User login
Doug Brunk is a San Diego-based award-winning reporter who began covering health care in 1991. Before joining the company, he wrote for the health sciences division of Columbia University and was an associate editor at Contemporary Long Term Care magazine when it won a Jesse H. Neal Award. His work has been syndicated by the Los Angeles Times and he is the author of two books related to the University of Kentucky Wildcats men's basketball program. Doug has a master’s degree in magazine journalism from the S.I. Newhouse School of Public Communications at Syracuse University. Follow him on Twitter @dougbrunk.
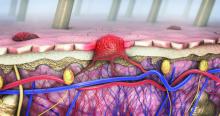
Imaging techniques will revolutionize cancer detection, expert predicts
PHOENIX –
In a lecture during a multispecialty roundup of cutting-edge energy-based device applications at the annual conference of the American Society for Laser Medicine and Surgery, Dr. Barton, a biomedical engineer who directs the BIO5 Institute at the University of Arizona, Tucson, said that while no current modality exists to enable physicians in dermatology and other specialties to view internal structures throughout the entire body with cellular resolution, refining existing technologies is a good way to start.
In 2011, renowned cancer researchers Douglas Hanahan, PhD, and Robert A. Weinberg, PhD, proposed six hallmarks of cancer, which include sustaining proliferative signaling, evading growth suppressors, resisting cell death, enabling replicative immortality, inducing angiogenesis, and activating invasion and metastasis. Each hallmark poses unique imaging challenges. For example, enabling replicative immortality “means that the cell nuclei change size and shape; they change their position,” said Dr. Barton, who is also professor of biomedical engineering and optical sciences at the university. “If we want to see that, we’re going to need an imaging modality that’s subcellular in resolution.”
Similarly, if clinicians want to view how proliferative signaling is changing, “that means being able to visualize the cell surface receptors; those are even smaller to actually visualize,” she said. “But we have technologies where we can target those receptors with fluorophores. And then we can look at large areas very quickly.” Meanwhile, the ability of cancer cells to resist cell death and evade growth suppressors often results in thickening of epithelium throughout the body. “So, if we can measure the thickness of the epithelium, we can see that there’s something wrong with that tissue,” she said.
As for cancer’s propensity for invasion and metastasis, “here, we’re looking at how the collagen structure [between the cells] has changed and whether there’s layer breakdown or not. Optical imaging can detect cancer. However, high resolution optical techniques can only image about 1 mm deep, so unless you’re looking at the skin or the eye, you’re going to have to develop an endoscope to be able to view these hallmarks.”
OCT images the tissue microstructure, generally in a resolution of 2-20 microns, at a depth of 1-2 mm, and it measures reflected light. When possible, Dr. Barton combines OCT with laser-induced fluorescence for enhanced accuracy of detection of cancer. Induced fluorescence senses molecular information with the natural fluorophores in the body or with targeted exogenous agents. Then there’s multiphoton microscopy, an advanced imaging technique that enables clinicians to view cellular and subcellular events within living tissue. Early models of this technology “took up entire benches” in physics labs, Dr. Barton said, but she and other investigators are designing smaller devices for use in clinics. “This is exciting, because not only do we [view] subcellular structure with this modality, but it can also be highly sensitive to collagen structure,” she said.
Ovarian cancer model
In a model of ovarian cancer, she and colleagues externalized the ovaries of a mouse, imaged the organs, put them back in, and reassessed them at 8 weeks. “This model develops cancer very quickly,” said Dr. Barton, who once worked for McDonnell Douglas on the Space Station program. At 8 weeks, using fluorescence and targeted agents with a tabletop multiphoton microscopy system, they observed that the proliferation signals of cancer had begun. “So, with an agent targeted to the folate receptor or to other receptors that are implicated in cancer development, we can see that ovaries and fallopian tubes are lighting up,” she said.
With proof of concept established with the mouse study, she and other researchers are drawing from technological advances to create tiny laser systems for use in the clinic to image a variety of structures in the human body. Optics advances include bulk optics and all-fiber designs where engineers can create an imaging probe that’s only 125 microns in diameter, “or maybe even as small as 70 microns in diameter,” she said. “We can do fabrications on the tips of endoscopes to redirect the light and focus it. We can also do 3-D printing and spiral scanning to create miniature devices to make new advances. That means that instead of just white light imaging of the colon or the lung like we have had in the past, we can start moving into smaller structures, such as the eustachian tube, the fallopian tube, the bile ducts, or making miniature devices for brain biopsies, lung biopsies, and maybe being able to get into bronchioles and arterioles.”
According to Dr. Barton, prior research has demonstrated that cerebral vasculature can be imaged with a catheter 400 microns in diameter, the spaces in the lungs can be imaged with a needle that is 310 microns in diameter, and the inner structures of the eustachian tube can be viewed with an endoscope 1 mm in diameter.
She and her colleagues are developing an OCT/fluorescence imaging falloposcope that is 0.8 mm in diameter, flexible, and steerable, as a tool for early detection of ovarian cancer in humans. “It’s now known that most ovarian cancer starts in the fallopian tubes,” Dr. Barton said. “It’s metastatic disease when those cells break off from the fallopian tubes and go to the ovaries. We wanted to create an imaging system where we created a fiber bundle that we could navigate with white light and with fluorescence so that we can see these early stages of cancer [and] how they fluoresce differently. We also wanted to have an OCT system so that we could image through the wall of the fallopian tube and look for that layer thickening and other precursors to ovarian cancer.”
To date, in vivo testing in healthy women has demonstrated that the miniature endoscope is able to reach the fallopian tubes through the natural orifice of the vagina and uterus. “That is pretty exciting,” she said. “The images may not be of the highest quality, but we are advancing.”
Dr. Barton reported having no relevant financial disclosures.
PHOENIX –
In a lecture during a multispecialty roundup of cutting-edge energy-based device applications at the annual conference of the American Society for Laser Medicine and Surgery, Dr. Barton, a biomedical engineer who directs the BIO5 Institute at the University of Arizona, Tucson, said that while no current modality exists to enable physicians in dermatology and other specialties to view internal structures throughout the entire body with cellular resolution, refining existing technologies is a good way to start.
In 2011, renowned cancer researchers Douglas Hanahan, PhD, and Robert A. Weinberg, PhD, proposed six hallmarks of cancer, which include sustaining proliferative signaling, evading growth suppressors, resisting cell death, enabling replicative immortality, inducing angiogenesis, and activating invasion and metastasis. Each hallmark poses unique imaging challenges. For example, enabling replicative immortality “means that the cell nuclei change size and shape; they change their position,” said Dr. Barton, who is also professor of biomedical engineering and optical sciences at the university. “If we want to see that, we’re going to need an imaging modality that’s subcellular in resolution.”
Similarly, if clinicians want to view how proliferative signaling is changing, “that means being able to visualize the cell surface receptors; those are even smaller to actually visualize,” she said. “But we have technologies where we can target those receptors with fluorophores. And then we can look at large areas very quickly.” Meanwhile, the ability of cancer cells to resist cell death and evade growth suppressors often results in thickening of epithelium throughout the body. “So, if we can measure the thickness of the epithelium, we can see that there’s something wrong with that tissue,” she said.
As for cancer’s propensity for invasion and metastasis, “here, we’re looking at how the collagen structure [between the cells] has changed and whether there’s layer breakdown or not. Optical imaging can detect cancer. However, high resolution optical techniques can only image about 1 mm deep, so unless you’re looking at the skin or the eye, you’re going to have to develop an endoscope to be able to view these hallmarks.”
OCT images the tissue microstructure, generally in a resolution of 2-20 microns, at a depth of 1-2 mm, and it measures reflected light. When possible, Dr. Barton combines OCT with laser-induced fluorescence for enhanced accuracy of detection of cancer. Induced fluorescence senses molecular information with the natural fluorophores in the body or with targeted exogenous agents. Then there’s multiphoton microscopy, an advanced imaging technique that enables clinicians to view cellular and subcellular events within living tissue. Early models of this technology “took up entire benches” in physics labs, Dr. Barton said, but she and other investigators are designing smaller devices for use in clinics. “This is exciting, because not only do we [view] subcellular structure with this modality, but it can also be highly sensitive to collagen structure,” she said.
Ovarian cancer model
In a model of ovarian cancer, she and colleagues externalized the ovaries of a mouse, imaged the organs, put them back in, and reassessed them at 8 weeks. “This model develops cancer very quickly,” said Dr. Barton, who once worked for McDonnell Douglas on the Space Station program. At 8 weeks, using fluorescence and targeted agents with a tabletop multiphoton microscopy system, they observed that the proliferation signals of cancer had begun. “So, with an agent targeted to the folate receptor or to other receptors that are implicated in cancer development, we can see that ovaries and fallopian tubes are lighting up,” she said.
With proof of concept established with the mouse study, she and other researchers are drawing from technological advances to create tiny laser systems for use in the clinic to image a variety of structures in the human body. Optics advances include bulk optics and all-fiber designs where engineers can create an imaging probe that’s only 125 microns in diameter, “or maybe even as small as 70 microns in diameter,” she said. “We can do fabrications on the tips of endoscopes to redirect the light and focus it. We can also do 3-D printing and spiral scanning to create miniature devices to make new advances. That means that instead of just white light imaging of the colon or the lung like we have had in the past, we can start moving into smaller structures, such as the eustachian tube, the fallopian tube, the bile ducts, or making miniature devices for brain biopsies, lung biopsies, and maybe being able to get into bronchioles and arterioles.”
According to Dr. Barton, prior research has demonstrated that cerebral vasculature can be imaged with a catheter 400 microns in diameter, the spaces in the lungs can be imaged with a needle that is 310 microns in diameter, and the inner structures of the eustachian tube can be viewed with an endoscope 1 mm in diameter.
She and her colleagues are developing an OCT/fluorescence imaging falloposcope that is 0.8 mm in diameter, flexible, and steerable, as a tool for early detection of ovarian cancer in humans. “It’s now known that most ovarian cancer starts in the fallopian tubes,” Dr. Barton said. “It’s metastatic disease when those cells break off from the fallopian tubes and go to the ovaries. We wanted to create an imaging system where we created a fiber bundle that we could navigate with white light and with fluorescence so that we can see these early stages of cancer [and] how they fluoresce differently. We also wanted to have an OCT system so that we could image through the wall of the fallopian tube and look for that layer thickening and other precursors to ovarian cancer.”
To date, in vivo testing in healthy women has demonstrated that the miniature endoscope is able to reach the fallopian tubes through the natural orifice of the vagina and uterus. “That is pretty exciting,” she said. “The images may not be of the highest quality, but we are advancing.”
Dr. Barton reported having no relevant financial disclosures.
PHOENIX –
In a lecture during a multispecialty roundup of cutting-edge energy-based device applications at the annual conference of the American Society for Laser Medicine and Surgery, Dr. Barton, a biomedical engineer who directs the BIO5 Institute at the University of Arizona, Tucson, said that while no current modality exists to enable physicians in dermatology and other specialties to view internal structures throughout the entire body with cellular resolution, refining existing technologies is a good way to start.
In 2011, renowned cancer researchers Douglas Hanahan, PhD, and Robert A. Weinberg, PhD, proposed six hallmarks of cancer, which include sustaining proliferative signaling, evading growth suppressors, resisting cell death, enabling replicative immortality, inducing angiogenesis, and activating invasion and metastasis. Each hallmark poses unique imaging challenges. For example, enabling replicative immortality “means that the cell nuclei change size and shape; they change their position,” said Dr. Barton, who is also professor of biomedical engineering and optical sciences at the university. “If we want to see that, we’re going to need an imaging modality that’s subcellular in resolution.”
Similarly, if clinicians want to view how proliferative signaling is changing, “that means being able to visualize the cell surface receptors; those are even smaller to actually visualize,” she said. “But we have technologies where we can target those receptors with fluorophores. And then we can look at large areas very quickly.” Meanwhile, the ability of cancer cells to resist cell death and evade growth suppressors often results in thickening of epithelium throughout the body. “So, if we can measure the thickness of the epithelium, we can see that there’s something wrong with that tissue,” she said.
As for cancer’s propensity for invasion and metastasis, “here, we’re looking at how the collagen structure [between the cells] has changed and whether there’s layer breakdown or not. Optical imaging can detect cancer. However, high resolution optical techniques can only image about 1 mm deep, so unless you’re looking at the skin or the eye, you’re going to have to develop an endoscope to be able to view these hallmarks.”
OCT images the tissue microstructure, generally in a resolution of 2-20 microns, at a depth of 1-2 mm, and it measures reflected light. When possible, Dr. Barton combines OCT with laser-induced fluorescence for enhanced accuracy of detection of cancer. Induced fluorescence senses molecular information with the natural fluorophores in the body or with targeted exogenous agents. Then there’s multiphoton microscopy, an advanced imaging technique that enables clinicians to view cellular and subcellular events within living tissue. Early models of this technology “took up entire benches” in physics labs, Dr. Barton said, but she and other investigators are designing smaller devices for use in clinics. “This is exciting, because not only do we [view] subcellular structure with this modality, but it can also be highly sensitive to collagen structure,” she said.
Ovarian cancer model
In a model of ovarian cancer, she and colleagues externalized the ovaries of a mouse, imaged the organs, put them back in, and reassessed them at 8 weeks. “This model develops cancer very quickly,” said Dr. Barton, who once worked for McDonnell Douglas on the Space Station program. At 8 weeks, using fluorescence and targeted agents with a tabletop multiphoton microscopy system, they observed that the proliferation signals of cancer had begun. “So, with an agent targeted to the folate receptor or to other receptors that are implicated in cancer development, we can see that ovaries and fallopian tubes are lighting up,” she said.
With proof of concept established with the mouse study, she and other researchers are drawing from technological advances to create tiny laser systems for use in the clinic to image a variety of structures in the human body. Optics advances include bulk optics and all-fiber designs where engineers can create an imaging probe that’s only 125 microns in diameter, “or maybe even as small as 70 microns in diameter,” she said. “We can do fabrications on the tips of endoscopes to redirect the light and focus it. We can also do 3-D printing and spiral scanning to create miniature devices to make new advances. That means that instead of just white light imaging of the colon or the lung like we have had in the past, we can start moving into smaller structures, such as the eustachian tube, the fallopian tube, the bile ducts, or making miniature devices for brain biopsies, lung biopsies, and maybe being able to get into bronchioles and arterioles.”
According to Dr. Barton, prior research has demonstrated that cerebral vasculature can be imaged with a catheter 400 microns in diameter, the spaces in the lungs can be imaged with a needle that is 310 microns in diameter, and the inner structures of the eustachian tube can be viewed with an endoscope 1 mm in diameter.
She and her colleagues are developing an OCT/fluorescence imaging falloposcope that is 0.8 mm in diameter, flexible, and steerable, as a tool for early detection of ovarian cancer in humans. “It’s now known that most ovarian cancer starts in the fallopian tubes,” Dr. Barton said. “It’s metastatic disease when those cells break off from the fallopian tubes and go to the ovaries. We wanted to create an imaging system where we created a fiber bundle that we could navigate with white light and with fluorescence so that we can see these early stages of cancer [and] how they fluoresce differently. We also wanted to have an OCT system so that we could image through the wall of the fallopian tube and look for that layer thickening and other precursors to ovarian cancer.”
