User login
Doug Brunk is a San Diego-based award-winning reporter who began covering health care in 1991. Before joining the company, he wrote for the health sciences division of Columbia University and was an associate editor at Contemporary Long Term Care magazine when it won a Jesse H. Neal Award. His work has been syndicated by the Los Angeles Times and he is the author of two books related to the University of Kentucky Wildcats men's basketball program. Doug has a master’s degree in magazine journalism from the S.I. Newhouse School of Public Communications at Syracuse University. Follow him on Twitter @dougbrunk.
FDA warns about fecal microbiota for transplantation
Officials at the Food and Drug Administration have issued a safety alert regarding the use of fecal microbiota for transplantation and the risk of serious adverse reactions because of transmission of multidrug-resistant organisms (MDROs).
According to the alert, which was issued on June 13, 2019, the agency became aware of two immunocompromised adult patients who received investigational fecal microbiota for transplantation (FMT) and developed extended-spectrum beta-lactamase (EBSL)–producing Escherichia coli. One of the patients died.
“This is certainly a theoretical risk that we’ve known about,” Lea Ann Chen, MD, a gastroenterologist at New York University, said in an interview. “This announcement is important, because we probably don’t counsel patients specifically about this risk. We say there is a risk for transmission of infectious agents in general, but I think that probably very few counsel patients about a risk for transmission of MDROs.”
The donor stool and FMT used in the two patients were not tested for ESBL-producing gram-negative organisms prior to use.
As a result of these serious adverse reactions, the FDA has determined that the following donor screening and stool testing protections are needed for any investigational use of FMT.
- Donor screening with questions that specifically address risk factors for colonization with MDROs, and exclusion of individuals at higher risk of colonization with MDROs.
- MDRO testing of donor stool and exclusion of stool that tests positive for MDRO. FDA scientists have determined the specific MDRO testing and frequency that should be implemented.
On June 14, the American Gastroenterological Association sent a communication about the FDA alert to its members, which stated that the AGA “is committed to advancing applications of the gut microbiome. Our top priority is ensuring patient safety from microbiome-based therapeutics, such as FMT. Through the AGA FMT National Registry, AGA is working with physicians and patients to track FMT usage, patient outcomes and adverse events. Associated with the registry is a biorepository of donor and patient stool samples, which will allow further investigation of unexpected events such as those described in FDA’s safety alert.”
Dr. Chen, who received the AGA Research Foundation’s 2016 Research Scholar Award for her work on the gut microbiome and inflammatory bowel disease, pointed out that FMT has also been studied as a way to prevent colonization and infection with certain drug resistant organisms, such as vancomycin-resistant Enterococcus.
“Therefore, it’s not that FMT is ‘bad;’ we just have to be more diligent about optimizing the safety of the procedure by screening for of multidrug-resistant organisms,” she said. “We also need to study the use of FMT more, so that we can fully understand the risks associated with the procedure. It’s an important and potentially lifesaving procedure for some, but it’s important that everyone go into the procedure understanding fully what the risks and benefits are.”
Suspected adverse events related to the administration of FMT products can be reported to the FDA at 1-800-332-1088 or via MedWatch.
Officials at the Food and Drug Administration have issued a safety alert regarding the use of fecal microbiota for transplantation and the risk of serious adverse reactions because of transmission of multidrug-resistant organisms (MDROs).
According to the alert, which was issued on June 13, 2019, the agency became aware of two immunocompromised adult patients who received investigational fecal microbiota for transplantation (FMT) and developed extended-spectrum beta-lactamase (EBSL)–producing Escherichia coli. One of the patients died.
“This is certainly a theoretical risk that we’ve known about,” Lea Ann Chen, MD, a gastroenterologist at New York University, said in an interview. “This announcement is important, because we probably don’t counsel patients specifically about this risk. We say there is a risk for transmission of infectious agents in general, but I think that probably very few counsel patients about a risk for transmission of MDROs.”
The donor stool and FMT used in the two patients were not tested for ESBL-producing gram-negative organisms prior to use.
As a result of these serious adverse reactions, the FDA has determined that the following donor screening and stool testing protections are needed for any investigational use of FMT.
- Donor screening with questions that specifically address risk factors for colonization with MDROs, and exclusion of individuals at higher risk of colonization with MDROs.
- MDRO testing of donor stool and exclusion of stool that tests positive for MDRO. FDA scientists have determined the specific MDRO testing and frequency that should be implemented.
On June 14, the American Gastroenterological Association sent a communication about the FDA alert to its members, which stated that the AGA “is committed to advancing applications of the gut microbiome. Our top priority is ensuring patient safety from microbiome-based therapeutics, such as FMT. Through the AGA FMT National Registry, AGA is working with physicians and patients to track FMT usage, patient outcomes and adverse events. Associated with the registry is a biorepository of donor and patient stool samples, which will allow further investigation of unexpected events such as those described in FDA’s safety alert.”
Dr. Chen, who received the AGA Research Foundation’s 2016 Research Scholar Award for her work on the gut microbiome and inflammatory bowel disease, pointed out that FMT has also been studied as a way to prevent colonization and infection with certain drug resistant organisms, such as vancomycin-resistant Enterococcus.
“Therefore, it’s not that FMT is ‘bad;’ we just have to be more diligent about optimizing the safety of the procedure by screening for of multidrug-resistant organisms,” she said. “We also need to study the use of FMT more, so that we can fully understand the risks associated with the procedure. It’s an important and potentially lifesaving procedure for some, but it’s important that everyone go into the procedure understanding fully what the risks and benefits are.”
Suspected adverse events related to the administration of FMT products can be reported to the FDA at 1-800-332-1088 or via MedWatch.
Officials at the Food and Drug Administration have issued a safety alert regarding the use of fecal microbiota for transplantation and the risk of serious adverse reactions because of transmission of multidrug-resistant organisms (MDROs).
According to the alert, which was issued on June 13, 2019, the agency became aware of two immunocompromised adult patients who received investigational fecal microbiota for transplantation (FMT) and developed extended-spectrum beta-lactamase (EBSL)–producing Escherichia coli. One of the patients died.
“This is certainly a theoretical risk that we’ve known about,” Lea Ann Chen, MD, a gastroenterologist at New York University, said in an interview. “This announcement is important, because we probably don’t counsel patients specifically about this risk. We say there is a risk for transmission of infectious agents in general, but I think that probably very few counsel patients about a risk for transmission of MDROs.”
The donor stool and FMT used in the two patients were not tested for ESBL-producing gram-negative organisms prior to use.
As a result of these serious adverse reactions, the FDA has determined that the following donor screening and stool testing protections are needed for any investigational use of FMT.
- Donor screening with questions that specifically address risk factors for colonization with MDROs, and exclusion of individuals at higher risk of colonization with MDROs.
- MDRO testing of donor stool and exclusion of stool that tests positive for MDRO. FDA scientists have determined the specific MDRO testing and frequency that should be implemented.
On June 14, the American Gastroenterological Association sent a communication about the FDA alert to its members, which stated that the AGA “is committed to advancing applications of the gut microbiome. Our top priority is ensuring patient safety from microbiome-based therapeutics, such as FMT. Through the AGA FMT National Registry, AGA is working with physicians and patients to track FMT usage, patient outcomes and adverse events. Associated with the registry is a biorepository of donor and patient stool samples, which will allow further investigation of unexpected events such as those described in FDA’s safety alert.”
Dr. Chen, who received the AGA Research Foundation’s 2016 Research Scholar Award for her work on the gut microbiome and inflammatory bowel disease, pointed out that FMT has also been studied as a way to prevent colonization and infection with certain drug resistant organisms, such as vancomycin-resistant Enterococcus.
“Therefore, it’s not that FMT is ‘bad;’ we just have to be more diligent about optimizing the safety of the procedure by screening for of multidrug-resistant organisms,” she said. “We also need to study the use of FMT more, so that we can fully understand the risks associated with the procedure. It’s an important and potentially lifesaving procedure for some, but it’s important that everyone go into the procedure understanding fully what the risks and benefits are.”
Suspected adverse events related to the administration of FMT products can be reported to the FDA at 1-800-332-1088 or via MedWatch.
Study IDs factors contributing to sleep clinic no-show rates
SAN ANTONIO – results from a single-center study showed.
“There are lots of people with sleep problems,” one of the study’s authors, Alvan Nzewuihe, MD, said in an interview at the annual meeting of the Associated Professional Sleep Societies. “However, we have to identify these patients to be able to help them. If they don’t come to the sleep clinic [for assessment], we are going nowhere.”
In an effort to better understand determinants of no-show rates among sleep clinics, Dr. Nzewuihe and colleagues performed a 10-month, retrospective chart review of 2,532 patients scheduled at Saint Louis University’s SLUCare Sleep Disorders Center during the months of July and October 2017 and April 2018. A no-show was determined if the patient failed to show up at their scheduled appointment on time or if they canceled their appointment. The researchers used multivariable logistic regression to determine which factors were independently associated with no-show rates.
Dr. Nzewuihe, a sleep medicine physician at the university, reported that the overall no-show rate during the study period was 21.2% and did not change with age, sex, or appointment factors such as time of day, day of week, or season of the year. Significant determinants of no-show rates included being a new patient (39.1% vs. 28.8% among established patients; P less than .0001) and having no health insurance (47.5% vs. public 28.3% vs private 24.2%; P less than .0001). Multivariate logistic regression confirmed the associations. New patients were 1.96 times more likely to not show up to the sleep clinic, compared with established patients, while patients with no health insurance coverage were 1.55 times more likely to not show up, compared with those with public health insurance.
The researchers wrote in their poster abstract, “Patients who are new to the clinic or have no insurance coverage have a higher odds of not showing up to their appointment and delaying their care. Efforts to prioritize high-risk patients of nonadherence will help contribute to better care and outcomes. Further studies are needed to develop methods to decrease no-show rates once high-risk appointments have been identified.”
Dr. Nzewuihe acknowledged certain limitations of the study, including its retrospective design and the fact that other possible contributing factors were not evaluated such as literacy level, employment status, and length of time between appointment booking and appointment date. The study’s first author was Julie Sahrmann, DO. The researchers reported having no financial disclosures.
