User login
Topical ruxolitinib looks good for facial vitiligo, in phase 2 study
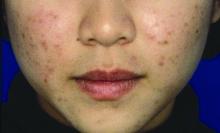
MILAN – Targeting the Janus kinase (JAK) 1 and 2 pathways in vitiligo resulted in significant reduction of facial depigmentation after 24 weeks of treatment, in a phase 2b trial of topical ruxolitinib cream.
compared with vehicle alone, said David Rosmarin, MD, speaking in a late-breaking abstracts session at the World Congress of Dermatology.
The highest response rate was seen with a higher dose: Among patients receiving ruxolitinib cream 1.5% once daily, 50% met the 50% clearing mark at 24 weeks, as did 45.5% of those with twice-daily 1.5% dosing of the 1.5% formulation. At 24 weeks, 3.1% of those receiving vehicle had 50% facial vitiligo resolution (P less than .0001, compared with vehicle for both doses).
Vitiligo affects about 3,000,000 people in the United States, and it is a plausible treatment target for the JAK inhibitor ruxolitinib, explained Dr. Rosmarin, a dermatologist at Tufts University, Boston. “Interferon-gamma, signaling through JAK1 and JAK2, is central to the pathogenesis of vitiligo,” he said. “Ruxolitinib is a potent inhibitor of JAK1 and JAK2, so it made sense to investigate it as a treatment for vitiligo.”
The 24-month randomized, double-blind, vehicle-controlled phase 2 study of ruxolitinib cream for vitiligo compared the vehicle to four different concentrations of ruxolitinib during the first phase of the study. For the first 24 weeks, patients were randomized to receive vehicle twice daily, or various doses of ruxolitinib ranging from 0.15% once daily to 1.5% twice daily.
At this point, the study’s primary endpoint was assessed, with investigators comparing the proportion of patients treated with ruxolitinib who had at least 50% improvement in facial repigmentation from baseline on the Facial Vitiligo Area Scoring Index (F-VASI50) compared with those who received vehicle. A secondary endpoint, also assessed at week 24, was the proportion of patients who were clear, or almost clear, of facial vitiligo; safety and tolerability were also assessed.
In addition to the F-VASI50 measure, Dr. Rosmarin and his coinvestigators also tracked 75% facial clearing (F-VASI75). Here, the 1.5% twice daily regimen topped the others, with 30% of those receiving that dose achieving F-VASI75, compared with almost 10%-17% of those on other doses.
Using another measure, More than one-third of patients using ruxolitinib (35.3%) had clear (no signs of vitiligo) or almost clear (only specks of depigmentation) facial skin at week 24, according to a clinician assessment tool. No patients on placebo had clear or almost clear facial skin at that point. “It is my hope that with continued use beyond week 24, more patients will meet this very stringent endpoint,” Dr. Rosmarin said.
The safety profile was good, with no serious treatment-related adverse events, and no application site reactions that reached clinical significance, although numerically more patients reported acne with ruxolitinib than with vehicle alone.
In the trial, patients aged 18-75 years with vitiligo were eligible if they had facial depigmentation that constituted at least half of their body surface area (BSA), as well as depigmentation of at least 3% of BSA on nonfacial areas. Patients were excluded if they had another dermatologic disease, infection, prior JAK inhibitor therapy, or recent use of biologic or experimental drugs, laser or light-based treatments, or immunomodulators. Of the 157 patients who were randomized, 18 patients (11.5%) had discontinued treatment by week 24, with 3 patients stopping for adverse events, 3 for protocol deviation or noncompliance, and 10 withdrawals. Two patients were lost to follow-up; all patients were included in analysis of the primary and secondary endpoints.
In the second year of the study, investigators rerandomized patients who had been receiving vehicle to an active arm of the study, and patients who had less than 25% improvement on a facial vitiligo scoring scale were rerandomized to one of the different doses. Twenty-eight weeks after rerandomization, all participants were given the opportunity to participate in a year-long open-label extension, receiving 1.5% ruxolitinib cream twice daily. Phototherapy was allowed in the extension arm, but not in the first year of the study.
Data beyond 24 weeks have not yet been reported, and the 2-year study plan acknowledged that “repigmentation takes a while,” Dr. Rosmarin said. He added that patients were allowed to use the study drug on body vitiligo as well, and many saw improvement there, although these results weren’t tracked in the study. “This isn’t a drug that’s meant just for the face,” he said.
Dr. Rosmarin and his coauthors reported financial arrangements with several pharmaceutical companies, including Incyte, which funded the study. An oral formulation of ruxolitinib (Jakafi), marketed by Incyte, was approved by the Food and Drug Administration in 2011, for myelofibrosis, and was recently approved for steroid-refractory acute graft-versus-host disease in adults and children aged 12 years and older.
MILAN – Targeting the Janus kinase (JAK) 1 and 2 pathways in vitiligo resulted in significant reduction of facial depigmentation after 24 weeks of treatment, in a phase 2b trial of topical ruxolitinib cream.
compared with vehicle alone, said David Rosmarin, MD, speaking in a late-breaking abstracts session at the World Congress of Dermatology.
The highest response rate was seen with a higher dose: Among patients receiving ruxolitinib cream 1.5% once daily, 50% met the 50% clearing mark at 24 weeks, as did 45.5% of those with twice-daily 1.5% dosing of the 1.5% formulation. At 24 weeks, 3.1% of those receiving vehicle had 50% facial vitiligo resolution (P less than .0001, compared with vehicle for both doses).
Vitiligo affects about 3,000,000 people in the United States, and it is a plausible treatment target for the JAK inhibitor ruxolitinib, explained Dr. Rosmarin, a dermatologist at Tufts University, Boston. “Interferon-gamma, signaling through JAK1 and JAK2, is central to the pathogenesis of vitiligo,” he said. “Ruxolitinib is a potent inhibitor of JAK1 and JAK2, so it made sense to investigate it as a treatment for vitiligo.”
The 24-month randomized, double-blind, vehicle-controlled phase 2 study of ruxolitinib cream for vitiligo compared the vehicle to four different concentrations of ruxolitinib during the first phase of the study. For the first 24 weeks, patients were randomized to receive vehicle twice daily, or various doses of ruxolitinib ranging from 0.15% once daily to 1.5% twice daily.
At this point, the study’s primary endpoint was assessed, with investigators comparing the proportion of patients treated with ruxolitinib who had at least 50% improvement in facial repigmentation from baseline on the Facial Vitiligo Area Scoring Index (F-VASI50) compared with those who received vehicle. A secondary endpoint, also assessed at week 24, was the proportion of patients who were clear, or almost clear, of facial vitiligo; safety and tolerability were also assessed.
In addition to the F-VASI50 measure, Dr. Rosmarin and his coinvestigators also tracked 75% facial clearing (F-VASI75). Here, the 1.5% twice daily regimen topped the others, with 30% of those receiving that dose achieving F-VASI75, compared with almost 10%-17% of those on other doses.
Using another measure, More than one-third of patients using ruxolitinib (35.3%) had clear (no signs of vitiligo) or almost clear (only specks of depigmentation) facial skin at week 24, according to a clinician assessment tool. No patients on placebo had clear or almost clear facial skin at that point. “It is my hope that with continued use beyond week 24, more patients will meet this very stringent endpoint,” Dr. Rosmarin said.
The safety profile was good, with no serious treatment-related adverse events, and no application site reactions that reached clinical significance, although numerically more patients reported acne with ruxolitinib than with vehicle alone.
In the trial, patients aged 18-75 years with vitiligo were eligible if they had facial depigmentation that constituted at least half of their body surface area (BSA), as well as depigmentation of at least 3% of BSA on nonfacial areas. Patients were excluded if they had another dermatologic disease, infection, prior JAK inhibitor therapy, or recent use of biologic or experimental drugs, laser or light-based treatments, or immunomodulators. Of the 157 patients who were randomized, 18 patients (11.5%) had discontinued treatment by week 24, with 3 patients stopping for adverse events, 3 for protocol deviation or noncompliance, and 10 withdrawals. Two patients were lost to follow-up; all patients were included in analysis of the primary and secondary endpoints.
In the second year of the study, investigators rerandomized patients who had been receiving vehicle to an active arm of the study, and patients who had less than 25% improvement on a facial vitiligo scoring scale were rerandomized to one of the different doses. Twenty-eight weeks after rerandomization, all participants were given the opportunity to participate in a year-long open-label extension, receiving 1.5% ruxolitinib cream twice daily. Phototherapy was allowed in the extension arm, but not in the first year of the study.
Data beyond 24 weeks have not yet been reported, and the 2-year study plan acknowledged that “repigmentation takes a while,” Dr. Rosmarin said. He added that patients were allowed to use the study drug on body vitiligo as well, and many saw improvement there, although these results weren’t tracked in the study. “This isn’t a drug that’s meant just for the face,” he said.
Dr. Rosmarin and his coauthors reported financial arrangements with several pharmaceutical companies, including Incyte, which funded the study. An oral formulation of ruxolitinib (Jakafi), marketed by Incyte, was approved by the Food and Drug Administration in 2011, for myelofibrosis, and was recently approved for steroid-refractory acute graft-versus-host disease in adults and children aged 12 years and older.
MILAN – Targeting the Janus kinase (JAK) 1 and 2 pathways in vitiligo resulted in significant reduction of facial depigmentation after 24 weeks of treatment, in a phase 2b trial of topical ruxolitinib cream.
compared with vehicle alone, said David Rosmarin, MD, speaking in a late-breaking abstracts session at the World Congress of Dermatology.
The highest response rate was seen with a higher dose: Among patients receiving ruxolitinib cream 1.5% once daily, 50% met the 50% clearing mark at 24 weeks, as did 45.5% of those with twice-daily 1.5% dosing of the 1.5% formulation. At 24 weeks, 3.1% of those receiving vehicle had 50% facial vitiligo resolution (P less than .0001, compared with vehicle for both doses).
Vitiligo affects about 3,000,000 people in the United States, and it is a plausible treatment target for the JAK inhibitor ruxolitinib, explained Dr. Rosmarin, a dermatologist at Tufts University, Boston. “Interferon-gamma, signaling through JAK1 and JAK2, is central to the pathogenesis of vitiligo,” he said. “Ruxolitinib is a potent inhibitor of JAK1 and JAK2, so it made sense to investigate it as a treatment for vitiligo.”
The 24-month randomized, double-blind, vehicle-controlled phase 2 study of ruxolitinib cream for vitiligo compared the vehicle to four different concentrations of ruxolitinib during the first phase of the study. For the first 24 weeks, patients were randomized to receive vehicle twice daily, or various doses of ruxolitinib ranging from 0.15% once daily to 1.5% twice daily.
At this point, the study’s primary endpoint was assessed, with investigators comparing the proportion of patients treated with ruxolitinib who had at least 50% improvement in facial repigmentation from baseline on the Facial Vitiligo Area Scoring Index (F-VASI50) compared with those who received vehicle. A secondary endpoint, also assessed at week 24, was the proportion of patients who were clear, or almost clear, of facial vitiligo; safety and tolerability were also assessed.
In addition to the F-VASI50 measure, Dr. Rosmarin and his coinvestigators also tracked 75% facial clearing (F-VASI75). Here, the 1.5% twice daily regimen topped the others, with 30% of those receiving that dose achieving F-VASI75, compared with almost 10%-17% of those on other doses.
Using another measure, More than one-third of patients using ruxolitinib (35.3%) had clear (no signs of vitiligo) or almost clear (only specks of depigmentation) facial skin at week 24, according to a clinician assessment tool. No patients on placebo had clear or almost clear facial skin at that point. “It is my hope that with continued use beyond week 24, more patients will meet this very stringent endpoint,” Dr. Rosmarin said.
The safety profile was good, with no serious treatment-related adverse events, and no application site reactions that reached clinical significance, although numerically more patients reported acne with ruxolitinib than with vehicle alone.
In the trial, patients aged 18-75 years with vitiligo were eligible if they had facial depigmentation that constituted at least half of their body surface area (BSA), as well as depigmentation of at least 3% of BSA on nonfacial areas. Patients were excluded if they had another dermatologic disease, infection, prior JAK inhibitor therapy, or recent use of biologic or experimental drugs, laser or light-based treatments, or immunomodulators. Of the 157 patients who were randomized, 18 patients (11.5%) had discontinued treatment by week 24, with 3 patients stopping for adverse events, 3 for protocol deviation or noncompliance, and 10 withdrawals. Two patients were lost to follow-up; all patients were included in analysis of the primary and secondary endpoints.
In the second year of the study, investigators rerandomized patients who had been receiving vehicle to an active arm of the study, and patients who had less than 25% improvement on a facial vitiligo scoring scale were rerandomized to one of the different doses. Twenty-eight weeks after rerandomization, all participants were given the opportunity to participate in a year-long open-label extension, receiving 1.5% ruxolitinib cream twice daily. Phototherapy was allowed in the extension arm, but not in the first year of the study.
Data beyond 24 weeks have not yet been reported, and the 2-year study plan acknowledged that “repigmentation takes a while,” Dr. Rosmarin said. He added that patients were allowed to use the study drug on body vitiligo as well, and many saw improvement there, although these results weren’t tracked in the study. “This isn’t a drug that’s meant just for the face,” he said.
Dr. Rosmarin and his coauthors reported financial arrangements with several pharmaceutical companies, including Incyte, which funded the study. An oral formulation of ruxolitinib (Jakafi), marketed by Incyte, was approved by the Food and Drug Administration in 2011, for myelofibrosis, and was recently approved for steroid-refractory acute graft-versus-host disease in adults and children aged 12 years and older.
REPORTING FROM WCD2019
Postholiday colonoscopies have lower rates of good bowel prep
SAN DIEGO – .
Of patients whose colonoscopies were performed the day after a holiday, 55.4% had inadequate bowel preparation, compared with 45.7% of those receiving colonoscopies on other days, for an odds ratio of 1.5 for inadequate preparation on the day after a holiday (95% confidence interval, 1.1-1.9; P = .006).
In addition to the lead finding, inadequate bowel prep was also more likely in the afternoon, and earlier in the week (OR, 1.6 and 1.3, respectively), said Ammar Nassri, MD, a gastroenterology fellow at the University of Florida, Jacksonville.
Patients who were male and white were more likely to have inadequate bowel preparation (OR, 1.3 and 2.7, respectively). Having Medicaid as opposed to other forms of insurance also upped the likelihood of inadequate bowel preparation (OR, 1.9).
It’s important to identify modifiable factors associated with inadequate bowel preparation for a number of reasons. Among them, said Dr. Nassri, is cost-effectiveness: Screening colonoscopy has been found to be cost effective, compared with fecal immunochemical testing only when the inadequate bowel prep rate is 13% or less.
Adenomas are more likely to be missed with inadequate bowel preparation as well, he noted, with one study finding missed adenomas on 33% of subsequent colonoscopies performed after an initial colonoscopy with inadequate preparation.
Also, inadequate preparation can mean longer procedures and increased likelihood of failed procedures – and higher costs, he said.
“Several studies have created prediction models to predict the likelihood of having an inadequate bowel preparation, but these models have not gained widespread acceptance,” said Dr. Nassri.
He and his collaborators aimed to identify the rate of inadequate bowel preparation in their patient population, and to examine the association of modifiable variables with adequacy of preparation. These included the day of the week, the time of day, and whether a colonoscopy followed a holiday.
Additionally, the investigators looked at various patient demographic variables to see whether they were associated with adequacy of bowel preparation. Adult patients who received outpatient colonoscopy over a 3-year period were included. Preparation was considered adequate if it was assigned a score of at least 6 on the Boston Bowel Preparation Scale, or at least “fair” on the Aronchik scale.
A total of 6,510 patients were included. The mean age was 56.3 years, and about 60% were female. Just over half (51.3%) were African American; 46.6% were white. Over half of patients (56.4%) had health insurance provided by city contract or Florida Medicaid; the remainder had either Medicare or commercial insurance.
