User login
Damian McNamara is a journalist for Medscape Medical News and MDedge. He worked full-time for MDedge as the Miami Bureau covering a dozen medical specialties during 2001-2012, then as a freelancer for Medscape and MDedge, before being hired on staff by Medscape in 2018. Now the two companies are one. He uses what he learned in school – Damian has a BS in chemistry and an MS in science, health and environmental reporting/journalism. He works out of a home office in Miami, with a 100-pound chocolate lab known to snore under his desk during work hours.
Moderna filing for FDA emergency COVID-19 vaccine approval, reports 94.1% efficacy
The Moderna COVID-19 vaccine in development was 94.1% effective in the final analysis of its 30,000-participant phase 3 study. Bolstered by the new findings, the company plans to file for an emergency use authorization (EUA) from the Food and Drug Administration (FDA) today, according to a company release.
A total of 11 people in the mRNA-1273 vaccinated group later tested positive for COVID-19, compared with 185 participants given two placebo injections, resulting in a point estimate of 94.1% efficacy. This finding aligns with the 94.5% efficacy in interim trial results announced on November 16, as reported by Medscape Medical News.
Furthermore, Moderna announced that the vaccine prevented serious cases of infection. All 30 severe infections occurred among those people randomly assigned to placebo.
The FDA plans to review the Moderna vaccine safety and efficacy data at the next Vaccines and Related Biological Products Advisory Committee (VRBPAC) meeting scheduled for December 17. If and when approved, healthcare providers can use the new 91301 CPT code specific to mRNA-1273 vaccination.
“This positive primary analysis confirms the ability of our vaccine to prevent COVID-19 disease with 94.1% efficacy and, importantly, the ability to prevent severe COVID-19 disease,” said Stéphane Bancel, MBA, MEng, chief executive officer of Moderna, in the news release. “We believe that our vaccine will provide a new and powerful tool that may change the course of this pandemic and help prevent severe disease, hospitalizations, and death.”
Vaccine efficacy remained consistent across different groups analyzed by age, race/ethnicity, and gender. The 196 COVID-19 cases in the trial included 33 adults older than 65 years and 42 people from diverse communities, including 29 Hispanic or Latinx, six Black or African Americans, four Asian Americans, and three multiracial participants, the company reported.
No serious vaccine-related safety issues
The mRNA-1273 vaccine was generally well tolerated and no serious safety concerns with the vaccine have been identified to date, the company reported.
Injection site pain, fatigue, myalgia, arthralgia, headache, and erythema/redness at the injection site were the most common solicited adverse events in a prior analysis. The company noted that these solicited adverse reactions increased in frequency and severity after the second vaccine dose. A continuous review of safety data is ongoing.
One COVID-19-related death in the study occurred in the placebo group.
Ready to start shipping
Moderna expects to have approximately 20 million doses of mRNA-1273 available in the United States by the end of this year. The company reports that it’s on track to manufacture 500 million to 1 billion doses globally in 2021.
The company also is seeking approval from nations and organizations worldwide, including a conditional approval from the European Medicines Agency (EMA). The study is being conducted in collaboration with the National Institute of Allergy and Infectious Diseases (NIAID) and the Biomedical Advanced Research and Development Authority (BARDA), part of the Office of the Assistant Secretary for Preparedness and Response at the US Department of Health and Human Services.
Moderna will be the second company to file an EUA with the FDA for a COVID vaccine, after Pfizer requested one for its mRNA vaccine earlier this month.
This article first appeared on Medscape.com.
The Moderna COVID-19 vaccine in development was 94.1% effective in the final analysis of its 30,000-participant phase 3 study. Bolstered by the new findings, the company plans to file for an emergency use authorization (EUA) from the Food and Drug Administration (FDA) today, according to a company release.
A total of 11 people in the mRNA-1273 vaccinated group later tested positive for COVID-19, compared with 185 participants given two placebo injections, resulting in a point estimate of 94.1% efficacy. This finding aligns with the 94.5% efficacy in interim trial results announced on November 16, as reported by Medscape Medical News.
Furthermore, Moderna announced that the vaccine prevented serious cases of infection. All 30 severe infections occurred among those people randomly assigned to placebo.
The FDA plans to review the Moderna vaccine safety and efficacy data at the next Vaccines and Related Biological Products Advisory Committee (VRBPAC) meeting scheduled for December 17. If and when approved, healthcare providers can use the new 91301 CPT code specific to mRNA-1273 vaccination.
“This positive primary analysis confirms the ability of our vaccine to prevent COVID-19 disease with 94.1% efficacy and, importantly, the ability to prevent severe COVID-19 disease,” said Stéphane Bancel, MBA, MEng, chief executive officer of Moderna, in the news release. “We believe that our vaccine will provide a new and powerful tool that may change the course of this pandemic and help prevent severe disease, hospitalizations, and death.”
Vaccine efficacy remained consistent across different groups analyzed by age, race/ethnicity, and gender. The 196 COVID-19 cases in the trial included 33 adults older than 65 years and 42 people from diverse communities, including 29 Hispanic or Latinx, six Black or African Americans, four Asian Americans, and three multiracial participants, the company reported.
No serious vaccine-related safety issues
The mRNA-1273 vaccine was generally well tolerated and no serious safety concerns with the vaccine have been identified to date, the company reported.
Injection site pain, fatigue, myalgia, arthralgia, headache, and erythema/redness at the injection site were the most common solicited adverse events in a prior analysis. The company noted that these solicited adverse reactions increased in frequency and severity after the second vaccine dose. A continuous review of safety data is ongoing.
One COVID-19-related death in the study occurred in the placebo group.
Ready to start shipping
Moderna expects to have approximately 20 million doses of mRNA-1273 available in the United States by the end of this year. The company reports that it’s on track to manufacture 500 million to 1 billion doses globally in 2021.
The company also is seeking approval from nations and organizations worldwide, including a conditional approval from the European Medicines Agency (EMA). The study is being conducted in collaboration with the National Institute of Allergy and Infectious Diseases (NIAID) and the Biomedical Advanced Research and Development Authority (BARDA), part of the Office of the Assistant Secretary for Preparedness and Response at the US Department of Health and Human Services.
Moderna will be the second company to file an EUA with the FDA for a COVID vaccine, after Pfizer requested one for its mRNA vaccine earlier this month.
This article first appeared on Medscape.com.
The Moderna COVID-19 vaccine in development was 94.1% effective in the final analysis of its 30,000-participant phase 3 study. Bolstered by the new findings, the company plans to file for an emergency use authorization (EUA) from the Food and Drug Administration (FDA) today, according to a company release.
A total of 11 people in the mRNA-1273 vaccinated group later tested positive for COVID-19, compared with 185 participants given two placebo injections, resulting in a point estimate of 94.1% efficacy. This finding aligns with the 94.5% efficacy in interim trial results announced on November 16, as reported by Medscape Medical News.
Furthermore, Moderna announced that the vaccine prevented serious cases of infection. All 30 severe infections occurred among those people randomly assigned to placebo.
The FDA plans to review the Moderna vaccine safety and efficacy data at the next Vaccines and Related Biological Products Advisory Committee (VRBPAC) meeting scheduled for December 17. If and when approved, healthcare providers can use the new 91301 CPT code specific to mRNA-1273 vaccination.
“This positive primary analysis confirms the ability of our vaccine to prevent COVID-19 disease with 94.1% efficacy and, importantly, the ability to prevent severe COVID-19 disease,” said Stéphane Bancel, MBA, MEng, chief executive officer of Moderna, in the news release. “We believe that our vaccine will provide a new and powerful tool that may change the course of this pandemic and help prevent severe disease, hospitalizations, and death.”
Vaccine efficacy remained consistent across different groups analyzed by age, race/ethnicity, and gender. The 196 COVID-19 cases in the trial included 33 adults older than 65 years and 42 people from diverse communities, including 29 Hispanic or Latinx, six Black or African Americans, four Asian Americans, and three multiracial participants, the company reported.
No serious vaccine-related safety issues
The mRNA-1273 vaccine was generally well tolerated and no serious safety concerns with the vaccine have been identified to date, the company reported.
Injection site pain, fatigue, myalgia, arthralgia, headache, and erythema/redness at the injection site were the most common solicited adverse events in a prior analysis. The company noted that these solicited adverse reactions increased in frequency and severity after the second vaccine dose. A continuous review of safety data is ongoing.
One COVID-19-related death in the study occurred in the placebo group.
Ready to start shipping
Moderna expects to have approximately 20 million doses of mRNA-1273 available in the United States by the end of this year. The company reports that it’s on track to manufacture 500 million to 1 billion doses globally in 2021.
The company also is seeking approval from nations and organizations worldwide, including a conditional approval from the European Medicines Agency (EMA). The study is being conducted in collaboration with the National Institute of Allergy and Infectious Diseases (NIAID) and the Biomedical Advanced Research and Development Authority (BARDA), part of the Office of the Assistant Secretary for Preparedness and Response at the US Department of Health and Human Services.
Moderna will be the second company to file an EUA with the FDA for a COVID vaccine, after Pfizer requested one for its mRNA vaccine earlier this month.
This article first appeared on Medscape.com.
IDSA updates COVID guidelines for antibodies, antivirals, other drugs
An infectious disease expert panel cautions against routine use of bamlanivimab (Eli Lilly) and notes that remdesivir (Veklury) can shorten the clinical course of COVID-19 – which could be critical “as hospitals fill up” across the United States.
The group also said the monoclonal antibodies approved for emergency use by the Food and Drug Administration and still in development hold promise, although more clinical trial data are needed.
These and other recommendations appear in updated guidelines from the Infectious Diseases Society of America, released Nov. 18 and Nov. 22.
A conditional ‘no’ on routine bamlanivimab
“The guideline panel gave a conditional recommendation against the routine use of bamlanivimab,” Adarsh Bhimraj, MD, cochair of the IDSA COVID-19 Treatment and Management Guidelines Expert Panel, said.
On Nov. 10, the FDA issued an emergency-use authorization (EUA) for bamlanivimab for use in ambulatory patients with mild to moderate COVID-19.
“We did have a remark that it may be used in patients who have increased risk of severe COVID-19, as it is outlined in the FDA emergency-use authorization issued last week,” he said. He added that use should follow an informed discussion between provider and patient, one in which “the patient puts a very high value on the uncertain benefits and a low value on uncertain adverse events.”
The panel’s rationale was based in part on interim analysis of the phase 2 BLAZE-1 trial, which found 1.6% of people randomly assigned to bamlanivimab had an emergency department visit or hospitalization compared with 6.3% of those receiving a placebo.
“We thought the estimate was too fragile because the number in each arm was very low. Even a small change in these numbers could make the difference nonsignificant,” said Bhimraj, head of the neurologic infectious diseases section in the department of infectious diseases at the Cleveland (Ohio) Clinic.
Awaiting more data on antibody combination
On November 21, the FDA granted an EUA to the casirivimab and imbdevimab monoclonal antibody combination (Regeneron), indicated to treated mild to moderate COVID-19.
“Surprisingly, the preliminary results released in the EUA look a lot like bamlanivimab,” Dr. Bhimraj said.
Unlike bamlanivimab, for which trial details were published, the panel does not yet have the totality of data on casirivimab and imbdevimab, and therefore is not yet making a recommendation. “We want to be cautious as a guideline panel. We are anxiously awaiting the full publication,” he added.
“I do think these monoclonal antibodies show potential for benefit, but as Dr. Bhimraj said, it’s very difficult with the relatively small numbers we’re talking about,” said Rajesh T. Gandhi, MD, cochair of the IDSA COVID-19 Treatment and Management Guidelines Expert Panel.
Remaining questions include the degree of efficacy these antibody therapies will have, as well as which patients are most likely to benefit, added Dr. Gandhi, who is also a professor of medicine at Harvard Medical School and director of HIV Clinical Services and Education at Massachusetts General Hospital, both in Boston.
Furthermore, although there appear to be adequate supplies of remdesivir and dexamethasone, for example, availability and distribution of monoclonal antibodies could present logistic challenges. Prioritizing which high-risk patients receive this therapy and ensuring equity and access to communities most affected by COVID-19, including minority and low socioeconomic populations, need to be addressed, Dr. Gandhi said.
Remdesivir recommended to shorten hospital stays
The panel’s recommendations regarding the use of remdesivir “has largely remained the same,” Dr. Gandhi said. Evidence indicates recovery is faster with remdesivir at 10 days vs 15 days in people taking a placebo.
In the ACTT-1 trial, for example, participants in the treatment group recovered in a median 10 days versus 15 days in the placebo group.
Therefore, the IDSA panel continues to recommend remdesivir treatment for hospitalized patients with COVID-19.
“As hospitals around the United States fill up, the IDSA panel believes the effect of remdesivir on speeding up recovery could be an important benefit, and that is why we continue to suggest its use,” Dr. Gandhi said.
When asked about the World Health Organization–sponsored trial that showed no benefit in terms of mortality, he replied, “Remdesivir is not a home run – we need better drugs.”
A recommendation against lopinavir and ritonavir
In contrast, the panel recommends against use of the lopinavir/ritonavir protease inhibitor combination therapy, based in part on data from a preprint of the Solidarity study.
The open-label Solidarity trial in 30 countries, sponsored by WHO, assessed hydroxychloroquine, interferon, lopinavir/ritonavir, and remdesivir in people hospitalized with COVID-19.
None of these drugs showed an effect on mortality, Dr. Gandhi said. “Better medicines that improve survival are clearly needed.”
Dexamethasone remains the only agent demonstrated to reduce mortality in people hospitalized with COVID-19, he added.
Tocilizumab not for routine use
After critical review of the studies that have emerged since the last IDSA recommendation regarding tocilizumab (Actemra) in September, “the panel still stood with the recommendation against routine use of tocilizumab in hospitalized patients with COVID-19,” Dr. Bhimraj said.
The guidance is based on trials including COVACTA and EMPACTA. Treatment with tocilizumab was not associated with significant differences in mortality. In these and other studies, “we did not really find a significant difference, and that was the reason for the conditional recommendation against routine use of tocilizumab in hospitalized patients,” Dr. Bhimraj said.
Also, although the trials were blinded, “we know treatment with tocilizumab can cause a reduction in C-reactive protein levels,” which could indicate to researchers which participants were receiving active treatment versus placebo, he said.
Jury still out on baricitinib, remdesivir combination
The FDA granted an EUA to the combination of remdesivir and baricitinib (Olumiant) on Nov. 19. However, the IDSA panel is reserving its recommendation on this therapeutic combination until more data emerge.
“We still don’t have complete results of the ACTT-2 study, and the information we do have is what is available in the EUA,” Dr. Bhimraj said. The panel expects to issue guidance once the totality of data become available.
Unanswered questions include why investigators chose a 4-mg dose of baricitinib – twice the 2-mg dose commonly used for treating rheumatoid arthritis – and how many patients in the trial also were treated with steroids.
Dr. Gandhi agreed that the proportion of patients taking a steroid is “really an important consideration.” He added that dexamethasone has become standard of care because it reduces mortality, as well as the number of people requiring oxygen. He said it will be important to know how the baricitinib/remdesivir combination compares with dexamethasone.
“You don’t want to give a drug with less certain benefit over a drug for which there is more certain benefit,” Dr. Gandhi said.
Future possibilities
“The monoclonal antibodies are important to continue studying, particularly in combinations,” Dr. Gandhi said. Researchers are investigating formulations other than IV infusion to make therapy more convenient. For example, a subcutaneous injection like insulin would make administration at home more of a possibility.
Investigators are also looking at oral antiviral therapy, inhaled antivirals, and the promise of using interferon therapy. Dr. Gandhi added there is also “a lot of work around medications to reduce the excess inflammation that drives very severe COVID-19.”
‘Exciting news’ on AstraZeneca vaccine
Although not part of the IDSA guidelines, “we saw the news from AstraZeneca this morning, which is exciting,” Dr. Gandhi said during a media briefing.
Unlike the Pfizer and Moderna messenger RNA vaccines, which use the genetic material of the virus to make the virus proteins that elicit an immune response, the AstraZeneca/Oxford University vaccine uses a viral vector to carry the SARS-CoV-2 protein, to which the body produces an immune response.
“I’m thrilled that several different vaccines are showing important effects at rates higher than the FDA benchmark of 50%, and these are well exceeding that,” Dr. Gandhi said.
“One interesting thing from the [AstraZeneca] press release is they show asymptomatic infection being reduced,” he added. “That is critical because we know a lot of transmission of SARS-CoV-2 comes from asymptomatic people.”
Reasons for optimism
In response to a question about whether the experts feel more optimistic about COVID-19, Dr. Bhimraj said he is cautiously optimistic. “We have made tremendous progress in therapeutic agents, and in how the world has come together in the middle of a catastrophe to collaborate, setting our differences apart, to do trials. That is commendable.”
Dr. Gandhi said he felt more optimistic than he did in the spring. He pointed out that physicians and researchers know a lot more about potential blood clotting complications, how to support patients through severe COVID-19 and keep them off a ventilator whenever possible, and how to provide dexamethasone to reduce the risk of death.
Those benefits are in hospitalized patients, however, and “we need ways to prevent people from getting into the hospital, and that is why we are looking at the monoclonal antibodies,” Dr. Gandhi said. “If borne out in larger trials, that will be a major advance.”
“We need to keep our focus on prevention and go back to our idea of flattening the curve. That is critical so our health care systems do not get overwhelmed during this massive surge we are in,” Dr. Gandhi said. “So masking and social distancing are just as important as they always have been.”
Dr. Bhimraj disclosed no relevant financial relationships. Dr. Gandhi has no disclosures for the past 12 months; in the past 3 years, he has served on scientific advisory boards for Gilead and Merck.
This article first appeared on Medscape.com.
An infectious disease expert panel cautions against routine use of bamlanivimab (Eli Lilly) and notes that remdesivir (Veklury) can shorten the clinical course of COVID-19 – which could be critical “as hospitals fill up” across the United States.
The group also said the monoclonal antibodies approved for emergency use by the Food and Drug Administration and still in development hold promise, although more clinical trial data are needed.
These and other recommendations appear in updated guidelines from the Infectious Diseases Society of America, released Nov. 18 and Nov. 22.
A conditional ‘no’ on routine bamlanivimab
“The guideline panel gave a conditional recommendation against the routine use of bamlanivimab,” Adarsh Bhimraj, MD, cochair of the IDSA COVID-19 Treatment and Management Guidelines Expert Panel, said.
On Nov. 10, the FDA issued an emergency-use authorization (EUA) for bamlanivimab for use in ambulatory patients with mild to moderate COVID-19.
“We did have a remark that it may be used in patients who have increased risk of severe COVID-19, as it is outlined in the FDA emergency-use authorization issued last week,” he said. He added that use should follow an informed discussion between provider and patient, one in which “the patient puts a very high value on the uncertain benefits and a low value on uncertain adverse events.”
The panel’s rationale was based in part on interim analysis of the phase 2 BLAZE-1 trial, which found 1.6% of people randomly assigned to bamlanivimab had an emergency department visit or hospitalization compared with 6.3% of those receiving a placebo.
“We thought the estimate was too fragile because the number in each arm was very low. Even a small change in these numbers could make the difference nonsignificant,” said Bhimraj, head of the neurologic infectious diseases section in the department of infectious diseases at the Cleveland (Ohio) Clinic.
Awaiting more data on antibody combination
On November 21, the FDA granted an EUA to the casirivimab and imbdevimab monoclonal antibody combination (Regeneron), indicated to treated mild to moderate COVID-19.
“Surprisingly, the preliminary results released in the EUA look a lot like bamlanivimab,” Dr. Bhimraj said.
Unlike bamlanivimab, for which trial details were published, the panel does not yet have the totality of data on casirivimab and imbdevimab, and therefore is not yet making a recommendation. “We want to be cautious as a guideline panel. We are anxiously awaiting the full publication,” he added.
“I do think these monoclonal antibodies show potential for benefit, but as Dr. Bhimraj said, it’s very difficult with the relatively small numbers we’re talking about,” said Rajesh T. Gandhi, MD, cochair of the IDSA COVID-19 Treatment and Management Guidelines Expert Panel.
