User login
Doug Brunk is a San Diego-based award-winning reporter who began covering health care in 1991. Before joining the company, he wrote for the health sciences division of Columbia University and was an associate editor at Contemporary Long Term Care magazine when it won a Jesse H. Neal Award. His work has been syndicated by the Los Angeles Times and he is the author of two books related to the University of Kentucky Wildcats men's basketball program. Doug has a master’s degree in magazine journalism from the S.I. Newhouse School of Public Communications at Syracuse University. Follow him on Twitter @dougbrunk.
Is Atopic Dermatitis Linked to Cognitive Impairment Symptoms in Children?
TOPLINE:
METHODOLOGY:
It remains unknown whether subpopulations of children with atopic dermatitis face a greater risk for cognitive impairment or not.
To determine the association, researchers drew from a weighted sample of 69,732,807 children with atopic dermatitis in the 2021 US National Health Interview Survey.
Main outcomes of interest were difficulty in learning or memory (cognitive impairment symptoms) as reported by the child’s caregiver.
The researchers performed logistic regression to compare the odds of learning or memory difficulties between 60,509,794 children without atopic dermatitis and 9,223,013 children with atopic dermatitis.
TAKEAWAY:
Children with versus without atopic dermatitis were more likely to experience difficulties with learning (10.8% [95% CI, 7.8%-15.8%] vs 5.9% [95% CI, 5.1%-6.9%]; P < .001) and difficulties with memory (11.1% [95% CI, 8.0%-15.9%] vs 5.8% [95% CI, 4.9%-6.9%]; P < .001).
On multivariable logistic regression adjusted for sociodemographic factors, asthma, food allergies, and seasonal allergies or hay fever, researchers found that having atopic dermatitis was associated with increased odds of difficulties in learning (adjusted odds ratio [aOR], 1.77; 95% CI, 1.28-2.45) and memory (aOR, 1.69; 95% CI, 1.19-2.41).
When stratified by neurodevelopmental comorbidities, having atopic dermatitis was associated with a 2- to 3-fold greater odds of memory difficulties among children with any neurodevelopmental disorder (aOR, 2.26; 95% CI, 1.43-3.57), which included ADHD (aOR, 2.90; 95% CI, 1.60-5.24) or learning disabilities (aOR, 2.04; 95% CI, 1.04-4.00).
Having atopic dermatitis was not associated with learning or memory difficulties among children without neurodevelopmental conditions.
IN PRACTICE:
“These findings may improve the risk stratification of children with atopic dermatitis for cognitive impairment and suggest that evaluation for cognitive impairment should be prioritized among children with atopic dermatitis and comorbid ADHD or learning disability,” the authors wrote.
SOURCE:
Corresponding author Joy Wan, MD, of the department of dermatology at Johns Hopkins University School of Medicine, Baltimore, and colleagues conducted the research, which was published on March 6, 2024, in JAMA Dermatology.
LIMITATIONS:
The study’s limitations were its cross-sectional design, reliance on caregiver reports, and the fact that National Health Interview Survey data do not include information on factors such as atopic dermatitis severity, age at atopic dermatitis diagnosis, and sleep.
DISCLOSURES:
The study was supported by a grant from the National Institutes of Health. Dr. Wan reported receiving a grant from Pfizer and personal fees from Sun Pharmaceutical Industries and Janssen Pharmaceuticals outside the submitted work. No other study authors had disclosures to report.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
It remains unknown whether subpopulations of children with atopic dermatitis face a greater risk for cognitive impairment or not.
To determine the association, researchers drew from a weighted sample of 69,732,807 children with atopic dermatitis in the 2021 US National Health Interview Survey.
Main outcomes of interest were difficulty in learning or memory (cognitive impairment symptoms) as reported by the child’s caregiver.
The researchers performed logistic regression to compare the odds of learning or memory difficulties between 60,509,794 children without atopic dermatitis and 9,223,013 children with atopic dermatitis.
TAKEAWAY:
Children with versus without atopic dermatitis were more likely to experience difficulties with learning (10.8% [95% CI, 7.8%-15.8%] vs 5.9% [95% CI, 5.1%-6.9%]; P < .001) and difficulties with memory (11.1% [95% CI, 8.0%-15.9%] vs 5.8% [95% CI, 4.9%-6.9%]; P < .001).
On multivariable logistic regression adjusted for sociodemographic factors, asthma, food allergies, and seasonal allergies or hay fever, researchers found that having atopic dermatitis was associated with increased odds of difficulties in learning (adjusted odds ratio [aOR], 1.77; 95% CI, 1.28-2.45) and memory (aOR, 1.69; 95% CI, 1.19-2.41).
When stratified by neurodevelopmental comorbidities, having atopic dermatitis was associated with a 2- to 3-fold greater odds of memory difficulties among children with any neurodevelopmental disorder (aOR, 2.26; 95% CI, 1.43-3.57), which included ADHD (aOR, 2.90; 95% CI, 1.60-5.24) or learning disabilities (aOR, 2.04; 95% CI, 1.04-4.00).
Having atopic dermatitis was not associated with learning or memory difficulties among children without neurodevelopmental conditions.
IN PRACTICE:
“These findings may improve the risk stratification of children with atopic dermatitis for cognitive impairment and suggest that evaluation for cognitive impairment should be prioritized among children with atopic dermatitis and comorbid ADHD or learning disability,” the authors wrote.
SOURCE:
Corresponding author Joy Wan, MD, of the department of dermatology at Johns Hopkins University School of Medicine, Baltimore, and colleagues conducted the research, which was published on March 6, 2024, in JAMA Dermatology.
LIMITATIONS:
The study’s limitations were its cross-sectional design, reliance on caregiver reports, and the fact that National Health Interview Survey data do not include information on factors such as atopic dermatitis severity, age at atopic dermatitis diagnosis, and sleep.
DISCLOSURES:
The study was supported by a grant from the National Institutes of Health. Dr. Wan reported receiving a grant from Pfizer and personal fees from Sun Pharmaceutical Industries and Janssen Pharmaceuticals outside the submitted work. No other study authors had disclosures to report.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
It remains unknown whether subpopulations of children with atopic dermatitis face a greater risk for cognitive impairment or not.
To determine the association, researchers drew from a weighted sample of 69,732,807 children with atopic dermatitis in the 2021 US National Health Interview Survey.
Main outcomes of interest were difficulty in learning or memory (cognitive impairment symptoms) as reported by the child’s caregiver.
The researchers performed logistic regression to compare the odds of learning or memory difficulties between 60,509,794 children without atopic dermatitis and 9,223,013 children with atopic dermatitis.
TAKEAWAY:
Children with versus without atopic dermatitis were more likely to experience difficulties with learning (10.8% [95% CI, 7.8%-15.8%] vs 5.9% [95% CI, 5.1%-6.9%]; P < .001) and difficulties with memory (11.1% [95% CI, 8.0%-15.9%] vs 5.8% [95% CI, 4.9%-6.9%]; P < .001).
On multivariable logistic regression adjusted for sociodemographic factors, asthma, food allergies, and seasonal allergies or hay fever, researchers found that having atopic dermatitis was associated with increased odds of difficulties in learning (adjusted odds ratio [aOR], 1.77; 95% CI, 1.28-2.45) and memory (aOR, 1.69; 95% CI, 1.19-2.41).
When stratified by neurodevelopmental comorbidities, having atopic dermatitis was associated with a 2- to 3-fold greater odds of memory difficulties among children with any neurodevelopmental disorder (aOR, 2.26; 95% CI, 1.43-3.57), which included ADHD (aOR, 2.90; 95% CI, 1.60-5.24) or learning disabilities (aOR, 2.04; 95% CI, 1.04-4.00).
Having atopic dermatitis was not associated with learning or memory difficulties among children without neurodevelopmental conditions.
IN PRACTICE:
“These findings may improve the risk stratification of children with atopic dermatitis for cognitive impairment and suggest that evaluation for cognitive impairment should be prioritized among children with atopic dermatitis and comorbid ADHD or learning disability,” the authors wrote.
SOURCE:
Corresponding author Joy Wan, MD, of the department of dermatology at Johns Hopkins University School of Medicine, Baltimore, and colleagues conducted the research, which was published on March 6, 2024, in JAMA Dermatology.
LIMITATIONS:
The study’s limitations were its cross-sectional design, reliance on caregiver reports, and the fact that National Health Interview Survey data do not include information on factors such as atopic dermatitis severity, age at atopic dermatitis diagnosis, and sleep.
DISCLOSURES:
The study was supported by a grant from the National Institutes of Health. Dr. Wan reported receiving a grant from Pfizer and personal fees from Sun Pharmaceutical Industries and Janssen Pharmaceuticals outside the submitted work. No other study authors had disclosures to report.
A version of this article appeared on Medscape.com.
Another Neurotoxin for Frown Lines Enters the Market
The Food and Drug Administration (FDA) has approved letibotulinumtoxinA-wlbg, an injectable neurotoxin long used in South Korea for the treatment of moderate to severe glabellar (frown) lines in adults. Developed by Hugel, the product is being marketed under the brand name Letybo.
The FDA’s approval was based on positive results from three phase 3 trials of letibotulinumtoxinA-wlbg that enrolled more than 1000 individuals in the United States and Europe. According to information in the package insert, the most common adverse reaction reported in the trials was headache, which occurred in 2% of trial participants. Other adverse events reported by fewer than 1% of trial participants included brow ptosis, eyelid ptosis, and blepharospasm, while the most frequently reported injection site reactions included administrative site swelling, facial pain, folliculitis, and periorbital hematoma.
According to a press release from the company, letibotulinumtoxinA-wlbg has been the leading neurotoxin brand in South Korea for 7 consecutive years, and the product has been sold in more than 50 different countries. Hugel plans to launch Letybo for US-based aesthetic clinicians in the latter half of 2024.
A version of this article appeared on Medscape.com.
The Food and Drug Administration (FDA) has approved letibotulinumtoxinA-wlbg, an injectable neurotoxin long used in South Korea for the treatment of moderate to severe glabellar (frown) lines in adults. Developed by Hugel, the product is being marketed under the brand name Letybo.