Overall, nearly half of patients (46%) had inadequate bowel preparation. Half of males overall had adequate bowel preparation, compared with 57% of females (P less than .001). As the hour of the colonoscopy grew later, the likelihood of adequacy of bowel preparation dropped. The inverse relationship was statistically significant (P less than .001), with over 60% of 7 a.m. colonoscopies having adequate preparation. By 3 p.m., over 60% of bowel preparations were inadequate in the University of Florida cohort.
Colonoscopies performed later in the week were most likely to have adequate bowel preparation, with rates nearing 60% by Friday, compared with rates just over or at 50% for the first 3 days of the week (P less than .001).
“This study showed that a colonoscopy on the day after a holiday has a higher rate of inadequate bowel preparation,” said Dr. Nassri. Conversely, he said, “Colonoscopy toward the end of the week has a higher likelihood of adequate bowel preparation.”
The present work, he said, “re-demonstrated that procedures done later in the day have a poorer bowel preparation.”
Dr. Nassri reported no conflicts of interest.
SAN DIEGO – .
Of patients whose colonoscopies were performed the day after a holiday, 55.4% had inadequate bowel preparation, compared with 45.7% of those receiving colonoscopies on other days, for an odds ratio of 1.5 for inadequate preparation on the day after a holiday (95% confidence interval, 1.1-1.9; P = .006).
In addition to the lead finding, inadequate bowel prep was also more likely in the afternoon, and earlier in the week (OR, 1.6 and 1.3, respectively), said Ammar Nassri, MD, a gastroenterology fellow at the University of Florida, Jacksonville.
Patients who were male and white were more likely to have inadequate bowel preparation (OR, 1.3 and 2.7, respectively). Having Medicaid as opposed to other forms of insurance also upped the likelihood of inadequate bowel preparation (OR, 1.9).
It’s important to identify modifiable factors associated with inadequate bowel preparation for a number of reasons. Among them, said Dr. Nassri, is cost-effectiveness: Screening colonoscopy has been found to be cost effective, compared with fecal immunochemical testing only when the inadequate bowel prep rate is 13% or less.
Adenomas are more likely to be missed with inadequate bowel preparation as well, he noted, with one study finding missed adenomas on 33% of subsequent colonoscopies performed after an initial colonoscopy with inadequate preparation.
Also, inadequate preparation can mean longer procedures and increased likelihood of failed procedures – and higher costs, he said.
“Several studies have created prediction models to predict the likelihood of having an inadequate bowel preparation, but these models have not gained widespread acceptance,” said Dr. Nassri.
He and his collaborators aimed to identify the rate of inadequate bowel preparation in their patient population, and to examine the association of modifiable variables with adequacy of preparation. These included the day of the week, the time of day, and whether a colonoscopy followed a holiday.
Additionally, the investigators looked at various patient demographic variables to see whether they were associated with adequacy of bowel preparation. Adult patients who received outpatient colonoscopy over a 3-year period were included. Preparation was considered adequate if it was assigned a score of at least 6 on the Boston Bowel Preparation Scale, or at least “fair” on the Aronchik scale.
A total of 6,510 patients were included. The mean age was 56.3 years, and about 60% were female. Just over half (51.3%) were African American; 46.6% were white. Over half of patients (56.4%) had health insurance provided by city contract or Florida Medicaid; the remainder had either Medicare or commercial insurance.
Overall, nearly half of patients (46%) had inadequate bowel preparation. Half of males overall had adequate bowel preparation, compared with 57% of females (P less than .001). As the hour of the colonoscopy grew later, the likelihood of adequacy of bowel preparation dropped. The inverse relationship was statistically significant (P less than .001), with over 60% of 7 a.m. colonoscopies having adequate preparation. By 3 p.m., over 60% of bowel preparations were inadequate in the University of Florida cohort.
Colonoscopies performed later in the week were most likely to have adequate bowel preparation, with rates nearing 60% by Friday, compared with rates just over or at 50% for the first 3 days of the week (P less than .001).
“This study showed that a colonoscopy on the day after a holiday has a higher rate of inadequate bowel preparation,” said Dr. Nassri. Conversely, he said, “Colonoscopy toward the end of the week has a higher likelihood of adequate bowel preparation.”
The present work, he said, “re-demonstrated that procedures done later in the day have a poorer bowel preparation.”
Dr. Nassri reported no conflicts of interest.
SAN DIEGO – .
Of patients whose colonoscopies were performed the day after a holiday, 55.4% had inadequate bowel preparation, compared with 45.7% of those receiving colonoscopies on other days, for an odds ratio of 1.5 for inadequate preparation on the day after a holiday (95% confidence interval, 1.1-1.9; P = .006).
In addition to the lead finding, inadequate bowel prep was also more likely in the afternoon, and earlier in the week (OR, 1.6 and 1.3, respectively), said Ammar Nassri, MD, a gastroenterology fellow at the University of Florida, Jacksonville.
Patients who were male and white were more likely to have inadequate bowel preparation (OR, 1.3 and 2.7, respectively). Having Medicaid as opposed to other forms of insurance also upped the likelihood of inadequate bowel preparation (OR, 1.9).
It’s important to identify modifiable factors associated with inadequate bowel preparation for a number of reasons. Among them, said Dr. Nassri, is cost-effectiveness: Screening colonoscopy has been found to be cost effective, compared with fecal immunochemical testing only when the inadequate bowel prep rate is 13% or less.
Adenomas are more likely to be missed with inadequate bowel preparation as well, he noted, with one study finding missed adenomas on 33% of subsequent colonoscopies performed after an initial colonoscopy with inadequate preparation.
Also, inadequate preparation can mean longer procedures and increased likelihood of failed procedures – and higher costs, he said.
“Several studies have created prediction models to predict the likelihood of having an inadequate bowel preparation, but these models have not gained widespread acceptance,” said Dr. Nassri.
He and his collaborators aimed to identify the rate of inadequate bowel preparation in their patient population, and to examine the association of modifiable variables with adequacy of preparation. These included the day of the week, the time of day, and whether a colonoscopy followed a holiday.
Additionally, the investigators looked at various patient demographic variables to see whether they were associated with adequacy of bowel preparation. Adult patients who received outpatient colonoscopy over a 3-year period were included. Preparation was considered adequate if it was assigned a score of at least 6 on the Boston Bowel Preparation Scale, or at least “fair” on the Aronchik scale.
A total of 6,510 patients were included. The mean age was 56.3 years, and about 60% were female. Just over half (51.3%) were African American; 46.6% were white. Over half of patients (56.4%) had health insurance provided by city contract or Florida Medicaid; the remainder had either Medicare or commercial insurance.
Overall, nearly half of patients (46%) had inadequate bowel preparation. Half of males overall had adequate bowel preparation, compared with 57% of females (P less than .001). As the hour of the colonoscopy grew later, the likelihood of adequacy of bowel preparation dropped. The inverse relationship was statistically significant (P less than .001), with over 60% of 7 a.m. colonoscopies having adequate preparation. By 3 p.m., over 60% of bowel preparations were inadequate in the University of Florida cohort.
Colonoscopies performed later in the week were most likely to have adequate bowel preparation, with rates nearing 60% by Friday, compared with rates just over or at 50% for the first 3 days of the week (P less than .001).
“This study showed that a colonoscopy on the day after a holiday has a higher rate of inadequate bowel preparation,” said Dr. Nassri. Conversely, he said, “Colonoscopy toward the end of the week has a higher likelihood of adequate bowel preparation.”
The present work, he said, “re-demonstrated that procedures done later in the day have a poorer bowel preparation.”
Dr. Nassri reported no conflicts of interest.
REPORTING FROM DDW 2019
Ovarian reserve markers fall on isotretinoin, but rebound after stopping treatment
MILAN – according to data presented at the World Congress of Dermatology.
Although markers for ovarian reserve, including anti-Müllerian hormone (AMH) serum levels, ovarian volume, and antral follicle count, were significantly lower during a period of isotretinoin use than at baseline, these values were were not significantly different from pretreatment levels by 1 month after stopping isotretinoin.
For patients taking isotretinoin at a dose of 0.5 mg/kg/day, AMH levels fell from a baseline level of 5.29 ng/mL to 4.16 ng/mL during treatment, but rebounded to 4.77 ng/mL 1 month after stopping treatment (P less than .001 for difference between baseline and on-drug values), Tuğba Özkök Akbulut, MD, said during a late-breaking abstracts session.
For women taking isotretinoin 1 mg/kg/day, AMH levels went from 5.14 ng/mL at baseline to 4.24 ng/mL on treatment, to 4.65 ng/mL 1 month after treatment (P less than .001 for difference between baseline and on-drug values), reported Dr. Akbulut a dermatologist at the Haseki Training Research Hospital, Istanbul.
Women on the higher dose of isotretinoin had a similar pattern of decline while on treatment and rebound after ceasing isotretinoin for ovarian volume and antral follicle count (P less than .001 for all values). These differences were not statistically significant for women taking 0.5 mg/kg/day of isotretinoin, except for right ovarian volume (P = 0.013).
Although values were numerically lower for many markers of ovarian reserve after ceasing treatment, compared with baseline figures, these differences were not statistically significantly different. Markers of ovarian reserve did not change significantly for a control group of women without acne.
Dr. Akbulut and her colleagues conducted this prospective case-control study of 42 women of reproductive age who sought dermatologist care for severe acne unresponsive to conservative therapy; 26 women who did not have acne constituted the control group. Smokers, patients with thyroid disease, and those with known polycystic ovary syndrome were excluded from participation.
The women with acne received oral isotretinoin dosed either at 0.5 or 1.0 mg/kg/day, with treatment lasting 5-9 months. For each patient, treatment was stopped when the cumulative dose reached 120 mg/kg.
After an initial visit at which blood was collected from all participants to measure serum AMH levels, those receiving isotretinoin were seen every 4 weeks to check serum lipid and liver enzyme levels.
At the 3-month mark during the study period and 1 month after the end of completing isotretinoin treatment, or at the end of the study period for the control group, blood samples also were drawn for AMH levels.
To measure hormone levels, also blood was drawn between days 2 and 5 of the follicular phase of the menstrual cycle. Participants received ultrasounds to measure antral follicle count and ovarian volume between days 2 and 5 of the menstrual cycle at the initial visit, at the 3-month visit, and at the final visit. Results were interpreted by a trained gynecologist.
Patients, who were mostly in their early 20s, had a mean body mass index of about 22 kg/m2. Hormone levels, ovarian volume, and antral follicle count did not differ among study arms at baseline.
“There are contradictory reports in the literature regarding the effect of retinoic acid on ovarian reserve,” noted Dr. Akbulut. Some preclinical studies found that retinoic acid increased fertility and ovarian reserve in rodents; however, some human studies had shown lower serum AMH concentrations in patients using isotretinoin.
This new demonstration of the reversibility of isotretinoin’s negative effect on ovarian reserve helps clarify a confused picture in the medical literature, said Dr. Akbulut. “The results of our study demonstrated that systemic isotretinoin had a reversible effect on ovarian reserve.”
Dr. Akbulut reported no outside sources of funding and that she had no relevant financial disclosures.
MILAN – according to data presented at the World Congress of Dermatology.
Although markers for ovarian reserve, including anti-Müllerian hormone (AMH) serum levels, ovarian volume, and antral follicle count, were significantly lower during a period of isotretinoin use than at baseline, these values were were not significantly different from pretreatment levels by 1 month after stopping isotretinoin.
For patients taking isotretinoin at a dose of 0.5 mg/kg/day, AMH levels fell from a baseline level of 5.29 ng/mL to 4.16 ng/mL during treatment, but rebounded to 4.77 ng/mL 1 month after stopping treatment (P less than .001 for difference between baseline and on-drug values), Tuğba Özkök Akbulut, MD, said during a late-breaking abstracts session.
For women taking isotretinoin 1 mg/kg/day, AMH levels went from 5.14 ng/mL at baseline to 4.24 ng/mL on treatment, to 4.65 ng/mL 1 month after treatment (P less than .001 for difference between baseline and on-drug values), reported Dr. Akbulut a dermatologist at the Haseki Training Research Hospital, Istanbul.
Women on the higher dose of isotretinoin had a similar pattern of decline while on treatment and rebound after ceasing isotretinoin for ovarian volume and antral follicle count (P less than .001 for all values). These differences were not statistically significant for women taking 0.5 mg/kg/day of isotretinoin, except for right ovarian volume (P = 0.013).
Although values were numerically lower for many markers of ovarian reserve after ceasing treatment, compared with baseline figures, these differences were not statistically significantly different. Markers of ovarian reserve did not change significantly for a control group of women without acne.
Dr. Akbulut and her colleagues conducted this prospective case-control study of 42 women of reproductive age who sought dermatologist care for severe acne unresponsive to conservative therapy; 26 women who did not have acne constituted the control group. Smokers, patients with thyroid disease, and those with known polycystic ovary syndrome were excluded from participation.
The women with acne received oral isotretinoin dosed either at 0.5 or 1.0 mg/kg/day, with treatment lasting 5-9 months. For each patient, treatment was stopped when the cumulative dose reached 120 mg/kg.
After an initial visit at which blood was collected from all participants to measure serum AMH levels, those receiving isotretinoin were seen every 4 weeks to check serum lipid and liver enzyme levels.
At the 3-month mark during the study period and 1 month after the end of completing isotretinoin treatment, or at the end of the study period for the control group, blood samples also were drawn for AMH levels.
To measure hormone levels, also blood was drawn between days 2 and 5 of the follicular phase of the menstrual cycle. Participants received ultrasounds to measure antral follicle count and ovarian volume between days 2 and 5 of the menstrual cycle at the initial visit, at the 3-month visit, and at the final visit. Results were interpreted by a trained gynecologist.
Patients, who were mostly in their early 20s, had a mean body mass index of about 22 kg/m2. Hormone levels, ovarian volume, and antral follicle count did not differ among study arms at baseline.
“There are contradictory reports in the literature regarding the effect of retinoic acid on ovarian reserve,” noted Dr. Akbulut. Some preclinical studies found that retinoic acid increased fertility and ovarian reserve in rodents; however, some human studies had shown lower serum AMH concentrations in patients using isotretinoin.
This new demonstration of the reversibility of isotretinoin’s negative effect on ovarian reserve helps clarify a confused picture in the medical literature, said Dr. Akbulut. “The results of our study demonstrated that systemic isotretinoin had a reversible effect on ovarian reserve.”
Dr. Akbulut reported no outside sources of funding and that she had no relevant financial disclosures.
MILAN – according to data presented at the World Congress of Dermatology.
Although markers for ovarian reserve, including anti-Müllerian hormone (AMH) serum levels, ovarian volume, and antral follicle count, were significantly lower during a period of isotretinoin use than at baseline, these values were were not significantly different from pretreatment levels by 1 month after stopping isotretinoin.
For patients taking isotretinoin at a dose of 0.5 mg/kg/day, AMH levels fell from a baseline level of 5.29 ng/mL to 4.16 ng/mL during treatment, but rebounded to 4.77 ng/mL 1 month after stopping treatment (P less than .001 for difference between baseline and on-drug values), Tuğba Özkök Akbulut, MD, said during a late-breaking abstracts session.
For women taking isotretinoin 1 mg/kg/day, AMH levels went from 5.14 ng/mL at baseline to 4.24 ng/mL on treatment, to 4.65 ng/mL 1 month after treatment (P less than .001 for difference between baseline and on-drug values), reported Dr. Akbulut a dermatologist at the Haseki Training Research Hospital, Istanbul.
Women on the higher dose of isotretinoin had a similar pattern of decline while on treatment and rebound after ceasing isotretinoin for ovarian volume and antral follicle count (P less than .001 for all values). These differences were not statistically significant for women taking 0.5 mg/kg/day of isotretinoin, except for right ovarian volume (P = 0.013).