Remaining questions include the degree of efficacy these antibody therapies will have, as well as which patients are most likely to benefit, added Dr. Gandhi, who is also a professor of medicine at Harvard Medical School and director of HIV Clinical Services and Education at Massachusetts General Hospital, both in Boston.
Furthermore, although there appear to be adequate supplies of remdesivir and dexamethasone, for example, availability and distribution of monoclonal antibodies could present logistic challenges. Prioritizing which high-risk patients receive this therapy and ensuring equity and access to communities most affected by COVID-19, including minority and low socioeconomic populations, need to be addressed, Dr. Gandhi said.
Remdesivir recommended to shorten hospital stays
The panel’s recommendations regarding the use of remdesivir “has largely remained the same,” Dr. Gandhi said. Evidence indicates recovery is faster with remdesivir at 10 days vs 15 days in people taking a placebo.
In the ACTT-1 trial, for example, participants in the treatment group recovered in a median 10 days versus 15 days in the placebo group.
Therefore, the IDSA panel continues to recommend remdesivir treatment for hospitalized patients with COVID-19.
“As hospitals around the United States fill up, the IDSA panel believes the effect of remdesivir on speeding up recovery could be an important benefit, and that is why we continue to suggest its use,” Dr. Gandhi said.
When asked about the World Health Organization–sponsored trial that showed no benefit in terms of mortality, he replied, “Remdesivir is not a home run – we need better drugs.”
A recommendation against lopinavir and ritonavir
In contrast, the panel recommends against use of the lopinavir/ritonavir protease inhibitor combination therapy, based in part on data from a preprint of the Solidarity study.
The open-label Solidarity trial in 30 countries, sponsored by WHO, assessed hydroxychloroquine, interferon, lopinavir/ritonavir, and remdesivir in people hospitalized with COVID-19.
None of these drugs showed an effect on mortality, Dr. Gandhi said. “Better medicines that improve survival are clearly needed.”
Dexamethasone remains the only agent demonstrated to reduce mortality in people hospitalized with COVID-19, he added.
Tocilizumab not for routine use
After critical review of the studies that have emerged since the last IDSA recommendation regarding tocilizumab (Actemra) in September, “the panel still stood with the recommendation against routine use of tocilizumab in hospitalized patients with COVID-19,” Dr. Bhimraj said.
The guidance is based on trials including COVACTA and EMPACTA. Treatment with tocilizumab was not associated with significant differences in mortality. In these and other studies, “we did not really find a significant difference, and that was the reason for the conditional recommendation against routine use of tocilizumab in hospitalized patients,” Dr. Bhimraj said.
Also, although the trials were blinded, “we know treatment with tocilizumab can cause a reduction in C-reactive protein levels,” which could indicate to researchers which participants were receiving active treatment versus placebo, he said.
Jury still out on baricitinib, remdesivir combination
The FDA granted an EUA to the combination of remdesivir and baricitinib (Olumiant) on Nov. 19. However, the IDSA panel is reserving its recommendation on this therapeutic combination until more data emerge.
“We still don’t have complete results of the ACTT-2 study, and the information we do have is what is available in the EUA,” Dr. Bhimraj said. The panel expects to issue guidance once the totality of data become available.
Unanswered questions include why investigators chose a 4-mg dose of baricitinib – twice the 2-mg dose commonly used for treating rheumatoid arthritis – and how many patients in the trial also were treated with steroids.
Dr. Gandhi agreed that the proportion of patients taking a steroid is “really an important consideration.” He added that dexamethasone has become standard of care because it reduces mortality, as well as the number of people requiring oxygen. He said it will be important to know how the baricitinib/remdesivir combination compares with dexamethasone.
“You don’t want to give a drug with less certain benefit over a drug for which there is more certain benefit,” Dr. Gandhi said.
Future possibilities
“The monoclonal antibodies are important to continue studying, particularly in combinations,” Dr. Gandhi said. Researchers are investigating formulations other than IV infusion to make therapy more convenient. For example, a subcutaneous injection like insulin would make administration at home more of a possibility.
Investigators are also looking at oral antiviral therapy, inhaled antivirals, and the promise of using interferon therapy. Dr. Gandhi added there is also “a lot of work around medications to reduce the excess inflammation that drives very severe COVID-19.”
‘Exciting news’ on AstraZeneca vaccine
Although not part of the IDSA guidelines, “we saw the news from AstraZeneca this morning, which is exciting,” Dr. Gandhi said during a media briefing.
Unlike the Pfizer and Moderna messenger RNA vaccines, which use the genetic material of the virus to make the virus proteins that elicit an immune response, the AstraZeneca/Oxford University vaccine uses a viral vector to carry the SARS-CoV-2 protein, to which the body produces an immune response.
“I’m thrilled that several different vaccines are showing important effects at rates higher than the FDA benchmark of 50%, and these are well exceeding that,” Dr. Gandhi said.
“One interesting thing from the [AstraZeneca] press release is they show asymptomatic infection being reduced,” he added. “That is critical because we know a lot of transmission of SARS-CoV-2 comes from asymptomatic people.”
Reasons for optimism
In response to a question about whether the experts feel more optimistic about COVID-19, Dr. Bhimraj said he is cautiously optimistic. “We have made tremendous progress in therapeutic agents, and in how the world has come together in the middle of a catastrophe to collaborate, setting our differences apart, to do trials. That is commendable.”
Dr. Gandhi said he felt more optimistic than he did in the spring. He pointed out that physicians and researchers know a lot more about potential blood clotting complications, how to support patients through severe COVID-19 and keep them off a ventilator whenever possible, and how to provide dexamethasone to reduce the risk of death.
Those benefits are in hospitalized patients, however, and “we need ways to prevent people from getting into the hospital, and that is why we are looking at the monoclonal antibodies,” Dr. Gandhi said. “If borne out in larger trials, that will be a major advance.”
“We need to keep our focus on prevention and go back to our idea of flattening the curve. That is critical so our health care systems do not get overwhelmed during this massive surge we are in,” Dr. Gandhi said. “So masking and social distancing are just as important as they always have been.”
Dr. Bhimraj disclosed no relevant financial relationships. Dr. Gandhi has no disclosures for the past 12 months; in the past 3 years, he has served on scientific advisory boards for Gilead and Merck.
This article first appeared on Medscape.com.
An infectious disease expert panel cautions against routine use of bamlanivimab (Eli Lilly) and notes that remdesivir (Veklury) can shorten the clinical course of COVID-19 – which could be critical “as hospitals fill up” across the United States.
The group also said the monoclonal antibodies approved for emergency use by the Food and Drug Administration and still in development hold promise, although more clinical trial data are needed.
These and other recommendations appear in updated guidelines from the Infectious Diseases Society of America, released Nov. 18 and Nov. 22.
A conditional ‘no’ on routine bamlanivimab
“The guideline panel gave a conditional recommendation against the routine use of bamlanivimab,” Adarsh Bhimraj, MD, cochair of the IDSA COVID-19 Treatment and Management Guidelines Expert Panel, said.
On Nov. 10, the FDA issued an emergency-use authorization (EUA) for bamlanivimab for use in ambulatory patients with mild to moderate COVID-19.
“We did have a remark that it may be used in patients who have increased risk of severe COVID-19, as it is outlined in the FDA emergency-use authorization issued last week,” he said. He added that use should follow an informed discussion between provider and patient, one in which “the patient puts a very high value on the uncertain benefits and a low value on uncertain adverse events.”
The panel’s rationale was based in part on interim analysis of the phase 2 BLAZE-1 trial, which found 1.6% of people randomly assigned to bamlanivimab had an emergency department visit or hospitalization compared with 6.3% of those receiving a placebo.
“We thought the estimate was too fragile because the number in each arm was very low. Even a small change in these numbers could make the difference nonsignificant,” said Bhimraj, head of the neurologic infectious diseases section in the department of infectious diseases at the Cleveland (Ohio) Clinic.
Awaiting more data on antibody combination
On November 21, the FDA granted an EUA to the casirivimab and imbdevimab monoclonal antibody combination (Regeneron), indicated to treated mild to moderate COVID-19.
“Surprisingly, the preliminary results released in the EUA look a lot like bamlanivimab,” Dr. Bhimraj said.
Unlike bamlanivimab, for which trial details were published, the panel does not yet have the totality of data on casirivimab and imbdevimab, and therefore is not yet making a recommendation. “We want to be cautious as a guideline panel. We are anxiously awaiting the full publication,” he added.
“I do think these monoclonal antibodies show potential for benefit, but as Dr. Bhimraj said, it’s very difficult with the relatively small numbers we’re talking about,” said Rajesh T. Gandhi, MD, cochair of the IDSA COVID-19 Treatment and Management Guidelines Expert Panel.
Remaining questions include the degree of efficacy these antibody therapies will have, as well as which patients are most likely to benefit, added Dr. Gandhi, who is also a professor of medicine at Harvard Medical School and director of HIV Clinical Services and Education at Massachusetts General Hospital, both in Boston.
Furthermore, although there appear to be adequate supplies of remdesivir and dexamethasone, for example, availability and distribution of monoclonal antibodies could present logistic challenges. Prioritizing which high-risk patients receive this therapy and ensuring equity and access to communities most affected by COVID-19, including minority and low socioeconomic populations, need to be addressed, Dr. Gandhi said.
Remdesivir recommended to shorten hospital stays
The panel’s recommendations regarding the use of remdesivir “has largely remained the same,” Dr. Gandhi said. Evidence indicates recovery is faster with remdesivir at 10 days vs 15 days in people taking a placebo.
In the ACTT-1 trial, for example, participants in the treatment group recovered in a median 10 days versus 15 days in the placebo group.
Therefore, the IDSA panel continues to recommend remdesivir treatment for hospitalized patients with COVID-19.
“As hospitals around the United States fill up, the IDSA panel believes the effect of remdesivir on speeding up recovery could be an important benefit, and that is why we continue to suggest its use,” Dr. Gandhi said.
When asked about the World Health Organization–sponsored trial that showed no benefit in terms of mortality, he replied, “Remdesivir is not a home run – we need better drugs.”
A recommendation against lopinavir and ritonavir
In contrast, the panel recommends against use of the lopinavir/ritonavir protease inhibitor combination therapy, based in part on data from a preprint of the Solidarity study.
The open-label Solidarity trial in 30 countries, sponsored by WHO, assessed hydroxychloroquine, interferon, lopinavir/ritonavir, and remdesivir in people hospitalized with COVID-19.
None of these drugs showed an effect on mortality, Dr. Gandhi said. “Better medicines that improve survival are clearly needed.”
Dexamethasone remains the only agent demonstrated to reduce mortality in people hospitalized with COVID-19, he added.
Tocilizumab not for routine use
After critical review of the studies that have emerged since the last IDSA recommendation regarding tocilizumab (Actemra) in September, “the panel still stood with the recommendation against routine use of tocilizumab in hospitalized patients with COVID-19,” Dr. Bhimraj said.
The guidance is based on trials including COVACTA and EMPACTA. Treatment with tocilizumab was not associated with significant differences in mortality. In these and other studies, “we did not really find a significant difference, and that was the reason for the conditional recommendation against routine use of tocilizumab in hospitalized patients,” Dr. Bhimraj said.
Also, although the trials were blinded, “we know treatment with tocilizumab can cause a reduction in C-reactive protein levels,” which could indicate to researchers which participants were receiving active treatment versus placebo, he said.
Jury still out on baricitinib, remdesivir combination
The FDA granted an EUA to the combination of remdesivir and baricitinib (Olumiant) on Nov. 19. However, the IDSA panel is reserving its recommendation on this therapeutic combination until more data emerge.
“We still don’t have complete results of the ACTT-2 study, and the information we do have is what is available in the EUA,” Dr. Bhimraj said. The panel expects to issue guidance once the totality of data become available.
Unanswered questions include why investigators chose a 4-mg dose of baricitinib – twice the 2-mg dose commonly used for treating rheumatoid arthritis – and how many patients in the trial also were treated with steroids.
Dr. Gandhi agreed that the proportion of patients taking a steroid is “really an important consideration.” He added that dexamethasone has become standard of care because it reduces mortality, as well as the number of people requiring oxygen. He said it will be important to know how the baricitinib/remdesivir combination compares with dexamethasone.
“You don’t want to give a drug with less certain benefit over a drug for which there is more certain benefit,” Dr. Gandhi said.
Future possibilities
“The monoclonal antibodies are important to continue studying, particularly in combinations,” Dr. Gandhi said. Researchers are investigating formulations other than IV infusion to make therapy more convenient. For example, a subcutaneous injection like insulin would make administration at home more of a possibility.
Investigators are also looking at oral antiviral therapy, inhaled antivirals, and the promise of using interferon therapy. Dr. Gandhi added there is also “a lot of work around medications to reduce the excess inflammation that drives very severe COVID-19.”
‘Exciting news’ on AstraZeneca vaccine
Although not part of the IDSA guidelines, “we saw the news from AstraZeneca this morning, which is exciting,” Dr. Gandhi said during a media briefing.
Unlike the Pfizer and Moderna messenger RNA vaccines, which use the genetic material of the virus to make the virus proteins that elicit an immune response, the AstraZeneca/Oxford University vaccine uses a viral vector to carry the SARS-CoV-2 protein, to which the body produces an immune response.
“I’m thrilled that several different vaccines are showing important effects at rates higher than the FDA benchmark of 50%, and these are well exceeding that,” Dr. Gandhi said.
“One interesting thing from the [AstraZeneca] press release is they show asymptomatic infection being reduced,” he added. “That is critical because we know a lot of transmission of SARS-CoV-2 comes from asymptomatic people.”
Reasons for optimism
In response to a question about whether the experts feel more optimistic about COVID-19, Dr. Bhimraj said he is cautiously optimistic. “We have made tremendous progress in therapeutic agents, and in how the world has come together in the middle of a catastrophe to collaborate, setting our differences apart, to do trials. That is commendable.”
Dr. Gandhi said he felt more optimistic than he did in the spring. He pointed out that physicians and researchers know a lot more about potential blood clotting complications, how to support patients through severe COVID-19 and keep them off a ventilator whenever possible, and how to provide dexamethasone to reduce the risk of death.
Those benefits are in hospitalized patients, however, and “we need ways to prevent people from getting into the hospital, and that is why we are looking at the monoclonal antibodies,” Dr. Gandhi said. “If borne out in larger trials, that will be a major advance.”
“We need to keep our focus on prevention and go back to our idea of flattening the curve. That is critical so our health care systems do not get overwhelmed during this massive surge we are in,” Dr. Gandhi said. “So masking and social distancing are just as important as they always have been.”
Dr. Bhimraj disclosed no relevant financial relationships. Dr. Gandhi has no disclosures for the past 12 months; in the past 3 years, he has served on scientific advisory boards for Gilead and Merck.
This article first appeared on Medscape.com.
Debate: After methotrexate failure, is JAK inhibitor or biologic next?
What is the next step in treatment after a person with rheumatoid arthritis fails to adequately respond to methotrexate – a Janus kinase (JAK) inhibitor or a biologic? That was the focus of a lively debate at the virtual annual meeting of the American College of Rheumatology.
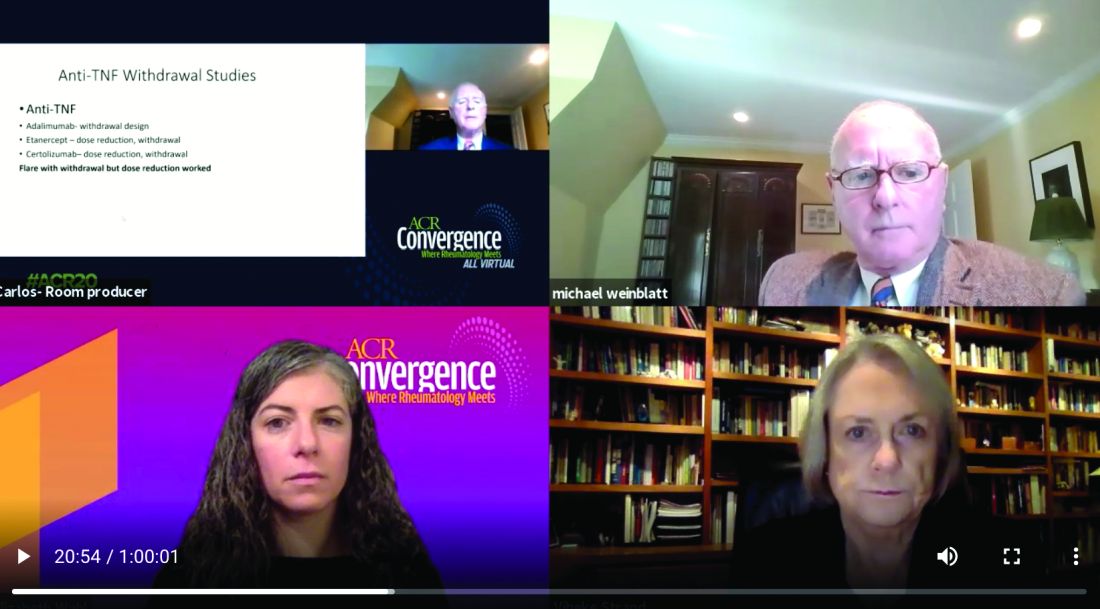
Vibeke Strand, MD, argued that JAK inhibitors offer the distinct advantage of a faster clinical response than biologics, meaning that decisions to change therapy based on nonresponse or adverse effects can be made earlier in a treatment plan.
Michael Weinblatt, MD, countered that the faster-response advantage is offset by potential adverse events associated with the JAK inhibitors, including increased risk of herpes zoster infection, venous thromboembolism (VTE), and arterial thromboembolism (ATE). He suggested switching patients to a biologic instead.
In addition, the debate was held just days before the ACR released a proposed guideline for the management of RA. This update to the 2015 guidance is the first to prioritize the order of RA treatments, emphasizing that clinicians should maximize methotrexate therapy before switching RA patients to a JAK inhibitor or a biologic. Release of the full guidelines is pending, and it remains unclear if the ACR provides any guidance regarding the “jakinib” versus biologic decision.
Interestingly, the debate did not hinge on any differences in efficacy. Both speakers pointed to similar efficacy between anti–tumor necrosis factor (TNF) agents and JAK inhibitors, and despite working on different pathways, among the individual JAK inhibitors as well.
Is timing of the essence?
Knowing whether a person with RA responds to a JAK inhibitor more quickly than to a biologic is a major advantage, said Dr. Strand, of the division of immunology and rheumatology at Stanford (Calif.) University. “The argument that I am making is that patients are more responsive if treated earlier in the disease process and they are less treatment-experienced.”
Dr. Strand said the advantages extend to remission as well. “When patients are aware of early improvements, their adherence is increased. Remission is more likely because it occurs earlier.”
“I will certainly grant it to Vibeke that jakinibs work much faster,” said Dr. Weinblatt, chair of rheumatology at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School in Boston. However, he added, “my bias is that you give patients an anti-TNF therapy first, and if they are not responding by 12 weeks, you move on to another class of drugs, perhaps even the jakinibs.”
Herpes zoster risk
Dr. Strand and Dr. Weinblatt addressed potential adverse events associated with both classes of agents. For the JAK inhibitors, concerns include herpes zoster infections, increased VTE and ATE incidence, and largely unknown risks during pregnancy and lactation. For the anti-TNF agents, safety concerns include reactivation of tuberculosis, fungal infections, demyelinating syndrome, and skin cancer.
With the shortest half-life of any therapeutic class in rheumatology, adverse events with JAK inhibitors often can resolve quickly, Dr. Strand said.
The increased risk of herpes zoster is important, she added, “but we have a recombinant vaccination that works. It’s quite effective.”
Dr. Weinblatt pointed out that all the JAK inhibitors carry this increase herpes zoster risk, which is related to their mechanism of action. There is a catch with the vaccine, however, he added. The vaccine is approved for treatment of patients 50 years and older. For younger people with RA starting a JAK inhibitor, the cost is out-of-pocket.
