User login
Surgical Pearls and Wellness Tips From the American Academy of Dermatology Annual Meeting
Attendees of the 2024 American Academy of Dermatology annual meeting in San Diego, California, were eager to delve into the latest trends and advancements in dermatology and dermatologic surgery. This article provides a few key takeaways for residents from a range of engaging sessions, with an emphasis on procedural dermatology and physician health and well-being.
Practical Applications of Surgical Enhancements
In an informative session dedicated to dermatologic surgeons and their patients, “Simple Tricks and Practical Tips to Optimize the Surgical Experience for You and Your Patients,” attendees learned practical tips for enhancing the surgical experience. The discussion spanned various aspects of surgery, from managing preoperative anxiety with anxiolytics such as midazolam to the strategic use of skin hooks for delicate tissue manipulation. Midazolam is fast acting and its use is tailored to patient factors such as weight, hepatic function, and prior use. An innovative anxiety management algorithm combining “talkesethesia” with other methods such as anodynes and benzodiazepines underscored the importance of a calm patient in successful surgical outcomes. Talkesthesia involves engaging patients in soothing and distracting conversation throughout the procedure. This technique can include discussing nonmedical topics of interest with the patient—such as their hobbies, family, or favorite movies—to divert their attention from the surgical process and reduce anxiety. By creating a friendly and reassuring atmosphere, talkesthesia helps to establish trust between the patient and the medical team, ultimately contributing to a more relaxed and cooperative patient.1
The utility of skin hooks also was discussed, with an emphasis on their role in ensuring gentle tissue handling. The modified buried vertical mattress technique was discussed for its added benefits in wound approximation and strength. Emphasis was placed on the importance of maintaining a clear surgical field by electrocautery to ensure optimal visibility.
Focusing on the treatment of skin cancer, curettage alone was touted as a viable alternative to electrodesiccation and curettage, especially in reducing postoperative hypopigmentation while maintaining high cure rates. This method was shown to be effective in treating basal cell carcinoma and well-differentiated squamous cell carcinoma.2,3
Suturing techniques such as pulley, purse-string, and buried sutures offer efficiencies in time, cost, and improved healing in high-tension areas. These methods can contribute to postsurgical aesthetic and functional outcomes. Additionally, Dr. Desiree Ratner shared her tips for painless local anesthesia techniques, emphasizing the importance of patient comfort through methods such as slow injection and buffering of lidocaine. The next time you give a local anesthetic, try this technique to minimize pain: using a 30-gauge needle, hold the syringe with the bevel up, insert only the bevel into the skin (needle tip goes into the papillary dermis), and numb superficially around the periphery using as little volume as possible. Keep pressure slow and steady without moving the needle, then insert the needle only in previously anesthetized areas, numbing deeply only after the entire periphery has been anesthetized.
The session concluded with the recommendation to provide patients with a goody bag containing postoperative supplies. This thoughtful gesture not only enhances patient satisfaction but also addresses the practical aspect of postsurgery care, offering an inexpensive yet impactful way to ensure patients have the necessary supplies for their recovery.
Take-Home Point—This session distilled essential surgical enhancements into practical applications, emphasizing the importance of anxiety management, delicate tissue handling, innovative suturing techniques, and thoughtful patient care postsurgery. The overarching message highlighted the synergy between technical skill and patient-centric approaches in optimizing surgical outcomes, underscoring the significance of attention to detail in every aspect of patient care, from preoperative preparation to postoperative recovery.
Optimizing Safety and Ergonomics in Surgical Practices
Understanding the dynamics of surgical plume is crucial to safety in the operating room. The carcinogenic risk associated with surgical smoke is not trivial: exposure to the plume generated by monopolar electrocautery in a single day can be equivalent to smoking approximately 30 cigarettes, and a surgeon’s lifetime cancer risk from polycyclic aromatic hydrocarbons exposure is alarmingly high.4 To mitigate these risks, several strategies were recommended, including using lower-energy settings, choosing indirect or bipolar cautery, and ensuring efficient room ventilation with HEPA (high-efficiency particulate absorbing) filters to turn over air frequently. Additionally, employing the use of smoke evacuators and suction devices with proper filters can reduce particulate matter in the operating room.
The importance of the surgeon’s posture during procedures also was emphasized for ergonomic benefits and to minimize fatigue. Maintaining a neutral stance with the core and glutes engaged, standing on the balls of the feet, and aligning the table height to keep the hands at the lower chest level were recommended; this not only helps in reducing strain but also in maintaining precision during surgical tasks.
The surgeons on the panel also highlighted the novel use of hydrocolloid dressings with tattoo lasers, electrodesiccation and curettage for treating rhinophyma, and purse-string closure for chest defects as evolving practices to enhance outcomes and safety.
