User login
Evaluation of VTE Prophylaxis in CLD
Chronic liver disease (CLD) or cirrhosis results in greater than 400,000 hospital admissions every year and accounted for approximately 29,000 deaths in 2007.1,2 CLD patients often have an elevated international normalized ratio (INR) due to disease‐associated coagulopathy resulting from a decrease in the production of most procoagulant factors. Due to INR elevations in CLD, clinicians are given a false sense of security surrounding the risk of developing a venous thromboembolism (VTE). The hypothesis that CLD patients are autoanticoagulated and therefore protected against VTE has not been proven.
In the United States, the total incidence of VTE is greater than 200,000 events per year accompanied by a significant number of events occurring in high‐risk hospitalized patients.[3] It has been suggested that patients with liver disease may have a reduced risk for VTE.[4] However, more recent studies report an increased risk with the incidence of VTE in CLD patients occurring in 0.5% to 6.3% of the population.[5, 6, 7, 8, 9, 10] The parallel reduction of anticoagulant factors, such as antithrombin and protein C, along with the reduction in procoagulant factors rebalances the coagulation system, possibly explaining why CLD patients are not protected from VTE.[11, 12] Other mechanistic possibilities include low serum albumin,[8, 9] an elevation of endogenous estrogen levels, immobility associated with the disease,[5] greater morbidity as reflected by high Child‐Pugh scores, and a chronic inflammatory state that results in poor flow and vasculopathy.[7]
Current guidelines for the prevention of VTE do not provide recommendations on the use of prophylaxis in the cirrhotic population,[13] although recent literature reviews suggest that strong consideration for pharmacologic prophylaxis be given when the benefit outweighs the risk.[14, 15] Limited studies have evaluated the use of VTE prophylaxis in CLD patients, whether pharmacologic or mechanical.[6, 7, 8, 16] These studies report that the utilization of VTE prophylaxis in CLD patients is suboptimal, with at least 75% of CLD patients receiving no prophylaxis.[6, 7, 8] The purpose of our study was to examine the use of prophylactic agents and the incidence of VTE and bleeding events in CLD patients.
METHODS
A retrospective chart review of patients diagnosed with CLD or cirrhosis at Methodist University Hospital between August 1, 2009 and July 31, 2011 was conducted. These patients were identified through the corporate patient financial services database using the International Classification of Diseases, 9th Revision, Clinical Modification code 571.xx for CLD/cirrhosis. Patients were included if they were 18 years or older, admitted for or with a history of CLD, and had an INR of 1.4 on admission. An elevated INR was chosen as inclusion criteria as this is often when the controversy of prophylaxis versus no prophylaxis emerges. CLD was defined based on previous histories or clinical presentations of past variceal bleed, presence of varices based on endoscopy report, hepatic encephalopathy, spontaneous bacterial peritonitis, ascites, liver biopsy proven cirrhosis, or imaging consistent with cirrhotic liver changes. CLD was classified as alcoholic, viral hepatitis (hepatitis B and C), and other, such as nonalcoholic steatohepatitis and autoimmune. Patients admitted with maintenance anticoagulation, suspected bleed or VTE, palliative care diagnosis, or history of/anticipated liver transplant were excluded. If a patient met inclusion criteria for an admission and was subsequently readmitted within 30 days, only the initial admission was included. Once patients were included they were assigned to 1 of 4 groups based on the type of prophylaxis received: pharmacologic, mechanical, combined pharmacologic and mechanical, and no prophylaxis. Patients who received pharmacologic or mechanical prophylaxis for at least 50% of their hospital stay were assigned to their corresponding groups accordingly. Patients who received pharmacologic and mechanical prophylaxis for at least 50% of their hospital stay were assigned to the combination group. Patients receiving either form of VTE prophylaxis for <50% of their hospital stay were considered to be without prophylaxis. Pharmacologic prophylaxis was defined by the use of unfractionated heparin (UFH) 5000 units subcutaneously (sq) 3 times daily or twice daily (bid), low molecular weight heparin (LMWH) 30 mg sq bid or 40 mg every day (qd), or fondaparinux 2.5 mg qd. Mechanical prophylaxis was defined by the use of a sequential compression device (SCD). The study was approved by the University of Tennessee Institutional Review Board.
Patient demographics including age, sex, race, height, and weight were documented with a body mass index (BMI) calculated for each patient. Obesity was defined as BMI 30 kg/m2. Risk factors for VTE including obesity, surgery, infection, trauma, malignancy, and history of VTE as well as the etiology of cirrhosis were collected and recorded whenever available based on documentation in the medical chart. Clinical data including lowest serum albumin, highest total bilirubin, highest INR, and platelets on admission were recorded. Severity of ascites and hepatic encephalopathy were documented. Child‐Pugh score and stage as well as Model for End‐Stage Liver Disease (MELD) score were calculated. In‐hospital VTE, bleeding events, length of stay, in‐hospital mortality, and the use, type, and number of days of VTE prophylaxis were documented. VTE was defined as deep venous thrombosis (DVT) or pulmonary embolism diagnosed by venous Doppler ultrasonography, spiral computed tomography (CT) of the chest, or ventilation/perfusion scan. Bleeding was defined by documentation in the medical record plus the administration of packed red blood cells, fresh frozen plasma, recombinant factor VIIa, or vitamin K. For patients who experienced a bleed, risk factors for in‐hospital bleeding as defined by American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines 2012 guidelines (CHEST) were documented.[13]
The primary outcome was to describe the use of VTE prophylaxis in CLD patients. Secondary outcomes were to determine the overall incidence of VTE in CLD patients, examine the incidence of VTE based on the utilization of prophylaxis, compare the occurrence of bleeding events in CLD patients based on type of prophylaxis, evaluate the use of mechanical versus pharmacologic prophylaxis based on INR, evaluate length of stay (LOS) and in‐hospital mortality for CLD patients with and without prophylaxis, and evaluate 30‐day readmission rate for VTE.
Patients were arbitrarily divided into 2 groups according to the highest INR (1.42.0 or >2.0). Baseline characteristics were compared between the 2 groups. Variables were expressed as mean or median with standard deviation or interquartile range. Categorical values were expressed as percentages and compared using the [2] test or Fisher exact test. Continuous data were compared using Mann‐Whitney U test for nonparametric data or Student t test for parametric data. Significance was defined as P<0.05. All statistical analyses were performed using SPSS Statistics (version 20.0; SPSS, Inc., Chicago, IL).
RESULTS
We identified 410 patients who met inclusion criteria during the study period. Baseline demographics were similar between the 2 groups with the exception of age, which was statistically higher in the INR 1.4 to 2.0 group. The most common etiology of CLD was hepatitis B or C, followed by alcohol, then other causes. Alcoholic CLD was associated with higher INR values (>2.0). Patients with INR >2.0 were found to exhibit lower serum albumin levels and platelets on admission as well as higher total bilirubin and INR values. There was also a significant difference in Child‐Pugh stages B and C, with the INR >2.0 group only having stage C. In addition, the higher INR group had a significantly higher average MELD score (Table 1).
| Characteristic | INR1.42.0, n=251 | INR>2.0, n=159 | P Value |
|---|---|---|---|
| |||
| Age, yearsSD | 55.710.4 | 53.310.1 | 0.017 |
| Male sex | 137 (54.6) | 99 (62.3) | 0.125 |
| BMISD | 29.17.3 | 30.37.7 | 0.103 |
| Race | |||
| African American | 99 (39.4) | 53 (33.3) | 0.212 |
| White | 139 (55.4) | 99 (62.3) | 0.169 |
| Other | 13 (5.2) | 7 (4.4) | 0.722 |
| Etiology of CLD | |||
| Hepatitis B or C | 127 (50.6) | 70 (44) | 0.194 |
| Alcohol | 59 (23.5) | 57 (35.9) | 0.007 |
| Other | 65 (25.9) | 32 (20.1) | 0.18 |
| VTE risk factors | |||
| Obesity, BMI 30 | 107 (42.6) | 71 (44.6) | 0.687 |
| Surgery | 21 (8.4) | 7 (4.1) | 0.121 |
| Infection | 81 (32.3) | 63 (39.6) | 0.129 |
| Trauma | 1 (0.4) | 1 (0.6) | 1.00 |
| Malignancy | 35 (13.9) | 24 (15.1) | 0.746 |
| History of VTE | 6 (2.4) | 4 (2.5) | 1.00 |
| Median number VTE risk factors (range) | 1 (03) | 1 (04) | 0.697 |
| Laboratory values | |||
| AlbuminSD | 2.20.58 | 2.00.53 | <0.001 |
| Tbili, median (IQR) | 2.8 (1.95.0) | 8.1 (5.013.3) | <0.001 |
| INR, median (IQR) | 1.7 (1.51.8) | 2.4 (2.22.9) | <0.001 |
| Admission platelets, median (IQR) | 92 (61141) | 79 (58121) | 0.008 |
| Child Pugh stage | |||
| Class A | 3 (1.2) | 0 (0) | 0.286 |
| Class B | 91 (36.3) | 0 (0) | <0.001 |
| Class C | 157 (62.5) | 159 (100) | <0.001 |
| MELD scoreSD | 18.55.1 | 28.36.3 | <0.001 |
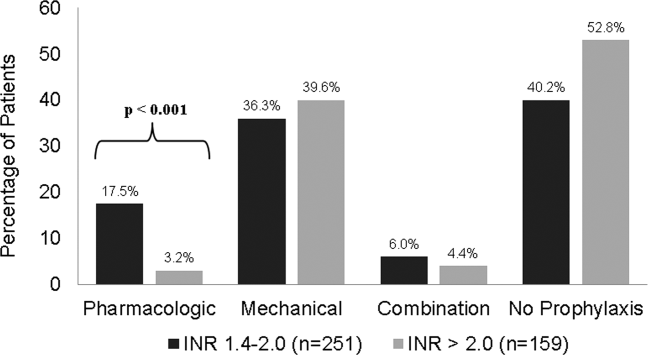
Of the 410 patients included, 225 (55%) patients received thromboprophylaxis. The majority of patients received mechanical prophylaxis (n=154), followed by pharmacologic (n=49), and then a combination of mechanical plus pharmacologic (n=22). For patients receiving pharmacologic either alone or in combination with SCDs, 30 received UFH, 33 received LMWH, 1 patient received fondaparinux, and the remaining 7 received a combination of the agents to total 50% of their hospital stay. For patients with INR >2.0, a significant decrease in overall thromboprophylaxis use was seen compared to those with INR 1.4 to 2.0 (47% vs 60%; P=0.013). Patients with INR >2.0 also received significantly less pharmacologic prophylaxis compared to those with INR 1.4 to 2.0 (3.2% vs 17.5%; P<0.001). No differences in the use of mechanical or combination prophylaxis was seen between the groups (Figure 1).

As shown in Table 2, in‐hospital VTE occurred in 3 patients (0.7%). All 3 patients had a DVT. Of the patients with documented VTE, 1 was Child‐Pugh stage B and 2 were stage C. Fifteen bleeding events occurred (3.7%), 9 on mechanical prophylaxis, 1 on pharmacologic, 3 on combination, and 2 with no prophylaxis. The majority of patients experiencing a bleeding event had an INR >2.0 (P=0.001). Eleven patients out of the 15 were considered to be at high risk of bleeding as defined per CHEST 2012 guidelines,[13] whereas 100% had Child‐Pugh stage C with an average MELD score of 31.77.5. It should be noted that 1 patient experienced a bleeding event after receiving pharmacologic treatment doses for VTE and was subsequently placed on a prophylactic dose without any bleeding complications.
| Characteristic | INR1.42.0, n=251 | INR>2.0, n=159 | P Value |
|---|---|---|---|
| |||
| In‐hospital VTE | 1 (0.4) | 2 (1.3) | 0.563 |
| Mechanical | 0 (0) | 1 (0.6) | 0.389 |
| Pharmacologic | 0 (0) | 1 (0.6) | 0.389 |
| Combination | 0 (0) | 0 (0) | 1.00 |
| No prophylaxis | 1 (0.4) | 0 (0) | 1.00 |
| Bleeding event | 3 (1.2) | 12 (7.5) | 0.001 |
| Mechanical | 2 (0.8) | 7 (4.4) | 0.033 |
| Pharmacologic* | 0 (0) | 1 (0.6) | 0.389 |
| Combination | 1 (0.4) | 2 (1.3) | 0.563 |
| No prophylaxis | 0 (0) | 2 (1.3) | 0.152 |
| LOS, median (IQR) | 5 (2.98) | 7.2 (413.1) | <0.001 |
| Hospital mortality | 6 (2.4) | 30 (18.9) | <0.001 |
| 30‐day readmission rate for VTE | 2 (0.8) | 0 (0) | 0.524 |
Longer LOS and higher mortality rates were seen in patients who received prophylaxis compared to those who received no prophylaxis (P<0.001 and P=0.001, respectively). Of the 36 patients who died, 22 received mechanical prophylaxis, 2 received pharmacologic, 5 received a combination, and 7 received no prophylaxis. Longer LOS and higher mortality rates were also seen in patients with INR >2 compared to patients with INR 1.4 to 2.0 (P<0.001 for both) (Table 2). Higher mortality rates were associated with greater severity of disease as defined by Child‐Pugh C classification in all 36 patients (P=0.001) and an average MELD score of 31.87.6. No differences in 30‐day readmission rates for VTE were seen between prophylaxis groups.
DISCUSSION/CONCLUSION
The use of thromboprophylaxis in our study was 55%, which is consistent with the reported rate of 30% to 70% in general hospitalized patients.[17] To our knowledge this is the first study to focus primarily on the use of both pharmacologic and mechanical thromboprophylaxis in CLD patients. Previous studies have focused on the incidence and risks of VTE in CLD patients,[5, 6, 7, 8, 9, 10] with only 3 of those studies evaluating the use of pharmacologic and mechanical thromboprophylaxis as a secondary outcome.[6, 7, 8] The reported use of thromboprophylaxis in these studies ranges from 21% to 25%. Pharmacologic prophylaxis rates were 7% in Northup et al.,[8] 12% in Aldawood et al.,[7] and 9% in Dabbagh et al.,[6] compared to 17% in our study (pharmacologic alone plus combination). Mechanical prophylaxis rates were 14%, 12%, and 16%, respectively, compared to our 38%. None of the previous studies gave a definition for prophylaxis. This is important to note because discrepancies in prophylaxis reporting could lead to significant differences in rates of prophylaxis when comparing these studies to our study.
Despite the higher rates of thromboprophylaxis, the incidence of VTE was 0.7%. Our VTE incidence falls within the reported incidence rate of 0.5% to 6.3%.[6, 7, 8, 9, 10] Similar to Aldawood et al. and Dabbagh et al., we found no significant differences in the incidence of VTE and prophylaxis use.[6, 7] Dabbagh et al. suggest that the incidence of VTE increases as disease severity increases.[6] However, with only 25% of their patients receiving thromboprophylaxis, it is hard to determine if the higher incidence of VTE was due to greater disease severity or the low use of thromboprophylaxis. It is expected that patients with more severe disease are less likely to receive VTE prophylaxis secondary to increases in INR and/or thrombocytopenia. As evidenced in our study, there was a significant decrease in the use of thromboprophylaxis in patients with INR >2.0, driven largely by the significant decrease in the use of pharmacologic prophylaxis. Due to the low incidence of VTE observed, our study lacks adequate power to truly determine the relationship between use of thromboprophylaxis or severity of disease and incidence of VTE.
Nonetheless, we did find a significant correlation between disease severity and bleeding in CLD patients. Although not a new finding in the literature, this result substantiates the claim that the delicate balance and unpredictability of coagulopathy in CLD leads to bleeding events as well as VTE. In our study we had an overall bleeding rate of 3.7%. Patients who experienced a bleeding event had greater disease severity, significantly higher INR, and 73% were considered to be at high risk for an event as defined by CHEST guidelines.[13] The majority of events happened while on mechanical or no prophylaxis. Four patients who received pharmacologic prophylaxis had a bleeding event; however, 1 of those patients bled on VTE pharmacologic treatment dose for VTE found on day 2 of hospital admission. In a recent study by Bechmann et al. looking at the use of LMWH in 84 cirrhotic patients, they report a bleeding rate of 8.3%, a rate that is similar to rates of bleeding in nonanticoagulated cirrhotic patients.[18] In comparison with our study, we had 71 patients receive pharmacologic prophylaxis either alone or in combination and 4 bleeding events, giving an event rate of 5.6%. This rate decreases to 4.2% when considering only prophylactic pharmacologic doses, suggesting that pharmacologic prophylaxis in CLD patients poses a low risk of bleeding. Interestingly enough, an association was found between alcoholic CLD and higher INR (>2.0) in our study. Given that patients with higher INR had increased bleeding events, this introduces a question of whether or not the specific cause of CLD (ie, alcoholic hepatitis) may represent a special risk for bleeding in this population. However, additional studies are needed to confirm this hypothesis.
To our knowledge, this study is also the first to look at the relationship of thromboprophylaxis use on LOS and mortality in CLD patients. At first glance, the fact that patients who received prophylaxis had both significantly longer LOS and higher mortality rates in our study is concerning. However, it is likely that the increased LOS and mortality in our study is attributed to greater disease severity, as evidenced by higher INRs and Child‐Pugh scores regardless of prophylaxis use or not. Also, a known risk factor for VTE is reduced mobility. Although no standard definition for reduced mobility exists, Barbar et al. define it as anticipated bed rest with bathroom privileges (either because of patient's limitations or on physician's order) for at least 3 days.[19] Due to this known increased risk for VTE, it is expected that patient's with a LOS of 3 days are more likely to receive thromboprophylaxis.