Evaluating risk of emboli
The incidence of VTE is about two times higher among people with RA, compared with the general population, Dr. Strand said, with research suggesting the majority of risk resides among people with a previous event. However, she added, an emerging profile of thromboembolic events associated with JAK inhibitors is “considered a class effect by the FDA.”
One exception in the JAK inhibitor class could be tofacitinib (Xeljanz), which might carry less risk because “most of the data with tofacitinib are quite good,” Dr. Weinblatt said. One study presented at ACR 2018, for example, showed a similar VTE rate between tofacitinib and TNF inhibitors.
Nevertheless, the FDA issued a boxed warning in July 2019 about elevated risks of blood clots and death at a higher dose of tofacitinib. The concerns stem from an open-label, endpoint-driven study mandated by the FDA to explore major adverse cardiac events. “There was a clinically important and statistically significant occurrence of pulmonary embolism and VTE, and an increase in mortality in the 10-mg dose group as opposed to the anti-TNF therapies,” Dr. Weinblatt said. As a result, the FDA requested patients on the 10-mg twice-daily dose be transitioned to the 5-mg twice-daily dose.
The package labeling for the JAK inhibitors baricitinib (Olumiant) and upadacitinib (Rinvoq) feature warnings about increased risk for thromboembolic events. Furthermore, the labeling for filgotinib, a JAK inhibitor in development that received a complete response letter from the FDA in August 2020, is expected to carry the same warning.An unanswered question remains on why this class of agents potentially increases risk of thromboembolism. “We’re all uncomfortable because there is no known mechanism of JAK inhibition that should lead to this,” he added. Another unresolved issue is whether or not patients prescribed a JAK inhibitor should also be prescribed an anticoagulant.
Anti-TNF adverse events
Infections, primarily reactivation of tuberculosis and an increased risk for fungal disease, are concerns with the anti-TNF agents. However, the risk is not restricted to this class. “Greater risk of infection is seen with all our immune-modulating therapies,” Dr. Weinblatt said.
Rare adverse events include demyelinating syndromes, hematologic toxicity, and a worsening of heart failure in some cases.
“Despite a concern about malignancy, the only defined cancer reported over 22 years of use was skin cancer,” he said. “It took more than a decade of ongoing registry data for skin cancer to be identified. It was not noted in randomized, placebo-controlled trials.”
Potential pregnancy concerns
When it comes to risk during reproduction, “there is a clear difference,” Dr. Weinblatt said. “We know anti-TNF therapy can be used safely in pregnant women. We know they can conceive on them and maintain them during pregnancy. They can also breastfeed on them.”
“Frankly, I’m not so ‘gung ho’ on TNF inhibitor safety in pregnancy and lactation with exception of certolizumab, which doesn’t result in high levels of antibody in the placenta or the mother’s milk,” Dr. Strand said.
The 2020 ACR Guideline for the Management of Reproductive Health in Rheumatic and Musculoskeletal Diseases states that all anti-TNF therapies can be used during pregnancy and lactation, Dr. Weinblatt said. “Although I agree certolizumab has the best safety profile, all of them can be used.”
“The same is not true with the jakinibs,” he added, pointing to warnings that women of reproductive age should use contraception while on JAK inhibitors and for 4 weeks after stopping treatment.
However, Dr. Strand defended the pregnancy risk with JAK inhibitors. She cited two publications, including a 2016 study where researchers evaluated the safety of tofacitinib during pregnancy in women with rheumatoid arthritis and psoriasis. “There was only one possible deformity, a pulmonary stenosis,” Dr. Strand said. “Essentially, the majority of patients delivered healthy babies. There was very little difference from what we know occurs in RA otherwise.”
A 2018 study assessed pregnancy outcomes with tofacitinib among people with ulcerative colitis, “again showing the majority of patients had normal deliveries.”
“There just aren’t enough data,” Dr. Weinblatt said. “Perhaps in 5 years, we will reach same conclusion with the jakinibs.”
Differences in cost?
“We have not benefited yet from the biosimilar costs. But in Europe, the cost of an adalimumab or etanercept biosimilar is about $5,000, versus about $50,000 to $60,000 in the U.S. for the JAK inhibitors,” Dr. Weinblatt said. “So there are major cost savings with biosimilars.”
“I can’t understand cost at all for our drugs,” he continued. “They’re not rational, and the price increases are clearly not rational. Potentially, a small molecule is going to be a lot easier to produce than a biologic, so you could argue that generic jakinibs ought to be less. But in the United States we have a distorted pricing model.”
“Until that changes, I don’t think we can predict [future costs]. One could predict that generics and biosimilars will be less than the orginators,” Dr. Weinblatt said.
“It is really criminal we don’t have biosimilars for most of our TNFs, but that is the way it is,” Dr. Strand said.
Summary statements
“The JAK-inhibitor class is an exciting development for rheumatology and a broad variety of autoimmune diseases,” Dr. Strand said.
“In rheumatoid arthritis, they should be used early,” she added. “Based on phase 3 trials, responses are better in progressively earlier disease with less treatment-experienced patients.”
She pointed out that many patients like the convenience of the oral JAK inhibitors.
Dr. Weinblatt stated the 22 years of clinical experience with the anti-TNF class versus about 8 years with jakinibs favors the biologics. “Virtually every approved drug has been tested versus methotrexate, in early studies, long-term studies, and most importantly, in reduction and withdrawal studies, which are not available with the JAK inhibitors.”
Anti-TNFs have impressive effects on clinical disease activity, functional outcomes, and radiographic progression, Dr. Weinblatt said. They work in early and longstanding disease among patients who are disease-modifying antirheumatic drug naive and after multiple DMARD failures, he added.
Adding up the vote
The question was: Should JAK inhibitors be used before TNF inhibitors? The results showed 69%-31% in favor of anti-TNF agents.
“So the majority are more comfortable using TNFs,” said debate moderator Elizabeth Wahl, MD, of the department of rheumatology at VA Puget Sound Healthcare System and the University of Washington in Seattle. Regarding a switch to JAK inhibitors, she interpreted the poll numbers to mean, “we are not there yet, it takes years and years of safety data.”
Both Dr. Strand and Dr. Weinblatt disclosed numerous financial relationships with pharmaceutical companies that market RA drugs.
What is the next step in treatment after a person with rheumatoid arthritis fails to adequately respond to methotrexate – a Janus kinase (JAK) inhibitor or a biologic? That was the focus of a lively debate at the virtual annual meeting of the American College of Rheumatology.

Vibeke Strand, MD, argued that JAK inhibitors offer the distinct advantage of a faster clinical response than biologics, meaning that decisions to change therapy based on nonresponse or adverse effects can be made earlier in a treatment plan.
Michael Weinblatt, MD, countered that the faster-response advantage is offset by potential adverse events associated with the JAK inhibitors, including increased risk of herpes zoster infection, venous thromboembolism (VTE), and arterial thromboembolism (ATE). He suggested switching patients to a biologic instead.
In addition, the debate was held just days before the ACR released a proposed guideline for the management of RA. This update to the 2015 guidance is the first to prioritize the order of RA treatments, emphasizing that clinicians should maximize methotrexate therapy before switching RA patients to a JAK inhibitor or a biologic. Release of the full guidelines is pending, and it remains unclear if the ACR provides any guidance regarding the “jakinib” versus biologic decision.
Interestingly, the debate did not hinge on any differences in efficacy. Both speakers pointed to similar efficacy between anti–tumor necrosis factor (TNF) agents and JAK inhibitors, and despite working on different pathways, among the individual JAK inhibitors as well.
Is timing of the essence?
Knowing whether a person with RA responds to a JAK inhibitor more quickly than to a biologic is a major advantage, said Dr. Strand, of the division of immunology and rheumatology at Stanford (Calif.) University. “The argument that I am making is that patients are more responsive if treated earlier in the disease process and they are less treatment-experienced.”
Dr. Strand said the advantages extend to remission as well. “When patients are aware of early improvements, their adherence is increased. Remission is more likely because it occurs earlier.”
“I will certainly grant it to Vibeke that jakinibs work much faster,” said Dr. Weinblatt, chair of rheumatology at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School in Boston. However, he added, “my bias is that you give patients an anti-TNF therapy first, and if they are not responding by 12 weeks, you move on to another class of drugs, perhaps even the jakinibs.”
Herpes zoster risk
Dr. Strand and Dr. Weinblatt addressed potential adverse events associated with both classes of agents. For the JAK inhibitors, concerns include herpes zoster infections, increased VTE and ATE incidence, and largely unknown risks during pregnancy and lactation. For the anti-TNF agents, safety concerns include reactivation of tuberculosis, fungal infections, demyelinating syndrome, and skin cancer.
With the shortest half-life of any therapeutic class in rheumatology, adverse events with JAK inhibitors often can resolve quickly, Dr. Strand said.
The increased risk of herpes zoster is important, she added, “but we have a recombinant vaccination that works. It’s quite effective.”
Dr. Weinblatt pointed out that all the JAK inhibitors carry this increase herpes zoster risk, which is related to their mechanism of action. There is a catch with the vaccine, however, he added. The vaccine is approved for treatment of patients 50 years and older. For younger people with RA starting a JAK inhibitor, the cost is out-of-pocket.
Evaluating risk of emboli
The incidence of VTE is about two times higher among people with RA, compared with the general population, Dr. Strand said, with research suggesting the majority of risk resides among people with a previous event. However, she added, an emerging profile of thromboembolic events associated with JAK inhibitors is “considered a class effect by the FDA.”
One exception in the JAK inhibitor class could be tofacitinib (Xeljanz), which might carry less risk because “most of the data with tofacitinib are quite good,” Dr. Weinblatt said. One study presented at ACR 2018, for example, showed a similar VTE rate between tofacitinib and TNF inhibitors.
Nevertheless, the FDA issued a boxed warning in July 2019 about elevated risks of blood clots and death at a higher dose of tofacitinib. The concerns stem from an open-label, endpoint-driven study mandated by the FDA to explore major adverse cardiac events. “There was a clinically important and statistically significant occurrence of pulmonary embolism and VTE, and an increase in mortality in the 10-mg dose group as opposed to the anti-TNF therapies,” Dr. Weinblatt said. As a result, the FDA requested patients on the 10-mg twice-daily dose be transitioned to the 5-mg twice-daily dose.
The package labeling for the JAK inhibitors baricitinib (Olumiant) and upadacitinib (Rinvoq) feature warnings about increased risk for thromboembolic events. Furthermore, the labeling for filgotinib, a JAK inhibitor in development that received a complete response letter from the FDA in August 2020, is expected to carry the same warning.An unanswered question remains on why this class of agents potentially increases risk of thromboembolism. “We’re all uncomfortable because there is no known mechanism of JAK inhibition that should lead to this,” he added. Another unresolved issue is whether or not patients prescribed a JAK inhibitor should also be prescribed an anticoagulant.
Anti-TNF adverse events
Infections, primarily reactivation of tuberculosis and an increased risk for fungal disease, are concerns with the anti-TNF agents. However, the risk is not restricted to this class. “Greater risk of infection is seen with all our immune-modulating therapies,” Dr. Weinblatt said.
Rare adverse events include demyelinating syndromes, hematologic toxicity, and a worsening of heart failure in some cases.
“Despite a concern about malignancy, the only defined cancer reported over 22 years of use was skin cancer,” he said. “It took more than a decade of ongoing registry data for skin cancer to be identified. It was not noted in randomized, placebo-controlled trials.”
Potential pregnancy concerns
When it comes to risk during reproduction, “there is a clear difference,” Dr. Weinblatt said. “We know anti-TNF therapy can be used safely in pregnant women. We know they can conceive on them and maintain them during pregnancy. They can also breastfeed on them.”
“Frankly, I’m not so ‘gung ho’ on TNF inhibitor safety in pregnancy and lactation with exception of certolizumab, which doesn’t result in high levels of antibody in the placenta or the mother’s milk,” Dr. Strand said.
The 2020 ACR Guideline for the Management of Reproductive Health in Rheumatic and Musculoskeletal Diseases states that all anti-TNF therapies can be used during pregnancy and lactation, Dr. Weinblatt said. “Although I agree certolizumab has the best safety profile, all of them can be used.”
“The same is not true with the jakinibs,” he added, pointing to warnings that women of reproductive age should use contraception while on JAK inhibitors and for 4 weeks after stopping treatment.
However, Dr. Strand defended the pregnancy risk with JAK inhibitors. She cited two publications, including a 2016 study where researchers evaluated the safety of tofacitinib during pregnancy in women with rheumatoid arthritis and psoriasis. “There was only one possible deformity, a pulmonary stenosis,” Dr. Strand said. “Essentially, the majority of patients delivered healthy babies. There was very little difference from what we know occurs in RA otherwise.”
A 2018 study assessed pregnancy outcomes with tofacitinib among people with ulcerative colitis, “again showing the majority of patients had normal deliveries.”
“There just aren’t enough data,” Dr. Weinblatt said. “Perhaps in 5 years, we will reach same conclusion with the jakinibs.”
Differences in cost?
“We have not benefited yet from the biosimilar costs. But in Europe, the cost of an adalimumab or etanercept biosimilar is about $5,000, versus about $50,000 to $60,000 in the U.S. for the JAK inhibitors,” Dr. Weinblatt said. “So there are major cost savings with biosimilars.”
“I can’t understand cost at all for our drugs,” he continued. “They’re not rational, and the price increases are clearly not rational. Potentially, a small molecule is going to be a lot easier to produce than a biologic, so you could argue that generic jakinibs ought to be less. But in the United States we have a distorted pricing model.”
“Until that changes, I don’t think we can predict [future costs]. One could predict that generics and biosimilars will be less than the orginators,” Dr. Weinblatt said.
“It is really criminal we don’t have biosimilars for most of our TNFs, but that is the way it is,” Dr. Strand said.
Summary statements
“The JAK-inhibitor class is an exciting development for rheumatology and a broad variety of autoimmune diseases,” Dr. Strand said.
“In rheumatoid arthritis, they should be used early,” she added. “Based on phase 3 trials, responses are better in progressively earlier disease with less treatment-experienced patients.”
She pointed out that many patients like the convenience of the oral JAK inhibitors.
Dr. Weinblatt stated the 22 years of clinical experience with the anti-TNF class versus about 8 years with jakinibs favors the biologics. “Virtually every approved drug has been tested versus methotrexate, in early studies, long-term studies, and most importantly, in reduction and withdrawal studies, which are not available with the JAK inhibitors.”
Anti-TNFs have impressive effects on clinical disease activity, functional outcomes, and radiographic progression, Dr. Weinblatt said. They work in early and longstanding disease among patients who are disease-modifying antirheumatic drug naive and after multiple DMARD failures, he added.
Adding up the vote
The question was: Should JAK inhibitors be used before TNF inhibitors? The results showed 69%-31% in favor of anti-TNF agents.
“So the majority are more comfortable using TNFs,” said debate moderator Elizabeth Wahl, MD, of the department of rheumatology at VA Puget Sound Healthcare System and the University of Washington in Seattle. Regarding a switch to JAK inhibitors, she interpreted the poll numbers to mean, “we are not there yet, it takes years and years of safety data.”
Both Dr. Strand and Dr. Weinblatt disclosed numerous financial relationships with pharmaceutical companies that market RA drugs.
What is the next step in treatment after a person with rheumatoid arthritis fails to adequately respond to methotrexate – a Janus kinase (JAK) inhibitor or a biologic? That was the focus of a lively debate at the virtual annual meeting of the American College of Rheumatology.

Vibeke Strand, MD, argued that JAK inhibitors offer the distinct advantage of a faster clinical response than biologics, meaning that decisions to change therapy based on nonresponse or adverse effects can be made earlier in a treatment plan.
Michael Weinblatt, MD, countered that the faster-response advantage is offset by potential adverse events associated with the JAK inhibitors, including increased risk of herpes zoster infection, venous thromboembolism (VTE), and arterial thromboembolism (ATE). He suggested switching patients to a biologic instead.
In addition, the debate was held just days before the ACR released a proposed guideline for the management of RA. This update to the 2015 guidance is the first to prioritize the order of RA treatments, emphasizing that clinicians should maximize methotrexate therapy before switching RA patients to a JAK inhibitor or a biologic. Release of the full guidelines is pending, and it remains unclear if the ACR provides any guidance regarding the “jakinib” versus biologic decision.
Interestingly, the debate did not hinge on any differences in efficacy. Both speakers pointed to similar efficacy between anti–tumor necrosis factor (TNF) agents and JAK inhibitors, and despite working on different pathways, among the individual JAK inhibitors as well.
Is timing of the essence?
Knowing whether a person with RA responds to a JAK inhibitor more quickly than to a biologic is a major advantage, said Dr. Strand, of the division of immunology and rheumatology at Stanford (Calif.) University. “The argument that I am making is that patients are more responsive if treated earlier in the disease process and they are less treatment-experienced.”
Dr. Strand said the advantages extend to remission as well. “When patients are aware of early improvements, their adherence is increased. Remission is more likely because it occurs earlier.”
“I will certainly grant it to Vibeke that jakinibs work much faster,” said Dr. Weinblatt, chair of rheumatology at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School in Boston. However, he added, “my bias is that you give patients an anti-TNF therapy first, and if they are not responding by 12 weeks, you move on to another class of drugs, perhaps even the jakinibs.”
Herpes zoster risk
Dr. Strand and Dr. Weinblatt addressed potential adverse events associated with both classes of agents. For the JAK inhibitors, concerns include herpes zoster infections, increased VTE and ATE incidence, and largely unknown risks during pregnancy and lactation. For the anti-TNF agents, safety concerns include reactivation of tuberculosis, fungal infections, demyelinating syndrome, and skin cancer.
With the shortest half-life of any therapeutic class in rheumatology, adverse events with JAK inhibitors often can resolve quickly, Dr. Strand said.
The increased risk of herpes zoster is important, she added, “but we have a recombinant vaccination that works. It’s quite effective.”
Dr. Weinblatt pointed out that all the JAK inhibitors carry this increase herpes zoster risk, which is related to their mechanism of action. There is a catch with the vaccine, however, he added. The vaccine is approved for treatment of patients 50 years and older. For younger people with RA starting a JAK inhibitor, the cost is out-of-pocket.
Evaluating risk of emboli
The incidence of VTE is about two times higher among people with RA, compared with the general population, Dr. Strand said, with research suggesting the majority of risk resides among people with a previous event. However, she added, an emerging profile of thromboembolic events associated with JAK inhibitors is “considered a class effect by the FDA.”
One exception in the JAK inhibitor class could be tofacitinib (Xeljanz), which might carry less risk because “most of the data with tofacitinib are quite good,” Dr. Weinblatt said. One study presented at ACR 2018, for example, showed a similar VTE rate between tofacitinib and TNF inhibitors.
Nevertheless, the FDA issued a boxed warning in July 2019 about elevated risks of blood clots and death at a higher dose of tofacitinib. The concerns stem from an open-label, endpoint-driven study mandated by the FDA to explore major adverse cardiac events. “There was a clinically important and statistically significant occurrence of pulmonary embolism and VTE, and an increase in mortality in the 10-mg dose group as opposed to the anti-TNF therapies,” Dr. Weinblatt said. As a result, the FDA requested patients on the 10-mg twice-daily dose be transitioned to the 5-mg twice-daily dose.
The package labeling for the JAK inhibitors baricitinib (Olumiant) and upadacitinib (Rinvoq) feature warnings about increased risk for thromboembolic events. Furthermore, the labeling for filgotinib, a JAK inhibitor in development that received a complete response letter from the FDA in August 2020, is expected to carry the same warning.An unanswered question remains on why this class of agents potentially increases risk of thromboembolism. “We’re all uncomfortable because there is no known mechanism of JAK inhibition that should lead to this,” he added. Another unresolved issue is whether or not patients prescribed a JAK inhibitor should also be prescribed an anticoagulant.
Anti-TNF adverse events
Infections, primarily reactivation of tuberculosis and an increased risk for fungal disease, are concerns with the anti-TNF agents. However, the risk is not restricted to this class. “Greater risk of infection is seen with all our immune-modulating therapies,” Dr. Weinblatt said.
