User login
'Energy Insecurity' Tied to Anxiety, Depression Risk
'Energy Insecurity' Tied to Anxiety, Depression Risk
TOPLINE:
Energy insecurity, the inability to meet household energy needs, was associated with more than twice the odds of having depression and anxiety symptoms than energy security in US adults, a new cross-sectional study showed.
METHODOLOGY:
- Using data from the US Census Bureau's online Household Pulse Survey, administered between 2022 and 2024, researchers conducted a cross-sectional study with a weighted population of > 187 million US adults (51% women; 64% White, 16% Hispanic, 10% Black, and 5% Asian). About a quarter of the population was in each of 4 age groups: 18-34 years, 35-49 years, 50-64 years, and ≥ 65 years.
- Three indicators of energy insecurity—inability to pay energy bills, maintaining unsafe/unhealthy home temperatures, and forgoing expenses on basic necessities to pay energy bills—were assessed individually and as a composite measure.
- Mental health was assessed using modified versions of the 2-item Patient Health Questionnaire for depression and the 2-item Generalized Anxiety Disorder scale for anxiety.
- The analysis was adjusted for other social determinants of health, including unemployment, housing instability, and food insecurity. Covariates included a wide range of factors, such as age, educational level, sex, and annual household income.
TAKEAWAY:
- In all, > 43% of the population reported having ≥ 1 form of energy security; around 22% reported being unable to pay energy bills, 22% maintained unsafe home temperatures, and nearly 34% forewent spending on basic necessities to pay energy bills.
- Individuals who gave up spending on basic necessities to pay energy bills had higher odds of anxiety (adjusted odds ratio [aOR], 1.79) and depression (aOR, 1.74) than those who did not.
- Adults with energy insecurity on the composite measure had higher odds for anxiety (aOR, 2.29) and depression (aOR, 2.31) than those with energy security.
- Food insecurity was also associated with poorer mental health, with higher odds for symptoms of depression (aOR, 2.05) and anxiety (aOR, 2.07).
IN PRACTICE:
"Despite its high prevalence, energy insecurity remains underrecognized in public health and policy intervention strategies," the investigators wrote.
"These findings suggest that energy insecurity is a widespread and important factor associated with mental health symptoms and may warrant consideration in efforts to reduce adverse mental health outcomes," they added.
SOURCE:
This study was led by Michelle Graf, PhD, Carter School of Public Policy, Georgia Institute of Technology, Atlanta. It was published online on October 27 in JAMA Network Open.
LIMITATIONS:
The cross-sectional nature of the data limited causal interference and increased the possibility of reverse causality. The questionnaire captured subjective interpretations of unsafe and unhealthy indoor temperatures, which may have varied among respondents. Additionally, the recall periods for energy insecurity and mental health outcomes were different.
DISCLOSURES:
The investigators reported no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Energy insecurity, the inability to meet household energy needs, was associated with more than twice the odds of having depression and anxiety symptoms than energy security in US adults, a new cross-sectional study showed.
METHODOLOGY:
- Using data from the US Census Bureau's online Household Pulse Survey, administered between 2022 and 2024, researchers conducted a cross-sectional study with a weighted population of > 187 million US adults (51% women; 64% White, 16% Hispanic, 10% Black, and 5% Asian). About a quarter of the population was in each of 4 age groups: 18-34 years, 35-49 years, 50-64 years, and ≥ 65 years.
- Three indicators of energy insecurity—inability to pay energy bills, maintaining unsafe/unhealthy home temperatures, and forgoing expenses on basic necessities to pay energy bills—were assessed individually and as a composite measure.
- Mental health was assessed using modified versions of the 2-item Patient Health Questionnaire for depression and the 2-item Generalized Anxiety Disorder scale for anxiety.
- The analysis was adjusted for other social determinants of health, including unemployment, housing instability, and food insecurity. Covariates included a wide range of factors, such as age, educational level, sex, and annual household income.
TAKEAWAY:
- In all, > 43% of the population reported having ≥ 1 form of energy security; around 22% reported being unable to pay energy bills, 22% maintained unsafe home temperatures, and nearly 34% forewent spending on basic necessities to pay energy bills.
- Individuals who gave up spending on basic necessities to pay energy bills had higher odds of anxiety (adjusted odds ratio [aOR], 1.79) and depression (aOR, 1.74) than those who did not.
- Adults with energy insecurity on the composite measure had higher odds for anxiety (aOR, 2.29) and depression (aOR, 2.31) than those with energy security.
- Food insecurity was also associated with poorer mental health, with higher odds for symptoms of depression (aOR, 2.05) and anxiety (aOR, 2.07).
IN PRACTICE:
"Despite its high prevalence, energy insecurity remains underrecognized in public health and policy intervention strategies," the investigators wrote.
"These findings suggest that energy insecurity is a widespread and important factor associated with mental health symptoms and may warrant consideration in efforts to reduce adverse mental health outcomes," they added.
SOURCE:
This study was led by Michelle Graf, PhD, Carter School of Public Policy, Georgia Institute of Technology, Atlanta. It was published online on October 27 in JAMA Network Open.
LIMITATIONS:
The cross-sectional nature of the data limited causal interference and increased the possibility of reverse causality. The questionnaire captured subjective interpretations of unsafe and unhealthy indoor temperatures, which may have varied among respondents. Additionally, the recall periods for energy insecurity and mental health outcomes were different.
DISCLOSURES:
The investigators reported no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Energy insecurity, the inability to meet household energy needs, was associated with more than twice the odds of having depression and anxiety symptoms than energy security in US adults, a new cross-sectional study showed.
METHODOLOGY:
- Using data from the US Census Bureau's online Household Pulse Survey, administered between 2022 and 2024, researchers conducted a cross-sectional study with a weighted population of > 187 million US adults (51% women; 64% White, 16% Hispanic, 10% Black, and 5% Asian). About a quarter of the population was in each of 4 age groups: 18-34 years, 35-49 years, 50-64 years, and ≥ 65 years.
- Three indicators of energy insecurity—inability to pay energy bills, maintaining unsafe/unhealthy home temperatures, and forgoing expenses on basic necessities to pay energy bills—were assessed individually and as a composite measure.
- Mental health was assessed using modified versions of the 2-item Patient Health Questionnaire for depression and the 2-item Generalized Anxiety Disorder scale for anxiety.
- The analysis was adjusted for other social determinants of health, including unemployment, housing instability, and food insecurity. Covariates included a wide range of factors, such as age, educational level, sex, and annual household income.
TAKEAWAY:
- In all, > 43% of the population reported having ≥ 1 form of energy security; around 22% reported being unable to pay energy bills, 22% maintained unsafe home temperatures, and nearly 34% forewent spending on basic necessities to pay energy bills.
- Individuals who gave up spending on basic necessities to pay energy bills had higher odds of anxiety (adjusted odds ratio [aOR], 1.79) and depression (aOR, 1.74) than those who did not.
- Adults with energy insecurity on the composite measure had higher odds for anxiety (aOR, 2.29) and depression (aOR, 2.31) than those with energy security.
- Food insecurity was also associated with poorer mental health, with higher odds for symptoms of depression (aOR, 2.05) and anxiety (aOR, 2.07).
IN PRACTICE:
"Despite its high prevalence, energy insecurity remains underrecognized in public health and policy intervention strategies," the investigators wrote.
"These findings suggest that energy insecurity is a widespread and important factor associated with mental health symptoms and may warrant consideration in efforts to reduce adverse mental health outcomes," they added.
SOURCE:
This study was led by Michelle Graf, PhD, Carter School of Public Policy, Georgia Institute of Technology, Atlanta. It was published online on October 27 in JAMA Network Open.
LIMITATIONS:
The cross-sectional nature of the data limited causal interference and increased the possibility of reverse causality. The questionnaire captured subjective interpretations of unsafe and unhealthy indoor temperatures, which may have varied among respondents. Additionally, the recall periods for energy insecurity and mental health outcomes were different.
DISCLOSURES:
The investigators reported no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
'Energy Insecurity' Tied to Anxiety, Depression Risk
'Energy Insecurity' Tied to Anxiety, Depression Risk
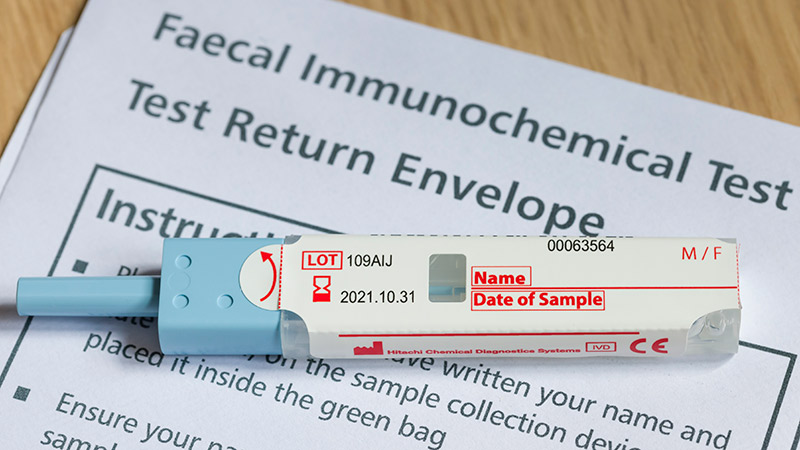
Patients With a Positive FIT Fail to Get Follow-Up Colonoscopies
Patients With a Positive FIT Fail to Get Follow-Up Colonoscopies
PHOENIX -- Patients with or without polyp removal in an index colonoscopy commonly receive follow-up surveillance with a fecal immunochemical test (FIT), yet many of these patients do not receive a recommended colonoscopy after a positive FIT.
"In this large US study, we found interval FITs are frequently performed in patients with and without prior polypectomy," said first author Natalie J. Wilson, MD, of the University of Minnesota in Minneapolis, while presenting the findings this week at the American College of Gastroenterology (ACG) 2025 Annual Scientific Meeting.
"These findings reinforce the importance of colonoscopy following positive interval FIT, given the high risk of advanced neoplasia and colorectal cancer, regardless of polypectomy history," Wilson said.
Guideline recommendations stress the need for follow-up surveillance with a colonoscopy, particularly in patients who have had a prior polypectomy, due to the higher risk.
Reasons patients may instead turn to FIT include cost or other factors.
To determine just how often that happens, how having a previous polypectomy affects FIT results, and how adherent patients are to follow up if a FIT result is positive, Wilson and her colleagues evaluated data from nearly 4.8 million individuals in the Veterans Health Administration Corporate Data Warehouse who underwent colonoscopy between 2000 and 2004.
Of the patients, 10.9% were found to have subsequently received interval FIT within 10 years of the index colonoscopy, and of those patients, nearly half (49.9%) had received a polypectomy at the index colonoscopy.
The average time from the colonoscopy/polypectomy to the interval FIT was 5.9 years (5.6 years in the polypectomy group vs 6.2 years in the nonpolypectomy group).
Among the FIT screenings, results were positive in 17.2% of postpolypectomy patients and 14.1% of patients who no prior polypectomy, indicating a history of polypectomy to be predictive of positive interval FIT (odds ratio [OR], 1.12; P < .0001).
Notably, while a follow-up colonoscopy is considered essential following a positive FIT result -- and having a previous polypectomy should add further emergency to the matter -- the study showed only 50.4% of those who had an earlier polypectomy went on to receive the recommended follow-up colonoscopy after a positive follow-up FIT, and the rate was 49.3% among those who had not received a polypectomy (P = .001).
For those who did receive a follow-up colonoscopy after a positive FIT, the duration of time to receiving the colonoscopy was longer among those who had a prior polypectomy, at 2.9 months compared with 2.5 months in the nonpolypectomy group (P < .001).
Colonoscopy results following a positive FIT showed higher rates of detections among patients who had prior polypectomies than among those with no prior polypectomy, including tubular adenomas (54.7% vs 45.8%), tubulovillous adenomas (5.6% vs 4.7%), adenomas with high-grade dysplasia (0.8% vs 0.7%), sessile serrated lesions (3.52% vs 2.4%), advanced colorectal neoplasia (9.2% vs 7.9%), and colorectal cancer (3.3% vs 3.0%).
However, a prior polypectomy was not independently predictive of colorectal cancer (OR, 0.96; P = .65) or advanced colorectal neoplasia (OR, 0.97; P = .57) in the postcolonoscopy interval FIT.
The findings underscore that "positive results carried a high risk of advanced neoplasia or cancer, irrespective or prior polypectomy history," Wilson said.
Commenting on the study, William D. Chey, MD, chief of the Division of Gastroenterology & Hepatology at the University of Michigan in Ann Arbor, Michigan, noted that the study "addresses one of the biggest challenges we face as a profession, which is making sure that patients who have a positive stool test get a colonoscopy."
He noted that the low rate of just 50% of recipients of positive FITs going on to receive a colonoscopy is consistent with what is observed in other trials.
"Other data suggest that the rate might even be significantly higher -- at 70% to 80%, depending upon the population and the test," Chey told Medscape Medical News.
Reasons for the failure to receive the follow-up testing range from income restrictions (due to the high cost of a colonoscopy, especially if not covered by insurance), education, speaking a foreign language, and other factors, he said.
The relatively high rates of colon cancers detected by FIT in the study, in those with and without a prior polypectomy, along with findings from other studies "should raise questions about whether there might be a role for FIT testing in addition to colonoscopy." However, much stronger evidence would be needed, Chey noted.
In the meantime, a key issue is "how do we do a better job of making sure that individuals who have a positive FIT test get a colonoscopy," he said.
"I think a lot of this is going to come down to how it's down at the primary care level."
Chey added that in that, and any other setting, "the main message that needs to get out to people who are undergoing stool-based screening is that the stool test is only the first part of the screening process, and if it's positive, a follow-up colonoscopy must be performed.
"Otherwise, the stool-based test is of no value."
Wilson had no disclosures to report. Chey's disclosures include consulting and/or other relationships with Ardelyx, Atmo, Biomerica, Commonwealth Diagnostics International, Corprata, Dieta, Evinature, Food Marble, Gemelli, Kiwi BioScience, Modify Health, Nestle, Phathom, Redhill, Salix/Valean, Takeda, and Vibrant.
A version of this article first appeared on Medscape.com.
PHOENIX -- Patients with or without polyp removal in an index colonoscopy commonly receive follow-up surveillance with a fecal immunochemical test (FIT), yet many of these patients do not receive a recommended colonoscopy after a positive FIT.
"In this large US study, we found interval FITs are frequently performed in patients with and without prior polypectomy," said first author Natalie J. Wilson, MD, of the University of Minnesota in Minneapolis, while presenting the findings this week at the American College of Gastroenterology (ACG) 2025 Annual Scientific Meeting.
"These findings reinforce the importance of colonoscopy following positive interval FIT, given the high risk of advanced neoplasia and colorectal cancer, regardless of polypectomy history," Wilson said.
Guideline recommendations stress the need for follow-up surveillance with a colonoscopy, particularly in patients who have had a prior polypectomy, due to the higher risk.
Reasons patients may instead turn to FIT include cost or other factors.
To determine just how often that happens, how having a previous polypectomy affects FIT results, and how adherent patients are to follow up if a FIT result is positive, Wilson and her colleagues evaluated data from nearly 4.8 million individuals in the Veterans Health Administration Corporate Data Warehouse who underwent colonoscopy between 2000 and 2004.
Of the patients, 10.9% were found to have subsequently received interval FIT within 10 years of the index colonoscopy, and of those patients, nearly half (49.9%) had received a polypectomy at the index colonoscopy.
The average time from the colonoscopy/polypectomy to the interval FIT was 5.9 years (5.6 years in the polypectomy group vs 6.2 years in the nonpolypectomy group).
Among the FIT screenings, results were positive in 17.2% of postpolypectomy patients and 14.1% of patients who no prior polypectomy, indicating a history of polypectomy to be predictive of positive interval FIT (odds ratio [OR], 1.12; P < .0001).
Notably, while a follow-up colonoscopy is considered essential following a positive FIT result -- and having a previous polypectomy should add further emergency to the matter -- the study showed only 50.4% of those who had an earlier polypectomy went on to receive the recommended follow-up colonoscopy after a positive follow-up FIT, and the rate was 49.3% among those who had not received a polypectomy (P = .001).
For those who did receive a follow-up colonoscopy after a positive FIT, the duration of time to receiving the colonoscopy was longer among those who had a prior polypectomy, at 2.9 months compared with 2.5 months in the nonpolypectomy group (P < .001).
Colonoscopy results following a positive FIT showed higher rates of detections among patients who had prior polypectomies than among those with no prior polypectomy, including tubular adenomas (54.7% vs 45.8%), tubulovillous adenomas (5.6% vs 4.7%), adenomas with high-grade dysplasia (0.8% vs 0.7%), sessile serrated lesions (3.52% vs 2.4%), advanced colorectal neoplasia (9.2% vs 7.9%), and colorectal cancer (3.3% vs 3.0%).
However, a prior polypectomy was not independently predictive of colorectal cancer (OR, 0.96; P = .65) or advanced colorectal neoplasia (OR, 0.97; P = .57) in the postcolonoscopy interval FIT.
The findings underscore that "positive results carried a high risk of advanced neoplasia or cancer, irrespective or prior polypectomy history," Wilson said.
Commenting on the study, William D. Chey, MD, chief of the Division of Gastroenterology & Hepatology at the University of Michigan in Ann Arbor, Michigan, noted that the study "addresses one of the biggest challenges we face as a profession, which is making sure that patients who have a positive stool test get a colonoscopy."
He noted that the low rate of just 50% of recipients of positive FITs going on to receive a colonoscopy is consistent with what is observed in other trials.
"Other data suggest that the rate might even be significantly higher -- at 70% to 80%, depending upon the population and the test," Chey told Medscape Medical News.
