User login
New study reveals a link between estrogen-progestin HRT and advanced breast cancer
“In the aftermath of the WHI, consider estrogen patient by patient”
Janelle Yates, Senior Editor
June 2010
Women who participated in the estrogen-progestin arm of the WHI and who were followed for approximately 11 years had an increased incidence of breast cancer—and those cancers were more likely to be advanced, with a higher risk of death. That is the finding of a new study led by Rowan T. Chlebowski, MD, PhD, of Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center in Torrance, Calif. The study was published in the October 20 issue of JAMA.
Chlebowski and colleagues analyzed data and report updated information on breast cancer incidence. For the first time, they also make information available on breast cancer mortality related to the use of combined hormone therapy in the WHI trial.
A total of 16,608 postmenopausal women 50 to 79 years old who had no history of hysterectomy were randomized, in 40 US clinical centers, to combined conjugated equine estrogens, 0.625 mg/d, plus medroxyprogesterone acetate, 2.5 mg/d, or placebo. After the original trial completion date (March 31, 2005), repeat consent was required for continued follow-up for breast cancer incidence and was obtained from 12,788 (83%) of the surviving participants.
In intention-to-treat analyses including all randomized participants (except for those who failed to consent to additional follow-up), researchers found that estrogen plus progestin increased the incidence of invasive breast cancer to a greater extent than placebo did (385 cases [0.42% per year] vs. 293 cases [0.34% per year], respectively). A significantly larger percentage of women in the combined hormone therapy group had breast cancers with positive lymph nodes, compared with women in the placebo group (81 [23.7%] vs. 43 [16.2%], respectively).
“More women died of breast cancer in the combined hormone therapy group compared with the placebo group (25 deaths [0.03% per year] vs. 12 deaths [0.01% per year]), representing 2.6 vs. 1.3 deaths per 10,000 women per year, respectively,” the investigators write. “Consideration of all-cause mortality after breast cancer diagnosis provided similar results; among women in the combined hormone therapy group, there were 51 deaths (0.05% per year) compared with 31 deaths (0.03% per year) among women in the placebo group, representing 5.3 vs. 3.4 deaths per 10,000 women per year, respectively.
“With some exceptions, the preponderance of observational studies have associated combined hormone therapy use with an increase in breast cancers that have favorable characteristics, lower stage, and longer survival compared with breast cancers diagnosed in nonusers of hormone therapy,” Chlebowski and colleagues write. However, in the WHI randomized trial, combined hormone therapy increased breast cancer risk and interfered with breast cancer detection, leading to cancers being diagnosed at more advanced stages.
“Now, with longer follow-up results available, there remains a cumulative, statistically significant increase in breast cancers in the combined hormone therapy group, and the cancers more commonly had lymph node involvement. The observed adverse influence on breast cancer mortality of combined hormone therapy can reasonably be explained by the influence on breast cancer incidence and stage.
“Following the initial report of results from the WHI trial,” the researchers note, “a substantial decrease in breast cancer incidence occurred in the United States, which was attributed to the marked decrease in postmenopausal hormone therapy use that occurred after publication of the trial results. The adverse influence of estrogen plus progestin on breast cancer mortality suggests that a future reduction in breast cancer mortality in the United States may be anticipated as well.”
We want to hear from you! Tell us what you think.
“In the aftermath of the WHI, consider estrogen patient by patient”
Janelle Yates, Senior Editor
June 2010
Women who participated in the estrogen-progestin arm of the WHI and who were followed for approximately 11 years had an increased incidence of breast cancer—and those cancers were more likely to be advanced, with a higher risk of death. That is the finding of a new study led by Rowan T. Chlebowski, MD, PhD, of Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center in Torrance, Calif. The study was published in the October 20 issue of JAMA.
Chlebowski and colleagues analyzed data and report updated information on breast cancer incidence. For the first time, they also make information available on breast cancer mortality related to the use of combined hormone therapy in the WHI trial.
A total of 16,608 postmenopausal women 50 to 79 years old who had no history of hysterectomy were randomized, in 40 US clinical centers, to combined conjugated equine estrogens, 0.625 mg/d, plus medroxyprogesterone acetate, 2.5 mg/d, or placebo. After the original trial completion date (March 31, 2005), repeat consent was required for continued follow-up for breast cancer incidence and was obtained from 12,788 (83%) of the surviving participants.
In intention-to-treat analyses including all randomized participants (except for those who failed to consent to additional follow-up), researchers found that estrogen plus progestin increased the incidence of invasive breast cancer to a greater extent than placebo did (385 cases [0.42% per year] vs. 293 cases [0.34% per year], respectively). A significantly larger percentage of women in the combined hormone therapy group had breast cancers with positive lymph nodes, compared with women in the placebo group (81 [23.7%] vs. 43 [16.2%], respectively).
“More women died of breast cancer in the combined hormone therapy group compared with the placebo group (25 deaths [0.03% per year] vs. 12 deaths [0.01% per year]), representing 2.6 vs. 1.3 deaths per 10,000 women per year, respectively,” the investigators write. “Consideration of all-cause mortality after breast cancer diagnosis provided similar results; among women in the combined hormone therapy group, there were 51 deaths (0.05% per year) compared with 31 deaths (0.03% per year) among women in the placebo group, representing 5.3 vs. 3.4 deaths per 10,000 women per year, respectively.
“With some exceptions, the preponderance of observational studies have associated combined hormone therapy use with an increase in breast cancers that have favorable characteristics, lower stage, and longer survival compared with breast cancers diagnosed in nonusers of hormone therapy,” Chlebowski and colleagues write. However, in the WHI randomized trial, combined hormone therapy increased breast cancer risk and interfered with breast cancer detection, leading to cancers being diagnosed at more advanced stages.
“Now, with longer follow-up results available, there remains a cumulative, statistically significant increase in breast cancers in the combined hormone therapy group, and the cancers more commonly had lymph node involvement. The observed adverse influence on breast cancer mortality of combined hormone therapy can reasonably be explained by the influence on breast cancer incidence and stage.
“Following the initial report of results from the WHI trial,” the researchers note, “a substantial decrease in breast cancer incidence occurred in the United States, which was attributed to the marked decrease in postmenopausal hormone therapy use that occurred after publication of the trial results. The adverse influence of estrogen plus progestin on breast cancer mortality suggests that a future reduction in breast cancer mortality in the United States may be anticipated as well.”
We want to hear from you! Tell us what you think.
“In the aftermath of the WHI, consider estrogen patient by patient”
Janelle Yates, Senior Editor
June 2010
Women who participated in the estrogen-progestin arm of the WHI and who were followed for approximately 11 years had an increased incidence of breast cancer—and those cancers were more likely to be advanced, with a higher risk of death. That is the finding of a new study led by Rowan T. Chlebowski, MD, PhD, of Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center in Torrance, Calif. The study was published in the October 20 issue of JAMA.
Chlebowski and colleagues analyzed data and report updated information on breast cancer incidence. For the first time, they also make information available on breast cancer mortality related to the use of combined hormone therapy in the WHI trial.
A total of 16,608 postmenopausal women 50 to 79 years old who had no history of hysterectomy were randomized, in 40 US clinical centers, to combined conjugated equine estrogens, 0.625 mg/d, plus medroxyprogesterone acetate, 2.5 mg/d, or placebo. After the original trial completion date (March 31, 2005), repeat consent was required for continued follow-up for breast cancer incidence and was obtained from 12,788 (83%) of the surviving participants.
In intention-to-treat analyses including all randomized participants (except for those who failed to consent to additional follow-up), researchers found that estrogen plus progestin increased the incidence of invasive breast cancer to a greater extent than placebo did (385 cases [0.42% per year] vs. 293 cases [0.34% per year], respectively). A significantly larger percentage of women in the combined hormone therapy group had breast cancers with positive lymph nodes, compared with women in the placebo group (81 [23.7%] vs. 43 [16.2%], respectively).
“More women died of breast cancer in the combined hormone therapy group compared with the placebo group (25 deaths [0.03% per year] vs. 12 deaths [0.01% per year]), representing 2.6 vs. 1.3 deaths per 10,000 women per year, respectively,” the investigators write. “Consideration of all-cause mortality after breast cancer diagnosis provided similar results; among women in the combined hormone therapy group, there were 51 deaths (0.05% per year) compared with 31 deaths (0.03% per year) among women in the placebo group, representing 5.3 vs. 3.4 deaths per 10,000 women per year, respectively.
“With some exceptions, the preponderance of observational studies have associated combined hormone therapy use with an increase in breast cancers that have favorable characteristics, lower stage, and longer survival compared with breast cancers diagnosed in nonusers of hormone therapy,” Chlebowski and colleagues write. However, in the WHI randomized trial, combined hormone therapy increased breast cancer risk and interfered with breast cancer detection, leading to cancers being diagnosed at more advanced stages.
“Now, with longer follow-up results available, there remains a cumulative, statistically significant increase in breast cancers in the combined hormone therapy group, and the cancers more commonly had lymph node involvement. The observed adverse influence on breast cancer mortality of combined hormone therapy can reasonably be explained by the influence on breast cancer incidence and stage.
“Following the initial report of results from the WHI trial,” the researchers note, “a substantial decrease in breast cancer incidence occurred in the United States, which was attributed to the marked decrease in postmenopausal hormone therapy use that occurred after publication of the trial results. The adverse influence of estrogen plus progestin on breast cancer mortality suggests that a future reduction in breast cancer mortality in the United States may be anticipated as well.”
We want to hear from you! Tell us what you think.
Why you should recommend flu vaccine to "every single patient"
OCTOBER 2010—ObGyns and other physicians who manage the care of pregnant women should routinely discuss the safety and benefits of seasonal influenza vaccination. That means raising the subject with “every single patient,” said Laura E. Riley, MD, who spoke on behalf of ACOG after a news conference sponsored by the National Foundation for Infectious Diseases (NFID) on October 7.
“That’s the job of providers, of doctors, to put it into perspective—for the patient’s sake as well as for the patient’s family,” said Dr. Riley, who is medical director of labor and delivery and director of ObGyn infectious disease at Massachusetts General Hospital in Boston.
Results of a recent consumer survey undertaken by NFID indicate that the advice of a physician strongly influences patient behavior. Among people who have already been vaccinated with the influenza vaccine or who plan to get vaccinated this season, 76% say they received a recommendation from a health-care provider; only 35% of those who do not plan to get vaccinated report receiving a recommendation from a health-care provider.
Expect flu to strike pregnant women hard
Vaccination against influenza is especially important for pregnant women, said Dr. Riley, for several reasons:
- Mortality from influenza is five times higher among pregnant women who have not been vaccinated than it is among nonpregnant counterparts
- Pregnant women who get the flu are more likely to be hospitalized for severe respiratory illness
- Influenza increases the risk of preterm delivery.
If a patient is concerned about risks associated with being vaccinated against influenza, she can be reassured that benefits far outweigh any potential risks, Dr. Riley said.
“The risks of getting vaccinated in pregnancy aren’t any different than vaccination outside of pregnancy,” she said. “We’ve been giving millions of doses of seasonal flu vaccine to pregnant women for years—at least 10 years. And there’s never been any increase in concern over safety in terms of maternal disease.” The vaccine also is safe for babies, she added.
A new year, a new protocol
This year, for the first time, influenza vaccine is recommended for everyone 6 months of age and older. Infants younger than 6 months cannot be vaccinated, but they can benefit from the antibodies they receive from their vaccinated mothers (if the mothers were vaccinated during pregnancy, or if the infant is breastfeeding).
This year’s vaccine is an “all-in-one” product; patients do not need to get a separate H1N1 vaccine. The 2010 vaccine provides coverage against three strains of virus:
- influenza B virus
- influenza A H3N2
- influenza A H1N1 (2009 variant).
These strains were chosen for inclusion in the vaccine because they are very similar to the viruses circulating in the United States and abroad,” said Daniel Jernigan, MD, MPH, who spoke at the news conference. Dr. Jernigan is deputy director of the influenza division at the National Center for Immunization and Respiratory Diseases at the Centers for Disease Control and Prevention.
The fact that the vaccine provides coverage so specific to what is happening in the community is “very good news,” he added. “We also know that the viruses that we’re seeing are susceptible to antiviral drugs.”
Don’t forget about pertussis
Although pertussis typically is not life-threatening in adults, adults and adolescents who have the disease can pass it to infants who have not been immunized—among whom it can be especially severe. In recent years, 91% of all deaths from pertussis have been in infants 6 months and younger. In addition, more than 50% of infants younger than 1 year who contract pertussis must be hospitalized.
For that reason, it is important that every woman be immunized with Tdap immediately postpartum if her last tetanus-diphtheria booster was more than 2 years earlier.
We want to hear from you! Tell us what you think.
OCTOBER 2010—ObGyns and other physicians who manage the care of pregnant women should routinely discuss the safety and benefits of seasonal influenza vaccination. That means raising the subject with “every single patient,” said Laura E. Riley, MD, who spoke on behalf of ACOG after a news conference sponsored by the National Foundation for Infectious Diseases (NFID) on October 7.
“That’s the job of providers, of doctors, to put it into perspective—for the patient’s sake as well as for the patient’s family,” said Dr. Riley, who is medical director of labor and delivery and director of ObGyn infectious disease at Massachusetts General Hospital in Boston.
Results of a recent consumer survey undertaken by NFID indicate that the advice of a physician strongly influences patient behavior. Among people who have already been vaccinated with the influenza vaccine or who plan to get vaccinated this season, 76% say they received a recommendation from a health-care provider; only 35% of those who do not plan to get vaccinated report receiving a recommendation from a health-care provider.
Expect flu to strike pregnant women hard
Vaccination against influenza is especially important for pregnant women, said Dr. Riley, for several reasons:
- Mortality from influenza is five times higher among pregnant women who have not been vaccinated than it is among nonpregnant counterparts
- Pregnant women who get the flu are more likely to be hospitalized for severe respiratory illness
- Influenza increases the risk of preterm delivery.
If a patient is concerned about risks associated with being vaccinated against influenza, she can be reassured that benefits far outweigh any potential risks, Dr. Riley said.
“The risks of getting vaccinated in pregnancy aren’t any different than vaccination outside of pregnancy,” she said. “We’ve been giving millions of doses of seasonal flu vaccine to pregnant women for years—at least 10 years. And there’s never been any increase in concern over safety in terms of maternal disease.” The vaccine also is safe for babies, she added.
A new year, a new protocol
This year, for the first time, influenza vaccine is recommended for everyone 6 months of age and older. Infants younger than 6 months cannot be vaccinated, but they can benefit from the antibodies they receive from their vaccinated mothers (if the mothers were vaccinated during pregnancy, or if the infant is breastfeeding).
This year’s vaccine is an “all-in-one” product; patients do not need to get a separate H1N1 vaccine. The 2010 vaccine provides coverage against three strains of virus:
- influenza B virus
- influenza A H3N2
- influenza A H1N1 (2009 variant).
These strains were chosen for inclusion in the vaccine because they are very similar to the viruses circulating in the United States and abroad,” said Daniel Jernigan, MD, MPH, who spoke at the news conference. Dr. Jernigan is deputy director of the influenza division at the National Center for Immunization and Respiratory Diseases at the Centers for Disease Control and Prevention.
The fact that the vaccine provides coverage so specific to what is happening in the community is “very good news,” he added. “We also know that the viruses that we’re seeing are susceptible to antiviral drugs.”
Don’t forget about pertussis
Although pertussis typically is not life-threatening in adults, adults and adolescents who have the disease can pass it to infants who have not been immunized—among whom it can be especially severe. In recent years, 91% of all deaths from pertussis have been in infants 6 months and younger. In addition, more than 50% of infants younger than 1 year who contract pertussis must be hospitalized.
For that reason, it is important that every woman be immunized with Tdap immediately postpartum if her last tetanus-diphtheria booster was more than 2 years earlier.
We want to hear from you! Tell us what you think.
OCTOBER 2010—ObGyns and other physicians who manage the care of pregnant women should routinely discuss the safety and benefits of seasonal influenza vaccination. That means raising the subject with “every single patient,” said Laura E. Riley, MD, who spoke on behalf of ACOG after a news conference sponsored by the National Foundation for Infectious Diseases (NFID) on October 7.
“That’s the job of providers, of doctors, to put it into perspective—for the patient’s sake as well as for the patient’s family,” said Dr. Riley, who is medical director of labor and delivery and director of ObGyn infectious disease at Massachusetts General Hospital in Boston.
Results of a recent consumer survey undertaken by NFID indicate that the advice of a physician strongly influences patient behavior. Among people who have already been vaccinated with the influenza vaccine or who plan to get vaccinated this season, 76% say they received a recommendation from a health-care provider; only 35% of those who do not plan to get vaccinated report receiving a recommendation from a health-care provider.
Expect flu to strike pregnant women hard
Vaccination against influenza is especially important for pregnant women, said Dr. Riley, for several reasons:
- Mortality from influenza is five times higher among pregnant women who have not been vaccinated than it is among nonpregnant counterparts
- Pregnant women who get the flu are more likely to be hospitalized for severe respiratory illness
- Influenza increases the risk of preterm delivery.
If a patient is concerned about risks associated with being vaccinated against influenza, she can be reassured that benefits far outweigh any potential risks, Dr. Riley said.
“The risks of getting vaccinated in pregnancy aren’t any different than vaccination outside of pregnancy,” she said. “We’ve been giving millions of doses of seasonal flu vaccine to pregnant women for years—at least 10 years. And there’s never been any increase in concern over safety in terms of maternal disease.” The vaccine also is safe for babies, she added.
A new year, a new protocol
This year, for the first time, influenza vaccine is recommended for everyone 6 months of age and older. Infants younger than 6 months cannot be vaccinated, but they can benefit from the antibodies they receive from their vaccinated mothers (if the mothers were vaccinated during pregnancy, or if the infant is breastfeeding).
This year’s vaccine is an “all-in-one” product; patients do not need to get a separate H1N1 vaccine. The 2010 vaccine provides coverage against three strains of virus:
- influenza B virus
- influenza A H3N2
- influenza A H1N1 (2009 variant).
These strains were chosen for inclusion in the vaccine because they are very similar to the viruses circulating in the United States and abroad,” said Daniel Jernigan, MD, MPH, who spoke at the news conference. Dr. Jernigan is deputy director of the influenza division at the National Center for Immunization and Respiratory Diseases at the Centers for Disease Control and Prevention.
The fact that the vaccine provides coverage so specific to what is happening in the community is “very good news,” he added. “We also know that the viruses that we’re seeing are susceptible to antiviral drugs.”
Don’t forget about pertussis
Although pertussis typically is not life-threatening in adults, adults and adolescents who have the disease can pass it to infants who have not been immunized—among whom it can be especially severe. In recent years, 91% of all deaths from pertussis have been in infants 6 months and younger. In addition, more than 50% of infants younger than 1 year who contract pertussis must be hospitalized.
For that reason, it is important that every woman be immunized with Tdap immediately postpartum if her last tetanus-diphtheria booster was more than 2 years earlier.
We want to hear from you! Tell us what you think.
An ObGyn’s guide to aromatase inhibitors as adjuvant therapy for breast CA
Think breast cancer survivors are unlikely to show up in your practice? You should think again.
At last official count, 2.5 million women in the United States had a history of breast cancer.1 Most of them are now free of malignancy, but others are still grappling with the disease in some form or fashion.2 All need continuing health care.
Roughly two thirds of women who have breast cancer have disease that is hormone-receptor–positive.2,3 Recently updated guidelines from the American Society of Clinical Oncology (ASCO) recommend that adjuvant therapy for postmenopausal women who have hormone-positive breast cancer include an aromatase inhibitor (AI) (see a summary of these guidelines on page 36). That makes it likely that a good number of breast cancer survivors who visit your practice are taking one of these medications: anastrozole (Arimidex), letrozole (Femara), or exemestane (Aromasin). These drugs are antiestrogens, given to postmenopausal women to reduce the likelihood of disease recurrence and progression.
The antiestrogenic properties of these drugs are what make them lifesavers. But the same qualities can create a range of health issues, from increased risk of osteoporosis and fracture to vasomotor and joint symptoms. And although ObGyns are not the physicians who prescribe these drugs, you may be the provider one of these women consults about their side effects and related issues.
To find out the latest on the management of women who are taking one of these agents, we inserted ourselves into the busy schedule of Andrew M. Kaunitz, MD, who agreed to address some fundamental—and some not so basic—questions about the drugs. In this extended Q&A, Dr. Kaunitz touches on mechanism of action, benefits versus risks, common side effects, compliance with therapy, and the ill effects of early discontinuation.
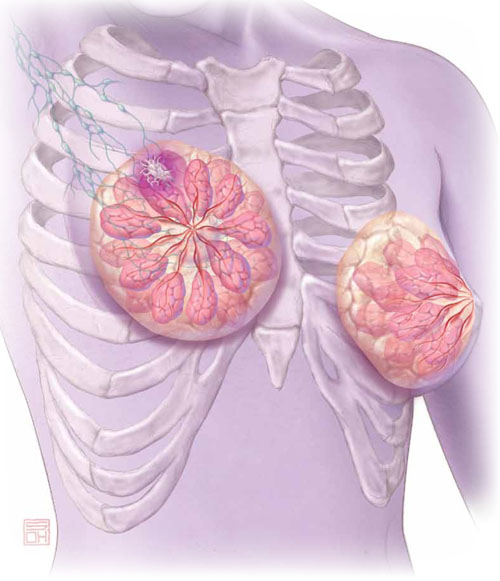
Aromatase inhibitors are better than tamoxifen at reducing the risk of breast cancer recurrence in postmenopausal women who have hormone-receptor–positive disease. But, they increase the risk of osteoporosis and fracture and often cause arthralgias and other complaints that ObGyn practitioners may be called upon to manage.
OBG Management: What is the overall aim of adjuvant endocrine therapy in the setting of breast cancer?
Dr. Kaunitz: Endocrine therapy—specifically, use of an AI—prevents the stimulation of breast cancer cells by endogenous estrogen. In other words, aromatase inhibitors suppress the growth of cancer cells that have estrogen receptors. These drugs also inhibit aromatase near any breast tumor and reduce estrogen levels in breast tissue.
OBG Management: What is the mechanism of action of adjuvant endocrine therapy?
Dr. Kaunitz: In postmenopausal women, androgens are converted to estrogens via the aromatase enzyme, which is present in adipose tissue and other sites. By blocking this enzyme, AIs reduce endogenous estrogen levels by as much as 95%.4
OBG Management: How does that differ from the mechanism of action of tamoxifen, another drug used in breast cancer patients?
Dr. Kaunitz: Tamoxifen is a selective estrogen receptor modulator (SERM). It blocks estrogen in breast tissue selectively, by competitively binding to estrogen receptors.5 However, tamoxifen has estrogenic effects in the uterus, bone, and liver, as well as other tissues.
The efficacy of AIs in preventing breast cancer recurrence in the first 2 years after breast cancer surgery is higher than that of tamoxifen. And unlike tamoxifen, the AIs do not increase the risk of venous thromboembolism or cause endometrial disease.
OBG Management: What effects do aromatase inhibitors have in premenopausal women?
Dr. Kaunitz: These agents are not recommended for use in premenopausal women because, in that population, the lion’s share of estrogen production takes place in the ovary rather than in adipose tissue and muscle. If you were to administer an AI to a premenopausal woman, the reduced hypothalamic and pituitary estrogen feedback could lead to ovarian stimulation—which could increase ovarian steroid production.
OBG Management: What about women who become amenorrheic as a result of chemotherapy or other cancer treatment? Do most oncologists assume that they are postmenopausal and prescribe an aromatase inhibitor?
Dr. Kaunitz: Clinicians should not assume that chemotherapy-induced amenorrhea signals permanent cessation of ovarian function. It is common for ovarian function to return in this setting. Accordingly, follicle-stimulating hormone (FSH) and estradiol levels should be assessed before an AI is considered as adjuvant therapy. Some investigators have suggested that the use of an AI in women who have chemotherapy-induced amenorrhea may actually increase the likelihood that ovarian function will return.6
OBG Management: Do all AIs produce the same effects?
Dr. Kaunitz: The AIs used in women with breast cancer are third-generation drugs. These AIs are classified as steroidal (type 1; exemestane) or nonsteroidal (type 2; anastrozole, letrozole). Exemestane, a steroid derived from androstenedione, inhibits the aromatase enzyme irreversibly. The nonsteroidal AIs are reversible.
Although all three AIs have numerous similarities, there are other distinctions between them in pharmacokinetics, mechanism of action, and toxicity—so they are not completely interchangeable.7 However, from our perspective as ObGyns caring for breast cancer survivors, we can assume that all three AIs will have similar effects on skeletal health and produce similar side effects in postmenopausal women.
- The American Society of Clinical Oncology recommends that adjuvant therapy for postmenopausal women who have hormone-positive breast cancer include an aromatase inhibitor (AI).
- AIs are not recommended for use in premenopausal women.
- By blocking the aromatase enzyme, AIs reduce endogenous estrogen levels by as much as 95%.4
- AIs are more effective than tamoxifen at preventing recurrence in the first 2 years after breast cancer surgery. Postmenopausal women taking an AI have a longer disease-free survival and time to recurrence than do women taking tamoxifen. They also have a lower incidence of contralateral breast cancer.
- The most prominent side effects of AI therapy include arthralgias and hot flushes, while the most serious health impact appears to be a decrease in bone mineral density (BMD). However, endometrial cancer, vaginal bleeding and discharge, cerebrovascular events, venous thromboembolic events, and hot flushes all are less common among women taking an AI than among those taking tamoxifen.
- The FDA strongly discourages the use of estrogen therapy—systemic or local—in women who are taking an AI. Accordingly, bisphospho-nate therapy is recommended as first-line treatment of low bone mineral density. Vaginal lubricants and moisturizers are the mainstay strategy for symptomatic genital atrophy. And gabapentin, selective serotonin reuptake inhibitors, and serotonin norepinephrine reuptake inhibitors are the mainstay of therapy for vasomotor flushes.
- Roughly 50% of women who are prescribed adjuvant endocrine therapy with tamoxifen or an AI discontinue the drug early.32 Early discontinuation is associated with an increase in mortality.33
How much do we know about these drugs?
OBG Management: How long does a woman typically take an AI?
Dr. Kaunitz: At present, in women treated for early-stage, hormone-positive breast cancer, the optimal duration of treatment is unknown. Most oncologists prescribe an AI for 5 years, the length of treatment in a prominent trial of the drugs.8
OBG Management: Is that duration likely to increase as more data come in?
Dr. Kaunitz: The optimal duration of adjuvant AI therapy will be determined by the findings of long-term clinical trials. The National Surgical Adjuvant Breast and Bowel Project B-42 trial may provide new insights into optimal duration of AI treatment after initial tamoxifen therapy.9
OBG Management: How thoroughly have AIs been studied in regard to their use in breast cancer survivors and women who have early-stage disease? How would you characterize the quantity and quality of data that we have so far?
Dr. Kaunitz: AIs have been extensively studied. The most important clinical trials of AIs in this setting, including the Anastrozole, Tamoxifen Alone or in Combination (ATAC) trial (over 6,000 participants, median follow-up of 100 months) and the Breast International Group (BIG) trial (almost 5,000 participants, median follow-up of 76 months) have been detailed in the recent ASCO report.10 These two large landmark trials, in particular, formed the basis for ASCO’s recommendations to routinely incorporate AIs into the therapy of postmenopausal women who have hormone-receptor–positive breast cancer.
OBG Management: In treating breast cancer, what other applications are AIs used for?
Dr. Kaunitz: AIs appear to be slightly more effective than tamoxifen in treating postmenopausal women who have metastatic breast cancer.11
They are approved as first-line therapy for breast cancer in:
- postmenopausal women who have hormone-receptor–positive disease
- postmenopausal women who have locally advanced disease when the hormone receptor is unknown
- postmenopausal women who have metastatic disease.
In addition, they are approved as second-line treatment of advanced breast cancer in postmenopausal women who have disease progression following tamoxifen therapy.12
How effective is AI therapy?
OBG Management: What do we know about the efficacy of these drugs?
Dr. Kaunitz: Most of the studies that have explored efficacy compare an AI with tamoxifen rather than with placebo. In the ATAC trial, after a median follow-up of 33 months, women who were taking anastrozole for early-stage breast cancer had longer disease-free survival and time to recurrence and a lower incidence of contralateral breast cancer than did women taking tamoxifen.8
After 4 years of follow-up in the ATAC trial, women taking anastrozole continued to have more favorable disease-free survival (86.9% vs 84.5% for anastrozole and tamoxifen, respectively; hazard ratio [HR], 0.86; 95% confidence interval [CI], 0.76–0.99; P =.03).13 They also had a more favorable time to recurrence than did women taking tamoxifen (HR, 0.83; 95% CI, 0.71–0.96; P =.015). And women taking anastrozole had a lower incidence of contralateral breast cancer, as well, although this different did not achieve statistical significance (HR, 0.62; 95% CI, 0.38–1.02; P =.062).13
In the BIG study, women taking letrozole had a 5-year disease-free survival estimate of 84.0%, compared with 81.4% for women taking tamoxifen.14 In addition, women taking letrozole were significantly less likely than those taking tamoxifen to experience an event that ended a period of disease-free survival (HR, 0.81; 95% CI, 0.70–0.93; P =.003), especially the event of distant recurrence (HR, 0.73; 95% CI, 0.60–0.88; P =.001).14
And a phase-3 study of exemestane versus tamoxifen in women who had metastatic breast cancer found that the AI produced a superior response rate (46% vs 31% for exemestane and tamoxifen, respectively; odds ratio [OR], 1.85; 95% CI, 1.21–2.82; P =.005). In addition, median progression-free survival was greater with exemestane (9.9 months; 95% CI, 8.7–11.8 months) than with tamoxifen (5.8 months; 95% CI, 5.3–8.1 months). However, there was no difference between arms in progression-free survival or overall survival.
Postmenopausal women who have hormone-receptor–positive breast cancer should consider taking an aromatase inhibitor (AI) to lengthen disease-free survival and lower the risk of recurrence. That’s one of the recommendations in updated guidelines issued earlier this year by the American Society of Clinical Oncology (ASCO). The guidelines suggest a duration of AI therapy of 5 years. In the event that a woman discontinues AI therapy before 5 years are up, she should consider using tamoxifen to bring the total duration of treatment to 5 years.
Other recommendations in the guidelines include:
- Women who have taken tamoxifen for 5 years stand to benefit from switching to an AI for as long as 5 additional years.
- When advising a woman about adjuvant therapy with an AI, clinicians should consider the potential adverse effects, which include osteoporosis, fracture, and arthralgias.
- The third-generation AIs on the market today have not been found to have clinically important differences between them. A woman who cannot tolerate a particular AI should consider switching to a different AI.
- Switching from an AI to tamoxifen (or vice versa) may be an appropriate option for patients who cannot tolerate a drug’s adverse effects. In the event of a switch to tamoxifen, the clinician should counsel the patient about its adverse effects, which include venous thromboembolism and endometrial polyps, hyperplasia, and cancer.
The full guidelines can be accessed at http://jco.ascopubs.org/content/early/2010/07/12/JCO.2009.26.3756.full.pdf.
—Andrew M. Kaunitz, MD
How well tolerated are AIs?
OBG Management: What adverse effects are associated with AIs?
Dr. Kaunitz: Although AIs, overall, are safe medications, their use is associated with a number of adverse events. The most prominent side effects include arthralgias and hot flushes, while the most serious health impact appears to be a decrease in bone mineral density (BMD).
However, the drugs are generally perceived as being easier to tolerate than tamoxifen. That’s because endometrial cancer, vaginal bleeding and discharge, cerebrovascular events, venous thromboembolic events, and hot flushes all are less common among women taking an AI than among those taking tamoxifen.8,13
For overweight women, who face an elevated baseline risk of thromboembolism, the availability of AIs represents a major advantage over tamoxifen. Similarly, AIs offer advantages over tamoxifen for women who have an intact uterus. In addition, postmenopausal women who are taking a selective serotonin reuptake inhibitor (SSRI) such as paroxetine should take an AI rather than tamoxifen, because the concomitant use of SSRIs attenuates the efficacy of tamoxifen.15
What can be done about the most prominent risks?
OBG Management: Let’s focus on what’s probably the best-known adverse effect of AIs—the heightened risk of osteoporosis and fracture. How significant is this effect?
Dr. Kaunitz: Because use of an AI is associated with a profound reduction in endogenous estrogen levels, it also decreases BMD and can lead to osteoporotic fractures. All major phase-3 trials of adjuvant use of AIs in women who have early breast cancer found an increased risk of fracture, with no significant differences between AIs.16
Fortunately, bisphosphonate therapy (oral or intravenous) has been found to reduce bone loss associated with AI therapy.17,18
Assessing baseline BMD is important as women initiate AI therapy. Although no consensus exists regarding follow-up BMD assessment in the setting of AI use, an interval of 2 years is prudent, with the follow-up study preferably performed at the same imaging center and by the same technician as the first. If baseline osteoporosis is observed at the lumbar spine or hip, bisphosphonate therapy is appropriate. If a woman taking an AI has low bone mass (osteopenia) but not osteoporosis, bisphosphonate therapy should be considered if any of the following risk factors are present:
- advanced age
- history of fracture
- glucocorticoid therapy
- parental history of hip fracture
- low body weight
- current smoking status
- excess alcohol consumption
- rheumatoid arthritis
- known risk factors for secondary osteoporosis.19
In breast cancer survivors initiating or continuing AI therapy, it is also appropriate to check a serum vitamin D level and ensure that intake of this nutrient is adequate.
Bisphosphonates may offer oncologic benefits, as well; preliminary evidence suggests that the drugs may prevent recurrence of the cancer and prolong survival.20
OBG Management: What can an ObGyn offer to a woman who complains of significant AI-related arthralgia?
Dr. Kaunitz: Bone and joint symptoms, including aches, pain, and stiffness that is bilateral and not associated with other evidence of rheumatologic disorders, are among the most common side effects of AI therapy. On the plus side of the equation, these symptoms are more likely to be mild to moderate than severe. On the negative side, no specific treatment has been found to be effective in relieving these symptoms, which usually resolve within 2 months or so after discontinuing AI therapy.10
OBG Management: Do AIs have a negative impact on cardiovascular health?
Dr. Kaunitz: Unlike tamoxifen, AIs do not increase the risk of thromboembolic disease. Although the use of an AI may modestly increase the risk of ischemic cardiovascular disease (and lipid changes), compared with tamoxifen, AIs do not appear to increase cardiovascular risk compared with placebo.21,22
OBG Management: Do the antiestrogenic effects of AIs have a significant impact on vaginal health and sexual desire?
Dr. Kaunitz: A review of published reports did not find that the use of AIs has a predictable impact on vaginal dryness or sexual desire.10 However, symptomatic genital atrophy is common in postmenopausal breast cancer survivors, whether or not they use adjuvant therapy.
Although the FDA considers the use of any estrogen (systemic or vaginal) following a diagnosis of breast cancer to be contraindicated, some breast cancer survivors who have symptomatic genital atrophy express an interest in the use of vaginal estrogen. Use of 25-μg estradiol tablets (Vagifem) is associated with a short-term increase in serum estradiol levels.23 This finding has reinforced caution among medical oncologists about the safety of vaginal estrogen in breast cancer survivors. (The 25-μg tablets are no longer marketed.) The lowest dosage of vaginal estrogen available for the treatment of genital atrophy is found in 10-μg estradiol tablets (Vagifem) and the estradiol (2-mg) 3-month vaginal ring (Estring). Nonetheless, in the absence of data, oncologists will likely continue to be concerned that even the lowest dosage of vaginal estrogen could attenuate the favorable impact of AIs on breast cancer. Accordingly, use of vaginal lubricants and moisturizers are the mainstay strategy for symptomatic genital atrophy.
OBG Management: What about the ubiquitous hot flush? Vasomotor symptoms may be more common in women who take tamoxifen, but women on AIs are also bothered by flushes. What are the alternatives to estrogen therapy?
Dr. Kaunitz: Both nonprescription and prescription alternatives are available. Nonprescription options include soy extract and red clover isoflavones, black cohosh, and Chinese herbs. However, none of these over-the-counter approaches has been found to be more effective than placebo in the treatment of menopausal hot flushes.24-26
As for prescription nonhormonal options, ObGyns should recognize that all such treatments are off-label and that none attain the efficacy of hormone therapy in the treatment of vasomotor symptoms. The best-studied and most effective medications include gabapentin, SSRIs (especially paroxetine), and serotonin-norepinephrine reuptake inhibitors (venlafaxine and desvenlafaxine).24,27
OBG Management: Is there any evidence that AIs impair cognitive function in postmenopausal women?
Dr. Kaunitz: Because estrogen is important for cognition, one might anticipate that the profound reduction in background estrogen associated with AI use would impair cognition. Fortunately, the evidence to date is reassuring. Substudies of the BIG trial and the Tamoxifen and Exemestane Adjuvant Multinational Trial indicate that, compared with tamoxifen (which is associated with declines in cognitive function in postmenopausal women), letrozole and exemestane do not diminish cognitive function.28,29
OBG Management: Overall, what is the typical impact of an AI on a woman’s quality of life?
Dr. Kaunitz: Most women do very well on an AI, finding it easier to tolerate than tamoxifen, as we have discussed. However, a significant minority of women is seriously bothered by the adverse effects, with arthralgias usually leading the pack of complaints.30,31
OBG Management: Do some women discontinue adjuvant endocrine therapy because of adverse effects?
Dr. Kaunitz: Regrettably, the answer is “Yes.” A recent study from Kaiser Permanente of northern California found that roughly 50% of women who are prescribed adjuvant endocrine therapy with tamoxifen or an AI discontinue the drug early.32
OBG Management: What can an ObGyn do to encourage compliance with and completion of AI therapy?
Dr. Kaunitz: First, it is critical that patients understand that AIs are lifesaving drugs. As a recent paper points out, early discontinuation or noncompliance with AI therapy is associated with higher mortality.33
Clinicians should also help breast cancer patients understand what common side effects to anticipate with these medications.
Finally, clinicians who understand the financial toll a breast cancer diagnosis and treatment can take are better positioned to help women overcome challenges that may interfere with long-term compliance with AI therapy.
OBG Management: Do you expect the use of AIs in breast cancer survivors to become more commonplace?
Dr. Kaunitz: Given how common breast cancer is, and given the new ASCO guidelines and the extensive literature upon which they are based, ObGyns will be seeing more women using AIs. Although we are not the physicians who prescribe AIs, we need to remain up to date on their benefits and side effects. This important class of drugs is positioned to improve outcomes for postmenopausal women with breast cancer.
- Do certain SSRIs reduce the benefits of tamoxifen in breast cancer survivors?
Examining the Evidence
Andrew M. Kaunitz, MD - Does the clinical breast exam boost the sensitivity of mammography?
Examining the Evidence
Jennifer Griffin, MD, and Mark Pearlman, MD - A guide to lotions and potions for treating vaginal atrophy
Danielle D. Marshall, MD, and Cheryl Iglesia, MD
We want to hear from you! Tell us what you think.
1. Horner MJ, Ries LAG, Krapcho M, et al. eds. SEER Cancer Statistics Review, 1975–2006. Bethesda, Md: National Cancer Institute; 2009.http://seer.cancer.gov/csr/1975_2006. Accessed August 18, 2010.
2. American Cancer Society. Breast cancer facts and figures, 2009–2010. Atlanta, Ga: American Cancer Society; 2010. http://www.cancer.org/Research/CancerFactsFigures/BreastCancerFactsFigures/breast-cancer-facts—figures-2009-2010. Accessed August 18, 2010.
3. Li CI, Daling JR, Malone KE. Incidence of invasive breast cancer by hormone receptor status from 1992 to 1998. J Clin Oncol. 2003;21(1):28-34.
4. Miller WR. Aromatase inhibitors: mechanism of action and role in the treatment of breast cancer. Semin Oncol. 2003;30(4 suppl 14):3-11.
5. Peng J, Sengupta S, Jordan VC. Potential of selective estrogen receptor modulators as treatments and preventives of breast cancer. Anticancer Agents Med Chem. 2009;9(5):481-499.
6. Smith IE, Dowsett M, Yap YS, et al. Adjuvant aromatase inhibitors for early breast cancer after chemotherapy-induced amenorrhea: caution and suggested guidelines. J Clin Oncol. 2006;24(16):2444-2447.
7. Nabholtz JM, Mouret-Reynier MA, Durando X, et al. Comparative review of anastrozole, letrozole and exemestane in the management of early breast cancer. Expert Opin Pharmacother. 2009;10(9):1435-1447.
8. Baum M, Budzar AU, Cuzick J, et al. ATAC Trialists’ Group. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomized trial. Lancet. 2002;359(9324):2131-2139.
9. Letrozole in treating postmenopausal women who have received hormone therapy for hormone receptor-positive breast cancer. National Cancer Institute Web site.http://www.cancer.gov/clinicaltrials/NSABP-B-42. Published August 16, 2010. Accessed August 18, 2010.
10. Burstein HJ, Prestrud AA, Seidenfeld J, et al. American Society of Clinical Oncology clinical practice guideline: update on adjuvant endocrine therapy for women with hormone-receptor–positive breast cancer. J Clin Oncol. 2010;28(23):3784-3796.
11. Mauri D, Pavlidis N, Polyzos NP, Ioanidis JP. Survival with aromatase inhibitors and inactivators versus standard hormonal therapy in advanced breast cancer: meta-analysis. J Natl Cancer Inst. 2006;98(18):1285-1291.
12. Arimidex [package insert]. AstraZeneca; 2009.
13. Baum M, Buzdar A, Cuzick J, et al. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early-stage breast cancer: results of the ATAC (Arimidex, Taxoxifen Alone or in Combination) trial efficacy and safety update analyses. Cancer. 2003;98(9):1802-1810.
