User login
Best inhaler for COPD is the one the patient will use
ORLANDO –
That’s according to Gabriel Ortiz, MPAS, PA-C, DFAAPA, a speaker at the Cardiovascular & Respiratory Summit by Global Academy for Medical Education.
There are several treatment options available in inhaler form for patients with COPD, including short-acting and long-acting beta2-agonists (SABA/LABA), short-acting and long-acting antimuscarinics (SAMA/LAMA), combination therapy, and triple therapy. But education and training on how to use an inhaler is also important, Mr. Ortiz said in his presentation.
Providers should help a patient choose an inhaler they are most likely to use. “It’s difficult to actuate and inhale, so there’s a lot of education that goes into that,” said Mr. Ortiz, of Pediatric Pulmonary Services in El Paso, Texas. “What we do for our patients is, we educate them, we tell them to demonstrate it, then bring [the] inhaler back on the next visit and demonstrate to us every time.”
“Make sure that they demonstrate because, as patients get older, they may lose their ability to actuate and inhale,” he added.
Adherence to therapy should also be considered before changing from a current therapy, he added. Mr. Ortiz described a scenario in which a prescription was filled, but because of the cost, the patient reduced the dose by half to make the therapy last longer.
“We could be the best providers in the world, prescribing the best medication in the world. If the medication doesn’t get to where we need it, it’s not going to help anybody,” he said.
Providers should also use nonpharmacological treatments to prevent or keep COPD from progressing. Smoking cessation is key to reducing the risk of developing COPD, and it is not clear whether e-cigarettes aid in smoking cessation, despite companies that market these products making that claim. “We have a huge e-cigarette epidemic here,” he said.
Instead, Mr. Ortiz recommended identifying which patients are current tobacco users, encouraging them to quit, determining whether the patient is willing to make a commitment to cut down on tobacco use or stop entirely, helping draft a quit plan and obtaining intra- and extratreatment social support for smoking cessation, and scheduling follow-up. Providers should discuss smoking cessation at each visit, and it may take multiple visits before a patient is willing to consider quitting, he said.
Another measure providers can take is making sure patients with COPD have received influenza and pneumococcal vaccinations because this can lower the risk of respiratory tract infections. Physical activity, training in exercise, nutritional support, and pulmonary rehabilitation can improve their health status, dyspnea, and exercise tolerance. While C-level evidence shows education alone is not effective according to Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines, providers working with patients through self-management interventions have been shown to improve health status and decrease COPD-related hospitalizations.
Oxygen therapy has been effective for patients with severe resting chronic hypoxia and improves survival, said Mr. Ortiz. For patients who gradually decline in health despite treatment, palliative, hospice, and end-of-life care may be an option. “Remember that as acute exacerbations continue, that increases the risk of death,” he said. “We want to try and prevent [those] exacerbations and improve quality of life.”
During follow-up visits, providers should continue performing spirometry tests annually to measure decline in forced expiratory volume in 1 second, information on symptoms that have presented since the previous visit, details of any exacerbations that occurred, and current smoking status. When making decisions to adjust therapy, providers should examine the effectiveness of the current regimen and consider the dose of prescribed medications, whether the patient is adhering to the regimen, inhaler technique, and any side effects.
Mr. Ortiz reports also being a paid employee for Thermo Fisher, a biotechnology product development company based in Waltham, Mass. Global Academy for Medical Education and this news organization are owned by the same parent company.
ORLANDO –
That’s according to Gabriel Ortiz, MPAS, PA-C, DFAAPA, a speaker at the Cardiovascular & Respiratory Summit by Global Academy for Medical Education.
There are several treatment options available in inhaler form for patients with COPD, including short-acting and long-acting beta2-agonists (SABA/LABA), short-acting and long-acting antimuscarinics (SAMA/LAMA), combination therapy, and triple therapy. But education and training on how to use an inhaler is also important, Mr. Ortiz said in his presentation.
Providers should help a patient choose an inhaler they are most likely to use. “It’s difficult to actuate and inhale, so there’s a lot of education that goes into that,” said Mr. Ortiz, of Pediatric Pulmonary Services in El Paso, Texas. “What we do for our patients is, we educate them, we tell them to demonstrate it, then bring [the] inhaler back on the next visit and demonstrate to us every time.”
“Make sure that they demonstrate because, as patients get older, they may lose their ability to actuate and inhale,” he added.
Adherence to therapy should also be considered before changing from a current therapy, he added. Mr. Ortiz described a scenario in which a prescription was filled, but because of the cost, the patient reduced the dose by half to make the therapy last longer.
“We could be the best providers in the world, prescribing the best medication in the world. If the medication doesn’t get to where we need it, it’s not going to help anybody,” he said.
Providers should also use nonpharmacological treatments to prevent or keep COPD from progressing. Smoking cessation is key to reducing the risk of developing COPD, and it is not clear whether e-cigarettes aid in smoking cessation, despite companies that market these products making that claim. “We have a huge e-cigarette epidemic here,” he said.
Instead, Mr. Ortiz recommended identifying which patients are current tobacco users, encouraging them to quit, determining whether the patient is willing to make a commitment to cut down on tobacco use or stop entirely, helping draft a quit plan and obtaining intra- and extratreatment social support for smoking cessation, and scheduling follow-up. Providers should discuss smoking cessation at each visit, and it may take multiple visits before a patient is willing to consider quitting, he said.
Another measure providers can take is making sure patients with COPD have received influenza and pneumococcal vaccinations because this can lower the risk of respiratory tract infections. Physical activity, training in exercise, nutritional support, and pulmonary rehabilitation can improve their health status, dyspnea, and exercise tolerance. While C-level evidence shows education alone is not effective according to Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines, providers working with patients through self-management interventions have been shown to improve health status and decrease COPD-related hospitalizations.
Oxygen therapy has been effective for patients with severe resting chronic hypoxia and improves survival, said Mr. Ortiz. For patients who gradually decline in health despite treatment, palliative, hospice, and end-of-life care may be an option. “Remember that as acute exacerbations continue, that increases the risk of death,” he said. “We want to try and prevent [those] exacerbations and improve quality of life.”
During follow-up visits, providers should continue performing spirometry tests annually to measure decline in forced expiratory volume in 1 second, information on symptoms that have presented since the previous visit, details of any exacerbations that occurred, and current smoking status. When making decisions to adjust therapy, providers should examine the effectiveness of the current regimen and consider the dose of prescribed medications, whether the patient is adhering to the regimen, inhaler technique, and any side effects.
Mr. Ortiz reports also being a paid employee for Thermo Fisher, a biotechnology product development company based in Waltham, Mass. Global Academy for Medical Education and this news organization are owned by the same parent company.
ORLANDO –
That’s according to Gabriel Ortiz, MPAS, PA-C, DFAAPA, a speaker at the Cardiovascular & Respiratory Summit by Global Academy for Medical Education.
There are several treatment options available in inhaler form for patients with COPD, including short-acting and long-acting beta2-agonists (SABA/LABA), short-acting and long-acting antimuscarinics (SAMA/LAMA), combination therapy, and triple therapy. But education and training on how to use an inhaler is also important, Mr. Ortiz said in his presentation.
Providers should help a patient choose an inhaler they are most likely to use. “It’s difficult to actuate and inhale, so there’s a lot of education that goes into that,” said Mr. Ortiz, of Pediatric Pulmonary Services in El Paso, Texas. “What we do for our patients is, we educate them, we tell them to demonstrate it, then bring [the] inhaler back on the next visit and demonstrate to us every time.”
“Make sure that they demonstrate because, as patients get older, they may lose their ability to actuate and inhale,” he added.
Adherence to therapy should also be considered before changing from a current therapy, he added. Mr. Ortiz described a scenario in which a prescription was filled, but because of the cost, the patient reduced the dose by half to make the therapy last longer.
“We could be the best providers in the world, prescribing the best medication in the world. If the medication doesn’t get to where we need it, it’s not going to help anybody,” he said.
Providers should also use nonpharmacological treatments to prevent or keep COPD from progressing. Smoking cessation is key to reducing the risk of developing COPD, and it is not clear whether e-cigarettes aid in smoking cessation, despite companies that market these products making that claim. “We have a huge e-cigarette epidemic here,” he said.
Instead, Mr. Ortiz recommended identifying which patients are current tobacco users, encouraging them to quit, determining whether the patient is willing to make a commitment to cut down on tobacco use or stop entirely, helping draft a quit plan and obtaining intra- and extratreatment social support for smoking cessation, and scheduling follow-up. Providers should discuss smoking cessation at each visit, and it may take multiple visits before a patient is willing to consider quitting, he said.
Another measure providers can take is making sure patients with COPD have received influenza and pneumococcal vaccinations because this can lower the risk of respiratory tract infections. Physical activity, training in exercise, nutritional support, and pulmonary rehabilitation can improve their health status, dyspnea, and exercise tolerance. While C-level evidence shows education alone is not effective according to Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines, providers working with patients through self-management interventions have been shown to improve health status and decrease COPD-related hospitalizations.
Oxygen therapy has been effective for patients with severe resting chronic hypoxia and improves survival, said Mr. Ortiz. For patients who gradually decline in health despite treatment, palliative, hospice, and end-of-life care may be an option. “Remember that as acute exacerbations continue, that increases the risk of death,” he said. “We want to try and prevent [those] exacerbations and improve quality of life.”
During follow-up visits, providers should continue performing spirometry tests annually to measure decline in forced expiratory volume in 1 second, information on symptoms that have presented since the previous visit, details of any exacerbations that occurred, and current smoking status. When making decisions to adjust therapy, providers should examine the effectiveness of the current regimen and consider the dose of prescribed medications, whether the patient is adhering to the regimen, inhaler technique, and any side effects.
Mr. Ortiz reports also being a paid employee for Thermo Fisher, a biotechnology product development company based in Waltham, Mass. Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM CARPS 2019
iPledge: Fetal exposure to isotretinoin continues
but pregnancy, abortions, and fetal defects associated with isotretinoin exposure are still occurring in women of reproductive age, according to a retrospective study published in JAMA Dermatology.
In 2006, the Food and Drug Administration implemented the iPledge program, with requirements that include women of childbearing age having a negative pregnancy test and evidence of using two forms of contraception monthly to use isotretinoin, a teratogen. “Although the number of pregnancy-related adverse events for patients taking isotretinoin has decreased since 2006, pregnancies, abortions, and fetal defects associated with isotretinoin exposure continue to be a problem,” Elizabeth Tkachenko, BS, from the University of Massachusetts Medical School, Worcester, and coauthors concluded. “Further research is required to determine the most efficacious system to reduce complications for patients and administrative requirements for physicians while at the same time maintaining access to this important drug.” (iPledge followed other Risk Evaluation and Mitigation Strategy systems for isotretinoin.)
She and her colleagues performed a retrospective evaluation of pregnancy-related adverse events related to isotretinoin that had occurred between January 1997 and December 2017 using the FDA Adverse Event Reporting System (FAERS), which receives reports from prescribers, consumers, and pharmaceutical manufacturers. While there could be many different classification terms for each individual, any number of adverse events reported by an individual was counted as one pregnancy. Ms. Tkachenko and colleagues classified abortions, pregnancies during contraception use, and pregnancy-related defects into separate subgroups for analysis.
From 1997 to 2017, there were 6,740 pregnancies among women (mean age, 24.6 years) during treatment with isotretinoin reported to FAERS, with 7 reports in 1997, and a peak of 768 pregnancies in 2006. Almost 70% (4,647) of the pregnancies were reported after iPledge was introduced. Between 2011 and 2017, there were 218-310 pregnancy reports each year.
Of the total number of pregnancy reports during the study period, 1,896 were abortions (28.1% of the total); 10.9% of the total number of pregnancy reports were spontaneous abortions (733). The number of abortions peaked in 2008, with 291 reports, of which 85% were therapeutic abortions. Also peaking in 2008 was the number of reports of pregnancies while taking a contraceptive (64). After 2008, pregnancies and abortions dropped.
Fetal defects peaked in 2000, with 34 cases reported, and dropped to four or fewer reports annually after 2008.
“Our findings demonstrate that reports of pregnancy among women taking isotretinoin are concentrated among those aged 20 to 29 years, peaked in 2006, and have been consistent since 2011,” the authors wrote.
Limitations of the study, they noted, include limitations of FAERS data and possible reporting fatigue among doctors and patients. The total number of isotretinoin courses prescribed to this patient population is also unknown, which affected their ability to determine the true rate of pregnancy-related adverse events, they noted.
The other authors for this study were from Harvard Medical School and the departments of dermatology at Brigham and Women’s Hospital, both in Boston, as well as the University of Pennsylvania, Philadelphia. One author reported support from an award by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health and salary support from a Pfizer Fellowship in Dermatology Patient Oriented Research grant to the trustees of the University of Pennsylvania. The other authors reported no relevant conflicts of interest.
SOURCE: Tkachenko E et al. JAMA Dermatol. 2019. doi: 10.1001/jamadermatol.2019.1388.
The rate of fetal exposure to isotretinoin has generally decreased since the implementation of the iPledge program, but rates have plateaued since 2011, and it is unclear why the exposure rate does not continue to decrease, Arielle R. Nagler, MD, wrote in a related editorial.
As noted by Tkachenko et al., it is not possible to infer that iPledge resulted in declines in fetal exposure, abortions, and pregnancy-related complications. Use of long-acting reversible contraception, education about contraception use, and reporting fatigue could be factors in the decline, Dr. Nagler noted. “The inability to clearly demonstrate causality, combined with the unexplained delay and plateau in the number of fetal exposures to isotretinoin after the implementation of iPledge, makes it difficult to draw firm conclusions about the role of iPledge in this reported trend,” she said.
The decrease in fetal exposure could also potentially be explained by effects of iPledge on the availability of isotretinoin for women of childbearing age. Indeed, studies have shown a significant decrease in isotretinoin prescriptions in this patient population after iPledge was implemented.
Despite lack of data, there is still too much fetal exposure to isotretinoin, wrote Dr. Nagler, which calls into question the efficacy of the iPledge program. “We can all agree that 1 fetal exposure to isotretinoin should be too many, but without taking isotretinoin off the market, we will never achieve zero fetal exposures to isotretinoin. Still, we can – and should – expect more from a REMS [Risk Evaluation and Mitigation Strategy] program,” Dr. Nagler concluded.
Dr. Nagler is with the department of dermatology at New York University. She reported no relevant conflicts of interest.