Reasons for the failure to receive the follow-up testing range from income restrictions (due to the high cost of a colonoscopy, especially if not covered by insurance), education, speaking a foreign language, and other factors, he said.
The relatively high rates of colon cancers detected by FIT in the study, in those with and without a prior polypectomy, along with findings from other studies "should raise questions about whether there might be a role for FIT testing in addition to colonoscopy." However, much stronger evidence would be needed, Chey noted.
In the meantime, a key issue is "how do we do a better job of making sure that individuals who have a positive FIT test get a colonoscopy," he said.
"I think a lot of this is going to come down to how it's down at the primary care level."
Chey added that in that, and any other setting, "the main message that needs to get out to people who are undergoing stool-based screening is that the stool test is only the first part of the screening process, and if it's positive, a follow-up colonoscopy must be performed.
"Otherwise, the stool-based test is of no value."
Wilson had no disclosures to report. Chey's disclosures include consulting and/or other relationships with Ardelyx, Atmo, Biomerica, Commonwealth Diagnostics International, Corprata, Dieta, Evinature, Food Marble, Gemelli, Kiwi BioScience, Modify Health, Nestle, Phathom, Redhill, Salix/Valean, Takeda, and Vibrant.
A version of this article first appeared on Medscape.com.
PHOENIX -- Patients with or without polyp removal in an index colonoscopy commonly receive follow-up surveillance with a fecal immunochemical test (FIT), yet many of these patients do not receive a recommended colonoscopy after a positive FIT.
"In this large US study, we found interval FITs are frequently performed in patients with and without prior polypectomy," said first author Natalie J. Wilson, MD, of the University of Minnesota in Minneapolis, while presenting the findings this week at the American College of Gastroenterology (ACG) 2025 Annual Scientific Meeting.
"These findings reinforce the importance of colonoscopy following positive interval FIT, given the high risk of advanced neoplasia and colorectal cancer, regardless of polypectomy history," Wilson said.
Guideline recommendations stress the need for follow-up surveillance with a colonoscopy, particularly in patients who have had a prior polypectomy, due to the higher risk.
Reasons patients may instead turn to FIT include cost or other factors.
To determine just how often that happens, how having a previous polypectomy affects FIT results, and how adherent patients are to follow up if a FIT result is positive, Wilson and her colleagues evaluated data from nearly 4.8 million individuals in the Veterans Health Administration Corporate Data Warehouse who underwent colonoscopy between 2000 and 2004.
Of the patients, 10.9% were found to have subsequently received interval FIT within 10 years of the index colonoscopy, and of those patients, nearly half (49.9%) had received a polypectomy at the index colonoscopy.
The average time from the colonoscopy/polypectomy to the interval FIT was 5.9 years (5.6 years in the polypectomy group vs 6.2 years in the nonpolypectomy group).
Among the FIT screenings, results were positive in 17.2% of postpolypectomy patients and 14.1% of patients who no prior polypectomy, indicating a history of polypectomy to be predictive of positive interval FIT (odds ratio [OR], 1.12; P < .0001).
Notably, while a follow-up colonoscopy is considered essential following a positive FIT result -- and having a previous polypectomy should add further emergency to the matter -- the study showed only 50.4% of those who had an earlier polypectomy went on to receive the recommended follow-up colonoscopy after a positive follow-up FIT, and the rate was 49.3% among those who had not received a polypectomy (P = .001).
For those who did receive a follow-up colonoscopy after a positive FIT, the duration of time to receiving the colonoscopy was longer among those who had a prior polypectomy, at 2.9 months compared with 2.5 months in the nonpolypectomy group (P < .001).
Colonoscopy results following a positive FIT showed higher rates of detections among patients who had prior polypectomies than among those with no prior polypectomy, including tubular adenomas (54.7% vs 45.8%), tubulovillous adenomas (5.6% vs 4.7%), adenomas with high-grade dysplasia (0.8% vs 0.7%), sessile serrated lesions (3.52% vs 2.4%), advanced colorectal neoplasia (9.2% vs 7.9%), and colorectal cancer (3.3% vs 3.0%).
However, a prior polypectomy was not independently predictive of colorectal cancer (OR, 0.96; P = .65) or advanced colorectal neoplasia (OR, 0.97; P = .57) in the postcolonoscopy interval FIT.
The findings underscore that "positive results carried a high risk of advanced neoplasia or cancer, irrespective or prior polypectomy history," Wilson said.
Commenting on the study, William D. Chey, MD, chief of the Division of Gastroenterology & Hepatology at the University of Michigan in Ann Arbor, Michigan, noted that the study "addresses one of the biggest challenges we face as a profession, which is making sure that patients who have a positive stool test get a colonoscopy."
He noted that the low rate of just 50% of recipients of positive FITs going on to receive a colonoscopy is consistent with what is observed in other trials.
"Other data suggest that the rate might even be significantly higher -- at 70% to 80%, depending upon the population and the test," Chey told Medscape Medical News.
Reasons for the failure to receive the follow-up testing range from income restrictions (due to the high cost of a colonoscopy, especially if not covered by insurance), education, speaking a foreign language, and other factors, he said.
The relatively high rates of colon cancers detected by FIT in the study, in those with and without a prior polypectomy, along with findings from other studies "should raise questions about whether there might be a role for FIT testing in addition to colonoscopy." However, much stronger evidence would be needed, Chey noted.
In the meantime, a key issue is "how do we do a better job of making sure that individuals who have a positive FIT test get a colonoscopy," he said.
"I think a lot of this is going to come down to how it's down at the primary care level."
Chey added that in that, and any other setting, "the main message that needs to get out to people who are undergoing stool-based screening is that the stool test is only the first part of the screening process, and if it's positive, a follow-up colonoscopy must be performed.
"Otherwise, the stool-based test is of no value."
Wilson had no disclosures to report. Chey's disclosures include consulting and/or other relationships with Ardelyx, Atmo, Biomerica, Commonwealth Diagnostics International, Corprata, Dieta, Evinature, Food Marble, Gemelli, Kiwi BioScience, Modify Health, Nestle, Phathom, Redhill, Salix/Valean, Takeda, and Vibrant.
A version of this article first appeared on Medscape.com.
Patients With a Positive FIT Fail to Get Follow-Up Colonoscopies
Patients With a Positive FIT Fail to Get Follow-Up Colonoscopies
What Drives Lung Cancer in Nonsmokers?
TOPLINE:
A comprehensive review of 92 studies found that 15% to 20% of lung cancers occurred among nonsmokers and were associated with environmental and germline risk factors. These cancers frequently harbored actionable genomic drivers, and targeted EGFR and ALK therapies produced significant diseasefree survival (DFS) and overall survival benefits.
METHODOLOGY:
- Lung cancer continues to be the leading cause of cancer death worldwide, causing about 1.8 million deaths in 2022, with smoking remaining the predominant risk factor. However, the incidence of lung cancer among nonsmokers (those who have smoked less than 100 cigarettes in their lifetime) is rising, varies by sex and geography, and is linked to environmental exposures and family history. The misperception that lung cancer is almost invariably caused by smoking may delay assessment and diagnosis.
- Researchers conducted a review of 92 studies on lung cancer in nonsmokers: 6 meta-analyses or systematic reviews, 16 randomized clinical trials, eight prospective cohort studies, seven retrospective cohort studies, three cross-sectional studies, four observational or case-control studies, 13 genomic studies, and 35 other studies.
- Overall, lung cancer among nonsmokers accounted for 15% to 20% of all lung cancer cases. Most lung cancers in nonsmokers were adenocarcinomas (60% to 80%), with a median age at diagnosis of 67 years in this group compared with 70 years in people with a history of smoking.
- Data analysis from three US hospital networks showed that the proportion of lung cancer among nonsmokers increased from 8.0% to 14.9% between 1990 and 2013. A pooled analysis of seven Finnish cohorts reported an absolute increase in lung cancer among nonsmokers from 6.9 per 100,000 person-years in 1972 to 12.9 per 100,000 person-years in 2015.
- The age-adjusted incidence rate of lung cancer in the US between 2000 and 2013 was 17.5 per 100,000 individuals among Asian female nonsmokers compared with 10.1 per 100,000 among non-Hispanic White female nonsmokers.
TAKEAWAY:
- Environmental and occupational risk factors were secondhand smoke, residential radon, outdoor and household air pollution (PM2.5), asbestos and silica exposure, and prior thoracic radiotherapy. Having a first-degree relative with lung cancer increased the risk of developing lung cancer, and genome-wide association studies identified susceptibility loci associated with lung cancer risk in nonsmokers.
- Family history and inherited susceptibility increased lung cancer risk in never smokers (odds ratio [OR] for lung cancer in those with a first–degree relative, 1.51), and clonal hematopoiesis was also associated with higher risk (OR, 1.43). Importantly, tumors in nonsmokers were frequently driven by actionable somatic alterations (EGFR mutations, 40% to 60% in nonsmokers compared with 10% in smokers) and enrichment of ALK/ROS1/RET/ERBB2/NTRK/NRG1 fusions; 78% to 92% of adenocarcinomas in nonsmokers harbored actionable drivers (compared with 49.5% in ever smokers), and nonsmokers had a substantially lower tumor mutational burden (10–fold lower).
- Similar to individuals with a history of smoking, nonsmokers with lung cancer presented with cough, pain, dyspnea, or weight loss or had disease detected incidentally. Surgical resection remained the preferred treatment for anatomically resectable lung cancer (stages I-III) in medically eligible patients, with follow-up CT screening recommended every 6 months for 2 to 3 years and then annually.
- Targeted adjuvant therapy substantially improved outcomes for resected EGFR–mutant or ALK–rearranged non-small cell lung cancer (NSCLC). Four-year DFS was increased to 70% with osimertinib compared with 29% with placebo (hazard ratio [HR], 0.23) and 5–year overall survival was increased to 85% compared with 73% (HR, 0.49). Two–year DFS was 93.8% with alectinib compared with 63% with placebo (HR, 0.24). In unresectable EGFR-mutated stage III NSCLC, median progression-free survival was 39.1 months with adjuvant osimertinib compared with 5.6 months with placebo. For resected ALKpositive disease, 2–year DFS was 93.8% with adjuvant alectinib compared with 63.0% with chemotherapy (HR, 0.24).
- However, singleagent single agent programmed cell death protein 1 inhibitors or programmed death-ligand 1 inhibitors demonstrated limited efficacy in EGFR or ALK–driven tumors, and benefit was attenuated in never smokers. Regarding screening and early detection, the US Preventive Services Task Force did not recommend lowdose CT screening for nonsmokers, whereas Taiwan implemented a biennial screening program for selected nonsmoking high–risk groups.
IN PRACTICE:
“Among patients with lung cancer, nonsmoking individuals are more likely to have genomic alterations, such as EGFR mutations or ALK gene rearrangements, and these patients have improved survival when treated with TKIs compared with chemotherapy,” the authors of the study wrote.
SOURCE:
The study, led by Cian Murphy, PhD, Cancer Evolution and Genome Instability Laboratory, Francis Crick Institute, London, England, was published online in JAMA.
LIMITATIONS:
Becausesmoking history was often not included in many databases, cancer registries, and trials, the incidence and prevalence of lung cancer in nonsmokers could not be accurately determined. Additionally, accurate quantification of environmental exposures, such as air pollution, presented significant challenges. The quality of the evidence was not formally evaluated, and some relevant articles may have been missed in the literature review.
DISCLOSURES:
The study received support from multiple organizations, including the Rosetrees Trust, Ruth Strauss Foundation, Cancer Research UK, and the National Health and Medical Research Council. Several authors reported receiving grants or personal fees from and having other ties with various sources. Full disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
A comprehensive review of 92 studies found that 15% to 20% of lung cancers occurred among nonsmokers and were associated with environmental and germline risk factors. These cancers frequently harbored actionable genomic drivers, and targeted EGFR and ALK therapies produced significant diseasefree survival (DFS) and overall survival benefits.
METHODOLOGY:
- Lung cancer continues to be the leading cause of cancer death worldwide, causing about 1.8 million deaths in 2022, with smoking remaining the predominant risk factor. However, the incidence of lung cancer among nonsmokers (those who have smoked less than 100 cigarettes in their lifetime) is rising, varies by sex and geography, and is linked to environmental exposures and family history. The misperception that lung cancer is almost invariably caused by smoking may delay assessment and diagnosis.
- Researchers conducted a review of 92 studies on lung cancer in nonsmokers: 6 meta-analyses or systematic reviews, 16 randomized clinical trials, eight prospective cohort studies, seven retrospective cohort studies, three cross-sectional studies, four observational or case-control studies, 13 genomic studies, and 35 other studies.
- Overall, lung cancer among nonsmokers accounted for 15% to 20% of all lung cancer cases. Most lung cancers in nonsmokers were adenocarcinomas (60% to 80%), with a median age at diagnosis of 67 years in this group compared with 70 years in people with a history of smoking.
- Data analysis from three US hospital networks showed that the proportion of lung cancer among nonsmokers increased from 8.0% to 14.9% between 1990 and 2013. A pooled analysis of seven Finnish cohorts reported an absolute increase in lung cancer among nonsmokers from 6.9 per 100,000 person-years in 1972 to 12.9 per 100,000 person-years in 2015.
- The age-adjusted incidence rate of lung cancer in the US between 2000 and 2013 was 17.5 per 100,000 individuals among Asian female nonsmokers compared with 10.1 per 100,000 among non-Hispanic White female nonsmokers.
TAKEAWAY:
- Environmental and occupational risk factors were secondhand smoke, residential radon, outdoor and household air pollution (PM2.5), asbestos and silica exposure, and prior thoracic radiotherapy. Having a first-degree relative with lung cancer increased the risk of developing lung cancer, and genome-wide association studies identified susceptibility loci associated with lung cancer risk in nonsmokers.
- Family history and inherited susceptibility increased lung cancer risk in never smokers (odds ratio [OR] for lung cancer in those with a first–degree relative, 1.51), and clonal hematopoiesis was also associated with higher risk (OR, 1.43). Importantly, tumors in nonsmokers were frequently driven by actionable somatic alterations (EGFR mutations, 40% to 60% in nonsmokers compared with 10% in smokers) and enrichment of ALK/ROS1/RET/ERBB2/NTRK/NRG1 fusions; 78% to 92% of adenocarcinomas in nonsmokers harbored actionable drivers (compared with 49.5% in ever smokers), and nonsmokers had a substantially lower tumor mutational burden (10–fold lower).
- Similar to individuals with a history of smoking, nonsmokers with lung cancer presented with cough, pain, dyspnea, or weight loss or had disease detected incidentally. Surgical resection remained the preferred treatment for anatomically resectable lung cancer (stages I-III) in medically eligible patients, with follow-up CT screening recommended every 6 months for 2 to 3 years and then annually.
- Targeted adjuvant therapy substantially improved outcomes for resected EGFR–mutant or ALK–rearranged non-small cell lung cancer (NSCLC). Four-year DFS was increased to 70% with osimertinib compared with 29% with placebo (hazard ratio [HR], 0.23) and 5–year overall survival was increased to 85% compared with 73% (HR, 0.49). Two–year DFS was 93.8% with alectinib compared with 63% with placebo (HR, 0.24). In unresectable EGFR-mutated stage III NSCLC, median progression-free survival was 39.1 months with adjuvant osimertinib compared with 5.6 months with placebo. For resected ALKpositive disease, 2–year DFS was 93.8% with adjuvant alectinib compared with 63.0% with chemotherapy (HR, 0.24).
- However, singleagent single agent programmed cell death protein 1 inhibitors or programmed death-ligand 1 inhibitors demonstrated limited efficacy in EGFR or ALK–driven tumors, and benefit was attenuated in never smokers. Regarding screening and early detection, the US Preventive Services Task Force did not recommend lowdose CT screening for nonsmokers, whereas Taiwan implemented a biennial screening program for selected nonsmoking high–risk groups.
IN PRACTICE:
“Among patients with lung cancer, nonsmoking individuals are more likely to have genomic alterations, such as EGFR mutations or ALK gene rearrangements, and these patients have improved survival when treated with TKIs compared with chemotherapy,” the authors of the study wrote.
SOURCE:
The study, led by Cian Murphy, PhD, Cancer Evolution and Genome Instability Laboratory, Francis Crick Institute, London, England, was published online in JAMA.
LIMITATIONS:
Becausesmoking history was often not included in many databases, cancer registries, and trials, the incidence and prevalence of lung cancer in nonsmokers could not be accurately determined. Additionally, accurate quantification of environmental exposures, such as air pollution, presented significant challenges. The quality of the evidence was not formally evaluated, and some relevant articles may have been missed in the literature review.
DISCLOSURES:
The study received support from multiple organizations, including the Rosetrees Trust, Ruth Strauss Foundation, Cancer Research UK, and the National Health and Medical Research Council. Several authors reported receiving grants or personal fees from and having other ties with various sources. Full disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
A comprehensive review of 92 studies found that 15% to 20% of lung cancers occurred among nonsmokers and were associated with environmental and germline risk factors. These cancers frequently harbored actionable genomic drivers, and targeted EGFR and ALK therapies produced significant diseasefree survival (DFS) and overall survival benefits.
METHODOLOGY:
- Lung cancer continues to be the leading cause of cancer death worldwide, causing about 1.8 million deaths in 2022, with smoking remaining the predominant risk factor. However, the incidence of lung cancer among nonsmokers (those who have smoked less than 100 cigarettes in their lifetime) is rising, varies by sex and geography, and is linked to environmental exposures and family history. The misperception that lung cancer is almost invariably caused by smoking may delay assessment and diagnosis.
- Researchers conducted a review of 92 studies on lung cancer in nonsmokers: 6 meta-analyses or systematic reviews, 16 randomized clinical trials, eight prospective cohort studies, seven retrospective cohort studies, three cross-sectional studies, four observational or case-control studies, 13 genomic studies, and 35 other studies.
