User login
Damian McNamara is a journalist for Medscape Medical News and MDedge. He worked full-time for MDedge as the Miami Bureau covering a dozen medical specialties during 2001-2012, then as a freelancer for Medscape and MDedge, before being hired on staff by Medscape in 2018. Now the two companies are one. He uses what he learned in school – Damian has a BS in chemistry and an MS in science, health and environmental reporting/journalism. He works out of a home office in Miami, with a 100-pound chocolate lab known to snore under his desk during work hours.
Immobility implicated in increased complications after bariatric surgery
NEW YORK –
“The importance of this study is to help us as an institution, but then also nationally, to try to focus on quality initiatives to improve the complication rate and safety profile of these patients, who are incredibly high risk for bariatric surgery,” said Rana Higgins, MD, a general surgeon at Froedtert Hospital and the Medical College of Wisconsin in Milwaukee.
Dr. Higgins and her colleagues compared 2,969 immobile patients with 145,741 who were ambulatory before surgery. The most common bariatric procedure was sleeve gastrectomy at 56%. Another 30% had gastric bypass, 3% had the gastric band, and the remaining 1% underwent other procedures, such as biliopancreatic diversion with duodenal switch. The MBSAQIP (Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program) defines immobility as a patient with limited ambulation who requires assistive devices, such as a scooter or wheelchair, to ambulate most or all of the time. In addition, with regard to negotiating stairs, immobile patients need a home lift or an elevator.
Only three complications evaluated by the researchers were not statistically different between groups: intraoperative or postoperative coma, stroke, and myocardial infarction.
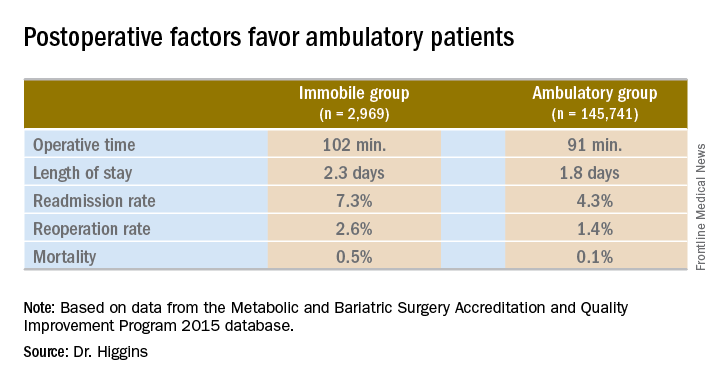
Operative time was longer in the immobile group, about 102 minutes vs. 91 minutes (P less than .001). A meeting attendee asked what accounted for the difference. Dr. Higgins replied, “We’ll have to go back and look at our data. My hypothesis is that the immobile patients had a higher BMI [body mass index]. They may also have had other comorbidities that contributed to increased operative time.”
Hospital length of stay was also significantly longer among immobile patients at 2.3 days vs. 1.8 days in the ambulatory group (P less than .001).
The readmission rate was higher among immobile patients – 7.3% vs. 4.3% for the ambulatory group. The reoperation rate was higher at 2.6% vs. 1.4%. Both these findings were statistically significant as well (P less than .001).
Immobile patients had a statistically higher risk of mortality at 0.5%, compared with 0.1% among ambulatory patients (OR, 4.6).
A meeting attendee asked Dr. Higgins if her institution addresses mobility issues. She replied that there is preoperative education about the importance of ambulation, but the interventions are focused on ambulation in the postoperative period. “We order physical therapy, immediately postoperatively; typically the patients will receive it that day or the next day. We make sure patients are up and moving as much as possible, but there are limitations if they have limited mobility.”
The same attendee suggested preoperative physical therapy could help, even if only 2-4 weeks prior to surgery. Dr. Higgins agreed that would be a good quality initiative to explore in the future.
She had no relevant financial disclosures.
NEW YORK –
“The importance of this study is to help us as an institution, but then also nationally, to try to focus on quality initiatives to improve the complication rate and safety profile of these patients, who are incredibly high risk for bariatric surgery,” said Rana Higgins, MD, a general surgeon at Froedtert Hospital and the Medical College of Wisconsin in Milwaukee.
Dr. Higgins and her colleagues compared 2,969 immobile patients with 145,741 who were ambulatory before surgery. The most common bariatric procedure was sleeve gastrectomy at 56%. Another 30% had gastric bypass, 3% had the gastric band, and the remaining 1% underwent other procedures, such as biliopancreatic diversion with duodenal switch. The MBSAQIP (Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program) defines immobility as a patient with limited ambulation who requires assistive devices, such as a scooter or wheelchair, to ambulate most or all of the time. In addition, with regard to negotiating stairs, immobile patients need a home lift or an elevator.
Only three complications evaluated by the researchers were not statistically different between groups: intraoperative or postoperative coma, stroke, and myocardial infarction.

Operative time was longer in the immobile group, about 102 minutes vs. 91 minutes (P less than .001). A meeting attendee asked what accounted for the difference. Dr. Higgins replied, “We’ll have to go back and look at our data. My hypothesis is that the immobile patients had a higher BMI [body mass index]. They may also have had other comorbidities that contributed to increased operative time.”
Hospital length of stay was also significantly longer among immobile patients at 2.3 days vs. 1.8 days in the ambulatory group (P less than .001).
The readmission rate was higher among immobile patients – 7.3% vs. 4.3% for the ambulatory group. The reoperation rate was higher at 2.6% vs. 1.4%. Both these findings were statistically significant as well (P less than .001).
Immobile patients had a statistically higher risk of mortality at 0.5%, compared with 0.1% among ambulatory patients (OR, 4.6).
A meeting attendee asked Dr. Higgins if her institution addresses mobility issues. She replied that there is preoperative education about the importance of ambulation, but the interventions are focused on ambulation in the postoperative period. “We order physical therapy, immediately postoperatively; typically the patients will receive it that day or the next day. We make sure patients are up and moving as much as possible, but there are limitations if they have limited mobility.”
The same attendee suggested preoperative physical therapy could help, even if only 2-4 weeks prior to surgery. Dr. Higgins agreed that would be a good quality initiative to explore in the future.
She had no relevant financial disclosures.
NEW YORK –
“The importance of this study is to help us as an institution, but then also nationally, to try to focus on quality initiatives to improve the complication rate and safety profile of these patients, who are incredibly high risk for bariatric surgery,” said Rana Higgins, MD, a general surgeon at Froedtert Hospital and the Medical College of Wisconsin in Milwaukee.
Dr. Higgins and her colleagues compared 2,969 immobile patients with 145,741 who were ambulatory before surgery. The most common bariatric procedure was sleeve gastrectomy at 56%. Another 30% had gastric bypass, 3% had the gastric band, and the remaining 1% underwent other procedures, such as biliopancreatic diversion with duodenal switch. The MBSAQIP (Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program) defines immobility as a patient with limited ambulation who requires assistive devices, such as a scooter or wheelchair, to ambulate most or all of the time. In addition, with regard to negotiating stairs, immobile patients need a home lift or an elevator.
Only three complications evaluated by the researchers were not statistically different between groups: intraoperative or postoperative coma, stroke, and myocardial infarction.

Operative time was longer in the immobile group, about 102 minutes vs. 91 minutes (P less than .001). A meeting attendee asked what accounted for the difference. Dr. Higgins replied, “We’ll have to go back and look at our data. My hypothesis is that the immobile patients had a higher BMI [body mass index]. They may also have had other comorbidities that contributed to increased operative time.”
Hospital length of stay was also significantly longer among immobile patients at 2.3 days vs. 1.8 days in the ambulatory group (P less than .001).
The readmission rate was higher among immobile patients – 7.3% vs. 4.3% for the ambulatory group. The reoperation rate was higher at 2.6% vs. 1.4%. Both these findings were statistically significant as well (P less than .001).
Immobile patients had a statistically higher risk of mortality at 0.5%, compared with 0.1% among ambulatory patients (OR, 4.6).
A meeting attendee asked Dr. Higgins if her institution addresses mobility issues. She replied that there is preoperative education about the importance of ambulation, but the interventions are focused on ambulation in the postoperative period. “We order physical therapy, immediately postoperatively; typically the patients will receive it that day or the next day. We make sure patients are up and moving as much as possible, but there are limitations if they have limited mobility.”
The same attendee suggested preoperative physical therapy could help, even if only 2-4 weeks prior to surgery. Dr. Higgins agreed that would be a good quality initiative to explore in the future.
She had no relevant financial disclosures.
AT THE ACS QUALITY & SAFETY CONFERENCE
Key clinical point: Patients immobile before bariatric surgery could require closer monitoring for postoperative complications.
Major finding: Thirty-day mortality after bariatric surgery in immobile patients was 0.5%, vs. 0.1% for an ambulatory group (P less than .0001).
Data source: A comparison of 2015 MBSAQIP data for 145,741 ambulatory patients and 2,969 immobile patients before bariatric surgery.
Disclosures: Dr. Higgins had no relevant financial disclosures.
Think beyond BMI to optimize bariatric patients presurgery
NEW YORK – A structured, four-pronged approach to get patients as fit and healthy as possible prior to bariatric surgery holds the potential to improve postoperative outcomes. In general, bariatric surgery patients are in a better position than most surgery candidates because of a longer preoperative period. During this time, surgeons can work with a multidisciplinary team to optimize any medical, nutritional, exercise-related, and mental health concerns.
“People focus on the size of our patients and the weight of our patients, but [body mass index] is only one factor. They can have many other comorbidities that are significant,” Dr. LaMasters said. Patients can present with cardiac and pulmonary issues, hypertension, sleep apnea, diabetes, asthma, reflux and “a very high incidence of anxiety and depression.”
“So we have a lot of challenges,” she added. “We take care of complex, high-risk patients, and our goal is to improve outcomes. Using presurgery optimization can be a key to that.”
Maximizing medical readiness
Multiple providers drive the medical intervention, Dr. LaMasters said, including surgeons and primary care doctors, as well as advanced practice providers, medical weight loss providers, and other specialists. “We do try to get patients to lose weight before surgery, but that’s not an absolute requirement. More important is adjustment of other risk factors like pulmonary risk factors, control of hypertension, treatment of sleep apnea, and control of hyperglycemia. We’d like to have their A1c [test results to be] under 8%. We want to start [proton pump inhibitors] early because there is a very high prevalence of reflux and gastritis in this population.”
Bariatric surgery patients “are uniquely positioned to have a substantial benefit from that ‘prehabilitation,’ but this only works if you have a multidisciplinary team,” Dr. LaMasters said at the American College of Surgeons Quality and Safety Conference. “Think of this as down-staging disease, like in a cancer model.”
“The message from this is there is an opportunity if we build it into the prehab phase of care. It’s a new way of thinking in surgery. You can change your results,” said session moderator David B. Hoyt, MD, FACS, Executive Director of the American College of Surgeons.
Nutritional know-how
Dietitians determine the second component – how to optimize nutrition before surgery. They focus on education, evaluation, setting goals, “and very importantly, supporting patients to attain those goals,” Dr. LaMasters said. Goals include increasing protein intake prior to surgery to a recommended 1.5 g/kg/day and starting nutritional supplements ahead of time.
Even though they typically consume an excess amount of calories, “many of our patients have baseline malnutrition,” Dr. LaMasters said. Establishing mindful behavior for meal planning, preparation, and eating is a potential solution, as is addressing any socioeconomic factors that can present challenges to healthy eating.
Emphasizing exercise
“The exercise piece is really key for our patients,” Dr. LaMasters said. Many candidates for bariatric surgery have mobility issues. “The first thing many say is ‘I can’t exercise.’ We instruct them that they can exercise. Our job is to find out what they can do – there are many different exercise modalities.”
A good baseline assessment is a 6-minute walk test to assess their distance limits, oxygen level, and any resulting symptoms.
“Our goal is to get them to walking – even those who can barely walk with a walker – for 5-10 minutes, six times a day,” Dr. LaMasters said. “We feel that is a minimum threshold to prevent blood clots after surgery.” Another recommendation is to get surgical candidates to do some activity 30 minutes a day, four times a week, at a minimum. “Eventually, after surgery and when they’ve lost weight and are healthier, the goal is going to be 1 hour, five days a week.”
Start the exercise program at least 4-8 weeks prior to surgery. Most studies show significant benefit if you start at least 4 weeks prior to surgery, Dr. LaMasters suggested. “In our own practice, we’ve seen if you can start a daily walking program even just 2 weeks prior to surgery, we see a significant benefit.”
Addressing anxiety or depression
The mental health piece is very important and should be guided by mental health providers on the multidisciplinary team, Dr. LaMasters said.
“Our patients have a high degree of stress in their lives, especially related to socioeconomic factors. A patient who does not have their anxiety or depression under control will not do as well after surgery.”
Optimization in other specialties
The benefits of a prehabilitation exercise program have been demonstrated across many other specialties, especially in colorectal surgery, cardiovascular surgery, and orthopedic surgery, Dr. LaMasters said. In randomized, controlled studies, this optimization is associated with decreased complications, mortality, and length of hospital stay.
“There is actually way less data from bariatric studies. I suggest to you that our bariatric surgery patients have similar comorbidities when compared with those other specialties – specialties that refer their patients to us for treatment,” Dr. LaMasters said.
