User login
Combo therapy outcomes for West syndrome prove no better than monotherapy
BANGKOK – Hiroki Nariai, MD, declared at the International Epilepsy Congress.
West syndrome, or infantile spasms with a hypsarrhythmic EEG, is a severe infantile epileptic encephalopathy. It has high morbidity and mortality, and it’s challenging to treat. So neurologists and pediatricians were thrilled by an earlier preliminary report from an open-label, randomized, controlled trial conducted by the International Collaborative Infantile Spasms Study (ICISS) investigators. They reported that a hormonal therapy and vigabatrin (Sabril) combination provided significantly better seizure control between days 14 and 42 of treatment than hormonal therapy alone, albeit at the cost of more side effects (Lancet Neurol. 2017 Jan;16[1]:33-42).
However, a sobering update from the 377-infant study conducted in Australia, Switzerland, Germany, New Zealand, and the United Kingdom concluded that combination therapy didn’t result in improved developmental or epilepsy outcomes at 18 months, Dr. Nariai said at the congress sponsored by the International League Against Epilepsy.
“We still have inconclusive evidence to support the routine use of combination therapy. Clearly we need a better disease-modifying therapy because our best results with hormonal therapy or vigabatrin are only a 50%-70% response rate. And having a biomarker to guide early therapy and follow treatment response would help in establishing a better therapy,” commented Dr. Nariai, a pediatric neurologist at the University of California, Los Angeles.
He wasn’t involved in the international trial. He is, however, active in the search for a biomarker that would aid in speedier diagnosis of West syndrome, which in turn would allow for earlier treatment and, potentially, better outcomes. Indeed, Dr. Nariai has done pioneering work in identifying several EEG abnormalities readily measurable noninvasively using scalp electrodes that show considerable promise in this regard. These candidate biomarkers include ictal or interictal high-frequency oscillations at 80 Hz or more, along with cross-frequency coupling of high-frequency oscillations and delta-wave activity.
The primary endpoint in the ICISS study was developmental outcome at 18 months as evaluated using the Vineland Adaptive Behavior Scales composite score. The mean score was 73.9 in the combination therapy group and closely similar at 72.7 in the children on hormonal therapy alone. At 18 months, 30% of children in the combination therapy group carried a diagnosis of epilepsy, as did 29.2% of controls randomized to either high-dose oral steroids or intramuscular depot tetracosactide. About 15% of children randomized to combination therapy still had spasms at 18 months, as did 15.7% on hormonal therapy alone (Lancet Child Adolesc Health. 2018 Oct;2[10]:715-25).
The chief side effects of hormonal therapy included hypertension, hypoglycemia, and immunosuppression. Vigabatrin’s side effects included dose- and duration-dependent peripheral vision loss, movement disorders, and undesirable MRI signal changes.
Dr. Nariai observed that, even though hormonal therapy is widely used as first-line therapy in West syndrome, it remains surrounded by important unanswered questions.
“We don’t have head-to-head comparative studies of ACTH versus high-dose steroids, the optimal dosing protocol is not established, and we really don’t even know the mechanism of action for hormonal therapy and vigabatrin,” he said.
The study was sponsored by the U.K. National Institute of Health Research and other noncommercial entities. Dr. Nariai reported having no financial conflicts regarding his presentation.
BANGKOK – Hiroki Nariai, MD, declared at the International Epilepsy Congress.
West syndrome, or infantile spasms with a hypsarrhythmic EEG, is a severe infantile epileptic encephalopathy. It has high morbidity and mortality, and it’s challenging to treat. So neurologists and pediatricians were thrilled by an earlier preliminary report from an open-label, randomized, controlled trial conducted by the International Collaborative Infantile Spasms Study (ICISS) investigators. They reported that a hormonal therapy and vigabatrin (Sabril) combination provided significantly better seizure control between days 14 and 42 of treatment than hormonal therapy alone, albeit at the cost of more side effects (Lancet Neurol. 2017 Jan;16[1]:33-42).
However, a sobering update from the 377-infant study conducted in Australia, Switzerland, Germany, New Zealand, and the United Kingdom concluded that combination therapy didn’t result in improved developmental or epilepsy outcomes at 18 months, Dr. Nariai said at the congress sponsored by the International League Against Epilepsy.
“We still have inconclusive evidence to support the routine use of combination therapy. Clearly we need a better disease-modifying therapy because our best results with hormonal therapy or vigabatrin are only a 50%-70% response rate. And having a biomarker to guide early therapy and follow treatment response would help in establishing a better therapy,” commented Dr. Nariai, a pediatric neurologist at the University of California, Los Angeles.
He wasn’t involved in the international trial. He is, however, active in the search for a biomarker that would aid in speedier diagnosis of West syndrome, which in turn would allow for earlier treatment and, potentially, better outcomes. Indeed, Dr. Nariai has done pioneering work in identifying several EEG abnormalities readily measurable noninvasively using scalp electrodes that show considerable promise in this regard. These candidate biomarkers include ictal or interictal high-frequency oscillations at 80 Hz or more, along with cross-frequency coupling of high-frequency oscillations and delta-wave activity.
The primary endpoint in the ICISS study was developmental outcome at 18 months as evaluated using the Vineland Adaptive Behavior Scales composite score. The mean score was 73.9 in the combination therapy group and closely similar at 72.7 in the children on hormonal therapy alone. At 18 months, 30% of children in the combination therapy group carried a diagnosis of epilepsy, as did 29.2% of controls randomized to either high-dose oral steroids or intramuscular depot tetracosactide. About 15% of children randomized to combination therapy still had spasms at 18 months, as did 15.7% on hormonal therapy alone (Lancet Child Adolesc Health. 2018 Oct;2[10]:715-25).
The chief side effects of hormonal therapy included hypertension, hypoglycemia, and immunosuppression. Vigabatrin’s side effects included dose- and duration-dependent peripheral vision loss, movement disorders, and undesirable MRI signal changes.
Dr. Nariai observed that, even though hormonal therapy is widely used as first-line therapy in West syndrome, it remains surrounded by important unanswered questions.
“We don’t have head-to-head comparative studies of ACTH versus high-dose steroids, the optimal dosing protocol is not established, and we really don’t even know the mechanism of action for hormonal therapy and vigabatrin,” he said.
The study was sponsored by the U.K. National Institute of Health Research and other noncommercial entities. Dr. Nariai reported having no financial conflicts regarding his presentation.
BANGKOK – Hiroki Nariai, MD, declared at the International Epilepsy Congress.
West syndrome, or infantile spasms with a hypsarrhythmic EEG, is a severe infantile epileptic encephalopathy. It has high morbidity and mortality, and it’s challenging to treat. So neurologists and pediatricians were thrilled by an earlier preliminary report from an open-label, randomized, controlled trial conducted by the International Collaborative Infantile Spasms Study (ICISS) investigators. They reported that a hormonal therapy and vigabatrin (Sabril) combination provided significantly better seizure control between days 14 and 42 of treatment than hormonal therapy alone, albeit at the cost of more side effects (Lancet Neurol. 2017 Jan;16[1]:33-42).
However, a sobering update from the 377-infant study conducted in Australia, Switzerland, Germany, New Zealand, and the United Kingdom concluded that combination therapy didn’t result in improved developmental or epilepsy outcomes at 18 months, Dr. Nariai said at the congress sponsored by the International League Against Epilepsy.
“We still have inconclusive evidence to support the routine use of combination therapy. Clearly we need a better disease-modifying therapy because our best results with hormonal therapy or vigabatrin are only a 50%-70% response rate. And having a biomarker to guide early therapy and follow treatment response would help in establishing a better therapy,” commented Dr. Nariai, a pediatric neurologist at the University of California, Los Angeles.
He wasn’t involved in the international trial. He is, however, active in the search for a biomarker that would aid in speedier diagnosis of West syndrome, which in turn would allow for earlier treatment and, potentially, better outcomes. Indeed, Dr. Nariai has done pioneering work in identifying several EEG abnormalities readily measurable noninvasively using scalp electrodes that show considerable promise in this regard. These candidate biomarkers include ictal or interictal high-frequency oscillations at 80 Hz or more, along with cross-frequency coupling of high-frequency oscillations and delta-wave activity.
The primary endpoint in the ICISS study was developmental outcome at 18 months as evaluated using the Vineland Adaptive Behavior Scales composite score. The mean score was 73.9 in the combination therapy group and closely similar at 72.7 in the children on hormonal therapy alone. At 18 months, 30% of children in the combination therapy group carried a diagnosis of epilepsy, as did 29.2% of controls randomized to either high-dose oral steroids or intramuscular depot tetracosactide. About 15% of children randomized to combination therapy still had spasms at 18 months, as did 15.7% on hormonal therapy alone (Lancet Child Adolesc Health. 2018 Oct;2[10]:715-25).
The chief side effects of hormonal therapy included hypertension, hypoglycemia, and immunosuppression. Vigabatrin’s side effects included dose- and duration-dependent peripheral vision loss, movement disorders, and undesirable MRI signal changes.
Dr. Nariai observed that, even though hormonal therapy is widely used as first-line therapy in West syndrome, it remains surrounded by important unanswered questions.
“We don’t have head-to-head comparative studies of ACTH versus high-dose steroids, the optimal dosing protocol is not established, and we really don’t even know the mechanism of action for hormonal therapy and vigabatrin,” he said.
The study was sponsored by the U.K. National Institute of Health Research and other noncommercial entities. Dr. Nariai reported having no financial conflicts regarding his presentation.
EXPERT ANALYSIS FROM IEC 2019
A new beverage aims to make ketogenic diets more palatable
BANGKOK –
Chief among those shortcomings is the notoriously poor compliance with these highly restrictive diets, which, as defining features, emphasize high fat intake and scrupulous restriction of carbohydrates in an effort to mimic the metabolic effects of starvation, J. Helen Cross, MD, explained at the International Epilepsy Congress.
She was a coauthor of a study led by Natasha E. Schoeler, PhD, a research dietician at the University College London Great Ormond Street Institute of Child Health, which demonstrated that children and adults with epilepsy who experience a significant antiseizure effect in response to ketogenic diet therapies have higher baseline blood levels of acetyl carnitine (Epilepsia. 2017 May;58(5):893-900).
The importance of this novel observation is twofold: It indicates a potential role for baseline acetyl carnitine level as a predictor of differential response to ketogenic diet therapies, a predictor for which there is an unmet need, and it is consistent with the hypothesis that an important potential mechanism of ketogenic diet effectiveness in epilepsy involves altered mitochondrial energy metabolism. That is because acetyl carnitine plays an essential role in mitochondrial uptake of long-chain fatty acids, noted Dr. Cross, professor of pediatric neurology and head of the developmental neurosciences program at the University College London Great Ormond Street Institute of Child Health.
At the congress sponsored by the International League Against Epilepsy, Dr. Cross and Dr. Schoeler presented the results of the initial 12-week tolerability study of Betashot, a ready-to-use, palatable blend of specific medium-chain triglycerides designed to be consumed three to four times daily with normal meals, limiting only intake of foods high in refined sugar. The Betashot beverage was developed in conjunction with Vitaflo International, a nutritional products company.
“It actually tastes good. It tastes like a strawberry shake,” according to Dr. Schoeler.
The 12-week study included 35 children with genetically caused forms of epilepsy and 26 adults with drug-resistant epilepsy. This was primarily a tolerability and compliance study, and the main finding was that two-thirds of the children and 69% of adults who started the study were still using Betashot at the 12-week mark. Moreover, 91% of the children and 56% of adults who completed the study elected to stay on Betashot afterwards. By week 12, after titrating their daily dose of Betashot upward as tolerated, the pediatric patients averaged 18% of their total daily energy intake from Betashot, the adults 24%.
The most common reasons for discontinuation among both children and adults were gastrointestinal side effects: abdominal discomfort, diarrhea, and/or vomiting.
“What’s exciting is that, even though the study is not powered to look at seizure response – it’s a tolerability study – we can report that there was a statistically significant reduction in the number of seizures in the group overall after 3 months of treatment,” Dr. Cross said.
She declined to provide specific data on seizure frequency because the study was underpowered for that endpoint. However, she added that further larger studies looking at a possible antiseizure effect of Betashot are ongoing.
The Betashot study was funded by Vitaflo International.
BANGKOK –
Chief among those shortcomings is the notoriously poor compliance with these highly restrictive diets, which, as defining features, emphasize high fat intake and scrupulous restriction of carbohydrates in an effort to mimic the metabolic effects of starvation, J. Helen Cross, MD, explained at the International Epilepsy Congress.
She was a coauthor of a study led by Natasha E. Schoeler, PhD, a research dietician at the University College London Great Ormond Street Institute of Child Health, which demonstrated that children and adults with epilepsy who experience a significant antiseizure effect in response to ketogenic diet therapies have higher baseline blood levels of acetyl carnitine (Epilepsia. 2017 May;58(5):893-900).
The importance of this novel observation is twofold: It indicates a potential role for baseline acetyl carnitine level as a predictor of differential response to ketogenic diet therapies, a predictor for which there is an unmet need, and it is consistent with the hypothesis that an important potential mechanism of ketogenic diet effectiveness in epilepsy involves altered mitochondrial energy metabolism. That is because acetyl carnitine plays an essential role in mitochondrial uptake of long-chain fatty acids, noted Dr. Cross, professor of pediatric neurology and head of the developmental neurosciences program at the University College London Great Ormond Street Institute of Child Health.
At the congress sponsored by the International League Against Epilepsy, Dr. Cross and Dr. Schoeler presented the results of the initial 12-week tolerability study of Betashot, a ready-to-use, palatable blend of specific medium-chain triglycerides designed to be consumed three to four times daily with normal meals, limiting only intake of foods high in refined sugar. The Betashot beverage was developed in conjunction with Vitaflo International, a nutritional products company.
“It actually tastes good. It tastes like a strawberry shake,” according to Dr. Schoeler.
The 12-week study included 35 children with genetically caused forms of epilepsy and 26 adults with drug-resistant epilepsy. This was primarily a tolerability and compliance study, and the main finding was that two-thirds of the children and 69% of adults who started the study were still using Betashot at the 12-week mark. Moreover, 91% of the children and 56% of adults who completed the study elected to stay on Betashot afterwards. By week 12, after titrating their daily dose of Betashot upward as tolerated, the pediatric patients averaged 18% of their total daily energy intake from Betashot, the adults 24%.
