User login
Engaging skeptical parents
While every day seems to bring extraordinary new advances in science – robotic surgery, individually targeted medications, and even gene therapy – there are many people who currently approach the science of medicine with skepticism.
While it is the right of legally competent adults in a free society to chose how best to care for their own health, to explore holistic or alternative therapies, or avoid medicine altogether, it is more complex when they are skeptical of accepted medical practice in managing the health of their children. For those parents who trust you enough to bring their children to you for care but remain skeptical of vaccines or other treatments, you have an opportunity to work with that trust and engage in a discussion so that they might reconsider their position on valuable and even life-saving treatments for their children.
In each of these cases, launching into an enthusiastic explanation of the advanced statistics that underpin your recommendation is unlikely to bridge the gap. Instead, you want to start with these parents by being curious. Resist the urge to tell, and listen instead. What is their understanding of the problem you are treating or preventing? What have they heard or read about the treatment or test in question? What do they most fear is going to happen to their child if they do or do not accept your recommendation? Are there specific events (with their child or with the health care system) that have informed this fear?
Respectfully listening to their experiences, thoughts, and feelings goes a long way toward building a trusting alliance. It can help overcome feelings of distrust or defensiveness around authority figures. And it models the thoughtful, respectful give and take that are essential to a healthy collaboration between pediatrician and parents.
Once you have information about what they think and some about how they think and make decisions, you then can offer your perspective. “You are the expert on your child, what I bring to this equation is experience with (this problem) and with assessing the scientific evidence that guides treatments in medicine. It is true that treatments often change as we learn more, but here is what the evidence currently supports.”
After learning something about how they think, you might offer more data or more warm acknowledgment of how difficult it can be to make medical decisions for your children with imperfect information. Be humble while also being accurate about your level of confidence in a recommendation. Humility is important because it is easy for parents to feel insecure and condescended to. You understand their greatest fear, now let them know what your greatest worry is for their child should they forgo a recommended treatment. Explaining all of this with humility and warmth makes it more likely that the parents will take in the facts you are trying to share with them and not be derailed by suspicion, defensiveness, or insecurity.
Make building an alliance with the parents your top priority. This does not mean that you do not offer your best recommendation for their child. Rather, it means that, if they still decline recommended treatment, you treat them with respect and invest your time in explaining what they should be watching or monitoring their child for without recommended treatment. Building trust is a long game. If you patiently stick with parents even when it’s not easy, they may be ready to trust you with a subsequent decision when the stakes are even higher.
Of course, all this thoughtful communication takes a lot of time! You may learn to block off more time for certain families. It also can be helpful to have these conversations as a team. If you and your nurse or social worker can meet with parents together, then some of the listening and learning can be done by the nurse or social worker alone, so that everyone’s time might be managed more efficiently. And managing skeptical parents as a team also can help to prevent frustration or burnout. It will not always succeed, but in some cases, your investment will pay off in a trusting alliance, mutual respect, and healthy patients.
Dr. Swick is an attending psychiatrist in the division of child psychiatry at Massachusetts General Hospital, Boston, and director of the Parenting at a Challenging Time (PACT) Program at the Vernon Cancer Center at Newton Wellesley Hospital, also in Boston. Dr. Jellinek is professor emeritus of psychiatry and pediatrics at Harvard Medical School, Boston. Email them at [email protected].
While every day seems to bring extraordinary new advances in science – robotic surgery, individually targeted medications, and even gene therapy – there are many people who currently approach the science of medicine with skepticism.
While it is the right of legally competent adults in a free society to chose how best to care for their own health, to explore holistic or alternative therapies, or avoid medicine altogether, it is more complex when they are skeptical of accepted medical practice in managing the health of their children. For those parents who trust you enough to bring their children to you for care but remain skeptical of vaccines or other treatments, you have an opportunity to work with that trust and engage in a discussion so that they might reconsider their position on valuable and even life-saving treatments for their children.
In each of these cases, launching into an enthusiastic explanation of the advanced statistics that underpin your recommendation is unlikely to bridge the gap. Instead, you want to start with these parents by being curious. Resist the urge to tell, and listen instead. What is their understanding of the problem you are treating or preventing? What have they heard or read about the treatment or test in question? What do they most fear is going to happen to their child if they do or do not accept your recommendation? Are there specific events (with their child or with the health care system) that have informed this fear?
Respectfully listening to their experiences, thoughts, and feelings goes a long way toward building a trusting alliance. It can help overcome feelings of distrust or defensiveness around authority figures. And it models the thoughtful, respectful give and take that are essential to a healthy collaboration between pediatrician and parents.
Once you have information about what they think and some about how they think and make decisions, you then can offer your perspective. “You are the expert on your child, what I bring to this equation is experience with (this problem) and with assessing the scientific evidence that guides treatments in medicine. It is true that treatments often change as we learn more, but here is what the evidence currently supports.”
After learning something about how they think, you might offer more data or more warm acknowledgment of how difficult it can be to make medical decisions for your children with imperfect information. Be humble while also being accurate about your level of confidence in a recommendation. Humility is important because it is easy for parents to feel insecure and condescended to. You understand their greatest fear, now let them know what your greatest worry is for their child should they forgo a recommended treatment. Explaining all of this with humility and warmth makes it more likely that the parents will take in the facts you are trying to share with them and not be derailed by suspicion, defensiveness, or insecurity.
Make building an alliance with the parents your top priority. This does not mean that you do not offer your best recommendation for their child. Rather, it means that, if they still decline recommended treatment, you treat them with respect and invest your time in explaining what they should be watching or monitoring their child for without recommended treatment. Building trust is a long game. If you patiently stick with parents even when it’s not easy, they may be ready to trust you with a subsequent decision when the stakes are even higher.
Of course, all this thoughtful communication takes a lot of time! You may learn to block off more time for certain families. It also can be helpful to have these conversations as a team. If you and your nurse or social worker can meet with parents together, then some of the listening and learning can be done by the nurse or social worker alone, so that everyone’s time might be managed more efficiently. And managing skeptical parents as a team also can help to prevent frustration or burnout. It will not always succeed, but in some cases, your investment will pay off in a trusting alliance, mutual respect, and healthy patients.
Dr. Swick is an attending psychiatrist in the division of child psychiatry at Massachusetts General Hospital, Boston, and director of the Parenting at a Challenging Time (PACT) Program at the Vernon Cancer Center at Newton Wellesley Hospital, also in Boston. Dr. Jellinek is professor emeritus of psychiatry and pediatrics at Harvard Medical School, Boston. Email them at [email protected].
While every day seems to bring extraordinary new advances in science – robotic surgery, individually targeted medications, and even gene therapy – there are many people who currently approach the science of medicine with skepticism.
While it is the right of legally competent adults in a free society to chose how best to care for their own health, to explore holistic or alternative therapies, or avoid medicine altogether, it is more complex when they are skeptical of accepted medical practice in managing the health of their children. For those parents who trust you enough to bring their children to you for care but remain skeptical of vaccines or other treatments, you have an opportunity to work with that trust and engage in a discussion so that they might reconsider their position on valuable and even life-saving treatments for their children.
In each of these cases, launching into an enthusiastic explanation of the advanced statistics that underpin your recommendation is unlikely to bridge the gap. Instead, you want to start with these parents by being curious. Resist the urge to tell, and listen instead. What is their understanding of the problem you are treating or preventing? What have they heard or read about the treatment or test in question? What do they most fear is going to happen to their child if they do or do not accept your recommendation? Are there specific events (with their child or with the health care system) that have informed this fear?
Respectfully listening to their experiences, thoughts, and feelings goes a long way toward building a trusting alliance. It can help overcome feelings of distrust or defensiveness around authority figures. And it models the thoughtful, respectful give and take that are essential to a healthy collaboration between pediatrician and parents.
Once you have information about what they think and some about how they think and make decisions, you then can offer your perspective. “You are the expert on your child, what I bring to this equation is experience with (this problem) and with assessing the scientific evidence that guides treatments in medicine. It is true that treatments often change as we learn more, but here is what the evidence currently supports.”
After learning something about how they think, you might offer more data or more warm acknowledgment of how difficult it can be to make medical decisions for your children with imperfect information. Be humble while also being accurate about your level of confidence in a recommendation. Humility is important because it is easy for parents to feel insecure and condescended to. You understand their greatest fear, now let them know what your greatest worry is for their child should they forgo a recommended treatment. Explaining all of this with humility and warmth makes it more likely that the parents will take in the facts you are trying to share with them and not be derailed by suspicion, defensiveness, or insecurity.
Make building an alliance with the parents your top priority. This does not mean that you do not offer your best recommendation for their child. Rather, it means that, if they still decline recommended treatment, you treat them with respect and invest your time in explaining what they should be watching or monitoring their child for without recommended treatment. Building trust is a long game. If you patiently stick with parents even when it’s not easy, they may be ready to trust you with a subsequent decision when the stakes are even higher.
Of course, all this thoughtful communication takes a lot of time! You may learn to block off more time for certain families. It also can be helpful to have these conversations as a team. If you and your nurse or social worker can meet with parents together, then some of the listening and learning can be done by the nurse or social worker alone, so that everyone’s time might be managed more efficiently. And managing skeptical parents as a team also can help to prevent frustration or burnout. It will not always succeed, but in some cases, your investment will pay off in a trusting alliance, mutual respect, and healthy patients.
Dr. Swick is an attending psychiatrist in the division of child psychiatry at Massachusetts General Hospital, Boston, and director of the Parenting at a Challenging Time (PACT) Program at the Vernon Cancer Center at Newton Wellesley Hospital, also in Boston. Dr. Jellinek is professor emeritus of psychiatry and pediatrics at Harvard Medical School, Boston. Email them at [email protected].
April 2018 - What's your diagnosis?
Answer to “What’s your diagnosis?” Chilaiditi syndrome
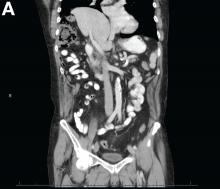
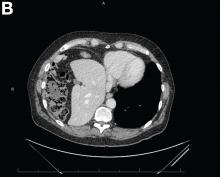
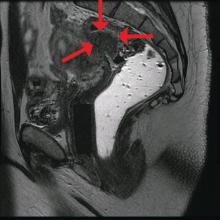
Abdominal CT images display the Chilaiditi sign, which is the radiographic term used to describe interposition of the colon, usually at the hepatic flexure, with the liver and right diaphragm.1 This is considered an incidental radiographic finding and is generally asymptomatic; however, when one develops clinical symptoms such as abdominal pain, bloating or distension, anorexia, constipation, or nausea it is called Chilaiditi syndrome.
First described by Greek radiologist Demetrius Chilaiditi in 1910, Chilaiditi syndrome is a rare occurrence with an incidence rate of 0.25%-0.28% in the general population.2 The etiology of Chilaiditi syndrome is felt to be congenital or acquired with predisposing congenital abnormalities such as absent suspensory or falciform ligaments, redundant colon, malposition of the colon, dolichocolon, and paralysis of the right diaphragm. Other risk factors for development of Chilaiditi syndrome include chronic constipation, cirrhosis, ascites, and obesity. Men are times times as likely as women to develop Chilaiditi syndrome and it is more common in the elderly, occurring in 1% of the elderly population.3 Chilaiditi sign is diagnosed with radiographic imaging meeting the following criteria: the right hemidiaphragm must be elevated above the liver by the intestine, the bowel must be distended by air to illustrate pseudopneumoperitoneum, and the superior margin of the liver must be depressed below the level of the left hemidiaphragm.1
Chilaiditi syndrome is managed conservatively with close observation. Recurrent symptoms can be treated with colopexy. This syndrome has been known to cause severe complications including volvulus of the cecum, splenic flexure, or transverse colon, cecal perforation, and subdiaphragmatic perforated appendicitis, which all require surgical intervention.3 It is important to recognize Chilaiditi syndrome on presentation to prevent unnecessary diagnostic studies and unwarranted surgical intervention.
References
1. Uygungul, E., Uygungul, D., Ayrik, C., et al. Chilaiditi sign: why are clinical findings more important in ED?. Am J Emerg Med. 2015;33:733.e1-733.e2.
2. Ho, M.P., Cheung, W.K., Tsai, K.C., et al. Chilaiditi syndrome mimicking subdiaphragmatic free air in an elderly adult. J Am Geriatr Soc. 2014;62:2019-21.
3. Kang, D., Pan, A.S., Lopez, M.A., et al. Acute abdominal pain secondary to Chilaiditi syndrome. Case Rep Surg. 2013;2013:756590.
Answer to “What’s your diagnosis?” Chilaiditi syndrome
Abdominal CT images display the Chilaiditi sign, which is the radiographic term used to describe interposition of the colon, usually at the hepatic flexure, with the liver and right diaphragm.1 This is considered an incidental radiographic finding and is generally asymptomatic; however, when one develops clinical symptoms such as abdominal pain, bloating or distension, anorexia, constipation, or nausea it is called Chilaiditi syndrome.
First described by Greek radiologist Demetrius Chilaiditi in 1910, Chilaiditi syndrome is a rare occurrence with an incidence rate of 0.25%-0.28% in the general population.2 The etiology of Chilaiditi syndrome is felt to be congenital or acquired with predisposing congenital abnormalities such as absent suspensory or falciform ligaments, redundant colon, malposition of the colon, dolichocolon, and paralysis of the right diaphragm. Other risk factors for development of Chilaiditi syndrome include chronic constipation, cirrhosis, ascites, and obesity. Men are times times as likely as women to develop Chilaiditi syndrome and it is more common in the elderly, occurring in 1% of the elderly population.3 Chilaiditi sign is diagnosed with radiographic imaging meeting the following criteria: the right hemidiaphragm must be elevated above the liver by the intestine, the bowel must be distended by air to illustrate pseudopneumoperitoneum, and the superior margin of the liver must be depressed below the level of the left hemidiaphragm.1
Chilaiditi syndrome is managed conservatively with close observation. Recurrent symptoms can be treated with colopexy. This syndrome has been known to cause severe complications including volvulus of the cecum, splenic flexure, or transverse colon, cecal perforation, and subdiaphragmatic perforated appendicitis, which all require surgical intervention.3 It is important to recognize Chilaiditi syndrome on presentation to prevent unnecessary diagnostic studies and unwarranted surgical intervention.
