User login
Doug Brunk is a San Diego-based award-winning reporter who began covering health care in 1991. Before joining the company, he wrote for the health sciences division of Columbia University and was an associate editor at Contemporary Long Term Care magazine when it won a Jesse H. Neal Award. His work has been syndicated by the Los Angeles Times and he is the author of two books related to the University of Kentucky Wildcats men's basketball program. Doug has a master’s degree in magazine journalism from the S.I. Newhouse School of Public Communications at Syracuse University. Follow him on Twitter @dougbrunk.
COVID-19 airway management: Expert tips on infection control
As continue to evolve, practicing vigilant transmission-based infection control precautions remains essential.
This starts with observing droplet precautions to prevent exposure to droplets larger than 5 microns in size, Charles Griffis, PhD, CRNA, said at a Society for Critical Care Medicine virtual meeting: COVID-19: What’s Next. “These are particles exhaled from infected persons and which fall within around 6 feet and involve an exposure time of 15 or more minutes of contact,” said Dr. Griffis, of the department of anesthesiology at the University of Southern California, Los Angeles. “We will always observe standard precautions, which include hand hygiene, gloves, hair and eye cover, medical mask, and face shield. We will observe these at all times for all patients and layer our transmission-based precautions on top.”
During aerosol-producing procedures such as airway management maneuvers, tracheostomies, and bronchoscopies, very fine microscopic particles less than 5 microns in size are produced, which remain airborne for potentially many hours and travel long distances. “We will add an N95 mask or a powered air-purifying respirator (PAPR) device to filter out tiny particles in addition to our ever-present standard precautions,” he said. “Contact precautions are indicated for direct contact with patient saliva, blood, urine, and stool. In addition to standard precautions, we’re going to add an impermeable gown and we’ll continue with gloves, eye protection, and shoe covers. The message is to all of us. We have to observe all of the infection precautions that all of us have learned and trained in to avoid exposure.”
In terms of airway management for infected patients for elective procedures and surgery, recommendations based on current and previous coronavirus outbreaks suggest that all patients get polymerase chain reaction (PCR) tested within 24-48 hours of elective procedures or surgeries. If positive, they should be quarantined for 10-14 days and then, if asymptomatic, these patients may be retested or they can be regarded as negative. “Patients who are PCR positive with active infection and active symptoms receive only urgent or emergent care in most settings,” said Dr. Griffis, a member of the American Association of Nurse Anesthetists Infection Control Advisory Panel. “The care provided to our patients, whether they’re positive or not, is individualized per patient needs and institutional policy. Some folks have made the decision to treat all patients as infected and to use airborne precautions for all aerosol-producing procedures for all patients all the time.”
When a COVID-19 patient requires emergent or urgent airway management because of respiratory failure or some other surgical or procedural intervention necessitating airway management, preprocedural planning is key, he continued. This means establishing the steps in airway management scenarios for infected patients and rehearsing those steps in each ICU setting with key personnel such as nurses, respiratory therapists, and medical staff. “You want to make sure that the PPE is readily available and determine and limit the number of personnel that are going to enter the patient’s room or area for airway management,” Dr. Griffis said. “Have all the airway equipment and drugs immediately available. Perhaps you could organize them in a cart which is decontaminated after every use.”
He also recommends forming an intubation team for ICUs and perhaps even for ORs, where the most experienced clinicians perform airway management. “This helps to avoid unnecessary airway manipulation and minimizes personnel exposure and time to airway establishment,” he said.
Always attempt to house the infected patient in an airborne isolation, negative-pressure room, with a minimum of 12 exchanges per hour and which will take 35 minutes for 99.99% removal of airborne contaminants after airway management. “These numbers are important to remember for room turnover safety,” he said.
Patient factors to review during airway management include assessing the past medical history, inspecting the airway and considering the patient’s current physiological status as time permits. Previously in the pandemic, intubation was used earlier in the disease course, but now data suggest that patients do better without intubation if possible (Am J Trop Med Hyg. 2020;102[6]. doi: 10.4269/aitmh.20-0283). “This is because the pathophysiology of COVID-19 is such that the lung tissue is predisposed to iatrogenic barotrauma damage from positive-pressure ventilation,” Dr. Griffis said. “In addition, COVID patients appear to tolerate significant hypoxemia without distress in many cases. Therefore, many clinicians now hold off on intubation until the hypoxemic patient begins exhibiting signs and symptoms of respiratory distress.”
Options for delivering noninvasive airway support for COVID-19 patients include high-flow nasal cannula and noninvasive positive-pressure ventilation via CPAP or BiPAP. To mitigate the associated aerosol production, consider applying a surgical mask, helmet, or face mask over the airway device/patient’s face. “Another measure that has proven helpful in general respiratory support is to actually put the patient in a prone position to help redistribute ventilation throughout the lungs,” Dr. Griffis said (see Resp Care. 2015;60[11]:1660-87).
To prepare for the actual intubation procedure, gather two expert intubators who are going to be entering the patient’s room. The team should perform hand hygiene and don full PPE prior to entry. “It’s recommended that you consider wearing double gloves for the intubation,” he said. “Have the airway equipment easily accessible in a central location on a cart or in a kit, and use disposable, single-use equipment if possible. All of the usual intubation equipment to maintain a clear airway and give positive pressure ventilation should be arranged for easy access. A video laryngoscope should be used, if possible, for greater accuracy and reduced procedure time. Ready access to sedation and muscle relaxant drugs must be assured at all times.”
For the intubation procedure itself, Dr. Griffis recommends ensuring that an oxygen source, positive-pressure ventilation, and suction and resuscitation drugs and equipment are available per institutional protocol. Assign one person outside the room to coordinate supplies and assistance. “Preoxygenate the patient as permitted by clinical status,” he said. “A nonrebreathing oxygen mask can be used if sufficient spontaneous ventilation is present. Assess the airway, check and arrange equipment for easy access, and develop the safest airway management plan. Consider a rapid sequence induction and intubation as the first option.” Avoid positive-pressure ventilation or awake fiber optic intubation unless absolutely necessary, thus avoiding aerosol production. “Only ventilate the patient after the endotracheal tube cuff is inflated, to avoid aerosol release,” he said.
For intubation, administer airway procedural drugs and insert the laryngoscope – ideally a video laryngoscope if available. Intubate the trachea under direct vision, inflate the cuff, and remove outer gloves. Then attach the Ambu bag with a 99% filtration efficiency, heat-and-moisture exchange filter; and proceed to ventilate the patient, checking for chest rise, breath sounds, and CO2 production. “Discard contaminated equipment in designated bins and secure the tube,” Dr. Griffis advised. “Attach the ventilator with an HMEF filter to protect the ventilator circuit and inner parts of the machine. Recheck your breath sounds, CO2 production, and oxygen saturation, and adjust your vent settings as indicated.”
For post intubation, Dr. Griffis recommends securing contaminated discardable equipment in biohazard-labeled bins or bags, safely doffing your PPE, and retaining your N95 mask in the room. Remove your inner gloves, perform hand hygiene with soap and water if available, with alcohol-based hand rub if not, then don clean gloves. Exit the room, safely transporting any contaminated equipment that will be reused such as a cart or video laryngoscope to decontamination areas for processing. “Once clear of the room, order your chest x-ray to confirm your tube position per institutional protocol, understanding that radiology techs are all going to be following infection control procedures and wearing their PPE,” he said.
For extubation, Dr. Griffis recommends excusing all nonessential personnel from the patient room and assigning an assistant outside the room for necessary help. An experienced airway management expert should evaluate the patient wearing full PPE and be double-gloved. “If the extubation criteria are met, suction the pharynx and extubate,” he said. “Remove outer gloves and apply desired oxygen delivery equipment to the patient and assess respiratory status and vital signs for stability.” Next, discard all contaminated equipment in designated bins, doff contaminated PPE, and retain your N95 mask. Doff inner gloves, perform hand hygiene, and don clean gloves. “Exit the room, hand off contaminated equipment that is reusable, doff your gloves outside, do hand hygiene, then proceed to change your scrubs and complete your own personal hygiene measures,” he said.
Dr. Griffis reported having no financial disclosures.
“While the PPE used for intubation of a coronavirus patient is certainly more than the typical droplet precautions observed when intubating any other patient, the process and best practices aren’t terribly different from usual standard of care: Ensuring all necessary equipment is readily available with backup plans should the airway be difficult,” said Megan Conroy, MD, assistant professor of clinical medicine at The Ohio State University.
“We’ve been streamlining the team that’s present in the room for intubations of COVID patients, but I’m always amazed at the team members that stand at the ready to lend additional assistance just from the other side of the door. So while fewer personnel may be exposed, I wouldn’t consider the team needed for intubation to actually be much smaller, we’re just functioning differently.
In my practice the decision of when to intubate, clinically, doesn’t vary too much from any other form of severe ARDS. We may tolerate higher FiO2 requirements on heated high-flow nasal cannula if the patient exhibits acceptable work of breathing, but I wouldn’t advise allowing a patient to remain hypoxemic with oxygen needs unmet by noninvasive methods out of fear of intubation or ventilator management. In my opinion, this simply delays a necessary therapy and only makes for a higher risk intubation. Certainly, the decision to intubate is never based on only one single data point, but takes an expert assessment of the whole clinical picture.
I’d assert that it’s true in every disease that patients do better if it’s possible to avoid intubation – but I would argue that the ability to avoid intubation is determined primarily by the disease course and clinical scenario, and not by whether the physician wishes to avoid intubation or not. If I can safely manage a patient off of a ventilator, I will always do so, COVID or otherwise. I think in this phase of the pandemic, patients ‘do better without intubation’ because those who didn’t require intubation were inherently doing better!”
As continue to evolve, practicing vigilant transmission-based infection control precautions remains essential.
This starts with observing droplet precautions to prevent exposure to droplets larger than 5 microns in size, Charles Griffis, PhD, CRNA, said at a Society for Critical Care Medicine virtual meeting: COVID-19: What’s Next. “These are particles exhaled from infected persons and which fall within around 6 feet and involve an exposure time of 15 or more minutes of contact,” said Dr. Griffis, of the department of anesthesiology at the University of Southern California, Los Angeles. “We will always observe standard precautions, which include hand hygiene, gloves, hair and eye cover, medical mask, and face shield. We will observe these at all times for all patients and layer our transmission-based precautions on top.”
During aerosol-producing procedures such as airway management maneuvers, tracheostomies, and bronchoscopies, very fine microscopic particles less than 5 microns in size are produced, which remain airborne for potentially many hours and travel long distances. “We will add an N95 mask or a powered air-purifying respirator (PAPR) device to filter out tiny particles in addition to our ever-present standard precautions,” he said. “Contact precautions are indicated for direct contact with patient saliva, blood, urine, and stool. In addition to standard precautions, we’re going to add an impermeable gown and we’ll continue with gloves, eye protection, and shoe covers. The message is to all of us. We have to observe all of the infection precautions that all of us have learned and trained in to avoid exposure.”
In terms of airway management for infected patients for elective procedures and surgery, recommendations based on current and previous coronavirus outbreaks suggest that all patients get polymerase chain reaction (PCR) tested within 24-48 hours of elective procedures or surgeries. If positive, they should be quarantined for 10-14 days and then, if asymptomatic, these patients may be retested or they can be regarded as negative. “Patients who are PCR positive with active infection and active symptoms receive only urgent or emergent care in most settings,” said Dr. Griffis, a member of the American Association of Nurse Anesthetists Infection Control Advisory Panel. “The care provided to our patients, whether they’re positive or not, is individualized per patient needs and institutional policy. Some folks have made the decision to treat all patients as infected and to use airborne precautions for all aerosol-producing procedures for all patients all the time.”
When a COVID-19 patient requires emergent or urgent airway management because of respiratory failure or some other surgical or procedural intervention necessitating airway management, preprocedural planning is key, he continued. This means establishing the steps in airway management scenarios for infected patients and rehearsing those steps in each ICU setting with key personnel such as nurses, respiratory therapists, and medical staff. “You want to make sure that the PPE is readily available and determine and limit the number of personnel that are going to enter the patient’s room or area for airway management,” Dr. Griffis said. “Have all the airway equipment and drugs immediately available. Perhaps you could organize them in a cart which is decontaminated after every use.”
He also recommends forming an intubation team for ICUs and perhaps even for ORs, where the most experienced clinicians perform airway management. “This helps to avoid unnecessary airway manipulation and minimizes personnel exposure and time to airway establishment,” he said.
Always attempt to house the infected patient in an airborne isolation, negative-pressure room, with a minimum of 12 exchanges per hour and which will take 35 minutes for 99.99% removal of airborne contaminants after airway management. “These numbers are important to remember for room turnover safety,” he said.
Patient factors to review during airway management include assessing the past medical history, inspecting the airway and considering the patient’s current physiological status as time permits. Previously in the pandemic, intubation was used earlier in the disease course, but now data suggest that patients do better without intubation if possible (Am J Trop Med Hyg. 2020;102[6]. doi: 10.4269/aitmh.20-0283). “This is because the pathophysiology of COVID-19 is such that the lung tissue is predisposed to iatrogenic barotrauma damage from positive-pressure ventilation,” Dr. Griffis said. “In addition, COVID patients appear to tolerate significant hypoxemia without distress in many cases. Therefore, many clinicians now hold off on intubation until the hypoxemic patient begins exhibiting signs and symptoms of respiratory distress.”
Options for delivering noninvasive airway support for COVID-19 patients include high-flow nasal cannula and noninvasive positive-pressure ventilation via CPAP or BiPAP. To mitigate the associated aerosol production, consider applying a surgical mask, helmet, or face mask over the airway device/patient’s face. “Another measure that has proven helpful in general respiratory support is to actually put the patient in a prone position to help redistribute ventilation throughout the lungs,” Dr. Griffis said (see Resp Care. 2015;60[11]:1660-87).
To prepare for the actual intubation procedure, gather two expert intubators who are going to be entering the patient’s room. The team should perform hand hygiene and don full PPE prior to entry. “It’s recommended that you consider wearing double gloves for the intubation,” he said. “Have the airway equipment easily accessible in a central location on a cart or in a kit, and use disposable, single-use equipment if possible. All of the usual intubation equipment to maintain a clear airway and give positive pressure ventilation should be arranged for easy access. A video laryngoscope should be used, if possible, for greater accuracy and reduced procedure time. Ready access to sedation and muscle relaxant drugs must be assured at all times.”
For the intubation procedure itself, Dr. Griffis recommends ensuring that an oxygen source, positive-pressure ventilation, and suction and resuscitation drugs and equipment are available per institutional protocol. Assign one person outside the room to coordinate supplies and assistance. “Preoxygenate the patient as permitted by clinical status,” he said. “A nonrebreathing oxygen mask can be used if sufficient spontaneous ventilation is present. Assess the airway, check and arrange equipment for easy access, and develop the safest airway management plan. Consider a rapid sequence induction and intubation as the first option.” Avoid positive-pressure ventilation or awake fiber optic intubation unless absolutely necessary, thus avoiding aerosol production. “Only ventilate the patient after the endotracheal tube cuff is inflated, to avoid aerosol release,” he said.
For intubation, administer airway procedural drugs and insert the laryngoscope – ideally a video laryngoscope if available. Intubate the trachea under direct vision, inflate the cuff, and remove outer gloves. Then attach the Ambu bag with a 99% filtration efficiency, heat-and-moisture exchange filter; and proceed to ventilate the patient, checking for chest rise, breath sounds, and CO2 production. “Discard contaminated equipment in designated bins and secure the tube,” Dr. Griffis advised. “Attach the ventilator with an HMEF filter to protect the ventilator circuit and inner parts of the machine. Recheck your breath sounds, CO2 production, and oxygen saturation, and adjust your vent settings as indicated.”
For post intubation, Dr. Griffis recommends securing contaminated discardable equipment in biohazard-labeled bins or bags, safely doffing your PPE, and retaining your N95 mask in the room. Remove your inner gloves, perform hand hygiene with soap and water if available, with alcohol-based hand rub if not, then don clean gloves. Exit the room, safely transporting any contaminated equipment that will be reused such as a cart or video laryngoscope to decontamination areas for processing. “Once clear of the room, order your chest x-ray to confirm your tube position per institutional protocol, understanding that radiology techs are all going to be following infection control procedures and wearing their PPE,” he said.
For extubation, Dr. Griffis recommends excusing all nonessential personnel from the patient room and assigning an assistant outside the room for necessary help. An experienced airway management expert should evaluate the patient wearing full PPE and be double-gloved. “If the extubation criteria are met, suction the pharynx and extubate,” he said. “Remove outer gloves and apply desired oxygen delivery equipment to the patient and assess respiratory status and vital signs for stability.” Next, discard all contaminated equipment in designated bins, doff contaminated PPE, and retain your N95 mask. Doff inner gloves, perform hand hygiene, and don clean gloves. “Exit the room, hand off contaminated equipment that is reusable, doff your gloves outside, do hand hygiene, then proceed to change your scrubs and complete your own personal hygiene measures,” he said.
Dr. Griffis reported having no financial disclosures.
“While the PPE used for intubation of a coronavirus patient is certainly more than the typical droplet precautions observed when intubating any other patient, the process and best practices aren’t terribly different from usual standard of care: Ensuring all necessary equipment is readily available with backup plans should the airway be difficult,” said Megan Conroy, MD, assistant professor of clinical medicine at The Ohio State University.
“We’ve been streamlining the team that’s present in the room for intubations of COVID patients, but I’m always amazed at the team members that stand at the ready to lend additional assistance just from the other side of the door. So while fewer personnel may be exposed, I wouldn’t consider the team needed for intubation to actually be much smaller, we’re just functioning differently.
In my practice the decision of when to intubate, clinically, doesn’t vary too much from any other form of severe ARDS. We may tolerate higher FiO2 requirements on heated high-flow nasal cannula if the patient exhibits acceptable work of breathing, but I wouldn’t advise allowing a patient to remain hypoxemic with oxygen needs unmet by noninvasive methods out of fear of intubation or ventilator management. In my opinion, this simply delays a necessary therapy and only makes for a higher risk intubation. Certainly, the decision to intubate is never based on only one single data point, but takes an expert assessment of the whole clinical picture.
I’d assert that it’s true in every disease that patients do better if it’s possible to avoid intubation – but I would argue that the ability to avoid intubation is determined primarily by the disease course and clinical scenario, and not by whether the physician wishes to avoid intubation or not. If I can safely manage a patient off of a ventilator, I will always do so, COVID or otherwise. I think in this phase of the pandemic, patients ‘do better without intubation’ because those who didn’t require intubation were inherently doing better!”
As continue to evolve, practicing vigilant transmission-based infection control precautions remains essential.
This starts with observing droplet precautions to prevent exposure to droplets larger than 5 microns in size, Charles Griffis, PhD, CRNA, said at a Society for Critical Care Medicine virtual meeting: COVID-19: What’s Next. “These are particles exhaled from infected persons and which fall within around 6 feet and involve an exposure time of 15 or more minutes of contact,” said Dr. Griffis, of the department of anesthesiology at the University of Southern California, Los Angeles. “We will always observe standard precautions, which include hand hygiene, gloves, hair and eye cover, medical mask, and face shield. We will observe these at all times for all patients and layer our transmission-based precautions on top.”
During aerosol-producing procedures such as airway management maneuvers, tracheostomies, and bronchoscopies, very fine microscopic particles less than 5 microns in size are produced, which remain airborne for potentially many hours and travel long distances. “We will add an N95 mask or a powered air-purifying respirator (PAPR) device to filter out tiny particles in addition to our ever-present standard precautions,” he said. “Contact precautions are indicated for direct contact with patient saliva, blood, urine, and stool. In addition to standard precautions, we’re going to add an impermeable gown and we’ll continue with gloves, eye protection, and shoe covers. The message is to all of us. We have to observe all of the infection precautions that all of us have learned and trained in to avoid exposure.”
In terms of airway management for infected patients for elective procedures and surgery, recommendations based on current and previous coronavirus outbreaks suggest that all patients get polymerase chain reaction (PCR) tested within 24-48 hours of elective procedures or surgeries. If positive, they should be quarantined for 10-14 days and then, if asymptomatic, these patients may be retested or they can be regarded as negative. “Patients who are PCR positive with active infection and active symptoms receive only urgent or emergent care in most settings,” said Dr. Griffis, a member of the American Association of Nurse Anesthetists Infection Control Advisory Panel. “The care provided to our patients, whether they’re positive or not, is individualized per patient needs and institutional policy. Some folks have made the decision to treat all patients as infected and to use airborne precautions for all aerosol-producing procedures for all patients all the time.”
When a COVID-19 patient requires emergent or urgent airway management because of respiratory failure or some other surgical or procedural intervention necessitating airway management, preprocedural planning is key, he continued. This means establishing the steps in airway management scenarios for infected patients and rehearsing those steps in each ICU setting with key personnel such as nurses, respiratory therapists, and medical staff. “You want to make sure that the PPE is readily available and determine and limit the number of personnel that are going to enter the patient’s room or area for airway management,” Dr. Griffis said. “Have all the airway equipment and drugs immediately available. Perhaps you could organize them in a cart which is decontaminated after every use.”
He also recommends forming an intubation team for ICUs and perhaps even for ORs, where the most experienced clinicians perform airway management. “This helps to avoid unnecessary airway manipulation and minimizes personnel exposure and time to airway establishment,” he said.
Always attempt to house the infected patient in an airborne isolation, negative-pressure room, with a minimum of 12 exchanges per hour and which will take 35 minutes for 99.99% removal of airborne contaminants after airway management. “These numbers are important to remember for room turnover safety,” he said.
Patient factors to review during airway management include assessing the past medical history, inspecting the airway and considering the patient’s current physiological status as time permits. Previously in the pandemic, intubation was used earlier in the disease course, but now data suggest that patients do better without intubation if possible (Am J Trop Med Hyg. 2020;102[6]. doi: 10.4269/aitmh.20-0283). “This is because the pathophysiology of COVID-19 is such that the lung tissue is predisposed to iatrogenic barotrauma damage from positive-pressure ventilation,” Dr. Griffis said. “In addition, COVID patients appear to tolerate significant hypoxemia without distress in many cases. Therefore, many clinicians now hold off on intubation until the hypoxemic patient begins exhibiting signs and symptoms of respiratory distress.”
Options for delivering noninvasive airway support for COVID-19 patients include high-flow nasal cannula and noninvasive positive-pressure ventilation via CPAP or BiPAP. To mitigate the associated aerosol production, consider applying a surgical mask, helmet, or face mask over the airway device/patient’s face. “Another measure that has proven helpful in general respiratory support is to actually put the patient in a prone position to help redistribute ventilation throughout the lungs,” Dr. Griffis said (see Resp Care. 2015;60[11]:1660-87).
To prepare for the actual intubation procedure, gather two expert intubators who are going to be entering the patient’s room. The team should perform hand hygiene and don full PPE prior to entry. “It’s recommended that you consider wearing double gloves for the intubation,” he said. “Have the airway equipment easily accessible in a central location on a cart or in a kit, and use disposable, single-use equipment if possible. All of the usual intubation equipment to maintain a clear airway and give positive pressure ventilation should be arranged for easy access. A video laryngoscope should be used, if possible, for greater accuracy and reduced procedure time. Ready access to sedation and muscle relaxant drugs must be assured at all times.”