The FDA’s approval was based on positive results from three phase 3 trials of letibotulinumtoxinA-wlbg that enrolled more than 1000 individuals in the United States and Europe. According to information in the package insert, the most common adverse reaction reported in the trials was headache, which occurred in 2% of trial participants. Other adverse events reported by fewer than 1% of trial participants included brow ptosis, eyelid ptosis, and blepharospasm, while the most frequently reported injection site reactions included administrative site swelling, facial pain, folliculitis, and periorbital hematoma.
According to a press release from the company, letibotulinumtoxinA-wlbg has been the leading neurotoxin brand in South Korea for 7 consecutive years, and the product has been sold in more than 50 different countries. Hugel plans to launch Letybo for US-based aesthetic clinicians in the latter half of 2024.
A version of this article appeared on Medscape.com.
The Food and Drug Administration (FDA) has approved letibotulinumtoxinA-wlbg, an injectable neurotoxin long used in South Korea for the treatment of moderate to severe glabellar (frown) lines in adults. Developed by Hugel, the product is being marketed under the brand name Letybo.
The FDA’s approval was based on positive results from three phase 3 trials of letibotulinumtoxinA-wlbg that enrolled more than 1000 individuals in the United States and Europe. According to information in the package insert, the most common adverse reaction reported in the trials was headache, which occurred in 2% of trial participants. Other adverse events reported by fewer than 1% of trial participants included brow ptosis, eyelid ptosis, and blepharospasm, while the most frequently reported injection site reactions included administrative site swelling, facial pain, folliculitis, and periorbital hematoma.
According to a press release from the company, letibotulinumtoxinA-wlbg has been the leading neurotoxin brand in South Korea for 7 consecutive years, and the product has been sold in more than 50 different countries. Hugel plans to launch Letybo for US-based aesthetic clinicians in the latter half of 2024.
A version of this article appeared on Medscape.com.
Older Age Confers a Higher Risk for Second Primary Melanoma: Study
TOPLINE:
.
METHODOLOGY:
- Knowledge about one’s risk for a second primary invasive melanoma over time remains incomplete.
- Using data from the Cancer Registry of Norway, researchers performed a cohort study of 19,196 individuals diagnosed with invasive melanomas during 2008-2020; 51% were women. The mean age at the first primary melanoma diagnosis was 62 years.
- The main outcome of interest was the incidence rate of second primary invasive melanoma after the first diagnosis.
- The researchers used accelerated failure time models to examine potential associations with patient and tumor characteristics. They also calculated the median time between first and second melanomas and the likelihood of second primary melanomas on the same or different site as the first primary melanoma.
TAKEAWAY:
- The incidence rate of a second primary melanoma in the year following a first primary melanoma was 16.8 (95% CI, 14.9-18.7) per 1000 person-years. This decreased to 7.3 (95% CI, 6.0-8.6) per 1000 person-years during the second year and remained stable after that.
- Patient age influenced the median time between first and second primaries. The median interval was 37 months (95% CI, 8-49) in patients aged < 40 years, 18 months (95% CI, 13-24) in patients aged 50-59 years, and 11 months (95% CI, 7-18) in patients aged ≥ 80 years.
- The body site of the second primary melanoma was the same as the first primary in 47% of patients but was located on a different body site in the remaining 53% of patients.
- Among patients who developed a second primary melanoma on the same site as the first, the median interval was shorter for men compared with women: A median of 12 (95% CI, 7-19) months vs 22 (95% CI, 11-35) months, respectively.
IN PRACTICE:
“Our findings suggest that increased surveillance may be considered for older patients, especially men, for at least the first 3 years after their initial diagnosis, regardless of the characteristics of their first melanoma,” the authors wrote.
SOURCE:
Corresponding author Reza Ghiasvand, PhD, of the Oslo Centre for Biostatistics and Epidemiology, Oslo University Hospital, Oslo, Norway, and colleagues conducted the research, published online on February 28, 2024, in JAMA Dermatology.
LIMITATIONS:
Information was lacking on phenotypic characteristics, personal ultraviolet radiation (UVR) exposure, genetic factors, and the number of follow-up skin examinations.
DISCLOSURES:
The study was supported by the South-Eastern Norway Regional Health Authority. The authors reported having no disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
.
METHODOLOGY:
- Knowledge about one’s risk for a second primary invasive melanoma over time remains incomplete.
- Using data from the Cancer Registry of Norway, researchers performed a cohort study of 19,196 individuals diagnosed with invasive melanomas during 2008-2020; 51% were women. The mean age at the first primary melanoma diagnosis was 62 years.
- The main outcome of interest was the incidence rate of second primary invasive melanoma after the first diagnosis.
- The researchers used accelerated failure time models to examine potential associations with patient and tumor characteristics. They also calculated the median time between first and second melanomas and the likelihood of second primary melanomas on the same or different site as the first primary melanoma.
TAKEAWAY:
- The incidence rate of a second primary melanoma in the year following a first primary melanoma was 16.8 (95% CI, 14.9-18.7) per 1000 person-years. This decreased to 7.3 (95% CI, 6.0-8.6) per 1000 person-years during the second year and remained stable after that.
- Patient age influenced the median time between first and second primaries. The median interval was 37 months (95% CI, 8-49) in patients aged < 40 years, 18 months (95% CI, 13-24) in patients aged 50-59 years, and 11 months (95% CI, 7-18) in patients aged ≥ 80 years.
- The body site of the second primary melanoma was the same as the first primary in 47% of patients but was located on a different body site in the remaining 53% of patients.
- Among patients who developed a second primary melanoma on the same site as the first, the median interval was shorter for men compared with women: A median of 12 (95% CI, 7-19) months vs 22 (95% CI, 11-35) months, respectively.
IN PRACTICE:
“Our findings suggest that increased surveillance may be considered for older patients, especially men, for at least the first 3 years after their initial diagnosis, regardless of the characteristics of their first melanoma,” the authors wrote.
SOURCE:
Corresponding author Reza Ghiasvand, PhD, of the Oslo Centre for Biostatistics and Epidemiology, Oslo University Hospital, Oslo, Norway, and colleagues conducted the research, published online on February 28, 2024, in JAMA Dermatology.
LIMITATIONS:
Information was lacking on phenotypic characteristics, personal ultraviolet radiation (UVR) exposure, genetic factors, and the number of follow-up skin examinations.
DISCLOSURES:
The study was supported by the South-Eastern Norway Regional Health Authority. The authors reported having no disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
.
METHODOLOGY:
- Knowledge about one’s risk for a second primary invasive melanoma over time remains incomplete.
- Using data from the Cancer Registry of Norway, researchers performed a cohort study of 19,196 individuals diagnosed with invasive melanomas during 2008-2020; 51% were women. The mean age at the first primary melanoma diagnosis was 62 years.
- The main outcome of interest was the incidence rate of second primary invasive melanoma after the first diagnosis.
- The researchers used accelerated failure time models to examine potential associations with patient and tumor characteristics. They also calculated the median time between first and second melanomas and the likelihood of second primary melanomas on the same or different site as the first primary melanoma.
TAKEAWAY:
- The incidence rate of a second primary melanoma in the year following a first primary melanoma was 16.8 (95% CI, 14.9-18.7) per 1000 person-years. This decreased to 7.3 (95% CI, 6.0-8.6) per 1000 person-years during the second year and remained stable after that.
- Patient age influenced the median time between first and second primaries. The median interval was 37 months (95% CI, 8-49) in patients aged < 40 years, 18 months (95% CI, 13-24) in patients aged 50-59 years, and 11 months (95% CI, 7-18) in patients aged ≥ 80 years.
- The body site of the second primary melanoma was the same as the first primary in 47% of patients but was located on a different body site in the remaining 53% of patients.
- Among patients who developed a second primary melanoma on the same site as the first, the median interval was shorter for men compared with women: A median of 12 (95% CI, 7-19) months vs 22 (95% CI, 11-35) months, respectively.
IN PRACTICE:
“Our findings suggest that increased surveillance may be considered for older patients, especially men, for at least the first 3 years after their initial diagnosis, regardless of the characteristics of their first melanoma,” the authors wrote.
SOURCE:
Corresponding author Reza Ghiasvand, PhD, of the Oslo Centre for Biostatistics and Epidemiology, Oslo University Hospital, Oslo, Norway, and colleagues conducted the research, published online on February 28, 2024, in JAMA Dermatology.
LIMITATIONS:
Information was lacking on phenotypic characteristics, personal ultraviolet radiation (UVR) exposure, genetic factors, and the number of follow-up skin examinations.
DISCLOSURES:
The study was supported by the South-Eastern Norway Regional Health Authority. The authors reported having no disclosures.
A version of this article appeared on Medscape.com.
Study Evaluates Factors Driving Fatigue in Patients With Psoriasis, PsA
TOPLINE:
Many factors may influence fatigue in patients with psoriasis and psoriatic arthritis (PsA), researchers report.
METHODOLOGY:
- The individual components of fatigue in psoriasis and PsA have not been examined thoroughly.
- Researchers drew from the nationwide prospective Danish Skin Cohort to identify 2741 adults with dermatologist-diagnosed psoriasis (of which 593 also had PsA) and 3788 controls in the general population.
- All adults in the analysis completed the multidimensional fatigue inventory (MIF-20), a validated 20-item tool that measures five dimensions of fatigue: General fatigue, physical fatigue, reduced activity, reduced motivation, and mental fatigue. A higher score indicates more severe fatigue.
- All adults were also asked about their current intensity of joint pain over the previous 7 days, severity of pruritus and skin pain over the previous 24 hours, and sleep problems over the previous 72 hours on a numerical rating scale (NRS). The researchers applied linear regression models to continuous outcomes and adjusted for age, sex, socioeconomic status, psoriasis severity, and joint pain intensity, and beta coefficients (β) for the slopes were estimated with 95% CIs.
TAKEAWAY:
- Compared with the general population, higher total MFI-20 scores were observed for psoriasis and PsA, respectively. However, on the adjusted analysis, the impact on total fatigue was greatest for those with PsA (β = 5.23; 95% CI, 3.55-6.90), followed by psoriasis (β = 2.10; 95% CI, 0.96-3.25) compared with the general population (P trend < .0001).
