User login
Intravascular Involvement of Cutaneous Squamous Cell Carcinoma
Cutaneous squamous cell carcinoma (cSCC) is the second most common form of skin cancer after basal cell carcinoma.1 With an estimated 700,000 cases reported annually in the United States, the incidence of cSCC continues to increase.2 Most patients with cSCC have an excellent prognosis after surgical clearance, with Mohs micrographic surgery (MMS) being the most successful treatment, followed by excision and electrodesiccation and curettage. A subset of patients with cSCC carry an increased risk of local recurrence, lymph node metastasis, and disease-specific death. A meta-analysis of 36 studies found that statistically significant risk factors for recurrence of cSCC included thickness greater than 2 mm (risk ratio [RR], 9.64; 95% CI, 1.30-1.52), invasion beyond the subcutaneous fat (RR, 7.61; 95% CI, 4.17-13.88), perineural invasion (RR, 4.30; 95% CI, 2.80-6.60), diameter greater than 20 mm (RR, 3.22; 95% CI, 1.91-5.45), location on temple (RR, 3.20; 95% CI, 1.12-9.15), and poor differentiation (RR, 2.66; 95% CI, 1.72-4.14).3 Additional risk factors for cSCC metastasis included location on the temple, ear, or lip, as well as a history of immunosuppression. Factors for disease-specific death were diameter greater than 20 mm, poor differentiation, location on the ear or lip, invasion beyond the subcutaneous fat, and perineural invasion.3 Perineural and/or lymphovascular invasion is considered high risk, but despite being linked to negative outcomes, there are no treatment guidelines based on lymphovascular (intravascular) invasion.4 We present a case of intravascular involvement found during MMS and treated with adjuvant radiotherapy after surgery. We share this case with the goal of discussing management in such cases and highlighting the need for improved definitive guidelines for high-risk cSCCs.
Case Report
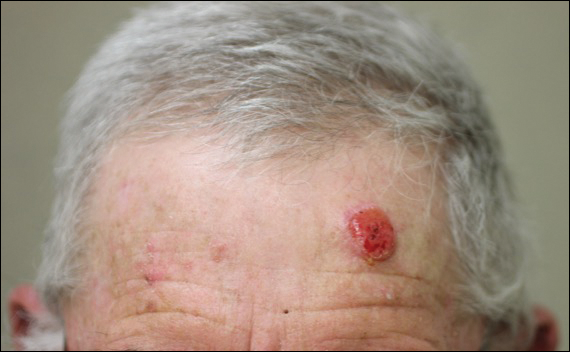