14. Thurlimann B, Keshaviah A, Coates AS, et al. Breast International Group (BIG) 1-98 Collaborative Group. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med. 2005;353(26):2747-2757.
15. Kelly CM, Juurlink DM, Gomes T, et al. Selective serotonin reuptake inhibitors and breast cancer mortality in women receiving tamoxifen: a population-based cohort study. 2010;340:c693.-doi: 10.1136/bmj.c693.
16. Geisler J, Lonning PE. Impact of aromatase inhibitors on bone health in breast cancer patients. J Steroid Biochem Mol Biol. 2010;118(4–5):294-299.
17. Van Poznak C, Hannon RA, Mackey JR, et al. Prevention of aromatase inhibitor-induced bone loss using risedronate: the SABRE trial. J Clin Oncol. 2010;28(6):967-975.
18. Brufsky AM, Bosserman LD, Caradonna RR, et al. Zoledronic acid effectively prevents aromatase-inhibitor associated bone loss in postmenopausal women with early breast cancer receiving adjuvant letrozole. Z-FAST study 36-month follow-up results. Clin Breast Cancer. 2009;9(2):77-85.
19. Kanis JA, Borgstrom F, De Laet C, et al. Assessment of fracture risk. Osteoporosis Int. 2005;16(6):581-589.
20. Reeder JG, Brufsky AM. The role of bisphosphonates in the adjuvant setting for breast cancer. Oncology. 2010;24(6):462-467,475.
21. Nabholtz JM. Long-term safety of aromatase inhibitors in the treatment of breast cancer. Ther Clin Risk Manag. 2008;4(1):189-204.
22. Cuppone F, Bria E, Verma S, et al. Do adjuvant aromatase inhibitors increase the cardiovascular risk in postmenopausal women with early breast cancer? Meta-analysis of randomized trials. Cancer. 2008;112(2):260-267.
23. Kendall A, Dowsett M, Folkerd E, Smith I. Caution: Vaginal estradiol appears to be contraindicated in postmenopausal women on adjuvant aromatase inhibitors. Ann Oncol. 2006;17(4):584-587.
24. Nelson HD. Menopause. Lancet. 2008;371(9614):760-770.
25. Geller SE, Shulman LP, van Breemen RB, et al. Safety and efficacy of black cohosh and red clover for the management of vasomotor symptoms: a randomized controlled trial. Menopause. 2009;16(6):1156-1166.
26. Kaunitz AM. Effective herbal treatment of vasomotor symptoms—are we any closer? Menopause. 2009;16(3):428-429.
27. Archer DF, Dupont CM, Constantine GD, Pickar JH, Olivier S. Study 319 Investigators. Desvenlafaxine for the treatment of vasomotor symptoms associated with menopause: a double-blind, randomized, placebo-controlled trial of efficacy and safety. Am J Obstet Gynecol. 2009;200(3):238.e1-e10.
28. Phillips KA, Ribi K, Sun Z, et al. Cognitive function in postmenopausal women receiving adjuvant letrozole or tamoxifen for breast cancer in the BIG 1-98 randomized trial [published online ahead of print April 10, 2010]. Breast. doi:10.1016/j.breast.2010.03.025.
29. Schilder CM, Seynaeve C, Beex LV, et al. Effects of tamoxifen and exemestane on cognitive functioning of postmenopausal patients with breast cancer: results from the neuropsychological side study of the Tamoxifen and Exemestane Adjuvant Multinational Trial. J Clin Oncol. 2010;28(8):1294-1300.
30. Din OS, Dodwell D, Wakefield RJ, Coleman RE. Aromatase inhibitor-induced arthralgia in early breast cancer: what do we know and how can we find out more? Breast Cancer Res Treat. 2010;120(3):525-538.
31. Burstein HJ. Aromatase inhibitor-associated arthralgia syndrome. Breast. 2007;16(3):223-234.
32. Hershman DL, Kushi LH, Shao T, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients [published online ahead of print June 28, 2010]. J Clin Oncol. doi: 10.1200/JCO.2009.25.9655.
33. Hershman DL, Shao T, Kushi LH, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer [published online ahead of print August 28, 2010]. Breast Cancer Res Treat. doi: 10.1007/ s10549-010-1132-4.
Think breast cancer survivors are unlikely to show up in your practice? You should think again.
At last official count, 2.5 million women in the United States had a history of breast cancer.1 Most of them are now free of malignancy, but others are still grappling with the disease in some form or fashion.2 All need continuing health care.
Roughly two thirds of women who have breast cancer have disease that is hormone-receptor–positive.2,3 Recently updated guidelines from the American Society of Clinical Oncology (ASCO) recommend that adjuvant therapy for postmenopausal women who have hormone-positive breast cancer include an aromatase inhibitor (AI) (see a summary of these guidelines on page 36). That makes it likely that a good number of breast cancer survivors who visit your practice are taking one of these medications: anastrozole (Arimidex), letrozole (Femara), or exemestane (Aromasin). These drugs are antiestrogens, given to postmenopausal women to reduce the likelihood of disease recurrence and progression.
The antiestrogenic properties of these drugs are what make them lifesavers. But the same qualities can create a range of health issues, from increased risk of osteoporosis and fracture to vasomotor and joint symptoms. And although ObGyns are not the physicians who prescribe these drugs, you may be the provider one of these women consults about their side effects and related issues.
To find out the latest on the management of women who are taking one of these agents, we inserted ourselves into the busy schedule of Andrew M. Kaunitz, MD, who agreed to address some fundamental—and some not so basic—questions about the drugs. In this extended Q&A, Dr. Kaunitz touches on mechanism of action, benefits versus risks, common side effects, compliance with therapy, and the ill effects of early discontinuation.

Aromatase inhibitors are better than tamoxifen at reducing the risk of breast cancer recurrence in postmenopausal women who have hormone-receptor–positive disease. But, they increase the risk of osteoporosis and fracture and often cause arthralgias and other complaints that ObGyn practitioners may be called upon to manage.
OBG Management: What is the overall aim of adjuvant endocrine therapy in the setting of breast cancer?
Dr. Kaunitz: Endocrine therapy—specifically, use of an AI—prevents the stimulation of breast cancer cells by endogenous estrogen. In other words, aromatase inhibitors suppress the growth of cancer cells that have estrogen receptors. These drugs also inhibit aromatase near any breast tumor and reduce estrogen levels in breast tissue.
OBG Management: What is the mechanism of action of adjuvant endocrine therapy?
Dr. Kaunitz: In postmenopausal women, androgens are converted to estrogens via the aromatase enzyme, which is present in adipose tissue and other sites. By blocking this enzyme, AIs reduce endogenous estrogen levels by as much as 95%.4
OBG Management: How does that differ from the mechanism of action of tamoxifen, another drug used in breast cancer patients?
Dr. Kaunitz: Tamoxifen is a selective estrogen receptor modulator (SERM). It blocks estrogen in breast tissue selectively, by competitively binding to estrogen receptors.5 However, tamoxifen has estrogenic effects in the uterus, bone, and liver, as well as other tissues.
The efficacy of AIs in preventing breast cancer recurrence in the first 2 years after breast cancer surgery is higher than that of tamoxifen. And unlike tamoxifen, the AIs do not increase the risk of venous thromboembolism or cause endometrial disease.
OBG Management: What effects do aromatase inhibitors have in premenopausal women?
Dr. Kaunitz: These agents are not recommended for use in premenopausal women because, in that population, the lion’s share of estrogen production takes place in the ovary rather than in adipose tissue and muscle. If you were to administer an AI to a premenopausal woman, the reduced hypothalamic and pituitary estrogen feedback could lead to ovarian stimulation—which could increase ovarian steroid production.
OBG Management: What about women who become amenorrheic as a result of chemotherapy or other cancer treatment? Do most oncologists assume that they are postmenopausal and prescribe an aromatase inhibitor?
Dr. Kaunitz: Clinicians should not assume that chemotherapy-induced amenorrhea signals permanent cessation of ovarian function. It is common for ovarian function to return in this setting. Accordingly, follicle-stimulating hormone (FSH) and estradiol levels should be assessed before an AI is considered as adjuvant therapy. Some investigators have suggested that the use of an AI in women who have chemotherapy-induced amenorrhea may actually increase the likelihood that ovarian function will return.6
OBG Management: Do all AIs produce the same effects?
Dr. Kaunitz: The AIs used in women with breast cancer are third-generation drugs. These AIs are classified as steroidal (type 1; exemestane) or nonsteroidal (type 2; anastrozole, letrozole). Exemestane, a steroid derived from androstenedione, inhibits the aromatase enzyme irreversibly. The nonsteroidal AIs are reversible.
Although all three AIs have numerous similarities, there are other distinctions between them in pharmacokinetics, mechanism of action, and toxicity—so they are not completely interchangeable.7 However, from our perspective as ObGyns caring for breast cancer survivors, we can assume that all three AIs will have similar effects on skeletal health and produce similar side effects in postmenopausal women.
- The American Society of Clinical Oncology recommends that adjuvant therapy for postmenopausal women who have hormone-positive breast cancer include an aromatase inhibitor (AI).
- AIs are not recommended for use in premenopausal women.
- By blocking the aromatase enzyme, AIs reduce endogenous estrogen levels by as much as 95%.4
- AIs are more effective than tamoxifen at preventing recurrence in the first 2 years after breast cancer surgery. Postmenopausal women taking an AI have a longer disease-free survival and time to recurrence than do women taking tamoxifen. They also have a lower incidence of contralateral breast cancer.
- The most prominent side effects of AI therapy include arthralgias and hot flushes, while the most serious health impact appears to be a decrease in bone mineral density (BMD). However, endometrial cancer, vaginal bleeding and discharge, cerebrovascular events, venous thromboembolic events, and hot flushes all are less common among women taking an AI than among those taking tamoxifen.
- The FDA strongly discourages the use of estrogen therapy—systemic or local—in women who are taking an AI. Accordingly, bisphospho-nate therapy is recommended as first-line treatment of low bone mineral density. Vaginal lubricants and moisturizers are the mainstay strategy for symptomatic genital atrophy. And gabapentin, selective serotonin reuptake inhibitors, and serotonin norepinephrine reuptake inhibitors are the mainstay of therapy for vasomotor flushes.
- Roughly 50% of women who are prescribed adjuvant endocrine therapy with tamoxifen or an AI discontinue the drug early.32 Early discontinuation is associated with an increase in mortality.33
How much do we know about these drugs?
OBG Management: How long does a woman typically take an AI?
Dr. Kaunitz: At present, in women treated for early-stage, hormone-positive breast cancer, the optimal duration of treatment is unknown. Most oncologists prescribe an AI for 5 years, the length of treatment in a prominent trial of the drugs.8
OBG Management: Is that duration likely to increase as more data come in?
Dr. Kaunitz: The optimal duration of adjuvant AI therapy will be determined by the findings of long-term clinical trials. The National Surgical Adjuvant Breast and Bowel Project B-42 trial may provide new insights into optimal duration of AI treatment after initial tamoxifen therapy.9
OBG Management: How thoroughly have AIs been studied in regard to their use in breast cancer survivors and women who have early-stage disease? How would you characterize the quantity and quality of data that we have so far?
Dr. Kaunitz: AIs have been extensively studied. The most important clinical trials of AIs in this setting, including the Anastrozole, Tamoxifen Alone or in Combination (ATAC) trial (over 6,000 participants, median follow-up of 100 months) and the Breast International Group (BIG) trial (almost 5,000 participants, median follow-up of 76 months) have been detailed in the recent ASCO report.10 These two large landmark trials, in particular, formed the basis for ASCO’s recommendations to routinely incorporate AIs into the therapy of postmenopausal women who have hormone-receptor–positive breast cancer.
OBG Management: In treating breast cancer, what other applications are AIs used for?
Dr. Kaunitz: AIs appear to be slightly more effective than tamoxifen in treating postmenopausal women who have metastatic breast cancer.11
They are approved as first-line therapy for breast cancer in:
- postmenopausal women who have hormone-receptor–positive disease
- postmenopausal women who have locally advanced disease when the hormone receptor is unknown
- postmenopausal women who have metastatic disease.
In addition, they are approved as second-line treatment of advanced breast cancer in postmenopausal women who have disease progression following tamoxifen therapy.12
How effective is AI therapy?
OBG Management: What do we know about the efficacy of these drugs?
Dr. Kaunitz: Most of the studies that have explored efficacy compare an AI with tamoxifen rather than with placebo. In the ATAC trial, after a median follow-up of 33 months, women who were taking anastrozole for early-stage breast cancer had longer disease-free survival and time to recurrence and a lower incidence of contralateral breast cancer than did women taking tamoxifen.8
After 4 years of follow-up in the ATAC trial, women taking anastrozole continued to have more favorable disease-free survival (86.9% vs 84.5% for anastrozole and tamoxifen, respectively; hazard ratio [HR], 0.86; 95% confidence interval [CI], 0.76–0.99; P =.03).13 They also had a more favorable time to recurrence than did women taking tamoxifen (HR, 0.83; 95% CI, 0.71–0.96; P =.015). And women taking anastrozole had a lower incidence of contralateral breast cancer, as well, although this different did not achieve statistical significance (HR, 0.62; 95% CI, 0.38–1.02; P =.062).13
In the BIG study, women taking letrozole had a 5-year disease-free survival estimate of 84.0%, compared with 81.4% for women taking tamoxifen.14 In addition, women taking letrozole were significantly less likely than those taking tamoxifen to experience an event that ended a period of disease-free survival (HR, 0.81; 95% CI, 0.70–0.93; P =.003), especially the event of distant recurrence (HR, 0.73; 95% CI, 0.60–0.88; P =.001).14
And a phase-3 study of exemestane versus tamoxifen in women who had metastatic breast cancer found that the AI produced a superior response rate (46% vs 31% for exemestane and tamoxifen, respectively; odds ratio [OR], 1.85; 95% CI, 1.21–2.82; P =.005). In addition, median progression-free survival was greater with exemestane (9.9 months; 95% CI, 8.7–11.8 months) than with tamoxifen (5.8 months; 95% CI, 5.3–8.1 months). However, there was no difference between arms in progression-free survival or overall survival.
Postmenopausal women who have hormone-receptor–positive breast cancer should consider taking an aromatase inhibitor (AI) to lengthen disease-free survival and lower the risk of recurrence. That’s one of the recommendations in updated guidelines issued earlier this year by the American Society of Clinical Oncology (ASCO). The guidelines suggest a duration of AI therapy of 5 years. In the event that a woman discontinues AI therapy before 5 years are up, she should consider using tamoxifen to bring the total duration of treatment to 5 years.
Other recommendations in the guidelines include:
- Women who have taken tamoxifen for 5 years stand to benefit from switching to an AI for as long as 5 additional years.
- When advising a woman about adjuvant therapy with an AI, clinicians should consider the potential adverse effects, which include osteoporosis, fracture, and arthralgias.
- The third-generation AIs on the market today have not been found to have clinically important differences between them. A woman who cannot tolerate a particular AI should consider switching to a different AI.
- Switching from an AI to tamoxifen (or vice versa) may be an appropriate option for patients who cannot tolerate a drug’s adverse effects. In the event of a switch to tamoxifen, the clinician should counsel the patient about its adverse effects, which include venous thromboembolism and endometrial polyps, hyperplasia, and cancer.
The full guidelines can be accessed at http://jco.ascopubs.org/content/early/2010/07/12/JCO.2009.26.3756.full.pdf.
—Andrew M. Kaunitz, MD
How well tolerated are AIs?
OBG Management: What adverse effects are associated with AIs?
Dr. Kaunitz: Although AIs, overall, are safe medications, their use is associated with a number of adverse events. The most prominent side effects include arthralgias and hot flushes, while the most serious health impact appears to be a decrease in bone mineral density (BMD).
However, the drugs are generally perceived as being easier to tolerate than tamoxifen. That’s because endometrial cancer, vaginal bleeding and discharge, cerebrovascular events, venous thromboembolic events, and hot flushes all are less common among women taking an AI than among those taking tamoxifen.8,13
For overweight women, who face an elevated baseline risk of thromboembolism, the availability of AIs represents a major advantage over tamoxifen. Similarly, AIs offer advantages over tamoxifen for women who have an intact uterus. In addition, postmenopausal women who are taking a selective serotonin reuptake inhibitor (SSRI) such as paroxetine should take an AI rather than tamoxifen, because the concomitant use of SSRIs attenuates the efficacy of tamoxifen.15
What can be done about the most prominent risks?
OBG Management: Let’s focus on what’s probably the best-known adverse effect of AIs—the heightened risk of osteoporosis and fracture. How significant is this effect?
Dr. Kaunitz: Because use of an AI is associated with a profound reduction in endogenous estrogen levels, it also decreases BMD and can lead to osteoporotic fractures. All major phase-3 trials of adjuvant use of AIs in women who have early breast cancer found an increased risk of fracture, with no significant differences between AIs.16
Fortunately, bisphosphonate therapy (oral or intravenous) has been found to reduce bone loss associated with AI therapy.17,18
Assessing baseline BMD is important as women initiate AI therapy. Although no consensus exists regarding follow-up BMD assessment in the setting of AI use, an interval of 2 years is prudent, with the follow-up study preferably performed at the same imaging center and by the same technician as the first. If baseline osteoporosis is observed at the lumbar spine or hip, bisphosphonate therapy is appropriate. If a woman taking an AI has low bone mass (osteopenia) but not osteoporosis, bisphosphonate therapy should be considered if any of the following risk factors are present:
- advanced age
- history of fracture
- glucocorticoid therapy
- parental history of hip fracture
- low body weight
- current smoking status
- excess alcohol consumption
- rheumatoid arthritis
- known risk factors for secondary osteoporosis.19
In breast cancer survivors initiating or continuing AI therapy, it is also appropriate to check a serum vitamin D level and ensure that intake of this nutrient is adequate.
Bisphosphonates may offer oncologic benefits, as well; preliminary evidence suggests that the drugs may prevent recurrence of the cancer and prolong survival.20
OBG Management: What can an ObGyn offer to a woman who complains of significant AI-related arthralgia?
Dr. Kaunitz: Bone and joint symptoms, including aches, pain, and stiffness that is bilateral and not associated with other evidence of rheumatologic disorders, are among the most common side effects of AI therapy. On the plus side of the equation, these symptoms are more likely to be mild to moderate than severe. On the negative side, no specific treatment has been found to be effective in relieving these symptoms, which usually resolve within 2 months or so after discontinuing AI therapy.10
OBG Management: Do AIs have a negative impact on cardiovascular health?
Dr. Kaunitz: Unlike tamoxifen, AIs do not increase the risk of thromboembolic disease. Although the use of an AI may modestly increase the risk of ischemic cardiovascular disease (and lipid changes), compared with tamoxifen, AIs do not appear to increase cardiovascular risk compared with placebo.21,22
OBG Management: Do the antiestrogenic effects of AIs have a significant impact on vaginal health and sexual desire?
Dr. Kaunitz: A review of published reports did not find that the use of AIs has a predictable impact on vaginal dryness or sexual desire.10 However, symptomatic genital atrophy is common in postmenopausal breast cancer survivors, whether or not they use adjuvant therapy.
Although the FDA considers the use of any estrogen (systemic or vaginal) following a diagnosis of breast cancer to be contraindicated, some breast cancer survivors who have symptomatic genital atrophy express an interest in the use of vaginal estrogen. Use of 25-μg estradiol tablets (Vagifem) is associated with a short-term increase in serum estradiol levels.23 This finding has reinforced caution among medical oncologists about the safety of vaginal estrogen in breast cancer survivors. (The 25-μg tablets are no longer marketed.) The lowest dosage of vaginal estrogen available for the treatment of genital atrophy is found in 10-μg estradiol tablets (Vagifem) and the estradiol (2-mg) 3-month vaginal ring (Estring). Nonetheless, in the absence of data, oncologists will likely continue to be concerned that even the lowest dosage of vaginal estrogen could attenuate the favorable impact of AIs on breast cancer. Accordingly, use of vaginal lubricants and moisturizers are the mainstay strategy for symptomatic genital atrophy.
OBG Management: What about the ubiquitous hot flush? Vasomotor symptoms may be more common in women who take tamoxifen, but women on AIs are also bothered by flushes. What are the alternatives to estrogen therapy?
Dr. Kaunitz: Both nonprescription and prescription alternatives are available. Nonprescription options include soy extract and red clover isoflavones, black cohosh, and Chinese herbs. However, none of these over-the-counter approaches has been found to be more effective than placebo in the treatment of menopausal hot flushes.24-26
As for prescription nonhormonal options, ObGyns should recognize that all such treatments are off-label and that none attain the efficacy of hormone therapy in the treatment of vasomotor symptoms. The best-studied and most effective medications include gabapentin, SSRIs (especially paroxetine), and serotonin-norepinephrine reuptake inhibitors (venlafaxine and desvenlafaxine).24,27
OBG Management: Is there any evidence that AIs impair cognitive function in postmenopausal women?
Dr. Kaunitz: Because estrogen is important for cognition, one might anticipate that the profound reduction in background estrogen associated with AI use would impair cognition. Fortunately, the evidence to date is reassuring. Substudies of the BIG trial and the Tamoxifen and Exemestane Adjuvant Multinational Trial indicate that, compared with tamoxifen (which is associated with declines in cognitive function in postmenopausal women), letrozole and exemestane do not diminish cognitive function.28,29
OBG Management: Overall, what is the typical impact of an AI on a woman’s quality of life?
Dr. Kaunitz: Most women do very well on an AI, finding it easier to tolerate than tamoxifen, as we have discussed. However, a significant minority of women is seriously bothered by the adverse effects, with arthralgias usually leading the pack of complaints.30,31
OBG Management: Do some women discontinue adjuvant endocrine therapy because of adverse effects?
Dr. Kaunitz: Regrettably, the answer is “Yes.” A recent study from Kaiser Permanente of northern California found that roughly 50% of women who are prescribed adjuvant endocrine therapy with tamoxifen or an AI discontinue the drug early.32
OBG Management: What can an ObGyn do to encourage compliance with and completion of AI therapy?
Dr. Kaunitz: First, it is critical that patients understand that AIs are lifesaving drugs. As a recent paper points out, early discontinuation or noncompliance with AI therapy is associated with higher mortality.33
Clinicians should also help breast cancer patients understand what common side effects to anticipate with these medications.
Finally, clinicians who understand the financial toll a breast cancer diagnosis and treatment can take are better positioned to help women overcome challenges that may interfere with long-term compliance with AI therapy.
OBG Management: Do you expect the use of AIs in breast cancer survivors to become more commonplace?
Dr. Kaunitz: Given how common breast cancer is, and given the new ASCO guidelines and the extensive literature upon which they are based, ObGyns will be seeing more women using AIs. Although we are not the physicians who prescribe AIs, we need to remain up to date on their benefits and side effects. This important class of drugs is positioned to improve outcomes for postmenopausal women with breast cancer.
- Do certain SSRIs reduce the benefits of tamoxifen in breast cancer survivors?
Examining the Evidence
Andrew M. Kaunitz, MD - Does the clinical breast exam boost the sensitivity of mammography?
Examining the Evidence
Jennifer Griffin, MD, and Mark Pearlman, MD - A guide to lotions and potions for treating vaginal atrophy
Danielle D. Marshall, MD, and Cheryl Iglesia, MD
We want to hear from you! Tell us what you think.
Think breast cancer survivors are unlikely to show up in your practice? You should think again.
At last official count, 2.5 million women in the United States had a history of breast cancer.1 Most of them are now free of malignancy, but others are still grappling with the disease in some form or fashion.2 All need continuing health care.
Roughly two thirds of women who have breast cancer have disease that is hormone-receptor–positive.2,3 Recently updated guidelines from the American Society of Clinical Oncology (ASCO) recommend that adjuvant therapy for postmenopausal women who have hormone-positive breast cancer include an aromatase inhibitor (AI) (see a summary of these guidelines on page 36). That makes it likely that a good number of breast cancer survivors who visit your practice are taking one of these medications: anastrozole (Arimidex), letrozole (Femara), or exemestane (Aromasin). These drugs are antiestrogens, given to postmenopausal women to reduce the likelihood of disease recurrence and progression.
The antiestrogenic properties of these drugs are what make them lifesavers. But the same qualities can create a range of health issues, from increased risk of osteoporosis and fracture to vasomotor and joint symptoms. And although ObGyns are not the physicians who prescribe these drugs, you may be the provider one of these women consults about their side effects and related issues.
To find out the latest on the management of women who are taking one of these agents, we inserted ourselves into the busy schedule of Andrew M. Kaunitz, MD, who agreed to address some fundamental—and some not so basic—questions about the drugs. In this extended Q&A, Dr. Kaunitz touches on mechanism of action, benefits versus risks, common side effects, compliance with therapy, and the ill effects of early discontinuation.

Aromatase inhibitors are better than tamoxifen at reducing the risk of breast cancer recurrence in postmenopausal women who have hormone-receptor–positive disease. But, they increase the risk of osteoporosis and fracture and often cause arthralgias and other complaints that ObGyn practitioners may be called upon to manage.
OBG Management: What is the overall aim of adjuvant endocrine therapy in the setting of breast cancer?
Dr. Kaunitz: Endocrine therapy—specifically, use of an AI—prevents the stimulation of breast cancer cells by endogenous estrogen. In other words, aromatase inhibitors suppress the growth of cancer cells that have estrogen receptors. These drugs also inhibit aromatase near any breast tumor and reduce estrogen levels in breast tissue.
OBG Management: What is the mechanism of action of adjuvant endocrine therapy?
Dr. Kaunitz: In postmenopausal women, androgens are converted to estrogens via the aromatase enzyme, which is present in adipose tissue and other sites. By blocking this enzyme, AIs reduce endogenous estrogen levels by as much as 95%.4
OBG Management: How does that differ from the mechanism of action of tamoxifen, another drug used in breast cancer patients?
Dr. Kaunitz: Tamoxifen is a selective estrogen receptor modulator (SERM). It blocks estrogen in breast tissue selectively, by competitively binding to estrogen receptors.5 However, tamoxifen has estrogenic effects in the uterus, bone, and liver, as well as other tissues.
The efficacy of AIs in preventing breast cancer recurrence in the first 2 years after breast cancer surgery is higher than that of tamoxifen. And unlike tamoxifen, the AIs do not increase the risk of venous thromboembolism or cause endometrial disease.
OBG Management: What effects do aromatase inhibitors have in premenopausal women?
Dr. Kaunitz: These agents are not recommended for use in premenopausal women because, in that population, the lion’s share of estrogen production takes place in the ovary rather than in adipose tissue and muscle. If you were to administer an AI to a premenopausal woman, the reduced hypothalamic and pituitary estrogen feedback could lead to ovarian stimulation—which could increase ovarian steroid production.
OBG Management: What about women who become amenorrheic as a result of chemotherapy or other cancer treatment? Do most oncologists assume that they are postmenopausal and prescribe an aromatase inhibitor?
Dr. Kaunitz: Clinicians should not assume that chemotherapy-induced amenorrhea signals permanent cessation of ovarian function. It is common for ovarian function to return in this setting. Accordingly, follicle-stimulating hormone (FSH) and estradiol levels should be assessed before an AI is considered as adjuvant therapy. Some investigators have suggested that the use of an AI in women who have chemotherapy-induced amenorrhea may actually increase the likelihood that ovarian function will return.6
OBG Management: Do all AIs produce the same effects?
Dr. Kaunitz: The AIs used in women with breast cancer are third-generation drugs. These AIs are classified as steroidal (type 1; exemestane) or nonsteroidal (type 2; anastrozole, letrozole). Exemestane, a steroid derived from androstenedione, inhibits the aromatase enzyme irreversibly. The nonsteroidal AIs are reversible.
Although all three AIs have numerous similarities, there are other distinctions between them in pharmacokinetics, mechanism of action, and toxicity—so they are not completely interchangeable.7 However, from our perspective as ObGyns caring for breast cancer survivors, we can assume that all three AIs will have similar effects on skeletal health and produce similar side effects in postmenopausal women.
- The American Society of Clinical Oncology recommends that adjuvant therapy for postmenopausal women who have hormone-positive breast cancer include an aromatase inhibitor (AI).
- AIs are not recommended for use in premenopausal women.
- By blocking the aromatase enzyme, AIs reduce endogenous estrogen levels by as much as 95%.4
- AIs are more effective than tamoxifen at preventing recurrence in the first 2 years after breast cancer surgery. Postmenopausal women taking an AI have a longer disease-free survival and time to recurrence than do women taking tamoxifen. They also have a lower incidence of contralateral breast cancer.
- The most prominent side effects of AI therapy include arthralgias and hot flushes, while the most serious health impact appears to be a decrease in bone mineral density (BMD). However, endometrial cancer, vaginal bleeding and discharge, cerebrovascular events, venous thromboembolic events, and hot flushes all are less common among women taking an AI than among those taking tamoxifen.
- The FDA strongly discourages the use of estrogen therapy—systemic or local—in women who are taking an AI. Accordingly, bisphospho-nate therapy is recommended as first-line treatment of low bone mineral density. Vaginal lubricants and moisturizers are the mainstay strategy for symptomatic genital atrophy. And gabapentin, selective serotonin reuptake inhibitors, and serotonin norepinephrine reuptake inhibitors are the mainstay of therapy for vasomotor flushes.
- Roughly 50% of women who are prescribed adjuvant endocrine therapy with tamoxifen or an AI discontinue the drug early.32 Early discontinuation is associated with an increase in mortality.33
How much do we know about these drugs?
OBG Management: How long does a woman typically take an AI?
Dr. Kaunitz: At present, in women treated for early-stage, hormone-positive breast cancer, the optimal duration of treatment is unknown. Most oncologists prescribe an AI for 5 years, the length of treatment in a prominent trial of the drugs.8
OBG Management: Is that duration likely to increase as more data come in?
Dr. Kaunitz: The optimal duration of adjuvant AI therapy will be determined by the findings of long-term clinical trials. The National Surgical Adjuvant Breast and Bowel Project B-42 trial may provide new insights into optimal duration of AI treatment after initial tamoxifen therapy.9
OBG Management: How thoroughly have AIs been studied in regard to their use in breast cancer survivors and women who have early-stage disease? How would you characterize the quantity and quality of data that we have so far?
Dr. Kaunitz: AIs have been extensively studied. The most important clinical trials of AIs in this setting, including the Anastrozole, Tamoxifen Alone or in Combination (ATAC) trial (over 6,000 participants, median follow-up of 100 months) and the Breast International Group (BIG) trial (almost 5,000 participants, median follow-up of 76 months) have been detailed in the recent ASCO report.10 These two large landmark trials, in particular, formed the basis for ASCO’s recommendations to routinely incorporate AIs into the therapy of postmenopausal women who have hormone-receptor–positive breast cancer.
OBG Management: In treating breast cancer, what other applications are AIs used for?
Dr. Kaunitz: AIs appear to be slightly more effective than tamoxifen in treating postmenopausal women who have metastatic breast cancer.11
They are approved as first-line therapy for breast cancer in:
- postmenopausal women who have hormone-receptor–positive disease
- postmenopausal women who have locally advanced disease when the hormone receptor is unknown
- postmenopausal women who have metastatic disease.
In addition, they are approved as second-line treatment of advanced breast cancer in postmenopausal women who have disease progression following tamoxifen therapy.12
How effective is AI therapy?
OBG Management: What do we know about the efficacy of these drugs?
Dr. Kaunitz: Most of the studies that have explored efficacy compare an AI with tamoxifen rather than with placebo. In the ATAC trial, after a median follow-up of 33 months, women who were taking anastrozole for early-stage breast cancer had longer disease-free survival and time to recurrence and a lower incidence of contralateral breast cancer than did women taking tamoxifen.8
After 4 years of follow-up in the ATAC trial, women taking anastrozole continued to have more favorable disease-free survival (86.9% vs 84.5% for anastrozole and tamoxifen, respectively; hazard ratio [HR], 0.86; 95% confidence interval [CI], 0.76–0.99; P =.03).13 They also had a more favorable time to recurrence than did women taking tamoxifen (HR, 0.83; 95% CI, 0.71–0.96; P =.015). And women taking anastrozole had a lower incidence of contralateral breast cancer, as well, although this different did not achieve statistical significance (HR, 0.62; 95% CI, 0.38–1.02; P =.062).13
In the BIG study, women taking letrozole had a 5-year disease-free survival estimate of 84.0%, compared with 81.4% for women taking tamoxifen.14 In addition, women taking letrozole were significantly less likely than those taking tamoxifen to experience an event that ended a period of disease-free survival (HR, 0.81; 95% CI, 0.70–0.93; P =.003), especially the event of distant recurrence (HR, 0.73; 95% CI, 0.60–0.88; P =.001).14
And a phase-3 study of exemestane versus tamoxifen in women who had metastatic breast cancer found that the AI produced a superior response rate (46% vs 31% for exemestane and tamoxifen, respectively; odds ratio [OR], 1.85; 95% CI, 1.21–2.82; P =.005). In addition, median progression-free survival was greater with exemestane (9.9 months; 95% CI, 8.7–11.8 months) than with tamoxifen (5.8 months; 95% CI, 5.3–8.1 months). However, there was no difference between arms in progression-free survival or overall survival.
Postmenopausal women who have hormone-receptor–positive breast cancer should consider taking an aromatase inhibitor (AI) to lengthen disease-free survival and lower the risk of recurrence. That’s one of the recommendations in updated guidelines issued earlier this year by the American Society of Clinical Oncology (ASCO). The guidelines suggest a duration of AI therapy of 5 years. In the event that a woman discontinues AI therapy before 5 years are up, she should consider using tamoxifen to bring the total duration of treatment to 5 years.
Other recommendations in the guidelines include:
- Women who have taken tamoxifen for 5 years stand to benefit from switching to an AI for as long as 5 additional years.
- When advising a woman about adjuvant therapy with an AI, clinicians should consider the potential adverse effects, which include osteoporosis, fracture, and arthralgias.
- The third-generation AIs on the market today have not been found to have clinically important differences between them. A woman who cannot tolerate a particular AI should consider switching to a different AI.
- Switching from an AI to tamoxifen (or vice versa) may be an appropriate option for patients who cannot tolerate a drug’s adverse effects. In the event of a switch to tamoxifen, the clinician should counsel the patient about its adverse effects, which include venous thromboembolism and endometrial polyps, hyperplasia, and cancer.
The full guidelines can be accessed at http://jco.ascopubs.org/content/early/2010/07/12/JCO.2009.26.3756.full.pdf.
—Andrew M. Kaunitz, MD
How well tolerated are AIs?
OBG Management: What adverse effects are associated with AIs?
Dr. Kaunitz: Although AIs, overall, are safe medications, their use is associated with a number of adverse events. The most prominent side effects include arthralgias and hot flushes, while the most serious health impact appears to be a decrease in bone mineral density (BMD).
However, the drugs are generally perceived as being easier to tolerate than tamoxifen. That’s because endometrial cancer, vaginal bleeding and discharge, cerebrovascular events, venous thromboembolic events, and hot flushes all are less common among women taking an AI than among those taking tamoxifen.8,13
For overweight women, who face an elevated baseline risk of thromboembolism, the availability of AIs represents a major advantage over tamoxifen. Similarly, AIs offer advantages over tamoxifen for women who have an intact uterus. In addition, postmenopausal women who are taking a selective serotonin reuptake inhibitor (SSRI) such as paroxetine should take an AI rather than tamoxifen, because the concomitant use of SSRIs attenuates the efficacy of tamoxifen.15
What can be done about the most prominent risks?
OBG Management: Let’s focus on what’s probably the best-known adverse effect of AIs—the heightened risk of osteoporosis and fracture. How significant is this effect?
Dr. Kaunitz: Because use of an AI is associated with a profound reduction in endogenous estrogen levels, it also decreases BMD and can lead to osteoporotic fractures. All major phase-3 trials of adjuvant use of AIs in women who have early breast cancer found an increased risk of fracture, with no significant differences between AIs.16
Fortunately, bisphosphonate therapy (oral or intravenous) has been found to reduce bone loss associated with AI therapy.17,18
Assessing baseline BMD is important as women initiate AI therapy. Although no consensus exists regarding follow-up BMD assessment in the setting of AI use, an interval of 2 years is prudent, with the follow-up study preferably performed at the same imaging center and by the same technician as the first. If baseline osteoporosis is observed at the lumbar spine or hip, bisphosphonate therapy is appropriate. If a woman taking an AI has low bone mass (osteopenia) but not osteoporosis, bisphosphonate therapy should be considered if any of the following risk factors are present:
- advanced age
- history of fracture
- glucocorticoid therapy
- parental history of hip fracture
- low body weight
- current smoking status
- excess alcohol consumption
- rheumatoid arthritis
- known risk factors for secondary osteoporosis.19
In breast cancer survivors initiating or continuing AI therapy, it is also appropriate to check a serum vitamin D level and ensure that intake of this nutrient is adequate.
Bisphosphonates may offer oncologic benefits, as well; preliminary evidence suggests that the drugs may prevent recurrence of the cancer and prolong survival.20
OBG Management: What can an ObGyn offer to a woman who complains of significant AI-related arthralgia?
Dr. Kaunitz: Bone and joint symptoms, including aches, pain, and stiffness that is bilateral and not associated with other evidence of rheumatologic disorders, are among the most common side effects of AI therapy. On the plus side of the equation, these symptoms are more likely to be mild to moderate than severe. On the negative side, no specific treatment has been found to be effective in relieving these symptoms, which usually resolve within 2 months or so after discontinuing AI therapy.10
OBG Management: Do AIs have a negative impact on cardiovascular health?
Dr. Kaunitz: Unlike tamoxifen, AIs do not increase the risk of thromboembolic disease. Although the use of an AI may modestly increase the risk of ischemic cardiovascular disease (and lipid changes), compared with tamoxifen, AIs do not appear to increase cardiovascular risk compared with placebo.21,22
OBG Management: Do the antiestrogenic effects of AIs have a significant impact on vaginal health and sexual desire?
Dr. Kaunitz: A review of published reports did not find that the use of AIs has a predictable impact on vaginal dryness or sexual desire.10 However, symptomatic genital atrophy is common in postmenopausal breast cancer survivors, whether or not they use adjuvant therapy.
Although the FDA considers the use of any estrogen (systemic or vaginal) following a diagnosis of breast cancer to be contraindicated, some breast cancer survivors who have symptomatic genital atrophy express an interest in the use of vaginal estrogen. Use of 25-μg estradiol tablets (Vagifem) is associated with a short-term increase in serum estradiol levels.23 This finding has reinforced caution among medical oncologists about the safety of vaginal estrogen in breast cancer survivors. (The 25-μg tablets are no longer marketed.) The lowest dosage of vaginal estrogen available for the treatment of genital atrophy is found in 10-μg estradiol tablets (Vagifem) and the estradiol (2-mg) 3-month vaginal ring (Estring). Nonetheless, in the absence of data, oncologists will likely continue to be concerned that even the lowest dosage of vaginal estrogen could attenuate the favorable impact of AIs on breast cancer. Accordingly, use of vaginal lubricants and moisturizers are the mainstay strategy for symptomatic genital atrophy.
OBG Management: What about the ubiquitous hot flush? Vasomotor symptoms may be more common in women who take tamoxifen, but women on AIs are also bothered by flushes. What are the alternatives to estrogen therapy?
Dr. Kaunitz: Both nonprescription and prescription alternatives are available. Nonprescription options include soy extract and red clover isoflavones, black cohosh, and Chinese herbs. However, none of these over-the-counter approaches has been found to be more effective than placebo in the treatment of menopausal hot flushes.24-26
As for prescription nonhormonal options, ObGyns should recognize that all such treatments are off-label and that none attain the efficacy of hormone therapy in the treatment of vasomotor symptoms. The best-studied and most effective medications include gabapentin, SSRIs (especially paroxetine), and serotonin-norepinephrine reuptake inhibitors (venlafaxine and desvenlafaxine).24,27
OBG Management: Is there any evidence that AIs impair cognitive function in postmenopausal women?
Dr. Kaunitz: Because estrogen is important for cognition, one might anticipate that the profound reduction in background estrogen associated with AI use would impair cognition. Fortunately, the evidence to date is reassuring. Substudies of the BIG trial and the Tamoxifen and Exemestane Adjuvant Multinational Trial indicate that, compared with tamoxifen (which is associated with declines in cognitive function in postmenopausal women), letrozole and exemestane do not diminish cognitive function.28,29
OBG Management: Overall, what is the typical impact of an AI on a woman’s quality of life?
Dr. Kaunitz: Most women do very well on an AI, finding it easier to tolerate than tamoxifen, as we have discussed. However, a significant minority of women is seriously bothered by the adverse effects, with arthralgias usually leading the pack of complaints.30,31
OBG Management: Do some women discontinue adjuvant endocrine therapy because of adverse effects?
Dr. Kaunitz: Regrettably, the answer is “Yes.” A recent study from Kaiser Permanente of northern California found that roughly 50% of women who are prescribed adjuvant endocrine therapy with tamoxifen or an AI discontinue the drug early.32
OBG Management: What can an ObGyn do to encourage compliance with and completion of AI therapy?
Dr. Kaunitz: First, it is critical that patients understand that AIs are lifesaving drugs. As a recent paper points out, early discontinuation or noncompliance with AI therapy is associated with higher mortality.33
Clinicians should also help breast cancer patients understand what common side effects to anticipate with these medications.
Finally, clinicians who understand the financial toll a breast cancer diagnosis and treatment can take are better positioned to help women overcome challenges that may interfere with long-term compliance with AI therapy.
OBG Management: Do you expect the use of AIs in breast cancer survivors to become more commonplace?
Dr. Kaunitz: Given how common breast cancer is, and given the new ASCO guidelines and the extensive literature upon which they are based, ObGyns will be seeing more women using AIs. Although we are not the physicians who prescribe AIs, we need to remain up to date on their benefits and side effects. This important class of drugs is positioned to improve outcomes for postmenopausal women with breast cancer.