For the intubation procedure itself, Dr. Griffis recommends ensuring that an oxygen source, positive-pressure ventilation, and suction and resuscitation drugs and equipment are available per institutional protocol. Assign one person outside the room to coordinate supplies and assistance. “Preoxygenate the patient as permitted by clinical status,” he said. “A nonrebreathing oxygen mask can be used if sufficient spontaneous ventilation is present. Assess the airway, check and arrange equipment for easy access, and develop the safest airway management plan. Consider a rapid sequence induction and intubation as the first option.” Avoid positive-pressure ventilation or awake fiber optic intubation unless absolutely necessary, thus avoiding aerosol production. “Only ventilate the patient after the endotracheal tube cuff is inflated, to avoid aerosol release,” he said.
For intubation, administer airway procedural drugs and insert the laryngoscope – ideally a video laryngoscope if available. Intubate the trachea under direct vision, inflate the cuff, and remove outer gloves. Then attach the Ambu bag with a 99% filtration efficiency, heat-and-moisture exchange filter; and proceed to ventilate the patient, checking for chest rise, breath sounds, and CO2 production. “Discard contaminated equipment in designated bins and secure the tube,” Dr. Griffis advised. “Attach the ventilator with an HMEF filter to protect the ventilator circuit and inner parts of the machine. Recheck your breath sounds, CO2 production, and oxygen saturation, and adjust your vent settings as indicated.”
For post intubation, Dr. Griffis recommends securing contaminated discardable equipment in biohazard-labeled bins or bags, safely doffing your PPE, and retaining your N95 mask in the room. Remove your inner gloves, perform hand hygiene with soap and water if available, with alcohol-based hand rub if not, then don clean gloves. Exit the room, safely transporting any contaminated equipment that will be reused such as a cart or video laryngoscope to decontamination areas for processing. “Once clear of the room, order your chest x-ray to confirm your tube position per institutional protocol, understanding that radiology techs are all going to be following infection control procedures and wearing their PPE,” he said.
For extubation, Dr. Griffis recommends excusing all nonessential personnel from the patient room and assigning an assistant outside the room for necessary help. An experienced airway management expert should evaluate the patient wearing full PPE and be double-gloved. “If the extubation criteria are met, suction the pharynx and extubate,” he said. “Remove outer gloves and apply desired oxygen delivery equipment to the patient and assess respiratory status and vital signs for stability.” Next, discard all contaminated equipment in designated bins, doff contaminated PPE, and retain your N95 mask. Doff inner gloves, perform hand hygiene, and don clean gloves. “Exit the room, hand off contaminated equipment that is reusable, doff your gloves outside, do hand hygiene, then proceed to change your scrubs and complete your own personal hygiene measures,” he said.
Dr. Griffis reported having no financial disclosures.
“While the PPE used for intubation of a coronavirus patient is certainly more than the typical droplet precautions observed when intubating any other patient, the process and best practices aren’t terribly different from usual standard of care: Ensuring all necessary equipment is readily available with backup plans should the airway be difficult,” said Megan Conroy, MD, assistant professor of clinical medicine at The Ohio State University.
“We’ve been streamlining the team that’s present in the room for intubations of COVID patients, but I’m always amazed at the team members that stand at the ready to lend additional assistance just from the other side of the door. So while fewer personnel may be exposed, I wouldn’t consider the team needed for intubation to actually be much smaller, we’re just functioning differently.
In my practice the decision of when to intubate, clinically, doesn’t vary too much from any other form of severe ARDS. We may tolerate higher FiO2 requirements on heated high-flow nasal cannula if the patient exhibits acceptable work of breathing, but I wouldn’t advise allowing a patient to remain hypoxemic with oxygen needs unmet by noninvasive methods out of fear of intubation or ventilator management. In my opinion, this simply delays a necessary therapy and only makes for a higher risk intubation. Certainly, the decision to intubate is never based on only one single data point, but takes an expert assessment of the whole clinical picture.
I’d assert that it’s true in every disease that patients do better if it’s possible to avoid intubation – but I would argue that the ability to avoid intubation is determined primarily by the disease course and clinical scenario, and not by whether the physician wishes to avoid intubation or not. If I can safely manage a patient off of a ventilator, I will always do so, COVID or otherwise. I think in this phase of the pandemic, patients ‘do better without intubation’ because those who didn’t require intubation were inherently doing better!”
FROM AN SCCM VIRTUAL MEETING
Dermatologists play a key role in the transformation of transgender patients
, according to Doris Day, MD.
While clinical management of this patient population has historically been limited to experts in mental health, endocrinology, and select surgeons with experience in sex reassignment surgery, “what dermatologists provide on an aesthetic level through noninvasive or minimally invasive procedures can have a big impact in helping that transformation,” Dr. Day, of the department of dermatology at New York University Langone Health, said during the virtual annual Masters of Aesthetics Symposium. “But, we have to go through a transformation of sorts as well as we care for these patients, because we need to help them in the way that best matches their needs. We need to know about their mental health and the medicines they’re taking as well as their goals for their outcomes. If they’re working with surgeons for sex reassignment, we should have discussions with those clinicians as well.”
Gender-affirming hormone therapy is the primary medical intervention sought by transgender people, she said. This allows the acquisition of secondary sex characteristics more aligned with their gender identity. Feminizing hormone therapy affects the skin by reducing sebaceous gland activity, “which can lead to fewer acne breakouts and smaller pores but also cause drier skin,” Dr. Day said. “We can slow down the growth of body and facial hair and we can perform hair removal treatments. We see decreased male-pattern scalp hair loss, and we see smoother skin as the fat under the skin becomes thicker and the pores become smaller. We can also have increased pigment production, which is always a good thing.”
In a 2016 survey of 327 transgender individuals led by Dr. Day’s mentee, Brian A. Ginsberg, MD, and published in the Journal of the American Academy of Dermatology, most transgender women indicated that their face was most important to have changed, while for men it was the chest. Hair removal was the most common women’s facial procedure, followed by surgery then injectables, mostly performed by plastic surgeons.
Limitations of hormone therapy include the fact that it can take 2 or more years for associated changes to fully develop. “At least here in New York, patients want everything in a New York minute, so that’s always an issue,” she said. “We often recommend that patients wait at least 2 years after beginning hormone therapy before considering drastic feminization surgeries, but there are many options we have for them while they’re waiting for that. Even with hormone therapy, the bone structure of the face is unaffected, so we need to be artistic in creating a more feminized balance in order to help them physically match their gender to their identity.”
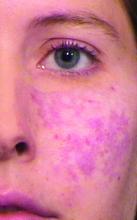
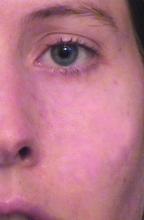
Noninvasive aesthetic procedures can compound the effects of hormone therapy, in addition to offering physical transformation beyond hormone therapy. She recalled assisting one of her patients transform from male to female. Over a period of 2 years, Dr. Day added Botox then Juvederm Voluma to the patient’s cheeks and chin, “and she started her transformation to a more feminized gender matching identity,” she said. Next came a hair transplant and the injection of more Voluma and fillers in the lips and cheeks on an as-needed basis.
“During one visit, I felt that we could still do more,” Dr. Day recalled. “She looked at me and said, ‘Actually, I feel so happy. This looks like me as I imagined I would look in my mind.’ I realized that my vision for her wasn’t the same as her vision for herself. She was thrilled with her transformation. I realized that as we see these patients, for all we learn about the science of gender transformation, the emotional aspects of our vision of what we can accomplish for our patients versus their vision of what their happiness level is may not entirely match. We have to be careful to help them celebrate their version of their femininity or masculinity, rather than trying to have our patients match what we think we can accomplish for them with our own sense of what femininity or masculinity is.”
Over time, Dr. Day said, the patient’s acne scars improved with fillers and microneedling treatments, and with the hormone therapy. “As we softened her appearance and as she made changes like the earrings that she wore and the hair style that she chose, she was in line with what her perception of her femininity was,” she said. “Little by little we’ve been watching her grow into her new self. It’s been a beautiful transformation. I was honored to be able to share in that journey with her.”
Dr. Day reported having no relevant financial disclosures.
, according to Doris Day, MD.
While clinical management of this patient population has historically been limited to experts in mental health, endocrinology, and select surgeons with experience in sex reassignment surgery, “what dermatologists provide on an aesthetic level through noninvasive or minimally invasive procedures can have a big impact in helping that transformation,” Dr. Day, of the department of dermatology at New York University Langone Health, said during the virtual annual Masters of Aesthetics Symposium. “But, we have to go through a transformation of sorts as well as we care for these patients, because we need to help them in the way that best matches their needs. We need to know about their mental health and the medicines they’re taking as well as their goals for their outcomes. If they’re working with surgeons for sex reassignment, we should have discussions with those clinicians as well.”
Gender-affirming hormone therapy is the primary medical intervention sought by transgender people, she said. This allows the acquisition of secondary sex characteristics more aligned with their gender identity. Feminizing hormone therapy affects the skin by reducing sebaceous gland activity, “which can lead to fewer acne breakouts and smaller pores but also cause drier skin,” Dr. Day said. “We can slow down the growth of body and facial hair and we can perform hair removal treatments. We see decreased male-pattern scalp hair loss, and we see smoother skin as the fat under the skin becomes thicker and the pores become smaller. We can also have increased pigment production, which is always a good thing.”
In a 2016 survey of 327 transgender individuals led by Dr. Day’s mentee, Brian A. Ginsberg, MD, and published in the Journal of the American Academy of Dermatology, most transgender women indicated that their face was most important to have changed, while for men it was the chest. Hair removal was the most common women’s facial procedure, followed by surgery then injectables, mostly performed by plastic surgeons.
Limitations of hormone therapy include the fact that it can take 2 or more years for associated changes to fully develop. “At least here in New York, patients want everything in a New York minute, so that’s always an issue,” she said. “We often recommend that patients wait at least 2 years after beginning hormone therapy before considering drastic feminization surgeries, but there are many options we have for them while they’re waiting for that. Even with hormone therapy, the bone structure of the face is unaffected, so we need to be artistic in creating a more feminized balance in order to help them physically match their gender to their identity.”
Noninvasive aesthetic procedures can compound the effects of hormone therapy, in addition to offering physical transformation beyond hormone therapy. She recalled assisting one of her patients transform from male to female. Over a period of 2 years, Dr. Day added Botox then Juvederm Voluma to the patient’s cheeks and chin, “and she started her transformation to a more feminized gender matching identity,” she said. Next came a hair transplant and the injection of more Voluma and fillers in the lips and cheeks on an as-needed basis.
“During one visit, I felt that we could still do more,” Dr. Day recalled. “She looked at me and said, ‘Actually, I feel so happy. This looks like me as I imagined I would look in my mind.’ I realized that my vision for her wasn’t the same as her vision for herself. She was thrilled with her transformation. I realized that as we see these patients, for all we learn about the science of gender transformation, the emotional aspects of our vision of what we can accomplish for our patients versus their vision of what their happiness level is may not entirely match. We have to be careful to help them celebrate their version of their femininity or masculinity, rather than trying to have our patients match what we think we can accomplish for them with our own sense of what femininity or masculinity is.”
Over time, Dr. Day said, the patient’s acne scars improved with fillers and microneedling treatments, and with the hormone therapy. “As we softened her appearance and as she made changes like the earrings that she wore and the hair style that she chose, she was in line with what her perception of her femininity was,” she said. “Little by little we’ve been watching her grow into her new self. It’s been a beautiful transformation. I was honored to be able to share in that journey with her.”
Dr. Day reported having no relevant financial disclosures.
, according to Doris Day, MD.
While clinical management of this patient population has historically been limited to experts in mental health, endocrinology, and select surgeons with experience in sex reassignment surgery, “what dermatologists provide on an aesthetic level through noninvasive or minimally invasive procedures can have a big impact in helping that transformation,” Dr. Day, of the department of dermatology at New York University Langone Health, said during the virtual annual Masters of Aesthetics Symposium. “But, we have to go through a transformation of sorts as well as we care for these patients, because we need to help them in the way that best matches their needs. We need to know about their mental health and the medicines they’re taking as well as their goals for their outcomes. If they’re working with surgeons for sex reassignment, we should have discussions with those clinicians as well.”
Gender-affirming hormone therapy is the primary medical intervention sought by transgender people, she said. This allows the acquisition of secondary sex characteristics more aligned with their gender identity. Feminizing hormone therapy affects the skin by reducing sebaceous gland activity, “which can lead to fewer acne breakouts and smaller pores but also cause drier skin,” Dr. Day said. “We can slow down the growth of body and facial hair and we can perform hair removal treatments. We see decreased male-pattern scalp hair loss, and we see smoother skin as the fat under the skin becomes thicker and the pores become smaller. We can also have increased pigment production, which is always a good thing.”
In a 2016 survey of 327 transgender individuals led by Dr. Day’s mentee, Brian A. Ginsberg, MD, and published in the Journal of the American Academy of Dermatology, most transgender women indicated that their face was most important to have changed, while for men it was the chest. Hair removal was the most common women’s facial procedure, followed by surgery then injectables, mostly performed by plastic surgeons.
Limitations of hormone therapy include the fact that it can take 2 or more years for associated changes to fully develop. “At least here in New York, patients want everything in a New York minute, so that’s always an issue,” she said. “We often recommend that patients wait at least 2 years after beginning hormone therapy before considering drastic feminization surgeries, but there are many options we have for them while they’re waiting for that. Even with hormone therapy, the bone structure of the face is unaffected, so we need to be artistic in creating a more feminized balance in order to help them physically match their gender to their identity.”
Noninvasive aesthetic procedures can compound the effects of hormone therapy, in addition to offering physical transformation beyond hormone therapy. She recalled assisting one of her patients transform from male to female. Over a period of 2 years, Dr. Day added Botox then Juvederm Voluma to the patient’s cheeks and chin, “and she started her transformation to a more feminized gender matching identity,” she said. Next came a hair transplant and the injection of more Voluma and fillers in the lips and cheeks on an as-needed basis.
“During one visit, I felt that we could still do more,” Dr. Day recalled. “She looked at me and said, ‘Actually, I feel so happy. This looks like me as I imagined I would look in my mind.’ I realized that my vision for her wasn’t the same as her vision for herself. She was thrilled with her transformation. I realized that as we see these patients, for all we learn about the science of gender transformation, the emotional aspects of our vision of what we can accomplish for our patients versus their vision of what their happiness level is may not entirely match. We have to be careful to help them celebrate their version of their femininity or masculinity, rather than trying to have our patients match what we think we can accomplish for them with our own sense of what femininity or masculinity is.”
Over time, Dr. Day said, the patient’s acne scars improved with fillers and microneedling treatments, and with the hormone therapy. “As we softened her appearance and as she made changes like the earrings that she wore and the hair style that she chose, she was in line with what her perception of her femininity was,” she said. “Little by little we’ve been watching her grow into her new self. It’s been a beautiful transformation. I was honored to be able to share in that journey with her.”
Dr. Day reported having no relevant financial disclosures.
FROM MOA 2020
‘Conservative parameters’ key to maximizing cosmetic laser results in skin of color
with special considerations, according to Andrew F. Alexis, MD, MPH.
“With the devices and approaches we have today, we can achieve safe and favorable outcomes, as long as we keep in mind that there is no one-size-fits all approach,” Dr. Alexis, chair of the department of dermatology at Mount Sinai Morningside and Mount Sinai West, New York, said during the virtual annual Masters of Aesthetics Symposium. “Conservative parameters are key.”
According to 2018 data from the American Society for Aesthetic Plastic Surgery, 30% of all aesthetic procedures in the United States are being performed on self-identified non-White racial ethnic groups. “This is projected to continue to increase given demographic changes as well as changes in our technologies and approaches to aesthetic procedures that allow for safer outcomes across a more diverse range of patients,” said Dr. Alexis, professor of dermatology at Icahn School of Medicine at Mount Sinai, New York. “That being said, even though we have many safe and effective options for all skin types today, we still have to consider that on the whole, there are higher risks of pigmentary and scarring complications when we perform most of our aesthetic procedures in darker skin types. The concept of limiting the degree of injury associated with a procedure remains paramount. Even when we pick the correct device for a give patient’s skin type, if our parameters aren’t optimal, or if our technique isn’t optimal, we can still end up with pigmentary and scarring complications.”
He offered key principles for maximining safety and optimal outcomes:
Know your device. Understand the range of parameters that are safe and effective for the given skin types that you see in your practice. “Don’t just rely on what the manufacturer provides in the manual, because you could have safe parameters as directed by the manual but undertreat some patients because the settings are too conservative,” Dr. Alexis said. “On the other hand, there might be scenarios where following recommended settings for a specific skin type might still wind up with a complication. Doing test spots is key in order to master the device that you are using.”
Know your patient. Don’t assume that you know a patient’s skin phototype or ancestry when that person first presents. “When we do that, we can arrive at erroneous conclusions with respect to phototype and with respect to ancestral background, and with respect to risk of pigmentary and scarring complications,” he said. “Treat your patient as an individual; no cookie-cutter responses, no assumptions.” He makes it a point to ask patients about their ancestry and about how their skin responds to sunlight in terms of tanning ability and to injury and inflammation such as insect bites, acne, and minor abrasions. “What happens to their skin when those things happen?” Dr. Alexis said. “Do they have a tendency to hyperpigment or not? You can easily ask for that or look for evidence of that on their skin. Similarly, asking about a personal or family history of keloids or hypertrophic scars is helpful in determining an overall risk assessment for a patient before you proceed with a given procedure.”
Recognize differences in preferred treatment options and parameters. Often, less is more. For example, he said, with laser hair removal, strive for longer wavelengths, lower fluences, longer pulse durations, and increased epidermal cooling. A study from 2002 in the Journal of the American Academy of Dermatology showed that the maximum tolerated fluence of type VI skin with the 1064 Nd: YAG laser was 50 J/cm2.
According to Dr. Alexis, nonablative fractional resurfacing “set the stage for being able to have safe outcomes for all skin types,” he said. “That being said, the higher the skin phototype, the higher the incidence of postinflammatory hyperpigmentation. How can we reduce this? The most important parameter is the treatment density, even though in a retrospective review from my center, high energies were associated with higher PIH rates too. Using conservative treatment densities lowers the risk of hyperpigmentation.”
Prophylactic use of hydroquinone prior to resurfacing with fractional lasers is another way to minimize the risk of postinflammatory hyperpigmentation. With this approach, Dr. Alexis asks patients to apply hydroquinone two weeks before treatment and for at least 4 weeks after. “Sun protection is key,” he said. “But when taking all of this into account, using conservative treatment densities in the range of 11%-20% coverage with a 1,550-nm Erbium-doped fractional laser, you can get favorable outcomes across skin types. But sometimes you can wind up with complications even if you do the right things.” He recalled a patient he treated for acne scarring and atrophic scars. After three treatments with the nonablative fractional 1,550-nm Erbium-doped laser set at level 4 (11% coverage), the patient developed hyperpigmentation of the treatment area. Dr. Alexis chose to continue treatment “with a few tweaks to reduce the risk of further hyperpigmentation,” he said. “I reduced the treatment density and the number of passes by half, so that the total energy delivered was halved. I also increased the concentration of hydroquinone from 4% to 6%. With that, the postinflammatory hyperpigmentation resolved.”
Another tool for resurfacing is the microsecond 1,064-nm Nd:YAG laser. “No anesthesia is required, there’s minimal down time, and you can treat all skin types,” Dr. Alexis said. “No pre- or posttreatment prophylaxis with bleaching agents are necessary, but multiple laser treatment sessions are required in order to achieve clinically meaningful results.” His approach to treating types V and VI skin involves a 1,064-nm Nd:YAG laser with a 5-mm spot size, a 0.3-microsecond pulse duration, a fluence of 12-14 J/cm2, a repetition rate of 5-8 Hz, 1,000-2,000 pulses per cosmetic unit, and avoidance of pulse stacking. He generally performs 4-6 treatment sessions 2-6 weeks apart.
An additional option for resurfacing is the 650-microsecond 1,064-nm Nd:YAG laser. The recommend fluence in skin of color is 14-21 J/cm2. A recent review article in the Journal of Drugs in Dermatology described clinical experience using this device for a wide range of conditions in darker skin types, including acne, hyperpigmentation, and melasma.
A more recent approach is using fractional radiofrequency devices, especially those that feature coated pin tips. These tips “protect the epidermis from heat injury and deliver heat to the deeper dermis where we want it, and minimize the risk to the epidermis,” Dr. Alexis said. In a 2018 study in the Journal of Drugs in Dermatology of 35 patients with skin type VI, participants received three sessions of facial treatments, 4 weeks apart using a fractional RF device with 24-pin coated tip. The researchers found that the regimen was safe and effective, and that it resulted in improved wrinkles, acne scars, and overall skin appearance.
Dr. Alexis disclosed that he has served as an adviser to or has received consulting fees from Leo, Novartis, Menlo, Galderma, Pfizer, Sanofi-Regeneron, Dermavant, Unilever, Celgene, Beiersdorf, Valeant, L’Oreal, BMS, Scientis, Bausch Health, UCB, Foamix, and Cassiopea.
with special considerations, according to Andrew F. Alexis, MD, MPH.
“With the devices and approaches we have today, we can achieve safe and favorable outcomes, as long as we keep in mind that there is no one-size-fits all approach,” Dr. Alexis, chair of the department of dermatology at Mount Sinai Morningside and Mount Sinai West, New York, said during the virtual annual Masters of Aesthetics Symposium. “Conservative parameters are key.”
According to 2018 data from the American Society for Aesthetic Plastic Surgery, 30% of all aesthetic procedures in the United States are being performed on self-identified non-White racial ethnic groups. “This is projected to continue to increase given demographic changes as well as changes in our technologies and approaches to aesthetic procedures that allow for safer outcomes across a more diverse range of patients,” said Dr. Alexis, professor of dermatology at Icahn School of Medicine at Mount Sinai, New York. “That being said, even though we have many safe and effective options for all skin types today, we still have to consider that on the whole, there are higher risks of pigmentary and scarring complications when we perform most of our aesthetic procedures in darker skin types. The concept of limiting the degree of injury associated with a procedure remains paramount. Even when we pick the correct device for a give patient’s skin type, if our parameters aren’t optimal, or if our technique isn’t optimal, we can still end up with pigmentary and scarring complications.”
He offered key principles for maximining safety and optimal outcomes:
Know your device. Understand the range of parameters that are safe and effective for the given skin types that you see in your practice. “Don’t just rely on what the manufacturer provides in the manual, because you could have safe parameters as directed by the manual but undertreat some patients because the settings are too conservative,” Dr. Alexis said. “On the other hand, there might be scenarios where following recommended settings for a specific skin type might still wind up with a complication. Doing test spots is key in order to master the device that you are using.”
Know your patient. Don’t assume that you know a patient’s skin phototype or ancestry when that person first presents. “When we do that, we can arrive at erroneous conclusions with respect to phototype and with respect to ancestral background, and with respect to risk of pigmentary and scarring complications,” he said. “Treat your patient as an individual; no cookie-cutter responses, no assumptions.” He makes it a point to ask patients about their ancestry and about how their skin responds to sunlight in terms of tanning ability and to injury and inflammation such as insect bites, acne, and minor abrasions. “What happens to their skin when those things happen?” Dr. Alexis said. “Do they have a tendency to hyperpigment or not? You can easily ask for that or look for evidence of that on their skin. Similarly, asking about a personal or family history of keloids or hypertrophic scars is helpful in determining an overall risk assessment for a patient before you proceed with a given procedure.”
Recognize differences in preferred treatment options and parameters. Often, less is more. For example, he said, with laser hair removal, strive for longer wavelengths, lower fluences, longer pulse durations, and increased epidermal cooling. A study from 2002 in the Journal of the American Academy of Dermatology showed that the maximum tolerated fluence of type VI skin with the 1064 Nd: YAG laser was 50 J/cm2.
