User login
Early BCC seen in teen kidney transplant patient
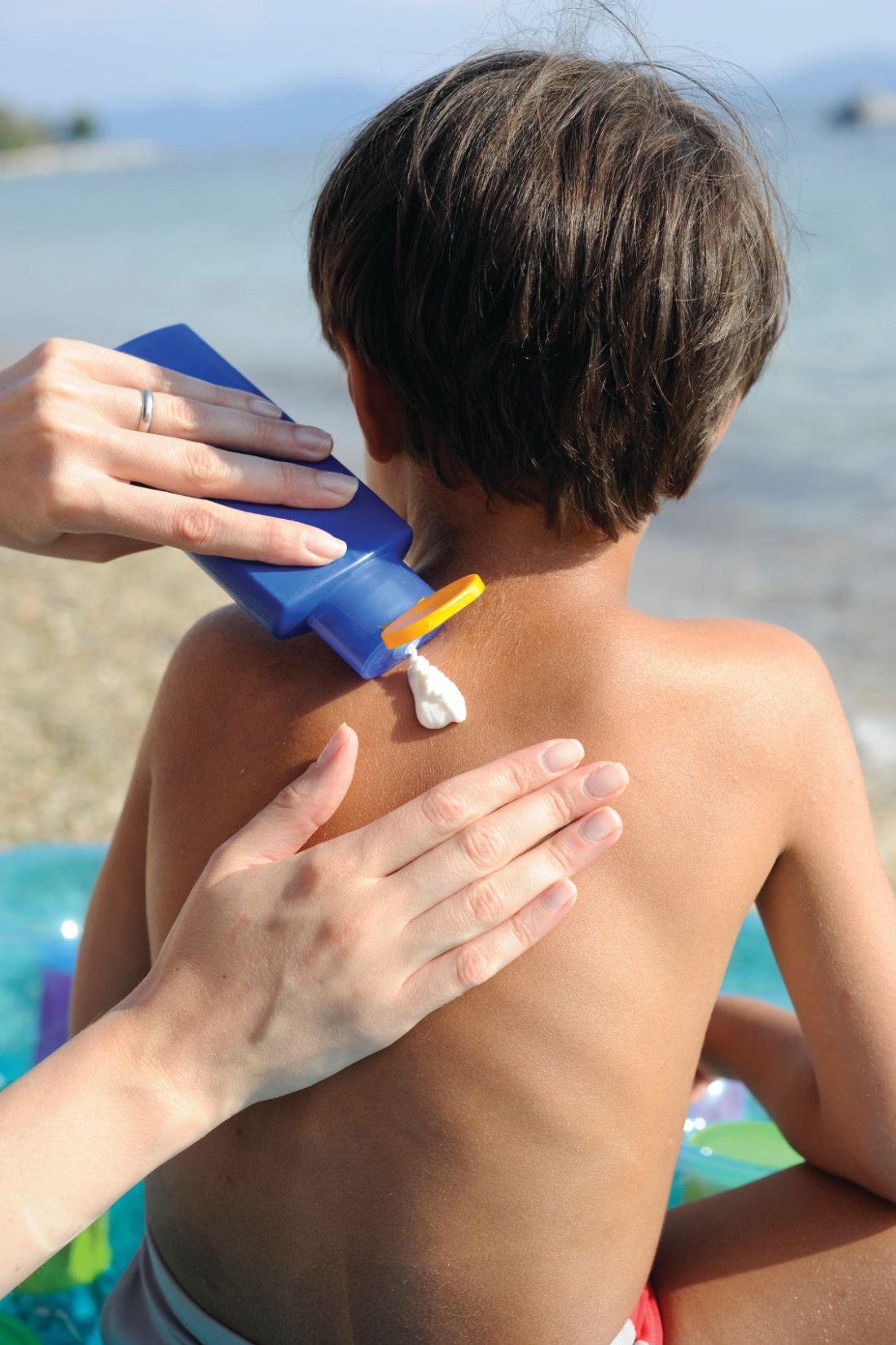
A 17-year-old girl seen in a Portuguese dermatology clinic was found to have a nodular basal cell carcinoma on the parietal region of her scalp. The nodule appeared 6 years after she had received a kidney transplant, according to João Borges-Costa, MD, PhD, who submitted the case report.
Since the transplant, the girl had been maintained on immunosuppressive medication of tacrolimus 1 mg twice daily, mycophenolate sodium 360 mg twice daily, and prednisolone 10 mg every other day. The 1-cm nodule was pigmented; dermatoscopy did not yield clarity about whether the lesion was melanocytic. An excisional biopsy with 0.5-cm margins was performed, and histology confirmed that the lesion was a nodular pigmented basal cell carcinoma that had been excised completely.
The case, said Dr. Borges-Costa, shows that skin cancers can develop earlier than the typical 12-18 years after pediatric transplantation. Most reported cases have been squamous cell cancers and melanomas, and often are associated with lack of appropriate sun protection behavior.
The patient, a Caucasian, was a sailor who used sunscreen but did not typically wear a hat while sailing, reported Dr. Borges-Costa, a dermatologist at the University of Lisbon. Her family history was significant for a grandparent with melanoma.
Dr. Borges noted that the parents and patient were given advice regarding the importance of the lifelong use of sun-protective clothing and headgear. “Education of pediatric organ recipients and their parents about sun protection is important because, as occurred with our patient, protective clothing and hats are frequently forgotten.”
Because of the ongoing potential for skin malignancies, early referral “after transplantation to specialized dermatology outpatient clinics, similar to what is now advocated for transplanted adults, could help in surveillance and improve adherence to sun-protective measures,” he added.
SOURCE: Borges-Costa J et al. Pediatr Dermatol. 2018. doi: 10.1111/pde.13537..
A 17-year-old girl seen in a Portuguese dermatology clinic was found to have a nodular basal cell carcinoma on the parietal region of her scalp. The nodule appeared 6 years after she had received a kidney transplant, according to João Borges-Costa, MD, PhD, who submitted the case report.
Since the transplant, the girl had been maintained on immunosuppressive medication of tacrolimus 1 mg twice daily, mycophenolate sodium 360 mg twice daily, and prednisolone 10 mg every other day. The 1-cm nodule was pigmented; dermatoscopy did not yield clarity about whether the lesion was melanocytic. An excisional biopsy with 0.5-cm margins was performed, and histology confirmed that the lesion was a nodular pigmented basal cell carcinoma that had been excised completely.
The case, said Dr. Borges-Costa, shows that skin cancers can develop earlier than the typical 12-18 years after pediatric transplantation. Most reported cases have been squamous cell cancers and melanomas, and often are associated with lack of appropriate sun protection behavior.
The patient, a Caucasian, was a sailor who used sunscreen but did not typically wear a hat while sailing, reported Dr. Borges-Costa, a dermatologist at the University of Lisbon. Her family history was significant for a grandparent with melanoma.
Dr. Borges noted that the parents and patient were given advice regarding the importance of the lifelong use of sun-protective clothing and headgear. “Education of pediatric organ recipients and their parents about sun protection is important because, as occurred with our patient, protective clothing and hats are frequently forgotten.”
Because of the ongoing potential for skin malignancies, early referral “after transplantation to specialized dermatology outpatient clinics, similar to what is now advocated for transplanted adults, could help in surveillance and improve adherence to sun-protective measures,” he added.
SOURCE: Borges-Costa J et al. Pediatr Dermatol. 2018. doi: 10.1111/pde.13537..
A 17-year-old girl seen in a Portuguese dermatology clinic was found to have a nodular basal cell carcinoma on the parietal region of her scalp. The nodule appeared 6 years after she had received a kidney transplant, according to João Borges-Costa, MD, PhD, who submitted the case report.
Since the transplant, the girl had been maintained on immunosuppressive medication of tacrolimus 1 mg twice daily, mycophenolate sodium 360 mg twice daily, and prednisolone 10 mg every other day. The 1-cm nodule was pigmented; dermatoscopy did not yield clarity about whether the lesion was melanocytic. An excisional biopsy with 0.5-cm margins was performed, and histology confirmed that the lesion was a nodular pigmented basal cell carcinoma that had been excised completely.
The case, said Dr. Borges-Costa, shows that skin cancers can develop earlier than the typical 12-18 years after pediatric transplantation. Most reported cases have been squamous cell cancers and melanomas, and often are associated with lack of appropriate sun protection behavior.
The patient, a Caucasian, was a sailor who used sunscreen but did not typically wear a hat while sailing, reported Dr. Borges-Costa, a dermatologist at the University of Lisbon. Her family history was significant for a grandparent with melanoma.
Dr. Borges noted that the parents and patient were given advice regarding the importance of the lifelong use of sun-protective clothing and headgear. “Education of pediatric organ recipients and their parents about sun protection is important because, as occurred with our patient, protective clothing and hats are frequently forgotten.”
Because of the ongoing potential for skin malignancies, early referral “after transplantation to specialized dermatology outpatient clinics, similar to what is now advocated for transplanted adults, could help in surveillance and improve adherence to sun-protective measures,” he added.
SOURCE: Borges-Costa J et al. Pediatr Dermatol. 2018. doi: 10.1111/pde.13537..
FROM PEDIATRIC DERMATOLOGY
Sunscreen use in grade schoolers: Wide racial, ethnic disparities seen
and the figures were much lower for non-Hispanic black children.
Just 23% of fifth graders almost always used sunscreen, according to data drawn from the Healthy Passages study, which surveyed the parents or caregivers of 5,119 fifth graders. That figure was similar in the 1,802 Hispanic respondents, but fell to just 6% of the 1,748 non-Hispanic black respondents.
Some other factors that were associated with less chance of adherence to sunscreen use included being male and having lower socioeconomic status, wrote Christina M. Correnti, MD, and her study coauthors. The report was published in in Pediatric Dermatology. Perhaps surprisingly, they said, “School-based sun-safety education and involvement in team sports were not significant factors.”
Healthy Passages is a prospective multisite cohort study of child and adolescent health. Dr. Correnti, a dermatology resident at the University of Maryland, Baltimore, and her colleagues used baseline Healthy Passages data collected from the period of 2004-2006. Children enrolled in fifth grade at public schools in Birmingham, Ala., Houston, and Los Angeles, together with their caregivers, participated in the survey. Deidentified demographic data were collected, and participants were asked about four preventive health behaviors in addition to sunscreen use and flossing teeth: brushing teeth, helmet use, seatbelt use, and well-child examinations.
Dr. Correnti and her colleagues used multivariable analysis to calculate odds ratios for the association between the various demographic factors and other preventive behaviors and sunscreen use. They found that sunscreen adherence was correlated with all other preventive behaviors (P less than .001), but that the interrelationship with helmet use was confounded by racial and ethnic variables. Seatbelt use was not significantly correlated with sunscreen use for non-Hispanic black or Hispanic respondents.
“Children from more-educated and affluent households were more likely to use sun protection. Perhaps they had greater parental awareness and practice of sun safe habits,” wrote Dr. Correnti and her colleagues, noting that other work has shown that even low-income parents generally don’t see the cost of sunscreen as a barrier to use.
Although overall use of sunscreen among non-Hispanic black children was low, both non-Hispanic black and Hispanic children were more likely to use sunscreen if they had three or more sunburns within the prior 12 months. “Although darker skin tones may afford some sun protection, melanoma incidence is growing in Hispanic populations,” the researchers wrote.
To address these overall low rates of sunscreen use, the investigators discussed the utility of a variety of education options. The well-child visit affords an opportunity to reinforce the importance of preventive behaviors, but physicians may run into a time crunch and forgo thorough sun safety education, they said. Written materials can be a useful adjunct for clinicians in this setting.
“Health care practitioners may use absence of other preventive behaviors as potential markers for inadequate sunscreen use, prompting a point-of-care sun-safety intervention,” they suggested.
A school-based public health approach offers another route for education. “School sun-safety programs may alleviate the primary care burden,” wrote Dr. Correnti and her coinvestigators. The opportunity to deliver repeated, age-tailored messages as children progress through school may be effective in promoting healthy sun behaviors. Messaging that focuses on the negative effects of sun exposure on appearance such as age spots and wrinkles have been more effective than those warning of the risk of skin cancer for teens; investigating appearance-based content for this age group might be a good idea, the authors said.
The fact that the survey sites were in southern cities may mean that national rates of consistent sunscreen use for elementary schoolers may be even lower, said Dr. Correnti and her coauthors. Many other real-world factors, such as frequency and amount of sunscreen applied and the use of sun-protective clothing, couldn’t be captured by the survey, they acknowledged.
“Even in the most adherent group, non-Hispanic whites, only 44.8% always used sunscreen,” the researchers wrote. The study’s findings leave plenty of room for implementation of broad-based programs, especially in low-resource communities.
The National Institutes of Health funded the research. Dr. Correnti was supported by NIH awards.
SOURCE: Correnti CM et al. Pediatr Dermatol. 2018. doi: 10.1111/pde.13550.
and the figures were much lower for non-Hispanic black children.
Just 23% of fifth graders almost always used sunscreen, according to data drawn from the Healthy Passages study, which surveyed the parents or caregivers of 5,119 fifth graders. That figure was similar in the 1,802 Hispanic respondents, but fell to just 6% of the 1,748 non-Hispanic black respondents.
Some other factors that were associated with less chance of adherence to sunscreen use included being male and having lower socioeconomic status, wrote Christina M. Correnti, MD, and her study coauthors. The report was published in in Pediatric Dermatology. Perhaps surprisingly, they said, “School-based sun-safety education and involvement in team sports were not significant factors.”
