User login
AGA clinical practice update: Expert review on managing refractory gastroparesis
Gastroparesis can be tricky to diagnose and treat, in part because its symptoms can be difficult to distinguish from functional dyspepsia. A new clinical practice update from the American Gastroenterological Association aims to help physicians treat medically refractory gastroparesis with practical advice stemming from expert opinion and a literature review.
Although gastroparesis can be caused by known factors such as diabetes and medications, the largest group is idiopathic. The authors define medically refractory gastroparesis as symptoms that are not due to medication use, that continue despite dietary changes and first-line treatment with metoclopramide.
Although the authors outline several best practice advice statements on symptom identification and management, they acknowledge that much uncertainty still exists. “Our knowledge gap remains vast, and areas for future research include study of pathophysiology and etiology, as well as identification of clinical and investigation-based predictors of response to each management approach,” the authors wrote. Their report is in Clinical Gastroenterology and Hepatology.
They also call for research to identify gastroparesis phenotypes that are most likely to respond to individual management approaches.
Common gastroparesis symptoms include nausea, vomiting, early satiety, bloating, postprandial fullness, abdominal pain, and weight loss. Many of these overlap with functional dyspepsia (FD). In fact, one study found that 42% of gastroparesis could be reclassified as having functional dyspepsia, and 37% of FD patients as having gastroparesis.
About 5 million adults in the United States, and 7.2% of the world population, report gastroparesis-like symptoms. The similarities between the two groups poses a significant diagnostic challenge. However, a careful history, physical exam, and appropriate diagnostic tests should allow the physician to rule out other conditions that may mimic gastroparesis. Repeating scintigraphy may change diagnosis from gastroparesis to FD or vice versa, but the authors note that this technique is often performed incorrectly and so should be conducted at centers that closely follow guidelines. They suggest a 4 hour meal-based test of gastric emptying over the wireless motility capsule because it provides a better physiological assessment.
They also suggest that treatment should focus on the most bothersome symptom, along with reducing the potential for complications such as esophagitis, malnutrition, and weight loss, as well as improving quality of life.
There are medications available for nausea and vomiting, although most have not been studied in large randomized controlled trials. These agents include domperidone, 5-hydroxytryptamine3 receptor antagonists, neurokinin receptor antagonists, and phenothiazine antipsychotics.
There are also medications available to increase the rate of gastric emptying. Erythromycin can be used intravenously or orally ahead of meals, while the 5-HT4 receptor agonist velusetrag improved gastric emptying in healthy volunteers with no sign of cardiac side effects. The commonly available 5-HT4 agonist prucalopride has also shown promise in improving gastric emptying.
For visceral pain, the authors suggest not using opioids because they may slow gastric emptying and increase pain perception. It is believed that neuromodulators such as tricyclic antidepressants (TCAs) and serotonin norepinephrine reuptake inhibitors (SNRIs) may reduce perception of pain, but there is limited high-quality evidence available for these therapies. The authors suggest that higher potency tertiary tricyclic amines such as amitriptyline or imipramine may be effective, particularly in diabetic gastroparesis since they provide relief in FD.
Nonpharmaceutical options include gastric electrical stimulation (GES), which improves refractory nausea and vomiting in some patients with gastroparesis, but does not accelerate gastric emptying. It may also improve glycemic control, nutritional status, and quality of life. The treatment may be well suited to opioid-free patients with refractory or intractable nausea and vomiting whose predominant symptom is not abdominal pain.
Other therapies focus on the pylorus and its role in gastric emptying, which can be impaired as a result of abnormalities of pyloric tone and pressure. Functional lumen imaging probe (FLIP) can be used to probe pyloric tone and pressure, but it is expensive, invasive, and not widely available.
Outside of clinical trial settings, the authors advise against the use of intrapyloric botulinum toxic injection and transpyloric stent placement. Per oral endoscopic myotomy (POEM) has shown some efficacy at improving symptoms and reducing gastric emptying times, but it has not been studied in sham-controlled trials. The authors call the technique intriguing, but say it should not be considered a first-line therapy, and should be performed only at tertiary centers with expert motility specialists and endoscopists.
In extreme cases, enteral nutrition may be necessary, and a transjejunal tube or combined gastrojejunostomy tube should be emplaced beyond the pylorus. In a retrospective case series, patients experienced weight recovery with acceptable morbidity and mortality, and the implant was removed at an average of 20 months.
The authors have consulted or been on scientific advisory boards for Salix, Ironwood, Allergan, Arena, Allakos, Medtronic, Diversatek, Takeda, Quintiles, and IsoThrive.
This article was updated Feb. 17, 2022.
Gastroparesis can be tricky to diagnose and treat, in part because its symptoms can be difficult to distinguish from functional dyspepsia. A new clinical practice update from the American Gastroenterological Association aims to help physicians treat medically refractory gastroparesis with practical advice stemming from expert opinion and a literature review.
Although gastroparesis can be caused by known factors such as diabetes and medications, the largest group is idiopathic. The authors define medically refractory gastroparesis as symptoms that are not due to medication use, that continue despite dietary changes and first-line treatment with metoclopramide.
Although the authors outline several best practice advice statements on symptom identification and management, they acknowledge that much uncertainty still exists. “Our knowledge gap remains vast, and areas for future research include study of pathophysiology and etiology, as well as identification of clinical and investigation-based predictors of response to each management approach,” the authors wrote. Their report is in Clinical Gastroenterology and Hepatology.
They also call for research to identify gastroparesis phenotypes that are most likely to respond to individual management approaches.
Common gastroparesis symptoms include nausea, vomiting, early satiety, bloating, postprandial fullness, abdominal pain, and weight loss. Many of these overlap with functional dyspepsia (FD). In fact, one study found that 42% of gastroparesis could be reclassified as having functional dyspepsia, and 37% of FD patients as having gastroparesis.
About 5 million adults in the United States, and 7.2% of the world population, report gastroparesis-like symptoms. The similarities between the two groups poses a significant diagnostic challenge. However, a careful history, physical exam, and appropriate diagnostic tests should allow the physician to rule out other conditions that may mimic gastroparesis. Repeating scintigraphy may change diagnosis from gastroparesis to FD or vice versa, but the authors note that this technique is often performed incorrectly and so should be conducted at centers that closely follow guidelines. They suggest a 4 hour meal-based test of gastric emptying over the wireless motility capsule because it provides a better physiological assessment.
They also suggest that treatment should focus on the most bothersome symptom, along with reducing the potential for complications such as esophagitis, malnutrition, and weight loss, as well as improving quality of life.
There are medications available for nausea and vomiting, although most have not been studied in large randomized controlled trials. These agents include domperidone, 5-hydroxytryptamine3 receptor antagonists, neurokinin receptor antagonists, and phenothiazine antipsychotics.
There are also medications available to increase the rate of gastric emptying. Erythromycin can be used intravenously or orally ahead of meals, while the 5-HT4 receptor agonist velusetrag improved gastric emptying in healthy volunteers with no sign of cardiac side effects. The commonly available 5-HT4 agonist prucalopride has also shown promise in improving gastric emptying.
For visceral pain, the authors suggest not using opioids because they may slow gastric emptying and increase pain perception. It is believed that neuromodulators such as tricyclic antidepressants (TCAs) and serotonin norepinephrine reuptake inhibitors (SNRIs) may reduce perception of pain, but there is limited high-quality evidence available for these therapies. The authors suggest that higher potency tertiary tricyclic amines such as amitriptyline or imipramine may be effective, particularly in diabetic gastroparesis since they provide relief in FD.
Nonpharmaceutical options include gastric electrical stimulation (GES), which improves refractory nausea and vomiting in some patients with gastroparesis, but does not accelerate gastric emptying. It may also improve glycemic control, nutritional status, and quality of life. The treatment may be well suited to opioid-free patients with refractory or intractable nausea and vomiting whose predominant symptom is not abdominal pain.
Other therapies focus on the pylorus and its role in gastric emptying, which can be impaired as a result of abnormalities of pyloric tone and pressure. Functional lumen imaging probe (FLIP) can be used to probe pyloric tone and pressure, but it is expensive, invasive, and not widely available.
Outside of clinical trial settings, the authors advise against the use of intrapyloric botulinum toxic injection and transpyloric stent placement. Per oral endoscopic myotomy (POEM) has shown some efficacy at improving symptoms and reducing gastric emptying times, but it has not been studied in sham-controlled trials. The authors call the technique intriguing, but say it should not be considered a first-line therapy, and should be performed only at tertiary centers with expert motility specialists and endoscopists.
In extreme cases, enteral nutrition may be necessary, and a transjejunal tube or combined gastrojejunostomy tube should be emplaced beyond the pylorus. In a retrospective case series, patients experienced weight recovery with acceptable morbidity and mortality, and the implant was removed at an average of 20 months.
The authors have consulted or been on scientific advisory boards for Salix, Ironwood, Allergan, Arena, Allakos, Medtronic, Diversatek, Takeda, Quintiles, and IsoThrive.
This article was updated Feb. 17, 2022.
Gastroparesis can be tricky to diagnose and treat, in part because its symptoms can be difficult to distinguish from functional dyspepsia. A new clinical practice update from the American Gastroenterological Association aims to help physicians treat medically refractory gastroparesis with practical advice stemming from expert opinion and a literature review.
Although gastroparesis can be caused by known factors such as diabetes and medications, the largest group is idiopathic. The authors define medically refractory gastroparesis as symptoms that are not due to medication use, that continue despite dietary changes and first-line treatment with metoclopramide.
Although the authors outline several best practice advice statements on symptom identification and management, they acknowledge that much uncertainty still exists. “Our knowledge gap remains vast, and areas for future research include study of pathophysiology and etiology, as well as identification of clinical and investigation-based predictors of response to each management approach,” the authors wrote. Their report is in Clinical Gastroenterology and Hepatology.
They also call for research to identify gastroparesis phenotypes that are most likely to respond to individual management approaches.
Common gastroparesis symptoms include nausea, vomiting, early satiety, bloating, postprandial fullness, abdominal pain, and weight loss. Many of these overlap with functional dyspepsia (FD). In fact, one study found that 42% of gastroparesis could be reclassified as having functional dyspepsia, and 37% of FD patients as having gastroparesis.
About 5 million adults in the United States, and 7.2% of the world population, report gastroparesis-like symptoms. The similarities between the two groups poses a significant diagnostic challenge. However, a careful history, physical exam, and appropriate diagnostic tests should allow the physician to rule out other conditions that may mimic gastroparesis. Repeating scintigraphy may change diagnosis from gastroparesis to FD or vice versa, but the authors note that this technique is often performed incorrectly and so should be conducted at centers that closely follow guidelines. They suggest a 4 hour meal-based test of gastric emptying over the wireless motility capsule because it provides a better physiological assessment.
They also suggest that treatment should focus on the most bothersome symptom, along with reducing the potential for complications such as esophagitis, malnutrition, and weight loss, as well as improving quality of life.
There are medications available for nausea and vomiting, although most have not been studied in large randomized controlled trials. These agents include domperidone, 5-hydroxytryptamine3 receptor antagonists, neurokinin receptor antagonists, and phenothiazine antipsychotics.
There are also medications available to increase the rate of gastric emptying. Erythromycin can be used intravenously or orally ahead of meals, while the 5-HT4 receptor agonist velusetrag improved gastric emptying in healthy volunteers with no sign of cardiac side effects. The commonly available 5-HT4 agonist prucalopride has also shown promise in improving gastric emptying.
For visceral pain, the authors suggest not using opioids because they may slow gastric emptying and increase pain perception. It is believed that neuromodulators such as tricyclic antidepressants (TCAs) and serotonin norepinephrine reuptake inhibitors (SNRIs) may reduce perception of pain, but there is limited high-quality evidence available for these therapies. The authors suggest that higher potency tertiary tricyclic amines such as amitriptyline or imipramine may be effective, particularly in diabetic gastroparesis since they provide relief in FD.
Nonpharmaceutical options include gastric electrical stimulation (GES), which improves refractory nausea and vomiting in some patients with gastroparesis, but does not accelerate gastric emptying. It may also improve glycemic control, nutritional status, and quality of life. The treatment may be well suited to opioid-free patients with refractory or intractable nausea and vomiting whose predominant symptom is not abdominal pain.
Other therapies focus on the pylorus and its role in gastric emptying, which can be impaired as a result of abnormalities of pyloric tone and pressure. Functional lumen imaging probe (FLIP) can be used to probe pyloric tone and pressure, but it is expensive, invasive, and not widely available.
Outside of clinical trial settings, the authors advise against the use of intrapyloric botulinum toxic injection and transpyloric stent placement. Per oral endoscopic myotomy (POEM) has shown some efficacy at improving symptoms and reducing gastric emptying times, but it has not been studied in sham-controlled trials. The authors call the technique intriguing, but say it should not be considered a first-line therapy, and should be performed only at tertiary centers with expert motility specialists and endoscopists.
In extreme cases, enteral nutrition may be necessary, and a transjejunal tube or combined gastrojejunostomy tube should be emplaced beyond the pylorus. In a retrospective case series, patients experienced weight recovery with acceptable morbidity and mortality, and the implant was removed at an average of 20 months.
The authors have consulted or been on scientific advisory boards for Salix, Ironwood, Allergan, Arena, Allakos, Medtronic, Diversatek, Takeda, Quintiles, and IsoThrive.
This article was updated Feb. 17, 2022.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Novel treatment shows early promise for gastric cancer
Chinese researchers reporting at the 2022 Gastrointestinal Cancers Symposium shared new results from a phase 1b/2 study showing that combination treatment with the antibody AK104 shows promise for patients with gastric and gastroesophageal junction cancer.
“AK-104 plus chemo presents a potential new first line treatment option for these patients,” said Jiafu Ji, MD, PhD, of Peking University Cancer Hospital and Institute Gastrointestinal Cancer Center, Beijing.
AK104 is a PD-1/CTLA4 antibody manufactured by Akeso Biopharma, which in 2020 received fast-track designation for the drug’s use as monotherapy for patients with recurrent or chemotherapy-resistant metastatic squamous cervical cancer.
The new trial was a multicenter, open-label study that combined chemotherapy (XELOX – capecitabine combined with oxaliplatin) with AK104 for use as first-line therapy for patients with gastric and gastroesophageal junction cancer.
Two previous studies showed that combination treatment with an anti–PD-1 and anti-CTLA4 AK104 produced a higher response rate and better long-term overall survival than anti–PD-1 therapy alone, but at a cost of greater toxicity.
“The toxicity can be really significant with the combination ... you see some severe immune related events. So with the bispecific antibody, the hope is that we can minimize that additive toxicity by bringing the CTLA4 inhibitor to antigen-experienced PD-1–positive T cells, and hopefully enhance the effect of blocking CTLA4 at the tumor-immune interface, rather than nonspecifically,” said Katherine Bever, MD, who was asked to comment on the study. Dr. Bever is an assistant professor of oncology at Johns Hopkins University, Baltimore, and moderated the panel where the study was presented.
The new results are encouraging, Dr. Bever said. “ to show exactly what the contribution of the bispecific antibody is to the chemotherapy that it’s being combined with, and how that compares to combination with anti–PD-1 alone, and also to understand more about what they saw in terms of immune toxicities.”
Dr. Bever said the combination of chemotherapy and the PD-1 inhibitor nivolumab is now a first-line treatment for gastric and gastroesophageal junction cancer based on results from the CheckMate 649 study, but relatively few patients appear to benefit from the PD-1 inhibitor compared to chemotherapy alone.
“I think there’s the potential that by incorporating PD-1 and CTLA4 targeting in the first line, we might further improve outcomes for these patients, but you need randomized data to show that,” she said.
Dr. Ji said that there is an ongoing phase 3 study of AK104 in combination with chemotherapy for first-line treatment of gastric or gastroesophageal junction cancer.
The phase 1b/2 clinical trial included 96 patients (median age, 62.7 years; 70.8% male) who were treated with AK-104 every 2-3 weeks, plus XELOX (capecitabine plus oxaliplatin) or modified XELOX.
After a median follow-up of 9.95 months the overall response rate was 65.9% (2.3% complete, 63.6% partial). The disease control rate was 92.0%. The median duration of response was 6.93 months, the median progression-free survival was 7.10 months, and median overall survival was 17.41 months. Treatment-related adverse events included reductions in platelet count (60.4%), white blood cells (58.3%), and neutrophils (56.3%), anemia (47.9%), nausea (30.2%), vomiting (30.2%), and increase in AST (30.2%); 62.5% had at least one grade 3 or higher TRAE.
The study was funded by Akeso Biopharma. Dr. Ji and Dr. Bever have no relevant financial disclosures.
Chinese researchers reporting at the 2022 Gastrointestinal Cancers Symposium shared new results from a phase 1b/2 study showing that combination treatment with the antibody AK104 shows promise for patients with gastric and gastroesophageal junction cancer.
“AK-104 plus chemo presents a potential new first line treatment option for these patients,” said Jiafu Ji, MD, PhD, of Peking University Cancer Hospital and Institute Gastrointestinal Cancer Center, Beijing.
AK104 is a PD-1/CTLA4 antibody manufactured by Akeso Biopharma, which in 2020 received fast-track designation for the drug’s use as monotherapy for patients with recurrent or chemotherapy-resistant metastatic squamous cervical cancer.
The new trial was a multicenter, open-label study that combined chemotherapy (XELOX – capecitabine combined with oxaliplatin) with AK104 for use as first-line therapy for patients with gastric and gastroesophageal junction cancer.