- Overall, lung cancer among nonsmokers accounted for 15% to 20% of all lung cancer cases. Most lung cancers in nonsmokers were adenocarcinomas (60% to 80%), with a median age at diagnosis of 67 years in this group compared with 70 years in people with a history of smoking.
- Data analysis from three US hospital networks showed that the proportion of lung cancer among nonsmokers increased from 8.0% to 14.9% between 1990 and 2013. A pooled analysis of seven Finnish cohorts reported an absolute increase in lung cancer among nonsmokers from 6.9 per 100,000 person-years in 1972 to 12.9 per 100,000 person-years in 2015.
- The age-adjusted incidence rate of lung cancer in the US between 2000 and 2013 was 17.5 per 100,000 individuals among Asian female nonsmokers compared with 10.1 per 100,000 among non-Hispanic White female nonsmokers.
TAKEAWAY:
- Environmental and occupational risk factors were secondhand smoke, residential radon, outdoor and household air pollution (PM2.5), asbestos and silica exposure, and prior thoracic radiotherapy. Having a first-degree relative with lung cancer increased the risk of developing lung cancer, and genome-wide association studies identified susceptibility loci associated with lung cancer risk in nonsmokers.
- Family history and inherited susceptibility increased lung cancer risk in never smokers (odds ratio [OR] for lung cancer in those with a first–degree relative, 1.51), and clonal hematopoiesis was also associated with higher risk (OR, 1.43). Importantly, tumors in nonsmokers were frequently driven by actionable somatic alterations (EGFR mutations, 40% to 60% in nonsmokers compared with 10% in smokers) and enrichment of ALK/ROS1/RET/ERBB2/NTRK/NRG1 fusions; 78% to 92% of adenocarcinomas in nonsmokers harbored actionable drivers (compared with 49.5% in ever smokers), and nonsmokers had a substantially lower tumor mutational burden (10–fold lower).
- Similar to individuals with a history of smoking, nonsmokers with lung cancer presented with cough, pain, dyspnea, or weight loss or had disease detected incidentally. Surgical resection remained the preferred treatment for anatomically resectable lung cancer (stages I-III) in medically eligible patients, with follow-up CT screening recommended every 6 months for 2 to 3 years and then annually.
- Targeted adjuvant therapy substantially improved outcomes for resected EGFR–mutant or ALK–rearranged non-small cell lung cancer (NSCLC). Four-year DFS was increased to 70% with osimertinib compared with 29% with placebo (hazard ratio [HR], 0.23) and 5–year overall survival was increased to 85% compared with 73% (HR, 0.49). Two–year DFS was 93.8% with alectinib compared with 63% with placebo (HR, 0.24). In unresectable EGFR-mutated stage III NSCLC, median progression-free survival was 39.1 months with adjuvant osimertinib compared with 5.6 months with placebo. For resected ALKpositive disease, 2–year DFS was 93.8% with adjuvant alectinib compared with 63.0% with chemotherapy (HR, 0.24).
- However, singleagent single agent programmed cell death protein 1 inhibitors or programmed death-ligand 1 inhibitors demonstrated limited efficacy in EGFR or ALK–driven tumors, and benefit was attenuated in never smokers. Regarding screening and early detection, the US Preventive Services Task Force did not recommend lowdose CT screening for nonsmokers, whereas Taiwan implemented a biennial screening program for selected nonsmoking high–risk groups.
IN PRACTICE:
“Among patients with lung cancer, nonsmoking individuals are more likely to have genomic alterations, such as EGFR mutations or ALK gene rearrangements, and these patients have improved survival when treated with TKIs compared with chemotherapy,” the authors of the study wrote.
SOURCE:
The study, led by Cian Murphy, PhD, Cancer Evolution and Genome Instability Laboratory, Francis Crick Institute, London, England, was published online in JAMA.
LIMITATIONS:
Becausesmoking history was often not included in many databases, cancer registries, and trials, the incidence and prevalence of lung cancer in nonsmokers could not be accurately determined. Additionally, accurate quantification of environmental exposures, such as air pollution, presented significant challenges. The quality of the evidence was not formally evaluated, and some relevant articles may have been missed in the literature review.
DISCLOSURES:
The study received support from multiple organizations, including the Rosetrees Trust, Ruth Strauss Foundation, Cancer Research UK, and the National Health and Medical Research Council. Several authors reported receiving grants or personal fees from and having other ties with various sources. Full disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
When in the Treatment Sequence Should Metastatic CRC Be Retreated With an Anti-EGFR?
BERLIN — Re-treatment with an antiepidermal growth factor receptor (EGFR) agent is effective in patients with chemorefractory metastatic colorectal cancer (mCRC) with RAS and BRAF wild-type tumors confirmed on circulating tumor DNA (ctDNA), although the sequencing of therapy does not seem to matter, suggest overall survival results from the crossover trial PARERE.
The findings nevertheless indicate that anti-EGFR rechallenge with panitumumab may prolong progression-free survival (PFS) over the multiple kinase inhibitor regorafenib. This suggests that “the most pragmatic choice” would be to give the anti-EGFR before regorafenib, said study presenter Marco Maria Germani, MD, Department of Translational Research and New Technologies in Medicine and Surgery, University of Pisa, Pisa, Italy.
The caveat, however, is in patients who have an anti-EGFR interval since previously receiving the drugs of < 6 months. Those patients appeared to do better if they had regorafenib first and then anti-EGFR rechallenge.
Overall, Germani said that “since [trifluridine/tipiracil] plus bevacizumab is today the third-line standard of care” in this patient population, “anti-EGFR re-treatment might be considered after progression” on that combination.
Germani presented the research on October 18 at the European Society for Medical Oncology (ESMO) Annual Meeting 2025, which was simultaneously published in the Annals of Oncology.
Michel P. Ducreux, MD, PhD, head of the Digestive Cancer Committee at Gustave Roussy, Villejuif, France, and invited discussant for the results, said, despite the study being negative, it is “very important to continue to perform this kind of trial to evaluate the [ideal] sequence in the treatment of our patients.”
He continued that the secondary endpoints in the trial of PFS and objective response and disease control rates were “fairly in favor of the use of rechallenge before regorafenib, and in my opinion, this is really quite convincing.”
Ducreux, who was not involved in PARERE trail, also pointed to the sex difference seen in the study, which suggested that women responded much better to having anti-EGFR retreatment before regorafenib than did men.
Similar findings have been reported in a number of other trials, and previous work has suggested that there are sex differences in the pharmacokinetics of several anticancer drugs. However, while this is “very important,” he said that “we never consider it, because we are not able to really explain [it].”
Overall, he concluded that, on the basis of these results, he would agree with the notion that it is better to propose a rechallenge with anti-EGFR treatment as the fourth-line therapy in this patient population, before administering regorafenib.
Ducreux explained that, after a partial response, tumors acquire resistance to EGFR inhibitors through alterations and mutations that occur during treatment, via nongenetic mechanisms, and through treatment-induced selection for preexisting mutations.
Previous work has shown that mutations, such as in the RAS gene, are detectable early during EGFR inhibitor therapy, but that they then decay exponentially once the drugs are stopped, with the potential that tumors regain their sensitivity to them.
Germani said that this means that ctDNA-guided retreatment with anti-EGFR therapies is a “promising approach” in pretreated patients with RAS and BRAF wild-type mCRC, and that the sequencing of the drugs may be important. Indeed, the REVERCE trial showed that giving regorafenib followed by the anti-EGFR drug cetuzximab was associated with longer overall survival than the other way around in anti-EGFR medication-naive patients.
Methods and Results
For PARERE, the researchers enrolled patients aged at least 18 years with RAS and BRAF wild-type mCRC who were previously treated with a first-line anti-EGFR-containing regimen and had at least a partial response or stable disease for at least 6 months.
The patients were also required to have had at least one intervening anti-EGFR-free line of therapy, and to have previously received treatment with fluoropyrimidine, oxaliplatin, irinotecan, and anti-angiogenics. At least 4 months were required to have passed between the end of anti-EGFR administration and screening for the study.
In all, 428 patients were screened between December 2020 and December 2024, with 213 patients with RAS and BRAF wild-type mCRC, as detected on ctDNA, enrolled. They were randomized to panitumumab or regorafenib until first progression, followed by regorafenib, if they started on panitumumab, or panitumumab, if they started on regorafenib, until second progression.
The median age of the patients was 61 years among those who started on panitumumab and 64 years among those initially given regorafenib in the trial, and 63% and 57%, respectively, were male. The median number of prior lines of therapy was two in both groups, and 65% and 69%, respectively, had received pantitumumab as their first-line anti-EGFR.
Initial findings from the study presented at the 2025 ASCO Annual Meeting indicated that, after a median follow-up of 23.5 months, there was no significant difference in the median first PFS between the two treatment arms.
However, patients who started with panitumumab had a significant improvement in both the objective response and disease control rates (P < .001), as well as a signal for a potentially longer median second PFS, than those who started with regorafenib, particularly on the per-protocol analysis.
Presenting the overall survival results, Germani said that there was no significant difference between the groups on the intention-to-treat analysis, at a stratified hazard ratio of 1.13 (P = .440), or on the per-protocol analysis, at a hazard ratio of 1.07 (P = .730).
“We then ran a subgroup analysis,” he continued, “and we found out that an anti-EGFR-free interval before liquid biopsy shorter than 6 months was associated with less benefit from a panitumumab [first] sequence, which is biologically sound.”
It was also observed that women did significantly better when having panitumumab first, whereas men did not, for which “we do not have a clear biological explanation,” Germani added.
Confining the analysis to so-called “hyperselected” patients, who not only were RAS and BRAF wild type but also had no pathogenic mutations associated with anti-EGFR resistance, did not reveal any significant overall survival differences between the treatment groups.
However, Ducreux took issue with the way in which hyperselection, which is turning up more and more regularly in trials, is defined, as the choice of which mutations to include varies widely. He suggested that a consensus group be assembled to resolve this issue.
Looking more broadly, the researchers were able to show that, in this updated analysis, anti-EGFR re-treatment was superior to regorafenib regardless of the treatment sequence in terms of PFS, at 4.2 months vs 2.4 months (P = .103) when given first in the trial, and 3.9 months vs 2.7 months (P = .019) when given second in the trial, as well as in terms of objective response and disease control rates.
Adverse Events
In terms of safety, the results showed that, as expected, acneiform rash, fatigue, and hypomagnesemia were the most common adverse events associated with panitumumb, while those with regorafenib were fatigue, hand-foot skin reactions, and hypertension.
There were no notable differences in the number of patients receiving a post-study treatment nor in the post-study therapeutic choices, between the study arms.
The study was sponsored by GONO Foundation and partially supported by Amgen and Bayer. Germani declared having relationships with MSD and Amgen. Ducreux declared having relationships with Amgen, Bayer, BeiGene, Incyte, Jazz, Merck KGaA, Merck Serono, Merck Sharp & Dohme, Pierre Fabre, Roche, Servier, Keocyt, AbbVie, Abcely, Arcus, Bayer, BMS, Boehringer, GlaxoSmithKline, Sanofi, Scandion, and Zymeworks.
A version of this article first appeared on Medscape.com.
BERLIN — Re-treatment with an antiepidermal growth factor receptor (EGFR) agent is effective in patients with chemorefractory metastatic colorectal cancer (mCRC) with RAS and BRAF wild-type tumors confirmed on circulating tumor DNA (ctDNA), although the sequencing of therapy does not seem to matter, suggest overall survival results from the crossover trial PARERE.
The findings nevertheless indicate that anti-EGFR rechallenge with panitumumab may prolong progression-free survival (PFS) over the multiple kinase inhibitor regorafenib. This suggests that “the most pragmatic choice” would be to give the anti-EGFR before regorafenib, said study presenter Marco Maria Germani, MD, Department of Translational Research and New Technologies in Medicine and Surgery, University of Pisa, Pisa, Italy.
The caveat, however, is in patients who have an anti-EGFR interval since previously receiving the drugs of < 6 months. Those patients appeared to do better if they had regorafenib first and then anti-EGFR rechallenge.
Overall, Germani said that “since [trifluridine/tipiracil] plus bevacizumab is today the third-line standard of care” in this patient population, “anti-EGFR re-treatment might be considered after progression” on that combination.
Germani presented the research on October 18 at the European Society for Medical Oncology (ESMO) Annual Meeting 2025, which was simultaneously published in the Annals of Oncology.
Michel P. Ducreux, MD, PhD, head of the Digestive Cancer Committee at Gustave Roussy, Villejuif, France, and invited discussant for the results, said, despite the study being negative, it is “very important to continue to perform this kind of trial to evaluate the [ideal] sequence in the treatment of our patients.”
He continued that the secondary endpoints in the trial of PFS and objective response and disease control rates were “fairly in favor of the use of rechallenge before regorafenib, and in my opinion, this is really quite convincing.”
Ducreux, who was not involved in PARERE trail, also pointed to the sex difference seen in the study, which suggested that women responded much better to having anti-EGFR retreatment before regorafenib than did men.
Similar findings have been reported in a number of other trials, and previous work has suggested that there are sex differences in the pharmacokinetics of several anticancer drugs. However, while this is “very important,” he said that “we never consider it, because we are not able to really explain [it].”
Overall, he concluded that, on the basis of these results, he would agree with the notion that it is better to propose a rechallenge with anti-EGFR treatment as the fourth-line therapy in this patient population, before administering regorafenib.
Ducreux explained that, after a partial response, tumors acquire resistance to EGFR inhibitors through alterations and mutations that occur during treatment, via nongenetic mechanisms, and through treatment-induced selection for preexisting mutations.
Previous work has shown that mutations, such as in the RAS gene, are detectable early during EGFR inhibitor therapy, but that they then decay exponentially once the drugs are stopped, with the potential that tumors regain their sensitivity to them.
Germani said that this means that ctDNA-guided retreatment with anti-EGFR therapies is a “promising approach” in pretreated patients with RAS and BRAF wild-type mCRC, and that the sequencing of the drugs may be important. Indeed, the REVERCE trial showed that giving regorafenib followed by the anti-EGFR drug cetuzximab was associated with longer overall survival than the other way around in anti-EGFR medication-naive patients.
Methods and Results
For PARERE, the researchers enrolled patients aged at least 18 years with RAS and BRAF wild-type mCRC who were previously treated with a first-line anti-EGFR-containing regimen and had at least a partial response or stable disease for at least 6 months.
The patients were also required to have had at least one intervening anti-EGFR-free line of therapy, and to have previously received treatment with fluoropyrimidine, oxaliplatin, irinotecan, and anti-angiogenics. At least 4 months were required to have passed between the end of anti-EGFR administration and screening for the study.
In all, 428 patients were screened between December 2020 and December 2024, with 213 patients with RAS and BRAF wild-type mCRC, as detected on ctDNA, enrolled. They were randomized to panitumumab or regorafenib until first progression, followed by regorafenib, if they started on panitumumab, or panitumumab, if they started on regorafenib, until second progression.
The median age of the patients was 61 years among those who started on panitumumab and 64 years among those initially given regorafenib in the trial, and 63% and 57%, respectively, were male. The median number of prior lines of therapy was two in both groups, and 65% and 69%, respectively, had received pantitumumab as their first-line anti-EGFR.
Initial findings from the study presented at the 2025 ASCO Annual Meeting indicated that, after a median follow-up of 23.5 months, there was no significant difference in the median first PFS between the two treatment arms.
However, patients who started with panitumumab had a significant improvement in both the objective response and disease control rates (P < .001), as well as a signal for a potentially longer median second PFS, than those who started with regorafenib, particularly on the per-protocol analysis.
Presenting the overall survival results, Germani said that there was no significant difference between the groups on the intention-to-treat analysis, at a stratified hazard ratio of 1.13 (P = .440), or on the per-protocol analysis, at a hazard ratio of 1.07 (P = .730).
“We then ran a subgroup analysis,” he continued, “and we found out that an anti-EGFR-free interval before liquid biopsy shorter than 6 months was associated with less benefit from a panitumumab [first] sequence, which is biologically sound.”
It was also observed that women did significantly better when having panitumumab first, whereas men did not, for which “we do not have a clear biological explanation,” Germani added.
Confining the analysis to so-called “hyperselected” patients, who not only were RAS and BRAF wild type but also had no pathogenic mutations associated with anti-EGFR resistance, did not reveal any significant overall survival differences between the treatment groups.
However, Ducreux took issue with the way in which hyperselection, which is turning up more and more regularly in trials, is defined, as the choice of which mutations to include varies widely. He suggested that a consensus group be assembled to resolve this issue.
Looking more broadly, the researchers were able to show that, in this updated analysis, anti-EGFR re-treatment was superior to regorafenib regardless of the treatment sequence in terms of PFS, at 4.2 months vs 2.4 months (P = .103) when given first in the trial, and 3.9 months vs 2.7 months (P = .019) when given second in the trial, as well as in terms of objective response and disease control rates.
Adverse Events
In terms of safety, the results showed that, as expected, acneiform rash, fatigue, and hypomagnesemia were the most common adverse events associated with panitumumb, while those with regorafenib were fatigue, hand-foot skin reactions, and hypertension.
There were no notable differences in the number of patients receiving a post-study treatment nor in the post-study therapeutic choices, between the study arms.
The study was sponsored by GONO Foundation and partially supported by Amgen and Bayer. Germani declared having relationships with MSD and Amgen. Ducreux declared having relationships with Amgen, Bayer, BeiGene, Incyte, Jazz, Merck KGaA, Merck Serono, Merck Sharp & Dohme, Pierre Fabre, Roche, Servier, Keocyt, AbbVie, Abcely, Arcus, Bayer, BMS, Boehringer, GlaxoSmithKline, Sanofi, Scandion, and Zymeworks.
