User login
Cost Drivers Associated withClostridium difficile-Associated Diarrhea in a Hospital Setting
From HealthCore, Wilmington, DE, and Cubist Pharma-ceuticals, San Diego, CA.
Abstract
- Objectives: To describe trends in inpatient resource utilization and potential cost drivers of Clostridium difficile-associated diarrhea (CDAD) treated in the hospital.
- Methods: Retrospective medical record review included 500 patients with ≥1 inpatient medical claim diagnosis of CDAD (ICD-9-CM: 008.45) between 01/01/2005-10/31/2010. Information was collected on patient demographics, admission diagnoses, laboratory data, and CDAD-related characteristics and discharge. Hospital costs were evaluated for the entire inpatient episode and prorated for the duration of the CDAD episode (ie, CDAD diagnosis date to diarrhea resolution/discharge date).
- Results: The cohort was mostly female (62%), Caucasian (72%), with mean (SD) age 66 (±17.6) years. 60% had diagnosis of CDAD or presence of diarrhea at admission. CDAD diagnosis was confirmed with laboratory test in 92% of patients. ~44% had mild CDAD, 35% had severe CDAD. Following CDAD diagnosis, approximately 53% of patients were isolated for ≥1 days, 12% transferred to the ICU for a median (Q1–Q3) length of stay of 8 (5–15) days. Two-thirds received gastrointestinal or infectious disease consult. Median time from CDAD diagnosis to discharge was 6 (4–9) days; 5.5 (4–8) days for patients admitted with CDAD, 6.5 (4–10) days for those with hospital-acquired CDAD. The mean and median costs (2011 USD) for CDAD-associated hospitalization were $35,621 and $13,153, respectively.
- Conclusion: Patients with CDAD utilize numerous expensive resources during hospitalization including laboratory tests, isolation, prolonged ICU stay, and specialist consultations.
Clostridium difficile, classified as an urgent public health threat by the Centers for Disease Control and Prevention (CDC), causes approximately 250,000 hospitalizations and an estimated 14,000 deaths per year in the United States [1]. An estimated 15% to 25% of patients with C. difficile-associated diarrhea (CDAD) will experience at least 1 recurrence [2-4], frequently requiring rehospitalization [5]. The high incidence of primary and recurrent infections contributes to a substantial burden associated with CDAD in terms of extended and repeat hospital stays [6,7].
Conservative estimates of the direct annual costs of CDAD in the United States over the past 15 years range from $1.1 billion [8] to $3.2 billion, with an average cost per stay of $10,212 for patients hospitalized with a principal diagnosis of CDAD or a CDAD-related symptom [5]. O’Brien et al estimated that costs associated with rehospitalizations accounted for 11% of overall CDAD-related hospital costs;when considering all CDAD-related hospitalizations, including both initial and subsequent rehospitalizations for recurrent infection and not accounting for post-acute or outpatient care, the 2-year cumulative cost was estimated to be $51.2 million. While studies have yielded varying assessments of the actual CDAD burden [5–10], they all suggest that CDAD burden is considerable and that extended hospital stays are the major component of CDAD-associated costs [9,10]. In a claims-based study by Quimbo et al [11], when multiple and diverse cohorts of CDAD patients at elevated risk for recurrence were matched with patients with similar underlying at-risk condition(s) but no CDAD, the CDAD at-risk groups had an incremental LOS per hospitalization ranging from approximately 3 to 18 days and an incremental cost burden ranging from a mean of $11,179 to $115,632 (2011 USD) per stay.
While it is recognized that CDAD carries significant cost burden and is driven by LOS, current literature is lacking regarding the characteristics of these hospital stays. Building on the Quimbo et al study, the current study was designed to probe further into the nature of the burden (ie, resource use) incurred during the course of CDAD hospitalizations. As such, the objective of this study was to identify the common trends in hospital-related resource utilization and describe the potential drivers that affect the cost burden of CDAD using hospital medical record data.
Methods
Population
Patients were selected for this retrospective medical record review from the HealthCore Integrated Research Database (HealthCore, Wilmington, DE). The database contains a broad, clinically rich and geographically diverse spectrum of longitudinal claims information from one of the largest commercially insured populations in the United States, representing 48 million lives. We identified 21,177 adult (≥ 18 years) patients with at least 1 inpatient claim with an International Classification of Diseases, 9th Edition, Clinical Modification (ICD-9-CM) diagnosis code for C. difficile infection (CDI; 008.45) between 1 January 2005 and 31 October 2010 (intake period). All patients had at least 12 months of prior and continuous medical and pharmacy health plan eligibility prior to the incident CDAD-associated hospitalization within the database. Additional details regarding this cohort identification has been published previously [11]. The study was undertaken in accordance with Health Insurance Portability and Accountability Act (HIPAA) guidelines and the necessary central institutional review board approval was obtained prior to medical record identification and abstraction.
Sampling Strategy
Medical Record Abstraction
During the record abstraction process, information was collected on patients’ race/ethnicity, body mass index (BMI), admission diagnosis and other conditions, point of entry and prior location, body temperature and laboratory data (eg, creatinine and albumin values, white blood cell [WBC] count), diarrhea and stool characteristics, CDAD diagnosis date, CDAD-specific characteristics, severity, complications, and related tests/procedures, CDAD treatments (eg, dose, duration, and formulation of medications), hospital LOS, including stays in the intensive care unit (ICU), cardiac care unit (CCU) following CDAD diagnosis; consultations provided by gastrointestinal, infectious disease, intensivists, or surgery care specialists, and discharge summary on disposition, CDAD status, and medications prescribed. Standardized data collection forms were used by trained nurses or pharmacists to collect information from the medical records and inter-rater reliability testing with a 0.9 cutoff was required to confirm accuracy. To ensure consistency, a pilot test of the first 20 abstracted records were re-abstracted by the research team. Last, quality checks were implemented throughout the abstraction process to identify any inconsistencies or data entry errors including coding errors and atypical, unrealistic data entry patterns (eg, identical values for a particular data field entered on multiple records; implausible or erratic inputs; or a high percentage of missing data points). Missing data were not imputed.
Study Definitions
Diarrhea was defined as 3 or more unformed (includes bloody, watery, thin, loose, soft, and/or unformed stool) bowel movements per day.CDAD severity was classified as mild (4–5 unformed bowel movements per day or WBC ≤ 2000/mm3); moderate (6–9 unformed bowel movements per day or WBC between 12,001/mm3 and 15,000/mm3); or severe (≥10 unformed bowel movements per day or WBC ≥15,001/mm3) [12,13]. Diarrhea was considered to be resolved when the patient had no more than 3 unformed stools for 2 consecutive days and lasting until treatment was completed, with no additional therapy required for CDAD as of the second day after the end of the course of therapy [2,14].CDAD episode was defined as the duration from the date of the CDAD diagnosis or confirmation (whichever occurred first), to the date of diarrhea resolution (where documented) or discharge date.