Rare adverse events include demyelinating syndromes, hematologic toxicity, and a worsening of heart failure in some cases.
“Despite a concern about malignancy, the only defined cancer reported over 22 years of use was skin cancer,” he said. “It took more than a decade of ongoing registry data for skin cancer to be identified. It was not noted in randomized, placebo-controlled trials.”
Potential pregnancy concerns
When it comes to risk during reproduction, “there is a clear difference,” Dr. Weinblatt said. “We know anti-TNF therapy can be used safely in pregnant women. We know they can conceive on them and maintain them during pregnancy. They can also breastfeed on them.”
“Frankly, I’m not so ‘gung ho’ on TNF inhibitor safety in pregnancy and lactation with exception of certolizumab, which doesn’t result in high levels of antibody in the placenta or the mother’s milk,” Dr. Strand said.
The 2020 ACR Guideline for the Management of Reproductive Health in Rheumatic and Musculoskeletal Diseases states that all anti-TNF therapies can be used during pregnancy and lactation, Dr. Weinblatt said. “Although I agree certolizumab has the best safety profile, all of them can be used.”
“The same is not true with the jakinibs,” he added, pointing to warnings that women of reproductive age should use contraception while on JAK inhibitors and for 4 weeks after stopping treatment.
However, Dr. Strand defended the pregnancy risk with JAK inhibitors. She cited two publications, including a 2016 study where researchers evaluated the safety of tofacitinib during pregnancy in women with rheumatoid arthritis and psoriasis. “There was only one possible deformity, a pulmonary stenosis,” Dr. Strand said. “Essentially, the majority of patients delivered healthy babies. There was very little difference from what we know occurs in RA otherwise.”
A 2018 study assessed pregnancy outcomes with tofacitinib among people with ulcerative colitis, “again showing the majority of patients had normal deliveries.”
“There just aren’t enough data,” Dr. Weinblatt said. “Perhaps in 5 years, we will reach same conclusion with the jakinibs.”
Differences in cost?
“We have not benefited yet from the biosimilar costs. But in Europe, the cost of an adalimumab or etanercept biosimilar is about $5,000, versus about $50,000 to $60,000 in the U.S. for the JAK inhibitors,” Dr. Weinblatt said. “So there are major cost savings with biosimilars.”
“I can’t understand cost at all for our drugs,” he continued. “They’re not rational, and the price increases are clearly not rational. Potentially, a small molecule is going to be a lot easier to produce than a biologic, so you could argue that generic jakinibs ought to be less. But in the United States we have a distorted pricing model.”
“Until that changes, I don’t think we can predict [future costs]. One could predict that generics and biosimilars will be less than the orginators,” Dr. Weinblatt said.
“It is really criminal we don’t have biosimilars for most of our TNFs, but that is the way it is,” Dr. Strand said.
Summary statements
“The JAK-inhibitor class is an exciting development for rheumatology and a broad variety of autoimmune diseases,” Dr. Strand said.
“In rheumatoid arthritis, they should be used early,” she added. “Based on phase 3 trials, responses are better in progressively earlier disease with less treatment-experienced patients.”
She pointed out that many patients like the convenience of the oral JAK inhibitors.
Dr. Weinblatt stated the 22 years of clinical experience with the anti-TNF class versus about 8 years with jakinibs favors the biologics. “Virtually every approved drug has been tested versus methotrexate, in early studies, long-term studies, and most importantly, in reduction and withdrawal studies, which are not available with the JAK inhibitors.”
Anti-TNFs have impressive effects on clinical disease activity, functional outcomes, and radiographic progression, Dr. Weinblatt said. They work in early and longstanding disease among patients who are disease-modifying antirheumatic drug naive and after multiple DMARD failures, he added.
Adding up the vote
The question was: Should JAK inhibitors be used before TNF inhibitors? The results showed 69%-31% in favor of anti-TNF agents.
“So the majority are more comfortable using TNFs,” said debate moderator Elizabeth Wahl, MD, of the department of rheumatology at VA Puget Sound Healthcare System and the University of Washington in Seattle. Regarding a switch to JAK inhibitors, she interpreted the poll numbers to mean, “we are not there yet, it takes years and years of safety data.”
Both Dr. Strand and Dr. Weinblatt disclosed numerous financial relationships with pharmaceutical companies that market RA drugs.
FROM ACR 2020
Pfizer’s COVID-19 vaccine 95% effective in final phase 3 results
After initial promising interim results on Nov. 9, Pfizer and BioNTech today announced that their mRNA vaccine, in development to prevent COVID-19, is 95% effective.
Final analysis of the randomized, phase 3 study of more than 43,000 people yielded 170 confirmed cases of COVID-19 – with 162 positive cases in the placebo group versus 8 in the BNT162b2 vaccine group.
Researchers reported 10 severe cases of COVID-19 in the trial, 9 of which occurred in the placebo group.
The study was ethnically diverse, and results were consistent across gender and age groups, with a 94% efficacy reported among participants aged older than 65 years.
Pfizer plans to file for an emergency-use authorization with the Food and Drug Administration “within days,” having now met all the FDA data endpoints, according to a news release from the two companies.
The vaccine was well tolerated with no serious safety concerns, the company stated. Two grade 3 adverse events were reported – fatigue in 3.8% of participants and headache in 2%.
The 95% efficacy places the Pfizer vaccine in the same neighborhood as the interim results of the Moderna vaccine, reported at 94.5%. Both products are two-dose mRNA vaccines.
As of Nov. 13, of 43,661 total participants in the Pfizer vaccine phase 3 trial, 41,135 received a second dose. The final results are based on two outcomes measured 7 days after the second dose: vaccine efficacy in people without prior SARS-CoV-2 infection as well as a secondary outcome in people both with and without prior SARS-CoV-2 infection.
The 95% vaccine efficacy was statistically significant, compared with placebo (P < .0001).
‘Historic 8-month journey’
The BNT162b2 vaccine candidate is a joint effort between Pfizer and BioNTech. “The study results mark an important step in this historic 8-month journey to bring forward a vaccine capable of helping to end this devastating pandemic,” Albert Bourla, DVM, PhD, Pfizer chairman and CEO, said in a statement. “With hundreds of thousands of people around the globe infected every day, we urgently need to get a safe and effective vaccine to the world.”
Ugur Sahin, MD, PhD, cofounder and CEO of BioNTech, added, “we are grateful that the first global trial to reach the final efficacy analysis mark indicates that a high rate of protection against COVID-19 can be achieved very fast after the first 30-mcg dose, underscoring the power of BNT162 in providing early protection.”
The two companies expect to produce up to 50 million vaccine doses in 2020 for global distribution. Projections for 2021 include up to 1.3 billion doses.
The companies also designed temperature-controlled thermal shipping containers with dry ice to maintain the required, approximate –70° C (–94° F) conditions. Clinicians can use the containers as temporary storage units for up to 15 days by replacing the dry ice.
This article first appeared on Medscape.com.
After initial promising interim results on Nov. 9, Pfizer and BioNTech today announced that their mRNA vaccine, in development to prevent COVID-19, is 95% effective.
Final analysis of the randomized, phase 3 study of more than 43,000 people yielded 170 confirmed cases of COVID-19 – with 162 positive cases in the placebo group versus 8 in the BNT162b2 vaccine group.
Researchers reported 10 severe cases of COVID-19 in the trial, 9 of which occurred in the placebo group.
The study was ethnically diverse, and results were consistent across gender and age groups, with a 94% efficacy reported among participants aged older than 65 years.
Pfizer plans to file for an emergency-use authorization with the Food and Drug Administration “within days,” having now met all the FDA data endpoints, according to a news release from the two companies.
The vaccine was well tolerated with no serious safety concerns, the company stated. Two grade 3 adverse events were reported – fatigue in 3.8% of participants and headache in 2%.
The 95% efficacy places the Pfizer vaccine in the same neighborhood as the interim results of the Moderna vaccine, reported at 94.5%. Both products are two-dose mRNA vaccines.
As of Nov. 13, of 43,661 total participants in the Pfizer vaccine phase 3 trial, 41,135 received a second dose. The final results are based on two outcomes measured 7 days after the second dose: vaccine efficacy in people without prior SARS-CoV-2 infection as well as a secondary outcome in people both with and without prior SARS-CoV-2 infection.
The 95% vaccine efficacy was statistically significant, compared with placebo (P < .0001).
‘Historic 8-month journey’
The BNT162b2 vaccine candidate is a joint effort between Pfizer and BioNTech. “The study results mark an important step in this historic 8-month journey to bring forward a vaccine capable of helping to end this devastating pandemic,” Albert Bourla, DVM, PhD, Pfizer chairman and CEO, said in a statement. “With hundreds of thousands of people around the globe infected every day, we urgently need to get a safe and effective vaccine to the world.”
Ugur Sahin, MD, PhD, cofounder and CEO of BioNTech, added, “we are grateful that the first global trial to reach the final efficacy analysis mark indicates that a high rate of protection against COVID-19 can be achieved very fast after the first 30-mcg dose, underscoring the power of BNT162 in providing early protection.”
The two companies expect to produce up to 50 million vaccine doses in 2020 for global distribution. Projections for 2021 include up to 1.3 billion doses.
The companies also designed temperature-controlled thermal shipping containers with dry ice to maintain the required, approximate –70° C (–94° F) conditions. Clinicians can use the containers as temporary storage units for up to 15 days by replacing the dry ice.
This article first appeared on Medscape.com.
After initial promising interim results on Nov. 9, Pfizer and BioNTech today announced that their mRNA vaccine, in development to prevent COVID-19, is 95% effective.
Final analysis of the randomized, phase 3 study of more than 43,000 people yielded 170 confirmed cases of COVID-19 – with 162 positive cases in the placebo group versus 8 in the BNT162b2 vaccine group.
Researchers reported 10 severe cases of COVID-19 in the trial, 9 of which occurred in the placebo group.
The study was ethnically diverse, and results were consistent across gender and age groups, with a 94% efficacy reported among participants aged older than 65 years.
Pfizer plans to file for an emergency-use authorization with the Food and Drug Administration “within days,” having now met all the FDA data endpoints, according to a news release from the two companies.
The vaccine was well tolerated with no serious safety concerns, the company stated. Two grade 3 adverse events were reported – fatigue in 3.8% of participants and headache in 2%.
The 95% efficacy places the Pfizer vaccine in the same neighborhood as the interim results of the Moderna vaccine, reported at 94.5%. Both products are two-dose mRNA vaccines.
As of Nov. 13, of 43,661 total participants in the Pfizer vaccine phase 3 trial, 41,135 received a second dose. The final results are based on two outcomes measured 7 days after the second dose: vaccine efficacy in people without prior SARS-CoV-2 infection as well as a secondary outcome in people both with and without prior SARS-CoV-2 infection.
The 95% vaccine efficacy was statistically significant, compared with placebo (P < .0001).
‘Historic 8-month journey’
The BNT162b2 vaccine candidate is a joint effort between Pfizer and BioNTech. “The study results mark an important step in this historic 8-month journey to bring forward a vaccine capable of helping to end this devastating pandemic,” Albert Bourla, DVM, PhD, Pfizer chairman and CEO, said in a statement. “With hundreds of thousands of people around the globe infected every day, we urgently need to get a safe and effective vaccine to the world.”
Ugur Sahin, MD, PhD, cofounder and CEO of BioNTech, added, “we are grateful that the first global trial to reach the final efficacy analysis mark indicates that a high rate of protection against COVID-19 can be achieved very fast after the first 30-mcg dose, underscoring the power of BNT162 in providing early protection.”
The two companies expect to produce up to 50 million vaccine doses in 2020 for global distribution. Projections for 2021 include up to 1.3 billion doses.
The companies also designed temperature-controlled thermal shipping containers with dry ice to maintain the required, approximate –70° C (–94° F) conditions. Clinicians can use the containers as temporary storage units for up to 15 days by replacing the dry ice.
This article first appeared on Medscape.com.
Moderna: Interim data show 94.5% efficacy for COVID-19 vaccine, will seek FDA EUA
The Moderna mRNA-1273 vaccine, in development to prevent COVID-19, yielded 94.5% efficacy in early results and is generally well tolerated, the company announced early Monday. The product can be stored at refrigeration temperatures common to many physician offices, pharmacies, and hospitals.
The first interim results of the phase 3 COVE trial included 95 participants with confirmed COVID-19. An independent data safety monitoring board, which was appointed by the National Institutes of Health, informed Moderna that 90 of the patients who were positive for COVID-19 were in a placebo group and that 5 patients were in the mRNA-1273 vaccine group, resulting in a vaccine efficacy of 94.5% (P < .0001).
Interim data included 11 patients with severe COVID-19, all of whom were in the placebo group.
“This positive interim analysis from our phase 3 study has given us the first clinical validation that our vaccine can prevent COVID-19 disease, including severe disease,” said Stéphane Bancel, CEO of Moderna, said in a statement.
The vaccine met its primary study endpoint, which was based on adjudicated data that were collected starting 2 weeks after the second dose of mRNA-1273. The interim study population included people who could be at higher risk for COVID-19, including 15 adults aged 65 years and older and 20 participants from diverse communities.
Safety data
The DSMB also reviewed safety data for the COVE study interim results. The vaccine was generally safe and well tolerated, as determined on the basis of solicited adverse events. Most adverse events were mild to moderate and were generally short-lived, according to a company news release.
Injection-site pain was reported in 2.7% of participants after the first dose. After the second dose, 9.7% of participants reported fatigue, 8.9% reported myalgia, 5.2% reported arthralgia, 4.5% reported headache, 4.1% reported pain, and 2.0% reported erythema or redness at the injection site.
Moderna plans to request emergency-use authorization (EUA) from the Food and Drug Administration in the coming weeks. The company expects that the EUA will be based on more data from the COVE study, including a final analysis of 151 patients with a median follow-up of more than 2 months. Moderna also plans to seek authorizations from global regulatory agencies.
The company expects to have approximately 20 million doses of mRNA-1273 ready to ship in the United States by the end of the year. In addition, the company says it remains on track to manufacture between 500 million and 1 billion doses globally in 2021.
Moderna is developing distribution plans in conjunction with the Centers for Disease Control and Prevention, the federal government’s Operation Warp Speed, and McKesson, a COVID-19 vaccine distributor contracted by the U.S. government.
Refrigeration requirements
The mRNA-1273 vaccine can be shipped and stored for up to 6 months at –20° C (about –4° F), a temperature maintained in most home or medical freezers, according to Moderna. The company expects that, after the product thaws, it will remain stable at standard refrigerator temperatures of 2°-8° C (36°-46° F) for up to 30 days within the 6-month shelf life.
Because the mRNA-1273 vaccine is stable at these refrigerator temperatures, it can be stored at most physicians’ offices, pharmacies, and hospitals, the company noted. In contrast, the similar Pfizer BTN162b2 vaccine – early results for which showed a 90% efficacy rate – requires shipment and storage at “deep-freeze” conditions of –70° C or –80° C, which is more challenging from a logistic point of view.
Moderna’s mRNA-1273 can be kept at room temperature for up to 12 hours after removal from a refrigerator for patient administration. The vaccine will not require dilution prior to use.
More than 30,000 people aged older than 18 years in the United States are enrolled in the COVE study. The research is being conducted in collaboration with the National Institute of Allergy and Infectious Diseases and the Biomedical Advanced Research and Development Authority, part of the Office of the Assistant Secretary for Preparedness and Response at the Department of Health & Human Services.
A version of this article originally appeared on Medscape.com.
The Moderna mRNA-1273 vaccine, in development to prevent COVID-19, yielded 94.5% efficacy in early results and is generally well tolerated, the company announced early Monday. The product can be stored at refrigeration temperatures common to many physician offices, pharmacies, and hospitals.
The first interim results of the phase 3 COVE trial included 95 participants with confirmed COVID-19. An independent data safety monitoring board, which was appointed by the National Institutes of Health, informed Moderna that 90 of the patients who were positive for COVID-19 were in a placebo group and that 5 patients were in the mRNA-1273 vaccine group, resulting in a vaccine efficacy of 94.5% (P < .0001).
Interim data included 11 patients with severe COVID-19, all of whom were in the placebo group.
“This positive interim analysis from our phase 3 study has given us the first clinical validation that our vaccine can prevent COVID-19 disease, including severe disease,” said Stéphane Bancel, CEO of Moderna, said in a statement.
The vaccine met its primary study endpoint, which was based on adjudicated data that were collected starting 2 weeks after the second dose of mRNA-1273. The interim study population included people who could be at higher risk for COVID-19, including 15 adults aged 65 years and older and 20 participants from diverse communities.
Safety data
The DSMB also reviewed safety data for the COVE study interim results. The vaccine was generally safe and well tolerated, as determined on the basis of solicited adverse events. Most adverse events were mild to moderate and were generally short-lived, according to a company news release.
Injection-site pain was reported in 2.7% of participants after the first dose. After the second dose, 9.7% of participants reported fatigue, 8.9% reported myalgia, 5.2% reported arthralgia, 4.5% reported headache, 4.1% reported pain, and 2.0% reported erythema or redness at the injection site.
Moderna plans to request emergency-use authorization (EUA) from the Food and Drug Administration in the coming weeks. The company expects that the EUA will be based on more data from the COVE study, including a final analysis of 151 patients with a median follow-up of more than 2 months. Moderna also plans to seek authorizations from global regulatory agencies.
The company expects to have approximately 20 million doses of mRNA-1273 ready to ship in the United States by the end of the year. In addition, the company says it remains on track to manufacture between 500 million and 1 billion doses globally in 2021.
Moderna is developing distribution plans in conjunction with the Centers for Disease Control and Prevention, the federal government’s Operation Warp Speed, and McKesson, a COVID-19 vaccine distributor contracted by the U.S. government.
Refrigeration requirements
The mRNA-1273 vaccine can be shipped and stored for up to 6 months at –20° C (about –4° F), a temperature maintained in most home or medical freezers, according to Moderna. The company expects that, after the product thaws, it will remain stable at standard refrigerator temperatures of 2°-8° C (36°-46° F) for up to 30 days within the 6-month shelf life.
Because the mRNA-1273 vaccine is stable at these refrigerator temperatures, it can be stored at most physicians’ offices, pharmacies, and hospitals, the company noted. In contrast, the similar Pfizer BTN162b2 vaccine – early results for which showed a 90% efficacy rate – requires shipment and storage at “deep-freeze” conditions of –70° C or –80° C, which is more challenging from a logistic point of view.
Moderna’s mRNA-1273 can be kept at room temperature for up to 12 hours after removal from a refrigerator for patient administration. The vaccine will not require dilution prior to use.
More than 30,000 people aged older than 18 years in the United States are enrolled in the COVE study. The research is being conducted in collaboration with the National Institute of Allergy and Infectious Diseases and the Biomedical Advanced Research and Development Authority, part of the Office of the Assistant Secretary for Preparedness and Response at the Department of Health & Human Services.
A version of this article originally appeared on Medscape.com.
The Moderna mRNA-1273 vaccine, in development to prevent COVID-19, yielded 94.5% efficacy in early results and is generally well tolerated, the company announced early Monday. The product can be stored at refrigeration temperatures common to many physician offices, pharmacies, and hospitals.
The first interim results of the phase 3 COVE trial included 95 participants with confirmed COVID-19. An independent data safety monitoring board, which was appointed by the National Institutes of Health, informed Moderna that 90 of the patients who were positive for COVID-19 were in a placebo group and that 5 patients were in the mRNA-1273 vaccine group, resulting in a vaccine efficacy of 94.5% (P < .0001).
Interim data included 11 patients with severe COVID-19, all of whom were in the placebo group.
“This positive interim analysis from our phase 3 study has given us the first clinical validation that our vaccine can prevent COVID-19 disease, including severe disease,” said Stéphane Bancel, CEO of Moderna, said in a statement.