References
1. Uygungul, E., Uygungul, D., Ayrik, C., et al. Chilaiditi sign: why are clinical findings more important in ED?. Am J Emerg Med. 2015;33:733.e1-733.e2.
2. Ho, M.P., Cheung, W.K., Tsai, K.C., et al. Chilaiditi syndrome mimicking subdiaphragmatic free air in an elderly adult. J Am Geriatr Soc. 2014;62:2019-21.
3. Kang, D., Pan, A.S., Lopez, M.A., et al. Acute abdominal pain secondary to Chilaiditi syndrome. Case Rep Surg. 2013;2013:756590.
Answer to “What’s your diagnosis?” Chilaiditi syndrome
Abdominal CT images display the Chilaiditi sign, which is the radiographic term used to describe interposition of the colon, usually at the hepatic flexure, with the liver and right diaphragm.1 This is considered an incidental radiographic finding and is generally asymptomatic; however, when one develops clinical symptoms such as abdominal pain, bloating or distension, anorexia, constipation, or nausea it is called Chilaiditi syndrome.
First described by Greek radiologist Demetrius Chilaiditi in 1910, Chilaiditi syndrome is a rare occurrence with an incidence rate of 0.25%-0.28% in the general population.2 The etiology of Chilaiditi syndrome is felt to be congenital or acquired with predisposing congenital abnormalities such as absent suspensory or falciform ligaments, redundant colon, malposition of the colon, dolichocolon, and paralysis of the right diaphragm. Other risk factors for development of Chilaiditi syndrome include chronic constipation, cirrhosis, ascites, and obesity. Men are times times as likely as women to develop Chilaiditi syndrome and it is more common in the elderly, occurring in 1% of the elderly population.3 Chilaiditi sign is diagnosed with radiographic imaging meeting the following criteria: the right hemidiaphragm must be elevated above the liver by the intestine, the bowel must be distended by air to illustrate pseudopneumoperitoneum, and the superior margin of the liver must be depressed below the level of the left hemidiaphragm.1
Chilaiditi syndrome is managed conservatively with close observation. Recurrent symptoms can be treated with colopexy. This syndrome has been known to cause severe complications including volvulus of the cecum, splenic flexure, or transverse colon, cecal perforation, and subdiaphragmatic perforated appendicitis, which all require surgical intervention.3 It is important to recognize Chilaiditi syndrome on presentation to prevent unnecessary diagnostic studies and unwarranted surgical intervention.
References
1. Uygungul, E., Uygungul, D., Ayrik, C., et al. Chilaiditi sign: why are clinical findings more important in ED?. Am J Emerg Med. 2015;33:733.e1-733.e2.
2. Ho, M.P., Cheung, W.K., Tsai, K.C., et al. Chilaiditi syndrome mimicking subdiaphragmatic free air in an elderly adult. J Am Geriatr Soc. 2014;62:2019-21.
3. Kang, D., Pan, A.S., Lopez, M.A., et al. Acute abdominal pain secondary to Chilaiditi syndrome. Case Rep Surg. 2013;2013:756590.
By Jordan Orr, MD, and Charles O. Elson III, MD. Published previously in Gastroenterology (2016;151[2]:241-2).
A 67-year-old man presented to the emergency department with complaints of subacute, right-sided flank pain with migratory pain to his right lower quadrant and suprapubic area of increasing intensity for 1 week. He described his pain as cramping in nature and of fluctuating intensity, acutely worse on the day of presentation. However, within 15 minutes of waiting in the emergency department his pain subsided completely. He further denied any associated nausea, vomiting, diarrhea, melena, hematochezia, dysuria, or hematuria. Vital signs and abdominal physical examination were normal. Further, laboratory testing was unremarkable including a normal urinalysis. A bedside ultrasound was negative for gallbladder pathology or nephrolithiasis; however, it revealed an abnormal appearing liver. As further diagnostic work up, an abdominopelvic computed tomography scan revealed the following images (Figures A, B). The patient was discharged from the emergency department with scheduled follow-up in the gastroenterology clinic.
What is your diagnosis and treatment?
Dr. T. Berry Brazelton was a pioneer of child-centered parenting
You may not realize it, but as you navigated through this morning’s hospital rounds and your busy office schedule, some of what you did and how you did it was the result of the pioneering work of Boston-based pediatrician T. Berry Brazelton, MD, who died March 13, 2018, at the age of 99.
You probably found the newborn you needed to examine in his mother’s hospital room. The 3-year-old in the croup tent was sharing his room with his father, who was sleeping on a cot at his crib side, and three out of the first four patients you saw in your office had been breastfed. These scenarios would have been unheard of 50 years ago. But Dr. Brazelton’s voice was the most widely heard, yet gentlest and persuasive in support of rooming-in and breastfeeding.
My fellow house officers and I had been accustomed to picking up infants to assess their tone. However, when Dr. Brazelton picked up a newborn, it was more like a conversation, an interview, and in a sense, it was a meeting of the minds.
It wasn’t that we had been rejecting the notion that a newborn could have a personality. It is just that we hadn’t been taught to look for it or to take it seriously. Dr. Brazelton taught us how to examine the person inside that little body and understand the importance of her temperament. By sharing what we learned from doing a Brazelton-style exam, we hoped to encourage the child’s parents to adopt more realistic expectations, and as a consequence, make parenting less mysterious and stressful.
When I first met Dr. Brazelton, he was in his mid-40s and just beginning on his trajectory toward national prominence. When we were assigned to take care of his hospitalized patients, it was obvious that his patient skills with sick children had taken a back seat to his interest in newborn temperament. He was more than willing to let us make the management decisions. In retrospect, that experience was a warning that I, like many other pediatricians, would face the similar challenge of maintaining my clinical skills in the face of a patient mix that was steadily acquiring a more behavioral and developmental flavor.
It is impossible to quantify the degree to which Dr. Brazelton’s ubiquity contributed to the popularity of a more child-centered parenting style. However, I think it would be unfair to blame him for the unfortunate phenomenon known as “helicopter parenting.”
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
You may not realize it, but as you navigated through this morning’s hospital rounds and your busy office schedule, some of what you did and how you did it was the result of the pioneering work of Boston-based pediatrician T. Berry Brazelton, MD, who died March 13, 2018, at the age of 99.
You probably found the newborn you needed to examine in his mother’s hospital room. The 3-year-old in the croup tent was sharing his room with his father, who was sleeping on a cot at his crib side, and three out of the first four patients you saw in your office had been breastfed. These scenarios would have been unheard of 50 years ago. But Dr. Brazelton’s voice was the most widely heard, yet gentlest and persuasive in support of rooming-in and breastfeeding.
My fellow house officers and I had been accustomed to picking up infants to assess their tone. However, when Dr. Brazelton picked up a newborn, it was more like a conversation, an interview, and in a sense, it was a meeting of the minds.
It wasn’t that we had been rejecting the notion that a newborn could have a personality. It is just that we hadn’t been taught to look for it or to take it seriously. Dr. Brazelton taught us how to examine the person inside that little body and understand the importance of her temperament. By sharing what we learned from doing a Brazelton-style exam, we hoped to encourage the child’s parents to adopt more realistic expectations, and as a consequence, make parenting less mysterious and stressful.
When I first met Dr. Brazelton, he was in his mid-40s and just beginning on his trajectory toward national prominence. When we were assigned to take care of his hospitalized patients, it was obvious that his patient skills with sick children had taken a back seat to his interest in newborn temperament. He was more than willing to let us make the management decisions. In retrospect, that experience was a warning that I, like many other pediatricians, would face the similar challenge of maintaining my clinical skills in the face of a patient mix that was steadily acquiring a more behavioral and developmental flavor.
It is impossible to quantify the degree to which Dr. Brazelton’s ubiquity contributed to the popularity of a more child-centered parenting style. However, I think it would be unfair to blame him for the unfortunate phenomenon known as “helicopter parenting.”
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
You may not realize it, but as you navigated through this morning’s hospital rounds and your busy office schedule, some of what you did and how you did it was the result of the pioneering work of Boston-based pediatrician T. Berry Brazelton, MD, who died March 13, 2018, at the age of 99.
You probably found the newborn you needed to examine in his mother’s hospital room. The 3-year-old in the croup tent was sharing his room with his father, who was sleeping on a cot at his crib side, and three out of the first four patients you saw in your office had been breastfed. These scenarios would have been unheard of 50 years ago. But Dr. Brazelton’s voice was the most widely heard, yet gentlest and persuasive in support of rooming-in and breastfeeding.
My fellow house officers and I had been accustomed to picking up infants to assess their tone. However, when Dr. Brazelton picked up a newborn, it was more like a conversation, an interview, and in a sense, it was a meeting of the minds.
It wasn’t that we had been rejecting the notion that a newborn could have a personality. It is just that we hadn’t been taught to look for it or to take it seriously. Dr. Brazelton taught us how to examine the person inside that little body and understand the importance of her temperament. By sharing what we learned from doing a Brazelton-style exam, we hoped to encourage the child’s parents to adopt more realistic expectations, and as a consequence, make parenting less mysterious and stressful.
When I first met Dr. Brazelton, he was in his mid-40s and just beginning on his trajectory toward national prominence. When we were assigned to take care of his hospitalized patients, it was obvious that his patient skills with sick children had taken a back seat to his interest in newborn temperament. He was more than willing to let us make the management decisions. In retrospect, that experience was a warning that I, like many other pediatricians, would face the similar challenge of maintaining my clinical skills in the face of a patient mix that was steadily acquiring a more behavioral and developmental flavor.
It is impossible to quantify the degree to which Dr. Brazelton’s ubiquity contributed to the popularity of a more child-centered parenting style. However, I think it would be unfair to blame him for the unfortunate phenomenon known as “helicopter parenting.”
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Anterior discoid resection using a ‘squeeze’ technique
Rectosigmoid endometriosis has been estimated to affect between 4% and 37% of patients with endometriosis and is one of the most advanced and complex forms of the disease. Bowel endometriosis can be asymptomatic but often involves severe dysmenorrhea, dyspareunia, and a spectrum of bowel symptoms such as dyschezia, diarrhea, constipation, bloating, and rectal bleeding. Deep infiltrating rectovaginal endometriosis causes persistent or recurrent pain and is best treated by surgical removal of nodular lesions.
I have found that laparoscopic full-thickness disc resection (anterior discoid resection) with primary two-layer closure is often feasible and avoids the need for a complete bowel reanastomosis. It may not be an option in cases of multifocal rectal involvement (which may affect between one-quarter and one-third of patients with bowel endometriosis) or in cases involving large rectal nodules or luminal stenosis secondary to advanced fibrosis. In these cases, segmental bowel resection (low anterior resection) is often necessary. When anterior discoid resection is feasible, however, patients face significantly less morbidity with comparable outcomes.
Less morbidity
Preoperative evaluation is far from straightforward, and practices vary. Transvaginal ultrasonography is used for diagnosing rectal endometriosis in select centers in certain regions of the world, but there are important limitations; not only is it highly operator dependent, but its limited range does not allow for the detection of endometriosis higher in the sigmoid colon. Endorectal ultrasonography can be an excellent tool for more fully evaluating rectal wall involvement, but it does not usually allow for the evaluation of disease elsewhere in the pelvis.
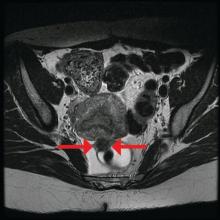
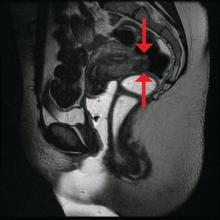
The preoperative tool we utilize most often along with clinical examination is MRI with vaginal and rectal contrast. MRI provides us with a superior anatomic perspective on the disease. Not only can we assess the depth of bowel wall infiltration and the distribution of the affected areas of the bowel, but we can see the bladder, the uterosacral ligaments, and how the uterus is situated relative to areas of disease. However, there are individualized limits to how high the contrast will travel, even with bowel preparation; disease that occurs significantly above the uterus often cannot be visualized as well as disease that occurs lower.
My general surgeon colleague and I have been working together for years, and we both are involved in counseling the patient suspected of having deep infiltrating disease. I typically talk with the patient about the probability of segmental resection based on my exam and preoperative MRI, and my colleague expands on this discussion with further explanation of the risks of bowel surgery.
Segmental resection has been associated with significant postoperative complications. In a single-center series of 436 laparoscopic colorectal resections for deep infiltrating endometriosis, rectovaginal and anastomotic fistula were among the most frequent postoperative complications (3.2% and 1.1%), along with transient urinary retention, which occurred in almost 20% (Surg Endosc. 2010 Jan;24:63-7).
Patients undergoing discoid resection for deep infiltrating endometriosis also had a significantly lower rate of temporary ileostomy (2.1% vs. 9.1%), a reduced rate of postoperative fever, and a reduced rate of gastrointestinal complications, mainly anastomotic leak or rectovaginal fistula (2.1% vs. 5.6%). There were no significant differences in the recurrence rate (13.8% vs. 11.5%).
A retrospective cohort study from our institution similarly showed decreased operative time, blood loss, hospital stay, and a lower rate of anastomotic strictures in patients who underwent laparoscopic anterior discoid resection between 2001 and 2009. The ADR group consistently had higher increments of improvement in bowel symptoms and dyspareunia, compared with patients who were selected to have segmental resection. Patients were followed for a mean of 41 months (JSLS. 2011;15[3]:331-8).
In general, there is agreement among surgeons that for consideration of discoid resection, nodule diameter should not exceed 3 cm, with a maximum of half of the bowel circumference and a maximum of 60% stenosis. I view these numbers as guiding principles, however, and not firm rules. Surgical decisions should be personalized based on the patient, the surgeon’s impression of the extent of the disease, and the ability to perform anterior discoid resection without compromising the rectal lumen with primary closure of the defect.