A version of this article first appeared on Medscape.com.
BERLIN — Re-treatment with an antiepidermal growth factor receptor (EGFR) agent is effective in patients with chemorefractory metastatic colorectal cancer (mCRC) with RAS and BRAF wild-type tumors confirmed on circulating tumor DNA (ctDNA), although the sequencing of therapy does not seem to matter, suggest overall survival results from the crossover trial PARERE.
The findings nevertheless indicate that anti-EGFR rechallenge with panitumumab may prolong progression-free survival (PFS) over the multiple kinase inhibitor regorafenib. This suggests that “the most pragmatic choice” would be to give the anti-EGFR before regorafenib, said study presenter Marco Maria Germani, MD, Department of Translational Research and New Technologies in Medicine and Surgery, University of Pisa, Pisa, Italy.
The caveat, however, is in patients who have an anti-EGFR interval since previously receiving the drugs of < 6 months. Those patients appeared to do better if they had regorafenib first and then anti-EGFR rechallenge.
Overall, Germani said that “since [trifluridine/tipiracil] plus bevacizumab is today the third-line standard of care” in this patient population, “anti-EGFR re-treatment might be considered after progression” on that combination.
Germani presented the research on October 18 at the European Society for Medical Oncology (ESMO) Annual Meeting 2025, which was simultaneously published in the Annals of Oncology.
Michel P. Ducreux, MD, PhD, head of the Digestive Cancer Committee at Gustave Roussy, Villejuif, France, and invited discussant for the results, said, despite the study being negative, it is “very important to continue to perform this kind of trial to evaluate the [ideal] sequence in the treatment of our patients.”
He continued that the secondary endpoints in the trial of PFS and objective response and disease control rates were “fairly in favor of the use of rechallenge before regorafenib, and in my opinion, this is really quite convincing.”
Ducreux, who was not involved in PARERE trail, also pointed to the sex difference seen in the study, which suggested that women responded much better to having anti-EGFR retreatment before regorafenib than did men.
Similar findings have been reported in a number of other trials, and previous work has suggested that there are sex differences in the pharmacokinetics of several anticancer drugs. However, while this is “very important,” he said that “we never consider it, because we are not able to really explain [it].”
Overall, he concluded that, on the basis of these results, he would agree with the notion that it is better to propose a rechallenge with anti-EGFR treatment as the fourth-line therapy in this patient population, before administering regorafenib.
Ducreux explained that, after a partial response, tumors acquire resistance to EGFR inhibitors through alterations and mutations that occur during treatment, via nongenetic mechanisms, and through treatment-induced selection for preexisting mutations.
Previous work has shown that mutations, such as in the RAS gene, are detectable early during EGFR inhibitor therapy, but that they then decay exponentially once the drugs are stopped, with the potential that tumors regain their sensitivity to them.
Germani said that this means that ctDNA-guided retreatment with anti-EGFR therapies is a “promising approach” in pretreated patients with RAS and BRAF wild-type mCRC, and that the sequencing of the drugs may be important. Indeed, the REVERCE trial showed that giving regorafenib followed by the anti-EGFR drug cetuzximab was associated with longer overall survival than the other way around in anti-EGFR medication-naive patients.
Methods and Results
For PARERE, the researchers enrolled patients aged at least 18 years with RAS and BRAF wild-type mCRC who were previously treated with a first-line anti-EGFR-containing regimen and had at least a partial response or stable disease for at least 6 months.
The patients were also required to have had at least one intervening anti-EGFR-free line of therapy, and to have previously received treatment with fluoropyrimidine, oxaliplatin, irinotecan, and anti-angiogenics. At least 4 months were required to have passed between the end of anti-EGFR administration and screening for the study.
In all, 428 patients were screened between December 2020 and December 2024, with 213 patients with RAS and BRAF wild-type mCRC, as detected on ctDNA, enrolled. They were randomized to panitumumab or regorafenib until first progression, followed by regorafenib, if they started on panitumumab, or panitumumab, if they started on regorafenib, until second progression.
The median age of the patients was 61 years among those who started on panitumumab and 64 years among those initially given regorafenib in the trial, and 63% and 57%, respectively, were male. The median number of prior lines of therapy was two in both groups, and 65% and 69%, respectively, had received pantitumumab as their first-line anti-EGFR.
Initial findings from the study presented at the 2025 ASCO Annual Meeting indicated that, after a median follow-up of 23.5 months, there was no significant difference in the median first PFS between the two treatment arms.
However, patients who started with panitumumab had a significant improvement in both the objective response and disease control rates (P < .001), as well as a signal for a potentially longer median second PFS, than those who started with regorafenib, particularly on the per-protocol analysis.
Presenting the overall survival results, Germani said that there was no significant difference between the groups on the intention-to-treat analysis, at a stratified hazard ratio of 1.13 (P = .440), or on the per-protocol analysis, at a hazard ratio of 1.07 (P = .730).
“We then ran a subgroup analysis,” he continued, “and we found out that an anti-EGFR-free interval before liquid biopsy shorter than 6 months was associated with less benefit from a panitumumab [first] sequence, which is biologically sound.”
It was also observed that women did significantly better when having panitumumab first, whereas men did not, for which “we do not have a clear biological explanation,” Germani added.
Confining the analysis to so-called “hyperselected” patients, who not only were RAS and BRAF wild type but also had no pathogenic mutations associated with anti-EGFR resistance, did not reveal any significant overall survival differences between the treatment groups.
However, Ducreux took issue with the way in which hyperselection, which is turning up more and more regularly in trials, is defined, as the choice of which mutations to include varies widely. He suggested that a consensus group be assembled to resolve this issue.
Looking more broadly, the researchers were able to show that, in this updated analysis, anti-EGFR re-treatment was superior to regorafenib regardless of the treatment sequence in terms of PFS, at 4.2 months vs 2.4 months (P = .103) when given first in the trial, and 3.9 months vs 2.7 months (P = .019) when given second in the trial, as well as in terms of objective response and disease control rates.
Adverse Events
In terms of safety, the results showed that, as expected, acneiform rash, fatigue, and hypomagnesemia were the most common adverse events associated with panitumumb, while those with regorafenib were fatigue, hand-foot skin reactions, and hypertension.
There were no notable differences in the number of patients receiving a post-study treatment nor in the post-study therapeutic choices, between the study arms.
The study was sponsored by GONO Foundation and partially supported by Amgen and Bayer. Germani declared having relationships with MSD and Amgen. Ducreux declared having relationships with Amgen, Bayer, BeiGene, Incyte, Jazz, Merck KGaA, Merck Serono, Merck Sharp & Dohme, Pierre Fabre, Roche, Servier, Keocyt, AbbVie, Abcely, Arcus, Bayer, BMS, Boehringer, GlaxoSmithKline, Sanofi, Scandion, and Zymeworks.
A version of this article first appeared on Medscape.com.
FROM ENDO 2025
NICE Endorses Oral Alternative to Chemo in Prostate Cancer
A faster, oral alternative to docetaxel is set to reach NHS clinics after the National Institute for Health and Care Excellence (NICE) recommended darolutamide (Nubeqa, Bayer) in combination with androgen deprivation therapy (ADT) for men with metastatic hormone-sensitive prostate cancer who are unable to receive or tolerate chemotherapy.
Detailed in NICE’s final draft guidance, the decision will make darolutamide available through the NHS in England and Wales to approximately 6000 patients, offering a new oral therapy for those who with limited alternatives to docetaxel or other androgen-receptor inhibitors.
New Option for Chemo-Ineligible Patients
Darolutamide functions by blocking hormones that fuel cancer growth, specifically depriving prostate cancer cells of testosterone required for multiplication and spread. Patients take two tablets twice daily alongside standard ADT.
Peter Johnson, national clinical director for cancer at NHS England, welcomed the decision and expects this approval to give clinicians and their patients “more flexibility to choose the approach best suited to individual circumstances and clinical needs.”
The guidance was finalised 5 weeks ahead of the standard review timeline, underscoring NICE’s commitment to accelerating access to effective prostate cancer treatments.
Clinical Trial Evidence
The NICE’s decision was supported by evidence from the phase 3 ARASENS trial (N = 1306).
The results showed that adding darolutamide to ADT and docetaxel significantly improved overall survival in metastatic hormone-sensitive prostate cancer, reducing the risk for death by 32% compared with ADT and docetaxel alone. Progression-free outcomes, measured by time to castration-resistant disease or death, also favoured darolutamide.
A NICE network meta-analysis of the TITAN, ARCHES, LATITUDE, and STAMPEDE trials suggested that combining ADT with androgen-receptor pathway inhibitors such as apalutamide, enzalutamide, and abiraterone provides comparable survival benefits in this disease setting.
Cost and Implementation
NICE determined that darolutamide plus ADT delivers similar or lower overall costs to the NHS compared with apalutamide plus ADT. The list price is £4040.00 for a 28-day supply (112 × 300-mg tablets), though Bayer has agreed to a confidential commercial discount.
The guidance requires healthcare providers to use the least expensive suitable treatment option, considering administration costs, dosages, price per dose, and commercial arrangements when choosing between darolutamide plus ADT and apalutamide plus ADT.
NHS England and integrated care boards must provide funding within 30 days of final publication, with routine commissioning beginning after this interim period.
A version of this article first appeared on Medscape.com.
A faster, oral alternative to docetaxel is set to reach NHS clinics after the National Institute for Health and Care Excellence (NICE) recommended darolutamide (Nubeqa, Bayer) in combination with androgen deprivation therapy (ADT) for men with metastatic hormone-sensitive prostate cancer who are unable to receive or tolerate chemotherapy.
Detailed in NICE’s final draft guidance, the decision will make darolutamide available through the NHS in England and Wales to approximately 6000 patients, offering a new oral therapy for those who with limited alternatives to docetaxel or other androgen-receptor inhibitors.
New Option for Chemo-Ineligible Patients
Darolutamide functions by blocking hormones that fuel cancer growth, specifically depriving prostate cancer cells of testosterone required for multiplication and spread. Patients take two tablets twice daily alongside standard ADT.
Peter Johnson, national clinical director for cancer at NHS England, welcomed the decision and expects this approval to give clinicians and their patients “more flexibility to choose the approach best suited to individual circumstances and clinical needs.”
The guidance was finalised 5 weeks ahead of the standard review timeline, underscoring NICE’s commitment to accelerating access to effective prostate cancer treatments.
Clinical Trial Evidence
The NICE’s decision was supported by evidence from the phase 3 ARASENS trial (N = 1306).
The results showed that adding darolutamide to ADT and docetaxel significantly improved overall survival in metastatic hormone-sensitive prostate cancer, reducing the risk for death by 32% compared with ADT and docetaxel alone. Progression-free outcomes, measured by time to castration-resistant disease or death, also favoured darolutamide.
A NICE network meta-analysis of the TITAN, ARCHES, LATITUDE, and STAMPEDE trials suggested that combining ADT with androgen-receptor pathway inhibitors such as apalutamide, enzalutamide, and abiraterone provides comparable survival benefits in this disease setting.
Cost and Implementation
NICE determined that darolutamide plus ADT delivers similar or lower overall costs to the NHS compared with apalutamide plus ADT. The list price is £4040.00 for a 28-day supply (112 × 300-mg tablets), though Bayer has agreed to a confidential commercial discount.
The guidance requires healthcare providers to use the least expensive suitable treatment option, considering administration costs, dosages, price per dose, and commercial arrangements when choosing between darolutamide plus ADT and apalutamide plus ADT.
NHS England and integrated care boards must provide funding within 30 days of final publication, with routine commissioning beginning after this interim period.
A version of this article first appeared on Medscape.com.
A faster, oral alternative to docetaxel is set to reach NHS clinics after the National Institute for Health and Care Excellence (NICE) recommended darolutamide (Nubeqa, Bayer) in combination with androgen deprivation therapy (ADT) for men with metastatic hormone-sensitive prostate cancer who are unable to receive or tolerate chemotherapy.
Detailed in NICE’s final draft guidance, the decision will make darolutamide available through the NHS in England and Wales to approximately 6000 patients, offering a new oral therapy for those who with limited alternatives to docetaxel or other androgen-receptor inhibitors.
New Option for Chemo-Ineligible Patients
Darolutamide functions by blocking hormones that fuel cancer growth, specifically depriving prostate cancer cells of testosterone required for multiplication and spread. Patients take two tablets twice daily alongside standard ADT.
Peter Johnson, national clinical director for cancer at NHS England, welcomed the decision and expects this approval to give clinicians and their patients “more flexibility to choose the approach best suited to individual circumstances and clinical needs.”
The guidance was finalised 5 weeks ahead of the standard review timeline, underscoring NICE’s commitment to accelerating access to effective prostate cancer treatments.
Clinical Trial Evidence
The NICE’s decision was supported by evidence from the phase 3 ARASENS trial (N = 1306).
The results showed that adding darolutamide to ADT and docetaxel significantly improved overall survival in metastatic hormone-sensitive prostate cancer, reducing the risk for death by 32% compared with ADT and docetaxel alone. Progression-free outcomes, measured by time to castration-resistant disease or death, also favoured darolutamide.
A NICE network meta-analysis of the TITAN, ARCHES, LATITUDE, and STAMPEDE trials suggested that combining ADT with androgen-receptor pathway inhibitors such as apalutamide, enzalutamide, and abiraterone provides comparable survival benefits in this disease setting.
Cost and Implementation
NICE determined that darolutamide plus ADT delivers similar or lower overall costs to the NHS compared with apalutamide plus ADT. The list price is £4040.00 for a 28-day supply (112 × 300-mg tablets), though Bayer has agreed to a confidential commercial discount.
The guidance requires healthcare providers to use the least expensive suitable treatment option, considering administration costs, dosages, price per dose, and commercial arrangements when choosing between darolutamide plus ADT and apalutamide plus ADT.
NHS England and integrated care boards must provide funding within 30 days of final publication, with routine commissioning beginning after this interim period.
A version of this article first appeared on Medscape.com.
Two ADCs Offer More Hope for Patients With Advanced TNBC
BERLIN — Patients with previously untreated locally recurrent inoperable or metastatic triple negative breast cancer (TNBC) who are not candidates for immunotherapy may experience improved survival outcomes with TROP2-directed antibody-drug conjugates (ADCs), suggested two trials presented at European Society for Medical Oncology (ESMO) Annual Meeting 2025 on October 19.
ASCENT-03 compared sacituzumab govitecan with standard of care chemotherapy, finding that the drug was associated with a 38% improvement in progression-free survival (PFS) in this patient population that has, traditionally, a poor prognosis. Overall survival data remain immature.
TROPION-Breast02 studied datopotamab deruxtecan (Dato-DXd) against investigator’s choice of chemotherapy. The PFS improvement with the ADC was 43%, while patients also experienced a 21% improvement in overall survival. In both cases, the safety profile of the experimental drugs was deemed to be manageable.
Discussant Ana C. Garrido-Castro, MD, director, Triple-Negative Breast Cancer Research, Dana-Farber Cancer Institute, Boston, who was not involved in either study, said that both sacituzumab govitecan and Dato-DXd showed a PFS benefit. The choice between them, leaving aside overall survival until the data are mature, will be largely based on factors such as the safety profile and the patient preference, she continued.
Sacituzumab govitecan is associated with an increase in neutropenia, nausea, and diarrhea, she pointed out, while Dato-DXd has increased rates of ocular surface toxicity, oral mucositis/stomatitis, and requires monitoring for interstitial lung disease.
Dato-DXd has a higher objective response rate than chemotherapy, unlike sacituzumab govitecan, but, crucially, requires one infusion vs 2 for sacituzumab govitecan per 21-day cycle, and has a shorter total infusion time.
There are nevertheless a number of unanswered questions about the drugs, including how the ADCs affect quality of life, and how common patient adherence to the recommended prophylaxis is. Patients with early relapse of < 12 months remain an “urgent unmet need,” Garrido-Castro said, and the role of immunotherapy rechallenge remains to be explored.
ADCs are also being tested in the neo-adjuvant TNBC setting, and the potential impact of that on the use of the drugs in the metastatic setting is currently unclear. In addition, there are questions around access to therapy.
“Ultimately, it will be very important to have a better understanding of the biomarkers of response and resistance and toxicity to these agents, and whether we should be sequencing antibody drug conjugates,” Garrido-Castro said. “All of this will help shape the next wave of treatment strategies for this patient population.”
She concluded: “Today, marks a paradigm shift of metastatic TNBC, in my opinion. ASCENT-03 and TROPION-Breast02 support TROP2 ADC therapy as the new preferred first-line regimen for this patient population.”
Method and Results of ASCENT-03
ASCENT-03 study presenter Javier C. Cortés, MD, PhD, International Breast Cancer Center, Pangaea Oncology, Quiron Group, Barcelona, Spain, said there is currently an unmet clinical need in the approximately 60% of patients with previously untreated metastatic TNBC who are not candidates for immune checkpoint inhibitors.
Median PFS in previous first-line studies was < 6 months with chemotherapy — the current standard of care — and Cortés said that around half of the patients who receive that in the first-line do not receive second-line therapy because of clinical deterioration or death.
“The sobering truth is that across studies in the US and Europe, approximately 25% to 30% of patients diagnosed with metastatic TNBC are no longer alive at 6 months from their metastatic diagnosis,” said Garrido-Castro. “So if there is a new drug that is able to significantly improve PFS with an acceptable toxicity profile, this should be sufficient to change the current standard of care in the first-line setting.”
As sacituzumab govitecan is already approved for second-line metastatic TNBC and for pretreated hormone receptor positive/HER2- metastatic breast cancer, the ASCENT-03 researchers studied the drug in patients with previously untreated locally advanced inoperable, or metastatic TNBC.