The vaccine met its primary study endpoint, which was based on adjudicated data that were collected starting 2 weeks after the second dose of mRNA-1273. The interim study population included people who could be at higher risk for COVID-19, including 15 adults aged 65 years and older and 20 participants from diverse communities.
Safety data
The DSMB also reviewed safety data for the COVE study interim results. The vaccine was generally safe and well tolerated, as determined on the basis of solicited adverse events. Most adverse events were mild to moderate and were generally short-lived, according to a company news release.
Injection-site pain was reported in 2.7% of participants after the first dose. After the second dose, 9.7% of participants reported fatigue, 8.9% reported myalgia, 5.2% reported arthralgia, 4.5% reported headache, 4.1% reported pain, and 2.0% reported erythema or redness at the injection site.
Moderna plans to request emergency-use authorization (EUA) from the Food and Drug Administration in the coming weeks. The company expects that the EUA will be based on more data from the COVE study, including a final analysis of 151 patients with a median follow-up of more than 2 months. Moderna also plans to seek authorizations from global regulatory agencies.
The company expects to have approximately 20 million doses of mRNA-1273 ready to ship in the United States by the end of the year. In addition, the company says it remains on track to manufacture between 500 million and 1 billion doses globally in 2021.
Moderna is developing distribution plans in conjunction with the Centers for Disease Control and Prevention, the federal government’s Operation Warp Speed, and McKesson, a COVID-19 vaccine distributor contracted by the U.S. government.
Refrigeration requirements
The mRNA-1273 vaccine can be shipped and stored for up to 6 months at –20° C (about –4° F), a temperature maintained in most home or medical freezers, according to Moderna. The company expects that, after the product thaws, it will remain stable at standard refrigerator temperatures of 2°-8° C (36°-46° F) for up to 30 days within the 6-month shelf life.
Because the mRNA-1273 vaccine is stable at these refrigerator temperatures, it can be stored at most physicians’ offices, pharmacies, and hospitals, the company noted. In contrast, the similar Pfizer BTN162b2 vaccine – early results for which showed a 90% efficacy rate – requires shipment and storage at “deep-freeze” conditions of –70° C or –80° C, which is more challenging from a logistic point of view.
Moderna’s mRNA-1273 can be kept at room temperature for up to 12 hours after removal from a refrigerator for patient administration. The vaccine will not require dilution prior to use.
More than 30,000 people aged older than 18 years in the United States are enrolled in the COVE study. The research is being conducted in collaboration with the National Institute of Allergy and Infectious Diseases and the Biomedical Advanced Research and Development Authority, part of the Office of the Assistant Secretary for Preparedness and Response at the Department of Health & Human Services.
A version of this article originally appeared on Medscape.com.
Methotrexate and hydroxychloroquine split on cardiovascular outcomes in RA
No significant differences in major adverse cardiovascular events (MACE) emerged between methotrexate and hydroxychloroquine (HCQ) treatment in a comparison of adults 65 years or older with rheumatoid arthritis. However, researchers reported some elevation in risk for stroke in the methotrexate group and for myocardial infarction and heart failure in the HCQ group.
The primary outcome, a composite of MI, stroke, or cardiovascular death, had an incidence of 23.39 per 1,000 person-years in the methotrexate group versus 24.33 in the HCQ group in this observational study of nearly 60,000 people.
“These results suggest an importance of looking at different individual events of cardiovascular disease rather than the whole ‘CV’ disease only,” Seoyoung Kim, MD, said in an interview. “The other important thing is that the mortality was not significantly different between the two groups.”
For example, the researchers reported 256 cardiovascular-related deaths in the methotrexate group and 263 such deaths in the HCQ cohort.
Addressing a recognized risk
“It is well known that patients with rheumatoid arthritis have excessive morbidity and mortality,” Dr. Kim, of the division of rheumatology at Brigham and Women’s Hospital and associate professor of medicine at Harvard Medical School in Boston, said at the virtual annual meeting of the American College of Rheumatology.
Among prior studies in this area, the Cardiovascular Inflammation Reduction Trial (CIRT) found no significant reduction in cardiovascular events among people taking methotrexate versus placebo. However, the study of 4,786 people was not specific to RA, Dr. Kim said. The lack of efficacy on this endpoint prompted researchers to stop CIRT early.
“So what does the conclusion of the CIRT trial mean for rheumatoid arthritis patients?” Dr. Kim asked.
To find out, she and colleagues compared risk of MACE among participants newly starting either methotrexate or HCQ. The study included 59,329 people aged 65 and older who were identified through Medicare claims data from 2008 to 2016. Mean age was 74 years, and 80% were women.
The investigators used propensity score matching to control for multiple covariates for demographics, other medications, and comorbidities. Use of other medications was similar between groups, including glucocorticoids, NSAIDs, and statins. Baseline cardiovascular morbidities likewise were well balanced, Dr. Kim said.
The hazard ratio for the primary MACE outcome was 0.96 (95% confidence interval, 0.86-1.08).
Secondary outcomes
MI was less common in the methotrexate group, for example, with an incidence of 8.49 per 1,000 person-years versus 10.68 per 1,000 person-years in the HCQ cohort. This finding was statically significant, Dr. Kim said, with a hazard ratio of 0.80 favoring methotrexate.
Heart failure also occurred less often in the methotrexate cohort, with an incidence rate of 8.57 per 1,000 person-years versus a rate of 14.24 in the HCQ group. The hazard ratio again favored methotrexate at 0.60.
In contrast, strokes were more common with methotrexate than with (incidence of 7.94 vs. 6.01 per 1,000 person-years).
Another secondary outcome, all-cause mortality, was not significantly different between groups. There were 821 deaths in the methotrexate group (28.65 per 1,000 person-years) and 796 deaths in the HCQ group (31.33 per 1,000 person-years).
Studying causality next?
Session moderator Maya Buch, MD, PhD, professor of rheumatology at the University of Manchester (England), asked Dr. Kim why she found significant differences in some secondary outcomes but not the primary composite endpoint.
“When we think of cardiovascular diseases, we tend to think of them all developing through the same mechanism. But perhaps the exact mechanism might not be identical,” Dr. Kim replied. The findings do not suggest causality because the study was observational, she added, “but maybe this will lead to a randomized, controlled trial.”
When asked for comment, Dr. Buch said that the study was “interesting” and “suggestive of differences in type of MACE between the two drugs evaluated,” but that there should be caution in interpreting the findings because of the lack of detailed information on RA disease and activity in claims databases, in addition to other factors, even though the investigators made adjustments for known differences through propensity score matching.
The division of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital supported the study. Dr. Kim has received support for Brigham and Women’s Hospital for unrelated research from Pfizer, AbbVie, Roche, and Bristol-Myers Squibb. Several other coauthors reported having financial relationships with pharmaceutical companies that make drugs for RA. Dr. Buch had no relevant disclosures.
SOURCE: He M et al. Arthritis Rheumatol. 2020;72(suppl 10): Abstract 1993.
No significant differences in major adverse cardiovascular events (MACE) emerged between methotrexate and hydroxychloroquine (HCQ) treatment in a comparison of adults 65 years or older with rheumatoid arthritis. However, researchers reported some elevation in risk for stroke in the methotrexate group and for myocardial infarction and heart failure in the HCQ group.
The primary outcome, a composite of MI, stroke, or cardiovascular death, had an incidence of 23.39 per 1,000 person-years in the methotrexate group versus 24.33 in the HCQ group in this observational study of nearly 60,000 people.
“These results suggest an importance of looking at different individual events of cardiovascular disease rather than the whole ‘CV’ disease only,” Seoyoung Kim, MD, said in an interview. “The other important thing is that the mortality was not significantly different between the two groups.”
For example, the researchers reported 256 cardiovascular-related deaths in the methotrexate group and 263 such deaths in the HCQ cohort.
Addressing a recognized risk
“It is well known that patients with rheumatoid arthritis have excessive morbidity and mortality,” Dr. Kim, of the division of rheumatology at Brigham and Women’s Hospital and associate professor of medicine at Harvard Medical School in Boston, said at the virtual annual meeting of the American College of Rheumatology.
Among prior studies in this area, the Cardiovascular Inflammation Reduction Trial (CIRT) found no significant reduction in cardiovascular events among people taking methotrexate versus placebo. However, the study of 4,786 people was not specific to RA, Dr. Kim said. The lack of efficacy on this endpoint prompted researchers to stop CIRT early.
“So what does the conclusion of the CIRT trial mean for rheumatoid arthritis patients?” Dr. Kim asked.
To find out, she and colleagues compared risk of MACE among participants newly starting either methotrexate or HCQ. The study included 59,329 people aged 65 and older who were identified through Medicare claims data from 2008 to 2016. Mean age was 74 years, and 80% were women.
The investigators used propensity score matching to control for multiple covariates for demographics, other medications, and comorbidities. Use of other medications was similar between groups, including glucocorticoids, NSAIDs, and statins. Baseline cardiovascular morbidities likewise were well balanced, Dr. Kim said.
The hazard ratio for the primary MACE outcome was 0.96 (95% confidence interval, 0.86-1.08).
Secondary outcomes
MI was less common in the methotrexate group, for example, with an incidence of 8.49 per 1,000 person-years versus 10.68 per 1,000 person-years in the HCQ cohort. This finding was statically significant, Dr. Kim said, with a hazard ratio of 0.80 favoring methotrexate.
Heart failure also occurred less often in the methotrexate cohort, with an incidence rate of 8.57 per 1,000 person-years versus a rate of 14.24 in the HCQ group. The hazard ratio again favored methotrexate at 0.60.
In contrast, strokes were more common with methotrexate than with (incidence of 7.94 vs. 6.01 per 1,000 person-years).
Another secondary outcome, all-cause mortality, was not significantly different between groups. There were 821 deaths in the methotrexate group (28.65 per 1,000 person-years) and 796 deaths in the HCQ group (31.33 per 1,000 person-years).
Studying causality next?
Session moderator Maya Buch, MD, PhD, professor of rheumatology at the University of Manchester (England), asked Dr. Kim why she found significant differences in some secondary outcomes but not the primary composite endpoint.
“When we think of cardiovascular diseases, we tend to think of them all developing through the same mechanism. But perhaps the exact mechanism might not be identical,” Dr. Kim replied. The findings do not suggest causality because the study was observational, she added, “but maybe this will lead to a randomized, controlled trial.”
When asked for comment, Dr. Buch said that the study was “interesting” and “suggestive of differences in type of MACE between the two drugs evaluated,” but that there should be caution in interpreting the findings because of the lack of detailed information on RA disease and activity in claims databases, in addition to other factors, even though the investigators made adjustments for known differences through propensity score matching.
The division of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital supported the study. Dr. Kim has received support for Brigham and Women’s Hospital for unrelated research from Pfizer, AbbVie, Roche, and Bristol-Myers Squibb. Several other coauthors reported having financial relationships with pharmaceutical companies that make drugs for RA. Dr. Buch had no relevant disclosures.
SOURCE: He M et al. Arthritis Rheumatol. 2020;72(suppl 10): Abstract 1993.
No significant differences in major adverse cardiovascular events (MACE) emerged between methotrexate and hydroxychloroquine (HCQ) treatment in a comparison of adults 65 years or older with rheumatoid arthritis. However, researchers reported some elevation in risk for stroke in the methotrexate group and for myocardial infarction and heart failure in the HCQ group.
The primary outcome, a composite of MI, stroke, or cardiovascular death, had an incidence of 23.39 per 1,000 person-years in the methotrexate group versus 24.33 in the HCQ group in this observational study of nearly 60,000 people.
“These results suggest an importance of looking at different individual events of cardiovascular disease rather than the whole ‘CV’ disease only,” Seoyoung Kim, MD, said in an interview. “The other important thing is that the mortality was not significantly different between the two groups.”
For example, the researchers reported 256 cardiovascular-related deaths in the methotrexate group and 263 such deaths in the HCQ cohort.
Addressing a recognized risk
“It is well known that patients with rheumatoid arthritis have excessive morbidity and mortality,” Dr. Kim, of the division of rheumatology at Brigham and Women’s Hospital and associate professor of medicine at Harvard Medical School in Boston, said at the virtual annual meeting of the American College of Rheumatology.
Among prior studies in this area, the Cardiovascular Inflammation Reduction Trial (CIRT) found no significant reduction in cardiovascular events among people taking methotrexate versus placebo. However, the study of 4,786 people was not specific to RA, Dr. Kim said. The lack of efficacy on this endpoint prompted researchers to stop CIRT early.
“So what does the conclusion of the CIRT trial mean for rheumatoid arthritis patients?” Dr. Kim asked.
To find out, she and colleagues compared risk of MACE among participants newly starting either methotrexate or HCQ. The study included 59,329 people aged 65 and older who were identified through Medicare claims data from 2008 to 2016. Mean age was 74 years, and 80% were women.
The investigators used propensity score matching to control for multiple covariates for demographics, other medications, and comorbidities. Use of other medications was similar between groups, including glucocorticoids, NSAIDs, and statins. Baseline cardiovascular morbidities likewise were well balanced, Dr. Kim said.
The hazard ratio for the primary MACE outcome was 0.96 (95% confidence interval, 0.86-1.08).
Secondary outcomes
MI was less common in the methotrexate group, for example, with an incidence of 8.49 per 1,000 person-years versus 10.68 per 1,000 person-years in the HCQ cohort. This finding was statically significant, Dr. Kim said, with a hazard ratio of 0.80 favoring methotrexate.
Heart failure also occurred less often in the methotrexate cohort, with an incidence rate of 8.57 per 1,000 person-years versus a rate of 14.24 in the HCQ group. The hazard ratio again favored methotrexate at 0.60.
In contrast, strokes were more common with methotrexate than with (incidence of 7.94 vs. 6.01 per 1,000 person-years).
Another secondary outcome, all-cause mortality, was not significantly different between groups. There were 821 deaths in the methotrexate group (28.65 per 1,000 person-years) and 796 deaths in the HCQ group (31.33 per 1,000 person-years).
Studying causality next?
Session moderator Maya Buch, MD, PhD, professor of rheumatology at the University of Manchester (England), asked Dr. Kim why she found significant differences in some secondary outcomes but not the primary composite endpoint.
“When we think of cardiovascular diseases, we tend to think of them all developing through the same mechanism. But perhaps the exact mechanism might not be identical,” Dr. Kim replied. The findings do not suggest causality because the study was observational, she added, “but maybe this will lead to a randomized, controlled trial.”
When asked for comment, Dr. Buch said that the study was “interesting” and “suggestive of differences in type of MACE between the two drugs evaluated,” but that there should be caution in interpreting the findings because of the lack of detailed information on RA disease and activity in claims databases, in addition to other factors, even though the investigators made adjustments for known differences through propensity score matching.
The division of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital supported the study. Dr. Kim has received support for Brigham and Women’s Hospital for unrelated research from Pfizer, AbbVie, Roche, and Bristol-Myers Squibb. Several other coauthors reported having financial relationships with pharmaceutical companies that make drugs for RA. Dr. Buch had no relevant disclosures.
SOURCE: He M et al. Arthritis Rheumatol. 2020;72(suppl 10): Abstract 1993.
FROM ACR 2020
Moral distress: COVID-19 shortages prompt tough decisions at bedside
Choosing which hospitalized COVID-19 patients receive potentially lifesaving care, making urgent calls for ventilators and other equipment, and triaging care based on patient age and comorbidities were among the challenges revealed in new feedback from health care leaders and frontline workers.
Even though many hospitals have contingency plans for how to allocate resources and triage patient care during crisis capacity, for many providers during the real-world COVID-19 trial of these protocols, they fell short.
Many hospital crisis capacity plans, for example, were too general to address all the specific challenges arising during the pandemic, investigators report in a study published online Nov. 6 in JAMA Network Open.
“Our research shows that the types of challenges and approach to resource limitation in real-world clinical settings during the pandemic differed in practice from how we had prepared in theory,” lead author Catherine Butler, MD, told Medscape Medical News. Insufficient dialysis treatment time, staff shortages, and routine supply scarcity are examples “for which there was not an established plan or approach for appropriate allocation.”
“This left frontline clinicians to determine what constituted an acceptable standard of care and to make difficult allocation decisions at the bedside,” added Butler, acting instructor in the Division of Nephrology at the University of Washington in Seattle and a research fellow at the VA Health Services Research and Development Seattle-Denver Center of Innovation.
The investigators conducted semistructured interviews in April and May with 61 clinicians and health leaders. Mean age was 46 years, 63% were women, and participants practiced in 15 states. Most participants hailed from locations hard-hit by the pandemic at the time, including Seattle, New York City, and New Orleans.
Triage tribulations
The qualitative study included comments from respondents on three major themes that emerged: planning for crisis capacity, adapting to resource limitation, and the multiple unprecedented barriers to care delivery.
Overall, planning and support from institutional leaders varied. One provider said, “Talking to administration, and they just seemed really disengaged with the problem. We asked multiple times if there was a triage command center or a plan for what would occur if we got to the point where we had to triage resources. They said there was, but they wouldn’t provide it to us.”
Another had a more positive experience. “The biggest deal in the ethics world in the last 2 months has been preparing in case we need to triage. So, we have a very detailed, elaborate, well thought-out triage policy … that was done at the highest levels of the system.”
Clinicians said they participate on triage teams – despite the moral weight and likely emotional burden – out of a sense of duty.
Interestingly, some providers on these teams also reported a reluctance to reveal their participation to colleagues. “I didn’t feel like I should tell anybody … even some of my close friends who are physicians and nurses here … that I’ve been asked to be on this [triage team],” one respondent said. “I didn’t feel like I should make it known.”
Adapting to scarce resources
Multiple providers said they faced difficult care decisions because of limited dialysis or supply shortages. “They felt that this patient had the greatest likelihood of benefiting from most aggressive therapy. … I think there was probably like 5 or 6 patients in the ICU … and then you had this 35-year-old with no comorbidities,” one respondent said. “That’s who the ICU dialyzed, and I couldn’t really disagree.”
“I emailed all of [my colleagues], and I said ‘Help! We need X, we need CRRT [continuous renal replacement therapy] machines, we need dialysates,’ “ another responded.
“One of the attendings had a tweet when we were running out of CRRT. He had a tweet about, ‘Can anybody give us supplies for CRRT?’ So, it got to that. You do anything. You get really desperate,” the clinician said.
Other providers reported getting innovative under the circumstances. “My partner’s son, he actually borrowed a couple of 3D printers. He printed some of these face shields, and then they got the formula, or the specifics as to how to make this particular connection to connect to a dialysis machine to generate dialysate. So, he also printed some of those from the 3D printer.”
Dire situations with dialysis
Another respondent understood the focus on ventilators and ICU beds throughout the crisis, but said “no one has acknowledged that dialysis has been one of the most, if not the most, limited resources.”
Another clinician expressed surprise at a decision made in the face of limited availability of traditional dialysis. “A month ago, people said we were going to do acute peritoneal dialysis [PD]. And I said, ‘No, we’re not going to do acute PD. PD, it’s not that great for acute patients, sick people in the ICUs. I don’t think we’re going to do PD.’
“Three days later we were doing acute PD. I mean, that was unbelievable!”
Some institutions rationed dialysis therapy. “We went through the entire list at the beginning of the week and [said], this person has to dialyze these days, this person would probably benefit from a dialysis session, a third group person we could probably just string along and medically manage if we needed to,” one provider said.
Another respondent reported a different strategy. “No one was not getting dialysis, but there were a lot of people getting minimal dialysis. Even though people were getting treated, resources were very stretched.”
Changing family dynamics
COVID-19 has naturally changed how clinicians speak with families. One respondent recalled looking at the ICU physician and being like, ‘Have you talked to the son this week?’ And she’s like, ‘Oh my God, no. … Did you talk to the son?’ I’m like, ‘Oh my God, no.’ “
They realized, the respondent added, “that none of us had called the family because it’s just not in your workflow. You’re so used to the family being there.”
Multiple providers also feared a conversation with family regarding necessary changes to care given the limitation of resources during the pandemic.
“Most families have been actually very understanding. This is a crisis, and we’re in a pandemic, and we’re all doing things we wouldn’t normally do.”
