User login
ACOG recommends that annual screening mammograms begin at age 40
Annual mammography screening should be offered to women when they reach 40 years, says a new Practice Bulletin on breast cancer screening from ACOG.1 Previous ACOG guidelines recommended mammograms every 1 to 2 years starting at age 40 and annually beginning at age 50.
According to Jennifer Griffin, MD, MPH, who coauthored the ACOG guidelines, the change in mammography screening for women beginning at age 40 is based on three variables:
- Incidence of breast cancer. The malignancy remains the second-leading cause of all cancer-related deaths.
- “Sojourn time” for breast cancer growth (the time it takes for a tumor that is identifiable by mammography to grow big enough to cause symptoms)
- Potential to reduce the number of deaths from breast cancer.
Although the sojourn time of individual cancers varies, the greatest predictor is age. Women 40 to 49 years old have the shortest average sojourn time (2–2.4 years), whereas women 70 to 74 years old have the longest average sojourn time (4–4.1 years).
“Although women in their 40s have a lower overall incidence of breast cancer compared with older women, the window to detect tumors before they become symptomatic is shorter, on average,” said Dr. Griffin. The 5-year survival rate is 98% for women whose breast cancer tumors are discovered at their earliest stage, before they are palpable and when they are small and confined to the breast. “If women in their 40s have annual mammograms, there is a better chance of detecting and treating the cancer before it has time to spread than if they wait 2 years between mammograms.”
The College continues to recommend annual clinical breast exams (CBE) for women 40 years and older, and CBE every 1 to 3 years for women ages 20 to 39. Additionally, ACOG encourages “breast self-awareness” for women 20 years and older. Breast self-awareness is a woman’s understanding of the normal appearance and feel of her breasts.
“The goal here is for women to be alert to any changes, no matter how small, in their breasts, and report them to their doctor,” said Dr. Griffin. “Although we’ve moved away from routinely recommending [breast self-exams], some women will want to continue doing them and that’s OK.”
Enhanced breast cancer screening, such as more frequent CBEs, annual magnetic resonance imaging (MRI), or mammograms before 40 years of age, may be recommended for women at high risk of breast cancer. Breast MRI is not recommended for women at average risk.
The “downside” of annual screening
“More frequent screening is associated with more false-positive findings,” says Andrew M. Kaunitz, MD, professor and associate chairman of obstetrics and gynecology at the University of Florida College of Medicine in Jacksonville, Fla.
“Due to the high rate of false-positive screens and the large number of screening mammograms needed to prevent the death of one woman in her 40s from breast cancer, the US Preventive Services Task Force recommended in late 2009 that routine screening mammography be deferred until age 50 and that screens be biennial,” he explains.2
“The new ACOG guidance acknowledges these concerns, as well as the potential for anxiety associated with false-positive findings. However, ACOG clearly concluded that, in general, US women cope well with such anxiety.
“When discussing mammograms with women in their 40s, it’s appropriate to acknowledge the high rate of false positives in this age group, which may lead to additional imaging, as well as biopsies,” Dr. Kaunitz suggests. “In my view, we can best serve our patients by making recommendations regarding screening mammograms in a manner that is sensitive to women’s personal values as they attempt to weigh the benefits of earlier and more frequent screening against the risks associated with false-positive screens.”
We want to hear from you! Tell us what you think.
1. American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG Practice Bulletin No. 122: Breast cancer screening. Obstet Gynecol. 2011;118(2 Part 1):372-382.
2. Nelson HD, Tyne K, Naik A, Bougatsos C, Chan BK, Humphrey L. US Preventive Services Task Force. Screening for breast cancer: an update for the US Preventive Services Task Force. Ann Intern Med. 2009;151(10):727-737.
Annual mammography screening should be offered to women when they reach 40 years, says a new Practice Bulletin on breast cancer screening from ACOG.1 Previous ACOG guidelines recommended mammograms every 1 to 2 years starting at age 40 and annually beginning at age 50.
According to Jennifer Griffin, MD, MPH, who coauthored the ACOG guidelines, the change in mammography screening for women beginning at age 40 is based on three variables:
- Incidence of breast cancer. The malignancy remains the second-leading cause of all cancer-related deaths.
- “Sojourn time” for breast cancer growth (the time it takes for a tumor that is identifiable by mammography to grow big enough to cause symptoms)
- Potential to reduce the number of deaths from breast cancer.
Although the sojourn time of individual cancers varies, the greatest predictor is age. Women 40 to 49 years old have the shortest average sojourn time (2–2.4 years), whereas women 70 to 74 years old have the longest average sojourn time (4–4.1 years).
“Although women in their 40s have a lower overall incidence of breast cancer compared with older women, the window to detect tumors before they become symptomatic is shorter, on average,” said Dr. Griffin. The 5-year survival rate is 98% for women whose breast cancer tumors are discovered at their earliest stage, before they are palpable and when they are small and confined to the breast. “If women in their 40s have annual mammograms, there is a better chance of detecting and treating the cancer before it has time to spread than if they wait 2 years between mammograms.”
The College continues to recommend annual clinical breast exams (CBE) for women 40 years and older, and CBE every 1 to 3 years for women ages 20 to 39. Additionally, ACOG encourages “breast self-awareness” for women 20 years and older. Breast self-awareness is a woman’s understanding of the normal appearance and feel of her breasts.
“The goal here is for women to be alert to any changes, no matter how small, in their breasts, and report them to their doctor,” said Dr. Griffin. “Although we’ve moved away from routinely recommending [breast self-exams], some women will want to continue doing them and that’s OK.”
Enhanced breast cancer screening, such as more frequent CBEs, annual magnetic resonance imaging (MRI), or mammograms before 40 years of age, may be recommended for women at high risk of breast cancer. Breast MRI is not recommended for women at average risk.
The “downside” of annual screening
“More frequent screening is associated with more false-positive findings,” says Andrew M. Kaunitz, MD, professor and associate chairman of obstetrics and gynecology at the University of Florida College of Medicine in Jacksonville, Fla.
“Due to the high rate of false-positive screens and the large number of screening mammograms needed to prevent the death of one woman in her 40s from breast cancer, the US Preventive Services Task Force recommended in late 2009 that routine screening mammography be deferred until age 50 and that screens be biennial,” he explains.2
“The new ACOG guidance acknowledges these concerns, as well as the potential for anxiety associated with false-positive findings. However, ACOG clearly concluded that, in general, US women cope well with such anxiety.
“When discussing mammograms with women in their 40s, it’s appropriate to acknowledge the high rate of false positives in this age group, which may lead to additional imaging, as well as biopsies,” Dr. Kaunitz suggests. “In my view, we can best serve our patients by making recommendations regarding screening mammograms in a manner that is sensitive to women’s personal values as they attempt to weigh the benefits of earlier and more frequent screening against the risks associated with false-positive screens.”
We want to hear from you! Tell us what you think.
Annual mammography screening should be offered to women when they reach 40 years, says a new Practice Bulletin on breast cancer screening from ACOG.1 Previous ACOG guidelines recommended mammograms every 1 to 2 years starting at age 40 and annually beginning at age 50.
According to Jennifer Griffin, MD, MPH, who coauthored the ACOG guidelines, the change in mammography screening for women beginning at age 40 is based on three variables:
- Incidence of breast cancer. The malignancy remains the second-leading cause of all cancer-related deaths.
- “Sojourn time” for breast cancer growth (the time it takes for a tumor that is identifiable by mammography to grow big enough to cause symptoms)
- Potential to reduce the number of deaths from breast cancer.
Although the sojourn time of individual cancers varies, the greatest predictor is age. Women 40 to 49 years old have the shortest average sojourn time (2–2.4 years), whereas women 70 to 74 years old have the longest average sojourn time (4–4.1 years).
“Although women in their 40s have a lower overall incidence of breast cancer compared with older women, the window to detect tumors before they become symptomatic is shorter, on average,” said Dr. Griffin. The 5-year survival rate is 98% for women whose breast cancer tumors are discovered at their earliest stage, before they are palpable and when they are small and confined to the breast. “If women in their 40s have annual mammograms, there is a better chance of detecting and treating the cancer before it has time to spread than if they wait 2 years between mammograms.”
The College continues to recommend annual clinical breast exams (CBE) for women 40 years and older, and CBE every 1 to 3 years for women ages 20 to 39. Additionally, ACOG encourages “breast self-awareness” for women 20 years and older. Breast self-awareness is a woman’s understanding of the normal appearance and feel of her breasts.
“The goal here is for women to be alert to any changes, no matter how small, in their breasts, and report them to their doctor,” said Dr. Griffin. “Although we’ve moved away from routinely recommending [breast self-exams], some women will want to continue doing them and that’s OK.”
Enhanced breast cancer screening, such as more frequent CBEs, annual magnetic resonance imaging (MRI), or mammograms before 40 years of age, may be recommended for women at high risk of breast cancer. Breast MRI is not recommended for women at average risk.
The “downside” of annual screening
“More frequent screening is associated with more false-positive findings,” says Andrew M. Kaunitz, MD, professor and associate chairman of obstetrics and gynecology at the University of Florida College of Medicine in Jacksonville, Fla.
“Due to the high rate of false-positive screens and the large number of screening mammograms needed to prevent the death of one woman in her 40s from breast cancer, the US Preventive Services Task Force recommended in late 2009 that routine screening mammography be deferred until age 50 and that screens be biennial,” he explains.2
“The new ACOG guidance acknowledges these concerns, as well as the potential for anxiety associated with false-positive findings. However, ACOG clearly concluded that, in general, US women cope well with such anxiety.
“When discussing mammograms with women in their 40s, it’s appropriate to acknowledge the high rate of false positives in this age group, which may lead to additional imaging, as well as biopsies,” Dr. Kaunitz suggests. “In my view, we can best serve our patients by making recommendations regarding screening mammograms in a manner that is sensitive to women’s personal values as they attempt to weigh the benefits of earlier and more frequent screening against the risks associated with false-positive screens.”
We want to hear from you! Tell us what you think.
1. American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG Practice Bulletin No. 122: Breast cancer screening. Obstet Gynecol. 2011;118(2 Part 1):372-382.
2. Nelson HD, Tyne K, Naik A, Bougatsos C, Chan BK, Humphrey L. US Preventive Services Task Force. Screening for breast cancer: an update for the US Preventive Services Task Force. Ann Intern Med. 2009;151(10):727-737.
1. American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG Practice Bulletin No. 122: Breast cancer screening. Obstet Gynecol. 2011;118(2 Part 1):372-382.
2. Nelson HD, Tyne K, Naik A, Bougatsos C, Chan BK, Humphrey L. US Preventive Services Task Force. Screening for breast cancer: an update for the US Preventive Services Task Force. Ann Intern Med. 2009;151(10):727-737.
Cancer survivors have many complaints not addressed by their physicians
![]()
How to talk to patients about sex
Barbara S. Levy, MD (September 2010)
Sex talk is ubiquitous in American culture—except in the doctor’s office.
So observed psychologist Sharon L. Bober, PhD, at the Cancer Survivorship and Sexual Health Symposium, held June 17–19, 2011, in Washington, DC. Dr. Bober is director of the Sexual Health Program in Pediatric Oncology at Dana-Farber Cancer Institute in Boston.
The survivorship symposium, sponsored by the International Society for Sexual Medicine and the Sexual Medicine Society of North America, offered background on the most common problems encountered by cancer survivors, as well as guidance on how to care for them in gynecology, primary care, oncology, and other fields. Sexual dysfunction, urinary incontinence, and fatigue figured prominently among those problems, yet few clinicians are asking about these conditions among cancer survivors—and even fewer are treating them, Dr. Bober said.
One in three women will be diagnosed with cancer in her lifetime, Dr. Bober noted, and the number of cancer survivors will double—from the current number of approximately 12 million—by the year 2016. The bulk of survivors seek care in the community after treatment, she added.
One reason sexual dysfunction is so widespread among cancer survivors: 64% of all cancer patients have a malignancy that directly affects sexual organs, said Stacy T. Lindau, MD, another speaker at the symposium. Dr. Lindau is associate professor of obstetrics and gynecology and of medicine and geriatrics at the University of Chicago.
According to a 2010 survey by the Lance Armstrong Foundation cited at the symposium, 43% of respondents reported problems with sexual function following treatment, with 29% of that group reporting “a lot” of functional impairment.1 Yet, only 13% of respondents who reported problems with sexual function received care. When respondents were asked why they did not receive care for their problem:
- 55% said they had learned to live with it
- 37% said they had been told it was a side effect of treatment
- 20% said they addressed the problem on their own
- 19% said they were told nothing could be done
- 14% said they expected to get care in the future.
“Discomfort around human sexuality is the main reason the issue doesn’t get raised by health-care providers,” Dr. Bober noted.
“If you’re going to wait for your doctor to bring up this topic, it’s like waiting for Godot.”
We want to hear from you! Tell us what you think.
Reference
1. 2010 LIVESTRONG Survey for People Affected by Cancer. Austin, Tex: Lance Armstrong Foundation; 2011.
![]()
How to talk to patients about sex
Barbara S. Levy, MD (September 2010)
Sex talk is ubiquitous in American culture—except in the doctor’s office.
So observed psychologist Sharon L. Bober, PhD, at the Cancer Survivorship and Sexual Health Symposium, held June 17–19, 2011, in Washington, DC. Dr. Bober is director of the Sexual Health Program in Pediatric Oncology at Dana-Farber Cancer Institute in Boston.
The survivorship symposium, sponsored by the International Society for Sexual Medicine and the Sexual Medicine Society of North America, offered background on the most common problems encountered by cancer survivors, as well as guidance on how to care for them in gynecology, primary care, oncology, and other fields. Sexual dysfunction, urinary incontinence, and fatigue figured prominently among those problems, yet few clinicians are asking about these conditions among cancer survivors—and even fewer are treating them, Dr. Bober said.
One in three women will be diagnosed with cancer in her lifetime, Dr. Bober noted, and the number of cancer survivors will double—from the current number of approximately 12 million—by the year 2016. The bulk of survivors seek care in the community after treatment, she added.
One reason sexual dysfunction is so widespread among cancer survivors: 64% of all cancer patients have a malignancy that directly affects sexual organs, said Stacy T. Lindau, MD, another speaker at the symposium. Dr. Lindau is associate professor of obstetrics and gynecology and of medicine and geriatrics at the University of Chicago.
According to a 2010 survey by the Lance Armstrong Foundation cited at the symposium, 43% of respondents reported problems with sexual function following treatment, with 29% of that group reporting “a lot” of functional impairment.1 Yet, only 13% of respondents who reported problems with sexual function received care. When respondents were asked why they did not receive care for their problem:
- 55% said they had learned to live with it
- 37% said they had been told it was a side effect of treatment
- 20% said they addressed the problem on their own
- 19% said they were told nothing could be done
- 14% said they expected to get care in the future.
“Discomfort around human sexuality is the main reason the issue doesn’t get raised by health-care providers,” Dr. Bober noted.
“If you’re going to wait for your doctor to bring up this topic, it’s like waiting for Godot.”
We want to hear from you! Tell us what you think.
![]()
How to talk to patients about sex
Barbara S. Levy, MD (September 2010)
Sex talk is ubiquitous in American culture—except in the doctor’s office.
So observed psychologist Sharon L. Bober, PhD, at the Cancer Survivorship and Sexual Health Symposium, held June 17–19, 2011, in Washington, DC. Dr. Bober is director of the Sexual Health Program in Pediatric Oncology at Dana-Farber Cancer Institute in Boston.
The survivorship symposium, sponsored by the International Society for Sexual Medicine and the Sexual Medicine Society of North America, offered background on the most common problems encountered by cancer survivors, as well as guidance on how to care for them in gynecology, primary care, oncology, and other fields. Sexual dysfunction, urinary incontinence, and fatigue figured prominently among those problems, yet few clinicians are asking about these conditions among cancer survivors—and even fewer are treating them, Dr. Bober said.
One in three women will be diagnosed with cancer in her lifetime, Dr. Bober noted, and the number of cancer survivors will double—from the current number of approximately 12 million—by the year 2016. The bulk of survivors seek care in the community after treatment, she added.
One reason sexual dysfunction is so widespread among cancer survivors: 64% of all cancer patients have a malignancy that directly affects sexual organs, said Stacy T. Lindau, MD, another speaker at the symposium. Dr. Lindau is associate professor of obstetrics and gynecology and of medicine and geriatrics at the University of Chicago.
According to a 2010 survey by the Lance Armstrong Foundation cited at the symposium, 43% of respondents reported problems with sexual function following treatment, with 29% of that group reporting “a lot” of functional impairment.1 Yet, only 13% of respondents who reported problems with sexual function received care. When respondents were asked why they did not receive care for their problem:
- 55% said they had learned to live with it
- 37% said they had been told it was a side effect of treatment
- 20% said they addressed the problem on their own
- 19% said they were told nothing could be done
- 14% said they expected to get care in the future.
“Discomfort around human sexuality is the main reason the issue doesn’t get raised by health-care providers,” Dr. Bober noted.
“If you’re going to wait for your doctor to bring up this topic, it’s like waiting for Godot.”
We want to hear from you! Tell us what you think.
Reference
1. 2010 LIVESTRONG Survey for People Affected by Cancer. Austin, Tex: Lance Armstrong Foundation; 2011.
Reference
1. 2010 LIVESTRONG Survey for People Affected by Cancer. Austin, Tex: Lance Armstrong Foundation; 2011.
Most OB malpractice claims involve cascading events, not isolated errors
- Read Medical Verdicts, Notable Judgments and Settlements
every month
(June 2011)
Errors in clinical judgment were cited in 77% of more than 800 clinically coded obstetric malpractice cases analyzed by CRICO Strategies, a division of CRICO. CRICO is the patient safety and medical malpractice insurance company owned by the Harvard medical community since 1976. The findings of the analysis were published in a 2010 report entitled Malpractice Risks in Obstetrics.1 The cases on which the report is based were asserted from 2005 to 2009.
According to the report, other prevalent areas of causation were:
- miscommunication (36% of cases)
- technical error (26%)
- inadequate documentation (26%)
- administrative failures (23%)
- ineffective supervision (15%).
The report also reveals the top three most common OB risks or allegations:
- delay in treatment of fetal distress
- improper performance of vaginal delivery
- improper management of pregnancy.
In the CRICO analysis, OB malpractice issues were rarely the result of a single act or omission by a single clinician. Rather, cases typically involved a series of missteps and mishandled decisions by a team of physicians and nurses who converged too late to resolve a rapidly devolving crisis.
“Obstetrics has some unique vulnerabilities, most often involving situations in which a sequence of errors or oversights cascade into a crisis that can put mother and baby in jeopardy,” said Robert Hanscom, senior vice president of CRICO Strategies. “Because there is rarely that standout ‘single event,’ it is absolutely paramount that OB practices understand how these missteps unfold, and then focus on education and training initiatives designed specifically to help clinicians avert those mistakes.”
Although the rate of OB claims is relatively infrequent—less than one case for every 1,000 births—the average malpractice payment is approximately $947,000. That figure is more than twice that of other clinical areas, and second only to surgery in total indemnity payments.
We want to hear from you! Tell us what you think.
Reference
1. 2010 Annual Benchmarking Report: Malpractice Risks in Obstetrics. Boston, Mass: CRICO Strategies; 2010.
- Read Medical Verdicts, Notable Judgments and Settlements
every month
(June 2011)
Errors in clinical judgment were cited in 77% of more than 800 clinically coded obstetric malpractice cases analyzed by CRICO Strategies, a division of CRICO. CRICO is the patient safety and medical malpractice insurance company owned by the Harvard medical community since 1976. The findings of the analysis were published in a 2010 report entitled Malpractice Risks in Obstetrics.1 The cases on which the report is based were asserted from 2005 to 2009.
According to the report, other prevalent areas of causation were:
- miscommunication (36% of cases)
- technical error (26%)
- inadequate documentation (26%)
- administrative failures (23%)
- ineffective supervision (15%).
The report also reveals the top three most common OB risks or allegations:
- delay in treatment of fetal distress
- improper performance of vaginal delivery
- improper management of pregnancy.
In the CRICO analysis, OB malpractice issues were rarely the result of a single act or omission by a single clinician. Rather, cases typically involved a series of missteps and mishandled decisions by a team of physicians and nurses who converged too late to resolve a rapidly devolving crisis.
“Obstetrics has some unique vulnerabilities, most often involving situations in which a sequence of errors or oversights cascade into a crisis that can put mother and baby in jeopardy,” said Robert Hanscom, senior vice president of CRICO Strategies. “Because there is rarely that standout ‘single event,’ it is absolutely paramount that OB practices understand how these missteps unfold, and then focus on education and training initiatives designed specifically to help clinicians avert those mistakes.”
Although the rate of OB claims is relatively infrequent—less than one case for every 1,000 births—the average malpractice payment is approximately $947,000. That figure is more than twice that of other clinical areas, and second only to surgery in total indemnity payments.
We want to hear from you! Tell us what you think.
- Read Medical Verdicts, Notable Judgments and Settlements
every month
(June 2011)
Errors in clinical judgment were cited in 77% of more than 800 clinically coded obstetric malpractice cases analyzed by CRICO Strategies, a division of CRICO. CRICO is the patient safety and medical malpractice insurance company owned by the Harvard medical community since 1976. The findings of the analysis were published in a 2010 report entitled Malpractice Risks in Obstetrics.1 The cases on which the report is based were asserted from 2005 to 2009.
According to the report, other prevalent areas of causation were:
- miscommunication (36% of cases)
- technical error (26%)
- inadequate documentation (26%)
- administrative failures (23%)
- ineffective supervision (15%).
The report also reveals the top three most common OB risks or allegations:
- delay in treatment of fetal distress
- improper performance of vaginal delivery
- improper management of pregnancy.
In the CRICO analysis, OB malpractice issues were rarely the result of a single act or omission by a single clinician. Rather, cases typically involved a series of missteps and mishandled decisions by a team of physicians and nurses who converged too late to resolve a rapidly devolving crisis.
“Obstetrics has some unique vulnerabilities, most often involving situations in which a sequence of errors or oversights cascade into a crisis that can put mother and baby in jeopardy,” said Robert Hanscom, senior vice president of CRICO Strategies. “Because there is rarely that standout ‘single event,’ it is absolutely paramount that OB practices understand how these missteps unfold, and then focus on education and training initiatives designed specifically to help clinicians avert those mistakes.”
Although the rate of OB claims is relatively infrequent—less than one case for every 1,000 births—the average malpractice payment is approximately $947,000. That figure is more than twice that of other clinical areas, and second only to surgery in total indemnity payments.
We want to hear from you! Tell us what you think.
Reference
1. 2010 Annual Benchmarking Report: Malpractice Risks in Obstetrics. Boston, Mass: CRICO Strategies; 2010.
Reference
1. 2010 Annual Benchmarking Report: Malpractice Risks in Obstetrics. Boston, Mass: CRICO Strategies; 2010.
Is the MOC process driving some physicians into early retirement?
Send us an e-mail and let us know your thoughts about the process, at [email protected]
A year has passed since the straightforwardly named organization Change Board Recertification was launched as a call to arms against Maintenance of Certification (MOC), but the debate among physicians over recertification shows no signs of being resolved any time soon. In fact, the summer 2011 issue of The Journal of American Physicians and Surgeons presents not one but two editorials that address the topic—challenging the utility, necessity, and cost of MOC. And a press release from the Association of American Physicians and Surgeons (AAPS) avers that the recertification process is so onerous that it may “drive our most seasoned, experienced physicians into early retirement”—or so AAPS Executive Director Jane M. Orient, MD, is quoted.
In one editorial, Martin Dubravec, MD, minces no words, calling board certification, recertification, and MOC “a malignant growth.”1 And in the other, Lee D. Hieb, MD, president of AAPS, writes that the process of recertification “benefits neither patients nor physicians, and certainly adds nothing to the time-honored practice of medicine.”2
In March 2010, the New England Journal of Medicine ran a clinical vignette and asked readers to vote on whether the internist described in the scenario should enroll in the current MOC program or not.3 Almost two thirds (63%) voted no. And an ongoing poll—admittedly unscientific—at the Change Board Recertification Web site reveals that 92.5% of respondents would abolish the process of recertification altogether.
In addition, a 2009 survey of 100 randomly selected members of AAPS found that only 30% felt that the process of recertification had improved their performance as a physician, and only 22% would voluntarily go through it again.
The American Board of Obstetrics and Gynecology has no figures on how many ObGyns choose not to pursue MOC. One reason for the lack of data: “It’s a 6-year process, and we’re only in year 4,” said MOC administrator Marsha Markham. She did confirm that more than 24,000 physicians are going through the MOC process.
“Right now, our numbers keep increasing each year,” she said.
We want to hear from you! Tell us what you think.
1. Dubravec M. Board certification/recertification/ maintenance of certification: a malignant growth. J Am Physicians Surgeons. 2011;16(2):52-53.
2. Hieb LD. Down the rabbit hole of recertification. J Am Physicians Surgeons. 2011;16(2):36-37.
3. Clinical decisions: American Board of Internal Medicine Maintenance of Certification program. N Engl J Med. 2010;362(10):948-952.
Send us an e-mail and let us know your thoughts about the process, at [email protected]
A year has passed since the straightforwardly named organization Change Board Recertification was launched as a call to arms against Maintenance of Certification (MOC), but the debate among physicians over recertification shows no signs of being resolved any time soon. In fact, the summer 2011 issue of The Journal of American Physicians and Surgeons presents not one but two editorials that address the topic—challenging the utility, necessity, and cost of MOC. And a press release from the Association of American Physicians and Surgeons (AAPS) avers that the recertification process is so onerous that it may “drive our most seasoned, experienced physicians into early retirement”—or so AAPS Executive Director Jane M. Orient, MD, is quoted.
In one editorial, Martin Dubravec, MD, minces no words, calling board certification, recertification, and MOC “a malignant growth.”1 And in the other, Lee D. Hieb, MD, president of AAPS, writes that the process of recertification “benefits neither patients nor physicians, and certainly adds nothing to the time-honored practice of medicine.”2
In March 2010, the New England Journal of Medicine ran a clinical vignette and asked readers to vote on whether the internist described in the scenario should enroll in the current MOC program or not.3 Almost two thirds (63%) voted no. And an ongoing poll—admittedly unscientific—at the Change Board Recertification Web site reveals that 92.5% of respondents would abolish the process of recertification altogether.
In addition, a 2009 survey of 100 randomly selected members of AAPS found that only 30% felt that the process of recertification had improved their performance as a physician, and only 22% would voluntarily go through it again.
The American Board of Obstetrics and Gynecology has no figures on how many ObGyns choose not to pursue MOC. One reason for the lack of data: “It’s a 6-year process, and we’re only in year 4,” said MOC administrator Marsha Markham. She did confirm that more than 24,000 physicians are going through the MOC process.
“Right now, our numbers keep increasing each year,” she said.
We want to hear from you! Tell us what you think.
Send us an e-mail and let us know your thoughts about the process, at [email protected]
A year has passed since the straightforwardly named organization Change Board Recertification was launched as a call to arms against Maintenance of Certification (MOC), but the debate among physicians over recertification shows no signs of being resolved any time soon. In fact, the summer 2011 issue of The Journal of American Physicians and Surgeons presents not one but two editorials that address the topic—challenging the utility, necessity, and cost of MOC. And a press release from the Association of American Physicians and Surgeons (AAPS) avers that the recertification process is so onerous that it may “drive our most seasoned, experienced physicians into early retirement”—or so AAPS Executive Director Jane M. Orient, MD, is quoted.
In one editorial, Martin Dubravec, MD, minces no words, calling board certification, recertification, and MOC “a malignant growth.”1 And in the other, Lee D. Hieb, MD, president of AAPS, writes that the process of recertification “benefits neither patients nor physicians, and certainly adds nothing to the time-honored practice of medicine.”2
In March 2010, the New England Journal of Medicine ran a clinical vignette and asked readers to vote on whether the internist described in the scenario should enroll in the current MOC program or not.3 Almost two thirds (63%) voted no. And an ongoing poll—admittedly unscientific—at the Change Board Recertification Web site reveals that 92.5% of respondents would abolish the process of recertification altogether.
In addition, a 2009 survey of 100 randomly selected members of AAPS found that only 30% felt that the process of recertification had improved their performance as a physician, and only 22% would voluntarily go through it again.
The American Board of Obstetrics and Gynecology has no figures on how many ObGyns choose not to pursue MOC. One reason for the lack of data: “It’s a 6-year process, and we’re only in year 4,” said MOC administrator Marsha Markham. She did confirm that more than 24,000 physicians are going through the MOC process.
“Right now, our numbers keep increasing each year,” she said.
We want to hear from you! Tell us what you think.
1. Dubravec M. Board certification/recertification/ maintenance of certification: a malignant growth. J Am Physicians Surgeons. 2011;16(2):52-53.
2. Hieb LD. Down the rabbit hole of recertification. J Am Physicians Surgeons. 2011;16(2):36-37.
3. Clinical decisions: American Board of Internal Medicine Maintenance of Certification program. N Engl J Med. 2010;362(10):948-952.
1. Dubravec M. Board certification/recertification/ maintenance of certification: a malignant growth. J Am Physicians Surgeons. 2011;16(2):52-53.
2. Hieb LD. Down the rabbit hole of recertification. J Am Physicians Surgeons. 2011;16(2):36-37.
3. Clinical decisions: American Board of Internal Medicine Maintenance of Certification program. N Engl J Med. 2010;362(10):948-952.
In women under 50 years, mammography detects smaller tumors than clinical examination does
- “Just how much does screening mammography reduce mortality from breast cancer?”
Andrew M. Kaunitz, MD (Examining the Evidence; November 2010) - “Access to screening mammography: Priceless”
Robert L. Barbieri, MD (Editorial; January 2010) - “Confused about mammography guidelines? 7 questions answered”
Janelle Yates (January 2010) - “I’ve been rethinking my zeal for breast cancer screening”
Andrew M. Kaunitz, MD (Editorial; December 2009) - “Does the clinical breast exam boost the sensitivity of mammography?”
Jennifer Griffin, MD, and Mark Pearlman, MD (Examining the Evidence; December 2009)
In women younger than 50 years, screening mammography detects smaller tumors that have less nodal involvement than the tumors detected by clinical exam. That’s a principal finding of a study presented this spring at the annual meeting of the American Society of Breast Surgeons in Washington, DC. The study also found that women whose tumors were identified through mammography generally had better outcomes after treatment than women whose tumors were found through a clinical exam, said researcher Paul Dale, MD, chief of surgical oncology at the Ellis Fischel Cancer Center at the University of Missouri School of Medicine in Columbia, Missouri.
The 10-year retrospective study, conducted at the University of Missouri, involved patients treated for breast cancer at the Ellis Fischel Cancer Center between 1998 and 2008. Of these 1,581 women, 20% were 40 to 49 years old. Forty-seven percent of these patients were given a diagnosis on the basis of mammography, and 53% were given a diagnosis on the basis of a clinical exam or other non-mammographic method.
In the group whose diagnosis was based on mammography, the mean tumor diameter was 20 mm; tumors identified by non-mammographic methods, on the other hand, had a mean diameter of 30 mm. This tumor size differential is “highly significant,” said Dr. Dale.
The study also found that lymph-node involvement among women whose diagnosis was based on non-mammographic methods was about twice as common as that of patients whose tumors were found by mammography.
Five-year disease-free survival was estimated to be 94% for the women receiving mammograms; 78%, for those who did not.
“This study found that 20% of women diagnosed with breast cancer in our institution are under age 50, and almost half of their tumors were detected through mammography,” said Dr. Dale. Under the new US Preventive Services Task Force (USPSTF) guidelines, which recommend against routine mammography screening in women under 50, “younger breast cancer patients not undergoing screening and early detection may miss out on important therapy that could significantly impact their survival.”
Many of the studies the USPSTF evaluated before issuing the new guidelines were published before the availability of digital mammography, observed Dr. Dale. He noted that full-field digital mammography has recognized benefits over plain film exams in younger patients who have dense breast tissue that may be difficult to image.
“One concern underlying the new recommendations is that mammography in younger women is less effective and results in too many biopsies that are unnecessary and potentially traumatic for patients,” said Dr. Dale. “Perhaps today’s advancing imaging technologies, as well as less invasive biopsies, will help to eliminate those concerns.”
should start at 40
A new Harris Interactive/HealthDay poll found that women in their 40s want their mammograms, regardless of the latest USPSTF guidelines, which recommend against routine screening mammography in this population. In fact, two thirds of women polled were unaware of the task force’s recommendation.
About 57% of women surveyed believe screening mammography should start at age 40, according to the poll of 1,083 US women 18 years and older. Just 12% thought 50 years was the right age to begin imaging.
When women were apprised of the USPSTF guidelines, 45% of them said the task force had pushed back the recommended age to begin screening to reduce health-care costs and avoid administering unnecessary tests. Thirty percent believed the task force made the recommendation because excessive screening produces too many so-called false-positive results, leading women to think they had cancer when they did not.
New recommendations notwithstanding, many women in their 40s are still getting mammograms—77% of women in their 40s have already had at least one mammogram, and 64% report annual screening, the poll found.
The American Cancer Society continues to recommend annual mammograms for women starting at 40 years.
We want to hear from you! Tell us what you think.
- “Just how much does screening mammography reduce mortality from breast cancer?”
Andrew M. Kaunitz, MD (Examining the Evidence; November 2010) - “Access to screening mammography: Priceless”
Robert L. Barbieri, MD (Editorial; January 2010) - “Confused about mammography guidelines? 7 questions answered”
Janelle Yates (January 2010) - “I’ve been rethinking my zeal for breast cancer screening”
Andrew M. Kaunitz, MD (Editorial; December 2009) - “Does the clinical breast exam boost the sensitivity of mammography?”
Jennifer Griffin, MD, and Mark Pearlman, MD (Examining the Evidence; December 2009)
In women younger than 50 years, screening mammography detects smaller tumors that have less nodal involvement than the tumors detected by clinical exam. That’s a principal finding of a study presented this spring at the annual meeting of the American Society of Breast Surgeons in Washington, DC. The study also found that women whose tumors were identified through mammography generally had better outcomes after treatment than women whose tumors were found through a clinical exam, said researcher Paul Dale, MD, chief of surgical oncology at the Ellis Fischel Cancer Center at the University of Missouri School of Medicine in Columbia, Missouri.
The 10-year retrospective study, conducted at the University of Missouri, involved patients treated for breast cancer at the Ellis Fischel Cancer Center between 1998 and 2008. Of these 1,581 women, 20% were 40 to 49 years old. Forty-seven percent of these patients were given a diagnosis on the basis of mammography, and 53% were given a diagnosis on the basis of a clinical exam or other non-mammographic method.
In the group whose diagnosis was based on mammography, the mean tumor diameter was 20 mm; tumors identified by non-mammographic methods, on the other hand, had a mean diameter of 30 mm. This tumor size differential is “highly significant,” said Dr. Dale.
The study also found that lymph-node involvement among women whose diagnosis was based on non-mammographic methods was about twice as common as that of patients whose tumors were found by mammography.
Five-year disease-free survival was estimated to be 94% for the women receiving mammograms; 78%, for those who did not.
“This study found that 20% of women diagnosed with breast cancer in our institution are under age 50, and almost half of their tumors were detected through mammography,” said Dr. Dale. Under the new US Preventive Services Task Force (USPSTF) guidelines, which recommend against routine mammography screening in women under 50, “younger breast cancer patients not undergoing screening and early detection may miss out on important therapy that could significantly impact their survival.”
Many of the studies the USPSTF evaluated before issuing the new guidelines were published before the availability of digital mammography, observed Dr. Dale. He noted that full-field digital mammography has recognized benefits over plain film exams in younger patients who have dense breast tissue that may be difficult to image.
“One concern underlying the new recommendations is that mammography in younger women is less effective and results in too many biopsies that are unnecessary and potentially traumatic for patients,” said Dr. Dale. “Perhaps today’s advancing imaging technologies, as well as less invasive biopsies, will help to eliminate those concerns.”
should start at 40
A new Harris Interactive/HealthDay poll found that women in their 40s want their mammograms, regardless of the latest USPSTF guidelines, which recommend against routine screening mammography in this population. In fact, two thirds of women polled were unaware of the task force’s recommendation.
About 57% of women surveyed believe screening mammography should start at age 40, according to the poll of 1,083 US women 18 years and older. Just 12% thought 50 years was the right age to begin imaging.
When women were apprised of the USPSTF guidelines, 45% of them said the task force had pushed back the recommended age to begin screening to reduce health-care costs and avoid administering unnecessary tests. Thirty percent believed the task force made the recommendation because excessive screening produces too many so-called false-positive results, leading women to think they had cancer when they did not.
New recommendations notwithstanding, many women in their 40s are still getting mammograms—77% of women in their 40s have already had at least one mammogram, and 64% report annual screening, the poll found.
The American Cancer Society continues to recommend annual mammograms for women starting at 40 years.
We want to hear from you! Tell us what you think.
- “Just how much does screening mammography reduce mortality from breast cancer?”
Andrew M. Kaunitz, MD (Examining the Evidence; November 2010) - “Access to screening mammography: Priceless”
Robert L. Barbieri, MD (Editorial; January 2010) - “Confused about mammography guidelines? 7 questions answered”
Janelle Yates (January 2010) - “I’ve been rethinking my zeal for breast cancer screening”
Andrew M. Kaunitz, MD (Editorial; December 2009) - “Does the clinical breast exam boost the sensitivity of mammography?”
Jennifer Griffin, MD, and Mark Pearlman, MD (Examining the Evidence; December 2009)
In women younger than 50 years, screening mammography detects smaller tumors that have less nodal involvement than the tumors detected by clinical exam. That’s a principal finding of a study presented this spring at the annual meeting of the American Society of Breast Surgeons in Washington, DC. The study also found that women whose tumors were identified through mammography generally had better outcomes after treatment than women whose tumors were found through a clinical exam, said researcher Paul Dale, MD, chief of surgical oncology at the Ellis Fischel Cancer Center at the University of Missouri School of Medicine in Columbia, Missouri.
The 10-year retrospective study, conducted at the University of Missouri, involved patients treated for breast cancer at the Ellis Fischel Cancer Center between 1998 and 2008. Of these 1,581 women, 20% were 40 to 49 years old. Forty-seven percent of these patients were given a diagnosis on the basis of mammography, and 53% were given a diagnosis on the basis of a clinical exam or other non-mammographic method.
In the group whose diagnosis was based on mammography, the mean tumor diameter was 20 mm; tumors identified by non-mammographic methods, on the other hand, had a mean diameter of 30 mm. This tumor size differential is “highly significant,” said Dr. Dale.
The study also found that lymph-node involvement among women whose diagnosis was based on non-mammographic methods was about twice as common as that of patients whose tumors were found by mammography.
Five-year disease-free survival was estimated to be 94% for the women receiving mammograms; 78%, for those who did not.
“This study found that 20% of women diagnosed with breast cancer in our institution are under age 50, and almost half of their tumors were detected through mammography,” said Dr. Dale. Under the new US Preventive Services Task Force (USPSTF) guidelines, which recommend against routine mammography screening in women under 50, “younger breast cancer patients not undergoing screening and early detection may miss out on important therapy that could significantly impact their survival.”
Many of the studies the USPSTF evaluated before issuing the new guidelines were published before the availability of digital mammography, observed Dr. Dale. He noted that full-field digital mammography has recognized benefits over plain film exams in younger patients who have dense breast tissue that may be difficult to image.
“One concern underlying the new recommendations is that mammography in younger women is less effective and results in too many biopsies that are unnecessary and potentially traumatic for patients,” said Dr. Dale. “Perhaps today’s advancing imaging technologies, as well as less invasive biopsies, will help to eliminate those concerns.”
should start at 40
A new Harris Interactive/HealthDay poll found that women in their 40s want their mammograms, regardless of the latest USPSTF guidelines, which recommend against routine screening mammography in this population. In fact, two thirds of women polled were unaware of the task force’s recommendation.
About 57% of women surveyed believe screening mammography should start at age 40, according to the poll of 1,083 US women 18 years and older. Just 12% thought 50 years was the right age to begin imaging.
When women were apprised of the USPSTF guidelines, 45% of them said the task force had pushed back the recommended age to begin screening to reduce health-care costs and avoid administering unnecessary tests. Thirty percent believed the task force made the recommendation because excessive screening produces too many so-called false-positive results, leading women to think they had cancer when they did not.
New recommendations notwithstanding, many women in their 40s are still getting mammograms—77% of women in their 40s have already had at least one mammogram, and 64% report annual screening, the poll found.
The American Cancer Society continues to recommend annual mammograms for women starting at 40 years.
We want to hear from you! Tell us what you think.
Is hormone therapy still a valid option? 12 ObGyns address this question
- Update on menopause
Andrew M. Kaunitz, MD (May 2011)
When the Women’s Health Initiative (WHI) published follow-up data on the association between estrogen-progestin hormone therapy (HT) and breast cancer last fall, it seemed, for a time, like another death knell had sounded for hormonal management of menopausal symptoms.1 The data showed that breast cancers in women who have used oral estrogen-progestin therapy are more likely to be node-positive and carry a higher death rate than breast cancers in nonusers.
Since then, a new WHI analysis from the estrogen-alone arm has found a protective effect against breast cancer among hysterectomized users of unopposed conjugated equine estrogens (CEE).2
So what are clinicians to make of all the data? And how should you counsel your menopausal patients who report bothersome vasomotor symptoms? We put these questions to members of the OBG Management Virtual Board of Editors, and they responded with a not-so-surprising diversity of opinion. Presented here are excerpts of their reflections on the role of HT in clinical practice today.
For a closer look at data from the WHI and other studies, see the Update on Menopause, by Andrew M. Kaunitz, MD, on the facing page.
Hormone therapy is alive and kicking