The session offered valuable insights into suturing techniques, advocating for the use of deep sutures—ideally Monocryl (Ethicon US, LLC)—for superficial closures and fast-absorbing gut sutures for patients who are not expected to return for suture removal. Keith LeBlanc Jr, MD, shared one of his favorite tricks for suturing fragile, sun-damaged skin on the forearm in elderly patients: apply adhesive skin closures aligned parallel to the suture line, then suture through them for extra support. This can help ensure a more secure closure.
In situations when no deep sutures are required, such as on the hair-bearing scalp, large bites through the galea using monofilament nonabsorbable sutures for up to 14 days or staples can offer favorable closures and enhanced hemostasis. Tranexamic acid has emerged as a versatile hemostatic agent—available in multiple forms ranging from direct injection to topical applications—and is cost-effective, enhancing its accessibility in various surgical settings.
A high proportion of patients are taken aback by the length of the scar following removal of what they perceive as a small skin cancer. Leslie Storey, MD, cleverly recommended using the back of a glove to mark surgical planning, giving the patient a visual guide for anticipating the size of the excision. This is a simple yet effective approach to enhance patient understanding and informed consent.
Lastly, the notion that “patients remember you if you don’t cause them pain” resonated deeply, underlining the importance of gentle techniques such as pinching the suture rather than pushing the wound edges together and asking assistants to maintain tension without obstructing the field. In the words of Seth Matarasso, MD: “If you pain ‘em, you won’t retain ‘em!”
Take-Home Point—The take-home message from the session was a comprehensive approach to surgical excellence that aligns technical proficiency with a strong emphasis on safety, patient comfort, and operative efficiency. Surgeons were advised to adopt practices that reduce the risks associated with surgical plume, maintain ergonomic discipline, and apply innovative suturing techniques to enhance patient outcomes. Compassionate patient care, innovative use of materials and methods, and a commitment to continual learning and adaptation of new evidence-based practices are paramount for the modern surgeon.
Approaches for Facial Reconstruction
The intricacies of multisubunit facial reconstruction were explored in a session that blended the pursuit of aesthetic harmony with functional restoration, “Simplifying the Complex: Reconstructing Multisubunit Defects.” The session began with an introduction to flap design principles, emphasizing the importance of thorough defect analysis and the strategic design of flaps. A key objective within this framework is the integration of the flap within existing cosmetic subunits to avoid unwanted effects such as unintended eyebrow elevation.
The concept of tissue reservoirs was discussed,focusing on regions such as the glabella as potential sources for skin recruitment. This then transitioned into a nuanced discussion on incision planning, underscoring the significance of aligning incision lines with relaxed skin tension lines to enhance healing and minimize scarring.
The topic of delayed reconstruction also was introduced as a deliberate tactic for high-risk tumor management. This approach allows for an in-depth pathologic examination and provides patients with more time for psychological adjustment, which may be particularly important for those with complex medical histories or those who require staged surgical interventions.
In a thorough examination of flap design techniques, the session highlighted the bilobed transposition flap as a versatile choice for nasal reconstruction, particularly apt for the distal third of the nose due to its design that harnesses skin from nonadjacent areas. Accompanying this was an exploration of Zitelli modifications, which enhance the bilobed flap by reducing issues such as pincushioning through a moderated rotation angle and the strategic incorporation of a Burow triangle.
Finally, the interpolated paranasal flap was discussed. This technique is designed to reduce the risk for cheek asymmetry and is suitable for patients with generous donor sites; however, this method requires diligent evaluation to avoid complications such as external nasal valve collapse.
Take-Home Point—This session highlighted approaches in facial reconstruction, emphasizing the necessity of strategic flap design and meticulous incision planning to maintain aesthetic harmony and functional integrity.
Strategies for Improving Physician Well-Being
Evidence-based recommendations to support physicians’ well-being are crucial as the health care system becomes increasingly demanding. Instead of focusing on aspects of the health care system that frequently are outside of physicians’ control, the session “A Realistic and Evidence-Based Roadmap for Thriving in Life and Career” discussed many practical, self-empowering tools and strategies to lead a happier and healthier life—both personally and professionally.
The speakers cautioned against the concept of an “unlimited ceiling” for achieving a certain goal, where an unlimited amount of time and energy is allowed to be dedicated to a given task over a period of time. They highlighted the potential consequences of this approach, such as stress, dissatisfaction, and ultimately burnout. The speakers explored the concept of well-being as a continuous journey rather than a destination, emphasizing that it is not the opposite of burnout. To promote well-being, the speakers advocated for utilizing concepts rooted in positive psychology to empower the individual rather than longing for a different environment. They hypothesized that changing one’s life can be accomplished by changing one’s mind, independent of the environment.
