodes will have patency issues.7
Consider arranging for a hysterosalpingogram (HSG) in all patients as part of an initial work-up for infertility.8 HSG is useful in evaluating tubal patency and the uterine cavity, and it can be therapeutic. HSG is not useful in detecting peritubal adhesions or endometriosis; patients in whom you suspect these conditions should undergo diagnostic laparoscopy. If abnormalities are found on HSG, refer patients to a reproductive endocrinologist to evaluate treatment options.
Chlamydia trachomatis IgG antibody testing can predict the presence of tubal disease. For women with low risk of tubal disease, it may be more cost effective to test for the Chlamydia antibody and proceed with HSG if the result is positive. Antibody testing is also useful for women with an allergy to contrast dye who cannot undergo an HSG. If the antibody test result is positive, consider arranging for a sonohysterogram to evaluate for the presence of fluid in the cul-de-sac, or an intrauterine infusion of saline to evaluate the patency of at least one tube.9
Ovulatory function. To assess ovulatory function, measure a midluteal-phase serum progesterone level, drawn 1 week before the expected day of menses (Day 21 of a 28-day cycle). A level >3 ng/mL is evidence of ovulation. Over-the-counter ovulation kits detect the luteinizing hormone (LH) surge but have false-positive and false-negative rates of 5% to 10%, respectively.10 Recording basal body temperature is a noninvasive and inexpensive means of evaluating ovulation. The patient must record temperatures at exactly the same time each day. Have her log the temperatures and watch for a spike that occurs 1 to 2 days after the LH surge. The average woman’s temperature rises above 98ºF in progressing from the follicular to the luteal phase. Since the spike occurs 1 to 2 days after ovulation, this method is best used for many months so the woman can predict her cycle.11
Once timing of ovulation has been established, you can check lab results at Day 3 of the woman’s cycle for follicle-stimulating hormone (FSH), LH, estradiol, thyroid-stimulating hormone (TSH), prolactin, and 2-hour fasting glucose tolerance. In addition to polycystic ovarian syndrome (PCOS), patients may have ovulatory dysfunction secondary to glucose intolerance.
A clomiphene (Clomid) challenge can help in assessing ovarian reserve. Administer 100 mg clomiphene on Days 5 through 9 of the patient’s cycle, and check FSH and estradiol levels on Day 10. With diminished ovarian reserve, FSH will increase to >12 mIU/mL and estradiol to >300 pg/mL.12 If this occurs, consider referring for an ultrasound measurement of antral follicle count. The presence of 4 to 10 follicles measuring 2 to 10 mm in diameter suggests adequate reserve.13
Although not widely available in the United States, the test for antimüllerian hormone (AMH) levels may be useful in reflecting the size of the primordial follicle pool. At menopause, the level is undetectable. A level above 0.5 ng/mL correlates with good ovarian reserve; levels <0.15 ng/mL suggest poor response to in vitro fertilization (IVF).14
Endocrine factors account for 45% to 55% of female infertility and include thyroid disease, PCOS, diabetes mellitus, prolactinemia, and luteal phase defects. Subclinical hypothyroidism, often evidenced only by high levels of TSH, decreases the chance of a successful pregnancy. This can occur even if the dysfunction is not severe enough to affect cycle regularity.15 Clinical hypo- or hyperthyroidism can affect ovulation by interfering with normal hormonal feedback loops, and correcting thyroid disease can improve fertility.
Galactorrhea discovered during the history and physical may be caused by elevated prolactin levels, which also inhibit normal ovulatory function. Chronically elevated prolactin levels in patients with PCOS can be attributed to elevated estrogen levels. Adiposity, acne, and hirsutism with menstrual irregularity can indicate PCOS as the primary cause, and your work-up should focus on a hyperandrogenic state.16 Low or normal FSH levels are common in patients with PCOS. Also test for 17a-hydroxyprogestrone and serum testosterone levels.17
Endometriosis. How endometriosis affects fertility is controversial. One hypothesis is that it is associated with overproduction of prostaglandins, metalloproteinases, cytokines, and chemokines. The inflammatory process impairs ovarian, peritoneal, tubal, and endometrial function.18
Discourage the use of the postcoital test. Patients may inquire about this test, in which the cervical mucus is obtained after intercourse to assess stretch ability and sperm motility. This test has been used for more than a century, but has poor predictive value and is not recommended.19
Or is it him?