Cost Measures
The total hospital health plan paid costs for the entire inpatient episode (includes treatment costs, diagnostics, services provided, etc.) were estimated using medical claims present in the database and pertaining to the hospitalization from where medical records were abstracted. Then the proportionate amount for the duration of the CDAD episode (from CDAD diagnosis to the diarrhea resolution date or the discharge date in cases where the resolution date could not be ascertained) was calculated to estimate the average CDAD associated in-hospital costs.
Analysis
Means (± standard deviation [SD]), medians (interquartile range Q1 to Q3), and relative frequencies were calculated for continuous and categorical data, respectively. This analysis was descriptive in nature; hence, no statistical tests to determine significance were conducted.
Results
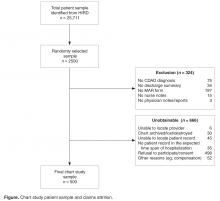
We had a 55.3% success rate in obtaining the medical records from the contacted hospitals with refusal to participate/consent by the hospital in question being the most frequent reason for failure in 3 out of 4 cases. An additional attrition of 39.3% was observed among the medical records received, with absence of a MAR form (23.9%) and confirmatory CDAD diagnosis or note (9.1%) being the most frequent criteria for discarding an available record prior to abstraction (Figure).
Patient Characteristics
CDAD Characteristics and Complications
Using a derived definition of severity, most CDAD cases were classified either as
CDAD-Related Resource Utilization
Following CDAD diagnosis, more than half of the study patients were isolated for 1 or more days. While the majority of patients with CDAD (74.0%) stayed in a general hospital room, 12.4% stayed in the ICU for a mean duration of 12.1 (± 12.3) days (Table 3). Half of these ICU patients required
About one-third of patients consulted a gastrointestinal or infectious disease specialist at least once. Among these patients, assuming that a patient following an initial specialist consultation would have follow-up visits at least once a day (formal or informal) for the remainder of the CDAD episode, we estimate that there were an average of 8.7 (± 15.6) and 11.6 (± 19.4) GI or ID specialist visits respectively during the CDAD episode.
Nearly all patients had their CDAD diagnosis confirmed by laboratory tests. CDAD virulence was identified as toxin A and/or toxin B in 47.6% of the samples. However, nearly three-fifths of patients also underwent 1 or more nondiagnostic tests including endoscopy, colonoscopy, computed axial tomography (CAT), or magnetic resonance imaging (MRI) scans, sigmoidoscopy, and/or other obstructive series tests during the CDAD episode.
CDAD Treatment
CDAD at Discharge
Hospitalization Costs
Based on claims data, the mean (±SD) and median (Q1–Q3) plan costs for the duration of a CDAD-associated hospitalization (2011 USD) for these 500 patients were found to be $35,621 (± $100,502) and $13,153 ($8,209–$26,893), respectively.
Discussion
While multiple studies have documented the considerable economic burden associated with CDAD [5–10], this study was the first to our knowledge to evaluate the specific hospital resources that are used during an extended hospital stay for CDAD. This real-world analysis, in conjunction with the Quimbo et al claims analysis, demonstrated the significant burden associated with CDAD in terms of both fixed costs (eg, hospital stay) as well as the variable components that drive these expenditures (eg, consultations, ICU stay).
The mean ($35,621) and median ($13,153) total costs associated with the CDAD segment of the hospitalization, as measured via the claims, were quite high despite a greater prevalence of mild CDAD rather than severe infection, and required only a general hospital room stay. Both of the above CDAD hospital cost measures were well above the mean US general hospitalization cost of $11,666 and the median cost of $7334 measured from Healthcare Cost and Utilization Project data [15]. However, the mean cost of hospitalization reported in the current study falls within the range of previously reported costs for CDAD-associated hospitalizations [5,8,10]. While the mean cost may have been disproportionately inflated by a few extreme cases, the median CDAD-associated hospitalization cost was nearly twice the median cost of an average general hospital stay in the US [15]. Our finding that these elevated costs were observed among patients with mild CDAD and its relative magnitude compared with the average hospitalization costs (approximately 3-fold higher) were also consistent with the literature. For instance, Pakyz and colleagues reported that relative to patients without CDAD, hospital costs were tripled for patients with low-severity CDAD and 10% higher for those with more severe CDAD, presumably because CDAD resulted in costly complications that prolonged what would have otherwise been a short, simple hospital stay [10].
Type of hospital room could also be an important driver of cost. While most patients stayed in general hospital rooms, more than half were isolated for at least a day, and 12% of patients required nearly 2 weeks of intensive care. Taken together, 26% of patients in the current study were required to stay in a special care unit or a non–general hospital room for 5.5 to 12.2 days. This is consistent with the 28% of patients with CDAD that required stay on a special care unit previously reported by O’Brien et al [5].Additionally, previous research from Canadian health care data has shown that a single ICU stay costs an average of $7000 more per patient per day than a general hospital room (1992 Canadian dollars) or $9589 (2013 USD calculated using historical exchange rate data and adjusted for inflation) [16].However, despite this additional cost and resource burden, it appears that overall only 53.4% of all patients received care within an isolated setting as guidelines recommended.
Repeated specialist visits, procedures and multiple testing (concomitant diagnostic EIA and nondiagnostic tests) potentially added to the health care resource utilization and costs, along with the extra resources associated with specialized hospital care. We found that roughly one-third of patients consulted a specialist, although we did not distinguish between ‘formal’ and ‘informal’ consultations. Numerous studies published over the past 2 decades have demonstrated increased costs and resource utilization associated with specialist consultations [17–21]. Although the focused knowledge and experience of specialists may reduce morbidity and mortality [18,21], specialists are more likely than generalists to order more diagnostic tests, perform more procedures, and keep patients hospitalized longer and in ICUs, all of which contribute to higher costs without necessarily leading to improved health outcomes [21].
Limitations
One major limitation of this study was the inability to assess the individual costs of the resources used for each individual patient either through the medical charts or via claims. Additionally, the burden of CDAD was found to continue beyond the hospital stay, with documented evidence of persisting infection in 84% of patients at the point of discharge. Since the medical records obtained were limited to a single hospitalization and a single place of service, the data capture of an entire CDAD episode remains potentially incomplete for a number of patients who had recurrences or who had visited multiple sites of care in addition to the hospital (ie, emergency department or outpatient facility). The transition to outpatient care is often multifaceted and challenging for patients, especially those who are elderly and have multiple underlying conditions [18]. Access to care become more difficult, and patients become wholly responsible for taking their medication as prescribed and following other post-discharge treatment stratagems. Furthermore, no differentiation was made between patients having a primary versus secondary CDAD diagnosis.
Another limitation is that the costs of the hospitalization was calculated from claims and as such do not include either patient paid costs (eg, deductible) or indirect costs (eg, lost work or productivity or caregiver costs) due to CDAD. This study likely underestimates the true costs associated with CDAD. Finally, the patients included in this analysis were all members of large commercial health plans in the US and who are also working and relatively healthy. Therefore, these results may not be generalizable to patients with other types of health insurance or no insurance or to those living outside of the United States.
