User login
Pharmacist‐Directed Anticoagulation
Anticoagulants are one of the most common drug classes involved in medication errors and adverse events. Warfarin, an anticoagulant that plays a key role in the management of many disease states, is implicated in approximately 30% of reported anticoagulant‐related errors.1 Anticoagulation with warfarin is complicated by inter‐individual variability in response to therapy, clinically significant drug interactions, a narrow therapeutic window, and the need for frequent and lifelong monitoring.2
In the hospital setting, warfarin use is complicated due to patient handoff among health care providers, and acute illnesses that impact sensitivity and response to warfarin. Common causes of errors with anticoagulants are knowledge deficits, failure to follow policy/procedure/protocol, and communication issues.1 An added opportunity for warfarin‐related medication errors is the risk associated with the transition from the inpatient‐to‐outpatient setting. Due to the risk and complexity associated with anticoagulant medications, the Joint Commission instituted National Patient Safety Goal (NPSG) 03.05.01 (formerly NPSG 3E): a series of requirements intended to Reduce the likelihood of patient harm with the use of anticoagulation therapy.3 In order to optimally address this National Patient Safety Goal, a systematic intervention would be required to impact each step of the medication use process for anticoagulants.
Several studies have suggested that dedicated anticoagulation management services or clinics improve anticoagulation management in the outpatient setting.2 Non‐physician providers, primarily pharmacists and nurses, frequently manage outpatient anticoagulation management services or clinics. However, very few studies have evaluated the impact of a warfarin management service in the inpatient hospital setting.48 While the few available studies suggest some benefit associated with an inpatient anticoagulation management service, a minority of these studies have assessed the role of these services in facilitating the transition of the anticoagulated patient to the outpatient setting.7
In order to improve anticoagulation management and safety, our institution implemented an inpatient Pharmacist‐Directed Anticoagulation Service (PDAS). The purpose of this study was to evaluate the impact of this service on both transition of care and safety of patients receiving warfarin anticoagulation.
METHODS
This study was completed at Henry Ford Hospital, an 802‐bed, tertiary care, level 1 trauma and academic medical center in Detroit, MI. The study was carried out between November 2007 and June 2009. The study was approved by the Henry Ford Hospital Institutional Review Board with waiver of consent.
Patients
This was a prospective cluster randomized study. All patients admitted to two internal medicine units (IM1 or IM2) or two cardiology units (Card1 or Card2), who received at least one inpatient dose of warfarin, were eligible for inclusion. Patients were included regardless of whether warfarin was newly initiated during the index admission (newly initiated patients) or was continuation of existing anticoagulation (existing warfarin patients). In order to ensure that patient data following discharge would be available for analysis, patients were excluded from this analysis if they were not scheduled to follow‐up in the Henry Ford Medical Group outpatient anticoagulation clinics after discharge, however, these patients were cared for by the PDAS service in the usual manner.
Study Design
Prior to implementation of the PDAS, one internal medicine and one cardiology unit was randomly selected to receive the PDAS intervention (IM1 and Card1), while the other two units (IM2 and Card2) served as control units. These hospital units were selected because anticoagulants are frequently used on these units and the patient population is generally similar between the two internal medicine and two cardiology unitswith exception that Card1 unit also contains a specialized service for advanced heart failure and left ventricular assist device (LVAD) patients. Of note, there was significant expansion of the heart failure service and LVAD program during the time frame of the study, accounting for a greater number of more complicated patients on the Card1 (PDAS) unit.
Specific responsibilities of the PDAS related to warfarin are detailed in Table 1. The PDAS was implemented in September 2007 as a system‐based change to improve anticoagulant safety at our institution. The goals of this service were to improve communication regarding anticoagulation; to improve safety as patients transition from the inpatient‐to‐outpatient settings; and to standardize anticoagulant dosing, monitoring, and patient education. For patients taking warfarin, who are cared for by a health system‐affiliated physician, the PDAS collaborates with our outpatient anticoagulation clinics in order to facilitate transition from the inpatient‐to‐outpatient setting. The Henry Ford Health System has an established, multisite outpatient Anticoagulation Clinic with >5000 patients actively receiving warfarin dosing and monitoring. The anticoagulation clinics are staffed by nurses and pharmacists who provide standardized management of warfarin for patients of all physicians within our health system and provide consistent high‐quality care (average time in international normalized ratio [INR] goal range = 68.2%). The anticoagulation clinics have been in existence since 1992. The PDAS is comprised of three full‐time and two part‐time pharmacists whose responsibilities are limited to the management of anticoagulation throughout the hospital.
| Inpatient Care | Patient Education | Transition of Care |
|---|---|---|
| ||
| Initial dose selection and daily dose adjustments after warfarin is initiated by primary team | Comprehensive education provided verbally and via written communication utilizing the Krames database. | Contact anticoagulation‐responsible physician and anticoagulation clinic via phone. |
| Provide written dosing regimen to patient and provide date for first INR postdischarge. | ||
| Daily laboratory monitoring | Education provided is standardized between inpatient and outpatient settings. | Create electronic Anticoagulation Discharge Summary. Document communication with the outpatient clinicians, reason for admission, steps taken to manage warfarin drug interactions, and warfarin doses administered during stay, discharge warfarin dose and follow‐up date. |
The PDAS was staffed by repurposing pharmacist staff. All pharmacists had either several years of general medicine‐based clinical practice experience or residency training, or both. Pharmacists were oriented to service responsibilities by spending approximately one week in the outpatient anticoagulation clinic and completing focused review of internal and external anticoagulation guidelines.
In the control group, management of anticoagulation and transition of care occurred at the discretion of the primary care team. The primary team had access to a clinical pharmacist, who was not part of the PDAS, seven days per week. However, the primary team was not able to consult the PDAS.
This study was primarily designed to assess the impact of the PDAS on both transition of care and patient safety. For study endpoint purposes, transition of care was assessed by satisfactory completion and documentation of four important metrics: 1) appropriate enrollment in the anticoagulation clinic; 2) documented communication between the inpatient service responsible for anticoagulation and the outpatient anticoagulation clinic prior to patient discharge; 3) documented communication between the inpatient service responsible for anticoagulation and the physician responsible for outpatient management of the patient; 4) INR drawn within five days of hospital discharge. Documentation of communication for metric #2 and #3 was obtained by reviewing the electronic medical record system, particularly electronic discharge summaries and telephone encounter notes.
The primary safety endpoint was defined as a composite of any INR >5, any episode of major bleeding, or development of new thrombosis. This endpoint was met if any of these events occurred either during the index hospitalization or within 30 days of hospital discharge. Major bleeding was identified by review of outpatient anticoagulation clinic encounters and the patient's electronic medical record (includes all inpatient and outpatient encounters within Henry Ford Health System) by using the International Society of Thrombosis and Haemostasis standard and was defined as fatal bleeding or symptomatic bleeding in a critical area or organ (intracranial, intraspinal, intraocular, retroperitoneal, intraarticular, pericardial, or intramuscular with compartment syndrome), or bleeding causing a reduction in hemoglobin levels of 2 g/dL or more, or leading to transfusion of two or more units of blood or red cells.9 New thrombosis was defined as documentation of any of the following: deep vein thrombosis, pulmonary embolism, or cardioembolic stroke. Need for dose adjustment at the first anticoagulation clinic visit after discharge was evaluated as a secondary endpoint.
All analyses compared the PDAS to the control group. In addition, a planned comparison of patients in the PDAS and control groups who were newly initiated to warfarin during the study hospitalization (newly initiated subgroup) and those who were taking warfarin on admission (existing warfarin subgroup) was also undertaken. It was expected that these subgroup analyses would likely be underpowered, however, the potential implications of a service such as this could differ based on history of warfarin use. Therefore, these analyses were planned for exploratory purposes. In order to determine the impact of risk factors for altered warfarin pharmacodynamic response on the safety endpoint, post hoc subgroup analyses were performed based on demographics and clinical characteristics.
Data Analysis
Data are presented as mean standard deviation or proportion, as appropriate. A P‐value of less than 0.05 was considered significant for all comparisons and all tests were two‐tailed.
Intervention and control groups were compared with Student's t test, MannWhitney U test, chi‐square or Fishers exact test, as appropriate. Relative risk (RR) and 95% confidence intervals (CI) were calculated for all primary analyses. All statistical analyses were performed with SPSS v.12.0 (SPSS Inc, Chicago, IL).