The rate of fetal exposure to isotretinoin has generally decreased since the implementation of the iPledge program, but rates have plateaued since 2011, and it is unclear why the exposure rate does not continue to decrease, Arielle R. Nagler, MD, wrote in a related editorial.
As noted by Tkachenko et al., it is not possible to infer that iPledge resulted in declines in fetal exposure, abortions, and pregnancy-related complications. Use of long-acting reversible contraception, education about contraception use, and reporting fatigue could be factors in the decline, Dr. Nagler noted. “The inability to clearly demonstrate causality, combined with the unexplained delay and plateau in the number of fetal exposures to isotretinoin after the implementation of iPledge, makes it difficult to draw firm conclusions about the role of iPledge in this reported trend,” she said.
The decrease in fetal exposure could also potentially be explained by effects of iPledge on the availability of isotretinoin for women of childbearing age. Indeed, studies have shown a significant decrease in isotretinoin prescriptions in this patient population after iPledge was implemented.
Despite lack of data, there is still too much fetal exposure to isotretinoin, wrote Dr. Nagler, which calls into question the efficacy of the iPledge program. “We can all agree that 1 fetal exposure to isotretinoin should be too many, but without taking isotretinoin off the market, we will never achieve zero fetal exposures to isotretinoin. Still, we can – and should – expect more from a REMS [Risk Evaluation and Mitigation Strategy] program,” Dr. Nagler concluded.
Dr. Nagler is with the department of dermatology at New York University. She reported no relevant conflicts of interest.
The rate of fetal exposure to isotretinoin has generally decreased since the implementation of the iPledge program, but rates have plateaued since 2011, and it is unclear why the exposure rate does not continue to decrease, Arielle R. Nagler, MD, wrote in a related editorial.
As noted by Tkachenko et al., it is not possible to infer that iPledge resulted in declines in fetal exposure, abortions, and pregnancy-related complications. Use of long-acting reversible contraception, education about contraception use, and reporting fatigue could be factors in the decline, Dr. Nagler noted. “The inability to clearly demonstrate causality, combined with the unexplained delay and plateau in the number of fetal exposures to isotretinoin after the implementation of iPledge, makes it difficult to draw firm conclusions about the role of iPledge in this reported trend,” she said.
The decrease in fetal exposure could also potentially be explained by effects of iPledge on the availability of isotretinoin for women of childbearing age. Indeed, studies have shown a significant decrease in isotretinoin prescriptions in this patient population after iPledge was implemented.
Despite lack of data, there is still too much fetal exposure to isotretinoin, wrote Dr. Nagler, which calls into question the efficacy of the iPledge program. “We can all agree that 1 fetal exposure to isotretinoin should be too many, but without taking isotretinoin off the market, we will never achieve zero fetal exposures to isotretinoin. Still, we can – and should – expect more from a REMS [Risk Evaluation and Mitigation Strategy] program,” Dr. Nagler concluded.
Dr. Nagler is with the department of dermatology at New York University. She reported no relevant conflicts of interest.
but pregnancy, abortions, and fetal defects associated with isotretinoin exposure are still occurring in women of reproductive age, according to a retrospective study published in JAMA Dermatology.
In 2006, the Food and Drug Administration implemented the iPledge program, with requirements that include women of childbearing age having a negative pregnancy test and evidence of using two forms of contraception monthly to use isotretinoin, a teratogen. “Although the number of pregnancy-related adverse events for patients taking isotretinoin has decreased since 2006, pregnancies, abortions, and fetal defects associated with isotretinoin exposure continue to be a problem,” Elizabeth Tkachenko, BS, from the University of Massachusetts Medical School, Worcester, and coauthors concluded. “Further research is required to determine the most efficacious system to reduce complications for patients and administrative requirements for physicians while at the same time maintaining access to this important drug.” (iPledge followed other Risk Evaluation and Mitigation Strategy systems for isotretinoin.)
She and her colleagues performed a retrospective evaluation of pregnancy-related adverse events related to isotretinoin that had occurred between January 1997 and December 2017 using the FDA Adverse Event Reporting System (FAERS), which receives reports from prescribers, consumers, and pharmaceutical manufacturers. While there could be many different classification terms for each individual, any number of adverse events reported by an individual was counted as one pregnancy. Ms. Tkachenko and colleagues classified abortions, pregnancies during contraception use, and pregnancy-related defects into separate subgroups for analysis.
From 1997 to 2017, there were 6,740 pregnancies among women (mean age, 24.6 years) during treatment with isotretinoin reported to FAERS, with 7 reports in 1997, and a peak of 768 pregnancies in 2006. Almost 70% (4,647) of the pregnancies were reported after iPledge was introduced. Between 2011 and 2017, there were 218-310 pregnancy reports each year.
Of the total number of pregnancy reports during the study period, 1,896 were abortions (28.1% of the total); 10.9% of the total number of pregnancy reports were spontaneous abortions (733). The number of abortions peaked in 2008, with 291 reports, of which 85% were therapeutic abortions. Also peaking in 2008 was the number of reports of pregnancies while taking a contraceptive (64). After 2008, pregnancies and abortions dropped.
Fetal defects peaked in 2000, with 34 cases reported, and dropped to four or fewer reports annually after 2008.
“Our findings demonstrate that reports of pregnancy among women taking isotretinoin are concentrated among those aged 20 to 29 years, peaked in 2006, and have been consistent since 2011,” the authors wrote.
Limitations of the study, they noted, include limitations of FAERS data and possible reporting fatigue among doctors and patients. The total number of isotretinoin courses prescribed to this patient population is also unknown, which affected their ability to determine the true rate of pregnancy-related adverse events, they noted.
The other authors for this study were from Harvard Medical School and the departments of dermatology at Brigham and Women’s Hospital, both in Boston, as well as the University of Pennsylvania, Philadelphia. One author reported support from an award by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health and salary support from a Pfizer Fellowship in Dermatology Patient Oriented Research grant to the trustees of the University of Pennsylvania. The other authors reported no relevant conflicts of interest.
SOURCE: Tkachenko E et al. JAMA Dermatol. 2019. doi: 10.1001/jamadermatol.2019.1388.
but pregnancy, abortions, and fetal defects associated with isotretinoin exposure are still occurring in women of reproductive age, according to a retrospective study published in JAMA Dermatology.
In 2006, the Food and Drug Administration implemented the iPledge program, with requirements that include women of childbearing age having a negative pregnancy test and evidence of using two forms of contraception monthly to use isotretinoin, a teratogen. “Although the number of pregnancy-related adverse events for patients taking isotretinoin has decreased since 2006, pregnancies, abortions, and fetal defects associated with isotretinoin exposure continue to be a problem,” Elizabeth Tkachenko, BS, from the University of Massachusetts Medical School, Worcester, and coauthors concluded. “Further research is required to determine the most efficacious system to reduce complications for patients and administrative requirements for physicians while at the same time maintaining access to this important drug.” (iPledge followed other Risk Evaluation and Mitigation Strategy systems for isotretinoin.)
She and her colleagues performed a retrospective evaluation of pregnancy-related adverse events related to isotretinoin that had occurred between January 1997 and December 2017 using the FDA Adverse Event Reporting System (FAERS), which receives reports from prescribers, consumers, and pharmaceutical manufacturers. While there could be many different classification terms for each individual, any number of adverse events reported by an individual was counted as one pregnancy. Ms. Tkachenko and colleagues classified abortions, pregnancies during contraception use, and pregnancy-related defects into separate subgroups for analysis.
From 1997 to 2017, there were 6,740 pregnancies among women (mean age, 24.6 years) during treatment with isotretinoin reported to FAERS, with 7 reports in 1997, and a peak of 768 pregnancies in 2006. Almost 70% (4,647) of the pregnancies were reported after iPledge was introduced. Between 2011 and 2017, there were 218-310 pregnancy reports each year.
Of the total number of pregnancy reports during the study period, 1,896 were abortions (28.1% of the total); 10.9% of the total number of pregnancy reports were spontaneous abortions (733). The number of abortions peaked in 2008, with 291 reports, of which 85% were therapeutic abortions. Also peaking in 2008 was the number of reports of pregnancies while taking a contraceptive (64). After 2008, pregnancies and abortions dropped.
Fetal defects peaked in 2000, with 34 cases reported, and dropped to four or fewer reports annually after 2008.
“Our findings demonstrate that reports of pregnancy among women taking isotretinoin are concentrated among those aged 20 to 29 years, peaked in 2006, and have been consistent since 2011,” the authors wrote.
Limitations of the study, they noted, include limitations of FAERS data and possible reporting fatigue among doctors and patients. The total number of isotretinoin courses prescribed to this patient population is also unknown, which affected their ability to determine the true rate of pregnancy-related adverse events, they noted.
The other authors for this study were from Harvard Medical School and the departments of dermatology at Brigham and Women’s Hospital, both in Boston, as well as the University of Pennsylvania, Philadelphia. One author reported support from an award by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health and salary support from a Pfizer Fellowship in Dermatology Patient Oriented Research grant to the trustees of the University of Pennsylvania. The other authors reported no relevant conflicts of interest.
SOURCE: Tkachenko E et al. JAMA Dermatol. 2019. doi: 10.1001/jamadermatol.2019.1388.
FROM JAMA DERMATOLOGY
Continuous anticoagulation plus cold snare colon polypectomy decreases bleeding, procedure time, hospital stay
, according to recent research published in the Annals of Internal Medicine.
“Guidelines on peripolypectomy management of anticoagulants vary greatly, and the current updated guidelines do not recommend heparin bridging (HB) for all patients; however, direct comparison of HB with continuous administration of oral anticoagulants (CA) has provided little evidence,” Yoji Takeuchi, MD, from the Department of Gastrointestinal Oncology at Osaka International Cancer Institute in Osaka, Japan, and colleagues wrote.
While cold snare polypectomy (CSP) has been recommended by the European Society of Gastrointestinal Endoscopy for subcentimeter polyps, anticoagulant delivery method has not been studied between these two poly removal methods. “Cold snare polypectomy with CA may be performed safely, without the complications of HB, while theoretically maintaining an anticoagulant effect,” the researchers said.
Dr. Takeuchi and colleagues performed a randomized controlled trial of 182 patients with subcentimeter colorectal polyps who underwent either CA with CSP (CA+CSP; 92 patients) or hot snare polypectomy (HSP) with HB (HB+HSP; 90 patients) at one of 30 different Japanese centers. Patients were between 20 and 80 years old and had preserved organ function, an Eastern Cooperative Oncology Group Performance Status score of 1 or less, and were taking warfarin or a direct oral anticoagulant (DOAC) such as dabigatran, rivaroxaban, apixaban, or edoxaban. Researchers assessed the level of bleeding at 28-day follow-up, and also measured procedure time per polyp and length of hospital stay for each group.
Overall, there were 611 polyps removed in 168 patients. The rate of major bleeding in the CA+CSP group was 4.7% (95% confidence interval [CI], 0.2%-9.2%) compared with 12.0% (95% CI, 5.0%-19.1%) in the HB+HSP group with an intergroup difference of 7.3% (95% CI, 1.0%-15.7%).
“[T]he Japanese guidelines consider all patients receiving anticoagulants to be at high risk for thromboembolism associated with antithrombotic withdrawal,” Dr. Takeuchi and colleagues said. “Our results suggest that discontinuing anticoagulant therapy before polypectomy for subcentimeter polyps may be unnecessary and support the Japanese guidelines, which recommend not withholding anticoagulants for procedures with low bleeding risk.”
The researchers declared CA+CSP to be non-inferior with a 0.4% lower limit of 2-sided 90% CI. “[W]e noted a higher number of total and right-sided polyps in the CA+CSP group, both of which may result in more frequent bleeding episodes, which suggests that CA+CSP may be a relatively safe approach,” the researchers said. “Therefore, we think that CSP may be the least risky polypectomy procedure.”
The mean procedure time per polyp was 59.6 seconds in the CA+CSP group (54.0-65.2 seconds) compared with 94.4 seconds in the HB+HSP group (87.1-101.7 seconds; P less than .001). Mean hospital stay for patients in the CA+CSP group was shorter at 2.9 days (1.8-4.0 days) compared with 5.1 days in the HB+HSP group (4.2-6.1 days; P equals .003).
The study examined patients receiving two different anticoagulant delivery methods and polyp removal procedures, which made it difficult to determine which intervention contributed to the results, the researchers said. In addition, the study was not blinded and polyp type was limited to only subcentimeter polyps.
“Although CA+CSP is considered standard treatment for subcentimeter colorectal polyps in patients receiving anticoagulants, a larger trial is needed to identify a better management strategy for patients receiving DOACs,” the researchers said.
This study was supported by a grant from the Japanese Gastroenterological Association. The authors report no relevant conflicts of interest.
SOURCE: Takeuchi Y et al. Ann Intern Med. 2019;doi: 10.7326/M19-0026 .
It is still an open question what the safest method to remove colon polyps is in patients taking continuous anticoagulants (CA), but the study by Takeuchi et al. shows cold snare polypectomy (CSP) has promise, Jeffrey L. Tokar, MD; and Michael J. Bartel, MD, wrote in a related editorial.
“[T]his study adds to emerging evidence that small colorectal polyps may be resected safely with CSP while oral anticoagulation continues and provides the first comparative evidence that this strategy may be safer than [heparin bridging with hot snare polypectomy] HB+HSP,” they said.
Another consideration of CA+CSP is the risk of intraprocedural postpolypectomy bleeding, but there were no cases of this kind of bleeding in the results by Takeuchi et al., which may give some clinicians reassurance about the method. However, the study did not take into account the risk in patients taking warfarin or direct oral anticoagulants who had incomplete polyp resection, and the difference in CA therapy between CSP and HSP, or the effect of not using heparin bridging in CSP or HSP was not studied.
“The results warrant confirmatory studies, preferably with blinding to the use of anticoagulation and assessment of several additional factors: incomplete polyp resection, the effect of prophylactic hemostatic actions (such as clipping), and the applicability of CA+CSP to the removal of larger polyps and to the use of other classes of antithrombotic medications (such as thienopyridines),” Dr. Tokar and Dr. Bartel concluded.
Dr. Tokar and Dr. Bartel are from the Fox Chase Cancer Center in Philadelphia. They report no relevant conflicts of interest.
It is still an open question what the safest method to remove colon polyps is in patients taking continuous anticoagulants (CA), but the study by Takeuchi et al. shows cold snare polypectomy (CSP) has promise, Jeffrey L. Tokar, MD; and Michael J. Bartel, MD, wrote in a related editorial.