It is important to note that the trends and drivers described in this study are “potential” influencers contributing to the burden of CDAD. Given that this study is descriptive in nature, formal analyses aimed at confirming these factors as “drivers” should be conducted in future. CDAD-related hospitalizations have previously been shown to be associated with increased inpatient LOS and a substantial economic burden. Our study demonstrates that the CDAD-associated cost burden in hospital settings may be driven by the use of numerous high-cost hospital resources including prolonged ICU stays, isolation, frequent GI and ID consultations, CDAD-related non-diagnostic tests/procedures, and symptomatic CDAD treatment.
Acknowledgments: The authors acknowledge Cheryl Jones for her editorial assistance in preparing this manuscript.
Corresponding author: Swetha Rao Palli, CTI Clinical Trial and Consulting, 1775 Lexington Ave, Ste. 200, Cincinnati, OH 45209, [email protected]
Funding/support: Funding for this study was provided Cubist Pharmaceuticals.
Financial disclosures: Ms. Palli and Mr. Quimbo are former and current employees of HealthCore, respectively. HealthCore is an independent research organization that received funding from Cubist Pharmaceuticals for the conduct of this study. Dr. Broderick is an employee of Cubist Pharmaceuticals. Ms. Strauss was an employee of Optimer Pharmaceuticals during the time the study was carried out.
1. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Available at: www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf. Accessed March 6, 2013.
2. Louie TJ, Miller MA, Mullane KM, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med 2011;364:422–31.
3. Lowy I, Molrine DC, Leav BA, et al. Treatment with monoclonal antibodies against Clostridium difficile toxins. N Engl J Med 2010;362:197–205.
4. Bouza E, Dryden M, Mohammed R, et al. Results of a phase III trial comparing tolevamer, vancomycin and metronidazole in patients with Clostridium difficile-associated diarrhoea [ECCMID abstract O464]. Clin Microbiol Infect 2008;14(Suppl s7):S103–4.
5. O’Brien JA, Betsy JL, Caro J, Davidson DM. The emerging infectious challenge of Clostridium difficile-associated disease in Massachusetts hospitals: clinical and economic consequences. Infect Control Hosp Epidemiol 2007;28:1219–27.
6. Dubberke ER, Wertheimer AI. Review of current literature on the economic burden of Clostridium difficile infection. Infect Control Hosp Epidemiol 2009;30:57–66.
7. Ghantoji SS, Sail K, Lairson DR, et al. Economic healthcare costs of Clostridium difficile infection: a systematic review. J Hosp Infect 2010;74:309–18.
8. Kyne L, Hamel MB, Polavaram R, Kelly CP. Health care costs and mortality associated with nosocomial diarrhea due to Clostridium difficile. Clin Infec Dis 2002;34:346–53.
9. Forster AJ, Taljaard M, Oake N, et al. The effect of hospital-acquired infection with Clostridium difficile on length of stay in hospital. CMAJ 2012;184:37–42.
10. Pakyz A, Carroll NV, Harpe SE, et al. Economic impact of Clostridium difficile infection in a multihospital cohort of academic health centers. Pharmacotherapy 2011;31:546–51.
11. Quimbo RA, Palli SR, Singer J, et al. Burden of Clostridium difficile-associated diarrhea among hospitalized patients at high risk of recurrent infection. J Clin Outcomes Manag 2013;20:544–54.
12. Golan Y, Mullane KM, Miller MA, et al. Low recurrence rate among patients with C. difficile infection treated with fidaxomicin. Poster presented at: 49th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy; 12–15 Sep 2009; San Francisco, CA.
13. Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol 1997;32:920–4.
14. Cornely OA, Crook DW, Esposito R, et al. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect Dis 2012;12:281–9.
15. Palli SR, Strauss M, Quimbo RA, et al. Cost drivers associated with Clostridium-difficile infection in a hospital setting. Poster presented at American Society of Health System Pharmacists Midyear Clinical Meeting; December 2012; Las Vegas, NV.
16. Noseworthy TW, Konopad E, Shustack A, et al. Cost accounting of adult intensive care: methods and human and capital inputs. Crit Care Med 1996;24:1168–72.
17. Classen DC, Burke JP, Wenzel RP. Infectious diseases consultation: impact on outcomes for hospitalized patients and results of a preliminary study. Clin Infect Dis 1997;24:468–70.
18. Petrak RM, Sexton DJ, Butera ML, et al. The value of an infectious diseases specialist. Clin Infect Dis 2003;36:1013–7.
19. Sellier E, Pavese P, Gennai S, et al. Factors and outcomes associated with physicians’ adherence to recommendations of infectious disease consultations for patients. J Antimicrob Chemother 2010;65:156–62.
20. Jollis JG, DeLong ER, Peterson ED, et al. Outcome of acute myocardial infarction according to the specialty of the admitting physician. N Engl J Med 1996;335:1880–7.
21. Harrold LR, Field TS, Gurwitz JH. Knowledge, patterns of care, and outcomes of care for generalists and specialists. J Gen Intern Med 1999;14:499–511.
From HealthCore, Wilmington, DE, and Cubist Pharma-ceuticals, San Diego, CA.
Abstract
- Objectives: To describe trends in inpatient resource utilization and potential cost drivers of Clostridium difficile-associated diarrhea (CDAD) treated in the hospital.
- Methods: Retrospective medical record review included 500 patients with ≥1 inpatient medical claim diagnosis of CDAD (ICD-9-CM: 008.45) between 01/01/2005-10/31/2010. Information was collected on patient demographics, admission diagnoses, laboratory data, and CDAD-related characteristics and discharge. Hospital costs were evaluated for the entire inpatient episode and prorated for the duration of the CDAD episode (ie, CDAD diagnosis date to diarrhea resolution/discharge date).
- Results: The cohort was mostly female (62%), Caucasian (72%), with mean (SD) age 66 (±17.6) years. 60% had diagnosis of CDAD or presence of diarrhea at admission. CDAD diagnosis was confirmed with laboratory test in 92% of patients. ~44% had mild CDAD, 35% had severe CDAD. Following CDAD diagnosis, approximately 53% of patients were isolated for ≥1 days, 12% transferred to the ICU for a median (Q1–Q3) length of stay of 8 (5–15) days. Two-thirds received gastrointestinal or infectious disease consult. Median time from CDAD diagnosis to discharge was 6 (4–9) days; 5.5 (4–8) days for patients admitted with CDAD, 6.5 (4–10) days for those with hospital-acquired CDAD. The mean and median costs (2011 USD) for CDAD-associated hospitalization were $35,621 and $13,153, respectively.
- Conclusion: Patients with CDAD utilize numerous expensive resources during hospitalization including laboratory tests, isolation, prolonged ICU stay, and specialist consultations.
Clostridium difficile, classified as an urgent public health threat by the Centers for Disease Control and Prevention (CDC), causes approximately 250,000 hospitalizations and an estimated 14,000 deaths per year in the United States [1]. An estimated 15% to 25% of patients with C. difficile-associated diarrhea (CDAD) will experience at least 1 recurrence [2-4], frequently requiring rehospitalization [5]. The high incidence of primary and recurrent infections contributes to a substantial burden associated with CDAD in terms of extended and repeat hospital stays [6,7].