Two previous studies showed that combination treatment with an anti–PD-1 and anti-CTLA4 AK104 produced a higher response rate and better long-term overall survival than anti–PD-1 therapy alone, but at a cost of greater toxicity.
“The toxicity can be really significant with the combination ... you see some severe immune related events. So with the bispecific antibody, the hope is that we can minimize that additive toxicity by bringing the CTLA4 inhibitor to antigen-experienced PD-1–positive T cells, and hopefully enhance the effect of blocking CTLA4 at the tumor-immune interface, rather than nonspecifically,” said Katherine Bever, MD, who was asked to comment on the study. Dr. Bever is an assistant professor of oncology at Johns Hopkins University, Baltimore, and moderated the panel where the study was presented.
The new results are encouraging, Dr. Bever said. “ to show exactly what the contribution of the bispecific antibody is to the chemotherapy that it’s being combined with, and how that compares to combination with anti–PD-1 alone, and also to understand more about what they saw in terms of immune toxicities.”
Dr. Bever said the combination of chemotherapy and the PD-1 inhibitor nivolumab is now a first-line treatment for gastric and gastroesophageal junction cancer based on results from the CheckMate 649 study, but relatively few patients appear to benefit from the PD-1 inhibitor compared to chemotherapy alone.
“I think there’s the potential that by incorporating PD-1 and CTLA4 targeting in the first line, we might further improve outcomes for these patients, but you need randomized data to show that,” she said.
Dr. Ji said that there is an ongoing phase 3 study of AK104 in combination with chemotherapy for first-line treatment of gastric or gastroesophageal junction cancer.
The phase 1b/2 clinical trial included 96 patients (median age, 62.7 years; 70.8% male) who were treated with AK-104 every 2-3 weeks, plus XELOX (capecitabine plus oxaliplatin) or modified XELOX.
After a median follow-up of 9.95 months the overall response rate was 65.9% (2.3% complete, 63.6% partial). The disease control rate was 92.0%. The median duration of response was 6.93 months, the median progression-free survival was 7.10 months, and median overall survival was 17.41 months. Treatment-related adverse events included reductions in platelet count (60.4%), white blood cells (58.3%), and neutrophils (56.3%), anemia (47.9%), nausea (30.2%), vomiting (30.2%), and increase in AST (30.2%); 62.5% had at least one grade 3 or higher TRAE.
The study was funded by Akeso Biopharma. Dr. Ji and Dr. Bever have no relevant financial disclosures.
Chinese researchers reporting at the 2022 Gastrointestinal Cancers Symposium shared new results from a phase 1b/2 study showing that combination treatment with the antibody AK104 shows promise for patients with gastric and gastroesophageal junction cancer.
“AK-104 plus chemo presents a potential new first line treatment option for these patients,” said Jiafu Ji, MD, PhD, of Peking University Cancer Hospital and Institute Gastrointestinal Cancer Center, Beijing.
AK104 is a PD-1/CTLA4 antibody manufactured by Akeso Biopharma, which in 2020 received fast-track designation for the drug’s use as monotherapy for patients with recurrent or chemotherapy-resistant metastatic squamous cervical cancer.
The new trial was a multicenter, open-label study that combined chemotherapy (XELOX – capecitabine combined with oxaliplatin) with AK104 for use as first-line therapy for patients with gastric and gastroesophageal junction cancer.
Two previous studies showed that combination treatment with an anti–PD-1 and anti-CTLA4 AK104 produced a higher response rate and better long-term overall survival than anti–PD-1 therapy alone, but at a cost of greater toxicity.
“The toxicity can be really significant with the combination ... you see some severe immune related events. So with the bispecific antibody, the hope is that we can minimize that additive toxicity by bringing the CTLA4 inhibitor to antigen-experienced PD-1–positive T cells, and hopefully enhance the effect of blocking CTLA4 at the tumor-immune interface, rather than nonspecifically,” said Katherine Bever, MD, who was asked to comment on the study. Dr. Bever is an assistant professor of oncology at Johns Hopkins University, Baltimore, and moderated the panel where the study was presented.
The new results are encouraging, Dr. Bever said. “ to show exactly what the contribution of the bispecific antibody is to the chemotherapy that it’s being combined with, and how that compares to combination with anti–PD-1 alone, and also to understand more about what they saw in terms of immune toxicities.”
Dr. Bever said the combination of chemotherapy and the PD-1 inhibitor nivolumab is now a first-line treatment for gastric and gastroesophageal junction cancer based on results from the CheckMate 649 study, but relatively few patients appear to benefit from the PD-1 inhibitor compared to chemotherapy alone.
“I think there’s the potential that by incorporating PD-1 and CTLA4 targeting in the first line, we might further improve outcomes for these patients, but you need randomized data to show that,” she said.
Dr. Ji said that there is an ongoing phase 3 study of AK104 in combination with chemotherapy for first-line treatment of gastric or gastroesophageal junction cancer.
The phase 1b/2 clinical trial included 96 patients (median age, 62.7 years; 70.8% male) who were treated with AK-104 every 2-3 weeks, plus XELOX (capecitabine plus oxaliplatin) or modified XELOX.
After a median follow-up of 9.95 months the overall response rate was 65.9% (2.3% complete, 63.6% partial). The disease control rate was 92.0%. The median duration of response was 6.93 months, the median progression-free survival was 7.10 months, and median overall survival was 17.41 months. Treatment-related adverse events included reductions in platelet count (60.4%), white blood cells (58.3%), and neutrophils (56.3%), anemia (47.9%), nausea (30.2%), vomiting (30.2%), and increase in AST (30.2%); 62.5% had at least one grade 3 or higher TRAE.
The study was funded by Akeso Biopharma. Dr. Ji and Dr. Bever have no relevant financial disclosures.
FROM GI CANCERS SYMPOSIUM 2022
Oxaliplatin add-on not recommended for elderly with colon cancer
A Japanese study finds that the addition of oxaliplatin (Eloxatin, Sanofi-Aventis) to fluoropyrimidine with bevacizumab, does not extend progression-free survival in elderly patients with metastatic colorectal cancer and in fact, can lead to more severe adverse events.
The results of the trial were presented by Tetsuya Hamaguchi, MD, PhD, at the 2022 Gastrointestinal Cancers Symposium.
Fluoropyrimidine with oxaliplatin and bevacizumab is a standard intensive initial therapy for metastatic colorectal cancer, but how well this combination works in elderly patients isn’t known because few clinical trials include senior-aged patients. It is known that the combination of fluoropyrimidine and bevacizumab can lead to significantly longer progression-free survival in elderly patients with metastatic colorectal cancer.
Dr. Hamaguchi, an oncologist with Saitama Medical University International Medical Center in Hidaka, Japan, included 251 patients (93% were at least 75 years old) with newly diagnosed metastatic colorectal cancer in a randomized, phase 3 trial of modified FOLFOX7 (folinic acid, fluorouracil, and oxaliplatin) or CapeOX (capecitabine and oxaliplatin) plus bevacizumab versus 5-fluorouracil/l-LV or capecitabine plus bevacizumab.
The patients were treated between September 2012 and March 2019. 125 patients received combination therapy and 126 received the addition of oxaliplatin. Researchers found the addition of oxaliplatin to fluoropyrimidine with bevacizumab did not extend progression-free survival and it was associated with more frequent and severe adverse events, including neutropenia, nausea, stomatitis, diarrhea, fatigue, sensory neuropathy, and hypertension.
Triggering sensory neuropathy
At issue is neuropathy caused by oxaliplatin. Grade 3 neuropathy can occur in 25% of patients after 12 cycles of FOLFOX. It has also been recorded in more than 20% of patients at 18 months, according to Gabriel Brooks, MD, an oncologist with the Dartmouth-Hitchcock Norris Cotton Cancer Center, Lebanon, N.H., who served as the discussant for the oral abstract session featuring the study presented by Dr. Hamaguchi.
“Even if we carefully ask our patients about their symptoms, and even if we stop oxaliplatin as soon as they have evidence of grade 2 neuropathy, many of those patients who didn’t have any neuropathy when the oxaliplatin is stopped will go on to develop it. And many of those patients who have grade 2 neuropathy will have worsening of their neuropathy and it will become a grade 3 neuropathy, even if we try to stop conscientiously,” Dr. Brooks said during his presentation.
The MOSAIC trial demonstrated that oxaliplatin improved disease-free and overall survival when added to 5-fluorouracil in the adjuvant treatment of stage II/III colon cancer. However, neuropathy concerns led to studies examining shortened regimens in an effort to reduce neuropathy incidence. In 2018, the IDEA study compared 3 months versus 6 months of oxaliplatin with fluoropyrimidine as adjuvant therapy for stage III colon cancer. The study found that the 6-month regimen was superior with respect to disease-free survival, but the benefit was very small, according to Dr. Brooks.
“What was not small was the difference in the incidence of [grade] 3-4 neuropathy, which was only 2.5% among patients who received 3 months of adjuvant chemotherapy with oxaliplatin, and it was 15.9% among people randomized to 6 months of adjuvant chemotherapy,” he said. The same study suggested that 3 months of the CapeOX regimen is noninferior to 6 months.
The new study included patients aged 70-74 years with Performance Status 2, or 75 or older with PS 0-2. They were randomized to receive fluoropyrimidine plus bevacizumab (arm A) or fluoropyrimidine, bevacizumab, and oxaliplatin (arm B). The study had to be terminated because of poor accrual, and the study was later amended to include a smaller required sample size. After a recruitment of 251 patients and a 2-year minimum follow-up, there was no significant difference in progression-free survival between the two groups (hazard ratio, 0.837; one-sided P = .086), overall survival, or objective response rate.
Adverse events were more common in the oxaliplatin group, including neutropenia (24% vs. 15%), nausea (22% vs. 10%), diarrhea (16% vs. 7%), fatigue (32% vs. 21%), and sensory neuropathy (57% vs. 15%).
Subgroup analyses did identify a subgroup of patients with KRAS wild type benefited from oxaliplatin (HR, 0.578; 95% confidence interval, 0.380-0.879), and Dr. Brooks said that this echoes findings from other studies.
Dr. Brooks advocated for deintensification of oxaliplatin. “The benefits of oxaliplatin are less than are often assumed in multiple settings, and the harms of oxaliplatin are well described. We are probably overly aggressive in our use of oxaliplatin. I submit that we should use oxaliplatin cautiously and sparingly in a couple of situations: We should use it very cautiously and sparingly beyond the third month of adjuvant therapy for colon cancer, and we should use it cautiously and sparingly in elderly or frail patients with metastatic colorectal cancer.”
The study was funded, in part, by the National Cancer Center Research and Development Fund, Grant-in-Aid for Clinical Cancer Research, and the Agency for Medical Research and Development in Japan. Dr. Brooks has consulted for CareCentrix, Ipsen, and UnitedHealthcare and has received research funding from Boston Biomedical and Roche/Genentech. Dr. Hamaguchi has received honoraria from Bayer, Bristol-Myers Squibb Japan, and other pharmaceutical companies. The Gastrointestinal Cancers Symposium is sponsored by the American Gastroenterological Association, the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
A Japanese study finds that the addition of oxaliplatin (Eloxatin, Sanofi-Aventis) to fluoropyrimidine with bevacizumab, does not extend progression-free survival in elderly patients with metastatic colorectal cancer and in fact, can lead to more severe adverse events.
The results of the trial were presented by Tetsuya Hamaguchi, MD, PhD, at the 2022 Gastrointestinal Cancers Symposium.
Fluoropyrimidine with oxaliplatin and bevacizumab is a standard intensive initial therapy for metastatic colorectal cancer, but how well this combination works in elderly patients isn’t known because few clinical trials include senior-aged patients. It is known that the combination of fluoropyrimidine and bevacizumab can lead to significantly longer progression-free survival in elderly patients with metastatic colorectal cancer.
Dr. Hamaguchi, an oncologist with Saitama Medical University International Medical Center in Hidaka, Japan, included 251 patients (93% were at least 75 years old) with newly diagnosed metastatic colorectal cancer in a randomized, phase 3 trial of modified FOLFOX7 (folinic acid, fluorouracil, and oxaliplatin) or CapeOX (capecitabine and oxaliplatin) plus bevacizumab versus 5-fluorouracil/l-LV or capecitabine plus bevacizumab.
The patients were treated between September 2012 and March 2019. 125 patients received combination therapy and 126 received the addition of oxaliplatin. Researchers found the addition of oxaliplatin to fluoropyrimidine with bevacizumab did not extend progression-free survival and it was associated with more frequent and severe adverse events, including neutropenia, nausea, stomatitis, diarrhea, fatigue, sensory neuropathy, and hypertension.
Triggering sensory neuropathy
At issue is neuropathy caused by oxaliplatin. Grade 3 neuropathy can occur in 25% of patients after 12 cycles of FOLFOX. It has also been recorded in more than 20% of patients at 18 months, according to Gabriel Brooks, MD, an oncologist with the Dartmouth-Hitchcock Norris Cotton Cancer Center, Lebanon, N.H., who served as the discussant for the oral abstract session featuring the study presented by Dr. Hamaguchi.
“Even if we carefully ask our patients about their symptoms, and even if we stop oxaliplatin as soon as they have evidence of grade 2 neuropathy, many of those patients who didn’t have any neuropathy when the oxaliplatin is stopped will go on to develop it. And many of those patients who have grade 2 neuropathy will have worsening of their neuropathy and it will become a grade 3 neuropathy, even if we try to stop conscientiously,” Dr. Brooks said during his presentation.
The MOSAIC trial demonstrated that oxaliplatin improved disease-free and overall survival when added to 5-fluorouracil in the adjuvant treatment of stage II/III colon cancer. However, neuropathy concerns led to studies examining shortened regimens in an effort to reduce neuropathy incidence. In 2018, the IDEA study compared 3 months versus 6 months of oxaliplatin with fluoropyrimidine as adjuvant therapy for stage III colon cancer. The study found that the 6-month regimen was superior with respect to disease-free survival, but the benefit was very small, according to Dr. Brooks.
“What was not small was the difference in the incidence of [grade] 3-4 neuropathy, which was only 2.5% among patients who received 3 months of adjuvant chemotherapy with oxaliplatin, and it was 15.9% among people randomized to 6 months of adjuvant chemotherapy,” he said. The same study suggested that 3 months of the CapeOX regimen is noninferior to 6 months.
The new study included patients aged 70-74 years with Performance Status 2, or 75 or older with PS 0-2. They were randomized to receive fluoropyrimidine plus bevacizumab (arm A) or fluoropyrimidine, bevacizumab, and oxaliplatin (arm B). The study had to be terminated because of poor accrual, and the study was later amended to include a smaller required sample size. After a recruitment of 251 patients and a 2-year minimum follow-up, there was no significant difference in progression-free survival between the two groups (hazard ratio, 0.837; one-sided P = .086), overall survival, or objective response rate.
Adverse events were more common in the oxaliplatin group, including neutropenia (24% vs. 15%), nausea (22% vs. 10%), diarrhea (16% vs. 7%), fatigue (32% vs. 21%), and sensory neuropathy (57% vs. 15%).
Subgroup analyses did identify a subgroup of patients with KRAS wild type benefited from oxaliplatin (HR, 0.578; 95% confidence interval, 0.380-0.879), and Dr. Brooks said that this echoes findings from other studies.
Dr. Brooks advocated for deintensification of oxaliplatin. “The benefits of oxaliplatin are less than are often assumed in multiple settings, and the harms of oxaliplatin are well described. We are probably overly aggressive in our use of oxaliplatin. I submit that we should use oxaliplatin cautiously and sparingly in a couple of situations: We should use it very cautiously and sparingly beyond the third month of adjuvant therapy for colon cancer, and we should use it cautiously and sparingly in elderly or frail patients with metastatic colorectal cancer.”
The study was funded, in part, by the National Cancer Center Research and Development Fund, Grant-in-Aid for Clinical Cancer Research, and the Agency for Medical Research and Development in Japan. Dr. Brooks has consulted for CareCentrix, Ipsen, and UnitedHealthcare and has received research funding from Boston Biomedical and Roche/Genentech. Dr. Hamaguchi has received honoraria from Bayer, Bristol-Myers Squibb Japan, and other pharmaceutical companies. The Gastrointestinal Cancers Symposium is sponsored by the American Gastroenterological Association, the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
A Japanese study finds that the addition of oxaliplatin (Eloxatin, Sanofi-Aventis) to fluoropyrimidine with bevacizumab, does not extend progression-free survival in elderly patients with metastatic colorectal cancer and in fact, can lead to more severe adverse events.
The results of the trial were presented by Tetsuya Hamaguchi, MD, PhD, at the 2022 Gastrointestinal Cancers Symposium.
Fluoropyrimidine with oxaliplatin and bevacizumab is a standard intensive initial therapy for metastatic colorectal cancer, but how well this combination works in elderly patients isn’t known because few clinical trials include senior-aged patients. It is known that the combination of fluoropyrimidine and bevacizumab can lead to significantly longer progression-free survival in elderly patients with metastatic colorectal cancer.
Dr. Hamaguchi, an oncologist with Saitama Medical University International Medical Center in Hidaka, Japan, included 251 patients (93% were at least 75 years old) with newly diagnosed metastatic colorectal cancer in a randomized, phase 3 trial of modified FOLFOX7 (folinic acid, fluorouracil, and oxaliplatin) or CapeOX (capecitabine and oxaliplatin) plus bevacizumab versus 5-fluorouracil/l-LV or capecitabine plus bevacizumab.
