User login
FDA takes more steps to keep kids off tobacco products
New regulations from the Food and Drug Administration ban the sale of all tobacco products – including e-cigarettes – to minors.
The final rule announced May 5 extends FDA regulatory authority to e-cigarettes, cigars, hookah tobacco, pipe tobacco, and other similar products. Prior to this, FDA regulated cigarettes, cigarette tobacco, roll-your-own tobacco, and smokeless tobacco under the Tobacco Control Act of 2009.
The new regulations requires manufacturers of all newly regulated products to show that their products meet applicable public health standards and that makers receive marketing authorization from the FDA. The agency now has the authority to evaluate ingredients, product design, and health risks of these products.
U.S. Department of Health & Human Services Secretary Sylvia Burwell said the move was critical to improve public health and protect future generations from the dangers of tobacco.
“As cigarette smoking among those under 18 has fallen, the use of other nicotine products, including e-cigarettes, has taken a drastic leap,” Secretary Burwell said in a statement. “All of this is creating a new generation of Americans who are at risk of addiction. Today’s announcement is an important step in the fight for a tobacco-free generation – it will help us catch up with changes in the marketplace, put into place rules that protect our kids and give adults information they need to make informed decisions.”
Before the rule, no federal law prohibited stores and websites from selling e-cigarettes, hookah tobacco, and cigars to minors, according to an FDA fact sheet. The new rule aims to deter youth access to the products by barring sales to persons under 18, requiring age verification of purchasers, preventing the selling of covered tobacco products in vending machines, and prohibiting the distribution of free samples.
In addition, manufacturers, importers, and retailers of newly regulated tobacco products must follow provisions, such as registering their manufacturing establishments and providing product listings to the FDA, reporting ingredients and potentially harmful constituents to the agency, and placing health warnings on packages and advertisements, among other guidelines. Manufacturers of newly regulated products must meet the applicable public health standards, unless the product was on the market as of Feb. 15, 2007.
FDA Commissioner Dr. Robert M. Califf said the rule marks a milestone in patient protection.
“As a physician, I’ve seen first hand the devastating health effects of tobacco use,” Dr. Califf said in a statement. “At the FDA, we must do our job under the Tobacco Control Act to reduce the harms caused by tobacco. That includes ensuring consumers have the information they need to make informed decisions about tobacco use and making sure that new tobacco products for purchase come under comprehensive FDA review.”
It’s about time that e-cigarettes are regulated like other tobacco products, said Dr. Roy Herbst, chief of medical oncology at Yale Cancer Center and Smilow Cancer Hospital at Yale-New Haven (Conn).
“It’s been quite some time that e-cigarettes have been gaining momentum in our communities and now to finally have them under the purview of the FDA is huge,” Dr. Herbst said in an interview. “It will force more science and research into these products. ... If these are so safe, they should go through clinical studies to prove that.”
Dr. Herbst noted that within 90 days of the published rule, retailers can no longer sell the products to minors, and that within 3 years, the products will be regulated like other tobacco products.
Manufacturers will be allowed to continue selling their products for up to 3 years while they submit a new tobacco product application and the FDA reviews the application, according to the FDA.
Physicians and other health advocates expressed support for the regulations.
“The AMA supports the FDA’s new rule and its efforts to ensure the public – especially young people – is aware of and protected from these harmful products,” AMA President Dr. Steven J. Stack said in a statement. “The new FDA rule, which fills the gap in federal regulations on purchasing, labeling, and packaging of e-cigarettes; cigars; and other tobacco products, is a notable and important step that will ban the sale of these products to minors and improve public health. However, we urge FDA to issue further regulations addressing marketing of these products and banning flavored e-cigarettes, which are particularly enticing to minors.”
The American Academy of Pediatrics also suggested more steps to curb tobacco use.
“The rule is a welcomed starting point, but it is only a framework upon which to build meaningful regulation to end the tobacco epidemic in the United States once and for all,” American Academy of Pediatrics President Dr. Benard P. Dreyer said in a statement. “Today’s action marks an historic step forward in helping to alleviate the threat of lifelong nicotine addiction for our youth, and should serve as a foundation for further progress when it comes to keeping children safe from dangerous tobacco products.”
The rule will be published in the Federal Register on May 10.
On Twitter @legal_med
New regulations from the Food and Drug Administration ban the sale of all tobacco products – including e-cigarettes – to minors.
The final rule announced May 5 extends FDA regulatory authority to e-cigarettes, cigars, hookah tobacco, pipe tobacco, and other similar products. Prior to this, FDA regulated cigarettes, cigarette tobacco, roll-your-own tobacco, and smokeless tobacco under the Tobacco Control Act of 2009.
The new regulations requires manufacturers of all newly regulated products to show that their products meet applicable public health standards and that makers receive marketing authorization from the FDA. The agency now has the authority to evaluate ingredients, product design, and health risks of these products.
U.S. Department of Health & Human Services Secretary Sylvia Burwell said the move was critical to improve public health and protect future generations from the dangers of tobacco.
“As cigarette smoking among those under 18 has fallen, the use of other nicotine products, including e-cigarettes, has taken a drastic leap,” Secretary Burwell said in a statement. “All of this is creating a new generation of Americans who are at risk of addiction. Today’s announcement is an important step in the fight for a tobacco-free generation – it will help us catch up with changes in the marketplace, put into place rules that protect our kids and give adults information they need to make informed decisions.”
Before the rule, no federal law prohibited stores and websites from selling e-cigarettes, hookah tobacco, and cigars to minors, according to an FDA fact sheet. The new rule aims to deter youth access to the products by barring sales to persons under 18, requiring age verification of purchasers, preventing the selling of covered tobacco products in vending machines, and prohibiting the distribution of free samples.
In addition, manufacturers, importers, and retailers of newly regulated tobacco products must follow provisions, such as registering their manufacturing establishments and providing product listings to the FDA, reporting ingredients and potentially harmful constituents to the agency, and placing health warnings on packages and advertisements, among other guidelines. Manufacturers of newly regulated products must meet the applicable public health standards, unless the product was on the market as of Feb. 15, 2007.
FDA Commissioner Dr. Robert M. Califf said the rule marks a milestone in patient protection.
“As a physician, I’ve seen first hand the devastating health effects of tobacco use,” Dr. Califf said in a statement. “At the FDA, we must do our job under the Tobacco Control Act to reduce the harms caused by tobacco. That includes ensuring consumers have the information they need to make informed decisions about tobacco use and making sure that new tobacco products for purchase come under comprehensive FDA review.”
It’s about time that e-cigarettes are regulated like other tobacco products, said Dr. Roy Herbst, chief of medical oncology at Yale Cancer Center and Smilow Cancer Hospital at Yale-New Haven (Conn).
“It’s been quite some time that e-cigarettes have been gaining momentum in our communities and now to finally have them under the purview of the FDA is huge,” Dr. Herbst said in an interview. “It will force more science and research into these products. ... If these are so safe, they should go through clinical studies to prove that.”
Dr. Herbst noted that within 90 days of the published rule, retailers can no longer sell the products to minors, and that within 3 years, the products will be regulated like other tobacco products.
Manufacturers will be allowed to continue selling their products for up to 3 years while they submit a new tobacco product application and the FDA reviews the application, according to the FDA.
Physicians and other health advocates expressed support for the regulations.
“The AMA supports the FDA’s new rule and its efforts to ensure the public – especially young people – is aware of and protected from these harmful products,” AMA President Dr. Steven J. Stack said in a statement. “The new FDA rule, which fills the gap in federal regulations on purchasing, labeling, and packaging of e-cigarettes; cigars; and other tobacco products, is a notable and important step that will ban the sale of these products to minors and improve public health. However, we urge FDA to issue further regulations addressing marketing of these products and banning flavored e-cigarettes, which are particularly enticing to minors.”
The American Academy of Pediatrics also suggested more steps to curb tobacco use.
“The rule is a welcomed starting point, but it is only a framework upon which to build meaningful regulation to end the tobacco epidemic in the United States once and for all,” American Academy of Pediatrics President Dr. Benard P. Dreyer said in a statement. “Today’s action marks an historic step forward in helping to alleviate the threat of lifelong nicotine addiction for our youth, and should serve as a foundation for further progress when it comes to keeping children safe from dangerous tobacco products.”
The rule will be published in the Federal Register on May 10.
On Twitter @legal_med
New regulations from the Food and Drug Administration ban the sale of all tobacco products – including e-cigarettes – to minors.
The final rule announced May 5 extends FDA regulatory authority to e-cigarettes, cigars, hookah tobacco, pipe tobacco, and other similar products. Prior to this, FDA regulated cigarettes, cigarette tobacco, roll-your-own tobacco, and smokeless tobacco under the Tobacco Control Act of 2009.
The new regulations requires manufacturers of all newly regulated products to show that their products meet applicable public health standards and that makers receive marketing authorization from the FDA. The agency now has the authority to evaluate ingredients, product design, and health risks of these products.
U.S. Department of Health & Human Services Secretary Sylvia Burwell said the move was critical to improve public health and protect future generations from the dangers of tobacco.
“As cigarette smoking among those under 18 has fallen, the use of other nicotine products, including e-cigarettes, has taken a drastic leap,” Secretary Burwell said in a statement. “All of this is creating a new generation of Americans who are at risk of addiction. Today’s announcement is an important step in the fight for a tobacco-free generation – it will help us catch up with changes in the marketplace, put into place rules that protect our kids and give adults information they need to make informed decisions.”
Before the rule, no federal law prohibited stores and websites from selling e-cigarettes, hookah tobacco, and cigars to minors, according to an FDA fact sheet. The new rule aims to deter youth access to the products by barring sales to persons under 18, requiring age verification of purchasers, preventing the selling of covered tobacco products in vending machines, and prohibiting the distribution of free samples.
In addition, manufacturers, importers, and retailers of newly regulated tobacco products must follow provisions, such as registering their manufacturing establishments and providing product listings to the FDA, reporting ingredients and potentially harmful constituents to the agency, and placing health warnings on packages and advertisements, among other guidelines. Manufacturers of newly regulated products must meet the applicable public health standards, unless the product was on the market as of Feb. 15, 2007.
FDA Commissioner Dr. Robert M. Califf said the rule marks a milestone in patient protection.
“As a physician, I’ve seen first hand the devastating health effects of tobacco use,” Dr. Califf said in a statement. “At the FDA, we must do our job under the Tobacco Control Act to reduce the harms caused by tobacco. That includes ensuring consumers have the information they need to make informed decisions about tobacco use and making sure that new tobacco products for purchase come under comprehensive FDA review.”
It’s about time that e-cigarettes are regulated like other tobacco products, said Dr. Roy Herbst, chief of medical oncology at Yale Cancer Center and Smilow Cancer Hospital at Yale-New Haven (Conn).
“It’s been quite some time that e-cigarettes have been gaining momentum in our communities and now to finally have them under the purview of the FDA is huge,” Dr. Herbst said in an interview. “It will force more science and research into these products. ... If these are so safe, they should go through clinical studies to prove that.”
Dr. Herbst noted that within 90 days of the published rule, retailers can no longer sell the products to minors, and that within 3 years, the products will be regulated like other tobacco products.
Manufacturers will be allowed to continue selling their products for up to 3 years while they submit a new tobacco product application and the FDA reviews the application, according to the FDA.
Physicians and other health advocates expressed support for the regulations.
“The AMA supports the FDA’s new rule and its efforts to ensure the public – especially young people – is aware of and protected from these harmful products,” AMA President Dr. Steven J. Stack said in a statement. “The new FDA rule, which fills the gap in federal regulations on purchasing, labeling, and packaging of e-cigarettes; cigars; and other tobacco products, is a notable and important step that will ban the sale of these products to minors and improve public health. However, we urge FDA to issue further regulations addressing marketing of these products and banning flavored e-cigarettes, which are particularly enticing to minors.”
The American Academy of Pediatrics also suggested more steps to curb tobacco use.
“The rule is a welcomed starting point, but it is only a framework upon which to build meaningful regulation to end the tobacco epidemic in the United States once and for all,” American Academy of Pediatrics President Dr. Benard P. Dreyer said in a statement. “Today’s action marks an historic step forward in helping to alleviate the threat of lifelong nicotine addiction for our youth, and should serve as a foundation for further progress when it comes to keeping children safe from dangerous tobacco products.”
