User login
New Trump travel order could disrupt meetings, trainees
President’s Trump’s revised executive order blocking travelers from six Muslim-majority countries from entering the United States could land a damaging blow to global cooperation in scientific research and impede assemblies of the world’s top medical experts.
The executive order, signed March 6, bars citizens of Iran, Libya, Somalia, Sudan, Syria, and Yemen from obtaining visas for 90 days and blocks refugees from the affected countries from entering the U.S. for 120 days. The new executive measure, which takes effect March 16, supersedes President Trump’s original Jan. 27 travel ban that has been blocked by federal courts.
The new order clarifies that citizens of the six countries who are legal permanent U.S. residents or who have current visas to enter the country are exempt from the travel prohibition.
The revised travel ban could disrupt the exchange of medical knowledge by barring foreign experts from traveling to medical and scientific conferences in the United States, and leaves the status of medical trainees from those countries in limbo, according to American Medical Association President Andrew W. Gurman, MD.
“Hundreds of physicians from six countries are subject to the revised executive order and have applied to U.S. training programs and requested visa sponsorship,” he said in a statement. “The new executive order leaves them in limbo and without an explicit waiver, these foreign physicians will be unable to provide care in the U.S. when training programs begin on July 1.”
The new order is already being challenged in court. On March 7, the state of Hawaii filed a lawsuit seeking to block the order, saying that it subjects a portion of Hawaii’s population to “discrimination and second-class treatment.”
When the original ban took effect, thousands of academics from around the world, including physicians, researchers, and professors, vowed to boycott U.S.-based conferences. A Google Docs petition started shortly after the ban was announced garnered more than 5,000 signatures by professionals acting in solidarity with those affected by the travel restrictions. The academicians who signed the petition said they would not attend international conferences in the United States until those restricted from participating could rejoin their colleagues and freely share their ideas.
The new executive order comes nearly 2 months after President Trump’s original travel ban caused nationwide protests and led to a series of legal challenges. The states of Washington and Minnesota, which sued President Trump over his original ban, argued that such a ban harms the teaching and research missions of their universities and prevents students and faculty from traveling for research and academic collaboration. In addition, the executive order restricts universities from hiring attractive candidates from countries affected by the ban, state officials said.
A federal court temporarily blocked the original travel ban on Feb. 3, a decision upheld by the 9th U.S. Circuit Court of Appeals on Feb. 9.
The new executive order excludes Iraq this time around and also removes language that had indefinitely banned Syrian refugees. In a March 6 memorandum, the White House said the purpose of the ban is to prevent “foreign nationals who may aid, support, or commit violent, criminal, or terrorist acts,” while the administration enhances the screening and vetting protocols and procedures for granting visas and admission to the United States.
“This nation cannot delay the immediate implementation of additional heightened screening and vetting protocols and procedures for issuing visas to ensure that we strengthen the safety and security of our country,” the memo states.
This article was updated March 8, 2017.
[email protected]
On Twitter @legal_med
President’s Trump’s revised executive order blocking travelers from six Muslim-majority countries from entering the United States could land a damaging blow to global cooperation in scientific research and impede assemblies of the world’s top medical experts.
The executive order, signed March 6, bars citizens of Iran, Libya, Somalia, Sudan, Syria, and Yemen from obtaining visas for 90 days and blocks refugees from the affected countries from entering the U.S. for 120 days. The new executive measure, which takes effect March 16, supersedes President Trump’s original Jan. 27 travel ban that has been blocked by federal courts.
The new order clarifies that citizens of the six countries who are legal permanent U.S. residents or who have current visas to enter the country are exempt from the travel prohibition.
The revised travel ban could disrupt the exchange of medical knowledge by barring foreign experts from traveling to medical and scientific conferences in the United States, and leaves the status of medical trainees from those countries in limbo, according to American Medical Association President Andrew W. Gurman, MD.
“Hundreds of physicians from six countries are subject to the revised executive order and have applied to U.S. training programs and requested visa sponsorship,” he said in a statement. “The new executive order leaves them in limbo and without an explicit waiver, these foreign physicians will be unable to provide care in the U.S. when training programs begin on July 1.”
The new order is already being challenged in court. On March 7, the state of Hawaii filed a lawsuit seeking to block the order, saying that it subjects a portion of Hawaii’s population to “discrimination and second-class treatment.”
When the original ban took effect, thousands of academics from around the world, including physicians, researchers, and professors, vowed to boycott U.S.-based conferences. A Google Docs petition started shortly after the ban was announced garnered more than 5,000 signatures by professionals acting in solidarity with those affected by the travel restrictions. The academicians who signed the petition said they would not attend international conferences in the United States until those restricted from participating could rejoin their colleagues and freely share their ideas.
The new executive order comes nearly 2 months after President Trump’s original travel ban caused nationwide protests and led to a series of legal challenges. The states of Washington and Minnesota, which sued President Trump over his original ban, argued that such a ban harms the teaching and research missions of their universities and prevents students and faculty from traveling for research and academic collaboration. In addition, the executive order restricts universities from hiring attractive candidates from countries affected by the ban, state officials said.
A federal court temporarily blocked the original travel ban on Feb. 3, a decision upheld by the 9th U.S. Circuit Court of Appeals on Feb. 9.
The new executive order excludes Iraq this time around and also removes language that had indefinitely banned Syrian refugees. In a March 6 memorandum, the White House said the purpose of the ban is to prevent “foreign nationals who may aid, support, or commit violent, criminal, or terrorist acts,” while the administration enhances the screening and vetting protocols and procedures for granting visas and admission to the United States.
“This nation cannot delay the immediate implementation of additional heightened screening and vetting protocols and procedures for issuing visas to ensure that we strengthen the safety and security of our country,” the memo states.
This article was updated March 8, 2017.
[email protected]
On Twitter @legal_med
President’s Trump’s revised executive order blocking travelers from six Muslim-majority countries from entering the United States could land a damaging blow to global cooperation in scientific research and impede assemblies of the world’s top medical experts.
The executive order, signed March 6, bars citizens of Iran, Libya, Somalia, Sudan, Syria, and Yemen from obtaining visas for 90 days and blocks refugees from the affected countries from entering the U.S. for 120 days. The new executive measure, which takes effect March 16, supersedes President Trump’s original Jan. 27 travel ban that has been blocked by federal courts.
The new order clarifies that citizens of the six countries who are legal permanent U.S. residents or who have current visas to enter the country are exempt from the travel prohibition.
The revised travel ban could disrupt the exchange of medical knowledge by barring foreign experts from traveling to medical and scientific conferences in the United States, and leaves the status of medical trainees from those countries in limbo, according to American Medical Association President Andrew W. Gurman, MD.
“Hundreds of physicians from six countries are subject to the revised executive order and have applied to U.S. training programs and requested visa sponsorship,” he said in a statement. “The new executive order leaves them in limbo and without an explicit waiver, these foreign physicians will be unable to provide care in the U.S. when training programs begin on July 1.”
The new order is already being challenged in court. On March 7, the state of Hawaii filed a lawsuit seeking to block the order, saying that it subjects a portion of Hawaii’s population to “discrimination and second-class treatment.”
When the original ban took effect, thousands of academics from around the world, including physicians, researchers, and professors, vowed to boycott U.S.-based conferences. A Google Docs petition started shortly after the ban was announced garnered more than 5,000 signatures by professionals acting in solidarity with those affected by the travel restrictions. The academicians who signed the petition said they would not attend international conferences in the United States until those restricted from participating could rejoin their colleagues and freely share their ideas.
The new executive order comes nearly 2 months after President Trump’s original travel ban caused nationwide protests and led to a series of legal challenges. The states of Washington and Minnesota, which sued President Trump over his original ban, argued that such a ban harms the teaching and research missions of their universities and prevents students and faculty from traveling for research and academic collaboration. In addition, the executive order restricts universities from hiring attractive candidates from countries affected by the ban, state officials said.
A federal court temporarily blocked the original travel ban on Feb. 3, a decision upheld by the 9th U.S. Circuit Court of Appeals on Feb. 9.
The new executive order excludes Iraq this time around and also removes language that had indefinitely banned Syrian refugees. In a March 6 memorandum, the White House said the purpose of the ban is to prevent “foreign nationals who may aid, support, or commit violent, criminal, or terrorist acts,” while the administration enhances the screening and vetting protocols and procedures for granting visas and admission to the United States.
“This nation cannot delay the immediate implementation of additional heightened screening and vetting protocols and procedures for issuing visas to ensure that we strengthen the safety and security of our country,” the memo states.
This article was updated March 8, 2017.
[email protected]
On Twitter @legal_med
CMS nominee Verma clears Senate Finance hurdle
Seema Verma has moved one step closer to becoming the administrator of the Centers for Medicare & Medicaid Services.
The Senate Finance Committee voted 13-12 on March 2 to approve Ms. Verma’s nomination after a delayed vote the day before. The vote, conducted during a meeting off the floor, was a straight party-line vote, with 13 Republicans voting for Ms. Verma and 12 Democrats voting against. One proxy vote was not counted into the final tally per Senate rules.
Her nomination will now be considered by the full Senate.
Senate Finance Committee Chairman Orrin Hatch (R-Utah) praised Ms. Verma as a qualified leader who will help improve CMS.
Senate Finance Committee Ranking Member Ron Wyden, (D-Ore.) denounced Ms. Verma, stressing that she failed to adequately answer questions during her nomination hearing and has presented no clear vision of her plans as the next CMS administrator.
During her nomination hearing on Feb. 16, Ms. Verma came under fire for past consulting agreements with states while working for Hewlett Packard, a company that had financial interests in the health programs she designed. The issue was raised again during a preliminary vote by the Finance Committee on March 1.
“Ms. Verma was on both sides of the deal, helping manage the state’s health programs while being paid by vendors to those same programs,” Sen. Wyden said during the hearing. “I am concerned that if Ms. Verma is confirmed to lead CMS, where many of the companies she worked for are major vendors, there will not be adequate scrutiny of her past relationships with them, just as there wasn’t in Indiana.”
Ms. Verma previously said she never negotiated on behalf of Hewlett Packard and that the work she conducted for the states did not overlap with work she completed for HP.
“I hold honesty and integrity and adherence to a high ethical standard as part of my personal philosophy. That’s for me, I demand that from my employees, and I set that example for my own children,” she said during the Feb. 16 hearing. “We were never in a position where we were negotiating on behalf of HP or any other contractor with the state that we had a relationship with. If there was the potential [for a conflict], we would recuse ourselves.”
Ms. Verma’s nomination will now move to the full Senate. No date has yet been set for the vote.
[email protected]
On Twitter @legal_med
Seema Verma has moved one step closer to becoming the administrator of the Centers for Medicare & Medicaid Services.
The Senate Finance Committee voted 13-12 on March 2 to approve Ms. Verma’s nomination after a delayed vote the day before. The vote, conducted during a meeting off the floor, was a straight party-line vote, with 13 Republicans voting for Ms. Verma and 12 Democrats voting against. One proxy vote was not counted into the final tally per Senate rules.
Her nomination will now be considered by the full Senate.
Senate Finance Committee Chairman Orrin Hatch (R-Utah) praised Ms. Verma as a qualified leader who will help improve CMS.
Senate Finance Committee Ranking Member Ron Wyden, (D-Ore.) denounced Ms. Verma, stressing that she failed to adequately answer questions during her nomination hearing and has presented no clear vision of her plans as the next CMS administrator.
During her nomination hearing on Feb. 16, Ms. Verma came under fire for past consulting agreements with states while working for Hewlett Packard, a company that had financial interests in the health programs she designed. The issue was raised again during a preliminary vote by the Finance Committee on March 1.
“Ms. Verma was on both sides of the deal, helping manage the state’s health programs while being paid by vendors to those same programs,” Sen. Wyden said during the hearing. “I am concerned that if Ms. Verma is confirmed to lead CMS, where many of the companies she worked for are major vendors, there will not be adequate scrutiny of her past relationships with them, just as there wasn’t in Indiana.”
Ms. Verma previously said she never negotiated on behalf of Hewlett Packard and that the work she conducted for the states did not overlap with work she completed for HP.
“I hold honesty and integrity and adherence to a high ethical standard as part of my personal philosophy. That’s for me, I demand that from my employees, and I set that example for my own children,” she said during the Feb. 16 hearing. “We were never in a position where we were negotiating on behalf of HP or any other contractor with the state that we had a relationship with. If there was the potential [for a conflict], we would recuse ourselves.”
Ms. Verma’s nomination will now move to the full Senate. No date has yet been set for the vote.
[email protected]
On Twitter @legal_med
Seema Verma has moved one step closer to becoming the administrator of the Centers for Medicare & Medicaid Services.
The Senate Finance Committee voted 13-12 on March 2 to approve Ms. Verma’s nomination after a delayed vote the day before. The vote, conducted during a meeting off the floor, was a straight party-line vote, with 13 Republicans voting for Ms. Verma and 12 Democrats voting against. One proxy vote was not counted into the final tally per Senate rules.
Her nomination will now be considered by the full Senate.
Senate Finance Committee Chairman Orrin Hatch (R-Utah) praised Ms. Verma as a qualified leader who will help improve CMS.
Senate Finance Committee Ranking Member Ron Wyden, (D-Ore.) denounced Ms. Verma, stressing that she failed to adequately answer questions during her nomination hearing and has presented no clear vision of her plans as the next CMS administrator.
During her nomination hearing on Feb. 16, Ms. Verma came under fire for past consulting agreements with states while working for Hewlett Packard, a company that had financial interests in the health programs she designed. The issue was raised again during a preliminary vote by the Finance Committee on March 1.
“Ms. Verma was on both sides of the deal, helping manage the state’s health programs while being paid by vendors to those same programs,” Sen. Wyden said during the hearing. “I am concerned that if Ms. Verma is confirmed to lead CMS, where many of the companies she worked for are major vendors, there will not be adequate scrutiny of her past relationships with them, just as there wasn’t in Indiana.”
Ms. Verma previously said she never negotiated on behalf of Hewlett Packard and that the work she conducted for the states did not overlap with work she completed for HP.
“I hold honesty and integrity and adherence to a high ethical standard as part of my personal philosophy. That’s for me, I demand that from my employees, and I set that example for my own children,” she said during the Feb. 16 hearing. “We were never in a position where we were negotiating on behalf of HP or any other contractor with the state that we had a relationship with. If there was the potential [for a conflict], we would recuse ourselves.”
Ms. Verma’s nomination will now move to the full Senate. No date has yet been set for the vote.
[email protected]
On Twitter @legal_med
Judge blocks Texas attempt to defund Planned Parenthood
A federal judge has blocked Texas from withholding Medicaid funds from Planned Parenthood centers in the state, ruling that state officials had no cause to terminate funding for the providers.
In his Feb. 21 decision, Judge Sam Sparks of the U.S. District Court for the Western District of Texas temporarily barred Texas from taking funds from Planned Parenthood while the case proceeds. Restricting the money would deprive Medicaid patients of their right to obtain health care from their chosen providers and would potentially disrupt care for 12,500 Medicaid patients, Judge Sparks wrote.
Texas Attorney General Ken Paxton (R) pledged to appeal the ruling.
“[The] decision is disappointing and flies in the face of basic human decency,” Mr. Paxton said in a statement. “The raw, unedited footage from undercover videos exposed a brazen willingness by Planned Parenthood officials to traffic in fetal body parts, as well as manipulate the timing and method of an abortion ... No taxpayer in Texas should have to subsidize this repugnant and illegal conduct. We should never lose sight of the fact that, as long as abortion is legal in the United States, the potential for these types of horrors will continue.”
Planned Parenthood representatives did not return messages seeking comment on the ruling.
The Texas Health and Human Services Commission (HHSC) terminated funding for its Planned Parenthood providers in the state following a 2015 video by two anti-abortion advocates that purported to show representatives of Planned Parenthood Gulf Coast (Houston) contracting to sell aborted human fetal tissue and body parts. State authorities investigated the facility and found no wrongdoing, but a grand jury indicted the two anti-abortion activists for using fake names and identities.
Separate investigations by the Texas Attorney General’s Office, the Texas Department of State Health Services, and HHSC also found no wrongdoing, according to court documents. In December 2016 however, HHSC sent termination letters to five Texas Planned Parenthood centers, stating that the facilities were not qualified to provide medical services in a “competent, safe, legal and ethical manner” under state and federal law, according to court records. The providers and several patients sued.
In his ruling, Judge Sparks said he found no evidence indicating that an actual program violation occurred warranting termination of funding for the providers.
“After reviewing the evidence currently in the record, the court finds the Inspector General, and thus HHSC, likely acted to disenroll qualified health care providers from Medicaid without cause,” Judge Sparks wrote. “The individual plaintiffs have met their burden to establish a likelihood of success on the merits. The Inspector General did not have prima facie of evidence, or even a scintilla of evidence, to conclude the basis of termination set forth in the final notice merited finding [the plaintiffs] were not qualified.”
Similar efforts were overturned in Virgina after Governor Terry McAuliffe (D) vetoed a bill that sought to restrict state and federal funding from Planned Parenthood providers in the state. In a statement, Gov. McAuliffe said the bill would have harmed Virginians who rely on health care services and programs provided by Planned Parenthood health centers by denying them access to affordable care.
Similar legislation to defund Planned Parenthood providers was introduced by Michigan lawmakers in February.
In addition, efforts are underway at the federal level. On Feb. 16, the House passed H.J.Res.43, which would allow states to withhold Title X family planning funds from providers that offer abortion services, overturning a rule put in place at the end of the Obama administration. The resolution passed 230-188, largely along party lines. It would strike down the Obama-era rule via the 1996 Congressional Review Act, which allows Congress to overturn new regulations within 60 days of their passage. H.J.Res.43 is currently before the Senate.
The American Congress of Obstetricians and Gynecologists expressed disappointment with the House resolution.