- Do certain SSRIs reduce the benefits of tamoxifen in breast cancer survivors?
Examining the Evidence
Andrew M. Kaunitz, MD - Does the clinical breast exam boost the sensitivity of mammography?
Examining the Evidence
Jennifer Griffin, MD, and Mark Pearlman, MD - A guide to lotions and potions for treating vaginal atrophy
Danielle D. Marshall, MD, and Cheryl Iglesia, MD
We want to hear from you! Tell us what you think.
1. Horner MJ, Ries LAG, Krapcho M, et al. eds. SEER Cancer Statistics Review, 1975–2006. Bethesda, Md: National Cancer Institute; 2009.http://seer.cancer.gov/csr/1975_2006. Accessed August 18, 2010.
2. American Cancer Society. Breast cancer facts and figures, 2009–2010. Atlanta, Ga: American Cancer Society; 2010. http://www.cancer.org/Research/CancerFactsFigures/BreastCancerFactsFigures/breast-cancer-facts—figures-2009-2010. Accessed August 18, 2010.
3. Li CI, Daling JR, Malone KE. Incidence of invasive breast cancer by hormone receptor status from 1992 to 1998. J Clin Oncol. 2003;21(1):28-34.
4. Miller WR. Aromatase inhibitors: mechanism of action and role in the treatment of breast cancer. Semin Oncol. 2003;30(4 suppl 14):3-11.
5. Peng J, Sengupta S, Jordan VC. Potential of selective estrogen receptor modulators as treatments and preventives of breast cancer. Anticancer Agents Med Chem. 2009;9(5):481-499.
6. Smith IE, Dowsett M, Yap YS, et al. Adjuvant aromatase inhibitors for early breast cancer after chemotherapy-induced amenorrhea: caution and suggested guidelines. J Clin Oncol. 2006;24(16):2444-2447.
7. Nabholtz JM, Mouret-Reynier MA, Durando X, et al. Comparative review of anastrozole, letrozole and exemestane in the management of early breast cancer. Expert Opin Pharmacother. 2009;10(9):1435-1447.
8. Baum M, Budzar AU, Cuzick J, et al. ATAC Trialists’ Group. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomized trial. Lancet. 2002;359(9324):2131-2139.
9. Letrozole in treating postmenopausal women who have received hormone therapy for hormone receptor-positive breast cancer. National Cancer Institute Web site.http://www.cancer.gov/clinicaltrials/NSABP-B-42. Published August 16, 2010. Accessed August 18, 2010.
10. Burstein HJ, Prestrud AA, Seidenfeld J, et al. American Society of Clinical Oncology clinical practice guideline: update on adjuvant endocrine therapy for women with hormone-receptor–positive breast cancer. J Clin Oncol. 2010;28(23):3784-3796.
11. Mauri D, Pavlidis N, Polyzos NP, Ioanidis JP. Survival with aromatase inhibitors and inactivators versus standard hormonal therapy in advanced breast cancer: meta-analysis. J Natl Cancer Inst. 2006;98(18):1285-1291.
12. Arimidex [package insert]. AstraZeneca; 2009.
13. Baum M, Buzdar A, Cuzick J, et al. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early-stage breast cancer: results of the ATAC (Arimidex, Taxoxifen Alone or in Combination) trial efficacy and safety update analyses. Cancer. 2003;98(9):1802-1810.
14. Thurlimann B, Keshaviah A, Coates AS, et al. Breast International Group (BIG) 1-98 Collaborative Group. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med. 2005;353(26):2747-2757.
15. Kelly CM, Juurlink DM, Gomes T, et al. Selective serotonin reuptake inhibitors and breast cancer mortality in women receiving tamoxifen: a population-based cohort study. 2010;340:c693.-doi: 10.1136/bmj.c693.
16. Geisler J, Lonning PE. Impact of aromatase inhibitors on bone health in breast cancer patients. J Steroid Biochem Mol Biol. 2010;118(4–5):294-299.
17. Van Poznak C, Hannon RA, Mackey JR, et al. Prevention of aromatase inhibitor-induced bone loss using risedronate: the SABRE trial. J Clin Oncol. 2010;28(6):967-975.
18. Brufsky AM, Bosserman LD, Caradonna RR, et al. Zoledronic acid effectively prevents aromatase-inhibitor associated bone loss in postmenopausal women with early breast cancer receiving adjuvant letrozole. Z-FAST study 36-month follow-up results. Clin Breast Cancer. 2009;9(2):77-85.
19. Kanis JA, Borgstrom F, De Laet C, et al. Assessment of fracture risk. Osteoporosis Int. 2005;16(6):581-589.
20. Reeder JG, Brufsky AM. The role of bisphosphonates in the adjuvant setting for breast cancer. Oncology. 2010;24(6):462-467,475.
21. Nabholtz JM. Long-term safety of aromatase inhibitors in the treatment of breast cancer. Ther Clin Risk Manag. 2008;4(1):189-204.
22. Cuppone F, Bria E, Verma S, et al. Do adjuvant aromatase inhibitors increase the cardiovascular risk in postmenopausal women with early breast cancer? Meta-analysis of randomized trials. Cancer. 2008;112(2):260-267.
23. Kendall A, Dowsett M, Folkerd E, Smith I. Caution: Vaginal estradiol appears to be contraindicated in postmenopausal women on adjuvant aromatase inhibitors. Ann Oncol. 2006;17(4):584-587.
24. Nelson HD. Menopause. Lancet. 2008;371(9614):760-770.
25. Geller SE, Shulman LP, van Breemen RB, et al. Safety and efficacy of black cohosh and red clover for the management of vasomotor symptoms: a randomized controlled trial. Menopause. 2009;16(6):1156-1166.
26. Kaunitz AM. Effective herbal treatment of vasomotor symptoms—are we any closer? Menopause. 2009;16(3):428-429.
27. Archer DF, Dupont CM, Constantine GD, Pickar JH, Olivier S. Study 319 Investigators. Desvenlafaxine for the treatment of vasomotor symptoms associated with menopause: a double-blind, randomized, placebo-controlled trial of efficacy and safety. Am J Obstet Gynecol. 2009;200(3):238.e1-e10.
28. Phillips KA, Ribi K, Sun Z, et al. Cognitive function in postmenopausal women receiving adjuvant letrozole or tamoxifen for breast cancer in the BIG 1-98 randomized trial [published online ahead of print April 10, 2010]. Breast. doi:10.1016/j.breast.2010.03.025.
29. Schilder CM, Seynaeve C, Beex LV, et al. Effects of tamoxifen and exemestane on cognitive functioning of postmenopausal patients with breast cancer: results from the neuropsychological side study of the Tamoxifen and Exemestane Adjuvant Multinational Trial. J Clin Oncol. 2010;28(8):1294-1300.
30. Din OS, Dodwell D, Wakefield RJ, Coleman RE. Aromatase inhibitor-induced arthralgia in early breast cancer: what do we know and how can we find out more? Breast Cancer Res Treat. 2010;120(3):525-538.
31. Burstein HJ. Aromatase inhibitor-associated arthralgia syndrome. Breast. 2007;16(3):223-234.
32. Hershman DL, Kushi LH, Shao T, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients [published online ahead of print June 28, 2010]. J Clin Oncol. doi: 10.1200/JCO.2009.25.9655.
33. Hershman DL, Shao T, Kushi LH, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer [published online ahead of print August 28, 2010]. Breast Cancer Res Treat. doi: 10.1007/ s10549-010-1132-4.
1. Horner MJ, Ries LAG, Krapcho M, et al. eds. SEER Cancer Statistics Review, 1975–2006. Bethesda, Md: National Cancer Institute; 2009.http://seer.cancer.gov/csr/1975_2006. Accessed August 18, 2010.
2. American Cancer Society. Breast cancer facts and figures, 2009–2010. Atlanta, Ga: American Cancer Society; 2010. http://www.cancer.org/Research/CancerFactsFigures/BreastCancerFactsFigures/breast-cancer-facts—figures-2009-2010. Accessed August 18, 2010.
3. Li CI, Daling JR, Malone KE. Incidence of invasive breast cancer by hormone receptor status from 1992 to 1998. J Clin Oncol. 2003;21(1):28-34.
4. Miller WR. Aromatase inhibitors: mechanism of action and role in the treatment of breast cancer. Semin Oncol. 2003;30(4 suppl 14):3-11.
5. Peng J, Sengupta S, Jordan VC. Potential of selective estrogen receptor modulators as treatments and preventives of breast cancer. Anticancer Agents Med Chem. 2009;9(5):481-499.
6. Smith IE, Dowsett M, Yap YS, et al. Adjuvant aromatase inhibitors for early breast cancer after chemotherapy-induced amenorrhea: caution and suggested guidelines. J Clin Oncol. 2006;24(16):2444-2447.
7. Nabholtz JM, Mouret-Reynier MA, Durando X, et al. Comparative review of anastrozole, letrozole and exemestane in the management of early breast cancer. Expert Opin Pharmacother. 2009;10(9):1435-1447.
8. Baum M, Budzar AU, Cuzick J, et al. ATAC Trialists’ Group. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomized trial. Lancet. 2002;359(9324):2131-2139.
9. Letrozole in treating postmenopausal women who have received hormone therapy for hormone receptor-positive breast cancer. National Cancer Institute Web site.http://www.cancer.gov/clinicaltrials/NSABP-B-42. Published August 16, 2010. Accessed August 18, 2010.
10. Burstein HJ, Prestrud AA, Seidenfeld J, et al. American Society of Clinical Oncology clinical practice guideline: update on adjuvant endocrine therapy for women with hormone-receptor–positive breast cancer. J Clin Oncol. 2010;28(23):3784-3796.
11. Mauri D, Pavlidis N, Polyzos NP, Ioanidis JP. Survival with aromatase inhibitors and inactivators versus standard hormonal therapy in advanced breast cancer: meta-analysis. J Natl Cancer Inst. 2006;98(18):1285-1291.
12. Arimidex [package insert]. AstraZeneca; 2009.
13. Baum M, Buzdar A, Cuzick J, et al. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early-stage breast cancer: results of the ATAC (Arimidex, Taxoxifen Alone or in Combination) trial efficacy and safety update analyses. Cancer. 2003;98(9):1802-1810.
14. Thurlimann B, Keshaviah A, Coates AS, et al. Breast International Group (BIG) 1-98 Collaborative Group. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med. 2005;353(26):2747-2757.
15. Kelly CM, Juurlink DM, Gomes T, et al. Selective serotonin reuptake inhibitors and breast cancer mortality in women receiving tamoxifen: a population-based cohort study. 2010;340:c693.-doi: 10.1136/bmj.c693.
16. Geisler J, Lonning PE. Impact of aromatase inhibitors on bone health in breast cancer patients. J Steroid Biochem Mol Biol. 2010;118(4–5):294-299.
17. Van Poznak C, Hannon RA, Mackey JR, et al. Prevention of aromatase inhibitor-induced bone loss using risedronate: the SABRE trial. J Clin Oncol. 2010;28(6):967-975.
18. Brufsky AM, Bosserman LD, Caradonna RR, et al. Zoledronic acid effectively prevents aromatase-inhibitor associated bone loss in postmenopausal women with early breast cancer receiving adjuvant letrozole. Z-FAST study 36-month follow-up results. Clin Breast Cancer. 2009;9(2):77-85.
19. Kanis JA, Borgstrom F, De Laet C, et al. Assessment of fracture risk. Osteoporosis Int. 2005;16(6):581-589.
20. Reeder JG, Brufsky AM. The role of bisphosphonates in the adjuvant setting for breast cancer. Oncology. 2010;24(6):462-467,475.
21. Nabholtz JM. Long-term safety of aromatase inhibitors in the treatment of breast cancer. Ther Clin Risk Manag. 2008;4(1):189-204.
22. Cuppone F, Bria E, Verma S, et al. Do adjuvant aromatase inhibitors increase the cardiovascular risk in postmenopausal women with early breast cancer? Meta-analysis of randomized trials. Cancer. 2008;112(2):260-267.
23. Kendall A, Dowsett M, Folkerd E, Smith I. Caution: Vaginal estradiol appears to be contraindicated in postmenopausal women on adjuvant aromatase inhibitors. Ann Oncol. 2006;17(4):584-587.
24. Nelson HD. Menopause. Lancet. 2008;371(9614):760-770.
25. Geller SE, Shulman LP, van Breemen RB, et al. Safety and efficacy of black cohosh and red clover for the management of vasomotor symptoms: a randomized controlled trial. Menopause. 2009;16(6):1156-1166.
26. Kaunitz AM. Effective herbal treatment of vasomotor symptoms—are we any closer? Menopause. 2009;16(3):428-429.
27. Archer DF, Dupont CM, Constantine GD, Pickar JH, Olivier S. Study 319 Investigators. Desvenlafaxine for the treatment of vasomotor symptoms associated with menopause: a double-blind, randomized, placebo-controlled trial of efficacy and safety. Am J Obstet Gynecol. 2009;200(3):238.e1-e10.
28. Phillips KA, Ribi K, Sun Z, et al. Cognitive function in postmenopausal women receiving adjuvant letrozole or tamoxifen for breast cancer in the BIG 1-98 randomized trial [published online ahead of print April 10, 2010]. Breast. doi:10.1016/j.breast.2010.03.025.
29. Schilder CM, Seynaeve C, Beex LV, et al. Effects of tamoxifen and exemestane on cognitive functioning of postmenopausal patients with breast cancer: results from the neuropsychological side study of the Tamoxifen and Exemestane Adjuvant Multinational Trial. J Clin Oncol. 2010;28(8):1294-1300.
30. Din OS, Dodwell D, Wakefield RJ, Coleman RE. Aromatase inhibitor-induced arthralgia in early breast cancer: what do we know and how can we find out more? Breast Cancer Res Treat. 2010;120(3):525-538.
31. Burstein HJ. Aromatase inhibitor-associated arthralgia syndrome. Breast. 2007;16(3):223-234.
32. Hershman DL, Kushi LH, Shao T, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients [published online ahead of print June 28, 2010]. J Clin Oncol. doi: 10.1200/JCO.2009.25.9655.
33. Hershman DL, Shao T, Kushi LH, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer [published online ahead of print August 28, 2010]. Breast Cancer Res Treat. doi: 10.1007/ s10549-010-1132-4.
Another reason to postpone delivery until 37 weeks
- Can we reduce the rate of scheduled births that occur earlier than 39 weeks of gestation? Examining the Evidence.
Commentary by George A. Macones, MD, MSCE
(OBG Management, June 2010)
AUGUST 2010—An analysis of more than 200,000 deliveries finds that babies born between 34 weeks and 37 weeks are more likely to have severe respiratory illness than those born at term, and that this risk decreases with each added week of gestational age during the late preterm period. The report of the study was published in the July 28 issue of JAMA.
Late preterm birth (34 0/7 to 36 6/7 weeks’ gestation) accounts for 9.1% of all deliveries and 75% of all preterm births in the United States. Considerable evidence has shown that short-term illnesses are prevalent; however, much of the supporting data for that evidence is more than a decade old or drawn from small populations, according to background information in the article.
Judith U. Hibbard, MD, of the University of Illinois at Chicago, and colleagues of the National Institutes of Health’s Consortium on Safe Labor, conducted a study to determine the rate of respiratory illness among late preterm births by analyzing recent data from a large group of late preterm infants. The study included electronic data from 12 institutions (19 hospitals) across the United States on 233,844 deliveries between 2002 and 2008. Charts were abstracted for all newborns with respiratory problems admitted to a neonatal intensive care unit (NICU), and late preterm births were compared with term births in regard to resuscitation, respiratory support, and respiratory diagnoses.
Of 19,334 late preterm births, 7,055 (36.5%) were admitted to a NICU and 2,032 had respiratory compromise. Of 165,993 term infants, 11,980 (7.2%) were admitted to a NICU, 1,874 with respiratory illness. The researchers found that respiratory distress syndrome (RDS) was the most common respiratory illness, occurring in 10.5% (n = 390) of 34-week deliveries, decreasing with gestational age to 0.3% (n = 140/41,764) at 38 weeks. Transient tachypnea of the newborn was the second most common morbidity at 6.4% (n = 236) at 34 weeks, reaching a low of 0.3% (n = 207/ 62,295) at 39 weeks. Also decreasing from 34 weeks were pneumonia, from 1.5% to 0.1% at 39 weeks, and overall respiratory failure, from 1.6% to 0.09% at 40 weeks. The percentage of infants who had respiratory illness decreased significantly as gestational age increased until 39 to 40 weeks.
Additional analysis found that newborns born at 34 weeks had a 40-fold increase in the odds of RDS; that risk decreased with each advancing week of gestation until 38 weeks.
“Even at 37 weeks, the odds of RDS were still 3-fold greater than that of a 39- or 40-week birth. Similar patterns were seen for transient tachypnea of the newborn, pneumonia, standard or high-frequency ventilator requirements, and respiratory failure,” according to the authors of the report.
“We suggest that future studies should focus on indications for late preterm birth. Only by more completely understanding reasons for rising rates of late preterm birth might clinicians be able to initiate salutary interventions to decrease neonatal respiratory morbidity. Improved pregnancy dating through early ultrasound confirmation of estimated due date may help prevent neonatal morbidity associated with erroneous delivery of a neonate that is actually at an earlier gestational age. Finally, a better understanding of the effect of mode of delivery on neonates may help with future interventions to decrease morbidity.”
We want to hear from you! Tell us what you think.
- Can we reduce the rate of scheduled births that occur earlier than 39 weeks of gestation? Examining the Evidence.
Commentary by George A. Macones, MD, MSCE
(OBG Management, June 2010)
AUGUST 2010—An analysis of more than 200,000 deliveries finds that babies born between 34 weeks and 37 weeks are more likely to have severe respiratory illness than those born at term, and that this risk decreases with each added week of gestational age during the late preterm period. The report of the study was published in the July 28 issue of JAMA.
Late preterm birth (34 0/7 to 36 6/7 weeks’ gestation) accounts for 9.1% of all deliveries and 75% of all preterm births in the United States. Considerable evidence has shown that short-term illnesses are prevalent; however, much of the supporting data for that evidence is more than a decade old or drawn from small populations, according to background information in the article.
Judith U. Hibbard, MD, of the University of Illinois at Chicago, and colleagues of the National Institutes of Health’s Consortium on Safe Labor, conducted a study to determine the rate of respiratory illness among late preterm births by analyzing recent data from a large group of late preterm infants. The study included electronic data from 12 institutions (19 hospitals) across the United States on 233,844 deliveries between 2002 and 2008. Charts were abstracted for all newborns with respiratory problems admitted to a neonatal intensive care unit (NICU), and late preterm births were compared with term births in regard to resuscitation, respiratory support, and respiratory diagnoses.
Of 19,334 late preterm births, 7,055 (36.5%) were admitted to a NICU and 2,032 had respiratory compromise. Of 165,993 term infants, 11,980 (7.2%) were admitted to a NICU, 1,874 with respiratory illness. The researchers found that respiratory distress syndrome (RDS) was the most common respiratory illness, occurring in 10.5% (n = 390) of 34-week deliveries, decreasing with gestational age to 0.3% (n = 140/41,764) at 38 weeks. Transient tachypnea of the newborn was the second most common morbidity at 6.4% (n = 236) at 34 weeks, reaching a low of 0.3% (n = 207/ 62,295) at 39 weeks. Also decreasing from 34 weeks were pneumonia, from 1.5% to 0.1% at 39 weeks, and overall respiratory failure, from 1.6% to 0.09% at 40 weeks. The percentage of infants who had respiratory illness decreased significantly as gestational age increased until 39 to 40 weeks.
Additional analysis found that newborns born at 34 weeks had a 40-fold increase in the odds of RDS; that risk decreased with each advancing week of gestation until 38 weeks.
“Even at 37 weeks, the odds of RDS were still 3-fold greater than that of a 39- or 40-week birth. Similar patterns were seen for transient tachypnea of the newborn, pneumonia, standard or high-frequency ventilator requirements, and respiratory failure,” according to the authors of the report.
“We suggest that future studies should focus on indications for late preterm birth. Only by more completely understanding reasons for rising rates of late preterm birth might clinicians be able to initiate salutary interventions to decrease neonatal respiratory morbidity. Improved pregnancy dating through early ultrasound confirmation of estimated due date may help prevent neonatal morbidity associated with erroneous delivery of a neonate that is actually at an earlier gestational age. Finally, a better understanding of the effect of mode of delivery on neonates may help with future interventions to decrease morbidity.”
We want to hear from you! Tell us what you think.
- Can we reduce the rate of scheduled births that occur earlier than 39 weeks of gestation? Examining the Evidence.
Commentary by George A. Macones, MD, MSCE
(OBG Management, June 2010)
AUGUST 2010—An analysis of more than 200,000 deliveries finds that babies born between 34 weeks and 37 weeks are more likely to have severe respiratory illness than those born at term, and that this risk decreases with each added week of gestational age during the late preterm period. The report of the study was published in the July 28 issue of JAMA.
Late preterm birth (34 0/7 to 36 6/7 weeks’ gestation) accounts for 9.1% of all deliveries and 75% of all preterm births in the United States. Considerable evidence has shown that short-term illnesses are prevalent; however, much of the supporting data for that evidence is more than a decade old or drawn from small populations, according to background information in the article.
Judith U. Hibbard, MD, of the University of Illinois at Chicago, and colleagues of the National Institutes of Health’s Consortium on Safe Labor, conducted a study to determine the rate of respiratory illness among late preterm births by analyzing recent data from a large group of late preterm infants. The study included electronic data from 12 institutions (19 hospitals) across the United States on 233,844 deliveries between 2002 and 2008. Charts were abstracted for all newborns with respiratory problems admitted to a neonatal intensive care unit (NICU), and late preterm births were compared with term births in regard to resuscitation, respiratory support, and respiratory diagnoses.
Of 19,334 late preterm births, 7,055 (36.5%) were admitted to a NICU and 2,032 had respiratory compromise. Of 165,993 term infants, 11,980 (7.2%) were admitted to a NICU, 1,874 with respiratory illness. The researchers found that respiratory distress syndrome (RDS) was the most common respiratory illness, occurring in 10.5% (n = 390) of 34-week deliveries, decreasing with gestational age to 0.3% (n = 140/41,764) at 38 weeks. Transient tachypnea of the newborn was the second most common morbidity at 6.4% (n = 236) at 34 weeks, reaching a low of 0.3% (n = 207/ 62,295) at 39 weeks. Also decreasing from 34 weeks were pneumonia, from 1.5% to 0.1% at 39 weeks, and overall respiratory failure, from 1.6% to 0.09% at 40 weeks. The percentage of infants who had respiratory illness decreased significantly as gestational age increased until 39 to 40 weeks.
Additional analysis found that newborns born at 34 weeks had a 40-fold increase in the odds of RDS; that risk decreased with each advancing week of gestation until 38 weeks.
“Even at 37 weeks, the odds of RDS were still 3-fold greater than that of a 39- or 40-week birth. Similar patterns were seen for transient tachypnea of the newborn, pneumonia, standard or high-frequency ventilator requirements, and respiratory failure,” according to the authors of the report.
“We suggest that future studies should focus on indications for late preterm birth. Only by more completely understanding reasons for rising rates of late preterm birth might clinicians be able to initiate salutary interventions to decrease neonatal respiratory morbidity. Improved pregnancy dating through early ultrasound confirmation of estimated due date may help prevent neonatal morbidity associated with erroneous delivery of a neonate that is actually at an earlier gestational age. Finally, a better understanding of the effect of mode of delivery on neonates may help with future interventions to decrease morbidity.”
We want to hear from you! Tell us what you think.
FDA warning: Don’t use unapproved IUDs
AUGUST 2010—Some intrauterine devices (IUDs) purchased from offshore Web sites are not the devices they purport to be. The FDA is disturbed enough about the problem to have issued a warning letter late last month to women’s health care providers outlining its concerns:
The FDA has become aware of the purchase, use, and distribution of unapproved [IUDs] and intrauterine systems (IUS) by some medical practices throughout the United States. The violative products include unapproved versions of FDA-approved products such as Mirena, Implanon, Copper-T, and ParaGard; as well as products not approved for use in the United States, such as T-Safe.
In an OBG Management Web-exclusive article in 2009, Andrew M. Kaunitz, MD, raised concerns about the practice of buying IUDs from offshore Web sites. Dr. Kaunitz is professor and associate chairman of obstetrics and gynecology at the University of Florida College of Medicine in Jacksonville, Fla. He serves on the OBG Management Board of Editors.
Dr. Kaunitz noted that some physicians have been lured by substantial discounts for IUDs—both the copper T380A (ParaGard) and the levonorgestrel-releasing intrauterine system (LNG-IUS; Mirena)—offered at some Web sites. The prices for these devices are lower, but the IUDs themselves are knockoffs—counterfeits. ParaGard costs approximately $494, but counterfeit models are offered online for as little as $199. As for Mirena, the legitimate device costs $717, compared with $270 for the online knockoff.
In the case of the copper IUD, what may at first appear to be the ParaGard device is typically the “T-Safe CU 380A QL.” Although ParaGard is approved by the FDA, has no labeling restrictions for use in nulliparous women, is indicated for a uterus that ranges in depth from 6 cm to 9 cm, and is effective for 7 years, that is not the case for the “T-Safe” device. Besides lacking FDA approval, the T-Safe is not intended for use in nulliparous women, is designed for use only if the uterus is at least 6.5 cm in depth, and is effective for 4 years.
“The common scenario is ordering these devices online from Canadian pharmacies or offshore Web sites,” Kaunitz said. “Most physicians who frequent these Web sites are just trying to save money,” he added.
FDA raises safety, fraud concerns
In its warning letter to providers, the FDA outlined three main concerns:
- “potential lack of safety and efficacy, and especially the risk of reduced efficacy for preventing pregnancy”
- harm to public health—namely, the fact that unapproved devices can come from unknown sources and “may not have been manufactured, transported or stored under conditions required as part of the FDA approval process”
- “the use of and subsequent billing for unapproved medical products, which raises the possibility of insurance fraud, particularly Medicaid fraud.”
The letter also warned against inserting an IUD provided by the patient without verifying that it is an FDA-approved device “that was purchased from a licensed pharmaceutical or device supplier in the US.”
Not all Canadian Web sites offer Canadian products
“To reduce the chance of receiving an unapproved or adulterated medical product, your Internet purchases should be made from state-licensed distributors or pharmacies located in the U.S.,” the FDA advises. “FDA is aware of several Web sites that appear to be Canadian Web sites but ship products from countries other than Canada.”
The FDA is not the only entity sending letters about the issue to providers. The problem has also come to the attention of the manufacturer of ParaGard. Last year, DuraMed, a subsidiary of Barr Pharmaceuticals, carried out a broad-based mailing to physicians, warning them of the problem of offshore Web sites offering the device at a significant discount.
“This is of particular concern with respect to intrauterine contraceptives because they are implanted into the human body for up to a decade, and because inferior quality and improper use may result in an increased risk of pelvic inflammatory disease, ectopic pregnancy, hysterectomy, infertility, and other serious complications,” the DuraMed letter said.
To avoid the risk, use conventional ordering
That is Kaunitz’s advice. The best way to ensure that the device you order is an FDA-approved IUD is to order it directly from a legitimate distributor, such as CareMark (www.caremark.com), he said.
“I think you put yourself and your patients at risk by using counterfeit and unapproved devices,” he added.
You can report the distribution of unapproved IUDs by contacting the FDA Office of Criminal Investigations at http://www.fda.gov/ICECI/CriminalInvestigations/default.htm.
We want to hear from you! Tell us what you think.
AUGUST 2010—Some intrauterine devices (IUDs) purchased from offshore Web sites are not the devices they purport to be. The FDA is disturbed enough about the problem to have issued a warning letter late last month to women’s health care providers outlining its concerns:
The FDA has become aware of the purchase, use, and distribution of unapproved [IUDs] and intrauterine systems (IUS) by some medical practices throughout the United States. The violative products include unapproved versions of FDA-approved products such as Mirena, Implanon, Copper-T, and ParaGard; as well as products not approved for use in the United States, such as T-Safe.
In an OBG Management Web-exclusive article in 2009, Andrew M. Kaunitz, MD, raised concerns about the practice of buying IUDs from offshore Web sites. Dr. Kaunitz is professor and associate chairman of obstetrics and gynecology at the University of Florida College of Medicine in Jacksonville, Fla. He serves on the OBG Management Board of Editors.
Dr. Kaunitz noted that some physicians have been lured by substantial discounts for IUDs—both the copper T380A (ParaGard) and the levonorgestrel-releasing intrauterine system (LNG-IUS; Mirena)—offered at some Web sites. The prices for these devices are lower, but the IUDs themselves are knockoffs—counterfeits. ParaGard costs approximately $494, but counterfeit models are offered online for as little as $199. As for Mirena, the legitimate device costs $717, compared with $270 for the online knockoff.
In the case of the copper IUD, what may at first appear to be the ParaGard device is typically the “T-Safe CU 380A QL.” Although ParaGard is approved by the FDA, has no labeling restrictions for use in nulliparous women, is indicated for a uterus that ranges in depth from 6 cm to 9 cm, and is effective for 7 years, that is not the case for the “T-Safe” device. Besides lacking FDA approval, the T-Safe is not intended for use in nulliparous women, is designed for use only if the uterus is at least 6.5 cm in depth, and is effective for 4 years.
“The common scenario is ordering these devices online from Canadian pharmacies or offshore Web sites,” Kaunitz said. “Most physicians who frequent these Web sites are just trying to save money,” he added.
FDA raises safety, fraud concerns
In its warning letter to providers, the FDA outlined three main concerns:
- “potential lack of safety and efficacy, and especially the risk of reduced efficacy for preventing pregnancy”
- harm to public health—namely, the fact that unapproved devices can come from unknown sources and “may not have been manufactured, transported or stored under conditions required as part of the FDA approval process”
- “the use of and subsequent billing for unapproved medical products, which raises the possibility of insurance fraud, particularly Medicaid fraud.”
The letter also warned against inserting an IUD provided by the patient without verifying that it is an FDA-approved device “that was purchased from a licensed pharmaceutical or device supplier in the US.”
Not all Canadian Web sites offer Canadian products
“To reduce the chance of receiving an unapproved or adulterated medical product, your Internet purchases should be made from state-licensed distributors or pharmacies located in the U.S.,” the FDA advises. “FDA is aware of several Web sites that appear to be Canadian Web sites but ship products from countries other than Canada.”
The FDA is not the only entity sending letters about the issue to providers. The problem has also come to the attention of the manufacturer of ParaGard. Last year, DuraMed, a subsidiary of Barr Pharmaceuticals, carried out a broad-based mailing to physicians, warning them of the problem of offshore Web sites offering the device at a significant discount.
“This is of particular concern with respect to intrauterine contraceptives because they are implanted into the human body for up to a decade, and because inferior quality and improper use may result in an increased risk of pelvic inflammatory disease, ectopic pregnancy, hysterectomy, infertility, and other serious complications,” the DuraMed letter said.
To avoid the risk, use conventional ordering
That is Kaunitz’s advice. The best way to ensure that the device you order is an FDA-approved IUD is to order it directly from a legitimate distributor, such as CareMark (www.caremark.com), he said.
“I think you put yourself and your patients at risk by using counterfeit and unapproved devices,” he added.
You can report the distribution of unapproved IUDs by contacting the FDA Office of Criminal Investigations at http://www.fda.gov/ICECI/CriminalInvestigations/default.htm.
We want to hear from you! Tell us what you think.
AUGUST 2010—Some intrauterine devices (IUDs) purchased from offshore Web sites are not the devices they purport to be. The FDA is disturbed enough about the problem to have issued a warning letter late last month to women’s health care providers outlining its concerns:
The FDA has become aware of the purchase, use, and distribution of unapproved [IUDs] and intrauterine systems (IUS) by some medical practices throughout the United States. The violative products include unapproved versions of FDA-approved products such as Mirena, Implanon, Copper-T, and ParaGard; as well as products not approved for use in the United States, such as T-Safe.
In an OBG Management Web-exclusive article in 2009, Andrew M. Kaunitz, MD, raised concerns about the practice of buying IUDs from offshore Web sites. Dr. Kaunitz is professor and associate chairman of obstetrics and gynecology at the University of Florida College of Medicine in Jacksonville, Fla. He serves on the OBG Management Board of Editors.
Dr. Kaunitz noted that some physicians have been lured by substantial discounts for IUDs—both the copper T380A (ParaGard) and the levonorgestrel-releasing intrauterine system (LNG-IUS; Mirena)—offered at some Web sites. The prices for these devices are lower, but the IUDs themselves are knockoffs—counterfeits. ParaGard costs approximately $494, but counterfeit models are offered online for as little as $199. As for Mirena, the legitimate device costs $717, compared with $270 for the online knockoff.
In the case of the copper IUD, what may at first appear to be the ParaGard device is typically the “T-Safe CU 380A QL.” Although ParaGard is approved by the FDA, has no labeling restrictions for use in nulliparous women, is indicated for a uterus that ranges in depth from 6 cm to 9 cm, and is effective for 7 years, that is not the case for the “T-Safe” device. Besides lacking FDA approval, the T-Safe is not intended for use in nulliparous women, is designed for use only if the uterus is at least 6.5 cm in depth, and is effective for 4 years.
“The common scenario is ordering these devices online from Canadian pharmacies or offshore Web sites,” Kaunitz said. “Most physicians who frequent these Web sites are just trying to save money,” he added.
FDA raises safety, fraud concerns
In its warning letter to providers, the FDA outlined three main concerns:
- “potential lack of safety and efficacy, and especially the risk of reduced efficacy for preventing pregnancy”
- harm to public health—namely, the fact that unapproved devices can come from unknown sources and “may not have been manufactured, transported or stored under conditions required as part of the FDA approval process”
- “the use of and subsequent billing for unapproved medical products, which raises the possibility of insurance fraud, particularly Medicaid fraud.”
The letter also warned against inserting an IUD provided by the patient without verifying that it is an FDA-approved device “that was purchased from a licensed pharmaceutical or device supplier in the US.”
Not all Canadian Web sites offer Canadian products
“To reduce the chance of receiving an unapproved or adulterated medical product, your Internet purchases should be made from state-licensed distributors or pharmacies located in the U.S.,” the FDA advises. “FDA is aware of several Web sites that appear to be Canadian Web sites but ship products from countries other than Canada.”
The FDA is not the only entity sending letters about the issue to providers. The problem has also come to the attention of the manufacturer of ParaGard. Last year, DuraMed, a subsidiary of Barr Pharmaceuticals, carried out a broad-based mailing to physicians, warning them of the problem of offshore Web sites offering the device at a significant discount.
“This is of particular concern with respect to intrauterine contraceptives because they are implanted into the human body for up to a decade, and because inferior quality and improper use may result in an increased risk of pelvic inflammatory disease, ectopic pregnancy, hysterectomy, infertility, and other serious complications,” the DuraMed letter said.
To avoid the risk, use conventional ordering
That is Kaunitz’s advice. The best way to ensure that the device you order is an FDA-approved IUD is to order it directly from a legitimate distributor, such as CareMark (www.caremark.com), he said.
“I think you put yourself and your patients at risk by using counterfeit and unapproved devices,” he added.
You can report the distribution of unapproved IUDs by contacting the FDA Office of Criminal Investigations at http://www.fda.gov/ICECI/CriminalInvestigations/default.htm.
We want to hear from you! Tell us what you think.
14 questions (and answers) about health reform and you
With passage of the Patient Protection and Affordable Care Act earlier this year, big changes are afoot in the way Americans practice medicine. In a plethora of articles, blogs, and broadcast spots, the media have focused on what the new law portends for the average employee, employers, and the uninsured—but what, exactly, does it entail for ObGyns and their patients?
To find an answer to that overarching question—and 13 others—we invited Lucia DiVenere, director of government relations at the American Congress of Obstetricians and Gynecologists, to join us in an extended discussion of the law and its ramifications. She offered insight into ACOG’s extensive lobbying efforts on behalf of women and the specialty and described the many ways ObGyn care will change in the near and proximal future, focusing on questions that you might find yourself asking, including:
- Will I see a lot more patients?
- What reforms are woman-specific?
- How will my practice change?
- Which of my services will be fully covered?
- Will expanded coverage improve birth outcomes?
- Is “femaleness” a preexisting condition?
- What happened to tort reform?
- Is the system repairable?
1. Will ObGyns see a lot more patients?
OBG Management: The most talked about change the new law heralds is the addition of roughly 32 million people to the insurance rolls. Is the most significant impact of the legislation for ObGyns likely to be an increase in the number of patients they will be seeing?
Lucia DiVenere: Congress wanted to increase the ranks of the insured and expand access to health care, and it addressed these goals with individual and employer mandates, state exchanges, Medicaid expansion, and insurance reforms.
But that isn’t the most significant change in store for us. Congress also wanted to reform our health care system in a number of fundamental ways, some of which are designed to change the way physicians provide care to their patients.
For example, Congress wanted to “bend the cost curve”—to reduce the expected rate of growth in health care spending over the long term. That doesn’t mean that health care costs in 2020 will be less than they were in 2018, but it does mean that annual and long-term growth rates should level off and become sustainable. To accomplish this goal, Congress created an Independent Payment Advisory Commission, which may prove to be extremely powerful in reducing health care costs and is likely to significantly affect all physicians. Greater protections against fraud and abuse, experiments with new kinds of payment and delivery systems, including “medical homes,” and increased reliance on nonphysician practitioners—all included in the law—are also expected to reduce costs.

OBG Management: What other changes are coming?
DiVenere: Congress was determined to alter the practice of health care, ensuring higher quality for each dollar spent and consistent delivery of care. It also sought to kick-start our health care system—especially in the physician arena—into greater and, theoretically, more efficient reliance on electronic health records (EHR). Medicare and Medicaid physician payments will be juggled to increase reimbursement for E&M services and for physicians who provide greater value in relation to cost. Physicians will be required to participate in the Physician Quality Reporting Initiative (PQRI) program in 2015 and beyond to avoid stiff penalties. And EHR systems are required to adopt uniform standards for electronic transactions.
2. What reforms are woman-specific?
OBG Management: What initiatives are planned for the care of women, in particular?
DiVenere: Congress recognized the importance of reforming women’s health and included many provisions advocated by ACOG in our “Health care for women, health care for all” campaign.
Probably the most important of these provisions is the guarantee of direct access to ObGyn care without need of a referral or pre-authorization from a primary care provider or insurance company. Nor can an insurance company restrict a patient’s direct access to her ObGyn to a certain number of visits or types of services.
This was a major ACOG victory. For 20 years, ObGyns have been waging battles in the states for direct access for patients. Last year, nine states did not require insurers to grant women direct access to ObGyns, and 16 states allowed insurers to restrict ObGyn visits and services. This part of the law, which is effective this year, provides national direct access to all women in all states, and is not tied to an ObGyn’s primary care designation.
Another area of reform concerns maternity care. In 2009, 13% of all pregnant women in the United States were uninsured, as were 20.4% of all women between the ages of 15 and 44, the childbearing years. The uninsured rate for nonelderly women in 2007 ranged from a high of 28% in New Mexico and Texas to a low of 8% in Massachusetts. Today, 42% of all pregnancies are covered by Medicaid. Women have been able, usually, to gain access to some kind of care—sometimes in emergency departments at the time of labor—but the nation clearly needs to do better.
Medicaid and new insurance plans will be required to offer maternity care and women’s preventive services, including mammography screening. The exact parameters of maternity care and other types of care in the essential benefits package will be determined by the Secretary of Health and Human Services (HHS), based on the typical package offered to employees in group health plans. The idea behind the law is that many women who are now covered by Medicaid will transfer to private insurance in their states’ exchanges.
3. How will ObGyn practice change?
OBG Management: What are some of the opportunities and challenges ObGyns will encounter?
DiVenere: There are three key areas of challenge and opportunity:
- Development of the “medical home.” A medical home is a practice designed to provide and coordinate comprehensive patient care. State Medicaid agencies are authorized to require certain beneficiaries, including those who have two or more chronic conditions, to join a medical home. Medicare will also experiment with medical homes, and both Medicaid and Medicare medical home practices will receive additional payments. Most medical homes are expected to be family practice, internal medicine, or pediatric care providers, but ObGyn practices can participate, too. ObGyns should look carefully at the opportunities this paradigm provides and consider having their practice designated as a medical home.
- Increased use of nonphysician providers. The new law strongly encourages this practice, including in the ObGyn specialty. Congress is determined to experiment with non-ObGyn deliveries in response to patient demand and midlevel assurances that nonphysicians can deliver babies with better outcomes at significantly lower cost. Our specialty’s cesarean delivery rate is under intense scrutiny. Skewed studies “prove” happier and healthier deliveries in homes and other out-of-hospital locations without an ObGyn in attendance. And midlevel practitioners are offering vaginal birth after cesarean delivery in many cases where ObGyns are restricted by hospital rules.
- The law extends Medicaid payments to free-standing birth centers and birth attendants and does not specify which kinds of practitioners can qualify as birth attendants. Free-standing birth centers can provide high-quality care if they are appropriately accredited and have an established transfer relationship with a nearby hospital. The law does not specify these criteria, either.
- Increasing payments to nonphysicians. Medicare payments to certified nurse midwives (CNMs) will reach the rate paid to physicians for the same services in January 2011, up from 65% of the physician rate. Medicare will also pay CNMs a 10% bonus if primary care services account for at least 60% of their allowed charges. And the law requires health plans in the state exchanges to pay for covered health services provided by any practitioner recognized under state law, whether or not the plan contracts with that individual or type of provider. Certified professional midwives (lay midwives) are licensed in 21 states, and this provision may give them significant new entry.
