User login
TAVR for bicuspid aortic stenosis gets selective thumbs up
NEW ORLEANS – Results of the largest-ever analysis of TAVR in patients with bicuspid aortic stenosis indicate that key 30-day and 1-year outcomes are similar to those of propensity-matched TAVR patients with tricuspid disease, Raj R. Makkar, MD, said at the annual meeting of the American College of Cardiology.
“Select bicuspid anatomy is amenable to TAVR with current-generation, balloon-expandable TAVR technology with acceptable clinical outcomes. These data provide an argument for TAVR to be a reasonable alternative for bicuspid AS [aortic stenosis] patients who are at intermediate or high risk for surgical aortic valve replacement, which are the patients that are enrolled in this registry, and provide a sound basis to conduct a randomized clinical trial in young patients with bicuspid AS who are at low risk for surgery,” declared Dr. Makkar, director of interventional cardiology and the cardiac catheterization laboratory at Cedars-Sinai Medical Center in Los Angeles.
The landmark randomized trials of TAVR versus SAVR (surgical aortic valve replacement) that established TAVR as the preferred treatment for patients with severe aortic stenosis who are at high, intermediate, or low surgical risk systematically excluded patients with bicuspid AS, even though bicuspid anatomy is common, particularly in younger patients with AS.
Despite the absence of supportive randomized trial data, TAVR is being done for bicuspid AS. To learn how patients with bicuspid disease have fared, Dr. Makkar and coinvestigators analyzed the real-world Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy (STS/ACC TVT) Registry of all patients who underwent TAVR with the balloon-expandable Sapien 3 stent in the United States during 2015-2018. They compared outcomes in 2,691 patients with high or intermediate surgical risk who underwent TAVR for bicuspid AS to an equal number of patients who had TAVR for tricuspid disease, with the two groups being propensity-matched across 25 variables.
Key outcomes were reassuringly similar in the two groups. For example, 30-day and 1-year all-cause mortality rates were 2.6% and 10.8% in patients with bicuspid valves and similar, at 2.5% and 12.1%, in those with tricuspid AS. Paravalvular leak rates at 30 days and 1 year were similar in the two groups. The Kansas City Cardiomyopathy Questionnaire scores, reflecting quality of life, improved dramatically – by nearly 30 points – from pre-TAVR baseline in both groups. The proportion of patients who were New York Heart Association functional class III or IV improved from nearly 85% at baseline to about 8% at 30 days and 1 year, again with no significant difference between the bicuspid and tricuspid AS groups. And there were other benefits, too.
“Despite the concerns regarding optimal expansion of these valves in a bicuspid anatomy, what we observed here was a significant and similar reduction in mean gradients and increase in valve area, both in the bicuspid and tricuspid AS patients. So there was no impact of bicuspid anatomy as seen here in terms of valve hemodynamics,” according to the cardiologist.
Conversion from TAVR to open surgery was required in 0.9% of bicuspid and 0.4% of tricuspid AS patients. Rates of aortic dissection and need for aortic valve reintervention were similarly low in both groups.
The 30-day stroke rate was significantly higher in the bicuspid patients – 2.4% versus 1.6% – but by 1 year there was no significant between-group difference, with stroke rates of 3.4% in the bicuspid and 3.1% in the tricuspid TAVR patients.
“I’d like to point out that more than 75% of strokes occurred in the first 3 days. These are periprocedural strokes, and there was no difference in the time distribution of strokes between the bicuspid and tricuspid groups,” Dr. Makkar said.
These stroke data make a compelling case for the routine use of cerebral protection devices in patients undergoing TAVR, something which now occurs in less than 10% of cases nationally, he continued.
“I would argue that, based on these data, it would be wise for us to use cerebral protection devices, especially when we are doing TAVR in patients with bicuspid AS, because their valves tend to be more heavily calcified than is often the case in tricuspid AS,” Dr. Makkar said.
Discussant Mayra Guerrero, MD, of the Mayo Clinic in Rochester, Minn., took issue with Dr. Makkar’s comment regarding the need for a randomized trial of TAVR in bicuspid AS patients with low surgical risk.
“Do we really need a randomized trial when we see in real-world experience with more than 2,600 patients that the outcomes are fairly similar?” she asked.
Affirmative, Dr. Makkar responded, in light of the fact that the STS/ACC TVT Registry doesn’t include low–surgical risk, typically relatively young bicuspid AS TAVR patients.
“I would say that these data are reassuring and encouraging, but we must not get carried away. I think that would be the important message that I must give,” Dr. Makkar replied. “I think for patients who are high risk and who are intermediate risk, with STS scores of what they were here – 5 and more – I think it’s reasonable to consider them for TAVR based upon CT anatomy. For young patients, as I concluded, I think we must do a randomized clinical trial to definitely establish the safety and efficacy in these patients.”
Dr. Makkar reported receiving research grants from and serving as a consultant to Edwards Lifesciences, which supported the study, as well as Abbott, Medtronic, and Boston Scientific.
[email protected]
SOURCE: Makkar RR. ACC 19, 404-15. Late-breaking clinical trials
NEW ORLEANS – Results of the largest-ever analysis of TAVR in patients with bicuspid aortic stenosis indicate that key 30-day and 1-year outcomes are similar to those of propensity-matched TAVR patients with tricuspid disease, Raj R. Makkar, MD, said at the annual meeting of the American College of Cardiology.
“Select bicuspid anatomy is amenable to TAVR with current-generation, balloon-expandable TAVR technology with acceptable clinical outcomes. These data provide an argument for TAVR to be a reasonable alternative for bicuspid AS [aortic stenosis] patients who are at intermediate or high risk for surgical aortic valve replacement, which are the patients that are enrolled in this registry, and provide a sound basis to conduct a randomized clinical trial in young patients with bicuspid AS who are at low risk for surgery,” declared Dr. Makkar, director of interventional cardiology and the cardiac catheterization laboratory at Cedars-Sinai Medical Center in Los Angeles.
The landmark randomized trials of TAVR versus SAVR (surgical aortic valve replacement) that established TAVR as the preferred treatment for patients with severe aortic stenosis who are at high, intermediate, or low surgical risk systematically excluded patients with bicuspid AS, even though bicuspid anatomy is common, particularly in younger patients with AS.
Despite the absence of supportive randomized trial data, TAVR is being done for bicuspid AS. To learn how patients with bicuspid disease have fared, Dr. Makkar and coinvestigators analyzed the real-world Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy (STS/ACC TVT) Registry of all patients who underwent TAVR with the balloon-expandable Sapien 3 stent in the United States during 2015-2018. They compared outcomes in 2,691 patients with high or intermediate surgical risk who underwent TAVR for bicuspid AS to an equal number of patients who had TAVR for tricuspid disease, with the two groups being propensity-matched across 25 variables.
Key outcomes were reassuringly similar in the two groups. For example, 30-day and 1-year all-cause mortality rates were 2.6% and 10.8% in patients with bicuspid valves and similar, at 2.5% and 12.1%, in those with tricuspid AS. Paravalvular leak rates at 30 days and 1 year were similar in the two groups. The Kansas City Cardiomyopathy Questionnaire scores, reflecting quality of life, improved dramatically – by nearly 30 points – from pre-TAVR baseline in both groups. The proportion of patients who were New York Heart Association functional class III or IV improved from nearly 85% at baseline to about 8% at 30 days and 1 year, again with no significant difference between the bicuspid and tricuspid AS groups. And there were other benefits, too.
“Despite the concerns regarding optimal expansion of these valves in a bicuspid anatomy, what we observed here was a significant and similar reduction in mean gradients and increase in valve area, both in the bicuspid and tricuspid AS patients. So there was no impact of bicuspid anatomy as seen here in terms of valve hemodynamics,” according to the cardiologist.
Conversion from TAVR to open surgery was required in 0.9% of bicuspid and 0.4% of tricuspid AS patients. Rates of aortic dissection and need for aortic valve reintervention were similarly low in both groups.
The 30-day stroke rate was significantly higher in the bicuspid patients – 2.4% versus 1.6% – but by 1 year there was no significant between-group difference, with stroke rates of 3.4% in the bicuspid and 3.1% in the tricuspid TAVR patients.
“I’d like to point out that more than 75% of strokes occurred in the first 3 days. These are periprocedural strokes, and there was no difference in the time distribution of strokes between the bicuspid and tricuspid groups,” Dr. Makkar said.
These stroke data make a compelling case for the routine use of cerebral protection devices in patients undergoing TAVR, something which now occurs in less than 10% of cases nationally, he continued.
“I would argue that, based on these data, it would be wise for us to use cerebral protection devices, especially when we are doing TAVR in patients with bicuspid AS, because their valves tend to be more heavily calcified than is often the case in tricuspid AS,” Dr. Makkar said.
Discussant Mayra Guerrero, MD, of the Mayo Clinic in Rochester, Minn., took issue with Dr. Makkar’s comment regarding the need for a randomized trial of TAVR in bicuspid AS patients with low surgical risk.
“Do we really need a randomized trial when we see in real-world experience with more than 2,600 patients that the outcomes are fairly similar?” she asked.
Affirmative, Dr. Makkar responded, in light of the fact that the STS/ACC TVT Registry doesn’t include low–surgical risk, typically relatively young bicuspid AS TAVR patients.
“I would say that these data are reassuring and encouraging, but we must not get carried away. I think that would be the important message that I must give,” Dr. Makkar replied. “I think for patients who are high risk and who are intermediate risk, with STS scores of what they were here – 5 and more – I think it’s reasonable to consider them for TAVR based upon CT anatomy. For young patients, as I concluded, I think we must do a randomized clinical trial to definitely establish the safety and efficacy in these patients.”
Dr. Makkar reported receiving research grants from and serving as a consultant to Edwards Lifesciences, which supported the study, as well as Abbott, Medtronic, and Boston Scientific.
[email protected]
SOURCE: Makkar RR. ACC 19, 404-15. Late-breaking clinical trials
NEW ORLEANS – Results of the largest-ever analysis of TAVR in patients with bicuspid aortic stenosis indicate that key 30-day and 1-year outcomes are similar to those of propensity-matched TAVR patients with tricuspid disease, Raj R. Makkar, MD, said at the annual meeting of the American College of Cardiology.
“Select bicuspid anatomy is amenable to TAVR with current-generation, balloon-expandable TAVR technology with acceptable clinical outcomes. These data provide an argument for TAVR to be a reasonable alternative for bicuspid AS [aortic stenosis] patients who are at intermediate or high risk for surgical aortic valve replacement, which are the patients that are enrolled in this registry, and provide a sound basis to conduct a randomized clinical trial in young patients with bicuspid AS who are at low risk for surgery,” declared Dr. Makkar, director of interventional cardiology and the cardiac catheterization laboratory at Cedars-Sinai Medical Center in Los Angeles.
The landmark randomized trials of TAVR versus SAVR (surgical aortic valve replacement) that established TAVR as the preferred treatment for patients with severe aortic stenosis who are at high, intermediate, or low surgical risk systematically excluded patients with bicuspid AS, even though bicuspid anatomy is common, particularly in younger patients with AS.
Despite the absence of supportive randomized trial data, TAVR is being done for bicuspid AS. To learn how patients with bicuspid disease have fared, Dr. Makkar and coinvestigators analyzed the real-world Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy (STS/ACC TVT) Registry of all patients who underwent TAVR with the balloon-expandable Sapien 3 stent in the United States during 2015-2018. They compared outcomes in 2,691 patients with high or intermediate surgical risk who underwent TAVR for bicuspid AS to an equal number of patients who had TAVR for tricuspid disease, with the two groups being propensity-matched across 25 variables.
Key outcomes were reassuringly similar in the two groups. For example, 30-day and 1-year all-cause mortality rates were 2.6% and 10.8% in patients with bicuspid valves and similar, at 2.5% and 12.1%, in those with tricuspid AS. Paravalvular leak rates at 30 days and 1 year were similar in the two groups. The Kansas City Cardiomyopathy Questionnaire scores, reflecting quality of life, improved dramatically – by nearly 30 points – from pre-TAVR baseline in both groups. The proportion of patients who were New York Heart Association functional class III or IV improved from nearly 85% at baseline to about 8% at 30 days and 1 year, again with no significant difference between the bicuspid and tricuspid AS groups. And there were other benefits, too.
“Despite the concerns regarding optimal expansion of these valves in a bicuspid anatomy, what we observed here was a significant and similar reduction in mean gradients and increase in valve area, both in the bicuspid and tricuspid AS patients. So there was no impact of bicuspid anatomy as seen here in terms of valve hemodynamics,” according to the cardiologist.
Conversion from TAVR to open surgery was required in 0.9% of bicuspid and 0.4% of tricuspid AS patients. Rates of aortic dissection and need for aortic valve reintervention were similarly low in both groups.
The 30-day stroke rate was significantly higher in the bicuspid patients – 2.4% versus 1.6% – but by 1 year there was no significant between-group difference, with stroke rates of 3.4% in the bicuspid and 3.1% in the tricuspid TAVR patients.
“I’d like to point out that more than 75% of strokes occurred in the first 3 days. These are periprocedural strokes, and there was no difference in the time distribution of strokes between the bicuspid and tricuspid groups,” Dr. Makkar said.
These stroke data make a compelling case for the routine use of cerebral protection devices in patients undergoing TAVR, something which now occurs in less than 10% of cases nationally, he continued.
“I would argue that, based on these data, it would be wise for us to use cerebral protection devices, especially when we are doing TAVR in patients with bicuspid AS, because their valves tend to be more heavily calcified than is often the case in tricuspid AS,” Dr. Makkar said.
Discussant Mayra Guerrero, MD, of the Mayo Clinic in Rochester, Minn., took issue with Dr. Makkar’s comment regarding the need for a randomized trial of TAVR in bicuspid AS patients with low surgical risk.
“Do we really need a randomized trial when we see in real-world experience with more than 2,600 patients that the outcomes are fairly similar?” she asked.
Affirmative, Dr. Makkar responded, in light of the fact that the STS/ACC TVT Registry doesn’t include low–surgical risk, typically relatively young bicuspid AS TAVR patients.
“I would say that these data are reassuring and encouraging, but we must not get carried away. I think that would be the important message that I must give,” Dr. Makkar replied. “I think for patients who are high risk and who are intermediate risk, with STS scores of what they were here – 5 and more – I think it’s reasonable to consider them for TAVR based upon CT anatomy. For young patients, as I concluded, I think we must do a randomized clinical trial to definitely establish the safety and efficacy in these patients.”