Inquire about sexual development and medical history, including mumps orchitis or other infections, sinopulmonary symptoms suggesting cystic fibrosis, sexually transmitted infections (STIs) and genitourinary infections, and surgical procedures of the inguinal and scrotal areas. Also ask about prescription and illicit drug use, environmental exposures, and sexual history.
Physical exam. Look for signs of androgen deficiency, such as an arm span >2 cm longer than height (eunuchoidal proportions), or loss of pubic, axillary, or facial hair.20 Examine the external genitalia to evaluate for complete sexual development (Tanner stage of 5). The scrotum can provide clues to disorders that can affect sperm maturation and transport. Examination may reveal absence of the vas deferens, epididymal thickening, varicocele, or hernia.21 Testicular volume, if <15 mL with testicular length <3.6 cm, can point to a decreased number of seminiferous tubules.21
Semen analysis. If the physical examination is normal, analyze semen for volume and pH; microscopic debris and agglutination; sperm concentration, motility, and morphology; leukocyte count; and immature germ cells. Have the man abstain from sex for 2 to 7 days before semen collection. If collection is not possible to do in the office, the patient can drop it off at a lab within an hour of collection. Analyze 2 samples at least 2 weeks apart.22
More detailed semen analysis can be done, especially if evaluation of the female partner does not reveal a cause of infertility. Tests include sperm autoantibodies, sperm biochemistry, semen culture, sperm function tests, and sperm-cervical mucus interaction. Typically, these tests and further evaluation of the male partner after an abnormal semen analysis are best done by a urologist specializing in reproduction.
Oligospermia or azoospermia point toward hypogonadism. Elevated morning FSH and low total testosterone correlate with primary hypogonadism, whereas low levels of both hormones correlate with secondary hypogonadism. Hyperprolactinemia is a cause of secondary hypogonadism.3 Low volume of semen can be further evaluated by testing a postejaculatory urinalysis and transrectal ultrasonography to rule out retrograde ejaculation and ejaculatory duct obstruction.23
Fixing the problem
Focus initial counseling for couples on lifestyle modifications. Advise patients to quit smoking, reduce excessive caffeine and alcohol consumption, and engage in intercourse every day or every other day around ovulation. Patients should also avoid lubricants and douching as they can interfere with sperm deposition.
Managing female infertility
Tubal, pelvic, and uterine infertility. Patients with bilateral tubal obstruction may wish to undergo tubal reconstruction, especially if IVF treatments are not readily available to them. Counsel them that surgery for proximal tubal occlusion is not effective and the risk of ectopic pregnancy in the future is high, at approximately 20%.24 Because of the low efficacy of surgery and high ectopic rate, most patients with tubal disease favor IVF. Patients with endometriosis sometimes benefit from laser ablation or surgical resection, but often do well with intrauterine insemination (IUI) or IVF in conjunction with ovulation induction.25 Uterine abnormalities including submucous fibroid, endometrial polyp, septate uterus, or uterine synechiae frequently benefit from surgical correction.26 Patients with irreparable defects may want to consider a surrogate.
Ovulatory dysfunction. Anovulation can be hypogonadotropic hypogonadal (secondary to functional factors such as exercise and weight), normogonadotropic normoestrogenic with PCOS, or hypergonadotropic hypoestrogenic infertility (premature ovarian failure).
A body mass index >17 and <27 kg/m2 is optimal to achieve fertility and to sustain a healthy pregnancy.27 Individuals who are obese or very thin or who overexercise and do not respond to behavioral modification are known to benefit from pulsatile gonadotropin-releasing hormone therapy. This treatment, however, is not available in the United States.28
Dopaminergic agents can restore normal ovulation in patients with hyperprolactinemia,29 but they should receive ovulation induction first. Patients who have glucose intolerance may benefit from an insulin-sensitizing agent such as metformin. It is particularly useful if patients also have PCOS; however, it is not an FDA-approved indication for the medication. Clomiphene has recently been shown to result in a higher rate of ovulation, but not pregnancy, than metformin.30
Most patients with ovulatory dysfunction are best treated with clomiphene.31 Give 50 mg of the drug on cycle Days 3 through 7; ovulation occurs between Days 10 through 15.12 If, after the first cycle, pregnancy has not occurred, increase the dose by 50 mg with each cycle, to a maximum of 150 mg daily.32 Higher doses are not FDA approved, nor are they more effective. Clomiphene is most effective in the first 6 cycles, and the American Congress of Obstetricians and Gynecologists recommends limiting its use to fewer than 12 cycles due to the risk of ovarian neoplasm.33 Clomiphene yields an ovulation rate of 73% and a pregnancy rate of 36% per cycle. Multiple births, primarily twinning, occur at a rate of 8% to 13%.33 If clomiphene is unsuccessful, refer patients to a reproductive endocrinologist for evaluation for IVF and injectable ovulation-inducing agents.