It was estimated that a sample size of 250 patients per group would provide greater than 80% power to detect at least a 50% improvement in both the transition of care and primary safety endpoints, with implementation of the PDAS. This calculation is based on the following assumptions: alpha = 0.05; expected control group achievement of the four transition of care metrics = 50%; rate of safety endpoint for the control group = 20%.4
RESULTS
Baseline Characteristics
During the study period, 1360 patients were admitted to the study units. A total of 377 and 483 patients were found to be ineligible for inclusion on the PDAS and control units, respectively. These patients were ineligible because they did not follow up in the Henry Ford Medical Group outpatient anticoagulation clinic. In total, 500 patients were included in the analysis. Patients (n = 145) who were newly initiated on warfarin made up 29% of the total population. Table 2 presents baseline clinical characteristics for patients in the PDAS and control groups, showing increased age, and a greater proportion of patients with heart failure and LVADs in the PDAS group. Patients in the PDAS group had significantly longer hospital stays, however, these increases were driven by a longer length of stay among the advanced heart failure service patients that were managed by the PDAS.
| PDAS (n = 250) | Control (n = 250) | P Value | |
|---|---|---|---|
| |||
| Demographic data | |||
| Age (mean SD) | 64.1 15.6 | 68.0 14.9 | 0.004 |
| Male gender | 54.0% | 56.4% | 0.589 |
| Caucasian race | 44.4% | 50.4% | 0.179 |
| Admitted to a cardiology unit | 78.8% | 74.8% | 0.289 |
| Length of stay (mean SD) | 8.13 7.04 | 6.29 5.63 | 0.001 |
| No heart failure history: length of stay (mean SD) | 6.83 4.53 | 6.15 5.14 | 0.288 |
| Heart failure history: length of stay (mean SD) | 9.09 8.31 | 6.45 6.15 | 0.004 |
| History of heart failure* | 57.6% | 47.6% | 0.025 |
| Heart failure with an LVAD | 14.0% | 0.4% | <0.001 |
| Indication for anticoagulation | |||
| Venous thromboembolism | 21.6% | 18.4% | 0.371 |
| Atrial fibrillation | 54.4% | 66.4% | 0.006 |
| Other | 24.0% | 15.2% | 0.013 |
| Primary admission diagnosis* | |||
| Heart failure | 25.6%* | 21.6% | 0.292 |
| Atrial fibrillation | 16.4% | 20.8% | 0.206 |
| Acute coronary syndrome | 13.6% | 17.6% | 0.218 |
| Venous thromboembolism | 4.8% | 4.8% | 1.00 |
| Infection | 12.4% | 10.0% | 0.395 |
| Bleeding | 1.6% | 1.2% | 0.703 |
Early Warfarin Management
Warfarin management metrics are presented in Table 3. The number of inpatient days prescribed warfarin was increased in the PDAS group by greater than one day while PDAS patients required significantly less dosage adjustment at first outpatient follow‐up visit. Similar to increases noted with length of stay, increases in inpatient warfarin days were likely driven by patients with severe heart failure managed by the PDAS.
| Warfarin Dosing | PDAS (n = 250) | Control (n = 250) | P Value |
|---|---|---|---|
| |||
| Initial dose (mean SD) | 5.23 2.37 | 4.99 2.07 | 0.245 |
| Discharge dose (mean SD) | 5.15 2.52 | 4.91 2.14 | 0.258 |
| INR at discharge (mean SD) | 2.07 0.73 | 2.04 0.73 | 0.660 |
| Therapeutic INR at discharge | 40.8% | 38.0% | 0.522 |
| Inpatient warfarin days (mean SD) | 4.97 4.30 | 3.68 2.69 | <0.001 |
| No heart failure: inpatient warfarin days (mean SD) | 4.09 2.49 | 3.60 2.67 | 0.148 |
| Heart failure: inpatient warfarin days (mean SD) | 5.62 5.16 | 3.76 2.71 | <0.001 |
| Dose change required at first follow‐up visit | 44.8% | 72.6% | <0.001 |
Transition of Care
Transition of care results are presented in Table 4. Full compliance and achievement of the transition of care metrics occurred significantly more often in the PDAS versus control patients with markedly increased rates of documented communication between inpatient providers and both outpatient anticoagulation clinic staff and outpatient physicians. Early follow‐up INR monitoring also occurred more frequently in the PDAS patients. The PDAS patients experienced greater compliance with the transition of care metrics regardless of whether they were in the newly initiated or existing warfarin subgroups (data not shown).
| Transition of Care | PDAS (n = 250) | Control (n = 250) | Relative Risk (95% CI) | P Value |
|---|---|---|---|---|
| ||||
| 100% Communication bundle* compliance, % (n) | 75.6% (189) | 2.8% (7) | 27.0 (13.056.2) | <0.001 |
| Appropriately enrolled in the AC clinic, % (n) | 97.2% (243) | 95.2% (238) | 1.02 (0.991.06) | 0.242 |
| Communication: inpatient service and outpatient physician, % (n) | 99.6% (249) | 12.4% (31) | 8.03 (5.7811.2) | <0.001 |
| Communication: inpatient clinicians and AC clinic staff, % (n) | 98.8% (247) | 14.8% (37) | 6.68 (4.969.00) | <0.001 |
| INR drawn within five days of hospital discharge, % (n) | 78.4% (196) | 66.4% (166) | 1.18 (1.061.32) | 0.003 |
| 30‐Day Composite safety endpoint, % (n) | 10.0% (25) | 14.8% (37) | 0.68 (0.421.09) | 0.103 |
| Inpatient + 30‐day INR >5, % (n) | 9.6% (24) | 14.8% (37) | 0.65 (0.401.05) | 0.076 |
| Inpatient + 30‐day major bleeding, % (n) | 0.8% (2) | 0.4% (1) | 2.00 (0.1821.9) | 0.563 |
| Inpatient + 30‐day thrombosis, % (n) | 0% (0) | 0% (0) | N/A | N/A |
Anticoagulant Safety
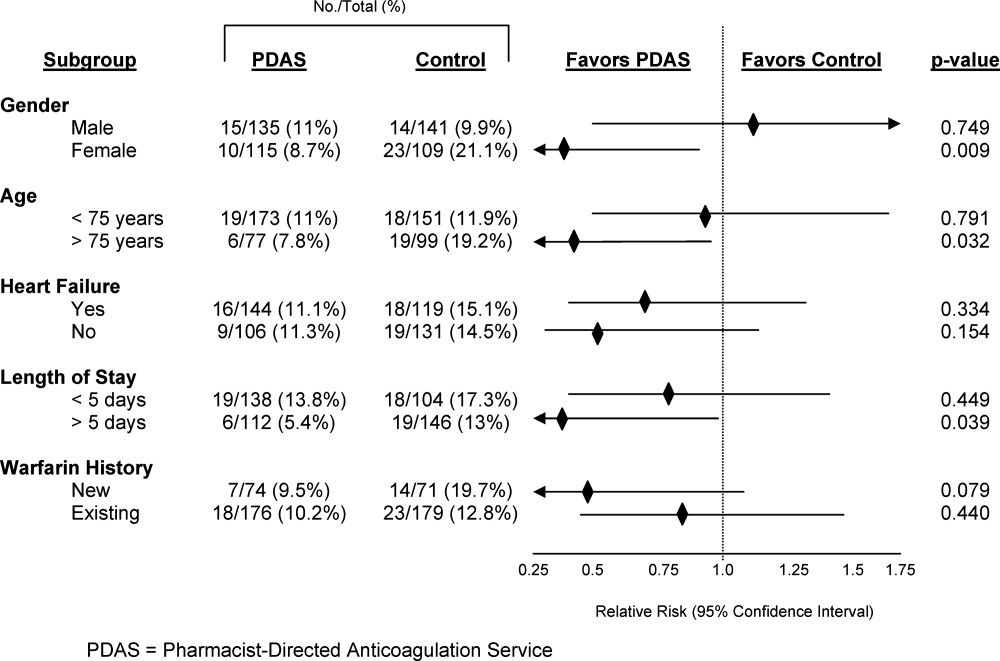
Safety endpoint data is presented in Table 4. The composite safety outcome of INR >5, major bleeding event, or thrombosis occurred in 12.4% of all patients with no early thrombotic events and only three major bleeding events recorded. Excessive INR values >5 occurred less frequently in the PDAS patients, however, differences in this metric and the composite safety outcome were not significantly different. Safety endpoint results in the overall population were driven by a reduction in INR values >5 among newly initiated warfarin patients in the PDAS group (PDAS: 9.5% vs control: 19.7%; P = 0.079; Figure 1). Other subgroup analyses relating to the safety endpoint are presented in Figure 1.
DISCUSSION
This article describes a systematic intervention designed to improve anticoagulation safety and efficacy in the hospital and during the transition to the postdischarge setting. Implementation of a PDAS did not impact patient bleeding and thrombotic outcomes, but did result in improved coordination and documentation of warfarin management and subsequent enhancement in the transition of the anticoagulated patient from the inpatient‐to‐outpatient setting with the Pharmacist‐Directed Anticoagulation Service.
Limited previous work has investigated the role of an anticoagulation service in inpatient management of anticoagulation.48 Only one published study has investigated the impact of this type of service on transition of care issues with warfarin, as was done in our study.7 In that study, management by an inpatient anticoagulation service resulted in a greater proportion of patients referred to an anticoagulation clinic for management (P = 0.001), more patients presenting to the anticoagulation clinic with a therapeutic INR (P = 0.001), and fewer patients presenting to the clinic with supratherapeutic levels of anticoagulation (P = 0.002). These results are somewhat analogous to our findings, in that patients in our study were less likely to require a dose change at the first clinic follow‐up visit after discharge or to have INR values 5.