Our study has several limitations. Like other retrospective studies, this study was conducted in 1 medical center and relies on the accuracy of documentation. We relied on patient history and clinical presentation to diagnose CLD without the requirement of histologic diagnosis. However, all patients included in the study had an unquestionable diagnosis by a physician. We used an arbitrary definition and assignment of patients into groups based on the method of VTE prophylaxis utilized due to lack of a definition in the medical literature. There was a possible selection bias for pharmacologic prophylaxis based on patient risk factors for bleeding, such as presence of varices and thrombocytopenia. Also, the inability to ensure that patients with an order for SCDs were actively wearing the device throughout their hospital stay is yet another limitation. Not all patients underwent testing for VTE; therefore, the actual incidence of VTE may be higher than what we found. Only those patients who experienced a bleeding event were assessed for risk factors that predisposed them to bleed, making it hard to correlate those risk factors with the risk of bleeding in all CLD patients.
Despite these limitations, our study has great strengths. This is the first study to focus primarily on the use of both pharmacologic and mechanical thromboprophylaxis in CLD patients. Therefore, it has the potential to influence and raise awareness on the decisions made involving the management of CLD patients in regard to VTE prophylaxis and will hopefully serve as an impetus for future prospective studies. When comparing this study to other studies looking at the incidence of VTE in CLD patients and the use of prophylaxis, our study sample size is relatively large. Also, by including only those patients with INR of at least 1.4 on admission, our study patients had greater severity of disease, making this study distinctly relevant in the clinical debate of whether or not CLD patients should receive thromboprophylaxis.
In conclusion, the use of thromboprophylaxis in CLD patients is higher in our study than previous reports but remains suboptimal. Although bleeding is an inherent risk factor in CLD independent of VTE prophylaxis, the use of VTE pharmacologic prophylaxis does not appear to increase bleeding in CLD patients with INR 2.0. Further studies focusing on baseline bleeding risks (ie, thrombocytopenia, presence of varices) and the use of pharmacologic prophylaxis are needed to provide additional safety data on the use of pharmacologic prophylaxis in this patient population.
Disclosures: All coauthors have seen and agree with the contents of the article. Submission is not under review by any other publication. All authors have not received notification of redundant or duplicate publication. All authors have no financial conflicts of interest. No funding was received for this study or article.
- , , . National hospital discharge survey: 2002 annual summary with detailed diagnosis and procedure data. Vital Health Stat 13. 2005;158:1–199.
- , , , . Deaths: final data for 2007. Natl Vital Stat Rep. 2010;58(19):1–135.
- , , , et al. The diagnostic approach to acute venous thromboembolism: clinical practice guideline. Am J Respir Crit Care Med. 1999;160(3):1043–1066.
- , , , , , . Risk factors for deep vein thrombosis and pulmonary embolism: a population‐based case‐control study. Arch Intern Med. 2000;160(6):809–815.
- , , , , , . Risk of venous thromboembolism in patients with liver disease: a nationwide population‐based case‐control study. Am J Gastroenterol. 2009;104(1):96–101.
- , , , , . Coagulopathy does not protect against venous thromboembolism in hospitalized patients with chronic liver disease. Chest. 2010;137(5):1145–1149.
- , , , et al. The incidence of venous thromboembolism and practice of deep venous thrombosis prophylaxis in hospitalized cirrhotic patients. Thromb J. 2011;9(1):1.
- , , , et al. Coagulopathy does not fully protect hospitalized cirrhosis patients from peripheral venous thromboembolism. Am J Gastroenterol. 2006;101(7):1524–1528.
- , , , , . Deep vein thrombosis and pulmonary embolism in cirrhosis patients. Dig Dis Sci. 2008;53(11):3012–3017.
- , , , , , . Venous thromboembolism and liver cirrhosis. Rev Esp Enferm Dig. 2008;100(5):259–262.
- , , . Should we give thromboprophylaxis to patients with liver cirrhosis and coagulopathy? HPB. 2009;11(6):459–464.
- , . The coagulopathy of chronic liver disease. N Engl J Med. 2011;365(2):147–156.
- , , , et al. Prevention of VTE in nonsurgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(suppl 2):e195S–e226S.
- , . Pharmacologic prophylaxis against venous thromboembolism in hospitalized patients with cirrhosis and associated coagulopathies. Am J Health Syst Pharm. 2012;69(8):658–63.
- , , . Risk of venous thromboembolism in patients with chronic liver disease and the utility of venous thromboembolism prophylaxis. Ann Pharmacother. 2012;46(6):873–878.
- , , , et al. A systematic review of venous thromboembolism prophylaxis strategies in patients with renal insufficiency, obesity, or on antiplatelet agents. J Hosp Med. 2013;8(7):394–401.
- , , , , , . Prevention of venous thromboembolism in the hospitalized medical patient. Cleve Clin J Med. 2008;75(suppl 3):S7–S16.
- , , , , , . Low‐molecular‐weight heparin in patients with advanced cirrhosis. Liver Int. 2011;31(1):75–82.
- , , , et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the Padua Prediction Score. J Thromb Haemost. 2010;8(11):2450–2457.
Chronic liver disease (CLD) or cirrhosis results in greater than 400,000 hospital admissions every year and accounted for approximately 29,000 deaths in 2007.1,2 CLD patients often have an elevated international normalized ratio (INR) due to disease‐associated coagulopathy resulting from a decrease in the production of most procoagulant factors. Due to INR elevations in CLD, clinicians are given a false sense of security surrounding the risk of developing a venous thromboembolism (VTE). The hypothesis that CLD patients are autoanticoagulated and therefore protected against VTE has not been proven.
In the United States, the total incidence of VTE is greater than 200,000 events per year accompanied by a significant number of events occurring in high‐risk hospitalized patients.[3] It has been suggested that patients with liver disease may have a reduced risk for VTE.[4] However, more recent studies report an increased risk with the incidence of VTE in CLD patients occurring in 0.5% to 6.3% of the population.[5, 6, 7, 8, 9, 10] The parallel reduction of anticoagulant factors, such as antithrombin and protein C, along with the reduction in procoagulant factors rebalances the coagulation system, possibly explaining why CLD patients are not protected from VTE.[11, 12] Other mechanistic possibilities include low serum albumin,[8, 9] an elevation of endogenous estrogen levels, immobility associated with the disease,[5] greater morbidity as reflected by high Child‐Pugh scores, and a chronic inflammatory state that results in poor flow and vasculopathy.[7]
Current guidelines for the prevention of VTE do not provide recommendations on the use of prophylaxis in the cirrhotic population,[13] although recent literature reviews suggest that strong consideration for pharmacologic prophylaxis be given when the benefit outweighs the risk.[14, 15] Limited studies have evaluated the use of VTE prophylaxis in CLD patients, whether pharmacologic or mechanical.[6, 7, 8, 16] These studies report that the utilization of VTE prophylaxis in CLD patients is suboptimal, with at least 75% of CLD patients receiving no prophylaxis.[6, 7, 8] The purpose of our study was to examine the use of prophylactic agents and the incidence of VTE and bleeding events in CLD patients.
METHODS
A retrospective chart review of patients diagnosed with CLD or cirrhosis at Methodist University Hospital between August 1, 2009 and July 31, 2011 was conducted. These patients were identified through the corporate patient financial services database using the International Classification of Diseases, 9th Revision, Clinical Modification code 571.xx for CLD/cirrhosis. Patients were included if they were 18 years or older, admitted for or with a history of CLD, and had an INR of 1.4 on admission. An elevated INR was chosen as inclusion criteria as this is often when the controversy of prophylaxis versus no prophylaxis emerges. CLD was defined based on previous histories or clinical presentations of past variceal bleed, presence of varices based on endoscopy report, hepatic encephalopathy, spontaneous bacterial peritonitis, ascites, liver biopsy proven cirrhosis, or imaging consistent with cirrhotic liver changes. CLD was classified as alcoholic, viral hepatitis (hepatitis B and C), and other, such as nonalcoholic steatohepatitis and autoimmune. Patients admitted with maintenance anticoagulation, suspected bleed or VTE, palliative care diagnosis, or history of/anticipated liver transplant were excluded. If a patient met inclusion criteria for an admission and was subsequently readmitted within 30 days, only the initial admission was included. Once patients were included they were assigned to 1 of 4 groups based on the type of prophylaxis received: pharmacologic, mechanical, combined pharmacologic and mechanical, and no prophylaxis. Patients who received pharmacologic or mechanical prophylaxis for at least 50% of their hospital stay were assigned to their corresponding groups accordingly. Patients who received pharmacologic and mechanical prophylaxis for at least 50% of their hospital stay were assigned to the combination group. Patients receiving either form of VTE prophylaxis for <50% of their hospital stay were considered to be without prophylaxis. Pharmacologic prophylaxis was defined by the use of unfractionated heparin (UFH) 5000 units subcutaneously (sq) 3 times daily or twice daily (bid), low molecular weight heparin (LMWH) 30 mg sq bid or 40 mg every day (qd), or fondaparinux 2.5 mg qd. Mechanical prophylaxis was defined by the use of a sequential compression device (SCD). The study was approved by the University of Tennessee Institutional Review Board.
Patient demographics including age, sex, race, height, and weight were documented with a body mass index (BMI) calculated for each patient. Obesity was defined as BMI 30 kg/m2. Risk factors for VTE including obesity, surgery, infection, trauma, malignancy, and history of VTE as well as the etiology of cirrhosis were collected and recorded whenever available based on documentation in the medical chart. Clinical data including lowest serum albumin, highest total bilirubin, highest INR, and platelets on admission were recorded. Severity of ascites and hepatic encephalopathy were documented. Child‐Pugh score and stage as well as Model for End‐Stage Liver Disease (MELD) score were calculated. In‐hospital VTE, bleeding events, length of stay, in‐hospital mortality, and the use, type, and number of days of VTE prophylaxis were documented. VTE was defined as deep venous thrombosis (DVT) or pulmonary embolism diagnosed by venous Doppler ultrasonography, spiral computed tomography (CT) of the chest, or ventilation/perfusion scan. Bleeding was defined by documentation in the medical record plus the administration of packed red blood cells, fresh frozen plasma, recombinant factor VIIa, or vitamin K. For patients who experienced a bleed, risk factors for in‐hospital bleeding as defined by American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines 2012 guidelines (CHEST) were documented.[13]
The primary outcome was to describe the use of VTE prophylaxis in CLD patients. Secondary outcomes were to determine the overall incidence of VTE in CLD patients, examine the incidence of VTE based on the utilization of prophylaxis, compare the occurrence of bleeding events in CLD patients based on type of prophylaxis, evaluate the use of mechanical versus pharmacologic prophylaxis based on INR, evaluate length of stay (LOS) and in‐hospital mortality for CLD patients with and without prophylaxis, and evaluate 30‐day readmission rate for VTE.
Patients were arbitrarily divided into 2 groups according to the highest INR (1.42.0 or >2.0). Baseline characteristics were compared between the 2 groups. Variables were expressed as mean or median with standard deviation or interquartile range. Categorical values were expressed as percentages and compared using the [2] test or Fisher exact test. Continuous data were compared using Mann‐Whitney U test for nonparametric data or Student t test for parametric data. Significance was defined as P<0.05. All statistical analyses were performed using SPSS Statistics (version 20.0; SPSS, Inc., Chicago, IL).
RESULTS
We identified 410 patients who met inclusion criteria during the study period. Baseline demographics were similar between the 2 groups with the exception of age, which was statistically higher in the INR 1.4 to 2.0 group. The most common etiology of CLD was hepatitis B or C, followed by alcohol, then other causes. Alcoholic CLD was associated with higher INR values (>2.0). Patients with INR >2.0 were found to exhibit lower serum albumin levels and platelets on admission as well as higher total bilirubin and INR values. There was also a significant difference in Child‐Pugh stages B and C, with the INR >2.0 group only having stage C. In addition, the higher INR group had a significantly higher average MELD score (Table 1).
| Characteristic | INR1.42.0, n=251 | INR>2.0, n=159 | P Value |
|---|---|---|---|
| |||
| Age, yearsSD | 55.710.4 | 53.310.1 | 0.017 |
| Male sex | 137 (54.6) | 99 (62.3) | 0.125 |
| BMISD | 29.17.3 | 30.37.7 | 0.103 |
| Race | |||
| African American | 99 (39.4) | 53 (33.3) | 0.212 |
| White | 139 (55.4) | 99 (62.3) | 0.169 |
| Other | 13 (5.2) | 7 (4.4) | 0.722 |
| Etiology of CLD | |||
| Hepatitis B or C | 127 (50.6) | 70 (44) | 0.194 |
| Alcohol | 59 (23.5) | 57 (35.9) | 0.007 |
| Other | 65 (25.9) | 32 (20.1) | 0.18 |
| VTE risk factors | |||
| Obesity, BMI 30 | 107 (42.6) | 71 (44.6) | 0.687 |
| Surgery | 21 (8.4) | 7 (4.1) | 0.121 |
| Infection | 81 (32.3) | 63 (39.6) | 0.129 |
| Trauma | 1 (0.4) | 1 (0.6) | 1.00 |
| Malignancy | 35 (13.9) | 24 (15.1) | 0.746 |
| History of VTE | 6 (2.4) | 4 (2.5) | 1.00 |
| Median number VTE risk factors (range) | 1 (03) | 1 (04) | 0.697 |
| Laboratory values | |||
| AlbuminSD | 2.20.58 | 2.00.53 | <0.001 |
| Tbili, median (IQR) | 2.8 (1.95.0) | 8.1 (5.013.3) | <0.001 |
| INR, median (IQR) | 1.7 (1.51.8) | 2.4 (2.22.9) | <0.001 |
| Admission platelets, median (IQR) | 92 (61141) | 79 (58121) | 0.008 |
| Child Pugh stage | |||
| Class A | 3 (1.2) | 0 (0) | 0.286 |
| Class B | 91 (36.3) | 0 (0) | <0.001 |
| Class C | 157 (62.5) | 159 (100) | <0.001 |
| MELD scoreSD | 18.55.1 | 28.36.3 | <0.001 |
Of the 410 patients included, 225 (55%) patients received thromboprophylaxis. The majority of patients received mechanical prophylaxis (n=154), followed by pharmacologic (n=49), and then a combination of mechanical plus pharmacologic (n=22). For patients receiving pharmacologic either alone or in combination with SCDs, 30 received UFH, 33 received LMWH, 1 patient received fondaparinux, and the remaining 7 received a combination of the agents to total 50% of their hospital stay. For patients with INR >2.0, a significant decrease in overall thromboprophylaxis use was seen compared to those with INR 1.4 to 2.0 (47% vs 60%; P=0.013). Patients with INR >2.0 also received significantly less pharmacologic prophylaxis compared to those with INR 1.4 to 2.0 (3.2% vs 17.5%; P<0.001). No differences in the use of mechanical or combination prophylaxis was seen between the groups (Figure 1).

As shown in Table 2, in‐hospital VTE occurred in 3 patients (0.7%). All 3 patients had a DVT. Of the patients with documented VTE, 1 was Child‐Pugh stage B and 2 were stage C. Fifteen bleeding events occurred (3.7%), 9 on mechanical prophylaxis, 1 on pharmacologic, 3 on combination, and 2 with no prophylaxis. The majority of patients experiencing a bleeding event had an INR >2.0 (P=0.001). Eleven patients out of the 15 were considered to be at high risk of bleeding as defined per CHEST 2012 guidelines,[13] whereas 100% had Child‐Pugh stage C with an average MELD score of 31.77.5. It should be noted that 1 patient experienced a bleeding event after receiving pharmacologic treatment doses for VTE and was subsequently placed on a prophylactic dose without any bleeding complications.
| Characteristic | INR1.42.0, n=251 | INR>2.0, n=159 | P Value |
|---|---|---|---|
| |||
| In‐hospital VTE | 1 (0.4) | 2 (1.3) | 0.563 |
| Mechanical | 0 (0) | 1 (0.6) | 0.389 |
| Pharmacologic | 0 (0) | 1 (0.6) | 0.389 |
| Combination | 0 (0) | 0 (0) | 1.00 |
| No prophylaxis | 1 (0.4) | 0 (0) | 1.00 |
| Bleeding event | 3 (1.2) | 12 (7.5) | 0.001 |
| Mechanical | 2 (0.8) | 7 (4.4) | 0.033 |
| Pharmacologic* | 0 (0) | 1 (0.6) | 0.389 |
| Combination | 1 (0.4) | 2 (1.3) | 0.563 |
| No prophylaxis | 0 (0) | 2 (1.3) | 0.152 |
| LOS, median (IQR) | 5 (2.98) | 7.2 (413.1) | <0.001 |
| Hospital mortality | 6 (2.4) | 30 (18.9) | <0.001 |
| 30‐day readmission rate for VTE | 2 (0.8) | 0 (0) | 0.524 |
Longer LOS and higher mortality rates were seen in patients who received prophylaxis compared to those who received no prophylaxis (P<0.001 and P=0.001, respectively). Of the 36 patients who died, 22 received mechanical prophylaxis, 2 received pharmacologic, 5 received a combination, and 7 received no prophylaxis. Longer LOS and higher mortality rates were also seen in patients with INR >2 compared to patients with INR 1.4 to 2.0 (P<0.001 for both) (Table 2). Higher mortality rates were associated with greater severity of disease as defined by Child‐Pugh C classification in all 36 patients (P=0.001) and an average MELD score of 31.87.6. No differences in 30‐day readmission rates for VTE were seen between prophylaxis groups.