“The resolution allows states to discriminate against women’s health care providers for reasons unrelated to qualifications or best practices,” according to an ACOG statement. “Under this resolution, states could disqualify health centers, including Planned Parenthood, from providing Title X contraceptive and preventive care to over 4 million individuals. The Title X program is the only federal grant program exclusively dedicated to providing low-income patients with access to effective family planning and related preventive health services, including contraceptive care. Contraceptive access is essential to helping women achieve greater educational, financial, and professional success and stability. It’s critical to the economic success of this population.”
House Speaker Paul Ryan (R-Wis.) has promised that the bill to repeal the Affordable Care Act will include a measure stripping funds from Planned Parenthood.
[email protected]
On Twitter @legal_med
A federal judge has blocked Texas from withholding Medicaid funds from Planned Parenthood centers in the state, ruling that state officials had no cause to terminate funding for the providers.
In his Feb. 21 decision, Judge Sam Sparks of the U.S. District Court for the Western District of Texas temporarily barred Texas from taking funds from Planned Parenthood while the case proceeds. Restricting the money would deprive Medicaid patients of their right to obtain health care from their chosen providers and would potentially disrupt care for 12,500 Medicaid patients, Judge Sparks wrote.
Texas Attorney General Ken Paxton (R) pledged to appeal the ruling.
“[The] decision is disappointing and flies in the face of basic human decency,” Mr. Paxton said in a statement. “The raw, unedited footage from undercover videos exposed a brazen willingness by Planned Parenthood officials to traffic in fetal body parts, as well as manipulate the timing and method of an abortion ... No taxpayer in Texas should have to subsidize this repugnant and illegal conduct. We should never lose sight of the fact that, as long as abortion is legal in the United States, the potential for these types of horrors will continue.”
Planned Parenthood representatives did not return messages seeking comment on the ruling.
The Texas Health and Human Services Commission (HHSC) terminated funding for its Planned Parenthood providers in the state following a 2015 video by two anti-abortion advocates that purported to show representatives of Planned Parenthood Gulf Coast (Houston) contracting to sell aborted human fetal tissue and body parts. State authorities investigated the facility and found no wrongdoing, but a grand jury indicted the two anti-abortion activists for using fake names and identities.
Separate investigations by the Texas Attorney General’s Office, the Texas Department of State Health Services, and HHSC also found no wrongdoing, according to court documents. In December 2016 however, HHSC sent termination letters to five Texas Planned Parenthood centers, stating that the facilities were not qualified to provide medical services in a “competent, safe, legal and ethical manner” under state and federal law, according to court records. The providers and several patients sued.
In his ruling, Judge Sparks said he found no evidence indicating that an actual program violation occurred warranting termination of funding for the providers.
“After reviewing the evidence currently in the record, the court finds the Inspector General, and thus HHSC, likely acted to disenroll qualified health care providers from Medicaid without cause,” Judge Sparks wrote. “The individual plaintiffs have met their burden to establish a likelihood of success on the merits. The Inspector General did not have prima facie of evidence, or even a scintilla of evidence, to conclude the basis of termination set forth in the final notice merited finding [the plaintiffs] were not qualified.”
Similar efforts were overturned in Virgina after Governor Terry McAuliffe (D) vetoed a bill that sought to restrict state and federal funding from Planned Parenthood providers in the state. In a statement, Gov. McAuliffe said the bill would have harmed Virginians who rely on health care services and programs provided by Planned Parenthood health centers by denying them access to affordable care.
Similar legislation to defund Planned Parenthood providers was introduced by Michigan lawmakers in February.
In addition, efforts are underway at the federal level. On Feb. 16, the House passed H.J.Res.43, which would allow states to withhold Title X family planning funds from providers that offer abortion services, overturning a rule put in place at the end of the Obama administration. The resolution passed 230-188, largely along party lines. It would strike down the Obama-era rule via the 1996 Congressional Review Act, which allows Congress to overturn new regulations within 60 days of their passage. H.J.Res.43 is currently before the Senate.
The American Congress of Obstetricians and Gynecologists expressed disappointment with the House resolution.
“The resolution allows states to discriminate against women’s health care providers for reasons unrelated to qualifications or best practices,” according to an ACOG statement. “Under this resolution, states could disqualify health centers, including Planned Parenthood, from providing Title X contraceptive and preventive care to over 4 million individuals. The Title X program is the only federal grant program exclusively dedicated to providing low-income patients with access to effective family planning and related preventive health services, including contraceptive care. Contraceptive access is essential to helping women achieve greater educational, financial, and professional success and stability. It’s critical to the economic success of this population.”
House Speaker Paul Ryan (R-Wis.) has promised that the bill to repeal the Affordable Care Act will include a measure stripping funds from Planned Parenthood.
[email protected]
On Twitter @legal_med
A federal judge has blocked Texas from withholding Medicaid funds from Planned Parenthood centers in the state, ruling that state officials had no cause to terminate funding for the providers.
In his Feb. 21 decision, Judge Sam Sparks of the U.S. District Court for the Western District of Texas temporarily barred Texas from taking funds from Planned Parenthood while the case proceeds. Restricting the money would deprive Medicaid patients of their right to obtain health care from their chosen providers and would potentially disrupt care for 12,500 Medicaid patients, Judge Sparks wrote.
Texas Attorney General Ken Paxton (R) pledged to appeal the ruling.
“[The] decision is disappointing and flies in the face of basic human decency,” Mr. Paxton said in a statement. “The raw, unedited footage from undercover videos exposed a brazen willingness by Planned Parenthood officials to traffic in fetal body parts, as well as manipulate the timing and method of an abortion ... No taxpayer in Texas should have to subsidize this repugnant and illegal conduct. We should never lose sight of the fact that, as long as abortion is legal in the United States, the potential for these types of horrors will continue.”
Planned Parenthood representatives did not return messages seeking comment on the ruling.
The Texas Health and Human Services Commission (HHSC) terminated funding for its Planned Parenthood providers in the state following a 2015 video by two anti-abortion advocates that purported to show representatives of Planned Parenthood Gulf Coast (Houston) contracting to sell aborted human fetal tissue and body parts. State authorities investigated the facility and found no wrongdoing, but a grand jury indicted the two anti-abortion activists for using fake names and identities.
Separate investigations by the Texas Attorney General’s Office, the Texas Department of State Health Services, and HHSC also found no wrongdoing, according to court documents. In December 2016 however, HHSC sent termination letters to five Texas Planned Parenthood centers, stating that the facilities were not qualified to provide medical services in a “competent, safe, legal and ethical manner” under state and federal law, according to court records. The providers and several patients sued.
In his ruling, Judge Sparks said he found no evidence indicating that an actual program violation occurred warranting termination of funding for the providers.
“After reviewing the evidence currently in the record, the court finds the Inspector General, and thus HHSC, likely acted to disenroll qualified health care providers from Medicaid without cause,” Judge Sparks wrote. “The individual plaintiffs have met their burden to establish a likelihood of success on the merits. The Inspector General did not have prima facie of evidence, or even a scintilla of evidence, to conclude the basis of termination set forth in the final notice merited finding [the plaintiffs] were not qualified.”
Similar efforts were overturned in Virgina after Governor Terry McAuliffe (D) vetoed a bill that sought to restrict state and federal funding from Planned Parenthood providers in the state. In a statement, Gov. McAuliffe said the bill would have harmed Virginians who rely on health care services and programs provided by Planned Parenthood health centers by denying them access to affordable care.
Similar legislation to defund Planned Parenthood providers was introduced by Michigan lawmakers in February.
In addition, efforts are underway at the federal level. On Feb. 16, the House passed H.J.Res.43, which would allow states to withhold Title X family planning funds from providers that offer abortion services, overturning a rule put in place at the end of the Obama administration. The resolution passed 230-188, largely along party lines. It would strike down the Obama-era rule via the 1996 Congressional Review Act, which allows Congress to overturn new regulations within 60 days of their passage. H.J.Res.43 is currently before the Senate.
The American Congress of Obstetricians and Gynecologists expressed disappointment with the House resolution.
“The resolution allows states to discriminate against women’s health care providers for reasons unrelated to qualifications or best practices,” according to an ACOG statement. “Under this resolution, states could disqualify health centers, including Planned Parenthood, from providing Title X contraceptive and preventive care to over 4 million individuals. The Title X program is the only federal grant program exclusively dedicated to providing low-income patients with access to effective family planning and related preventive health services, including contraceptive care. Contraceptive access is essential to helping women achieve greater educational, financial, and professional success and stability. It’s critical to the economic success of this population.”
House Speaker Paul Ryan (R-Wis.) has promised that the bill to repeal the Affordable Care Act will include a measure stripping funds from Planned Parenthood.
[email protected]
On Twitter @legal_med
Megamerger rulings may chill future consolidations
Expect a chilling effect on future health care consolidations after two health insurance megamergers were blocked by federal courts, analysts say.
A federal judge barred a merger between Aetna and Humana on Jan. 23, followed by a Feb. 8 decision that blocked Anthem and Cigna from consolidating. Both rulings called the deals anticompetitive.
“Both of these decisions are likely to chill future mergers,” Mr. Dahlquist said in an interview. “We have the FTC [Federal Trade Commission] winning on the provider side. Now we have the DOJ [Department of Justice] winning on the insurer side. While they are different players in the marketplace, the law that is being applied is very similar, if not the same. The one-sided victories by the government are creating a situation of imbalance.”
Ruling positive and negative for providers
Anthem’s proposed $54 billion merger with Cigna would have been the largest insurer consolidation in history. The merger, along with Aetna’s $37 billion plan to purchase Humana, would have reduced the number of major U.S. health insurers from five to three, reshaping the industry. The Department of Justice and a number of states immediately opposed the mergers, arguing the consolidations would significantly reduce competition to the detriment of patients. Judges ultimately agreed.
“The [insurers] already have a huge market position so they can already hammer [providers] with low rates,” Mr. Greer said in an interview. “The reason why [the rulings are] such a big win for providers, is there won’t be this huge concentration of power. Anthem may not be able to hammer them quite as much as if they would’ve merged with Cigna.”
However, the rulings may discourage future health care consolidations between insurers and among health providers, Mr. Dahlquist noted. In recent years, the government has scored a series of legal wins that have blocked provider mergers. In 2013 for example, the Federal Trade Commission successfully stopped the acquisition of Palmyra Medical Center by Phoebe Putney Memorial Hospital (Albany, Ga.), followed by a 2014 win that barred St. Luke’s Health System’s acquisition of Saltzer Medical Group (Nampa, Idaho), an independent physician practice. In 2016, the FTC successfully blocked the merger of Penn State Hershey (Pa.) Medical Center’s merger with PinnacleHealth System (Harrisburg, Penn.).
“Every merger needs to be looked at [based on] the facts of that merger, and the facts of that case, the markets they compete in, [and] the markets they want to expand into,” Mr. Dahlquist said. “That’s why you shouldn’t look at all mergers of health care providers or health insurers as inherently bad, and I think that’s what these [decisions] are unfortunately driving toward.”
A broader takeaway is that the insurance industry needs to develop a better business model, said Barak D. Richman, PhD, an antitrust expert and law professor at Duke University in Durham, N.C. Mr. Richman consulted with several state departments of insurance as they were reviewing the Anthem-Cigna mergers.
“The general story here is that the insurance industry, over a number of years, has been consolidating and has reached a point where it now has passed a threshold where antitrust scrutiny will be very real,” Mr. Richman said in an interview. “One thing that the court cases revealed is that there is a certain prevailing business model in insurance which is: Get as big as you can, balance risk through volume, and use buying leverage to negotiate favorable prices from providers. It looks like that business model has reached its limits and really is not going to be sustainable any longer.”
The current market demands business models that center on value, not volume, Mr. Richman continued. Insurance companies will need to be more deliberate and analytical about how to provide value to patients and generate cost effective care.
“That really is the takeaway, that we need a real shift in the business of health insurance,” he said.
Anthem banking on new administration
Since the rulings, Aetna and Humana have chosen not to appeal and have mutually ended their merger agreement. Cigna is seeking to terminate its agreement with Anthem, but Anthem is fighting the dissolution. The insurance giant is looking toward the Trump administration to potentially turn the case around.
To enforce the termination, Cigna is suing Anthem in the Delaware Court of Chancery seeking a declaratory judgment that Cigna has lawfully terminated the contract, according to an announcement by Cigna. Anthem, meanwhile, is seeking a temporary restraining order to enjoin Cigna from terminating its contract.
“Anthem believes there is still sufficient time and a viable path forward potentially to complete the transaction that will save millions of Americans more than $2 billion in annual medical costs and deliver significant value to shareholders,” according to a company statement. “In addition to filing this lawsuit, Anthem is pursuing an expedited appeal of the District Court’s decision and is committed to completing this value-creating merger either through a successful appeal or through settlement with the new leadership at the Department of Justice.”
Part of that new leadership could include Makan Delrahim, a former Anthem lobbyist now serving as deputy White House counsel. He is rumored to be a top contender to lead the DOJ’s antitrust division.
Records also show that Indiana insurance regulators were supportive of the Anthem-Cigna deal while Vice President Pence was governor of Indiana and that Anthem contributed $100,000 to President Trump’s inaugural committee, according to Senate lobbying reports.
“A lot of folks are talking about the possibility that Trump will appoint people at both the FTC and DOJ antitrust devision [who] are Republicans and tend to be more business friendly, which would mean that maybe some of these mergers would be more likely to make it and less likely to be challenged,” he said.
On the other hand, a couple of leadership changes will not likely reverse the country’s historic antitrust enforcement, Mr. Dahlquist said.
“I don’t believe you’re going to see a systemic shift in the antitrust enforcement policy of this country,” he said. “Even one change at the top is not going to [create] upheaval within the ranks at the FTC and DOJ. The government is on a winning streak; I think that’s going to continue in the short term.”
“I don’t expect to see any change in the government’s approach in continuing to challenge anticompetitive health care transactions,” Ms. Feinstein said during at interview at the meeting.
[email protected]
On Twitter @legal_med
Expect a chilling effect on future health care consolidations after two health insurance megamergers were blocked by federal courts, analysts say.
A federal judge barred a merger between Aetna and Humana on Jan. 23, followed by a Feb. 8 decision that blocked Anthem and Cigna from consolidating. Both rulings called the deals anticompetitive.
“Both of these decisions are likely to chill future mergers,” Mr. Dahlquist said in an interview. “We have the FTC [Federal Trade Commission] winning on the provider side. Now we have the DOJ [Department of Justice] winning on the insurer side. While they are different players in the marketplace, the law that is being applied is very similar, if not the same. The one-sided victories by the government are creating a situation of imbalance.”
Ruling positive and negative for providers
Anthem’s proposed $54 billion merger with Cigna would have been the largest insurer consolidation in history. The merger, along with Aetna’s $37 billion plan to purchase Humana, would have reduced the number of major U.S. health insurers from five to three, reshaping the industry. The Department of Justice and a number of states immediately opposed the mergers, arguing the consolidations would significantly reduce competition to the detriment of patients. Judges ultimately agreed.
“The [insurers] already have a huge market position so they can already hammer [providers] with low rates,” Mr. Greer said in an interview. “The reason why [the rulings are] such a big win for providers, is there won’t be this huge concentration of power. Anthem may not be able to hammer them quite as much as if they would’ve merged with Cigna.”
However, the rulings may discourage future health care consolidations between insurers and among health providers, Mr. Dahlquist noted. In recent years, the government has scored a series of legal wins that have blocked provider mergers. In 2013 for example, the Federal Trade Commission successfully stopped the acquisition of Palmyra Medical Center by Phoebe Putney Memorial Hospital (Albany, Ga.), followed by a 2014 win that barred St. Luke’s Health System’s acquisition of Saltzer Medical Group (Nampa, Idaho), an independent physician practice. In 2016, the FTC successfully blocked the merger of Penn State Hershey (Pa.) Medical Center’s merger with PinnacleHealth System (Harrisburg, Penn.).
“Every merger needs to be looked at [based on] the facts of that merger, and the facts of that case, the markets they compete in, [and] the markets they want to expand into,” Mr. Dahlquist said. “That’s why you shouldn’t look at all mergers of health care providers or health insurers as inherently bad, and I think that’s what these [decisions] are unfortunately driving toward.”
A broader takeaway is that the insurance industry needs to develop a better business model, said Barak D. Richman, PhD, an antitrust expert and law professor at Duke University in Durham, N.C. Mr. Richman consulted with several state departments of insurance as they were reviewing the Anthem-Cigna mergers.
“The general story here is that the insurance industry, over a number of years, has been consolidating and has reached a point where it now has passed a threshold where antitrust scrutiny will be very real,” Mr. Richman said in an interview. “One thing that the court cases revealed is that there is a certain prevailing business model in insurance which is: Get as big as you can, balance risk through volume, and use buying leverage to negotiate favorable prices from providers. It looks like that business model has reached its limits and really is not going to be sustainable any longer.”
The current market demands business models that center on value, not volume, Mr. Richman continued. Insurance companies will need to be more deliberate and analytical about how to provide value to patients and generate cost effective care.
“That really is the takeaway, that we need a real shift in the business of health insurance,” he said.
Anthem banking on new administration
Since the rulings, Aetna and Humana have chosen not to appeal and have mutually ended their merger agreement. Cigna is seeking to terminate its agreement with Anthem, but Anthem is fighting the dissolution. The insurance giant is looking toward the Trump administration to potentially turn the case around.
To enforce the termination, Cigna is suing Anthem in the Delaware Court of Chancery seeking a declaratory judgment that Cigna has lawfully terminated the contract, according to an announcement by Cigna. Anthem, meanwhile, is seeking a temporary restraining order to enjoin Cigna from terminating its contract.
“Anthem believes there is still sufficient time and a viable path forward potentially to complete the transaction that will save millions of Americans more than $2 billion in annual medical costs and deliver significant value to shareholders,” according to a company statement. “In addition to filing this lawsuit, Anthem is pursuing an expedited appeal of the District Court’s decision and is committed to completing this value-creating merger either through a successful appeal or through settlement with the new leadership at the Department of Justice.”