Although values were numerically lower for many markers of ovarian reserve after ceasing treatment, compared with baseline figures, these differences were not statistically significantly different. Markers of ovarian reserve did not change significantly for a control group of women without acne.
Dr. Akbulut and her colleagues conducted this prospective case-control study of 42 women of reproductive age who sought dermatologist care for severe acne unresponsive to conservative therapy; 26 women who did not have acne constituted the control group. Smokers, patients with thyroid disease, and those with known polycystic ovary syndrome were excluded from participation.
The women with acne received oral isotretinoin dosed either at 0.5 or 1.0 mg/kg/day, with treatment lasting 5-9 months. For each patient, treatment was stopped when the cumulative dose reached 120 mg/kg.
After an initial visit at which blood was collected from all participants to measure serum AMH levels, those receiving isotretinoin were seen every 4 weeks to check serum lipid and liver enzyme levels.
At the 3-month mark during the study period and 1 month after the end of completing isotretinoin treatment, or at the end of the study period for the control group, blood samples also were drawn for AMH levels.
To measure hormone levels, also blood was drawn between days 2 and 5 of the follicular phase of the menstrual cycle. Participants received ultrasounds to measure antral follicle count and ovarian volume between days 2 and 5 of the menstrual cycle at the initial visit, at the 3-month visit, and at the final visit. Results were interpreted by a trained gynecologist.
Patients, who were mostly in their early 20s, had a mean body mass index of about 22 kg/m2. Hormone levels, ovarian volume, and antral follicle count did not differ among study arms at baseline.
“There are contradictory reports in the literature regarding the effect of retinoic acid on ovarian reserve,” noted Dr. Akbulut. Some preclinical studies found that retinoic acid increased fertility and ovarian reserve in rodents; however, some human studies had shown lower serum AMH concentrations in patients using isotretinoin.
This new demonstration of the reversibility of isotretinoin’s negative effect on ovarian reserve helps clarify a confused picture in the medical literature, said Dr. Akbulut. “The results of our study demonstrated that systemic isotretinoin had a reversible effect on ovarian reserve.”
Dr. Akbulut reported no outside sources of funding and that she had no relevant financial disclosures.
REPORTING FROM WCD2019
Visual examinations yield signs to guide vitiligo treatment
MILAN – Subtle signs beyond depigmentation alone can guide management of vitiligo, Michelle Rodrigues, MBBS, said at the World Congress of Dermatology.
Signs of high disease activity can be visually observed and, when found, can compel urgent treatment, Dr. Rodrigues said. “If we identify and understand these [signs, they] can change our management plan, and the patient’s outcomes ... picking these up quickly, getting the best response you can, can help our patients tremendously.”
To assess clinical signs of severity in vitiligo, “use the tools that you have in your practice – your dermatoscope, your Wood’s lamp.”
Showing an image of the leg of a patient with vitiligo, Dr. Rodrigues said, “I know this patient’s vitiligo is very, very active. Why?” Clues come when there are areas of hypopigmentation at the rim of lesions, with depigmentation at the center. The presence of pigmentation, hypopigmentation, and depigmentation within the same lesion indicates high disease activity. This finding is the trichrome sign, also called the “blurry borders” sign in some regions, said Dr. Rodrigues, a dermatologist in Melbourne and the founder of Chroma Dermatology, which specializes in treating pigment problems and diagnosing and managing skin conditions in patients with skin of color.
Next, Dr. Rodrigues said, look at hair growth within the vitiliginous area. “If you’re unable to see that clinically, it’s really important to get that dermatoscope onto the patient, and look within a patch, to see whether or not you can actually see white hairs or normal colored hairs,” she said. This finding will help to determine both treatment plan and prognosis, since leukotrichia is a marker of disease severity in vitiligo.
Be alert to Koebnerization, said Dr. Rodrigues; the presentation may be subtle. As an example, she shared an image of a patient with depigmented patches on the dorsum of each foot. It wasn’t until the patient removed her foot gear – rubber slide-type sandals with a single broad strap over the dorsum – that Dr. Rodrigues recognized that “there was clear Koebnerization from the constant friction as a result of the wearing of the shoes.
“This can also be seen when patients scratch themselves, as can be seen with the itch that vitiligo can sometimes cause,” she said.
She noted that about 10% of patients with vitiligo have pruritus as a prominent symptom. Here, she said, is where a Wood’s lamp can be helpful as well. “Sometimes we can’t appreciate the very, very subtle Koebnerization, especially in patients with lighter skin. Getting out that Wood’s lamp and looking at other areas of involvement is really important,” she said. Areas of high disease activity and signs of progression that might otherwise be missed will be more obvious under the ultraviolet light.
It’s important to look beyond the obvious patches of vitiligo to examine the surrounding skin. Searching for “confetti depigmentation” – tiny white dots of depigmentation scattered over the otherwise normally pigmented skin – also marks high disease activity. An area with these dots – each often only a few millimeters in diameter – is likely destined for rapid depigmentation unless aggressive treatment is started. “We know that without treating these areas there will be very, very rapid and aggressive depigmentation. And remember that in areas that have a paucity of hair follicles, it might be irreversible ... so recognizing these signs is absolutely critical.”
The final clue to highly active disease that’s likely to move quickly without intervention can be found at the border of a vitiligo lesion. Look for a fine rim of erythema and some scale, Dr. Rodrigues said. This sign is common, and often seen early in the disease course. When this erythematous region is biopsied, ”You’ll see an intense inflammatory response, with an interface dermatitis. Again, this tells us that the patient may have a poorer prognosis if we don’t commence treatment early on.”
As a final clinical tip, Dr. Rodrigues reminded attendees that when one sign of disease activity is seen, others are often present. A thorough clinical examination is needed to document aggressive disease. “Please make sure that if you find one, you’re looking for other signs of disease severity as well.”
Dr. Rodrigues reported that she had no disclosures relevant to her presentation.
MILAN – Subtle signs beyond depigmentation alone can guide management of vitiligo, Michelle Rodrigues, MBBS, said at the World Congress of Dermatology.
Signs of high disease activity can be visually observed and, when found, can compel urgent treatment, Dr. Rodrigues said. “If we identify and understand these [signs, they] can change our management plan, and the patient’s outcomes ... picking these up quickly, getting the best response you can, can help our patients tremendously.”
To assess clinical signs of severity in vitiligo, “use the tools that you have in your practice – your dermatoscope, your Wood’s lamp.”
Showing an image of the leg of a patient with vitiligo, Dr. Rodrigues said, “I know this patient’s vitiligo is very, very active. Why?” Clues come when there are areas of hypopigmentation at the rim of lesions, with depigmentation at the center. The presence of pigmentation, hypopigmentation, and depigmentation within the same lesion indicates high disease activity. This finding is the trichrome sign, also called the “blurry borders” sign in some regions, said Dr. Rodrigues, a dermatologist in Melbourne and the founder of Chroma Dermatology, which specializes in treating pigment problems and diagnosing and managing skin conditions in patients with skin of color.
Next, Dr. Rodrigues said, look at hair growth within the vitiliginous area. “If you’re unable to see that clinically, it’s really important to get that dermatoscope onto the patient, and look within a patch, to see whether or not you can actually see white hairs or normal colored hairs,” she said. This finding will help to determine both treatment plan and prognosis, since leukotrichia is a marker of disease severity in vitiligo.
Be alert to Koebnerization, said Dr. Rodrigues; the presentation may be subtle. As an example, she shared an image of a patient with depigmented patches on the dorsum of each foot. It wasn’t until the patient removed her foot gear – rubber slide-type sandals with a single broad strap over the dorsum – that Dr. Rodrigues recognized that “there was clear Koebnerization from the constant friction as a result of the wearing of the shoes.
“This can also be seen when patients scratch themselves, as can be seen with the itch that vitiligo can sometimes cause,” she said.
She noted that about 10% of patients with vitiligo have pruritus as a prominent symptom. Here, she said, is where a Wood’s lamp can be helpful as well. “Sometimes we can’t appreciate the very, very subtle Koebnerization, especially in patients with lighter skin. Getting out that Wood’s lamp and looking at other areas of involvement is really important,” she said. Areas of high disease activity and signs of progression that might otherwise be missed will be more obvious under the ultraviolet light.
It’s important to look beyond the obvious patches of vitiligo to examine the surrounding skin. Searching for “confetti depigmentation” – tiny white dots of depigmentation scattered over the otherwise normally pigmented skin – also marks high disease activity. An area with these dots – each often only a few millimeters in diameter – is likely destined for rapid depigmentation unless aggressive treatment is started. “We know that without treating these areas there will be very, very rapid and aggressive depigmentation. And remember that in areas that have a paucity of hair follicles, it might be irreversible ... so recognizing these signs is absolutely critical.”
The final clue to highly active disease that’s likely to move quickly without intervention can be found at the border of a vitiligo lesion. Look for a fine rim of erythema and some scale, Dr. Rodrigues said. This sign is common, and often seen early in the disease course. When this erythematous region is biopsied, ”You’ll see an intense inflammatory response, with an interface dermatitis. Again, this tells us that the patient may have a poorer prognosis if we don’t commence treatment early on.”
As a final clinical tip, Dr. Rodrigues reminded attendees that when one sign of disease activity is seen, others are often present. A thorough clinical examination is needed to document aggressive disease. “Please make sure that if you find one, you’re looking for other signs of disease severity as well.”
Dr. Rodrigues reported that she had no disclosures relevant to her presentation.
MILAN – Subtle signs beyond depigmentation alone can guide management of vitiligo, Michelle Rodrigues, MBBS, said at the World Congress of Dermatology.
Signs of high disease activity can be visually observed and, when found, can compel urgent treatment, Dr. Rodrigues said. “If we identify and understand these [signs, they] can change our management plan, and the patient’s outcomes ... picking these up quickly, getting the best response you can, can help our patients tremendously.”
To assess clinical signs of severity in vitiligo, “use the tools that you have in your practice – your dermatoscope, your Wood’s lamp.”
Showing an image of the leg of a patient with vitiligo, Dr. Rodrigues said, “I know this patient’s vitiligo is very, very active. Why?” Clues come when there are areas of hypopigmentation at the rim of lesions, with depigmentation at the center. The presence of pigmentation, hypopigmentation, and depigmentation within the same lesion indicates high disease activity. This finding is the trichrome sign, also called the “blurry borders” sign in some regions, said Dr. Rodrigues, a dermatologist in Melbourne and the founder of Chroma Dermatology, which specializes in treating pigment problems and diagnosing and managing skin conditions in patients with skin of color.
Next, Dr. Rodrigues said, look at hair growth within the vitiliginous area. “If you’re unable to see that clinically, it’s really important to get that dermatoscope onto the patient, and look within a patch, to see whether or not you can actually see white hairs or normal colored hairs,” she said. This finding will help to determine both treatment plan and prognosis, since leukotrichia is a marker of disease severity in vitiligo.
Be alert to Koebnerization, said Dr. Rodrigues; the presentation may be subtle. As an example, she shared an image of a patient with depigmented patches on the dorsum of each foot. It wasn’t until the patient removed her foot gear – rubber slide-type sandals with a single broad strap over the dorsum – that Dr. Rodrigues recognized that “there was clear Koebnerization from the constant friction as a result of the wearing of the shoes.
“This can also be seen when patients scratch themselves, as can be seen with the itch that vitiligo can sometimes cause,” she said.
She noted that about 10% of patients with vitiligo have pruritus as a prominent symptom. Here, she said, is where a Wood’s lamp can be helpful as well. “Sometimes we can’t appreciate the very, very subtle Koebnerization, especially in patients with lighter skin. Getting out that Wood’s lamp and looking at other areas of involvement is really important,” she said. Areas of high disease activity and signs of progression that might otherwise be missed will be more obvious under the ultraviolet light.
It’s important to look beyond the obvious patches of vitiligo to examine the surrounding skin. Searching for “confetti depigmentation” – tiny white dots of depigmentation scattered over the otherwise normally pigmented skin – also marks high disease activity. An area with these dots – each often only a few millimeters in diameter – is likely destined for rapid depigmentation unless aggressive treatment is started. “We know that without treating these areas there will be very, very rapid and aggressive depigmentation. And remember that in areas that have a paucity of hair follicles, it might be irreversible ... so recognizing these signs is absolutely critical.”
The final clue to highly active disease that’s likely to move quickly without intervention can be found at the border of a vitiligo lesion. Look for a fine rim of erythema and some scale, Dr. Rodrigues said. This sign is common, and often seen early in the disease course. When this erythematous region is biopsied, ”You’ll see an intense inflammatory response, with an interface dermatitis. Again, this tells us that the patient may have a poorer prognosis if we don’t commence treatment early on.”
As a final clinical tip, Dr. Rodrigues reminded attendees that when one sign of disease activity is seen, others are often present. A thorough clinical examination is needed to document aggressive disease. “Please make sure that if you find one, you’re looking for other signs of disease severity as well.”
Dr. Rodrigues reported that she had no disclosures relevant to her presentation.
EXPERT ANALYSIS FROM WCD2019
Teletriage connects uninsured with timely dermatologist care
MILAN – and optimized primary care physicians’ care of nonreferred patients, Cory Simpson, MD, PhD, reported at the World Congress of Dermatology.
With implementation of teledermatology, patient wait times for specialist input dropped from 13.9 days to 1.6 days (P less than .00001).
By allowing dermatologists to evaluate photographs of lesions and perform their own triage of referrals from primary care physicians (PCPs), the teletriage pilot program reduced the number of patients for whom dermatology consults were deemed necessary and also allowed optimal management for the nonreferred patients, said Dr. Simpson, of the University of Pennsylvania, Philadelphia.
“Teledermatology has the potential to increase access to dermatologist-level care, especially for underserved patients,” he commented. “It allows us to educate primary care physicians in resource-limited settings, and it also allows us to avoid suboptimal care of skin disease by nonspecialists – especially the more judicious use of antimicrobial agents and corticosteroids.”
Dr. Simpson explained to the international audience that, for many in the United States, access to a dermatologist requires a lengthy wait that can extend to months.
In Philadelphia, University of Pennsylvania dermatology residents and attending physicians volunteer in an outreach program that serves an uninsured population of primarily Latino immigrants. Operating 1 or 2 evenings a month, the medical and surgical dermatology clinics can accommodate from 8-12 appointments per clinic.
The clinic had been overwhelmed with referrals from PCPs, but Dr. Simpson and his colleagues realized that many of the conditions they were seeing – verruca vulgaris, hand dermatitis, and psoriasis, for example – did not necessarily need a face-to-face dermatologic evaluation.
The AccessDerm app, available at no cost by the American Academy of Dermatology, allows PCPs and dermatologists to communicate and collaborate. “This is a store-and-forward program, meaning the primary physician takes the photos and sends them to an off-site dermatologist who can then review them at his or her convenience,” Dr. Simpson said. “It’s a smartphone-based app, so actually, while I was at this conference, even though I’m thousands of miles from Philadelphia, I got through three consults this morning on my smartphone. It’s a very convenient way to be a volunteer.”
The consultation is between the PCP and the dermatologist, he added. “It’s the dermatologist talking to the PCP, and the patient receives the care recommendations from their primary doctor – so there’s no direct communication with the patient.”
Using the app, PCPs photographed skin lesions and completed simple history and physical exam modules within the app. Then, Dr. Simpson and his dermatology colleagues reviewed the photos and pertinent information.
If diagnostic uncertainty persisted after the teledermatology review, or if Dr. Simpson and his colleagues judged that a procedure such as a biopsy or lesion destruction was required, then the patient was scheduled for an appointment, with an interim plan put in place. Otherwise, patients were managed by teledermatology alone.
Of the 131 patients involved in the pilot study, 48 (37%) were female; the average patient age was 31.7 years (range, 1-92 years).
About 40% of patients were seen for inflammatory conditions, and another 20% for nonpigmented neoplasms. Almost 18% were seen for infectious reasons, with the remainder divided between pigmented neoplasms, hair disorders, and other conditions.