In a study of cardiorespiratory fitness before bariatric surgery, other researchers found that the most serious postoperative complications occurred more often among patients who were less fit preoperatively (Chest. 2006 Aug;130[2]:517-25). These investigators measured peak oxygen consumption (VO2) preoperatively in 109 patients. “Each unit increase in peak VO2 rate was associated with 61% decrease in overall complications,” Dr. LaMasters said. “So a small increase in fitness led to a big decrease in complications.”
Other researchers compared optimization of exercise, nutrition, and psychological factors before and after surgery in 185 patients with colorectal cancer (Acta Oncol. 2017 Feb;56[2]:295-300). A control group received the interventions postoperatively. “They found a statistically significant difference in the prehabilitation group in increased functional capacity, with more than a 30-meter improvement in 6-minute walk test before surgery,” Dr. LaMasters said. Although the 6-minute walk test results decreased 4 weeks after surgery, as might be expected, by 8 weeks the prehabilitation patients performed better than controls – and even better than their own baseline, she added. “This model of optimization can be very well applied in bariatric surgery.”
“The goal is safe surgery with outstanding long-term outcomes,” Dr. LaMasters said. “It is really not enough in this era to ‘get a patient through surgery.’ We really need to optimize the risk factors we can and identify any areas where they will have additional needs after surgery,” she added. “This will allow us to have excellent outcomes in this complex patient population.”
Dr. LaMasters and Dr. Hoyt had no relevant financial disclosures.
NEW YORK – A structured, four-pronged approach to get patients as fit and healthy as possible prior to bariatric surgery holds the potential to improve postoperative outcomes. In general, bariatric surgery patients are in a better position than most surgery candidates because of a longer preoperative period. During this time, surgeons can work with a multidisciplinary team to optimize any medical, nutritional, exercise-related, and mental health concerns.
“People focus on the size of our patients and the weight of our patients, but [body mass index] is only one factor. They can have many other comorbidities that are significant,” Dr. LaMasters said. Patients can present with cardiac and pulmonary issues, hypertension, sleep apnea, diabetes, asthma, reflux and “a very high incidence of anxiety and depression.”
“So we have a lot of challenges,” she added. “We take care of complex, high-risk patients, and our goal is to improve outcomes. Using presurgery optimization can be a key to that.”
Maximizing medical readiness
Multiple providers drive the medical intervention, Dr. LaMasters said, including surgeons and primary care doctors, as well as advanced practice providers, medical weight loss providers, and other specialists. “We do try to get patients to lose weight before surgery, but that’s not an absolute requirement. More important is adjustment of other risk factors like pulmonary risk factors, control of hypertension, treatment of sleep apnea, and control of hyperglycemia. We’d like to have their A1c [test results to be] under 8%. We want to start [proton pump inhibitors] early because there is a very high prevalence of reflux and gastritis in this population.”
Bariatric surgery patients “are uniquely positioned to have a substantial benefit from that ‘prehabilitation,’ but this only works if you have a multidisciplinary team,” Dr. LaMasters said at the American College of Surgeons Quality and Safety Conference. “Think of this as down-staging disease, like in a cancer model.”
“The message from this is there is an opportunity if we build it into the prehab phase of care. It’s a new way of thinking in surgery. You can change your results,” said session moderator David B. Hoyt, MD, FACS, Executive Director of the American College of Surgeons.
Nutritional know-how
Dietitians determine the second component – how to optimize nutrition before surgery. They focus on education, evaluation, setting goals, “and very importantly, supporting patients to attain those goals,” Dr. LaMasters said. Goals include increasing protein intake prior to surgery to a recommended 1.5 g/kg/day and starting nutritional supplements ahead of time.
Even though they typically consume an excess amount of calories, “many of our patients have baseline malnutrition,” Dr. LaMasters said. Establishing mindful behavior for meal planning, preparation, and eating is a potential solution, as is addressing any socioeconomic factors that can present challenges to healthy eating.
Emphasizing exercise
“The exercise piece is really key for our patients,” Dr. LaMasters said. Many candidates for bariatric surgery have mobility issues. “The first thing many say is ‘I can’t exercise.’ We instruct them that they can exercise. Our job is to find out what they can do – there are many different exercise modalities.”
A good baseline assessment is a 6-minute walk test to assess their distance limits, oxygen level, and any resulting symptoms.
“Our goal is to get them to walking – even those who can barely walk with a walker – for 5-10 minutes, six times a day,” Dr. LaMasters said. “We feel that is a minimum threshold to prevent blood clots after surgery.” Another recommendation is to get surgical candidates to do some activity 30 minutes a day, four times a week, at a minimum. “Eventually, after surgery and when they’ve lost weight and are healthier, the goal is going to be 1 hour, five days a week.”
Start the exercise program at least 4-8 weeks prior to surgery. Most studies show significant benefit if you start at least 4 weeks prior to surgery, Dr. LaMasters suggested. “In our own practice, we’ve seen if you can start a daily walking program even just 2 weeks prior to surgery, we see a significant benefit.”
Addressing anxiety or depression
The mental health piece is very important and should be guided by mental health providers on the multidisciplinary team, Dr. LaMasters said.
“Our patients have a high degree of stress in their lives, especially related to socioeconomic factors. A patient who does not have their anxiety or depression under control will not do as well after surgery.”
Optimization in other specialties
The benefits of a prehabilitation exercise program have been demonstrated across many other specialties, especially in colorectal surgery, cardiovascular surgery, and orthopedic surgery, Dr. LaMasters said. In randomized, controlled studies, this optimization is associated with decreased complications, mortality, and length of hospital stay.
“There is actually way less data from bariatric studies. I suggest to you that our bariatric surgery patients have similar comorbidities when compared with those other specialties – specialties that refer their patients to us for treatment,” Dr. LaMasters said.
In a study of cardiorespiratory fitness before bariatric surgery, other researchers found that the most serious postoperative complications occurred more often among patients who were less fit preoperatively (Chest. 2006 Aug;130[2]:517-25). These investigators measured peak oxygen consumption (VO2) preoperatively in 109 patients. “Each unit increase in peak VO2 rate was associated with 61% decrease in overall complications,” Dr. LaMasters said. “So a small increase in fitness led to a big decrease in complications.”
Other researchers compared optimization of exercise, nutrition, and psychological factors before and after surgery in 185 patients with colorectal cancer (Acta Oncol. 2017 Feb;56[2]:295-300). A control group received the interventions postoperatively. “They found a statistically significant difference in the prehabilitation group in increased functional capacity, with more than a 30-meter improvement in 6-minute walk test before surgery,” Dr. LaMasters said. Although the 6-minute walk test results decreased 4 weeks after surgery, as might be expected, by 8 weeks the prehabilitation patients performed better than controls – and even better than their own baseline, she added. “This model of optimization can be very well applied in bariatric surgery.”
“The goal is safe surgery with outstanding long-term outcomes,” Dr. LaMasters said. “It is really not enough in this era to ‘get a patient through surgery.’ We really need to optimize the risk factors we can and identify any areas where they will have additional needs after surgery,” she added. “This will allow us to have excellent outcomes in this complex patient population.”
Dr. LaMasters and Dr. Hoyt had no relevant financial disclosures.
NEW YORK – A structured, four-pronged approach to get patients as fit and healthy as possible prior to bariatric surgery holds the potential to improve postoperative outcomes. In general, bariatric surgery patients are in a better position than most surgery candidates because of a longer preoperative period. During this time, surgeons can work with a multidisciplinary team to optimize any medical, nutritional, exercise-related, and mental health concerns.
“People focus on the size of our patients and the weight of our patients, but [body mass index] is only one factor. They can have many other comorbidities that are significant,” Dr. LaMasters said. Patients can present with cardiac and pulmonary issues, hypertension, sleep apnea, diabetes, asthma, reflux and “a very high incidence of anxiety and depression.”
“So we have a lot of challenges,” she added. “We take care of complex, high-risk patients, and our goal is to improve outcomes. Using presurgery optimization can be a key to that.”
Maximizing medical readiness
Multiple providers drive the medical intervention, Dr. LaMasters said, including surgeons and primary care doctors, as well as advanced practice providers, medical weight loss providers, and other specialists. “We do try to get patients to lose weight before surgery, but that’s not an absolute requirement. More important is adjustment of other risk factors like pulmonary risk factors, control of hypertension, treatment of sleep apnea, and control of hyperglycemia. We’d like to have their A1c [test results to be] under 8%. We want to start [proton pump inhibitors] early because there is a very high prevalence of reflux and gastritis in this population.”
Bariatric surgery patients “are uniquely positioned to have a substantial benefit from that ‘prehabilitation,’ but this only works if you have a multidisciplinary team,” Dr. LaMasters said at the American College of Surgeons Quality and Safety Conference. “Think of this as down-staging disease, like in a cancer model.”
“The message from this is there is an opportunity if we build it into the prehab phase of care. It’s a new way of thinking in surgery. You can change your results,” said session moderator David B. Hoyt, MD, FACS, Executive Director of the American College of Surgeons.
Nutritional know-how
Dietitians determine the second component – how to optimize nutrition before surgery. They focus on education, evaluation, setting goals, “and very importantly, supporting patients to attain those goals,” Dr. LaMasters said. Goals include increasing protein intake prior to surgery to a recommended 1.5 g/kg/day and starting nutritional supplements ahead of time.
Even though they typically consume an excess amount of calories, “many of our patients have baseline malnutrition,” Dr. LaMasters said. Establishing mindful behavior for meal planning, preparation, and eating is a potential solution, as is addressing any socioeconomic factors that can present challenges to healthy eating.
Emphasizing exercise
“The exercise piece is really key for our patients,” Dr. LaMasters said. Many candidates for bariatric surgery have mobility issues. “The first thing many say is ‘I can’t exercise.’ We instruct them that they can exercise. Our job is to find out what they can do – there are many different exercise modalities.”
A good baseline assessment is a 6-minute walk test to assess their distance limits, oxygen level, and any resulting symptoms.
“Our goal is to get them to walking – even those who can barely walk with a walker – for 5-10 minutes, six times a day,” Dr. LaMasters said. “We feel that is a minimum threshold to prevent blood clots after surgery.” Another recommendation is to get surgical candidates to do some activity 30 minutes a day, four times a week, at a minimum. “Eventually, after surgery and when they’ve lost weight and are healthier, the goal is going to be 1 hour, five days a week.”
Start the exercise program at least 4-8 weeks prior to surgery. Most studies show significant benefit if you start at least 4 weeks prior to surgery, Dr. LaMasters suggested. “In our own practice, we’ve seen if you can start a daily walking program even just 2 weeks prior to surgery, we see a significant benefit.”
Addressing anxiety or depression
The mental health piece is very important and should be guided by mental health providers on the multidisciplinary team, Dr. LaMasters said.
“Our patients have a high degree of stress in their lives, especially related to socioeconomic factors. A patient who does not have their anxiety or depression under control will not do as well after surgery.”
Optimization in other specialties
The benefits of a prehabilitation exercise program have been demonstrated across many other specialties, especially in colorectal surgery, cardiovascular surgery, and orthopedic surgery, Dr. LaMasters said. In randomized, controlled studies, this optimization is associated with decreased complications, mortality, and length of hospital stay.
“There is actually way less data from bariatric studies. I suggest to you that our bariatric surgery patients have similar comorbidities when compared with those other specialties – specialties that refer their patients to us for treatment,” Dr. LaMasters said.
In a study of cardiorespiratory fitness before bariatric surgery, other researchers found that the most serious postoperative complications occurred more often among patients who were less fit preoperatively (Chest. 2006 Aug;130[2]:517-25). These investigators measured peak oxygen consumption (VO2) preoperatively in 109 patients. “Each unit increase in peak VO2 rate was associated with 61% decrease in overall complications,” Dr. LaMasters said. “So a small increase in fitness led to a big decrease in complications.”
Other researchers compared optimization of exercise, nutrition, and psychological factors before and after surgery in 185 patients with colorectal cancer (Acta Oncol. 2017 Feb;56[2]:295-300). A control group received the interventions postoperatively. “They found a statistically significant difference in the prehabilitation group in increased functional capacity, with more than a 30-meter improvement in 6-minute walk test before surgery,” Dr. LaMasters said. Although the 6-minute walk test results decreased 4 weeks after surgery, as might be expected, by 8 weeks the prehabilitation patients performed better than controls – and even better than their own baseline, she added. “This model of optimization can be very well applied in bariatric surgery.”
“The goal is safe surgery with outstanding long-term outcomes,” Dr. LaMasters said. “It is really not enough in this era to ‘get a patient through surgery.’ We really need to optimize the risk factors we can and identify any areas where they will have additional needs after surgery,” she added. “This will allow us to have excellent outcomes in this complex patient population.”
Dr. LaMasters and Dr. Hoyt had no relevant financial disclosures.
AT THE ACS QUALITY & SAFETY CONFERENCE
MBSAQIP data helped target problem areas to cut readmissions
NEW YORK – Targeted interventions aimed at reducing patient readmission after bariatric surgery at a high-volume academic medical center led to a 61% overall decrease year over year. The center also saw a substantial reduction in readmissions linked to the top three factors of readmission identified by the Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program, as well as a precipitous drop in the revisional surgery readmission rate.