San Mateo, Calif
To borrow from Mark Twain: Rumors of the death of HT have been greatly exaggerated. With every new spinoff report from the WHI, the tide of panic rises again.
In my private practice in gynecology, I see many patients who seek my care because another physician (usually another gynecologist) has declined to prescribe HT. Sometimes the HT is refused because the patient has reached 5 years of therapy, and the doctor is simply not comfortable continuing.
What is the guiding principle here? Beneficence? Paternalism (or maternalism)? Risk-aversion? All pharmaceutical therapies have risks. Penicillin can cause anaphylaxis; should we advise patients to avoid antibiotics?
When I counsel women about treatment of vasomotor symptoms, I review herbal and botanical remedies and neurotransmitter modulators as well as estrogen and progestin HT options. I believe that these are all valid options, and I take time to give the patient realistic expectations of efficacy and risks for each one, so that she can make a well-informed decision. But which is the most effective for relief of vasomotor symptoms?
Yes, it’s still HT.
In 2011, we have reached an age of enlightenment with regard to HT. We are using lower dosages of estrogen than ever to address menopausal symptoms. We are preferentially prescribing non-oral HT to reduce thromboembolic complications. To prevent endometrial hyperplasia, we are looking to native (dare I say “bioidentical”?) progesterone, as it appears that different progestins carry different levels of breast cancer risk.3
An enlightened approach means addressing the patient’s symptoms while minimizing the risk of adverse effects. Let’s not regress back to the age of panic.
Dr. Spencer reports no relevant financial relationships.