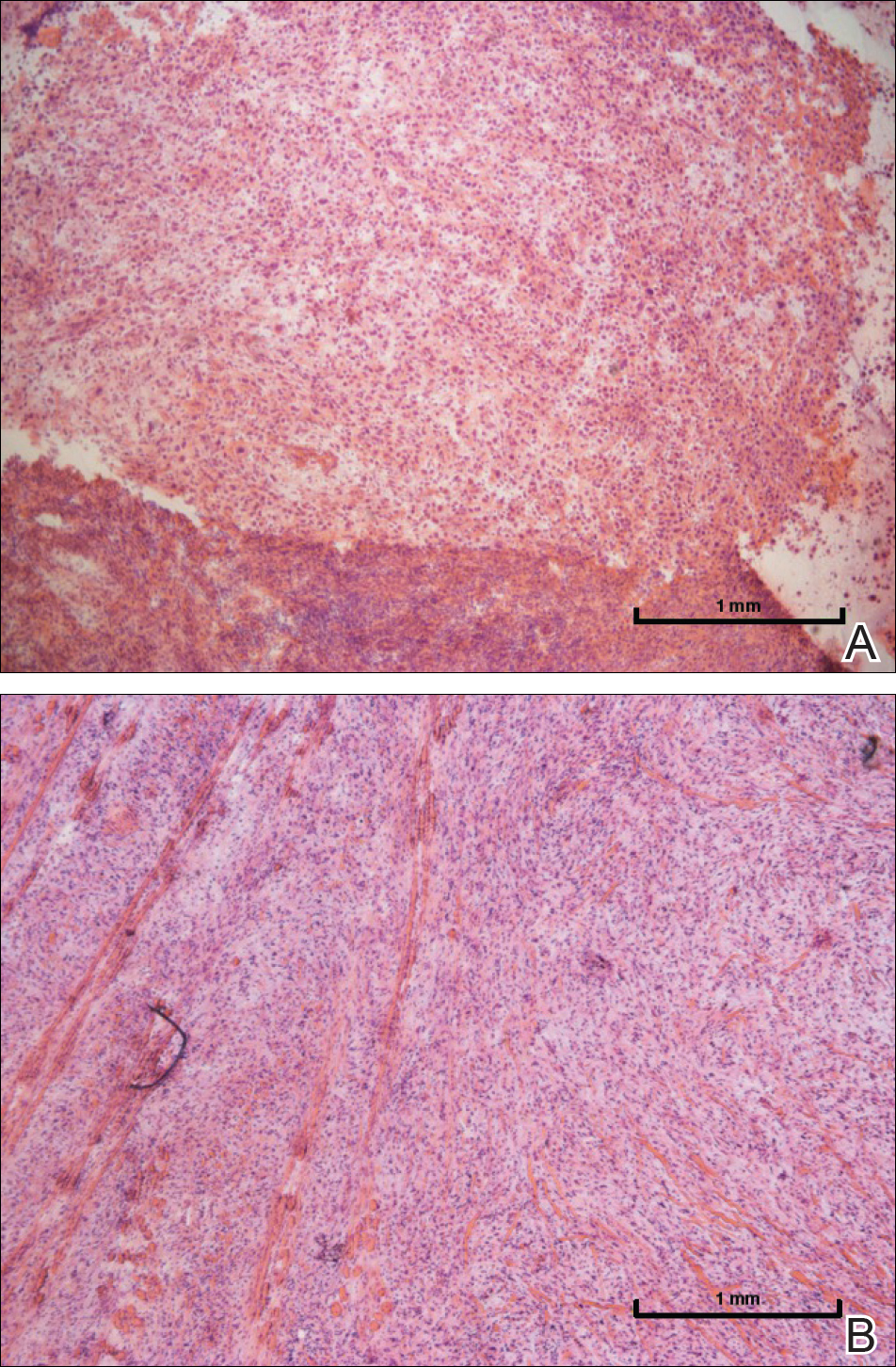
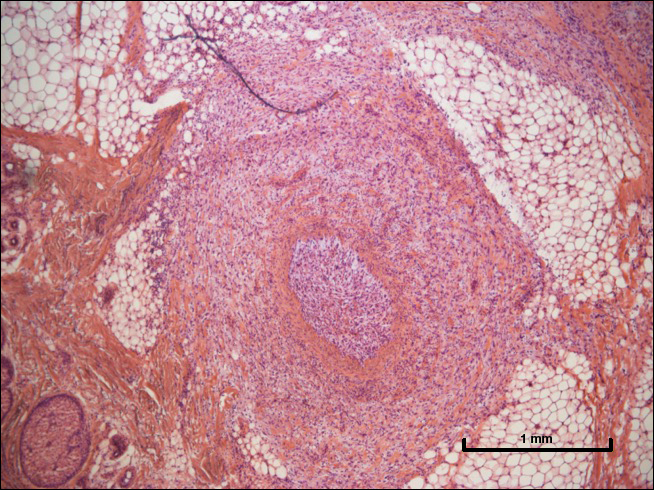
A 72-year-old man presented with a rapidly growing lesion on the left side of the forehead of 1 year’s duration. His medical history was remarkable for B-cell lymphoma, which was currently in remission following chemotherapy 10 years prior. The lesion started as a small, red, dry patch that the patient initially thought was eczema. The site progressively enlarged to a red tumor measuring 2.4×2.0 cm (Figure 1), and the patient presented to the dermatology department for further evaluation. There was no clinical evidence of lymphadenopathy. A skin biopsy confirmed a moderately differentiated cSCC with a positive deep margin (Figure 2). Due to the tumor’s location, histology, size, and poorly defined borders, the patient was referred for treatment with MMS. The lesion was removed in a total of 2 stages and 4 sections. In addition to a proliferation of spindled tumor cells seen during surgery, which was consistent with cSCC, an intravascular component was noted despite clear margins after the surgery (Figure 3). The aggressive histology of intravascular involvement was subsequently confirmed by the academic dermatopathologist at our institution. With the evidence of an intravascular component of this patient’s cSCC, there was concern about further metastatic disease. After discussing the more aggressive histology type and size of the cSCC with the patient, he underwent subsequent computed tomography of the head, neck, and chest. Fortunately, this imaging did not show evidence of metastatic disease; thus, final staging of the cSCC was cT2N0M0. After interdisciplinary discussion and consultation with radiation oncology, the site of the cSCC was treated with adjuvant radiotherapy. The patient received a total of 6600 cGy delivered in 33 fractions of 200 cGy, each using an en face technique and 6 eV over a total treatment course of 48 days.