Part of that new leadership could include Makan Delrahim, a former Anthem lobbyist now serving as deputy White House counsel. He is rumored to be a top contender to lead the DOJ’s antitrust division.
Records also show that Indiana insurance regulators were supportive of the Anthem-Cigna deal while Vice President Pence was governor of Indiana and that Anthem contributed $100,000 to President Trump’s inaugural committee, according to Senate lobbying reports.
“A lot of folks are talking about the possibility that Trump will appoint people at both the FTC and DOJ antitrust devision [who] are Republicans and tend to be more business friendly, which would mean that maybe some of these mergers would be more likely to make it and less likely to be challenged,” he said.
On the other hand, a couple of leadership changes will not likely reverse the country’s historic antitrust enforcement, Mr. Dahlquist said.
“I don’t believe you’re going to see a systemic shift in the antitrust enforcement policy of this country,” he said. “Even one change at the top is not going to [create] upheaval within the ranks at the FTC and DOJ. The government is on a winning streak; I think that’s going to continue in the short term.”
“I don’t expect to see any change in the government’s approach in continuing to challenge anticompetitive health care transactions,” Ms. Feinstein said during at interview at the meeting.
[email protected]
On Twitter @legal_med
Expect a chilling effect on future health care consolidations after two health insurance megamergers were blocked by federal courts, analysts say.
A federal judge barred a merger between Aetna and Humana on Jan. 23, followed by a Feb. 8 decision that blocked Anthem and Cigna from consolidating. Both rulings called the deals anticompetitive.
“Both of these decisions are likely to chill future mergers,” Mr. Dahlquist said in an interview. “We have the FTC [Federal Trade Commission] winning on the provider side. Now we have the DOJ [Department of Justice] winning on the insurer side. While they are different players in the marketplace, the law that is being applied is very similar, if not the same. The one-sided victories by the government are creating a situation of imbalance.”
Ruling positive and negative for providers
Anthem’s proposed $54 billion merger with Cigna would have been the largest insurer consolidation in history. The merger, along with Aetna’s $37 billion plan to purchase Humana, would have reduced the number of major U.S. health insurers from five to three, reshaping the industry. The Department of Justice and a number of states immediately opposed the mergers, arguing the consolidations would significantly reduce competition to the detriment of patients. Judges ultimately agreed.
“The [insurers] already have a huge market position so they can already hammer [providers] with low rates,” Mr. Greer said in an interview. “The reason why [the rulings are] such a big win for providers, is there won’t be this huge concentration of power. Anthem may not be able to hammer them quite as much as if they would’ve merged with Cigna.”
However, the rulings may discourage future health care consolidations between insurers and among health providers, Mr. Dahlquist noted. In recent years, the government has scored a series of legal wins that have blocked provider mergers. In 2013 for example, the Federal Trade Commission successfully stopped the acquisition of Palmyra Medical Center by Phoebe Putney Memorial Hospital (Albany, Ga.), followed by a 2014 win that barred St. Luke’s Health System’s acquisition of Saltzer Medical Group (Nampa, Idaho), an independent physician practice. In 2016, the FTC successfully blocked the merger of Penn State Hershey (Pa.) Medical Center’s merger with PinnacleHealth System (Harrisburg, Penn.).
“Every merger needs to be looked at [based on] the facts of that merger, and the facts of that case, the markets they compete in, [and] the markets they want to expand into,” Mr. Dahlquist said. “That’s why you shouldn’t look at all mergers of health care providers or health insurers as inherently bad, and I think that’s what these [decisions] are unfortunately driving toward.”
A broader takeaway is that the insurance industry needs to develop a better business model, said Barak D. Richman, PhD, an antitrust expert and law professor at Duke University in Durham, N.C. Mr. Richman consulted with several state departments of insurance as they were reviewing the Anthem-Cigna mergers.
“The general story here is that the insurance industry, over a number of years, has been consolidating and has reached a point where it now has passed a threshold where antitrust scrutiny will be very real,” Mr. Richman said in an interview. “One thing that the court cases revealed is that there is a certain prevailing business model in insurance which is: Get as big as you can, balance risk through volume, and use buying leverage to negotiate favorable prices from providers. It looks like that business model has reached its limits and really is not going to be sustainable any longer.”
The current market demands business models that center on value, not volume, Mr. Richman continued. Insurance companies will need to be more deliberate and analytical about how to provide value to patients and generate cost effective care.
“That really is the takeaway, that we need a real shift in the business of health insurance,” he said.
Anthem banking on new administration
Since the rulings, Aetna and Humana have chosen not to appeal and have mutually ended their merger agreement. Cigna is seeking to terminate its agreement with Anthem, but Anthem is fighting the dissolution. The insurance giant is looking toward the Trump administration to potentially turn the case around.
To enforce the termination, Cigna is suing Anthem in the Delaware Court of Chancery seeking a declaratory judgment that Cigna has lawfully terminated the contract, according to an announcement by Cigna. Anthem, meanwhile, is seeking a temporary restraining order to enjoin Cigna from terminating its contract.
“Anthem believes there is still sufficient time and a viable path forward potentially to complete the transaction that will save millions of Americans more than $2 billion in annual medical costs and deliver significant value to shareholders,” according to a company statement. “In addition to filing this lawsuit, Anthem is pursuing an expedited appeal of the District Court’s decision and is committed to completing this value-creating merger either through a successful appeal or through settlement with the new leadership at the Department of Justice.”
Part of that new leadership could include Makan Delrahim, a former Anthem lobbyist now serving as deputy White House counsel. He is rumored to be a top contender to lead the DOJ’s antitrust division.
Records also show that Indiana insurance regulators were supportive of the Anthem-Cigna deal while Vice President Pence was governor of Indiana and that Anthem contributed $100,000 to President Trump’s inaugural committee, according to Senate lobbying reports.
“A lot of folks are talking about the possibility that Trump will appoint people at both the FTC and DOJ antitrust devision [who] are Republicans and tend to be more business friendly, which would mean that maybe some of these mergers would be more likely to make it and less likely to be challenged,” he said.
On the other hand, a couple of leadership changes will not likely reverse the country’s historic antitrust enforcement, Mr. Dahlquist said.
“I don’t believe you’re going to see a systemic shift in the antitrust enforcement policy of this country,” he said. “Even one change at the top is not going to [create] upheaval within the ranks at the FTC and DOJ. The government is on a winning streak; I think that’s going to continue in the short term.”
“I don’t expect to see any change in the government’s approach in continuing to challenge anticompetitive health care transactions,” Ms. Feinstein said during at interview at the meeting.
[email protected]
On Twitter @legal_med
Appeals court strikes down doctor gun gag law
An appeals court has struck down a Florida law that banned physicians from asking patients about firearms, ruling that the so-called gun gag law violates doctors’ First Amendment rights.
The decision by the 11th U.S. Circuit Court of Appeals enables Florida physicians to once again query patients and families about gun ownership and record firearm information in their records. The court, however, left intact a provision that allows patients to refuse answering such questions and upheld another rule that physicians cannot discriminate against patients based on firearm status.
”While the chapter does not wish to impinge on the rights of gun owners, it has fought this legislation in the legislature and in the courts because it is essential that physicians and patients have the right to an open dialogue, free from government restrictions,” Dr. Goldman said in a statement. “[The] ruling is a victory for patients and the profession.”
Florida Gov. Rick Scott (R) did not respond to a request for comment.
The Florida Firearms Owners’ Privacy Act (FOPA) was enacted in 2011 after complaints from patients that physicians were inquiring about firearms in the home and in some cases, refusing to provide care if patients declined to answer. The law barred physicians from asking about firearms and from recording information about firearms in patient records. The statute also precluded doctors from unnecessarily harassing patients about firearm ownership during an examination and prevented physicians from discriminating against patients based solely on firearm ownership. Violations of the law could result in a $10,000 fine per offense, a letter of reprimand, probation, suspension, compulsory remedial education, or permanent license revocation.
Shortly after the law passed, the ACP Florida Chapter sued the state; the suit was joined by the American Academy of Family Physicians Florida Chapter, the American Academy of Pediatrics Florida chapter, and a group of physicians. The plaintiffs argued that doctors routinely ask patients about potential health and safety risks, including firearms, to assess safety risks, educate patients and parents, and encourage gun safety. The gag law violated doctors’ free speech protections, according to the plaintiffs. The state argued that FOPA ensures patient privacy and protects gun owners’ right to own and bear arms from private encumbrances. A district court ruled in favor of the plaintiffs.
In the ruling, judges for the 11th U.S. Circuit Court of Appeals wrote that the right to own and possess firearms does not preclude questions about, commentary on, or criticism for the exercise of that right.
“As the district court aptly noted, there is no actual conflict between the First Amendment rights of doctors and medical professionals and the Second Amendment rights of patients that justifies FOPA’s speaker-focused and content-based restrictions on speech,” judges wrote. “Even if there were some possible conflict between the First Amendment rights of doctors and medical professionals and the Second Amendment rights of patients, the record-keeping, inquiry, and anti-harassment provisions do not advance [the legislative goals] in a permissible way.”
“The legislature has every right to regulate any profession to protect the public from discrimination and abuse,” Ms. Hammer said in an interview. “Doctors are businessmen, not gods. This activist decision attempts to use the First Amendment as a sword to terrorize the Second Amendment and completely disregards the rights and the will of the elected representatives of the people of Florida.”
The American Medical Association called the court ruling a clear victory against censorship of private medical discussions between patients and physicians.
“The court agreed that in the fields of medicine and public health, information saves lives,” according to an AMA statement. “Studies show that patients who received physician counseling on firearm safety are more likely to adopt one or more safe gun-storage practices. … Counseling patients we care for makes a difference in preventing gun-related injuries and deaths.”
[email protected]
On Twitter @legal_med
An appeals court has struck down a Florida law that banned physicians from asking patients about firearms, ruling that the so-called gun gag law violates doctors’ First Amendment rights.
The decision by the 11th U.S. Circuit Court of Appeals enables Florida physicians to once again query patients and families about gun ownership and record firearm information in their records. The court, however, left intact a provision that allows patients to refuse answering such questions and upheld another rule that physicians cannot discriminate against patients based on firearm status.
”While the chapter does not wish to impinge on the rights of gun owners, it has fought this legislation in the legislature and in the courts because it is essential that physicians and patients have the right to an open dialogue, free from government restrictions,” Dr. Goldman said in a statement. “[The] ruling is a victory for patients and the profession.”
Florida Gov. Rick Scott (R) did not respond to a request for comment.
The Florida Firearms Owners’ Privacy Act (FOPA) was enacted in 2011 after complaints from patients that physicians were inquiring about firearms in the home and in some cases, refusing to provide care if patients declined to answer. The law barred physicians from asking about firearms and from recording information about firearms in patient records. The statute also precluded doctors from unnecessarily harassing patients about firearm ownership during an examination and prevented physicians from discriminating against patients based solely on firearm ownership. Violations of the law could result in a $10,000 fine per offense, a letter of reprimand, probation, suspension, compulsory remedial education, or permanent license revocation.
Shortly after the law passed, the ACP Florida Chapter sued the state; the suit was joined by the American Academy of Family Physicians Florida Chapter, the American Academy of Pediatrics Florida chapter, and a group of physicians. The plaintiffs argued that doctors routinely ask patients about potential health and safety risks, including firearms, to assess safety risks, educate patients and parents, and encourage gun safety. The gag law violated doctors’ free speech protections, according to the plaintiffs. The state argued that FOPA ensures patient privacy and protects gun owners’ right to own and bear arms from private encumbrances. A district court ruled in favor of the plaintiffs.
In the ruling, judges for the 11th U.S. Circuit Court of Appeals wrote that the right to own and possess firearms does not preclude questions about, commentary on, or criticism for the exercise of that right.
“As the district court aptly noted, there is no actual conflict between the First Amendment rights of doctors and medical professionals and the Second Amendment rights of patients that justifies FOPA’s speaker-focused and content-based restrictions on speech,” judges wrote. “Even if there were some possible conflict between the First Amendment rights of doctors and medical professionals and the Second Amendment rights of patients, the record-keeping, inquiry, and anti-harassment provisions do not advance [the legislative goals] in a permissible way.”
“The legislature has every right to regulate any profession to protect the public from discrimination and abuse,” Ms. Hammer said in an interview. “Doctors are businessmen, not gods. This activist decision attempts to use the First Amendment as a sword to terrorize the Second Amendment and completely disregards the rights and the will of the elected representatives of the people of Florida.”
The American Medical Association called the court ruling a clear victory against censorship of private medical discussions between patients and physicians.
“The court agreed that in the fields of medicine and public health, information saves lives,” according to an AMA statement. “Studies show that patients who received physician counseling on firearm safety are more likely to adopt one or more safe gun-storage practices. … Counseling patients we care for makes a difference in preventing gun-related injuries and deaths.”
[email protected]
On Twitter @legal_med
An appeals court has struck down a Florida law that banned physicians from asking patients about firearms, ruling that the so-called gun gag law violates doctors’ First Amendment rights.
The decision by the 11th U.S. Circuit Court of Appeals enables Florida physicians to once again query patients and families about gun ownership and record firearm information in their records. The court, however, left intact a provision that allows patients to refuse answering such questions and upheld another rule that physicians cannot discriminate against patients based on firearm status.
”While the chapter does not wish to impinge on the rights of gun owners, it has fought this legislation in the legislature and in the courts because it is essential that physicians and patients have the right to an open dialogue, free from government restrictions,” Dr. Goldman said in a statement. “[The] ruling is a victory for patients and the profession.”
Florida Gov. Rick Scott (R) did not respond to a request for comment.
The Florida Firearms Owners’ Privacy Act (FOPA) was enacted in 2011 after complaints from patients that physicians were inquiring about firearms in the home and in some cases, refusing to provide care if patients declined to answer. The law barred physicians from asking about firearms and from recording information about firearms in patient records. The statute also precluded doctors from unnecessarily harassing patients about firearm ownership during an examination and prevented physicians from discriminating against patients based solely on firearm ownership. Violations of the law could result in a $10,000 fine per offense, a letter of reprimand, probation, suspension, compulsory remedial education, or permanent license revocation.
Shortly after the law passed, the ACP Florida Chapter sued the state; the suit was joined by the American Academy of Family Physicians Florida Chapter, the American Academy of Pediatrics Florida chapter, and a group of physicians. The plaintiffs argued that doctors routinely ask patients about potential health and safety risks, including firearms, to assess safety risks, educate patients and parents, and encourage gun safety. The gag law violated doctors’ free speech protections, according to the plaintiffs. The state argued that FOPA ensures patient privacy and protects gun owners’ right to own and bear arms from private encumbrances. A district court ruled in favor of the plaintiffs.
In the ruling, judges for the 11th U.S. Circuit Court of Appeals wrote that the right to own and possess firearms does not preclude questions about, commentary on, or criticism for the exercise of that right.
“As the district court aptly noted, there is no actual conflict between the First Amendment rights of doctors and medical professionals and the Second Amendment rights of patients that justifies FOPA’s speaker-focused and content-based restrictions on speech,” judges wrote. “Even if there were some possible conflict between the First Amendment rights of doctors and medical professionals and the Second Amendment rights of patients, the record-keeping, inquiry, and anti-harassment provisions do not advance [the legislative goals] in a permissible way.”
“The legislature has every right to regulate any profession to protect the public from discrimination and abuse,” Ms. Hammer said in an interview. “Doctors are businessmen, not gods. This activist decision attempts to use the First Amendment as a sword to terrorize the Second Amendment and completely disregards the rights and the will of the elected representatives of the people of Florida.”
The American Medical Association called the court ruling a clear victory against censorship of private medical discussions between patients and physicians.
“The court agreed that in the fields of medicine and public health, information saves lives,” according to an AMA statement. “Studies show that patients who received physician counseling on firearm safety are more likely to adopt one or more safe gun-storage practices. … Counseling patients we care for makes a difference in preventing gun-related injuries and deaths.”
[email protected]
On Twitter @legal_med
Seema Verma dodges questions on how to improve CMS
If confirmed as Centers for Medicare & Medicaid Services administrator, Seema Verma vowed to modernize CMS programs, improve Medicaid access, and leverage technology to drive better care, but she stopped short of explaining how she would do so during her confirmation hearing Feb. 16 before the Senate Finance Committee.
Legislators grilled Ms. Verma on Medicare improvements, the fate of the Affordable Care Act (ACA), Medicaid reform, and the execution of value-based care. At every turn. Ms. Verma pledged to review current processes and work toward enhancing programs, but she declined to support or oppose specific changes. Instead, she promised to help make health care more affordable and to allow patients more flexibility in making health insurance decisions.
Much of the committee’s questioning centered on Ms. Verma’s work on the Healthy Indiana Plan (HIP), Indiana’s Medicaid expansion under the ACA. The conservative plan requires patients to pay a small amount to receive health coverage and includes a lockout period if payments are missed. Legislators repeatedly asked if Ms. Verma planned to use HIP as a model to alter the Medicaid program.
Ms. Verma countered that each state has different needs and should be allowed to develop individualized Medicaid programs that provide flexibility.
“This is about putting states in a leadership role so that they can manage their programs better,” she said. “States are closer to the people that they serve and have a better understanding of what can work in their state than the federal government. I think states should have that flexibility.”
Legislators raised concerns about Ms. Verma’s past consulting agreements with states while working for Hewlett Packard (HP), a company that had financial interests in the health programs she designed. Ms. Verma’s company, SVC, advises clients on Medicaid waivers and state plan amendment development.
Ms. Verma argued that she never negotiated on behalf of Hewlett Packard, and that the work she conducted for the states did not overlap with work she completed for HP. Her company sought an ethics opinion to ensure the arrangement was not problematic, she said.
“I hold honesty and integrity and adherence to a high ethical standard as part of my personal philosophy. That’s for me, I demand that from my employees, and I set that example for my own children,” she said. “We were never in a position where we were negotiating on behalf of HP or any other contractor with the state that we had a relationship with. If there was the potential [for a conflict], we would recuse ourselves.”