“[T]his study adds to emerging evidence that small colorectal polyps may be resected safely with CSP while oral anticoagulation continues and provides the first comparative evidence that this strategy may be safer than [heparin bridging with hot snare polypectomy] HB+HSP,” they said.
Another consideration of CA+CSP is the risk of intraprocedural postpolypectomy bleeding, but there were no cases of this kind of bleeding in the results by Takeuchi et al., which may give some clinicians reassurance about the method. However, the study did not take into account the risk in patients taking warfarin or direct oral anticoagulants who had incomplete polyp resection, and the difference in CA therapy between CSP and HSP, or the effect of not using heparin bridging in CSP or HSP was not studied.
“The results warrant confirmatory studies, preferably with blinding to the use of anticoagulation and assessment of several additional factors: incomplete polyp resection, the effect of prophylactic hemostatic actions (such as clipping), and the applicability of CA+CSP to the removal of larger polyps and to the use of other classes of antithrombotic medications (such as thienopyridines),” Dr. Tokar and Dr. Bartel concluded.
Dr. Tokar and Dr. Bartel are from the Fox Chase Cancer Center in Philadelphia. They report no relevant conflicts of interest.
It is still an open question what the safest method to remove colon polyps is in patients taking continuous anticoagulants (CA), but the study by Takeuchi et al. shows cold snare polypectomy (CSP) has promise, Jeffrey L. Tokar, MD; and Michael J. Bartel, MD, wrote in a related editorial.
“[T]his study adds to emerging evidence that small colorectal polyps may be resected safely with CSP while oral anticoagulation continues and provides the first comparative evidence that this strategy may be safer than [heparin bridging with hot snare polypectomy] HB+HSP,” they said.
Another consideration of CA+CSP is the risk of intraprocedural postpolypectomy bleeding, but there were no cases of this kind of bleeding in the results by Takeuchi et al., which may give some clinicians reassurance about the method. However, the study did not take into account the risk in patients taking warfarin or direct oral anticoagulants who had incomplete polyp resection, and the difference in CA therapy between CSP and HSP, or the effect of not using heparin bridging in CSP or HSP was not studied.
“The results warrant confirmatory studies, preferably with blinding to the use of anticoagulation and assessment of several additional factors: incomplete polyp resection, the effect of prophylactic hemostatic actions (such as clipping), and the applicability of CA+CSP to the removal of larger polyps and to the use of other classes of antithrombotic medications (such as thienopyridines),” Dr. Tokar and Dr. Bartel concluded.
Dr. Tokar and Dr. Bartel are from the Fox Chase Cancer Center in Philadelphia. They report no relevant conflicts of interest.
, according to recent research published in the Annals of Internal Medicine.
“Guidelines on peripolypectomy management of anticoagulants vary greatly, and the current updated guidelines do not recommend heparin bridging (HB) for all patients; however, direct comparison of HB with continuous administration of oral anticoagulants (CA) has provided little evidence,” Yoji Takeuchi, MD, from the Department of Gastrointestinal Oncology at Osaka International Cancer Institute in Osaka, Japan, and colleagues wrote.
While cold snare polypectomy (CSP) has been recommended by the European Society of Gastrointestinal Endoscopy for subcentimeter polyps, anticoagulant delivery method has not been studied between these two poly removal methods. “Cold snare polypectomy with CA may be performed safely, without the complications of HB, while theoretically maintaining an anticoagulant effect,” the researchers said.
Dr. Takeuchi and colleagues performed a randomized controlled trial of 182 patients with subcentimeter colorectal polyps who underwent either CA with CSP (CA+CSP; 92 patients) or hot snare polypectomy (HSP) with HB (HB+HSP; 90 patients) at one of 30 different Japanese centers. Patients were between 20 and 80 years old and had preserved organ function, an Eastern Cooperative Oncology Group Performance Status score of 1 or less, and were taking warfarin or a direct oral anticoagulant (DOAC) such as dabigatran, rivaroxaban, apixaban, or edoxaban. Researchers assessed the level of bleeding at 28-day follow-up, and also measured procedure time per polyp and length of hospital stay for each group.
Overall, there were 611 polyps removed in 168 patients. The rate of major bleeding in the CA+CSP group was 4.7% (95% confidence interval [CI], 0.2%-9.2%) compared with 12.0% (95% CI, 5.0%-19.1%) in the HB+HSP group with an intergroup difference of 7.3% (95% CI, 1.0%-15.7%).
“[T]he Japanese guidelines consider all patients receiving anticoagulants to be at high risk for thromboembolism associated with antithrombotic withdrawal,” Dr. Takeuchi and colleagues said. “Our results suggest that discontinuing anticoagulant therapy before polypectomy for subcentimeter polyps may be unnecessary and support the Japanese guidelines, which recommend not withholding anticoagulants for procedures with low bleeding risk.”
The researchers declared CA+CSP to be non-inferior with a 0.4% lower limit of 2-sided 90% CI. “[W]e noted a higher number of total and right-sided polyps in the CA+CSP group, both of which may result in more frequent bleeding episodes, which suggests that CA+CSP may be a relatively safe approach,” the researchers said. “Therefore, we think that CSP may be the least risky polypectomy procedure.”
The mean procedure time per polyp was 59.6 seconds in the CA+CSP group (54.0-65.2 seconds) compared with 94.4 seconds in the HB+HSP group (87.1-101.7 seconds; P less than .001). Mean hospital stay for patients in the CA+CSP group was shorter at 2.9 days (1.8-4.0 days) compared with 5.1 days in the HB+HSP group (4.2-6.1 days; P equals .003).
The study examined patients receiving two different anticoagulant delivery methods and polyp removal procedures, which made it difficult to determine which intervention contributed to the results, the researchers said. In addition, the study was not blinded and polyp type was limited to only subcentimeter polyps.
“Although CA+CSP is considered standard treatment for subcentimeter colorectal polyps in patients receiving anticoagulants, a larger trial is needed to identify a better management strategy for patients receiving DOACs,” the researchers said.
This study was supported by a grant from the Japanese Gastroenterological Association. The authors report no relevant conflicts of interest.
SOURCE: Takeuchi Y et al. Ann Intern Med. 2019;doi: 10.7326/M19-0026 .
, according to recent research published in the Annals of Internal Medicine.
“Guidelines on peripolypectomy management of anticoagulants vary greatly, and the current updated guidelines do not recommend heparin bridging (HB) for all patients; however, direct comparison of HB with continuous administration of oral anticoagulants (CA) has provided little evidence,” Yoji Takeuchi, MD, from the Department of Gastrointestinal Oncology at Osaka International Cancer Institute in Osaka, Japan, and colleagues wrote.
While cold snare polypectomy (CSP) has been recommended by the European Society of Gastrointestinal Endoscopy for subcentimeter polyps, anticoagulant delivery method has not been studied between these two poly removal methods. “Cold snare polypectomy with CA may be performed safely, without the complications of HB, while theoretically maintaining an anticoagulant effect,” the researchers said.
Dr. Takeuchi and colleagues performed a randomized controlled trial of 182 patients with subcentimeter colorectal polyps who underwent either CA with CSP (CA+CSP; 92 patients) or hot snare polypectomy (HSP) with HB (HB+HSP; 90 patients) at one of 30 different Japanese centers. Patients were between 20 and 80 years old and had preserved organ function, an Eastern Cooperative Oncology Group Performance Status score of 1 or less, and were taking warfarin or a direct oral anticoagulant (DOAC) such as dabigatran, rivaroxaban, apixaban, or edoxaban. Researchers assessed the level of bleeding at 28-day follow-up, and also measured procedure time per polyp and length of hospital stay for each group.
Overall, there were 611 polyps removed in 168 patients. The rate of major bleeding in the CA+CSP group was 4.7% (95% confidence interval [CI], 0.2%-9.2%) compared with 12.0% (95% CI, 5.0%-19.1%) in the HB+HSP group with an intergroup difference of 7.3% (95% CI, 1.0%-15.7%).
“[T]he Japanese guidelines consider all patients receiving anticoagulants to be at high risk for thromboembolism associated with antithrombotic withdrawal,” Dr. Takeuchi and colleagues said. “Our results suggest that discontinuing anticoagulant therapy before polypectomy for subcentimeter polyps may be unnecessary and support the Japanese guidelines, which recommend not withholding anticoagulants for procedures with low bleeding risk.”
The researchers declared CA+CSP to be non-inferior with a 0.4% lower limit of 2-sided 90% CI. “[W]e noted a higher number of total and right-sided polyps in the CA+CSP group, both of which may result in more frequent bleeding episodes, which suggests that CA+CSP may be a relatively safe approach,” the researchers said. “Therefore, we think that CSP may be the least risky polypectomy procedure.”
The mean procedure time per polyp was 59.6 seconds in the CA+CSP group (54.0-65.2 seconds) compared with 94.4 seconds in the HB+HSP group (87.1-101.7 seconds; P less than .001). Mean hospital stay for patients in the CA+CSP group was shorter at 2.9 days (1.8-4.0 days) compared with 5.1 days in the HB+HSP group (4.2-6.1 days; P equals .003).
The study examined patients receiving two different anticoagulant delivery methods and polyp removal procedures, which made it difficult to determine which intervention contributed to the results, the researchers said. In addition, the study was not blinded and polyp type was limited to only subcentimeter polyps.
“Although CA+CSP is considered standard treatment for subcentimeter colorectal polyps in patients receiving anticoagulants, a larger trial is needed to identify a better management strategy for patients receiving DOACs,” the researchers said.
This study was supported by a grant from the Japanese Gastroenterological Association. The authors report no relevant conflicts of interest.
SOURCE: Takeuchi Y et al. Ann Intern Med. 2019;doi: 10.7326/M19-0026 .
FROM THE ANNALS OF INTERNAL MEDICINE
Key clinical point: Cold snare polypectomy (CSP) with continuous administration of anticoagulants (CA) used to remove colon polyps appears to result in less bleeding, a lower procedure time and shorter hospital stay than heparin bridging (HB) with hot snare polypectomy (HSP).
Major finding: The rate of major bleeding in the CA+CSP group was 4.7% compared with 12.0% in the HB+HSP group.
Study details: A prospective, open-label, parallel, multicenter randomized controlled trial of 182 patients who underwent CA+CSP or HB+HSP at 30 Japanese institutions between June 2016 and April 2018.
Disclosures: This study was supported by a grant from the Japanese Gastroenterological Association. The authors report no relevant conflicts of interest.
Source: Takeuchi Y, et al. Ann Intern Med. 2019;doi:10.7326/M19-0026.
Cathepsin Z identified as a potential biomarker for osteoporosis
The presence of cathepsin Z messenger RNA in peripheral blood mononuclear cells of people with osteopenia, osteoporosis, and women with osteoporosis and older than 50 years could be used as a biomarker to help diagnose osteoporosis, according to a recent study published in Scientific Reports.
Dong L. Barraclough, PhD, of the Institute of Ageing and Chronic Disease at the University of Liverpool, England, and colleagues studied the expression of cathepsin Z messenger RNA (mRNA) in peripheral blood mononuclear cells (PBMCs) of 88 participants (71 women, 17 men). The participants were grouped according to their bone mineral density and T score, where a T score of −1.0 or higher was considered nonosteoporotic, a score between −1.0 and −2.5 was classified as osteopenia, and −2.5 or less was classified as osteoporosis.
Overall, there were 48 participants with osteopenia (38 women, 10 men; 55% of total participants; average age, 65 years), 23 participants with osteoporosis (19 women, 4 men; 26%; 69 years), and 17 participants in the nonosteoporotic control group (14 women, 3 men; 19%; 56 years), with 88% of the total number of participants aged 50 years and older (82% women, 18% men).
The researchers found significantly higher differential expression of cathepsin Z mRNA in PBMCs when comparing the nonosteoporotic control group and participants with osteopenia (95% confidence interval, −0.32 to −0.053; P = .0067), the control group with participants with osteoporosis (95% CI, −0.543 to −0.24; P less than .0001), and participants with osteopenia and those with osteoporosis (95% CI, −0.325 to −0.084; P = .0011).
That association also was seen in women with osteoporosis who were older than 50 years (P = .0016) and did not change when participants were excluded for receiving treatment for osteoporosis, the authors wrote.
There also was an inverse association between cathepsin Z mRNA levels and bone mineral density (P = .0149) as well as inversely associated with lumbar spine L2-L4 and femoral neck T-scores (P = .0002 and P = .0139, respectively) and fragility fracture (P = .0018) in participants with osteopenia, osteoporosis, and women with osteoporosis older than 50 years.
Patients with chronic inflammatory disease sometimes have “osteoporosis-like conditions,” the authors noted. “However, there was no significant difference in cathepsin Z mRNA levels between osteopenia and osteoporosis patients who were also suffering from chronic inflammatory disorders and those [who] were not,” either when all osteopenia and osteoporosis participants were included (P = .774), or when only women participants with osteopenia or osteoporosis and older than 50 years were included (P = .666).
“The observation that [participants] with osteopenia also showed a significant increase in cathepsin Z mRNA, compared [with] nonosteoporotic controls, strongly suggests that, if replicated in a larger study, the cathepsin Z mRNA in patients’ PBMC preparations could form the basis of a test for osteoporosis, which could aid in the detection of osteoporosis before a critical and expensive fragility fracture occurs,” the authors wrote.
The authors reported no relevant conflicts of interest.
SOURCE: Dera AA et al. Sci Rep. 2019 Jul 5. doi: 10.1038/s41598-019-46068-0.
The presence of cathepsin Z messenger RNA in peripheral blood mononuclear cells of people with osteopenia, osteoporosis, and women with osteoporosis and older than 50 years could be used as a biomarker to help diagnose osteoporosis, according to a recent study published in Scientific Reports.
Dong L. Barraclough, PhD, of the Institute of Ageing and Chronic Disease at the University of Liverpool, England, and colleagues studied the expression of cathepsin Z messenger RNA (mRNA) in peripheral blood mononuclear cells (PBMCs) of 88 participants (71 women, 17 men). The participants were grouped according to their bone mineral density and T score, where a T score of −1.0 or higher was considered nonosteoporotic, a score between −1.0 and −2.5 was classified as osteopenia, and −2.5 or less was classified as osteoporosis.
Overall, there were 48 participants with osteopenia (38 women, 10 men; 55% of total participants; average age, 65 years), 23 participants with osteoporosis (19 women, 4 men; 26%; 69 years), and 17 participants in the nonosteoporotic control group (14 women, 3 men; 19%; 56 years), with 88% of the total number of participants aged 50 years and older (82% women, 18% men).