One year after undergoing MMS and adjuvant radiotherapy, the patient remains free of cSCC recurrence or metastases and still undergoes regular interdisciplinary monitoring. Without clear guidelines on the treatment of patients with intravascular involvement of cSCC, we relied on prior experience with similar cases.
Comment
This case highlights the challenge in managing patients with high-risk cSCC, as the current guidelines provided by the American Joint Committee on Cancer (AJCC) and the National Comprehensive Cancer Network (NCCN) vary on the inclusion of intravascular involvement of cSCC as high risk and treatment is at the discretion of the provider in such circumstances.5-7 Both the AJCC and the NCCN have defined high-risk factors and staging for cSCC. The AJCC 8th edition (AJCC-8) revised guidelines include several high-risk factors of cSCC, including tumor diameter of 4 cm or larger leading to upstaging of a tumor from T2 to T3, invasion into or beyond the level of the subcutaneous tissue, depth of invasion greater than 6 mm, and large-caliber perineural invasion, and removed poorly differentiated histology from the AJCC-8 guidelines compared to the AJCC-7 guidelines. According to the AJCC-8 guidelines, location on the ear or lip, desmoplastic or spindle cell features, lymphovascular invasion, and immunosuppression do not affect tumor staging. The AJCC’s criteria for its TNM staging system strictly focus on features of the primary tumor and do not include clinical risk factors such as recurrence or immunosuppression. In contrast, the NCCN does include lymphovascular invasion as a high-risk factor of cSCC.
Intravascular invasion plays a considerable role in patient survival in certain cancers (eg, breast, gastric, prostate). In cutaneous malignancies, such as melanoma and SCC, metastasis more commonly occurs via lymphatic spread. When present, vascular invasion typically coexists with lymphatic involvement. The presence of microscopic lymphovascular invasion in cSCCs has not been definitively proven to increase the risk of metastases.8 However, multivariate analysis has shown that lymphovascular invasion independently predicts nodal metastasis and disease-specific death.9 As such, there are no guidelines on sentinel lymph node biopsy or adjuvant therapy in the setting of lymphovascular involvement of cSCCs. A survey-based study of 117 Mohs surgeons found a lack of consistency in their approaches to evaluation and management of high-risk SCCs. Most respondents noted perineural invasion and in-transit metastasis as the main findings that would lead to radiologic nodal staging, sentinel lymph node biopsy, or adjuvant radiotherapy, but they highlighted the lack of evidence-based treatment guidelines.4 High-risk cSCC can be treated via MMS or conventional surgery with safe excision margins. Adjuvant radiotherapy can reduce tumor recurrence and improve survival and therefore should be considered in cases of advanced or high-risk cSCCs, such as in our case.
The lack of consensus over the definition of high-risk cSCCs, a lack of high-quality therapeutic studies, and the absence of a prognostic model that integrates multiple risk factors all have made the prediction of outcomes and the formation of definitive management of cSCCs challenging. Multidisciplinary teams and vigilant monitoring are crucial in the successful management of high-risk cSCC, but further studies and reports are needed to develop definitive treatment algorithms.
- Karia PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012. J Am Acad Dermatol. 2013;68:957-966.
- Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283-287.
- Thompson AK, Kelley BF, Prokop LJ, et al. Risk factors for cutaneous squamous cell carcinoma recurrence, metastasis, and disease-specific death: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:419-428.
- Jambusaria-Pahlajani A, Hess SD, Katz KA, et al. Uncertainty in the perioperative management of high-risk cutaneous squamous cell carcinoma among Mohs surgeons. Arch Dermatol. 2010;146:1225-1231.