The rule will be published in the Federal Register on May 10.
On Twitter @legal_med
Supreme Court case could expand false claims liability
A case before the U.S. Supreme Court could expand physicians’ liability under the False Claims Act (FCA).
The case of Escobar v. Universal Health Services centers on the theory of implied certification and how that legal test should be used to determine whether a claim for payment is fraudulent.
The case “is an opportunity for the Supreme Court to figure out how far the False Claims Act is going to stretch,” said Lawrence M. Kraus, a Boston health law attorney who attended the April 19 oral arguments. “On the practical level, it may have an impact as to whether [such] cases get dismissed at an early stage or whether they go into the discovery phase, which can be quite long, unpleasant, and expensive.”
The Escobar case arises from the death of a patient who was treated at a Lawrence, Mass., mental health clinic operated by Universal Health Services. The patient died from an alleged adverse reaction to medication prescribed for her by clinic staff, according to allegations by her family. The patient’s father, Julio Escobar, later learned counselors and psychologists involved in his daughter’s treatment were not licensed, were not properly supervised by a physician, and had lied about their medical credentials, according to court documents.
The Massachusetts Department of Public Health found the clinic had violated 14 distinct regulations, including those relating to staff licensure and supervision. As a result of the investigation, the clinic entered into a correction plan with the agency and paid a civil fine.
Mr. Escobar and his wife then filed suit under the FCA and the Massachusetts False Claims Act, claiming that Universal had presented false claims to Medicaid by seeking payments for services provided by unlicensed, unsupervised health care providers. Although the reimbursement claims submitted to the government accurately described the services provided and cited the correct charges, the plaintiffs alleged that because the clinic’s operations violated state requirements to participate in Medicaid, Universal had also violated the FCA. The federal government intervened in the case on behalf of the Escobars.
Universal countered that the FCA suit was invalid because a reimbursement claim cannot be false unless its details are untrue or inaccurate.
The plaintiffs, however, contend that a claim does not have to include explicit false statements to be fraudulent. Rather, their complaint relies on “implied certification,” a theory holding that any submission for government payment includes an implicit certification that the health provider has complied with all applicable contract requirements, laws, and regulations that could be a condition of payment. Universal falsely claimed entitlement when it submitted reimbursement requests that did not conform to applicable laws, the plaintiffs argued.
The 1st U.S. Circuit Court of Appeals ruled in favor of Escobar, and Universal appealed to the Supreme Court.
Circuit courts across the country have split on the issue, Mr. Kraus noted.
“There have been a number of different approaches from appeals courts in the country,” he said. “This is not a new issue, but one that the Supreme Court found important enough to decide.”
Why should doctors care about this case?
A ruling for the plaintiff could increase the chances that physicians are accused of an FCA violation after submitting a claim for payment, said William W. Horton, a Birmingham, Ala., health law attorney and chair of the American Bar Association Health Law Section.
“The problem that this raises for health care providers is: There is an enormous web of laws and regulations out there, many of which don’t have anything to do with whether a particular service was rendered or not,” Mr. Horton said in an interview “If you adopt the implied certification theory and take a broad view, than you significantly enhance the scope of claims that could be pursued under the False Claims Act.”
Mr. Horton provides this example: Take a physician group that has an in-office lab, and assume that for some technical reason, the group doesn’t satisfy the Stark Law exception for in-office ancillary services. If a physician in the group refers a Medicare patient to the lab and the group bills Medicare, that’s a Stark Law violation because the group didn’t meet the Stark exception, even if there’s no dispute over whether the patient needed the test or whether the test was done correctly, or whether the Medicare claim accurately reflected the charges, he said. By broadly applying the implied certification theory to this scenario, a case could be made that the practice violated the FCA in submitting the claim because the group was implicitly certifying that the claim did not result from a referral that violated the Stark Law.
“The group could be found liable for the enormous penalties available under the False Claims Act even though the services rendered were medically necessary and appropriate, and even though the group did not expressly certify, in so many words, that the claim did not result from a referral that violated the Stark Law,” Mr. Horton said.
Medical associations, including the American Medical Association and American Hospital Association have weighed in on the case in favor of Universal Health Services. In its brief, the AMA said there is a “sharp distinction” between statutory, regulatory, or contractual violations and false or fraudulent claims.
“Implied certification claims find no support in the statute and do not resemble claims Congress had in mind when enacting or amending the FCA,” according to the brief. “They deprive contractors of their constitutional rights to have notice that they are engaging in conduct subject to heightened sanctions.”
How might the Supreme Court rule?
During oral arguments on April 19, some justices appeared to indicate which way they are leaning, Mr. Kraus said.
Chief Justice John Roberts seemed concerned about the reach of the FCA under the implied certification theory. He raised questions about how people conducting business with the government would know about each and every regulation that could apply as a condition of payment.
Associate Justice Sonia Sotomayer and Associate Justice Elena Kagan appeared in favor of implied certification, while Associate Justice Samuel Alito Jr., Associate Justice Clarence Thomas, and Associate Justice Ruth Bader-Ginsberg did not display a strong opinion either way, Mr. Kraus said. Associate Justice Stephen Breyer appeared to be conflicted, asking for guidance from Roy T. Englert, an attorney for Universal Health Services.
“I’m asking for advice from you, from your point of view,” Justice Breyer said to Mr. Englert. “What the sentence in the opinion should say that describes the circumstances under which the person who submits a form saying, ‘I want a thousand dollars. I just supplied the guns or the medical care.’ ... When has that person committed fraud? – Or that’s what I want. What is the sentence you want me to write?”
Justices could rule a number of ways. They could uphold the appeals court decision, which would affirm a broad interpretation of implied certification theory. They could rule that the implied certification theory is valid, but it cannot be stretched as far as the appeals court expanded it. Justices could choose to reject the implied certification theory altogether and decide that the government must expressly identify every condition of payment in which a health provider is certifying compliance when they submit a claim, either on the claim form or by regulation. The high court could also split on the issue four to four, leaving intact the range of circuit court interpretations on implied certification across the country.
“There’s a very real question as to whether they’re going to be able to get a majority on any of those decisions because this is not an easy question,” Mr. Horton said. “The court has a pretty wide range of potential rulings available to it, but I don’t know what they’re going to be able to majority around, if they’re going to be able get a majority around any result at all.”
A decision in the case is expected by June.
On Twitter @legal_med
A case before the U.S. Supreme Court could expand physicians’ liability under the False Claims Act (FCA).
The case of Escobar v. Universal Health Services centers on the theory of implied certification and how that legal test should be used to determine whether a claim for payment is fraudulent.
The case “is an opportunity for the Supreme Court to figure out how far the False Claims Act is going to stretch,” said Lawrence M. Kraus, a Boston health law attorney who attended the April 19 oral arguments. “On the practical level, it may have an impact as to whether [such] cases get dismissed at an early stage or whether they go into the discovery phase, which can be quite long, unpleasant, and expensive.”
The Escobar case arises from the death of a patient who was treated at a Lawrence, Mass., mental health clinic operated by Universal Health Services. The patient died from an alleged adverse reaction to medication prescribed for her by clinic staff, according to allegations by her family. The patient’s father, Julio Escobar, later learned counselors and psychologists involved in his daughter’s treatment were not licensed, were not properly supervised by a physician, and had lied about their medical credentials, according to court documents.
The Massachusetts Department of Public Health found the clinic had violated 14 distinct regulations, including those relating to staff licensure and supervision. As a result of the investigation, the clinic entered into a correction plan with the agency and paid a civil fine.
Mr. Escobar and his wife then filed suit under the FCA and the Massachusetts False Claims Act, claiming that Universal had presented false claims to Medicaid by seeking payments for services provided by unlicensed, unsupervised health care providers. Although the reimbursement claims submitted to the government accurately described the services provided and cited the correct charges, the plaintiffs alleged that because the clinic’s operations violated state requirements to participate in Medicaid, Universal had also violated the FCA. The federal government intervened in the case on behalf of the Escobars.
Universal countered that the FCA suit was invalid because a reimbursement claim cannot be false unless its details are untrue or inaccurate.
The plaintiffs, however, contend that a claim does not have to include explicit false statements to be fraudulent. Rather, their complaint relies on “implied certification,” a theory holding that any submission for government payment includes an implicit certification that the health provider has complied with all applicable contract requirements, laws, and regulations that could be a condition of payment. Universal falsely claimed entitlement when it submitted reimbursement requests that did not conform to applicable laws, the plaintiffs argued.
The 1st U.S. Circuit Court of Appeals ruled in favor of Escobar, and Universal appealed to the Supreme Court.
Circuit courts across the country have split on the issue, Mr. Kraus noted.
“There have been a number of different approaches from appeals courts in the country,” he said. “This is not a new issue, but one that the Supreme Court found important enough to decide.”
Why should doctors care about this case?
A ruling for the plaintiff could increase the chances that physicians are accused of an FCA violation after submitting a claim for payment, said William W. Horton, a Birmingham, Ala., health law attorney and chair of the American Bar Association Health Law Section.
“The problem that this raises for health care providers is: There is an enormous web of laws and regulations out there, many of which don’t have anything to do with whether a particular service was rendered or not,” Mr. Horton said in an interview “If you adopt the implied certification theory and take a broad view, than you significantly enhance the scope of claims that could be pursued under the False Claims Act.”
Mr. Horton provides this example: Take a physician group that has an in-office lab, and assume that for some technical reason, the group doesn’t satisfy the Stark Law exception for in-office ancillary services. If a physician in the group refers a Medicare patient to the lab and the group bills Medicare, that’s a Stark Law violation because the group didn’t meet the Stark exception, even if there’s no dispute over whether the patient needed the test or whether the test was done correctly, or whether the Medicare claim accurately reflected the charges, he said. By broadly applying the implied certification theory to this scenario, a case could be made that the practice violated the FCA in submitting the claim because the group was implicitly certifying that the claim did not result from a referral that violated the Stark Law.
“The group could be found liable for the enormous penalties available under the False Claims Act even though the services rendered were medically necessary and appropriate, and even though the group did not expressly certify, in so many words, that the claim did not result from a referral that violated the Stark Law,” Mr. Horton said.
Medical associations, including the American Medical Association and American Hospital Association have weighed in on the case in favor of Universal Health Services. In its brief, the AMA said there is a “sharp distinction” between statutory, regulatory, or contractual violations and false or fraudulent claims.
“Implied certification claims find no support in the statute and do not resemble claims Congress had in mind when enacting or amending the FCA,” according to the brief. “They deprive contractors of their constitutional rights to have notice that they are engaging in conduct subject to heightened sanctions.”
How might the Supreme Court rule?
During oral arguments on April 19, some justices appeared to indicate which way they are leaning, Mr. Kraus said.
Chief Justice John Roberts seemed concerned about the reach of the FCA under the implied certification theory. He raised questions about how people conducting business with the government would know about each and every regulation that could apply as a condition of payment.
Associate Justice Sonia Sotomayer and Associate Justice Elena Kagan appeared in favor of implied certification, while Associate Justice Samuel Alito Jr., Associate Justice Clarence Thomas, and Associate Justice Ruth Bader-Ginsberg did not display a strong opinion either way, Mr. Kraus said. Associate Justice Stephen Breyer appeared to be conflicted, asking for guidance from Roy T. Englert, an attorney for Universal Health Services.
“I’m asking for advice from you, from your point of view,” Justice Breyer said to Mr. Englert. “What the sentence in the opinion should say that describes the circumstances under which the person who submits a form saying, ‘I want a thousand dollars. I just supplied the guns or the medical care.’ ... When has that person committed fraud? – Or that’s what I want. What is the sentence you want me to write?”
Justices could rule a number of ways. They could uphold the appeals court decision, which would affirm a broad interpretation of implied certification theory. They could rule that the implied certification theory is valid, but it cannot be stretched as far as the appeals court expanded it. Justices could choose to reject the implied certification theory altogether and decide that the government must expressly identify every condition of payment in which a health provider is certifying compliance when they submit a claim, either on the claim form or by regulation. The high court could also split on the issue four to four, leaving intact the range of circuit court interpretations on implied certification across the country.