Dr. Makkar reported receiving research grants from and serving as a consultant to Edwards Lifesciences, which supported the study, as well as Abbott, Medtronic, and Boston Scientific.
[email protected]
SOURCE: Makkar RR. ACC 19, 404-15. Late-breaking clinical trials
REPORTING FROM ACC 19
WISE sheds light on angina in INOCA
NEW ORLEANS – A higher baseline average coronary peak flow velocity is an independent predictor of angina in women with symptomatic ischemia and nonobstructive coronary artery disease (INOCA), according to a new report from the WISE-CVD study.
WISE-CVD (the Women’s Ischemia Syndrome Evaluation: Coronary Vascular Dysfunction) project is a National Institutes of Health–sponsored series of studies. WISE investigators have previously shown that higher baseline average peak flow velocity (BAPV) is correlated with volumetric flow and is an independent predictor of major adverse cardiovascular events. However, until now the relationship between BAPV and anginal symptoms hadn’t been investigated, Nissi S. Suppogu, MD, observed at the annual meeting of the American College of Cardiology.
She reported on 260 women with angiographically evaluated symptomatic INOCA who participated in WISE-CVD. They were divided into two groups based upon their BAPV: 123 had a BAPV of 22 cm/sec or more, and 137 had a BAPV of less than 22 cm/sec.
Women in the high BAPV group had more frequent angina as shown by their average score of 50 on that domain of the Seattle Angina Questionnaire, compared with 60 in the low-BAPV group. The high-BAPV group also had significantly worse angina-related quality of life as reflected in their lower score on that dimension of a related instrument, the Seattle Angina Questionnaire–7.
Further support for the notion that high-BAPV women with INOCA have more severe angina than those with low BAPV comes from the finding that they were significantly more likely to use nitrates (37.6% of them did so, compared with 22.6% of low-BAPV women) and ranolazine, or Ranexa (7.9% versus 1.7%). In addition, the high-BAPV patients had numerically greater usage of other antianginal agents – beta-blockers, calcium channel blockers, and ACE inhibitors or angiotensin receptor blockers – although these differences didn’t reach statistical significance, reported Dr. Suppogu of Cedars-Sinai Medical Center in Los Angeles.
She reported having no financial conflicts regarding her presentation.
NEW ORLEANS – A higher baseline average coronary peak flow velocity is an independent predictor of angina in women with symptomatic ischemia and nonobstructive coronary artery disease (INOCA), according to a new report from the WISE-CVD study.
WISE-CVD (the Women’s Ischemia Syndrome Evaluation: Coronary Vascular Dysfunction) project is a National Institutes of Health–sponsored series of studies. WISE investigators have previously shown that higher baseline average peak flow velocity (BAPV) is correlated with volumetric flow and is an independent predictor of major adverse cardiovascular events. However, until now the relationship between BAPV and anginal symptoms hadn’t been investigated, Nissi S. Suppogu, MD, observed at the annual meeting of the American College of Cardiology.
She reported on 260 women with angiographically evaluated symptomatic INOCA who participated in WISE-CVD. They were divided into two groups based upon their BAPV: 123 had a BAPV of 22 cm/sec or more, and 137 had a BAPV of less than 22 cm/sec.
Women in the high BAPV group had more frequent angina as shown by their average score of 50 on that domain of the Seattle Angina Questionnaire, compared with 60 in the low-BAPV group. The high-BAPV group also had significantly worse angina-related quality of life as reflected in their lower score on that dimension of a related instrument, the Seattle Angina Questionnaire–7.
Further support for the notion that high-BAPV women with INOCA have more severe angina than those with low BAPV comes from the finding that they were significantly more likely to use nitrates (37.6% of them did so, compared with 22.6% of low-BAPV women) and ranolazine, or Ranexa (7.9% versus 1.7%). In addition, the high-BAPV patients had numerically greater usage of other antianginal agents – beta-blockers, calcium channel blockers, and ACE inhibitors or angiotensin receptor blockers – although these differences didn’t reach statistical significance, reported Dr. Suppogu of Cedars-Sinai Medical Center in Los Angeles.
She reported having no financial conflicts regarding her presentation.
NEW ORLEANS – A higher baseline average coronary peak flow velocity is an independent predictor of angina in women with symptomatic ischemia and nonobstructive coronary artery disease (INOCA), according to a new report from the WISE-CVD study.
WISE-CVD (the Women’s Ischemia Syndrome Evaluation: Coronary Vascular Dysfunction) project is a National Institutes of Health–sponsored series of studies. WISE investigators have previously shown that higher baseline average peak flow velocity (BAPV) is correlated with volumetric flow and is an independent predictor of major adverse cardiovascular events. However, until now the relationship between BAPV and anginal symptoms hadn’t been investigated, Nissi S. Suppogu, MD, observed at the annual meeting of the American College of Cardiology.
She reported on 260 women with angiographically evaluated symptomatic INOCA who participated in WISE-CVD. They were divided into two groups based upon their BAPV: 123 had a BAPV of 22 cm/sec or more, and 137 had a BAPV of less than 22 cm/sec.
Women in the high BAPV group had more frequent angina as shown by their average score of 50 on that domain of the Seattle Angina Questionnaire, compared with 60 in the low-BAPV group. The high-BAPV group also had significantly worse angina-related quality of life as reflected in their lower score on that dimension of a related instrument, the Seattle Angina Questionnaire–7.
Further support for the notion that high-BAPV women with INOCA have more severe angina than those with low BAPV comes from the finding that they were significantly more likely to use nitrates (37.6% of them did so, compared with 22.6% of low-BAPV women) and ranolazine, or Ranexa (7.9% versus 1.7%). In addition, the high-BAPV patients had numerically greater usage of other antianginal agents – beta-blockers, calcium channel blockers, and ACE inhibitors or angiotensin receptor blockers – although these differences didn’t reach statistical significance, reported Dr. Suppogu of Cedars-Sinai Medical Center in Los Angeles.
She reported having no financial conflicts regarding her presentation.
REPORTING FROM ACC 19
SGLT2 inhibitors prevent HF hospitalization regardless of baseline LVEF
NEW ORLEANS – based on data from a large real-world patient registry.
“The observed beneficial effects of SGLT2 inhibitors on heart failure may extend across the range of baseline ejection fractions,” Mikhail Kosiborod, MD, observed at the annual meeting of the American College of Cardiology.
This is an important new insight. The major randomized cardiovascular outcome trials that showed lower risks of heart failure hospitalization and all-cause mortality in type 2 diabetic patients on an SGLT2 inhibitor, such as EMPA-REG OUTCOME for empagliflozin (Jardiance) and CANVAS for canagliflozin (Invokana), didn’t include information on baseline LVEF. So until now it has been unclear whether the beneficial effects of the SGLT2 inhibitors preventing heart failure hospitalization vary depending upon LVEF, explained Dr. Kosiborod, a cardiologist at Saint Luke’s Mid America Heart Institute in Kansas City, Mo.
He presented an analysis drawn from the patient database kept by Maccabi Healthcare Services in Israel. The study included 5,307 patients with type 2 diabetes and an LVEF measurement recorded in their chart at the time they started on either empagliflozin or dapagliflozin (Farxiga) and an equal number of propensity-matched type 2 diabetic controls who started on other glucose-lowering drugs, most commonly an oral dipeptidyl peptidase-4 inhibitor.
During roughly 16,000 person-years of follow-up, 239 deaths occurred. Compared with patients on another glucose-lowering drug, the risk of death from all causes was reduced by 47% among patients who were on an SGLT2 inhibitor and had a baseline LVEF of 50% or greater and by 62% among the 9% of subjects who had a baseline LVEF less than 50%.
Similarly, the risk of heart failure hospitalization was reduced by 29% in SGLT2 inhibitor users with a preserved LVEF and by 27% if they had a reduced LVEF.
For the composite endpoint of heart failure hospitalization or all-cause mortality, the risk reductions associated with SGLT2 inhibitor therapy were 45% with preserved and 39% with reduced LVEF.
Session comoderator Prakash C. Deedwania, MD, noted that there are ongoing major randomized trials of various SGLT2 inhibitors in patients with known heart failure, with cardiovascular death and heart failure hospitalization as primary endpoints. He asked Dr. Kosiborod whether, given that the results of these studies aren’t in yet, he thinks clinicians should be prescribing SGLT2 inhibitors to diabetic or prediabetic patients who don’t have clinical symptoms of heart failure but may have a marker of increased risk, such as an elevated B-type natriuretic peptide.
“At least in my mind, we have more than enough evidence at this point to say that SGLT2 inhibitors are effective in preventing heart failure,” Dr. Kosiborod replied.
“Obviously, if your risk for developing a condition is higher at baseline, then the absolute benefit that you’re going to get from using an agent that’s effective in preventing that event is going to be higher and the number needed to treat is going to be lower. So if you have a patient at high risk for heart failure by whatever risk predictor you’re using and the patient doesn’t yet have heart failure but does have diabetes, which is already a risk factor for heart failure, I think we have pretty solid data now that SGLT2 inhibitors will likely be effective in preventing heart failure in that kind of patient population. But I don’t think we have definitive data at this point to say that the drugs are effective in treating heart failure in people who already have a manifest clinical syndrome of heart failure, which is why we’re doing all these clinical trials now,” he continued.
Dr. Deedwania urged audience members to make the effort to become comfortable in prescribing SGLT2 inhibitors for their patients with type 2 diabetes.
“Many different surveys show that these drugs are not being utilized effectively by cardiologists,” noted Dr. Deedwania, professor of medicine at the University of California, San Francisco, and director of the heart failure program at the university’s Fresno campus.
“As cardiologists, we may not want to own diabetes, but we at least have to feel that we have the ownership of treating the diabetic patient with cardiovascular disease with appropriate drugs. We don’t need to depend on endocrinologists because if we do these patients may become lost,” he said.
Dr. Kosiborod concurred, citing evidence that diabetic patients with cardiovascular disease are much more likely to see a cardiologist than an endocrinologist in the course of usual care.
“There’s definitely a golden opportunity here to intervene to reduce risk,” he said.
Dr. Kosiborod reported serving as a consultant to roughly a dozen pharmaceutical companies.
SOURCE: Kosiborod M. ACC 19, Abstract #1024-07.
NEW ORLEANS – based on data from a large real-world patient registry.
“The observed beneficial effects of SGLT2 inhibitors on heart failure may extend across the range of baseline ejection fractions,” Mikhail Kosiborod, MD, observed at the annual meeting of the American College of Cardiology.
This is an important new insight. The major randomized cardiovascular outcome trials that showed lower risks of heart failure hospitalization and all-cause mortality in type 2 diabetic patients on an SGLT2 inhibitor, such as EMPA-REG OUTCOME for empagliflozin (Jardiance) and CANVAS for canagliflozin (Invokana), didn’t include information on baseline LVEF. So until now it has been unclear whether the beneficial effects of the SGLT2 inhibitors preventing heart failure hospitalization vary depending upon LVEF, explained Dr. Kosiborod, a cardiologist at Saint Luke’s Mid America Heart Institute in Kansas City, Mo.
He presented an analysis drawn from the patient database kept by Maccabi Healthcare Services in Israel. The study included 5,307 patients with type 2 diabetes and an LVEF measurement recorded in their chart at the time they started on either empagliflozin or dapagliflozin (Farxiga) and an equal number of propensity-matched type 2 diabetic controls who started on other glucose-lowering drugs, most commonly an oral dipeptidyl peptidase-4 inhibitor.
During roughly 16,000 person-years of follow-up, 239 deaths occurred. Compared with patients on another glucose-lowering drug, the risk of death from all causes was reduced by 47% among patients who were on an SGLT2 inhibitor and had a baseline LVEF of 50% or greater and by 62% among the 9% of subjects who had a baseline LVEF less than 50%.
Similarly, the risk of heart failure hospitalization was reduced by 29% in SGLT2 inhibitor users with a preserved LVEF and by 27% if they had a reduced LVEF.
For the composite endpoint of heart failure hospitalization or all-cause mortality, the risk reductions associated with SGLT2 inhibitor therapy were 45% with preserved and 39% with reduced LVEF.
Session comoderator Prakash C. Deedwania, MD, noted that there are ongoing major randomized trials of various SGLT2 inhibitors in patients with known heart failure, with cardiovascular death and heart failure hospitalization as primary endpoints. He asked Dr. Kosiborod whether, given that the results of these studies aren’t in yet, he thinks clinicians should be prescribing SGLT2 inhibitors to diabetic or prediabetic patients who don’t have clinical symptoms of heart failure but may have a marker of increased risk, such as an elevated B-type natriuretic peptide.
“At least in my mind, we have more than enough evidence at this point to say that SGLT2 inhibitors are effective in preventing heart failure,” Dr. Kosiborod replied.
“Obviously, if your risk for developing a condition is higher at baseline, then the absolute benefit that you’re going to get from using an agent that’s effective in preventing that event is going to be higher and the number needed to treat is going to be lower. So if you have a patient at high risk for heart failure by whatever risk predictor you’re using and the patient doesn’t yet have heart failure but does have diabetes, which is already a risk factor for heart failure, I think we have pretty solid data now that SGLT2 inhibitors will likely be effective in preventing heart failure in that kind of patient population. But I don’t think we have definitive data at this point to say that the drugs are effective in treating heart failure in people who already have a manifest clinical syndrome of heart failure, which is why we’re doing all these clinical trials now,” he continued.
Dr. Deedwania urged audience members to make the effort to become comfortable in prescribing SGLT2 inhibitors for their patients with type 2 diabetes.
“Many different surveys show that these drugs are not being utilized effectively by cardiologists,” noted Dr. Deedwania, professor of medicine at the University of California, San Francisco, and director of the heart failure program at the university’s Fresno campus.
“As cardiologists, we may not want to own diabetes, but we at least have to feel that we have the ownership of treating the diabetic patient with cardiovascular disease with appropriate drugs. We don’t need to depend on endocrinologists because if we do these patients may become lost,” he said.
Dr. Kosiborod concurred, citing evidence that diabetic patients with cardiovascular disease are much more likely to see a cardiologist than an endocrinologist in the course of usual care.
“There’s definitely a golden opportunity here to intervene to reduce risk,” he said.
Dr. Kosiborod reported serving as a consultant to roughly a dozen pharmaceutical companies.
SOURCE: Kosiborod M. ACC 19, Abstract #1024-07.
NEW ORLEANS – based on data from a large real-world patient registry.
