User login
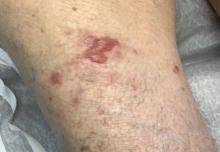
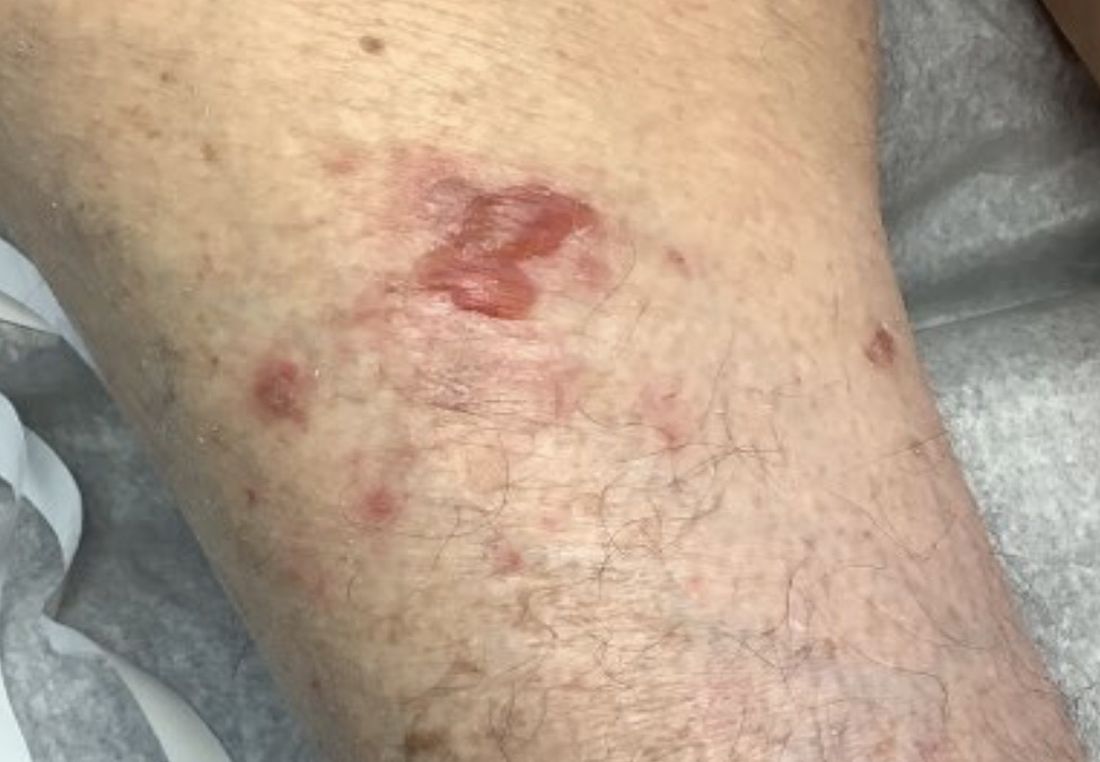
A 95-year-old White male with hypertension presented with itchy patches and bullae on the trunk and extremities
and is associated with various predisposing factors, including HLA genes, comorbidities, aging, and trigger factors such as drugs, trauma, radiation, chemotherapy, and infections. The autoimmune reaction is mediated by a dysregulation of T cells in which IgG and IgE autoantibodies form against hemidesmosomal proteins (BP180 and BP230). These autoantibodies induce neutrophil activation, recruitment, and degradation in the basement membrane of the skin.
Typically, patients present with intense pruritus followed by an urticarial or eczematous eruption. Tense blisters and bullae occur commonly on the trunk and extremities. Drug-associated bullous pemphigoid (DABP) is a common manifestation of the disease with histologic and immunologic features similar to those of the idiopathic version. Eruptions can be triggered by systemic or topical medications, and incidence of these reactions may be related to a genetic predisposition for the disease.
Some research suggests that drug-induced changes to the antigenic properties of the epidermal basement membrane result in an augmented immune response, while others point to structural modification in these zones that stimulate the immune system. Thiol- and phenol-based drugs have been largely implicated in the development of DABP because they are capable of structural modification and disruption of the dermo-epidermal junction in the basement membrane.
DABP often presents with patients taking multiple medications. Some of the most common medications are gliptins, PD-1 inhibitors, diuretics, antibiotics, anti-inflammatory drugs, and ACE-inhibitors, and other cardiovascular drugs. DABP may present with mucosal eruptions unlike its idiopathic counterpart that is mostly contained to the skin.
On this patient, two punch biopsies were taken. Histopathology revealed an eosinophil-rich subepidermal blister with a smooth epidermal undersurface consistent with bullous pemphigoid. Direct immunofluorescence was positive with a deposition of IgG and C3 at the epidermal side of salt split basement membrane zone.
Treatment for BP includes high potency topical and systemic steroids. Tetracyclines and niacinamide have been reported to improve the condition. Treatment is tailored to allow for cutaneous healing and control pruritus, but the physician must be mindful of the patient’s comorbidities and capacity for self-care. Prognosis is often better for DABP as withdrawal of the medication greatly accelerates clearance of the lesions. Worse prognosis is related to increased number of comorbidities and older age. Our patient’s BP is controlled currently with topical steroids and oral doxycycline.
This case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University College of Osteopathic Medicine, Tampa, and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Miyamoto D et al. An Bras Dermatol. 2019 Mar-Apr;94(2):133-46.
2. Moro et al. Biomolecules. 2020 Oct 10;10(10):1432.
3. Verheyden M et al. Acta Derm Venereol. 2020 Aug 17;100(15):adv00224.
and is associated with various predisposing factors, including HLA genes, comorbidities, aging, and trigger factors such as drugs, trauma, radiation, chemotherapy, and infections. The autoimmune reaction is mediated by a dysregulation of T cells in which IgG and IgE autoantibodies form against hemidesmosomal proteins (BP180 and BP230). These autoantibodies induce neutrophil activation, recruitment, and degradation in the basement membrane of the skin.
Typically, patients present with intense pruritus followed by an urticarial or eczematous eruption. Tense blisters and bullae occur commonly on the trunk and extremities. Drug-associated bullous pemphigoid (DABP) is a common manifestation of the disease with histologic and immunologic features similar to those of the idiopathic version. Eruptions can be triggered by systemic or topical medications, and incidence of these reactions may be related to a genetic predisposition for the disease.
Some research suggests that drug-induced changes to the antigenic properties of the epidermal basement membrane result in an augmented immune response, while others point to structural modification in these zones that stimulate the immune system. Thiol- and phenol-based drugs have been largely implicated in the development of DABP because they are capable of structural modification and disruption of the dermo-epidermal junction in the basement membrane.
DABP often presents with patients taking multiple medications. Some of the most common medications are gliptins, PD-1 inhibitors, diuretics, antibiotics, anti-inflammatory drugs, and ACE-inhibitors, and other cardiovascular drugs. DABP may present with mucosal eruptions unlike its idiopathic counterpart that is mostly contained to the skin.
On this patient, two punch biopsies were taken. Histopathology revealed an eosinophil-rich subepidermal blister with a smooth epidermal undersurface consistent with bullous pemphigoid. Direct immunofluorescence was positive with a deposition of IgG and C3 at the epidermal side of salt split basement membrane zone.
Treatment for BP includes high potency topical and systemic steroids. Tetracyclines and niacinamide have been reported to improve the condition. Treatment is tailored to allow for cutaneous healing and control pruritus, but the physician must be mindful of the patient’s comorbidities and capacity for self-care. Prognosis is often better for DABP as withdrawal of the medication greatly accelerates clearance of the lesions. Worse prognosis is related to increased number of comorbidities and older age. Our patient’s BP is controlled currently with topical steroids and oral doxycycline.
This case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University College of Osteopathic Medicine, Tampa, and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Miyamoto D et al. An Bras Dermatol. 2019 Mar-Apr;94(2):133-46.
2. Moro et al. Biomolecules. 2020 Oct 10;10(10):1432.
3. Verheyden M et al. Acta Derm Venereol. 2020 Aug 17;100(15):adv00224.
and is associated with various predisposing factors, including HLA genes, comorbidities, aging, and trigger factors such as drugs, trauma, radiation, chemotherapy, and infections. The autoimmune reaction is mediated by a dysregulation of T cells in which IgG and IgE autoantibodies form against hemidesmosomal proteins (BP180 and BP230). These autoantibodies induce neutrophil activation, recruitment, and degradation in the basement membrane of the skin.
Typically, patients present with intense pruritus followed by an urticarial or eczematous eruption. Tense blisters and bullae occur commonly on the trunk and extremities. Drug-associated bullous pemphigoid (DABP) is a common manifestation of the disease with histologic and immunologic features similar to those of the idiopathic version. Eruptions can be triggered by systemic or topical medications, and incidence of these reactions may be related to a genetic predisposition for the disease.
Some research suggests that drug-induced changes to the antigenic properties of the epidermal basement membrane result in an augmented immune response, while others point to structural modification in these zones that stimulate the immune system. Thiol- and phenol-based drugs have been largely implicated in the development of DABP because they are capable of structural modification and disruption of the dermo-epidermal junction in the basement membrane.
DABP often presents with patients taking multiple medications. Some of the most common medications are gliptins, PD-1 inhibitors, diuretics, antibiotics, anti-inflammatory drugs, and ACE-inhibitors, and other cardiovascular drugs. DABP may present with mucosal eruptions unlike its idiopathic counterpart that is mostly contained to the skin.
On this patient, two punch biopsies were taken. Histopathology revealed an eosinophil-rich subepidermal blister with a smooth epidermal undersurface consistent with bullous pemphigoid. Direct immunofluorescence was positive with a deposition of IgG and C3 at the epidermal side of salt split basement membrane zone.
Treatment for BP includes high potency topical and systemic steroids. Tetracyclines and niacinamide have been reported to improve the condition. Treatment is tailored to allow for cutaneous healing and control pruritus, but the physician must be mindful of the patient’s comorbidities and capacity for self-care. Prognosis is often better for DABP as withdrawal of the medication greatly accelerates clearance of the lesions. Worse prognosis is related to increased number of comorbidities and older age. Our patient’s BP is controlled currently with topical steroids and oral doxycycline.
This case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University College of Osteopathic Medicine, Tampa, and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Miyamoto D et al. An Bras Dermatol. 2019 Mar-Apr;94(2):133-46.
2. Moro et al. Biomolecules. 2020 Oct 10;10(10):1432.
3. Verheyden M et al. Acta Derm Venereol. 2020 Aug 17;100(15):adv00224.
Four methods to chip away at imposter syndrome
Regardless of the setting, one of the most frequently discussed topics in health care is imposter syndrome.
Imposter syndrome was first defined by Clance and Imes as an inability to internalize success, and the tendency to attribute success to external causes such as luck, error, or knowing the appropriate individual.1 This definition is essential because most health care professionals have had a sense of doubt or questioned the full extent of their competencies in various situations. I would argue that this is normal and – within reason – helpful to the practice of medicine. The problem with true imposter syndrome is that the individual does not incorporate success in a way that builds healthy self-esteem and self-efficacy.2
Imposter syndrome has a very nasty way of interacting with burnout. Studies have shown that imposter syndrome can be associated with high levels of emotional exhaustion at work.3 In my experience, this makes clinical sense. Professionals suffering from imposter syndrome can spend a great deal of time and energy trying to maintain a particular image.4 They are acting a part 24/7. Have you ever seriously tried to act? It’s arduous work. A friend once asked me to read a role for a play because “you’d be great; you’re a natural.” By the time I was done with rehearsal, I felt like I had run a 4-by-400-meter relay, by myself, in Victoria, Tex.
And any talk of imposter syndrome must include its running mate, perfectionism. These two conditions exist together so commonly it can be a bit of a chicken or egg question as to which came first.
Imposter syndrome, perfectionism, and burnout can form a deadly triad if not recognized and addressed quickly. In medicine, perfectionism can be a coping strategy that sets up unrelenting standards. Failure to meet unrelenting standards then serves as fuel and validation for imposter syndrome and emotional exhaustion. The consequences of this cycle going unchecked over a health care professional’s career are seismic and can include downstream effects ranging from depression to suicide.
Some readers will relate to this, while others will shrug their shoulders and say that this has never happened in their professional life. I get it. However, I would now ask if you have ever felt like an imposter in your personal life. I’ll make a cup of tea and wait for you to figure out precisely what is the boundary between your personal and professional life. Okay, all done? Great. Now I’ll give you some more time to sincerely reflect if any of the traits of imposter syndrome have described you at times in your personal life. Hmmm, interesting to think about, isn’t it?
I believe that health care professionals frequently use one credit card to pay off another, but the debt remains the same. So even if things are going well at work, we may have just shifted the debt to our personal lives. (At some point in the future, I’ll share my 10 greatest father fails to date to elucidate my point.)
In my work at the GW Resiliency and Well-Being Center, I’ve gravitated toward a few methods supported by evidence that help alleviate imposter syndrome symptoms and potentially serve as protective factors against the future development of imposter syndrome.4 These include but are not limited to:
- Keep a record of small personal success that is yours alone.
- Have a mentor to share failures with.
- Use personal reflection to examine what it means to successfully reach your goals and fulfill your purpose, not a relative value unit target.
- Share experiences with each other, so you know you’re not alone.
The last method is one of my favorites because it involves connecting to others and shining a light on our shared experiences and, coincidentally, our collective strengths. Once this collective strength is realized, the circumstances of that 4-by-400-meter relay change drastically. Be safe and well, everyone.
Lorenzo Norris, MD, is a psychiatrist and chief wellness officer for the George Washington University Medical Enterprise and serves as associate dean of student affairs and administration for the George Washington University School of Medicine and Health Sciences. A version of this article first appeared on Medscape.com.
References
1. Clance PR, Imes SA. The imposter phenomenon in high achieving women: Dynamics and therapeutic intervention. Psychotherapy: Theory, Research & Practice. 1978;15(3): 241-7. doi: 10.1037/h0086006.
2. Thomas M, Bigatti S. Perfectionism, impostor phenomenon, and mental health in medicine: A literature review. Int J Med Educ. 2020 Sep 28;11:201-3. doi: 10.5116/ijme.5f54.c8f8.
3. Liu RQ et al. Impostorism and anxiety contribute to burnout among resident physicians. Med Teach. 2022 Jul;44(7):758-64. doi: 10.1080/0142159X.2022.2028751.
4. Gottlieb M et al. Impostor syndrome among physicians and physicians in training: A scoping review. Med Educ. 2020 Feb;54(2):116-24. doi: 10.1111/medu.13956.
Regardless of the setting, one of the most frequently discussed topics in health care is imposter syndrome.
Imposter syndrome was first defined by Clance and Imes as an inability to internalize success, and the tendency to attribute success to external causes such as luck, error, or knowing the appropriate individual.1 This definition is essential because most health care professionals have had a sense of doubt or questioned the full extent of their competencies in various situations. I would argue that this is normal and – within reason – helpful to the practice of medicine. The problem with true imposter syndrome is that the individual does not incorporate success in a way that builds healthy self-esteem and self-efficacy.2
Imposter syndrome has a very nasty way of interacting with burnout. Studies have shown that imposter syndrome can be associated with high levels of emotional exhaustion at work.3 In my experience, this makes clinical sense. Professionals suffering from imposter syndrome can spend a great deal of time and energy trying to maintain a particular image.4 They are acting a part 24/7. Have you ever seriously tried to act? It’s arduous work. A friend once asked me to read a role for a play because “you’d be great; you’re a natural.” By the time I was done with rehearsal, I felt like I had run a 4-by-400-meter relay, by myself, in Victoria, Tex.
And any talk of imposter syndrome must include its running mate, perfectionism. These two conditions exist together so commonly it can be a bit of a chicken or egg question as to which came first.
Imposter syndrome, perfectionism, and burnout can form a deadly triad if not recognized and addressed quickly. In medicine, perfectionism can be a coping strategy that sets up unrelenting standards. Failure to meet unrelenting standards then serves as fuel and validation for imposter syndrome and emotional exhaustion. The consequences of this cycle going unchecked over a health care professional’s career are seismic and can include downstream effects ranging from depression to suicide.
Some readers will relate to this, while others will shrug their shoulders and say that this has never happened in their professional life. I get it. However, I would now ask if you have ever felt like an imposter in your personal life. I’ll make a cup of tea and wait for you to figure out precisely what is the boundary between your personal and professional life. Okay, all done? Great. Now I’ll give you some more time to sincerely reflect if any of the traits of imposter syndrome have described you at times in your personal life. Hmmm, interesting to think about, isn’t it?
I believe that health care professionals frequently use one credit card to pay off another, but the debt remains the same. So even if things are going well at work, we may have just shifted the debt to our personal lives. (At some point in the future, I’ll share my 10 greatest father fails to date to elucidate my point.)
In my work at the GW Resiliency and Well-Being Center, I’ve gravitated toward a few methods supported by evidence that help alleviate imposter syndrome symptoms and potentially serve as protective factors against the future development of imposter syndrome.4 These include but are not limited to:
- Keep a record of small personal success that is yours alone.
- Have a mentor to share failures with.
- Use personal reflection to examine what it means to successfully reach your goals and fulfill your purpose, not a relative value unit target.
- Share experiences with each other, so you know you’re not alone.
The last method is one of my favorites because it involves connecting to others and shining a light on our shared experiences and, coincidentally, our collective strengths. Once this collective strength is realized, the circumstances of that 4-by-400-meter relay change drastically. Be safe and well, everyone.
Lorenzo Norris, MD, is a psychiatrist and chief wellness officer for the George Washington University Medical Enterprise and serves as associate dean of student affairs and administration for the George Washington University School of Medicine and Health Sciences. A version of this article first appeared on Medscape.com.
References
1. Clance PR, Imes SA. The imposter phenomenon in high achieving women: Dynamics and therapeutic intervention. Psychotherapy: Theory, Research & Practice. 1978;15(3): 241-7. doi: 10.1037/h0086006.
2. Thomas M, Bigatti S. Perfectionism, impostor phenomenon, and mental health in medicine: A literature review. Int J Med Educ. 2020 Sep 28;11:201-3. doi: 10.5116/ijme.5f54.c8f8.
3. Liu RQ et al. Impostorism and anxiety contribute to burnout among resident physicians. Med Teach. 2022 Jul;44(7):758-64. doi: 10.1080/0142159X.2022.2028751.
4. Gottlieb M et al. Impostor syndrome among physicians and physicians in training: A scoping review. Med Educ. 2020 Feb;54(2):116-24. doi: 10.1111/medu.13956.
Regardless of the setting, one of the most frequently discussed topics in health care is imposter syndrome.