Healthy Passages is a prospective multisite cohort study of child and adolescent health. Dr. Correnti, a dermatology resident at the University of Maryland, Baltimore, and her colleagues used baseline Healthy Passages data collected from the period of 2004-2006. Children enrolled in fifth grade at public schools in Birmingham, Ala., Houston, and Los Angeles, together with their caregivers, participated in the survey. Deidentified demographic data were collected, and participants were asked about four preventive health behaviors in addition to sunscreen use and flossing teeth: brushing teeth, helmet use, seatbelt use, and well-child examinations.
Dr. Correnti and her colleagues used multivariable analysis to calculate odds ratios for the association between the various demographic factors and other preventive behaviors and sunscreen use. They found that sunscreen adherence was correlated with all other preventive behaviors (P less than .001), but that the interrelationship with helmet use was confounded by racial and ethnic variables. Seatbelt use was not significantly correlated with sunscreen use for non-Hispanic black or Hispanic respondents.
“Children from more-educated and affluent households were more likely to use sun protection. Perhaps they had greater parental awareness and practice of sun safe habits,” wrote Dr. Correnti and her colleagues, noting that other work has shown that even low-income parents generally don’t see the cost of sunscreen as a barrier to use.
Although overall use of sunscreen among non-Hispanic black children was low, both non-Hispanic black and Hispanic children were more likely to use sunscreen if they had three or more sunburns within the prior 12 months. “Although darker skin tones may afford some sun protection, melanoma incidence is growing in Hispanic populations,” the researchers wrote.
To address these overall low rates of sunscreen use, the investigators discussed the utility of a variety of education options. The well-child visit affords an opportunity to reinforce the importance of preventive behaviors, but physicians may run into a time crunch and forgo thorough sun safety education, they said. Written materials can be a useful adjunct for clinicians in this setting.
“Health care practitioners may use absence of other preventive behaviors as potential markers for inadequate sunscreen use, prompting a point-of-care sun-safety intervention,” they suggested.
A school-based public health approach offers another route for education. “School sun-safety programs may alleviate the primary care burden,” wrote Dr. Correnti and her coinvestigators. The opportunity to deliver repeated, age-tailored messages as children progress through school may be effective in promoting healthy sun behaviors. Messaging that focuses on the negative effects of sun exposure on appearance such as age spots and wrinkles have been more effective than those warning of the risk of skin cancer for teens; investigating appearance-based content for this age group might be a good idea, the authors said.
The fact that the survey sites were in southern cities may mean that national rates of consistent sunscreen use for elementary schoolers may be even lower, said Dr. Correnti and her coauthors. Many other real-world factors, such as frequency and amount of sunscreen applied and the use of sun-protective clothing, couldn’t be captured by the survey, they acknowledged.
“Even in the most adherent group, non-Hispanic whites, only 44.8% always used sunscreen,” the researchers wrote. The study’s findings leave plenty of room for implementation of broad-based programs, especially in low-resource communities.
The National Institutes of Health funded the research. Dr. Correnti was supported by NIH awards.
SOURCE: Correnti CM et al. Pediatr Dermatol. 2018. doi: 10.1111/pde.13550.
and the figures were much lower for non-Hispanic black children.
Just 23% of fifth graders almost always used sunscreen, according to data drawn from the Healthy Passages study, which surveyed the parents or caregivers of 5,119 fifth graders. That figure was similar in the 1,802 Hispanic respondents, but fell to just 6% of the 1,748 non-Hispanic black respondents.
Some other factors that were associated with less chance of adherence to sunscreen use included being male and having lower socioeconomic status, wrote Christina M. Correnti, MD, and her study coauthors. The report was published in in Pediatric Dermatology. Perhaps surprisingly, they said, “School-based sun-safety education and involvement in team sports were not significant factors.”
Healthy Passages is a prospective multisite cohort study of child and adolescent health. Dr. Correnti, a dermatology resident at the University of Maryland, Baltimore, and her colleagues used baseline Healthy Passages data collected from the period of 2004-2006. Children enrolled in fifth grade at public schools in Birmingham, Ala., Houston, and Los Angeles, together with their caregivers, participated in the survey. Deidentified demographic data were collected, and participants were asked about four preventive health behaviors in addition to sunscreen use and flossing teeth: brushing teeth, helmet use, seatbelt use, and well-child examinations.
Dr. Correnti and her colleagues used multivariable analysis to calculate odds ratios for the association between the various demographic factors and other preventive behaviors and sunscreen use. They found that sunscreen adherence was correlated with all other preventive behaviors (P less than .001), but that the interrelationship with helmet use was confounded by racial and ethnic variables. Seatbelt use was not significantly correlated with sunscreen use for non-Hispanic black or Hispanic respondents.
“Children from more-educated and affluent households were more likely to use sun protection. Perhaps they had greater parental awareness and practice of sun safe habits,” wrote Dr. Correnti and her colleagues, noting that other work has shown that even low-income parents generally don’t see the cost of sunscreen as a barrier to use.
Although overall use of sunscreen among non-Hispanic black children was low, both non-Hispanic black and Hispanic children were more likely to use sunscreen if they had three or more sunburns within the prior 12 months. “Although darker skin tones may afford some sun protection, melanoma incidence is growing in Hispanic populations,” the researchers wrote.
To address these overall low rates of sunscreen use, the investigators discussed the utility of a variety of education options. The well-child visit affords an opportunity to reinforce the importance of preventive behaviors, but physicians may run into a time crunch and forgo thorough sun safety education, they said. Written materials can be a useful adjunct for clinicians in this setting.
“Health care practitioners may use absence of other preventive behaviors as potential markers for inadequate sunscreen use, prompting a point-of-care sun-safety intervention,” they suggested.
A school-based public health approach offers another route for education. “School sun-safety programs may alleviate the primary care burden,” wrote Dr. Correnti and her coinvestigators. The opportunity to deliver repeated, age-tailored messages as children progress through school may be effective in promoting healthy sun behaviors. Messaging that focuses on the negative effects of sun exposure on appearance such as age spots and wrinkles have been more effective than those warning of the risk of skin cancer for teens; investigating appearance-based content for this age group might be a good idea, the authors said.
The fact that the survey sites were in southern cities may mean that national rates of consistent sunscreen use for elementary schoolers may be even lower, said Dr. Correnti and her coauthors. Many other real-world factors, such as frequency and amount of sunscreen applied and the use of sun-protective clothing, couldn’t be captured by the survey, they acknowledged.
“Even in the most adherent group, non-Hispanic whites, only 44.8% always used sunscreen,” the researchers wrote. The study’s findings leave plenty of room for implementation of broad-based programs, especially in low-resource communities.
The National Institutes of Health funded the research. Dr. Correnti was supported by NIH awards.
SOURCE: Correnti CM et al. Pediatr Dermatol. 2018. doi: 10.1111/pde.13550.
FROM PEDIATRIC DERMATOLOGY
Key clinical point: Most parents surveyed said their children didn’t use sunscreen consistently.
Major finding: Of non-Hispanic black children, 6% almost always used sunscreen.
Study details: Data drawn from Healthy Passages, a prospective cohort study of 5,119 fifth-graders and their parents or caregivers.
Disclosures: The National Institutes of Health funded the research. Dr. Correnti was supported by NIH awards.
Source: Correnti C et al. Pediatr Dermatol. 2018. doi: 10.1111/pde.13550.
Many actionable mutations may be missed in current testing of advanced RCC
Germline mutations in patients with advanced renal cell carcinoma may be more common than previously suspected.
In a single-center cohort of 254 patients with advanced renal cell carcinoma (RCC) who received matched tumor-germline DNA sequencing, over a third (35.7%) of patients who had mutations in genes associated with RCC had not met current clinical criteria for testing.
In all, pathogenic germline mutations were identified in 41 patients (16.1%), with 14 patients’ mutations (5.5%) in genes known to be associated with RCC. For the remaining 27 patients (10.5%), the mutations were in non–RCC-associated genes, investigators reported in JAMA Oncology.
Of the non–RCC-associated mutations, CHEK2 was particularly common among patients with clear cell RCC (ccRCC), occurring in eight patients with ccRCC and two with non ccRCC (nccRCC). The overall odds ratio for this mutation among the study cohort was 3.0, compared with the general population (95% confidence interval 1.3-5.8; P = .003). “Although there are currently no RCC-specific screening recommendations for individuals with CHEK2 mutations, there may be incremental screening for other cancers, justifying including this gene on RCC panel tests,” wrote Maria Carlo, MD, and her coauthors.
Germline FH mutations were seen in seven patients, all with nccRCC. This higher rate of hereditary leiomyomatosis and RCC (HLRCC) was higher than previously reported in the literature, and clinical cues to the diagnosis were few among the study patients. Even though clues pointing to HLRCC were seen when tumor samples were submitted for histopathology to the genitourinary specialists at the study site, “it is unclear whether nonspecialist pathologists would be able to draw the same conclusions,” wrote Dr. Carlo and her colleagues.
Renal cell cancer–associated mutations were significantly more common in patients with nccRCC than in the ccRCC group: 9/74 (11.7%) nccRCC patients had an RCC-associated mutation, compared with 3/177 of the ccRCC group (P = .001).
The patient’s course of therapy could be guided by the mutation identified in 10% (eight) of the nccRCC patients, “none of which would have been identified with somatic-only sequencing,” wrote Dr. Carlo and associates. “Our results suggest that germline mutations in cancer-associated genes in patients with advanced RCC may be prevalent, and many of these mutations can be used to guide therapy.”
The 254 patients (median age 56 years, 70.5% male, 83.1% non-Hispanic white) were drawn from 267 patients with American Joint Committee on Cancer (AJCC) stage III or IV RCC participating in clinical trials at Memorial Sloan Kettering Cancer Center, New York, where Dr. Carlo practices as an oncologist. The patients included in the cohort were those who consented to germline sequencing and results disclosure.
To determine which pathogenic variants were identified by the study protocol that would have been missed by current testing standards, the investigators assumed that for those who met guidelines, the multigene test panel would probe for VHL, VH, FLCN, MET, SDHB, SDHD, BAP1, TSC1, TSC2, TP53, and MITF. If another mutation was picked up by the next-generation sequencing used in the study, or if a mutation was found in an individual who otherwise would not have been tested, the finding was considered incremental and attributable to the study protocol.
Implications of the additional mutations picked up by the tumor-germline sequencing approach go beyond the patient, said the researchers, who have seen several of the study participants’ family members receive positive test results for cancer-associated mutations as well. “Relatives who are also found to carry FH mutations should be considered for RCC screening. Early detection may increase the likelihood of cure and survivorship,” wrote Dr. Carlo and her coinvestigators.
Dr. Carlo reported serving as a consultant for Pfizer. Other authors reported multiple associations with pharmaceutical companies. The study was funded by the National Institutes of Health, the J. Randall and Kathleen L. MacDonald Kidney Cancer Research Fund, and the Robert and Kate Niehaus Center for Inherited Cancer Genomics at Memorial Sloan Kettering Cancer Center.
SOURCE: Carlo M et al. JAMA Oncol. 2018 Jul 5. doi: 10.1001/jamaoncol.2018.1986.
In the present study, the number of patients who did not meet current criteria for genetic testing, but who had germline RCC-associated mutations, should prompt reevaluation of testing criteria for individuals with advanced RCC.
Particularly for patients with advanced nccRCC, a genetic referral should be considered to weigh germline testing as well as testing for an expanded set of mutations. Patients with advanced ccRCC may also benefit from a broader testing panel that may include some non-RCC related genes.
Further research is needed to elucidate the genotype-phenotype association in some of the non-RCC mutations seen in this cohort, particularly in CHEK2 mutations. There are currently no screening guidelines for CHEK2 in regard to RCC, and the risk for RCC among those with these mutations is not known. RCC patients who have biallelic loss of DNA damage repair genes such as CHEK2 may benefit from treatment that targets these pathways, though these therapies are not currently offered for RCC.
The study population were individuals with advanced RCC, and the increased numbers of pathogenic germline mutations seen in this population are consistent with other studies finding higher rates of these mutations in patients who have other cancer with advanced disease. As this body of knowledge accumulates, interdisciplinary teams will be able to give more accurate information about risk and prognosis to patients and families and, increasingly, offer optimized care.
Dr. Patrick Pilié is an oncologist at the University of Texas MD Anderson Cancer Center, Houston; Dr. Kathleen Cooney is chair of the department of internal medicine and H.A. and Edna Benning Presidential Endowed Chair at the University of Utah, Salt Lake City. These remarks are drawn from a jointly authored editorial accompanying the study’s publication.
In the present study, the number of patients who did not meet current criteria for genetic testing, but who had germline RCC-associated mutations, should prompt reevaluation of testing criteria for individuals with advanced RCC.
Particularly for patients with advanced nccRCC, a genetic referral should be considered to weigh germline testing as well as testing for an expanded set of mutations. Patients with advanced ccRCC may also benefit from a broader testing panel that may include some non-RCC related genes.
Further research is needed to elucidate the genotype-phenotype association in some of the non-RCC mutations seen in this cohort, particularly in CHEK2 mutations. There are currently no screening guidelines for CHEK2 in regard to RCC, and the risk for RCC among those with these mutations is not known. RCC patients who have biallelic loss of DNA damage repair genes such as CHEK2 may benefit from treatment that targets these pathways, though these therapies are not currently offered for RCC.
The study population were individuals with advanced RCC, and the increased numbers of pathogenic germline mutations seen in this population are consistent with other studies finding higher rates of these mutations in patients who have other cancer with advanced disease. As this body of knowledge accumulates, interdisciplinary teams will be able to give more accurate information about risk and prognosis to patients and families and, increasingly, offer optimized care.
Dr. Patrick Pilié is an oncologist at the University of Texas MD Anderson Cancer Center, Houston; Dr. Kathleen Cooney is chair of the department of internal medicine and H.A. and Edna Benning Presidential Endowed Chair at the University of Utah, Salt Lake City. These remarks are drawn from a jointly authored editorial accompanying the study’s publication.