Managing male infertility
Men who have hyperprolactinemic infertility can often be treated with dopaminergic agents such as bromocriptine. Inform them that normal spermatogenesis can take 3 to 6 months. Gonadotropin therapy may be effective for patients with hypothalamic or pituitary diseases. Surgery may correct obstruction, but may not actually increase pregnancy rates. Repairing a varicocele, for instance, increases sperm counts but not conception rates.34 Other obstructive problems may need sperm extraction followed by IUI or IVF, with or without intracytoplasmic sperm injection, where the sperm is injected into the ovum in the lab before implantation.34
Managing unexplained infertility
Fifteen percent of infertility is unexplained.35 Assisting these patients is challenging. Performing IUI with or without clomiphene, or giving clomiphene alone is often attempted. Pregnancy rates are 2% for expectant management, 5% for IUI alone, 9.5% for clomiphene alone, and 19% for combined IUI with clomiphene.36 Gonadotropins are no more effective in achieving conception than clomiphene, but gonadotropin injection and IUI together are more effective than no treatment.37 IVF, if successful, leads to pregnancy in the shortest amount of time. But it is the most costly intervention and the most likely to result in multiple births. In randomized controlled trials, however, IVF has not proved beneficial for unexplained infertility.38
Trends likely to affect fertility treatment
Currently in the United States, there is little regulation to guide reproductive technologies. But there is a trend, varying by state, toward legislation similar to child protection laws and adoption services, under which couples are evaluated for suitability as parents for the potential child’s safety.39 Other countries have acts regulating reproductive technologies and infertility services. England focuses on the child’s welfare; Australia restricts access by eligibility requirements.39 In the United States we may see similar policies, especially as controversy grows regarding multiple births. Cost is a factor in the treatment of infertility. Education and household income correlate with the amount of money spent on fertility care.
CORRESPONDENCE Heather Bell, MD, Center for Family Medicine, 1115 East 20th Street, Sioux Falls, SD 57105; [email protected]
1. Practice Committee of the American Society for Reproductive Medicine. Definitions of infertility and recurrent pregnancy loss. Fertil Steril. 2008;90(suppl):S60.-
2. Boivin J, Bunting L, Collins JA, et al. International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum Reprod. 2007;22:1506-1512.
3. Hull MGR, Glazener CMJ, Kelly NJ, et al. Population studies of causes, treatment, and outcome of infertility. BMJ. 1985;291:1693-1697.
4. Carlsen E, Giwercman A, Keiding N, et al. Evidence for decreasing quality of semen during past 50 years. BMJ. 1992;305:609-613.
5. de La Rochebrochard E, Thonneau P. Paternal age >or=40 years: an important risk factor for infertility. Am J Obstet Gynecol. 2003;189:901-905.
6. Behre HM, Kuhlage J, Gassner C, et al. Prediction of ovulation by urinary hormone measurements with the home use ClearPlan Fertility Monitor: comparison with transvaginal ultrasound scans and serum hormone measurements. Hum Reprod. 2000;15:2478-2482.
7. Brassard M, AinMelk Y, Baillargeon J. Basic infertility including polycystic ovary syndrome. Med Clin North Am. 2008;92:1163-1192.
8. Papaioannou S, Bourdrez P, Varma R, et al. Tubal evaluation in the investigation of subfertility: a structured comparison of tests. BJOG. 2004;111:1313-1321.
9. Mol BW, Collins JA, Van Der Veen F, et al. Cost-effectiveness of hysterosalpingography, laparoscopy, and Chlamydia antibody testing in subfertile couples. Fertil Steril. 2001;75:571-580.