The roadmap for physician well-being, as presented by clinical psychologist Amy MacDonald, PsyD, commenced with urging the audience to introspect on situations and experiences, categorizing them into “feel good” and “feel bad” buckets. For every feel-good event, Dr. MacDonald proposed 5 mental exercises for optimized well-being: (1) control/increase: evaluate whether one can control or increase the frequency of the event; (2) consider: reflect on why this event feels good and explore other aspects to gain any additional joy from the event; (3) share: recognize that some feel goods are more joyous when shared; (4) value: connect the feel-good experiences with personal core values, as research shows value affirmations can buffer neuroendocrine and psychological stress responses; and (5) savor: deliberately relish each small or notable feel-good moment.
Similarly, after labeling an event as a feel-bad experience, Dr. MacDonald encouraged the audience to go through mental exercises to strengthen their well-being journey; however, before proceeding, she highlighted the importance of arming ourselves with self-compassion. The 5 mental exercises to address feel bads include (1) solve: assess whether we have control over the situation and attempt to make changes if possible; (2) reframe: explore new perspectives and assess assumptions without minimizing the situation; (3) connect: embrace the positive impact of safe human connections on our stress response; (4) reflect: search curiously using a compassionate lens for any existing patterns of reactions; and (5) accept and pivot: allow thoughts and feelings to exist and pivot to values-based engagement without waiting for the environment to change. Consistently seeking and appreciating feel goods while addressing rather than suppressing the feel bads can lead to joyful satisfaction and overall well-being.
Additional pearls for optimizing physician well-being included accurately labeling emotions rather than lumping them into an overarching theme (eg, stressed), avoiding comparisons with others, choosing courage over comfort, celebrating vulnerability, and embracing the ability to say no to prioritize engagements aligned with one’s purpose and values. Additional resources were shared for further reading, including Emotional Agility by Susan David, Daring Greatly and Rising Strong by Brené Brown, and Self-Compassion by Kristin Neff.
Take-Home Point—This lecture highlighted key strategies for physicians to improve their well-being, emphasizing self-empowerment and practical tools over external circumstances. It distinguished between productive and destructive influences on satisfaction, and emphasized decision-making aligned with personal values. The concept of well-being as a journey, not a destination, was central, encouraging positive psychology and self-reflection to enhance fulfillment. By focusing on amplifying feel-good experiences and addressing feel-bad experiences with resilience, the lecture advocated for internal over external change, offering a pathway to a balanced and satisfying professional and personal life for physicians.
Final Thoughts
The recent American Academy of Dermatology meeting offered valuable insights and practical pearls to enhance surgical practices and promote physician well-being, in addition to a wide range of topics beyond what is mentioned in this article. From optimizing surgical techniques to prioritizing patient care and safety, the sessions underscored the importance of continuous learning and adaptation in the ever-evolving field of dermatology. As we reflect on the lessons learned and the camaraderie shared during this gathering, let us carry forward these teachings to improve patient outcomes, foster innovation, and cultivate resilience in our pursuit of excellence. Together, we can continue to push the boundaries of dermatologic care while nurturing our own well-being and that of our colleagues, ensuring a brighter future for both patients and practitioners alike.
Acknowledgments—Sultan H. Qiblawi, MD, MBA; Eva Shelton, MD; and Christy T. Behnam, MD (all from Madison, Wisconsin), shared their insights and key takeaways from American Academy of Dermatology lecturers, which enriched the content of this article.
- Hills LS. Putting patients at ease with conversation. J Med Pract Manage. 2006;22:168-170.
- Barlow JO, Zalla MJ, Kyle A, et al. Treatment of basal cell carcinoma with curettage alone. J Am Acad Dermatol. 2006;54:1039-1045.
- Yakish K, Graham J, Hossler EW. Efficacy of curettage alone for invasive cutaneous squamous cell carcinoma: a retrospective cohort study. J Am Acad Dermatol. 2017;77:582-584.
- Shah NR. Commentary on: “surgical smoke—a health hazard in the operating theatre: a study to quantify exposure and a survey of the use of smoke extractor systems in UK plastic surgery units.”Ann Med Surg (Lond). 2012;1:23-24.
Attendees of the 2024 American Academy of Dermatology annual meeting in San Diego, California, were eager to delve into the latest trends and advancements in dermatology and dermatologic surgery. This article provides a few key takeaways for residents from a range of engaging sessions, with an emphasis on procedural dermatology and physician health and well-being.
Practical Applications of Surgical Enhancements
In an informative session dedicated to dermatologic surgeons and their patients, “Simple Tricks and Practical Tips to Optimize the Surgical Experience for You and Your Patients,” attendees learned practical tips for enhancing the surgical experience. The discussion spanned various aspects of surgery, from managing preoperative anxiety with anxiolytics such as midazolam to the strategic use of skin hooks for delicate tissue manipulation. Midazolam is fast acting and its use is tailored to patient factors such as weight, hepatic function, and prior use. An innovative anxiety management algorithm combining “talkesethesia” with other methods such as anodynes and benzodiazepines underscored the importance of a calm patient in successful surgical outcomes. Talkesthesia involves engaging patients in soothing and distracting conversation throughout the procedure. This technique can include discussing nonmedical topics of interest with the patient—such as their hobbies, family, or favorite movies—to divert their attention from the surgical process and reduce anxiety. By creating a friendly and reassuring atmosphere, talkesthesia helps to establish trust between the patient and the medical team, ultimately contributing to a more relaxed and cooperative patient.1
The utility of skin hooks also was discussed, with an emphasis on their role in ensuring gentle tissue handling. The modified buried vertical mattress technique was discussed for its added benefits in wound approximation and strength. Emphasis was placed on the importance of maintaining a clear surgical field by electrocautery to ensure optimal visibility.