We completed several subgroup analyses to thoroughly explore the impact of the PDAS on the safety endpoint. While firm conclusions cannot be drawn from these subgroup analyses, some hypothesis‐generating observations can be made. First, there was a greater impact of the PDAS on the safety endpoint in patients who are usually more sensitive to the effects of warfarin and therefore more challenging to manage.2 The impact of the PDAS was also greater among patients whose length of stay was more than five days (population median). This is significant because it suggests that when the opportunity for adverse events and miscommunication is greatest (ie, during hospitalizations of longer duration), there appears to be improvement in the safety endpoint with the PDAS.
To our knowledge, this study was the first to explore the impact of an inpatient anticoagulation service on the care of both newly initiated and existing warfarin patients, rather than only patients newly initiated on warfarin. As expected, the greatest influence of the PDAS on the safety endpoint was observed among the newly initiated patients. While the safety impact of the PDAS was noted most significantly among the newly initiated patients, the PDAS had a positive effect on the transition of care metrics regardless of previous warfarin use.
A limitation of our study should be mentioned. While we employed a design in which randomly selected units were exposed to the PDAS, individual patients were not randomized to the service. This cluster randomized design was chosen because it mimics quality improvement processes that would be rolled out to a hospital nursing unit. While lack of randomization at the patient level is a limitation, our cluster randomized study design is pragmatic and represents an improvement over the existing published before and after quasi‐experimental studies in this area.48
An improved system for documentation of communication was built into the PDAS processes on implementation of the service. However, it should also be noted that inadequate communication between inpatient and outpatient providers was an identified root cause for adverse events with warfarin in our institution prior to implementation of the PDAS. Therefore, improvement in the transition of care metrics with the PDAS were likely due to a combination of both improved documentation and true improvement in communication.
The approach of the PDAS was refined in several ways early after implementation of the service. Some major notable improvements included the development of a systematic approach to ensuring appropriate follow‐up and transition of care for patients being discharged to a skilled nursing facility, and creation of a system that required a mandatory conversation between the PDAS and a surgical service if anticoagulation is ordered within 48 hours of a major procedure. An upcoming improvement to the service will be to transition from a home grown electronic database, which was built for the purposes of streamlining clinical workflow and data collection, to a commercially available software program that has recently become available for management of inpatient anticoagulation. The major advantage of the new program will be the clinical decision support capabilities that will help to further streamline the service and allow for greater efficiency.
To understand implications of our PDAS intervention, it is important to remember that the majority of study patients were prescribed warfarin prior to hospital admission, that patients in both study groups were enrolled in an established, multisite anticoagulation clinic, and that patients in both groups were managed with a comprehensive inpatientoutpatient electronic medical record. Therefore, all providers caring for these patients had real‐time access to all warfarin dosing and dose adjustments, INR results, and anticoagulation clinic encounters, even though formal communication between providers was infrequently documented for control group patients in our electronic medical record. Study patients had low rates of bleeding and no adverse thrombotic outcomes across both treatment groups. Therefore, this type of model is likely to produce larger gains in communication and safety outcomes in health care systems without established anticoagulation clinics or comprehensive electronic medical records.
The PDAS was enthusiastically accepted by providers at our institution and expanded hospital‐wide after completion of this pilot. The PDAS model is a viable approach to standardize anticoagulant management with a goal of improving anticoagulant safety in the inpatient setting. Assessment of the effectiveness of models such as the PDAS for improving anticoagulant safety in the inpatient setting is particularly relevant with the current expectations for hospitals set by The Joint Commission's NPSG.03.05.01.3 More importantly the PDAS model can be an option for improving the transition of the anticoagulated patient from the inpatient‐to‐outpatient setting. Follow‐up with the anticoagulation clinic occurred earlier with the PDAS and, while this study was not designed to evaluate the impact of this new service on rehospitalization, recent literature suggests that earlier follow‐up after discharge leads to less rehospitalization.10 Finally, it may be possible to adapt this model to provide more intensive medication therapy management and monitoring for hospitalized patients with other complicated medication regimens or chronic disease.
CONCLUSION
The clinical pharmacist is uniquely prepared to manage inter‐individual variability in pharmacodynamic response to drug therapy, as well as to provide high‐quality patient education. This study evaluated a new model of inpatient warfarin management, in which warfarin dosing, monitoring, patient education, and transition of care was coordinated by a specialized team of clinical pharmacists that worked in collaboration with physicians and outpatient anticoagulation clinic staff. Safety and efficiency of the care provided by this new service was improved in certain subsets of more complex patients. The major advantage of this service was improvement in patient handoff, improved communication, and earlier patient follow‐up after discharge. Therefore, implementation of a Pharmacist‐Directed Anticoagulation Service provides a net improvement in quality of care for the patient taking warfarin in the inpatient setting.
Acknowledgements
The authors acknowledge the efforts of the PDAS staff: Nassif Abi‐Samra, Pam Holland, Sara Lanfear, and Gail Washington. This work would not have been possible without the dedication of these pharmacists. All authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
- U.S. Pharmacopeia. USP Patient Safety CapsLink: January 2008. Available at: http://www.usp.org/pdf/EN/patientSafety/capsLink2008–01‐01.pdf. Accessed March 19,2010.
- ,,,,,.Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians evidence‐based clinical practice guidelines (8th ed.).Chest.2008;133:160S–198S.
- The Joint Commission. 2009 National Patient Safety Goals. Available at: http://www.jointcommission.org/NR/rdonlyres/31666E86‐E7F4–423E‐9BE8‐F05BD1CB0AA8/0/HAP_NPSG.pdf. Accessed May 6,2010.
- ,,, et al.Optimization of inpatient warfarin therapy: impact of a daily consultation by a pharmacist‐managed anticoagulation service.Ann Pharmacother.2000;34:567–572.
- ,,, et al.Pharmacy‐managed protocol for warfarin use in orthopedic surgery patients.Am J Health‐Syst Pharm.1995;52:1310–1316.
- .Pharmacist involvement with warfarin dosing for inpatients.Pharm World Sci.2001;23:31–35.
- ,,.Evaluation of a pharmacy‐managed warfarin‐monitoring service to coordinate inpatient and outpatient therapy.Am J Hosp Pharm.1992;49:387–394.
- ,.Implementation and evaluation of a pharmacist‐assisted warfarin dosing program.Can J Hosp Pharm.1997;50:169–175.
- ,.Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non‐surgical patients.J Thromb Haemostasis.2005;3:692–694.
- ,,.Rehospitalizations among patients in the Medicare fee‐for‐service program.N Engl J Med.2009;360:1418–1428.
Anticoagulants are one of the most common drug classes involved in medication errors and adverse events. Warfarin, an anticoagulant that plays a key role in the management of many disease states, is implicated in approximately 30% of reported anticoagulant‐related errors.1 Anticoagulation with warfarin is complicated by inter‐individual variability in response to therapy, clinically significant drug interactions, a narrow therapeutic window, and the need for frequent and lifelong monitoring.2
In the hospital setting, warfarin use is complicated due to patient handoff among health care providers, and acute illnesses that impact sensitivity and response to warfarin. Common causes of errors with anticoagulants are knowledge deficits, failure to follow policy/procedure/protocol, and communication issues.1 An added opportunity for warfarin‐related medication errors is the risk associated with the transition from the inpatient‐to‐outpatient setting. Due to the risk and complexity associated with anticoagulant medications, the Joint Commission instituted National Patient Safety Goal (NPSG) 03.05.01 (formerly NPSG 3E): a series of requirements intended to Reduce the likelihood of patient harm with the use of anticoagulation therapy.3 In order to optimally address this National Patient Safety Goal, a systematic intervention would be required to impact each step of the medication use process for anticoagulants.
Several studies have suggested that dedicated anticoagulation management services or clinics improve anticoagulation management in the outpatient setting.2 Non‐physician providers, primarily pharmacists and nurses, frequently manage outpatient anticoagulation management services or clinics. However, very few studies have evaluated the impact of a warfarin management service in the inpatient hospital setting.48 While the few available studies suggest some benefit associated with an inpatient anticoagulation management service, a minority of these studies have assessed the role of these services in facilitating the transition of the anticoagulated patient to the outpatient setting.7
In order to improve anticoagulation management and safety, our institution implemented an inpatient Pharmacist‐Directed Anticoagulation Service (PDAS). The purpose of this study was to evaluate the impact of this service on both transition of care and safety of patients receiving warfarin anticoagulation.
METHODS
This study was completed at Henry Ford Hospital, an 802‐bed, tertiary care, level 1 trauma and academic medical center in Detroit, MI. The study was carried out between November 2007 and June 2009. The study was approved by the Henry Ford Hospital Institutional Review Board with waiver of consent.