DISCUSSION/CONCLUSION
The use of thromboprophylaxis in our study was 55%, which is consistent with the reported rate of 30% to 70% in general hospitalized patients.[17] To our knowledge this is the first study to focus primarily on the use of both pharmacologic and mechanical thromboprophylaxis in CLD patients. Previous studies have focused on the incidence and risks of VTE in CLD patients,[5, 6, 7, 8, 9, 10] with only 3 of those studies evaluating the use of pharmacologic and mechanical thromboprophylaxis as a secondary outcome.[6, 7, 8] The reported use of thromboprophylaxis in these studies ranges from 21% to 25%. Pharmacologic prophylaxis rates were 7% in Northup et al.,[8] 12% in Aldawood et al.,[7] and 9% in Dabbagh et al.,[6] compared to 17% in our study (pharmacologic alone plus combination). Mechanical prophylaxis rates were 14%, 12%, and 16%, respectively, compared to our 38%. None of the previous studies gave a definition for prophylaxis. This is important to note because discrepancies in prophylaxis reporting could lead to significant differences in rates of prophylaxis when comparing these studies to our study.
Despite the higher rates of thromboprophylaxis, the incidence of VTE was 0.7%. Our VTE incidence falls within the reported incidence rate of 0.5% to 6.3%.[6, 7, 8, 9, 10] Similar to Aldawood et al. and Dabbagh et al., we found no significant differences in the incidence of VTE and prophylaxis use.[6, 7] Dabbagh et al. suggest that the incidence of VTE increases as disease severity increases.[6] However, with only 25% of their patients receiving thromboprophylaxis, it is hard to determine if the higher incidence of VTE was due to greater disease severity or the low use of thromboprophylaxis. It is expected that patients with more severe disease are less likely to receive VTE prophylaxis secondary to increases in INR and/or thrombocytopenia. As evidenced in our study, there was a significant decrease in the use of thromboprophylaxis in patients with INR >2.0, driven largely by the significant decrease in the use of pharmacologic prophylaxis. Due to the low incidence of VTE observed, our study lacks adequate power to truly determine the relationship between use of thromboprophylaxis or severity of disease and incidence of VTE.
Nonetheless, we did find a significant correlation between disease severity and bleeding in CLD patients. Although not a new finding in the literature, this result substantiates the claim that the delicate balance and unpredictability of coagulopathy in CLD leads to bleeding events as well as VTE. In our study we had an overall bleeding rate of 3.7%. Patients who experienced a bleeding event had greater disease severity, significantly higher INR, and 73% were considered to be at high risk for an event as defined by CHEST guidelines.[13] The majority of events happened while on mechanical or no prophylaxis. Four patients who received pharmacologic prophylaxis had a bleeding event; however, 1 of those patients bled on VTE pharmacologic treatment dose for VTE found on day 2 of hospital admission. In a recent study by Bechmann et al. looking at the use of LMWH in 84 cirrhotic patients, they report a bleeding rate of 8.3%, a rate that is similar to rates of bleeding in nonanticoagulated cirrhotic patients.[18] In comparison with our study, we had 71 patients receive pharmacologic prophylaxis either alone or in combination and 4 bleeding events, giving an event rate of 5.6%. This rate decreases to 4.2% when considering only prophylactic pharmacologic doses, suggesting that pharmacologic prophylaxis in CLD patients poses a low risk of bleeding. Interestingly enough, an association was found between alcoholic CLD and higher INR (>2.0) in our study. Given that patients with higher INR had increased bleeding events, this introduces a question of whether or not the specific cause of CLD (ie, alcoholic hepatitis) may represent a special risk for bleeding in this population. However, additional studies are needed to confirm this hypothesis.
To our knowledge, this study is also the first to look at the relationship of thromboprophylaxis use on LOS and mortality in CLD patients. At first glance, the fact that patients who received prophylaxis had both significantly longer LOS and higher mortality rates in our study is concerning. However, it is likely that the increased LOS and mortality in our study is attributed to greater disease severity, as evidenced by higher INRs and Child‐Pugh scores regardless of prophylaxis use or not. Also, a known risk factor for VTE is reduced mobility. Although no standard definition for reduced mobility exists, Barbar et al. define it as anticipated bed rest with bathroom privileges (either because of patient's limitations or on physician's order) for at least 3 days.[19] Due to this known increased risk for VTE, it is expected that patient's with a LOS of 3 days are more likely to receive thromboprophylaxis.
Our study has several limitations. Like other retrospective studies, this study was conducted in 1 medical center and relies on the accuracy of documentation. We relied on patient history and clinical presentation to diagnose CLD without the requirement of histologic diagnosis. However, all patients included in the study had an unquestionable diagnosis by a physician. We used an arbitrary definition and assignment of patients into groups based on the method of VTE prophylaxis utilized due to lack of a definition in the medical literature. There was a possible selection bias for pharmacologic prophylaxis based on patient risk factors for bleeding, such as presence of varices and thrombocytopenia. Also, the inability to ensure that patients with an order for SCDs were actively wearing the device throughout their hospital stay is yet another limitation. Not all patients underwent testing for VTE; therefore, the actual incidence of VTE may be higher than what we found. Only those patients who experienced a bleeding event were assessed for risk factors that predisposed them to bleed, making it hard to correlate those risk factors with the risk of bleeding in all CLD patients.
Despite these limitations, our study has great strengths. This is the first study to focus primarily on the use of both pharmacologic and mechanical thromboprophylaxis in CLD patients. Therefore, it has the potential to influence and raise awareness on the decisions made involving the management of CLD patients in regard to VTE prophylaxis and will hopefully serve as an impetus for future prospective studies. When comparing this study to other studies looking at the incidence of VTE in CLD patients and the use of prophylaxis, our study sample size is relatively large. Also, by including only those patients with INR of at least 1.4 on admission, our study patients had greater severity of disease, making this study distinctly relevant in the clinical debate of whether or not CLD patients should receive thromboprophylaxis.
In conclusion, the use of thromboprophylaxis in CLD patients is higher in our study than previous reports but remains suboptimal. Although bleeding is an inherent risk factor in CLD independent of VTE prophylaxis, the use of VTE pharmacologic prophylaxis does not appear to increase bleeding in CLD patients with INR 2.0. Further studies focusing on baseline bleeding risks (ie, thrombocytopenia, presence of varices) and the use of pharmacologic prophylaxis are needed to provide additional safety data on the use of pharmacologic prophylaxis in this patient population.
Disclosures: All coauthors have seen and agree with the contents of the article. Submission is not under review by any other publication. All authors have not received notification of redundant or duplicate publication. All authors have no financial conflicts of interest. No funding was received for this study or article.
Chronic liver disease (CLD) or cirrhosis results in greater than 400,000 hospital admissions every year and accounted for approximately 29,000 deaths in 2007.1,2 CLD patients often have an elevated international normalized ratio (INR) due to disease‐associated coagulopathy resulting from a decrease in the production of most procoagulant factors. Due to INR elevations in CLD, clinicians are given a false sense of security surrounding the risk of developing a venous thromboembolism (VTE). The hypothesis that CLD patients are autoanticoagulated and therefore protected against VTE has not been proven.
In the United States, the total incidence of VTE is greater than 200,000 events per year accompanied by a significant number of events occurring in high‐risk hospitalized patients.[3] It has been suggested that patients with liver disease may have a reduced risk for VTE.[4] However, more recent studies report an increased risk with the incidence of VTE in CLD patients occurring in 0.5% to 6.3% of the population.[5, 6, 7, 8, 9, 10] The parallel reduction of anticoagulant factors, such as antithrombin and protein C, along with the reduction in procoagulant factors rebalances the coagulation system, possibly explaining why CLD patients are not protected from VTE.[11, 12] Other mechanistic possibilities include low serum albumin,[8, 9] an elevation of endogenous estrogen levels, immobility associated with the disease,[5] greater morbidity as reflected by high Child‐Pugh scores, and a chronic inflammatory state that results in poor flow and vasculopathy.[7]
Current guidelines for the prevention of VTE do not provide recommendations on the use of prophylaxis in the cirrhotic population,[13] although recent literature reviews suggest that strong consideration for pharmacologic prophylaxis be given when the benefit outweighs the risk.[14, 15] Limited studies have evaluated the use of VTE prophylaxis in CLD patients, whether pharmacologic or mechanical.[6, 7, 8, 16] These studies report that the utilization of VTE prophylaxis in CLD patients is suboptimal, with at least 75% of CLD patients receiving no prophylaxis.[6, 7, 8] The purpose of our study was to examine the use of prophylactic agents and the incidence of VTE and bleeding events in CLD patients.
METHODS
A retrospective chart review of patients diagnosed with CLD or cirrhosis at Methodist University Hospital between August 1, 2009 and July 31, 2011 was conducted. These patients were identified through the corporate patient financial services database using the International Classification of Diseases, 9th Revision, Clinical Modification code 571.xx for CLD/cirrhosis. Patients were included if they were 18 years or older, admitted for or with a history of CLD, and had an INR of 1.4 on admission. An elevated INR was chosen as inclusion criteria as this is often when the controversy of prophylaxis versus no prophylaxis emerges. CLD was defined based on previous histories or clinical presentations of past variceal bleed, presence of varices based on endoscopy report, hepatic encephalopathy, spontaneous bacterial peritonitis, ascites, liver biopsy proven cirrhosis, or imaging consistent with cirrhotic liver changes. CLD was classified as alcoholic, viral hepatitis (hepatitis B and C), and other, such as nonalcoholic steatohepatitis and autoimmune. Patients admitted with maintenance anticoagulation, suspected bleed or VTE, palliative care diagnosis, or history of/anticipated liver transplant were excluded. If a patient met inclusion criteria for an admission and was subsequently readmitted within 30 days, only the initial admission was included. Once patients were included they were assigned to 1 of 4 groups based on the type of prophylaxis received: pharmacologic, mechanical, combined pharmacologic and mechanical, and no prophylaxis. Patients who received pharmacologic or mechanical prophylaxis for at least 50% of their hospital stay were assigned to their corresponding groups accordingly. Patients who received pharmacologic and mechanical prophylaxis for at least 50% of their hospital stay were assigned to the combination group. Patients receiving either form of VTE prophylaxis for <50% of their hospital stay were considered to be without prophylaxis. Pharmacologic prophylaxis was defined by the use of unfractionated heparin (UFH) 5000 units subcutaneously (sq) 3 times daily or twice daily (bid), low molecular weight heparin (LMWH) 30 mg sq bid or 40 mg every day (qd), or fondaparinux 2.5 mg qd. Mechanical prophylaxis was defined by the use of a sequential compression device (SCD). The study was approved by the University of Tennessee Institutional Review Board.
Patient demographics including age, sex, race, height, and weight were documented with a body mass index (BMI) calculated for each patient. Obesity was defined as BMI 30 kg/m2. Risk factors for VTE including obesity, surgery, infection, trauma, malignancy, and history of VTE as well as the etiology of cirrhosis were collected and recorded whenever available based on documentation in the medical chart. Clinical data including lowest serum albumin, highest total bilirubin, highest INR, and platelets on admission were recorded. Severity of ascites and hepatic encephalopathy were documented. Child‐Pugh score and stage as well as Model for End‐Stage Liver Disease (MELD) score were calculated. In‐hospital VTE, bleeding events, length of stay, in‐hospital mortality, and the use, type, and number of days of VTE prophylaxis were documented. VTE was defined as deep venous thrombosis (DVT) or pulmonary embolism diagnosed by venous Doppler ultrasonography, spiral computed tomography (CT) of the chest, or ventilation/perfusion scan. Bleeding was defined by documentation in the medical record plus the administration of packed red blood cells, fresh frozen plasma, recombinant factor VIIa, or vitamin K. For patients who experienced a bleed, risk factors for in‐hospital bleeding as defined by American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines 2012 guidelines (CHEST) were documented.[13]
The primary outcome was to describe the use of VTE prophylaxis in CLD patients. Secondary outcomes were to determine the overall incidence of VTE in CLD patients, examine the incidence of VTE based on the utilization of prophylaxis, compare the occurrence of bleeding events in CLD patients based on type of prophylaxis, evaluate the use of mechanical versus pharmacologic prophylaxis based on INR, evaluate length of stay (LOS) and in‐hospital mortality for CLD patients with and without prophylaxis, and evaluate 30‐day readmission rate for VTE.
Patients were arbitrarily divided into 2 groups according to the highest INR (1.42.0 or >2.0). Baseline characteristics were compared between the 2 groups. Variables were expressed as mean or median with standard deviation or interquartile range. Categorical values were expressed as percentages and compared using the [2] test or Fisher exact test. Continuous data were compared using Mann‐Whitney U test for nonparametric data or Student t test for parametric data. Significance was defined as P<0.05. All statistical analyses were performed using SPSS Statistics (version 20.0; SPSS, Inc., Chicago, IL).
RESULTS
We identified 410 patients who met inclusion criteria during the study period. Baseline demographics were similar between the 2 groups with the exception of age, which was statistically higher in the INR 1.4 to 2.0 group. The most common etiology of CLD was hepatitis B or C, followed by alcohol, then other causes. Alcoholic CLD was associated with higher INR values (>2.0). Patients with INR >2.0 were found to exhibit lower serum albumin levels and platelets on admission as well as higher total bilirubin and INR values. There was also a significant difference in Child‐Pugh stages B and C, with the INR >2.0 group only having stage C. In addition, the higher INR group had a significantly higher average MELD score (Table 1).
| Characteristic | INR1.42.0, n=251 | INR>2.0, n=159 | P Value |
|---|---|---|---|
| |||
| Age, yearsSD | 55.710.4 | 53.310.1 | 0.017 |
| Male sex | 137 (54.6) | 99 (62.3) | 0.125 |
| BMISD | 29.17.3 | 30.37.7 | 0.103 |
| Race | |||
| African American | 99 (39.4) | 53 (33.3) | 0.212 |
| White | 139 (55.4) | 99 (62.3) | 0.169 |
| Other | 13 (5.2) | 7 (4.4) | 0.722 |
| Etiology of CLD | |||
| Hepatitis B or C | 127 (50.6) | 70 (44) | 0.194 |
| Alcohol | 59 (23.5) | 57 (35.9) | 0.007 |
| Other | 65 (25.9) | 32 (20.1) | 0.18 |
| VTE risk factors | |||
| Obesity, BMI 30 | 107 (42.6) | 71 (44.6) | 0.687 |
| Surgery | 21 (8.4) | 7 (4.1) | 0.121 |
| Infection | 81 (32.3) | 63 (39.6) | 0.129 |
| Trauma | 1 (0.4) | 1 (0.6) | 1.00 |
| Malignancy | 35 (13.9) | 24 (15.1) | 0.746 |
| History of VTE | 6 (2.4) | 4 (2.5) | 1.00 |
| Median number VTE risk factors (range) | 1 (03) | 1 (04) | 0.697 |
| Laboratory values | |||
| AlbuminSD | 2.20.58 | 2.00.53 | <0.001 |
| Tbili, median (IQR) | 2.8 (1.95.0) | 8.1 (5.013.3) | <0.001 |
| INR, median (IQR) | 1.7 (1.51.8) | 2.4 (2.22.9) | <0.001 |
| Admission platelets, median (IQR) | 92 (61141) | 79 (58121) | 0.008 |
| Child Pugh stage | |||
| Class A | 3 (1.2) | 0 (0) | 0.286 |
| Class B | 91 (36.3) | 0 (0) | <0.001 |
| Class C | 157 (62.5) | 159 (100) | <0.001 |
| MELD scoreSD | 18.55.1 | 28.36.3 | <0.001 |
Of the 410 patients included, 225 (55%) patients received thromboprophylaxis. The majority of patients received mechanical prophylaxis (n=154), followed by pharmacologic (n=49), and then a combination of mechanical plus pharmacologic (n=22). For patients receiving pharmacologic either alone or in combination with SCDs, 30 received UFH, 33 received LMWH, 1 patient received fondaparinux, and the remaining 7 received a combination of the agents to total 50% of their hospital stay. For patients with INR >2.0, a significant decrease in overall thromboprophylaxis use was seen compared to those with INR 1.4 to 2.0 (47% vs 60%; P=0.013). Patients with INR >2.0 also received significantly less pharmacologic prophylaxis compared to those with INR 1.4 to 2.0 (3.2% vs 17.5%; P<0.001). No differences in the use of mechanical or combination prophylaxis was seen between the groups (Figure 1).