ObGyns stop delivering babies at increasingly early points in their career, and only 13% of family physicians deliver babies today. So we need to find ways to extend our care—and increasing collaborative practice between ObGyns and CNMs and certified midwives may help close this gap. The increased focus on midlevel providers in the law may present us with both a challenge and an important opportunity.
4. What services will be fully covered now?
OBG Management: Beginning this year, health plans will be required to provide a minimum level of coverage without cost-sharing for preventive care and screenings for women, among others. What will this requirement encompass?
DiVenere: Congress emphasized prevention in the reform law as part of its strategy to bend the cost curve, investing in prevention in order to reduce higher spending on illness.
Beginning in September 2010, all plans—including those that existed before this law was passed—must cover preventive health services without any patient cost sharing, whether copayments or deductibles. These services include women’s preventive care and screening included in comprehensive guidelines supported by the Health Resources and Services Administration (HRSA), even if they are more extensive than services recommended by the Centers for Disease Control and Prevention (CDC) and US Preventive Services Task Force (USPSTF). Breast cancer screening, mammography, and prevention services are covered as though the November 2009 USPSTF recommendations suggesting limits on mammography screening for certain age groups did not exist.
The mammography screening coverage was a big win for ACOG. We worked closely with Senator Barbara Mikulski (D-Md.) on this amendment, and it was the first Democratic amendment offered. It passed on the Senate floor during a contentious floor fight.
ACOG continues to recommend screening mammography every 1 to 2 years for women 40 to 49 years old; annual screening for women 50 and older; clinical breast examination every year for women 19 years and older; and regular breast self-examination.
The Senate bill that was brought to the floor would have limited women’s preventive care to USPSTF recommendations only. Working with Senator Mikulski, we made sure that women younger than 50 will be covered for mammography every 1 to 2 years.
OBG Management: Are there other important benefits for women included in the law?
DiVenere: Yes. One provision will improve research, screening, and treatment for postpartum depression, a signature issue of ACOG President Gerald F. Joseph Jr., MD, during his presidential year. ACOG and Dr. Joseph worked closely with Senator Bob Menendez (D-NJ) to introduce the Moms Act and win its inclusion in the health reform law.
Under this section, HHS will:
- conduct research into the causes of, and treatments for, postpartum conditions
- create a national public awareness campaign to increase knowledge of postpartum depression and postpartum psychosis
- provide grants to study the benefits of screening for postpartum depression and postpartum psychosis
- establish grants to deliver or enhance outpatient, inpatient, and home-based health and support services, including case management and comprehensive treatment services for women with or at risk of postpartum conditions.
5. Will expanded coverage improve birth outcomes?
OBG Management: Do you expect that guaranteed coverage of pregnancy will increase the number of women who seek prenatal care—as opposed to waiting until labor begins—to see a doctor? Will guaranteed coverage of pregnancy improve birth outcomes over the long term?
DiVenere: Those are certainly the goals. And guaranteed coverage of pregnancy was one of ACOG’s essential elements in health care reform. Prenatal care has been shown to save $3 for every $1 spent in the Medicaid program and continues to be the primary way to identify problems during pregnancy, giving ObGyns the opportunity to assess and manage the risk of preterm labor and other threats to the health of the mother and baby.
The health reform law recognizes that better prenatal care can lead to healthier babies—both in its coverage of maternity and preventive care, and by new Medicaid coverage of smoking-cessation counseling and family planning, both beginning this year.
Medicaid will now cover the costs of diagnostic, therapeutic, and counseling services, as well as pharmacotherapy for pregnant women covered by Medicaid, at no cost to the patient. Before health reform passed, only 24 states reimbursed ObGyns and other physicians for smoking-cessation counseling for pregnant women. Five states didn’t cover any smoking-cessation services at all.
Also beginning this year, states can provide family planning services to nonpregnant women up to the same eligibility levels to which they cover pregnant women, without the need to apply for federal waivers or permission. Forty-five states extend Medicaid coverage to pregnant women who have incomes above the regular Medicaid eligibility levels, from a low of 150% to a high of 300% of the federal poverty levels.
Before this new law, 27 states had federal waivers to provide family planning to women who had an income above the Medicaid eligibility levels, most of them at or near 200% of the federal poverty level. Eleven of these waivers expire this year.
6. Is femaleness a “preexisting condition”?
OBG Management: During the debate on health reform, many people claimed, somewhat facetiously, that female sex has been a preexisting condition. The new law will ensure that patients can’t be dropped by their insurance company—or denied coverage—for arbitrary or unfair reasons, such as preexisting conditions. How are these changes likely to affect women and their ObGyns?
DiVenere: The insurance reforms in the new law are very important to women and to ObGyn practices. In fact, the prohibition on preexisting conditions was a top priority of ACOG’s “Health care for women, health care for all” campaign, and Congress included this provision with women’s health in mind.
Many members of Congress were shocked to learn that it was not unusual for insurers to deny coverage to women who were pregnant, who had had a previous cesarean delivery, or who had been the victim of domestic violence at some point in their history. In fact, almost any medical history, genetic information, disability, or current health condition was grounds for denial of coverage.
Women were also often charged higher premiums than men for the same coverage. And insurance companies would sometimes require waiting periods for coverage—sometimes as long as 9 months.
All of these practices are outlawed by the health reform law, which prohibits plans from using preexisting condition exclusions to deny children coverage as of September 1, 2010 and adults as of January 1, 2014. Beginning on January 1, 2014, women cannot be denied coverage due to pregnancy, previous cesarean delivery, or domestic violence, or medical history, among many other reasons.
Effective March 23, 2010 and ending January 1, 2014, a high-risk pool insurance program has been created for people who have been uninsured for 6 months and who have a preexisting condition. Funding for the temporary risk pool is capped at $5 billion.
Insurers in the small and individual markets and in the exchanges cannot discriminate on the basis of medical history or other variables; may only charge limited premium differentials for age, family size, and smoking, but not for gender; and cannot mandate a waiting period longer than 90 days.
Insurance plans that were in existence before enactment must comply with reforms on waiting periods; lifetime limits; rescission; extension of dependent coverage; uniform explanation of coverage; and loss ratio reporting and premium rebates. Group grandfather plans must also comply with restrictions on annual limits and preexisting conditions.
All these protections should benefit ObGyn practices by ensuring coverage and continuity of care for their patients.
7. What happened to tort reform?
OBG Management: No tort reform was included in the law. Why not?
DiVenere: The law authorizes HHS to award $50 million over 5 years, up to $500,000 per state, to develop, implement, and evaluate alternative medical liability reform initiatives that meet several specific criteria. Medical liability protections under the Federal Tort Claims Act are extended to officers, governing board members, employees, and contractors of free clinics.
We at ACOG were very disappointed that Congress didn’t take a serious step toward medical liability reform in this bill. Liability reform was one of ACOG’s five essential elements of health reform, and its absence from the final bill was a prominent reason why we ultimately “reluctantly opposed” passage. We, and the rest of the House of Medicine, were clear that health reform wouldn’t work without meaningful medical liability reform.
ACOG supports caps on noneconomic damages and other reforms in California and Texas law. We also support testing alternatives, including health courts, alternative dispute resolution, “Sorry Works!” programs, and birth injury compensation funds. But this part of the health reform law requires that tests be linked to patient safety, an association that is impossible to establish in cases of neonatal encephalopathy. The law also requires that patients be allowed to opt out of a system if they choose to go to court. Both of these requirements hamper the development of meaningful alternatives for the ObGyn specialty.
8. Is the system repairable?
OBG Management: Can the US health care system be fixed in one fell swoop?
DiVenere: ACOG pursued two integral missions in reform efforts: improving women’s health and advocating for practicing ObGyns. Our mission in women’s health included guaranteed maternity care, important insurance reforms, and direct access to ObGyns. Our mission in regard to practicing ObGyns included the protection of ultrasonography, the reform of medical liability laws, and repeal of the Medicare sustainable growth rate, along with an array of other issues, all of which were shared by the entire House of Medicine.
We see these missions as integral; Congress saw them as separable. We were largely successful on the women’s health side of the ledger. But Congress responded to the House of Medicine issues with little interest.
We believe that we can fulfill our mission to women’s health only if the issues of practicing ObGyns are addressed in the process. You can’t build a new health care system on a broken medical liability system or a broken Medicare physician payment system, and we still have both. We have a lot more work to do on these issues and the myriad of other issues that need to be addressed. This is really just the beginning of health reform.
9. Has PQRI regained the limelight?
OBG Management: The Medicare quality reporting incentive payments under the Physician Quality Reporting Initiative (PQRI) have been extended. In fact, physicians will be penalized, beginning in 2015, if they do not participate. Are the incentive payments a good thing for ObGyns?
DiVenere: Yes, a big change is coming in this program. ObGyns who participate in PQRI will be eligible to receive bonus payments of 1% in 2011 and 0.5% from 2012 to 2014. Payments will be reduced by 1.5% in 2015 and by 2.0% in 2016 for physicians who don’t participate in the PQRI program.
Beginning in 2012, PQRI participation becomes a meaningful use qualifier for EHR grants.
In 2011 to 2014, physicians who complete Maintenance of Certification (MOC) are eligible for an additional 1% bonus in 2011 and 0.5% bonus in 2012 to 2014. Data on a physician’s quality measures must be submitted on the physician’s behalf by the MOC program. After 2014, the Secretary of HHS can add MOC completion to the quality measures used for the value-based payment modifier. The American Board of Obstetrics and Gynecology hasn’t yet qualified its MOC for this part of the program.
ObGyn participation in the PQRI program is very limited (less than 10%). While only about 25 of the 215 PQRI quality measures apply to ObGyn care, most are easily applicable, and a physician needs to report on only three to five measures to qualify for the program.
The very low participation rate is likely because many ObGyn practices just didn’t think the incentive payment was worth the trouble. They may need to rethink that math once they’re faced with payment cuts in 2015.
ObGyns should also be aware that the Secretary of HHS, with input from stakeholders, will set up a Physician Compare Web site (modeled after the program that already exists for hospitals) using PQRI data. Data will be made public on January 1, 2013, comparing physicians in terms of quality of care and patient experience.
The Secretary must ensure that the data are statistically valid and risk-adjusted. In addition, the physician must be given time to review the information before it becomes public, and data must ensure appropriate attribution of care when multiple physicians and other providers are involved. The Secretary must also give physicians timely performance feedback.
For all these reasons, ACOG is working with the physician community to make a number of improvements to the PQRI program, doing our best to make it as easy as possible for our members to participate and benefit.
10. What effect will the expansion of Medicaid have on ObGyn practice?
DiVenere: Starting in 2014, the same year that state exchanges are expected to be established, Medicaid eligibility will be broadened to cover all individuals younger than 65 years who have incomes up to 133% of the federal poverty level. All newly eligible adults will be guaranteed a benchmark benefit package that provides the essential health benefits.
States that have already expanded eligibility to adults who have incomes up to 100% of the federal poverty level will receive a phased-in increase in the federal medical assistance percentage so that, by 2019, they will receive the same federal financing as other states (93% in 2019 and 90% in 2020 and later). And states have the option to expand Medicaid eligibility to childless adults as of April 1, 2010, but will receive their regular federal medical assistance percentage until 2014.
Although these changes will broaden the range and increase the number of individuals who will be eligible for Medicaid, the effect on ObGyn practice remains to be seen, especially as pregnant women who were covered by Medicaid at income levels above 133% of the federal poverty level transition off of Medicaid and into private health insurance offered in the exchanges.
Today, about 38% of all ObGyns accept Medicaid gynecologic patients, and 44% accept Medicaid obstetric patients. Medicaid accounts for 18% of revenues of the average ObGyn practice.
11. Will the extension of benefits to young adults have a measurable impact on ObGyn practice?
DiVenere: Congress included two provisions to target “young immortals,” young adults who don’t think they need health insurance because they’re young and healthy and never need to see a doctor. Many young adults are not offered employer-based health insurance, and many see no advantage in buying coverage that they don’t expect to use. But we all know that someone pays when any uninsured person falls sick or has an accident that necessitates medical care.
Beginning this year, adult children as old as 26 years can go onto their parents’ health insurance plan. In addition, catastrophic plans will soon be available to individuals younger than 30 who want to purchase a higher deductible plan through their state exchange or on the individual and small group markets. These catastrophic plans are not required to include the essential benefits package, including maternity care. Nevertheless, both of these provisions should be helpful to ObGyn practices.
12. Will the mandate for employers to provide health insurance affect many ObGyns?
DiVenere: The employer mandate takes effect in 2014, when employers with more than 50 employees, at least one of whom receives a premium tax credit, are required to offer health insurance coverage to employees or be assessed a range of fees. Employers that have 50 or fewer employees are exempt from this requirement.
In 2007, 75% of ObGyn practices had fewer than 42 full-time employees, with an average number of full-time employees, including physicians, of 34.4. So this mandate should not apply to the average ObGyn practice.
A range of small business tax credits for employers that contribute at least 50% of the cost of coverage for their employees will also be available, with credits phasing out as the size of the firm and the average employee wage increase.
13. Who will benefit from the Medicare geographic payment adjustments?
DiVenere: The increased Medicare geographic practice cost index (GPCI) payments and new Frontier payments won’t affect many ObGyns nationally, but they are likely to affect most ObGyns in the related rural locations.
The law reestablishes the national average floor on Medicare’s GPCI for physician work. In 2010 and 2011, Medicare makes a separate adjustment for the practice expense portion of physician payments that will benefit physicians in rural and low-cost areas.
Beginning in 2011, a third adjustment will increase the practice expense GPCI for physicians in frontier states. A frontier state is one in which at least half of its counties have populations smaller than six people per square mile. Frontier states are expected to be Montana, North Dakota, South Dakota, Utah, and Wyoming.
Physicians in 51 localities in 42 states, Puerto Rico, and the Virgin Islands will benefit from the two practice expense adjustments.
ObGyns should also know about two other payment changes:
- The HHS Secretary will create and apply to Medicare provider payments a value-based modifier that will result in higher Medicare payments for high-quality, low-cost physicians and lower payments for high-cost, low-quality physicians. The modifier is to be based on a composite quality score and a composite cost score determined by measures selected by the HHS Secretary and endorsed by a consensus organization. This change begins with 2015 Medicare payments and applies only to physicians in 2015. In 2017, it will also apply to other health professionals.
- Effective immediately, the HHS Secretary has the authority to increase or decrease Medicare relative values, and payments for services, with special attention focused on:
- – services that have high growth rates
- – services that have seen substantial changes in the practice expense or work components
- – services for which new technology has reduced costs
- – instances in which multiple codes are frequently billed for a single service
- – codes that have not been reviewed since implementation of the resource-based relative value scale (RBRVS).
ObGyns who participate in Medicare will start receiving individual physician resource use reports in 2012. These reports will compare per capita utilization of physicians (or physician groups) with the utilization rate of physicians who see similar patients. Reports are required to be risk-adjusted and standardized to take into account local health-care costs.
14. Do you expect the law’s requirements for “administrative simplification” to reduce overhead and increase efficiency?
DiVenere: Don’t we all hope so.
The bill contains several requirements such as:
- establishment of a standardized claim form
- streamlining of claims processing
- improvement of interoperability to allow for more electronic information sharing. These changes will not be implemented until 2013 at the earliest.
Today, about 34% of all ObGyn practices use electronic health records. The systemwide benefits of health information technology (HIT) can be many. Insurers can save by reducing unnecessary tests. Patients can benefit from better coordination of care and fewer medical errors. But these advantages don’t necessarily translate into savings or revenue for physician practices.
Instead, physicians face Medicare and private insurance payment cuts. Little assistance is available for the investment in HIT. And uncertain interoperability standards and rapid technological changes can very quickly make this year’s investment obsolete. Many physicians in solo and small practices are understandably reluctant to take the HIT plunge.
The initial cost of purchasing HIT for a small practice is typically at least $50,000 per physician. Physicians face additional, ongoing costs in staff training and hardware and software updates as well. And many physicians see significant efficiency losses for months and sometimes years after upgrading to an electronic health record system.
Still, with interoperable, shareable electronic records, all physicians treating a particular patient can have the full story. A patient’s paper record kept in her physician’s office shows only a slice of her medical history, potentially missing important information from the patient’s other physicians, including allergies to medication, test results, and the results of particular therapies.
Without a shared electronic record, a physician relies on the recollection of each patient, which is often unintentionally incomplete. A patient may be uncertain about the name or dosage of a medication, fail to remember the date of a screening examination, or lack results of lab tests ordered by another physician.
A physician’s access to the full story with shareable electronic records is important to the care of all patients and can be particularly relevant for patients who have inconsistent contact with health care providers. Often, these patients get care in various settings, including physician offices, community clinics, and emergency departments. Because these patients tend to have a higher incidence of chronic disease, they may greatly benefit from the sharing of medical information.
Clearly, Congress wants to move us to full adoption of HIT. Beginning in 2013, all plans must comply with a uniform standard for electronic transactions, including eligibility verification and health claims status.
In 2014, uniform standards must allow automatic reconciliation of electronic funds transfers and HIPAA payment and remittance; use standardized and consistent methods of health plan enrollment and claim edits; use unique health plan identifiers to simplify and improve routing of health care transactions; and use standard electronic claims attachments.
Uniformity and standardization can help address one of the major roadblocks to physician adoption of health information technology.
With passage of the Patient Protection and Affordable Care Act earlier this year, big changes are afoot in the way Americans practice medicine. In a plethora of articles, blogs, and broadcast spots, the media have focused on what the new law portends for the average employee, employers, and the uninsured—but what, exactly, does it entail for ObGyns and their patients?
To find an answer to that overarching question—and 13 others—we invited Lucia DiVenere, director of government relations at the American Congress of Obstetricians and Gynecologists, to join us in an extended discussion of the law and its ramifications. She offered insight into ACOG’s extensive lobbying efforts on behalf of women and the specialty and described the many ways ObGyn care will change in the near and proximal future, focusing on questions that you might find yourself asking, including:
- Will I see a lot more patients?
- What reforms are woman-specific?
- How will my practice change?
- Which of my services will be fully covered?
- Will expanded coverage improve birth outcomes?
- Is “femaleness” a preexisting condition?
- What happened to tort reform?
- Is the system repairable?
1. Will ObGyns see a lot more patients?
OBG Management: The most talked about change the new law heralds is the addition of roughly 32 million people to the insurance rolls. Is the most significant impact of the legislation for ObGyns likely to be an increase in the number of patients they will be seeing?
Lucia DiVenere: Congress wanted to increase the ranks of the insured and expand access to health care, and it addressed these goals with individual and employer mandates, state exchanges, Medicaid expansion, and insurance reforms.
But that isn’t the most significant change in store for us. Congress also wanted to reform our health care system in a number of fundamental ways, some of which are designed to change the way physicians provide care to their patients.
For example, Congress wanted to “bend the cost curve”—to reduce the expected rate of growth in health care spending over the long term. That doesn’t mean that health care costs in 2020 will be less than they were in 2018, but it does mean that annual and long-term growth rates should level off and become sustainable. To accomplish this goal, Congress created an Independent Payment Advisory Commission, which may prove to be extremely powerful in reducing health care costs and is likely to significantly affect all physicians. Greater protections against fraud and abuse, experiments with new kinds of payment and delivery systems, including “medical homes,” and increased reliance on nonphysician practitioners—all included in the law—are also expected to reduce costs.

OBG Management: What other changes are coming?
DiVenere: Congress was determined to alter the practice of health care, ensuring higher quality for each dollar spent and consistent delivery of care. It also sought to kick-start our health care system—especially in the physician arena—into greater and, theoretically, more efficient reliance on electronic health records (EHR). Medicare and Medicaid physician payments will be juggled to increase reimbursement for E&M services and for physicians who provide greater value in relation to cost. Physicians will be required to participate in the Physician Quality Reporting Initiative (PQRI) program in 2015 and beyond to avoid stiff penalties. And EHR systems are required to adopt uniform standards for electronic transactions.
2. What reforms are woman-specific?
OBG Management: What initiatives are planned for the care of women, in particular?
DiVenere: Congress recognized the importance of reforming women’s health and included many provisions advocated by ACOG in our “Health care for women, health care for all” campaign.
Probably the most important of these provisions is the guarantee of direct access to ObGyn care without need of a referral or pre-authorization from a primary care provider or insurance company. Nor can an insurance company restrict a patient’s direct access to her ObGyn to a certain number of visits or types of services.
This was a major ACOG victory. For 20 years, ObGyns have been waging battles in the states for direct access for patients. Last year, nine states did not require insurers to grant women direct access to ObGyns, and 16 states allowed insurers to restrict ObGyn visits and services. This part of the law, which is effective this year, provides national direct access to all women in all states, and is not tied to an ObGyn’s primary care designation.
Another area of reform concerns maternity care. In 2009, 13% of all pregnant women in the United States were uninsured, as were 20.4% of all women between the ages of 15 and 44, the childbearing years. The uninsured rate for nonelderly women in 2007 ranged from a high of 28% in New Mexico and Texas to a low of 8% in Massachusetts. Today, 42% of all pregnancies are covered by Medicaid. Women have been able, usually, to gain access to some kind of care—sometimes in emergency departments at the time of labor—but the nation clearly needs to do better.
Medicaid and new insurance plans will be required to offer maternity care and women’s preventive services, including mammography screening. The exact parameters of maternity care and other types of care in the essential benefits package will be determined by the Secretary of Health and Human Services (HHS), based on the typical package offered to employees in group health plans. The idea behind the law is that many women who are now covered by Medicaid will transfer to private insurance in their states’ exchanges.
3. How will ObGyn practice change?
OBG Management: What are some of the opportunities and challenges ObGyns will encounter?
DiVenere: There are three key areas of challenge and opportunity:
- Development of the “medical home.” A medical home is a practice designed to provide and coordinate comprehensive patient care. State Medicaid agencies are authorized to require certain beneficiaries, including those who have two or more chronic conditions, to join a medical home. Medicare will also experiment with medical homes, and both Medicaid and Medicare medical home practices will receive additional payments. Most medical homes are expected to be family practice, internal medicine, or pediatric care providers, but ObGyn practices can participate, too. ObGyns should look carefully at the opportunities this paradigm provides and consider having their practice designated as a medical home.
- Increased use of nonphysician providers. The new law strongly encourages this practice, including in the ObGyn specialty. Congress is determined to experiment with non-ObGyn deliveries in response to patient demand and midlevel assurances that nonphysicians can deliver babies with better outcomes at significantly lower cost. Our specialty’s cesarean delivery rate is under intense scrutiny. Skewed studies “prove” happier and healthier deliveries in homes and other out-of-hospital locations without an ObGyn in attendance. And midlevel practitioners are offering vaginal birth after cesarean delivery in many cases where ObGyns are restricted by hospital rules.
- The law extends Medicaid payments to free-standing birth centers and birth attendants and does not specify which kinds of practitioners can qualify as birth attendants. Free-standing birth centers can provide high-quality care if they are appropriately accredited and have an established transfer relationship with a nearby hospital. The law does not specify these criteria, either.
- Increasing payments to nonphysicians. Medicare payments to certified nurse midwives (CNMs) will reach the rate paid to physicians for the same services in January 2011, up from 65% of the physician rate. Medicare will also pay CNMs a 10% bonus if primary care services account for at least 60% of their allowed charges. And the law requires health plans in the state exchanges to pay for covered health services provided by any practitioner recognized under state law, whether or not the plan contracts with that individual or type of provider. Certified professional midwives (lay midwives) are licensed in 21 states, and this provision may give them significant new entry.
ObGyns stop delivering babies at increasingly early points in their career, and only 13% of family physicians deliver babies today. So we need to find ways to extend our care—and increasing collaborative practice between ObGyns and CNMs and certified midwives may help close this gap. The increased focus on midlevel providers in the law may present us with both a challenge and an important opportunity.
4. What services will be fully covered now?
OBG Management: Beginning this year, health plans will be required to provide a minimum level of coverage without cost-sharing for preventive care and screenings for women, among others. What will this requirement encompass?
DiVenere: Congress emphasized prevention in the reform law as part of its strategy to bend the cost curve, investing in prevention in order to reduce higher spending on illness.
Beginning in September 2010, all plans—including those that existed before this law was passed—must cover preventive health services without any patient cost sharing, whether copayments or deductibles. These services include women’s preventive care and screening included in comprehensive guidelines supported by the Health Resources and Services Administration (HRSA), even if they are more extensive than services recommended by the Centers for Disease Control and Prevention (CDC) and US Preventive Services Task Force (USPSTF). Breast cancer screening, mammography, and prevention services are covered as though the November 2009 USPSTF recommendations suggesting limits on mammography screening for certain age groups did not exist.
The mammography screening coverage was a big win for ACOG. We worked closely with Senator Barbara Mikulski (D-Md.) on this amendment, and it was the first Democratic amendment offered. It passed on the Senate floor during a contentious floor fight.
ACOG continues to recommend screening mammography every 1 to 2 years for women 40 to 49 years old; annual screening for women 50 and older; clinical breast examination every year for women 19 years and older; and regular breast self-examination.
The Senate bill that was brought to the floor would have limited women’s preventive care to USPSTF recommendations only. Working with Senator Mikulski, we made sure that women younger than 50 will be covered for mammography every 1 to 2 years.
OBG Management: Are there other important benefits for women included in the law?
DiVenere: Yes. One provision will improve research, screening, and treatment for postpartum depression, a signature issue of ACOG President Gerald F. Joseph Jr., MD, during his presidential year. ACOG and Dr. Joseph worked closely with Senator Bob Menendez (D-NJ) to introduce the Moms Act and win its inclusion in the health reform law.
Under this section, HHS will:
- conduct research into the causes of, and treatments for, postpartum conditions
- create a national public awareness campaign to increase knowledge of postpartum depression and postpartum psychosis
- provide grants to study the benefits of screening for postpartum depression and postpartum psychosis
- establish grants to deliver or enhance outpatient, inpatient, and home-based health and support services, including case management and comprehensive treatment services for women with or at risk of postpartum conditions.
5. Will expanded coverage improve birth outcomes?
OBG Management: Do you expect that guaranteed coverage of pregnancy will increase the number of women who seek prenatal care—as opposed to waiting until labor begins—to see a doctor? Will guaranteed coverage of pregnancy improve birth outcomes over the long term?
DiVenere: Those are certainly the goals. And guaranteed coverage of pregnancy was one of ACOG’s essential elements in health care reform. Prenatal care has been shown to save $3 for every $1 spent in the Medicaid program and continues to be the primary way to identify problems during pregnancy, giving ObGyns the opportunity to assess and manage the risk of preterm labor and other threats to the health of the mother and baby.
The health reform law recognizes that better prenatal care can lead to healthier babies—both in its coverage of maternity and preventive care, and by new Medicaid coverage of smoking-cessation counseling and family planning, both beginning this year.
Medicaid will now cover the costs of diagnostic, therapeutic, and counseling services, as well as pharmacotherapy for pregnant women covered by Medicaid, at no cost to the patient. Before health reform passed, only 24 states reimbursed ObGyns and other physicians for smoking-cessation counseling for pregnant women. Five states didn’t cover any smoking-cessation services at all.
Also beginning this year, states can provide family planning services to nonpregnant women up to the same eligibility levels to which they cover pregnant women, without the need to apply for federal waivers or permission. Forty-five states extend Medicaid coverage to pregnant women who have incomes above the regular Medicaid eligibility levels, from a low of 150% to a high of 300% of the federal poverty levels.
Before this new law, 27 states had federal waivers to provide family planning to women who had an income above the Medicaid eligibility levels, most of them at or near 200% of the federal poverty level. Eleven of these waivers expire this year.
6. Is femaleness a “preexisting condition”?
OBG Management: During the debate on health reform, many people claimed, somewhat facetiously, that female sex has been a preexisting condition. The new law will ensure that patients can’t be dropped by their insurance company—or denied coverage—for arbitrary or unfair reasons, such as preexisting conditions. How are these changes likely to affect women and their ObGyns?
DiVenere: The insurance reforms in the new law are very important to women and to ObGyn practices. In fact, the prohibition on preexisting conditions was a top priority of ACOG’s “Health care for women, health care for all” campaign, and Congress included this provision with women’s health in mind.
Many members of Congress were shocked to learn that it was not unusual for insurers to deny coverage to women who were pregnant, who had had a previous cesarean delivery, or who had been the victim of domestic violence at some point in their history. In fact, almost any medical history, genetic information, disability, or current health condition was grounds for denial of coverage.
Women were also often charged higher premiums than men for the same coverage. And insurance companies would sometimes require waiting periods for coverage—sometimes as long as 9 months.
All of these practices are outlawed by the health reform law, which prohibits plans from using preexisting condition exclusions to deny children coverage as of September 1, 2010 and adults as of January 1, 2014. Beginning on January 1, 2014, women cannot be denied coverage due to pregnancy, previous cesarean delivery, or domestic violence, or medical history, among many other reasons.
Effective March 23, 2010 and ending January 1, 2014, a high-risk pool insurance program has been created for people who have been uninsured for 6 months and who have a preexisting condition. Funding for the temporary risk pool is capped at $5 billion.
Insurers in the small and individual markets and in the exchanges cannot discriminate on the basis of medical history or other variables; may only charge limited premium differentials for age, family size, and smoking, but not for gender; and cannot mandate a waiting period longer than 90 days.
Insurance plans that were in existence before enactment must comply with reforms on waiting periods; lifetime limits; rescission; extension of dependent coverage; uniform explanation of coverage; and loss ratio reporting and premium rebates. Group grandfather plans must also comply with restrictions on annual limits and preexisting conditions.
All these protections should benefit ObGyn practices by ensuring coverage and continuity of care for their patients.
7. What happened to tort reform?
OBG Management: No tort reform was included in the law. Why not?
DiVenere: The law authorizes HHS to award $50 million over 5 years, up to $500,000 per state, to develop, implement, and evaluate alternative medical liability reform initiatives that meet several specific criteria. Medical liability protections under the Federal Tort Claims Act are extended to officers, governing board members, employees, and contractors of free clinics.
We at ACOG were very disappointed that Congress didn’t take a serious step toward medical liability reform in this bill. Liability reform was one of ACOG’s five essential elements of health reform, and its absence from the final bill was a prominent reason why we ultimately “reluctantly opposed” passage. We, and the rest of the House of Medicine, were clear that health reform wouldn’t work without meaningful medical liability reform.
ACOG supports caps on noneconomic damages and other reforms in California and Texas law. We also support testing alternatives, including health courts, alternative dispute resolution, “Sorry Works!” programs, and birth injury compensation funds. But this part of the health reform law requires that tests be linked to patient safety, an association that is impossible to establish in cases of neonatal encephalopathy. The law also requires that patients be allowed to opt out of a system if they choose to go to court. Both of these requirements hamper the development of meaningful alternatives for the ObGyn specialty.
8. Is the system repairable?
OBG Management: Can the US health care system be fixed in one fell swoop?
DiVenere: ACOG pursued two integral missions in reform efforts: improving women’s health and advocating for practicing ObGyns. Our mission in women’s health included guaranteed maternity care, important insurance reforms, and direct access to ObGyns. Our mission in regard to practicing ObGyns included the protection of ultrasonography, the reform of medical liability laws, and repeal of the Medicare sustainable growth rate, along with an array of other issues, all of which were shared by the entire House of Medicine.
We see these missions as integral; Congress saw them as separable. We were largely successful on the women’s health side of the ledger. But Congress responded to the House of Medicine issues with little interest.
We believe that we can fulfill our mission to women’s health only if the issues of practicing ObGyns are addressed in the process. You can’t build a new health care system on a broken medical liability system or a broken Medicare physician payment system, and we still have both. We have a lot more work to do on these issues and the myriad of other issues that need to be addressed. This is really just the beginning of health reform.
9. Has PQRI regained the limelight?
OBG Management: The Medicare quality reporting incentive payments under the Physician Quality Reporting Initiative (PQRI) have been extended. In fact, physicians will be penalized, beginning in 2015, if they do not participate. Are the incentive payments a good thing for ObGyns?
DiVenere: Yes, a big change is coming in this program. ObGyns who participate in PQRI will be eligible to receive bonus payments of 1% in 2011 and 0.5% from 2012 to 2014. Payments will be reduced by 1.5% in 2015 and by 2.0% in 2016 for physicians who don’t participate in the PQRI program.
Beginning in 2012, PQRI participation becomes a meaningful use qualifier for EHR grants.
In 2011 to 2014, physicians who complete Maintenance of Certification (MOC) are eligible for an additional 1% bonus in 2011 and 0.5% bonus in 2012 to 2014. Data on a physician’s quality measures must be submitted on the physician’s behalf by the MOC program. After 2014, the Secretary of HHS can add MOC completion to the quality measures used for the value-based payment modifier. The American Board of Obstetrics and Gynecology hasn’t yet qualified its MOC for this part of the program.
ObGyn participation in the PQRI program is very limited (less than 10%). While only about 25 of the 215 PQRI quality measures apply to ObGyn care, most are easily applicable, and a physician needs to report on only three to five measures to qualify for the program.
The very low participation rate is likely because many ObGyn practices just didn’t think the incentive payment was worth the trouble. They may need to rethink that math once they’re faced with payment cuts in 2015.
ObGyns should also be aware that the Secretary of HHS, with input from stakeholders, will set up a Physician Compare Web site (modeled after the program that already exists for hospitals) using PQRI data. Data will be made public on January 1, 2013, comparing physicians in terms of quality of care and patient experience.
The Secretary must ensure that the data are statistically valid and risk-adjusted. In addition, the physician must be given time to review the information before it becomes public, and data must ensure appropriate attribution of care when multiple physicians and other providers are involved. The Secretary must also give physicians timely performance feedback.
For all these reasons, ACOG is working with the physician community to make a number of improvements to the PQRI program, doing our best to make it as easy as possible for our members to participate and benefit.
10. What effect will the expansion of Medicaid have on ObGyn practice?
DiVenere: Starting in 2014, the same year that state exchanges are expected to be established, Medicaid eligibility will be broadened to cover all individuals younger than 65 years who have incomes up to 133% of the federal poverty level. All newly eligible adults will be guaranteed a benchmark benefit package that provides the essential health benefits.
States that have already expanded eligibility to adults who have incomes up to 100% of the federal poverty level will receive a phased-in increase in the federal medical assistance percentage so that, by 2019, they will receive the same federal financing as other states (93% in 2019 and 90% in 2020 and later). And states have the option to expand Medicaid eligibility to childless adults as of April 1, 2010, but will receive their regular federal medical assistance percentage until 2014.
Although these changes will broaden the range and increase the number of individuals who will be eligible for Medicaid, the effect on ObGyn practice remains to be seen, especially as pregnant women who were covered by Medicaid at income levels above 133% of the federal poverty level transition off of Medicaid and into private health insurance offered in the exchanges.
Today, about 38% of all ObGyns accept Medicaid gynecologic patients, and 44% accept Medicaid obstetric patients. Medicaid accounts for 18% of revenues of the average ObGyn practice.
11. Will the extension of benefits to young adults have a measurable impact on ObGyn practice?
DiVenere: Congress included two provisions to target “young immortals,” young adults who don’t think they need health insurance because they’re young and healthy and never need to see a doctor. Many young adults are not offered employer-based health insurance, and many see no advantage in buying coverage that they don’t expect to use. But we all know that someone pays when any uninsured person falls sick or has an accident that necessitates medical care.
Beginning this year, adult children as old as 26 years can go onto their parents’ health insurance plan. In addition, catastrophic plans will soon be available to individuals younger than 30 who want to purchase a higher deductible plan through their state exchange or on the individual and small group markets. These catastrophic plans are not required to include the essential benefits package, including maternity care. Nevertheless, both of these provisions should be helpful to ObGyn practices.
12. Will the mandate for employers to provide health insurance affect many ObGyns?
DiVenere: The employer mandate takes effect in 2014, when employers with more than 50 employees, at least one of whom receives a premium tax credit, are required to offer health insurance coverage to employees or be assessed a range of fees. Employers that have 50 or fewer employees are exempt from this requirement.
In 2007, 75% of ObGyn practices had fewer than 42 full-time employees, with an average number of full-time employees, including physicians, of 34.4. So this mandate should not apply to the average ObGyn practice.
A range of small business tax credits for employers that contribute at least 50% of the cost of coverage for their employees will also be available, with credits phasing out as the size of the firm and the average employee wage increase.
13. Who will benefit from the Medicare geographic payment adjustments?
DiVenere: The increased Medicare geographic practice cost index (GPCI) payments and new Frontier payments won’t affect many ObGyns nationally, but they are likely to affect most ObGyns in the related rural locations.
The law reestablishes the national average floor on Medicare’s GPCI for physician work. In 2010 and 2011, Medicare makes a separate adjustment for the practice expense portion of physician payments that will benefit physicians in rural and low-cost areas.
Beginning in 2011, a third adjustment will increase the practice expense GPCI for physicians in frontier states. A frontier state is one in which at least half of its counties have populations smaller than six people per square mile. Frontier states are expected to be Montana, North Dakota, South Dakota, Utah, and Wyoming.
Physicians in 51 localities in 42 states, Puerto Rico, and the Virgin Islands will benefit from the two practice expense adjustments.
ObGyns should also know about two other payment changes:
- The HHS Secretary will create and apply to Medicare provider payments a value-based modifier that will result in higher Medicare payments for high-quality, low-cost physicians and lower payments for high-cost, low-quality physicians. The modifier is to be based on a composite quality score and a composite cost score determined by measures selected by the HHS Secretary and endorsed by a consensus organization. This change begins with 2015 Medicare payments and applies only to physicians in 2015. In 2017, it will also apply to other health professionals.
- Effective immediately, the HHS Secretary has the authority to increase or decrease Medicare relative values, and payments for services, with special attention focused on:
- – services that have high growth rates
- – services that have seen substantial changes in the practice expense or work components
- – services for which new technology has reduced costs
- – instances in which multiple codes are frequently billed for a single service
- – codes that have not been reviewed since implementation of the resource-based relative value scale (RBRVS).
ObGyns who participate in Medicare will start receiving individual physician resource use reports in 2012. These reports will compare per capita utilization of physicians (or physician groups) with the utilization rate of physicians who see similar patients. Reports are required to be risk-adjusted and standardized to take into account local health-care costs.
14. Do you expect the law’s requirements for “administrative simplification” to reduce overhead and increase efficiency?
DiVenere: Don’t we all hope so.
The bill contains several requirements such as:
- establishment of a standardized claim form
- streamlining of claims processing
- improvement of interoperability to allow for more electronic information sharing. These changes will not be implemented until 2013 at the earliest.
Today, about 34% of all ObGyn practices use electronic health records. The systemwide benefits of health information technology (HIT) can be many. Insurers can save by reducing unnecessary tests. Patients can benefit from better coordination of care and fewer medical errors. But these advantages don’t necessarily translate into savings or revenue for physician practices.
Instead, physicians face Medicare and private insurance payment cuts. Little assistance is available for the investment in HIT. And uncertain interoperability standards and rapid technological changes can very quickly make this year’s investment obsolete. Many physicians in solo and small practices are understandably reluctant to take the HIT plunge.
The initial cost of purchasing HIT for a small practice is typically at least $50,000 per physician. Physicians face additional, ongoing costs in staff training and hardware and software updates as well. And many physicians see significant efficiency losses for months and sometimes years after upgrading to an electronic health record system.
Still, with interoperable, shareable electronic records, all physicians treating a particular patient can have the full story. A patient’s paper record kept in her physician’s office shows only a slice of her medical history, potentially missing important information from the patient’s other physicians, including allergies to medication, test results, and the results of particular therapies.
Without a shared electronic record, a physician relies on the recollection of each patient, which is often unintentionally incomplete. A patient may be uncertain about the name or dosage of a medication, fail to remember the date of a screening examination, or lack results of lab tests ordered by another physician.
A physician’s access to the full story with shareable electronic records is important to the care of all patients and can be particularly relevant for patients who have inconsistent contact with health care providers. Often, these patients get care in various settings, including physician offices, community clinics, and emergency departments. Because these patients tend to have a higher incidence of chronic disease, they may greatly benefit from the sharing of medical information.
Clearly, Congress wants to move us to full adoption of HIT. Beginning in 2013, all plans must comply with a uniform standard for electronic transactions, including eligibility verification and health claims status.
In 2014, uniform standards must allow automatic reconciliation of electronic funds transfers and HIPAA payment and remittance; use standardized and consistent methods of health plan enrollment and claim edits; use unique health plan identifiers to simplify and improve routing of health care transactions; and use standard electronic claims attachments.
Uniformity and standardization can help address one of the major roadblocks to physician adoption of health information technology.
With passage of the Patient Protection and Affordable Care Act earlier this year, big changes are afoot in the way Americans practice medicine. In a plethora of articles, blogs, and broadcast spots, the media have focused on what the new law portends for the average employee, employers, and the uninsured—but what, exactly, does it entail for ObGyns and their patients?
To find an answer to that overarching question—and 13 others—we invited Lucia DiVenere, director of government relations at the American Congress of Obstetricians and Gynecologists, to join us in an extended discussion of the law and its ramifications. She offered insight into ACOG’s extensive lobbying efforts on behalf of women and the specialty and described the many ways ObGyn care will change in the near and proximal future, focusing on questions that you might find yourself asking, including:
- Will I see a lot more patients?
- What reforms are woman-specific?
- How will my practice change?
- Which of my services will be fully covered?
- Will expanded coverage improve birth outcomes?
- Is “femaleness” a preexisting condition?
- What happened to tort reform?
- Is the system repairable?
1. Will ObGyns see a lot more patients?
OBG Management: The most talked about change the new law heralds is the addition of roughly 32 million people to the insurance rolls. Is the most significant impact of the legislation for ObGyns likely to be an increase in the number of patients they will be seeing?
Lucia DiVenere: Congress wanted to increase the ranks of the insured and expand access to health care, and it addressed these goals with individual and employer mandates, state exchanges, Medicaid expansion, and insurance reforms.