“Our center, like so many others, has quarterly meetings in accordance with the MBSAQIP to look at our data. And this led to recognition of some common reasons for readmission,” said Chetan V. Aher, MD, a general surgeon at the department of surgery at Vanderbilt University Medical Center in Nashville, Tenn. Oral (PO) intolerance, dehydration, and nonemergent abdominal pain were the top reasons flagged by the MBSAQIP registry data at the medical center. Dr. Aher and his colleagues moved to focus on postoperative diet, administration of medications, management of patients who return to the hospital after surgery, and optimal staffing.
“Notably, the readmission rate for revisional procedures decreased by a whopping 90%,” Dr. Aher said. “I think a lot of these targeted interventions just really helped these patients who were at a higher risk to begin with to be readmitted.”
New dietary dos and don’ts
“We changed our postoperative diet,” Dr. Aher said. Instead of a soft food diet a couple of days after surgery, the full liquid diet was extended to 3 weeks post surgery.
The clinicians also implemented what they called a ‘no MEALS’ policy, which stands for no Meat, Eggs And Leftovers. “We were having problems with meat, although tender fish was okay, and some other things that went down easily,” Dr. Aher said at the American College of Surgeons Quality and Safety Conference. “We had some complaints about no eggs after surgery. A lot of patients love eggs,” he added. But they recommended avoiding eggs for 1 month after bariatric surgery to avoid nausea.
“Avoiding leftovers was also a big deal for patients,” Dr. Aher said. But patients who microwaved leftovers would “then come into the hospital with problems.”
Medication modifications
Another frequent cause of nausea was a “terrible and off-putting” taste when crushed tablets or medication capsules were added to the patient’s diet. Changing how patients took their medication “was a big help.” At the same time, there was a large institutional effort at Vanderbilt to start providing discharge medications in the hospital to increase postoperative compliance. “Bariatric surgery was one of the pilot programs for this,” Dr. Aher said. Discharge medications were filled by the pharmacy at Vanderbilt and delivered to the patient’s room, and a pharmacist or pharmacy intern explained how to use them. Compliance on medications increased, which may in turn have had an impact on readmissions.
Changes to patient management
Dr. Aher and his colleagues also changed where they treated patients who returned with problems. “Previously, when patients called in, the clinic diverted them to the emergency room. We stopped doing that, and increased our capacity to see these patients in the clinic instead.” This led to an increase in use of IV hydration in the clinic.
A meeting attendee asked if providing this service led to any problems with clinic capacity.
“Sometimes,” Dr. Aher said. “We don’t have a huge number of patients coming in for IV hydration, but when we had two come in on the same day, it did take up a couple of exam rooms.” To address this, the clinicians found other space in the clinic that would offer privacy for patients while not tying up exam rooms.
In addition, the clinic expanded nurse practitioner availability to 5 days a week to make the discharge process more consistent. “Of course, as we rolled all these things out, we made sure our educational material was updated accordingly,” Dr. Aher said.
The study demonstrates that a collaborative team effort and targeted interventions can result in a significant reduction in readmissions, Dr. Aher said. “Regular quality focused meetings are really important to facilitate recognition of various areas for improvement, especially in a high-volume center. Introducing an MBSAQIP registry serves as an excellent tool to effect these changes,” he said.
Dr. Aher had no relevant financial disclosures.
NEW YORK – Targeted interventions aimed at reducing patient readmission after bariatric surgery at a high-volume academic medical center led to a 61% overall decrease year over year. The center also saw a substantial reduction in readmissions linked to the top three factors of readmission identified by the Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program, as well as a precipitous drop in the revisional surgery readmission rate.
“Our center, like so many others, has quarterly meetings in accordance with the MBSAQIP to look at our data. And this led to recognition of some common reasons for readmission,” said Chetan V. Aher, MD, a general surgeon at the department of surgery at Vanderbilt University Medical Center in Nashville, Tenn. Oral (PO) intolerance, dehydration, and nonemergent abdominal pain were the top reasons flagged by the MBSAQIP registry data at the medical center. Dr. Aher and his colleagues moved to focus on postoperative diet, administration of medications, management of patients who return to the hospital after surgery, and optimal staffing.
“Notably, the readmission rate for revisional procedures decreased by a whopping 90%,” Dr. Aher said. “I think a lot of these targeted interventions just really helped these patients who were at a higher risk to begin with to be readmitted.”
New dietary dos and don’ts
“We changed our postoperative diet,” Dr. Aher said. Instead of a soft food diet a couple of days after surgery, the full liquid diet was extended to 3 weeks post surgery.
The clinicians also implemented what they called a ‘no MEALS’ policy, which stands for no Meat, Eggs And Leftovers. “We were having problems with meat, although tender fish was okay, and some other things that went down easily,” Dr. Aher said at the American College of Surgeons Quality and Safety Conference. “We had some complaints about no eggs after surgery. A lot of patients love eggs,” he added. But they recommended avoiding eggs for 1 month after bariatric surgery to avoid nausea.
“Avoiding leftovers was also a big deal for patients,” Dr. Aher said. But patients who microwaved leftovers would “then come into the hospital with problems.”
Medication modifications
Another frequent cause of nausea was a “terrible and off-putting” taste when crushed tablets or medication capsules were added to the patient’s diet. Changing how patients took their medication “was a big help.” At the same time, there was a large institutional effort at Vanderbilt to start providing discharge medications in the hospital to increase postoperative compliance. “Bariatric surgery was one of the pilot programs for this,” Dr. Aher said. Discharge medications were filled by the pharmacy at Vanderbilt and delivered to the patient’s room, and a pharmacist or pharmacy intern explained how to use them. Compliance on medications increased, which may in turn have had an impact on readmissions.
Changes to patient management
Dr. Aher and his colleagues also changed where they treated patients who returned with problems. “Previously, when patients called in, the clinic diverted them to the emergency room. We stopped doing that, and increased our capacity to see these patients in the clinic instead.” This led to an increase in use of IV hydration in the clinic.
A meeting attendee asked if providing this service led to any problems with clinic capacity.
“Sometimes,” Dr. Aher said. “We don’t have a huge number of patients coming in for IV hydration, but when we had two come in on the same day, it did take up a couple of exam rooms.” To address this, the clinicians found other space in the clinic that would offer privacy for patients while not tying up exam rooms.
In addition, the clinic expanded nurse practitioner availability to 5 days a week to make the discharge process more consistent. “Of course, as we rolled all these things out, we made sure our educational material was updated accordingly,” Dr. Aher said.
The study demonstrates that a collaborative team effort and targeted interventions can result in a significant reduction in readmissions, Dr. Aher said. “Regular quality focused meetings are really important to facilitate recognition of various areas for improvement, especially in a high-volume center. Introducing an MBSAQIP registry serves as an excellent tool to effect these changes,” he said.
Dr. Aher had no relevant financial disclosures.
NEW YORK – Targeted interventions aimed at reducing patient readmission after bariatric surgery at a high-volume academic medical center led to a 61% overall decrease year over year. The center also saw a substantial reduction in readmissions linked to the top three factors of readmission identified by the Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program, as well as a precipitous drop in the revisional surgery readmission rate.
“Our center, like so many others, has quarterly meetings in accordance with the MBSAQIP to look at our data. And this led to recognition of some common reasons for readmission,” said Chetan V. Aher, MD, a general surgeon at the department of surgery at Vanderbilt University Medical Center in Nashville, Tenn. Oral (PO) intolerance, dehydration, and nonemergent abdominal pain were the top reasons flagged by the MBSAQIP registry data at the medical center. Dr. Aher and his colleagues moved to focus on postoperative diet, administration of medications, management of patients who return to the hospital after surgery, and optimal staffing.
“Notably, the readmission rate for revisional procedures decreased by a whopping 90%,” Dr. Aher said. “I think a lot of these targeted interventions just really helped these patients who were at a higher risk to begin with to be readmitted.”
New dietary dos and don’ts
“We changed our postoperative diet,” Dr. Aher said. Instead of a soft food diet a couple of days after surgery, the full liquid diet was extended to 3 weeks post surgery.
The clinicians also implemented what they called a ‘no MEALS’ policy, which stands for no Meat, Eggs And Leftovers. “We were having problems with meat, although tender fish was okay, and some other things that went down easily,” Dr. Aher said at the American College of Surgeons Quality and Safety Conference. “We had some complaints about no eggs after surgery. A lot of patients love eggs,” he added. But they recommended avoiding eggs for 1 month after bariatric surgery to avoid nausea.
“Avoiding leftovers was also a big deal for patients,” Dr. Aher said. But patients who microwaved leftovers would “then come into the hospital with problems.”
Medication modifications
Another frequent cause of nausea was a “terrible and off-putting” taste when crushed tablets or medication capsules were added to the patient’s diet. Changing how patients took their medication “was a big help.” At the same time, there was a large institutional effort at Vanderbilt to start providing discharge medications in the hospital to increase postoperative compliance. “Bariatric surgery was one of the pilot programs for this,” Dr. Aher said. Discharge medications were filled by the pharmacy at Vanderbilt and delivered to the patient’s room, and a pharmacist or pharmacy intern explained how to use them. Compliance on medications increased, which may in turn have had an impact on readmissions.
Changes to patient management
Dr. Aher and his colleagues also changed where they treated patients who returned with problems. “Previously, when patients called in, the clinic diverted them to the emergency room. We stopped doing that, and increased our capacity to see these patients in the clinic instead.” This led to an increase in use of IV hydration in the clinic.
A meeting attendee asked if providing this service led to any problems with clinic capacity.
“Sometimes,” Dr. Aher said. “We don’t have a huge number of patients coming in for IV hydration, but when we had two come in on the same day, it did take up a couple of exam rooms.” To address this, the clinicians found other space in the clinic that would offer privacy for patients while not tying up exam rooms.
In addition, the clinic expanded nurse practitioner availability to 5 days a week to make the discharge process more consistent. “Of course, as we rolled all these things out, we made sure our educational material was updated accordingly,” Dr. Aher said.
The study demonstrates that a collaborative team effort and targeted interventions can result in a significant reduction in readmissions, Dr. Aher said. “Regular quality focused meetings are really important to facilitate recognition of various areas for improvement, especially in a high-volume center. Introducing an MBSAQIP registry serves as an excellent tool to effect these changes,” he said.
Dr. Aher had no relevant financial disclosures.
AT THE ACS QUALITY & SAFETY CONFERENCE
Key clinical point: A collaborative effort and target interventions can successfully reduce bariatric surgery readmissions.
Major finding: The overall bariatric surgery readmission rate dropped 61% in the year after intervention compared to the previous year.
Data source: Comparison of 471 bariatric procedures in 2015 to 539 others in 2016 at Vanderbilt University Medical Center.
Disclosures: Dr. Aher had no relevant financial disclosures.
ERAS program cuts complications after radical cystectomy
NEW YORK – Prior to October 2014, urology patients undergoing radical cystectomy at a 950-bed, tertiary care hospital experienced postoperative morbidity at a relative high rate, according to NSQIP data.
“Despite improvements in surgical techniques and perioperative care protocols, the rate of the overall morbidity for radical cystectomy was higher than we would like to see,” said Tracey Hong, RN, BScN, of the Clinical Quality and Patient Safety Department at Vancouver (B.C.) General Hospital. “We took this as an opportunity to improve our patient outcomes and experience.”
Vancouver General joined the American College of Surgeons National Surgical Improvement Quality Program (ACS NSQIP) in 2011. The enhanced recovery after surgery (ERAS) perioperative protocol the institution adopted in late 2014 was associated with a 32% decrease in overall morbidity. The rate dropped from 31.3% in the pre-ERAS study period from May 2011 to September 2014, to 21.1% after implementation, from October 2014 to September 2016, according to a study Ms. Hong presented at the American College of Surgeons Quality and Safety Conference.
The investigators compared outcomes between all 92 people undergoing elective radical cystectomy during the first time period to 152 consecutive patients treated under the ERAS protocol. Median length of stay decreased from 8 days before ERAS to 7 days after, a significant difference (P less than .05).
The researchers also assessed outcomes based on how adherent clinicians were to 12 key elements of the 26-item ERAS initiative. These elements included preoperative counseling, preoperative anesthesia consultation, and carbohydrate loading on the morning of surgery. Intraoperatively, they tracked normothermia, use of multimodal anesthesia, use of goal-directed fluid therapy using a monitor, timely antibiotics, and adequate postoperative nausea and vomiting prophylaxis. The four postoperative key measures were mobilization at least once by postoperative day 0, full fluids and mobilization twice on postoperative day 1, and starting solid food by postoperative day 4.
A total 52% of the ERAS cases were associated with 75% or greater adherence to these 12 key items. Adherence with the intraoperative fluid therapy and all the postoperative elements proved to be the most challenging, Ms. Hong said.
The more adherent cases experienced a lower overall postoperative morbidity rate, 15.2%, compared with 27.4% among the less adherent group. The 15.2% morbidity among the more adherent cases also compared favorably with the 31.1% rate for cases prior to ERAS adoption.
“We will continue working on improving compliance,” Ms. Hong said. “We need to increase adherence to goal-directed fluid therapy and the postoperative components,” Ms. Hong said.
Three main strategies remain essential to the ongoing success of the ERAS program, Ms. Hong said. Empowering patients to be active participants and to engage in their own health outcomes is one. “Second, we involve a multidisciplinary team at an early stage so they take ownership and get engaged in the program,” she said. “Last but not least, we continue to measure the outcomes in 100% of cases.”