Imposter syndrome was first defined by Clance and Imes as an inability to internalize success, and the tendency to attribute success to external causes such as luck, error, or knowing the appropriate individual.1 This definition is essential because most health care professionals have had a sense of doubt or questioned the full extent of their competencies in various situations. I would argue that this is normal and – within reason – helpful to the practice of medicine. The problem with true imposter syndrome is that the individual does not incorporate success in a way that builds healthy self-esteem and self-efficacy.2
Imposter syndrome has a very nasty way of interacting with burnout. Studies have shown that imposter syndrome can be associated with high levels of emotional exhaustion at work.3 In my experience, this makes clinical sense. Professionals suffering from imposter syndrome can spend a great deal of time and energy trying to maintain a particular image.4 They are acting a part 24/7. Have you ever seriously tried to act? It’s arduous work. A friend once asked me to read a role for a play because “you’d be great; you’re a natural.” By the time I was done with rehearsal, I felt like I had run a 4-by-400-meter relay, by myself, in Victoria, Tex.
And any talk of imposter syndrome must include its running mate, perfectionism. These two conditions exist together so commonly it can be a bit of a chicken or egg question as to which came first.
Imposter syndrome, perfectionism, and burnout can form a deadly triad if not recognized and addressed quickly. In medicine, perfectionism can be a coping strategy that sets up unrelenting standards. Failure to meet unrelenting standards then serves as fuel and validation for imposter syndrome and emotional exhaustion. The consequences of this cycle going unchecked over a health care professional’s career are seismic and can include downstream effects ranging from depression to suicide.
Some readers will relate to this, while others will shrug their shoulders and say that this has never happened in their professional life. I get it. However, I would now ask if you have ever felt like an imposter in your personal life. I’ll make a cup of tea and wait for you to figure out precisely what is the boundary between your personal and professional life. Okay, all done? Great. Now I’ll give you some more time to sincerely reflect if any of the traits of imposter syndrome have described you at times in your personal life. Hmmm, interesting to think about, isn’t it?
I believe that health care professionals frequently use one credit card to pay off another, but the debt remains the same. So even if things are going well at work, we may have just shifted the debt to our personal lives. (At some point in the future, I’ll share my 10 greatest father fails to date to elucidate my point.)
In my work at the GW Resiliency and Well-Being Center, I’ve gravitated toward a few methods supported by evidence that help alleviate imposter syndrome symptoms and potentially serve as protective factors against the future development of imposter syndrome.4 These include but are not limited to:
- Keep a record of small personal success that is yours alone.
- Have a mentor to share failures with.
- Use personal reflection to examine what it means to successfully reach your goals and fulfill your purpose, not a relative value unit target.
- Share experiences with each other, so you know you’re not alone.
The last method is one of my favorites because it involves connecting to others and shining a light on our shared experiences and, coincidentally, our collective strengths. Once this collective strength is realized, the circumstances of that 4-by-400-meter relay change drastically. Be safe and well, everyone.
Lorenzo Norris, MD, is a psychiatrist and chief wellness officer for the George Washington University Medical Enterprise and serves as associate dean of student affairs and administration for the George Washington University School of Medicine and Health Sciences. A version of this article first appeared on Medscape.com.
References
1. Clance PR, Imes SA. The imposter phenomenon in high achieving women: Dynamics and therapeutic intervention. Psychotherapy: Theory, Research & Practice. 1978;15(3): 241-7. doi: 10.1037/h0086006.
2. Thomas M, Bigatti S. Perfectionism, impostor phenomenon, and mental health in medicine: A literature review. Int J Med Educ. 2020 Sep 28;11:201-3. doi: 10.5116/ijme.5f54.c8f8.
3. Liu RQ et al. Impostorism and anxiety contribute to burnout among resident physicians. Med Teach. 2022 Jul;44(7):758-64. doi: 10.1080/0142159X.2022.2028751.
4. Gottlieb M et al. Impostor syndrome among physicians and physicians in training: A scoping review. Med Educ. 2020 Feb;54(2):116-24. doi: 10.1111/medu.13956.
Fitness trackers: Useful in sleep medicine?
Who doesn’t love data, especially their own? With that thought in mind, over the years I have owned several activity trackers, including at least two Fitbits, and I frequently check my iPhone to see how far I’ve walked or how many steps I have taken. My most recent acquisition is an Oura (smart ring, third generation), which includes my first sleep tracker.
Sleep trackers are not unique to the Oura Ring; they are included on many of the newer activity trackers and smart watches, but the design and breakdown of daily sleep, activity, and readiness scores are hallmarks of Oura Rings.
The ring generates data for different phases of sleep, movements, oxygen saturation, disturbances in breathing, heart rate, and heart rate variability. I began to wonder how useful this information would be clinically and whether it might be helpful in either the diagnosis or treatment of sleep disorders.
David Neubauer, MD, is a psychiatrist at the Johns Hopkins Sleep Disorders Center. “Sleep tracking devices are more than just toys but less than medical devices. They do have clinical utility and might show findings that warrant further medical workup,” Dr. Neubauer said. “It is impressive that these devices estimate sleep as well as they do, but there is a problem with how they divide sleep stages that can lead people to believe their sleep is worse than it really is.”
For more than 50 years, he explained, sleep researchers and clinicians have categorized sleep as non–rapid eye movement (NREM) sleep stages 1-4 and REM sleep. More recently, sleep was reorganized to N1, N2, and N3 (which combines the older stages 3 and 4, representing “deep sleep” or “slow wave sleep”) and REM sleep. We normally spend more time in N2 than the other stages. However, the device companies often categorize their sleep estimates as “light sleep,” “deep sleep,” or “REM.” With “light sleep,” they are lumping together N1 and N2 sleep, and this is misleading, said Dr. Neubauer. “Understandably, people often think that there is something wrong if their tracker reports they are spending a lot of time in light sleep, when actually their sleep may be entirely normal.”
Sleep tracker validity
A study by Massimiliano de Zambotti, PhD, and colleagues, “The Sleep of the Ring: Comparison of the ŌURA Sleep Tracker Against Polysomnography”, looked at sleep patterns of 41 adolescents and young adults and concluded that the second-generation tracker was accurate in terms of total sleep but underestimated time spent in N3 stage sleep by approximately 20 minutes while overestimating time spent in REM sleep by 17 minutes. They concluded that the ring had potential to be clinically useful but that further studies and validation were needed.
A larger study of the newest, third-generation Oura tracker, conducted by Altini and Kinnunen at Oura Health, found that the added sensors with the newer-generation ring led to improved accuracy, but they noted that the study was done with a healthy population and might not generalize to clinical populations.
Fernando Goes, MD, and Matthew Reid, PhD, both at Johns Hopkins, are working on a multicenter study using the Oura Ring and the mindLAMP app to look at the impact of sleep on mood in people with mood disorders as well as healthy controls. Dr. Reid said that “validation of sleep stages takes a hit when the ring is used in people with insomnia. We find it useful for total sleep time, but when you look at sleep architecture, the concordance is only 60%. And oxygen saturation measures are less accurate in people with dark skin.”
Clinical uses for sleep trackers
More accurate information might prove reassuring to patients. Dr. Goes added, “One use, for example, might be to help patients to limit or come off of long-term hypnotics with a more benign intervention that incorporates passive monitoring such as that in the Oura Ring. Some patients worry excessively about not being able to sleep, and sleep monitoring data can be helpful to reduce some of these concerns so patients can focus on safer interventions, such as cognitive behavioral therapy for insomnia.” Dr. Reid believes that wearable trackers have potential usefulness in monitoring sleep in patients with insomnia. “In insomnia, sleep state misperception is common. They are hyper-aroused, and they perceive that they are awake when in fact they are sleeping.”
Dr. Goes mentioned another use for sleep trackers in clinical settings: “In our inpatient units, the nurses open the door to look in on patients every hour to monitor and document if they are sleeping. If they look in and the patient isn’t moving, they will ask the patient to raise their hand, which of course is not going to help someone to fall back asleep.” Wearable devices might provide data on sleep without the risk of waking patients every hour through the night.
Not medical devices
However, Dr. Neubauer emphasized that current sleep trackers are not medical devices, saying “they may be measuring the same parameters that are measured with medical devices, for example pulse oximetry or sleep states, but there’s no simple answer yet to the question of whether the devices provide reliable data for clinical decision-making.”
Dr. Neubauer is skeptical about the accuracy of some of the measures the device provides. “I would not use the information from a consumer device to rule out obstructive sleep apnea based on good oxygen saturation numbers. So much depends on the history – snoring, gasping awakenings, reports from bed partners, and daytime sleepiness. These devices do not measure respiratory effort or nasal airflow as sleep studies do. But big drops in oxygen saturation from a consumer device certainly warrant attention for further evaluation.” Dr. Neubauer also noted that the parameters on sleep trackers do not differentiate between central or obstructive sleep apnea and that insurers won’t pay for continuous positive airway pressure to treat sleep apnea without a sleep study.
I enjoy looking at the data, even knowing that they are not entirely accurate. and we may find more clinical uses for these devices. For now, I’m off to get more exercise, at the suggestion of my tracker!
Dinah Miller, MD, is assistant professor of psychiatry and behavioral sciences, Johns Hopkins Medicine, Baltimore.
A version of this article first appeared on Medscape.com.
Who doesn’t love data, especially their own? With that thought in mind, over the years I have owned several activity trackers, including at least two Fitbits, and I frequently check my iPhone to see how far I’ve walked or how many steps I have taken. My most recent acquisition is an Oura (smart ring, third generation), which includes my first sleep tracker.
Sleep trackers are not unique to the Oura Ring; they are included on many of the newer activity trackers and smart watches, but the design and breakdown of daily sleep, activity, and readiness scores are hallmarks of Oura Rings.
The ring generates data for different phases of sleep, movements, oxygen saturation, disturbances in breathing, heart rate, and heart rate variability. I began to wonder how useful this information would be clinically and whether it might be helpful in either the diagnosis or treatment of sleep disorders.
David Neubauer, MD, is a psychiatrist at the Johns Hopkins Sleep Disorders Center. “Sleep tracking devices are more than just toys but less than medical devices. They do have clinical utility and might show findings that warrant further medical workup,” Dr. Neubauer said. “It is impressive that these devices estimate sleep as well as they do, but there is a problem with how they divide sleep stages that can lead people to believe their sleep is worse than it really is.”
For more than 50 years, he explained, sleep researchers and clinicians have categorized sleep as non–rapid eye movement (NREM) sleep stages 1-4 and REM sleep. More recently, sleep was reorganized to N1, N2, and N3 (which combines the older stages 3 and 4, representing “deep sleep” or “slow wave sleep”) and REM sleep. We normally spend more time in N2 than the other stages. However, the device companies often categorize their sleep estimates as “light sleep,” “deep sleep,” or “REM.” With “light sleep,” they are lumping together N1 and N2 sleep, and this is misleading, said Dr. Neubauer. “Understandably, people often think that there is something wrong if their tracker reports they are spending a lot of time in light sleep, when actually their sleep may be entirely normal.”
Sleep tracker validity
A study by Massimiliano de Zambotti, PhD, and colleagues, “The Sleep of the Ring: Comparison of the ŌURA Sleep Tracker Against Polysomnography”, looked at sleep patterns of 41 adolescents and young adults and concluded that the second-generation tracker was accurate in terms of total sleep but underestimated time spent in N3 stage sleep by approximately 20 minutes while overestimating time spent in REM sleep by 17 minutes. They concluded that the ring had potential to be clinically useful but that further studies and validation were needed.
A larger study of the newest, third-generation Oura tracker, conducted by Altini and Kinnunen at Oura Health, found that the added sensors with the newer-generation ring led to improved accuracy, but they noted that the study was done with a healthy population and might not generalize to clinical populations.
Fernando Goes, MD, and Matthew Reid, PhD, both at Johns Hopkins, are working on a multicenter study using the Oura Ring and the mindLAMP app to look at the impact of sleep on mood in people with mood disorders as well as healthy controls. Dr. Reid said that “validation of sleep stages takes a hit when the ring is used in people with insomnia. We find it useful for total sleep time, but when you look at sleep architecture, the concordance is only 60%. And oxygen saturation measures are less accurate in people with dark skin.”
Clinical uses for sleep trackers
More accurate information might prove reassuring to patients. Dr. Goes added, “One use, for example, might be to help patients to limit or come off of long-term hypnotics with a more benign intervention that incorporates passive monitoring such as that in the Oura Ring. Some patients worry excessively about not being able to sleep, and sleep monitoring data can be helpful to reduce some of these concerns so patients can focus on safer interventions, such as cognitive behavioral therapy for insomnia.” Dr. Reid believes that wearable trackers have potential usefulness in monitoring sleep in patients with insomnia. “In insomnia, sleep state misperception is common. They are hyper-aroused, and they perceive that they are awake when in fact they are sleeping.”
Dr. Goes mentioned another use for sleep trackers in clinical settings: “In our inpatient units, the nurses open the door to look in on patients every hour to monitor and document if they are sleeping. If they look in and the patient isn’t moving, they will ask the patient to raise their hand, which of course is not going to help someone to fall back asleep.” Wearable devices might provide data on sleep without the risk of waking patients every hour through the night.
Not medical devices
However, Dr. Neubauer emphasized that current sleep trackers are not medical devices, saying “they may be measuring the same parameters that are measured with medical devices, for example pulse oximetry or sleep states, but there’s no simple answer yet to the question of whether the devices provide reliable data for clinical decision-making.”
Dr. Neubauer is skeptical about the accuracy of some of the measures the device provides. “I would not use the information from a consumer device to rule out obstructive sleep apnea based on good oxygen saturation numbers. So much depends on the history – snoring, gasping awakenings, reports from bed partners, and daytime sleepiness. These devices do not measure respiratory effort or nasal airflow as sleep studies do. But big drops in oxygen saturation from a consumer device certainly warrant attention for further evaluation.” Dr. Neubauer also noted that the parameters on sleep trackers do not differentiate between central or obstructive sleep apnea and that insurers won’t pay for continuous positive airway pressure to treat sleep apnea without a sleep study.
I enjoy looking at the data, even knowing that they are not entirely accurate. and we may find more clinical uses for these devices. For now, I’m off to get more exercise, at the suggestion of my tracker!
Dinah Miller, MD, is assistant professor of psychiatry and behavioral sciences, Johns Hopkins Medicine, Baltimore.
A version of this article first appeared on Medscape.com.
Who doesn’t love data, especially their own? With that thought in mind, over the years I have owned several activity trackers, including at least two Fitbits, and I frequently check my iPhone to see how far I’ve walked or how many steps I have taken. My most recent acquisition is an Oura (smart ring, third generation), which includes my first sleep tracker.
Sleep trackers are not unique to the Oura Ring; they are included on many of the newer activity trackers and smart watches, but the design and breakdown of daily sleep, activity, and readiness scores are hallmarks of Oura Rings.
The ring generates data for different phases of sleep, movements, oxygen saturation, disturbances in breathing, heart rate, and heart rate variability. I began to wonder how useful this information would be clinically and whether it might be helpful in either the diagnosis or treatment of sleep disorders.
David Neubauer, MD, is a psychiatrist at the Johns Hopkins Sleep Disorders Center. “Sleep tracking devices are more than just toys but less than medical devices. They do have clinical utility and might show findings that warrant further medical workup,” Dr. Neubauer said. “It is impressive that these devices estimate sleep as well as they do, but there is a problem with how they divide sleep stages that can lead people to believe their sleep is worse than it really is.”
For more than 50 years, he explained, sleep researchers and clinicians have categorized sleep as non–rapid eye movement (NREM) sleep stages 1-4 and REM sleep. More recently, sleep was reorganized to N1, N2, and N3 (which combines the older stages 3 and 4, representing “deep sleep” or “slow wave sleep”) and REM sleep. We normally spend more time in N2 than the other stages. However, the device companies often categorize their sleep estimates as “light sleep,” “deep sleep,” or “REM.” With “light sleep,” they are lumping together N1 and N2 sleep, and this is misleading, said Dr. Neubauer. “Understandably, people often think that there is something wrong if their tracker reports they are spending a lot of time in light sleep, when actually their sleep may be entirely normal.”
Sleep tracker validity
A study by Massimiliano de Zambotti, PhD, and colleagues, “The Sleep of the Ring: Comparison of the ŌURA Sleep Tracker Against Polysomnography”, looked at sleep patterns of 41 adolescents and young adults and concluded that the second-generation tracker was accurate in terms of total sleep but underestimated time spent in N3 stage sleep by approximately 20 minutes while overestimating time spent in REM sleep by 17 minutes. They concluded that the ring had potential to be clinically useful but that further studies and validation were needed.
A larger study of the newest, third-generation Oura tracker, conducted by Altini and Kinnunen at Oura Health, found that the added sensors with the newer-generation ring led to improved accuracy, but they noted that the study was done with a healthy population and might not generalize to clinical populations.
Fernando Goes, MD, and Matthew Reid, PhD, both at Johns Hopkins, are working on a multicenter study using the Oura Ring and the mindLAMP app to look at the impact of sleep on mood in people with mood disorders as well as healthy controls. Dr. Reid said that “validation of sleep stages takes a hit when the ring is used in people with insomnia. We find it useful for total sleep time, but when you look at sleep architecture, the concordance is only 60%. And oxygen saturation measures are less accurate in people with dark skin.”
Clinical uses for sleep trackers
More accurate information might prove reassuring to patients. Dr. Goes added, “One use, for example, might be to help patients to limit or come off of long-term hypnotics with a more benign intervention that incorporates passive monitoring such as that in the Oura Ring. Some patients worry excessively about not being able to sleep, and sleep monitoring data can be helpful to reduce some of these concerns so patients can focus on safer interventions, such as cognitive behavioral therapy for insomnia.” Dr. Reid believes that wearable trackers have potential usefulness in monitoring sleep in patients with insomnia. “In insomnia, sleep state misperception is common. They are hyper-aroused, and they perceive that they are awake when in fact they are sleeping.”
Dr. Goes mentioned another use for sleep trackers in clinical settings: “In our inpatient units, the nurses open the door to look in on patients every hour to monitor and document if they are sleeping. If they look in and the patient isn’t moving, they will ask the patient to raise their hand, which of course is not going to help someone to fall back asleep.” Wearable devices might provide data on sleep without the risk of waking patients every hour through the night.
Not medical devices
However, Dr. Neubauer emphasized that current sleep trackers are not medical devices, saying “they may be measuring the same parameters that are measured with medical devices, for example pulse oximetry or sleep states, but there’s no simple answer yet to the question of whether the devices provide reliable data for clinical decision-making.”