To date, in vivo testing in healthy women has demonstrated that the miniature endoscope is able to reach the fallopian tubes through the natural orifice of the vagina and uterus. “That is pretty exciting,” she said. “The images may not be of the highest quality, but we are advancing.”
Dr. Barton reported having no relevant financial disclosures.
AT ASLMS 2023
Prognostic factors of SCCs in organ transplant recipients worse compared with general population
, results from a dual cohort study demonstrated.
The findings build on previous research and underscore the need for early diagnosis and aggressive surveillance in this patient population, corresponding author Adele C. Green, MBBS, PhD, professor and senior scientist at the QIMR Berghofer Medical Research Institute, Brisbane, Queensland, Australia, and colleagues wrote in the study, which was published online in JAMA Dermatology. “Squamous cell carcinomas (SCCs) of the skin develop up to 77 times more frequently in immunosuppressed organ transplant recipients (OTRs) than the general population,” they wrote. “Because SCCs cause substantially more morbidity and death in the former, they are postulated to be innately more aggressive than in immunocompetent patients, but OTRs’ higher SCC mortality may simply reflect greater SCC tumor burdens per patient.”
In what is believed to be the first study of its kind, Dr. Green and colleagues drew data from two cohort studies to evaluate five key clinicopathologic indicators of poor SCC outcomes in organ transplant recipients, and in those from the general population in Queensland, Australia: cephalic location, perineural invasion, invasion to/beyond subcutaneous fat, poor differentiation, and tumor size greater than 20 mm. The study population included organ transplant recipients at high risk of skin cancer, who were enrolled in the Skin Tumours in Allograft Recipients (STAR) study, and those from a population-based cohort, the QSkin Sun and Health Study. STAR consisted of lung transplant recipients and kidney and liver transplant recipients at high risk of skin cancer who were recruited from tertiary centers and diagnosed with histopathologically confirmed SCC from 2012 to 2015. QSkin consisted of individuals from Queensland’s general adult population diagnosed with SCCs from 2012 to 2015.
SCC cases in QSkin were ascertained through Australia’s universal health insurance agency and linked with histopathology records. Next, the researchers performed data analysis from both cohort studies to determine the prevalence ratio (PR) of head/neck location, perineural invasion, tumor invasion to/beyond subcutaneous fat, poor cellular differentiation, and tumor diameter greater than 20 mm among SCCs among organ transplant recipients compared with the general population.
After combining the two studies, the researchers compared 741 SCCs excised from 191 organ transplant recipients and 2,558 SCCs excised from 1,507 individuals in the general population. Their median ages were similar (62.7 and 63.7 years, respectively) and most were male (78% and 63.4%, respectively).
As for site of involvement, SCCs developed most often on the head and neck in the transplant recipients (38.6%) and on the arms and hands in the general population (35.2%). After adjustment for age and sex, perineural invasion of SCCs was more than twice as common in transplant recipients than among cases in the general population, as was invasion to/beyond subcutaneous fat (PR of 2.37 for both associations).
In other findings, compared with SCCs in the general population, poorly vs. well-differentiated SCCs were more than threefold more common in transplant recipients (PR, 3.45), while the prevalence of tumors greater than 20 mm vs. 20 mm or smaller was moderately higher in transplant recipients (PR, 1.52).
“These findings are considered generalizable, confirming that OTRs’ poorer SCC outcomes are associated with not only their sheer numbers of SCC tumors, but also with a strong shift toward more invasive, less differentiated, and larger SCC tumors, in agreement with previous findings,” the researchers wrote. “This shift is likely associated with decreased immunosurveillance resulting from immunosuppressive therapy (since carcinogenesis decelerates with therapy cessation) interacting with effects of high UV radiation exposure.”
They acknowledged certain limitations of their analysis, chiefly the lack of central review of SCCs to ensure standard assessment of histopathologic features “including caliber of nerves with perineural invasion and cell differentiation; such a review would not have been feasible logistically.”
The study was supported by grants from the National Health and Medical Research Council of Australia. The researchers reported having no disclosures related to the submitted work.
, results from a dual cohort study demonstrated.
The findings build on previous research and underscore the need for early diagnosis and aggressive surveillance in this patient population, corresponding author Adele C. Green, MBBS, PhD, professor and senior scientist at the QIMR Berghofer Medical Research Institute, Brisbane, Queensland, Australia, and colleagues wrote in the study, which was published online in JAMA Dermatology. “Squamous cell carcinomas (SCCs) of the skin develop up to 77 times more frequently in immunosuppressed organ transplant recipients (OTRs) than the general population,” they wrote. “Because SCCs cause substantially more morbidity and death in the former, they are postulated to be innately more aggressive than in immunocompetent patients, but OTRs’ higher SCC mortality may simply reflect greater SCC tumor burdens per patient.”
In what is believed to be the first study of its kind, Dr. Green and colleagues drew data from two cohort studies to evaluate five key clinicopathologic indicators of poor SCC outcomes in organ transplant recipients, and in those from the general population in Queensland, Australia: cephalic location, perineural invasion, invasion to/beyond subcutaneous fat, poor differentiation, and tumor size greater than 20 mm. The study population included organ transplant recipients at high risk of skin cancer, who were enrolled in the Skin Tumours in Allograft Recipients (STAR) study, and those from a population-based cohort, the QSkin Sun and Health Study. STAR consisted of lung transplant recipients and kidney and liver transplant recipients at high risk of skin cancer who were recruited from tertiary centers and diagnosed with histopathologically confirmed SCC from 2012 to 2015. QSkin consisted of individuals from Queensland’s general adult population diagnosed with SCCs from 2012 to 2015.
SCC cases in QSkin were ascertained through Australia’s universal health insurance agency and linked with histopathology records. Next, the researchers performed data analysis from both cohort studies to determine the prevalence ratio (PR) of head/neck location, perineural invasion, tumor invasion to/beyond subcutaneous fat, poor cellular differentiation, and tumor diameter greater than 20 mm among SCCs among organ transplant recipients compared with the general population.
After combining the two studies, the researchers compared 741 SCCs excised from 191 organ transplant recipients and 2,558 SCCs excised from 1,507 individuals in the general population. Their median ages were similar (62.7 and 63.7 years, respectively) and most were male (78% and 63.4%, respectively).
As for site of involvement, SCCs developed most often on the head and neck in the transplant recipients (38.6%) and on the arms and hands in the general population (35.2%). After adjustment for age and sex, perineural invasion of SCCs was more than twice as common in transplant recipients than among cases in the general population, as was invasion to/beyond subcutaneous fat (PR of 2.37 for both associations).
In other findings, compared with SCCs in the general population, poorly vs. well-differentiated SCCs were more than threefold more common in transplant recipients (PR, 3.45), while the prevalence of tumors greater than 20 mm vs. 20 mm or smaller was moderately higher in transplant recipients (PR, 1.52).
“These findings are considered generalizable, confirming that OTRs’ poorer SCC outcomes are associated with not only their sheer numbers of SCC tumors, but also with a strong shift toward more invasive, less differentiated, and larger SCC tumors, in agreement with previous findings,” the researchers wrote. “This shift is likely associated with decreased immunosurveillance resulting from immunosuppressive therapy (since carcinogenesis decelerates with therapy cessation) interacting with effects of high UV radiation exposure.”
They acknowledged certain limitations of their analysis, chiefly the lack of central review of SCCs to ensure standard assessment of histopathologic features “including caliber of nerves with perineural invasion and cell differentiation; such a review would not have been feasible logistically.”
The study was supported by grants from the National Health and Medical Research Council of Australia. The researchers reported having no disclosures related to the submitted work.
, results from a dual cohort study demonstrated.
The findings build on previous research and underscore the need for early diagnosis and aggressive surveillance in this patient population, corresponding author Adele C. Green, MBBS, PhD, professor and senior scientist at the QIMR Berghofer Medical Research Institute, Brisbane, Queensland, Australia, and colleagues wrote in the study, which was published online in JAMA Dermatology. “Squamous cell carcinomas (SCCs) of the skin develop up to 77 times more frequently in immunosuppressed organ transplant recipients (OTRs) than the general population,” they wrote. “Because SCCs cause substantially more morbidity and death in the former, they are postulated to be innately more aggressive than in immunocompetent patients, but OTRs’ higher SCC mortality may simply reflect greater SCC tumor burdens per patient.”
In what is believed to be the first study of its kind, Dr. Green and colleagues drew data from two cohort studies to evaluate five key clinicopathologic indicators of poor SCC outcomes in organ transplant recipients, and in those from the general population in Queensland, Australia: cephalic location, perineural invasion, invasion to/beyond subcutaneous fat, poor differentiation, and tumor size greater than 20 mm. The study population included organ transplant recipients at high risk of skin cancer, who were enrolled in the Skin Tumours in Allograft Recipients (STAR) study, and those from a population-based cohort, the QSkin Sun and Health Study. STAR consisted of lung transplant recipients and kidney and liver transplant recipients at high risk of skin cancer who were recruited from tertiary centers and diagnosed with histopathologically confirmed SCC from 2012 to 2015. QSkin consisted of individuals from Queensland’s general adult population diagnosed with SCCs from 2012 to 2015.
SCC cases in QSkin were ascertained through Australia’s universal health insurance agency and linked with histopathology records. Next, the researchers performed data analysis from both cohort studies to determine the prevalence ratio (PR) of head/neck location, perineural invasion, tumor invasion to/beyond subcutaneous fat, poor cellular differentiation, and tumor diameter greater than 20 mm among SCCs among organ transplant recipients compared with the general population.
After combining the two studies, the researchers compared 741 SCCs excised from 191 organ transplant recipients and 2,558 SCCs excised from 1,507 individuals in the general population. Their median ages were similar (62.7 and 63.7 years, respectively) and most were male (78% and 63.4%, respectively).
As for site of involvement, SCCs developed most often on the head and neck in the transplant recipients (38.6%) and on the arms and hands in the general population (35.2%). After adjustment for age and sex, perineural invasion of SCCs was more than twice as common in transplant recipients than among cases in the general population, as was invasion to/beyond subcutaneous fat (PR of 2.37 for both associations).
In other findings, compared with SCCs in the general population, poorly vs. well-differentiated SCCs were more than threefold more common in transplant recipients (PR, 3.45), while the prevalence of tumors greater than 20 mm vs. 20 mm or smaller was moderately higher in transplant recipients (PR, 1.52).
“These findings are considered generalizable, confirming that OTRs’ poorer SCC outcomes are associated with not only their sheer numbers of SCC tumors, but also with a strong shift toward more invasive, less differentiated, and larger SCC tumors, in agreement with previous findings,” the researchers wrote. “This shift is likely associated with decreased immunosurveillance resulting from immunosuppressive therapy (since carcinogenesis decelerates with therapy cessation) interacting with effects of high UV radiation exposure.”
They acknowledged certain limitations of their analysis, chiefly the lack of central review of SCCs to ensure standard assessment of histopathologic features “including caliber of nerves with perineural invasion and cell differentiation; such a review would not have been feasible logistically.”
The study was supported by grants from the National Health and Medical Research Council of Australia. The researchers reported having no disclosures related to the submitted work.
FROM JAMA DERMATOLOGY
Experts share their sun protection tips for children
“I basically say, ‘sun protection means clothing, shade, [considering the] time of day of exposure, and sunscreen if you are going to be otherwise exposed,’ ” Dr. Eichenfield, chief of pediatric and adolescent dermatology at Rady’s Children’s Hospital, San Diego, said during a panel discussion about sunscreen use at the Hawaii Dermatology Seminar provided by MedscapeLIVE! He recommends photoprotective gear such as rash guards for surfers and other water sport enthusiasts. When patients ask him if they should use sunscreen, he often replies with a question of his own.
“Do you brush your teeth?” he’ll ask.
“Yes, I do.”
“Well, you should put sunscreen on every day.”
Another panelist, Adelaide A. Hebert, MD, professor of dermatology and pediatrics and chief of pediatric dermatology at the University of Texas, Houston, said that she advises new parents to start sun protection efforts early. “Most sunscreens are not approved for use in children under the age of 6 months because testing has not been done in this age group, but I do recommend protective clothing. I also recommend wrap-around sunglasses, which offer 5% more protection from the sun than regular sunglasses.”
In her opinion, stick sunscreens are “a good add-on,” especially for under the eyes and the backs of the hands, but she is not a fan of spray sunscreens, which can leave large areas of skin unprotected if not applied properly.
Fellow panelist Jennifer Huang, MD, a pediatric dermatologist at Boston Children’s Hospital, who has a special interest in taking care of dermatologic conditions of children with cancer, generally recommends mineral-based sunscreens. “There is data to suggest that nonmineral sunscreens are less safe than mineral sunscreens for humans, and mineral sunscreens are considered to be better for the environment,” Dr. Huang said. “Plus, there are more elegant versions of mineral sunscreens that don’t make your skin pasty white.” However, for patients with darker skin tones, “it can be hard to apply a pasty white sunscreen, so I lean on some recommendations for tinted sunscreens, too, so there are options. I specifically recommend sunscreens that have iron oxides in them so that it can block physical rays and help with the cosmetic appearance.”
Moise Levy, MD, professor of internal medicine and pediatrics at the University of Texas at Austin, said that his approach to imparting sunscreen advice to children and their parents involves a mix of spoken information, printed information, and sunscreen samples for children to try in the office, in the presence of a parent. To help patients choose among different samples, be they ointments, gels, or lotions, he will often ask the child: “‘What do you like the feel of better?’ If the child says, ‘I like this one,’ I make sure the parent hears that,” Dr. Levy said.
Next, Dr. Eichenfield, who moderated the discussion, asked his fellow panelists how they would counsel someone who comes to their practice for evaluation of moles and has a family history of nonmelanoma skin cancer. “I think this is one of the easier counseling sessions, because there are enough kids who are asked about the moles on their skin when they’re at school,” Dr. Hebert said. “I think they’re very ready to wear sun protective clothing and I certainly don’t want any sun exposure that would pose an increased risk for their child.”
In addition to routine sun protection, Dr. Huang recommends annual mole checks for children who have a first-degree relative with a history of malignant melanoma. Other high-risk groups that should undergo annual skin exams include anyone who has received high doses of radiation, bone marrow transplants, prolonged use of voriconazole, or prolonged systemic immunosuppression. Without a known genetic predisposition syndrome, a family history of nonmelanoma skin cancer would not raise concern for melanoma in an otherwise healthy child.
Dr. Eichenfield added that freckling used to be the secondary risk factor for melanoma, “but it’s flipped over to a primary risk factor. A history of immunosuppression or prior cancer is a major risk factor in childhood and teenage years.”
Dr. Eichenfield disclosed that he is a consultant or adviser for numerous pharmaceutical companies. He has also received research funding from AbbVie, Bausch & Lomb, Galderma Laboratories, and Pfizer. Dr. Hebert disclosed that she is a consultant or adviser for AbbVie, Almirall, Amryt Pharma, Arcutis Biotherapeutics, Beiersdorf, Dermavant Sciences, Galderma Laboratories, L’Oreal, Novan, Ortho Dermatologics, Pfizer, and Verrica. Dr. Levy disclosed that he is consultant or adviser for Abeona, Castle Creek, Dusa Pharma, Krystal Bio, Novan, Regeneron, and Sanofi Genzyme. Dr. Huang disclosed that she is an adviser for EllaOla.
MedscapeLive! and this news organization are owned by the same parent company.