The researchers found significantly higher differential expression of cathepsin Z mRNA in PBMCs when comparing the nonosteoporotic control group and participants with osteopenia (95% confidence interval, −0.32 to −0.053; P = .0067), the control group with participants with osteoporosis (95% CI, −0.543 to −0.24; P less than .0001), and participants with osteopenia and those with osteoporosis (95% CI, −0.325 to −0.084; P = .0011).
That association also was seen in women with osteoporosis who were older than 50 years (P = .0016) and did not change when participants were excluded for receiving treatment for osteoporosis, the authors wrote.
There also was an inverse association between cathepsin Z mRNA levels and bone mineral density (P = .0149) as well as inversely associated with lumbar spine L2-L4 and femoral neck T-scores (P = .0002 and P = .0139, respectively) and fragility fracture (P = .0018) in participants with osteopenia, osteoporosis, and women with osteoporosis older than 50 years.
Patients with chronic inflammatory disease sometimes have “osteoporosis-like conditions,” the authors noted. “However, there was no significant difference in cathepsin Z mRNA levels between osteopenia and osteoporosis patients who were also suffering from chronic inflammatory disorders and those [who] were not,” either when all osteopenia and osteoporosis participants were included (P = .774), or when only women participants with osteopenia or osteoporosis and older than 50 years were included (P = .666).
“The observation that [participants] with osteopenia also showed a significant increase in cathepsin Z mRNA, compared [with] nonosteoporotic controls, strongly suggests that, if replicated in a larger study, the cathepsin Z mRNA in patients’ PBMC preparations could form the basis of a test for osteoporosis, which could aid in the detection of osteoporosis before a critical and expensive fragility fracture occurs,” the authors wrote.
The authors reported no relevant conflicts of interest.
SOURCE: Dera AA et al. Sci Rep. 2019 Jul 5. doi: 10.1038/s41598-019-46068-0.
The presence of cathepsin Z messenger RNA in peripheral blood mononuclear cells of people with osteopenia, osteoporosis, and women with osteoporosis and older than 50 years could be used as a biomarker to help diagnose osteoporosis, according to a recent study published in Scientific Reports.
Dong L. Barraclough, PhD, of the Institute of Ageing and Chronic Disease at the University of Liverpool, England, and colleagues studied the expression of cathepsin Z messenger RNA (mRNA) in peripheral blood mononuclear cells (PBMCs) of 88 participants (71 women, 17 men). The participants were grouped according to their bone mineral density and T score, where a T score of −1.0 or higher was considered nonosteoporotic, a score between −1.0 and −2.5 was classified as osteopenia, and −2.5 or less was classified as osteoporosis.
Overall, there were 48 participants with osteopenia (38 women, 10 men; 55% of total participants; average age, 65 years), 23 participants with osteoporosis (19 women, 4 men; 26%; 69 years), and 17 participants in the nonosteoporotic control group (14 women, 3 men; 19%; 56 years), with 88% of the total number of participants aged 50 years and older (82% women, 18% men).
The researchers found significantly higher differential expression of cathepsin Z mRNA in PBMCs when comparing the nonosteoporotic control group and participants with osteopenia (95% confidence interval, −0.32 to −0.053; P = .0067), the control group with participants with osteoporosis (95% CI, −0.543 to −0.24; P less than .0001), and participants with osteopenia and those with osteoporosis (95% CI, −0.325 to −0.084; P = .0011).
That association also was seen in women with osteoporosis who were older than 50 years (P = .0016) and did not change when participants were excluded for receiving treatment for osteoporosis, the authors wrote.
There also was an inverse association between cathepsin Z mRNA levels and bone mineral density (P = .0149) as well as inversely associated with lumbar spine L2-L4 and femoral neck T-scores (P = .0002 and P = .0139, respectively) and fragility fracture (P = .0018) in participants with osteopenia, osteoporosis, and women with osteoporosis older than 50 years.
Patients with chronic inflammatory disease sometimes have “osteoporosis-like conditions,” the authors noted. “However, there was no significant difference in cathepsin Z mRNA levels between osteopenia and osteoporosis patients who were also suffering from chronic inflammatory disorders and those [who] were not,” either when all osteopenia and osteoporosis participants were included (P = .774), or when only women participants with osteopenia or osteoporosis and older than 50 years were included (P = .666).
“The observation that [participants] with osteopenia also showed a significant increase in cathepsin Z mRNA, compared [with] nonosteoporotic controls, strongly suggests that, if replicated in a larger study, the cathepsin Z mRNA in patients’ PBMC preparations could form the basis of a test for osteoporosis, which could aid in the detection of osteoporosis before a critical and expensive fragility fracture occurs,” the authors wrote.
The authors reported no relevant conflicts of interest.
SOURCE: Dera AA et al. Sci Rep. 2019 Jul 5. doi: 10.1038/s41598-019-46068-0.
FROM SCIENTIFIC REPORTS
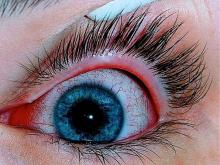
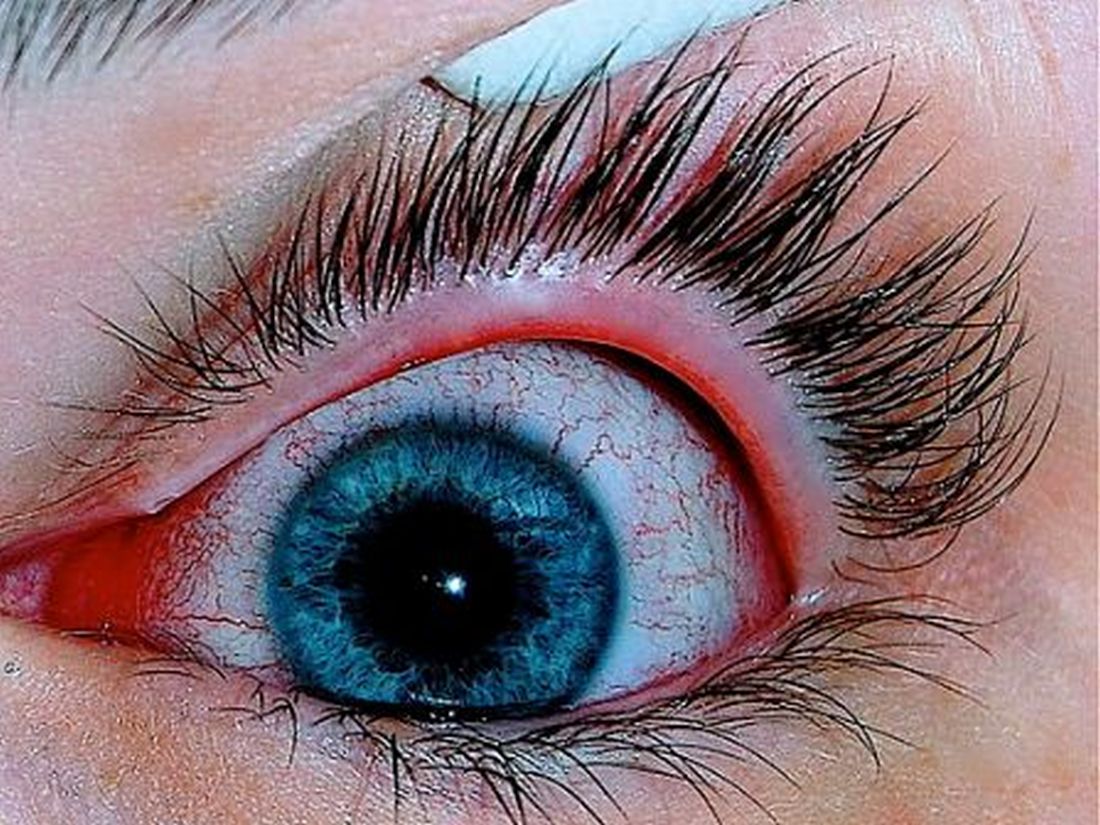
Patients with AD should routinely be asked about conjunctivitis
according to a recent position statement from the International Eczema Council.
Patients with AD who develop conjunctivitis during dupilumab treatment may experience “bilateral inflammation of the anterior conjunctiva and hyperaemia of the limbus, which may cause nodular swelling.”according to the statement, which pertains to conjunctivitis in AD patients, “with and without dupilumab therapy.” (J Eur Acad Dermatol Venereol. 2019 May 6. doi: 10.1111/jdv.15608). Currently, there are no predictive factors of conjunctivitis and no guidance in the literature on how to manage conjunctivitis associated with dupilumab, which in some cases can make it necessary to stop treatment, the authors wrote.
The International Eczema Council (IEC) polled 86 dermatologists in 22 countries who are experts in AD; 46 members responded from 19 countries, including dermatologists from Australia, Canada, Denmark, France, Germany, Japan, Korea, the Netherlands, the United Kingdom, and the United States. The questions centered on the diagnosis and management of conjunctivitis in AD patients, and whether to refer cases to an ophthalmologist. Consensus was achieved if less than 30% of participants disagreed with a statement. IEC members then met in person at a European Academy of Dermatology and Venereology meeting in Paris to discuss the results of the survey. Survey respondents noted they had seen dupilumab-associated conjunctivitis in both pediatric and adult patients.
The IEC members recommended that:
- Patients should be informed about the risks of conjunctivitis before being prescribed dupilumab.
- AD patients should be asked “routinely” about ocular complaints or symptoms.
- AD patients with conjunctivitis should be referred to an ophthalmologist for diagnosis and treatment.
- AD patients with new-onset conjunctivitis during dupilumab treatment always should be referred to an ophthalmologist, especially in more severe cases such as when they do not respond to treatment with antihistamine or artificial tears.
- Dermatologists also should rule out keratoconjunctivitis before treating with dupilumab, as it may cause keratitis and blindness.
- Patients who have had keratoconjunctivitis in the past should not be precluded from treatment with dupilumab, and those who develop conjunctivitis during treatment should be referred to an ophthalmologist – but should stay on treatment while waiting for the consult.
“It was stressed that at this moment there are also no reliable data on the course of atopic keratoconjunctivitis and vernal keratoconjunctivitis during dupilumab treatment,” according to Jacob P. Thyssen, MD, PhD, Herlev and Gentofte Hospital, Hellerup, Denmark, and coauthors. These patients “should be carefully monitored by an ophthalmologist before and during treatment with dupilumab.”
The recommendations also centered around which treatments should be initiated by dermatologists, and which should be referred to ophthalmologists. Those patients with conjunctivitis should receive eye drops, eye ointment, or oral antihistamines from their dermatologists before an ophthalmology referral, the IEC members said. Dermatologists also should perform, or refer patients for, skin prick testing or specific IgE testing for aeroallergens in patients with AD who have conjunctivitis, and patch testing with an “ophthalmologic series, and native eye drops/ointments to diagnose possible delayed type hypersensitivity reactions to topical ingredients,” they added.
Among the treatments for conjunctivitis best left to ophthalmologists are cyclosporine, tacrolimus, or corticosteroid eye drops.
“Despite the more limited experience with eye drops by dermatologists, rapid access to ophthalmological service may be difficult, sometimes warranting a short course of corticosteroid eye drops without ophthalmological consultations,” Dr. Thyssen and associates said. “However, persistent or recurrent conjunctivitis requiring repeated or prolonged use of corticosteroid, tacrolimus, and ciclosporin-containing eye drops, must be managed by an ophthalmologist, given the risk of glaucoma, cataract, and infections.”
“The AD severity, conjunctivitis severity, possible contraindications, possible effect of dupilumab therapy on concomitant asthma or other comorbidities, as well as other treatment options, should be considered on an individual patient basis,” the authors concluded.
The IEC survey was limited by the small survey response and reliance on expert opinion.
The authors reported personal and institutional relationships in the form of grants, corporate sponsorships, advisory board memberships, investigator appointments, speakers bureau positions, and consultancies for a variety of pharmaceutical companies, agencies, societies, and other organizations. No funding was obtained for the study.
This article was updated 7/17/19.
according to a recent position statement from the International Eczema Council.
Patients with AD who develop conjunctivitis during dupilumab treatment may experience “bilateral inflammation of the anterior conjunctiva and hyperaemia of the limbus, which may cause nodular swelling.”according to the statement, which pertains to conjunctivitis in AD patients, “with and without dupilumab therapy.” (J Eur Acad Dermatol Venereol. 2019 May 6. doi: 10.1111/jdv.15608). Currently, there are no predictive factors of conjunctivitis and no guidance in the literature on how to manage conjunctivitis associated with dupilumab, which in some cases can make it necessary to stop treatment, the authors wrote.
The International Eczema Council (IEC) polled 86 dermatologists in 22 countries who are experts in AD; 46 members responded from 19 countries, including dermatologists from Australia, Canada, Denmark, France, Germany, Japan, Korea, the Netherlands, the United Kingdom, and the United States. The questions centered on the diagnosis and management of conjunctivitis in AD patients, and whether to refer cases to an ophthalmologist. Consensus was achieved if less than 30% of participants disagreed with a statement. IEC members then met in person at a European Academy of Dermatology and Venereology meeting in Paris to discuss the results of the survey. Survey respondents noted they had seen dupilumab-associated conjunctivitis in both pediatric and adult patients.
The IEC members recommended that:
- Patients should be informed about the risks of conjunctivitis before being prescribed dupilumab.
- AD patients should be asked “routinely” about ocular complaints or symptoms.
- AD patients with conjunctivitis should be referred to an ophthalmologist for diagnosis and treatment.
- AD patients with new-onset conjunctivitis during dupilumab treatment always should be referred to an ophthalmologist, especially in more severe cases such as when they do not respond to treatment with antihistamine or artificial tears.
- Dermatologists also should rule out keratoconjunctivitis before treating with dupilumab, as it may cause keratitis and blindness.
- Patients who have had keratoconjunctivitis in the past should not be precluded from treatment with dupilumab, and those who develop conjunctivitis during treatment should be referred to an ophthalmologist – but should stay on treatment while waiting for the consult.
“It was stressed that at this moment there are also no reliable data on the course of atopic keratoconjunctivitis and vernal keratoconjunctivitis during dupilumab treatment,” according to Jacob P. Thyssen, MD, PhD, Herlev and Gentofte Hospital, Hellerup, Denmark, and coauthors. These patients “should be carefully monitored by an ophthalmologist before and during treatment with dupilumab.”