The most common reasons for discontinuation among both children and adults were gastrointestinal side effects: abdominal discomfort, diarrhea, and/or vomiting.
“What’s exciting is that, even though the study is not powered to look at seizure response – it’s a tolerability study – we can report that there was a statistically significant reduction in the number of seizures in the group overall after 3 months of treatment,” Dr. Cross said.
She declined to provide specific data on seizure frequency because the study was underpowered for that endpoint. However, she added that further larger studies looking at a possible antiseizure effect of Betashot are ongoing.
The Betashot study was funded by Vitaflo International.
BANGKOK –
Chief among those shortcomings is the notoriously poor compliance with these highly restrictive diets, which, as defining features, emphasize high fat intake and scrupulous restriction of carbohydrates in an effort to mimic the metabolic effects of starvation, J. Helen Cross, MD, explained at the International Epilepsy Congress.
She was a coauthor of a study led by Natasha E. Schoeler, PhD, a research dietician at the University College London Great Ormond Street Institute of Child Health, which demonstrated that children and adults with epilepsy who experience a significant antiseizure effect in response to ketogenic diet therapies have higher baseline blood levels of acetyl carnitine (Epilepsia. 2017 May;58(5):893-900).
The importance of this novel observation is twofold: It indicates a potential role for baseline acetyl carnitine level as a predictor of differential response to ketogenic diet therapies, a predictor for which there is an unmet need, and it is consistent with the hypothesis that an important potential mechanism of ketogenic diet effectiveness in epilepsy involves altered mitochondrial energy metabolism. That is because acetyl carnitine plays an essential role in mitochondrial uptake of long-chain fatty acids, noted Dr. Cross, professor of pediatric neurology and head of the developmental neurosciences program at the University College London Great Ormond Street Institute of Child Health.
At the congress sponsored by the International League Against Epilepsy, Dr. Cross and Dr. Schoeler presented the results of the initial 12-week tolerability study of Betashot, a ready-to-use, palatable blend of specific medium-chain triglycerides designed to be consumed three to four times daily with normal meals, limiting only intake of foods high in refined sugar. The Betashot beverage was developed in conjunction with Vitaflo International, a nutritional products company.
“It actually tastes good. It tastes like a strawberry shake,” according to Dr. Schoeler.
The 12-week study included 35 children with genetically caused forms of epilepsy and 26 adults with drug-resistant epilepsy. This was primarily a tolerability and compliance study, and the main finding was that two-thirds of the children and 69% of adults who started the study were still using Betashot at the 12-week mark. Moreover, 91% of the children and 56% of adults who completed the study elected to stay on Betashot afterwards. By week 12, after titrating their daily dose of Betashot upward as tolerated, the pediatric patients averaged 18% of their total daily energy intake from Betashot, the adults 24%.
The most common reasons for discontinuation among both children and adults were gastrointestinal side effects: abdominal discomfort, diarrhea, and/or vomiting.
“What’s exciting is that, even though the study is not powered to look at seizure response – it’s a tolerability study – we can report that there was a statistically significant reduction in the number of seizures in the group overall after 3 months of treatment,” Dr. Cross said.
She declined to provide specific data on seizure frequency because the study was underpowered for that endpoint. However, she added that further larger studies looking at a possible antiseizure effect of Betashot are ongoing.
The Betashot study was funded by Vitaflo International.
REPORTING FROM IEC 2019
Intranasal midazolam as first line for status epilepticus
BANGKOK – Lara Kay, MD, said at the International Epilepsy Congress.
Why? Because status epilepticus is a major medical emergency. It’s associated with substantial morbidity and mortality. And of the various factors that influence outcome in status epilepticus – including age, underlying etiology, and level of consciousness – only one is potentially within physician control: time to treatment, she noted at the congress sponsored by the International League Against Epilepsy.
“Time is brain,” observed Dr. Kay, a neurologist at the epilepsy center at University Hospital Frankfurt.
While intravenous benzodiazepines – for example, lorazepam at 2-4 mg – are widely accepted as the time-honored first-line treatment for status epilepticus, trying to place a line in a patient experiencing this emergency can be a tricky, time-consuming business. Multiple studies have demonstrated that various nonintravenous formulations of benzodiazepines, such as rectal diazepam or buccal or intramuscular midazolam, can be administered much faster and are as effective as intravenous benzodiazepines. But buccal midazolam is quite expensive in Germany, and the ready-to-use intramuscular midazolam applicator that’s available in the United States isn’t marketed in Germany. So several years ago Dr. Kay and her fellow neurologists started having their university hospital pharmacy manufacture intranasal midazolam.
Dr. Kay presented an observational study of 42 consecutive patients with status epilepticus who received intranasal midazolam as first-line treatment. The patients had a mean age of nearly 53 years and 23 were women. The starting dose was 2.5 mg per nostril, moving up to 5 mg per nostril after waiting 5 minutes in initial nonresponders.
Status epilepticus ceased both clinically and by EEG in 24 of the 42 patients, or 57%, in an average of 5 minutes after administration of the intranasal medication at a mean dose of 5.6 mg. Nonresponders received a mean dose of 7.5 mg. There were no significant differences between responders and nonresponders in terms of the proportion presenting with preexisting epilepsy or the epilepsy etiology. However, responders presented at a mean of 54 minutes in status epilepticus, while nonresponders had been in status for 17 minutes.
The 57% response rate with intranasal midazolam is comparable with other investigators’ reported success rates using other benzodiazepines and routes of administration, she noted.
Session cochair Gregory Krauss, MD, commented that he thought the Frankfurt neurologists may have been too cautious in their dosing of intranasal midazolam for status epilepticus.
“Often in the U.S. 5 mg is initially used in each nostril,” according to Dr. Krauss, professor of neurology at Johns Hopkins University, Baltimore.
Dr. Kay reported having no financial conflicts of interest regarding her study.
SOURCE: Kay L et al. IEC 2019, Abstract P029.
BANGKOK – Lara Kay, MD, said at the International Epilepsy Congress.
Why? Because status epilepticus is a major medical emergency. It’s associated with substantial morbidity and mortality. And of the various factors that influence outcome in status epilepticus – including age, underlying etiology, and level of consciousness – only one is potentially within physician control: time to treatment, she noted at the congress sponsored by the International League Against Epilepsy.
“Time is brain,” observed Dr. Kay, a neurologist at the epilepsy center at University Hospital Frankfurt.
While intravenous benzodiazepines – for example, lorazepam at 2-4 mg – are widely accepted as the time-honored first-line treatment for status epilepticus, trying to place a line in a patient experiencing this emergency can be a tricky, time-consuming business. Multiple studies have demonstrated that various nonintravenous formulations of benzodiazepines, such as rectal diazepam or buccal or intramuscular midazolam, can be administered much faster and are as effective as intravenous benzodiazepines. But buccal midazolam is quite expensive in Germany, and the ready-to-use intramuscular midazolam applicator that’s available in the United States isn’t marketed in Germany. So several years ago Dr. Kay and her fellow neurologists started having their university hospital pharmacy manufacture intranasal midazolam.
Dr. Kay presented an observational study of 42 consecutive patients with status epilepticus who received intranasal midazolam as first-line treatment. The patients had a mean age of nearly 53 years and 23 were women. The starting dose was 2.5 mg per nostril, moving up to 5 mg per nostril after waiting 5 minutes in initial nonresponders.
Status epilepticus ceased both clinically and by EEG in 24 of the 42 patients, or 57%, in an average of 5 minutes after administration of the intranasal medication at a mean dose of 5.6 mg. Nonresponders received a mean dose of 7.5 mg. There were no significant differences between responders and nonresponders in terms of the proportion presenting with preexisting epilepsy or the epilepsy etiology. However, responders presented at a mean of 54 minutes in status epilepticus, while nonresponders had been in status for 17 minutes.
The 57% response rate with intranasal midazolam is comparable with other investigators’ reported success rates using other benzodiazepines and routes of administration, she noted.
Session cochair Gregory Krauss, MD, commented that he thought the Frankfurt neurologists may have been too cautious in their dosing of intranasal midazolam for status epilepticus.
“Often in the U.S. 5 mg is initially used in each nostril,” according to Dr. Krauss, professor of neurology at Johns Hopkins University, Baltimore.
Dr. Kay reported having no financial conflicts of interest regarding her study.
SOURCE: Kay L et al. IEC 2019, Abstract P029.
BANGKOK – Lara Kay, MD, said at the International Epilepsy Congress.
Why? Because status epilepticus is a major medical emergency. It’s associated with substantial morbidity and mortality. And of the various factors that influence outcome in status epilepticus – including age, underlying etiology, and level of consciousness – only one is potentially within physician control: time to treatment, she noted at the congress sponsored by the International League Against Epilepsy.
“Time is brain,” observed Dr. Kay, a neurologist at the epilepsy center at University Hospital Frankfurt.
While intravenous benzodiazepines – for example, lorazepam at 2-4 mg – are widely accepted as the time-honored first-line treatment for status epilepticus, trying to place a line in a patient experiencing this emergency can be a tricky, time-consuming business. Multiple studies have demonstrated that various nonintravenous formulations of benzodiazepines, such as rectal diazepam or buccal or intramuscular midazolam, can be administered much faster and are as effective as intravenous benzodiazepines. But buccal midazolam is quite expensive in Germany, and the ready-to-use intramuscular midazolam applicator that’s available in the United States isn’t marketed in Germany. So several years ago Dr. Kay and her fellow neurologists started having their university hospital pharmacy manufacture intranasal midazolam.
Dr. Kay presented an observational study of 42 consecutive patients with status epilepticus who received intranasal midazolam as first-line treatment. The patients had a mean age of nearly 53 years and 23 were women. The starting dose was 2.5 mg per nostril, moving up to 5 mg per nostril after waiting 5 minutes in initial nonresponders.
Status epilepticus ceased both clinically and by EEG in 24 of the 42 patients, or 57%, in an average of 5 minutes after administration of the intranasal medication at a mean dose of 5.6 mg. Nonresponders received a mean dose of 7.5 mg. There were no significant differences between responders and nonresponders in terms of the proportion presenting with preexisting epilepsy or the epilepsy etiology. However, responders presented at a mean of 54 minutes in status epilepticus, while nonresponders had been in status for 17 minutes.
The 57% response rate with intranasal midazolam is comparable with other investigators’ reported success rates using other benzodiazepines and routes of administration, she noted.
Session cochair Gregory Krauss, MD, commented that he thought the Frankfurt neurologists may have been too cautious in their dosing of intranasal midazolam for status epilepticus.
“Often in the U.S. 5 mg is initially used in each nostril,” according to Dr. Krauss, professor of neurology at Johns Hopkins University, Baltimore.
Dr. Kay reported having no financial conflicts of interest regarding her study.
SOURCE: Kay L et al. IEC 2019, Abstract P029.
REPORTING FROM IEC 2019
A practical tool predicts childhood epilepsy diagnosis
BANGKOK – A prediction tool that determines the risk of a pediatric epilepsy diagnosis eventually being made in a child who has had one or more paroxysmal events of possible epileptic origin is now available, and the clarity it provides makes life considerably easier for physicians and worried parents, Kees P. Braun, MD, PhD, said at the International Epilepsy Congress.
This prediction tool is highly practical. It relies upon certain clinical characteristics and a first interictal EEG, all information readily available at the time of the family’s first consultation with a neurologist or pediatrician with access to EEG, noted Dr. Braun, professor of neurology at Utrecht (the Netherlands) University.
The tool is freely available online (http://epilepsypredictiontools.info/first-consultation). The details of how Dr. Braun and coinvestigators developed the prediction tool have been published (Pediatrics. 2018 Dec;142[6]:e20180931. doi: 10.1542/peds.2018-0931), he said at the congress sponsored by the International League Against Epilepsy.
Early and accurate diagnosis or exclusion of epilepsy following a suspicious paroxysmal event deserves to be a high priority. Diagnostic delay is common, with resultant unrecognized recurrent epileptic seizures that can cause cognitive and behavioral impairments. And overdiagnosis of pediatric epilepsy unnecessarily exposes a child to the risks of antiepileptic drug therapy, not to mention the potential social stigma.
The predictive tool was developed through retrospective, multidimensional analysis of detailed data on 451 children who visited the outpatient pediatric neurology clinic at University Medical Center Utrecht for a diagnostic work-up after one or more paroxysmal events that might have been seizures, all of whom were subsequently followed for a year or longer. The resultant predictive model was then independently validated in a separate cohort of 187 children seen for the same reason at another Dutch university.
The model had an area under the receiver operating characteristic curve of 0.86, which statisticians consider to be excellent discriminatory power. The tool’s sensitivity and specificity varied according to the diagnostic probability threshold selected by the parents and physicians. For example, the predictive tool had a sensitivity of 18%, specificity of 99%, positive predictive value of 94%, and negative predictive value of 80% for identification of individuals with a greater than 80% probability of being diagnosed with epilepsy. For identification of all patients with a greater than 20% likelihood of receiving the diagnosis, the sensitivity was 73%, specificity 82%, positive predictive value 76%, and negative predictive value 79%.
The clinical characteristics incorporated in the predictive model include age at first seizure, gender, details of the paroxysmal event, and specifics of the child’s medical history. The relevant features of the standard interictal EEG recorded at the time of consultation include the presence or absence of focal epileptiform abnormalities if focal spikes or spike-wave complexes were detected, generalized epileptiform abnormalities in the presence of generalized spikes or spike-wave complexes, and nonspecific nonepileptiform abnormalities.
Future predictive refinements are under study
Dr. Braun and coworkers have reported that examining EEG functional network characteristics – that is, the functional networks of correlated brain activity in an individual patient’s brain – improves the EEG’s predictive value for epilepsy (PLoS One. 2013;8[4]:e59764. doi: 10.1371/journal.pone.0059764), a conclusion further reinforced in their systematic review and meta-analysis incorporating 11 additional studies (PLoS One. 2014 Dec 10;9[12]:e114606. doi: 10.1371/journal.pone.0114606).