As shown in Table 2, in‐hospital VTE occurred in 3 patients (0.7%). All 3 patients had a DVT. Of the patients with documented VTE, 1 was Child‐Pugh stage B and 2 were stage C. Fifteen bleeding events occurred (3.7%), 9 on mechanical prophylaxis, 1 on pharmacologic, 3 on combination, and 2 with no prophylaxis. The majority of patients experiencing a bleeding event had an INR >2.0 (P=0.001). Eleven patients out of the 15 were considered to be at high risk of bleeding as defined per CHEST 2012 guidelines,[13] whereas 100% had Child‐Pugh stage C with an average MELD score of 31.77.5. It should be noted that 1 patient experienced a bleeding event after receiving pharmacologic treatment doses for VTE and was subsequently placed on a prophylactic dose without any bleeding complications.
| Characteristic | INR1.42.0, n=251 | INR>2.0, n=159 | P Value |
|---|---|---|---|
| |||
| In‐hospital VTE | 1 (0.4) | 2 (1.3) | 0.563 |
| Mechanical | 0 (0) | 1 (0.6) | 0.389 |
| Pharmacologic | 0 (0) | 1 (0.6) | 0.389 |
| Combination | 0 (0) | 0 (0) | 1.00 |
| No prophylaxis | 1 (0.4) | 0 (0) | 1.00 |
| Bleeding event | 3 (1.2) | 12 (7.5) | 0.001 |
| Mechanical | 2 (0.8) | 7 (4.4) | 0.033 |
| Pharmacologic* | 0 (0) | 1 (0.6) | 0.389 |
| Combination | 1 (0.4) | 2 (1.3) | 0.563 |
| No prophylaxis | 0 (0) | 2 (1.3) | 0.152 |
| LOS, median (IQR) | 5 (2.98) | 7.2 (413.1) | <0.001 |
| Hospital mortality | 6 (2.4) | 30 (18.9) | <0.001 |
| 30‐day readmission rate for VTE | 2 (0.8) | 0 (0) | 0.524 |
Longer LOS and higher mortality rates were seen in patients who received prophylaxis compared to those who received no prophylaxis (P<0.001 and P=0.001, respectively). Of the 36 patients who died, 22 received mechanical prophylaxis, 2 received pharmacologic, 5 received a combination, and 7 received no prophylaxis. Longer LOS and higher mortality rates were also seen in patients with INR >2 compared to patients with INR 1.4 to 2.0 (P<0.001 for both) (Table 2). Higher mortality rates were associated with greater severity of disease as defined by Child‐Pugh C classification in all 36 patients (P=0.001) and an average MELD score of 31.87.6. No differences in 30‐day readmission rates for VTE were seen between prophylaxis groups.
DISCUSSION/CONCLUSION
The use of thromboprophylaxis in our study was 55%, which is consistent with the reported rate of 30% to 70% in general hospitalized patients.[17] To our knowledge this is the first study to focus primarily on the use of both pharmacologic and mechanical thromboprophylaxis in CLD patients. Previous studies have focused on the incidence and risks of VTE in CLD patients,[5, 6, 7, 8, 9, 10] with only 3 of those studies evaluating the use of pharmacologic and mechanical thromboprophylaxis as a secondary outcome.[6, 7, 8] The reported use of thromboprophylaxis in these studies ranges from 21% to 25%. Pharmacologic prophylaxis rates were 7% in Northup et al.,[8] 12% in Aldawood et al.,[7] and 9% in Dabbagh et al.,[6] compared to 17% in our study (pharmacologic alone plus combination). Mechanical prophylaxis rates were 14%, 12%, and 16%, respectively, compared to our 38%. None of the previous studies gave a definition for prophylaxis. This is important to note because discrepancies in prophylaxis reporting could lead to significant differences in rates of prophylaxis when comparing these studies to our study.
Despite the higher rates of thromboprophylaxis, the incidence of VTE was 0.7%. Our VTE incidence falls within the reported incidence rate of 0.5% to 6.3%.[6, 7, 8, 9, 10] Similar to Aldawood et al. and Dabbagh et al., we found no significant differences in the incidence of VTE and prophylaxis use.[6, 7] Dabbagh et al. suggest that the incidence of VTE increases as disease severity increases.[6] However, with only 25% of their patients receiving thromboprophylaxis, it is hard to determine if the higher incidence of VTE was due to greater disease severity or the low use of thromboprophylaxis. It is expected that patients with more severe disease are less likely to receive VTE prophylaxis secondary to increases in INR and/or thrombocytopenia. As evidenced in our study, there was a significant decrease in the use of thromboprophylaxis in patients with INR >2.0, driven largely by the significant decrease in the use of pharmacologic prophylaxis. Due to the low incidence of VTE observed, our study lacks adequate power to truly determine the relationship between use of thromboprophylaxis or severity of disease and incidence of VTE.
Nonetheless, we did find a significant correlation between disease severity and bleeding in CLD patients. Although not a new finding in the literature, this result substantiates the claim that the delicate balance and unpredictability of coagulopathy in CLD leads to bleeding events as well as VTE. In our study we had an overall bleeding rate of 3.7%. Patients who experienced a bleeding event had greater disease severity, significantly higher INR, and 73% were considered to be at high risk for an event as defined by CHEST guidelines.[13] The majority of events happened while on mechanical or no prophylaxis. Four patients who received pharmacologic prophylaxis had a bleeding event; however, 1 of those patients bled on VTE pharmacologic treatment dose for VTE found on day 2 of hospital admission. In a recent study by Bechmann et al. looking at the use of LMWH in 84 cirrhotic patients, they report a bleeding rate of 8.3%, a rate that is similar to rates of bleeding in nonanticoagulated cirrhotic patients.[18] In comparison with our study, we had 71 patients receive pharmacologic prophylaxis either alone or in combination and 4 bleeding events, giving an event rate of 5.6%. This rate decreases to 4.2% when considering only prophylactic pharmacologic doses, suggesting that pharmacologic prophylaxis in CLD patients poses a low risk of bleeding. Interestingly enough, an association was found between alcoholic CLD and higher INR (>2.0) in our study. Given that patients with higher INR had increased bleeding events, this introduces a question of whether or not the specific cause of CLD (ie, alcoholic hepatitis) may represent a special risk for bleeding in this population. However, additional studies are needed to confirm this hypothesis.
To our knowledge, this study is also the first to look at the relationship of thromboprophylaxis use on LOS and mortality in CLD patients. At first glance, the fact that patients who received prophylaxis had both significantly longer LOS and higher mortality rates in our study is concerning. However, it is likely that the increased LOS and mortality in our study is attributed to greater disease severity, as evidenced by higher INRs and Child‐Pugh scores regardless of prophylaxis use or not. Also, a known risk factor for VTE is reduced mobility. Although no standard definition for reduced mobility exists, Barbar et al. define it as anticipated bed rest with bathroom privileges (either because of patient's limitations or on physician's order) for at least 3 days.[19] Due to this known increased risk for VTE, it is expected that patient's with a LOS of 3 days are more likely to receive thromboprophylaxis.
Our study has several limitations. Like other retrospective studies, this study was conducted in 1 medical center and relies on the accuracy of documentation. We relied on patient history and clinical presentation to diagnose CLD without the requirement of histologic diagnosis. However, all patients included in the study had an unquestionable diagnosis by a physician. We used an arbitrary definition and assignment of patients into groups based on the method of VTE prophylaxis utilized due to lack of a definition in the medical literature. There was a possible selection bias for pharmacologic prophylaxis based on patient risk factors for bleeding, such as presence of varices and thrombocytopenia. Also, the inability to ensure that patients with an order for SCDs were actively wearing the device throughout their hospital stay is yet another limitation. Not all patients underwent testing for VTE; therefore, the actual incidence of VTE may be higher than what we found. Only those patients who experienced a bleeding event were assessed for risk factors that predisposed them to bleed, making it hard to correlate those risk factors with the risk of bleeding in all CLD patients.
Despite these limitations, our study has great strengths. This is the first study to focus primarily on the use of both pharmacologic and mechanical thromboprophylaxis in CLD patients. Therefore, it has the potential to influence and raise awareness on the decisions made involving the management of CLD patients in regard to VTE prophylaxis and will hopefully serve as an impetus for future prospective studies. When comparing this study to other studies looking at the incidence of VTE in CLD patients and the use of prophylaxis, our study sample size is relatively large. Also, by including only those patients with INR of at least 1.4 on admission, our study patients had greater severity of disease, making this study distinctly relevant in the clinical debate of whether or not CLD patients should receive thromboprophylaxis.
In conclusion, the use of thromboprophylaxis in CLD patients is higher in our study than previous reports but remains suboptimal. Although bleeding is an inherent risk factor in CLD independent of VTE prophylaxis, the use of VTE pharmacologic prophylaxis does not appear to increase bleeding in CLD patients with INR 2.0. Further studies focusing on baseline bleeding risks (ie, thrombocytopenia, presence of varices) and the use of pharmacologic prophylaxis are needed to provide additional safety data on the use of pharmacologic prophylaxis in this patient population.
Disclosures: All coauthors have seen and agree with the contents of the article. Submission is not under review by any other publication. All authors have not received notification of redundant or duplicate publication. All authors have no financial conflicts of interest. No funding was received for this study or article.
- , , . National hospital discharge survey: 2002 annual summary with detailed diagnosis and procedure data. Vital Health Stat 13. 2005;158:1–199.
- , , , . Deaths: final data for 2007. Natl Vital Stat Rep. 2010;58(19):1–135.
- , , , et al. The diagnostic approach to acute venous thromboembolism: clinical practice guideline. Am J Respir Crit Care Med. 1999;160(3):1043–1066.
- , , , , , . Risk factors for deep vein thrombosis and pulmonary embolism: a population‐based case‐control study. Arch Intern Med. 2000;160(6):809–815.
- , , , , , . Risk of venous thromboembolism in patients with liver disease: a nationwide population‐based case‐control study. Am J Gastroenterol. 2009;104(1):96–101.
- , , , , . Coagulopathy does not protect against venous thromboembolism in hospitalized patients with chronic liver disease. Chest. 2010;137(5):1145–1149.
- , , , et al. The incidence of venous thromboembolism and practice of deep venous thrombosis prophylaxis in hospitalized cirrhotic patients. Thromb J. 2011;9(1):1.
- , , , et al. Coagulopathy does not fully protect hospitalized cirrhosis patients from peripheral venous thromboembolism. Am J Gastroenterol. 2006;101(7):1524–1528.
- , , , , . Deep vein thrombosis and pulmonary embolism in cirrhosis patients. Dig Dis Sci. 2008;53(11):3012–3017.
- , , , , , . Venous thromboembolism and liver cirrhosis. Rev Esp Enferm Dig. 2008;100(5):259–262.
- , , . Should we give thromboprophylaxis to patients with liver cirrhosis and coagulopathy? HPB. 2009;11(6):459–464.
- , . The coagulopathy of chronic liver disease. N Engl J Med. 2011;365(2):147–156.
- , , , et al. Prevention of VTE in nonsurgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(suppl 2):e195S–e226S.
- , . Pharmacologic prophylaxis against venous thromboembolism in hospitalized patients with cirrhosis and associated coagulopathies. Am J Health Syst Pharm. 2012;69(8):658–63.
- , , . Risk of venous thromboembolism in patients with chronic liver disease and the utility of venous thromboembolism prophylaxis. Ann Pharmacother. 2012;46(6):873–878.
- , , , et al. A systematic review of venous thromboembolism prophylaxis strategies in patients with renal insufficiency, obesity, or on antiplatelet agents. J Hosp Med. 2013;8(7):394–401.
- , , , , , . Prevention of venous thromboembolism in the hospitalized medical patient. Cleve Clin J Med. 2008;75(suppl 3):S7–S16.
- , , , , , . Low‐molecular‐weight heparin in patients with advanced cirrhosis. Liver Int. 2011;31(1):75–82.
- , , , et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the Padua Prediction Score. J Thromb Haemost. 2010;8(11):2450–2457.
- , , . National hospital discharge survey: 2002 annual summary with detailed diagnosis and procedure data. Vital Health Stat 13. 2005;158:1–199.
- , , , . Deaths: final data for 2007. Natl Vital Stat Rep. 2010;58(19):1–135.
- , , , et al. The diagnostic approach to acute venous thromboembolism: clinical practice guideline. Am J Respir Crit Care Med. 1999;160(3):1043–1066.
- , , , , , . Risk factors for deep vein thrombosis and pulmonary embolism: a population‐based case‐control study. Arch Intern Med. 2000;160(6):809–815.
- , , , , , . Risk of venous thromboembolism in patients with liver disease: a nationwide population‐based case‐control study. Am J Gastroenterol. 2009;104(1):96–101.
- , , , , . Coagulopathy does not protect against venous thromboembolism in hospitalized patients with chronic liver disease. Chest. 2010;137(5):1145–1149.
- , , , et al. The incidence of venous thromboembolism and practice of deep venous thrombosis prophylaxis in hospitalized cirrhotic patients. Thromb J. 2011;9(1):1.
- , , , et al. Coagulopathy does not fully protect hospitalized cirrhosis patients from peripheral venous thromboembolism. Am J Gastroenterol. 2006;101(7):1524–1528.
- , , , , . Deep vein thrombosis and pulmonary embolism in cirrhosis patients. Dig Dis Sci. 2008;53(11):3012–3017.
- , , , , , . Venous thromboembolism and liver cirrhosis. Rev Esp Enferm Dig. 2008;100(5):259–262.
- , , . Should we give thromboprophylaxis to patients with liver cirrhosis and coagulopathy? HPB. 2009;11(6):459–464.
- , . The coagulopathy of chronic liver disease. N Engl J Med. 2011;365(2):147–156.
- , , , et al. Prevention of VTE in nonsurgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(suppl 2):e195S–e226S.
- , . Pharmacologic prophylaxis against venous thromboembolism in hospitalized patients with cirrhosis and associated coagulopathies. Am J Health Syst Pharm. 2012;69(8):658–63.
- , , . Risk of venous thromboembolism in patients with chronic liver disease and the utility of venous thromboembolism prophylaxis. Ann Pharmacother. 2012;46(6):873–878.
- , , , et al. A systematic review of venous thromboembolism prophylaxis strategies in patients with renal insufficiency, obesity, or on antiplatelet agents. J Hosp Med. 2013;8(7):394–401.
- , , , , , . Prevention of venous thromboembolism in the hospitalized medical patient. Cleve Clin J Med. 2008;75(suppl 3):S7–S16.
- , , , , , . Low‐molecular‐weight heparin in patients with advanced cirrhosis. Liver Int. 2011;31(1):75–82.
- , , , et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the Padua Prediction Score. J Thromb Haemost. 2010;8(11):2450–2457.
© 2013 Society of Hospital Medicine
VRE Bacteremia Treatment With Linezolid or Daptomycin
Enterococci have been identified as a causative organism in approximately 10% of all nosocomial bloodstream infections (BSIs).1, 2 In 2006, the Infectious Diseases Society of America identified vancomycin‐ resistant Enterococcus faecium (VRE) as 1 of 6 microbes considered to be among the most dangerous due to high rates of resistance and a limited number of effective antimicrobials.3 E. faecium has exhibited high rates of glycopeptide resistance with as many as 60% of isolates from BSIs being resistant to vancomycin.2, 4 Due to increasing resistance to glycopeptides, vancomycin has become obsolete in the treatment of E. faecium infections.5
A limited number of antimicrobials are available for the treatment of infections due to VRE. Agents active in vitro are quinupristin‐dalfopristin, tigecycline, linezolid, and daptomycin. Quinupristin‐dalfopristin was one of the first agents approved for use in VRE infections; however, treatment with this agent has been limited because of mediocre clinical response rates, undesirable adverse effects, high cost, and insufficient E. faecalis activity.6, 7 Tigecycline is not an optimal antibiotic for the treatment of VRE bacteremia, because serum concentrations achieved after administration are inadequate to treat BSIs.7 In contrast, linezolid and daptomycin have evinced efficacy against VRE bacteremia, with reported microbiologic response rates of 85% and 80%, respectively.7, 8 One inherent difference between these antibiotics that may theoretically affect their use in immunocompromised patients is that linezolid is bacteriostatic, whereas daptomycin is bactericidal. It has been postulated that by using a bactericidal antibiotic such as daptomycin in the immunocompromised host, one may achieve superior clinical and microbiologic response rates.3, 7, 9, 10
Since the introduction of the oxazolidinone linezolid in 2000, widespread use has led to reports of linezolid‐resistant VRE as well as nosocomial transmission of linezolid‐resistant VRE in hospitals.4, 1114 Despite linezolid being a key antibiotic for the treatment of VRE infections over the last 10 years, development of resistance along with potential hematologic and neurologic toxicity during long‐term use remains a concern.7, 8 Although daptomycin is active against several resistant organisms, including VRE, the evidence supporting use of daptomycin for VRE BSI is limited to case reports or small case series.7, 9 Moreover, daptomycin has not received US Food and Drug Administration approval for the treatment of VRE infections,15 and emerging data regarding daptomycin‐nonsusceptible enterococci (Minimum Inhibitory Concentration, MIC >4 mg/L) highlight a new problem for this multidrug‐resistant pathogen.16, 17 Few studies in recent years have compared these 2 antibiotics in the treatment of VRE BSIs.4, 18, 19 Due to the high rates of vancomycin resistance reported at our institution and the ubiquitous use of linezolid and daptomycin in the treatment of VRE bacteremia, we chose to evaluate response rates for these antibiotics in an effort to add to previously published literature on this topic.
MATERIALS AND METHODS
Patient Selection
Methodist University Hospital (MUH) in Memphis, Tennessee, is part of a 7‐hospital system with 697 licensed beds. MUH is a tertiary teaching hospital with centers of excellence in neuroscience and transplantation. Patients admitted to MUH diagnosed with VRE bacteremia between January 1, 2004, and July 31, 2009, were identified by the microbiology laboratory. All patients who were 18 years of age, had 1 documented positive VRE blood culture, and received linezolid or daptomycin for 5 days were eligible. Patients were excluded if they were treated simultaneously with more than 1 agent active against VRE. This study was approved by the MUH Institutional Review Board. Of note, use of linezolid or daptomycin at MUH is restricted to an infectious disease physician or pulmonologist. Currently, there are no protocols at our institution for treating VRE infections.