- Increasing age was associated with a lower impact on total fatigue in psoriasis (β = −0.13; 95% CI, −0.18 to −0.08) and in PsA (β = −0.10; 95% CI, −0.19 to −0.01).
- Among patients with psoriasis with or without PsA, increasing joint pain intensity was associated with overall fatigue (β = 2.23; 95% CI, 2.03-2.44) for each one-point increase in joint pain on the NRS.
- In other findings, greater intensity of itch was associated with higher fatigue scores for both psoriasis and PsA, while skin pain was significantly associated with fatigue in PsA (β = 0.65; 95% CI, 0.08-1.22) but not in psoriasis without PsA (P = .2043).
IN PRACTICE:
“The when treating psoriasis, rather than focusing on objective severity measures alone,” the authors wrote.
SOURCE:
Corresponding author Alexander Egeberg, MD, of the Department of Dermatology at Bispebjerg Hospital, University of Copenhagen, Copenhagen, Denmark, and colleagues conducted the research, which was published in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The researchers were unable to assess whether the pain was inflammatory or noninflammatory or the number of affected joints. They also lacked information about the use of methotrexate, which commonly causes fatigue.
DISCLOSURES:
Dr. Egeberg is now an employee at LEO Pharma. He has received research funding from Pfizer, Eli Lilly, the Danish National Psoriasis Foundation, and the Royal Hofbundtmager Aage Bang Foundation, and honoraria as a consultant and/or speaker from AbbVie, Almirall, Bristol-Myers Squibb, Leo Pharma, Samsung Bioepis Co., Ltd., Pfizer, Eli Lilly, Novartis, UCB, Union Therapeutics, Horizon Therapeutics, Galderma, and Janssen Pharmaceuticals. Three of the coauthors reported being a consultant to, an adviser for, and/or having received research support from many pharmaceutical companies.
A version of this article appeared on Medscape.com.
TOPLINE:
Many factors may influence fatigue in patients with psoriasis and psoriatic arthritis (PsA), researchers report.
METHODOLOGY:
- The individual components of fatigue in psoriasis and PsA have not been examined thoroughly.
- Researchers drew from the nationwide prospective Danish Skin Cohort to identify 2741 adults with dermatologist-diagnosed psoriasis (of which 593 also had PsA) and 3788 controls in the general population.
- All adults in the analysis completed the multidimensional fatigue inventory (MIF-20), a validated 20-item tool that measures five dimensions of fatigue: General fatigue, physical fatigue, reduced activity, reduced motivation, and mental fatigue. A higher score indicates more severe fatigue.
- All adults were also asked about their current intensity of joint pain over the previous 7 days, severity of pruritus and skin pain over the previous 24 hours, and sleep problems over the previous 72 hours on a numerical rating scale (NRS). The researchers applied linear regression models to continuous outcomes and adjusted for age, sex, socioeconomic status, psoriasis severity, and joint pain intensity, and beta coefficients (β) for the slopes were estimated with 95% CIs.
TAKEAWAY:
- Compared with the general population, higher total MFI-20 scores were observed for psoriasis and PsA, respectively. However, on the adjusted analysis, the impact on total fatigue was greatest for those with PsA (β = 5.23; 95% CI, 3.55-6.90), followed by psoriasis (β = 2.10; 95% CI, 0.96-3.25) compared with the general population (P trend < .0001).
- Increasing age was associated with a lower impact on total fatigue in psoriasis (β = −0.13; 95% CI, −0.18 to −0.08) and in PsA (β = −0.10; 95% CI, −0.19 to −0.01).
- Among patients with psoriasis with or without PsA, increasing joint pain intensity was associated with overall fatigue (β = 2.23; 95% CI, 2.03-2.44) for each one-point increase in joint pain on the NRS.
- In other findings, greater intensity of itch was associated with higher fatigue scores for both psoriasis and PsA, while skin pain was significantly associated with fatigue in PsA (β = 0.65; 95% CI, 0.08-1.22) but not in psoriasis without PsA (P = .2043).
IN PRACTICE:
“The when treating psoriasis, rather than focusing on objective severity measures alone,” the authors wrote.
SOURCE:
Corresponding author Alexander Egeberg, MD, of the Department of Dermatology at Bispebjerg Hospital, University of Copenhagen, Copenhagen, Denmark, and colleagues conducted the research, which was published in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The researchers were unable to assess whether the pain was inflammatory or noninflammatory or the number of affected joints. They also lacked information about the use of methotrexate, which commonly causes fatigue.
DISCLOSURES:
Dr. Egeberg is now an employee at LEO Pharma. He has received research funding from Pfizer, Eli Lilly, the Danish National Psoriasis Foundation, and the Royal Hofbundtmager Aage Bang Foundation, and honoraria as a consultant and/or speaker from AbbVie, Almirall, Bristol-Myers Squibb, Leo Pharma, Samsung Bioepis Co., Ltd., Pfizer, Eli Lilly, Novartis, UCB, Union Therapeutics, Horizon Therapeutics, Galderma, and Janssen Pharmaceuticals. Three of the coauthors reported being a consultant to, an adviser for, and/or having received research support from many pharmaceutical companies.
A version of this article appeared on Medscape.com.
TOPLINE:
Many factors may influence fatigue in patients with psoriasis and psoriatic arthritis (PsA), researchers report.
METHODOLOGY:
- The individual components of fatigue in psoriasis and PsA have not been examined thoroughly.
- Researchers drew from the nationwide prospective Danish Skin Cohort to identify 2741 adults with dermatologist-diagnosed psoriasis (of which 593 also had PsA) and 3788 controls in the general population.
- All adults in the analysis completed the multidimensional fatigue inventory (MIF-20), a validated 20-item tool that measures five dimensions of fatigue: General fatigue, physical fatigue, reduced activity, reduced motivation, and mental fatigue. A higher score indicates more severe fatigue.
- All adults were also asked about their current intensity of joint pain over the previous 7 days, severity of pruritus and skin pain over the previous 24 hours, and sleep problems over the previous 72 hours on a numerical rating scale (NRS). The researchers applied linear regression models to continuous outcomes and adjusted for age, sex, socioeconomic status, psoriasis severity, and joint pain intensity, and beta coefficients (β) for the slopes were estimated with 95% CIs.
TAKEAWAY:
- Compared with the general population, higher total MFI-20 scores were observed for psoriasis and PsA, respectively. However, on the adjusted analysis, the impact on total fatigue was greatest for those with PsA (β = 5.23; 95% CI, 3.55-6.90), followed by psoriasis (β = 2.10; 95% CI, 0.96-3.25) compared with the general population (P trend < .0001).
- Increasing age was associated with a lower impact on total fatigue in psoriasis (β = −0.13; 95% CI, −0.18 to −0.08) and in PsA (β = −0.10; 95% CI, −0.19 to −0.01).
- Among patients with psoriasis with or without PsA, increasing joint pain intensity was associated with overall fatigue (β = 2.23; 95% CI, 2.03-2.44) for each one-point increase in joint pain on the NRS.
- In other findings, greater intensity of itch was associated with higher fatigue scores for both psoriasis and PsA, while skin pain was significantly associated with fatigue in PsA (β = 0.65; 95% CI, 0.08-1.22) but not in psoriasis without PsA (P = .2043).
IN PRACTICE:
“The when treating psoriasis, rather than focusing on objective severity measures alone,” the authors wrote.
SOURCE:
Corresponding author Alexander Egeberg, MD, of the Department of Dermatology at Bispebjerg Hospital, University of Copenhagen, Copenhagen, Denmark, and colleagues conducted the research, which was published in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The researchers were unable to assess whether the pain was inflammatory or noninflammatory or the number of affected joints. They also lacked information about the use of methotrexate, which commonly causes fatigue.
DISCLOSURES:
Dr. Egeberg is now an employee at LEO Pharma. He has received research funding from Pfizer, Eli Lilly, the Danish National Psoriasis Foundation, and the Royal Hofbundtmager Aage Bang Foundation, and honoraria as a consultant and/or speaker from AbbVie, Almirall, Bristol-Myers Squibb, Leo Pharma, Samsung Bioepis Co., Ltd., Pfizer, Eli Lilly, Novartis, UCB, Union Therapeutics, Horizon Therapeutics, Galderma, and Janssen Pharmaceuticals. Three of the coauthors reported being a consultant to, an adviser for, and/or having received research support from many pharmaceutical companies.
A version of this article appeared on Medscape.com.
Be Wary of TikTok Content on Infantile Hemangiomas: Study
TOPLINE:
.
METHODOLOGY:
- New parents may turn to TikTok for information about IHs, but little is known about the quality of videos on the social media platform related to the topic.
- Using the search term “hemangioma,” researchers reviewed the top 50 English-language TikTok videos that resulted from the query in November 2022.
- The researchers analyzed the videos for their content source, accuracy, and purpose and used Infantile Hemangioma Referral Score criteria to determine if the lesions pictured on the videos met criteria for referral to a specialist or not.
TAKEAWAY:
- Combined, the 50 videos were viewed 25.1 million times, had 2.6 million likes, and received 17,600 comments.
- Only 36 were considered likely to be IH. Of those 36 videos, the researchers deemed 33 (92%) to be potentially problematic, meriting referral to a specialist. The remaining three lesions could not be classified because of insufficient information.
- Of the 50 videos, 45 were created by individuals personally affected by IH (parents of a child with IH or young adults living with residual impacts), and only three were created by physicians (two by plastic surgeons and one by a neonatologist).
- In terms of content, 2 of the 45 videos created by someone personally affected by IH contained inaccurate information, while all three of videos created by physicians contained inaccurate information, such as oversimplification of the prognosis or incorrect nomenclature.
IN PRACTICE:
“Providers should be aware that TikTok may be useful for promoting birthmark awareness, but that it should not be relied on for accurate information about IHs,” the authors wrote.
SOURCE:
First author Sonora Yun, a medical student at Columbia University College of Physicians and Surgeons, New York City, conducted the research with Maria C. Garzon, MD, and Kimberly D. Morel, MD, who are board-certified pediatric dermatologists at Columbia. The study was published in Pediatric Dermatology.