“There’s a very real question as to whether they’re going to be able to get a majority on any of those decisions because this is not an easy question,” Mr. Horton said. “The court has a pretty wide range of potential rulings available to it, but I don’t know what they’re going to be able to majority around, if they’re going to be able get a majority around any result at all.”
A decision in the case is expected by June.
On Twitter @legal_med
A case before the U.S. Supreme Court could expand physicians’ liability under the False Claims Act (FCA).
The case of Escobar v. Universal Health Services centers on the theory of implied certification and how that legal test should be used to determine whether a claim for payment is fraudulent.
The case “is an opportunity for the Supreme Court to figure out how far the False Claims Act is going to stretch,” said Lawrence M. Kraus, a Boston health law attorney who attended the April 19 oral arguments. “On the practical level, it may have an impact as to whether [such] cases get dismissed at an early stage or whether they go into the discovery phase, which can be quite long, unpleasant, and expensive.”
The Escobar case arises from the death of a patient who was treated at a Lawrence, Mass., mental health clinic operated by Universal Health Services. The patient died from an alleged adverse reaction to medication prescribed for her by clinic staff, according to allegations by her family. The patient’s father, Julio Escobar, later learned counselors and psychologists involved in his daughter’s treatment were not licensed, were not properly supervised by a physician, and had lied about their medical credentials, according to court documents.
The Massachusetts Department of Public Health found the clinic had violated 14 distinct regulations, including those relating to staff licensure and supervision. As a result of the investigation, the clinic entered into a correction plan with the agency and paid a civil fine.
Mr. Escobar and his wife then filed suit under the FCA and the Massachusetts False Claims Act, claiming that Universal had presented false claims to Medicaid by seeking payments for services provided by unlicensed, unsupervised health care providers. Although the reimbursement claims submitted to the government accurately described the services provided and cited the correct charges, the plaintiffs alleged that because the clinic’s operations violated state requirements to participate in Medicaid, Universal had also violated the FCA. The federal government intervened in the case on behalf of the Escobars.
Universal countered that the FCA suit was invalid because a reimbursement claim cannot be false unless its details are untrue or inaccurate.
The plaintiffs, however, contend that a claim does not have to include explicit false statements to be fraudulent. Rather, their complaint relies on “implied certification,” a theory holding that any submission for government payment includes an implicit certification that the health provider has complied with all applicable contract requirements, laws, and regulations that could be a condition of payment. Universal falsely claimed entitlement when it submitted reimbursement requests that did not conform to applicable laws, the plaintiffs argued.
The 1st U.S. Circuit Court of Appeals ruled in favor of Escobar, and Universal appealed to the Supreme Court.
Circuit courts across the country have split on the issue, Mr. Kraus noted.
“There have been a number of different approaches from appeals courts in the country,” he said. “This is not a new issue, but one that the Supreme Court found important enough to decide.”
Why should doctors care about this case?
A ruling for the plaintiff could increase the chances that physicians are accused of an FCA violation after submitting a claim for payment, said William W. Horton, a Birmingham, Ala., health law attorney and chair of the American Bar Association Health Law Section.
“The problem that this raises for health care providers is: There is an enormous web of laws and regulations out there, many of which don’t have anything to do with whether a particular service was rendered or not,” Mr. Horton said in an interview “If you adopt the implied certification theory and take a broad view, than you significantly enhance the scope of claims that could be pursued under the False Claims Act.”
Mr. Horton provides this example: Take a physician group that has an in-office lab, and assume that for some technical reason, the group doesn’t satisfy the Stark Law exception for in-office ancillary services. If a physician in the group refers a Medicare patient to the lab and the group bills Medicare, that’s a Stark Law violation because the group didn’t meet the Stark exception, even if there’s no dispute over whether the patient needed the test or whether the test was done correctly, or whether the Medicare claim accurately reflected the charges, he said. By broadly applying the implied certification theory to this scenario, a case could be made that the practice violated the FCA in submitting the claim because the group was implicitly certifying that the claim did not result from a referral that violated the Stark Law.
“The group could be found liable for the enormous penalties available under the False Claims Act even though the services rendered were medically necessary and appropriate, and even though the group did not expressly certify, in so many words, that the claim did not result from a referral that violated the Stark Law,” Mr. Horton said.
Medical associations, including the American Medical Association and American Hospital Association have weighed in on the case in favor of Universal Health Services. In its brief, the AMA said there is a “sharp distinction” between statutory, regulatory, or contractual violations and false or fraudulent claims.
“Implied certification claims find no support in the statute and do not resemble claims Congress had in mind when enacting or amending the FCA,” according to the brief. “They deprive contractors of their constitutional rights to have notice that they are engaging in conduct subject to heightened sanctions.”
How might the Supreme Court rule?
During oral arguments on April 19, some justices appeared to indicate which way they are leaning, Mr. Kraus said.
Chief Justice John Roberts seemed concerned about the reach of the FCA under the implied certification theory. He raised questions about how people conducting business with the government would know about each and every regulation that could apply as a condition of payment.
Associate Justice Sonia Sotomayer and Associate Justice Elena Kagan appeared in favor of implied certification, while Associate Justice Samuel Alito Jr., Associate Justice Clarence Thomas, and Associate Justice Ruth Bader-Ginsberg did not display a strong opinion either way, Mr. Kraus said. Associate Justice Stephen Breyer appeared to be conflicted, asking for guidance from Roy T. Englert, an attorney for Universal Health Services.
“I’m asking for advice from you, from your point of view,” Justice Breyer said to Mr. Englert. “What the sentence in the opinion should say that describes the circumstances under which the person who submits a form saying, ‘I want a thousand dollars. I just supplied the guns or the medical care.’ ... When has that person committed fraud? – Or that’s what I want. What is the sentence you want me to write?”
Justices could rule a number of ways. They could uphold the appeals court decision, which would affirm a broad interpretation of implied certification theory. They could rule that the implied certification theory is valid, but it cannot be stretched as far as the appeals court expanded it. Justices could choose to reject the implied certification theory altogether and decide that the government must expressly identify every condition of payment in which a health provider is certifying compliance when they submit a claim, either on the claim form or by regulation. The high court could also split on the issue four to four, leaving intact the range of circuit court interpretations on implied certification across the country.
“There’s a very real question as to whether they’re going to be able to get a majority on any of those decisions because this is not an easy question,” Mr. Horton said. “The court has a pretty wide range of potential rulings available to it, but I don’t know what they’re going to be able to majority around, if they’re going to be able get a majority around any result at all.”
A decision in the case is expected by June.
On Twitter @legal_med
HHS guidance facilitates Medicaid access for former inmates
New guidance from the U.S. Health and Human Services Department clarifies that individuals on parole, probation, or in-home confinement are not considered inmates and therefore are eligible for Medicaid coverage.
“Today’s actions will immediately begin to give as many as 96,000 of American’s most vulnerable citizens access to needed health care through Medicaid, including mental health and substance use disorder treatment, reducing the risk they will be re-incarcerated or hurt,” Richard G. Frank, HHS Assistant Secretary for Planning and Evaluation, said in a statement.
Historically, most former inmates transitioning back into their communities have been uninsured and have experienced disproportionately high rates of chronic conditions, infectious disease, and mental health issues, according to the HHS. Access to health care through the Medicaid program plays a key role in improving the health of these patients, HHS officials said during an April 28 press conference.
The guidance also makes clear that incarcerated individuals are not prevented from enrolling in Medicaid or renewing their Medicaid enrollment. State Medicaid agencies must accept applications from inmates while they are incarcerated. Once they are enrolled however, the state may suspend the inmates’ eligibility status until they are released from prison or jail. A suspension of enrollment makes it easier for former inmates to resume coverage after they are released, said Rae Woods, a senior counsel at the Department of Justice.
“Inmates do not need to wait to apply for health coverage until after they leave prison [or a] halfway house,” Ms. Woods said during the press conference. “This will prevent former inmates from having gaps during the most crucial time of reentry; the first few days, weeks, and months as they navigate reentry obstacles and are at the most danger at falling back into addiction or crime.”
Currently, 2.2 million people are incarcerated in the United States and 4.7 million people are on probation or parole, a new HHS report finds. More than 95% of incarcerated individuals will return to the community.
On Twitter @legal_med
New guidance from the U.S. Health and Human Services Department clarifies that individuals on parole, probation, or in-home confinement are not considered inmates and therefore are eligible for Medicaid coverage.
“Today’s actions will immediately begin to give as many as 96,000 of American’s most vulnerable citizens access to needed health care through Medicaid, including mental health and substance use disorder treatment, reducing the risk they will be re-incarcerated or hurt,” Richard G. Frank, HHS Assistant Secretary for Planning and Evaluation, said in a statement.
Historically, most former inmates transitioning back into their communities have been uninsured and have experienced disproportionately high rates of chronic conditions, infectious disease, and mental health issues, according to the HHS. Access to health care through the Medicaid program plays a key role in improving the health of these patients, HHS officials said during an April 28 press conference.
The guidance also makes clear that incarcerated individuals are not prevented from enrolling in Medicaid or renewing their Medicaid enrollment. State Medicaid agencies must accept applications from inmates while they are incarcerated. Once they are enrolled however, the state may suspend the inmates’ eligibility status until they are released from prison or jail. A suspension of enrollment makes it easier for former inmates to resume coverage after they are released, said Rae Woods, a senior counsel at the Department of Justice.
“Inmates do not need to wait to apply for health coverage until after they leave prison [or a] halfway house,” Ms. Woods said during the press conference. “This will prevent former inmates from having gaps during the most crucial time of reentry; the first few days, weeks, and months as they navigate reentry obstacles and are at the most danger at falling back into addiction or crime.”
Currently, 2.2 million people are incarcerated in the United States and 4.7 million people are on probation or parole, a new HHS report finds. More than 95% of incarcerated individuals will return to the community.
On Twitter @legal_med
New guidance from the U.S. Health and Human Services Department clarifies that individuals on parole, probation, or in-home confinement are not considered inmates and therefore are eligible for Medicaid coverage.
“Today’s actions will immediately begin to give as many as 96,000 of American’s most vulnerable citizens access to needed health care through Medicaid, including mental health and substance use disorder treatment, reducing the risk they will be re-incarcerated or hurt,” Richard G. Frank, HHS Assistant Secretary for Planning and Evaluation, said in a statement.
Historically, most former inmates transitioning back into their communities have been uninsured and have experienced disproportionately high rates of chronic conditions, infectious disease, and mental health issues, according to the HHS. Access to health care through the Medicaid program plays a key role in improving the health of these patients, HHS officials said during an April 28 press conference.
The guidance also makes clear that incarcerated individuals are not prevented from enrolling in Medicaid or renewing their Medicaid enrollment. State Medicaid agencies must accept applications from inmates while they are incarcerated. Once they are enrolled however, the state may suspend the inmates’ eligibility status until they are released from prison or jail. A suspension of enrollment makes it easier for former inmates to resume coverage after they are released, said Rae Woods, a senior counsel at the Department of Justice.
“Inmates do not need to wait to apply for health coverage until after they leave prison [or a] halfway house,” Ms. Woods said during the press conference. “This will prevent former inmates from having gaps during the most crucial time of reentry; the first few days, weeks, and months as they navigate reentry obstacles and are at the most danger at falling back into addiction or crime.”
Currently, 2.2 million people are incarcerated in the United States and 4.7 million people are on probation or parole, a new HHS report finds. More than 95% of incarcerated individuals will return to the community.
On Twitter @legal_med
VP Biden to AACR: Help me help you
Stronger teamwork among researchers, sharing data, and realignment of incentives for scientific breakthroughs, in addition to more funding, are key steps needed to advance cancer research, Vice President Joe Biden said during the annual meeting of the American Association for Cancer Research (AACR).
During a plenary speech to close the meeting, Vice President Biden praised the dedication of current cancer researchers and pledged to break down the walls that prevent them from achieving more progress in the field.
“I made a commitment that I will – as I gain this information and knowledge – I will eliminate the barriers that get in your way, get in the way of science and research and development,” he said. “I had to ... learn from all of you how we can proceed, how we can break down silos, how we can accommodate more rapidly the efforts you’re making.”