Conservative estimates of the direct annual costs of CDAD in the United States over the past 15 years range from $1.1 billion [8] to $3.2 billion, with an average cost per stay of $10,212 for patients hospitalized with a principal diagnosis of CDAD or a CDAD-related symptom [5]. O’Brien et al estimated that costs associated with rehospitalizations accounted for 11% of overall CDAD-related hospital costs;when considering all CDAD-related hospitalizations, including both initial and subsequent rehospitalizations for recurrent infection and not accounting for post-acute or outpatient care, the 2-year cumulative cost was estimated to be $51.2 million. While studies have yielded varying assessments of the actual CDAD burden [5–10], they all suggest that CDAD burden is considerable and that extended hospital stays are the major component of CDAD-associated costs [9,10]. In a claims-based study by Quimbo et al [11], when multiple and diverse cohorts of CDAD patients at elevated risk for recurrence were matched with patients with similar underlying at-risk condition(s) but no CDAD, the CDAD at-risk groups had an incremental LOS per hospitalization ranging from approximately 3 to 18 days and an incremental cost burden ranging from a mean of $11,179 to $115,632 (2011 USD) per stay.
While it is recognized that CDAD carries significant cost burden and is driven by LOS, current literature is lacking regarding the characteristics of these hospital stays. Building on the Quimbo et al study, the current study was designed to probe further into the nature of the burden (ie, resource use) incurred during the course of CDAD hospitalizations. As such, the objective of this study was to identify the common trends in hospital-related resource utilization and describe the potential drivers that affect the cost burden of CDAD using hospital medical record data.
Methods
Population
Patients were selected for this retrospective medical record review from the HealthCore Integrated Research Database (HealthCore, Wilmington, DE). The database contains a broad, clinically rich and geographically diverse spectrum of longitudinal claims information from one of the largest commercially insured populations in the United States, representing 48 million lives. We identified 21,177 adult (≥ 18 years) patients with at least 1 inpatient claim with an International Classification of Diseases, 9th Edition, Clinical Modification (ICD-9-CM) diagnosis code for C. difficile infection (CDI; 008.45) between 1 January 2005 and 31 October 2010 (intake period). All patients had at least 12 months of prior and continuous medical and pharmacy health plan eligibility prior to the incident CDAD-associated hospitalization within the database. Additional details regarding this cohort identification has been published previously [11]. The study was undertaken in accordance with Health Insurance Portability and Accountability Act (HIPAA) guidelines and the necessary central institutional review board approval was obtained prior to medical record identification and abstraction.
Sampling Strategy
Medical Record Abstraction
During the record abstraction process, information was collected on patients’ race/ethnicity, body mass index (BMI), admission diagnosis and other conditions, point of entry and prior location, body temperature and laboratory data (eg, creatinine and albumin values, white blood cell [WBC] count), diarrhea and stool characteristics, CDAD diagnosis date, CDAD-specific characteristics, severity, complications, and related tests/procedures, CDAD treatments (eg, dose, duration, and formulation of medications), hospital LOS, including stays in the intensive care unit (ICU), cardiac care unit (CCU) following CDAD diagnosis; consultations provided by gastrointestinal, infectious disease, intensivists, or surgery care specialists, and discharge summary on disposition, CDAD status, and medications prescribed. Standardized data collection forms were used by trained nurses or pharmacists to collect information from the medical records and inter-rater reliability testing with a 0.9 cutoff was required to confirm accuracy. To ensure consistency, a pilot test of the first 20 abstracted records were re-abstracted by the research team. Last, quality checks were implemented throughout the abstraction process to identify any inconsistencies or data entry errors including coding errors and atypical, unrealistic data entry patterns (eg, identical values for a particular data field entered on multiple records; implausible or erratic inputs; or a high percentage of missing data points). Missing data were not imputed.
Study Definitions
Diarrhea was defined as 3 or more unformed (includes bloody, watery, thin, loose, soft, and/or unformed stool) bowel movements per day.CDAD severity was classified as mild (4–5 unformed bowel movements per day or WBC ≤ 2000/mm3); moderate (6–9 unformed bowel movements per day or WBC between 12,001/mm3 and 15,000/mm3); or severe (≥10 unformed bowel movements per day or WBC ≥15,001/mm3) [12,13]. Diarrhea was considered to be resolved when the patient had no more than 3 unformed stools for 2 consecutive days and lasting until treatment was completed, with no additional therapy required for CDAD as of the second day after the end of the course of therapy [2,14].CDAD episode was defined as the duration from the date of the CDAD diagnosis or confirmation (whichever occurred first), to the date of diarrhea resolution (where documented) or discharge date.
Cost Measures
The total hospital health plan paid costs for the entire inpatient episode (includes treatment costs, diagnostics, services provided, etc.) were estimated using medical claims present in the database and pertaining to the hospitalization from where medical records were abstracted. Then the proportionate amount for the duration of the CDAD episode (from CDAD diagnosis to the diarrhea resolution date or the discharge date in cases where the resolution date could not be ascertained) was calculated to estimate the average CDAD associated in-hospital costs.
Analysis
Means (± standard deviation [SD]), medians (interquartile range Q1 to Q3), and relative frequencies were calculated for continuous and categorical data, respectively. This analysis was descriptive in nature; hence, no statistical tests to determine significance were conducted.
Results
We had a 55.3% success rate in obtaining the medical records from the contacted hospitals with refusal to participate/consent by the hospital in question being the most frequent reason for failure in 3 out of 4 cases. An additional attrition of 39.3% was observed among the medical records received, with absence of a MAR form (23.9%) and confirmatory CDAD diagnosis or note (9.1%) being the most frequent criteria for discarding an available record prior to abstraction (Figure).
Patient Characteristics
CDAD Characteristics and Complications
Using a derived definition of severity, most CDAD cases were classified either as
CDAD-Related Resource Utilization
Following CDAD diagnosis, more than half of the study patients were isolated for 1 or more days. While the majority of patients with CDAD (74.0%) stayed in a general hospital room, 12.4% stayed in the ICU for a mean duration of 12.1 (± 12.3) days (Table 3). Half of these ICU patients required
About one-third of patients consulted a gastrointestinal or infectious disease specialist at least once. Among these patients, assuming that a patient following an initial specialist consultation would have follow-up visits at least once a day (formal or informal) for the remainder of the CDAD episode, we estimate that there were an average of 8.7 (± 15.6) and 11.6 (± 19.4) GI or ID specialist visits respectively during the CDAD episode.
Nearly all patients had their CDAD diagnosis confirmed by laboratory tests. CDAD virulence was identified as toxin A and/or toxin B in 47.6% of the samples. However, nearly three-fifths of patients also underwent 1 or more nondiagnostic tests including endoscopy, colonoscopy, computed axial tomography (CAT), or magnetic resonance imaging (MRI) scans, sigmoidoscopy, and/or other obstructive series tests during the CDAD episode.