LIMITATIONS:
The authors noted no specific limitations to the study.
DISCLOSURES:
The researchers reported having no disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
.
METHODOLOGY:
- New parents may turn to TikTok for information about IHs, but little is known about the quality of videos on the social media platform related to the topic.
- Using the search term “hemangioma,” researchers reviewed the top 50 English-language TikTok videos that resulted from the query in November 2022.
- The researchers analyzed the videos for their content source, accuracy, and purpose and used Infantile Hemangioma Referral Score criteria to determine if the lesions pictured on the videos met criteria for referral to a specialist or not.
TAKEAWAY:
- Combined, the 50 videos were viewed 25.1 million times, had 2.6 million likes, and received 17,600 comments.
- Only 36 were considered likely to be IH. Of those 36 videos, the researchers deemed 33 (92%) to be potentially problematic, meriting referral to a specialist. The remaining three lesions could not be classified because of insufficient information.
- Of the 50 videos, 45 were created by individuals personally affected by IH (parents of a child with IH or young adults living with residual impacts), and only three were created by physicians (two by plastic surgeons and one by a neonatologist).
- In terms of content, 2 of the 45 videos created by someone personally affected by IH contained inaccurate information, while all three of videos created by physicians contained inaccurate information, such as oversimplification of the prognosis or incorrect nomenclature.
IN PRACTICE:
“Providers should be aware that TikTok may be useful for promoting birthmark awareness, but that it should not be relied on for accurate information about IHs,” the authors wrote.
SOURCE:
First author Sonora Yun, a medical student at Columbia University College of Physicians and Surgeons, New York City, conducted the research with Maria C. Garzon, MD, and Kimberly D. Morel, MD, who are board-certified pediatric dermatologists at Columbia. The study was published in Pediatric Dermatology.
LIMITATIONS:
The authors noted no specific limitations to the study.
DISCLOSURES:
The researchers reported having no disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
.
METHODOLOGY:
- New parents may turn to TikTok for information about IHs, but little is known about the quality of videos on the social media platform related to the topic.
- Using the search term “hemangioma,” researchers reviewed the top 50 English-language TikTok videos that resulted from the query in November 2022.
- The researchers analyzed the videos for their content source, accuracy, and purpose and used Infantile Hemangioma Referral Score criteria to determine if the lesions pictured on the videos met criteria for referral to a specialist or not.
TAKEAWAY:
- Combined, the 50 videos were viewed 25.1 million times, had 2.6 million likes, and received 17,600 comments.
- Only 36 were considered likely to be IH. Of those 36 videos, the researchers deemed 33 (92%) to be potentially problematic, meriting referral to a specialist. The remaining three lesions could not be classified because of insufficient information.
- Of the 50 videos, 45 were created by individuals personally affected by IH (parents of a child with IH or young adults living with residual impacts), and only three were created by physicians (two by plastic surgeons and one by a neonatologist).
- In terms of content, 2 of the 45 videos created by someone personally affected by IH contained inaccurate information, while all three of videos created by physicians contained inaccurate information, such as oversimplification of the prognosis or incorrect nomenclature.
IN PRACTICE:
“Providers should be aware that TikTok may be useful for promoting birthmark awareness, but that it should not be relied on for accurate information about IHs,” the authors wrote.
SOURCE:
First author Sonora Yun, a medical student at Columbia University College of Physicians and Surgeons, New York City, conducted the research with Maria C. Garzon, MD, and Kimberly D. Morel, MD, who are board-certified pediatric dermatologists at Columbia. The study was published in Pediatric Dermatology.
LIMITATIONS:
The authors noted no specific limitations to the study.
DISCLOSURES:
The researchers reported having no disclosures.
A version of this article appeared on Medscape.com.
Use of Biologics for Psoriasis Found to Confer a Survival Benefit
Among patients with psoriasis, the risk of mortality was strongly associated with hepatic injury, cardiovascular disease, and psychiatric affective disorders, but was reduced among those who received systemic therapy with biologics, researchers from Canada report.
Those are key findings from a large retrospective registry study of patients with psoriasis, published in The Journal of the American Academy of Dermatology.
“Psoriasis, a chronic inflammatory condition affecting approximately 3% of the western populations, bears a higher risk of mortality compared to healthy individuals, possibly by inducing systemic inflammation associated with numerous comorbidities, especially cardiovascular diseases, metabolic syndrome, and others,” wrote corresponding author Robert Gniadecki, MD, PhD, of the division of dermatology at the University of Alberta, Canada, and colleagues. “It has been argued that the use of systemic immunomodulatory agents quenches systemic inflammation and potentially improves patient survival. However, the evidence to support this hypothesis is limited.”
To investigate the impact of comorbidities and systemic therapies on all-cause mortality in psoriasis, the researchers used the Alberta Health Services Data Repository of Reporting database from January 1, 2012, to June 1, 2019, which represents a population base of 4.47 million individuals. They extracted data on 18,618 psoriasis cases and 55,854 controls, stratified cases according to the Charlson Comorbidity Index (CCI), a surrogate measure for comorbidity burden, and by the type of therapy received, and conducted statistical analyses including Cox proportional hazards regression to determine absolute hazard ratios representing relative effects of specific demographic and comorbidity factors on mortality within groups.
The median age in both cohorts was 48 years, and 51% were male. The researchers observed that mortality in the psoriasis cohort was significantly higher than in the controls (5.7% vs. 3.8%, respectively; P < .05), with a median age at the time of death of 72 vs. 74.4 years.
The CCI and comorbidities strongly predicted mortality, especially drug-induced liver injury (hazard ratio [HR], 1.78), bipolar disorder and suicidal ideation (HR, 1.24-1.58), and major cardiovascular diseases, which included myocardial infarction (MI), congestive heart failure (CHF), and cerebrovascular disease (CVA) (HR, 1.2-1.4).
Among patients in the psoriasis cohort, survival of those treated with biologic agents was higher than in controls, even after matching for CCI (3.2% vs. 4.4%, respectively, P < .05). “These patients also exhibit reduced overall mortality compared to those treated with methotrexate or topical agents,” Dr. Gniadecki and colleagues wrote. “There was no difference in mortality between methotrexate patients and the topical therapy patients, but any of those treatment groups had superior survival compared to the no-treatment cohort.”
They added that despite better survival among patients treated with biologic agents, no significant improvements were detected in their comorbidity profiles. “Notably, the frequency of major cardiovascular disease (MI, CHF, CVA) was the same as in the controls, and overall, the frequency of diseases coded as cardiovascular was slightly increased,” they wrote.
The fact that some factors could not be measured, including the type and severity of psoriasis, response to treatment, smoking history, and alcohol intake, was a study limitation, they noted.
Joel M. Gelfand, MD, director of the psoriasis and phototherapy treatment center at the University of Pennsylvania, Philadelphia, who was asked to comment on the analysis, said the study confirms prior work indicating that having psoriasis is a predictor of mortality. In addition, “there is a strong healthy user affect among patients who take and stay on biologics for psoriasis,” he told this news organization.
“The results are encouraging but are not able to establish a causal relationship between treating psoriasis with biologics and lowering mortality risk. Ultimately, randomized comparative trials will be needed to determine which approach or approaches to treating psoriasis, if any, lower the risk of psoriatic arthritis, cardiovascular disease, and mortality,” said Dr. Gelfand, who was not involved with the study.
Asked to comment on the results, Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was not involved with the study, said that “data such as these enable us to rationalize the cost of our fleet of biologics, as managing the outpatient/inpatient burden of many of these comorbidities will actually drain the healthcare system, more so than managing psoriasis in the first place. Certainly other interventions to address the well known comorbidities, such as cardiovascular and hepatic, are warranted, but what if you could prevent the problem in the first place? To be continued for that answer.”
The study was funded by Canadian Dermatology Foundation, Alberta Innovates, and by a Health Sciences TD Bank Studentship Award. Dr. Gniadecki reported conducting clinical trials for Bausch Health, AbbVie and Janssen, and he has received honoraria as consultant and/or speaker from AbbVie, Bausch Health, Eli Lilly, Janssen, Mallinckrodt, Novartis, Kyowa Kirin, Sun Pharma and Sanofi. The other authors had no disclosures. Dr. Gelfand reported serving as a consultant for AbbVie, Artax, Bristol-Myers Squibb, GlaxoSmithKline, and other companies. He is on the board of directors for the International Psoriasis Council and the Medical Dermatology Society. Dr. Friedman disclosed that he is a speaker for Janssen and Bristol Myers Squibb. He has received grants from Janssen, Pfizer, Bristol Myers Squibb, and Lilly, and has served as an advisor for Arcutis, Dermavant, and Janssen.
Among patients with psoriasis, the risk of mortality was strongly associated with hepatic injury, cardiovascular disease, and psychiatric affective disorders, but was reduced among those who received systemic therapy with biologics, researchers from Canada report.
Those are key findings from a large retrospective registry study of patients with psoriasis, published in The Journal of the American Academy of Dermatology.
“Psoriasis, a chronic inflammatory condition affecting approximately 3% of the western populations, bears a higher risk of mortality compared to healthy individuals, possibly by inducing systemic inflammation associated with numerous comorbidities, especially cardiovascular diseases, metabolic syndrome, and others,” wrote corresponding author Robert Gniadecki, MD, PhD, of the division of dermatology at the University of Alberta, Canada, and colleagues. “It has been argued that the use of systemic immunomodulatory agents quenches systemic inflammation and potentially improves patient survival. However, the evidence to support this hypothesis is limited.”
To investigate the impact of comorbidities and systemic therapies on all-cause mortality in psoriasis, the researchers used the Alberta Health Services Data Repository of Reporting database from January 1, 2012, to June 1, 2019, which represents a population base of 4.47 million individuals. They extracted data on 18,618 psoriasis cases and 55,854 controls, stratified cases according to the Charlson Comorbidity Index (CCI), a surrogate measure for comorbidity burden, and by the type of therapy received, and conducted statistical analyses including Cox proportional hazards regression to determine absolute hazard ratios representing relative effects of specific demographic and comorbidity factors on mortality within groups.