Patients
This was a prospective cluster randomized study. All patients admitted to two internal medicine units (IM1 or IM2) or two cardiology units (Card1 or Card2), who received at least one inpatient dose of warfarin, were eligible for inclusion. Patients were included regardless of whether warfarin was newly initiated during the index admission (newly initiated patients) or was continuation of existing anticoagulation (existing warfarin patients). In order to ensure that patient data following discharge would be available for analysis, patients were excluded from this analysis if they were not scheduled to follow‐up in the Henry Ford Medical Group outpatient anticoagulation clinics after discharge, however, these patients were cared for by the PDAS service in the usual manner.
Study Design
Prior to implementation of the PDAS, one internal medicine and one cardiology unit was randomly selected to receive the PDAS intervention (IM1 and Card1), while the other two units (IM2 and Card2) served as control units. These hospital units were selected because anticoagulants are frequently used on these units and the patient population is generally similar between the two internal medicine and two cardiology unitswith exception that Card1 unit also contains a specialized service for advanced heart failure and left ventricular assist device (LVAD) patients. Of note, there was significant expansion of the heart failure service and LVAD program during the time frame of the study, accounting for a greater number of more complicated patients on the Card1 (PDAS) unit.
Specific responsibilities of the PDAS related to warfarin are detailed in Table 1. The PDAS was implemented in September 2007 as a system‐based change to improve anticoagulant safety at our institution. The goals of this service were to improve communication regarding anticoagulation; to improve safety as patients transition from the inpatient‐to‐outpatient settings; and to standardize anticoagulant dosing, monitoring, and patient education. For patients taking warfarin, who are cared for by a health system‐affiliated physician, the PDAS collaborates with our outpatient anticoagulation clinics in order to facilitate transition from the inpatient‐to‐outpatient setting. The Henry Ford Health System has an established, multisite outpatient Anticoagulation Clinic with >5000 patients actively receiving warfarin dosing and monitoring. The anticoagulation clinics are staffed by nurses and pharmacists who provide standardized management of warfarin for patients of all physicians within our health system and provide consistent high‐quality care (average time in international normalized ratio [INR] goal range = 68.2%). The anticoagulation clinics have been in existence since 1992. The PDAS is comprised of three full‐time and two part‐time pharmacists whose responsibilities are limited to the management of anticoagulation throughout the hospital.
| Inpatient Care | Patient Education | Transition of Care |
|---|---|---|
| ||
| Initial dose selection and daily dose adjustments after warfarin is initiated by primary team | Comprehensive education provided verbally and via written communication utilizing the Krames database. | Contact anticoagulation‐responsible physician and anticoagulation clinic via phone. |
| Provide written dosing regimen to patient and provide date for first INR postdischarge. | ||
| Daily laboratory monitoring | Education provided is standardized between inpatient and outpatient settings. | Create electronic Anticoagulation Discharge Summary. Document communication with the outpatient clinicians, reason for admission, steps taken to manage warfarin drug interactions, and warfarin doses administered during stay, discharge warfarin dose and follow‐up date. |
The PDAS was staffed by repurposing pharmacist staff. All pharmacists had either several years of general medicine‐based clinical practice experience or residency training, or both. Pharmacists were oriented to service responsibilities by spending approximately one week in the outpatient anticoagulation clinic and completing focused review of internal and external anticoagulation guidelines.
In the control group, management of anticoagulation and transition of care occurred at the discretion of the primary care team. The primary team had access to a clinical pharmacist, who was not part of the PDAS, seven days per week. However, the primary team was not able to consult the PDAS.
This study was primarily designed to assess the impact of the PDAS on both transition of care and patient safety. For study endpoint purposes, transition of care was assessed by satisfactory completion and documentation of four important metrics: 1) appropriate enrollment in the anticoagulation clinic; 2) documented communication between the inpatient service responsible for anticoagulation and the outpatient anticoagulation clinic prior to patient discharge; 3) documented communication between the inpatient service responsible for anticoagulation and the physician responsible for outpatient management of the patient; 4) INR drawn within five days of hospital discharge. Documentation of communication for metric #2 and #3 was obtained by reviewing the electronic medical record system, particularly electronic discharge summaries and telephone encounter notes.
The primary safety endpoint was defined as a composite of any INR >5, any episode of major bleeding, or development of new thrombosis. This endpoint was met if any of these events occurred either during the index hospitalization or within 30 days of hospital discharge. Major bleeding was identified by review of outpatient anticoagulation clinic encounters and the patient's electronic medical record (includes all inpatient and outpatient encounters within Henry Ford Health System) by using the International Society of Thrombosis and Haemostasis standard and was defined as fatal bleeding or symptomatic bleeding in a critical area or organ (intracranial, intraspinal, intraocular, retroperitoneal, intraarticular, pericardial, or intramuscular with compartment syndrome), or bleeding causing a reduction in hemoglobin levels of 2 g/dL or more, or leading to transfusion of two or more units of blood or red cells.9 New thrombosis was defined as documentation of any of the following: deep vein thrombosis, pulmonary embolism, or cardioembolic stroke. Need for dose adjustment at the first anticoagulation clinic visit after discharge was evaluated as a secondary endpoint.
All analyses compared the PDAS to the control group. In addition, a planned comparison of patients in the PDAS and control groups who were newly initiated to warfarin during the study hospitalization (newly initiated subgroup) and those who were taking warfarin on admission (existing warfarin subgroup) was also undertaken. It was expected that these subgroup analyses would likely be underpowered, however, the potential implications of a service such as this could differ based on history of warfarin use. Therefore, these analyses were planned for exploratory purposes. In order to determine the impact of risk factors for altered warfarin pharmacodynamic response on the safety endpoint, post hoc subgroup analyses were performed based on demographics and clinical characteristics.
Data Analysis
Data are presented as mean standard deviation or proportion, as appropriate. A P‐value of less than 0.05 was considered significant for all comparisons and all tests were two‐tailed.
Intervention and control groups were compared with Student's t test, MannWhitney U test, chi‐square or Fishers exact test, as appropriate. Relative risk (RR) and 95% confidence intervals (CI) were calculated for all primary analyses. All statistical analyses were performed with SPSS v.12.0 (SPSS Inc, Chicago, IL).
It was estimated that a sample size of 250 patients per group would provide greater than 80% power to detect at least a 50% improvement in both the transition of care and primary safety endpoints, with implementation of the PDAS. This calculation is based on the following assumptions: alpha = 0.05; expected control group achievement of the four transition of care metrics = 50%; rate of safety endpoint for the control group = 20%.4
RESULTS
Baseline Characteristics
During the study period, 1360 patients were admitted to the study units. A total of 377 and 483 patients were found to be ineligible for inclusion on the PDAS and control units, respectively. These patients were ineligible because they did not follow up in the Henry Ford Medical Group outpatient anticoagulation clinic. In total, 500 patients were included in the analysis. Patients (n = 145) who were newly initiated on warfarin made up 29% of the total population. Table 2 presents baseline clinical characteristics for patients in the PDAS and control groups, showing increased age, and a greater proportion of patients with heart failure and LVADs in the PDAS group. Patients in the PDAS group had significantly longer hospital stays, however, these increases were driven by a longer length of stay among the advanced heart failure service patients that were managed by the PDAS.
| PDAS (n = 250) | Control (n = 250) | P Value | |
|---|---|---|---|
| |||
| Demographic data | |||
| Age (mean SD) | 64.1 15.6 | 68.0 14.9 | 0.004 |
| Male gender | 54.0% | 56.4% | 0.589 |
| Caucasian race | 44.4% | 50.4% | 0.179 |
| Admitted to a cardiology unit | 78.8% | 74.8% | 0.289 |
| Length of stay (mean SD) | 8.13 7.04 | 6.29 5.63 | 0.001 |
| No heart failure history: length of stay (mean SD) | 6.83 4.53 | 6.15 5.14 | 0.288 |
| Heart failure history: length of stay (mean SD) | 9.09 8.31 | 6.45 6.15 | 0.004 |
| History of heart failure* | 57.6% | 47.6% | 0.025 |
| Heart failure with an LVAD | 14.0% | 0.4% | <0.001 |
| Indication for anticoagulation | |||
| Venous thromboembolism | 21.6% | 18.4% | 0.371 |
| Atrial fibrillation | 54.4% | 66.4% | 0.006 |
| Other | 24.0% | 15.2% | 0.013 |
| Primary admission diagnosis* | |||
| Heart failure | 25.6%* | 21.6% | 0.292 |
| Atrial fibrillation | 16.4% | 20.8% | 0.206 |
| Acute coronary syndrome | 13.6% | 17.6% | 0.218 |
| Venous thromboembolism | 4.8% | 4.8% | 1.00 |
| Infection | 12.4% | 10.0% | 0.395 |
| Bleeding | 1.6% | 1.2% | 0.703 |
Early Warfarin Management
Warfarin management metrics are presented in Table 3. The number of inpatient days prescribed warfarin was increased in the PDAS group by greater than one day while PDAS patients required significantly less dosage adjustment at first outpatient follow‐up visit. Similar to increases noted with length of stay, increases in inpatient warfarin days were likely driven by patients with severe heart failure managed by the PDAS.