SOURCE: Sahrmann J et al. Sleep 2019, Abstract 0965.
SAN ANTONIO – results from a single-center study showed.
“There are lots of people with sleep problems,” one of the study’s authors, Alvan Nzewuihe, MD, said in an interview at the annual meeting of the Associated Professional Sleep Societies. “However, we have to identify these patients to be able to help them. If they don’t come to the sleep clinic [for assessment], we are going nowhere.”
In an effort to better understand determinants of no-show rates among sleep clinics, Dr. Nzewuihe and colleagues performed a 10-month, retrospective chart review of 2,532 patients scheduled at Saint Louis University’s SLUCare Sleep Disorders Center during the months of July and October 2017 and April 2018. A no-show was determined if the patient failed to show up at their scheduled appointment on time or if they canceled their appointment. The researchers used multivariable logistic regression to determine which factors were independently associated with no-show rates.
Dr. Nzewuihe, a sleep medicine physician at the university, reported that the overall no-show rate during the study period was 21.2% and did not change with age, sex, or appointment factors such as time of day, day of week, or season of the year. Significant determinants of no-show rates included being a new patient (39.1% vs. 28.8% among established patients; P less than .0001) and having no health insurance (47.5% vs. public 28.3% vs private 24.2%; P less than .0001). Multivariate logistic regression confirmed the associations. New patients were 1.96 times more likely to not show up to the sleep clinic, compared with established patients, while patients with no health insurance coverage were 1.55 times more likely to not show up, compared with those with public health insurance.
The researchers wrote in their poster abstract, “Patients who are new to the clinic or have no insurance coverage have a higher odds of not showing up to their appointment and delaying their care. Efforts to prioritize high-risk patients of nonadherence will help contribute to better care and outcomes. Further studies are needed to develop methods to decrease no-show rates once high-risk appointments have been identified.”
Dr. Nzewuihe acknowledged certain limitations of the study, including its retrospective design and the fact that other possible contributing factors were not evaluated such as literacy level, employment status, and length of time between appointment booking and appointment date. The study’s first author was Julie Sahrmann, DO. The researchers reported having no financial disclosures.
SOURCE: Sahrmann J et al. Sleep 2019, Abstract 0965.
SAN ANTONIO – results from a single-center study showed.
“There are lots of people with sleep problems,” one of the study’s authors, Alvan Nzewuihe, MD, said in an interview at the annual meeting of the Associated Professional Sleep Societies. “However, we have to identify these patients to be able to help them. If they don’t come to the sleep clinic [for assessment], we are going nowhere.”
In an effort to better understand determinants of no-show rates among sleep clinics, Dr. Nzewuihe and colleagues performed a 10-month, retrospective chart review of 2,532 patients scheduled at Saint Louis University’s SLUCare Sleep Disorders Center during the months of July and October 2017 and April 2018. A no-show was determined if the patient failed to show up at their scheduled appointment on time or if they canceled their appointment. The researchers used multivariable logistic regression to determine which factors were independently associated with no-show rates.
Dr. Nzewuihe, a sleep medicine physician at the university, reported that the overall no-show rate during the study period was 21.2% and did not change with age, sex, or appointment factors such as time of day, day of week, or season of the year. Significant determinants of no-show rates included being a new patient (39.1% vs. 28.8% among established patients; P less than .0001) and having no health insurance (47.5% vs. public 28.3% vs private 24.2%; P less than .0001). Multivariate logistic regression confirmed the associations. New patients were 1.96 times more likely to not show up to the sleep clinic, compared with established patients, while patients with no health insurance coverage were 1.55 times more likely to not show up, compared with those with public health insurance.
The researchers wrote in their poster abstract, “Patients who are new to the clinic or have no insurance coverage have a higher odds of not showing up to their appointment and delaying their care. Efforts to prioritize high-risk patients of nonadherence will help contribute to better care and outcomes. Further studies are needed to develop methods to decrease no-show rates once high-risk appointments have been identified.”
Dr. Nzewuihe acknowledged certain limitations of the study, including its retrospective design and the fact that other possible contributing factors were not evaluated such as literacy level, employment status, and length of time between appointment booking and appointment date. The study’s first author was Julie Sahrmann, DO. The researchers reported having no financial disclosures.
SOURCE: Sahrmann J et al. Sleep 2019, Abstract 0965.
REPORTING FROM SLEEP 2019
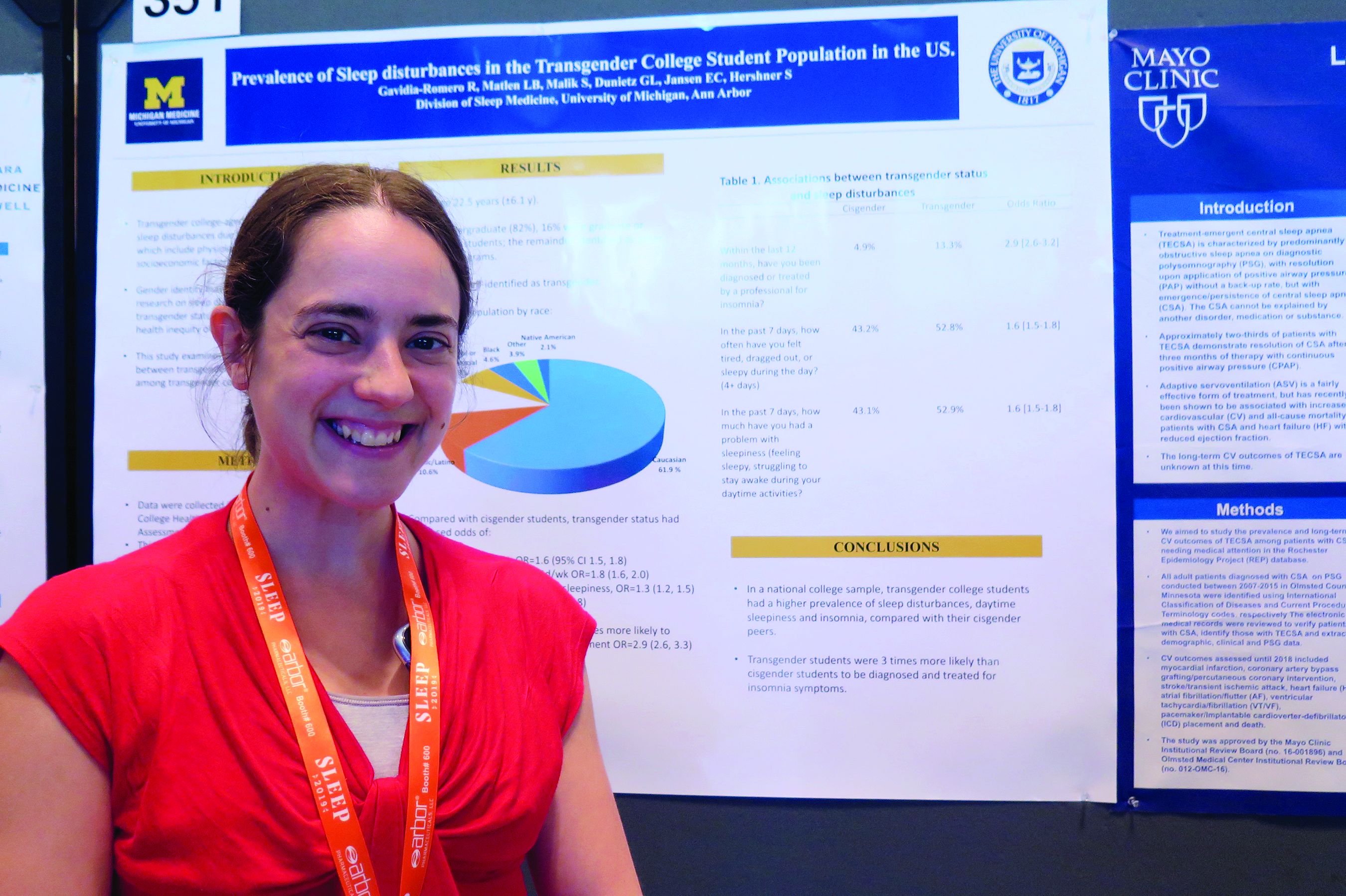
Insomnia common among transgender college students
SAN ANTONIO – Compared with their cisgender counterparts, , results from a large national population-based survey showed.
“That was a stronger association than we expected,” one of the study’s researchers, Lisa B. Matlen, MD, said during an interview at the annual meeting of the Associated Professional Sleep Societies.
According to Dr. Matlen, a fellow in the division of sleep medicine at the University of Michigan, Ann Arbor, the transgender population is “extremely understudied” when it comes to research on sleep disturbances. In an effort to examine the prevalence of sleep disturbances and the association between transgender identity and sleep disturbances among transgender college students in the United States, she and her colleagues drew from the 2016 and 2017 American College Health Association National College Health Assessment II, a confidential, voluntary, electronically administered survey of college and university students. In all, 224,233 students were polled, and the researchers analyzed their responses to questions about gender identity, sleep symptoms, and diagnoses.
The mean age of the respondents was 23 years, and most (82%) were undergraduate students. Of the 224,233 students, 3,471 (1.6%) self-identified as transgender. More than half of the transgender population (61.9%) was white, 10.6% were Hispanic/Latino, 10.5% were Asian or Pacific Islander, 6.3% were biracial or multiracial, 4.6% were black, and the rest were from other ethnicities. Compared with cisgender students, transgender students had increased odds of sleep disturbances (odds ratio, 1.6), not feeling well rested on 4 or more days per week (OR, 1.8), going to bed early on 3 or more days per week due to sleepiness (OR, 1.3), and having insomnia 3 or more days per week (OR, 1.7). In addition, transgender students were nearly three times more likely to have an insomnia diagnosis and treatment, compared with their cisgender counterparts (OR, 2.9).
Dr. Matlen acknowledged certain limitations of the study, including the fact that it drew from a population-based sample and that the survey was based on self-reported information. The study’s first author was Ronald R. Gavidia Romero, MD. The researchers reported having no financial disclosures.
SAN ANTONIO – Compared with their cisgender counterparts, , results from a large national population-based survey showed.