The median age in both cohorts was 48 years, and 51% were male. The researchers observed that mortality in the psoriasis cohort was significantly higher than in the controls (5.7% vs. 3.8%, respectively; P < .05), with a median age at the time of death of 72 vs. 74.4 years.
The CCI and comorbidities strongly predicted mortality, especially drug-induced liver injury (hazard ratio [HR], 1.78), bipolar disorder and suicidal ideation (HR, 1.24-1.58), and major cardiovascular diseases, which included myocardial infarction (MI), congestive heart failure (CHF), and cerebrovascular disease (CVA) (HR, 1.2-1.4).
Among patients in the psoriasis cohort, survival of those treated with biologic agents was higher than in controls, even after matching for CCI (3.2% vs. 4.4%, respectively, P < .05). “These patients also exhibit reduced overall mortality compared to those treated with methotrexate or topical agents,” Dr. Gniadecki and colleagues wrote. “There was no difference in mortality between methotrexate patients and the topical therapy patients, but any of those treatment groups had superior survival compared to the no-treatment cohort.”
They added that despite better survival among patients treated with biologic agents, no significant improvements were detected in their comorbidity profiles. “Notably, the frequency of major cardiovascular disease (MI, CHF, CVA) was the same as in the controls, and overall, the frequency of diseases coded as cardiovascular was slightly increased,” they wrote.
The fact that some factors could not be measured, including the type and severity of psoriasis, response to treatment, smoking history, and alcohol intake, was a study limitation, they noted.
Joel M. Gelfand, MD, director of the psoriasis and phototherapy treatment center at the University of Pennsylvania, Philadelphia, who was asked to comment on the analysis, said the study confirms prior work indicating that having psoriasis is a predictor of mortality. In addition, “there is a strong healthy user affect among patients who take and stay on biologics for psoriasis,” he told this news organization.
“The results are encouraging but are not able to establish a causal relationship between treating psoriasis with biologics and lowering mortality risk. Ultimately, randomized comparative trials will be needed to determine which approach or approaches to treating psoriasis, if any, lower the risk of psoriatic arthritis, cardiovascular disease, and mortality,” said Dr. Gelfand, who was not involved with the study.
Asked to comment on the results, Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was not involved with the study, said that “data such as these enable us to rationalize the cost of our fleet of biologics, as managing the outpatient/inpatient burden of many of these comorbidities will actually drain the healthcare system, more so than managing psoriasis in the first place. Certainly other interventions to address the well known comorbidities, such as cardiovascular and hepatic, are warranted, but what if you could prevent the problem in the first place? To be continued for that answer.”
The study was funded by Canadian Dermatology Foundation, Alberta Innovates, and by a Health Sciences TD Bank Studentship Award. Dr. Gniadecki reported conducting clinical trials for Bausch Health, AbbVie and Janssen, and he has received honoraria as consultant and/or speaker from AbbVie, Bausch Health, Eli Lilly, Janssen, Mallinckrodt, Novartis, Kyowa Kirin, Sun Pharma and Sanofi. The other authors had no disclosures. Dr. Gelfand reported serving as a consultant for AbbVie, Artax, Bristol-Myers Squibb, GlaxoSmithKline, and other companies. He is on the board of directors for the International Psoriasis Council and the Medical Dermatology Society. Dr. Friedman disclosed that he is a speaker for Janssen and Bristol Myers Squibb. He has received grants from Janssen, Pfizer, Bristol Myers Squibb, and Lilly, and has served as an advisor for Arcutis, Dermavant, and Janssen.
Among patients with psoriasis, the risk of mortality was strongly associated with hepatic injury, cardiovascular disease, and psychiatric affective disorders, but was reduced among those who received systemic therapy with biologics, researchers from Canada report.
Those are key findings from a large retrospective registry study of patients with psoriasis, published in The Journal of the American Academy of Dermatology.
“Psoriasis, a chronic inflammatory condition affecting approximately 3% of the western populations, bears a higher risk of mortality compared to healthy individuals, possibly by inducing systemic inflammation associated with numerous comorbidities, especially cardiovascular diseases, metabolic syndrome, and others,” wrote corresponding author Robert Gniadecki, MD, PhD, of the division of dermatology at the University of Alberta, Canada, and colleagues. “It has been argued that the use of systemic immunomodulatory agents quenches systemic inflammation and potentially improves patient survival. However, the evidence to support this hypothesis is limited.”
To investigate the impact of comorbidities and systemic therapies on all-cause mortality in psoriasis, the researchers used the Alberta Health Services Data Repository of Reporting database from January 1, 2012, to June 1, 2019, which represents a population base of 4.47 million individuals. They extracted data on 18,618 psoriasis cases and 55,854 controls, stratified cases according to the Charlson Comorbidity Index (CCI), a surrogate measure for comorbidity burden, and by the type of therapy received, and conducted statistical analyses including Cox proportional hazards regression to determine absolute hazard ratios representing relative effects of specific demographic and comorbidity factors on mortality within groups.
The median age in both cohorts was 48 years, and 51% were male. The researchers observed that mortality in the psoriasis cohort was significantly higher than in the controls (5.7% vs. 3.8%, respectively; P < .05), with a median age at the time of death of 72 vs. 74.4 years.
The CCI and comorbidities strongly predicted mortality, especially drug-induced liver injury (hazard ratio [HR], 1.78), bipolar disorder and suicidal ideation (HR, 1.24-1.58), and major cardiovascular diseases, which included myocardial infarction (MI), congestive heart failure (CHF), and cerebrovascular disease (CVA) (HR, 1.2-1.4).
Among patients in the psoriasis cohort, survival of those treated with biologic agents was higher than in controls, even after matching for CCI (3.2% vs. 4.4%, respectively, P < .05). “These patients also exhibit reduced overall mortality compared to those treated with methotrexate or topical agents,” Dr. Gniadecki and colleagues wrote. “There was no difference in mortality between methotrexate patients and the topical therapy patients, but any of those treatment groups had superior survival compared to the no-treatment cohort.”
They added that despite better survival among patients treated with biologic agents, no significant improvements were detected in their comorbidity profiles. “Notably, the frequency of major cardiovascular disease (MI, CHF, CVA) was the same as in the controls, and overall, the frequency of diseases coded as cardiovascular was slightly increased,” they wrote.
The fact that some factors could not be measured, including the type and severity of psoriasis, response to treatment, smoking history, and alcohol intake, was a study limitation, they noted.
Joel M. Gelfand, MD, director of the psoriasis and phototherapy treatment center at the University of Pennsylvania, Philadelphia, who was asked to comment on the analysis, said the study confirms prior work indicating that having psoriasis is a predictor of mortality. In addition, “there is a strong healthy user affect among patients who take and stay on biologics for psoriasis,” he told this news organization.
“The results are encouraging but are not able to establish a causal relationship between treating psoriasis with biologics and lowering mortality risk. Ultimately, randomized comparative trials will be needed to determine which approach or approaches to treating psoriasis, if any, lower the risk of psoriatic arthritis, cardiovascular disease, and mortality,” said Dr. Gelfand, who was not involved with the study.
Asked to comment on the results, Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was not involved with the study, said that “data such as these enable us to rationalize the cost of our fleet of biologics, as managing the outpatient/inpatient burden of many of these comorbidities will actually drain the healthcare system, more so than managing psoriasis in the first place. Certainly other interventions to address the well known comorbidities, such as cardiovascular and hepatic, are warranted, but what if you could prevent the problem in the first place? To be continued for that answer.”
The study was funded by Canadian Dermatology Foundation, Alberta Innovates, and by a Health Sciences TD Bank Studentship Award. Dr. Gniadecki reported conducting clinical trials for Bausch Health, AbbVie and Janssen, and he has received honoraria as consultant and/or speaker from AbbVie, Bausch Health, Eli Lilly, Janssen, Mallinckrodt, Novartis, Kyowa Kirin, Sun Pharma and Sanofi. The other authors had no disclosures. Dr. Gelfand reported serving as a consultant for AbbVie, Artax, Bristol-Myers Squibb, GlaxoSmithKline, and other companies. He is on the board of directors for the International Psoriasis Council and the Medical Dermatology Society. Dr. Friedman disclosed that he is a speaker for Janssen and Bristol Myers Squibb. He has received grants from Janssen, Pfizer, Bristol Myers Squibb, and Lilly, and has served as an advisor for Arcutis, Dermavant, and Janssen.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
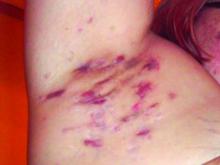
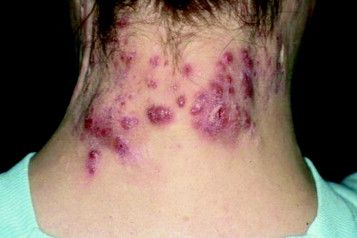
Study Eyes Longer IV Ertapenem for Recalcitrant Hidradenitis Suppurativa
at the completion of treatment, a retrospective study showed.
“These findings suggest a course of 12 to 16 weeks of ertapenem may be appropriate as a new standard length of therapy in HS patients, which is at least twice the current recommendation of the North American treatment guidelines,” wrote corresponding author Steven R. Cohen, MD, MPH, of the departments of dermatology at Weill Cornell Medicine and Albert Einstein College of Medicine, New York, and his coauthors. The results were published online February 14, 2024, in JAMA Dermatology.
In an earlier study , some of the same researchers evaluated the efficacy of daily IV ertapenem for 6 weeks in seven patients with HS. The patients experienced “notable remediation of disease that was rapidly lost within 1 month of withdrawal.”
Treatment guidelines published in 2019 recommend ertapenem as a highly effective third-line therapy limited to one 6-week course “as rescue therapy or during surgical planning, given the practical barriers to home infusions and concerns about antibiotic resistance” .
For the current analysis, Dr. Cohen and colleagues explored the effects of a longer duration of treatment with ertapenem in this patient population. They retrospectively reviewed the medical records of 98 patients with HS who received care at Albert Einstein College of Medicine’s Montefiore HS Center between 2018 and 2022. Each patient used an elastomeric pump to self-administer 1 g IV ertapenem daily for 12-16 weeks.