The patients were treated between September 2012 and March 2019. 125 patients received combination therapy and 126 received the addition of oxaliplatin. Researchers found the addition of oxaliplatin to fluoropyrimidine with bevacizumab did not extend progression-free survival and it was associated with more frequent and severe adverse events, including neutropenia, nausea, stomatitis, diarrhea, fatigue, sensory neuropathy, and hypertension.
Triggering sensory neuropathy
At issue is neuropathy caused by oxaliplatin. Grade 3 neuropathy can occur in 25% of patients after 12 cycles of FOLFOX. It has also been recorded in more than 20% of patients at 18 months, according to Gabriel Brooks, MD, an oncologist with the Dartmouth-Hitchcock Norris Cotton Cancer Center, Lebanon, N.H., who served as the discussant for the oral abstract session featuring the study presented by Dr. Hamaguchi.
“Even if we carefully ask our patients about their symptoms, and even if we stop oxaliplatin as soon as they have evidence of grade 2 neuropathy, many of those patients who didn’t have any neuropathy when the oxaliplatin is stopped will go on to develop it. And many of those patients who have grade 2 neuropathy will have worsening of their neuropathy and it will become a grade 3 neuropathy, even if we try to stop conscientiously,” Dr. Brooks said during his presentation.
The MOSAIC trial demonstrated that oxaliplatin improved disease-free and overall survival when added to 5-fluorouracil in the adjuvant treatment of stage II/III colon cancer. However, neuropathy concerns led to studies examining shortened regimens in an effort to reduce neuropathy incidence. In 2018, the IDEA study compared 3 months versus 6 months of oxaliplatin with fluoropyrimidine as adjuvant therapy for stage III colon cancer. The study found that the 6-month regimen was superior with respect to disease-free survival, but the benefit was very small, according to Dr. Brooks.
“What was not small was the difference in the incidence of [grade] 3-4 neuropathy, which was only 2.5% among patients who received 3 months of adjuvant chemotherapy with oxaliplatin, and it was 15.9% among people randomized to 6 months of adjuvant chemotherapy,” he said. The same study suggested that 3 months of the CapeOX regimen is noninferior to 6 months.
The new study included patients aged 70-74 years with Performance Status 2, or 75 or older with PS 0-2. They were randomized to receive fluoropyrimidine plus bevacizumab (arm A) or fluoropyrimidine, bevacizumab, and oxaliplatin (arm B). The study had to be terminated because of poor accrual, and the study was later amended to include a smaller required sample size. After a recruitment of 251 patients and a 2-year minimum follow-up, there was no significant difference in progression-free survival between the two groups (hazard ratio, 0.837; one-sided P = .086), overall survival, or objective response rate.
Adverse events were more common in the oxaliplatin group, including neutropenia (24% vs. 15%), nausea (22% vs. 10%), diarrhea (16% vs. 7%), fatigue (32% vs. 21%), and sensory neuropathy (57% vs. 15%).
Subgroup analyses did identify a subgroup of patients with KRAS wild type benefited from oxaliplatin (HR, 0.578; 95% confidence interval, 0.380-0.879), and Dr. Brooks said that this echoes findings from other studies.
Dr. Brooks advocated for deintensification of oxaliplatin. “The benefits of oxaliplatin are less than are often assumed in multiple settings, and the harms of oxaliplatin are well described. We are probably overly aggressive in our use of oxaliplatin. I submit that we should use oxaliplatin cautiously and sparingly in a couple of situations: We should use it very cautiously and sparingly beyond the third month of adjuvant therapy for colon cancer, and we should use it cautiously and sparingly in elderly or frail patients with metastatic colorectal cancer.”
The study was funded, in part, by the National Cancer Center Research and Development Fund, Grant-in-Aid for Clinical Cancer Research, and the Agency for Medical Research and Development in Japan. Dr. Brooks has consulted for CareCentrix, Ipsen, and UnitedHealthcare and has received research funding from Boston Biomedical and Roche/Genentech. Dr. Hamaguchi has received honoraria from Bayer, Bristol-Myers Squibb Japan, and other pharmaceutical companies. The Gastrointestinal Cancers Symposium is sponsored by the American Gastroenterological Association, the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
FROM GI CANCERS SYMPOSIUM 2022
Abraxane still in short supply for cancer patients
forcing physicians to find alternatives for a drug once lauded for being easier to tolerate.
Abraxane (Bristol-Myers Squibb) is a paclitaxel albumin-bound injectable. It is different from alternative chemotherapy treatments like Taxol (paclitaxel) because it doesn’t use the solvents that can make Taxol difficult to tolerate. It was described as a “next-generation taxane” because it didn’t rely on solvents. It was approved in 2005 for metastatic breast cancer, then in 2012 for advanced non–small cell lung cancer, in 2013 for late-stage pancreatic cancer and in 2019 for people with PD-L1–positive metastatic triple-negative breast cancer.
The shortage, which was announced on Oct. 5, 2021, by the Food and Drug Administration, has led to some difficult decisions for patients and physicians. How long the shortage will last isn’t clear.
“I printed out [an] allotment sheet 2 days ago, and all it says [for Abraxane] is allocated,” said Kathy Oubre, MS, CEO of Pontchartrain Cancer Center, Hammond, La. “Everyone is keeping what they’ve got for their own patients, so there really isn’t anything available.”
The Pontchartrain Cancer Center sent two patients to the University of Texas MD Anderson Cancer Center, Houston, for continued treatment with Abraxane, but that option is costly and time consuming for patients. The two patients had the means to travel, but Ms. Oubre said that many others cannot afford to travel for treatment. “Everyone has patients who are living paycheck to paycheck who certainly couldn’t afford to do that. There are going to be patients across the nation that are not going to be able to have care as a result of these things.”
The supply problems are causing difficult decisions for physicians, who may have to switch a patient from an unavailable drug to an alternative that isn’t as effective, Ms. Oubre said. “I can’t imagine the stress and the sadness that the physicians have to feel when they have to go explain that to a patient. That runs counter to everything they are as physicians.”
Other strategies include chemo holidays and rounding down doses in patients with metastatic cancer, according to Camille Hill, PharmD, vice president of oncology pharmacy services, West Cancer Center, Germantown, Tenn.
Shortages and allocations are growing at an alarming rate, Ms. Oubre said. In her 15 years of working in the industry, “I don’t recall it ever being this challenging.” During a Zoom interview, she held up a lengthy list of drugs on allocation or unavailable that her pharmacy group purchasing organization sent her the previous week. “I don’t ever recall getting this kind of list. Every 3 days, I’m getting this. If it were just that one product, I can live with that. We figure it out. But it’s bigger than that.”
Worker shortages are exacerbating the issue. Ms. Oubre received a letter from a drug company describing its employee issues, which included chemists, plant workers, and loading dock staff. On top of that, delivery companies are experiencing staff shortages, which can result in more delays and complicate matters further. “It’s just compounding. These things can get really difficult very quickly. I don’t want to say we’re in crisis, and we’re not rationing care. We’re not in those buckets yet. But I would say that if these things don’t get better, it’s the first time in my work career that we are having those conversations of: ‘How we are going to plan for that it does come to that?’ ” she said.
“In general, with the pandemic, we have seen all sorts of just disruptions to the supply chain. So, I think you just do your best, you find alternatives for those patients that you can, and you come up with strategies. I don’t know that for Abraxane, or any other product, that I’d be particularly confident that we may not see another shortage,” Dr. Hill said.
forcing physicians to find alternatives for a drug once lauded for being easier to tolerate.
Abraxane (Bristol-Myers Squibb) is a paclitaxel albumin-bound injectable. It is different from alternative chemotherapy treatments like Taxol (paclitaxel) because it doesn’t use the solvents that can make Taxol difficult to tolerate. It was described as a “next-generation taxane” because it didn’t rely on solvents. It was approved in 2005 for metastatic breast cancer, then in 2012 for advanced non–small cell lung cancer, in 2013 for late-stage pancreatic cancer and in 2019 for people with PD-L1–positive metastatic triple-negative breast cancer.
The shortage, which was announced on Oct. 5, 2021, by the Food and Drug Administration, has led to some difficult decisions for patients and physicians. How long the shortage will last isn’t clear.
“I printed out [an] allotment sheet 2 days ago, and all it says [for Abraxane] is allocated,” said Kathy Oubre, MS, CEO of Pontchartrain Cancer Center, Hammond, La. “Everyone is keeping what they’ve got for their own patients, so there really isn’t anything available.”
The Pontchartrain Cancer Center sent two patients to the University of Texas MD Anderson Cancer Center, Houston, for continued treatment with Abraxane, but that option is costly and time consuming for patients. The two patients had the means to travel, but Ms. Oubre said that many others cannot afford to travel for treatment. “Everyone has patients who are living paycheck to paycheck who certainly couldn’t afford to do that. There are going to be patients across the nation that are not going to be able to have care as a result of these things.”
The supply problems are causing difficult decisions for physicians, who may have to switch a patient from an unavailable drug to an alternative that isn’t as effective, Ms. Oubre said. “I can’t imagine the stress and the sadness that the physicians have to feel when they have to go explain that to a patient. That runs counter to everything they are as physicians.”
Other strategies include chemo holidays and rounding down doses in patients with metastatic cancer, according to Camille Hill, PharmD, vice president of oncology pharmacy services, West Cancer Center, Germantown, Tenn.
Shortages and allocations are growing at an alarming rate, Ms. Oubre said. In her 15 years of working in the industry, “I don’t recall it ever being this challenging.” During a Zoom interview, she held up a lengthy list of drugs on allocation or unavailable that her pharmacy group purchasing organization sent her the previous week. “I don’t ever recall getting this kind of list. Every 3 days, I’m getting this. If it were just that one product, I can live with that. We figure it out. But it’s bigger than that.”
Worker shortages are exacerbating the issue. Ms. Oubre received a letter from a drug company describing its employee issues, which included chemists, plant workers, and loading dock staff. On top of that, delivery companies are experiencing staff shortages, which can result in more delays and complicate matters further. “It’s just compounding. These things can get really difficult very quickly. I don’t want to say we’re in crisis, and we’re not rationing care. We’re not in those buckets yet. But I would say that if these things don’t get better, it’s the first time in my work career that we are having those conversations of: ‘How we are going to plan for that it does come to that?’ ” she said.
“In general, with the pandemic, we have seen all sorts of just disruptions to the supply chain. So, I think you just do your best, you find alternatives for those patients that you can, and you come up with strategies. I don’t know that for Abraxane, or any other product, that I’d be particularly confident that we may not see another shortage,” Dr. Hill said.
forcing physicians to find alternatives for a drug once lauded for being easier to tolerate.
Abraxane (Bristol-Myers Squibb) is a paclitaxel albumin-bound injectable. It is different from alternative chemotherapy treatments like Taxol (paclitaxel) because it doesn’t use the solvents that can make Taxol difficult to tolerate. It was described as a “next-generation taxane” because it didn’t rely on solvents. It was approved in 2005 for metastatic breast cancer, then in 2012 for advanced non–small cell lung cancer, in 2013 for late-stage pancreatic cancer and in 2019 for people with PD-L1–positive metastatic triple-negative breast cancer.
The shortage, which was announced on Oct. 5, 2021, by the Food and Drug Administration, has led to some difficult decisions for patients and physicians. How long the shortage will last isn’t clear.
“I printed out [an] allotment sheet 2 days ago, and all it says [for Abraxane] is allocated,” said Kathy Oubre, MS, CEO of Pontchartrain Cancer Center, Hammond, La. “Everyone is keeping what they’ve got for their own patients, so there really isn’t anything available.”
The Pontchartrain Cancer Center sent two patients to the University of Texas MD Anderson Cancer Center, Houston, for continued treatment with Abraxane, but that option is costly and time consuming for patients. The two patients had the means to travel, but Ms. Oubre said that many others cannot afford to travel for treatment. “Everyone has patients who are living paycheck to paycheck who certainly couldn’t afford to do that. There are going to be patients across the nation that are not going to be able to have care as a result of these things.”
The supply problems are causing difficult decisions for physicians, who may have to switch a patient from an unavailable drug to an alternative that isn’t as effective, Ms. Oubre said. “I can’t imagine the stress and the sadness that the physicians have to feel when they have to go explain that to a patient. That runs counter to everything they are as physicians.”
Other strategies include chemo holidays and rounding down doses in patients with metastatic cancer, according to Camille Hill, PharmD, vice president of oncology pharmacy services, West Cancer Center, Germantown, Tenn.
Shortages and allocations are growing at an alarming rate, Ms. Oubre said. In her 15 years of working in the industry, “I don’t recall it ever being this challenging.” During a Zoom interview, she held up a lengthy list of drugs on allocation or unavailable that her pharmacy group purchasing organization sent her the previous week. “I don’t ever recall getting this kind of list. Every 3 days, I’m getting this. If it were just that one product, I can live with that. We figure it out. But it’s bigger than that.”
Worker shortages are exacerbating the issue. Ms. Oubre received a letter from a drug company describing its employee issues, which included chemists, plant workers, and loading dock staff. On top of that, delivery companies are experiencing staff shortages, which can result in more delays and complicate matters further. “It’s just compounding. These things can get really difficult very quickly. I don’t want to say we’re in crisis, and we’re not rationing care. We’re not in those buckets yet. But I would say that if these things don’t get better, it’s the first time in my work career that we are having those conversations of: ‘How we are going to plan for that it does come to that?’ ” she said.
“In general, with the pandemic, we have seen all sorts of just disruptions to the supply chain. So, I think you just do your best, you find alternatives for those patients that you can, and you come up with strategies. I don’t know that for Abraxane, or any other product, that I’d be particularly confident that we may not see another shortage,” Dr. Hill said.
Long COVID associated with risk of metabolic liver disease
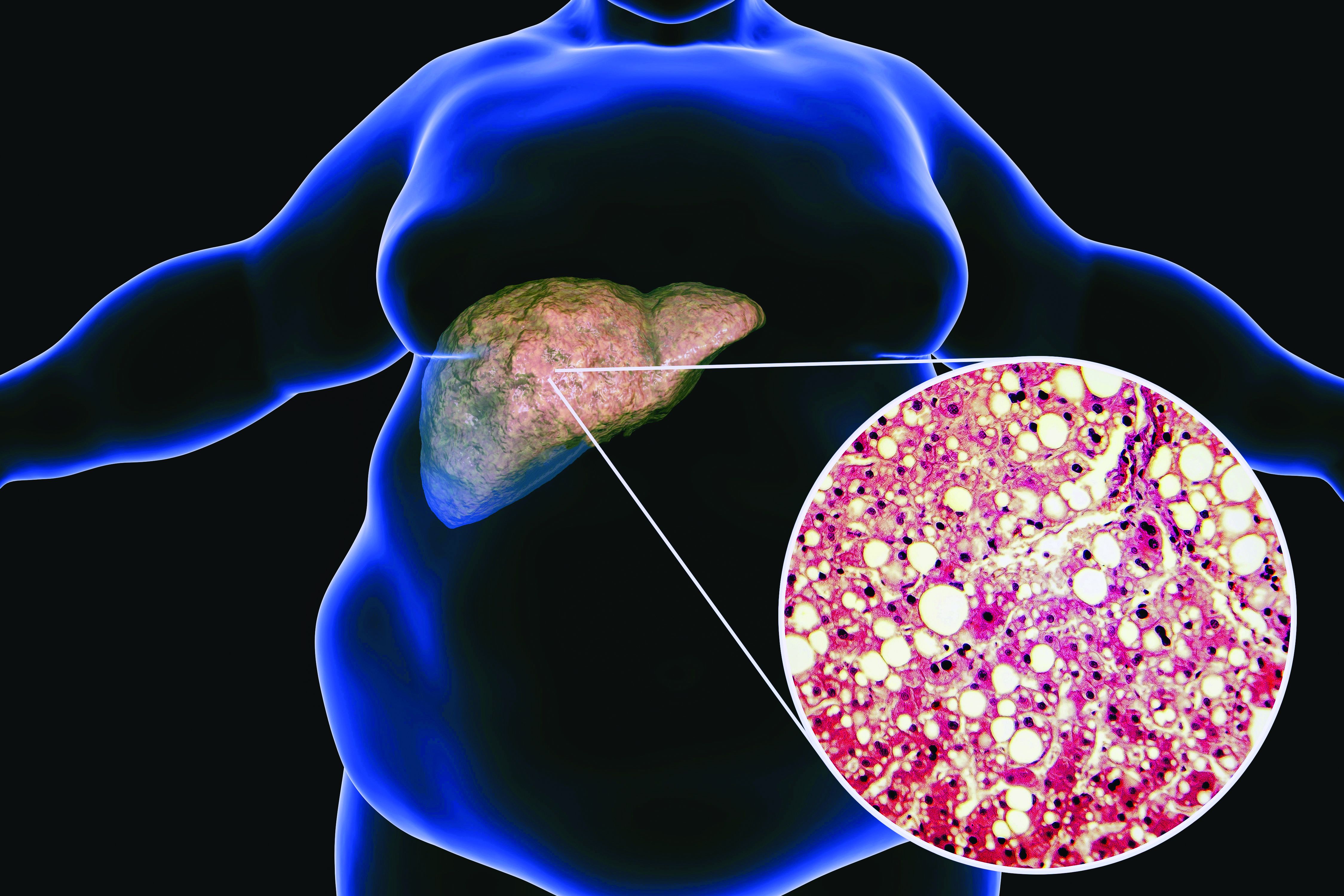
Postacute COVID syndrome (PACS), an ongoing inflammatory state following infection with SARS-CoV-2, is associated with greater risk of metabolic-associated fatty liver disease (MAFLD), according to an analysis of patients at a single clinic in Canada published in Open Forum Infectious Diseases.