The patients were deemed not to be candidates for PD-L1 inhibitors through having PD-L1-negative tumors, by having PD-L1-positive tumors that had previously been treated with PD-L1 inhibitors in the curative setting, or by having a comorbidity that precluded PD-L1 inhibitor use.
The patients were required to have finished any prior treatment in the curative setting at least 6 months previously. Previously treated, stable central nervous system metastases were allowed.
They were randomized to sacituzumab govitecan or chemotherapy, comprising paclitaxel or nab-paclitaxel, or gemcitabine plus carboplatin, until progression, as verified by blinded independent central review (BICR), or unacceptable toxicity. Patients who progressed on chemotherapy were offered crossover to second-line sacituzumab govitecan.
In all, 558 patients were randomized. The median age was 56 years in the sacituzumab govitecan group vs 54 years in the chemotherapy group. The majority (64% in both groups) of patients were White individuals. The most common metastatic site was the lung (59% vs 61%), and 58% of patients in both groups had previously received a taxane.
Cortés reported that sacituzumab govitecan was associated with a “statistically significant and clinically meaningful” improvement in PFS by BICR, at a median of 9.7 months vs 6.9 months, or a hazard ratio (HR) of 0.62 (P < .0001). This benefit was seen across prespecified subgroups.
The objective response rate was almost identical between the two treatment groups, at 48% with sacituzumab govitecan vs 46% with chemotherapy, although the median duration of response was longer with the ADC, at 12.2 months vs 7.2 months.
Cortés showed the latest results on overall survival. This showed no significant difference between the two treatments, although he underlined that the data are not yet mature.
He also reported that the rates of grade ≥ 3 treatment-emergent adverse events (TEAEs) were similar in the two groups, at 66% with sacituzumab govitecan vs 62% with chemotherapy. However, the rates of TEAEs leading to treatment discontinuation (4% vs 12%) or dose reduction (37% vs 45%) were lower with the ADC.
Cortés concluded that the results suggest that sacituzumab govitecan “is a good option for patients with triple negative breast cancer when they develop metastasis and are unable to receive immune checkpoint inhibitors.”
TROPION-Breast02 Methods and Results
Presenting TROPION-Breast02, Rebecca A. Dent, MD, MSc, National Cancer Center Singapore and Duke-NUS Medical School, Singapore, explained that the trial looked at a patient population similar to that of ASCENT-03, here focusing instead on Dato-DXd.
Patients were included if they had histologically or cytologically documented locally recurrent inoperable or metastatic TNBC, no prior chemotherapy or targeted systemic therapy in this setting, and in whom immunotherapy was not an option.
They were randomized to Dato-DXd or the investigator’s choice of chemotherapy, with treatment continued until investigator-assessed progressive disease on RECIST v1.1, unacceptable toxicity, or another criterion for discontinuation was met.
In total, 642 patients were enrolled. The median age was 56 years for those in the Dato-DXd group and 57 years for those in chemotherapy group, and less than half (41% in the Dato-DXd group and 48% in the chemotherapy group) were White individuals. The number of metastatic sites was less than three in 64% and 67% of patients, respectively.
Dent showed that Dato-DXd was associated with a statistically significant and clinically meaningful improvement in BICR-assessed PFS, at a median of 10.8 months vs 5.6 months with chemotherapy, at a HR of 0.57 (P < .0001). The findings were replicated across the prespecified subgroups.
There was a marked overall survival benefit with Dato-DXd, at a median of 23.7 months vs 18.7 months, at a HR of 0.79 (P = .0291). Dent reported that, at 18 months, 61.2% of patients in the Dato-DXd group were still alive vs 51.3% in the chemotherapy group. Again, the benefit was seen across subgroups.
The confirmed objective response rate with Dato-DXd was far higher than that with chemotherapy, at 62.5% vs 29.3%, or an odds ratio of 4.24. The duration of response was also longer, at 12.3 months vs 7.1 months.
Rates of grade ≥ 3 adverse events were comparable, at 33% with Dato-DXd vs 29% with chemotherapy, although there were more events associated with dose reduction (27% vs 18%) and dose interruption (24% vs 19%) with the ADC.
“These results support Dato-DXd as the first new first-line standard of care for patients with locally recurrent inoperable or metastatic TNBC for whom immunotherapy is not an option,” Dent said.
“What’s important is the patients enrolled into this trial are clearly representative of real world patients that we are treating in our clinics every day. These patients are often excluded from our current clinical trials,” she said.
ASCENT-03 was funded by Gilead Sciences.
TROPION-Breast02 was funded by AstraZeneca.Cortés declared relationships with Roche, AstraZeneca, Seattle Genetics, Daiichi Sankyo, Lilly, Merck Sharpe & Dohme, Leuko, Bioasis, Clovis oncology, Boehringer Ingelheim, Ellipses, HiberCell, BioInvent, GEMoaB, Gilead, Menarini, Zymeworks, Reveal Genomics, Expres2ion Biotechnologies, Jazz Pharmaceuticals, AbbVie, Scorpion Therapeutics, Bridgebio, Biocon, Biontech, Circle Pharma, Delcath Systems, Hexagon Bio, Novartis, Eisai, Pfizer, Stemline Therapeutics, MAJ3 Capital, Leuko, Ariad Pharmaceuticals, Baxalta GmbH/Servier Affaires, Bayer healthcare, Guardant Health, and PIQUR Therapeutics.
Dent declared relationships with AstraZeneca, MSD, Pfizer, Eisai, Novartis, Daiichi Sankyo/AstraZeneca, Roche, and Gilead Sciences.
Garrido-Castro declared relationships with AstraZeneca, MSD, Pfizer, Eisai, Novartis, Daiichi Sankyo/AstraZeneca, Roche, Gilead Sciences, Pfizer, TD Cowen, and Roche/Genentech.
A version of this article first appeared on Medscape.com.
BERLIN — Patients with previously untreated locally recurrent inoperable or metastatic triple negative breast cancer (TNBC) who are not candidates for immunotherapy may experience improved survival outcomes with TROP2-directed antibody-drug conjugates (ADCs), suggested two trials presented at European Society for Medical Oncology (ESMO) Annual Meeting 2025 on October 19.
ASCENT-03 compared sacituzumab govitecan with standard of care chemotherapy, finding that the drug was associated with a 38% improvement in progression-free survival (PFS) in this patient population that has, traditionally, a poor prognosis. Overall survival data remain immature.
TROPION-Breast02 studied datopotamab deruxtecan (Dato-DXd) against investigator’s choice of chemotherapy. The PFS improvement with the ADC was 43%, while patients also experienced a 21% improvement in overall survival. In both cases, the safety profile of the experimental drugs was deemed to be manageable.
Discussant Ana C. Garrido-Castro, MD, director, Triple-Negative Breast Cancer Research, Dana-Farber Cancer Institute, Boston, who was not involved in either study, said that both sacituzumab govitecan and Dato-DXd showed a PFS benefit. The choice between them, leaving aside overall survival until the data are mature, will be largely based on factors such as the safety profile and the patient preference, she continued.
Sacituzumab govitecan is associated with an increase in neutropenia, nausea, and diarrhea, she pointed out, while Dato-DXd has increased rates of ocular surface toxicity, oral mucositis/stomatitis, and requires monitoring for interstitial lung disease.
Dato-DXd has a higher objective response rate than chemotherapy, unlike sacituzumab govitecan, but, crucially, requires one infusion vs 2 for sacituzumab govitecan per 21-day cycle, and has a shorter total infusion time.
There are nevertheless a number of unanswered questions about the drugs, including how the ADCs affect quality of life, and how common patient adherence to the recommended prophylaxis is. Patients with early relapse of < 12 months remain an “urgent unmet need,” Garrido-Castro said, and the role of immunotherapy rechallenge remains to be explored.
ADCs are also being tested in the neo-adjuvant TNBC setting, and the potential impact of that on the use of the drugs in the metastatic setting is currently unclear. In addition, there are questions around access to therapy.
“Ultimately, it will be very important to have a better understanding of the biomarkers of response and resistance and toxicity to these agents, and whether we should be sequencing antibody drug conjugates,” Garrido-Castro said. “All of this will help shape the next wave of treatment strategies for this patient population.”
She concluded: “Today, marks a paradigm shift of metastatic TNBC, in my opinion. ASCENT-03 and TROPION-Breast02 support TROP2 ADC therapy as the new preferred first-line regimen for this patient population.”
Method and Results of ASCENT-03
ASCENT-03 study presenter Javier C. Cortés, MD, PhD, International Breast Cancer Center, Pangaea Oncology, Quiron Group, Barcelona, Spain, said there is currently an unmet clinical need in the approximately 60% of patients with previously untreated metastatic TNBC who are not candidates for immune checkpoint inhibitors.
Median PFS in previous first-line studies was < 6 months with chemotherapy — the current standard of care — and Cortés said that around half of the patients who receive that in the first-line do not receive second-line therapy because of clinical deterioration or death.
“The sobering truth is that across studies in the US and Europe, approximately 25% to 30% of patients diagnosed with metastatic TNBC are no longer alive at 6 months from their metastatic diagnosis,” said Garrido-Castro. “So if there is a new drug that is able to significantly improve PFS with an acceptable toxicity profile, this should be sufficient to change the current standard of care in the first-line setting.”
As sacituzumab govitecan is already approved for second-line metastatic TNBC and for pretreated hormone receptor positive/HER2- metastatic breast cancer, the ASCENT-03 researchers studied the drug in patients with previously untreated locally advanced inoperable, or metastatic TNBC.
The patients were deemed not to be candidates for PD-L1 inhibitors through having PD-L1-negative tumors, by having PD-L1-positive tumors that had previously been treated with PD-L1 inhibitors in the curative setting, or by having a comorbidity that precluded PD-L1 inhibitor use.
The patients were required to have finished any prior treatment in the curative setting at least 6 months previously. Previously treated, stable central nervous system metastases were allowed.
They were randomized to sacituzumab govitecan or chemotherapy, comprising paclitaxel or nab-paclitaxel, or gemcitabine plus carboplatin, until progression, as verified by blinded independent central review (BICR), or unacceptable toxicity. Patients who progressed on chemotherapy were offered crossover to second-line sacituzumab govitecan.
In all, 558 patients were randomized. The median age was 56 years in the sacituzumab govitecan group vs 54 years in the chemotherapy group. The majority (64% in both groups) of patients were White individuals. The most common metastatic site was the lung (59% vs 61%), and 58% of patients in both groups had previously received a taxane.
Cortés reported that sacituzumab govitecan was associated with a “statistically significant and clinically meaningful” improvement in PFS by BICR, at a median of 9.7 months vs 6.9 months, or a hazard ratio (HR) of 0.62 (P < .0001). This benefit was seen across prespecified subgroups.
The objective response rate was almost identical between the two treatment groups, at 48% with sacituzumab govitecan vs 46% with chemotherapy, although the median duration of response was longer with the ADC, at 12.2 months vs 7.2 months.
Cortés showed the latest results on overall survival. This showed no significant difference between the two treatments, although he underlined that the data are not yet mature.
He also reported that the rates of grade ≥ 3 treatment-emergent adverse events (TEAEs) were similar in the two groups, at 66% with sacituzumab govitecan vs 62% with chemotherapy. However, the rates of TEAEs leading to treatment discontinuation (4% vs 12%) or dose reduction (37% vs 45%) were lower with the ADC.
Cortés concluded that the results suggest that sacituzumab govitecan “is a good option for patients with triple negative breast cancer when they develop metastasis and are unable to receive immune checkpoint inhibitors.”
TROPION-Breast02 Methods and Results
Presenting TROPION-Breast02, Rebecca A. Dent, MD, MSc, National Cancer Center Singapore and Duke-NUS Medical School, Singapore, explained that the trial looked at a patient population similar to that of ASCENT-03, here focusing instead on Dato-DXd.
Patients were included if they had histologically or cytologically documented locally recurrent inoperable or metastatic TNBC, no prior chemotherapy or targeted systemic therapy in this setting, and in whom immunotherapy was not an option.
They were randomized to Dato-DXd or the investigator’s choice of chemotherapy, with treatment continued until investigator-assessed progressive disease on RECIST v1.1, unacceptable toxicity, or another criterion for discontinuation was met.
In total, 642 patients were enrolled. The median age was 56 years for those in the Dato-DXd group and 57 years for those in chemotherapy group, and less than half (41% in the Dato-DXd group and 48% in the chemotherapy group) were White individuals. The number of metastatic sites was less than three in 64% and 67% of patients, respectively.
Dent showed that Dato-DXd was associated with a statistically significant and clinically meaningful improvement in BICR-assessed PFS, at a median of 10.8 months vs 5.6 months with chemotherapy, at a HR of 0.57 (P < .0001). The findings were replicated across the prespecified subgroups.
There was a marked overall survival benefit with Dato-DXd, at a median of 23.7 months vs 18.7 months, at a HR of 0.79 (P = .0291). Dent reported that, at 18 months, 61.2% of patients in the Dato-DXd group were still alive vs 51.3% in the chemotherapy group. Again, the benefit was seen across subgroups.
The confirmed objective response rate with Dato-DXd was far higher than that with chemotherapy, at 62.5% vs 29.3%, or an odds ratio of 4.24. The duration of response was also longer, at 12.3 months vs 7.1 months.
Rates of grade ≥ 3 adverse events were comparable, at 33% with Dato-DXd vs 29% with chemotherapy, although there were more events associated with dose reduction (27% vs 18%) and dose interruption (24% vs 19%) with the ADC.
“These results support Dato-DXd as the first new first-line standard of care for patients with locally recurrent inoperable or metastatic TNBC for whom immunotherapy is not an option,” Dent said.
“What’s important is the patients enrolled into this trial are clearly representative of real world patients that we are treating in our clinics every day. These patients are often excluded from our current clinical trials,” she said.
ASCENT-03 was funded by Gilead Sciences.
TROPION-Breast02 was funded by AstraZeneca.Cortés declared relationships with Roche, AstraZeneca, Seattle Genetics, Daiichi Sankyo, Lilly, Merck Sharpe & Dohme, Leuko, Bioasis, Clovis oncology, Boehringer Ingelheim, Ellipses, HiberCell, BioInvent, GEMoaB, Gilead, Menarini, Zymeworks, Reveal Genomics, Expres2ion Biotechnologies, Jazz Pharmaceuticals, AbbVie, Scorpion Therapeutics, Bridgebio, Biocon, Biontech, Circle Pharma, Delcath Systems, Hexagon Bio, Novartis, Eisai, Pfizer, Stemline Therapeutics, MAJ3 Capital, Leuko, Ariad Pharmaceuticals, Baxalta GmbH/Servier Affaires, Bayer healthcare, Guardant Health, and PIQUR Therapeutics.
Dent declared relationships with AstraZeneca, MSD, Pfizer, Eisai, Novartis, Daiichi Sankyo/AstraZeneca, Roche, and Gilead Sciences.
Garrido-Castro declared relationships with AstraZeneca, MSD, Pfizer, Eisai, Novartis, Daiichi Sankyo/AstraZeneca, Roche, Gilead Sciences, Pfizer, TD Cowen, and Roche/Genentech.
A version of this article first appeared on Medscape.com.
BERLIN — Patients with previously untreated locally recurrent inoperable or metastatic triple negative breast cancer (TNBC) who are not candidates for immunotherapy may experience improved survival outcomes with TROP2-directed antibody-drug conjugates (ADCs), suggested two trials presented at European Society for Medical Oncology (ESMO) Annual Meeting 2025 on October 19.
ASCENT-03 compared sacituzumab govitecan with standard of care chemotherapy, finding that the drug was associated with a 38% improvement in progression-free survival (PFS) in this patient population that has, traditionally, a poor prognosis. Overall survival data remain immature.
TROPION-Breast02 studied datopotamab deruxtecan (Dato-DXd) against investigator’s choice of chemotherapy. The PFS improvement with the ADC was 43%, while patients also experienced a 21% improvement in overall survival. In both cases, the safety profile of the experimental drugs was deemed to be manageable.
Discussant Ana C. Garrido-Castro, MD, director, Triple-Negative Breast Cancer Research, Dana-Farber Cancer Institute, Boston, who was not involved in either study, said that both sacituzumab govitecan and Dato-DXd showed a PFS benefit. The choice between them, leaving aside overall survival until the data are mature, will be largely based on factors such as the safety profile and the patient preference, she continued.
Sacituzumab govitecan is associated with an increase in neutropenia, nausea, and diarrhea, she pointed out, while Dato-DXd has increased rates of ocular surface toxicity, oral mucositis/stomatitis, and requires monitoring for interstitial lung disease.
Dato-DXd has a higher objective response rate than chemotherapy, unlike sacituzumab govitecan, but, crucially, requires one infusion vs 2 for sacituzumab govitecan per 21-day cycle, and has a shorter total infusion time.
There are nevertheless a number of unanswered questions about the drugs, including how the ADCs affect quality of life, and how common patient adherence to the recommended prophylaxis is. Patients with early relapse of < 12 months remain an “urgent unmet need,” Garrido-Castro said, and the role of immunotherapy rechallenge remains to be explored.
ADCs are also being tested in the neo-adjuvant TNBC setting, and the potential impact of that on the use of the drugs in the metastatic setting is currently unclear. In addition, there are questions around access to therapy.
“Ultimately, it will be very important to have a better understanding of the biomarkers of response and resistance and toxicity to these agents, and whether we should be sequencing antibody drug conjugates,” Garrido-Castro said. “All of this will help shape the next wave of treatment strategies for this patient population.”
She concluded: “Today, marks a paradigm shift of metastatic TNBC, in my opinion. ASCENT-03 and TROPION-Breast02 support TROP2 ADC therapy as the new preferred first-line regimen for this patient population.”