In addition, the Dutch investigators have shown that ripples superimposed on rolandic spikes seen in scalp EEG recordings have prognostic significance. An absence of ripples superimposed on rolandic spikes identified children without epilepsy. In contrast, more than five ripples predicted atypical and symptomatic rolandic epilepsy with a substantial seizure risk warranting consideration of antiepileptic drug therapy (Epilepsia. 2016 Jul;57[7]:1179-89).
A Boston group using a fully automated spike ripple detector subsequently confirmed that ripples occurring in conjunction with epileptiform discharges on scalp EEG constitute a noninvasive biomarker for seizure risk that outperforms analysis of spikes alone and could potentially be useful in guiding medication tapering decisions in children (Brain. 2019 May 1;142[5]:1296-1309).
Dr. Braun reported having no financial conflicts regarding his presentation.
BANGKOK – A prediction tool that determines the risk of a pediatric epilepsy diagnosis eventually being made in a child who has had one or more paroxysmal events of possible epileptic origin is now available, and the clarity it provides makes life considerably easier for physicians and worried parents, Kees P. Braun, MD, PhD, said at the International Epilepsy Congress.
This prediction tool is highly practical. It relies upon certain clinical characteristics and a first interictal EEG, all information readily available at the time of the family’s first consultation with a neurologist or pediatrician with access to EEG, noted Dr. Braun, professor of neurology at Utrecht (the Netherlands) University.
The tool is freely available online (http://epilepsypredictiontools.info/first-consultation). The details of how Dr. Braun and coinvestigators developed the prediction tool have been published (Pediatrics. 2018 Dec;142[6]:e20180931. doi: 10.1542/peds.2018-0931), he said at the congress sponsored by the International League Against Epilepsy.
Early and accurate diagnosis or exclusion of epilepsy following a suspicious paroxysmal event deserves to be a high priority. Diagnostic delay is common, with resultant unrecognized recurrent epileptic seizures that can cause cognitive and behavioral impairments. And overdiagnosis of pediatric epilepsy unnecessarily exposes a child to the risks of antiepileptic drug therapy, not to mention the potential social stigma.
The predictive tool was developed through retrospective, multidimensional analysis of detailed data on 451 children who visited the outpatient pediatric neurology clinic at University Medical Center Utrecht for a diagnostic work-up after one or more paroxysmal events that might have been seizures, all of whom were subsequently followed for a year or longer. The resultant predictive model was then independently validated in a separate cohort of 187 children seen for the same reason at another Dutch university.
The model had an area under the receiver operating characteristic curve of 0.86, which statisticians consider to be excellent discriminatory power. The tool’s sensitivity and specificity varied according to the diagnostic probability threshold selected by the parents and physicians. For example, the predictive tool had a sensitivity of 18%, specificity of 99%, positive predictive value of 94%, and negative predictive value of 80% for identification of individuals with a greater than 80% probability of being diagnosed with epilepsy. For identification of all patients with a greater than 20% likelihood of receiving the diagnosis, the sensitivity was 73%, specificity 82%, positive predictive value 76%, and negative predictive value 79%.
The clinical characteristics incorporated in the predictive model include age at first seizure, gender, details of the paroxysmal event, and specifics of the child’s medical history. The relevant features of the standard interictal EEG recorded at the time of consultation include the presence or absence of focal epileptiform abnormalities if focal spikes or spike-wave complexes were detected, generalized epileptiform abnormalities in the presence of generalized spikes or spike-wave complexes, and nonspecific nonepileptiform abnormalities.
Future predictive refinements are under study
Dr. Braun and coworkers have reported that examining EEG functional network characteristics – that is, the functional networks of correlated brain activity in an individual patient’s brain – improves the EEG’s predictive value for epilepsy (PLoS One. 2013;8[4]:e59764. doi: 10.1371/journal.pone.0059764), a conclusion further reinforced in their systematic review and meta-analysis incorporating 11 additional studies (PLoS One. 2014 Dec 10;9[12]:e114606. doi: 10.1371/journal.pone.0114606).
In addition, the Dutch investigators have shown that ripples superimposed on rolandic spikes seen in scalp EEG recordings have prognostic significance. An absence of ripples superimposed on rolandic spikes identified children without epilepsy. In contrast, more than five ripples predicted atypical and symptomatic rolandic epilepsy with a substantial seizure risk warranting consideration of antiepileptic drug therapy (Epilepsia. 2016 Jul;57[7]:1179-89).
A Boston group using a fully automated spike ripple detector subsequently confirmed that ripples occurring in conjunction with epileptiform discharges on scalp EEG constitute a noninvasive biomarker for seizure risk that outperforms analysis of spikes alone and could potentially be useful in guiding medication tapering decisions in children (Brain. 2019 May 1;142[5]:1296-1309).
Dr. Braun reported having no financial conflicts regarding his presentation.
BANGKOK – A prediction tool that determines the risk of a pediatric epilepsy diagnosis eventually being made in a child who has had one or more paroxysmal events of possible epileptic origin is now available, and the clarity it provides makes life considerably easier for physicians and worried parents, Kees P. Braun, MD, PhD, said at the International Epilepsy Congress.
This prediction tool is highly practical. It relies upon certain clinical characteristics and a first interictal EEG, all information readily available at the time of the family’s first consultation with a neurologist or pediatrician with access to EEG, noted Dr. Braun, professor of neurology at Utrecht (the Netherlands) University.
The tool is freely available online (http://epilepsypredictiontools.info/first-consultation). The details of how Dr. Braun and coinvestigators developed the prediction tool have been published (Pediatrics. 2018 Dec;142[6]:e20180931. doi: 10.1542/peds.2018-0931), he said at the congress sponsored by the International League Against Epilepsy.
Early and accurate diagnosis or exclusion of epilepsy following a suspicious paroxysmal event deserves to be a high priority. Diagnostic delay is common, with resultant unrecognized recurrent epileptic seizures that can cause cognitive and behavioral impairments. And overdiagnosis of pediatric epilepsy unnecessarily exposes a child to the risks of antiepileptic drug therapy, not to mention the potential social stigma.
The predictive tool was developed through retrospective, multidimensional analysis of detailed data on 451 children who visited the outpatient pediatric neurology clinic at University Medical Center Utrecht for a diagnostic work-up after one or more paroxysmal events that might have been seizures, all of whom were subsequently followed for a year or longer. The resultant predictive model was then independently validated in a separate cohort of 187 children seen for the same reason at another Dutch university.
The model had an area under the receiver operating characteristic curve of 0.86, which statisticians consider to be excellent discriminatory power. The tool’s sensitivity and specificity varied according to the diagnostic probability threshold selected by the parents and physicians. For example, the predictive tool had a sensitivity of 18%, specificity of 99%, positive predictive value of 94%, and negative predictive value of 80% for identification of individuals with a greater than 80% probability of being diagnosed with epilepsy. For identification of all patients with a greater than 20% likelihood of receiving the diagnosis, the sensitivity was 73%, specificity 82%, positive predictive value 76%, and negative predictive value 79%.
The clinical characteristics incorporated in the predictive model include age at first seizure, gender, details of the paroxysmal event, and specifics of the child’s medical history. The relevant features of the standard interictal EEG recorded at the time of consultation include the presence or absence of focal epileptiform abnormalities if focal spikes or spike-wave complexes were detected, generalized epileptiform abnormalities in the presence of generalized spikes or spike-wave complexes, and nonspecific nonepileptiform abnormalities.
Future predictive refinements are under study
Dr. Braun and coworkers have reported that examining EEG functional network characteristics – that is, the functional networks of correlated brain activity in an individual patient’s brain – improves the EEG’s predictive value for epilepsy (PLoS One. 2013;8[4]:e59764. doi: 10.1371/journal.pone.0059764), a conclusion further reinforced in their systematic review and meta-analysis incorporating 11 additional studies (PLoS One. 2014 Dec 10;9[12]:e114606. doi: 10.1371/journal.pone.0114606).
In addition, the Dutch investigators have shown that ripples superimposed on rolandic spikes seen in scalp EEG recordings have prognostic significance. An absence of ripples superimposed on rolandic spikes identified children without epilepsy. In contrast, more than five ripples predicted atypical and symptomatic rolandic epilepsy with a substantial seizure risk warranting consideration of antiepileptic drug therapy (Epilepsia. 2016 Jul;57[7]:1179-89).
A Boston group using a fully automated spike ripple detector subsequently confirmed that ripples occurring in conjunction with epileptiform discharges on scalp EEG constitute a noninvasive biomarker for seizure risk that outperforms analysis of spikes alone and could potentially be useful in guiding medication tapering decisions in children (Brain. 2019 May 1;142[5]:1296-1309).
Dr. Braun reported having no financial conflicts regarding his presentation.
REPORTING FROM IEC 2019
‘Pot’ is still hot for Dravet, Lennox-Gastaut
BANGKOK – Interim results of long-term, open-label extension trials of add-on prescription cannabidiol in patients with Dravet syndrome or Lennox-Gastaut syndrome show sustained, clinically meaningful seizure reductions with no new safety concerns, Anup D. Patel, MD, reported at the International Epilepsy Congress.
“Overall, this is a very promising and sustainable result that we were happy to see,” said Dr. Patel, chief of child neurology at Nationwide Children’s Hospital in Columbus, Ohio.
Epidiolex is the brand name for the plant-derived, highly purified cannabidiol (CBD) in an oil-based oral solution at 100 mg/mL. Dr. Patel has been involved in the medication’s development program since the earliest open-label compassionate use study, which was followed by rigorous phase 3, double-blind, placebo-controlled randomized trials, eventually leading to Food and Drug Administration marketing approval for the treatment of Dravet syndrome and Lennox-Gastaut syndrome in patients 2 years of age or older.
“On June 25th, 2018, history was made: for the first time in United States history, a plant-based derivative of marijuana was approved for use as a medication, and it was also the first FDA-approved treatment for Dravet syndrome,” Dr. Patel noted at the congress sponsored by the International League Against Epilepsy.
A total of 96% of the 289 children with Dravet syndrome who completed the 14-week, double-blind, controlled randomized trials enrolled in the open-label, long-term extension study, during which they were on a median of three concurrent antiepileptic drugs along with a mean modal dose of CBD at 22 mg/kg/day. Although the target maintenance dose of CBD was 20 mg/kg/day, as advised in the product labeling, physicians could reduce or increase the dose up to 30 mg/kg/day.
“In the initial compassionate-use study, our site could go up to 50 mg/kg/day,” according to Dr. Patel. “We have plenty of data showing efficacy and continued safety beyond the FDA-recommended dose.”
In the open-label extension study, the median reduction from baseline in monthly seizure frequency assessed in 12-week intervals up to a maximum of week 72 was 44%-57% for convulsive seizures and 49%-67% for total seizures. More than 80% of patients and/or caregivers reported improvement in the patient’s overall condition as assessed on the Subject/Caregiver Global Impression of Change scale.
The pattern of adverse events associated with CBD has been consistent across all of the studies. The most common side effects are diarrhea in about one-third of patients, sleepiness in one-quarter, and decreased appetite in about one-quarter. Seven percent of patients discontinued the long-term extension trial because of adverse events.
Seventy percent of patients remained in the long-term extension study at 1 year.
Twenty-six patients developed liver transaminase levels greater than three times the upper limit of normal, and of note, 23 of the 26 were on concomitant valproic acid. None met criteria for severe drug-induced liver injury, and all recovered either spontaneously or after a reduction in the dose of CBD or valproic acid. But this association between CBD, valproic acid, and increased risk of mild liver injury has been a consistent finding across the clinical trials program.
“This is a very important clinical pearl to take away,” commented Dr. Patel, who is also a pediatric neurologist at Ohio State University.
The interim results of the long-term, open-label extension study of add-on CBD in patients with Lennox-Gastaut syndrome are similar to the Dravet syndrome study. Overall, 99% of the 368 patients with Lennox-Gastaut syndrome who completed the 14-week, double-blind, randomized trials signed up for the open-label extension. During a median follow-up of 61 weeks, the median percent reduction in seizure frequency as assessed in serial 12-week windows was 48%-70% for drop seizures and 48%-63% for total seizures. Twenty-four percent of patients withdrew from the study. Eighty-eight percent of patients or caregivers reported an improvement in overall condition when assessed at weeks 24 and 48. Forty-seven patients developed elevated transaminase levels – typically within the first 2 months on CBD – and 35 of them were on concomitant valproic acid.
More on drug-drug interactions
Elsewhere at IEC 2019, Gilmour Morrison of GW Pharmaceuticals, the Cambridge, England, company that markets Epidiolex, presented the findings of a series of drug-drug interaction studies involving coadministration of their CBD with clobazam (Sympazan and Onfi), valproate, stiripentol (Diacomit), or midazolam (Versed) in adult epilepsy patients and healthy volunteers. The researchers reported a bidirectional drug-drug interaction between Epidiolex and clobazam resulting in increased levels of the active metabolites of both drugs. The mechanism is believed to involve inhibition of cytochrome P450 2C19. However, there were no interactions with midazolam or valproate, and the slight bump in stiripentol levels when given with CBD didn’t reach the level of a clinically meaningful drug-drug interaction, according to the investigators.
On the horizon, Canadian researchers are investigating the possibility that since both the tetrahydrocannabinol (THC) and CBD components of marijuana have been shown to have anticonvulsant effects, adding a bit of THC to CBD will result in even better seizure control than with pure CBD in patients with Dravet syndrome. Investigators at Toronto’s Hospital for Sick Children have conducted a prospective, open-label study of a product containing CBD and THC in a 50:1 ratio as add-on therapy in 20 children with Dravet syndrome. The dose was 2-16 mg/kg/day of CBD and 0.04-0.32 mg/kg/day of THC. The cannabis plant extract used in the study was produced by Tilray, a Canadian pharmaceutical company.
Nineteen of the 20 patients completed the 20-week study. The sole noncompleter died of SUDEP (sudden unexpected death in epilepsy) deemed treatment unrelated. Patients experienced a median 71% reduction in motor seizures, compared with baseline. Sixty-three percent of patients had at least a 50% reduction in seizure frequency. Elevated liver transaminases occurred in patients on concomitant valproic acid, as did platelet abnormalities, which have not been seen in the Epidiolex studies, noted Dr. Patel, who was not involved in the Canadian study (Ann Clin Transl Neurol. 2018 Aug 1;5[9]:1077-88).