“I basically say, ‘sun protection means clothing, shade, [considering the] time of day of exposure, and sunscreen if you are going to be otherwise exposed,’ ” Dr. Eichenfield, chief of pediatric and adolescent dermatology at Rady’s Children’s Hospital, San Diego, said during a panel discussion about sunscreen use at the Hawaii Dermatology Seminar provided by MedscapeLIVE! He recommends photoprotective gear such as rash guards for surfers and other water sport enthusiasts. When patients ask him if they should use sunscreen, he often replies with a question of his own.
“Do you brush your teeth?” he’ll ask.
“Yes, I do.”
“Well, you should put sunscreen on every day.”
Another panelist, Adelaide A. Hebert, MD, professor of dermatology and pediatrics and chief of pediatric dermatology at the University of Texas, Houston, said that she advises new parents to start sun protection efforts early. “Most sunscreens are not approved for use in children under the age of 6 months because testing has not been done in this age group, but I do recommend protective clothing. I also recommend wrap-around sunglasses, which offer 5% more protection from the sun than regular sunglasses.”
In her opinion, stick sunscreens are “a good add-on,” especially for under the eyes and the backs of the hands, but she is not a fan of spray sunscreens, which can leave large areas of skin unprotected if not applied properly.
Fellow panelist Jennifer Huang, MD, a pediatric dermatologist at Boston Children’s Hospital, who has a special interest in taking care of dermatologic conditions of children with cancer, generally recommends mineral-based sunscreens. “There is data to suggest that nonmineral sunscreens are less safe than mineral sunscreens for humans, and mineral sunscreens are considered to be better for the environment,” Dr. Huang said. “Plus, there are more elegant versions of mineral sunscreens that don’t make your skin pasty white.” However, for patients with darker skin tones, “it can be hard to apply a pasty white sunscreen, so I lean on some recommendations for tinted sunscreens, too, so there are options. I specifically recommend sunscreens that have iron oxides in them so that it can block physical rays and help with the cosmetic appearance.”
Moise Levy, MD, professor of internal medicine and pediatrics at the University of Texas at Austin, said that his approach to imparting sunscreen advice to children and their parents involves a mix of spoken information, printed information, and sunscreen samples for children to try in the office, in the presence of a parent. To help patients choose among different samples, be they ointments, gels, or lotions, he will often ask the child: “‘What do you like the feel of better?’ If the child says, ‘I like this one,’ I make sure the parent hears that,” Dr. Levy said.
Next, Dr. Eichenfield, who moderated the discussion, asked his fellow panelists how they would counsel someone who comes to their practice for evaluation of moles and has a family history of nonmelanoma skin cancer. “I think this is one of the easier counseling sessions, because there are enough kids who are asked about the moles on their skin when they’re at school,” Dr. Hebert said. “I think they’re very ready to wear sun protective clothing and I certainly don’t want any sun exposure that would pose an increased risk for their child.”
In addition to routine sun protection, Dr. Huang recommends annual mole checks for children who have a first-degree relative with a history of malignant melanoma. Other high-risk groups that should undergo annual skin exams include anyone who has received high doses of radiation, bone marrow transplants, prolonged use of voriconazole, or prolonged systemic immunosuppression. Without a known genetic predisposition syndrome, a family history of nonmelanoma skin cancer would not raise concern for melanoma in an otherwise healthy child.
Dr. Eichenfield added that freckling used to be the secondary risk factor for melanoma, “but it’s flipped over to a primary risk factor. A history of immunosuppression or prior cancer is a major risk factor in childhood and teenage years.”
Dr. Eichenfield disclosed that he is a consultant or adviser for numerous pharmaceutical companies. He has also received research funding from AbbVie, Bausch & Lomb, Galderma Laboratories, and Pfizer. Dr. Hebert disclosed that she is a consultant or adviser for AbbVie, Almirall, Amryt Pharma, Arcutis Biotherapeutics, Beiersdorf, Dermavant Sciences, Galderma Laboratories, L’Oreal, Novan, Ortho Dermatologics, Pfizer, and Verrica. Dr. Levy disclosed that he is consultant or adviser for Abeona, Castle Creek, Dusa Pharma, Krystal Bio, Novan, Regeneron, and Sanofi Genzyme. Dr. Huang disclosed that she is an adviser for EllaOla.
MedscapeLive! and this news organization are owned by the same parent company.
“I basically say, ‘sun protection means clothing, shade, [considering the] time of day of exposure, and sunscreen if you are going to be otherwise exposed,’ ” Dr. Eichenfield, chief of pediatric and adolescent dermatology at Rady’s Children’s Hospital, San Diego, said during a panel discussion about sunscreen use at the Hawaii Dermatology Seminar provided by MedscapeLIVE! He recommends photoprotective gear such as rash guards for surfers and other water sport enthusiasts. When patients ask him if they should use sunscreen, he often replies with a question of his own.
“Do you brush your teeth?” he’ll ask.
“Yes, I do.”
“Well, you should put sunscreen on every day.”
Another panelist, Adelaide A. Hebert, MD, professor of dermatology and pediatrics and chief of pediatric dermatology at the University of Texas, Houston, said that she advises new parents to start sun protection efforts early. “Most sunscreens are not approved for use in children under the age of 6 months because testing has not been done in this age group, but I do recommend protective clothing. I also recommend wrap-around sunglasses, which offer 5% more protection from the sun than regular sunglasses.”
In her opinion, stick sunscreens are “a good add-on,” especially for under the eyes and the backs of the hands, but she is not a fan of spray sunscreens, which can leave large areas of skin unprotected if not applied properly.
Fellow panelist Jennifer Huang, MD, a pediatric dermatologist at Boston Children’s Hospital, who has a special interest in taking care of dermatologic conditions of children with cancer, generally recommends mineral-based sunscreens. “There is data to suggest that nonmineral sunscreens are less safe than mineral sunscreens for humans, and mineral sunscreens are considered to be better for the environment,” Dr. Huang said. “Plus, there are more elegant versions of mineral sunscreens that don’t make your skin pasty white.” However, for patients with darker skin tones, “it can be hard to apply a pasty white sunscreen, so I lean on some recommendations for tinted sunscreens, too, so there are options. I specifically recommend sunscreens that have iron oxides in them so that it can block physical rays and help with the cosmetic appearance.”
Moise Levy, MD, professor of internal medicine and pediatrics at the University of Texas at Austin, said that his approach to imparting sunscreen advice to children and their parents involves a mix of spoken information, printed information, and sunscreen samples for children to try in the office, in the presence of a parent. To help patients choose among different samples, be they ointments, gels, or lotions, he will often ask the child: “‘What do you like the feel of better?’ If the child says, ‘I like this one,’ I make sure the parent hears that,” Dr. Levy said.
Next, Dr. Eichenfield, who moderated the discussion, asked his fellow panelists how they would counsel someone who comes to their practice for evaluation of moles and has a family history of nonmelanoma skin cancer. “I think this is one of the easier counseling sessions, because there are enough kids who are asked about the moles on their skin when they’re at school,” Dr. Hebert said. “I think they’re very ready to wear sun protective clothing and I certainly don’t want any sun exposure that would pose an increased risk for their child.”
In addition to routine sun protection, Dr. Huang recommends annual mole checks for children who have a first-degree relative with a history of malignant melanoma. Other high-risk groups that should undergo annual skin exams include anyone who has received high doses of radiation, bone marrow transplants, prolonged use of voriconazole, or prolonged systemic immunosuppression. Without a known genetic predisposition syndrome, a family history of nonmelanoma skin cancer would not raise concern for melanoma in an otherwise healthy child.
Dr. Eichenfield added that freckling used to be the secondary risk factor for melanoma, “but it’s flipped over to a primary risk factor. A history of immunosuppression or prior cancer is a major risk factor in childhood and teenage years.”
Dr. Eichenfield disclosed that he is a consultant or adviser for numerous pharmaceutical companies. He has also received research funding from AbbVie, Bausch & Lomb, Galderma Laboratories, and Pfizer. Dr. Hebert disclosed that she is a consultant or adviser for AbbVie, Almirall, Amryt Pharma, Arcutis Biotherapeutics, Beiersdorf, Dermavant Sciences, Galderma Laboratories, L’Oreal, Novan, Ortho Dermatologics, Pfizer, and Verrica. Dr. Levy disclosed that he is consultant or adviser for Abeona, Castle Creek, Dusa Pharma, Krystal Bio, Novan, Regeneron, and Sanofi Genzyme. Dr. Huang disclosed that she is an adviser for EllaOla.
MedscapeLive! and this news organization are owned by the same parent company.
FROM THE MEDSCAPELIVE! HAWAII DERMATOLOGY SEMINAR
Report eyes complications from microwave energy devices for hyperhidrosis
database showed.
While microwave energy devices (MEDs) are used to treat hyperhidrosis, the largest MED clinical trial included only 101 patients, Samantha Jo Albucker and Shari Lipner, MD, PhD, wrote in a research letter reporting the results.
For the study, published online in the Journal of the American Academy of Dermatology, Ms. Albucker, a student at Tulane University, New Orleans, and Dr. Lipner, associate professor of clinical dermatology at Weill Cornell Medicine, New York, searched the MAUDE database between Feb. 28, 2013, and Dec. 29, 2022, for adverse events (AEs) involving MEDs for hyperhidrosis treatment. Of the 502 medical device reports identified over the study period, the axilla was the most frequent injury site in 50.4% of cases. The three most common complications were infections (45.4%); neurological symptoms including neuropathy, nerve damage, and numbness (21.7%); and burns/ulcerations/erosions (19.1%).
In other findings, 2.4% of patients required hospitalization, most often because of infection (83.3%), followed by burn and coma (8.3% each). The average symptom onset was 2 months postprocedure, and the most common treatment was antibiotics in 62.2% of cases, followed by incision and drainage/aspiration in 21.7% of cases.
A codiagnosis of hidradenitis suppurativa (HS) was reported in 5.4% of all medical device reports. The researchers noted that in a published randomized clinical trial of eight HS patients undergoing MED treatment to assess the effect on HS symptoms, the treatment showed no clinical advantage. In addition, they referred to two case reports describing new-onset HS after MED treatment for hyperhidrosis.
“Therefore, we recommend questioning patients about HS history and examining for HS clinical findings before performing MED for hyperhidrosis,” they wrote, adding that the data, “taken together, suggests that avoidance of MED treatment of hyperhidrosis in HS patients is prudent and alternative treatments may be prescribed.”
The researchers acknowledged certain limitations of their analysis, including uncompleted medical device reports, patient reporting, and unverified causes of adverse events. “Large multicenter studies are needed to corroborate our results,” they concluded.
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the study, said that primary idiopathic hyperhidrosis is a common medical condition that is often overlooked as a legitimate concern, and causes a quality-of-life burden. “Even with the striking numbers in the millions, there are limited treatment options available for axillary let alone other forms of primary hyperhidrosis,” said Dr. Friedman, who was not involved with the study.
“Therefore, for the short treatment list we have, it is important to have some predictive power with respect to clinical impact to provide realistic expectations as well as potential adverse events to ensure best practices and meaningful patient guidance. In this research letter, our colleagues highlight complications that can ensue from microwave therapy for hyperhidrosis and the frequency of said adverse events. Knowing these data is half the battle, and I for one would not have assumed infection was number one on the list of adverse events.”
Ms. Albucker had no relevant conflicts of interest to disclose. Dr. Lipner disclosed that she has served as a consultant for Ortho Dermatologics, Hoth Therapeutics, BelleTorus Corporation, and Moberg Pharmaceuticals.
Dr. Friedman disclosed that he is a consultant and/or advisory board member for Medscape/SanovaWorks, Oakstone Institute, L’Oréal, La Roche Posay, Galderma, Aveeno, Ortho Dermatologic, Microcures, Pfizer, Novartis, Lilly, Hoth Therapeutics, Zylo Therapeutics, BMS, Vial, Janssen, Novocure, Dermavant, Regeneron/Sanofi, and Incyte. He has also received grants from Pfizer, the Dermatology Foundation, Lilly, Janssen, Incyte, and Galderma.
database showed.
While microwave energy devices (MEDs) are used to treat hyperhidrosis, the largest MED clinical trial included only 101 patients, Samantha Jo Albucker and Shari Lipner, MD, PhD, wrote in a research letter reporting the results.
For the study, published online in the Journal of the American Academy of Dermatology, Ms. Albucker, a student at Tulane University, New Orleans, and Dr. Lipner, associate professor of clinical dermatology at Weill Cornell Medicine, New York, searched the MAUDE database between Feb. 28, 2013, and Dec. 29, 2022, for adverse events (AEs) involving MEDs for hyperhidrosis treatment. Of the 502 medical device reports identified over the study period, the axilla was the most frequent injury site in 50.4% of cases. The three most common complications were infections (45.4%); neurological symptoms including neuropathy, nerve damage, and numbness (21.7%); and burns/ulcerations/erosions (19.1%).
In other findings, 2.4% of patients required hospitalization, most often because of infection (83.3%), followed by burn and coma (8.3% each). The average symptom onset was 2 months postprocedure, and the most common treatment was antibiotics in 62.2% of cases, followed by incision and drainage/aspiration in 21.7% of cases.
A codiagnosis of hidradenitis suppurativa (HS) was reported in 5.4% of all medical device reports. The researchers noted that in a published randomized clinical trial of eight HS patients undergoing MED treatment to assess the effect on HS symptoms, the treatment showed no clinical advantage. In addition, they referred to two case reports describing new-onset HS after MED treatment for hyperhidrosis.
“Therefore, we recommend questioning patients about HS history and examining for HS clinical findings before performing MED for hyperhidrosis,” they wrote, adding that the data, “taken together, suggests that avoidance of MED treatment of hyperhidrosis in HS patients is prudent and alternative treatments may be prescribed.”
The researchers acknowledged certain limitations of their analysis, including uncompleted medical device reports, patient reporting, and unverified causes of adverse events. “Large multicenter studies are needed to corroborate our results,” they concluded.
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the study, said that primary idiopathic hyperhidrosis is a common medical condition that is often overlooked as a legitimate concern, and causes a quality-of-life burden. “Even with the striking numbers in the millions, there are limited treatment options available for axillary let alone other forms of primary hyperhidrosis,” said Dr. Friedman, who was not involved with the study.
“Therefore, for the short treatment list we have, it is important to have some predictive power with respect to clinical impact to provide realistic expectations as well as potential adverse events to ensure best practices and meaningful patient guidance. In this research letter, our colleagues highlight complications that can ensue from microwave therapy for hyperhidrosis and the frequency of said adverse events. Knowing these data is half the battle, and I for one would not have assumed infection was number one on the list of adverse events.”
Ms. Albucker had no relevant conflicts of interest to disclose. Dr. Lipner disclosed that she has served as a consultant for Ortho Dermatologics, Hoth Therapeutics, BelleTorus Corporation, and Moberg Pharmaceuticals.
Dr. Friedman disclosed that he is a consultant and/or advisory board member for Medscape/SanovaWorks, Oakstone Institute, L’Oréal, La Roche Posay, Galderma, Aveeno, Ortho Dermatologic, Microcures, Pfizer, Novartis, Lilly, Hoth Therapeutics, Zylo Therapeutics, BMS, Vial, Janssen, Novocure, Dermavant, Regeneron/Sanofi, and Incyte. He has also received grants from Pfizer, the Dermatology Foundation, Lilly, Janssen, Incyte, and Galderma.
database showed.