Dr. Neubauer is skeptical about the accuracy of some of the measures the device provides. “I would not use the information from a consumer device to rule out obstructive sleep apnea based on good oxygen saturation numbers. So much depends on the history – snoring, gasping awakenings, reports from bed partners, and daytime sleepiness. These devices do not measure respiratory effort or nasal airflow as sleep studies do. But big drops in oxygen saturation from a consumer device certainly warrant attention for further evaluation.” Dr. Neubauer also noted that the parameters on sleep trackers do not differentiate between central or obstructive sleep apnea and that insurers won’t pay for continuous positive airway pressure to treat sleep apnea without a sleep study.
I enjoy looking at the data, even knowing that they are not entirely accurate. and we may find more clinical uses for these devices. For now, I’m off to get more exercise, at the suggestion of my tracker!
Dinah Miller, MD, is assistant professor of psychiatry and behavioral sciences, Johns Hopkins Medicine, Baltimore.
A version of this article first appeared on Medscape.com.
Major depression treatments boost brain connectivity
VIENNA – , new research suggests.
In a “repeat” MRI study, adult participants with MDD had significantly lower brain connectivity compared with their healthy peers at baseline – but showed significant improvement at the 6-week follow-up. These improvements were associated with decreases in symptom severity, independent of whether they received electroconvulsive therapy (ECT) or other treatment modalities.
“This means that the brain structure of patients with serious clinical depression is not as fixed as we thought, and we can improve brain structure within a short time frame [of] around 6 weeks,” lead author Jonathan Repple, MD, now professor of predictive psychiatry at the University of Frankfurt, Germany, said in a release.
“This gives hope to patients who believe nothing can change and they have to live with a disease forever because it is ‘set in stone’ in their brain,” he added.
The findings were presented at the 35th European College of Neuropsychopharmacology (ECNP) Congress.
‘Easily understandable picture’
Dr. Repple said in an interview that the investigators “were surprised to see how plastic” the brain could be.
“I’ve done a lot of imaging studies in the past where we looked at differences in depression vs. healthy controls, and then maybe had tiny effects. But we’ve never seen such a clear and easily understandable picture, where we see a deficit at the beginning and then a significant increase in whatever biomarker we were looking at, that even correlated with how successful the treatment was,” he said.
Dr. Repple noted that “this is the thing everyone is looking for when we’re talking about a biomarker: That we see this exact pattern” – and it is why they are so excited about the results.
However, he cautioned that the study included a “small sample” and the results need to be independently replicated.
“If this can be replicated, this might be a very good target for future intervention studies,” Dr. Repple said.
The investigators noted that altered brain structural connectivity has been implicated before in the pathophysiology of MDD.
However, it is not clear whether these changes are stable over time and indicate a biological predisposition, or are markers of current disease severity and can be altered by effective treatment.
To investigate further, the researchers used gray matter T1-weighted MRI to define nodes in the brain and diffusion-weighted imaging (DWI)-based tractography to determine connections between the nodes, to create a structural connectome or white matter network.
They performed assessments at baseline and at 6 weeks’ follow-up in 123 participants diagnosed with current MDD and receiving inpatient treatment, and 55 participants who acted as the healthy controls group.
Among the patients with MDD, 56 were treated with ECT and 67 received other antidepressant care, including psychological therapy or medications. Some patients had received all three treatment modalities.
Significant interactions
Results showed a significant interaction by group and time between the baseline and 6-week follow-up assessments (P < .05).
This was partly driven by the MDD group having a significantly lower connectivity strength at baseline than the healthy controls group (P < .05).
It was also partly driven by patients showing a significant improvement in connectivity strength between the baseline and follow-up assessments (P < .05), a pattern that was not seen in the nonpatients.
This increase in connectivity strength was associated with a significant decrease in depression symptom severity (P < .05). This was independent of the treatment modality, indicating that it was not linked to the use of ECT.
Dr. Repple acknowledged the relatively short follow-up period of the study, and added that he is not aware of longitudinal studies of the structural connectome with a longer follow-up.
He pointed out that the structural connectivity of the brain decreases with age, but there have been no studies that have assessed patients with depression and “measured the same person again after 2, 4, 6, or 8 years.”
Dr. Repple reported that the investigators will be following up with their participants, “so hopefully in a few years we’ll have more information on that.
“One thing I also need to stress is that, when we’re looking at the MRI brain scans, we see an increase in connectivity strength, but we really can’t say what the molecular mechanisms behind it are,” he said. “This is a black box for us.”
Several unanswered questions
Commenting in the release, Eric Ruhe, MD, PhD, Radboud University Medical Center, Nijmegen, the Netherlands, said this was a “very interesting and difficult study to perform.”
However, Dr. Ruhe, who was not involved in the research, told this news organization that it is “very difficult to connect the lack of brain connectivity to the patient symptomatology because there is a huge gap between them.”
The problem is that, despite “lots of evidence” that they are effective, “we currently don’t know how antidepressant therapies work” in terms of their underlying mechanisms of action, he said.
“We think that these types of therapies all modulate the plasticity of the brain,” said Dr. Ruhe. “What this study showed is there are changes that you can detect even in 6 weeks,” although they may have been observed even sooner with a shorter follow-up.
He noted that big questions are whether the change is specific to the treatment given, and “can you modulate different brain network dysfunctions with different treatments?”
Moreover, he wondered if a brain scan could indicate which type of treatment should be used. “This is, of course, very new and very challenging, and we don’t know yet, but we should be pursuing this,” Dr. Ruhe said.
Another question is whether or not the brain connectivity changes shown in the study represent a persistent change – “and whether this is a persistent change that is associated with a consistent and persistent relief of depression.
“Again, this is something that needs to be followed up,” said Dr. Ruhe.
No funding was declared. The study authors and Dr. Ruhe report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
VIENNA – , new research suggests.
In a “repeat” MRI study, adult participants with MDD had significantly lower brain connectivity compared with their healthy peers at baseline – but showed significant improvement at the 6-week follow-up. These improvements were associated with decreases in symptom severity, independent of whether they received electroconvulsive therapy (ECT) or other treatment modalities.
“This means that the brain structure of patients with serious clinical depression is not as fixed as we thought, and we can improve brain structure within a short time frame [of] around 6 weeks,” lead author Jonathan Repple, MD, now professor of predictive psychiatry at the University of Frankfurt, Germany, said in a release.
“This gives hope to patients who believe nothing can change and they have to live with a disease forever because it is ‘set in stone’ in their brain,” he added.
The findings were presented at the 35th European College of Neuropsychopharmacology (ECNP) Congress.
‘Easily understandable picture’
Dr. Repple said in an interview that the investigators “were surprised to see how plastic” the brain could be.
“I’ve done a lot of imaging studies in the past where we looked at differences in depression vs. healthy controls, and then maybe had tiny effects. But we’ve never seen such a clear and easily understandable picture, where we see a deficit at the beginning and then a significant increase in whatever biomarker we were looking at, that even correlated with how successful the treatment was,” he said.
Dr. Repple noted that “this is the thing everyone is looking for when we’re talking about a biomarker: That we see this exact pattern” – and it is why they are so excited about the results.
However, he cautioned that the study included a “small sample” and the results need to be independently replicated.
“If this can be replicated, this might be a very good target for future intervention studies,” Dr. Repple said.
The investigators noted that altered brain structural connectivity has been implicated before in the pathophysiology of MDD.
However, it is not clear whether these changes are stable over time and indicate a biological predisposition, or are markers of current disease severity and can be altered by effective treatment.
To investigate further, the researchers used gray matter T1-weighted MRI to define nodes in the brain and diffusion-weighted imaging (DWI)-based tractography to determine connections between the nodes, to create a structural connectome or white matter network.
They performed assessments at baseline and at 6 weeks’ follow-up in 123 participants diagnosed with current MDD and receiving inpatient treatment, and 55 participants who acted as the healthy controls group.
Among the patients with MDD, 56 were treated with ECT and 67 received other antidepressant care, including psychological therapy or medications. Some patients had received all three treatment modalities.
Significant interactions
Results showed a significant interaction by group and time between the baseline and 6-week follow-up assessments (P < .05).
This was partly driven by the MDD group having a significantly lower connectivity strength at baseline than the healthy controls group (P < .05).
It was also partly driven by patients showing a significant improvement in connectivity strength between the baseline and follow-up assessments (P < .05), a pattern that was not seen in the nonpatients.
This increase in connectivity strength was associated with a significant decrease in depression symptom severity (P < .05). This was independent of the treatment modality, indicating that it was not linked to the use of ECT.
Dr. Repple acknowledged the relatively short follow-up period of the study, and added that he is not aware of longitudinal studies of the structural connectome with a longer follow-up.
He pointed out that the structural connectivity of the brain decreases with age, but there have been no studies that have assessed patients with depression and “measured the same person again after 2, 4, 6, or 8 years.”
Dr. Repple reported that the investigators will be following up with their participants, “so hopefully in a few years we’ll have more information on that.
“One thing I also need to stress is that, when we’re looking at the MRI brain scans, we see an increase in connectivity strength, but we really can’t say what the molecular mechanisms behind it are,” he said. “This is a black box for us.”
Several unanswered questions
Commenting in the release, Eric Ruhe, MD, PhD, Radboud University Medical Center, Nijmegen, the Netherlands, said this was a “very interesting and difficult study to perform.”
However, Dr. Ruhe, who was not involved in the research, told this news organization that it is “very difficult to connect the lack of brain connectivity to the patient symptomatology because there is a huge gap between them.”
The problem is that, despite “lots of evidence” that they are effective, “we currently don’t know how antidepressant therapies work” in terms of their underlying mechanisms of action, he said.
“We think that these types of therapies all modulate the plasticity of the brain,” said Dr. Ruhe. “What this study showed is there are changes that you can detect even in 6 weeks,” although they may have been observed even sooner with a shorter follow-up.
He noted that big questions are whether the change is specific to the treatment given, and “can you modulate different brain network dysfunctions with different treatments?”
Moreover, he wondered if a brain scan could indicate which type of treatment should be used. “This is, of course, very new and very challenging, and we don’t know yet, but we should be pursuing this,” Dr. Ruhe said.
Another question is whether or not the brain connectivity changes shown in the study represent a persistent change – “and whether this is a persistent change that is associated with a consistent and persistent relief of depression.
“Again, this is something that needs to be followed up,” said Dr. Ruhe.
No funding was declared. The study authors and Dr. Ruhe report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
VIENNA – , new research suggests.
In a “repeat” MRI study, adult participants with MDD had significantly lower brain connectivity compared with their healthy peers at baseline – but showed significant improvement at the 6-week follow-up. These improvements were associated with decreases in symptom severity, independent of whether they received electroconvulsive therapy (ECT) or other treatment modalities.
“This means that the brain structure of patients with serious clinical depression is not as fixed as we thought, and we can improve brain structure within a short time frame [of] around 6 weeks,” lead author Jonathan Repple, MD, now professor of predictive psychiatry at the University of Frankfurt, Germany, said in a release.
“This gives hope to patients who believe nothing can change and they have to live with a disease forever because it is ‘set in stone’ in their brain,” he added.
The findings were presented at the 35th European College of Neuropsychopharmacology (ECNP) Congress.
‘Easily understandable picture’
Dr. Repple said in an interview that the investigators “were surprised to see how plastic” the brain could be.
“I’ve done a lot of imaging studies in the past where we looked at differences in depression vs. healthy controls, and then maybe had tiny effects. But we’ve never seen such a clear and easily understandable picture, where we see a deficit at the beginning and then a significant increase in whatever biomarker we were looking at, that even correlated with how successful the treatment was,” he said.
Dr. Repple noted that “this is the thing everyone is looking for when we’re talking about a biomarker: That we see this exact pattern” – and it is why they are so excited about the results.
However, he cautioned that the study included a “small sample” and the results need to be independently replicated.
“If this can be replicated, this might be a very good target for future intervention studies,” Dr. Repple said.
The investigators noted that altered brain structural connectivity has been implicated before in the pathophysiology of MDD.
However, it is not clear whether these changes are stable over time and indicate a biological predisposition, or are markers of current disease severity and can be altered by effective treatment.
To investigate further, the researchers used gray matter T1-weighted MRI to define nodes in the brain and diffusion-weighted imaging (DWI)-based tractography to determine connections between the nodes, to create a structural connectome or white matter network.
They performed assessments at baseline and at 6 weeks’ follow-up in 123 participants diagnosed with current MDD and receiving inpatient treatment, and 55 participants who acted as the healthy controls group.
Among the patients with MDD, 56 were treated with ECT and 67 received other antidepressant care, including psychological therapy or medications. Some patients had received all three treatment modalities.
Significant interactions
Results showed a significant interaction by group and time between the baseline and 6-week follow-up assessments (P < .05).
This was partly driven by the MDD group having a significantly lower connectivity strength at baseline than the healthy controls group (P < .05).
It was also partly driven by patients showing a significant improvement in connectivity strength between the baseline and follow-up assessments (P < .05), a pattern that was not seen in the nonpatients.
This increase in connectivity strength was associated with a significant decrease in depression symptom severity (P < .05). This was independent of the treatment modality, indicating that it was not linked to the use of ECT.
Dr. Repple acknowledged the relatively short follow-up period of the study, and added that he is not aware of longitudinal studies of the structural connectome with a longer follow-up.
He pointed out that the structural connectivity of the brain decreases with age, but there have been no studies that have assessed patients with depression and “measured the same person again after 2, 4, 6, or 8 years.”
Dr. Repple reported that the investigators will be following up with their participants, “so hopefully in a few years we’ll have more information on that.
“One thing I also need to stress is that, when we’re looking at the MRI brain scans, we see an increase in connectivity strength, but we really can’t say what the molecular mechanisms behind it are,” he said. “This is a black box for us.”
Several unanswered questions
Commenting in the release, Eric Ruhe, MD, PhD, Radboud University Medical Center, Nijmegen, the Netherlands, said this was a “very interesting and difficult study to perform.”
However, Dr. Ruhe, who was not involved in the research, told this news organization that it is “very difficult to connect the lack of brain connectivity to the patient symptomatology because there is a huge gap between them.”
The problem is that, despite “lots of evidence” that they are effective, “we currently don’t know how antidepressant therapies work” in terms of their underlying mechanisms of action, he said.
“We think that these types of therapies all modulate the plasticity of the brain,” said Dr. Ruhe. “What this study showed is there are changes that you can detect even in 6 weeks,” although they may have been observed even sooner with a shorter follow-up.
He noted that big questions are whether the change is specific to the treatment given, and “can you modulate different brain network dysfunctions with different treatments?”
Moreover, he wondered if a brain scan could indicate which type of treatment should be used. “This is, of course, very new and very challenging, and we don’t know yet, but we should be pursuing this,” Dr. Ruhe said.
Another question is whether or not the brain connectivity changes shown in the study represent a persistent change – “and whether this is a persistent change that is associated with a consistent and persistent relief of depression.
“Again, this is something that needs to be followed up,” said Dr. Ruhe.
No funding was declared. The study authors and Dr. Ruhe report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ECNP 2022
Should health care be a right?
Is health care a human right?
This year voters in Oregon are being asked to decide that.
It brings up some interesting questions. Should it be a right? Food, water, shelter, and oxygen aren’t, as far as I know, considered such. So why health care?
Probably the main argument against the idea is that, if it’s a right, shouldn’t the government (and therefore taxpayers) be tasked with paying for it all?
Good question, and not one that I can answer. If my neighbor refuses to buy insurance, then has a health crisis he can’t afford, why should I have to pay for his obstinacy and lack of foresight? Isn’t it his problem?
Of course, the truth is that not everyone can afford health care, or insurance. They ain’t cheap. Even if you get coverage through your job, part of your earnings, and part of the company’s profits, are being taken out to pay for it.
This raises the question of whether health care is something that should be rationed only to the working, successfully retired, or wealthy. Heaven knows I have plenty of patients tell me that. Their point is that if you’re not contributing to society, why should society contribute to you?
One even said that our distant ancestors didn’t see an issue with this: If you were unable to hunt, or outrun a cave lion, you probably weren’t helping the rest of the tribe anyway and deserved what happened to you.
Perhaps true, but we aren’t our distant ancestors. Over the millennia we’ve developed into a remarkably social, and increasingly interconnected, species. Somewhat paradoxically we often care more about famines on the other side of the world than we do in our own cities. If you’re going to use the argument of “we didn’t used to do this,” we also didn’t used to have cars, planes, or computers, but I don’t see anyone giving them up.
Another thing to keep in mind is that we are all paying for the uninsured under pretty much any system of health care there is. Whether it’s through taxes, insurance premiums, or both, our own costs go up to pay the bills of those who don’t have coverage. So in that respect the financial aspect of declaring it a right probably doesn’t change the de facto truth of the situation. It just makes it more official-ish.
Maybe the statement has more philosophical or political meaning than it does practical. If it passes it may change a lot of things, or nothing at all, depending how it’s legally interpreted.
Like so many things, we won’t know where it goes unless it happens. And even then it’s uncertain where it will lead.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Is health care a human right?
This year voters in Oregon are being asked to decide that.
It brings up some interesting questions. Should it be a right? Food, water, shelter, and oxygen aren’t, as far as I know, considered such. So why health care?
Probably the main argument against the idea is that, if it’s a right, shouldn’t the government (and therefore taxpayers) be tasked with paying for it all?
Good question, and not one that I can answer. If my neighbor refuses to buy insurance, then has a health crisis he can’t afford, why should I have to pay for his obstinacy and lack of foresight? Isn’t it his problem?
Of course, the truth is that not everyone can afford health care, or insurance. They ain’t cheap. Even if you get coverage through your job, part of your earnings, and part of the company’s profits, are being taken out to pay for it.
This raises the question of whether health care is something that should be rationed only to the working, successfully retired, or wealthy. Heaven knows I have plenty of patients tell me that. Their point is that if you’re not contributing to society, why should society contribute to you?
One even said that our distant ancestors didn’t see an issue with this: If you were unable to hunt, or outrun a cave lion, you probably weren’t helping the rest of the tribe anyway and deserved what happened to you.
Perhaps true, but we aren’t our distant ancestors. Over the millennia we’ve developed into a remarkably social, and increasingly interconnected, species. Somewhat paradoxically we often care more about famines on the other side of the world than we do in our own cities. If you’re going to use the argument of “we didn’t used to do this,” we also didn’t used to have cars, planes, or computers, but I don’t see anyone giving them up.
Another thing to keep in mind is that we are all paying for the uninsured under pretty much any system of health care there is. Whether it’s through taxes, insurance premiums, or both, our own costs go up to pay the bills of those who don’t have coverage. So in that respect the financial aspect of declaring it a right probably doesn’t change the de facto truth of the situation. It just makes it more official-ish.
Maybe the statement has more philosophical or political meaning than it does practical. If it passes it may change a lot of things, or nothing at all, depending how it’s legally interpreted.