“The observed beneficial effects of SGLT2 inhibitors on heart failure may extend across the range of baseline ejection fractions,” Mikhail Kosiborod, MD, observed at the annual meeting of the American College of Cardiology.
This is an important new insight. The major randomized cardiovascular outcome trials that showed lower risks of heart failure hospitalization and all-cause mortality in type 2 diabetic patients on an SGLT2 inhibitor, such as EMPA-REG OUTCOME for empagliflozin (Jardiance) and CANVAS for canagliflozin (Invokana), didn’t include information on baseline LVEF. So until now it has been unclear whether the beneficial effects of the SGLT2 inhibitors preventing heart failure hospitalization vary depending upon LVEF, explained Dr. Kosiborod, a cardiologist at Saint Luke’s Mid America Heart Institute in Kansas City, Mo.
He presented an analysis drawn from the patient database kept by Maccabi Healthcare Services in Israel. The study included 5,307 patients with type 2 diabetes and an LVEF measurement recorded in their chart at the time they started on either empagliflozin or dapagliflozin (Farxiga) and an equal number of propensity-matched type 2 diabetic controls who started on other glucose-lowering drugs, most commonly an oral dipeptidyl peptidase-4 inhibitor.
During roughly 16,000 person-years of follow-up, 239 deaths occurred. Compared with patients on another glucose-lowering drug, the risk of death from all causes was reduced by 47% among patients who were on an SGLT2 inhibitor and had a baseline LVEF of 50% or greater and by 62% among the 9% of subjects who had a baseline LVEF less than 50%.
Similarly, the risk of heart failure hospitalization was reduced by 29% in SGLT2 inhibitor users with a preserved LVEF and by 27% if they had a reduced LVEF.
For the composite endpoint of heart failure hospitalization or all-cause mortality, the risk reductions associated with SGLT2 inhibitor therapy were 45% with preserved and 39% with reduced LVEF.
Session comoderator Prakash C. Deedwania, MD, noted that there are ongoing major randomized trials of various SGLT2 inhibitors in patients with known heart failure, with cardiovascular death and heart failure hospitalization as primary endpoints. He asked Dr. Kosiborod whether, given that the results of these studies aren’t in yet, he thinks clinicians should be prescribing SGLT2 inhibitors to diabetic or prediabetic patients who don’t have clinical symptoms of heart failure but may have a marker of increased risk, such as an elevated B-type natriuretic peptide.
“At least in my mind, we have more than enough evidence at this point to say that SGLT2 inhibitors are effective in preventing heart failure,” Dr. Kosiborod replied.
“Obviously, if your risk for developing a condition is higher at baseline, then the absolute benefit that you’re going to get from using an agent that’s effective in preventing that event is going to be higher and the number needed to treat is going to be lower. So if you have a patient at high risk for heart failure by whatever risk predictor you’re using and the patient doesn’t yet have heart failure but does have diabetes, which is already a risk factor for heart failure, I think we have pretty solid data now that SGLT2 inhibitors will likely be effective in preventing heart failure in that kind of patient population. But I don’t think we have definitive data at this point to say that the drugs are effective in treating heart failure in people who already have a manifest clinical syndrome of heart failure, which is why we’re doing all these clinical trials now,” he continued.
Dr. Deedwania urged audience members to make the effort to become comfortable in prescribing SGLT2 inhibitors for their patients with type 2 diabetes.
“Many different surveys show that these drugs are not being utilized effectively by cardiologists,” noted Dr. Deedwania, professor of medicine at the University of California, San Francisco, and director of the heart failure program at the university’s Fresno campus.
“As cardiologists, we may not want to own diabetes, but we at least have to feel that we have the ownership of treating the diabetic patient with cardiovascular disease with appropriate drugs. We don’t need to depend on endocrinologists because if we do these patients may become lost,” he said.
Dr. Kosiborod concurred, citing evidence that diabetic patients with cardiovascular disease are much more likely to see a cardiologist than an endocrinologist in the course of usual care.
“There’s definitely a golden opportunity here to intervene to reduce risk,” he said.
Dr. Kosiborod reported serving as a consultant to roughly a dozen pharmaceutical companies.
SOURCE: Kosiborod M. ACC 19, Abstract #1024-07.
REPORTING FROM ACC 19
Unrecognized focal stenosis after angiographically successful PCI
Moreover, in 82% of DEFINE PCI participants with post-PCI residual ischemia as defined by instantaneous wave-free ratio (iFR) with pullback evaluation of the whole coronary vessel, the impaired physiology was due to an angiographically unrecognized focal stenosis that’s usually potentially treatable, Allen Jeremias, MD, observed in presenting the DEFINE PCI results at the annual meeting of the American College of Cardiology.
“We estimated that if all residual focal lesions could be treated with additional PCI, the rate of significant ischemia could theoretically be reduced from 24% to 5%, said Dr. Jeremias, director of the physiology core laboratory at the Cardiovascular Research Foundation in New York.
Post-PCI ischemia has been associated with recurrent angina and repeat PCI. The 24% prevalence of residual impaired physiology and ischemia despite a successful angiographic result may seem startlingly high to some, but it really shouldn’t be, according to Dr. Jeremias, who is also director of interventional cardiology research and associate director of the cardiac catheterization laboratory at St. Francis Hospital in Roslyn, N.Y.
“There are a lot of physiologic studies looking at FFR [fractional flow reserve] before PCI to determine if we should do it. And we learned how unreliable the angiogram is to make those decisions. So I think obviously we shouldn’t be surprised if the angiogram afterwards is just as unreliable,” he said.
DEFINE PCI was a prospective observational study of 500 patients who underwent PCI for stable or unstable angina at 27 U.S. and European sites. An iFR was done prior to PCI in all vessels with an angiographic lesion severity of 40% or more. Participating interventionalists performed angiographically guided PCI and confirmed their procedural success with post-PCI angiography before the patient left the cath lab. They also performed an iFR manual pullback interrogation of the entire treated vessel. Although the iFR data are linked to the angiographic images via a technology known as co-registration, the operators were blinded to the iFR results, which along with the angiograms were interpreted in a core laboratory. All patients received guideline-directed medical therapy.
The iFR improved on average from 0.69 pre-PCI to 0.93 post treatment. To put that in perspective, an iFR value of 0.89 or less defines hemodynamically significant ischemia.
Residual physiologic impairment post PCI was deemed due to a missed focal stenosis in 82% of cases and to diffuse atherosclerotic disease in the other 18%. Untreated focal lesions were located within the stent in 38% of cases, proximally in 31%, and distal to the stent in the remainder.
Dr. Jeremias said the investigators looked in vain for possible predictors of post-PCI residual impaired physiology. Post-PCI angiographic results were poorly correlated with iFR. For example, 30% of patients with a residual diameter stenosis of 50% or more had a post-PCI iFR of 0.89 or less, as did 21% of those with a residual diameter stenosis of less than 50%, a nonsignificant difference. Moreover, there were no procedural predictors of poor physiologic outcome.
“I don’t think that the answer is more angiograms or procedural changes guided by the angiogram, but rather guiding of the procedure with physiology and also intravascular imaging,” the cardiologist said.
Session cochair J. Dawn Abbott, MD, an interventional cardiologist at Brown University in Providence, R.I., said DEFINE PCI “really brings up the importance of co-registration of iFR, be it with optical coherence tomography or angiography, because we need the information together. If we can’t see these lesions on the angiogram, we need to be doing more complicated combined physiology and anatomy.”
“This is a very interesting study that I think generates a lot of provocative information,” said discussant John J. Warner, MD.
“It certainly challenges, once again, our definition of angiographic success.” The key remaining question is whether additional PCI addressing the residual focal stenoses causing ischemia will result in improved clinical outcomes, added Dr. Warner, an interventional cardiologist and CEO of the University of Texas Southwestern Medical Center Hospitals, Dallas.
Dr. Jeremias noted that DEFINE PCI participants are in the process of being followed through 12 months to see the impact of residual ischemia on recurrent angina, major adverse cardiovascular events, and quality of life. Moreover, a large randomized trial known as DEFINE GPS (Guided Physiologic Stenting) will soon get underway. Participants will be randomized to unblinded iFR-guided therapy with pullback in order to optimize the physiologic result or to conventional angiographically guided PCI. This trial will define the clinical value of PCI with iFR pullback and should answer the question of whether the more important iFR number is the magnitude of the iFR gain achieved via revascularization or the absolute iFR number achieved at the end.
DEFINE PCI and DEFINE GPS are funded by Volcano/Philips. Dr. Jeremias reported serving as a consultant to that company and a handful of others.
SOURCE: Jeremias A. ACC 19 Abstract 408-10.
Moreover, in 82% of DEFINE PCI participants with post-PCI residual ischemia as defined by instantaneous wave-free ratio (iFR) with pullback evaluation of the whole coronary vessel, the impaired physiology was due to an angiographically unrecognized focal stenosis that’s usually potentially treatable, Allen Jeremias, MD, observed in presenting the DEFINE PCI results at the annual meeting of the American College of Cardiology.
“We estimated that if all residual focal lesions could be treated with additional PCI, the rate of significant ischemia could theoretically be reduced from 24% to 5%, said Dr. Jeremias, director of the physiology core laboratory at the Cardiovascular Research Foundation in New York.
Post-PCI ischemia has been associated with recurrent angina and repeat PCI. The 24% prevalence of residual impaired physiology and ischemia despite a successful angiographic result may seem startlingly high to some, but it really shouldn’t be, according to Dr. Jeremias, who is also director of interventional cardiology research and associate director of the cardiac catheterization laboratory at St. Francis Hospital in Roslyn, N.Y.
“There are a lot of physiologic studies looking at FFR [fractional flow reserve] before PCI to determine if we should do it. And we learned how unreliable the angiogram is to make those decisions. So I think obviously we shouldn’t be surprised if the angiogram afterwards is just as unreliable,” he said.
DEFINE PCI was a prospective observational study of 500 patients who underwent PCI for stable or unstable angina at 27 U.S. and European sites. An iFR was done prior to PCI in all vessels with an angiographic lesion severity of 40% or more. Participating interventionalists performed angiographically guided PCI and confirmed their procedural success with post-PCI angiography before the patient left the cath lab. They also performed an iFR manual pullback interrogation of the entire treated vessel. Although the iFR data are linked to the angiographic images via a technology known as co-registration, the operators were blinded to the iFR results, which along with the angiograms were interpreted in a core laboratory. All patients received guideline-directed medical therapy.
The iFR improved on average from 0.69 pre-PCI to 0.93 post treatment. To put that in perspective, an iFR value of 0.89 or less defines hemodynamically significant ischemia.
Residual physiologic impairment post PCI was deemed due to a missed focal stenosis in 82% of cases and to diffuse atherosclerotic disease in the other 18%. Untreated focal lesions were located within the stent in 38% of cases, proximally in 31%, and distal to the stent in the remainder.
Dr. Jeremias said the investigators looked in vain for possible predictors of post-PCI residual impaired physiology. Post-PCI angiographic results were poorly correlated with iFR. For example, 30% of patients with a residual diameter stenosis of 50% or more had a post-PCI iFR of 0.89 or less, as did 21% of those with a residual diameter stenosis of less than 50%, a nonsignificant difference. Moreover, there were no procedural predictors of poor physiologic outcome.
“I don’t think that the answer is more angiograms or procedural changes guided by the angiogram, but rather guiding of the procedure with physiology and also intravascular imaging,” the cardiologist said.
Session cochair J. Dawn Abbott, MD, an interventional cardiologist at Brown University in Providence, R.I., said DEFINE PCI “really brings up the importance of co-registration of iFR, be it with optical coherence tomography or angiography, because we need the information together. If we can’t see these lesions on the angiogram, we need to be doing more complicated combined physiology and anatomy.”
“This is a very interesting study that I think generates a lot of provocative information,” said discussant John J. Warner, MD.
“It certainly challenges, once again, our definition of angiographic success.” The key remaining question is whether additional PCI addressing the residual focal stenoses causing ischemia will result in improved clinical outcomes, added Dr. Warner, an interventional cardiologist and CEO of the University of Texas Southwestern Medical Center Hospitals, Dallas.
Dr. Jeremias noted that DEFINE PCI participants are in the process of being followed through 12 months to see the impact of residual ischemia on recurrent angina, major adverse cardiovascular events, and quality of life. Moreover, a large randomized trial known as DEFINE GPS (Guided Physiologic Stenting) will soon get underway. Participants will be randomized to unblinded iFR-guided therapy with pullback in order to optimize the physiologic result or to conventional angiographically guided PCI. This trial will define the clinical value of PCI with iFR pullback and should answer the question of whether the more important iFR number is the magnitude of the iFR gain achieved via revascularization or the absolute iFR number achieved at the end.
DEFINE PCI and DEFINE GPS are funded by Volcano/Philips. Dr. Jeremias reported serving as a consultant to that company and a handful of others.
SOURCE: Jeremias A. ACC 19 Abstract 408-10.
Moreover, in 82% of DEFINE PCI participants with post-PCI residual ischemia as defined by instantaneous wave-free ratio (iFR) with pullback evaluation of the whole coronary vessel, the impaired physiology was due to an angiographically unrecognized focal stenosis that’s usually potentially treatable, Allen Jeremias, MD, observed in presenting the DEFINE PCI results at the annual meeting of the American College of Cardiology.
“We estimated that if all residual focal lesions could be treated with additional PCI, the rate of significant ischemia could theoretically be reduced from 24% to 5%, said Dr. Jeremias, director of the physiology core laboratory at the Cardiovascular Research Foundation in New York.
Post-PCI ischemia has been associated with recurrent angina and repeat PCI. The 24% prevalence of residual impaired physiology and ischemia despite a successful angiographic result may seem startlingly high to some, but it really shouldn’t be, according to Dr. Jeremias, who is also director of interventional cardiology research and associate director of the cardiac catheterization laboratory at St. Francis Hospital in Roslyn, N.Y.
“There are a lot of physiologic studies looking at FFR [fractional flow reserve] before PCI to determine if we should do it. And we learned how unreliable the angiogram is to make those decisions. So I think obviously we shouldn’t be surprised if the angiogram afterwards is just as unreliable,” he said.