Focusing on the treatment of skin cancer, curettage alone was touted as a viable alternative to electrodesiccation and curettage, especially in reducing postoperative hypopigmentation while maintaining high cure rates. This method was shown to be effective in treating basal cell carcinoma and well-differentiated squamous cell carcinoma.2,3
Suturing techniques such as pulley, purse-string, and buried sutures offer efficiencies in time, cost, and improved healing in high-tension areas. These methods can contribute to postsurgical aesthetic and functional outcomes. Additionally, Dr. Desiree Ratner shared her tips for painless local anesthesia techniques, emphasizing the importance of patient comfort through methods such as slow injection and buffering of lidocaine. The next time you give a local anesthetic, try this technique to minimize pain: using a 30-gauge needle, hold the syringe with the bevel up, insert only the bevel into the skin (needle tip goes into the papillary dermis), and numb superficially around the periphery using as little volume as possible. Keep pressure slow and steady without moving the needle, then insert the needle only in previously anesthetized areas, numbing deeply only after the entire periphery has been anesthetized.
The session concluded with the recommendation to provide patients with a goody bag containing postoperative supplies. This thoughtful gesture not only enhances patient satisfaction but also addresses the practical aspect of postsurgery care, offering an inexpensive yet impactful way to ensure patients have the necessary supplies for their recovery.
Take-Home Point—This session distilled essential surgical enhancements into practical applications, emphasizing the importance of anxiety management, delicate tissue handling, innovative suturing techniques, and thoughtful patient care postsurgery. The overarching message highlighted the synergy between technical skill and patient-centric approaches in optimizing surgical outcomes, underscoring the significance of attention to detail in every aspect of patient care, from preoperative preparation to postoperative recovery.
Optimizing Safety and Ergonomics in Surgical Practices
Understanding the dynamics of surgical plume is crucial to safety in the operating room. The carcinogenic risk associated with surgical smoke is not trivial: exposure to the plume generated by monopolar electrocautery in a single day can be equivalent to smoking approximately 30 cigarettes, and a surgeon’s lifetime cancer risk from polycyclic aromatic hydrocarbons exposure is alarmingly high.4 To mitigate these risks, several strategies were recommended, including using lower-energy settings, choosing indirect or bipolar cautery, and ensuring efficient room ventilation with HEPA (high-efficiency particulate absorbing) filters to turn over air frequently. Additionally, employing the use of smoke evacuators and suction devices with proper filters can reduce particulate matter in the operating room.
The importance of the surgeon’s posture during procedures also was emphasized for ergonomic benefits and to minimize fatigue. Maintaining a neutral stance with the core and glutes engaged, standing on the balls of the feet, and aligning the table height to keep the hands at the lower chest level were recommended; this not only helps in reducing strain but also in maintaining precision during surgical tasks.
The surgeons on the panel also highlighted the novel use of hydrocolloid dressings with tattoo lasers, electrodesiccation and curettage for treating rhinophyma, and purse-string closure for chest defects as evolving practices to enhance outcomes and safety.
The session offered valuable insights into suturing techniques, advocating for the use of deep sutures—ideally Monocryl (Ethicon US, LLC)—for superficial closures and fast-absorbing gut sutures for patients who are not expected to return for suture removal. Keith LeBlanc Jr, MD, shared one of his favorite tricks for suturing fragile, sun-damaged skin on the forearm in elderly patients: apply adhesive skin closures aligned parallel to the suture line, then suture through them for extra support. This can help ensure a more secure closure.
In situations when no deep sutures are required, such as on the hair-bearing scalp, large bites through the galea using monofilament nonabsorbable sutures for up to 14 days or staples can offer favorable closures and enhanced hemostasis. Tranexamic acid has emerged as a versatile hemostatic agent—available in multiple forms ranging from direct injection to topical applications—and is cost-effective, enhancing its accessibility in various surgical settings.
A high proportion of patients are taken aback by the length of the scar following removal of what they perceive as a small skin cancer. Leslie Storey, MD, cleverly recommended using the back of a glove to mark surgical planning, giving the patient a visual guide for anticipating the size of the excision. This is a simple yet effective approach to enhance patient understanding and informed consent.