Like so many things, we won’t know where it goes unless it happens. And even then it’s uncertain where it will lead.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Is health care a human right?
This year voters in Oregon are being asked to decide that.
It brings up some interesting questions. Should it be a right? Food, water, shelter, and oxygen aren’t, as far as I know, considered such. So why health care?
Probably the main argument against the idea is that, if it’s a right, shouldn’t the government (and therefore taxpayers) be tasked with paying for it all?
Good question, and not one that I can answer. If my neighbor refuses to buy insurance, then has a health crisis he can’t afford, why should I have to pay for his obstinacy and lack of foresight? Isn’t it his problem?
Of course, the truth is that not everyone can afford health care, or insurance. They ain’t cheap. Even if you get coverage through your job, part of your earnings, and part of the company’s profits, are being taken out to pay for it.
This raises the question of whether health care is something that should be rationed only to the working, successfully retired, or wealthy. Heaven knows I have plenty of patients tell me that. Their point is that if you’re not contributing to society, why should society contribute to you?
One even said that our distant ancestors didn’t see an issue with this: If you were unable to hunt, or outrun a cave lion, you probably weren’t helping the rest of the tribe anyway and deserved what happened to you.
Perhaps true, but we aren’t our distant ancestors. Over the millennia we’ve developed into a remarkably social, and increasingly interconnected, species. Somewhat paradoxically we often care more about famines on the other side of the world than we do in our own cities. If you’re going to use the argument of “we didn’t used to do this,” we also didn’t used to have cars, planes, or computers, but I don’t see anyone giving them up.
Another thing to keep in mind is that we are all paying for the uninsured under pretty much any system of health care there is. Whether it’s through taxes, insurance premiums, or both, our own costs go up to pay the bills of those who don’t have coverage. So in that respect the financial aspect of declaring it a right probably doesn’t change the de facto truth of the situation. It just makes it more official-ish.
Maybe the statement has more philosophical or political meaning than it does practical. If it passes it may change a lot of things, or nothing at all, depending how it’s legally interpreted.
Like so many things, we won’t know where it goes unless it happens. And even then it’s uncertain where it will lead.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Rheumatic diseases and assisted reproductive technology: Things to consider
The field of “reproductive rheumatology” has received growing attention in recent years as we learn more about how autoimmune rheumatic diseases and their treatment affect women of reproductive age. In 2020, the American College of Rheumatology published a comprehensive guideline that includes recommendations and supporting evidence for managing issues related to reproductive health in patients with rheumatic diseases and has since launched an ongoing Reproductive Health Initiative, with the goal of translating established guidelines into practice through various education and awareness campaigns. One area addressed by the guideline that comes up commonly in practice but receives less attention and research is the use of assisted reproductive technology (ART) in patients with rheumatic diseases.
Literature is conflicting regarding whether patients with autoimmune rheumatic diseases are inherently at increased risk for infertility, defined as failure to achieve a clinical pregnancy after 12 months or more of regular unprotected intercourse, or subfertility, defined as a delay in conception. Regardless, several factors indirectly contribute to a disproportionate risk for infertility or subfertility in this patient population, including active inflammatory disease, reduced ovarian reserve, and medications.
Patients with subfertility or infertility who desire pregnancy may pursue ovulation induction with timed intercourse or intrauterine insemination, in vitro fertilization (IVF)/intracytoplasmic sperm injection with either embryo transfer, or gestational surrogacy. Those who require treatment with cyclophosphamide or who plan to defer pregnancy for whatever reason can opt for oocyte cryopreservation (colloquially known as “egg freezing”). For IVF and oocyte cryopreservation, controlled ovarian stimulation is typically the first step (except in unstimulated, or “natural cycle,” IVF).
Various protocols are used for ovarian stimulation and ovulation induction, the nuances of which are beyond the scope of this article. In general, ovarian stimulation involves gonadotropin therapy (follicle-stimulating hormone and/or human menopausal gonadotropin) administered via scheduled subcutaneous injections to stimulate follicular growth, as well as gonadotropin-releasing hormone (GnRH) agonists or antagonists to suppress luteinizing hormone, preventing ovulation. Adjunctive oral therapy (clomiphene citrate or letrozole, an aromatase inhibitor) may be used as well. The patient has frequent lab monitoring of hormone levels and transvaginal ultrasounds to measure follicle number and size and, when the timing is right, receives an “ovulation trigger” – either human chorionic gonadotropin or GnRH agonist, depending on the protocol. At this point, transvaginal ultrasound–guided egg retrieval is done under sedation. Recovered oocytes are then either frozen for later use or fertilized in the lab for embryo transfer. Lastly, exogenous hormones are often used: estrogen to support frozen embryo transfers and progesterone for so-called luteal phase support.
ART is not contraindicated in patients with autoimmune rheumatic diseases, but there may be additional factors to consider, particularly for those with systemic lupus erythematosus (SLE), antiphospholipid syndrome (APS), and antiphospholipid antibodies (aPL) without clinical APS.
Ovarian stimulation elevates estrogen levels to varying degrees depending on the patient and the medications used. In all cases, though, peak levels are significantly lower than levels reached during pregnancy. It is well established that elevated estrogen – whether from hormone therapies or pregnancy – significantly increases thrombotic risk, even in healthy people. High-risk patients should receive low-molecular-weight heparin – a prophylactic dose for patients with either positive aPL without clinical APS (including those with SLE) or with obstetric APS, and a therapeutic dose for those with thrombotic APS – during ART procedures.
In patients with SLE, another concern is that increased estrogen will cause disease flare. One case series published in 2017 reported 37 patients with SLE and/or APS who underwent 97 IVF cycles, of which 8% were complicated by flare or thrombotic events. Notably, half of these complications occurred in patients who stopped prescribed therapies (immunomodulatory therapy in two patients with SLE, anticoagulation in two patients with APS) after failure to conceive. In a separate study from 2000 including 19 patients with SLE, APS, or high-titer aPL who underwent 68 IVF cycles, 19% of cycles in patients with SLE were complicated by flare, and no thrombotic events occurred in the cohort. The authors concluded that ovulation induction does not exacerbate SLE or APS. In these studies, the overall pregnancy rates were felt to be consistent with those achieved by the general population through IVF. Although obstetric complications, such as preeclampsia and preterm delivery, were reported in about half of the pregnancies described, these are known to occur more frequently in those with SLE and APS, especially when active disease or other risk factors are present. There are no large-scale, controlled studies evaluating ART outcomes in patients with autoimmune rheumatic diseases to date.
Finally, ovarian hyperstimulation syndrome (OHSS) is an increasingly rare but severe complication of ovarian stimulation. OHSS is characterized by capillary leak, fluid overload, and cytokine release syndrome and can lead to thromboembolic events. Comorbidities like hypertension and renal failure, which can go along with autoimmune rheumatic diseases, are risk factors for OHSS. The use of human chorionic gonadotropin to trigger ovulation is also associated with an increased risk for OHSS, so a GnRH agonist trigger may be preferable.
The ACR guideline recommends that individuals with any of these underlying conditions undergo ART only in expert centers. The ovarian stimulation protocol needs to be tailored to the individual patient to minimize risk and optimize outcomes. The overall goal when managing patients with autoimmune rheumatic diseases during ART is to establish and maintain disease control with pregnancy-compatible medications (when pregnancy is the goal). With adequate planning, appropriate treatment, and collaboration between obstetricians and rheumatologists, individuals with autoimmune rheumatic diseases can safely pursue ART and go on to have successful pregnancies.
Dr. Siegel is a 2022-2023 UCB Women’s Health rheumatology fellow in the rheumatology reproductive health program of the Barbara Volcker Center for Women and Rheumatic Diseases at Hospital for Special Surgery/Weill Cornell Medicine, New York. Her clinical and research focus is on reproductive health issues in individuals with rheumatic disease. Dr. Chan is an assistant professor at Weill Cornell Medical College and an attending physician at Hospital for Special Surgery and Memorial Sloan Kettering Cancer Center in New York. Before moving to New York City, she spent 7 years in private practice in Rhode Island and was a columnist for a monthly rheumatology publication, writing about the challenges of starting life as a full-fledged rheumatologist in a private practice. Follow Dr Chan on Twitter. Dr. Siegel and Dr. Chan disclosed no relevant financial relationships.
A version of this article – an editorial collaboration between Medscape and the Hospital for Special Surgery – first appeared on Medscape.com.
The field of “reproductive rheumatology” has received growing attention in recent years as we learn more about how autoimmune rheumatic diseases and their treatment affect women of reproductive age. In 2020, the American College of Rheumatology published a comprehensive guideline that includes recommendations and supporting evidence for managing issues related to reproductive health in patients with rheumatic diseases and has since launched an ongoing Reproductive Health Initiative, with the goal of translating established guidelines into practice through various education and awareness campaigns. One area addressed by the guideline that comes up commonly in practice but receives less attention and research is the use of assisted reproductive technology (ART) in patients with rheumatic diseases.
Literature is conflicting regarding whether patients with autoimmune rheumatic diseases are inherently at increased risk for infertility, defined as failure to achieve a clinical pregnancy after 12 months or more of regular unprotected intercourse, or subfertility, defined as a delay in conception. Regardless, several factors indirectly contribute to a disproportionate risk for infertility or subfertility in this patient population, including active inflammatory disease, reduced ovarian reserve, and medications.
Patients with subfertility or infertility who desire pregnancy may pursue ovulation induction with timed intercourse or intrauterine insemination, in vitro fertilization (IVF)/intracytoplasmic sperm injection with either embryo transfer, or gestational surrogacy. Those who require treatment with cyclophosphamide or who plan to defer pregnancy for whatever reason can opt for oocyte cryopreservation (colloquially known as “egg freezing”). For IVF and oocyte cryopreservation, controlled ovarian stimulation is typically the first step (except in unstimulated, or “natural cycle,” IVF).
Various protocols are used for ovarian stimulation and ovulation induction, the nuances of which are beyond the scope of this article. In general, ovarian stimulation involves gonadotropin therapy (follicle-stimulating hormone and/or human menopausal gonadotropin) administered via scheduled subcutaneous injections to stimulate follicular growth, as well as gonadotropin-releasing hormone (GnRH) agonists or antagonists to suppress luteinizing hormone, preventing ovulation. Adjunctive oral therapy (clomiphene citrate or letrozole, an aromatase inhibitor) may be used as well. The patient has frequent lab monitoring of hormone levels and transvaginal ultrasounds to measure follicle number and size and, when the timing is right, receives an “ovulation trigger” – either human chorionic gonadotropin or GnRH agonist, depending on the protocol. At this point, transvaginal ultrasound–guided egg retrieval is done under sedation. Recovered oocytes are then either frozen for later use or fertilized in the lab for embryo transfer. Lastly, exogenous hormones are often used: estrogen to support frozen embryo transfers and progesterone for so-called luteal phase support.
ART is not contraindicated in patients with autoimmune rheumatic diseases, but there may be additional factors to consider, particularly for those with systemic lupus erythematosus (SLE), antiphospholipid syndrome (APS), and antiphospholipid antibodies (aPL) without clinical APS.
Ovarian stimulation elevates estrogen levels to varying degrees depending on the patient and the medications used. In all cases, though, peak levels are significantly lower than levels reached during pregnancy. It is well established that elevated estrogen – whether from hormone therapies or pregnancy – significantly increases thrombotic risk, even in healthy people. High-risk patients should receive low-molecular-weight heparin – a prophylactic dose for patients with either positive aPL without clinical APS (including those with SLE) or with obstetric APS, and a therapeutic dose for those with thrombotic APS – during ART procedures.
In patients with SLE, another concern is that increased estrogen will cause disease flare. One case series published in 2017 reported 37 patients with SLE and/or APS who underwent 97 IVF cycles, of which 8% were complicated by flare or thrombotic events. Notably, half of these complications occurred in patients who stopped prescribed therapies (immunomodulatory therapy in two patients with SLE, anticoagulation in two patients with APS) after failure to conceive. In a separate study from 2000 including 19 patients with SLE, APS, or high-titer aPL who underwent 68 IVF cycles, 19% of cycles in patients with SLE were complicated by flare, and no thrombotic events occurred in the cohort. The authors concluded that ovulation induction does not exacerbate SLE or APS. In these studies, the overall pregnancy rates were felt to be consistent with those achieved by the general population through IVF. Although obstetric complications, such as preeclampsia and preterm delivery, were reported in about half of the pregnancies described, these are known to occur more frequently in those with SLE and APS, especially when active disease or other risk factors are present. There are no large-scale, controlled studies evaluating ART outcomes in patients with autoimmune rheumatic diseases to date.
Finally, ovarian hyperstimulation syndrome (OHSS) is an increasingly rare but severe complication of ovarian stimulation. OHSS is characterized by capillary leak, fluid overload, and cytokine release syndrome and can lead to thromboembolic events. Comorbidities like hypertension and renal failure, which can go along with autoimmune rheumatic diseases, are risk factors for OHSS. The use of human chorionic gonadotropin to trigger ovulation is also associated with an increased risk for OHSS, so a GnRH agonist trigger may be preferable.
The ACR guideline recommends that individuals with any of these underlying conditions undergo ART only in expert centers. The ovarian stimulation protocol needs to be tailored to the individual patient to minimize risk and optimize outcomes. The overall goal when managing patients with autoimmune rheumatic diseases during ART is to establish and maintain disease control with pregnancy-compatible medications (when pregnancy is the goal). With adequate planning, appropriate treatment, and collaboration between obstetricians and rheumatologists, individuals with autoimmune rheumatic diseases can safely pursue ART and go on to have successful pregnancies.
Dr. Siegel is a 2022-2023 UCB Women’s Health rheumatology fellow in the rheumatology reproductive health program of the Barbara Volcker Center for Women and Rheumatic Diseases at Hospital for Special Surgery/Weill Cornell Medicine, New York. Her clinical and research focus is on reproductive health issues in individuals with rheumatic disease. Dr. Chan is an assistant professor at Weill Cornell Medical College and an attending physician at Hospital for Special Surgery and Memorial Sloan Kettering Cancer Center in New York. Before moving to New York City, she spent 7 years in private practice in Rhode Island and was a columnist for a monthly rheumatology publication, writing about the challenges of starting life as a full-fledged rheumatologist in a private practice. Follow Dr Chan on Twitter. Dr. Siegel and Dr. Chan disclosed no relevant financial relationships.
A version of this article – an editorial collaboration between Medscape and the Hospital for Special Surgery – first appeared on Medscape.com.
The field of “reproductive rheumatology” has received growing attention in recent years as we learn more about how autoimmune rheumatic diseases and their treatment affect women of reproductive age. In 2020, the American College of Rheumatology published a comprehensive guideline that includes recommendations and supporting evidence for managing issues related to reproductive health in patients with rheumatic diseases and has since launched an ongoing Reproductive Health Initiative, with the goal of translating established guidelines into practice through various education and awareness campaigns. One area addressed by the guideline that comes up commonly in practice but receives less attention and research is the use of assisted reproductive technology (ART) in patients with rheumatic diseases.
Literature is conflicting regarding whether patients with autoimmune rheumatic diseases are inherently at increased risk for infertility, defined as failure to achieve a clinical pregnancy after 12 months or more of regular unprotected intercourse, or subfertility, defined as a delay in conception. Regardless, several factors indirectly contribute to a disproportionate risk for infertility or subfertility in this patient population, including active inflammatory disease, reduced ovarian reserve, and medications.
Patients with subfertility or infertility who desire pregnancy may pursue ovulation induction with timed intercourse or intrauterine insemination, in vitro fertilization (IVF)/intracytoplasmic sperm injection with either embryo transfer, or gestational surrogacy. Those who require treatment with cyclophosphamide or who plan to defer pregnancy for whatever reason can opt for oocyte cryopreservation (colloquially known as “egg freezing”). For IVF and oocyte cryopreservation, controlled ovarian stimulation is typically the first step (except in unstimulated, or “natural cycle,” IVF).
Various protocols are used for ovarian stimulation and ovulation induction, the nuances of which are beyond the scope of this article. In general, ovarian stimulation involves gonadotropin therapy (follicle-stimulating hormone and/or human menopausal gonadotropin) administered via scheduled subcutaneous injections to stimulate follicular growth, as well as gonadotropin-releasing hormone (GnRH) agonists or antagonists to suppress luteinizing hormone, preventing ovulation. Adjunctive oral therapy (clomiphene citrate or letrozole, an aromatase inhibitor) may be used as well. The patient has frequent lab monitoring of hormone levels and transvaginal ultrasounds to measure follicle number and size and, when the timing is right, receives an “ovulation trigger” – either human chorionic gonadotropin or GnRH agonist, depending on the protocol. At this point, transvaginal ultrasound–guided egg retrieval is done under sedation. Recovered oocytes are then either frozen for later use or fertilized in the lab for embryo transfer. Lastly, exogenous hormones are often used: estrogen to support frozen embryo transfers and progesterone for so-called luteal phase support.
ART is not contraindicated in patients with autoimmune rheumatic diseases, but there may be additional factors to consider, particularly for those with systemic lupus erythematosus (SLE), antiphospholipid syndrome (APS), and antiphospholipid antibodies (aPL) without clinical APS.
Ovarian stimulation elevates estrogen levels to varying degrees depending on the patient and the medications used. In all cases, though, peak levels are significantly lower than levels reached during pregnancy. It is well established that elevated estrogen – whether from hormone therapies or pregnancy – significantly increases thrombotic risk, even in healthy people. High-risk patients should receive low-molecular-weight heparin – a prophylactic dose for patients with either positive aPL without clinical APS (including those with SLE) or with obstetric APS, and a therapeutic dose for those with thrombotic APS – during ART procedures.
In patients with SLE, another concern is that increased estrogen will cause disease flare. One case series published in 2017 reported 37 patients with SLE and/or APS who underwent 97 IVF cycles, of which 8% were complicated by flare or thrombotic events. Notably, half of these complications occurred in patients who stopped prescribed therapies (immunomodulatory therapy in two patients with SLE, anticoagulation in two patients with APS) after failure to conceive. In a separate study from 2000 including 19 patients with SLE, APS, or high-titer aPL who underwent 68 IVF cycles, 19% of cycles in patients with SLE were complicated by flare, and no thrombotic events occurred in the cohort. The authors concluded that ovulation induction does not exacerbate SLE or APS. In these studies, the overall pregnancy rates were felt to be consistent with those achieved by the general population through IVF. Although obstetric complications, such as preeclampsia and preterm delivery, were reported in about half of the pregnancies described, these are known to occur more frequently in those with SLE and APS, especially when active disease or other risk factors are present. There are no large-scale, controlled studies evaluating ART outcomes in patients with autoimmune rheumatic diseases to date.