Vice President Biden, who is leading a new $1 billion initiative to eliminate cancer called “Moonshot,” outlined the top obstacles to cancer research he has garnered from recent visits with renowned cancer scientists and research leaders around the world. This includes a lack of unity among researchers, poor rewards for novel research, and limited data sharing, he said.
“The way the system now is set up, researchers are not incentivized to share their data,” Vice President Biden said, acknowledging that some medical experts are against the idea. “But every expert I’ve spoken to said you need to share these data to move this process rapidly.”
Involving patients earlier in clinical trials design is also a primary focus, he said. Patients should understand more about trials and be more open to signing up.
He noted the “incredible” research currently being conducted by various entities, such as AACR’s Project Genie, Orion Foundation, and The Parker Institute. Mr. Biden stressed however, that such efforts are too isolated.
“It raises [the] question: ‘Why is all this being done separately?’ ” Vice President Biden said. “Why is so much money being spent when if it’s aggregated, everyone acknowledges, the answers would come more quickly?”
Incentives for new research and the way in which funding is alloted must also be redesigned, he stressed. Today, it takes too long for researchers to get projects approved by the government and funding dispersed. He acknowledged the difficulty researchers face in obtaining grants and the fact that those who think “outside the box” are less likely to receive funding.
“It seems to me that we slow down our best minds by making them spend years in the lab before they can get their own grants and, when they do, they spend a third of their time writing a grant that takes months to be approved and awarded,” he said. “It’s like asking Derek Jeter to take several years off to sell bonds to build Yankee stadium.”
The Vice President did not purport to have all the answers, and asked those at the AARC meeting to provide feedback on his suggestions.
“The question I’d ask you to contemplate, because I’d like you to communicate with us, is, ‘Does it require realigning incentives; changing behaviors to take advantage of this inflection point? Does it require sharing more knowledge, treatment, and understanding? Or does that slow the process up?’ ”
He added,“I hope you all know it, but you’re one of the most valuable resources that our great country has, those of you sitting in this room. So ask your institutions, your colleagues, your mentors, your administrators: How can we move your ideas faster together in the interest of patients?”
The Vice President’s Moonshot initiative was announced during President Obama’s 2016 State of the Union Address. The effort includes a new Cancer Moonshot Task Force that will focus on federal investments, targeted incentives, private sector efforts from industry and philanthropy, patient engagement initiatives, and other mechanisms to support cancer research and enable progress in treatment and care, according to the White House. As part of the plan, the President’s fiscal 2017 budget proposes $755 million in mandatory funds for new cancer-related research activities at the National Institutes of Health and the Food and Drug Administration. The initiative also includes increased investments by the Department of Defense and the Department of Veterans Affairs in cancer research, including through funding centers of excellence focused on specific cancers and conducting longitudinal studies to determine risk factors and enhance treatment.
On Twitter @legal_med
Stronger teamwork among researchers, sharing data, and realignment of incentives for scientific breakthroughs, in addition to more funding, are key steps needed to advance cancer research, Vice President Joe Biden said during the annual meeting of the American Association for Cancer Research (AACR).
During a plenary speech to close the meeting, Vice President Biden praised the dedication of current cancer researchers and pledged to break down the walls that prevent them from achieving more progress in the field.
“I made a commitment that I will – as I gain this information and knowledge – I will eliminate the barriers that get in your way, get in the way of science and research and development,” he said. “I had to ... learn from all of you how we can proceed, how we can break down silos, how we can accommodate more rapidly the efforts you’re making.”
Vice President Biden, who is leading a new $1 billion initiative to eliminate cancer called “Moonshot,” outlined the top obstacles to cancer research he has garnered from recent visits with renowned cancer scientists and research leaders around the world. This includes a lack of unity among researchers, poor rewards for novel research, and limited data sharing, he said.
“The way the system now is set up, researchers are not incentivized to share their data,” Vice President Biden said, acknowledging that some medical experts are against the idea. “But every expert I’ve spoken to said you need to share these data to move this process rapidly.”
Involving patients earlier in clinical trials design is also a primary focus, he said. Patients should understand more about trials and be more open to signing up.
He noted the “incredible” research currently being conducted by various entities, such as AACR’s Project Genie, Orion Foundation, and The Parker Institute. Mr. Biden stressed however, that such efforts are too isolated.
“It raises [the] question: ‘Why is all this being done separately?’ ” Vice President Biden said. “Why is so much money being spent when if it’s aggregated, everyone acknowledges, the answers would come more quickly?”
Incentives for new research and the way in which funding is alloted must also be redesigned, he stressed. Today, it takes too long for researchers to get projects approved by the government and funding dispersed. He acknowledged the difficulty researchers face in obtaining grants and the fact that those who think “outside the box” are less likely to receive funding.
“It seems to me that we slow down our best minds by making them spend years in the lab before they can get their own grants and, when they do, they spend a third of their time writing a grant that takes months to be approved and awarded,” he said. “It’s like asking Derek Jeter to take several years off to sell bonds to build Yankee stadium.”
The Vice President did not purport to have all the answers, and asked those at the AARC meeting to provide feedback on his suggestions.
“The question I’d ask you to contemplate, because I’d like you to communicate with us, is, ‘Does it require realigning incentives; changing behaviors to take advantage of this inflection point? Does it require sharing more knowledge, treatment, and understanding? Or does that slow the process up?’ ”
He added,“I hope you all know it, but you’re one of the most valuable resources that our great country has, those of you sitting in this room. So ask your institutions, your colleagues, your mentors, your administrators: How can we move your ideas faster together in the interest of patients?”
The Vice President’s Moonshot initiative was announced during President Obama’s 2016 State of the Union Address. The effort includes a new Cancer Moonshot Task Force that will focus on federal investments, targeted incentives, private sector efforts from industry and philanthropy, patient engagement initiatives, and other mechanisms to support cancer research and enable progress in treatment and care, according to the White House. As part of the plan, the President’s fiscal 2017 budget proposes $755 million in mandatory funds for new cancer-related research activities at the National Institutes of Health and the Food and Drug Administration. The initiative also includes increased investments by the Department of Defense and the Department of Veterans Affairs in cancer research, including through funding centers of excellence focused on specific cancers and conducting longitudinal studies to determine risk factors and enhance treatment.
On Twitter @legal_med
Stronger teamwork among researchers, sharing data, and realignment of incentives for scientific breakthroughs, in addition to more funding, are key steps needed to advance cancer research, Vice President Joe Biden said during the annual meeting of the American Association for Cancer Research (AACR).
During a plenary speech to close the meeting, Vice President Biden praised the dedication of current cancer researchers and pledged to break down the walls that prevent them from achieving more progress in the field.
“I made a commitment that I will – as I gain this information and knowledge – I will eliminate the barriers that get in your way, get in the way of science and research and development,” he said. “I had to ... learn from all of you how we can proceed, how we can break down silos, how we can accommodate more rapidly the efforts you’re making.”
Vice President Biden, who is leading a new $1 billion initiative to eliminate cancer called “Moonshot,” outlined the top obstacles to cancer research he has garnered from recent visits with renowned cancer scientists and research leaders around the world. This includes a lack of unity among researchers, poor rewards for novel research, and limited data sharing, he said.
“The way the system now is set up, researchers are not incentivized to share their data,” Vice President Biden said, acknowledging that some medical experts are against the idea. “But every expert I’ve spoken to said you need to share these data to move this process rapidly.”
Involving patients earlier in clinical trials design is also a primary focus, he said. Patients should understand more about trials and be more open to signing up.
He noted the “incredible” research currently being conducted by various entities, such as AACR’s Project Genie, Orion Foundation, and The Parker Institute. Mr. Biden stressed however, that such efforts are too isolated.
“It raises [the] question: ‘Why is all this being done separately?’ ” Vice President Biden said. “Why is so much money being spent when if it’s aggregated, everyone acknowledges, the answers would come more quickly?”
Incentives for new research and the way in which funding is alloted must also be redesigned, he stressed. Today, it takes too long for researchers to get projects approved by the government and funding dispersed. He acknowledged the difficulty researchers face in obtaining grants and the fact that those who think “outside the box” are less likely to receive funding.
“It seems to me that we slow down our best minds by making them spend years in the lab before they can get their own grants and, when they do, they spend a third of their time writing a grant that takes months to be approved and awarded,” he said. “It’s like asking Derek Jeter to take several years off to sell bonds to build Yankee stadium.”
The Vice President did not purport to have all the answers, and asked those at the AARC meeting to provide feedback on his suggestions.
“The question I’d ask you to contemplate, because I’d like you to communicate with us, is, ‘Does it require realigning incentives; changing behaviors to take advantage of this inflection point? Does it require sharing more knowledge, treatment, and understanding? Or does that slow the process up?’ ”
He added,“I hope you all know it, but you’re one of the most valuable resources that our great country has, those of you sitting in this room. So ask your institutions, your colleagues, your mentors, your administrators: How can we move your ideas faster together in the interest of patients?”
The Vice President’s Moonshot initiative was announced during President Obama’s 2016 State of the Union Address. The effort includes a new Cancer Moonshot Task Force that will focus on federal investments, targeted incentives, private sector efforts from industry and philanthropy, patient engagement initiatives, and other mechanisms to support cancer research and enable progress in treatment and care, according to the White House. As part of the plan, the President’s fiscal 2017 budget proposes $755 million in mandatory funds for new cancer-related research activities at the National Institutes of Health and the Food and Drug Administration. The initiative also includes increased investments by the Department of Defense and the Department of Veterans Affairs in cancer research, including through funding centers of excellence focused on specific cancers and conducting longitudinal studies to determine risk factors and enhance treatment.
On Twitter @legal_med
FROM THE AACR ANNUAL MEETING
Both sides open to compromise in Zubik birth control case
In new court briefs, a group of religious employers and the federal government both say they’re open to a compromise in the case regarding the Affordable Care Act birth control mandate that has landed them before the U.S. Supreme Court. Each side, however, has a different picture of what a resolution would entail.
Justices in late March ordered both sides to provide new briefs in Zubik v. Burwell, a religious freedom case that centers on the ACA provision requiring copayment-free health care coverage for contraception. The high court wants to know whether and how contraceptive coverage could be obtained by employees of religious nonprofits through their health plans in a way that does not require employer involvement.
For the federal government, Solicitor General Donald B. Verrilli Jr. wrote in an April 12 brief that an alternative to the current accommodation could be acceptable, but only if the affected women still receive seamless birth control coverage and if the resolution finally ends litigation over the issue.
“A decision requiring a modification to the accommodation while leaving open the possibility that even the arrangement as so modified might itself be deemed insufficient, would lead to years of additional litigation, during which tens of thousands of women would likely continue to be denied the coverage to which they are legally entitled,” Mr. Verrilli wrote.
The plaintiffs – part of seven consolidated cases that include a Catholic bishop and an order of nuns – also expressed willingness to compromise, but only if they can be fully excluded from the process.
“There are many ways in which the employees could receive cost-free contraceptive coverage through the same insurance company that would not require further involvement by the petitioner, including the way described in the court’s order,” plaintiffs’ attorney Paul D. Clement wrote in his brief. “And each one of those ways is a less restrictive alternative that dooms the government’s ongoing effort to use the threat of massive penalties to compel petitioners to forsake their sincerely held religious beliefs.”
The ACA’s current accommodation applies to organizations that oppose coverage for contraceptives but are not exempted entities, such as churches. The plaintiffs argue that the opt-out process makes them complicit in offering contraception coverage indirectly. Forcing them to cooperate with the accommodation violates their rights under the federal Religious Freedom Restoration Act, according to the plaintiffs.
The government contends that the exception does not impose a burden on the groups and that courts should not disregard the interest of employees who may not share their employers’ religious beliefs. The 8th U.S. Circuit Court of Appeals struck down the accommodation twice, ruling that forcing organizations to offer contraceptive coverage – even indirectly – violates their religious rights. The 8th Circuit’s decisions are at odds with rulings by the 2nd and 5th Circuit courts.
Supreme Court arguments were heard March 23, and the justices appeared split over the accommodation’s constitutionality. Rather than employers having to provide separate written notice to the government to become exempt from the coverage regulations, justices proposed that the plaintiffs could instead “inform their insurance company that they do not want their health plan to include contraceptive coverage of the type to which they object on religious grounds.” The insurance companies would then separately notify employees that they will provide cost-free contraceptive coverage, and that such coverage is not paid for by their employers nor is it offered through their employer’s health plan.