According to Dr. Alexis, nonablative fractional resurfacing “set the stage for being able to have safe outcomes for all skin types,” he said. “That being said, the higher the skin phototype, the higher the incidence of postinflammatory hyperpigmentation. How can we reduce this? The most important parameter is the treatment density, even though in a retrospective review from my center, high energies were associated with higher PIH rates too. Using conservative treatment densities lowers the risk of hyperpigmentation.”
Prophylactic use of hydroquinone prior to resurfacing with fractional lasers is another way to minimize the risk of postinflammatory hyperpigmentation. With this approach, Dr. Alexis asks patients to apply hydroquinone two weeks before treatment and for at least 4 weeks after. “Sun protection is key,” he said. “But when taking all of this into account, using conservative treatment densities in the range of 11%-20% coverage with a 1,550-nm Erbium-doped fractional laser, you can get favorable outcomes across skin types. But sometimes you can wind up with complications even if you do the right things.” He recalled a patient he treated for acne scarring and atrophic scars. After three treatments with the nonablative fractional 1,550-nm Erbium-doped laser set at level 4 (11% coverage), the patient developed hyperpigmentation of the treatment area. Dr. Alexis chose to continue treatment “with a few tweaks to reduce the risk of further hyperpigmentation,” he said. “I reduced the treatment density and the number of passes by half, so that the total energy delivered was halved. I also increased the concentration of hydroquinone from 4% to 6%. With that, the postinflammatory hyperpigmentation resolved.”
Another tool for resurfacing is the microsecond 1,064-nm Nd:YAG laser. “No anesthesia is required, there’s minimal down time, and you can treat all skin types,” Dr. Alexis said. “No pre- or posttreatment prophylaxis with bleaching agents are necessary, but multiple laser treatment sessions are required in order to achieve clinically meaningful results.” His approach to treating types V and VI skin involves a 1,064-nm Nd:YAG laser with a 5-mm spot size, a 0.3-microsecond pulse duration, a fluence of 12-14 J/cm2, a repetition rate of 5-8 Hz, 1,000-2,000 pulses per cosmetic unit, and avoidance of pulse stacking. He generally performs 4-6 treatment sessions 2-6 weeks apart.
An additional option for resurfacing is the 650-microsecond 1,064-nm Nd:YAG laser. The recommend fluence in skin of color is 14-21 J/cm2. A recent review article in the Journal of Drugs in Dermatology described clinical experience using this device for a wide range of conditions in darker skin types, including acne, hyperpigmentation, and melasma.
A more recent approach is using fractional radiofrequency devices, especially those that feature coated pin tips. These tips “protect the epidermis from heat injury and deliver heat to the deeper dermis where we want it, and minimize the risk to the epidermis,” Dr. Alexis said. In a 2018 study in the Journal of Drugs in Dermatology of 35 patients with skin type VI, participants received three sessions of facial treatments, 4 weeks apart using a fractional RF device with 24-pin coated tip. The researchers found that the regimen was safe and effective, and that it resulted in improved wrinkles, acne scars, and overall skin appearance.
Dr. Alexis disclosed that he has served as an adviser to or has received consulting fees from Leo, Novartis, Menlo, Galderma, Pfizer, Sanofi-Regeneron, Dermavant, Unilever, Celgene, Beiersdorf, Valeant, L’Oreal, BMS, Scientis, Bausch Health, UCB, Foamix, and Cassiopea.
with special considerations, according to Andrew F. Alexis, MD, MPH.
“With the devices and approaches we have today, we can achieve safe and favorable outcomes, as long as we keep in mind that there is no one-size-fits all approach,” Dr. Alexis, chair of the department of dermatology at Mount Sinai Morningside and Mount Sinai West, New York, said during the virtual annual Masters of Aesthetics Symposium. “Conservative parameters are key.”
According to 2018 data from the American Society for Aesthetic Plastic Surgery, 30% of all aesthetic procedures in the United States are being performed on self-identified non-White racial ethnic groups. “This is projected to continue to increase given demographic changes as well as changes in our technologies and approaches to aesthetic procedures that allow for safer outcomes across a more diverse range of patients,” said Dr. Alexis, professor of dermatology at Icahn School of Medicine at Mount Sinai, New York. “That being said, even though we have many safe and effective options for all skin types today, we still have to consider that on the whole, there are higher risks of pigmentary and scarring complications when we perform most of our aesthetic procedures in darker skin types. The concept of limiting the degree of injury associated with a procedure remains paramount. Even when we pick the correct device for a give patient’s skin type, if our parameters aren’t optimal, or if our technique isn’t optimal, we can still end up with pigmentary and scarring complications.”
He offered key principles for maximining safety and optimal outcomes:
Know your device. Understand the range of parameters that are safe and effective for the given skin types that you see in your practice. “Don’t just rely on what the manufacturer provides in the manual, because you could have safe parameters as directed by the manual but undertreat some patients because the settings are too conservative,” Dr. Alexis said. “On the other hand, there might be scenarios where following recommended settings for a specific skin type might still wind up with a complication. Doing test spots is key in order to master the device that you are using.”
Know your patient. Don’t assume that you know a patient’s skin phototype or ancestry when that person first presents. “When we do that, we can arrive at erroneous conclusions with respect to phototype and with respect to ancestral background, and with respect to risk of pigmentary and scarring complications,” he said. “Treat your patient as an individual; no cookie-cutter responses, no assumptions.” He makes it a point to ask patients about their ancestry and about how their skin responds to sunlight in terms of tanning ability and to injury and inflammation such as insect bites, acne, and minor abrasions. “What happens to their skin when those things happen?” Dr. Alexis said. “Do they have a tendency to hyperpigment or not? You can easily ask for that or look for evidence of that on their skin. Similarly, asking about a personal or family history of keloids or hypertrophic scars is helpful in determining an overall risk assessment for a patient before you proceed with a given procedure.”
Recognize differences in preferred treatment options and parameters. Often, less is more. For example, he said, with laser hair removal, strive for longer wavelengths, lower fluences, longer pulse durations, and increased epidermal cooling. A study from 2002 in the Journal of the American Academy of Dermatology showed that the maximum tolerated fluence of type VI skin with the 1064 Nd: YAG laser was 50 J/cm2.
According to Dr. Alexis, nonablative fractional resurfacing “set the stage for being able to have safe outcomes for all skin types,” he said. “That being said, the higher the skin phototype, the higher the incidence of postinflammatory hyperpigmentation. How can we reduce this? The most important parameter is the treatment density, even though in a retrospective review from my center, high energies were associated with higher PIH rates too. Using conservative treatment densities lowers the risk of hyperpigmentation.”
Prophylactic use of hydroquinone prior to resurfacing with fractional lasers is another way to minimize the risk of postinflammatory hyperpigmentation. With this approach, Dr. Alexis asks patients to apply hydroquinone two weeks before treatment and for at least 4 weeks after. “Sun protection is key,” he said. “But when taking all of this into account, using conservative treatment densities in the range of 11%-20% coverage with a 1,550-nm Erbium-doped fractional laser, you can get favorable outcomes across skin types. But sometimes you can wind up with complications even if you do the right things.” He recalled a patient he treated for acne scarring and atrophic scars. After three treatments with the nonablative fractional 1,550-nm Erbium-doped laser set at level 4 (11% coverage), the patient developed hyperpigmentation of the treatment area. Dr. Alexis chose to continue treatment “with a few tweaks to reduce the risk of further hyperpigmentation,” he said. “I reduced the treatment density and the number of passes by half, so that the total energy delivered was halved. I also increased the concentration of hydroquinone from 4% to 6%. With that, the postinflammatory hyperpigmentation resolved.”
Another tool for resurfacing is the microsecond 1,064-nm Nd:YAG laser. “No anesthesia is required, there’s minimal down time, and you can treat all skin types,” Dr. Alexis said. “No pre- or posttreatment prophylaxis with bleaching agents are necessary, but multiple laser treatment sessions are required in order to achieve clinically meaningful results.” His approach to treating types V and VI skin involves a 1,064-nm Nd:YAG laser with a 5-mm spot size, a 0.3-microsecond pulse duration, a fluence of 12-14 J/cm2, a repetition rate of 5-8 Hz, 1,000-2,000 pulses per cosmetic unit, and avoidance of pulse stacking. He generally performs 4-6 treatment sessions 2-6 weeks apart.
An additional option for resurfacing is the 650-microsecond 1,064-nm Nd:YAG laser. The recommend fluence in skin of color is 14-21 J/cm2. A recent review article in the Journal of Drugs in Dermatology described clinical experience using this device for a wide range of conditions in darker skin types, including acne, hyperpigmentation, and melasma.
A more recent approach is using fractional radiofrequency devices, especially those that feature coated pin tips. These tips “protect the epidermis from heat injury and deliver heat to the deeper dermis where we want it, and minimize the risk to the epidermis,” Dr. Alexis said. In a 2018 study in the Journal of Drugs in Dermatology of 35 patients with skin type VI, participants received three sessions of facial treatments, 4 weeks apart using a fractional RF device with 24-pin coated tip. The researchers found that the regimen was safe and effective, and that it resulted in improved wrinkles, acne scars, and overall skin appearance.
Dr. Alexis disclosed that he has served as an adviser to or has received consulting fees from Leo, Novartis, Menlo, Galderma, Pfizer, Sanofi-Regeneron, Dermavant, Unilever, Celgene, Beiersdorf, Valeant, L’Oreal, BMS, Scientis, Bausch Health, UCB, Foamix, and Cassiopea.
AT MOA 2020
Noninvasive ventilation: Options and cautions for patients with COVID-19
Early on in the COVID-19 pandemic,
“We were concerned that, if we put them on high-flow nasal cannula or a noninvasive ventilation, that we would create aerosols that would then be a risk to clinicians,” Meghan Lane-Fall, MD, MSHP, FCCM, said at a Society for Critical Care Medicine virtual meeting called COVID-19: What’s Next. “However, we’ve gotten much more comfortable with infection control. We’ve gotten much more comfortable with controlling these aerosols, with making sure that our clinicians are protected with the appropriate protective equipment. We’ve also realized that patients who end up becoming intubated have really poor outcomes, so we’ve looked at our practice critically and tried to figure out how to support patients noninvasively when that’s possible.”
Respiratory support options
According to Dr. Lane-Fall, an associate professor of anesthesiology and critical care at the University of Pennsylvania, Philadelphia, there are two basic types of respiratory support in patients with moderate, severe, or critical COVID-19: noninvasive and invasive. Noninvasive options include CPAP or BiPAP which can be delivered through nasal pillows, masks, and helmets, as well as high-flow nasal oxygen. Invasive options include endotracheal intubation, tracheostomy, and extracorporeal membrane oxygenation (ECMO), usually the veno-venous (VV) form. “But it’s uncommon to need VV ECMO, even in patients who have critical COVID-19,” she said.
Factors that favor noninvasive ventilation include stably high oxygen requirements, normal mental status, ward location of care, and moderate to severe COVID-19. Factors that favor invasive ventilation include someone who’s deteriorating rapidly, “whose oxygen requirements aren’t stable or who is cardiopulmonary compromised,” said Dr. Lane-Fall, who is also co–medical director of the Trauma Surgery Intensive Care Unit at Penn Presbyterian Medical Center, also in Philadelphia. Other factors include the need for other invasive procedures such as surgery or if they have severe to critical COVID-19, “not just pneumonia, but [illness that’s] progressing into [acute respiratory distress syndrome],” she said.
Indications for urgent endotracheal intubation as opposed to giving a trial of noninvasive ventilation or high-flow nasal oxygen include altered mental status, inability to protect airway, copious amounts of secretions, a Glasgow Coma Scale score of less than 8, severe respiratory acidosis, hypopnea or apnea, shock, or an inability to tolerate noninvasive support. “This is a relative contraindication,” Dr. Lane-Fall said. “I’ve certainly talked people through the BiPAP mask or the helmet. If you tell a patient, ‘I don’t want to have to put in a breathing tube; I want to maintain you on this,’ often they’ll be able to work through it.”
Safety precautions
Aerosolizing procedures require attention to location, personnel, and equipment, including personal protective equipment (PPE), said Dr. Lane-Fall, who is an anesthesiologist by training. “When you are intubating someone, whether they have COVID-19 or not, you are sort of in the belly of the beast,” she said. “You are very exposed to secretions that occur at the time of endotracheal intubation. That’s why it’s important for us to have PPE and barriers to protect ourselves from potential exposure to aerosols during the care of patients with COVID-19.”
In February 2020, the non-for-profit Anesthesia Patient Safety Foundation published recommendations for airway management in patients with suspected COVID-19. A separate guidance was published the British Journal of Anaesthesiology based on emergency tracheal intubation in 202 patients with COVID-19 in Wuhan, China. “The idea here is that you want to intubate under controlled conditions,” said Dr. Lane-Fall, who is an author of the guidance. “You want to use the most experienced operator. You want to have full PPE, including an N95 mask, or something more protective like a powered air purifying respirator or an N95 mask with a face shield. You want the eyes, nose, and mouth of the operator covered completely.”
CPR, another aerosolizing procedure, requires vigilant safety precautions as well. “We struggled with this a little bit at our institution, because our inclination as intensivists when someone is pulseless is to run into the room and start chest compressions and to start resuscitation,” Dr. Lane-Fall said. “But the act of chest compression itself can create aerosols that can present risk to clinicians. We had to tell our clinicians that they have to put on PPE before they do CPR. The buzz phrase here is that there is no emergency in a pandemic. The idea here is that the good of that one patient is outweighed by the good of all the other patients that you could care for if you didn’t have COVID-19 as a clinician. So we have had to encourage our staff to put on PPE first before attending to patients first, even if it delays patient care. Once you have donned PPE, when you’re administering CPR, the number of staff should be minimized. You should have a compressor, and someone to relieve the compressor, and a code leader, someone tending to the airway. But in general, anyone who’s not actively involved should not be in the room.”
Risks during extubation
Extubation of COVID-19 patients is also an aerosolizing procedure not just because you’re pulling an endotracheal tube out of the airway but because coughing is a normal part of extubation. “We’ve had to be careful with how we approach extubation in COVID-19 patients,” Dr. Lane-Fall said. “Ideally you’re doing this in a negative pressure environment. We have also had to use full PPE, covering the eyes and face, and putting on a gown for precaution.”
Reintubation of COVID-19 patients is not uncommon. She and her colleagues at Penn Medicine created procedures for having intubators at the ready outside the room in case the patient were to decompensate clinically. “Another thing we learned is that it’s useful to do a leak test prior to extubation, because there may be airway edema related to prolonged intubation in these patients,” Dr. Lane-Fall said. “We found that, if a leak is absent on checking the cuff leak, the use of steroids for a day or 2 may help decrease airway edema. That improves the chances of extubation success.”
Strategies for aerosol containment
She concluded her remarks by reviewing airway control adjuncts and clinician safety. This includes physically isolating COVID-19 patients in negative pressure rooms and avoiding and minimizing aerosols, including the use of rapid intubation, “where we induce anesthesia for intubation but we don’t bag-mask the patient because that creates aerosols,” she said. The Anesthesia Patient Safety Foundation guidelines advocate for the use of video laryngoscopy so that you can visualize the glottis easily “and make sure that you successfully intubate the glottis and not the esophagus,” she said.
A smart strategy for aerosol containment is to use the most experienced laryngoscopist available. “If you are in a teaching program, ideally you’re using your most experienced resident, or you’re using fellows or attending physicians,” Dr. Lane-Fall said. “This is not the space for an inexperienced learner.”
Another way to make intubation faster and easier in COVID-19 patients is to use an intubation box, which features a plexiglass shield that enables the intubator to use their hands to get in the patient’s airway while being protected from viral droplets generated during intubation. The box can be cleaned after each use. Blueprints for an open source intubation box can be found at http://www.intubationbox.com.
Expert view on aerosol containment in COVID-19
“While there is a dearth of evidence from controlled trials, recommendations mentioned in this story are based on the best available evidence and are in agreement with guidelines from several expert groups,” said David L. Bowton, MD, FCCP, FCCM, of the department of anesthesiology at Wake Forest Baptist Health in Winston-Salem, NC. “The recommendation of Dr. Lane-Fall’s that is perhaps most controversial is the use of an intubation box. Multiple designs for these intubation/aerosol containment devices have been proposed, and the data supporting their ease of use and efficacy has been mixed [See Anaesthesia 2020;75(8):1014-21 and Anaesthesia. 2020. doi: 10.1111/anae.15188]. While bag valve mask ventilation should be avoided if possible, it may be a valuable rescue tool in the severely hypoxemic patient when used with two-person technique to achieve a tight seal and a PEEP valve and an HME over the exhalation port to minimize aerosol spread.
“It cannot be stressed enough that the most skilled individual should be tasked with intubating the patient and as few providers as possible [usually three] should be in the room and have donned full PPE. Negative pressure rooms should be used whenever feasible. Noninvasive ventilation appears safer from an infection control standpoint than initially feared and its use has become more widespread. However, noninvasive ventilation is not without its hazards, and Dr. Lane-Fall’s enumeration of the patient characteristics applicable to the selection of patients for noninvasive ventilation are extremely important. At our institution, the use of noninvasive ventilation and especially high-flow oxygen therapy has increased. Staff have become more comfortable with the donning and doffing of PPE.”
Dr. Lane-Fall reported having no financial disclosures.
Early on in the COVID-19 pandemic,
“We were concerned that, if we put them on high-flow nasal cannula or a noninvasive ventilation, that we would create aerosols that would then be a risk to clinicians,” Meghan Lane-Fall, MD, MSHP, FCCM, said at a Society for Critical Care Medicine virtual meeting called COVID-19: What’s Next. “However, we’ve gotten much more comfortable with infection control. We’ve gotten much more comfortable with controlling these aerosols, with making sure that our clinicians are protected with the appropriate protective equipment. We’ve also realized that patients who end up becoming intubated have really poor outcomes, so we’ve looked at our practice critically and tried to figure out how to support patients noninvasively when that’s possible.”
Respiratory support options
According to Dr. Lane-Fall, an associate professor of anesthesiology and critical care at the University of Pennsylvania, Philadelphia, there are two basic types of respiratory support in patients with moderate, severe, or critical COVID-19: noninvasive and invasive. Noninvasive options include CPAP or BiPAP which can be delivered through nasal pillows, masks, and helmets, as well as high-flow nasal oxygen. Invasive options include endotracheal intubation, tracheostomy, and extracorporeal membrane oxygenation (ECMO), usually the veno-venous (VV) form. “But it’s uncommon to need VV ECMO, even in patients who have critical COVID-19,” she said.
Factors that favor noninvasive ventilation include stably high oxygen requirements, normal mental status, ward location of care, and moderate to severe COVID-19. Factors that favor invasive ventilation include someone who’s deteriorating rapidly, “whose oxygen requirements aren’t stable or who is cardiopulmonary compromised,” said Dr. Lane-Fall, who is also co–medical director of the Trauma Surgery Intensive Care Unit at Penn Presbyterian Medical Center, also in Philadelphia. Other factors include the need for other invasive procedures such as surgery or if they have severe to critical COVID-19, “not just pneumonia, but [illness that’s] progressing into [acute respiratory distress syndrome],” she said.
Indications for urgent endotracheal intubation as opposed to giving a trial of noninvasive ventilation or high-flow nasal oxygen include altered mental status, inability to protect airway, copious amounts of secretions, a Glasgow Coma Scale score of less than 8, severe respiratory acidosis, hypopnea or apnea, shock, or an inability to tolerate noninvasive support. “This is a relative contraindication,” Dr. Lane-Fall said. “I’ve certainly talked people through the BiPAP mask or the helmet. If you tell a patient, ‘I don’t want to have to put in a breathing tube; I want to maintain you on this,’ often they’ll be able to work through it.”
Safety precautions
Aerosolizing procedures require attention to location, personnel, and equipment, including personal protective equipment (PPE), said Dr. Lane-Fall, who is an anesthesiologist by training. “When you are intubating someone, whether they have COVID-19 or not, you are sort of in the belly of the beast,” she said. “You are very exposed to secretions that occur at the time of endotracheal intubation. That’s why it’s important for us to have PPE and barriers to protect ourselves from potential exposure to aerosols during the care of patients with COVID-19.”
In February 2020, the non-for-profit Anesthesia Patient Safety Foundation published recommendations for airway management in patients with suspected COVID-19. A separate guidance was published the British Journal of Anaesthesiology based on emergency tracheal intubation in 202 patients with COVID-19 in Wuhan, China. “The idea here is that you want to intubate under controlled conditions,” said Dr. Lane-Fall, who is an author of the guidance. “You want to use the most experienced operator. You want to have full PPE, including an N95 mask, or something more protective like a powered air purifying respirator or an N95 mask with a face shield. You want the eyes, nose, and mouth of the operator covered completely.”
CPR, another aerosolizing procedure, requires vigilant safety precautions as well. “We struggled with this a little bit at our institution, because our inclination as intensivists when someone is pulseless is to run into the room and start chest compressions and to start resuscitation,” Dr. Lane-Fall said. “But the act of chest compression itself can create aerosols that can present risk to clinicians. We had to tell our clinicians that they have to put on PPE before they do CPR. The buzz phrase here is that there is no emergency in a pandemic. The idea here is that the good of that one patient is outweighed by the good of all the other patients that you could care for if you didn’t have COVID-19 as a clinician. So we have had to encourage our staff to put on PPE first before attending to patients first, even if it delays patient care. Once you have donned PPE, when you’re administering CPR, the number of staff should be minimized. You should have a compressor, and someone to relieve the compressor, and a code leader, someone tending to the airway. But in general, anyone who’s not actively involved should not be in the room.”
Risks during extubation
Extubation of COVID-19 patients is also an aerosolizing procedure not just because you’re pulling an endotracheal tube out of the airway but because coughing is a normal part of extubation. “We’ve had to be careful with how we approach extubation in COVID-19 patients,” Dr. Lane-Fall said. “Ideally you’re doing this in a negative pressure environment. We have also had to use full PPE, covering the eyes and face, and putting on a gown for precaution.”
Reintubation of COVID-19 patients is not uncommon. She and her colleagues at Penn Medicine created procedures for having intubators at the ready outside the room in case the patient were to decompensate clinically. “Another thing we learned is that it’s useful to do a leak test prior to extubation, because there may be airway edema related to prolonged intubation in these patients,” Dr. Lane-Fall said. “We found that, if a leak is absent on checking the cuff leak, the use of steroids for a day or 2 may help decrease airway edema. That improves the chances of extubation success.”
Strategies for aerosol containment
She concluded her remarks by reviewing airway control adjuncts and clinician safety. This includes physically isolating COVID-19 patients in negative pressure rooms and avoiding and minimizing aerosols, including the use of rapid intubation, “where we induce anesthesia for intubation but we don’t bag-mask the patient because that creates aerosols,” she said. The Anesthesia Patient Safety Foundation guidelines advocate for the use of video laryngoscopy so that you can visualize the glottis easily “and make sure that you successfully intubate the glottis and not the esophagus,” she said.
A smart strategy for aerosol containment is to use the most experienced laryngoscopist available. “If you are in a teaching program, ideally you’re using your most experienced resident, or you’re using fellows or attending physicians,” Dr. Lane-Fall said. “This is not the space for an inexperienced learner.”
Another way to make intubation faster and easier in COVID-19 patients is to use an intubation box, which features a plexiglass shield that enables the intubator to use their hands to get in the patient’s airway while being protected from viral droplets generated during intubation. The box can be cleaned after each use. Blueprints for an open source intubation box can be found at http://www.intubationbox.com.