While microwave energy devices (MEDs) are used to treat hyperhidrosis, the largest MED clinical trial included only 101 patients, Samantha Jo Albucker and Shari Lipner, MD, PhD, wrote in a research letter reporting the results.
For the study, published online in the Journal of the American Academy of Dermatology, Ms. Albucker, a student at Tulane University, New Orleans, and Dr. Lipner, associate professor of clinical dermatology at Weill Cornell Medicine, New York, searched the MAUDE database between Feb. 28, 2013, and Dec. 29, 2022, for adverse events (AEs) involving MEDs for hyperhidrosis treatment. Of the 502 medical device reports identified over the study period, the axilla was the most frequent injury site in 50.4% of cases. The three most common complications were infections (45.4%); neurological symptoms including neuropathy, nerve damage, and numbness (21.7%); and burns/ulcerations/erosions (19.1%).
In other findings, 2.4% of patients required hospitalization, most often because of infection (83.3%), followed by burn and coma (8.3% each). The average symptom onset was 2 months postprocedure, and the most common treatment was antibiotics in 62.2% of cases, followed by incision and drainage/aspiration in 21.7% of cases.
A codiagnosis of hidradenitis suppurativa (HS) was reported in 5.4% of all medical device reports. The researchers noted that in a published randomized clinical trial of eight HS patients undergoing MED treatment to assess the effect on HS symptoms, the treatment showed no clinical advantage. In addition, they referred to two case reports describing new-onset HS after MED treatment for hyperhidrosis.
“Therefore, we recommend questioning patients about HS history and examining for HS clinical findings before performing MED for hyperhidrosis,” they wrote, adding that the data, “taken together, suggests that avoidance of MED treatment of hyperhidrosis in HS patients is prudent and alternative treatments may be prescribed.”
The researchers acknowledged certain limitations of their analysis, including uncompleted medical device reports, patient reporting, and unverified causes of adverse events. “Large multicenter studies are needed to corroborate our results,” they concluded.
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the study, said that primary idiopathic hyperhidrosis is a common medical condition that is often overlooked as a legitimate concern, and causes a quality-of-life burden. “Even with the striking numbers in the millions, there are limited treatment options available for axillary let alone other forms of primary hyperhidrosis,” said Dr. Friedman, who was not involved with the study.
“Therefore, for the short treatment list we have, it is important to have some predictive power with respect to clinical impact to provide realistic expectations as well as potential adverse events to ensure best practices and meaningful patient guidance. In this research letter, our colleagues highlight complications that can ensue from microwave therapy for hyperhidrosis and the frequency of said adverse events. Knowing these data is half the battle, and I for one would not have assumed infection was number one on the list of adverse events.”
Ms. Albucker had no relevant conflicts of interest to disclose. Dr. Lipner disclosed that she has served as a consultant for Ortho Dermatologics, Hoth Therapeutics, BelleTorus Corporation, and Moberg Pharmaceuticals.
Dr. Friedman disclosed that he is a consultant and/or advisory board member for Medscape/SanovaWorks, Oakstone Institute, L’Oréal, La Roche Posay, Galderma, Aveeno, Ortho Dermatologic, Microcures, Pfizer, Novartis, Lilly, Hoth Therapeutics, Zylo Therapeutics, BMS, Vial, Janssen, Novocure, Dermavant, Regeneron/Sanofi, and Incyte. He has also received grants from Pfizer, the Dermatology Foundation, Lilly, Janssen, Incyte, and Galderma.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
How does psoriasis affect fertility and birth outcomes?
in a U.K. cohort study.
Those are key findings from what is believed to be one of the largest studies to investigate fertility and obstetric outcomes in patients with psoriasis.
“Studies that have examined fertility and pregnancy outcomes in women with psoriasis have reported conflicting findings,” lead author Teng-Chou Chen, PhD, of the Centre for Pharmacoepidemiology and Drug Safety at the University of Manchester (England), and colleagues from the Global Psoriasis Atlas wrote in the study, published in JAMA Dermatology. Most of the studies were small, with under 100 women, “and are thus likely underpowered to detect a difference in pregnancy outcomes. The majority of those studies used disease registry data or lacked a matched comparison group and hence were unable to estimate the association of fertility and adverse pregnancy outcomes in women with psoriasis when compared with the general population.”
To determine fertility rates and birth outcomes in female patients with psoriasis, compared with age- and practice-matched patients without psoriasis, the researchers evaluated EHR data from a large U.K. primary care database, the Clinical Practice Research Datalink GOLD, from 1998 to 2019. They limited the analysis to patients aged 15-44 years and used relevant codes from clinical consultations to identify those with psoriasis. Then, for each patient with psoriasis, the researchers selected five comparators without psoriasis from the same primary care practice and matched for year of birth.
Both sets of patients were followed from the index date to age 45 years, death, transfer out of practice, last date of data collection, or end of the study period (Dec. 31, 2019), whichever came first. Pregnancy records were extracted for both sets of patients, and birth outcomes were categorized as pregnancy loss, live birth, stillbirth, and preterm birth. Adverse pregnancy outcomes were also collected. Finally, Dr. Chen and colleagues used a negative binomial model to examine the association between psoriasis and the fertility rate, and they applied logistic regression to compare the association between psoriasis and obstetric outcomes.
The analysis included 63,681 patients with psoriasis and 318,405 comparators whose median age on the index date was 30 years and who were followed for a median of 4.1 years. Among patients with psoriasis, 5.1% met criteria for moderate to severe disease in the follow-up period. The researchers observed that, compared with their age- and practice-matched counterparts, patients with psoriasis were more likely to be current smokers, alcohol drinkers, or overweight on the index date. They were also more often diagnosed with diabetes, hypertension, inflammatory bowel disease, thyroid disorders, and respiratory diseases such as asthma and chronic obstructive pulmonary disease.
Fertility, birth outcomes
When they looked at fertility outcomes, the researchers found that, compared with their matched peers without psoriasis, those with psoriasis had higher rates of fertility (risk ratio, 1.30; 95% confidence interval, 1.27-1.33; P < .001). But after the researchers stratified patients based on psoriasis severity, those with moderate to severe disease had significantly lower rates of fertility (RR, 0.75; 95% CI, 0.69-0.83; P < .001), compared those who did not have psoriasis.
As for adverse birth outcomes, compared with their matched comparators, pregnancies in patients with psoriasis were less likely to end in a live birth (odds ratio, 0.91; 95% CI, 0.88-0.93; P < .001). They also had a higher risk of pregnancy loss (OR, 1.06; 95% CI, 1.03-1.10; P < .001), most during the first trimester, at a gestation period of under 91 days.
In addition to psoriasis, patients younger than age 20 (OR, 2.04; 95% CI, 1.94-2.15; P < .011) and those aged between 20 and 24 years (OR, 1.35; 95% CI, 1.31-1.40; P < .001) had a higher risk of pregnancy loss, compared with those aged between 25 and 34 years.
However, no increases in the risks of antenatal hemorrhage, preeclampsia, or gestational diabetes were observed in patients with psoriasis, and no statistically significant differences in the odds of stillbirth and preterm birth were found between patients with psoriasis and matched comparators who did not have psoriasis.
“The mechanism to link the higher risk of pregnancy loss in patients with psoriasis is not clear, but there might be potential explanations,” the researchers wrote. “Psoriasis is characterized by the increased activity of [interleukin]-17, IL-23, and tumor necrosis factor–alpha. Those proinflammatory cytokines may negatively affect the placenta and cause impaired fetal growth.”
They recommended that further studies “evaluate the effects of better management of psoriasis and close monitoring during pregnancy on pregnancy loss.” In particular, “patients with psoriasis were more likely to have comorbidities that may be related to poor pregnancy outcomes, and hence increased emphasis of managing comorbidities as part of the routine management plan is also warranted.”
Asked to comment on the study, Alexa B. Kimball, MD, MPH, who has been involved with research on this topic, said that she and other investigators had observed some years ago that fertility rates for women with moderate to severe psoriasis might be lower than expected.
This trend was observed in some psoriasis registries, some pregnancy registries, and in clinical practice, Dr. Kimball, professor of dermatology at Harvard Medical School, Boston, said in an interview. “This study clearly demonstrates that lower fertility rates in the moderate to severe psoriasis population occurs and compels further exploration of the reason why.” The reasons could be biologic, she continued, including difficulty conceiving or an increased risk of miscarriage, sociobehavioral issues, or a combination.
“Behavioral examples could include that some women with moderate to severe psoriasis can flare during pregnancy, which might affect their choice” to become pregnant, Dr. Kimball said. “Stigma may also play a role in how women with moderate to severe psoriasis form relationships. Now that there are much better treatments for moderate to severe psoriasis and better knowledge about managing psoriasis during pregnancy, it will also be important to explore whether these trends change over time.”
The study was funded by the International League of Dermatological Societies on behalf of the Global Psoriasis Atlas. Two of the study authors reported receiving consulting fees and grant support from many pharmaceutical companies. Dr. Kimball disclosed that she serves or has served on several Organization of Teratology Information Specialists advisory board pregnancy registries, is a consultant and investigator for Abbvie, Janssen, Lilly, Bristol-Myers Squibb, Moonlake, UCB, and Amgen; has fellowship funding from Janssen; and serves on the board of Almirall.
in a U.K. cohort study.
Those are key findings from what is believed to be one of the largest studies to investigate fertility and obstetric outcomes in patients with psoriasis.
“Studies that have examined fertility and pregnancy outcomes in women with psoriasis have reported conflicting findings,” lead author Teng-Chou Chen, PhD, of the Centre for Pharmacoepidemiology and Drug Safety at the University of Manchester (England), and colleagues from the Global Psoriasis Atlas wrote in the study, published in JAMA Dermatology. Most of the studies were small, with under 100 women, “and are thus likely underpowered to detect a difference in pregnancy outcomes. The majority of those studies used disease registry data or lacked a matched comparison group and hence were unable to estimate the association of fertility and adverse pregnancy outcomes in women with psoriasis when compared with the general population.”
To determine fertility rates and birth outcomes in female patients with psoriasis, compared with age- and practice-matched patients without psoriasis, the researchers evaluated EHR data from a large U.K. primary care database, the Clinical Practice Research Datalink GOLD, from 1998 to 2019. They limited the analysis to patients aged 15-44 years and used relevant codes from clinical consultations to identify those with psoriasis. Then, for each patient with psoriasis, the researchers selected five comparators without psoriasis from the same primary care practice and matched for year of birth.
Both sets of patients were followed from the index date to age 45 years, death, transfer out of practice, last date of data collection, or end of the study period (Dec. 31, 2019), whichever came first. Pregnancy records were extracted for both sets of patients, and birth outcomes were categorized as pregnancy loss, live birth, stillbirth, and preterm birth. Adverse pregnancy outcomes were also collected. Finally, Dr. Chen and colleagues used a negative binomial model to examine the association between psoriasis and the fertility rate, and they applied logistic regression to compare the association between psoriasis and obstetric outcomes.
The analysis included 63,681 patients with psoriasis and 318,405 comparators whose median age on the index date was 30 years and who were followed for a median of 4.1 years. Among patients with psoriasis, 5.1% met criteria for moderate to severe disease in the follow-up period. The researchers observed that, compared with their age- and practice-matched counterparts, patients with psoriasis were more likely to be current smokers, alcohol drinkers, or overweight on the index date. They were also more often diagnosed with diabetes, hypertension, inflammatory bowel disease, thyroid disorders, and respiratory diseases such as asthma and chronic obstructive pulmonary disease.
Fertility, birth outcomes
When they looked at fertility outcomes, the researchers found that, compared with their matched peers without psoriasis, those with psoriasis had higher rates of fertility (risk ratio, 1.30; 95% confidence interval, 1.27-1.33; P < .001). But after the researchers stratified patients based on psoriasis severity, those with moderate to severe disease had significantly lower rates of fertility (RR, 0.75; 95% CI, 0.69-0.83; P < .001), compared those who did not have psoriasis.
As for adverse birth outcomes, compared with their matched comparators, pregnancies in patients with psoriasis were less likely to end in a live birth (odds ratio, 0.91; 95% CI, 0.88-0.93; P < .001). They also had a higher risk of pregnancy loss (OR, 1.06; 95% CI, 1.03-1.10; P < .001), most during the first trimester, at a gestation period of under 91 days.
In addition to psoriasis, patients younger than age 20 (OR, 2.04; 95% CI, 1.94-2.15; P < .011) and those aged between 20 and 24 years (OR, 1.35; 95% CI, 1.31-1.40; P < .001) had a higher risk of pregnancy loss, compared with those aged between 25 and 34 years.
However, no increases in the risks of antenatal hemorrhage, preeclampsia, or gestational diabetes were observed in patients with psoriasis, and no statistically significant differences in the odds of stillbirth and preterm birth were found between patients with psoriasis and matched comparators who did not have psoriasis.
“The mechanism to link the higher risk of pregnancy loss in patients with psoriasis is not clear, but there might be potential explanations,” the researchers wrote. “Psoriasis is characterized by the increased activity of [interleukin]-17, IL-23, and tumor necrosis factor–alpha. Those proinflammatory cytokines may negatively affect the placenta and cause impaired fetal growth.”
They recommended that further studies “evaluate the effects of better management of psoriasis and close monitoring during pregnancy on pregnancy loss.” In particular, “patients with psoriasis were more likely to have comorbidities that may be related to poor pregnancy outcomes, and hence increased emphasis of managing comorbidities as part of the routine management plan is also warranted.”
Asked to comment on the study, Alexa B. Kimball, MD, MPH, who has been involved with research on this topic, said that she and other investigators had observed some years ago that fertility rates for women with moderate to severe psoriasis might be lower than expected.
This trend was observed in some psoriasis registries, some pregnancy registries, and in clinical practice, Dr. Kimball, professor of dermatology at Harvard Medical School, Boston, said in an interview. “This study clearly demonstrates that lower fertility rates in the moderate to severe psoriasis population occurs and compels further exploration of the reason why.” The reasons could be biologic, she continued, including difficulty conceiving or an increased risk of miscarriage, sociobehavioral issues, or a combination.
“Behavioral examples could include that some women with moderate to severe psoriasis can flare during pregnancy, which might affect their choice” to become pregnant, Dr. Kimball said. “Stigma may also play a role in how women with moderate to severe psoriasis form relationships. Now that there are much better treatments for moderate to severe psoriasis and better knowledge about managing psoriasis during pregnancy, it will also be important to explore whether these trends change over time.”
The study was funded by the International League of Dermatological Societies on behalf of the Global Psoriasis Atlas. Two of the study authors reported receiving consulting fees and grant support from many pharmaceutical companies. Dr. Kimball disclosed that she serves or has served on several Organization of Teratology Information Specialists advisory board pregnancy registries, is a consultant and investigator for Abbvie, Janssen, Lilly, Bristol-Myers Squibb, Moonlake, UCB, and Amgen; has fellowship funding from Janssen; and serves on the board of Almirall.
in a U.K. cohort study.
Those are key findings from what is believed to be one of the largest studies to investigate fertility and obstetric outcomes in patients with psoriasis.