Data Collection and Definitions
Cerner Millennium was used to collect all pertinent patient information. Patient records were reviewed to determine demographic data, comorbid illnesses, laboratory data (from admission to discharge), medications, and discharge status (home, long‐term care facility, or death). Comorbid illnesses evaluated included: chronic obstructive pulmonary disease, diabetes mellitus, malignancy (solid or hematologic), transplant (liver or kidney), end‐stage renal disease (ESRD) (hemodialysis or nonhemodialysis), cirrhosis, and endocarditis. ESRD and endocarditis were defined per chart diagnosis. Laboratory data collected included serum creatinine, creatine phosphokinase, absolute neutrophil count (neutropenia defined as absolute neutrophil count <1000), and number and site (intravenous line or peripheral blood draw) of positive VRE blood cultures. Other data collected were (1) time elapsed to adequate antibiotic coverage, which was defined as microbiologic documentation of an infection that was being effectively treated at the time of its identification, and (2) time to appropriate antibiotic coverage, which was defined as antimicrobial treatment selected for efficacy based on presumptive identification of the causative pathogen, the antimicrobial agent's spectrum of activity, and local microbial resistance patterns.20 Doses of daptomycin and linezolid used in patients with VRE bacteremia were also documented.
Clinical cure was defined as a resolution of signs and/or symptoms of infection (white blood cell count <10,000/mm3, bands <5%, heart rate <90 beats per minute, respiratory rate <20 breaths per minute, and maximum oral temperature <38C) after gram‐positive therapy was discontinued. The definition of microbiologic cure was lack of positive blood cultures for VRE at least 14 days after cessation of gram‐positive therapy. Microbiologic failure was defined as positive VRE blood cultures obtained on gram‐positive therapy necessitating a change in treatment. Recurrence was defined as VRE bacteremia within 30 days after discontinuation of gram‐positive therapy. Reinfection was defined as VRE bacteremia that appeared 30 days after completion of primary gram‐positive therapy.
All isolates were tested for susceptibility to linezolid using the MicroScan system, whereas daptomycin susceptibility patterns were obtained by either the Etest or MicroScan system. Of importance, our laboratory did not routinely report isolate susceptibility for daptomycin until 2008. Clinical Laboratory Standards Institute breakpoint guidelines were used to delineate minimum inhibitory concentrations for linezolid and daptomycin.
Outcomes
The primary objective was to determine the cure rate, both clinical and microbiologic, of VRE bacteremia with the use of linezolid and daptomycin. Secondary outcomes were rates of recurrence and reinfection as well as 30‐day mortality. Clinical and microbiologic response rates for subsets of the patient population that were deemed immunocompromised or at an increased risk for VRE infections (neutropenic, transplant, malignancy, and ESRD on hemodialysis) were also evaluated.
Statistical Analysis
Data were analyzed using SAS version 9.2 (SAS Inc, Cary, NC). Patients with categorical characteristics were compared using a chi‐square test or Fisher's exact test. Continuous data were analyzed using a Student t test and are expressed as the mean standard deviation. The mean duration of initial antibiotics, time to appropriate antibiotics, time to adequate antibiotic therapy, and LOS were all calculated for the linezolid and daptomycin group with a Student t test used to compare the differences. Multivariate logistic regression was used for the following outcomes: clinical cure, microbiologic cure, mortality, reinfection, and recurrence. For the interval variable, LOS, stepwise multiple regression was used to choose significant independent variables. P < 0.05 was considered statistically significant.
RESULTS
Patient Characteristics
Of the 361 patients identified with a positive VRE blood culture, 201 were included in the study. The remaining 160 patients were excluded for one of the following reasons: <5 days of therapy (n = 87), no documented gram‐positive therapy (n = 49), simultaneous gram‐positive therapy (n = 10), or insufficient data to evaluate response rates (n = 14). For the treatment of VRE bacteremia, 138 patients received linezolid and 63 patients received daptomycin. Demographics, comorbid illnesses, and patient characteristics are shown in Table 1. There was a statistically significant difference in the average age, with the linezolid group consisting of older patients. The daptomycin group had more patients with hematologic malignancies than the linezolid group (33% vs 14%; P = 0.0021) and more patients who received liver transplants (13% vs 4%; P = 0.0264).
| Linezolid (n = 138) | Daptomycin (n = 63) | P Value | |
|---|---|---|---|
| |||
| Average age, years, mean SD | 60 16 | 5315 | 0.0028 |
| Male, No. (%) | 59 (43) | 36 (57) | 0.0682 |
| Race, No. (%) | |||
| Caucasian | 34 (25) | 23 (37) | 0.1043 |
| African American | 103 (75) | 39 (62) | |
| Other | 1 (1) | 1 (2) | |
| COPD, No. (%) | 8 (6) | 2 (3) | 0.7277 |
| Diabetes mellitus, No. (%) | 61 (44) | 21 (33) | 0.1655 |
| Hemodialysis, No. (%) | 35 (25) | 17 (27) | 0.8627 |
| Malignancy, No. (%) | |||
| Solid organ | 26 (19) | 16 (25) | 0.3499 |
| Hematologic | 19 (14) | 21 (33) | 0.0021 |
| Transplant, No. (%) | |||
| Liver | 5 (4) | 8 (13) | 0.0264 |
| Kidney | 3 (2) | 0 | 0.5533 |
| Endocarditis,* No. (%) | 4 (3) | 3 (5) | 0.6801 |
| Species of VRE (%) | |||
| Enterococcus faecalis | 33 (24) | 10 (16) | 0.2658 |
| Enterococcus faecium | 105 (76) | 53 (84) | 0.2658 |
| Time to appropriate abx therapy, hours, mean SD | 12.4 26.9 | 818.8 | 0.1851 |
| Time to adequate abx therapy, days, mean SD | 2.3 1.8 | 1.81.5 | 0.0554 |
| Duration of initial abx, days, mean SD | 11.1 6.0 | 14.114.6 | 0.0401 |
| Abx before initial therapy, No. (%) | 85 (62) | 34 (54) | 0.3541 |
| Average dose, mg/kg, mean SD | NA | 6.11.5 | NA |
| Mortality, No. (%) | 25 (18) | 15 (24) | 0.3481 |
| LOS (days) | 37.527.7 | 40.827.9 | 0.4336 |
From the microbiology laboratory report of initial blood cultures, 78.6% of the isolates were noted as being E. faecium, with the remainder being E. faecalis (21.4%). One patient was classified as having linezolid‐resistant E. faecium (MIC >4 mg/L) upon repeat blood culture. Daptomycin MICs were obtained for 44 isolates using the Etest or MicroScan system; all isolates were susceptible with MICs ranging from 0.254 mg/L. As mentioned previously, our laboratory did not routinely report isolate susceptibility to daptomycin until 2008.
There were no statistically significant differences between the treatment groups with regard to time to appropriate or adequate antibiotic therapy (Table 1). However, there was a statistically significant difference in the mean duration of initial antibiotics between linezolid and daptomycin (11.1 days vs 14.1 days; P = 0.0401). Dosing strategies used in these patients were also evaluated. All linezolid patients received a dose of 600 mg every 12 hours by mouth or intravenously. The average dose of daptomycin was 6.1 mg/kg (range, 3.410.4 mg/kg; median, 6 mg/kg). The average LOS was 37 days for linezolid vs 40 days for daptomycin, which did not confer statistical significance. Overall mortality was 20%, occurring in 25 linezolid patients versus 15 daptomycin patients (P = 0.3481). The stepwise multiple regression analysis did not identify any statistically significant variables in patients treated with linezolid or daptomycin that affected any of the outcomes.
Outcomes and Analysis
As shown in Table 2, there were no statistically significant differences in clinical or microbiologic cure between the linezolid and daptomycin groups (74% vs 75% and 94% vs 94%, respectively). However, the linezolid group compared with the daptomycin group had fewer patients that developed a positive blood culture while on their initial antibiotic therapy (8% vs 22%; P = 0.0097). Follow‐up cultures were required to determine rates of recurrence and reinfection. Only 107/138 patients in the linezolid group and 51/63 patients in the daptomycin group had follow‐up cultures collected. Recurrence was documented in 3% of linezolid patients vs 12% of daptomycin patients (P = 0.0321). The odds ratio for developing a recurrent infection with daptomycin versus linezolid was 5.51 (95% confidence interval, 1.2524.28). Out of 6 patients that developed a recurrent VRE infection in the daptomycin group, 2 were prescribed doses <4 mg/kg with no reported MICs, and 2 patients received 6 mg/kg with reported MICs of 4 mg/L. No statistically significant difference existed for the rate of reinfection between linezolid and daptomycin (1% vs 6%; P = 0.0992).
| Linezolid (n = 138) | Daptomycin (n = 63) | P Value | |
|---|---|---|---|
| |||
| Patients with positive culture on G+ therapy | 11 (8) | 14 (22) | 0.0097 |
| Clinical cure | 102 (74) | 47 (75) | 1 |
| Microbiologic cure | 130 (94) | 59 (94) | 1 |
| Recurrence* | 3 (3) | 6 (12) | 0.0321 |
| Reinfection* | 1 (1) | 3 (6) | 0.0992 |
Table 3 provides information on subsets of the patient population deemed high‐risk for VRE infection or immunocompromised. There was no statistically significant difference between the 2 antibiotic groups in clinical or microbiologic cure. In the subsets of immunocompromised patients, there was no difference in recurrence or reinfection between the linezolid and daptomycin patients. Furthermore, all groups had similar LOS regardless of the antibiotic used to treat the VRE BSI. Moreover, there were no statistically significant differences in 30‐day mortality in these subsets of the population with regard to initial antibiotic choice. No significant independent variables were found between linezolid or daptomycin that affected any of the outcomes listed in Table 3.
| Neutropenia (%) | Hematologic Malignancy (%) | ESRD on Hemodialysis (%) | Liver Transplant (%) | |||||
|---|---|---|---|---|---|---|---|---|
| LZD (n = 16) | Dapto (n = 5) | LZD (n = 19) | Dapto (n = 21) | LZD (n = 35) | Dapto (n = 17) | LZD (n = 5) | Dapto (n = 8) | |
| ||||||||
| Clinical cure, No. (%) | 12 (75) | 5 (100) | 18 (95) | 17 (81) | 24 (69) | 12 (71) | 4 (80) | 3 (38) |
| Microbiologic cure,* No. (%) | 13 (81) | 5 (100) | 18 (95) | 20 (95) | 33 (94) | 16 (94) | 5 (100) | 8 (100) |
| Recurrence,* No. (%) | 2 (13) | 1 (20) | 1 (6) | 2 (11) | 0 | 2 (15) | 0 | 1 (14) |
| Reinfection,* No. (%) | 0 | 0 | 0 | 0 | 1 (4) | 2 (15) | 0 | 1 (14) |
| Mortality, No. (%) | 3 (19) | 1 (20) | 4 (21) | 3 (14) | 7 (20) | 4 (24) | 1 (20) | 4 (50) |
| LOS, days, meanSD | 57.422 | 39.412.4 | 47.626.2 | 4127.1 | 38.833.8 | 39.640.4 | 5034.5 | 73.338.8 |
DISCUSSION
Vergis et al21 reported that infections with VRE compared with vancomycin‐sensitive infections were associated with a higher rate of mortality and that the chosen antimicrobial therapy may play a pivotal role in the risk of death. Our retrospective study suggests that linezolid and daptomycin appear to be equally efficacious for the treatment of VRE BSIs. The results from our study for clinical and microbiologic cure rates for linezolid and daptomycin are similar to previously published data.7, 8 In accordance with previous studies,4 our data demonstrate that there is a higher rate of recurrence in patients treated with daptomycin. This finding may be explained by the fact that the daptomycin group was comprised of more complex patients with a greater disease burden versus the linezolid group; therefore, they were more susceptible to a recurrent VRE infection. In our study, patients who were treated with daptomycin were 5.5 times more likely to have a recurrent infection than linezolid‐treated patients. However, this finding must be scrutinized, because over half of the patients with recurrence either received an inappropriate dose or had high MICs to daptomycin.
Despite there being few clinical and microbiologic outcome data with daptomycin, our study proposes that a bactericidal antibiotic and a bacteriostatic antibiotic have comparable efficacy in the treatment of VRE BSIs. Previous literature has mainly comprised case studies or series that have evaluated clinical outcomes with daptomycin in the treatment of VRE BSIs. Gallagher et al7 reported the results of a retrospective case series of 30 patients with VRE bacteremia who were treated with daptomycin. In this study, microbiologic cure was achieved in 80% of patients, with clinical success in 59% of the patients. In 2009, Mave et al4 compared clinical outcomes between daptomycin and linezolid in the treatment of VRE bacteremia. Reported results demonstrated a microbiologic cure rate of 90% for daptomycin versus 88% for linezolid.4 Moreover, there were no differences in mortality between the groups in our study. In 2010, Crank et al18 reported no differences in mortality (in‐hospital) for hospitalized patients with VRE BSIs treated with linezolid or daptomycin. Our results seem to be consistent with what has been published previously concerning clinical outcomes associated with linezolid or daptomycin in the treatment of VRE BSI.
The average daptomycin dose received in our patients was 6.1 mg/kg with doses ranging from 3.410.4 mg/kg. The underdosing as well as higher MICs to daptomycin may have contributed to a higher rate of recurrence. Previous reports state that Enterococcus species may have higher MICs to daptomycin than Staphylococcus or Streptococcus species; consequently, higher doses may be needed to adequately treat enterococcal infections.7 In the aforementioned study by Gallagher et al,7 doses of daptomycin 6 mg/kg were associated with a positive clinical outcome in 81% of patients compared with 31% if the dose used was <6 mg/kg. Linezolid is dosed 600 mg every 12 hours by mouth or intravenously, with no variations. There have been no studies comparing the uniform dosing of linezolid to the weight‐based dosing of daptomycin and their effects on outcomes.
Patients particularly susceptible to VRE infections include those with neutropenia and/or cancer, patients receiving long‐term hemodialysis, and liver transplant recipients.3, 22, 23 Upon review of this immunocompromised population, we noted no statistically significant differences in overall outcomes. A study by Kraft et al.24 supports the findings in our study that both drugs appear useful in the treatment of VRE bacteremia in patients with hematologic malignancy. We did identify a difference, albeit nonsignificant, in LOS for daptomycin versus linezolid in patients with a history of liver transplantation. Again, the level of care that these patients needed compared with the general population may explain this difference. As mentioned previously, another pertinent factor would be the dose of daptomycin used in these patients, because the dose can affect clinical success. Because all of the other patients had a similar LOS, we cannot determine that the increased LOS seen in liver transplant patients treated with daptomycin was solely due to daptomycin use. The reason for the increased LOS seems to be multifactorial. In the neutropenic population, a difference in LOS was also recognized, but follow‐up complete blood count values were not collected for these patients to determine whether linezolid contributed to further bone marrow suppression leading to an increase in LOS. For both of these patient populations, the number of patients included is very small (n = 21 for neutropenia total, n = 13 for liver transplant total), which can lead to a high degree of variance.
This study has several limitations. This was a retrospective review; therefore, we had no control over the selection of therapy. This may be reflected in an apparent preferential use of daptomycin in immunocompromised patients. Furthermore, 62% of linezolid patients and 54% of daptomycin patients received an antibiotic before initial therapy that could have potentially altered response rates. Due to the paucity of documentation surrounding initial site of infection, some of the positive cultures may represent potential contamination, because VRE may contaminate skin.25 Contamination seems implausible, however, because patients were seen by an infectious disease physician and had at least 1 documented positive VRE blood culture. We chose arbitrary definitions for clinical cure, microbiologic cure, microbiologic failure, recurrence, and reinfection. Previous studies have used their own definitions leading to discrepancies in reporting. Another limitation was that follow‐up cultures were not obtained on all of the patients, which was needed to determine rates of recurrence, reinfection, and microbiologic cure. MICs to daptomycin were not reported in 30% of our patients, potentially altering the recurrence rate seen in the daptomycin‐treated patients. Because clinical cure was not documented in the chart, it was inferred from the laboratory values and vital sign information. One investigator analyzed all of the values and made the determination of clinical cure, allowing for a consistent approach to data review.
In the face of the imposing threat of a highly resistant organism such as VRE with a limited number of efficacious antibiotics, antimicrobial selection becomes increasingly important and is requisite to clinical and microbiological success. To our knowledge, this is one of the largest studies to date comparing the efficacy of linezolid with that of daptomycin in the treatment of VRE bacteremia. Both of these agents are effective for the treatment of VRE BSIs. Nevertheless, specific factors related to the medication (eg, dose, route of administration) as well as the patient (eg, comorbid conditions, acuity of illness) should be taken into consideration when selecting an initial antimicrobial agent. Because the treatment of VRE BSIs continues to be a challenge, larger prospective randomized controlled trials are needed to corroborate our results and determine the optimal therapy for this serious infection.
Acknowledgements
Disclosures: Michael S. Gelfand is on the speaker's bureau for Cubist and Pfizer.
- ,,,,,.Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study.Clin Infect Dis.2004;39:309–317.
- ,,, et al.Clinical practice guidelines for the management of intravascular catheter‐related infection: 2009 update by the Infectious Diseases Society of America.Clin Infect Dis.2009;49:1–45.
- ,,,,,.Bad bugs need drugs: an update on the development pipeline from the antimicrobial availability task force of the infectious diseases society of America.Clin Infect Dis.2006;42:657–668.
- ,,,.Vancomycin‐resistant enterococcal bacteraemia: is daptomycin as effective as linezolid?J Antimicrob Chemother.2009;64:175–180.