The court’s suggestion could work for employers that contract with third-party insurers, but not for employers that self-insure and who rely on third-party administrators, Mr. Verrilli wrote. In this context, the current accommodation is necessary given the requirements of the Employee Retirement Income Security Act.
Attorneys are required to issue additional briefs by April 20 reacting to the other’s position. A decision in the case is expected by June.
The Feb. 13 death of Justice Antonin Scalia could mean a 4-4 decision in the case. If such a division occurs, the lower court rulings would stand. The justices could choose to rehear arguments at a later date.
On Twitter @legal_med
In new court briefs, a group of religious employers and the federal government both say they’re open to a compromise in the case regarding the Affordable Care Act birth control mandate that has landed them before the U.S. Supreme Court. Each side, however, has a different picture of what a resolution would entail.
Justices in late March ordered both sides to provide new briefs in Zubik v. Burwell, a religious freedom case that centers on the ACA provision requiring copayment-free health care coverage for contraception. The high court wants to know whether and how contraceptive coverage could be obtained by employees of religious nonprofits through their health plans in a way that does not require employer involvement.
For the federal government, Solicitor General Donald B. Verrilli Jr. wrote in an April 12 brief that an alternative to the current accommodation could be acceptable, but only if the affected women still receive seamless birth control coverage and if the resolution finally ends litigation over the issue.
“A decision requiring a modification to the accommodation while leaving open the possibility that even the arrangement as so modified might itself be deemed insufficient, would lead to years of additional litigation, during which tens of thousands of women would likely continue to be denied the coverage to which they are legally entitled,” Mr. Verrilli wrote.
The plaintiffs – part of seven consolidated cases that include a Catholic bishop and an order of nuns – also expressed willingness to compromise, but only if they can be fully excluded from the process.
“There are many ways in which the employees could receive cost-free contraceptive coverage through the same insurance company that would not require further involvement by the petitioner, including the way described in the court’s order,” plaintiffs’ attorney Paul D. Clement wrote in his brief. “And each one of those ways is a less restrictive alternative that dooms the government’s ongoing effort to use the threat of massive penalties to compel petitioners to forsake their sincerely held religious beliefs.”
The ACA’s current accommodation applies to organizations that oppose coverage for contraceptives but are not exempted entities, such as churches. The plaintiffs argue that the opt-out process makes them complicit in offering contraception coverage indirectly. Forcing them to cooperate with the accommodation violates their rights under the federal Religious Freedom Restoration Act, according to the plaintiffs.
The government contends that the exception does not impose a burden on the groups and that courts should not disregard the interest of employees who may not share their employers’ religious beliefs. The 8th U.S. Circuit Court of Appeals struck down the accommodation twice, ruling that forcing organizations to offer contraceptive coverage – even indirectly – violates their religious rights. The 8th Circuit’s decisions are at odds with rulings by the 2nd and 5th Circuit courts.
Supreme Court arguments were heard March 23, and the justices appeared split over the accommodation’s constitutionality. Rather than employers having to provide separate written notice to the government to become exempt from the coverage regulations, justices proposed that the plaintiffs could instead “inform their insurance company that they do not want their health plan to include contraceptive coverage of the type to which they object on religious grounds.” The insurance companies would then separately notify employees that they will provide cost-free contraceptive coverage, and that such coverage is not paid for by their employers nor is it offered through their employer’s health plan.
The court’s suggestion could work for employers that contract with third-party insurers, but not for employers that self-insure and who rely on third-party administrators, Mr. Verrilli wrote. In this context, the current accommodation is necessary given the requirements of the Employee Retirement Income Security Act.
Attorneys are required to issue additional briefs by April 20 reacting to the other’s position. A decision in the case is expected by June.
The Feb. 13 death of Justice Antonin Scalia could mean a 4-4 decision in the case. If such a division occurs, the lower court rulings would stand. The justices could choose to rehear arguments at a later date.
On Twitter @legal_med
In new court briefs, a group of religious employers and the federal government both say they’re open to a compromise in the case regarding the Affordable Care Act birth control mandate that has landed them before the U.S. Supreme Court. Each side, however, has a different picture of what a resolution would entail.
Justices in late March ordered both sides to provide new briefs in Zubik v. Burwell, a religious freedom case that centers on the ACA provision requiring copayment-free health care coverage for contraception. The high court wants to know whether and how contraceptive coverage could be obtained by employees of religious nonprofits through their health plans in a way that does not require employer involvement.
For the federal government, Solicitor General Donald B. Verrilli Jr. wrote in an April 12 brief that an alternative to the current accommodation could be acceptable, but only if the affected women still receive seamless birth control coverage and if the resolution finally ends litigation over the issue.
“A decision requiring a modification to the accommodation while leaving open the possibility that even the arrangement as so modified might itself be deemed insufficient, would lead to years of additional litigation, during which tens of thousands of women would likely continue to be denied the coverage to which they are legally entitled,” Mr. Verrilli wrote.
The plaintiffs – part of seven consolidated cases that include a Catholic bishop and an order of nuns – also expressed willingness to compromise, but only if they can be fully excluded from the process.
“There are many ways in which the employees could receive cost-free contraceptive coverage through the same insurance company that would not require further involvement by the petitioner, including the way described in the court’s order,” plaintiffs’ attorney Paul D. Clement wrote in his brief. “And each one of those ways is a less restrictive alternative that dooms the government’s ongoing effort to use the threat of massive penalties to compel petitioners to forsake their sincerely held religious beliefs.”
The ACA’s current accommodation applies to organizations that oppose coverage for contraceptives but are not exempted entities, such as churches. The plaintiffs argue that the opt-out process makes them complicit in offering contraception coverage indirectly. Forcing them to cooperate with the accommodation violates their rights under the federal Religious Freedom Restoration Act, according to the plaintiffs.
The government contends that the exception does not impose a burden on the groups and that courts should not disregard the interest of employees who may not share their employers’ religious beliefs. The 8th U.S. Circuit Court of Appeals struck down the accommodation twice, ruling that forcing organizations to offer contraceptive coverage – even indirectly – violates their religious rights. The 8th Circuit’s decisions are at odds with rulings by the 2nd and 5th Circuit courts.
Supreme Court arguments were heard March 23, and the justices appeared split over the accommodation’s constitutionality. Rather than employers having to provide separate written notice to the government to become exempt from the coverage regulations, justices proposed that the plaintiffs could instead “inform their insurance company that they do not want their health plan to include contraceptive coverage of the type to which they object on religious grounds.” The insurance companies would then separately notify employees that they will provide cost-free contraceptive coverage, and that such coverage is not paid for by their employers nor is it offered through their employer’s health plan.
The court’s suggestion could work for employers that contract with third-party insurers, but not for employers that self-insure and who rely on third-party administrators, Mr. Verrilli wrote. In this context, the current accommodation is necessary given the requirements of the Employee Retirement Income Security Act.
Attorneys are required to issue additional briefs by April 20 reacting to the other’s position. A decision in the case is expected by June.
The Feb. 13 death of Justice Antonin Scalia could mean a 4-4 decision in the case. If such a division occurs, the lower court rulings would stand. The justices could choose to rehear arguments at a later date.
On Twitter @legal_med
New research institute to advance cancer immunotherapy
A new research institute launched by tech billionaire Sean Parker aims to advance the field of cancer immunotherapy by uniting the country’s top scientists, including Dr. Carl June, Dr. Jedd Wolchok, and Dr. James Allison, who are leaders in the field.
Best known for founding music-sharing service Napster, Mr. Parker has provided a $250 million grant to develop the Parker Institute for Cancer Immunotherapy, a collaboration between researchers, clinicians, and other stakeholders that focuses on expanding immunotherapy research efforts, according to an April announcement by the Parker Foundation.
“We are at an inflection point in cancer research and now is the time to maximize immunotherapy’s unique potential to transform all cancers into manageable diseases, saving millions of lives,” Mr. Parker said in a statement. “We believe that the creation of a new funding and research model can overcome many of the obstacles that currently prevent research breakthroughs. Working closely with our scientists and more than 30 industry partners, the Parker Institute is positioned to broadly disseminate discoveries and, most importantly, more rapidly deliver treatments to patients.”
The Parker Institute for Cancer Immunotherapy will include 40 laboratories and more than 300 researchers from leading cancer centers, including Memorial Sloan Kettering Cancer Center; Stanford Medicine; the University of California, Los Angeles; the University of California, San Francisco; the University of Pennsylvania; and the University of Texas MD Anderson Cancer Center. Each research center will receive comprehensive funding, clinical resources, and key technologies needed to accelerate research discoveries, according to the foundation. In a unique agreement, the administration of intellectual property will be shared between the research centers, allowing scientists to have immediate access to the broad spectrum of core discoveries.
Scientists involved with the institute call immunotherapy “one of the most important medical advances of our time,” and the first approach that could generate long-lasting remissions for all cancer types and stages. The relatively new treatment area mobilizes the body’s own defense systems to fight cancer cells.
“Immunotherapy represents a fundamentally new, breakthrough treatment paradigm in the fight against cancer – it harnesses the body’s own powerful immune system to mobilize its highly refined disease-fighting arsenal to engage and eliminate the cancer cells,” Parker Institute CEO and President Jeff Bluestone, Ph.D., said in a statement. “Our scientists are leaders in the field and will now work together to make discoveries to treat and potentially cure cancer.”
In the 1990s, Dr. Bluestone of the University of California, San Francisco, was one of two scientists who discovered that, to prevent autoimmunity and overreactions, a molecule called CTLA-4 acts as a “brake,” or checkpoint, on the immune response. The finding fueled a new class of checkpoint-blockade treatments for multiple cancers.
The Parker Institute will focus on several initial objectives, including developing novel approaches to modify T-cells to enhance function and creating a new generation of more effective T-cell therapies. Researchers also will work to improve the rates of durable responses and broaden the use of checkpoint-blocking agents, alone or in combination to treat more types of cancer. Finally, scientists will conduct research to advance DNA sequencing, antigenic peptide discovery efforts, and immune monitoring technologies. Their goal is to understand how to more effectively target tumors and ultimately develop new vaccines and T-cell therapies.
A major key of the institute is to remove competition among researchers that silo breakthroughs and instead enhance teamwork, said James P. Allison, Ph.D., of the University of Texas MD Anderson Cancer Center, Houston.
“One of the goals is to try to remove that competitive edge and have everybody bring their skills together,” Dr. Allison said in a video. “Still, you’re trying to do more than the other guy, but you’re doing it with them. So you use that competitive edge to an advantage.”
On Twitter @legal_med
A new research institute launched by tech billionaire Sean Parker aims to advance the field of cancer immunotherapy by uniting the country’s top scientists, including Dr. Carl June, Dr. Jedd Wolchok, and Dr. James Allison, who are leaders in the field.
Best known for founding music-sharing service Napster, Mr. Parker has provided a $250 million grant to develop the Parker Institute for Cancer Immunotherapy, a collaboration between researchers, clinicians, and other stakeholders that focuses on expanding immunotherapy research efforts, according to an April announcement by the Parker Foundation.
“We are at an inflection point in cancer research and now is the time to maximize immunotherapy’s unique potential to transform all cancers into manageable diseases, saving millions of lives,” Mr. Parker said in a statement. “We believe that the creation of a new funding and research model can overcome many of the obstacles that currently prevent research breakthroughs. Working closely with our scientists and more than 30 industry partners, the Parker Institute is positioned to broadly disseminate discoveries and, most importantly, more rapidly deliver treatments to patients.”
The Parker Institute for Cancer Immunotherapy will include 40 laboratories and more than 300 researchers from leading cancer centers, including Memorial Sloan Kettering Cancer Center; Stanford Medicine; the University of California, Los Angeles; the University of California, San Francisco; the University of Pennsylvania; and the University of Texas MD Anderson Cancer Center. Each research center will receive comprehensive funding, clinical resources, and key technologies needed to accelerate research discoveries, according to the foundation. In a unique agreement, the administration of intellectual property will be shared between the research centers, allowing scientists to have immediate access to the broad spectrum of core discoveries.