Dr. Patel reported serving as a consultant to Greenwich Biosciences, a U.S. offshoot of GW Pharmaceuticals. He receives research grants from that company as well as from the National Institutes of Health and the Pediatric Epilepsy Research Foundation.
BANGKOK – Interim results of long-term, open-label extension trials of add-on prescription cannabidiol in patients with Dravet syndrome or Lennox-Gastaut syndrome show sustained, clinically meaningful seizure reductions with no new safety concerns, Anup D. Patel, MD, reported at the International Epilepsy Congress.
“Overall, this is a very promising and sustainable result that we were happy to see,” said Dr. Patel, chief of child neurology at Nationwide Children’s Hospital in Columbus, Ohio.
Epidiolex is the brand name for the plant-derived, highly purified cannabidiol (CBD) in an oil-based oral solution at 100 mg/mL. Dr. Patel has been involved in the medication’s development program since the earliest open-label compassionate use study, which was followed by rigorous phase 3, double-blind, placebo-controlled randomized trials, eventually leading to Food and Drug Administration marketing approval for the treatment of Dravet syndrome and Lennox-Gastaut syndrome in patients 2 years of age or older.
“On June 25th, 2018, history was made: for the first time in United States history, a plant-based derivative of marijuana was approved for use as a medication, and it was also the first FDA-approved treatment for Dravet syndrome,” Dr. Patel noted at the congress sponsored by the International League Against Epilepsy.
A total of 96% of the 289 children with Dravet syndrome who completed the 14-week, double-blind, controlled randomized trials enrolled in the open-label, long-term extension study, during which they were on a median of three concurrent antiepileptic drugs along with a mean modal dose of CBD at 22 mg/kg/day. Although the target maintenance dose of CBD was 20 mg/kg/day, as advised in the product labeling, physicians could reduce or increase the dose up to 30 mg/kg/day.
“In the initial compassionate-use study, our site could go up to 50 mg/kg/day,” according to Dr. Patel. “We have plenty of data showing efficacy and continued safety beyond the FDA-recommended dose.”
In the open-label extension study, the median reduction from baseline in monthly seizure frequency assessed in 12-week intervals up to a maximum of week 72 was 44%-57% for convulsive seizures and 49%-67% for total seizures. More than 80% of patients and/or caregivers reported improvement in the patient’s overall condition as assessed on the Subject/Caregiver Global Impression of Change scale.
The pattern of adverse events associated with CBD has been consistent across all of the studies. The most common side effects are diarrhea in about one-third of patients, sleepiness in one-quarter, and decreased appetite in about one-quarter. Seven percent of patients discontinued the long-term extension trial because of adverse events.
Seventy percent of patients remained in the long-term extension study at 1 year.
Twenty-six patients developed liver transaminase levels greater than three times the upper limit of normal, and of note, 23 of the 26 were on concomitant valproic acid. None met criteria for severe drug-induced liver injury, and all recovered either spontaneously or after a reduction in the dose of CBD or valproic acid. But this association between CBD, valproic acid, and increased risk of mild liver injury has been a consistent finding across the clinical trials program.
“This is a very important clinical pearl to take away,” commented Dr. Patel, who is also a pediatric neurologist at Ohio State University.
The interim results of the long-term, open-label extension study of add-on CBD in patients with Lennox-Gastaut syndrome are similar to the Dravet syndrome study. Overall, 99% of the 368 patients with Lennox-Gastaut syndrome who completed the 14-week, double-blind, randomized trials signed up for the open-label extension. During a median follow-up of 61 weeks, the median percent reduction in seizure frequency as assessed in serial 12-week windows was 48%-70% for drop seizures and 48%-63% for total seizures. Twenty-four percent of patients withdrew from the study. Eighty-eight percent of patients or caregivers reported an improvement in overall condition when assessed at weeks 24 and 48. Forty-seven patients developed elevated transaminase levels – typically within the first 2 months on CBD – and 35 of them were on concomitant valproic acid.
More on drug-drug interactions
Elsewhere at IEC 2019, Gilmour Morrison of GW Pharmaceuticals, the Cambridge, England, company that markets Epidiolex, presented the findings of a series of drug-drug interaction studies involving coadministration of their CBD with clobazam (Sympazan and Onfi), valproate, stiripentol (Diacomit), or midazolam (Versed) in adult epilepsy patients and healthy volunteers. The researchers reported a bidirectional drug-drug interaction between Epidiolex and clobazam resulting in increased levels of the active metabolites of both drugs. The mechanism is believed to involve inhibition of cytochrome P450 2C19. However, there were no interactions with midazolam or valproate, and the slight bump in stiripentol levels when given with CBD didn’t reach the level of a clinically meaningful drug-drug interaction, according to the investigators.
On the horizon, Canadian researchers are investigating the possibility that since both the tetrahydrocannabinol (THC) and CBD components of marijuana have been shown to have anticonvulsant effects, adding a bit of THC to CBD will result in even better seizure control than with pure CBD in patients with Dravet syndrome. Investigators at Toronto’s Hospital for Sick Children have conducted a prospective, open-label study of a product containing CBD and THC in a 50:1 ratio as add-on therapy in 20 children with Dravet syndrome. The dose was 2-16 mg/kg/day of CBD and 0.04-0.32 mg/kg/day of THC. The cannabis plant extract used in the study was produced by Tilray, a Canadian pharmaceutical company.
Nineteen of the 20 patients completed the 20-week study. The sole noncompleter died of SUDEP (sudden unexpected death in epilepsy) deemed treatment unrelated. Patients experienced a median 71% reduction in motor seizures, compared with baseline. Sixty-three percent of patients had at least a 50% reduction in seizure frequency. Elevated liver transaminases occurred in patients on concomitant valproic acid, as did platelet abnormalities, which have not been seen in the Epidiolex studies, noted Dr. Patel, who was not involved in the Canadian study (Ann Clin Transl Neurol. 2018 Aug 1;5[9]:1077-88).
Dr. Patel reported serving as a consultant to Greenwich Biosciences, a U.S. offshoot of GW Pharmaceuticals. He receives research grants from that company as well as from the National Institutes of Health and the Pediatric Epilepsy Research Foundation.
BANGKOK – Interim results of long-term, open-label extension trials of add-on prescription cannabidiol in patients with Dravet syndrome or Lennox-Gastaut syndrome show sustained, clinically meaningful seizure reductions with no new safety concerns, Anup D. Patel, MD, reported at the International Epilepsy Congress.
“Overall, this is a very promising and sustainable result that we were happy to see,” said Dr. Patel, chief of child neurology at Nationwide Children’s Hospital in Columbus, Ohio.
Epidiolex is the brand name for the plant-derived, highly purified cannabidiol (CBD) in an oil-based oral solution at 100 mg/mL. Dr. Patel has been involved in the medication’s development program since the earliest open-label compassionate use study, which was followed by rigorous phase 3, double-blind, placebo-controlled randomized trials, eventually leading to Food and Drug Administration marketing approval for the treatment of Dravet syndrome and Lennox-Gastaut syndrome in patients 2 years of age or older.
“On June 25th, 2018, history was made: for the first time in United States history, a plant-based derivative of marijuana was approved for use as a medication, and it was also the first FDA-approved treatment for Dravet syndrome,” Dr. Patel noted at the congress sponsored by the International League Against Epilepsy.
A total of 96% of the 289 children with Dravet syndrome who completed the 14-week, double-blind, controlled randomized trials enrolled in the open-label, long-term extension study, during which they were on a median of three concurrent antiepileptic drugs along with a mean modal dose of CBD at 22 mg/kg/day. Although the target maintenance dose of CBD was 20 mg/kg/day, as advised in the product labeling, physicians could reduce or increase the dose up to 30 mg/kg/day.
“In the initial compassionate-use study, our site could go up to 50 mg/kg/day,” according to Dr. Patel. “We have plenty of data showing efficacy and continued safety beyond the FDA-recommended dose.”
In the open-label extension study, the median reduction from baseline in monthly seizure frequency assessed in 12-week intervals up to a maximum of week 72 was 44%-57% for convulsive seizures and 49%-67% for total seizures. More than 80% of patients and/or caregivers reported improvement in the patient’s overall condition as assessed on the Subject/Caregiver Global Impression of Change scale.
The pattern of adverse events associated with CBD has been consistent across all of the studies. The most common side effects are diarrhea in about one-third of patients, sleepiness in one-quarter, and decreased appetite in about one-quarter. Seven percent of patients discontinued the long-term extension trial because of adverse events.
Seventy percent of patients remained in the long-term extension study at 1 year.
Twenty-six patients developed liver transaminase levels greater than three times the upper limit of normal, and of note, 23 of the 26 were on concomitant valproic acid. None met criteria for severe drug-induced liver injury, and all recovered either spontaneously or after a reduction in the dose of CBD or valproic acid. But this association between CBD, valproic acid, and increased risk of mild liver injury has been a consistent finding across the clinical trials program.
“This is a very important clinical pearl to take away,” commented Dr. Patel, who is also a pediatric neurologist at Ohio State University.
The interim results of the long-term, open-label extension study of add-on CBD in patients with Lennox-Gastaut syndrome are similar to the Dravet syndrome study. Overall, 99% of the 368 patients with Lennox-Gastaut syndrome who completed the 14-week, double-blind, randomized trials signed up for the open-label extension. During a median follow-up of 61 weeks, the median percent reduction in seizure frequency as assessed in serial 12-week windows was 48%-70% for drop seizures and 48%-63% for total seizures. Twenty-four percent of patients withdrew from the study. Eighty-eight percent of patients or caregivers reported an improvement in overall condition when assessed at weeks 24 and 48. Forty-seven patients developed elevated transaminase levels – typically within the first 2 months on CBD – and 35 of them were on concomitant valproic acid.
More on drug-drug interactions
Elsewhere at IEC 2019, Gilmour Morrison of GW Pharmaceuticals, the Cambridge, England, company that markets Epidiolex, presented the findings of a series of drug-drug interaction studies involving coadministration of their CBD with clobazam (Sympazan and Onfi), valproate, stiripentol (Diacomit), or midazolam (Versed) in adult epilepsy patients and healthy volunteers. The researchers reported a bidirectional drug-drug interaction between Epidiolex and clobazam resulting in increased levels of the active metabolites of both drugs. The mechanism is believed to involve inhibition of cytochrome P450 2C19. However, there were no interactions with midazolam or valproate, and the slight bump in stiripentol levels when given with CBD didn’t reach the level of a clinically meaningful drug-drug interaction, according to the investigators.
On the horizon, Canadian researchers are investigating the possibility that since both the tetrahydrocannabinol (THC) and CBD components of marijuana have been shown to have anticonvulsant effects, adding a bit of THC to CBD will result in even better seizure control than with pure CBD in patients with Dravet syndrome. Investigators at Toronto’s Hospital for Sick Children have conducted a prospective, open-label study of a product containing CBD and THC in a 50:1 ratio as add-on therapy in 20 children with Dravet syndrome. The dose was 2-16 mg/kg/day of CBD and 0.04-0.32 mg/kg/day of THC. The cannabis plant extract used in the study was produced by Tilray, a Canadian pharmaceutical company.
Nineteen of the 20 patients completed the 20-week study. The sole noncompleter died of SUDEP (sudden unexpected death in epilepsy) deemed treatment unrelated. Patients experienced a median 71% reduction in motor seizures, compared with baseline. Sixty-three percent of patients had at least a 50% reduction in seizure frequency. Elevated liver transaminases occurred in patients on concomitant valproic acid, as did platelet abnormalities, which have not been seen in the Epidiolex studies, noted Dr. Patel, who was not involved in the Canadian study (Ann Clin Transl Neurol. 2018 Aug 1;5[9]:1077-88).
Dr. Patel reported serving as a consultant to Greenwich Biosciences, a U.S. offshoot of GW Pharmaceuticals. He receives research grants from that company as well as from the National Institutes of Health and the Pediatric Epilepsy Research Foundation.
REPORTING FROM IEC 2019
FUO, pneumonia often distinguishes influenza from RSV in hospitalized young children
LJUBLJANA, SLOVENIA – as the cause of hospitalization in infants and young children, Cihan Papan, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
Dr. Papan, a pediatrician at University Children’s Hospital Mannheim (Germany) and Heidelberg (Germany) University, presented a retrospective single-center study of all 573 children aged under 2 years hospitalized over the course of several seasons for respiratory syncytial virus (RSV) or influenza as confirmed by rapid antigen testing. Even though these are two of the leading causes of hospitalization among young children, there is surprisingly sparse data comparing the two in terms of disease severity and hospital resource utilization, including antibiotic consumption. That information gap provided the basis for this study.
There were 476 children with confirmed RSV, 96 with influenza, and 1 RSV/influenza coinfection. Notably, even though the RSV group had lower temperatures and C-reactive protein levels, they were nevertheless more likely to be treated with antibiotics, by a margin of 29% to 23%.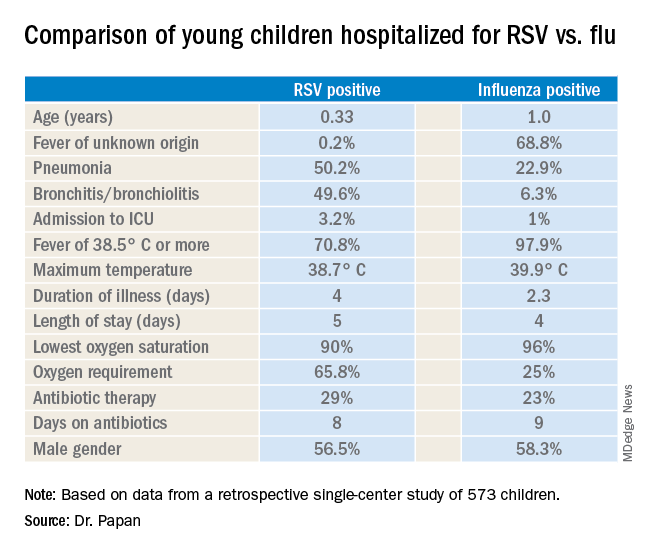
“These findings open new possibilities for antimicrobial stewardship in these groups of virally infected children,” observed Dr. Papan.