Expert view on aerosol containment in COVID-19
“While there is a dearth of evidence from controlled trials, recommendations mentioned in this story are based on the best available evidence and are in agreement with guidelines from several expert groups,” said David L. Bowton, MD, FCCP, FCCM, of the department of anesthesiology at Wake Forest Baptist Health in Winston-Salem, NC. “The recommendation of Dr. Lane-Fall’s that is perhaps most controversial is the use of an intubation box. Multiple designs for these intubation/aerosol containment devices have been proposed, and the data supporting their ease of use and efficacy has been mixed [See Anaesthesia 2020;75(8):1014-21 and Anaesthesia. 2020. doi: 10.1111/anae.15188]. While bag valve mask ventilation should be avoided if possible, it may be a valuable rescue tool in the severely hypoxemic patient when used with two-person technique to achieve a tight seal and a PEEP valve and an HME over the exhalation port to minimize aerosol spread.
“It cannot be stressed enough that the most skilled individual should be tasked with intubating the patient and as few providers as possible [usually three] should be in the room and have donned full PPE. Negative pressure rooms should be used whenever feasible. Noninvasive ventilation appears safer from an infection control standpoint than initially feared and its use has become more widespread. However, noninvasive ventilation is not without its hazards, and Dr. Lane-Fall’s enumeration of the patient characteristics applicable to the selection of patients for noninvasive ventilation are extremely important. At our institution, the use of noninvasive ventilation and especially high-flow oxygen therapy has increased. Staff have become more comfortable with the donning and doffing of PPE.”
Dr. Lane-Fall reported having no financial disclosures.
Early on in the COVID-19 pandemic,
“We were concerned that, if we put them on high-flow nasal cannula or a noninvasive ventilation, that we would create aerosols that would then be a risk to clinicians,” Meghan Lane-Fall, MD, MSHP, FCCM, said at a Society for Critical Care Medicine virtual meeting called COVID-19: What’s Next. “However, we’ve gotten much more comfortable with infection control. We’ve gotten much more comfortable with controlling these aerosols, with making sure that our clinicians are protected with the appropriate protective equipment. We’ve also realized that patients who end up becoming intubated have really poor outcomes, so we’ve looked at our practice critically and tried to figure out how to support patients noninvasively when that’s possible.”
Respiratory support options
According to Dr. Lane-Fall, an associate professor of anesthesiology and critical care at the University of Pennsylvania, Philadelphia, there are two basic types of respiratory support in patients with moderate, severe, or critical COVID-19: noninvasive and invasive. Noninvasive options include CPAP or BiPAP which can be delivered through nasal pillows, masks, and helmets, as well as high-flow nasal oxygen. Invasive options include endotracheal intubation, tracheostomy, and extracorporeal membrane oxygenation (ECMO), usually the veno-venous (VV) form. “But it’s uncommon to need VV ECMO, even in patients who have critical COVID-19,” she said.
Factors that favor noninvasive ventilation include stably high oxygen requirements, normal mental status, ward location of care, and moderate to severe COVID-19. Factors that favor invasive ventilation include someone who’s deteriorating rapidly, “whose oxygen requirements aren’t stable or who is cardiopulmonary compromised,” said Dr. Lane-Fall, who is also co–medical director of the Trauma Surgery Intensive Care Unit at Penn Presbyterian Medical Center, also in Philadelphia. Other factors include the need for other invasive procedures such as surgery or if they have severe to critical COVID-19, “not just pneumonia, but [illness that’s] progressing into [acute respiratory distress syndrome],” she said.
Indications for urgent endotracheal intubation as opposed to giving a trial of noninvasive ventilation or high-flow nasal oxygen include altered mental status, inability to protect airway, copious amounts of secretions, a Glasgow Coma Scale score of less than 8, severe respiratory acidosis, hypopnea or apnea, shock, or an inability to tolerate noninvasive support. “This is a relative contraindication,” Dr. Lane-Fall said. “I’ve certainly talked people through the BiPAP mask or the helmet. If you tell a patient, ‘I don’t want to have to put in a breathing tube; I want to maintain you on this,’ often they’ll be able to work through it.”
Safety precautions
Aerosolizing procedures require attention to location, personnel, and equipment, including personal protective equipment (PPE), said Dr. Lane-Fall, who is an anesthesiologist by training. “When you are intubating someone, whether they have COVID-19 or not, you are sort of in the belly of the beast,” she said. “You are very exposed to secretions that occur at the time of endotracheal intubation. That’s why it’s important for us to have PPE and barriers to protect ourselves from potential exposure to aerosols during the care of patients with COVID-19.”
In February 2020, the non-for-profit Anesthesia Patient Safety Foundation published recommendations for airway management in patients with suspected COVID-19. A separate guidance was published the British Journal of Anaesthesiology based on emergency tracheal intubation in 202 patients with COVID-19 in Wuhan, China. “The idea here is that you want to intubate under controlled conditions,” said Dr. Lane-Fall, who is an author of the guidance. “You want to use the most experienced operator. You want to have full PPE, including an N95 mask, or something more protective like a powered air purifying respirator or an N95 mask with a face shield. You want the eyes, nose, and mouth of the operator covered completely.”
CPR, another aerosolizing procedure, requires vigilant safety precautions as well. “We struggled with this a little bit at our institution, because our inclination as intensivists when someone is pulseless is to run into the room and start chest compressions and to start resuscitation,” Dr. Lane-Fall said. “But the act of chest compression itself can create aerosols that can present risk to clinicians. We had to tell our clinicians that they have to put on PPE before they do CPR. The buzz phrase here is that there is no emergency in a pandemic. The idea here is that the good of that one patient is outweighed by the good of all the other patients that you could care for if you didn’t have COVID-19 as a clinician. So we have had to encourage our staff to put on PPE first before attending to patients first, even if it delays patient care. Once you have donned PPE, when you’re administering CPR, the number of staff should be minimized. You should have a compressor, and someone to relieve the compressor, and a code leader, someone tending to the airway. But in general, anyone who’s not actively involved should not be in the room.”
Risks during extubation
Extubation of COVID-19 patients is also an aerosolizing procedure not just because you’re pulling an endotracheal tube out of the airway but because coughing is a normal part of extubation. “We’ve had to be careful with how we approach extubation in COVID-19 patients,” Dr. Lane-Fall said. “Ideally you’re doing this in a negative pressure environment. We have also had to use full PPE, covering the eyes and face, and putting on a gown for precaution.”
Reintubation of COVID-19 patients is not uncommon. She and her colleagues at Penn Medicine created procedures for having intubators at the ready outside the room in case the patient were to decompensate clinically. “Another thing we learned is that it’s useful to do a leak test prior to extubation, because there may be airway edema related to prolonged intubation in these patients,” Dr. Lane-Fall said. “We found that, if a leak is absent on checking the cuff leak, the use of steroids for a day or 2 may help decrease airway edema. That improves the chances of extubation success.”
Strategies for aerosol containment
She concluded her remarks by reviewing airway control adjuncts and clinician safety. This includes physically isolating COVID-19 patients in negative pressure rooms and avoiding and minimizing aerosols, including the use of rapid intubation, “where we induce anesthesia for intubation but we don’t bag-mask the patient because that creates aerosols,” she said. The Anesthesia Patient Safety Foundation guidelines advocate for the use of video laryngoscopy so that you can visualize the glottis easily “and make sure that you successfully intubate the glottis and not the esophagus,” she said.
A smart strategy for aerosol containment is to use the most experienced laryngoscopist available. “If you are in a teaching program, ideally you’re using your most experienced resident, or you’re using fellows or attending physicians,” Dr. Lane-Fall said. “This is not the space for an inexperienced learner.”
Another way to make intubation faster and easier in COVID-19 patients is to use an intubation box, which features a plexiglass shield that enables the intubator to use their hands to get in the patient’s airway while being protected from viral droplets generated during intubation. The box can be cleaned after each use. Blueprints for an open source intubation box can be found at http://www.intubationbox.com.
Expert view on aerosol containment in COVID-19
“While there is a dearth of evidence from controlled trials, recommendations mentioned in this story are based on the best available evidence and are in agreement with guidelines from several expert groups,” said David L. Bowton, MD, FCCP, FCCM, of the department of anesthesiology at Wake Forest Baptist Health in Winston-Salem, NC. “The recommendation of Dr. Lane-Fall’s that is perhaps most controversial is the use of an intubation box. Multiple designs for these intubation/aerosol containment devices have been proposed, and the data supporting their ease of use and efficacy has been mixed [See Anaesthesia 2020;75(8):1014-21 and Anaesthesia. 2020. doi: 10.1111/anae.15188]. While bag valve mask ventilation should be avoided if possible, it may be a valuable rescue tool in the severely hypoxemic patient when used with two-person technique to achieve a tight seal and a PEEP valve and an HME over the exhalation port to minimize aerosol spread.
“It cannot be stressed enough that the most skilled individual should be tasked with intubating the patient and as few providers as possible [usually three] should be in the room and have donned full PPE. Negative pressure rooms should be used whenever feasible. Noninvasive ventilation appears safer from an infection control standpoint than initially feared and its use has become more widespread. However, noninvasive ventilation is not without its hazards, and Dr. Lane-Fall’s enumeration of the patient characteristics applicable to the selection of patients for noninvasive ventilation are extremely important. At our institution, the use of noninvasive ventilation and especially high-flow oxygen therapy has increased. Staff have become more comfortable with the donning and doffing of PPE.”
Dr. Lane-Fall reported having no financial disclosures.
FROM AN SCCM VIRTUAL MEETING
RAP device being investigated as a way to improve appearance of cellulite
.
“The procedure is relatively painless, without anesthesia and can easily be delegated with physician oversight,” Mathew M. Avram, MD, JD, said during the virtual annual Masters of Aesthetics Symposium. “Side effects have been minimal and transient to date. There is no down time.”
According to Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital, Boston, the RAP device emits rapid acoustic pulses (shock waves) that are transmitted through the skin to rupture or “shear” the fibrotic septa. This causes the release of septa, which results in a smoothening of skin dimples.
“Basically, what you have is a repetition rate and very short rise time that provide microscopic mechanical disruption to the targeted cellular level structures and vacuoles,” Dr. Avram explained. “There’s a high leak pressure and fast repetition rate that exploits the viscoelastic nature of the tissue. You get compressed pulses from electronic filtering and the reflector shape eliminates cavitation, heat, and pain.”
The procedure takes 20-30 minutes to perform and it generates minimal heat and pain, “which is an advantage of the treatment,” he said. “It is completely noninvasive, with no incision whatsoever. No anesthetic is required. There can be physician oversight of delivery, so it is delegable, and there is no recovery time. More study is needed, and we need to stay tuned.”
Dr. Avram disclosed that he has received consulting fees from Allergan, Merz, Sciton, and Soliton. He also reported having ownership and/or shareholder interest in Cytrellis.
.
“The procedure is relatively painless, without anesthesia and can easily be delegated with physician oversight,” Mathew M. Avram, MD, JD, said during the virtual annual Masters of Aesthetics Symposium. “Side effects have been minimal and transient to date. There is no down time.”
According to Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital, Boston, the RAP device emits rapid acoustic pulses (shock waves) that are transmitted through the skin to rupture or “shear” the fibrotic septa. This causes the release of septa, which results in a smoothening of skin dimples.
“Basically, what you have is a repetition rate and very short rise time that provide microscopic mechanical disruption to the targeted cellular level structures and vacuoles,” Dr. Avram explained. “There’s a high leak pressure and fast repetition rate that exploits the viscoelastic nature of the tissue. You get compressed pulses from electronic filtering and the reflector shape eliminates cavitation, heat, and pain.”
The procedure takes 20-30 minutes to perform and it generates minimal heat and pain, “which is an advantage of the treatment,” he said. “It is completely noninvasive, with no incision whatsoever. No anesthetic is required. There can be physician oversight of delivery, so it is delegable, and there is no recovery time. More study is needed, and we need to stay tuned.”
Dr. Avram disclosed that he has received consulting fees from Allergan, Merz, Sciton, and Soliton. He also reported having ownership and/or shareholder interest in Cytrellis.
.
“The procedure is relatively painless, without anesthesia and can easily be delegated with physician oversight,” Mathew M. Avram, MD, JD, said during the virtual annual Masters of Aesthetics Symposium. “Side effects have been minimal and transient to date. There is no down time.”
According to Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital, Boston, the RAP device emits rapid acoustic pulses (shock waves) that are transmitted through the skin to rupture or “shear” the fibrotic septa. This causes the release of septa, which results in a smoothening of skin dimples.
“Basically, what you have is a repetition rate and very short rise time that provide microscopic mechanical disruption to the targeted cellular level structures and vacuoles,” Dr. Avram explained. “There’s a high leak pressure and fast repetition rate that exploits the viscoelastic nature of the tissue. You get compressed pulses from electronic filtering and the reflector shape eliminates cavitation, heat, and pain.”
The procedure takes 20-30 minutes to perform and it generates minimal heat and pain, “which is an advantage of the treatment,” he said. “It is completely noninvasive, with no incision whatsoever. No anesthetic is required. There can be physician oversight of delivery, so it is delegable, and there is no recovery time. More study is needed, and we need to stay tuned.”
Dr. Avram disclosed that he has received consulting fees from Allergan, Merz, Sciton, and Soliton. He also reported having ownership and/or shareholder interest in Cytrellis.
FROM MOA 2020
No one-size-fits-all approach to tissue-tightening devices
.
“There are many devices on the market, but their efficacy is not consistent,” Catherine M. DiGiorgio, MS, MD, said during the virtual annual Masters of Aesthetics Symposium. “The key to maximizing patient satisfaction is patient selection and setting realistic expectations.”
She avoids recommending the use of tissue-tightening devices for patients who require surgical correction and for those who find the idea of minimal improvement unacceptable. “These are not the treatments for them,” she said. “I also find that when a patient uses her fingers to pull her face back and says, ‘I want to look like this,’ this is not the right patient for these devices. They can get a good amount of improvement, but efficacy is not consistent.”
Still, patients favor noninvasive or minimally invasive procedures for skin tightening now more than ever before. “They are not willing to undergo surgical treatments, and they want something with low downtime,” she said.
Dr. DiGiorgio, who practices at the Boston Center for Facial Rejuvenation, began a review of tissue-tightening devices on the market by discussing the role of ablative fractional lasers such as the carbon dioxide 10,600-nm laser and the Erbium:YAG 2,940-nm laser, which carry risks and downtime. “I don’t view these lasers as a tissue-tightening devices, but they are included because they can provide a little bit of tightening,” she said.
The ideal candidate is someone with skin type I-II and mild skin laxity. “These lasers are really good at improving rhytides,” she noted. “The patient needs to be able to tolerate the discomfort and manage the healing process. Sometimes you can get blepharoplastylike results with some patients. This can be combined with vascular lasers and pigment-targeting lasers to improve the overall texture and tone of the skin. Many combine this with a face-lift or a blepharoplasty. You should wait at least 6-8 weeks after a face-lift before performing this procedure. Some plastic surgeons do combine this with blepharoplasty in the same visit.”
A less invasive option for skin tightening is the delivery of radiofrequency energy, which disrupts hydrogen bonds of the collagen triple helix. This occurs in temperatures greater than 60° C and results in collagen contraction and tightening and neocollagenesis. There are several devices available including transcutaneous monopolar radiofrequency (Thermage, TempSure), subsurface thermistor–controlled monopolar radiofrequency (ThermiTight), and fractional microneedling radiofrequency (Profound RF, Genius RF, Vivace, and Secret RF). The transcutaneous monopolar radiofrequency device delivers energy uniformly via a treatment tip that has contact cooling and coupling fluid. Collagen is denatured at 65° C and fibroblasts are stimulated to form new collagen. The healing process provides additional tightening.
“These treatments are noninvasive; there’s no downtime, and there’s mild discomfort,” Dr. DiGiorgio commented. “Treatments can be done around the eyes, on the face and body. When treating around the eyes with these devices you want to use a corneal plastic eye shield. Contraindications include having a pacemaker, defibrillator, or other electronic implantable device.”
In her opinion, the ideal patient for this device has mild skin laxity or is younger and seeking to maintain a youthful appearance. “It’s great for mild upper eyelid laxity and for temporary improvement of cellulite appearance,” she said. “The patient should not require surgical intervention and the patient should also agree to undergo multiple treatment sessions. Just one treatment session is not going to cut it.”
Another device in this class of technology is subsurface thermistor–controlled monopolar radiofrequency, “which is basically a probe that’s inserted into the skin, most commonly in the submental area,” Dr. DiGiorgio said. An external infrared camera monitors the epidermal temperature, which should not exceed 45°C. This results in a controlled deep dermal and subdermal delivery of thermal energy. “It requires light tumescent anesthesia, and it can be combined with liposuction,” she said. “Common side effects include erythema, edema, and bruising, and sometimes contour irregularities or nodules.” In her opinion, the ideal candidate for this device is someone with mild to moderate skin laxity who does not require surgical correction. “You can combine this with liposuction, but you can achieve good results without it,” she said.
The next device in this class of technology that Dr. DiGiorgio discussed is fractional microneedling radiofrequency. Of several such devices on the market, some have adjustable depths up to 4 mm while others have fixed depths. The energy is adjustable, and the tips can be insulated or noninsulated. “Insulated tips make it safer to perform in darker skin types because the proximal portion of the needle is insulated and the epidermis is spared from damage,” she explained. “Some devices are a bit more painful than others. It does require topical anesthesia; some require local injection anesthesia. Patients have erythema for about 24 hours, and treatments are recommended monthly.” In her opinion, the ideal candidate for this device is someone with mild to moderate skin laxity who does not require surgical intervention but who seeks to maintain a youthful appearance. “Patients should understand that multiple treatments will be required to achieve optimal results,” she said. “I find that there is less improvement in older patients. This can be combined with thread lifts, vascular lasers, pigment-targeting lasers, and CO2 lasers.”
The next device for skin tightening that she discussed is microfocused ultrasound (Ultherapy), which delivers millisecond domain pulses at three different depths that are determined by the transducer that you use. It can go as deep as 4.5 mm. “Each pulse delivers a focal zone of coagulation to achieve tissue contraction,” Dr. DiGiorgio said. “There’s an ultrasound-imaging device attached to it to ensure proper skin contact and the delivery of energy at an appropriate depth. Patients can have a little bit of pain and erythema and edema, sometime bruising. Usually there is not much downtime with these treatments.”
A newcomer in this class of technology is SoftWave, an intense ultrasound beam array (IUB), which delivers energy precisely to the middermis at a depth of 1.5 mm. “With each pulse, the hand piece has seven transducers that deliver energy in 3-dimensional cylindrical thermal zones,” Dr. DiGiorgio said. “You get greater than 25% tissue coverage in one treatment, and there is no injury to the epidermis or deeper structures. It has unique vectors that are along the lines of facial wrinkles, so you get tightening along those lines.”
The procedure takes about 30 minutes, there is no downtime, and it causes no pain, she said. Pretreatment, patients receive topical anesthesia. “This device has active skin cooling and has an ultrasound gel,” she added. “It does not have an imaging platform like the microfocused ultrasound does, because the depth is fixed. You get significant wrinkle reduction and decrease in submental fullness with improvement in jawline definition, eyebrow position, fine lines, and texture.” In her opinion, the ideal candidate for this device is a patient in the mid-40s to early 50s with mild to moderate elastosis, fullness, texture irregularities, laxity, rhytids, elastosis, and photoaging.
She reported having no financial disclosures.
.
“There are many devices on the market, but their efficacy is not consistent,” Catherine M. DiGiorgio, MS, MD, said during the virtual annual Masters of Aesthetics Symposium. “The key to maximizing patient satisfaction is patient selection and setting realistic expectations.”
She avoids recommending the use of tissue-tightening devices for patients who require surgical correction and for those who find the idea of minimal improvement unacceptable. “These are not the treatments for them,” she said. “I also find that when a patient uses her fingers to pull her face back and says, ‘I want to look like this,’ this is not the right patient for these devices. They can get a good amount of improvement, but efficacy is not consistent.”
Still, patients favor noninvasive or minimally invasive procedures for skin tightening now more than ever before. “They are not willing to undergo surgical treatments, and they want something with low downtime,” she said.
Dr. DiGiorgio, who practices at the Boston Center for Facial Rejuvenation, began a review of tissue-tightening devices on the market by discussing the role of ablative fractional lasers such as the carbon dioxide 10,600-nm laser and the Erbium:YAG 2,940-nm laser, which carry risks and downtime. “I don’t view these lasers as a tissue-tightening devices, but they are included because they can provide a little bit of tightening,” she said.
The ideal candidate is someone with skin type I-II and mild skin laxity. “These lasers are really good at improving rhytides,” she noted. “The patient needs to be able to tolerate the discomfort and manage the healing process. Sometimes you can get blepharoplastylike results with some patients. This can be combined with vascular lasers and pigment-targeting lasers to improve the overall texture and tone of the skin. Many combine this with a face-lift or a blepharoplasty. You should wait at least 6-8 weeks after a face-lift before performing this procedure. Some plastic surgeons do combine this with blepharoplasty in the same visit.”
A less invasive option for skin tightening is the delivery of radiofrequency energy, which disrupts hydrogen bonds of the collagen triple helix. This occurs in temperatures greater than 60° C and results in collagen contraction and tightening and neocollagenesis. There are several devices available including transcutaneous monopolar radiofrequency (Thermage, TempSure), subsurface thermistor–controlled monopolar radiofrequency (ThermiTight), and fractional microneedling radiofrequency (Profound RF, Genius RF, Vivace, and Secret RF). The transcutaneous monopolar radiofrequency device delivers energy uniformly via a treatment tip that has contact cooling and coupling fluid. Collagen is denatured at 65° C and fibroblasts are stimulated to form new collagen. The healing process provides additional tightening.
“These treatments are noninvasive; there’s no downtime, and there’s mild discomfort,” Dr. DiGiorgio commented. “Treatments can be done around the eyes, on the face and body. When treating around the eyes with these devices you want to use a corneal plastic eye shield. Contraindications include having a pacemaker, defibrillator, or other electronic implantable device.”
In her opinion, the ideal patient for this device has mild skin laxity or is younger and seeking to maintain a youthful appearance. “It’s great for mild upper eyelid laxity and for temporary improvement of cellulite appearance,” she said. “The patient should not require surgical intervention and the patient should also agree to undergo multiple treatment sessions. Just one treatment session is not going to cut it.”
Another device in this class of technology is subsurface thermistor–controlled monopolar radiofrequency, “which is basically a probe that’s inserted into the skin, most commonly in the submental area,” Dr. DiGiorgio said. An external infrared camera monitors the epidermal temperature, which should not exceed 45°C. This results in a controlled deep dermal and subdermal delivery of thermal energy. “It requires light tumescent anesthesia, and it can be combined with liposuction,” she said. “Common side effects include erythema, edema, and bruising, and sometimes contour irregularities or nodules.” In her opinion, the ideal candidate for this device is someone with mild to moderate skin laxity who does not require surgical correction. “You can combine this with liposuction, but you can achieve good results without it,” she said.
The next device in this class of technology that Dr. DiGiorgio discussed is fractional microneedling radiofrequency. Of several such devices on the market, some have adjustable depths up to 4 mm while others have fixed depths. The energy is adjustable, and the tips can be insulated or noninsulated. “Insulated tips make it safer to perform in darker skin types because the proximal portion of the needle is insulated and the epidermis is spared from damage,” she explained. “Some devices are a bit more painful than others. It does require topical anesthesia; some require local injection anesthesia. Patients have erythema for about 24 hours, and treatments are recommended monthly.” In her opinion, the ideal candidate for this device is someone with mild to moderate skin laxity who does not require surgical intervention but who seeks to maintain a youthful appearance. “Patients should understand that multiple treatments will be required to achieve optimal results,” she said. “I find that there is less improvement in older patients. This can be combined with thread lifts, vascular lasers, pigment-targeting lasers, and CO2 lasers.”