Key outcome measures of interest were the HS Physician Global Assessment (PGA) score (a 6-point scale ranging from clear to very severe) and a numerical rating scale (NRS) for pain (an 11-point scale in which a score of 0 indicates no pain and a score of 10 indicates the worst possible pain) and markers of inflammation such as leukocytes, erythrocyte sedimentation rate, C-reactive protein (CRP), and interleukin (IL)-6. The researchers measured these outcomes at baseline, the midcourse of IV ertapenem treatment, at the end of the course, and post therapy.
The mean age of the patients was 35.8 years, 62.2% were female, and 60.2% were Black. The mean treatment duration was 13.1 weeks and the mean posttherapy follow-up occurred after a mean of 7.8 weeks.
Between baseline and posttherapy follow-up, the HS PGA scores dropped from a mean of 3.9 to 2.7 and the NRS for pain dropped from 4.2 to 1.8 (P < .001 for both associations). Markers of inflammation also dropped between baseline and post therapy.
Specifically, values for CRP dropped from 5.4 to 2.4 mg/dL; IL-6 dropped from 25.2 to 13.7, and leukocytes dropped from 11.3 to 10.0 (P < .001 for all associations). Among the 76 patients who participated in a follow-up telephone survey, 63 (80.3%) reported medium to high satisfaction with their course of ertapenem, and 69 (90.8%) said they would recommend the treatment to other patients with HS.
The authors noted certain limitations of their study, including its retrospective, single-center design, the lack of a control group, and the fact that the HS-PGA scores at each visit did not meet the threshold of a 2-point decrease that is considered a clinically meaningful in the medical literature.
The definitive mechanism of ertapenem efficacy remains elusive, the authors pointed out. “Although oral antibiotics are generally accepted as a core therapeutic approach to HS, much less is known about the efficacy of IV antibiotics, especially ertapenem, a parenteral carbapenem possessing activity against many gram-positive bacteria, gram-negative bacteria, and anaerobic organisms,” they wrote.
In an accompanying editorial, Haley B. Naik, MD, MHSc, a dermatologist at the University of California, San Francisco, said that adopting prolonged courses of ertapenem treatment “comes with substantial individual and public health considerations”.
“Even though HS is a noninfectious disease, microbes might play a role in inciting HS immune dysregulation, prompting the inclusion of antimicrobial therapy in treatment regimens. However, broad-spectrum antibiotics for HS are associated with high levels of antibiotic resistance,” she wrote. Prolonged use of ertapenem and other carbapenems in HS treatment “will likely increase antimicrobial resistance, thereby limiting management of both HS and comorbid infections.”
Jennifer L. Hsiao, MD, a dermatologist who directs the HS clinic at the University of Southern California, Los Angeles, who was asked to comment on the study, said that, despite significant advances in the management of HS over the past decade, there are still patients who do not respond adequately to standard treatments.
For these patients, IV ertapenem can serve as a valuable bridge to a longer-term therapeutic option, “be it surgery or escalated immunomodulation,” such as dual biologic therapy, she said. “In my personal experience, IV ertapenem, which like the authors I also typically use for a 12-week course, delivers impressive and fast results even in the worst disease cases.
“It can be difficult to maintain the therapeutic benefit of ertapenem after it is discontinued, which is why patients should be on concomitant medications as they were in this study and have a post-ertapenem treatment plan in place,” said Dr. Hsiao, who was not involved with the study. “Hopefully, we will be able to one day understand why ertapenem is so effective for HS and be able to harness that benefit for patients without concern for antimicrobial resistance.”
Dr. Cohen reported receiving personal fees from Verrica Pharmaceuticals and belonging to the Board of Trustees of the American Skin Association outside the submitted work. No other disclosures were reported. Dr. Naik reported having received grants from AbbVie and the National Institutes of Health; personal fees from Novartis, UCB, Boehringer Ingelheim, 23andMe, Aristea Therapeutics, Medscape, Sonoma Biotherapeutics, DAVA Oncology, and Pfizer; and shares from Radera during the conduct of the study. She is a board member of the Hidradenitis Suppurativa Foundation. Dr. Hsiao disclosed that she is a member of the board of directors for the Hidradenitis Suppurativa Foundation. She has served as a consultant for AbbVie, Aclaris, Boehringer Ingelheim, Incyte, Novartis, UCB, as a speaker for AbbVie, Novartis, and UCB, and as an investigator for Amgen, Boehringer Ingelheim, and Incyte.
at the completion of treatment, a retrospective study showed.
“These findings suggest a course of 12 to 16 weeks of ertapenem may be appropriate as a new standard length of therapy in HS patients, which is at least twice the current recommendation of the North American treatment guidelines,” wrote corresponding author Steven R. Cohen, MD, MPH, of the departments of dermatology at Weill Cornell Medicine and Albert Einstein College of Medicine, New York, and his coauthors. The results were published online February 14, 2024, in JAMA Dermatology.
In an earlier study , some of the same researchers evaluated the efficacy of daily IV ertapenem for 6 weeks in seven patients with HS. The patients experienced “notable remediation of disease that was rapidly lost within 1 month of withdrawal.”
Treatment guidelines published in 2019 recommend ertapenem as a highly effective third-line therapy limited to one 6-week course “as rescue therapy or during surgical planning, given the practical barriers to home infusions and concerns about antibiotic resistance” .
For the current analysis, Dr. Cohen and colleagues explored the effects of a longer duration of treatment with ertapenem in this patient population. They retrospectively reviewed the medical records of 98 patients with HS who received care at Albert Einstein College of Medicine’s Montefiore HS Center between 2018 and 2022. Each patient used an elastomeric pump to self-administer 1 g IV ertapenem daily for 12-16 weeks.
Key outcome measures of interest were the HS Physician Global Assessment (PGA) score (a 6-point scale ranging from clear to very severe) and a numerical rating scale (NRS) for pain (an 11-point scale in which a score of 0 indicates no pain and a score of 10 indicates the worst possible pain) and markers of inflammation such as leukocytes, erythrocyte sedimentation rate, C-reactive protein (CRP), and interleukin (IL)-6. The researchers measured these outcomes at baseline, the midcourse of IV ertapenem treatment, at the end of the course, and post therapy.
The mean age of the patients was 35.8 years, 62.2% were female, and 60.2% were Black. The mean treatment duration was 13.1 weeks and the mean posttherapy follow-up occurred after a mean of 7.8 weeks.
Between baseline and posttherapy follow-up, the HS PGA scores dropped from a mean of 3.9 to 2.7 and the NRS for pain dropped from 4.2 to 1.8 (P < .001 for both associations). Markers of inflammation also dropped between baseline and post therapy.
Specifically, values for CRP dropped from 5.4 to 2.4 mg/dL; IL-6 dropped from 25.2 to 13.7, and leukocytes dropped from 11.3 to 10.0 (P < .001 for all associations). Among the 76 patients who participated in a follow-up telephone survey, 63 (80.3%) reported medium to high satisfaction with their course of ertapenem, and 69 (90.8%) said they would recommend the treatment to other patients with HS.
The authors noted certain limitations of their study, including its retrospective, single-center design, the lack of a control group, and the fact that the HS-PGA scores at each visit did not meet the threshold of a 2-point decrease that is considered a clinically meaningful in the medical literature.
The definitive mechanism of ertapenem efficacy remains elusive, the authors pointed out. “Although oral antibiotics are generally accepted as a core therapeutic approach to HS, much less is known about the efficacy of IV antibiotics, especially ertapenem, a parenteral carbapenem possessing activity against many gram-positive bacteria, gram-negative bacteria, and anaerobic organisms,” they wrote.
In an accompanying editorial, Haley B. Naik, MD, MHSc, a dermatologist at the University of California, San Francisco, said that adopting prolonged courses of ertapenem treatment “comes with substantial individual and public health considerations”.
“Even though HS is a noninfectious disease, microbes might play a role in inciting HS immune dysregulation, prompting the inclusion of antimicrobial therapy in treatment regimens. However, broad-spectrum antibiotics for HS are associated with high levels of antibiotic resistance,” she wrote. Prolonged use of ertapenem and other carbapenems in HS treatment “will likely increase antimicrobial resistance, thereby limiting management of both HS and comorbid infections.”
Jennifer L. Hsiao, MD, a dermatologist who directs the HS clinic at the University of Southern California, Los Angeles, who was asked to comment on the study, said that, despite significant advances in the management of HS over the past decade, there are still patients who do not respond adequately to standard treatments.
For these patients, IV ertapenem can serve as a valuable bridge to a longer-term therapeutic option, “be it surgery or escalated immunomodulation,” such as dual biologic therapy, she said. “In my personal experience, IV ertapenem, which like the authors I also typically use for a 12-week course, delivers impressive and fast results even in the worst disease cases.
“It can be difficult to maintain the therapeutic benefit of ertapenem after it is discontinued, which is why patients should be on concomitant medications as they were in this study and have a post-ertapenem treatment plan in place,” said Dr. Hsiao, who was not involved with the study. “Hopefully, we will be able to one day understand why ertapenem is so effective for HS and be able to harness that benefit for patients without concern for antimicrobial resistance.”
Dr. Cohen reported receiving personal fees from Verrica Pharmaceuticals and belonging to the Board of Trustees of the American Skin Association outside the submitted work. No other disclosures were reported. Dr. Naik reported having received grants from AbbVie and the National Institutes of Health; personal fees from Novartis, UCB, Boehringer Ingelheim, 23andMe, Aristea Therapeutics, Medscape, Sonoma Biotherapeutics, DAVA Oncology, and Pfizer; and shares from Radera during the conduct of the study. She is a board member of the Hidradenitis Suppurativa Foundation. Dr. Hsiao disclosed that she is a member of the board of directors for the Hidradenitis Suppurativa Foundation. She has served as a consultant for AbbVie, Aclaris, Boehringer Ingelheim, Incyte, Novartis, UCB, as a speaker for AbbVie, Novartis, and UCB, and as an investigator for Amgen, Boehringer Ingelheim, and Incyte.
at the completion of treatment, a retrospective study showed.