“Studies that have examined fertility and pregnancy outcomes in women with psoriasis have reported conflicting findings,” lead author Teng-Chou Chen, PhD, of the Centre for Pharmacoepidemiology and Drug Safety at the University of Manchester (England), and colleagues from the Global Psoriasis Atlas wrote in the study, published in JAMA Dermatology. Most of the studies were small, with under 100 women, “and are thus likely underpowered to detect a difference in pregnancy outcomes. The majority of those studies used disease registry data or lacked a matched comparison group and hence were unable to estimate the association of fertility and adverse pregnancy outcomes in women with psoriasis when compared with the general population.”
To determine fertility rates and birth outcomes in female patients with psoriasis, compared with age- and practice-matched patients without psoriasis, the researchers evaluated EHR data from a large U.K. primary care database, the Clinical Practice Research Datalink GOLD, from 1998 to 2019. They limited the analysis to patients aged 15-44 years and used relevant codes from clinical consultations to identify those with psoriasis. Then, for each patient with psoriasis, the researchers selected five comparators without psoriasis from the same primary care practice and matched for year of birth.
Both sets of patients were followed from the index date to age 45 years, death, transfer out of practice, last date of data collection, or end of the study period (Dec. 31, 2019), whichever came first. Pregnancy records were extracted for both sets of patients, and birth outcomes were categorized as pregnancy loss, live birth, stillbirth, and preterm birth. Adverse pregnancy outcomes were also collected. Finally, Dr. Chen and colleagues used a negative binomial model to examine the association between psoriasis and the fertility rate, and they applied logistic regression to compare the association between psoriasis and obstetric outcomes.
The analysis included 63,681 patients with psoriasis and 318,405 comparators whose median age on the index date was 30 years and who were followed for a median of 4.1 years. Among patients with psoriasis, 5.1% met criteria for moderate to severe disease in the follow-up period. The researchers observed that, compared with their age- and practice-matched counterparts, patients with psoriasis were more likely to be current smokers, alcohol drinkers, or overweight on the index date. They were also more often diagnosed with diabetes, hypertension, inflammatory bowel disease, thyroid disorders, and respiratory diseases such as asthma and chronic obstructive pulmonary disease.
Fertility, birth outcomes
When they looked at fertility outcomes, the researchers found that, compared with their matched peers without psoriasis, those with psoriasis had higher rates of fertility (risk ratio, 1.30; 95% confidence interval, 1.27-1.33; P < .001). But after the researchers stratified patients based on psoriasis severity, those with moderate to severe disease had significantly lower rates of fertility (RR, 0.75; 95% CI, 0.69-0.83; P < .001), compared those who did not have psoriasis.
As for adverse birth outcomes, compared with their matched comparators, pregnancies in patients with psoriasis were less likely to end in a live birth (odds ratio, 0.91; 95% CI, 0.88-0.93; P < .001). They also had a higher risk of pregnancy loss (OR, 1.06; 95% CI, 1.03-1.10; P < .001), most during the first trimester, at a gestation period of under 91 days.
In addition to psoriasis, patients younger than age 20 (OR, 2.04; 95% CI, 1.94-2.15; P < .011) and those aged between 20 and 24 years (OR, 1.35; 95% CI, 1.31-1.40; P < .001) had a higher risk of pregnancy loss, compared with those aged between 25 and 34 years.
However, no increases in the risks of antenatal hemorrhage, preeclampsia, or gestational diabetes were observed in patients with psoriasis, and no statistically significant differences in the odds of stillbirth and preterm birth were found between patients with psoriasis and matched comparators who did not have psoriasis.
“The mechanism to link the higher risk of pregnancy loss in patients with psoriasis is not clear, but there might be potential explanations,” the researchers wrote. “Psoriasis is characterized by the increased activity of [interleukin]-17, IL-23, and tumor necrosis factor–alpha. Those proinflammatory cytokines may negatively affect the placenta and cause impaired fetal growth.”
They recommended that further studies “evaluate the effects of better management of psoriasis and close monitoring during pregnancy on pregnancy loss.” In particular, “patients with psoriasis were more likely to have comorbidities that may be related to poor pregnancy outcomes, and hence increased emphasis of managing comorbidities as part of the routine management plan is also warranted.”
Asked to comment on the study, Alexa B. Kimball, MD, MPH, who has been involved with research on this topic, said that she and other investigators had observed some years ago that fertility rates for women with moderate to severe psoriasis might be lower than expected.
This trend was observed in some psoriasis registries, some pregnancy registries, and in clinical practice, Dr. Kimball, professor of dermatology at Harvard Medical School, Boston, said in an interview. “This study clearly demonstrates that lower fertility rates in the moderate to severe psoriasis population occurs and compels further exploration of the reason why.” The reasons could be biologic, she continued, including difficulty conceiving or an increased risk of miscarriage, sociobehavioral issues, or a combination.
“Behavioral examples could include that some women with moderate to severe psoriasis can flare during pregnancy, which might affect their choice” to become pregnant, Dr. Kimball said. “Stigma may also play a role in how women with moderate to severe psoriasis form relationships. Now that there are much better treatments for moderate to severe psoriasis and better knowledge about managing psoriasis during pregnancy, it will also be important to explore whether these trends change over time.”
The study was funded by the International League of Dermatological Societies on behalf of the Global Psoriasis Atlas. Two of the study authors reported receiving consulting fees and grant support from many pharmaceutical companies. Dr. Kimball disclosed that she serves or has served on several Organization of Teratology Information Specialists advisory board pregnancy registries, is a consultant and investigator for Abbvie, Janssen, Lilly, Bristol-Myers Squibb, Moonlake, UCB, and Amgen; has fellowship funding from Janssen; and serves on the board of Almirall.
FROM JAMA DERMATOLOGY
Lower racial disparity in melanoma diagnoses in vets than U.S. men overall, study finds
a new analysis shows.
“The trend of a lower racial disparity in the VA in the proportion of melanomas with local disease and in the proportion of distant metastasis at presentation was observed across age groups,” wrote Martin A. Weinstock MD, PhD, and Rachel K. Lim, of the department of dermatology at Brown University, Providence, R.I., and the Center for Dermatoepidemiology at the VA Providence Healthcare System. The study was published online in the Journal of the American Academy of Dermatology.
“Melanoma was the fourth-most common cancer [diagnosed] in male VA patients in 2010,” wrote the authors, who also pointed out that “prior surveys found that 11%-13% of U.S. active-duty personnel routinely use sunscreen despite significant occupational sun exposure. Racial disparities are important concerns in the VA and elsewhere.”
To compare the stage of melanoma at presentation among White and non-Whites patients in the VA and in the general U.S. population, the researchers identified invasive cutaneous melanoma cases from 2000 to 2019 in the VA Corporate Data Warehouse and the Surveillance, Epidemiology and End Results Program (SEER).
They restricted the analysis to men because of the small proportion of women in the at-risk veteran population and excluded cases with an age younger than 20, those with unknown histology, and melanoma in situ. The researchers performed two-tailed z-tests to evaluate the difference in proportions of melanoma stages between the veteran population and the general population.
The analysis included 44,077 cases of invasive melanoma in the VA and 217,030 in SEER. Racial disparities in melanoma staging were substantially less pronounced in the VA than in SEER.
In the VA, localized disease represented 77.9% of melanomas among Whites versus 71.0% among non-Whites. But in SEER, localized disease represented 80.7% of melanomas among Whites versus 61.5% in non-Whites – over double the VA disparity (P < .0001).
Likewise, the disparity between Whites and nonwhites observed for regional or distant metastatic disease at presentation in the VA was lower than the disparity observed in SEER. For example, in the VA, distant metastatic disease at presentation represented 6.1% of melanomas among Whites versus 8.6% among non-Whites, while in SEER it represented 4.8% of melanomas among Whites versus 11.3% in non-Whites – again, more than double the VA disparity (P < .0001).
“These differences between the VA and SEER were less marked” among those older than 65 years, the researchers wrote. “Notably, the differences between VA and SEER in racial disparities among those greater than 65 in age were still significant for localized disease and for distant metastasis.”
The findings suggest that the VA “may be more effective in reducing racial disparities in melanoma stage at diagnosis, potentially due to all patients in the VA dataset having insured access to health care, regardless of socioeconomic status,” the researchers concluded. Similarly, the decreased difference in racial disparities observed in patients older than 65 across systems “may be related to the availability of Medicare to the older general populations. The authors acknowledged several study limitations, such as the predominantly elderly and male VA population, potentially underreported utilization of non-VA dermatologic care, and variation in geographic regions covered by each database.
Travis W. Blalock, MD, director of dermatologic surgery, Mohs micrographic surgery, and cutaneous oncology at Emory University, Atlanta, who was asked to comment on the work, said in an interview he would have liked to see a more detailed breakdown of the younger patients, “for those in their 30s and 40s, to see if this trend held up.”
He would have also liked to see how the data trended over time, adding, “while this, broadly, may be good news for our veterans, attributing this finding to a reduction in access disparity or some other organizational intervention seems a little premature. Regardless, Dr. Weinstock has given us, once again, information from our veterans to probe for the betterment of all patients.”
The researchers reported having no relevant disclosures and the study had no funding. Dr. Blalock disclosed that he has served as a principal investigator for Castle Biosciences.
a new analysis shows.
“The trend of a lower racial disparity in the VA in the proportion of melanomas with local disease and in the proportion of distant metastasis at presentation was observed across age groups,” wrote Martin A. Weinstock MD, PhD, and Rachel K. Lim, of the department of dermatology at Brown University, Providence, R.I., and the Center for Dermatoepidemiology at the VA Providence Healthcare System. The study was published online in the Journal of the American Academy of Dermatology.
“Melanoma was the fourth-most common cancer [diagnosed] in male VA patients in 2010,” wrote the authors, who also pointed out that “prior surveys found that 11%-13% of U.S. active-duty personnel routinely use sunscreen despite significant occupational sun exposure. Racial disparities are important concerns in the VA and elsewhere.”
To compare the stage of melanoma at presentation among White and non-Whites patients in the VA and in the general U.S. population, the researchers identified invasive cutaneous melanoma cases from 2000 to 2019 in the VA Corporate Data Warehouse and the Surveillance, Epidemiology and End Results Program (SEER).
They restricted the analysis to men because of the small proportion of women in the at-risk veteran population and excluded cases with an age younger than 20, those with unknown histology, and melanoma in situ. The researchers performed two-tailed z-tests to evaluate the difference in proportions of melanoma stages between the veteran population and the general population.
The analysis included 44,077 cases of invasive melanoma in the VA and 217,030 in SEER. Racial disparities in melanoma staging were substantially less pronounced in the VA than in SEER.
In the VA, localized disease represented 77.9% of melanomas among Whites versus 71.0% among non-Whites. But in SEER, localized disease represented 80.7% of melanomas among Whites versus 61.5% in non-Whites – over double the VA disparity (P < .0001).
Likewise, the disparity between Whites and nonwhites observed for regional or distant metastatic disease at presentation in the VA was lower than the disparity observed in SEER. For example, in the VA, distant metastatic disease at presentation represented 6.1% of melanomas among Whites versus 8.6% among non-Whites, while in SEER it represented 4.8% of melanomas among Whites versus 11.3% in non-Whites – again, more than double the VA disparity (P < .0001).
“These differences between the VA and SEER were less marked” among those older than 65 years, the researchers wrote. “Notably, the differences between VA and SEER in racial disparities among those greater than 65 in age were still significant for localized disease and for distant metastasis.”
The findings suggest that the VA “may be more effective in reducing racial disparities in melanoma stage at diagnosis, potentially due to all patients in the VA dataset having insured access to health care, regardless of socioeconomic status,” the researchers concluded. Similarly, the decreased difference in racial disparities observed in patients older than 65 across systems “may be related to the availability of Medicare to the older general populations. The authors acknowledged several study limitations, such as the predominantly elderly and male VA population, potentially underreported utilization of non-VA dermatologic care, and variation in geographic regions covered by each database.
Travis W. Blalock, MD, director of dermatologic surgery, Mohs micrographic surgery, and cutaneous oncology at Emory University, Atlanta, who was asked to comment on the work, said in an interview he would have liked to see a more detailed breakdown of the younger patients, “for those in their 30s and 40s, to see if this trend held up.”
He would have also liked to see how the data trended over time, adding, “while this, broadly, may be good news for our veterans, attributing this finding to a reduction in access disparity or some other organizational intervention seems a little premature. Regardless, Dr. Weinstock has given us, once again, information from our veterans to probe for the betterment of all patients.”
The researchers reported having no relevant disclosures and the study had no funding. Dr. Blalock disclosed that he has served as a principal investigator for Castle Biosciences.
a new analysis shows.
“The trend of a lower racial disparity in the VA in the proportion of melanomas with local disease and in the proportion of distant metastasis at presentation was observed across age groups,” wrote Martin A. Weinstock MD, PhD, and Rachel K. Lim, of the department of dermatology at Brown University, Providence, R.I., and the Center for Dermatoepidemiology at the VA Providence Healthcare System. The study was published online in the Journal of the American Academy of Dermatology.
“Melanoma was the fourth-most common cancer [diagnosed] in male VA patients in 2010,” wrote the authors, who also pointed out that “prior surveys found that 11%-13% of U.S. active-duty personnel routinely use sunscreen despite significant occupational sun exposure. Racial disparities are important concerns in the VA and elsewhere.”
To compare the stage of melanoma at presentation among White and non-Whites patients in the VA and in the general U.S. population, the researchers identified invasive cutaneous melanoma cases from 2000 to 2019 in the VA Corporate Data Warehouse and the Surveillance, Epidemiology and End Results Program (SEER).
They restricted the analysis to men because of the small proportion of women in the at-risk veteran population and excluded cases with an age younger than 20, those with unknown histology, and melanoma in situ. The researchers performed two-tailed z-tests to evaluate the difference in proportions of melanoma stages between the veteran population and the general population.
The analysis included 44,077 cases of invasive melanoma in the VA and 217,030 in SEER. Racial disparities in melanoma staging were substantially less pronounced in the VA than in SEER.
In the VA, localized disease represented 77.9% of melanomas among Whites versus 71.0% among non-Whites. But in SEER, localized disease represented 80.7% of melanomas among Whites versus 61.5% in non-Whites – over double the VA disparity (P < .0001).
Likewise, the disparity between Whites and nonwhites observed for regional or distant metastatic disease at presentation in the VA was lower than the disparity observed in SEER. For example, in the VA, distant metastatic disease at presentation represented 6.1% of melanomas among Whites versus 8.6% among non-Whites, while in SEER it represented 4.8% of melanomas among Whites versus 11.3% in non-Whites – again, more than double the VA disparity (P < .0001).
“These differences between the VA and SEER were less marked” among those older than 65 years, the researchers wrote. “Notably, the differences between VA and SEER in racial disparities among those greater than 65 in age were still significant for localized disease and for distant metastasis.”
The findings suggest that the VA “may be more effective in reducing racial disparities in melanoma stage at diagnosis, potentially due to all patients in the VA dataset having insured access to health care, regardless of socioeconomic status,” the researchers concluded. Similarly, the decreased difference in racial disparities observed in patients older than 65 across systems “may be related to the availability of Medicare to the older general populations. The authors acknowledged several study limitations, such as the predominantly elderly and male VA population, potentially underreported utilization of non-VA dermatologic care, and variation in geographic regions covered by each database.