- ,,.Management of multidrug‐resistant enterococcal Infections.Clin Microbiol Infect.2010;16:555–562.
- ,,, et al.Treatment of vancomycin‐resistant Enterococcus faecium infections with quinupristin/dalfopristin.Clin Infect Dis.2001;33:1816–1823.
- ,,, et al.Daptomycin for vancomycin‐resistant enterococcol bacteremia: a retrospective case series of 30 patients.Pharmacotherapy.2009;29:792–799.
- ,,, et al.Safety, efficacy and pharmacokinetics of linezolid for treatment of resistant gram positive infections in cancer patients with neutropenia.Ann Oncol.2003;14:795–801.
- ,,,,.Daptomycin for the treatment of vancomycin resistant Enterococcus faecium bacteremia.Scand J Infect Dis.2003;38:290–292.
- ,.New antimicrobial agents for the treatment of gram positive bacterial infections.Clin Microbiol Infec.2008;14:411–420.
- ,.Emergence and management of drug‐resistant enterococcal infections.Expert Rev Anti Infect Ther.2008;6:637–655.
- ,,.Nosocomial spread of linezolid‐resistant, Vancomycin resistant Enterococcus faecium.N Engl J Med.2002;346:867–869.
- ,, et al.Nosocomial superinfections due to linezolid‐resitant Enterococcus faecalis: evidence for a gene dosage effect of linezolid MICs.Diagn Microbiol Infect Dis.2003;47:511–513.
- .Quinupristin‐dalfopristin and linezolid: evidence and opinion.Clin Infect Dis.2003;36:473–481.
- Cubicin (Daptomycin for injection) [package insert].Lexington, MA:Cubist Pharmaceuticals;2010.
- ,,,.Daptomycin nonsusceptible Enterococci: an emerging challenge for clinicians.Clin Infect Dis.2011;52:228–234.
- ,,,.A potential role for daptomycin in enterococcal infections: what is the evidence?J Antimicrob Chemother.2010;65:1126–1136.
- ,,, et al.Comparison of outcomes from daptomycin or linezolid for vancomycin‐resistant enterococcal bloodstream infection: a retrospective, multicenter, cohort study.Clin Ther.2010;32:1713–1719.
- ,,,,,.Observational study of the epidemiology and outcomes of vancomycin‐resistant Enterococcus bacteraemia treated with newer antimicrobial agents.Epidemiol Infect.2010;15:1–9.
- .Inadequate antimicrobial treatment: an important determinant of outcome for hospitalized patients.Clin Infect Dis.2000;31:S131–S138.
- ,,, et al.Determinants of vancomycin resistance and mortality rates in enterococcal bacteremia: a prospective multicenter study.Ann Intern Med.2001;135:484–492.
- ,,,,,.Vancomycin resistant enterococci among chronic hemodialysis patients: a prospective study of acquisition.Clin Infect Dis.2001;32:23–29.
- ,,, et al.A nationwide, multicenter, case control study comparing risk factors, treatment, and outcome for vancomycin resistant andsusceptible enterococcal bacteremia.Diagn Microbiol Infect Dis.2000;36:145–158.
- ,,,,.Outcomes of therapy: vancomycin‐resistant enterococcal bacteremia in hematology and bone marrow transplant patients [published online ahead of print November 26, 2010].Support Care Cancer. doi: 10.1007/s00520–010‐1038‐z.
- ,,, et al.Skin colonization with vancomycin‐resistant enterococci among hospitalized patients with bacteremia.Clin Infect Dis.1997;24:704–706.
Enterococci have been identified as a causative organism in approximately 10% of all nosocomial bloodstream infections (BSIs).1, 2 In 2006, the Infectious Diseases Society of America identified vancomycin‐ resistant Enterococcus faecium (VRE) as 1 of 6 microbes considered to be among the most dangerous due to high rates of resistance and a limited number of effective antimicrobials.3 E. faecium has exhibited high rates of glycopeptide resistance with as many as 60% of isolates from BSIs being resistant to vancomycin.2, 4 Due to increasing resistance to glycopeptides, vancomycin has become obsolete in the treatment of E. faecium infections.5
A limited number of antimicrobials are available for the treatment of infections due to VRE. Agents active in vitro are quinupristin‐dalfopristin, tigecycline, linezolid, and daptomycin. Quinupristin‐dalfopristin was one of the first agents approved for use in VRE infections; however, treatment with this agent has been limited because of mediocre clinical response rates, undesirable adverse effects, high cost, and insufficient E. faecalis activity.6, 7 Tigecycline is not an optimal antibiotic for the treatment of VRE bacteremia, because serum concentrations achieved after administration are inadequate to treat BSIs.7 In contrast, linezolid and daptomycin have evinced efficacy against VRE bacteremia, with reported microbiologic response rates of 85% and 80%, respectively.7, 8 One inherent difference between these antibiotics that may theoretically affect their use in immunocompromised patients is that linezolid is bacteriostatic, whereas daptomycin is bactericidal. It has been postulated that by using a bactericidal antibiotic such as daptomycin in the immunocompromised host, one may achieve superior clinical and microbiologic response rates.3, 7, 9, 10
Since the introduction of the oxazolidinone linezolid in 2000, widespread use has led to reports of linezolid‐resistant VRE as well as nosocomial transmission of linezolid‐resistant VRE in hospitals.4, 1114 Despite linezolid being a key antibiotic for the treatment of VRE infections over the last 10 years, development of resistance along with potential hematologic and neurologic toxicity during long‐term use remains a concern.7, 8 Although daptomycin is active against several resistant organisms, including VRE, the evidence supporting use of daptomycin for VRE BSI is limited to case reports or small case series.7, 9 Moreover, daptomycin has not received US Food and Drug Administration approval for the treatment of VRE infections,15 and emerging data regarding daptomycin‐nonsusceptible enterococci (Minimum Inhibitory Concentration, MIC >4 mg/L) highlight a new problem for this multidrug‐resistant pathogen.16, 17 Few studies in recent years have compared these 2 antibiotics in the treatment of VRE BSIs.4, 18, 19 Due to the high rates of vancomycin resistance reported at our institution and the ubiquitous use of linezolid and daptomycin in the treatment of VRE bacteremia, we chose to evaluate response rates for these antibiotics in an effort to add to previously published literature on this topic.
MATERIALS AND METHODS
Patient Selection
Methodist University Hospital (MUH) in Memphis, Tennessee, is part of a 7‐hospital system with 697 licensed beds. MUH is a tertiary teaching hospital with centers of excellence in neuroscience and transplantation. Patients admitted to MUH diagnosed with VRE bacteremia between January 1, 2004, and July 31, 2009, were identified by the microbiology laboratory. All patients who were 18 years of age, had 1 documented positive VRE blood culture, and received linezolid or daptomycin for 5 days were eligible. Patients were excluded if they were treated simultaneously with more than 1 agent active against VRE. This study was approved by the MUH Institutional Review Board. Of note, use of linezolid or daptomycin at MUH is restricted to an infectious disease physician or pulmonologist. Currently, there are no protocols at our institution for treating VRE infections.
Data Collection and Definitions
Cerner Millennium was used to collect all pertinent patient information. Patient records were reviewed to determine demographic data, comorbid illnesses, laboratory data (from admission to discharge), medications, and discharge status (home, long‐term care facility, or death). Comorbid illnesses evaluated included: chronic obstructive pulmonary disease, diabetes mellitus, malignancy (solid or hematologic), transplant (liver or kidney), end‐stage renal disease (ESRD) (hemodialysis or nonhemodialysis), cirrhosis, and endocarditis. ESRD and endocarditis were defined per chart diagnosis. Laboratory data collected included serum creatinine, creatine phosphokinase, absolute neutrophil count (neutropenia defined as absolute neutrophil count <1000), and number and site (intravenous line or peripheral blood draw) of positive VRE blood cultures. Other data collected were (1) time elapsed to adequate antibiotic coverage, which was defined as microbiologic documentation of an infection that was being effectively treated at the time of its identification, and (2) time to appropriate antibiotic coverage, which was defined as antimicrobial treatment selected for efficacy based on presumptive identification of the causative pathogen, the antimicrobial agent's spectrum of activity, and local microbial resistance patterns.20 Doses of daptomycin and linezolid used in patients with VRE bacteremia were also documented.
Clinical cure was defined as a resolution of signs and/or symptoms of infection (white blood cell count <10,000/mm3, bands <5%, heart rate <90 beats per minute, respiratory rate <20 breaths per minute, and maximum oral temperature <38C) after gram‐positive therapy was discontinued. The definition of microbiologic cure was lack of positive blood cultures for VRE at least 14 days after cessation of gram‐positive therapy. Microbiologic failure was defined as positive VRE blood cultures obtained on gram‐positive therapy necessitating a change in treatment. Recurrence was defined as VRE bacteremia within 30 days after discontinuation of gram‐positive therapy. Reinfection was defined as VRE bacteremia that appeared 30 days after completion of primary gram‐positive therapy.
All isolates were tested for susceptibility to linezolid using the MicroScan system, whereas daptomycin susceptibility patterns were obtained by either the Etest or MicroScan system. Of importance, our laboratory did not routinely report isolate susceptibility for daptomycin until 2008. Clinical Laboratory Standards Institute breakpoint guidelines were used to delineate minimum inhibitory concentrations for linezolid and daptomycin.
Outcomes
The primary objective was to determine the cure rate, both clinical and microbiologic, of VRE bacteremia with the use of linezolid and daptomycin. Secondary outcomes were rates of recurrence and reinfection as well as 30‐day mortality. Clinical and microbiologic response rates for subsets of the patient population that were deemed immunocompromised or at an increased risk for VRE infections (neutropenic, transplant, malignancy, and ESRD on hemodialysis) were also evaluated.
Statistical Analysis
Data were analyzed using SAS version 9.2 (SAS Inc, Cary, NC). Patients with categorical characteristics were compared using a chi‐square test or Fisher's exact test. Continuous data were analyzed using a Student t test and are expressed as the mean standard deviation. The mean duration of initial antibiotics, time to appropriate antibiotics, time to adequate antibiotic therapy, and LOS were all calculated for the linezolid and daptomycin group with a Student t test used to compare the differences. Multivariate logistic regression was used for the following outcomes: clinical cure, microbiologic cure, mortality, reinfection, and recurrence. For the interval variable, LOS, stepwise multiple regression was used to choose significant independent variables. P < 0.05 was considered statistically significant.
RESULTS
Patient Characteristics
Of the 361 patients identified with a positive VRE blood culture, 201 were included in the study. The remaining 160 patients were excluded for one of the following reasons: <5 days of therapy (n = 87), no documented gram‐positive therapy (n = 49), simultaneous gram‐positive therapy (n = 10), or insufficient data to evaluate response rates (n = 14). For the treatment of VRE bacteremia, 138 patients received linezolid and 63 patients received daptomycin. Demographics, comorbid illnesses, and patient characteristics are shown in Table 1. There was a statistically significant difference in the average age, with the linezolid group consisting of older patients. The daptomycin group had more patients with hematologic malignancies than the linezolid group (33% vs 14%; P = 0.0021) and more patients who received liver transplants (13% vs 4%; P = 0.0264).
| Linezolid (n = 138) | Daptomycin (n = 63) | P Value | |
|---|---|---|---|
| |||
| Average age, years, mean SD | 60 16 | 5315 | 0.0028 |
| Male, No. (%) | 59 (43) | 36 (57) | 0.0682 |
| Race, No. (%) | |||
| Caucasian | 34 (25) | 23 (37) | 0.1043 |
| African American | 103 (75) | 39 (62) | |
| Other | 1 (1) | 1 (2) | |
| COPD, No. (%) | 8 (6) | 2 (3) | 0.7277 |
| Diabetes mellitus, No. (%) | 61 (44) | 21 (33) | 0.1655 |
| Hemodialysis, No. (%) | 35 (25) | 17 (27) | 0.8627 |
| Malignancy, No. (%) | |||
| Solid organ | 26 (19) | 16 (25) | 0.3499 |
| Hematologic | 19 (14) | 21 (33) | 0.0021 |
| Transplant, No. (%) | |||
| Liver | 5 (4) | 8 (13) | 0.0264 |
| Kidney | 3 (2) | 0 | 0.5533 |
| Endocarditis,* No. (%) | 4 (3) | 3 (5) | 0.6801 |
| Species of VRE (%) | |||
| Enterococcus faecalis | 33 (24) | 10 (16) | 0.2658 |
| Enterococcus faecium | 105 (76) | 53 (84) | 0.2658 |
| Time to appropriate abx therapy, hours, mean SD | 12.4 26.9 | 818.8 | 0.1851 |
| Time to adequate abx therapy, days, mean SD | 2.3 1.8 | 1.81.5 | 0.0554 |
| Duration of initial abx, days, mean SD | 11.1 6.0 | 14.114.6 | 0.0401 |
| Abx before initial therapy, No. (%) | 85 (62) | 34 (54) | 0.3541 |
| Average dose, mg/kg, mean SD | NA | 6.11.5 | NA |
| Mortality, No. (%) | 25 (18) | 15 (24) | 0.3481 |
| LOS (days) | 37.527.7 | 40.827.9 | 0.4336 |
From the microbiology laboratory report of initial blood cultures, 78.6% of the isolates were noted as being E. faecium, with the remainder being E. faecalis (21.4%). One patient was classified as having linezolid‐resistant E. faecium (MIC >4 mg/L) upon repeat blood culture. Daptomycin MICs were obtained for 44 isolates using the Etest or MicroScan system; all isolates were susceptible with MICs ranging from 0.254 mg/L. As mentioned previously, our laboratory did not routinely report isolate susceptibility to daptomycin until 2008.
There were no statistically significant differences between the treatment groups with regard to time to appropriate or adequate antibiotic therapy (Table 1). However, there was a statistically significant difference in the mean duration of initial antibiotics between linezolid and daptomycin (11.1 days vs 14.1 days; P = 0.0401). Dosing strategies used in these patients were also evaluated. All linezolid patients received a dose of 600 mg every 12 hours by mouth or intravenously. The average dose of daptomycin was 6.1 mg/kg (range, 3.410.4 mg/kg; median, 6 mg/kg). The average LOS was 37 days for linezolid vs 40 days for daptomycin, which did not confer statistical significance. Overall mortality was 20%, occurring in 25 linezolid patients versus 15 daptomycin patients (P = 0.3481). The stepwise multiple regression analysis did not identify any statistically significant variables in patients treated with linezolid or daptomycin that affected any of the outcomes.
Outcomes and Analysis
As shown in Table 2, there were no statistically significant differences in clinical or microbiologic cure between the linezolid and daptomycin groups (74% vs 75% and 94% vs 94%, respectively). However, the linezolid group compared with the daptomycin group had fewer patients that developed a positive blood culture while on their initial antibiotic therapy (8% vs 22%; P = 0.0097). Follow‐up cultures were required to determine rates of recurrence and reinfection. Only 107/138 patients in the linezolid group and 51/63 patients in the daptomycin group had follow‐up cultures collected. Recurrence was documented in 3% of linezolid patients vs 12% of daptomycin patients (P = 0.0321). The odds ratio for developing a recurrent infection with daptomycin versus linezolid was 5.51 (95% confidence interval, 1.2524.28). Out of 6 patients that developed a recurrent VRE infection in the daptomycin group, 2 were prescribed doses <4 mg/kg with no reported MICs, and 2 patients received 6 mg/kg with reported MICs of 4 mg/L. No statistically significant difference existed for the rate of reinfection between linezolid and daptomycin (1% vs 6%; P = 0.0992).
| Linezolid (n = 138) | Daptomycin (n = 63) | P Value | |
|---|---|---|---|
| |||
| Patients with positive culture on G+ therapy | 11 (8) | 14 (22) | 0.0097 |
| Clinical cure | 102 (74) | 47 (75) | 1 |
| Microbiologic cure | 130 (94) | 59 (94) | 1 |
| Recurrence* | 3 (3) | 6 (12) | 0.0321 |
| Reinfection* | 1 (1) | 3 (6) | 0.0992 |
Table 3 provides information on subsets of the patient population deemed high‐risk for VRE infection or immunocompromised. There was no statistically significant difference between the 2 antibiotic groups in clinical or microbiologic cure. In the subsets of immunocompromised patients, there was no difference in recurrence or reinfection between the linezolid and daptomycin patients. Furthermore, all groups had similar LOS regardless of the antibiotic used to treat the VRE BSI. Moreover, there were no statistically significant differences in 30‐day mortality in these subsets of the population with regard to initial antibiotic choice. No significant independent variables were found between linezolid or daptomycin that affected any of the outcomes listed in Table 3.
| Neutropenia (%) | Hematologic Malignancy (%) | ESRD on Hemodialysis (%) | Liver Transplant (%) | |||||
|---|---|---|---|---|---|---|---|---|
| LZD (n = 16) | Dapto (n = 5) | LZD (n = 19) | Dapto (n = 21) | LZD (n = 35) | Dapto (n = 17) | LZD (n = 5) | Dapto (n = 8) | |
| ||||||||
| Clinical cure, No. (%) | 12 (75) | 5 (100) | 18 (95) | 17 (81) | 24 (69) | 12 (71) | 4 (80) | 3 (38) |
| Microbiologic cure,* No. (%) | 13 (81) | 5 (100) | 18 (95) | 20 (95) | 33 (94) | 16 (94) | 5 (100) | 8 (100) |
| Recurrence,* No. (%) | 2 (13) | 1 (20) | 1 (6) | 2 (11) | 0 | 2 (15) | 0 | 1 (14) |
| Reinfection,* No. (%) | 0 | 0 | 0 | 0 | 1 (4) | 2 (15) | 0 | 1 (14) |
| Mortality, No. (%) | 3 (19) | 1 (20) | 4 (21) | 3 (14) | 7 (20) | 4 (24) | 1 (20) | 4 (50) |
| LOS, days, meanSD | 57.422 | 39.412.4 | 47.626.2 | 4127.1 | 38.833.8 | 39.640.4 | 5034.5 | 73.338.8 |
DISCUSSION
Vergis et al21 reported that infections with VRE compared with vancomycin‐sensitive infections were associated with a higher rate of mortality and that the chosen antimicrobial therapy may play a pivotal role in the risk of death. Our retrospective study suggests that linezolid and daptomycin appear to be equally efficacious for the treatment of VRE BSIs. The results from our study for clinical and microbiologic cure rates for linezolid and daptomycin are similar to previously published data.7, 8 In accordance with previous studies,4 our data demonstrate that there is a higher rate of recurrence in patients treated with daptomycin. This finding may be explained by the fact that the daptomycin group was comprised of more complex patients with a greater disease burden versus the linezolid group; therefore, they were more susceptible to a recurrent VRE infection. In our study, patients who were treated with daptomycin were 5.5 times more likely to have a recurrent infection than linezolid‐treated patients. However, this finding must be scrutinized, because over half of the patients with recurrence either received an inappropriate dose or had high MICs to daptomycin.