Cleveland, Ohio
As a staff physician in Specialized Women’s Health at the Cleveland Clinic, I manage menopausal women on a regular basis. I find that many of these patients—and their physicians—are poorly informed about the actual risks and benefits of HT. They are unaware of the difference between a prevention trial and a risk trial. And they grossly overestimate the risk of an adverse effect. For example, women who used combination estrogen-progestin in the WHI experienced an increase of 8 cases of breast cancer for every 10,000 woman-years of use. In contrast, women who do not exercise regularly suffer an increase of 35 cases of breast cancer for every 10,000 woman-years of use. In short, the use of HT in the average woman poses far less risk of breast cancer than a poor lifestyle does.
Furthermore, women are not aware that we have a great deal of evidence that early initiation of HT minimizes cardiovascular risk. They are unaware that this early initiation of therapy may well confer a decrease in overall mortality as high as 30%. Patients do not realize that the WHI studied only oral HT and that the use of lower-dose transdermal estrogen most likely minimizes the risks of blood clots, stroke, and hypertension.
I believe that we have allowed the sensationalized coverage of the WHI to cloud the actual data showing that the risks of HT are small. There appears to be some gender bias involved. We allow men to have a drug marketed to them that carries a risk of blindness, heart attack, hypertension, and 4-hour erections—we simply conclude that the benefit is worth the risk. Why don’t we look at HT in the same risk-benefit light? Perhaps it’s because we do not believe that treating a woman’s disabling vasomotor symptoms; her silent, progressive bone loss; or her painful vaginal dryness is worthy of our medical attention.
When we approach the problem of hypertension, we do not prescribe the same dosage of the same medication for all patients. Nor do we assume that any medical path is risk-free. My approach to the menopausal patient is the same: I treat her symptoms as I would any other medical condition that I manage. I conduct an individualized risk-benefit assessment, taking into account the patient’s family history, cardiovascular and lipid status, and risks of breast cancer and osteoporosis. Each patient is prescribed a unique dosage individualized for her symptomatology. And I reevaluate the patient routinely and make any necessary adjustment in the drug or dosage, or both.
As clinicians, we are charged with guiding our patients through the media frenzy to help them differentiate reality and hype. Our patients deserve evidence-based management of their real menopausal symptoms.
Dr. Volkar reports no relevant financial relationships.
Some patients demand HT

Fernandina Beach, Fla
HT still plays a significant role in my practice. At every annual visit, I review and document the updated risks and benefits of HT for the patient, as well as the alternatives. In recent years, there has been a decline in patient interest in hormones, but it hasn’t been as significant as I expected: My patients tend to be more interested in quality of life than the research I quote to them on the complications of HT.
Patients who have new-onset vasomotor instability seldom request HT as first-line therapy. Usually, they request guidance and recommendations for over-the-counter remedies out of concern about and fear of HT. The only patients who specifically request HT are symptomatic patients who have not responded to nonprescription treatment and established patients doing well on HT.
As expected, I have observed a significant increase in symptomatic urogenital atrophy in patients who are not taking systemic HT, so I am prescribing more local vaginal estrogen than ever before.
Despite my annual review of the HT warnings, most of my established patients demand to continue using HT, often commenting, “Doc, are you trying to ruin my marriage?” or “Doc, I need my hormones or I might kill somebody.” These particular patients are not fearful of HT—they are fearful of life without it.
As long as HT is FDA-approved and available for use, I will continue to prescribe it for patients when it is appropriate. However, as more potential adverse effects come to light, I am giving strong consideration to having the patient sign a consent form each time I start or renew HT, for obvious liability concerns.
Dr. McGrath reports no relevant financial relationships.
Hormones pose a real legal risk

Bethpage, NY
I have not prescribed HT since 2002. The reason is simple: No woman is going to sue me for not prescribing hormones for menopausal symptoms. She may not be happy. She may switch to another ObGyn. But she will not sue.
Forget about medical literature and scientific data. Every 6 months, it seems, some new article comes out with new recommendations. We ObGyns are like puppets dangling at the end of a string, swinging from one side to another, depending on which way the medical winds blow. Unfortunately, in this day and age, we no longer work for the patients, but for the lawyers.
So heed the following recommendation, and you may get some unhappy patients, but you won’t get sued: Do not prescribe hormones for menopausal symptoms. No woman has died from lack of hormones, but all you need is one case of breast cancer, or a fatal heart attack, stroke, or pulmonary embolism, for some lawyer to link the catastrophe to HT, and there goes your practice.
It’s just not worth it.
Dr. Zandieh reports no relevant financial relationships.
Many women turn to alternative therapies

Boca Raton, Fla
Many of my patients pursue alternative interventions that do not involve formal estrogen supplementation. These options include both lifestyle changes and phytoestrogens (plant-based supplements with estrogen-like properties). Phytoestrogen products often include black cohosh or soy isoflavones such as genistein that claim SERM-like activity (selective estrogen receptor modulator) to manage hot flashes, night sweats, vaginal dryness, and other menopausal symptoms.
Despite research showing a lack of effectiveness for most phytoestrogen-based products, a surprisingly large percentage of patients utilize these products, often without the knowledge of their provider. It is important to ask about these products because they can interfere with other medications and, in the case of black cohosh, may be contraindicated in patients who have liver disorders.
Although data have been lacking with respect to the use of phytoestrogen-based products, some of these formulations may provide a level of effectiveness for a variety of patients.
Despite the botanical nature of these products, I counsel my patients that there is a potential for estrogen-like activity. Therefore, these products may carry some of the same risks as the estrogen they seek to avoid.
Dr. Bernick reports that he is a consultant for vitaWebMD.
New data make it easier to tailor HT

Camp Hill, Pa
I completed my ObGyn residency during the mid-1990s, at a time when it was common to begin almost every menopausal woman on HT. As data from the WHI trial and Heart and Estrogen/progestin Replacement Study (HERS) exploded in the media, a small percentage of my patients stopped taking their hormones immediately.4,5 The majority of my patients turned to me for interpretation of the studies and guidance on how they applied to their particular clinical scenario.
I believe that my patients are better served by having an extensive discussion of their general health and behavioral habits as a means of addressing their menopausal symptoms. I must admit, before the WHI and HERS trials, I gave this kind of counseling short shrift. Now, when I talk with patients, I find it easiest to discuss HT from a risk-benefit standpoint in light of the data to date. Before the WHI and HERS trials, I did not treat hysterectomized women any differently than those who had an intact uterus. Nor did I think in terms of initiating treatment in early versus late menopause or pay much attention to risk factors for breast cancer or heart disease. Now, we have data on these considerations that enable me to more accurately determine a woman’s unique risk-benefit profile as she contemplates HT. ACOG’s analysis and perspective have also helped.6
Once beyond this first level of discussion, if the patient elects to initiate HT, the focus shifts to “What dosage and for how long?” At her annual visit, we revisit “the numbers” and discuss how they apply to her case. Most important, I assess how HT is affecting her quality of life. I explain to my patients that the concept of the lowest dosage for the shortest duration is one we should embrace not only with HT but with all of their medications on a yearly basis.
Today, my patients run the spectrum of HT use. I have 80-year-old hysterectomized patients with a 30-year history of HT use who look at me pointedly and say, “You’re not gonna stop my hormones, are you?” And I have 52-year-old patients who proudly inform me that their symptoms are manageable without HT now that they have started yoga.
Dr. delRosario reports no relevant financial relationships.
More patients are declining HT

Kansas City, Mo
I routinely advise my patients about the increased risk of breast cancer and positive nodes when I prescribe estrogen-progestin HT, based on the recent publication from the WHI study.1 I tell them straight up that it is a defined risk, but short-term usage of HT for vasomotor symptoms may be acceptable, along with yearly mammograms. They are comfortable knowing the risks and are declining, in increasing numbers, to start or maintain HT.
Alternatives that I recommend are multivitamins and supplemental vitamin D and daily calcium for osteopenia prevention. I suggest using a serotonin reuptake inhibitor for vasomotor symptom control.
Dr. Schnee reports no relevant financial relationships.
Individualizing therapy is a priority

Long Branch, NJ
I doubt that any gynecologist in active practice has forgotten the day in July 2002 when the startling news about the WHI study broke. I remember clearly that I was inundated with questions from anxious women—as well as my residents—wondering about the immediate implications. Suddenly, what had been a panacea for menopausal vasomotor symptoms had become a deadly poison, and women wanted to know with certainty whether they would develop breast cancer.
Since that time, as small aliquots of new information have been published periodically, we have learned to look at HT in a new light. Not all the news is positive, and not all of it is negative—and we are certainly far from the last word on this controversy.
My practice with a Federally Qualified Health Care Center brings patients of different ethnic and racial groups to my office. Most of them (~55%) have Spanish as their primary language, and a significant minority (~30%) are English-speaking. My patients are generally not forthcoming about symptoms that they consider a “normal” part of menopause. I therefore question perimenopausal and menopausal women specifically about vasomotor symptoms and vaginal dryness and dyspareunia. The options I offer them depend on the most troubling symptoms.
Besides estrogen, I offer fluoxetine and desvenlafaxine for vasomotor symptoms. For vaginal dryness and dyspareunia, I offer short-term local conjugated estrogen cream. My patients tend to be more accepting of the estrogen cream than the antidepressants. For perimenopausal women who also need contraception, I offer the low-dose oral contraceptive. Of course, I also suggest lifestyle adjustments such as avoidance of caffeine and increased physical activity.
Numerous reports have noted that over-weight and obese women experience more hot flushes and vasomotor symptoms than their counterparts of normal weight, but I find that thin Caucasian women complain of hot flushes most often. These patients are generally aware of HT but reluctant to use it. Many of these women are taking St. John’s wort or black cohosh as self-medication but do not necessarily report this use. Now I specifically ask about these remedies.
In short, I listen actively, take a thorough history, try to be culturally sensitive, and individualize my advice and pharmacotherapy to suit each patient’s needs.
Dr. Joshi reports no relevant financial relationships.
Transdermal and vaginal estrogen are mainstays

Winchester, Mass
Denying a woman HT when she is suffering from vasomotor symptoms is heartless. I typically recommend vaginal administration of estrogen and progesterone. Reports from the WHI suggest that it is best to avoid a first pass through the liver, and oral medroxyprogesterone acetate is implicated in unwanted heart and breast effects of HT, so I generally prescribe transdermal estrogen, the vaginal ring, or estrogen cream to relieve symptoms. A Prometrium capsule inserted vaginally twice a week protects the endometrium nicely. In my practice, an endometrial sample verified benign endometrium in every case of breakthrough bleeding with this program.
If a patient cannot take estrogen because of breast cancer or concerns about it, I typically offer oral gabapentin for vasomotor symptoms and local tamoxifen (one tablet, ground up, with KY jelly, inserted vaginally twice weekly) for symptoms in the pudendal region. This local tamoxifen improves clinical appearance, vaginal pH, and the cytologic cornification index.
Dr. Shirley reports no relevant financial relationships.
A turn away from hormones

Wichita, Kan
Very few of my patients accept hormonal therapy for their menopausal symptoms these days. A couple of patients have asked for bioidentical hormones, and a few others have been candidates for a low-dose oral contraceptive. Some patients ask about blood tests to determine their menopausal status, but they usually agree with me after I explain why these tests are not helpful.
In my practice, the most common menopausal symptom is vaginal dryness—but I usually have to ask about it before the patient acknowledges the problem. I recommend vaginal lubricants more often than local estrogen, and I try to keep a good supply of lubricants on hand.
Overall, patients are fearful of hormones. I try to counsel them that the benefits and risks of hormones vary according to age and route of administration. I rarely prescribe combination estrogen-progestin HT anymore. And I prefer the transdermal route rather than oral administration. In women who have a uterus, I prescribe quarterly progesterone (Prometrium). Otherwise, I recommend unopposed estrogen.
Dr. Goyle reports no relevant financial relationships.
Stress the benefits of HT!