CDAD Treatment
CDAD at Discharge
Hospitalization Costs
Based on claims data, the mean (±SD) and median (Q1–Q3) plan costs for the duration of a CDAD-associated hospitalization (2011 USD) for these 500 patients were found to be $35,621 (± $100,502) and $13,153 ($8,209–$26,893), respectively.
Discussion
While multiple studies have documented the considerable economic burden associated with CDAD [5–10], this study was the first to our knowledge to evaluate the specific hospital resources that are used during an extended hospital stay for CDAD. This real-world analysis, in conjunction with the Quimbo et al claims analysis, demonstrated the significant burden associated with CDAD in terms of both fixed costs (eg, hospital stay) as well as the variable components that drive these expenditures (eg, consultations, ICU stay).
The mean ($35,621) and median ($13,153) total costs associated with the CDAD segment of the hospitalization, as measured via the claims, were quite high despite a greater prevalence of mild CDAD rather than severe infection, and required only a general hospital room stay. Both of the above CDAD hospital cost measures were well above the mean US general hospitalization cost of $11,666 and the median cost of $7334 measured from Healthcare Cost and Utilization Project data [15]. However, the mean cost of hospitalization reported in the current study falls within the range of previously reported costs for CDAD-associated hospitalizations [5,8,10]. While the mean cost may have been disproportionately inflated by a few extreme cases, the median CDAD-associated hospitalization cost was nearly twice the median cost of an average general hospital stay in the US [15]. Our finding that these elevated costs were observed among patients with mild CDAD and its relative magnitude compared with the average hospitalization costs (approximately 3-fold higher) were also consistent with the literature. For instance, Pakyz and colleagues reported that relative to patients without CDAD, hospital costs were tripled for patients with low-severity CDAD and 10% higher for those with more severe CDAD, presumably because CDAD resulted in costly complications that prolonged what would have otherwise been a short, simple hospital stay [10].
Type of hospital room could also be an important driver of cost. While most patients stayed in general hospital rooms, more than half were isolated for at least a day, and 12% of patients required nearly 2 weeks of intensive care. Taken together, 26% of patients in the current study were required to stay in a special care unit or a non–general hospital room for 5.5 to 12.2 days. This is consistent with the 28% of patients with CDAD that required stay on a special care unit previously reported by O’Brien et al [5].Additionally, previous research from Canadian health care data has shown that a single ICU stay costs an average of $7000 more per patient per day than a general hospital room (1992 Canadian dollars) or $9589 (2013 USD calculated using historical exchange rate data and adjusted for inflation) [16].However, despite this additional cost and resource burden, it appears that overall only 53.4% of all patients received care within an isolated setting as guidelines recommended.
Repeated specialist visits, procedures and multiple testing (concomitant diagnostic EIA and nondiagnostic tests) potentially added to the health care resource utilization and costs, along with the extra resources associated with specialized hospital care. We found that roughly one-third of patients consulted a specialist, although we did not distinguish between ‘formal’ and ‘informal’ consultations. Numerous studies published over the past 2 decades have demonstrated increased costs and resource utilization associated with specialist consultations [17–21]. Although the focused knowledge and experience of specialists may reduce morbidity and mortality [18,21], specialists are more likely than generalists to order more diagnostic tests, perform more procedures, and keep patients hospitalized longer and in ICUs, all of which contribute to higher costs without necessarily leading to improved health outcomes [21].
Limitations
One major limitation of this study was the inability to assess the individual costs of the resources used for each individual patient either through the medical charts or via claims. Additionally, the burden of CDAD was found to continue beyond the hospital stay, with documented evidence of persisting infection in 84% of patients at the point of discharge. Since the medical records obtained were limited to a single hospitalization and a single place of service, the data capture of an entire CDAD episode remains potentially incomplete for a number of patients who had recurrences or who had visited multiple sites of care in addition to the hospital (ie, emergency department or outpatient facility). The transition to outpatient care is often multifaceted and challenging for patients, especially those who are elderly and have multiple underlying conditions [18]. Access to care become more difficult, and patients become wholly responsible for taking their medication as prescribed and following other post-discharge treatment stratagems. Furthermore, no differentiation was made between patients having a primary versus secondary CDAD diagnosis.
Another limitation is that the costs of the hospitalization was calculated from claims and as such do not include either patient paid costs (eg, deductible) or indirect costs (eg, lost work or productivity or caregiver costs) due to CDAD. This study likely underestimates the true costs associated with CDAD. Finally, the patients included in this analysis were all members of large commercial health plans in the US and who are also working and relatively healthy. Therefore, these results may not be generalizable to patients with other types of health insurance or no insurance or to those living outside of the United States.
It is important to note that the trends and drivers described in this study are “potential” influencers contributing to the burden of CDAD. Given that this study is descriptive in nature, formal analyses aimed at confirming these factors as “drivers” should be conducted in future. CDAD-related hospitalizations have previously been shown to be associated with increased inpatient LOS and a substantial economic burden. Our study demonstrates that the CDAD-associated cost burden in hospital settings may be driven by the use of numerous high-cost hospital resources including prolonged ICU stays, isolation, frequent GI and ID consultations, CDAD-related non-diagnostic tests/procedures, and symptomatic CDAD treatment.
Acknowledgments: The authors acknowledge Cheryl Jones for her editorial assistance in preparing this manuscript.
Corresponding author: Swetha Rao Palli, CTI Clinical Trial and Consulting, 1775 Lexington Ave, Ste. 200, Cincinnati, OH 45209, [email protected]
Funding/support: Funding for this study was provided Cubist Pharmaceuticals.
Financial disclosures: Ms. Palli and Mr. Quimbo are former and current employees of HealthCore, respectively. HealthCore is an independent research organization that received funding from Cubist Pharmaceuticals for the conduct of this study. Dr. Broderick is an employee of Cubist Pharmaceuticals. Ms. Strauss was an employee of Optimer Pharmaceuticals during the time the study was carried out.
From HealthCore, Wilmington, DE, and Cubist Pharma-ceuticals, San Diego, CA.
Abstract
- Objectives: To describe trends in inpatient resource utilization and potential cost drivers of Clostridium difficile-associated diarrhea (CDAD) treated in the hospital.
- Methods: Retrospective medical record review included 500 patients with ≥1 inpatient medical claim diagnosis of CDAD (ICD-9-CM: 008.45) between 01/01/2005-10/31/2010. Information was collected on patient demographics, admission diagnoses, laboratory data, and CDAD-related characteristics and discharge. Hospital costs were evaluated for the entire inpatient episode and prorated for the duration of the CDAD episode (ie, CDAD diagnosis date to diarrhea resolution/discharge date).
- Results: The cohort was mostly female (62%), Caucasian (72%), with mean (SD) age 66 (±17.6) years. 60% had diagnosis of CDAD or presence of diarrhea at admission. CDAD diagnosis was confirmed with laboratory test in 92% of patients. ~44% had mild CDAD, 35% had severe CDAD. Following CDAD diagnosis, approximately 53% of patients were isolated for ≥1 days, 12% transferred to the ICU for a median (Q1–Q3) length of stay of 8 (5–15) days. Two-thirds received gastrointestinal or infectious disease consult. Median time from CDAD diagnosis to discharge was 6 (4–9) days; 5.5 (4–8) days for patients admitted with CDAD, 6.5 (4–10) days for those with hospital-acquired CDAD. The mean and median costs (2011 USD) for CDAD-associated hospitalization were $35,621 and $13,153, respectively.