| Warfarin Dosing | PDAS (n = 250) | Control (n = 250) | P Value |
|---|---|---|---|
| |||
| Initial dose (mean SD) | 5.23 2.37 | 4.99 2.07 | 0.245 |
| Discharge dose (mean SD) | 5.15 2.52 | 4.91 2.14 | 0.258 |
| INR at discharge (mean SD) | 2.07 0.73 | 2.04 0.73 | 0.660 |
| Therapeutic INR at discharge | 40.8% | 38.0% | 0.522 |
| Inpatient warfarin days (mean SD) | 4.97 4.30 | 3.68 2.69 | <0.001 |
| No heart failure: inpatient warfarin days (mean SD) | 4.09 2.49 | 3.60 2.67 | 0.148 |
| Heart failure: inpatient warfarin days (mean SD) | 5.62 5.16 | 3.76 2.71 | <0.001 |
| Dose change required at first follow‐up visit | 44.8% | 72.6% | <0.001 |
Transition of Care
Transition of care results are presented in Table 4. Full compliance and achievement of the transition of care metrics occurred significantly more often in the PDAS versus control patients with markedly increased rates of documented communication between inpatient providers and both outpatient anticoagulation clinic staff and outpatient physicians. Early follow‐up INR monitoring also occurred more frequently in the PDAS patients. The PDAS patients experienced greater compliance with the transition of care metrics regardless of whether they were in the newly initiated or existing warfarin subgroups (data not shown).
| Transition of Care | PDAS (n = 250) | Control (n = 250) | Relative Risk (95% CI) | P Value |
|---|---|---|---|---|
| ||||
| 100% Communication bundle* compliance, % (n) | 75.6% (189) | 2.8% (7) | 27.0 (13.056.2) | <0.001 |
| Appropriately enrolled in the AC clinic, % (n) | 97.2% (243) | 95.2% (238) | 1.02 (0.991.06) | 0.242 |
| Communication: inpatient service and outpatient physician, % (n) | 99.6% (249) | 12.4% (31) | 8.03 (5.7811.2) | <0.001 |
| Communication: inpatient clinicians and AC clinic staff, % (n) | 98.8% (247) | 14.8% (37) | 6.68 (4.969.00) | <0.001 |
| INR drawn within five days of hospital discharge, % (n) | 78.4% (196) | 66.4% (166) | 1.18 (1.061.32) | 0.003 |
| 30‐Day Composite safety endpoint, % (n) | 10.0% (25) | 14.8% (37) | 0.68 (0.421.09) | 0.103 |
| Inpatient + 30‐day INR >5, % (n) | 9.6% (24) | 14.8% (37) | 0.65 (0.401.05) | 0.076 |
| Inpatient + 30‐day major bleeding, % (n) | 0.8% (2) | 0.4% (1) | 2.00 (0.1821.9) | 0.563 |
| Inpatient + 30‐day thrombosis, % (n) | 0% (0) | 0% (0) | N/A | N/A |
Anticoagulant Safety
Safety endpoint data is presented in Table 4. The composite safety outcome of INR >5, major bleeding event, or thrombosis occurred in 12.4% of all patients with no early thrombotic events and only three major bleeding events recorded. Excessive INR values >5 occurred less frequently in the PDAS patients, however, differences in this metric and the composite safety outcome were not significantly different. Safety endpoint results in the overall population were driven by a reduction in INR values >5 among newly initiated warfarin patients in the PDAS group (PDAS: 9.5% vs control: 19.7%; P = 0.079; Figure 1). Other subgroup analyses relating to the safety endpoint are presented in Figure 1.

DISCUSSION
This article describes a systematic intervention designed to improve anticoagulation safety and efficacy in the hospital and during the transition to the postdischarge setting. Implementation of a PDAS did not impact patient bleeding and thrombotic outcomes, but did result in improved coordination and documentation of warfarin management and subsequent enhancement in the transition of the anticoagulated patient from the inpatient‐to‐outpatient setting with the Pharmacist‐Directed Anticoagulation Service.
Limited previous work has investigated the role of an anticoagulation service in inpatient management of anticoagulation.48 Only one published study has investigated the impact of this type of service on transition of care issues with warfarin, as was done in our study.7 In that study, management by an inpatient anticoagulation service resulted in a greater proportion of patients referred to an anticoagulation clinic for management (P = 0.001), more patients presenting to the anticoagulation clinic with a therapeutic INR (P = 0.001), and fewer patients presenting to the clinic with supratherapeutic levels of anticoagulation (P = 0.002). These results are somewhat analogous to our findings, in that patients in our study were less likely to require a dose change at the first clinic follow‐up visit after discharge or to have INR values 5.
We completed several subgroup analyses to thoroughly explore the impact of the PDAS on the safety endpoint. While firm conclusions cannot be drawn from these subgroup analyses, some hypothesis‐generating observations can be made. First, there was a greater impact of the PDAS on the safety endpoint in patients who are usually more sensitive to the effects of warfarin and therefore more challenging to manage.2 The impact of the PDAS was also greater among patients whose length of stay was more than five days (population median). This is significant because it suggests that when the opportunity for adverse events and miscommunication is greatest (ie, during hospitalizations of longer duration), there appears to be improvement in the safety endpoint with the PDAS.
To our knowledge, this study was the first to explore the impact of an inpatient anticoagulation service on the care of both newly initiated and existing warfarin patients, rather than only patients newly initiated on warfarin. As expected, the greatest influence of the PDAS on the safety endpoint was observed among the newly initiated patients. While the safety impact of the PDAS was noted most significantly among the newly initiated patients, the PDAS had a positive effect on the transition of care metrics regardless of previous warfarin use.
A limitation of our study should be mentioned. While we employed a design in which randomly selected units were exposed to the PDAS, individual patients were not randomized to the service. This cluster randomized design was chosen because it mimics quality improvement processes that would be rolled out to a hospital nursing unit. While lack of randomization at the patient level is a limitation, our cluster randomized study design is pragmatic and represents an improvement over the existing published before and after quasi‐experimental studies in this area.48
An improved system for documentation of communication was built into the PDAS processes on implementation of the service. However, it should also be noted that inadequate communication between inpatient and outpatient providers was an identified root cause for adverse events with warfarin in our institution prior to implementation of the PDAS. Therefore, improvement in the transition of care metrics with the PDAS were likely due to a combination of both improved documentation and true improvement in communication.
The approach of the PDAS was refined in several ways early after implementation of the service. Some major notable improvements included the development of a systematic approach to ensuring appropriate follow‐up and transition of care for patients being discharged to a skilled nursing facility, and creation of a system that required a mandatory conversation between the PDAS and a surgical service if anticoagulation is ordered within 48 hours of a major procedure. An upcoming improvement to the service will be to transition from a home grown electronic database, which was built for the purposes of streamlining clinical workflow and data collection, to a commercially available software program that has recently become available for management of inpatient anticoagulation. The major advantage of the new program will be the clinical decision support capabilities that will help to further streamline the service and allow for greater efficiency.
To understand implications of our PDAS intervention, it is important to remember that the majority of study patients were prescribed warfarin prior to hospital admission, that patients in both study groups were enrolled in an established, multisite anticoagulation clinic, and that patients in both groups were managed with a comprehensive inpatientoutpatient electronic medical record. Therefore, all providers caring for these patients had real‐time access to all warfarin dosing and dose adjustments, INR results, and anticoagulation clinic encounters, even though formal communication between providers was infrequently documented for control group patients in our electronic medical record. Study patients had low rates of bleeding and no adverse thrombotic outcomes across both treatment groups. Therefore, this type of model is likely to produce larger gains in communication and safety outcomes in health care systems without established anticoagulation clinics or comprehensive electronic medical records.
The PDAS was enthusiastically accepted by providers at our institution and expanded hospital‐wide after completion of this pilot. The PDAS model is a viable approach to standardize anticoagulant management with a goal of improving anticoagulant safety in the inpatient setting. Assessment of the effectiveness of models such as the PDAS for improving anticoagulant safety in the inpatient setting is particularly relevant with the current expectations for hospitals set by The Joint Commission's NPSG.03.05.01.3 More importantly the PDAS model can be an option for improving the transition of the anticoagulated patient from the inpatient‐to‐outpatient setting. Follow‐up with the anticoagulation clinic occurred earlier with the PDAS and, while this study was not designed to evaluate the impact of this new service on rehospitalization, recent literature suggests that earlier follow‐up after discharge leads to less rehospitalization.10 Finally, it may be possible to adapt this model to provide more intensive medication therapy management and monitoring for hospitalized patients with other complicated medication regimens or chronic disease.