MAFLD, also known as nonalcoholic fatty liver disease (NAFLD), is considered an indicator of general health and is in turn linked to greater risk of cardiovascular complications and mortality. It may be a multisystem disorder with various underlying causes.
PACS includes symptoms that affect various organ systems, with neurocognitive, autonomic, gastrointestinal, respiratory, musculoskeletal, psychological, sensory, and dermatologic clusters. An estimated 50%-80% of COVID-19 patients experience one or more clusters of symptoms 3 months after leaving the hospital.
But liver problems also appear in the acute phase, said Paul Martin, MD, who was asked to comment on the study. “Up to about half the patients during the acute illness may have elevated liver tests, but there seems to be a subset of patients in whom the abnormality persists. And then there are some reports in the literature of patients developing injury to their bile ducts in the liver over the long term, apparently as a consequence of COVID infection. What this paper suggests is that there may be some metabolic derangements associated with COVID infection, which in turn can accentuate or possibly cause fatty liver,” said Dr. Martin in an interview. He is chief of digestive health and liver diseases and a professor of medicine at the University of Miami.
“It highlights the need to get vaccinated against COVID and to take appropriate precautions because contracting the infection may lead to all sorts of consequences quite apart from having a respiratory illness,” said Dr. Martin.
The researchers retrospectively identified 235 patients hospitalized with COVID-19 between July 2020 and April 2021. Overall, 69% were men, and the median age was 61 years; 19.2% underwent mechanical ventilation and the mean duration of hospitalization was 11.7 days. They were seen for PACS symptoms a median 143 days after COVID-19 symptoms began, with 77.5% having symptoms of at least one PACS cluster. Of these clusters, 34.9% were neurocognitive, 53.2% were respiratory, 26.4% were musculoskeletal, 29.4% were psychological, 25.1% were dermatologic, and 17.5% were sensory.
At the later clinical visit for PACS symptoms, all patients underwent screening for MAFLD, which was defined as the presence of liver steatosis plus overweight/obesity or type 2 diabetes. Hepatic steatosis was determined from controlled attenuation parameter using transient elastrography. The analysis excluded patients with significant alcohol intake or hepatitis B or C. All patients with liver steatosis also had MAFLD, and this included 55.3% of the study population.
The hospital was able to obtain hepatic steatosis index (HSI) scores for 103 of 235 patients. Of these, 50% had MAFLD on admission for acute COVID-19, and 48.1% had MAFLD upon discharge based on this criterion. At the PACS follow-up visit, 71.3% were diagnosed with MAFLD. There was no statistically significant difference in the use of glucocorticoids or tocilizumab during hospitalization between those with and without MAFLD, and remdesivir use was insignificant in the patient population.
Given that the prevalence of MAFLD among the study population is more than double that in the general population, the authors suggest that MAFLD may be a new PACS cluster phenotype that could lead to long-term metabolic and cardiovascular complications. A potential explanation is loss of lean body mass during COVID-19 hospitalization followed by liver fat accumulation during recovery.
Other infections have also shown an association with increased MAFLD incidence, including HIV, Heliobacter pylori, and viral hepatitis. The authors worry that COVID-19 infection could exacerbate underlying conditions to a more severe MAFLD disease state.
The study is limited by a small sample size, limited follow-up, and the lack of a control group. Its retrospective nature leaves it vulnerable to biases.
“The natural history of MAFLD in the context of PACS is unknown at this time, and careful follow-up of these patients is needed to understand the clinical implications of this syndrome in the context of long COVID,” the authors wrote. “We speculate that [MAFLD] may be considered as an independent PACS-cluster phenotype, potentially affecting the metabolic and cardiovascular health of patients with PACS.”
One author has relationships with several pharmaceutical companies, but the remaining authors reported no conflicts of interest. Dr. Martin has no relevant financial disclosures.
Postacute COVID syndrome (PACS), an ongoing inflammatory state following infection with SARS-CoV-2, is associated with greater risk of metabolic-associated fatty liver disease (MAFLD), according to an analysis of patients at a single clinic in Canada published in Open Forum Infectious Diseases.
MAFLD, also known as nonalcoholic fatty liver disease (NAFLD), is considered an indicator of general health and is in turn linked to greater risk of cardiovascular complications and mortality. It may be a multisystem disorder with various underlying causes.
PACS includes symptoms that affect various organ systems, with neurocognitive, autonomic, gastrointestinal, respiratory, musculoskeletal, psychological, sensory, and dermatologic clusters. An estimated 50%-80% of COVID-19 patients experience one or more clusters of symptoms 3 months after leaving the hospital.
But liver problems also appear in the acute phase, said Paul Martin, MD, who was asked to comment on the study. “Up to about half the patients during the acute illness may have elevated liver tests, but there seems to be a subset of patients in whom the abnormality persists. And then there are some reports in the literature of patients developing injury to their bile ducts in the liver over the long term, apparently as a consequence of COVID infection. What this paper suggests is that there may be some metabolic derangements associated with COVID infection, which in turn can accentuate or possibly cause fatty liver,” said Dr. Martin in an interview. He is chief of digestive health and liver diseases and a professor of medicine at the University of Miami.
“It highlights the need to get vaccinated against COVID and to take appropriate precautions because contracting the infection may lead to all sorts of consequences quite apart from having a respiratory illness,” said Dr. Martin.
The researchers retrospectively identified 235 patients hospitalized with COVID-19 between July 2020 and April 2021. Overall, 69% were men, and the median age was 61 years; 19.2% underwent mechanical ventilation and the mean duration of hospitalization was 11.7 days. They were seen for PACS symptoms a median 143 days after COVID-19 symptoms began, with 77.5% having symptoms of at least one PACS cluster. Of these clusters, 34.9% were neurocognitive, 53.2% were respiratory, 26.4% were musculoskeletal, 29.4% were psychological, 25.1% were dermatologic, and 17.5% were sensory.
At the later clinical visit for PACS symptoms, all patients underwent screening for MAFLD, which was defined as the presence of liver steatosis plus overweight/obesity or type 2 diabetes. Hepatic steatosis was determined from controlled attenuation parameter using transient elastrography. The analysis excluded patients with significant alcohol intake or hepatitis B or C. All patients with liver steatosis also had MAFLD, and this included 55.3% of the study population.
The hospital was able to obtain hepatic steatosis index (HSI) scores for 103 of 235 patients. Of these, 50% had MAFLD on admission for acute COVID-19, and 48.1% had MAFLD upon discharge based on this criterion. At the PACS follow-up visit, 71.3% were diagnosed with MAFLD. There was no statistically significant difference in the use of glucocorticoids or tocilizumab during hospitalization between those with and without MAFLD, and remdesivir use was insignificant in the patient population.
Given that the prevalence of MAFLD among the study population is more than double that in the general population, the authors suggest that MAFLD may be a new PACS cluster phenotype that could lead to long-term metabolic and cardiovascular complications. A potential explanation is loss of lean body mass during COVID-19 hospitalization followed by liver fat accumulation during recovery.
Other infections have also shown an association with increased MAFLD incidence, including HIV, Heliobacter pylori, and viral hepatitis. The authors worry that COVID-19 infection could exacerbate underlying conditions to a more severe MAFLD disease state.
The study is limited by a small sample size, limited follow-up, and the lack of a control group. Its retrospective nature leaves it vulnerable to biases.
“The natural history of MAFLD in the context of PACS is unknown at this time, and careful follow-up of these patients is needed to understand the clinical implications of this syndrome in the context of long COVID,” the authors wrote. “We speculate that [MAFLD] may be considered as an independent PACS-cluster phenotype, potentially affecting the metabolic and cardiovascular health of patients with PACS.”
One author has relationships with several pharmaceutical companies, but the remaining authors reported no conflicts of interest. Dr. Martin has no relevant financial disclosures.
Postacute COVID syndrome (PACS), an ongoing inflammatory state following infection with SARS-CoV-2, is associated with greater risk of metabolic-associated fatty liver disease (MAFLD), according to an analysis of patients at a single clinic in Canada published in Open Forum Infectious Diseases.
MAFLD, also known as nonalcoholic fatty liver disease (NAFLD), is considered an indicator of general health and is in turn linked to greater risk of cardiovascular complications and mortality. It may be a multisystem disorder with various underlying causes.
PACS includes symptoms that affect various organ systems, with neurocognitive, autonomic, gastrointestinal, respiratory, musculoskeletal, psychological, sensory, and dermatologic clusters. An estimated 50%-80% of COVID-19 patients experience one or more clusters of symptoms 3 months after leaving the hospital.
But liver problems also appear in the acute phase, said Paul Martin, MD, who was asked to comment on the study. “Up to about half the patients during the acute illness may have elevated liver tests, but there seems to be a subset of patients in whom the abnormality persists. And then there are some reports in the literature of patients developing injury to their bile ducts in the liver over the long term, apparently as a consequence of COVID infection. What this paper suggests is that there may be some metabolic derangements associated with COVID infection, which in turn can accentuate or possibly cause fatty liver,” said Dr. Martin in an interview. He is chief of digestive health and liver diseases and a professor of medicine at the University of Miami.
“It highlights the need to get vaccinated against COVID and to take appropriate precautions because contracting the infection may lead to all sorts of consequences quite apart from having a respiratory illness,” said Dr. Martin.
The researchers retrospectively identified 235 patients hospitalized with COVID-19 between July 2020 and April 2021. Overall, 69% were men, and the median age was 61 years; 19.2% underwent mechanical ventilation and the mean duration of hospitalization was 11.7 days. They were seen for PACS symptoms a median 143 days after COVID-19 symptoms began, with 77.5% having symptoms of at least one PACS cluster. Of these clusters, 34.9% were neurocognitive, 53.2% were respiratory, 26.4% were musculoskeletal, 29.4% were psychological, 25.1% were dermatologic, and 17.5% were sensory.
At the later clinical visit for PACS symptoms, all patients underwent screening for MAFLD, which was defined as the presence of liver steatosis plus overweight/obesity or type 2 diabetes. Hepatic steatosis was determined from controlled attenuation parameter using transient elastrography. The analysis excluded patients with significant alcohol intake or hepatitis B or C. All patients with liver steatosis also had MAFLD, and this included 55.3% of the study population.
The hospital was able to obtain hepatic steatosis index (HSI) scores for 103 of 235 patients. Of these, 50% had MAFLD on admission for acute COVID-19, and 48.1% had MAFLD upon discharge based on this criterion. At the PACS follow-up visit, 71.3% were diagnosed with MAFLD. There was no statistically significant difference in the use of glucocorticoids or tocilizumab during hospitalization between those with and without MAFLD, and remdesivir use was insignificant in the patient population.
Given that the prevalence of MAFLD among the study population is more than double that in the general population, the authors suggest that MAFLD may be a new PACS cluster phenotype that could lead to long-term metabolic and cardiovascular complications. A potential explanation is loss of lean body mass during COVID-19 hospitalization followed by liver fat accumulation during recovery.
Other infections have also shown an association with increased MAFLD incidence, including HIV, Heliobacter pylori, and viral hepatitis. The authors worry that COVID-19 infection could exacerbate underlying conditions to a more severe MAFLD disease state.
The study is limited by a small sample size, limited follow-up, and the lack of a control group. Its retrospective nature leaves it vulnerable to biases.
“The natural history of MAFLD in the context of PACS is unknown at this time, and careful follow-up of these patients is needed to understand the clinical implications of this syndrome in the context of long COVID,” the authors wrote. “We speculate that [MAFLD] may be considered as an independent PACS-cluster phenotype, potentially affecting the metabolic and cardiovascular health of patients with PACS.”
One author has relationships with several pharmaceutical companies, but the remaining authors reported no conflicts of interest. Dr. Martin has no relevant financial disclosures.
FROM OPEN FORUM INFECTIOUS DISEASES
Single-use duodenoscope is cost effective in ERCP
, according to a new analysis.
The study compared the EXALT Model-D, HLD, culture-and-quarantine (CQ), and ethylene oxide sterilization (ETO). The results came from a simulated cohort of patients undergoing endoscopic retrograde cholangiopancreatography (ERCP) to treat choledocholithiasis.
Although EXALT was the costliest option and HLD the cheapest, EXALT produced the most quality-adjusted life years (QALYs) and allowed the hospital to decrease net costs, and sensitivity analysis showed that it was a better option than HLD over a range of willingness-to-pay values.
“When evaluating technologies based on cost-effectiveness and additionally in the context of TPT [transitional passthrough] or NTAP [new technology add-on payment], the EXALT approach meets typically used cost-effectiveness thresholds compared to all other evaluated strategies and should be considered for standard practice,” wrote the authors, who were led by Ananya Das, MD, of the Arizona Centers for Digestive Health, Gilbert. The study was published in Techniques and Innovations in Gastrointestinal Endoscopy.
Duodenoscope contamination has resulted in outbreaks of various multidrug-resistant organisms in hospital settings, which has led to the publication of various reprocessing guidelines. Although many hospitals have adopted HLD protocols, others use additional or alternative reprocessing methods such as CQ or ETO. Despite these efforts, a recent Food and Drug Administration study found that 1.9%-22% of samples taken from duodenoscopes tested positive for bacteria of concern, such as pathogens. Those and other findings have led some to suggest that it would be best to move away from HLD, and instead employ sterilizable or disposable endoscopes.
In another study, The EXALT Model-D (Boston Scientific) had been shown to be a good alternative to standard reusable duodenoscopes.
The researchers used a Markov-model to determine the cost-effectiveness of EXALT Model-D against other approaches in a simulated cohort. They found that EXALT Model-D created the most QALYs (21.9265) at the highest cost ($3,000), and HLD the fewest QALYs (21.8938) at the lowest cost ($962). Compared with HLD, the incremental cost-effectiveness ratio (ICER) of EXALT was $62,185, and $38,461 for ETO gas sterilization. CQ was dominated, indicating that it had a higher cost but was not more effective than HLD.
The researchers conducted a subanalysis of ERCP and Medicare patients to consider the recently approved TPT payment and the NTAP, in both hospital outpatient and inpatient settings. With TPT, EXALT had no cost after reimbursement, with a net saving of $962 per patient when compared with HLD, plus an increase in 0.033 QALYs (0.15%). The other procedures cost more and were less effective. With NTAP, EXALT had a net cost of $323 versus HLD, with a similar QALY benefit.
A Monte Carlo analysis of EXALT versus HLD found reductions in duodenoscope infection-related ICU admission (relative risk reduction, 0.996; 95% confidence interval, 0.936-1.0; number needed to treat, 79; 95% CI, 67-95) and death (RRR, 0.973; 95% CI, 0.552-0.998; number needed to treat, 556; 95% CI, 350-997).
In willingness-to-pay estimates from $50,000 to $100,000, EXALT was cost effective in 67.28% of trials with ICER under $100,000 per QALY.
The study did not consider medicolegal costs, which could lead to an underestimation of EXALT’s cost-effectiveness. The study also relied on available published information to determine cost per patient of hospital outbreaks in the United States and Europe since 2012, but the authors did not include costs of administrative sanctions, litigation, and poor publicity due to inconsistencies in the literature.
“While more research is needed to understand and quantify the determinants of the natural history after exposure to contaminated duodenoscopes, such as the risk of transmission and the subsequent development of serious clinical infections, this economic analysis demonstrates an approach using EXALT Model-D is cost effective in the U.S. health care system when compared to the currently utilized strategies of duodenoscope reprocessing,” the researchers concluded.
The study did not receive any funding. One of the authors is an employee and stockholder of Boston Scientific, which manufactures and markets EXALT. The other two authors have consulted for Boston Scientific.
Consider for a moment: The single-use duodenoscope (SUD) represents a revolutionary approach to duodenoscope infection control. Who, even 10 years ago, would have imagined that a disposable duodenoscope would even be technically achievable, much less economically feasible? Notwithstanding, determining how to incorporate such a revolutionary new technology and its associated capital and recurring costs can be every bit as complex and challenging as conceiving and developing the SUD. The authors provide insights into answering these questions through Markov modeling, comparing cost-effectiveness of SUDs to traditional duodenoscopes (TD) using available data on TD and SUD performance, and extrapolating from nonendoscopic infection management data.
This analysis is helpful because it demonstrates that, despite SUD cost approaching $3,000, Centers for Medicare & Medicaid Services inpatient and outpatient cost-defrayment payments may result in SUDs being cost-effective within limits and assumptions the study incorporates. This information is also timely, because these CMS subsidies are guaranteed only through mid-2022 for Medicare inpatients and 2023 for Medicare outpatients.
Though useful and timely, this study does make assumptions that narrow its applicability to real-world endoscopic retrograde cholangiopancreatography (ERCP). Clinically, it considers only patients with uncomplicated common bile duct stones. While choledocholithiasis is the indication for ERCP in the majority of patients, over 40% of ERCPs in the United States are performed for other, often more complex applications. While most procedures in the referenced studies were performed by high-volume ERCP experts, a substantial proportion of ERCPs are performed by lower-volume ERCP proceduralists, who actually perform a substantial proportion of straight-forward ERCPs addressing uncomplicated choledocholithiasis.
This study focuses on cost implications on CMS Transitional Pass-through (TPT) and New Technology Add-On Payment (NTAP) subsidies available only for Medicare inpatients and outpatients, respectively. These reimbursements are set to expire in 2022 (inpatients) and 2023 (outpatients). What will happen after that? Also, the amount of TPT and NTAP cost defrayments are institution-dependent, because cost-to-charge ratio (CCR), an important factor in calculating these subsidies, varies substantially between institutions and regions. Looking to the future, how will the cost of SUDs be incorporated into the hospital business model when TPT and NTAP are over?