Continuous auditing and sharing results with the team on a regular basis will be necessary to maintain engagement in the ERAS protocol going forward, Ms. Hong added. “Tenacity is vital.”
Ms. Hong had no relevant financial disclosures.
NEW YORK – Prior to October 2014, urology patients undergoing radical cystectomy at a 950-bed, tertiary care hospital experienced postoperative morbidity at a relative high rate, according to NSQIP data.
“Despite improvements in surgical techniques and perioperative care protocols, the rate of the overall morbidity for radical cystectomy was higher than we would like to see,” said Tracey Hong, RN, BScN, of the Clinical Quality and Patient Safety Department at Vancouver (B.C.) General Hospital. “We took this as an opportunity to improve our patient outcomes and experience.”
Vancouver General joined the American College of Surgeons National Surgical Improvement Quality Program (ACS NSQIP) in 2011. The enhanced recovery after surgery (ERAS) perioperative protocol the institution adopted in late 2014 was associated with a 32% decrease in overall morbidity. The rate dropped from 31.3% in the pre-ERAS study period from May 2011 to September 2014, to 21.1% after implementation, from October 2014 to September 2016, according to a study Ms. Hong presented at the American College of Surgeons Quality and Safety Conference.
The investigators compared outcomes between all 92 people undergoing elective radical cystectomy during the first time period to 152 consecutive patients treated under the ERAS protocol. Median length of stay decreased from 8 days before ERAS to 7 days after, a significant difference (P less than .05).
The researchers also assessed outcomes based on how adherent clinicians were to 12 key elements of the 26-item ERAS initiative. These elements included preoperative counseling, preoperative anesthesia consultation, and carbohydrate loading on the morning of surgery. Intraoperatively, they tracked normothermia, use of multimodal anesthesia, use of goal-directed fluid therapy using a monitor, timely antibiotics, and adequate postoperative nausea and vomiting prophylaxis. The four postoperative key measures were mobilization at least once by postoperative day 0, full fluids and mobilization twice on postoperative day 1, and starting solid food by postoperative day 4.
A total 52% of the ERAS cases were associated with 75% or greater adherence to these 12 key items. Adherence with the intraoperative fluid therapy and all the postoperative elements proved to be the most challenging, Ms. Hong said.
The more adherent cases experienced a lower overall postoperative morbidity rate, 15.2%, compared with 27.4% among the less adherent group. The 15.2% morbidity among the more adherent cases also compared favorably with the 31.1% rate for cases prior to ERAS adoption.
“We will continue working on improving compliance,” Ms. Hong said. “We need to increase adherence to goal-directed fluid therapy and the postoperative components,” Ms. Hong said.
Three main strategies remain essential to the ongoing success of the ERAS program, Ms. Hong said. Empowering patients to be active participants and to engage in their own health outcomes is one. “Second, we involve a multidisciplinary team at an early stage so they take ownership and get engaged in the program,” she said. “Last but not least, we continue to measure the outcomes in 100% of cases.”
Continuous auditing and sharing results with the team on a regular basis will be necessary to maintain engagement in the ERAS protocol going forward, Ms. Hong added. “Tenacity is vital.”
Ms. Hong had no relevant financial disclosures.
NEW YORK – Prior to October 2014, urology patients undergoing radical cystectomy at a 950-bed, tertiary care hospital experienced postoperative morbidity at a relative high rate, according to NSQIP data.
“Despite improvements in surgical techniques and perioperative care protocols, the rate of the overall morbidity for radical cystectomy was higher than we would like to see,” said Tracey Hong, RN, BScN, of the Clinical Quality and Patient Safety Department at Vancouver (B.C.) General Hospital. “We took this as an opportunity to improve our patient outcomes and experience.”
Vancouver General joined the American College of Surgeons National Surgical Improvement Quality Program (ACS NSQIP) in 2011. The enhanced recovery after surgery (ERAS) perioperative protocol the institution adopted in late 2014 was associated with a 32% decrease in overall morbidity. The rate dropped from 31.3% in the pre-ERAS study period from May 2011 to September 2014, to 21.1% after implementation, from October 2014 to September 2016, according to a study Ms. Hong presented at the American College of Surgeons Quality and Safety Conference.
The investigators compared outcomes between all 92 people undergoing elective radical cystectomy during the first time period to 152 consecutive patients treated under the ERAS protocol. Median length of stay decreased from 8 days before ERAS to 7 days after, a significant difference (P less than .05).
The researchers also assessed outcomes based on how adherent clinicians were to 12 key elements of the 26-item ERAS initiative. These elements included preoperative counseling, preoperative anesthesia consultation, and carbohydrate loading on the morning of surgery. Intraoperatively, they tracked normothermia, use of multimodal anesthesia, use of goal-directed fluid therapy using a monitor, timely antibiotics, and adequate postoperative nausea and vomiting prophylaxis. The four postoperative key measures were mobilization at least once by postoperative day 0, full fluids and mobilization twice on postoperative day 1, and starting solid food by postoperative day 4.
A total 52% of the ERAS cases were associated with 75% or greater adherence to these 12 key items. Adherence with the intraoperative fluid therapy and all the postoperative elements proved to be the most challenging, Ms. Hong said.
The more adherent cases experienced a lower overall postoperative morbidity rate, 15.2%, compared with 27.4% among the less adherent group. The 15.2% morbidity among the more adherent cases also compared favorably with the 31.1% rate for cases prior to ERAS adoption.
“We will continue working on improving compliance,” Ms. Hong said. “We need to increase adherence to goal-directed fluid therapy and the postoperative components,” Ms. Hong said.
Three main strategies remain essential to the ongoing success of the ERAS program, Ms. Hong said. Empowering patients to be active participants and to engage in their own health outcomes is one. “Second, we involve a multidisciplinary team at an early stage so they take ownership and get engaged in the program,” she said. “Last but not least, we continue to measure the outcomes in 100% of cases.”
Continuous auditing and sharing results with the team on a regular basis will be necessary to maintain engagement in the ERAS protocol going forward, Ms. Hong added. “Tenacity is vital.”
Ms. Hong had no relevant financial disclosures.
AT THE ACS QUALITY & SAFETY CONFERENCE
Key clinical point: An enhanced recovery after surgery protocol can reduce postoperative morbidity after radical cystectomy.
Major finding: Investigators report a 32% decrease in overall morbidity after adoption of ERAS pathways.
Data source: Comparison between 92 patients before and 152 patients after implementation of ERAS protocol.
Disclosures: Tracey Hong, BScN, had no relevant financial disclosures.
Sodium fusidate noninferior to linezolid for acute skin infections
NEW ORLEANS – An oral antibiotic in development in the United States, fusidic acid (oral formulation, sodium fusidate) was noninferior to linezolid based on early clinical response in a randomized, double-blind, multicenter trial of 716 people with acute bacterial skin and skin structure infections (ABSSSI), including cellulitis, wound infection, and major cutaneous abscesses.
Early clinical response was defined as a 20% or greater reduction from baseline in the surface area of redness, edema, or induration at 48-72 hours after starting treatment with the study drugs. In an intent-to-treat analysis, 87.2% of patients randomized to fusidic acid and 86.6% of the linezolid group met this primary endpoint of the phase 3 study.
“Fusidic acid showed similar efficacy and comparable safety” that persisted through treatment, said Andy Strayer, PharmD, vice president of clinical programs at Cempra Pharmaceuticals, which is developing sodium fusidate as an oral agent to treat ABSSSI patients in the United States. Leo Pharmaceuticals has marketed sodium fusidate outside the United States in various formulations for decades.
Fusidic acid has potent activity against gram-positive aerobic organisms, including methicillin-resistant Staphylococcus aureus (MRSA). “Strikingly, fusidic acid showed 100% success in patients with MRSA in the microbiologically evaluable population at the end of treatment and posttherapy evaluation time points,” Dr. Strayer said at the annual meeting of the American Society for Microbiology. “Fusidic acid may offer an important oral therapy alternative for MRSA infection.”
“Fusidic acid, a drug long used in other parts of the world, has been demonstrated in this first phase 3 trial, to be a potential new option for the treatment of MRSA skin and skin structure infections in the U.S.,” said Carrie Cardenas, MD, lead study author and a principal investigator at eStudySite, San Diego, and an internist in private practice in La Mesa, California.
There was a microbiological diagnosis established in 75% of patients. S. aureus was the most commonly detected pathogen (422 patients; 59%), and the study included 235 patients diagnosed with MRSA infection.
About two-thirds, 65%, of participants were men. Mean age was 45 years. Infections were classified as wounds in 61%, cellulitis in 26%, and abscess in 13%. Notably, 68% of the recruited participants had ABSSSI associated with intravenous drug use, a “sometimes overlooked consequence of the ongoing epidemic of IV drug use in the U.S.,” Dr. Strayer said.
In terms of safety, treatment-emergent adverse event rates were comparable between the two groups (37.9% with fusidic acid versus 36.1% with linezolid). Gastrointestinal events were the most common adverse events, 22.8% versus 18.2%, respectively.
“Considering complicated skin infections are one of the most rapidly growing reasons for hospitalizations and emergency department visits each year, we anticipate that fusidic acid, if approved, may help clinicians decrease the length of inpatient stay or avoid hospitalization altogether,” Dr. Strayer said.
Cempra sponsored the study. Dr. Strayer is a Cempra employee and shareholder. Dr. Carrie Cardenas is a principal investigator at eStudySite, San Diego, and performs research for Cempra, Paratek, Debiopharm, Motif, Durata, MicuRx, Bristol-Myers Squibb, and Bayer.
NEW ORLEANS – An oral antibiotic in development in the United States, fusidic acid (oral formulation, sodium fusidate) was noninferior to linezolid based on early clinical response in a randomized, double-blind, multicenter trial of 716 people with acute bacterial skin and skin structure infections (ABSSSI), including cellulitis, wound infection, and major cutaneous abscesses.
Early clinical response was defined as a 20% or greater reduction from baseline in the surface area of redness, edema, or induration at 48-72 hours after starting treatment with the study drugs. In an intent-to-treat analysis, 87.2% of patients randomized to fusidic acid and 86.6% of the linezolid group met this primary endpoint of the phase 3 study.
“Fusidic acid showed similar efficacy and comparable safety” that persisted through treatment, said Andy Strayer, PharmD, vice president of clinical programs at Cempra Pharmaceuticals, which is developing sodium fusidate as an oral agent to treat ABSSSI patients in the United States. Leo Pharmaceuticals has marketed sodium fusidate outside the United States in various formulations for decades.
Fusidic acid has potent activity against gram-positive aerobic organisms, including methicillin-resistant Staphylococcus aureus (MRSA). “Strikingly, fusidic acid showed 100% success in patients with MRSA in the microbiologically evaluable population at the end of treatment and posttherapy evaluation time points,” Dr. Strayer said at the annual meeting of the American Society for Microbiology. “Fusidic acid may offer an important oral therapy alternative for MRSA infection.”
“Fusidic acid, a drug long used in other parts of the world, has been demonstrated in this first phase 3 trial, to be a potential new option for the treatment of MRSA skin and skin structure infections in the U.S.,” said Carrie Cardenas, MD, lead study author and a principal investigator at eStudySite, San Diego, and an internist in private practice in La Mesa, California.
There was a microbiological diagnosis established in 75% of patients. S. aureus was the most commonly detected pathogen (422 patients; 59%), and the study included 235 patients diagnosed with MRSA infection.
About two-thirds, 65%, of participants were men. Mean age was 45 years. Infections were classified as wounds in 61%, cellulitis in 26%, and abscess in 13%. Notably, 68% of the recruited participants had ABSSSI associated with intravenous drug use, a “sometimes overlooked consequence of the ongoing epidemic of IV drug use in the U.S.,” Dr. Strayer said.
In terms of safety, treatment-emergent adverse event rates were comparable between the two groups (37.9% with fusidic acid versus 36.1% with linezolid). Gastrointestinal events were the most common adverse events, 22.8% versus 18.2%, respectively.
“Considering complicated skin infections are one of the most rapidly growing reasons for hospitalizations and emergency department visits each year, we anticipate that fusidic acid, if approved, may help clinicians decrease the length of inpatient stay or avoid hospitalization altogether,” Dr. Strayer said.
Cempra sponsored the study. Dr. Strayer is a Cempra employee and shareholder. Dr. Carrie Cardenas is a principal investigator at eStudySite, San Diego, and performs research for Cempra, Paratek, Debiopharm, Motif, Durata, MicuRx, Bristol-Myers Squibb, and Bayer.
NEW ORLEANS – An oral antibiotic in development in the United States, fusidic acid (oral formulation, sodium fusidate) was noninferior to linezolid based on early clinical response in a randomized, double-blind, multicenter trial of 716 people with acute bacterial skin and skin structure infections (ABSSSI), including cellulitis, wound infection, and major cutaneous abscesses.
Early clinical response was defined as a 20% or greater reduction from baseline in the surface area of redness, edema, or induration at 48-72 hours after starting treatment with the study drugs. In an intent-to-treat analysis, 87.2% of patients randomized to fusidic acid and 86.6% of the linezolid group met this primary endpoint of the phase 3 study.