The technique
Rectosigmoid endometriotic nodules may present within the context of an obliterated posterior cul-de-sac, but the avascular pararectal space can be used to approach the nodules. Detailed knowledge of the avascular planes of this space, as well as the rectovaginal space, is crucial. Development of the rectovaginal space frees the bowel from its attachments to the posterior uterus and vagina. Judicious use of energized instruments in sharp dissection, and frequently sharp cold cutting, should be used near the bowel serosa to prevent thermal injury.
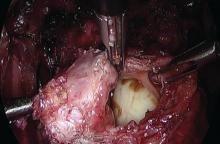
Presurgical imaging usually offers a good assessment of a nodule’s size and location, but intraoperatively, I typically use an atraumatic grasper to further assess size and contour and to determine if the nodule is suitable for discoid resection. If so, a suture is placed through the nodule to improve manipulation, and enucleation of the nodule itself is achieved through a “squeeze” technique in which an advanced bipolar device is used to circumscribe the lesion, dissecting the nodule as the device bounces off the thick endometriotic tissue.
The ENSEAL bipolar device (Ethicon, Somerville, N.J.) was designed as a vessel sealer, but because it will not cut through hard tissue as will other laparoscopic cutting devices, it serves as a useful tool for resecting endometriotic nodules while minimizing the removal of healthy rectal tissue. The device bounces off the nodule because it will avoid cutting through the thick tissue; in the process, it facilitates a fairly complete enucleation of the endometriotic nodule, starting with dissection until an intentional colotomy/enterotomy is made and followed by circumscription of the lesion once the rectum is entered.
Gentle traction and counter-traction increase the efficiency of dissection and minimize the amount of normal rectal tissue removed. Quick cutting with short bursts of energy allows for good hemostasis and minimizes thermal spread, which will maximize tissue healing from subsequent repair.
I then use a rectal probe as a template for repair. The probe is advanced underneath the defect between the distal and proximal portions, and the tissue is moved over the probe to ensure that the repair will be tension free. An ability to reapproximate the defect while keeping the probe in place indicates that the defect can be safely closed. (For a video presentation of the surgery, see www.surgeryu.com/leeobgyn.) If suturing is not feasible, the general surgeon is called to perform segmental resection.
The integrity of the repair is then thoroughly assessed with an air leak test. A bowel clamp is placed across the rectum and the pelvis is filled with sterile saline. Air is placed into the rectum with a rigid proctoscope while the operative field is inspected for evidence of an air leak.
Discoid resection may also be performed with a circular stapler. While this technique is faster than suturing, its use is limited by nodule size and has the potential to compromise complete excision of the nodule.
Dr. Lee is director of minimally invasive gynecologic surgery, Magee-Women’s Hospital of the University of Pittsburgh Medical Center.
Rectosigmoid endometriosis has been estimated to affect between 4% and 37% of patients with endometriosis and is one of the most advanced and complex forms of the disease. Bowel endometriosis can be asymptomatic but often involves severe dysmenorrhea, dyspareunia, and a spectrum of bowel symptoms such as dyschezia, diarrhea, constipation, bloating, and rectal bleeding. Deep infiltrating rectovaginal endometriosis causes persistent or recurrent pain and is best treated by surgical removal of nodular lesions.
I have found that laparoscopic full-thickness disc resection (anterior discoid resection) with primary two-layer closure is often feasible and avoids the need for a complete bowel reanastomosis. It may not be an option in cases of multifocal rectal involvement (which may affect between one-quarter and one-third of patients with bowel endometriosis) or in cases involving large rectal nodules or luminal stenosis secondary to advanced fibrosis. In these cases, segmental bowel resection (low anterior resection) is often necessary. When anterior discoid resection is feasible, however, patients face significantly less morbidity with comparable outcomes.
Less morbidity
Preoperative evaluation is far from straightforward, and practices vary. Transvaginal ultrasonography is used for diagnosing rectal endometriosis in select centers in certain regions of the world, but there are important limitations; not only is it highly operator dependent, but its limited range does not allow for the detection of endometriosis higher in the sigmoid colon. Endorectal ultrasonography can be an excellent tool for more fully evaluating rectal wall involvement, but it does not usually allow for the evaluation of disease elsewhere in the pelvis.
The preoperative tool we utilize most often along with clinical examination is MRI with vaginal and rectal contrast. MRI provides us with a superior anatomic perspective on the disease. Not only can we assess the depth of bowel wall infiltration and the distribution of the affected areas of the bowel, but we can see the bladder, the uterosacral ligaments, and how the uterus is situated relative to areas of disease. However, there are individualized limits to how high the contrast will travel, even with bowel preparation; disease that occurs significantly above the uterus often cannot be visualized as well as disease that occurs lower.
My general surgeon colleague and I have been working together for years, and we both are involved in counseling the patient suspected of having deep infiltrating disease. I typically talk with the patient about the probability of segmental resection based on my exam and preoperative MRI, and my colleague expands on this discussion with further explanation of the risks of bowel surgery.
Segmental resection has been associated with significant postoperative complications. In a single-center series of 436 laparoscopic colorectal resections for deep infiltrating endometriosis, rectovaginal and anastomotic fistula were among the most frequent postoperative complications (3.2% and 1.1%), along with transient urinary retention, which occurred in almost 20% (Surg Endosc. 2010 Jan;24:63-7).
Patients undergoing discoid resection for deep infiltrating endometriosis also had a significantly lower rate of temporary ileostomy (2.1% vs. 9.1%), a reduced rate of postoperative fever, and a reduced rate of gastrointestinal complications, mainly anastomotic leak or rectovaginal fistula (2.1% vs. 5.6%). There were no significant differences in the recurrence rate (13.8% vs. 11.5%).
A retrospective cohort study from our institution similarly showed decreased operative time, blood loss, hospital stay, and a lower rate of anastomotic strictures in patients who underwent laparoscopic anterior discoid resection between 2001 and 2009. The ADR group consistently had higher increments of improvement in bowel symptoms and dyspareunia, compared with patients who were selected to have segmental resection. Patients were followed for a mean of 41 months (JSLS. 2011;15[3]:331-8).
In general, there is agreement among surgeons that for consideration of discoid resection, nodule diameter should not exceed 3 cm, with a maximum of half of the bowel circumference and a maximum of 60% stenosis. I view these numbers as guiding principles, however, and not firm rules. Surgical decisions should be personalized based on the patient, the surgeon’s impression of the extent of the disease, and the ability to perform anterior discoid resection without compromising the rectal lumen with primary closure of the defect.
The technique
Rectosigmoid endometriotic nodules may present within the context of an obliterated posterior cul-de-sac, but the avascular pararectal space can be used to approach the nodules. Detailed knowledge of the avascular planes of this space, as well as the rectovaginal space, is crucial. Development of the rectovaginal space frees the bowel from its attachments to the posterior uterus and vagina. Judicious use of energized instruments in sharp dissection, and frequently sharp cold cutting, should be used near the bowel serosa to prevent thermal injury.
Presurgical imaging usually offers a good assessment of a nodule’s size and location, but intraoperatively, I typically use an atraumatic grasper to further assess size and contour and to determine if the nodule is suitable for discoid resection. If so, a suture is placed through the nodule to improve manipulation, and enucleation of the nodule itself is achieved through a “squeeze” technique in which an advanced bipolar device is used to circumscribe the lesion, dissecting the nodule as the device bounces off the thick endometriotic tissue.
The ENSEAL bipolar device (Ethicon, Somerville, N.J.) was designed as a vessel sealer, but because it will not cut through hard tissue as will other laparoscopic cutting devices, it serves as a useful tool for resecting endometriotic nodules while minimizing the removal of healthy rectal tissue. The device bounces off the nodule because it will avoid cutting through the thick tissue; in the process, it facilitates a fairly complete enucleation of the endometriotic nodule, starting with dissection until an intentional colotomy/enterotomy is made and followed by circumscription of the lesion once the rectum is entered.
Gentle traction and counter-traction increase the efficiency of dissection and minimize the amount of normal rectal tissue removed. Quick cutting with short bursts of energy allows for good hemostasis and minimizes thermal spread, which will maximize tissue healing from subsequent repair.
I then use a rectal probe as a template for repair. The probe is advanced underneath the defect between the distal and proximal portions, and the tissue is moved over the probe to ensure that the repair will be tension free. An ability to reapproximate the defect while keeping the probe in place indicates that the defect can be safely closed. (For a video presentation of the surgery, see www.surgeryu.com/leeobgyn.) If suturing is not feasible, the general surgeon is called to perform segmental resection.
The integrity of the repair is then thoroughly assessed with an air leak test. A bowel clamp is placed across the rectum and the pelvis is filled with sterile saline. Air is placed into the rectum with a rigid proctoscope while the operative field is inspected for evidence of an air leak.
Discoid resection may also be performed with a circular stapler. While this technique is faster than suturing, its use is limited by nodule size and has the potential to compromise complete excision of the nodule.
Dr. Lee is director of minimally invasive gynecologic surgery, Magee-Women’s Hospital of the University of Pittsburgh Medical Center.
Rectosigmoid endometriosis has been estimated to affect between 4% and 37% of patients with endometriosis and is one of the most advanced and complex forms of the disease. Bowel endometriosis can be asymptomatic but often involves severe dysmenorrhea, dyspareunia, and a spectrum of bowel symptoms such as dyschezia, diarrhea, constipation, bloating, and rectal bleeding. Deep infiltrating rectovaginal endometriosis causes persistent or recurrent pain and is best treated by surgical removal of nodular lesions.
I have found that laparoscopic full-thickness disc resection (anterior discoid resection) with primary two-layer closure is often feasible and avoids the need for a complete bowel reanastomosis. It may not be an option in cases of multifocal rectal involvement (which may affect between one-quarter and one-third of patients with bowel endometriosis) or in cases involving large rectal nodules or luminal stenosis secondary to advanced fibrosis. In these cases, segmental bowel resection (low anterior resection) is often necessary. When anterior discoid resection is feasible, however, patients face significantly less morbidity with comparable outcomes.
Less morbidity
Preoperative evaluation is far from straightforward, and practices vary. Transvaginal ultrasonography is used for diagnosing rectal endometriosis in select centers in certain regions of the world, but there are important limitations; not only is it highly operator dependent, but its limited range does not allow for the detection of endometriosis higher in the sigmoid colon. Endorectal ultrasonography can be an excellent tool for more fully evaluating rectal wall involvement, but it does not usually allow for the evaluation of disease elsewhere in the pelvis.
The preoperative tool we utilize most often along with clinical examination is MRI with vaginal and rectal contrast. MRI provides us with a superior anatomic perspective on the disease. Not only can we assess the depth of bowel wall infiltration and the distribution of the affected areas of the bowel, but we can see the bladder, the uterosacral ligaments, and how the uterus is situated relative to areas of disease. However, there are individualized limits to how high the contrast will travel, even with bowel preparation; disease that occurs significantly above the uterus often cannot be visualized as well as disease that occurs lower.
My general surgeon colleague and I have been working together for years, and we both are involved in counseling the patient suspected of having deep infiltrating disease. I typically talk with the patient about the probability of segmental resection based on my exam and preoperative MRI, and my colleague expands on this discussion with further explanation of the risks of bowel surgery.
Segmental resection has been associated with significant postoperative complications. In a single-center series of 436 laparoscopic colorectal resections for deep infiltrating endometriosis, rectovaginal and anastomotic fistula were among the most frequent postoperative complications (3.2% and 1.1%), along with transient urinary retention, which occurred in almost 20% (Surg Endosc. 2010 Jan;24:63-7).
Patients undergoing discoid resection for deep infiltrating endometriosis also had a significantly lower rate of temporary ileostomy (2.1% vs. 9.1%), a reduced rate of postoperative fever, and a reduced rate of gastrointestinal complications, mainly anastomotic leak or rectovaginal fistula (2.1% vs. 5.6%). There were no significant differences in the recurrence rate (13.8% vs. 11.5%).
A retrospective cohort study from our institution similarly showed decreased operative time, blood loss, hospital stay, and a lower rate of anastomotic strictures in patients who underwent laparoscopic anterior discoid resection between 2001 and 2009. The ADR group consistently had higher increments of improvement in bowel symptoms and dyspareunia, compared with patients who were selected to have segmental resection. Patients were followed for a mean of 41 months (JSLS. 2011;15[3]:331-8).
In general, there is agreement among surgeons that for consideration of discoid resection, nodule diameter should not exceed 3 cm, with a maximum of half of the bowel circumference and a maximum of 60% stenosis. I view these numbers as guiding principles, however, and not firm rules. Surgical decisions should be personalized based on the patient, the surgeon’s impression of the extent of the disease, and the ability to perform anterior discoid resection without compromising the rectal lumen with primary closure of the defect.
The technique
Rectosigmoid endometriotic nodules may present within the context of an obliterated posterior cul-de-sac, but the avascular pararectal space can be used to approach the nodules. Detailed knowledge of the avascular planes of this space, as well as the rectovaginal space, is crucial. Development of the rectovaginal space frees the bowel from its attachments to the posterior uterus and vagina. Judicious use of energized instruments in sharp dissection, and frequently sharp cold cutting, should be used near the bowel serosa to prevent thermal injury.
Presurgical imaging usually offers a good assessment of a nodule’s size and location, but intraoperatively, I typically use an atraumatic grasper to further assess size and contour and to determine if the nodule is suitable for discoid resection. If so, a suture is placed through the nodule to improve manipulation, and enucleation of the nodule itself is achieved through a “squeeze” technique in which an advanced bipolar device is used to circumscribe the lesion, dissecting the nodule as the device bounces off the thick endometriotic tissue.