CONCLUSION
The clinical pharmacist is uniquely prepared to manage inter‐individual variability in pharmacodynamic response to drug therapy, as well as to provide high‐quality patient education. This study evaluated a new model of inpatient warfarin management, in which warfarin dosing, monitoring, patient education, and transition of care was coordinated by a specialized team of clinical pharmacists that worked in collaboration with physicians and outpatient anticoagulation clinic staff. Safety and efficiency of the care provided by this new service was improved in certain subsets of more complex patients. The major advantage of this service was improvement in patient handoff, improved communication, and earlier patient follow‐up after discharge. Therefore, implementation of a Pharmacist‐Directed Anticoagulation Service provides a net improvement in quality of care for the patient taking warfarin in the inpatient setting.
Acknowledgements
The authors acknowledge the efforts of the PDAS staff: Nassif Abi‐Samra, Pam Holland, Sara Lanfear, and Gail Washington. This work would not have been possible without the dedication of these pharmacists. All authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Anticoagulants are one of the most common drug classes involved in medication errors and adverse events. Warfarin, an anticoagulant that plays a key role in the management of many disease states, is implicated in approximately 30% of reported anticoagulant‐related errors.1 Anticoagulation with warfarin is complicated by inter‐individual variability in response to therapy, clinically significant drug interactions, a narrow therapeutic window, and the need for frequent and lifelong monitoring.2
In the hospital setting, warfarin use is complicated due to patient handoff among health care providers, and acute illnesses that impact sensitivity and response to warfarin. Common causes of errors with anticoagulants are knowledge deficits, failure to follow policy/procedure/protocol, and communication issues.1 An added opportunity for warfarin‐related medication errors is the risk associated with the transition from the inpatient‐to‐outpatient setting. Due to the risk and complexity associated with anticoagulant medications, the Joint Commission instituted National Patient Safety Goal (NPSG) 03.05.01 (formerly NPSG 3E): a series of requirements intended to Reduce the likelihood of patient harm with the use of anticoagulation therapy.3 In order to optimally address this National Patient Safety Goal, a systematic intervention would be required to impact each step of the medication use process for anticoagulants.
Several studies have suggested that dedicated anticoagulation management services or clinics improve anticoagulation management in the outpatient setting.2 Non‐physician providers, primarily pharmacists and nurses, frequently manage outpatient anticoagulation management services or clinics. However, very few studies have evaluated the impact of a warfarin management service in the inpatient hospital setting.48 While the few available studies suggest some benefit associated with an inpatient anticoagulation management service, a minority of these studies have assessed the role of these services in facilitating the transition of the anticoagulated patient to the outpatient setting.7
In order to improve anticoagulation management and safety, our institution implemented an inpatient Pharmacist‐Directed Anticoagulation Service (PDAS). The purpose of this study was to evaluate the impact of this service on both transition of care and safety of patients receiving warfarin anticoagulation.
METHODS
This study was completed at Henry Ford Hospital, an 802‐bed, tertiary care, level 1 trauma and academic medical center in Detroit, MI. The study was carried out between November 2007 and June 2009. The study was approved by the Henry Ford Hospital Institutional Review Board with waiver of consent.
Patients
This was a prospective cluster randomized study. All patients admitted to two internal medicine units (IM1 or IM2) or two cardiology units (Card1 or Card2), who received at least one inpatient dose of warfarin, were eligible for inclusion. Patients were included regardless of whether warfarin was newly initiated during the index admission (newly initiated patients) or was continuation of existing anticoagulation (existing warfarin patients). In order to ensure that patient data following discharge would be available for analysis, patients were excluded from this analysis if they were not scheduled to follow‐up in the Henry Ford Medical Group outpatient anticoagulation clinics after discharge, however, these patients were cared for by the PDAS service in the usual manner.
Study Design
Prior to implementation of the PDAS, one internal medicine and one cardiology unit was randomly selected to receive the PDAS intervention (IM1 and Card1), while the other two units (IM2 and Card2) served as control units. These hospital units were selected because anticoagulants are frequently used on these units and the patient population is generally similar between the two internal medicine and two cardiology unitswith exception that Card1 unit also contains a specialized service for advanced heart failure and left ventricular assist device (LVAD) patients. Of note, there was significant expansion of the heart failure service and LVAD program during the time frame of the study, accounting for a greater number of more complicated patients on the Card1 (PDAS) unit.
Specific responsibilities of the PDAS related to warfarin are detailed in Table 1. The PDAS was implemented in September 2007 as a system‐based change to improve anticoagulant safety at our institution. The goals of this service were to improve communication regarding anticoagulation; to improve safety as patients transition from the inpatient‐to‐outpatient settings; and to standardize anticoagulant dosing, monitoring, and patient education. For patients taking warfarin, who are cared for by a health system‐affiliated physician, the PDAS collaborates with our outpatient anticoagulation clinics in order to facilitate transition from the inpatient‐to‐outpatient setting. The Henry Ford Health System has an established, multisite outpatient Anticoagulation Clinic with >5000 patients actively receiving warfarin dosing and monitoring. The anticoagulation clinics are staffed by nurses and pharmacists who provide standardized management of warfarin for patients of all physicians within our health system and provide consistent high‐quality care (average time in international normalized ratio [INR] goal range = 68.2%). The anticoagulation clinics have been in existence since 1992. The PDAS is comprised of three full‐time and two part‐time pharmacists whose responsibilities are limited to the management of anticoagulation throughout the hospital.
| Inpatient Care | Patient Education | Transition of Care |
|---|---|---|
| ||
| Initial dose selection and daily dose adjustments after warfarin is initiated by primary team | Comprehensive education provided verbally and via written communication utilizing the Krames database. | Contact anticoagulation‐responsible physician and anticoagulation clinic via phone. |
| Provide written dosing regimen to patient and provide date for first INR postdischarge. | ||
| Daily laboratory monitoring | Education provided is standardized between inpatient and outpatient settings. | Create electronic Anticoagulation Discharge Summary. Document communication with the outpatient clinicians, reason for admission, steps taken to manage warfarin drug interactions, and warfarin doses administered during stay, discharge warfarin dose and follow‐up date. |
The PDAS was staffed by repurposing pharmacist staff. All pharmacists had either several years of general medicine‐based clinical practice experience or residency training, or both. Pharmacists were oriented to service responsibilities by spending approximately one week in the outpatient anticoagulation clinic and completing focused review of internal and external anticoagulation guidelines.
In the control group, management of anticoagulation and transition of care occurred at the discretion of the primary care team. The primary team had access to a clinical pharmacist, who was not part of the PDAS, seven days per week. However, the primary team was not able to consult the PDAS.
This study was primarily designed to assess the impact of the PDAS on both transition of care and patient safety. For study endpoint purposes, transition of care was assessed by satisfactory completion and documentation of four important metrics: 1) appropriate enrollment in the anticoagulation clinic; 2) documented communication between the inpatient service responsible for anticoagulation and the outpatient anticoagulation clinic prior to patient discharge; 3) documented communication between the inpatient service responsible for anticoagulation and the physician responsible for outpatient management of the patient; 4) INR drawn within five days of hospital discharge. Documentation of communication for metric #2 and #3 was obtained by reviewing the electronic medical record system, particularly electronic discharge summaries and telephone encounter notes.
The primary safety endpoint was defined as a composite of any INR >5, any episode of major bleeding, or development of new thrombosis. This endpoint was met if any of these events occurred either during the index hospitalization or within 30 days of hospital discharge. Major bleeding was identified by review of outpatient anticoagulation clinic encounters and the patient's electronic medical record (includes all inpatient and outpatient encounters within Henry Ford Health System) by using the International Society of Thrombosis and Haemostasis standard and was defined as fatal bleeding or symptomatic bleeding in a critical area or organ (intracranial, intraspinal, intraocular, retroperitoneal, intraarticular, pericardial, or intramuscular with compartment syndrome), or bleeding causing a reduction in hemoglobin levels of 2 g/dL or more, or leading to transfusion of two or more units of blood or red cells.9 New thrombosis was defined as documentation of any of the following: deep vein thrombosis, pulmonary embolism, or cardioembolic stroke. Need for dose adjustment at the first anticoagulation clinic visit after discharge was evaluated as a secondary endpoint.
All analyses compared the PDAS to the control group. In addition, a planned comparison of patients in the PDAS and control groups who were newly initiated to warfarin during the study hospitalization (newly initiated subgroup) and those who were taking warfarin on admission (existing warfarin subgroup) was also undertaken. It was expected that these subgroup analyses would likely be underpowered, however, the potential implications of a service such as this could differ based on history of warfarin use. Therefore, these analyses were planned for exploratory purposes. In order to determine the impact of risk factors for altered warfarin pharmacodynamic response on the safety endpoint, post hoc subgroup analyses were performed based on demographics and clinical characteristics.
Data Analysis
Data are presented as mean standard deviation or proportion, as appropriate. A P‐value of less than 0.05 was considered significant for all comparisons and all tests were two‐tailed.
Intervention and control groups were compared with Student's t test, MannWhitney U test, chi‐square or Fishers exact test, as appropriate. Relative risk (RR) and 95% confidence intervals (CI) were calculated for all primary analyses. All statistical analyses were performed with SPSS v.12.0 (SPSS Inc, Chicago, IL).