Lastly, the notion that “patients remember you if you don’t cause them pain” resonated deeply, underlining the importance of gentle techniques such as pinching the suture rather than pushing the wound edges together and asking assistants to maintain tension without obstructing the field. In the words of Seth Matarasso, MD: “If you pain ‘em, you won’t retain ‘em!”
Take-Home Point—The take-home message from the session was a comprehensive approach to surgical excellence that aligns technical proficiency with a strong emphasis on safety, patient comfort, and operative efficiency. Surgeons were advised to adopt practices that reduce the risks associated with surgical plume, maintain ergonomic discipline, and apply innovative suturing techniques to enhance patient outcomes. Compassionate patient care, innovative use of materials and methods, and a commitment to continual learning and adaptation of new evidence-based practices are paramount for the modern surgeon.
Approaches for Facial Reconstruction
The intricacies of multisubunit facial reconstruction were explored in a session that blended the pursuit of aesthetic harmony with functional restoration, “Simplifying the Complex: Reconstructing Multisubunit Defects.” The session began with an introduction to flap design principles, emphasizing the importance of thorough defect analysis and the strategic design of flaps. A key objective within this framework is the integration of the flap within existing cosmetic subunits to avoid unwanted effects such as unintended eyebrow elevation.
The concept of tissue reservoirs was discussed,focusing on regions such as the glabella as potential sources for skin recruitment. This then transitioned into a nuanced discussion on incision planning, underscoring the significance of aligning incision lines with relaxed skin tension lines to enhance healing and minimize scarring.
The topic of delayed reconstruction also was introduced as a deliberate tactic for high-risk tumor management. This approach allows for an in-depth pathologic examination and provides patients with more time for psychological adjustment, which may be particularly important for those with complex medical histories or those who require staged surgical interventions.
In a thorough examination of flap design techniques, the session highlighted the bilobed transposition flap as a versatile choice for nasal reconstruction, particularly apt for the distal third of the nose due to its design that harnesses skin from nonadjacent areas. Accompanying this was an exploration of Zitelli modifications, which enhance the bilobed flap by reducing issues such as pincushioning through a moderated rotation angle and the strategic incorporation of a Burow triangle.
Finally, the interpolated paranasal flap was discussed. This technique is designed to reduce the risk for cheek asymmetry and is suitable for patients with generous donor sites; however, this method requires diligent evaluation to avoid complications such as external nasal valve collapse.
Take-Home Point—This session highlighted approaches in facial reconstruction, emphasizing the necessity of strategic flap design and meticulous incision planning to maintain aesthetic harmony and functional integrity.
Strategies for Improving Physician Well-Being
Evidence-based recommendations to support physicians’ well-being are crucial as the health care system becomes increasingly demanding. Instead of focusing on aspects of the health care system that frequently are outside of physicians’ control, the session “A Realistic and Evidence-Based Roadmap for Thriving in Life and Career” discussed many practical, self-empowering tools and strategies to lead a happier and healthier life—both personally and professionally.
The speakers cautioned against the concept of an “unlimited ceiling” for achieving a certain goal, where an unlimited amount of time and energy is allowed to be dedicated to a given task over a period of time. They highlighted the potential consequences of this approach, such as stress, dissatisfaction, and ultimately burnout. The speakers explored the concept of well-being as a continuous journey rather than a destination, emphasizing that it is not the opposite of burnout. To promote well-being, the speakers advocated for utilizing concepts rooted in positive psychology to empower the individual rather than longing for a different environment. They hypothesized that changing one’s life can be accomplished by changing one’s mind, independent of the environment.
The roadmap for physician well-being, as presented by clinical psychologist Amy MacDonald, PsyD, commenced with urging the audience to introspect on situations and experiences, categorizing them into “feel good” and “feel bad” buckets. For every feel-good event, Dr. MacDonald proposed 5 mental exercises for optimized well-being: (1) control/increase: evaluate whether one can control or increase the frequency of the event; (2) consider: reflect on why this event feels good and explore other aspects to gain any additional joy from the event; (3) share: recognize that some feel goods are more joyous when shared; (4) value: connect the feel-good experiences with personal core values, as research shows value affirmations can buffer neuroendocrine and psychological stress responses; and (5) savor: deliberately relish each small or notable feel-good moment.
Similarly, after labeling an event as a feel-bad experience, Dr. MacDonald encouraged the audience to go through mental exercises to strengthen their well-being journey; however, before proceeding, she highlighted the importance of arming ourselves with self-compassion. The 5 mental exercises to address feel bads include (1) solve: assess whether we have control over the situation and attempt to make changes if possible; (2) reframe: explore new perspectives and assess assumptions without minimizing the situation; (3) connect: embrace the positive impact of safe human connections on our stress response; (4) reflect: search curiously using a compassionate lens for any existing patterns of reactions; and (5) accept and pivot: allow thoughts and feelings to exist and pivot to values-based engagement without waiting for the environment to change. Consistently seeking and appreciating feel goods while addressing rather than suppressing the feel bads can lead to joyful satisfaction and overall well-being.