The ENSEAL bipolar device (Ethicon, Somerville, N.J.) was designed as a vessel sealer, but because it will not cut through hard tissue as will other laparoscopic cutting devices, it serves as a useful tool for resecting endometriotic nodules while minimizing the removal of healthy rectal tissue. The device bounces off the nodule because it will avoid cutting through the thick tissue; in the process, it facilitates a fairly complete enucleation of the endometriotic nodule, starting with dissection until an intentional colotomy/enterotomy is made and followed by circumscription of the lesion once the rectum is entered.
Gentle traction and counter-traction increase the efficiency of dissection and minimize the amount of normal rectal tissue removed. Quick cutting with short bursts of energy allows for good hemostasis and minimizes thermal spread, which will maximize tissue healing from subsequent repair.
I then use a rectal probe as a template for repair. The probe is advanced underneath the defect between the distal and proximal portions, and the tissue is moved over the probe to ensure that the repair will be tension free. An ability to reapproximate the defect while keeping the probe in place indicates that the defect can be safely closed. (For a video presentation of the surgery, see www.surgeryu.com/leeobgyn.) If suturing is not feasible, the general surgeon is called to perform segmental resection.
The integrity of the repair is then thoroughly assessed with an air leak test. A bowel clamp is placed across the rectum and the pelvis is filled with sterile saline. Air is placed into the rectum with a rigid proctoscope while the operative field is inspected for evidence of an air leak.
Discoid resection may also be performed with a circular stapler. While this technique is faster than suturing, its use is limited by nodule size and has the potential to compromise complete excision of the nodule.
Dr. Lee is director of minimally invasive gynecologic surgery, Magee-Women’s Hospital of the University of Pittsburgh Medical Center.
Discoid resection of rectal endometriotic nodules
The treatment of the rectovaginal endometriotic nodule continues to be controversial. While proponents of “shaving” the nodule are quick to point out that compared with segmental bowel resection, pelvic pain, dyspareunia, dysmenorrhea, and postoperative pregnancy rates are similarly reduced, most comparative studies are retrospective and are not randomized. That is, patients with larger nodules or multifocal disease with deep infiltration into the muscularis layer of the bowel, or involving more than half of the bowel wall circumference, with surrounding severe fibrosis, invariably are more likely to undergo segmental bowel resection. Even with performance of segmental bowel resection to treat more extensive disease, there is a trend toward greater improvement of pain-related symptoms when compared with the “shaving” technique. Furthermore, the risk of rectal recurrence is acknowledged to be greater in patients undergoing endometriotic rectal nodule shaving.
Concern must be raised with segmental bowel resection. Not only is the risk of temporary ileostomy increased, but subsequent anastomotic leakage and rectovaginal fistula is noted in up to 10% of women. Although reduced with nerve sparing techniques, bladder denervation secondary to damage of the parasympathetic plexus causes urinary retention. In a study of 436 cases of laparoscopic colorectal resection, 9.5% presented after 30 days with persistent urinary retention and 4.2% with constipation (Surg Endosc. 2010 Jan;24:63-7).
For this edition of the Master Class in Gynecologic Surgery, I have invited Ted Lee, MD, director of minimally invasive gynecologic surgery, Magee-Womens Hospital of the University of Pittsburgh Medical Center, to discuss laparoscopic rectosigmoid resection for a deep endometriotic nodule. While many surgeons utilize a single-use curved circular stapler, I appreciate Dr. Lee’s innovative technique, for both its ease of use and its safety.
Dr. Lee has received multiple awards for his efforts, including best surgical video presentation by the AAGL. He is also the only five-time winner of the prestigious Golden Laparoscope Award for best surgical video from the AAGL.
A highly-regarded lecturer and surgeon, Dr. Lee has taught and performed live surgeries around the world.
Dr. Lee’s practice is entirely dedicated to minimally invasive surgical options for women. He is a firm believer that virtually all benign gynecologic surgical conditions should be treated using a minimally invasive approach. Dr. Lee’s clinical expertise includes minimally invasive surgery for treatments of endometriosis (including severe endometriosis involving bowel, bladder, and ureter); fibroids; abnormal uterine bleeding; urinary incontinence; and pelvic organ prolapse.
It is a great honor for the Master Class in Gynecologic Surgery to have Dr. Lee as guest author for this important area of our surgical arena.
Dr. Miller is a minimally invasive gynecologic surgeon in Naperville, Ill., and a past president of the AAGL.
The treatment of the rectovaginal endometriotic nodule continues to be controversial. While proponents of “shaving” the nodule are quick to point out that compared with segmental bowel resection, pelvic pain, dyspareunia, dysmenorrhea, and postoperative pregnancy rates are similarly reduced, most comparative studies are retrospective and are not randomized. That is, patients with larger nodules or multifocal disease with deep infiltration into the muscularis layer of the bowel, or involving more than half of the bowel wall circumference, with surrounding severe fibrosis, invariably are more likely to undergo segmental bowel resection. Even with performance of segmental bowel resection to treat more extensive disease, there is a trend toward greater improvement of pain-related symptoms when compared with the “shaving” technique. Furthermore, the risk of rectal recurrence is acknowledged to be greater in patients undergoing endometriotic rectal nodule shaving.
Concern must be raised with segmental bowel resection. Not only is the risk of temporary ileostomy increased, but subsequent anastomotic leakage and rectovaginal fistula is noted in up to 10% of women. Although reduced with nerve sparing techniques, bladder denervation secondary to damage of the parasympathetic plexus causes urinary retention. In a study of 436 cases of laparoscopic colorectal resection, 9.5% presented after 30 days with persistent urinary retention and 4.2% with constipation (Surg Endosc. 2010 Jan;24:63-7).
For this edition of the Master Class in Gynecologic Surgery, I have invited Ted Lee, MD, director of minimally invasive gynecologic surgery, Magee-Womens Hospital of the University of Pittsburgh Medical Center, to discuss laparoscopic rectosigmoid resection for a deep endometriotic nodule. While many surgeons utilize a single-use curved circular stapler, I appreciate Dr. Lee’s innovative technique, for both its ease of use and its safety.
Dr. Lee has received multiple awards for his efforts, including best surgical video presentation by the AAGL. He is also the only five-time winner of the prestigious Golden Laparoscope Award for best surgical video from the AAGL.
A highly-regarded lecturer and surgeon, Dr. Lee has taught and performed live surgeries around the world.
Dr. Lee’s practice is entirely dedicated to minimally invasive surgical options for women. He is a firm believer that virtually all benign gynecologic surgical conditions should be treated using a minimally invasive approach. Dr. Lee’s clinical expertise includes minimally invasive surgery for treatments of endometriosis (including severe endometriosis involving bowel, bladder, and ureter); fibroids; abnormal uterine bleeding; urinary incontinence; and pelvic organ prolapse.
It is a great honor for the Master Class in Gynecologic Surgery to have Dr. Lee as guest author for this important area of our surgical arena.
Dr. Miller is a minimally invasive gynecologic surgeon in Naperville, Ill., and a past president of the AAGL.
The treatment of the rectovaginal endometriotic nodule continues to be controversial. While proponents of “shaving” the nodule are quick to point out that compared with segmental bowel resection, pelvic pain, dyspareunia, dysmenorrhea, and postoperative pregnancy rates are similarly reduced, most comparative studies are retrospective and are not randomized. That is, patients with larger nodules or multifocal disease with deep infiltration into the muscularis layer of the bowel, or involving more than half of the bowel wall circumference, with surrounding severe fibrosis, invariably are more likely to undergo segmental bowel resection. Even with performance of segmental bowel resection to treat more extensive disease, there is a trend toward greater improvement of pain-related symptoms when compared with the “shaving” technique. Furthermore, the risk of rectal recurrence is acknowledged to be greater in patients undergoing endometriotic rectal nodule shaving.
Concern must be raised with segmental bowel resection. Not only is the risk of temporary ileostomy increased, but subsequent anastomotic leakage and rectovaginal fistula is noted in up to 10% of women. Although reduced with nerve sparing techniques, bladder denervation secondary to damage of the parasympathetic plexus causes urinary retention. In a study of 436 cases of laparoscopic colorectal resection, 9.5% presented after 30 days with persistent urinary retention and 4.2% with constipation (Surg Endosc. 2010 Jan;24:63-7).
For this edition of the Master Class in Gynecologic Surgery, I have invited Ted Lee, MD, director of minimally invasive gynecologic surgery, Magee-Womens Hospital of the University of Pittsburgh Medical Center, to discuss laparoscopic rectosigmoid resection for a deep endometriotic nodule. While many surgeons utilize a single-use curved circular stapler, I appreciate Dr. Lee’s innovative technique, for both its ease of use and its safety.
Dr. Lee has received multiple awards for his efforts, including best surgical video presentation by the AAGL. He is also the only five-time winner of the prestigious Golden Laparoscope Award for best surgical video from the AAGL.
A highly-regarded lecturer and surgeon, Dr. Lee has taught and performed live surgeries around the world.
Dr. Lee’s practice is entirely dedicated to minimally invasive surgical options for women. He is a firm believer that virtually all benign gynecologic surgical conditions should be treated using a minimally invasive approach. Dr. Lee’s clinical expertise includes minimally invasive surgery for treatments of endometriosis (including severe endometriosis involving bowel, bladder, and ureter); fibroids; abnormal uterine bleeding; urinary incontinence; and pelvic organ prolapse.
It is a great honor for the Master Class in Gynecologic Surgery to have Dr. Lee as guest author for this important area of our surgical arena.
Dr. Miller is a minimally invasive gynecologic surgeon in Naperville, Ill., and a past president of the AAGL.
Sexual harassment, violence is our problem, too
This past year has seen an incredible transformation in our awareness and understanding of the extent of sexual harassment and assault in our society. The #metoo campaign and the brave women throughout the country who have come out and told us their stories have truly made a difference.
For years, I, and many other pediatricians like me, have counseled teenage girls on how to stay safe as they prepare to enter college and universities. So many times, I have mentioned the shocking statistics on rape and sexual assault in college. I have cautioned these young women on how to stay safe, to stay close to their girlfriends, to not accept drinks from other people, and if drinking, not to drink so much that they don’t know what is going on around them … and so on and so forth with many dos and don’ts.
But here is something I only recently noticed: In the last 18 years of practice, my counsel to the boys was limited to how to stay safe, protect themselves against sexually transmitted infections, and how to avoid pregnancy. It did not cross my mind to counsel the teenage boys on their respective dos and don’ts, especially when it comes to their behavior with women. For example, stern advice on how to respect women, that only yes means yes, and frankly how to make sure they don’t become sexual harassers or worse.
I have questioned myself since then, wondering if I was alone with this oversight. I asked several other pediatricians as well to see if they had spoken about sexual harassment and assault in this context with their teenage male patients. The vast majority had the same experience as I did. They had all counseled the girls on how to protect themselves from becoming victims, but somehow not the boys (or girls for that matter) on how to help them not become the aggressors.
It has occurred to me that our focus as physicians has largely been limited to what the victims can do to not become victims.
As pediatricians, we counsel parents on how to keep their children safe, and how to protect them. It is our obligation to help, to guide, and to teach parents. We teach parents about discipline, limit setting, sleeping, feeding, and so many other things. We need to teach them how to talk to their boys and girls about appropriate behavior with whomever they may be interested in, about sexual harassment, and assault, and how not to become part of the problem.
It is our obligation as pediatricians to teach the children we see growing up in front of us how to behave in an adult, sexual world. We can make a bigger difference than we might think. As physicians, we promised to do no harm when we took our oath. It is now our turn also to take proactive steps to stop the harm caused by others.
Dr. Rimawi is a pediatrician in private practice in Atherton, Calif. Email her at [email protected].
This past year has seen an incredible transformation in our awareness and understanding of the extent of sexual harassment and assault in our society. The #metoo campaign and the brave women throughout the country who have come out and told us their stories have truly made a difference.
For years, I, and many other pediatricians like me, have counseled teenage girls on how to stay safe as they prepare to enter college and universities. So many times, I have mentioned the shocking statistics on rape and sexual assault in college. I have cautioned these young women on how to stay safe, to stay close to their girlfriends, to not accept drinks from other people, and if drinking, not to drink so much that they don’t know what is going on around them … and so on and so forth with many dos and don’ts.
But here is something I only recently noticed: In the last 18 years of practice, my counsel to the boys was limited to how to stay safe, protect themselves against sexually transmitted infections, and how to avoid pregnancy. It did not cross my mind to counsel the teenage boys on their respective dos and don’ts, especially when it comes to their behavior with women. For example, stern advice on how to respect women, that only yes means yes, and frankly how to make sure they don’t become sexual harassers or worse.
I have questioned myself since then, wondering if I was alone with this oversight. I asked several other pediatricians as well to see if they had spoken about sexual harassment and assault in this context with their teenage male patients. The vast majority had the same experience as I did. They had all counseled the girls on how to protect themselves from becoming victims, but somehow not the boys (or girls for that matter) on how to help them not become the aggressors.
It has occurred to me that our focus as physicians has largely been limited to what the victims can do to not become victims.
As pediatricians, we counsel parents on how to keep their children safe, and how to protect them. It is our obligation to help, to guide, and to teach parents. We teach parents about discipline, limit setting, sleeping, feeding, and so many other things. We need to teach them how to talk to their boys and girls about appropriate behavior with whomever they may be interested in, about sexual harassment, and assault, and how not to become part of the problem.
It is our obligation as pediatricians to teach the children we see growing up in front of us how to behave in an adult, sexual world. We can make a bigger difference than we might think. As physicians, we promised to do no harm when we took our oath. It is now our turn also to take proactive steps to stop the harm caused by others.
Dr. Rimawi is a pediatrician in private practice in Atherton, Calif. Email her at [email protected].
This past year has seen an incredible transformation in our awareness and understanding of the extent of sexual harassment and assault in our society. The #metoo campaign and the brave women throughout the country who have come out and told us their stories have truly made a difference.