Scientists involved with the institute call immunotherapy “one of the most important medical advances of our time,” and the first approach that could generate long-lasting remissions for all cancer types and stages. The relatively new treatment area mobilizes the body’s own defense systems to fight cancer cells.
“Immunotherapy represents a fundamentally new, breakthrough treatment paradigm in the fight against cancer – it harnesses the body’s own powerful immune system to mobilize its highly refined disease-fighting arsenal to engage and eliminate the cancer cells,” Parker Institute CEO and President Jeff Bluestone, Ph.D., said in a statement. “Our scientists are leaders in the field and will now work together to make discoveries to treat and potentially cure cancer.”
In the 1990s, Dr. Bluestone of the University of California, San Francisco, was one of two scientists who discovered that, to prevent autoimmunity and overreactions, a molecule called CTLA-4 acts as a “brake,” or checkpoint, on the immune response. The finding fueled a new class of checkpoint-blockade treatments for multiple cancers.
The Parker Institute will focus on several initial objectives, including developing novel approaches to modify T-cells to enhance function and creating a new generation of more effective T-cell therapies. Researchers also will work to improve the rates of durable responses and broaden the use of checkpoint-blocking agents, alone or in combination to treat more types of cancer. Finally, scientists will conduct research to advance DNA sequencing, antigenic peptide discovery efforts, and immune monitoring technologies. Their goal is to understand how to more effectively target tumors and ultimately develop new vaccines and T-cell therapies.
A major key of the institute is to remove competition among researchers that silo breakthroughs and instead enhance teamwork, said James P. Allison, Ph.D., of the University of Texas MD Anderson Cancer Center, Houston.
“One of the goals is to try to remove that competitive edge and have everybody bring their skills together,” Dr. Allison said in a video. “Still, you’re trying to do more than the other guy, but you’re doing it with them. So you use that competitive edge to an advantage.”
On Twitter @legal_med
A new research institute launched by tech billionaire Sean Parker aims to advance the field of cancer immunotherapy by uniting the country’s top scientists, including Dr. Carl June, Dr. Jedd Wolchok, and Dr. James Allison, who are leaders in the field.
Best known for founding music-sharing service Napster, Mr. Parker has provided a $250 million grant to develop the Parker Institute for Cancer Immunotherapy, a collaboration between researchers, clinicians, and other stakeholders that focuses on expanding immunotherapy research efforts, according to an April announcement by the Parker Foundation.
“We are at an inflection point in cancer research and now is the time to maximize immunotherapy’s unique potential to transform all cancers into manageable diseases, saving millions of lives,” Mr. Parker said in a statement. “We believe that the creation of a new funding and research model can overcome many of the obstacles that currently prevent research breakthroughs. Working closely with our scientists and more than 30 industry partners, the Parker Institute is positioned to broadly disseminate discoveries and, most importantly, more rapidly deliver treatments to patients.”
The Parker Institute for Cancer Immunotherapy will include 40 laboratories and more than 300 researchers from leading cancer centers, including Memorial Sloan Kettering Cancer Center; Stanford Medicine; the University of California, Los Angeles; the University of California, San Francisco; the University of Pennsylvania; and the University of Texas MD Anderson Cancer Center. Each research center will receive comprehensive funding, clinical resources, and key technologies needed to accelerate research discoveries, according to the foundation. In a unique agreement, the administration of intellectual property will be shared between the research centers, allowing scientists to have immediate access to the broad spectrum of core discoveries.
Scientists involved with the institute call immunotherapy “one of the most important medical advances of our time,” and the first approach that could generate long-lasting remissions for all cancer types and stages. The relatively new treatment area mobilizes the body’s own defense systems to fight cancer cells.
“Immunotherapy represents a fundamentally new, breakthrough treatment paradigm in the fight against cancer – it harnesses the body’s own powerful immune system to mobilize its highly refined disease-fighting arsenal to engage and eliminate the cancer cells,” Parker Institute CEO and President Jeff Bluestone, Ph.D., said in a statement. “Our scientists are leaders in the field and will now work together to make discoveries to treat and potentially cure cancer.”
In the 1990s, Dr. Bluestone of the University of California, San Francisco, was one of two scientists who discovered that, to prevent autoimmunity and overreactions, a molecule called CTLA-4 acts as a “brake,” or checkpoint, on the immune response. The finding fueled a new class of checkpoint-blockade treatments for multiple cancers.
The Parker Institute will focus on several initial objectives, including developing novel approaches to modify T-cells to enhance function and creating a new generation of more effective T-cell therapies. Researchers also will work to improve the rates of durable responses and broaden the use of checkpoint-blocking agents, alone or in combination to treat more types of cancer. Finally, scientists will conduct research to advance DNA sequencing, antigenic peptide discovery efforts, and immune monitoring technologies. Their goal is to understand how to more effectively target tumors and ultimately develop new vaccines and T-cell therapies.
A major key of the institute is to remove competition among researchers that silo breakthroughs and instead enhance teamwork, said James P. Allison, Ph.D., of the University of Texas MD Anderson Cancer Center, Houston.
“One of the goals is to try to remove that competitive edge and have everybody bring their skills together,” Dr. Allison said in a video. “Still, you’re trying to do more than the other guy, but you’re doing it with them. So you use that competitive edge to an advantage.”
On Twitter @legal_med
SURVEY: Telemedicine high priority, but reimbursement remains challenging
Nearly two-thirds of health care providers rank telemedicine as a top priority in 2016, a 10% increase from last year, according to a survey.
Telemedicine software company REACH Health surveyed 390 U.S. health care professionals between November 2015 and December 2015, including physicians, nurses, and health care executives. Participants answered questions related to their objectives, challenges, telemedicine program models, and management structures, among other inquiries.
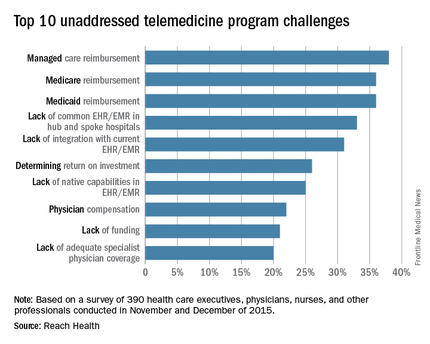
Of those polled, 96% of respondents said improving patient outcome was a top objective in developing telemedicine programs, according to the survey. Increasing patient convenience (87%) and improving patient engagement (86%) also rated highly. Other objectives included providing remote and rural patients with access to specialists (83%) and improving leverage of limited physician resources (81%). Percentages do not equal 100% because respondents could choose more than one objective.
The maturity of telemedicine programs varied widely depending on care setting. In general, settings requiring highly specialized treatment had more mature telemedicine programs than those requiring more generalized treatment. Stroke, neurology, and psychiatric/behavioral health settings had the most mature telemedicine programs, according to the survey.
Reimbursement ranked as the top barrier to telemedicine. Respondents rated private plan payment as the No. 1 challenge (38%), followed by Medicare reimbursement (36%) and Medicaid reimbursement (36%). Electronic health record incapabilities and liability risks also ranked as primary challenges.
“Telemedicine reimbursement poses the primary obstacle to success, but EMR-related challenges are persistent and widely noted in the survey,” Steve McGraw, president and CEO of REACH Health said in a statement. “There is clearly a high demand in the industry for EMR integration, specifically the two-way flow of individual data elements between telemedicine platforms and EMR systems.”
On Twitter @legal_med
Nearly two-thirds of health care providers rank telemedicine as a top priority in 2016, a 10% increase from last year, according to a survey.
Telemedicine software company REACH Health surveyed 390 U.S. health care professionals between November 2015 and December 2015, including physicians, nurses, and health care executives. Participants answered questions related to their objectives, challenges, telemedicine program models, and management structures, among other inquiries.

Of those polled, 96% of respondents said improving patient outcome was a top objective in developing telemedicine programs, according to the survey. Increasing patient convenience (87%) and improving patient engagement (86%) also rated highly. Other objectives included providing remote and rural patients with access to specialists (83%) and improving leverage of limited physician resources (81%). Percentages do not equal 100% because respondents could choose more than one objective.
The maturity of telemedicine programs varied widely depending on care setting. In general, settings requiring highly specialized treatment had more mature telemedicine programs than those requiring more generalized treatment. Stroke, neurology, and psychiatric/behavioral health settings had the most mature telemedicine programs, according to the survey.
Reimbursement ranked as the top barrier to telemedicine. Respondents rated private plan payment as the No. 1 challenge (38%), followed by Medicare reimbursement (36%) and Medicaid reimbursement (36%). Electronic health record incapabilities and liability risks also ranked as primary challenges.
“Telemedicine reimbursement poses the primary obstacle to success, but EMR-related challenges are persistent and widely noted in the survey,” Steve McGraw, president and CEO of REACH Health said in a statement. “There is clearly a high demand in the industry for EMR integration, specifically the two-way flow of individual data elements between telemedicine platforms and EMR systems.”
On Twitter @legal_med
Nearly two-thirds of health care providers rank telemedicine as a top priority in 2016, a 10% increase from last year, according to a survey.
Telemedicine software company REACH Health surveyed 390 U.S. health care professionals between November 2015 and December 2015, including physicians, nurses, and health care executives. Participants answered questions related to their objectives, challenges, telemedicine program models, and management structures, among other inquiries.

Of those polled, 96% of respondents said improving patient outcome was a top objective in developing telemedicine programs, according to the survey. Increasing patient convenience (87%) and improving patient engagement (86%) also rated highly. Other objectives included providing remote and rural patients with access to specialists (83%) and improving leverage of limited physician resources (81%). Percentages do not equal 100% because respondents could choose more than one objective.
The maturity of telemedicine programs varied widely depending on care setting. In general, settings requiring highly specialized treatment had more mature telemedicine programs than those requiring more generalized treatment. Stroke, neurology, and psychiatric/behavioral health settings had the most mature telemedicine programs, according to the survey.
Reimbursement ranked as the top barrier to telemedicine. Respondents rated private plan payment as the No. 1 challenge (38%), followed by Medicare reimbursement (36%) and Medicaid reimbursement (36%). Electronic health record incapabilities and liability risks also ranked as primary challenges.
“Telemedicine reimbursement poses the primary obstacle to success, but EMR-related challenges are persistent and widely noted in the survey,” Steve McGraw, president and CEO of REACH Health said in a statement. “There is clearly a high demand in the industry for EMR integration, specifically the two-way flow of individual data elements between telemedicine platforms and EMR systems.”
On Twitter @legal_med
Could value-based care raise False Claims Act liability?
As you begin to consider the switch to value-based care systems, be sure to safeguard against risks that could fuel false claims scrutiny by the government, legal experts advise.
A primary consideration is arrangements that include shared savings through coordinated care, said George B. Breen, a Washington-based health law attorney. For example, he said that the Stark Law could be implicated if a physician within a shared savings model receives a bonus payment for referring patients to specific providers. The Stark Law prohibits a physician from referring Medicare patients for designated health services to an entity with which the physician has a financial relationship.
“While there are a number of exceptions and safe harbors which would validate any such relationship, it’s something that needs to be thought through” from the start, Mr. Breen said in an interview. “You have to look at each factual circumstance separately because each is fact and circumstance dependent.”
The Anti-Kickback Statute also could come into play if value-based arrangements generate renumeration. The Anti-Kickback Statute prohibits offering, paying, soliciting, or receiving anything of value to induce or reward referrals or generate federal health care program business. Exceptions to the statute can be applied and should also be examined during arrangement development, Mr. Breen said.
Data collection and reporting also may present a problem, according to Seattle-based health law attorney Robert G. Homchick. Inaccurate data that become the basis for quality-based payments could lead to overpayment liability and indirect False Claims Act (FCA) exposure, he said.
In addition, “If the facts support that you were acting with intentional or deliberate ignorance or reckless disregard for how the data were gathered and reported that supported your value-based comp kicker, there could be direct False Claims Act liability,” Mr. Homchick said in an interview.
But Mr. Homchick stressed there are still many unknowns when it comes to how data-driven measurements will unfold.
“With MACRA, this is such a moving target as to exactly what type of data is going to form the basis for the metrics, and how the data need to be gathered and reported,” he said. “Many of those issues are still in play or still being developed at the agency level in terms of regulatory guidance.”