It was estimated that a sample size of 250 patients per group would provide greater than 80% power to detect at least a 50% improvement in both the transition of care and primary safety endpoints, with implementation of the PDAS. This calculation is based on the following assumptions: alpha = 0.05; expected control group achievement of the four transition of care metrics = 50%; rate of safety endpoint for the control group = 20%.4
RESULTS
Baseline Characteristics
During the study period, 1360 patients were admitted to the study units. A total of 377 and 483 patients were found to be ineligible for inclusion on the PDAS and control units, respectively. These patients were ineligible because they did not follow up in the Henry Ford Medical Group outpatient anticoagulation clinic. In total, 500 patients were included in the analysis. Patients (n = 145) who were newly initiated on warfarin made up 29% of the total population. Table 2 presents baseline clinical characteristics for patients in the PDAS and control groups, showing increased age, and a greater proportion of patients with heart failure and LVADs in the PDAS group. Patients in the PDAS group had significantly longer hospital stays, however, these increases were driven by a longer length of stay among the advanced heart failure service patients that were managed by the PDAS.
| PDAS (n = 250) | Control (n = 250) | P Value | |
|---|---|---|---|
| |||
| Demographic data | |||
| Age (mean SD) | 64.1 15.6 | 68.0 14.9 | 0.004 |
| Male gender | 54.0% | 56.4% | 0.589 |
| Caucasian race | 44.4% | 50.4% | 0.179 |
| Admitted to a cardiology unit | 78.8% | 74.8% | 0.289 |
| Length of stay (mean SD) | 8.13 7.04 | 6.29 5.63 | 0.001 |
| No heart failure history: length of stay (mean SD) | 6.83 4.53 | 6.15 5.14 | 0.288 |
| Heart failure history: length of stay (mean SD) | 9.09 8.31 | 6.45 6.15 | 0.004 |
| History of heart failure* | 57.6% | 47.6% | 0.025 |
| Heart failure with an LVAD | 14.0% | 0.4% | <0.001 |
| Indication for anticoagulation | |||
| Venous thromboembolism | 21.6% | 18.4% | 0.371 |
| Atrial fibrillation | 54.4% | 66.4% | 0.006 |
| Other | 24.0% | 15.2% | 0.013 |
| Primary admission diagnosis* | |||
| Heart failure | 25.6%* | 21.6% | 0.292 |
| Atrial fibrillation | 16.4% | 20.8% | 0.206 |
| Acute coronary syndrome | 13.6% | 17.6% | 0.218 |
| Venous thromboembolism | 4.8% | 4.8% | 1.00 |
| Infection | 12.4% | 10.0% | 0.395 |
| Bleeding | 1.6% | 1.2% | 0.703 |
Early Warfarin Management
Warfarin management metrics are presented in Table 3. The number of inpatient days prescribed warfarin was increased in the PDAS group by greater than one day while PDAS patients required significantly less dosage adjustment at first outpatient follow‐up visit. Similar to increases noted with length of stay, increases in inpatient warfarin days were likely driven by patients with severe heart failure managed by the PDAS.
| Warfarin Dosing | PDAS (n = 250) | Control (n = 250) | P Value |
|---|---|---|---|
| |||
| Initial dose (mean SD) | 5.23 2.37 | 4.99 2.07 | 0.245 |
| Discharge dose (mean SD) | 5.15 2.52 | 4.91 2.14 | 0.258 |
| INR at discharge (mean SD) | 2.07 0.73 | 2.04 0.73 | 0.660 |
| Therapeutic INR at discharge | 40.8% | 38.0% | 0.522 |
| Inpatient warfarin days (mean SD) | 4.97 4.30 | 3.68 2.69 | <0.001 |
| No heart failure: inpatient warfarin days (mean SD) | 4.09 2.49 | 3.60 2.67 | 0.148 |
| Heart failure: inpatient warfarin days (mean SD) | 5.62 5.16 | 3.76 2.71 | <0.001 |
| Dose change required at first follow‐up visit | 44.8% | 72.6% | <0.001 |
Transition of Care
Transition of care results are presented in Table 4. Full compliance and achievement of the transition of care metrics occurred significantly more often in the PDAS versus control patients with markedly increased rates of documented communication between inpatient providers and both outpatient anticoagulation clinic staff and outpatient physicians. Early follow‐up INR monitoring also occurred more frequently in the PDAS patients. The PDAS patients experienced greater compliance with the transition of care metrics regardless of whether they were in the newly initiated or existing warfarin subgroups (data not shown).
| Transition of Care | PDAS (n = 250) | Control (n = 250) | Relative Risk (95% CI) | P Value |
|---|---|---|---|---|
| ||||
| 100% Communication bundle* compliance, % (n) | 75.6% (189) | 2.8% (7) | 27.0 (13.056.2) | <0.001 |
| Appropriately enrolled in the AC clinic, % (n) | 97.2% (243) | 95.2% (238) | 1.02 (0.991.06) | 0.242 |
| Communication: inpatient service and outpatient physician, % (n) | 99.6% (249) | 12.4% (31) | 8.03 (5.7811.2) | <0.001 |
| Communication: inpatient clinicians and AC clinic staff, % (n) | 98.8% (247) | 14.8% (37) | 6.68 (4.969.00) | <0.001 |
| INR drawn within five days of hospital discharge, % (n) | 78.4% (196) | 66.4% (166) | 1.18 (1.061.32) | 0.003 |
| 30‐Day Composite safety endpoint, % (n) | 10.0% (25) | 14.8% (37) | 0.68 (0.421.09) | 0.103 |
| Inpatient + 30‐day INR >5, % (n) | 9.6% (24) | 14.8% (37) | 0.65 (0.401.05) | 0.076 |
| Inpatient + 30‐day major bleeding, % (n) | 0.8% (2) | 0.4% (1) | 2.00 (0.1821.9) | 0.563 |
| Inpatient + 30‐day thrombosis, % (n) | 0% (0) | 0% (0) | N/A | N/A |
Anticoagulant Safety
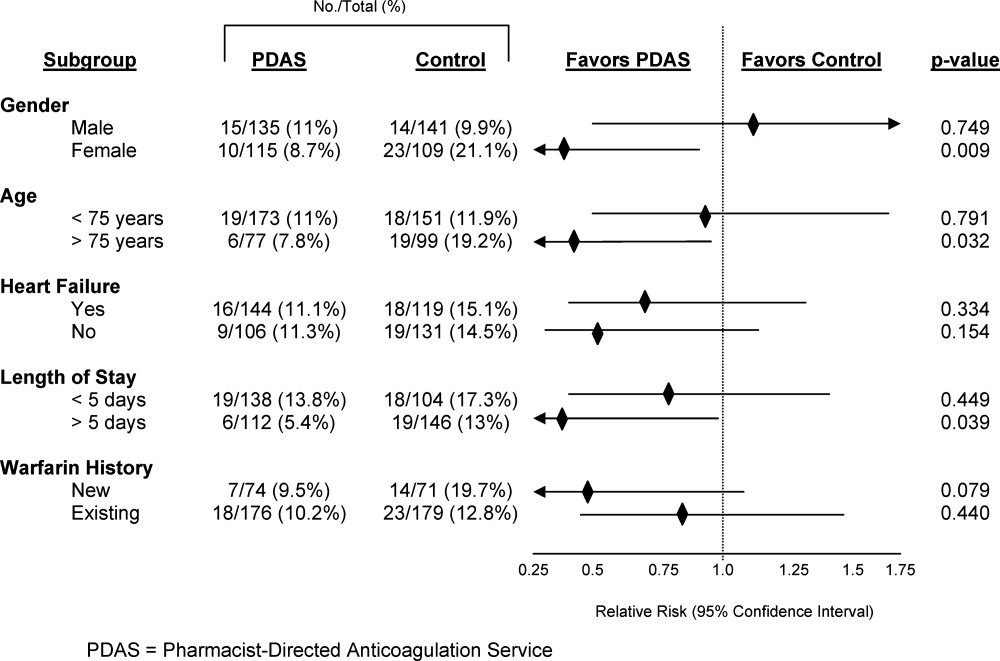
Safety endpoint data is presented in Table 4. The composite safety outcome of INR >5, major bleeding event, or thrombosis occurred in 12.4% of all patients with no early thrombotic events and only three major bleeding events recorded. Excessive INR values >5 occurred less frequently in the PDAS patients, however, differences in this metric and the composite safety outcome were not significantly different. Safety endpoint results in the overall population were driven by a reduction in INR values >5 among newly initiated warfarin patients in the PDAS group (PDAS: 9.5% vs control: 19.7%; P = 0.079; Figure 1). Other subgroup analyses relating to the safety endpoint are presented in Figure 1.

DISCUSSION
This article describes a systematic intervention designed to improve anticoagulation safety and efficacy in the hospital and during the transition to the postdischarge setting. Implementation of a PDAS did not impact patient bleeding and thrombotic outcomes, but did result in improved coordination and documentation of warfarin management and subsequent enhancement in the transition of the anticoagulated patient from the inpatient‐to‐outpatient setting with the Pharmacist‐Directed Anticoagulation Service.