- Motaparthi K, Kapil JP, Velazquez EF. Cutaneous squamous cell carcinoma: review of the eighth edition of the American Joint Committee on Cancer Staging Guidelines, prognostic factors, and histopathologic variants. Adv Anat Pathol. 2017;24:171-194.
- Amin MD, Edge SB, Greene FL, et al, eds. AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer; 2017.
- National Comprehensive Cancer Network. Squamous Cell Skin Cancer (Version 2.2018). https://www.nccn.org/professionals/physician_gls/pdf/squamous.pdf. Accessed June 27, 2018.
- Lonie S, Niumsawatt V, Castley A. A prognostic dilemma of basal cell carcinoma with intravascular invasion. Plast Reconstr Surg Glob Open. 2016;4:e1046.
- Carter JB, Johnson MM, Chua TL, et al. Outcomes of primary cutaneous squamous cell carcinoma with perineural invasion: an 11-year cohort study. JAMA Dermatol. 2013;149:35-41.
Cutaneous squamous cell carcinoma (cSCC) is the second most common form of skin cancer after basal cell carcinoma.1 With an estimated 700,000 cases reported annually in the United States, the incidence of cSCC continues to increase.2 Most patients with cSCC have an excellent prognosis after surgical clearance, with Mohs micrographic surgery (MMS) being the most successful treatment, followed by excision and electrodesiccation and curettage. A subset of patients with cSCC carry an increased risk of local recurrence, lymph node metastasis, and disease-specific death. A meta-analysis of 36 studies found that statistically significant risk factors for recurrence of cSCC included thickness greater than 2 mm (risk ratio [RR], 9.64; 95% CI, 1.30-1.52), invasion beyond the subcutaneous fat (RR, 7.61; 95% CI, 4.17-13.88), perineural invasion (RR, 4.30; 95% CI, 2.80-6.60), diameter greater than 20 mm (RR, 3.22; 95% CI, 1.91-5.45), location on temple (RR, 3.20; 95% CI, 1.12-9.15), and poor differentiation (RR, 2.66; 95% CI, 1.72-4.14).3 Additional risk factors for cSCC metastasis included location on the temple, ear, or lip, as well as a history of immunosuppression. Factors for disease-specific death were diameter greater than 20 mm, poor differentiation, location on the ear or lip, invasion beyond the subcutaneous fat, and perineural invasion.3 Perineural and/or lymphovascular invasion is considered high risk, but despite being linked to negative outcomes, there are no treatment guidelines based on lymphovascular (intravascular) invasion.4 We present a case of intravascular involvement found during MMS and treated with adjuvant radiotherapy after surgery. We share this case with the goal of discussing management in such cases and highlighting the need for improved definitive guidelines for high-risk cSCCs.
Case Report
A 72-year-old man presented with a rapidly growing lesion on the left side of the forehead of 1 year’s duration. His medical history was remarkable for B-cell lymphoma, which was currently in remission following chemotherapy 10 years prior. The lesion started as a small, red, dry patch that the patient initially thought was eczema. The site progressively enlarged to a red tumor measuring 2.4×2.0 cm (Figure 1), and the patient presented to the dermatology department for further evaluation. There was no clinical evidence of lymphadenopathy. A skin biopsy confirmed a moderately differentiated cSCC with a positive deep margin (Figure 2). Due to the tumor’s location, histology, size, and poorly defined borders, the patient was referred for treatment with MMS. The lesion was removed in a total of 2 stages and 4 sections. In addition to a proliferation of spindled tumor cells seen during surgery, which was consistent with cSCC, an intravascular component was noted despite clear margins after the surgery (Figure 3). The aggressive histology of intravascular involvement was subsequently confirmed by the academic dermatopathologist at our institution. With the evidence of an intravascular component of this patient’s cSCC, there was concern about further metastatic disease. After discussing the more aggressive histology type and size of the cSCC with the patient, he underwent subsequent computed tomography of the head, neck, and chest. Fortunately, this imaging did not show evidence of metastatic disease; thus, final staging of the cSCC was cT2N0M0. After interdisciplinary discussion and consultation with radiation oncology, the site of the cSCC was treated with adjuvant radiotherapy. The patient received a total of 6600 cGy delivered in 33 fractions of 200 cGy, each using an en face technique and 6 eV over a total treatment course of 48 days.