Lewisville, Tex
You only get one shot! One shot to sell symptomatic menopausal women on the benefits and use of estrogen. If you drop the ball by not anticipating and explaining the side effects, your patient will quit and buy the junk over the counter, which is usually worse than useless! If you are a firm believer in the four “S”s of HT—sleep, sex, skin, and sanity—you must be positive and stress them to your patient.
Sleep is obviously better when the patient doesn’t wake up drenched in sweat. Sex is better because it doesn’t hurt. (Ask your patient whether she would like a plum or a prune for a vagina! She will instantly grasp the physiologic concept!) Skin is better because of the slowdown in collagen loss. Sanity is improved because of the increase in well being, improved thought processes, and enjoyment of life.
For heaven’s sakes, don’t stop HT after 5 or 6 years! Keep it going with gels, patches, or intravaginal cream forever. After all, women spend more than one third of their life in the postmenopausal phase—make it a wonderful life! Your patients will be appreciative. More important, they will reward you by coming back to see you year after year and singing your praises.
Dr. Franklin reports no relevant financial relationships.
Scare headlines grab attention
Saul R. Berg, MD
Pittsburgh, Pa
I believe that the tide will turn in regard to HT in the not-too-distant future. It takes time for the real truth to get out. In the meantime, scare headlines tend to grab attention.
I hope that, in the near future, we will be able to genetically identify women who should not use HT. Until then, I discuss the risks and benefits of HT with my patients and honor their decision. Transdermal estrogen and bimonthly or quarterly progestin—I typically use Prometrium—are my preference.
At present, there don’t seem to be any outstanding alternatives to hormonal therapy.
Dr. Berg reports no relevant financial relationships.
INSTANT POLL
How do you counsel and treat your menopausal patients who report bothersome vasomotor symptoms?
To enter your response, click here.
We want to hear from you! Tell us what you think.
1. `Chlebowski RT, Anderson GL, Gass M, et al; WHI Investigators. Estrogen plus progestin and breast cancer incidence and mortality in postmenopausal women. JAMA. 2010;304(15):1684-1692.
2. LaCroix AZ, Chlebowski RT, Manson JE, et al; WHI Investigators. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy: a randomized controlled trial. JAMA. 2011;305(13):1305-1314.
3. Fournier A, Berrino F, Clavel-Chapelon F. Unequal risks for breast cancer associated with different hormone replacement therapies: results from the E3N cohort study. Breast Cancer Res Treat. 2008;107(1):103-111.
4. Rossouw JE, Anderson GL, Prentice RL, et al. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized trial. JAMA. 2002;288(3):321-333.
5. Hlatky MA, Boothroyd D, Vittinghoff D, Sharp P, Whooley MA. Heart and Estrogen/Progestin Replacement Study (HERS) Research Group. Quality-of-life and depressive symptoms in postmenopausal women after receiving hormone therapy. Results from the Heart and Estrogen/ Progestin Replacement Study (HERS) trial. JAMA. 2002;287(5):591-597.
6. American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG Committee Opinion No. 420: Hormone therapy and heart disease. Obstet Gynecol. 2008;112(5):1189-1192.
- Update on menopause
Andrew M. Kaunitz, MD (May 2011)
When the Women’s Health Initiative (WHI) published follow-up data on the association between estrogen-progestin hormone therapy (HT) and breast cancer last fall, it seemed, for a time, like another death knell had sounded for hormonal management of menopausal symptoms.1 The data showed that breast cancers in women who have used oral estrogen-progestin therapy are more likely to be node-positive and carry a higher death rate than breast cancers in nonusers.
Since then, a new WHI analysis from the estrogen-alone arm has found a protective effect against breast cancer among hysterectomized users of unopposed conjugated equine estrogens (CEE).2
So what are clinicians to make of all the data? And how should you counsel your menopausal patients who report bothersome vasomotor symptoms? We put these questions to members of the OBG Management Virtual Board of Editors, and they responded with a not-so-surprising diversity of opinion. Presented here are excerpts of their reflections on the role of HT in clinical practice today.
For a closer look at data from the WHI and other studies, see the Update on Menopause, by Andrew M. Kaunitz, MD, on the facing page.
Hormone therapy is alive and kicking

San Mateo, Calif
To borrow from Mark Twain: Rumors of the death of HT have been greatly exaggerated. With every new spinoff report from the WHI, the tide of panic rises again.
In my private practice in gynecology, I see many patients who seek my care because another physician (usually another gynecologist) has declined to prescribe HT. Sometimes the HT is refused because the patient has reached 5 years of therapy, and the doctor is simply not comfortable continuing.
What is the guiding principle here? Beneficence? Paternalism (or maternalism)? Risk-aversion? All pharmaceutical therapies have risks. Penicillin can cause anaphylaxis; should we advise patients to avoid antibiotics?
When I counsel women about treatment of vasomotor symptoms, I review herbal and botanical remedies and neurotransmitter modulators as well as estrogen and progestin HT options. I believe that these are all valid options, and I take time to give the patient realistic expectations of efficacy and risks for each one, so that she can make a well-informed decision. But which is the most effective for relief of vasomotor symptoms?
Yes, it’s still HT.
In 2011, we have reached an age of enlightenment with regard to HT. We are using lower dosages of estrogen than ever to address menopausal symptoms. We are preferentially prescribing non-oral HT to reduce thromboembolic complications. To prevent endometrial hyperplasia, we are looking to native (dare I say “bioidentical”?) progesterone, as it appears that different progestins carry different levels of breast cancer risk.3
An enlightened approach means addressing the patient’s symptoms while minimizing the risk of adverse effects. Let’s not regress back to the age of panic.
Dr. Spencer reports no relevant financial relationships.

Cleveland, Ohio
As a staff physician in Specialized Women’s Health at the Cleveland Clinic, I manage menopausal women on a regular basis. I find that many of these patients—and their physicians—are poorly informed about the actual risks and benefits of HT. They are unaware of the difference between a prevention trial and a risk trial. And they grossly overestimate the risk of an adverse effect. For example, women who used combination estrogen-progestin in the WHI experienced an increase of 8 cases of breast cancer for every 10,000 woman-years of use. In contrast, women who do not exercise regularly suffer an increase of 35 cases of breast cancer for every 10,000 woman-years of use. In short, the use of HT in the average woman poses far less risk of breast cancer than a poor lifestyle does.
Furthermore, women are not aware that we have a great deal of evidence that early initiation of HT minimizes cardiovascular risk. They are unaware that this early initiation of therapy may well confer a decrease in overall mortality as high as 30%. Patients do not realize that the WHI studied only oral HT and that the use of lower-dose transdermal estrogen most likely minimizes the risks of blood clots, stroke, and hypertension.
I believe that we have allowed the sensationalized coverage of the WHI to cloud the actual data showing that the risks of HT are small. There appears to be some gender bias involved. We allow men to have a drug marketed to them that carries a risk of blindness, heart attack, hypertension, and 4-hour erections—we simply conclude that the benefit is worth the risk. Why don’t we look at HT in the same risk-benefit light? Perhaps it’s because we do not believe that treating a woman’s disabling vasomotor symptoms; her silent, progressive bone loss; or her painful vaginal dryness is worthy of our medical attention.
When we approach the problem of hypertension, we do not prescribe the same dosage of the same medication for all patients. Nor do we assume that any medical path is risk-free. My approach to the menopausal patient is the same: I treat her symptoms as I would any other medical condition that I manage. I conduct an individualized risk-benefit assessment, taking into account the patient’s family history, cardiovascular and lipid status, and risks of breast cancer and osteoporosis. Each patient is prescribed a unique dosage individualized for her symptomatology. And I reevaluate the patient routinely and make any necessary adjustment in the drug or dosage, or both.
As clinicians, we are charged with guiding our patients through the media frenzy to help them differentiate reality and hype. Our patients deserve evidence-based management of their real menopausal symptoms.
Dr. Volkar reports no relevant financial relationships.
Some patients demand HT

Fernandina Beach, Fla
HT still plays a significant role in my practice. At every annual visit, I review and document the updated risks and benefits of HT for the patient, as well as the alternatives. In recent years, there has been a decline in patient interest in hormones, but it hasn’t been as significant as I expected: My patients tend to be more interested in quality of life than the research I quote to them on the complications of HT.
Patients who have new-onset vasomotor instability seldom request HT as first-line therapy. Usually, they request guidance and recommendations for over-the-counter remedies out of concern about and fear of HT. The only patients who specifically request HT are symptomatic patients who have not responded to nonprescription treatment and established patients doing well on HT.
As expected, I have observed a significant increase in symptomatic urogenital atrophy in patients who are not taking systemic HT, so I am prescribing more local vaginal estrogen than ever before.
Despite my annual review of the HT warnings, most of my established patients demand to continue using HT, often commenting, “Doc, are you trying to ruin my marriage?” or “Doc, I need my hormones or I might kill somebody.” These particular patients are not fearful of HT—they are fearful of life without it.
As long as HT is FDA-approved and available for use, I will continue to prescribe it for patients when it is appropriate. However, as more potential adverse effects come to light, I am giving strong consideration to having the patient sign a consent form each time I start or renew HT, for obvious liability concerns.
Dr. McGrath reports no relevant financial relationships.
Hormones pose a real legal risk

Bethpage, NY
I have not prescribed HT since 2002. The reason is simple: No woman is going to sue me for not prescribing hormones for menopausal symptoms. She may not be happy. She may switch to another ObGyn. But she will not sue.
Forget about medical literature and scientific data. Every 6 months, it seems, some new article comes out with new recommendations. We ObGyns are like puppets dangling at the end of a string, swinging from one side to another, depending on which way the medical winds blow. Unfortunately, in this day and age, we no longer work for the patients, but for the lawyers.
So heed the following recommendation, and you may get some unhappy patients, but you won’t get sued: Do not prescribe hormones for menopausal symptoms. No woman has died from lack of hormones, but all you need is one case of breast cancer, or a fatal heart attack, stroke, or pulmonary embolism, for some lawyer to link the catastrophe to HT, and there goes your practice.
It’s just not worth it.
Dr. Zandieh reports no relevant financial relationships.
Many women turn to alternative therapies

Boca Raton, Fla
Many of my patients pursue alternative interventions that do not involve formal estrogen supplementation. These options include both lifestyle changes and phytoestrogens (plant-based supplements with estrogen-like properties). Phytoestrogen products often include black cohosh or soy isoflavones such as genistein that claim SERM-like activity (selective estrogen receptor modulator) to manage hot flashes, night sweats, vaginal dryness, and other menopausal symptoms.
Despite research showing a lack of effectiveness for most phytoestrogen-based products, a surprisingly large percentage of patients utilize these products, often without the knowledge of their provider. It is important to ask about these products because they can interfere with other medications and, in the case of black cohosh, may be contraindicated in patients who have liver disorders.
Although data have been lacking with respect to the use of phytoestrogen-based products, some of these formulations may provide a level of effectiveness for a variety of patients.
Despite the botanical nature of these products, I counsel my patients that there is a potential for estrogen-like activity. Therefore, these products may carry some of the same risks as the estrogen they seek to avoid.
Dr. Bernick reports that he is a consultant for vitaWebMD.
New data make it easier to tailor HT

Camp Hill, Pa
I completed my ObGyn residency during the mid-1990s, at a time when it was common to begin almost every menopausal woman on HT. As data from the WHI trial and Heart and Estrogen/progestin Replacement Study (HERS) exploded in the media, a small percentage of my patients stopped taking their hormones immediately.4,5 The majority of my patients turned to me for interpretation of the studies and guidance on how they applied to their particular clinical scenario.
I believe that my patients are better served by having an extensive discussion of their general health and behavioral habits as a means of addressing their menopausal symptoms. I must admit, before the WHI and HERS trials, I gave this kind of counseling short shrift. Now, when I talk with patients, I find it easiest to discuss HT from a risk-benefit standpoint in light of the data to date. Before the WHI and HERS trials, I did not treat hysterectomized women any differently than those who had an intact uterus. Nor did I think in terms of initiating treatment in early versus late menopause or pay much attention to risk factors for breast cancer or heart disease. Now, we have data on these considerations that enable me to more accurately determine a woman’s unique risk-benefit profile as she contemplates HT. ACOG’s analysis and perspective have also helped.6
Once beyond this first level of discussion, if the patient elects to initiate HT, the focus shifts to “What dosage and for how long?” At her annual visit, we revisit “the numbers” and discuss how they apply to her case. Most important, I assess how HT is affecting her quality of life. I explain to my patients that the concept of the lowest dosage for the shortest duration is one we should embrace not only with HT but with all of their medications on a yearly basis.
Today, my patients run the spectrum of HT use. I have 80-year-old hysterectomized patients with a 30-year history of HT use who look at me pointedly and say, “You’re not gonna stop my hormones, are you?” And I have 52-year-old patients who proudly inform me that their symptoms are manageable without HT now that they have started yoga.
Dr. delRosario reports no relevant financial relationships.
More patients are declining HT

Kansas City, Mo
I routinely advise my patients about the increased risk of breast cancer and positive nodes when I prescribe estrogen-progestin HT, based on the recent publication from the WHI study.1 I tell them straight up that it is a defined risk, but short-term usage of HT for vasomotor symptoms may be acceptable, along with yearly mammograms. They are comfortable knowing the risks and are declining, in increasing numbers, to start or maintain HT.
Alternatives that I recommend are multivitamins and supplemental vitamin D and daily calcium for osteopenia prevention. I suggest using a serotonin reuptake inhibitor for vasomotor symptom control.
Dr. Schnee reports no relevant financial relationships.
Individualizing therapy is a priority

Long Branch, NJ
I doubt that any gynecologist in active practice has forgotten the day in July 2002 when the startling news about the WHI study broke. I remember clearly that I was inundated with questions from anxious women—as well as my residents—wondering about the immediate implications. Suddenly, what had been a panacea for menopausal vasomotor symptoms had become a deadly poison, and women wanted to know with certainty whether they would develop breast cancer.
Since that time, as small aliquots of new information have been published periodically, we have learned to look at HT in a new light. Not all the news is positive, and not all of it is negative—and we are certainly far from the last word on this controversy.
My practice with a Federally Qualified Health Care Center brings patients of different ethnic and racial groups to my office. Most of them (~55%) have Spanish as their primary language, and a significant minority (~30%) are English-speaking. My patients are generally not forthcoming about symptoms that they consider a “normal” part of menopause. I therefore question perimenopausal and menopausal women specifically about vasomotor symptoms and vaginal dryness and dyspareunia. The options I offer them depend on the most troubling symptoms.
Besides estrogen, I offer fluoxetine and desvenlafaxine for vasomotor symptoms. For vaginal dryness and dyspareunia, I offer short-term local conjugated estrogen cream. My patients tend to be more accepting of the estrogen cream than the antidepressants. For perimenopausal women who also need contraception, I offer the low-dose oral contraceptive. Of course, I also suggest lifestyle adjustments such as avoidance of caffeine and increased physical activity.
Numerous reports have noted that over-weight and obese women experience more hot flushes and vasomotor symptoms than their counterparts of normal weight, but I find that thin Caucasian women complain of hot flushes most often. These patients are generally aware of HT but reluctant to use it. Many of these women are taking St. John’s wort or black cohosh as self-medication but do not necessarily report this use. Now I specifically ask about these remedies.
In short, I listen actively, take a thorough history, try to be culturally sensitive, and individualize my advice and pharmacotherapy to suit each patient’s needs.
Dr. Joshi reports no relevant financial relationships.
Transdermal and vaginal estrogen are mainstays

Winchester, Mass
Denying a woman HT when she is suffering from vasomotor symptoms is heartless. I typically recommend vaginal administration of estrogen and progesterone. Reports from the WHI suggest that it is best to avoid a first pass through the liver, and oral medroxyprogesterone acetate is implicated in unwanted heart and breast effects of HT, so I generally prescribe transdermal estrogen, the vaginal ring, or estrogen cream to relieve symptoms. A Prometrium capsule inserted vaginally twice a week protects the endometrium nicely. In my practice, an endometrial sample verified benign endometrium in every case of breakthrough bleeding with this program.
If a patient cannot take estrogen because of breast cancer or concerns about it, I typically offer oral gabapentin for vasomotor symptoms and local tamoxifen (one tablet, ground up, with KY jelly, inserted vaginally twice weekly) for symptoms in the pudendal region. This local tamoxifen improves clinical appearance, vaginal pH, and the cytologic cornification index.
Dr. Shirley reports no relevant financial relationships.
A turn away from hormones

Wichita, Kan
Very few of my patients accept hormonal therapy for their menopausal symptoms these days. A couple of patients have asked for bioidentical hormones, and a few others have been candidates for a low-dose oral contraceptive. Some patients ask about blood tests to determine their menopausal status, but they usually agree with me after I explain why these tests are not helpful.
In my practice, the most common menopausal symptom is vaginal dryness—but I usually have to ask about it before the patient acknowledges the problem. I recommend vaginal lubricants more often than local estrogen, and I try to keep a good supply of lubricants on hand.
Overall, patients are fearful of hormones. I try to counsel them that the benefits and risks of hormones vary according to age and route of administration. I rarely prescribe combination estrogen-progestin HT anymore. And I prefer the transdermal route rather than oral administration. In women who have a uterus, I prescribe quarterly progesterone (Prometrium). Otherwise, I recommend unopposed estrogen.
Dr. Goyle reports no relevant financial relationships.
Stress the benefits of HT!