In the present study, the number of patients who did not meet current criteria for genetic testing, but who had germline RCC-associated mutations, should prompt reevaluation of testing criteria for individuals with advanced RCC.
Particularly for patients with advanced nccRCC, a genetic referral should be considered to weigh germline testing as well as testing for an expanded set of mutations. Patients with advanced ccRCC may also benefit from a broader testing panel that may include some non-RCC related genes.
Further research is needed to elucidate the genotype-phenotype association in some of the non-RCC mutations seen in this cohort, particularly in CHEK2 mutations. There are currently no screening guidelines for CHEK2 in regard to RCC, and the risk for RCC among those with these mutations is not known. RCC patients who have biallelic loss of DNA damage repair genes such as CHEK2 may benefit from treatment that targets these pathways, though these therapies are not currently offered for RCC.
The study population were individuals with advanced RCC, and the increased numbers of pathogenic germline mutations seen in this population are consistent with other studies finding higher rates of these mutations in patients who have other cancer with advanced disease. As this body of knowledge accumulates, interdisciplinary teams will be able to give more accurate information about risk and prognosis to patients and families and, increasingly, offer optimized care.
Dr. Patrick Pilié is an oncologist at the University of Texas MD Anderson Cancer Center, Houston; Dr. Kathleen Cooney is chair of the department of internal medicine and H.A. and Edna Benning Presidential Endowed Chair at the University of Utah, Salt Lake City. These remarks are drawn from a jointly authored editorial accompanying the study’s publication.
Germline mutations in patients with advanced renal cell carcinoma may be more common than previously suspected.
In a single-center cohort of 254 patients with advanced renal cell carcinoma (RCC) who received matched tumor-germline DNA sequencing, over a third (35.7%) of patients who had mutations in genes associated with RCC had not met current clinical criteria for testing.
In all, pathogenic germline mutations were identified in 41 patients (16.1%), with 14 patients’ mutations (5.5%) in genes known to be associated with RCC. For the remaining 27 patients (10.5%), the mutations were in non–RCC-associated genes, investigators reported in JAMA Oncology.
Of the non–RCC-associated mutations, CHEK2 was particularly common among patients with clear cell RCC (ccRCC), occurring in eight patients with ccRCC and two with non ccRCC (nccRCC). The overall odds ratio for this mutation among the study cohort was 3.0, compared with the general population (95% confidence interval 1.3-5.8; P = .003). “Although there are currently no RCC-specific screening recommendations for individuals with CHEK2 mutations, there may be incremental screening for other cancers, justifying including this gene on RCC panel tests,” wrote Maria Carlo, MD, and her coauthors.
Germline FH mutations were seen in seven patients, all with nccRCC. This higher rate of hereditary leiomyomatosis and RCC (HLRCC) was higher than previously reported in the literature, and clinical cues to the diagnosis were few among the study patients. Even though clues pointing to HLRCC were seen when tumor samples were submitted for histopathology to the genitourinary specialists at the study site, “it is unclear whether nonspecialist pathologists would be able to draw the same conclusions,” wrote Dr. Carlo and her colleagues.
Renal cell cancer–associated mutations were significantly more common in patients with nccRCC than in the ccRCC group: 9/74 (11.7%) nccRCC patients had an RCC-associated mutation, compared with 3/177 of the ccRCC group (P = .001).
The patient’s course of therapy could be guided by the mutation identified in 10% (eight) of the nccRCC patients, “none of which would have been identified with somatic-only sequencing,” wrote Dr. Carlo and associates. “Our results suggest that germline mutations in cancer-associated genes in patients with advanced RCC may be prevalent, and many of these mutations can be used to guide therapy.”
The 254 patients (median age 56 years, 70.5% male, 83.1% non-Hispanic white) were drawn from 267 patients with American Joint Committee on Cancer (AJCC) stage III or IV RCC participating in clinical trials at Memorial Sloan Kettering Cancer Center, New York, where Dr. Carlo practices as an oncologist. The patients included in the cohort were those who consented to germline sequencing and results disclosure.
To determine which pathogenic variants were identified by the study protocol that would have been missed by current testing standards, the investigators assumed that for those who met guidelines, the multigene test panel would probe for VHL, VH, FLCN, MET, SDHB, SDHD, BAP1, TSC1, TSC2, TP53, and MITF. If another mutation was picked up by the next-generation sequencing used in the study, or if a mutation was found in an individual who otherwise would not have been tested, the finding was considered incremental and attributable to the study protocol.
Implications of the additional mutations picked up by the tumor-germline sequencing approach go beyond the patient, said the researchers, who have seen several of the study participants’ family members receive positive test results for cancer-associated mutations as well. “Relatives who are also found to carry FH mutations should be considered for RCC screening. Early detection may increase the likelihood of cure and survivorship,” wrote Dr. Carlo and her coinvestigators.
Dr. Carlo reported serving as a consultant for Pfizer. Other authors reported multiple associations with pharmaceutical companies. The study was funded by the National Institutes of Health, the J. Randall and Kathleen L. MacDonald Kidney Cancer Research Fund, and the Robert and Kate Niehaus Center for Inherited Cancer Genomics at Memorial Sloan Kettering Cancer Center.
SOURCE: Carlo M et al. JAMA Oncol. 2018 Jul 5. doi: 10.1001/jamaoncol.2018.1986.
Germline mutations in patients with advanced renal cell carcinoma may be more common than previously suspected.
In a single-center cohort of 254 patients with advanced renal cell carcinoma (RCC) who received matched tumor-germline DNA sequencing, over a third (35.7%) of patients who had mutations in genes associated with RCC had not met current clinical criteria for testing.
In all, pathogenic germline mutations were identified in 41 patients (16.1%), with 14 patients’ mutations (5.5%) in genes known to be associated with RCC. For the remaining 27 patients (10.5%), the mutations were in non–RCC-associated genes, investigators reported in JAMA Oncology.
Of the non–RCC-associated mutations, CHEK2 was particularly common among patients with clear cell RCC (ccRCC), occurring in eight patients with ccRCC and two with non ccRCC (nccRCC). The overall odds ratio for this mutation among the study cohort was 3.0, compared with the general population (95% confidence interval 1.3-5.8; P = .003). “Although there are currently no RCC-specific screening recommendations for individuals with CHEK2 mutations, there may be incremental screening for other cancers, justifying including this gene on RCC panel tests,” wrote Maria Carlo, MD, and her coauthors.
Germline FH mutations were seen in seven patients, all with nccRCC. This higher rate of hereditary leiomyomatosis and RCC (HLRCC) was higher than previously reported in the literature, and clinical cues to the diagnosis were few among the study patients. Even though clues pointing to HLRCC were seen when tumor samples were submitted for histopathology to the genitourinary specialists at the study site, “it is unclear whether nonspecialist pathologists would be able to draw the same conclusions,” wrote Dr. Carlo and her colleagues.
Renal cell cancer–associated mutations were significantly more common in patients with nccRCC than in the ccRCC group: 9/74 (11.7%) nccRCC patients had an RCC-associated mutation, compared with 3/177 of the ccRCC group (P = .001).
The patient’s course of therapy could be guided by the mutation identified in 10% (eight) of the nccRCC patients, “none of which would have been identified with somatic-only sequencing,” wrote Dr. Carlo and associates. “Our results suggest that germline mutations in cancer-associated genes in patients with advanced RCC may be prevalent, and many of these mutations can be used to guide therapy.”
The 254 patients (median age 56 years, 70.5% male, 83.1% non-Hispanic white) were drawn from 267 patients with American Joint Committee on Cancer (AJCC) stage III or IV RCC participating in clinical trials at Memorial Sloan Kettering Cancer Center, New York, where Dr. Carlo practices as an oncologist. The patients included in the cohort were those who consented to germline sequencing and results disclosure.
To determine which pathogenic variants were identified by the study protocol that would have been missed by current testing standards, the investigators assumed that for those who met guidelines, the multigene test panel would probe for VHL, VH, FLCN, MET, SDHB, SDHD, BAP1, TSC1, TSC2, TP53, and MITF. If another mutation was picked up by the next-generation sequencing used in the study, or if a mutation was found in an individual who otherwise would not have been tested, the finding was considered incremental and attributable to the study protocol.
Implications of the additional mutations picked up by the tumor-germline sequencing approach go beyond the patient, said the researchers, who have seen several of the study participants’ family members receive positive test results for cancer-associated mutations as well. “Relatives who are also found to carry FH mutations should be considered for RCC screening. Early detection may increase the likelihood of cure and survivorship,” wrote Dr. Carlo and her coinvestigators.
Dr. Carlo reported serving as a consultant for Pfizer. Other authors reported multiple associations with pharmaceutical companies. The study was funded by the National Institutes of Health, the J. Randall and Kathleen L. MacDonald Kidney Cancer Research Fund, and the Robert and Kate Niehaus Center for Inherited Cancer Genomics at Memorial Sloan Kettering Cancer Center.
SOURCE: Carlo M et al. JAMA Oncol. 2018 Jul 5. doi: 10.1001/jamaoncol.2018.1986.
FROM JAMA ONCOLOGY
Key clinical point: A broader approach to sequencing of patients with advanced RCC may identify patients for targeted therapy.
Major finding: Pathogenic germline mutations were seen in 16% of patients with advanced RCC
Study details: Prospective single-center cohort study of 254 patients with advanced RCC.
Disclosures: Dr. Carlo reported serving as a consultant for Pfizer. Other authors reported multiple associations with pharmaceutical companies. The study was funded by the National Institutes of Health, the J.Randall and Kathleen L. MacDonald Kidney Cancer Research Fund, and the Robert and Kate Niehaus Center for Inherited Cancer Genomics at Memorial Sloan Kettering Cancer Center.
Source: Carlo M et al. JAMA Oncol. 2018 July 5. doi: 10.1001/jamaoncol.2018.1986.
Hydroxyurea well tolerated in longitudinal European study
WASHINGTON – European patients with sickle cell disease (SCD) are tolerating hydroxyurea treatment well, 9 years into a 10-year prospective cohort study.
ESCORT-HU (European Sickle Cell Disease Cohort – Hydroxyurea) is slated to enroll 2,000 children and adults over the study period to assess the long-term safety and efficacy of hydroxyurea (HU) as a treatment for SCD.
“Hydroxyurea is still underused in sickle cell disease” both in Europe and the United States, said Bernard Dauvergne, PharmD, executive director of Addmedica, which markets hydroxyurea as Siklos. In part, fears about toxicity and side effects contribute to this underuse, Dr. Dauvergne said at a top abstracts session of the annual meeting of the Foundation for Sickle Cell Disease Research.
ESCORT-HU was designed as a purely observational, noninterventional study of patients treated with HU. It was initiated as part of the European Medicines Agency risk management plan, conceived at the time of the drug’s approval for SCD, explained Dr. Dauvergne.
“This prospective cohort study could enable the identification of delayed rare adverse events in real life, occurring in between 1 in 10,000 and 1 in 1,000 patients,” Dr. Dauvergne said. However, “no new safety information has been detected ... and no secondary cancer has been reported.”
At the last data analysis cutpoint of June 2017, 1,921 patients had been enrolled. Patients have come from 63 centers in France, Germany, Greece, and Italy, Dr. Dauvergne reported.
Of the 1,829 patients with data available for analysis, 804 were under the age of 18. The pediatric patients were a mean 9 years of age, and the adult patients were a mean 33 years old at enrollment. Most enrollees (84%) have the HbSS genotype.
At baseline, the pediatric patients were dosed at a mean 17.2 mg/kg per day of HU; for adult patients, the figure was 15.54 mg/kg per day. More than one-third (35.4%) of pediatric patients had been on HU at the time of study enrollment, for a mean of 4.5 years. In contrast, more than half (60.4%) of adults had been taking hydroxyurea for a mean of 6.25 years at enrollment.
The 1,002 adult patients with recently reported safety data were on a mean HU dose of 20.2 mg/kg per day. About 40% of adults reported at least one adverse event (AE), and a total of 334 AEs in 16.3% of adult patients were judged to be related to HU.
Skin and subcutaneous tissue reactions occurred in about 1 in 10 patients, with 153 reported incidences of skin dryness, skin reactions, alopecia, or changes in skin pigmentation. The next most common adverse events were blood and lymphatic system disorders, mostly neutropenia or thrombocytopenia, occurring in 9.6% of patients.
Discontinuation rates have been low, with an overall dropout rate of 3.8%.
The ESCORT-HU data “confirm that HU is an efficient and well-tolerated drug in both children and adults,” Dr. Dauvergne said. Over the short term, adverse events are related to the expected dose dependent myelosuppression that’s key to the drug’s mechanism of action. This makes sense given the narrow therapeutic index, with a tight range between an efficacious dose and a cytotoxic one, he said. “And the lower the [patient] weight is, the more sensitive the dose variations become,” Dr. Dauvergne said.
Being flexible about fine-tuning doses is key to finding the balance between tolerability and efficacy, “and consequently, for compliance,” he said.
Data collection for the study will end in 2018, with safety and efficacy data expected in 2019.
WASHINGTON – European patients with sickle cell disease (SCD) are tolerating hydroxyurea treatment well, 9 years into a 10-year prospective cohort study.
ESCORT-HU (European Sickle Cell Disease Cohort – Hydroxyurea) is slated to enroll 2,000 children and adults over the study period to assess the long-term safety and efficacy of hydroxyurea (HU) as a treatment for SCD.
“Hydroxyurea is still underused in sickle cell disease” both in Europe and the United States, said Bernard Dauvergne, PharmD, executive director of Addmedica, which markets hydroxyurea as Siklos. In part, fears about toxicity and side effects contribute to this underuse, Dr. Dauvergne said at a top abstracts session of the annual meeting of the Foundation for Sickle Cell Disease Research.