The next device for skin tightening that she discussed is microfocused ultrasound (Ultherapy), which delivers millisecond domain pulses at three different depths that are determined by the transducer that you use. It can go as deep as 4.5 mm. “Each pulse delivers a focal zone of coagulation to achieve tissue contraction,” Dr. DiGiorgio said. “There’s an ultrasound-imaging device attached to it to ensure proper skin contact and the delivery of energy at an appropriate depth. Patients can have a little bit of pain and erythema and edema, sometime bruising. Usually there is not much downtime with these treatments.”
A newcomer in this class of technology is SoftWave, an intense ultrasound beam array (IUB), which delivers energy precisely to the middermis at a depth of 1.5 mm. “With each pulse, the hand piece has seven transducers that deliver energy in 3-dimensional cylindrical thermal zones,” Dr. DiGiorgio said. “You get greater than 25% tissue coverage in one treatment, and there is no injury to the epidermis or deeper structures. It has unique vectors that are along the lines of facial wrinkles, so you get tightening along those lines.”
The procedure takes about 30 minutes, there is no downtime, and it causes no pain, she said. Pretreatment, patients receive topical anesthesia. “This device has active skin cooling and has an ultrasound gel,” she added. “It does not have an imaging platform like the microfocused ultrasound does, because the depth is fixed. You get significant wrinkle reduction and decrease in submental fullness with improvement in jawline definition, eyebrow position, fine lines, and texture.” In her opinion, the ideal candidate for this device is a patient in the mid-40s to early 50s with mild to moderate elastosis, fullness, texture irregularities, laxity, rhytids, elastosis, and photoaging.
She reported having no financial disclosures.
.
“There are many devices on the market, but their efficacy is not consistent,” Catherine M. DiGiorgio, MS, MD, said during the virtual annual Masters of Aesthetics Symposium. “The key to maximizing patient satisfaction is patient selection and setting realistic expectations.”
She avoids recommending the use of tissue-tightening devices for patients who require surgical correction and for those who find the idea of minimal improvement unacceptable. “These are not the treatments for them,” she said. “I also find that when a patient uses her fingers to pull her face back and says, ‘I want to look like this,’ this is not the right patient for these devices. They can get a good amount of improvement, but efficacy is not consistent.”
Still, patients favor noninvasive or minimally invasive procedures for skin tightening now more than ever before. “They are not willing to undergo surgical treatments, and they want something with low downtime,” she said.
Dr. DiGiorgio, who practices at the Boston Center for Facial Rejuvenation, began a review of tissue-tightening devices on the market by discussing the role of ablative fractional lasers such as the carbon dioxide 10,600-nm laser and the Erbium:YAG 2,940-nm laser, which carry risks and downtime. “I don’t view these lasers as a tissue-tightening devices, but they are included because they can provide a little bit of tightening,” she said.
The ideal candidate is someone with skin type I-II and mild skin laxity. “These lasers are really good at improving rhytides,” she noted. “The patient needs to be able to tolerate the discomfort and manage the healing process. Sometimes you can get blepharoplastylike results with some patients. This can be combined with vascular lasers and pigment-targeting lasers to improve the overall texture and tone of the skin. Many combine this with a face-lift or a blepharoplasty. You should wait at least 6-8 weeks after a face-lift before performing this procedure. Some plastic surgeons do combine this with blepharoplasty in the same visit.”
A less invasive option for skin tightening is the delivery of radiofrequency energy, which disrupts hydrogen bonds of the collagen triple helix. This occurs in temperatures greater than 60° C and results in collagen contraction and tightening and neocollagenesis. There are several devices available including transcutaneous monopolar radiofrequency (Thermage, TempSure), subsurface thermistor–controlled monopolar radiofrequency (ThermiTight), and fractional microneedling radiofrequency (Profound RF, Genius RF, Vivace, and Secret RF). The transcutaneous monopolar radiofrequency device delivers energy uniformly via a treatment tip that has contact cooling and coupling fluid. Collagen is denatured at 65° C and fibroblasts are stimulated to form new collagen. The healing process provides additional tightening.
“These treatments are noninvasive; there’s no downtime, and there’s mild discomfort,” Dr. DiGiorgio commented. “Treatments can be done around the eyes, on the face and body. When treating around the eyes with these devices you want to use a corneal plastic eye shield. Contraindications include having a pacemaker, defibrillator, or other electronic implantable device.”
In her opinion, the ideal patient for this device has mild skin laxity or is younger and seeking to maintain a youthful appearance. “It’s great for mild upper eyelid laxity and for temporary improvement of cellulite appearance,” she said. “The patient should not require surgical intervention and the patient should also agree to undergo multiple treatment sessions. Just one treatment session is not going to cut it.”
Another device in this class of technology is subsurface thermistor–controlled monopolar radiofrequency, “which is basically a probe that’s inserted into the skin, most commonly in the submental area,” Dr. DiGiorgio said. An external infrared camera monitors the epidermal temperature, which should not exceed 45°C. This results in a controlled deep dermal and subdermal delivery of thermal energy. “It requires light tumescent anesthesia, and it can be combined with liposuction,” she said. “Common side effects include erythema, edema, and bruising, and sometimes contour irregularities or nodules.” In her opinion, the ideal candidate for this device is someone with mild to moderate skin laxity who does not require surgical correction. “You can combine this with liposuction, but you can achieve good results without it,” she said.
The next device in this class of technology that Dr. DiGiorgio discussed is fractional microneedling radiofrequency. Of several such devices on the market, some have adjustable depths up to 4 mm while others have fixed depths. The energy is adjustable, and the tips can be insulated or noninsulated. “Insulated tips make it safer to perform in darker skin types because the proximal portion of the needle is insulated and the epidermis is spared from damage,” she explained. “Some devices are a bit more painful than others. It does require topical anesthesia; some require local injection anesthesia. Patients have erythema for about 24 hours, and treatments are recommended monthly.” In her opinion, the ideal candidate for this device is someone with mild to moderate skin laxity who does not require surgical intervention but who seeks to maintain a youthful appearance. “Patients should understand that multiple treatments will be required to achieve optimal results,” she said. “I find that there is less improvement in older patients. This can be combined with thread lifts, vascular lasers, pigment-targeting lasers, and CO2 lasers.”
The next device for skin tightening that she discussed is microfocused ultrasound (Ultherapy), which delivers millisecond domain pulses at three different depths that are determined by the transducer that you use. It can go as deep as 4.5 mm. “Each pulse delivers a focal zone of coagulation to achieve tissue contraction,” Dr. DiGiorgio said. “There’s an ultrasound-imaging device attached to it to ensure proper skin contact and the delivery of energy at an appropriate depth. Patients can have a little bit of pain and erythema and edema, sometime bruising. Usually there is not much downtime with these treatments.”
A newcomer in this class of technology is SoftWave, an intense ultrasound beam array (IUB), which delivers energy precisely to the middermis at a depth of 1.5 mm. “With each pulse, the hand piece has seven transducers that deliver energy in 3-dimensional cylindrical thermal zones,” Dr. DiGiorgio said. “You get greater than 25% tissue coverage in one treatment, and there is no injury to the epidermis or deeper structures. It has unique vectors that are along the lines of facial wrinkles, so you get tightening along those lines.”
The procedure takes about 30 minutes, there is no downtime, and it causes no pain, she said. Pretreatment, patients receive topical anesthesia. “This device has active skin cooling and has an ultrasound gel,” she added. “It does not have an imaging platform like the microfocused ultrasound does, because the depth is fixed. You get significant wrinkle reduction and decrease in submental fullness with improvement in jawline definition, eyebrow position, fine lines, and texture.” In her opinion, the ideal candidate for this device is a patient in the mid-40s to early 50s with mild to moderate elastosis, fullness, texture irregularities, laxity, rhytids, elastosis, and photoaging.
She reported having no financial disclosures.
REPORTING FROM MOA 2020
Innovator banks on ‘truly smart’ robotic lasers in dermatology
Dr. Anderson, director of the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston, conceived and developed many of the nonscarring laser treatments now widely used in dermatology. These include selective photothermolysis for birthmarks, microvascular and pigmented lesions, and tattoo and permanent hair removal. He also contributed to laser lithotripsy, laser angioplasty, photodynamic therapy, and optical diagnostics. The highest-resolution imaging device approved for human use, an infrared confocal microscope, came from his laboratory. Dr. Anderson has also contributed to basic knowledge of human photobiology, drug photosensitization mechanisms, tissue optics and laser-tissue interactions. In this Q&A with Doug Brunk, he reflects on his achievements and on the future of lasers in dermatology.
In published interviews you have described yourself as more of a problem solver than an inventor. How did your upbringing foster your affinity for problem solving?
I grew up in Central Illinois during the 1950s and early 1960s, an area known for corn, soybeans, and hogs. At an early age I learned to be interested in other things because it’s possible to die of boredom there. By the time I was 12 years old, I was an amateur radio operator, and I was building rockets to see how high they would go.
Problem solving comes naturally to me. I enjoy very much finding a problem that is worth solving, which means getting passionate about it and brainstorming. Half the time you don’t come up with a potential route to solve the problem. I attended the Massachusetts Institute of Technology at the age of 17, which was a real eye-opener. I had never been east of the Wabash River prior to that. I studied physics for a while, then decided to flip into biology. That combination has served me well. My special sauce is to have some intuitive and academic rigorous feeling for physical processes. But we physicians have a front row seat to nature’s human drama. There is no lack of problems to solve. I can sit around and obsess about things theoretically, but at the end of the day I want to work on things that ultimately benefit people.
What inspired you most early in your career as a physician scientist?
After I made a commitment to medicine, Dr. John A. Parrish, and Dr. Thomas B. Fitzpatrick were key mentors to me. I was 30 years old when I started medical school, but they took me under their wing even before that. I took a part-time, temporary job with them, which turned into a permanent job. That turned into a love for the work they did. Instead of going into a graduate program in a laboratory and studying bacteria and genetics, the whole idea of working with people and on people was awesome. Dr. Parrish really mentored me. I won a lifetime achievement award from the American Academy of Dermatology a few years ago. I found myself on stage and it rolled out of my mouth that John Parrish believed in me before I believed in myself. It’s really true. He somehow recognized that I had some talents. I was very young and a combination of naive and humble, I guess.
What was the initial genesis for your idea of selective photothermolysis?
I was interested in going to medical school and working with Dr. Fitzpatrick and Dr. Parrish on things related to light. They were mostly interested in PUVA and UVB; it was the heyday of modern phototherapy. I attended a lecture at the Beth Israel Hospital in Boston given by a plastic surgeon, Dr. Joel Mark Noe. He was talking about using lasers to treat port-wine stains in children. The gist of the talk was that argon lasers were being used, and that the results were sometimes decent, but not great. Often children would have burn scars after the treatment. Dr. Noe was talking about how you had to choose the color of the wavelength of the laser to be absorbed by hemoglobin, but he wasn’t talking about what happens to the heat once it’s created. My background in physics led me to recognize that he wasn’t capturing the full picture. Selective disruption of a target in the skin by light is half of the story. The confinement of heat in the target is the other half of the story. Literally on a bus on the way home from that lecture to my apartment in Cambridge, I hatched the idea for selective photothermolysis and wrote down some equations. I also wrote down the ideal wavelength region, how much energy was needed, and what the pulse duration would have to be like to damage target vessels that small. I showed John Parrish what I had written. He took me seriously and said, “Let’s see if we can find a light source that can accomplish this.” We traveled around the country looking at various lasers, but we wound up building the first pulsed dye laser for treating port-wine stains. To me, the surprise was that we didn’t kill the skin. If you treat an area of skin with a laser and hurt all the blood vessels, you think, “Wait a minute. Are we going to kill the skin because it has no blood supply?” The questions of the day were so basic, and we just got lucky. It took 6-8 years before we ramped up the clinical studies showing efficacy and safety of this technology.
I presume that you experimented on your own skin while developing some of the nonscarring laser treatments now widely used in dermatology. What “war story” stands out to you most from that part of your work?
I’m right handed, so I’d grab a laser with my right hand and treat my left arm, so that arm sports a bit of history. In 1994, while working with Dr. Melanie Grossman on the development of laser hair removal, I used a ruby laser to self-treat a patch of hair on my left arm. I still have the world’s oldest laser-induced bald spot on that arm. It’s been 26 years now. I still look at it and count the hairs, because one of the big questions is, is laser hair removal permanent? In all these years I have grown two hairs.
What technology that you conceived of or developed has most surprised you, in term of its ultimate clinical impact?
I would say confocal microscopy. In the mid-1990s I worked with a physicist named Robert H. Webb, who invented an imaging system for the retina. We got together, noodled about it, and decided we would modify his ophthalmoscope system to see if we could get images from inside the skin. It worked pretty well. It was truly surprising from many points of view. First, it wasn’t clear at all that we’d get any images this way. Now, reflectance confocal microscopy is a standard tool in both clinical and research dermatology. But there were odd discoveries early on. For example, the darker your skin, the brighter it appeared in the microscope. You might think that melanin absorbs light and that you would get poor images in dark skin. It was the exact opposite; melanin acts as a natural contrast agent.
We worked with a small company to make the first confocal microscope. Initially, it had no clinical applications but what was fascinating to me was the incredible value of being able to see inside human skin harmlessly, and just see what’s going on. It became a potent research tool, and recently CPT codes were established for its use in evaluating skin cancer margins. I wouldn’t be surprised if 30 years from now, taking a skin biopsy is seemingly barbaric. A forerunner of all these new imaging tools for the skin was the confocal microscope developed in my lab in 1994.
During a 2011 TED talk, you said that nevus of Ota is your favorite thing to treat, because the outcome is usually perfect skin. Are there other technologies or devices you played a role in developing that make you proud at this stage in your career?
The reason I love treating nevus of Ota is that you have a lifelong facial disfigurement, and the only treatment for it is a laser we came up with, and it always works. How perfect could it be? The flip side of the same coin is, there are lesions of the skin that just don’t respond. One of the things we don’t know enough about is the connection between the biologic aspects of repair of various lesions and the treatments that we come up with. The most recent example of selective photothermolysis is a new laser we’re building right now for acne that is based on sebaceous gland injury. You’ll see this coming out in the next year or two. My heart goes out to people with nodular cystic acne. For young men it’s highly associated with suicide. So, I’m excited about optimizing and learning what happens when we target sebaceous glands.
One of the other big stories in laser dermatology is the fractional laser. I developed this with Dr. Dieter Manstein when he was a postdoc in my lab. One of the most pleasing things from this technology is how well you can rehabilitate scars, particularly burn scars in children. Over the last few years, I have trained plastic surgeons at the Shriners Hospital for Children in Boston on how to use fractional lasers to improve the lives of these kids. Another technology I developed with Dr. Manstein is cryolipolysis, which is removing fat from the body by cooling it. There are no lasers involved with this technology. I like to say that I’ve spent most of my career studying light and heat, and now we’ve come up with something that’s cold in the dark. We are now working on derivatives of cryolipolysis, to determine if what we’ve learned about targeting fat that might be applicable elsewhere.
Who inspires you most in your work today?
In addition to Dr. John Parrish and Dr. Thomas Fitzpatrick, the late Dr. Albert M. Kligman also influenced me. He never accepted dogma and he loved to ask questions, like, “What if?” as opposed to just accumulating a fund of knowledge. Understanding things is not just based on how much you know. It’s based on critical thinking and the ability to question. I also admire Albert Einstein, his ability to sit down with nothing more than pencil and paper and change our view of the universe. I love physics because it’s the science of everything. I also love poetry. My favorite poet is Stanley Kunitz. He had amazing insight and was named United States Poet Laureate in 1974 and in 2000. I have plenty of antiheroes as well, mostly politicians.
I understand that you play the banjo. How long have you been playing, and what do you enjoy about it?
You cannot sit down and play the banjo and have your mind on much else. It’s a wonderful moving meditation. Before my medical career, I was a schoolteacher in Vermont. There was a guy on the staff there who played banjo. He came from a small town in Georgia. I just picked it up and started plunking. It’s a happy instrument. It’s awfully hard to make the banjo sound melancholy.
What novel use of lasers and light in dermatology are you most excited about in the next 5 years?
The marriage of therapeutic devices with diagnostic and imaging devices has not happened yet. They are not even in the honeymoon moment. But I think that having truly smart robotic systems in our hands for treating patients will become a reality. These days, dermatologists have to buy a certain type of laser to treat a certain type of lesion. For example, the Q-switched alexandrite laser you buy for treating Nevus of Ota won’t do anything for a port-wine stain; it’s the wrong pulse duration. This means that clinicians who practice a lot of laser dermatology end up with a dozen lasers in their practice. In the future, I think it will be possible to have a software laser, so when you want to acquire another target, you load an App as opposed to buying a new laser. This means that you would have software programmable targeting, and you would not have the requirement of having selective absorption. So, I’m excited by the idea of guided fractional lasers. None of them exist now. We have to start from scratch.
Dr. Anderson, director of the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston, conceived and developed many of the nonscarring laser treatments now widely used in dermatology. These include selective photothermolysis for birthmarks, microvascular and pigmented lesions, and tattoo and permanent hair removal. He also contributed to laser lithotripsy, laser angioplasty, photodynamic therapy, and optical diagnostics. The highest-resolution imaging device approved for human use, an infrared confocal microscope, came from his laboratory. Dr. Anderson has also contributed to basic knowledge of human photobiology, drug photosensitization mechanisms, tissue optics and laser-tissue interactions. In this Q&A with Doug Brunk, he reflects on his achievements and on the future of lasers in dermatology.
In published interviews you have described yourself as more of a problem solver than an inventor. How did your upbringing foster your affinity for problem solving?
I grew up in Central Illinois during the 1950s and early 1960s, an area known for corn, soybeans, and hogs. At an early age I learned to be interested in other things because it’s possible to die of boredom there. By the time I was 12 years old, I was an amateur radio operator, and I was building rockets to see how high they would go.
Problem solving comes naturally to me. I enjoy very much finding a problem that is worth solving, which means getting passionate about it and brainstorming. Half the time you don’t come up with a potential route to solve the problem. I attended the Massachusetts Institute of Technology at the age of 17, which was a real eye-opener. I had never been east of the Wabash River prior to that. I studied physics for a while, then decided to flip into biology. That combination has served me well. My special sauce is to have some intuitive and academic rigorous feeling for physical processes. But we physicians have a front row seat to nature’s human drama. There is no lack of problems to solve. I can sit around and obsess about things theoretically, but at the end of the day I want to work on things that ultimately benefit people.
What inspired you most early in your career as a physician scientist?
After I made a commitment to medicine, Dr. John A. Parrish, and Dr. Thomas B. Fitzpatrick were key mentors to me. I was 30 years old when I started medical school, but they took me under their wing even before that. I took a part-time, temporary job with them, which turned into a permanent job. That turned into a love for the work they did. Instead of going into a graduate program in a laboratory and studying bacteria and genetics, the whole idea of working with people and on people was awesome. Dr. Parrish really mentored me. I won a lifetime achievement award from the American Academy of Dermatology a few years ago. I found myself on stage and it rolled out of my mouth that John Parrish believed in me before I believed in myself. It’s really true. He somehow recognized that I had some talents. I was very young and a combination of naive and humble, I guess.
What was the initial genesis for your idea of selective photothermolysis?
I was interested in going to medical school and working with Dr. Fitzpatrick and Dr. Parrish on things related to light. They were mostly interested in PUVA and UVB; it was the heyday of modern phototherapy. I attended a lecture at the Beth Israel Hospital in Boston given by a plastic surgeon, Dr. Joel Mark Noe. He was talking about using lasers to treat port-wine stains in children. The gist of the talk was that argon lasers were being used, and that the results were sometimes decent, but not great. Often children would have burn scars after the treatment. Dr. Noe was talking about how you had to choose the color of the wavelength of the laser to be absorbed by hemoglobin, but he wasn’t talking about what happens to the heat once it’s created. My background in physics led me to recognize that he wasn’t capturing the full picture. Selective disruption of a target in the skin by light is half of the story. The confinement of heat in the target is the other half of the story. Literally on a bus on the way home from that lecture to my apartment in Cambridge, I hatched the idea for selective photothermolysis and wrote down some equations. I also wrote down the ideal wavelength region, how much energy was needed, and what the pulse duration would have to be like to damage target vessels that small. I showed John Parrish what I had written. He took me seriously and said, “Let’s see if we can find a light source that can accomplish this.” We traveled around the country looking at various lasers, but we wound up building the first pulsed dye laser for treating port-wine stains. To me, the surprise was that we didn’t kill the skin. If you treat an area of skin with a laser and hurt all the blood vessels, you think, “Wait a minute. Are we going to kill the skin because it has no blood supply?” The questions of the day were so basic, and we just got lucky. It took 6-8 years before we ramped up the clinical studies showing efficacy and safety of this technology.
I presume that you experimented on your own skin while developing some of the nonscarring laser treatments now widely used in dermatology. What “war story” stands out to you most from that part of your work?
I’m right handed, so I’d grab a laser with my right hand and treat my left arm, so that arm sports a bit of history. In 1994, while working with Dr. Melanie Grossman on the development of laser hair removal, I used a ruby laser to self-treat a patch of hair on my left arm. I still have the world’s oldest laser-induced bald spot on that arm. It’s been 26 years now. I still look at it and count the hairs, because one of the big questions is, is laser hair removal permanent? In all these years I have grown two hairs.
What technology that you conceived of or developed has most surprised you, in term of its ultimate clinical impact?
I would say confocal microscopy. In the mid-1990s I worked with a physicist named Robert H. Webb, who invented an imaging system for the retina. We got together, noodled about it, and decided we would modify his ophthalmoscope system to see if we could get images from inside the skin. It worked pretty well. It was truly surprising from many points of view. First, it wasn’t clear at all that we’d get any images this way. Now, reflectance confocal microscopy is a standard tool in both clinical and research dermatology. But there were odd discoveries early on. For example, the darker your skin, the brighter it appeared in the microscope. You might think that melanin absorbs light and that you would get poor images in dark skin. It was the exact opposite; melanin acts as a natural contrast agent.
We worked with a small company to make the first confocal microscope. Initially, it had no clinical applications but what was fascinating to me was the incredible value of being able to see inside human skin harmlessly, and just see what’s going on. It became a potent research tool, and recently CPT codes were established for its use in evaluating skin cancer margins. I wouldn’t be surprised if 30 years from now, taking a skin biopsy is seemingly barbaric. A forerunner of all these new imaging tools for the skin was the confocal microscope developed in my lab in 1994.
During a 2011 TED talk, you said that nevus of Ota is your favorite thing to treat, because the outcome is usually perfect skin. Are there other technologies or devices you played a role in developing that make you proud at this stage in your career?
The reason I love treating nevus of Ota is that you have a lifelong facial disfigurement, and the only treatment for it is a laser we came up with, and it always works. How perfect could it be? The flip side of the same coin is, there are lesions of the skin that just don’t respond. One of the things we don’t know enough about is the connection between the biologic aspects of repair of various lesions and the treatments that we come up with. The most recent example of selective photothermolysis is a new laser we’re building right now for acne that is based on sebaceous gland injury. You’ll see this coming out in the next year or two. My heart goes out to people with nodular cystic acne. For young men it’s highly associated with suicide. So, I’m excited about optimizing and learning what happens when we target sebaceous glands.