“These findings suggest a course of 12 to 16 weeks of ertapenem may be appropriate as a new standard length of therapy in HS patients, which is at least twice the current recommendation of the North American treatment guidelines,” wrote corresponding author Steven R. Cohen, MD, MPH, of the departments of dermatology at Weill Cornell Medicine and Albert Einstein College of Medicine, New York, and his coauthors. The results were published online February 14, 2024, in JAMA Dermatology.
In an earlier study , some of the same researchers evaluated the efficacy of daily IV ertapenem for 6 weeks in seven patients with HS. The patients experienced “notable remediation of disease that was rapidly lost within 1 month of withdrawal.”
Treatment guidelines published in 2019 recommend ertapenem as a highly effective third-line therapy limited to one 6-week course “as rescue therapy or during surgical planning, given the practical barriers to home infusions and concerns about antibiotic resistance” .
For the current analysis, Dr. Cohen and colleagues explored the effects of a longer duration of treatment with ertapenem in this patient population. They retrospectively reviewed the medical records of 98 patients with HS who received care at Albert Einstein College of Medicine’s Montefiore HS Center between 2018 and 2022. Each patient used an elastomeric pump to self-administer 1 g IV ertapenem daily for 12-16 weeks.
Key outcome measures of interest were the HS Physician Global Assessment (PGA) score (a 6-point scale ranging from clear to very severe) and a numerical rating scale (NRS) for pain (an 11-point scale in which a score of 0 indicates no pain and a score of 10 indicates the worst possible pain) and markers of inflammation such as leukocytes, erythrocyte sedimentation rate, C-reactive protein (CRP), and interleukin (IL)-6. The researchers measured these outcomes at baseline, the midcourse of IV ertapenem treatment, at the end of the course, and post therapy.
The mean age of the patients was 35.8 years, 62.2% were female, and 60.2% were Black. The mean treatment duration was 13.1 weeks and the mean posttherapy follow-up occurred after a mean of 7.8 weeks.
Between baseline and posttherapy follow-up, the HS PGA scores dropped from a mean of 3.9 to 2.7 and the NRS for pain dropped from 4.2 to 1.8 (P < .001 for both associations). Markers of inflammation also dropped between baseline and post therapy.
Specifically, values for CRP dropped from 5.4 to 2.4 mg/dL; IL-6 dropped from 25.2 to 13.7, and leukocytes dropped from 11.3 to 10.0 (P < .001 for all associations). Among the 76 patients who participated in a follow-up telephone survey, 63 (80.3%) reported medium to high satisfaction with their course of ertapenem, and 69 (90.8%) said they would recommend the treatment to other patients with HS.
The authors noted certain limitations of their study, including its retrospective, single-center design, the lack of a control group, and the fact that the HS-PGA scores at each visit did not meet the threshold of a 2-point decrease that is considered a clinically meaningful in the medical literature.
The definitive mechanism of ertapenem efficacy remains elusive, the authors pointed out. “Although oral antibiotics are generally accepted as a core therapeutic approach to HS, much less is known about the efficacy of IV antibiotics, especially ertapenem, a parenteral carbapenem possessing activity against many gram-positive bacteria, gram-negative bacteria, and anaerobic organisms,” they wrote.
In an accompanying editorial, Haley B. Naik, MD, MHSc, a dermatologist at the University of California, San Francisco, said that adopting prolonged courses of ertapenem treatment “comes with substantial individual and public health considerations”.
“Even though HS is a noninfectious disease, microbes might play a role in inciting HS immune dysregulation, prompting the inclusion of antimicrobial therapy in treatment regimens. However, broad-spectrum antibiotics for HS are associated with high levels of antibiotic resistance,” she wrote. Prolonged use of ertapenem and other carbapenems in HS treatment “will likely increase antimicrobial resistance, thereby limiting management of both HS and comorbid infections.”
Jennifer L. Hsiao, MD, a dermatologist who directs the HS clinic at the University of Southern California, Los Angeles, who was asked to comment on the study, said that, despite significant advances in the management of HS over the past decade, there are still patients who do not respond adequately to standard treatments.
For these patients, IV ertapenem can serve as a valuable bridge to a longer-term therapeutic option, “be it surgery or escalated immunomodulation,” such as dual biologic therapy, she said. “In my personal experience, IV ertapenem, which like the authors I also typically use for a 12-week course, delivers impressive and fast results even in the worst disease cases.
“It can be difficult to maintain the therapeutic benefit of ertapenem after it is discontinued, which is why patients should be on concomitant medications as they were in this study and have a post-ertapenem treatment plan in place,” said Dr. Hsiao, who was not involved with the study. “Hopefully, we will be able to one day understand why ertapenem is so effective for HS and be able to harness that benefit for patients without concern for antimicrobial resistance.”
Dr. Cohen reported receiving personal fees from Verrica Pharmaceuticals and belonging to the Board of Trustees of the American Skin Association outside the submitted work. No other disclosures were reported. Dr. Naik reported having received grants from AbbVie and the National Institutes of Health; personal fees from Novartis, UCB, Boehringer Ingelheim, 23andMe, Aristea Therapeutics, Medscape, Sonoma Biotherapeutics, DAVA Oncology, and Pfizer; and shares from Radera during the conduct of the study. She is a board member of the Hidradenitis Suppurativa Foundation. Dr. Hsiao disclosed that she is a member of the board of directors for the Hidradenitis Suppurativa Foundation. She has served as a consultant for AbbVie, Aclaris, Boehringer Ingelheim, Incyte, Novartis, UCB, as a speaker for AbbVie, Novartis, and UCB, and as an investigator for Amgen, Boehringer Ingelheim, and Incyte.
FROM JAMA DERMATOLOGY
What Skin Manifestations Are Associated With Pediatric IBD?
TOPLINE:
Skin conditions burden many children with inflammatory bowel disease (IBD), according to the authors of a single-center study.
METHODOLOGY:
- Researchers retrospectively reviewed the medical charts of 425 children and adolescents with (CD) or ulcerative (UC) at one or more dermatologic diagnoses who were seen at Mayo Clinic, Rochester, Minnesota, between 1999 and 2017.
- Of the children studied, 53% were male, 64.9% had CD, and 42.8% had one or more cutaneous infections.
- They used the chi-square/Fischer’s exact test to compare categorical outcomes between patients with CD and UC and to detect differences in IBD/CD/UC disease severity and skin conditions.
- Researchers retrospectively reviewed the medical charts of 425 children and adolescents with Crohn’s disease (CD) or ulcerative colitis (UC) at one or more dermatologic diagnoses who were seen at Mayo Clinic, Rochester, Minnesota, between 1999 and 2017.
- Of the children studied, 53% were male, 64.9% had CD, and 42.8% had one or more cutaneous infections.
- They used the chi-square/Fischer’s exact test to compare categorical outcomes between patients with CD and UC and to detect differences in IBD/CD/UC disease severity and skin conditions.
TAKEAWAY:
- The most common noninfectious dermatologic condition among the 425 children and adolescents was (30.8%), followed by eczema (15.8%) and perianal skin tags (14.6%).
- Angular cheilitis was more common among those with CD than those with UC (7.2% vs 2%, respectively; P = .024) as was keratosis pilaris (6.9% vs 0.7%; P = .003), and perianal skin complications such as skin tags (20.3% vs 4%), fistulas (13.4% vs 2.7%), and abscesses (13.4% vs 2%; P < .001 for all associations).
- Fungal skin infections were more frequently diagnosed in children with UC than those with CD (15.4% vs 8%; P = .017).
- The researchers observed that the severity of IBD correlated with a higher prevalence of perianal fistula (P = .003), perianal region abscess (P = .041), psoriasis (P < .001), and pyoderma gangrenosum (P = .003).
IN PRACTICE:
“Early identification of common dermatologic conditions in children and adolescents with IBD and recognizing their characteristic associations may alter management and improve skin-related outcomes in this patient population,” the authors wrote.
SOURCE:
Corresponding author Megha M. Tollefson, MD, of the Department of Dermatology at Mayo Clinic, Rochester, Minnesota, and colleagues conducted the research, which was published in Pediatric Dermatology.
LIMITATIONS:
The single-center design and the fact that database studies are subject to extraction error. There was no age- and sex-matched cohort to determine whether the prevalence of cutaneous infections, acne, eczema, and other inflammatory disorders was truly increased in IBD.
DISCLOSURES:
The researchers reported having no disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
Skin conditions burden many children with inflammatory bowel disease (IBD), according to the authors of a single-center study.
METHODOLOGY:
- Researchers retrospectively reviewed the medical charts of 425 children and adolescents with (CD) or ulcerative (UC) at one or more dermatologic diagnoses who were seen at Mayo Clinic, Rochester, Minnesota, between 1999 and 2017.
- Of the children studied, 53% were male, 64.9% had CD, and 42.8% had one or more cutaneous infections.
- They used the chi-square/Fischer’s exact test to compare categorical outcomes between patients with CD and UC and to detect differences in IBD/CD/UC disease severity and skin conditions.
- Researchers retrospectively reviewed the medical charts of 425 children and adolescents with Crohn’s disease (CD) or ulcerative colitis (UC) at one or more dermatologic diagnoses who were seen at Mayo Clinic, Rochester, Minnesota, between 1999 and 2017.
- Of the children studied, 53% were male, 64.9% had CD, and 42.8% had one or more cutaneous infections.
- They used the chi-square/Fischer’s exact test to compare categorical outcomes between patients with CD and UC and to detect differences in IBD/CD/UC disease severity and skin conditions.
TAKEAWAY:
- The most common noninfectious dermatologic condition among the 425 children and adolescents was (30.8%), followed by eczema (15.8%) and perianal skin tags (14.6%).
- Angular cheilitis was more common among those with CD than those with UC (7.2% vs 2%, respectively; P = .024) as was keratosis pilaris (6.9% vs 0.7%; P = .003), and perianal skin complications such as skin tags (20.3% vs 4%), fistulas (13.4% vs 2.7%), and abscesses (13.4% vs 2%; P < .001 for all associations).
- Fungal skin infections were more frequently diagnosed in children with UC than those with CD (15.4% vs 8%; P = .017).
- The researchers observed that the severity of IBD correlated with a higher prevalence of perianal fistula (P = .003), perianal region abscess (P = .041), psoriasis (P < .001), and pyoderma gangrenosum (P = .003).