“That was a stronger association than we expected,” one of the study’s researchers, Lisa B. Matlen, MD, said during an interview at the annual meeting of the Associated Professional Sleep Societies.
According to Dr. Matlen, a fellow in the division of sleep medicine at the University of Michigan, Ann Arbor, the transgender population is “extremely understudied” when it comes to research on sleep disturbances. In an effort to examine the prevalence of sleep disturbances and the association between transgender identity and sleep disturbances among transgender college students in the United States, she and her colleagues drew from the 2016 and 2017 American College Health Association National College Health Assessment II, a confidential, voluntary, electronically administered survey of college and university students. In all, 224,233 students were polled, and the researchers analyzed their responses to questions about gender identity, sleep symptoms, and diagnoses.
The mean age of the respondents was 23 years, and most (82%) were undergraduate students. Of the 224,233 students, 3,471 (1.6%) self-identified as transgender. More than half of the transgender population (61.9%) was white, 10.6% were Hispanic/Latino, 10.5% were Asian or Pacific Islander, 6.3% were biracial or multiracial, 4.6% were black, and the rest were from other ethnicities. Compared with cisgender students, transgender students had increased odds of sleep disturbances (odds ratio, 1.6), not feeling well rested on 4 or more days per week (OR, 1.8), going to bed early on 3 or more days per week due to sleepiness (OR, 1.3), and having insomnia 3 or more days per week (OR, 1.7). In addition, transgender students were nearly three times more likely to have an insomnia diagnosis and treatment, compared with their cisgender counterparts (OR, 2.9).
Dr. Matlen acknowledged certain limitations of the study, including the fact that it drew from a population-based sample and that the survey was based on self-reported information. The study’s first author was Ronald R. Gavidia Romero, MD. The researchers reported having no financial disclosures.
SAN ANTONIO – Compared with their cisgender counterparts, , results from a large national population-based survey showed.
“That was a stronger association than we expected,” one of the study’s researchers, Lisa B. Matlen, MD, said during an interview at the annual meeting of the Associated Professional Sleep Societies.
According to Dr. Matlen, a fellow in the division of sleep medicine at the University of Michigan, Ann Arbor, the transgender population is “extremely understudied” when it comes to research on sleep disturbances. In an effort to examine the prevalence of sleep disturbances and the association between transgender identity and sleep disturbances among transgender college students in the United States, she and her colleagues drew from the 2016 and 2017 American College Health Association National College Health Assessment II, a confidential, voluntary, electronically administered survey of college and university students. In all, 224,233 students were polled, and the researchers analyzed their responses to questions about gender identity, sleep symptoms, and diagnoses.
The mean age of the respondents was 23 years, and most (82%) were undergraduate students. Of the 224,233 students, 3,471 (1.6%) self-identified as transgender. More than half of the transgender population (61.9%) was white, 10.6% were Hispanic/Latino, 10.5% were Asian or Pacific Islander, 6.3% were biracial or multiracial, 4.6% were black, and the rest were from other ethnicities. Compared with cisgender students, transgender students had increased odds of sleep disturbances (odds ratio, 1.6), not feeling well rested on 4 or more days per week (OR, 1.8), going to bed early on 3 or more days per week due to sleepiness (OR, 1.3), and having insomnia 3 or more days per week (OR, 1.7). In addition, transgender students were nearly three times more likely to have an insomnia diagnosis and treatment, compared with their cisgender counterparts (OR, 2.9).
Dr. Matlen acknowledged certain limitations of the study, including the fact that it drew from a population-based sample and that the survey was based on self-reported information. The study’s first author was Ronald R. Gavidia Romero, MD. The researchers reported having no financial disclosures.
REPORTING FROM SLEEP 2019
Estimated prevalence of OSA in the Americas stands at 170 million
, results from a novel epidemiologic analysis showed.
“I would not have thought that there are 170 million people in the Americas with clinically important sleep apnea based on our conservative estimates,” the study’s first author, Atul Malhotra, MD, said in an interview in advance of the annual meeting of the Associated Professional Sleep Societies. “Even if we restrict the conversation to moderate to severe sleep apnea, we still see 81 million people afflicted in the Americas alone. We have recently estimated almost 1 billion patients afflicted with OSA worldwide.”
In an effort to estimate the Americas’ prevalence of adult OSA using existing data from epidemiologic studies, Dr. Malhotra, director of sleep medicine at the University of California, San Diego, senior author Adam V. Benjafield, PhD, and their colleagues contacted authors of important analyses on the topic following an exhaustive review of the literature. For countries where no measurement had been made, they used publicly available data to obtain estimates of age, sex, race, and body mass index. Next, they developed an algorithm to match countries without prevalence estimates with countries from which OSA epidemiologic studies exist. “The situation was complicated given the variable age of the existing studies, the differences in technology used (e.g., nasal pressure vs. thermistor), the changing scoring criteria, and other sources of variability,” the researchers wrote in their abstract.
Dr. Malhotra reported on data from 38 of 40 countries in the Americas. Drawing from American Academy of Sleep Medicine 2012 criteria and using what they characterized as a “somewhat conservative” approach, the researchers estimated the prevalence of adult OSA in the Americas to be 170 million, or 37% of the population. In addition, they estimate that 81 million adults, or 18% of the population, suffer from moderate to severe OSA based on an apnea hypopnea index of 15 or more per hour. The countries with the greatest burden of OSA are the United States (54 million), Brazil (49 million), and Colombia (11 million).
“The findings will hopefully help to raise awareness about the disease but also encourage a strategic conversation regarding how best to address this large burden,” Dr. Malhotra said. “We are unaware of prior efforts to estimate OSA prevalence on a large scale.”
He acknowledged certain limitations of the study, including that the methods, equipment, definitions, and criteria used in existing studies in the medial literature varied widely. “We did our best to harmonize these methods across studies but obviously we can’t change the equipment that was used in previous studies,” he said. “Thus, we view our findings as an estimate requiring further efforts to corroborate.”
The research stemmed from an academic/industry partnership with ResMed, which provided a donation the UCSD Sleep Medicine Center. Dr. Malhotra reported having no financial disclosures. Dr. Benjafield is an employee of ResMed, a medical equipment company that specializes in sleep-related breathing devices.
SOURCE: Malhotra A et al. SLEEP 2019, Abstract 0477.
, results from a novel epidemiologic analysis showed.
“I would not have thought that there are 170 million people in the Americas with clinically important sleep apnea based on our conservative estimates,” the study’s first author, Atul Malhotra, MD, said in an interview in advance of the annual meeting of the Associated Professional Sleep Societies. “Even if we restrict the conversation to moderate to severe sleep apnea, we still see 81 million people afflicted in the Americas alone. We have recently estimated almost 1 billion patients afflicted with OSA worldwide.”
In an effort to estimate the Americas’ prevalence of adult OSA using existing data from epidemiologic studies, Dr. Malhotra, director of sleep medicine at the University of California, San Diego, senior author Adam V. Benjafield, PhD, and their colleagues contacted authors of important analyses on the topic following an exhaustive review of the literature. For countries where no measurement had been made, they used publicly available data to obtain estimates of age, sex, race, and body mass index. Next, they developed an algorithm to match countries without prevalence estimates with countries from which OSA epidemiologic studies exist. “The situation was complicated given the variable age of the existing studies, the differences in technology used (e.g., nasal pressure vs. thermistor), the changing scoring criteria, and other sources of variability,” the researchers wrote in their abstract.
Dr. Malhotra reported on data from 38 of 40 countries in the Americas. Drawing from American Academy of Sleep Medicine 2012 criteria and using what they characterized as a “somewhat conservative” approach, the researchers estimated the prevalence of adult OSA in the Americas to be 170 million, or 37% of the population. In addition, they estimate that 81 million adults, or 18% of the population, suffer from moderate to severe OSA based on an apnea hypopnea index of 15 or more per hour. The countries with the greatest burden of OSA are the United States (54 million), Brazil (49 million), and Colombia (11 million).
“The findings will hopefully help to raise awareness about the disease but also encourage a strategic conversation regarding how best to address this large burden,” Dr. Malhotra said. “We are unaware of prior efforts to estimate OSA prevalence on a large scale.”
He acknowledged certain limitations of the study, including that the methods, equipment, definitions, and criteria used in existing studies in the medial literature varied widely. “We did our best to harmonize these methods across studies but obviously we can’t change the equipment that was used in previous studies,” he said. “Thus, we view our findings as an estimate requiring further efforts to corroborate.”
The research stemmed from an academic/industry partnership with ResMed, which provided a donation the UCSD Sleep Medicine Center. Dr. Malhotra reported having no financial disclosures. Dr. Benjafield is an employee of ResMed, a medical equipment company that specializes in sleep-related breathing devices.
SOURCE: Malhotra A et al. SLEEP 2019, Abstract 0477.
, results from a novel epidemiologic analysis showed.
“I would not have thought that there are 170 million people in the Americas with clinically important sleep apnea based on our conservative estimates,” the study’s first author, Atul Malhotra, MD, said in an interview in advance of the annual meeting of the Associated Professional Sleep Societies. “Even if we restrict the conversation to moderate to severe sleep apnea, we still see 81 million people afflicted in the Americas alone. We have recently estimated almost 1 billion patients afflicted with OSA worldwide.”
In an effort to estimate the Americas’ prevalence of adult OSA using existing data from epidemiologic studies, Dr. Malhotra, director of sleep medicine at the University of California, San Diego, senior author Adam V. Benjafield, PhD, and their colleagues contacted authors of important analyses on the topic following an exhaustive review of the literature. For countries where no measurement had been made, they used publicly available data to obtain estimates of age, sex, race, and body mass index. Next, they developed an algorithm to match countries without prevalence estimates with countries from which OSA epidemiologic studies exist. “The situation was complicated given the variable age of the existing studies, the differences in technology used (e.g., nasal pressure vs. thermistor), the changing scoring criteria, and other sources of variability,” the researchers wrote in their abstract.