One of the other big stories in laser dermatology is the fractional laser. I developed this with Dr. Dieter Manstein when he was a postdoc in my lab. One of the most pleasing things from this technology is how well you can rehabilitate scars, particularly burn scars in children. Over the last few years, I have trained plastic surgeons at the Shriners Hospital for Children in Boston on how to use fractional lasers to improve the lives of these kids. Another technology I developed with Dr. Manstein is cryolipolysis, which is removing fat from the body by cooling it. There are no lasers involved with this technology. I like to say that I’ve spent most of my career studying light and heat, and now we’ve come up with something that’s cold in the dark. We are now working on derivatives of cryolipolysis, to determine if what we’ve learned about targeting fat that might be applicable elsewhere.
Who inspires you most in your work today?
In addition to Dr. John Parrish and Dr. Thomas Fitzpatrick, the late Dr. Albert M. Kligman also influenced me. He never accepted dogma and he loved to ask questions, like, “What if?” as opposed to just accumulating a fund of knowledge. Understanding things is not just based on how much you know. It’s based on critical thinking and the ability to question. I also admire Albert Einstein, his ability to sit down with nothing more than pencil and paper and change our view of the universe. I love physics because it’s the science of everything. I also love poetry. My favorite poet is Stanley Kunitz. He had amazing insight and was named United States Poet Laureate in 1974 and in 2000. I have plenty of antiheroes as well, mostly politicians.
I understand that you play the banjo. How long have you been playing, and what do you enjoy about it?
You cannot sit down and play the banjo and have your mind on much else. It’s a wonderful moving meditation. Before my medical career, I was a schoolteacher in Vermont. There was a guy on the staff there who played banjo. He came from a small town in Georgia. I just picked it up and started plunking. It’s a happy instrument. It’s awfully hard to make the banjo sound melancholy.
What novel use of lasers and light in dermatology are you most excited about in the next 5 years?
The marriage of therapeutic devices with diagnostic and imaging devices has not happened yet. They are not even in the honeymoon moment. But I think that having truly smart robotic systems in our hands for treating patients will become a reality. These days, dermatologists have to buy a certain type of laser to treat a certain type of lesion. For example, the Q-switched alexandrite laser you buy for treating Nevus of Ota won’t do anything for a port-wine stain; it’s the wrong pulse duration. This means that clinicians who practice a lot of laser dermatology end up with a dozen lasers in their practice. In the future, I think it will be possible to have a software laser, so when you want to acquire another target, you load an App as opposed to buying a new laser. This means that you would have software programmable targeting, and you would not have the requirement of having selective absorption. So, I’m excited by the idea of guided fractional lasers. None of them exist now. We have to start from scratch.
Dr. Anderson, director of the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston, conceived and developed many of the nonscarring laser treatments now widely used in dermatology. These include selective photothermolysis for birthmarks, microvascular and pigmented lesions, and tattoo and permanent hair removal. He also contributed to laser lithotripsy, laser angioplasty, photodynamic therapy, and optical diagnostics. The highest-resolution imaging device approved for human use, an infrared confocal microscope, came from his laboratory. Dr. Anderson has also contributed to basic knowledge of human photobiology, drug photosensitization mechanisms, tissue optics and laser-tissue interactions. In this Q&A with Doug Brunk, he reflects on his achievements and on the future of lasers in dermatology.
In published interviews you have described yourself as more of a problem solver than an inventor. How did your upbringing foster your affinity for problem solving?
I grew up in Central Illinois during the 1950s and early 1960s, an area known for corn, soybeans, and hogs. At an early age I learned to be interested in other things because it’s possible to die of boredom there. By the time I was 12 years old, I was an amateur radio operator, and I was building rockets to see how high they would go.
Problem solving comes naturally to me. I enjoy very much finding a problem that is worth solving, which means getting passionate about it and brainstorming. Half the time you don’t come up with a potential route to solve the problem. I attended the Massachusetts Institute of Technology at the age of 17, which was a real eye-opener. I had never been east of the Wabash River prior to that. I studied physics for a while, then decided to flip into biology. That combination has served me well. My special sauce is to have some intuitive and academic rigorous feeling for physical processes. But we physicians have a front row seat to nature’s human drama. There is no lack of problems to solve. I can sit around and obsess about things theoretically, but at the end of the day I want to work on things that ultimately benefit people.
What inspired you most early in your career as a physician scientist?
After I made a commitment to medicine, Dr. John A. Parrish, and Dr. Thomas B. Fitzpatrick were key mentors to me. I was 30 years old when I started medical school, but they took me under their wing even before that. I took a part-time, temporary job with them, which turned into a permanent job. That turned into a love for the work they did. Instead of going into a graduate program in a laboratory and studying bacteria and genetics, the whole idea of working with people and on people was awesome. Dr. Parrish really mentored me. I won a lifetime achievement award from the American Academy of Dermatology a few years ago. I found myself on stage and it rolled out of my mouth that John Parrish believed in me before I believed in myself. It’s really true. He somehow recognized that I had some talents. I was very young and a combination of naive and humble, I guess.
What was the initial genesis for your idea of selective photothermolysis?
I was interested in going to medical school and working with Dr. Fitzpatrick and Dr. Parrish on things related to light. They were mostly interested in PUVA and UVB; it was the heyday of modern phototherapy. I attended a lecture at the Beth Israel Hospital in Boston given by a plastic surgeon, Dr. Joel Mark Noe. He was talking about using lasers to treat port-wine stains in children. The gist of the talk was that argon lasers were being used, and that the results were sometimes decent, but not great. Often children would have burn scars after the treatment. Dr. Noe was talking about how you had to choose the color of the wavelength of the laser to be absorbed by hemoglobin, but he wasn’t talking about what happens to the heat once it’s created. My background in physics led me to recognize that he wasn’t capturing the full picture. Selective disruption of a target in the skin by light is half of the story. The confinement of heat in the target is the other half of the story. Literally on a bus on the way home from that lecture to my apartment in Cambridge, I hatched the idea for selective photothermolysis and wrote down some equations. I also wrote down the ideal wavelength region, how much energy was needed, and what the pulse duration would have to be like to damage target vessels that small. I showed John Parrish what I had written. He took me seriously and said, “Let’s see if we can find a light source that can accomplish this.” We traveled around the country looking at various lasers, but we wound up building the first pulsed dye laser for treating port-wine stains. To me, the surprise was that we didn’t kill the skin. If you treat an area of skin with a laser and hurt all the blood vessels, you think, “Wait a minute. Are we going to kill the skin because it has no blood supply?” The questions of the day were so basic, and we just got lucky. It took 6-8 years before we ramped up the clinical studies showing efficacy and safety of this technology.
I presume that you experimented on your own skin while developing some of the nonscarring laser treatments now widely used in dermatology. What “war story” stands out to you most from that part of your work?
I’m right handed, so I’d grab a laser with my right hand and treat my left arm, so that arm sports a bit of history. In 1994, while working with Dr. Melanie Grossman on the development of laser hair removal, I used a ruby laser to self-treat a patch of hair on my left arm. I still have the world’s oldest laser-induced bald spot on that arm. It’s been 26 years now. I still look at it and count the hairs, because one of the big questions is, is laser hair removal permanent? In all these years I have grown two hairs.
What technology that you conceived of or developed has most surprised you, in term of its ultimate clinical impact?
I would say confocal microscopy. In the mid-1990s I worked with a physicist named Robert H. Webb, who invented an imaging system for the retina. We got together, noodled about it, and decided we would modify his ophthalmoscope system to see if we could get images from inside the skin. It worked pretty well. It was truly surprising from many points of view. First, it wasn’t clear at all that we’d get any images this way. Now, reflectance confocal microscopy is a standard tool in both clinical and research dermatology. But there were odd discoveries early on. For example, the darker your skin, the brighter it appeared in the microscope. You might think that melanin absorbs light and that you would get poor images in dark skin. It was the exact opposite; melanin acts as a natural contrast agent.
We worked with a small company to make the first confocal microscope. Initially, it had no clinical applications but what was fascinating to me was the incredible value of being able to see inside human skin harmlessly, and just see what’s going on. It became a potent research tool, and recently CPT codes were established for its use in evaluating skin cancer margins. I wouldn’t be surprised if 30 years from now, taking a skin biopsy is seemingly barbaric. A forerunner of all these new imaging tools for the skin was the confocal microscope developed in my lab in 1994.
During a 2011 TED talk, you said that nevus of Ota is your favorite thing to treat, because the outcome is usually perfect skin. Are there other technologies or devices you played a role in developing that make you proud at this stage in your career?
The reason I love treating nevus of Ota is that you have a lifelong facial disfigurement, and the only treatment for it is a laser we came up with, and it always works. How perfect could it be? The flip side of the same coin is, there are lesions of the skin that just don’t respond. One of the things we don’t know enough about is the connection between the biologic aspects of repair of various lesions and the treatments that we come up with. The most recent example of selective photothermolysis is a new laser we’re building right now for acne that is based on sebaceous gland injury. You’ll see this coming out in the next year or two. My heart goes out to people with nodular cystic acne. For young men it’s highly associated with suicide. So, I’m excited about optimizing and learning what happens when we target sebaceous glands.
One of the other big stories in laser dermatology is the fractional laser. I developed this with Dr. Dieter Manstein when he was a postdoc in my lab. One of the most pleasing things from this technology is how well you can rehabilitate scars, particularly burn scars in children. Over the last few years, I have trained plastic surgeons at the Shriners Hospital for Children in Boston on how to use fractional lasers to improve the lives of these kids. Another technology I developed with Dr. Manstein is cryolipolysis, which is removing fat from the body by cooling it. There are no lasers involved with this technology. I like to say that I’ve spent most of my career studying light and heat, and now we’ve come up with something that’s cold in the dark. We are now working on derivatives of cryolipolysis, to determine if what we’ve learned about targeting fat that might be applicable elsewhere.
Who inspires you most in your work today?
In addition to Dr. John Parrish and Dr. Thomas Fitzpatrick, the late Dr. Albert M. Kligman also influenced me. He never accepted dogma and he loved to ask questions, like, “What if?” as opposed to just accumulating a fund of knowledge. Understanding things is not just based on how much you know. It’s based on critical thinking and the ability to question. I also admire Albert Einstein, his ability to sit down with nothing more than pencil and paper and change our view of the universe. I love physics because it’s the science of everything. I also love poetry. My favorite poet is Stanley Kunitz. He had amazing insight and was named United States Poet Laureate in 1974 and in 2000. I have plenty of antiheroes as well, mostly politicians.
I understand that you play the banjo. How long have you been playing, and what do you enjoy about it?
You cannot sit down and play the banjo and have your mind on much else. It’s a wonderful moving meditation. Before my medical career, I was a schoolteacher in Vermont. There was a guy on the staff there who played banjo. He came from a small town in Georgia. I just picked it up and started plunking. It’s a happy instrument. It’s awfully hard to make the banjo sound melancholy.
What novel use of lasers and light in dermatology are you most excited about in the next 5 years?
The marriage of therapeutic devices with diagnostic and imaging devices has not happened yet. They are not even in the honeymoon moment. But I think that having truly smart robotic systems in our hands for treating patients will become a reality. These days, dermatologists have to buy a certain type of laser to treat a certain type of lesion. For example, the Q-switched alexandrite laser you buy for treating Nevus of Ota won’t do anything for a port-wine stain; it’s the wrong pulse duration. This means that clinicians who practice a lot of laser dermatology end up with a dozen lasers in their practice. In the future, I think it will be possible to have a software laser, so when you want to acquire another target, you load an App as opposed to buying a new laser. This means that you would have software programmable targeting, and you would not have the requirement of having selective absorption. So, I’m excited by the idea of guided fractional lasers. None of them exist now. We have to start from scratch.
Visionary reflects on the importance of teamwork in advancing technology
When John A. Parrish, MD, worked with R. Rox Anderson, MD, and a team of clinicians and scientists in the early 1980s to develop the first pulsed dye laser for dermatologic use, it became clear that the Food and Drug Administration required convincing that their prototype would be safe.
“Laser medicine was new, and lasers had some specific frightening risks like blindness and bleeding from laser suturing,” recalled Dr. Parrish, founder of the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston. “The main issue was eye risk. Because the operator and the patient were at risk for eye injury, the FDA was reluctant to press on with laser treatments of skin.”
To make the FDA more comfortable with their efforts, Dr. Parrish and his colleagues drew from the published work of ophthalmologists, who were ahead of dermatologists in the clinical use of lasers. “A lot of the animal experiments and the human understanding of laser-tissue interactions came from ophthalmologists,” he said. “We worked with a fellow named David H. Sliney, PhD. He was very interested in laser safety of the eye, so we worked closely with him to measure the boundary conditions that could be used without injuring the eye.”
To Dr. Parrish, forging that partnership illustrated a key principle in developing novel diagnostics and therapeutics that use lasers and light: You need a multidisciplinary team. “You need a pathologist, clinicians, physicists, technologists, and engineers, because all of the barriers to figure out how to deliver a new treatment safely often don’t rest in one person’s mind, so early on we had to be very collaborative and find experts who would help us solve problems,” he said. “That’s how the Wellman Labs got started. All of the new treatments were explored by multidisciplinary teams so that we didn’t have to hope that the expertise to get past all the barriers was in one person’s mind. That was often not the case.”
Dr. Parrish credits his mentor, the late Thomas B. Fitzpatrick, MD, PhD, who in 1975 devised the Fitzpatrick scale of skin phototypes, with inspiring his career path. Dr. Fitzpatrick, who is widely considered the father of modern academic dermatology, was professor and chief of dermatology at Harvard Medical School when Dr. Parrish began his dermatology training there. “He was a great clinician who loved patient care and he was a very curious investigator,” said Dr. Parrish, who cofounded the Consortia for Improving Medicine with Innovation and Technology (CIMIT). “He not only trained me, but I became his collaborator during my early faculty time. What I learned most from him was the joy of work, curiosity, and serious commitment to patient care. It was almost contagious.”
Of all the devices he’s played a role in developing in the past 50 years, Dr. Parrish said that he remains most surprised by the impact of pulsed lasers in dermatology. “It took us a while to understand the capabilities of pulsed lasers in that they could confine injury to small spots and treat multiple areas at once,” he said. “A lot of that did not come because we were so wise to think about that, but we did a lot of work in the early days with a free-electron laser, a pulsed laser which had a tunable wavelength and a tunable pulse duration. That gave us the capability of looking at very specific injuries and the host responses that heal without scarring.”
Dr. Parrish’s interest in dermatology was piqued in 1968, when he was assigned to Oak Knoll Naval Hospital in Oakland, Calif., after a year of serving in the U.S. Marine Corps as a battlefield doctor in Vietnam. (He wrote about his wartime experience in two books, most recently “Autopsy of War: A Personal History” [New York: Thomas Dunne Books, 2012].) Prior to serving in Vietnam he had completed early internal medicine training, but once at Oak Knoll he discovered that he had a propensity for diagnosing and treating disorders of the skin. “When I came back to resume my residency, I asked if I could train in dermatology,” he said. “It was by happenstance. I felt like I could understand skin disease and that I could make a difference. In internal medicine you often change blood pressure medicines around. I felt like I was a better diagnostician than in internal medicine and that I could most often make a difference. I liked seeing all ages of patients, and most of them got better, so it was more fun.”
When John A. Parrish, MD, worked with R. Rox Anderson, MD, and a team of clinicians and scientists in the early 1980s to develop the first pulsed dye laser for dermatologic use, it became clear that the Food and Drug Administration required convincing that their prototype would be safe.
“Laser medicine was new, and lasers had some specific frightening risks like blindness and bleeding from laser suturing,” recalled Dr. Parrish, founder of the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston. “The main issue was eye risk. Because the operator and the patient were at risk for eye injury, the FDA was reluctant to press on with laser treatments of skin.”
To make the FDA more comfortable with their efforts, Dr. Parrish and his colleagues drew from the published work of ophthalmologists, who were ahead of dermatologists in the clinical use of lasers. “A lot of the animal experiments and the human understanding of laser-tissue interactions came from ophthalmologists,” he said. “We worked with a fellow named David H. Sliney, PhD. He was very interested in laser safety of the eye, so we worked closely with him to measure the boundary conditions that could be used without injuring the eye.”
To Dr. Parrish, forging that partnership illustrated a key principle in developing novel diagnostics and therapeutics that use lasers and light: You need a multidisciplinary team. “You need a pathologist, clinicians, physicists, technologists, and engineers, because all of the barriers to figure out how to deliver a new treatment safely often don’t rest in one person’s mind, so early on we had to be very collaborative and find experts who would help us solve problems,” he said. “That’s how the Wellman Labs got started. All of the new treatments were explored by multidisciplinary teams so that we didn’t have to hope that the expertise to get past all the barriers was in one person’s mind. That was often not the case.”
Dr. Parrish credits his mentor, the late Thomas B. Fitzpatrick, MD, PhD, who in 1975 devised the Fitzpatrick scale of skin phototypes, with inspiring his career path. Dr. Fitzpatrick, who is widely considered the father of modern academic dermatology, was professor and chief of dermatology at Harvard Medical School when Dr. Parrish began his dermatology training there. “He was a great clinician who loved patient care and he was a very curious investigator,” said Dr. Parrish, who cofounded the Consortia for Improving Medicine with Innovation and Technology (CIMIT). “He not only trained me, but I became his collaborator during my early faculty time. What I learned most from him was the joy of work, curiosity, and serious commitment to patient care. It was almost contagious.”
Of all the devices he’s played a role in developing in the past 50 years, Dr. Parrish said that he remains most surprised by the impact of pulsed lasers in dermatology. “It took us a while to understand the capabilities of pulsed lasers in that they could confine injury to small spots and treat multiple areas at once,” he said. “A lot of that did not come because we were so wise to think about that, but we did a lot of work in the early days with a free-electron laser, a pulsed laser which had a tunable wavelength and a tunable pulse duration. That gave us the capability of looking at very specific injuries and the host responses that heal without scarring.”
Dr. Parrish’s interest in dermatology was piqued in 1968, when he was assigned to Oak Knoll Naval Hospital in Oakland, Calif., after a year of serving in the U.S. Marine Corps as a battlefield doctor in Vietnam. (He wrote about his wartime experience in two books, most recently “Autopsy of War: A Personal History” [New York: Thomas Dunne Books, 2012].) Prior to serving in Vietnam he had completed early internal medicine training, but once at Oak Knoll he discovered that he had a propensity for diagnosing and treating disorders of the skin. “When I came back to resume my residency, I asked if I could train in dermatology,” he said. “It was by happenstance. I felt like I could understand skin disease and that I could make a difference. In internal medicine you often change blood pressure medicines around. I felt like I was a better diagnostician than in internal medicine and that I could most often make a difference. I liked seeing all ages of patients, and most of them got better, so it was more fun.”
When John A. Parrish, MD, worked with R. Rox Anderson, MD, and a team of clinicians and scientists in the early 1980s to develop the first pulsed dye laser for dermatologic use, it became clear that the Food and Drug Administration required convincing that their prototype would be safe.
“Laser medicine was new, and lasers had some specific frightening risks like blindness and bleeding from laser suturing,” recalled Dr. Parrish, founder of the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston. “The main issue was eye risk. Because the operator and the patient were at risk for eye injury, the FDA was reluctant to press on with laser treatments of skin.”
To make the FDA more comfortable with their efforts, Dr. Parrish and his colleagues drew from the published work of ophthalmologists, who were ahead of dermatologists in the clinical use of lasers. “A lot of the animal experiments and the human understanding of laser-tissue interactions came from ophthalmologists,” he said. “We worked with a fellow named David H. Sliney, PhD. He was very interested in laser safety of the eye, so we worked closely with him to measure the boundary conditions that could be used without injuring the eye.”
To Dr. Parrish, forging that partnership illustrated a key principle in developing novel diagnostics and therapeutics that use lasers and light: You need a multidisciplinary team. “You need a pathologist, clinicians, physicists, technologists, and engineers, because all of the barriers to figure out how to deliver a new treatment safely often don’t rest in one person’s mind, so early on we had to be very collaborative and find experts who would help us solve problems,” he said. “That’s how the Wellman Labs got started. All of the new treatments were explored by multidisciplinary teams so that we didn’t have to hope that the expertise to get past all the barriers was in one person’s mind. That was often not the case.”
Dr. Parrish credits his mentor, the late Thomas B. Fitzpatrick, MD, PhD, who in 1975 devised the Fitzpatrick scale of skin phototypes, with inspiring his career path. Dr. Fitzpatrick, who is widely considered the father of modern academic dermatology, was professor and chief of dermatology at Harvard Medical School when Dr. Parrish began his dermatology training there. “He was a great clinician who loved patient care and he was a very curious investigator,” said Dr. Parrish, who cofounded the Consortia for Improving Medicine with Innovation and Technology (CIMIT). “He not only trained me, but I became his collaborator during my early faculty time. What I learned most from him was the joy of work, curiosity, and serious commitment to patient care. It was almost contagious.”
Of all the devices he’s played a role in developing in the past 50 years, Dr. Parrish said that he remains most surprised by the impact of pulsed lasers in dermatology. “It took us a while to understand the capabilities of pulsed lasers in that they could confine injury to small spots and treat multiple areas at once,” he said. “A lot of that did not come because we were so wise to think about that, but we did a lot of work in the early days with a free-electron laser, a pulsed laser which had a tunable wavelength and a tunable pulse duration. That gave us the capability of looking at very specific injuries and the host responses that heal without scarring.”
Dr. Parrish’s interest in dermatology was piqued in 1968, when he was assigned to Oak Knoll Naval Hospital in Oakland, Calif., after a year of serving in the U.S. Marine Corps as a battlefield doctor in Vietnam. (He wrote about his wartime experience in two books, most recently “Autopsy of War: A Personal History” [New York: Thomas Dunne Books, 2012].) Prior to serving in Vietnam he had completed early internal medicine training, but once at Oak Knoll he discovered that he had a propensity for diagnosing and treating disorders of the skin. “When I came back to resume my residency, I asked if I could train in dermatology,” he said. “It was by happenstance. I felt like I could understand skin disease and that I could make a difference. In internal medicine you often change blood pressure medicines around. I felt like I was a better diagnostician than in internal medicine and that I could most often make a difference. I liked seeing all ages of patients, and most of them got better, so it was more fun.”
Experts reflect on the past 50 years of lasers in dermatology
During her dermatology residency at Yale University in the late 1980s, Tina S. Alster, MD, met a 44-year-old woman who changed the trajectory of her professional career.
During her clinic visit, the woman explained that she always wore heavy facial makeup to hide her port-wine stain birthmark – a vascular malformation that she kept secret from her husband and teenage son. “She was very good about covering it,” recalled Dr. Alster, who is the founding director of the Washington Institute of Dermatologic Laser Surgery and clinical professor of dermatology at Georgetown University, Washington. “She removed a small amount of makeup for me so I could take a look at it. I had just finished reading an article about using a laser for birthmarks; it had just been published. I told her, ‘There’s something new; we don’t have it at Yale, but I read about treatment that could hone in on birthmarks.’ I promised her I would find out more details.”
A few days later, Dr. Alster pored through stacks of medical journals at Yale’s library and relocated the article she’d seen by first author Oon Tian Tan, MD, PhD, of the department of dermatology at Boston University Medical Center. It described use of the flashpump-pulsed tunable dye laser to treat port-wine stains in 35 children (N Engl J Med. 1989;320:416-21). After giving the article a more thorough read, Dr. Alster became so intrigued by the technology it described that she moved to Boston the following year for a dermatology fellowship with Dr. Tan and joined the ranks of early clinicians who used lasers for treating port-wine stains and other dermatologic conditions.