For years, I, and many other pediatricians like me, have counseled teenage girls on how to stay safe as they prepare to enter college and universities. So many times, I have mentioned the shocking statistics on rape and sexual assault in college. I have cautioned these young women on how to stay safe, to stay close to their girlfriends, to not accept drinks from other people, and if drinking, not to drink so much that they don’t know what is going on around them … and so on and so forth with many dos and don’ts.
But here is something I only recently noticed: In the last 18 years of practice, my counsel to the boys was limited to how to stay safe, protect themselves against sexually transmitted infections, and how to avoid pregnancy. It did not cross my mind to counsel the teenage boys on their respective dos and don’ts, especially when it comes to their behavior with women. For example, stern advice on how to respect women, that only yes means yes, and frankly how to make sure they don’t become sexual harassers or worse.
I have questioned myself since then, wondering if I was alone with this oversight. I asked several other pediatricians as well to see if they had spoken about sexual harassment and assault in this context with their teenage male patients. The vast majority had the same experience as I did. They had all counseled the girls on how to protect themselves from becoming victims, but somehow not the boys (or girls for that matter) on how to help them not become the aggressors.
It has occurred to me that our focus as physicians has largely been limited to what the victims can do to not become victims.
As pediatricians, we counsel parents on how to keep their children safe, and how to protect them. It is our obligation to help, to guide, and to teach parents. We teach parents about discipline, limit setting, sleeping, feeding, and so many other things. We need to teach them how to talk to their boys and girls about appropriate behavior with whomever they may be interested in, about sexual harassment, and assault, and how not to become part of the problem.
It is our obligation as pediatricians to teach the children we see growing up in front of us how to behave in an adult, sexual world. We can make a bigger difference than we might think. As physicians, we promised to do no harm when we took our oath. It is now our turn also to take proactive steps to stop the harm caused by others.
Dr. Rimawi is a pediatrician in private practice in Atherton, Calif. Email her at [email protected].
Why isn’t smart gun technology on Parkland activists’ agenda?
The evening before the March For Our Lives rally in Washington, I was hard at work on my sign. Making a statement is important, but at the Women’s March last January, I discovered that a sign was invaluable for keeping friends together in a crowd. Since my Women’s March sign read “Make America Kind Again,” I opted to keep with the theme, and I created a “Make America Safe Again” sign for gun control. My daughter looked at my sign skeptically. “It’s too vague and nondirective,” she declared. Now challenged, I added the following directives: “Universal Background Checks,” “Ban Assault Weapons,” and “Smart Guns.” My sign was now complete.
I was surprised when my daughter – our family Jeopardy! whiz – asked, “What’s a smart gun?” When we arrived to join friends, their daughter – a doctoral student – also looked at the sign and asked me what a smart gun is. I explained to both young women that a smart gun – like an iPhone – relies on a biometric such as a fingerprint so that it can be used only by authorized users. This technology would reduce the flow of firearms to illegal owners, prevent accidental discharge by children, protect rightful owners and law enforcement officers from having their own weapons used against them by criminals, and decrease the number of suicides by family members of gun owners. In my state of Maryland, there have been two school shootings, one as recently as March 20, and both were committed by boys who took a parent’s firearm to school.
We arrived at the rally and found a spot in front of the Newseum, with two jumbotrons in sight, right in the middle of the crowd. The young people who spoke were inspirational. Regardless of one’s political views or thoughts on gun control, they were bright, articulate, fearless, passionate, and determined. The show was stolen by Naomi Wadler, a precocious 11-year-old girl who is destined to be a member of Congress (if not president) one day, and by Dr. Martin Luther King Jr.’s 9-year-old granddaughter, Yolanda Renee King.
The rest of the speakers were teenagers, all of whom had lost someone to gunfire. There were speakers from Parkland, Fla., who talked about the raw losses they were feeling, and the student who wasn’t celebrating his 18th birthday on March 24. Matthew Soto from Newtown, Conn., talked about losing his sister, a first-grade teacher, in the Sandy Hook shooting.
But the rally wasn’t just about mass murders and school shootings: Zion Kelly talked about the death of his twin, a teen who was killed when he stopped at a convenience store on the way home from school in Washington, only to have his promising life snuffed out during a holdup. We heard about shootings in the bullet-riddled streets of Chicago and Los Angeles and the toll they have taken. The losses to gun violence have been felt most strongly in communities of color. While it was mentioned that the majority of gun deaths are suicides, there were no speakers from families of people who died from suicide. The final speaker was Emma Gonzalez, a young woman from Parkland who took the stage for 6 minutes and 20 seconds - the duration of the shooter’s rampage - and closed with a powerful moment of silence.* The rally closed with a performance of Bob Dylan’s “The Times They Are a-Changin’ ” by Jennifer Hudson, whose mother, brother, and nephew were murdered by a gunman.
The teens talked about what changes they wanted to see enacted. They talked about closing loopholes to background checks, and I was pleased that one young man specifically mentioned keeping guns from those who are violent, but did not mention mental illness as a reason to block gun ownership. A call to resume a ban on assault weapons was made repeatedly as was a call to raise the age (to 21) at which an individual can purchase a gun. Military-style weapons are not necessary for hunting or self-defense, and they enable the rapid-fire assassinations we have seen in mass shootings, so they remain an easy target of gun control advocacy. But in terms of numbers, these firearms are responsible for a small fraction of gun deaths. I was surprised, but the teens did not mention “smart gun” legislation as a way to reduce gun deaths.
Guns, and gun deaths, are a part of American society. While the majority of Americans favor stronger regulation of gun ownership, legislation that would end gun ownership is not likely to go anywhere. Smart guns, however, are different. Forty percent of polled gun owners have said they would swap their firearm for a smart firearm. So given the appeal of a firearm that can’t be diverted or stolen, used against the owner, discharged accidentally by a child, or used for suicide or homicide by a distressed family member, why don’t these weapons exist for use by the American people? . People want these guns and the protections they offer, yet they have never been produced and made available to either the American public or to our law enforcement officers.
So where are these firearms, and why aren’t our Parkland teens asking for them? The answer to the first part of that question lies with the National Rifle Association (NRA) and the state of New Jersey. In 2003, New Jersey passed the Childproof Handgun bill, which requires that all guns sold in the state be smart guns within 3 years of their availability. The NRA has vigorously opposed any legislation that would require all guns to be smart guns. Because the availability of these weapons would trigger the New Jersey bill, California and Maryland have been prevented from importing smart firearms from a German company. Perhaps, however, New Jersey does not need to bear all the blame; in 1999, 4 years before the passage of the bill, the NRA and its members boycotted Smith & Wesson when the gun manufacturer revealed plans to develop a smart gun for the government. The NRA’s public stance is that it does not oppose smart guns for those who want them, but it opposes legislation that would eliminate the sale of conventional firearms. The organization has voiced concerns that technology fails and that it potentially slows down firing the weapon. It doesn’t talk about dead toddlers, or about police officers who’ve been killed when their weapons were taken from them.
As for the Parkland students, I don’t know why they aren’t asking for smart gun technology while they have the attention of the country. Perhaps they, like the young women I was with, don’t know it’s an option. Perhaps it’s too removed from the issue of mass murders and an assault weapon ban feels more attainable. Or perhaps the NRA’s mission has too much of a stronghold in Florida. I don’t know why they aren’t asking for smart gun production, but I know they should be.
*Correction, 3/27/2018: An earlier version of this story misstated the duration of Emma Gonzalez's moment of silence.
Dr. Miller is coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press), 2016. She practices in Baltimore.
The evening before the March For Our Lives rally in Washington, I was hard at work on my sign. Making a statement is important, but at the Women’s March last January, I discovered that a sign was invaluable for keeping friends together in a crowd. Since my Women’s March sign read “Make America Kind Again,” I opted to keep with the theme, and I created a “Make America Safe Again” sign for gun control. My daughter looked at my sign skeptically. “It’s too vague and nondirective,” she declared. Now challenged, I added the following directives: “Universal Background Checks,” “Ban Assault Weapons,” and “Smart Guns.” My sign was now complete.
I was surprised when my daughter – our family Jeopardy! whiz – asked, “What’s a smart gun?” When we arrived to join friends, their daughter – a doctoral student – also looked at the sign and asked me what a smart gun is. I explained to both young women that a smart gun – like an iPhone – relies on a biometric such as a fingerprint so that it can be used only by authorized users. This technology would reduce the flow of firearms to illegal owners, prevent accidental discharge by children, protect rightful owners and law enforcement officers from having their own weapons used against them by criminals, and decrease the number of suicides by family members of gun owners. In my state of Maryland, there have been two school shootings, one as recently as March 20, and both were committed by boys who took a parent’s firearm to school.
We arrived at the rally and found a spot in front of the Newseum, with two jumbotrons in sight, right in the middle of the crowd. The young people who spoke were inspirational. Regardless of one’s political views or thoughts on gun control, they were bright, articulate, fearless, passionate, and determined. The show was stolen by Naomi Wadler, a precocious 11-year-old girl who is destined to be a member of Congress (if not president) one day, and by Dr. Martin Luther King Jr.’s 9-year-old granddaughter, Yolanda Renee King.
The rest of the speakers were teenagers, all of whom had lost someone to gunfire. There were speakers from Parkland, Fla., who talked about the raw losses they were feeling, and the student who wasn’t celebrating his 18th birthday on March 24. Matthew Soto from Newtown, Conn., talked about losing his sister, a first-grade teacher, in the Sandy Hook shooting.
But the rally wasn’t just about mass murders and school shootings: Zion Kelly talked about the death of his twin, a teen who was killed when he stopped at a convenience store on the way home from school in Washington, only to have his promising life snuffed out during a holdup. We heard about shootings in the bullet-riddled streets of Chicago and Los Angeles and the toll they have taken. The losses to gun violence have been felt most strongly in communities of color. While it was mentioned that the majority of gun deaths are suicides, there were no speakers from families of people who died from suicide. The final speaker was Emma Gonzalez, a young woman from Parkland who took the stage for 6 minutes and 20 seconds - the duration of the shooter’s rampage - and closed with a powerful moment of silence.* The rally closed with a performance of Bob Dylan’s “The Times They Are a-Changin’ ” by Jennifer Hudson, whose mother, brother, and nephew were murdered by a gunman.
The teens talked about what changes they wanted to see enacted. They talked about closing loopholes to background checks, and I was pleased that one young man specifically mentioned keeping guns from those who are violent, but did not mention mental illness as a reason to block gun ownership. A call to resume a ban on assault weapons was made repeatedly as was a call to raise the age (to 21) at which an individual can purchase a gun. Military-style weapons are not necessary for hunting or self-defense, and they enable the rapid-fire assassinations we have seen in mass shootings, so they remain an easy target of gun control advocacy. But in terms of numbers, these firearms are responsible for a small fraction of gun deaths. I was surprised, but the teens did not mention “smart gun” legislation as a way to reduce gun deaths.
Guns, and gun deaths, are a part of American society. While the majority of Americans favor stronger regulation of gun ownership, legislation that would end gun ownership is not likely to go anywhere. Smart guns, however, are different. Forty percent of polled gun owners have said they would swap their firearm for a smart firearm. So given the appeal of a firearm that can’t be diverted or stolen, used against the owner, discharged accidentally by a child, or used for suicide or homicide by a distressed family member, why don’t these weapons exist for use by the American people? . People want these guns and the protections they offer, yet they have never been produced and made available to either the American public or to our law enforcement officers.
So where are these firearms, and why aren’t our Parkland teens asking for them? The answer to the first part of that question lies with the National Rifle Association (NRA) and the state of New Jersey. In 2003, New Jersey passed the Childproof Handgun bill, which requires that all guns sold in the state be smart guns within 3 years of their availability. The NRA has vigorously opposed any legislation that would require all guns to be smart guns. Because the availability of these weapons would trigger the New Jersey bill, California and Maryland have been prevented from importing smart firearms from a German company. Perhaps, however, New Jersey does not need to bear all the blame; in 1999, 4 years before the passage of the bill, the NRA and its members boycotted Smith & Wesson when the gun manufacturer revealed plans to develop a smart gun for the government. The NRA’s public stance is that it does not oppose smart guns for those who want them, but it opposes legislation that would eliminate the sale of conventional firearms. The organization has voiced concerns that technology fails and that it potentially slows down firing the weapon. It doesn’t talk about dead toddlers, or about police officers who’ve been killed when their weapons were taken from them.
As for the Parkland students, I don’t know why they aren’t asking for smart gun technology while they have the attention of the country. Perhaps they, like the young women I was with, don’t know it’s an option. Perhaps it’s too removed from the issue of mass murders and an assault weapon ban feels more attainable. Or perhaps the NRA’s mission has too much of a stronghold in Florida. I don’t know why they aren’t asking for smart gun production, but I know they should be.
*Correction, 3/27/2018: An earlier version of this story misstated the duration of Emma Gonzalez's moment of silence.
Dr. Miller is coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press), 2016. She practices in Baltimore.
The evening before the March For Our Lives rally in Washington, I was hard at work on my sign. Making a statement is important, but at the Women’s March last January, I discovered that a sign was invaluable for keeping friends together in a crowd. Since my Women’s March sign read “Make America Kind Again,” I opted to keep with the theme, and I created a “Make America Safe Again” sign for gun control. My daughter looked at my sign skeptically. “It’s too vague and nondirective,” she declared. Now challenged, I added the following directives: “Universal Background Checks,” “Ban Assault Weapons,” and “Smart Guns.” My sign was now complete.
I was surprised when my daughter – our family Jeopardy! whiz – asked, “What’s a smart gun?” When we arrived to join friends, their daughter – a doctoral student – also looked at the sign and asked me what a smart gun is. I explained to both young women that a smart gun – like an iPhone – relies on a biometric such as a fingerprint so that it can be used only by authorized users. This technology would reduce the flow of firearms to illegal owners, prevent accidental discharge by children, protect rightful owners and law enforcement officers from having their own weapons used against them by criminals, and decrease the number of suicides by family members of gun owners. In my state of Maryland, there have been two school shootings, one as recently as March 20, and both were committed by boys who took a parent’s firearm to school.