Dr. Malhotra reported on data from 38 of 40 countries in the Americas. Drawing from American Academy of Sleep Medicine 2012 criteria and using what they characterized as a “somewhat conservative” approach, the researchers estimated the prevalence of adult OSA in the Americas to be 170 million, or 37% of the population. In addition, they estimate that 81 million adults, or 18% of the population, suffer from moderate to severe OSA based on an apnea hypopnea index of 15 or more per hour. The countries with the greatest burden of OSA are the United States (54 million), Brazil (49 million), and Colombia (11 million).
“The findings will hopefully help to raise awareness about the disease but also encourage a strategic conversation regarding how best to address this large burden,” Dr. Malhotra said. “We are unaware of prior efforts to estimate OSA prevalence on a large scale.”
He acknowledged certain limitations of the study, including that the methods, equipment, definitions, and criteria used in existing studies in the medial literature varied widely. “We did our best to harmonize these methods across studies but obviously we can’t change the equipment that was used in previous studies,” he said. “Thus, we view our findings as an estimate requiring further efforts to corroborate.”
The research stemmed from an academic/industry partnership with ResMed, which provided a donation the UCSD Sleep Medicine Center. Dr. Malhotra reported having no financial disclosures. Dr. Benjafield is an employee of ResMed, a medical equipment company that specializes in sleep-related breathing devices.
SOURCE: Malhotra A et al. SLEEP 2019, Abstract 0477.
REPORTING FROM SLEEP 2019
Key clinical point: The large burden of OSA in the Americas has not been widely appreciated.
Major finding: The estimated prevalence of adult OSA in the Americas is 170 million, or 37% of the population.
Study details: An analysis of epidemiologic studies that included data on 38 countries in the Americas.
Disclosures: The research stemmed from an academic/industry partnership with ResMed, a medical equipment company that specializes in sleep-related breathing devices, which provided a donation the UCSD Sleep Medicine Center. Dr. Malhotra reported having no financial disclosures. Dr. Benjafield is an employee of ResMed.
Source: Malhotra A et al. SLEEP 2019, Abstract 0477.
Novel molecular panel found to aid in eosinophilic gastritis diagnosis
SAN DIEGO – A new scoring system based on a set of 18 informative genes was sufficient to diagnose cases of eosinophilic gastritis, results from a molecular analysis showed.
The system, known as EG diagnostic panel 18 (EGDP18), “can provide clinicians with a better diagnostic classification of ambiguous cases of eosinophilic gastritis, indicating a strong correlation with disease severity,” lead study author Tetsuo Shoda, MD, PhD, said at the annual Digestive Disease Week®.“The EG molecular profile also strongly correlates with particular endoscopic and histological features, thus providing insight into pathogenesis for EG.”
In an effort to develop an EG diagnostic panel, to validate its utility for EG diagnosis and management, and to better understand disease pathogenesis, Dr. Shoda and his colleagues used RNA sequencing to generate genome-wide gene expression profiles from gastric biopsies. Next, they developed an EG diagnostic panel focusing on a set of 48 informative genes, and analyzed its performance in a discovery cohort (55 EG and 39 controls) and subsequently an independent validation cohort (67 EG and 27 controls). The EGDP score was calculated by summation of delta CT values of the most highly dysregulated 18 genes. For diagnosis, the researchers calculated the area under the receiver operating characteristic curve (AUC), and used Spearman correlation to analyze associations.
Dr. Shoda, a research fellow at Cincinnati Children’s Hospital Medical Center, reported that the EGDP18 score identified active EG patients in both cohorts (P less than .0001, AUC equal to or greater than 0.95). In the discovery cohort, a score of less than zero resulted in a sensitivity of 95.2%, a specificity of 100%, a positive predictive value of 100%, and a negative predictive value of 95.8%. In the validation cohort, a score of less than zero resulted in a sensitivity of 88.9%, a specificity of 100%, a positive predictive value of 100%, and a negative predictive value of 94.1%.
The researchers observed a significant inverse correlation between the EGDP18 score and gastric eosinophil counts cross-sectionally and longitudinally. The score also showed comparable levels and high correlation between the gastric antrum and body. In addition, when analyzed by EGDP18 score, 63% of ambiguous tissue eosinophils were found to be molecularly equivalent to active EG, “suggesting the capacity to offer an objective cutoff for EG diagnosis,” Dr. Shoda said.
The researchers reported having no financial disclosures.
SOURCE: Shoda T et al. DDW 2019, Abstract 165. doi: 10.1016/S0016-5085(19)36878-7.
SAN DIEGO – A new scoring system based on a set of 18 informative genes was sufficient to diagnose cases of eosinophilic gastritis, results from a molecular analysis showed.
The system, known as EG diagnostic panel 18 (EGDP18), “can provide clinicians with a better diagnostic classification of ambiguous cases of eosinophilic gastritis, indicating a strong correlation with disease severity,” lead study author Tetsuo Shoda, MD, PhD, said at the annual Digestive Disease Week®.“The EG molecular profile also strongly correlates with particular endoscopic and histological features, thus providing insight into pathogenesis for EG.”
In an effort to develop an EG diagnostic panel, to validate its utility for EG diagnosis and management, and to better understand disease pathogenesis, Dr. Shoda and his colleagues used RNA sequencing to generate genome-wide gene expression profiles from gastric biopsies. Next, they developed an EG diagnostic panel focusing on a set of 48 informative genes, and analyzed its performance in a discovery cohort (55 EG and 39 controls) and subsequently an independent validation cohort (67 EG and 27 controls). The EGDP score was calculated by summation of delta CT values of the most highly dysregulated 18 genes. For diagnosis, the researchers calculated the area under the receiver operating characteristic curve (AUC), and used Spearman correlation to analyze associations.
Dr. Shoda, a research fellow at Cincinnati Children’s Hospital Medical Center, reported that the EGDP18 score identified active EG patients in both cohorts (P less than .0001, AUC equal to or greater than 0.95). In the discovery cohort, a score of less than zero resulted in a sensitivity of 95.2%, a specificity of 100%, a positive predictive value of 100%, and a negative predictive value of 95.8%. In the validation cohort, a score of less than zero resulted in a sensitivity of 88.9%, a specificity of 100%, a positive predictive value of 100%, and a negative predictive value of 94.1%.
The researchers observed a significant inverse correlation between the EGDP18 score and gastric eosinophil counts cross-sectionally and longitudinally. The score also showed comparable levels and high correlation between the gastric antrum and body. In addition, when analyzed by EGDP18 score, 63% of ambiguous tissue eosinophils were found to be molecularly equivalent to active EG, “suggesting the capacity to offer an objective cutoff for EG diagnosis,” Dr. Shoda said.
The researchers reported having no financial disclosures.
SOURCE: Shoda T et al. DDW 2019, Abstract 165. doi: 10.1016/S0016-5085(19)36878-7.
SAN DIEGO – A new scoring system based on a set of 18 informative genes was sufficient to diagnose cases of eosinophilic gastritis, results from a molecular analysis showed.
The system, known as EG diagnostic panel 18 (EGDP18), “can provide clinicians with a better diagnostic classification of ambiguous cases of eosinophilic gastritis, indicating a strong correlation with disease severity,” lead study author Tetsuo Shoda, MD, PhD, said at the annual Digestive Disease Week®.“The EG molecular profile also strongly correlates with particular endoscopic and histological features, thus providing insight into pathogenesis for EG.”
In an effort to develop an EG diagnostic panel, to validate its utility for EG diagnosis and management, and to better understand disease pathogenesis, Dr. Shoda and his colleagues used RNA sequencing to generate genome-wide gene expression profiles from gastric biopsies. Next, they developed an EG diagnostic panel focusing on a set of 48 informative genes, and analyzed its performance in a discovery cohort (55 EG and 39 controls) and subsequently an independent validation cohort (67 EG and 27 controls). The EGDP score was calculated by summation of delta CT values of the most highly dysregulated 18 genes. For diagnosis, the researchers calculated the area under the receiver operating characteristic curve (AUC), and used Spearman correlation to analyze associations.
Dr. Shoda, a research fellow at Cincinnati Children’s Hospital Medical Center, reported that the EGDP18 score identified active EG patients in both cohorts (P less than .0001, AUC equal to or greater than 0.95). In the discovery cohort, a score of less than zero resulted in a sensitivity of 95.2%, a specificity of 100%, a positive predictive value of 100%, and a negative predictive value of 95.8%. In the validation cohort, a score of less than zero resulted in a sensitivity of 88.9%, a specificity of 100%, a positive predictive value of 100%, and a negative predictive value of 94.1%.
The researchers observed a significant inverse correlation between the EGDP18 score and gastric eosinophil counts cross-sectionally and longitudinally. The score also showed comparable levels and high correlation between the gastric antrum and body. In addition, when analyzed by EGDP18 score, 63% of ambiguous tissue eosinophils were found to be molecularly equivalent to active EG, “suggesting the capacity to offer an objective cutoff for EG diagnosis,” Dr. Shoda said.
The researchers reported having no financial disclosures.
SOURCE: Shoda T et al. DDW 2019, Abstract 165. doi: 10.1016/S0016-5085(19)36878-7.
REPORTING FROM DDW 2019
Persistent fatigue plagues many IBD patients
SAN DIEGO – .
“Fatigue is one of the most heard complaints in the clinic,” lead study author Nynke Z. Borren, MD, said in an interview at the annual Digestive Disease Week.® “In the past few years there has been more interest because we know there is a communication system between the gut and the brain. Some studies suggest that biologic therapy improves fatigue symptoms, but it’s really correlated with disease activity.”
In an effort to define the longitudinal trajectory of fatigue in IBD patients initiating treatment with infliximab, adalimumab, vedolizumab, or ustekinumab, Dr. Borren, a research fellow at the Massachusetts General Hospital Crohn’s and Colitis Center, Boston, and colleagues prospectively enrolled 206 patients with Crohn’s disease and 120 patients with ulcerative colitis. They used the seven-point fatigue question in the Short Inflammatory Bowel Disease Questionnaire (SIBDQ) to define fatigue. A score of four or less for this question was used to define fatigue. To validate this question, the researchers used two widely used questionnaires: the Functional Assessment of Chronic Illness Therapy–Fatigue (FACIT-F), and the Multidimensional Fatigue inventory (MFI). Next, they used multivariable regression models to examine the independent association between attainment of clinical remission and the resolution of fatigue.