ESCORT-HU was designed as a purely observational, noninterventional study of patients treated with HU. It was initiated as part of the European Medicines Agency risk management plan, conceived at the time of the drug’s approval for SCD, explained Dr. Dauvergne.
“This prospective cohort study could enable the identification of delayed rare adverse events in real life, occurring in between 1 in 10,000 and 1 in 1,000 patients,” Dr. Dauvergne said. However, “no new safety information has been detected ... and no secondary cancer has been reported.”
At the last data analysis cutpoint of June 2017, 1,921 patients had been enrolled. Patients have come from 63 centers in France, Germany, Greece, and Italy, Dr. Dauvergne reported.
Of the 1,829 patients with data available for analysis, 804 were under the age of 18. The pediatric patients were a mean 9 years of age, and the adult patients were a mean 33 years old at enrollment. Most enrollees (84%) have the HbSS genotype.
At baseline, the pediatric patients were dosed at a mean 17.2 mg/kg per day of HU; for adult patients, the figure was 15.54 mg/kg per day. More than one-third (35.4%) of pediatric patients had been on HU at the time of study enrollment, for a mean of 4.5 years. In contrast, more than half (60.4%) of adults had been taking hydroxyurea for a mean of 6.25 years at enrollment.
The 1,002 adult patients with recently reported safety data were on a mean HU dose of 20.2 mg/kg per day. About 40% of adults reported at least one adverse event (AE), and a total of 334 AEs in 16.3% of adult patients were judged to be related to HU.
Skin and subcutaneous tissue reactions occurred in about 1 in 10 patients, with 153 reported incidences of skin dryness, skin reactions, alopecia, or changes in skin pigmentation. The next most common adverse events were blood and lymphatic system disorders, mostly neutropenia or thrombocytopenia, occurring in 9.6% of patients.
Discontinuation rates have been low, with an overall dropout rate of 3.8%.
The ESCORT-HU data “confirm that HU is an efficient and well-tolerated drug in both children and adults,” Dr. Dauvergne said. Over the short term, adverse events are related to the expected dose dependent myelosuppression that’s key to the drug’s mechanism of action. This makes sense given the narrow therapeutic index, with a tight range between an efficacious dose and a cytotoxic one, he said. “And the lower the [patient] weight is, the more sensitive the dose variations become,” Dr. Dauvergne said.
Being flexible about fine-tuning doses is key to finding the balance between tolerability and efficacy, “and consequently, for compliance,” he said.
Data collection for the study will end in 2018, with safety and efficacy data expected in 2019.
WASHINGTON – European patients with sickle cell disease (SCD) are tolerating hydroxyurea treatment well, 9 years into a 10-year prospective cohort study.
ESCORT-HU (European Sickle Cell Disease Cohort – Hydroxyurea) is slated to enroll 2,000 children and adults over the study period to assess the long-term safety and efficacy of hydroxyurea (HU) as a treatment for SCD.
“Hydroxyurea is still underused in sickle cell disease” both in Europe and the United States, said Bernard Dauvergne, PharmD, executive director of Addmedica, which markets hydroxyurea as Siklos. In part, fears about toxicity and side effects contribute to this underuse, Dr. Dauvergne said at a top abstracts session of the annual meeting of the Foundation for Sickle Cell Disease Research.
ESCORT-HU was designed as a purely observational, noninterventional study of patients treated with HU. It was initiated as part of the European Medicines Agency risk management plan, conceived at the time of the drug’s approval for SCD, explained Dr. Dauvergne.
“This prospective cohort study could enable the identification of delayed rare adverse events in real life, occurring in between 1 in 10,000 and 1 in 1,000 patients,” Dr. Dauvergne said. However, “no new safety information has been detected ... and no secondary cancer has been reported.”
At the last data analysis cutpoint of June 2017, 1,921 patients had been enrolled. Patients have come from 63 centers in France, Germany, Greece, and Italy, Dr. Dauvergne reported.
Of the 1,829 patients with data available for analysis, 804 were under the age of 18. The pediatric patients were a mean 9 years of age, and the adult patients were a mean 33 years old at enrollment. Most enrollees (84%) have the HbSS genotype.
At baseline, the pediatric patients were dosed at a mean 17.2 mg/kg per day of HU; for adult patients, the figure was 15.54 mg/kg per day. More than one-third (35.4%) of pediatric patients had been on HU at the time of study enrollment, for a mean of 4.5 years. In contrast, more than half (60.4%) of adults had been taking hydroxyurea for a mean of 6.25 years at enrollment.
The 1,002 adult patients with recently reported safety data were on a mean HU dose of 20.2 mg/kg per day. About 40% of adults reported at least one adverse event (AE), and a total of 334 AEs in 16.3% of adult patients were judged to be related to HU.
Skin and subcutaneous tissue reactions occurred in about 1 in 10 patients, with 153 reported incidences of skin dryness, skin reactions, alopecia, or changes in skin pigmentation. The next most common adverse events were blood and lymphatic system disorders, mostly neutropenia or thrombocytopenia, occurring in 9.6% of patients.
Discontinuation rates have been low, with an overall dropout rate of 3.8%.
The ESCORT-HU data “confirm that HU is an efficient and well-tolerated drug in both children and adults,” Dr. Dauvergne said. Over the short term, adverse events are related to the expected dose dependent myelosuppression that’s key to the drug’s mechanism of action. This makes sense given the narrow therapeutic index, with a tight range between an efficacious dose and a cytotoxic one, he said. “And the lower the [patient] weight is, the more sensitive the dose variations become,” Dr. Dauvergne said.
Being flexible about fine-tuning doses is key to finding the balance between tolerability and efficacy, “and consequently, for compliance,” he said.
Data collection for the study will end in 2018, with safety and efficacy data expected in 2019.
REPORTING FROM FSCDR 2018
Key clinical point:
Major finding: Of adult participants, 16.9% have experienced hydroxyurea-related adverse events.
Study details: Observational 10-year study of 2,000 adults and children with SCD who were taking hydroxyurea.
Disclosures: The study was sponsored by Addmedica, which markets hydroxyurea as Siklos. Dr. Dauvergne is employed by Addmedica.
Source: Dauvergne B et al. FSCDR 2018, presentation JSCDH-D-18-00052.
Low platelets linked to pregnancy complications
A study that characterized the occurrence and frequency of thrombocytopenia throughout the course of pregnancy found a significant decline in platelet counts during the course of pregnancy, and significant differences between pregnant and nonpregnant women. However, the study – published in the New England Journal of Medicine – also found that women with pregnancy-related complications were more likely to have platelet counts less than 150,000/mm3, even in the absence of known causes of thrombocytopenia.
Jessica Reese, PhD, and her coinvestigators at the University of Oklahoma, Oklahoma City, used data from pregnant women who delivered at a single site from 2011 to 2014. In all, 4,568 women from the study group had uncomplicated pregnancies, and 2,586 had pregnancy-related complications. To be included in the complicated pregnancy group, women needed a diagnosis of hypertension, diabetes, eclampsia or preeclampsia, or abnormal placentation. Another 197 women had preexisting disorders known to be associated with thrombocytopenia.
For the women with uncomplicated pregnancies, Dr. Reese and her colleagues compared platelet counts with those of nonpregnant women who participated in the National Health and Nutrition Examination Survey (NHANES) from 1999 to 2012, using a stratified analysis that accounted for age and racial or ethnic background and excluding NHANES participants with cancer, diabetes, or hypertension.
To look at platelet levels across types of pregnancies and in comparison with nonpregnant women, the investigators established three cutpoints, grouping women into those who had a platelet count of at least 150,000/mm3, those with platelet counts less than 100,000/mm3 but at least 80,000/mm3, and those with platelet counts less than 80,000/mm3.
Only 1% of women with uncomplicated pregnancies had platelet counts less than 100,000/mm3 during pregnancy or at delivery, and just 5 women (0.1%) had unexplained platelet counts below 80,000/mm3. Seven more women with platelet counts less than 80,000/mm3 had an identified cause for their thrombocytopenia.
Overall, mean platelet counts were lower for the women with uncomplicated pregnancies during the first trimester than for nonpregnant women (251,000 vs. 273,000/mm3). These values fell throughout pregnancy to a mean of 217,000/mm3 by the time of delivery at a mean gestation of 39.0 weeks (P less than .001 for all time points). However, mean platelet counts rebounded by the time a postpartum value was obtained at a mean 7.1 weeks after delivery, to 264,000/mm3, a value that wasn’t significantly different from the nonpregnant cohort’s platelet counts.
When the investigators looked at mean platelet counts by trimester, they saw no difference between those with uncomplicated and complicated pregnancies until the third trimester. Then, “mean platelet counts decreased at a greater rate among women with pregnancy-related complications,” wrote Dr. Reese and her colleagues; 11.9% of women with complicated pregnancies had platelet counts below 150,000/mm3, while this level was seen in 9.9% of women without complications of pregnancy (P = .01).
At delivery, 2.3% (n = 59) of women with complicated pregnancies had platelet counts below 100,000/mm3, and 31 of these women had counts below 80,000/mm3, representing a significantly higher rate of thrombocytopenia at delivery than seen in the uncomplicated group (P less than .001).
In discussion, Dr. Reese and her coauthors examined the possible mechanisms for decreased levels of circulating platelets during pregnancy. Volume dilution from increased plasma volume is one well-accepted reason. Others include accumulation of platelets within the spleen, which increases in size by about 50% during pregnancy; similarly, the placenta’s circulation is similar to that of the spleen, so platelets may also accumulate there, the authors said. Further support for the placental mechanism comes from the lower average platelet counts for women with twin pregnancies.
The study’s relatively broad definition of pregnancy-related complications may have had the effect of lessening the difference in mean platelet counts between the complicated and uncomplicated pregnancy groups, the investigators acknowledged. Still, their study population had rates of these complications similar to those of the United States population, they said. “Therefore, our data may accurately reflect the platelet counts in women with these pregnancy-related complications,” they noted.
“Severe thrombocytopenia is rare, even in women with pregnancy-related complications,” concluded Dr. Reese and her colleagues. “Our data suggest that, for women with an uncomplicated pregnancy who have a platelet count of less than 100,000/mm3, a cause of thrombocytopenia other than the pregnancy itself should be considered.”
SOURCE: Reese J et al. N Engl J Med. 2018;379:32-43.
A study that characterized the occurrence and frequency of thrombocytopenia throughout the course of pregnancy found a significant decline in platelet counts during the course of pregnancy, and significant differences between pregnant and nonpregnant women. However, the study – published in the New England Journal of Medicine – also found that women with pregnancy-related complications were more likely to have platelet counts less than 150,000/mm3, even in the absence of known causes of thrombocytopenia.
Jessica Reese, PhD, and her coinvestigators at the University of Oklahoma, Oklahoma City, used data from pregnant women who delivered at a single site from 2011 to 2014. In all, 4,568 women from the study group had uncomplicated pregnancies, and 2,586 had pregnancy-related complications. To be included in the complicated pregnancy group, women needed a diagnosis of hypertension, diabetes, eclampsia or preeclampsia, or abnormal placentation. Another 197 women had preexisting disorders known to be associated with thrombocytopenia.
For the women with uncomplicated pregnancies, Dr. Reese and her colleagues compared platelet counts with those of nonpregnant women who participated in the National Health and Nutrition Examination Survey (NHANES) from 1999 to 2012, using a stratified analysis that accounted for age and racial or ethnic background and excluding NHANES participants with cancer, diabetes, or hypertension.
To look at platelet levels across types of pregnancies and in comparison with nonpregnant women, the investigators established three cutpoints, grouping women into those who had a platelet count of at least 150,000/mm3, those with platelet counts less than 100,000/mm3 but at least 80,000/mm3, and those with platelet counts less than 80,000/mm3.
Only 1% of women with uncomplicated pregnancies had platelet counts less than 100,000/mm3 during pregnancy or at delivery, and just 5 women (0.1%) had unexplained platelet counts below 80,000/mm3. Seven more women with platelet counts less than 80,000/mm3 had an identified cause for their thrombocytopenia.
Overall, mean platelet counts were lower for the women with uncomplicated pregnancies during the first trimester than for nonpregnant women (251,000 vs. 273,000/mm3). These values fell throughout pregnancy to a mean of 217,000/mm3 by the time of delivery at a mean gestation of 39.0 weeks (P less than .001 for all time points). However, mean platelet counts rebounded by the time a postpartum value was obtained at a mean 7.1 weeks after delivery, to 264,000/mm3, a value that wasn’t significantly different from the nonpregnant cohort’s platelet counts.
When the investigators looked at mean platelet counts by trimester, they saw no difference between those with uncomplicated and complicated pregnancies until the third trimester. Then, “mean platelet counts decreased at a greater rate among women with pregnancy-related complications,” wrote Dr. Reese and her colleagues; 11.9% of women with complicated pregnancies had platelet counts below 150,000/mm3, while this level was seen in 9.9% of women without complications of pregnancy (P = .01).
At delivery, 2.3% (n = 59) of women with complicated pregnancies had platelet counts below 100,000/mm3, and 31 of these women had counts below 80,000/mm3, representing a significantly higher rate of thrombocytopenia at delivery than seen in the uncomplicated group (P less than .001).
In discussion, Dr. Reese and her coauthors examined the possible mechanisms for decreased levels of circulating platelets during pregnancy. Volume dilution from increased plasma volume is one well-accepted reason. Others include accumulation of platelets within the spleen, which increases in size by about 50% during pregnancy; similarly, the placenta’s circulation is similar to that of the spleen, so platelets may also accumulate there, the authors said. Further support for the placental mechanism comes from the lower average platelet counts for women with twin pregnancies.
The study’s relatively broad definition of pregnancy-related complications may have had the effect of lessening the difference in mean platelet counts between the complicated and uncomplicated pregnancy groups, the investigators acknowledged. Still, their study population had rates of these complications similar to those of the United States population, they said. “Therefore, our data may accurately reflect the platelet counts in women with these pregnancy-related complications,” they noted.