DEFINE PCI was a prospective observational study of 500 patients who underwent PCI for stable or unstable angina at 27 U.S. and European sites. An iFR was done prior to PCI in all vessels with an angiographic lesion severity of 40% or more. Participating interventionalists performed angiographically guided PCI and confirmed their procedural success with post-PCI angiography before the patient left the cath lab. They also performed an iFR manual pullback interrogation of the entire treated vessel. Although the iFR data are linked to the angiographic images via a technology known as co-registration, the operators were blinded to the iFR results, which along with the angiograms were interpreted in a core laboratory. All patients received guideline-directed medical therapy.
The iFR improved on average from 0.69 pre-PCI to 0.93 post treatment. To put that in perspective, an iFR value of 0.89 or less defines hemodynamically significant ischemia.
Residual physiologic impairment post PCI was deemed due to a missed focal stenosis in 82% of cases and to diffuse atherosclerotic disease in the other 18%. Untreated focal lesions were located within the stent in 38% of cases, proximally in 31%, and distal to the stent in the remainder.
Dr. Jeremias said the investigators looked in vain for possible predictors of post-PCI residual impaired physiology. Post-PCI angiographic results were poorly correlated with iFR. For example, 30% of patients with a residual diameter stenosis of 50% or more had a post-PCI iFR of 0.89 or less, as did 21% of those with a residual diameter stenosis of less than 50%, a nonsignificant difference. Moreover, there were no procedural predictors of poor physiologic outcome.
“I don’t think that the answer is more angiograms or procedural changes guided by the angiogram, but rather guiding of the procedure with physiology and also intravascular imaging,” the cardiologist said.
Session cochair J. Dawn Abbott, MD, an interventional cardiologist at Brown University in Providence, R.I., said DEFINE PCI “really brings up the importance of co-registration of iFR, be it with optical coherence tomography or angiography, because we need the information together. If we can’t see these lesions on the angiogram, we need to be doing more complicated combined physiology and anatomy.”
“This is a very interesting study that I think generates a lot of provocative information,” said discussant John J. Warner, MD.
“It certainly challenges, once again, our definition of angiographic success.” The key remaining question is whether additional PCI addressing the residual focal stenoses causing ischemia will result in improved clinical outcomes, added Dr. Warner, an interventional cardiologist and CEO of the University of Texas Southwestern Medical Center Hospitals, Dallas.
Dr. Jeremias noted that DEFINE PCI participants are in the process of being followed through 12 months to see the impact of residual ischemia on recurrent angina, major adverse cardiovascular events, and quality of life. Moreover, a large randomized trial known as DEFINE GPS (Guided Physiologic Stenting) will soon get underway. Participants will be randomized to unblinded iFR-guided therapy with pullback in order to optimize the physiologic result or to conventional angiographically guided PCI. This trial will define the clinical value of PCI with iFR pullback and should answer the question of whether the more important iFR number is the magnitude of the iFR gain achieved via revascularization or the absolute iFR number achieved at the end.
DEFINE PCI and DEFINE GPS are funded by Volcano/Philips. Dr. Jeremias reported serving as a consultant to that company and a handful of others.
SOURCE: Jeremias A. ACC 19 Abstract 408-10.
REPORTING FROM ACC 19
Patch testing in atopic dermatitis: when and how
WAIKOLOA, HAWAII – The according to Jonathan I. Silverberg, MD, PhD.
“What are atopic dermatitis patients allergic to? It’s all coming from their personal care products and the things being used to treat their atopic dermatitis,” Dr. Silverberg said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Dr. Silverberg, of the department of dermatology at Northwestern University, Chicago, coauthored a systematic review and meta-analysis that examined the association between AD and contact sensitization. In their examination of 74 published studies, the investigators found that the likelihood of allergic contact dermatitis was 1.5-fold greater in adults and children with AD than in healthy individuals from the general population (J Am Acad Dermatol. 2017 Jul;77[1]:70-8).
This finding is at odds with an earlier widespread belief that AD patients should not be at increased risk because the immune profile of their primarily Th2-mediated disease would have a suppressant effect on Th1-mediated hypersensitivity.
“Recent data are calling into question old dogmas and reshaping the way we think about this. And this is not just an academic exercise, this is highly clinically relevant,” the dermatologist asserted.
The results of the meta-analysis prompted Dr. Silverberg and colleagues to conduct a retrospective study of more than 500 adults patch tested to an expanded allergen series at Northwestern’s patch test clinic with the purpose of identifying the common offending allergens in patients with AD. The key finding: The patients with AD were significantly more likely to have positive patch test reactions to ingredients in their repetitively used personal care products, topical corticosteroids, and topical antibiotics than the individuals without AD. The probable explanation for this results is that the skin barrier disruption inherent in AD allows for easier passage of weak allergens through the skin (J Am Acad Dermatol. 2018 Dec;79[6]:1028-33.e6).
Lanolin was identified as a particularly common allergen in the AD group. “Lanolin is found in one of the most commonly used moisturizers we recommend to patients: Aquaphor. It’s also found in tons of lip balms and emollients. Pretty much every soft soap out there contains lanolin, and it’s in a variety of other personal care products,” Dr. Silverberg noted.
Other common offenders in the AD population included fragrance mix II, cinnamal, quaternium-15, budesonide, tixocortol, carba mix, neomycin, bacitracin, rubber mix, and chlorhexidine. Relevance was established in more than 90% of the positive reactions.
“You can patch test them directly to their personal care products and make that connection beautifully and see how they’re reacting to them,” he said.
When to patch test atopic dermatitis patients
Dr. Silverberg was a coauthor of multidisciplinary expert consensus guidelines on when to consider patch testing in AD (Dermatitis. 2016 Jul-Aug;27[4]:186-92). “We had to go consensus because we don’t have nearly enough studies to provide true evidence-based recommendations,” he explained.
Because allergic contact dermatitis is a potentially curable comorbid condition in AD patients, it’s important to recognize the scenarios in which patch testing should be considered. These include AD refractory to topical therapy; adolescent- or adult-onset atopic dermatitis; and in AD patients with an atypical or evolving lesional distribution, such as localized dermatitis on the eyelids, head and neck, or hands and feet. Patch testing is also warranted before initiating systemic therapy for AD.
“If you’re about to put a patient on a biologic or phototherapy and step them up to a whole new class of risk of adverse events, that’s an ideal time to think about reversible options,” Dr. Silverberg advised.
Another situation in which he considers patch testing advisable, although this one isn’t covered in the consensus guidelines, is in AD patients with prominent nummular eczema lesions. “Widespread nummular eczema lesions may be a sign of allergic contact dermatitis in atopic dermatitis patients. I’m not saying everyone with nummular lesions is going to have a positive patch test, but it’s definitely a situation you want to think about,” he said.
How to patch test atopic dermatitis patients
Most of the common topical allergens in AD patients are not included in the T.R.U.E. Test. An expanded allergen series, such as the American Contact Dermatitis Society core 80 series, is the better way to go.
Once the dermatologist determines that a patient’s positive patch test reaction is relevant, it’s important to recommend the use of personal care products that are “pretty clean,” Dr. Silverberg said.
“Clean in my opinion is not a matter of ‘It should be all organic and all natural,’ ” he emphasized. “I’m not anti- any of that, but clean means having the fewest ingredients possible and trying to steer clear of those really common allergens that patients are highly likely to have been exposed to and potentially sensitized to over the many years of their tenure of atopic dermatitis.”
Dr. Silverberg reported receiving research grants from Galderma and GlaxoSmithKline and serving as a consultant to more than a dozen pharmaceutical companies.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – The according to Jonathan I. Silverberg, MD, PhD.
“What are atopic dermatitis patients allergic to? It’s all coming from their personal care products and the things being used to treat their atopic dermatitis,” Dr. Silverberg said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Dr. Silverberg, of the department of dermatology at Northwestern University, Chicago, coauthored a systematic review and meta-analysis that examined the association between AD and contact sensitization. In their examination of 74 published studies, the investigators found that the likelihood of allergic contact dermatitis was 1.5-fold greater in adults and children with AD than in healthy individuals from the general population (J Am Acad Dermatol. 2017 Jul;77[1]:70-8).
This finding is at odds with an earlier widespread belief that AD patients should not be at increased risk because the immune profile of their primarily Th2-mediated disease would have a suppressant effect on Th1-mediated hypersensitivity.
“Recent data are calling into question old dogmas and reshaping the way we think about this. And this is not just an academic exercise, this is highly clinically relevant,” the dermatologist asserted.
The results of the meta-analysis prompted Dr. Silverberg and colleagues to conduct a retrospective study of more than 500 adults patch tested to an expanded allergen series at Northwestern’s patch test clinic with the purpose of identifying the common offending allergens in patients with AD. The key finding: The patients with AD were significantly more likely to have positive patch test reactions to ingredients in their repetitively used personal care products, topical corticosteroids, and topical antibiotics than the individuals without AD. The probable explanation for this results is that the skin barrier disruption inherent in AD allows for easier passage of weak allergens through the skin (J Am Acad Dermatol. 2018 Dec;79[6]:1028-33.e6).
Lanolin was identified as a particularly common allergen in the AD group. “Lanolin is found in one of the most commonly used moisturizers we recommend to patients: Aquaphor. It’s also found in tons of lip balms and emollients. Pretty much every soft soap out there contains lanolin, and it’s in a variety of other personal care products,” Dr. Silverberg noted.
Other common offenders in the AD population included fragrance mix II, cinnamal, quaternium-15, budesonide, tixocortol, carba mix, neomycin, bacitracin, rubber mix, and chlorhexidine. Relevance was established in more than 90% of the positive reactions.
“You can patch test them directly to their personal care products and make that connection beautifully and see how they’re reacting to them,” he said.
When to patch test atopic dermatitis patients
Dr. Silverberg was a coauthor of multidisciplinary expert consensus guidelines on when to consider patch testing in AD (Dermatitis. 2016 Jul-Aug;27[4]:186-92). “We had to go consensus because we don’t have nearly enough studies to provide true evidence-based recommendations,” he explained.
Because allergic contact dermatitis is a potentially curable comorbid condition in AD patients, it’s important to recognize the scenarios in which patch testing should be considered. These include AD refractory to topical therapy; adolescent- or adult-onset atopic dermatitis; and in AD patients with an atypical or evolving lesional distribution, such as localized dermatitis on the eyelids, head and neck, or hands and feet. Patch testing is also warranted before initiating systemic therapy for AD.
“If you’re about to put a patient on a biologic or phototherapy and step them up to a whole new class of risk of adverse events, that’s an ideal time to think about reversible options,” Dr. Silverberg advised.
Another situation in which he considers patch testing advisable, although this one isn’t covered in the consensus guidelines, is in AD patients with prominent nummular eczema lesions. “Widespread nummular eczema lesions may be a sign of allergic contact dermatitis in atopic dermatitis patients. I’m not saying everyone with nummular lesions is going to have a positive patch test, but it’s definitely a situation you want to think about,” he said.
How to patch test atopic dermatitis patients
Most of the common topical allergens in AD patients are not included in the T.R.U.E. Test. An expanded allergen series, such as the American Contact Dermatitis Society core 80 series, is the better way to go.
Once the dermatologist determines that a patient’s positive patch test reaction is relevant, it’s important to recommend the use of personal care products that are “pretty clean,” Dr. Silverberg said.
“Clean in my opinion is not a matter of ‘It should be all organic and all natural,’ ” he emphasized. “I’m not anti- any of that, but clean means having the fewest ingredients possible and trying to steer clear of those really common allergens that patients are highly likely to have been exposed to and potentially sensitized to over the many years of their tenure of atopic dermatitis.”
Dr. Silverberg reported receiving research grants from Galderma and GlaxoSmithKline and serving as a consultant to more than a dozen pharmaceutical companies.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – The according to Jonathan I. Silverberg, MD, PhD.
“What are atopic dermatitis patients allergic to? It’s all coming from their personal care products and the things being used to treat their atopic dermatitis,” Dr. Silverberg said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Dr. Silverberg, of the department of dermatology at Northwestern University, Chicago, coauthored a systematic review and meta-analysis that examined the association between AD and contact sensitization. In their examination of 74 published studies, the investigators found that the likelihood of allergic contact dermatitis was 1.5-fold greater in adults and children with AD than in healthy individuals from the general population (J Am Acad Dermatol. 2017 Jul;77[1]:70-8).
This finding is at odds with an earlier widespread belief that AD patients should not be at increased risk because the immune profile of their primarily Th2-mediated disease would have a suppressant effect on Th1-mediated hypersensitivity.
“Recent data are calling into question old dogmas and reshaping the way we think about this. And this is not just an academic exercise, this is highly clinically relevant,” the dermatologist asserted.
The results of the meta-analysis prompted Dr. Silverberg and colleagues to conduct a retrospective study of more than 500 adults patch tested to an expanded allergen series at Northwestern’s patch test clinic with the purpose of identifying the common offending allergens in patients with AD. The key finding: The patients with AD were significantly more likely to have positive patch test reactions to ingredients in their repetitively used personal care products, topical corticosteroids, and topical antibiotics than the individuals without AD. The probable explanation for this results is that the skin barrier disruption inherent in AD allows for easier passage of weak allergens through the skin (J Am Acad Dermatol. 2018 Dec;79[6]:1028-33.e6).
Lanolin was identified as a particularly common allergen in the AD group. “Lanolin is found in one of the most commonly used moisturizers we recommend to patients: Aquaphor. It’s also found in tons of lip balms and emollients. Pretty much every soft soap out there contains lanolin, and it’s in a variety of other personal care products,” Dr. Silverberg noted.
Other common offenders in the AD population included fragrance mix II, cinnamal, quaternium-15, budesonide, tixocortol, carba mix, neomycin, bacitracin, rubber mix, and chlorhexidine. Relevance was established in more than 90% of the positive reactions.
“You can patch test them directly to their personal care products and make that connection beautifully and see how they’re reacting to them,” he said.
When to patch test atopic dermatitis patients
Dr. Silverberg was a coauthor of multidisciplinary expert consensus guidelines on when to consider patch testing in AD (Dermatitis. 2016 Jul-Aug;27[4]:186-92). “We had to go consensus because we don’t have nearly enough studies to provide true evidence-based recommendations,” he explained.
Because allergic contact dermatitis is a potentially curable comorbid condition in AD patients, it’s important to recognize the scenarios in which patch testing should be considered. These include AD refractory to topical therapy; adolescent- or adult-onset atopic dermatitis; and in AD patients with an atypical or evolving lesional distribution, such as localized dermatitis on the eyelids, head and neck, or hands and feet. Patch testing is also warranted before initiating systemic therapy for AD.