We arrived at the rally and found a spot in front of the Newseum, with two jumbotrons in sight, right in the middle of the crowd. The young people who spoke were inspirational. Regardless of one’s political views or thoughts on gun control, they were bright, articulate, fearless, passionate, and determined. The show was stolen by Naomi Wadler, a precocious 11-year-old girl who is destined to be a member of Congress (if not president) one day, and by Dr. Martin Luther King Jr.’s 9-year-old granddaughter, Yolanda Renee King.
The rest of the speakers were teenagers, all of whom had lost someone to gunfire. There were speakers from Parkland, Fla., who talked about the raw losses they were feeling, and the student who wasn’t celebrating his 18th birthday on March 24. Matthew Soto from Newtown, Conn., talked about losing his sister, a first-grade teacher, in the Sandy Hook shooting.
But the rally wasn’t just about mass murders and school shootings: Zion Kelly talked about the death of his twin, a teen who was killed when he stopped at a convenience store on the way home from school in Washington, only to have his promising life snuffed out during a holdup. We heard about shootings in the bullet-riddled streets of Chicago and Los Angeles and the toll they have taken. The losses to gun violence have been felt most strongly in communities of color. While it was mentioned that the majority of gun deaths are suicides, there were no speakers from families of people who died from suicide. The final speaker was Emma Gonzalez, a young woman from Parkland who took the stage for 6 minutes and 20 seconds - the duration of the shooter’s rampage - and closed with a powerful moment of silence.* The rally closed with a performance of Bob Dylan’s “The Times They Are a-Changin’ ” by Jennifer Hudson, whose mother, brother, and nephew were murdered by a gunman.
The teens talked about what changes they wanted to see enacted. They talked about closing loopholes to background checks, and I was pleased that one young man specifically mentioned keeping guns from those who are violent, but did not mention mental illness as a reason to block gun ownership. A call to resume a ban on assault weapons was made repeatedly as was a call to raise the age (to 21) at which an individual can purchase a gun. Military-style weapons are not necessary for hunting or self-defense, and they enable the rapid-fire assassinations we have seen in mass shootings, so they remain an easy target of gun control advocacy. But in terms of numbers, these firearms are responsible for a small fraction of gun deaths. I was surprised, but the teens did not mention “smart gun” legislation as a way to reduce gun deaths.
Guns, and gun deaths, are a part of American society. While the majority of Americans favor stronger regulation of gun ownership, legislation that would end gun ownership is not likely to go anywhere. Smart guns, however, are different. Forty percent of polled gun owners have said they would swap their firearm for a smart firearm. So given the appeal of a firearm that can’t be diverted or stolen, used against the owner, discharged accidentally by a child, or used for suicide or homicide by a distressed family member, why don’t these weapons exist for use by the American people? . People want these guns and the protections they offer, yet they have never been produced and made available to either the American public or to our law enforcement officers.
So where are these firearms, and why aren’t our Parkland teens asking for them? The answer to the first part of that question lies with the National Rifle Association (NRA) and the state of New Jersey. In 2003, New Jersey passed the Childproof Handgun bill, which requires that all guns sold in the state be smart guns within 3 years of their availability. The NRA has vigorously opposed any legislation that would require all guns to be smart guns. Because the availability of these weapons would trigger the New Jersey bill, California and Maryland have been prevented from importing smart firearms from a German company. Perhaps, however, New Jersey does not need to bear all the blame; in 1999, 4 years before the passage of the bill, the NRA and its members boycotted Smith & Wesson when the gun manufacturer revealed plans to develop a smart gun for the government. The NRA’s public stance is that it does not oppose smart guns for those who want them, but it opposes legislation that would eliminate the sale of conventional firearms. The organization has voiced concerns that technology fails and that it potentially slows down firing the weapon. It doesn’t talk about dead toddlers, or about police officers who’ve been killed when their weapons were taken from them.
As for the Parkland students, I don’t know why they aren’t asking for smart gun technology while they have the attention of the country. Perhaps they, like the young women I was with, don’t know it’s an option. Perhaps it’s too removed from the issue of mass murders and an assault weapon ban feels more attainable. Or perhaps the NRA’s mission has too much of a stronghold in Florida. I don’t know why they aren’t asking for smart gun production, but I know they should be.
*Correction, 3/27/2018: An earlier version of this story misstated the duration of Emma Gonzalez's moment of silence.
Dr. Miller is coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press), 2016. She practices in Baltimore.
Congressional budget includes AGA wins
AGA spends a lot of time on Capitol Hill advocating to help gastroenterologists in practice better care for their patients and receive fair reimbursement. Therefore, we were pleased that the budget deal passed by Congress and signed by the president in February included several policy victories that AGA has been working diligently on for many years.
IPAB repeal
AGA, and all of organized medicine, have long opposed the Independent Payment Advisory Board (IPAB) that was created as part of the Affordable Care Act. IPAB is an unelected, unaccountable board whose sole purpose is to cut Medicare spending from providers should Medicare reach a certain threshold of spending. Since hospitals are exempt from their purview, physicians would be particularly vulnerable to cuts. However, repealing IPAB has had bipartisan support over the years, and we applaud Congress for listening to us and the medical community and taking action.
Misvalued codes
AGA and the physician community were also successful in removing a provision that would have extended the misvalued codes initiative for the next two years to reallocate savings from potentially overvalued codes. AGA, the Alliance of Specialty Medicine and the AMA opposed the original provision expanding the misvalued codes initiative and have argued that virtually all codes under the fee schedule, including gastroenterology, have been reevaluated and have already faced significant cuts. In the final agreement, Congress eliminated recapturing savings from the misvalued codes initiative and instead lowered overall updates for physician reimbursement under Medicare by .25 percent for 1 year. Although AGA would prefer this reduction not be included, it is much better than the misvalued codes provision, which disproportionately impacts specialties, like gastroenterology.
Geographic Practice Cost Index
The budget agreement extends the work for the Geographic Practice Cost Index (GPCI) floor for two additional years, which avoids a decrease in Medicare reimbursement for physicians that practice in rural areas. The work GPCI is a variable that Medicare uses to adjust the work component of physician payment based on where they live. A work GPCI floor of 1.0 protects physicians in low-cost, often rural areas, from being paid less for the work they do.
Meaningful use standards
The package addresses electronic health record (EHR) standards and eases requirements for physicians. The language removes the mandate that meaningful use standards become more stringent over time, which is a major financial burden for physician practices. The language also gives physicians more time to submit and receive a hardship exemption from the current EHR standards that would apply to meaningful use and the Quality Payment Program’s advancing care information performance category.
Biosimilars coverage under Medicare Part D
The agreement also levels the playing field between biologics and biosimilars by adding biosimilars to the Medicare Coverage Gap Discount Program. Additionally, by providing the 50 percent discount equally, beneficiary out-of-pocket costs will be reduced and the Medicare program will save money as a result of covering the less expensive medication.
AGA and the medical community have fought long and hard for these provisions and are happy to see them finally being implemented. We thank all of our members who have worked along with us to ensure that the voice of gastroenterology continues to be heard on Capitol Hill.
AGA spends a lot of time on Capitol Hill advocating to help gastroenterologists in practice better care for their patients and receive fair reimbursement. Therefore, we were pleased that the budget deal passed by Congress and signed by the president in February included several policy victories that AGA has been working diligently on for many years.
IPAB repeal
AGA, and all of organized medicine, have long opposed the Independent Payment Advisory Board (IPAB) that was created as part of the Affordable Care Act. IPAB is an unelected, unaccountable board whose sole purpose is to cut Medicare spending from providers should Medicare reach a certain threshold of spending. Since hospitals are exempt from their purview, physicians would be particularly vulnerable to cuts. However, repealing IPAB has had bipartisan support over the years, and we applaud Congress for listening to us and the medical community and taking action.
Misvalued codes
AGA and the physician community were also successful in removing a provision that would have extended the misvalued codes initiative for the next two years to reallocate savings from potentially overvalued codes. AGA, the Alliance of Specialty Medicine and the AMA opposed the original provision expanding the misvalued codes initiative and have argued that virtually all codes under the fee schedule, including gastroenterology, have been reevaluated and have already faced significant cuts. In the final agreement, Congress eliminated recapturing savings from the misvalued codes initiative and instead lowered overall updates for physician reimbursement under Medicare by .25 percent for 1 year. Although AGA would prefer this reduction not be included, it is much better than the misvalued codes provision, which disproportionately impacts specialties, like gastroenterology.
Geographic Practice Cost Index
The budget agreement extends the work for the Geographic Practice Cost Index (GPCI) floor for two additional years, which avoids a decrease in Medicare reimbursement for physicians that practice in rural areas. The work GPCI is a variable that Medicare uses to adjust the work component of physician payment based on where they live. A work GPCI floor of 1.0 protects physicians in low-cost, often rural areas, from being paid less for the work they do.
Meaningful use standards
The package addresses electronic health record (EHR) standards and eases requirements for physicians. The language removes the mandate that meaningful use standards become more stringent over time, which is a major financial burden for physician practices. The language also gives physicians more time to submit and receive a hardship exemption from the current EHR standards that would apply to meaningful use and the Quality Payment Program’s advancing care information performance category.
Biosimilars coverage under Medicare Part D
The agreement also levels the playing field between biologics and biosimilars by adding biosimilars to the Medicare Coverage Gap Discount Program. Additionally, by providing the 50 percent discount equally, beneficiary out-of-pocket costs will be reduced and the Medicare program will save money as a result of covering the less expensive medication.
AGA and the medical community have fought long and hard for these provisions and are happy to see them finally being implemented. We thank all of our members who have worked along with us to ensure that the voice of gastroenterology continues to be heard on Capitol Hill.
AGA spends a lot of time on Capitol Hill advocating to help gastroenterologists in practice better care for their patients and receive fair reimbursement. Therefore, we were pleased that the budget deal passed by Congress and signed by the president in February included several policy victories that AGA has been working diligently on for many years.
IPAB repeal
AGA, and all of organized medicine, have long opposed the Independent Payment Advisory Board (IPAB) that was created as part of the Affordable Care Act. IPAB is an unelected, unaccountable board whose sole purpose is to cut Medicare spending from providers should Medicare reach a certain threshold of spending. Since hospitals are exempt from their purview, physicians would be particularly vulnerable to cuts. However, repealing IPAB has had bipartisan support over the years, and we applaud Congress for listening to us and the medical community and taking action.
Misvalued codes
AGA and the physician community were also successful in removing a provision that would have extended the misvalued codes initiative for the next two years to reallocate savings from potentially overvalued codes. AGA, the Alliance of Specialty Medicine and the AMA opposed the original provision expanding the misvalued codes initiative and have argued that virtually all codes under the fee schedule, including gastroenterology, have been reevaluated and have already faced significant cuts. In the final agreement, Congress eliminated recapturing savings from the misvalued codes initiative and instead lowered overall updates for physician reimbursement under Medicare by .25 percent for 1 year. Although AGA would prefer this reduction not be included, it is much better than the misvalued codes provision, which disproportionately impacts specialties, like gastroenterology.
Geographic Practice Cost Index
The budget agreement extends the work for the Geographic Practice Cost Index (GPCI) floor for two additional years, which avoids a decrease in Medicare reimbursement for physicians that practice in rural areas. The work GPCI is a variable that Medicare uses to adjust the work component of physician payment based on where they live. A work GPCI floor of 1.0 protects physicians in low-cost, often rural areas, from being paid less for the work they do.
Meaningful use standards
The package addresses electronic health record (EHR) standards and eases requirements for physicians. The language removes the mandate that meaningful use standards become more stringent over time, which is a major financial burden for physician practices. The language also gives physicians more time to submit and receive a hardship exemption from the current EHR standards that would apply to meaningful use and the Quality Payment Program’s advancing care information performance category.
Biosimilars coverage under Medicare Part D
The agreement also levels the playing field between biologics and biosimilars by adding biosimilars to the Medicare Coverage Gap Discount Program. Additionally, by providing the 50 percent discount equally, beneficiary out-of-pocket costs will be reduced and the Medicare program will save money as a result of covering the less expensive medication.
AGA and the medical community have fought long and hard for these provisions and are happy to see them finally being implemented. We thank all of our members who have worked along with us to ensure that the voice of gastroenterology continues to be heard on Capitol Hill.
Same-day discharge for hysterectomy
There is an increased focus on reducing the costs of health care delivery, and one major driver of surgical cost is length of hospitalization. A minimally invasive surgical approach to hysterectomy is a strategy that significantly enhances recovery and shortens hospital stay, although many patients who can safely be considered for same-day discharge (SDD), including many with cancer, are still admitted to the hospital overnight. Much has been published on the predictors and pathways for successful same-day discharge after minimally invasive hysterectomy, and in this column we will review how to best predict who is a good candidate for SDD and how to optimize the success of this approach with respect to safety and patient satisfaction.
What are the benefits to SDD?
Certainly, decreased hospitalization costs are an attractive feature of SDD following hysterectomy, although surgeons should also be mindful that patient-centered outcomes, such as pain control, managing nausea, and patient satisfaction, also are considered with equal emphasis. Several studies have shown that, in appropriate candidates and when proactive pathways are used, patient satisfaction is preserved with SDD following hysterectomy.1
Choosing patient candidates
Same day discharge is most successfully accomplished in patients of good general baseline health.2 Diabetic patients, particularly those on insulin, are generally not good candidates for SDD because it is important to monitor and intervene in blood glucose changes that are influenced by a nothing-by-mouth status and surgical stress. We recommend observing patients overnight with a history of pulmonary disease who may have transient increased postoperative O2 needs. Similarly, patients with significant cardiac disease (including heart failure and coronary disease) may benefit from prolonged overnight observation.
Particular caution should be paid to patients with obstructive sleep apnea, which may be occult but anticipated in patients with very high body mass indexes (greater than 40 kg/m2). General anesthetic drugs, the trauma of intubation, and opioids all couple with the underlying airway compromise such that these patients are at risk for postoperative apnea, which, in severe cases, can result in anoxia and death. These patients should be considered for continuous pulse-ox monitoring for at least 12-24 hours postoperatively and are not good candidates for same-day discharge.