Additional pearls for optimizing physician well-being included accurately labeling emotions rather than lumping them into an overarching theme (eg, stressed), avoiding comparisons with others, choosing courage over comfort, celebrating vulnerability, and embracing the ability to say no to prioritize engagements aligned with one’s purpose and values. Additional resources were shared for further reading, including Emotional Agility by Susan David, Daring Greatly and Rising Strong by Brené Brown, and Self-Compassion by Kristin Neff.
Take-Home Point—This lecture highlighted key strategies for physicians to improve their well-being, emphasizing self-empowerment and practical tools over external circumstances. It distinguished between productive and destructive influences on satisfaction, and emphasized decision-making aligned with personal values. The concept of well-being as a journey, not a destination, was central, encouraging positive psychology and self-reflection to enhance fulfillment. By focusing on amplifying feel-good experiences and addressing feel-bad experiences with resilience, the lecture advocated for internal over external change, offering a pathway to a balanced and satisfying professional and personal life for physicians.
Final Thoughts
The recent American Academy of Dermatology meeting offered valuable insights and practical pearls to enhance surgical practices and promote physician well-being, in addition to a wide range of topics beyond what is mentioned in this article. From optimizing surgical techniques to prioritizing patient care and safety, the sessions underscored the importance of continuous learning and adaptation in the ever-evolving field of dermatology. As we reflect on the lessons learned and the camaraderie shared during this gathering, let us carry forward these teachings to improve patient outcomes, foster innovation, and cultivate resilience in our pursuit of excellence. Together, we can continue to push the boundaries of dermatologic care while nurturing our own well-being and that of our colleagues, ensuring a brighter future for both patients and practitioners alike.
Acknowledgments—Sultan H. Qiblawi, MD, MBA; Eva Shelton, MD; and Christy T. Behnam, MD (all from Madison, Wisconsin), shared their insights and key takeaways from American Academy of Dermatology lecturers, which enriched the content of this article.
Attendees of the 2024 American Academy of Dermatology annual meeting in San Diego, California, were eager to delve into the latest trends and advancements in dermatology and dermatologic surgery. This article provides a few key takeaways for residents from a range of engaging sessions, with an emphasis on procedural dermatology and physician health and well-being.
Practical Applications of Surgical Enhancements
In an informative session dedicated to dermatologic surgeons and their patients, “Simple Tricks and Practical Tips to Optimize the Surgical Experience for You and Your Patients,” attendees learned practical tips for enhancing the surgical experience. The discussion spanned various aspects of surgery, from managing preoperative anxiety with anxiolytics such as midazolam to the strategic use of skin hooks for delicate tissue manipulation. Midazolam is fast acting and its use is tailored to patient factors such as weight, hepatic function, and prior use. An innovative anxiety management algorithm combining “talkesethesia” with other methods such as anodynes and benzodiazepines underscored the importance of a calm patient in successful surgical outcomes. Talkesthesia involves engaging patients in soothing and distracting conversation throughout the procedure. This technique can include discussing nonmedical topics of interest with the patient—such as their hobbies, family, or favorite movies—to divert their attention from the surgical process and reduce anxiety. By creating a friendly and reassuring atmosphere, talkesthesia helps to establish trust between the patient and the medical team, ultimately contributing to a more relaxed and cooperative patient.1
The utility of skin hooks also was discussed, with an emphasis on their role in ensuring gentle tissue handling. The modified buried vertical mattress technique was discussed for its added benefits in wound approximation and strength. Emphasis was placed on the importance of maintaining a clear surgical field by electrocautery to ensure optimal visibility.
Focusing on the treatment of skin cancer, curettage alone was touted as a viable alternative to electrodesiccation and curettage, especially in reducing postoperative hypopigmentation while maintaining high cure rates. This method was shown to be effective in treating basal cell carcinoma and well-differentiated squamous cell carcinoma.2,3
Suturing techniques such as pulley, purse-string, and buried sutures offer efficiencies in time, cost, and improved healing in high-tension areas. These methods can contribute to postsurgical aesthetic and functional outcomes. Additionally, Dr. Desiree Ratner shared her tips for painless local anesthesia techniques, emphasizing the importance of patient comfort through methods such as slow injection and buffering of lidocaine. The next time you give a local anesthetic, try this technique to minimize pain: using a 30-gauge needle, hold the syringe with the bevel up, insert only the bevel into the skin (needle tip goes into the papillary dermis), and numb superficially around the periphery using as little volume as possible. Keep pressure slow and steady without moving the needle, then insert the needle only in previously anesthetized areas, numbing deeply only after the entire periphery has been anesthetized.