One year after undergoing MMS and adjuvant radiotherapy, the patient remains free of cSCC recurrence or metastases and still undergoes regular interdisciplinary monitoring. Without clear guidelines on the treatment of patients with intravascular involvement of cSCC, we relied on prior experience with similar cases.
Comment
This case highlights the challenge in managing patients with high-risk cSCC, as the current guidelines provided by the American Joint Committee on Cancer (AJCC) and the National Comprehensive Cancer Network (NCCN) vary on the inclusion of intravascular involvement of cSCC as high risk and treatment is at the discretion of the provider in such circumstances.5-7 Both the AJCC and the NCCN have defined high-risk factors and staging for cSCC. The AJCC 8th edition (AJCC-8) revised guidelines include several high-risk factors of cSCC, including tumor diameter of 4 cm or larger leading to upstaging of a tumor from T2 to T3, invasion into or beyond the level of the subcutaneous tissue, depth of invasion greater than 6 mm, and large-caliber perineural invasion, and removed poorly differentiated histology from the AJCC-8 guidelines compared to the AJCC-7 guidelines. According to the AJCC-8 guidelines, location on the ear or lip, desmoplastic or spindle cell features, lymphovascular invasion, and immunosuppression do not affect tumor staging. The AJCC’s criteria for its TNM staging system strictly focus on features of the primary tumor and do not include clinical risk factors such as recurrence or immunosuppression. In contrast, the NCCN does include lymphovascular invasion as a high-risk factor of cSCC.
Intravascular invasion plays a considerable role in patient survival in certain cancers (eg, breast, gastric, prostate). In cutaneous malignancies, such as melanoma and SCC, metastasis more commonly occurs via lymphatic spread. When present, vascular invasion typically coexists with lymphatic involvement. The presence of microscopic lymphovascular invasion in cSCCs has not been definitively proven to increase the risk of metastases.8 However, multivariate analysis has shown that lymphovascular invasion independently predicts nodal metastasis and disease-specific death.9 As such, there are no guidelines on sentinel lymph node biopsy or adjuvant therapy in the setting of lymphovascular involvement of cSCCs. A survey-based study of 117 Mohs surgeons found a lack of consistency in their approaches to evaluation and management of high-risk SCCs. Most respondents noted perineural invasion and in-transit metastasis as the main findings that would lead to radiologic nodal staging, sentinel lymph node biopsy, or adjuvant radiotherapy, but they highlighted the lack of evidence-based treatment guidelines.4 High-risk cSCC can be treated via MMS or conventional surgery with safe excision margins. Adjuvant radiotherapy can reduce tumor recurrence and improve survival and therefore should be considered in cases of advanced or high-risk cSCCs, such as in our case.
The lack of consensus over the definition of high-risk cSCCs, a lack of high-quality therapeutic studies, and the absence of a prognostic model that integrates multiple risk factors all have made the prediction of outcomes and the formation of definitive management of cSCCs challenging. Multidisciplinary teams and vigilant monitoring are crucial in the successful management of high-risk cSCC, but further studies and reports are needed to develop definitive treatment algorithms.
Cutaneous squamous cell carcinoma (cSCC) is the second most common form of skin cancer after basal cell carcinoma.1 With an estimated 700,000 cases reported annually in the United States, the incidence of cSCC continues to increase.2 Most patients with cSCC have an excellent prognosis after surgical clearance, with Mohs micrographic surgery (MMS) being the most successful treatment, followed by excision and electrodesiccation and curettage. A subset of patients with cSCC carry an increased risk of local recurrence, lymph node metastasis, and disease-specific death. A meta-analysis of 36 studies found that statistically significant risk factors for recurrence of cSCC included thickness greater than 2 mm (risk ratio [RR], 9.64; 95% CI, 1.30-1.52), invasion beyond the subcutaneous fat (RR, 7.61; 95% CI, 4.17-13.88), perineural invasion (RR, 4.30; 95% CI, 2.80-6.60), diameter greater than 20 mm (RR, 3.22; 95% CI, 1.91-5.45), location on temple (RR, 3.20; 95% CI, 1.12-9.15), and poor differentiation (RR, 2.66; 95% CI, 1.72-4.14).3 Additional risk factors for cSCC metastasis included location on the temple, ear, or lip, as well as a history of immunosuppression. Factors for disease-specific death were diameter greater than 20 mm, poor differentiation, location on the ear or lip, invasion beyond the subcutaneous fat, and perineural invasion.3 Perineural and/or lymphovascular invasion is considered high risk, but despite being linked to negative outcomes, there are no treatment guidelines based on lymphovascular (intravascular) invasion.4 We present a case of intravascular involvement found during MMS and treated with adjuvant radiotherapy after surgery. We share this case with the goal of discussing management in such cases and highlighting the need for improved definitive guidelines for high-risk cSCCs.