Method and Results of ASCENT-03
ASCENT-03 study presenter Javier C. Cortés, MD, PhD, International Breast Cancer Center, Pangaea Oncology, Quiron Group, Barcelona, Spain, said there is currently an unmet clinical need in the approximately 60% of patients with previously untreated metastatic TNBC who are not candidates for immune checkpoint inhibitors.
Median PFS in previous first-line studies was < 6 months with chemotherapy — the current standard of care — and Cortés said that around half of the patients who receive that in the first-line do not receive second-line therapy because of clinical deterioration or death.
“The sobering truth is that across studies in the US and Europe, approximately 25% to 30% of patients diagnosed with metastatic TNBC are no longer alive at 6 months from their metastatic diagnosis,” said Garrido-Castro. “So if there is a new drug that is able to significantly improve PFS with an acceptable toxicity profile, this should be sufficient to change the current standard of care in the first-line setting.”
As sacituzumab govitecan is already approved for second-line metastatic TNBC and for pretreated hormone receptor positive/HER2- metastatic breast cancer, the ASCENT-03 researchers studied the drug in patients with previously untreated locally advanced inoperable, or metastatic TNBC.
The patients were deemed not to be candidates for PD-L1 inhibitors through having PD-L1-negative tumors, by having PD-L1-positive tumors that had previously been treated with PD-L1 inhibitors in the curative setting, or by having a comorbidity that precluded PD-L1 inhibitor use.
The patients were required to have finished any prior treatment in the curative setting at least 6 months previously. Previously treated, stable central nervous system metastases were allowed.
They were randomized to sacituzumab govitecan or chemotherapy, comprising paclitaxel or nab-paclitaxel, or gemcitabine plus carboplatin, until progression, as verified by blinded independent central review (BICR), or unacceptable toxicity. Patients who progressed on chemotherapy were offered crossover to second-line sacituzumab govitecan.
In all, 558 patients were randomized. The median age was 56 years in the sacituzumab govitecan group vs 54 years in the chemotherapy group. The majority (64% in both groups) of patients were White individuals. The most common metastatic site was the lung (59% vs 61%), and 58% of patients in both groups had previously received a taxane.
Cortés reported that sacituzumab govitecan was associated with a “statistically significant and clinically meaningful” improvement in PFS by BICR, at a median of 9.7 months vs 6.9 months, or a hazard ratio (HR) of 0.62 (P < .0001). This benefit was seen across prespecified subgroups.
The objective response rate was almost identical between the two treatment groups, at 48% with sacituzumab govitecan vs 46% with chemotherapy, although the median duration of response was longer with the ADC, at 12.2 months vs 7.2 months.
Cortés showed the latest results on overall survival. This showed no significant difference between the two treatments, although he underlined that the data are not yet mature.
He also reported that the rates of grade ≥ 3 treatment-emergent adverse events (TEAEs) were similar in the two groups, at 66% with sacituzumab govitecan vs 62% with chemotherapy. However, the rates of TEAEs leading to treatment discontinuation (4% vs 12%) or dose reduction (37% vs 45%) were lower with the ADC.
Cortés concluded that the results suggest that sacituzumab govitecan “is a good option for patients with triple negative breast cancer when they develop metastasis and are unable to receive immune checkpoint inhibitors.”
TROPION-Breast02 Methods and Results
Presenting TROPION-Breast02, Rebecca A. Dent, MD, MSc, National Cancer Center Singapore and Duke-NUS Medical School, Singapore, explained that the trial looked at a patient population similar to that of ASCENT-03, here focusing instead on Dato-DXd.
Patients were included if they had histologically or cytologically documented locally recurrent inoperable or metastatic TNBC, no prior chemotherapy or targeted systemic therapy in this setting, and in whom immunotherapy was not an option.
They were randomized to Dato-DXd or the investigator’s choice of chemotherapy, with treatment continued until investigator-assessed progressive disease on RECIST v1.1, unacceptable toxicity, or another criterion for discontinuation was met.
In total, 642 patients were enrolled. The median age was 56 years for those in the Dato-DXd group and 57 years for those in chemotherapy group, and less than half (41% in the Dato-DXd group and 48% in the chemotherapy group) were White individuals. The number of metastatic sites was less than three in 64% and 67% of patients, respectively.
Dent showed that Dato-DXd was associated with a statistically significant and clinically meaningful improvement in BICR-assessed PFS, at a median of 10.8 months vs 5.6 months with chemotherapy, at a HR of 0.57 (P < .0001). The findings were replicated across the prespecified subgroups.
There was a marked overall survival benefit with Dato-DXd, at a median of 23.7 months vs 18.7 months, at a HR of 0.79 (P = .0291). Dent reported that, at 18 months, 61.2% of patients in the Dato-DXd group were still alive vs 51.3% in the chemotherapy group. Again, the benefit was seen across subgroups.
The confirmed objective response rate with Dato-DXd was far higher than that with chemotherapy, at 62.5% vs 29.3%, or an odds ratio of 4.24. The duration of response was also longer, at 12.3 months vs 7.1 months.
Rates of grade ≥ 3 adverse events were comparable, at 33% with Dato-DXd vs 29% with chemotherapy, although there were more events associated with dose reduction (27% vs 18%) and dose interruption (24% vs 19%) with the ADC.
“These results support Dato-DXd as the first new first-line standard of care for patients with locally recurrent inoperable or metastatic TNBC for whom immunotherapy is not an option,” Dent said.
“What’s important is the patients enrolled into this trial are clearly representative of real world patients that we are treating in our clinics every day. These patients are often excluded from our current clinical trials,” she said.
ASCENT-03 was funded by Gilead Sciences.
TROPION-Breast02 was funded by AstraZeneca.Cortés declared relationships with Roche, AstraZeneca, Seattle Genetics, Daiichi Sankyo, Lilly, Merck Sharpe & Dohme, Leuko, Bioasis, Clovis oncology, Boehringer Ingelheim, Ellipses, HiberCell, BioInvent, GEMoaB, Gilead, Menarini, Zymeworks, Reveal Genomics, Expres2ion Biotechnologies, Jazz Pharmaceuticals, AbbVie, Scorpion Therapeutics, Bridgebio, Biocon, Biontech, Circle Pharma, Delcath Systems, Hexagon Bio, Novartis, Eisai, Pfizer, Stemline Therapeutics, MAJ3 Capital, Leuko, Ariad Pharmaceuticals, Baxalta GmbH/Servier Affaires, Bayer healthcare, Guardant Health, and PIQUR Therapeutics.
Dent declared relationships with AstraZeneca, MSD, Pfizer, Eisai, Novartis, Daiichi Sankyo/AstraZeneca, Roche, and Gilead Sciences.
Garrido-Castro declared relationships with AstraZeneca, MSD, Pfizer, Eisai, Novartis, Daiichi Sankyo/AstraZeneca, Roche, Gilead Sciences, Pfizer, TD Cowen, and Roche/Genentech.
A version of this article first appeared on Medscape.com.
FROM ESMO 2025
Is High Quality VA Psychiatric Care Keeping Readmissions Rates Low?
Repeated and frequent hospitalizations—sometimes referred to as the revolving door phenomenon— are a particular risk for patients during the first month after discharge. Early psychiatric readmission is a standard indicator of adverse outcomes. However, the results
The quality of previous care has long been thought to be a driver of readmission. If that’s the case, a 2025 study suggests that on average veterans received high-quality inpatient psychiatric services at Veterans Health Administration (VHA) facilities across the nation and that may have been key to keeping readmissions down. Analyzing data from 88,954 veterans who received care at VHA Inpatient Mental Health (IMH) services, the researchers found a “relatively low” rate of readmission within 30 days: 7.1% compared with 8% to 31% of other psychiatric patients in the US. With 40,220 unique patients receiving IMH care per year on average between October 2019 and September 2022, a 7.1% readmission rate means > 2800 30-day readmissions annually.
Research has found that veterans who receive care at the VA have better outcomes than those treated in the private sector. Part of that has to do with practitioners who understand the unique needs of their patients. Veterans may have posttraumatic stress disorder or multiple diagnoses, such as depression, panic disorder, and a substance use disorder. Their mental health issues may also coexist with physical health problems, such as traumatic brain injuries due to explosions.
“If you’re trained at the VA, you learn something important about veteran mental health care that you’ll never get if you’re trained someplace else,” Rodney R. Baker, PhD, retired mental health director and chief of psychology for the South Texas VA Health Care System, said recently. Community clinicians may not know how to collect and incorporate information about a patient’s military history, including details about deployments, combat exposure, injuries, military sexual trauma, and unit culture. They may also lack expertise in navigating the transition between military and veteran life, now considered a critical adjustment period.
“This is a unique population,” said Conwell Smith, the American Psychological Association’s deputy chief of military and veteran policy. “Sending veterans out to the community without requiring that mental health care providers understand them is concerning.”
IMH services aim to stabilize mental health crises and improve veterans’ functioning through patient-centered, evidence-based, and recovery-oriented approaches shown to reduce readmission rates. Treatment generally involves a minimum of 4 hours of interdisciplinary, therapeutic programming each day. And upon discharge, the inpatient care team facilitates the patient’s transition to appropriate outpatient services.
Follow-up care, particularly during the first 30 days, has proved critical in reducing readmissions. In studies that have analyzed postdischarge interventions (psychoeducation, mentoring, community-based hospital treatment, use of continuous follow-up and compulsory community treatment), all found fewer hospitalizations when compared to a control group, or a smaller number of admissions after the intervention.
Mental health care for veterans should be provided by experienced practitioners—but those practitioners are leaving VA. According to the VA Office of Inspector General, 57% of medical centers report a shortage of psychologists. And according to the VA’s monthly Workforce Dashboard, the VHA lost 234 psychologists in the first 9 months of 2025. The VA has also announced plans to cut 30,000 jobs by the end of the year and impose caps on staff at every medical center.
“This approach locks in permanent VA understaffing just as demand for mental health services is projected to continue growing through 2030,” said Russell Lemle, PhD, a clinical psychologist and senior policy analyst for the Veterans Healthcare Policy Institute. “The private sector can’t fill this gap either—over a third of Americans live in areas already facing mental health professional shortages. That’s not taking care of our veterans.
“Unless actions are taken quickly to reverse the trend, its mental health services could easily diminish substantially within 10 to 20 years.”
Repeated and frequent hospitalizations—sometimes referred to as the revolving door phenomenon— are a particular risk for patients during the first month after discharge. Early psychiatric readmission is a standard indicator of adverse outcomes. However, the results
The quality of previous care has long been thought to be a driver of readmission. If that’s the case, a 2025 study suggests that on average veterans received high-quality inpatient psychiatric services at Veterans Health Administration (VHA) facilities across the nation and that may have been key to keeping readmissions down. Analyzing data from 88,954 veterans who received care at VHA Inpatient Mental Health (IMH) services, the researchers found a “relatively low” rate of readmission within 30 days: 7.1% compared with 8% to 31% of other psychiatric patients in the US. With 40,220 unique patients receiving IMH care per year on average between October 2019 and September 2022, a 7.1% readmission rate means > 2800 30-day readmissions annually.
Research has found that veterans who receive care at the VA have better outcomes than those treated in the private sector. Part of that has to do with practitioners who understand the unique needs of their patients. Veterans may have posttraumatic stress disorder or multiple diagnoses, such as depression, panic disorder, and a substance use disorder. Their mental health issues may also coexist with physical health problems, such as traumatic brain injuries due to explosions.
“If you’re trained at the VA, you learn something important about veteran mental health care that you’ll never get if you’re trained someplace else,” Rodney R. Baker, PhD, retired mental health director and chief of psychology for the South Texas VA Health Care System, said recently. Community clinicians may not know how to collect and incorporate information about a patient’s military history, including details about deployments, combat exposure, injuries, military sexual trauma, and unit culture. They may also lack expertise in navigating the transition between military and veteran life, now considered a critical adjustment period.
“This is a unique population,” said Conwell Smith, the American Psychological Association’s deputy chief of military and veteran policy. “Sending veterans out to the community without requiring that mental health care providers understand them is concerning.”
IMH services aim to stabilize mental health crises and improve veterans’ functioning through patient-centered, evidence-based, and recovery-oriented approaches shown to reduce readmission rates. Treatment generally involves a minimum of 4 hours of interdisciplinary, therapeutic programming each day. And upon discharge, the inpatient care team facilitates the patient’s transition to appropriate outpatient services.
Follow-up care, particularly during the first 30 days, has proved critical in reducing readmissions. In studies that have analyzed postdischarge interventions (psychoeducation, mentoring, community-based hospital treatment, use of continuous follow-up and compulsory community treatment), all found fewer hospitalizations when compared to a control group, or a smaller number of admissions after the intervention.
Mental health care for veterans should be provided by experienced practitioners—but those practitioners are leaving VA. According to the VA Office of Inspector General, 57% of medical centers report a shortage of psychologists. And according to the VA’s monthly Workforce Dashboard, the VHA lost 234 psychologists in the first 9 months of 2025. The VA has also announced plans to cut 30,000 jobs by the end of the year and impose caps on staff at every medical center.
“This approach locks in permanent VA understaffing just as demand for mental health services is projected to continue growing through 2030,” said Russell Lemle, PhD, a clinical psychologist and senior policy analyst for the Veterans Healthcare Policy Institute. “The private sector can’t fill this gap either—over a third of Americans live in areas already facing mental health professional shortages. That’s not taking care of our veterans.
“Unless actions are taken quickly to reverse the trend, its mental health services could easily diminish substantially within 10 to 20 years.”
Repeated and frequent hospitalizations—sometimes referred to as the revolving door phenomenon— are a particular risk for patients during the first month after discharge. Early psychiatric readmission is a standard indicator of adverse outcomes. However, the results
The quality of previous care has long been thought to be a driver of readmission. If that’s the case, a 2025 study suggests that on average veterans received high-quality inpatient psychiatric services at Veterans Health Administration (VHA) facilities across the nation and that may have been key to keeping readmissions down. Analyzing data from 88,954 veterans who received care at VHA Inpatient Mental Health (IMH) services, the researchers found a “relatively low” rate of readmission within 30 days: 7.1% compared with 8% to 31% of other psychiatric patients in the US. With 40,220 unique patients receiving IMH care per year on average between October 2019 and September 2022, a 7.1% readmission rate means > 2800 30-day readmissions annually.
Research has found that veterans who receive care at the VA have better outcomes than those treated in the private sector. Part of that has to do with practitioners who understand the unique needs of their patients. Veterans may have posttraumatic stress disorder or multiple diagnoses, such as depression, panic disorder, and a substance use disorder. Their mental health issues may also coexist with physical health problems, such as traumatic brain injuries due to explosions.
“If you’re trained at the VA, you learn something important about veteran mental health care that you’ll never get if you’re trained someplace else,” Rodney R. Baker, PhD, retired mental health director and chief of psychology for the South Texas VA Health Care System, said recently. Community clinicians may not know how to collect and incorporate information about a patient’s military history, including details about deployments, combat exposure, injuries, military sexual trauma, and unit culture. They may also lack expertise in navigating the transition between military and veteran life, now considered a critical adjustment period.
“This is a unique population,” said Conwell Smith, the American Psychological Association’s deputy chief of military and veteran policy. “Sending veterans out to the community without requiring that mental health care providers understand them is concerning.”
IMH services aim to stabilize mental health crises and improve veterans’ functioning through patient-centered, evidence-based, and recovery-oriented approaches shown to reduce readmission rates. Treatment generally involves a minimum of 4 hours of interdisciplinary, therapeutic programming each day. And upon discharge, the inpatient care team facilitates the patient’s transition to appropriate outpatient services.
Follow-up care, particularly during the first 30 days, has proved critical in reducing readmissions. In studies that have analyzed postdischarge interventions (psychoeducation, mentoring, community-based hospital treatment, use of continuous follow-up and compulsory community treatment), all found fewer hospitalizations when compared to a control group, or a smaller number of admissions after the intervention.
Mental health care for veterans should be provided by experienced practitioners—but those practitioners are leaving VA. According to the VA Office of Inspector General, 57% of medical centers report a shortage of psychologists. And according to the VA’s monthly Workforce Dashboard, the VHA lost 234 psychologists in the first 9 months of 2025. The VA has also announced plans to cut 30,000 jobs by the end of the year and impose caps on staff at every medical center.
“This approach locks in permanent VA understaffing just as demand for mental health services is projected to continue growing through 2030,” said Russell Lemle, PhD, a clinical psychologist and senior policy analyst for the Veterans Healthcare Policy Institute. “The private sector can’t fill this gap either—over a third of Americans live in areas already facing mental health professional shortages. That’s not taking care of our veterans.
“Unless actions are taken quickly to reverse the trend, its mental health services could easily diminish substantially within 10 to 20 years.”
As Federal Cuts Deepen Mental Health Crisis, Philanthropy Scrambles to Fill the Gap
As Federal Cuts Deepen Mental Health Crisis, Philanthropy Scrambles to Fill the Gap
It's hardly news that the United States is experiencing a mental health crisis -- the CDC says as much. But experts in the field say that the current administration has severely compounded the problem by eliminating agency funding and national programs, slashing research grants and data resources, and creating new barriers to behavioral health care.
Philanthropic foundations aim to do what they can to address the shortfall. The numbers, however, just don't add up.
"Some big foundations and philanthropies have said they're going to increase what they give out in the next 4 years, but they'll never be able to fill the gap," said Morgan F. McDonald, MD, national director of population health at the Milbank Memorial Fund in New York City, which works with states on health policy. "Even if every one of them were to spend down their endowments, they still couldn't."
Given the financial limitations, some foundations are taking a different tack. While looking for ways to join forces with fellow nonprofits, they are providing emergency grants to bridge funding in the short term to keep research from grinding to a halt.
Budget Cuts Reach Far and Wide
Mental health research certainly didn't escape the extensive grant cancellations at the National Institutes of Health and the National Science Foundation.