Ms. Verma dodged many specific questions, including whether she supported block grants for Medicaid, how she might improve the problem of prescription drug prices, and whether she supported Medicare as a voucher program. When asked by Sen. Sherrod Campbell Brown (D-Ohio) whether she supported an extension of the current Children’s Health Insurance Program (CHIP) for another 8 years, Ms. Verma said she supported “the reauthorization of CHIP for as long as possible.”
When asked about the value-based reforms included in MACRA (the Medicare Access & CHIP Reauthorization Act of 2015), Ms. Verma said she applauded passage of the law, but she would not go into detail about potential changes to the statute.
“I think it’s an important step forward, not only to providing more stability for providers, but also moving us to better outcomes,” she said.
Sen. Wyden grew visibly frustrated with Ms. Verma’s vague answers, saying he was disappointed that, after many questions, the stances she took in many areas were still unclear.
“You’ve been asked a lot of questions and they were not ‘gotcha’ questions,” Sen. Wyden said during the hearing. “These were questions that were appropriate given the fact that if you’re confirmed, you’re going to head an agency that’s involved with a trillion [dollars] of spending in the health care of 100 million people. We’re not really getting much of a sense of how you’d approach [these issues]. I think this committee needs answers and I think the public needs answers.”
“It is critical that we get a strong, skilled leader as CMS administrator,” he said. “Here you are, somebody who has proven to be a tremendous leader in health care, not only to Indiana but as an example to the rest of the states. All I can say is you will be a strong, skilled leader as CMS administrator.”
A relative unknown before her nomination, Ms. Verma spent 20 years designing policy projects involving Medicaid, including HIP, the nation’s first consumer-directed Medicaid program under Indiana Governor Mitch Daniels and then-Gov. Mike Pence’s HIP 2.0 waiver proposal.
Prior to consulting, Ms. Verma served as vice president of planning for the Health and Hospital Corporation of Marion County (Ind.) and as a director with the Association of State and Territorial Health Officials in Washington.
Senators have asked that Ms. Verma submit written answers to their questions, which they will review before voting on her nomination.
[email protected]
On Twitter @legal_med
If confirmed as Centers for Medicare & Medicaid Services administrator, Seema Verma vowed to modernize CMS programs, improve Medicaid access, and leverage technology to drive better care, but she stopped short of explaining how she would do so during her confirmation hearing Feb. 16 before the Senate Finance Committee.
Legislators grilled Ms. Verma on Medicare improvements, the fate of the Affordable Care Act (ACA), Medicaid reform, and the execution of value-based care. At every turn. Ms. Verma pledged to review current processes and work toward enhancing programs, but she declined to support or oppose specific changes. Instead, she promised to help make health care more affordable and to allow patients more flexibility in making health insurance decisions.
Much of the committee’s questioning centered on Ms. Verma’s work on the Healthy Indiana Plan (HIP), Indiana’s Medicaid expansion under the ACA. The conservative plan requires patients to pay a small amount to receive health coverage and includes a lockout period if payments are missed. Legislators repeatedly asked if Ms. Verma planned to use HIP as a model to alter the Medicaid program.
Ms. Verma countered that each state has different needs and should be allowed to develop individualized Medicaid programs that provide flexibility.
“This is about putting states in a leadership role so that they can manage their programs better,” she said. “States are closer to the people that they serve and have a better understanding of what can work in their state than the federal government. I think states should have that flexibility.”
Legislators raised concerns about Ms. Verma’s past consulting agreements with states while working for Hewlett Packard (HP), a company that had financial interests in the health programs she designed. Ms. Verma’s company, SVC, advises clients on Medicaid waivers and state plan amendment development.
Ms. Verma argued that she never negotiated on behalf of Hewlett Packard, and that the work she conducted for the states did not overlap with work she completed for HP. Her company sought an ethics opinion to ensure the arrangement was not problematic, she said.
“I hold honesty and integrity and adherence to a high ethical standard as part of my personal philosophy. That’s for me, I demand that from my employees, and I set that example for my own children,” she said. “We were never in a position where we were negotiating on behalf of HP or any other contractor with the state that we had a relationship with. If there was the potential [for a conflict], we would recuse ourselves.”
Ms. Verma dodged many specific questions, including whether she supported block grants for Medicaid, how she might improve the problem of prescription drug prices, and whether she supported Medicare as a voucher program. When asked by Sen. Sherrod Campbell Brown (D-Ohio) whether she supported an extension of the current Children’s Health Insurance Program (CHIP) for another 8 years, Ms. Verma said she supported “the reauthorization of CHIP for as long as possible.”
When asked about the value-based reforms included in MACRA (the Medicare Access & CHIP Reauthorization Act of 2015), Ms. Verma said she applauded passage of the law, but she would not go into detail about potential changes to the statute.
“I think it’s an important step forward, not only to providing more stability for providers, but also moving us to better outcomes,” she said.
Sen. Wyden grew visibly frustrated with Ms. Verma’s vague answers, saying he was disappointed that, after many questions, the stances she took in many areas were still unclear.
“You’ve been asked a lot of questions and they were not ‘gotcha’ questions,” Sen. Wyden said during the hearing. “These were questions that were appropriate given the fact that if you’re confirmed, you’re going to head an agency that’s involved with a trillion [dollars] of spending in the health care of 100 million people. We’re not really getting much of a sense of how you’d approach [these issues]. I think this committee needs answers and I think the public needs answers.”
“It is critical that we get a strong, skilled leader as CMS administrator,” he said. “Here you are, somebody who has proven to be a tremendous leader in health care, not only to Indiana but as an example to the rest of the states. All I can say is you will be a strong, skilled leader as CMS administrator.”
A relative unknown before her nomination, Ms. Verma spent 20 years designing policy projects involving Medicaid, including HIP, the nation’s first consumer-directed Medicaid program under Indiana Governor Mitch Daniels and then-Gov. Mike Pence’s HIP 2.0 waiver proposal.
Prior to consulting, Ms. Verma served as vice president of planning for the Health and Hospital Corporation of Marion County (Ind.) and as a director with the Association of State and Territorial Health Officials in Washington.
Senators have asked that Ms. Verma submit written answers to their questions, which they will review before voting on her nomination.
[email protected]
On Twitter @legal_med
If confirmed as Centers for Medicare & Medicaid Services administrator, Seema Verma vowed to modernize CMS programs, improve Medicaid access, and leverage technology to drive better care, but she stopped short of explaining how she would do so during her confirmation hearing Feb. 16 before the Senate Finance Committee.
Legislators grilled Ms. Verma on Medicare improvements, the fate of the Affordable Care Act (ACA), Medicaid reform, and the execution of value-based care. At every turn. Ms. Verma pledged to review current processes and work toward enhancing programs, but she declined to support or oppose specific changes. Instead, she promised to help make health care more affordable and to allow patients more flexibility in making health insurance decisions.
Much of the committee’s questioning centered on Ms. Verma’s work on the Healthy Indiana Plan (HIP), Indiana’s Medicaid expansion under the ACA. The conservative plan requires patients to pay a small amount to receive health coverage and includes a lockout period if payments are missed. Legislators repeatedly asked if Ms. Verma planned to use HIP as a model to alter the Medicaid program.
Ms. Verma countered that each state has different needs and should be allowed to develop individualized Medicaid programs that provide flexibility.
“This is about putting states in a leadership role so that they can manage their programs better,” she said. “States are closer to the people that they serve and have a better understanding of what can work in their state than the federal government. I think states should have that flexibility.”
Legislators raised concerns about Ms. Verma’s past consulting agreements with states while working for Hewlett Packard (HP), a company that had financial interests in the health programs she designed. Ms. Verma’s company, SVC, advises clients on Medicaid waivers and state plan amendment development.
Ms. Verma argued that she never negotiated on behalf of Hewlett Packard, and that the work she conducted for the states did not overlap with work she completed for HP. Her company sought an ethics opinion to ensure the arrangement was not problematic, she said.
“I hold honesty and integrity and adherence to a high ethical standard as part of my personal philosophy. That’s for me, I demand that from my employees, and I set that example for my own children,” she said. “We were never in a position where we were negotiating on behalf of HP or any other contractor with the state that we had a relationship with. If there was the potential [for a conflict], we would recuse ourselves.”
Ms. Verma dodged many specific questions, including whether she supported block grants for Medicaid, how she might improve the problem of prescription drug prices, and whether she supported Medicare as a voucher program. When asked by Sen. Sherrod Campbell Brown (D-Ohio) whether she supported an extension of the current Children’s Health Insurance Program (CHIP) for another 8 years, Ms. Verma said she supported “the reauthorization of CHIP for as long as possible.”
When asked about the value-based reforms included in MACRA (the Medicare Access & CHIP Reauthorization Act of 2015), Ms. Verma said she applauded passage of the law, but she would not go into detail about potential changes to the statute.
“I think it’s an important step forward, not only to providing more stability for providers, but also moving us to better outcomes,” she said.
Sen. Wyden grew visibly frustrated with Ms. Verma’s vague answers, saying he was disappointed that, after many questions, the stances she took in many areas were still unclear.
“You’ve been asked a lot of questions and they were not ‘gotcha’ questions,” Sen. Wyden said during the hearing. “These were questions that were appropriate given the fact that if you’re confirmed, you’re going to head an agency that’s involved with a trillion [dollars] of spending in the health care of 100 million people. We’re not really getting much of a sense of how you’d approach [these issues]. I think this committee needs answers and I think the public needs answers.”
“It is critical that we get a strong, skilled leader as CMS administrator,” he said. “Here you are, somebody who has proven to be a tremendous leader in health care, not only to Indiana but as an example to the rest of the states. All I can say is you will be a strong, skilled leader as CMS administrator.”
A relative unknown before her nomination, Ms. Verma spent 20 years designing policy projects involving Medicaid, including HIP, the nation’s first consumer-directed Medicaid program under Indiana Governor Mitch Daniels and then-Gov. Mike Pence’s HIP 2.0 waiver proposal.
Prior to consulting, Ms. Verma served as vice president of planning for the Health and Hospital Corporation of Marion County (Ind.) and as a director with the Association of State and Territorial Health Officials in Washington.
Senators have asked that Ms. Verma submit written answers to their questions, which they will review before voting on her nomination.
[email protected]
On Twitter @legal_med
Malpractice 2017: Do we need reform?
Malpractice reforms long espoused by Thomas E. Price, MD, Health & Human Services secretary, are fostering debate on whether national fixes are necessary in an improving liability landscape. Claims against health providers have decreased twofold and doctors nationwide have seen their premiums decrease steadily for a decade. But the numbers tell just part of the story, liability experts say.
“Most health policy and medical malpractice scholars would not describe the environment we’re in right now as a state of crisis, at least not compared to what we observed a decade ago,” said Anupam B. Jena, MD, PhD, health care policy professor at Harvard University, Boston, and a medical liability researcher.“But I would argue that malpractice is still a salient fact for most physicians. What’s much more important than focusing on changes over time is taking a static perspective and understanding the risk that a physician faces when it comes to malpractice.”
During just over 12 years in Congress, Dr. Price (R-Ga.) has consistently advocated for tort reform, proposing legislation that would restructure the lawsuit process. He supports damage caps and has recommended the creation of health care tribunals that would hear cases only after medical review. He has also proposed the development of national practice guidelines for doctors, which if followed could be used defensively.
“From exorbitant malpractice insurance premiums to the remarkably expensive practice of defensive medicine, it is my experience that the current culture of litigation costs patients hundreds of billions of dollars,” Dr. Price wrote in a 2009 commentary about his Empowering Patients First Act. “These costs do nothing to provide better care, but rather serve only as a defense against unyielding personal injury lawyers ...When malpractice suits are brought through specialized courts and viewed through the perspective of medically appropriate care, rather than a lottery mentality, we will see a decline in frivolous lawsuits.”
Data show lawsuits down
Claims data however, show that lawsuits against doctors are on the decline – and have been for more than 10 years. The rate of paid claims against physicians dropped from 18.6 to 9.9 paid claims per 1,000 physicians between 2002 and 2013, according to 2014 study by Michelle Mello of Stanford (Calif.) University and colleagues (JAMA. 2014;312(20):2146-2155. doi:10.1001/jama.2014.10705). Another analysis found that claims decreased from 12 claims per 100 doctors in 2003 to 6 per 100 doctors in 2016, according to a study of claims from The Doctors Company database.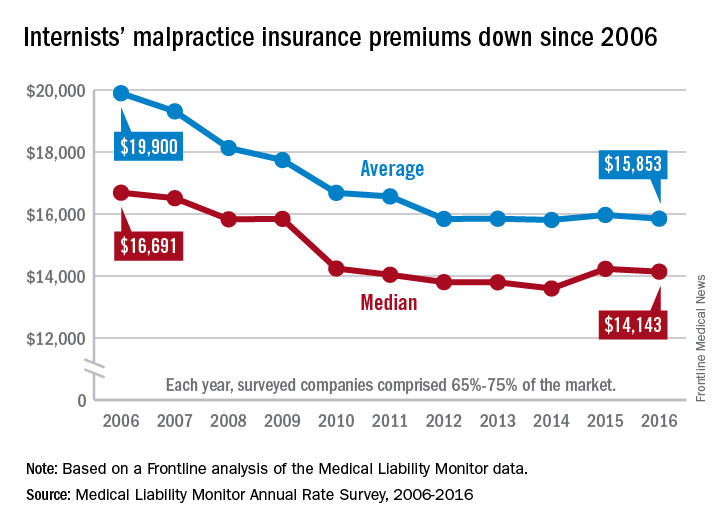
The sharp decline in claims is directly affecting premiums, which have remained nearly unchanged for years, said Michael Matray, editor of the Medical Liability Monitor (MLM), which tracks premium rates. In 2003, rate increases of 49% were commonplace, he noted, with some states reporting rises of 100%. But premiums started to fall in 2006, he said.
“We are in the midst of a 10-year soft market, the longest we’ve ever had,” Mr. Matray said in an interview. “In 2006, we started to soften. We have been soft or flat with no change since. It’s definitely unprecedented.”
In 2016, U.S. internists paid an average premium of $15,853, compared to an average premium payment of $19,900 in 2006 without inflation adjustment, according to this news organization’s analysis of MLM data. General surgeons meanwhile, paid an average of $52,905 in premiums in 2016, compared to a 2006 average of $68,186. Ob.gyns. paid an average premium of $72,999 in 2016, a drop from $93,230 in 2006.
“Generally speaking, it’s great for doctors,” Mr. Matray said. “Even if you remove the inflationary factor, there are some states where they are paying less now than they were 10 years ago.”
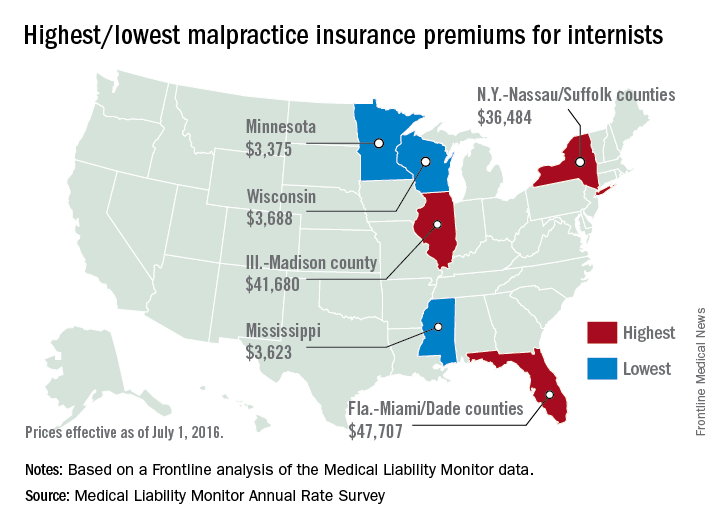
But stable premiums do not mean inexpensive insurance, noted Mike Stinson, vice president of government relations and public policy for the PIAA, a national trade association for medical liability insurers.
“They’ve certainly come down over the last few years,” Mr. Stinson said in an interview. “But they certainly are not low.”
South Florida doctors for example, are paying among the highest premiums in the nation despite an overall drop in the last 10 years. Internists in Dade County, Fla., paid $47,707 for insurance in 2016, $27,000 less than in 2006, according to MLM data. Coverage is still is not affordable, said Jason M. Goldman, MD, an internist in private practice in Coral Springs, Fla., and governor for the American College of Physicians Florida chapter.
The premiums are ridiculous,” he said in an interview. “That’s one of the reasons I went bare. Having medical insurance makes you a target, because lawyers think, ‘We can sue the insurance company and the insurance company is going to pay.’ ”
State reforms driving lawsuit decline
A combination of state reforms and patient safety initiatives are fueling the lawsuit down slide, said Paul A. Greve Jr. senior vice president/senior consultant for Willis Towers Watson Health Care Practice and coauthor of the 2016 MLM Survey report.
More than 25 states now have laws that enforce caps on noneconomic damages in medical liability cases, and in the last 15 years, a large majority have upheld such limits, Mr. Greve said. In addition, a number of states require a certificate of merit before suits can move forward, which mandate that a qualified physician verify that a defendant’s actions were likely negligent.
Patient safety programs such as internal patient safety committees, enhanced provider education, safety protocols, and communication and resolution programs, are also making an impact.
“There have been many efforts toward thinking innovatively about how we should be compensating patients more fairly and more quickly,” said Harvard’s Dr. Jena, who practices at Massachusetts General Hospital. “There are early disclosure programs that many hospitals have adopted as a movement toward that goal. The system is evolving.”
The high expense of filing a medical malpractice lawsuit is another factor detouring lawsuits, Mr. Greve added.
“It’s very expensive for [plaintiffs’ attorneys] to pursue these cases,” he said. “They have to invest a large amount of money into them and they can’t afford to take cases that have questionable liability, or they’re going to end up shelling out a lot of money and not getting anything in return.”