“Fusidic acid showed similar efficacy and comparable safety” that persisted through treatment, said Andy Strayer, PharmD, vice president of clinical programs at Cempra Pharmaceuticals, which is developing sodium fusidate as an oral agent to treat ABSSSI patients in the United States. Leo Pharmaceuticals has marketed sodium fusidate outside the United States in various formulations for decades.
Fusidic acid has potent activity against gram-positive aerobic organisms, including methicillin-resistant Staphylococcus aureus (MRSA). “Strikingly, fusidic acid showed 100% success in patients with MRSA in the microbiologically evaluable population at the end of treatment and posttherapy evaluation time points,” Dr. Strayer said at the annual meeting of the American Society for Microbiology. “Fusidic acid may offer an important oral therapy alternative for MRSA infection.”
“Fusidic acid, a drug long used in other parts of the world, has been demonstrated in this first phase 3 trial, to be a potential new option for the treatment of MRSA skin and skin structure infections in the U.S.,” said Carrie Cardenas, MD, lead study author and a principal investigator at eStudySite, San Diego, and an internist in private practice in La Mesa, California.
There was a microbiological diagnosis established in 75% of patients. S. aureus was the most commonly detected pathogen (422 patients; 59%), and the study included 235 patients diagnosed with MRSA infection.
About two-thirds, 65%, of participants were men. Mean age was 45 years. Infections were classified as wounds in 61%, cellulitis in 26%, and abscess in 13%. Notably, 68% of the recruited participants had ABSSSI associated with intravenous drug use, a “sometimes overlooked consequence of the ongoing epidemic of IV drug use in the U.S.,” Dr. Strayer said.
In terms of safety, treatment-emergent adverse event rates were comparable between the two groups (37.9% with fusidic acid versus 36.1% with linezolid). Gastrointestinal events were the most common adverse events, 22.8% versus 18.2%, respectively.
“Considering complicated skin infections are one of the most rapidly growing reasons for hospitalizations and emergency department visits each year, we anticipate that fusidic acid, if approved, may help clinicians decrease the length of inpatient stay or avoid hospitalization altogether,” Dr. Strayer said.
Cempra sponsored the study. Dr. Strayer is a Cempra employee and shareholder. Dr. Carrie Cardenas is a principal investigator at eStudySite, San Diego, and performs research for Cempra, Paratek, Debiopharm, Motif, Durata, MicuRx, Bristol-Myers Squibb, and Bayer.
AT ASM MICROBE 2017
Key clinical point: Sodium fusidate, active as fusidic acid, showed noninferiority to linezolid for early clinical response in ABSSI patients.
Major finding: 87.2% of patients given sodium fusidate and 86.6% of those receiving linezolid achieved an early clinical response.
Data source: Randomized, controlled, double-blind, phase 3 study with 716 participants.
Disclosures: Cempra sponsored the study. Dr. Carrier Cardenas is a researcher for Cempra, Paratek, Debiopharm, Motif, Durata, MicuRx, Bristol-Myers Squibb, and Bayer. Dr. Strayer is a Cempra employee and shareholder.
Fluid protocol takes aim at bariatric surgery readmissions
NEW YORK – With dehydration considered a contributor to hospital readmission after bariatric weight loss surgery, a multidisciplinary team of clinicians searched for a way to get patients to drink up. Specifically,
The project results were presented at a poster session at the American College of Surgeons Quality and Safety Conference.
“Our QI project was to streamline that process to ultimately eliminate or decrease those readmits,” Ms. Melei said.
They used 8-ounce water bottles. To track water consumption, they also numbered the bottles one through six for each patient. One-ounce cups were used during mealtimes. RNs, certified nursing assistants, and food service staff were educated about the fluid intake initiative. Only fluids provided by nursing were permitted and patients also received a clear message about fluid goals.
Another focus was standardizing communication with patients. They were not getting a consistent message from staff on what to expect before, during, and after surgery. “So we had to make sure [we] were all saying the same thing.”
The nurses and the other staff now ask the patients “Did you finish the bottle?” or “Where are you with the bottles?” Ms. Melei said. “At the end of a shift, the nurses document fluid consumption.”
During a 2-month baseline period, 12 patients drank an average 381.5 mL over 24 hours. Since the close of the project, the average daily fluid intake for patients undergoing bariatric surgery is 1,007 mL. In 12 months post implementation, average fluid intake for 39 patients jumped to 1,109.5 mL over 24 hours. “It was just such a hugely successful program. We had buy-in from every department, it worked really well, and we had great results,” said Cheryl Williams, the current bariatric program coordinator at Greenwich Hospital.
Sometimes dehydrated patients present to an emergency department complaining of vomiting, headache, and dizziness if they are not properly hydrated after surgery, Ms. Williams said. “Preventing dehydration helps to improve the patient’s recovery, as well as decrease emergency room visits and hospital admissions.”
Ashutosh Kaul, MD, FACS, medical director of the bariatric surgery program at Greenwich Hospital, said this study shows the importance of team building to improve patient care. “We built a core team that implemented a simple, low-cost, structured, and well-defined water distribution and documentation process, which resulted in better compliance of water intake. This reduced variability and improved postoperative intake progression, and we are presently studying its effect on reduction of readmissions and facilitating early discharge.”
Greenwich Hospital’s bariatric surgery center is accredited by the Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program (MBSAQIP), a collaboration between the American College of Surgeons and the American Society for Metabolic and Bariatric Surgery. Ms. Williams and Ms. Melei had no relevant financial disclosures.
NEW YORK – With dehydration considered a contributor to hospital readmission after bariatric weight loss surgery, a multidisciplinary team of clinicians searched for a way to get patients to drink up. Specifically,
The project results were presented at a poster session at the American College of Surgeons Quality and Safety Conference.
“Our QI project was to streamline that process to ultimately eliminate or decrease those readmits,” Ms. Melei said.
They used 8-ounce water bottles. To track water consumption, they also numbered the bottles one through six for each patient. One-ounce cups were used during mealtimes. RNs, certified nursing assistants, and food service staff were educated about the fluid intake initiative. Only fluids provided by nursing were permitted and patients also received a clear message about fluid goals.
Another focus was standardizing communication with patients. They were not getting a consistent message from staff on what to expect before, during, and after surgery. “So we had to make sure [we] were all saying the same thing.”
The nurses and the other staff now ask the patients “Did you finish the bottle?” or “Where are you with the bottles?” Ms. Melei said. “At the end of a shift, the nurses document fluid consumption.”
During a 2-month baseline period, 12 patients drank an average 381.5 mL over 24 hours. Since the close of the project, the average daily fluid intake for patients undergoing bariatric surgery is 1,007 mL. In 12 months post implementation, average fluid intake for 39 patients jumped to 1,109.5 mL over 24 hours. “It was just such a hugely successful program. We had buy-in from every department, it worked really well, and we had great results,” said Cheryl Williams, the current bariatric program coordinator at Greenwich Hospital.
Sometimes dehydrated patients present to an emergency department complaining of vomiting, headache, and dizziness if they are not properly hydrated after surgery, Ms. Williams said. “Preventing dehydration helps to improve the patient’s recovery, as well as decrease emergency room visits and hospital admissions.”
Ashutosh Kaul, MD, FACS, medical director of the bariatric surgery program at Greenwich Hospital, said this study shows the importance of team building to improve patient care. “We built a core team that implemented a simple, low-cost, structured, and well-defined water distribution and documentation process, which resulted in better compliance of water intake. This reduced variability and improved postoperative intake progression, and we are presently studying its effect on reduction of readmissions and facilitating early discharge.”
Greenwich Hospital’s bariatric surgery center is accredited by the Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program (MBSAQIP), a collaboration between the American College of Surgeons and the American Society for Metabolic and Bariatric Surgery. Ms. Williams and Ms. Melei had no relevant financial disclosures.
NEW YORK – With dehydration considered a contributor to hospital readmission after bariatric weight loss surgery, a multidisciplinary team of clinicians searched for a way to get patients to drink up. Specifically,
The project results were presented at a poster session at the American College of Surgeons Quality and Safety Conference.
“Our QI project was to streamline that process to ultimately eliminate or decrease those readmits,” Ms. Melei said.
They used 8-ounce water bottles. To track water consumption, they also numbered the bottles one through six for each patient. One-ounce cups were used during mealtimes. RNs, certified nursing assistants, and food service staff were educated about the fluid intake initiative. Only fluids provided by nursing were permitted and patients also received a clear message about fluid goals.
Another focus was standardizing communication with patients. They were not getting a consistent message from staff on what to expect before, during, and after surgery. “So we had to make sure [we] were all saying the same thing.”
The nurses and the other staff now ask the patients “Did you finish the bottle?” or “Where are you with the bottles?” Ms. Melei said. “At the end of a shift, the nurses document fluid consumption.”
During a 2-month baseline period, 12 patients drank an average 381.5 mL over 24 hours. Since the close of the project, the average daily fluid intake for patients undergoing bariatric surgery is 1,007 mL. In 12 months post implementation, average fluid intake for 39 patients jumped to 1,109.5 mL over 24 hours. “It was just such a hugely successful program. We had buy-in from every department, it worked really well, and we had great results,” said Cheryl Williams, the current bariatric program coordinator at Greenwich Hospital.
Sometimes dehydrated patients present to an emergency department complaining of vomiting, headache, and dizziness if they are not properly hydrated after surgery, Ms. Williams said. “Preventing dehydration helps to improve the patient’s recovery, as well as decrease emergency room visits and hospital admissions.”
Ashutosh Kaul, MD, FACS, medical director of the bariatric surgery program at Greenwich Hospital, said this study shows the importance of team building to improve patient care. “We built a core team that implemented a simple, low-cost, structured, and well-defined water distribution and documentation process, which resulted in better compliance of water intake. This reduced variability and improved postoperative intake progression, and we are presently studying its effect on reduction of readmissions and facilitating early discharge.”
Greenwich Hospital’s bariatric surgery center is accredited by the Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program (MBSAQIP), a collaboration between the American College of Surgeons and the American Society for Metabolic and Bariatric Surgery. Ms. Williams and Ms. Melei had no relevant financial disclosures.
AT THE ACS QUALITY & SAFETY CONFERENCE
Key clinical point: QI project to increase water intake after bariatric surgery could decrease hospital readmission rates.
Major finding: Multidisciplinary protocol increases 24-hour fluid intake from 382 mL to 1,110 mL on average.
Data source: Comparison of water consumed by 12 patients before versus 39 patients after implementation.
Disclosures: Ms. Williams and Ms. Melei had no relevant financial disclosures.
Multidisciplinary bundle drives drop in colorectal SSIs
NEW YORK – Facing an “unacceptably high” rate of surgical site infections associated with colorectal surgery at their community hospital, surgeons searched for solutions. They created a perioperative bundle of interventions that ultimately dropped their infection rates enough to achieve the highest ranking in the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP).
“The Centers for Disease Control and Prevention recommends we use a robust surveillance program to monitor surgical site infection data. The system gives us feedback, and that will [help us] reduce surgical site infection (SSI) risk,” said Christopher Wolff, MD, a PGY4 resident at the Cleveland Clinic Akron General Hospital. “NSQIP and the National Healthcare Safety Network from the CDC are two programs that do just that.”
The effort paid off, with the number of SSIs going from 16 cases in 2013 to 10 cases in 2014 and then 5 cases in 2015. Since the bundle was implemented in the last quarter of 2014, “we’ve seen a consistent downtrend since that point in our total infections, and we kept that in the background of a consistent number of cases.
“We have good outcomes by incidence, but that is not the whole story,” Dr. Wolff said. “With respect to colorectal infections, we are now performing in the ‘exemplary’ category, compared with our peers” according to the ACS NSQIP data. In addition, “we are performing at or below the SIR [standardized infection ratio] or expected number consistently since the implementation.”
The bundle addresses actions in five domains: preoperative, anesthesia, operating room, post–anesthesia care unit, and postoperative floor interventions. Preoperative elements include patient education, use of chlorhexidine wipes before surgery, and antibiotics noted on the chart, for example. Additional features include prewarming preoperatively and maintaining normothermia, requiring all surgeons scrub traditionally instead of “foaming,” use of wound protectors in the OR, and close monitoring of blood glucose in diabetics postoperatively. “There also is education of floor nurses on how to take care of these patients specifically,” Dr. Wolff noted.
To identify these areas for improvement, Dr. Wolff and his colleagues initially reviewed the literature to find individual and bundle elements demonstrated to improve outcomes. Then, a surgeon group “think tank” discussed the possibilities. However, reaching agreement was not easy, Dr. Wolff said. “They had a hard time agreeing on best practices, even within our own specialty. We did finally come to a consensus.
“We took those bundled protocols through to other areas and said ‘here are the things we want you to work on, things we want you to improve.’ That did not necessarily go over so well,” Dr. Wolff said. Because of resistance from their colleagues, they changed strategies. “We brought other people to the table and changed our work groups from being surgeons only to [being] a multidisciplinary team.”
The process took months and months of deliberation. It’s important to have a champion behind the project, said Dr. Wolff. “I have to thank my chairman, Mark C. Horattas, MD, FACS, who had the vision to see this through.
“We implemented tried-and-true measures to reduce surgical site infections. We did so in a team manner and had multidisciplinary buy-in, and that created a culture change in our program over time,” Dr. Wolff said.
This study also shows, Dr. Wolff added, that “a successful multidisciplinary quality improvement program can be implemented in a community hospital setting.”