“If you’re about to put a patient on a biologic or phototherapy and step them up to a whole new class of risk of adverse events, that’s an ideal time to think about reversible options,” Dr. Silverberg advised.
Another situation in which he considers patch testing advisable, although this one isn’t covered in the consensus guidelines, is in AD patients with prominent nummular eczema lesions. “Widespread nummular eczema lesions may be a sign of allergic contact dermatitis in atopic dermatitis patients. I’m not saying everyone with nummular lesions is going to have a positive patch test, but it’s definitely a situation you want to think about,” he said.
How to patch test atopic dermatitis patients
Most of the common topical allergens in AD patients are not included in the T.R.U.E. Test. An expanded allergen series, such as the American Contact Dermatitis Society core 80 series, is the better way to go.
Once the dermatologist determines that a patient’s positive patch test reaction is relevant, it’s important to recommend the use of personal care products that are “pretty clean,” Dr. Silverberg said.
“Clean in my opinion is not a matter of ‘It should be all organic and all natural,’ ” he emphasized. “I’m not anti- any of that, but clean means having the fewest ingredients possible and trying to steer clear of those really common allergens that patients are highly likely to have been exposed to and potentially sensitized to over the many years of their tenure of atopic dermatitis.”
Dr. Silverberg reported receiving research grants from Galderma and GlaxoSmithKline and serving as a consultant to more than a dozen pharmaceutical companies.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Interosseous tendon inflammation is common prior to RA
MAUI, HAWAII – Inflammation of the hand interosseous tendons found on MRI is a novel target in efforts to preempt the development and progression of rheumatoid arthritis, Paul Emery, MD, said at the 2019 Rheumatology Winter Clinical Symposium.
He and his coinvestigators have previously shown there is a high prevalence of interosseous tendon inflammation in the hands of patients with established RA, but now they’ve demonstrated that this phenomenon also occurs in anti–cyclic citrullinated peptide (CCP)–positive individuals at increased risk for RA, even before onset of clinical synovitis.
This finding is consistent with the notion that, even though RA is classically considered a disease of the synovial joints, the joint involvement is a relatively late phenomenon in the disease development process and extracapsular structures may be important early targets of RA-related inflammation. Indeed, the MRI finding of tenosynovitis of the wrist and finger flexor tendons is known to be the strongest predictor of progression to arthritis in patients with recent-onset arthralgia or other musculoskeletal symptoms but no clinical synovitis, according to Dr. Emery, professor of rheumatology and director of the University of Leeds (England) Musculoskeletal Biomedical Research Center.
Because the interosseous muscles of the hands play a critical role in hand function – pianists and other musicians not infrequently present to rheumatologists with overuse injuries of the muscles and their tendons – Dr. Emery and his coworkers decided to take a comprehensive look at interosseous tendon inflammation across the full spectrum of RA and pre-RA. They conducted a retrospective study of clinical and hand MRI data on 93 CCP-positive patients who presented with new-onset musculoskeletal symptoms but no clinical synovitis; 47 patients with early RA, all of whom were disease-modifying antirheumatic drug–naive; 28 patients with late RA as defined by at least 1 year of symptoms, anti-CCP and/or rheumatoid factor positivity, a Disease Activity Score in 28 joints (DAS28) of 3.2 or more, plus a history of exposure to one or more DMARDs at the time of their hand imaging; and 20 healthy controls.
The key finding is that the proportion of subjects with MRI evidence of interosseous tendon inflammation rose along the advancing RA continuum. It was present in 19% of the CCP-positive patients without clinical synovitis; 49% of the DMARD-naive early RA group; 57% of the late RA group; and in none of the healthy controls. Moreover, the number of inflamed interosseous tendons per patient also increased with RA progression.
A total of 12% of 507 nontender metacarpophalangeal joints showed MRI evidence of interosseous tendon inflammation, as did 28% of 141 tender ones (Ann Rheum Dis. 2019 Mar 23. doi: 10.1136/annrheumdis-2018-214331).
As part of the study, Dr. Emery and coinvestigators performed cadaveric dissections that demonstrated that the interosseous tendons don’t possess a tendon sheath and don’t directly communicate with the joint capsule.
A prospective study is warranted in order to confirm the observed association between interosseous tendon inflammation and clinical and subclinical synovitis and to establish the predictive value of hand MRI as a harbinger of RA, he noted.
Dr. Emery reported having no financial conflicts regarding his presentation.
MAUI, HAWAII – Inflammation of the hand interosseous tendons found on MRI is a novel target in efforts to preempt the development and progression of rheumatoid arthritis, Paul Emery, MD, said at the 2019 Rheumatology Winter Clinical Symposium.
He and his coinvestigators have previously shown there is a high prevalence of interosseous tendon inflammation in the hands of patients with established RA, but now they’ve demonstrated that this phenomenon also occurs in anti–cyclic citrullinated peptide (CCP)–positive individuals at increased risk for RA, even before onset of clinical synovitis.
This finding is consistent with the notion that, even though RA is classically considered a disease of the synovial joints, the joint involvement is a relatively late phenomenon in the disease development process and extracapsular structures may be important early targets of RA-related inflammation. Indeed, the MRI finding of tenosynovitis of the wrist and finger flexor tendons is known to be the strongest predictor of progression to arthritis in patients with recent-onset arthralgia or other musculoskeletal symptoms but no clinical synovitis, according to Dr. Emery, professor of rheumatology and director of the University of Leeds (England) Musculoskeletal Biomedical Research Center.
Because the interosseous muscles of the hands play a critical role in hand function – pianists and other musicians not infrequently present to rheumatologists with overuse injuries of the muscles and their tendons – Dr. Emery and his coworkers decided to take a comprehensive look at interosseous tendon inflammation across the full spectrum of RA and pre-RA. They conducted a retrospective study of clinical and hand MRI data on 93 CCP-positive patients who presented with new-onset musculoskeletal symptoms but no clinical synovitis; 47 patients with early RA, all of whom were disease-modifying antirheumatic drug–naive; 28 patients with late RA as defined by at least 1 year of symptoms, anti-CCP and/or rheumatoid factor positivity, a Disease Activity Score in 28 joints (DAS28) of 3.2 or more, plus a history of exposure to one or more DMARDs at the time of their hand imaging; and 20 healthy controls.
The key finding is that the proportion of subjects with MRI evidence of interosseous tendon inflammation rose along the advancing RA continuum. It was present in 19% of the CCP-positive patients without clinical synovitis; 49% of the DMARD-naive early RA group; 57% of the late RA group; and in none of the healthy controls. Moreover, the number of inflamed interosseous tendons per patient also increased with RA progression.
A total of 12% of 507 nontender metacarpophalangeal joints showed MRI evidence of interosseous tendon inflammation, as did 28% of 141 tender ones (Ann Rheum Dis. 2019 Mar 23. doi: 10.1136/annrheumdis-2018-214331).
As part of the study, Dr. Emery and coinvestigators performed cadaveric dissections that demonstrated that the interosseous tendons don’t possess a tendon sheath and don’t directly communicate with the joint capsule.
A prospective study is warranted in order to confirm the observed association between interosseous tendon inflammation and clinical and subclinical synovitis and to establish the predictive value of hand MRI as a harbinger of RA, he noted.
Dr. Emery reported having no financial conflicts regarding his presentation.
MAUI, HAWAII – Inflammation of the hand interosseous tendons found on MRI is a novel target in efforts to preempt the development and progression of rheumatoid arthritis, Paul Emery, MD, said at the 2019 Rheumatology Winter Clinical Symposium.
He and his coinvestigators have previously shown there is a high prevalence of interosseous tendon inflammation in the hands of patients with established RA, but now they’ve demonstrated that this phenomenon also occurs in anti–cyclic citrullinated peptide (CCP)–positive individuals at increased risk for RA, even before onset of clinical synovitis.
This finding is consistent with the notion that, even though RA is classically considered a disease of the synovial joints, the joint involvement is a relatively late phenomenon in the disease development process and extracapsular structures may be important early targets of RA-related inflammation. Indeed, the MRI finding of tenosynovitis of the wrist and finger flexor tendons is known to be the strongest predictor of progression to arthritis in patients with recent-onset arthralgia or other musculoskeletal symptoms but no clinical synovitis, according to Dr. Emery, professor of rheumatology and director of the University of Leeds (England) Musculoskeletal Biomedical Research Center.
Because the interosseous muscles of the hands play a critical role in hand function – pianists and other musicians not infrequently present to rheumatologists with overuse injuries of the muscles and their tendons – Dr. Emery and his coworkers decided to take a comprehensive look at interosseous tendon inflammation across the full spectrum of RA and pre-RA. They conducted a retrospective study of clinical and hand MRI data on 93 CCP-positive patients who presented with new-onset musculoskeletal symptoms but no clinical synovitis; 47 patients with early RA, all of whom were disease-modifying antirheumatic drug–naive; 28 patients with late RA as defined by at least 1 year of symptoms, anti-CCP and/or rheumatoid factor positivity, a Disease Activity Score in 28 joints (DAS28) of 3.2 or more, plus a history of exposure to one or more DMARDs at the time of their hand imaging; and 20 healthy controls.
The key finding is that the proportion of subjects with MRI evidence of interosseous tendon inflammation rose along the advancing RA continuum. It was present in 19% of the CCP-positive patients without clinical synovitis; 49% of the DMARD-naive early RA group; 57% of the late RA group; and in none of the healthy controls. Moreover, the number of inflamed interosseous tendons per patient also increased with RA progression.
A total of 12% of 507 nontender metacarpophalangeal joints showed MRI evidence of interosseous tendon inflammation, as did 28% of 141 tender ones (Ann Rheum Dis. 2019 Mar 23. doi: 10.1136/annrheumdis-2018-214331).
As part of the study, Dr. Emery and coinvestigators performed cadaveric dissections that demonstrated that the interosseous tendons don’t possess a tendon sheath and don’t directly communicate with the joint capsule.
A prospective study is warranted in order to confirm the observed association between interosseous tendon inflammation and clinical and subclinical synovitis and to establish the predictive value of hand MRI as a harbinger of RA, he noted.
Dr. Emery reported having no financial conflicts regarding his presentation.
REPORTING FROM RWCS 2019
Weight loss improves psoriatic arthritis
MAUI, HAWAII – Serious weight loss brings big improvement in psoriatic arthritis in obese patients, at least short term, according to a Swedish, single-arm, prospective, proof-of-concept study.
A dose-response effect was evident: the greater the lost poundage, the bigger the improvement across multiple dimensions of psoriatic arthritis.
The short-term efficacy was eye-catching, especially in view of the well-recognized increased prevalence of obesity in psoriatic arthritis patients. But the jury is still out as to the long-term impact of this nonpharmacologic therapy, Eric M. Ruderman, MD, said at the 2019 Rheumatology Winter Clinical Symposium.
He has spoken with the Swedish investigators and was happy to learn they’re continuing to follow study participants long term.
“That’s going to be the key, right? Because if you do this for 12 weeks, like every other fad crash diet, and then you let the weight go right back on again, you haven’t really accomplished anything. I think the key will be what happens at a year,” according to Dr. Ruderman, professor of medicine and associate chief for clinical affairs in the division of rheumatology at Northwestern University, Chicago.
The study included 46 obese psoriatic arthritis patients who signed on for a structured, medically supervised very-low-energy diet lasting 12-16 weeks, depending upon their baseline obesity level. The commercially available liquid diet (Cambridge Weight Plan Limited) is a type of therapy widely prescribed by Swedish physicians, clocking in at a mere 640 kcal/day.
“I don’t know about you, but I ate that at breakfast this morning,” quipped symposium director Arthur Kavanaugh, MD, professor of medicine at the University of California, San Diego.
Following completion of the strict very-low-energy diet, patients were gradually reintroduced to a less-draconian, solid-food, energy-restricted diet, to be followed through the 12-month mark. The full 12-month protocol was supervised by staff in the obesity unit at Sahlgrenska University Hospital in Gothenburg, Sweden. The 12-month results will be presented at the annual European Congress of Rheumatology in Madrid.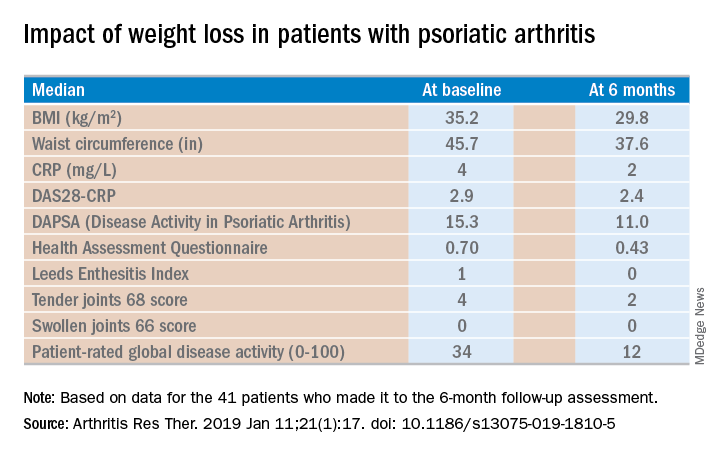
Of the 46 starters, 41 made it to the 6-month follow-up assessment. At that point they’d lost a median of 18.2 kg, or 18.6% of their baseline body weight. Their body mass index had dropped from an average of 35.2 to 29.8 kg/m2. And their psoriatic arthritis had improved significantly. For example, their median Disease Activity Score using 28 joint counts based upon C-reactive protein (DAS28-CRP) decreased from 2.9 at baseline to 2.4 at 6 months, with ACR 20, -50, and -70 responses of 51.2%, 34.1%, and 7.3% while disease-directed medications were held constant (Arthritis Res Ther. 2019 Jan 11;21[1]:17. doi: 10.1186/s13075-019-1810-5).
The investigators reported the very-low-energy diet phase was generally well tolerated. A total of 34 of the 41 patients deemed it “easier or much easier” than expected, prompting Dr. Ruderman to comment: “Because they thought it was going to be awful.”
Dr. Ruderman and Dr. Kavanaugh reported serving as consultants to numerous pharmaceutical companies.
MAUI, HAWAII – Serious weight loss brings big improvement in psoriatic arthritis in obese patients, at least short term, according to a Swedish, single-arm, prospective, proof-of-concept study.
A dose-response effect was evident: the greater the lost poundage, the bigger the improvement across multiple dimensions of psoriatic arthritis.