Lewisville, Tex
You only get one shot! One shot to sell symptomatic menopausal women on the benefits and use of estrogen. If you drop the ball by not anticipating and explaining the side effects, your patient will quit and buy the junk over the counter, which is usually worse than useless! If you are a firm believer in the four “S”s of HT—sleep, sex, skin, and sanity—you must be positive and stress them to your patient.
Sleep is obviously better when the patient doesn’t wake up drenched in sweat. Sex is better because it doesn’t hurt. (Ask your patient whether she would like a plum or a prune for a vagina! She will instantly grasp the physiologic concept!) Skin is better because of the slowdown in collagen loss. Sanity is improved because of the increase in well being, improved thought processes, and enjoyment of life.
For heaven’s sakes, don’t stop HT after 5 or 6 years! Keep it going with gels, patches, or intravaginal cream forever. After all, women spend more than one third of their life in the postmenopausal phase—make it a wonderful life! Your patients will be appreciative. More important, they will reward you by coming back to see you year after year and singing your praises.
Dr. Franklin reports no relevant financial relationships.
Scare headlines grab attention
Saul R. Berg, MD
Pittsburgh, Pa
I believe that the tide will turn in regard to HT in the not-too-distant future. It takes time for the real truth to get out. In the meantime, scare headlines tend to grab attention.
I hope that, in the near future, we will be able to genetically identify women who should not use HT. Until then, I discuss the risks and benefits of HT with my patients and honor their decision. Transdermal estrogen and bimonthly or quarterly progestin—I typically use Prometrium—are my preference.
At present, there don’t seem to be any outstanding alternatives to hormonal therapy.
Dr. Berg reports no relevant financial relationships.
INSTANT POLL
How do you counsel and treat your menopausal patients who report bothersome vasomotor symptoms?
To enter your response, click here.
We want to hear from you! Tell us what you think.
- Update on menopause
Andrew M. Kaunitz, MD (May 2011)
When the Women’s Health Initiative (WHI) published follow-up data on the association between estrogen-progestin hormone therapy (HT) and breast cancer last fall, it seemed, for a time, like another death knell had sounded for hormonal management of menopausal symptoms.1 The data showed that breast cancers in women who have used oral estrogen-progestin therapy are more likely to be node-positive and carry a higher death rate than breast cancers in nonusers.
Since then, a new WHI analysis from the estrogen-alone arm has found a protective effect against breast cancer among hysterectomized users of unopposed conjugated equine estrogens (CEE).2
So what are clinicians to make of all the data? And how should you counsel your menopausal patients who report bothersome vasomotor symptoms? We put these questions to members of the OBG Management Virtual Board of Editors, and they responded with a not-so-surprising diversity of opinion. Presented here are excerpts of their reflections on the role of HT in clinical practice today.
For a closer look at data from the WHI and other studies, see the Update on Menopause, by Andrew M. Kaunitz, MD, on the facing page.
Hormone therapy is alive and kicking

San Mateo, Calif
To borrow from Mark Twain: Rumors of the death of HT have been greatly exaggerated. With every new spinoff report from the WHI, the tide of panic rises again.
In my private practice in gynecology, I see many patients who seek my care because another physician (usually another gynecologist) has declined to prescribe HT. Sometimes the HT is refused because the patient has reached 5 years of therapy, and the doctor is simply not comfortable continuing.
What is the guiding principle here? Beneficence? Paternalism (or maternalism)? Risk-aversion? All pharmaceutical therapies have risks. Penicillin can cause anaphylaxis; should we advise patients to avoid antibiotics?
When I counsel women about treatment of vasomotor symptoms, I review herbal and botanical remedies and neurotransmitter modulators as well as estrogen and progestin HT options. I believe that these are all valid options, and I take time to give the patient realistic expectations of efficacy and risks for each one, so that she can make a well-informed decision. But which is the most effective for relief of vasomotor symptoms?
Yes, it’s still HT.
In 2011, we have reached an age of enlightenment with regard to HT. We are using lower dosages of estrogen than ever to address menopausal symptoms. We are preferentially prescribing non-oral HT to reduce thromboembolic complications. To prevent endometrial hyperplasia, we are looking to native (dare I say “bioidentical”?) progesterone, as it appears that different progestins carry different levels of breast cancer risk.3
An enlightened approach means addressing the patient’s symptoms while minimizing the risk of adverse effects. Let’s not regress back to the age of panic.
Dr. Spencer reports no relevant financial relationships.

Cleveland, Ohio
As a staff physician in Specialized Women’s Health at the Cleveland Clinic, I manage menopausal women on a regular basis. I find that many of these patients—and their physicians—are poorly informed about the actual risks and benefits of HT. They are unaware of the difference between a prevention trial and a risk trial. And they grossly overestimate the risk of an adverse effect. For example, women who used combination estrogen-progestin in the WHI experienced an increase of 8 cases of breast cancer for every 10,000 woman-years of use. In contrast, women who do not exercise regularly suffer an increase of 35 cases of breast cancer for every 10,000 woman-years of use. In short, the use of HT in the average woman poses far less risk of breast cancer than a poor lifestyle does.
Furthermore, women are not aware that we have a great deal of evidence that early initiation of HT minimizes cardiovascular risk. They are unaware that this early initiation of therapy may well confer a decrease in overall mortality as high as 30%. Patients do not realize that the WHI studied only oral HT and that the use of lower-dose transdermal estrogen most likely minimizes the risks of blood clots, stroke, and hypertension.
I believe that we have allowed the sensationalized coverage of the WHI to cloud the actual data showing that the risks of HT are small. There appears to be some gender bias involved. We allow men to have a drug marketed to them that carries a risk of blindness, heart attack, hypertension, and 4-hour erections—we simply conclude that the benefit is worth the risk. Why don’t we look at HT in the same risk-benefit light? Perhaps it’s because we do not believe that treating a woman’s disabling vasomotor symptoms; her silent, progressive bone loss; or her painful vaginal dryness is worthy of our medical attention.
When we approach the problem of hypertension, we do not prescribe the same dosage of the same medication for all patients. Nor do we assume that any medical path is risk-free. My approach to the menopausal patient is the same: I treat her symptoms as I would any other medical condition that I manage. I conduct an individualized risk-benefit assessment, taking into account the patient’s family history, cardiovascular and lipid status, and risks of breast cancer and osteoporosis. Each patient is prescribed a unique dosage individualized for her symptomatology. And I reevaluate the patient routinely and make any necessary adjustment in the drug or dosage, or both.
As clinicians, we are charged with guiding our patients through the media frenzy to help them differentiate reality and hype. Our patients deserve evidence-based management of their real menopausal symptoms.
Dr. Volkar reports no relevant financial relationships.
Some patients demand HT

Fernandina Beach, Fla
HT still plays a significant role in my practice. At every annual visit, I review and document the updated risks and benefits of HT for the patient, as well as the alternatives. In recent years, there has been a decline in patient interest in hormones, but it hasn’t been as significant as I expected: My patients tend to be more interested in quality of life than the research I quote to them on the complications of HT.
Patients who have new-onset vasomotor instability seldom request HT as first-line therapy. Usually, they request guidance and recommendations for over-the-counter remedies out of concern about and fear of HT. The only patients who specifically request HT are symptomatic patients who have not responded to nonprescription treatment and established patients doing well on HT.
As expected, I have observed a significant increase in symptomatic urogenital atrophy in patients who are not taking systemic HT, so I am prescribing more local vaginal estrogen than ever before.
Despite my annual review of the HT warnings, most of my established patients demand to continue using HT, often commenting, “Doc, are you trying to ruin my marriage?” or “Doc, I need my hormones or I might kill somebody.” These particular patients are not fearful of HT—they are fearful of life without it.
As long as HT is FDA-approved and available for use, I will continue to prescribe it for patients when it is appropriate. However, as more potential adverse effects come to light, I am giving strong consideration to having the patient sign a consent form each time I start or renew HT, for obvious liability concerns.
Dr. McGrath reports no relevant financial relationships.
Hormones pose a real legal risk

Bethpage, NY
I have not prescribed HT since 2002. The reason is simple: No woman is going to sue me for not prescribing hormones for menopausal symptoms. She may not be happy. She may switch to another ObGyn. But she will not sue.
Forget about medical literature and scientific data. Every 6 months, it seems, some new article comes out with new recommendations. We ObGyns are like puppets dangling at the end of a string, swinging from one side to another, depending on which way the medical winds blow. Unfortunately, in this day and age, we no longer work for the patients, but for the lawyers.
So heed the following recommendation, and you may get some unhappy patients, but you won’t get sued: Do not prescribe hormones for menopausal symptoms. No woman has died from lack of hormones, but all you need is one case of breast cancer, or a fatal heart attack, stroke, or pulmonary embolism, for some lawyer to link the catastrophe to HT, and there goes your practice.
It’s just not worth it.
Dr. Zandieh reports no relevant financial relationships.
Many women turn to alternative therapies

Boca Raton, Fla
Many of my patients pursue alternative interventions that do not involve formal estrogen supplementation. These options include both lifestyle changes and phytoestrogens (plant-based supplements with estrogen-like properties). Phytoestrogen products often include black cohosh or soy isoflavones such as genistein that claim SERM-like activity (selective estrogen receptor modulator) to manage hot flashes, night sweats, vaginal dryness, and other menopausal symptoms.
Despite research showing a lack of effectiveness for most phytoestrogen-based products, a surprisingly large percentage of patients utilize these products, often without the knowledge of their provider. It is important to ask about these products because they can interfere with other medications and, in the case of black cohosh, may be contraindicated in patients who have liver disorders.
Although data have been lacking with respect to the use of phytoestrogen-based products, some of these formulations may provide a level of effectiveness for a variety of patients.
Despite the botanical nature of these products, I counsel my patients that there is a potential for estrogen-like activity. Therefore, these products may carry some of the same risks as the estrogen they seek to avoid.
Dr. Bernick reports that he is a consultant for vitaWebMD.
New data make it easier to tailor HT

Camp Hill, Pa
I completed my ObGyn residency during the mid-1990s, at a time when it was common to begin almost every menopausal woman on HT. As data from the WHI trial and Heart and Estrogen/progestin Replacement Study (HERS) exploded in the media, a small percentage of my patients stopped taking their hormones immediately.4,5 The majority of my patients turned to me for interpretation of the studies and guidance on how they applied to their particular clinical scenario.
I believe that my patients are better served by having an extensive discussion of their general health and behavioral habits as a means of addressing their menopausal symptoms. I must admit, before the WHI and HERS trials, I gave this kind of counseling short shrift. Now, when I talk with patients, I find it easiest to discuss HT from a risk-benefit standpoint in light of the data to date. Before the WHI and HERS trials, I did not treat hysterectomized women any differently than those who had an intact uterus. Nor did I think in terms of initiating treatment in early versus late menopause or pay much attention to risk factors for breast cancer or heart disease. Now, we have data on these considerations that enable me to more accurately determine a woman’s unique risk-benefit profile as she contemplates HT. ACOG’s analysis and perspective have also helped.6
Once beyond this first level of discussion, if the patient elects to initiate HT, the focus shifts to “What dosage and for how long?” At her annual visit, we revisit “the numbers” and discuss how they apply to her case. Most important, I assess how HT is affecting her quality of life. I explain to my patients that the concept of the lowest dosage for the shortest duration is one we should embrace not only with HT but with all of their medications on a yearly basis.
Today, my patients run the spectrum of HT use. I have 80-year-old hysterectomized patients with a 30-year history of HT use who look at me pointedly and say, “You’re not gonna stop my hormones, are you?” And I have 52-year-old patients who proudly inform me that their symptoms are manageable without HT now that they have started yoga.
Dr. delRosario reports no relevant financial relationships.
More patients are declining HT

Kansas City, Mo
I routinely advise my patients about the increased risk of breast cancer and positive nodes when I prescribe estrogen-progestin HT, based on the recent publication from the WHI study.1 I tell them straight up that it is a defined risk, but short-term usage of HT for vasomotor symptoms may be acceptable, along with yearly mammograms. They are comfortable knowing the risks and are declining, in increasing numbers, to start or maintain HT.
Alternatives that I recommend are multivitamins and supplemental vitamin D and daily calcium for osteopenia prevention. I suggest using a serotonin reuptake inhibitor for vasomotor symptom control.
Dr. Schnee reports no relevant financial relationships.
Individualizing therapy is a priority

Long Branch, NJ
I doubt that any gynecologist in active practice has forgotten the day in July 2002 when the startling news about the WHI study broke. I remember clearly that I was inundated with questions from anxious women—as well as my residents—wondering about the immediate implications. Suddenly, what had been a panacea for menopausal vasomotor symptoms had become a deadly poison, and women wanted to know with certainty whether they would develop breast cancer.
Since that time, as small aliquots of new information have been published periodically, we have learned to look at HT in a new light. Not all the news is positive, and not all of it is negative—and we are certainly far from the last word on this controversy.
My practice with a Federally Qualified Health Care Center brings patients of different ethnic and racial groups to my office. Most of them (~55%) have Spanish as their primary language, and a significant minority (~30%) are English-speaking. My patients are generally not forthcoming about symptoms that they consider a “normal” part of menopause. I therefore question perimenopausal and menopausal women specifically about vasomotor symptoms and vaginal dryness and dyspareunia. The options I offer them depend on the most troubling symptoms.
Besides estrogen, I offer fluoxetine and desvenlafaxine for vasomotor symptoms. For vaginal dryness and dyspareunia, I offer short-term local conjugated estrogen cream. My patients tend to be more accepting of the estrogen cream than the antidepressants. For perimenopausal women who also need contraception, I offer the low-dose oral contraceptive. Of course, I also suggest lifestyle adjustments such as avoidance of caffeine and increased physical activity.
Numerous reports have noted that over-weight and obese women experience more hot flushes and vasomotor symptoms than their counterparts of normal weight, but I find that thin Caucasian women complain of hot flushes most often. These patients are generally aware of HT but reluctant to use it. Many of these women are taking St. John’s wort or black cohosh as self-medication but do not necessarily report this use. Now I specifically ask about these remedies.
In short, I listen actively, take a thorough history, try to be culturally sensitive, and individualize my advice and pharmacotherapy to suit each patient’s needs.
Dr. Joshi reports no relevant financial relationships.
Transdermal and vaginal estrogen are mainstays

Winchester, Mass
Denying a woman HT when she is suffering from vasomotor symptoms is heartless. I typically recommend vaginal administration of estrogen and progesterone. Reports from the WHI suggest that it is best to avoid a first pass through the liver, and oral medroxyprogesterone acetate is implicated in unwanted heart and breast effects of HT, so I generally prescribe transdermal estrogen, the vaginal ring, or estrogen cream to relieve symptoms. A Prometrium capsule inserted vaginally twice a week protects the endometrium nicely. In my practice, an endometrial sample verified benign endometrium in every case of breakthrough bleeding with this program.
If a patient cannot take estrogen because of breast cancer or concerns about it, I typically offer oral gabapentin for vasomotor symptoms and local tamoxifen (one tablet, ground up, with KY jelly, inserted vaginally twice weekly) for symptoms in the pudendal region. This local tamoxifen improves clinical appearance, vaginal pH, and the cytologic cornification index.
Dr. Shirley reports no relevant financial relationships.
A turn away from hormones

Wichita, Kan
Very few of my patients accept hormonal therapy for their menopausal symptoms these days. A couple of patients have asked for bioidentical hormones, and a few others have been candidates for a low-dose oral contraceptive. Some patients ask about blood tests to determine their menopausal status, but they usually agree with me after I explain why these tests are not helpful.
In my practice, the most common menopausal symptom is vaginal dryness—but I usually have to ask about it before the patient acknowledges the problem. I recommend vaginal lubricants more often than local estrogen, and I try to keep a good supply of lubricants on hand.
Overall, patients are fearful of hormones. I try to counsel them that the benefits and risks of hormones vary according to age and route of administration. I rarely prescribe combination estrogen-progestin HT anymore. And I prefer the transdermal route rather than oral administration. In women who have a uterus, I prescribe quarterly progesterone (Prometrium). Otherwise, I recommend unopposed estrogen.
Dr. Goyle reports no relevant financial relationships.
Stress the benefits of HT!