Patients who have baseline anticoagulation that has been stopped or bridged preoperatively should have prolonged observation with recheck of their postoperative hemoglobin prior to discharge.
Patients who live alone or are very elderly with baseline frailty are poor candidates for SDD and may benefit from nursing observation overnight while they metabolize their anesthesia. Patients who have chronic opioid dependency present a greater challenge to control postoperative pain; these patients are generally less good candidates for SDD.
Studies have shown that the indication for the procedure (for example, cancer with staging, fibroids, endometriosis) is less critical in determining who is a good candidate for SDD.3 However, successful SDD rates are highest in more straightforward cases with few or no prior surgeries, small uteri (less than 14 weeks), a surgical duration of less than 3 hours, and a surgical start time before 2 p.m. Longer, more complex cases are typically associated with more blood loss, higher risk for occult complications, and more time under anesthesia (and in Trendelenburg), which can exacerbate airway edema. In preparation for such cases, it might be wise to prepare patients for the possibility that they may not be good candidates for discharge on the same day. In general, most SDD pathways exclude patients with very high BMI (greater than 50 kg/m2) because of concern for airway patency and because these cases may be more complex with higher underlying risk. In addition, many of these patients have diabetes and require perioperative metabolic interventions.
Patient preparation
A key component to successful SDD is setting patient expectations. Patients should be informed at their preoperative visit that, unless there is an unexpected occurrence or response to the surgery, they will be discharged to home the same day. This allows them to prepare their home (including transportation needs) in advance. They should be provided with information about what to expect that first night after surgery (including potential residual drowsiness or nausea from anesthesia and immediate postoperative pain).
On the day of surgery, under the influence of anesthesia and pain medication, patients will have difficulty retaining complex discharge instructions. The preoperative visit is critically important because it’s the best time to provide them with this information, including postoperative activity limitations, wound and dressing care, and follow-up instructions. This is also the best time to provide prescriptions for postoperative pain, nausea, and constipation prophylaxis with detailed instructions about best use. Patients should be encouraged to fill these prescriptions preoperatively so that they have these medications on hand on the evening of their discharge.
Many programs utilize a combination of educational strategies (in person, written, video) to maximize the likelihood of retention.1 It is also important to offer an opportunity for patients to ask questions about this information after they have received it (for example, by phoning the patients prior to their procedure).
Preoperative strategies

Intraoperative strategies
Consider in-and-out catheterization rather than placement of an indwelling catheter for anticipated short cases without complex bladder dissection.5 Minimize blood loss and maximally evacuate blood and clots with suction because hemoperitoneum can induce nausea and pain.
Pain from retained gas under the diaphragm can be reduced by bathing the diaphragms with 400 cc of dilute local anesthetic made by mixing 50 mL of 0.5% bupivacaine in 1000 mL normal saline prior to removal of pneumoperitoneum and while still in Trendelenburg. Ensure there is minimal retained intraperitoneal CO2 at the completion of the surgery by asking the anesthesiologists to perform positive pressure ventilations prior to fascial closure. Consider injecting port sites (including the peritoneal and fascial layers) with a mixture of immediate and long-acting local anesthetics. Request that the anesthesia staff administer intraoperative doses of IV ketorolac, acetaminophen, and tramadol (in preference to opioids) and an aggressive perioperative cocktail of antiemetics.
Management in the recovery room
Surgeons should ensure that recovery room staff are well versed in the pathway for patients who are selected for SDD to ensure proactive implementation of analgesic and antiemetic regimens and to fast-track the various tasks and education required for discharge.5
Patients should be started on their home postoperative medication regimen in the recovery room, including an anti-inflammatory such as diclofenac, sublingual tramadol (in preference to an opioid, such as hydrocodone), docusate, and sennosides. IV opioids should be avoided because they can result in somnolence and nausea.
If placed intraoperatively, the Foley catheter should be removed early to allow adequate time to void. Backfilling the bladder prior to removal can hasten the urge to void and help objectively document completeness of evacuation. All patients should be seen by the anesthesiologist and/or surgeon prior to discharge.
For patients who are discharged same day, a follow-up phone call on postoperative day 1 is valuable to ensure that they have continued their successful postoperative transition to the home and to intervene early if there are concerns for patient satisfaction.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Fountain CR et al. Promoting same-day discharge for gynecologic oncology patients in minimally invasive hysterectomy. J Minim Invasive Gynecol. 2017 Sep-Oct;24(6):932-9.
2. Rivard C et al. Factors influencing same-day hospital discharge and risk factors for readmission after robotic surgery in the gynecologic oncology patient population. J Minim Invasive Gynecol. 2015 Feb;22(2):219-26.
3. Lee SJ et al. The feasibility and safety of same-day discharge after robotic-assisted hysterectomy alone or with other procedures for benign and malignant indications. Gynecol Oncol. 2014 Jun;133(3):552-5.
4. Elia N et al. Does multimodal analgesia with acetaminophen, nonsteroidal antiinflammatory drugs, or selective cyclooxygenase-2 inhibitors and patient-controlled analgesia morphine offer advantages over morphine alone? Meta-analyses of randomized trials. Anesthesiology. 2005 Dec;103(6):1296-304.
5. Donnez O et al. Low pain score after total laparoscopic hysterectomy and same-day discharge within less than 5 hours: Results of a prospective observational study. J Minim Invasive Gynecol. 2015 Nov-Dec;22(7):1293-9.
There is an increased focus on reducing the costs of health care delivery, and one major driver of surgical cost is length of hospitalization. A minimally invasive surgical approach to hysterectomy is a strategy that significantly enhances recovery and shortens hospital stay, although many patients who can safely be considered for same-day discharge (SDD), including many with cancer, are still admitted to the hospital overnight. Much has been published on the predictors and pathways for successful same-day discharge after minimally invasive hysterectomy, and in this column we will review how to best predict who is a good candidate for SDD and how to optimize the success of this approach with respect to safety and patient satisfaction.
What are the benefits to SDD?
Certainly, decreased hospitalization costs are an attractive feature of SDD following hysterectomy, although surgeons should also be mindful that patient-centered outcomes, such as pain control, managing nausea, and patient satisfaction, also are considered with equal emphasis. Several studies have shown that, in appropriate candidates and when proactive pathways are used, patient satisfaction is preserved with SDD following hysterectomy.1
Choosing patient candidates
Same day discharge is most successfully accomplished in patients of good general baseline health.2 Diabetic patients, particularly those on insulin, are generally not good candidates for SDD because it is important to monitor and intervene in blood glucose changes that are influenced by a nothing-by-mouth status and surgical stress. We recommend observing patients overnight with a history of pulmonary disease who may have transient increased postoperative O2 needs. Similarly, patients with significant cardiac disease (including heart failure and coronary disease) may benefit from prolonged overnight observation.
Particular caution should be paid to patients with obstructive sleep apnea, which may be occult but anticipated in patients with very high body mass indexes (greater than 40 kg/m2). General anesthetic drugs, the trauma of intubation, and opioids all couple with the underlying airway compromise such that these patients are at risk for postoperative apnea, which, in severe cases, can result in anoxia and death. These patients should be considered for continuous pulse-ox monitoring for at least 12-24 hours postoperatively and are not good candidates for same-day discharge.
Patients who have baseline anticoagulation that has been stopped or bridged preoperatively should have prolonged observation with recheck of their postoperative hemoglobin prior to discharge.
Patients who live alone or are very elderly with baseline frailty are poor candidates for SDD and may benefit from nursing observation overnight while they metabolize their anesthesia. Patients who have chronic opioid dependency present a greater challenge to control postoperative pain; these patients are generally less good candidates for SDD.
Studies have shown that the indication for the procedure (for example, cancer with staging, fibroids, endometriosis) is less critical in determining who is a good candidate for SDD.3 However, successful SDD rates are highest in more straightforward cases with few or no prior surgeries, small uteri (less than 14 weeks), a surgical duration of less than 3 hours, and a surgical start time before 2 p.m. Longer, more complex cases are typically associated with more blood loss, higher risk for occult complications, and more time under anesthesia (and in Trendelenburg), which can exacerbate airway edema. In preparation for such cases, it might be wise to prepare patients for the possibility that they may not be good candidates for discharge on the same day. In general, most SDD pathways exclude patients with very high BMI (greater than 50 kg/m2) because of concern for airway patency and because these cases may be more complex with higher underlying risk. In addition, many of these patients have diabetes and require perioperative metabolic interventions.
Patient preparation
A key component to successful SDD is setting patient expectations. Patients should be informed at their preoperative visit that, unless there is an unexpected occurrence or response to the surgery, they will be discharged to home the same day. This allows them to prepare their home (including transportation needs) in advance. They should be provided with information about what to expect that first night after surgery (including potential residual drowsiness or nausea from anesthesia and immediate postoperative pain).
On the day of surgery, under the influence of anesthesia and pain medication, patients will have difficulty retaining complex discharge instructions. The preoperative visit is critically important because it’s the best time to provide them with this information, including postoperative activity limitations, wound and dressing care, and follow-up instructions. This is also the best time to provide prescriptions for postoperative pain, nausea, and constipation prophylaxis with detailed instructions about best use. Patients should be encouraged to fill these prescriptions preoperatively so that they have these medications on hand on the evening of their discharge.
Many programs utilize a combination of educational strategies (in person, written, video) to maximize the likelihood of retention.1 It is also important to offer an opportunity for patients to ask questions about this information after they have received it (for example, by phoning the patients prior to their procedure).
Preoperative strategies

Intraoperative strategies
Consider in-and-out catheterization rather than placement of an indwelling catheter for anticipated short cases without complex bladder dissection.5 Minimize blood loss and maximally evacuate blood and clots with suction because hemoperitoneum can induce nausea and pain.
Pain from retained gas under the diaphragm can be reduced by bathing the diaphragms with 400 cc of dilute local anesthetic made by mixing 50 mL of 0.5% bupivacaine in 1000 mL normal saline prior to removal of pneumoperitoneum and while still in Trendelenburg. Ensure there is minimal retained intraperitoneal CO2 at the completion of the surgery by asking the anesthesiologists to perform positive pressure ventilations prior to fascial closure. Consider injecting port sites (including the peritoneal and fascial layers) with a mixture of immediate and long-acting local anesthetics. Request that the anesthesia staff administer intraoperative doses of IV ketorolac, acetaminophen, and tramadol (in preference to opioids) and an aggressive perioperative cocktail of antiemetics.
Management in the recovery room
Surgeons should ensure that recovery room staff are well versed in the pathway for patients who are selected for SDD to ensure proactive implementation of analgesic and antiemetic regimens and to fast-track the various tasks and education required for discharge.5
Patients should be started on their home postoperative medication regimen in the recovery room, including an anti-inflammatory such as diclofenac, sublingual tramadol (in preference to an opioid, such as hydrocodone), docusate, and sennosides. IV opioids should be avoided because they can result in somnolence and nausea.
If placed intraoperatively, the Foley catheter should be removed early to allow adequate time to void. Backfilling the bladder prior to removal can hasten the urge to void and help objectively document completeness of evacuation. All patients should be seen by the anesthesiologist and/or surgeon prior to discharge.
For patients who are discharged same day, a follow-up phone call on postoperative day 1 is valuable to ensure that they have continued their successful postoperative transition to the home and to intervene early if there are concerns for patient satisfaction.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Fountain CR et al. Promoting same-day discharge for gynecologic oncology patients in minimally invasive hysterectomy. J Minim Invasive Gynecol. 2017 Sep-Oct;24(6):932-9.
2. Rivard C et al. Factors influencing same-day hospital discharge and risk factors for readmission after robotic surgery in the gynecologic oncology patient population. J Minim Invasive Gynecol. 2015 Feb;22(2):219-26.
3. Lee SJ et al. The feasibility and safety of same-day discharge after robotic-assisted hysterectomy alone or with other procedures for benign and malignant indications. Gynecol Oncol. 2014 Jun;133(3):552-5.
4. Elia N et al. Does multimodal analgesia with acetaminophen, nonsteroidal antiinflammatory drugs, or selective cyclooxygenase-2 inhibitors and patient-controlled analgesia morphine offer advantages over morphine alone? Meta-analyses of randomized trials. Anesthesiology. 2005 Dec;103(6):1296-304.
5. Donnez O et al. Low pain score after total laparoscopic hysterectomy and same-day discharge within less than 5 hours: Results of a prospective observational study. J Minim Invasive Gynecol. 2015 Nov-Dec;22(7):1293-9.
There is an increased focus on reducing the costs of health care delivery, and one major driver of surgical cost is length of hospitalization. A minimally invasive surgical approach to hysterectomy is a strategy that significantly enhances recovery and shortens hospital stay, although many patients who can safely be considered for same-day discharge (SDD), including many with cancer, are still admitted to the hospital overnight. Much has been published on the predictors and pathways for successful same-day discharge after minimally invasive hysterectomy, and in this column we will review how to best predict who is a good candidate for SDD and how to optimize the success of this approach with respect to safety and patient satisfaction.
What are the benefits to SDD?
Certainly, decreased hospitalization costs are an attractive feature of SDD following hysterectomy, although surgeons should also be mindful that patient-centered outcomes, such as pain control, managing nausea, and patient satisfaction, also are considered with equal emphasis. Several studies have shown that, in appropriate candidates and when proactive pathways are used, patient satisfaction is preserved with SDD following hysterectomy.1
Choosing patient candidates
Same day discharge is most successfully accomplished in patients of good general baseline health.2 Diabetic patients, particularly those on insulin, are generally not good candidates for SDD because it is important to monitor and intervene in blood glucose changes that are influenced by a nothing-by-mouth status and surgical stress. We recommend observing patients overnight with a history of pulmonary disease who may have transient increased postoperative O2 needs. Similarly, patients with significant cardiac disease (including heart failure and coronary disease) may benefit from prolonged overnight observation.