Case Report
A 72-year-old man presented with a rapidly growing lesion on the left side of the forehead of 1 year’s duration. His medical history was remarkable for B-cell lymphoma, which was currently in remission following chemotherapy 10 years prior. The lesion started as a small, red, dry patch that the patient initially thought was eczema. The site progressively enlarged to a red tumor measuring 2.4×2.0 cm (Figure 1), and the patient presented to the dermatology department for further evaluation. There was no clinical evidence of lymphadenopathy. A skin biopsy confirmed a moderately differentiated cSCC with a positive deep margin (Figure 2). Due to the tumor’s location, histology, size, and poorly defined borders, the patient was referred for treatment with MMS. The lesion was removed in a total of 2 stages and 4 sections. In addition to a proliferation of spindled tumor cells seen during surgery, which was consistent with cSCC, an intravascular component was noted despite clear margins after the surgery (Figure 3). The aggressive histology of intravascular involvement was subsequently confirmed by the academic dermatopathologist at our institution. With the evidence of an intravascular component of this patient’s cSCC, there was concern about further metastatic disease. After discussing the more aggressive histology type and size of the cSCC with the patient, he underwent subsequent computed tomography of the head, neck, and chest. Fortunately, this imaging did not show evidence of metastatic disease; thus, final staging of the cSCC was cT2N0M0. After interdisciplinary discussion and consultation with radiation oncology, the site of the cSCC was treated with adjuvant radiotherapy. The patient received a total of 6600 cGy delivered in 33 fractions of 200 cGy, each using an en face technique and 6 eV over a total treatment course of 48 days.



One year after undergoing MMS and adjuvant radiotherapy, the patient remains free of cSCC recurrence or metastases and still undergoes regular interdisciplinary monitoring. Without clear guidelines on the treatment of patients with intravascular involvement of cSCC, we relied on prior experience with similar cases.
Comment
This case highlights the challenge in managing patients with high-risk cSCC, as the current guidelines provided by the American Joint Committee on Cancer (AJCC) and the National Comprehensive Cancer Network (NCCN) vary on the inclusion of intravascular involvement of cSCC as high risk and treatment is at the discretion of the provider in such circumstances.5-7 Both the AJCC and the NCCN have defined high-risk factors and staging for cSCC. The AJCC 8th edition (AJCC-8) revised guidelines include several high-risk factors of cSCC, including tumor diameter of 4 cm or larger leading to upstaging of a tumor from T2 to T3, invasion into or beyond the level of the subcutaneous tissue, depth of invasion greater than 6 mm, and large-caliber perineural invasion, and removed poorly differentiated histology from the AJCC-8 guidelines compared to the AJCC-7 guidelines. According to the AJCC-8 guidelines, location on the ear or lip, desmoplastic or spindle cell features, lymphovascular invasion, and immunosuppression do not affect tumor staging. The AJCC’s criteria for its TNM staging system strictly focus on features of the primary tumor and do not include clinical risk factors such as recurrence or immunosuppression. In contrast, the NCCN does include lymphovascular invasion as a high-risk factor of cSCC.