Another respondent said, “We were pretty honest about how resources were limited and how we were doing with this COVID-19 surge. And I think we talked about how the usual ability to provide aggressive dialysis was not the case with COVID-19. There was a lot of understanding, sometimes to my surprise. I would think people would be more upset when hearing something like that.”
Many clinicians facing these challenges experience moral distress, the researchers noted.
“Early in the pandemic, it became quickly apparent that possible resource limitation, such as scarce ventilators, was a major ethical concern. There was robust debate and discussion published in medical journals and the popular press about how to appropriately allocate health care resources,” the University of Washington’s Butler said.
“Transparency, accountability, and standardized processes for rationing these resources in ‘crisis capacity’ settings were seen as key to avoiding the impact of implicit bias and moral distress for clinicians,” she added.
Lessons learned
In terms of potential solutions that could mitigate these challenges in the future, health care leaders “could develop standardized protocols or guidelines for allocating a broader range of potentially scarce health care resources even before ‘crisis capacity’ is declared,” Butler said.
Furthermore, no frontline worker should have to go it alone. “Medical ethicists and/or other clinicians familiar with ethical considerations in settings of scarce health care resources might provide bedside consultation and collaborate with frontline providers who must grapple with the impact of more subtle forms of resource limitation on clinical decision-making.”
The study was partially funded by grants from the National Institute of Diabetes and Digestive and Kidney Diseases and a COVID-19 Research Award from the University of Washington Institute of Translational Health Sciences given to Butler.
This article first appeared on Medscape.com.
Choosing which hospitalized COVID-19 patients receive potentially lifesaving care, making urgent calls for ventilators and other equipment, and triaging care based on patient age and comorbidities were among the challenges revealed in new feedback from health care leaders and frontline workers.
Even though many hospitals have contingency plans for how to allocate resources and triage patient care during crisis capacity, for many providers during the real-world COVID-19 trial of these protocols, they fell short.
Many hospital crisis capacity plans, for example, were too general to address all the specific challenges arising during the pandemic, investigators report in a study published online Nov. 6 in JAMA Network Open.
“Our research shows that the types of challenges and approach to resource limitation in real-world clinical settings during the pandemic differed in practice from how we had prepared in theory,” lead author Catherine Butler, MD, told Medscape Medical News. Insufficient dialysis treatment time, staff shortages, and routine supply scarcity are examples “for which there was not an established plan or approach for appropriate allocation.”
“This left frontline clinicians to determine what constituted an acceptable standard of care and to make difficult allocation decisions at the bedside,” added Butler, acting instructor in the Division of Nephrology at the University of Washington in Seattle and a research fellow at the VA Health Services Research and Development Seattle-Denver Center of Innovation.
The investigators conducted semistructured interviews in April and May with 61 clinicians and health leaders. Mean age was 46 years, 63% were women, and participants practiced in 15 states. Most participants hailed from locations hard-hit by the pandemic at the time, including Seattle, New York City, and New Orleans.
Triage tribulations
The qualitative study included comments from respondents on three major themes that emerged: planning for crisis capacity, adapting to resource limitation, and the multiple unprecedented barriers to care delivery.
Overall, planning and support from institutional leaders varied. One provider said, “Talking to administration, and they just seemed really disengaged with the problem. We asked multiple times if there was a triage command center or a plan for what would occur if we got to the point where we had to triage resources. They said there was, but they wouldn’t provide it to us.”
Another had a more positive experience. “The biggest deal in the ethics world in the last 2 months has been preparing in case we need to triage. So, we have a very detailed, elaborate, well thought-out triage policy … that was done at the highest levels of the system.”
Clinicians said they participate on triage teams – despite the moral weight and likely emotional burden – out of a sense of duty.
Interestingly, some providers on these teams also reported a reluctance to reveal their participation to colleagues. “I didn’t feel like I should tell anybody … even some of my close friends who are physicians and nurses here … that I’ve been asked to be on this [triage team],” one respondent said. “I didn’t feel like I should make it known.”
Adapting to scarce resources
Multiple providers said they faced difficult care decisions because of limited dialysis or supply shortages. “They felt that this patient had the greatest likelihood of benefiting from most aggressive therapy. … I think there was probably like 5 or 6 patients in the ICU … and then you had this 35-year-old with no comorbidities,” one respondent said. “That’s who the ICU dialyzed, and I couldn’t really disagree.”
“I emailed all of [my colleagues], and I said ‘Help! We need X, we need CRRT [continuous renal replacement therapy] machines, we need dialysates,’ “ another responded.
“One of the attendings had a tweet when we were running out of CRRT. He had a tweet about, ‘Can anybody give us supplies for CRRT?’ So, it got to that. You do anything. You get really desperate,” the clinician said.
Other providers reported getting innovative under the circumstances. “My partner’s son, he actually borrowed a couple of 3D printers. He printed some of these face shields, and then they got the formula, or the specifics as to how to make this particular connection to connect to a dialysis machine to generate dialysate. So, he also printed some of those from the 3D printer.”
Dire situations with dialysis
Another respondent understood the focus on ventilators and ICU beds throughout the crisis, but said “no one has acknowledged that dialysis has been one of the most, if not the most, limited resources.”
Another clinician expressed surprise at a decision made in the face of limited availability of traditional dialysis. “A month ago, people said we were going to do acute peritoneal dialysis [PD]. And I said, ‘No, we’re not going to do acute PD. PD, it’s not that great for acute patients, sick people in the ICUs. I don’t think we’re going to do PD.’
“Three days later we were doing acute PD. I mean, that was unbelievable!”
Some institutions rationed dialysis therapy. “We went through the entire list at the beginning of the week and [said], this person has to dialyze these days, this person would probably benefit from a dialysis session, a third group person we could probably just string along and medically manage if we needed to,” one provider said.
Another respondent reported a different strategy. “No one was not getting dialysis, but there were a lot of people getting minimal dialysis. Even though people were getting treated, resources were very stretched.”
Changing family dynamics
COVID-19 has naturally changed how clinicians speak with families. One respondent recalled looking at the ICU physician and being like, ‘Have you talked to the son this week?’ And she’s like, ‘Oh my God, no. … Did you talk to the son?’ I’m like, ‘Oh my God, no.’ “
They realized, the respondent added, “that none of us had called the family because it’s just not in your workflow. You’re so used to the family being there.”
Multiple providers also feared a conversation with family regarding necessary changes to care given the limitation of resources during the pandemic.
“Most families have been actually very understanding. This is a crisis, and we’re in a pandemic, and we’re all doing things we wouldn’t normally do.”
Another respondent said, “We were pretty honest about how resources were limited and how we were doing with this COVID-19 surge. And I think we talked about how the usual ability to provide aggressive dialysis was not the case with COVID-19. There was a lot of understanding, sometimes to my surprise. I would think people would be more upset when hearing something like that.”
Many clinicians facing these challenges experience moral distress, the researchers noted.
“Early in the pandemic, it became quickly apparent that possible resource limitation, such as scarce ventilators, was a major ethical concern. There was robust debate and discussion published in medical journals and the popular press about how to appropriately allocate health care resources,” the University of Washington’s Butler said.
“Transparency, accountability, and standardized processes for rationing these resources in ‘crisis capacity’ settings were seen as key to avoiding the impact of implicit bias and moral distress for clinicians,” she added.
Lessons learned
In terms of potential solutions that could mitigate these challenges in the future, health care leaders “could develop standardized protocols or guidelines for allocating a broader range of potentially scarce health care resources even before ‘crisis capacity’ is declared,” Butler said.
Furthermore, no frontline worker should have to go it alone. “Medical ethicists and/or other clinicians familiar with ethical considerations in settings of scarce health care resources might provide bedside consultation and collaborate with frontline providers who must grapple with the impact of more subtle forms of resource limitation on clinical decision-making.”
The study was partially funded by grants from the National Institute of Diabetes and Digestive and Kidney Diseases and a COVID-19 Research Award from the University of Washington Institute of Translational Health Sciences given to Butler.
This article first appeared on Medscape.com.
Choosing which hospitalized COVID-19 patients receive potentially lifesaving care, making urgent calls for ventilators and other equipment, and triaging care based on patient age and comorbidities were among the challenges revealed in new feedback from health care leaders and frontline workers.
Even though many hospitals have contingency plans for how to allocate resources and triage patient care during crisis capacity, for many providers during the real-world COVID-19 trial of these protocols, they fell short.
Many hospital crisis capacity plans, for example, were too general to address all the specific challenges arising during the pandemic, investigators report in a study published online Nov. 6 in JAMA Network Open.
“Our research shows that the types of challenges and approach to resource limitation in real-world clinical settings during the pandemic differed in practice from how we had prepared in theory,” lead author Catherine Butler, MD, told Medscape Medical News. Insufficient dialysis treatment time, staff shortages, and routine supply scarcity are examples “for which there was not an established plan or approach for appropriate allocation.”
“This left frontline clinicians to determine what constituted an acceptable standard of care and to make difficult allocation decisions at the bedside,” added Butler, acting instructor in the Division of Nephrology at the University of Washington in Seattle and a research fellow at the VA Health Services Research and Development Seattle-Denver Center of Innovation.
The investigators conducted semistructured interviews in April and May with 61 clinicians and health leaders. Mean age was 46 years, 63% were women, and participants practiced in 15 states. Most participants hailed from locations hard-hit by the pandemic at the time, including Seattle, New York City, and New Orleans.
Triage tribulations
The qualitative study included comments from respondents on three major themes that emerged: planning for crisis capacity, adapting to resource limitation, and the multiple unprecedented barriers to care delivery.
Overall, planning and support from institutional leaders varied. One provider said, “Talking to administration, and they just seemed really disengaged with the problem. We asked multiple times if there was a triage command center or a plan for what would occur if we got to the point where we had to triage resources. They said there was, but they wouldn’t provide it to us.”
Another had a more positive experience. “The biggest deal in the ethics world in the last 2 months has been preparing in case we need to triage. So, we have a very detailed, elaborate, well thought-out triage policy … that was done at the highest levels of the system.”
Clinicians said they participate on triage teams – despite the moral weight and likely emotional burden – out of a sense of duty.
Interestingly, some providers on these teams also reported a reluctance to reveal their participation to colleagues. “I didn’t feel like I should tell anybody … even some of my close friends who are physicians and nurses here … that I’ve been asked to be on this [triage team],” one respondent said. “I didn’t feel like I should make it known.”
Adapting to scarce resources
Multiple providers said they faced difficult care decisions because of limited dialysis or supply shortages. “They felt that this patient had the greatest likelihood of benefiting from most aggressive therapy. … I think there was probably like 5 or 6 patients in the ICU … and then you had this 35-year-old with no comorbidities,” one respondent said. “That’s who the ICU dialyzed, and I couldn’t really disagree.”
“I emailed all of [my colleagues], and I said ‘Help! We need X, we need CRRT [continuous renal replacement therapy] machines, we need dialysates,’ “ another responded.
“One of the attendings had a tweet when we were running out of CRRT. He had a tweet about, ‘Can anybody give us supplies for CRRT?’ So, it got to that. You do anything. You get really desperate,” the clinician said.
Other providers reported getting innovative under the circumstances. “My partner’s son, he actually borrowed a couple of 3D printers. He printed some of these face shields, and then they got the formula, or the specifics as to how to make this particular connection to connect to a dialysis machine to generate dialysate. So, he also printed some of those from the 3D printer.”
Dire situations with dialysis
Another respondent understood the focus on ventilators and ICU beds throughout the crisis, but said “no one has acknowledged that dialysis has been one of the most, if not the most, limited resources.”
Another clinician expressed surprise at a decision made in the face of limited availability of traditional dialysis. “A month ago, people said we were going to do acute peritoneal dialysis [PD]. And I said, ‘No, we’re not going to do acute PD. PD, it’s not that great for acute patients, sick people in the ICUs. I don’t think we’re going to do PD.’
“Three days later we were doing acute PD. I mean, that was unbelievable!”
Some institutions rationed dialysis therapy. “We went through the entire list at the beginning of the week and [said], this person has to dialyze these days, this person would probably benefit from a dialysis session, a third group person we could probably just string along and medically manage if we needed to,” one provider said.
Another respondent reported a different strategy. “No one was not getting dialysis, but there were a lot of people getting minimal dialysis. Even though people were getting treated, resources were very stretched.”
Changing family dynamics
COVID-19 has naturally changed how clinicians speak with families. One respondent recalled looking at the ICU physician and being like, ‘Have you talked to the son this week?’ And she’s like, ‘Oh my God, no. … Did you talk to the son?’ I’m like, ‘Oh my God, no.’ “
They realized, the respondent added, “that none of us had called the family because it’s just not in your workflow. You’re so used to the family being there.”
Multiple providers also feared a conversation with family regarding necessary changes to care given the limitation of resources during the pandemic.
“Most families have been actually very understanding. This is a crisis, and we’re in a pandemic, and we’re all doing things we wouldn’t normally do.”
Another respondent said, “We were pretty honest about how resources were limited and how we were doing with this COVID-19 surge. And I think we talked about how the usual ability to provide aggressive dialysis was not the case with COVID-19. There was a lot of understanding, sometimes to my surprise. I would think people would be more upset when hearing something like that.”
Many clinicians facing these challenges experience moral distress, the researchers noted.
“Early in the pandemic, it became quickly apparent that possible resource limitation, such as scarce ventilators, was a major ethical concern. There was robust debate and discussion published in medical journals and the popular press about how to appropriately allocate health care resources,” the University of Washington’s Butler said.
“Transparency, accountability, and standardized processes for rationing these resources in ‘crisis capacity’ settings were seen as key to avoiding the impact of implicit bias and moral distress for clinicians,” she added.
Lessons learned
In terms of potential solutions that could mitigate these challenges in the future, health care leaders “could develop standardized protocols or guidelines for allocating a broader range of potentially scarce health care resources even before ‘crisis capacity’ is declared,” Butler said.
Furthermore, no frontline worker should have to go it alone. “Medical ethicists and/or other clinicians familiar with ethical considerations in settings of scarce health care resources might provide bedside consultation and collaborate with frontline providers who must grapple with the impact of more subtle forms of resource limitation on clinical decision-making.”
The study was partially funded by grants from the National Institute of Diabetes and Digestive and Kidney Diseases and a COVID-19 Research Award from the University of Washington Institute of Translational Health Sciences given to Butler.
This article first appeared on Medscape.com.
FDA grants emergency use authorization to Lilly’s antibody COVID-19 therapy
The monoclonal antibody therapy has emergency authorization for treating patients who have tested positive for SARS-CoV-2 infection and who are considered to be at high risk for progression to severe COVID-19 or hospitalization. To be eligible for treatment with bamlanivimab, patients must be at least 12 years of age and weigh at least 40 kg (approximately 88 lb). The agency notes that this includes patients aged 65 years and older or people with certain chronic conditions.
Bamlanivimab is not authorized for use in patients who are hospitalized or who require oxygen therapy because of COVID-19. The FDA’s action comes less than 2 weeks after Eli Lilly halted the ACTIV-3 study of the therapy for severe, hospitalized COVID-19 patients after evidence showed that adding the antibody therapy to standard care did not improve outcomes over standard care alone for patients with advanced COVID-19.
The government contract with Eli Lilly involves the purchase of 300,000 doses through December, with the option to procure another 650,000 doses through June 2021.
Because of Operation Warp Speed, “we have supplies to distribute now. Product distribution will begin this week,” US Health & Human Services (HHS) Secretary Alex Azar said at a news conference today.
“We talked about building the bridge to safe and effective vaccines” for COVID-19, Azar added. “With this therapeutic, the bridge is taking shape.”
Bamlanivimab 700 mg will be administered as a 1-hour infusion followed by a 1-hour observation period for detecting any infusion-related side effects. The authorized dose is 700 mg, which was on the lower end of the dose range evaluated in studies.
During the press conference, a reporter asked whether the lower dose was chosen in order that more doses of the antibody could be made available. “The lower dose is a rational choice in this situation because we don’t want to give more of a drug than you need,” said Janet Woodcock, MD, the therapeutics lead for Operation Warp Speed. “I think we could probably go lower.”
Bamlanivimab works by attaching to the virus and blocking its entry into the cells and possibly by helping the patients’ immune system clear the virus, said Woodcock, who is also director of the FDA’s Center for Drug Evaluation and Research.
“The goal is to treat high-risk people as soon as possible after they show symptoms and are diagnosed,” she added.
Infusions an initial challenge?
There could be some logistic challenges at first because the antibody is administered via infusion. “We expect there will initially be a challenge in administering ... these infusions and setting up infusion centers,” Woodcock said.
Outpatient intravenous infusions are normally performed at infusion centers for patients with cancer and immune disorders, she noted. “You really don’t want them mixing with people who have COVID-19 disease, so we will need to set up separate sites.”
Bamlanivimab will be provided free of cost to patients, Azar said. Patients should be aware that coinsurance may be required for the infusion.
“Fair and equitable” distribution planned
During phase 1 of distribution, the agent will first be allocated to hospitals and hospital-affiliated locations only, John Redd, MD, MPH, chief medical officer, Office of the Assistant Secretary for Preparedness and Response at HHS, said at the press conference.
During phase 2, “there will be expanded distribution to outpatient sites,” he said. In an effort to keep the process transparent, a new website features the latest updates on the distribution of bamlanivimab.
Allocation will be based on two factors: the number of new cases reported in a state or territory in the prior 7 days, and rates of COVID-19 hospitalization during the same period.
Asked why the government would determine distribution of the antibody on the basis of the number of hospitalized patients when the indication includes prevention of admission, Woodcock replied that hospitalization is a surrogate measure that can reflect risk factors in a particular state population, such as obesity, diabetes, or the proportion of older people.
Furthermore, the confirmed cases are a “leading indicator,” she said, that can help identify a steep rise in COVID-19 cases that could indicate more hospitalizations are likely soon. “We don’t want to miss that.”
Data underlying the EUA decision
A decrease in hospitalizations or emergency department visits within 28 days of treatment in preclinical studies was “the most important evidence that bamlanivimab may be effective,” the agency noted in the press release announcing the EUA. Among patients at high risk for progression, 3% required such interventions, compared with 10% of placebo-treated patients.
Potential side effects of bamlanivimab include anaphylaxis, infusion-related reactions, nausea, diarrhea, dizziness, headache, itching, and vomiting.
“As illustrated by today’s action, the FDA remains committed to expediting the development and availability of potential COVID-19 treatments and providing sick patients timely access to new therapies where appropriate,” FDA Commissioner Stephen M. Hahn, MD, said in the news release.
Healthcare providers can download a detailed FDA fact sheet on the EUA for bamlanivimab, which includes dosing instructions.
This article first appeared on Medscape.com.
The monoclonal antibody therapy has emergency authorization for treating patients who have tested positive for SARS-CoV-2 infection and who are considered to be at high risk for progression to severe COVID-19 or hospitalization. To be eligible for treatment with bamlanivimab, patients must be at least 12 years of age and weigh at least 40 kg (approximately 88 lb). The agency notes that this includes patients aged 65 years and older or people with certain chronic conditions.
Bamlanivimab is not authorized for use in patients who are hospitalized or who require oxygen therapy because of COVID-19. The FDA’s action comes less than 2 weeks after Eli Lilly halted the ACTIV-3 study of the therapy for severe, hospitalized COVID-19 patients after evidence showed that adding the antibody therapy to standard care did not improve outcomes over standard care alone for patients with advanced COVID-19.
The government contract with Eli Lilly involves the purchase of 300,000 doses through December, with the option to procure another 650,000 doses through June 2021.
Because of Operation Warp Speed, “we have supplies to distribute now. Product distribution will begin this week,” US Health & Human Services (HHS) Secretary Alex Azar said at a news conference today.
“We talked about building the bridge to safe and effective vaccines” for COVID-19, Azar added. “With this therapeutic, the bridge is taking shape.”
Bamlanivimab 700 mg will be administered as a 1-hour infusion followed by a 1-hour observation period for detecting any infusion-related side effects. The authorized dose is 700 mg, which was on the lower end of the dose range evaluated in studies.