Malpractice system remains broken
While lawsuit and premium data paint a positive picture, the medical malpractice system remains dysfunctional for doctors and patients, said PIAA’s Mr. Stinson.
A majority of medical malpractice claims against physicians are determined to be nonmeritorious yet an average lawsuit takes roughly 4 years to resolve, he noted. Suits against doctors are dismissed by the court 54% of the time across all specialties, and among cases that go to trial, 80% end in the physician’s favor, according to a 2012 study by Dr. Jena and colleagues (JAMA Intern Med. 2012 Jun. 11 doi: 10.1001/jamainternmed.2012.1416)
“We think that’s a huge amount of resources both in time and money and human capital to get poured into cases where no payment is ever deemed appropriate,” Mr. Stinson said. “We would like to see [malpractice] reforms in part because we think you can weed out some of these claims in advance, so more of the resources can be used to determine whether or not there was negligence and if so, making that patient whole as fast as possible.”
Regardless of declining claims, most physicians will still be sued in their lifetime, Dr. Jena said. By age 65, 75% of physicians in low-risk specialties have faced a malpractice claim, and 99% of doctors in high-risk specialties have been sued, according to a 2011 study by Dr. Jena and colleagues (N Engl J Med. 2011 Aug. 18. doi: 10.1056/NEJMsa1012370)
“What really matters for physicians is not only whether they win or lose a malpractice suit, but whether they are sued in the first place,” Dr. Jena said. “Lawsuits happen quite often and over the course of a physician’s career they are quite common. So whether or not we’re in a state of malpractice crisis or not, the lifetime risk for physicians is quite real.”
The cost of defending claims continues to rise, noted Richard E. Anderson, MD, chair and CEO of The Doctors Company. At the same time, multi-million dollar malpractice verdicts have become more common.
“There’s still way too much malpractice litigation,” Dr. Anderson said. “The majority of it is fruitless, and the total cost burden borne by physicians has not decreased as much as the frequency because of the ongoing increase in severity.”
“The bottom line is defensive medicine is a huge and growing cost to all Americans,” he said. “We’re all on the hook for America’s rising health care bills. And as my increasingly infirm Baby Boomer generation gets older and weaker and sicker, those bills are going to rise that much faster. So the notion advanced by some so-called experts that we can nonetheless afford to spend a half trillion dollars a year or more on largely unnecessary testing driven in significant part by fear of litigation and giant outlier verdicts seems rather inane, and it suggests perhaps that those experts aren’t so expert.”
What would Dr. Price do?
Opinions are mixed about whether Dr. Price’s proposed reforms are the right changes for the medical liability system.
Expert panels to review claims for validity are a promising suggestion, Dr. Jena said. “Administrative courts to help identify malpractice cases that are truly malpractice early on is a good idea,” he said. “We want to have a system that prevents less meritorious cases from soaking up resources.”
But the idea may be easier said than done, said Dr. Anderson of The Doctors Company. “The devil is always in the details There is a lot of merit in [health courts] and they make a lot of sense. However, the notion of going from where we are today to an untested system, which would have to be a compromise between adversaries, would be a very challenging one.”
National clinical standards for physicians to follow and use as a safeguard could backfire, Mr. Stinson said. Bureaucracy could slow the guidelines from being promptly updated, and the standards could fail to keep up with the latest medical developments. As doctors know, medicine is not a one-size-fits-all approach, he added.
“[Guidelines] could encourage doctors to practice cookbook medicine, where they’re just going to follow the standard along without being given the opportunity to use their training and clinical experience to see whether that’s actually in the patient’s best interest,” Mr. Stinson said. “We certainly wouldn’t want to see a situation where a doctor feels diverting from a guidelines is in a patient’s best interest, but they don’t dare do it because it could subject them to a lawsuit.”
When enacting malpractice reforms, it will be critical to evaluate the intervention first to ensure the right objections are met, Dr. Jena added.
“At the end of the day, if the [legal] environment is such that it’s uncomfortable to practice medicine, that’s going to have implications for who goes into medicine and implications for ordering tests and procedures,” he said. “Any reform that attempts to make the process more efficient is a good idea because it lowers cost to the system and makes the experience better for patients and physicians.”
This article was updated 2/15/17.
[email protected]
On Twitter @legal_med
Malpractice reforms long espoused by Thomas E. Price, MD, Health & Human Services secretary, are fostering debate on whether national fixes are necessary in an improving liability landscape. Claims against health providers have decreased twofold and doctors nationwide have seen their premiums decrease steadily for a decade. But the numbers tell just part of the story, liability experts say.
“Most health policy and medical malpractice scholars would not describe the environment we’re in right now as a state of crisis, at least not compared to what we observed a decade ago,” said Anupam B. Jena, MD, PhD, health care policy professor at Harvard University, Boston, and a medical liability researcher.“But I would argue that malpractice is still a salient fact for most physicians. What’s much more important than focusing on changes over time is taking a static perspective and understanding the risk that a physician faces when it comes to malpractice.”
During just over 12 years in Congress, Dr. Price (R-Ga.) has consistently advocated for tort reform, proposing legislation that would restructure the lawsuit process. He supports damage caps and has recommended the creation of health care tribunals that would hear cases only after medical review. He has also proposed the development of national practice guidelines for doctors, which if followed could be used defensively.
“From exorbitant malpractice insurance premiums to the remarkably expensive practice of defensive medicine, it is my experience that the current culture of litigation costs patients hundreds of billions of dollars,” Dr. Price wrote in a 2009 commentary about his Empowering Patients First Act. “These costs do nothing to provide better care, but rather serve only as a defense against unyielding personal injury lawyers ...When malpractice suits are brought through specialized courts and viewed through the perspective of medically appropriate care, rather than a lottery mentality, we will see a decline in frivolous lawsuits.”
Data show lawsuits down
Claims data however, show that lawsuits against doctors are on the decline – and have been for more than 10 years. The rate of paid claims against physicians dropped from 18.6 to 9.9 paid claims per 1,000 physicians between 2002 and 2013, according to 2014 study by Michelle Mello of Stanford (Calif.) University and colleagues (JAMA. 2014;312(20):2146-2155. doi:10.1001/jama.2014.10705). Another analysis found that claims decreased from 12 claims per 100 doctors in 2003 to 6 per 100 doctors in 2016, according to a study of claims from The Doctors Company database.
The sharp decline in claims is directly affecting premiums, which have remained nearly unchanged for years, said Michael Matray, editor of the Medical Liability Monitor (MLM), which tracks premium rates. In 2003, rate increases of 49% were commonplace, he noted, with some states reporting rises of 100%. But premiums started to fall in 2006, he said.
“We are in the midst of a 10-year soft market, the longest we’ve ever had,” Mr. Matray said in an interview. “In 2006, we started to soften. We have been soft or flat with no change since. It’s definitely unprecedented.”
In 2016, U.S. internists paid an average premium of $15,853, compared to an average premium payment of $19,900 in 2006 without inflation adjustment, according to this news organization’s analysis of MLM data. General surgeons meanwhile, paid an average of $52,905 in premiums in 2016, compared to a 2006 average of $68,186. Ob.gyns. paid an average premium of $72,999 in 2016, a drop from $93,230 in 2006.
“Generally speaking, it’s great for doctors,” Mr. Matray said. “Even if you remove the inflationary factor, there are some states where they are paying less now than they were 10 years ago.”

But stable premiums do not mean inexpensive insurance, noted Mike Stinson, vice president of government relations and public policy for the PIAA, a national trade association for medical liability insurers.
“They’ve certainly come down over the last few years,” Mr. Stinson said in an interview. “But they certainly are not low.”
South Florida doctors for example, are paying among the highest premiums in the nation despite an overall drop in the last 10 years. Internists in Dade County, Fla., paid $47,707 for insurance in 2016, $27,000 less than in 2006, according to MLM data. Coverage is still is not affordable, said Jason M. Goldman, MD, an internist in private practice in Coral Springs, Fla., and governor for the American College of Physicians Florida chapter.
The premiums are ridiculous,” he said in an interview. “That’s one of the reasons I went bare. Having medical insurance makes you a target, because lawyers think, ‘We can sue the insurance company and the insurance company is going to pay.’ ”
State reforms driving lawsuit decline
A combination of state reforms and patient safety initiatives are fueling the lawsuit down slide, said Paul A. Greve Jr. senior vice president/senior consultant for Willis Towers Watson Health Care Practice and coauthor of the 2016 MLM Survey report.
More than 25 states now have laws that enforce caps on noneconomic damages in medical liability cases, and in the last 15 years, a large majority have upheld such limits, Mr. Greve said. In addition, a number of states require a certificate of merit before suits can move forward, which mandate that a qualified physician verify that a defendant’s actions were likely negligent.
Patient safety programs such as internal patient safety committees, enhanced provider education, safety protocols, and communication and resolution programs, are also making an impact.
“There have been many efforts toward thinking innovatively about how we should be compensating patients more fairly and more quickly,” said Harvard’s Dr. Jena, who practices at Massachusetts General Hospital. “There are early disclosure programs that many hospitals have adopted as a movement toward that goal. The system is evolving.”
The high expense of filing a medical malpractice lawsuit is another factor detouring lawsuits, Mr. Greve added.
“It’s very expensive for [plaintiffs’ attorneys] to pursue these cases,” he said. “They have to invest a large amount of money into them and they can’t afford to take cases that have questionable liability, or they’re going to end up shelling out a lot of money and not getting anything in return.”
Malpractice system remains broken
While lawsuit and premium data paint a positive picture, the medical malpractice system remains dysfunctional for doctors and patients, said PIAA’s Mr. Stinson.
A majority of medical malpractice claims against physicians are determined to be nonmeritorious yet an average lawsuit takes roughly 4 years to resolve, he noted. Suits against doctors are dismissed by the court 54% of the time across all specialties, and among cases that go to trial, 80% end in the physician’s favor, according to a 2012 study by Dr. Jena and colleagues (JAMA Intern Med. 2012 Jun. 11 doi: 10.1001/jamainternmed.2012.1416)
“We think that’s a huge amount of resources both in time and money and human capital to get poured into cases where no payment is ever deemed appropriate,” Mr. Stinson said. “We would like to see [malpractice] reforms in part because we think you can weed out some of these claims in advance, so more of the resources can be used to determine whether or not there was negligence and if so, making that patient whole as fast as possible.”
Regardless of declining claims, most physicians will still be sued in their lifetime, Dr. Jena said. By age 65, 75% of physicians in low-risk specialties have faced a malpractice claim, and 99% of doctors in high-risk specialties have been sued, according to a 2011 study by Dr. Jena and colleagues (N Engl J Med. 2011 Aug. 18. doi: 10.1056/NEJMsa1012370)
“What really matters for physicians is not only whether they win or lose a malpractice suit, but whether they are sued in the first place,” Dr. Jena said. “Lawsuits happen quite often and over the course of a physician’s career they are quite common. So whether or not we’re in a state of malpractice crisis or not, the lifetime risk for physicians is quite real.”
The cost of defending claims continues to rise, noted Richard E. Anderson, MD, chair and CEO of The Doctors Company. At the same time, multi-million dollar malpractice verdicts have become more common.
“There’s still way too much malpractice litigation,” Dr. Anderson said. “The majority of it is fruitless, and the total cost burden borne by physicians has not decreased as much as the frequency because of the ongoing increase in severity.”
“The bottom line is defensive medicine is a huge and growing cost to all Americans,” he said. “We’re all on the hook for America’s rising health care bills. And as my increasingly infirm Baby Boomer generation gets older and weaker and sicker, those bills are going to rise that much faster. So the notion advanced by some so-called experts that we can nonetheless afford to spend a half trillion dollars a year or more on largely unnecessary testing driven in significant part by fear of litigation and giant outlier verdicts seems rather inane, and it suggests perhaps that those experts aren’t so expert.”
What would Dr. Price do?
Opinions are mixed about whether Dr. Price’s proposed reforms are the right changes for the medical liability system.
Expert panels to review claims for validity are a promising suggestion, Dr. Jena said. “Administrative courts to help identify malpractice cases that are truly malpractice early on is a good idea,” he said. “We want to have a system that prevents less meritorious cases from soaking up resources.”
But the idea may be easier said than done, said Dr. Anderson of The Doctors Company. “The devil is always in the details There is a lot of merit in [health courts] and they make a lot of sense. However, the notion of going from where we are today to an untested system, which would have to be a compromise between adversaries, would be a very challenging one.”
National clinical standards for physicians to follow and use as a safeguard could backfire, Mr. Stinson said. Bureaucracy could slow the guidelines from being promptly updated, and the standards could fail to keep up with the latest medical developments. As doctors know, medicine is not a one-size-fits-all approach, he added.
“[Guidelines] could encourage doctors to practice cookbook medicine, where they’re just going to follow the standard along without being given the opportunity to use their training and clinical experience to see whether that’s actually in the patient’s best interest,” Mr. Stinson said. “We certainly wouldn’t want to see a situation where a doctor feels diverting from a guidelines is in a patient’s best interest, but they don’t dare do it because it could subject them to a lawsuit.”
When enacting malpractice reforms, it will be critical to evaluate the intervention first to ensure the right objections are met, Dr. Jena added.
“At the end of the day, if the [legal] environment is such that it’s uncomfortable to practice medicine, that’s going to have implications for who goes into medicine and implications for ordering tests and procedures,” he said. “Any reform that attempts to make the process more efficient is a good idea because it lowers cost to the system and makes the experience better for patients and physicians.”
This article was updated 2/15/17.
[email protected]
On Twitter @legal_med
Malpractice reforms long espoused by Thomas E. Price, MD, Health & Human Services secretary, are fostering debate on whether national fixes are necessary in an improving liability landscape. Claims against health providers have decreased twofold and doctors nationwide have seen their premiums decrease steadily for a decade. But the numbers tell just part of the story, liability experts say.
“Most health policy and medical malpractice scholars would not describe the environment we’re in right now as a state of crisis, at least not compared to what we observed a decade ago,” said Anupam B. Jena, MD, PhD, health care policy professor at Harvard University, Boston, and a medical liability researcher.“But I would argue that malpractice is still a salient fact for most physicians. What’s much more important than focusing on changes over time is taking a static perspective and understanding the risk that a physician faces when it comes to malpractice.”
During just over 12 years in Congress, Dr. Price (R-Ga.) has consistently advocated for tort reform, proposing legislation that would restructure the lawsuit process. He supports damage caps and has recommended the creation of health care tribunals that would hear cases only after medical review. He has also proposed the development of national practice guidelines for doctors, which if followed could be used defensively.
“From exorbitant malpractice insurance premiums to the remarkably expensive practice of defensive medicine, it is my experience that the current culture of litigation costs patients hundreds of billions of dollars,” Dr. Price wrote in a 2009 commentary about his Empowering Patients First Act. “These costs do nothing to provide better care, but rather serve only as a defense against unyielding personal injury lawyers ...When malpractice suits are brought through specialized courts and viewed through the perspective of medically appropriate care, rather than a lottery mentality, we will see a decline in frivolous lawsuits.”
Data show lawsuits down
Claims data however, show that lawsuits against doctors are on the decline – and have been for more than 10 years. The rate of paid claims against physicians dropped from 18.6 to 9.9 paid claims per 1,000 physicians between 2002 and 2013, according to 2014 study by Michelle Mello of Stanford (Calif.) University and colleagues (JAMA. 2014;312(20):2146-2155. doi:10.1001/jama.2014.10705). Another analysis found that claims decreased from 12 claims per 100 doctors in 2003 to 6 per 100 doctors in 2016, according to a study of claims from The Doctors Company database.
The sharp decline in claims is directly affecting premiums, which have remained nearly unchanged for years, said Michael Matray, editor of the Medical Liability Monitor (MLM), which tracks premium rates. In 2003, rate increases of 49% were commonplace, he noted, with some states reporting rises of 100%. But premiums started to fall in 2006, he said.
“We are in the midst of a 10-year soft market, the longest we’ve ever had,” Mr. Matray said in an interview. “In 2006, we started to soften. We have been soft or flat with no change since. It’s definitely unprecedented.”
In 2016, U.S. internists paid an average premium of $15,853, compared to an average premium payment of $19,900 in 2006 without inflation adjustment, according to this news organization’s analysis of MLM data. General surgeons meanwhile, paid an average of $52,905 in premiums in 2016, compared to a 2006 average of $68,186. Ob.gyns. paid an average premium of $72,999 in 2016, a drop from $93,230 in 2006.
“Generally speaking, it’s great for doctors,” Mr. Matray said. “Even if you remove the inflationary factor, there are some states where they are paying less now than they were 10 years ago.”

But stable premiums do not mean inexpensive insurance, noted Mike Stinson, vice president of government relations and public policy for the PIAA, a national trade association for medical liability insurers.
“They’ve certainly come down over the last few years,” Mr. Stinson said in an interview. “But they certainly are not low.”
South Florida doctors for example, are paying among the highest premiums in the nation despite an overall drop in the last 10 years. Internists in Dade County, Fla., paid $47,707 for insurance in 2016, $27,000 less than in 2006, according to MLM data. Coverage is still is not affordable, said Jason M. Goldman, MD, an internist in private practice in Coral Springs, Fla., and governor for the American College of Physicians Florida chapter.
The premiums are ridiculous,” he said in an interview. “That’s one of the reasons I went bare. Having medical insurance makes you a target, because lawyers think, ‘We can sue the insurance company and the insurance company is going to pay.’ ”
State reforms driving lawsuit decline
A combination of state reforms and patient safety initiatives are fueling the lawsuit down slide, said Paul A. Greve Jr. senior vice president/senior consultant for Willis Towers Watson Health Care Practice and coauthor of the 2016 MLM Survey report.
More than 25 states now have laws that enforce caps on noneconomic damages in medical liability cases, and in the last 15 years, a large majority have upheld such limits, Mr. Greve said. In addition, a number of states require a certificate of merit before suits can move forward, which mandate that a qualified physician verify that a defendant’s actions were likely negligent.