It turned out, said Dr. Simpson, that about two-thirds (65%) of the teletriage consultations ended in a definitive plan not requiring a face-to-face dermatology appointment. About a quarter (23%) were deferred to an in-person dermatology appointment, and the remaining 12% had an interim plan while more information was gathered.
Of the 32 neoplasms addressed by the teletriage strategy, 21 (66%) were deferred to an in-person visit. By contrast, 24 of the 95 non–neoplastic teletriage encounters were deferred to an in-person visit (P less than .001).
Overall, the strategy opened up 18% more appointment slots for new patients, Dr. Simpson said.
As part of the teletriage process, PCPs provided their proposed plan of care before receiving a dermatologist’s advice. When comparing the PCP’s plan to the dermatologist’s final plan, he and his colleagues found that there was a complete change of plan for three-quarters of visits (76%). A partial change happened 14% of the time, and only one in ten patients had no change in treatment plan as a result of the teledermatology consult. “This indicates again that specialist input matters,” he noted.
“This also gives us an opportunity to educate primary care physicians,” Dr. Simpson said, pointing out that in replies, he and his dermatologist colleagues included information about common diagnoses, including first-line treatments and “worrisome features they should be thinking about.”
He and his collaborators found that proper treatment would have been provided 30% of the time without a teledermatology consult, but that patients would have been undertreated 27% of the time. Overtreatment would have occurred at a rate of 11%, and care would have been unnecessarily delayed for about one in four patients. Unnecessary ED visits were averted for 6% of patients with the teletriage approach.
Examples of undertreatment included use of a weak topical steroid, missing infections or the need for referral, and using a suboptimal acne regimen. On the other hand, Dr. Simpson said, overtreatment with unnecessary antibiotics, antifungals, and antivirals also was averted; on some occasions, the PCP plan for an oral corticosteroid or an overly potent topical steroid was shifted to a more appropriate plan by teledermatology.
In sum, said Dr. Simpson, “teletriage via AccessDerm allowed us to reduce by tenfold the wait time for specialist input in dermatology cases. We were able to remove almost two-thirds of people from the queue ... waiting for dermatology appointments, which was very helpful to our clinic.”
And most importantly, he added, “this allowed us to allocate the limited number of in-person appointments that we had at this volunteer clinic to those that were more complicated cases.”
Dr. Simpson reported that he had no relevant disclosures. The project was funded by Penn Medicine and the American Academy of Dermatology.
MILAN – and optimized primary care physicians’ care of nonreferred patients, Cory Simpson, MD, PhD, reported at the World Congress of Dermatology.
With implementation of teledermatology, patient wait times for specialist input dropped from 13.9 days to 1.6 days (P less than .00001).
By allowing dermatologists to evaluate photographs of lesions and perform their own triage of referrals from primary care physicians (PCPs), the teletriage pilot program reduced the number of patients for whom dermatology consults were deemed necessary and also allowed optimal management for the nonreferred patients, said Dr. Simpson, of the University of Pennsylvania, Philadelphia.
“Teledermatology has the potential to increase access to dermatologist-level care, especially for underserved patients,” he commented. “It allows us to educate primary care physicians in resource-limited settings, and it also allows us to avoid suboptimal care of skin disease by nonspecialists – especially the more judicious use of antimicrobial agents and corticosteroids.”
Dr. Simpson explained to the international audience that, for many in the United States, access to a dermatologist requires a lengthy wait that can extend to months.
In Philadelphia, University of Pennsylvania dermatology residents and attending physicians volunteer in an outreach program that serves an uninsured population of primarily Latino immigrants. Operating 1 or 2 evenings a month, the medical and surgical dermatology clinics can accommodate from 8-12 appointments per clinic.
The clinic had been overwhelmed with referrals from PCPs, but Dr. Simpson and his colleagues realized that many of the conditions they were seeing – verruca vulgaris, hand dermatitis, and psoriasis, for example – did not necessarily need a face-to-face dermatologic evaluation.
The AccessDerm app, available at no cost by the American Academy of Dermatology, allows PCPs and dermatologists to communicate and collaborate. “This is a store-and-forward program, meaning the primary physician takes the photos and sends them to an off-site dermatologist who can then review them at his or her convenience,” Dr. Simpson said. “It’s a smartphone-based app, so actually, while I was at this conference, even though I’m thousands of miles from Philadelphia, I got through three consults this morning on my smartphone. It’s a very convenient way to be a volunteer.”
The consultation is between the PCP and the dermatologist, he added. “It’s the dermatologist talking to the PCP, and the patient receives the care recommendations from their primary doctor – so there’s no direct communication with the patient.”
Using the app, PCPs photographed skin lesions and completed simple history and physical exam modules within the app. Then, Dr. Simpson and his dermatology colleagues reviewed the photos and pertinent information.
If diagnostic uncertainty persisted after the teledermatology review, or if Dr. Simpson and his colleagues judged that a procedure such as a biopsy or lesion destruction was required, then the patient was scheduled for an appointment, with an interim plan put in place. Otherwise, patients were managed by teledermatology alone.
Of the 131 patients involved in the pilot study, 48 (37%) were female; the average patient age was 31.7 years (range, 1-92 years).
About 40% of patients were seen for inflammatory conditions, and another 20% for nonpigmented neoplasms. Almost 18% were seen for infectious reasons, with the remainder divided between pigmented neoplasms, hair disorders, and other conditions.
It turned out, said Dr. Simpson, that about two-thirds (65%) of the teletriage consultations ended in a definitive plan not requiring a face-to-face dermatology appointment. About a quarter (23%) were deferred to an in-person dermatology appointment, and the remaining 12% had an interim plan while more information was gathered.
Of the 32 neoplasms addressed by the teletriage strategy, 21 (66%) were deferred to an in-person visit. By contrast, 24 of the 95 non–neoplastic teletriage encounters were deferred to an in-person visit (P less than .001).
Overall, the strategy opened up 18% more appointment slots for new patients, Dr. Simpson said.
As part of the teletriage process, PCPs provided their proposed plan of care before receiving a dermatologist’s advice. When comparing the PCP’s plan to the dermatologist’s final plan, he and his colleagues found that there was a complete change of plan for three-quarters of visits (76%). A partial change happened 14% of the time, and only one in ten patients had no change in treatment plan as a result of the teledermatology consult. “This indicates again that specialist input matters,” he noted.
“This also gives us an opportunity to educate primary care physicians,” Dr. Simpson said, pointing out that in replies, he and his dermatologist colleagues included information about common diagnoses, including first-line treatments and “worrisome features they should be thinking about.”
He and his collaborators found that proper treatment would have been provided 30% of the time without a teledermatology consult, but that patients would have been undertreated 27% of the time. Overtreatment would have occurred at a rate of 11%, and care would have been unnecessarily delayed for about one in four patients. Unnecessary ED visits were averted for 6% of patients with the teletriage approach.
Examples of undertreatment included use of a weak topical steroid, missing infections or the need for referral, and using a suboptimal acne regimen. On the other hand, Dr. Simpson said, overtreatment with unnecessary antibiotics, antifungals, and antivirals also was averted; on some occasions, the PCP plan for an oral corticosteroid or an overly potent topical steroid was shifted to a more appropriate plan by teledermatology.
In sum, said Dr. Simpson, “teletriage via AccessDerm allowed us to reduce by tenfold the wait time for specialist input in dermatology cases. We were able to remove almost two-thirds of people from the queue ... waiting for dermatology appointments, which was very helpful to our clinic.”
And most importantly, he added, “this allowed us to allocate the limited number of in-person appointments that we had at this volunteer clinic to those that were more complicated cases.”
Dr. Simpson reported that he had no relevant disclosures. The project was funded by Penn Medicine and the American Academy of Dermatology.
MILAN – and optimized primary care physicians’ care of nonreferred patients, Cory Simpson, MD, PhD, reported at the World Congress of Dermatology.
With implementation of teledermatology, patient wait times for specialist input dropped from 13.9 days to 1.6 days (P less than .00001).
By allowing dermatologists to evaluate photographs of lesions and perform their own triage of referrals from primary care physicians (PCPs), the teletriage pilot program reduced the number of patients for whom dermatology consults were deemed necessary and also allowed optimal management for the nonreferred patients, said Dr. Simpson, of the University of Pennsylvania, Philadelphia.
“Teledermatology has the potential to increase access to dermatologist-level care, especially for underserved patients,” he commented. “It allows us to educate primary care physicians in resource-limited settings, and it also allows us to avoid suboptimal care of skin disease by nonspecialists – especially the more judicious use of antimicrobial agents and corticosteroids.”
Dr. Simpson explained to the international audience that, for many in the United States, access to a dermatologist requires a lengthy wait that can extend to months.
In Philadelphia, University of Pennsylvania dermatology residents and attending physicians volunteer in an outreach program that serves an uninsured population of primarily Latino immigrants. Operating 1 or 2 evenings a month, the medical and surgical dermatology clinics can accommodate from 8-12 appointments per clinic.
The clinic had been overwhelmed with referrals from PCPs, but Dr. Simpson and his colleagues realized that many of the conditions they were seeing – verruca vulgaris, hand dermatitis, and psoriasis, for example – did not necessarily need a face-to-face dermatologic evaluation.
The AccessDerm app, available at no cost by the American Academy of Dermatology, allows PCPs and dermatologists to communicate and collaborate. “This is a store-and-forward program, meaning the primary physician takes the photos and sends them to an off-site dermatologist who can then review them at his or her convenience,” Dr. Simpson said. “It’s a smartphone-based app, so actually, while I was at this conference, even though I’m thousands of miles from Philadelphia, I got through three consults this morning on my smartphone. It’s a very convenient way to be a volunteer.”
The consultation is between the PCP and the dermatologist, he added. “It’s the dermatologist talking to the PCP, and the patient receives the care recommendations from their primary doctor – so there’s no direct communication with the patient.”
Using the app, PCPs photographed skin lesions and completed simple history and physical exam modules within the app. Then, Dr. Simpson and his dermatology colleagues reviewed the photos and pertinent information.
If diagnostic uncertainty persisted after the teledermatology review, or if Dr. Simpson and his colleagues judged that a procedure such as a biopsy or lesion destruction was required, then the patient was scheduled for an appointment, with an interim plan put in place. Otherwise, patients were managed by teledermatology alone.
Of the 131 patients involved in the pilot study, 48 (37%) were female; the average patient age was 31.7 years (range, 1-92 years).
About 40% of patients were seen for inflammatory conditions, and another 20% for nonpigmented neoplasms. Almost 18% were seen for infectious reasons, with the remainder divided between pigmented neoplasms, hair disorders, and other conditions.
It turned out, said Dr. Simpson, that about two-thirds (65%) of the teletriage consultations ended in a definitive plan not requiring a face-to-face dermatology appointment. About a quarter (23%) were deferred to an in-person dermatology appointment, and the remaining 12% had an interim plan while more information was gathered.
Of the 32 neoplasms addressed by the teletriage strategy, 21 (66%) were deferred to an in-person visit. By contrast, 24 of the 95 non–neoplastic teletriage encounters were deferred to an in-person visit (P less than .001).
Overall, the strategy opened up 18% more appointment slots for new patients, Dr. Simpson said.
As part of the teletriage process, PCPs provided their proposed plan of care before receiving a dermatologist’s advice. When comparing the PCP’s plan to the dermatologist’s final plan, he and his colleagues found that there was a complete change of plan for three-quarters of visits (76%). A partial change happened 14% of the time, and only one in ten patients had no change in treatment plan as a result of the teledermatology consult. “This indicates again that specialist input matters,” he noted.
“This also gives us an opportunity to educate primary care physicians,” Dr. Simpson said, pointing out that in replies, he and his dermatologist colleagues included information about common diagnoses, including first-line treatments and “worrisome features they should be thinking about.”
He and his collaborators found that proper treatment would have been provided 30% of the time without a teledermatology consult, but that patients would have been undertreated 27% of the time. Overtreatment would have occurred at a rate of 11%, and care would have been unnecessarily delayed for about one in four patients. Unnecessary ED visits were averted for 6% of patients with the teletriage approach.
Examples of undertreatment included use of a weak topical steroid, missing infections or the need for referral, and using a suboptimal acne regimen. On the other hand, Dr. Simpson said, overtreatment with unnecessary antibiotics, antifungals, and antivirals also was averted; on some occasions, the PCP plan for an oral corticosteroid or an overly potent topical steroid was shifted to a more appropriate plan by teledermatology.
In sum, said Dr. Simpson, “teletriage via AccessDerm allowed us to reduce by tenfold the wait time for specialist input in dermatology cases. We were able to remove almost two-thirds of people from the queue ... waiting for dermatology appointments, which was very helpful to our clinic.”
And most importantly, he added, “this allowed us to allocate the limited number of in-person appointments that we had at this volunteer clinic to those that were more complicated cases.”
Dr. Simpson reported that he had no relevant disclosures. The project was funded by Penn Medicine and the American Academy of Dermatology.
REPORTING FROM WCD2019
Scabies rates plummeted with community mass drug administration
MILAN – In a region where scabies is endemic, a , findings that may have implications for future treatment of scabies or other infestations in other regions, dermatologist Margot Whitfield, MD, said at the World Congress of Dermatology.
“Mass drug administration is highly effective and safe in the treatment of endemic scabies,” she said.
Using a strategy of directly observed treatment (DOT) with oral ivermectin or topical permethrin for all residents of two separate island groups in Fiji, Dr. Whitfield, together with epidemiologist Lucia Romani, PhD, both of the University of New South Wales, Sydney, and coinvestigators, demonstrated large and sustained decreases in the rates of scabies and impetigo (N Engl J Med. 2015 Dec 10;373[24]:2305-13).
Across study arms, which included a usual care arm, the baseline rate for scabies ranged from 30% to 40%. With usual care, the rate dropped from 36.6% to 18.8% at the end of 12 months, a relative reduction of 49%. However, the 15.8% prevalence rate 12 months after permethrin DOT (from 41.7%), and the 1.9% rate 12 months after ivermectin DOT (from 32.1%) – reductions of 62% and 94%, respectively – represented much larger decreases, “especially since these reductions were seen without any further interventions,” Dr. Whitfield said. “This was extremely exciting, and a game-changer as far as the management of endemic scabies is concerned.”
At baseline, impetigo rates hovered around 20%-25%, and usual care resulted in a 32% reduction at 12 months. With permethrin DOT, the impetigo rate dropped by 54%; with ivermectin DOT, the impetigo rate dropped by 67%. “The community level of impetigo went down, purely as a result of treating the scabies,” Dr. Whitfield said.
The outcomes of this study, she noted, “have contributed to the global discussion of the treatment of scabies.”
Two years after the mass drug administration (MDA) campaign, scabies prevalence remained much lower than at baseline, with clinical scabies diagnosed in 15.2% of the usual care group, 13.5% of the permethrin group, and just 3.6% of the ivermectin group. “The exciting thing for us was that these levels ... were able to be sustained at 2 years,” Dr. Whitfield noted.
The islands that had received ivermectin saw a continued decline in impetigo prevalence as well: By 24 months, impetigo was seen in 2.6% of participants in that arm.
Scabies is a neglected – but highly treatable – tropical disease, she noted. It is associated with intense pruritus, which results in reduced quality of life, and excoriations predispose those affected to bacterial superinfections, commonly impetigo in the young.
In Fiji, the scabies mite infests nearly 40% of those aged 5-9 years, and over one-third of those younger than 5 years. Rates drop steeply with increasing age and then climb again for the elderly; still, prevalence tops 10% for all Fijian age groups, Dr. Whitfield pointed out. Overall, scabies prevalence is 23% in Fiji, with resultant impetigo affecting 19% of the population.
Providing more details about the study, she said that she and her collaborators – working in conjunction with the Fijian Ministry of Health – took advantage of the geography of the island country, whose 850,000 residents live on 300 islands, to compare mass drug treatment with either ivermectin or permethrin with usual care. “We actually didn’t look for ‘infected scabies,’ ” she explained. “We looked for scabies as one outcome, and infection as another.”