The recommendations also centered around which treatments should be initiated by dermatologists, and which should be referred to ophthalmologists. Those patients with conjunctivitis should receive eye drops, eye ointment, or oral antihistamines from their dermatologists before an ophthalmology referral, the IEC members said. Dermatologists also should perform, or refer patients for, skin prick testing or specific IgE testing for aeroallergens in patients with AD who have conjunctivitis, and patch testing with an “ophthalmologic series, and native eye drops/ointments to diagnose possible delayed type hypersensitivity reactions to topical ingredients,” they added.
Among the treatments for conjunctivitis best left to ophthalmologists are cyclosporine, tacrolimus, or corticosteroid eye drops.
“Despite the more limited experience with eye drops by dermatologists, rapid access to ophthalmological service may be difficult, sometimes warranting a short course of corticosteroid eye drops without ophthalmological consultations,” Dr. Thyssen and associates said. “However, persistent or recurrent conjunctivitis requiring repeated or prolonged use of corticosteroid, tacrolimus, and ciclosporin-containing eye drops, must be managed by an ophthalmologist, given the risk of glaucoma, cataract, and infections.”
“The AD severity, conjunctivitis severity, possible contraindications, possible effect of dupilumab therapy on concomitant asthma or other comorbidities, as well as other treatment options, should be considered on an individual patient basis,” the authors concluded.
The IEC survey was limited by the small survey response and reliance on expert opinion.
The authors reported personal and institutional relationships in the form of grants, corporate sponsorships, advisory board memberships, investigator appointments, speakers bureau positions, and consultancies for a variety of pharmaceutical companies, agencies, societies, and other organizations. No funding was obtained for the study.
This article was updated 7/17/19.
according to a recent position statement from the International Eczema Council.
Patients with AD who develop conjunctivitis during dupilumab treatment may experience “bilateral inflammation of the anterior conjunctiva and hyperaemia of the limbus, which may cause nodular swelling.”according to the statement, which pertains to conjunctivitis in AD patients, “with and without dupilumab therapy.” (J Eur Acad Dermatol Venereol. 2019 May 6. doi: 10.1111/jdv.15608). Currently, there are no predictive factors of conjunctivitis and no guidance in the literature on how to manage conjunctivitis associated with dupilumab, which in some cases can make it necessary to stop treatment, the authors wrote.
The International Eczema Council (IEC) polled 86 dermatologists in 22 countries who are experts in AD; 46 members responded from 19 countries, including dermatologists from Australia, Canada, Denmark, France, Germany, Japan, Korea, the Netherlands, the United Kingdom, and the United States. The questions centered on the diagnosis and management of conjunctivitis in AD patients, and whether to refer cases to an ophthalmologist. Consensus was achieved if less than 30% of participants disagreed with a statement. IEC members then met in person at a European Academy of Dermatology and Venereology meeting in Paris to discuss the results of the survey. Survey respondents noted they had seen dupilumab-associated conjunctivitis in both pediatric and adult patients.
The IEC members recommended that:
- Patients should be informed about the risks of conjunctivitis before being prescribed dupilumab.
- AD patients should be asked “routinely” about ocular complaints or symptoms.
- AD patients with conjunctivitis should be referred to an ophthalmologist for diagnosis and treatment.
- AD patients with new-onset conjunctivitis during dupilumab treatment always should be referred to an ophthalmologist, especially in more severe cases such as when they do not respond to treatment with antihistamine or artificial tears.
- Dermatologists also should rule out keratoconjunctivitis before treating with dupilumab, as it may cause keratitis and blindness.
- Patients who have had keratoconjunctivitis in the past should not be precluded from treatment with dupilumab, and those who develop conjunctivitis during treatment should be referred to an ophthalmologist – but should stay on treatment while waiting for the consult.
“It was stressed that at this moment there are also no reliable data on the course of atopic keratoconjunctivitis and vernal keratoconjunctivitis during dupilumab treatment,” according to Jacob P. Thyssen, MD, PhD, Herlev and Gentofte Hospital, Hellerup, Denmark, and coauthors. These patients “should be carefully monitored by an ophthalmologist before and during treatment with dupilumab.”
The recommendations also centered around which treatments should be initiated by dermatologists, and which should be referred to ophthalmologists. Those patients with conjunctivitis should receive eye drops, eye ointment, or oral antihistamines from their dermatologists before an ophthalmology referral, the IEC members said. Dermatologists also should perform, or refer patients for, skin prick testing or specific IgE testing for aeroallergens in patients with AD who have conjunctivitis, and patch testing with an “ophthalmologic series, and native eye drops/ointments to diagnose possible delayed type hypersensitivity reactions to topical ingredients,” they added.
Among the treatments for conjunctivitis best left to ophthalmologists are cyclosporine, tacrolimus, or corticosteroid eye drops.
“Despite the more limited experience with eye drops by dermatologists, rapid access to ophthalmological service may be difficult, sometimes warranting a short course of corticosteroid eye drops without ophthalmological consultations,” Dr. Thyssen and associates said. “However, persistent or recurrent conjunctivitis requiring repeated or prolonged use of corticosteroid, tacrolimus, and ciclosporin-containing eye drops, must be managed by an ophthalmologist, given the risk of glaucoma, cataract, and infections.”
“The AD severity, conjunctivitis severity, possible contraindications, possible effect of dupilumab therapy on concomitant asthma or other comorbidities, as well as other treatment options, should be considered on an individual patient basis,” the authors concluded.
The IEC survey was limited by the small survey response and reliance on expert opinion.
The authors reported personal and institutional relationships in the form of grants, corporate sponsorships, advisory board memberships, investigator appointments, speakers bureau positions, and consultancies for a variety of pharmaceutical companies, agencies, societies, and other organizations. No funding was obtained for the study.
This article was updated 7/17/19.
FROM THE JOURNAL OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Perinatal depression screening improves screening, treatment for postpartum depression
A policy of universal screening of perinatal depression for women receiving prenatal care at an academic medical center led to more regular screening of depression, and made it more likely that women with postpartum depression would be referred for treatment, according to recent research published in Obstetrics & Gynecology.
Emily S. Miller, MD, MPH, at Northwestern University, Chicago, and colleagues performed a retrospective study of 5,127 women receiving prenatal care at the center between 2008 and 2015. They divided the group into those who were at the center before (n = 1,122) and after (n = 4,005) initiation of a policy on universal perinatal depression screening, which consisted of two antenatal screenings at the first prenatal visit and third trimester, and one postpartum screening.
After initiation of the policy, screening increased during the first trimester (0.1% vs. 66%; P less than .001), the third trimester (0% vs. 43%; P less than .001), and at the postpartum visit (70% vs. 90%; P less than .001). Screening continued to increase at both prenatal visits, while screening prevalence remained the same for the postpartum visit. in the post-policy group (30% vs. 65%).
Katrina S. Mark, MD, associate professor of the department of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, said in an interview that the study “brings attention to an incredibly important topic.
“The researchers in this study found that, after implementation of a new policy regarding antenatal and postpartum depression screening, there was a significant increase in women who were screened during and after pregnancy as well as an increase in those who were appropriately treated,” she said. “Importantly, however, their intervention was not only a policy, but also provided education and resources to providers to increase awareness and knowledge surrounding the subject of depression and how to screen and treat this common condition.”
Dr. Miller and colleagues noted their study was limited because they were unable to determine whether prescriptions were filled or if referrals led to actual provider visits. Other obstacles to mental health care in the perinatal period also exist in the form of logistic barriers to appointments and stigma about mental health treatment.
“Depression is common, and screening and treatment during pregnancy and the postpartum period are extremely important to improve maternal and child health. As the authors point out, there has historically been a hesitation among obstetric providers to screen for depression,” Dr. Mark said. “My suspicion is that this hesitation is not because of a lack of awareness, but rather due to a lack of knowledge of what to do when a woman has a positive screen. In my opinion, the take-home message from this study is that implementation of a policy is possible and can lead to real change if it is accompanied by the appropriate resources and education.”
This study was funded by the Maternal-Fetal Medicine/Lumara Health Policy Award, and grants from the Eunice Kennedy Shriver National Institute of Child and Human Development and from the National Institutes of Health’s National Center for Advancing Translational Sciences. The authors reported no conflicts of interest.
SOURCE: Miller ES et al. Obstet Gynecol. 2019. doi: 10.1097/AOG.0000000000003369.
A policy of universal screening of perinatal depression for women receiving prenatal care at an academic medical center led to more regular screening of depression, and made it more likely that women with postpartum depression would be referred for treatment, according to recent research published in Obstetrics & Gynecology.
Emily S. Miller, MD, MPH, at Northwestern University, Chicago, and colleagues performed a retrospective study of 5,127 women receiving prenatal care at the center between 2008 and 2015. They divided the group into those who were at the center before (n = 1,122) and after (n = 4,005) initiation of a policy on universal perinatal depression screening, which consisted of two antenatal screenings at the first prenatal visit and third trimester, and one postpartum screening.
After initiation of the policy, screening increased during the first trimester (0.1% vs. 66%; P less than .001), the third trimester (0% vs. 43%; P less than .001), and at the postpartum visit (70% vs. 90%; P less than .001). Screening continued to increase at both prenatal visits, while screening prevalence remained the same for the postpartum visit. in the post-policy group (30% vs. 65%).
Katrina S. Mark, MD, associate professor of the department of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, said in an interview that the study “brings attention to an incredibly important topic.
“The researchers in this study found that, after implementation of a new policy regarding antenatal and postpartum depression screening, there was a significant increase in women who were screened during and after pregnancy as well as an increase in those who were appropriately treated,” she said. “Importantly, however, their intervention was not only a policy, but also provided education and resources to providers to increase awareness and knowledge surrounding the subject of depression and how to screen and treat this common condition.”
Dr. Miller and colleagues noted their study was limited because they were unable to determine whether prescriptions were filled or if referrals led to actual provider visits. Other obstacles to mental health care in the perinatal period also exist in the form of logistic barriers to appointments and stigma about mental health treatment.
“Depression is common, and screening and treatment during pregnancy and the postpartum period are extremely important to improve maternal and child health. As the authors point out, there has historically been a hesitation among obstetric providers to screen for depression,” Dr. Mark said. “My suspicion is that this hesitation is not because of a lack of awareness, but rather due to a lack of knowledge of what to do when a woman has a positive screen. In my opinion, the take-home message from this study is that implementation of a policy is possible and can lead to real change if it is accompanied by the appropriate resources and education.”
This study was funded by the Maternal-Fetal Medicine/Lumara Health Policy Award, and grants from the Eunice Kennedy Shriver National Institute of Child and Human Development and from the National Institutes of Health’s National Center for Advancing Translational Sciences. The authors reported no conflicts of interest.
SOURCE: Miller ES et al. Obstet Gynecol. 2019. doi: 10.1097/AOG.0000000000003369.
A policy of universal screening of perinatal depression for women receiving prenatal care at an academic medical center led to more regular screening of depression, and made it more likely that women with postpartum depression would be referred for treatment, according to recent research published in Obstetrics & Gynecology.
Emily S. Miller, MD, MPH, at Northwestern University, Chicago, and colleagues performed a retrospective study of 5,127 women receiving prenatal care at the center between 2008 and 2015. They divided the group into those who were at the center before (n = 1,122) and after (n = 4,005) initiation of a policy on universal perinatal depression screening, which consisted of two antenatal screenings at the first prenatal visit and third trimester, and one postpartum screening.
After initiation of the policy, screening increased during the first trimester (0.1% vs. 66%; P less than .001), the third trimester (0% vs. 43%; P less than .001), and at the postpartum visit (70% vs. 90%; P less than .001). Screening continued to increase at both prenatal visits, while screening prevalence remained the same for the postpartum visit. in the post-policy group (30% vs. 65%).
Katrina S. Mark, MD, associate professor of the department of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, said in an interview that the study “brings attention to an incredibly important topic.
“The researchers in this study found that, after implementation of a new policy regarding antenatal and postpartum depression screening, there was a significant increase in women who were screened during and after pregnancy as well as an increase in those who were appropriately treated,” she said. “Importantly, however, their intervention was not only a policy, but also provided education and resources to providers to increase awareness and knowledge surrounding the subject of depression and how to screen and treat this common condition.”
Dr. Miller and colleagues noted their study was limited because they were unable to determine whether prescriptions were filled or if referrals led to actual provider visits. Other obstacles to mental health care in the perinatal period also exist in the form of logistic barriers to appointments and stigma about mental health treatment.
“Depression is common, and screening and treatment during pregnancy and the postpartum period are extremely important to improve maternal and child health. As the authors point out, there has historically been a hesitation among obstetric providers to screen for depression,” Dr. Mark said. “My suspicion is that this hesitation is not because of a lack of awareness, but rather due to a lack of knowledge of what to do when a woman has a positive screen. In my opinion, the take-home message from this study is that implementation of a policy is possible and can lead to real change if it is accompanied by the appropriate resources and education.”
This study was funded by the Maternal-Fetal Medicine/Lumara Health Policy Award, and grants from the Eunice Kennedy Shriver National Institute of Child and Human Development and from the National Institutes of Health’s National Center for Advancing Translational Sciences. The authors reported no conflicts of interest.
SOURCE: Miller ES et al. Obstet Gynecol. 2019. doi: 10.1097/AOG.0000000000003369.
FROM OBSTETRICS & GYNECOLOGY
Key clinical point: A policy of universal perinatal screening improved adherence to screening and treatment for women with postpartum depression.
Major finding: After initiation of the policy, screening increased during the first prenatal visit (0.1% vs. 66%), the third trimester (0% vs. 43%), and at a postpartum visit (70% vs. 90%). Women who had a positive result after postpartum depression screening were more than twice as likely to receive treatment or a referral for their depression in the post-policy group (30% vs. 65%).
Study details: A retrospective cohort study of 5,127 women at a single academic center undergoing perinatal care before and after an institutional policy for perinatal depression screening between 2008 and 2015.
Disclosures: This study was funded by the Maternal-Fetal Medicine/Lumara Health Policy Award, and grants from the Eunice Kennedy Shriver National Institute of Child and Human Development and from the National Institutes of Health’s National Center for Advancing Translational Sciences. The authors reported no conflicts of interest.
Source: Miller ES et al. Obstet Gynecol. 2019. doi: 10.1097/AOG.0000000000003369.
Medication abortion with telemedicine is comparable to in-person visit
Patients who underwent medication abortion under the care of a clinician through a telemedicine service did not have any difference in outcomes, compared with patients who saw a clinician in person, according to a study in Obstetrics & Gynecology.