"It's already affecting our ability to stay on the cutting edge of research, best practices, and treatment approaches," said Zainab Okolo, EdD, senior vice president of policy, advocacy, and government relations at The Jed Foundation in New York City, which focuses on the emotional health of teens and young adults.
The upheaval is evident in an array of government agencies. The Health Resources and Services Administration, which last year awarded $12 billion in grants to community health centers and addiction treatment services, has seen > one-fourth of its staff eliminated. The Substance Abuse and Mental Health Services Administration has lost more than a third of its staff as federal cuts took a $1 billion bite out of its operating budget. The Education Department has halted $1 billion in grants used to hire mental health workers in school districts nationwide.
"We're very, very concerned about cuts to behavioral health systems," said Alonzo Plough, PhD, chief science officer at the Robert Wood Johnson Foundation in Princeton, New Jersey. "Doctors and nurses working in safety-net clinics are seeing tremendous reductions."
All in all, the new tax and spending law means $1 trillion in cuts to health care programs including Medicaid -- the nation's largest payer for mental health services -- Medicare, and Affordable Care Act insurance. An estimated 10 million Americans are expected to lose their health coverage as a result.
"When accessibility to care goes down, there's a chance that more people will die by suicide," said Jill Harkavy-Friedman, PhD, senior vice president of research at the American Foundation for Suicide Prevention. "But it also means people will come into care later in the course of their difficulties. Health professionals will be dealing with worse problems."
Foundations Take Emergency Measures
Even if private dollars can't replace what's been lost, philanthropic and medical foundations are stepping up.
We're seeing a lot of foundations and funders that are shifting their funding," said Alyson Niemann, CEO of Mindful Philanthropy, an organization that works with > 1000 private funders to marshal resources for mental health. This year, in response to federal cuts, "many increased funding to health and well-being, doubling or even tripling it," Niemann noted.
"They're making a great deal of effort to respond with emergency funds, really getting in the trenches and being good partners to their grantees," she said. "We've seen them asking deliberate questions, thinking about where their funding can have the most impact."
The American Psychological Foundation (APF), a longtime supporter of research and innovation, is addressing the current crisis with 2 initiatives, Michelle Quist Ryder, PhD, the organization's CEO, explained in an email. The first is APF Director Action, which funds innovative interventions at the community level. The second, Direct Action Crisis Funding Grants, will help continue research that is at risk of stalling because of budget cuts.
"Studies that are 'paused' or lose funding often cannot simply pick back up where they left off. Having to halt progress on a project can invalidate the work already completed," Ryder wrote. "These Direct Action Crisis Grants help bridge funding gaps and keep research viable."
At the same time, collaboration between foundations is becoming more widespread as they seek to maximize their impact. Philanthropic organizations are sharing ideas and best practices as well as pooling fundings.
"The goal of philanthropy is to help people," Harkavy-Friedman said. "There's strength in numbers and more dollars in numbers."
Some See Hope in Raised Voices
Despite the emergency scrambling, many of those in the trenches remain surprisingly optimistic. Some point out that the current turmoil has put a helpful spotlight on behavioral health care. Practitioners, meanwhile, have an essential role to play.
"There's a reason that things were the way they were: People advocated for many years to get where we've gotten," Harkavy-Friedman said, citing veterans' mental health care, the national violent death reporting system, and 988 as examples. "We have to raise our voices louder -- professionals in particular, because they know the impact a person in the general public many not fully grasp."
As a growing numbers of health professionals call attention to the damage wrought by deep cuts in the federal budget, foundation executives see an opportunity.
"In the mental health field, there's a deficit in the narrative, where there's a lot of focus on crisis. What we're hoping to do is shift the narrative toward 'How do we flourish together?'" Niemann said. "Sometimes deficits are where the most incredible innovations appear."
Debbie Koenig is a health writer whose work has been published by WebMD, The New York Times, and The Washington Post.
A version of this article first appeared on Medscape.com.
It's hardly news that the United States is experiencing a mental health crisis -- the CDC says as much. But experts in the field say that the current administration has severely compounded the problem by eliminating agency funding and national programs, slashing research grants and data resources, and creating new barriers to behavioral health care.
Philanthropic foundations aim to do what they can to address the shortfall. The numbers, however, just don't add up.
"Some big foundations and philanthropies have said they're going to increase what they give out in the next 4 years, but they'll never be able to fill the gap," said Morgan F. McDonald, MD, national director of population health at the Milbank Memorial Fund in New York City, which works with states on health policy. "Even if every one of them were to spend down their endowments, they still couldn't."
Given the financial limitations, some foundations are taking a different tack. While looking for ways to join forces with fellow nonprofits, they are providing emergency grants to bridge funding in the short term to keep research from grinding to a halt.
Budget Cuts Reach Far and Wide
Mental health research certainly didn't escape the extensive grant cancellations at the National Institutes of Health and the National Science Foundation.
"It's already affecting our ability to stay on the cutting edge of research, best practices, and treatment approaches," said Zainab Okolo, EdD, senior vice president of policy, advocacy, and government relations at The Jed Foundation in New York City, which focuses on the emotional health of teens and young adults.
The upheaval is evident in an array of government agencies. The Health Resources and Services Administration, which last year awarded $12 billion in grants to community health centers and addiction treatment services, has seen > one-fourth of its staff eliminated. The Substance Abuse and Mental Health Services Administration has lost more than a third of its staff as federal cuts took a $1 billion bite out of its operating budget. The Education Department has halted $1 billion in grants used to hire mental health workers in school districts nationwide.
"We're very, very concerned about cuts to behavioral health systems," said Alonzo Plough, PhD, chief science officer at the Robert Wood Johnson Foundation in Princeton, New Jersey. "Doctors and nurses working in safety-net clinics are seeing tremendous reductions."
All in all, the new tax and spending law means $1 trillion in cuts to health care programs including Medicaid -- the nation's largest payer for mental health services -- Medicare, and Affordable Care Act insurance. An estimated 10 million Americans are expected to lose their health coverage as a result.
"When accessibility to care goes down, there's a chance that more people will die by suicide," said Jill Harkavy-Friedman, PhD, senior vice president of research at the American Foundation for Suicide Prevention. "But it also means people will come into care later in the course of their difficulties. Health professionals will be dealing with worse problems."
Foundations Take Emergency Measures
Even if private dollars can't replace what's been lost, philanthropic and medical foundations are stepping up.
We're seeing a lot of foundations and funders that are shifting their funding," said Alyson Niemann, CEO of Mindful Philanthropy, an organization that works with > 1000 private funders to marshal resources for mental health. This year, in response to federal cuts, "many increased funding to health and well-being, doubling or even tripling it," Niemann noted.
"They're making a great deal of effort to respond with emergency funds, really getting in the trenches and being good partners to their grantees," she said. "We've seen them asking deliberate questions, thinking about where their funding can have the most impact."
The American Psychological Foundation (APF), a longtime supporter of research and innovation, is addressing the current crisis with 2 initiatives, Michelle Quist Ryder, PhD, the organization's CEO, explained in an email. The first is APF Director Action, which funds innovative interventions at the community level. The second, Direct Action Crisis Funding Grants, will help continue research that is at risk of stalling because of budget cuts.
"Studies that are 'paused' or lose funding often cannot simply pick back up where they left off. Having to halt progress on a project can invalidate the work already completed," Ryder wrote. "These Direct Action Crisis Grants help bridge funding gaps and keep research viable."
At the same time, collaboration between foundations is becoming more widespread as they seek to maximize their impact. Philanthropic organizations are sharing ideas and best practices as well as pooling fundings.
"The goal of philanthropy is to help people," Harkavy-Friedman said. "There's strength in numbers and more dollars in numbers."
Some See Hope in Raised Voices
Despite the emergency scrambling, many of those in the trenches remain surprisingly optimistic. Some point out that the current turmoil has put a helpful spotlight on behavioral health care. Practitioners, meanwhile, have an essential role to play.
"There's a reason that things were the way they were: People advocated for many years to get where we've gotten," Harkavy-Friedman said, citing veterans' mental health care, the national violent death reporting system, and 988 as examples. "We have to raise our voices louder -- professionals in particular, because they know the impact a person in the general public many not fully grasp."
As a growing numbers of health professionals call attention to the damage wrought by deep cuts in the federal budget, foundation executives see an opportunity.
"In the mental health field, there's a deficit in the narrative, where there's a lot of focus on crisis. What we're hoping to do is shift the narrative toward 'How do we flourish together?'" Niemann said. "Sometimes deficits are where the most incredible innovations appear."
Debbie Koenig is a health writer whose work has been published by WebMD, The New York Times, and The Washington Post.
A version of this article first appeared on Medscape.com.
It's hardly news that the United States is experiencing a mental health crisis -- the CDC says as much. But experts in the field say that the current administration has severely compounded the problem by eliminating agency funding and national programs, slashing research grants and data resources, and creating new barriers to behavioral health care.
Philanthropic foundations aim to do what they can to address the shortfall. The numbers, however, just don't add up.
"Some big foundations and philanthropies have said they're going to increase what they give out in the next 4 years, but they'll never be able to fill the gap," said Morgan F. McDonald, MD, national director of population health at the Milbank Memorial Fund in New York City, which works with states on health policy. "Even if every one of them were to spend down their endowments, they still couldn't."
Given the financial limitations, some foundations are taking a different tack. While looking for ways to join forces with fellow nonprofits, they are providing emergency grants to bridge funding in the short term to keep research from grinding to a halt.
Budget Cuts Reach Far and Wide
Mental health research certainly didn't escape the extensive grant cancellations at the National Institutes of Health and the National Science Foundation.
"It's already affecting our ability to stay on the cutting edge of research, best practices, and treatment approaches," said Zainab Okolo, EdD, senior vice president of policy, advocacy, and government relations at The Jed Foundation in New York City, which focuses on the emotional health of teens and young adults.
The upheaval is evident in an array of government agencies. The Health Resources and Services Administration, which last year awarded $12 billion in grants to community health centers and addiction treatment services, has seen > one-fourth of its staff eliminated. The Substance Abuse and Mental Health Services Administration has lost more than a third of its staff as federal cuts took a $1 billion bite out of its operating budget. The Education Department has halted $1 billion in grants used to hire mental health workers in school districts nationwide.
"We're very, very concerned about cuts to behavioral health systems," said Alonzo Plough, PhD, chief science officer at the Robert Wood Johnson Foundation in Princeton, New Jersey. "Doctors and nurses working in safety-net clinics are seeing tremendous reductions."
All in all, the new tax and spending law means $1 trillion in cuts to health care programs including Medicaid -- the nation's largest payer for mental health services -- Medicare, and Affordable Care Act insurance. An estimated 10 million Americans are expected to lose their health coverage as a result.
"When accessibility to care goes down, there's a chance that more people will die by suicide," said Jill Harkavy-Friedman, PhD, senior vice president of research at the American Foundation for Suicide Prevention. "But it also means people will come into care later in the course of their difficulties. Health professionals will be dealing with worse problems."
Foundations Take Emergency Measures
Even if private dollars can't replace what's been lost, philanthropic and medical foundations are stepping up.
We're seeing a lot of foundations and funders that are shifting their funding," said Alyson Niemann, CEO of Mindful Philanthropy, an organization that works with > 1000 private funders to marshal resources for mental health. This year, in response to federal cuts, "many increased funding to health and well-being, doubling or even tripling it," Niemann noted.
"They're making a great deal of effort to respond with emergency funds, really getting in the trenches and being good partners to their grantees," she said. "We've seen them asking deliberate questions, thinking about where their funding can have the most impact."
The American Psychological Foundation (APF), a longtime supporter of research and innovation, is addressing the current crisis with 2 initiatives, Michelle Quist Ryder, PhD, the organization's CEO, explained in an email. The first is APF Director Action, which funds innovative interventions at the community level. The second, Direct Action Crisis Funding Grants, will help continue research that is at risk of stalling because of budget cuts.
"Studies that are 'paused' or lose funding often cannot simply pick back up where they left off. Having to halt progress on a project can invalidate the work already completed," Ryder wrote. "These Direct Action Crisis Grants help bridge funding gaps and keep research viable."
At the same time, collaboration between foundations is becoming more widespread as they seek to maximize their impact. Philanthropic organizations are sharing ideas and best practices as well as pooling fundings.
"The goal of philanthropy is to help people," Harkavy-Friedman said. "There's strength in numbers and more dollars in numbers."
Some See Hope in Raised Voices
Despite the emergency scrambling, many of those in the trenches remain surprisingly optimistic. Some point out that the current turmoil has put a helpful spotlight on behavioral health care. Practitioners, meanwhile, have an essential role to play.
"There's a reason that things were the way they were: People advocated for many years to get where we've gotten," Harkavy-Friedman said, citing veterans' mental health care, the national violent death reporting system, and 988 as examples. "We have to raise our voices louder -- professionals in particular, because they know the impact a person in the general public many not fully grasp."
As a growing numbers of health professionals call attention to the damage wrought by deep cuts in the federal budget, foundation executives see an opportunity.
"In the mental health field, there's a deficit in the narrative, where there's a lot of focus on crisis. What we're hoping to do is shift the narrative toward 'How do we flourish together?'" Niemann said. "Sometimes deficits are where the most incredible innovations appear."
Debbie Koenig is a health writer whose work has been published by WebMD, The New York Times, and The Washington Post.
A version of this article first appeared on Medscape.com.
As Federal Cuts Deepen Mental Health Crisis, Philanthropy Scrambles to Fill the Gap
As Federal Cuts Deepen Mental Health Crisis, Philanthropy Scrambles to Fill the Gap
Taking Therapy Home With Mobile Mental Health Apps
For Kelly, a retired Navy operations specialist, coping with depression and anxiety hindered her ability to enjoy everyday life. Then she elected to enter therapy, a decision she calls “transformative.”
“When I started doing therapy, it was like releasing the toxins, releasing the buildup of the fear or the rage or the overwhelming feelings of shame,” she says. “We can’t just hold on to it. Just telling the truth, it helps me every single day. It is so worth it.”
Kurt, an Army veteran, tried to power through his anxiety, depression, and survivor guilt. He didn’t have much faith in mental health therapy, thinking no one could relate to him. He was surprised, though, once he started treatment, how much his life improved. He now encourages other veterans to face their own mental health challenges, be it through virtual/mental health apps or in-person care.
“From getting help, every day of my life is better,” he says, “and I couldn’t be more grateful for it.”
Stories from Kelly and Kurt are 2 of 7 the US Department of Veterans Affairs (VA) highlighted during National Recovery Month, outlining how their lives were forever changed with the support of mental health care.
But for every Kelly and Kurt, there are thousands of individuals reluctant to seek mental health care. A analysis of 2019-2020 data from the National Health and Resilience in Veterans Study found that 924 (26%) of 4069 veterans met criteria for ≥ 1 psychological disorders, but only 12% reported engagement in mental health care. The researchers considered the role of protective psychosocial characteristics, such as grit (ie, “trait perseverance that extends to one’s decision or commitment to address mental health needs on one’s own; dispositional optimism; and purpose in life”). Veterans who reported mental dysfunction but scored highly on grit were less likely to be engaged in treatment. This pattern suggests higher levels of grit may reduce the likelihood of seeking treatment, “even in the presence of clinically meaningful distress.”
A 2004 study found only 23% to 40% of service members who screened positive for a mental disorder sought care. They often believed they would be seen as weak, or their unit leadership might treat them differently, and unit members would have less confidence in them.
Given that military members and veterans are at increased risk of posttraumatic stress disorder (PTSD) in addition to mood, anxiety, and substance use disorders, any alternatives that increase their access to support and services are crucial. For those who aren’t disposed to office visits and group therapy, the answer may lie in mobile apps.
In a recent randomized controlled trial, 201 veterans who screened positive for PTSD and alcohol use disorder were divided into 2 groups: a mobile mindfulness-based intervention group enhanced with brief alcohol intervention content (Mind Guide), and an active stress management program group. Mind Guide engagement was excellent, according to the study, with averages of > 31 logins and 5 hours of app use. At 16 weeks, the Mind Guide group showed significant reductions in PTSD symptoms (no differences emerged for alcohol use frequency). Mind Guide may be a valuable adjunct to more intensive in-person PTSD treatment by facilitating interest in services, integration into care, and/or sustainment of posttreatment improvements. The VA currently offers 16 apps, including MHA for Veterans, an app designed for patients to complete mental health assessments after their clinician assigned them. Other apps address a variety of issues, such as anger management, insomnia, chronic pain, and PTSD.
Two apps were created with an eye toward specific communities. One, Veterans Wellness Path, was designed for American Indians and Alaska Natives with input from those veterans, their family members, and health care practitioners. It supports the transition from military service to home and encourages balance and connection with self, family, community, and environment. Similarly, WellWithin Coach was designed by the VA National Center for PTSD with input from women veterans and subject matter experts in women’s mental health.
Whatever form it takes—in-person or virtual—finding support that works can make all the difference for veterans. Kelly founded and serves as the executive director of Acta Non Verba: Youth Urban Farm Project, an organization that brings together > 3000 low-income youth and families annually to learn about urban farming, aiming to fill a gap in an area known as a food desert: “We do have the power and the right to wake up the next day and try to do something different,” she said.
For Kelly, a retired Navy operations specialist, coping with depression and anxiety hindered her ability to enjoy everyday life. Then she elected to enter therapy, a decision she calls “transformative.”
“When I started doing therapy, it was like releasing the toxins, releasing the buildup of the fear or the rage or the overwhelming feelings of shame,” she says. “We can’t just hold on to it. Just telling the truth, it helps me every single day. It is so worth it.”