But that isn’t the most significant change in store for us. Congress also wanted to reform our health care system in a number of fundamental ways, some of which are designed to change the way physicians provide care to their patients.
For example, Congress wanted to “bend the cost curve”—to reduce the expected rate of growth in health care spending over the long term. That doesn’t mean that health care costs in 2020 will be less than they were in 2018, but it does mean that annual and long-term growth rates should level off and become sustainable. To accomplish this goal, Congress created an Independent Payment Advisory Commission, which may prove to be extremely powerful in reducing health care costs and is likely to significantly affect all physicians. Greater protections against fraud and abuse, experiments with new kinds of payment and delivery systems, including “medical homes,” and increased reliance on nonphysician practitioners—all included in the law—are also expected to reduce costs.

OBG Management: What other changes are coming?
DiVenere: Congress was determined to alter the practice of health care, ensuring higher quality for each dollar spent and consistent delivery of care. It also sought to kick-start our health care system—especially in the physician arena—into greater and, theoretically, more efficient reliance on electronic health records (EHR). Medicare and Medicaid physician payments will be juggled to increase reimbursement for E&M services and for physicians who provide greater value in relation to cost. Physicians will be required to participate in the Physician Quality Reporting Initiative (PQRI) program in 2015 and beyond to avoid stiff penalties. And EHR systems are required to adopt uniform standards for electronic transactions.
2. What reforms are woman-specific?
OBG Management: What initiatives are planned for the care of women, in particular?
DiVenere: Congress recognized the importance of reforming women’s health and included many provisions advocated by ACOG in our “Health care for women, health care for all” campaign.
Probably the most important of these provisions is the guarantee of direct access to ObGyn care without need of a referral or pre-authorization from a primary care provider or insurance company. Nor can an insurance company restrict a patient’s direct access to her ObGyn to a certain number of visits or types of services.
This was a major ACOG victory. For 20 years, ObGyns have been waging battles in the states for direct access for patients. Last year, nine states did not require insurers to grant women direct access to ObGyns, and 16 states allowed insurers to restrict ObGyn visits and services. This part of the law, which is effective this year, provides national direct access to all women in all states, and is not tied to an ObGyn’s primary care designation.
Another area of reform concerns maternity care. In 2009, 13% of all pregnant women in the United States were uninsured, as were 20.4% of all women between the ages of 15 and 44, the childbearing years. The uninsured rate for nonelderly women in 2007 ranged from a high of 28% in New Mexico and Texas to a low of 8% in Massachusetts. Today, 42% of all pregnancies are covered by Medicaid. Women have been able, usually, to gain access to some kind of care—sometimes in emergency departments at the time of labor—but the nation clearly needs to do better.
Medicaid and new insurance plans will be required to offer maternity care and women’s preventive services, including mammography screening. The exact parameters of maternity care and other types of care in the essential benefits package will be determined by the Secretary of Health and Human Services (HHS), based on the typical package offered to employees in group health plans. The idea behind the law is that many women who are now covered by Medicaid will transfer to private insurance in their states’ exchanges.
3. How will ObGyn practice change?
OBG Management: What are some of the opportunities and challenges ObGyns will encounter?
DiVenere: There are three key areas of challenge and opportunity:
- Development of the “medical home.” A medical home is a practice designed to provide and coordinate comprehensive patient care. State Medicaid agencies are authorized to require certain beneficiaries, including those who have two or more chronic conditions, to join a medical home. Medicare will also experiment with medical homes, and both Medicaid and Medicare medical home practices will receive additional payments. Most medical homes are expected to be family practice, internal medicine, or pediatric care providers, but ObGyn practices can participate, too. ObGyns should look carefully at the opportunities this paradigm provides and consider having their practice designated as a medical home.
- Increased use of nonphysician providers. The new law strongly encourages this practice, including in the ObGyn specialty. Congress is determined to experiment with non-ObGyn deliveries in response to patient demand and midlevel assurances that nonphysicians can deliver babies with better outcomes at significantly lower cost. Our specialty’s cesarean delivery rate is under intense scrutiny. Skewed studies “prove” happier and healthier deliveries in homes and other out-of-hospital locations without an ObGyn in attendance. And midlevel practitioners are offering vaginal birth after cesarean delivery in many cases where ObGyns are restricted by hospital rules.
- The law extends Medicaid payments to free-standing birth centers and birth attendants and does not specify which kinds of practitioners can qualify as birth attendants. Free-standing birth centers can provide high-quality care if they are appropriately accredited and have an established transfer relationship with a nearby hospital. The law does not specify these criteria, either.
- Increasing payments to nonphysicians. Medicare payments to certified nurse midwives (CNMs) will reach the rate paid to physicians for the same services in January 2011, up from 65% of the physician rate. Medicare will also pay CNMs a 10% bonus if primary care services account for at least 60% of their allowed charges. And the law requires health plans in the state exchanges to pay for covered health services provided by any practitioner recognized under state law, whether or not the plan contracts with that individual or type of provider. Certified professional midwives (lay midwives) are licensed in 21 states, and this provision may give them significant new entry.
ObGyns stop delivering babies at increasingly early points in their career, and only 13% of family physicians deliver babies today. So we need to find ways to extend our care—and increasing collaborative practice between ObGyns and CNMs and certified midwives may help close this gap. The increased focus on midlevel providers in the law may present us with both a challenge and an important opportunity.
4. What services will be fully covered now?
OBG Management: Beginning this year, health plans will be required to provide a minimum level of coverage without cost-sharing for preventive care and screenings for women, among others. What will this requirement encompass?
DiVenere: Congress emphasized prevention in the reform law as part of its strategy to bend the cost curve, investing in prevention in order to reduce higher spending on illness.
Beginning in September 2010, all plans—including those that existed before this law was passed—must cover preventive health services without any patient cost sharing, whether copayments or deductibles. These services include women’s preventive care and screening included in comprehensive guidelines supported by the Health Resources and Services Administration (HRSA), even if they are more extensive than services recommended by the Centers for Disease Control and Prevention (CDC) and US Preventive Services Task Force (USPSTF). Breast cancer screening, mammography, and prevention services are covered as though the November 2009 USPSTF recommendations suggesting limits on mammography screening for certain age groups did not exist.
The mammography screening coverage was a big win for ACOG. We worked closely with Senator Barbara Mikulski (D-Md.) on this amendment, and it was the first Democratic amendment offered. It passed on the Senate floor during a contentious floor fight.
ACOG continues to recommend screening mammography every 1 to 2 years for women 40 to 49 years old; annual screening for women 50 and older; clinical breast examination every year for women 19 years and older; and regular breast self-examination.
The Senate bill that was brought to the floor would have limited women’s preventive care to USPSTF recommendations only. Working with Senator Mikulski, we made sure that women younger than 50 will be covered for mammography every 1 to 2 years.
OBG Management: Are there other important benefits for women included in the law?
DiVenere: Yes. One provision will improve research, screening, and treatment for postpartum depression, a signature issue of ACOG President Gerald F. Joseph Jr., MD, during his presidential year. ACOG and Dr. Joseph worked closely with Senator Bob Menendez (D-NJ) to introduce the Moms Act and win its inclusion in the health reform law.
Under this section, HHS will:
- conduct research into the causes of, and treatments for, postpartum conditions
- create a national public awareness campaign to increase knowledge of postpartum depression and postpartum psychosis
- provide grants to study the benefits of screening for postpartum depression and postpartum psychosis
- establish grants to deliver or enhance outpatient, inpatient, and home-based health and support services, including case management and comprehensive treatment services for women with or at risk of postpartum conditions.
5. Will expanded coverage improve birth outcomes?
OBG Management: Do you expect that guaranteed coverage of pregnancy will increase the number of women who seek prenatal care—as opposed to waiting until labor begins—to see a doctor? Will guaranteed coverage of pregnancy improve birth outcomes over the long term?
DiVenere: Those are certainly the goals. And guaranteed coverage of pregnancy was one of ACOG’s essential elements in health care reform. Prenatal care has been shown to save $3 for every $1 spent in the Medicaid program and continues to be the primary way to identify problems during pregnancy, giving ObGyns the opportunity to assess and manage the risk of preterm labor and other threats to the health of the mother and baby.
The health reform law recognizes that better prenatal care can lead to healthier babies—both in its coverage of maternity and preventive care, and by new Medicaid coverage of smoking-cessation counseling and family planning, both beginning this year.
Medicaid will now cover the costs of diagnostic, therapeutic, and counseling services, as well as pharmacotherapy for pregnant women covered by Medicaid, at no cost to the patient. Before health reform passed, only 24 states reimbursed ObGyns and other physicians for smoking-cessation counseling for pregnant women. Five states didn’t cover any smoking-cessation services at all.
Also beginning this year, states can provide family planning services to nonpregnant women up to the same eligibility levels to which they cover pregnant women, without the need to apply for federal waivers or permission. Forty-five states extend Medicaid coverage to pregnant women who have incomes above the regular Medicaid eligibility levels, from a low of 150% to a high of 300% of the federal poverty levels.
Before this new law, 27 states had federal waivers to provide family planning to women who had an income above the Medicaid eligibility levels, most of them at or near 200% of the federal poverty level. Eleven of these waivers expire this year.
6. Is femaleness a “preexisting condition”?
OBG Management: During the debate on health reform, many people claimed, somewhat facetiously, that female sex has been a preexisting condition. The new law will ensure that patients can’t be dropped by their insurance company—or denied coverage—for arbitrary or unfair reasons, such as preexisting conditions. How are these changes likely to affect women and their ObGyns?
DiVenere: The insurance reforms in the new law are very important to women and to ObGyn practices. In fact, the prohibition on preexisting conditions was a top priority of ACOG’s “Health care for women, health care for all” campaign, and Congress included this provision with women’s health in mind.
Many members of Congress were shocked to learn that it was not unusual for insurers to deny coverage to women who were pregnant, who had had a previous cesarean delivery, or who had been the victim of domestic violence at some point in their history. In fact, almost any medical history, genetic information, disability, or current health condition was grounds for denial of coverage.
Women were also often charged higher premiums than men for the same coverage. And insurance companies would sometimes require waiting periods for coverage—sometimes as long as 9 months.
All of these practices are outlawed by the health reform law, which prohibits plans from using preexisting condition exclusions to deny children coverage as of September 1, 2010 and adults as of January 1, 2014. Beginning on January 1, 2014, women cannot be denied coverage due to pregnancy, previous cesarean delivery, or domestic violence, or medical history, among many other reasons.
Effective March 23, 2010 and ending January 1, 2014, a high-risk pool insurance program has been created for people who have been uninsured for 6 months and who have a preexisting condition. Funding for the temporary risk pool is capped at $5 billion.
Insurers in the small and individual markets and in the exchanges cannot discriminate on the basis of medical history or other variables; may only charge limited premium differentials for age, family size, and smoking, but not for gender; and cannot mandate a waiting period longer than 90 days.
Insurance plans that were in existence before enactment must comply with reforms on waiting periods; lifetime limits; rescission; extension of dependent coverage; uniform explanation of coverage; and loss ratio reporting and premium rebates. Group grandfather plans must also comply with restrictions on annual limits and preexisting conditions.
All these protections should benefit ObGyn practices by ensuring coverage and continuity of care for their patients.
7. What happened to tort reform?
OBG Management: No tort reform was included in the law. Why not?
DiVenere: The law authorizes HHS to award $50 million over 5 years, up to $500,000 per state, to develop, implement, and evaluate alternative medical liability reform initiatives that meet several specific criteria. Medical liability protections under the Federal Tort Claims Act are extended to officers, governing board members, employees, and contractors of free clinics.
We at ACOG were very disappointed that Congress didn’t take a serious step toward medical liability reform in this bill. Liability reform was one of ACOG’s five essential elements of health reform, and its absence from the final bill was a prominent reason why we ultimately “reluctantly opposed” passage. We, and the rest of the House of Medicine, were clear that health reform wouldn’t work without meaningful medical liability reform.
ACOG supports caps on noneconomic damages and other reforms in California and Texas law. We also support testing alternatives, including health courts, alternative dispute resolution, “Sorry Works!” programs, and birth injury compensation funds. But this part of the health reform law requires that tests be linked to patient safety, an association that is impossible to establish in cases of neonatal encephalopathy. The law also requires that patients be allowed to opt out of a system if they choose to go to court. Both of these requirements hamper the development of meaningful alternatives for the ObGyn specialty.
8. Is the system repairable?
OBG Management: Can the US health care system be fixed in one fell swoop?
DiVenere: ACOG pursued two integral missions in reform efforts: improving women’s health and advocating for practicing ObGyns. Our mission in women’s health included guaranteed maternity care, important insurance reforms, and direct access to ObGyns. Our mission in regard to practicing ObGyns included the protection of ultrasonography, the reform of medical liability laws, and repeal of the Medicare sustainable growth rate, along with an array of other issues, all of which were shared by the entire House of Medicine.
We see these missions as integral; Congress saw them as separable. We were largely successful on the women’s health side of the ledger. But Congress responded to the House of Medicine issues with little interest.
We believe that we can fulfill our mission to women’s health only if the issues of practicing ObGyns are addressed in the process. You can’t build a new health care system on a broken medical liability system or a broken Medicare physician payment system, and we still have both. We have a lot more work to do on these issues and the myriad of other issues that need to be addressed. This is really just the beginning of health reform.
9. Has PQRI regained the limelight?
OBG Management: The Medicare quality reporting incentive payments under the Physician Quality Reporting Initiative (PQRI) have been extended. In fact, physicians will be penalized, beginning in 2015, if they do not participate. Are the incentive payments a good thing for ObGyns?
DiVenere: Yes, a big change is coming in this program. ObGyns who participate in PQRI will be eligible to receive bonus payments of 1% in 2011 and 0.5% from 2012 to 2014. Payments will be reduced by 1.5% in 2015 and by 2.0% in 2016 for physicians who don’t participate in the PQRI program.
Beginning in 2012, PQRI participation becomes a meaningful use qualifier for EHR grants.
In 2011 to 2014, physicians who complete Maintenance of Certification (MOC) are eligible for an additional 1% bonus in 2011 and 0.5% bonus in 2012 to 2014. Data on a physician’s quality measures must be submitted on the physician’s behalf by the MOC program. After 2014, the Secretary of HHS can add MOC completion to the quality measures used for the value-based payment modifier. The American Board of Obstetrics and Gynecology hasn’t yet qualified its MOC for this part of the program.
ObGyn participation in the PQRI program is very limited (less than 10%). While only about 25 of the 215 PQRI quality measures apply to ObGyn care, most are easily applicable, and a physician needs to report on only three to five measures to qualify for the program.
The very low participation rate is likely because many ObGyn practices just didn’t think the incentive payment was worth the trouble. They may need to rethink that math once they’re faced with payment cuts in 2015.
ObGyns should also be aware that the Secretary of HHS, with input from stakeholders, will set up a Physician Compare Web site (modeled after the program that already exists for hospitals) using PQRI data. Data will be made public on January 1, 2013, comparing physicians in terms of quality of care and patient experience.
The Secretary must ensure that the data are statistically valid and risk-adjusted. In addition, the physician must be given time to review the information before it becomes public, and data must ensure appropriate attribution of care when multiple physicians and other providers are involved. The Secretary must also give physicians timely performance feedback.
For all these reasons, ACOG is working with the physician community to make a number of improvements to the PQRI program, doing our best to make it as easy as possible for our members to participate and benefit.
10. What effect will the expansion of Medicaid have on ObGyn practice?
DiVenere: Starting in 2014, the same year that state exchanges are expected to be established, Medicaid eligibility will be broadened to cover all individuals younger than 65 years who have incomes up to 133% of the federal poverty level. All newly eligible adults will be guaranteed a benchmark benefit package that provides the essential health benefits.
States that have already expanded eligibility to adults who have incomes up to 100% of the federal poverty level will receive a phased-in increase in the federal medical assistance percentage so that, by 2019, they will receive the same federal financing as other states (93% in 2019 and 90% in 2020 and later). And states have the option to expand Medicaid eligibility to childless adults as of April 1, 2010, but will receive their regular federal medical assistance percentage until 2014.
Although these changes will broaden the range and increase the number of individuals who will be eligible for Medicaid, the effect on ObGyn practice remains to be seen, especially as pregnant women who were covered by Medicaid at income levels above 133% of the federal poverty level transition off of Medicaid and into private health insurance offered in the exchanges.
Today, about 38% of all ObGyns accept Medicaid gynecologic patients, and 44% accept Medicaid obstetric patients. Medicaid accounts for 18% of revenues of the average ObGyn practice.
11. Will the extension of benefits to young adults have a measurable impact on ObGyn practice?
DiVenere: Congress included two provisions to target “young immortals,” young adults who don’t think they need health insurance because they’re young and healthy and never need to see a doctor. Many young adults are not offered employer-based health insurance, and many see no advantage in buying coverage that they don’t expect to use. But we all know that someone pays when any uninsured person falls sick or has an accident that necessitates medical care.
Beginning this year, adult children as old as 26 years can go onto their parents’ health insurance plan. In addition, catastrophic plans will soon be available to individuals younger than 30 who want to purchase a higher deductible plan through their state exchange or on the individual and small group markets. These catastrophic plans are not required to include the essential benefits package, including maternity care. Nevertheless, both of these provisions should be helpful to ObGyn practices.
12. Will the mandate for employers to provide health insurance affect many ObGyns?
DiVenere: The employer mandate takes effect in 2014, when employers with more than 50 employees, at least one of whom receives a premium tax credit, are required to offer health insurance coverage to employees or be assessed a range of fees. Employers that have 50 or fewer employees are exempt from this requirement.
In 2007, 75% of ObGyn practices had fewer than 42 full-time employees, with an average number of full-time employees, including physicians, of 34.4. So this mandate should not apply to the average ObGyn practice.
A range of small business tax credits for employers that contribute at least 50% of the cost of coverage for their employees will also be available, with credits phasing out as the size of the firm and the average employee wage increase.
13. Who will benefit from the Medicare geographic payment adjustments?
DiVenere: The increased Medicare geographic practice cost index (GPCI) payments and new Frontier payments won’t affect many ObGyns nationally, but they are likely to affect most ObGyns in the related rural locations.
The law reestablishes the national average floor on Medicare’s GPCI for physician work. In 2010 and 2011, Medicare makes a separate adjustment for the practice expense portion of physician payments that will benefit physicians in rural and low-cost areas.
Beginning in 2011, a third adjustment will increase the practice expense GPCI for physicians in frontier states. A frontier state is one in which at least half of its counties have populations smaller than six people per square mile. Frontier states are expected to be Montana, North Dakota, South Dakota, Utah, and Wyoming.
Physicians in 51 localities in 42 states, Puerto Rico, and the Virgin Islands will benefit from the two practice expense adjustments.
ObGyns should also know about two other payment changes:
- The HHS Secretary will create and apply to Medicare provider payments a value-based modifier that will result in higher Medicare payments for high-quality, low-cost physicians and lower payments for high-cost, low-quality physicians. The modifier is to be based on a composite quality score and a composite cost score determined by measures selected by the HHS Secretary and endorsed by a consensus organization. This change begins with 2015 Medicare payments and applies only to physicians in 2015. In 2017, it will also apply to other health professionals.
- Effective immediately, the HHS Secretary has the authority to increase or decrease Medicare relative values, and payments for services, with special attention focused on:
- – services that have high growth rates
- – services that have seen substantial changes in the practice expense or work components
- – services for which new technology has reduced costs
- – instances in which multiple codes are frequently billed for a single service
- – codes that have not been reviewed since implementation of the resource-based relative value scale (RBRVS).
ObGyns who participate in Medicare will start receiving individual physician resource use reports in 2012. These reports will compare per capita utilization of physicians (or physician groups) with the utilization rate of physicians who see similar patients. Reports are required to be risk-adjusted and standardized to take into account local health-care costs.
14. Do you expect the law’s requirements for “administrative simplification” to reduce overhead and increase efficiency?
DiVenere: Don’t we all hope so.
The bill contains several requirements such as:
- establishment of a standardized claim form
- streamlining of claims processing
- improvement of interoperability to allow for more electronic information sharing. These changes will not be implemented until 2013 at the earliest.
Today, about 34% of all ObGyn practices use electronic health records. The systemwide benefits of health information technology (HIT) can be many. Insurers can save by reducing unnecessary tests. Patients can benefit from better coordination of care and fewer medical errors. But these advantages don’t necessarily translate into savings or revenue for physician practices.
Instead, physicians face Medicare and private insurance payment cuts. Little assistance is available for the investment in HIT. And uncertain interoperability standards and rapid technological changes can very quickly make this year’s investment obsolete. Many physicians in solo and small practices are understandably reluctant to take the HIT plunge.
The initial cost of purchasing HIT for a small practice is typically at least $50,000 per physician. Physicians face additional, ongoing costs in staff training and hardware and software updates as well. And many physicians see significant efficiency losses for months and sometimes years after upgrading to an electronic health record system.
Still, with interoperable, shareable electronic records, all physicians treating a particular patient can have the full story. A patient’s paper record kept in her physician’s office shows only a slice of her medical history, potentially missing important information from the patient’s other physicians, including allergies to medication, test results, and the results of particular therapies.
Without a shared electronic record, a physician relies on the recollection of each patient, which is often unintentionally incomplete. A patient may be uncertain about the name or dosage of a medication, fail to remember the date of a screening examination, or lack results of lab tests ordered by another physician.
A physician’s access to the full story with shareable electronic records is important to the care of all patients and can be particularly relevant for patients who have inconsistent contact with health care providers. Often, these patients get care in various settings, including physician offices, community clinics, and emergency departments. Because these patients tend to have a higher incidence of chronic disease, they may greatly benefit from the sharing of medical information.
Clearly, Congress wants to move us to full adoption of HIT. Beginning in 2013, all plans must comply with a uniform standard for electronic transactions, including eligibility verification and health claims status.
In 2014, uniform standards must allow automatic reconciliation of electronic funds transfers and HIPAA payment and remittance; use standardized and consistent methods of health plan enrollment and claim edits; use unique health plan identifiers to simplify and improve routing of health care transactions; and use standard electronic claims attachments.
Uniformity and standardization can help address one of the major roadblocks to physician adoption of health information technology.
Why the FDA asked for more testing on flibanserin for HSDD
When the FDA Advisory Committee for Reproductive Health Drugs convened in mid-June to consider approving the nonhormonal agent flibanserin for the treatment of hypoactive sexual desire disorder (HSDD) in premenopausal women, there was no FDA-approved drug for the disorder.
There still isn’t.
After examining data from two US-based Phase-3 trials (and a third trial conducted in Europe), the committee 1) decided that evidence of flibanserin’s efficacy is lacking, and 2) found the prevalence of side effects worrisome. It did agree with drug maker Boehringer Ingelheim that there is “widespread unmet need” for a medical therapy for HSDD.
The two primary US efficacy studies of flibanserin failed to achieve significance for the prespecified co-primary endpoint of sexual desire, as measured by a daily eDiary that recorded the number of sexually satisfying events and asked patients to rate the intensity of desire as “none,” “low,” “moderate,” or “strong.” At the same time, 15% of women taking 100 mg of the drug—the recommended dosage—discontinued treatment due to adverse events such as somnolence, nausea, dizziness, insomnia, and anxiety, compared with 7% of those taking placebo.
The committee’s decision does not mean that the FDA will ultimately reject the new drug application of German manufacturer Boehringer Ingelheim, but approval appears unlikely based on the data reviewed at the committee hearing.
After voting down the drug, Acting Committee Chair Julia Johnson, MD, recommended that Boehringer Ingelheim continue to study the agent because of its “promising” potential—and several other panelists supported that recommendation. Dr. Johnson is Professor and Chair of Obstetrics and Gynecology at the University of Massachusetts Medical School and UMass Memorial Medical Center in Worcester, Mass.
Boehringer Ingelheim is currently recruiting participants for a Phase 3 trial of flibanserin in postmenopausal women in North America (NCT01057901).
When the FDA Advisory Committee for Reproductive Health Drugs convened in mid-June to consider approving the nonhormonal agent flibanserin for the treatment of hypoactive sexual desire disorder (HSDD) in premenopausal women, there was no FDA-approved drug for the disorder.
There still isn’t.
After examining data from two US-based Phase-3 trials (and a third trial conducted in Europe), the committee 1) decided that evidence of flibanserin’s efficacy is lacking, and 2) found the prevalence of side effects worrisome. It did agree with drug maker Boehringer Ingelheim that there is “widespread unmet need” for a medical therapy for HSDD.
The two primary US efficacy studies of flibanserin failed to achieve significance for the prespecified co-primary endpoint of sexual desire, as measured by a daily eDiary that recorded the number of sexually satisfying events and asked patients to rate the intensity of desire as “none,” “low,” “moderate,” or “strong.” At the same time, 15% of women taking 100 mg of the drug—the recommended dosage—discontinued treatment due to adverse events such as somnolence, nausea, dizziness, insomnia, and anxiety, compared with 7% of those taking placebo.
The committee’s decision does not mean that the FDA will ultimately reject the new drug application of German manufacturer Boehringer Ingelheim, but approval appears unlikely based on the data reviewed at the committee hearing.
After voting down the drug, Acting Committee Chair Julia Johnson, MD, recommended that Boehringer Ingelheim continue to study the agent because of its “promising” potential—and several other panelists supported that recommendation. Dr. Johnson is Professor and Chair of Obstetrics and Gynecology at the University of Massachusetts Medical School and UMass Memorial Medical Center in Worcester, Mass.
Boehringer Ingelheim is currently recruiting participants for a Phase 3 trial of flibanserin in postmenopausal women in North America (NCT01057901).
When the FDA Advisory Committee for Reproductive Health Drugs convened in mid-June to consider approving the nonhormonal agent flibanserin for the treatment of hypoactive sexual desire disorder (HSDD) in premenopausal women, there was no FDA-approved drug for the disorder.
There still isn’t.
After examining data from two US-based Phase-3 trials (and a third trial conducted in Europe), the committee 1) decided that evidence of flibanserin’s efficacy is lacking, and 2) found the prevalence of side effects worrisome. It did agree with drug maker Boehringer Ingelheim that there is “widespread unmet need” for a medical therapy for HSDD.
The two primary US efficacy studies of flibanserin failed to achieve significance for the prespecified co-primary endpoint of sexual desire, as measured by a daily eDiary that recorded the number of sexually satisfying events and asked patients to rate the intensity of desire as “none,” “low,” “moderate,” or “strong.” At the same time, 15% of women taking 100 mg of the drug—the recommended dosage—discontinued treatment due to adverse events such as somnolence, nausea, dizziness, insomnia, and anxiety, compared with 7% of those taking placebo.
The committee’s decision does not mean that the FDA will ultimately reject the new drug application of German manufacturer Boehringer Ingelheim, but approval appears unlikely based on the data reviewed at the committee hearing.
After voting down the drug, Acting Committee Chair Julia Johnson, MD, recommended that Boehringer Ingelheim continue to study the agent because of its “promising” potential—and several other panelists supported that recommendation. Dr. Johnson is Professor and Chair of Obstetrics and Gynecology at the University of Massachusetts Medical School and UMass Memorial Medical Center in Worcester, Mass.
Boehringer Ingelheim is currently recruiting participants for a Phase 3 trial of flibanserin in postmenopausal women in North America (NCT01057901).
14 questions (and answers) about health reform and you
With passage of the Patient Protection and Affordable Care Act earlier this year, big changes are afoot in the way Americans practice medicine. In a plethora of articles, blogs, and broadcast spots, the media have focused on what the new law portends for the average employee, employers, and the uninsured—but what, exactly, does it entail for ObGyns and their patients?
To find an answer to that overarching question—and 13 others—we invited Lucia DiVenere, director of government relations at the American Congress of Obstetricians and Gynecologists, to join us in an extended discussion of the law and its ramifications. She offered insight into ACOG’s extensive lobbying efforts on behalf of women and the specialty and described the many ways ObGyn care will change in the near and proximal future, focusing on questions that you might find yourself asking, including:
- Will I see a lot more patients?
- What reforms are woman-specific?
- How will my practice change?
- Which of my services will be fully covered?
- Will expanded coverage improve birth outcomes?
- Is “femaleness” a preexisting condition?
- What happened to tort reform?
- Is the system repairable?
1 Will ObGyns see a lot more patients?
OBG Management: The most talked about change the new law heralds is the addition of roughly 32 million people to the insurance rolls. Is the most significant impact of the legislation for ObGyns likely to be an increase in the number of patients they will be seeing?
Lucia DiVenere: Congress wanted to increase the ranks of the insured and expand access to health care, and it addressed these goals with individual and employer mandates, state exchanges, Medicaid expansion, and insurance reforms.
But that isn’t the most significant change in store for us. Congress also wanted to reform our health care system in a number of fundamental ways, some of which are designed to change the way physicians provide care to their patients.
For example, Congress wanted to “bend the cost curve”—to reduce the expected rate of growth in health care spending over the long term. That doesn’t mean that health care costs in 2020 will be less than they were in 2018, but it does mean that annual and long-term growth rates should level off and become sustainable. To accomplish this goal, Congress created an Independent Payment Advisory Commission, which may prove to be extremely powerful in reducing health care costs and is likely to significantly affect all physicians. Greater protections against fraud and abuse, experiments with new kinds of payment and delivery systems, including “medical homes,” and increased reliance on nonphysician practitioners—all included in the law—are also expected to reduce costs.
OBG Management: What other changes are coming?
DiVenere: Congress was determined to alter the practice of health care, ensuring higher quality for each dollar spent and consistent delivery of care. It also sought to kick-start our health care system—especially in the physician arena—into greater and, theoretically, more efficient reliance on electronic health records (EHR). Medicare and Medicaid physician payments will be juggled to increase reimbursement for E&M services and for physicians who provide greater value in relation to cost. Physicians will be required to participate in the Physician Quality Reporting Initiative (PQRI) program in 2015 and beyond to avoid stiff penalties. And EHR systems are required to adopt uniform standards for electronic transactions.
2 What reforms are woman-specific?
OBG Management: What initiatives are planned for the care of women, in particular?
DiVenere: Congress recognized the importance of reforming women’s health and included many provisions advocated by ACOG in our “Health care for women, health care for all” campaign.
Probably the most important of these provisions is the guarantee of direct access to ObGyn care without need of a referral or pre-authorization from a primary care provider or insurance company. Nor can an insurance company restrict a patient’s direct access to her ObGyn to a certain number of visits or types of services.
This was a major ACOG victory. For 20 years, ObGyns have been waging battles in the states for direct access for patients. Last year, nine states did not require insurers to grant women direct access to ObGyns, and 16 states allowed insurers to restrict ObGyn visits and services. This part of the law, which is effective this year, provides national direct access to all women in all states, and is not tied to an ObGyn’s primary care designation.
Another area of reform concerns maternity care. In 2009, 13% of all pregnant women in the United States were uninsured, as were 20.4% of all women between the ages of 15 and 44, the childbearing years. The uninsured rate for nonelderly women in 2007 ranged from a high of 28% in New Mexico and Texas to a low of 8% in Massachusetts. Today, 42% of all pregnancies are covered by Medicaid. Women have been able, usually, to gain access to some kind of care—sometimes in emergency departments at the time of labor—but the nation clearly needs to do better.
Medicaid and new insurance plans will be required to offer maternity care and women’s preventive services, including mammography screening. The exact parameters of maternity care and other types of care in the essential benefits package will be determined by the Secretary of Health and Human Services (HHS), based on the typical package offered to employees in group health plans. The idea behind the law is that many women who are now covered by Medicaid will transfer to private insurance in their states’ exchanges.
3 How will ObGyn practice change?
OBG Management: What are some of the opportunities and challenges ObGyns will encounter?
DiVenere: There are three key areas of challenge and opportunity:
- Development of the “medical home.” A medical home is a practice designed to provide and coordinate comprehensive patient care. State Medicaid agencies are authorized to require certain beneficiaries, including those who have two or more chronic conditions, to join a medical home. Medicare will also experiment with medical homes, and both Medicaid and Medicare medical home practices will receive additional payments. Most medical homes are expected to be family practice, internal medicine, or pediatric care providers, but ObGyn practices can participate, too. ObGyns should look carefully at the opportunities this paradigm provides and consider having their practice designated as a medical home.
- Increased use of nonphysician providers. The new law strongly encourages this practice, including in the ObGyn specialty. Congress is determined to experiment with non-ObGyn deliveries in response to patient demand and midlevel assurances that nonphysicians can deliver babies with better outcomes at significantly lower cost. Our specialty’s cesarean delivery rate is under intense scrutiny. Skewed studies “prove” happier and healthier deliveries in homes and other out-of-hospital locations without an ObGyn in attendance. And midlevel practitioners are offering vaginal birth after cesarean delivery in many cases where ObGyns are restricted by hospital rules.
- The law extends Medicaid payments to free-standing birth centers and birth attendants and does not specify which kinds of practitioners can qualify as birth attendants. Free-standing birth centers can provide high-quality care if they are appropriately accredited and have an established transfer relationship with a nearby hospital. The law does not specify these criteria, either.
- Increasing payments to nonphysicians. Medicare payments to certified nurse midwives (CNMs) will reach the rate paid to physicians for the same services in January 2011, up from 65% of the physician rate. Medicare will also pay CNMs a 10% bonus if primary care services account for at least 60% of their allowed charges. And the law requires health plans in the state exchanges to pay for covered health services provided by any practitioner recognized under state law, whether or not the plan contracts with that individual or type of provider. Certified professional midwives (lay midwives) are licensed in 21 states, and this provision may give them significant new entry.
ObGyns stop delivering babies at increasingly early points in their career, and only 13% of family physicians deliver babies today. So we need to find ways to extend our care—and increasing collaborative practice between ObGyns and CNMs and certified midwives may help close this gap. The increased focus on midlevel providers in the law may present us with both a challenge and an important opportunity.
4 What services will be fully covered now?
OBG Management: Beginning this year, health plans will be required to provide a minimum level of coverage without cost-sharing for preventive care and screenings for women, among others. What will this requirement encompass?
DiVenere: Congress emphasized prevention in the reform law as part of its strategy to bend the cost curve, investing in prevention in order to reduce higher spending on illness.
Beginning in September 2010, all plans—including those that existed before this law was passed—must cover preventive health services without any patient cost sharing, whether copayments or deductibles. These services include women’s preventive care and screening included in comprehensive guidelines supported by the Health Resources and Services Administration (HRSA), even if they are more extensive than services recommended by the Centers for Disease Control and Prevention (CDC) and US Preventive Services Task Force (USPSTF). Breast cancer screening, mammography, and prevention services are covered as though the November 2009 USPSTF recommendations suggesting limits on mammography screening for certain age groups did not exist.
The mammography screening coverage was a big win for ACOG. We worked closely with Senator Barbara Mikulski (D-Md.) on this amendment, and it was the first Democratic amendment offered. It passed on the Senate floor during a contentious floor fight.
ACOG continues to recommend screening mammography every 1 to 2 years for women 40 to 49 years old; annual screening for women 50 and older; clinical breast examination every year for women 19 years and older; and regular breast self-examination.
The Senate bill that was brought to the floor would have limited women’s preventive care to USPSTF recommendations only. Working with Senator Mikulski, we made sure that women younger than 50 will be covered for mammography every 1 to 2 years.
OBG Management: Are there other important benefits for women included in the law?
DiVenere: Yes. One provision will improve research, screening, and treatment for postpartum depression, a signature issue of ACOG President Gerald F. Joseph Jr., MD, during his presidential year. ACOG and Dr. Joseph worked closely with Senator Bob Menendez (D-NJ) to introduce the Moms Act and win its inclusion in the health reform law.
Under this section, HHS will:
- conduct research into the causes of, and treatments for, postpartum conditions
- create a national public awareness campaign to increase knowledge of postpartum depression and postpartum psychosis
- provide grants to study the benefits of screening for postpartum depression and postpartum psychosis
- establish grants to deliver or enhance outpatient, inpatient, and home-based health and support services, including case management and comprehensive treatment services for women with or at risk of postpartum conditions.
5 Will expanded coverage improve birth outcomes?
OBG Management: Do you expect that guaranteed coverage of pregnancy will increase the number of women who seek prenatal care—as opposed to waiting until labor begins—to see a doctor? Will guaranteed coverage of pregnancy improve birth outcomes over the long term?
DiVenere: Those are certainly the goals. And guaranteed coverage of pregnancy was one of ACOG’s essential elements in health care reform. Prenatal care has been shown to save $3 for every $1 spent in the Medicaid program and continues to be the primary way to identify problems during pregnancy, giving ObGyns the opportunity to assess and manage the risk of preterm labor and other threats to the health of the mother and baby.
The health reform law recognizes that better prenatal care can lead to healthier babies—both in its coverage of maternity and preventive care, and by new Medicaid coverage of smoking-cessation counseling and family planning, both beginning this year.
Medicaid will now cover the costs of diagnostic, therapeutic, and counseling services, as well as pharmacotherapy for pregnant women covered by Medicaid, at no cost to the patient. Before health reform passed, only 24 states reimbursed ObGyns and other physicians for smoking-cessation counseling for pregnant women. Five states didn’t cover any smoking-cessation services at all.
Also beginning this year, states can provide family planning services to nonpregnant women up to the same eligibility levels to which they cover pregnant women, without the need to apply for federal waivers or permission. Forty-five states extend Medicaid coverage to pregnant women who have incomes above the regular Medicaid eligibility levels, from a low of 150% to a high of 300% of the federal poverty levels.
Before this new law, 27 states had federal waivers to provide family planning to women who had an income above the Medicaid eligibility levels, most of them at or near 200% of the federal poverty level. Eleven of these waivers expire this year.
6 Is femaleness a “preexisting condition”?
OBG Management: During the debate on health reform, many people claimed, somewhat facetiously, that female sex has been a preexisting condition. The new law will ensure that patients can’t be dropped by their insurance company—or denied coverage—for arbitrary or unfair reasons, such as preexisting conditions. How are these changes likely to affect women and their ObGyns?
DiVenere: The insurance reforms in the new law are very important to women and to ObGyn practices. In fact, the prohibition on preexisting conditions was a top priority of ACOG’s “Health care for women, health care for all” campaign, and Congress included this provision with women’s health in mind.
Many members of Congress were shocked to learn that it was not unusual for insurers to deny coverage to women who were pregnant, who had had a previous cesarean delivery, or who had been the victim of domestic violence at some point in their history. In fact, almost any medical history, genetic information, disability, or current health condition was grounds for denial of coverage.
Women were also often charged higher premiums than men for the same coverage. And insurance companies would sometimes require waiting periods for coverage—sometimes as long as 9 months.
All of these practices are outlawed by the health reform law, which prohibits plans from using preexisting condition exclusions to deny children coverage as of September 1, 2010 and adults as of January 1, 2014. Beginning on January 1, 2014, women cannot be denied coverage due to pregnancy, previous cesarean delivery, or domestic violence, or medical history, among many other reasons.
Effective March 23, 2010 and ending January 1, 2014, a high-risk pool insurance program has been created for people who have been uninsured for 6 months and who have a preexisting condition. Funding for the temporary risk pool is capped at $5 billion.
Insurers in the small and individual markets and in the exchanges cannot discriminate on the basis of medical history or other variables; may only charge limited premium differentials for age, family size, and smoking, but not for gender; and cannot mandate a waiting period longer than 90 days.
Insurance plans that were in existence before enactment must comply with reforms on waiting periods; lifetime limits; rescission; extension of dependent coverage; uniform explanation of coverage; and loss ratio reporting and premium rebates. Group grandfather plans must also comply with restrictions on annual limits and preexisting conditions.
All these protections should benefit ObGyn practices by ensuring coverage and continuity of care for their patients.
7 What happened to tort reform?
OBG Management: No tort reform was included in the law. Why not?
DiVenere: The law authorizes HHS to award $50 million over 5 years, up to $500,000 per state, to develop, implement, and evaluate alternative medical liability reform initiatives that meet several specific criteria. Medical liability protections under the Federal Tort Claims Act are extended to officers, governing board members, employees, and contractors of free clinics.
We at ACOG were very disappointed that Congress didn’t take a serious step toward medical liability reform in this bill. Liability reform was one of ACOG’s five essential elements of health reform, and its absence from the final bill was a prominent reason why we ultimately “reluctantly opposed” passage. We, and the rest of the House of Medicine, were clear that health reform wouldn’t work without meaningful medical liability reform.
ACOG supports caps on noneconomic damages and other reforms in California and Texas law. We also support testing alternatives, including health courts, alternative dispute resolution, “Sorry Works!” programs, and birth injury compensation funds. But this part of the health reform law requires that tests be linked to patient safety, an association that is impossible to establish in cases of neonatal encephalopathy. The law also requires that patients be allowed to opt out of a system if they choose to go to court. Both of these requirements hamper the development of meaningful alternatives for the ObGyn specialty.
8 Is the system repairable?
OBG Management: Can the US health care system be fixed in one fell swoop?
DiVenere: ACOG pursued two integral missions in reform efforts: improving women’s health and advocating for practicing ObGyns. Our mission in women’s health included guaranteed maternity care, important insurance reforms, and direct access to ObGyns. Our mission in regard to practicing ObGyns included the protection of ultrasonography, the reform of medical liability laws, and repeal of the Medicare sustainable growth rate, along with an array of other issues, all of which were shared by the entire House of Medicine.
We see these missions as integral; Congress saw them as separable. We were largely successful on the women’s health side of the ledger. But Congress responded to the House of Medicine issues with little interest.
We believe that we can fulfill our mission to women’s health only if the issues of practicing ObGyns are addressed in the process. You can’t build a new health care system on a broken medical liability system or a broken Medicare physician payment system, and we still have both. We have a lot more work to do on these issues and the myriad of other issues that need to be addressed. This is really just the beginning of health reform.
9 Has PQRI regained the limelight?
OBG Management: The Medicare quality reporting incentive payments under the Physician Quality Reporting Initiative (PQRI) have been extended. In fact, physicians will be penalized, beginning in 2015, if they do not participate. Are the incentive payments a good thing for ObGyns?