- Conclusion: Patients with CDAD utilize numerous expensive resources during hospitalization including laboratory tests, isolation, prolonged ICU stay, and specialist consultations.
Clostridium difficile, classified as an urgent public health threat by the Centers for Disease Control and Prevention (CDC), causes approximately 250,000 hospitalizations and an estimated 14,000 deaths per year in the United States [1]. An estimated 15% to 25% of patients with C. difficile-associated diarrhea (CDAD) will experience at least 1 recurrence [2-4], frequently requiring rehospitalization [5]. The high incidence of primary and recurrent infections contributes to a substantial burden associated with CDAD in terms of extended and repeat hospital stays [6,7].
Conservative estimates of the direct annual costs of CDAD in the United States over the past 15 years range from $1.1 billion [8] to $3.2 billion, with an average cost per stay of $10,212 for patients hospitalized with a principal diagnosis of CDAD or a CDAD-related symptom [5]. O’Brien et al estimated that costs associated with rehospitalizations accounted for 11% of overall CDAD-related hospital costs;when considering all CDAD-related hospitalizations, including both initial and subsequent rehospitalizations for recurrent infection and not accounting for post-acute or outpatient care, the 2-year cumulative cost was estimated to be $51.2 million. While studies have yielded varying assessments of the actual CDAD burden [5–10], they all suggest that CDAD burden is considerable and that extended hospital stays are the major component of CDAD-associated costs [9,10]. In a claims-based study by Quimbo et al [11], when multiple and diverse cohorts of CDAD patients at elevated risk for recurrence were matched with patients with similar underlying at-risk condition(s) but no CDAD, the CDAD at-risk groups had an incremental LOS per hospitalization ranging from approximately 3 to 18 days and an incremental cost burden ranging from a mean of $11,179 to $115,632 (2011 USD) per stay.
While it is recognized that CDAD carries significant cost burden and is driven by LOS, current literature is lacking regarding the characteristics of these hospital stays. Building on the Quimbo et al study, the current study was designed to probe further into the nature of the burden (ie, resource use) incurred during the course of CDAD hospitalizations. As such, the objective of this study was to identify the common trends in hospital-related resource utilization and describe the potential drivers that affect the cost burden of CDAD using hospital medical record data.
Methods
Population
Patients were selected for this retrospective medical record review from the HealthCore Integrated Research Database (HealthCore, Wilmington, DE). The database contains a broad, clinically rich and geographically diverse spectrum of longitudinal claims information from one of the largest commercially insured populations in the United States, representing 48 million lives. We identified 21,177 adult (≥ 18 years) patients with at least 1 inpatient claim with an International Classification of Diseases, 9th Edition, Clinical Modification (ICD-9-CM) diagnosis code for C. difficile infection (CDI; 008.45) between 1 January 2005 and 31 October 2010 (intake period). All patients had at least 12 months of prior and continuous medical and pharmacy health plan eligibility prior to the incident CDAD-associated hospitalization within the database. Additional details regarding this cohort identification has been published previously [11]. The study was undertaken in accordance with Health Insurance Portability and Accountability Act (HIPAA) guidelines and the necessary central institutional review board approval was obtained prior to medical record identification and abstraction.
Sampling Strategy
Medical Record Abstraction
During the record abstraction process, information was collected on patients’ race/ethnicity, body mass index (BMI), admission diagnosis and other conditions, point of entry and prior location, body temperature and laboratory data (eg, creatinine and albumin values, white blood cell [WBC] count), diarrhea and stool characteristics, CDAD diagnosis date, CDAD-specific characteristics, severity, complications, and related tests/procedures, CDAD treatments (eg, dose, duration, and formulation of medications), hospital LOS, including stays in the intensive care unit (ICU), cardiac care unit (CCU) following CDAD diagnosis; consultations provided by gastrointestinal, infectious disease, intensivists, or surgery care specialists, and discharge summary on disposition, CDAD status, and medications prescribed. Standardized data collection forms were used by trained nurses or pharmacists to collect information from the medical records and inter-rater reliability testing with a 0.9 cutoff was required to confirm accuracy. To ensure consistency, a pilot test of the first 20 abstracted records were re-abstracted by the research team. Last, quality checks were implemented throughout the abstraction process to identify any inconsistencies or data entry errors including coding errors and atypical, unrealistic data entry patterns (eg, identical values for a particular data field entered on multiple records; implausible or erratic inputs; or a high percentage of missing data points). Missing data were not imputed.
Study Definitions
Diarrhea was defined as 3 or more unformed (includes bloody, watery, thin, loose, soft, and/or unformed stool) bowel movements per day.CDAD severity was classified as mild (4–5 unformed bowel movements per day or WBC ≤ 2000/mm3); moderate (6–9 unformed bowel movements per day or WBC between 12,001/mm3 and 15,000/mm3); or severe (≥10 unformed bowel movements per day or WBC ≥15,001/mm3) [12,13]. Diarrhea was considered to be resolved when the patient had no more than 3 unformed stools for 2 consecutive days and lasting until treatment was completed, with no additional therapy required for CDAD as of the second day after the end of the course of therapy [2,14].CDAD episode was defined as the duration from the date of the CDAD diagnosis or confirmation (whichever occurred first), to the date of diarrhea resolution (where documented) or discharge date.
Cost Measures
The total hospital health plan paid costs for the entire inpatient episode (includes treatment costs, diagnostics, services provided, etc.) were estimated using medical claims present in the database and pertaining to the hospitalization from where medical records were abstracted. Then the proportionate amount for the duration of the CDAD episode (from CDAD diagnosis to the diarrhea resolution date or the discharge date in cases where the resolution date could not be ascertained) was calculated to estimate the average CDAD associated in-hospital costs.
Analysis
Means (± standard deviation [SD]), medians (interquartile range Q1 to Q3), and relative frequencies were calculated for continuous and categorical data, respectively. This analysis was descriptive in nature; hence, no statistical tests to determine significance were conducted.
Results
We had a 55.3% success rate in obtaining the medical records from the contacted hospitals with refusal to participate/consent by the hospital in question being the most frequent reason for failure in 3 out of 4 cases. An additional attrition of 39.3% was observed among the medical records received, with absence of a MAR form (23.9%) and confirmatory CDAD diagnosis or note (9.1%) being the most frequent criteria for discarding an available record prior to abstraction (Figure).
Patient Characteristics
CDAD Characteristics and Complications
Using a derived definition of severity, most CDAD cases were classified either as
CDAD-Related Resource Utilization
Following CDAD diagnosis, more than half of the study patients were isolated for 1 or more days. While the majority of patients with CDAD (74.0%) stayed in a general hospital room, 12.4% stayed in the ICU for a mean duration of 12.1 (± 12.3) days (Table 3). Half of these ICU patients required
About one-third of patients consulted a gastrointestinal or infectious disease specialist at least once. Among these patients, assuming that a patient following an initial specialist consultation would have follow-up visits at least once a day (formal or informal) for the remainder of the CDAD episode, we estimate that there were an average of 8.7 (± 15.6) and 11.6 (± 19.4) GI or ID specialist visits respectively during the CDAD episode.