Going forward, continuous monitoring will identify any areas that need improvement over time. The preoperative bundle also will be integrated into an Enhanced Recovery After Surgery protocol.
The Akron Hospital is now ranked by ACS NSQIP in the top 10% of hospitals for their colorectal SSI rate. “It’s nice to meet someone in the first decile,” session moderator Timothy D. Jackson, MD, FACS, of the University of Toronto said after Dr. Wolff’s presentation. “I’ve never done that before, and I took notes for what to do at my hospital.”
Dr. Wolff had no relevant financial disclosures.
NEW YORK – Facing an “unacceptably high” rate of surgical site infections associated with colorectal surgery at their community hospital, surgeons searched for solutions. They created a perioperative bundle of interventions that ultimately dropped their infection rates enough to achieve the highest ranking in the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP).
“The Centers for Disease Control and Prevention recommends we use a robust surveillance program to monitor surgical site infection data. The system gives us feedback, and that will [help us] reduce surgical site infection (SSI) risk,” said Christopher Wolff, MD, a PGY4 resident at the Cleveland Clinic Akron General Hospital. “NSQIP and the National Healthcare Safety Network from the CDC are two programs that do just that.”
The effort paid off, with the number of SSIs going from 16 cases in 2013 to 10 cases in 2014 and then 5 cases in 2015. Since the bundle was implemented in the last quarter of 2014, “we’ve seen a consistent downtrend since that point in our total infections, and we kept that in the background of a consistent number of cases.
“We have good outcomes by incidence, but that is not the whole story,” Dr. Wolff said. “With respect to colorectal infections, we are now performing in the ‘exemplary’ category, compared with our peers” according to the ACS NSQIP data. In addition, “we are performing at or below the SIR [standardized infection ratio] or expected number consistently since the implementation.”
The bundle addresses actions in five domains: preoperative, anesthesia, operating room, post–anesthesia care unit, and postoperative floor interventions. Preoperative elements include patient education, use of chlorhexidine wipes before surgery, and antibiotics noted on the chart, for example. Additional features include prewarming preoperatively and maintaining normothermia, requiring all surgeons scrub traditionally instead of “foaming,” use of wound protectors in the OR, and close monitoring of blood glucose in diabetics postoperatively. “There also is education of floor nurses on how to take care of these patients specifically,” Dr. Wolff noted.
To identify these areas for improvement, Dr. Wolff and his colleagues initially reviewed the literature to find individual and bundle elements demonstrated to improve outcomes. Then, a surgeon group “think tank” discussed the possibilities. However, reaching agreement was not easy, Dr. Wolff said. “They had a hard time agreeing on best practices, even within our own specialty. We did finally come to a consensus.
“We took those bundled protocols through to other areas and said ‘here are the things we want you to work on, things we want you to improve.’ That did not necessarily go over so well,” Dr. Wolff said. Because of resistance from their colleagues, they changed strategies. “We brought other people to the table and changed our work groups from being surgeons only to [being] a multidisciplinary team.”
The process took months and months of deliberation. It’s important to have a champion behind the project, said Dr. Wolff. “I have to thank my chairman, Mark C. Horattas, MD, FACS, who had the vision to see this through.
“We implemented tried-and-true measures to reduce surgical site infections. We did so in a team manner and had multidisciplinary buy-in, and that created a culture change in our program over time,” Dr. Wolff said.
This study also shows, Dr. Wolff added, that “a successful multidisciplinary quality improvement program can be implemented in a community hospital setting.”
Going forward, continuous monitoring will identify any areas that need improvement over time. The preoperative bundle also will be integrated into an Enhanced Recovery After Surgery protocol.
The Akron Hospital is now ranked by ACS NSQIP in the top 10% of hospitals for their colorectal SSI rate. “It’s nice to meet someone in the first decile,” session moderator Timothy D. Jackson, MD, FACS, of the University of Toronto said after Dr. Wolff’s presentation. “I’ve never done that before, and I took notes for what to do at my hospital.”
Dr. Wolff had no relevant financial disclosures.
NEW YORK – Facing an “unacceptably high” rate of surgical site infections associated with colorectal surgery at their community hospital, surgeons searched for solutions. They created a perioperative bundle of interventions that ultimately dropped their infection rates enough to achieve the highest ranking in the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP).
“The Centers for Disease Control and Prevention recommends we use a robust surveillance program to monitor surgical site infection data. The system gives us feedback, and that will [help us] reduce surgical site infection (SSI) risk,” said Christopher Wolff, MD, a PGY4 resident at the Cleveland Clinic Akron General Hospital. “NSQIP and the National Healthcare Safety Network from the CDC are two programs that do just that.”
The effort paid off, with the number of SSIs going from 16 cases in 2013 to 10 cases in 2014 and then 5 cases in 2015. Since the bundle was implemented in the last quarter of 2014, “we’ve seen a consistent downtrend since that point in our total infections, and we kept that in the background of a consistent number of cases.
“We have good outcomes by incidence, but that is not the whole story,” Dr. Wolff said. “With respect to colorectal infections, we are now performing in the ‘exemplary’ category, compared with our peers” according to the ACS NSQIP data. In addition, “we are performing at or below the SIR [standardized infection ratio] or expected number consistently since the implementation.”
The bundle addresses actions in five domains: preoperative, anesthesia, operating room, post–anesthesia care unit, and postoperative floor interventions. Preoperative elements include patient education, use of chlorhexidine wipes before surgery, and antibiotics noted on the chart, for example. Additional features include prewarming preoperatively and maintaining normothermia, requiring all surgeons scrub traditionally instead of “foaming,” use of wound protectors in the OR, and close monitoring of blood glucose in diabetics postoperatively. “There also is education of floor nurses on how to take care of these patients specifically,” Dr. Wolff noted.
To identify these areas for improvement, Dr. Wolff and his colleagues initially reviewed the literature to find individual and bundle elements demonstrated to improve outcomes. Then, a surgeon group “think tank” discussed the possibilities. However, reaching agreement was not easy, Dr. Wolff said. “They had a hard time agreeing on best practices, even within our own specialty. We did finally come to a consensus.
“We took those bundled protocols through to other areas and said ‘here are the things we want you to work on, things we want you to improve.’ That did not necessarily go over so well,” Dr. Wolff said. Because of resistance from their colleagues, they changed strategies. “We brought other people to the table and changed our work groups from being surgeons only to [being] a multidisciplinary team.”
The process took months and months of deliberation. It’s important to have a champion behind the project, said Dr. Wolff. “I have to thank my chairman, Mark C. Horattas, MD, FACS, who had the vision to see this through.
“We implemented tried-and-true measures to reduce surgical site infections. We did so in a team manner and had multidisciplinary buy-in, and that created a culture change in our program over time,” Dr. Wolff said.
This study also shows, Dr. Wolff added, that “a successful multidisciplinary quality improvement program can be implemented in a community hospital setting.”
Going forward, continuous monitoring will identify any areas that need improvement over time. The preoperative bundle also will be integrated into an Enhanced Recovery After Surgery protocol.
The Akron Hospital is now ranked by ACS NSQIP in the top 10% of hospitals for their colorectal SSI rate. “It’s nice to meet someone in the first decile,” session moderator Timothy D. Jackson, MD, FACS, of the University of Toronto said after Dr. Wolff’s presentation. “I’ve never done that before, and I took notes for what to do at my hospital.”
Dr. Wolff had no relevant financial disclosures.
AT THE ACS QUALITY AND SAFETY CONFERENCE
Key clinical point: A multidisciplinary team initiative successfully reduced surgical site infections after colorectal surgery in a community hospital.
Major finding: The number of annual SSIs dropped from 16 in the calendar year before the intervention to 5 afterward.
Data source: Comparison of SSI rates before and after a bundled intervention in late 2014.
Disclosures: Dr. Wolff had no relevant financial disclosures.
Surgeons can take steps to reduce excess opioids
NEW YORK – Surgeons prescribe approximately 10% of the 280 million schedule II prescriptions written every year. Because patients report taking on average 20%-30% of their opioid pills, a large amount of these drugs end up being diverted to nonpatients and contributing to the opioid epidemic in the United States, said Jonah J. Stulberg, MD.
”You can’t turn on the television, pick up the newspaper, do anything on social media without seeing a lot of headlines about the tragedies occurring because of opioid abuse,” said Dr. Stulberg, assistant professor of surgery at Northwestern University in Chicago.
The majority of opioid abusers are obtaining the drugs through diversion, he said. “So the prescriptions we’re writing and the excess pills we have available, they do make a difference. They’re making it down the pipeline.”
Some practical steps
There are practical actions surgeons can take to try to reduce the sheer volume of prescription opioids that end up in the hands of people for whom they are not intended, Dr. Stulberg said at the American College of Surgeons Quality and Safety Conference.
Sign up for a prescription monitoring program, he suggested. These programs are now available in 49 states (Missouri is the exception). “It’s really easy to create an account as a provider.” Just search online for your state’s prescription monitoring program and enter your contact information, he said. The state will then ask you for some basic information.
Take it one step further and integrate your prescription monitoring program into your electronic medical records, Dr. Stulberg said. “We’re starting to integrate it in all hospitals in Illinois. That means when the surgeon or the nurse providers look up the patient, they will know if the patient got two other prescriptions in the last 2 weeks.” He added, “You can ask if they need more pills before adding to the surplus.”
Another practical strategy is to give patients better access to opioid disposal. Find out where the nearest drug return receptacle or kiosk is located – a pharmacy, police station, or elsewhere – and inform your patients. “We put opioid retrieval boxes in our clinic. When patients are educated about this, they bring [excess pills] back.”
Minimizing opioid prescribing in surgery
“This is not a problem of one individual specialty,” Dr. Stulberg said. “We have a problem of culture, a problem of mindset. So we need to approach solving this problem, in my opinion, from that perspective.”
Start with setting patient expectations in the preoperative period. Explain what the surgery entails and the amount of pain they should expect. “Patients have to understand what we’re doing hurts but we can manage that pain. It can make a big difference in their satisfaction … and also in how much medication they end up taking.”
In addition, perform preoperative risk screening for opioid abuse. “There is very good evidence that patients with addictions to other drugs, alcohol, or who have previous drug use, are much more likely to continue using prescription pain medications after a surgical encounter – 6 months to 12 months later.”
In addition, a paradigm shift in thinking is needed. “When we’re talking about pain control in the clinic and minimizing opioids during a surgical episode, we should be focusing on function,” he said. Orthopedic surgery is a prime example, where physicians encourage patients to start mobility exercises the same day of surgery because evidence shows it helps restore function faster.
Surgeons should tell patients that “the goal of how we are treating your pain is getting you to your optimal function.” Then check on function recovery postoperatively, he added. “We don’t follow-up – that is not part of our standard postoperative pathway in any way.”
Consider prescribing fewer pills postoperatively
If surgeons have a prescription feedback mechanism in place, they might know how many opioid pills patients are actually taking. “If I gave 20 pills to the last 10 patients but they only took 8 on average – can I go lower?” Dr. Stulberg said.
Researchers conducted a study to determine opioid needs for 80% of the patients at their institution after certain types of surgery (Ann Surg. 2017;265:709-14).
The study determined the number of pills that 80% of patients found controlled their pain: after partial mastectomy, 5; after partial mastectomy with sentinel lymph node biopsy, 10; after laparoscopic cholecystectomy, 15; after laparoscopic inguinal hernia repair, 15; and after open inguinal hernia repair, 15. These numbers represent about 43% of the actual number prescribed overall, so many patients are getting more medications than they require.
Dr. Stulberg suggested that surgeons could use the data from this study to counter any pushback from others at their institution reluctant to prescribe fewer opioids for their patients.
Dr. Stulberg did not have any relevant disclosures.
NEW YORK – Surgeons prescribe approximately 10% of the 280 million schedule II prescriptions written every year. Because patients report taking on average 20%-30% of their opioid pills, a large amount of these drugs end up being diverted to nonpatients and contributing to the opioid epidemic in the United States, said Jonah J. Stulberg, MD.
”You can’t turn on the television, pick up the newspaper, do anything on social media without seeing a lot of headlines about the tragedies occurring because of opioid abuse,” said Dr. Stulberg, assistant professor of surgery at Northwestern University in Chicago.
The majority of opioid abusers are obtaining the drugs through diversion, he said. “So the prescriptions we’re writing and the excess pills we have available, they do make a difference. They’re making it down the pipeline.”
Some practical steps
There are practical actions surgeons can take to try to reduce the sheer volume of prescription opioids that end up in the hands of people for whom they are not intended, Dr. Stulberg said at the American College of Surgeons Quality and Safety Conference.
Sign up for a prescription monitoring program, he suggested. These programs are now available in 49 states (Missouri is the exception). “It’s really easy to create an account as a provider.” Just search online for your state’s prescription monitoring program and enter your contact information, he said. The state will then ask you for some basic information.
Take it one step further and integrate your prescription monitoring program into your electronic medical records, Dr. Stulberg said. “We’re starting to integrate it in all hospitals in Illinois. That means when the surgeon or the nurse providers look up the patient, they will know if the patient got two other prescriptions in the last 2 weeks.” He added, “You can ask if they need more pills before adding to the surplus.”