Lewisville, Tex
You only get one shot! One shot to sell symptomatic menopausal women on the benefits and use of estrogen. If you drop the ball by not anticipating and explaining the side effects, your patient will quit and buy the junk over the counter, which is usually worse than useless! If you are a firm believer in the four “S”s of HT—sleep, sex, skin, and sanity—you must be positive and stress them to your patient.
Sleep is obviously better when the patient doesn’t wake up drenched in sweat. Sex is better because it doesn’t hurt. (Ask your patient whether she would like a plum or a prune for a vagina! She will instantly grasp the physiologic concept!) Skin is better because of the slowdown in collagen loss. Sanity is improved because of the increase in well being, improved thought processes, and enjoyment of life.
For heaven’s sakes, don’t stop HT after 5 or 6 years! Keep it going with gels, patches, or intravaginal cream forever. After all, women spend more than one third of their life in the postmenopausal phase—make it a wonderful life! Your patients will be appreciative. More important, they will reward you by coming back to see you year after year and singing your praises.
Dr. Franklin reports no relevant financial relationships.
Scare headlines grab attention
Saul R. Berg, MD
Pittsburgh, Pa
I believe that the tide will turn in regard to HT in the not-too-distant future. It takes time for the real truth to get out. In the meantime, scare headlines tend to grab attention.
I hope that, in the near future, we will be able to genetically identify women who should not use HT. Until then, I discuss the risks and benefits of HT with my patients and honor their decision. Transdermal estrogen and bimonthly or quarterly progestin—I typically use Prometrium—are my preference.
At present, there don’t seem to be any outstanding alternatives to hormonal therapy.
Dr. Berg reports no relevant financial relationships.
INSTANT POLL
How do you counsel and treat your menopausal patients who report bothersome vasomotor symptoms?
To enter your response, click here.
We want to hear from you! Tell us what you think.
1. `Chlebowski RT, Anderson GL, Gass M, et al; WHI Investigators. Estrogen plus progestin and breast cancer incidence and mortality in postmenopausal women. JAMA. 2010;304(15):1684-1692.
2. LaCroix AZ, Chlebowski RT, Manson JE, et al; WHI Investigators. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy: a randomized controlled trial. JAMA. 2011;305(13):1305-1314.
3. Fournier A, Berrino F, Clavel-Chapelon F. Unequal risks for breast cancer associated with different hormone replacement therapies: results from the E3N cohort study. Breast Cancer Res Treat. 2008;107(1):103-111.
4. Rossouw JE, Anderson GL, Prentice RL, et al. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized trial. JAMA. 2002;288(3):321-333.
5. Hlatky MA, Boothroyd D, Vittinghoff D, Sharp P, Whooley MA. Heart and Estrogen/Progestin Replacement Study (HERS) Research Group. Quality-of-life and depressive symptoms in postmenopausal women after receiving hormone therapy. Results from the Heart and Estrogen/ Progestin Replacement Study (HERS) trial. JAMA. 2002;287(5):591-597.
6. American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG Committee Opinion No. 420: Hormone therapy and heart disease. Obstet Gynecol. 2008;112(5):1189-1192.
1. `Chlebowski RT, Anderson GL, Gass M, et al; WHI Investigators. Estrogen plus progestin and breast cancer incidence and mortality in postmenopausal women. JAMA. 2010;304(15):1684-1692.
2. LaCroix AZ, Chlebowski RT, Manson JE, et al; WHI Investigators. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy: a randomized controlled trial. JAMA. 2011;305(13):1305-1314.
3. Fournier A, Berrino F, Clavel-Chapelon F. Unequal risks for breast cancer associated with different hormone replacement therapies: results from the E3N cohort study. Breast Cancer Res Treat. 2008;107(1):103-111.
4. Rossouw JE, Anderson GL, Prentice RL, et al. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized trial. JAMA. 2002;288(3):321-333.
5. Hlatky MA, Boothroyd D, Vittinghoff D, Sharp P, Whooley MA. Heart and Estrogen/Progestin Replacement Study (HERS) Research Group. Quality-of-life and depressive symptoms in postmenopausal women after receiving hormone therapy. Results from the Heart and Estrogen/ Progestin Replacement Study (HERS) trial. JAMA. 2002;287(5):591-597.
6. American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG Committee Opinion No. 420: Hormone therapy and heart disease. Obstet Gynecol. 2008;112(5):1189-1192.
New group B strep guidelines clarify management of key groups
- Neonatal death from group B strep
(Medical Verdicts, March 2011)
Before widespread intrapartum prophylaxis against group B Streptococcus (GBS) was initiated in the late 1990s, roughly 7,500 newborns developed invasive GBS disease every year in the United States, and the case-fatality rate reached an astonishing—and disheartening—50%.1 Now that all pregnant women undergo culture-based screening at 35 to 37 weeks’ gestation, the incidence of early-onset neonatal GBS disease has declined precipitously.
According to a report issued late last year by the Centers for Disease Control and Prevention (CDC), GBS now causes roughly 1,200 cases of early-onset invasive disease every year, approximately 70% of them among infants born at or after 37 weeks’ gestation, and the case-fatality rate is 4% to 6%.2 Mortality is higher among preterm infants, with a case-fatality rate of 20% to 30% for infants born at or before 33 weeks’ gestation, compared with 2% to 3% for full-term infants.2
Despite progress, GBS remains the leading cause of early-onset neonatal sepsis in the United States. In November 2010, to spur further improvement, the CDC updated its guidelines on prevention of perinatal GBS, and ACOG and other professional organizations endorsed the new recommendations. This article highlights changes to the guidelines—the first since 2002—in four critical areas:
- clarification of who should receive GBS prophylaxis, and when
- updated algorithms for screening and intrapartum prophylaxis for women who experience preterm labor or pre-term premature rupture of membranes (pPROM)
- new recommended dosage of penicillin G for prophylaxis
- updated regimens for prophylaxis among women who are allergic to penicillin.2
When is intrapartum antibiotic prophylaxis indicated? When is it not?
| Indicated | Not indicated |
|---|---|
Previous infant with invasive GBS disease GBS bacteriuria during any trimester of the current pregnancy* Positive GBS vaginal-rectal screening culture in late gestation† during current pregnancy* Unknown GBS status at the onset of labor (culture not done, incomplete, or results unknown) and any of the following:
| Colonization with GBS during a previous pregnancy (unless an indication for GBS prophylaxis is present for current pregnancy) GBS bacteriuria during previous pregnancy (unless an indication for GBS prophylaxis is present for current pregnancy) Negative vaginal and rectal GBS screening culture in late gestation† during the current pregnancy, regardless of intrapartum risk factors Cesarean delivery performed before onset of labor on a woman who has intact amniotic membranes, regardless of GBS colonization status or gestational age |
| SOURCE: CDC2 * Intrapartum antibiotic prophylaxis is not indicated in this circumstance if a cesarean delivery is performed before onset of labor on a woman who has intact amniotic membranes. † Optimal timing for prenatal GBS screening is at 35–37 weeks’ gestation. § Recommendations for the use of intrapartum antibiotics for prevention of early-onset GBS disease in the setting of threatened preterm delivery are presented in FIGURES 1 and 2. ¶ If amnionitis is suspected, broad-spectrum antibiotic therapy that includes an agent known to be active against GBS should replace GBS prophylaxis. ** NAAT testing for GBS is optional and might not be available in all settings. If intrapartum NAAT is negative for GBS but any other intrapartum risk factor (delivery at <37 weeks’ gestation, amniotic membrane rupture at ≥18 hours, or temperature ≥100.4°F [≥38.0°C]) is present, then intrapartum antibiotic prophylaxis is indicated. | |
Who should receive prophylaxis?
In its report, the CDC reiterated the indications and “nonindications” for intrapartum prophylaxis (TABLE). Among the clarifications:
- Women who have GBS isolated from the urine at any time during pregnancy should undergo intrapartum prophylaxis. They do not need third-trimester screening for GBS.
- Women who had a previous infant with invasive GBS disease should also undergo intrapartum prophylaxis, with no need for third-trimester screening
- All other pregnant women should undergo screening at 35 to 37 weeks’ gestation. If results are positive, intrapartum prophylaxis is indicated.
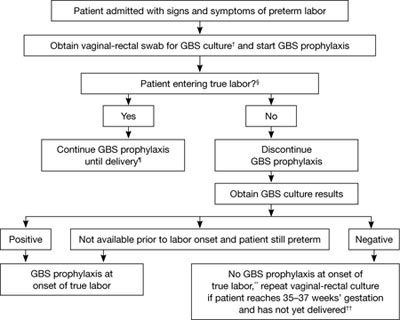
FIGURE 1 Recommended management when a patient experiences preterm labor*
SOURCE: CDC2
*At <37 weeks and 0 days’ gestation.
† If patient has undergone vaginal-rectal GBS culture within the preceding 5 weeks, the results of that culture should guide management. GBS-colonized women should receive intrapartum antibiotic prophylaxis. No antibiotics are indicated for GBS prophylaxis if a vaginal-rectal screen within 5 weeks was negative.
§ Patient should be regularly assessed for progression to true labor; if the patient is considered not to be in true labor, discontinue GBS prophylaxis.
¶ If GBS culture results become available prior to delivery and are negative, discontinue GBS prophylaxis.
** Unless subsequent GBS culture prior to delivery is positive.
†† A negative GBS screen is considered valid for 5 weeks. If a patient with a history of preterm labor is readmitted with signs and symptoms of preterm labor and had a negative GBS screen >5 weeks earlier, she should be rescreened and managed according to this algorithm at that time.
CDC now offers distinct algorithms for preterm labor and pPROM
To clarify the management of two distinct groups of women, the CDC developed separate algorithms for GBS prophylaxis in the setting of threatened preterm delivery—one for spontaneous preterm labor (FIGURE 1) and another for pPROM (FIGURE 2). In addition, it now recommends:
- When GBS prophylaxis is given to a woman who has signs and symptoms of preterm labor, it should be discontinued if it is later determined that she is not in true labor
- If antibiotics given to prolong latency for pPROM include adequate coverage for GBS (i.e., 2 g intravenous [IV] ampicillin followed by 1 g IV ampicillin every 6 hours for 48 hours), no additional prophylaxis for GBS is necessary, provided delivery occurs during administration of that antibiotic regimen. Oral antibiotics alone are not adequate for GBS prophylaxis.
- When a woman who has pPROM is not in labor and is receiving antibiotics with adequate GBS coverage to prolong latency, she should be managed according to the standard of care for pPROM. GBS testing results should not affect the duration of antibiotics.
- When a woman who has pPROM is not in labor and is not receiving antibiotics to prolong latency (or is receiving antibiotics that do not have adequate GBS coverage), she should undergo GBS prophylaxis for 48 hours unless a GBS screen performed within 5 weeks was negative.
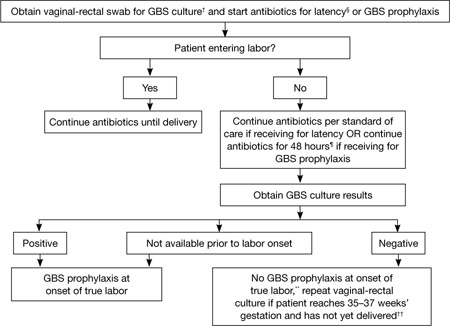
FIGURE 2 GBS screening and prophylaxis for preterm premature rupture of membranes (pPROM)*
SOURCE: CDC2
* At <37 weeks and 0 days’ gestation.
† If patient has undergone vaginal-rectal GBS culture within the preceding 5 weeks, the results of that culture should guide management. GBS-colonized women should receive intrapartum antibiotic prophylaxis. No antibiotics are indicated for GBS prophylaxis if a vaginal-rectal screen within 5 weeks was negative.
§ Antibiotics given for latency in the setting of pPROM that include ampicillin 2 g IV once, followed by 1 g IV every 6 hours for at least 48 hours are adequate for GBS prophylaxis. If other regimens are used, GBS prophylaxis should be initiated in addition.
¶ GBS prophylaxis should be discontinued at 48 hours for women with pPROM who are not in labor. If results from a GBS screen performed at admission become available during the 48-hour period and are negative, GBS prophylaxis should be discontinued at that time.
** Unless subsequent GBS culture prior to delivery is positive.
†† A negative GBS screen is considered valid for 5 weeks. If a patient with pPROM is entering labor and had a negative GBS screen >5 weeks earlier, she should be rescreened and managed according to this algorithm at that time.
New dosage allows room for flexibility
The CDC now recommends a dosage of 5 million units of IV penicillin G for GBS prophylaxis, followed by 2.5 to 3.0 million units IV every 4 hours. The range of 2.5 to 3.0 million units is recommended to ensure that the drug reaches an adequate concentration in the fetal circulation and amniotic fluid without being neurotoxic. The choice of dosage within that range should be guided by which formulations of penicillin G are readily available, says the CDC.
Penicillin remains the agent of choice for intrapartum prophylaxis, but ampicillin is an acceptable alternative.
If a woman is allergic to penicillin but has no history of anaphylaxis, angioedema, respiratory distress, or urticaria following administration of a penicillin or cephalosporin, she should be given 2 g IV cefazolin, followed by 1 g IV cefazolin every 8 hours until delivery. If she does have a history of anaphylaxis or is at high risk for anaphylaxis, ask the laboratory for antimicrobial susceptibility testing on the antenatal GBS culture. If the isolate is susceptible to clindamycin, give her 900 mg IV clindamycin every 8 hours until delivery. If it is not susceptible to clindamycin, give her 1 g IV vancomycin every 12 hours until the time of delivery.
The CDC no longer considers erythromycin to be an acceptable alternative for intrapartum GBS prophylaxis for penicillin-allergic women at high risk of anaphylaxis.
Where we go from here
Although early-onset GBS disease has become relatively uncommon, the rate of maternal GBS colonization remains unchanged since the 1970s. Therefore, it is important to continue efforts to sustain and improve on the progress that has been made. There is also a need to monitor for potential adverse consequences of intrapartum antibiotic prophylaxis, such as emergence of bacterial antimicrobial resistance or an increased incidence or severity of nonGBS neonatal pathogens, the CDC observes. “In the absence of a licensed GBS vaccine, universal screening and intrapartum antibiotic prophylaxis continue to be the cornerstones of early-onset GBS disease prevention."
We want to hear from you! Tell us what you think.
1. Baker CJ, Barrett FF. Group B streptococcal infections in infants. The importance of the various serotypes. JAMA. 1974;230(8):1158-1160.
2. Verani JR, McGee L, Schrag SJ. Centers for Disease Control and Prevention. Prevention of Perinatal Group B Streptococcal Disease: Revised Guidelines from CDC, 2010. MMWR. 2010;59(RR-10):1-36.
- Neonatal death from group B strep
(Medical Verdicts, March 2011)
Before widespread intrapartum prophylaxis against group B Streptococcus (GBS) was initiated in the late 1990s, roughly 7,500 newborns developed invasive GBS disease every year in the United States, and the case-fatality rate reached an astonishing—and disheartening—50%.1 Now that all pregnant women undergo culture-based screening at 35 to 37 weeks’ gestation, the incidence of early-onset neonatal GBS disease has declined precipitously.
According to a report issued late last year by the Centers for Disease Control and Prevention (CDC), GBS now causes roughly 1,200 cases of early-onset invasive disease every year, approximately 70% of them among infants born at or after 37 weeks’ gestation, and the case-fatality rate is 4% to 6%.2 Mortality is higher among preterm infants, with a case-fatality rate of 20% to 30% for infants born at or before 33 weeks’ gestation, compared with 2% to 3% for full-term infants.2
Despite progress, GBS remains the leading cause of early-onset neonatal sepsis in the United States. In November 2010, to spur further improvement, the CDC updated its guidelines on prevention of perinatal GBS, and ACOG and other professional organizations endorsed the new recommendations. This article highlights changes to the guidelines—the first since 2002—in four critical areas:
- clarification of who should receive GBS prophylaxis, and when
- updated algorithms for screening and intrapartum prophylaxis for women who experience preterm labor or pre-term premature rupture of membranes (pPROM)
- new recommended dosage of penicillin G for prophylaxis
- updated regimens for prophylaxis among women who are allergic to penicillin.2
When is intrapartum antibiotic prophylaxis indicated? When is it not?
| Indicated | Not indicated |
|---|---|
Previous infant with invasive GBS disease GBS bacteriuria during any trimester of the current pregnancy* Positive GBS vaginal-rectal screening culture in late gestation† during current pregnancy* Unknown GBS status at the onset of labor (culture not done, incomplete, or results unknown) and any of the following:
| Colonization with GBS during a previous pregnancy (unless an indication for GBS prophylaxis is present for current pregnancy) GBS bacteriuria during previous pregnancy (unless an indication for GBS prophylaxis is present for current pregnancy) Negative vaginal and rectal GBS screening culture in late gestation† during the current pregnancy, regardless of intrapartum risk factors Cesarean delivery performed before onset of labor on a woman who has intact amniotic membranes, regardless of GBS colonization status or gestational age |
| SOURCE: CDC2 * Intrapartum antibiotic prophylaxis is not indicated in this circumstance if a cesarean delivery is performed before onset of labor on a woman who has intact amniotic membranes. † Optimal timing for prenatal GBS screening is at 35–37 weeks’ gestation. § Recommendations for the use of intrapartum antibiotics for prevention of early-onset GBS disease in the setting of threatened preterm delivery are presented in FIGURES 1 and 2. ¶ If amnionitis is suspected, broad-spectrum antibiotic therapy that includes an agent known to be active against GBS should replace GBS prophylaxis. ** NAAT testing for GBS is optional and might not be available in all settings. If intrapartum NAAT is negative for GBS but any other intrapartum risk factor (delivery at <37 weeks’ gestation, amniotic membrane rupture at ≥18 hours, or temperature ≥100.4°F [≥38.0°C]) is present, then intrapartum antibiotic prophylaxis is indicated. | |
Who should receive prophylaxis?
In its report, the CDC reiterated the indications and “nonindications” for intrapartum prophylaxis (TABLE). Among the clarifications:
- Women who have GBS isolated from the urine at any time during pregnancy should undergo intrapartum prophylaxis. They do not need third-trimester screening for GBS.
- Women who had a previous infant with invasive GBS disease should also undergo intrapartum prophylaxis, with no need for third-trimester screening
- All other pregnant women should undergo screening at 35 to 37 weeks’ gestation. If results are positive, intrapartum prophylaxis is indicated.