Nearly all patients had their CDAD diagnosis confirmed by laboratory tests. CDAD virulence was identified as toxin A and/or toxin B in 47.6% of the samples. However, nearly three-fifths of patients also underwent 1 or more nondiagnostic tests including endoscopy, colonoscopy, computed axial tomography (CAT), or magnetic resonance imaging (MRI) scans, sigmoidoscopy, and/or other obstructive series tests during the CDAD episode.
CDAD Treatment
CDAD at Discharge
Hospitalization Costs
Based on claims data, the mean (±SD) and median (Q1–Q3) plan costs for the duration of a CDAD-associated hospitalization (2011 USD) for these 500 patients were found to be $35,621 (± $100,502) and $13,153 ($8,209–$26,893), respectively.
Discussion
While multiple studies have documented the considerable economic burden associated with CDAD [5–10], this study was the first to our knowledge to evaluate the specific hospital resources that are used during an extended hospital stay for CDAD. This real-world analysis, in conjunction with the Quimbo et al claims analysis, demonstrated the significant burden associated with CDAD in terms of both fixed costs (eg, hospital stay) as well as the variable components that drive these expenditures (eg, consultations, ICU stay).
The mean ($35,621) and median ($13,153) total costs associated with the CDAD segment of the hospitalization, as measured via the claims, were quite high despite a greater prevalence of mild CDAD rather than severe infection, and required only a general hospital room stay. Both of the above CDAD hospital cost measures were well above the mean US general hospitalization cost of $11,666 and the median cost of $7334 measured from Healthcare Cost and Utilization Project data [15]. However, the mean cost of hospitalization reported in the current study falls within the range of previously reported costs for CDAD-associated hospitalizations [5,8,10]. While the mean cost may have been disproportionately inflated by a few extreme cases, the median CDAD-associated hospitalization cost was nearly twice the median cost of an average general hospital stay in the US [15]. Our finding that these elevated costs were observed among patients with mild CDAD and its relative magnitude compared with the average hospitalization costs (approximately 3-fold higher) were also consistent with the literature. For instance, Pakyz and colleagues reported that relative to patients without CDAD, hospital costs were tripled for patients with low-severity CDAD and 10% higher for those with more severe CDAD, presumably because CDAD resulted in costly complications that prolonged what would have otherwise been a short, simple hospital stay [10].
Type of hospital room could also be an important driver of cost. While most patients stayed in general hospital rooms, more than half were isolated for at least a day, and 12% of patients required nearly 2 weeks of intensive care. Taken together, 26% of patients in the current study were required to stay in a special care unit or a non–general hospital room for 5.5 to 12.2 days. This is consistent with the 28% of patients with CDAD that required stay on a special care unit previously reported by O’Brien et al [5].Additionally, previous research from Canadian health care data has shown that a single ICU stay costs an average of $7000 more per patient per day than a general hospital room (1992 Canadian dollars) or $9589 (2013 USD calculated using historical exchange rate data and adjusted for inflation) [16].However, despite this additional cost and resource burden, it appears that overall only 53.4% of all patients received care within an isolated setting as guidelines recommended.
Repeated specialist visits, procedures and multiple testing (concomitant diagnostic EIA and nondiagnostic tests) potentially added to the health care resource utilization and costs, along with the extra resources associated with specialized hospital care. We found that roughly one-third of patients consulted a specialist, although we did not distinguish between ‘formal’ and ‘informal’ consultations. Numerous studies published over the past 2 decades have demonstrated increased costs and resource utilization associated with specialist consultations [17–21]. Although the focused knowledge and experience of specialists may reduce morbidity and mortality [18,21], specialists are more likely than generalists to order more diagnostic tests, perform more procedures, and keep patients hospitalized longer and in ICUs, all of which contribute to higher costs without necessarily leading to improved health outcomes [21].
Limitations
One major limitation of this study was the inability to assess the individual costs of the resources used for each individual patient either through the medical charts or via claims. Additionally, the burden of CDAD was found to continue beyond the hospital stay, with documented evidence of persisting infection in 84% of patients at the point of discharge. Since the medical records obtained were limited to a single hospitalization and a single place of service, the data capture of an entire CDAD episode remains potentially incomplete for a number of patients who had recurrences or who had visited multiple sites of care in addition to the hospital (ie, emergency department or outpatient facility). The transition to outpatient care is often multifaceted and challenging for patients, especially those who are elderly and have multiple underlying conditions [18]. Access to care become more difficult, and patients become wholly responsible for taking their medication as prescribed and following other post-discharge treatment stratagems. Furthermore, no differentiation was made between patients having a primary versus secondary CDAD diagnosis.
Another limitation is that the costs of the hospitalization was calculated from claims and as such do not include either patient paid costs (eg, deductible) or indirect costs (eg, lost work or productivity or caregiver costs) due to CDAD. This study likely underestimates the true costs associated with CDAD. Finally, the patients included in this analysis were all members of large commercial health plans in the US and who are also working and relatively healthy. Therefore, these results may not be generalizable to patients with other types of health insurance or no insurance or to those living outside of the United States.
It is important to note that the trends and drivers described in this study are “potential” influencers contributing to the burden of CDAD. Given that this study is descriptive in nature, formal analyses aimed at confirming these factors as “drivers” should be conducted in future. CDAD-related hospitalizations have previously been shown to be associated with increased inpatient LOS and a substantial economic burden. Our study demonstrates that the CDAD-associated cost burden in hospital settings may be driven by the use of numerous high-cost hospital resources including prolonged ICU stays, isolation, frequent GI and ID consultations, CDAD-related non-diagnostic tests/procedures, and symptomatic CDAD treatment.
Acknowledgments: The authors acknowledge Cheryl Jones for her editorial assistance in preparing this manuscript.
Corresponding author: Swetha Rao Palli, CTI Clinical Trial and Consulting, 1775 Lexington Ave, Ste. 200, Cincinnati, OH 45209, [email protected]
Funding/support: Funding for this study was provided Cubist Pharmaceuticals.
Financial disclosures: Ms. Palli and Mr. Quimbo are former and current employees of HealthCore, respectively. HealthCore is an independent research organization that received funding from Cubist Pharmaceuticals for the conduct of this study. Dr. Broderick is an employee of Cubist Pharmaceuticals. Ms. Strauss was an employee of Optimer Pharmaceuticals during the time the study was carried out.
1. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Available at: www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf. Accessed March 6, 2013.
2. Louie TJ, Miller MA, Mullane KM, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med 2011;364:422–31.
3. Lowy I, Molrine DC, Leav BA, et al. Treatment with monoclonal antibodies against Clostridium difficile toxins. N Engl J Med 2010;362:197–205.