Patient safety programs such as internal patient safety committees, enhanced provider education, safety protocols, and communication and resolution programs, are also making an impact.
“There have been many efforts toward thinking innovatively about how we should be compensating patients more fairly and more quickly,” said Harvard’s Dr. Jena, who practices at Massachusetts General Hospital. “There are early disclosure programs that many hospitals have adopted as a movement toward that goal. The system is evolving.”
The high expense of filing a medical malpractice lawsuit is another factor detouring lawsuits, Mr. Greve added.
“It’s very expensive for [plaintiffs’ attorneys] to pursue these cases,” he said. “They have to invest a large amount of money into them and they can’t afford to take cases that have questionable liability, or they’re going to end up shelling out a lot of money and not getting anything in return.”
Malpractice system remains broken
While lawsuit and premium data paint a positive picture, the medical malpractice system remains dysfunctional for doctors and patients, said PIAA’s Mr. Stinson.
A majority of medical malpractice claims against physicians are determined to be nonmeritorious yet an average lawsuit takes roughly 4 years to resolve, he noted. Suits against doctors are dismissed by the court 54% of the time across all specialties, and among cases that go to trial, 80% end in the physician’s favor, according to a 2012 study by Dr. Jena and colleagues (JAMA Intern Med. 2012 Jun. 11 doi: 10.1001/jamainternmed.2012.1416)
“We think that’s a huge amount of resources both in time and money and human capital to get poured into cases where no payment is ever deemed appropriate,” Mr. Stinson said. “We would like to see [malpractice] reforms in part because we think you can weed out some of these claims in advance, so more of the resources can be used to determine whether or not there was negligence and if so, making that patient whole as fast as possible.”
Regardless of declining claims, most physicians will still be sued in their lifetime, Dr. Jena said. By age 65, 75% of physicians in low-risk specialties have faced a malpractice claim, and 99% of doctors in high-risk specialties have been sued, according to a 2011 study by Dr. Jena and colleagues (N Engl J Med. 2011 Aug. 18. doi: 10.1056/NEJMsa1012370)
“What really matters for physicians is not only whether they win or lose a malpractice suit, but whether they are sued in the first place,” Dr. Jena said. “Lawsuits happen quite often and over the course of a physician’s career they are quite common. So whether or not we’re in a state of malpractice crisis or not, the lifetime risk for physicians is quite real.”
The cost of defending claims continues to rise, noted Richard E. Anderson, MD, chair and CEO of The Doctors Company. At the same time, multi-million dollar malpractice verdicts have become more common.
“There’s still way too much malpractice litigation,” Dr. Anderson said. “The majority of it is fruitless, and the total cost burden borne by physicians has not decreased as much as the frequency because of the ongoing increase in severity.”
“The bottom line is defensive medicine is a huge and growing cost to all Americans,” he said. “We’re all on the hook for America’s rising health care bills. And as my increasingly infirm Baby Boomer generation gets older and weaker and sicker, those bills are going to rise that much faster. So the notion advanced by some so-called experts that we can nonetheless afford to spend a half trillion dollars a year or more on largely unnecessary testing driven in significant part by fear of litigation and giant outlier verdicts seems rather inane, and it suggests perhaps that those experts aren’t so expert.”
What would Dr. Price do?
Opinions are mixed about whether Dr. Price’s proposed reforms are the right changes for the medical liability system.
Expert panels to review claims for validity are a promising suggestion, Dr. Jena said. “Administrative courts to help identify malpractice cases that are truly malpractice early on is a good idea,” he said. “We want to have a system that prevents less meritorious cases from soaking up resources.”
But the idea may be easier said than done, said Dr. Anderson of The Doctors Company. “The devil is always in the details There is a lot of merit in [health courts] and they make a lot of sense. However, the notion of going from where we are today to an untested system, which would have to be a compromise between adversaries, would be a very challenging one.”
National clinical standards for physicians to follow and use as a safeguard could backfire, Mr. Stinson said. Bureaucracy could slow the guidelines from being promptly updated, and the standards could fail to keep up with the latest medical developments. As doctors know, medicine is not a one-size-fits-all approach, he added.
“[Guidelines] could encourage doctors to practice cookbook medicine, where they’re just going to follow the standard along without being given the opportunity to use their training and clinical experience to see whether that’s actually in the patient’s best interest,” Mr. Stinson said. “We certainly wouldn’t want to see a situation where a doctor feels diverting from a guidelines is in a patient’s best interest, but they don’t dare do it because it could subject them to a lawsuit.”
When enacting malpractice reforms, it will be critical to evaluate the intervention first to ensure the right objections are met, Dr. Jena added.
“At the end of the day, if the [legal] environment is such that it’s uncomfortable to practice medicine, that’s going to have implications for who goes into medicine and implications for ordering tests and procedures,” he said. “Any reform that attempts to make the process more efficient is a good idea because it lowers cost to the system and makes the experience better for patients and physicians.”
This article was updated 2/15/17.
[email protected]
On Twitter @legal_med
Federal judge blocks merger between Anthem and Cigna
A federal district court judge has blocked health insurer Anthem from acquiring Cigna, ruling the megamerger would violate antitrust laws and stifle competition.
The decision comes weeks after another U.S. district court judge barred a merger between health insurance giants Aetna and Humana.
“This merger would have stifled competition, harming consumers by increasing health insurance prices and slowing innovation aimed at lowering the costs of health care,” Acting Assistant Attorney General Brent Snyder said in a statement.
Anthem intends to appeal the decision, said Joseph R. Swedish, Anthem’s chair, president, and chief executive officer.
“Anthem is significantly disappointed by the decision, as combining Anthem and Cigna would positively impact the health and well-being of millions of Americans – saving them more than $2 billion in medical costs annually,” Mr. Swedish said in a statement.“If not overturned, the consequences of the decision are far reaching and will hurt American consumers by limiting their access to high-quality affordable care, slowing the industry’s shift to value-based care and improved outcomes for patients, and restricting innovation, which is critical to meeting the evolving needs of health care consumers.”
In a statement, a Cigna official said the company intends to carefully review the opinion and evaluate its options in accordance with the merger agreement.
“Cigna remains focused on helping to improve health care by delivering value to our customers and clients and expanding our business around the world,” the statement said.
The DOJ, 11 states, and the District of Columbia sued Anthem and Cigna in July over their proposed $54 billion consolidation in what would have been the largest merger in history.
The DOJ argued the merger would substantially harm competition and negatively impact the entire insurance industry if allowed to proceed. The consolidation would enhance Anthem’s power to profit at the expense of consumers and the doctors and hospitals who provide their medical care, DOJ attorneys said in their complaint.
Anthem and Cigna argued the proposed acquisition was “procompetitive,” and that the merger would result in efficiencies that would directly benefit consumers via greater access to affordable health care. The benefits of the merger outweigh any alleged anticompetitive effects, according to Anthem.
A trial before Judge Amy Berman Jackson of the U.S. District Court for the District of Columbia ran from November through January.
Judge Berman’s opinion is temporarily under seal to allow parties to review for confidentiality.
The ruling is the second victory for the DOJ in as many weeks. In a Jan. 23 decision, Judge John D. Bates of the U.S. District Court for the District of Columbia denied Aetna’s $37 billion plan to purchase Humana, following a month-long trial that began in early December. Judge Bates ruled the consolidation would violate antitrust laws and reduce competition.
[email protected]
On Twitter @legal_med
A federal district court judge has blocked health insurer Anthem from acquiring Cigna, ruling the megamerger would violate antitrust laws and stifle competition.
The decision comes weeks after another U.S. district court judge barred a merger between health insurance giants Aetna and Humana.
“This merger would have stifled competition, harming consumers by increasing health insurance prices and slowing innovation aimed at lowering the costs of health care,” Acting Assistant Attorney General Brent Snyder said in a statement.
Anthem intends to appeal the decision, said Joseph R. Swedish, Anthem’s chair, president, and chief executive officer.
“Anthem is significantly disappointed by the decision, as combining Anthem and Cigna would positively impact the health and well-being of millions of Americans – saving them more than $2 billion in medical costs annually,” Mr. Swedish said in a statement.“If not overturned, the consequences of the decision are far reaching and will hurt American consumers by limiting their access to high-quality affordable care, slowing the industry’s shift to value-based care and improved outcomes for patients, and restricting innovation, which is critical to meeting the evolving needs of health care consumers.”
In a statement, a Cigna official said the company intends to carefully review the opinion and evaluate its options in accordance with the merger agreement.
“Cigna remains focused on helping to improve health care by delivering value to our customers and clients and expanding our business around the world,” the statement said.
The DOJ, 11 states, and the District of Columbia sued Anthem and Cigna in July over their proposed $54 billion consolidation in what would have been the largest merger in history.
The DOJ argued the merger would substantially harm competition and negatively impact the entire insurance industry if allowed to proceed. The consolidation would enhance Anthem’s power to profit at the expense of consumers and the doctors and hospitals who provide their medical care, DOJ attorneys said in their complaint.
Anthem and Cigna argued the proposed acquisition was “procompetitive,” and that the merger would result in efficiencies that would directly benefit consumers via greater access to affordable health care. The benefits of the merger outweigh any alleged anticompetitive effects, according to Anthem.
A trial before Judge Amy Berman Jackson of the U.S. District Court for the District of Columbia ran from November through January.
Judge Berman’s opinion is temporarily under seal to allow parties to review for confidentiality.
The ruling is the second victory for the DOJ in as many weeks. In a Jan. 23 decision, Judge John D. Bates of the U.S. District Court for the District of Columbia denied Aetna’s $37 billion plan to purchase Humana, following a month-long trial that began in early December. Judge Bates ruled the consolidation would violate antitrust laws and reduce competition.
[email protected]
On Twitter @legal_med
A federal district court judge has blocked health insurer Anthem from acquiring Cigna, ruling the megamerger would violate antitrust laws and stifle competition.
The decision comes weeks after another U.S. district court judge barred a merger between health insurance giants Aetna and Humana.
“This merger would have stifled competition, harming consumers by increasing health insurance prices and slowing innovation aimed at lowering the costs of health care,” Acting Assistant Attorney General Brent Snyder said in a statement.
Anthem intends to appeal the decision, said Joseph R. Swedish, Anthem’s chair, president, and chief executive officer.
“Anthem is significantly disappointed by the decision, as combining Anthem and Cigna would positively impact the health and well-being of millions of Americans – saving them more than $2 billion in medical costs annually,” Mr. Swedish said in a statement.“If not overturned, the consequences of the decision are far reaching and will hurt American consumers by limiting their access to high-quality affordable care, slowing the industry’s shift to value-based care and improved outcomes for patients, and restricting innovation, which is critical to meeting the evolving needs of health care consumers.”
In a statement, a Cigna official said the company intends to carefully review the opinion and evaluate its options in accordance with the merger agreement.
“Cigna remains focused on helping to improve health care by delivering value to our customers and clients and expanding our business around the world,” the statement said.
The DOJ, 11 states, and the District of Columbia sued Anthem and Cigna in July over their proposed $54 billion consolidation in what would have been the largest merger in history.
The DOJ argued the merger would substantially harm competition and negatively impact the entire insurance industry if allowed to proceed. The consolidation would enhance Anthem’s power to profit at the expense of consumers and the doctors and hospitals who provide their medical care, DOJ attorneys said in their complaint.
Anthem and Cigna argued the proposed acquisition was “procompetitive,” and that the merger would result in efficiencies that would directly benefit consumers via greater access to affordable health care. The benefits of the merger outweigh any alleged anticompetitive effects, according to Anthem.
A trial before Judge Amy Berman Jackson of the U.S. District Court for the District of Columbia ran from November through January.
Judge Berman’s opinion is temporarily under seal to allow parties to review for confidentiality.
The ruling is the second victory for the DOJ in as many weeks. In a Jan. 23 decision, Judge John D. Bates of the U.S. District Court for the District of Columbia denied Aetna’s $37 billion plan to purchase Humana, following a month-long trial that began in early December. Judge Bates ruled the consolidation would violate antitrust laws and reduce competition.
[email protected]
On Twitter @legal_med
Tele-rheumatology reaches patients who lack access to care
By the time Christine Peoples, MD, examined the patient, the middle-aged man had been treated inadequately for psoriatic arthritis for years.
Poor mobility made it difficult for him to travel and gaps in his care had exacerbated his condition. But a bidirectional video system at the University of Pittsburgh Medical Center (UPMC) allowed Dr. Peoples to assess the patient, correct his therapeutic regimen, and establish consistent follow up.
Without telemedicine, the patient would have likely continued to deteriorate, said Dr. Peoples, a rheumatologist with the UPMC Teleconsult Centers.
Across the country, more rheumatology practices are employing telemedicine to treat patients, particularly physicians at academic medicine centers that have the resources to launch comprehensive units. The technology is a way to bridge the shortage of rheumatologists in rural areas and reach patients who cannot access specialty care for rheumatic conditions, proponents say. A 2013 study in Arthritis & Rheumatism found that many towns and small cities with populations up to 50,000 have no practicing rheumatologists, with some patients having to travel more than 200 miles to get specialty care (2013 Dec;65[12]:3017-25).
“Telemedicine is expanding both in terms of the number of consultations and the breadth of services involved,” he said. “Tele-rheumatology was certainly not a big area to be looked at in years past, but it’s one more area that is starting to expand.”
Research is lacking regarding how prevalent tele-rheumatology has become in the United States and whether it’s as effective as face-to-face visits. In a recent analysis of 1,468 potentially eligible tele-rheumatology studies and literature, only 20 addressed direct provider to patient contact that influenced or had the potential to influence clinical care, according to a November 2016 review in Arthritis Care & Research (doi: 10.1002/acr.23153). Of the 20 studies, the majority of articles involved tele-rheumatology use in Europe or Great Britain, said study author John Allen McDougall, MD, a postdoctoral fellow at Yale University, New Haven, Conn. According to the literature, the most common condition treated through telemedicine was rheumatoid arthritis. Little information existed on telemedicine use for gout or the treatment of connective tissue diseases, Dr. McDougall said.
Increasing access, saving time
UPMC has operated a tele-rheumatology program for nearly 3 years that connects rural patients with their specialists. Patients present for video examination at teleconsult centers located in underserved areas within western and central Pennsylvania. On the other end of the screen, Dr. Peoples uses high-level audio and video technology to discuss the patient’s history, direct nurses, make diagnoses, answer questions, and prescribe treatment to patients.
“Patients comment about how much easier it is for them and what a difference this makes in their health care,” Dr. Peoples said. “[Tele-rheumatology also increases] the ability to have regular follow-up care. I see them every few months for their condition and manage it. You can really establish a long relationship with patients just as you would if you saw patients in person.”
Rheumatologists at Dartmouth–Hitchcock Medical Center (DHMC) in Lebanon, N.H., are achieving similar results.
The medical center’s tele-rheumatology unit began in 2011 with the aim of increasing specialty access for patients in northern New Hampshire. Dartmouth also employed a telemedicine clinic from 2012 to 2013 in southern Vermont while seeking to replace a well-established rheumatologist who had retired.
Between October 2011 and December 2014, 176 patients were seen via tele-rheumatology between the two sites over the course of 244 tele-rheumatology patient visits with Dartmouth providers, according to a study published in the June 2016 Seminars in Arthritis & Rheumatism (2016 Dec;46[3]:380-5). Patients lived on average 99 miles from DHMC and 22 miles from the remote clinic site, the study found. The top diagnosis was inflammatory arthritis.
For most of doctors and patients involved in the study, tele-rheumatology visits were positive and aided patient access, said Daniel Albert, MD, section chief of rheumatology at DHMC.
“There are some limitations, there’s no question about that,” he said. ... [However], right now there are way too few rheumatologists to care for patients with rheumatic disease. We’ve tried to improve that with [nurse practitioners and physician assistants], but this is another way of addressing that need.”
Pediatric rheumatologists are also using the benefits of telemedicine. Children’s Mercy in Kansas City, Mo., launched a pediatric rheumatology telemedicine clinic in 2014 that links children in Joplin, Mo., with rheumatologists at Children’s Mercy, about 160 miles away. Patients and their parents/guardians treated via telemedicine at the Joplin site missed less work and school and saved money on food, compared with traveling to Kansas City for treatment, according to a 2016 study published in Pediatric Rheumatology (doi: 10.1186/s12969-016-0116-2).
“This is much more convenient for patients,” said Elizabeth A. Kessler, MD, a pediatric rheumatologist at Children’s Mercy and coauthor of the study. “It saves them time and money. With the training of the tele-facilitator, I believe that we’re able to provide them with equivalent care compared with if they were in-person.”
Is tele-rheumatology right for all settings?
But tele-rheumatology may not be appropriate in all practice settings or the right method for all rheumatology patients, doctors say.
As the Dartmouth-Hitchcock study noted, patients with complex diseases or unclear underlying conditions do not make good telemedicine patients. In addition, opinions are mixed about whether physicians and patients should have an established relationship before telemedicine visits occur and what such visits should entail.
Anchorage, Alaska-based rheumatologist Elizabeth Ferucci, MD, uses telemedicine only for follow up. Like many rheumatologists in Alaska, she travels to field clinics in larger communities throughout the state twice a year to treat patients, said Dr. Ferucci, a rheumatologist for the Alaska Native Tribal Health Consortium. Because some patients need to be seen more than twice a year, Dr. Ferucci conducts follow-up visits every 2-3 months via video.
However, Dr. Ferucci notes that having a trained telemedicine presenter capable of performing a joint examination in the room with the patient is not always possible because of high staff turnover and the more than 200 villages served. A nurse or staff member remains in the room with the patient, but they are not always trained to perform joint exams.
At Dartmouth-Hitchcock, Dr. Albert’s practice accepts both initial and follow-up patient visits. He acknowledges that tele-rheumatology is best used for follow-up care, but explains that limiting the center’s telemedicine use would leave some patients without treatment.
“Because the constraints of getting to Dartmouth are so great, we don’t want to impose that restriction on patients,” Dr. Albert said. “However, in general, it is preferable to do an in-person consultation first to clarify the physical findings.”