FIGURE 1 Recommended management when a patient experiences preterm labor*
SOURCE: CDC2
*At <37 weeks and 0 days’ gestation.
† If patient has undergone vaginal-rectal GBS culture within the preceding 5 weeks, the results of that culture should guide management. GBS-colonized women should receive intrapartum antibiotic prophylaxis. No antibiotics are indicated for GBS prophylaxis if a vaginal-rectal screen within 5 weeks was negative.
§ Patient should be regularly assessed for progression to true labor; if the patient is considered not to be in true labor, discontinue GBS prophylaxis.
¶ If GBS culture results become available prior to delivery and are negative, discontinue GBS prophylaxis.
** Unless subsequent GBS culture prior to delivery is positive.
†† A negative GBS screen is considered valid for 5 weeks. If a patient with a history of preterm labor is readmitted with signs and symptoms of preterm labor and had a negative GBS screen >5 weeks earlier, she should be rescreened and managed according to this algorithm at that time.
CDC now offers distinct algorithms for preterm labor and pPROM
To clarify the management of two distinct groups of women, the CDC developed separate algorithms for GBS prophylaxis in the setting of threatened preterm delivery—one for spontaneous preterm labor (FIGURE 1) and another for pPROM (FIGURE 2). In addition, it now recommends:
- When GBS prophylaxis is given to a woman who has signs and symptoms of preterm labor, it should be discontinued if it is later determined that she is not in true labor
- If antibiotics given to prolong latency for pPROM include adequate coverage for GBS (i.e., 2 g intravenous [IV] ampicillin followed by 1 g IV ampicillin every 6 hours for 48 hours), no additional prophylaxis for GBS is necessary, provided delivery occurs during administration of that antibiotic regimen. Oral antibiotics alone are not adequate for GBS prophylaxis.
- When a woman who has pPROM is not in labor and is receiving antibiotics with adequate GBS coverage to prolong latency, she should be managed according to the standard of care for pPROM. GBS testing results should not affect the duration of antibiotics.
- When a woman who has pPROM is not in labor and is not receiving antibiotics to prolong latency (or is receiving antibiotics that do not have adequate GBS coverage), she should undergo GBS prophylaxis for 48 hours unless a GBS screen performed within 5 weeks was negative.

FIGURE 2 GBS screening and prophylaxis for preterm premature rupture of membranes (pPROM)*
SOURCE: CDC2
* At <37 weeks and 0 days’ gestation.
† If patient has undergone vaginal-rectal GBS culture within the preceding 5 weeks, the results of that culture should guide management. GBS-colonized women should receive intrapartum antibiotic prophylaxis. No antibiotics are indicated for GBS prophylaxis if a vaginal-rectal screen within 5 weeks was negative.
§ Antibiotics given for latency in the setting of pPROM that include ampicillin 2 g IV once, followed by 1 g IV every 6 hours for at least 48 hours are adequate for GBS prophylaxis. If other regimens are used, GBS prophylaxis should be initiated in addition.
¶ GBS prophylaxis should be discontinued at 48 hours for women with pPROM who are not in labor. If results from a GBS screen performed at admission become available during the 48-hour period and are negative, GBS prophylaxis should be discontinued at that time.
** Unless subsequent GBS culture prior to delivery is positive.
†† A negative GBS screen is considered valid for 5 weeks. If a patient with pPROM is entering labor and had a negative GBS screen >5 weeks earlier, she should be rescreened and managed according to this algorithm at that time.
New dosage allows room for flexibility
The CDC now recommends a dosage of 5 million units of IV penicillin G for GBS prophylaxis, followed by 2.5 to 3.0 million units IV every 4 hours. The range of 2.5 to 3.0 million units is recommended to ensure that the drug reaches an adequate concentration in the fetal circulation and amniotic fluid without being neurotoxic. The choice of dosage within that range should be guided by which formulations of penicillin G are readily available, says the CDC.
Penicillin remains the agent of choice for intrapartum prophylaxis, but ampicillin is an acceptable alternative.
If a woman is allergic to penicillin but has no history of anaphylaxis, angioedema, respiratory distress, or urticaria following administration of a penicillin or cephalosporin, she should be given 2 g IV cefazolin, followed by 1 g IV cefazolin every 8 hours until delivery. If she does have a history of anaphylaxis or is at high risk for anaphylaxis, ask the laboratory for antimicrobial susceptibility testing on the antenatal GBS culture. If the isolate is susceptible to clindamycin, give her 900 mg IV clindamycin every 8 hours until delivery. If it is not susceptible to clindamycin, give her 1 g IV vancomycin every 12 hours until the time of delivery.
The CDC no longer considers erythromycin to be an acceptable alternative for intrapartum GBS prophylaxis for penicillin-allergic women at high risk of anaphylaxis.
Where we go from here
Although early-onset GBS disease has become relatively uncommon, the rate of maternal GBS colonization remains unchanged since the 1970s. Therefore, it is important to continue efforts to sustain and improve on the progress that has been made. There is also a need to monitor for potential adverse consequences of intrapartum antibiotic prophylaxis, such as emergence of bacterial antimicrobial resistance or an increased incidence or severity of nonGBS neonatal pathogens, the CDC observes. “In the absence of a licensed GBS vaccine, universal screening and intrapartum antibiotic prophylaxis continue to be the cornerstones of early-onset GBS disease prevention."
We want to hear from you! Tell us what you think.
- Neonatal death from group B strep
(Medical Verdicts, March 2011)
Before widespread intrapartum prophylaxis against group B Streptococcus (GBS) was initiated in the late 1990s, roughly 7,500 newborns developed invasive GBS disease every year in the United States, and the case-fatality rate reached an astonishing—and disheartening—50%.1 Now that all pregnant women undergo culture-based screening at 35 to 37 weeks’ gestation, the incidence of early-onset neonatal GBS disease has declined precipitously.
According to a report issued late last year by the Centers for Disease Control and Prevention (CDC), GBS now causes roughly 1,200 cases of early-onset invasive disease every year, approximately 70% of them among infants born at or after 37 weeks’ gestation, and the case-fatality rate is 4% to 6%.2 Mortality is higher among preterm infants, with a case-fatality rate of 20% to 30% for infants born at or before 33 weeks’ gestation, compared with 2% to 3% for full-term infants.2
Despite progress, GBS remains the leading cause of early-onset neonatal sepsis in the United States. In November 2010, to spur further improvement, the CDC updated its guidelines on prevention of perinatal GBS, and ACOG and other professional organizations endorsed the new recommendations. This article highlights changes to the guidelines—the first since 2002—in four critical areas:
- clarification of who should receive GBS prophylaxis, and when
- updated algorithms for screening and intrapartum prophylaxis for women who experience preterm labor or pre-term premature rupture of membranes (pPROM)
- new recommended dosage of penicillin G for prophylaxis
- updated regimens for prophylaxis among women who are allergic to penicillin.2
When is intrapartum antibiotic prophylaxis indicated? When is it not?
| Indicated | Not indicated |
|---|---|
Previous infant with invasive GBS disease GBS bacteriuria during any trimester of the current pregnancy* Positive GBS vaginal-rectal screening culture in late gestation† during current pregnancy* Unknown GBS status at the onset of labor (culture not done, incomplete, or results unknown) and any of the following:
| Colonization with GBS during a previous pregnancy (unless an indication for GBS prophylaxis is present for current pregnancy) GBS bacteriuria during previous pregnancy (unless an indication for GBS prophylaxis is present for current pregnancy) Negative vaginal and rectal GBS screening culture in late gestation† during the current pregnancy, regardless of intrapartum risk factors Cesarean delivery performed before onset of labor on a woman who has intact amniotic membranes, regardless of GBS colonization status or gestational age |
| SOURCE: CDC2 * Intrapartum antibiotic prophylaxis is not indicated in this circumstance if a cesarean delivery is performed before onset of labor on a woman who has intact amniotic membranes. † Optimal timing for prenatal GBS screening is at 35–37 weeks’ gestation. § Recommendations for the use of intrapartum antibiotics for prevention of early-onset GBS disease in the setting of threatened preterm delivery are presented in FIGURES 1 and 2. ¶ If amnionitis is suspected, broad-spectrum antibiotic therapy that includes an agent known to be active against GBS should replace GBS prophylaxis. ** NAAT testing for GBS is optional and might not be available in all settings. If intrapartum NAAT is negative for GBS but any other intrapartum risk factor (delivery at <37 weeks’ gestation, amniotic membrane rupture at ≥18 hours, or temperature ≥100.4°F [≥38.0°C]) is present, then intrapartum antibiotic prophylaxis is indicated. | |
Who should receive prophylaxis?
In its report, the CDC reiterated the indications and “nonindications” for intrapartum prophylaxis (TABLE). Among the clarifications:
- Women who have GBS isolated from the urine at any time during pregnancy should undergo intrapartum prophylaxis. They do not need third-trimester screening for GBS.
- Women who had a previous infant with invasive GBS disease should also undergo intrapartum prophylaxis, with no need for third-trimester screening
- All other pregnant women should undergo screening at 35 to 37 weeks’ gestation. If results are positive, intrapartum prophylaxis is indicated.

FIGURE 1 Recommended management when a patient experiences preterm labor*
SOURCE: CDC2
*At <37 weeks and 0 days’ gestation.
† If patient has undergone vaginal-rectal GBS culture within the preceding 5 weeks, the results of that culture should guide management. GBS-colonized women should receive intrapartum antibiotic prophylaxis. No antibiotics are indicated for GBS prophylaxis if a vaginal-rectal screen within 5 weeks was negative.
§ Patient should be regularly assessed for progression to true labor; if the patient is considered not to be in true labor, discontinue GBS prophylaxis.
¶ If GBS culture results become available prior to delivery and are negative, discontinue GBS prophylaxis.
** Unless subsequent GBS culture prior to delivery is positive.
†† A negative GBS screen is considered valid for 5 weeks. If a patient with a history of preterm labor is readmitted with signs and symptoms of preterm labor and had a negative GBS screen >5 weeks earlier, she should be rescreened and managed according to this algorithm at that time.
CDC now offers distinct algorithms for preterm labor and pPROM
To clarify the management of two distinct groups of women, the CDC developed separate algorithms for GBS prophylaxis in the setting of threatened preterm delivery—one for spontaneous preterm labor (FIGURE 1) and another for pPROM (FIGURE 2). In addition, it now recommends:
- When GBS prophylaxis is given to a woman who has signs and symptoms of preterm labor, it should be discontinued if it is later determined that she is not in true labor
- If antibiotics given to prolong latency for pPROM include adequate coverage for GBS (i.e., 2 g intravenous [IV] ampicillin followed by 1 g IV ampicillin every 6 hours for 48 hours), no additional prophylaxis for GBS is necessary, provided delivery occurs during administration of that antibiotic regimen. Oral antibiotics alone are not adequate for GBS prophylaxis.
- When a woman who has pPROM is not in labor and is receiving antibiotics with adequate GBS coverage to prolong latency, she should be managed according to the standard of care for pPROM. GBS testing results should not affect the duration of antibiotics.
- When a woman who has pPROM is not in labor and is not receiving antibiotics to prolong latency (or is receiving antibiotics that do not have adequate GBS coverage), she should undergo GBS prophylaxis for 48 hours unless a GBS screen performed within 5 weeks was negative.