Finally, ovarian hyperstimulation syndrome (OHSS) is an increasingly rare but severe complication of ovarian stimulation. OHSS is characterized by capillary leak, fluid overload, and cytokine release syndrome and can lead to thromboembolic events. Comorbidities like hypertension and renal failure, which can go along with autoimmune rheumatic diseases, are risk factors for OHSS. The use of human chorionic gonadotropin to trigger ovulation is also associated with an increased risk for OHSS, so a GnRH agonist trigger may be preferable.
The ACR guideline recommends that individuals with any of these underlying conditions undergo ART only in expert centers. The ovarian stimulation protocol needs to be tailored to the individual patient to minimize risk and optimize outcomes. The overall goal when managing patients with autoimmune rheumatic diseases during ART is to establish and maintain disease control with pregnancy-compatible medications (when pregnancy is the goal). With adequate planning, appropriate treatment, and collaboration between obstetricians and rheumatologists, individuals with autoimmune rheumatic diseases can safely pursue ART and go on to have successful pregnancies.
Dr. Siegel is a 2022-2023 UCB Women’s Health rheumatology fellow in the rheumatology reproductive health program of the Barbara Volcker Center for Women and Rheumatic Diseases at Hospital for Special Surgery/Weill Cornell Medicine, New York. Her clinical and research focus is on reproductive health issues in individuals with rheumatic disease. Dr. Chan is an assistant professor at Weill Cornell Medical College and an attending physician at Hospital for Special Surgery and Memorial Sloan Kettering Cancer Center in New York. Before moving to New York City, she spent 7 years in private practice in Rhode Island and was a columnist for a monthly rheumatology publication, writing about the challenges of starting life as a full-fledged rheumatologist in a private practice. Follow Dr Chan on Twitter. Dr. Siegel and Dr. Chan disclosed no relevant financial relationships.
A version of this article – an editorial collaboration between Medscape and the Hospital for Special Surgery – first appeared on Medscape.com.
Then and now: Gut microbiome
The HMP, which was supported by “only” approximately $20 million of funding in its first year, served as a catalyst for the development of computational tools, clinical protocols, and reference datasets for an emerging field that now approaches nearly $2 billion per year in market value of diagnostics and therapeutics.
Over the past 15 years, many important discoveries about the microbiome have been made, particularly in the fields of gastroenterology, hepatology, and nutrition. The transplantation of gut microbiome from one person to another has been shown to be more than 90% effective in the treatment of recurrent C. difficile infection, disrupting our current therapeutic algorithms of repetitive antibiotics. Other exciting discoveries have included the relationship between the gut microbiome and enteric nervous system, and its roles in the regulation of metabolism and obesity and in the progression of liver fibrosis and cancer.
Looking ahead, several exciting areas related to digestive health and the microbiome are being prioritized, including the role of probiotics in nutrition, the complex relationship of the bidirectional “gut-brain” axis, and further development of analytics to define and deliver precision medicine across a wide range of digestive disorders. Without a doubt, emerging microbiome discoveries will be prominently featured in the pages of GI & Hepatology News over the coming years to keep our readers informed of these cutting-edge findings.
Dr. Rosenberg is medical director of the North Shore Endoscopy Center and director of clinical research at GI Alliance of Illinois in Gurnee, Ill. Dr. Rosenberg is a consultant for Aimmune Therapeutics and performs clinical research with Ferring Pharmaceuticals.
The HMP, which was supported by “only” approximately $20 million of funding in its first year, served as a catalyst for the development of computational tools, clinical protocols, and reference datasets for an emerging field that now approaches nearly $2 billion per year in market value of diagnostics and therapeutics.
Over the past 15 years, many important discoveries about the microbiome have been made, particularly in the fields of gastroenterology, hepatology, and nutrition. The transplantation of gut microbiome from one person to another has been shown to be more than 90% effective in the treatment of recurrent C. difficile infection, disrupting our current therapeutic algorithms of repetitive antibiotics. Other exciting discoveries have included the relationship between the gut microbiome and enteric nervous system, and its roles in the regulation of metabolism and obesity and in the progression of liver fibrosis and cancer.
Looking ahead, several exciting areas related to digestive health and the microbiome are being prioritized, including the role of probiotics in nutrition, the complex relationship of the bidirectional “gut-brain” axis, and further development of analytics to define and deliver precision medicine across a wide range of digestive disorders. Without a doubt, emerging microbiome discoveries will be prominently featured in the pages of GI & Hepatology News over the coming years to keep our readers informed of these cutting-edge findings.
Dr. Rosenberg is medical director of the North Shore Endoscopy Center and director of clinical research at GI Alliance of Illinois in Gurnee, Ill. Dr. Rosenberg is a consultant for Aimmune Therapeutics and performs clinical research with Ferring Pharmaceuticals.
The HMP, which was supported by “only” approximately $20 million of funding in its first year, served as a catalyst for the development of computational tools, clinical protocols, and reference datasets for an emerging field that now approaches nearly $2 billion per year in market value of diagnostics and therapeutics.
Over the past 15 years, many important discoveries about the microbiome have been made, particularly in the fields of gastroenterology, hepatology, and nutrition. The transplantation of gut microbiome from one person to another has been shown to be more than 90% effective in the treatment of recurrent C. difficile infection, disrupting our current therapeutic algorithms of repetitive antibiotics. Other exciting discoveries have included the relationship between the gut microbiome and enteric nervous system, and its roles in the regulation of metabolism and obesity and in the progression of liver fibrosis and cancer.
Looking ahead, several exciting areas related to digestive health and the microbiome are being prioritized, including the role of probiotics in nutrition, the complex relationship of the bidirectional “gut-brain” axis, and further development of analytics to define and deliver precision medicine across a wide range of digestive disorders. Without a doubt, emerging microbiome discoveries will be prominently featured in the pages of GI & Hepatology News over the coming years to keep our readers informed of these cutting-edge findings.
Dr. Rosenberg is medical director of the North Shore Endoscopy Center and director of clinical research at GI Alliance of Illinois in Gurnee, Ill. Dr. Rosenberg is a consultant for Aimmune Therapeutics and performs clinical research with Ferring Pharmaceuticals.
I’m a physician battling long COVID. I can assure you it’s real
One in 5. It almost seems unimaginable that this is the real number of people who are struggling with long COVID, especially considering how many people in the United States have had COVID-19 at this point (more than 96 million).
Even more unimaginable at this time is that it’s happening to me. I’ve experienced not only the disabling effects of long COVID, but I’ve also seen, firsthand, the frustration of navigating diagnosis and treatment. It’s given me a taste of what millions of other patients are going through.
Vaxxed, masked, and (too) relaxed
I caught COVID-19 (probably Omicron BA.5) that presented as sniffles, making me think it was probably just allergies. However, my resting heart rate was up on my Garmin watch, so of course I got tested and was positive.
With my symptoms virtually nonexistent, it seemed, at the time, merely an inconvenience, because I was forced to isolate away from family and friends, who all stayed negative.
But 2 weeks later, I began to have urticaria – hives – after physical exertion. Did that mean my mast cells were angry? There’s some evidence these immune cells become overactivated in some patients with COVID. Next, I began to experience lightheadedness and the rapid heartbeat of tachycardia. The tachycardia was especially bad any time I physically exerted myself, including on a walk. Imagine me – a lover of all bargain shopping – cutting short a trip to the outlet mall on a particularly bad day when my heart rate was 140 after taking just a few steps. This was orthostatic intolerance.
Then came the severe worsening of my migraines – which are often vestibular, making me nauseated and dizzy on top of the throbbing.
I was of course familiar with these symptoms, as professor and chair of the department of rehabilitation medicine at the Joe R. and Teresa Lozano Long School of Medicine at University of Texas Health Science Center, San Antonio. I developed a post-COVID recovery clinic to help patients.
So I knew about postexertional malaise (PEM) and postexertional symptom exacerbation (PESE), but I was now experiencing these distressing symptoms firsthand.
Clinicians really need to look for this cardinal sign of long COVID as well as evidence of myalgic encephalomyelitis or chronic fatigue syndrome (ME/CFS). ME/CFS is marked by exacerbation of fatigue or symptoms after an activity that could previously be done without these aftereffects. In my case, as an All-American Masters miler with several marathons under my belt, running 5 miles is a walk in the park. But now, I pay for those 5 miles for the rest of the day on the couch or with palpitations, dizziness, and fatigue the following day. Busy clinic day full of procedures? I would have to be sitting by the end of it. Bed by 9 PM was not always early enough.
Becoming a statistic
Here I am, one of the leading experts in the country on caring for people with long COVID, featured in the national news and having testified in front of Congress, and now I am part of that lived experience. Me – a healthy athlete, with no comorbidities, a normal BMI, vaccinated and boosted, and after an almost asymptomatic bout of COVID-19, a victim to long COVID.
You just never know how your body is going to react. Neuroinflammation occurred in studies with mice with mild respiratory COVID and could be happening to me. I did not want a chronic immune-mediated vasculopathy.
So, I did what any other hyperaware physician-researcher would do. I enrolled in the RECOVER trial – a study my own institution is taking part in and one that I recommend to my own patients.
I also decided that I need to access care and not just ignore my symptoms or try to treat them myself.
That’s when things got difficult. There was a wait of at least a month to see my primary care provider – but I was able to use my privileged position as a physician to get in sooner.
My provider said that she had limited knowledge of long COVID, and she hesitated to order some of the tests and treatments that I recommended because they were not yet considered standard of care. I can understand the hesitation. It is engrained in medical education to follow evidence based on the highest-quality research studies. We are slowly learning more about long COVID, but acknowledging the learning curve offers little to patients who need help now.
This has made me realize that we cannot wait on an evidence-based approach – which can take decades to develop – while people are suffering. And it’s important that everyone on the front line learn about some of the manifestations and disease management of long COVID.
I left this first physician visit feeling more defeated than anything and decided to try to push through. That, I quickly realized, was not the right thing to do.
So again, after a couple of significant crashes and days of severe migraines, I phoned a friend: Ratna Bhavaraju-Sanka, MD, the amazing neurologist who treats patients with long COVID alongside me. She squeezed me in on a non-clinic day. Again, I had the privilege to see a specialist most people wait half a year to see. I was diagnosed with both autonomic dysfunction and intractable migraine.
She ordered some intravenous fluids and IV magnesium that would probably help both. But then another obstacle arose. My institution’s infusion center is focused on patients with cancer, and I was unable to schedule treatments there.
Luckily, I knew about the concierge mobile IV hydration therapy companies that come to your house – mostly offering a hangover treatment service. And I am thankful that I had the health literacy and financial ability to pay for some fluids at home.
On another particularly bad day, I phoned other friends – higher-ups at the hospital – who expedited a slot at the hospital infusion center and approval for the IV magnesium.
Thanks to my access, knowledge, and other privileges, I got fairly quick if imperfect care, enrolled in a research trial, and received medications. I knew to pace myself. The vast majority of others with long COVID lack these advantages.
The patient with long COVID
Things I have learned that others can learn, too:
- Acknowledge and recognize that long COVID is a disease that is affecting 1 in 5 Americans who catch COVID. Many look completely “normal on the outside.” Please listen to your patients.
- Autonomic dysfunction is a common manifestation of long COVID. A 10-minute stand test goes a long way in diagnosing this condition, from the American Academy of Physical Medicine and Rehabilitation. It is not just anxiety.
- “That’s only in research” is dismissive and harmful. Think outside the box. Follow guidelines. Consider encouraging patients to sign up for trials.
- Screen for PEM/PESE and teach your patients to pace themselves, because pushing through it or doing graded exercises will be harmful.
- We need to train more physicians to treat postacute sequelae of SARS-CoV-2 infection () and other postinfectious conditions, such as ME/CFS.
If long COVID is hard for physicians to understand and deal with, imagine how difficult it is for patients with no expertise in this area.
It is exponentially harder for those with fewer resources, time, and health literacy. My lived experience with long COVID has shown me that being a patient is never easy. You put your body and fate into the hands of trusted professionals and expect validation and assistance, not gaslighting or gatekeeping.
Along with millions of others, I am tired of waiting.
Dr. Gutierrez is Professor and Distinguished Chair, department of rehabilitation medicine, University of Texas Health Science Center at San Antonio. She reported receiving honoraria for lecturing on long COVID and receiving a research grant from Co-PI for the NIH RECOVER trial.
A version of this article first appeared on Medscape.com.
One in 5. It almost seems unimaginable that this is the real number of people who are struggling with long COVID, especially considering how many people in the United States have had COVID-19 at this point (more than 96 million).
Even more unimaginable at this time is that it’s happening to me. I’ve experienced not only the disabling effects of long COVID, but I’ve also seen, firsthand, the frustration of navigating diagnosis and treatment. It’s given me a taste of what millions of other patients are going through.
Vaxxed, masked, and (too) relaxed
I caught COVID-19 (probably Omicron BA.5) that presented as sniffles, making me think it was probably just allergies. However, my resting heart rate was up on my Garmin watch, so of course I got tested and was positive.
With my symptoms virtually nonexistent, it seemed, at the time, merely an inconvenience, because I was forced to isolate away from family and friends, who all stayed negative.
But 2 weeks later, I began to have urticaria – hives – after physical exertion. Did that mean my mast cells were angry? There’s some evidence these immune cells become overactivated in some patients with COVID. Next, I began to experience lightheadedness and the rapid heartbeat of tachycardia. The tachycardia was especially bad any time I physically exerted myself, including on a walk. Imagine me – a lover of all bargain shopping – cutting short a trip to the outlet mall on a particularly bad day when my heart rate was 140 after taking just a few steps. This was orthostatic intolerance.
Then came the severe worsening of my migraines – which are often vestibular, making me nauseated and dizzy on top of the throbbing.
I was of course familiar with these symptoms, as professor and chair of the department of rehabilitation medicine at the Joe R. and Teresa Lozano Long School of Medicine at University of Texas Health Science Center, San Antonio. I developed a post-COVID recovery clinic to help patients.
So I knew about postexertional malaise (PEM) and postexertional symptom exacerbation (PESE), but I was now experiencing these distressing symptoms firsthand.
Clinicians really need to look for this cardinal sign of long COVID as well as evidence of myalgic encephalomyelitis or chronic fatigue syndrome (ME/CFS). ME/CFS is marked by exacerbation of fatigue or symptoms after an activity that could previously be done without these aftereffects. In my case, as an All-American Masters miler with several marathons under my belt, running 5 miles is a walk in the park. But now, I pay for those 5 miles for the rest of the day on the couch or with palpitations, dizziness, and fatigue the following day. Busy clinic day full of procedures? I would have to be sitting by the end of it. Bed by 9 PM was not always early enough.
Becoming a statistic
Here I am, one of the leading experts in the country on caring for people with long COVID, featured in the national news and having testified in front of Congress, and now I am part of that lived experience. Me – a healthy athlete, with no comorbidities, a normal BMI, vaccinated and boosted, and after an almost asymptomatic bout of COVID-19, a victim to long COVID.
You just never know how your body is going to react. Neuroinflammation occurred in studies with mice with mild respiratory COVID and could be happening to me. I did not want a chronic immune-mediated vasculopathy.
So, I did what any other hyperaware physician-researcher would do. I enrolled in the RECOVER trial – a study my own institution is taking part in and one that I recommend to my own patients.
I also decided that I need to access care and not just ignore my symptoms or try to treat them myself.
That’s when things got difficult. There was a wait of at least a month to see my primary care provider – but I was able to use my privileged position as a physician to get in sooner.
My provider said that she had limited knowledge of long COVID, and she hesitated to order some of the tests and treatments that I recommended because they were not yet considered standard of care. I can understand the hesitation. It is engrained in medical education to follow evidence based on the highest-quality research studies. We are slowly learning more about long COVID, but acknowledging the learning curve offers little to patients who need help now.
This has made me realize that we cannot wait on an evidence-based approach – which can take decades to develop – while people are suffering. And it’s important that everyone on the front line learn about some of the manifestations and disease management of long COVID.
I left this first physician visit feeling more defeated than anything and decided to try to push through. That, I quickly realized, was not the right thing to do.
So again, after a couple of significant crashes and days of severe migraines, I phoned a friend: Ratna Bhavaraju-Sanka, MD, the amazing neurologist who treats patients with long COVID alongside me. She squeezed me in on a non-clinic day. Again, I had the privilege to see a specialist most people wait half a year to see. I was diagnosed with both autonomic dysfunction and intractable migraine.
She ordered some intravenous fluids and IV magnesium that would probably help both. But then another obstacle arose. My institution’s infusion center is focused on patients with cancer, and I was unable to schedule treatments there.
Luckily, I knew about the concierge mobile IV hydration therapy companies that come to your house – mostly offering a hangover treatment service. And I am thankful that I had the health literacy and financial ability to pay for some fluids at home.
On another particularly bad day, I phoned other friends – higher-ups at the hospital – who expedited a slot at the hospital infusion center and approval for the IV magnesium.
Thanks to my access, knowledge, and other privileges, I got fairly quick if imperfect care, enrolled in a research trial, and received medications. I knew to pace myself. The vast majority of others with long COVID lack these advantages.
The patient with long COVID
Things I have learned that others can learn, too:
- Acknowledge and recognize that long COVID is a disease that is affecting 1 in 5 Americans who catch COVID. Many look completely “normal on the outside.” Please listen to your patients.
- Autonomic dysfunction is a common manifestation of long COVID. A 10-minute stand test goes a long way in diagnosing this condition, from the American Academy of Physical Medicine and Rehabilitation. It is not just anxiety.
- “That’s only in research” is dismissive and harmful. Think outside the box. Follow guidelines. Consider encouraging patients to sign up for trials.
- Screen for PEM/PESE and teach your patients to pace themselves, because pushing through it or doing graded exercises will be harmful.
- We need to train more physicians to treat postacute sequelae of SARS-CoV-2 infection () and other postinfectious conditions, such as ME/CFS.
If long COVID is hard for physicians to understand and deal with, imagine how difficult it is for patients with no expertise in this area.
It is exponentially harder for those with fewer resources, time, and health literacy. My lived experience with long COVID has shown me that being a patient is never easy. You put your body and fate into the hands of trusted professionals and expect validation and assistance, not gaslighting or gatekeeping.
Along with millions of others, I am tired of waiting.
Dr. Gutierrez is Professor and Distinguished Chair, department of rehabilitation medicine, University of Texas Health Science Center at San Antonio. She reported receiving honoraria for lecturing on long COVID and receiving a research grant from Co-PI for the NIH RECOVER trial.
A version of this article first appeared on Medscape.com.