The study was designed to take advantage of lessons from previous public health work addressing filariasis and soil-transmitted helminths, and addressed the following question: In Fiji, could a single round of MDA for scabies control lead to sustained reductions in scabies and impetigo prevalence 12 months later, compared with standard care?
The study applied standard-of-care scabies treatment to residents of one island; here, all residents of the island were assessed for scabies, and those who received a clinical diagnosis of scabies, along with family members and close contacts, were treated. Another group of three small islands received permethrin MDA. A third pair of neighboring islands received ivermectin MDA.
For one MDA arm, island residents received oral ivermectin via DOT. A second DOT dose was administered for those with clinically diagnosed scabies. For pregnant and breastfeeding women, children weighing less than 15 kg, and those with ivermectin hypersensitivity, permethrin was used, Dr. Whitfield said.
The individuals in the permethrin MDA arm received one topical dose via DOT, with a second round of topical permethrin for those with topical scabies.
In all, 803 Fijians were assigned to receive standard of care, 532 permethrin MDA, and 716 ivermectin MDA. Of these, 623 received ivermectin DOT, and 93 received permethrin. In all, DOT was achieved for 96% of those receiving the first dose. At baseline, 230 patients had scabies, with 200 receiving ivermectin and 30 permethrin; the DOT rate was 100% for the second dose.
For the permethrin arm, just 307 of 532 participants (58%) had DOT, though all were given permethrin. Scabies was present at baseline for 222 participants, and of these, 181 had DOT. “It’s much easier to do the direct observed therapy with an oral medication than with a cream,” Dr. Whitfield said. Data were not collected for the Fijians who received usual care at community health centers.
Outcomes were clinically determined via the child skin assessment algorithm of the World Health Organization’s International Management of Childhood Illness (IMCI) guidelines.
Dr. Whitfield acknowledged that the study was not a true cluster-randomized trial, and differences existed between the communities studies. Also, “dermatoscopy was not a practical option” for this real-world trial in a resource-limited setting, but validated clinical criteria were used, she said.
Going forward, she and her colleagues are continuing to track durability of reduced scabies rates, as well as downstream sequelae such as impetigo and septicemia. Also, “we need to see whether this community- and island-based project could be scaled up to a national or regional level,” she said.
The burden of disease from scabies globally is probably underestimated, and changing migration patterns may bring endemic scabies to the doorsteps of more developed nations, prompting consideration of MDA as a strategy in expanded circumstances.
Dr. Whitfield reported that she had no relevant conflicts of interest.
MILAN – In a region where scabies is endemic, a , findings that may have implications for future treatment of scabies or other infestations in other regions, dermatologist Margot Whitfield, MD, said at the World Congress of Dermatology.
“Mass drug administration is highly effective and safe in the treatment of endemic scabies,” she said.
Using a strategy of directly observed treatment (DOT) with oral ivermectin or topical permethrin for all residents of two separate island groups in Fiji, Dr. Whitfield, together with epidemiologist Lucia Romani, PhD, both of the University of New South Wales, Sydney, and coinvestigators, demonstrated large and sustained decreases in the rates of scabies and impetigo (N Engl J Med. 2015 Dec 10;373[24]:2305-13).
Across study arms, which included a usual care arm, the baseline rate for scabies ranged from 30% to 40%. With usual care, the rate dropped from 36.6% to 18.8% at the end of 12 months, a relative reduction of 49%. However, the 15.8% prevalence rate 12 months after permethrin DOT (from 41.7%), and the 1.9% rate 12 months after ivermectin DOT (from 32.1%) – reductions of 62% and 94%, respectively – represented much larger decreases, “especially since these reductions were seen without any further interventions,” Dr. Whitfield said. “This was extremely exciting, and a game-changer as far as the management of endemic scabies is concerned.”
At baseline, impetigo rates hovered around 20%-25%, and usual care resulted in a 32% reduction at 12 months. With permethrin DOT, the impetigo rate dropped by 54%; with ivermectin DOT, the impetigo rate dropped by 67%. “The community level of impetigo went down, purely as a result of treating the scabies,” Dr. Whitfield said.
The outcomes of this study, she noted, “have contributed to the global discussion of the treatment of scabies.”
Two years after the mass drug administration (MDA) campaign, scabies prevalence remained much lower than at baseline, with clinical scabies diagnosed in 15.2% of the usual care group, 13.5% of the permethrin group, and just 3.6% of the ivermectin group. “The exciting thing for us was that these levels ... were able to be sustained at 2 years,” Dr. Whitfield noted.
The islands that had received ivermectin saw a continued decline in impetigo prevalence as well: By 24 months, impetigo was seen in 2.6% of participants in that arm.
Scabies is a neglected – but highly treatable – tropical disease, she noted. It is associated with intense pruritus, which results in reduced quality of life, and excoriations predispose those affected to bacterial superinfections, commonly impetigo in the young.
In Fiji, the scabies mite infests nearly 40% of those aged 5-9 years, and over one-third of those younger than 5 years. Rates drop steeply with increasing age and then climb again for the elderly; still, prevalence tops 10% for all Fijian age groups, Dr. Whitfield pointed out. Overall, scabies prevalence is 23% in Fiji, with resultant impetigo affecting 19% of the population.
Providing more details about the study, she said that she and her collaborators – working in conjunction with the Fijian Ministry of Health – took advantage of the geography of the island country, whose 850,000 residents live on 300 islands, to compare mass drug treatment with either ivermectin or permethrin with usual care. “We actually didn’t look for ‘infected scabies,’ ” she explained. “We looked for scabies as one outcome, and infection as another.”
The study was designed to take advantage of lessons from previous public health work addressing filariasis and soil-transmitted helminths, and addressed the following question: In Fiji, could a single round of MDA for scabies control lead to sustained reductions in scabies and impetigo prevalence 12 months later, compared with standard care?
The study applied standard-of-care scabies treatment to residents of one island; here, all residents of the island were assessed for scabies, and those who received a clinical diagnosis of scabies, along with family members and close contacts, were treated. Another group of three small islands received permethrin MDA. A third pair of neighboring islands received ivermectin MDA.
For one MDA arm, island residents received oral ivermectin via DOT. A second DOT dose was administered for those with clinically diagnosed scabies. For pregnant and breastfeeding women, children weighing less than 15 kg, and those with ivermectin hypersensitivity, permethrin was used, Dr. Whitfield said.
The individuals in the permethrin MDA arm received one topical dose via DOT, with a second round of topical permethrin for those with topical scabies.
In all, 803 Fijians were assigned to receive standard of care, 532 permethrin MDA, and 716 ivermectin MDA. Of these, 623 received ivermectin DOT, and 93 received permethrin. In all, DOT was achieved for 96% of those receiving the first dose. At baseline, 230 patients had scabies, with 200 receiving ivermectin and 30 permethrin; the DOT rate was 100% for the second dose.
For the permethrin arm, just 307 of 532 participants (58%) had DOT, though all were given permethrin. Scabies was present at baseline for 222 participants, and of these, 181 had DOT. “It’s much easier to do the direct observed therapy with an oral medication than with a cream,” Dr. Whitfield said. Data were not collected for the Fijians who received usual care at community health centers.
Outcomes were clinically determined via the child skin assessment algorithm of the World Health Organization’s International Management of Childhood Illness (IMCI) guidelines.
Dr. Whitfield acknowledged that the study was not a true cluster-randomized trial, and differences existed between the communities studies. Also, “dermatoscopy was not a practical option” for this real-world trial in a resource-limited setting, but validated clinical criteria were used, she said.
Going forward, she and her colleagues are continuing to track durability of reduced scabies rates, as well as downstream sequelae such as impetigo and septicemia. Also, “we need to see whether this community- and island-based project could be scaled up to a national or regional level,” she said.
The burden of disease from scabies globally is probably underestimated, and changing migration patterns may bring endemic scabies to the doorsteps of more developed nations, prompting consideration of MDA as a strategy in expanded circumstances.
Dr. Whitfield reported that she had no relevant conflicts of interest.
MILAN – In a region where scabies is endemic, a , findings that may have implications for future treatment of scabies or other infestations in other regions, dermatologist Margot Whitfield, MD, said at the World Congress of Dermatology.
“Mass drug administration is highly effective and safe in the treatment of endemic scabies,” she said.
Using a strategy of directly observed treatment (DOT) with oral ivermectin or topical permethrin for all residents of two separate island groups in Fiji, Dr. Whitfield, together with epidemiologist Lucia Romani, PhD, both of the University of New South Wales, Sydney, and coinvestigators, demonstrated large and sustained decreases in the rates of scabies and impetigo (N Engl J Med. 2015 Dec 10;373[24]:2305-13).
Across study arms, which included a usual care arm, the baseline rate for scabies ranged from 30% to 40%. With usual care, the rate dropped from 36.6% to 18.8% at the end of 12 months, a relative reduction of 49%. However, the 15.8% prevalence rate 12 months after permethrin DOT (from 41.7%), and the 1.9% rate 12 months after ivermectin DOT (from 32.1%) – reductions of 62% and 94%, respectively – represented much larger decreases, “especially since these reductions were seen without any further interventions,” Dr. Whitfield said. “This was extremely exciting, and a game-changer as far as the management of endemic scabies is concerned.”
At baseline, impetigo rates hovered around 20%-25%, and usual care resulted in a 32% reduction at 12 months. With permethrin DOT, the impetigo rate dropped by 54%; with ivermectin DOT, the impetigo rate dropped by 67%. “The community level of impetigo went down, purely as a result of treating the scabies,” Dr. Whitfield said.
The outcomes of this study, she noted, “have contributed to the global discussion of the treatment of scabies.”
Two years after the mass drug administration (MDA) campaign, scabies prevalence remained much lower than at baseline, with clinical scabies diagnosed in 15.2% of the usual care group, 13.5% of the permethrin group, and just 3.6% of the ivermectin group. “The exciting thing for us was that these levels ... were able to be sustained at 2 years,” Dr. Whitfield noted.
The islands that had received ivermectin saw a continued decline in impetigo prevalence as well: By 24 months, impetigo was seen in 2.6% of participants in that arm.
Scabies is a neglected – but highly treatable – tropical disease, she noted. It is associated with intense pruritus, which results in reduced quality of life, and excoriations predispose those affected to bacterial superinfections, commonly impetigo in the young.
In Fiji, the scabies mite infests nearly 40% of those aged 5-9 years, and over one-third of those younger than 5 years. Rates drop steeply with increasing age and then climb again for the elderly; still, prevalence tops 10% for all Fijian age groups, Dr. Whitfield pointed out. Overall, scabies prevalence is 23% in Fiji, with resultant impetigo affecting 19% of the population.
Providing more details about the study, she said that she and her collaborators – working in conjunction with the Fijian Ministry of Health – took advantage of the geography of the island country, whose 850,000 residents live on 300 islands, to compare mass drug treatment with either ivermectin or permethrin with usual care. “We actually didn’t look for ‘infected scabies,’ ” she explained. “We looked for scabies as one outcome, and infection as another.”
The study was designed to take advantage of lessons from previous public health work addressing filariasis and soil-transmitted helminths, and addressed the following question: In Fiji, could a single round of MDA for scabies control lead to sustained reductions in scabies and impetigo prevalence 12 months later, compared with standard care?
The study applied standard-of-care scabies treatment to residents of one island; here, all residents of the island were assessed for scabies, and those who received a clinical diagnosis of scabies, along with family members and close contacts, were treated. Another group of three small islands received permethrin MDA. A third pair of neighboring islands received ivermectin MDA.
For one MDA arm, island residents received oral ivermectin via DOT. A second DOT dose was administered for those with clinically diagnosed scabies. For pregnant and breastfeeding women, children weighing less than 15 kg, and those with ivermectin hypersensitivity, permethrin was used, Dr. Whitfield said.
The individuals in the permethrin MDA arm received one topical dose via DOT, with a second round of topical permethrin for those with topical scabies.
In all, 803 Fijians were assigned to receive standard of care, 532 permethrin MDA, and 716 ivermectin MDA. Of these, 623 received ivermectin DOT, and 93 received permethrin. In all, DOT was achieved for 96% of those receiving the first dose. At baseline, 230 patients had scabies, with 200 receiving ivermectin and 30 permethrin; the DOT rate was 100% for the second dose.
For the permethrin arm, just 307 of 532 participants (58%) had DOT, though all were given permethrin. Scabies was present at baseline for 222 participants, and of these, 181 had DOT. “It’s much easier to do the direct observed therapy with an oral medication than with a cream,” Dr. Whitfield said. Data were not collected for the Fijians who received usual care at community health centers.
Outcomes were clinically determined via the child skin assessment algorithm of the World Health Organization’s International Management of Childhood Illness (IMCI) guidelines.
Dr. Whitfield acknowledged that the study was not a true cluster-randomized trial, and differences existed between the communities studies. Also, “dermatoscopy was not a practical option” for this real-world trial in a resource-limited setting, but validated clinical criteria were used, she said.
Going forward, she and her colleagues are continuing to track durability of reduced scabies rates, as well as downstream sequelae such as impetigo and septicemia. Also, “we need to see whether this community- and island-based project could be scaled up to a national or regional level,” she said.
The burden of disease from scabies globally is probably underestimated, and changing migration patterns may bring endemic scabies to the doorsteps of more developed nations, prompting consideration of MDA as a strategy in expanded circumstances.
Dr. Whitfield reported that she had no relevant conflicts of interest.
EXPERT ANALYSIS FROM WCD2019
Fewer antibiotics prescribed with PCR than conventional stool testing
SAN DIEGO – However, antibiotics were still prescribed for more than one in three patients tested by any method.
“A positive test by any modality did result in decreased utilization of endoscopy, radiology, and antibiotic prescribing, but this effect appeared to be much greater for the GI PCR assay,” said Jordan Axelrad, MD, speaking at the annual Digestive Disease Week.
“Overall, patients who received GI PCR were 12% less likely to undergo endoscopy, 7% less likely to undergo abdominal radiography, and 11% less likely to be prescribed any antibiotic,” compared with patients who were tested by conventional stool culture, said Dr. Axelrad, a gastroenterologist at New York University.
In a cross-sectional study, Dr. Axelrad and his coauthors looked at patients who underwent stool testing for the 26 months before (n = 5,986) and after (n = 9,402) March 2015, when Dr. Axelrad’s home institution switched from conventional stool culture to the GI PCR panel. For the earlier time period, the investigators included patients who received stool culture both with and without an ova and parasites exam, as well as those who underwent enzyme-linked immunosorbent assay viral testing for rotavirus and adenovirus.
Patient demographic data were included as study variables; additionally, the study tracked utilization of endoscopy, abdominal, or other radiology studies, and ED visits for 30 days after testing. They also included any antibiotic prescribing within the 14 days post testing.
Roughly one-third of patients were tested as outpatients, 1 in 10 in the ED, and the remainder as inpatients. Patient age was a mean 46.7 years for the culture group, and 45.5 years for the GI PCR group.
The multiplex PCR test used in the study tested for 12 gastrointestinal pathogenic bacteria, 4 parasites, and 5 viruses.
As expected, PCR testing yielded a higher positive test rate than conventional stool testing, even when EIA tests were included (29.2% vs. 4.1%). In the 2,746 patients with a positive GI PCR test, a total of 3,804 pathogens were identified. Adenovirus accounted for 39% of these positive results. Positive bacterial results were seen in about 65.0% of the positive subgroup, with Escherichia coli subtypes seen in 51.7% of the positive tests.
Overall, positive results for viruses, bacteria, and multiple pathogens were more likely with GI PCR testing, compared with conventional testing (P = .001 for all). Parasites accounted for only 8.2% of the positive PCR test results, but this was significantly more than the 3.7% seen with conventional testing (P = .011).