The session concluded with the recommendation to provide patients with a goody bag containing postoperative supplies. This thoughtful gesture not only enhances patient satisfaction but also addresses the practical aspect of postsurgery care, offering an inexpensive yet impactful way to ensure patients have the necessary supplies for their recovery.
Take-Home Point—This session distilled essential surgical enhancements into practical applications, emphasizing the importance of anxiety management, delicate tissue handling, innovative suturing techniques, and thoughtful patient care postsurgery. The overarching message highlighted the synergy between technical skill and patient-centric approaches in optimizing surgical outcomes, underscoring the significance of attention to detail in every aspect of patient care, from preoperative preparation to postoperative recovery.
Optimizing Safety and Ergonomics in Surgical Practices
Understanding the dynamics of surgical plume is crucial to safety in the operating room. The carcinogenic risk associated with surgical smoke is not trivial: exposure to the plume generated by monopolar electrocautery in a single day can be equivalent to smoking approximately 30 cigarettes, and a surgeon’s lifetime cancer risk from polycyclic aromatic hydrocarbons exposure is alarmingly high.4 To mitigate these risks, several strategies were recommended, including using lower-energy settings, choosing indirect or bipolar cautery, and ensuring efficient room ventilation with HEPA (high-efficiency particulate absorbing) filters to turn over air frequently. Additionally, employing the use of smoke evacuators and suction devices with proper filters can reduce particulate matter in the operating room.
The importance of the surgeon’s posture during procedures also was emphasized for ergonomic benefits and to minimize fatigue. Maintaining a neutral stance with the core and glutes engaged, standing on the balls of the feet, and aligning the table height to keep the hands at the lower chest level were recommended; this not only helps in reducing strain but also in maintaining precision during surgical tasks.
The surgeons on the panel also highlighted the novel use of hydrocolloid dressings with tattoo lasers, electrodesiccation and curettage for treating rhinophyma, and purse-string closure for chest defects as evolving practices to enhance outcomes and safety.
The session offered valuable insights into suturing techniques, advocating for the use of deep sutures—ideally Monocryl (Ethicon US, LLC)—for superficial closures and fast-absorbing gut sutures for patients who are not expected to return for suture removal. Keith LeBlanc Jr, MD, shared one of his favorite tricks for suturing fragile, sun-damaged skin on the forearm in elderly patients: apply adhesive skin closures aligned parallel to the suture line, then suture through them for extra support. This can help ensure a more secure closure.
In situations when no deep sutures are required, such as on the hair-bearing scalp, large bites through the galea using monofilament nonabsorbable sutures for up to 14 days or staples can offer favorable closures and enhanced hemostasis. Tranexamic acid has emerged as a versatile hemostatic agent—available in multiple forms ranging from direct injection to topical applications—and is cost-effective, enhancing its accessibility in various surgical settings.
A high proportion of patients are taken aback by the length of the scar following removal of what they perceive as a small skin cancer. Leslie Storey, MD, cleverly recommended using the back of a glove to mark surgical planning, giving the patient a visual guide for anticipating the size of the excision. This is a simple yet effective approach to enhance patient understanding and informed consent.
Lastly, the notion that “patients remember you if you don’t cause them pain” resonated deeply, underlining the importance of gentle techniques such as pinching the suture rather than pushing the wound edges together and asking assistants to maintain tension without obstructing the field. In the words of Seth Matarasso, MD: “If you pain ‘em, you won’t retain ‘em!”
Take-Home Point—The take-home message from the session was a comprehensive approach to surgical excellence that aligns technical proficiency with a strong emphasis on safety, patient comfort, and operative efficiency. Surgeons were advised to adopt practices that reduce the risks associated with surgical plume, maintain ergonomic discipline, and apply innovative suturing techniques to enhance patient outcomes. Compassionate patient care, innovative use of materials and methods, and a commitment to continual learning and adaptation of new evidence-based practices are paramount for the modern surgeon.
Approaches for Facial Reconstruction
The intricacies of multisubunit facial reconstruction were explored in a session that blended the pursuit of aesthetic harmony with functional restoration, “Simplifying the Complex: Reconstructing Multisubunit Defects.” The session began with an introduction to flap design principles, emphasizing the importance of thorough defect analysis and the strategic design of flaps. A key objective within this framework is the integration of the flap within existing cosmetic subunits to avoid unwanted effects such as unintended eyebrow elevation.
The concept of tissue reservoirs was discussed,focusing on regions such as the glabella as potential sources for skin recruitment. This then transitioned into a nuanced discussion on incision planning, underscoring the significance of aligning incision lines with relaxed skin tension lines to enhance healing and minimize scarring.
The topic of delayed reconstruction also was introduced as a deliberate tactic for high-risk tumor management. This approach allows for an in-depth pathologic examination and provides patients with more time for psychological adjustment, which may be particularly important for those with complex medical histories or those who require staged surgical interventions.