Particular caution should be paid to patients with obstructive sleep apnea, which may be occult but anticipated in patients with very high body mass indexes (greater than 40 kg/m2). General anesthetic drugs, the trauma of intubation, and opioids all couple with the underlying airway compromise such that these patients are at risk for postoperative apnea, which, in severe cases, can result in anoxia and death. These patients should be considered for continuous pulse-ox monitoring for at least 12-24 hours postoperatively and are not good candidates for same-day discharge.
Patients who have baseline anticoagulation that has been stopped or bridged preoperatively should have prolonged observation with recheck of their postoperative hemoglobin prior to discharge.
Patients who live alone or are very elderly with baseline frailty are poor candidates for SDD and may benefit from nursing observation overnight while they metabolize their anesthesia. Patients who have chronic opioid dependency present a greater challenge to control postoperative pain; these patients are generally less good candidates for SDD.
Studies have shown that the indication for the procedure (for example, cancer with staging, fibroids, endometriosis) is less critical in determining who is a good candidate for SDD.3 However, successful SDD rates are highest in more straightforward cases with few or no prior surgeries, small uteri (less than 14 weeks), a surgical duration of less than 3 hours, and a surgical start time before 2 p.m. Longer, more complex cases are typically associated with more blood loss, higher risk for occult complications, and more time under anesthesia (and in Trendelenburg), which can exacerbate airway edema. In preparation for such cases, it might be wise to prepare patients for the possibility that they may not be good candidates for discharge on the same day. In general, most SDD pathways exclude patients with very high BMI (greater than 50 kg/m2) because of concern for airway patency and because these cases may be more complex with higher underlying risk. In addition, many of these patients have diabetes and require perioperative metabolic interventions.
Patient preparation
A key component to successful SDD is setting patient expectations. Patients should be informed at their preoperative visit that, unless there is an unexpected occurrence or response to the surgery, they will be discharged to home the same day. This allows them to prepare their home (including transportation needs) in advance. They should be provided with information about what to expect that first night after surgery (including potential residual drowsiness or nausea from anesthesia and immediate postoperative pain).
On the day of surgery, under the influence of anesthesia and pain medication, patients will have difficulty retaining complex discharge instructions. The preoperative visit is critically important because it’s the best time to provide them with this information, including postoperative activity limitations, wound and dressing care, and follow-up instructions. This is also the best time to provide prescriptions for postoperative pain, nausea, and constipation prophylaxis with detailed instructions about best use. Patients should be encouraged to fill these prescriptions preoperatively so that they have these medications on hand on the evening of their discharge.
Many programs utilize a combination of educational strategies (in person, written, video) to maximize the likelihood of retention.1 It is also important to offer an opportunity for patients to ask questions about this information after they have received it (for example, by phoning the patients prior to their procedure).
Preoperative strategies

Intraoperative strategies
Consider in-and-out catheterization rather than placement of an indwelling catheter for anticipated short cases without complex bladder dissection.5 Minimize blood loss and maximally evacuate blood and clots with suction because hemoperitoneum can induce nausea and pain.
Pain from retained gas under the diaphragm can be reduced by bathing the diaphragms with 400 cc of dilute local anesthetic made by mixing 50 mL of 0.5% bupivacaine in 1000 mL normal saline prior to removal of pneumoperitoneum and while still in Trendelenburg. Ensure there is minimal retained intraperitoneal CO2 at the completion of the surgery by asking the anesthesiologists to perform positive pressure ventilations prior to fascial closure. Consider injecting port sites (including the peritoneal and fascial layers) with a mixture of immediate and long-acting local anesthetics. Request that the anesthesia staff administer intraoperative doses of IV ketorolac, acetaminophen, and tramadol (in preference to opioids) and an aggressive perioperative cocktail of antiemetics.
Management in the recovery room
Surgeons should ensure that recovery room staff are well versed in the pathway for patients who are selected for SDD to ensure proactive implementation of analgesic and antiemetic regimens and to fast-track the various tasks and education required for discharge.5
Patients should be started on their home postoperative medication regimen in the recovery room, including an anti-inflammatory such as diclofenac, sublingual tramadol (in preference to an opioid, such as hydrocodone), docusate, and sennosides. IV opioids should be avoided because they can result in somnolence and nausea.
If placed intraoperatively, the Foley catheter should be removed early to allow adequate time to void. Backfilling the bladder prior to removal can hasten the urge to void and help objectively document completeness of evacuation. All patients should be seen by the anesthesiologist and/or surgeon prior to discharge.
For patients who are discharged same day, a follow-up phone call on postoperative day 1 is valuable to ensure that they have continued their successful postoperative transition to the home and to intervene early if there are concerns for patient satisfaction.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Fountain CR et al. Promoting same-day discharge for gynecologic oncology patients in minimally invasive hysterectomy. J Minim Invasive Gynecol. 2017 Sep-Oct;24(6):932-9.
2. Rivard C et al. Factors influencing same-day hospital discharge and risk factors for readmission after robotic surgery in the gynecologic oncology patient population. J Minim Invasive Gynecol. 2015 Feb;22(2):219-26.
3. Lee SJ et al. The feasibility and safety of same-day discharge after robotic-assisted hysterectomy alone or with other procedures for benign and malignant indications. Gynecol Oncol. 2014 Jun;133(3):552-5.
4. Elia N et al. Does multimodal analgesia with acetaminophen, nonsteroidal antiinflammatory drugs, or selective cyclooxygenase-2 inhibitors and patient-controlled analgesia morphine offer advantages over morphine alone? Meta-analyses of randomized trials. Anesthesiology. 2005 Dec;103(6):1296-304.
5. Donnez O et al. Low pain score after total laparoscopic hysterectomy and same-day discharge within less than 5 hours: Results of a prospective observational study. J Minim Invasive Gynecol. 2015 Nov-Dec;22(7):1293-9.
Tardive dyskinesia is theme of awards competition for early career psychiatrists
Important advances in neuroscience and clinical psychiatry have been achieved in recent years, but there are significant gaps in knowledge and much that we don’t understand about the brain and behavior. Further advances depend on cultivating and supporting a new generation of dedicated basic science and clinical investigators. While there is a compelling need to attract, recruit, and encourage talented individuals to pursue scholarly interests, competing life and career demands often prove daunting.
The theme of the competition this year concerning tardive dyskinesia is timely and consistent with the mission of NMSIS to promote knowledge on neurologic side effects of antipsychotic drugs. Tardive dyskinesia can have a negative impact on the social, psychological, and physical well-being of patients; it remains a legacy of past treatment with antipsychotics; it is an increasing concern among an ever widening population of patients receiving even newer antipsychotics; and there are now two Food and Drug Administration–approved treatments for the disorder. Early career psychiatrists may have had limited instruction on tardive dyskinesia, which has not received prominent attention in curricular programs in recent years. Thus, in addition to supporting scholarly work and research experience, the 2018 Promising Scholars Award Program aims to promote knowledge and skills in managing patients with tardive dyskinesia.
Specific learning objectives are:
- Participants will learn the steps necessary to prepare a scientific manuscript for publication.
- Participants will review comments by expert referees and learn to incorporate and respond to the peer review process.
- Participants will review the evidence related to the diagnosis and treatment of tardive dyskinesia.
- Participants will be introduced to the spectrum of educational and networking opportunities at the Institute for Psychiatric Services conference.
In the past, this program was very popular and gained national recognition among psychiatric trainees. Numerous submitted papers were accepted for publication in peer-reviewed journals after the competition was completed.
Instructions for manuscript preparation are:
- First author must be a student, resident, or fellow.
- Papers should address specific issues related to the theme of tardive dyskinesia and be no longer than 15 double-spaced typed pages in length (excluding references and illustrations).
- Literature reviews, case reports, or studies that are original and newly developed or recently published are acceptable.
- Reviews and feedback will be provided by a panel of academic psychiatrists.
- Papers will be judged on relevance to tardive dyskinesia, originality, scholarship, scientific rigor, valid methodology, clinical significance, and organization.
To participate, papers and curriculum vitae of the first author must be submitted by July 1, 2018, to Dianne Daugherty by email at [email protected]. Winners will be announced by Aug. 10, 2018. For additional information, write to [email protected] or visit www.mhaus.org/nmsis/about-us/what-is-nmsis.
Dr. Caroff, professor of psychiatry, Corporal Michael J. Crescenz VA Medical Center and at the University of Pennsylvania, both in Philadelphia, is director of the NMSIS. He served as consultant to Neurocrine Biosciences and Teva Pharmaceutical Industries, and receives research grant funding from Neurocrine Biosciences.
Important advances in neuroscience and clinical psychiatry have been achieved in recent years, but there are significant gaps in knowledge and much that we don’t understand about the brain and behavior. Further advances depend on cultivating and supporting a new generation of dedicated basic science and clinical investigators. While there is a compelling need to attract, recruit, and encourage talented individuals to pursue scholarly interests, competing life and career demands often prove daunting.
The theme of the competition this year concerning tardive dyskinesia is timely and consistent with the mission of NMSIS to promote knowledge on neurologic side effects of antipsychotic drugs. Tardive dyskinesia can have a negative impact on the social, psychological, and physical well-being of patients; it remains a legacy of past treatment with antipsychotics; it is an increasing concern among an ever widening population of patients receiving even newer antipsychotics; and there are now two Food and Drug Administration–approved treatments for the disorder. Early career psychiatrists may have had limited instruction on tardive dyskinesia, which has not received prominent attention in curricular programs in recent years. Thus, in addition to supporting scholarly work and research experience, the 2018 Promising Scholars Award Program aims to promote knowledge and skills in managing patients with tardive dyskinesia.
Specific learning objectives are:
- Participants will learn the steps necessary to prepare a scientific manuscript for publication.
- Participants will review comments by expert referees and learn to incorporate and respond to the peer review process.
- Participants will review the evidence related to the diagnosis and treatment of tardive dyskinesia.
- Participants will be introduced to the spectrum of educational and networking opportunities at the Institute for Psychiatric Services conference.
In the past, this program was very popular and gained national recognition among psychiatric trainees. Numerous submitted papers were accepted for publication in peer-reviewed journals after the competition was completed.
Instructions for manuscript preparation are:
- First author must be a student, resident, or fellow.
- Papers should address specific issues related to the theme of tardive dyskinesia and be no longer than 15 double-spaced typed pages in length (excluding references and illustrations).
- Literature reviews, case reports, or studies that are original and newly developed or recently published are acceptable.
- Reviews and feedback will be provided by a panel of academic psychiatrists.
- Papers will be judged on relevance to tardive dyskinesia, originality, scholarship, scientific rigor, valid methodology, clinical significance, and organization.
To participate, papers and curriculum vitae of the first author must be submitted by July 1, 2018, to Dianne Daugherty by email at [email protected]. Winners will be announced by Aug. 10, 2018. For additional information, write to [email protected] or visit www.mhaus.org/nmsis/about-us/what-is-nmsis.
Dr. Caroff, professor of psychiatry, Corporal Michael J. Crescenz VA Medical Center and at the University of Pennsylvania, both in Philadelphia, is director of the NMSIS. He served as consultant to Neurocrine Biosciences and Teva Pharmaceutical Industries, and receives research grant funding from Neurocrine Biosciences.
Important advances in neuroscience and clinical psychiatry have been achieved in recent years, but there are significant gaps in knowledge and much that we don’t understand about the brain and behavior. Further advances depend on cultivating and supporting a new generation of dedicated basic science and clinical investigators. While there is a compelling need to attract, recruit, and encourage talented individuals to pursue scholarly interests, competing life and career demands often prove daunting.
The theme of the competition this year concerning tardive dyskinesia is timely and consistent with the mission of NMSIS to promote knowledge on neurologic side effects of antipsychotic drugs. Tardive dyskinesia can have a negative impact on the social, psychological, and physical well-being of patients; it remains a legacy of past treatment with antipsychotics; it is an increasing concern among an ever widening population of patients receiving even newer antipsychotics; and there are now two Food and Drug Administration–approved treatments for the disorder. Early career psychiatrists may have had limited instruction on tardive dyskinesia, which has not received prominent attention in curricular programs in recent years. Thus, in addition to supporting scholarly work and research experience, the 2018 Promising Scholars Award Program aims to promote knowledge and skills in managing patients with tardive dyskinesia.
Specific learning objectives are:
- Participants will learn the steps necessary to prepare a scientific manuscript for publication.
- Participants will review comments by expert referees and learn to incorporate and respond to the peer review process.
- Participants will review the evidence related to the diagnosis and treatment of tardive dyskinesia.
- Participants will be introduced to the spectrum of educational and networking opportunities at the Institute for Psychiatric Services conference.
In the past, this program was very popular and gained national recognition among psychiatric trainees. Numerous submitted papers were accepted for publication in peer-reviewed journals after the competition was completed.
Instructions for manuscript preparation are:
- First author must be a student, resident, or fellow.
- Papers should address specific issues related to the theme of tardive dyskinesia and be no longer than 15 double-spaced typed pages in length (excluding references and illustrations).
- Literature reviews, case reports, or studies that are original and newly developed or recently published are acceptable.
- Reviews and feedback will be provided by a panel of academic psychiatrists.
- Papers will be judged on relevance to tardive dyskinesia, originality, scholarship, scientific rigor, valid methodology, clinical significance, and organization.
To participate, papers and curriculum vitae of the first author must be submitted by July 1, 2018, to Dianne Daugherty by email at [email protected]. Winners will be announced by Aug. 10, 2018. For additional information, write to [email protected] or visit www.mhaus.org/nmsis/about-us/what-is-nmsis.
Dr. Caroff, professor of psychiatry, Corporal Michael J. Crescenz VA Medical Center and at the University of Pennsylvania, both in Philadelphia, is director of the NMSIS. He served as consultant to Neurocrine Biosciences and Teva Pharmaceutical Industries, and receives research grant funding from Neurocrine Biosciences.