DiVenere: Yes, a big change is coming in this program. ObGyns who participate in PQRI will be eligible to receive bonus payments of 1% in 2011 and 0.5% from 2012 to 2014. Payments will be reduced by 1.5% in 2015 and by 2.0% in 2016 for physicians who don’t participate in the PQRI program.
Beginning in 2012, PQRI participation becomes a meaningful use qualifier for EHR grants.
In 2011 to 2014, physicians who complete Maintenance of Certification (MOC) are eligible for an additional 1% bonus in 2011 and 0.5% bonus in 2012 to 2014. Data on a physician’s quality measures must be submitted on the physician’s behalf by the MOC program. After 2014, the Secretary of HHS can add MOC completion to the quality measures used for the value-based payment modifier. The American Board of Obstetrics and Gynecology hasn’t yet qualified its MOC for this part of the program.
ObGyn participation in the PQRI program is very limited (less than 10%). While only about 25 of the 215 PQRI quality measures apply to ObGyn care, most are easily applicable, and a physician needs to report on only three to five measures to qualify for the program.
The very low participation rate is likely because many ObGyn practices just didn’t think the incentive payment was worth the trouble. They may need to rethink that math once they’re faced with payment cuts in 2015.
ObGyns should also be aware that the Secretary of HHS, with input from stakeholders, will set up a Physician Compare Web site (modeled after the program that already exists for hospitals) using PQRI data. Data will be made public on January 1, 2013, comparing physicians in terms of quality of care and patient experience.
The Secretary must ensure that the data are statistically valid and risk-adjusted. In addition, the physician must be given time to review the information before it becomes public, and data must ensure appropriate attribution of care when multiple physicians and other providers are involved. The Secretary must also give physicians timely performance feedback.
For all these reasons, ACOG is working with the physician community to make a number of improvements to the PQRI program, doing our best to make it as easy as possible for our members to participate and benefit.
10 What effect will the expansion of Medicaid have on ObGyn practice?
DiVenere: Starting in 2014, the same year that state exchanges are expected to be established, Medicaid eligibility will be broadened to cover all individuals younger than 65 years who have incomes up to 133% of the federal poverty level. All newly eligible adults will be guaranteed a benchmark benefit package that provides the essential health benefits.
States that have already expanded eligibility to adults who have incomes up to 100% of the federal poverty level will receive a phased-in increase in the federal medical assistance percentage so that, by 2019, they will receive the same federal financing as other states (93% in 2019 and 90% in 2020 and later). And states have the option to expand Medicaid eligibility to childless adults as of April 1, 2010, but will receive their regular federal medical assistance percentage until 2014.
Although these changes will broaden the range and increase the number of individuals who will be eligible for Medicaid, the effect on ObGyn practice remains to be seen, especially as pregnant women who were covered by Medicaid at income levels above 133% of the federal poverty level transition off of Medicaid and into private health insurance offered in the exchanges.
Today, about 38% of all ObGyns accept Medicaid gynecologic patients, and 44% accept Medicaid obstetric patients. Medicaid accounts for 18% of revenues of the average ObGyn practice.
11 Will the extension of benefits to young adults have a measurable impact on ObGyn practice?
DiVenere: Congress included two provisions to target “young immortals,” young adults who don’t think they need health insurance because they’re young and healthy and never need to see a doctor. Many young adults are not offered employer-based health insurance, and many see no advantage in buying coverage that they don’t expect to use. But we all know that someone pays when any uninsured person falls sick or has an accident that necessitates medical care.
Beginning this year, adult children as old as 26 years can go onto their parents’ health insurance plan. In addition, catastrophic plans will soon be available to individuals younger than 30 who want to purchase a higher deductible plan through their state exchange or on the individual and small group markets. These catastrophic plans are not required to include the essential benefits package, including maternity care. Nevertheless, both of these provisions should be helpful to ObGyn practices.
12 Will the mandate for employers to provide health insurance affect many ObGyns?
DiVenere: The employer mandate takes effect in 2014, when employers with more than 50 employees, at least one of whom receives a premium tax credit, are required to offer health insurance coverage to employees or be assessed a range of fees. Employers that have 50 or fewer employees are exempt from this requirement.
In 2007, 75% of ObGyn practices had fewer than 42 full-time employees, with an average number of full-time employees, including physicians, of 34.4. So this mandate should not apply to the average ObGyn practice.
A range of small business tax credits for employers that contribute at least 50% of the cost of coverage for their employees will also be available, with credits phasing out as the size of the firm and the average employee wage increase.
13 Who will benefit from the Medicare geographic payment adjustments?
DiVenere: The increased Medicare geographic practice cost index (GPCI) payments and new Frontier payments won’t affect many ObGyns nationally, but they are likely to affect most ObGyns in the related rural locations.
The law reestablishes the national average floor on Medicare’s GPCI for physician work. In 2010 and 2011, Medicare makes a separate adjustment for the practice expense portion of physician payments that will benefit physicians in rural and low-cost areas.
Beginning in 2011, a third adjustment will increase the practice expense GPCI for physicians in frontier states. A frontier state is one in which at least half of its counties have populations smaller than six people per square mile. Frontier states are expected to be Montana, North Dakota, South Dakota, Utah, and Wyoming.
Physicians in 51 localities in 42 states, Puerto Rico, and the Virgin Islands will benefit from the two practice expense adjustments.
ObGyns should also know about two other payment changes:
- The HHS Secretary will create and apply to Medicare provider payments a value-based modifier that will result in higher Medicare payments for high-quality, low-cost physicians and lower payments for high-cost, low-quality physicians. The modifier is to be based on a composite quality score and a composite cost score determined by measures selected by the HHS Secretary and endorsed by a consensus organization. This change begins with 2015 Medicare payments and applies only to physicians in 2015. In 2017, it will also apply to other health professionals.
- Effective immediately, the HHS Secretary has the authority to increase or decrease Medicare relative values, and payments for services, with special attention focused on:
- – services that have high growth rates
- – services that have seen substantial changes in the practice expense or work components
- – services for which new technology has reduced costs
- – instances in which multiple codes are frequently billed for a single service
- – codes that have not been reviewed since implementation of the resource-based relative value scale (RBRVS).
ObGyns who participate in Medicare will start receiving individual physician resource use reports in 2012. These reports will compare per capita utilization of physicians (or physician groups) with the utilization rate of physicians who see similar patients. Reports are required to be risk-adjusted and standardized to take into account local health-care costs.
14 Do you expect the law’s requirements for “administrative simplification” to reduce overhead and increase efficiency?
DiVenere: Don’t we all hope so.
The bill contains several requirements such as:
- establishment of a standardized claim form
- streamlining of claims processing
- improvement of interoperability to allow for more electronic information sharing. These changes will not be implemented until 2013 at the earliest.
Today, about 34% of all ObGyn practices use electronic health records. The systemwide benefits of health information technology (HIT) can be many. Insurers can save by reducing unnecessary tests. Patients can benefit from better coordination of care and fewer medical errors. But these advantages don’t necessarily translate into savings or revenue for physician practices.
Instead, physicians face Medicare and private insurance payment cuts. Little assistance is available for the investment in HIT. And uncertain interoperability standards and rapid technological changes can very quickly make this year’s investment obsolete. Many physicians in solo and small practices are understandably reluctant to take the HIT plunge.
The initial cost of purchasing HIT for a small practice is typically at least $50,000 per physician. Physicians face additional, ongoing costs in staff training and hardware and software updates as well. And many physicians see significant efficiency losses for months and sometimes years after upgrading to an electronic health record system.
Still, with interoperable, shareable electronic records, all physicians treating a particular patient can have the full story. A patient’s paper record kept in her physician’s office shows only a slice of her medical history, potentially missing important information from the patient’s other physicians, including allergies to medication, test results, and the results of particular therapies.
Without a shared electronic record, a physician relies on the recollection of each patient, which is often unintentionally incomplete. A patient may be uncertain about the name or dosage of a medication, fail to remember the date of a screening examination, or lack results of lab tests ordered by another physician.
A physician’s access to the full story with shareable electronic records is important to the care of all patients and can be particularly relevant for patients who have inconsistent contact with health care providers. Often, these patients get care in various settings, including physician offices, community clinics, and emergency departments. Because these patients tend to have a higher incidence of chronic disease, they may greatly benefit from the sharing of medical information.
Clearly, Congress wants to move us to full adoption of HIT. Beginning in 2013, all plans must comply with a uniform standard for electronic transactions, including eligibility verification and health claims status.
In 2014, uniform standards must allow automatic reconciliation of electronic funds transfers and HIPAA payment and remittance; use standardized and consistent methods of health plan enrollment and claim edits; use unique health plan identifiers to simplify and improve routing of health care transactions; and use standard electronic claims attachments.
Uniformity and standardization can help address one of the major roadblocks to physician adoption of health information technology.
With passage of the Patient Protection and Affordable Care Act earlier this year, big changes are afoot in the way Americans practice medicine. In a plethora of articles, blogs, and broadcast spots, the media have focused on what the new law portends for the average employee, employers, and the uninsured—but what, exactly, does it entail for ObGyns and their patients?
To find an answer to that overarching question—and 13 others—we invited Lucia DiVenere, director of government relations at the American Congress of Obstetricians and Gynecologists, to join us in an extended discussion of the law and its ramifications. She offered insight into ACOG’s extensive lobbying efforts on behalf of women and the specialty and described the many ways ObGyn care will change in the near and proximal future, focusing on questions that you might find yourself asking, including:
- Will I see a lot more patients?
- What reforms are woman-specific?
- How will my practice change?
- Which of my services will be fully covered?
- Will expanded coverage improve birth outcomes?
- Is “femaleness” a preexisting condition?
- What happened to tort reform?
- Is the system repairable?
1 Will ObGyns see a lot more patients?
OBG Management: The most talked about change the new law heralds is the addition of roughly 32 million people to the insurance rolls. Is the most significant impact of the legislation for ObGyns likely to be an increase in the number of patients they will be seeing?
Lucia DiVenere: Congress wanted to increase the ranks of the insured and expand access to health care, and it addressed these goals with individual and employer mandates, state exchanges, Medicaid expansion, and insurance reforms.
But that isn’t the most significant change in store for us. Congress also wanted to reform our health care system in a number of fundamental ways, some of which are designed to change the way physicians provide care to their patients.
For example, Congress wanted to “bend the cost curve”—to reduce the expected rate of growth in health care spending over the long term. That doesn’t mean that health care costs in 2020 will be less than they were in 2018, but it does mean that annual and long-term growth rates should level off and become sustainable. To accomplish this goal, Congress created an Independent Payment Advisory Commission, which may prove to be extremely powerful in reducing health care costs and is likely to significantly affect all physicians. Greater protections against fraud and abuse, experiments with new kinds of payment and delivery systems, including “medical homes,” and increased reliance on nonphysician practitioners—all included in the law—are also expected to reduce costs.
OBG Management: What other changes are coming?
DiVenere: Congress was determined to alter the practice of health care, ensuring higher quality for each dollar spent and consistent delivery of care. It also sought to kick-start our health care system—especially in the physician arena—into greater and, theoretically, more efficient reliance on electronic health records (EHR). Medicare and Medicaid physician payments will be juggled to increase reimbursement for E&M services and for physicians who provide greater value in relation to cost. Physicians will be required to participate in the Physician Quality Reporting Initiative (PQRI) program in 2015 and beyond to avoid stiff penalties. And EHR systems are required to adopt uniform standards for electronic transactions.
2 What reforms are woman-specific?
OBG Management: What initiatives are planned for the care of women, in particular?
DiVenere: Congress recognized the importance of reforming women’s health and included many provisions advocated by ACOG in our “Health care for women, health care for all” campaign.
Probably the most important of these provisions is the guarantee of direct access to ObGyn care without need of a referral or pre-authorization from a primary care provider or insurance company. Nor can an insurance company restrict a patient’s direct access to her ObGyn to a certain number of visits or types of services.
This was a major ACOG victory. For 20 years, ObGyns have been waging battles in the states for direct access for patients. Last year, nine states did not require insurers to grant women direct access to ObGyns, and 16 states allowed insurers to restrict ObGyn visits and services. This part of the law, which is effective this year, provides national direct access to all women in all states, and is not tied to an ObGyn’s primary care designation.
Another area of reform concerns maternity care. In 2009, 13% of all pregnant women in the United States were uninsured, as were 20.4% of all women between the ages of 15 and 44, the childbearing years. The uninsured rate for nonelderly women in 2007 ranged from a high of 28% in New Mexico and Texas to a low of 8% in Massachusetts. Today, 42% of all pregnancies are covered by Medicaid. Women have been able, usually, to gain access to some kind of care—sometimes in emergency departments at the time of labor—but the nation clearly needs to do better.
Medicaid and new insurance plans will be required to offer maternity care and women’s preventive services, including mammography screening. The exact parameters of maternity care and other types of care in the essential benefits package will be determined by the Secretary of Health and Human Services (HHS), based on the typical package offered to employees in group health plans. The idea behind the law is that many women who are now covered by Medicaid will transfer to private insurance in their states’ exchanges.
3 How will ObGyn practice change?
OBG Management: What are some of the opportunities and challenges ObGyns will encounter?
DiVenere: There are three key areas of challenge and opportunity:
- Development of the “medical home.” A medical home is a practice designed to provide and coordinate comprehensive patient care. State Medicaid agencies are authorized to require certain beneficiaries, including those who have two or more chronic conditions, to join a medical home. Medicare will also experiment with medical homes, and both Medicaid and Medicare medical home practices will receive additional payments. Most medical homes are expected to be family practice, internal medicine, or pediatric care providers, but ObGyn practices can participate, too. ObGyns should look carefully at the opportunities this paradigm provides and consider having their practice designated as a medical home.
- Increased use of nonphysician providers. The new law strongly encourages this practice, including in the ObGyn specialty. Congress is determined to experiment with non-ObGyn deliveries in response to patient demand and midlevel assurances that nonphysicians can deliver babies with better outcomes at significantly lower cost. Our specialty’s cesarean delivery rate is under intense scrutiny. Skewed studies “prove” happier and healthier deliveries in homes and other out-of-hospital locations without an ObGyn in attendance. And midlevel practitioners are offering vaginal birth after cesarean delivery in many cases where ObGyns are restricted by hospital rules.
- The law extends Medicaid payments to free-standing birth centers and birth attendants and does not specify which kinds of practitioners can qualify as birth attendants. Free-standing birth centers can provide high-quality care if they are appropriately accredited and have an established transfer relationship with a nearby hospital. The law does not specify these criteria, either.
- Increasing payments to nonphysicians. Medicare payments to certified nurse midwives (CNMs) will reach the rate paid to physicians for the same services in January 2011, up from 65% of the physician rate. Medicare will also pay CNMs a 10% bonus if primary care services account for at least 60% of their allowed charges. And the law requires health plans in the state exchanges to pay for covered health services provided by any practitioner recognized under state law, whether or not the plan contracts with that individual or type of provider. Certified professional midwives (lay midwives) are licensed in 21 states, and this provision may give them significant new entry.
ObGyns stop delivering babies at increasingly early points in their career, and only 13% of family physicians deliver babies today. So we need to find ways to extend our care—and increasing collaborative practice between ObGyns and CNMs and certified midwives may help close this gap. The increased focus on midlevel providers in the law may present us with both a challenge and an important opportunity.
4 What services will be fully covered now?
OBG Management: Beginning this year, health plans will be required to provide a minimum level of coverage without cost-sharing for preventive care and screenings for women, among others. What will this requirement encompass?
DiVenere: Congress emphasized prevention in the reform law as part of its strategy to bend the cost curve, investing in prevention in order to reduce higher spending on illness.
Beginning in September 2010, all plans—including those that existed before this law was passed—must cover preventive health services without any patient cost sharing, whether copayments or deductibles. These services include women’s preventive care and screening included in comprehensive guidelines supported by the Health Resources and Services Administration (HRSA), even if they are more extensive than services recommended by the Centers for Disease Control and Prevention (CDC) and US Preventive Services Task Force (USPSTF). Breast cancer screening, mammography, and prevention services are covered as though the November 2009 USPSTF recommendations suggesting limits on mammography screening for certain age groups did not exist.
The mammography screening coverage was a big win for ACOG. We worked closely with Senator Barbara Mikulski (D-Md.) on this amendment, and it was the first Democratic amendment offered. It passed on the Senate floor during a contentious floor fight.
ACOG continues to recommend screening mammography every 1 to 2 years for women 40 to 49 years old; annual screening for women 50 and older; clinical breast examination every year for women 19 years and older; and regular breast self-examination.
The Senate bill that was brought to the floor would have limited women’s preventive care to USPSTF recommendations only. Working with Senator Mikulski, we made sure that women younger than 50 will be covered for mammography every 1 to 2 years.
OBG Management: Are there other important benefits for women included in the law?
DiVenere: Yes. One provision will improve research, screening, and treatment for postpartum depression, a signature issue of ACOG President Gerald F. Joseph Jr., MD, during his presidential year. ACOG and Dr. Joseph worked closely with Senator Bob Menendez (D-NJ) to introduce the Moms Act and win its inclusion in the health reform law.
Under this section, HHS will:
- conduct research into the causes of, and treatments for, postpartum conditions
- create a national public awareness campaign to increase knowledge of postpartum depression and postpartum psychosis
- provide grants to study the benefits of screening for postpartum depression and postpartum psychosis
- establish grants to deliver or enhance outpatient, inpatient, and home-based health and support services, including case management and comprehensive treatment services for women with or at risk of postpartum conditions.
5 Will expanded coverage improve birth outcomes?
OBG Management: Do you expect that guaranteed coverage of pregnancy will increase the number of women who seek prenatal care—as opposed to waiting until labor begins—to see a doctor? Will guaranteed coverage of pregnancy improve birth outcomes over the long term?
DiVenere: Those are certainly the goals. And guaranteed coverage of pregnancy was one of ACOG’s essential elements in health care reform. Prenatal care has been shown to save $3 for every $1 spent in the Medicaid program and continues to be the primary way to identify problems during pregnancy, giving ObGyns the opportunity to assess and manage the risk of preterm labor and other threats to the health of the mother and baby.
The health reform law recognizes that better prenatal care can lead to healthier babies—both in its coverage of maternity and preventive care, and by new Medicaid coverage of smoking-cessation counseling and family planning, both beginning this year.
Medicaid will now cover the costs of diagnostic, therapeutic, and counseling services, as well as pharmacotherapy for pregnant women covered by Medicaid, at no cost to the patient. Before health reform passed, only 24 states reimbursed ObGyns and other physicians for smoking-cessation counseling for pregnant women. Five states didn’t cover any smoking-cessation services at all.
Also beginning this year, states can provide family planning services to nonpregnant women up to the same eligibility levels to which they cover pregnant women, without the need to apply for federal waivers or permission. Forty-five states extend Medicaid coverage to pregnant women who have incomes above the regular Medicaid eligibility levels, from a low of 150% to a high of 300% of the federal poverty levels.
Before this new law, 27 states had federal waivers to provide family planning to women who had an income above the Medicaid eligibility levels, most of them at or near 200% of the federal poverty level. Eleven of these waivers expire this year.
6 Is femaleness a “preexisting condition”?
OBG Management: During the debate on health reform, many people claimed, somewhat facetiously, that female sex has been a preexisting condition. The new law will ensure that patients can’t be dropped by their insurance company—or denied coverage—for arbitrary or unfair reasons, such as preexisting conditions. How are these changes likely to affect women and their ObGyns?
DiVenere: The insurance reforms in the new law are very important to women and to ObGyn practices. In fact, the prohibition on preexisting conditions was a top priority of ACOG’s “Health care for women, health care for all” campaign, and Congress included this provision with women’s health in mind.
Many members of Congress were shocked to learn that it was not unusual for insurers to deny coverage to women who were pregnant, who had had a previous cesarean delivery, or who had been the victim of domestic violence at some point in their history. In fact, almost any medical history, genetic information, disability, or current health condition was grounds for denial of coverage.
Women were also often charged higher premiums than men for the same coverage. And insurance companies would sometimes require waiting periods for coverage—sometimes as long as 9 months.
All of these practices are outlawed by the health reform law, which prohibits plans from using preexisting condition exclusions to deny children coverage as of September 1, 2010 and adults as of January 1, 2014. Beginning on January 1, 2014, women cannot be denied coverage due to pregnancy, previous cesarean delivery, or domestic violence, or medical history, among many other reasons.
Effective March 23, 2010 and ending January 1, 2014, a high-risk pool insurance program has been created for people who have been uninsured for 6 months and who have a preexisting condition. Funding for the temporary risk pool is capped at $5 billion.
Insurers in the small and individual markets and in the exchanges cannot discriminate on the basis of medical history or other variables; may only charge limited premium differentials for age, family size, and smoking, but not for gender; and cannot mandate a waiting period longer than 90 days.
Insurance plans that were in existence before enactment must comply with reforms on waiting periods; lifetime limits; rescission; extension of dependent coverage; uniform explanation of coverage; and loss ratio reporting and premium rebates. Group grandfather plans must also comply with restrictions on annual limits and preexisting conditions.
All these protections should benefit ObGyn practices by ensuring coverage and continuity of care for their patients.
7 What happened to tort reform?
OBG Management: No tort reform was included in the law. Why not?
DiVenere: The law authorizes HHS to award $50 million over 5 years, up to $500,000 per state, to develop, implement, and evaluate alternative medical liability reform initiatives that meet several specific criteria. Medical liability protections under the Federal Tort Claims Act are extended to officers, governing board members, employees, and contractors of free clinics.
We at ACOG were very disappointed that Congress didn’t take a serious step toward medical liability reform in this bill. Liability reform was one of ACOG’s five essential elements of health reform, and its absence from the final bill was a prominent reason why we ultimately “reluctantly opposed” passage. We, and the rest of the House of Medicine, were clear that health reform wouldn’t work without meaningful medical liability reform.
ACOG supports caps on noneconomic damages and other reforms in California and Texas law. We also support testing alternatives, including health courts, alternative dispute resolution, “Sorry Works!” programs, and birth injury compensation funds. But this part of the health reform law requires that tests be linked to patient safety, an association that is impossible to establish in cases of neonatal encephalopathy. The law also requires that patients be allowed to opt out of a system if they choose to go to court. Both of these requirements hamper the development of meaningful alternatives for the ObGyn specialty.
8 Is the system repairable?
OBG Management: Can the US health care system be fixed in one fell swoop?
DiVenere: ACOG pursued two integral missions in reform efforts: improving women’s health and advocating for practicing ObGyns. Our mission in women’s health included guaranteed maternity care, important insurance reforms, and direct access to ObGyns. Our mission in regard to practicing ObGyns included the protection of ultrasonography, the reform of medical liability laws, and repeal of the Medicare sustainable growth rate, along with an array of other issues, all of which were shared by the entire House of Medicine.
We see these missions as integral; Congress saw them as separable. We were largely successful on the women’s health side of the ledger. But Congress responded to the House of Medicine issues with little interest.
We believe that we can fulfill our mission to women’s health only if the issues of practicing ObGyns are addressed in the process. You can’t build a new health care system on a broken medical liability system or a broken Medicare physician payment system, and we still have both. We have a lot more work to do on these issues and the myriad of other issues that need to be addressed. This is really just the beginning of health reform.
9 Has PQRI regained the limelight?
OBG Management: The Medicare quality reporting incentive payments under the Physician Quality Reporting Initiative (PQRI) have been extended. In fact, physicians will be penalized, beginning in 2015, if they do not participate. Are the incentive payments a good thing for ObGyns?
DiVenere: Yes, a big change is coming in this program. ObGyns who participate in PQRI will be eligible to receive bonus payments of 1% in 2011 and 0.5% from 2012 to 2014. Payments will be reduced by 1.5% in 2015 and by 2.0% in 2016 for physicians who don’t participate in the PQRI program.
Beginning in 2012, PQRI participation becomes a meaningful use qualifier for EHR grants.
In 2011 to 2014, physicians who complete Maintenance of Certification (MOC) are eligible for an additional 1% bonus in 2011 and 0.5% bonus in 2012 to 2014. Data on a physician’s quality measures must be submitted on the physician’s behalf by the MOC program. After 2014, the Secretary of HHS can add MOC completion to the quality measures used for the value-based payment modifier. The American Board of Obstetrics and Gynecology hasn’t yet qualified its MOC for this part of the program.
ObGyn participation in the PQRI program is very limited (less than 10%). While only about 25 of the 215 PQRI quality measures apply to ObGyn care, most are easily applicable, and a physician needs to report on only three to five measures to qualify for the program.
The very low participation rate is likely because many ObGyn practices just didn’t think the incentive payment was worth the trouble. They may need to rethink that math once they’re faced with payment cuts in 2015.
ObGyns should also be aware that the Secretary of HHS, with input from stakeholders, will set up a Physician Compare Web site (modeled after the program that already exists for hospitals) using PQRI data. Data will be made public on January 1, 2013, comparing physicians in terms of quality of care and patient experience.
The Secretary must ensure that the data are statistically valid and risk-adjusted. In addition, the physician must be given time to review the information before it becomes public, and data must ensure appropriate attribution of care when multiple physicians and other providers are involved. The Secretary must also give physicians timely performance feedback.
For all these reasons, ACOG is working with the physician community to make a number of improvements to the PQRI program, doing our best to make it as easy as possible for our members to participate and benefit.
10 What effect will the expansion of Medicaid have on ObGyn practice?
DiVenere: Starting in 2014, the same year that state exchanges are expected to be established, Medicaid eligibility will be broadened to cover all individuals younger than 65 years who have incomes up to 133% of the federal poverty level. All newly eligible adults will be guaranteed a benchmark benefit package that provides the essential health benefits.
States that have already expanded eligibility to adults who have incomes up to 100% of the federal poverty level will receive a phased-in increase in the federal medical assistance percentage so that, by 2019, they will receive the same federal financing as other states (93% in 2019 and 90% in 2020 and later). And states have the option to expand Medicaid eligibility to childless adults as of April 1, 2010, but will receive their regular federal medical assistance percentage until 2014.
Although these changes will broaden the range and increase the number of individuals who will be eligible for Medicaid, the effect on ObGyn practice remains to be seen, especially as pregnant women who were covered by Medicaid at income levels above 133% of the federal poverty level transition off of Medicaid and into private health insurance offered in the exchanges.
Today, about 38% of all ObGyns accept Medicaid gynecologic patients, and 44% accept Medicaid obstetric patients. Medicaid accounts for 18% of revenues of the average ObGyn practice.
11 Will the extension of benefits to young adults have a measurable impact on ObGyn practice?
DiVenere: Congress included two provisions to target “young immortals,” young adults who don’t think they need health insurance because they’re young and healthy and never need to see a doctor. Many young adults are not offered employer-based health insurance, and many see no advantage in buying coverage that they don’t expect to use. But we all know that someone pays when any uninsured person falls sick or has an accident that necessitates medical care.
Beginning this year, adult children as old as 26 years can go onto their parents’ health insurance plan. In addition, catastrophic plans will soon be available to individuals younger than 30 who want to purchase a higher deductible plan through their state exchange or on the individual and small group markets. These catastrophic plans are not required to include the essential benefits package, including maternity care. Nevertheless, both of these provisions should be helpful to ObGyn practices.
12 Will the mandate for employers to provide health insurance affect many ObGyns?
DiVenere: The employer mandate takes effect in 2014, when employers with more than 50 employees, at least one of whom receives a premium tax credit, are required to offer health insurance coverage to employees or be assessed a range of fees. Employers that have 50 or fewer employees are exempt from this requirement.
In 2007, 75% of ObGyn practices had fewer than 42 full-time employees, with an average number of full-time employees, including physicians, of 34.4. So this mandate should not apply to the average ObGyn practice.
A range of small business tax credits for employers that contribute at least 50% of the cost of coverage for their employees will also be available, with credits phasing out as the size of the firm and the average employee wage increase.
13 Who will benefit from the Medicare geographic payment adjustments?
DiVenere: The increased Medicare geographic practice cost index (GPCI) payments and new Frontier payments won’t affect many ObGyns nationally, but they are likely to affect most ObGyns in the related rural locations.
The law reestablishes the national average floor on Medicare’s GPCI for physician work. In 2010 and 2011, Medicare makes a separate adjustment for the practice expense portion of physician payments that will benefit physicians in rural and low-cost areas.
Beginning in 2011, a third adjustment will increase the practice expense GPCI for physicians in frontier states. A frontier state is one in which at least half of its counties have populations smaller than six people per square mile. Frontier states are expected to be Montana, North Dakota, South Dakota, Utah, and Wyoming.
Physicians in 51 localities in 42 states, Puerto Rico, and the Virgin Islands will benefit from the two practice expense adjustments.
ObGyns should also know about two other payment changes:
- The HHS Secretary will create and apply to Medicare provider payments a value-based modifier that will result in higher Medicare payments for high-quality, low-cost physicians and lower payments for high-cost, low-quality physicians. The modifier is to be based on a composite quality score and a composite cost score determined by measures selected by the HHS Secretary and endorsed by a consensus organization. This change begins with 2015 Medicare payments and applies only to physicians in 2015. In 2017, it will also apply to other health professionals.
- Effective immediately, the HHS Secretary has the authority to increase or decrease Medicare relative values, and payments for services, with special attention focused on:
- – services that have high growth rates
- – services that have seen substantial changes in the practice expense or work components
- – services for which new technology has reduced costs
- – instances in which multiple codes are frequently billed for a single service
- – codes that have not been reviewed since implementation of the resource-based relative value scale (RBRVS).
ObGyns who participate in Medicare will start receiving individual physician resource use reports in 2012. These reports will compare per capita utilization of physicians (or physician groups) with the utilization rate of physicians who see similar patients. Reports are required to be risk-adjusted and standardized to take into account local health-care costs.
14 Do you expect the law’s requirements for “administrative simplification” to reduce overhead and increase efficiency?
DiVenere: Don’t we all hope so.
The bill contains several requirements such as:
- establishment of a standardized claim form
- streamlining of claims processing
- improvement of interoperability to allow for more electronic information sharing. These changes will not be implemented until 2013 at the earliest.
Today, about 34% of all ObGyn practices use electronic health records. The systemwide benefits of health information technology (HIT) can be many. Insurers can save by reducing unnecessary tests. Patients can benefit from better coordination of care and fewer medical errors. But these advantages don’t necessarily translate into savings or revenue for physician practices.
Instead, physicians face Medicare and private insurance payment cuts. Little assistance is available for the investment in HIT. And uncertain interoperability standards and rapid technological changes can very quickly make this year’s investment obsolete. Many physicians in solo and small practices are understandably reluctant to take the HIT plunge.
The initial cost of purchasing HIT for a small practice is typically at least $50,000 per physician. Physicians face additional, ongoing costs in staff training and hardware and software updates as well. And many physicians see significant efficiency losses for months and sometimes years after upgrading to an electronic health record system.
Still, with interoperable, shareable electronic records, all physicians treating a particular patient can have the full story. A patient’s paper record kept in her physician’s office shows only a slice of her medical history, potentially missing important information from the patient’s other physicians, including allergies to medication, test results, and the results of particular therapies.
Without a shared electronic record, a physician relies on the recollection of each patient, which is often unintentionally incomplete. A patient may be uncertain about the name or dosage of a medication, fail to remember the date of a screening examination, or lack results of lab tests ordered by another physician.
A physician’s access to the full story with shareable electronic records is important to the care of all patients and can be particularly relevant for patients who have inconsistent contact with health care providers. Often, these patients get care in various settings, including physician offices, community clinics, and emergency departments. Because these patients tend to have a higher incidence of chronic disease, they may greatly benefit from the sharing of medical information.
Clearly, Congress wants to move us to full adoption of HIT. Beginning in 2013, all plans must comply with a uniform standard for electronic transactions, including eligibility verification and health claims status.
In 2014, uniform standards must allow automatic reconciliation of electronic funds transfers and HIPAA payment and remittance; use standardized and consistent methods of health plan enrollment and claim edits; use unique health plan identifiers to simplify and improve routing of health care transactions; and use standard electronic claims attachments.
Uniformity and standardization can help address one of the major roadblocks to physician adoption of health information technology.
With passage of the Patient Protection and Affordable Care Act earlier this year, big changes are afoot in the way Americans practice medicine. In a plethora of articles, blogs, and broadcast spots, the media have focused on what the new law portends for the average employee, employers, and the uninsured—but what, exactly, does it entail for ObGyns and their patients?
To find an answer to that overarching question—and 13 others—we invited Lucia DiVenere, director of government relations at the American Congress of Obstetricians and Gynecologists, to join us in an extended discussion of the law and its ramifications. She offered insight into ACOG’s extensive lobbying efforts on behalf of women and the specialty and described the many ways ObGyn care will change in the near and proximal future, focusing on questions that you might find yourself asking, including:
- Will I see a lot more patients?
- What reforms are woman-specific?
- How will my practice change?
- Which of my services will be fully covered?
- Will expanded coverage improve birth outcomes?
- Is “femaleness” a preexisting condition?
- What happened to tort reform?
- Is the system repairable?
1 Will ObGyns see a lot more patients?
OBG Management: The most talked about change the new law heralds is the addition of roughly 32 million people to the insurance rolls. Is the most significant impact of the legislation for ObGyns likely to be an increase in the number of patients they will be seeing?
Lucia DiVenere: Congress wanted to increase the ranks of the insured and expand access to health care, and it addressed these goals with individual and employer mandates, state exchanges, Medicaid expansion, and insurance reforms.
But that isn’t the most significant change in store for us. Congress also wanted to reform our health care system in a number of fundamental ways, some of which are designed to change the way physicians provide care to their patients.
For example, Congress wanted to “bend the cost curve”—to reduce the expected rate of growth in health care spending over the long term. That doesn’t mean that health care costs in 2020 will be less than they were in 2018, but it does mean that annual and long-term growth rates should level off and become sustainable. To accomplish this goal, Congress created an Independent Payment Advisory Commission, which may prove to be extremely powerful in reducing health care costs and is likely to significantly affect all physicians. Greater protections against fraud and abuse, experiments with new kinds of payment and delivery systems, including “medical homes,” and increased reliance on nonphysician practitioners—all included in the law—are also expected to reduce costs.
OBG Management: What other changes are coming?
DiVenere: Congress was determined to alter the practice of health care, ensuring higher quality for each dollar spent and consistent delivery of care. It also sought to kick-start our health care system—especially in the physician arena—into greater and, theoretically, more efficient reliance on electronic health records (EHR). Medicare and Medicaid physician payments will be juggled to increase reimbursement for E&M services and for physicians who provide greater value in relation to cost. Physicians will be required to participate in the Physician Quality Reporting Initiative (PQRI) program in 2015 and beyond to avoid stiff penalties. And EHR systems are required to adopt uniform standards for electronic transactions.
2 What reforms are woman-specific?
OBG Management: What initiatives are planned for the care of women, in particular?
DiVenere: Congress recognized the importance of reforming women’s health and included many provisions advocated by ACOG in our “Health care for women, health care for all” campaign.
Probably the most important of these provisions is the guarantee of direct access to ObGyn care without need of a referral or pre-authorization from a primary care provider or insurance company. Nor can an insurance company restrict a patient’s direct access to her ObGyn to a certain number of visits or types of services.
This was a major ACOG victory. For 20 years, ObGyns have been waging battles in the states for direct access for patients. Last year, nine states did not require insurers to grant women direct access to ObGyns, and 16 states allowed insurers to restrict ObGyn visits and services. This part of the law, which is effective this year, provides national direct access to all women in all states, and is not tied to an ObGyn’s primary care designation.
Another area of reform concerns maternity care. In 2009, 13% of all pregnant women in the United States were uninsured, as were 20.4% of all women between the ages of 15 and 44, the childbearing years. The uninsured rate for nonelderly women in 2007 ranged from a high of 28% in New Mexico and Texas to a low of 8% in Massachusetts. Today, 42% of all pregnancies are covered by Medicaid. Women have been able, usually, to gain access to some kind of care—sometimes in emergency departments at the time of labor—but the nation clearly needs to do better.
Medicaid and new insurance plans will be required to offer maternity care and women’s preventive services, including mammography screening. The exact parameters of maternity care and other types of care in the essential benefits package will be determined by the Secretary of Health and Human Services (HHS), based on the typical package offered to employees in group health plans. The idea behind the law is that many women who are now covered by Medicaid will transfer to private insurance in their states’ exchanges.
3 How will ObGyn practice change?
OBG Management: What are some of the opportunities and challenges ObGyns will encounter?
DiVenere: There are three key areas of challenge and opportunity:
- Development of the “medical home.” A medical home is a practice designed to provide and coordinate comprehensive patient care. State Medicaid agencies are authorized to require certain beneficiaries, including those who have two or more chronic conditions, to join a medical home. Medicare will also experiment with medical homes, and both Medicaid and Medicare medical home practices will receive additional payments. Most medical homes are expected to be family practice, internal medicine, or pediatric care providers, but ObGyn practices can participate, too. ObGyns should look carefully at the opportunities this paradigm provides and consider having their practice designated as a medical home.
- Increased use of nonphysician providers. The new law strongly encourages this practice, including in the ObGyn specialty. Congress is determined to experiment with non-ObGyn deliveries in response to patient demand and midlevel assurances that nonphysicians can deliver babies with better outcomes at significantly lower cost. Our specialty’s cesarean delivery rate is under intense scrutiny. Skewed studies “prove” happier and healthier deliveries in homes and other out-of-hospital locations without an ObGyn in attendance. And midlevel practitioners are offering vaginal birth after cesarean delivery in many cases where ObGyns are restricted by hospital rules.
- The law extends Medicaid payments to free-standing birth centers and birth attendants and does not specify which kinds of practitioners can qualify as birth attendants. Free-standing birth centers can provide high-quality care if they are appropriately accredited and have an established transfer relationship with a nearby hospital. The law does not specify these criteria, either.
- Increasing payments to nonphysicians. Medicare payments to certified nurse midwives (CNMs) will reach the rate paid to physicians for the same services in January 2011, up from 65% of the physician rate. Medicare will also pay CNMs a 10% bonus if primary care services account for at least 60% of their allowed charges. And the law requires health plans in the state exchanges to pay for covered health services provided by any practitioner recognized under state law, whether or not the plan contracts with that individual or type of provider. Certified professional midwives (lay midwives) are licensed in 21 states, and this provision may give them significant new entry.
ObGyns stop delivering babies at increasingly early points in their career, and only 13% of family physicians deliver babies today. So we need to find ways to extend our care—and increasing collaborative practice between ObGyns and CNMs and certified midwives may help close this gap. The increased focus on midlevel providers in the law may present us with both a challenge and an important opportunity.
4 What services will be fully covered now?
OBG Management: Beginning this year, health plans will be required to provide a minimum level of coverage without cost-sharing for preventive care and screenings for women, among others. What will this requirement encompass?
DiVenere: Congress emphasized prevention in the reform law as part of its strategy to bend the cost curve, investing in prevention in order to reduce higher spending on illness.
Beginning in September 2010, all plans—including those that existed before this law was passed—must cover preventive health services without any patient cost sharing, whether copayments or deductibles. These services include women’s preventive care and screening included in comprehensive guidelines supported by the Health Resources and Services Administration (HRSA), even if they are more extensive than services recommended by the Centers for Disease Control and Prevention (CDC) and US Preventive Services Task Force (USPSTF). Breast cancer screening, mammography, and prevention services are covered as though the November 2009 USPSTF recommendations suggesting limits on mammography screening for certain age groups did not exist.
The mammography screening coverage was a big win for ACOG. We worked closely with Senator Barbara Mikulski (D-Md.) on this amendment, and it was the first Democratic amendment offered. It passed on the Senate floor during a contentious floor fight.
ACOG continues to recommend screening mammography every 1 to 2 years for women 40 to 49 years old; annual screening for women 50 and older; clinical breast examination every year for women 19 years and older; and regular breast self-examination.
The Senate bill that was brought to the floor would have limited women’s preventive care to USPSTF recommendations only. Working with Senator Mikulski, we made sure that women younger than 50 will be covered for mammography every 1 to 2 years.
OBG Management: Are there other important benefits for women included in the law?
DiVenere: Yes. One provision will improve research, screening, and treatment for postpartum depression, a signature issue of ACOG President Gerald F. Joseph Jr., MD, during his presidential year. ACOG and Dr. Joseph worked closely with Senator Bob Menendez (D-NJ) to introduce the Moms Act and win its inclusion in the health reform law.
Under this section, HHS will:
- conduct research into the causes of, and treatments for, postpartum conditions
- create a national public awareness campaign to increase knowledge of postpartum depression and postpartum psychosis
- provide grants to study the benefits of screening for postpartum depression and postpartum psychosis
- establish grants to deliver or enhance outpatient, inpatient, and home-based health and support services, including case management and comprehensive treatment services for women with or at risk of postpartum conditions.
5 Will expanded coverage improve birth outcomes?
OBG Management: Do you expect that guaranteed coverage of pregnancy will increase the number of women who seek prenatal care—as opposed to waiting until labor begins—to see a doctor? Will guaranteed coverage of pregnancy improve birth outcomes over the long term?
DiVenere: Those are certainly the goals. And guaranteed coverage of pregnancy was one of ACOG’s essential elements in health care reform. Prenatal care has been shown to save $3 for every $1 spent in the Medicaid program and continues to be the primary way to identify problems during pregnancy, giving ObGyns the opportunity to assess and manage the risk of preterm labor and other threats to the health of the mother and baby.
The health reform law recognizes that better prenatal care can lead to healthier babies—both in its coverage of maternity and preventive care, and by new Medicaid coverage of smoking-cessation counseling and family planning, both beginning this year.