Despite there being few clinical and microbiologic outcome data with daptomycin, our study proposes that a bactericidal antibiotic and a bacteriostatic antibiotic have comparable efficacy in the treatment of VRE BSIs. Previous literature has mainly comprised case studies or series that have evaluated clinical outcomes with daptomycin in the treatment of VRE BSIs. Gallagher et al7 reported the results of a retrospective case series of 30 patients with VRE bacteremia who were treated with daptomycin. In this study, microbiologic cure was achieved in 80% of patients, with clinical success in 59% of the patients. In 2009, Mave et al4 compared clinical outcomes between daptomycin and linezolid in the treatment of VRE bacteremia. Reported results demonstrated a microbiologic cure rate of 90% for daptomycin versus 88% for linezolid.4 Moreover, there were no differences in mortality between the groups in our study. In 2010, Crank et al18 reported no differences in mortality (in‐hospital) for hospitalized patients with VRE BSIs treated with linezolid or daptomycin. Our results seem to be consistent with what has been published previously concerning clinical outcomes associated with linezolid or daptomycin in the treatment of VRE BSI.
The average daptomycin dose received in our patients was 6.1 mg/kg with doses ranging from 3.410.4 mg/kg. The underdosing as well as higher MICs to daptomycin may have contributed to a higher rate of recurrence. Previous reports state that Enterococcus species may have higher MICs to daptomycin than Staphylococcus or Streptococcus species; consequently, higher doses may be needed to adequately treat enterococcal infections.7 In the aforementioned study by Gallagher et al,7 doses of daptomycin 6 mg/kg were associated with a positive clinical outcome in 81% of patients compared with 31% if the dose used was <6 mg/kg. Linezolid is dosed 600 mg every 12 hours by mouth or intravenously, with no variations. There have been no studies comparing the uniform dosing of linezolid to the weight‐based dosing of daptomycin and their effects on outcomes.
Patients particularly susceptible to VRE infections include those with neutropenia and/or cancer, patients receiving long‐term hemodialysis, and liver transplant recipients.3, 22, 23 Upon review of this immunocompromised population, we noted no statistically significant differences in overall outcomes. A study by Kraft et al.24 supports the findings in our study that both drugs appear useful in the treatment of VRE bacteremia in patients with hematologic malignancy. We did identify a difference, albeit nonsignificant, in LOS for daptomycin versus linezolid in patients with a history of liver transplantation. Again, the level of care that these patients needed compared with the general population may explain this difference. As mentioned previously, another pertinent factor would be the dose of daptomycin used in these patients, because the dose can affect clinical success. Because all of the other patients had a similar LOS, we cannot determine that the increased LOS seen in liver transplant patients treated with daptomycin was solely due to daptomycin use. The reason for the increased LOS seems to be multifactorial. In the neutropenic population, a difference in LOS was also recognized, but follow‐up complete blood count values were not collected for these patients to determine whether linezolid contributed to further bone marrow suppression leading to an increase in LOS. For both of these patient populations, the number of patients included is very small (n = 21 for neutropenia total, n = 13 for liver transplant total), which can lead to a high degree of variance.
This study has several limitations. This was a retrospective review; therefore, we had no control over the selection of therapy. This may be reflected in an apparent preferential use of daptomycin in immunocompromised patients. Furthermore, 62% of linezolid patients and 54% of daptomycin patients received an antibiotic before initial therapy that could have potentially altered response rates. Due to the paucity of documentation surrounding initial site of infection, some of the positive cultures may represent potential contamination, because VRE may contaminate skin.25 Contamination seems implausible, however, because patients were seen by an infectious disease physician and had at least 1 documented positive VRE blood culture. We chose arbitrary definitions for clinical cure, microbiologic cure, microbiologic failure, recurrence, and reinfection. Previous studies have used their own definitions leading to discrepancies in reporting. Another limitation was that follow‐up cultures were not obtained on all of the patients, which was needed to determine rates of recurrence, reinfection, and microbiologic cure. MICs to daptomycin were not reported in 30% of our patients, potentially altering the recurrence rate seen in the daptomycin‐treated patients. Because clinical cure was not documented in the chart, it was inferred from the laboratory values and vital sign information. One investigator analyzed all of the values and made the determination of clinical cure, allowing for a consistent approach to data review.
In the face of the imposing threat of a highly resistant organism such as VRE with a limited number of efficacious antibiotics, antimicrobial selection becomes increasingly important and is requisite to clinical and microbiological success. To our knowledge, this is one of the largest studies to date comparing the efficacy of linezolid with that of daptomycin in the treatment of VRE bacteremia. Both of these agents are effective for the treatment of VRE BSIs. Nevertheless, specific factors related to the medication (eg, dose, route of administration) as well as the patient (eg, comorbid conditions, acuity of illness) should be taken into consideration when selecting an initial antimicrobial agent. Because the treatment of VRE BSIs continues to be a challenge, larger prospective randomized controlled trials are needed to corroborate our results and determine the optimal therapy for this serious infection.
Acknowledgements
Disclosures: Michael S. Gelfand is on the speaker's bureau for Cubist and Pfizer.
Enterococci have been identified as a causative organism in approximately 10% of all nosocomial bloodstream infections (BSIs).1, 2 In 2006, the Infectious Diseases Society of America identified vancomycin‐ resistant Enterococcus faecium (VRE) as 1 of 6 microbes considered to be among the most dangerous due to high rates of resistance and a limited number of effective antimicrobials.3 E. faecium has exhibited high rates of glycopeptide resistance with as many as 60% of isolates from BSIs being resistant to vancomycin.2, 4 Due to increasing resistance to glycopeptides, vancomycin has become obsolete in the treatment of E. faecium infections.5
A limited number of antimicrobials are available for the treatment of infections due to VRE. Agents active in vitro are quinupristin‐dalfopristin, tigecycline, linezolid, and daptomycin. Quinupristin‐dalfopristin was one of the first agents approved for use in VRE infections; however, treatment with this agent has been limited because of mediocre clinical response rates, undesirable adverse effects, high cost, and insufficient E. faecalis activity.6, 7 Tigecycline is not an optimal antibiotic for the treatment of VRE bacteremia, because serum concentrations achieved after administration are inadequate to treat BSIs.7 In contrast, linezolid and daptomycin have evinced efficacy against VRE bacteremia, with reported microbiologic response rates of 85% and 80%, respectively.7, 8 One inherent difference between these antibiotics that may theoretically affect their use in immunocompromised patients is that linezolid is bacteriostatic, whereas daptomycin is bactericidal. It has been postulated that by using a bactericidal antibiotic such as daptomycin in the immunocompromised host, one may achieve superior clinical and microbiologic response rates.3, 7, 9, 10
Since the introduction of the oxazolidinone linezolid in 2000, widespread use has led to reports of linezolid‐resistant VRE as well as nosocomial transmission of linezolid‐resistant VRE in hospitals.4, 1114 Despite linezolid being a key antibiotic for the treatment of VRE infections over the last 10 years, development of resistance along with potential hematologic and neurologic toxicity during long‐term use remains a concern.7, 8 Although daptomycin is active against several resistant organisms, including VRE, the evidence supporting use of daptomycin for VRE BSI is limited to case reports or small case series.7, 9 Moreover, daptomycin has not received US Food and Drug Administration approval for the treatment of VRE infections,15 and emerging data regarding daptomycin‐nonsusceptible enterococci (Minimum Inhibitory Concentration, MIC >4 mg/L) highlight a new problem for this multidrug‐resistant pathogen.16, 17 Few studies in recent years have compared these 2 antibiotics in the treatment of VRE BSIs.4, 18, 19 Due to the high rates of vancomycin resistance reported at our institution and the ubiquitous use of linezolid and daptomycin in the treatment of VRE bacteremia, we chose to evaluate response rates for these antibiotics in an effort to add to previously published literature on this topic.
MATERIALS AND METHODS
Patient Selection
Methodist University Hospital (MUH) in Memphis, Tennessee, is part of a 7‐hospital system with 697 licensed beds. MUH is a tertiary teaching hospital with centers of excellence in neuroscience and transplantation. Patients admitted to MUH diagnosed with VRE bacteremia between January 1, 2004, and July 31, 2009, were identified by the microbiology laboratory. All patients who were 18 years of age, had 1 documented positive VRE blood culture, and received linezolid or daptomycin for 5 days were eligible. Patients were excluded if they were treated simultaneously with more than 1 agent active against VRE. This study was approved by the MUH Institutional Review Board. Of note, use of linezolid or daptomycin at MUH is restricted to an infectious disease physician or pulmonologist. Currently, there are no protocols at our institution for treating VRE infections.
Data Collection and Definitions
Cerner Millennium was used to collect all pertinent patient information. Patient records were reviewed to determine demographic data, comorbid illnesses, laboratory data (from admission to discharge), medications, and discharge status (home, long‐term care facility, or death). Comorbid illnesses evaluated included: chronic obstructive pulmonary disease, diabetes mellitus, malignancy (solid or hematologic), transplant (liver or kidney), end‐stage renal disease (ESRD) (hemodialysis or nonhemodialysis), cirrhosis, and endocarditis. ESRD and endocarditis were defined per chart diagnosis. Laboratory data collected included serum creatinine, creatine phosphokinase, absolute neutrophil count (neutropenia defined as absolute neutrophil count <1000), and number and site (intravenous line or peripheral blood draw) of positive VRE blood cultures. Other data collected were (1) time elapsed to adequate antibiotic coverage, which was defined as microbiologic documentation of an infection that was being effectively treated at the time of its identification, and (2) time to appropriate antibiotic coverage, which was defined as antimicrobial treatment selected for efficacy based on presumptive identification of the causative pathogen, the antimicrobial agent's spectrum of activity, and local microbial resistance patterns.20 Doses of daptomycin and linezolid used in patients with VRE bacteremia were also documented.
Clinical cure was defined as a resolution of signs and/or symptoms of infection (white blood cell count <10,000/mm3, bands <5%, heart rate <90 beats per minute, respiratory rate <20 breaths per minute, and maximum oral temperature <38C) after gram‐positive therapy was discontinued. The definition of microbiologic cure was lack of positive blood cultures for VRE at least 14 days after cessation of gram‐positive therapy. Microbiologic failure was defined as positive VRE blood cultures obtained on gram‐positive therapy necessitating a change in treatment. Recurrence was defined as VRE bacteremia within 30 days after discontinuation of gram‐positive therapy. Reinfection was defined as VRE bacteremia that appeared 30 days after completion of primary gram‐positive therapy.
All isolates were tested for susceptibility to linezolid using the MicroScan system, whereas daptomycin susceptibility patterns were obtained by either the Etest or MicroScan system. Of importance, our laboratory did not routinely report isolate susceptibility for daptomycin until 2008. Clinical Laboratory Standards Institute breakpoint guidelines were used to delineate minimum inhibitory concentrations for linezolid and daptomycin.
Outcomes
The primary objective was to determine the cure rate, both clinical and microbiologic, of VRE bacteremia with the use of linezolid and daptomycin. Secondary outcomes were rates of recurrence and reinfection as well as 30‐day mortality. Clinical and microbiologic response rates for subsets of the patient population that were deemed immunocompromised or at an increased risk for VRE infections (neutropenic, transplant, malignancy, and ESRD on hemodialysis) were also evaluated.
Statistical Analysis
Data were analyzed using SAS version 9.2 (SAS Inc, Cary, NC). Patients with categorical characteristics were compared using a chi‐square test or Fisher's exact test. Continuous data were analyzed using a Student t test and are expressed as the mean standard deviation. The mean duration of initial antibiotics, time to appropriate antibiotics, time to adequate antibiotic therapy, and LOS were all calculated for the linezolid and daptomycin group with a Student t test used to compare the differences. Multivariate logistic regression was used for the following outcomes: clinical cure, microbiologic cure, mortality, reinfection, and recurrence. For the interval variable, LOS, stepwise multiple regression was used to choose significant independent variables. P < 0.05 was considered statistically significant.
RESULTS
Patient Characteristics
Of the 361 patients identified with a positive VRE blood culture, 201 were included in the study. The remaining 160 patients were excluded for one of the following reasons: <5 days of therapy (n = 87), no documented gram‐positive therapy (n = 49), simultaneous gram‐positive therapy (n = 10), or insufficient data to evaluate response rates (n = 14). For the treatment of VRE bacteremia, 138 patients received linezolid and 63 patients received daptomycin. Demographics, comorbid illnesses, and patient characteristics are shown in Table 1. There was a statistically significant difference in the average age, with the linezolid group consisting of older patients. The daptomycin group had more patients with hematologic malignancies than the linezolid group (33% vs 14%; P = 0.0021) and more patients who received liver transplants (13% vs 4%; P = 0.0264).
| Linezolid (n = 138) | Daptomycin (n = 63) | P Value | |
|---|---|---|---|
| |||
| Average age, years, mean SD | 60 16 | 5315 | 0.0028 |
| Male, No. (%) | 59 (43) | 36 (57) | 0.0682 |
| Race, No. (%) | |||
| Caucasian | 34 (25) | 23 (37) | 0.1043 |
| African American | 103 (75) | 39 (62) | |
| Other | 1 (1) | 1 (2) | |
| COPD, No. (%) | 8 (6) | 2 (3) | 0.7277 |
| Diabetes mellitus, No. (%) | 61 (44) | 21 (33) | 0.1655 |
| Hemodialysis, No. (%) | 35 (25) | 17 (27) | 0.8627 |
| Malignancy, No. (%) | |||
| Solid organ | 26 (19) | 16 (25) | 0.3499 |
| Hematologic | 19 (14) | 21 (33) | 0.0021 |
| Transplant, No. (%) | |||
| Liver | 5 (4) | 8 (13) | 0.0264 |
| Kidney | 3 (2) | 0 | 0.5533 |
| Endocarditis,* No. (%) | 4 (3) | 3 (5) | 0.6801 |
| Species of VRE (%) | |||
| Enterococcus faecalis | 33 (24) | 10 (16) | 0.2658 |
| Enterococcus faecium | 105 (76) | 53 (84) | 0.2658 |
| Time to appropriate abx therapy, hours, mean SD | 12.4 26.9 | 818.8 | 0.1851 |
| Time to adequate abx therapy, days, mean SD | 2.3 1.8 | 1.81.5 | 0.0554 |
| Duration of initial abx, days, mean SD | 11.1 6.0 | 14.114.6 | 0.0401 |
| Abx before initial therapy, No. (%) | 85 (62) | 34 (54) | 0.3541 |
| Average dose, mg/kg, mean SD | NA | 6.11.5 | NA |
| Mortality, No. (%) | 25 (18) | 15 (24) | 0.3481 |
| LOS (days) | 37.527.7 | 40.827.9 | 0.4336 |
From the microbiology laboratory report of initial blood cultures, 78.6% of the isolates were noted as being E. faecium, with the remainder being E. faecalis (21.4%). One patient was classified as having linezolid‐resistant E. faecium (MIC >4 mg/L) upon repeat blood culture. Daptomycin MICs were obtained for 44 isolates using the Etest or MicroScan system; all isolates were susceptible with MICs ranging from 0.254 mg/L. As mentioned previously, our laboratory did not routinely report isolate susceptibility to daptomycin until 2008.
There were no statistically significant differences between the treatment groups with regard to time to appropriate or adequate antibiotic therapy (Table 1). However, there was a statistically significant difference in the mean duration of initial antibiotics between linezolid and daptomycin (11.1 days vs 14.1 days; P = 0.0401). Dosing strategies used in these patients were also evaluated. All linezolid patients received a dose of 600 mg every 12 hours by mouth or intravenously. The average dose of daptomycin was 6.1 mg/kg (range, 3.410.4 mg/kg; median, 6 mg/kg). The average LOS was 37 days for linezolid vs 40 days for daptomycin, which did not confer statistical significance. Overall mortality was 20%, occurring in 25 linezolid patients versus 15 daptomycin patients (P = 0.3481). The stepwise multiple regression analysis did not identify any statistically significant variables in patients treated with linezolid or daptomycin that affected any of the outcomes.