Current lawsuit could influence future cases
While it is too early to know every legal theory that could intersect with quality-based care, legal experts are closely watching a case that could offer insight into future claims.
In Duffy v. Lawrence Memorial Hospital, a former employee turned whistle-blower alleges that the Lawrence, Kan.–based hospital inflated its performance scores under the Hospital Value-Based Purchasing Program to increase federal incentive payments. Hospital leaders deny they falsified data and claim the allegations are based on the whistle-blower’s “improper understanding of acceptable reporting times for patient arrival,” according the hospital’s court response. The FCA lawsuit is before the U.S. District Court for the District of Kansas.
Notably, the government has declined to intervene in the lawsuit twice, Mr. Breen said. The case is continuing without government intervention, a trend that has become more common in recent years, he said. In 2015, whistle-blower cases in which U.S. Department of Justice declined to intervene led to $1.1 billion in recoveries for the government and $335 million in rewards for whistle-blowers, according to government data.
Mr. Homchick called the Lawrence Memorial lawsuit “troubling.”
“Providers are struggling with the complexity of the reporting requirements imposed by the layers of value-based payment programs implemented by both government and private payers,” he said. “The Lawrence Memorial case illustrates that the whistle-blower community will likely exploit the inevitable mistakes or missteps of providers attempting to comply with the increasingly byzantine quality-reporting requirements.”
The outcome of the Lawrence Memorial case could influence similar lawsuits involving value-based programs, Mr. Breen said.
“I think this theory that there is some false reporting, or false certification, is a theory that you will see being pursed in connection with some of these quality-based programs,” Mr. Breen said.
Early steps can curb legal risk
Asking questions and being proactive as new value-based models develop is key to mitigating legal dangers, experts said.
Ensure that new arrangements are analyzed for fraud and abuse risk exposure before finalizing, Mr. Breen advised.
“Have a comfort level about the arrangement” that’s being entered into, he added, and “have those arrangements vetted.”
Pay attention to data, added Michael E. Paulhus, an Atlanta-based health law attorney who specializes in FCA cases.
“The more data they collect, the more the government is paying attention to where you are in the range,” he said in an interview. “If you stick out on either end, that would be a risk profile that, as a physician, I would want to know. I would want to know where I sit in the data.”
When making reports regarding quality measures, include such reports in internal audits as part of regular compliance efforts, the experts suggested.
In addition, seek out resources early that can help prepare the practice for new quality-based regulations, Mr. Homchick said.
“There will be guidance coming out [regarding] eligibility for these value-based incentives,” he said. “That will require you and your staff to really pay attention and seek out resources and guidance to try to do this right. If you have the bandwidth to get out ahead of this, that would certainly be the best approach.”
On Twitter @legal_med
As you begin to consider the switch to value-based care systems, be sure to safeguard against risks that could fuel false claims scrutiny by the government, legal experts advise.
A primary consideration is arrangements that include shared savings through coordinated care, said George B. Breen, a Washington-based health law attorney. For example, he said that the Stark Law could be implicated if a physician within a shared savings model receives a bonus payment for referring patients to specific providers. The Stark Law prohibits a physician from referring Medicare patients for designated health services to an entity with which the physician has a financial relationship.
“While there are a number of exceptions and safe harbors which would validate any such relationship, it’s something that needs to be thought through” from the start, Mr. Breen said in an interview. “You have to look at each factual circumstance separately because each is fact and circumstance dependent.”
The Anti-Kickback Statute also could come into play if value-based arrangements generate renumeration. The Anti-Kickback Statute prohibits offering, paying, soliciting, or receiving anything of value to induce or reward referrals or generate federal health care program business. Exceptions to the statute can be applied and should also be examined during arrangement development, Mr. Breen said.
Data collection and reporting also may present a problem, according to Seattle-based health law attorney Robert G. Homchick. Inaccurate data that become the basis for quality-based payments could lead to overpayment liability and indirect False Claims Act (FCA) exposure, he said.
In addition, “If the facts support that you were acting with intentional or deliberate ignorance or reckless disregard for how the data were gathered and reported that supported your value-based comp kicker, there could be direct False Claims Act liability,” Mr. Homchick said in an interview.
But Mr. Homchick stressed there are still many unknowns when it comes to how data-driven measurements will unfold.
“With MACRA, this is such a moving target as to exactly what type of data is going to form the basis for the metrics, and how the data need to be gathered and reported,” he said. “Many of those issues are still in play or still being developed at the agency level in terms of regulatory guidance.”
Current lawsuit could influence future cases
While it is too early to know every legal theory that could intersect with quality-based care, legal experts are closely watching a case that could offer insight into future claims.
In Duffy v. Lawrence Memorial Hospital, a former employee turned whistle-blower alleges that the Lawrence, Kan.–based hospital inflated its performance scores under the Hospital Value-Based Purchasing Program to increase federal incentive payments. Hospital leaders deny they falsified data and claim the allegations are based on the whistle-blower’s “improper understanding of acceptable reporting times for patient arrival,” according the hospital’s court response. The FCA lawsuit is before the U.S. District Court for the District of Kansas.
Notably, the government has declined to intervene in the lawsuit twice, Mr. Breen said. The case is continuing without government intervention, a trend that has become more common in recent years, he said. In 2015, whistle-blower cases in which U.S. Department of Justice declined to intervene led to $1.1 billion in recoveries for the government and $335 million in rewards for whistle-blowers, according to government data.
Mr. Homchick called the Lawrence Memorial lawsuit “troubling.”
“Providers are struggling with the complexity of the reporting requirements imposed by the layers of value-based payment programs implemented by both government and private payers,” he said. “The Lawrence Memorial case illustrates that the whistle-blower community will likely exploit the inevitable mistakes or missteps of providers attempting to comply with the increasingly byzantine quality-reporting requirements.”
The outcome of the Lawrence Memorial case could influence similar lawsuits involving value-based programs, Mr. Breen said.
“I think this theory that there is some false reporting, or false certification, is a theory that you will see being pursed in connection with some of these quality-based programs,” Mr. Breen said.
Early steps can curb legal risk
Asking questions and being proactive as new value-based models develop is key to mitigating legal dangers, experts said.
Ensure that new arrangements are analyzed for fraud and abuse risk exposure before finalizing, Mr. Breen advised.
“Have a comfort level about the arrangement” that’s being entered into, he added, and “have those arrangements vetted.”
Pay attention to data, added Michael E. Paulhus, an Atlanta-based health law attorney who specializes in FCA cases.
“The more data they collect, the more the government is paying attention to where you are in the range,” he said in an interview. “If you stick out on either end, that would be a risk profile that, as a physician, I would want to know. I would want to know where I sit in the data.”
When making reports regarding quality measures, include such reports in internal audits as part of regular compliance efforts, the experts suggested.
In addition, seek out resources early that can help prepare the practice for new quality-based regulations, Mr. Homchick said.
“There will be guidance coming out [regarding] eligibility for these value-based incentives,” he said. “That will require you and your staff to really pay attention and seek out resources and guidance to try to do this right. If you have the bandwidth to get out ahead of this, that would certainly be the best approach.”
On Twitter @legal_med
As you begin to consider the switch to value-based care systems, be sure to safeguard against risks that could fuel false claims scrutiny by the government, legal experts advise.
A primary consideration is arrangements that include shared savings through coordinated care, said George B. Breen, a Washington-based health law attorney. For example, he said that the Stark Law could be implicated if a physician within a shared savings model receives a bonus payment for referring patients to specific providers. The Stark Law prohibits a physician from referring Medicare patients for designated health services to an entity with which the physician has a financial relationship.
“While there are a number of exceptions and safe harbors which would validate any such relationship, it’s something that needs to be thought through” from the start, Mr. Breen said in an interview. “You have to look at each factual circumstance separately because each is fact and circumstance dependent.”
The Anti-Kickback Statute also could come into play if value-based arrangements generate renumeration. The Anti-Kickback Statute prohibits offering, paying, soliciting, or receiving anything of value to induce or reward referrals or generate federal health care program business. Exceptions to the statute can be applied and should also be examined during arrangement development, Mr. Breen said.
Data collection and reporting also may present a problem, according to Seattle-based health law attorney Robert G. Homchick. Inaccurate data that become the basis for quality-based payments could lead to overpayment liability and indirect False Claims Act (FCA) exposure, he said.
In addition, “If the facts support that you were acting with intentional or deliberate ignorance or reckless disregard for how the data were gathered and reported that supported your value-based comp kicker, there could be direct False Claims Act liability,” Mr. Homchick said in an interview.
But Mr. Homchick stressed there are still many unknowns when it comes to how data-driven measurements will unfold.
“With MACRA, this is such a moving target as to exactly what type of data is going to form the basis for the metrics, and how the data need to be gathered and reported,” he said. “Many of those issues are still in play or still being developed at the agency level in terms of regulatory guidance.”
Current lawsuit could influence future cases
While it is too early to know every legal theory that could intersect with quality-based care, legal experts are closely watching a case that could offer insight into future claims.
In Duffy v. Lawrence Memorial Hospital, a former employee turned whistle-blower alleges that the Lawrence, Kan.–based hospital inflated its performance scores under the Hospital Value-Based Purchasing Program to increase federal incentive payments. Hospital leaders deny they falsified data and claim the allegations are based on the whistle-blower’s “improper understanding of acceptable reporting times for patient arrival,” according the hospital’s court response. The FCA lawsuit is before the U.S. District Court for the District of Kansas.
Notably, the government has declined to intervene in the lawsuit twice, Mr. Breen said. The case is continuing without government intervention, a trend that has become more common in recent years, he said. In 2015, whistle-blower cases in which U.S. Department of Justice declined to intervene led to $1.1 billion in recoveries for the government and $335 million in rewards for whistle-blowers, according to government data.
Mr. Homchick called the Lawrence Memorial lawsuit “troubling.”
“Providers are struggling with the complexity of the reporting requirements imposed by the layers of value-based payment programs implemented by both government and private payers,” he said. “The Lawrence Memorial case illustrates that the whistle-blower community will likely exploit the inevitable mistakes or missteps of providers attempting to comply with the increasingly byzantine quality-reporting requirements.”
The outcome of the Lawrence Memorial case could influence similar lawsuits involving value-based programs, Mr. Breen said.
“I think this theory that there is some false reporting, or false certification, is a theory that you will see being pursed in connection with some of these quality-based programs,” Mr. Breen said.
Early steps can curb legal risk
Asking questions and being proactive as new value-based models develop is key to mitigating legal dangers, experts said.
Ensure that new arrangements are analyzed for fraud and abuse risk exposure before finalizing, Mr. Breen advised.
“Have a comfort level about the arrangement” that’s being entered into, he added, and “have those arrangements vetted.”
Pay attention to data, added Michael E. Paulhus, an Atlanta-based health law attorney who specializes in FCA cases.
“The more data they collect, the more the government is paying attention to where you are in the range,” he said in an interview. “If you stick out on either end, that would be a risk profile that, as a physician, I would want to know. I would want to know where I sit in the data.”
When making reports regarding quality measures, include such reports in internal audits as part of regular compliance efforts, the experts suggested.
In addition, seek out resources early that can help prepare the practice for new quality-based regulations, Mr. Homchick said.
“There will be guidance coming out [regarding] eligibility for these value-based incentives,” he said. “That will require you and your staff to really pay attention and seek out resources and guidance to try to do this right. If you have the bandwidth to get out ahead of this, that would certainly be the best approach.”
On Twitter @legal_med
State board discipline of physicians varies widely by state
The rate at which medical boards discipline physicians varies widely by state, with Delaware doctors facing four times more disciplinary actions that Massachusetts physicians, according to a review of physicians in all 50 states.
Dr. John Alexander Harris of the University of Michigan, Ann Arbor, and colleagues reviewed 21,647 physician disciplinary actions recorded in the National Practitioner Data Bank from 2010 to 2014 across all 50 states and the District of Columbia (BMJ Qual Saf. 2016 Mar 23; doi: 10.1136/bmjqs-2015-004974).