“To the extent that state bans on telemedicine for abortion rest on arguments of improved patient safety, the findings of this and previous studies do not support such contentions,” Julia E. Kohn, PhD, MPA, from Planned Parenthood Federation of America in New York and colleagues wrote.
Dr. Kohn, with colleagues from Ibis Reproductive Health, Bixby Center for Global Reproductive Health, and the University of California, San Francisco, assessed the outcomes of 5,952 patients who underwent medication abortion either through a telemedicine visit (738 patients) or in-person visit (5,214 patients). In the telemedicine group, the patients took mifepristone in view of the clinician over a secure videoconference platform followed by misoprostol 48 hours later as dispensed by a health center. Patients in the telemedicine group had a slightly older gestational age (50 days), compared with patients in the standard-care group (49 days).
Telemedicine patients received the same on-site care as those patients who saw a clinician in person, including informed consent, lab testing, and ultrasound scans. Patients who received care over telemedicine also received the same follow-up instructions as those who received standard of care, which consisted of an ultrasound evaluation 1-2 weeks after the visit, or human chorionic gonadotropin (hCG) testing.
While telemedicine patients were less likely to follow up at 45 days than were patients who received standard care (60% vs. 77%; prevalence ratio, 0.83; 95% confidence interval, 0.78-0.88), they also were less likely to have an ongoing pregnancy at follow-up (0.5% vs. 1.8%; adjusted odds ratio, 0.23; 95% CI, 0.14–0.39) or undergo an aspiration procedure (1% vs. 5%; aOR, 0.28; 95% CI, 0.17–0.46) than were standard-of-care patients. With regard to adverse events, the rate was less than 1% for each group, and the researchers reported no maternal deaths in either group.
Eve Espey, MD, MPH, professor and chair of the department of obstetrics and gynecology and director of the family planning fellowship at the University of New Mexico, Albuquerque, commented that this study expands the evidence of positive outcomes of telemedicine abortion to four new states: Alaska, Idaho, Nevada, and Washington.
“Abortion access is limited in the large rural states in which the study was conducted; across the country, abortion access is increasingly limited by restrictive legislation including telemedicine abortion bans,” she said in an interview. “This reassuring study helps demonstrate the safety of telemedicine medication abortion and highlights the role of telemedicine in improving health equity by increasing access to a critical health care service.”
The researchers said the results were limited in that most telemedicine care was centered in one state, Nevada, and the sample size was inadequate to do per-state comparisons of in-person visits and telemedicine. In addition, follow-up data was available for 75% of patients, which meant approximately one-fourth of patients did not follow up with the health center.
The Susan T. Buffett Foundation provided a grant for this study. Dr. Grossman receives consulting payments from Planned Parenthood Federation of America for work related to telemedicine for medication abortion. The other authors reported no relevant conflicts of interest.
SOURCE: Kohn JE et al. Obstet Gynecol. 2019. doi: 10.1097/AOG.0000000000003357.
Patients who underwent medication abortion under the care of a clinician through a telemedicine service did not have any difference in outcomes, compared with patients who saw a clinician in person, according to a study in Obstetrics & Gynecology.
“To the extent that state bans on telemedicine for abortion rest on arguments of improved patient safety, the findings of this and previous studies do not support such contentions,” Julia E. Kohn, PhD, MPA, from Planned Parenthood Federation of America in New York and colleagues wrote.
Dr. Kohn, with colleagues from Ibis Reproductive Health, Bixby Center for Global Reproductive Health, and the University of California, San Francisco, assessed the outcomes of 5,952 patients who underwent medication abortion either through a telemedicine visit (738 patients) or in-person visit (5,214 patients). In the telemedicine group, the patients took mifepristone in view of the clinician over a secure videoconference platform followed by misoprostol 48 hours later as dispensed by a health center. Patients in the telemedicine group had a slightly older gestational age (50 days), compared with patients in the standard-care group (49 days).
Telemedicine patients received the same on-site care as those patients who saw a clinician in person, including informed consent, lab testing, and ultrasound scans. Patients who received care over telemedicine also received the same follow-up instructions as those who received standard of care, which consisted of an ultrasound evaluation 1-2 weeks after the visit, or human chorionic gonadotropin (hCG) testing.
While telemedicine patients were less likely to follow up at 45 days than were patients who received standard care (60% vs. 77%; prevalence ratio, 0.83; 95% confidence interval, 0.78-0.88), they also were less likely to have an ongoing pregnancy at follow-up (0.5% vs. 1.8%; adjusted odds ratio, 0.23; 95% CI, 0.14–0.39) or undergo an aspiration procedure (1% vs. 5%; aOR, 0.28; 95% CI, 0.17–0.46) than were standard-of-care patients. With regard to adverse events, the rate was less than 1% for each group, and the researchers reported no maternal deaths in either group.
Eve Espey, MD, MPH, professor and chair of the department of obstetrics and gynecology and director of the family planning fellowship at the University of New Mexico, Albuquerque, commented that this study expands the evidence of positive outcomes of telemedicine abortion to four new states: Alaska, Idaho, Nevada, and Washington.
“Abortion access is limited in the large rural states in which the study was conducted; across the country, abortion access is increasingly limited by restrictive legislation including telemedicine abortion bans,” she said in an interview. “This reassuring study helps demonstrate the safety of telemedicine medication abortion and highlights the role of telemedicine in improving health equity by increasing access to a critical health care service.”
The researchers said the results were limited in that most telemedicine care was centered in one state, Nevada, and the sample size was inadequate to do per-state comparisons of in-person visits and telemedicine. In addition, follow-up data was available for 75% of patients, which meant approximately one-fourth of patients did not follow up with the health center.
The Susan T. Buffett Foundation provided a grant for this study. Dr. Grossman receives consulting payments from Planned Parenthood Federation of America for work related to telemedicine for medication abortion. The other authors reported no relevant conflicts of interest.
SOURCE: Kohn JE et al. Obstet Gynecol. 2019. doi: 10.1097/AOG.0000000000003357.
Patients who underwent medication abortion under the care of a clinician through a telemedicine service did not have any difference in outcomes, compared with patients who saw a clinician in person, according to a study in Obstetrics & Gynecology.
“To the extent that state bans on telemedicine for abortion rest on arguments of improved patient safety, the findings of this and previous studies do not support such contentions,” Julia E. Kohn, PhD, MPA, from Planned Parenthood Federation of America in New York and colleagues wrote.
Dr. Kohn, with colleagues from Ibis Reproductive Health, Bixby Center for Global Reproductive Health, and the University of California, San Francisco, assessed the outcomes of 5,952 patients who underwent medication abortion either through a telemedicine visit (738 patients) or in-person visit (5,214 patients). In the telemedicine group, the patients took mifepristone in view of the clinician over a secure videoconference platform followed by misoprostol 48 hours later as dispensed by a health center. Patients in the telemedicine group had a slightly older gestational age (50 days), compared with patients in the standard-care group (49 days).
Telemedicine patients received the same on-site care as those patients who saw a clinician in person, including informed consent, lab testing, and ultrasound scans. Patients who received care over telemedicine also received the same follow-up instructions as those who received standard of care, which consisted of an ultrasound evaluation 1-2 weeks after the visit, or human chorionic gonadotropin (hCG) testing.
While telemedicine patients were less likely to follow up at 45 days than were patients who received standard care (60% vs. 77%; prevalence ratio, 0.83; 95% confidence interval, 0.78-0.88), they also were less likely to have an ongoing pregnancy at follow-up (0.5% vs. 1.8%; adjusted odds ratio, 0.23; 95% CI, 0.14–0.39) or undergo an aspiration procedure (1% vs. 5%; aOR, 0.28; 95% CI, 0.17–0.46) than were standard-of-care patients. With regard to adverse events, the rate was less than 1% for each group, and the researchers reported no maternal deaths in either group.
Eve Espey, MD, MPH, professor and chair of the department of obstetrics and gynecology and director of the family planning fellowship at the University of New Mexico, Albuquerque, commented that this study expands the evidence of positive outcomes of telemedicine abortion to four new states: Alaska, Idaho, Nevada, and Washington.
“Abortion access is limited in the large rural states in which the study was conducted; across the country, abortion access is increasingly limited by restrictive legislation including telemedicine abortion bans,” she said in an interview. “This reassuring study helps demonstrate the safety of telemedicine medication abortion and highlights the role of telemedicine in improving health equity by increasing access to a critical health care service.”
The researchers said the results were limited in that most telemedicine care was centered in one state, Nevada, and the sample size was inadequate to do per-state comparisons of in-person visits and telemedicine. In addition, follow-up data was available for 75% of patients, which meant approximately one-fourth of patients did not follow up with the health center.
The Susan T. Buffett Foundation provided a grant for this study. Dr. Grossman receives consulting payments from Planned Parenthood Federation of America for work related to telemedicine for medication abortion. The other authors reported no relevant conflicts of interest.
SOURCE: Kohn JE et al. Obstet Gynecol. 2019. doi: 10.1097/AOG.0000000000003357.
FROM OBSTETRICS & GYNECOLOGY
Key clinical point:
Major finding: Telemedicine patients were less likely to have an ongoing pregnancy at follow-up (0.5% vs. 1.8%; adjusted odds ratio, 0.23; 95% confidence interval, 0.14–0.39) or undergo an aspiration procedure (1% vs. 5%; aOR, 0.28; 95% CI, 0.17–0.46) than standard-of-care patients.
Study details: A retrospective cohort study of 5,932 patients who underwent medical abortion at Planned Parenthood health centers in Alaska, Idaho, Nevada, and Washington.
Disclosures: The Susan T. Buffett Foundation provided a grant for this study. Dr. Grossman receives consulting payments from Planned Parenthood Federation of America for work related to telemedicine for medication abortion. The other authors reported no relevant conflicts of interest.
Source: Kohn JE et al. Obstet Gynecol. 2019. doi: 10.1097/AOG.0000000000003357.
Teen mothers using long-acting reversible contraception are least likely to use condoms
such as the birth control pill, vaginal ring, contraceptive patch, or injection, according to research in JAMA Pediatrics.
This highlights a need for education to lower the risk of sexually transmitted infections in this population.
“Our finding that less than 30% of sexually active teenage mothers using LARC or non-LARC hormonal methods also reported using condoms suggests the need for enhanced efforts to increase condom use among teenage mothers,” wrote Katherine Kortsmit, PhD, MPH, of the National Center for Chronic Disease Prevention and Health Promotion at the Centers for Disease Control and Prevention, Atlanta, and colleagues.
The researchers performed a cross-sectional analysis of contraceptive use among 5,480 new teenage mothers between 2012 and 2015 who were aged 19 years or younger in the Pregnancy Risk Assessment Monitoring System (PRAMS). Participants were mainly first-time teenage mothers between ages 18 and 19 years (46% non-Hispanic white), current Medicaid users, and reported an unintended pregnancy. Dr. Kortsmit and colleagues monitored use of LARC and non-LARC hormonal methods, including condom use, among participants in PRAMS from 37 different sites.
Among teenage mothers in PRAM, 29% reported using condoms; 18% of mothers using LARC said they also used condoms, compared with 36% of mothers who used non-LARC hormonal methods (adjusted prevalence ratio, 0.50; 95% confidence interval, 0.41-0.60). Participants with IUDs were least likely to report using condoms (15%), compared with participants using implants (22%; aPR, 0.70; 95% CI, 0.51-0.98), participants using the patch, ring, or injection (25%; aPR, 0.61; 95% CI, 0.47-0.79), or the pill (47%; aPR, 0.32; 95% CI, 0.25-0.40).
“These findings can be used to inform clinician counseling that sexually active teenage mothers have low uptake of condom use combined with more effective contraceptive methods and may need additional counseling on the importance of consistent and correct condom use for the prevention of STIs,” Dr. Kortsmit and associates wrote.
Limitations included the self-reported nature of the study, and lack of information on baseline condom use prior to pregnancy, relationship characteristics, and sexual partners during the postpartum period.
Education on contraceptive methods by clinicians is an important part of an adolescent’s contextualization of the benefits and risks of those methods, especially for women of color and marginalized groups, Andrea J. Hoopes, MD, MPH; and Gina S. Sucato, MD, MPH, wrote in an editorial related to the study by Kortsmit and colleagues.
In particular, these groups have higher rates of unplanned pregnancy, may have a history of being coerced to use contraception, and may be reluctant to discuss their sexual history or contraception use. “Many young women, including teenage mothers, remain at risk for coercion from partners, family members, and health care clinicians, so adopting a stance that ensures autonomy while eliciting unique developmental perspectives is paramount,” they said.
It is critically important to give women access to LARCs that are effective and easily used, and patients have a right to choose the contraception method that best fits their situation. It is through integrated programs, made available by Title X funding, that clinicians may be able to monitor their patients’ sexual, reproductive, and psychological health needs, and have conversations about the importance of contraception and prevention of sexually transmitted infections.
“Future studies should examine specific interventions aimed at promoting all adolescents’ motivations to remain safe and healthy by using condoms consistently and by seeking comprehensive sexual health care services, regardless of contraceptive method,” concluded Dr. Hoopes and Dr. Sucato, of the Adolescent Center at Kaiser Permanente Washington in Seattle. “In addition to receiving counseling about, and access to, condoms, adolescents need to develop the skills to negotiate condom use with partners.”
Dr. Kortsmit received support in the form of an appointment to the Research Participation Program at Centers for Disease Control and Prevention through an interagency agreement. The other authors reported no conflicts of interest.
Dr. Hoopes reported previous grant support from Bayer and the North American Society for Pediatric and Adolescent Gynecology. Dr. Sucato reported previous grant and other research support from Teva.
SOURCE: Kortsmit K et al. JAMA Pediatr. 2019. doi: 10.1001/jamapediatrics.2019.1136; Hoopes AJ et al. JAMA Pediatr. 2019. doi: 10.1001/jamapediatrics.2019.1133.
such as the birth control pill, vaginal ring, contraceptive patch, or injection, according to research in JAMA Pediatrics.
This highlights a need for education to lower the risk of sexually transmitted infections in this population.
“Our finding that less than 30% of sexually active teenage mothers using LARC or non-LARC hormonal methods also reported using condoms suggests the need for enhanced efforts to increase condom use among teenage mothers,” wrote Katherine Kortsmit, PhD, MPH, of the National Center for Chronic Disease Prevention and Health Promotion at the Centers for Disease Control and Prevention, Atlanta, and colleagues.