SUDs are a technological marvel and a remarkable advance in endoscopic infection control. But innovations in medical technology are expectedly accompanied by new operational challenges: How to incorporate them into day-to-day practice and develop a business model that avails valuable new resources to patients. Such operational challenges require as much heavy lifting as the technological innovation needed to produce innovative devices like SUDs. The authors’ vision and effort in ideating and executing this study give us a head-start on this path by helping us to imagine what is possible.
John A. Martin, MD, is associate professor and consultant at the Mayo Clinic, Rochester, Minn. He is a former member of the editorial board for GI & Hepatology News, but has no relevant conflicts to disclose.
Consider for a moment: The single-use duodenoscope (SUD) represents a revolutionary approach to duodenoscope infection control. Who, even 10 years ago, would have imagined that a disposable duodenoscope would even be technically achievable, much less economically feasible? Notwithstanding, determining how to incorporate such a revolutionary new technology and its associated capital and recurring costs can be every bit as complex and challenging as conceiving and developing the SUD. The authors provide insights into answering these questions through Markov modeling, comparing cost-effectiveness of SUDs to traditional duodenoscopes (TD) using available data on TD and SUD performance, and extrapolating from nonendoscopic infection management data.
This analysis is helpful because it demonstrates that, despite SUD cost approaching $3,000, Centers for Medicare & Medicaid Services inpatient and outpatient cost-defrayment payments may result in SUDs being cost-effective within limits and assumptions the study incorporates. This information is also timely, because these CMS subsidies are guaranteed only through mid-2022 for Medicare inpatients and 2023 for Medicare outpatients.
Though useful and timely, this study does make assumptions that narrow its applicability to real-world endoscopic retrograde cholangiopancreatography (ERCP). Clinically, it considers only patients with uncomplicated common bile duct stones. While choledocholithiasis is the indication for ERCP in the majority of patients, over 40% of ERCPs in the United States are performed for other, often more complex applications. While most procedures in the referenced studies were performed by high-volume ERCP experts, a substantial proportion of ERCPs are performed by lower-volume ERCP proceduralists, who actually perform a substantial proportion of straight-forward ERCPs addressing uncomplicated choledocholithiasis.
This study focuses on cost implications on CMS Transitional Pass-through (TPT) and New Technology Add-On Payment (NTAP) subsidies available only for Medicare inpatients and outpatients, respectively. These reimbursements are set to expire in 2022 (inpatients) and 2023 (outpatients). What will happen after that? Also, the amount of TPT and NTAP cost defrayments are institution-dependent, because cost-to-charge ratio (CCR), an important factor in calculating these subsidies, varies substantially between institutions and regions. Looking to the future, how will the cost of SUDs be incorporated into the hospital business model when TPT and NTAP are over?
SUDs are a technological marvel and a remarkable advance in endoscopic infection control. But innovations in medical technology are expectedly accompanied by new operational challenges: How to incorporate them into day-to-day practice and develop a business model that avails valuable new resources to patients. Such operational challenges require as much heavy lifting as the technological innovation needed to produce innovative devices like SUDs. The authors’ vision and effort in ideating and executing this study give us a head-start on this path by helping us to imagine what is possible.
John A. Martin, MD, is associate professor and consultant at the Mayo Clinic, Rochester, Minn. He is a former member of the editorial board for GI & Hepatology News, but has no relevant conflicts to disclose.
Consider for a moment: The single-use duodenoscope (SUD) represents a revolutionary approach to duodenoscope infection control. Who, even 10 years ago, would have imagined that a disposable duodenoscope would even be technically achievable, much less economically feasible? Notwithstanding, determining how to incorporate such a revolutionary new technology and its associated capital and recurring costs can be every bit as complex and challenging as conceiving and developing the SUD. The authors provide insights into answering these questions through Markov modeling, comparing cost-effectiveness of SUDs to traditional duodenoscopes (TD) using available data on TD and SUD performance, and extrapolating from nonendoscopic infection management data.
This analysis is helpful because it demonstrates that, despite SUD cost approaching $3,000, Centers for Medicare & Medicaid Services inpatient and outpatient cost-defrayment payments may result in SUDs being cost-effective within limits and assumptions the study incorporates. This information is also timely, because these CMS subsidies are guaranteed only through mid-2022 for Medicare inpatients and 2023 for Medicare outpatients.
Though useful and timely, this study does make assumptions that narrow its applicability to real-world endoscopic retrograde cholangiopancreatography (ERCP). Clinically, it considers only patients with uncomplicated common bile duct stones. While choledocholithiasis is the indication for ERCP in the majority of patients, over 40% of ERCPs in the United States are performed for other, often more complex applications. While most procedures in the referenced studies were performed by high-volume ERCP experts, a substantial proportion of ERCPs are performed by lower-volume ERCP proceduralists, who actually perform a substantial proportion of straight-forward ERCPs addressing uncomplicated choledocholithiasis.
This study focuses on cost implications on CMS Transitional Pass-through (TPT) and New Technology Add-On Payment (NTAP) subsidies available only for Medicare inpatients and outpatients, respectively. These reimbursements are set to expire in 2022 (inpatients) and 2023 (outpatients). What will happen after that? Also, the amount of TPT and NTAP cost defrayments are institution-dependent, because cost-to-charge ratio (CCR), an important factor in calculating these subsidies, varies substantially between institutions and regions. Looking to the future, how will the cost of SUDs be incorporated into the hospital business model when TPT and NTAP are over?
SUDs are a technological marvel and a remarkable advance in endoscopic infection control. But innovations in medical technology are expectedly accompanied by new operational challenges: How to incorporate them into day-to-day practice and develop a business model that avails valuable new resources to patients. Such operational challenges require as much heavy lifting as the technological innovation needed to produce innovative devices like SUDs. The authors’ vision and effort in ideating and executing this study give us a head-start on this path by helping us to imagine what is possible.
John A. Martin, MD, is associate professor and consultant at the Mayo Clinic, Rochester, Minn. He is a former member of the editorial board for GI & Hepatology News, but has no relevant conflicts to disclose.
, according to a new analysis.
The study compared the EXALT Model-D, HLD, culture-and-quarantine (CQ), and ethylene oxide sterilization (ETO). The results came from a simulated cohort of patients undergoing endoscopic retrograde cholangiopancreatography (ERCP) to treat choledocholithiasis.
Although EXALT was the costliest option and HLD the cheapest, EXALT produced the most quality-adjusted life years (QALYs) and allowed the hospital to decrease net costs, and sensitivity analysis showed that it was a better option than HLD over a range of willingness-to-pay values.
“When evaluating technologies based on cost-effectiveness and additionally in the context of TPT [transitional passthrough] or NTAP [new technology add-on payment], the EXALT approach meets typically used cost-effectiveness thresholds compared to all other evaluated strategies and should be considered for standard practice,” wrote the authors, who were led by Ananya Das, MD, of the Arizona Centers for Digestive Health, Gilbert. The study was published in Techniques and Innovations in Gastrointestinal Endoscopy.
Duodenoscope contamination has resulted in outbreaks of various multidrug-resistant organisms in hospital settings, which has led to the publication of various reprocessing guidelines. Although many hospitals have adopted HLD protocols, others use additional or alternative reprocessing methods such as CQ or ETO. Despite these efforts, a recent Food and Drug Administration study found that 1.9%-22% of samples taken from duodenoscopes tested positive for bacteria of concern, such as pathogens. Those and other findings have led some to suggest that it would be best to move away from HLD, and instead employ sterilizable or disposable endoscopes.
In another study, The EXALT Model-D (Boston Scientific) had been shown to be a good alternative to standard reusable duodenoscopes.
The researchers used a Markov-model to determine the cost-effectiveness of EXALT Model-D against other approaches in a simulated cohort. They found that EXALT Model-D created the most QALYs (21.9265) at the highest cost ($3,000), and HLD the fewest QALYs (21.8938) at the lowest cost ($962). Compared with HLD, the incremental cost-effectiveness ratio (ICER) of EXALT was $62,185, and $38,461 for ETO gas sterilization. CQ was dominated, indicating that it had a higher cost but was not more effective than HLD.
The researchers conducted a subanalysis of ERCP and Medicare patients to consider the recently approved TPT payment and the NTAP, in both hospital outpatient and inpatient settings. With TPT, EXALT had no cost after reimbursement, with a net saving of $962 per patient when compared with HLD, plus an increase in 0.033 QALYs (0.15%). The other procedures cost more and were less effective. With NTAP, EXALT had a net cost of $323 versus HLD, with a similar QALY benefit.
A Monte Carlo analysis of EXALT versus HLD found reductions in duodenoscope infection-related ICU admission (relative risk reduction, 0.996; 95% confidence interval, 0.936-1.0; number needed to treat, 79; 95% CI, 67-95) and death (RRR, 0.973; 95% CI, 0.552-0.998; number needed to treat, 556; 95% CI, 350-997).
In willingness-to-pay estimates from $50,000 to $100,000, EXALT was cost effective in 67.28% of trials with ICER under $100,000 per QALY.
The study did not consider medicolegal costs, which could lead to an underestimation of EXALT’s cost-effectiveness. The study also relied on available published information to determine cost per patient of hospital outbreaks in the United States and Europe since 2012, but the authors did not include costs of administrative sanctions, litigation, and poor publicity due to inconsistencies in the literature.
“While more research is needed to understand and quantify the determinants of the natural history after exposure to contaminated duodenoscopes, such as the risk of transmission and the subsequent development of serious clinical infections, this economic analysis demonstrates an approach using EXALT Model-D is cost effective in the U.S. health care system when compared to the currently utilized strategies of duodenoscope reprocessing,” the researchers concluded.
The study did not receive any funding. One of the authors is an employee and stockholder of Boston Scientific, which manufactures and markets EXALT. The other two authors have consulted for Boston Scientific.
, according to a new analysis.
The study compared the EXALT Model-D, HLD, culture-and-quarantine (CQ), and ethylene oxide sterilization (ETO). The results came from a simulated cohort of patients undergoing endoscopic retrograde cholangiopancreatography (ERCP) to treat choledocholithiasis.
Although EXALT was the costliest option and HLD the cheapest, EXALT produced the most quality-adjusted life years (QALYs) and allowed the hospital to decrease net costs, and sensitivity analysis showed that it was a better option than HLD over a range of willingness-to-pay values.
“When evaluating technologies based on cost-effectiveness and additionally in the context of TPT [transitional passthrough] or NTAP [new technology add-on payment], the EXALT approach meets typically used cost-effectiveness thresholds compared to all other evaluated strategies and should be considered for standard practice,” wrote the authors, who were led by Ananya Das, MD, of the Arizona Centers for Digestive Health, Gilbert. The study was published in Techniques and Innovations in Gastrointestinal Endoscopy.
Duodenoscope contamination has resulted in outbreaks of various multidrug-resistant organisms in hospital settings, which has led to the publication of various reprocessing guidelines. Although many hospitals have adopted HLD protocols, others use additional or alternative reprocessing methods such as CQ or ETO. Despite these efforts, a recent Food and Drug Administration study found that 1.9%-22% of samples taken from duodenoscopes tested positive for bacteria of concern, such as pathogens. Those and other findings have led some to suggest that it would be best to move away from HLD, and instead employ sterilizable or disposable endoscopes.
In another study, The EXALT Model-D (Boston Scientific) had been shown to be a good alternative to standard reusable duodenoscopes.
The researchers used a Markov-model to determine the cost-effectiveness of EXALT Model-D against other approaches in a simulated cohort. They found that EXALT Model-D created the most QALYs (21.9265) at the highest cost ($3,000), and HLD the fewest QALYs (21.8938) at the lowest cost ($962). Compared with HLD, the incremental cost-effectiveness ratio (ICER) of EXALT was $62,185, and $38,461 for ETO gas sterilization. CQ was dominated, indicating that it had a higher cost but was not more effective than HLD.
The researchers conducted a subanalysis of ERCP and Medicare patients to consider the recently approved TPT payment and the NTAP, in both hospital outpatient and inpatient settings. With TPT, EXALT had no cost after reimbursement, with a net saving of $962 per patient when compared with HLD, plus an increase in 0.033 QALYs (0.15%). The other procedures cost more and were less effective. With NTAP, EXALT had a net cost of $323 versus HLD, with a similar QALY benefit.
A Monte Carlo analysis of EXALT versus HLD found reductions in duodenoscope infection-related ICU admission (relative risk reduction, 0.996; 95% confidence interval, 0.936-1.0; number needed to treat, 79; 95% CI, 67-95) and death (RRR, 0.973; 95% CI, 0.552-0.998; number needed to treat, 556; 95% CI, 350-997).
In willingness-to-pay estimates from $50,000 to $100,000, EXALT was cost effective in 67.28% of trials with ICER under $100,000 per QALY.
The study did not consider medicolegal costs, which could lead to an underestimation of EXALT’s cost-effectiveness. The study also relied on available published information to determine cost per patient of hospital outbreaks in the United States and Europe since 2012, but the authors did not include costs of administrative sanctions, litigation, and poor publicity due to inconsistencies in the literature.
“While more research is needed to understand and quantify the determinants of the natural history after exposure to contaminated duodenoscopes, such as the risk of transmission and the subsequent development of serious clinical infections, this economic analysis demonstrates an approach using EXALT Model-D is cost effective in the U.S. health care system when compared to the currently utilized strategies of duodenoscope reprocessing,” the researchers concluded.
The study did not receive any funding. One of the authors is an employee and stockholder of Boston Scientific, which manufactures and markets EXALT. The other two authors have consulted for Boston Scientific.
FROM TECHNIQUES AND INNOVATIONS IN GASTROINTESTINAL ENDOSCOPY
GERD: Upper endoscopy may reduce GI cancer mortality
Among individuals with gastroesophageal reflux disease (GERD), a negative upper endoscopy is associated with decreased risk in incidence and mortality from gastrointestinal cancer. The benefit persisted through 5-10 years following the procedure.
The finding is similar to the survival benefit seen with colonoscopies and colorectal cancer, and may be attributable to endoscopic treatment of premalignant lesions.
“The relatively high incidence rate of upper gastrointestinal cancer in patients with GERD indicates that a one-time upper endoscopy may be beneficial,” wrote the authors, who were led by Dag Holmberg, MD, PhD, of the department of molecular medicine and surgery at the Karolinska Institutet and Karolinska University Hospital, both in Stockholm. The study was published in Gastroenterology.
GERD is the most frequent reason patients undergo an upper endoscopy, but the results are often negative. It is generally a benign condition, but can lead to Barrett’s esophagus, as well as esophageal and gastric cardia adenocarcinoma. Upper endoscopy can identify other esophageal cancers like gastric noncardia cancer and duodenal cancer, which may cause dyspepsia or GERD-like symptoms.
To determine the potential benefit of upper endoscopy, the researchers conducted a population-based, four-nation cohort study that included 1,062,740 individuals with GERD in Denmark, Finland, Norway, and Sweden. The data were gathered from national patient registries, cancer registries, and cause of death registries. The study encompassed data from 1979 through the end of 2018.
The median age was 58 years, and 52% of participants were women.
The researchers defined a negative endoscopy as no diagnosis of gastrointestinal cancer within 6 months of the procedure; 69.3% of procedures were negative.
During the follow-up period, 0.34% of participants developed and 0.27% died of upper gastrointestinal cancer. Among those with negative endoscopies, 0.23% developed and 0.22% died from upper gastrointestinal cancer.
Participants with a negative endoscopy had a lower risk of being diagnosed with upper gastrointestinal cancer during the follow-up period (adjusted hazard ratio, 0.45; 95% confidence interval, 0.43-0.48). The reduction in risk was similar across age sexes and age groups, but among procedures performed after 2008, the risk reduction was even higher (aHR, 0.34; P < .001).
The effect was strongest in the first year after the procedure, but it persisted out to 5 years before returning to baseline risk levels.
A negative endoscopy was also associated with decreased mortality risk from upper gastrointestinal cancer versus those who hadn’t had an endoscopy (aHR, 0.39; 95% CI, 0.37-0.42). The protective value continued for at least 10 years.
Esophageal adenocarcinoma developed in 0.12% of participants, and 0.10% died of the disease. Among those with a negative endoscopy, 0.09% developed adenocarcinoma, and 0.07% died (aHR vs. no upper endoscopy, 0.33; 95% CI, 0.30-0.37).
The rapid return to baseline risk was notable, and different from what occurs after negative colonoscopies. However, new tumors can readily form within one year, and the risk may reflect early malignant or premalignant lesions that were missed during the procedure.
In fact, a meta-analysis found that 11.3% of upper gastrointestinal cancers had escaped detection during an endoscopy in the previous 3 years before diagnosis, and case reviews of patients diagnosed with gastrointestinal cancer soon after an upper endoscopy usually reveal suspicious or indeterminate results that the endoscopist or pathologist missed.
Quality indicators for upper endoscopy include procedure time, rate of targeted biopsies, and computer-aided detection, but it isn’t clear what impact these measures have on outcomes. However, the greater risk reduction found with endoscopies performed more recently suggests that newer quality indicators and technological improvements may be improving outcomes.
The relatively low incidence of esophageal and gastric cancer in Western countries has discouraged widespread adoption of endoscopic screening, but the researchers point out that the risk of gastrointestinal cancer among individuals with GERD is similar to the risk of colorectal cancer in the 60-69 age group in the United States, for whom colonoscopy is recommended.