During the press conference, a reporter asked whether the lower dose was chosen in order that more doses of the antibody could be made available. “The lower dose is a rational choice in this situation because we don’t want to give more of a drug than you need,” said Janet Woodcock, MD, the therapeutics lead for Operation Warp Speed. “I think we could probably go lower.”
Bamlanivimab works by attaching to the virus and blocking its entry into the cells and possibly by helping the patients’ immune system clear the virus, said Woodcock, who is also director of the FDA’s Center for Drug Evaluation and Research.
“The goal is to treat high-risk people as soon as possible after they show symptoms and are diagnosed,” she added.
Infusions an initial challenge?
There could be some logistic challenges at first because the antibody is administered via infusion. “We expect there will initially be a challenge in administering ... these infusions and setting up infusion centers,” Woodcock said.
Outpatient intravenous infusions are normally performed at infusion centers for patients with cancer and immune disorders, she noted. “You really don’t want them mixing with people who have COVID-19 disease, so we will need to set up separate sites.”
Bamlanivimab will be provided free of cost to patients, Azar said. Patients should be aware that coinsurance may be required for the infusion.
“Fair and equitable” distribution planned
During phase 1 of distribution, the agent will first be allocated to hospitals and hospital-affiliated locations only, John Redd, MD, MPH, chief medical officer, Office of the Assistant Secretary for Preparedness and Response at HHS, said at the press conference.
During phase 2, “there will be expanded distribution to outpatient sites,” he said. In an effort to keep the process transparent, a new website features the latest updates on the distribution of bamlanivimab.
Allocation will be based on two factors: the number of new cases reported in a state or territory in the prior 7 days, and rates of COVID-19 hospitalization during the same period.
Asked why the government would determine distribution of the antibody on the basis of the number of hospitalized patients when the indication includes prevention of admission, Woodcock replied that hospitalization is a surrogate measure that can reflect risk factors in a particular state population, such as obesity, diabetes, or the proportion of older people.
Furthermore, the confirmed cases are a “leading indicator,” she said, that can help identify a steep rise in COVID-19 cases that could indicate more hospitalizations are likely soon. “We don’t want to miss that.”
Data underlying the EUA decision
A decrease in hospitalizations or emergency department visits within 28 days of treatment in preclinical studies was “the most important evidence that bamlanivimab may be effective,” the agency noted in the press release announcing the EUA. Among patients at high risk for progression, 3% required such interventions, compared with 10% of placebo-treated patients.
Potential side effects of bamlanivimab include anaphylaxis, infusion-related reactions, nausea, diarrhea, dizziness, headache, itching, and vomiting.
“As illustrated by today’s action, the FDA remains committed to expediting the development and availability of potential COVID-19 treatments and providing sick patients timely access to new therapies where appropriate,” FDA Commissioner Stephen M. Hahn, MD, said in the news release.
Healthcare providers can download a detailed FDA fact sheet on the EUA for bamlanivimab, which includes dosing instructions.
This article first appeared on Medscape.com.
The monoclonal antibody therapy has emergency authorization for treating patients who have tested positive for SARS-CoV-2 infection and who are considered to be at high risk for progression to severe COVID-19 or hospitalization. To be eligible for treatment with bamlanivimab, patients must be at least 12 years of age and weigh at least 40 kg (approximately 88 lb). The agency notes that this includes patients aged 65 years and older or people with certain chronic conditions.
Bamlanivimab is not authorized for use in patients who are hospitalized or who require oxygen therapy because of COVID-19. The FDA’s action comes less than 2 weeks after Eli Lilly halted the ACTIV-3 study of the therapy for severe, hospitalized COVID-19 patients after evidence showed that adding the antibody therapy to standard care did not improve outcomes over standard care alone for patients with advanced COVID-19.
The government contract with Eli Lilly involves the purchase of 300,000 doses through December, with the option to procure another 650,000 doses through June 2021.
Because of Operation Warp Speed, “we have supplies to distribute now. Product distribution will begin this week,” US Health & Human Services (HHS) Secretary Alex Azar said at a news conference today.
“We talked about building the bridge to safe and effective vaccines” for COVID-19, Azar added. “With this therapeutic, the bridge is taking shape.”
Bamlanivimab 700 mg will be administered as a 1-hour infusion followed by a 1-hour observation period for detecting any infusion-related side effects. The authorized dose is 700 mg, which was on the lower end of the dose range evaluated in studies.
During the press conference, a reporter asked whether the lower dose was chosen in order that more doses of the antibody could be made available. “The lower dose is a rational choice in this situation because we don’t want to give more of a drug than you need,” said Janet Woodcock, MD, the therapeutics lead for Operation Warp Speed. “I think we could probably go lower.”
Bamlanivimab works by attaching to the virus and blocking its entry into the cells and possibly by helping the patients’ immune system clear the virus, said Woodcock, who is also director of the FDA’s Center for Drug Evaluation and Research.
“The goal is to treat high-risk people as soon as possible after they show symptoms and are diagnosed,” she added.
Infusions an initial challenge?
There could be some logistic challenges at first because the antibody is administered via infusion. “We expect there will initially be a challenge in administering ... these infusions and setting up infusion centers,” Woodcock said.
Outpatient intravenous infusions are normally performed at infusion centers for patients with cancer and immune disorders, she noted. “You really don’t want them mixing with people who have COVID-19 disease, so we will need to set up separate sites.”
Bamlanivimab will be provided free of cost to patients, Azar said. Patients should be aware that coinsurance may be required for the infusion.
“Fair and equitable” distribution planned
During phase 1 of distribution, the agent will first be allocated to hospitals and hospital-affiliated locations only, John Redd, MD, MPH, chief medical officer, Office of the Assistant Secretary for Preparedness and Response at HHS, said at the press conference.
During phase 2, “there will be expanded distribution to outpatient sites,” he said. In an effort to keep the process transparent, a new website features the latest updates on the distribution of bamlanivimab.
Allocation will be based on two factors: the number of new cases reported in a state or territory in the prior 7 days, and rates of COVID-19 hospitalization during the same period.
Asked why the government would determine distribution of the antibody on the basis of the number of hospitalized patients when the indication includes prevention of admission, Woodcock replied that hospitalization is a surrogate measure that can reflect risk factors in a particular state population, such as obesity, diabetes, or the proportion of older people.
Furthermore, the confirmed cases are a “leading indicator,” she said, that can help identify a steep rise in COVID-19 cases that could indicate more hospitalizations are likely soon. “We don’t want to miss that.”
Data underlying the EUA decision
A decrease in hospitalizations or emergency department visits within 28 days of treatment in preclinical studies was “the most important evidence that bamlanivimab may be effective,” the agency noted in the press release announcing the EUA. Among patients at high risk for progression, 3% required such interventions, compared with 10% of placebo-treated patients.
Potential side effects of bamlanivimab include anaphylaxis, infusion-related reactions, nausea, diarrhea, dizziness, headache, itching, and vomiting.
“As illustrated by today’s action, the FDA remains committed to expediting the development and availability of potential COVID-19 treatments and providing sick patients timely access to new therapies where appropriate,” FDA Commissioner Stephen M. Hahn, MD, said in the news release.
Healthcare providers can download a detailed FDA fact sheet on the EUA for bamlanivimab, which includes dosing instructions.
This article first appeared on Medscape.com.
FAST trial clears febuxostat of increased mortality in gout
Febuxostat (Uloric) emerged as noninferior to allopurinol regarding risk of cardiovascular death among people 60 years and older with gout and at least one additional cardiovascular risk factor, results of the Febuxostat versus Allopurinol Streamlined Trial (FAST) suggest.
This primary outcome of the FAST trial stands in contrast to results of the CARES trial in 2018. The CARES researchers previously reported a 4.3% increased risk of cardiovascular death associated with febuxostat, compared with a 3.2% rate with allopurinol, a statistically significant 34% increase in the relative risk.
“In contrast to previous studies, there was no evidence of increased mortality with febuxostat, and we believe the regulators should review febuxostat licensing restrictions,” senior author Thomas MacDonald, MD, of the University of Dundee (Scotland), said during a late-breaking abstract session at the virtual annual meeting of the American College of Rheumatology.
The results of the FAST trial were simultaneously published online in The Lancet.
Both febuxostat and allopurinol treat gout by lowering urate levels. Concerns about the cardiovascular safety of febuxostat led to two post-licensing studies: the Cardiovascular Safety of Febuxostat and Allopurinol in Participants with Gout and Cardiovascular Comorbidities (CARES) study, mandated by the U.S. Food and Drug Administration, and FAST, requested by the European Medicines Agency. In February 2019, the FDA added a warning about elevated cardiovascular death and death risk associated with febuxostat.
“When CARES was published, it was somewhat of a threat to our study,” Dr. MacDonald said. “After hearing from our data-monitoring committee, we were told we could continue the trial.”
Some switched from allopurinol to febuxostat
So Dr. MacDonald, lead author Isla Mackenzie, MBChB, and their colleagues enrolled 6,128 people with gout in the United Kingdom, Sweden, and Denmark between December 2011 and January 2018. They followed patients for a median of 4 years. Participants had a mean age of 71 years, 85% were men, and 33% had a history of cardiovascular disease. The investigators excluded anyone with a stroke or myocardial infarction in the previous 6 months.
All participants were being treated with allopurinol. The investigators titrated those not at target up to an ideal dose that achieved a serum urate concentration of less than 0.357 mmol/L (< 6 mg/dL). Next, they randomly assigned 3,065 people to continue allopurinol and another 3,063 to switch to 80-120 mg of febuxostat.
The primary outcome of the multicenter, prospective, randomized, open-label FAST trial was a composite of hospitalization for nonfatal MI or biomarker positive for acute coronary syndrome, nonfatal stroke, or cardiovascular death.
Key findings
“There was definitely a noninferior primary outcome,” Dr. MacDonald said. In the on-treatment analysis, 172 patients in the febuxostat group reached the composite endpoint versus 241 patients in the allopurinol group. There were 1.72 events per 100 patient-years in the febuxostat group versus 2.05 events in the allopurinol group (adjusted hazard ratio, 0.85; 95% confidence interval, 0.70-1.03). An intent-to-treat analysis also found that febuxostat was noninferior to allopurinol on this measure.
Urate levels were approximately 80 micromoles lower in the febuxostat group versus the allopurinol group each year of the study, Dr. MacDonald said.
At least one gout flare was experienced by 1,017 patients in the febuxostat group and by 1,044 participants in the allopurinol group. “However, there was no placebo group, so we don’t know the effectiveness of either of these agents at preventing flares” based on this research, he said.
Both the on-treatment and intention-to-treat (ITT) secondary analyses demonstrated the noninferiority of febuxostat, compared with allopurinol, for all-cause death, each individual component of the composite primary outcome – cardiovascular death, hospitalization for heart failure, and hospitalization for new, unstable, or worsening angina.
In contrast, the ITT analysis revealed a “nominally significant increase” in hospitalization for arrhythmia with no evidence of ischemia in the febuxostat group. The 0.583 events per 100 patient-years in this group versus 0.385 events in the allopurinol cohort generated an adjusted HR of 1.51 (95% CI, 1.05-2.17).
In terms of all-cause mortality, 222 participants (7.2%) in the febuxostat group died, compared with 263 people (8.6%) in the allopurinol group.
Adverse events and withdrawals
A total 1,720 participants (57.3%) in the febuxostat group experienced at least one serious adverse event, as did 1,812 participants (59.4%) in the allopurinol group. Less than 1% of serious adverse events in each group were considered treatment-related.
Dr. MacDonald said that 6.2% of the febuxostat patients and 5.5% of the allopurinol group withdrew from the study. “We had pretty good follow-up [94%],” Dr. MacDonald said. “I don’t want to criticize CARES, but 47% did drop out of that study, and they could not follow them anymore.”
Limitations of FAST include its open-label design and lack of a placebo group, although Dr. MacDonald pointed out that a placebo group would have been unethical. Strengths included its large randomized trial design and good external validity, he added. “This is what will happen in clinical practice if you switch people from allopurinol to febuxostat.”
When asked how he would treat people with gout now given the FAST findings, Dr. MacDonald said, “I’m not a rheumatologist, I’m a cardiovascular physician. But I would say from the evidence from the FAST trial, it appears to be safe to give patients febuxostat whether or not they have cardiovascular risk factors or prior cardiovascular disease.”
“The FAST study indicates that febuxostat is similar to allopurinol in terms of cardiovascular events during the treatment period. The strengths of this study are its large sample size, excellent follow-up rate, and the relatively long follow-up time,” session moderator Shervin Assassi, MD, said when asked for comment. Dr. Assassi, director of the division of rheumatology at the University of Texas Health Science Center at Houston, was not involved in the research.
Menarini, Ipsen, and Teijin Pharma funded the study. The University of Dundee receives research funds from Menarini. Dr. MacDonald disclosed that he received speaker or consultant fees from Menarini. Dr. Assassi had no relevant disclosures.
SOURCE: MacDonald T et al. Arthritis Rheumatol. 2020;72(suppl 10). ACR 2020, Abstract L08.
Febuxostat (Uloric) emerged as noninferior to allopurinol regarding risk of cardiovascular death among people 60 years and older with gout and at least one additional cardiovascular risk factor, results of the Febuxostat versus Allopurinol Streamlined Trial (FAST) suggest.
This primary outcome of the FAST trial stands in contrast to results of the CARES trial in 2018. The CARES researchers previously reported a 4.3% increased risk of cardiovascular death associated with febuxostat, compared with a 3.2% rate with allopurinol, a statistically significant 34% increase in the relative risk.
“In contrast to previous studies, there was no evidence of increased mortality with febuxostat, and we believe the regulators should review febuxostat licensing restrictions,” senior author Thomas MacDonald, MD, of the University of Dundee (Scotland), said during a late-breaking abstract session at the virtual annual meeting of the American College of Rheumatology.
The results of the FAST trial were simultaneously published online in The Lancet.
Both febuxostat and allopurinol treat gout by lowering urate levels. Concerns about the cardiovascular safety of febuxostat led to two post-licensing studies: the Cardiovascular Safety of Febuxostat and Allopurinol in Participants with Gout and Cardiovascular Comorbidities (CARES) study, mandated by the U.S. Food and Drug Administration, and FAST, requested by the European Medicines Agency. In February 2019, the FDA added a warning about elevated cardiovascular death and death risk associated with febuxostat.
“When CARES was published, it was somewhat of a threat to our study,” Dr. MacDonald said. “After hearing from our data-monitoring committee, we were told we could continue the trial.”
Some switched from allopurinol to febuxostat
So Dr. MacDonald, lead author Isla Mackenzie, MBChB, and their colleagues enrolled 6,128 people with gout in the United Kingdom, Sweden, and Denmark between December 2011 and January 2018. They followed patients for a median of 4 years. Participants had a mean age of 71 years, 85% were men, and 33% had a history of cardiovascular disease. The investigators excluded anyone with a stroke or myocardial infarction in the previous 6 months.
All participants were being treated with allopurinol. The investigators titrated those not at target up to an ideal dose that achieved a serum urate concentration of less than 0.357 mmol/L (< 6 mg/dL). Next, they randomly assigned 3,065 people to continue allopurinol and another 3,063 to switch to 80-120 mg of febuxostat.
The primary outcome of the multicenter, prospective, randomized, open-label FAST trial was a composite of hospitalization for nonfatal MI or biomarker positive for acute coronary syndrome, nonfatal stroke, or cardiovascular death.
Key findings
“There was definitely a noninferior primary outcome,” Dr. MacDonald said. In the on-treatment analysis, 172 patients in the febuxostat group reached the composite endpoint versus 241 patients in the allopurinol group. There were 1.72 events per 100 patient-years in the febuxostat group versus 2.05 events in the allopurinol group (adjusted hazard ratio, 0.85; 95% confidence interval, 0.70-1.03). An intent-to-treat analysis also found that febuxostat was noninferior to allopurinol on this measure.
Urate levels were approximately 80 micromoles lower in the febuxostat group versus the allopurinol group each year of the study, Dr. MacDonald said.
At least one gout flare was experienced by 1,017 patients in the febuxostat group and by 1,044 participants in the allopurinol group. “However, there was no placebo group, so we don’t know the effectiveness of either of these agents at preventing flares” based on this research, he said.
Both the on-treatment and intention-to-treat (ITT) secondary analyses demonstrated the noninferiority of febuxostat, compared with allopurinol, for all-cause death, each individual component of the composite primary outcome – cardiovascular death, hospitalization for heart failure, and hospitalization for new, unstable, or worsening angina.
In contrast, the ITT analysis revealed a “nominally significant increase” in hospitalization for arrhythmia with no evidence of ischemia in the febuxostat group. The 0.583 events per 100 patient-years in this group versus 0.385 events in the allopurinol cohort generated an adjusted HR of 1.51 (95% CI, 1.05-2.17).
In terms of all-cause mortality, 222 participants (7.2%) in the febuxostat group died, compared with 263 people (8.6%) in the allopurinol group.
Adverse events and withdrawals
A total 1,720 participants (57.3%) in the febuxostat group experienced at least one serious adverse event, as did 1,812 participants (59.4%) in the allopurinol group. Less than 1% of serious adverse events in each group were considered treatment-related.
Dr. MacDonald said that 6.2% of the febuxostat patients and 5.5% of the allopurinol group withdrew from the study. “We had pretty good follow-up [94%],” Dr. MacDonald said. “I don’t want to criticize CARES, but 47% did drop out of that study, and they could not follow them anymore.”
Limitations of FAST include its open-label design and lack of a placebo group, although Dr. MacDonald pointed out that a placebo group would have been unethical. Strengths included its large randomized trial design and good external validity, he added. “This is what will happen in clinical practice if you switch people from allopurinol to febuxostat.”
When asked how he would treat people with gout now given the FAST findings, Dr. MacDonald said, “I’m not a rheumatologist, I’m a cardiovascular physician. But I would say from the evidence from the FAST trial, it appears to be safe to give patients febuxostat whether or not they have cardiovascular risk factors or prior cardiovascular disease.”
“The FAST study indicates that febuxostat is similar to allopurinol in terms of cardiovascular events during the treatment period. The strengths of this study are its large sample size, excellent follow-up rate, and the relatively long follow-up time,” session moderator Shervin Assassi, MD, said when asked for comment. Dr. Assassi, director of the division of rheumatology at the University of Texas Health Science Center at Houston, was not involved in the research.
Menarini, Ipsen, and Teijin Pharma funded the study. The University of Dundee receives research funds from Menarini. Dr. MacDonald disclosed that he received speaker or consultant fees from Menarini. Dr. Assassi had no relevant disclosures.
SOURCE: MacDonald T et al. Arthritis Rheumatol. 2020;72(suppl 10). ACR 2020, Abstract L08.
Febuxostat (Uloric) emerged as noninferior to allopurinol regarding risk of cardiovascular death among people 60 years and older with gout and at least one additional cardiovascular risk factor, results of the Febuxostat versus Allopurinol Streamlined Trial (FAST) suggest.
This primary outcome of the FAST trial stands in contrast to results of the CARES trial in 2018. The CARES researchers previously reported a 4.3% increased risk of cardiovascular death associated with febuxostat, compared with a 3.2% rate with allopurinol, a statistically significant 34% increase in the relative risk.
“In contrast to previous studies, there was no evidence of increased mortality with febuxostat, and we believe the regulators should review febuxostat licensing restrictions,” senior author Thomas MacDonald, MD, of the University of Dundee (Scotland), said during a late-breaking abstract session at the virtual annual meeting of the American College of Rheumatology.
The results of the FAST trial were simultaneously published online in The Lancet.
Both febuxostat and allopurinol treat gout by lowering urate levels. Concerns about the cardiovascular safety of febuxostat led to two post-licensing studies: the Cardiovascular Safety of Febuxostat and Allopurinol in Participants with Gout and Cardiovascular Comorbidities (CARES) study, mandated by the U.S. Food and Drug Administration, and FAST, requested by the European Medicines Agency. In February 2019, the FDA added a warning about elevated cardiovascular death and death risk associated with febuxostat.