One in 5. It almost seems unimaginable that this is the real number of people who are struggling with long COVID, especially considering how many people in the United States have had COVID-19 at this point (more than 96 million).
Even more unimaginable at this time is that it’s happening to me. I’ve experienced not only the disabling effects of long COVID, but I’ve also seen, firsthand, the frustration of navigating diagnosis and treatment. It’s given me a taste of what millions of other patients are going through.
Vaxxed, masked, and (too) relaxed
I caught COVID-19 (probably Omicron BA.5) that presented as sniffles, making me think it was probably just allergies. However, my resting heart rate was up on my Garmin watch, so of course I got tested and was positive.
With my symptoms virtually nonexistent, it seemed, at the time, merely an inconvenience, because I was forced to isolate away from family and friends, who all stayed negative.
But 2 weeks later, I began to have urticaria – hives – after physical exertion. Did that mean my mast cells were angry? There’s some evidence these immune cells become overactivated in some patients with COVID. Next, I began to experience lightheadedness and the rapid heartbeat of tachycardia. The tachycardia was especially bad any time I physically exerted myself, including on a walk. Imagine me – a lover of all bargain shopping – cutting short a trip to the outlet mall on a particularly bad day when my heart rate was 140 after taking just a few steps. This was orthostatic intolerance.
Then came the severe worsening of my migraines – which are often vestibular, making me nauseated and dizzy on top of the throbbing.
I was of course familiar with these symptoms, as professor and chair of the department of rehabilitation medicine at the Joe R. and Teresa Lozano Long School of Medicine at University of Texas Health Science Center, San Antonio. I developed a post-COVID recovery clinic to help patients.
So I knew about postexertional malaise (PEM) and postexertional symptom exacerbation (PESE), but I was now experiencing these distressing symptoms firsthand.
Clinicians really need to look for this cardinal sign of long COVID as well as evidence of myalgic encephalomyelitis or chronic fatigue syndrome (ME/CFS). ME/CFS is marked by exacerbation of fatigue or symptoms after an activity that could previously be done without these aftereffects. In my case, as an All-American Masters miler with several marathons under my belt, running 5 miles is a walk in the park. But now, I pay for those 5 miles for the rest of the day on the couch or with palpitations, dizziness, and fatigue the following day. Busy clinic day full of procedures? I would have to be sitting by the end of it. Bed by 9 PM was not always early enough.
Becoming a statistic
Here I am, one of the leading experts in the country on caring for people with long COVID, featured in the national news and having testified in front of Congress, and now I am part of that lived experience. Me – a healthy athlete, with no comorbidities, a normal BMI, vaccinated and boosted, and after an almost asymptomatic bout of COVID-19, a victim to long COVID.
You just never know how your body is going to react. Neuroinflammation occurred in studies with mice with mild respiratory COVID and could be happening to me. I did not want a chronic immune-mediated vasculopathy.
So, I did what any other hyperaware physician-researcher would do. I enrolled in the RECOVER trial – a study my own institution is taking part in and one that I recommend to my own patients.
I also decided that I need to access care and not just ignore my symptoms or try to treat them myself.
That’s when things got difficult. There was a wait of at least a month to see my primary care provider – but I was able to use my privileged position as a physician to get in sooner.
My provider said that she had limited knowledge of long COVID, and she hesitated to order some of the tests and treatments that I recommended because they were not yet considered standard of care. I can understand the hesitation. It is engrained in medical education to follow evidence based on the highest-quality research studies. We are slowly learning more about long COVID, but acknowledging the learning curve offers little to patients who need help now.
This has made me realize that we cannot wait on an evidence-based approach – which can take decades to develop – while people are suffering. And it’s important that everyone on the front line learn about some of the manifestations and disease management of long COVID.
I left this first physician visit feeling more defeated than anything and decided to try to push through. That, I quickly realized, was not the right thing to do.
So again, after a couple of significant crashes and days of severe migraines, I phoned a friend: Ratna Bhavaraju-Sanka, MD, the amazing neurologist who treats patients with long COVID alongside me. She squeezed me in on a non-clinic day. Again, I had the privilege to see a specialist most people wait half a year to see. I was diagnosed with both autonomic dysfunction and intractable migraine.
She ordered some intravenous fluids and IV magnesium that would probably help both. But then another obstacle arose. My institution’s infusion center is focused on patients with cancer, and I was unable to schedule treatments there.
Luckily, I knew about the concierge mobile IV hydration therapy companies that come to your house – mostly offering a hangover treatment service. And I am thankful that I had the health literacy and financial ability to pay for some fluids at home.
On another particularly bad day, I phoned other friends – higher-ups at the hospital – who expedited a slot at the hospital infusion center and approval for the IV magnesium.
Thanks to my access, knowledge, and other privileges, I got fairly quick if imperfect care, enrolled in a research trial, and received medications. I knew to pace myself. The vast majority of others with long COVID lack these advantages.
The patient with long COVID
Things I have learned that others can learn, too:
- Acknowledge and recognize that long COVID is a disease that is affecting 1 in 5 Americans who catch COVID. Many look completely “normal on the outside.” Please listen to your patients.
- Autonomic dysfunction is a common manifestation of long COVID. A 10-minute stand test goes a long way in diagnosing this condition, from the American Academy of Physical Medicine and Rehabilitation. It is not just anxiety.
- “That’s only in research” is dismissive and harmful. Think outside the box. Follow guidelines. Consider encouraging patients to sign up for trials.
- Screen for PEM/PESE and teach your patients to pace themselves, because pushing through it or doing graded exercises will be harmful.
- We need to train more physicians to treat postacute sequelae of SARS-CoV-2 infection () and other postinfectious conditions, such as ME/CFS.
If long COVID is hard for physicians to understand and deal with, imagine how difficult it is for patients with no expertise in this area.
It is exponentially harder for those with fewer resources, time, and health literacy. My lived experience with long COVID has shown me that being a patient is never easy. You put your body and fate into the hands of trusted professionals and expect validation and assistance, not gaslighting or gatekeeping.
Along with millions of others, I am tired of waiting.
Dr. Gutierrez is Professor and Distinguished Chair, department of rehabilitation medicine, University of Texas Health Science Center at San Antonio. She reported receiving honoraria for lecturing on long COVID and receiving a research grant from Co-PI for the NIH RECOVER trial.
A version of this article first appeared on Medscape.com.
BMI and reproduction – weighing the evidence
Arguably, no topic during an infertility consultation generates more of an emotional reaction than discussing body mass index (BMI), particularly when it is high. Patients have become increasingly sensitive to weight discussions with their physicians because of concerns about body shaming. Among patients with an elevated BMI, criticism on social media of health care professionals’ counseling and a preemptive presentation of “Don’t Weigh Me” cards have become popular responses. Despite the medical evidence on impaired reproduction with an abnormal BMI, patients are choosing to forgo the topic. Research has demonstrated “extensive evidence [of] strong weight bias” in a wide range of health staff.1 A “viral” TikTok study revealed that medical “gaslighting” founded in weight stigma and bias is harmful, as reported on KevinMD.com.2 This month, we review the effect of abnormal BMI, both high and low, on reproduction and pregnancy.
A method to assess relative weight was first described in 1832 as its ratio in kilograms divided by the square of the height in meters, or the Quetelet Index. The search for a functional assessment of relative body weight began after World War II when reports by actuaries noted the increased mortality of overweight policyholders. The relationship between weight and cardiovascular disease was further revealed in epidemiologic studies. The Quetelet Index became the BMI in 1972.3
Weight measurement is a mainstay in the assessment of a patient’s vital signs along with blood pressure, pulse rate, respiration rate, and temperature. Weight is vital to the calculation of medication dosage – for instance, administration of conscious sedative drugs, methotrexate, and gonadotropins. Some state boards of medicine, such as Florida, have a limitation on patient BMI at office-based surgery centers (40 kg/m2).
Obesity is a disease
As reported by the World Health Organization in 2022, the disease of obesity is an epidemic afflicting more than 1 billion people worldwide, or 1 in 8 individuals globally.4 The health implications of an elevated BMI include increased mortality, diabetes, heart disease, and stroke, physical limitations to activities of daily living, and complications affecting reproduction.
Female obesity is related to poorer outcomes in natural and assisted conception, including an increased risk of miscarriage. Compared with normal-weight women, those with obesity are three times more likely to have ovulatory dysfunction,5 infertility,6 a lower chance for conception,7 higher rate of miscarriage, and low birth weight.8,9During pregnancy, women with obesity have three to four times higher rates of gestational diabetes and preeclampsia,10 as well as likelihood of delivering preterm,11 having a fetus with macrosomia and birth defects, and a 1.3- to 2.1-times higher risk of stillbirth.12
Obesity is present in 40%-80% of women with polycystic ovary syndrome,13 the most common cause of ovulatory dysfunction from dysregulation of the hypothalamic-pituitary-ovarian axis. While PCOS is associated with reproductive and metabolic consequences, even in regularly ovulating women, increasing obesity appears to be associated with decreasing spontaneous pregnancy rates and increased time to pregnancy.14
Obesity and IVF
Women with obesity have reduced success with assisted reproductive technology, an increased number of canceled cycles, and poorer quality oocytes retrieved. A prospective cohort study of nearly 2,000 women reported that every 5 kg of body weight increase (from the patient’s baseline weight at age 18) was associated with a 5% increase in the mean duration of time required for conception (95% confidence interval, 3%-7%).15 Given that approximately 90% of these women had regular menstrual cycles, ovulatory dysfunction was not the suspected pathophysiology.
A meta-analysis of 21 cohort studies reported a lower likelihood of live birth following in vitro fertilization for women with obesity, compared with normal-weight women (risk ratio, 0.85; 95% CI, 0.82-0.87).16 A further subgroup analysis that evaluated only women with PCOS showed a reduction in the live birth rate following IVF for individuals with obesity, compared with normal-weight individuals (RR, 0.78; 95% CI, 0.74-0.82).
In a retrospective study of almost 500,000 fresh autologous IVF cycles, women with obesity had a 6% reduction in pregnancy rates and a 13% reduction in live birth rates, compared with normal-weight women. Both high and low BMI were associated with an increased risk of low birth weight and preterm delivery.17 The live birth rates per transfer for normal-weight and higher-weight women were 38% and 33%, respectively.
Contrarily, a randomized controlled trial showed that an intensive weight-reduction program resulted in a large weight loss but did not substantially affect live birth rates in women with obesity scheduled for IVF.18
Low BMI
A noteworthy cause of low BMI is functional hypothalamic amenorrhea (FHA), a disorder with low energy availability either from decreased caloric intake and/or excessive energy expenditure associated with eating disorders, excessive exercise, and stress. Consequently, a reduced GnRH drive results in a decreased pulse frequency and amplitude leading to low levels of follicle-stimulating hormone and luteinizing hormone, resulting in anovulation. Correction of lifestyle behaviors related to FHA can restore menstrual cycles. After normal weight is achieved, it appears unlikely that fertility is affected.19 In 47% of adolescent patients with anorexia, menses spontaneously returned within the first 12 months after admission, with an improved prognosis in secondary over primary amenorrhea.20,21 Interestingly, mildly and significantly underweight infertile women have pregnancy and live birth rates similar to normal-weight patients after IVF treatment.22
Pregnancy is complicated in underweight women, resulting in an increased risk of anemia, fetal growth retardation, and low birth weight, as well as preterm birth.21
Take-home message
The extremes of BMI both impair natural reproduction. Elevated BMI reduces success with IVF but rapid weight loss prior to IVF does not improve outcomes. A normal BMI is the goal for optimal reproductive and pregnancy health.
Dr. Trolice is director of the IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Talumaa B et al. Obesity Rev. 2022;23:e13494.
2. https://bit.ly/3rHCivE.
3. Eknoyan G. Nephrol Dial Transplant. 2008;23:47-51.
4. Wells JCK. Dis Models Mech. 2012;5:595-607.
5. Brewer CJ and Balen AH. Reproduction. 2010;140:347-64.
6. Silvestris E et al. Reprod Biol Endocrinol. 2018;16:22.
7. Wise LA et al. Hum Reprod. 2010;25:253-64.
8. Bellver J. Curr Opin Obstet Gynecol. 2022;34:114-21.
9. Dickey RP et al. Am J Obstet Gynecol. 2013;209:349.e1.
10. Alwash SM et al. Obes Res Clin Pract. 2021;15:425-30.
11. Cnattingius S et al. JAMA. 2013;309:2362-70.
12. Aune D et al. JAMA. 2014;311:1536-46.
13. Sam S. Obes Manag. 2007;3:69-73.
14. van der Steeg JW et al. Hum Reprod. 2008;23:324-8.
15. Gaskins AJ et al. Obstet Gynecol. 2015;126:850-8.
16. Sermondade N et al. Hum Reprod Update. 2019;25:439-519.
17. Kawwass JF et al. Fertil Steril. 2016;106[7]:1742-50.
18. Einarsson S et al. Hum Reprod. 2017;32:1621-30.
19. Chaer R et al. Diseases. 2020;8:46.
20. Dempfle A et al. Psychiatry. 2013;13:308.
21. Verma A and Shrimali L. J Clin Diagn Res. 2012;6:1531-3.
22. Romanski PA et al. Reprod Biomed Online. 2020;42:366-74.
Arguably, no topic during an infertility consultation generates more of an emotional reaction than discussing body mass index (BMI), particularly when it is high. Patients have become increasingly sensitive to weight discussions with their physicians because of concerns about body shaming. Among patients with an elevated BMI, criticism on social media of health care professionals’ counseling and a preemptive presentation of “Don’t Weigh Me” cards have become popular responses. Despite the medical evidence on impaired reproduction with an abnormal BMI, patients are choosing to forgo the topic. Research has demonstrated “extensive evidence [of] strong weight bias” in a wide range of health staff.1 A “viral” TikTok study revealed that medical “gaslighting” founded in weight stigma and bias is harmful, as reported on KevinMD.com.2 This month, we review the effect of abnormal BMI, both high and low, on reproduction and pregnancy.
A method to assess relative weight was first described in 1832 as its ratio in kilograms divided by the square of the height in meters, or the Quetelet Index. The search for a functional assessment of relative body weight began after World War II when reports by actuaries noted the increased mortality of overweight policyholders. The relationship between weight and cardiovascular disease was further revealed in epidemiologic studies. The Quetelet Index became the BMI in 1972.3
Weight measurement is a mainstay in the assessment of a patient’s vital signs along with blood pressure, pulse rate, respiration rate, and temperature. Weight is vital to the calculation of medication dosage – for instance, administration of conscious sedative drugs, methotrexate, and gonadotropins. Some state boards of medicine, such as Florida, have a limitation on patient BMI at office-based surgery centers (40 kg/m2).
Obesity is a disease
As reported by the World Health Organization in 2022, the disease of obesity is an epidemic afflicting more than 1 billion people worldwide, or 1 in 8 individuals globally.4 The health implications of an elevated BMI include increased mortality, diabetes, heart disease, and stroke, physical limitations to activities of daily living, and complications affecting reproduction.
Female obesity is related to poorer outcomes in natural and assisted conception, including an increased risk of miscarriage. Compared with normal-weight women, those with obesity are three times more likely to have ovulatory dysfunction,5 infertility,6 a lower chance for conception,7 higher rate of miscarriage, and low birth weight.8,9During pregnancy, women with obesity have three to four times higher rates of gestational diabetes and preeclampsia,10 as well as likelihood of delivering preterm,11 having a fetus with macrosomia and birth defects, and a 1.3- to 2.1-times higher risk of stillbirth.12
Obesity is present in 40%-80% of women with polycystic ovary syndrome,13 the most common cause of ovulatory dysfunction from dysregulation of the hypothalamic-pituitary-ovarian axis. While PCOS is associated with reproductive and metabolic consequences, even in regularly ovulating women, increasing obesity appears to be associated with decreasing spontaneous pregnancy rates and increased time to pregnancy.14
Obesity and IVF
Women with obesity have reduced success with assisted reproductive technology, an increased number of canceled cycles, and poorer quality oocytes retrieved. A prospective cohort study of nearly 2,000 women reported that every 5 kg of body weight increase (from the patient’s baseline weight at age 18) was associated with a 5% increase in the mean duration of time required for conception (95% confidence interval, 3%-7%).15 Given that approximately 90% of these women had regular menstrual cycles, ovulatory dysfunction was not the suspected pathophysiology.
A meta-analysis of 21 cohort studies reported a lower likelihood of live birth following in vitro fertilization for women with obesity, compared with normal-weight women (risk ratio, 0.85; 95% CI, 0.82-0.87).16 A further subgroup analysis that evaluated only women with PCOS showed a reduction in the live birth rate following IVF for individuals with obesity, compared with normal-weight individuals (RR, 0.78; 95% CI, 0.74-0.82).
In a retrospective study of almost 500,000 fresh autologous IVF cycles, women with obesity had a 6% reduction in pregnancy rates and a 13% reduction in live birth rates, compared with normal-weight women. Both high and low BMI were associated with an increased risk of low birth weight and preterm delivery.17 The live birth rates per transfer for normal-weight and higher-weight women were 38% and 33%, respectively.
Contrarily, a randomized controlled trial showed that an intensive weight-reduction program resulted in a large weight loss but did not substantially affect live birth rates in women with obesity scheduled for IVF.18
Low BMI
A noteworthy cause of low BMI is functional hypothalamic amenorrhea (FHA), a disorder with low energy availability either from decreased caloric intake and/or excessive energy expenditure associated with eating disorders, excessive exercise, and stress. Consequently, a reduced GnRH drive results in a decreased pulse frequency and amplitude leading to low levels of follicle-stimulating hormone and luteinizing hormone, resulting in anovulation. Correction of lifestyle behaviors related to FHA can restore menstrual cycles. After normal weight is achieved, it appears unlikely that fertility is affected.19 In 47% of adolescent patients with anorexia, menses spontaneously returned within the first 12 months after admission, with an improved prognosis in secondary over primary amenorrhea.20,21 Interestingly, mildly and significantly underweight infertile women have pregnancy and live birth rates similar to normal-weight patients after IVF treatment.22
Pregnancy is complicated in underweight women, resulting in an increased risk of anemia, fetal growth retardation, and low birth weight, as well as preterm birth.21
Take-home message
The extremes of BMI both impair natural reproduction. Elevated BMI reduces success with IVF but rapid weight loss prior to IVF does not improve outcomes. A normal BMI is the goal for optimal reproductive and pregnancy health.
Dr. Trolice is director of the IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Talumaa B et al. Obesity Rev. 2022;23:e13494.
2. https://bit.ly/3rHCivE.