“That was at a time when there were only a handful of pulsed dye lasers in the world, and the first time I used it was when I went to Boston,” she said. “It was life-changing. You think, ‘Isn’t this great for children with port-wine stains.’ Your heart breaks for them, but I also felt compassion for adults who had suffered a lifetime of stares, including the woman who propelled me to look into this. She ended up coming to Boston during my fellowship and had her birthmark removed, so I changed her life, but she changed mine as well.”
The real credit for that series of events, Dr. Alster continued, belongs to John A. Parrish, MD, and R. Rox Anderson, MD, who in 1983 published the concept of selective photothermolysis, a seminal work that shifted the paradigm for how lasers and other light sources are designed for skin diseases and conditions (Science. 1983 Apr 29;220(4596):524-7). The first pulsed dye laser was built on this concept, an approach that minimized or eliminated the unwanted tissue damage and significant scarring that impeded therapeutic use of laser energy for port-wine stains and other lesions prior to that time. “Lasers that were built subsequent to that seminal paper focused our attention on building lasers that were specific for treatment of certain skin conditions,” Dr. Alster said. “Selective photothermolysis catapulted not only our understanding of how lasers interact with the skin, but allowed us to identify things in the skin that we could potentially target with this new laser technology, and to build laser systems that were specific to those purposes.”
In the late 1970s, Dr. Parrish, who played a key role in making psoralens plus ultraviolet A safe and effective for patients with severe psoriasis, turned his attention to studying lasers in his lab at Harvard Medical School. He hired R. Rox Anderson, a recent graduate of the Massachusetts Institute of Technology, as a technician. “Rox then got interested in medicine and went to medical school at Harvard, got interested in dermatology, and then worked in my lab a little bit more,” said Dr. Parrish, who founded the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston.
“Rox was interested in port-wine stains because of his rotation through pediatrics and was theorizing about how lasers could improve port-wine stains and hemangiomas. I think he first thought of that through the physics of what would be needed. He was thinking, ‘What are these hemangiomas under the microscope? What does the target look like, and what do you need to do to promote healing without scarring? You would have to be able to heat for this duration and this time and at this wavelength.’ He matched the physics of lasers with the pathophysiology of port-wine stains, and together we figured out how to deliver the right energy at the right wavelength at the right time. In fact, at the time, there was no ideal laser. We had to convince a laser manufacturer to build a tunable dye laser, which is what we ended up using around the specifications that we wanted for this treatment.”
Prior to the theory of selective photothermolysis, lasers were a blunt instrument. “They would target the skin but you wouldn’t just selectively target something; you’d get a result you didn’t want,” said Mathew M. Avram, MD, JD, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital.
Once pulsed dye lasers that incorporated principles of selective photothermolysis hit the marketplace, clinicians could treat and improve port-wine stains without scarring the skin. They could even improve scarring from port-wine stains that had been previously treated with the argon laser, the subject of early published work by Dr. Alster (Lasers Surg Med. 1993;13[3]:368-73). “When we treated port-wine stains with the pulsed dye laser on top of the argon laser scars, we observed that the scars were looking better,” Dr. Alster said. “From that observation, we were able to demonstrate improvement of a wide range of scars: traumatic and burn scars, surgical scars, acne scars, and scars caused by other lasers. But it all started with the pulsed dye laser for treating port-wine stains that had scars in them.”
, which enabled the user to deliver even shorter pulse widths in the nanosecond domain. “That changed tattoo treatment,” said Dr. Avram, who is also a past president of the American Society for Laser Medicine and Surgery. “Prior to that, for tattoos and brown spots you would use ablative lasers like CO2 or dermabrasion. They would cause scarring and not really get rid of the tattoo ink or the brown spots. With the Q-switched nanosecond lasers and the picosecond lasers, which came about 15 years later, you had the ability to remove spots with a week of down time, and [they worked] for things like Nevus of Ota, where someone has a disfiguring blue-brown discoloration of their cheek. There’s no surgical treatment for that whatsoever. It’s not like you can take it out.”
Another key advancement was the introduction of “scanning” technology in the early 1990s for CO2 and erbium YAG lasers, which enabled precise computerized control of laser beams. Dr. Avram characterized the CO2 laser as “the gold standard for facial rejuvenation, for sun-damaged skin. The downside of CO2 lasers is that they really need to be in skilled hands. There can be serious side effects such as scarring if it’s not done appropriately or there is not appropriate follow-up. The CO2 lasers have been used in fractional modes for scars. I think it’s the best treatment for scars.”
Dr. Anderson and Melanie Grossman, MD, who practices in New York City, developed the ruby laser for hair removal in the 1990s, and today that procedure ranks as the most common laser treatment in medicine, according to Dr. Avram. He described it as “safe and effective in skilled hands,” requiring about six treatments. Indications are for hypertrichosis, hirsutism (sometimes in the setting of polycystic ovary syndrome), pseudofolliculitis barbae, pilonidal cysts, and gender reassignment surgery.
Another game-changing technology developed by Dr. Anderson came in the early 2000s with the Food and Drug Administration clearance of the Fraxel laser, which is based on the concept of fractional photothermolysis. With this technology, “instead of treating skin to a certain depth, you treat a fraction of it, anywhere from 5% to 40% of the skin,” Dr. Avram explained. “You go in deeper, but you leave surrounding viable tissue that is not affected by the laser. That serves as viable tissue to promote healing. The laser goes in deeper but it’s fractional, so there are skip zones in between the lasers that are going into the skin. You can do this with the CO2 and erbium YAG lasers.” Since hitting the marketplace, the FDA has cleared the use of Fraxel for a number of indications, from periorbital wrinkles and acne scars to surgical scars and melasma.
Dr. Parrish predicted that the next frontier for the advancement of lasers in dermatology will involve the treatment of photodamaged skin. “I’m not sure which technology is going to win,” he said.
Dr. Avram anticipates that dermatologic lasers of the future are going to be more effective, safer, and result in less downtime for patients. “I think we are going to be able to treat skin of color more safely and more effectively, and I think we’re going to become much more successful,” he said. “At some point, the standard of care of treatment for skin cancer will involve lasers and light sources. With all the advances that have happened in the last 50 years, sometimes you wonder, are we at a time to pause, or is most of the story behind us? I think that the advances in innovation that are occurring are going to accelerate greatly as we pass the 50th anniversary. In due credit, laser therapy has completely revolutionized the field of dermatology and has completely revolutionized the way we practice medicine. That will only accelerate in the future.”
Dr. Alster emphasized a “safety first” approach to her hopes for the future. “My wish is that we educate people to know that, while lasers have become ubiquitous and we’ve made them safe, they’re still only safe in the right hands,” she said. “There’s not a day that goes by when I don’t have somebody referred to me who’s been mishandled. There’s no reason for that. With proper training, the risk of bad side effects or complications is markedly reduced.”
During her dermatology residency at Yale University in the late 1980s, Tina S. Alster, MD, met a 44-year-old woman who changed the trajectory of her professional career.
During her clinic visit, the woman explained that she always wore heavy facial makeup to hide her port-wine stain birthmark – a vascular malformation that she kept secret from her husband and teenage son. “She was very good about covering it,” recalled Dr. Alster, who is the founding director of the Washington Institute of Dermatologic Laser Surgery and clinical professor of dermatology at Georgetown University, Washington. “She removed a small amount of makeup for me so I could take a look at it. I had just finished reading an article about using a laser for birthmarks; it had just been published. I told her, ‘There’s something new; we don’t have it at Yale, but I read about treatment that could hone in on birthmarks.’ I promised her I would find out more details.”
A few days later, Dr. Alster pored through stacks of medical journals at Yale’s library and relocated the article she’d seen by first author Oon Tian Tan, MD, PhD, of the department of dermatology at Boston University Medical Center. It described use of the flashpump-pulsed tunable dye laser to treat port-wine stains in 35 children (N Engl J Med. 1989;320:416-21). After giving the article a more thorough read, Dr. Alster became so intrigued by the technology it described that she moved to Boston the following year for a dermatology fellowship with Dr. Tan and joined the ranks of early clinicians who used lasers for treating port-wine stains and other dermatologic conditions.
“That was at a time when there were only a handful of pulsed dye lasers in the world, and the first time I used it was when I went to Boston,” she said. “It was life-changing. You think, ‘Isn’t this great for children with port-wine stains.’ Your heart breaks for them, but I also felt compassion for adults who had suffered a lifetime of stares, including the woman who propelled me to look into this. She ended up coming to Boston during my fellowship and had her birthmark removed, so I changed her life, but she changed mine as well.”
The real credit for that series of events, Dr. Alster continued, belongs to John A. Parrish, MD, and R. Rox Anderson, MD, who in 1983 published the concept of selective photothermolysis, a seminal work that shifted the paradigm for how lasers and other light sources are designed for skin diseases and conditions (Science. 1983 Apr 29;220(4596):524-7). The first pulsed dye laser was built on this concept, an approach that minimized or eliminated the unwanted tissue damage and significant scarring that impeded therapeutic use of laser energy for port-wine stains and other lesions prior to that time. “Lasers that were built subsequent to that seminal paper focused our attention on building lasers that were specific for treatment of certain skin conditions,” Dr. Alster said. “Selective photothermolysis catapulted not only our understanding of how lasers interact with the skin, but allowed us to identify things in the skin that we could potentially target with this new laser technology, and to build laser systems that were specific to those purposes.”
In the late 1970s, Dr. Parrish, who played a key role in making psoralens plus ultraviolet A safe and effective for patients with severe psoriasis, turned his attention to studying lasers in his lab at Harvard Medical School. He hired R. Rox Anderson, a recent graduate of the Massachusetts Institute of Technology, as a technician. “Rox then got interested in medicine and went to medical school at Harvard, got interested in dermatology, and then worked in my lab a little bit more,” said Dr. Parrish, who founded the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston.
“Rox was interested in port-wine stains because of his rotation through pediatrics and was theorizing about how lasers could improve port-wine stains and hemangiomas. I think he first thought of that through the physics of what would be needed. He was thinking, ‘What are these hemangiomas under the microscope? What does the target look like, and what do you need to do to promote healing without scarring? You would have to be able to heat for this duration and this time and at this wavelength.’ He matched the physics of lasers with the pathophysiology of port-wine stains, and together we figured out how to deliver the right energy at the right wavelength at the right time. In fact, at the time, there was no ideal laser. We had to convince a laser manufacturer to build a tunable dye laser, which is what we ended up using around the specifications that we wanted for this treatment.”
Prior to the theory of selective photothermolysis, lasers were a blunt instrument. “They would target the skin but you wouldn’t just selectively target something; you’d get a result you didn’t want,” said Mathew M. Avram, MD, JD, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital.
Once pulsed dye lasers that incorporated principles of selective photothermolysis hit the marketplace, clinicians could treat and improve port-wine stains without scarring the skin. They could even improve scarring from port-wine stains that had been previously treated with the argon laser, the subject of early published work by Dr. Alster (Lasers Surg Med. 1993;13[3]:368-73). “When we treated port-wine stains with the pulsed dye laser on top of the argon laser scars, we observed that the scars were looking better,” Dr. Alster said. “From that observation, we were able to demonstrate improvement of a wide range of scars: traumatic and burn scars, surgical scars, acne scars, and scars caused by other lasers. But it all started with the pulsed dye laser for treating port-wine stains that had scars in them.”
, which enabled the user to deliver even shorter pulse widths in the nanosecond domain. “That changed tattoo treatment,” said Dr. Avram, who is also a past president of the American Society for Laser Medicine and Surgery. “Prior to that, for tattoos and brown spots you would use ablative lasers like CO2 or dermabrasion. They would cause scarring and not really get rid of the tattoo ink or the brown spots. With the Q-switched nanosecond lasers and the picosecond lasers, which came about 15 years later, you had the ability to remove spots with a week of down time, and [they worked] for things like Nevus of Ota, where someone has a disfiguring blue-brown discoloration of their cheek. There’s no surgical treatment for that whatsoever. It’s not like you can take it out.”
Another key advancement was the introduction of “scanning” technology in the early 1990s for CO2 and erbium YAG lasers, which enabled precise computerized control of laser beams. Dr. Avram characterized the CO2 laser as “the gold standard for facial rejuvenation, for sun-damaged skin. The downside of CO2 lasers is that they really need to be in skilled hands. There can be serious side effects such as scarring if it’s not done appropriately or there is not appropriate follow-up. The CO2 lasers have been used in fractional modes for scars. I think it’s the best treatment for scars.”
Dr. Anderson and Melanie Grossman, MD, who practices in New York City, developed the ruby laser for hair removal in the 1990s, and today that procedure ranks as the most common laser treatment in medicine, according to Dr. Avram. He described it as “safe and effective in skilled hands,” requiring about six treatments. Indications are for hypertrichosis, hirsutism (sometimes in the setting of polycystic ovary syndrome), pseudofolliculitis barbae, pilonidal cysts, and gender reassignment surgery.
Another game-changing technology developed by Dr. Anderson came in the early 2000s with the Food and Drug Administration clearance of the Fraxel laser, which is based on the concept of fractional photothermolysis. With this technology, “instead of treating skin to a certain depth, you treat a fraction of it, anywhere from 5% to 40% of the skin,” Dr. Avram explained. “You go in deeper, but you leave surrounding viable tissue that is not affected by the laser. That serves as viable tissue to promote healing. The laser goes in deeper but it’s fractional, so there are skip zones in between the lasers that are going into the skin. You can do this with the CO2 and erbium YAG lasers.” Since hitting the marketplace, the FDA has cleared the use of Fraxel for a number of indications, from periorbital wrinkles and acne scars to surgical scars and melasma.
Dr. Parrish predicted that the next frontier for the advancement of lasers in dermatology will involve the treatment of photodamaged skin. “I’m not sure which technology is going to win,” he said.
Dr. Avram anticipates that dermatologic lasers of the future are going to be more effective, safer, and result in less downtime for patients. “I think we are going to be able to treat skin of color more safely and more effectively, and I think we’re going to become much more successful,” he said. “At some point, the standard of care of treatment for skin cancer will involve lasers and light sources. With all the advances that have happened in the last 50 years, sometimes you wonder, are we at a time to pause, or is most of the story behind us? I think that the advances in innovation that are occurring are going to accelerate greatly as we pass the 50th anniversary. In due credit, laser therapy has completely revolutionized the field of dermatology and has completely revolutionized the way we practice medicine. That will only accelerate in the future.”
Dr. Alster emphasized a “safety first” approach to her hopes for the future. “My wish is that we educate people to know that, while lasers have become ubiquitous and we’ve made them safe, they’re still only safe in the right hands,” she said. “There’s not a day that goes by when I don’t have somebody referred to me who’s been mishandled. There’s no reason for that. With proper training, the risk of bad side effects or complications is markedly reduced.”
During her dermatology residency at Yale University in the late 1980s, Tina S. Alster, MD, met a 44-year-old woman who changed the trajectory of her professional career.
During her clinic visit, the woman explained that she always wore heavy facial makeup to hide her port-wine stain birthmark – a vascular malformation that she kept secret from her husband and teenage son. “She was very good about covering it,” recalled Dr. Alster, who is the founding director of the Washington Institute of Dermatologic Laser Surgery and clinical professor of dermatology at Georgetown University, Washington. “She removed a small amount of makeup for me so I could take a look at it. I had just finished reading an article about using a laser for birthmarks; it had just been published. I told her, ‘There’s something new; we don’t have it at Yale, but I read about treatment that could hone in on birthmarks.’ I promised her I would find out more details.”
A few days later, Dr. Alster pored through stacks of medical journals at Yale’s library and relocated the article she’d seen by first author Oon Tian Tan, MD, PhD, of the department of dermatology at Boston University Medical Center. It described use of the flashpump-pulsed tunable dye laser to treat port-wine stains in 35 children (N Engl J Med. 1989;320:416-21). After giving the article a more thorough read, Dr. Alster became so intrigued by the technology it described that she moved to Boston the following year for a dermatology fellowship with Dr. Tan and joined the ranks of early clinicians who used lasers for treating port-wine stains and other dermatologic conditions.
“That was at a time when there were only a handful of pulsed dye lasers in the world, and the first time I used it was when I went to Boston,” she said. “It was life-changing. You think, ‘Isn’t this great for children with port-wine stains.’ Your heart breaks for them, but I also felt compassion for adults who had suffered a lifetime of stares, including the woman who propelled me to look into this. She ended up coming to Boston during my fellowship and had her birthmark removed, so I changed her life, but she changed mine as well.”
The real credit for that series of events, Dr. Alster continued, belongs to John A. Parrish, MD, and R. Rox Anderson, MD, who in 1983 published the concept of selective photothermolysis, a seminal work that shifted the paradigm for how lasers and other light sources are designed for skin diseases and conditions (Science. 1983 Apr 29;220(4596):524-7). The first pulsed dye laser was built on this concept, an approach that minimized or eliminated the unwanted tissue damage and significant scarring that impeded therapeutic use of laser energy for port-wine stains and other lesions prior to that time. “Lasers that were built subsequent to that seminal paper focused our attention on building lasers that were specific for treatment of certain skin conditions,” Dr. Alster said. “Selective photothermolysis catapulted not only our understanding of how lasers interact with the skin, but allowed us to identify things in the skin that we could potentially target with this new laser technology, and to build laser systems that were specific to those purposes.”
In the late 1970s, Dr. Parrish, who played a key role in making psoralens plus ultraviolet A safe and effective for patients with severe psoriasis, turned his attention to studying lasers in his lab at Harvard Medical School. He hired R. Rox Anderson, a recent graduate of the Massachusetts Institute of Technology, as a technician. “Rox then got interested in medicine and went to medical school at Harvard, got interested in dermatology, and then worked in my lab a little bit more,” said Dr. Parrish, who founded the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston.
“Rox was interested in port-wine stains because of his rotation through pediatrics and was theorizing about how lasers could improve port-wine stains and hemangiomas. I think he first thought of that through the physics of what would be needed. He was thinking, ‘What are these hemangiomas under the microscope? What does the target look like, and what do you need to do to promote healing without scarring? You would have to be able to heat for this duration and this time and at this wavelength.’ He matched the physics of lasers with the pathophysiology of port-wine stains, and together we figured out how to deliver the right energy at the right wavelength at the right time. In fact, at the time, there was no ideal laser. We had to convince a laser manufacturer to build a tunable dye laser, which is what we ended up using around the specifications that we wanted for this treatment.”
Prior to the theory of selective photothermolysis, lasers were a blunt instrument. “They would target the skin but you wouldn’t just selectively target something; you’d get a result you didn’t want,” said Mathew M. Avram, MD, JD, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital.
Once pulsed dye lasers that incorporated principles of selective photothermolysis hit the marketplace, clinicians could treat and improve port-wine stains without scarring the skin. They could even improve scarring from port-wine stains that had been previously treated with the argon laser, the subject of early published work by Dr. Alster (Lasers Surg Med. 1993;13[3]:368-73). “When we treated port-wine stains with the pulsed dye laser on top of the argon laser scars, we observed that the scars were looking better,” Dr. Alster said. “From that observation, we were able to demonstrate improvement of a wide range of scars: traumatic and burn scars, surgical scars, acne scars, and scars caused by other lasers. But it all started with the pulsed dye laser for treating port-wine stains that had scars in them.”
, which enabled the user to deliver even shorter pulse widths in the nanosecond domain. “That changed tattoo treatment,” said Dr. Avram, who is also a past president of the American Society for Laser Medicine and Surgery. “Prior to that, for tattoos and brown spots you would use ablative lasers like CO2 or dermabrasion. They would cause scarring and not really get rid of the tattoo ink or the brown spots. With the Q-switched nanosecond lasers and the picosecond lasers, which came about 15 years later, you had the ability to remove spots with a week of down time, and [they worked] for things like Nevus of Ota, where someone has a disfiguring blue-brown discoloration of their cheek. There’s no surgical treatment for that whatsoever. It’s not like you can take it out.”
Another key advancement was the introduction of “scanning” technology in the early 1990s for CO2 and erbium YAG lasers, which enabled precise computerized control of laser beams. Dr. Avram characterized the CO2 laser as “the gold standard for facial rejuvenation, for sun-damaged skin. The downside of CO2 lasers is that they really need to be in skilled hands. There can be serious side effects such as scarring if it’s not done appropriately or there is not appropriate follow-up. The CO2 lasers have been used in fractional modes for scars. I think it’s the best treatment for scars.”
Dr. Anderson and Melanie Grossman, MD, who practices in New York City, developed the ruby laser for hair removal in the 1990s, and today that procedure ranks as the most common laser treatment in medicine, according to Dr. Avram. He described it as “safe and effective in skilled hands,” requiring about six treatments. Indications are for hypertrichosis, hirsutism (sometimes in the setting of polycystic ovary syndrome), pseudofolliculitis barbae, pilonidal cysts, and gender reassignment surgery.
Another game-changing technology developed by Dr. Anderson came in the early 2000s with the Food and Drug Administration clearance of the Fraxel laser, which is based on the concept of fractional photothermolysis. With this technology, “instead of treating skin to a certain depth, you treat a fraction of it, anywhere from 5% to 40% of the skin,” Dr. Avram explained. “You go in deeper, but you leave surrounding viable tissue that is not affected by the laser. That serves as viable tissue to promote healing. The laser goes in deeper but it’s fractional, so there are skip zones in between the lasers that are going into the skin. You can do this with the CO2 and erbium YAG lasers.” Since hitting the marketplace, the FDA has cleared the use of Fraxel for a number of indications, from periorbital wrinkles and acne scars to surgical scars and melasma.
Dr. Parrish predicted that the next frontier for the advancement of lasers in dermatology will involve the treatment of photodamaged skin. “I’m not sure which technology is going to win,” he said.
Dr. Avram anticipates that dermatologic lasers of the future are going to be more effective, safer, and result in less downtime for patients. “I think we are going to be able to treat skin of color more safely and more effectively, and I think we’re going to become much more successful,” he said. “At some point, the standard of care of treatment for skin cancer will involve lasers and light sources. With all the advances that have happened in the last 50 years, sometimes you wonder, are we at a time to pause, or is most of the story behind us? I think that the advances in innovation that are occurring are going to accelerate greatly as we pass the 50th anniversary. In due credit, laser therapy has completely revolutionized the field of dermatology and has completely revolutionized the way we practice medicine. That will only accelerate in the future.”
Dr. Alster emphasized a “safety first” approach to her hopes for the future. “My wish is that we educate people to know that, while lasers have become ubiquitous and we’ve made them safe, they’re still only safe in the right hands,” she said. “There’s not a day that goes by when I don’t have somebody referred to me who’s been mishandled. There’s no reason for that. With proper training, the risk of bad side effects or complications is markedly reduced.”
Combination approach to melasma treatment yields best results
When establishing a treatment plan for patients with melasma, counseling them about realistic expectations is key.
“It’s important that they understand that this is a chronic condition, so it does require long-term maintenance therapy,” Arisa E. Ortiz, MD, said at the virtual annual Masters of Aesthetics Symposium. “We can improve melasma, but it’s difficult to cure melasma.”
While hydroquinone and other bleaching agents are typical treatment mainstays, chemical peels with glycolic acid, trichloroacetic acid, and salicylic acid can benefit some individuals. “For chemical peels, I really like glycolic acid peels because there is no downtime; it peels at the microscopic level,” said Dr. Ortiz, who is director of laser and cosmetic dermatology at the University of California, San Diego. “This is something they may need to repeat monthly, and having a week of peeling may be difficult to go through every month.”