10. Corson SL. Self-prediction of ovulation using a urinary luteinizing hormone test. J Reprod Med. 1986;31(8 suppl):760-763.
11. Kambic R, Gray RH. Interobserver variation in estimation of day of conception intercourse using selected natural family planning charts. Fertil Steril. 1989;51:430-434.
12. Wu CH, Winkel CA. The effect of therapy initiation day on clomiphene citrate therapy. Fertil Steril. 1989;52:564-568.
13. Chang MY, Chiang CH, Hsieh TT, et al. Use of the antral follicle count to predict the outcome of assisted reproductive technologies. Fertil Steril. 1998;69:505-510.
14. de Vet A, Laven JS, de Jong FH, et al. Antimüllerian hormone serum levels: a putative marker for ovarian aging. Fertil Steril. 2002;77:357-362.
15. De Sutter P. Rational diagnosis and treatment in infertility. Best Pract Res Clin Obstet Gynaecol. 2006;20:647-664.
16. Ehrmann DA. Polycystic ovary syndrome. N Engl J Med. 2005;352:1223-1236.
17. Azziz R, Zacur HA. 21-Hydroxylase deficiency in female hyperandrogenism, screening and diagnosis. J Clin Endocrinol Metab. 1989;69:577-584.
18. Bulun SE. Endometriosis. N Engl J Med. 2009;360:268-279.
19. van der Steeg JW, Steures P, Eijkemans MJ, et al. Should the post-coital test (PCT) be part of the routine fertility work-up? Hum Reprod. 2004;19:1373-1379.
20. Themmen APN, Huhtaniemi IT. Mutations of gonadotropins and gonadotropin receptors: elucidating the physiology and pathophysiology of pituitary-gonadal function. Endocr Rev. 2000;21:551-583.
21. Rowe PJ. WHO Manual for the Standardization Investigation and Diagnosis of the Infertile Couple. New York, NY: Cambridge University Press; 1993.
22. World Health Organization Department of Reproductive Health and Research World Health Organization Laboratory Manual for the Examination and Processing of Human Semen. 5th ed. Geneva, Switzerland: World Health Organization; 2010.
23. Male Infertility Best Practice Policy Committee of the American Urological Association; Practical Committee of the American Society for Reproductive Medicine. Report on optimal evaluation of the infertile male. Fertil Steril. 2004;82(suppl 1):S123-S130.
24. Honoré GM, Holden AE, Schenken RS. Pathophysiology and management of proximal tubal blockage. Fertil Steril. 1999;71:785-795.
25. Tummon IS, Asher LF, Martin JS, et al. Randomized controlled trial of superovulation and insemination for infertility associated with minimal or mild endometriosis. Fertil Steril. 1997;68:8-12.
26. Heinonen PK, Saarikoski S, Pystynen P. Reproductive performance of women with uterine anomalies. An evaluation of 182 cases. Acta Obstet Gynecol Scand. 1982;61:157-162.
27. Frisch RE. The right weight: body fat, menarche and ovulation. Baillieres Clin Obstet Gynaecol. 1990;4:419-439.
28. Abraham S, Mira M, Llewellyn-Jones D. Should ovulation be induced in women recovering from an eating disorder or who are compulsive exercisers? Fertil Steril. 1990;53:566-568.
29. Crosignan PG. Management of hyperprolactinemia in infertility. J Reprod Med. 1999;44(12 suppl):1116-1120.
30. Baran S, Api M, Godsedef BP, et al. Comparison of metformin and clomiphene citrate therapy for induction in the polycystic ovary syndrome. Arch Gynecol Obstet. 2010;282:439-443.
31. Kerin JF, Liu JH, Phillipou G, et al. Evidence for a hypothalamic site of action of clomiphene citrate in women. J Clin Endocrinol Metab. 1985;61:265-268.
32. Huang KE. The primary treatment of luteal phase inadequacy: progesterone versus clomiphene citrate. Am J Obstet Gynecol. 1986;155:824-828.
33. Homburg R. Clomiphene citrate—end of an era? A mini-review. Hum Reprod. 2005;20:2043-2051.
34. Hirsh A. Male subfertility. BMJ. 2003;327:669-672.
35. Collins JA, Crosignani PG. Unexplained infertility: a review of diagnosis, prognosis, treatment efficacy and management. Int J Gynaecol Obstet. 1992;39:267-275.