Another practical strategy is to give patients better access to opioid disposal. Find out where the nearest drug return receptacle or kiosk is located – a pharmacy, police station, or elsewhere – and inform your patients. “We put opioid retrieval boxes in our clinic. When patients are educated about this, they bring [excess pills] back.”
Minimizing opioid prescribing in surgery
“This is not a problem of one individual specialty,” Dr. Stulberg said. “We have a problem of culture, a problem of mindset. So we need to approach solving this problem, in my opinion, from that perspective.”
Start with setting patient expectations in the preoperative period. Explain what the surgery entails and the amount of pain they should expect. “Patients have to understand what we’re doing hurts but we can manage that pain. It can make a big difference in their satisfaction … and also in how much medication they end up taking.”
In addition, perform preoperative risk screening for opioid abuse. “There is very good evidence that patients with addictions to other drugs, alcohol, or who have previous drug use, are much more likely to continue using prescription pain medications after a surgical encounter – 6 months to 12 months later.”
In addition, a paradigm shift in thinking is needed. “When we’re talking about pain control in the clinic and minimizing opioids during a surgical episode, we should be focusing on function,” he said. Orthopedic surgery is a prime example, where physicians encourage patients to start mobility exercises the same day of surgery because evidence shows it helps restore function faster.
Surgeons should tell patients that “the goal of how we are treating your pain is getting you to your optimal function.” Then check on function recovery postoperatively, he added. “We don’t follow-up – that is not part of our standard postoperative pathway in any way.”
Consider prescribing fewer pills postoperatively
If surgeons have a prescription feedback mechanism in place, they might know how many opioid pills patients are actually taking. “If I gave 20 pills to the last 10 patients but they only took 8 on average – can I go lower?” Dr. Stulberg said.
Researchers conducted a study to determine opioid needs for 80% of the patients at their institution after certain types of surgery (Ann Surg. 2017;265:709-14).
The study determined the number of pills that 80% of patients found controlled their pain: after partial mastectomy, 5; after partial mastectomy with sentinel lymph node biopsy, 10; after laparoscopic cholecystectomy, 15; after laparoscopic inguinal hernia repair, 15; and after open inguinal hernia repair, 15. These numbers represent about 43% of the actual number prescribed overall, so many patients are getting more medications than they require.
Dr. Stulberg suggested that surgeons could use the data from this study to counter any pushback from others at their institution reluctant to prescribe fewer opioids for their patients.
Dr. Stulberg did not have any relevant disclosures.
NEW YORK – Surgeons prescribe approximately 10% of the 280 million schedule II prescriptions written every year. Because patients report taking on average 20%-30% of their opioid pills, a large amount of these drugs end up being diverted to nonpatients and contributing to the opioid epidemic in the United States, said Jonah J. Stulberg, MD.
”You can’t turn on the television, pick up the newspaper, do anything on social media without seeing a lot of headlines about the tragedies occurring because of opioid abuse,” said Dr. Stulberg, assistant professor of surgery at Northwestern University in Chicago.
The majority of opioid abusers are obtaining the drugs through diversion, he said. “So the prescriptions we’re writing and the excess pills we have available, they do make a difference. They’re making it down the pipeline.”
Some practical steps
There are practical actions surgeons can take to try to reduce the sheer volume of prescription opioids that end up in the hands of people for whom they are not intended, Dr. Stulberg said at the American College of Surgeons Quality and Safety Conference.
Sign up for a prescription monitoring program, he suggested. These programs are now available in 49 states (Missouri is the exception). “It’s really easy to create an account as a provider.” Just search online for your state’s prescription monitoring program and enter your contact information, he said. The state will then ask you for some basic information.
Take it one step further and integrate your prescription monitoring program into your electronic medical records, Dr. Stulberg said. “We’re starting to integrate it in all hospitals in Illinois. That means when the surgeon or the nurse providers look up the patient, they will know if the patient got two other prescriptions in the last 2 weeks.” He added, “You can ask if they need more pills before adding to the surplus.”
Another practical strategy is to give patients better access to opioid disposal. Find out where the nearest drug return receptacle or kiosk is located – a pharmacy, police station, or elsewhere – and inform your patients. “We put opioid retrieval boxes in our clinic. When patients are educated about this, they bring [excess pills] back.”
Minimizing opioid prescribing in surgery
“This is not a problem of one individual specialty,” Dr. Stulberg said. “We have a problem of culture, a problem of mindset. So we need to approach solving this problem, in my opinion, from that perspective.”
Start with setting patient expectations in the preoperative period. Explain what the surgery entails and the amount of pain they should expect. “Patients have to understand what we’re doing hurts but we can manage that pain. It can make a big difference in their satisfaction … and also in how much medication they end up taking.”
In addition, perform preoperative risk screening for opioid abuse. “There is very good evidence that patients with addictions to other drugs, alcohol, or who have previous drug use, are much more likely to continue using prescription pain medications after a surgical encounter – 6 months to 12 months later.”
In addition, a paradigm shift in thinking is needed. “When we’re talking about pain control in the clinic and minimizing opioids during a surgical episode, we should be focusing on function,” he said. Orthopedic surgery is a prime example, where physicians encourage patients to start mobility exercises the same day of surgery because evidence shows it helps restore function faster.
Surgeons should tell patients that “the goal of how we are treating your pain is getting you to your optimal function.” Then check on function recovery postoperatively, he added. “We don’t follow-up – that is not part of our standard postoperative pathway in any way.”
Consider prescribing fewer pills postoperatively
If surgeons have a prescription feedback mechanism in place, they might know how many opioid pills patients are actually taking. “If I gave 20 pills to the last 10 patients but they only took 8 on average – can I go lower?” Dr. Stulberg said.
Researchers conducted a study to determine opioid needs for 80% of the patients at their institution after certain types of surgery (Ann Surg. 2017;265:709-14).
The study determined the number of pills that 80% of patients found controlled their pain: after partial mastectomy, 5; after partial mastectomy with sentinel lymph node biopsy, 10; after laparoscopic cholecystectomy, 15; after laparoscopic inguinal hernia repair, 15; and after open inguinal hernia repair, 15. These numbers represent about 43% of the actual number prescribed overall, so many patients are getting more medications than they require.
Dr. Stulberg suggested that surgeons could use the data from this study to counter any pushback from others at their institution reluctant to prescribe fewer opioids for their patients.
Dr. Stulberg did not have any relevant disclosures.
EXPERT ANALYSIS AT THE ACS QUALITY & SAFETY CONFERENCE
Outcomes improved with ERAS pathway tailored to colorectal surgery
NEW YORK – A protocol that standardizes care before, during, and after colorectal surgery cut average hospital stays from almost 6 days to less than 3, reduced complications, and slashed costs an estimated $11,227 per procedure.
“We decided to take a look at our own data, and we saw that a couple of variables were higher than we wanted them to be,” said Deepa Bhat, MD, a second year surgery resident at Advocate Illinois Masonic Medical Center in Chicago. Length of stay was one. Colorectal surgery patients were staying in the hospital an average 5.65 days, for example.
Their surgical site infection rate also warranted attention, Dr. Bhat said during a poster session at the American College of Surgeons Quality and Safety Conference. The overall complication rate was 6.45%.
“So, we decided to implement this enhanced recovery pathway in the hope that it would, one, get patients out of the hospital faster and allow them to recover at home and, two, decrease the rate of complications, including surgical site infections.”
Prior to the protocol, individual surgeons chose when to initiate fluids, when to discharge a patient, and many other preoperative factors. “Now, care is standardized so that every patient experiences the same pre-, intra-, and postoperative protocol, which leads to better outcomes,” Dr. Bhat said.
The multidisciplinary Enhanced Recovery After Colorectal Surgery (ERACS) pathway also now emphasizes more patient education prior to surgery. “The patients go into surgery having a very clear idea of what they can expect, such as how their pain will be controlled, when they can start liquids, and what their expectations are for ambulation,” she said. “By making patients active participants in their own care, they tend to do better.”
The investigators studied 246 elective colorectal surgery patients at their large, urban, community teaching hospital. They compared outcomes in 2014, versus 2015, to gauge the effectiveness of the ERACS. “The change in length of stay was really rather remarkable,” Dr. Bhat said. In fact, the typical number of days in the hospital decreased by about half from 5.65 to 2.89 days, a statistically significant difference (P less than .0001).
Historically, “the whole reason we don’t send patients home sooner is we’re worried they’re going to bounce right back, and that’s an issue for the 30-day readmission rate,” Dr. Bhat said. However, the enhanced recovery protocol was designed to minimize that risk, and the study verified that it works. “We wanted to show that you can send patients home safely and also not worry that they would come back more often.”
Patient satisfaction seems higher too. Dr. Bhat added, “Who doesn’t feel better at home?”
“It is pretty remarkable that you can send somebody home in less than 3 days and they don’t come back to the hospital with complications, versus having them stay double that amount of time,” Dr. Bhat said. The direct variable cost was approximately $3,705 lower with the ERACS, and total hospitalization costs decreased by up to $11,227 per patient. For the institution overall, that outcome translated into savings of approximately $1 million for the year.
Dr. Bhat continues to see increasing gains from the protocol ERACS since the study period ended. “Our enhanced recovery pathway is getting better and better and more efficient.” The institution is now looking to expand enhanced recovery protocols to other types of surgery.
Dr. Deepa Bhat had no relevant financial disclosures.
NEW YORK – A protocol that standardizes care before, during, and after colorectal surgery cut average hospital stays from almost 6 days to less than 3, reduced complications, and slashed costs an estimated $11,227 per procedure.
“We decided to take a look at our own data, and we saw that a couple of variables were higher than we wanted them to be,” said Deepa Bhat, MD, a second year surgery resident at Advocate Illinois Masonic Medical Center in Chicago. Length of stay was one. Colorectal surgery patients were staying in the hospital an average 5.65 days, for example.
Their surgical site infection rate also warranted attention, Dr. Bhat said during a poster session at the American College of Surgeons Quality and Safety Conference. The overall complication rate was 6.45%.
“So, we decided to implement this enhanced recovery pathway in the hope that it would, one, get patients out of the hospital faster and allow them to recover at home and, two, decrease the rate of complications, including surgical site infections.”
Prior to the protocol, individual surgeons chose when to initiate fluids, when to discharge a patient, and many other preoperative factors. “Now, care is standardized so that every patient experiences the same pre-, intra-, and postoperative protocol, which leads to better outcomes,” Dr. Bhat said.
The multidisciplinary Enhanced Recovery After Colorectal Surgery (ERACS) pathway also now emphasizes more patient education prior to surgery. “The patients go into surgery having a very clear idea of what they can expect, such as how their pain will be controlled, when they can start liquids, and what their expectations are for ambulation,” she said. “By making patients active participants in their own care, they tend to do better.”
The investigators studied 246 elective colorectal surgery patients at their large, urban, community teaching hospital. They compared outcomes in 2014, versus 2015, to gauge the effectiveness of the ERACS. “The change in length of stay was really rather remarkable,” Dr. Bhat said. In fact, the typical number of days in the hospital decreased by about half from 5.65 to 2.89 days, a statistically significant difference (P less than .0001).
Historically, “the whole reason we don’t send patients home sooner is we’re worried they’re going to bounce right back, and that’s an issue for the 30-day readmission rate,” Dr. Bhat said. However, the enhanced recovery protocol was designed to minimize that risk, and the study verified that it works. “We wanted to show that you can send patients home safely and also not worry that they would come back more often.”
Patient satisfaction seems higher too. Dr. Bhat added, “Who doesn’t feel better at home?”
“It is pretty remarkable that you can send somebody home in less than 3 days and they don’t come back to the hospital with complications, versus having them stay double that amount of time,” Dr. Bhat said. The direct variable cost was approximately $3,705 lower with the ERACS, and total hospitalization costs decreased by up to $11,227 per patient. For the institution overall, that outcome translated into savings of approximately $1 million for the year.
Dr. Bhat continues to see increasing gains from the protocol ERACS since the study period ended. “Our enhanced recovery pathway is getting better and better and more efficient.” The institution is now looking to expand enhanced recovery protocols to other types of surgery.
Dr. Deepa Bhat had no relevant financial disclosures.
NEW YORK – A protocol that standardizes care before, during, and after colorectal surgery cut average hospital stays from almost 6 days to less than 3, reduced complications, and slashed costs an estimated $11,227 per procedure.
“We decided to take a look at our own data, and we saw that a couple of variables were higher than we wanted them to be,” said Deepa Bhat, MD, a second year surgery resident at Advocate Illinois Masonic Medical Center in Chicago. Length of stay was one. Colorectal surgery patients were staying in the hospital an average 5.65 days, for example.
Their surgical site infection rate also warranted attention, Dr. Bhat said during a poster session at the American College of Surgeons Quality and Safety Conference. The overall complication rate was 6.45%.
“So, we decided to implement this enhanced recovery pathway in the hope that it would, one, get patients out of the hospital faster and allow them to recover at home and, two, decrease the rate of complications, including surgical site infections.”
Prior to the protocol, individual surgeons chose when to initiate fluids, when to discharge a patient, and many other preoperative factors. “Now, care is standardized so that every patient experiences the same pre-, intra-, and postoperative protocol, which leads to better outcomes,” Dr. Bhat said.