Intravascular invasion plays a considerable role in patient survival in certain cancers (eg, breast, gastric, prostate). In cutaneous malignancies, such as melanoma and SCC, metastasis more commonly occurs via lymphatic spread. When present, vascular invasion typically coexists with lymphatic involvement. The presence of microscopic lymphovascular invasion in cSCCs has not been definitively proven to increase the risk of metastases.8 However, multivariate analysis has shown that lymphovascular invasion independently predicts nodal metastasis and disease-specific death.9 As such, there are no guidelines on sentinel lymph node biopsy or adjuvant therapy in the setting of lymphovascular involvement of cSCCs. A survey-based study of 117 Mohs surgeons found a lack of consistency in their approaches to evaluation and management of high-risk SCCs. Most respondents noted perineural invasion and in-transit metastasis as the main findings that would lead to radiologic nodal staging, sentinel lymph node biopsy, or adjuvant radiotherapy, but they highlighted the lack of evidence-based treatment guidelines.4 High-risk cSCC can be treated via MMS or conventional surgery with safe excision margins. Adjuvant radiotherapy can reduce tumor recurrence and improve survival and therefore should be considered in cases of advanced or high-risk cSCCs, such as in our case.
The lack of consensus over the definition of high-risk cSCCs, a lack of high-quality therapeutic studies, and the absence of a prognostic model that integrates multiple risk factors all have made the prediction of outcomes and the formation of definitive management of cSCCs challenging. Multidisciplinary teams and vigilant monitoring are crucial in the successful management of high-risk cSCC, but further studies and reports are needed to develop definitive treatment algorithms.
- Karia PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012. J Am Acad Dermatol. 2013;68:957-966.
- Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283-287.
- Thompson AK, Kelley BF, Prokop LJ, et al. Risk factors for cutaneous squamous cell carcinoma recurrence, metastasis, and disease-specific death: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:419-428.
- Jambusaria-Pahlajani A, Hess SD, Katz KA, et al. Uncertainty in the perioperative management of high-risk cutaneous squamous cell carcinoma among Mohs surgeons. Arch Dermatol. 2010;146:1225-1231.
- Motaparthi K, Kapil JP, Velazquez EF. Cutaneous squamous cell carcinoma: review of the eighth edition of the American Joint Committee on Cancer Staging Guidelines, prognostic factors, and histopathologic variants. Adv Anat Pathol. 2017;24:171-194.
- Amin MD, Edge SB, Greene FL, et al, eds. AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer; 2017.
- National Comprehensive Cancer Network. Squamous Cell Skin Cancer (Version 2.2018). https://www.nccn.org/professionals/physician_gls/pdf/squamous.pdf. Accessed June 27, 2018.
- Lonie S, Niumsawatt V, Castley A. A prognostic dilemma of basal cell carcinoma with intravascular invasion. Plast Reconstr Surg Glob Open. 2016;4:e1046.
- Carter JB, Johnson MM, Chua TL, et al. Outcomes of primary cutaneous squamous cell carcinoma with perineural invasion: an 11-year cohort study. JAMA Dermatol. 2013;149:35-41.
- Karia PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012. J Am Acad Dermatol. 2013;68:957-966.
- Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283-287.
- Thompson AK, Kelley BF, Prokop LJ, et al. Risk factors for cutaneous squamous cell carcinoma recurrence, metastasis, and disease-specific death: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:419-428.
- Jambusaria-Pahlajani A, Hess SD, Katz KA, et al. Uncertainty in the perioperative management of high-risk cutaneous squamous cell carcinoma among Mohs surgeons. Arch Dermatol. 2010;146:1225-1231.
- Motaparthi K, Kapil JP, Velazquez EF. Cutaneous squamous cell carcinoma: review of the eighth edition of the American Joint Committee on Cancer Staging Guidelines, prognostic factors, and histopathologic variants. Adv Anat Pathol. 2017;24:171-194.
- Amin MD, Edge SB, Greene FL, et al, eds. AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer; 2017.
- National Comprehensive Cancer Network. Squamous Cell Skin Cancer (Version 2.2018). https://www.nccn.org/professionals/physician_gls/pdf/squamous.pdf. Accessed June 27, 2018.
- Lonie S, Niumsawatt V, Castley A. A prognostic dilemma of basal cell carcinoma with intravascular invasion. Plast Reconstr Surg Glob Open. 2016;4:e1046.
- Carter JB, Johnson MM, Chua TL, et al. Outcomes of primary cutaneous squamous cell carcinoma with perineural invasion: an 11-year cohort study. JAMA Dermatol. 2013;149:35-41.
Resident Pearl
- Intravascular (also referred to as lymphovascular when involving vessels and/or lymphatics) invasion of cutaneous squamous cell carcinoma can be considered a high-risk factor that may warrant adjuvant therapy.