3. Eknoyan G. Nephrol Dial Transplant. 2008;23:47-51.
4. Wells JCK. Dis Models Mech. 2012;5:595-607.
5. Brewer CJ and Balen AH. Reproduction. 2010;140:347-64.
6. Silvestris E et al. Reprod Biol Endocrinol. 2018;16:22.
7. Wise LA et al. Hum Reprod. 2010;25:253-64.
8. Bellver J. Curr Opin Obstet Gynecol. 2022;34:114-21.
9. Dickey RP et al. Am J Obstet Gynecol. 2013;209:349.e1.
10. Alwash SM et al. Obes Res Clin Pract. 2021;15:425-30.
11. Cnattingius S et al. JAMA. 2013;309:2362-70.
12. Aune D et al. JAMA. 2014;311:1536-46.
13. Sam S. Obes Manag. 2007;3:69-73.
14. van der Steeg JW et al. Hum Reprod. 2008;23:324-8.
15. Gaskins AJ et al. Obstet Gynecol. 2015;126:850-8.
16. Sermondade N et al. Hum Reprod Update. 2019;25:439-519.
17. Kawwass JF et al. Fertil Steril. 2016;106[7]:1742-50.
18. Einarsson S et al. Hum Reprod. 2017;32:1621-30.
19. Chaer R et al. Diseases. 2020;8:46.
20. Dempfle A et al. Psychiatry. 2013;13:308.
21. Verma A and Shrimali L. J Clin Diagn Res. 2012;6:1531-3.
22. Romanski PA et al. Reprod Biomed Online. 2020;42:366-74.
Arguably, no topic during an infertility consultation generates more of an emotional reaction than discussing body mass index (BMI), particularly when it is high. Patients have become increasingly sensitive to weight discussions with their physicians because of concerns about body shaming. Among patients with an elevated BMI, criticism on social media of health care professionals’ counseling and a preemptive presentation of “Don’t Weigh Me” cards have become popular responses. Despite the medical evidence on impaired reproduction with an abnormal BMI, patients are choosing to forgo the topic. Research has demonstrated “extensive evidence [of] strong weight bias” in a wide range of health staff.1 A “viral” TikTok study revealed that medical “gaslighting” founded in weight stigma and bias is harmful, as reported on KevinMD.com.2 This month, we review the effect of abnormal BMI, both high and low, on reproduction and pregnancy.
A method to assess relative weight was first described in 1832 as its ratio in kilograms divided by the square of the height in meters, or the Quetelet Index. The search for a functional assessment of relative body weight began after World War II when reports by actuaries noted the increased mortality of overweight policyholders. The relationship between weight and cardiovascular disease was further revealed in epidemiologic studies. The Quetelet Index became the BMI in 1972.3
Weight measurement is a mainstay in the assessment of a patient’s vital signs along with blood pressure, pulse rate, respiration rate, and temperature. Weight is vital to the calculation of medication dosage – for instance, administration of conscious sedative drugs, methotrexate, and gonadotropins. Some state boards of medicine, such as Florida, have a limitation on patient BMI at office-based surgery centers (40 kg/m2).
Obesity is a disease
As reported by the World Health Organization in 2022, the disease of obesity is an epidemic afflicting more than 1 billion people worldwide, or 1 in 8 individuals globally.4 The health implications of an elevated BMI include increased mortality, diabetes, heart disease, and stroke, physical limitations to activities of daily living, and complications affecting reproduction.
Female obesity is related to poorer outcomes in natural and assisted conception, including an increased risk of miscarriage. Compared with normal-weight women, those with obesity are three times more likely to have ovulatory dysfunction,5 infertility,6 a lower chance for conception,7 higher rate of miscarriage, and low birth weight.8,9During pregnancy, women with obesity have three to four times higher rates of gestational diabetes and preeclampsia,10 as well as likelihood of delivering preterm,11 having a fetus with macrosomia and birth defects, and a 1.3- to 2.1-times higher risk of stillbirth.12
Obesity is present in 40%-80% of women with polycystic ovary syndrome,13 the most common cause of ovulatory dysfunction from dysregulation of the hypothalamic-pituitary-ovarian axis. While PCOS is associated with reproductive and metabolic consequences, even in regularly ovulating women, increasing obesity appears to be associated with decreasing spontaneous pregnancy rates and increased time to pregnancy.14
Obesity and IVF
Women with obesity have reduced success with assisted reproductive technology, an increased number of canceled cycles, and poorer quality oocytes retrieved. A prospective cohort study of nearly 2,000 women reported that every 5 kg of body weight increase (from the patient’s baseline weight at age 18) was associated with a 5% increase in the mean duration of time required for conception (95% confidence interval, 3%-7%).15 Given that approximately 90% of these women had regular menstrual cycles, ovulatory dysfunction was not the suspected pathophysiology.
A meta-analysis of 21 cohort studies reported a lower likelihood of live birth following in vitro fertilization for women with obesity, compared with normal-weight women (risk ratio, 0.85; 95% CI, 0.82-0.87).16 A further subgroup analysis that evaluated only women with PCOS showed a reduction in the live birth rate following IVF for individuals with obesity, compared with normal-weight individuals (RR, 0.78; 95% CI, 0.74-0.82).
In a retrospective study of almost 500,000 fresh autologous IVF cycles, women with obesity had a 6% reduction in pregnancy rates and a 13% reduction in live birth rates, compared with normal-weight women. Both high and low BMI were associated with an increased risk of low birth weight and preterm delivery.17 The live birth rates per transfer for normal-weight and higher-weight women were 38% and 33%, respectively.
Contrarily, a randomized controlled trial showed that an intensive weight-reduction program resulted in a large weight loss but did not substantially affect live birth rates in women with obesity scheduled for IVF.18
Low BMI
A noteworthy cause of low BMI is functional hypothalamic amenorrhea (FHA), a disorder with low energy availability either from decreased caloric intake and/or excessive energy expenditure associated with eating disorders, excessive exercise, and stress. Consequently, a reduced GnRH drive results in a decreased pulse frequency and amplitude leading to low levels of follicle-stimulating hormone and luteinizing hormone, resulting in anovulation. Correction of lifestyle behaviors related to FHA can restore menstrual cycles. After normal weight is achieved, it appears unlikely that fertility is affected.19 In 47% of adolescent patients with anorexia, menses spontaneously returned within the first 12 months after admission, with an improved prognosis in secondary over primary amenorrhea.20,21 Interestingly, mildly and significantly underweight infertile women have pregnancy and live birth rates similar to normal-weight patients after IVF treatment.22
Pregnancy is complicated in underweight women, resulting in an increased risk of anemia, fetal growth retardation, and low birth weight, as well as preterm birth.21
Take-home message
The extremes of BMI both impair natural reproduction. Elevated BMI reduces success with IVF but rapid weight loss prior to IVF does not improve outcomes. A normal BMI is the goal for optimal reproductive and pregnancy health.
Dr. Trolice is director of the IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Talumaa B et al. Obesity Rev. 2022;23:e13494.
2. https://bit.ly/3rHCivE.
3. Eknoyan G. Nephrol Dial Transplant. 2008;23:47-51.
4. Wells JCK. Dis Models Mech. 2012;5:595-607.
5. Brewer CJ and Balen AH. Reproduction. 2010;140:347-64.
6. Silvestris E et al. Reprod Biol Endocrinol. 2018;16:22.
7. Wise LA et al. Hum Reprod. 2010;25:253-64.
8. Bellver J. Curr Opin Obstet Gynecol. 2022;34:114-21.
9. Dickey RP et al. Am J Obstet Gynecol. 2013;209:349.e1.
10. Alwash SM et al. Obes Res Clin Pract. 2021;15:425-30.
11. Cnattingius S et al. JAMA. 2013;309:2362-70.
12. Aune D et al. JAMA. 2014;311:1536-46.
13. Sam S. Obes Manag. 2007;3:69-73.
14. van der Steeg JW et al. Hum Reprod. 2008;23:324-8.
15. Gaskins AJ et al. Obstet Gynecol. 2015;126:850-8.
16. Sermondade N et al. Hum Reprod Update. 2019;25:439-519.
17. Kawwass JF et al. Fertil Steril. 2016;106[7]:1742-50.
18. Einarsson S et al. Hum Reprod. 2017;32:1621-30.
19. Chaer R et al. Diseases. 2020;8:46.
20. Dempfle A et al. Psychiatry. 2013;13:308.
21. Verma A and Shrimali L. J Clin Diagn Res. 2012;6:1531-3.
22. Romanski PA et al. Reprod Biomed Online. 2020;42:366-74.
Decoding mechanisms of diabetic embryopathy suggests therapeutic targets
Before the introduction of insulin, there were few reported cases of pregnancy complicated by diabetes because women with the disease too often did not live to childbearing age, and when they did, they were often counseled to terminate their pregnancies. Perinatal and maternal mortality in the limited number of reported pregnancies were 70% and 40%, respectively,1 making the risks of continuing the pregnancy quite high.
After insulin became available, maternal mortality dropped dramatically, down to a few percent. Perinatal mortality also declined, but it took several decades to achieve a similar magnitude of reduction.2 Today, with insulin therapy and tight glucose control as well as improved perinatal care, almost all women with diabetes can contemplate pregnancy with greater hope for normal outcomes.
Problems persist, however. Maternal diabetes continues to cause a variety of adverse outcomes, including infants large for gestational age, prematurity, and structural birth defects. Birth defects and prematurity, in fact, are the top causes of the unacceptably high infant mortality rate in the United States – a rate that is about 70% higher than the average in comparable developed countries.3
Infant mortality is considered an indicator of population health and of the development of a country; to reduce its rate, we must address these two areas.
Women with type 1 and type 2 diabetes are five times more likely to have a child with birth defects than are nondiabetic women.4 Up to 10% of women with preexisting diabetes will have fetuses with a major congenital malformation.5
Over the years we have been striving in our Center for Birth Defects Research to understand the pathomechanisms and the molecular and epigenetic alterations behind the high rates of birth defects in the offspring of women with preexisting diabetes. We have focused on heart defects and neural tube defects (particularly the latter), which together cause significant mortality, morbidity, disability, and human suffering.
Using animal models that mimic human diabetic pregnancy, we have made significant strides in our understanding of the mechanisms, uncovering molecular pathways involving oxidative stress, senescence/premature cellular aging, and epigenetic modifications (Figure 1). Understanding these pathways is providing us, in turn, with potential therapeutic targets and approaches that may be used in the future to prevent birth defects in women who enter pregnancy with type 1 or type 2 diabetes.
Unraveling the role of oxidative stress
Our mouse models accurately reflect the human conditions of diabetes in pregnancy and diabetic embryopathy. Offspring of mice with type 1 and type 2 diabetes have a similarly higher rate of neural tube defects and congenital heart disease, compared to mice without diabetes. We observe a similar incidence of anencephaly and spina bifida, and of cardiac septation defects in the mouse embryo hearts, for instance.
A primary mechanism and causal event of diabetic embryopathy is hyperglycemia-induced apoptosis in embryonic cells. Excessive cell death in the neural epithelium or in the developing heart leads to abnormal organogenesis and dysfunctional developmental events that cause birth defects. We have identified pathways leading to apoptosis, and have found that many of these pathways crosstalk with each other.
Hyperglycemia induces oxidative stress – one of these pathways – by causing sustained generation of reactive oxygen species. The cells’ mitochondrial function is significantly impaired by the hyperglycemia response, and this diabetes-induced mitochondrial dysfunction further increases the production of reactive oxygen species and a weakening of the endogenous cellular antioxidant systems, both of which then exacerbate oxidative stress.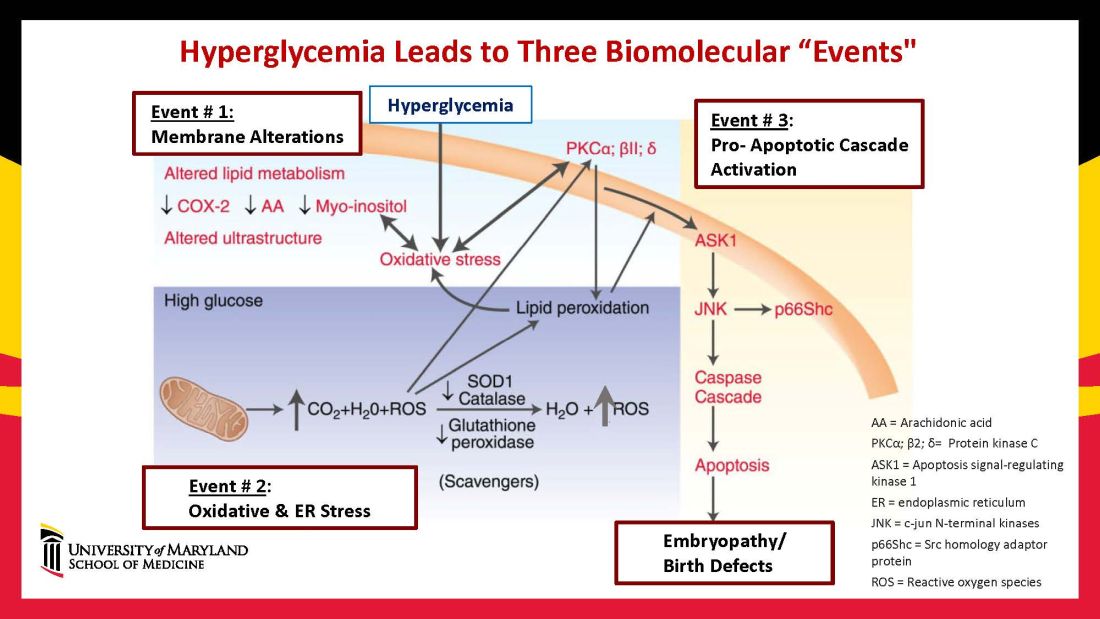
Our research has detailed what happens downstream. We’ve learned that oxidative stress in embryos exposed to maternal diabetes activates a cascade of proapoptotic kinase signaling molecules – for example, protein kinase C isoforms such as PKCalpha; apoptosis signal-regulating kinase 1; and c-Jun-N-terminal kinases – that ultimately lead to abnormal cell death in the neuroepithelium before neural tube closure (Figure 2).5
Hyperglycemia also alters membrane biochemistry in the developing embryo, suppressing lipids including arachidonic acid and myoinositol, and induces the elevation of other molecules that cause newly synthesized proteins to be misfolded. A build-up of misfolded/unfolded proteins triggers or exacerbates endoplasmic reticulum stress, which, like oxidative stress, plays a role in the activation of proapoptotic kinase signaling and apoptosis.6
When we’ve deleted genes for some of the proapoptotic kinase–signaling intermediates, or otherwise inhibited oxidative and endoplasmic reticulum stresses, we’ve been able to ameliorate neural cell apoptosis and the formation of neural tube defects. Studying the processes both forward and backward gives us confidence that the pathways are real and important, and that altering the pathways can alter the outcomes.
Reduced autophagy and induction of cellular senescence
Just as mitochondria are negatively affected by hyperglycemic conditions, so are autophagosomes – organelles that play a key role in removing abnormal or damaged stem cells and cellular components (including unfolded protein aggregates) and in maintaining cellular homeostasis. A high level of autophagy is essential for neural tube closure as well as cardiac morphogenesis.
In our models, maternal diabetes significantly suppressed the process of autophagy in neuroepithelial cells. We have identified responsible molecular intermediates and a key regulating gene for autophagy impairment and have found that deletion of the gene restores autophagy and reduces the development of neural tube defects.4 Administration of a naturally occurring compound, trehalose, which reactivates autophagy, had a similar effect.7Exposure to hyperglycemia not only causes cell death and suppresses autophagy, it also impairs other aspects of cellular function. More recently, we have shown that cells in the neuroepithelium become quiescent and cease proliferating. The quiescent cells, those cells with premature aging markers, also produce cytokines that influence the functioning and development of neighboring cells, causing additional cell death.
All told, premature senescence in the neuroepithelium adversely affects the neurulation process, leading to neural tube defects. In our mouse model, the senomorphic agent rapamycin suppressed cellular senescence, reduced the number of apoptotic neuroepithelial cells, and reduced the formation of neural tube defects.8
The role of epigenetics, future interventions
Epigenetics – the process by which gene expression and function can be modified by environmental conditions without modification of the DNA sequence – has become an additional area of focus in diabetic embryopathy. Our lab has studied the overexpression of both DNA methyltransferases (DNMTs) that cause DNA hypermethylation, and of microRNAs (miRNAs) that can suppress gene expression at the posttranscriptional level. Both are considered to be primary epigenetic mechanisms involved in human diseases and it appears that they are influential in the incidence of birth defects in diabetic mothers.
In our mouse models, maternal diabetes induces DNA hypermethylation via the increase of DNMTs, leading to the silencing of genes essential for neural tube closure and formation of the developing heart. MiRNAs also play a role; in addition to finding altered DNMT activity in the neural epithelium and other tissues of diabetes-exposed embryos, we also found altered miRNA expression. By deleting miRNA genes or by inhibiting DNMT activity through treatment with antioxidants, we saw significant reductions in birth defects.
In one study of the green tea polyphenol epigallocatechin gallate (EGCG), we demonstrated inhibition of diabetes-elevated DNMT expression and activity and suppression of DNA hypermethylation. The expression of genes essential for neural tube closure was restored, with a subsequent reduction in neural tube defects from 29.5% to 2% in embryos treated with EGCG.9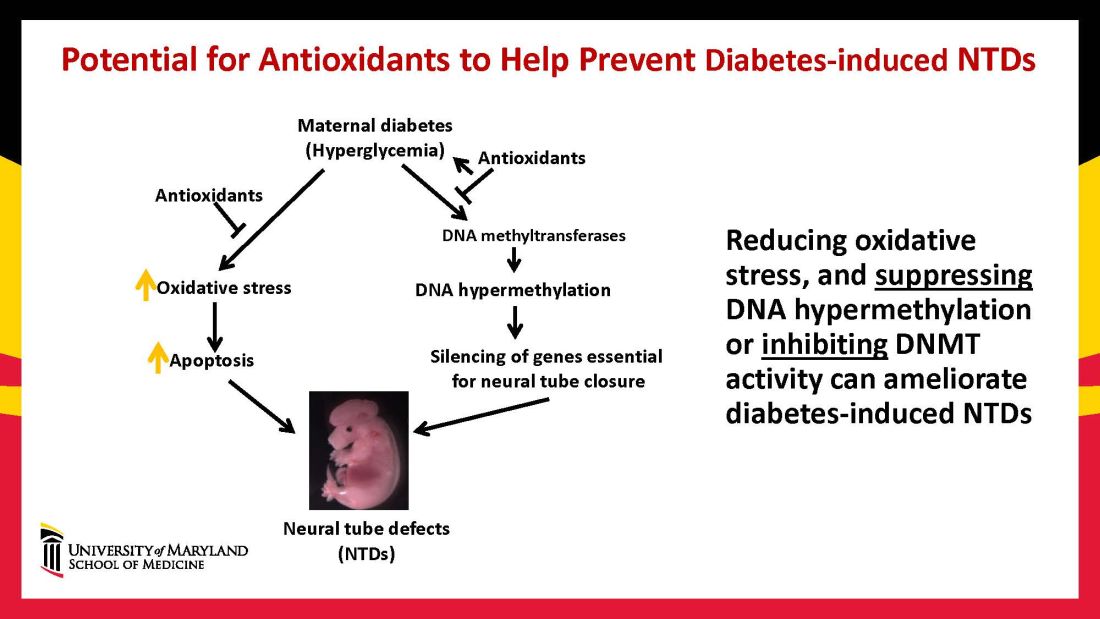
Our interventions to reverse or alter the mechanisms and pathways leading to birth defects have not only helped prove causation, but have given us hope for the future. Antioxidants are among the compounds that could be used as dietary supplements during pregnancy to prevent structural birth defects (Figure 3). Other compounds could activate the process of autophagy (for example, trehalose) and antisenescence compounds similar to rapamycin could be used to reduce numbers of senescent cells in the neuroepithelium or the developing heart.