The short-term efficacy was eye-catching, especially in view of the well-recognized increased prevalence of obesity in psoriatic arthritis patients. But the jury is still out as to the long-term impact of this nonpharmacologic therapy, Eric M. Ruderman, MD, said at the 2019 Rheumatology Winter Clinical Symposium.
He has spoken with the Swedish investigators and was happy to learn they’re continuing to follow study participants long term.
“That’s going to be the key, right? Because if you do this for 12 weeks, like every other fad crash diet, and then you let the weight go right back on again, you haven’t really accomplished anything. I think the key will be what happens at a year,” according to Dr. Ruderman, professor of medicine and associate chief for clinical affairs in the division of rheumatology at Northwestern University, Chicago.
The study included 46 obese psoriatic arthritis patients who signed on for a structured, medically supervised very-low-energy diet lasting 12-16 weeks, depending upon their baseline obesity level. The commercially available liquid diet (Cambridge Weight Plan Limited) is a type of therapy widely prescribed by Swedish physicians, clocking in at a mere 640 kcal/day.
“I don’t know about you, but I ate that at breakfast this morning,” quipped symposium director Arthur Kavanaugh, MD, professor of medicine at the University of California, San Diego.
Following completion of the strict very-low-energy diet, patients were gradually reintroduced to a less-draconian, solid-food, energy-restricted diet, to be followed through the 12-month mark. The full 12-month protocol was supervised by staff in the obesity unit at Sahlgrenska University Hospital in Gothenburg, Sweden. The 12-month results will be presented at the annual European Congress of Rheumatology in Madrid.
Of the 46 starters, 41 made it to the 6-month follow-up assessment. At that point they’d lost a median of 18.2 kg, or 18.6% of their baseline body weight. Their body mass index had dropped from an average of 35.2 to 29.8 kg/m2. And their psoriatic arthritis had improved significantly. For example, their median Disease Activity Score using 28 joint counts based upon C-reactive protein (DAS28-CRP) decreased from 2.9 at baseline to 2.4 at 6 months, with ACR 20, -50, and -70 responses of 51.2%, 34.1%, and 7.3% while disease-directed medications were held constant (Arthritis Res Ther. 2019 Jan 11;21[1]:17. doi: 10.1186/s13075-019-1810-5).
The investigators reported the very-low-energy diet phase was generally well tolerated. A total of 34 of the 41 patients deemed it “easier or much easier” than expected, prompting Dr. Ruderman to comment: “Because they thought it was going to be awful.”
Dr. Ruderman and Dr. Kavanaugh reported serving as consultants to numerous pharmaceutical companies.
MAUI, HAWAII – Serious weight loss brings big improvement in psoriatic arthritis in obese patients, at least short term, according to a Swedish, single-arm, prospective, proof-of-concept study.
A dose-response effect was evident: the greater the lost poundage, the bigger the improvement across multiple dimensions of psoriatic arthritis.
The short-term efficacy was eye-catching, especially in view of the well-recognized increased prevalence of obesity in psoriatic arthritis patients. But the jury is still out as to the long-term impact of this nonpharmacologic therapy, Eric M. Ruderman, MD, said at the 2019 Rheumatology Winter Clinical Symposium.
He has spoken with the Swedish investigators and was happy to learn they’re continuing to follow study participants long term.
“That’s going to be the key, right? Because if you do this for 12 weeks, like every other fad crash diet, and then you let the weight go right back on again, you haven’t really accomplished anything. I think the key will be what happens at a year,” according to Dr. Ruderman, professor of medicine and associate chief for clinical affairs in the division of rheumatology at Northwestern University, Chicago.
The study included 46 obese psoriatic arthritis patients who signed on for a structured, medically supervised very-low-energy diet lasting 12-16 weeks, depending upon their baseline obesity level. The commercially available liquid diet (Cambridge Weight Plan Limited) is a type of therapy widely prescribed by Swedish physicians, clocking in at a mere 640 kcal/day.
“I don’t know about you, but I ate that at breakfast this morning,” quipped symposium director Arthur Kavanaugh, MD, professor of medicine at the University of California, San Diego.
Following completion of the strict very-low-energy diet, patients were gradually reintroduced to a less-draconian, solid-food, energy-restricted diet, to be followed through the 12-month mark. The full 12-month protocol was supervised by staff in the obesity unit at Sahlgrenska University Hospital in Gothenburg, Sweden. The 12-month results will be presented at the annual European Congress of Rheumatology in Madrid.
Of the 46 starters, 41 made it to the 6-month follow-up assessment. At that point they’d lost a median of 18.2 kg, or 18.6% of their baseline body weight. Their body mass index had dropped from an average of 35.2 to 29.8 kg/m2. And their psoriatic arthritis had improved significantly. For example, their median Disease Activity Score using 28 joint counts based upon C-reactive protein (DAS28-CRP) decreased from 2.9 at baseline to 2.4 at 6 months, with ACR 20, -50, and -70 responses of 51.2%, 34.1%, and 7.3% while disease-directed medications were held constant (Arthritis Res Ther. 2019 Jan 11;21[1]:17. doi: 10.1186/s13075-019-1810-5).
The investigators reported the very-low-energy diet phase was generally well tolerated. A total of 34 of the 41 patients deemed it “easier or much easier” than expected, prompting Dr. Ruderman to comment: “Because they thought it was going to be awful.”
Dr. Ruderman and Dr. Kavanaugh reported serving as consultants to numerous pharmaceutical companies.
REPORTING FROM RWCS 2019
Methotrexate pneumonitis called ‘super rare’
MAUI, HAWAII – The incidence of methotrexate pneumonitis has been reported as ranging from 3.5% to 7.6% among patients taking the disease-modifying antirheumatic drug. It’s an estimate that Aryeh Fischer, MD, counters with a one-word response: “Nonsense!”
“There’s just no way that methotrexate is causing that much lung disease,” he declared at the 2019 Rheumatology Winter Clinical Symposium.
Dr. Fischer, a rheumatologist with joint appointments to the divisions of rheumatology and pulmonary sciences and critical care medicine at the University of Colorado at Denver, Aurora, noted that his opinion is considered controversial in the pulmonology world.
“I’m not allowed to talk about methotrexate at lung conferences. They stop you at the gate. They’re convinced in lung circles that methotrexate is the worst drug known to mankind,” he said.
“My take home on methotrexate lung toxicity is this: I would just say, yes, it can occur, but it’s super rare and most often we’re not really sure that it was methotrexate pneumonitis. The diagnosis is not definitive, it’s exclusionary. We know that patients with interstitial lung disease of all types get acute exacerbations, and in idiopathic pulmonary fibrosis it’s actually the leading cause of mortality,” the rheumatologist said.
He highlighted a meta-analysis of 22 randomized, double-blind clinical trials published in 1990-2013 of methotrexate versus placebo or active comparators in 8,584 RA patients. The Irish investigators of that meta-analysis found that methotrexate was associated with a small albeit statistically significant 10% increase in the risk of all adverse respiratory events and an 11% increase in the risk of respiratory infection. However, patients on methotrexate were not at increased risk of mortality because of lung disease. And not a single case of methotrexate pneumonitis was reported after 2002 (Arthritis Rheumatol. 2014 Apr;66[4]:803-12).
Methotrexate pneumonitis is not dose dependent, nor is it related to treatment duration.
“Just because your patient has been on methotrexate for years does not mean they won’t get methotrexate lung toxicity,” he cautioned. “But this is not a chronic fibrotic interstitial lung disease, this is an acute onset of peripheral infiltrates and ground glass opacifications on chest imaging.”
Bronchoalveolar lavage classically shows a hypersensitivity pneumonitis with lymphocytosis. Transbronchial or surgical lung biopsy may show an organizing pneumonia or airway-based nonnecrotizing granulomas, again indicative of a hypersensitivity reaction.
Because the diagnostic picture is so often cloudy, Dr. Fischer generally tries to avoid methotrexate in patients with moderate or severe interstitial lung disease. “I have the luxury of avoiding it because we have so many great arthritis drugs these days,” he noted.
“That being said, the notion that we’re going to stop methotrexate in an 80-year-old who’s been on it for years and has mild bibasilar fibrotic interstitial lung disease so that her lung doc can sleep better at night is not very helpful for our patients. If the patient is doing well on methotrexate and the interstitial lung disease is mild, I continue [the methotrexate],” Dr. Fischer said.
He reported receiving research grants from Boehringer Ingelheim and Corbus Pharmaceuticals and serving as a consultant to Boehringer Ingelheim and other pharmaceutical companies.
MAUI, HAWAII – The incidence of methotrexate pneumonitis has been reported as ranging from 3.5% to 7.6% among patients taking the disease-modifying antirheumatic drug. It’s an estimate that Aryeh Fischer, MD, counters with a one-word response: “Nonsense!”
“There’s just no way that methotrexate is causing that much lung disease,” he declared at the 2019 Rheumatology Winter Clinical Symposium.
Dr. Fischer, a rheumatologist with joint appointments to the divisions of rheumatology and pulmonary sciences and critical care medicine at the University of Colorado at Denver, Aurora, noted that his opinion is considered controversial in the pulmonology world.
“I’m not allowed to talk about methotrexate at lung conferences. They stop you at the gate. They’re convinced in lung circles that methotrexate is the worst drug known to mankind,” he said.
“My take home on methotrexate lung toxicity is this: I would just say, yes, it can occur, but it’s super rare and most often we’re not really sure that it was methotrexate pneumonitis. The diagnosis is not definitive, it’s exclusionary. We know that patients with interstitial lung disease of all types get acute exacerbations, and in idiopathic pulmonary fibrosis it’s actually the leading cause of mortality,” the rheumatologist said.
He highlighted a meta-analysis of 22 randomized, double-blind clinical trials published in 1990-2013 of methotrexate versus placebo or active comparators in 8,584 RA patients. The Irish investigators of that meta-analysis found that methotrexate was associated with a small albeit statistically significant 10% increase in the risk of all adverse respiratory events and an 11% increase in the risk of respiratory infection. However, patients on methotrexate were not at increased risk of mortality because of lung disease. And not a single case of methotrexate pneumonitis was reported after 2002 (Arthritis Rheumatol. 2014 Apr;66[4]:803-12).
Methotrexate pneumonitis is not dose dependent, nor is it related to treatment duration.
“Just because your patient has been on methotrexate for years does not mean they won’t get methotrexate lung toxicity,” he cautioned. “But this is not a chronic fibrotic interstitial lung disease, this is an acute onset of peripheral infiltrates and ground glass opacifications on chest imaging.”
Bronchoalveolar lavage classically shows a hypersensitivity pneumonitis with lymphocytosis. Transbronchial or surgical lung biopsy may show an organizing pneumonia or airway-based nonnecrotizing granulomas, again indicative of a hypersensitivity reaction.
Because the diagnostic picture is so often cloudy, Dr. Fischer generally tries to avoid methotrexate in patients with moderate or severe interstitial lung disease. “I have the luxury of avoiding it because we have so many great arthritis drugs these days,” he noted.
“That being said, the notion that we’re going to stop methotrexate in an 80-year-old who’s been on it for years and has mild bibasilar fibrotic interstitial lung disease so that her lung doc can sleep better at night is not very helpful for our patients. If the patient is doing well on methotrexate and the interstitial lung disease is mild, I continue [the methotrexate],” Dr. Fischer said.
He reported receiving research grants from Boehringer Ingelheim and Corbus Pharmaceuticals and serving as a consultant to Boehringer Ingelheim and other pharmaceutical companies.
MAUI, HAWAII – The incidence of methotrexate pneumonitis has been reported as ranging from 3.5% to 7.6% among patients taking the disease-modifying antirheumatic drug. It’s an estimate that Aryeh Fischer, MD, counters with a one-word response: “Nonsense!”
“There’s just no way that methotrexate is causing that much lung disease,” he declared at the 2019 Rheumatology Winter Clinical Symposium.
Dr. Fischer, a rheumatologist with joint appointments to the divisions of rheumatology and pulmonary sciences and critical care medicine at the University of Colorado at Denver, Aurora, noted that his opinion is considered controversial in the pulmonology world.
“I’m not allowed to talk about methotrexate at lung conferences. They stop you at the gate. They’re convinced in lung circles that methotrexate is the worst drug known to mankind,” he said.
“My take home on methotrexate lung toxicity is this: I would just say, yes, it can occur, but it’s super rare and most often we’re not really sure that it was methotrexate pneumonitis. The diagnosis is not definitive, it’s exclusionary. We know that patients with interstitial lung disease of all types get acute exacerbations, and in idiopathic pulmonary fibrosis it’s actually the leading cause of mortality,” the rheumatologist said.
He highlighted a meta-analysis of 22 randomized, double-blind clinical trials published in 1990-2013 of methotrexate versus placebo or active comparators in 8,584 RA patients. The Irish investigators of that meta-analysis found that methotrexate was associated with a small albeit statistically significant 10% increase in the risk of all adverse respiratory events and an 11% increase in the risk of respiratory infection. However, patients on methotrexate were not at increased risk of mortality because of lung disease. And not a single case of methotrexate pneumonitis was reported after 2002 (Arthritis Rheumatol. 2014 Apr;66[4]:803-12).
Methotrexate pneumonitis is not dose dependent, nor is it related to treatment duration.
“Just because your patient has been on methotrexate for years does not mean they won’t get methotrexate lung toxicity,” he cautioned. “But this is not a chronic fibrotic interstitial lung disease, this is an acute onset of peripheral infiltrates and ground glass opacifications on chest imaging.”
Bronchoalveolar lavage classically shows a hypersensitivity pneumonitis with lymphocytosis. Transbronchial or surgical lung biopsy may show an organizing pneumonia or airway-based nonnecrotizing granulomas, again indicative of a hypersensitivity reaction.
Because the diagnostic picture is so often cloudy, Dr. Fischer generally tries to avoid methotrexate in patients with moderate or severe interstitial lung disease. “I have the luxury of avoiding it because we have so many great arthritis drugs these days,” he noted.
“That being said, the notion that we’re going to stop methotrexate in an 80-year-old who’s been on it for years and has mild bibasilar fibrotic interstitial lung disease so that her lung doc can sleep better at night is not very helpful for our patients. If the patient is doing well on methotrexate and the interstitial lung disease is mild, I continue [the methotrexate],” Dr. Fischer said.
He reported receiving research grants from Boehringer Ingelheim and Corbus Pharmaceuticals and serving as a consultant to Boehringer Ingelheim and other pharmaceutical companies.