“The present study indicates that upper endoscopy may be beneficial for patients with GERD, but to make upper endoscopy screening more cost beneficial at its initiation, the target group may be limited to include patients at highest risk of cancer. Such previous cost-effectiveness studies have indicated that endoscopy is cost effective in men at aged 50 years or older with chronic GERD,” the authors wrote.
The study was funded by Swedish Research Council and Swedish Cancer Society. The authors disclosed no relevant conflicts of interest.
This study from Holmberg and colleagues has the potential to revolutionize future clinical guidelines determining endoscopic investigations for GERD patients.
The cohort for analysis is staggering in magnitude: The authors analyzed real-world data from over 1 million participants with GERD in four Scandinavian databases. The results show strong and precise reductions in both risk and mortality from upper gastrointestinal cancer in the whole cohort. This reduction was consistent across all subgroup and sensitivity analyses.
These findings are important as GERD alone does not necessarily warrant an upper endoscopy investigation in current practice. This study provides strong evidence that a one-off endoscopic investigation in patients with GERD could bring meaningful opportunities for early detection of esophageal and gastric cancers – and in turn lead to fewer patients dying from these tumors. The immediacy of the return for investment is also impressive; with the risk reduction being strongest in the first few years of follow-up.
The elusive next step, as highlighted by the authors, is to ensure implementation of endoscopic screening can be done in a cost-effective manner. This is even more important because many health care systems across the world struggle with endoscopy capacity during the COVID-19 pandemic.
Helen Coleman, PhD, BSc(Hons), is a professor of cancer epidemiology at Queen’s University Belfast (Northern Ireland); joint deputy director of the Northern Ireland Cancer Registry; a Cancer Research UK Fellow; and a visiting scientist with the Fitzgerald Lab at the University of Cambridge (England). She has no conflicts.
This study from Holmberg and colleagues has the potential to revolutionize future clinical guidelines determining endoscopic investigations for GERD patients.
The cohort for analysis is staggering in magnitude: The authors analyzed real-world data from over 1 million participants with GERD in four Scandinavian databases. The results show strong and precise reductions in both risk and mortality from upper gastrointestinal cancer in the whole cohort. This reduction was consistent across all subgroup and sensitivity analyses.
These findings are important as GERD alone does not necessarily warrant an upper endoscopy investigation in current practice. This study provides strong evidence that a one-off endoscopic investigation in patients with GERD could bring meaningful opportunities for early detection of esophageal and gastric cancers – and in turn lead to fewer patients dying from these tumors. The immediacy of the return for investment is also impressive; with the risk reduction being strongest in the first few years of follow-up.
The elusive next step, as highlighted by the authors, is to ensure implementation of endoscopic screening can be done in a cost-effective manner. This is even more important because many health care systems across the world struggle with endoscopy capacity during the COVID-19 pandemic.
Helen Coleman, PhD, BSc(Hons), is a professor of cancer epidemiology at Queen’s University Belfast (Northern Ireland); joint deputy director of the Northern Ireland Cancer Registry; a Cancer Research UK Fellow; and a visiting scientist with the Fitzgerald Lab at the University of Cambridge (England). She has no conflicts.
This study from Holmberg and colleagues has the potential to revolutionize future clinical guidelines determining endoscopic investigations for GERD patients.
The cohort for analysis is staggering in magnitude: The authors analyzed real-world data from over 1 million participants with GERD in four Scandinavian databases. The results show strong and precise reductions in both risk and mortality from upper gastrointestinal cancer in the whole cohort. This reduction was consistent across all subgroup and sensitivity analyses.
These findings are important as GERD alone does not necessarily warrant an upper endoscopy investigation in current practice. This study provides strong evidence that a one-off endoscopic investigation in patients with GERD could bring meaningful opportunities for early detection of esophageal and gastric cancers – and in turn lead to fewer patients dying from these tumors. The immediacy of the return for investment is also impressive; with the risk reduction being strongest in the first few years of follow-up.
The elusive next step, as highlighted by the authors, is to ensure implementation of endoscopic screening can be done in a cost-effective manner. This is even more important because many health care systems across the world struggle with endoscopy capacity during the COVID-19 pandemic.
Helen Coleman, PhD, BSc(Hons), is a professor of cancer epidemiology at Queen’s University Belfast (Northern Ireland); joint deputy director of the Northern Ireland Cancer Registry; a Cancer Research UK Fellow; and a visiting scientist with the Fitzgerald Lab at the University of Cambridge (England). She has no conflicts.
Among individuals with gastroesophageal reflux disease (GERD), a negative upper endoscopy is associated with decreased risk in incidence and mortality from gastrointestinal cancer. The benefit persisted through 5-10 years following the procedure.
The finding is similar to the survival benefit seen with colonoscopies and colorectal cancer, and may be attributable to endoscopic treatment of premalignant lesions.
“The relatively high incidence rate of upper gastrointestinal cancer in patients with GERD indicates that a one-time upper endoscopy may be beneficial,” wrote the authors, who were led by Dag Holmberg, MD, PhD, of the department of molecular medicine and surgery at the Karolinska Institutet and Karolinska University Hospital, both in Stockholm. The study was published in Gastroenterology.
GERD is the most frequent reason patients undergo an upper endoscopy, but the results are often negative. It is generally a benign condition, but can lead to Barrett’s esophagus, as well as esophageal and gastric cardia adenocarcinoma. Upper endoscopy can identify other esophageal cancers like gastric noncardia cancer and duodenal cancer, which may cause dyspepsia or GERD-like symptoms.
To determine the potential benefit of upper endoscopy, the researchers conducted a population-based, four-nation cohort study that included 1,062,740 individuals with GERD in Denmark, Finland, Norway, and Sweden. The data were gathered from national patient registries, cancer registries, and cause of death registries. The study encompassed data from 1979 through the end of 2018.
The median age was 58 years, and 52% of participants were women.
The researchers defined a negative endoscopy as no diagnosis of gastrointestinal cancer within 6 months of the procedure; 69.3% of procedures were negative.
During the follow-up period, 0.34% of participants developed and 0.27% died of upper gastrointestinal cancer. Among those with negative endoscopies, 0.23% developed and 0.22% died from upper gastrointestinal cancer.
Participants with a negative endoscopy had a lower risk of being diagnosed with upper gastrointestinal cancer during the follow-up period (adjusted hazard ratio, 0.45; 95% confidence interval, 0.43-0.48). The reduction in risk was similar across age sexes and age groups, but among procedures performed after 2008, the risk reduction was even higher (aHR, 0.34; P < .001).
The effect was strongest in the first year after the procedure, but it persisted out to 5 years before returning to baseline risk levels.
A negative endoscopy was also associated with decreased mortality risk from upper gastrointestinal cancer versus those who hadn’t had an endoscopy (aHR, 0.39; 95% CI, 0.37-0.42). The protective value continued for at least 10 years.
Esophageal adenocarcinoma developed in 0.12% of participants, and 0.10% died of the disease. Among those with a negative endoscopy, 0.09% developed adenocarcinoma, and 0.07% died (aHR vs. no upper endoscopy, 0.33; 95% CI, 0.30-0.37).
The rapid return to baseline risk was notable, and different from what occurs after negative colonoscopies. However, new tumors can readily form within one year, and the risk may reflect early malignant or premalignant lesions that were missed during the procedure.
In fact, a meta-analysis found that 11.3% of upper gastrointestinal cancers had escaped detection during an endoscopy in the previous 3 years before diagnosis, and case reviews of patients diagnosed with gastrointestinal cancer soon after an upper endoscopy usually reveal suspicious or indeterminate results that the endoscopist or pathologist missed.
Quality indicators for upper endoscopy include procedure time, rate of targeted biopsies, and computer-aided detection, but it isn’t clear what impact these measures have on outcomes. However, the greater risk reduction found with endoscopies performed more recently suggests that newer quality indicators and technological improvements may be improving outcomes.
The relatively low incidence of esophageal and gastric cancer in Western countries has discouraged widespread adoption of endoscopic screening, but the researchers point out that the risk of gastrointestinal cancer among individuals with GERD is similar to the risk of colorectal cancer in the 60-69 age group in the United States, for whom colonoscopy is recommended.
“The present study indicates that upper endoscopy may be beneficial for patients with GERD, but to make upper endoscopy screening more cost beneficial at its initiation, the target group may be limited to include patients at highest risk of cancer. Such previous cost-effectiveness studies have indicated that endoscopy is cost effective in men at aged 50 years or older with chronic GERD,” the authors wrote.
The study was funded by Swedish Research Council and Swedish Cancer Society. The authors disclosed no relevant conflicts of interest.
Among individuals with gastroesophageal reflux disease (GERD), a negative upper endoscopy is associated with decreased risk in incidence and mortality from gastrointestinal cancer. The benefit persisted through 5-10 years following the procedure.
The finding is similar to the survival benefit seen with colonoscopies and colorectal cancer, and may be attributable to endoscopic treatment of premalignant lesions.
“The relatively high incidence rate of upper gastrointestinal cancer in patients with GERD indicates that a one-time upper endoscopy may be beneficial,” wrote the authors, who were led by Dag Holmberg, MD, PhD, of the department of molecular medicine and surgery at the Karolinska Institutet and Karolinska University Hospital, both in Stockholm. The study was published in Gastroenterology.
GERD is the most frequent reason patients undergo an upper endoscopy, but the results are often negative. It is generally a benign condition, but can lead to Barrett’s esophagus, as well as esophageal and gastric cardia adenocarcinoma. Upper endoscopy can identify other esophageal cancers like gastric noncardia cancer and duodenal cancer, which may cause dyspepsia or GERD-like symptoms.
To determine the potential benefit of upper endoscopy, the researchers conducted a population-based, four-nation cohort study that included 1,062,740 individuals with GERD in Denmark, Finland, Norway, and Sweden. The data were gathered from national patient registries, cancer registries, and cause of death registries. The study encompassed data from 1979 through the end of 2018.
The median age was 58 years, and 52% of participants were women.
The researchers defined a negative endoscopy as no diagnosis of gastrointestinal cancer within 6 months of the procedure; 69.3% of procedures were negative.
During the follow-up period, 0.34% of participants developed and 0.27% died of upper gastrointestinal cancer. Among those with negative endoscopies, 0.23% developed and 0.22% died from upper gastrointestinal cancer.
Participants with a negative endoscopy had a lower risk of being diagnosed with upper gastrointestinal cancer during the follow-up period (adjusted hazard ratio, 0.45; 95% confidence interval, 0.43-0.48). The reduction in risk was similar across age sexes and age groups, but among procedures performed after 2008, the risk reduction was even higher (aHR, 0.34; P < .001).
The effect was strongest in the first year after the procedure, but it persisted out to 5 years before returning to baseline risk levels.
A negative endoscopy was also associated with decreased mortality risk from upper gastrointestinal cancer versus those who hadn’t had an endoscopy (aHR, 0.39; 95% CI, 0.37-0.42). The protective value continued for at least 10 years.
Esophageal adenocarcinoma developed in 0.12% of participants, and 0.10% died of the disease. Among those with a negative endoscopy, 0.09% developed adenocarcinoma, and 0.07% died (aHR vs. no upper endoscopy, 0.33; 95% CI, 0.30-0.37).
The rapid return to baseline risk was notable, and different from what occurs after negative colonoscopies. However, new tumors can readily form within one year, and the risk may reflect early malignant or premalignant lesions that were missed during the procedure.
In fact, a meta-analysis found that 11.3% of upper gastrointestinal cancers had escaped detection during an endoscopy in the previous 3 years before diagnosis, and case reviews of patients diagnosed with gastrointestinal cancer soon after an upper endoscopy usually reveal suspicious or indeterminate results that the endoscopist or pathologist missed.
Quality indicators for upper endoscopy include procedure time, rate of targeted biopsies, and computer-aided detection, but it isn’t clear what impact these measures have on outcomes. However, the greater risk reduction found with endoscopies performed more recently suggests that newer quality indicators and technological improvements may be improving outcomes.
The relatively low incidence of esophageal and gastric cancer in Western countries has discouraged widespread adoption of endoscopic screening, but the researchers point out that the risk of gastrointestinal cancer among individuals with GERD is similar to the risk of colorectal cancer in the 60-69 age group in the United States, for whom colonoscopy is recommended.
“The present study indicates that upper endoscopy may be beneficial for patients with GERD, but to make upper endoscopy screening more cost beneficial at its initiation, the target group may be limited to include patients at highest risk of cancer. Such previous cost-effectiveness studies have indicated that endoscopy is cost effective in men at aged 50 years or older with chronic GERD,” the authors wrote.
The study was funded by Swedish Research Council and Swedish Cancer Society. The authors disclosed no relevant conflicts of interest.
FROM GASTROENTEROLOGY
Flexible sigmoidoscopy ADR linked to long-term survival
Gastroenterology centers with higher adenoma detection rates (ADR) with the use of flexible sigmoidoscopy (FS) had a lower long-term colorectal cancer incidence and lower CRC mortality among its patients, according to a new study.
Detection and removal of polyps during colonoscopy screening is vital to the prevention of CRC, and previous research has shown that centers with higher detection rates are associated with lower rates of CRC diagnosis within 3-5 years after a negative screen.
In Clinical Gastroenterology and Hepatology, researchers led by Amanda J. Cross, PhD, a professor of cancer epidemiology at Imperial College London, published an analysis of the UK Flexible Sigmoidoscopy Screening Trial, which found that FS screening between the ages 55 and 64 led to a 35% reduction of CRC incidence and a 41% reduction in CRC over a mean follow-up 17.1 years. The screening program had no apparent effect on incidence and mortality of proximal cancers. The researchers speculated that this was because few patients underwent proximal examination during follow-up colonoscopy.
“Considering only 5% of participants were referred for follow-up colonoscopy and 4% were referred for surveillance, we conclude that the improved detection of adenomas at FS has a measurable impact on long-term distal CRC outcomes, even when there is infrequent colonoscopy use. It is possible that high detectors also were more adept at polypectomy than intermediate or low detectors, and achieved more complete resection of detected lesions,” the authors wrote.
The researchers analyzed data from 38,550 patients who underwent screening at 14 U.K. hospitals, between 1994 and 1999. A single endoscopist was responsible for nearly all FS screens performed at each participating hospital.
The mean patient age was 60 years, and 49% were male. The researchers calculated ADRs for each center using the percentage of patients who had at least one adenoma detected during screening, which included any distal adenomas discovered during follow-up colonoscopy.
The ADR overall was 12%. The researchers used multivariate logistic regression to rank individual centers as having high (15%; five centers), intermediate (12%; four centers), or low (9%; four centers) detection rates.
There was a strong association between detection rates of small adenomas and a center’s ADR (P < .001), but not for large or advanced adenomas. In the high-detector group, 6.2% of patients screened were referred to colonoscopy versus 4.5% in the intermediate group and 4.5% in the low group. About half of colonoscopies were conducted by the same endoscopist who performed FS.
During follow-up, the distal CRC incidence was 1.5% in the high ADR group, 1.4% in the intermediate group, and 1.7% in the low group, and mortality rates were 0.4%, 0.4%, and 0.5%, respectively.
Compared with unscreened controls, risk of distal CRC was lowest among individuals who underwent screening in the high ADR group (hazard ratio, 0.34; 95% confidence interval, 0.27-0.42), followed by the intermediate group (HR, 0.46; 95% CI, 0.36-0.59), and the low ADR group (HR, 0.55; 95% CI, 0.44-0.68; P < .05 for all).
Compared with unscreened controls, CRC mortality was lower among individuals who underwent screening in the high ADR group (HR, 0.22; 95% CI, 0.13-0.37), followed by the intermediate group (HR, 0.30; 95% CI, 0.17-0.55), and the low ADR group (HR, 0.54; 95% CI, 0.34-0.86; P < .05 for between group differences).
All-site CRC incidence followed similar trends, with the lowest risks in the high ADR group (HR, 0.58; 95% CI, 0.50-0.67), followed by intermediate ADR (HR, 0.65; 95% CI, 0.55-0.77) and low ADR groups (HR, 0.72; 95% CI, 0.61-0.85; between group differences not statistically significant).
All-site CRC mortality was lowest in the high ADR group (HR, 0.52; 95% CI, 0.39-0.69), followed by the intermediate group (HR, 0.53; 95% CI, 0.38-0.73), and the low ADR group (HR, 0.68; 95% CI, 0.51-0.92; between-group differences not statistically significant).
The number needed to screen (NNS) to prevent one CRC diagnosis was 78 in the high ADR group (95% CI, 61-106), 103 in the intermediate group (95% CI, 74-171), and 125 in the low ADR group (95% CI, 82-256). The NNS to prevent one CRC death was 226 (95% CI, 159-387), 247 (95% CI, 165-490), and 349 respectively (95% CI, 192-1,904).
However, the researchers also pointed out that efforts to increase ADR could result in more complications, such as perforations or gastrointestinal bleeding, as well as more frequent diagnosis and recommended surveillance for diminutive adenomas.
The study is limited by the fact that endoscopists were either gastroenterologists or surgeons and the study population was made up of individuals who desired screening.
The UK Flexible Sigmoidoscopy Screening Trial was funded by the UK Medical Research Council and the National Institute for Health Research. The authors disclosed no conflicts of interest.