Limited previous work has investigated the role of an anticoagulation service in inpatient management of anticoagulation.48 Only one published study has investigated the impact of this type of service on transition of care issues with warfarin, as was done in our study.7 In that study, management by an inpatient anticoagulation service resulted in a greater proportion of patients referred to an anticoagulation clinic for management (P = 0.001), more patients presenting to the anticoagulation clinic with a therapeutic INR (P = 0.001), and fewer patients presenting to the clinic with supratherapeutic levels of anticoagulation (P = 0.002). These results are somewhat analogous to our findings, in that patients in our study were less likely to require a dose change at the first clinic follow‐up visit after discharge or to have INR values 5.
We completed several subgroup analyses to thoroughly explore the impact of the PDAS on the safety endpoint. While firm conclusions cannot be drawn from these subgroup analyses, some hypothesis‐generating observations can be made. First, there was a greater impact of the PDAS on the safety endpoint in patients who are usually more sensitive to the effects of warfarin and therefore more challenging to manage.2 The impact of the PDAS was also greater among patients whose length of stay was more than five days (population median). This is significant because it suggests that when the opportunity for adverse events and miscommunication is greatest (ie, during hospitalizations of longer duration), there appears to be improvement in the safety endpoint with the PDAS.
To our knowledge, this study was the first to explore the impact of an inpatient anticoagulation service on the care of both newly initiated and existing warfarin patients, rather than only patients newly initiated on warfarin. As expected, the greatest influence of the PDAS on the safety endpoint was observed among the newly initiated patients. While the safety impact of the PDAS was noted most significantly among the newly initiated patients, the PDAS had a positive effect on the transition of care metrics regardless of previous warfarin use.
A limitation of our study should be mentioned. While we employed a design in which randomly selected units were exposed to the PDAS, individual patients were not randomized to the service. This cluster randomized design was chosen because it mimics quality improvement processes that would be rolled out to a hospital nursing unit. While lack of randomization at the patient level is a limitation, our cluster randomized study design is pragmatic and represents an improvement over the existing published before and after quasi‐experimental studies in this area.48
An improved system for documentation of communication was built into the PDAS processes on implementation of the service. However, it should also be noted that inadequate communication between inpatient and outpatient providers was an identified root cause for adverse events with warfarin in our institution prior to implementation of the PDAS. Therefore, improvement in the transition of care metrics with the PDAS were likely due to a combination of both improved documentation and true improvement in communication.
The approach of the PDAS was refined in several ways early after implementation of the service. Some major notable improvements included the development of a systematic approach to ensuring appropriate follow‐up and transition of care for patients being discharged to a skilled nursing facility, and creation of a system that required a mandatory conversation between the PDAS and a surgical service if anticoagulation is ordered within 48 hours of a major procedure. An upcoming improvement to the service will be to transition from a home grown electronic database, which was built for the purposes of streamlining clinical workflow and data collection, to a commercially available software program that has recently become available for management of inpatient anticoagulation. The major advantage of the new program will be the clinical decision support capabilities that will help to further streamline the service and allow for greater efficiency.
To understand implications of our PDAS intervention, it is important to remember that the majority of study patients were prescribed warfarin prior to hospital admission, that patients in both study groups were enrolled in an established, multisite anticoagulation clinic, and that patients in both groups were managed with a comprehensive inpatientoutpatient electronic medical record. Therefore, all providers caring for these patients had real‐time access to all warfarin dosing and dose adjustments, INR results, and anticoagulation clinic encounters, even though formal communication between providers was infrequently documented for control group patients in our electronic medical record. Study patients had low rates of bleeding and no adverse thrombotic outcomes across both treatment groups. Therefore, this type of model is likely to produce larger gains in communication and safety outcomes in health care systems without established anticoagulation clinics or comprehensive electronic medical records.
The PDAS was enthusiastically accepted by providers at our institution and expanded hospital‐wide after completion of this pilot. The PDAS model is a viable approach to standardize anticoagulant management with a goal of improving anticoagulant safety in the inpatient setting. Assessment of the effectiveness of models such as the PDAS for improving anticoagulant safety in the inpatient setting is particularly relevant with the current expectations for hospitals set by The Joint Commission's NPSG.03.05.01.3 More importantly the PDAS model can be an option for improving the transition of the anticoagulated patient from the inpatient‐to‐outpatient setting. Follow‐up with the anticoagulation clinic occurred earlier with the PDAS and, while this study was not designed to evaluate the impact of this new service on rehospitalization, recent literature suggests that earlier follow‐up after discharge leads to less rehospitalization.10 Finally, it may be possible to adapt this model to provide more intensive medication therapy management and monitoring for hospitalized patients with other complicated medication regimens or chronic disease.
CONCLUSION
The clinical pharmacist is uniquely prepared to manage inter‐individual variability in pharmacodynamic response to drug therapy, as well as to provide high‐quality patient education. This study evaluated a new model of inpatient warfarin management, in which warfarin dosing, monitoring, patient education, and transition of care was coordinated by a specialized team of clinical pharmacists that worked in collaboration with physicians and outpatient anticoagulation clinic staff. Safety and efficiency of the care provided by this new service was improved in certain subsets of more complex patients. The major advantage of this service was improvement in patient handoff, improved communication, and earlier patient follow‐up after discharge. Therefore, implementation of a Pharmacist‐Directed Anticoagulation Service provides a net improvement in quality of care for the patient taking warfarin in the inpatient setting.
Acknowledgements
The authors acknowledge the efforts of the PDAS staff: Nassif Abi‐Samra, Pam Holland, Sara Lanfear, and Gail Washington. This work would not have been possible without the dedication of these pharmacists. All authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
- U.S. Pharmacopeia. USP Patient Safety CapsLink: January 2008. Available at: http://www.usp.org/pdf/EN/patientSafety/capsLink2008–01‐01.pdf. Accessed March 19,2010.
- ,,,,,.Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians evidence‐based clinical practice guidelines (8th ed.).Chest.2008;133:160S–198S.
- The Joint Commission. 2009 National Patient Safety Goals. Available at: http://www.jointcommission.org/NR/rdonlyres/31666E86‐E7F4–423E‐9BE8‐F05BD1CB0AA8/0/HAP_NPSG.pdf. Accessed May 6,2010.
- ,,, et al.Optimization of inpatient warfarin therapy: impact of a daily consultation by a pharmacist‐managed anticoagulation service.Ann Pharmacother.2000;34:567–572.
- ,,, et al.Pharmacy‐managed protocol for warfarin use in orthopedic surgery patients.Am J Health‐Syst Pharm.1995;52:1310–1316.
- .Pharmacist involvement with warfarin dosing for inpatients.Pharm World Sci.2001;23:31–35.
- ,,.Evaluation of a pharmacy‐managed warfarin‐monitoring service to coordinate inpatient and outpatient therapy.Am J Hosp Pharm.1992;49:387–394.
- ,.Implementation and evaluation of a pharmacist‐assisted warfarin dosing program.Can J Hosp Pharm.1997;50:169–175.
- ,.Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non‐surgical patients.J Thromb Haemostasis.2005;3:692–694.
- ,,.Rehospitalizations among patients in the Medicare fee‐for‐service program.N Engl J Med.2009;360:1418–1428.
- U.S. Pharmacopeia. USP Patient Safety CapsLink: January 2008. Available at: http://www.usp.org/pdf/EN/patientSafety/capsLink2008–01‐01.pdf. Accessed March 19,2010.
- ,,,,,.Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians evidence‐based clinical practice guidelines (8th ed.).Chest.2008;133:160S–198S.
- The Joint Commission. 2009 National Patient Safety Goals. Available at: http://www.jointcommission.org/NR/rdonlyres/31666E86‐E7F4–423E‐9BE8‐F05BD1CB0AA8/0/HAP_NPSG.pdf. Accessed May 6,2010.
- ,,, et al.Optimization of inpatient warfarin therapy: impact of a daily consultation by a pharmacist‐managed anticoagulation service.Ann Pharmacother.2000;34:567–572.
- ,,, et al.Pharmacy‐managed protocol for warfarin use in orthopedic surgery patients.Am J Health‐Syst Pharm.1995;52:1310–1316.
- .Pharmacist involvement with warfarin dosing for inpatients.Pharm World Sci.2001;23:31–35.
- ,,.Evaluation of a pharmacy‐managed warfarin‐monitoring service to coordinate inpatient and outpatient therapy.Am J Hosp Pharm.1992;49:387–394.
- ,.Implementation and evaluation of a pharmacist‐assisted warfarin dosing program.Can J Hosp Pharm.1997;50:169–175.
- ,.Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non‐surgical patients.J Thromb Haemostasis.2005;3:692–694.
- ,,.Rehospitalizations among patients in the Medicare fee‐for‐service program.N Engl J Med.2009;360:1418–1428.
Copyright © 2011 Society of Hospital Medicine