The multidisciplinary Enhanced Recovery After Colorectal Surgery (ERACS) pathway also now emphasizes more patient education prior to surgery. “The patients go into surgery having a very clear idea of what they can expect, such as how their pain will be controlled, when they can start liquids, and what their expectations are for ambulation,” she said. “By making patients active participants in their own care, they tend to do better.”
The investigators studied 246 elective colorectal surgery patients at their large, urban, community teaching hospital. They compared outcomes in 2014, versus 2015, to gauge the effectiveness of the ERACS. “The change in length of stay was really rather remarkable,” Dr. Bhat said. In fact, the typical number of days in the hospital decreased by about half from 5.65 to 2.89 days, a statistically significant difference (P less than .0001).
Historically, “the whole reason we don’t send patients home sooner is we’re worried they’re going to bounce right back, and that’s an issue for the 30-day readmission rate,” Dr. Bhat said. However, the enhanced recovery protocol was designed to minimize that risk, and the study verified that it works. “We wanted to show that you can send patients home safely and also not worry that they would come back more often.”
Patient satisfaction seems higher too. Dr. Bhat added, “Who doesn’t feel better at home?”
“It is pretty remarkable that you can send somebody home in less than 3 days and they don’t come back to the hospital with complications, versus having them stay double that amount of time,” Dr. Bhat said. The direct variable cost was approximately $3,705 lower with the ERACS, and total hospitalization costs decreased by up to $11,227 per patient. For the institution overall, that outcome translated into savings of approximately $1 million for the year.
Dr. Bhat continues to see increasing gains from the protocol ERACS since the study period ended. “Our enhanced recovery pathway is getting better and better and more efficient.” The institution is now looking to expand enhanced recovery protocols to other types of surgery.
Dr. Deepa Bhat had no relevant financial disclosures.
AT THE ACS QUALITY AND SAFETY CONFERENCE
Key clinical point: An Enhanced Recovery After Colorectal Surgery (ERACS) protocol cut colorectal surgical site infections and reduced length of stay.
Major finding: The intervention decreased the hospital stays from an average of 6 days to 3 days and saved an estimated $11,227 per surgery.
Data source: A retrospective study of 246 patients undergoing elective colorectal surgery at a large, urban, community teaching hospital.
Disclosures: Dr. Deepa Bhat had no relevant financial disclosures.
Ventricular assist devices linked to sepsis
NEW ORLEANS – Back in 2008, there was only one case.
Since then, however, the number of patients with ventricular assist devices who developed sepsis while being treated in the cardiac unit at Queen Elizabeth Hospital in Birmingham, England, appeared to be noticeably growing. So, investigators launched a study to confirm their suspicions and to learn more about the underlying causes.
“Bloodstream infection is a serious infection, so I thought, ‘Let’s see what’s happening,’ ” explained Ira Das, MD, a consultant microbiologist at Queen Elizabeth Hospital.
Coagulase-negative staphylococci were the most common cause, present in 32% of the 25 cases. Sepsis was caused by Enterococcus faecium in 12%, Candida parapsilosis in 8%, and Staphylococcus aureus in 2%. Another 4% were either Enterococcus faecalis, Serratia marcescens, Pseudomonas aeruginosa, C. guilliermondii, or C. orthopsilosis. The remaining 16% of bloodstream infections were polymicrobial.
Less certain was the source of these infections.
“In the majority of cases, we didn’t know where it was coming from,” Dr. Das said at the annual meeting of the American Society for Microbiology. In 6 of the 25 cases, VAD was confirmed to be the focus of infection, either through imaging or because a failing component of the explanted device was examined later. An intravascular catheter was the source in another 5 patients, and in 14 cases, the source remained a mystery.
“Some of these infections just might have been hard to see,” Dr. Das said. “If the infection is inside the device, it’s not always easy to visualize.”
The study supports earlier findings from a review article that points to a significant infection risk associated with the implantation of VADs (Expert Rev Med Devices. 2011 Sep;8[5]:627-34). That article’s authors noted, “Despite recent improvements in outcomes, device-related infections remain a significant complication of LVAD [left ventricular assist device] therapy.”
In a previous study of people with end-stage heart failure, other investigators noted that, “despite the substantial survival benefit, the morbidity and mortality associated with the use of the left ventricular assist device were considerable. In particular, infection and mechanical failure of the device were major factors in the 2-year survival rate of only 23%” (N Engl J Med. 2001 Nov 15;345[20]:1435-43).
Similarly, in the current study, mortality was higher among those with sepsis and a VAD. Mortality was 39% – including eight patients who died with a VAD in situ and one following cardiac transplantation. However, Dr. Das cautioned, “It’s a small number, and there are other factors that could have contributed. They all go on anticoagulants so they have bleeding tendencies, and many of the patients are in the ICU with multiorgan failure.”
Infection prevention remains paramount to minimize mortality and other adverse events associated with a patient’s having a VAD. “We have to make sure that infection control procedures and our treatments are up to the optimal standard,” Dr. Das said. “It’s not easy to remove the device.”
Of the 129 VADs implanted, 68 were long-term LVADs, 11 were short-term LVADs, 15 were right ventricular devices, and 35 were biventricular devices.
The study is ongoing. The data presented at the meeting were collected up until December 2016.
“Since then, I’ve seen two more cases, and – very interestingly – one was Haemophilus influenzae,” Dr. Das said. “The patient was on the device, he was at home, and he came in with bacteremia.” Again, the source of infection proved elusive. “With H. influenzae, you would think it was coming from his chest, but the chest x-ray was normal.”
The second case, a patient with a coagulase-negative staphylococci bloodstream infection, was scheduled for a PET scan at the time of Dr. Das’ presentation to try to identify the source of infection.
Dr. Das had no relevant disclosures.
Modern technology saves our patients' lives, but there is always another side to the coin. Reports that LVAD devices are associated with a high incidence of bloodstream infections is important for future clinical practice. The fact that the causes and risk factors for these infections are unknown make this phenomena one of high interest.
Modern technology saves our patients' lives, but there is always another side to the coin. Reports that LVAD devices are associated with a high incidence of bloodstream infections is important for future clinical practice. The fact that the causes and risk factors for these infections are unknown make this phenomena one of high interest.
Modern technology saves our patients' lives, but there is always another side to the coin. Reports that LVAD devices are associated with a high incidence of bloodstream infections is important for future clinical practice. The fact that the causes and risk factors for these infections are unknown make this phenomena one of high interest.
NEW ORLEANS – Back in 2008, there was only one case.
Since then, however, the number of patients with ventricular assist devices who developed sepsis while being treated in the cardiac unit at Queen Elizabeth Hospital in Birmingham, England, appeared to be noticeably growing. So, investigators launched a study to confirm their suspicions and to learn more about the underlying causes.
“Bloodstream infection is a serious infection, so I thought, ‘Let’s see what’s happening,’ ” explained Ira Das, MD, a consultant microbiologist at Queen Elizabeth Hospital.
Coagulase-negative staphylococci were the most common cause, present in 32% of the 25 cases. Sepsis was caused by Enterococcus faecium in 12%, Candida parapsilosis in 8%, and Staphylococcus aureus in 2%. Another 4% were either Enterococcus faecalis, Serratia marcescens, Pseudomonas aeruginosa, C. guilliermondii, or C. orthopsilosis. The remaining 16% of bloodstream infections were polymicrobial.
Less certain was the source of these infections.
“In the majority of cases, we didn’t know where it was coming from,” Dr. Das said at the annual meeting of the American Society for Microbiology. In 6 of the 25 cases, VAD was confirmed to be the focus of infection, either through imaging or because a failing component of the explanted device was examined later. An intravascular catheter was the source in another 5 patients, and in 14 cases, the source remained a mystery.
“Some of these infections just might have been hard to see,” Dr. Das said. “If the infection is inside the device, it’s not always easy to visualize.”
The study supports earlier findings from a review article that points to a significant infection risk associated with the implantation of VADs (Expert Rev Med Devices. 2011 Sep;8[5]:627-34). That article’s authors noted, “Despite recent improvements in outcomes, device-related infections remain a significant complication of LVAD [left ventricular assist device] therapy.”
In a previous study of people with end-stage heart failure, other investigators noted that, “despite the substantial survival benefit, the morbidity and mortality associated with the use of the left ventricular assist device were considerable. In particular, infection and mechanical failure of the device were major factors in the 2-year survival rate of only 23%” (N Engl J Med. 2001 Nov 15;345[20]:1435-43).
Similarly, in the current study, mortality was higher among those with sepsis and a VAD. Mortality was 39% – including eight patients who died with a VAD in situ and one following cardiac transplantation. However, Dr. Das cautioned, “It’s a small number, and there are other factors that could have contributed. They all go on anticoagulants so they have bleeding tendencies, and many of the patients are in the ICU with multiorgan failure.”
Infection prevention remains paramount to minimize mortality and other adverse events associated with a patient’s having a VAD. “We have to make sure that infection control procedures and our treatments are up to the optimal standard,” Dr. Das said. “It’s not easy to remove the device.”
Of the 129 VADs implanted, 68 were long-term LVADs, 11 were short-term LVADs, 15 were right ventricular devices, and 35 were biventricular devices.
The study is ongoing. The data presented at the meeting were collected up until December 2016.
“Since then, I’ve seen two more cases, and – very interestingly – one was Haemophilus influenzae,” Dr. Das said. “The patient was on the device, he was at home, and he came in with bacteremia.” Again, the source of infection proved elusive. “With H. influenzae, you would think it was coming from his chest, but the chest x-ray was normal.”
The second case, a patient with a coagulase-negative staphylococci bloodstream infection, was scheduled for a PET scan at the time of Dr. Das’ presentation to try to identify the source of infection.
Dr. Das had no relevant disclosures.
NEW ORLEANS – Back in 2008, there was only one case.
Since then, however, the number of patients with ventricular assist devices who developed sepsis while being treated in the cardiac unit at Queen Elizabeth Hospital in Birmingham, England, appeared to be noticeably growing. So, investigators launched a study to confirm their suspicions and to learn more about the underlying causes.
“Bloodstream infection is a serious infection, so I thought, ‘Let’s see what’s happening,’ ” explained Ira Das, MD, a consultant microbiologist at Queen Elizabeth Hospital.
Coagulase-negative staphylococci were the most common cause, present in 32% of the 25 cases. Sepsis was caused by Enterococcus faecium in 12%, Candida parapsilosis in 8%, and Staphylococcus aureus in 2%. Another 4% were either Enterococcus faecalis, Serratia marcescens, Pseudomonas aeruginosa, C. guilliermondii, or C. orthopsilosis. The remaining 16% of bloodstream infections were polymicrobial.
Less certain was the source of these infections.
“In the majority of cases, we didn’t know where it was coming from,” Dr. Das said at the annual meeting of the American Society for Microbiology. In 6 of the 25 cases, VAD was confirmed to be the focus of infection, either through imaging or because a failing component of the explanted device was examined later. An intravascular catheter was the source in another 5 patients, and in 14 cases, the source remained a mystery.
“Some of these infections just might have been hard to see,” Dr. Das said. “If the infection is inside the device, it’s not always easy to visualize.”
The study supports earlier findings from a review article that points to a significant infection risk associated with the implantation of VADs (Expert Rev Med Devices. 2011 Sep;8[5]:627-34). That article’s authors noted, “Despite recent improvements in outcomes, device-related infections remain a significant complication of LVAD [left ventricular assist device] therapy.”
In a previous study of people with end-stage heart failure, other investigators noted that, “despite the substantial survival benefit, the morbidity and mortality associated with the use of the left ventricular assist device were considerable. In particular, infection and mechanical failure of the device were major factors in the 2-year survival rate of only 23%” (N Engl J Med. 2001 Nov 15;345[20]:1435-43).
Similarly, in the current study, mortality was higher among those with sepsis and a VAD. Mortality was 39% – including eight patients who died with a VAD in situ and one following cardiac transplantation. However, Dr. Das cautioned, “It’s a small number, and there are other factors that could have contributed. They all go on anticoagulants so they have bleeding tendencies, and many of the patients are in the ICU with multiorgan failure.”
Infection prevention remains paramount to minimize mortality and other adverse events associated with a patient’s having a VAD. “We have to make sure that infection control procedures and our treatments are up to the optimal standard,” Dr. Das said. “It’s not easy to remove the device.”
Of the 129 VADs implanted, 68 were long-term LVADs, 11 were short-term LVADs, 15 were right ventricular devices, and 35 were biventricular devices.
The study is ongoing. The data presented at the meeting were collected up until December 2016.
“Since then, I’ve seen two more cases, and – very interestingly – one was Haemophilus influenzae,” Dr. Das said. “The patient was on the device, he was at home, and he came in with bacteremia.” Again, the source of infection proved elusive. “With H. influenzae, you would think it was coming from his chest, but the chest x-ray was normal.”
The second case, a patient with a coagulase-negative staphylococci bloodstream infection, was scheduled for a PET scan at the time of Dr. Das’ presentation to try to identify the source of infection.
Dr. Das had no relevant disclosures.
AT ASM MICROBE 2017
Key clinical point: There may be a significant rate of bloodstream infections among people with a ventricular assist device.
Major finding: A total of 20% of the 118 people with a VAD had a bloodstream infection.
Data source: A retrospective study of 129 ventricular assist devices placed in 118 people between 2008 and 2016.
Disclosures: Dr. Das had no relevant disclosures.