“When CARES was published, it was somewhat of a threat to our study,” Dr. MacDonald said. “After hearing from our data-monitoring committee, we were told we could continue the trial.”
Some switched from allopurinol to febuxostat
So Dr. MacDonald, lead author Isla Mackenzie, MBChB, and their colleagues enrolled 6,128 people with gout in the United Kingdom, Sweden, and Denmark between December 2011 and January 2018. They followed patients for a median of 4 years. Participants had a mean age of 71 years, 85% were men, and 33% had a history of cardiovascular disease. The investigators excluded anyone with a stroke or myocardial infarction in the previous 6 months.
All participants were being treated with allopurinol. The investigators titrated those not at target up to an ideal dose that achieved a serum urate concentration of less than 0.357 mmol/L (< 6 mg/dL). Next, they randomly assigned 3,065 people to continue allopurinol and another 3,063 to switch to 80-120 mg of febuxostat.
The primary outcome of the multicenter, prospective, randomized, open-label FAST trial was a composite of hospitalization for nonfatal MI or biomarker positive for acute coronary syndrome, nonfatal stroke, or cardiovascular death.
Key findings
“There was definitely a noninferior primary outcome,” Dr. MacDonald said. In the on-treatment analysis, 172 patients in the febuxostat group reached the composite endpoint versus 241 patients in the allopurinol group. There were 1.72 events per 100 patient-years in the febuxostat group versus 2.05 events in the allopurinol group (adjusted hazard ratio, 0.85; 95% confidence interval, 0.70-1.03). An intent-to-treat analysis also found that febuxostat was noninferior to allopurinol on this measure.
Urate levels were approximately 80 micromoles lower in the febuxostat group versus the allopurinol group each year of the study, Dr. MacDonald said.
At least one gout flare was experienced by 1,017 patients in the febuxostat group and by 1,044 participants in the allopurinol group. “However, there was no placebo group, so we don’t know the effectiveness of either of these agents at preventing flares” based on this research, he said.
Both the on-treatment and intention-to-treat (ITT) secondary analyses demonstrated the noninferiority of febuxostat, compared with allopurinol, for all-cause death, each individual component of the composite primary outcome – cardiovascular death, hospitalization for heart failure, and hospitalization for new, unstable, or worsening angina.
In contrast, the ITT analysis revealed a “nominally significant increase” in hospitalization for arrhythmia with no evidence of ischemia in the febuxostat group. The 0.583 events per 100 patient-years in this group versus 0.385 events in the allopurinol cohort generated an adjusted HR of 1.51 (95% CI, 1.05-2.17).
In terms of all-cause mortality, 222 participants (7.2%) in the febuxostat group died, compared with 263 people (8.6%) in the allopurinol group.
Adverse events and withdrawals
A total 1,720 participants (57.3%) in the febuxostat group experienced at least one serious adverse event, as did 1,812 participants (59.4%) in the allopurinol group. Less than 1% of serious adverse events in each group were considered treatment-related.
Dr. MacDonald said that 6.2% of the febuxostat patients and 5.5% of the allopurinol group withdrew from the study. “We had pretty good follow-up [94%],” Dr. MacDonald said. “I don’t want to criticize CARES, but 47% did drop out of that study, and they could not follow them anymore.”
Limitations of FAST include its open-label design and lack of a placebo group, although Dr. MacDonald pointed out that a placebo group would have been unethical. Strengths included its large randomized trial design and good external validity, he added. “This is what will happen in clinical practice if you switch people from allopurinol to febuxostat.”
When asked how he would treat people with gout now given the FAST findings, Dr. MacDonald said, “I’m not a rheumatologist, I’m a cardiovascular physician. But I would say from the evidence from the FAST trial, it appears to be safe to give patients febuxostat whether or not they have cardiovascular risk factors or prior cardiovascular disease.”
“The FAST study indicates that febuxostat is similar to allopurinol in terms of cardiovascular events during the treatment period. The strengths of this study are its large sample size, excellent follow-up rate, and the relatively long follow-up time,” session moderator Shervin Assassi, MD, said when asked for comment. Dr. Assassi, director of the division of rheumatology at the University of Texas Health Science Center at Houston, was not involved in the research.
Menarini, Ipsen, and Teijin Pharma funded the study. The University of Dundee receives research funds from Menarini. Dr. MacDonald disclosed that he received speaker or consultant fees from Menarini. Dr. Assassi had no relevant disclosures.
SOURCE: MacDonald T et al. Arthritis Rheumatol. 2020;72(suppl 10). ACR 2020, Abstract L08.
FROM ACR 2020
Denosumab favored over alendronate for BMD protection in glucocorticoid-induced osteoporosis
Denosumab boosted bone mineral density (BMD) over 12 months to a greater extent than did alendronate in a randomized, 12-month study. The investigator-initiated research compared BMD at the lumbar spine and elsewhere among people with systemic lupus erythematosus (SLE) and other autoimmune conditions. Long-term glucocorticoid therapy places some people in this group at higher risk for adverse effects of bone density loss.
“Glucocorticoids remain the mainstay of treatment of rheumatic diseases, but [they are] a major risk factor for osteoporosis and fracture,” study author Chi Chiu Mok, MD, said in an interview.
Compared with baseline, adults randomly assigned to denosumab had a 3.5% increase in lumbar spine BMD at 12 months, compared with 2.5% among those taking alendronate, a significant difference. Dr. Mok, a consultant and honorary associate professor in the department of medicine and nuclear medicine at Tuen Mun Hospital in Hong Kong, presented the study results at the virtual annual meeting of the American College of Rheumatology.
“Given the knowledge that denosumab is more effective than alendronate in raising spinal BMD in chronic users of GCs without increasing adverse events, this drug may be considered as an alternative first-line therapy in higher-risk patients and in those who are contraindicated for the oral bisphosphonates,” he said.
Cost considerations
Denosumab is a human monoclonal antibody administered as a subcutaneous injection, available under the brand names Prolia and Xgeva. Alendronate is an oral agent available as both generic and brand name formulations.
“Yes, denosumab is more expensive, more costly than oral alendronate, but our study shows efficacy is better for steroid users,” Dr. Mok said in answer to a question about cost disparity between the two agents during his presentation at the meeting. “For patients who are contraindicated or have low compliance for bisphosphonate, or are high-risk patients, I recommend first-line use of denosumab.”
Researchers previously studied these agents, including a smaller study by Dr. Mok and colleagues that showed a BMD benefit after switching people on an oral bisphosphonate to denosumab. However, he said, “There is a paucity of data regarding comparative efficacy of denosumab and the bisphosphonates in long-term steroid users.”
To explore any differences in a larger patient population, the investigators randomly assigned adults with SLE and other autoimmune conditions to the two treatments: denosumab 60 mg subcutaneoulsy every 6 months or oral alendronate 70 mg/week. All patients also received 3,000 mg calcium and 1,000 IU vitamin D3 (cholecalciferol) each day.
After three discontinuations in denosumab cohort and four in the alendronate group, the researchers evaluated 69 people taking denosumab and 70 others taking alendronate. The discontinuations were caused by noncompliance, Dr. Mok said, not by adverse events.
Adverse events were reported, but the rate did not differ significantly between groups. Dr. Mok highlighted some notable differences, including more minor infections and arthralgias reported in the denosumab cohort. Chest discomfort was reported in one denosumab recipient versus no patients in the alendronate group. Dyspepsia/upper GI symptoms and dizziness/vertigo occurred more often in the alendronate group.
Women were 96% of the study population, and mean age was 50 years. A majority, 81%, had underlying SLE. Other diagnoses included rheumatoid arthritis, myositis, antineutrophil cytoplasmic antibody–associated vasculitis, and polymyalgia rheumatica. The mean dose of prednisolone at study entry was 5.1 mg/day.
Key BMD and biomarker findings
BMD increased significantly in the spine, hip, and femoral neck in both treatment groups by 12 months. However, after adjustment for baseline BMD and covariates including age, menopause, and history of fracture, the gains in the denosumab group were significantly higher.
The increase in lumbar spine BMD at 12 months of 3.5% in the denosumab group versus 2.5% in the alendronate group was statistically significant (P = .045). Less significant was a 0.9% increase at the hip in the denosumab patients versus 1.6% in the alendronate group (P = .10), as well as femoral neck BMD gains of 1% in the denosumab group versus 1.5% in the alendronate group (P = .86).
Furthermore, “denosumab was more potent in suppressing the bone markers at 12 months,” Dr. Mok said.
Specifically, the percentage decrease in serum PINP (procollagen type I N-terminal propeptide) levels in the denosumab group was significantly greater than in the alendronate group (P = .001). Likewise, the decrease in CTX (C-terminal telopeptide of type I collagen) was significantly greater in the denosumab cohort versus the alendronate cohort (P < .001).
“Dr. Mok’s study was a well-controlled investigation. The superiority of denosumab was impressive, especially given the small group sizes of 69 and 70,” session comoderator Gregg Silverman, MD, professor in the department of internal medicine and the department of pathology at New York University, said when asked for comment.
“However, bone density measurements may not tell the whole story. These results support a bigger and much larger-scale study to confirm that rates of fracture on denosumab are also reduced.”
No new symptomatic fractures occurred in either group during the study. The investigators are evaluating for any new radiologic fractures, with results pending.
Dr. Mok said “results of our study in Asian patients are largely confirmatory” of a previous 2018 comparison study and a 2019 comparison study, each sponsored by Amgen.
A small sample size, short duration of treatment, and the open-label design were limitations of the study.
The trial was an investigator-initiated study. Dr. Mok and colleagues had no relevant financial disclosures. Dr. Silverman had no relevant financial disclosures.
SOURCE: Mok CC et al. Arthritis Rheumatol. 2020;72(suppl 10). ACR 2020, Abstract 1442.
Denosumab boosted bone mineral density (BMD) over 12 months to a greater extent than did alendronate in a randomized, 12-month study. The investigator-initiated research compared BMD at the lumbar spine and elsewhere among people with systemic lupus erythematosus (SLE) and other autoimmune conditions. Long-term glucocorticoid therapy places some people in this group at higher risk for adverse effects of bone density loss.
“Glucocorticoids remain the mainstay of treatment of rheumatic diseases, but [they are] a major risk factor for osteoporosis and fracture,” study author Chi Chiu Mok, MD, said in an interview.
Compared with baseline, adults randomly assigned to denosumab had a 3.5% increase in lumbar spine BMD at 12 months, compared with 2.5% among those taking alendronate, a significant difference. Dr. Mok, a consultant and honorary associate professor in the department of medicine and nuclear medicine at Tuen Mun Hospital in Hong Kong, presented the study results at the virtual annual meeting of the American College of Rheumatology.
“Given the knowledge that denosumab is more effective than alendronate in raising spinal BMD in chronic users of GCs without increasing adverse events, this drug may be considered as an alternative first-line therapy in higher-risk patients and in those who are contraindicated for the oral bisphosphonates,” he said.
Cost considerations
Denosumab is a human monoclonal antibody administered as a subcutaneous injection, available under the brand names Prolia and Xgeva. Alendronate is an oral agent available as both generic and brand name formulations.
“Yes, denosumab is more expensive, more costly than oral alendronate, but our study shows efficacy is better for steroid users,” Dr. Mok said in answer to a question about cost disparity between the two agents during his presentation at the meeting. “For patients who are contraindicated or have low compliance for bisphosphonate, or are high-risk patients, I recommend first-line use of denosumab.”
Researchers previously studied these agents, including a smaller study by Dr. Mok and colleagues that showed a BMD benefit after switching people on an oral bisphosphonate to denosumab. However, he said, “There is a paucity of data regarding comparative efficacy of denosumab and the bisphosphonates in long-term steroid users.”
To explore any differences in a larger patient population, the investigators randomly assigned adults with SLE and other autoimmune conditions to the two treatments: denosumab 60 mg subcutaneoulsy every 6 months or oral alendronate 70 mg/week. All patients also received 3,000 mg calcium and 1,000 IU vitamin D3 (cholecalciferol) each day.
After three discontinuations in denosumab cohort and four in the alendronate group, the researchers evaluated 69 people taking denosumab and 70 others taking alendronate. The discontinuations were caused by noncompliance, Dr. Mok said, not by adverse events.
Adverse events were reported, but the rate did not differ significantly between groups. Dr. Mok highlighted some notable differences, including more minor infections and arthralgias reported in the denosumab cohort. Chest discomfort was reported in one denosumab recipient versus no patients in the alendronate group. Dyspepsia/upper GI symptoms and dizziness/vertigo occurred more often in the alendronate group.
Women were 96% of the study population, and mean age was 50 years. A majority, 81%, had underlying SLE. Other diagnoses included rheumatoid arthritis, myositis, antineutrophil cytoplasmic antibody–associated vasculitis, and polymyalgia rheumatica. The mean dose of prednisolone at study entry was 5.1 mg/day.
Key BMD and biomarker findings
BMD increased significantly in the spine, hip, and femoral neck in both treatment groups by 12 months. However, after adjustment for baseline BMD and covariates including age, menopause, and history of fracture, the gains in the denosumab group were significantly higher.
The increase in lumbar spine BMD at 12 months of 3.5% in the denosumab group versus 2.5% in the alendronate group was statistically significant (P = .045). Less significant was a 0.9% increase at the hip in the denosumab patients versus 1.6% in the alendronate group (P = .10), as well as femoral neck BMD gains of 1% in the denosumab group versus 1.5% in the alendronate group (P = .86).
Furthermore, “denosumab was more potent in suppressing the bone markers at 12 months,” Dr. Mok said.
Specifically, the percentage decrease in serum PINP (procollagen type I N-terminal propeptide) levels in the denosumab group was significantly greater than in the alendronate group (P = .001). Likewise, the decrease in CTX (C-terminal telopeptide of type I collagen) was significantly greater in the denosumab cohort versus the alendronate cohort (P < .001).
“Dr. Mok’s study was a well-controlled investigation. The superiority of denosumab was impressive, especially given the small group sizes of 69 and 70,” session comoderator Gregg Silverman, MD, professor in the department of internal medicine and the department of pathology at New York University, said when asked for comment.
“However, bone density measurements may not tell the whole story. These results support a bigger and much larger-scale study to confirm that rates of fracture on denosumab are also reduced.”
No new symptomatic fractures occurred in either group during the study. The investigators are evaluating for any new radiologic fractures, with results pending.
Dr. Mok said “results of our study in Asian patients are largely confirmatory” of a previous 2018 comparison study and a 2019 comparison study, each sponsored by Amgen.
A small sample size, short duration of treatment, and the open-label design were limitations of the study.
The trial was an investigator-initiated study. Dr. Mok and colleagues had no relevant financial disclosures. Dr. Silverman had no relevant financial disclosures.
SOURCE: Mok CC et al. Arthritis Rheumatol. 2020;72(suppl 10). ACR 2020, Abstract 1442.
Denosumab boosted bone mineral density (BMD) over 12 months to a greater extent than did alendronate in a randomized, 12-month study. The investigator-initiated research compared BMD at the lumbar spine and elsewhere among people with systemic lupus erythematosus (SLE) and other autoimmune conditions. Long-term glucocorticoid therapy places some people in this group at higher risk for adverse effects of bone density loss.
“Glucocorticoids remain the mainstay of treatment of rheumatic diseases, but [they are] a major risk factor for osteoporosis and fracture,” study author Chi Chiu Mok, MD, said in an interview.
Compared with baseline, adults randomly assigned to denosumab had a 3.5% increase in lumbar spine BMD at 12 months, compared with 2.5% among those taking alendronate, a significant difference. Dr. Mok, a consultant and honorary associate professor in the department of medicine and nuclear medicine at Tuen Mun Hospital in Hong Kong, presented the study results at the virtual annual meeting of the American College of Rheumatology.
“Given the knowledge that denosumab is more effective than alendronate in raising spinal BMD in chronic users of GCs without increasing adverse events, this drug may be considered as an alternative first-line therapy in higher-risk patients and in those who are contraindicated for the oral bisphosphonates,” he said.
Cost considerations
Denosumab is a human monoclonal antibody administered as a subcutaneous injection, available under the brand names Prolia and Xgeva. Alendronate is an oral agent available as both generic and brand name formulations.
“Yes, denosumab is more expensive, more costly than oral alendronate, but our study shows efficacy is better for steroid users,” Dr. Mok said in answer to a question about cost disparity between the two agents during his presentation at the meeting. “For patients who are contraindicated or have low compliance for bisphosphonate, or are high-risk patients, I recommend first-line use of denosumab.”
Researchers previously studied these agents, including a smaller study by Dr. Mok and colleagues that showed a BMD benefit after switching people on an oral bisphosphonate to denosumab. However, he said, “There is a paucity of data regarding comparative efficacy of denosumab and the bisphosphonates in long-term steroid users.”
To explore any differences in a larger patient population, the investigators randomly assigned adults with SLE and other autoimmune conditions to the two treatments: denosumab 60 mg subcutaneoulsy every 6 months or oral alendronate 70 mg/week. All patients also received 3,000 mg calcium and 1,000 IU vitamin D3 (cholecalciferol) each day.
After three discontinuations in denosumab cohort and four in the alendronate group, the researchers evaluated 69 people taking denosumab and 70 others taking alendronate. The discontinuations were caused by noncompliance, Dr. Mok said, not by adverse events.
Adverse events were reported, but the rate did not differ significantly between groups. Dr. Mok highlighted some notable differences, including more minor infections and arthralgias reported in the denosumab cohort. Chest discomfort was reported in one denosumab recipient versus no patients in the alendronate group. Dyspepsia/upper GI symptoms and dizziness/vertigo occurred more often in the alendronate group.
Women were 96% of the study population, and mean age was 50 years. A majority, 81%, had underlying SLE. Other diagnoses included rheumatoid arthritis, myositis, antineutrophil cytoplasmic antibody–associated vasculitis, and polymyalgia rheumatica. The mean dose of prednisolone at study entry was 5.1 mg/day.
Key BMD and biomarker findings
BMD increased significantly in the spine, hip, and femoral neck in both treatment groups by 12 months. However, after adjustment for baseline BMD and covariates including age, menopause, and history of fracture, the gains in the denosumab group were significantly higher.
The increase in lumbar spine BMD at 12 months of 3.5% in the denosumab group versus 2.5% in the alendronate group was statistically significant (P = .045). Less significant was a 0.9% increase at the hip in the denosumab patients versus 1.6% in the alendronate group (P = .10), as well as femoral neck BMD gains of 1% in the denosumab group versus 1.5% in the alendronate group (P = .86).
Furthermore, “denosumab was more potent in suppressing the bone markers at 12 months,” Dr. Mok said.
Specifically, the percentage decrease in serum PINP (procollagen type I N-terminal propeptide) levels in the denosumab group was significantly greater than in the alendronate group (P = .001). Likewise, the decrease in CTX (C-terminal telopeptide of type I collagen) was significantly greater in the denosumab cohort versus the alendronate cohort (P < .001).
“Dr. Mok’s study was a well-controlled investigation. The superiority of denosumab was impressive, especially given the small group sizes of 69 and 70,” session comoderator Gregg Silverman, MD, professor in the department of internal medicine and the department of pathology at New York University, said when asked for comment.
“However, bone density measurements may not tell the whole story. These results support a bigger and much larger-scale study to confirm that rates of fracture on denosumab are also reduced.”
No new symptomatic fractures occurred in either group during the study. The investigators are evaluating for any new radiologic fractures, with results pending.
Dr. Mok said “results of our study in Asian patients are largely confirmatory” of a previous 2018 comparison study and a 2019 comparison study, each sponsored by Amgen.
A small sample size, short duration of treatment, and the open-label design were limitations of the study.
The trial was an investigator-initiated study. Dr. Mok and colleagues had no relevant financial disclosures. Dr. Silverman had no relevant financial disclosures.
SOURCE: Mok CC et al. Arthritis Rheumatol. 2020;72(suppl 10). ACR 2020, Abstract 1442.
FROM ACR 2020