EXPERT ANALYSIS FROM RWCS 2019
Surprise! MTX proves effective in psoriatic arthritis
MAUI, HAWAII – The first-ever, double-blind, randomized, controlled clinical trial evidence demonstrating that methotrexate indeed has therapeutic efficacy in psoriatic arthritis has come at an awkward time – on the heels of a basically negative Cochrane Collaboration systematic review as well as the latest American College of Rheumatology/National Psoriasis Foundation guidelines for treatment of psoriatic arthritis, which recommend anti–tumor necrosis factor therapy as first line, ahead of methotrexate.
The timing of the release of the SEAM-PsA randomized trial results was such that neither the Cochrane group nor the ACR/NPF guideline committee was able to consider the new, potentially game-changing study findings.
“I look at SEAM-PsA and have to say, methotrexate does seem to be an effective therapy. I think it calls into question the new guidelines, which were developed before the data were out. Now you look at this and have to ask, can you really say you should use a TNF inhibitor before methotrexate based on these results? I don’t know,” Eric M. Ruderman, MD, said at the 2019 Rheumatology Winter Clinical Symposium.
He also shared other problems he has with the new guidelines, which he considers seriously flawed.
The Cochrane Collaboration Systematic Review
The Cochrane group cast a net for all randomized, controlled clinical trials of methotrexate versus placebo or another disease-modifying antirheumatic drug (DMARD). They found eight, which they judged to be of poor quality. Their conclusion: “Low-quality evidence suggests that low-dose (15 mg or less) oral methotrexate might be slightly more effective than placebo when taken for 6 months; however, we are uncertain if it is more harmful” (Cochrane Database Syst Rev. 2019 Jan 18;1:CD012722. doi: 10.1002/14651858.CD012722.pub2).
“The new Cochrane Review concludes methotrexate doesn’t seem to work that well,” observed symposium director Arthur Kavanaugh, MD, professor of medicine at the University of California, San Diego.
“That’s because it’s based on published data, and there’s been very little of that,” said Dr. Ruderman, professor of medicine and associate chief for clinical affairs in the division of rheumatology at Northwestern University in Chicago.
“I think most people assume, based on clinical experience, that it does work well. It’s all we had for years and years and years,” he added.
That is, until SEAM-PsA.
SEAM-PsA
SEAM-PsA randomized 851 DMARD- and biologic-naive patients with a median 0.6-year duration of psoriatic arthritis to one of three treatment arms for 48 weeks: once-weekly etanercept at 50 mg plus oral methotrexate at 20 mg, etanercept plus oral placebo, or methotrexate plus injectable placebo.
This is a study that will reshape clinical practice for many rheumatologists, according to Dr. Kavanaugh. The hypothesis was that in psoriatic arthritis, just as has been shown to be the case in rheumatoid arthritis, the combination of a TNF inhibitor plus methotrexate would have greater efficacy than either agent alone. But the study brought a couple of major surprises.
“Methotrexate didn’t do so badly,” Dr. Kavanaugh observed. “And the combination did nothing. I would have bet that the combination would have shown methotrexate had a synergistic effect with the TNF inhibitor, especially for x-ray changes. But the combination didn’t do any better than etanercept alone.”
Make no mistake: Etanercept monotherapy significantly outperformed methotrexate monotherapy for the primary endpoint, the ACR 20 response at week 24, by a margin of 60.9% versus 50.7%. Dr. Ruderman deemed that methotrexate response rate to be quite respectable, although he bemoaned the absence of a double-placebo comparator arm. And the key secondary endpoint, the minimal disease activity response rate at week 24, was also significantly better with etanercept, at 35.9% compared with 22.9%. Moreover, both etanercept arms showed significantly less radiographic progression than with methotrexate alone.
However, that was it. There were no significant differences between etanercept and methotrexate in other secondary endpoints, including the Spondyloarthritis Research Consortium of Canada Enthesitis Index (SPARCC), the Disease Activity in PSoriatic Arthritis (DAPSA) score, the Leeds Dactylitis Instrument (LDI), and quality of life as assessed by the 36-item Short Form Health Survey total score.
“Methotrexate showed generally good efficacy across multiple domains,” the investigators concluded (Arthritis Rheumatol. 2019 Feb 12. doi: 10.1002/art.40851).
“Another intriguing thing to come out of this study for me were the enthesitis and dactylitis results. My clinical experience suggested methotrexate wasn’t so great for that, but this study suggests that’s not true,” Dr. Ruderman said.
“There are a couple of key take-home points from this study,” according to Dr. Kavanaugh. “One is that the combination is not synergistic. When you start a rheumatoid arthritis patient on methotrexate, you try to keep him on methotrexate when you add a TNF inhibitor. This study would say there doesn’t seem like there’s a reason to do that in your psoriatic arthritis patient. And the second message is that methotrexate seems to work.”
New ACR/NPF psoriatic arthritis guidelines under fire
“The new guidelines are fuzzy, aren’t they?” Dr. Kavanaugh said in lobbing the topic over to Dr. Ruderman.
“Where do we start?” he replied, shaking his head. “These are evidence-based guidelines in an area in which there was virtually no evidence.”
Indeed, the guidelines committee proudly employed the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) methodology, which forces committee members to issue “conditional” recommendations when there’s not enough evidence to make a “strong” recommendation.
“You’re not allowed to say, ‘We don’t know, there’s not enough evidence to make a choice,’ ” Dr. Ruderman said. “The problem with these guidelines is virtually everything in it is a conditional recommendation except ‘stop smoking,’ which was a strong recommendation.
“A conditional recommendation is pretty much a fancy term for expert opinion. It’s basically everybody in the room saying, ‘This is what we think.’ And that makes guidelines challenging because as a rheumatologist, you’re an expert. The people in the room have perhaps looked at the data more carefully than you’ve drilled down into the studies, but ultimately they’ve taken care of these patients and you’ve taken care of these patients, so why is their opinion better than your opinion, if it’s an informed opinion?”
His other critique of the 28-page guidelines is they don’t include the reasoning behind the conditional recommendations.
“If the conditional recommendation is, ‘In this situation, a TNF inhibitor is preferred over an IL-17 inhibitor,’ that would be great if they had also said, ‘This is why we thought that.’ But that’s not in the paper,” Dr. Ruderman said.
He and Dr. Kavanaugh reported serving as consultants to numerous pharmaceutical companies.
MAUI, HAWAII – The first-ever, double-blind, randomized, controlled clinical trial evidence demonstrating that methotrexate indeed has therapeutic efficacy in psoriatic arthritis has come at an awkward time – on the heels of a basically negative Cochrane Collaboration systematic review as well as the latest American College of Rheumatology/National Psoriasis Foundation guidelines for treatment of psoriatic arthritis, which recommend anti–tumor necrosis factor therapy as first line, ahead of methotrexate.
The timing of the release of the SEAM-PsA randomized trial results was such that neither the Cochrane group nor the ACR/NPF guideline committee was able to consider the new, potentially game-changing study findings.
“I look at SEAM-PsA and have to say, methotrexate does seem to be an effective therapy. I think it calls into question the new guidelines, which were developed before the data were out. Now you look at this and have to ask, can you really say you should use a TNF inhibitor before methotrexate based on these results? I don’t know,” Eric M. Ruderman, MD, said at the 2019 Rheumatology Winter Clinical Symposium.
He also shared other problems he has with the new guidelines, which he considers seriously flawed.
The Cochrane Collaboration Systematic Review
The Cochrane group cast a net for all randomized, controlled clinical trials of methotrexate versus placebo or another disease-modifying antirheumatic drug (DMARD). They found eight, which they judged to be of poor quality. Their conclusion: “Low-quality evidence suggests that low-dose (15 mg or less) oral methotrexate might be slightly more effective than placebo when taken for 6 months; however, we are uncertain if it is more harmful” (Cochrane Database Syst Rev. 2019 Jan 18;1:CD012722. doi: 10.1002/14651858.CD012722.pub2).
“The new Cochrane Review concludes methotrexate doesn’t seem to work that well,” observed symposium director Arthur Kavanaugh, MD, professor of medicine at the University of California, San Diego.
“That’s because it’s based on published data, and there’s been very little of that,” said Dr. Ruderman, professor of medicine and associate chief for clinical affairs in the division of rheumatology at Northwestern University in Chicago.
“I think most people assume, based on clinical experience, that it does work well. It’s all we had for years and years and years,” he added.
That is, until SEAM-PsA.
SEAM-PsA
SEAM-PsA randomized 851 DMARD- and biologic-naive patients with a median 0.6-year duration of psoriatic arthritis to one of three treatment arms for 48 weeks: once-weekly etanercept at 50 mg plus oral methotrexate at 20 mg, etanercept plus oral placebo, or methotrexate plus injectable placebo.
This is a study that will reshape clinical practice for many rheumatologists, according to Dr. Kavanaugh. The hypothesis was that in psoriatic arthritis, just as has been shown to be the case in rheumatoid arthritis, the combination of a TNF inhibitor plus methotrexate would have greater efficacy than either agent alone. But the study brought a couple of major surprises.
“Methotrexate didn’t do so badly,” Dr. Kavanaugh observed. “And the combination did nothing. I would have bet that the combination would have shown methotrexate had a synergistic effect with the TNF inhibitor, especially for x-ray changes. But the combination didn’t do any better than etanercept alone.”
Make no mistake: Etanercept monotherapy significantly outperformed methotrexate monotherapy for the primary endpoint, the ACR 20 response at week 24, by a margin of 60.9% versus 50.7%. Dr. Ruderman deemed that methotrexate response rate to be quite respectable, although he bemoaned the absence of a double-placebo comparator arm. And the key secondary endpoint, the minimal disease activity response rate at week 24, was also significantly better with etanercept, at 35.9% compared with 22.9%. Moreover, both etanercept arms showed significantly less radiographic progression than with methotrexate alone.
However, that was it. There were no significant differences between etanercept and methotrexate in other secondary endpoints, including the Spondyloarthritis Research Consortium of Canada Enthesitis Index (SPARCC), the Disease Activity in PSoriatic Arthritis (DAPSA) score, the Leeds Dactylitis Instrument (LDI), and quality of life as assessed by the 36-item Short Form Health Survey total score.
“Methotrexate showed generally good efficacy across multiple domains,” the investigators concluded (Arthritis Rheumatol. 2019 Feb 12. doi: 10.1002/art.40851).
“Another intriguing thing to come out of this study for me were the enthesitis and dactylitis results. My clinical experience suggested methotrexate wasn’t so great for that, but this study suggests that’s not true,” Dr. Ruderman said.
“There are a couple of key take-home points from this study,” according to Dr. Kavanaugh. “One is that the combination is not synergistic. When you start a rheumatoid arthritis patient on methotrexate, you try to keep him on methotrexate when you add a TNF inhibitor. This study would say there doesn’t seem like there’s a reason to do that in your psoriatic arthritis patient. And the second message is that methotrexate seems to work.”
New ACR/NPF psoriatic arthritis guidelines under fire
“The new guidelines are fuzzy, aren’t they?” Dr. Kavanaugh said in lobbing the topic over to Dr. Ruderman.
“Where do we start?” he replied, shaking his head. “These are evidence-based guidelines in an area in which there was virtually no evidence.”
Indeed, the guidelines committee proudly employed the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) methodology, which forces committee members to issue “conditional” recommendations when there’s not enough evidence to make a “strong” recommendation.
“You’re not allowed to say, ‘We don’t know, there’s not enough evidence to make a choice,’ ” Dr. Ruderman said. “The problem with these guidelines is virtually everything in it is a conditional recommendation except ‘stop smoking,’ which was a strong recommendation.
“A conditional recommendation is pretty much a fancy term for expert opinion. It’s basically everybody in the room saying, ‘This is what we think.’ And that makes guidelines challenging because as a rheumatologist, you’re an expert. The people in the room have perhaps looked at the data more carefully than you’ve drilled down into the studies, but ultimately they’ve taken care of these patients and you’ve taken care of these patients, so why is their opinion better than your opinion, if it’s an informed opinion?”
His other critique of the 28-page guidelines is they don’t include the reasoning behind the conditional recommendations.
“If the conditional recommendation is, ‘In this situation, a TNF inhibitor is preferred over an IL-17 inhibitor,’ that would be great if they had also said, ‘This is why we thought that.’ But that’s not in the paper,” Dr. Ruderman said.
He and Dr. Kavanaugh reported serving as consultants to numerous pharmaceutical companies.
MAUI, HAWAII – The first-ever, double-blind, randomized, controlled clinical trial evidence demonstrating that methotrexate indeed has therapeutic efficacy in psoriatic arthritis has come at an awkward time – on the heels of a basically negative Cochrane Collaboration systematic review as well as the latest American College of Rheumatology/National Psoriasis Foundation guidelines for treatment of psoriatic arthritis, which recommend anti–tumor necrosis factor therapy as first line, ahead of methotrexate.
The timing of the release of the SEAM-PsA randomized trial results was such that neither the Cochrane group nor the ACR/NPF guideline committee was able to consider the new, potentially game-changing study findings.
“I look at SEAM-PsA and have to say, methotrexate does seem to be an effective therapy. I think it calls into question the new guidelines, which were developed before the data were out. Now you look at this and have to ask, can you really say you should use a TNF inhibitor before methotrexate based on these results? I don’t know,” Eric M. Ruderman, MD, said at the 2019 Rheumatology Winter Clinical Symposium.
He also shared other problems he has with the new guidelines, which he considers seriously flawed.
The Cochrane Collaboration Systematic Review
The Cochrane group cast a net for all randomized, controlled clinical trials of methotrexate versus placebo or another disease-modifying antirheumatic drug (DMARD). They found eight, which they judged to be of poor quality. Their conclusion: “Low-quality evidence suggests that low-dose (15 mg or less) oral methotrexate might be slightly more effective than placebo when taken for 6 months; however, we are uncertain if it is more harmful” (Cochrane Database Syst Rev. 2019 Jan 18;1:CD012722. doi: 10.1002/14651858.CD012722.pub2).
“The new Cochrane Review concludes methotrexate doesn’t seem to work that well,” observed symposium director Arthur Kavanaugh, MD, professor of medicine at the University of California, San Diego.
“That’s because it’s based on published data, and there’s been very little of that,” said Dr. Ruderman, professor of medicine and associate chief for clinical affairs in the division of rheumatology at Northwestern University in Chicago.
“I think most people assume, based on clinical experience, that it does work well. It’s all we had for years and years and years,” he added.
That is, until SEAM-PsA.