4. Bouza E, Dryden M, Mohammed R, et al. Results of a phase III trial comparing tolevamer, vancomycin and metronidazole in patients with Clostridium difficile-associated diarrhoea [ECCMID abstract O464]. Clin Microbiol Infect 2008;14(Suppl s7):S103–4.
5. O’Brien JA, Betsy JL, Caro J, Davidson DM. The emerging infectious challenge of Clostridium difficile-associated disease in Massachusetts hospitals: clinical and economic consequences. Infect Control Hosp Epidemiol 2007;28:1219–27.
6. Dubberke ER, Wertheimer AI. Review of current literature on the economic burden of Clostridium difficile infection. Infect Control Hosp Epidemiol 2009;30:57–66.
7. Ghantoji SS, Sail K, Lairson DR, et al. Economic healthcare costs of Clostridium difficile infection: a systematic review. J Hosp Infect 2010;74:309–18.
8. Kyne L, Hamel MB, Polavaram R, Kelly CP. Health care costs and mortality associated with nosocomial diarrhea due to Clostridium difficile. Clin Infec Dis 2002;34:346–53.
9. Forster AJ, Taljaard M, Oake N, et al. The effect of hospital-acquired infection with Clostridium difficile on length of stay in hospital. CMAJ 2012;184:37–42.
10. Pakyz A, Carroll NV, Harpe SE, et al. Economic impact of Clostridium difficile infection in a multihospital cohort of academic health centers. Pharmacotherapy 2011;31:546–51.
11. Quimbo RA, Palli SR, Singer J, et al. Burden of Clostridium difficile-associated diarrhea among hospitalized patients at high risk of recurrent infection. J Clin Outcomes Manag 2013;20:544–54.
12. Golan Y, Mullane KM, Miller MA, et al. Low recurrence rate among patients with C. difficile infection treated with fidaxomicin. Poster presented at: 49th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy; 12–15 Sep 2009; San Francisco, CA.
13. Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol 1997;32:920–4.
14. Cornely OA, Crook DW, Esposito R, et al. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect Dis 2012;12:281–9.
15. Palli SR, Strauss M, Quimbo RA, et al. Cost drivers associated with Clostridium-difficile infection in a hospital setting. Poster presented at American Society of Health System Pharmacists Midyear Clinical Meeting; December 2012; Las Vegas, NV.
16. Noseworthy TW, Konopad E, Shustack A, et al. Cost accounting of adult intensive care: methods and human and capital inputs. Crit Care Med 1996;24:1168–72.
17. Classen DC, Burke JP, Wenzel RP. Infectious diseases consultation: impact on outcomes for hospitalized patients and results of a preliminary study. Clin Infect Dis 1997;24:468–70.
18. Petrak RM, Sexton DJ, Butera ML, et al. The value of an infectious diseases specialist. Clin Infect Dis 2003;36:1013–7.
19. Sellier E, Pavese P, Gennai S, et al. Factors and outcomes associated with physicians’ adherence to recommendations of infectious disease consultations for patients. J Antimicrob Chemother 2010;65:156–62.
20. Jollis JG, DeLong ER, Peterson ED, et al. Outcome of acute myocardial infarction according to the specialty of the admitting physician. N Engl J Med 1996;335:1880–7.
21. Harrold LR, Field TS, Gurwitz JH. Knowledge, patterns of care, and outcomes of care for generalists and specialists. J Gen Intern Med 1999;14:499–511.
1. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Available at: www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf. Accessed March 6, 2013.
2. Louie TJ, Miller MA, Mullane KM, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med 2011;364:422–31.
3. Lowy I, Molrine DC, Leav BA, et al. Treatment with monoclonal antibodies against Clostridium difficile toxins. N Engl J Med 2010;362:197–205.
4. Bouza E, Dryden M, Mohammed R, et al. Results of a phase III trial comparing tolevamer, vancomycin and metronidazole in patients with Clostridium difficile-associated diarrhoea [ECCMID abstract O464]. Clin Microbiol Infect 2008;14(Suppl s7):S103–4.
5. O’Brien JA, Betsy JL, Caro J, Davidson DM. The emerging infectious challenge of Clostridium difficile-associated disease in Massachusetts hospitals: clinical and economic consequences. Infect Control Hosp Epidemiol 2007;28:1219–27.
6. Dubberke ER, Wertheimer AI. Review of current literature on the economic burden of Clostridium difficile infection. Infect Control Hosp Epidemiol 2009;30:57–66.
7. Ghantoji SS, Sail K, Lairson DR, et al. Economic healthcare costs of Clostridium difficile infection: a systematic review. J Hosp Infect 2010;74:309–18.
8. Kyne L, Hamel MB, Polavaram R, Kelly CP. Health care costs and mortality associated with nosocomial diarrhea due to Clostridium difficile. Clin Infec Dis 2002;34:346–53.
9. Forster AJ, Taljaard M, Oake N, et al. The effect of hospital-acquired infection with Clostridium difficile on length of stay in hospital. CMAJ 2012;184:37–42.
10. Pakyz A, Carroll NV, Harpe SE, et al. Economic impact of Clostridium difficile infection in a multihospital cohort of academic health centers. Pharmacotherapy 2011;31:546–51.
11. Quimbo RA, Palli SR, Singer J, et al. Burden of Clostridium difficile-associated diarrhea among hospitalized patients at high risk of recurrent infection. J Clin Outcomes Manag 2013;20:544–54.
12. Golan Y, Mullane KM, Miller MA, et al. Low recurrence rate among patients with C. difficile infection treated with fidaxomicin. Poster presented at: 49th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy; 12–15 Sep 2009; San Francisco, CA.
13. Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol 1997;32:920–4.
14. Cornely OA, Crook DW, Esposito R, et al. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect Dis 2012;12:281–9.
15. Palli SR, Strauss M, Quimbo RA, et al. Cost drivers associated with Clostridium-difficile infection in a hospital setting. Poster presented at American Society of Health System Pharmacists Midyear Clinical Meeting; December 2012; Las Vegas, NV.
16. Noseworthy TW, Konopad E, Shustack A, et al. Cost accounting of adult intensive care: methods and human and capital inputs. Crit Care Med 1996;24:1168–72.
17. Classen DC, Burke JP, Wenzel RP. Infectious diseases consultation: impact on outcomes for hospitalized patients and results of a preliminary study. Clin Infect Dis 1997;24:468–70.
18. Petrak RM, Sexton DJ, Butera ML, et al. The value of an infectious diseases specialist. Clin Infect Dis 2003;36:1013–7.
19. Sellier E, Pavese P, Gennai S, et al. Factors and outcomes associated with physicians’ adherence to recommendations of infectious disease consultations for patients. J Antimicrob Chemother 2010;65:156–62.
20. Jollis JG, DeLong ER, Peterson ED, et al. Outcome of acute myocardial infarction according to the specialty of the admitting physician. N Engl J Med 1996;335:1880–7.
21. Harrold LR, Field TS, Gurwitz JH. Knowledge, patterns of care, and outcomes of care for generalists and specialists. J Gen Intern Med 1999;14:499–511.