Dartmouth has used both nurses and medical assistants as presenters during tele-rheumatology after developing a web-based video series to train presenters. Nurses at UPMC are also trained as telemedicine presenters, and telemedicine visits are only used for follow-up care, Dr. Peoples said.
At Children’s Mercy in Kansas City, telemedicine visits are restricted to follow-up care and include a nurse facilitator at the virtual site. However, the type of conditions and stages of disease examined through the technology has grown since the telemedicine services started, Dr. Kessler said.
“What we feel comfortable seeing has expanded since the time we initially started the clinic because some of the patients, when they were having flares, could not come to Kansas City for financial reasons,” she said, adding that she has since examined children with lupus, vasculitis, and active arthritis through telemedicine.
She stresses that in-person visits should depend on the patient, the circumstances, and the physician’s comfort level. ”You need to be realistic,” she said. “There are times when I’ve said to families, ‘I would like you to come to Kansas City because I’m a little unsure about your exam findings.’ There are definitely some patients who do more of a hybrid approach where they come to Kansas City and are also seen by telemedicine.”
Barriers slow telemedicine progress
Amid its benefits, challenges for tele-rheumatology, such as reimbursement and licensure obstacles, remain.
Commercial insurers pay for telemedicine at varying rates, making it difficult for doctors to consistently be paid, Dr. Peoples said.
“A lot of insurance companies do cover telemedicine visits, but still, a lot don’t,” she said. “I do think that in order to remain competitive, insurance companies overall are going to have to start covering these services.”
At least 32 states and the District of Columbia have parity laws that require commercial health insurers to cover services provided through telehealth to the same extent as those services are covered in person, according to an Aug. 15, 2016 Health Affairs policy brief. However, how private insurers pay and what services they cover vary widely.
Medicaid reimbursement for telehealth depends on individual state Medicaid programs. Only 9 states Medicaid programs reimburse for store-and-forward services, while at least 16 states have some sort of Medicaid reimbursement for remote patient monitoring, according to the Health Affairs report.
Medicare payment for telehealth is clouded in restrictions. The Centers for Medicare & Medicaid Services reimburses for synchronous communications only and does not cover store-and-forward services or remote patient monitoring for chronic diseases, except in Alaska and Hawaii. The patient must be present at an originating site for the visit and cannot be home to receive the services. In addition, CMS reimburses for telehealth only when the originating site is in a Health Professional Shortage Area or within a county outside a Metropolitan Statistical Area. In 2016, CMS expanded its coverage of telehealth to include several new codes including, end-stage renal disease–related services for dialysis, advance care planning services, and critical care consultations furnished via telehealth using new Medicare G-codes.
It’s worth noting that rheumatologists who are salaried employees of academic medical centers, as is the case for all rheumatologists interviewed here, don’t handle the billing for their telemedicine services, and so have not experienced the payment challenges that smaller practices may face.
Mr. Linkous of the American Telemedicine Association said that he believes that reimbursement for telemedicine will improve as health care heads toward value-based payment systems.
“In terms of reimbursement, with the move away from fee-based systems to value-based systems, it really opens the door because for many of the services, you no longer write out a bill with a code associated with it,” he said. “You are paid on the basis of keeping people well. That’s a great positive incentive for using telemedicine.”
Differing state licensure requirements also pose a challenge. The process to obtain licenses and navigate varying credentialing processes can be a headache for health providers and delay approval.
The barrier has led to model legislation by the Federation of State Medical Boards (FSMB) that aims to make it easier for telemedicine physicians to gain licenses in multiple states. Under the Interstate Medical Licensure Compact, physicians designate a member state as the state of principal licensure and select the other states in which they want to license. The state of principal licensure then verifies the physician’s eligibility and provides credential information to the interstate commission, which collects applicable fees and transmits the doctor’s information to the other states. Upon receipt in the additional states, the physician would be granted a license.
As of January, 18 states had enacted the compact legislation, and at least 4 states had introduced the legislation. In 2015, the Health Resources and Services Administration awarded the FSMB a grant to support establishment of the commission and aid with the compact’s infrastructure.
A new normal?
Dr. Albert notes that tele-rheumatology programs can succeed only with adequate resources, time, and staff.
“To set up Skype or Facetime is nothing; you just click on it,” he said. “To set up tele-rheumatology requires a fair amount of administrative substructure that has to be generated in terms of who’s going to do the documentation? Who is going to do the billing? Who is going to do the credentialing? Where is the credentialing going to be validated?”
The equipment required for telemedicine visits also requires a significant amount of time and regular maintenance, Dr. Peoples said. At UPMC for instance, the tele-rheumatology program has an information technology team that assists with technology glitches, changes, and updates, she said. That’s why it’s helpful to have the support of a large academic medical center with the sufficient resources to build a telemedicine program, she added.
In order for more practices to consider tele-rheumatology, more research about cost-effectiveness and best uses of the technology use would be useful, Dr. McDougall said.
“The main question that policy makers are going to want to answer is, ‘What’s the return on investment? Does this make sense for my practice?’ ” he said. “The methods reporting in tele-rheumatologist [literature] is lacking.” But regardless of barriers, telemedicine experts say the technology will likely continue to expand and transform the way rheumatologists are practicing and patients are receiving care.
“Tele-rheumatology will never replace an in-person exam,” Dr. Ferucci said. “But my vision is that it will be able to improve the quality of care for patients living in rural and remote locations, by allowing for more frequent visits and adjustment of medications, which are necessary to achieve the goal of treat-to-target for RA and other rheumatologic conditions.”
* This story was updated on 2/10/2017.
On Twitter @legal_med
By the time Christine Peoples, MD, examined the patient, the middle-aged man had been treated inadequately for psoriatic arthritis for years.
Poor mobility made it difficult for him to travel and gaps in his care had exacerbated his condition. But a bidirectional video system at the University of Pittsburgh Medical Center (UPMC) allowed Dr. Peoples to assess the patient, correct his therapeutic regimen, and establish consistent follow up.
Without telemedicine, the patient would have likely continued to deteriorate, said Dr. Peoples, a rheumatologist with the UPMC Teleconsult Centers.
Across the country, more rheumatology practices are employing telemedicine to treat patients, particularly physicians at academic medicine centers that have the resources to launch comprehensive units. The technology is a way to bridge the shortage of rheumatologists in rural areas and reach patients who cannot access specialty care for rheumatic conditions, proponents say. A 2013 study in Arthritis & Rheumatism found that many towns and small cities with populations up to 50,000 have no practicing rheumatologists, with some patients having to travel more than 200 miles to get specialty care (2013 Dec;65[12]:3017-25).
“Telemedicine is expanding both in terms of the number of consultations and the breadth of services involved,” he said. “Tele-rheumatology was certainly not a big area to be looked at in years past, but it’s one more area that is starting to expand.”
Research is lacking regarding how prevalent tele-rheumatology has become in the United States and whether it’s as effective as face-to-face visits. In a recent analysis of 1,468 potentially eligible tele-rheumatology studies and literature, only 20 addressed direct provider to patient contact that influenced or had the potential to influence clinical care, according to a November 2016 review in Arthritis Care & Research (doi: 10.1002/acr.23153). Of the 20 studies, the majority of articles involved tele-rheumatology use in Europe or Great Britain, said study author John Allen McDougall, MD, a postdoctoral fellow at Yale University, New Haven, Conn. According to the literature, the most common condition treated through telemedicine was rheumatoid arthritis. Little information existed on telemedicine use for gout or the treatment of connective tissue diseases, Dr. McDougall said.
Increasing access, saving time
UPMC has operated a tele-rheumatology program for nearly 3 years that connects rural patients with their specialists. Patients present for video examination at teleconsult centers located in underserved areas within western and central Pennsylvania. On the other end of the screen, Dr. Peoples uses high-level audio and video technology to discuss the patient’s history, direct nurses, make diagnoses, answer questions, and prescribe treatment to patients.
“Patients comment about how much easier it is for them and what a difference this makes in their health care,” Dr. Peoples said. “[Tele-rheumatology also increases] the ability to have regular follow-up care. I see them every few months for their condition and manage it. You can really establish a long relationship with patients just as you would if you saw patients in person.”
Rheumatologists at Dartmouth–Hitchcock Medical Center (DHMC) in Lebanon, N.H., are achieving similar results.
The medical center’s tele-rheumatology unit began in 2011 with the aim of increasing specialty access for patients in northern New Hampshire. Dartmouth also employed a telemedicine clinic from 2012 to 2013 in southern Vermont while seeking to replace a well-established rheumatologist who had retired.
Between October 2011 and December 2014, 176 patients were seen via tele-rheumatology between the two sites over the course of 244 tele-rheumatology patient visits with Dartmouth providers, according to a study published in the June 2016 Seminars in Arthritis & Rheumatism (2016 Dec;46[3]:380-5). Patients lived on average 99 miles from DHMC and 22 miles from the remote clinic site, the study found. The top diagnosis was inflammatory arthritis.
For most of doctors and patients involved in the study, tele-rheumatology visits were positive and aided patient access, said Daniel Albert, MD, section chief of rheumatology at DHMC.
“There are some limitations, there’s no question about that,” he said. ... [However], right now there are way too few rheumatologists to care for patients with rheumatic disease. We’ve tried to improve that with [nurse practitioners and physician assistants], but this is another way of addressing that need.”
Pediatric rheumatologists are also using the benefits of telemedicine. Children’s Mercy in Kansas City, Mo., launched a pediatric rheumatology telemedicine clinic in 2014 that links children in Joplin, Mo., with rheumatologists at Children’s Mercy, about 160 miles away. Patients and their parents/guardians treated via telemedicine at the Joplin site missed less work and school and saved money on food, compared with traveling to Kansas City for treatment, according to a 2016 study published in Pediatric Rheumatology (doi: 10.1186/s12969-016-0116-2).
“This is much more convenient for patients,” said Elizabeth A. Kessler, MD, a pediatric rheumatologist at Children’s Mercy and coauthor of the study. “It saves them time and money. With the training of the tele-facilitator, I believe that we’re able to provide them with equivalent care compared with if they were in-person.”
Is tele-rheumatology right for all settings?
But tele-rheumatology may not be appropriate in all practice settings or the right method for all rheumatology patients, doctors say.
As the Dartmouth-Hitchcock study noted, patients with complex diseases or unclear underlying conditions do not make good telemedicine patients. In addition, opinions are mixed about whether physicians and patients should have an established relationship before telemedicine visits occur and what such visits should entail.
Anchorage, Alaska-based rheumatologist Elizabeth Ferucci, MD, uses telemedicine only for follow up. Like many rheumatologists in Alaska, she travels to field clinics in larger communities throughout the state twice a year to treat patients, said Dr. Ferucci, a rheumatologist for the Alaska Native Tribal Health Consortium. Because some patients need to be seen more than twice a year, Dr. Ferucci conducts follow-up visits every 2-3 months via video.
However, Dr. Ferucci notes that having a trained telemedicine presenter capable of performing a joint examination in the room with the patient is not always possible because of high staff turnover and the more than 200 villages served. A nurse or staff member remains in the room with the patient, but they are not always trained to perform joint exams.
At Dartmouth-Hitchcock, Dr. Albert’s practice accepts both initial and follow-up patient visits. He acknowledges that tele-rheumatology is best used for follow-up care, but explains that limiting the center’s telemedicine use would leave some patients without treatment.
“Because the constraints of getting to Dartmouth are so great, we don’t want to impose that restriction on patients,” Dr. Albert said. “However, in general, it is preferable to do an in-person consultation first to clarify the physical findings.”
Dartmouth has used both nurses and medical assistants as presenters during tele-rheumatology after developing a web-based video series to train presenters. Nurses at UPMC are also trained as telemedicine presenters, and telemedicine visits are only used for follow-up care, Dr. Peoples said.
At Children’s Mercy in Kansas City, telemedicine visits are restricted to follow-up care and include a nurse facilitator at the virtual site. However, the type of conditions and stages of disease examined through the technology has grown since the telemedicine services started, Dr. Kessler said.
“What we feel comfortable seeing has expanded since the time we initially started the clinic because some of the patients, when they were having flares, could not come to Kansas City for financial reasons,” she said, adding that she has since examined children with lupus, vasculitis, and active arthritis through telemedicine.
She stresses that in-person visits should depend on the patient, the circumstances, and the physician’s comfort level. ”You need to be realistic,” she said. “There are times when I’ve said to families, ‘I would like you to come to Kansas City because I’m a little unsure about your exam findings.’ There are definitely some patients who do more of a hybrid approach where they come to Kansas City and are also seen by telemedicine.”
Barriers slow telemedicine progress
Amid its benefits, challenges for tele-rheumatology, such as reimbursement and licensure obstacles, remain.
Commercial insurers pay for telemedicine at varying rates, making it difficult for doctors to consistently be paid, Dr. Peoples said.
“A lot of insurance companies do cover telemedicine visits, but still, a lot don’t,” she said. “I do think that in order to remain competitive, insurance companies overall are going to have to start covering these services.”
At least 32 states and the District of Columbia have parity laws that require commercial health insurers to cover services provided through telehealth to the same extent as those services are covered in person, according to an Aug. 15, 2016 Health Affairs policy brief. However, how private insurers pay and what services they cover vary widely.
Medicaid reimbursement for telehealth depends on individual state Medicaid programs. Only 9 states Medicaid programs reimburse for store-and-forward services, while at least 16 states have some sort of Medicaid reimbursement for remote patient monitoring, according to the Health Affairs report.
Medicare payment for telehealth is clouded in restrictions. The Centers for Medicare & Medicaid Services reimburses for synchronous communications only and does not cover store-and-forward services or remote patient monitoring for chronic diseases, except in Alaska and Hawaii. The patient must be present at an originating site for the visit and cannot be home to receive the services. In addition, CMS reimburses for telehealth only when the originating site is in a Health Professional Shortage Area or within a county outside a Metropolitan Statistical Area. In 2016, CMS expanded its coverage of telehealth to include several new codes including, end-stage renal disease–related services for dialysis, advance care planning services, and critical care consultations furnished via telehealth using new Medicare G-codes.
It’s worth noting that rheumatologists who are salaried employees of academic medical centers, as is the case for all rheumatologists interviewed here, don’t handle the billing for their telemedicine services, and so have not experienced the payment challenges that smaller practices may face.
Mr. Linkous of the American Telemedicine Association said that he believes that reimbursement for telemedicine will improve as health care heads toward value-based payment systems.
“In terms of reimbursement, with the move away from fee-based systems to value-based systems, it really opens the door because for many of the services, you no longer write out a bill with a code associated with it,” he said. “You are paid on the basis of keeping people well. That’s a great positive incentive for using telemedicine.”
Differing state licensure requirements also pose a challenge. The process to obtain licenses and navigate varying credentialing processes can be a headache for health providers and delay approval.
The barrier has led to model legislation by the Federation of State Medical Boards (FSMB) that aims to make it easier for telemedicine physicians to gain licenses in multiple states. Under the Interstate Medical Licensure Compact, physicians designate a member state as the state of principal licensure and select the other states in which they want to license. The state of principal licensure then verifies the physician’s eligibility and provides credential information to the interstate commission, which collects applicable fees and transmits the doctor’s information to the other states. Upon receipt in the additional states, the physician would be granted a license.
As of January, 18 states had enacted the compact legislation, and at least 4 states had introduced the legislation. In 2015, the Health Resources and Services Administration awarded the FSMB a grant to support establishment of the commission and aid with the compact’s infrastructure.
A new normal?
Dr. Albert notes that tele-rheumatology programs can succeed only with adequate resources, time, and staff.
“To set up Skype or Facetime is nothing; you just click on it,” he said. “To set up tele-rheumatology requires a fair amount of administrative substructure that has to be generated in terms of who’s going to do the documentation? Who is going to do the billing? Who is going to do the credentialing? Where is the credentialing going to be validated?”
The equipment required for telemedicine visits also requires a significant amount of time and regular maintenance, Dr. Peoples said. At UPMC for instance, the tele-rheumatology program has an information technology team that assists with technology glitches, changes, and updates, she said. That’s why it’s helpful to have the support of a large academic medical center with the sufficient resources to build a telemedicine program, she added.
In order for more practices to consider tele-rheumatology, more research about cost-effectiveness and best uses of the technology use would be useful, Dr. McDougall said.
“The main question that policy makers are going to want to answer is, ‘What’s the return on investment? Does this make sense for my practice?’ ” he said. “The methods reporting in tele-rheumatologist [literature] is lacking.” But regardless of barriers, telemedicine experts say the technology will likely continue to expand and transform the way rheumatologists are practicing and patients are receiving care.
“Tele-rheumatology will never replace an in-person exam,” Dr. Ferucci said. “But my vision is that it will be able to improve the quality of care for patients living in rural and remote locations, by allowing for more frequent visits and adjustment of medications, which are necessary to achieve the goal of treat-to-target for RA and other rheumatologic conditions.”
* This story was updated on 2/10/2017.
On Twitter @legal_med
By the time Christine Peoples, MD, examined the patient, the middle-aged man had been treated inadequately for psoriatic arthritis for years.
Poor mobility made it difficult for him to travel and gaps in his care had exacerbated his condition. But a bidirectional video system at the University of Pittsburgh Medical Center (UPMC) allowed Dr. Peoples to assess the patient, correct his therapeutic regimen, and establish consistent follow up.
Without telemedicine, the patient would have likely continued to deteriorate, said Dr. Peoples, a rheumatologist with the UPMC Teleconsult Centers.
Across the country, more rheumatology practices are employing telemedicine to treat patients, particularly physicians at academic medicine centers that have the resources to launch comprehensive units. The technology is a way to bridge the shortage of rheumatologists in rural areas and reach patients who cannot access specialty care for rheumatic conditions, proponents say. A 2013 study in Arthritis & Rheumatism found that many towns and small cities with populations up to 50,000 have no practicing rheumatologists, with some patients having to travel more than 200 miles to get specialty care (2013 Dec;65[12]:3017-25).