Travis W. Blalock, MD, director of dermatologic surgery, Mohs micrographic surgery, and cutaneous oncology at Emory University, Atlanta, who was asked to comment on the work, said in an interview he would have liked to see a more detailed breakdown of the younger patients, “for those in their 30s and 40s, to see if this trend held up.”
He would have also liked to see how the data trended over time, adding, “while this, broadly, may be good news for our veterans, attributing this finding to a reduction in access disparity or some other organizational intervention seems a little premature. Regardless, Dr. Weinstock has given us, once again, information from our veterans to probe for the betterment of all patients.”
The researchers reported having no relevant disclosures and the study had no funding. Dr. Blalock disclosed that he has served as a principal investigator for Castle Biosciences.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Men underrepresented in clinical trials of laser hair removal, review finds
PHOENIX – .
To characterize the sex of patients in trials evaluating hair removal with energy-based devices, Dr. Lee, an internal medicine intern at Beth Israel Deaconess Medical Center, Boston, and Jessica Labadie, MD, director of lasers and cosmetic surgery at the Icahn School of Medicine at Mount Sinai, New York, conducted a systematic review using PubMed with the search query hair AND laser AND removal AND (dermatology OR skin OR cutaneous). They limited the analysis to English-language clinical trials that investigated a laser and light-based therapy as an intervention and if hair reduction was an outcome, and excluded studies that did not include the face as a treatment area and laser hair removal for diseases with disproportionate occurrence in females or males, such as polycystic ovarian syndrome or pseudofolliculitis barbae.
Of 121 articles identified from the PubMed search, 28 studies involving 3,882 patients treated with lasers or intense pulsed light (IPL) for hair removal were included in the final analysis. Of these 28 articles, 22 (79%) reported the sex of trial participants. The population of these 22 studies included 3,104 (88.7%) females, 384 (11.0%) males, and 11 (0.003%) nonbinary identifying patients. None of the studies evaluated laser hair removal outcomes by sex.
“This study adds to the current knowledge of laser hair removal as a part of gender-affirming care by characterizing the representation of assigned sexes of patients in clinical trials evaluating the effectiveness of laser hair removal,” Dr. Lee told this news organization. “It highlights the underrepresentation of people assigned to male sex at birth in these clinical trials, despite this population’s potential interest in laser hair removal as a part of gender-affirming care.”
She acknowledged certain limitations of the review, including the absence of reporting on sex in the demographic sections of many trials and the exclusion of trials that did not include treatment of the face. “Clinicians need to be aware of the underrepresentation of men in clinical trials evaluating laser hair removal, and this may limit their understanding of treatment outcomes in this particular cohort,” she concluded. “Clinicians should emphasize inclusivity in future laser hair removal clinical trials and include outcomes by sex.”
The study “looks at an important aspect of clinical trials in the device-based space,” said Omar A. Ibrahimi, MD, PhD, medical director of the Connecticut Skin Institute, Stamford, who was not involved in the study and was asked to comment on the results. “Laser hair removal is the most commonly performed procedure in aesthetic energy-based device dermatology. While these trials are often very small compared to drug trials, it highlights that men are a very underrepresented cohort in laser hair removal trials,” he said. “More recently, there is an increased interest in gender-affirming procedures, and this has highlighted the need to ensure we include a diverse spectrum of patients in devices-based research studies. This is a very challenging mandate but certainly one we should strive for to make efforts to be more inclusive when designing these clinical studies so that the information we gain from these studies is more broadly applicable.”
The researchers reported having no financial disclosures. Dr. Ibrahimi disclosed that he is a member of the advisory board for Accure Acne, AbbVie, Cutera, Lutronic, Blueberry Therapeutics, Cytrellis, and Quthero, and holds stock in many device and pharmaceutical companies.
PHOENIX – .
To characterize the sex of patients in trials evaluating hair removal with energy-based devices, Dr. Lee, an internal medicine intern at Beth Israel Deaconess Medical Center, Boston, and Jessica Labadie, MD, director of lasers and cosmetic surgery at the Icahn School of Medicine at Mount Sinai, New York, conducted a systematic review using PubMed with the search query hair AND laser AND removal AND (dermatology OR skin OR cutaneous). They limited the analysis to English-language clinical trials that investigated a laser and light-based therapy as an intervention and if hair reduction was an outcome, and excluded studies that did not include the face as a treatment area and laser hair removal for diseases with disproportionate occurrence in females or males, such as polycystic ovarian syndrome or pseudofolliculitis barbae.
Of 121 articles identified from the PubMed search, 28 studies involving 3,882 patients treated with lasers or intense pulsed light (IPL) for hair removal were included in the final analysis. Of these 28 articles, 22 (79%) reported the sex of trial participants. The population of these 22 studies included 3,104 (88.7%) females, 384 (11.0%) males, and 11 (0.003%) nonbinary identifying patients. None of the studies evaluated laser hair removal outcomes by sex.
“This study adds to the current knowledge of laser hair removal as a part of gender-affirming care by characterizing the representation of assigned sexes of patients in clinical trials evaluating the effectiveness of laser hair removal,” Dr. Lee told this news organization. “It highlights the underrepresentation of people assigned to male sex at birth in these clinical trials, despite this population’s potential interest in laser hair removal as a part of gender-affirming care.”
She acknowledged certain limitations of the review, including the absence of reporting on sex in the demographic sections of many trials and the exclusion of trials that did not include treatment of the face. “Clinicians need to be aware of the underrepresentation of men in clinical trials evaluating laser hair removal, and this may limit their understanding of treatment outcomes in this particular cohort,” she concluded. “Clinicians should emphasize inclusivity in future laser hair removal clinical trials and include outcomes by sex.”
The study “looks at an important aspect of clinical trials in the device-based space,” said Omar A. Ibrahimi, MD, PhD, medical director of the Connecticut Skin Institute, Stamford, who was not involved in the study and was asked to comment on the results. “Laser hair removal is the most commonly performed procedure in aesthetic energy-based device dermatology. While these trials are often very small compared to drug trials, it highlights that men are a very underrepresented cohort in laser hair removal trials,” he said. “More recently, there is an increased interest in gender-affirming procedures, and this has highlighted the need to ensure we include a diverse spectrum of patients in devices-based research studies. This is a very challenging mandate but certainly one we should strive for to make efforts to be more inclusive when designing these clinical studies so that the information we gain from these studies is more broadly applicable.”
The researchers reported having no financial disclosures. Dr. Ibrahimi disclosed that he is a member of the advisory board for Accure Acne, AbbVie, Cutera, Lutronic, Blueberry Therapeutics, Cytrellis, and Quthero, and holds stock in many device and pharmaceutical companies.
PHOENIX – .
To characterize the sex of patients in trials evaluating hair removal with energy-based devices, Dr. Lee, an internal medicine intern at Beth Israel Deaconess Medical Center, Boston, and Jessica Labadie, MD, director of lasers and cosmetic surgery at the Icahn School of Medicine at Mount Sinai, New York, conducted a systematic review using PubMed with the search query hair AND laser AND removal AND (dermatology OR skin OR cutaneous). They limited the analysis to English-language clinical trials that investigated a laser and light-based therapy as an intervention and if hair reduction was an outcome, and excluded studies that did not include the face as a treatment area and laser hair removal for diseases with disproportionate occurrence in females or males, such as polycystic ovarian syndrome or pseudofolliculitis barbae.
Of 121 articles identified from the PubMed search, 28 studies involving 3,882 patients treated with lasers or intense pulsed light (IPL) for hair removal were included in the final analysis. Of these 28 articles, 22 (79%) reported the sex of trial participants. The population of these 22 studies included 3,104 (88.7%) females, 384 (11.0%) males, and 11 (0.003%) nonbinary identifying patients. None of the studies evaluated laser hair removal outcomes by sex.
“This study adds to the current knowledge of laser hair removal as a part of gender-affirming care by characterizing the representation of assigned sexes of patients in clinical trials evaluating the effectiveness of laser hair removal,” Dr. Lee told this news organization. “It highlights the underrepresentation of people assigned to male sex at birth in these clinical trials, despite this population’s potential interest in laser hair removal as a part of gender-affirming care.”
She acknowledged certain limitations of the review, including the absence of reporting on sex in the demographic sections of many trials and the exclusion of trials that did not include treatment of the face. “Clinicians need to be aware of the underrepresentation of men in clinical trials evaluating laser hair removal, and this may limit their understanding of treatment outcomes in this particular cohort,” she concluded. “Clinicians should emphasize inclusivity in future laser hair removal clinical trials and include outcomes by sex.”
The study “looks at an important aspect of clinical trials in the device-based space,” said Omar A. Ibrahimi, MD, PhD, medical director of the Connecticut Skin Institute, Stamford, who was not involved in the study and was asked to comment on the results. “Laser hair removal is the most commonly performed procedure in aesthetic energy-based device dermatology. While these trials are often very small compared to drug trials, it highlights that men are a very underrepresented cohort in laser hair removal trials,” he said. “More recently, there is an increased interest in gender-affirming procedures, and this has highlighted the need to ensure we include a diverse spectrum of patients in devices-based research studies. This is a very challenging mandate but certainly one we should strive for to make efforts to be more inclusive when designing these clinical studies so that the information we gain from these studies is more broadly applicable.”
The researchers reported having no financial disclosures. Dr. Ibrahimi disclosed that he is a member of the advisory board for Accure Acne, AbbVie, Cutera, Lutronic, Blueberry Therapeutics, Cytrellis, and Quthero, and holds stock in many device and pharmaceutical companies.
AT ASLMS 2023
Picosecond laser applications continue to expand
PHOENIX – Ever since PicoSure became the first picosecond laser cleared by the Food and Drug Administration for the treatment of unwanted tattoos and pigmented lesions in 2012, new uses for this technology continue to expand.
Now, These include PicoWay, PicoSure, Enlighten, PicoPlus, PiQo4, and Quanta Pico, among others.
“PicoWay technology has integrated nicely into my practice in Houston, the most ethnically diverse city in the country, with its ability to safely treat a number of various benign, congenital, and acquired epidermal and dermal pigmented lesions with ultrashort pulse duration and low thermal impact, which greatly reduces the risk of postinflammatory hyperpigmentation even in darker skin types,” Paul M. Friedman, MD, director of the Dermatology and Laser Surgery Center, Houston, said at the annual conference of the American Society for Laser Medicine and Surgery.
He emphasized the importance of therapeutic clinical endpoints, noting that with q-switched lasers, “you’re looking for immediate whitening, whereas with picosecond lasers, your endpoint is slight whitening or slight darkening depending on wavelength, indication, and skin type. The ability to fractionate picosecond pulses has also allowed us to utilize this technology for photoaging as well as acne scarring.”
The PicoWay system includes a 730-nm picosecond titanium sapphire handpiece, which is FDA cleared for treatment of benign pigmented lesions and blue and green tattoo removal. Dr. Friedman said that he has seen good clinical results using the handpiece for café-au-lait macules, particularly in skin of color.
In an abstract presented at the ASLMS meeting, he and his colleagues presented a retrospective review of 12 patients with café-au-lait macules with Fitzpatrick skin types III-VI who were treated with the PicoWay 730 nm handpiece between April 2021 and January 2023. Patients received a mean of 3.1 treatments at intervals that ranged from 5 to 40 weeks. Clinical photographs were graded by three board-certified dermatologists using a 5-point visual analogue scale.
Overall, patients were rated to have a mean improvement of 26%-50%. Two patients achieved 100% clearance after four to five treatment sessions. “Café-au-lait macules with smooth borders responded less well to laser treatment, confirming prior studies at our center,” he said. “We often educate parents that café-au-lait macules may recur over time, especially with repeated sun exposure.”
Treating melasma
Dr. Friedman’s go-to devices for melasma include the low-density, low-energy 1,927-nm fractional diode laser; the 1,064 nm picosecond Nd:YAG, the low-fluence 1,064 nm Q-switched Nd:YAG with a nanosecond pulse duration, and the 595-nm pulsed dye laser for lesions exhibiting underlying vascularity. He said that combining therapies that target pigment and vasculature may be ideal to prevent relapses. “Melasma is a multifactorial condition so by improving patient education and expectation alongside advances in laser treatment of melasma, we have ultimately improved our ability to treat this condition,” he said.
“We’re approaching it from all angles, with ultraviolet photography and spectrocolorimetry, behavioral modifications, topical skin-lightening agents, broad spectrum sunscreens with protection against visible light, and oral tranexamic acid in advanced cases. Then, we intervene with these energy-based modalities, and the bottom line is, less energy and density is more, with lengthened treatment intervals. In 2023, we’re better than we’ve ever been in terms of our ability to safely and effectively improve melasma.”
Novel lasers
Dr. Friedman also described the UltraClear, a novel ablative fractional 2,910-nm erbium-doped glass fiber laser that delivers a customized blend of ablation and coagulation based on the patient’s condition, skin type, and tolerability for down time. He provided an overview of the versatility of what he described as highly customizable technology for conditions such as photoaging and dyschromia in patients of various skin types, making it a very versatile platform in his practice.
The AVAVA MIRIA system is a “next generation” laser “where you’re able to use a focal point. Basically, you’re treating the skin from the inside out in a 3D manner and you’re able to focus intradermally up to 1 mm with high energy 1,064 nm or 1,550 nm,” he said. “It’s a unique conical geometry that spares the epidermis, combined with sapphire tip cooling and images the skin at the same time with the potential for personalized treatments of dyschromia and photoaging in all skin types. It’s truly remarkable where the technology is heading.”
Dr. Friedman disclosed that he has received consulting fees from Allergan, Galderma, Acclaro, Merz Aesthetics, Solta Medical, and Cytrellis. He has conducted contracted research for Sofwave and is a member of the speakers bureau for Solta Medical and Candela.
PHOENIX – Ever since PicoSure became the first picosecond laser cleared by the Food and Drug Administration for the treatment of unwanted tattoos and pigmented lesions in 2012, new uses for this technology continue to expand.
Now, These include PicoWay, PicoSure, Enlighten, PicoPlus, PiQo4, and Quanta Pico, among others.
“PicoWay technology has integrated nicely into my practice in Houston, the most ethnically diverse city in the country, with its ability to safely treat a number of various benign, congenital, and acquired epidermal and dermal pigmented lesions with ultrashort pulse duration and low thermal impact, which greatly reduces the risk of postinflammatory hyperpigmentation even in darker skin types,” Paul M. Friedman, MD, director of the Dermatology and Laser Surgery Center, Houston, said at the annual conference of the American Society for Laser Medicine and Surgery.
He emphasized the importance of therapeutic clinical endpoints, noting that with q-switched lasers, “you’re looking for immediate whitening, whereas with picosecond lasers, your endpoint is slight whitening or slight darkening depending on wavelength, indication, and skin type. The ability to fractionate picosecond pulses has also allowed us to utilize this technology for photoaging as well as acne scarring.”
The PicoWay system includes a 730-nm picosecond titanium sapphire handpiece, which is FDA cleared for treatment of benign pigmented lesions and blue and green tattoo removal. Dr. Friedman said that he has seen good clinical results using the handpiece for café-au-lait macules, particularly in skin of color.
In an abstract presented at the ASLMS meeting, he and his colleagues presented a retrospective review of 12 patients with café-au-lait macules with Fitzpatrick skin types III-VI who were treated with the PicoWay 730 nm handpiece between April 2021 and January 2023. Patients received a mean of 3.1 treatments at intervals that ranged from 5 to 40 weeks. Clinical photographs were graded by three board-certified dermatologists using a 5-point visual analogue scale.