36. Fisch P, Casper RF, Brown SE, et al. Unexplained infertility: evaluation of treatment with clomiphene citrate and human chorionic gonadotropin. Fertil Steril. 1989;51:828-833.
37. Verhulst SM, Cohlen BJ, Hughes E, et al. Intra-uterine insemination for unexplained subfertility. Cochrane Database Syst Rev. 2006;(4):CD001838.-
38. Pandian Z, Bhattacharya S, Vale L, et al. In vitro fertilization for unexplained subfertility. Cochrane Database Syst Rev. 2005;(2):CD003357.-
39. Liu C. Restricting access to infertility services: what is a justified limitation on reproductive freedom? Minn J Law Sci Technol. 2009;10:291. Available at: http://mjlst.umn.edu/uploads/QJ/WD/QJWDUZ1D-pV28sHvArqp6A/101_liu.pdf. Accessed May 4, 2011.
40. Practice Committee of the American Society for Reproductive Medicine. Optimal evaluation of the infertile female. Fertil Steril. 2006;86(suppl 1):S264-S267.
41. Practice Committee of the American Society for Reproductive Medicine. Current evaluation of amenorrhea. Fertil Steril. 2004;82(suppl 1):S33-S39.
1. Practice Committee of the American Society for Reproductive Medicine. Definitions of infertility and recurrent pregnancy loss. Fertil Steril. 2008;90(suppl):S60.-
2. Boivin J, Bunting L, Collins JA, et al. International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum Reprod. 2007;22:1506-1512.
3. Hull MGR, Glazener CMJ, Kelly NJ, et al. Population studies of causes, treatment, and outcome of infertility. BMJ. 1985;291:1693-1697.
4. Carlsen E, Giwercman A, Keiding N, et al. Evidence for decreasing quality of semen during past 50 years. BMJ. 1992;305:609-613.
5. de La Rochebrochard E, Thonneau P. Paternal age >or=40 years: an important risk factor for infertility. Am J Obstet Gynecol. 2003;189:901-905.
6. Behre HM, Kuhlage J, Gassner C, et al. Prediction of ovulation by urinary hormone measurements with the home use ClearPlan Fertility Monitor: comparison with transvaginal ultrasound scans and serum hormone measurements. Hum Reprod. 2000;15:2478-2482.
7. Brassard M, AinMelk Y, Baillargeon J. Basic infertility including polycystic ovary syndrome. Med Clin North Am. 2008;92:1163-1192.
8. Papaioannou S, Bourdrez P, Varma R, et al. Tubal evaluation in the investigation of subfertility: a structured comparison of tests. BJOG. 2004;111:1313-1321.
9. Mol BW, Collins JA, Van Der Veen F, et al. Cost-effectiveness of hysterosalpingography, laparoscopy, and Chlamydia antibody testing in subfertile couples. Fertil Steril. 2001;75:571-580.
10. Corson SL. Self-prediction of ovulation using a urinary luteinizing hormone test. J Reprod Med. 1986;31(8 suppl):760-763.
11. Kambic R, Gray RH. Interobserver variation in estimation of day of conception intercourse using selected natural family planning charts. Fertil Steril. 1989;51:430-434.
12. Wu CH, Winkel CA. The effect of therapy initiation day on clomiphene citrate therapy. Fertil Steril. 1989;52:564-568.
13. Chang MY, Chiang CH, Hsieh TT, et al. Use of the antral follicle count to predict the outcome of assisted reproductive technologies. Fertil Steril. 1998;69:505-510.
14. de Vet A, Laven JS, de Jong FH, et al. Antimüllerian hormone serum levels: a putative marker for ovarian aging. Fertil Steril. 2002;77:357-362.
15. De Sutter P. Rational diagnosis and treatment in infertility. Best Pract Res Clin Obstet Gynaecol. 2006;20:647-664.
16. Ehrmann DA. Polycystic ovary syndrome. N Engl J Med. 2005;352:1223-1236.
17. Azziz R, Zacur HA. 21-Hydroxylase deficiency in female hyperandrogenism, screening and diagnosis. J Clin Endocrinol Metab. 1989;69:577-584.