Fever of unknown origin was present in 68.8% of the influenza-positive patients, compared with just 0.2% of the RSV-positive children. In contrast, 50.2% of the RSV group had pneumonia and 49.6% had bronchitis or bronchiolitis, versus just 22.9% and 6.3% of the influenza patients, respectively. A larger proportion of the young children with RSV infection presented in a severely ill–looking condition. Children with RSV infection also were significantly younger.
Dr. Papan reported having no financial conflicts regarding his study.
LJUBLJANA, SLOVENIA – as the cause of hospitalization in infants and young children, Cihan Papan, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
Dr. Papan, a pediatrician at University Children’s Hospital Mannheim (Germany) and Heidelberg (Germany) University, presented a retrospective single-center study of all 573 children aged under 2 years hospitalized over the course of several seasons for respiratory syncytial virus (RSV) or influenza as confirmed by rapid antigen testing. Even though these are two of the leading causes of hospitalization among young children, there is surprisingly sparse data comparing the two in terms of disease severity and hospital resource utilization, including antibiotic consumption. That information gap provided the basis for this study.
There were 476 children with confirmed RSV, 96 with influenza, and 1 RSV/influenza coinfection. Notably, even though the RSV group had lower temperatures and C-reactive protein levels, they were nevertheless more likely to be treated with antibiotics, by a margin of 29% to 23%.
“These findings open new possibilities for antimicrobial stewardship in these groups of virally infected children,” observed Dr. Papan.
Fever of unknown origin was present in 68.8% of the influenza-positive patients, compared with just 0.2% of the RSV-positive children. In contrast, 50.2% of the RSV group had pneumonia and 49.6% had bronchitis or bronchiolitis, versus just 22.9% and 6.3% of the influenza patients, respectively. A larger proportion of the young children with RSV infection presented in a severely ill–looking condition. Children with RSV infection also were significantly younger.
Dr. Papan reported having no financial conflicts regarding his study.
LJUBLJANA, SLOVENIA – as the cause of hospitalization in infants and young children, Cihan Papan, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
Dr. Papan, a pediatrician at University Children’s Hospital Mannheim (Germany) and Heidelberg (Germany) University, presented a retrospective single-center study of all 573 children aged under 2 years hospitalized over the course of several seasons for respiratory syncytial virus (RSV) or influenza as confirmed by rapid antigen testing. Even though these are two of the leading causes of hospitalization among young children, there is surprisingly sparse data comparing the two in terms of disease severity and hospital resource utilization, including antibiotic consumption. That information gap provided the basis for this study.
There were 476 children with confirmed RSV, 96 with influenza, and 1 RSV/influenza coinfection. Notably, even though the RSV group had lower temperatures and C-reactive protein levels, they were nevertheless more likely to be treated with antibiotics, by a margin of 29% to 23%.
“These findings open new possibilities for antimicrobial stewardship in these groups of virally infected children,” observed Dr. Papan.
Fever of unknown origin was present in 68.8% of the influenza-positive patients, compared with just 0.2% of the RSV-positive children. In contrast, 50.2% of the RSV group had pneumonia and 49.6% had bronchitis or bronchiolitis, versus just 22.9% and 6.3% of the influenza patients, respectively. A larger proportion of the young children with RSV infection presented in a severely ill–looking condition. Children with RSV infection also were significantly younger.
Dr. Papan reported having no financial conflicts regarding his study.
REPORTING FROM ESPID 2019
No teen herd immunity for 4CMenB in landmark trial
LJUBLJANA, SLOVENIA – The 4CMenB vaccine didn’t affect carriage of disease-causing genogroups of Neisseria meningitidis in adolescents in the landmark Australian cluster-randomized trial of herd immunity known as the “B Part of It” study, Helen S. Marshall, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
This was the largest-ever randomized trial of adolescents vaccinated against meningococcal disease, and the message, albeit somewhat disappointing, is clear: “MenB [Meningococcal serogroup B] vaccine programs should be designed to provide direct protection for those at highest risk of disease,” declared Dr. Marshall, professor of vaccinology and deputy director of the Robinson Research Institute at the University of Adelaide.
In other words, Youths in the age groups at highest risk of disease – infants and adolescents – need to routinely receive the vaccine.
The B Part of It study, whose sheer scope and rigor drew the attention of infectious disease clinical trialists the world over, randomized nearly 35,000 students at all high schools in the state of South Australia – whether urban, rural, or remote – to two doses of the 4CMenB vaccine known as Bexsero or to a nonvaccinated control group. This massive trial entailed training more than 250 nurses in the study procedures and involved 3,100 miles of travel to transport oropharyngeal swab samples obtained from students in outlying areas for centralized laboratory analysis using real-time polymerase chain reaction with meningococcal genotyping, culture for N. meningitidis, and whole-genome sequencing. Samples were obtained on day 1 of the study and 12 months later.
The investigators created widespread regional enthusiasm for this project through adept use of social media and other methods. As a result, 99.5% of students randomized to the intervention arm received one dose, while 97% got two doses. A gratifying unintended consequence of the study was that parents who’d never previously vaccinated their children enrolled them in B Part of It, Dr. Marshall noted.
The impetus for B Part of It was that, while the Australian national health insurance program covers a single dose of meningococcal conjugate MenACWY vaccine given at age 12 months and 14-19 years, MenB vaccine isn’t covered because of uncertainties about cost effectiveness and the vaccine’s impact on meningococcal carriage and herd immunity. B Part of It was designed to resolve those uncertainties.
South Australia has the highest rate of invasive meningococcal disease in the country, and more than 80% of cases there are caused by meningococcal serogroup B. Moreover, 75% of group B cases in South Australia involve the nasty hypervirulent New Zealand strain known as CC 41/44.
The primary outcome in B Part of It was the difference in carriage of the major disease-causing serotypes – groups A, B, C, W, X, and Y – between vaccinated and unvaccinated students at the 1-year follow-up mark. The carriage prevalence of all N. meningitidis in the vaccinated students went from 2.8% at baseline to 4.0% at 12 months, and similarly from 2.6% to 4.7% in unvaccinated controls. More importantly, the prevalence of disease-causing genotypes rose from 1.3% at baseline to 2.4% at follow-up in the vaccinated subjects, with a near-identical pattern seen in controls, where the prevalence rose from 1.4% to 2.4%. In an as-treated analysis, the rate of acquisition of carriage of disease-causing genotypes was identical at 2.0% in both study arms.
The 4CMenB vaccine proved reassuringly safe and effective in preventing meningococcal disease in vaccinated teens. With more than 58,000 doses of the vaccine given in the study, no new safety concerns or signals emerged. And the observed number of cases of invasive meningococcal disease in South Australian adolescent vaccine recipients to date has been significantly lower than expected.
Secondary and exploratory outcomes
Independent risk factors associated with N. meningitidis carriage in the study participants at the 1-year mark included smoking cigarettes or hookah, intimate kissing within the last week, and being in grades 11-12, as opposed to grade 10.
The vaccine had no significant impact on the carriage rate of the hypervirulent New Zealand serogroup B strain. Nor was there a vaccine impact on carriage density, as Mark McMillan, MD, reported elsewhere at ESPID 2019. But while the 4CMenB vaccine had minimal impact upon N. meningitidis carriage density, it was associated with a significant 41% increase in the likelihood of cleared carriage of disease-causing strains at 12 months, added Dr. McMillan, Dr. Marshall’s coinvestigator at University of Adelaide.
What’s next
The ongoing B Part of It School Leaver study is assessing carriage prevalence in vaccinated versus unvaccinated high schoolers in their first year after graduating.
In addition, the B Part of It investigators plan to prospectively study the impact of the 4CMen B vaccine on N. gonorrhoeae disease in an effort to confirm the intriguing findings of an earlier large, retrospective New Zealand case-control study. The Kiwis found that recipients of an outer membrane vesicle MenB vaccine had an adjusted 31% reduction in the risk of gonorrhea. This was the first-ever report of any vaccine effectiveness against this major global public health problem, in which antibiotic resistance is a growing concern (Lancet. 2017 Sep 30;390[10102]:1603-10). Dr. Marshall reported receiving research funding from GlaxoSmithKline, which markets Bexsero and was the major financial supporter of the B Part of It study.
But wait a minute...
Following Dr. Marshall’s report on the B Part of It study, outgoing ESPID president Adam Finn, MD, PhD, presented longitudinal data that he believes raise the possibility that protein-antigen vaccines such as Bexsero, which promote naturally acquired mucosal immunity, may impact on transmission population wide without reliably preventing acquisition. This would stand in stark contrast to conjugate meningococcus vaccines, which have a well-established massive impact on carriage and acquisition of N. meningitidis.
It may be that in studying throat carriage rates once in individuals immunized 12 months earlier, as in the B Part of It study, investigators are not asking the right question, proposed Dr. Finn, professor of pediatrics at the University of Bristol (England).
His research team has been obtaining throat swabs at monthly intervals in a population of 917 high schoolers aged 16-17 years. In 416 of the students, they also have collected saliva samples weekly both before and after immunization with 4CMenB vaccine, analyzing the samples for N. meningitidis by polymerase chain reaction. This is a novel method of studying meningococcal carriage they have found to be both reliable and far more acceptable to patients than oropharyngeal swabbing, which adolescents balk at if asked to do with any frequency (PLoS One. 2019 Feb 11;14[2]:e0209905).
Dr. Finn said that their findings, which need confirmation, suggest that N. meningitidis carriage is usually brief and dynamic. They also have found that carriage density varies markedly from month to month.
“We see much higher-density carriage in the adolescent population in the early months of the year in conjunction, we think, with viral infection with influenza and so forth,” he said, adding that this could have clinical implications. “It feels sort of intuitive that someone walking around with 1,000 or 10,000 times as many meningococci in their throat is more likely to be more infectious to people around them with a very small number, although this hasn’t been formally proven.”
He hopes that the Be on the TEAM (Teenagers Against Meningitis) study will help provide answers. The study is randomizing 24,000 U.K. high school students to vaccination with the meningococcal B protein–antigen vaccines Bexsero or Trumenba or to no vaccine in order to learn if there are significant herd immunity effects.
Dr. Finn’s meningococcal carriage research is funded by the Meningitis Research Foundation and the National Institute for Health Research. Dr. Marshall reported receiving research funding from GlaxoSmithKline, the major sponsor of the B Part of It study.
LJUBLJANA, SLOVENIA – The 4CMenB vaccine didn’t affect carriage of disease-causing genogroups of Neisseria meningitidis in adolescents in the landmark Australian cluster-randomized trial of herd immunity known as the “B Part of It” study, Helen S. Marshall, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
This was the largest-ever randomized trial of adolescents vaccinated against meningococcal disease, and the message, albeit somewhat disappointing, is clear: “MenB [Meningococcal serogroup B] vaccine programs should be designed to provide direct protection for those at highest risk of disease,” declared Dr. Marshall, professor of vaccinology and deputy director of the Robinson Research Institute at the University of Adelaide.
In other words, Youths in the age groups at highest risk of disease – infants and adolescents – need to routinely receive the vaccine.
The B Part of It study, whose sheer scope and rigor drew the attention of infectious disease clinical trialists the world over, randomized nearly 35,000 students at all high schools in the state of South Australia – whether urban, rural, or remote – to two doses of the 4CMenB vaccine known as Bexsero or to a nonvaccinated control group. This massive trial entailed training more than 250 nurses in the study procedures and involved 3,100 miles of travel to transport oropharyngeal swab samples obtained from students in outlying areas for centralized laboratory analysis using real-time polymerase chain reaction with meningococcal genotyping, culture for N. meningitidis, and whole-genome sequencing. Samples were obtained on day 1 of the study and 12 months later.
The investigators created widespread regional enthusiasm for this project through adept use of social media and other methods. As a result, 99.5% of students randomized to the intervention arm received one dose, while 97% got two doses. A gratifying unintended consequence of the study was that parents who’d never previously vaccinated their children enrolled them in B Part of It, Dr. Marshall noted.
The impetus for B Part of It was that, while the Australian national health insurance program covers a single dose of meningococcal conjugate MenACWY vaccine given at age 12 months and 14-19 years, MenB vaccine isn’t covered because of uncertainties about cost effectiveness and the vaccine’s impact on meningococcal carriage and herd immunity. B Part of It was designed to resolve those uncertainties.
South Australia has the highest rate of invasive meningococcal disease in the country, and more than 80% of cases there are caused by meningococcal serogroup B. Moreover, 75% of group B cases in South Australia involve the nasty hypervirulent New Zealand strain known as CC 41/44.
The primary outcome in B Part of It was the difference in carriage of the major disease-causing serotypes – groups A, B, C, W, X, and Y – between vaccinated and unvaccinated students at the 1-year follow-up mark. The carriage prevalence of all N. meningitidis in the vaccinated students went from 2.8% at baseline to 4.0% at 12 months, and similarly from 2.6% to 4.7% in unvaccinated controls. More importantly, the prevalence of disease-causing genotypes rose from 1.3% at baseline to 2.4% at follow-up in the vaccinated subjects, with a near-identical pattern seen in controls, where the prevalence rose from 1.4% to 2.4%. In an as-treated analysis, the rate of acquisition of carriage of disease-causing genotypes was identical at 2.0% in both study arms.
The 4CMenB vaccine proved reassuringly safe and effective in preventing meningococcal disease in vaccinated teens. With more than 58,000 doses of the vaccine given in the study, no new safety concerns or signals emerged. And the observed number of cases of invasive meningococcal disease in South Australian adolescent vaccine recipients to date has been significantly lower than expected.
Secondary and exploratory outcomes
Independent risk factors associated with N. meningitidis carriage in the study participants at the 1-year mark included smoking cigarettes or hookah, intimate kissing within the last week, and being in grades 11-12, as opposed to grade 10.
The vaccine had no significant impact on the carriage rate of the hypervirulent New Zealand serogroup B strain. Nor was there a vaccine impact on carriage density, as Mark McMillan, MD, reported elsewhere at ESPID 2019. But while the 4CMenB vaccine had minimal impact upon N. meningitidis carriage density, it was associated with a significant 41% increase in the likelihood of cleared carriage of disease-causing strains at 12 months, added Dr. McMillan, Dr. Marshall’s coinvestigator at University of Adelaide.