Investigators evaluated American Medical Association demographic data from the same time frame to estimate rates of disciplinary actions while controlling for the number of physicians in each state. Of the actions studied, 24% were major disciplinary actions involving revocation, suspension, or license surrender. Researchers also controlled for data reliability, year to year variation, physician labor supply, and malpractice climate in each state.
Delaware experienced the highest rate of medical board discipline with eight actions per 1,000 physicians, while Massachusetts had the lowest rate with two actions per 1,000 physicians, the investigators found. Kentucky and Ohio were among the states with the highest rates of disciplinary actions. New York, Connecticut, and Pennsylvania doctors experienced among the lowest rates of medical board disciplinary actions.
Researchers presumed there would be differences in the level of medical board disciplinary actions, but they were surprised at the fourfold gap between states, Dr. Harris said in an interview.
“The biggest takeaway is that there is a large, unexplained variation between the rate at which medical boards discipline physicians from state to state,” he said. “We should focus more on understanding what’s causing this variation.”
Investigators found no significant connection between state medical malpractice climate and the rate of medical board disciplinary actions. They also found no marked association between the rate of disciplinary actions and physician supply or the year of the discipline. No correlation was found between the rate of major and minor disciplinary action. In other words, states that had higher rates of major actions did not necessarily have higher rates of minor actions.
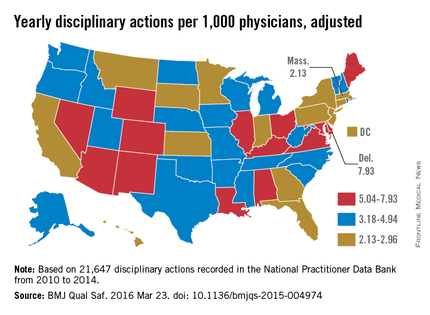
Potential reasons behind the state differences could include variations in the volume of physician misconduct per state, differences in the reporting of misconduct, or disparities in how often medical boards investigate incidents, Dr. Harris said.
“The process of physician disciplinary actions is complex,” he noted. “There are several different steps where the system can change the outcome significantly.”
For more than one-third of actions studied, the basis for the disciplinary action was classified as “unspecified” (38%). Specified reasons included negligence (9%), illegal activity (8%), license action (7%), failure to comply with medical board (6%), fraud (6%), unprofessional conduct (4%), substance abuse (4%), sexual or boundary misconduct (2%), failure to maintain adequate records (2%), immediate threat to public health and safety (1%), and “other” (14%). A total of 5,242 actions had no information regarding the reason for the action.
State medical boards should consider policies aimed at “improving standardization and coordination to provide consistent supervision to physicians and ensure public safety,” the authors wrote. In the meantime, more research is needed in the area, Dr. Harris said.
On Twitter @legal_med
The rate at which medical boards discipline physicians varies widely by state, with Delaware doctors facing four times more disciplinary actions that Massachusetts physicians, according to a review of physicians in all 50 states.
Dr. John Alexander Harris of the University of Michigan, Ann Arbor, and colleagues reviewed 21,647 physician disciplinary actions recorded in the National Practitioner Data Bank from 2010 to 2014 across all 50 states and the District of Columbia (BMJ Qual Saf. 2016 Mar 23; doi: 10.1136/bmjqs-2015-004974).
Investigators evaluated American Medical Association demographic data from the same time frame to estimate rates of disciplinary actions while controlling for the number of physicians in each state. Of the actions studied, 24% were major disciplinary actions involving revocation, suspension, or license surrender. Researchers also controlled for data reliability, year to year variation, physician labor supply, and malpractice climate in each state.
Delaware experienced the highest rate of medical board discipline with eight actions per 1,000 physicians, while Massachusetts had the lowest rate with two actions per 1,000 physicians, the investigators found. Kentucky and Ohio were among the states with the highest rates of disciplinary actions. New York, Connecticut, and Pennsylvania doctors experienced among the lowest rates of medical board disciplinary actions.
Researchers presumed there would be differences in the level of medical board disciplinary actions, but they were surprised at the fourfold gap between states, Dr. Harris said in an interview.
“The biggest takeaway is that there is a large, unexplained variation between the rate at which medical boards discipline physicians from state to state,” he said. “We should focus more on understanding what’s causing this variation.”
Investigators found no significant connection between state medical malpractice climate and the rate of medical board disciplinary actions. They also found no marked association between the rate of disciplinary actions and physician supply or the year of the discipline. No correlation was found between the rate of major and minor disciplinary action. In other words, states that had higher rates of major actions did not necessarily have higher rates of minor actions.

Potential reasons behind the state differences could include variations in the volume of physician misconduct per state, differences in the reporting of misconduct, or disparities in how often medical boards investigate incidents, Dr. Harris said.
“The process of physician disciplinary actions is complex,” he noted. “There are several different steps where the system can change the outcome significantly.”
For more than one-third of actions studied, the basis for the disciplinary action was classified as “unspecified” (38%). Specified reasons included negligence (9%), illegal activity (8%), license action (7%), failure to comply with medical board (6%), fraud (6%), unprofessional conduct (4%), substance abuse (4%), sexual or boundary misconduct (2%), failure to maintain adequate records (2%), immediate threat to public health and safety (1%), and “other” (14%). A total of 5,242 actions had no information regarding the reason for the action.
State medical boards should consider policies aimed at “improving standardization and coordination to provide consistent supervision to physicians and ensure public safety,” the authors wrote. In the meantime, more research is needed in the area, Dr. Harris said.
On Twitter @legal_med
The rate at which medical boards discipline physicians varies widely by state, with Delaware doctors facing four times more disciplinary actions that Massachusetts physicians, according to a review of physicians in all 50 states.
Dr. John Alexander Harris of the University of Michigan, Ann Arbor, and colleagues reviewed 21,647 physician disciplinary actions recorded in the National Practitioner Data Bank from 2010 to 2014 across all 50 states and the District of Columbia (BMJ Qual Saf. 2016 Mar 23; doi: 10.1136/bmjqs-2015-004974).
Investigators evaluated American Medical Association demographic data from the same time frame to estimate rates of disciplinary actions while controlling for the number of physicians in each state. Of the actions studied, 24% were major disciplinary actions involving revocation, suspension, or license surrender. Researchers also controlled for data reliability, year to year variation, physician labor supply, and malpractice climate in each state.
Delaware experienced the highest rate of medical board discipline with eight actions per 1,000 physicians, while Massachusetts had the lowest rate with two actions per 1,000 physicians, the investigators found. Kentucky and Ohio were among the states with the highest rates of disciplinary actions. New York, Connecticut, and Pennsylvania doctors experienced among the lowest rates of medical board disciplinary actions.
Researchers presumed there would be differences in the level of medical board disciplinary actions, but they were surprised at the fourfold gap between states, Dr. Harris said in an interview.
“The biggest takeaway is that there is a large, unexplained variation between the rate at which medical boards discipline physicians from state to state,” he said. “We should focus more on understanding what’s causing this variation.”
Investigators found no significant connection between state medical malpractice climate and the rate of medical board disciplinary actions. They also found no marked association between the rate of disciplinary actions and physician supply or the year of the discipline. No correlation was found between the rate of major and minor disciplinary action. In other words, states that had higher rates of major actions did not necessarily have higher rates of minor actions.

Potential reasons behind the state differences could include variations in the volume of physician misconduct per state, differences in the reporting of misconduct, or disparities in how often medical boards investigate incidents, Dr. Harris said.
“The process of physician disciplinary actions is complex,” he noted. “There are several different steps where the system can change the outcome significantly.”
For more than one-third of actions studied, the basis for the disciplinary action was classified as “unspecified” (38%). Specified reasons included negligence (9%), illegal activity (8%), license action (7%), failure to comply with medical board (6%), fraud (6%), unprofessional conduct (4%), substance abuse (4%), sexual or boundary misconduct (2%), failure to maintain adequate records (2%), immediate threat to public health and safety (1%), and “other” (14%). A total of 5,242 actions had no information regarding the reason for the action.
State medical boards should consider policies aimed at “improving standardization and coordination to provide consistent supervision to physicians and ensure public safety,” the authors wrote. In the meantime, more research is needed in the area, Dr. Harris said.
On Twitter @legal_med
What are the best, worst states for physicians?
Should your future include a move to the South? A new report finds that Mississippi ranks as the best state to practice medicine, while the District of Columbia and New York are the least doctor-friendly areas in the United States.
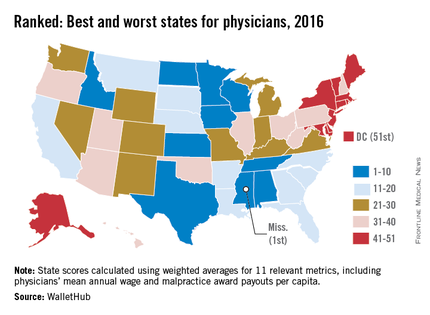
The survey, conducted by personal finance website WalletHub, compares all 50 states and D.C. across 11 metrics, including physician starting salary, medical malpractice climate, provider competition, and annual wages – adjusted for cost of living. Data was derived from the U.S. Census Bureau, the Bureau of Labor Statistics, the U.S. Department of Health and Human Services, and the Missouri Economic Research & Information Center, among other sources.
Researchers gave each metric a value between 0 and 100 and then calculated an overall score for each state using the weighted average across all metrics. Behind Mississippi, Iowa, Minnesota, and North Dakota ranked among the best states to practice medicine, according to the report. Rhode Island, Maryland, and Connecticut ranked among the worst, just slightly better than New York and D.C.
“There are an abundance of differences in terms of the working environments faced by doctors across the nation,” WalletHub analyst Jill Gonzalez said in an interview. “The results, while not too surprising, may certainly be eye opening for many new or soon-to-be doctors. Doctors should understand what they’re signing up for in terms of wages, malpractice rates, and job security when they move to another state to practice.”
View the entire WalletHub analysis here.
On Twitter @legal_med
Should your future include a move to the South? A new report finds that Mississippi ranks as the best state to practice medicine, while the District of Columbia and New York are the least doctor-friendly areas in the United States.

The survey, conducted by personal finance website WalletHub, compares all 50 states and D.C. across 11 metrics, including physician starting salary, medical malpractice climate, provider competition, and annual wages – adjusted for cost of living. Data was derived from the U.S. Census Bureau, the Bureau of Labor Statistics, the U.S. Department of Health and Human Services, and the Missouri Economic Research & Information Center, among other sources.
Researchers gave each metric a value between 0 and 100 and then calculated an overall score for each state using the weighted average across all metrics. Behind Mississippi, Iowa, Minnesota, and North Dakota ranked among the best states to practice medicine, according to the report. Rhode Island, Maryland, and Connecticut ranked among the worst, just slightly better than New York and D.C.
“There are an abundance of differences in terms of the working environments faced by doctors across the nation,” WalletHub analyst Jill Gonzalez said in an interview. “The results, while not too surprising, may certainly be eye opening for many new or soon-to-be doctors. Doctors should understand what they’re signing up for in terms of wages, malpractice rates, and job security when they move to another state to practice.”
View the entire WalletHub analysis here.
On Twitter @legal_med
Should your future include a move to the South? A new report finds that Mississippi ranks as the best state to practice medicine, while the District of Columbia and New York are the least doctor-friendly areas in the United States.

The survey, conducted by personal finance website WalletHub, compares all 50 states and D.C. across 11 metrics, including physician starting salary, medical malpractice climate, provider competition, and annual wages – adjusted for cost of living. Data was derived from the U.S. Census Bureau, the Bureau of Labor Statistics, the U.S. Department of Health and Human Services, and the Missouri Economic Research & Information Center, among other sources.
Researchers gave each metric a value between 0 and 100 and then calculated an overall score for each state using the weighted average across all metrics. Behind Mississippi, Iowa, Minnesota, and North Dakota ranked among the best states to practice medicine, according to the report. Rhode Island, Maryland, and Connecticut ranked among the worst, just slightly better than New York and D.C.
“There are an abundance of differences in terms of the working environments faced by doctors across the nation,” WalletHub analyst Jill Gonzalez said in an interview. “The results, while not too surprising, may certainly be eye opening for many new or soon-to-be doctors. Doctors should understand what they’re signing up for in terms of wages, malpractice rates, and job security when they move to another state to practice.”
View the entire WalletHub analysis here.
On Twitter @legal_med