The researchers performed a cross-sectional analysis of contraceptive use among 5,480 new teenage mothers between 2012 and 2015 who were aged 19 years or younger in the Pregnancy Risk Assessment Monitoring System (PRAMS). Participants were mainly first-time teenage mothers between ages 18 and 19 years (46% non-Hispanic white), current Medicaid users, and reported an unintended pregnancy. Dr. Kortsmit and colleagues monitored use of LARC and non-LARC hormonal methods, including condom use, among participants in PRAMS from 37 different sites.
Among teenage mothers in PRAM, 29% reported using condoms; 18% of mothers using LARC said they also used condoms, compared with 36% of mothers who used non-LARC hormonal methods (adjusted prevalence ratio, 0.50; 95% confidence interval, 0.41-0.60). Participants with IUDs were least likely to report using condoms (15%), compared with participants using implants (22%; aPR, 0.70; 95% CI, 0.51-0.98), participants using the patch, ring, or injection (25%; aPR, 0.61; 95% CI, 0.47-0.79), or the pill (47%; aPR, 0.32; 95% CI, 0.25-0.40).
“These findings can be used to inform clinician counseling that sexually active teenage mothers have low uptake of condom use combined with more effective contraceptive methods and may need additional counseling on the importance of consistent and correct condom use for the prevention of STIs,” Dr. Kortsmit and associates wrote.
Limitations included the self-reported nature of the study, and lack of information on baseline condom use prior to pregnancy, relationship characteristics, and sexual partners during the postpartum period.
Education on contraceptive methods by clinicians is an important part of an adolescent’s contextualization of the benefits and risks of those methods, especially for women of color and marginalized groups, Andrea J. Hoopes, MD, MPH; and Gina S. Sucato, MD, MPH, wrote in an editorial related to the study by Kortsmit and colleagues.
In particular, these groups have higher rates of unplanned pregnancy, may have a history of being coerced to use contraception, and may be reluctant to discuss their sexual history or contraception use. “Many young women, including teenage mothers, remain at risk for coercion from partners, family members, and health care clinicians, so adopting a stance that ensures autonomy while eliciting unique developmental perspectives is paramount,” they said.
It is critically important to give women access to LARCs that are effective and easily used, and patients have a right to choose the contraception method that best fits their situation. It is through integrated programs, made available by Title X funding, that clinicians may be able to monitor their patients’ sexual, reproductive, and psychological health needs, and have conversations about the importance of contraception and prevention of sexually transmitted infections.
“Future studies should examine specific interventions aimed at promoting all adolescents’ motivations to remain safe and healthy by using condoms consistently and by seeking comprehensive sexual health care services, regardless of contraceptive method,” concluded Dr. Hoopes and Dr. Sucato, of the Adolescent Center at Kaiser Permanente Washington in Seattle. “In addition to receiving counseling about, and access to, condoms, adolescents need to develop the skills to negotiate condom use with partners.”
Dr. Kortsmit received support in the form of an appointment to the Research Participation Program at Centers for Disease Control and Prevention through an interagency agreement. The other authors reported no conflicts of interest.
Dr. Hoopes reported previous grant support from Bayer and the North American Society for Pediatric and Adolescent Gynecology. Dr. Sucato reported previous grant and other research support from Teva.
SOURCE: Kortsmit K et al. JAMA Pediatr. 2019. doi: 10.1001/jamapediatrics.2019.1136; Hoopes AJ et al. JAMA Pediatr. 2019. doi: 10.1001/jamapediatrics.2019.1133.
such as the birth control pill, vaginal ring, contraceptive patch, or injection, according to research in JAMA Pediatrics.
This highlights a need for education to lower the risk of sexually transmitted infections in this population.
“Our finding that less than 30% of sexually active teenage mothers using LARC or non-LARC hormonal methods also reported using condoms suggests the need for enhanced efforts to increase condom use among teenage mothers,” wrote Katherine Kortsmit, PhD, MPH, of the National Center for Chronic Disease Prevention and Health Promotion at the Centers for Disease Control and Prevention, Atlanta, and colleagues.
The researchers performed a cross-sectional analysis of contraceptive use among 5,480 new teenage mothers between 2012 and 2015 who were aged 19 years or younger in the Pregnancy Risk Assessment Monitoring System (PRAMS). Participants were mainly first-time teenage mothers between ages 18 and 19 years (46% non-Hispanic white), current Medicaid users, and reported an unintended pregnancy. Dr. Kortsmit and colleagues monitored use of LARC and non-LARC hormonal methods, including condom use, among participants in PRAMS from 37 different sites.
Among teenage mothers in PRAM, 29% reported using condoms; 18% of mothers using LARC said they also used condoms, compared with 36% of mothers who used non-LARC hormonal methods (adjusted prevalence ratio, 0.50; 95% confidence interval, 0.41-0.60). Participants with IUDs were least likely to report using condoms (15%), compared with participants using implants (22%; aPR, 0.70; 95% CI, 0.51-0.98), participants using the patch, ring, or injection (25%; aPR, 0.61; 95% CI, 0.47-0.79), or the pill (47%; aPR, 0.32; 95% CI, 0.25-0.40).
“These findings can be used to inform clinician counseling that sexually active teenage mothers have low uptake of condom use combined with more effective contraceptive methods and may need additional counseling on the importance of consistent and correct condom use for the prevention of STIs,” Dr. Kortsmit and associates wrote.
Limitations included the self-reported nature of the study, and lack of information on baseline condom use prior to pregnancy, relationship characteristics, and sexual partners during the postpartum period.
Education on contraceptive methods by clinicians is an important part of an adolescent’s contextualization of the benefits and risks of those methods, especially for women of color and marginalized groups, Andrea J. Hoopes, MD, MPH; and Gina S. Sucato, MD, MPH, wrote in an editorial related to the study by Kortsmit and colleagues.
In particular, these groups have higher rates of unplanned pregnancy, may have a history of being coerced to use contraception, and may be reluctant to discuss their sexual history or contraception use. “Many young women, including teenage mothers, remain at risk for coercion from partners, family members, and health care clinicians, so adopting a stance that ensures autonomy while eliciting unique developmental perspectives is paramount,” they said.
It is critically important to give women access to LARCs that are effective and easily used, and patients have a right to choose the contraception method that best fits their situation. It is through integrated programs, made available by Title X funding, that clinicians may be able to monitor their patients’ sexual, reproductive, and psychological health needs, and have conversations about the importance of contraception and prevention of sexually transmitted infections.
“Future studies should examine specific interventions aimed at promoting all adolescents’ motivations to remain safe and healthy by using condoms consistently and by seeking comprehensive sexual health care services, regardless of contraceptive method,” concluded Dr. Hoopes and Dr. Sucato, of the Adolescent Center at Kaiser Permanente Washington in Seattle. “In addition to receiving counseling about, and access to, condoms, adolescents need to develop the skills to negotiate condom use with partners.”
Dr. Kortsmit received support in the form of an appointment to the Research Participation Program at Centers for Disease Control and Prevention through an interagency agreement. The other authors reported no conflicts of interest.
Dr. Hoopes reported previous grant support from Bayer and the North American Society for Pediatric and Adolescent Gynecology. Dr. Sucato reported previous grant and other research support from Teva.
SOURCE: Kortsmit K et al. JAMA Pediatr. 2019. doi: 10.1001/jamapediatrics.2019.1136; Hoopes AJ et al. JAMA Pediatr. 2019. doi: 10.1001/jamapediatrics.2019.1133.
FROM JAMA PEDIATRICS
CDC: Look for early symptoms of acute flaccid myelitis, report suspected cases
the CDC said in a telebriefing.
Acute flaccid myelitis (AFM) is defined as acute, flaccid muscle weakness that occurs less than 1 week after a fever or respiratory illness. Viruses, including enterovirus, are believed to play a role in AFM, but the cause still is unknown. The disease appears mostly in children, and the average age of a patient diagnosed with AFM is 5 years.
“Doctors and other clinicians in the United States play a critical role,” Anne Schuchat, MD, principal deputy director of the Centers for Disease Control and Prevention, said in the telebriefing. “We ask for your help with early recognition of patients with AFM symptoms, prompt specimen collection for testing, and immediate reporting of suspected AFM cases to health departments.”
While there is no proven treatment for AFM, early diagnosis is critical to getting patients the best care possible, according to a Vital Signs report released today. This means that clinicians should not wait for the CDC’s case definition before diagnosis, the CDC said.
“When specimens are collected as soon as possible after symptom onset, we have a better chance of understanding the causes of AFM, these recurrent outbreaks, and developing a diagnostic test,” Dr. Schuchat said. “Rapid reporting also helps us to identify and respond to outbreaks early and alert other clinicians and the public.”
AFM appears to follow a seasonal and biennial pattern, with the number of cases increasing mainly in the late summer and early fall. As the season approaches where AFM cases increase, CDC is asking clinicians to look out for patients with suspected AFM so cases can be reported as early as possible.
Since the CDC began tracking AFM, the number of cases has risen every 2 years. In 2018, there were 233 cases in 41 states, the highest number of reported cases since the CDC began tracking AFM following an outbreak in 2014, according to a Vital Signs report. Overall, there have been 570 cases of AFM reported in 48 states and the District of Columbia since 2014.
There is yet to be a confirmatory test for AFM, but clinicians should obtain cerebrospinal fluid, serum, stool and nasopharyngeal swab from patients with suspected AFM as soon as possible, followed by an MRI. AFM has unique MRI features , such as gray matter involvement, that can help distinguish it from other diseases characterized by acute weakness.
In the Vital Signs report, which examined AFM in 2018, 92% of confirmed cases had respiratory symptoms or fever, and 42% of confirmed cases had upper limb involvement. The median time from limb weakness to hospitalization was 1 day, and time from weakness to MRI was 2 days. Cases were reported to the CDC a median of 18 days from onset of limb weakness, but time to reporting ranged between 18 days and 36 days, said Tom Clark, MD, MPH, deputy director of the division of viral diseases at CDC.
“This delay hampers our ability to understand the causes AFM,” he said. “We believe that recognizing AFM early is critical and can lead to better patient management.”
In lieu of a diagnostic test for AFM, clinicians should make management decisions through review of patient symptoms, exam findings, MRI, other test results, and in consulting with neurology experts. The Transverse Myelitis Association also has created a support portal for 24/7 physician consultation in AFM cases.
SOURCE: Lopez A et al. MMWR Morb Mortal Wkly Rep. 2019;68:1-7 .
the CDC said in a telebriefing.

Acute flaccid myelitis (AFM) is defined as acute, flaccid muscle weakness that occurs less than 1 week after a fever or respiratory illness. Viruses, including enterovirus, are believed to play a role in AFM, but the cause still is unknown. The disease appears mostly in children, and the average age of a patient diagnosed with AFM is 5 years.
“Doctors and other clinicians in the United States play a critical role,” Anne Schuchat, MD, principal deputy director of the Centers for Disease Control and Prevention, said in the telebriefing. “We ask for your help with early recognition of patients with AFM symptoms, prompt specimen collection for testing, and immediate reporting of suspected AFM cases to health departments.”
While there is no proven treatment for AFM, early diagnosis is critical to getting patients the best care possible, according to a Vital Signs report released today. This means that clinicians should not wait for the CDC’s case definition before diagnosis, the CDC said.
“When specimens are collected as soon as possible after symptom onset, we have a better chance of understanding the causes of AFM, these recurrent outbreaks, and developing a diagnostic test,” Dr. Schuchat said. “Rapid reporting also helps us to identify and respond to outbreaks early and alert other clinicians and the public.”
AFM appears to follow a seasonal and biennial pattern, with the number of cases increasing mainly in the late summer and early fall. As the season approaches where AFM cases increase, CDC is asking clinicians to look out for patients with suspected AFM so cases can be reported as early as possible.
Since the CDC began tracking AFM, the number of cases has risen every 2 years. In 2018, there were 233 cases in 41 states, the highest number of reported cases since the CDC began tracking AFM following an outbreak in 2014, according to a Vital Signs report. Overall, there have been 570 cases of AFM reported in 48 states and the District of Columbia since 2014.
There is yet to be a confirmatory test for AFM, but clinicians should obtain cerebrospinal fluid, serum, stool and nasopharyngeal swab from patients with suspected AFM as soon as possible, followed by an MRI. AFM has unique MRI features , such as gray matter involvement, that can help distinguish it from other diseases characterized by acute weakness.
In the Vital Signs report, which examined AFM in 2018, 92% of confirmed cases had respiratory symptoms or fever, and 42% of confirmed cases had upper limb involvement. The median time from limb weakness to hospitalization was 1 day, and time from weakness to MRI was 2 days. Cases were reported to the CDC a median of 18 days from onset of limb weakness, but time to reporting ranged between 18 days and 36 days, said Tom Clark, MD, MPH, deputy director of the division of viral diseases at CDC.
“This delay hampers our ability to understand the causes AFM,” he said. “We believe that recognizing AFM early is critical and can lead to better patient management.”
In lieu of a diagnostic test for AFM, clinicians should make management decisions through review of patient symptoms, exam findings, MRI, other test results, and in consulting with neurology experts. The Transverse Myelitis Association also has created a support portal for 24/7 physician consultation in AFM cases.
SOURCE: Lopez A et al. MMWR Morb Mortal Wkly Rep. 2019;68:1-7 .
the CDC said in a telebriefing.

Acute flaccid myelitis (AFM) is defined as acute, flaccid muscle weakness that occurs less than 1 week after a fever or respiratory illness. Viruses, including enterovirus, are believed to play a role in AFM, but the cause still is unknown. The disease appears mostly in children, and the average age of a patient diagnosed with AFM is 5 years.
“Doctors and other clinicians in the United States play a critical role,” Anne Schuchat, MD, principal deputy director of the Centers for Disease Control and Prevention, said in the telebriefing. “We ask for your help with early recognition of patients with AFM symptoms, prompt specimen collection for testing, and immediate reporting of suspected AFM cases to health departments.”
While there is no proven treatment for AFM, early diagnosis is critical to getting patients the best care possible, according to a Vital Signs report released today. This means that clinicians should not wait for the CDC’s case definition before diagnosis, the CDC said.
“When specimens are collected as soon as possible after symptom onset, we have a better chance of understanding the causes of AFM, these recurrent outbreaks, and developing a diagnostic test,” Dr. Schuchat said. “Rapid reporting also helps us to identify and respond to outbreaks early and alert other clinicians and the public.”
AFM appears to follow a seasonal and biennial pattern, with the number of cases increasing mainly in the late summer and early fall. As the season approaches where AFM cases increase, CDC is asking clinicians to look out for patients with suspected AFM so cases can be reported as early as possible.