Other common melasma treatments include lasers, intense pulsed light (IPL), and oral medications. “I personally am not impressed with microdermabrasion for melasma, so I don’t use that very much,” she said. “With laser treatment, you want to make sure you’re using low-energy lasers so that it doesn’t exacerbate or make them relapse or rebound.”
While hydroquinone is a mainstay of therapy, “you can’t use it chronically because of the risk of ochronosis (permanent darkening), so you do need to take drug holidays,” Dr. Ortiz said. “During those drug holidays, you want to make sure patients have a nonhydroquinone bleaching agent so that they don’t flare.” Options include lignin peroxidase, oligopeptide, Lytera, Melaplex, 4-n-butylresorcinol, Cysteamine cream, tranexamic acid, and oral antioxidants.
In a study sponsored by SkinMedica, investigators conducted a randomized, double-blind, half-face study in females with moderate to severe facial hyperpigmentation to assess the efficacy and tolerability of three new skin brightener formulations containing SMA-432, a prostaglandin E2 inhibitor, compared with topical 4% hydroquinone (J Drugs Dermatol 2012 Dec;11[12]:1478-82). They found that the nonhydroquinone skin formulations were better tolerated and were just as effective as 4% hydroquinone.
In a separate unpublished study of 22 females, investigators assessed the efficacy of the U.SK Advanced Defense Booster, which contains ferulic acid, maslinic acid, peptides, and olive leaf extract. They observed that 98% of patients saw improvement after 28 days of treatment.
When it comes to using lasers for melasma treatment, low-energy devices provide the best outcomes. “I prefer using something like the 1927-nm fractional diode lasers at 3.75% density, really low densities because there’s less risk for rebound,” Dr. Ortiz said. “They also enhance skin permeability for the use of topicals.”
In an observational study of 27 female patients with refractory melasma, Arielle Kauvar, MD, director of New York Laser & Skin Care, combined microdermabrasion with the Q-switched Nd:YAG (Lasers in Surgery and Medicine 2012; 44:117-24). “The settings she used were very low fluence, so there was no clinical endpoint or no whitening,” Dr. Ortiz said. Specifically, she used a laser at 1.6-2 J/cm2 with a 5- or 6-mm spot size immediately following microdermabrasion for 4 weeks. “She got a good improvement using a skin care regimen of sunscreen, hydroquinone, and tretinoin or vitamin C,” she said. “Remission lasted at least 6 months.”
In a study presented at the 2019 annual meeting of the America Society for Laser Medicine and Surgery, Dr. Ortiz and Tanya Greywal, MD, of the University of California, San Diego, used three passes of the 10764-nm Nd:YAG laser to treat 10 subjects with melasma skin types 2-5. The device has a 650-microsecond pulse duration, a 6-mm spot size, and an energy mode of 11-14 J/cm3. “There was no downtime with these patients, and they saw a mean improvement of 26%-50% as early as 3 weeks,” she said. “Patients did require multiple treatments to see adequate resolution, but no anesthesia or numbing cream was required. This is a good option for patients who need chronic maintenance treatment.”
Topicals also play a key role following the laser treatment of melasma. Dr. Ortiz characterized clobetasol as “kind of like the magic ointment.” She uses one application immediately post procedure “whenever I’m worried about a patient having postinflammatory hyperpigmentation or if I don’t want melasma patients to rebound. It can help reduce swelling and inflammation to decrease the risk of postinflammatory hyperpigmentation.”
Researchers have discovered that there is a vascular component to melasma. Paul M. Friedman, MD, of the Dermatology and Laser Surgery Center, Houston, and his colleagues used spectrocolorimetry to detect an underlying prominent vascular component in 11 patients with melasma (Lasers Surg Med 2017 Jan;49[1]:20-6). They determined that melasma lesions exhibiting subtle or subclinical telangiectatic erythema may be improved by combined vascular-targeted laser therapy together with fractional low-powered diode laser therapy. “A parallel improvement in telangiectatic erythema suggests a relationship between the underlying vasculature and hyperpigmentation,” said Dr. Ortiz, who was not affiliated with the study. “So, patients who have a vascular component to their melasma actually can get improved efficacy.”
Another strategy for melasma patients involves oral treatment with Polypodium leucotomos extract (PLE), a fern from the Polypodiaceae family with antioxidant properties that has been shown to be photoprotective against UVA and UVB radiation. “I like to think of it as an internal sunscreen,” Dr. Ortiz said. “It does not replace your external sunscreen, but it adds extra protection. It has been shown to significantly reduce the severity of sunburn and decrease the risk of UV radiation–induced skin cancer, as well as prevent skin aging.” The purported mechanism of action includes decreasing UV-mediated oxidative damage to DNA, enhancing the activity of endogenous antioxidant systems, increasing the minimal erythema dose, blocking UV radiation–induced cyclooxygenase-2 expression, reducing UV-induced immune suppression, and promoting p53 suppressor gene expression.
In a pilot placebo-controlled study of melasma patients on their normal regimen of hydroquinone and sunscreen, 40 Asian patients with melasma were randomized to receive either oral PLE supplementation or placebo for 12 weeks (J Clin Aesthet Dermatol 2018 Mar;11[3]:14-9). They found that PLE significantly improved and accelerated the outcome reached with hydroquinone and sunscreen from the first month of treatment, compared with placebo.
Dr. Ortiz next discussed the role of oral tranexamic acid, an antifibrinolytic, procoagulant agent that is approved by the Food and Drug Administration for the treatment of menorrhagia and for prevention of hemorrhage in patients with hemophilia undergoing tooth extractions. “It is a synthetic lysine derivative that inhibits plasminogen activation by blocking lysine-binding sites on the plasminogen molecule, and it’s a game changer for melasma treatment,” she said. “One of the side effects is that it inhibits melanogenesis and neovascularization. It’s been effective for melasma, but its use is limited by the risk for thromboembolism. It’s a slight increased risk, something patients should be aware of, but not something that should scare us away from prescribing it.”
In a study of 561 patients with melasma, 90% improved after a median treatment duration of 4 months, and only 7% had side effects (J Am Acad Dermatol 2016;75:385-92). The most common side effects were abdominal bloating and pain. One patient developed a DVT during treatment, but that person was found to have a protein S deficiency.
The daily dosing of tranexamic acid for menorrhagia is 3,900 mg daily, while the dose for melasma has ranged from 500 mg-1,500 mg per day, Dr. Ortiz said. It’s available as a 650-mg pill in the United States. “I prescribe 325 mg twice a day, but studies have shown that 650 mg once a day is just as effective,” she said.
Prior to prescribing tranexamic acid, Dr. Ortiz does not order labs, but she performs an extensive history of present illness. She does not prescribe it in patients with an increased risk of clotting, including people who smoke and those who take oral contraceptives or are on hormone supplementation. Use is also contraindicated in people with a current malignancy, those with a history of stroke or DVT, and those who have any clotting disorder.
She concluded her presentation by noting that she favors a combination approach to treating melasma patients that starts with a broad spectrum sunscreen and PLE. “For bleaching, I like to use 12% hydroquinone with 6% kojic acid in VersaBase,” she said. “Once I get them in better control, then I switch them to 4% hydroquinone for maintenance. I use glycolic peels, low-energy lasers, and tranexamic acid if the melasma is severe, and they have no contraindications. A combination approach really achieves the best results, and counseling is key.”
Dr. Ortiz disclosed having financial relationships with numerous pharmaceutical and device companies. She is also cochair of MOA.
When establishing a treatment plan for patients with melasma, counseling them about realistic expectations is key.
“It’s important that they understand that this is a chronic condition, so it does require long-term maintenance therapy,” Arisa E. Ortiz, MD, said at the virtual annual Masters of Aesthetics Symposium. “We can improve melasma, but it’s difficult to cure melasma.”
While hydroquinone and other bleaching agents are typical treatment mainstays, chemical peels with glycolic acid, trichloroacetic acid, and salicylic acid can benefit some individuals. “For chemical peels, I really like glycolic acid peels because there is no downtime; it peels at the microscopic level,” said Dr. Ortiz, who is director of laser and cosmetic dermatology at the University of California, San Diego. “This is something they may need to repeat monthly, and having a week of peeling may be difficult to go through every month.”
Other common melasma treatments include lasers, intense pulsed light (IPL), and oral medications. “I personally am not impressed with microdermabrasion for melasma, so I don’t use that very much,” she said. “With laser treatment, you want to make sure you’re using low-energy lasers so that it doesn’t exacerbate or make them relapse or rebound.”
While hydroquinone is a mainstay of therapy, “you can’t use it chronically because of the risk of ochronosis (permanent darkening), so you do need to take drug holidays,” Dr. Ortiz said. “During those drug holidays, you want to make sure patients have a nonhydroquinone bleaching agent so that they don’t flare.” Options include lignin peroxidase, oligopeptide, Lytera, Melaplex, 4-n-butylresorcinol, Cysteamine cream, tranexamic acid, and oral antioxidants.
In a study sponsored by SkinMedica, investigators conducted a randomized, double-blind, half-face study in females with moderate to severe facial hyperpigmentation to assess the efficacy and tolerability of three new skin brightener formulations containing SMA-432, a prostaglandin E2 inhibitor, compared with topical 4% hydroquinone (J Drugs Dermatol 2012 Dec;11[12]:1478-82). They found that the nonhydroquinone skin formulations were better tolerated and were just as effective as 4% hydroquinone.
In a separate unpublished study of 22 females, investigators assessed the efficacy of the U.SK Advanced Defense Booster, which contains ferulic acid, maslinic acid, peptides, and olive leaf extract. They observed that 98% of patients saw improvement after 28 days of treatment.
When it comes to using lasers for melasma treatment, low-energy devices provide the best outcomes. “I prefer using something like the 1927-nm fractional diode lasers at 3.75% density, really low densities because there’s less risk for rebound,” Dr. Ortiz said. “They also enhance skin permeability for the use of topicals.”
In an observational study of 27 female patients with refractory melasma, Arielle Kauvar, MD, director of New York Laser & Skin Care, combined microdermabrasion with the Q-switched Nd:YAG (Lasers in Surgery and Medicine 2012; 44:117-24). “The settings she used were very low fluence, so there was no clinical endpoint or no whitening,” Dr. Ortiz said. Specifically, she used a laser at 1.6-2 J/cm2 with a 5- or 6-mm spot size immediately following microdermabrasion for 4 weeks. “She got a good improvement using a skin care regimen of sunscreen, hydroquinone, and tretinoin or vitamin C,” she said. “Remission lasted at least 6 months.”
In a study presented at the 2019 annual meeting of the America Society for Laser Medicine and Surgery, Dr. Ortiz and Tanya Greywal, MD, of the University of California, San Diego, used three passes of the 10764-nm Nd:YAG laser to treat 10 subjects with melasma skin types 2-5. The device has a 650-microsecond pulse duration, a 6-mm spot size, and an energy mode of 11-14 J/cm3. “There was no downtime with these patients, and they saw a mean improvement of 26%-50% as early as 3 weeks,” she said. “Patients did require multiple treatments to see adequate resolution, but no anesthesia or numbing cream was required. This is a good option for patients who need chronic maintenance treatment.”
Topicals also play a key role following the laser treatment of melasma. Dr. Ortiz characterized clobetasol as “kind of like the magic ointment.” She uses one application immediately post procedure “whenever I’m worried about a patient having postinflammatory hyperpigmentation or if I don’t want melasma patients to rebound. It can help reduce swelling and inflammation to decrease the risk of postinflammatory hyperpigmentation.”
Researchers have discovered that there is a vascular component to melasma. Paul M. Friedman, MD, of the Dermatology and Laser Surgery Center, Houston, and his colleagues used spectrocolorimetry to detect an underlying prominent vascular component in 11 patients with melasma (Lasers Surg Med 2017 Jan;49[1]:20-6). They determined that melasma lesions exhibiting subtle or subclinical telangiectatic erythema may be improved by combined vascular-targeted laser therapy together with fractional low-powered diode laser therapy. “A parallel improvement in telangiectatic erythema suggests a relationship between the underlying vasculature and hyperpigmentation,” said Dr. Ortiz, who was not affiliated with the study. “So, patients who have a vascular component to their melasma actually can get improved efficacy.”
Another strategy for melasma patients involves oral treatment with Polypodium leucotomos extract (PLE), a fern from the Polypodiaceae family with antioxidant properties that has been shown to be photoprotective against UVA and UVB radiation. “I like to think of it as an internal sunscreen,” Dr. Ortiz said. “It does not replace your external sunscreen, but it adds extra protection. It has been shown to significantly reduce the severity of sunburn and decrease the risk of UV radiation–induced skin cancer, as well as prevent skin aging.” The purported mechanism of action includes decreasing UV-mediated oxidative damage to DNA, enhancing the activity of endogenous antioxidant systems, increasing the minimal erythema dose, blocking UV radiation–induced cyclooxygenase-2 expression, reducing UV-induced immune suppression, and promoting p53 suppressor gene expression.
In a pilot placebo-controlled study of melasma patients on their normal regimen of hydroquinone and sunscreen, 40 Asian patients with melasma were randomized to receive either oral PLE supplementation or placebo for 12 weeks (J Clin Aesthet Dermatol 2018 Mar;11[3]:14-9). They found that PLE significantly improved and accelerated the outcome reached with hydroquinone and sunscreen from the first month of treatment, compared with placebo.
Dr. Ortiz next discussed the role of oral tranexamic acid, an antifibrinolytic, procoagulant agent that is approved by the Food and Drug Administration for the treatment of menorrhagia and for prevention of hemorrhage in patients with hemophilia undergoing tooth extractions. “It is a synthetic lysine derivative that inhibits plasminogen activation by blocking lysine-binding sites on the plasminogen molecule, and it’s a game changer for melasma treatment,” she said. “One of the side effects is that it inhibits melanogenesis and neovascularization. It’s been effective for melasma, but its use is limited by the risk for thromboembolism. It’s a slight increased risk, something patients should be aware of, but not something that should scare us away from prescribing it.”
In a study of 561 patients with melasma, 90% improved after a median treatment duration of 4 months, and only 7% had side effects (J Am Acad Dermatol 2016;75:385-92). The most common side effects were abdominal bloating and pain. One patient developed a DVT during treatment, but that person was found to have a protein S deficiency.
The daily dosing of tranexamic acid for menorrhagia is 3,900 mg daily, while the dose for melasma has ranged from 500 mg-1,500 mg per day, Dr. Ortiz said. It’s available as a 650-mg pill in the United States. “I prescribe 325 mg twice a day, but studies have shown that 650 mg once a day is just as effective,” she said.
Prior to prescribing tranexamic acid, Dr. Ortiz does not order labs, but she performs an extensive history of present illness. She does not prescribe it in patients with an increased risk of clotting, including people who smoke and those who take oral contraceptives or are on hormone supplementation. Use is also contraindicated in people with a current malignancy, those with a history of stroke or DVT, and those who have any clotting disorder.
She concluded her presentation by noting that she favors a combination approach to treating melasma patients that starts with a broad spectrum sunscreen and PLE. “For bleaching, I like to use 12% hydroquinone with 6% kojic acid in VersaBase,” she said. “Once I get them in better control, then I switch them to 4% hydroquinone for maintenance. I use glycolic peels, low-energy lasers, and tranexamic acid if the melasma is severe, and they have no contraindications. A combination approach really achieves the best results, and counseling is key.”
Dr. Ortiz disclosed having financial relationships with numerous pharmaceutical and device companies. She is also cochair of MOA.
When establishing a treatment plan for patients with melasma, counseling them about realistic expectations is key.
“It’s important that they understand that this is a chronic condition, so it does require long-term maintenance therapy,” Arisa E. Ortiz, MD, said at the virtual annual Masters of Aesthetics Symposium. “We can improve melasma, but it’s difficult to cure melasma.”
While hydroquinone and other bleaching agents are typical treatment mainstays, chemical peels with glycolic acid, trichloroacetic acid, and salicylic acid can benefit some individuals. “For chemical peels, I really like glycolic acid peels because there is no downtime; it peels at the microscopic level,” said Dr. Ortiz, who is director of laser and cosmetic dermatology at the University of California, San Diego. “This is something they may need to repeat monthly, and having a week of peeling may be difficult to go through every month.”
Other common melasma treatments include lasers, intense pulsed light (IPL), and oral medications. “I personally am not impressed with microdermabrasion for melasma, so I don’t use that very much,” she said. “With laser treatment, you want to make sure you’re using low-energy lasers so that it doesn’t exacerbate or make them relapse or rebound.”
While hydroquinone is a mainstay of therapy, “you can’t use it chronically because of the risk of ochronosis (permanent darkening), so you do need to take drug holidays,” Dr. Ortiz said. “During those drug holidays, you want to make sure patients have a nonhydroquinone bleaching agent so that they don’t flare.” Options include lignin peroxidase, oligopeptide, Lytera, Melaplex, 4-n-butylresorcinol, Cysteamine cream, tranexamic acid, and oral antioxidants.
In a study sponsored by SkinMedica, investigators conducted a randomized, double-blind, half-face study in females with moderate to severe facial hyperpigmentation to assess the efficacy and tolerability of three new skin brightener formulations containing SMA-432, a prostaglandin E2 inhibitor, compared with topical 4% hydroquinone (J Drugs Dermatol 2012 Dec;11[12]:1478-82). They found that the nonhydroquinone skin formulations were better tolerated and were just as effective as 4% hydroquinone.
In a separate unpublished study of 22 females, investigators assessed the efficacy of the U.SK Advanced Defense Booster, which contains ferulic acid, maslinic acid, peptides, and olive leaf extract. They observed that 98% of patients saw improvement after 28 days of treatment.
When it comes to using lasers for melasma treatment, low-energy devices provide the best outcomes. “I prefer using something like the 1927-nm fractional diode lasers at 3.75% density, really low densities because there’s less risk for rebound,” Dr. Ortiz said. “They also enhance skin permeability for the use of topicals.”
In an observational study of 27 female patients with refractory melasma, Arielle Kauvar, MD, director of New York Laser & Skin Care, combined microdermabrasion with the Q-switched Nd:YAG (Lasers in Surgery and Medicine 2012; 44:117-24). “The settings she used were very low fluence, so there was no clinical endpoint or no whitening,” Dr. Ortiz said. Specifically, she used a laser at 1.6-2 J/cm2 with a 5- or 6-mm spot size immediately following microdermabrasion for 4 weeks. “She got a good improvement using a skin care regimen of sunscreen, hydroquinone, and tretinoin or vitamin C,” she said. “Remission lasted at least 6 months.”
In a study presented at the 2019 annual meeting of the America Society for Laser Medicine and Surgery, Dr. Ortiz and Tanya Greywal, MD, of the University of California, San Diego, used three passes of the 10764-nm Nd:YAG laser to treat 10 subjects with melasma skin types 2-5. The device has a 650-microsecond pulse duration, a 6-mm spot size, and an energy mode of 11-14 J/cm3. “There was no downtime with these patients, and they saw a mean improvement of 26%-50% as early as 3 weeks,” she said. “Patients did require multiple treatments to see adequate resolution, but no anesthesia or numbing cream was required. This is a good option for patients who need chronic maintenance treatment.”
Topicals also play a key role following the laser treatment of melasma. Dr. Ortiz characterized clobetasol as “kind of like the magic ointment.” She uses one application immediately post procedure “whenever I’m worried about a patient having postinflammatory hyperpigmentation or if I don’t want melasma patients to rebound. It can help reduce swelling and inflammation to decrease the risk of postinflammatory hyperpigmentation.”
Researchers have discovered that there is a vascular component to melasma. Paul M. Friedman, MD, of the Dermatology and Laser Surgery Center, Houston, and his colleagues used spectrocolorimetry to detect an underlying prominent vascular component in 11 patients with melasma (Lasers Surg Med 2017 Jan;49[1]:20-6). They determined that melasma lesions exhibiting subtle or subclinical telangiectatic erythema may be improved by combined vascular-targeted laser therapy together with fractional low-powered diode laser therapy. “A parallel improvement in telangiectatic erythema suggests a relationship between the underlying vasculature and hyperpigmentation,” said Dr. Ortiz, who was not affiliated with the study. “So, patients who have a vascular component to their melasma actually can get improved efficacy.”
Another strategy for melasma patients involves oral treatment with Polypodium leucotomos extract (PLE), a fern from the Polypodiaceae family with antioxidant properties that has been shown to be photoprotective against UVA and UVB radiation. “I like to think of it as an internal sunscreen,” Dr. Ortiz said. “It does not replace your external sunscreen, but it adds extra protection. It has been shown to significantly reduce the severity of sunburn and decrease the risk of UV radiation–induced skin cancer, as well as prevent skin aging.” The purported mechanism of action includes decreasing UV-mediated oxidative damage to DNA, enhancing the activity of endogenous antioxidant systems, increasing the minimal erythema dose, blocking UV radiation–induced cyclooxygenase-2 expression, reducing UV-induced immune suppression, and promoting p53 suppressor gene expression.
In a pilot placebo-controlled study of melasma patients on their normal regimen of hydroquinone and sunscreen, 40 Asian patients with melasma were randomized to receive either oral PLE supplementation or placebo for 12 weeks (J Clin Aesthet Dermatol 2018 Mar;11[3]:14-9). They found that PLE significantly improved and accelerated the outcome reached with hydroquinone and sunscreen from the first month of treatment, compared with placebo.
Dr. Ortiz next discussed the role of oral tranexamic acid, an antifibrinolytic, procoagulant agent that is approved by the Food and Drug Administration for the treatment of menorrhagia and for prevention of hemorrhage in patients with hemophilia undergoing tooth extractions. “It is a synthetic lysine derivative that inhibits plasminogen activation by blocking lysine-binding sites on the plasminogen molecule, and it’s a game changer for melasma treatment,” she said. “One of the side effects is that it inhibits melanogenesis and neovascularization. It’s been effective for melasma, but its use is limited by the risk for thromboembolism. It’s a slight increased risk, something patients should be aware of, but not something that should scare us away from prescribing it.”
In a study of 561 patients with melasma, 90% improved after a median treatment duration of 4 months, and only 7% had side effects (J Am Acad Dermatol 2016;75:385-92). The most common side effects were abdominal bloating and pain. One patient developed a DVT during treatment, but that person was found to have a protein S deficiency.
The daily dosing of tranexamic acid for menorrhagia is 3,900 mg daily, while the dose for melasma has ranged from 500 mg-1,500 mg per day, Dr. Ortiz said. It’s available as a 650-mg pill in the United States. “I prescribe 325 mg twice a day, but studies have shown that 650 mg once a day is just as effective,” she said.
Prior to prescribing tranexamic acid, Dr. Ortiz does not order labs, but she performs an extensive history of present illness. She does not prescribe it in patients with an increased risk of clotting, including people who smoke and those who take oral contraceptives or are on hormone supplementation. Use is also contraindicated in people with a current malignancy, those with a history of stroke or DVT, and those who have any clotting disorder.
She concluded her presentation by noting that she favors a combination approach to treating melasma patients that starts with a broad spectrum sunscreen and PLE. “For bleaching, I like to use 12% hydroquinone with 6% kojic acid in VersaBase,” she said. “Once I get them in better control, then I switch them to 4% hydroquinone for maintenance. I use glycolic peels, low-energy lasers, and tranexamic acid if the melasma is severe, and they have no contraindications. A combination approach really achieves the best results, and counseling is key.”
Dr. Ortiz disclosed having financial relationships with numerous pharmaceutical and device companies. She is also cochair of MOA.
EXPERT ANALYSIS FROM MOA 2020