What’s next
The ongoing B Part of It School Leaver study is assessing carriage prevalence in vaccinated versus unvaccinated high schoolers in their first year after graduating.
In addition, the B Part of It investigators plan to prospectively study the impact of the 4CMen B vaccine on N. gonorrhoeae disease in an effort to confirm the intriguing findings of an earlier large, retrospective New Zealand case-control study. The Kiwis found that recipients of an outer membrane vesicle MenB vaccine had an adjusted 31% reduction in the risk of gonorrhea. This was the first-ever report of any vaccine effectiveness against this major global public health problem, in which antibiotic resistance is a growing concern (Lancet. 2017 Sep 30;390[10102]:1603-10). Dr. Marshall reported receiving research funding from GlaxoSmithKline, which markets Bexsero and was the major financial supporter of the B Part of It study.
But wait a minute...
Following Dr. Marshall’s report on the B Part of It study, outgoing ESPID president Adam Finn, MD, PhD, presented longitudinal data that he believes raise the possibility that protein-antigen vaccines such as Bexsero, which promote naturally acquired mucosal immunity, may impact on transmission population wide without reliably preventing acquisition. This would stand in stark contrast to conjugate meningococcus vaccines, which have a well-established massive impact on carriage and acquisition of N. meningitidis.
It may be that in studying throat carriage rates once in individuals immunized 12 months earlier, as in the B Part of It study, investigators are not asking the right question, proposed Dr. Finn, professor of pediatrics at the University of Bristol (England).
His research team has been obtaining throat swabs at monthly intervals in a population of 917 high schoolers aged 16-17 years. In 416 of the students, they also have collected saliva samples weekly both before and after immunization with 4CMenB vaccine, analyzing the samples for N. meningitidis by polymerase chain reaction. This is a novel method of studying meningococcal carriage they have found to be both reliable and far more acceptable to patients than oropharyngeal swabbing, which adolescents balk at if asked to do with any frequency (PLoS One. 2019 Feb 11;14[2]:e0209905).
Dr. Finn said that their findings, which need confirmation, suggest that N. meningitidis carriage is usually brief and dynamic. They also have found that carriage density varies markedly from month to month.
“We see much higher-density carriage in the adolescent population in the early months of the year in conjunction, we think, with viral infection with influenza and so forth,” he said, adding that this could have clinical implications. “It feels sort of intuitive that someone walking around with 1,000 or 10,000 times as many meningococci in their throat is more likely to be more infectious to people around them with a very small number, although this hasn’t been formally proven.”
He hopes that the Be on the TEAM (Teenagers Against Meningitis) study will help provide answers. The study is randomizing 24,000 U.K. high school students to vaccination with the meningococcal B protein–antigen vaccines Bexsero or Trumenba or to no vaccine in order to learn if there are significant herd immunity effects.
Dr. Finn’s meningococcal carriage research is funded by the Meningitis Research Foundation and the National Institute for Health Research. Dr. Marshall reported receiving research funding from GlaxoSmithKline, the major sponsor of the B Part of It study.
LJUBLJANA, SLOVENIA – The 4CMenB vaccine didn’t affect carriage of disease-causing genogroups of Neisseria meningitidis in adolescents in the landmark Australian cluster-randomized trial of herd immunity known as the “B Part of It” study, Helen S. Marshall, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
This was the largest-ever randomized trial of adolescents vaccinated against meningococcal disease, and the message, albeit somewhat disappointing, is clear: “MenB [Meningococcal serogroup B] vaccine programs should be designed to provide direct protection for those at highest risk of disease,” declared Dr. Marshall, professor of vaccinology and deputy director of the Robinson Research Institute at the University of Adelaide.
In other words, Youths in the age groups at highest risk of disease – infants and adolescents – need to routinely receive the vaccine.
The B Part of It study, whose sheer scope and rigor drew the attention of infectious disease clinical trialists the world over, randomized nearly 35,000 students at all high schools in the state of South Australia – whether urban, rural, or remote – to two doses of the 4CMenB vaccine known as Bexsero or to a nonvaccinated control group. This massive trial entailed training more than 250 nurses in the study procedures and involved 3,100 miles of travel to transport oropharyngeal swab samples obtained from students in outlying areas for centralized laboratory analysis using real-time polymerase chain reaction with meningococcal genotyping, culture for N. meningitidis, and whole-genome sequencing. Samples were obtained on day 1 of the study and 12 months later.
The investigators created widespread regional enthusiasm for this project through adept use of social media and other methods. As a result, 99.5% of students randomized to the intervention arm received one dose, while 97% got two doses. A gratifying unintended consequence of the study was that parents who’d never previously vaccinated their children enrolled them in B Part of It, Dr. Marshall noted.
The impetus for B Part of It was that, while the Australian national health insurance program covers a single dose of meningococcal conjugate MenACWY vaccine given at age 12 months and 14-19 years, MenB vaccine isn’t covered because of uncertainties about cost effectiveness and the vaccine’s impact on meningococcal carriage and herd immunity. B Part of It was designed to resolve those uncertainties.
South Australia has the highest rate of invasive meningococcal disease in the country, and more than 80% of cases there are caused by meningococcal serogroup B. Moreover, 75% of group B cases in South Australia involve the nasty hypervirulent New Zealand strain known as CC 41/44.
The primary outcome in B Part of It was the difference in carriage of the major disease-causing serotypes – groups A, B, C, W, X, and Y – between vaccinated and unvaccinated students at the 1-year follow-up mark. The carriage prevalence of all N. meningitidis in the vaccinated students went from 2.8% at baseline to 4.0% at 12 months, and similarly from 2.6% to 4.7% in unvaccinated controls. More importantly, the prevalence of disease-causing genotypes rose from 1.3% at baseline to 2.4% at follow-up in the vaccinated subjects, with a near-identical pattern seen in controls, where the prevalence rose from 1.4% to 2.4%. In an as-treated analysis, the rate of acquisition of carriage of disease-causing genotypes was identical at 2.0% in both study arms.
The 4CMenB vaccine proved reassuringly safe and effective in preventing meningococcal disease in vaccinated teens. With more than 58,000 doses of the vaccine given in the study, no new safety concerns or signals emerged. And the observed number of cases of invasive meningococcal disease in South Australian adolescent vaccine recipients to date has been significantly lower than expected.
Secondary and exploratory outcomes
Independent risk factors associated with N. meningitidis carriage in the study participants at the 1-year mark included smoking cigarettes or hookah, intimate kissing within the last week, and being in grades 11-12, as opposed to grade 10.
The vaccine had no significant impact on the carriage rate of the hypervirulent New Zealand serogroup B strain. Nor was there a vaccine impact on carriage density, as Mark McMillan, MD, reported elsewhere at ESPID 2019. But while the 4CMenB vaccine had minimal impact upon N. meningitidis carriage density, it was associated with a significant 41% increase in the likelihood of cleared carriage of disease-causing strains at 12 months, added Dr. McMillan, Dr. Marshall’s coinvestigator at University of Adelaide.
What’s next
The ongoing B Part of It School Leaver study is assessing carriage prevalence in vaccinated versus unvaccinated high schoolers in their first year after graduating.
In addition, the B Part of It investigators plan to prospectively study the impact of the 4CMen B vaccine on N. gonorrhoeae disease in an effort to confirm the intriguing findings of an earlier large, retrospective New Zealand case-control study. The Kiwis found that recipients of an outer membrane vesicle MenB vaccine had an adjusted 31% reduction in the risk of gonorrhea. This was the first-ever report of any vaccine effectiveness against this major global public health problem, in which antibiotic resistance is a growing concern (Lancet. 2017 Sep 30;390[10102]:1603-10). Dr. Marshall reported receiving research funding from GlaxoSmithKline, which markets Bexsero and was the major financial supporter of the B Part of It study.
But wait a minute...
Following Dr. Marshall’s report on the B Part of It study, outgoing ESPID president Adam Finn, MD, PhD, presented longitudinal data that he believes raise the possibility that protein-antigen vaccines such as Bexsero, which promote naturally acquired mucosal immunity, may impact on transmission population wide without reliably preventing acquisition. This would stand in stark contrast to conjugate meningococcus vaccines, which have a well-established massive impact on carriage and acquisition of N. meningitidis.
It may be that in studying throat carriage rates once in individuals immunized 12 months earlier, as in the B Part of It study, investigators are not asking the right question, proposed Dr. Finn, professor of pediatrics at the University of Bristol (England).
His research team has been obtaining throat swabs at monthly intervals in a population of 917 high schoolers aged 16-17 years. In 416 of the students, they also have collected saliva samples weekly both before and after immunization with 4CMenB vaccine, analyzing the samples for N. meningitidis by polymerase chain reaction. This is a novel method of studying meningococcal carriage they have found to be both reliable and far more acceptable to patients than oropharyngeal swabbing, which adolescents balk at if asked to do with any frequency (PLoS One. 2019 Feb 11;14[2]:e0209905).
Dr. Finn said that their findings, which need confirmation, suggest that N. meningitidis carriage is usually brief and dynamic. They also have found that carriage density varies markedly from month to month.
“We see much higher-density carriage in the adolescent population in the early months of the year in conjunction, we think, with viral infection with influenza and so forth,” he said, adding that this could have clinical implications. “It feels sort of intuitive that someone walking around with 1,000 or 10,000 times as many meningococci in their throat is more likely to be more infectious to people around them with a very small number, although this hasn’t been formally proven.”
He hopes that the Be on the TEAM (Teenagers Against Meningitis) study will help provide answers. The study is randomizing 24,000 U.K. high school students to vaccination with the meningococcal B protein–antigen vaccines Bexsero or Trumenba or to no vaccine in order to learn if there are significant herd immunity effects.
Dr. Finn’s meningococcal carriage research is funded by the Meningitis Research Foundation and the National Institute for Health Research. Dr. Marshall reported receiving research funding from GlaxoSmithKline, the major sponsor of the B Part of It study.
REPORTING FROM ESPID 2019
Differential monocytic HLA-DR expression prognostically useful in PICU
LJUBLJANA, SLOVENIA – During their first 4 days in the pediatric ICU, critically ill children have significantly reduced human leukocyte antigen (HLA)–DR expression within all three major subsets of monocytes. The reductions are seen regardless of whether the children were admitted for sepsis, trauma, or after surgery, Navin Boeddha, MD, PhD, reported in his PIDJ Award Lecture at the annual meeting of the European Society for Paediatric Infectious Diseases.
The PIDJ Award is given annually by the editors of the Pediatric Infectious Disease Journal in recognition of what they deem the most important study published in the journal during the prior year. This one stood out because it identified promising potential laboratory markers that have been sought as a prerequisite to developing immunostimulatory therapies aimed at improving outcomes in severely immunosuppressed children.
Researchers are particularly eager to explore this investigative treatment strategy because the mortality and long-term morbidity of pediatric sepsis, in particular, remain unacceptably high. The hope now is that HLA-DR expression on monocyte subsets will be helpful in directing granulocyte-macrophage colony-stimulating factor, interferon-gamma, and other immunostimulatory therapies to the pediatric ICU patients with the most favorable benefit/risk ratio, according to Dr. Boeddha of Sophia Children’s Hospital and Erasmus University, Rotterdam, the Netherlands.
He reported on 37 critically ill children admitted to a pediatric ICU – 12 for sepsis, 11 post surgery, 10 for trauma, and 4 for other reasons – as well as 37 healthy controls. HLA-DR expression on monocyte subsets was measured by flow cytometry upon admission and again on each of the following 3 days.
The impetus for this study is that severe infection, major surgery, and severe trauma are often associated with immunosuppression. And while prior work in septic adults has concluded that decreased monocytic HLA-DR expression is a marker for immunosuppression – and that the lower the level of such expression, the greater the risk of nosocomial infection and death – this phenomenon hasn’t been well studied in critically ill children, he explained.
Dr. Boeddha and coinvestigators found that monocytic HLA-DR expression, which plays a major role in presenting antigens to T cells, decreased over time during the critically ill children’s first 4 days in the pediatric ICU. Moreover, it was lower than in controls at all four time points. This was true both for the percentage of HLA-DR–expressing monocytes of all subsets, as well as for HLA-DR mean fluorescence intensity.
In the critically ill study population as a whole, the percentage of classical monocytes – that is, CD14++ CD16– monocytes – was significantly greater at admission than in healthy controls by margins of 95% and 87%, while the percentage of nonclassical CD14+/-CD16++ monocytes was markedly lower at 2% than the 9% figure in controls.
The biggest discrepancy in monocyte subset distribution was seen in patients admitted for sepsis. Their percentage of classical monocytes was lower than in controls by a margin of 82% versus 87%; however, their proportion of intermediate monocytes (CD14++ CD16+) upon admission was twice that of controls, and it climbed further to 14% on day 2.
Among the key findings in the Rotterdam study: 13 of 37 critically ill patients experienced at least one nosocomial infection while in the pediatric ICU. Their day 2 percentage of HLA-DR–expressing classical monocytes was 42%, strikingly lower than the 78% figure in patients who didn’t develop an infection. Also, the 6 patients who died had only a 33% rate of HLA-DR–expressing classical monocytes on day 3 after pediatric ICU admission versus a 63% rate in survivors of their critical illness.
Thus, low HLA-DR expression on classical monocytes early during the course of a pediatric ICU stay may be the sought-after biomarker that identifies a particularly high-risk subgroup of critically ill children in whom immunostimulatory therapies should be studied. However, future confirmatory studies should monitor monocytic HLA-DR expression in a larger critically ill patient population for a longer period in order to establish the time to recovery of low expression and its impact on long-term complications, the physician said.
Dr. Boeddha reported having no financial conflicts regarding the award-winning study, supported by the European Union and Erasmus University.
SOURCE: Boeddha NP et al. Pediatr Infect Dis J. 2018 Oct;37(10):1034-40.