Of the 326 patients, 134 initiated biologic therapy with infliximab or adalimumab, 129 with vedolizumab, and 63 with ustekinumab. Nearly two-thirds of all patients (198, or 61%) reported significant fatigue at baseline, which was associated with female sex, depressive symptoms, and disturbed sleep (P less than .001). Those reporting significant fatigue at baseline also had higher disease activity scores, compared with those without fatigue (P less than .001).
Among the 198 patients who reported fatigue at baseline, 70% remained fatigued at week 14, while 63% remained fatigued at week 30, and 61% remained fatigued at week 54. Dr. Borren and associates observed that at each of these time points, achieving clinical remission was associated with threefold lower likelihood of remaining fatigued. However, 35% of patients who achieved clinical remission experienced persistent fatigue at week 14, compared with 37% of patients at week 30 and 35% of patients at week 54.
The researchers observed no significant differences between the different therapies in the proportion of patients who remained fatigued. In addition to disease activity, disturbed sleep at baseline was associated with persistent fatigue at week 14 (OR 9.7) and at week 30 (OR 3.7).
“We think that gut dysbiosis might be involved in inducing fatigue,” Dr. Borren said. “In the beginning, we thought that it might be due to ongoing inflammation, but our research has shown that we find a less diverse gut microbiome in those patients with fatigue compared to patients without fatigue while they were in remission. There is something in the gut that influences the central nervous system. We are still exploring this.”
The researchers reported having no financial disclosures. The abstract received a “poster of distinction” honor at the meeting.
SAN DIEGO – .
“Fatigue is one of the most heard complaints in the clinic,” lead study author Nynke Z. Borren, MD, said in an interview at the annual Digestive Disease Week.® “In the past few years there has been more interest because we know there is a communication system between the gut and the brain. Some studies suggest that biologic therapy improves fatigue symptoms, but it’s really correlated with disease activity.”
In an effort to define the longitudinal trajectory of fatigue in IBD patients initiating treatment with infliximab, adalimumab, vedolizumab, or ustekinumab, Dr. Borren, a research fellow at the Massachusetts General Hospital Crohn’s and Colitis Center, Boston, and colleagues prospectively enrolled 206 patients with Crohn’s disease and 120 patients with ulcerative colitis. They used the seven-point fatigue question in the Short Inflammatory Bowel Disease Questionnaire (SIBDQ) to define fatigue. A score of four or less for this question was used to define fatigue. To validate this question, the researchers used two widely used questionnaires: the Functional Assessment of Chronic Illness Therapy–Fatigue (FACIT-F), and the Multidimensional Fatigue inventory (MFI). Next, they used multivariable regression models to examine the independent association between attainment of clinical remission and the resolution of fatigue.
Of the 326 patients, 134 initiated biologic therapy with infliximab or adalimumab, 129 with vedolizumab, and 63 with ustekinumab. Nearly two-thirds of all patients (198, or 61%) reported significant fatigue at baseline, which was associated with female sex, depressive symptoms, and disturbed sleep (P less than .001). Those reporting significant fatigue at baseline also had higher disease activity scores, compared with those without fatigue (P less than .001).
Among the 198 patients who reported fatigue at baseline, 70% remained fatigued at week 14, while 63% remained fatigued at week 30, and 61% remained fatigued at week 54. Dr. Borren and associates observed that at each of these time points, achieving clinical remission was associated with threefold lower likelihood of remaining fatigued. However, 35% of patients who achieved clinical remission experienced persistent fatigue at week 14, compared with 37% of patients at week 30 and 35% of patients at week 54.
The researchers observed no significant differences between the different therapies in the proportion of patients who remained fatigued. In addition to disease activity, disturbed sleep at baseline was associated with persistent fatigue at week 14 (OR 9.7) and at week 30 (OR 3.7).
“We think that gut dysbiosis might be involved in inducing fatigue,” Dr. Borren said. “In the beginning, we thought that it might be due to ongoing inflammation, but our research has shown that we find a less diverse gut microbiome in those patients with fatigue compared to patients without fatigue while they were in remission. There is something in the gut that influences the central nervous system. We are still exploring this.”
The researchers reported having no financial disclosures. The abstract received a “poster of distinction” honor at the meeting.
SAN DIEGO – .
“Fatigue is one of the most heard complaints in the clinic,” lead study author Nynke Z. Borren, MD, said in an interview at the annual Digestive Disease Week.® “In the past few years there has been more interest because we know there is a communication system between the gut and the brain. Some studies suggest that biologic therapy improves fatigue symptoms, but it’s really correlated with disease activity.”
In an effort to define the longitudinal trajectory of fatigue in IBD patients initiating treatment with infliximab, adalimumab, vedolizumab, or ustekinumab, Dr. Borren, a research fellow at the Massachusetts General Hospital Crohn’s and Colitis Center, Boston, and colleagues prospectively enrolled 206 patients with Crohn’s disease and 120 patients with ulcerative colitis. They used the seven-point fatigue question in the Short Inflammatory Bowel Disease Questionnaire (SIBDQ) to define fatigue. A score of four or less for this question was used to define fatigue. To validate this question, the researchers used two widely used questionnaires: the Functional Assessment of Chronic Illness Therapy–Fatigue (FACIT-F), and the Multidimensional Fatigue inventory (MFI). Next, they used multivariable regression models to examine the independent association between attainment of clinical remission and the resolution of fatigue.
Of the 326 patients, 134 initiated biologic therapy with infliximab or adalimumab, 129 with vedolizumab, and 63 with ustekinumab. Nearly two-thirds of all patients (198, or 61%) reported significant fatigue at baseline, which was associated with female sex, depressive symptoms, and disturbed sleep (P less than .001). Those reporting significant fatigue at baseline also had higher disease activity scores, compared with those without fatigue (P less than .001).
Among the 198 patients who reported fatigue at baseline, 70% remained fatigued at week 14, while 63% remained fatigued at week 30, and 61% remained fatigued at week 54. Dr. Borren and associates observed that at each of these time points, achieving clinical remission was associated with threefold lower likelihood of remaining fatigued. However, 35% of patients who achieved clinical remission experienced persistent fatigue at week 14, compared with 37% of patients at week 30 and 35% of patients at week 54.
The researchers observed no significant differences between the different therapies in the proportion of patients who remained fatigued. In addition to disease activity, disturbed sleep at baseline was associated with persistent fatigue at week 14 (OR 9.7) and at week 30 (OR 3.7).
“We think that gut dysbiosis might be involved in inducing fatigue,” Dr. Borren said. “In the beginning, we thought that it might be due to ongoing inflammation, but our research has shown that we find a less diverse gut microbiome in those patients with fatigue compared to patients without fatigue while they were in remission. There is something in the gut that influences the central nervous system. We are still exploring this.”
The researchers reported having no financial disclosures. The abstract received a “poster of distinction” honor at the meeting.
REPORTING FROM DDW 2019
Asymptomatic gallstones seldom require surgical intervention
“Most patients with asymptomatic gallstones never develop symptoms and probably don’t need surgical intervention,” lead study author Gareth Morris-Stiff, MD, PhD, said at the annual Digestive Disease Week.
Dr. Morris-Stiff, of the department of general surgery at Cleveland Clinic, said that, while previous studies have evaluated the time to development of gallstone-related complications following identification of asymptomatic gallstones, factors associated with the need for surgical intervention in this population have not been documented. The aims of the current study were to perform a big data analysis to evaluate risk factors associated with intervention in asymptomatic gallstones and to develop a risk stratification tool to aid in patient consultations by predicting individuals likely to need future intervention for their gallstones.
The researchers included Cleveland Clinic patients with CT/US reports containing “cholelithiasis” or “gallstones” between January 1996 and December 2016. Patients were excluded if they had a concurrent or prior event, had an event within 2 months, or lacked follow-up. Data collection included demographic characteristics, comorbid conditions or surgeries, imaging features, and medication use.
Dr. Morris-Stiff and his colleagues constructed Kaplan-Meier curves to analyze time to intervention and calculated cumulative incidence ratios. They used automated forward stepwise competing risk regression to create their model and receiver operating characteristics curves to analyze it.
Of the 49,414 patients identified with asymptomatic gallstones, 22,257 met criteria for analysis. Slightly more than half (51%) were female, their mean age was 61 years, 80% were white, 16% were black, and the rest were from other racial and ethnic groups. The median follow-up was 4.5 years, and the median follow-up of patients undergoing intervention was 3.9 years. This translated to 112,111 total years of observation.
The researchers found that the cumulative incidence of intervention at 15 years was 25% and it increased linearly from the time of initial diagnosis of asymptomatic gallstones. A total of 1,762 patients (7.9%) underwent a surgical procedure, most often cholecystectomy (5.7%). Three factors were associated with a reduced risk for surgical intervention: increasing age (hazard ratio, 0.94; P less than 0.001), male gender (HR, 0.78; P less than 0.001), and statin use (HR, 0.67; P less than 0.001).
Patient variables associated with an increased need for surgical intervention included obesity (HR, 1.44; P less than 0.001) and having a hemolytic disorder (HR, 2.42; P less than 0.001). Gallstone-specific characteristics that increased the need for surgical intervention included a stone size of greater than 9 mm (HR, 1.56; P less than 0.001), the presence of sludge (HR, 1.46; P less than 0.001), the presence of a polyp (HR, 1.68; P less than 0.001), and having multiple stones (HR, 1.69; P less than 0.001).
The analysis enabled Dr. Morris-Stiff and colleagues to generate a Web-based risk score to reliably identify these patients and provide prognostic information for counseling. An app for smartphones based on the score is being developed. The researchers reported having no financial disclosures.
“Most patients with asymptomatic gallstones never develop symptoms and probably don’t need surgical intervention,” lead study author Gareth Morris-Stiff, MD, PhD, said at the annual Digestive Disease Week.
Dr. Morris-Stiff, of the department of general surgery at Cleveland Clinic, said that, while previous studies have evaluated the time to development of gallstone-related complications following identification of asymptomatic gallstones, factors associated with the need for surgical intervention in this population have not been documented. The aims of the current study were to perform a big data analysis to evaluate risk factors associated with intervention in asymptomatic gallstones and to develop a risk stratification tool to aid in patient consultations by predicting individuals likely to need future intervention for their gallstones.