Overall, patients were rated to have a mean improvement of 26%-50%. Two patients achieved 100% clearance after four to five treatment sessions. “Café-au-lait macules with smooth borders responded less well to laser treatment, confirming prior studies at our center,” he said. “We often educate parents that café-au-lait macules may recur over time, especially with repeated sun exposure.”
Treating melasma
Dr. Friedman’s go-to devices for melasma include the low-density, low-energy 1,927-nm fractional diode laser; the 1,064 nm picosecond Nd:YAG, the low-fluence 1,064 nm Q-switched Nd:YAG with a nanosecond pulse duration, and the 595-nm pulsed dye laser for lesions exhibiting underlying vascularity. He said that combining therapies that target pigment and vasculature may be ideal to prevent relapses. “Melasma is a multifactorial condition so by improving patient education and expectation alongside advances in laser treatment of melasma, we have ultimately improved our ability to treat this condition,” he said.
“We’re approaching it from all angles, with ultraviolet photography and spectrocolorimetry, behavioral modifications, topical skin-lightening agents, broad spectrum sunscreens with protection against visible light, and oral tranexamic acid in advanced cases. Then, we intervene with these energy-based modalities, and the bottom line is, less energy and density is more, with lengthened treatment intervals. In 2023, we’re better than we’ve ever been in terms of our ability to safely and effectively improve melasma.”
Novel lasers
Dr. Friedman also described the UltraClear, a novel ablative fractional 2,910-nm erbium-doped glass fiber laser that delivers a customized blend of ablation and coagulation based on the patient’s condition, skin type, and tolerability for down time. He provided an overview of the versatility of what he described as highly customizable technology for conditions such as photoaging and dyschromia in patients of various skin types, making it a very versatile platform in his practice.
The AVAVA MIRIA system is a “next generation” laser “where you’re able to use a focal point. Basically, you’re treating the skin from the inside out in a 3D manner and you’re able to focus intradermally up to 1 mm with high energy 1,064 nm or 1,550 nm,” he said. “It’s a unique conical geometry that spares the epidermis, combined with sapphire tip cooling and images the skin at the same time with the potential for personalized treatments of dyschromia and photoaging in all skin types. It’s truly remarkable where the technology is heading.”
Dr. Friedman disclosed that he has received consulting fees from Allergan, Galderma, Acclaro, Merz Aesthetics, Solta Medical, and Cytrellis. He has conducted contracted research for Sofwave and is a member of the speakers bureau for Solta Medical and Candela.
PHOENIX – Ever since PicoSure became the first picosecond laser cleared by the Food and Drug Administration for the treatment of unwanted tattoos and pigmented lesions in 2012, new uses for this technology continue to expand.
Now, These include PicoWay, PicoSure, Enlighten, PicoPlus, PiQo4, and Quanta Pico, among others.
“PicoWay technology has integrated nicely into my practice in Houston, the most ethnically diverse city in the country, with its ability to safely treat a number of various benign, congenital, and acquired epidermal and dermal pigmented lesions with ultrashort pulse duration and low thermal impact, which greatly reduces the risk of postinflammatory hyperpigmentation even in darker skin types,” Paul M. Friedman, MD, director of the Dermatology and Laser Surgery Center, Houston, said at the annual conference of the American Society for Laser Medicine and Surgery.
He emphasized the importance of therapeutic clinical endpoints, noting that with q-switched lasers, “you’re looking for immediate whitening, whereas with picosecond lasers, your endpoint is slight whitening or slight darkening depending on wavelength, indication, and skin type. The ability to fractionate picosecond pulses has also allowed us to utilize this technology for photoaging as well as acne scarring.”
The PicoWay system includes a 730-nm picosecond titanium sapphire handpiece, which is FDA cleared for treatment of benign pigmented lesions and blue and green tattoo removal. Dr. Friedman said that he has seen good clinical results using the handpiece for café-au-lait macules, particularly in skin of color.
In an abstract presented at the ASLMS meeting, he and his colleagues presented a retrospective review of 12 patients with café-au-lait macules with Fitzpatrick skin types III-VI who were treated with the PicoWay 730 nm handpiece between April 2021 and January 2023. Patients received a mean of 3.1 treatments at intervals that ranged from 5 to 40 weeks. Clinical photographs were graded by three board-certified dermatologists using a 5-point visual analogue scale.
Overall, patients were rated to have a mean improvement of 26%-50%. Two patients achieved 100% clearance after four to five treatment sessions. “Café-au-lait macules with smooth borders responded less well to laser treatment, confirming prior studies at our center,” he said. “We often educate parents that café-au-lait macules may recur over time, especially with repeated sun exposure.”
Treating melasma
Dr. Friedman’s go-to devices for melasma include the low-density, low-energy 1,927-nm fractional diode laser; the 1,064 nm picosecond Nd:YAG, the low-fluence 1,064 nm Q-switched Nd:YAG with a nanosecond pulse duration, and the 595-nm pulsed dye laser for lesions exhibiting underlying vascularity. He said that combining therapies that target pigment and vasculature may be ideal to prevent relapses. “Melasma is a multifactorial condition so by improving patient education and expectation alongside advances in laser treatment of melasma, we have ultimately improved our ability to treat this condition,” he said.
“We’re approaching it from all angles, with ultraviolet photography and spectrocolorimetry, behavioral modifications, topical skin-lightening agents, broad spectrum sunscreens with protection against visible light, and oral tranexamic acid in advanced cases. Then, we intervene with these energy-based modalities, and the bottom line is, less energy and density is more, with lengthened treatment intervals. In 2023, we’re better than we’ve ever been in terms of our ability to safely and effectively improve melasma.”
Novel lasers
Dr. Friedman also described the UltraClear, a novel ablative fractional 2,910-nm erbium-doped glass fiber laser that delivers a customized blend of ablation and coagulation based on the patient’s condition, skin type, and tolerability for down time. He provided an overview of the versatility of what he described as highly customizable technology for conditions such as photoaging and dyschromia in patients of various skin types, making it a very versatile platform in his practice.
The AVAVA MIRIA system is a “next generation” laser “where you’re able to use a focal point. Basically, you’re treating the skin from the inside out in a 3D manner and you’re able to focus intradermally up to 1 mm with high energy 1,064 nm or 1,550 nm,” he said. “It’s a unique conical geometry that spares the epidermis, combined with sapphire tip cooling and images the skin at the same time with the potential for personalized treatments of dyschromia and photoaging in all skin types. It’s truly remarkable where the technology is heading.”
Dr. Friedman disclosed that he has received consulting fees from Allergan, Galderma, Acclaro, Merz Aesthetics, Solta Medical, and Cytrellis. He has conducted contracted research for Sofwave and is a member of the speakers bureau for Solta Medical and Candela.
FROM ASLMS 2023
Two phase 3 trials show benefits of dupilumab for prurigo nodularis
The results, which were published online in Nature Medicine, were the basis for the FDA approval of dupilumab (Dupixent) for adults with PN in September 2022, the first treatment approved for treating PN in the United States.
“These positive studies support the involvement of type 2 cytokines in driving PN disease pathogenesis and the targeting of the [interleukin]-4/IL-13 axis as a novel therapeutic paradigm for patients with PN,” wrote the researchers, who were led by principal investigator Gil Yosipovitch, MD, professor of dermatology at the University of Miami, Fla. Dupilumab, an IL-4 receptor alpha antagonist, blocks the shared receptor component (IL-4R alpha) for IL-4 and IL-13.
For the two phase 3 trials, which were called LIBERTY-PN PRIME and PRIME2 and were sponsored by Sanofi and Regeneron Pharmaceuticals, researchers randomized adults with PN with 20 or more nodules and severe itch uncontrolled with topical therapies 1:1 to 300 mg dupilumab or placebo subcutaneously every 2 weeks for 24 weeks. The primary endpoint was pruritus improvement, which was measured by the proportion of patients with a 4-point or greater reduction in Worst Itch Numeric Rating Scale (WI-NRS) from baseline at week 24 (PRIME) or week 12 (PRIME2). Key secondary endpoints included a reduction in the number of nodules to 5 or fewer at week 24.
PRIME and PRIME2 enrolled 151 and 160 patients, respectively. In PRIME, 60% of patients in the dupilumab arm achieved a 4-point or greater reduction in the WI-NRS at week 24, compared with 18.4% of patients in the placebo arm (P < .001). In PRIME2, 37.2% of patients in the dupilumab arm achieved a 4-point or greater reduction in the WI-NRS at week 12, compared with 22% of patients in the placebo arm (P = .022).
The researchers also reported that, from an initial baseline of 20 to greater than 100 nodules, 32.0% of dupilumab-treated patients in PRIME and 25.6% in PRIME2 showed a reduction to 5 nodules or fewer, which corresponded to a response of “clear” or “almost clear” skin at week 12, compared with 11.8% and 12.2% of placebo-treated patients, respectively. This treatment effect on skin lesions continued to improve after week 12, with 48% of dupilumab-treated patients in PRIME and 44.9% in PRIME2 having five nodules or fewer at week 24, compared with 18.4% and 15.9% of placebo-treated patients, respectively. Safety was consistent with the known dupilumab safety profile.
“Validation is the first success of this paper,” said Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the study. “While both the safety and efficacy of dupilumab in these two phase 3 programs is the meat of the matter, nuanced highlights for me include the rigid nature of the exclusion criteria to ensure a study population that truly has PN as a stand-alone disease, rather than a secondary finding as we once believed to be the entire story. I think it’s important for us to recognize that it’s not one or the other, rather there is both ‘primary’ prurigo nodularis, and then there is secondary prurigo nodularis associated with something else [a wide range of underlying medical conditions], just like we divide primary and secondary hyperhidrosis.”
Dr. Yosipovitch reported having competing interests with several pharmaceutical companies, including Regeneron and Sanofi. Dr. Friedman disclosed that he is a consultant to and a speaker for Regeneron.
The results, which were published online in Nature Medicine, were the basis for the FDA approval of dupilumab (Dupixent) for adults with PN in September 2022, the first treatment approved for treating PN in the United States.
“These positive studies support the involvement of type 2 cytokines in driving PN disease pathogenesis and the targeting of the [interleukin]-4/IL-13 axis as a novel therapeutic paradigm for patients with PN,” wrote the researchers, who were led by principal investigator Gil Yosipovitch, MD, professor of dermatology at the University of Miami, Fla. Dupilumab, an IL-4 receptor alpha antagonist, blocks the shared receptor component (IL-4R alpha) for IL-4 and IL-13.
For the two phase 3 trials, which were called LIBERTY-PN PRIME and PRIME2 and were sponsored by Sanofi and Regeneron Pharmaceuticals, researchers randomized adults with PN with 20 or more nodules and severe itch uncontrolled with topical therapies 1:1 to 300 mg dupilumab or placebo subcutaneously every 2 weeks for 24 weeks. The primary endpoint was pruritus improvement, which was measured by the proportion of patients with a 4-point or greater reduction in Worst Itch Numeric Rating Scale (WI-NRS) from baseline at week 24 (PRIME) or week 12 (PRIME2). Key secondary endpoints included a reduction in the number of nodules to 5 or fewer at week 24.
PRIME and PRIME2 enrolled 151 and 160 patients, respectively. In PRIME, 60% of patients in the dupilumab arm achieved a 4-point or greater reduction in the WI-NRS at week 24, compared with 18.4% of patients in the placebo arm (P < .001). In PRIME2, 37.2% of patients in the dupilumab arm achieved a 4-point or greater reduction in the WI-NRS at week 12, compared with 22% of patients in the placebo arm (P = .022).
The researchers also reported that, from an initial baseline of 20 to greater than 100 nodules, 32.0% of dupilumab-treated patients in PRIME and 25.6% in PRIME2 showed a reduction to 5 nodules or fewer, which corresponded to a response of “clear” or “almost clear” skin at week 12, compared with 11.8% and 12.2% of placebo-treated patients, respectively. This treatment effect on skin lesions continued to improve after week 12, with 48% of dupilumab-treated patients in PRIME and 44.9% in PRIME2 having five nodules or fewer at week 24, compared with 18.4% and 15.9% of placebo-treated patients, respectively. Safety was consistent with the known dupilumab safety profile.
“Validation is the first success of this paper,” said Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the study. “While both the safety and efficacy of dupilumab in these two phase 3 programs is the meat of the matter, nuanced highlights for me include the rigid nature of the exclusion criteria to ensure a study population that truly has PN as a stand-alone disease, rather than a secondary finding as we once believed to be the entire story. I think it’s important for us to recognize that it’s not one or the other, rather there is both ‘primary’ prurigo nodularis, and then there is secondary prurigo nodularis associated with something else [a wide range of underlying medical conditions], just like we divide primary and secondary hyperhidrosis.”
Dr. Yosipovitch reported having competing interests with several pharmaceutical companies, including Regeneron and Sanofi. Dr. Friedman disclosed that he is a consultant to and a speaker for Regeneron.
The results, which were published online in Nature Medicine, were the basis for the FDA approval of dupilumab (Dupixent) for adults with PN in September 2022, the first treatment approved for treating PN in the United States.
“These positive studies support the involvement of type 2 cytokines in driving PN disease pathogenesis and the targeting of the [interleukin]-4/IL-13 axis as a novel therapeutic paradigm for patients with PN,” wrote the researchers, who were led by principal investigator Gil Yosipovitch, MD, professor of dermatology at the University of Miami, Fla. Dupilumab, an IL-4 receptor alpha antagonist, blocks the shared receptor component (IL-4R alpha) for IL-4 and IL-13.
For the two phase 3 trials, which were called LIBERTY-PN PRIME and PRIME2 and were sponsored by Sanofi and Regeneron Pharmaceuticals, researchers randomized adults with PN with 20 or more nodules and severe itch uncontrolled with topical therapies 1:1 to 300 mg dupilumab or placebo subcutaneously every 2 weeks for 24 weeks. The primary endpoint was pruritus improvement, which was measured by the proportion of patients with a 4-point or greater reduction in Worst Itch Numeric Rating Scale (WI-NRS) from baseline at week 24 (PRIME) or week 12 (PRIME2). Key secondary endpoints included a reduction in the number of nodules to 5 or fewer at week 24.
PRIME and PRIME2 enrolled 151 and 160 patients, respectively. In PRIME, 60% of patients in the dupilumab arm achieved a 4-point or greater reduction in the WI-NRS at week 24, compared with 18.4% of patients in the placebo arm (P < .001). In PRIME2, 37.2% of patients in the dupilumab arm achieved a 4-point or greater reduction in the WI-NRS at week 12, compared with 22% of patients in the placebo arm (P = .022).
The researchers also reported that, from an initial baseline of 20 to greater than 100 nodules, 32.0% of dupilumab-treated patients in PRIME and 25.6% in PRIME2 showed a reduction to 5 nodules or fewer, which corresponded to a response of “clear” or “almost clear” skin at week 12, compared with 11.8% and 12.2% of placebo-treated patients, respectively. This treatment effect on skin lesions continued to improve after week 12, with 48% of dupilumab-treated patients in PRIME and 44.9% in PRIME2 having five nodules or fewer at week 24, compared with 18.4% and 15.9% of placebo-treated patients, respectively. Safety was consistent with the known dupilumab safety profile.