At the 14-day mark post testing, “Patients who underwent a GI panel were less likely to be prescribed any antibiotic. But overall, antibiotics were fairly common in both groups,” said Dr. Axelrad, noting that 41% of patients who underwent stool culture received an antibiotic by 14 days, compared with 36% for patients who underwent a GI PCR panel (P = .001).
By the end of 30 days, most patients in each group had not received an endoscopic procedure, with significantly more procedure-free patients in the PCR group (91.6% vs. 90.4%; P = .008).
Against a backdrop of slightly higher overall radiology utilization in the PCR group – potentially attributable to practice trends over time – abdominal radiology was less likely for these patients than for the culture group (11.4% vs. 12.8%; P = .011).
The 30-day ED visit rate was low and similar between groups (11.4% for PCR vs. 12.8% for culture; P = .116).
The much quicker turnaround for the GI PCR panel didn’t translate into a shorter length of stay, though: Inpatient length of stay was a median 5 days in both groups.
“We feel that the outcomes that we noted were likely due to the increased sensitivity and specificity” of the PCR-based testing, said Dr. Axelrad. “Obviously, if you have more pathogen-positive findings, you may be less likely to order extensive testing. And if you’ve identified something like norovirus, you may feel reassured, and not order further testing.”
Dr. Axelrad pointed out that his institution’s overall PCR positivity rates were lower than the 70% rates some other studies have reported. “We feel that, given our large sample size, our results may more accurately reflect clinical practice, and perhaps that lower positivity rate may reflect increased use of this test in an inpatient setting,” he said. “We’re looking at that.”
Study limitations included the retrospective nature of the study. “Also, as we all know, PCR testing fails to discriminate between active infection and asymptomatic colonization,” raising questions about whether a positive PCR test really indicates true infection, noted Dr. Axelrad.
“Coupled with a high-sensitivity rapid turnaround, there’s the potential to reduce costs, but the cost-effectiveness of these assays has not been fully determined. There are several studies looking at this,” with results still to come, he said.
The notable reduction in antibiotic prescribing for those patients who received PCR-based testing means that GI PCR panels could be a useful tool to promote antibiotic stewardship, though Dr. Axelrad also noted that “antibiotics were still used in about a third of all patients.”
Dr. Axelrad reported no outside sources of funding. He has performed consulting services for and received research funding from BioFire, which manufactured the GI PCR assay used in the study, but BioFire did not fund this research.
SOURCE: Axelrad J et al. DDW 2019, Presentation 978.
SAN DIEGO – However, antibiotics were still prescribed for more than one in three patients tested by any method.
“A positive test by any modality did result in decreased utilization of endoscopy, radiology, and antibiotic prescribing, but this effect appeared to be much greater for the GI PCR assay,” said Jordan Axelrad, MD, speaking at the annual Digestive Disease Week.
“Overall, patients who received GI PCR were 12% less likely to undergo endoscopy, 7% less likely to undergo abdominal radiography, and 11% less likely to be prescribed any antibiotic,” compared with patients who were tested by conventional stool culture, said Dr. Axelrad, a gastroenterologist at New York University.
In a cross-sectional study, Dr. Axelrad and his coauthors looked at patients who underwent stool testing for the 26 months before (n = 5,986) and after (n = 9,402) March 2015, when Dr. Axelrad’s home institution switched from conventional stool culture to the GI PCR panel. For the earlier time period, the investigators included patients who received stool culture both with and without an ova and parasites exam, as well as those who underwent enzyme-linked immunosorbent assay viral testing for rotavirus and adenovirus.
Patient demographic data were included as study variables; additionally, the study tracked utilization of endoscopy, abdominal, or other radiology studies, and ED visits for 30 days after testing. They also included any antibiotic prescribing within the 14 days post testing.
Roughly one-third of patients were tested as outpatients, 1 in 10 in the ED, and the remainder as inpatients. Patient age was a mean 46.7 years for the culture group, and 45.5 years for the GI PCR group.
The multiplex PCR test used in the study tested for 12 gastrointestinal pathogenic bacteria, 4 parasites, and 5 viruses.
As expected, PCR testing yielded a higher positive test rate than conventional stool testing, even when EIA tests were included (29.2% vs. 4.1%). In the 2,746 patients with a positive GI PCR test, a total of 3,804 pathogens were identified. Adenovirus accounted for 39% of these positive results. Positive bacterial results were seen in about 65.0% of the positive subgroup, with Escherichia coli subtypes seen in 51.7% of the positive tests.
Overall, positive results for viruses, bacteria, and multiple pathogens were more likely with GI PCR testing, compared with conventional testing (P = .001 for all). Parasites accounted for only 8.2% of the positive PCR test results, but this was significantly more than the 3.7% seen with conventional testing (P = .011).
At the 14-day mark post testing, “Patients who underwent a GI panel were less likely to be prescribed any antibiotic. But overall, antibiotics were fairly common in both groups,” said Dr. Axelrad, noting that 41% of patients who underwent stool culture received an antibiotic by 14 days, compared with 36% for patients who underwent a GI PCR panel (P = .001).
By the end of 30 days, most patients in each group had not received an endoscopic procedure, with significantly more procedure-free patients in the PCR group (91.6% vs. 90.4%; P = .008).
Against a backdrop of slightly higher overall radiology utilization in the PCR group – potentially attributable to practice trends over time – abdominal radiology was less likely for these patients than for the culture group (11.4% vs. 12.8%; P = .011).
The 30-day ED visit rate was low and similar between groups (11.4% for PCR vs. 12.8% for culture; P = .116).
The much quicker turnaround for the GI PCR panel didn’t translate into a shorter length of stay, though: Inpatient length of stay was a median 5 days in both groups.
“We feel that the outcomes that we noted were likely due to the increased sensitivity and specificity” of the PCR-based testing, said Dr. Axelrad. “Obviously, if you have more pathogen-positive findings, you may be less likely to order extensive testing. And if you’ve identified something like norovirus, you may feel reassured, and not order further testing.”
Dr. Axelrad pointed out that his institution’s overall PCR positivity rates were lower than the 70% rates some other studies have reported. “We feel that, given our large sample size, our results may more accurately reflect clinical practice, and perhaps that lower positivity rate may reflect increased use of this test in an inpatient setting,” he said. “We’re looking at that.”
Study limitations included the retrospective nature of the study. “Also, as we all know, PCR testing fails to discriminate between active infection and asymptomatic colonization,” raising questions about whether a positive PCR test really indicates true infection, noted Dr. Axelrad.
“Coupled with a high-sensitivity rapid turnaround, there’s the potential to reduce costs, but the cost-effectiveness of these assays has not been fully determined. There are several studies looking at this,” with results still to come, he said.
The notable reduction in antibiotic prescribing for those patients who received PCR-based testing means that GI PCR panels could be a useful tool to promote antibiotic stewardship, though Dr. Axelrad also noted that “antibiotics were still used in about a third of all patients.”
Dr. Axelrad reported no outside sources of funding. He has performed consulting services for and received research funding from BioFire, which manufactured the GI PCR assay used in the study, but BioFire did not fund this research.
SOURCE: Axelrad J et al. DDW 2019, Presentation 978.
SAN DIEGO – However, antibiotics were still prescribed for more than one in three patients tested by any method.
“A positive test by any modality did result in decreased utilization of endoscopy, radiology, and antibiotic prescribing, but this effect appeared to be much greater for the GI PCR assay,” said Jordan Axelrad, MD, speaking at the annual Digestive Disease Week.
“Overall, patients who received GI PCR were 12% less likely to undergo endoscopy, 7% less likely to undergo abdominal radiography, and 11% less likely to be prescribed any antibiotic,” compared with patients who were tested by conventional stool culture, said Dr. Axelrad, a gastroenterologist at New York University.
In a cross-sectional study, Dr. Axelrad and his coauthors looked at patients who underwent stool testing for the 26 months before (n = 5,986) and after (n = 9,402) March 2015, when Dr. Axelrad’s home institution switched from conventional stool culture to the GI PCR panel. For the earlier time period, the investigators included patients who received stool culture both with and without an ova and parasites exam, as well as those who underwent enzyme-linked immunosorbent assay viral testing for rotavirus and adenovirus.
Patient demographic data were included as study variables; additionally, the study tracked utilization of endoscopy, abdominal, or other radiology studies, and ED visits for 30 days after testing. They also included any antibiotic prescribing within the 14 days post testing.
Roughly one-third of patients were tested as outpatients, 1 in 10 in the ED, and the remainder as inpatients. Patient age was a mean 46.7 years for the culture group, and 45.5 years for the GI PCR group.
The multiplex PCR test used in the study tested for 12 gastrointestinal pathogenic bacteria, 4 parasites, and 5 viruses.
As expected, PCR testing yielded a higher positive test rate than conventional stool testing, even when EIA tests were included (29.2% vs. 4.1%). In the 2,746 patients with a positive GI PCR test, a total of 3,804 pathogens were identified. Adenovirus accounted for 39% of these positive results. Positive bacterial results were seen in about 65.0% of the positive subgroup, with Escherichia coli subtypes seen in 51.7% of the positive tests.
Overall, positive results for viruses, bacteria, and multiple pathogens were more likely with GI PCR testing, compared with conventional testing (P = .001 for all). Parasites accounted for only 8.2% of the positive PCR test results, but this was significantly more than the 3.7% seen with conventional testing (P = .011).
At the 14-day mark post testing, “Patients who underwent a GI panel were less likely to be prescribed any antibiotic. But overall, antibiotics were fairly common in both groups,” said Dr. Axelrad, noting that 41% of patients who underwent stool culture received an antibiotic by 14 days, compared with 36% for patients who underwent a GI PCR panel (P = .001).
By the end of 30 days, most patients in each group had not received an endoscopic procedure, with significantly more procedure-free patients in the PCR group (91.6% vs. 90.4%; P = .008).
Against a backdrop of slightly higher overall radiology utilization in the PCR group – potentially attributable to practice trends over time – abdominal radiology was less likely for these patients than for the culture group (11.4% vs. 12.8%; P = .011).
The 30-day ED visit rate was low and similar between groups (11.4% for PCR vs. 12.8% for culture; P = .116).
The much quicker turnaround for the GI PCR panel didn’t translate into a shorter length of stay, though: Inpatient length of stay was a median 5 days in both groups.
“We feel that the outcomes that we noted were likely due to the increased sensitivity and specificity” of the PCR-based testing, said Dr. Axelrad. “Obviously, if you have more pathogen-positive findings, you may be less likely to order extensive testing. And if you’ve identified something like norovirus, you may feel reassured, and not order further testing.”
Dr. Axelrad pointed out that his institution’s overall PCR positivity rates were lower than the 70% rates some other studies have reported. “We feel that, given our large sample size, our results may more accurately reflect clinical practice, and perhaps that lower positivity rate may reflect increased use of this test in an inpatient setting,” he said. “We’re looking at that.”
Study limitations included the retrospective nature of the study. “Also, as we all know, PCR testing fails to discriminate between active infection and asymptomatic colonization,” raising questions about whether a positive PCR test really indicates true infection, noted Dr. Axelrad.
“Coupled with a high-sensitivity rapid turnaround, there’s the potential to reduce costs, but the cost-effectiveness of these assays has not been fully determined. There are several studies looking at this,” with results still to come, he said.
The notable reduction in antibiotic prescribing for those patients who received PCR-based testing means that GI PCR panels could be a useful tool to promote antibiotic stewardship, though Dr. Axelrad also noted that “antibiotics were still used in about a third of all patients.”
Dr. Axelrad reported no outside sources of funding. He has performed consulting services for and received research funding from BioFire, which manufactured the GI PCR assay used in the study, but BioFire did not fund this research.
SOURCE: Axelrad J et al. DDW 2019, Presentation 978.
REPORTING FROM DDW 2019
Novel oral drug shows early promise for IBD
SAN DIEGO – A novel oral drug for inflammatory bowel disease showed good safety and efficacy data in preliminary clinical trial results.
Among a group of 32 patients with ulcerative colitis, the investigative drug ABX464 showed a decrease in Mayo score of over 50% and a drop in fecal calprotectin to near-normal levels. The safety profile was reassuring, and results were durable at the 9-month mark.
Coauthor Jean-Marc Steens, MD, presented results of the randomized, double-blind, placebo-controlled phase 2a study at the annual Digestive Disease Week®, and noted that ABX464 is also being investigated as antiviral therapy for individuals with HIV/AIDS.
“Despite the major advances in the last 10 years with the introduction of biologics and [Janus kinase] inhibitors, there is still a huge unmet medical need for these patients,” said Dr. Steens, chief medical officer of Abivax (Paris), in an interview. “Large phase 3 studies with these recent drugs have shown that about two-thirds of the patients show a clinical response during induction, but that half of these responders will lose their response within the next 6-12 months. The safety profile of these drugs also includes severe infections, which is a major concern,” he said.
Dr. Steens presented the findings on behalf of first author Severine Vermeire, MD, chair of the department of chronic diseases, metabolism, and aging at Catholic University, Leuven, Belgium.
ABX464, a small-molecule oral medication, has been evaluated for safety among more than 180 patients with HIV as well as the patients with ulcerative colitis (UC) studied in the current trial. The drug increases expression of the microRNA precursor miR-124, with the result that “the inflammatory brake is applied,” explained Dr. Steens.
In the present study, whose primary outcome was safety, 23 patients with moderate to severe active UC were randomized to ABX464 50 mg once daily, and 9 to placebo. Patients were included if they had failed or were intolerant to immunomodulators, anti–tumor necrosis factor–alpha therapies, vedolizumab, or corticosteroids; the two groups had balanced disease and demographic characteristics. At baseline, patients had a total Mayo score of 6-12, and an endoscopic subscore of 2 or 3.
Three patients withdrew from the ABX464 arm by the end of 8 weeks: one because of adverse events (AEs), one withdrew consent, and the third declined to undergo endoscopy at the 8-week mark.
All treatment-emergent AEs were mild or moderate, with gastrointestinal disorders occurring in eight of the ABX464 patients and two placebo patients (34.8% and 22.2%, respectively.) Five ABX-464 patients (21.7%) experienced nervous system symptoms – mostly headaches, said Dr. Steens. No patients in the placebo arm had headache or other neurological AEs.
By the end of 8 weeks, 30% of the intention-to-treat ABX464 group was in clinical remission, compared with 11% of the placebo group; this was not a statistically significant difference (P = .16). The proportion of ABX464 patients who had a clinical response just missed statistical significance, compared with placebo (61% versus 33%; P = 06).
However, significant endoscopic improvement was seen in the ABX464 arm, with 43% having a Mayo endoscopy subscore of 0 or 1, compared with 11% in the placebo arm (P = .03).
The total Mayo score dropped by 53% in the ABX464 group, compared with 27% in the placebo group (P = .03); a partial Mayo score dropped by 62% for those in the active arm, compared with 32% in the placebo arm (P = .02).
“The major finding from the induction study was that all endpoints were going in the same direction in favor of ABX464, even reaching statistical significance for endoscopy as well, and total and partial Mayo score,” said Dr. Steens.
Patients underwent rectal biopsies at the end of 8 weeks, and miR-124 expression increased more than sevenfold from baseline for those taking ABX464, compared with a small increase in the placebo group (7.69- versus 1.46-fold; P = .004). Expression of miR-124 in total blood also increased – by over 800-fold – at study day 28 for the ABX464 arm. Levels were sustained at more than 700-fold at study day 56 in this group. Placebo arm participants saw an insignificant rise in miR-124 blood levels.
Dr. Steens reported that 22 patients, including 7 who had originally been placebo arm participants, continued into the maintenance phase of the study. Nineteen patients have now had a median of over 400 days of exposure to ABX464, with sustained significant improvement in partial Mayo scores from a baseline of 6 to scores below 2 at 6 and 9 months. Fecal calprotectin scores have dropped from a median 1,044 mcg/g at baseline to 23.5 mcg/g at 9 months.