IN PRACTICE:
“Early identification of common dermatologic conditions in children and adolescents with IBD and recognizing their characteristic associations may alter management and improve skin-related outcomes in this patient population,” the authors wrote.
SOURCE:
Corresponding author Megha M. Tollefson, MD, of the Department of Dermatology at Mayo Clinic, Rochester, Minnesota, and colleagues conducted the research, which was published in Pediatric Dermatology.
LIMITATIONS:
The single-center design and the fact that database studies are subject to extraction error. There was no age- and sex-matched cohort to determine whether the prevalence of cutaneous infections, acne, eczema, and other inflammatory disorders was truly increased in IBD.
DISCLOSURES:
The researchers reported having no disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
Skin conditions burden many children with inflammatory bowel disease (IBD), according to the authors of a single-center study.
METHODOLOGY:
- Researchers retrospectively reviewed the medical charts of 425 children and adolescents with (CD) or ulcerative (UC) at one or more dermatologic diagnoses who were seen at Mayo Clinic, Rochester, Minnesota, between 1999 and 2017.
- Of the children studied, 53% were male, 64.9% had CD, and 42.8% had one or more cutaneous infections.
- They used the chi-square/Fischer’s exact test to compare categorical outcomes between patients with CD and UC and to detect differences in IBD/CD/UC disease severity and skin conditions.
- Researchers retrospectively reviewed the medical charts of 425 children and adolescents with Crohn’s disease (CD) or ulcerative colitis (UC) at one or more dermatologic diagnoses who were seen at Mayo Clinic, Rochester, Minnesota, between 1999 and 2017.
- Of the children studied, 53% were male, 64.9% had CD, and 42.8% had one or more cutaneous infections.
- They used the chi-square/Fischer’s exact test to compare categorical outcomes between patients with CD and UC and to detect differences in IBD/CD/UC disease severity and skin conditions.
TAKEAWAY:
- The most common noninfectious dermatologic condition among the 425 children and adolescents was (30.8%), followed by eczema (15.8%) and perianal skin tags (14.6%).
- Angular cheilitis was more common among those with CD than those with UC (7.2% vs 2%, respectively; P = .024) as was keratosis pilaris (6.9% vs 0.7%; P = .003), and perianal skin complications such as skin tags (20.3% vs 4%), fistulas (13.4% vs 2.7%), and abscesses (13.4% vs 2%; P < .001 for all associations).
- Fungal skin infections were more frequently diagnosed in children with UC than those with CD (15.4% vs 8%; P = .017).
- The researchers observed that the severity of IBD correlated with a higher prevalence of perianal fistula (P = .003), perianal region abscess (P = .041), psoriasis (P < .001), and pyoderma gangrenosum (P = .003).
IN PRACTICE:
“Early identification of common dermatologic conditions in children and adolescents with IBD and recognizing their characteristic associations may alter management and improve skin-related outcomes in this patient population,” the authors wrote.
SOURCE:
Corresponding author Megha M. Tollefson, MD, of the Department of Dermatology at Mayo Clinic, Rochester, Minnesota, and colleagues conducted the research, which was published in Pediatric Dermatology.
LIMITATIONS:
The single-center design and the fact that database studies are subject to extraction error. There was no age- and sex-matched cohort to determine whether the prevalence of cutaneous infections, acne, eczema, and other inflammatory disorders was truly increased in IBD.
DISCLOSURES:
The researchers reported having no disclosures.
A version of this article appeared on Medscape.com.
Company Announces Regulatory Filing for Nemolizumab for Two Indications
On February 14, 2024, Galderma announced that .
A first-in-class investigational monoclonal antibody specifically designed to inhibit interleukin (IL) IL-31 signaling, nemolizumab has also been granted FDA Priority Review for prurigo nodularis, according to a press release from the company. The European Medicines Agency has also accepted Galderma’s Marketing Authorization Applications for nemolizumab for both prurigo nodularis and atopic dermatitis.
The regulatory developments follow data from the phase III OLYMPIA clinical trial program, which evaluated the efficacy and safety of nemolizumab administered subcutaneously every 4 weeks in patients with prurigo nodularis (NCT04501679 and NCT04501666). According to the press release, in OLYMPIA 1 and 2, 58% and 56% of patients, respectively, achieved at least a least four-point reduction in itch intensity as measured by the peak-pruritus numerical rating scale (PP-NRS), compared with 17% and 21% in the placebo groups (P < .0001). At the same time, 26% and 38% of nemolizumab-treated patients reached clearance or almost-clearance of skin lesions on the investigator’s global assessment (IGA) score, compared with 7% and 11% in the placebo groups (P < .0001).
On February 14, 2024, Galderma announced that .
A first-in-class investigational monoclonal antibody specifically designed to inhibit interleukin (IL) IL-31 signaling, nemolizumab has also been granted FDA Priority Review for prurigo nodularis, according to a press release from the company. The European Medicines Agency has also accepted Galderma’s Marketing Authorization Applications for nemolizumab for both prurigo nodularis and atopic dermatitis.
The regulatory developments follow data from the phase III OLYMPIA clinical trial program, which evaluated the efficacy and safety of nemolizumab administered subcutaneously every 4 weeks in patients with prurigo nodularis (NCT04501679 and NCT04501666). According to the press release, in OLYMPIA 1 and 2, 58% and 56% of patients, respectively, achieved at least a least four-point reduction in itch intensity as measured by the peak-pruritus numerical rating scale (PP-NRS), compared with 17% and 21% in the placebo groups (P < .0001). At the same time, 26% and 38% of nemolizumab-treated patients reached clearance or almost-clearance of skin lesions on the investigator’s global assessment (IGA) score, compared with 7% and 11% in the placebo groups (P < .0001).
On February 14, 2024, Galderma announced that .
A first-in-class investigational monoclonal antibody specifically designed to inhibit interleukin (IL) IL-31 signaling, nemolizumab has also been granted FDA Priority Review for prurigo nodularis, according to a press release from the company. The European Medicines Agency has also accepted Galderma’s Marketing Authorization Applications for nemolizumab for both prurigo nodularis and atopic dermatitis.
The regulatory developments follow data from the phase III OLYMPIA clinical trial program, which evaluated the efficacy and safety of nemolizumab administered subcutaneously every 4 weeks in patients with prurigo nodularis (NCT04501679 and NCT04501666). According to the press release, in OLYMPIA 1 and 2, 58% and 56% of patients, respectively, achieved at least a least four-point reduction in itch intensity as measured by the peak-pruritus numerical rating scale (PP-NRS), compared with 17% and 21% in the placebo groups (P < .0001). At the same time, 26% and 38% of nemolizumab-treated patients reached clearance or almost-clearance of skin lesions on the investigator’s global assessment (IGA) score, compared with 7% and 11% in the placebo groups (P < .0001).
Tapinarof Cream Under FDA Review for Atopic Dermatitis Indication
On February 14, Dermavant Sciences announced that the company had submitted a supplemental New Drug Application (sNDA) to the Food and Drug Administration for tapinarof cream, 1%, for treating atopic dermatitis (AD) in adults and children 2 years of age and older.
Tapinarof cream, 1%, is an aryl hydrocarbon receptor agonist marketed under the brand name VTAMA that was approved in 2022 for treating plaque psoriasis in adults.
According to a Dermavant press release, the sNDA is based on positive data from the phase 3 ADORING 1 and ADORING 2 pivotal trials and interim results from the phase 3 ADORING 3 open-label, long-term extension 48-week trial. In ADORING 1 and ADORING 2, tapinarof cream demonstrated statistically significant improvements in the primary endpoint of Validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD) treatment success, defined as a vIGA-AD score of 0 (clear) or 1 (almost clear) with at least a 2-grade improvement from baseline; demonstrated treatment success over vehicle at week 8; and met all key secondary endpoints with statistical significance, according to the company.
The most common adverse reactions in patients treated with VTAMA cream include folliculitis, nasopharyngitis, contact dermatitis, headache, and pruritus.
On February 14, Dermavant Sciences announced that the company had submitted a supplemental New Drug Application (sNDA) to the Food and Drug Administration for tapinarof cream, 1%, for treating atopic dermatitis (AD) in adults and children 2 years of age and older.
Tapinarof cream, 1%, is an aryl hydrocarbon receptor agonist marketed under the brand name VTAMA that was approved in 2022 for treating plaque psoriasis in adults.
According to a Dermavant press release, the sNDA is based on positive data from the phase 3 ADORING 1 and ADORING 2 pivotal trials and interim results from the phase 3 ADORING 3 open-label, long-term extension 48-week trial. In ADORING 1 and ADORING 2, tapinarof cream demonstrated statistically significant improvements in the primary endpoint of Validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD) treatment success, defined as a vIGA-AD score of 0 (clear) or 1 (almost clear) with at least a 2-grade improvement from baseline; demonstrated treatment success over vehicle at week 8; and met all key secondary endpoints with statistical significance, according to the company.
The most common adverse reactions in patients treated with VTAMA cream include folliculitis, nasopharyngitis, contact dermatitis, headache, and pruritus.
On February 14, Dermavant Sciences announced that the company had submitted a supplemental New Drug Application (sNDA) to the Food and Drug Administration for tapinarof cream, 1%, for treating atopic dermatitis (AD) in adults and children 2 years of age and older.
Tapinarof cream, 1%, is an aryl hydrocarbon receptor agonist marketed under the brand name VTAMA that was approved in 2022 for treating plaque psoriasis in adults.
According to a Dermavant press release, the sNDA is based on positive data from the phase 3 ADORING 1 and ADORING 2 pivotal trials and interim results from the phase 3 ADORING 3 open-label, long-term extension 48-week trial. In ADORING 1 and ADORING 2, tapinarof cream demonstrated statistically significant improvements in the primary endpoint of Validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD) treatment success, defined as a vIGA-AD score of 0 (clear) or 1 (almost clear) with at least a 2-grade improvement from baseline; demonstrated treatment success over vehicle at week 8; and met all key secondary endpoints with statistical significance, according to the company.
The most common adverse reactions in patients treated with VTAMA cream include folliculitis, nasopharyngitis, contact dermatitis, headache, and pruritus.