Kurt, an Army veteran, tried to power through his anxiety, depression, and survivor guilt. He didn’t have much faith in mental health therapy, thinking no one could relate to him. He was surprised, though, once he started treatment, how much his life improved. He now encourages other veterans to face their own mental health challenges, be it through virtual/mental health apps or in-person care.
“From getting help, every day of my life is better,” he says, “and I couldn’t be more grateful for it.”
Stories from Kelly and Kurt are 2 of 7 the US Department of Veterans Affairs (VA) highlighted during National Recovery Month, outlining how their lives were forever changed with the support of mental health care.
But for every Kelly and Kurt, there are thousands of individuals reluctant to seek mental health care. A analysis of 2019-2020 data from the National Health and Resilience in Veterans Study found that 924 (26%) of 4069 veterans met criteria for ≥ 1 psychological disorders, but only 12% reported engagement in mental health care. The researchers considered the role of protective psychosocial characteristics, such as grit (ie, “trait perseverance that extends to one’s decision or commitment to address mental health needs on one’s own; dispositional optimism; and purpose in life”). Veterans who reported mental dysfunction but scored highly on grit were less likely to be engaged in treatment. This pattern suggests higher levels of grit may reduce the likelihood of seeking treatment, “even in the presence of clinically meaningful distress.”
A 2004 study found only 23% to 40% of service members who screened positive for a mental disorder sought care. They often believed they would be seen as weak, or their unit leadership might treat them differently, and unit members would have less confidence in them.
Given that military members and veterans are at increased risk of posttraumatic stress disorder (PTSD) in addition to mood, anxiety, and substance use disorders, any alternatives that increase their access to support and services are crucial. For those who aren’t disposed to office visits and group therapy, the answer may lie in mobile apps.
In a recent randomized controlled trial, 201 veterans who screened positive for PTSD and alcohol use disorder were divided into 2 groups: a mobile mindfulness-based intervention group enhanced with brief alcohol intervention content (Mind Guide), and an active stress management program group. Mind Guide engagement was excellent, according to the study, with averages of > 31 logins and 5 hours of app use. At 16 weeks, the Mind Guide group showed significant reductions in PTSD symptoms (no differences emerged for alcohol use frequency). Mind Guide may be a valuable adjunct to more intensive in-person PTSD treatment by facilitating interest in services, integration into care, and/or sustainment of posttreatment improvements. The VA currently offers 16 apps, including MHA for Veterans, an app designed for patients to complete mental health assessments after their clinician assigned them. Other apps address a variety of issues, such as anger management, insomnia, chronic pain, and PTSD.
Two apps were created with an eye toward specific communities. One, Veterans Wellness Path, was designed for American Indians and Alaska Natives with input from those veterans, their family members, and health care practitioners. It supports the transition from military service to home and encourages balance and connection with self, family, community, and environment. Similarly, WellWithin Coach was designed by the VA National Center for PTSD with input from women veterans and subject matter experts in women’s mental health.
Whatever form it takes—in-person or virtual—finding support that works can make all the difference for veterans. Kelly founded and serves as the executive director of Acta Non Verba: Youth Urban Farm Project, an organization that brings together > 3000 low-income youth and families annually to learn about urban farming, aiming to fill a gap in an area known as a food desert: “We do have the power and the right to wake up the next day and try to do something different,” she said.
For Kelly, a retired Navy operations specialist, coping with depression and anxiety hindered her ability to enjoy everyday life. Then she elected to enter therapy, a decision she calls “transformative.”
“When I started doing therapy, it was like releasing the toxins, releasing the buildup of the fear or the rage or the overwhelming feelings of shame,” she says. “We can’t just hold on to it. Just telling the truth, it helps me every single day. It is so worth it.”
Kurt, an Army veteran, tried to power through his anxiety, depression, and survivor guilt. He didn’t have much faith in mental health therapy, thinking no one could relate to him. He was surprised, though, once he started treatment, how much his life improved. He now encourages other veterans to face their own mental health challenges, be it through virtual/mental health apps or in-person care.
“From getting help, every day of my life is better,” he says, “and I couldn’t be more grateful for it.”
Stories from Kelly and Kurt are 2 of 7 the US Department of Veterans Affairs (VA) highlighted during National Recovery Month, outlining how their lives were forever changed with the support of mental health care.
But for every Kelly and Kurt, there are thousands of individuals reluctant to seek mental health care. A analysis of 2019-2020 data from the National Health and Resilience in Veterans Study found that 924 (26%) of 4069 veterans met criteria for ≥ 1 psychological disorders, but only 12% reported engagement in mental health care. The researchers considered the role of protective psychosocial characteristics, such as grit (ie, “trait perseverance that extends to one’s decision or commitment to address mental health needs on one’s own; dispositional optimism; and purpose in life”). Veterans who reported mental dysfunction but scored highly on grit were less likely to be engaged in treatment. This pattern suggests higher levels of grit may reduce the likelihood of seeking treatment, “even in the presence of clinically meaningful distress.”
A 2004 study found only 23% to 40% of service members who screened positive for a mental disorder sought care. They often believed they would be seen as weak, or their unit leadership might treat them differently, and unit members would have less confidence in them.
Given that military members and veterans are at increased risk of posttraumatic stress disorder (PTSD) in addition to mood, anxiety, and substance use disorders, any alternatives that increase their access to support and services are crucial. For those who aren’t disposed to office visits and group therapy, the answer may lie in mobile apps.
In a recent randomized controlled trial, 201 veterans who screened positive for PTSD and alcohol use disorder were divided into 2 groups: a mobile mindfulness-based intervention group enhanced with brief alcohol intervention content (Mind Guide), and an active stress management program group. Mind Guide engagement was excellent, according to the study, with averages of > 31 logins and 5 hours of app use. At 16 weeks, the Mind Guide group showed significant reductions in PTSD symptoms (no differences emerged for alcohol use frequency). Mind Guide may be a valuable adjunct to more intensive in-person PTSD treatment by facilitating interest in services, integration into care, and/or sustainment of posttreatment improvements. The VA currently offers 16 apps, including MHA for Veterans, an app designed for patients to complete mental health assessments after their clinician assigned them. Other apps address a variety of issues, such as anger management, insomnia, chronic pain, and PTSD.
Two apps were created with an eye toward specific communities. One, Veterans Wellness Path, was designed for American Indians and Alaska Natives with input from those veterans, their family members, and health care practitioners. It supports the transition from military service to home and encourages balance and connection with self, family, community, and environment. Similarly, WellWithin Coach was designed by the VA National Center for PTSD with input from women veterans and subject matter experts in women’s mental health.
Whatever form it takes—in-person or virtual—finding support that works can make all the difference for veterans. Kelly founded and serves as the executive director of Acta Non Verba: Youth Urban Farm Project, an organization that brings together > 3000 low-income youth and families annually to learn about urban farming, aiming to fill a gap in an area known as a food desert: “We do have the power and the right to wake up the next day and try to do something different,” she said.
Durvalumab Plus FLOT Ups Survival in Early Upper-GI Cancer
BERLIN — , according to findings presented at the 2025 annual meeting of the European Society for Medical Oncology (ESMO).
Experts said the survival benefit further supports perioperative durvalumab plus FLOT (fluorouracil, leucovorin, oxaliplatin, and docetaxel) as the new standard of care for patients with localized gastric or gastroesophageal adenocarcinoma. Earlier results from the phase 3 MATTERHORN trial, reported at the American Society of Clinical Oncology meeting (ASCO) in June, showed that the addition of durvalumab improved event-free survival compared with FLOT alone.
The findings presented at ESMO show that at 36 months, overall survival was 68.6% among patients who received durvalumab + FLOT vs 61.9% among those given FLOT plus a placebo. After a median of 43 months, the survival advantage in the durvalumab group was statistically significant (hazard ratio [HR], 0.78; 95% CI, 0.63-0.96; P = .021) and “more importantly, clinically meaningful,” said lead investigator Josep Tabernero, MD, PhD, of Vall d’Hebron University Hospital in Barcelona.
The results “strongly support the use of perioperative durvalumab plus chemotherapy with FLOT as a new global standard of care for patients with localized gastric and gastroesophageal adenocarcinoma,” Tabernero said.
Speaking as discussant for the session, Sylvie Lorenzen, MD, PhD, Technische Universität München in Munich, Germany, was enthusiastic that the previously reported trends in MATTERHORN held strong.
“The shape of the curves presented at ASCO was already very positive,” she said. “And now, with a longer follow-up, more events, and a higher overall survival maturity, they reach statistical significance. It looks like the magnitude of the effect increases with longer follow-up, and this is important for our patients.”
The trial randomly assigned 948 patients with resectable gastric or gastroesophageal adenocarcinoma to receive either durvalumab (1500 mg) or placebo every 4 weeks, plus FLOT for 2 cycles before surgery and then again after, followed by durvalumab or placebo every 4 weeks for 10 cycles.
Patients were stratified according to lymph node status, as well as PD-L1 expression (≥ 1% or < 1%, according to the Tumor Area Positivity score.)
The improvement in overall survival with durvalumab was seen regardless of PD-L1 expression, Tabernero said, with the same hazard ratios (0.79) in both the positive and negative subgroups.
However, there was no clear overall survival benefit in certain other subgroups, including women (n = 266; HR, 0.91), those with node-negative disease (n = 277; HR, 1.01), and those with diffuse histology (n = 249; HR, 0.98).
Lorenzen said that clinicians should “pay attention” to those patient subgroups, as they seem to benefit less from the addition of durvalumab. However, she cautioned that the findings were based on small patient numbers and the confidence intervals were wide.
Tabernero also reported additional data on event-free survival (EFS). Overall, the durvalumab/FLOT combination improved EFS among patients with any degree of pathological response and irrespective of lymph node status at surgery.
Regarding nodal staging, which was done in 800 patients, the percentage who achieved negative nodal status was higher in the durvalumab group (58.2%) vs the placebo group (44.8%). However, the improvement in EFS with durvalumab was comparable for node-negative (HR, 0.74) and node-positive (HR, 0.77) patients.
Lorenzen said that overall, the results provide a solid answer to the question, “Is it time to change practice?”
“I think MATTERHORN gives us the largest dataset and answers this question satisfactorily,” she said. Given that overall survival improved regardless of PD-L1 expression, she added, the combination of durvalumab and FLOT should be offered to “all our patient subgroups.”
The study was funded by AstraZeneca. Tabernero made numerous disclosures, including relationships with AstraZeneca, Boehringer Ingelheim, Chugai, and Daichii Sankyo. Lorenzen disclosed financial interests in or serving as an invited speaker for Servier, Lilly, MSD, and BMS.
A version of this article appeared on Medscape.com .
BERLIN — , according to findings presented at the 2025 annual meeting of the European Society for Medical Oncology (ESMO).
Experts said the survival benefit further supports perioperative durvalumab plus FLOT (fluorouracil, leucovorin, oxaliplatin, and docetaxel) as the new standard of care for patients with localized gastric or gastroesophageal adenocarcinoma. Earlier results from the phase 3 MATTERHORN trial, reported at the American Society of Clinical Oncology meeting (ASCO) in June, showed that the addition of durvalumab improved event-free survival compared with FLOT alone.
The findings presented at ESMO show that at 36 months, overall survival was 68.6% among patients who received durvalumab + FLOT vs 61.9% among those given FLOT plus a placebo. After a median of 43 months, the survival advantage in the durvalumab group was statistically significant (hazard ratio [HR], 0.78; 95% CI, 0.63-0.96; P = .021) and “more importantly, clinically meaningful,” said lead investigator Josep Tabernero, MD, PhD, of Vall d’Hebron University Hospital in Barcelona.
The results “strongly support the use of perioperative durvalumab plus chemotherapy with FLOT as a new global standard of care for patients with localized gastric and gastroesophageal adenocarcinoma,” Tabernero said.
Speaking as discussant for the session, Sylvie Lorenzen, MD, PhD, Technische Universität München in Munich, Germany, was enthusiastic that the previously reported trends in MATTERHORN held strong.
“The shape of the curves presented at ASCO was already very positive,” she said. “And now, with a longer follow-up, more events, and a higher overall survival maturity, they reach statistical significance. It looks like the magnitude of the effect increases with longer follow-up, and this is important for our patients.”
The trial randomly assigned 948 patients with resectable gastric or gastroesophageal adenocarcinoma to receive either durvalumab (1500 mg) or placebo every 4 weeks, plus FLOT for 2 cycles before surgery and then again after, followed by durvalumab or placebo every 4 weeks for 10 cycles.
Patients were stratified according to lymph node status, as well as PD-L1 expression (≥ 1% or < 1%, according to the Tumor Area Positivity score.)
The improvement in overall survival with durvalumab was seen regardless of PD-L1 expression, Tabernero said, with the same hazard ratios (0.79) in both the positive and negative subgroups.
However, there was no clear overall survival benefit in certain other subgroups, including women (n = 266; HR, 0.91), those with node-negative disease (n = 277; HR, 1.01), and those with diffuse histology (n = 249; HR, 0.98).
Lorenzen said that clinicians should “pay attention” to those patient subgroups, as they seem to benefit less from the addition of durvalumab. However, she cautioned that the findings were based on small patient numbers and the confidence intervals were wide.
Tabernero also reported additional data on event-free survival (EFS). Overall, the durvalumab/FLOT combination improved EFS among patients with any degree of pathological response and irrespective of lymph node status at surgery.
Regarding nodal staging, which was done in 800 patients, the percentage who achieved negative nodal status was higher in the durvalumab group (58.2%) vs the placebo group (44.8%). However, the improvement in EFS with durvalumab was comparable for node-negative (HR, 0.74) and node-positive (HR, 0.77) patients.
Lorenzen said that overall, the results provide a solid answer to the question, “Is it time to change practice?”
“I think MATTERHORN gives us the largest dataset and answers this question satisfactorily,” she said. Given that overall survival improved regardless of PD-L1 expression, she added, the combination of durvalumab and FLOT should be offered to “all our patient subgroups.”
The study was funded by AstraZeneca. Tabernero made numerous disclosures, including relationships with AstraZeneca, Boehringer Ingelheim, Chugai, and Daichii Sankyo. Lorenzen disclosed financial interests in or serving as an invited speaker for Servier, Lilly, MSD, and BMS.
A version of this article appeared on Medscape.com .
BERLIN — , according to findings presented at the 2025 annual meeting of the European Society for Medical Oncology (ESMO).
Experts said the survival benefit further supports perioperative durvalumab plus FLOT (fluorouracil, leucovorin, oxaliplatin, and docetaxel) as the new standard of care for patients with localized gastric or gastroesophageal adenocarcinoma. Earlier results from the phase 3 MATTERHORN trial, reported at the American Society of Clinical Oncology meeting (ASCO) in June, showed that the addition of durvalumab improved event-free survival compared with FLOT alone.
The findings presented at ESMO show that at 36 months, overall survival was 68.6% among patients who received durvalumab + FLOT vs 61.9% among those given FLOT plus a placebo. After a median of 43 months, the survival advantage in the durvalumab group was statistically significant (hazard ratio [HR], 0.78; 95% CI, 0.63-0.96; P = .021) and “more importantly, clinically meaningful,” said lead investigator Josep Tabernero, MD, PhD, of Vall d’Hebron University Hospital in Barcelona.
The results “strongly support the use of perioperative durvalumab plus chemotherapy with FLOT as a new global standard of care for patients with localized gastric and gastroesophageal adenocarcinoma,” Tabernero said.
Speaking as discussant for the session, Sylvie Lorenzen, MD, PhD, Technische Universität München in Munich, Germany, was enthusiastic that the previously reported trends in MATTERHORN held strong.
“The shape of the curves presented at ASCO was already very positive,” she said. “And now, with a longer follow-up, more events, and a higher overall survival maturity, they reach statistical significance. It looks like the magnitude of the effect increases with longer follow-up, and this is important for our patients.”
The trial randomly assigned 948 patients with resectable gastric or gastroesophageal adenocarcinoma to receive either durvalumab (1500 mg) or placebo every 4 weeks, plus FLOT for 2 cycles before surgery and then again after, followed by durvalumab or placebo every 4 weeks for 10 cycles.
Patients were stratified according to lymph node status, as well as PD-L1 expression (≥ 1% or < 1%, according to the Tumor Area Positivity score.)
The improvement in overall survival with durvalumab was seen regardless of PD-L1 expression, Tabernero said, with the same hazard ratios (0.79) in both the positive and negative subgroups.
However, there was no clear overall survival benefit in certain other subgroups, including women (n = 266; HR, 0.91), those with node-negative disease (n = 277; HR, 1.01), and those with diffuse histology (n = 249; HR, 0.98).
Lorenzen said that clinicians should “pay attention” to those patient subgroups, as they seem to benefit less from the addition of durvalumab. However, she cautioned that the findings were based on small patient numbers and the confidence intervals were wide.
Tabernero also reported additional data on event-free survival (EFS). Overall, the durvalumab/FLOT combination improved EFS among patients with any degree of pathological response and irrespective of lymph node status at surgery.
Regarding nodal staging, which was done in 800 patients, the percentage who achieved negative nodal status was higher in the durvalumab group (58.2%) vs the placebo group (44.8%). However, the improvement in EFS with durvalumab was comparable for node-negative (HR, 0.74) and node-positive (HR, 0.77) patients.
Lorenzen said that overall, the results provide a solid answer to the question, “Is it time to change practice?”
“I think MATTERHORN gives us the largest dataset and answers this question satisfactorily,” she said. Given that overall survival improved regardless of PD-L1 expression, she added, the combination of durvalumab and FLOT should be offered to “all our patient subgroups.”
The study was funded by AstraZeneca. Tabernero made numerous disclosures, including relationships with AstraZeneca, Boehringer Ingelheim, Chugai, and Daichii Sankyo. Lorenzen disclosed financial interests in or serving as an invited speaker for Servier, Lilly, MSD, and BMS.
A version of this article appeared on Medscape.com .