Dr. Reece and Dr. Yang reported no relevant disclosures.
Dr. Reece, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president, endowed professor and director of CARTI, and codirector of the Center for Birth Defects.
*This story was updated on Nov. 3, 2022
References
1. Z Zhiyong and Reece EA. Clin Lab Med. 2013;33(2)207-33.
2. Reece EA and Coustan DR. Diabetes and obesity in women. Wolters Kluwer: 2019. 4th ed. (https://www.amazon.com/Diabetes-Obesity-Women-Albert-Reece/dp/1496390547).
3. The Peterson-KFF Health System Tracker. www.healthsystemtracker.org.
4. Wang F et al. Nat. Commun. 2017;8:15182.
5. Yang P et al. Am J Obstet Gynecol. 2015;212(5):569-79.
6. Li X et al. Diabetes. 2013 Feb;62(2):599-608.
7. Xu C et al. Am J Physiol Endocrinol Metab. 2013 Sep 1;305(5):E667-78.
8. Xu C et al. Sci Adv. 2021;7(27):eabf5089.
9. Zhong J et al. Am J Obstet Gynecol. 2016 Sep;215(3):368.e1-10.
Before the introduction of insulin, there were few reported cases of pregnancy complicated by diabetes because women with the disease too often did not live to childbearing age, and when they did, they were often counseled to terminate their pregnancies. Perinatal and maternal mortality in the limited number of reported pregnancies were 70% and 40%, respectively,1 making the risks of continuing the pregnancy quite high.
After insulin became available, maternal mortality dropped dramatically, down to a few percent. Perinatal mortality also declined, but it took several decades to achieve a similar magnitude of reduction.2 Today, with insulin therapy and tight glucose control as well as improved perinatal care, almost all women with diabetes can contemplate pregnancy with greater hope for normal outcomes.
Problems persist, however. Maternal diabetes continues to cause a variety of adverse outcomes, including infants large for gestational age, prematurity, and structural birth defects. Birth defects and prematurity, in fact, are the top causes of the unacceptably high infant mortality rate in the United States – a rate that is about 70% higher than the average in comparable developed countries.3
Infant mortality is considered an indicator of population health and of the development of a country; to reduce its rate, we must address these two areas.
Women with type 1 and type 2 diabetes are five times more likely to have a child with birth defects than are nondiabetic women.4 Up to 10% of women with preexisting diabetes will have fetuses with a major congenital malformation.5
Over the years we have been striving in our Center for Birth Defects Research to understand the pathomechanisms and the molecular and epigenetic alterations behind the high rates of birth defects in the offspring of women with preexisting diabetes. We have focused on heart defects and neural tube defects (particularly the latter), which together cause significant mortality, morbidity, disability, and human suffering.
Using animal models that mimic human diabetic pregnancy, we have made significant strides in our understanding of the mechanisms, uncovering molecular pathways involving oxidative stress, senescence/premature cellular aging, and epigenetic modifications (Figure 1). Understanding these pathways is providing us, in turn, with potential therapeutic targets and approaches that may be used in the future to prevent birth defects in women who enter pregnancy with type 1 or type 2 diabetes.
Unraveling the role of oxidative stress
Our mouse models accurately reflect the human conditions of diabetes in pregnancy and diabetic embryopathy. Offspring of mice with type 1 and type 2 diabetes have a similarly higher rate of neural tube defects and congenital heart disease, compared to mice without diabetes. We observe a similar incidence of anencephaly and spina bifida, and of cardiac septation defects in the mouse embryo hearts, for instance.
A primary mechanism and causal event of diabetic embryopathy is hyperglycemia-induced apoptosis in embryonic cells. Excessive cell death in the neural epithelium or in the developing heart leads to abnormal organogenesis and dysfunctional developmental events that cause birth defects. We have identified pathways leading to apoptosis, and have found that many of these pathways crosstalk with each other.
Hyperglycemia induces oxidative stress – one of these pathways – by causing sustained generation of reactive oxygen species. The cells’ mitochondrial function is significantly impaired by the hyperglycemia response, and this diabetes-induced mitochondrial dysfunction further increases the production of reactive oxygen species and a weakening of the endogenous cellular antioxidant systems, both of which then exacerbate oxidative stress.
Our research has detailed what happens downstream. We’ve learned that oxidative stress in embryos exposed to maternal diabetes activates a cascade of proapoptotic kinase signaling molecules – for example, protein kinase C isoforms such as PKCalpha; apoptosis signal-regulating kinase 1; and c-Jun-N-terminal kinases – that ultimately lead to abnormal cell death in the neuroepithelium before neural tube closure (Figure 2).5
Hyperglycemia also alters membrane biochemistry in the developing embryo, suppressing lipids including arachidonic acid and myoinositol, and induces the elevation of other molecules that cause newly synthesized proteins to be misfolded. A build-up of misfolded/unfolded proteins triggers or exacerbates endoplasmic reticulum stress, which, like oxidative stress, plays a role in the activation of proapoptotic kinase signaling and apoptosis.6
When we’ve deleted genes for some of the proapoptotic kinase–signaling intermediates, or otherwise inhibited oxidative and endoplasmic reticulum stresses, we’ve been able to ameliorate neural cell apoptosis and the formation of neural tube defects. Studying the processes both forward and backward gives us confidence that the pathways are real and important, and that altering the pathways can alter the outcomes.
Reduced autophagy and induction of cellular senescence
Just as mitochondria are negatively affected by hyperglycemic conditions, so are autophagosomes – organelles that play a key role in removing abnormal or damaged stem cells and cellular components (including unfolded protein aggregates) and in maintaining cellular homeostasis. A high level of autophagy is essential for neural tube closure as well as cardiac morphogenesis.
In our models, maternal diabetes significantly suppressed the process of autophagy in neuroepithelial cells. We have identified responsible molecular intermediates and a key regulating gene for autophagy impairment and have found that deletion of the gene restores autophagy and reduces the development of neural tube defects.4 Administration of a naturally occurring compound, trehalose, which reactivates autophagy, had a similar effect.7Exposure to hyperglycemia not only causes cell death and suppresses autophagy, it also impairs other aspects of cellular function. More recently, we have shown that cells in the neuroepithelium become quiescent and cease proliferating. The quiescent cells, those cells with premature aging markers, also produce cytokines that influence the functioning and development of neighboring cells, causing additional cell death.
All told, premature senescence in the neuroepithelium adversely affects the neurulation process, leading to neural tube defects. In our mouse model, the senomorphic agent rapamycin suppressed cellular senescence, reduced the number of apoptotic neuroepithelial cells, and reduced the formation of neural tube defects.8
The role of epigenetics, future interventions
Epigenetics – the process by which gene expression and function can be modified by environmental conditions without modification of the DNA sequence – has become an additional area of focus in diabetic embryopathy. Our lab has studied the overexpression of both DNA methyltransferases (DNMTs) that cause DNA hypermethylation, and of microRNAs (miRNAs) that can suppress gene expression at the posttranscriptional level. Both are considered to be primary epigenetic mechanisms involved in human diseases and it appears that they are influential in the incidence of birth defects in diabetic mothers.
In our mouse models, maternal diabetes induces DNA hypermethylation via the increase of DNMTs, leading to the silencing of genes essential for neural tube closure and formation of the developing heart. MiRNAs also play a role; in addition to finding altered DNMT activity in the neural epithelium and other tissues of diabetes-exposed embryos, we also found altered miRNA expression. By deleting miRNA genes or by inhibiting DNMT activity through treatment with antioxidants, we saw significant reductions in birth defects.
In one study of the green tea polyphenol epigallocatechin gallate (EGCG), we demonstrated inhibition of diabetes-elevated DNMT expression and activity and suppression of DNA hypermethylation. The expression of genes essential for neural tube closure was restored, with a subsequent reduction in neural tube defects from 29.5% to 2% in embryos treated with EGCG.9
Our interventions to reverse or alter the mechanisms and pathways leading to birth defects have not only helped prove causation, but have given us hope for the future. Antioxidants are among the compounds that could be used as dietary supplements during pregnancy to prevent structural birth defects (Figure 3). Other compounds could activate the process of autophagy (for example, trehalose) and antisenescence compounds similar to rapamycin could be used to reduce numbers of senescent cells in the neuroepithelium or the developing heart.
Dr. Reece and Dr. Yang reported no relevant disclosures.
Dr. Reece, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president, endowed professor and director of CARTI, and codirector of the Center for Birth Defects.
*This story was updated on Nov. 3, 2022
References
1. Z Zhiyong and Reece EA. Clin Lab Med. 2013;33(2)207-33.
2. Reece EA and Coustan DR. Diabetes and obesity in women. Wolters Kluwer: 2019. 4th ed. (https://www.amazon.com/Diabetes-Obesity-Women-Albert-Reece/dp/1496390547).
3. The Peterson-KFF Health System Tracker. www.healthsystemtracker.org.
4. Wang F et al. Nat. Commun. 2017;8:15182.
5. Yang P et al. Am J Obstet Gynecol. 2015;212(5):569-79.
6. Li X et al. Diabetes. 2013 Feb;62(2):599-608.
7. Xu C et al. Am J Physiol Endocrinol Metab. 2013 Sep 1;305(5):E667-78.
8. Xu C et al. Sci Adv. 2021;7(27):eabf5089.
9. Zhong J et al. Am J Obstet Gynecol. 2016 Sep;215(3):368.e1-10.
Before the introduction of insulin, there were few reported cases of pregnancy complicated by diabetes because women with the disease too often did not live to childbearing age, and when they did, they were often counseled to terminate their pregnancies. Perinatal and maternal mortality in the limited number of reported pregnancies were 70% and 40%, respectively,1 making the risks of continuing the pregnancy quite high.
After insulin became available, maternal mortality dropped dramatically, down to a few percent. Perinatal mortality also declined, but it took several decades to achieve a similar magnitude of reduction.2 Today, with insulin therapy and tight glucose control as well as improved perinatal care, almost all women with diabetes can contemplate pregnancy with greater hope for normal outcomes.
Problems persist, however. Maternal diabetes continues to cause a variety of adverse outcomes, including infants large for gestational age, prematurity, and structural birth defects. Birth defects and prematurity, in fact, are the top causes of the unacceptably high infant mortality rate in the United States – a rate that is about 70% higher than the average in comparable developed countries.3
Infant mortality is considered an indicator of population health and of the development of a country; to reduce its rate, we must address these two areas.
Women with type 1 and type 2 diabetes are five times more likely to have a child with birth defects than are nondiabetic women.4 Up to 10% of women with preexisting diabetes will have fetuses with a major congenital malformation.5
Over the years we have been striving in our Center for Birth Defects Research to understand the pathomechanisms and the molecular and epigenetic alterations behind the high rates of birth defects in the offspring of women with preexisting diabetes. We have focused on heart defects and neural tube defects (particularly the latter), which together cause significant mortality, morbidity, disability, and human suffering.
Using animal models that mimic human diabetic pregnancy, we have made significant strides in our understanding of the mechanisms, uncovering molecular pathways involving oxidative stress, senescence/premature cellular aging, and epigenetic modifications (Figure 1). Understanding these pathways is providing us, in turn, with potential therapeutic targets and approaches that may be used in the future to prevent birth defects in women who enter pregnancy with type 1 or type 2 diabetes.
Unraveling the role of oxidative stress
Our mouse models accurately reflect the human conditions of diabetes in pregnancy and diabetic embryopathy. Offspring of mice with type 1 and type 2 diabetes have a similarly higher rate of neural tube defects and congenital heart disease, compared to mice without diabetes. We observe a similar incidence of anencephaly and spina bifida, and of cardiac septation defects in the mouse embryo hearts, for instance.
A primary mechanism and causal event of diabetic embryopathy is hyperglycemia-induced apoptosis in embryonic cells. Excessive cell death in the neural epithelium or in the developing heart leads to abnormal organogenesis and dysfunctional developmental events that cause birth defects. We have identified pathways leading to apoptosis, and have found that many of these pathways crosstalk with each other.
Hyperglycemia induces oxidative stress – one of these pathways – by causing sustained generation of reactive oxygen species. The cells’ mitochondrial function is significantly impaired by the hyperglycemia response, and this diabetes-induced mitochondrial dysfunction further increases the production of reactive oxygen species and a weakening of the endogenous cellular antioxidant systems, both of which then exacerbate oxidative stress.
Our research has detailed what happens downstream. We’ve learned that oxidative stress in embryos exposed to maternal diabetes activates a cascade of proapoptotic kinase signaling molecules – for example, protein kinase C isoforms such as PKCalpha; apoptosis signal-regulating kinase 1; and c-Jun-N-terminal kinases – that ultimately lead to abnormal cell death in the neuroepithelium before neural tube closure (Figure 2).5
Hyperglycemia also alters membrane biochemistry in the developing embryo, suppressing lipids including arachidonic acid and myoinositol, and induces the elevation of other molecules that cause newly synthesized proteins to be misfolded. A build-up of misfolded/unfolded proteins triggers or exacerbates endoplasmic reticulum stress, which, like oxidative stress, plays a role in the activation of proapoptotic kinase signaling and apoptosis.6
When we’ve deleted genes for some of the proapoptotic kinase–signaling intermediates, or otherwise inhibited oxidative and endoplasmic reticulum stresses, we’ve been able to ameliorate neural cell apoptosis and the formation of neural tube defects. Studying the processes both forward and backward gives us confidence that the pathways are real and important, and that altering the pathways can alter the outcomes.
Reduced autophagy and induction of cellular senescence
Just as mitochondria are negatively affected by hyperglycemic conditions, so are autophagosomes – organelles that play a key role in removing abnormal or damaged stem cells and cellular components (including unfolded protein aggregates) and in maintaining cellular homeostasis. A high level of autophagy is essential for neural tube closure as well as cardiac morphogenesis.
In our models, maternal diabetes significantly suppressed the process of autophagy in neuroepithelial cells. We have identified responsible molecular intermediates and a key regulating gene for autophagy impairment and have found that deletion of the gene restores autophagy and reduces the development of neural tube defects.4 Administration of a naturally occurring compound, trehalose, which reactivates autophagy, had a similar effect.7Exposure to hyperglycemia not only causes cell death and suppresses autophagy, it also impairs other aspects of cellular function. More recently, we have shown that cells in the neuroepithelium become quiescent and cease proliferating. The quiescent cells, those cells with premature aging markers, also produce cytokines that influence the functioning and development of neighboring cells, causing additional cell death.
All told, premature senescence in the neuroepithelium adversely affects the neurulation process, leading to neural tube defects. In our mouse model, the senomorphic agent rapamycin suppressed cellular senescence, reduced the number of apoptotic neuroepithelial cells, and reduced the formation of neural tube defects.8
The role of epigenetics, future interventions
Epigenetics – the process by which gene expression and function can be modified by environmental conditions without modification of the DNA sequence – has become an additional area of focus in diabetic embryopathy. Our lab has studied the overexpression of both DNA methyltransferases (DNMTs) that cause DNA hypermethylation, and of microRNAs (miRNAs) that can suppress gene expression at the posttranscriptional level. Both are considered to be primary epigenetic mechanisms involved in human diseases and it appears that they are influential in the incidence of birth defects in diabetic mothers.
In our mouse models, maternal diabetes induces DNA hypermethylation via the increase of DNMTs, leading to the silencing of genes essential for neural tube closure and formation of the developing heart. MiRNAs also play a role; in addition to finding altered DNMT activity in the neural epithelium and other tissues of diabetes-exposed embryos, we also found altered miRNA expression. By deleting miRNA genes or by inhibiting DNMT activity through treatment with antioxidants, we saw significant reductions in birth defects.
In one study of the green tea polyphenol epigallocatechin gallate (EGCG), we demonstrated inhibition of diabetes-elevated DNMT expression and activity and suppression of DNA hypermethylation. The expression of genes essential for neural tube closure was restored, with a subsequent reduction in neural tube defects from 29.5% to 2% in embryos treated with EGCG.9
Our interventions to reverse or alter the mechanisms and pathways leading to birth defects have not only helped prove causation, but have given us hope for the future. Antioxidants are among the compounds that could be used as dietary supplements during pregnancy to prevent structural birth defects (Figure 3). Other compounds could activate the process of autophagy (for example, trehalose) and antisenescence compounds similar to rapamycin could be used to reduce numbers of senescent cells in the neuroepithelium or the developing heart.
Dr. Reece and Dr. Yang reported no relevant disclosures.
Dr. Reece, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president, endowed professor and director of CARTI, and codirector of the Center for Birth Defects.
*This story was updated on Nov. 3, 2022
References
1. Z Zhiyong and Reece EA. Clin Lab Med. 2013;33(2)207-33.
2. Reece EA and Coustan DR. Diabetes and obesity in women. Wolters Kluwer: 2019. 4th ed. (https://www.amazon.com/Diabetes-Obesity-Women-Albert-Reece/dp/1496390547).
3. The Peterson-KFF Health System Tracker. www.healthsystemtracker.org.
4. Wang F et al. Nat. Commun. 2017;8:15182.
5. Yang P et al. Am J Obstet Gynecol. 2015;212(5):569-79.
6. Li X et al. Diabetes. 2013 Feb;62(2):599-608.
7. Xu C et al. Am J Physiol Endocrinol Metab. 2013 Sep 1;305(5):E667-78.
8. Xu C et al. Sci Adv. 2021;7(27):eabf5089.
9. Zhong J et al. Am J Obstet Gynecol. 2016 Sep;215(3):368.e1-10.