“Severe thrombocytopenia is rare, even in women with pregnancy-related complications,” concluded Dr. Reese and her colleagues. “Our data suggest that, for women with an uncomplicated pregnancy who have a platelet count of less than 100,000/mm3, a cause of thrombocytopenia other than the pregnancy itself should be considered.”
SOURCE: Reese J et al. N Engl J Med. 2018;379:32-43.
A study that characterized the occurrence and frequency of thrombocytopenia throughout the course of pregnancy found a significant decline in platelet counts during the course of pregnancy, and significant differences between pregnant and nonpregnant women. However, the study – published in the New England Journal of Medicine – also found that women with pregnancy-related complications were more likely to have platelet counts less than 150,000/mm3, even in the absence of known causes of thrombocytopenia.
Jessica Reese, PhD, and her coinvestigators at the University of Oklahoma, Oklahoma City, used data from pregnant women who delivered at a single site from 2011 to 2014. In all, 4,568 women from the study group had uncomplicated pregnancies, and 2,586 had pregnancy-related complications. To be included in the complicated pregnancy group, women needed a diagnosis of hypertension, diabetes, eclampsia or preeclampsia, or abnormal placentation. Another 197 women had preexisting disorders known to be associated with thrombocytopenia.
For the women with uncomplicated pregnancies, Dr. Reese and her colleagues compared platelet counts with those of nonpregnant women who participated in the National Health and Nutrition Examination Survey (NHANES) from 1999 to 2012, using a stratified analysis that accounted for age and racial or ethnic background and excluding NHANES participants with cancer, diabetes, or hypertension.
To look at platelet levels across types of pregnancies and in comparison with nonpregnant women, the investigators established three cutpoints, grouping women into those who had a platelet count of at least 150,000/mm3, those with platelet counts less than 100,000/mm3 but at least 80,000/mm3, and those with platelet counts less than 80,000/mm3.
Only 1% of women with uncomplicated pregnancies had platelet counts less than 100,000/mm3 during pregnancy or at delivery, and just 5 women (0.1%) had unexplained platelet counts below 80,000/mm3. Seven more women with platelet counts less than 80,000/mm3 had an identified cause for their thrombocytopenia.
Overall, mean platelet counts were lower for the women with uncomplicated pregnancies during the first trimester than for nonpregnant women (251,000 vs. 273,000/mm3). These values fell throughout pregnancy to a mean of 217,000/mm3 by the time of delivery at a mean gestation of 39.0 weeks (P less than .001 for all time points). However, mean platelet counts rebounded by the time a postpartum value was obtained at a mean 7.1 weeks after delivery, to 264,000/mm3, a value that wasn’t significantly different from the nonpregnant cohort’s platelet counts.
When the investigators looked at mean platelet counts by trimester, they saw no difference between those with uncomplicated and complicated pregnancies until the third trimester. Then, “mean platelet counts decreased at a greater rate among women with pregnancy-related complications,” wrote Dr. Reese and her colleagues; 11.9% of women with complicated pregnancies had platelet counts below 150,000/mm3, while this level was seen in 9.9% of women without complications of pregnancy (P = .01).
At delivery, 2.3% (n = 59) of women with complicated pregnancies had platelet counts below 100,000/mm3, and 31 of these women had counts below 80,000/mm3, representing a significantly higher rate of thrombocytopenia at delivery than seen in the uncomplicated group (P less than .001).
In discussion, Dr. Reese and her coauthors examined the possible mechanisms for decreased levels of circulating platelets during pregnancy. Volume dilution from increased plasma volume is one well-accepted reason. Others include accumulation of platelets within the spleen, which increases in size by about 50% during pregnancy; similarly, the placenta’s circulation is similar to that of the spleen, so platelets may also accumulate there, the authors said. Further support for the placental mechanism comes from the lower average platelet counts for women with twin pregnancies.
The study’s relatively broad definition of pregnancy-related complications may have had the effect of lessening the difference in mean platelet counts between the complicated and uncomplicated pregnancy groups, the investigators acknowledged. Still, their study population had rates of these complications similar to those of the United States population, they said. “Therefore, our data may accurately reflect the platelet counts in women with these pregnancy-related complications,” they noted.
“Severe thrombocytopenia is rare, even in women with pregnancy-related complications,” concluded Dr. Reese and her colleagues. “Our data suggest that, for women with an uncomplicated pregnancy who have a platelet count of less than 100,000/mm3, a cause of thrombocytopenia other than the pregnancy itself should be considered.”
SOURCE: Reese J et al. N Engl J Med. 2018;379:32-43.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Platelet counts less than 150,000/mm3 were more common in complicated pregnancies.
Major finding: Platelet counts were below 150,000/mm3 in 11.9% of complicated versus 9.9% of uncomplicated pregnancies at the time of delivery (P = .01).
Study details: Review of records of 7,351 pregnant women delivering at a single site, compared with NHANES data for 8,885 nonpregnant women.
Disclosures: The National Institutes of Health funded the study. The authors reported having no conflicts of interest.
Source: Reese J et al. N Engl J Med. 2018;379:32-43.
Voxelotor cut transfusions in compassionate use sickle cell cohort
WASHINGTON – An investigational drug for sickle cell disease (SCD) boosted hemoglobin levels while reducing hospitalizations and transfusion needs by approximately two-thirds in a small cohort of severely affected patients.
Lanetta Bronté, MD, who presented the findings at the annual meeting of the Foundation for Sickle Cell Disease Research, reviewed the FDA’s requirements for allowing expanded access to an investigational drug. The key point, she said, is that expanded access may be granted “for treatment of patients with serious or immediately life-threatening diseases or conditions that lack therapeutic alternatives.” And, she said, the potential for benefit should outweigh potential risk of taking the investigational drug.
Current treatments don’t really address serious disease-related complications for patients with advanced SCD, she said. Furthermore, these patients will often be excluded from clinical trials of new SCD therapies.
Voxelotor is a novel small molecule that stabilizes the sickle hemoglobin molecule as a monomer in its high oxygen state. Thus, polymerization of the hemoglobin molecules is inhibited, which decreases the amount of red blood cell damage. Other beneficial effects of voxelotor include improved rheology and reduced hemolysis, as well as a boost to the oxygen-carrying capacity of the sickle hemoglobin molecules, Dr. Bronté explained.
For the seven patients in Dr. Bronté’s clinic who were granted expanded access to voxelator, the disease burden of their end-stage SCD was heavy. All participants had iron overload, five were receiving frequent transfusions, two required chronic oxygen supplementation, and four had severe fatigue. One patient had end-stage renal disease, and another had experienced multiple organ failure and prolonged hospitalizations.
None of the patients qualified for participation in ongoing clinical trials of voxelotor, said Dr. Bronté, who is president of the Foundation for Sickle Cell Disease Research. She also maintains a private practice in Hollywood, Fla.
The four women and three men, aged 22-67 years, were treated with voxelotor for a range of 6-17 months under the FDA’s Expanded Access Program. All patients saw rapid increases in serum hemoglobin, with increases of at least 1 g/dL in five of the seven. Across the participants, increases ranged from 0.5-5.4 g/dL at 24 weeks, up from baseline values of 5.2-7.8 g/dL.
One marker of clinical efficacy that Dr. Bronté and her coauthors examined was the number of hospitalizations for pain from vaso-occlusive crises (VOCs). In the 24 weeks before beginning voxelotor, participants had a summed total of 28 hospitalizations. In the first 24 weeks of treatment, there were a total of nine VOC-related hospitalizations among the participants, a 67% decrease.
The total number of red blood cell transfusions required by the study population declined by a similar proportion, from 33 during the 24 weeks before voxelotor treatment to 13 during the first 24 weeks of treatment, a decrease of 60%.
Individual patients saw improvements related to some of their most troublesome SCD complications, Dr. Bronté reported. All four patients whose oxygen saturation levels had been below 95% on room air saw oxygen saturations improve to 98%-99% on voxelotor. The two patients who had moderate or moderately severe depression, as assessed by the Patient Health Questionnaire 9-item, had minimal or no depression on retest after 24 weeks of voxelotor treatment.
Voxelotor was generally well tolerated at a 900-mg once-daily oral dose. One patient developed grade 2 diarrhea after increasing the dose to 1,500 mg, but symptoms resolved after returning to the 900-mg dose. Another patient had transient mild diarrhea on 900 mg of voxelotor; the symptoms resolved without changing or stopping the drug, Dr. Bronté said. There were no serious treatment-related adverse events.
Among this seriously ill population, two patients died after beginning voxelotor treatment, but both deaths were judged to be unrelated to the treatment. Dr. Bronté reported that the two deceased patients, one of whom was on voxelotor for 16 months and the other for 7 months, did experience reduced transfusion needs and reduced VOC-related hospitalizations while on the drug, experiences that were similar to the surviving members of the cohort.
“Voxelotor administered via compassionate use demonstrated large improvements in anemia and hemolysis, including in patients with lower baseline hemoglobin than studied in clinical trials to date,” Dr. Bronté said.
Taken together with clinical improvements and improved patient-focused outcomes among a severely affected population, “these data … support ongoing investigation in controlled clinical trials to confirm the benefits of voxelotor in a broad range of patients with SCD,” she said.
The study was supported by Global Blood Therapeutics, the manufacturer of voxelotor. Dr. Bronté reported having no other conflicts of interest.
WASHINGTON – An investigational drug for sickle cell disease (SCD) boosted hemoglobin levels while reducing hospitalizations and transfusion needs by approximately two-thirds in a small cohort of severely affected patients.
Lanetta Bronté, MD, who presented the findings at the annual meeting of the Foundation for Sickle Cell Disease Research, reviewed the FDA’s requirements for allowing expanded access to an investigational drug. The key point, she said, is that expanded access may be granted “for treatment of patients with serious or immediately life-threatening diseases or conditions that lack therapeutic alternatives.” And, she said, the potential for benefit should outweigh potential risk of taking the investigational drug.
Current treatments don’t really address serious disease-related complications for patients with advanced SCD, she said. Furthermore, these patients will often be excluded from clinical trials of new SCD therapies.
Voxelotor is a novel small molecule that stabilizes the sickle hemoglobin molecule as a monomer in its high oxygen state. Thus, polymerization of the hemoglobin molecules is inhibited, which decreases the amount of red blood cell damage. Other beneficial effects of voxelotor include improved rheology and reduced hemolysis, as well as a boost to the oxygen-carrying capacity of the sickle hemoglobin molecules, Dr. Bronté explained.
For the seven patients in Dr. Bronté’s clinic who were granted expanded access to voxelator, the disease burden of their end-stage SCD was heavy. All participants had iron overload, five were receiving frequent transfusions, two required chronic oxygen supplementation, and four had severe fatigue. One patient had end-stage renal disease, and another had experienced multiple organ failure and prolonged hospitalizations.
None of the patients qualified for participation in ongoing clinical trials of voxelotor, said Dr. Bronté, who is president of the Foundation for Sickle Cell Disease Research. She also maintains a private practice in Hollywood, Fla.
The four women and three men, aged 22-67 years, were treated with voxelotor for a range of 6-17 months under the FDA’s Expanded Access Program. All patients saw rapid increases in serum hemoglobin, with increases of at least 1 g/dL in five of the seven. Across the participants, increases ranged from 0.5-5.4 g/dL at 24 weeks, up from baseline values of 5.2-7.8 g/dL.
One marker of clinical efficacy that Dr. Bronté and her coauthors examined was the number of hospitalizations for pain from vaso-occlusive crises (VOCs). In the 24 weeks before beginning voxelotor, participants had a summed total of 28 hospitalizations. In the first 24 weeks of treatment, there were a total of nine VOC-related hospitalizations among the participants, a 67% decrease.
The total number of red blood cell transfusions required by the study population declined by a similar proportion, from 33 during the 24 weeks before voxelotor treatment to 13 during the first 24 weeks of treatment, a decrease of 60%.
Individual patients saw improvements related to some of their most troublesome SCD complications, Dr. Bronté reported. All four patients whose oxygen saturation levels had been below 95% on room air saw oxygen saturations improve to 98%-99% on voxelotor. The two patients who had moderate or moderately severe depression, as assessed by the Patient Health Questionnaire 9-item, had minimal or no depression on retest after 24 weeks of voxelotor treatment.
Voxelotor was generally well tolerated at a 900-mg once-daily oral dose. One patient developed grade 2 diarrhea after increasing the dose to 1,500 mg, but symptoms resolved after returning to the 900-mg dose. Another patient had transient mild diarrhea on 900 mg of voxelotor; the symptoms resolved without changing or stopping the drug, Dr. Bronté said. There were no serious treatment-related adverse events.
Among this seriously ill population, two patients died after beginning voxelotor treatment, but both deaths were judged to be unrelated to the treatment. Dr. Bronté reported that the two deceased patients, one of whom was on voxelotor for 16 months and the other for 7 months, did experience reduced transfusion needs and reduced VOC-related hospitalizations while on the drug, experiences that were similar to the surviving members of the cohort.
“Voxelotor administered via compassionate use demonstrated large improvements in anemia and hemolysis, including in patients with lower baseline hemoglobin than studied in clinical trials to date,” Dr. Bronté said.
Taken together with clinical improvements and improved patient-focused outcomes among a severely affected population, “these data … support ongoing investigation in controlled clinical trials to confirm the benefits of voxelotor in a broad range of patients with SCD,” she said.
The study was supported by Global Blood Therapeutics, the manufacturer of voxelotor. Dr. Bronté reported having no other conflicts of interest.
WASHINGTON – An investigational drug for sickle cell disease (SCD) boosted hemoglobin levels while reducing hospitalizations and transfusion needs by approximately two-thirds in a small cohort of severely affected patients.