Next steps include the 12-month assessment, which includes another endoscopy, said Dr. Steens. Also, a phase 2b study is seeking to enroll 232 patients who have moderate to severe ulcerative colitis, with room within the enrollment scheme for new study sites, said Dr. Steens. This larger study will have arms in which the current 50-mg oral dose is doubled and halved, as well as a placebo arm, he said. The medication will also be trialed for Crohn’s disease and rheumatoid arthritis.
The small sample size is an inherent limitation of this early-stage clinical trial, noted Dr. Steens.
Dr. Steens reported being an employee and holding shares in Abivax, which funded the study.
SOURCE: Vermeire S et al. DDW 2019, Abstract 1007.
SAN DIEGO – A novel oral drug for inflammatory bowel disease showed good safety and efficacy data in preliminary clinical trial results.
Among a group of 32 patients with ulcerative colitis, the investigative drug ABX464 showed a decrease in Mayo score of over 50% and a drop in fecal calprotectin to near-normal levels. The safety profile was reassuring, and results were durable at the 9-month mark.
Coauthor Jean-Marc Steens, MD, presented results of the randomized, double-blind, placebo-controlled phase 2a study at the annual Digestive Disease Week®, and noted that ABX464 is also being investigated as antiviral therapy for individuals with HIV/AIDS.
“Despite the major advances in the last 10 years with the introduction of biologics and [Janus kinase] inhibitors, there is still a huge unmet medical need for these patients,” said Dr. Steens, chief medical officer of Abivax (Paris), in an interview. “Large phase 3 studies with these recent drugs have shown that about two-thirds of the patients show a clinical response during induction, but that half of these responders will lose their response within the next 6-12 months. The safety profile of these drugs also includes severe infections, which is a major concern,” he said.
Dr. Steens presented the findings on behalf of first author Severine Vermeire, MD, chair of the department of chronic diseases, metabolism, and aging at Catholic University, Leuven, Belgium.
ABX464, a small-molecule oral medication, has been evaluated for safety among more than 180 patients with HIV as well as the patients with ulcerative colitis (UC) studied in the current trial. The drug increases expression of the microRNA precursor miR-124, with the result that “the inflammatory brake is applied,” explained Dr. Steens.
In the present study, whose primary outcome was safety, 23 patients with moderate to severe active UC were randomized to ABX464 50 mg once daily, and 9 to placebo. Patients were included if they had failed or were intolerant to immunomodulators, anti–tumor necrosis factor–alpha therapies, vedolizumab, or corticosteroids; the two groups had balanced disease and demographic characteristics. At baseline, patients had a total Mayo score of 6-12, and an endoscopic subscore of 2 or 3.
Three patients withdrew from the ABX464 arm by the end of 8 weeks: one because of adverse events (AEs), one withdrew consent, and the third declined to undergo endoscopy at the 8-week mark.
All treatment-emergent AEs were mild or moderate, with gastrointestinal disorders occurring in eight of the ABX464 patients and two placebo patients (34.8% and 22.2%, respectively.) Five ABX-464 patients (21.7%) experienced nervous system symptoms – mostly headaches, said Dr. Steens. No patients in the placebo arm had headache or other neurological AEs.
By the end of 8 weeks, 30% of the intention-to-treat ABX464 group was in clinical remission, compared with 11% of the placebo group; this was not a statistically significant difference (P = .16). The proportion of ABX464 patients who had a clinical response just missed statistical significance, compared with placebo (61% versus 33%; P = 06).
However, significant endoscopic improvement was seen in the ABX464 arm, with 43% having a Mayo endoscopy subscore of 0 or 1, compared with 11% in the placebo arm (P = .03).
The total Mayo score dropped by 53% in the ABX464 group, compared with 27% in the placebo group (P = .03); a partial Mayo score dropped by 62% for those in the active arm, compared with 32% in the placebo arm (P = .02).
“The major finding from the induction study was that all endpoints were going in the same direction in favor of ABX464, even reaching statistical significance for endoscopy as well, and total and partial Mayo score,” said Dr. Steens.
Patients underwent rectal biopsies at the end of 8 weeks, and miR-124 expression increased more than sevenfold from baseline for those taking ABX464, compared with a small increase in the placebo group (7.69- versus 1.46-fold; P = .004). Expression of miR-124 in total blood also increased – by over 800-fold – at study day 28 for the ABX464 arm. Levels were sustained at more than 700-fold at study day 56 in this group. Placebo arm participants saw an insignificant rise in miR-124 blood levels.
Dr. Steens reported that 22 patients, including 7 who had originally been placebo arm participants, continued into the maintenance phase of the study. Nineteen patients have now had a median of over 400 days of exposure to ABX464, with sustained significant improvement in partial Mayo scores from a baseline of 6 to scores below 2 at 6 and 9 months. Fecal calprotectin scores have dropped from a median 1,044 mcg/g at baseline to 23.5 mcg/g at 9 months.
Next steps include the 12-month assessment, which includes another endoscopy, said Dr. Steens. Also, a phase 2b study is seeking to enroll 232 patients who have moderate to severe ulcerative colitis, with room within the enrollment scheme for new study sites, said Dr. Steens. This larger study will have arms in which the current 50-mg oral dose is doubled and halved, as well as a placebo arm, he said. The medication will also be trialed for Crohn’s disease and rheumatoid arthritis.
The small sample size is an inherent limitation of this early-stage clinical trial, noted Dr. Steens.
Dr. Steens reported being an employee and holding shares in Abivax, which funded the study.
SOURCE: Vermeire S et al. DDW 2019, Abstract 1007.
SAN DIEGO – A novel oral drug for inflammatory bowel disease showed good safety and efficacy data in preliminary clinical trial results.
Among a group of 32 patients with ulcerative colitis, the investigative drug ABX464 showed a decrease in Mayo score of over 50% and a drop in fecal calprotectin to near-normal levels. The safety profile was reassuring, and results were durable at the 9-month mark.
Coauthor Jean-Marc Steens, MD, presented results of the randomized, double-blind, placebo-controlled phase 2a study at the annual Digestive Disease Week®, and noted that ABX464 is also being investigated as antiviral therapy for individuals with HIV/AIDS.
“Despite the major advances in the last 10 years with the introduction of biologics and [Janus kinase] inhibitors, there is still a huge unmet medical need for these patients,” said Dr. Steens, chief medical officer of Abivax (Paris), in an interview. “Large phase 3 studies with these recent drugs have shown that about two-thirds of the patients show a clinical response during induction, but that half of these responders will lose their response within the next 6-12 months. The safety profile of these drugs also includes severe infections, which is a major concern,” he said.
Dr. Steens presented the findings on behalf of first author Severine Vermeire, MD, chair of the department of chronic diseases, metabolism, and aging at Catholic University, Leuven, Belgium.
ABX464, a small-molecule oral medication, has been evaluated for safety among more than 180 patients with HIV as well as the patients with ulcerative colitis (UC) studied in the current trial. The drug increases expression of the microRNA precursor miR-124, with the result that “the inflammatory brake is applied,” explained Dr. Steens.
In the present study, whose primary outcome was safety, 23 patients with moderate to severe active UC were randomized to ABX464 50 mg once daily, and 9 to placebo. Patients were included if they had failed or were intolerant to immunomodulators, anti–tumor necrosis factor–alpha therapies, vedolizumab, or corticosteroids; the two groups had balanced disease and demographic characteristics. At baseline, patients had a total Mayo score of 6-12, and an endoscopic subscore of 2 or 3.
Three patients withdrew from the ABX464 arm by the end of 8 weeks: one because of adverse events (AEs), one withdrew consent, and the third declined to undergo endoscopy at the 8-week mark.
All treatment-emergent AEs were mild or moderate, with gastrointestinal disorders occurring in eight of the ABX464 patients and two placebo patients (34.8% and 22.2%, respectively.) Five ABX-464 patients (21.7%) experienced nervous system symptoms – mostly headaches, said Dr. Steens. No patients in the placebo arm had headache or other neurological AEs.
By the end of 8 weeks, 30% of the intention-to-treat ABX464 group was in clinical remission, compared with 11% of the placebo group; this was not a statistically significant difference (P = .16). The proportion of ABX464 patients who had a clinical response just missed statistical significance, compared with placebo (61% versus 33%; P = 06).
However, significant endoscopic improvement was seen in the ABX464 arm, with 43% having a Mayo endoscopy subscore of 0 or 1, compared with 11% in the placebo arm (P = .03).
The total Mayo score dropped by 53% in the ABX464 group, compared with 27% in the placebo group (P = .03); a partial Mayo score dropped by 62% for those in the active arm, compared with 32% in the placebo arm (P = .02).
“The major finding from the induction study was that all endpoints were going in the same direction in favor of ABX464, even reaching statistical significance for endoscopy as well, and total and partial Mayo score,” said Dr. Steens.
Patients underwent rectal biopsies at the end of 8 weeks, and miR-124 expression increased more than sevenfold from baseline for those taking ABX464, compared with a small increase in the placebo group (7.69- versus 1.46-fold; P = .004). Expression of miR-124 in total blood also increased – by over 800-fold – at study day 28 for the ABX464 arm. Levels were sustained at more than 700-fold at study day 56 in this group. Placebo arm participants saw an insignificant rise in miR-124 blood levels.
Dr. Steens reported that 22 patients, including 7 who had originally been placebo arm participants, continued into the maintenance phase of the study. Nineteen patients have now had a median of over 400 days of exposure to ABX464, with sustained significant improvement in partial Mayo scores from a baseline of 6 to scores below 2 at 6 and 9 months. Fecal calprotectin scores have dropped from a median 1,044 mcg/g at baseline to 23.5 mcg/g at 9 months.
Next steps include the 12-month assessment, which includes another endoscopy, said Dr. Steens. Also, a phase 2b study is seeking to enroll 232 patients who have moderate to severe ulcerative colitis, with room within the enrollment scheme for new study sites, said Dr. Steens. This larger study will have arms in which the current 50-mg oral dose is doubled and halved, as well as a placebo arm, he said. The medication will also be trialed for Crohn’s disease and rheumatoid arthritis.
The small sample size is an inherent limitation of this early-stage clinical trial, noted Dr. Steens.
Dr. Steens reported being an employee and holding shares in Abivax, which funded the study.
SOURCE: Vermeire S et al. DDW 2019, Abstract 1007.
REPORTING FROM DDW 2019
Key clinical point: A novel oral small molecule’s potent anti-inflammatory effect over 8 weeks was associated with significant endoscopic improvement, reduced Mayo scores, and a trend toward clinical response, compared with placebo.
Major finding: Study details: Randomized, double-blind, placebo-controlled study of 32 patients with moderate to severe ulcerative colitis.
Disclosures: The study was sponsored by Abivax. Dr. Steens is an employee of and holds shares of Abivax.
Source: Vermeire S et al. DDW, Abstract 1007.
Coffee, tea, and soda all up GERD risk
SAN DIEGO – .
In an interview following the oral presentation, Raaj S. Mehta, MD, said that patients in his primary care panel at Massachusetts General Hospital, Boston, where he’s a senior resident, frequently came to him with GERD. In addition to questions about diet, patients frequently wanted to know which beverages might provoke or exacerbate their GERD.

In trying to help his patients, Dr. Mehta said he realized that there wasn’t a prospective evidence base to answer their questions about beverages and GERD, so he and his colleagues used data from the Nurses’ Health Study II (NHS II), a prospective cohort study, to look at the association between various beverages and the incidence of GERD.
“What’s exciting is that we were able to find that coffee, tea, and soda – all three – increase your risk for gastroesophageal reflux disease,” Dr. Mehta said in a video interview. “At the highest quintile level, so looking at people who consume six or more cups per day, you’re looking at maybe a 25%-35% increase in risk of reflux disease.”
There was a dose-response relationship as well: “You do see a slight increase as you go from one cup, to two, to three, and so on, all the way up to six cups” of the offending beverages, said Dr. Mehta.
Overall, the risk for GERD rose from 1.17 to 1.34 with coffee consumption as servings per day increased from less than one to six or more (P for trend less than .0001). Tea consumption was associated with increased GERD risk ranging from 1.08 to 1.26 as consumption rose (P for trend .001). For soda, the increased risk went from 1.12 at less than one serving daily, to 1.41 at four to five servings daily, and then fell to 1.29 at six or more daily servings (P for trend less than .0001).
Whether the beverages were caffeinated or not, said Dr. Mehta, only made a “minimal difference” in GERD risk.
“In contrast, we didn’t see an association for beverages like water, juice, and milk,” he said – reassuring findings in light of fruit juice’s anecdotal status as a GERD culprit.
The NHS II collected data every 2 years from 48,308 female nurses aged 42-62 years at the beginning of the study. Every 4 years dietary information was collected, and on the opposite 4-year cycle, participants answered questions about GERD. Medication use, including the incident use of proton pump inhibitors, was collected every 2 years.
Patients with baseline GERD or use of PPIs or H2 receptor antagonists were excluded from participation.
The quantity and type of beverages were assessed by food frequency questionnaires; other demographic, dietary, and medication variables were also gathered and used to adjust the statistical analysis.
A substitution analysis answered the “what-if” question of the effect of substituting two glasses of plain water daily for either coffee, tea, or soda. Dr. Mehta and colleagues saw a modest reduction in risk for GERD with this strategy.
In addition to the prospective nature of the study (abstract 514, doi: 10.1016/S0016-5085(19)37044-1), the large sample size, high follow-up rates, and well validated dietary data were all strengths, said Dr. Mehta. However, the study’s population is relatively homogeneous, and residual confounding couldn’t be excluded. Also, GERD was defined by self-report, though participants were asked to respond to clear, validated criteria.
For Dr. Mehta, he’s glad to have a clear answer to a common clinic question. “I think that this is one additional thing that I can recommend as a primary care provider to my patients when they come into my office,” he said.
Dr. Mehta reported no conflicts of interest.
Encourage your patients to visit the AGA GI Patient Center for education by specialists for patients about GERD symptoms and treatments at https://www.gastro.org/practice-guidance/gi-patient-center/topic/gastroesophageal-reflux-disease-gerd.
SAN DIEGO – .
In an interview following the oral presentation, Raaj S. Mehta, MD, said that patients in his primary care panel at Massachusetts General Hospital, Boston, where he’s a senior resident, frequently came to him with GERD. In addition to questions about diet, patients frequently wanted to know which beverages might provoke or exacerbate their GERD.

In trying to help his patients, Dr. Mehta said he realized that there wasn’t a prospective evidence base to answer their questions about beverages and GERD, so he and his colleagues used data from the Nurses’ Health Study II (NHS II), a prospective cohort study, to look at the association between various beverages and the incidence of GERD.
“What’s exciting is that we were able to find that coffee, tea, and soda – all three – increase your risk for gastroesophageal reflux disease,” Dr. Mehta said in a video interview. “At the highest quintile level, so looking at people who consume six or more cups per day, you’re looking at maybe a 25%-35% increase in risk of reflux disease.”
There was a dose-response relationship as well: “You do see a slight increase as you go from one cup, to two, to three, and so on, all the way up to six cups” of the offending beverages, said Dr. Mehta.
Overall, the risk for GERD rose from 1.17 to 1.34 with coffee consumption as servings per day increased from less than one to six or more (P for trend less than .0001). Tea consumption was associated with increased GERD risk ranging from 1.08 to 1.26 as consumption rose (P for trend .001). For soda, the increased risk went from 1.12 at less than one serving daily, to 1.41 at four to five servings daily, and then fell to 1.29 at six or more daily servings (P for trend less than .0001).
Whether the beverages were caffeinated or not, said Dr. Mehta, only made a “minimal difference” in GERD risk.
“In contrast, we didn’t see an association for beverages like water, juice, and milk,” he said – reassuring findings in light of fruit juice’s anecdotal status as a GERD culprit.
The NHS II collected data every 2 years from 48,308 female nurses aged 42-62 years at the beginning of the study. Every 4 years dietary information was collected, and on the opposite 4-year cycle, participants answered questions about GERD. Medication use, including the incident use of proton pump inhibitors, was collected every 2 years.