“Validation is the first success of this paper,” said Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the study. “While both the safety and efficacy of dupilumab in these two phase 3 programs is the meat of the matter, nuanced highlights for me include the rigid nature of the exclusion criteria to ensure a study population that truly has PN as a stand-alone disease, rather than a secondary finding as we once believed to be the entire story. I think it’s important for us to recognize that it’s not one or the other, rather there is both ‘primary’ prurigo nodularis, and then there is secondary prurigo nodularis associated with something else [a wide range of underlying medical conditions], just like we divide primary and secondary hyperhidrosis.”
Dr. Yosipovitch reported having competing interests with several pharmaceutical companies, including Regeneron and Sanofi. Dr. Friedman disclosed that he is a consultant to and a speaker for Regeneron.
FROM NATURE MEDICINE
1,726-nm lasers poised to revolutionize acne treatment, expert predicts
PHOENIX – When Jeffrey Dover, MD, addressed audience members gathered for a session on cutting-edge technologies at the annual conference of the American Society for Laser Medicine and Surgery, he reflected on a conversation he had with R. Rox Anderson, MD, almost 40 years ago, about eventually finding a cure for acne.
“Despite the fact that we have over-the-counter therapies, prescription therapies, and all kinds of devices available to treat acne, there are still barriers to care that get in the way of treatment,” said Dr. Dover, director of SkinCare Physicians in Chestnut Hill, Mass. “If we had a device based on innovative light science that could meet the needs of the acne patient to get rid of these barriers, wouldn’t that be something wonderful?”
The answer to this question, he said, is now “yes,” because of advances in lasers that target sebaceous glands.
In a seminal paper published in 2012, Fernanda H. Sakamoto, MD, PhD, Dr. Anderson, and colleagues demonstrated the potential for a free electron laser to target sebaceous glands . Following several years of refinement, Dr. Dover said.
“With the 1,726-nm laser, there is some selective absorption in sebum in skin, which beats out absorption in the other chromophores,” he said. “But it’s not a big difference like it is, for example, for pulsed-dye lasers and vascular targets. ... This means that the therapeutic window is relatively small and protecting the rest of the epidermis and dermis is crucial to be able to target these lesions or the sebaceous gland without unnecessary damage. If we can protect the epidermis and heat just the sebaceous glands, we should be able to get Accutane-like results if we get durability [by] shrinking sebaceous glands.”
Effective cooling, whether contact cooling, bulk cooling, or air cooling, is crucial to success, he continued. “It’s got to be robust and highly specific to protect the skin, so you don’t end up with side effects that are worse than the disease.”
The AviClear laser delivers seven 3-mm spots, which takes into account the thermal relaxation times of the sebaceous glands. The algorithm delivers a treatment imprint at roughly 0.3 Hz and a 1.5-mm depth of penetration, and the device relies on contact cooling. In pivotal data submitted to the FDA, 104 individuals with moderate to severe acne received three treatments with the AviClear 1 month apart, with follow-up at 1, 3, 6, and 12 months post treatment. They had no other treatment regimens, and the primary endpoint was the percentage of patients who achieved a 50% reduction in inflammatory lesion count 3 months after the final treatment. The secondary endpoint was an Investigator’s Global Assessment (IGA) improvement of 2 or greater.
Dr. Dover, who helped design the study, said that, at 3 months, 80% of those treated achieved a 50% or greater reduction in inflammatory lesion count (P < .001). As for secondary endpoints, 36% of individuals were assessed as having clear or almost clear skin; 47% achieved a 2-point or greater improvement in IGA score, compared with baseline, and 87% achieved a 1-point or greater improvement in IGA score, compared with baseline. By 6 months, 88% of individuals achieved a 50% or greater reduction in inflammatory lesion count; this improved to 92% by 12 months (P < .001).
“All of these procedures were done with no topical anesthetic, no intralesional anesthetic, and they tolerated these quite well,” he said. “There was no down time that required medical intervention after the treatments. All posttreatment erythema and swelling resolved quickly,” and 75% of the patients were “very satisfied” with the treatments.
The Accure Laser System features a proprietary technology that precisely controls thermal gradient depth. “So instead of guessing whether you are delivering the correct amount of heat, it actually tells you,” said Dr. Dover, a past president of the ASLMS and the American Society for Dermatologic Surgery. “It correlates surface and at-depth temperatures, and there’s an infrared camera for real-time accurate temperature monitoring.” The device features highly controlled air cooling and a pulsing pattern that ensures treatment of sebaceous glands of all sizes and at all depths. The clinical end marker is peak epidermal temperature.
In a study supported by Accure, the manufacturer, researchers evaluated the efficacy of the Accure Laser System in 35 subjects with types I to VI skin, who received four monthly treatments 30-45 minutes each, and were followed 12, 26, 39, and 52 weeks following their last treatment. To date, data out to 52 weeks is available for 17 study participants. According to Dr. Dover, the researchers found 80% clearance at 12 weeks following the last treatment, with continued improvement at 52 weeks. One hundred percent of subjects responded. Side effects included erythema, edema, crusting, blisters, and inflammatory papules. “None of these were medically significant,” he said.
As dermatologists begin to incorporate the AviClear and Accure devices into their practices, Dr. Dover said that he is reminded of the conversation he had some 40 years ago with Dr. Anderson about finding a cure for acne, and he feels a bit awestruck. “These 1,726-nm lasers are effective for treating acne. I personally think they are going to revolutionize the way we treat at least some of our patients with acne. They may both be effective for treating facial acne scars. Time will tell. Further study of both scarring and acne are needed to fully categorize the benefit and to optimize treatments.”
To date no direct clinical comparisons have been made between the AviClear and Accure devices.
Dr. Dover reported that he is a consultant for Cutera, the manufacturer for AviClear. He also performs research for the company.
PHOENIX – When Jeffrey Dover, MD, addressed audience members gathered for a session on cutting-edge technologies at the annual conference of the American Society for Laser Medicine and Surgery, he reflected on a conversation he had with R. Rox Anderson, MD, almost 40 years ago, about eventually finding a cure for acne.
“Despite the fact that we have over-the-counter therapies, prescription therapies, and all kinds of devices available to treat acne, there are still barriers to care that get in the way of treatment,” said Dr. Dover, director of SkinCare Physicians in Chestnut Hill, Mass. “If we had a device based on innovative light science that could meet the needs of the acne patient to get rid of these barriers, wouldn’t that be something wonderful?”
The answer to this question, he said, is now “yes,” because of advances in lasers that target sebaceous glands.
In a seminal paper published in 2012, Fernanda H. Sakamoto, MD, PhD, Dr. Anderson, and colleagues demonstrated the potential for a free electron laser to target sebaceous glands . Following several years of refinement, Dr. Dover said.
“With the 1,726-nm laser, there is some selective absorption in sebum in skin, which beats out absorption in the other chromophores,” he said. “But it’s not a big difference like it is, for example, for pulsed-dye lasers and vascular targets. ... This means that the therapeutic window is relatively small and protecting the rest of the epidermis and dermis is crucial to be able to target these lesions or the sebaceous gland without unnecessary damage. If we can protect the epidermis and heat just the sebaceous glands, we should be able to get Accutane-like results if we get durability [by] shrinking sebaceous glands.”
Effective cooling, whether contact cooling, bulk cooling, or air cooling, is crucial to success, he continued. “It’s got to be robust and highly specific to protect the skin, so you don’t end up with side effects that are worse than the disease.”
The AviClear laser delivers seven 3-mm spots, which takes into account the thermal relaxation times of the sebaceous glands. The algorithm delivers a treatment imprint at roughly 0.3 Hz and a 1.5-mm depth of penetration, and the device relies on contact cooling. In pivotal data submitted to the FDA, 104 individuals with moderate to severe acne received three treatments with the AviClear 1 month apart, with follow-up at 1, 3, 6, and 12 months post treatment. They had no other treatment regimens, and the primary endpoint was the percentage of patients who achieved a 50% reduction in inflammatory lesion count 3 months after the final treatment. The secondary endpoint was an Investigator’s Global Assessment (IGA) improvement of 2 or greater.
Dr. Dover, who helped design the study, said that, at 3 months, 80% of those treated achieved a 50% or greater reduction in inflammatory lesion count (P < .001). As for secondary endpoints, 36% of individuals were assessed as having clear or almost clear skin; 47% achieved a 2-point or greater improvement in IGA score, compared with baseline, and 87% achieved a 1-point or greater improvement in IGA score, compared with baseline. By 6 months, 88% of individuals achieved a 50% or greater reduction in inflammatory lesion count; this improved to 92% by 12 months (P < .001).
“All of these procedures were done with no topical anesthetic, no intralesional anesthetic, and they tolerated these quite well,” he said. “There was no down time that required medical intervention after the treatments. All posttreatment erythema and swelling resolved quickly,” and 75% of the patients were “very satisfied” with the treatments.
The Accure Laser System features a proprietary technology that precisely controls thermal gradient depth. “So instead of guessing whether you are delivering the correct amount of heat, it actually tells you,” said Dr. Dover, a past president of the ASLMS and the American Society for Dermatologic Surgery. “It correlates surface and at-depth temperatures, and there’s an infrared camera for real-time accurate temperature monitoring.” The device features highly controlled air cooling and a pulsing pattern that ensures treatment of sebaceous glands of all sizes and at all depths. The clinical end marker is peak epidermal temperature.
In a study supported by Accure, the manufacturer, researchers evaluated the efficacy of the Accure Laser System in 35 subjects with types I to VI skin, who received four monthly treatments 30-45 minutes each, and were followed 12, 26, 39, and 52 weeks following their last treatment. To date, data out to 52 weeks is available for 17 study participants. According to Dr. Dover, the researchers found 80% clearance at 12 weeks following the last treatment, with continued improvement at 52 weeks. One hundred percent of subjects responded. Side effects included erythema, edema, crusting, blisters, and inflammatory papules. “None of these were medically significant,” he said.
As dermatologists begin to incorporate the AviClear and Accure devices into their practices, Dr. Dover said that he is reminded of the conversation he had some 40 years ago with Dr. Anderson about finding a cure for acne, and he feels a bit awestruck. “These 1,726-nm lasers are effective for treating acne. I personally think they are going to revolutionize the way we treat at least some of our patients with acne. They may both be effective for treating facial acne scars. Time will tell. Further study of both scarring and acne are needed to fully categorize the benefit and to optimize treatments.”
To date no direct clinical comparisons have been made between the AviClear and Accure devices.
Dr. Dover reported that he is a consultant for Cutera, the manufacturer for AviClear. He also performs research for the company.
PHOENIX – When Jeffrey Dover, MD, addressed audience members gathered for a session on cutting-edge technologies at the annual conference of the American Society for Laser Medicine and Surgery, he reflected on a conversation he had with R. Rox Anderson, MD, almost 40 years ago, about eventually finding a cure for acne.
“Despite the fact that we have over-the-counter therapies, prescription therapies, and all kinds of devices available to treat acne, there are still barriers to care that get in the way of treatment,” said Dr. Dover, director of SkinCare Physicians in Chestnut Hill, Mass. “If we had a device based on innovative light science that could meet the needs of the acne patient to get rid of these barriers, wouldn’t that be something wonderful?”
The answer to this question, he said, is now “yes,” because of advances in lasers that target sebaceous glands.
In a seminal paper published in 2012, Fernanda H. Sakamoto, MD, PhD, Dr. Anderson, and colleagues demonstrated the potential for a free electron laser to target sebaceous glands . Following several years of refinement, Dr. Dover said.
“With the 1,726-nm laser, there is some selective absorption in sebum in skin, which beats out absorption in the other chromophores,” he said. “But it’s not a big difference like it is, for example, for pulsed-dye lasers and vascular targets. ... This means that the therapeutic window is relatively small and protecting the rest of the epidermis and dermis is crucial to be able to target these lesions or the sebaceous gland without unnecessary damage. If we can protect the epidermis and heat just the sebaceous glands, we should be able to get Accutane-like results if we get durability [by] shrinking sebaceous glands.”
Effective cooling, whether contact cooling, bulk cooling, or air cooling, is crucial to success, he continued. “It’s got to be robust and highly specific to protect the skin, so you don’t end up with side effects that are worse than the disease.”
The AviClear laser delivers seven 3-mm spots, which takes into account the thermal relaxation times of the sebaceous glands. The algorithm delivers a treatment imprint at roughly 0.3 Hz and a 1.5-mm depth of penetration, and the device relies on contact cooling. In pivotal data submitted to the FDA, 104 individuals with moderate to severe acne received three treatments with the AviClear 1 month apart, with follow-up at 1, 3, 6, and 12 months post treatment. They had no other treatment regimens, and the primary endpoint was the percentage of patients who achieved a 50% reduction in inflammatory lesion count 3 months after the final treatment. The secondary endpoint was an Investigator’s Global Assessment (IGA) improvement of 2 or greater.
Dr. Dover, who helped design the study, said that, at 3 months, 80% of those treated achieved a 50% or greater reduction in inflammatory lesion count (P < .001). As for secondary endpoints, 36% of individuals were assessed as having clear or almost clear skin; 47% achieved a 2-point or greater improvement in IGA score, compared with baseline, and 87% achieved a 1-point or greater improvement in IGA score, compared with baseline. By 6 months, 88% of individuals achieved a 50% or greater reduction in inflammatory lesion count; this improved to 92% by 12 months (P < .001).
“All of these procedures were done with no topical anesthetic, no intralesional anesthetic, and they tolerated these quite well,” he said. “There was no down time that required medical intervention after the treatments. All posttreatment erythema and swelling resolved quickly,” and 75% of the patients were “very satisfied” with the treatments.
The Accure Laser System features a proprietary technology that precisely controls thermal gradient depth. “So instead of guessing whether you are delivering the correct amount of heat, it actually tells you,” said Dr. Dover, a past president of the ASLMS and the American Society for Dermatologic Surgery. “It correlates surface and at-depth temperatures, and there’s an infrared camera for real-time accurate temperature monitoring.” The device features highly controlled air cooling and a pulsing pattern that ensures treatment of sebaceous glands of all sizes and at all depths. The clinical end marker is peak epidermal temperature.
In a study supported by Accure, the manufacturer, researchers evaluated the efficacy of the Accure Laser System in 35 subjects with types I to VI skin, who received four monthly treatments 30-45 minutes each, and were followed 12, 26, 39, and 52 weeks following their last treatment. To date, data out to 52 weeks is available for 17 study participants. According to Dr. Dover, the researchers found 80% clearance at 12 weeks following the last treatment, with continued improvement at 52 weeks. One hundred percent of subjects responded. Side effects included erythema, edema, crusting, blisters, and inflammatory papules. “None of these were medically significant,” he said.
As dermatologists begin to incorporate the AviClear and Accure devices into their practices, Dr. Dover said that he is reminded of the conversation he had some 40 years ago with Dr. Anderson about finding a cure for acne, and he feels a bit awestruck. “These 1,726-nm lasers are effective for treating acne. I personally think they are going to revolutionize the way we treat at least some of our patients with acne. They may both be effective for treating facial acne scars. Time will tell. Further study of both scarring and acne are needed to fully categorize the benefit and to optimize treatments.”
To date no direct clinical comparisons have been made between the AviClear and Accure devices.
Dr. Dover reported that he is a consultant for Cutera, the manufacturer for AviClear. He also performs research for the company.
AT ASLMS 2023