Lanetta Bronté, MD, who presented the findings at the annual meeting of the Foundation for Sickle Cell Disease Research, reviewed the FDA’s requirements for allowing expanded access to an investigational drug. The key point, she said, is that expanded access may be granted “for treatment of patients with serious or immediately life-threatening diseases or conditions that lack therapeutic alternatives.” And, she said, the potential for benefit should outweigh potential risk of taking the investigational drug.
Current treatments don’t really address serious disease-related complications for patients with advanced SCD, she said. Furthermore, these patients will often be excluded from clinical trials of new SCD therapies.
Voxelotor is a novel small molecule that stabilizes the sickle hemoglobin molecule as a monomer in its high oxygen state. Thus, polymerization of the hemoglobin molecules is inhibited, which decreases the amount of red blood cell damage. Other beneficial effects of voxelotor include improved rheology and reduced hemolysis, as well as a boost to the oxygen-carrying capacity of the sickle hemoglobin molecules, Dr. Bronté explained.
For the seven patients in Dr. Bronté’s clinic who were granted expanded access to voxelator, the disease burden of their end-stage SCD was heavy. All participants had iron overload, five were receiving frequent transfusions, two required chronic oxygen supplementation, and four had severe fatigue. One patient had end-stage renal disease, and another had experienced multiple organ failure and prolonged hospitalizations.
None of the patients qualified for participation in ongoing clinical trials of voxelotor, said Dr. Bronté, who is president of the Foundation for Sickle Cell Disease Research. She also maintains a private practice in Hollywood, Fla.
The four women and three men, aged 22-67 years, were treated with voxelotor for a range of 6-17 months under the FDA’s Expanded Access Program. All patients saw rapid increases in serum hemoglobin, with increases of at least 1 g/dL in five of the seven. Across the participants, increases ranged from 0.5-5.4 g/dL at 24 weeks, up from baseline values of 5.2-7.8 g/dL.
One marker of clinical efficacy that Dr. Bronté and her coauthors examined was the number of hospitalizations for pain from vaso-occlusive crises (VOCs). In the 24 weeks before beginning voxelotor, participants had a summed total of 28 hospitalizations. In the first 24 weeks of treatment, there were a total of nine VOC-related hospitalizations among the participants, a 67% decrease.
The total number of red blood cell transfusions required by the study population declined by a similar proportion, from 33 during the 24 weeks before voxelotor treatment to 13 during the first 24 weeks of treatment, a decrease of 60%.
Individual patients saw improvements related to some of their most troublesome SCD complications, Dr. Bronté reported. All four patients whose oxygen saturation levels had been below 95% on room air saw oxygen saturations improve to 98%-99% on voxelotor. The two patients who had moderate or moderately severe depression, as assessed by the Patient Health Questionnaire 9-item, had minimal or no depression on retest after 24 weeks of voxelotor treatment.
Voxelotor was generally well tolerated at a 900-mg once-daily oral dose. One patient developed grade 2 diarrhea after increasing the dose to 1,500 mg, but symptoms resolved after returning to the 900-mg dose. Another patient had transient mild diarrhea on 900 mg of voxelotor; the symptoms resolved without changing or stopping the drug, Dr. Bronté said. There were no serious treatment-related adverse events.
Among this seriously ill population, two patients died after beginning voxelotor treatment, but both deaths were judged to be unrelated to the treatment. Dr. Bronté reported that the two deceased patients, one of whom was on voxelotor for 16 months and the other for 7 months, did experience reduced transfusion needs and reduced VOC-related hospitalizations while on the drug, experiences that were similar to the surviving members of the cohort.
“Voxelotor administered via compassionate use demonstrated large improvements in anemia and hemolysis, including in patients with lower baseline hemoglobin than studied in clinical trials to date,” Dr. Bronté said.
Taken together with clinical improvements and improved patient-focused outcomes among a severely affected population, “these data … support ongoing investigation in controlled clinical trials to confirm the benefits of voxelotor in a broad range of patients with SCD,” she said.
The study was supported by Global Blood Therapeutics, the manufacturer of voxelotor. Dr. Bronté reported having no other conflicts of interest.
REPORTING FROM FSCDR 2018
Key clinical point:
Major finding: Transfusion requirements were cut by 60% in the first 24 weeks on voxelotor.
Study details: Open label case series of seven patients with end-stage SCD at a single center.
Disclosures: The study was funded by Global Blood Therapeutics, which manufactures voxelotor. Dr. Bronté reported having no other conflicts of interest.
Ob.gyn. workforce shortage looms
The average age of ob.gyns. is rising across the country, signaling physician shortages that are projected to worsen over the next several decades. The pinch may be felt especially in areas with high birth rates and few younger ob.gyns., according to a report from Doximity.
Doximity’s geographic projections paint a fine-grained picture in agreement with the American College of Obstetricians and Gynecologists’ projections of a national shortfall of up to 8,800 ob.gyns. by 2020, with the deficit of ob.gyns. potentially climbing to 22,000 by 2050.
Pittsburgh and Bridgeport, Conn. top the list of metropolitan areas with the oldest average age of practicing ob.gyns., with average ages of 52.32 and 52.12 years, respectively. Las Vegas, Detroit, Miami, Los Angeles, New York, Boston, and Chicago all also made the top 15 cities in this list.
Because ob.gyns. start to leave the work force at age 59 years and retire at a median age of 64 years, this aging physician population could affect access to women’s health services within the decade, said Dr. Whaley. The fact that the burnout rate for ob.gyns. is only topped by emergency room physicians represents another area for concern, said Dr. Whaley of the University of California, Berkeley.
The report drew on data from the Centers for Medicare and Medicaid Services and board certification data. Doximity’s self-reported information from about 43,000 full-time board-certified ob.gyns. was also factored in. The top metropolitan areas were determined by 2010 census data and birth statistics came from federal databases.
With the data sliced another way, Pittsburgh also had the highest percentage of ob.gyns. aged 55 years or older, with 41.92% falling into this age group. Of the 50 metropolitan areas included in the Doximity report, 32 have an ob.gyn. workforce with at least one-third aged 55 years and up.
And there are many metropolitan areas where there’s a paucity of young ob.gyns., signaling future serious shortages, according to Doximity. In 12 of the metropolitan areas studied, less than 15% of the ob.gyn. workforce is aged 40 years or younger. Some of the cities with a potential “double hit” of older ob.gyns. and few younger physicians entering the profession include Las Vegas, Miami, Los Angeles, New York, and Chicago.
The Doximity methodology also took into account the average workload, in terms of the number of live births per physician per year, for ob.gyns. in the various metropolitan areas. There was a large variation in workload calculated this way: St. Louis has 247 live births per ob.gyn per year, while Louisville, Ky., has just 64, according to the report.
Factoring in workload information as well, the report gives a “shortage risk index” listing. Here, Las Vegas, Los Angeles, and Miami top the list. Cities considered at lowest risk for shortages according to this methodology include Baltimore, Denver, Portland, Ore., and Cleveland.
According to a 2017 ACOG workforce report, though women make up nearly half of those entering medical school, over four in five physicians entering ob.gyn. residencies (82.3% in 2016) are women. Further, women made up 58.7% of the ob.gyns. in active practice in 2017, outstripping all other surgical and medical specialties save pediatrics. Within 10 years, two-thirds of all ob.gyns. will be female, according to ACOG.
“While this study cannot determine causation for the variation in workloads, compensation, or shortages across metropolitan areas, we hope it will continue to serve as a baseline for the size of the challenge and prove helpful to health care employers, policymakers, patient advocates, and others interested in further study of this topic,” wrote Dr. Whaley and the report’s coauthors. “This information may also be helpful for ob.gyns. looking to live in areas with an increasing need for their expertise,” they said.
Dr. Whaley has received consulting fees from Castlight Health, Crossover Health, Doximity, Livongo, and Norwest Venture Partners. Doximity funded the production of the report.
The average age of ob.gyns. is rising across the country, signaling physician shortages that are projected to worsen over the next several decades. The pinch may be felt especially in areas with high birth rates and few younger ob.gyns., according to a report from Doximity.
Doximity’s geographic projections paint a fine-grained picture in agreement with the American College of Obstetricians and Gynecologists’ projections of a national shortfall of up to 8,800 ob.gyns. by 2020, with the deficit of ob.gyns. potentially climbing to 22,000 by 2050.
Pittsburgh and Bridgeport, Conn. top the list of metropolitan areas with the oldest average age of practicing ob.gyns., with average ages of 52.32 and 52.12 years, respectively. Las Vegas, Detroit, Miami, Los Angeles, New York, Boston, and Chicago all also made the top 15 cities in this list.
Because ob.gyns. start to leave the work force at age 59 years and retire at a median age of 64 years, this aging physician population could affect access to women’s health services within the decade, said Dr. Whaley. The fact that the burnout rate for ob.gyns. is only topped by emergency room physicians represents another area for concern, said Dr. Whaley of the University of California, Berkeley.
The report drew on data from the Centers for Medicare and Medicaid Services and board certification data. Doximity’s self-reported information from about 43,000 full-time board-certified ob.gyns. was also factored in. The top metropolitan areas were determined by 2010 census data and birth statistics came from federal databases.
With the data sliced another way, Pittsburgh also had the highest percentage of ob.gyns. aged 55 years or older, with 41.92% falling into this age group. Of the 50 metropolitan areas included in the Doximity report, 32 have an ob.gyn. workforce with at least one-third aged 55 years and up.
And there are many metropolitan areas where there’s a paucity of young ob.gyns., signaling future serious shortages, according to Doximity. In 12 of the metropolitan areas studied, less than 15% of the ob.gyn. workforce is aged 40 years or younger. Some of the cities with a potential “double hit” of older ob.gyns. and few younger physicians entering the profession include Las Vegas, Miami, Los Angeles, New York, and Chicago.
The Doximity methodology also took into account the average workload, in terms of the number of live births per physician per year, for ob.gyns. in the various metropolitan areas. There was a large variation in workload calculated this way: St. Louis has 247 live births per ob.gyn per year, while Louisville, Ky., has just 64, according to the report.
Factoring in workload information as well, the report gives a “shortage risk index” listing. Here, Las Vegas, Los Angeles, and Miami top the list. Cities considered at lowest risk for shortages according to this methodology include Baltimore, Denver, Portland, Ore., and Cleveland.
According to a 2017 ACOG workforce report, though women make up nearly half of those entering medical school, over four in five physicians entering ob.gyn. residencies (82.3% in 2016) are women. Further, women made up 58.7% of the ob.gyns. in active practice in 2017, outstripping all other surgical and medical specialties save pediatrics. Within 10 years, two-thirds of all ob.gyns. will be female, according to ACOG.
“While this study cannot determine causation for the variation in workloads, compensation, or shortages across metropolitan areas, we hope it will continue to serve as a baseline for the size of the challenge and prove helpful to health care employers, policymakers, patient advocates, and others interested in further study of this topic,” wrote Dr. Whaley and the report’s coauthors. “This information may also be helpful for ob.gyns. looking to live in areas with an increasing need for their expertise,” they said.
Dr. Whaley has received consulting fees from Castlight Health, Crossover Health, Doximity, Livongo, and Norwest Venture Partners. Doximity funded the production of the report.
The average age of ob.gyns. is rising across the country, signaling physician shortages that are projected to worsen over the next several decades. The pinch may be felt especially in areas with high birth rates and few younger ob.gyns., according to a report from Doximity.
Doximity’s geographic projections paint a fine-grained picture in agreement with the American College of Obstetricians and Gynecologists’ projections of a national shortfall of up to 8,800 ob.gyns. by 2020, with the deficit of ob.gyns. potentially climbing to 22,000 by 2050.
Pittsburgh and Bridgeport, Conn. top the list of metropolitan areas with the oldest average age of practicing ob.gyns., with average ages of 52.32 and 52.12 years, respectively. Las Vegas, Detroit, Miami, Los Angeles, New York, Boston, and Chicago all also made the top 15 cities in this list.
Because ob.gyns. start to leave the work force at age 59 years and retire at a median age of 64 years, this aging physician population could affect access to women’s health services within the decade, said Dr. Whaley. The fact that the burnout rate for ob.gyns. is only topped by emergency room physicians represents another area for concern, said Dr. Whaley of the University of California, Berkeley.
The report drew on data from the Centers for Medicare and Medicaid Services and board certification data. Doximity’s self-reported information from about 43,000 full-time board-certified ob.gyns. was also factored in. The top metropolitan areas were determined by 2010 census data and birth statistics came from federal databases.
With the data sliced another way, Pittsburgh also had the highest percentage of ob.gyns. aged 55 years or older, with 41.92% falling into this age group. Of the 50 metropolitan areas included in the Doximity report, 32 have an ob.gyn. workforce with at least one-third aged 55 years and up.
And there are many metropolitan areas where there’s a paucity of young ob.gyns., signaling future serious shortages, according to Doximity. In 12 of the metropolitan areas studied, less than 15% of the ob.gyn. workforce is aged 40 years or younger. Some of the cities with a potential “double hit” of older ob.gyns. and few younger physicians entering the profession include Las Vegas, Miami, Los Angeles, New York, and Chicago.
The Doximity methodology also took into account the average workload, in terms of the number of live births per physician per year, for ob.gyns. in the various metropolitan areas. There was a large variation in workload calculated this way: St. Louis has 247 live births per ob.gyn per year, while Louisville, Ky., has just 64, according to the report.
Factoring in workload information as well, the report gives a “shortage risk index” listing. Here, Las Vegas, Los Angeles, and Miami top the list. Cities considered at lowest risk for shortages according to this methodology include Baltimore, Denver, Portland, Ore., and Cleveland.