Adenoma detection rate (ADR) is an important quality indicator for colonoscopy. A higher ADR is associated with a lower risk of postcolonoscopy colorectal cancer (CRC). Flexible sigmoidoscopy (FS) is an evidence-based CRC screening modality, supported by multiple randomized trials reporting long-term reduction in CRC incidence and mortality. However, the impact of ADR of endoscopist performing FS on long-term outcomes is not known.
In this post hoc analysis from the UK Flexible Sigmoidoscopy Screening Trial the authors stratified the 13 endoscopy centers performing screening FS on 40,085 average-risk individuals aged between 55 and 64 years by their ADR into high, intermediate, and low with ADRs of 15%, 12%, and 9% respectively, and compared the relative reduction in CRC incidence and mortality with 113,195 controls over a median of 17 years. The authors reported greater reduction in both CRC incidence and mortality for CRC between high and low detectors (relative reduction of 42% versus 28% for CRC incidence and 48% versus 32% for CRC mortality respectively). Differences by ADR for distal CRC were more pronounced between high and low ADR centers (66% versus 45% for CRC incidence and 78% versus 46% for CRC mortality respectively); however, the test for interaction was not statistically significant, suggesting the three ADR groups cannot be differentiated from each other for the outcomes.
While FS is rarely used for screening in the United States, and U.K. guidelines also recently moved away from FS, the study illustrates that quality of FS is important, and that ADR can be a valid quality indicator for flexible sigmoidoscopy.
Aasma Shaukat, MD MPH AGAF, is Robert M. and Mary H. Glickman Professor of Medicine and Population Health and director of GI outcomes research at New York University. She reported having no relevant conflicts of interest.
Adenoma detection rate (ADR) is an important quality indicator for colonoscopy. A higher ADR is associated with a lower risk of postcolonoscopy colorectal cancer (CRC). Flexible sigmoidoscopy (FS) is an evidence-based CRC screening modality, supported by multiple randomized trials reporting long-term reduction in CRC incidence and mortality. However, the impact of ADR of endoscopist performing FS on long-term outcomes is not known.
In this post hoc analysis from the UK Flexible Sigmoidoscopy Screening Trial the authors stratified the 13 endoscopy centers performing screening FS on 40,085 average-risk individuals aged between 55 and 64 years by their ADR into high, intermediate, and low with ADRs of 15%, 12%, and 9% respectively, and compared the relative reduction in CRC incidence and mortality with 113,195 controls over a median of 17 years. The authors reported greater reduction in both CRC incidence and mortality for CRC between high and low detectors (relative reduction of 42% versus 28% for CRC incidence and 48% versus 32% for CRC mortality respectively). Differences by ADR for distal CRC were more pronounced between high and low ADR centers (66% versus 45% for CRC incidence and 78% versus 46% for CRC mortality respectively); however, the test for interaction was not statistically significant, suggesting the three ADR groups cannot be differentiated from each other for the outcomes.
While FS is rarely used for screening in the United States, and U.K. guidelines also recently moved away from FS, the study illustrates that quality of FS is important, and that ADR can be a valid quality indicator for flexible sigmoidoscopy.
Aasma Shaukat, MD MPH AGAF, is Robert M. and Mary H. Glickman Professor of Medicine and Population Health and director of GI outcomes research at New York University. She reported having no relevant conflicts of interest.
Adenoma detection rate (ADR) is an important quality indicator for colonoscopy. A higher ADR is associated with a lower risk of postcolonoscopy colorectal cancer (CRC). Flexible sigmoidoscopy (FS) is an evidence-based CRC screening modality, supported by multiple randomized trials reporting long-term reduction in CRC incidence and mortality. However, the impact of ADR of endoscopist performing FS on long-term outcomes is not known.
In this post hoc analysis from the UK Flexible Sigmoidoscopy Screening Trial the authors stratified the 13 endoscopy centers performing screening FS on 40,085 average-risk individuals aged between 55 and 64 years by their ADR into high, intermediate, and low with ADRs of 15%, 12%, and 9% respectively, and compared the relative reduction in CRC incidence and mortality with 113,195 controls over a median of 17 years. The authors reported greater reduction in both CRC incidence and mortality for CRC between high and low detectors (relative reduction of 42% versus 28% for CRC incidence and 48% versus 32% for CRC mortality respectively). Differences by ADR for distal CRC were more pronounced between high and low ADR centers (66% versus 45% for CRC incidence and 78% versus 46% for CRC mortality respectively); however, the test for interaction was not statistically significant, suggesting the three ADR groups cannot be differentiated from each other for the outcomes.
While FS is rarely used for screening in the United States, and U.K. guidelines also recently moved away from FS, the study illustrates that quality of FS is important, and that ADR can be a valid quality indicator for flexible sigmoidoscopy.
Aasma Shaukat, MD MPH AGAF, is Robert M. and Mary H. Glickman Professor of Medicine and Population Health and director of GI outcomes research at New York University. She reported having no relevant conflicts of interest.
Gastroenterology centers with higher adenoma detection rates (ADR) with the use of flexible sigmoidoscopy (FS) had a lower long-term colorectal cancer incidence and lower CRC mortality among its patients, according to a new study.
Detection and removal of polyps during colonoscopy screening is vital to the prevention of CRC, and previous research has shown that centers with higher detection rates are associated with lower rates of CRC diagnosis within 3-5 years after a negative screen.
In Clinical Gastroenterology and Hepatology, researchers led by Amanda J. Cross, PhD, a professor of cancer epidemiology at Imperial College London, published an analysis of the UK Flexible Sigmoidoscopy Screening Trial, which found that FS screening between the ages 55 and 64 led to a 35% reduction of CRC incidence and a 41% reduction in CRC over a mean follow-up 17.1 years. The screening program had no apparent effect on incidence and mortality of proximal cancers. The researchers speculated that this was because few patients underwent proximal examination during follow-up colonoscopy.
“Considering only 5% of participants were referred for follow-up colonoscopy and 4% were referred for surveillance, we conclude that the improved detection of adenomas at FS has a measurable impact on long-term distal CRC outcomes, even when there is infrequent colonoscopy use. It is possible that high detectors also were more adept at polypectomy than intermediate or low detectors, and achieved more complete resection of detected lesions,” the authors wrote.
The researchers analyzed data from 38,550 patients who underwent screening at 14 U.K. hospitals, between 1994 and 1999. A single endoscopist was responsible for nearly all FS screens performed at each participating hospital.
The mean patient age was 60 years, and 49% were male. The researchers calculated ADRs for each center using the percentage of patients who had at least one adenoma detected during screening, which included any distal adenomas discovered during follow-up colonoscopy.
The ADR overall was 12%. The researchers used multivariate logistic regression to rank individual centers as having high (15%; five centers), intermediate (12%; four centers), or low (9%; four centers) detection rates.
There was a strong association between detection rates of small adenomas and a center’s ADR (P < .001), but not for large or advanced adenomas. In the high-detector group, 6.2% of patients screened were referred to colonoscopy versus 4.5% in the intermediate group and 4.5% in the low group. About half of colonoscopies were conducted by the same endoscopist who performed FS.
During follow-up, the distal CRC incidence was 1.5% in the high ADR group, 1.4% in the intermediate group, and 1.7% in the low group, and mortality rates were 0.4%, 0.4%, and 0.5%, respectively.
Compared with unscreened controls, risk of distal CRC was lowest among individuals who underwent screening in the high ADR group (hazard ratio, 0.34; 95% confidence interval, 0.27-0.42), followed by the intermediate group (HR, 0.46; 95% CI, 0.36-0.59), and the low ADR group (HR, 0.55; 95% CI, 0.44-0.68; P < .05 for all).
Compared with unscreened controls, CRC mortality was lower among individuals who underwent screening in the high ADR group (HR, 0.22; 95% CI, 0.13-0.37), followed by the intermediate group (HR, 0.30; 95% CI, 0.17-0.55), and the low ADR group (HR, 0.54; 95% CI, 0.34-0.86; P < .05 for between group differences).
All-site CRC incidence followed similar trends, with the lowest risks in the high ADR group (HR, 0.58; 95% CI, 0.50-0.67), followed by intermediate ADR (HR, 0.65; 95% CI, 0.55-0.77) and low ADR groups (HR, 0.72; 95% CI, 0.61-0.85; between group differences not statistically significant).
All-site CRC mortality was lowest in the high ADR group (HR, 0.52; 95% CI, 0.39-0.69), followed by the intermediate group (HR, 0.53; 95% CI, 0.38-0.73), and the low ADR group (HR, 0.68; 95% CI, 0.51-0.92; between-group differences not statistically significant).
The number needed to screen (NNS) to prevent one CRC diagnosis was 78 in the high ADR group (95% CI, 61-106), 103 in the intermediate group (95% CI, 74-171), and 125 in the low ADR group (95% CI, 82-256). The NNS to prevent one CRC death was 226 (95% CI, 159-387), 247 (95% CI, 165-490), and 349 respectively (95% CI, 192-1,904).
However, the researchers also pointed out that efforts to increase ADR could result in more complications, such as perforations or gastrointestinal bleeding, as well as more frequent diagnosis and recommended surveillance for diminutive adenomas.
The study is limited by the fact that endoscopists were either gastroenterologists or surgeons and the study population was made up of individuals who desired screening.
The UK Flexible Sigmoidoscopy Screening Trial was funded by the UK Medical Research Council and the National Institute for Health Research. The authors disclosed no conflicts of interest.
Gastroenterology centers with higher adenoma detection rates (ADR) with the use of flexible sigmoidoscopy (FS) had a lower long-term colorectal cancer incidence and lower CRC mortality among its patients, according to a new study.
Detection and removal of polyps during colonoscopy screening is vital to the prevention of CRC, and previous research has shown that centers with higher detection rates are associated with lower rates of CRC diagnosis within 3-5 years after a negative screen.
In Clinical Gastroenterology and Hepatology, researchers led by Amanda J. Cross, PhD, a professor of cancer epidemiology at Imperial College London, published an analysis of the UK Flexible Sigmoidoscopy Screening Trial, which found that FS screening between the ages 55 and 64 led to a 35% reduction of CRC incidence and a 41% reduction in CRC over a mean follow-up 17.1 years. The screening program had no apparent effect on incidence and mortality of proximal cancers. The researchers speculated that this was because few patients underwent proximal examination during follow-up colonoscopy.
“Considering only 5% of participants were referred for follow-up colonoscopy and 4% were referred for surveillance, we conclude that the improved detection of adenomas at FS has a measurable impact on long-term distal CRC outcomes, even when there is infrequent colonoscopy use. It is possible that high detectors also were more adept at polypectomy than intermediate or low detectors, and achieved more complete resection of detected lesions,” the authors wrote.
The researchers analyzed data from 38,550 patients who underwent screening at 14 U.K. hospitals, between 1994 and 1999. A single endoscopist was responsible for nearly all FS screens performed at each participating hospital.
The mean patient age was 60 years, and 49% were male. The researchers calculated ADRs for each center using the percentage of patients who had at least one adenoma detected during screening, which included any distal adenomas discovered during follow-up colonoscopy.
The ADR overall was 12%. The researchers used multivariate logistic regression to rank individual centers as having high (15%; five centers), intermediate (12%; four centers), or low (9%; four centers) detection rates.
There was a strong association between detection rates of small adenomas and a center’s ADR (P < .001), but not for large or advanced adenomas. In the high-detector group, 6.2% of patients screened were referred to colonoscopy versus 4.5% in the intermediate group and 4.5% in the low group. About half of colonoscopies were conducted by the same endoscopist who performed FS.
During follow-up, the distal CRC incidence was 1.5% in the high ADR group, 1.4% in the intermediate group, and 1.7% in the low group, and mortality rates were 0.4%, 0.4%, and 0.5%, respectively.
Compared with unscreened controls, risk of distal CRC was lowest among individuals who underwent screening in the high ADR group (hazard ratio, 0.34; 95% confidence interval, 0.27-0.42), followed by the intermediate group (HR, 0.46; 95% CI, 0.36-0.59), and the low ADR group (HR, 0.55; 95% CI, 0.44-0.68; P < .05 for all).
Compared with unscreened controls, CRC mortality was lower among individuals who underwent screening in the high ADR group (HR, 0.22; 95% CI, 0.13-0.37), followed by the intermediate group (HR, 0.30; 95% CI, 0.17-0.55), and the low ADR group (HR, 0.54; 95% CI, 0.34-0.86; P < .05 for between group differences).
All-site CRC incidence followed similar trends, with the lowest risks in the high ADR group (HR, 0.58; 95% CI, 0.50-0.67), followed by intermediate ADR (HR, 0.65; 95% CI, 0.55-0.77) and low ADR groups (HR, 0.72; 95% CI, 0.61-0.85; between group differences not statistically significant).
All-site CRC mortality was lowest in the high ADR group (HR, 0.52; 95% CI, 0.39-0.69), followed by the intermediate group (HR, 0.53; 95% CI, 0.38-0.73), and the low ADR group (HR, 0.68; 95% CI, 0.51-0.92; between-group differences not statistically significant).
The number needed to screen (NNS) to prevent one CRC diagnosis was 78 in the high ADR group (95% CI, 61-106), 103 in the intermediate group (95% CI, 74-171), and 125 in the low ADR group (95% CI, 82-256). The NNS to prevent one CRC death was 226 (95% CI, 159-387), 247 (95% CI, 165-490), and 349 respectively (95% CI, 192-1,904).
However, the researchers also pointed out that efforts to increase ADR could result in more complications, such as perforations or gastrointestinal bleeding, as well as more frequent diagnosis and recommended surveillance for diminutive adenomas.
The study is limited by the fact that endoscopists were either gastroenterologists or surgeons and the study population was made up of individuals who desired screening.
The UK Flexible Sigmoidoscopy Screening Trial was funded by the UK Medical Research Council and the National Institute for Health Research. The authors disclosed no conflicts of interest.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Sleeve, RYGB reduce liver fat in type 2 diabetes
Both Roux-en-Y gastric bypass (RYGB) and sleeve gastrectomy (SG) are effective at improving hepatic steatosis in type 2 diabetes patients, according to a new analysis of a randomized, controlled trial.
Both procedures resulted in near elimination of liver fat 1 year after the surgery, but the effect on liver fibrosis was less clear. The authors called for more research to examine longer-term effects on fibrosis.
“Both gastric bypass and the sleeve had complete resolution of the liver fat based on their MRI findings. That’s impressive,” said Ali Aminian, MD, who was asked to comment on the study. Dr. Aminian is a professor of surgery and director of the Bariatric & Metabolic Institute at the Cleveland Clinic.
About 25% of the general population, and about 90% of people with type 2 diabetes and obesity have nonalcoholic fatty liver disease (NAFLD), which can lead to liver failure or hepatocellular carcinoma. Hepatic steatosis can combine with obesity, insulin resistance, and inflammation to heighten the risk of cardiovascular disease.
Moderate weight loss can clear liver fat and lead to histologic improvement of hepatic steatosis, and retrospective studies have suggested that RYGB may be more effective than SG and gastric banding in countering hepatic steatosis and steatohepatitis.
In fact, Dr. Aminian recently coauthored a paper describing results from the SPLENDOR study, which looked at 650 adults with obesity and nonalcoholic steatohepatitis (NASH) who underwent bariatric surgery at U.S. hospitals between 2004 and 2016, and compared liver biopsy outcomes to 508 patients who went through nonsurgical weight loss protocols.
After a median follow-up of 7 years, 2.3% In the bariatric surgery group had major adverse liver outcomes, compared with 9.6% in the nonsurgical group (adjusted hazard ratio, 0.12; P = .01). The cumulative incidence of major adverse cardiovascular events (MACE) was 8.5% in the bariatric surgery group and 15.7% in the nonsurgery group (aHR, 0.30; P = .007). 0.6% of the surgical group died within the first year after surgery from surgical complications.
Still, the question has not been tested in a randomized, controlled trial.
In the study published online in the Annals of Internal Medicine, researchers led by Kathrine Aglen Seeberg, MD, and Jens Kristoffer Hertel, PhD, of Vestfold Hospital Trust, Tønsberg, Norway, conducted a prespecified secondary analysis of data from 100 patients (65% female, mean age, 47.5 years) with type 2 diabetes who had been randomized to undergo RYGB or SG between January 2013 and February 2018 at their center.
Prior to surgery, the mean liver fat fraction (LFF) was 19% (stand deviation, 12%). In the SG and RYGB groups, 24% and 26% of patients had no or low-grade steatosis (LFF ≤ 10%). LFF declined by 13% in both groups at 5 weeks, and by 20% and 22% at 1 year, respectively, with no significant difference between the two groups.
At 1 year, 100% of the RYGB group had no or low-grade steatosis, as did 94% in the SG group (no significant difference). At 1 year, both groups had similar percentage decreases in the NAFLD liver fat score (between group difference, –0.05) and NAFLD liver fat percentage (between-group difference, –0.3; no significant difference for either).
At baseline, 6% of the RYGB group and 8% of the SG group had severe fibrosis as measured by the enhanced liver fibrosis (ELF) test. At 1 year, the respective frequencies were 9% and 15%, which were not statistically significant changes.
There was much variation in ELF score changes between Individuals, but 18% moved to a higher ELF category and only 5% improved to a lower ELF category at 1 year.
Limitations of the study include the fact that it was conducted at a single center and in a predominantly White population. The study also did not use liver biopsy, which is the standard for measuring fibrosis. Individuals with type 2 diabetes may have more severe NAFLD, which could limit the applicability to individuals without type 2 diabetes.
Together, the studies produce a clear clinical message, according to Dr. Aminian. “It provides compelling evidence for patients and medical providers that, if we can help patients lose weight, we can reverse fatty liver disease,” he said.