Medicaid will now cover the costs of diagnostic, therapeutic, and counseling services, as well as pharmacotherapy for pregnant women covered by Medicaid, at no cost to the patient. Before health reform passed, only 24 states reimbursed ObGyns and other physicians for smoking-cessation counseling for pregnant women. Five states didn’t cover any smoking-cessation services at all.
Also beginning this year, states can provide family planning services to nonpregnant women up to the same eligibility levels to which they cover pregnant women, without the need to apply for federal waivers or permission. Forty-five states extend Medicaid coverage to pregnant women who have incomes above the regular Medicaid eligibility levels, from a low of 150% to a high of 300% of the federal poverty levels.
Before this new law, 27 states had federal waivers to provide family planning to women who had an income above the Medicaid eligibility levels, most of them at or near 200% of the federal poverty level. Eleven of these waivers expire this year.
6 Is femaleness a “preexisting condition”?
OBG Management: During the debate on health reform, many people claimed, somewhat facetiously, that female sex has been a preexisting condition. The new law will ensure that patients can’t be dropped by their insurance company—or denied coverage—for arbitrary or unfair reasons, such as preexisting conditions. How are these changes likely to affect women and their ObGyns?
DiVenere: The insurance reforms in the new law are very important to women and to ObGyn practices. In fact, the prohibition on preexisting conditions was a top priority of ACOG’s “Health care for women, health care for all” campaign, and Congress included this provision with women’s health in mind.
Many members of Congress were shocked to learn that it was not unusual for insurers to deny coverage to women who were pregnant, who had had a previous cesarean delivery, or who had been the victim of domestic violence at some point in their history. In fact, almost any medical history, genetic information, disability, or current health condition was grounds for denial of coverage.
Women were also often charged higher premiums than men for the same coverage. And insurance companies would sometimes require waiting periods for coverage—sometimes as long as 9 months.
All of these practices are outlawed by the health reform law, which prohibits plans from using preexisting condition exclusions to deny children coverage as of September 1, 2010 and adults as of January 1, 2014. Beginning on January 1, 2014, women cannot be denied coverage due to pregnancy, previous cesarean delivery, or domestic violence, or medical history, among many other reasons.
Effective March 23, 2010 and ending January 1, 2014, a high-risk pool insurance program has been created for people who have been uninsured for 6 months and who have a preexisting condition. Funding for the temporary risk pool is capped at $5 billion.
Insurers in the small and individual markets and in the exchanges cannot discriminate on the basis of medical history or other variables; may only charge limited premium differentials for age, family size, and smoking, but not for gender; and cannot mandate a waiting period longer than 90 days.
Insurance plans that were in existence before enactment must comply with reforms on waiting periods; lifetime limits; rescission; extension of dependent coverage; uniform explanation of coverage; and loss ratio reporting and premium rebates. Group grandfather plans must also comply with restrictions on annual limits and preexisting conditions.
All these protections should benefit ObGyn practices by ensuring coverage and continuity of care for their patients.
7 What happened to tort reform?
OBG Management: No tort reform was included in the law. Why not?
DiVenere: The law authorizes HHS to award $50 million over 5 years, up to $500,000 per state, to develop, implement, and evaluate alternative medical liability reform initiatives that meet several specific criteria. Medical liability protections under the Federal Tort Claims Act are extended to officers, governing board members, employees, and contractors of free clinics.
We at ACOG were very disappointed that Congress didn’t take a serious step toward medical liability reform in this bill. Liability reform was one of ACOG’s five essential elements of health reform, and its absence from the final bill was a prominent reason why we ultimately “reluctantly opposed” passage. We, and the rest of the House of Medicine, were clear that health reform wouldn’t work without meaningful medical liability reform.
ACOG supports caps on noneconomic damages and other reforms in California and Texas law. We also support testing alternatives, including health courts, alternative dispute resolution, “Sorry Works!” programs, and birth injury compensation funds. But this part of the health reform law requires that tests be linked to patient safety, an association that is impossible to establish in cases of neonatal encephalopathy. The law also requires that patients be allowed to opt out of a system if they choose to go to court. Both of these requirements hamper the development of meaningful alternatives for the ObGyn specialty.
8 Is the system repairable?
OBG Management: Can the US health care system be fixed in one fell swoop?
DiVenere: ACOG pursued two integral missions in reform efforts: improving women’s health and advocating for practicing ObGyns. Our mission in women’s health included guaranteed maternity care, important insurance reforms, and direct access to ObGyns. Our mission in regard to practicing ObGyns included the protection of ultrasonography, the reform of medical liability laws, and repeal of the Medicare sustainable growth rate, along with an array of other issues, all of which were shared by the entire House of Medicine.
We see these missions as integral; Congress saw them as separable. We were largely successful on the women’s health side of the ledger. But Congress responded to the House of Medicine issues with little interest.
We believe that we can fulfill our mission to women’s health only if the issues of practicing ObGyns are addressed in the process. You can’t build a new health care system on a broken medical liability system or a broken Medicare physician payment system, and we still have both. We have a lot more work to do on these issues and the myriad of other issues that need to be addressed. This is really just the beginning of health reform.
9 Has PQRI regained the limelight?
OBG Management: The Medicare quality reporting incentive payments under the Physician Quality Reporting Initiative (PQRI) have been extended. In fact, physicians will be penalized, beginning in 2015, if they do not participate. Are the incentive payments a good thing for ObGyns?
DiVenere: Yes, a big change is coming in this program. ObGyns who participate in PQRI will be eligible to receive bonus payments of 1% in 2011 and 0.5% from 2012 to 2014. Payments will be reduced by 1.5% in 2015 and by 2.0% in 2016 for physicians who don’t participate in the PQRI program.
Beginning in 2012, PQRI participation becomes a meaningful use qualifier for EHR grants.
In 2011 to 2014, physicians who complete Maintenance of Certification (MOC) are eligible for an additional 1% bonus in 2011 and 0.5% bonus in 2012 to 2014. Data on a physician’s quality measures must be submitted on the physician’s behalf by the MOC program. After 2014, the Secretary of HHS can add MOC completion to the quality measures used for the value-based payment modifier. The American Board of Obstetrics and Gynecology hasn’t yet qualified its MOC for this part of the program.
ObGyn participation in the PQRI program is very limited (less than 10%). While only about 25 of the 215 PQRI quality measures apply to ObGyn care, most are easily applicable, and a physician needs to report on only three to five measures to qualify for the program.
The very low participation rate is likely because many ObGyn practices just didn’t think the incentive payment was worth the trouble. They may need to rethink that math once they’re faced with payment cuts in 2015.
ObGyns should also be aware that the Secretary of HHS, with input from stakeholders, will set up a Physician Compare Web site (modeled after the program that already exists for hospitals) using PQRI data. Data will be made public on January 1, 2013, comparing physicians in terms of quality of care and patient experience.
The Secretary must ensure that the data are statistically valid and risk-adjusted. In addition, the physician must be given time to review the information before it becomes public, and data must ensure appropriate attribution of care when multiple physicians and other providers are involved. The Secretary must also give physicians timely performance feedback.
For all these reasons, ACOG is working with the physician community to make a number of improvements to the PQRI program, doing our best to make it as easy as possible for our members to participate and benefit.
10 What effect will the expansion of Medicaid have on ObGyn practice?
DiVenere: Starting in 2014, the same year that state exchanges are expected to be established, Medicaid eligibility will be broadened to cover all individuals younger than 65 years who have incomes up to 133% of the federal poverty level. All newly eligible adults will be guaranteed a benchmark benefit package that provides the essential health benefits.
States that have already expanded eligibility to adults who have incomes up to 100% of the federal poverty level will receive a phased-in increase in the federal medical assistance percentage so that, by 2019, they will receive the same federal financing as other states (93% in 2019 and 90% in 2020 and later). And states have the option to expand Medicaid eligibility to childless adults as of April 1, 2010, but will receive their regular federal medical assistance percentage until 2014.
Although these changes will broaden the range and increase the number of individuals who will be eligible for Medicaid, the effect on ObGyn practice remains to be seen, especially as pregnant women who were covered by Medicaid at income levels above 133% of the federal poverty level transition off of Medicaid and into private health insurance offered in the exchanges.
Today, about 38% of all ObGyns accept Medicaid gynecologic patients, and 44% accept Medicaid obstetric patients. Medicaid accounts for 18% of revenues of the average ObGyn practice.
11 Will the extension of benefits to young adults have a measurable impact on ObGyn practice?
DiVenere: Congress included two provisions to target “young immortals,” young adults who don’t think they need health insurance because they’re young and healthy and never need to see a doctor. Many young adults are not offered employer-based health insurance, and many see no advantage in buying coverage that they don’t expect to use. But we all know that someone pays when any uninsured person falls sick or has an accident that necessitates medical care.
Beginning this year, adult children as old as 26 years can go onto their parents’ health insurance plan. In addition, catastrophic plans will soon be available to individuals younger than 30 who want to purchase a higher deductible plan through their state exchange or on the individual and small group markets. These catastrophic plans are not required to include the essential benefits package, including maternity care. Nevertheless, both of these provisions should be helpful to ObGyn practices.
12 Will the mandate for employers to provide health insurance affect many ObGyns?
DiVenere: The employer mandate takes effect in 2014, when employers with more than 50 employees, at least one of whom receives a premium tax credit, are required to offer health insurance coverage to employees or be assessed a range of fees. Employers that have 50 or fewer employees are exempt from this requirement.
In 2007, 75% of ObGyn practices had fewer than 42 full-time employees, with an average number of full-time employees, including physicians, of 34.4. So this mandate should not apply to the average ObGyn practice.
A range of small business tax credits for employers that contribute at least 50% of the cost of coverage for their employees will also be available, with credits phasing out as the size of the firm and the average employee wage increase.
13 Who will benefit from the Medicare geographic payment adjustments?
DiVenere: The increased Medicare geographic practice cost index (GPCI) payments and new Frontier payments won’t affect many ObGyns nationally, but they are likely to affect most ObGyns in the related rural locations.
The law reestablishes the national average floor on Medicare’s GPCI for physician work. In 2010 and 2011, Medicare makes a separate adjustment for the practice expense portion of physician payments that will benefit physicians in rural and low-cost areas.
Beginning in 2011, a third adjustment will increase the practice expense GPCI for physicians in frontier states. A frontier state is one in which at least half of its counties have populations smaller than six people per square mile. Frontier states are expected to be Montana, North Dakota, South Dakota, Utah, and Wyoming.
Physicians in 51 localities in 42 states, Puerto Rico, and the Virgin Islands will benefit from the two practice expense adjustments.
ObGyns should also know about two other payment changes:
- The HHS Secretary will create and apply to Medicare provider payments a value-based modifier that will result in higher Medicare payments for high-quality, low-cost physicians and lower payments for high-cost, low-quality physicians. The modifier is to be based on a composite quality score and a composite cost score determined by measures selected by the HHS Secretary and endorsed by a consensus organization. This change begins with 2015 Medicare payments and applies only to physicians in 2015. In 2017, it will also apply to other health professionals.
- Effective immediately, the HHS Secretary has the authority to increase or decrease Medicare relative values, and payments for services, with special attention focused on:
- – services that have high growth rates
- – services that have seen substantial changes in the practice expense or work components
- – services for which new technology has reduced costs
- – instances in which multiple codes are frequently billed for a single service
- – codes that have not been reviewed since implementation of the resource-based relative value scale (RBRVS).
ObGyns who participate in Medicare will start receiving individual physician resource use reports in 2012. These reports will compare per capita utilization of physicians (or physician groups) with the utilization rate of physicians who see similar patients. Reports are required to be risk-adjusted and standardized to take into account local health-care costs.
14 Do you expect the law’s requirements for “administrative simplification” to reduce overhead and increase efficiency?
DiVenere: Don’t we all hope so.
The bill contains several requirements such as:
- establishment of a standardized claim form
- streamlining of claims processing
- improvement of interoperability to allow for more electronic information sharing. These changes will not be implemented until 2013 at the earliest.
Today, about 34% of all ObGyn practices use electronic health records. The systemwide benefits of health information technology (HIT) can be many. Insurers can save by reducing unnecessary tests. Patients can benefit from better coordination of care and fewer medical errors. But these advantages don’t necessarily translate into savings or revenue for physician practices.
Instead, physicians face Medicare and private insurance payment cuts. Little assistance is available for the investment in HIT. And uncertain interoperability standards and rapid technological changes can very quickly make this year’s investment obsolete. Many physicians in solo and small practices are understandably reluctant to take the HIT plunge.
The initial cost of purchasing HIT for a small practice is typically at least $50,000 per physician. Physicians face additional, ongoing costs in staff training and hardware and software updates as well. And many physicians see significant efficiency losses for months and sometimes years after upgrading to an electronic health record system.
Still, with interoperable, shareable electronic records, all physicians treating a particular patient can have the full story. A patient’s paper record kept in her physician’s office shows only a slice of her medical history, potentially missing important information from the patient’s other physicians, including allergies to medication, test results, and the results of particular therapies.
Without a shared electronic record, a physician relies on the recollection of each patient, which is often unintentionally incomplete. A patient may be uncertain about the name or dosage of a medication, fail to remember the date of a screening examination, or lack results of lab tests ordered by another physician.
A physician’s access to the full story with shareable electronic records is important to the care of all patients and can be particularly relevant for patients who have inconsistent contact with health care providers. Often, these patients get care in various settings, including physician offices, community clinics, and emergency departments. Because these patients tend to have a higher incidence of chronic disease, they may greatly benefit from the sharing of medical information.
Clearly, Congress wants to move us to full adoption of HIT. Beginning in 2013, all plans must comply with a uniform standard for electronic transactions, including eligibility verification and health claims status.
In 2014, uniform standards must allow automatic reconciliation of electronic funds transfers and HIPAA payment and remittance; use standardized and consistent methods of health plan enrollment and claim edits; use unique health plan identifiers to simplify and improve routing of health care transactions; and use standard electronic claims attachments.
Uniformity and standardization can help address one of the major roadblocks to physician adoption of health information technology.
In the aftermath of the WHI, consider estrogen patient by patient
CASE: Vasomotor symptoms and a request for relief
A 54-year-old woman with a family history of osteoporosis visits your office complaining of mild vasomotor symptoms. She reports that it has been 3 years since her last menstrual period. She also points out that she has type 2 diabetes.
Is she a good candidate for estrogen therapy?
How would you manage her vasomotor symptoms?
In the 8 years since initial publication of the Women’s Health Initiative (WHI), ideas about when to use estrogen—and whom to treat—have evolved considerably. Today, we know that hormone therapy remains an appropriate and effective option in properly selected cases, said Isaac Schiff, MD, one of two experts selected to deliver the Morton and Diane Stenchever Lecture at the ACOG Annual Clinical Meeting last month in San Francisco. Along with JoAnn E. Manson, MD, DrPH, Dr. Schiff spoke on “Hormone therapy in the post-WHI era.”
Dr. Schiff is chief of the Vincent Obstetrics and Gynecology Service at Massachusetts General Hospital in Boston and the Joe Vincent Meigs Professor of Gynecology at Harvard Medical School.
Symptoms are the main requisite for estrogen
Estrogen remains the most effective therapy for vasomotor flushes and vaginal atrophy, Dr. Schiff said.
The most important requirement for its use?
The patient must have bothersome symptoms.
Menopausal status is also key—estrogen is appropriate only in recently menopausal women. In a woman who has never used estrogen and who is more than 10 years past the menopausal transition, vasomotor flushes are not very common. If vaginal atrophy is a problem, however, local estrogen therapy may be an option.
Estrogen is not recommended for prevention of cardiovascular events or osteoporosis, but it is appropriate for the patient who is bothered by moderate or severe vasomotor flushes or urogenital symptoms—provided it is prescribed at the lowest effective dosage and for a short duration.
Indication #1: Alleviating the vasomotor flush
Among the nonhormonal options many women use for relief of vasomotor flushes are complementary and alternative preparations such as black cohosh, dong quai, red clover, and ginseng, but research into these therapies has revealed that they yield mixed results, with herbal remedies usually having an effect similar to that of placebo, Dr. Schiff said.
“But if it works, so be it,” he added.
Selective serotonin reuptake inhibitors (SSRIs) and other antidepressants have been somewhat effective, he noted. In particular, paroxetine (Paxil) and venlafaxine (Effexor) have outperformed placebo—though paroxetine should be avoided in women who are taking tamoxifen because it diminishes the efficacy of tamoxifen.1,2 Paroxetine and venlafaxine are not FDA-approved for hot flashes, he noted.
The gold standard for relief of vasomotor flushes is estrogen, said Dr. Schiff. Although the efficacy of estrogen in regard to vasomotor flushes appears to increase with the dosage, progestin has an additive effect. Therefore, when both estrogen and a progestin are prescribed, the estrogen dosage can be kept at a lower range, Dr. Schiff said.
Among the options are transdermal estrogen gel, cream, and a patch; and oral estrogen. Because observational studies have shown that venous thromboembolic events are four times more likely when oral estrogen is used, compared with transdermal formulations, the latter could be considered, said Dr. Schiff.3
There is no evidence that compounded bioidentical formulations of estrogen are any safer than their manufactured counterparts, he noted.
Indication #2: Urogenital atrophy
Research has demonstrated that local estrogen therapy thickens the vaginal wall and stimulates glycogen formation, thereby increasing lactic acid and lowering the pH level, which may reduce vaginal infections.4
Because vaginal atrophy can significantly impair a woman’s quality of life, estrogen therapy may be warranted, said Dr. Schiff. The good news is that local estrogen appears to be amply effective and lacks many of the risks inherent in oral administration.
More good news: Regular sexual activity has a positive effect on vaginal lubrication and elasticity and promotes natural maintenance of urogenital health. For that reason, any woman being treated with estrogen for urogenital issues should have the dosage reassessed once therapy has allowed her to resume or increase sexual activity, Dr. Schiff noted.
If a patient wants to avoid hormonal therapy for urogenital atrophy, a number of other options are available, such as the polycarbophil-based vaginal moisturizer Replens, which has been shown to increase vaginal moisture and fluid volume and lower the vaginal pH level. It is not quite as effective as estrogen, Dr. Schiff noted, but does provide some relief.5,6
Estrogen is not first-line therapy for osteoporosis
One of the principal findings of the WHI is that estrogen reduces the risk of fracture. Dr. Schiff noted, however, that estrogen should not be prescribed for that indication, as a host of other medications are available that lack the risks of estrogen therapy. Those medications include alendronate and the other bisphosphonates; raloxifene; and zoledronic acid.
What’s the bottom line?
There is no single “right” answer to the question of when estrogen therapy is appropriate. Each case should be individualized, Dr. Schiff said. If a patient is healthy, recently menopausal, and bothered by moderate or severe vasomotor flushes or urogenital symptoms, then estrogen is one option that should be presented, along with its benefits and risks. Ultimately, it is the patient, in partnership with her physician, who must decide for or against estrogen therapy.
CASE: Resolved
This patient may not require estrogen, although it is certainly an option. Although she is young, symptomatic, and recently menopausal, her vasomotor symptoms are mild. Because her symptoms are not severe, time alone may be sufficient “treatment,” or she may want to try a simple lifestyle adjustment such as the wearing of layers of clothing that can be removed when a vasomotor flush occurs.
If vaginal atrophy is a problem, local estrogen or a topical vaginal agent such as Replens may provide relief.
1. Soares CN, Joffe H, Viguera AC, et al. Paroxetine versus placebo for women in midlife after hormone therapy discontinuation. Am J Med. 2008;121(2):159-162.e1.
2. Cheema D, Coomarasamy A, El-Toukhy T. Nonhormonal therapy of postmenopausal vasomotor symptoms: a structured evidence-based review. Arch Gynecol Obstet. 2007;276(5):463-469.
3. Canonico M, Plu-Bureau G, Lowe GDO, Scarabin PY. Hormone replacement therapy and risk of venous thromboembolism in postmenopausal women: systematic review and meta-analysis. BMJ. 2008;336(7655):1227-1231.
4. Archer DF. Efficacy and tolerability of local estrogen therapy for urogenital atrophy. Menopause. 2010;17(1):194-203.
5. Bygdeman M, Swahn ML. Replens versus dienoestrol cream in the symptomatic treatment of vaginal atrophy in postmenopausal women. Maturitas. 1996;23(3):259-263.
6. Nachtigall LE. Comparative study: Replens versus local estrogen in menopausal women. Fertil Steril. 1994;61(1):178-180.
CASE: Vasomotor symptoms and a request for relief
A 54-year-old woman with a family history of osteoporosis visits your office complaining of mild vasomotor symptoms. She reports that it has been 3 years since her last menstrual period. She also points out that she has type 2 diabetes.
Is she a good candidate for estrogen therapy?
How would you manage her vasomotor symptoms?
In the 8 years since initial publication of the Women’s Health Initiative (WHI), ideas about when to use estrogen—and whom to treat—have evolved considerably. Today, we know that hormone therapy remains an appropriate and effective option in properly selected cases, said Isaac Schiff, MD, one of two experts selected to deliver the Morton and Diane Stenchever Lecture at the ACOG Annual Clinical Meeting last month in San Francisco. Along with JoAnn E. Manson, MD, DrPH, Dr. Schiff spoke on “Hormone therapy in the post-WHI era.”
Dr. Schiff is chief of the Vincent Obstetrics and Gynecology Service at Massachusetts General Hospital in Boston and the Joe Vincent Meigs Professor of Gynecology at Harvard Medical School.
Symptoms are the main requisite for estrogen
Estrogen remains the most effective therapy for vasomotor flushes and vaginal atrophy, Dr. Schiff said.
The most important requirement for its use?
The patient must have bothersome symptoms.
Menopausal status is also key—estrogen is appropriate only in recently menopausal women. In a woman who has never used estrogen and who is more than 10 years past the menopausal transition, vasomotor flushes are not very common. If vaginal atrophy is a problem, however, local estrogen therapy may be an option.
Estrogen is not recommended for prevention of cardiovascular events or osteoporosis, but it is appropriate for the patient who is bothered by moderate or severe vasomotor flushes or urogenital symptoms—provided it is prescribed at the lowest effective dosage and for a short duration.
Indication #1: Alleviating the vasomotor flush
Among the nonhormonal options many women use for relief of vasomotor flushes are complementary and alternative preparations such as black cohosh, dong quai, red clover, and ginseng, but research into these therapies has revealed that they yield mixed results, with herbal remedies usually having an effect similar to that of placebo, Dr. Schiff said.
“But if it works, so be it,” he added.
Selective serotonin reuptake inhibitors (SSRIs) and other antidepressants have been somewhat effective, he noted. In particular, paroxetine (Paxil) and venlafaxine (Effexor) have outperformed placebo—though paroxetine should be avoided in women who are taking tamoxifen because it diminishes the efficacy of tamoxifen.1,2 Paroxetine and venlafaxine are not FDA-approved for hot flashes, he noted.
The gold standard for relief of vasomotor flushes is estrogen, said Dr. Schiff. Although the efficacy of estrogen in regard to vasomotor flushes appears to increase with the dosage, progestin has an additive effect. Therefore, when both estrogen and a progestin are prescribed, the estrogen dosage can be kept at a lower range, Dr. Schiff said.
Among the options are transdermal estrogen gel, cream, and a patch; and oral estrogen. Because observational studies have shown that venous thromboembolic events are four times more likely when oral estrogen is used, compared with transdermal formulations, the latter could be considered, said Dr. Schiff.3
There is no evidence that compounded bioidentical formulations of estrogen are any safer than their manufactured counterparts, he noted.
Indication #2: Urogenital atrophy
Research has demonstrated that local estrogen therapy thickens the vaginal wall and stimulates glycogen formation, thereby increasing lactic acid and lowering the pH level, which may reduce vaginal infections.4
Because vaginal atrophy can significantly impair a woman’s quality of life, estrogen therapy may be warranted, said Dr. Schiff. The good news is that local estrogen appears to be amply effective and lacks many of the risks inherent in oral administration.
More good news: Regular sexual activity has a positive effect on vaginal lubrication and elasticity and promotes natural maintenance of urogenital health. For that reason, any woman being treated with estrogen for urogenital issues should have the dosage reassessed once therapy has allowed her to resume or increase sexual activity, Dr. Schiff noted.
If a patient wants to avoid hormonal therapy for urogenital atrophy, a number of other options are available, such as the polycarbophil-based vaginal moisturizer Replens, which has been shown to increase vaginal moisture and fluid volume and lower the vaginal pH level. It is not quite as effective as estrogen, Dr. Schiff noted, but does provide some relief.5,6
Estrogen is not first-line therapy for osteoporosis
One of the principal findings of the WHI is that estrogen reduces the risk of fracture. Dr. Schiff noted, however, that estrogen should not be prescribed for that indication, as a host of other medications are available that lack the risks of estrogen therapy. Those medications include alendronate and the other bisphosphonates; raloxifene; and zoledronic acid.
What’s the bottom line?
There is no single “right” answer to the question of when estrogen therapy is appropriate. Each case should be individualized, Dr. Schiff said. If a patient is healthy, recently menopausal, and bothered by moderate or severe vasomotor flushes or urogenital symptoms, then estrogen is one option that should be presented, along with its benefits and risks. Ultimately, it is the patient, in partnership with her physician, who must decide for or against estrogen therapy.
CASE: Resolved
This patient may not require estrogen, although it is certainly an option. Although she is young, symptomatic, and recently menopausal, her vasomotor symptoms are mild. Because her symptoms are not severe, time alone may be sufficient “treatment,” or she may want to try a simple lifestyle adjustment such as the wearing of layers of clothing that can be removed when a vasomotor flush occurs.
If vaginal atrophy is a problem, local estrogen or a topical vaginal agent such as Replens may provide relief.
CASE: Vasomotor symptoms and a request for relief
A 54-year-old woman with a family history of osteoporosis visits your office complaining of mild vasomotor symptoms. She reports that it has been 3 years since her last menstrual period. She also points out that she has type 2 diabetes.
Is she a good candidate for estrogen therapy?
How would you manage her vasomotor symptoms?
In the 8 years since initial publication of the Women’s Health Initiative (WHI), ideas about when to use estrogen—and whom to treat—have evolved considerably. Today, we know that hormone therapy remains an appropriate and effective option in properly selected cases, said Isaac Schiff, MD, one of two experts selected to deliver the Morton and Diane Stenchever Lecture at the ACOG Annual Clinical Meeting last month in San Francisco. Along with JoAnn E. Manson, MD, DrPH, Dr. Schiff spoke on “Hormone therapy in the post-WHI era.”
Dr. Schiff is chief of the Vincent Obstetrics and Gynecology Service at Massachusetts General Hospital in Boston and the Joe Vincent Meigs Professor of Gynecology at Harvard Medical School.
Symptoms are the main requisite for estrogen
Estrogen remains the most effective therapy for vasomotor flushes and vaginal atrophy, Dr. Schiff said.
The most important requirement for its use?
The patient must have bothersome symptoms.
Menopausal status is also key—estrogen is appropriate only in recently menopausal women. In a woman who has never used estrogen and who is more than 10 years past the menopausal transition, vasomotor flushes are not very common. If vaginal atrophy is a problem, however, local estrogen therapy may be an option.
Estrogen is not recommended for prevention of cardiovascular events or osteoporosis, but it is appropriate for the patient who is bothered by moderate or severe vasomotor flushes or urogenital symptoms—provided it is prescribed at the lowest effective dosage and for a short duration.
Indication #1: Alleviating the vasomotor flush
Among the nonhormonal options many women use for relief of vasomotor flushes are complementary and alternative preparations such as black cohosh, dong quai, red clover, and ginseng, but research into these therapies has revealed that they yield mixed results, with herbal remedies usually having an effect similar to that of placebo, Dr. Schiff said.
“But if it works, so be it,” he added.
Selective serotonin reuptake inhibitors (SSRIs) and other antidepressants have been somewhat effective, he noted. In particular, paroxetine (Paxil) and venlafaxine (Effexor) have outperformed placebo—though paroxetine should be avoided in women who are taking tamoxifen because it diminishes the efficacy of tamoxifen.1,2 Paroxetine and venlafaxine are not FDA-approved for hot flashes, he noted.
The gold standard for relief of vasomotor flushes is estrogen, said Dr. Schiff. Although the efficacy of estrogen in regard to vasomotor flushes appears to increase with the dosage, progestin has an additive effect. Therefore, when both estrogen and a progestin are prescribed, the estrogen dosage can be kept at a lower range, Dr. Schiff said.
Among the options are transdermal estrogen gel, cream, and a patch; and oral estrogen. Because observational studies have shown that venous thromboembolic events are four times more likely when oral estrogen is used, compared with transdermal formulations, the latter could be considered, said Dr. Schiff.3
There is no evidence that compounded bioidentical formulations of estrogen are any safer than their manufactured counterparts, he noted.
Indication #2: Urogenital atrophy
Research has demonstrated that local estrogen therapy thickens the vaginal wall and stimulates glycogen formation, thereby increasing lactic acid and lowering the pH level, which may reduce vaginal infections.4
Because vaginal atrophy can significantly impair a woman’s quality of life, estrogen therapy may be warranted, said Dr. Schiff. The good news is that local estrogen appears to be amply effective and lacks many of the risks inherent in oral administration.
More good news: Regular sexual activity has a positive effect on vaginal lubrication and elasticity and promotes natural maintenance of urogenital health. For that reason, any woman being treated with estrogen for urogenital issues should have the dosage reassessed once therapy has allowed her to resume or increase sexual activity, Dr. Schiff noted.
If a patient wants to avoid hormonal therapy for urogenital atrophy, a number of other options are available, such as the polycarbophil-based vaginal moisturizer Replens, which has been shown to increase vaginal moisture and fluid volume and lower the vaginal pH level. It is not quite as effective as estrogen, Dr. Schiff noted, but does provide some relief.5,6
Estrogen is not first-line therapy for osteoporosis
One of the principal findings of the WHI is that estrogen reduces the risk of fracture. Dr. Schiff noted, however, that estrogen should not be prescribed for that indication, as a host of other medications are available that lack the risks of estrogen therapy. Those medications include alendronate and the other bisphosphonates; raloxifene; and zoledronic acid.
What’s the bottom line?
There is no single “right” answer to the question of when estrogen therapy is appropriate. Each case should be individualized, Dr. Schiff said. If a patient is healthy, recently menopausal, and bothered by moderate or severe vasomotor flushes or urogenital symptoms, then estrogen is one option that should be presented, along with its benefits and risks. Ultimately, it is the patient, in partnership with her physician, who must decide for or against estrogen therapy.
CASE: Resolved
This patient may not require estrogen, although it is certainly an option. Although she is young, symptomatic, and recently menopausal, her vasomotor symptoms are mild. Because her symptoms are not severe, time alone may be sufficient “treatment,” or she may want to try a simple lifestyle adjustment such as the wearing of layers of clothing that can be removed when a vasomotor flush occurs.
If vaginal atrophy is a problem, local estrogen or a topical vaginal agent such as Replens may provide relief.
1. Soares CN, Joffe H, Viguera AC, et al. Paroxetine versus placebo for women in midlife after hormone therapy discontinuation. Am J Med. 2008;121(2):159-162.e1.
2. Cheema D, Coomarasamy A, El-Toukhy T. Nonhormonal therapy of postmenopausal vasomotor symptoms: a structured evidence-based review. Arch Gynecol Obstet. 2007;276(5):463-469.
3. Canonico M, Plu-Bureau G, Lowe GDO, Scarabin PY. Hormone replacement therapy and risk of venous thromboembolism in postmenopausal women: systematic review and meta-analysis. BMJ. 2008;336(7655):1227-1231.
4. Archer DF. Efficacy and tolerability of local estrogen therapy for urogenital atrophy. Menopause. 2010;17(1):194-203.
5. Bygdeman M, Swahn ML. Replens versus dienoestrol cream in the symptomatic treatment of vaginal atrophy in postmenopausal women. Maturitas. 1996;23(3):259-263.
6. Nachtigall LE. Comparative study: Replens versus local estrogen in menopausal women. Fertil Steril. 1994;61(1):178-180.
1. Soares CN, Joffe H, Viguera AC, et al. Paroxetine versus placebo for women in midlife after hormone therapy discontinuation. Am J Med. 2008;121(2):159-162.e1.
2. Cheema D, Coomarasamy A, El-Toukhy T. Nonhormonal therapy of postmenopausal vasomotor symptoms: a structured evidence-based review. Arch Gynecol Obstet. 2007;276(5):463-469.
3. Canonico M, Plu-Bureau G, Lowe GDO, Scarabin PY. Hormone replacement therapy and risk of venous thromboembolism in postmenopausal women: systematic review and meta-analysis. BMJ. 2008;336(7655):1227-1231.
4. Archer DF. Efficacy and tolerability of local estrogen therapy for urogenital atrophy. Menopause. 2010;17(1):194-203.
5. Bygdeman M, Swahn ML. Replens versus dienoestrol cream in the symptomatic treatment of vaginal atrophy in postmenopausal women. Maturitas. 1996;23(3):259-263.
6. Nachtigall LE. Comparative study: Replens versus local estrogen in menopausal women. Fertil Steril. 1994;61(1):178-180.
IN THIS ARTICLE
Confused about mammography guidelines? 7 questions answered
Some clinicians were reconsidering the need for an annual mammogram even before the US Preventive Services Task Force (USPSTF) issued new guidelines late last year.1
Andrew M. Kaunitz, MD, is one of those clinicians. In an editorial in the December issue of OBG Management, he was bold enough to declare: “My plan is to be more acquiescent when a woman says ‘No’ to an annual mammogram.”2
Among the evidence he cited to justify that acquiescence was a recent article in the Journal of the American Medical Association that expressed concern about the high number of early cancers—including ductal carcinoma in situ—that are detected by mammography and treated even though many are unlikely to progress or ever become clinically significant.3 This phenomenon—termed “over-diagnosis”—is one of the risks of breast cancer screening.
Dr. Kaunitz is professor and associate chairman of obstetrics and gynecology at the University of Florida College of Medicine–Jacksonville. He also serves on the OBG Management Board of Editors.
Although the USPSTF is the only official body to revise its recommendations on breast cancer screening so far, more changes seem likely. This article aims to sift through the static on the airwaves of late and offer concrete recommendations for practice. In the process, it addresses seven questions:
- How did USPSTF guidelines change?
- Why did they change?
- Why did the changes attract so much attention?
- What is ACOG’s position?
- What do thought leaders make of the new guidelines?
- Are the USPSTF recommendations likely to affect insurance coverage for mammography?
- What should you tell your patients about breast cancer screening?
1. How did USPSTF guidelines change?
In an article published November 16, the USPSTF made a number of revisions to earlier breast cancer screening guidelines for women at average risk of the disease:
Approximately 39 million women undergo mammography each year in the United States, costing the health-care system more than $5 billion.
- Routine screening mammography is no longer recommended in women 40 to 49 years old. Rather, the decision about when to begin regular screening should be individualized and should “take into account patient context, including the patient’s values regarding specific benefits and harms” (Grade C recommendation).
- Screening mammography in women 50 to 74 years old should be biennial rather than annual (Grade B recommendation).
- Breast self-examination (BSE) is not recommended for any age group (Grade D recommendation).1
2. Why did the USPSTF guidelines change?
The changes were based on new data and analysis in the following areas:
- Mortality among women 40 to 49 years old. Although mammography screening reduces breast cancer mortality by 15% in this age group, the USPSTF concluded that “there is moderate certainty that the net benefit is small” in this population.1,4
- The effectiveness of BSE in decreasing breast cancer mortality among women of any age. Studies of BSE published since 2002 found no significant differences in breast cancer mortality between women who perform BSE and those who don’t.4
- The magnitude of harms of screening with mammography. Mammography screening in women 40 to 49 years old involves a significant risk of harms.4 Although the USPSTF observed that the benefits of mammography in women 40 to 49 years old appear to be equivalent to the benefits of mammography among women 50 to 59 years old, it concluded that the harms outweigh benefits in the younger women.
Harms cited by the USPSTF include:
- radiation exposure
- pain during the procedure
- anxiety and distress
- an increased rate of false-positive results
- greater need for additional imaging and biopsies.4
The USPSTF conceded that the radiation exposure from a mammogram is minimal, but questioned whether cumulative exposure in young women might be problematic. It also noted that “many women experience pain during the procedure (range, 1% to 77%), but few would consider this a deterrent from future screening.”4
As for false-positive results, the group observed: “Data from the [Breast Cancer Screening Consortium (BCSC)] for regularly screened women…indicate that false-positive mammography results are common in all age groups but are most common among women aged 40 to 49 years (97.8 per 1,000 women per screening round).”4
“The BCSC results indicate that for every case of invasive breast cancer detected by mammography screening in women aged 40 to 49 years, 556 women have mammography, 47 have additional imaging, and five have biopsies.”4
It is the significant rate of false positives that creates the need for additional screening, diagnostic imaging, and biopsy. These additional imaging and invasive procedures increase anxiety and distress among many women. The USPSTF concluded that these harms outweighed the benefits of mammography screening in women 40 to 49 years old.
After publication of the new US Preventive Services Task Force (USPSTF) breast cancer screening guidelines late last year, it was only a matter of hours before official bodies and professional organizations began to weigh in on the changes, and the verdict was unanimous—disagreement. Among those chiming in were the American Cancer Society (ACS), the American College of Obstetricians and Gynecologists (ACOG), the American College of Radiology, the American Society of Breast Surgeons, the Society for Breast Imaging (SBI), and Susan G. Komen for the Cure, among others. Here are excerpts from their statements.
American Cancer Society
The ACS immediately refuted the USPSTF recommendations:
The American Cancer Society continues to recommend annual screening using mammography and clinical breast examination for all women beginning at age 40. Our experts make this recommendation having reviewed virtually all the same data reviewed by the USPSTF, but also additional data that the USPSTF did not consider….[T]he American Cancer Society’s medical staff and volunteer experts overwhelmingly believe the benefits of screening women aged 40 to 49 outweigh its limitations.7
ACOG
The College reaffirmed its support for screening mammography every 1 to 2 years in women 40 to 49 years old and every year for women 50 and older, as well as breast self-examination for women of all ages:
At this time, The American College of Obstetricians and Gynecologists recommends that Fellows continue to follow current College guidelines for breast cancer screening. Evaluation of the new USPSTF recommendations is under way. Should the College update its guidelines in the future, Fellows would be alerted and such revised guidelines would be published in Obstetrics & Gynecology.5
American College of Radiology
The College minced no words in opposing the changes:
If cost-cutting US Preventive Services Task Force (USPSTF) mammography recommendations are adopted as policy, two decades of decline in breast cancer mortality could be reversed and countless American women may die needlessly from breast cancer each year.
These new recommendations seem to reflect a conscious decision to ration care. If Medicare and private insurers adopt these incredibly flawed USPSTF recommendations as a rationale for refusing women coverage of these life-saving exams, it could have deadly effects for American women,” said Carol H. Lee, MD, chair of the American College of Radiology Breast Imaging Commission.8
American Society of Breast Surgeons
The organization released a statement describing its position as “strongly opposed” to the USPSTF recommendations:
We believe there is sufficient data to support annual mammography screening for women age 40 and older. We also believe the breast cancer survival rate of women between 40 and 50 will improve from the increased use of digital mammographic screening, which is superior to older plain film techniques in detecting breast cancer in that age group.
While we recognize that there will be a number of benign biopsies, we also recognize that mammography is the optimal screening tool for the early diagnosis of breast cancer in terms of cost-effectiveness, practical use, and accuracy.9
Society for Breast Imaging
In its statement, the SBI noted the confusion caused by revision of the USPSTF guidelines, calling it “unnecessary and potentially deadly”:
Mammography has been shown unequivocally to save lives and is primarily responsible for the 30% decline in breast cancer mortality in the United States over the past 20 years. The USPSTF conclusion—that women under age 50 should not undergo routine screening—conflicts with their own report, which confirms a benefit of mammography to women age 40–49 that is statistically significant.
We strongly urge women and their physicians to adhere to the American Cancer Society recommendations of yearly screening beginning at age 40.10
Susan G. Komen for the Cure
This public advocacy group issued a statement in late November acknowledging “mass confusion and justifiable outrage” in the aftermath of the USPSTF changes:
”We have worked so hard to build public trust and urge people to get screened,” said Nancy G. Brinker, founder of Susan G. Komen for the Cure, “and now they hear that maybe they shouldn’t bother. That is dangerous….Let me say this as clearly as I can: Mammography saves lives, even this report says that. Keep doing what you are doing. And always, talk with your doctor.” Brinker also noted that Komen for the Cure was not changing its guidelines, continuing to recommend annual mammograms beginning at age 40.11
3. Why have the guidelines captured so much media attention?
Most of the controversy that has arisen since publication of the new guidelines has centered on the recommendation against screening mammography in women 40 to 49 years old. A number of media outlets have highlighted women whose breast cancer was detected by screening mammography when they were in their 40s, and many survivors with a similar history have spoken out against the new recommendations.
In addition, the American Cancer Society (ACS), the American College of Radiology, Susan G. Komen for the Cure, and other groups have publicly opposed the new guidelines. (See “Among professional organizations, a resounding chorus of disagreement”)
4. What is ACOG’s position on the new recommendations?
The American College of Obstetricians and Gynecologists (ACOG) was quick to weigh in on the new USPSTF guidelines, emphasizing that the College’s recommendations have not changed. They include:
- screening mammography every 1 to 2 years for women 40 to 49 years old
- screening mammography every year for women 50 years and older
- BSE for all women.
ACOG did note, however, that “the College is continuing to evaluate in detail the new USPSTF recommendations and the new evidence considered by the USPSTF.”5
5. What do thought leaders make of the USPSTF changes?
Although the USPSTF guidelines sparked a firestorm of media coverage, the change did not come as a shock to leaders in the ObGyn specialty.

Legitimate concerns about screening mammography have increasingly been raised by experts in the field.