LJUBLJANA, SLOVENIA – During their first 4 days in the pediatric ICU, critically ill children have significantly reduced human leukocyte antigen (HLA)–DR expression within all three major subsets of monocytes. The reductions are seen regardless of whether the children were admitted for sepsis, trauma, or after surgery, Navin Boeddha, MD, PhD, reported in his PIDJ Award Lecture at the annual meeting of the European Society for Paediatric Infectious Diseases.
The PIDJ Award is given annually by the editors of the Pediatric Infectious Disease Journal in recognition of what they deem the most important study published in the journal during the prior year. This one stood out because it identified promising potential laboratory markers that have been sought as a prerequisite to developing immunostimulatory therapies aimed at improving outcomes in severely immunosuppressed children.
Researchers are particularly eager to explore this investigative treatment strategy because the mortality and long-term morbidity of pediatric sepsis, in particular, remain unacceptably high. The hope now is that HLA-DR expression on monocyte subsets will be helpful in directing granulocyte-macrophage colony-stimulating factor, interferon-gamma, and other immunostimulatory therapies to the pediatric ICU patients with the most favorable benefit/risk ratio, according to Dr. Boeddha of Sophia Children’s Hospital and Erasmus University, Rotterdam, the Netherlands.
He reported on 37 critically ill children admitted to a pediatric ICU – 12 for sepsis, 11 post surgery, 10 for trauma, and 4 for other reasons – as well as 37 healthy controls. HLA-DR expression on monocyte subsets was measured by flow cytometry upon admission and again on each of the following 3 days.
The impetus for this study is that severe infection, major surgery, and severe trauma are often associated with immunosuppression. And while prior work in septic adults has concluded that decreased monocytic HLA-DR expression is a marker for immunosuppression – and that the lower the level of such expression, the greater the risk of nosocomial infection and death – this phenomenon hasn’t been well studied in critically ill children, he explained.
Dr. Boeddha and coinvestigators found that monocytic HLA-DR expression, which plays a major role in presenting antigens to T cells, decreased over time during the critically ill children’s first 4 days in the pediatric ICU. Moreover, it was lower than in controls at all four time points. This was true both for the percentage of HLA-DR–expressing monocytes of all subsets, as well as for HLA-DR mean fluorescence intensity.
In the critically ill study population as a whole, the percentage of classical monocytes – that is, CD14++ CD16– monocytes – was significantly greater at admission than in healthy controls by margins of 95% and 87%, while the percentage of nonclassical CD14+/-CD16++ monocytes was markedly lower at 2% than the 9% figure in controls.
The biggest discrepancy in monocyte subset distribution was seen in patients admitted for sepsis. Their percentage of classical monocytes was lower than in controls by a margin of 82% versus 87%; however, their proportion of intermediate monocytes (CD14++ CD16+) upon admission was twice that of controls, and it climbed further to 14% on day 2.
Among the key findings in the Rotterdam study: 13 of 37 critically ill patients experienced at least one nosocomial infection while in the pediatric ICU. Their day 2 percentage of HLA-DR–expressing classical monocytes was 42%, strikingly lower than the 78% figure in patients who didn’t develop an infection. Also, the 6 patients who died had only a 33% rate of HLA-DR–expressing classical monocytes on day 3 after pediatric ICU admission versus a 63% rate in survivors of their critical illness.
Thus, low HLA-DR expression on classical monocytes early during the course of a pediatric ICU stay may be the sought-after biomarker that identifies a particularly high-risk subgroup of critically ill children in whom immunostimulatory therapies should be studied. However, future confirmatory studies should monitor monocytic HLA-DR expression in a larger critically ill patient population for a longer period in order to establish the time to recovery of low expression and its impact on long-term complications, the physician said.
Dr. Boeddha reported having no financial conflicts regarding the award-winning study, supported by the European Union and Erasmus University.
SOURCE: Boeddha NP et al. Pediatr Infect Dis J. 2018 Oct;37(10):1034-40.
LJUBLJANA, SLOVENIA – During their first 4 days in the pediatric ICU, critically ill children have significantly reduced human leukocyte antigen (HLA)–DR expression within all three major subsets of monocytes. The reductions are seen regardless of whether the children were admitted for sepsis, trauma, or after surgery, Navin Boeddha, MD, PhD, reported in his PIDJ Award Lecture at the annual meeting of the European Society for Paediatric Infectious Diseases.
The PIDJ Award is given annually by the editors of the Pediatric Infectious Disease Journal in recognition of what they deem the most important study published in the journal during the prior year. This one stood out because it identified promising potential laboratory markers that have been sought as a prerequisite to developing immunostimulatory therapies aimed at improving outcomes in severely immunosuppressed children.
Researchers are particularly eager to explore this investigative treatment strategy because the mortality and long-term morbidity of pediatric sepsis, in particular, remain unacceptably high. The hope now is that HLA-DR expression on monocyte subsets will be helpful in directing granulocyte-macrophage colony-stimulating factor, interferon-gamma, and other immunostimulatory therapies to the pediatric ICU patients with the most favorable benefit/risk ratio, according to Dr. Boeddha of Sophia Children’s Hospital and Erasmus University, Rotterdam, the Netherlands.
He reported on 37 critically ill children admitted to a pediatric ICU – 12 for sepsis, 11 post surgery, 10 for trauma, and 4 for other reasons – as well as 37 healthy controls. HLA-DR expression on monocyte subsets was measured by flow cytometry upon admission and again on each of the following 3 days.
The impetus for this study is that severe infection, major surgery, and severe trauma are often associated with immunosuppression. And while prior work in septic adults has concluded that decreased monocytic HLA-DR expression is a marker for immunosuppression – and that the lower the level of such expression, the greater the risk of nosocomial infection and death – this phenomenon hasn’t been well studied in critically ill children, he explained.
Dr. Boeddha and coinvestigators found that monocytic HLA-DR expression, which plays a major role in presenting antigens to T cells, decreased over time during the critically ill children’s first 4 days in the pediatric ICU. Moreover, it was lower than in controls at all four time points. This was true both for the percentage of HLA-DR–expressing monocytes of all subsets, as well as for HLA-DR mean fluorescence intensity.
In the critically ill study population as a whole, the percentage of classical monocytes – that is, CD14++ CD16– monocytes – was significantly greater at admission than in healthy controls by margins of 95% and 87%, while the percentage of nonclassical CD14+/-CD16++ monocytes was markedly lower at 2% than the 9% figure in controls.
The biggest discrepancy in monocyte subset distribution was seen in patients admitted for sepsis. Their percentage of classical monocytes was lower than in controls by a margin of 82% versus 87%; however, their proportion of intermediate monocytes (CD14++ CD16+) upon admission was twice that of controls, and it climbed further to 14% on day 2.
Among the key findings in the Rotterdam study: 13 of 37 critically ill patients experienced at least one nosocomial infection while in the pediatric ICU. Their day 2 percentage of HLA-DR–expressing classical monocytes was 42%, strikingly lower than the 78% figure in patients who didn’t develop an infection. Also, the 6 patients who died had only a 33% rate of HLA-DR–expressing classical monocytes on day 3 after pediatric ICU admission versus a 63% rate in survivors of their critical illness.
Thus, low HLA-DR expression on classical monocytes early during the course of a pediatric ICU stay may be the sought-after biomarker that identifies a particularly high-risk subgroup of critically ill children in whom immunostimulatory therapies should be studied. However, future confirmatory studies should monitor monocytic HLA-DR expression in a larger critically ill patient population for a longer period in order to establish the time to recovery of low expression and its impact on long-term complications, the physician said.
Dr. Boeddha reported having no financial conflicts regarding the award-winning study, supported by the European Union and Erasmus University.
SOURCE: Boeddha NP et al. Pediatr Infect Dis J. 2018 Oct;37(10):1034-40.
REPORTING FROM ESPID 2019
Presepsin can rule out invasive bacterial infection in infants
LJUBLJANA, SLOVENIA – A point-of-care presepsin measurement in the emergency department displayed powerful accuracy for early rule-out of invasive bacterial infection in infants less than 3 months old presenting with fever without a source, based on results of a phase 3 multicenter Italian study.
“P-SEP [presepsin] is a promising new biomarker. P-SEP accuracy for invasive bacterial infection is comparable to procalcitonin, even though P-SEP, like procalcitonin, is probably not accurate enough to be used as a stand-alone marker to rule-in an invasive bacterial infection,” Luca Pierantoni, MD, said in presenting the preliminary study results at the annual meeting of the European Society for Paediatric Infectious Diseases.
The presepsin test is a rapid point-of-care test well-suited for the ED setting, with a cost equal to that of point-of-care procalcitonin.
Presepsin is a form of soluble CD14 that is released from the surface of macrophages, monocytes, and neutrophils when these immune cells are stimulated by pathogens. “We think it may be a reliable diagnostic and prognostic marker of sepsis in adults and neonates,” explained Dr. Pierantoni of the University of Bologna, Italy.
Indeed, studies in adults suggest presepsin has better sensitivity and specificity than other biomarkers for early diagnosis of sepsis, and that it provides useful information on severity and prognosis as well. But, Dr. Pierantoni and his coworkers wondered, how does it perform in febrile young infants?
The Italian study was designed to address an unmet need: Fever accounts for about one-third of ED visits in infants up to age 3 months, 20% of whom are initially categorized as having fever without source. Yet ultimately 10%-20% of those youngsters having fever without source are found to have an invasive bacterial infection – that is, sepsis or meningitis – or a severe bacterial infection such as pneumonia, a urinary tract infection, or an infected umbilical cord. The sooner these infants can be identified and appropriately treated, the better.
The study enrolled 284 children less than 3 months old who had fever without cause of a mean 10.5 hours duration and presented to the emergency departments of six Italian medical centers. Children were eligible for the study regardless of whether they appeared toxic or well. Presepsin, procalcitonin, and C-reactive protein levels were immediately measured in all participants. Ultimately, 5.6% of subjects were diagnosed with an invasive bacterial infection, and another 21.2% had a severe bacterial infection.
Using a cutoff value of 449 pg/mL, P-SEP had good diagnostic accuracy for invasive bacterial infection, with an area under the receiver operating characteristics curve of 0.81, essentially the same as the 0.82 value for procalcitonin. P-SEP had a sensitivity and specificity of 87% and 75%, respectively, placing it in the same ballpark as the 82% and 86% values for procalcitonin. The strong point for P-SEP was its 99% negative predictive value, as compared to 91% for procalcitonin. The positive predictive values were 17% for P-SEP and 20% for procalcitonin.
In response to an audience question, Dr. Pierantoni speculated that the best use for P-SEP in the setting of fever of unknown origin may be in combination with procalcitonin rather than as a replacement for it. The research team is now in the process of analyzing their study data to see if that is indeed the case.
He reported having no financial conflicts regarding his study, conducted free of commercial support.
LJUBLJANA, SLOVENIA – A point-of-care presepsin measurement in the emergency department displayed powerful accuracy for early rule-out of invasive bacterial infection in infants less than 3 months old presenting with fever without a source, based on results of a phase 3 multicenter Italian study.
“P-SEP [presepsin] is a promising new biomarker. P-SEP accuracy for invasive bacterial infection is comparable to procalcitonin, even though P-SEP, like procalcitonin, is probably not accurate enough to be used as a stand-alone marker to rule-in an invasive bacterial infection,” Luca Pierantoni, MD, said in presenting the preliminary study results at the annual meeting of the European Society for Paediatric Infectious Diseases.
The presepsin test is a rapid point-of-care test well-suited for the ED setting, with a cost equal to that of point-of-care procalcitonin.
Presepsin is a form of soluble CD14 that is released from the surface of macrophages, monocytes, and neutrophils when these immune cells are stimulated by pathogens. “We think it may be a reliable diagnostic and prognostic marker of sepsis in adults and neonates,” explained Dr. Pierantoni of the University of Bologna, Italy.
Indeed, studies in adults suggest presepsin has better sensitivity and specificity than other biomarkers for early diagnosis of sepsis, and that it provides useful information on severity and prognosis as well. But, Dr. Pierantoni and his coworkers wondered, how does it perform in febrile young infants?
The Italian study was designed to address an unmet need: Fever accounts for about one-third of ED visits in infants up to age 3 months, 20% of whom are initially categorized as having fever without source. Yet ultimately 10%-20% of those youngsters having fever without source are found to have an invasive bacterial infection – that is, sepsis or meningitis – or a severe bacterial infection such as pneumonia, a urinary tract infection, or an infected umbilical cord. The sooner these infants can be identified and appropriately treated, the better.
The study enrolled 284 children less than 3 months old who had fever without cause of a mean 10.5 hours duration and presented to the emergency departments of six Italian medical centers. Children were eligible for the study regardless of whether they appeared toxic or well. Presepsin, procalcitonin, and C-reactive protein levels were immediately measured in all participants. Ultimately, 5.6% of subjects were diagnosed with an invasive bacterial infection, and another 21.2% had a severe bacterial infection.
Using a cutoff value of 449 pg/mL, P-SEP had good diagnostic accuracy for invasive bacterial infection, with an area under the receiver operating characteristics curve of 0.81, essentially the same as the 0.82 value for procalcitonin. P-SEP had a sensitivity and specificity of 87% and 75%, respectively, placing it in the same ballpark as the 82% and 86% values for procalcitonin. The strong point for P-SEP was its 99% negative predictive value, as compared to 91% for procalcitonin. The positive predictive values were 17% for P-SEP and 20% for procalcitonin.
In response to an audience question, Dr. Pierantoni speculated that the best use for P-SEP in the setting of fever of unknown origin may be in combination with procalcitonin rather than as a replacement for it. The research team is now in the process of analyzing their study data to see if that is indeed the case.
He reported having no financial conflicts regarding his study, conducted free of commercial support.
LJUBLJANA, SLOVENIA – A point-of-care presepsin measurement in the emergency department displayed powerful accuracy for early rule-out of invasive bacterial infection in infants less than 3 months old presenting with fever without a source, based on results of a phase 3 multicenter Italian study.
“P-SEP [presepsin] is a promising new biomarker. P-SEP accuracy for invasive bacterial infection is comparable to procalcitonin, even though P-SEP, like procalcitonin, is probably not accurate enough to be used as a stand-alone marker to rule-in an invasive bacterial infection,” Luca Pierantoni, MD, said in presenting the preliminary study results at the annual meeting of the European Society for Paediatric Infectious Diseases.