According to a 2017 ACOG workforce report, though women make up nearly half of those entering medical school, over four in five physicians entering ob.gyn. residencies (82.3% in 2016) are women. Further, women made up 58.7% of the ob.gyns. in active practice in 2017, outstripping all other surgical and medical specialties save pediatrics. Within 10 years, two-thirds of all ob.gyns. will be female, according to ACOG.
“While this study cannot determine causation for the variation in workloads, compensation, or shortages across metropolitan areas, we hope it will continue to serve as a baseline for the size of the challenge and prove helpful to health care employers, policymakers, patient advocates, and others interested in further study of this topic,” wrote Dr. Whaley and the report’s coauthors. “This information may also be helpful for ob.gyns. looking to live in areas with an increasing need for their expertise,” they said.
Dr. Whaley has received consulting fees from Castlight Health, Crossover Health, Doximity, Livongo, and Norwest Venture Partners. Doximity funded the production of the report.
FDA approves Epidiolex for Lennox-Gastaut syndrome and Dravet syndrome
The Food and Drug Administration has approved cannabidiol oral solution (Epidiolex, GW Pharmaceuticals) for the treatment of two rare pediatric seizure disorders.
“This product approval demonstrates that advancing sound scientific research to investigate ingredients derived from marijuana can lead to important therapies. This new treatment provides new options for patients,” said FDA Commissioner Scott Gottlieb, MD, in a statement.
However, he cautioned, “This is an important medical advance. But it’s also important to note that this is not an approval of marijuana or all of its components. This is the approval of one specific CBD medication for a specific use. And it was based on well-controlled clinical trials evaluating the use of this compound in the treatment of a specific condition.”
The FDA Peripheral and Central Nervous System Drugs Advisory Committee’s earlier positive recommendation was based on three randomized, double-blind, placebo-controlled clinical trials. These trials showed a 50% reduction of drop seizure frequency in 40%-44% of patients with Lennox-Gastaut syndrome, and a 39% decrease in convulsive seizure frequency for trial participants with Dravet Syndrome. A total of 516 patients with one of the two seizure disorders participated in the clinical trials.
“In addition to another important treatment option for Lennox-Gastaut patients, this first-ever approval of a drug specifically for Dravet patients will provide a significant and needed improvement in the therapeutic approach to caring for people with this condition,” said Billy Dunn, MD, director of the Division of Neurology Products in the FDA Center for Drug Evaluation and Research, in a statement.
After reviewing information provided by the drug’s sponsor and the FDA, the advisory committee judged that CBD-OS, derived from a non-psychoactive chemical found in marijuana, was very unlikely to have potential for abuse.
Sedation, sleepiness, and lethargy were among the most frequently reported adverse events for the patients taking CBD-OS. In data pooled from the clinical trials, 16.3% of patients taking CBD-OS at the higher dose of 20 mg/kg/day had liver transaminase elevations above three times the upper limit of normal; this level of transaminase elevation was seen in 0.9% of patients taking placebo.
A patient medication guide detailing risks and how the drug should be used will accompany CBD-OS when it is dispensed, according to the FDA approval.
In his statement, Dr. Gottlieb put the approval in the context of the FDA’s broader efforts to encourage a strong clinical development program for marijuana-derived drugs that does not compromise standards for ensuring safety and efficacy of drugs approved by the agency. He also noted that ongoing efforts to support high quality research into marijuana-based therapies involve other federal agencies, including the National Institute on Drug Abuse and the Drug Enforcement Administration.
The FDA’s actions against companies distributing unapproved products that contain cannabidiol and making unproven marketing claims will continue, said Dr. Gottlieb. Still, “Today’s approval demonstrates our commitment to the scientific process and working with product developers to bring marijuana-based products to market,” he said.
The Food and Drug Administration has approved cannabidiol oral solution (Epidiolex, GW Pharmaceuticals) for the treatment of two rare pediatric seizure disorders.
“This product approval demonstrates that advancing sound scientific research to investigate ingredients derived from marijuana can lead to important therapies. This new treatment provides new options for patients,” said FDA Commissioner Scott Gottlieb, MD, in a statement.
However, he cautioned, “This is an important medical advance. But it’s also important to note that this is not an approval of marijuana or all of its components. This is the approval of one specific CBD medication for a specific use. And it was based on well-controlled clinical trials evaluating the use of this compound in the treatment of a specific condition.”
The FDA Peripheral and Central Nervous System Drugs Advisory Committee’s earlier positive recommendation was based on three randomized, double-blind, placebo-controlled clinical trials. These trials showed a 50% reduction of drop seizure frequency in 40%-44% of patients with Lennox-Gastaut syndrome, and a 39% decrease in convulsive seizure frequency for trial participants with Dravet Syndrome. A total of 516 patients with one of the two seizure disorders participated in the clinical trials.
“In addition to another important treatment option for Lennox-Gastaut patients, this first-ever approval of a drug specifically for Dravet patients will provide a significant and needed improvement in the therapeutic approach to caring for people with this condition,” said Billy Dunn, MD, director of the Division of Neurology Products in the FDA Center for Drug Evaluation and Research, in a statement.
After reviewing information provided by the drug’s sponsor and the FDA, the advisory committee judged that CBD-OS, derived from a non-psychoactive chemical found in marijuana, was very unlikely to have potential for abuse.
Sedation, sleepiness, and lethargy were among the most frequently reported adverse events for the patients taking CBD-OS. In data pooled from the clinical trials, 16.3% of patients taking CBD-OS at the higher dose of 20 mg/kg/day had liver transaminase elevations above three times the upper limit of normal; this level of transaminase elevation was seen in 0.9% of patients taking placebo.
A patient medication guide detailing risks and how the drug should be used will accompany CBD-OS when it is dispensed, according to the FDA approval.
In his statement, Dr. Gottlieb put the approval in the context of the FDA’s broader efforts to encourage a strong clinical development program for marijuana-derived drugs that does not compromise standards for ensuring safety and efficacy of drugs approved by the agency. He also noted that ongoing efforts to support high quality research into marijuana-based therapies involve other federal agencies, including the National Institute on Drug Abuse and the Drug Enforcement Administration.
The FDA’s actions against companies distributing unapproved products that contain cannabidiol and making unproven marketing claims will continue, said Dr. Gottlieb. Still, “Today’s approval demonstrates our commitment to the scientific process and working with product developers to bring marijuana-based products to market,” he said.
The Food and Drug Administration has approved cannabidiol oral solution (Epidiolex, GW Pharmaceuticals) for the treatment of two rare pediatric seizure disorders.
“This product approval demonstrates that advancing sound scientific research to investigate ingredients derived from marijuana can lead to important therapies. This new treatment provides new options for patients,” said FDA Commissioner Scott Gottlieb, MD, in a statement.
However, he cautioned, “This is an important medical advance. But it’s also important to note that this is not an approval of marijuana or all of its components. This is the approval of one specific CBD medication for a specific use. And it was based on well-controlled clinical trials evaluating the use of this compound in the treatment of a specific condition.”
The FDA Peripheral and Central Nervous System Drugs Advisory Committee’s earlier positive recommendation was based on three randomized, double-blind, placebo-controlled clinical trials. These trials showed a 50% reduction of drop seizure frequency in 40%-44% of patients with Lennox-Gastaut syndrome, and a 39% decrease in convulsive seizure frequency for trial participants with Dravet Syndrome. A total of 516 patients with one of the two seizure disorders participated in the clinical trials.
“In addition to another important treatment option for Lennox-Gastaut patients, this first-ever approval of a drug specifically for Dravet patients will provide a significant and needed improvement in the therapeutic approach to caring for people with this condition,” said Billy Dunn, MD, director of the Division of Neurology Products in the FDA Center for Drug Evaluation and Research, in a statement.
After reviewing information provided by the drug’s sponsor and the FDA, the advisory committee judged that CBD-OS, derived from a non-psychoactive chemical found in marijuana, was very unlikely to have potential for abuse.
Sedation, sleepiness, and lethargy were among the most frequently reported adverse events for the patients taking CBD-OS. In data pooled from the clinical trials, 16.3% of patients taking CBD-OS at the higher dose of 20 mg/kg/day had liver transaminase elevations above three times the upper limit of normal; this level of transaminase elevation was seen in 0.9% of patients taking placebo.
A patient medication guide detailing risks and how the drug should be used will accompany CBD-OS when it is dispensed, according to the FDA approval.
In his statement, Dr. Gottlieb put the approval in the context of the FDA’s broader efforts to encourage a strong clinical development program for marijuana-derived drugs that does not compromise standards for ensuring safety and efficacy of drugs approved by the agency. He also noted that ongoing efforts to support high quality research into marijuana-based therapies involve other federal agencies, including the National Institute on Drug Abuse and the Drug Enforcement Administration.
The FDA’s actions against companies distributing unapproved products that contain cannabidiol and making unproven marketing claims will continue, said Dr. Gottlieb. Still, “Today’s approval demonstrates our commitment to the scientific process and working with product developers to bring marijuana-based products to market,” he said.
FDA okays fully implantable continuous glucose monitor/mobile app combo for diabetes
to transmit continuous information about blood glucose levels for people with diabetes.
The sensor-mobile app combo, called the Eversense Continuous Glucose Monitoring (CGM) system, is designed to supplant the need for frequent blood sampling to monitor blood glucose levels.
“The FDA is committed to advancing novel products that leverage digital technology to improve patient care,” said FDA commissioner Scott Gottlieb, MD, in the agency’s press release announcing the approval. The sensor, which is roughly 1.5 cm long, is coated with a material that fluoresces when exposed to glucose; the sensor uses the amount of light emitted to calculate blood glucose levels. Patients use an adhesive patch, changed daily, to attach a “smart” transmitter that overlies the area where the sensor is implanted. This rechargeable transmitter sends blood glucose levels to the mobile app every 5 minutes, and also powers the sensor.
The FDA’s approval was based on data from 125 patients with type 1 and type 2 diabetes who used the CGM system. The bulk of clinical data was acquired from PRECISE II, which enrolled 90 patients with type 1 and type 2 diabetes. When compared with levels returned from concurrently performed conventional home glucose monitoring, the CGM system achieved a mean absolute relative difference (MARD) of 8.8% (95% confidence interval, 8.1%-9.3%). This was less than the prespecified accuracy goal of 20% MARD (P less than .0001).
During the nonrandomized, blinded, prospective PRECISE II trial, 91% of the implanted sensors were functioning through the end of 90 days. A variation of the Eversense CGM, the Eversense CGM XL, has been approved for use up to 180 days in Europe.
The overall rate of serious adverse events among patients participating in the Eversense CGM trials was less than 1%. “The safety of this novel system will also be evaluated in a post-approval study,” wrote FDA officials in the press release.
In addition to adverse effects related to the outpatient procedure in which the glucose sensor is implanted subcutaneously, the FDA said that allergic reactions, ongoing pain, discomfort, scarring, and skin changes are possible with use of the CGM. Though the system sends frequent blood glucose measurements to the accompanying mobile app, missed alerts might still result in hypo- or hyperglycemia.
The Eversense CGM is marketed by Senseonics, which funded the studies underpinning approval.
to transmit continuous information about blood glucose levels for people with diabetes.
The sensor-mobile app combo, called the Eversense Continuous Glucose Monitoring (CGM) system, is designed to supplant the need for frequent blood sampling to monitor blood glucose levels.
“The FDA is committed to advancing novel products that leverage digital technology to improve patient care,” said FDA commissioner Scott Gottlieb, MD, in the agency’s press release announcing the approval. The sensor, which is roughly 1.5 cm long, is coated with a material that fluoresces when exposed to glucose; the sensor uses the amount of light emitted to calculate blood glucose levels. Patients use an adhesive patch, changed daily, to attach a “smart” transmitter that overlies the area where the sensor is implanted. This rechargeable transmitter sends blood glucose levels to the mobile app every 5 minutes, and also powers the sensor.
The FDA’s approval was based on data from 125 patients with type 1 and type 2 diabetes who used the CGM system. The bulk of clinical data was acquired from PRECISE II, which enrolled 90 patients with type 1 and type 2 diabetes. When compared with levels returned from concurrently performed conventional home glucose monitoring, the CGM system achieved a mean absolute relative difference (MARD) of 8.8% (95% confidence interval, 8.1%-9.3%). This was less than the prespecified accuracy goal of 20% MARD (P less than .0001).
During the nonrandomized, blinded, prospective PRECISE II trial, 91% of the implanted sensors were functioning through the end of 90 days. A variation of the Eversense CGM, the Eversense CGM XL, has been approved for use up to 180 days in Europe.
The overall rate of serious adverse events among patients participating in the Eversense CGM trials was less than 1%. “The safety of this novel system will also be evaluated in a post-approval study,” wrote FDA officials in the press release.
In addition to adverse effects related to the outpatient procedure in which the glucose sensor is implanted subcutaneously, the FDA said that allergic reactions, ongoing pain, discomfort, scarring, and skin changes are possible with use of the CGM. Though the system sends frequent blood glucose measurements to the accompanying mobile app, missed alerts might still result in hypo- or hyperglycemia.
The Eversense CGM is marketed by Senseonics, which funded the studies underpinning approval.
to transmit continuous information about blood glucose levels for people with diabetes.
The sensor-mobile app combo, called the Eversense Continuous Glucose Monitoring (CGM) system, is designed to supplant the need for frequent blood sampling to monitor blood glucose levels.
“The FDA is committed to advancing novel products that leverage digital technology to improve patient care,” said FDA commissioner Scott Gottlieb, MD, in the agency’s press release announcing the approval. The sensor, which is roughly 1.5 cm long, is coated with a material that fluoresces when exposed to glucose; the sensor uses the amount of light emitted to calculate blood glucose levels. Patients use an adhesive patch, changed daily, to attach a “smart” transmitter that overlies the area where the sensor is implanted. This rechargeable transmitter sends blood glucose levels to the mobile app every 5 minutes, and also powers the sensor.