FIGURE 2 GBS screening and prophylaxis for preterm premature rupture of membranes (pPROM)*
SOURCE: CDC2
* At <37 weeks and 0 days’ gestation.
† If patient has undergone vaginal-rectal GBS culture within the preceding 5 weeks, the results of that culture should guide management. GBS-colonized women should receive intrapartum antibiotic prophylaxis. No antibiotics are indicated for GBS prophylaxis if a vaginal-rectal screen within 5 weeks was negative.
§ Antibiotics given for latency in the setting of pPROM that include ampicillin 2 g IV once, followed by 1 g IV every 6 hours for at least 48 hours are adequate for GBS prophylaxis. If other regimens are used, GBS prophylaxis should be initiated in addition.
¶ GBS prophylaxis should be discontinued at 48 hours for women with pPROM who are not in labor. If results from a GBS screen performed at admission become available during the 48-hour period and are negative, GBS prophylaxis should be discontinued at that time.
** Unless subsequent GBS culture prior to delivery is positive.
†† A negative GBS screen is considered valid for 5 weeks. If a patient with pPROM is entering labor and had a negative GBS screen >5 weeks earlier, she should be rescreened and managed according to this algorithm at that time.
New dosage allows room for flexibility
The CDC now recommends a dosage of 5 million units of IV penicillin G for GBS prophylaxis, followed by 2.5 to 3.0 million units IV every 4 hours. The range of 2.5 to 3.0 million units is recommended to ensure that the drug reaches an adequate concentration in the fetal circulation and amniotic fluid without being neurotoxic. The choice of dosage within that range should be guided by which formulations of penicillin G are readily available, says the CDC.
Penicillin remains the agent of choice for intrapartum prophylaxis, but ampicillin is an acceptable alternative.
If a woman is allergic to penicillin but has no history of anaphylaxis, angioedema, respiratory distress, or urticaria following administration of a penicillin or cephalosporin, she should be given 2 g IV cefazolin, followed by 1 g IV cefazolin every 8 hours until delivery. If she does have a history of anaphylaxis or is at high risk for anaphylaxis, ask the laboratory for antimicrobial susceptibility testing on the antenatal GBS culture. If the isolate is susceptible to clindamycin, give her 900 mg IV clindamycin every 8 hours until delivery. If it is not susceptible to clindamycin, give her 1 g IV vancomycin every 12 hours until the time of delivery.
The CDC no longer considers erythromycin to be an acceptable alternative for intrapartum GBS prophylaxis for penicillin-allergic women at high risk of anaphylaxis.
Where we go from here
Although early-onset GBS disease has become relatively uncommon, the rate of maternal GBS colonization remains unchanged since the 1970s. Therefore, it is important to continue efforts to sustain and improve on the progress that has been made. There is also a need to monitor for potential adverse consequences of intrapartum antibiotic prophylaxis, such as emergence of bacterial antimicrobial resistance or an increased incidence or severity of nonGBS neonatal pathogens, the CDC observes. “In the absence of a licensed GBS vaccine, universal screening and intrapartum antibiotic prophylaxis continue to be the cornerstones of early-onset GBS disease prevention."
We want to hear from you! Tell us what you think.
1. Baker CJ, Barrett FF. Group B streptococcal infections in infants. The importance of the various serotypes. JAMA. 1974;230(8):1158-1160.
2. Verani JR, McGee L, Schrag SJ. Centers for Disease Control and Prevention. Prevention of Perinatal Group B Streptococcal Disease: Revised Guidelines from CDC, 2010. MMWR. 2010;59(RR-10):1-36.
1. Baker CJ, Barrett FF. Group B streptococcal infections in infants. The importance of the various serotypes. JAMA. 1974;230(8):1158-1160.
2. Verani JR, McGee L, Schrag SJ. Centers for Disease Control and Prevention. Prevention of Perinatal Group B Streptococcal Disease: Revised Guidelines from CDC, 2010. MMWR. 2010;59(RR-10):1-36.
Quadrivalent HPV vaccine now FDA-approved to prevent anal cancer
2 HPV vaccines, 7 questions that you need answered
Neal M. Lonky, MD, MPH, and an expert panel
The FDA recently approved the quadrivalent formulation of the human papillomavirus (HPV) vaccine (Merck’s Gardasil) for prevention of anal cancer and associated precancerous lesions caused by HPV types 6, 11, 16, and 18 in people 9 to 26 years old.
The quadrivalent HPV vaccine is already approved for the same age population for the prevention of cervical, vulvar, and vaginal cancer and the associated precancerous lesions caused by HPV types 6, 11, 16, and 18 in females. In addition, it is approved for the prevention of genital warts caused by types 6 and 11 in both males and females.
The indication for anal cancer prevention does not extend to the other FDA-approved HPV vaccine (GlaxoSmithKline’s bivalent [types 16 and 18] formulation, Cervarix).
“Treatment for anal cancer is challenging; the use of Gardasil as a method of prevention is important, as it may result in fewer diagnoses and the subsequent surgery, radiation or chemotherapy that individuals need to endure,” said Karen Midthun, MD, director of the FDA’s Center for Biologics Evaluation and Research.
Anal cancer is uncommon in the general population, but incidence is increasing. HPV is associated with approximately 90% of anal cancer cases. The American Cancer Society estimates that approximately 5,300 people are given a diagnosis of anal cancer each year in the United States—more often women than men.
Gardasil will not prevent development of anal precancerous lesions associated with HPV infection that is already present at the time of vaccination. Its full potential for benefit—across all FDA-approved indications—is obtained by people who are vaccinated before they are exposed to HPV strains contained in the vaccine.
People who undergo regular screening for anal cancer on the recommendation of their health care provider should not discontinue screening after they receive the quadrivalent HPV vaccine.
2 HPV vaccines, 7 questions that you need answered
Neal M. Lonky, MD, MPH, and an expert panel
The FDA recently approved the quadrivalent formulation of the human papillomavirus (HPV) vaccine (Merck’s Gardasil) for prevention of anal cancer and associated precancerous lesions caused by HPV types 6, 11, 16, and 18 in people 9 to 26 years old.
The quadrivalent HPV vaccine is already approved for the same age population for the prevention of cervical, vulvar, and vaginal cancer and the associated precancerous lesions caused by HPV types 6, 11, 16, and 18 in females. In addition, it is approved for the prevention of genital warts caused by types 6 and 11 in both males and females.
The indication for anal cancer prevention does not extend to the other FDA-approved HPV vaccine (GlaxoSmithKline’s bivalent [types 16 and 18] formulation, Cervarix).
“Treatment for anal cancer is challenging; the use of Gardasil as a method of prevention is important, as it may result in fewer diagnoses and the subsequent surgery, radiation or chemotherapy that individuals need to endure,” said Karen Midthun, MD, director of the FDA’s Center for Biologics Evaluation and Research.
Anal cancer is uncommon in the general population, but incidence is increasing. HPV is associated with approximately 90% of anal cancer cases. The American Cancer Society estimates that approximately 5,300 people are given a diagnosis of anal cancer each year in the United States—more often women than men.
Gardasil will not prevent development of anal precancerous lesions associated with HPV infection that is already present at the time of vaccination. Its full potential for benefit—across all FDA-approved indications—is obtained by people who are vaccinated before they are exposed to HPV strains contained in the vaccine.
People who undergo regular screening for anal cancer on the recommendation of their health care provider should not discontinue screening after they receive the quadrivalent HPV vaccine.
2 HPV vaccines, 7 questions that you need answered
Neal M. Lonky, MD, MPH, and an expert panel
The FDA recently approved the quadrivalent formulation of the human papillomavirus (HPV) vaccine (Merck’s Gardasil) for prevention of anal cancer and associated precancerous lesions caused by HPV types 6, 11, 16, and 18 in people 9 to 26 years old.
The quadrivalent HPV vaccine is already approved for the same age population for the prevention of cervical, vulvar, and vaginal cancer and the associated precancerous lesions caused by HPV types 6, 11, 16, and 18 in females. In addition, it is approved for the prevention of genital warts caused by types 6 and 11 in both males and females.
The indication for anal cancer prevention does not extend to the other FDA-approved HPV vaccine (GlaxoSmithKline’s bivalent [types 16 and 18] formulation, Cervarix).
“Treatment for anal cancer is challenging; the use of Gardasil as a method of prevention is important, as it may result in fewer diagnoses and the subsequent surgery, radiation or chemotherapy that individuals need to endure,” said Karen Midthun, MD, director of the FDA’s Center for Biologics Evaluation and Research.
Anal cancer is uncommon in the general population, but incidence is increasing. HPV is associated with approximately 90% of anal cancer cases. The American Cancer Society estimates that approximately 5,300 people are given a diagnosis of anal cancer each year in the United States—more often women than men.
Gardasil will not prevent development of anal precancerous lesions associated with HPV infection that is already present at the time of vaccination. Its full potential for benefit—across all FDA-approved indications—is obtained by people who are vaccinated before they are exposed to HPV strains contained in the vaccine.
People who undergo regular screening for anal cancer on the recommendation of their health care provider should not discontinue screening after they receive the quadrivalent HPV vaccine.
Cease the practice of early elective delivery, says March of Dimes
“39 weeks is the rule, provided delivery is truly elective”
“If you give magnesium sulfate for fetal neuroprotection, adhere to a protocol”
(Update on Obstetrics; January 2011)
John T. Repke, MD, and Jaimey M. Pauli, MD
“More strategies to avoid malpractice hazards on labor and delivery”
(Second of two parts; Focus on Professional Liability; January 2011)
Martin L. Gimovsky, MD, and Alexis C. Gimovsky, MD
Obstetricians and other providers of intrapartum care can improve birth outcomes significantly by eliminating the practice of elective delivery before 39 full weeks of gestation. That’s a key recommendation in a report issued by the March of Dimes at the end of 2010.1
In tandem with the report and accompanying formal recommendations for clinical care, the March of Dimes is expanding a quality improvement program to reduce unnecessary inductions and cesarean deliveries, noted Scott D. Berns, MD, MPH, at a presentation by the organization in New York on December 15. Dr. Berns is senior vice president for Chapter Programs of the March of Dimes and editor of the report, Toward Improving the Outcome of Pregnancy III (TIOP III). He is also clinical professor of pediatrics at the Warren Alpert Medical School at Brown University.
“It’s about babies being born at the right time for the right reasons,” Dr. Berns said.
Medical inductions are too common
A key focus of TIOP III is the need to curtail the practice of elective “term” delivery at 37 and 38 weeks of gestation. As the report notes, although “there are many valid medical and obstetric indications for delivery before 39 weeks of gestation, medical justification for a significant proportion of early deliveries is questionable.”1
Of particular concern is the use of medical induction of labor at 37 to 39 weeks without a legitimate indication—a practice that has increased dramatically over the past decade and that raises the rate of cesarean delivery, said Mark R. Chassin, MD, MPP, MPH, who spoke at the New York release of the report. Dr. Chassin is President of the Joint Commission.
Morbidity rises with early delivery
Early term delivery is widespread. As many as 30% of all births in the United States are performed electively (“without identifiable medical or obstetric indication”) before 39 weeks’ gestation, said Dr. Berns. This statistic includes elective induction of labor and elective primary and repeat cesarean delivery, he added.
The morbidity associated with these early births is significant:
- The rate of admission to a newborn intensive care unit (NICU) doubles in infants born electively at 38 to 39 weeks of gestation, compared with those delivered at or beyond 39 weeks
- “Infants born before 39 completed weeks of gestation also have a higher incidence of respiratory distress syndrome and infant death than those delivered later”1
- There is evidence that neonatal morbidity increases even after fetal lung maturity is confirmed when elective delivery takes place before 39 weeks.1
ACOG has also warned against early elective delivery.2
The March of Dimes Foundation offers a toolkit on its Web site for clinicians to use to reduce the rate of elective delivery before 39 full weeks of gestation. It’s available at http://www.marchofdimes.com/professionals/less-than-39-weeks-toolkit.aspx.
Other intrapartum actions can boost outcomes
Other recommendations for improving intrapartum care and pregnancy outcomes included in TIOP III:
- Introduce facility-based protocols and develop effective leadership to eliminate elective deliveries before 39 weeks’ gestation
- Use standardized, low-dose oxytocin protocols for induction and augmentation of labor. (According to TIOP III: “Oxtyocin is the drug most commonly associated with preventable adverse events during childbirth and is also the drug most frequently implicated in professional liability claims.”1
- Uniformly implement unambiguous protocols for monitoring oxytocin infusion
- Avoid “inappropriate” cesarean delivery and de-emphasize the cesarean delivery rate as a primary quality indicator
- Adopt protocols for administration of magnesium sulfate for fetal neuroprotection in preterm infants
- Enhance and support a team approach to obstetric emergencies, and promote clinician understanding of intermediate and abnormal fetal heart rate patterns
- Use available checklists to document maneuvers—including those avoided—in the management of shoulder dystocia
- Develop a “robust quality improvement program” for intrapartum care processes.1
“39 weeks is the rule, provided delivery is truly elective”
“If you give magnesium sulfate for fetal neuroprotection, adhere to a protocol”
(Update on Obstetrics; January 2011)
John T. Repke, MD, and Jaimey M. Pauli, MD
“More strategies to avoid malpractice hazards on labor and delivery”
(Second of two parts; Focus on Professional Liability; January 2011)
Martin L. Gimovsky, MD, and Alexis C. Gimovsky, MD
Obstetricians and other providers of intrapartum care can improve birth outcomes significantly by eliminating the practice of elective delivery before 39 full weeks of gestation. That’s a key recommendation in a report issued by the March of Dimes at the end of 2010.1
In tandem with the report and accompanying formal recommendations for clinical care, the March of Dimes is expanding a quality improvement program to reduce unnecessary inductions and cesarean deliveries, noted Scott D. Berns, MD, MPH, at a presentation by the organization in New York on December 15. Dr. Berns is senior vice president for Chapter Programs of the March of Dimes and editor of the report, Toward Improving the Outcome of Pregnancy III (TIOP III). He is also clinical professor of pediatrics at the Warren Alpert Medical School at Brown University.
“It’s about babies being born at the right time for the right reasons,” Dr. Berns said.
Medical inductions are too common
A key focus of TIOP III is the need to curtail the practice of elective “term” delivery at 37 and 38 weeks of gestation. As the report notes, although “there are many valid medical and obstetric indications for delivery before 39 weeks of gestation, medical justification for a significant proportion of early deliveries is questionable.”1
Of particular concern is the use of medical induction of labor at 37 to 39 weeks without a legitimate indication—a practice that has increased dramatically over the past decade and that raises the rate of cesarean delivery, said Mark R. Chassin, MD, MPP, MPH, who spoke at the New York release of the report. Dr. Chassin is President of the Joint Commission.
Morbidity rises with early delivery
Early term delivery is widespread. As many as 30% of all births in the United States are performed electively (“without identifiable medical or obstetric indication”) before 39 weeks’ gestation, said Dr. Berns. This statistic includes elective induction of labor and elective primary and repeat cesarean delivery, he added.
The morbidity associated with these early births is significant:
- The rate of admission to a newborn intensive care unit (NICU) doubles in infants born electively at 38 to 39 weeks of gestation, compared with those delivered at or beyond 39 weeks
- “Infants born before 39 completed weeks of gestation also have a higher incidence of respiratory distress syndrome and infant death than those delivered later”1
- There is evidence that neonatal morbidity increases even after fetal lung maturity is confirmed when elective delivery takes place before 39 weeks.1
ACOG has also warned against early elective delivery.2
The March of Dimes Foundation offers a toolkit on its Web site for clinicians to use to reduce the rate of elective delivery before 39 full weeks of gestation. It’s available at http://www.marchofdimes.com/professionals/less-than-39-weeks-toolkit.aspx.
Other intrapartum actions can boost outcomes
Other recommendations for improving intrapartum care and pregnancy outcomes included in TIOP III:
- Introduce facility-based protocols and develop effective leadership to eliminate elective deliveries before 39 weeks’ gestation
- Use standardized, low-dose oxytocin protocols for induction and augmentation of labor. (According to TIOP III: “Oxtyocin is the drug most commonly associated with preventable adverse events during childbirth and is also the drug most frequently implicated in professional liability claims.”1
- Uniformly implement unambiguous protocols for monitoring oxytocin infusion
- Avoid “inappropriate” cesarean delivery and de-emphasize the cesarean delivery rate as a primary quality indicator
- Adopt protocols for administration of magnesium sulfate for fetal neuroprotection in preterm infants
- Enhance and support a team approach to obstetric emergencies, and promote clinician understanding of intermediate and abnormal fetal heart rate patterns
- Use available checklists to document maneuvers—including those avoided—in the management of shoulder dystocia
- Develop a “robust quality improvement program” for intrapartum care processes.1
“39 weeks is the rule, provided delivery is truly elective”
“If you give magnesium sulfate for fetal neuroprotection, adhere to a protocol”
(Update on Obstetrics; January 2011)
John T. Repke, MD, and Jaimey M. Pauli, MD
“More strategies to avoid malpractice hazards on labor and delivery”
(Second of two parts; Focus on Professional Liability; January 2011)
Martin L. Gimovsky, MD, and Alexis C. Gimovsky, MD
Obstetricians and other providers of intrapartum care can improve birth outcomes significantly by eliminating the practice of elective delivery before 39 full weeks of gestation. That’s a key recommendation in a report issued by the March of Dimes at the end of 2010.1
In tandem with the report and accompanying formal recommendations for clinical care, the March of Dimes is expanding a quality improvement program to reduce unnecessary inductions and cesarean deliveries, noted Scott D. Berns, MD, MPH, at a presentation by the organization in New York on December 15. Dr. Berns is senior vice president for Chapter Programs of the March of Dimes and editor of the report, Toward Improving the Outcome of Pregnancy III (TIOP III). He is also clinical professor of pediatrics at the Warren Alpert Medical School at Brown University.
“It’s about babies being born at the right time for the right reasons,” Dr. Berns said.
Medical inductions are too common
A key focus of TIOP III is the need to curtail the practice of elective “term” delivery at 37 and 38 weeks of gestation. As the report notes, although “there are many valid medical and obstetric indications for delivery before 39 weeks of gestation, medical justification for a significant proportion of early deliveries is questionable.”1
Of particular concern is the use of medical induction of labor at 37 to 39 weeks without a legitimate indication—a practice that has increased dramatically over the past decade and that raises the rate of cesarean delivery, said Mark R. Chassin, MD, MPP, MPH, who spoke at the New York release of the report. Dr. Chassin is President of the Joint Commission.
Morbidity rises with early delivery
Early term delivery is widespread. As many as 30% of all births in the United States are performed electively (“without identifiable medical or obstetric indication”) before 39 weeks’ gestation, said Dr. Berns. This statistic includes elective induction of labor and elective primary and repeat cesarean delivery, he added.
The morbidity associated with these early births is significant:
- The rate of admission to a newborn intensive care unit (NICU) doubles in infants born electively at 38 to 39 weeks of gestation, compared with those delivered at or beyond 39 weeks
- “Infants born before 39 completed weeks of gestation also have a higher incidence of respiratory distress syndrome and infant death than those delivered later”1
- There is evidence that neonatal morbidity increases even after fetal lung maturity is confirmed when elective delivery takes place before 39 weeks.1
ACOG has also warned against early elective delivery.2
The March of Dimes Foundation offers a toolkit on its Web site for clinicians to use to reduce the rate of elective delivery before 39 full weeks of gestation. It’s available at http://www.marchofdimes.com/professionals/less-than-39-weeks-toolkit.aspx.
Other intrapartum actions can boost outcomes
Other recommendations for improving intrapartum care and pregnancy outcomes included in TIOP III:
- Introduce facility-based protocols and develop effective leadership to eliminate elective deliveries before 39 weeks’ gestation
- Use standardized, low-dose oxytocin protocols for induction and augmentation of labor. (According to TIOP III: “Oxtyocin is the drug most commonly associated with preventable adverse events during childbirth and is also the drug most frequently implicated in professional liability claims.”1
- Uniformly implement unambiguous protocols for monitoring oxytocin infusion
- Avoid “inappropriate” cesarean delivery and de-emphasize the cesarean delivery rate as a primary quality indicator
- Adopt protocols for administration of magnesium sulfate for fetal neuroprotection in preterm infants
- Enhance and support a team approach to obstetric emergencies, and promote clinician understanding of intermediate and abnormal fetal heart rate patterns
- Use available checklists to document maneuvers—including those avoided—in the management of shoulder dystocia
- Develop a “robust quality improvement program” for intrapartum care processes.1
Women who have low sexual arousal may respond to simple measures
- Update on Sexual Dysfunction: What can you do for your patients?
Barbara S. Levy, MD (September 2010)
A new study reveals that women who have low sexual arousal may experience a clinically significant change in symptoms after taking a placebo.
Does this finding suggest that ObGyns should use placebo when managing women who have sexual dysfunction?
No.
But it does suggest that even simple interventions can make a difference in the management of these women, the authors say.
Andrea Bradford, PhD, a psychologist at Baylor College of Medicine, and coauthor Cindy Meston, PhD, a psychologist at the University of Texas at Austin, analyzed the behaviors and symptoms of 50 women who were randomly assigned to receive placebo in a large clinical trial of a drug treatment for low sexual arousal. Neither the women nor the study physicians knew whether they were taking the real drug or placebo.
After 12 weeks of treatment, symptoms in about one in three of these women improved to a degree that most clinicians would consider a meaningful change. Most of that improvement seemed to happen during the first four weeks of the study. Results were published online in the September 2010 issue of the Journal of Sexual Medicine.
The most important predictor of symptom change was an increase in the frequency of satisfying sexual encounters during the treatment. Many women even reported that they received more stimulation during sexual activity while they participated in the trial, even though their partners were not given any special instructions.
“It’s important to note that, even though these women received placebo, they all had an opportunity to talk to a health provider about their difficulties and were asked to closely monitor their sexual behavior and feelings over a 12-week period. Just taking part in this study probably started some meaningful conversations,” said Bradford. “Our study shows that even a limited intervention can have a positive effect in many women with sexual dysfunction. This comes as no surprise to sex therapists, but it does suggest a need to investigate behavioral factors more closely in clinical trials.”
We want to hear from you! Tell us what you think.
- Update on Sexual Dysfunction: What can you do for your patients?
Barbara S. Levy, MD (September 2010)
A new study reveals that women who have low sexual arousal may experience a clinically significant change in symptoms after taking a placebo.
Does this finding suggest that ObGyns should use placebo when managing women who have sexual dysfunction?
No.
But it does suggest that even simple interventions can make a difference in the management of these women, the authors say.
Andrea Bradford, PhD, a psychologist at Baylor College of Medicine, and coauthor Cindy Meston, PhD, a psychologist at the University of Texas at Austin, analyzed the behaviors and symptoms of 50 women who were randomly assigned to receive placebo in a large clinical trial of a drug treatment for low sexual arousal. Neither the women nor the study physicians knew whether they were taking the real drug or placebo.
After 12 weeks of treatment, symptoms in about one in three of these women improved to a degree that most clinicians would consider a meaningful change. Most of that improvement seemed to happen during the first four weeks of the study. Results were published online in the September 2010 issue of the Journal of Sexual Medicine.
The most important predictor of symptom change was an increase in the frequency of satisfying sexual encounters during the treatment. Many women even reported that they received more stimulation during sexual activity while they participated in the trial, even though their partners were not given any special instructions.
“It’s important to note that, even though these women received placebo, they all had an opportunity to talk to a health provider about their difficulties and were asked to closely monitor their sexual behavior and feelings over a 12-week period. Just taking part in this study probably started some meaningful conversations,” said Bradford. “Our study shows that even a limited intervention can have a positive effect in many women with sexual dysfunction. This comes as no surprise to sex therapists, but it does suggest a need to investigate behavioral factors more closely in clinical trials.”
We want to hear from you! Tell us what you think.
- Update on Sexual Dysfunction: What can you do for your patients?
Barbara S. Levy, MD (September 2010)
A new study reveals that women who have low sexual arousal may experience a clinically significant change in symptoms after taking a placebo.
Does this finding suggest that ObGyns should use placebo when managing women who have sexual dysfunction?
No.
But it does suggest that even simple interventions can make a difference in the management of these women, the authors say.
Andrea Bradford, PhD, a psychologist at Baylor College of Medicine, and coauthor Cindy Meston, PhD, a psychologist at the University of Texas at Austin, analyzed the behaviors and symptoms of 50 women who were randomly assigned to receive placebo in a large clinical trial of a drug treatment for low sexual arousal. Neither the women nor the study physicians knew whether they were taking the real drug or placebo.
After 12 weeks of treatment, symptoms in about one in three of these women improved to a degree that most clinicians would consider a meaningful change. Most of that improvement seemed to happen during the first four weeks of the study. Results were published online in the September 2010 issue of the Journal of Sexual Medicine.
The most important predictor of symptom change was an increase in the frequency of satisfying sexual encounters during the treatment. Many women even reported that they received more stimulation during sexual activity while they participated in the trial, even though their partners were not given any special instructions.
“It’s important to note that, even though these women received placebo, they all had an opportunity to talk to a health provider about their difficulties and were asked to closely monitor their sexual behavior and feelings over a 12-week period. Just taking part in this study probably started some meaningful conversations,” said Bradford. “Our study shows that even a limited intervention can have a positive effect in many women with sexual dysfunction. This comes as no surprise to sex therapists, but it does suggest a need to investigate behavioral factors more closely in clinical trials.”
We want to hear from you! Tell us what you think.