Patients with baseline GERD or use of PPIs or H2 receptor antagonists were excluded from participation.
The quantity and type of beverages were assessed by food frequency questionnaires; other demographic, dietary, and medication variables were also gathered and used to adjust the statistical analysis.
A substitution analysis answered the “what-if” question of the effect of substituting two glasses of plain water daily for either coffee, tea, or soda. Dr. Mehta and colleagues saw a modest reduction in risk for GERD with this strategy.
In addition to the prospective nature of the study (abstract 514, doi: 10.1016/S0016-5085(19)37044-1), the large sample size, high follow-up rates, and well validated dietary data were all strengths, said Dr. Mehta. However, the study’s population is relatively homogeneous, and residual confounding couldn’t be excluded. Also, GERD was defined by self-report, though participants were asked to respond to clear, validated criteria.
For Dr. Mehta, he’s glad to have a clear answer to a common clinic question. “I think that this is one additional thing that I can recommend as a primary care provider to my patients when they come into my office,” he said.
Dr. Mehta reported no conflicts of interest.
Encourage your patients to visit the AGA GI Patient Center for education by specialists for patients about GERD symptoms and treatments at https://www.gastro.org/practice-guidance/gi-patient-center/topic/gastroesophageal-reflux-disease-gerd.
SAN DIEGO – .
In an interview following the oral presentation, Raaj S. Mehta, MD, said that patients in his primary care panel at Massachusetts General Hospital, Boston, where he’s a senior resident, frequently came to him with GERD. In addition to questions about diet, patients frequently wanted to know which beverages might provoke or exacerbate their GERD.

In trying to help his patients, Dr. Mehta said he realized that there wasn’t a prospective evidence base to answer their questions about beverages and GERD, so he and his colleagues used data from the Nurses’ Health Study II (NHS II), a prospective cohort study, to look at the association between various beverages and the incidence of GERD.
“What’s exciting is that we were able to find that coffee, tea, and soda – all three – increase your risk for gastroesophageal reflux disease,” Dr. Mehta said in a video interview. “At the highest quintile level, so looking at people who consume six or more cups per day, you’re looking at maybe a 25%-35% increase in risk of reflux disease.”
There was a dose-response relationship as well: “You do see a slight increase as you go from one cup, to two, to three, and so on, all the way up to six cups” of the offending beverages, said Dr. Mehta.
Overall, the risk for GERD rose from 1.17 to 1.34 with coffee consumption as servings per day increased from less than one to six or more (P for trend less than .0001). Tea consumption was associated with increased GERD risk ranging from 1.08 to 1.26 as consumption rose (P for trend .001). For soda, the increased risk went from 1.12 at less than one serving daily, to 1.41 at four to five servings daily, and then fell to 1.29 at six or more daily servings (P for trend less than .0001).
Whether the beverages were caffeinated or not, said Dr. Mehta, only made a “minimal difference” in GERD risk.
“In contrast, we didn’t see an association for beverages like water, juice, and milk,” he said – reassuring findings in light of fruit juice’s anecdotal status as a GERD culprit.
The NHS II collected data every 2 years from 48,308 female nurses aged 42-62 years at the beginning of the study. Every 4 years dietary information was collected, and on the opposite 4-year cycle, participants answered questions about GERD. Medication use, including the incident use of proton pump inhibitors, was collected every 2 years.
Patients with baseline GERD or use of PPIs or H2 receptor antagonists were excluded from participation.
The quantity and type of beverages were assessed by food frequency questionnaires; other demographic, dietary, and medication variables were also gathered and used to adjust the statistical analysis.
A substitution analysis answered the “what-if” question of the effect of substituting two glasses of plain water daily for either coffee, tea, or soda. Dr. Mehta and colleagues saw a modest reduction in risk for GERD with this strategy.
In addition to the prospective nature of the study (abstract 514, doi: 10.1016/S0016-5085(19)37044-1), the large sample size, high follow-up rates, and well validated dietary data were all strengths, said Dr. Mehta. However, the study’s population is relatively homogeneous, and residual confounding couldn’t be excluded. Also, GERD was defined by self-report, though participants were asked to respond to clear, validated criteria.
For Dr. Mehta, he’s glad to have a clear answer to a common clinic question. “I think that this is one additional thing that I can recommend as a primary care provider to my patients when they come into my office,” he said.
Dr. Mehta reported no conflicts of interest.
Encourage your patients to visit the AGA GI Patient Center for education by specialists for patients about GERD symptoms and treatments at https://www.gastro.org/practice-guidance/gi-patient-center/topic/gastroesophageal-reflux-disease-gerd.
REPORTING FROM DDW 2019
Slow breathing: An effective, pragmatic analgesic technique?
MILWAUKEE – Mindfulness-based practices are effective in reducing pain perceptions, but a more easily taught breath control technique also showed efficacy in a recent study. Slow, rhythmic breathing alone, even without the additional attentional components of mindfulness, had significant analgesic effects in a human experimental model of pain.
“Slow breathing is much easier to perform” than mindfulness-based meditation, Fadel Zeidan, PhD, said at the scientific meeting of the American Pain Society. More research into the technique may offer a “clinically pragmatic” nonpharmacologic option for pain control, he said. And there may be some similarities between how the two techniques work: like mindfulness meditation, slow, rhythmic breathing’s analgesic properties are not dependent on the endogenous opioid system, said Dr. Zeidan, assistant professor of anesthesiology at the University of California, San Diego. His interests include mindfulness meditation–based pain relief.
In previous work, Dr. Zeidan and his collaborators had shown that the analgesic effect of mindfulness practices is not mediated by endogenous opioids. Participants in a study were trained in mindfulness meditation, and then exposed to a pain stimulus. Compared with a control group who listened to an audiobook rather than using mindfulness practices when exposed to pain, the meditators experienced a significant reduction in pain unpleasantness (J Neurosci. 16 March 2016;36[11]:3391-7).
In the experiment, both the meditation and the control group received first an intravenous saline solution, and then the opioid antagonist naloxone, which blocks endogenous opioids. When receiving naloxone, the meditators experienced reductions in the perceived unpleasantness of pain that were similar to what they experienced when they had received saline, showing that endogenous opioids weren’t responsible for meditation’s analgesic effects.
After verifying those findings, said Dr. Zeidan, he became interested in conducting a “graded analytical dissection of mindfulness,” to see exactly which components of the practice are nonopioidergic.
With mindfulness meditation, participants engage in slow, rhythmic breathing, and they learn about observation and appraisal practices, which can briefly be described as “the awareness of arising sensory events without reaction,” Dr. Zeidan said.
Mere belief in meditation in combination with the slow rhythmic breathing might have an analgesic effect, he said. In effect, this is sham mindfulness.
To try to tease out the contributions of each component of mindfulness meditation, Dr. Zeidan and his colleagues devised an experiment that trained participants in one of three ways. Over the course of four 20-minute sessions, randomized participants were trained in slow breathing techniques, with a goal respiratory rate of 6 breaths per minute; in mindfulness meditation techniques; or in a sham mindfulness technique that did not teach specific mindfulness principles.
The randomized participants were subject to a painful heat stimulus before the training to establish a baseline.
After training, they returned for two further sessions. At each visit, they experienced the noxious stimulus with no medication. After a rest period, they then received either high-dose intravenous naloxone or saline. The allocation was randomized and administration of the study drug was double-blinded.
With naloxone or saline infusion ongoing, participants were then again subjected to the painful heat stimulus.
“All manipulations effectively reduced the respiration rate,” by 18%-21%, Dr. Zeidan said.
However, with the introduction of naloxone, both the slow-breathing group and the mindfulness group maintained reductions in pain unpleasantness, while those in the sham group had significant increases in pain unpleasantness. Reductions in pain unpleasantness ranged from 11% to 18% for these two groups, while the initial 8% reduction for the sham group climbed to a 13% increase in pain unpleasantness when this group received naloxone.
An unexpected finding was how effective slow breathing alone was as an analgesic. “There’s really something here,” said Dr. Zeidan, in reference to the analgesic effect of breath control. He explained that the slow breathing technique training was done with the aid of a device that emitted a blue glow that dimmed and brightened at the target respiratory rate.
Dr. Zeidan added that few participants were able to slow their breathing to 6 respirations per minute, but that the average rate did slow to about 12 from the normal 16 or so breaths per minute.
Dr. Zeidan reported no conflicts of interest. The National Institutes of Health funded the research.
MILWAUKEE – Mindfulness-based practices are effective in reducing pain perceptions, but a more easily taught breath control technique also showed efficacy in a recent study. Slow, rhythmic breathing alone, even without the additional attentional components of mindfulness, had significant analgesic effects in a human experimental model of pain.
“Slow breathing is much easier to perform” than mindfulness-based meditation, Fadel Zeidan, PhD, said at the scientific meeting of the American Pain Society. More research into the technique may offer a “clinically pragmatic” nonpharmacologic option for pain control, he said. And there may be some similarities between how the two techniques work: like mindfulness meditation, slow, rhythmic breathing’s analgesic properties are not dependent on the endogenous opioid system, said Dr. Zeidan, assistant professor of anesthesiology at the University of California, San Diego. His interests include mindfulness meditation–based pain relief.
In previous work, Dr. Zeidan and his collaborators had shown that the analgesic effect of mindfulness practices is not mediated by endogenous opioids. Participants in a study were trained in mindfulness meditation, and then exposed to a pain stimulus. Compared with a control group who listened to an audiobook rather than using mindfulness practices when exposed to pain, the meditators experienced a significant reduction in pain unpleasantness (J Neurosci. 16 March 2016;36[11]:3391-7).
In the experiment, both the meditation and the control group received first an intravenous saline solution, and then the opioid antagonist naloxone, which blocks endogenous opioids. When receiving naloxone, the meditators experienced reductions in the perceived unpleasantness of pain that were similar to what they experienced when they had received saline, showing that endogenous opioids weren’t responsible for meditation’s analgesic effects.
After verifying those findings, said Dr. Zeidan, he became interested in conducting a “graded analytical dissection of mindfulness,” to see exactly which components of the practice are nonopioidergic.
With mindfulness meditation, participants engage in slow, rhythmic breathing, and they learn about observation and appraisal practices, which can briefly be described as “the awareness of arising sensory events without reaction,” Dr. Zeidan said.
Mere belief in meditation in combination with the slow rhythmic breathing might have an analgesic effect, he said. In effect, this is sham mindfulness.
To try to tease out the contributions of each component of mindfulness meditation, Dr. Zeidan and his colleagues devised an experiment that trained participants in one of three ways. Over the course of four 20-minute sessions, randomized participants were trained in slow breathing techniques, with a goal respiratory rate of 6 breaths per minute; in mindfulness meditation techniques; or in a sham mindfulness technique that did not teach specific mindfulness principles.
The randomized participants were subject to a painful heat stimulus before the training to establish a baseline.
After training, they returned for two further sessions. At each visit, they experienced the noxious stimulus with no medication. After a rest period, they then received either high-dose intravenous naloxone or saline. The allocation was randomized and administration of the study drug was double-blinded.
With naloxone or saline infusion ongoing, participants were then again subjected to the painful heat stimulus.
“All manipulations effectively reduced the respiration rate,” by 18%-21%, Dr. Zeidan said.
However, with the introduction of naloxone, both the slow-breathing group and the mindfulness group maintained reductions in pain unpleasantness, while those in the sham group had significant increases in pain unpleasantness. Reductions in pain unpleasantness ranged from 11% to 18% for these two groups, while the initial 8% reduction for the sham group climbed to a 13% increase in pain unpleasantness when this group received naloxone.
An unexpected finding was how effective slow breathing alone was as an analgesic. “There’s really something here,” said Dr. Zeidan, in reference to the analgesic effect of breath control. He explained that the slow breathing technique training was done with the aid of a device that emitted a blue glow that dimmed and brightened at the target respiratory rate.
Dr. Zeidan added that few participants were able to slow their breathing to 6 respirations per minute, but that the average rate did slow to about 12 from the normal 16 or so breaths per minute.
Dr. Zeidan reported no conflicts of interest. The National Institutes of Health funded the research.
MILWAUKEE – Mindfulness-based practices are effective in reducing pain perceptions, but a more easily taught breath control technique also showed efficacy in a recent study. Slow, rhythmic breathing alone, even without the additional attentional components of mindfulness, had significant analgesic effects in a human experimental model of pain.
“Slow breathing is much easier to perform” than mindfulness-based meditation, Fadel Zeidan, PhD, said at the scientific meeting of the American Pain Society. More research into the technique may offer a “clinically pragmatic” nonpharmacologic option for pain control, he said. And there may be some similarities between how the two techniques work: like mindfulness meditation, slow, rhythmic breathing’s analgesic properties are not dependent on the endogenous opioid system, said Dr. Zeidan, assistant professor of anesthesiology at the University of California, San Diego. His interests include mindfulness meditation–based pain relief.
In previous work, Dr. Zeidan and his collaborators had shown that the analgesic effect of mindfulness practices is not mediated by endogenous opioids. Participants in a study were trained in mindfulness meditation, and then exposed to a pain stimulus. Compared with a control group who listened to an audiobook rather than using mindfulness practices when exposed to pain, the meditators experienced a significant reduction in pain unpleasantness (J Neurosci. 16 March 2016;36[11]:3391-7).
In the experiment, both the meditation and the control group received first an intravenous saline solution, and then the opioid antagonist naloxone, which blocks endogenous opioids. When receiving naloxone, the meditators experienced reductions in the perceived unpleasantness of pain that were similar to what they experienced when they had received saline, showing that endogenous opioids weren’t responsible for meditation’s analgesic effects.
After verifying those findings, said Dr. Zeidan, he became interested in conducting a “graded analytical dissection of mindfulness,” to see exactly which components of the practice are nonopioidergic.
With mindfulness meditation, participants engage in slow, rhythmic breathing, and they learn about observation and appraisal practices, which can briefly be described as “the awareness of arising sensory events without reaction,” Dr. Zeidan said.
Mere belief in meditation in combination with the slow rhythmic breathing might have an analgesic effect, he said. In effect, this is sham mindfulness.
To try to tease out the contributions of each component of mindfulness meditation, Dr. Zeidan and his colleagues devised an experiment that trained participants in one of three ways. Over the course of four 20-minute sessions, randomized participants were trained in slow breathing techniques, with a goal respiratory rate of 6 breaths per minute; in mindfulness meditation techniques; or in a sham mindfulness technique that did not teach specific mindfulness principles.
The randomized participants were subject to a painful heat stimulus before the training to establish a baseline.
After training, they returned for two further sessions. At each visit, they experienced the noxious stimulus with no medication. After a rest period, they then received either high-dose intravenous naloxone or saline. The allocation was randomized and administration of the study drug was double-blinded.
With naloxone or saline infusion ongoing, participants were then again subjected to the painful heat stimulus.
“All manipulations effectively reduced the respiration rate,” by 18%-21%, Dr. Zeidan said.
However, with the introduction of naloxone, both the slow-breathing group and the mindfulness group maintained reductions in pain unpleasantness, while those in the sham group had significant increases in pain unpleasantness. Reductions in pain unpleasantness ranged from 11% to 18% for these two groups, while the initial 8% reduction for the sham group climbed to a 13% increase in pain unpleasantness when this group received naloxone.
An unexpected finding was how effective slow breathing alone was as an analgesic. “There’s really something here,” said Dr. Zeidan, in reference to the analgesic effect of breath control. He explained that the slow breathing technique training was done with the aid of a device that emitted a blue glow that dimmed and brightened at the target respiratory rate.
Dr. Zeidan added that few participants were able to slow their breathing to 6 respirations per minute, but that the average rate did slow to about 12 from the normal 16 or so breaths per minute.
Dr. Zeidan reported no conflicts of interest. The National Institutes of Health funded the research.
REPORTING FROM APS 2019