ANDREW M. KAUNITZ, MD “I was not surprised,” said Dr. Kaunitz. “As I pointed out in my editorial in OBG Management, legitimate concerns about screening mammography have increasingly been raised by experts in the field.2 Proposals to stop routinely screening women in their 40s were made earlier in this decade, but were met with major pushback from the ACS, breast cancer advocacy organizations, and medical specialty groups. These same groups are now pushing back against the new USPSTF guidelines,” he added.
Robert L. Barbieri, MD, was not taken aback by the guidelines themselves, but he was surprised by the manner and timing of their release. Dr. Barbieri is Kate Macy Ladd professor of obstetrics, gynecology, and reproductive biology at Harvard Medical School and chief of obstetrics and gynecology at Brigham and Women’s Hospital in Boston. He serves as editor-in-chief of OBG Management.
“I was surprised that the USPSTF did not weigh the potential impact of its analysis on the key stakeholders: patients, disease-based coalitions such as the American Cancer Society and Susan G. Komen for the Cure, and professional societies such as the American College of Radiology and ACOG,” he said. “If I were supervising the process, I would have asked for a comment period before releasing the report. I would have included the comments from key stakeholders in an appendix to the report.”
Are other organizations—besides the USPSTF—likely to change their recommendations for mammography screening in the near future? In the case of ACOG, Dr. Barbieri doesn’t think so.
“I don’t think ACOG will change the age at which to initiate screening,” he said. “I believe it will stick to its recommendation to start screening at 40 and continue every 1 to 2 years from 40 to 50 years of age. However, I could see ACOG becoming a bit more flexible on the question of whether screening should take place at 1- or 2-year intervals after age 50.”
Dr. Kaunitz sees things differently.
“It seems possible that, going forward, the College will give Fellows and their patients permission to implement the new guidelines without mandating their implementation. For example, if women in their 40s wish to defer screening, that would be OK, as would biennial screening for women in their 50s and 60s.”
6. Are the USPSTF recommendations likely to affect insurance coverage?
In a press release issued soon after the new guidelines were published, US Health and Human Services Secretary Kathleen Sebelius addressed Americans directly to reaffirm her support for mammography in women 40 to 49 years old: “There is no question that the US Preventive Services Task Force recommendations have caused a great deal of confusion and worry among women and their families,” her statement read.6 She made it clear that the new recommendations are unlikely to affect federal coverage of mammography.
“The US Preventive Services Task Force is an outside independent panel of doctors and scientists who make recommendations. They do not set federal policy and they don’t determine what services are covered by the federal government,” she said.6
But Dr. Barbieri thinks some changes in insurance coverage are inevitable.
“Any claims that the new guidelines do not represent a major change would be disingenuous,” he said. Because the USPSTF rated its recommendation against mammography for women 40 to 49 years old as grade ‘C,’ that change in guidelines is likely to trigger at least some change in coverage.
“In reality, the ‘C’ rating will require many insurance companies—by their own rule—to stop reimbursing for this screening test,” he said. “The ‘C’ rating means that the test has little benefit.”
ACOG also deems it likely that insurance coverage may be affected for some women.
“Fellows should be aware that the new USPSTF recommendations against routine screening mammography for women aged 40–49 (a grade C recommendation) has implications for insurance coverage, as some insurers will cover only preventive services rated as an ‘A’ or a ‘B’ by the USPSTF. Fellows should counsel their patients that insurance coverage for ‘routine screening’ mammography may become variable and that patients should address this question with their insurers. These recommendations do not apply to high-risk women or patients with clinical findings, and they should be managed accordingly.”5
7. What should you tell your patients?
With all the media attention devoted to the change in guidelines, it’s little surprise that patients are asking questions.
“Patients are aware of the USPSTF report,” said Dr. Barbieri. “They are largely ignoring the recommendations and sticking with annual mammograms.”
“I think, as always, women are looking to their ObGyn for guidance,” added Dr. Kaunitz.
So what are these clinicians telling patients about mammography screening?
As he was to begin with, Dr. Kaunitz is acquiescent if patients prefer to defer mammography screening to their 50s.
“Because it seems that insurance coverage, over the short term, is unlikely to restrict current access to mammograms,” said Dr. Kaunitz, “my evolving philosophy is that the new USPSTF guidelines, along with ACOG and other existing guidelines, give ObGyns and their patients permission to:
- proceed or not proceed with mammograms for women in their 40s, with the decision based on issues such as patient preference, family history of breast cancer, and body mass index (BMI)
- be flexible regarding 1- to 2-year screening intervals among women in their 50s, 60s, and 70s, with the decision based on issues such as patient preference, use or non-use of estrogen-progestin hormone therapy, family history of breast cancer, and BMI.”
Dr. Barbieri believes some effort to integrate the ACOG and USPSTF recommendations is called for. “Accordingly,” he said, “I suggest the following:

I suggest actively recommending biennial mammography for women 40 to 75 years old. Offer annual mammography to women 40 to 75 years old if they prefer that option.
ROBERT L. BARBIERI, MD
- Actively recommend biennial mammography for women 40 to 75 years old. Offer annual mammography to women 40 to 75 years old if they prefer that option.
- Aggressively search for high-risk women, with high risk defined as a lifetime risk of breast cancer exceeding 15%. Among the variables contributing to high-risk status are a history of thoracic radiotherapy, a strong family history of breast cancer, and BRCA mutation. For these women, I would recommend annual mammography and biennial MRI of the breasts.
- Perform annual or biennial clinical breast exam.
- Obtain imaging for any woman who has a palpable breast lump, and resect or biopsy the lump even if that imaging is negative.”
1. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151:716-726.
2. Kaunitz AM. I’ve been rethinking my zeal for breast cancer screening. OBG Management. 2009;21(12):6-8.
3. Esserman L, Shieh Y, Thompson I. Rethinking screening for breast cancer and prostate cancer. JAMA. 2009;302:1685-1692.
4. Nelson HD, Tyne K, Nalk A, Bougatsos C, Chan BK, Humphrey L. Screening for breast cancer: an update for the US Preventive Services Task Force. Ann Intern Med. 2009;151:727-737.
5. American College of Obstetricians and Gynecologists. Response of the American College of Obstetricians and Gynecologists to new breast cancer screening recommendations from the US Preventive Services Task Force. Available at: http://www.acog.org/from_home/Misc/uspstfResponse.cfm. Accessed Nov. 25, 2009.
6. US Department of Health and Human Services. Secretary Sebelius statement on new breast cancer recommendations [news release]. Nov. 18, 2009. Available at: http://www.hhs.gov/news/press/2009pres/11/20091118a.html. Accessed Dec. 4, 2009.
7. American Cancer Society responds to changes to USPSTF mammography guidelines [news release]. American Cancer Society. Nov. 16, 2009. Available at: http://www.cancer.org/docroot/MED/content/MED_2_1x_American_Cancer_Society_
Responds_to_Changes_to_USPSTF_Mammography_Guidelines.asp. Accessed Dec. 4, 2009.
8. American College of Radiology. USPSTF mammography recommendations will result in countless unnecessary breast cancer deaths each year [news release]. Nov. 16, 2009. Available at: www.acr.org/MainMenuCategories/media_room/FeaturedCategories/PressReleases/USPSTFMammoRecs.aspx. Accessed Dec. 4, 2009.
9. American Society of Breast Surgeons. Society responds to USPSTF changes in mammography guidelines [news release]. Available at: http://www.breastsurgeons.org/news/article.php?id=57. Accessed Dec. 4, 2009.
10. Society of Breast Imaging. Official Society of Breast Imaging response to the announcement by HHS Secretary Sebelius regarding USPSTF mammography recommendations. Available at:http://www.sbi-online.org/associations/8199/files/OFFICIAL%20SOCIETY%20OF%20
BREAST%20IMAGING%20RESPONSE%20TO%20THE%20ANNOUNCEMENT%20
BY%20HHS%20SECRETARY%20
SEBELIUS%20REGARDING%20USPSTF%20MAMMOGRAPHY%20RECOMMENDATIONS.pdf. Accessed Dec. 4, 2009.
11. Susan G. Komen for the Cure founder, Nancy G. Brinker, calls new mammography guidelines a “set back”; makes call to action [news release]. Nov. 23, 2009. Available at: http://ww5.komen.org/KomenNewsArticle.aspx?id=6442451516. Accessed Dec. 7, 2009.
Some clinicians were reconsidering the need for an annual mammogram even before the US Preventive Services Task Force (USPSTF) issued new guidelines late last year.1
Andrew M. Kaunitz, MD, is one of those clinicians. In an editorial in the December issue of OBG Management, he was bold enough to declare: “My plan is to be more acquiescent when a woman says ‘No’ to an annual mammogram.”2
Among the evidence he cited to justify that acquiescence was a recent article in the Journal of the American Medical Association that expressed concern about the high number of early cancers—including ductal carcinoma in situ—that are detected by mammography and treated even though many are unlikely to progress or ever become clinically significant.3 This phenomenon—termed “over-diagnosis”—is one of the risks of breast cancer screening.
Dr. Kaunitz is professor and associate chairman of obstetrics and gynecology at the University of Florida College of Medicine–Jacksonville. He also serves on the OBG Management Board of Editors.
Although the USPSTF is the only official body to revise its recommendations on breast cancer screening so far, more changes seem likely. This article aims to sift through the static on the airwaves of late and offer concrete recommendations for practice. In the process, it addresses seven questions:
- How did USPSTF guidelines change?
- Why did they change?
- Why did the changes attract so much attention?
- What is ACOG’s position?
- What do thought leaders make of the new guidelines?
- Are the USPSTF recommendations likely to affect insurance coverage for mammography?
- What should you tell your patients about breast cancer screening?
1. How did USPSTF guidelines change?
In an article published November 16, the USPSTF made a number of revisions to earlier breast cancer screening guidelines for women at average risk of the disease:

Approximately 39 million women undergo mammography each year in the United States, costing the health-care system more than $5 billion.
- Routine screening mammography is no longer recommended in women 40 to 49 years old. Rather, the decision about when to begin regular screening should be individualized and should “take into account patient context, including the patient’s values regarding specific benefits and harms” (Grade C recommendation).
- Screening mammography in women 50 to 74 years old should be biennial rather than annual (Grade B recommendation).
- Breast self-examination (BSE) is not recommended for any age group (Grade D recommendation).1
2. Why did the USPSTF guidelines change?
The changes were based on new data and analysis in the following areas:
- Mortality among women 40 to 49 years old. Although mammography screening reduces breast cancer mortality by 15% in this age group, the USPSTF concluded that “there is moderate certainty that the net benefit is small” in this population.1,4
- The effectiveness of BSE in decreasing breast cancer mortality among women of any age. Studies of BSE published since 2002 found no significant differences in breast cancer mortality between women who perform BSE and those who don’t.4
- The magnitude of harms of screening with mammography. Mammography screening in women 40 to 49 years old involves a significant risk of harms.4 Although the USPSTF observed that the benefits of mammography in women 40 to 49 years old appear to be equivalent to the benefits of mammography among women 50 to 59 years old, it concluded that the harms outweigh benefits in the younger women.
Harms cited by the USPSTF include:
- radiation exposure
- pain during the procedure
- anxiety and distress
- an increased rate of false-positive results
- greater need for additional imaging and biopsies.4
The USPSTF conceded that the radiation exposure from a mammogram is minimal, but questioned whether cumulative exposure in young women might be problematic. It also noted that “many women experience pain during the procedure (range, 1% to 77%), but few would consider this a deterrent from future screening.”4
As for false-positive results, the group observed: “Data from the [Breast Cancer Screening Consortium (BCSC)] for regularly screened women…indicate that false-positive mammography results are common in all age groups but are most common among women aged 40 to 49 years (97.8 per 1,000 women per screening round).”4
“The BCSC results indicate that for every case of invasive breast cancer detected by mammography screening in women aged 40 to 49 years, 556 women have mammography, 47 have additional imaging, and five have biopsies.”4
It is the significant rate of false positives that creates the need for additional screening, diagnostic imaging, and biopsy. These additional imaging and invasive procedures increase anxiety and distress among many women. The USPSTF concluded that these harms outweighed the benefits of mammography screening in women 40 to 49 years old.
After publication of the new US Preventive Services Task Force (USPSTF) breast cancer screening guidelines late last year, it was only a matter of hours before official bodies and professional organizations began to weigh in on the changes, and the verdict was unanimous—disagreement. Among those chiming in were the American Cancer Society (ACS), the American College of Obstetricians and Gynecologists (ACOG), the American College of Radiology, the American Society of Breast Surgeons, the Society for Breast Imaging (SBI), and Susan G. Komen for the Cure, among others. Here are excerpts from their statements.
American Cancer Society
The ACS immediately refuted the USPSTF recommendations:
The American Cancer Society continues to recommend annual screening using mammography and clinical breast examination for all women beginning at age 40. Our experts make this recommendation having reviewed virtually all the same data reviewed by the USPSTF, but also additional data that the USPSTF did not consider….[T]he American Cancer Society’s medical staff and volunteer experts overwhelmingly believe the benefits of screening women aged 40 to 49 outweigh its limitations.7
ACOG
The College reaffirmed its support for screening mammography every 1 to 2 years in women 40 to 49 years old and every year for women 50 and older, as well as breast self-examination for women of all ages:
At this time, The American College of Obstetricians and Gynecologists recommends that Fellows continue to follow current College guidelines for breast cancer screening. Evaluation of the new USPSTF recommendations is under way. Should the College update its guidelines in the future, Fellows would be alerted and such revised guidelines would be published in Obstetrics & Gynecology.5
American College of Radiology
The College minced no words in opposing the changes:
If cost-cutting US Preventive Services Task Force (USPSTF) mammography recommendations are adopted as policy, two decades of decline in breast cancer mortality could be reversed and countless American women may die needlessly from breast cancer each year.
These new recommendations seem to reflect a conscious decision to ration care. If Medicare and private insurers adopt these incredibly flawed USPSTF recommendations as a rationale for refusing women coverage of these life-saving exams, it could have deadly effects for American women,” said Carol H. Lee, MD, chair of the American College of Radiology Breast Imaging Commission.8
American Society of Breast Surgeons
The organization released a statement describing its position as “strongly opposed” to the USPSTF recommendations:
We believe there is sufficient data to support annual mammography screening for women age 40 and older. We also believe the breast cancer survival rate of women between 40 and 50 will improve from the increased use of digital mammographic screening, which is superior to older plain film techniques in detecting breast cancer in that age group.
While we recognize that there will be a number of benign biopsies, we also recognize that mammography is the optimal screening tool for the early diagnosis of breast cancer in terms of cost-effectiveness, practical use, and accuracy.9
Society for Breast Imaging
In its statement, the SBI noted the confusion caused by revision of the USPSTF guidelines, calling it “unnecessary and potentially deadly”:
Mammography has been shown unequivocally to save lives and is primarily responsible for the 30% decline in breast cancer mortality in the United States over the past 20 years. The USPSTF conclusion—that women under age 50 should not undergo routine screening—conflicts with their own report, which confirms a benefit of mammography to women age 40–49 that is statistically significant.
We strongly urge women and their physicians to adhere to the American Cancer Society recommendations of yearly screening beginning at age 40.10
Susan G. Komen for the Cure
This public advocacy group issued a statement in late November acknowledging “mass confusion and justifiable outrage” in the aftermath of the USPSTF changes:
”We have worked so hard to build public trust and urge people to get screened,” said Nancy G. Brinker, founder of Susan G. Komen for the Cure, “and now they hear that maybe they shouldn’t bother. That is dangerous….Let me say this as clearly as I can: Mammography saves lives, even this report says that. Keep doing what you are doing. And always, talk with your doctor.” Brinker also noted that Komen for the Cure was not changing its guidelines, continuing to recommend annual mammograms beginning at age 40.11
3. Why have the guidelines captured so much media attention?
Most of the controversy that has arisen since publication of the new guidelines has centered on the recommendation against screening mammography in women 40 to 49 years old. A number of media outlets have highlighted women whose breast cancer was detected by screening mammography when they were in their 40s, and many survivors with a similar history have spoken out against the new recommendations.
In addition, the American Cancer Society (ACS), the American College of Radiology, Susan G. Komen for the Cure, and other groups have publicly opposed the new guidelines. (See “Among professional organizations, a resounding chorus of disagreement”)
4. What is ACOG’s position on the new recommendations?
The American College of Obstetricians and Gynecologists (ACOG) was quick to weigh in on the new USPSTF guidelines, emphasizing that the College’s recommendations have not changed. They include:
- screening mammography every 1 to 2 years for women 40 to 49 years old
- screening mammography every year for women 50 years and older
- BSE for all women.
ACOG did note, however, that “the College is continuing to evaluate in detail the new USPSTF recommendations and the new evidence considered by the USPSTF.”5
5. What do thought leaders make of the USPSTF changes?
Although the USPSTF guidelines sparked a firestorm of media coverage, the change did not come as a shock to leaders in the ObGyn specialty.

Legitimate concerns about screening mammography have increasingly been raised by experts in the field.
ANDREW M. KAUNITZ, MD “I was not surprised,” said Dr. Kaunitz. “As I pointed out in my editorial in OBG Management, legitimate concerns about screening mammography have increasingly been raised by experts in the field.2 Proposals to stop routinely screening women in their 40s were made earlier in this decade, but were met with major pushback from the ACS, breast cancer advocacy organizations, and medical specialty groups. These same groups are now pushing back against the new USPSTF guidelines,” he added.
Robert L. Barbieri, MD, was not taken aback by the guidelines themselves, but he was surprised by the manner and timing of their release. Dr. Barbieri is Kate Macy Ladd professor of obstetrics, gynecology, and reproductive biology at Harvard Medical School and chief of obstetrics and gynecology at Brigham and Women’s Hospital in Boston. He serves as editor-in-chief of OBG Management.
“I was surprised that the USPSTF did not weigh the potential impact of its analysis on the key stakeholders: patients, disease-based coalitions such as the American Cancer Society and Susan G. Komen for the Cure, and professional societies such as the American College of Radiology and ACOG,” he said. “If I were supervising the process, I would have asked for a comment period before releasing the report. I would have included the comments from key stakeholders in an appendix to the report.”
Are other organizations—besides the USPSTF—likely to change their recommendations for mammography screening in the near future? In the case of ACOG, Dr. Barbieri doesn’t think so.
“I don’t think ACOG will change the age at which to initiate screening,” he said. “I believe it will stick to its recommendation to start screening at 40 and continue every 1 to 2 years from 40 to 50 years of age. However, I could see ACOG becoming a bit more flexible on the question of whether screening should take place at 1- or 2-year intervals after age 50.”
Dr. Kaunitz sees things differently.
“It seems possible that, going forward, the College will give Fellows and their patients permission to implement the new guidelines without mandating their implementation. For example, if women in their 40s wish to defer screening, that would be OK, as would biennial screening for women in their 50s and 60s.”
6. Are the USPSTF recommendations likely to affect insurance coverage?
In a press release issued soon after the new guidelines were published, US Health and Human Services Secretary Kathleen Sebelius addressed Americans directly to reaffirm her support for mammography in women 40 to 49 years old: “There is no question that the US Preventive Services Task Force recommendations have caused a great deal of confusion and worry among women and their families,” her statement read.6 She made it clear that the new recommendations are unlikely to affect federal coverage of mammography.
“The US Preventive Services Task Force is an outside independent panel of doctors and scientists who make recommendations. They do not set federal policy and they don’t determine what services are covered by the federal government,” she said.6
But Dr. Barbieri thinks some changes in insurance coverage are inevitable.
“Any claims that the new guidelines do not represent a major change would be disingenuous,” he said. Because the USPSTF rated its recommendation against mammography for women 40 to 49 years old as grade ‘C,’ that change in guidelines is likely to trigger at least some change in coverage.
“In reality, the ‘C’ rating will require many insurance companies—by their own rule—to stop reimbursing for this screening test,” he said. “The ‘C’ rating means that the test has little benefit.”
ACOG also deems it likely that insurance coverage may be affected for some women.
“Fellows should be aware that the new USPSTF recommendations against routine screening mammography for women aged 40–49 (a grade C recommendation) has implications for insurance coverage, as some insurers will cover only preventive services rated as an ‘A’ or a ‘B’ by the USPSTF. Fellows should counsel their patients that insurance coverage for ‘routine screening’ mammography may become variable and that patients should address this question with their insurers. These recommendations do not apply to high-risk women or patients with clinical findings, and they should be managed accordingly.”5
7. What should you tell your patients?
With all the media attention devoted to the change in guidelines, it’s little surprise that patients are asking questions.
“Patients are aware of the USPSTF report,” said Dr. Barbieri. “They are largely ignoring the recommendations and sticking with annual mammograms.”
“I think, as always, women are looking to their ObGyn for guidance,” added Dr. Kaunitz.
So what are these clinicians telling patients about mammography screening?
As he was to begin with, Dr. Kaunitz is acquiescent if patients prefer to defer mammography screening to their 50s.
“Because it seems that insurance coverage, over the short term, is unlikely to restrict current access to mammograms,” said Dr. Kaunitz, “my evolving philosophy is that the new USPSTF guidelines, along with ACOG and other existing guidelines, give ObGyns and their patients permission to:
- proceed or not proceed with mammograms for women in their 40s, with the decision based on issues such as patient preference, family history of breast cancer, and body mass index (BMI)
- be flexible regarding 1- to 2-year screening intervals among women in their 50s, 60s, and 70s, with the decision based on issues such as patient preference, use or non-use of estrogen-progestin hormone therapy, family history of breast cancer, and BMI.”
Dr. Barbieri believes some effort to integrate the ACOG and USPSTF recommendations is called for. “Accordingly,” he said, “I suggest the following:

I suggest actively recommending biennial mammography for women 40 to 75 years old. Offer annual mammography to women 40 to 75 years old if they prefer that option.
ROBERT L. BARBIERI, MD
- Actively recommend biennial mammography for women 40 to 75 years old. Offer annual mammography to women 40 to 75 years old if they prefer that option.
- Aggressively search for high-risk women, with high risk defined as a lifetime risk of breast cancer exceeding 15%. Among the variables contributing to high-risk status are a history of thoracic radiotherapy, a strong family history of breast cancer, and BRCA mutation. For these women, I would recommend annual mammography and biennial MRI of the breasts.
- Perform annual or biennial clinical breast exam.
- Obtain imaging for any woman who has a palpable breast lump, and resect or biopsy the lump even if that imaging is negative.”
Some clinicians were reconsidering the need for an annual mammogram even before the US Preventive Services Task Force (USPSTF) issued new guidelines late last year.1
Andrew M. Kaunitz, MD, is one of those clinicians. In an editorial in the December issue of OBG Management, he was bold enough to declare: “My plan is to be more acquiescent when a woman says ‘No’ to an annual mammogram.”2
Among the evidence he cited to justify that acquiescence was a recent article in the Journal of the American Medical Association that expressed concern about the high number of early cancers—including ductal carcinoma in situ—that are detected by mammography and treated even though many are unlikely to progress or ever become clinically significant.3 This phenomenon—termed “over-diagnosis”—is one of the risks of breast cancer screening.
Dr. Kaunitz is professor and associate chairman of obstetrics and gynecology at the University of Florida College of Medicine–Jacksonville. He also serves on the OBG Management Board of Editors.
Although the USPSTF is the only official body to revise its recommendations on breast cancer screening so far, more changes seem likely. This article aims to sift through the static on the airwaves of late and offer concrete recommendations for practice. In the process, it addresses seven questions:
- How did USPSTF guidelines change?
- Why did they change?
- Why did the changes attract so much attention?
- What is ACOG’s position?
- What do thought leaders make of the new guidelines?
- Are the USPSTF recommendations likely to affect insurance coverage for mammography?
- What should you tell your patients about breast cancer screening?
1. How did USPSTF guidelines change?
In an article published November 16, the USPSTF made a number of revisions to earlier breast cancer screening guidelines for women at average risk of the disease:

Approximately 39 million women undergo mammography each year in the United States, costing the health-care system more than $5 billion.
- Routine screening mammography is no longer recommended in women 40 to 49 years old. Rather, the decision about when to begin regular screening should be individualized and should “take into account patient context, including the patient’s values regarding specific benefits and harms” (Grade C recommendation).
- Screening mammography in women 50 to 74 years old should be biennial rather than annual (Grade B recommendation).
- Breast self-examination (BSE) is not recommended for any age group (Grade D recommendation).1
2. Why did the USPSTF guidelines change?
The changes were based on new data and analysis in the following areas:
- Mortality among women 40 to 49 years old. Although mammography screening reduces breast cancer mortality by 15% in this age group, the USPSTF concluded that “there is moderate certainty that the net benefit is small” in this population.1,4
- The effectiveness of BSE in decreasing breast cancer mortality among women of any age. Studies of BSE published since 2002 found no significant differences in breast cancer mortality between women who perform BSE and those who don’t.4
- The magnitude of harms of screening with mammography. Mammography screening in women 40 to 49 years old involves a significant risk of harms.4 Although the USPSTF observed that the benefits of mammography in women 40 to 49 years old appear to be equivalent to the benefits of mammography among women 50 to 59 years old, it concluded that the harms outweigh benefits in the younger women.
Harms cited by the USPSTF include:
- radiation exposure
- pain during the procedure
- anxiety and distress
- an increased rate of false-positive results
- greater need for additional imaging and biopsies.4
The USPSTF conceded that the radiation exposure from a mammogram is minimal, but questioned whether cumulative exposure in young women might be problematic. It also noted that “many women experience pain during the procedure (range, 1% to 77%), but few would consider this a deterrent from future screening.”4
As for false-positive results, the group observed: “Data from the [Breast Cancer Screening Consortium (BCSC)] for regularly screened women…indicate that false-positive mammography results are common in all age groups but are most common among women aged 40 to 49 years (97.8 per 1,000 women per screening round).”4
“The BCSC results indicate that for every case of invasive breast cancer detected by mammography screening in women aged 40 to 49 years, 556 women have mammography, 47 have additional imaging, and five have biopsies.”4
It is the significant rate of false positives that creates the need for additional screening, diagnostic imaging, and biopsy. These additional imaging and invasive procedures increase anxiety and distress among many women. The USPSTF concluded that these harms outweighed the benefits of mammography screening in women 40 to 49 years old.
After publication of the new US Preventive Services Task Force (USPSTF) breast cancer screening guidelines late last year, it was only a matter of hours before official bodies and professional organizations began to weigh in on the changes, and the verdict was unanimous—disagreement. Among those chiming in were the American Cancer Society (ACS), the American College of Obstetricians and Gynecologists (ACOG), the American College of Radiology, the American Society of Breast Surgeons, the Society for Breast Imaging (SBI), and Susan G. Komen for the Cure, among others. Here are excerpts from their statements.
American Cancer Society
The ACS immediately refuted the USPSTF recommendations:
The American Cancer Society continues to recommend annual screening using mammography and clinical breast examination for all women beginning at age 40. Our experts make this recommendation having reviewed virtually all the same data reviewed by the USPSTF, but also additional data that the USPSTF did not consider….[T]he American Cancer Society’s medical staff and volunteer experts overwhelmingly believe the benefits of screening women aged 40 to 49 outweigh its limitations.7
ACOG
The College reaffirmed its support for screening mammography every 1 to 2 years in women 40 to 49 years old and every year for women 50 and older, as well as breast self-examination for women of all ages:
At this time, The American College of Obstetricians and Gynecologists recommends that Fellows continue to follow current College guidelines for breast cancer screening. Evaluation of the new USPSTF recommendations is under way. Should the College update its guidelines in the future, Fellows would be alerted and such revised guidelines would be published in Obstetrics & Gynecology.5
American College of Radiology
The College minced no words in opposing the changes:
If cost-cutting US Preventive Services Task Force (USPSTF) mammography recommendations are adopted as policy, two decades of decline in breast cancer mortality could be reversed and countless American women may die needlessly from breast cancer each year.
These new recommendations seem to reflect a conscious decision to ration care. If Medicare and private insurers adopt these incredibly flawed USPSTF recommendations as a rationale for refusing women coverage of these life-saving exams, it could have deadly effects for American women,” said Carol H. Lee, MD, chair of the American College of Radiology Breast Imaging Commission.8
American Society of Breast Surgeons
The organization released a statement describing its position as “strongly opposed” to the USPSTF recommendations:
We believe there is sufficient data to support annual mammography screening for women age 40 and older. We also believe the breast cancer survival rate of women between 40 and 50 will improve from the increased use of digital mammographic screening, which is superior to older plain film techniques in detecting breast cancer in that age group.
While we recognize that there will be a number of benign biopsies, we also recognize that mammography is the optimal screening tool for the early diagnosis of breast cancer in terms of cost-effectiveness, practical use, and accuracy.9
Society for Breast Imaging
In its statement, the SBI noted the confusion caused by revision of the USPSTF guidelines, calling it “unnecessary and potentially deadly”:
Mammography has been shown unequivocally to save lives and is primarily responsible for the 30% decline in breast cancer mortality in the United States over the past 20 years. The USPSTF conclusion—that women under age 50 should not undergo routine screening—conflicts with their own report, which confirms a benefit of mammography to women age 40–49 that is statistically significant.
We strongly urge women and their physicians to adhere to the American Cancer Society recommendations of yearly screening beginning at age 40.10
Susan G. Komen for the Cure
This public advocacy group issued a statement in late November acknowledging “mass confusion and justifiable outrage” in the aftermath of the USPSTF changes:
”We have worked so hard to build public trust and urge people to get screened,” said Nancy G. Brinker, founder of Susan G. Komen for the Cure, “and now they hear that maybe they shouldn’t bother. That is dangerous….Let me say this as clearly as I can: Mammography saves lives, even this report says that. Keep doing what you are doing. And always, talk with your doctor.” Brinker also noted that Komen for the Cure was not changing its guidelines, continuing to recommend annual mammograms beginning at age 40.11
3. Why have the guidelines captured so much media attention?
Most of the controversy that has arisen since publication of the new guidelines has centered on the recommendation against screening mammography in women 40 to 49 years old. A number of media outlets have highlighted women whose breast cancer was detected by screening mammography when they were in their 40s, and many survivors with a similar history have spoken out against the new recommendations.
In addition, the American Cancer Society (ACS), the American College of Radiology, Susan G. Komen for the Cure, and other groups have publicly opposed the new guidelines. (See “Among professional organizations, a resounding chorus of disagreement”)
4. What is ACOG’s position on the new recommendations?
The American College of Obstetricians and Gynecologists (ACOG) was quick to weigh in on the new USPSTF guidelines, emphasizing that the College’s recommendations have not changed. They include:
- screening mammography every 1 to 2 years for women 40 to 49 years old
- screening mammography every year for women 50 years and older
- BSE for all women.
ACOG did note, however, that “the College is continuing to evaluate in detail the new USPSTF recommendations and the new evidence considered by the USPSTF.”5
5. What do thought leaders make of the USPSTF changes?
Although the USPSTF guidelines sparked a firestorm of media coverage, the change did not come as a shock to leaders in the ObGyn specialty.

Legitimate concerns about screening mammography have increasingly been raised by experts in the field.
ANDREW M. KAUNITZ, MD “I was not surprised,” said Dr. Kaunitz. “As I pointed out in my editorial in OBG Management, legitimate concerns about screening mammography have increasingly been raised by experts in the field.2 Proposals to stop routinely screening women in their 40s were made earlier in this decade, but were met with major pushback from the ACS, breast cancer advocacy organizations, and medical specialty groups. These same groups are now pushing back against the new USPSTF guidelines,” he added.
Robert L. Barbieri, MD, was not taken aback by the guidelines themselves, but he was surprised by the manner and timing of their release. Dr. Barbieri is Kate Macy Ladd professor of obstetrics, gynecology, and reproductive biology at Harvard Medical School and chief of obstetrics and gynecology at Brigham and Women’s Hospital in Boston. He serves as editor-in-chief of OBG Management.
“I was surprised that the USPSTF did not weigh the potential impact of its analysis on the key stakeholders: patients, disease-based coalitions such as the American Cancer Society and Susan G. Komen for the Cure, and professional societies such as the American College of Radiology and ACOG,” he said. “If I were supervising the process, I would have asked for a comment period before releasing the report. I would have included the comments from key stakeholders in an appendix to the report.”
Are other organizations—besides the USPSTF—likely to change their recommendations for mammography screening in the near future? In the case of ACOG, Dr. Barbieri doesn’t think so.
“I don’t think ACOG will change the age at which to initiate screening,” he said. “I believe it will stick to its recommendation to start screening at 40 and continue every 1 to 2 years from 40 to 50 years of age. However, I could see ACOG becoming a bit more flexible on the question of whether screening should take place at 1- or 2-year intervals after age 50.”
Dr. Kaunitz sees things differently.
“It seems possible that, going forward, the College will give Fellows and their patients permission to implement the new guidelines without mandating their implementation. For example, if women in their 40s wish to defer screening, that would be OK, as would biennial screening for women in their 50s and 60s.”
6. Are the USPSTF recommendations likely to affect insurance coverage?
In a press release issued soon after the new guidelines were published, US Health and Human Services Secretary Kathleen Sebelius addressed Americans directly to reaffirm her support for mammography in women 40 to 49 years old: “There is no question that the US Preventive Services Task Force recommendations have caused a great deal of confusion and worry among women and their families,” her statement read.6 She made it clear that the new recommendations are unlikely to affect federal coverage of mammography.
“The US Preventive Services Task Force is an outside independent panel of doctors and scientists who make recommendations. They do not set federal policy and they don’t determine what services are covered by the federal government,” she said.6
But Dr. Barbieri thinks some changes in insurance coverage are inevitable.
“Any claims that the new guidelines do not represent a major change would be disingenuous,” he said. Because the USPSTF rated its recommendation against mammography for women 40 to 49 years old as grade ‘C,’ that change in guidelines is likely to trigger at least some change in coverage.
“In reality, the ‘C’ rating will require many insurance companies—by their own rule—to stop reimbursing for this screening test,” he said. “The ‘C’ rating means that the test has little benefit.”
ACOG also deems it likely that insurance coverage may be affected for some women.
“Fellows should be aware that the new USPSTF recommendations against routine screening mammography for women aged 40–49 (a grade C recommendation) has implications for insurance coverage, as some insurers will cover only preventive services rated as an ‘A’ or a ‘B’ by the USPSTF. Fellows should counsel their patients that insurance coverage for ‘routine screening’ mammography may become variable and that patients should address this question with their insurers. These recommendations do not apply to high-risk women or patients with clinical findings, and they should be managed accordingly.”5
7. What should you tell your patients?
With all the media attention devoted to the change in guidelines, it’s little surprise that patients are asking questions.
“Patients are aware of the USPSTF report,” said Dr. Barbieri. “They are largely ignoring the recommendations and sticking with annual mammograms.”
“I think, as always, women are looking to their ObGyn for guidance,” added Dr. Kaunitz.
So what are these clinicians telling patients about mammography screening?
As he was to begin with, Dr. Kaunitz is acquiescent if patients prefer to defer mammography screening to their 50s.
“Because it seems that insurance coverage, over the short term, is unlikely to restrict current access to mammograms,” said Dr. Kaunitz, “my evolving philosophy is that the new USPSTF guidelines, along with ACOG and other existing guidelines, give ObGyns and their patients permission to:
- proceed or not proceed with mammograms for women in their 40s, with the decision based on issues such as patient preference, family history of breast cancer, and body mass index (BMI)
- be flexible regarding 1- to 2-year screening intervals among women in their 50s, 60s, and 70s, with the decision based on issues such as patient preference, use or non-use of estrogen-progestin hormone therapy, family history of breast cancer, and BMI.”
Dr. Barbieri believes some effort to integrate the ACOG and USPSTF recommendations is called for. “Accordingly,” he said, “I suggest the following:

I suggest actively recommending biennial mammography for women 40 to 75 years old. Offer annual mammography to women 40 to 75 years old if they prefer that option.
ROBERT L. BARBIERI, MD
- Actively recommend biennial mammography for women 40 to 75 years old. Offer annual mammography to women 40 to 75 years old if they prefer that option.
- Aggressively search for high-risk women, with high risk defined as a lifetime risk of breast cancer exceeding 15%. Among the variables contributing to high-risk status are a history of thoracic radiotherapy, a strong family history of breast cancer, and BRCA mutation. For these women, I would recommend annual mammography and biennial MRI of the breasts.
- Perform annual or biennial clinical breast exam.
- Obtain imaging for any woman who has a palpable breast lump, and resect or biopsy the lump even if that imaging is negative.”
1. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151:716-726.
2. Kaunitz AM. I’ve been rethinking my zeal for breast cancer screening. OBG Management. 2009;21(12):6-8.
3. Esserman L, Shieh Y, Thompson I. Rethinking screening for breast cancer and prostate cancer. JAMA. 2009;302:1685-1692.
4. Nelson HD, Tyne K, Nalk A, Bougatsos C, Chan BK, Humphrey L. Screening for breast cancer: an update for the US Preventive Services Task Force. Ann Intern Med. 2009;151:727-737.
5. American College of Obstetricians and Gynecologists. Response of the American College of Obstetricians and Gynecologists to new breast cancer screening recommendations from the US Preventive Services Task Force. Available at: http://www.acog.org/from_home/Misc/uspstfResponse.cfm. Accessed Nov. 25, 2009.
6. US Department of Health and Human Services. Secretary Sebelius statement on new breast cancer recommendations [news release]. Nov. 18, 2009. Available at: http://www.hhs.gov/news/press/2009pres/11/20091118a.html. Accessed Dec. 4, 2009.
7. American Cancer Society responds to changes to USPSTF mammography guidelines [news release]. American Cancer Society. Nov. 16, 2009. Available at: http://www.cancer.org/docroot/MED/content/MED_2_1x_American_Cancer_Society_
Responds_to_Changes_to_USPSTF_Mammography_Guidelines.asp. Accessed Dec. 4, 2009.
8. American College of Radiology. USPSTF mammography recommendations will result in countless unnecessary breast cancer deaths each year [news release]. Nov. 16, 2009. Available at: www.acr.org/MainMenuCategories/media_room/FeaturedCategories/PressReleases/USPSTFMammoRecs.aspx. Accessed Dec. 4, 2009.
9. American Society of Breast Surgeons. Society responds to USPSTF changes in mammography guidelines [news release]. Available at: http://www.breastsurgeons.org/news/article.php?id=57. Accessed Dec. 4, 2009.
10. Society of Breast Imaging. Official Society of Breast Imaging response to the announcement by HHS Secretary Sebelius regarding USPSTF mammography recommendations. Available at:http://www.sbi-online.org/associations/8199/files/OFFICIAL%20SOCIETY%20OF%20
BREAST%20IMAGING%20RESPONSE%20TO%20THE%20ANNOUNCEMENT%20
BY%20HHS%20SECRETARY%20
SEBELIUS%20REGARDING%20USPSTF%20MAMMOGRAPHY%20RECOMMENDATIONS.pdf. Accessed Dec. 4, 2009.
11. Susan G. Komen for the Cure founder, Nancy G. Brinker, calls new mammography guidelines a “set back”; makes call to action [news release]. Nov. 23, 2009. Available at: http://ww5.komen.org/KomenNewsArticle.aspx?id=6442451516. Accessed Dec. 7, 2009.
1. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151:716-726.
2. Kaunitz AM. I’ve been rethinking my zeal for breast cancer screening. OBG Management. 2009;21(12):6-8.
3. Esserman L, Shieh Y, Thompson I. Rethinking screening for breast cancer and prostate cancer. JAMA. 2009;302:1685-1692.
4. Nelson HD, Tyne K, Nalk A, Bougatsos C, Chan BK, Humphrey L. Screening for breast cancer: an update for the US Preventive Services Task Force. Ann Intern Med. 2009;151:727-737.
5. American College of Obstetricians and Gynecologists. Response of the American College of Obstetricians and Gynecologists to new breast cancer screening recommendations from the US Preventive Services Task Force. Available at: http://www.acog.org/from_home/Misc/uspstfResponse.cfm. Accessed Nov. 25, 2009.
6. US Department of Health and Human Services. Secretary Sebelius statement on new breast cancer recommendations [news release]. Nov. 18, 2009. Available at: http://www.hhs.gov/news/press/2009pres/11/20091118a.html. Accessed Dec. 4, 2009.
7. American Cancer Society responds to changes to USPSTF mammography guidelines [news release]. American Cancer Society. Nov. 16, 2009. Available at: http://www.cancer.org/docroot/MED/content/MED_2_1x_American_Cancer_Society_
Responds_to_Changes_to_USPSTF_Mammography_Guidelines.asp. Accessed Dec. 4, 2009.
8. American College of Radiology. USPSTF mammography recommendations will result in countless unnecessary breast cancer deaths each year [news release]. Nov. 16, 2009. Available at: www.acr.org/MainMenuCategories/media_room/FeaturedCategories/PressReleases/USPSTFMammoRecs.aspx. Accessed Dec. 4, 2009.
9. American Society of Breast Surgeons. Society responds to USPSTF changes in mammography guidelines [news release]. Available at: http://www.breastsurgeons.org/news/article.php?id=57. Accessed Dec. 4, 2009.
10. Society of Breast Imaging. Official Society of Breast Imaging response to the announcement by HHS Secretary Sebelius regarding USPSTF mammography recommendations. Available at:http://www.sbi-online.org/associations/8199/files/OFFICIAL%20SOCIETY%20OF%20
BREAST%20IMAGING%20RESPONSE%20TO%20THE%20ANNOUNCEMENT%20
BY%20HHS%20SECRETARY%20
SEBELIUS%20REGARDING%20USPSTF%20MAMMOGRAPHY%20RECOMMENDATIONS.pdf. Accessed Dec. 4, 2009.
11. Susan G. Komen for the Cure founder, Nancy G. Brinker, calls new mammography guidelines a “set back”; makes call to action [news release]. Nov. 23, 2009. Available at: http://ww5.komen.org/KomenNewsArticle.aspx?id=6442451516. Accessed Dec. 7, 2009.