The study was funded by the Southeastern Norway Regional Health Authority. Dr. Aminian has received research support from Medtronic.
Both Roux-en-Y gastric bypass (RYGB) and sleeve gastrectomy (SG) are effective at improving hepatic steatosis in type 2 diabetes patients, according to a new analysis of a randomized, controlled trial.
Both procedures resulted in near elimination of liver fat 1 year after the surgery, but the effect on liver fibrosis was less clear. The authors called for more research to examine longer-term effects on fibrosis.
“Both gastric bypass and the sleeve had complete resolution of the liver fat based on their MRI findings. That’s impressive,” said Ali Aminian, MD, who was asked to comment on the study. Dr. Aminian is a professor of surgery and director of the Bariatric & Metabolic Institute at the Cleveland Clinic.
About 25% of the general population, and about 90% of people with type 2 diabetes and obesity have nonalcoholic fatty liver disease (NAFLD), which can lead to liver failure or hepatocellular carcinoma. Hepatic steatosis can combine with obesity, insulin resistance, and inflammation to heighten the risk of cardiovascular disease.
Moderate weight loss can clear liver fat and lead to histologic improvement of hepatic steatosis, and retrospective studies have suggested that RYGB may be more effective than SG and gastric banding in countering hepatic steatosis and steatohepatitis.
In fact, Dr. Aminian recently coauthored a paper describing results from the SPLENDOR study, which looked at 650 adults with obesity and nonalcoholic steatohepatitis (NASH) who underwent bariatric surgery at U.S. hospitals between 2004 and 2016, and compared liver biopsy outcomes to 508 patients who went through nonsurgical weight loss protocols.
After a median follow-up of 7 years, 2.3% In the bariatric surgery group had major adverse liver outcomes, compared with 9.6% in the nonsurgical group (adjusted hazard ratio, 0.12; P = .01). The cumulative incidence of major adverse cardiovascular events (MACE) was 8.5% in the bariatric surgery group and 15.7% in the nonsurgery group (aHR, 0.30; P = .007). 0.6% of the surgical group died within the first year after surgery from surgical complications.
Still, the question has not been tested in a randomized, controlled trial.
In the study published online in the Annals of Internal Medicine, researchers led by Kathrine Aglen Seeberg, MD, and Jens Kristoffer Hertel, PhD, of Vestfold Hospital Trust, Tønsberg, Norway, conducted a prespecified secondary analysis of data from 100 patients (65% female, mean age, 47.5 years) with type 2 diabetes who had been randomized to undergo RYGB or SG between January 2013 and February 2018 at their center.
Prior to surgery, the mean liver fat fraction (LFF) was 19% (stand deviation, 12%). In the SG and RYGB groups, 24% and 26% of patients had no or low-grade steatosis (LFF ≤ 10%). LFF declined by 13% in both groups at 5 weeks, and by 20% and 22% at 1 year, respectively, with no significant difference between the two groups.
At 1 year, 100% of the RYGB group had no or low-grade steatosis, as did 94% in the SG group (no significant difference). At 1 year, both groups had similar percentage decreases in the NAFLD liver fat score (between group difference, –0.05) and NAFLD liver fat percentage (between-group difference, –0.3; no significant difference for either).
At baseline, 6% of the RYGB group and 8% of the SG group had severe fibrosis as measured by the enhanced liver fibrosis (ELF) test. At 1 year, the respective frequencies were 9% and 15%, which were not statistically significant changes.
There was much variation in ELF score changes between Individuals, but 18% moved to a higher ELF category and only 5% improved to a lower ELF category at 1 year.
Limitations of the study include the fact that it was conducted at a single center and in a predominantly White population. The study also did not use liver biopsy, which is the standard for measuring fibrosis. Individuals with type 2 diabetes may have more severe NAFLD, which could limit the applicability to individuals without type 2 diabetes.
Together, the studies produce a clear clinical message, according to Dr. Aminian. “It provides compelling evidence for patients and medical providers that, if we can help patients lose weight, we can reverse fatty liver disease,” he said.
The study was funded by the Southeastern Norway Regional Health Authority. Dr. Aminian has received research support from Medtronic.
Both Roux-en-Y gastric bypass (RYGB) and sleeve gastrectomy (SG) are effective at improving hepatic steatosis in type 2 diabetes patients, according to a new analysis of a randomized, controlled trial.
Both procedures resulted in near elimination of liver fat 1 year after the surgery, but the effect on liver fibrosis was less clear. The authors called for more research to examine longer-term effects on fibrosis.
“Both gastric bypass and the sleeve had complete resolution of the liver fat based on their MRI findings. That’s impressive,” said Ali Aminian, MD, who was asked to comment on the study. Dr. Aminian is a professor of surgery and director of the Bariatric & Metabolic Institute at the Cleveland Clinic.
About 25% of the general population, and about 90% of people with type 2 diabetes and obesity have nonalcoholic fatty liver disease (NAFLD), which can lead to liver failure or hepatocellular carcinoma. Hepatic steatosis can combine with obesity, insulin resistance, and inflammation to heighten the risk of cardiovascular disease.
Moderate weight loss can clear liver fat and lead to histologic improvement of hepatic steatosis, and retrospective studies have suggested that RYGB may be more effective than SG and gastric banding in countering hepatic steatosis and steatohepatitis.
In fact, Dr. Aminian recently coauthored a paper describing results from the SPLENDOR study, which looked at 650 adults with obesity and nonalcoholic steatohepatitis (NASH) who underwent bariatric surgery at U.S. hospitals between 2004 and 2016, and compared liver biopsy outcomes to 508 patients who went through nonsurgical weight loss protocols.
After a median follow-up of 7 years, 2.3% In the bariatric surgery group had major adverse liver outcomes, compared with 9.6% in the nonsurgical group (adjusted hazard ratio, 0.12; P = .01). The cumulative incidence of major adverse cardiovascular events (MACE) was 8.5% in the bariatric surgery group and 15.7% in the nonsurgery group (aHR, 0.30; P = .007). 0.6% of the surgical group died within the first year after surgery from surgical complications.
Still, the question has not been tested in a randomized, controlled trial.
In the study published online in the Annals of Internal Medicine, researchers led by Kathrine Aglen Seeberg, MD, and Jens Kristoffer Hertel, PhD, of Vestfold Hospital Trust, Tønsberg, Norway, conducted a prespecified secondary analysis of data from 100 patients (65% female, mean age, 47.5 years) with type 2 diabetes who had been randomized to undergo RYGB or SG between January 2013 and February 2018 at their center.
Prior to surgery, the mean liver fat fraction (LFF) was 19% (stand deviation, 12%). In the SG and RYGB groups, 24% and 26% of patients had no or low-grade steatosis (LFF ≤ 10%). LFF declined by 13% in both groups at 5 weeks, and by 20% and 22% at 1 year, respectively, with no significant difference between the two groups.
At 1 year, 100% of the RYGB group had no or low-grade steatosis, as did 94% in the SG group (no significant difference). At 1 year, both groups had similar percentage decreases in the NAFLD liver fat score (between group difference, –0.05) and NAFLD liver fat percentage (between-group difference, –0.3; no significant difference for either).
At baseline, 6% of the RYGB group and 8% of the SG group had severe fibrosis as measured by the enhanced liver fibrosis (ELF) test. At 1 year, the respective frequencies were 9% and 15%, which were not statistically significant changes.
There was much variation in ELF score changes between Individuals, but 18% moved to a higher ELF category and only 5% improved to a lower ELF category at 1 year.
Limitations of the study include the fact that it was conducted at a single center and in a predominantly White population. The study also did not use liver biopsy, which is the standard for measuring fibrosis. Individuals with type 2 diabetes may have more severe NAFLD, which could limit the applicability to individuals without type 2 diabetes.
Together, the studies produce a clear clinical message, according to Dr. Aminian. “It provides compelling evidence for patients and medical providers that, if we can help patients lose weight, we can reverse fatty liver disease,” he said.
The study was funded by the Southeastern Norway Regional Health Authority. Dr. Aminian has received research support from Medtronic.
FROM ANNALS OF INTERNAL MEDICINE
For equality in prostate cancer outcomes, seek equality in treatment
Black men who received radiation therapy for localized prostate cancer fared better.
Overall, Black men have a 50% higher risk of being diagnosed with prostate cancer, and an 80% greater risk of death than White men. Those numbers have complicated roots: There are differences in access to medical care, clinical trial enrollment, access to screening, and frequency of definitive treatment.
The new study, published online Dec. 29, 2021, in JAMA Network Open, was a meta-analysis of 8,814 men (18.5% Black, 81.5% White) who participated in 7 randomized, clinical trials that compared definitive radiotherapy with or without short- or long-term androgen deprivation therapy. The researchers found that Black men had more features of high-risk disease, but they were less likely than White men to experience biochemical recurrence (subdistribution hazard ratio, 0.79; P < .001), distant metastasis (sHR, 0.69; P = .002), or prostate cancer-specific mortality (sHR, 0.68; P = .01).
“These results provide high-level evidence challenging the common belief that Black men who are diagnosed with prostate cancer will necessarily have a worse prognosis than White men,” said study coauthor Amar Kishan, MD, in a press release. Dr. Kishan is associate professor and vice chair of clinical and translational research at the University of California, Los Angeles, and a researcher at the UCLA Jonsson Comprehensive Cancer Center.
“This is especially important because an unfounded belief can inadvertently contribute to ‘cancer injustice,’ leading to the use of more aggressive treatments than might be necessary – potentially reducing quality of life and diverting attention away from other important factors that can influence outcome, including access to more comprehensive health care,” Dr. Kishan said.
Better health care coverage may indeed be the driving force behind the benefit, according to an accompanying editorial authored by Bogdana Schmidt, MD, MPH and Neeraj Agarwal, MD, of the Huntsman Cancer Institute at the University of Utah, Salt Lake City. The results suggest that, when Black men with prostate cancer get the high quality of care seen in clinical trials and receive definitive therapy, they achieve good results.
It also suggests a path toward improving outcomes. “Through a multidisciplinary effort of enriching cohort studies with Black men, enrolling Black men into clinical trials and continuing the search for tumor-specific genomic factors, treatment-specific response factors, and pharmacologic response differences, as a community we can unequivocally improve prostate cancer care for Black men,” the editorial authors wrote.
Enrollment in clinical trials has also been linked to improved outcomes in studies of docetaxel and prednisone, enzalutamide and androgen deprivation therapy, and abiraterone acetate and prednisone. Other studies have shown that Black men in clinical trials or who get treated in high-volume centers are less likely to experience the adverse outcomes seen more widely among Black men.
The new finding that Black men have better outcomes with radiotherapy may also have a biological basis, as a retrospective study of patients undergoing prostatectomy for prostate cancer found that Black men had lower levels of mismatch repair genes and DNA repair activity.
The study isn’t the first to implicate access to care in outcome differential between Black and White men with prostate cancer. A 2019 study compared outcomes between White and Black men within registries that have standardized access, which is expected to minimize racial disparities. The researchers found no differences in prostate cancer–specific mortality within these databases. However, the differences in outcomes surfaced between Black and White men when they examined data from a large federal registry that reflects social and economic barriers to health care.
The authors of both the study and the editorial have extensive financial relationships with pharmaceutical companies.
Black men who received radiation therapy for localized prostate cancer fared better.
Overall, Black men have a 50% higher risk of being diagnosed with prostate cancer, and an 80% greater risk of death than White men. Those numbers have complicated roots: There are differences in access to medical care, clinical trial enrollment, access to screening, and frequency of definitive treatment.
The new study, published online Dec. 29, 2021, in JAMA Network Open, was a meta-analysis of 8,814 men (18.5% Black, 81.5% White) who participated in 7 randomized, clinical trials that compared definitive radiotherapy with or without short- or long-term androgen deprivation therapy. The researchers found that Black men had more features of high-risk disease, but they were less likely than White men to experience biochemical recurrence (subdistribution hazard ratio, 0.79; P < .001), distant metastasis (sHR, 0.69; P = .002), or prostate cancer-specific mortality (sHR, 0.68; P = .01).
“These results provide high-level evidence challenging the common belief that Black men who are diagnosed with prostate cancer will necessarily have a worse prognosis than White men,” said study coauthor Amar Kishan, MD, in a press release. Dr. Kishan is associate professor and vice chair of clinical and translational research at the University of California, Los Angeles, and a researcher at the UCLA Jonsson Comprehensive Cancer Center.
“This is especially important because an unfounded belief can inadvertently contribute to ‘cancer injustice,’ leading to the use of more aggressive treatments than might be necessary – potentially reducing quality of life and diverting attention away from other important factors that can influence outcome, including access to more comprehensive health care,” Dr. Kishan said.
Better health care coverage may indeed be the driving force behind the benefit, according to an accompanying editorial authored by Bogdana Schmidt, MD, MPH and Neeraj Agarwal, MD, of the Huntsman Cancer Institute at the University of Utah, Salt Lake City. The results suggest that, when Black men with prostate cancer get the high quality of care seen in clinical trials and receive definitive therapy, they achieve good results.
It also suggests a path toward improving outcomes. “Through a multidisciplinary effort of enriching cohort studies with Black men, enrolling Black men into clinical trials and continuing the search for tumor-specific genomic factors, treatment-specific response factors, and pharmacologic response differences, as a community we can unequivocally improve prostate cancer care for Black men,” the editorial authors wrote.
Enrollment in clinical trials has also been linked to improved outcomes in studies of docetaxel and prednisone, enzalutamide and androgen deprivation therapy, and abiraterone acetate and prednisone. Other studies have shown that Black men in clinical trials or who get treated in high-volume centers are less likely to experience the adverse outcomes seen more widely among Black men.
The new finding that Black men have better outcomes with radiotherapy may also have a biological basis, as a retrospective study of patients undergoing prostatectomy for prostate cancer found that Black men had lower levels of mismatch repair genes and DNA repair activity.
The study isn’t the first to implicate access to care in outcome differential between Black and White men with prostate cancer. A 2019 study compared outcomes between White and Black men within registries that have standardized access, which is expected to minimize racial disparities. The researchers found no differences in prostate cancer–specific mortality within these databases. However, the differences in outcomes surfaced between Black and White men when they examined data from a large federal registry that reflects social and economic barriers to health care.
The authors of both the study and the editorial have extensive financial relationships with pharmaceutical companies.
Black men who received radiation therapy for localized prostate cancer fared better.
Overall, Black men have a 50% higher risk of being diagnosed with prostate cancer, and an 80% greater risk of death than White men. Those numbers have complicated roots: There are differences in access to medical care, clinical trial enrollment, access to screening, and frequency of definitive treatment.
The new study, published online Dec. 29, 2021, in JAMA Network Open, was a meta-analysis of 8,814 men (18.5% Black, 81.5% White) who participated in 7 randomized, clinical trials that compared definitive radiotherapy with or without short- or long-term androgen deprivation therapy. The researchers found that Black men had more features of high-risk disease, but they were less likely than White men to experience biochemical recurrence (subdistribution hazard ratio, 0.79; P < .001), distant metastasis (sHR, 0.69; P = .002), or prostate cancer-specific mortality (sHR, 0.68; P = .01).
“These results provide high-level evidence challenging the common belief that Black men who are diagnosed with prostate cancer will necessarily have a worse prognosis than White men,” said study coauthor Amar Kishan, MD, in a press release. Dr. Kishan is associate professor and vice chair of clinical and translational research at the University of California, Los Angeles, and a researcher at the UCLA Jonsson Comprehensive Cancer Center.
“This is especially important because an unfounded belief can inadvertently contribute to ‘cancer injustice,’ leading to the use of more aggressive treatments than might be necessary – potentially reducing quality of life and diverting attention away from other important factors that can influence outcome, including access to more comprehensive health care,” Dr. Kishan said.
Better health care coverage may indeed be the driving force behind the benefit, according to an accompanying editorial authored by Bogdana Schmidt, MD, MPH and Neeraj Agarwal, MD, of the Huntsman Cancer Institute at the University of Utah, Salt Lake City. The results suggest that, when Black men with prostate cancer get the high quality of care seen in clinical trials and receive definitive therapy, they achieve good results.
It also suggests a path toward improving outcomes. “Through a multidisciplinary effort of enriching cohort studies with Black men, enrolling Black men into clinical trials and continuing the search for tumor-specific genomic factors, treatment-specific response factors, and pharmacologic response differences, as a community we can unequivocally improve prostate cancer care for Black men,” the editorial authors wrote.
Enrollment in clinical trials has also been linked to improved outcomes in studies of docetaxel and prednisone, enzalutamide and androgen deprivation therapy, and abiraterone acetate and prednisone. Other studies have shown that Black men in clinical trials or who get treated in high-volume centers are less likely to experience the adverse outcomes seen more widely among Black men.
The new finding that Black men have better outcomes with radiotherapy may also have a biological basis, as a retrospective study of patients undergoing prostatectomy for prostate cancer found that Black men had lower levels of mismatch repair genes and DNA repair activity.
The study isn’t the first to implicate access to care in outcome differential between Black and White men with prostate cancer. A 2019 study compared outcomes between White and Black men within registries that have standardized access, which is expected to minimize racial disparities. The researchers found no differences in prostate cancer–specific mortality within these databases. However, the differences in outcomes surfaced between Black and White men when they examined data from a large federal registry that reflects social and economic barriers to health care.
The authors of both the study and the editorial have extensive financial relationships with pharmaceutical companies.
FROM JAMA NETWORK OPEN