18. Bulun SE. Endometriosis. N Engl J Med. 2009;360:268-279.
19. van der Steeg JW, Steures P, Eijkemans MJ, et al. Should the post-coital test (PCT) be part of the routine fertility work-up? Hum Reprod. 2004;19:1373-1379.
20. Themmen APN, Huhtaniemi IT. Mutations of gonadotropins and gonadotropin receptors: elucidating the physiology and pathophysiology of pituitary-gonadal function. Endocr Rev. 2000;21:551-583.
21. Rowe PJ. WHO Manual for the Standardization Investigation and Diagnosis of the Infertile Couple. New York, NY: Cambridge University Press; 1993.
22. World Health Organization Department of Reproductive Health and Research World Health Organization Laboratory Manual for the Examination and Processing of Human Semen. 5th ed. Geneva, Switzerland: World Health Organization; 2010.
23. Male Infertility Best Practice Policy Committee of the American Urological Association; Practical Committee of the American Society for Reproductive Medicine. Report on optimal evaluation of the infertile male. Fertil Steril. 2004;82(suppl 1):S123-S130.
24. Honoré GM, Holden AE, Schenken RS. Pathophysiology and management of proximal tubal blockage. Fertil Steril. 1999;71:785-795.
25. Tummon IS, Asher LF, Martin JS, et al. Randomized controlled trial of superovulation and insemination for infertility associated with minimal or mild endometriosis. Fertil Steril. 1997;68:8-12.
26. Heinonen PK, Saarikoski S, Pystynen P. Reproductive performance of women with uterine anomalies. An evaluation of 182 cases. Acta Obstet Gynecol Scand. 1982;61:157-162.
27. Frisch RE. The right weight: body fat, menarche and ovulation. Baillieres Clin Obstet Gynaecol. 1990;4:419-439.
28. Abraham S, Mira M, Llewellyn-Jones D. Should ovulation be induced in women recovering from an eating disorder or who are compulsive exercisers? Fertil Steril. 1990;53:566-568.
29. Crosignan PG. Management of hyperprolactinemia in infertility. J Reprod Med. 1999;44(12 suppl):1116-1120.
30. Baran S, Api M, Godsedef BP, et al. Comparison of metformin and clomiphene citrate therapy for induction in the polycystic ovary syndrome. Arch Gynecol Obstet. 2010;282:439-443.
31. Kerin JF, Liu JH, Phillipou G, et al. Evidence for a hypothalamic site of action of clomiphene citrate in women. J Clin Endocrinol Metab. 1985;61:265-268.
32. Huang KE. The primary treatment of luteal phase inadequacy: progesterone versus clomiphene citrate. Am J Obstet Gynecol. 1986;155:824-828.
33. Homburg R. Clomiphene citrate—end of an era? A mini-review. Hum Reprod. 2005;20:2043-2051.
34. Hirsh A. Male subfertility. BMJ. 2003;327:669-672.
35. Collins JA, Crosignani PG. Unexplained infertility: a review of diagnosis, prognosis, treatment efficacy and management. Int J Gynaecol Obstet. 1992;39:267-275.
36. Fisch P, Casper RF, Brown SE, et al. Unexplained infertility: evaluation of treatment with clomiphene citrate and human chorionic gonadotropin. Fertil Steril. 1989;51:828-833.
37. Verhulst SM, Cohlen BJ, Hughes E, et al. Intra-uterine insemination for unexplained subfertility. Cochrane Database Syst Rev. 2006;(4):CD001838.-
38. Pandian Z, Bhattacharya S, Vale L, et al. In vitro fertilization for unexplained subfertility. Cochrane Database Syst Rev. 2005;(2):CD003357.-
39. Liu C. Restricting access to infertility services: what is a justified limitation on reproductive freedom? Minn J Law Sci Technol. 2009;10:291. Available at: http://mjlst.umn.edu/uploads/QJ/WD/QJWDUZ1D-pV28sHvArqp6A/101_liu.pdf. Accessed May 4, 2011.
40. Practice Committee of the American Society for Reproductive Medicine. Optimal evaluation of the infertile female. Fertil Steril. 2006;86(suppl 1):S264-S267.
41. Practice Committee of the American Society for Reproductive Medicine. Current evaluation of amenorrhea. Fertil Steril. 2004;82(suppl 1):S33-S39.