“Telemedicine is expanding both in terms of the number of consultations and the breadth of services involved,” he said. “Tele-rheumatology was certainly not a big area to be looked at in years past, but it’s one more area that is starting to expand.”
Research is lacking regarding how prevalent tele-rheumatology has become in the United States and whether it’s as effective as face-to-face visits. In a recent analysis of 1,468 potentially eligible tele-rheumatology studies and literature, only 20 addressed direct provider to patient contact that influenced or had the potential to influence clinical care, according to a November 2016 review in Arthritis Care & Research (doi: 10.1002/acr.23153). Of the 20 studies, the majority of articles involved tele-rheumatology use in Europe or Great Britain, said study author John Allen McDougall, MD, a postdoctoral fellow at Yale University, New Haven, Conn. According to the literature, the most common condition treated through telemedicine was rheumatoid arthritis. Little information existed on telemedicine use for gout or the treatment of connective tissue diseases, Dr. McDougall said.
Increasing access, saving time
UPMC has operated a tele-rheumatology program for nearly 3 years that connects rural patients with their specialists. Patients present for video examination at teleconsult centers located in underserved areas within western and central Pennsylvania. On the other end of the screen, Dr. Peoples uses high-level audio and video technology to discuss the patient’s history, direct nurses, make diagnoses, answer questions, and prescribe treatment to patients.
“Patients comment about how much easier it is for them and what a difference this makes in their health care,” Dr. Peoples said. “[Tele-rheumatology also increases] the ability to have regular follow-up care. I see them every few months for their condition and manage it. You can really establish a long relationship with patients just as you would if you saw patients in person.”
Rheumatologists at Dartmouth–Hitchcock Medical Center (DHMC) in Lebanon, N.H., are achieving similar results.
The medical center’s tele-rheumatology unit began in 2011 with the aim of increasing specialty access for patients in northern New Hampshire. Dartmouth also employed a telemedicine clinic from 2012 to 2013 in southern Vermont while seeking to replace a well-established rheumatologist who had retired.
Between October 2011 and December 2014, 176 patients were seen via tele-rheumatology between the two sites over the course of 244 tele-rheumatology patient visits with Dartmouth providers, according to a study published in the June 2016 Seminars in Arthritis & Rheumatism (2016 Dec;46[3]:380-5). Patients lived on average 99 miles from DHMC and 22 miles from the remote clinic site, the study found. The top diagnosis was inflammatory arthritis.
For most of doctors and patients involved in the study, tele-rheumatology visits were positive and aided patient access, said Daniel Albert, MD, section chief of rheumatology at DHMC.
“There are some limitations, there’s no question about that,” he said. ... [However], right now there are way too few rheumatologists to care for patients with rheumatic disease. We’ve tried to improve that with [nurse practitioners and physician assistants], but this is another way of addressing that need.”
Pediatric rheumatologists are also using the benefits of telemedicine. Children’s Mercy in Kansas City, Mo., launched a pediatric rheumatology telemedicine clinic in 2014 that links children in Joplin, Mo., with rheumatologists at Children’s Mercy, about 160 miles away. Patients and their parents/guardians treated via telemedicine at the Joplin site missed less work and school and saved money on food, compared with traveling to Kansas City for treatment, according to a 2016 study published in Pediatric Rheumatology (doi: 10.1186/s12969-016-0116-2).
“This is much more convenient for patients,” said Elizabeth A. Kessler, MD, a pediatric rheumatologist at Children’s Mercy and coauthor of the study. “It saves them time and money. With the training of the tele-facilitator, I believe that we’re able to provide them with equivalent care compared with if they were in-person.”
Is tele-rheumatology right for all settings?
But tele-rheumatology may not be appropriate in all practice settings or the right method for all rheumatology patients, doctors say.
As the Dartmouth-Hitchcock study noted, patients with complex diseases or unclear underlying conditions do not make good telemedicine patients. In addition, opinions are mixed about whether physicians and patients should have an established relationship before telemedicine visits occur and what such visits should entail.
Anchorage, Alaska-based rheumatologist Elizabeth Ferucci, MD, uses telemedicine only for follow up. Like many rheumatologists in Alaska, she travels to field clinics in larger communities throughout the state twice a year to treat patients, said Dr. Ferucci, a rheumatologist for the Alaska Native Tribal Health Consortium. Because some patients need to be seen more than twice a year, Dr. Ferucci conducts follow-up visits every 2-3 months via video.
However, Dr. Ferucci notes that having a trained telemedicine presenter capable of performing a joint examination in the room with the patient is not always possible because of high staff turnover and the more than 200 villages served. A nurse or staff member remains in the room with the patient, but they are not always trained to perform joint exams.
At Dartmouth-Hitchcock, Dr. Albert’s practice accepts both initial and follow-up patient visits. He acknowledges that tele-rheumatology is best used for follow-up care, but explains that limiting the center’s telemedicine use would leave some patients without treatment.
“Because the constraints of getting to Dartmouth are so great, we don’t want to impose that restriction on patients,” Dr. Albert said. “However, in general, it is preferable to do an in-person consultation first to clarify the physical findings.”
Dartmouth has used both nurses and medical assistants as presenters during tele-rheumatology after developing a web-based video series to train presenters. Nurses at UPMC are also trained as telemedicine presenters, and telemedicine visits are only used for follow-up care, Dr. Peoples said.
At Children’s Mercy in Kansas City, telemedicine visits are restricted to follow-up care and include a nurse facilitator at the virtual site. However, the type of conditions and stages of disease examined through the technology has grown since the telemedicine services started, Dr. Kessler said.
“What we feel comfortable seeing has expanded since the time we initially started the clinic because some of the patients, when they were having flares, could not come to Kansas City for financial reasons,” she said, adding that she has since examined children with lupus, vasculitis, and active arthritis through telemedicine.
She stresses that in-person visits should depend on the patient, the circumstances, and the physician’s comfort level. ”You need to be realistic,” she said. “There are times when I’ve said to families, ‘I would like you to come to Kansas City because I’m a little unsure about your exam findings.’ There are definitely some patients who do more of a hybrid approach where they come to Kansas City and are also seen by telemedicine.”
Barriers slow telemedicine progress
Amid its benefits, challenges for tele-rheumatology, such as reimbursement and licensure obstacles, remain.
Commercial insurers pay for telemedicine at varying rates, making it difficult for doctors to consistently be paid, Dr. Peoples said.
“A lot of insurance companies do cover telemedicine visits, but still, a lot don’t,” she said. “I do think that in order to remain competitive, insurance companies overall are going to have to start covering these services.”
At least 32 states and the District of Columbia have parity laws that require commercial health insurers to cover services provided through telehealth to the same extent as those services are covered in person, according to an Aug. 15, 2016 Health Affairs policy brief. However, how private insurers pay and what services they cover vary widely.
Medicaid reimbursement for telehealth depends on individual state Medicaid programs. Only 9 states Medicaid programs reimburse for store-and-forward services, while at least 16 states have some sort of Medicaid reimbursement for remote patient monitoring, according to the Health Affairs report.
Medicare payment for telehealth is clouded in restrictions. The Centers for Medicare & Medicaid Services reimburses for synchronous communications only and does not cover store-and-forward services or remote patient monitoring for chronic diseases, except in Alaska and Hawaii. The patient must be present at an originating site for the visit and cannot be home to receive the services. In addition, CMS reimburses for telehealth only when the originating site is in a Health Professional Shortage Area or within a county outside a Metropolitan Statistical Area. In 2016, CMS expanded its coverage of telehealth to include several new codes including, end-stage renal disease–related services for dialysis, advance care planning services, and critical care consultations furnished via telehealth using new Medicare G-codes.
It’s worth noting that rheumatologists who are salaried employees of academic medical centers, as is the case for all rheumatologists interviewed here, don’t handle the billing for their telemedicine services, and so have not experienced the payment challenges that smaller practices may face.
Mr. Linkous of the American Telemedicine Association said that he believes that reimbursement for telemedicine will improve as health care heads toward value-based payment systems.
“In terms of reimbursement, with the move away from fee-based systems to value-based systems, it really opens the door because for many of the services, you no longer write out a bill with a code associated with it,” he said. “You are paid on the basis of keeping people well. That’s a great positive incentive for using telemedicine.”
Differing state licensure requirements also pose a challenge. The process to obtain licenses and navigate varying credentialing processes can be a headache for health providers and delay approval.
The barrier has led to model legislation by the Federation of State Medical Boards (FSMB) that aims to make it easier for telemedicine physicians to gain licenses in multiple states. Under the Interstate Medical Licensure Compact, physicians designate a member state as the state of principal licensure and select the other states in which they want to license. The state of principal licensure then verifies the physician’s eligibility and provides credential information to the interstate commission, which collects applicable fees and transmits the doctor’s information to the other states. Upon receipt in the additional states, the physician would be granted a license.
As of January, 18 states had enacted the compact legislation, and at least 4 states had introduced the legislation. In 2015, the Health Resources and Services Administration awarded the FSMB a grant to support establishment of the commission and aid with the compact’s infrastructure.
A new normal?
Dr. Albert notes that tele-rheumatology programs can succeed only with adequate resources, time, and staff.
“To set up Skype or Facetime is nothing; you just click on it,” he said. “To set up tele-rheumatology requires a fair amount of administrative substructure that has to be generated in terms of who’s going to do the documentation? Who is going to do the billing? Who is going to do the credentialing? Where is the credentialing going to be validated?”
The equipment required for telemedicine visits also requires a significant amount of time and regular maintenance, Dr. Peoples said. At UPMC for instance, the tele-rheumatology program has an information technology team that assists with technology glitches, changes, and updates, she said. That’s why it’s helpful to have the support of a large academic medical center with the sufficient resources to build a telemedicine program, she added.
In order for more practices to consider tele-rheumatology, more research about cost-effectiveness and best uses of the technology use would be useful, Dr. McDougall said.
“The main question that policy makers are going to want to answer is, ‘What’s the return on investment? Does this make sense for my practice?’ ” he said. “The methods reporting in tele-rheumatologist [literature] is lacking.” But regardless of barriers, telemedicine experts say the technology will likely continue to expand and transform the way rheumatologists are practicing and patients are receiving care.
“Tele-rheumatology will never replace an in-person exam,” Dr. Ferucci said. “But my vision is that it will be able to improve the quality of care for patients living in rural and remote locations, by allowing for more frequent visits and adjustment of medications, which are necessary to achieve the goal of treat-to-target for RA and other rheumatologic conditions.”
* This story was updated on 2/10/2017.
On Twitter @legal_med
Travel ban affects international medical meetings
President Trump’s executive order blocking travelers from seven Muslim-majority countries from entering the United States has landed a damaging blow to global cooperation in scientific research and could impede assemblies of the world’s top medical experts.
“We are offering assistance as needed, and as each unique situation requires,” IAS-USA executive director and president Donna M. Jacobsen said in an interview. “Apart from that, we are establishing a list of individuals who may have contacts at the U.S. embassies, and will seek assistance from them as needed. Also, we are providing a letter documenting the individual as having been vetted as a legitimate scientist or researcher in the field, and having been approved to attend CROI.”
“We are monitoring the situation closely, however, and will offer whatever assistance we can to conference attendees, regardless of their point of origin, to ensure the free exchange of scientific information,” she said.
The travel ban could have detrimental effects on future collaboration between U.S. and international scientists and ultimately endanger the health and well-being of patients, Ms. Jacobsen said.
“Beyond the immediate possible impact on individuals intending to attend CROI who might be directly affected by the ban, there is serious reason for concern that the policy will dissuade other scientists and researchers from traveling to the U.S. in the future overall and sharing their work with colleagues here,” she said in an interview. “Such a response to the U.S. action would needlessly damage our global leadership in science and medicine.”
Thousands of academics from around the world, including physicians, researchers, and professors, have already vowed to boycott U.S.-based conferences until the travel ban is lifted. A Google Docs petition started shortly after the ban was announced has garnered more than 5,000 signatures by professionals acting in solidarity with those affected by the travel restrictions. It’s unclear who started the petition.
The academicians who signed the petition say they will not attend international conferences in the United States until those restricted from participating can rejoin their colleagues and freely share their ideas, said Charles R. Rogers, PhD, of the University of Minnesota, Minneapolis.
President Trump’s executive order, signed Jan. 27, bars refugees from entering the country for 120 days and bans immigrants from seven predominantly Muslim nations out for 3 months. The countries include Iran, Iraq, Syria, Sudan, Libya, Yemen and Somalia.
In a Feb. 1 press conference, White House press secretary Sean Spicer said the president’s intent is to ensure national security and prevent terrorists from entering the country.
“The president has been very clear that his No. 1 goal is not to target any one religion but places and areas where we believe that there is an issue,” Mr. Spicer said. “The president’s No. 1 goal has always been to focus on the safety of America, not the religion. He understands that it’s not a religious problem, it’s a radicalization problem. There’s a big difference between Islam, the religion, and radical Islamic terrorists that come here to seek to do us harm.”
[email protected]
On Twitter @legal_med
President Trump’s executive order blocking travelers from seven Muslim-majority countries from entering the United States has landed a damaging blow to global cooperation in scientific research and could impede assemblies of the world’s top medical experts.
“We are offering assistance as needed, and as each unique situation requires,” IAS-USA executive director and president Donna M. Jacobsen said in an interview. “Apart from that, we are establishing a list of individuals who may have contacts at the U.S. embassies, and will seek assistance from them as needed. Also, we are providing a letter documenting the individual as having been vetted as a legitimate scientist or researcher in the field, and having been approved to attend CROI.”
“We are monitoring the situation closely, however, and will offer whatever assistance we can to conference attendees, regardless of their point of origin, to ensure the free exchange of scientific information,” she said.
The travel ban could have detrimental effects on future collaboration between U.S. and international scientists and ultimately endanger the health and well-being of patients, Ms. Jacobsen said.
“Beyond the immediate possible impact on individuals intending to attend CROI who might be directly affected by the ban, there is serious reason for concern that the policy will dissuade other scientists and researchers from traveling to the U.S. in the future overall and sharing their work with colleagues here,” she said in an interview. “Such a response to the U.S. action would needlessly damage our global leadership in science and medicine.”
Thousands of academics from around the world, including physicians, researchers, and professors, have already vowed to boycott U.S.-based conferences until the travel ban is lifted. A Google Docs petition started shortly after the ban was announced has garnered more than 5,000 signatures by professionals acting in solidarity with those affected by the travel restrictions. It’s unclear who started the petition.
The academicians who signed the petition say they will not attend international conferences in the United States until those restricted from participating can rejoin their colleagues and freely share their ideas, said Charles R. Rogers, PhD, of the University of Minnesota, Minneapolis.
President Trump’s executive order, signed Jan. 27, bars refugees from entering the country for 120 days and bans immigrants from seven predominantly Muslim nations out for 3 months. The countries include Iran, Iraq, Syria, Sudan, Libya, Yemen and Somalia.
In a Feb. 1 press conference, White House press secretary Sean Spicer said the president’s intent is to ensure national security and prevent terrorists from entering the country.
“The president has been very clear that his No. 1 goal is not to target any one religion but places and areas where we believe that there is an issue,” Mr. Spicer said. “The president’s No. 1 goal has always been to focus on the safety of America, not the religion. He understands that it’s not a religious problem, it’s a radicalization problem. There’s a big difference between Islam, the religion, and radical Islamic terrorists that come here to seek to do us harm.”
[email protected]
On Twitter @legal_med
President Trump’s executive order blocking travelers from seven Muslim-majority countries from entering the United States has landed a damaging blow to global cooperation in scientific research and could impede assemblies of the world’s top medical experts.
“We are offering assistance as needed, and as each unique situation requires,” IAS-USA executive director and president Donna M. Jacobsen said in an interview. “Apart from that, we are establishing a list of individuals who may have contacts at the U.S. embassies, and will seek assistance from them as needed. Also, we are providing a letter documenting the individual as having been vetted as a legitimate scientist or researcher in the field, and having been approved to attend CROI.”
“We are monitoring the situation closely, however, and will offer whatever assistance we can to conference attendees, regardless of their point of origin, to ensure the free exchange of scientific information,” she said.
The travel ban could have detrimental effects on future collaboration between U.S. and international scientists and ultimately endanger the health and well-being of patients, Ms. Jacobsen said.
“Beyond the immediate possible impact on individuals intending to attend CROI who might be directly affected by the ban, there is serious reason for concern that the policy will dissuade other scientists and researchers from traveling to the U.S. in the future overall and sharing their work with colleagues here,” she said in an interview. “Such a response to the U.S. action would needlessly damage our global leadership in science and medicine.”
Thousands of academics from around the world, including physicians, researchers, and professors, have already vowed to boycott U.S.-based conferences until the travel ban is lifted. A Google Docs petition started shortly after the ban was announced has garnered more than 5,000 signatures by professionals acting in solidarity with those affected by the travel restrictions. It’s unclear who started the petition.
The academicians who signed the petition say they will not attend international conferences in the United States until those restricted from participating can rejoin their colleagues and freely share their ideas, said Charles R. Rogers, PhD, of the University of Minnesota, Minneapolis.
President Trump’s executive order, signed Jan. 27, bars refugees from entering the country for 120 days and bans immigrants from seven predominantly Muslim nations out for 3 months. The countries include Iran, Iraq, Syria, Sudan, Libya, Yemen and Somalia.
In a Feb. 1 press conference, White House press secretary Sean Spicer said the president’s intent is to ensure national security and prevent terrorists from entering the country.
“The president has been very clear that his No. 1 goal is not to target any one religion but places and areas where we believe that there is an issue,” Mr. Spicer said. “The president’s No. 1 goal has always been to focus on the safety of America, not the religion. He understands that it’s not a religious problem, it’s a radicalization problem. There’s a big difference between Islam, the religion, and radical Islamic terrorists that come here to seek to do us harm.”
[email protected]
On Twitter @legal_med