The researchers included Cleveland Clinic patients with CT/US reports containing “cholelithiasis” or “gallstones” between January 1996 and December 2016. Patients were excluded if they had a concurrent or prior event, had an event within 2 months, or lacked follow-up. Data collection included demographic characteristics, comorbid conditions or surgeries, imaging features, and medication use.
Dr. Morris-Stiff and his colleagues constructed Kaplan-Meier curves to analyze time to intervention and calculated cumulative incidence ratios. They used automated forward stepwise competing risk regression to create their model and receiver operating characteristics curves to analyze it.
Of the 49,414 patients identified with asymptomatic gallstones, 22,257 met criteria for analysis. Slightly more than half (51%) were female, their mean age was 61 years, 80% were white, 16% were black, and the rest were from other racial and ethnic groups. The median follow-up was 4.5 years, and the median follow-up of patients undergoing intervention was 3.9 years. This translated to 112,111 total years of observation.
The researchers found that the cumulative incidence of intervention at 15 years was 25% and it increased linearly from the time of initial diagnosis of asymptomatic gallstones. A total of 1,762 patients (7.9%) underwent a surgical procedure, most often cholecystectomy (5.7%). Three factors were associated with a reduced risk for surgical intervention: increasing age (hazard ratio, 0.94; P less than 0.001), male gender (HR, 0.78; P less than 0.001), and statin use (HR, 0.67; P less than 0.001).
Patient variables associated with an increased need for surgical intervention included obesity (HR, 1.44; P less than 0.001) and having a hemolytic disorder (HR, 2.42; P less than 0.001). Gallstone-specific characteristics that increased the need for surgical intervention included a stone size of greater than 9 mm (HR, 1.56; P less than 0.001), the presence of sludge (HR, 1.46; P less than 0.001), the presence of a polyp (HR, 1.68; P less than 0.001), and having multiple stones (HR, 1.69; P less than 0.001).
The analysis enabled Dr. Morris-Stiff and colleagues to generate a Web-based risk score to reliably identify these patients and provide prognostic information for counseling. An app for smartphones based on the score is being developed. The researchers reported having no financial disclosures.
“Most patients with asymptomatic gallstones never develop symptoms and probably don’t need surgical intervention,” lead study author Gareth Morris-Stiff, MD, PhD, said at the annual Digestive Disease Week.
Dr. Morris-Stiff, of the department of general surgery at Cleveland Clinic, said that, while previous studies have evaluated the time to development of gallstone-related complications following identification of asymptomatic gallstones, factors associated with the need for surgical intervention in this population have not been documented. The aims of the current study were to perform a big data analysis to evaluate risk factors associated with intervention in asymptomatic gallstones and to develop a risk stratification tool to aid in patient consultations by predicting individuals likely to need future intervention for their gallstones.
The researchers included Cleveland Clinic patients with CT/US reports containing “cholelithiasis” or “gallstones” between January 1996 and December 2016. Patients were excluded if they had a concurrent or prior event, had an event within 2 months, or lacked follow-up. Data collection included demographic characteristics, comorbid conditions or surgeries, imaging features, and medication use.
Dr. Morris-Stiff and his colleagues constructed Kaplan-Meier curves to analyze time to intervention and calculated cumulative incidence ratios. They used automated forward stepwise competing risk regression to create their model and receiver operating characteristics curves to analyze it.
Of the 49,414 patients identified with asymptomatic gallstones, 22,257 met criteria for analysis. Slightly more than half (51%) were female, their mean age was 61 years, 80% were white, 16% were black, and the rest were from other racial and ethnic groups. The median follow-up was 4.5 years, and the median follow-up of patients undergoing intervention was 3.9 years. This translated to 112,111 total years of observation.
The researchers found that the cumulative incidence of intervention at 15 years was 25% and it increased linearly from the time of initial diagnosis of asymptomatic gallstones. A total of 1,762 patients (7.9%) underwent a surgical procedure, most often cholecystectomy (5.7%). Three factors were associated with a reduced risk for surgical intervention: increasing age (hazard ratio, 0.94; P less than 0.001), male gender (HR, 0.78; P less than 0.001), and statin use (HR, 0.67; P less than 0.001).
Patient variables associated with an increased need for surgical intervention included obesity (HR, 1.44; P less than 0.001) and having a hemolytic disorder (HR, 2.42; P less than 0.001). Gallstone-specific characteristics that increased the need for surgical intervention included a stone size of greater than 9 mm (HR, 1.56; P less than 0.001), the presence of sludge (HR, 1.46; P less than 0.001), the presence of a polyp (HR, 1.68; P less than 0.001), and having multiple stones (HR, 1.69; P less than 0.001).
The analysis enabled Dr. Morris-Stiff and colleagues to generate a Web-based risk score to reliably identify these patients and provide prognostic information for counseling. An app for smartphones based on the score is being developed. The researchers reported having no financial disclosures.
REPORTING FROM DDW 2019
Meta-analysis finds no link between PPI use and risk of dementia
The finding runs counter to recent studies, including a large pharmacoepidemiological claims data analysis from Germany, that propose an association between proton pump inhibitor (PPI) use and the development of dementia (JAMA Neurol. 2016;73[4]:410-6). “The issue with these studies is that they’re based on retrospective claims data and pharmacoepidemiological studies and insurance databases that don’t really give you a good causality basis,” lead study author Saad Alrajhi, MD, said in an interview at the annual Digestive Disease Week.
In an effort to better characterize the association between PPI exposure and dementia, Dr. Alrajhi, a gastroenterology fellow at McGill University, Montreal, and colleagues conducted a meta-analysis of all fully published randomized clinical trials or observational studies comparing use of PPIs and occurrence of dementia. The researchers queried Embase, MEDLINE, and ISI Web of Knowledge for relevant studies that were published from 1995 through September 2018. Next, they assessed the quality of the studies by using the Cochrane risk assessment tool for RCTs or the Newcastle-Ottawa Scale for observational studies.
As the primary outcome, the researchers compared dementia incidence after PPI exposure (experimental group) versus no PPI exposure (control group). Development of Alzheimer’s dementia was a secondary outcome. Sensitivity analyses consisted of excluding one study at a time, and assessing results among studies of highest qualities. Subgroup analyses included stratifying patients by age. To report odds ratios, Dr. Alrajhi and colleagues used fixed or random effects models based on the absence or presence of heterogeneity.
Of 549 studies assessed, 5 met the criteria for inclusion in the final analysis: 3 case-control studies and 2 cohort studies, with a total of 472,933 patients. All of the studies scored 8 or 9 on the Newcastle-Ottawa scale, indicating high quality. Significant heterogeneity was noted for all analyses. The researchers found that the incidence of dementia was not significantly increased among patients in the PPI-exposed group (odd ratio, 1.08 (95% confidence interval, 0.97-1.20; P = .18). Sensitivity analyses confirmed the robustness of the results. Subgroup analysis showed no between-group differences among studies that included a minimum age above 65 years (three studies) or less than age 65 (two studies). PPI exposure was not associated with the development of Alzheimer’s dementia (two studies) (OR, 1.32 (95% CI, 0.80-2.17; P = .27).
“In the absence of randomized trial evidence, a PPI prescribing approach based on appropriate utilization of guideline-based prescription should be done without the extra fear of the association of dementia,” Dr. Alrajhi said.
The researchers reported having no financial disclosures.
The finding runs counter to recent studies, including a large pharmacoepidemiological claims data analysis from Germany, that propose an association between proton pump inhibitor (PPI) use and the development of dementia (JAMA Neurol. 2016;73[4]:410-6). “The issue with these studies is that they’re based on retrospective claims data and pharmacoepidemiological studies and insurance databases that don’t really give you a good causality basis,” lead study author Saad Alrajhi, MD, said in an interview at the annual Digestive Disease Week.
In an effort to better characterize the association between PPI exposure and dementia, Dr. Alrajhi, a gastroenterology fellow at McGill University, Montreal, and colleagues conducted a meta-analysis of all fully published randomized clinical trials or observational studies comparing use of PPIs and occurrence of dementia. The researchers queried Embase, MEDLINE, and ISI Web of Knowledge for relevant studies that were published from 1995 through September 2018. Next, they assessed the quality of the studies by using the Cochrane risk assessment tool for RCTs or the Newcastle-Ottawa Scale for observational studies.
As the primary outcome, the researchers compared dementia incidence after PPI exposure (experimental group) versus no PPI exposure (control group). Development of Alzheimer’s dementia was a secondary outcome. Sensitivity analyses consisted of excluding one study at a time, and assessing results among studies of highest qualities. Subgroup analyses included stratifying patients by age. To report odds ratios, Dr. Alrajhi and colleagues used fixed or random effects models based on the absence or presence of heterogeneity.
Of 549 studies assessed, 5 met the criteria for inclusion in the final analysis: 3 case-control studies and 2 cohort studies, with a total of 472,933 patients. All of the studies scored 8 or 9 on the Newcastle-Ottawa scale, indicating high quality. Significant heterogeneity was noted for all analyses. The researchers found that the incidence of dementia was not significantly increased among patients in the PPI-exposed group (odd ratio, 1.08 (95% confidence interval, 0.97-1.20; P = .18). Sensitivity analyses confirmed the robustness of the results. Subgroup analysis showed no between-group differences among studies that included a minimum age above 65 years (three studies) or less than age 65 (two studies). PPI exposure was not associated with the development of Alzheimer’s dementia (two studies) (OR, 1.32 (95% CI, 0.80-2.17; P = .27).
“In the absence of randomized trial evidence, a PPI prescribing approach based on appropriate utilization of guideline-based prescription should be done without the extra fear of the association of dementia,” Dr. Alrajhi said.
The researchers reported having no financial disclosures.
The finding runs counter to recent studies, including a large pharmacoepidemiological claims data analysis from Germany, that propose an association between proton pump inhibitor (PPI) use and the development of dementia (JAMA Neurol. 2016;73[4]:410-6). “The issue with these studies is that they’re based on retrospective claims data and pharmacoepidemiological studies and insurance databases that don’t really give you a good causality basis,” lead study author Saad Alrajhi, MD, said in an interview at the annual Digestive Disease Week.