Since the CDC began tracking AFM, the number of cases has risen every 2 years. In 2018, there were 233 cases in 41 states, the highest number of reported cases since the CDC began tracking AFM following an outbreak in 2014, according to a Vital Signs report. Overall, there have been 570 cases of AFM reported in 48 states and the District of Columbia since 2014.
There is yet to be a confirmatory test for AFM, but clinicians should obtain cerebrospinal fluid, serum, stool and nasopharyngeal swab from patients with suspected AFM as soon as possible, followed by an MRI. AFM has unique MRI features , such as gray matter involvement, that can help distinguish it from other diseases characterized by acute weakness.
In the Vital Signs report, which examined AFM in 2018, 92% of confirmed cases had respiratory symptoms or fever, and 42% of confirmed cases had upper limb involvement. The median time from limb weakness to hospitalization was 1 day, and time from weakness to MRI was 2 days. Cases were reported to the CDC a median of 18 days from onset of limb weakness, but time to reporting ranged between 18 days and 36 days, said Tom Clark, MD, MPH, deputy director of the division of viral diseases at CDC.
“This delay hampers our ability to understand the causes AFM,” he said. “We believe that recognizing AFM early is critical and can lead to better patient management.”
In lieu of a diagnostic test for AFM, clinicians should make management decisions through review of patient symptoms, exam findings, MRI, other test results, and in consulting with neurology experts. The Transverse Myelitis Association also has created a support portal for 24/7 physician consultation in AFM cases.
SOURCE: Lopez A et al. MMWR Morb Mortal Wkly Rep. 2019;68:1-7 .
NEWS FROM THE FDA/CDC
Sicker COPD patients may be more likely to initiate arformoterol
according to study to identify predictors of its use. In addition to being sicker, being treated by a pulmonologist rather than a primary care physician and being white were factors that increased a patient’s likelihood of receiving nebulized arformoterol.
Patients less likely to receive the nebulized version of this long-acting beta2 adrenoreceptor agonist (LABA) were African Americans, patients with psychiatric comorbidities, and patients eligible for both Medicare and Medicaid.
“Studies have shown that 40% to 71% of Medicare beneficiaries receive no maintenance treatment for COPD. Although a recent longitudinal study on Medicare populations reported that use of maintenance medications has been improving, in general, it is recognized that Medicare beneficiaries with COPD remain undertreated,” Todd P. Gilmer, PhD, from the department of family medicine and public health at the University of California, San Diego, and colleagues wrote.
The investigators identified patients with COPD using Medicare administrative data; of these patients, 11,887 were arformoterol users, and 450,178 were control patients who did not use arformoterol. Patients were included in the study if they had at least one claim for COPD medication and were continuously enrolled in Medicare Parts A, B, and D. The cohort consisted of mostly white women aged 70 years or older, and 47% were dual-eligible to receive both Medicare and Medicaid benefits. A subgroup of 1,778 arformoterol users were also identified for analysis who were hospitalized and discharged within 30 days of using arformoterol, as well as a subgroup of 21,910 control patients with hospitalizations.
The researchers found COPD-related hospitalization (odds ratio, 1.31; 95% confidence interval, 1.24-1.39; P less than .001), exacerbation (OR, 1.33; 95% CI, 1.26-1.41; P less than .001), use of a systemic corticosteroid (OR, 1.50; 95% CI, 1.43-1.57; P less than .001) or methylxanthine (OR, 1.37; 95% CI, 1.28-1.47; P less than .001), use of oxygen therapy (OR, 2.01; 95% CI, 1.93-2.09; P less than .001), pulmonologist care (OR, 1.40; 95% CI, 1.34-1.46; P less than .001), and respiratory therapist care (OR, 1.23; 95% CI, 1.11-1.36; P less than .001) strongly predicted arformoterol use, while racial/ethnic minority status, psychiatric comorbidity (OR, 0.65; 95% CI, 0.56-0.76; P less than .001), acquired immune deficiency syndrome (OR, 0.69; 95% CI, 0.52-0.94; P less than .01), and dual-eligibility for Medicare and Medicaid (OR, 0.73; 95% CI, 0.70-0.77; P less than .001) lowered the odds of arformoterol use (P less than .001). In the subgroup of patients with hospitalizations, COPD-related admission (OR, 1.83; 95% CI, 1.55-2.14; P less than .001), exacerbation (OR, 2.62; 95% CI, 1.88-3.63; P less than .001)m and inpatient care from a pulmonologist (OR, 1.78; 95% CI, 1.58-2.01; P less than .001) predicted arformoterol use.
“Given the results of this study, increasing access to nebulized maintenance therapy is warranted for select populations with COPD including racial/ethnic minorities, the dual-eligible, and those with certain comorbidities, such as psychiatric disorders,” Dr. Gilmer and colleagues wrote in their study. “Future studies are needed to explore the optimal time to initiate nebulized maintenance therapy, and the potential differential impact of early versus late initiation on patient outcomes.”
Researchers noted that, although their results may seem initially counterintuitive given that COPD has a higher prevalence in men, 56% of the beneficiaries in their Medicare data were women who were 65 years or older, and the results are consistent with other studies that show similar gender distribution findings for maintenance treatment patterns among COPD patients receiving Medicare.
“Since most Medicare beneficiaries with COPD are older than 70 years of age, the higher percentage of women than men in our two cohorts can be explained by the age distributions that ensued as a result of applying our various inclusion and exclusion criteria,” they said.
This study was funded by Sunovian. Dr. Gilmer and one coauthor are paid employees of University of California San Diego, which receives research funding from Advance Health Solutions. Another coauthor is an advisory board member for Advance Health Solutions and a consultant for GlaxoSmithKline, Boehringer-Ingelheim, Astra Zeneca, Novartis, and Pulmonix. Two other coauthors are paid employees of Advance Health Solutions, and another is a paid employee of Sunovion.
SOURCE: Gilmer TP et al. COPD. 2019 Jun 19. doi: 10.1080/15412555.2019.1618256.
according to study to identify predictors of its use. In addition to being sicker, being treated by a pulmonologist rather than a primary care physician and being white were factors that increased a patient’s likelihood of receiving nebulized arformoterol.
Patients less likely to receive the nebulized version of this long-acting beta2 adrenoreceptor agonist (LABA) were African Americans, patients with psychiatric comorbidities, and patients eligible for both Medicare and Medicaid.
“Studies have shown that 40% to 71% of Medicare beneficiaries receive no maintenance treatment for COPD. Although a recent longitudinal study on Medicare populations reported that use of maintenance medications has been improving, in general, it is recognized that Medicare beneficiaries with COPD remain undertreated,” Todd P. Gilmer, PhD, from the department of family medicine and public health at the University of California, San Diego, and colleagues wrote.
The investigators identified patients with COPD using Medicare administrative data; of these patients, 11,887 were arformoterol users, and 450,178 were control patients who did not use arformoterol. Patients were included in the study if they had at least one claim for COPD medication and were continuously enrolled in Medicare Parts A, B, and D. The cohort consisted of mostly white women aged 70 years or older, and 47% were dual-eligible to receive both Medicare and Medicaid benefits. A subgroup of 1,778 arformoterol users were also identified for analysis who were hospitalized and discharged within 30 days of using arformoterol, as well as a subgroup of 21,910 control patients with hospitalizations.
The researchers found COPD-related hospitalization (odds ratio, 1.31; 95% confidence interval, 1.24-1.39; P less than .001), exacerbation (OR, 1.33; 95% CI, 1.26-1.41; P less than .001), use of a systemic corticosteroid (OR, 1.50; 95% CI, 1.43-1.57; P less than .001) or methylxanthine (OR, 1.37; 95% CI, 1.28-1.47; P less than .001), use of oxygen therapy (OR, 2.01; 95% CI, 1.93-2.09; P less than .001), pulmonologist care (OR, 1.40; 95% CI, 1.34-1.46; P less than .001), and respiratory therapist care (OR, 1.23; 95% CI, 1.11-1.36; P less than .001) strongly predicted arformoterol use, while racial/ethnic minority status, psychiatric comorbidity (OR, 0.65; 95% CI, 0.56-0.76; P less than .001), acquired immune deficiency syndrome (OR, 0.69; 95% CI, 0.52-0.94; P less than .01), and dual-eligibility for Medicare and Medicaid (OR, 0.73; 95% CI, 0.70-0.77; P less than .001) lowered the odds of arformoterol use (P less than .001). In the subgroup of patients with hospitalizations, COPD-related admission (OR, 1.83; 95% CI, 1.55-2.14; P less than .001), exacerbation (OR, 2.62; 95% CI, 1.88-3.63; P less than .001)m and inpatient care from a pulmonologist (OR, 1.78; 95% CI, 1.58-2.01; P less than .001) predicted arformoterol use.
“Given the results of this study, increasing access to nebulized maintenance therapy is warranted for select populations with COPD including racial/ethnic minorities, the dual-eligible, and those with certain comorbidities, such as psychiatric disorders,” Dr. Gilmer and colleagues wrote in their study. “Future studies are needed to explore the optimal time to initiate nebulized maintenance therapy, and the potential differential impact of early versus late initiation on patient outcomes.”
Researchers noted that, although their results may seem initially counterintuitive given that COPD has a higher prevalence in men, 56% of the beneficiaries in their Medicare data were women who were 65 years or older, and the results are consistent with other studies that show similar gender distribution findings for maintenance treatment patterns among COPD patients receiving Medicare.
“Since most Medicare beneficiaries with COPD are older than 70 years of age, the higher percentage of women than men in our two cohorts can be explained by the age distributions that ensued as a result of applying our various inclusion and exclusion criteria,” they said.
This study was funded by Sunovian. Dr. Gilmer and one coauthor are paid employees of University of California San Diego, which receives research funding from Advance Health Solutions. Another coauthor is an advisory board member for Advance Health Solutions and a consultant for GlaxoSmithKline, Boehringer-Ingelheim, Astra Zeneca, Novartis, and Pulmonix. Two other coauthors are paid employees of Advance Health Solutions, and another is a paid employee of Sunovion.
SOURCE: Gilmer TP et al. COPD. 2019 Jun 19. doi: 10.1080/15412555.2019.1618256.
according to study to identify predictors of its use. In addition to being sicker, being treated by a pulmonologist rather than a primary care physician and being white were factors that increased a patient’s likelihood of receiving nebulized arformoterol.
Patients less likely to receive the nebulized version of this long-acting beta2 adrenoreceptor agonist (LABA) were African Americans, patients with psychiatric comorbidities, and patients eligible for both Medicare and Medicaid.
“Studies have shown that 40% to 71% of Medicare beneficiaries receive no maintenance treatment for COPD. Although a recent longitudinal study on Medicare populations reported that use of maintenance medications has been improving, in general, it is recognized that Medicare beneficiaries with COPD remain undertreated,” Todd P. Gilmer, PhD, from the department of family medicine and public health at the University of California, San Diego, and colleagues wrote.
The investigators identified patients with COPD using Medicare administrative data; of these patients, 11,887 were arformoterol users, and 450,178 were control patients who did not use arformoterol. Patients were included in the study if they had at least one claim for COPD medication and were continuously enrolled in Medicare Parts A, B, and D. The cohort consisted of mostly white women aged 70 years or older, and 47% were dual-eligible to receive both Medicare and Medicaid benefits. A subgroup of 1,778 arformoterol users were also identified for analysis who were hospitalized and discharged within 30 days of using arformoterol, as well as a subgroup of 21,910 control patients with hospitalizations.
The researchers found COPD-related hospitalization (odds ratio, 1.31; 95% confidence interval, 1.24-1.39; P less than .001), exacerbation (OR, 1.33; 95% CI, 1.26-1.41; P less than .001), use of a systemic corticosteroid (OR, 1.50; 95% CI, 1.43-1.57; P less than .001) or methylxanthine (OR, 1.37; 95% CI, 1.28-1.47; P less than .001), use of oxygen therapy (OR, 2.01; 95% CI, 1.93-2.09; P less than .001), pulmonologist care (OR, 1.40; 95% CI, 1.34-1.46; P less than .001), and respiratory therapist care (OR, 1.23; 95% CI, 1.11-1.36; P less than .001) strongly predicted arformoterol use, while racial/ethnic minority status, psychiatric comorbidity (OR, 0.65; 95% CI, 0.56-0.76; P less than .001), acquired immune deficiency syndrome (OR, 0.69; 95% CI, 0.52-0.94; P less than .01), and dual-eligibility for Medicare and Medicaid (OR, 0.73; 95% CI, 0.70-0.77; P less than .001) lowered the odds of arformoterol use (P less than .001). In the subgroup of patients with hospitalizations, COPD-related admission (OR, 1.83; 95% CI, 1.55-2.14; P less than .001), exacerbation (OR, 2.62; 95% CI, 1.88-3.63; P less than .001)m and inpatient care from a pulmonologist (OR, 1.78; 95% CI, 1.58-2.01; P less than .001) predicted arformoterol use.
“Given the results of this study, increasing access to nebulized maintenance therapy is warranted for select populations with COPD including racial/ethnic minorities, the dual-eligible, and those with certain comorbidities, such as psychiatric disorders,” Dr. Gilmer and colleagues wrote in their study. “Future studies are needed to explore the optimal time to initiate nebulized maintenance therapy, and the potential differential impact of early versus late initiation on patient outcomes.”
Researchers noted that, although their results may seem initially counterintuitive given that COPD has a higher prevalence in men, 56% of the beneficiaries in their Medicare data were women who were 65 years or older, and the results are consistent with other studies that show similar gender distribution findings for maintenance treatment patterns among COPD patients receiving Medicare.
“Since most Medicare beneficiaries with COPD are older than 70 years of age, the higher percentage of women than men in our two cohorts can be explained by the age distributions that ensued as a result of applying our various inclusion and exclusion criteria,” they said.
This study was funded by Sunovian. Dr. Gilmer and one coauthor are paid employees of University of California San Diego, which receives research funding from Advance Health Solutions. Another coauthor is an advisory board member for Advance Health Solutions and a consultant for GlaxoSmithKline, Boehringer-Ingelheim, Astra Zeneca, Novartis, and Pulmonix. Two other coauthors are paid employees of Advance Health Solutions, and another is a paid employee of Sunovion.
SOURCE: Gilmer TP et al. COPD. 2019 Jun 19. doi: 10.1080/15412555.2019.1618256.
FROM COPD: JOURNAL OF CHRONIC OBSTRUCTIVE PULMONARY DISEASE