Outcomes and Analysis
As shown in Table 2, there were no statistically significant differences in clinical or microbiologic cure between the linezolid and daptomycin groups (74% vs 75% and 94% vs 94%, respectively). However, the linezolid group compared with the daptomycin group had fewer patients that developed a positive blood culture while on their initial antibiotic therapy (8% vs 22%; P = 0.0097). Follow‐up cultures were required to determine rates of recurrence and reinfection. Only 107/138 patients in the linezolid group and 51/63 patients in the daptomycin group had follow‐up cultures collected. Recurrence was documented in 3% of linezolid patients vs 12% of daptomycin patients (P = 0.0321). The odds ratio for developing a recurrent infection with daptomycin versus linezolid was 5.51 (95% confidence interval, 1.2524.28). Out of 6 patients that developed a recurrent VRE infection in the daptomycin group, 2 were prescribed doses <4 mg/kg with no reported MICs, and 2 patients received 6 mg/kg with reported MICs of 4 mg/L. No statistically significant difference existed for the rate of reinfection between linezolid and daptomycin (1% vs 6%; P = 0.0992).
| Linezolid (n = 138) | Daptomycin (n = 63) | P Value | |
|---|---|---|---|
| |||
| Patients with positive culture on G+ therapy | 11 (8) | 14 (22) | 0.0097 |
| Clinical cure | 102 (74) | 47 (75) | 1 |
| Microbiologic cure | 130 (94) | 59 (94) | 1 |
| Recurrence* | 3 (3) | 6 (12) | 0.0321 |
| Reinfection* | 1 (1) | 3 (6) | 0.0992 |
Table 3 provides information on subsets of the patient population deemed high‐risk for VRE infection or immunocompromised. There was no statistically significant difference between the 2 antibiotic groups in clinical or microbiologic cure. In the subsets of immunocompromised patients, there was no difference in recurrence or reinfection between the linezolid and daptomycin patients. Furthermore, all groups had similar LOS regardless of the antibiotic used to treat the VRE BSI. Moreover, there were no statistically significant differences in 30‐day mortality in these subsets of the population with regard to initial antibiotic choice. No significant independent variables were found between linezolid or daptomycin that affected any of the outcomes listed in Table 3.
| Neutropenia (%) | Hematologic Malignancy (%) | ESRD on Hemodialysis (%) | Liver Transplant (%) | |||||
|---|---|---|---|---|---|---|---|---|
| LZD (n = 16) | Dapto (n = 5) | LZD (n = 19) | Dapto (n = 21) | LZD (n = 35) | Dapto (n = 17) | LZD (n = 5) | Dapto (n = 8) | |
| ||||||||
| Clinical cure, No. (%) | 12 (75) | 5 (100) | 18 (95) | 17 (81) | 24 (69) | 12 (71) | 4 (80) | 3 (38) |
| Microbiologic cure,* No. (%) | 13 (81) | 5 (100) | 18 (95) | 20 (95) | 33 (94) | 16 (94) | 5 (100) | 8 (100) |
| Recurrence,* No. (%) | 2 (13) | 1 (20) | 1 (6) | 2 (11) | 0 | 2 (15) | 0 | 1 (14) |
| Reinfection,* No. (%) | 0 | 0 | 0 | 0 | 1 (4) | 2 (15) | 0 | 1 (14) |
| Mortality, No. (%) | 3 (19) | 1 (20) | 4 (21) | 3 (14) | 7 (20) | 4 (24) | 1 (20) | 4 (50) |
| LOS, days, meanSD | 57.422 | 39.412.4 | 47.626.2 | 4127.1 | 38.833.8 | 39.640.4 | 5034.5 | 73.338.8 |
DISCUSSION
Vergis et al21 reported that infections with VRE compared with vancomycin‐sensitive infections were associated with a higher rate of mortality and that the chosen antimicrobial therapy may play a pivotal role in the risk of death. Our retrospective study suggests that linezolid and daptomycin appear to be equally efficacious for the treatment of VRE BSIs. The results from our study for clinical and microbiologic cure rates for linezolid and daptomycin are similar to previously published data.7, 8 In accordance with previous studies,4 our data demonstrate that there is a higher rate of recurrence in patients treated with daptomycin. This finding may be explained by the fact that the daptomycin group was comprised of more complex patients with a greater disease burden versus the linezolid group; therefore, they were more susceptible to a recurrent VRE infection. In our study, patients who were treated with daptomycin were 5.5 times more likely to have a recurrent infection than linezolid‐treated patients. However, this finding must be scrutinized, because over half of the patients with recurrence either received an inappropriate dose or had high MICs to daptomycin.
Despite there being few clinical and microbiologic outcome data with daptomycin, our study proposes that a bactericidal antibiotic and a bacteriostatic antibiotic have comparable efficacy in the treatment of VRE BSIs. Previous literature has mainly comprised case studies or series that have evaluated clinical outcomes with daptomycin in the treatment of VRE BSIs. Gallagher et al7 reported the results of a retrospective case series of 30 patients with VRE bacteremia who were treated with daptomycin. In this study, microbiologic cure was achieved in 80% of patients, with clinical success in 59% of the patients. In 2009, Mave et al4 compared clinical outcomes between daptomycin and linezolid in the treatment of VRE bacteremia. Reported results demonstrated a microbiologic cure rate of 90% for daptomycin versus 88% for linezolid.4 Moreover, there were no differences in mortality between the groups in our study. In 2010, Crank et al18 reported no differences in mortality (in‐hospital) for hospitalized patients with VRE BSIs treated with linezolid or daptomycin. Our results seem to be consistent with what has been published previously concerning clinical outcomes associated with linezolid or daptomycin in the treatment of VRE BSI.
The average daptomycin dose received in our patients was 6.1 mg/kg with doses ranging from 3.410.4 mg/kg. The underdosing as well as higher MICs to daptomycin may have contributed to a higher rate of recurrence. Previous reports state that Enterococcus species may have higher MICs to daptomycin than Staphylococcus or Streptococcus species; consequently, higher doses may be needed to adequately treat enterococcal infections.7 In the aforementioned study by Gallagher et al,7 doses of daptomycin 6 mg/kg were associated with a positive clinical outcome in 81% of patients compared with 31% if the dose used was <6 mg/kg. Linezolid is dosed 600 mg every 12 hours by mouth or intravenously, with no variations. There have been no studies comparing the uniform dosing of linezolid to the weight‐based dosing of daptomycin and their effects on outcomes.
Patients particularly susceptible to VRE infections include those with neutropenia and/or cancer, patients receiving long‐term hemodialysis, and liver transplant recipients.3, 22, 23 Upon review of this immunocompromised population, we noted no statistically significant differences in overall outcomes. A study by Kraft et al.24 supports the findings in our study that both drugs appear useful in the treatment of VRE bacteremia in patients with hematologic malignancy. We did identify a difference, albeit nonsignificant, in LOS for daptomycin versus linezolid in patients with a history of liver transplantation. Again, the level of care that these patients needed compared with the general population may explain this difference. As mentioned previously, another pertinent factor would be the dose of daptomycin used in these patients, because the dose can affect clinical success. Because all of the other patients had a similar LOS, we cannot determine that the increased LOS seen in liver transplant patients treated with daptomycin was solely due to daptomycin use. The reason for the increased LOS seems to be multifactorial. In the neutropenic population, a difference in LOS was also recognized, but follow‐up complete blood count values were not collected for these patients to determine whether linezolid contributed to further bone marrow suppression leading to an increase in LOS. For both of these patient populations, the number of patients included is very small (n = 21 for neutropenia total, n = 13 for liver transplant total), which can lead to a high degree of variance.
This study has several limitations. This was a retrospective review; therefore, we had no control over the selection of therapy. This may be reflected in an apparent preferential use of daptomycin in immunocompromised patients. Furthermore, 62% of linezolid patients and 54% of daptomycin patients received an antibiotic before initial therapy that could have potentially altered response rates. Due to the paucity of documentation surrounding initial site of infection, some of the positive cultures may represent potential contamination, because VRE may contaminate skin.25 Contamination seems implausible, however, because patients were seen by an infectious disease physician and had at least 1 documented positive VRE blood culture. We chose arbitrary definitions for clinical cure, microbiologic cure, microbiologic failure, recurrence, and reinfection. Previous studies have used their own definitions leading to discrepancies in reporting. Another limitation was that follow‐up cultures were not obtained on all of the patients, which was needed to determine rates of recurrence, reinfection, and microbiologic cure. MICs to daptomycin were not reported in 30% of our patients, potentially altering the recurrence rate seen in the daptomycin‐treated patients. Because clinical cure was not documented in the chart, it was inferred from the laboratory values and vital sign information. One investigator analyzed all of the values and made the determination of clinical cure, allowing for a consistent approach to data review.
In the face of the imposing threat of a highly resistant organism such as VRE with a limited number of efficacious antibiotics, antimicrobial selection becomes increasingly important and is requisite to clinical and microbiological success. To our knowledge, this is one of the largest studies to date comparing the efficacy of linezolid with that of daptomycin in the treatment of VRE bacteremia. Both of these agents are effective for the treatment of VRE BSIs. Nevertheless, specific factors related to the medication (eg, dose, route of administration) as well as the patient (eg, comorbid conditions, acuity of illness) should be taken into consideration when selecting an initial antimicrobial agent. Because the treatment of VRE BSIs continues to be a challenge, larger prospective randomized controlled trials are needed to corroborate our results and determine the optimal therapy for this serious infection.
Acknowledgements
Disclosures: Michael S. Gelfand is on the speaker's bureau for Cubist and Pfizer.
- ,,,,,.Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study.Clin Infect Dis.2004;39:309–317.
- ,,, et al.Clinical practice guidelines for the management of intravascular catheter‐related infection: 2009 update by the Infectious Diseases Society of America.Clin Infect Dis.2009;49:1–45.
- ,,,,,.Bad bugs need drugs: an update on the development pipeline from the antimicrobial availability task force of the infectious diseases society of America.Clin Infect Dis.2006;42:657–668.
- ,,,.Vancomycin‐resistant enterococcal bacteraemia: is daptomycin as effective as linezolid?J Antimicrob Chemother.2009;64:175–180.
- ,,.Management of multidrug‐resistant enterococcal Infections.Clin Microbiol Infect.2010;16:555–562.
- ,,, et al.Treatment of vancomycin‐resistant Enterococcus faecium infections with quinupristin/dalfopristin.Clin Infect Dis.2001;33:1816–1823.
- ,,, et al.Daptomycin for vancomycin‐resistant enterococcol bacteremia: a retrospective case series of 30 patients.Pharmacotherapy.2009;29:792–799.
- ,,, et al.Safety, efficacy and pharmacokinetics of linezolid for treatment of resistant gram positive infections in cancer patients with neutropenia.Ann Oncol.2003;14:795–801.
- ,,,,.Daptomycin for the treatment of vancomycin resistant Enterococcus faecium bacteremia.Scand J Infect Dis.2003;38:290–292.
- ,.New antimicrobial agents for the treatment of gram positive bacterial infections.Clin Microbiol Infec.2008;14:411–420.
- ,.Emergence and management of drug‐resistant enterococcal infections.Expert Rev Anti Infect Ther.2008;6:637–655.
- ,,.Nosocomial spread of linezolid‐resistant, Vancomycin resistant Enterococcus faecium.N Engl J Med.2002;346:867–869.
- ,, et al.Nosocomial superinfections due to linezolid‐resitant Enterococcus faecalis: evidence for a gene dosage effect of linezolid MICs.Diagn Microbiol Infect Dis.2003;47:511–513.
- .Quinupristin‐dalfopristin and linezolid: evidence and opinion.Clin Infect Dis.2003;36:473–481.
- Cubicin (Daptomycin for injection) [package insert].Lexington, MA:Cubist Pharmaceuticals;2010.
- ,,,.Daptomycin nonsusceptible Enterococci: an emerging challenge for clinicians.Clin Infect Dis.2011;52:228–234.
- ,,,.A potential role for daptomycin in enterococcal infections: what is the evidence?J Antimicrob Chemother.2010;65:1126–1136.
- ,,, et al.Comparison of outcomes from daptomycin or linezolid for vancomycin‐resistant enterococcal bloodstream infection: a retrospective, multicenter, cohort study.Clin Ther.2010;32:1713–1719.
- ,,,,,.Observational study of the epidemiology and outcomes of vancomycin‐resistant Enterococcus bacteraemia treated with newer antimicrobial agents.Epidemiol Infect.2010;15:1–9.
- .Inadequate antimicrobial treatment: an important determinant of outcome for hospitalized patients.Clin Infect Dis.2000;31:S131–S138.
- ,,, et al.Determinants of vancomycin resistance and mortality rates in enterococcal bacteremia: a prospective multicenter study.Ann Intern Med.2001;135:484–492.
- ,,,,,.Vancomycin resistant enterococci among chronic hemodialysis patients: a prospective study of acquisition.Clin Infect Dis.2001;32:23–29.
- ,,, et al.A nationwide, multicenter, case control study comparing risk factors, treatment, and outcome for vancomycin resistant andsusceptible enterococcal bacteremia.Diagn Microbiol Infect Dis.2000;36:145–158.
- ,,,,.Outcomes of therapy: vancomycin‐resistant enterococcal bacteremia in hematology and bone marrow transplant patients [published online ahead of print November 26, 2010].Support Care Cancer. doi: 10.1007/s00520–010‐1038‐z.
- ,,, et al.Skin colonization with vancomycin‐resistant enterococci among hospitalized patients with bacteremia.Clin Infect Dis.1997;24:704–706.
- ,,,,,.Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study.Clin Infect Dis.2004;39:309–317.
- ,,, et al.Clinical practice guidelines for the management of intravascular catheter‐related infection: 2009 update by the Infectious Diseases Society of America.Clin Infect Dis.2009;49:1–45.
- ,,,,,.Bad bugs need drugs: an update on the development pipeline from the antimicrobial availability task force of the infectious diseases society of America.Clin Infect Dis.2006;42:657–668.
- ,,,.Vancomycin‐resistant enterococcal bacteraemia: is daptomycin as effective as linezolid?J Antimicrob Chemother.2009;64:175–180.
- ,,.Management of multidrug‐resistant enterococcal Infections.Clin Microbiol Infect.2010;16:555–562.
- ,,, et al.Treatment of vancomycin‐resistant Enterococcus faecium infections with quinupristin/dalfopristin.Clin Infect Dis.2001;33:1816–1823.
- ,,, et al.Daptomycin for vancomycin‐resistant enterococcol bacteremia: a retrospective case series of 30 patients.Pharmacotherapy.2009;29:792–799.
- ,,, et al.Safety, efficacy and pharmacokinetics of linezolid for treatment of resistant gram positive infections in cancer patients with neutropenia.Ann Oncol.2003;14:795–801.
- ,,,,.Daptomycin for the treatment of vancomycin resistant Enterococcus faecium bacteremia.Scand J Infect Dis.2003;38:290–292.
- ,.New antimicrobial agents for the treatment of gram positive bacterial infections.Clin Microbiol Infec.2008;14:411–420.
- ,.Emergence and management of drug‐resistant enterococcal infections.Expert Rev Anti Infect Ther.2008;6:637–655.
- ,,.Nosocomial spread of linezolid‐resistant, Vancomycin resistant Enterococcus faecium.N Engl J Med.2002;346:867–869.
- ,, et al.Nosocomial superinfections due to linezolid‐resitant Enterococcus faecalis: evidence for a gene dosage effect of linezolid MICs.Diagn Microbiol Infect Dis.2003;47:511–513.
- .Quinupristin‐dalfopristin and linezolid: evidence and opinion.Clin Infect Dis.2003;36:473–481.
- Cubicin (Daptomycin for injection) [package insert].Lexington, MA:Cubist Pharmaceuticals;2010.
- ,,,.Daptomycin nonsusceptible Enterococci: an emerging challenge for clinicians.Clin Infect Dis.2011;52:228–234.
- ,,,.A potential role for daptomycin in enterococcal infections: what is the evidence?J Antimicrob Chemother.2010;65:1126–1136.
- ,,, et al.Comparison of outcomes from daptomycin or linezolid for vancomycin‐resistant enterococcal bloodstream infection: a retrospective, multicenter, cohort study.Clin Ther.2010;32:1713–1719.
- ,,,,,.Observational study of the epidemiology and outcomes of vancomycin‐resistant Enterococcus bacteraemia treated with newer antimicrobial agents.Epidemiol Infect.2010;15:1–9.
- .Inadequate antimicrobial treatment: an important determinant of outcome for hospitalized patients.Clin Infect Dis.2000;31:S131–S138.
- ,,, et al.Determinants of vancomycin resistance and mortality rates in enterococcal bacteremia: a prospective multicenter study.Ann Intern Med.2001;135:484–492.
- ,,,,,.Vancomycin resistant enterococci among chronic hemodialysis patients: a prospective study of acquisition.Clin Infect Dis.2001;32:23–29.
- ,,, et al.A nationwide, multicenter, case control study comparing risk factors, treatment, and outcome for vancomycin resistant andsusceptible enterococcal bacteremia.Diagn Microbiol Infect Dis.2000;36:145–158.
- ,,,,.Outcomes of therapy: vancomycin‐resistant enterococcal bacteremia in hematology and bone marrow transplant patients [published online ahead of print November 26, 2010].Support Care Cancer. doi: 10.1007/s00520–010‐1038‐z.
- ,,, et al.Skin colonization with vancomycin‐resistant enterococci among hospitalized patients with bacteremia.Clin Infect Dis.1997;24:704–706.
Copyright © 2011 Society of Hospital Medicine