In an effort to better characterize the association between PPI exposure and dementia, Dr. Alrajhi, a gastroenterology fellow at McGill University, Montreal, and colleagues conducted a meta-analysis of all fully published randomized clinical trials or observational studies comparing use of PPIs and occurrence of dementia. The researchers queried Embase, MEDLINE, and ISI Web of Knowledge for relevant studies that were published from 1995 through September 2018. Next, they assessed the quality of the studies by using the Cochrane risk assessment tool for RCTs or the Newcastle-Ottawa Scale for observational studies.
As the primary outcome, the researchers compared dementia incidence after PPI exposure (experimental group) versus no PPI exposure (control group). Development of Alzheimer’s dementia was a secondary outcome. Sensitivity analyses consisted of excluding one study at a time, and assessing results among studies of highest qualities. Subgroup analyses included stratifying patients by age. To report odds ratios, Dr. Alrajhi and colleagues used fixed or random effects models based on the absence or presence of heterogeneity.
Of 549 studies assessed, 5 met the criteria for inclusion in the final analysis: 3 case-control studies and 2 cohort studies, with a total of 472,933 patients. All of the studies scored 8 or 9 on the Newcastle-Ottawa scale, indicating high quality. Significant heterogeneity was noted for all analyses. The researchers found that the incidence of dementia was not significantly increased among patients in the PPI-exposed group (odd ratio, 1.08 (95% confidence interval, 0.97-1.20; P = .18). Sensitivity analyses confirmed the robustness of the results. Subgroup analysis showed no between-group differences among studies that included a minimum age above 65 years (three studies) or less than age 65 (two studies). PPI exposure was not associated with the development of Alzheimer’s dementia (two studies) (OR, 1.32 (95% CI, 0.80-2.17; P = .27).
“In the absence of randomized trial evidence, a PPI prescribing approach based on appropriate utilization of guideline-based prescription should be done without the extra fear of the association of dementia,” Dr. Alrajhi said.
The researchers reported having no financial disclosures.
REPORTING FROM DDW 2019
Cultural competence behaviors linked to higher patient satisfaction scores
results from a single-center study showed.
“Cultural competence is valued by patients, and there is a potential for training providers to be more culturally competent, focusing on their behaviors,” lead study author Miquell O. Miller, MD, said at the annual Digestive Disease Week.
While the Society for Surgery of the Alimentary Tract (J Gastrointest Surg 2016;20[5]:879-84) and other medical organizations have recognized the importance of workforce diversity and cultural competence of providers, little is known of the relationship between cultural competence and patient-reported outcomes in surgery, said Dr. Miller, who is a general surgery resident at Stanford (Calif.) University. To investigate this relationship, she and her colleagues recruited surgeons, oncologists, gastroenterologists, and advanced practice providers to complete a validated online survey that measured two domains of cultural competency: awareness and behaviors. They matched these scores with the 10-item Press Ganey provider care scores from 2017 to 2018. Next, the researchers conducted a linear regression analysis with mixed effects to account for clustering of patients within providers. They also adjusted for provider bias by measuring social desirability, “which is the tendency for respondents to put a more socially appropriate answer as opposed to the true answer on the cultural competence survey,” Dr. Miller explained.
A total of 1,322 Press Ganey satisfaction surveys were available for 29 providers. Their mean age was 48 years, 59% were white, 72% were physicians, and 28% were advanced practice providers. They practiced in GI oncology (41%), gastroenterology (31%) and colorectal surgery (28%). Dr. Miller reported that providers who participated in the survey had a mean cultural awareness score of 6.2 out of a possible 7 points, while the mean cultural behavior score was a 4.1 out of a possible 7 points. She and her colleagues observed that providers who had high levels of cultural competence on the behavioral assessment were positively associated with Press Ganey patient satisfaction (P = .039).
“I think we do a poor job of training our providers to be culturally competent, but there are multiple ways to improve behaviors, by teaching people and by having real training for our providers,” Dr. Miller said.
She acknowledged certain limitations of the study, including its single-center design and the fact that not all providers had Press Ganey scores available. The study was funded by the Society for Surgery of the Alimentary Tract and the Black Academic Surgeons Resident Research Award. Dr. Miller reported having no financial disclosures.
results from a single-center study showed.
“Cultural competence is valued by patients, and there is a potential for training providers to be more culturally competent, focusing on their behaviors,” lead study author Miquell O. Miller, MD, said at the annual Digestive Disease Week.
While the Society for Surgery of the Alimentary Tract (J Gastrointest Surg 2016;20[5]:879-84) and other medical organizations have recognized the importance of workforce diversity and cultural competence of providers, little is known of the relationship between cultural competence and patient-reported outcomes in surgery, said Dr. Miller, who is a general surgery resident at Stanford (Calif.) University. To investigate this relationship, she and her colleagues recruited surgeons, oncologists, gastroenterologists, and advanced practice providers to complete a validated online survey that measured two domains of cultural competency: awareness and behaviors. They matched these scores with the 10-item Press Ganey provider care scores from 2017 to 2018. Next, the researchers conducted a linear regression analysis with mixed effects to account for clustering of patients within providers. They also adjusted for provider bias by measuring social desirability, “which is the tendency for respondents to put a more socially appropriate answer as opposed to the true answer on the cultural competence survey,” Dr. Miller explained.
A total of 1,322 Press Ganey satisfaction surveys were available for 29 providers. Their mean age was 48 years, 59% were white, 72% were physicians, and 28% were advanced practice providers. They practiced in GI oncology (41%), gastroenterology (31%) and colorectal surgery (28%). Dr. Miller reported that providers who participated in the survey had a mean cultural awareness score of 6.2 out of a possible 7 points, while the mean cultural behavior score was a 4.1 out of a possible 7 points. She and her colleagues observed that providers who had high levels of cultural competence on the behavioral assessment were positively associated with Press Ganey patient satisfaction (P = .039).
“I think we do a poor job of training our providers to be culturally competent, but there are multiple ways to improve behaviors, by teaching people and by having real training for our providers,” Dr. Miller said.
She acknowledged certain limitations of the study, including its single-center design and the fact that not all providers had Press Ganey scores available. The study was funded by the Society for Surgery of the Alimentary Tract and the Black Academic Surgeons Resident Research Award. Dr. Miller reported having no financial disclosures.
results from a single-center study showed.
“Cultural competence is valued by patients, and there is a potential for training providers to be more culturally competent, focusing on their behaviors,” lead study author Miquell O. Miller, MD, said at the annual Digestive Disease Week.
While the Society for Surgery of the Alimentary Tract (J Gastrointest Surg 2016;20[5]:879-84) and other medical organizations have recognized the importance of workforce diversity and cultural competence of providers, little is known of the relationship between cultural competence and patient-reported outcomes in surgery, said Dr. Miller, who is a general surgery resident at Stanford (Calif.) University. To investigate this relationship, she and her colleagues recruited surgeons, oncologists, gastroenterologists, and advanced practice providers to complete a validated online survey that measured two domains of cultural competency: awareness and behaviors. They matched these scores with the 10-item Press Ganey provider care scores from 2017 to 2018. Next, the researchers conducted a linear regression analysis with mixed effects to account for clustering of patients within providers. They also adjusted for provider bias by measuring social desirability, “which is the tendency for respondents to put a more socially appropriate answer as opposed to the true answer on the cultural competence survey,” Dr. Miller explained.
A total of 1,322 Press Ganey satisfaction surveys were available for 29 providers. Their mean age was 48 years, 59% were white, 72% were physicians, and 28% were advanced practice providers. They practiced in GI oncology (41%), gastroenterology (31%) and colorectal surgery (28%). Dr. Miller reported that providers who participated in the survey had a mean cultural awareness score of 6.2 out of a possible 7 points, while the mean cultural behavior score was a 4.1 out of a possible 7 points. She and her colleagues observed that providers who had high levels of cultural competence on the behavioral assessment were positively associated with Press Ganey patient satisfaction (P = .039).
“I think we do a poor job of training our providers to be culturally competent, but there are multiple ways to improve behaviors, by teaching people and by having real training for our providers,” Dr. Miller said.
She acknowledged certain limitations of the study, including its single-center design and the fact that not all providers had Press Ganey scores available. The study was funded by the Society for Surgery of the Alimentary Tract and the Black Academic Surgeons Resident Research Award. Dr. Miller reported having no financial disclosures.
REPORTING FROM DDW 2019
Can acid suppression prevent recurrent idiopathic gastroduodenal ulcer bleeding?
SAN DIEGO – However, these patients face a considerable risk of overall mortality, despite a low incidence of recurrent GI bleeding.
“Peptic ulcer disease is a commonly encountered condition in our daily clinical practice,” one of the study authors, Louis Lau, MD, said at the annual Digestive Disease Week. “Most of the ulcers are due to H. pylori and NSAIDs or aspirin. However, idiopathic peptic ulcers remain an area to be investigated.”
According to Dr. Lau of the division of gastroenterology and hepatology at Prince of Wales Hospital, Hong Kong, H. pylori–negative idiopathic ulcers have increased in prevalence in the Asian Pacific region, from 1%-4% in 2000 to up to 20% in recent data. These ulcers have been shown to have a bleeding rate of up to 13% within 1 year (Gastroenterology. 2005;128:1845-50) and have been associated with a mortality rate of up to 88% in 7 years of follow-up (Gastroenterology. 2009;137:525-31).
“There is no consensus for the optimal management of this particular ulcer,” he continued. “One proposed management was maintenance PPI [proton pump inhibitors]. However, we would like to look for the best optimal prevention strategy.”
Dr. Lau and associates conducted a double-blind, randomized trial at Prince of Wales Hospital to determine if there were any differences between the PPI lansoprazole and the histamine2 receptor antagonist famotidine for the prevention of recurrent bleeding in patients with H. pylori–negative idiopathic peptic ulcers. It involved a 2-year follow-up period and included a screening and end-of-study esophagogastroduodenoscopy. The drugs were repackaged into identical green capsules.