The FDA’s approval was based on data from 125 patients with type 1 and type 2 diabetes who used the CGM system. The bulk of clinical data was acquired from PRECISE II, which enrolled 90 patients with type 1 and type 2 diabetes. When compared with levels returned from concurrently performed conventional home glucose monitoring, the CGM system achieved a mean absolute relative difference (MARD) of 8.8% (95% confidence interval, 8.1%-9.3%). This was less than the prespecified accuracy goal of 20% MARD (P less than .0001).
During the nonrandomized, blinded, prospective PRECISE II trial, 91% of the implanted sensors were functioning through the end of 90 days. A variation of the Eversense CGM, the Eversense CGM XL, has been approved for use up to 180 days in Europe.
The overall rate of serious adverse events among patients participating in the Eversense CGM trials was less than 1%. “The safety of this novel system will also be evaluated in a post-approval study,” wrote FDA officials in the press release.
In addition to adverse effects related to the outpatient procedure in which the glucose sensor is implanted subcutaneously, the FDA said that allergic reactions, ongoing pain, discomfort, scarring, and skin changes are possible with use of the CGM. Though the system sends frequent blood glucose measurements to the accompanying mobile app, missed alerts might still result in hypo- or hyperglycemia.
The Eversense CGM is marketed by Senseonics, which funded the studies underpinning approval.
Sickle cell disease exacts a heavy vocational toll
WASHINGTON – Three-quarters of patients with sickle cell disease (SCD) reported missing work in the last year because of disease symptoms, according to results from a single-center study.
While the direct costs of SCD are easy to measure, it’s harder to capture the indirect costs patients may incur from this chronic, progressive disease, which range from lost days at work to the downstream consequences of “presenteeism.”
“Indirect costs are related to things that have value, but it’s a little bit harder to apply an exact value to it,” said Nicholas Vendetti of Pfizer. But this is a critical piece for understanding SCD, he said. “The burden of illness is unknown without productivity costs.”
Mr. Vendetti and his collaborators attempted to capture the indirect costs of SCD, and reported the results of a single-site study at the annual meeting of the Foundation for Sickle Cell Disease Research.
They recruited patients from Virginia Commonwealth University’s adult sickle cell clinic and trained interviewers to conduct structured interviews using the Institute for Medical Technology Assessment Productivity Cost Questionnaire. The interviewers asked about absenteeism, lost work, unpaid work activity, and “presenteeism,” defined as days when participants were at work but experienced decreased work output because of disease symptoms.
In the end, the study enrolled 186 patients aged 18 and older, a figure that “really exceeded what we expected when we started the protocol,” Mr. Vendetti said. Most participants were between the ages of 20 and 60 years – the most productive working years.
About 58% of participants were female. Nearly half (46%) had the HbSS genotype, while 30% had the HbSC genotype. About half (52%) were high school graduates, and about a third had some college. There were no advanced degrees earned in the study population, and 11.5% had not finished high school.
Initial questions about educational status and employment status “highlighted a very interesting aspect of the disease: 43.8% reported that they were currently unable to work as part of their disease process,” Mr. Vendetti said. Just 28% were employed for wages, 3% were self-employed, and about 7% reported being homemakers. The remainder were out of work, were students, or were retired.
Three-quarters of patients reported missing work in the last year because of SCD symptoms. This group reported missing a mean 36.75 days yearly. Assuming the average Virginia hourly wage of $25.53 per hour, this comes to an average of $7,506 in lost wages each year, Mr. Vendetti said.
Presenteeism had a large impact as well. Nearly 73% of patients said they were bothered at work – either psychologically or physically – by their symptoms in the last 4 weeks, and 90% over the past year. These patients estimated they were affected for about 100 working days yearly.
When asked on a scale of 0-10 how much work they were able to get done on days when their SCD was affecting productivity, “most patients are falling into that middle range” of a score of 4-6, Mr. Vendetti said. “Most patients are moderately affected.
“It’s hard to apply a dollar value to that, but it’s easy to see how it could affect the trajectory of your career,” he added.
Another aspect of the indirect cost of the sickle cell disease burden that’s even harder to tease out is whether those affected are unable to complete a significant amount of unpaid work. Again, about three-quarters of patients reported that SCD had affected their ability to do this kind of work, and these patients said this happened on an average 105 days each year.
Even though patients may not be hiring others to do housework they’re unable to complete, or to care for children on days when they’re too unwell to do so, that doesn’t mean there’s no impact on the patient and those around them, Mr. Vendetti said. “If you ask a family member or a friend for help, that creates a strain in the relationship.”
In terms of resources to address the indirect burden of SCD on careers, Mr. Vendetti pointed out that many states have vocational rehabilitation programs that offer a significant amount of support and assistance to help find a productive work path that still accommodates a chronic illness such as SCD. In Virginia, he said, individuals need to be on disability to avail themselves of the program.
Health care providers can educate themselves about these and other programs. “Most adult sickle cell disease patients didn’t even know they might be eligible” for vocational assistance, he said.
During discussion after the presentation, an audience member pointed out that parents and caregivers of children with SCD are probably also incurring significant indirect costs because of their care-giving burden and that this population should also be studied. Mr. Vendetti agreed. “This is potential that isn’t fulfilled” for all patients and families whose work and personal lives are so profoundly affected by SCD, he said. “This is a dream deferred.”
Mr. Vendetti is employed by Pfizer and is a Pfizer stockholder. A coauthor of the study is a Pfizer consultant.
WASHINGTON – Three-quarters of patients with sickle cell disease (SCD) reported missing work in the last year because of disease symptoms, according to results from a single-center study.
While the direct costs of SCD are easy to measure, it’s harder to capture the indirect costs patients may incur from this chronic, progressive disease, which range from lost days at work to the downstream consequences of “presenteeism.”
“Indirect costs are related to things that have value, but it’s a little bit harder to apply an exact value to it,” said Nicholas Vendetti of Pfizer. But this is a critical piece for understanding SCD, he said. “The burden of illness is unknown without productivity costs.”
Mr. Vendetti and his collaborators attempted to capture the indirect costs of SCD, and reported the results of a single-site study at the annual meeting of the Foundation for Sickle Cell Disease Research.
They recruited patients from Virginia Commonwealth University’s adult sickle cell clinic and trained interviewers to conduct structured interviews using the Institute for Medical Technology Assessment Productivity Cost Questionnaire. The interviewers asked about absenteeism, lost work, unpaid work activity, and “presenteeism,” defined as days when participants were at work but experienced decreased work output because of disease symptoms.
In the end, the study enrolled 186 patients aged 18 and older, a figure that “really exceeded what we expected when we started the protocol,” Mr. Vendetti said. Most participants were between the ages of 20 and 60 years – the most productive working years.
About 58% of participants were female. Nearly half (46%) had the HbSS genotype, while 30% had the HbSC genotype. About half (52%) were high school graduates, and about a third had some college. There were no advanced degrees earned in the study population, and 11.5% had not finished high school.
Initial questions about educational status and employment status “highlighted a very interesting aspect of the disease: 43.8% reported that they were currently unable to work as part of their disease process,” Mr. Vendetti said. Just 28% were employed for wages, 3% were self-employed, and about 7% reported being homemakers. The remainder were out of work, were students, or were retired.
Three-quarters of patients reported missing work in the last year because of SCD symptoms. This group reported missing a mean 36.75 days yearly. Assuming the average Virginia hourly wage of $25.53 per hour, this comes to an average of $7,506 in lost wages each year, Mr. Vendetti said.
Presenteeism had a large impact as well. Nearly 73% of patients said they were bothered at work – either psychologically or physically – by their symptoms in the last 4 weeks, and 90% over the past year. These patients estimated they were affected for about 100 working days yearly.
When asked on a scale of 0-10 how much work they were able to get done on days when their SCD was affecting productivity, “most patients are falling into that middle range” of a score of 4-6, Mr. Vendetti said. “Most patients are moderately affected.
“It’s hard to apply a dollar value to that, but it’s easy to see how it could affect the trajectory of your career,” he added.
Another aspect of the indirect cost of the sickle cell disease burden that’s even harder to tease out is whether those affected are unable to complete a significant amount of unpaid work. Again, about three-quarters of patients reported that SCD had affected their ability to do this kind of work, and these patients said this happened on an average 105 days each year.
Even though patients may not be hiring others to do housework they’re unable to complete, or to care for children on days when they’re too unwell to do so, that doesn’t mean there’s no impact on the patient and those around them, Mr. Vendetti said. “If you ask a family member or a friend for help, that creates a strain in the relationship.”
In terms of resources to address the indirect burden of SCD on careers, Mr. Vendetti pointed out that many states have vocational rehabilitation programs that offer a significant amount of support and assistance to help find a productive work path that still accommodates a chronic illness such as SCD. In Virginia, he said, individuals need to be on disability to avail themselves of the program.
Health care providers can educate themselves about these and other programs. “Most adult sickle cell disease patients didn’t even know they might be eligible” for vocational assistance, he said.
During discussion after the presentation, an audience member pointed out that parents and caregivers of children with SCD are probably also incurring significant indirect costs because of their care-giving burden and that this population should also be studied. Mr. Vendetti agreed. “This is potential that isn’t fulfilled” for all patients and families whose work and personal lives are so profoundly affected by SCD, he said. “This is a dream deferred.”
Mr. Vendetti is employed by Pfizer and is a Pfizer stockholder. A coauthor of the study is a Pfizer consultant.
WASHINGTON – Three-quarters of patients with sickle cell disease (SCD) reported missing work in the last year because of disease symptoms, according to results from a single-center study.
While the direct costs of SCD are easy to measure, it’s harder to capture the indirect costs patients may incur from this chronic, progressive disease, which range from lost days at work to the downstream consequences of “presenteeism.”
“Indirect costs are related to things that have value, but it’s a little bit harder to apply an exact value to it,” said Nicholas Vendetti of Pfizer. But this is a critical piece for understanding SCD, he said. “The burden of illness is unknown without productivity costs.”
Mr. Vendetti and his collaborators attempted to capture the indirect costs of SCD, and reported the results of a single-site study at the annual meeting of the Foundation for Sickle Cell Disease Research.
They recruited patients from Virginia Commonwealth University’s adult sickle cell clinic and trained interviewers to conduct structured interviews using the Institute for Medical Technology Assessment Productivity Cost Questionnaire. The interviewers asked about absenteeism, lost work, unpaid work activity, and “presenteeism,” defined as days when participants were at work but experienced decreased work output because of disease symptoms.
In the end, the study enrolled 186 patients aged 18 and older, a figure that “really exceeded what we expected when we started the protocol,” Mr. Vendetti said. Most participants were between the ages of 20 and 60 years – the most productive working years.
About 58% of participants were female. Nearly half (46%) had the HbSS genotype, while 30% had the HbSC genotype. About half (52%) were high school graduates, and about a third had some college. There were no advanced degrees earned in the study population, and 11.5% had not finished high school.
Initial questions about educational status and employment status “highlighted a very interesting aspect of the disease: 43.8% reported that they were currently unable to work as part of their disease process,” Mr. Vendetti said. Just 28% were employed for wages, 3% were self-employed, and about 7% reported being homemakers. The remainder were out of work, were students, or were retired.
Three-quarters of patients reported missing work in the last year because of SCD symptoms. This group reported missing a mean 36.75 days yearly. Assuming the average Virginia hourly wage of $25.53 per hour, this comes to an average of $7,506 in lost wages each year, Mr. Vendetti said.
Presenteeism had a large impact as well. Nearly 73% of patients said they were bothered at work – either psychologically or physically – by their symptoms in the last 4 weeks, and 90% over the past year. These patients estimated they were affected for about 100 working days yearly.
When asked on a scale of 0-10 how much work they were able to get done on days when their SCD was affecting productivity, “most patients are falling into that middle range” of a score of 4-6, Mr. Vendetti said. “Most patients are moderately affected.
“It’s hard to apply a dollar value to that, but it’s easy to see how it could affect the trajectory of your career,” he added.
Another aspect of the indirect cost of the sickle cell disease burden that’s even harder to tease out is whether those affected are unable to complete a significant amount of unpaid work. Again, about three-quarters of patients reported that SCD had affected their ability to do this kind of work, and these patients said this happened on an average 105 days each year.
Even though patients may not be hiring others to do housework they’re unable to complete, or to care for children on days when they’re too unwell to do so, that doesn’t mean there’s no impact on the patient and those around them, Mr. Vendetti said. “If you ask a family member or a friend for help, that creates a strain in the relationship.”
In terms of resources to address the indirect burden of SCD on careers, Mr. Vendetti pointed out that many states have vocational rehabilitation programs that offer a significant amount of support and assistance to help find a productive work path that still accommodates a chronic illness such as SCD. In Virginia, he said, individuals need to be on disability to avail themselves of the program.
Health care providers can educate themselves about these and other programs. “Most adult sickle cell disease patients didn’t even know they might be eligible” for vocational assistance, he said.
During discussion after the presentation, an audience member pointed out that parents and caregivers of children with SCD are probably also incurring significant indirect costs because of their care-giving burden and that this population should also be studied. Mr. Vendetti agreed. “This is potential that isn’t fulfilled” for all patients and families whose work and personal lives are so profoundly affected by SCD, he said. “This is a dream deferred.”
Mr. Vendetti is employed by Pfizer and is a Pfizer stockholder. A coauthor of the study is a Pfizer consultant.
REPORTING FROM FSCDR 2018
Key clinical point:
Major finding: Three-quarters of patients reported missing work in the last year because of SCD symptoms. This group reported missing a mean 37 days yearly.
Study details: Single-site survey-based study of 186 adults with SCD.
Disclosures: Pfizer sponsored the study. Mr. Vendetti is employed by Pfizer and holds Pfizer stock. A study coauthor is a Pfizer consultant.