The presepsin test is a rapid point-of-care test well-suited for the ED setting, with a cost equal to that of point-of-care procalcitonin.
Presepsin is a form of soluble CD14 that is released from the surface of macrophages, monocytes, and neutrophils when these immune cells are stimulated by pathogens. “We think it may be a reliable diagnostic and prognostic marker of sepsis in adults and neonates,” explained Dr. Pierantoni of the University of Bologna, Italy.
Indeed, studies in adults suggest presepsin has better sensitivity and specificity than other biomarkers for early diagnosis of sepsis, and that it provides useful information on severity and prognosis as well. But, Dr. Pierantoni and his coworkers wondered, how does it perform in febrile young infants?
The Italian study was designed to address an unmet need: Fever accounts for about one-third of ED visits in infants up to age 3 months, 20% of whom are initially categorized as having fever without source. Yet ultimately 10%-20% of those youngsters having fever without source are found to have an invasive bacterial infection – that is, sepsis or meningitis – or a severe bacterial infection such as pneumonia, a urinary tract infection, or an infected umbilical cord. The sooner these infants can be identified and appropriately treated, the better.
The study enrolled 284 children less than 3 months old who had fever without cause of a mean 10.5 hours duration and presented to the emergency departments of six Italian medical centers. Children were eligible for the study regardless of whether they appeared toxic or well. Presepsin, procalcitonin, and C-reactive protein levels were immediately measured in all participants. Ultimately, 5.6% of subjects were diagnosed with an invasive bacterial infection, and another 21.2% had a severe bacterial infection.
Using a cutoff value of 449 pg/mL, P-SEP had good diagnostic accuracy for invasive bacterial infection, with an area under the receiver operating characteristics curve of 0.81, essentially the same as the 0.82 value for procalcitonin. P-SEP had a sensitivity and specificity of 87% and 75%, respectively, placing it in the same ballpark as the 82% and 86% values for procalcitonin. The strong point for P-SEP was its 99% negative predictive value, as compared to 91% for procalcitonin. The positive predictive values were 17% for P-SEP and 20% for procalcitonin.
In response to an audience question, Dr. Pierantoni speculated that the best use for P-SEP in the setting of fever of unknown origin may be in combination with procalcitonin rather than as a replacement for it. The research team is now in the process of analyzing their study data to see if that is indeed the case.
He reported having no financial conflicts regarding his study, conducted free of commercial support.
REPORTING FROM ESPID 2019
Key clinical point: A rapid point-of-care measurement of presepsin in the ED can rule out invasive bacterial infection with 99% accuracy in young infants with fever of unknown source.
Major finding: The negative predictive value of a presepsin level below the cutoff value of 449 pg/mL was 99%.
Study details: This was a multicenter Italian observational study of 284 infants less than 3 months old who presented to emergency departments with fever without source.
Disclosures: The presenter reported having no financial conflicts regarding his study, conducted free of commercial support.
‘Substantial burden’ of enterovirus meningitis in young infants
LJUBLJANA, SLOVENIA – A prospective international surveillance study has provided new insights into the surprisingly substantial clinical burden of viral meningitis caused by enteroviruses and human parechoviruses in young infants, Seilesh Kadambari, MBBS, PhD, said in his ESPID Young Investigator Award Lecture at the annual meeting of the European Society for Paediatric Infectious Diseases.
This comprehensive study captured all cases of laboratory-confirmed enterovirus (EV) and human parechovirus (HPeV) meningitis in infants less than 90 days old seen by pediatricians in the United Kingdom and Ireland during a 13-month period starting in July 2014, a time free of outbreaks. Dr. Kadambari, a pediatrician at the University of Oxford (England), was first author of the study. It was for this project, as well as his earlier studies shedding light on congenital viral infections, that he received the Young Investigator honor.
Among the key findings of the U.K./Ireland surveillance study: The incidence of EV/HPeV meningitis was more than twice that of bacterial meningitis in the same age group and more than fivefold higher than that of group B streptococcal meningitis, the No. 1 cause of bacterial meningitis in early infancy. Moreover, more than one-half of infants with EV/HPeV meningitis had low levels of inflammatory markers and no cerebrospinal fluid pleocytosis, which underscores the importance of routinely testing the cerebrospinal fluid for viral causes of meningitis in such patients using modern molecular tools such as multiplex polymerase chain reaction, according to Dr. Kadambari.
“Also, not a single one of the patients with EV/HPeV meningitis had a secondary bacterial infection – and that has important implications for management of our antibiotic stewardship programs,” he observed.
The study (Arch Dis Child. 2019 Jun;104(6):552-7) identified 668 cases of EV meningitis and 35 of HPeV meningitis, for an incidence of 0.79 and 0.04 per 1,000 live births, respectively. The most common clinical presentations were those generally seen in meningitis: fever, irritability, and reduced feeding. Circulatory shock was present in 43% of the infants with HPeV and 27% of the infants with EV infections.
Of infants with EV meningitis, 11% required admission to an intensive care unit, as did 23% of those with HPeV meningitis. Two babies with EV meningitis died and four others had continued neurologic complications at 12 months of follow-up. In contrast, all infants with HPeV survived without long-term sequelae.
Reassuringly, none of the 189 infants who underwent formal hearing testing had sensorineural hearing loss.
The surveillance study data have played an influential role in evidence-based guidelines for EV diagnosis and characterization published by the European Society of Clinical Virology (J Clin Virol. 2018 Apr;101:11-7).
An earlier study led by Dr. Kadambari documented a hefty sevenfold increase in the rate of laboratory-confirmed viral meningo-encephalitis in England and Wales during 2004-2013 across all age groups (J Infect. 2014 Oct;69[4]:326-32).
He attributed this increase to improved diagnosis of viral forms of meningitis through greater use of polymerase chain reaction. The study, based upon National Health Service hospital records, showed that more than 90% of all cases of viral meningo-encephalitis in infants less than 90 days old were caused by EV, a finding that prompted the subsequent prospective U.K./Ireland surveillance study.
Dr. Kadambari closed by noting the past decade had seen a greatly improved ability to diagnose congenital viral infections, but those improvements are not good enough.
“In the decade ahead, we hope to improve the management of this poorly understood group of infections,” the pediatrician promised.
Planned efforts include a cost-effectiveness analysis of a cytomegalovirus vaccine, an ESPID-funded research project aimed at identifying which EV/HPeV strains are most responsible for outbreaks and isolated severe disease, and gaining insight into the host-immunity factors associated with a proclivity to develop EV/HPeV meningitis in early infancy.
Dr. Kadambari reported having no financial conflicts regarding his studies, which was funded largely by Public Health England and university grants.
SOURCE: Kadambari S et al. Arch Dis Child. 2019;104:552-7.
LJUBLJANA, SLOVENIA – A prospective international surveillance study has provided new insights into the surprisingly substantial clinical burden of viral meningitis caused by enteroviruses and human parechoviruses in young infants, Seilesh Kadambari, MBBS, PhD, said in his ESPID Young Investigator Award Lecture at the annual meeting of the European Society for Paediatric Infectious Diseases.
This comprehensive study captured all cases of laboratory-confirmed enterovirus (EV) and human parechovirus (HPeV) meningitis in infants less than 90 days old seen by pediatricians in the United Kingdom and Ireland during a 13-month period starting in July 2014, a time free of outbreaks. Dr. Kadambari, a pediatrician at the University of Oxford (England), was first author of the study. It was for this project, as well as his earlier studies shedding light on congenital viral infections, that he received the Young Investigator honor.
Among the key findings of the U.K./Ireland surveillance study: The incidence of EV/HPeV meningitis was more than twice that of bacterial meningitis in the same age group and more than fivefold higher than that of group B streptococcal meningitis, the No. 1 cause of bacterial meningitis in early infancy. Moreover, more than one-half of infants with EV/HPeV meningitis had low levels of inflammatory markers and no cerebrospinal fluid pleocytosis, which underscores the importance of routinely testing the cerebrospinal fluid for viral causes of meningitis in such patients using modern molecular tools such as multiplex polymerase chain reaction, according to Dr. Kadambari.
“Also, not a single one of the patients with EV/HPeV meningitis had a secondary bacterial infection – and that has important implications for management of our antibiotic stewardship programs,” he observed.
The study (Arch Dis Child. 2019 Jun;104(6):552-7) identified 668 cases of EV meningitis and 35 of HPeV meningitis, for an incidence of 0.79 and 0.04 per 1,000 live births, respectively. The most common clinical presentations were those generally seen in meningitis: fever, irritability, and reduced feeding. Circulatory shock was present in 43% of the infants with HPeV and 27% of the infants with EV infections.
Of infants with EV meningitis, 11% required admission to an intensive care unit, as did 23% of those with HPeV meningitis. Two babies with EV meningitis died and four others had continued neurologic complications at 12 months of follow-up. In contrast, all infants with HPeV survived without long-term sequelae.
Reassuringly, none of the 189 infants who underwent formal hearing testing had sensorineural hearing loss.
The surveillance study data have played an influential role in evidence-based guidelines for EV diagnosis and characterization published by the European Society of Clinical Virology (J Clin Virol. 2018 Apr;101:11-7).
An earlier study led by Dr. Kadambari documented a hefty sevenfold increase in the rate of laboratory-confirmed viral meningo-encephalitis in England and Wales during 2004-2013 across all age groups (J Infect. 2014 Oct;69[4]:326-32).
He attributed this increase to improved diagnosis of viral forms of meningitis through greater use of polymerase chain reaction. The study, based upon National Health Service hospital records, showed that more than 90% of all cases of viral meningo-encephalitis in infants less than 90 days old were caused by EV, a finding that prompted the subsequent prospective U.K./Ireland surveillance study.
Dr. Kadambari closed by noting the past decade had seen a greatly improved ability to diagnose congenital viral infections, but those improvements are not good enough.
“In the decade ahead, we hope to improve the management of this poorly understood group of infections,” the pediatrician promised.
Planned efforts include a cost-effectiveness analysis of a cytomegalovirus vaccine, an ESPID-funded research project aimed at identifying which EV/HPeV strains are most responsible for outbreaks and isolated severe disease, and gaining insight into the host-immunity factors associated with a proclivity to develop EV/HPeV meningitis in early infancy.
Dr. Kadambari reported having no financial conflicts regarding his studies, which was funded largely by Public Health England and university grants.
SOURCE: Kadambari S et al. Arch Dis Child. 2019;104:552-7.
LJUBLJANA, SLOVENIA – A prospective international surveillance study has provided new insights into the surprisingly substantial clinical burden of viral meningitis caused by enteroviruses and human parechoviruses in young infants, Seilesh Kadambari, MBBS, PhD, said in his ESPID Young Investigator Award Lecture at the annual meeting of the European Society for Paediatric Infectious Diseases.
This comprehensive study captured all cases of laboratory-confirmed enterovirus (EV) and human parechovirus (HPeV) meningitis in infants less than 90 days old seen by pediatricians in the United Kingdom and Ireland during a 13-month period starting in July 2014, a time free of outbreaks. Dr. Kadambari, a pediatrician at the University of Oxford (England), was first author of the study. It was for this project, as well as his earlier studies shedding light on congenital viral infections, that he received the Young Investigator honor.
Among the key findings of the U.K./Ireland surveillance study: The incidence of EV/HPeV meningitis was more than twice that of bacterial meningitis in the same age group and more than fivefold higher than that of group B streptococcal meningitis, the No. 1 cause of bacterial meningitis in early infancy. Moreover, more than one-half of infants with EV/HPeV meningitis had low levels of inflammatory markers and no cerebrospinal fluid pleocytosis, which underscores the importance of routinely testing the cerebrospinal fluid for viral causes of meningitis in such patients using modern molecular tools such as multiplex polymerase chain reaction, according to Dr. Kadambari.
“Also, not a single one of the patients with EV/HPeV meningitis had a secondary bacterial infection – and that has important implications for management of our antibiotic stewardship programs,” he observed.
The study (Arch Dis Child. 2019 Jun;104(6):552-7) identified 668 cases of EV meningitis and 35 of HPeV meningitis, for an incidence of 0.79 and 0.04 per 1,000 live births, respectively. The most common clinical presentations were those generally seen in meningitis: fever, irritability, and reduced feeding. Circulatory shock was present in 43% of the infants with HPeV and 27% of the infants with EV infections.
Of infants with EV meningitis, 11% required admission to an intensive care unit, as did 23% of those with HPeV meningitis. Two babies with EV meningitis died and four others had continued neurologic complications at 12 months of follow-up. In contrast, all infants with HPeV survived without long-term sequelae.
Reassuringly, none of the 189 infants who underwent formal hearing testing had sensorineural hearing loss.
The surveillance study data have played an influential role in evidence-based guidelines for EV diagnosis and characterization published by the European Society of Clinical Virology (J Clin Virol. 2018 Apr;101:11-7).
An earlier study led by Dr. Kadambari documented a hefty sevenfold increase in the rate of laboratory-confirmed viral meningo-encephalitis in England and Wales during 2004-2013 across all age groups (J Infect. 2014 Oct;69[4]:326-32).
He attributed this increase to improved diagnosis of viral forms of meningitis through greater use of polymerase chain reaction. The study, based upon National Health Service hospital records, showed that more than 90% of all cases of viral meningo-encephalitis in infants less than 90 days old were caused by EV, a finding that prompted the subsequent prospective U.K./Ireland surveillance study.
Dr. Kadambari closed by noting the past decade had seen a greatly improved ability to diagnose congenital viral infections, but those improvements are not good enough.
“In the decade ahead, we hope to improve the management of this poorly understood group of infections,” the pediatrician promised.
Planned efforts include a cost-effectiveness analysis of a cytomegalovirus vaccine, an ESPID-funded research project aimed at identifying which EV/HPeV strains are most responsible for outbreaks and isolated severe disease, and gaining insight into the host-immunity factors associated with a proclivity to develop EV/HPeV meningitis in early infancy.
Dr. Kadambari reported having no financial conflicts regarding his studies, which was funded largely by Public Health England and university grants.
SOURCE: Kadambari S et al. Arch Dis Child. 2019;104:552-7.
REPORTING FROM ESPID 2019

