The researchers limited the analysis to 228 patients over the age 18 with H. pylori–negative idiopathic ulcers, defined as having endoscopic biopsies negative for both rapid urease test and histology for H. pylori; no exposure to aspirin, NSAIDs, or drugs of unknown nature including traditional Chinese medicine within 4 weeks; and no other causes of ulceration identified. Exclusion criteria included patients with a strong indication for maintenance therapy with PPIs, those with prior gastric surgery, pregnant patients, and those with advanced malignancy or comorbidity.
The primary endpoint was clinical GI bleeding or reduction in hemoglobin level of 2 g/dL or more and confirmation of peptic ulcers by endoscopy. The secondary endpoint was recurrent ulcer detected by end-of-study endoscopy, with or without clinical symptoms.
Of the 228 patients, 114 received lansoprazole 30 mg daily and 114 received famotidine 40 mg daily. Recurrent upper GI bleeding was suspected in 10 patients in each of the treatment groups. Ulcer confirmation via endoscopy detected one ulcer in the lansoprazole group and three in the famotidine group (0.9% vs. 2.6%, respectively; P = .62). For the secondary endpoint, there were five recurrent ulcers in the lansoprazole group and nine in the famotidine group, (4.4% vs. 7.9%, respectively; P = .27).
Dr. Lau and colleagues observed eight deaths in the lansoprazole group (7%), compared with five (4.4%) in the famotidine group (P = .39), mainly caused by pneumonia, renal failure, and other chronic medical conditions. Intention-to-treat analysis at 2 years found that both groups were comparable in terms of time to analysis of recurrent upper GI bleeding, adjusted for death as a competing risk (Gray’s test, P = .313).
“In this randomized, controlled trial, we found that both a PPI and an H2 blocker are comparable in the prevention of idiopathic ulcer recurrent bleeding,” Dr. Lau concluded. “The rebleeding rate was five times lower than a previous cohort reported with no or irregular acid suppression therapy [Gastroenterology. 2005;128[7]:1845-50]. With all this, there is still a considerable risk of recurrent ulcers and death. We postulate that there is an underlying mechanism for this type of idiopathic ulcer.”
The study was funded in part by the General Research Fund–Research Grant Council. Dr. Lau reported having no financial disclosures. Many of his coauthors reported having numerous financial ties to the pharmaceutical industry.
SOURCE: Lau L et al. DDW 2019, Abstract 322.
SAN DIEGO – However, these patients face a considerable risk of overall mortality, despite a low incidence of recurrent GI bleeding.
“Peptic ulcer disease is a commonly encountered condition in our daily clinical practice,” one of the study authors, Louis Lau, MD, said at the annual Digestive Disease Week. “Most of the ulcers are due to H. pylori and NSAIDs or aspirin. However, idiopathic peptic ulcers remain an area to be investigated.”
According to Dr. Lau of the division of gastroenterology and hepatology at Prince of Wales Hospital, Hong Kong, H. pylori–negative idiopathic ulcers have increased in prevalence in the Asian Pacific region, from 1%-4% in 2000 to up to 20% in recent data. These ulcers have been shown to have a bleeding rate of up to 13% within 1 year (Gastroenterology. 2005;128:1845-50) and have been associated with a mortality rate of up to 88% in 7 years of follow-up (Gastroenterology. 2009;137:525-31).
“There is no consensus for the optimal management of this particular ulcer,” he continued. “One proposed management was maintenance PPI [proton pump inhibitors]. However, we would like to look for the best optimal prevention strategy.”
Dr. Lau and associates conducted a double-blind, randomized trial at Prince of Wales Hospital to determine if there were any differences between the PPI lansoprazole and the histamine2 receptor antagonist famotidine for the prevention of recurrent bleeding in patients with H. pylori–negative idiopathic peptic ulcers. It involved a 2-year follow-up period and included a screening and end-of-study esophagogastroduodenoscopy. The drugs were repackaged into identical green capsules.
The researchers limited the analysis to 228 patients over the age 18 with H. pylori–negative idiopathic ulcers, defined as having endoscopic biopsies negative for both rapid urease test and histology for H. pylori; no exposure to aspirin, NSAIDs, or drugs of unknown nature including traditional Chinese medicine within 4 weeks; and no other causes of ulceration identified. Exclusion criteria included patients with a strong indication for maintenance therapy with PPIs, those with prior gastric surgery, pregnant patients, and those with advanced malignancy or comorbidity.
The primary endpoint was clinical GI bleeding or reduction in hemoglobin level of 2 g/dL or more and confirmation of peptic ulcers by endoscopy. The secondary endpoint was recurrent ulcer detected by end-of-study endoscopy, with or without clinical symptoms.
Of the 228 patients, 114 received lansoprazole 30 mg daily and 114 received famotidine 40 mg daily. Recurrent upper GI bleeding was suspected in 10 patients in each of the treatment groups. Ulcer confirmation via endoscopy detected one ulcer in the lansoprazole group and three in the famotidine group (0.9% vs. 2.6%, respectively; P = .62). For the secondary endpoint, there were five recurrent ulcers in the lansoprazole group and nine in the famotidine group, (4.4% vs. 7.9%, respectively; P = .27).
Dr. Lau and colleagues observed eight deaths in the lansoprazole group (7%), compared with five (4.4%) in the famotidine group (P = .39), mainly caused by pneumonia, renal failure, and other chronic medical conditions. Intention-to-treat analysis at 2 years found that both groups were comparable in terms of time to analysis of recurrent upper GI bleeding, adjusted for death as a competing risk (Gray’s test, P = .313).
“In this randomized, controlled trial, we found that both a PPI and an H2 blocker are comparable in the prevention of idiopathic ulcer recurrent bleeding,” Dr. Lau concluded. “The rebleeding rate was five times lower than a previous cohort reported with no or irregular acid suppression therapy [Gastroenterology. 2005;128[7]:1845-50]. With all this, there is still a considerable risk of recurrent ulcers and death. We postulate that there is an underlying mechanism for this type of idiopathic ulcer.”
The study was funded in part by the General Research Fund–Research Grant Council. Dr. Lau reported having no financial disclosures. Many of his coauthors reported having numerous financial ties to the pharmaceutical industry.
SOURCE: Lau L et al. DDW 2019, Abstract 322.
SAN DIEGO – However, these patients face a considerable risk of overall mortality, despite a low incidence of recurrent GI bleeding.
“Peptic ulcer disease is a commonly encountered condition in our daily clinical practice,” one of the study authors, Louis Lau, MD, said at the annual Digestive Disease Week. “Most of the ulcers are due to H. pylori and NSAIDs or aspirin. However, idiopathic peptic ulcers remain an area to be investigated.”
According to Dr. Lau of the division of gastroenterology and hepatology at Prince of Wales Hospital, Hong Kong, H. pylori–negative idiopathic ulcers have increased in prevalence in the Asian Pacific region, from 1%-4% in 2000 to up to 20% in recent data. These ulcers have been shown to have a bleeding rate of up to 13% within 1 year (Gastroenterology. 2005;128:1845-50) and have been associated with a mortality rate of up to 88% in 7 years of follow-up (Gastroenterology. 2009;137:525-31).
“There is no consensus for the optimal management of this particular ulcer,” he continued. “One proposed management was maintenance PPI [proton pump inhibitors]. However, we would like to look for the best optimal prevention strategy.”
Dr. Lau and associates conducted a double-blind, randomized trial at Prince of Wales Hospital to determine if there were any differences between the PPI lansoprazole and the histamine2 receptor antagonist famotidine for the prevention of recurrent bleeding in patients with H. pylori–negative idiopathic peptic ulcers. It involved a 2-year follow-up period and included a screening and end-of-study esophagogastroduodenoscopy. The drugs were repackaged into identical green capsules.
The researchers limited the analysis to 228 patients over the age 18 with H. pylori–negative idiopathic ulcers, defined as having endoscopic biopsies negative for both rapid urease test and histology for H. pylori; no exposure to aspirin, NSAIDs, or drugs of unknown nature including traditional Chinese medicine within 4 weeks; and no other causes of ulceration identified. Exclusion criteria included patients with a strong indication for maintenance therapy with PPIs, those with prior gastric surgery, pregnant patients, and those with advanced malignancy or comorbidity.
The primary endpoint was clinical GI bleeding or reduction in hemoglobin level of 2 g/dL or more and confirmation of peptic ulcers by endoscopy. The secondary endpoint was recurrent ulcer detected by end-of-study endoscopy, with or without clinical symptoms.
Of the 228 patients, 114 received lansoprazole 30 mg daily and 114 received famotidine 40 mg daily. Recurrent upper GI bleeding was suspected in 10 patients in each of the treatment groups. Ulcer confirmation via endoscopy detected one ulcer in the lansoprazole group and three in the famotidine group (0.9% vs. 2.6%, respectively; P = .62). For the secondary endpoint, there were five recurrent ulcers in the lansoprazole group and nine in the famotidine group, (4.4% vs. 7.9%, respectively; P = .27).
Dr. Lau and colleagues observed eight deaths in the lansoprazole group (7%), compared with five (4.4%) in the famotidine group (P = .39), mainly caused by pneumonia, renal failure, and other chronic medical conditions. Intention-to-treat analysis at 2 years found that both groups were comparable in terms of time to analysis of recurrent upper GI bleeding, adjusted for death as a competing risk (Gray’s test, P = .313).
“In this randomized, controlled trial, we found that both a PPI and an H2 blocker are comparable in the prevention of idiopathic ulcer recurrent bleeding,” Dr. Lau concluded. “The rebleeding rate was five times lower than a previous cohort reported with no or irregular acid suppression therapy [Gastroenterology. 2005;128[7]:1845-50]. With all this, there is still a considerable risk of recurrent ulcers and death. We postulate that there is an underlying mechanism for this type of idiopathic ulcer.”
The study was funded in part by the General Research Fund–Research Grant Council. Dr. Lau reported having no financial disclosures. Many of his coauthors reported having numerous financial ties to the pharmaceutical industry.
SOURCE: Lau L et al. DDW 2019, Abstract 322.
REPORTING FROM DDW 2019