SEAM-PsA
SEAM-PsA randomized 851 DMARD- and biologic-naive patients with a median 0.6-year duration of psoriatic arthritis to one of three treatment arms for 48 weeks: once-weekly etanercept at 50 mg plus oral methotrexate at 20 mg, etanercept plus oral placebo, or methotrexate plus injectable placebo.
This is a study that will reshape clinical practice for many rheumatologists, according to Dr. Kavanaugh. The hypothesis was that in psoriatic arthritis, just as has been shown to be the case in rheumatoid arthritis, the combination of a TNF inhibitor plus methotrexate would have greater efficacy than either agent alone. But the study brought a couple of major surprises.
“Methotrexate didn’t do so badly,” Dr. Kavanaugh observed. “And the combination did nothing. I would have bet that the combination would have shown methotrexate had a synergistic effect with the TNF inhibitor, especially for x-ray changes. But the combination didn’t do any better than etanercept alone.”
Make no mistake: Etanercept monotherapy significantly outperformed methotrexate monotherapy for the primary endpoint, the ACR 20 response at week 24, by a margin of 60.9% versus 50.7%. Dr. Ruderman deemed that methotrexate response rate to be quite respectable, although he bemoaned the absence of a double-placebo comparator arm. And the key secondary endpoint, the minimal disease activity response rate at week 24, was also significantly better with etanercept, at 35.9% compared with 22.9%. Moreover, both etanercept arms showed significantly less radiographic progression than with methotrexate alone.
However, that was it. There were no significant differences between etanercept and methotrexate in other secondary endpoints, including the Spondyloarthritis Research Consortium of Canada Enthesitis Index (SPARCC), the Disease Activity in PSoriatic Arthritis (DAPSA) score, the Leeds Dactylitis Instrument (LDI), and quality of life as assessed by the 36-item Short Form Health Survey total score.
“Methotrexate showed generally good efficacy across multiple domains,” the investigators concluded (Arthritis Rheumatol. 2019 Feb 12. doi: 10.1002/art.40851).
“Another intriguing thing to come out of this study for me were the enthesitis and dactylitis results. My clinical experience suggested methotrexate wasn’t so great for that, but this study suggests that’s not true,” Dr. Ruderman said.
“There are a couple of key take-home points from this study,” according to Dr. Kavanaugh. “One is that the combination is not synergistic. When you start a rheumatoid arthritis patient on methotrexate, you try to keep him on methotrexate when you add a TNF inhibitor. This study would say there doesn’t seem like there’s a reason to do that in your psoriatic arthritis patient. And the second message is that methotrexate seems to work.”
New ACR/NPF psoriatic arthritis guidelines under fire
“The new guidelines are fuzzy, aren’t they?” Dr. Kavanaugh said in lobbing the topic over to Dr. Ruderman.
“Where do we start?” he replied, shaking his head. “These are evidence-based guidelines in an area in which there was virtually no evidence.”
Indeed, the guidelines committee proudly employed the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) methodology, which forces committee members to issue “conditional” recommendations when there’s not enough evidence to make a “strong” recommendation.
“You’re not allowed to say, ‘We don’t know, there’s not enough evidence to make a choice,’ ” Dr. Ruderman said. “The problem with these guidelines is virtually everything in it is a conditional recommendation except ‘stop smoking,’ which was a strong recommendation.
“A conditional recommendation is pretty much a fancy term for expert opinion. It’s basically everybody in the room saying, ‘This is what we think.’ And that makes guidelines challenging because as a rheumatologist, you’re an expert. The people in the room have perhaps looked at the data more carefully than you’ve drilled down into the studies, but ultimately they’ve taken care of these patients and you’ve taken care of these patients, so why is their opinion better than your opinion, if it’s an informed opinion?”
His other critique of the 28-page guidelines is they don’t include the reasoning behind the conditional recommendations.
“If the conditional recommendation is, ‘In this situation, a TNF inhibitor is preferred over an IL-17 inhibitor,’ that would be great if they had also said, ‘This is why we thought that.’ But that’s not in the paper,” Dr. Ruderman said.
He and Dr. Kavanaugh reported serving as consultants to numerous pharmaceutical companies.
REPORTING FROM RWCS 2019
Lymphoma rate in RA patients is falling
MAUI, HAWAII – The incidence of lymphoma in patients with RA appears to have been dropping during the past 2 decades – and for rheumatologists, that’s news you can use.

“I think this is encouraging data about where we’re headed with therapy. And it’s encouraging data for your patients, that maybe more effective therapies can lead to a lower risk of cancer,” John J. Cush, MD, commented at the 2019 Rheumatology Winter Clinical Symposium.
“Patients are always worried about cancer,” observed symposium director Arthur Kavanaugh, MD. “I think this is very useful data to bring to a discussion with patients.”
The study they highlighted was presented at the 2018 annual meeting of the American College of Rheumatology by Namrata Singh, MD, of the University of Iowa, Iowa City and coinvestigators from Veterans Affairs medical centers around the country. They analyzed the incidence of lymphomas as well as all-site cancers in 50,870 men with RA in the national VA health care system during 2001-2015 and compared the rates with the background rates in the general U.S. population as captured in the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program.
The key finding: While the standardized incidence ratio for the development of lymphoma in the RA patients during 2001-2005 was 190% greater than in the SEER population, the SIR dropped to 1.6 in 2006-2010 and stayed low in 2011-2015.
“These are the only data I’m aware of that say maybe lymphomas are becoming less frequent among RA patients,” said Dr. Kavanaugh, professor of medicine at the University of California, San Diego.
Historically, RA has been associated with roughly a 100% increased risk of lymphoma. The source of the increased risk has been a matter of controversy: Is it the result of immunostimulation triggered by high RA disease activity, or a side effect of the drugs employed in treatment of the disease? The clear implication of the VA study is that it’s all about disease activity.
“The lymphoma rate is higher early in the use of our new therapies, in 2001-2005, because the patients who went on TNF [tumor necrosis factor] inhibitors then had the most disease activity. But with time, patients are getting those treatments earlier. Does this [lower lymphoma rate] reflect a change in the practice of rheumatology? I think it does,” according to Dr. Cush, professor of medicine and rheumatology at Baylor University Medical Center, Dallas.
Dr. Kavanaugh agreed. “Now, if we’re treating early and treating to target, we should see less lymphomas than we did back in the day.”
The rate of cancers at all sites in the VA RA patients has been going down as well, with the SIR dropping from 1.8 in 2001-2005 to close to 1, the background rate in the general population.
“What’s great about this study is this is a large data set. You really can’t compare an RA population on and off treatment. The right comparison is to a normal population – and SEER accounts for something like 14% of the U.S. population,” Dr. Cush said.
Previous support for the notion that the increased lymphoma risk associated with RA was a function of disease activity came from a Swedish study of 378 RA patients in the prebiologic era who developed lymphoma and a matched cohort of 378 others without lymphoma. The investigators found that patients with moderate overall RA disease activity were at a 700% increased risk of lymphoma, compared with those with low overall disease activity, and that patients with high RA disease activity were at a 6,900% increased risk (Arthritis Rheum. 2006 Mar;54[3]:692-701). But that was a cross-sectional study, whereas the VA study examined trends over time.
The VA RA cohort had a mean age of 64 years. About 60% were current or ex-smokers, 65% were positive for rheumatoid factor, and 62% were positive for anticyclic citrullinated peptide.
Dr. Kavanaugh said that, because of the potential for referral bias in the VA study, he’s eager to see the findings reproduced in another data set.
Both Dr. Cush and Dr. Kavanaugh reported serving as a consultant to and/or receiving research funding from numerous pharmaceutical companies.
MAUI, HAWAII – The incidence of lymphoma in patients with RA appears to have been dropping during the past 2 decades – and for rheumatologists, that’s news you can use.

“I think this is encouraging data about where we’re headed with therapy. And it’s encouraging data for your patients, that maybe more effective therapies can lead to a lower risk of cancer,” John J. Cush, MD, commented at the 2019 Rheumatology Winter Clinical Symposium.
“Patients are always worried about cancer,” observed symposium director Arthur Kavanaugh, MD. “I think this is very useful data to bring to a discussion with patients.”
The study they highlighted was presented at the 2018 annual meeting of the American College of Rheumatology by Namrata Singh, MD, of the University of Iowa, Iowa City and coinvestigators from Veterans Affairs medical centers around the country. They analyzed the incidence of lymphomas as well as all-site cancers in 50,870 men with RA in the national VA health care system during 2001-2015 and compared the rates with the background rates in the general U.S. population as captured in the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program.
The key finding: While the standardized incidence ratio for the development of lymphoma in the RA patients during 2001-2005 was 190% greater than in the SEER population, the SIR dropped to 1.6 in 2006-2010 and stayed low in 2011-2015.
“These are the only data I’m aware of that say maybe lymphomas are becoming less frequent among RA patients,” said Dr. Kavanaugh, professor of medicine at the University of California, San Diego.
Historically, RA has been associated with roughly a 100% increased risk of lymphoma. The source of the increased risk has been a matter of controversy: Is it the result of immunostimulation triggered by high RA disease activity, or a side effect of the drugs employed in treatment of the disease? The clear implication of the VA study is that it’s all about disease activity.
“The lymphoma rate is higher early in the use of our new therapies, in 2001-2005, because the patients who went on TNF [tumor necrosis factor] inhibitors then had the most disease activity. But with time, patients are getting those treatments earlier. Does this [lower lymphoma rate] reflect a change in the practice of rheumatology? I think it does,” according to Dr. Cush, professor of medicine and rheumatology at Baylor University Medical Center, Dallas.
Dr. Kavanaugh agreed. “Now, if we’re treating early and treating to target, we should see less lymphomas than we did back in the day.”
The rate of cancers at all sites in the VA RA patients has been going down as well, with the SIR dropping from 1.8 in 2001-2005 to close to 1, the background rate in the general population.
“What’s great about this study is this is a large data set. You really can’t compare an RA population on and off treatment. The right comparison is to a normal population – and SEER accounts for something like 14% of the U.S. population,” Dr. Cush said.
Previous support for the notion that the increased lymphoma risk associated with RA was a function of disease activity came from a Swedish study of 378 RA patients in the prebiologic era who developed lymphoma and a matched cohort of 378 others without lymphoma. The investigators found that patients with moderate overall RA disease activity were at a 700% increased risk of lymphoma, compared with those with low overall disease activity, and that patients with high RA disease activity were at a 6,900% increased risk (Arthritis Rheum. 2006 Mar;54[3]:692-701). But that was a cross-sectional study, whereas the VA study examined trends over time.
The VA RA cohort had a mean age of 64 years. About 60% were current or ex-smokers, 65% were positive for rheumatoid factor, and 62% were positive for anticyclic citrullinated peptide.
Dr. Kavanaugh said that, because of the potential for referral bias in the VA study, he’s eager to see the findings reproduced in another data set.
Both Dr. Cush and Dr. Kavanaugh reported serving as a consultant to and/or receiving research funding from numerous pharmaceutical companies.
MAUI, HAWAII – The incidence of lymphoma in patients with RA appears to have been dropping during the past 2 decades – and for rheumatologists, that’s news you can use.

“I think this is encouraging data about where we’re headed with therapy. And it’s encouraging data for your patients, that maybe more effective therapies can lead to a lower risk of cancer,” John J. Cush, MD, commented at the 2019 Rheumatology Winter Clinical Symposium.
“Patients are always worried about cancer,” observed symposium director Arthur Kavanaugh, MD. “I think this is very useful data to bring to a discussion with patients.”
The study they highlighted was presented at the 2018 annual meeting of the American College of Rheumatology by Namrata Singh, MD, of the University of Iowa, Iowa City and coinvestigators from Veterans Affairs medical centers around the country. They analyzed the incidence of lymphomas as well as all-site cancers in 50,870 men with RA in the national VA health care system during 2001-2015 and compared the rates with the background rates in the general U.S. population as captured in the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program.
The key finding: While the standardized incidence ratio for the development of lymphoma in the RA patients during 2001-2005 was 190% greater than in the SEER population, the SIR dropped to 1.6 in 2006-2010 and stayed low in 2011-2015.
“These are the only data I’m aware of that say maybe lymphomas are becoming less frequent among RA patients,” said Dr. Kavanaugh, professor of medicine at the University of California, San Diego.
Historically, RA has been associated with roughly a 100% increased risk of lymphoma. The source of the increased risk has been a matter of controversy: Is it the result of immunostimulation triggered by high RA disease activity, or a side effect of the drugs employed in treatment of the disease? The clear implication of the VA study is that it’s all about disease activity.
“The lymphoma rate is higher early in the use of our new therapies, in 2001-2005, because the patients who went on TNF [tumor necrosis factor] inhibitors then had the most disease activity. But with time, patients are getting those treatments earlier. Does this [lower lymphoma rate] reflect a change in the practice of rheumatology? I think it does,” according to Dr. Cush, professor of medicine and rheumatology at Baylor University Medical Center, Dallas.
Dr. Kavanaugh agreed. “Now, if we’re treating early and treating to target, we should see less lymphomas than we did back in the day.”
The rate of cancers at all sites in the VA RA patients has been going down as well, with the SIR dropping from 1.8 in 2001-2005 to close to 1, the background rate in the general population.
“What’s great about this study is this is a large data set. You really can’t compare an RA population on and off treatment. The right comparison is to a normal population – and SEER accounts for something like 14% of the U.S. population,” Dr. Cush said.
Previous support for the notion that the increased lymphoma risk associated with RA was a function of disease activity came from a Swedish study of 378 RA patients in the prebiologic era who developed lymphoma and a matched cohort of 378 others without lymphoma. The investigators found that patients with moderate overall RA disease activity were at a 700% increased risk of lymphoma, compared with those with low overall disease activity, and that patients with high RA disease activity were at a 6,900% increased risk (Arthritis Rheum. 2006 Mar;54[3]:692-701). But that was a cross-sectional study, whereas the VA study examined trends over time.
The VA RA cohort had a mean age of 64 years. About 60% were current or ex-smokers, 65% were positive for rheumatoid factor, and 62% were positive for anticyclic citrullinated peptide.
Dr. Kavanaugh said that, because of the potential for referral bias in the VA study, he’s eager to see the findings reproduced in another data set.
Both Dr. Cush and Dr. Kavanaugh reported serving as a consultant to and/or receiving research funding from numerous pharmaceutical companies.
REPORTING FROM RWCS 2019





