In a thorough examination of flap design techniques, the session highlighted the bilobed transposition flap as a versatile choice for nasal reconstruction, particularly apt for the distal third of the nose due to its design that harnesses skin from nonadjacent areas. Accompanying this was an exploration of Zitelli modifications, which enhance the bilobed flap by reducing issues such as pincushioning through a moderated rotation angle and the strategic incorporation of a Burow triangle.
Finally, the interpolated paranasal flap was discussed. This technique is designed to reduce the risk for cheek asymmetry and is suitable for patients with generous donor sites; however, this method requires diligent evaluation to avoid complications such as external nasal valve collapse.
Take-Home Point—This session highlighted approaches in facial reconstruction, emphasizing the necessity of strategic flap design and meticulous incision planning to maintain aesthetic harmony and functional integrity.
Strategies for Improving Physician Well-Being
Evidence-based recommendations to support physicians’ well-being are crucial as the health care system becomes increasingly demanding. Instead of focusing on aspects of the health care system that frequently are outside of physicians’ control, the session “A Realistic and Evidence-Based Roadmap for Thriving in Life and Career” discussed many practical, self-empowering tools and strategies to lead a happier and healthier life—both personally and professionally.
The speakers cautioned against the concept of an “unlimited ceiling” for achieving a certain goal, where an unlimited amount of time and energy is allowed to be dedicated to a given task over a period of time. They highlighted the potential consequences of this approach, such as stress, dissatisfaction, and ultimately burnout. The speakers explored the concept of well-being as a continuous journey rather than a destination, emphasizing that it is not the opposite of burnout. To promote well-being, the speakers advocated for utilizing concepts rooted in positive psychology to empower the individual rather than longing for a different environment. They hypothesized that changing one’s life can be accomplished by changing one’s mind, independent of the environment.
The roadmap for physician well-being, as presented by clinical psychologist Amy MacDonald, PsyD, commenced with urging the audience to introspect on situations and experiences, categorizing them into “feel good” and “feel bad” buckets. For every feel-good event, Dr. MacDonald proposed 5 mental exercises for optimized well-being: (1) control/increase: evaluate whether one can control or increase the frequency of the event; (2) consider: reflect on why this event feels good and explore other aspects to gain any additional joy from the event; (3) share: recognize that some feel goods are more joyous when shared; (4) value: connect the feel-good experiences with personal core values, as research shows value affirmations can buffer neuroendocrine and psychological stress responses; and (5) savor: deliberately relish each small or notable feel-good moment.
Similarly, after labeling an event as a feel-bad experience, Dr. MacDonald encouraged the audience to go through mental exercises to strengthen their well-being journey; however, before proceeding, she highlighted the importance of arming ourselves with self-compassion. The 5 mental exercises to address feel bads include (1) solve: assess whether we have control over the situation and attempt to make changes if possible; (2) reframe: explore new perspectives and assess assumptions without minimizing the situation; (3) connect: embrace the positive impact of safe human connections on our stress response; (4) reflect: search curiously using a compassionate lens for any existing patterns of reactions; and (5) accept and pivot: allow thoughts and feelings to exist and pivot to values-based engagement without waiting for the environment to change. Consistently seeking and appreciating feel goods while addressing rather than suppressing the feel bads can lead to joyful satisfaction and overall well-being.
Additional pearls for optimizing physician well-being included accurately labeling emotions rather than lumping them into an overarching theme (eg, stressed), avoiding comparisons with others, choosing courage over comfort, celebrating vulnerability, and embracing the ability to say no to prioritize engagements aligned with one’s purpose and values. Additional resources were shared for further reading, including Emotional Agility by Susan David, Daring Greatly and Rising Strong by Brené Brown, and Self-Compassion by Kristin Neff.
Take-Home Point—This lecture highlighted key strategies for physicians to improve their well-being, emphasizing self-empowerment and practical tools over external circumstances. It distinguished between productive and destructive influences on satisfaction, and emphasized decision-making aligned with personal values. The concept of well-being as a journey, not a destination, was central, encouraging positive psychology and self-reflection to enhance fulfillment. By focusing on amplifying feel-good experiences and addressing feel-bad experiences with resilience, the lecture advocated for internal over external change, offering a pathway to a balanced and satisfying professional and personal life for physicians.
Final Thoughts
The recent American Academy of Dermatology meeting offered valuable insights and practical pearls to enhance surgical practices and promote physician well-being, in addition to a wide range of topics beyond what is mentioned in this article. From optimizing surgical techniques to prioritizing patient care and safety, the sessions underscored the importance of continuous learning and adaptation in the ever-evolving field of dermatology. As we reflect on the lessons learned and the camaraderie shared during this gathering, let us carry forward these teachings to improve patient outcomes, foster innovation, and cultivate resilience in our pursuit of excellence. Together, we can continue to push the boundaries of dermatologic care while nurturing our own well-being and that of our colleagues, ensuring a brighter future for both patients and practitioners alike.
Acknowledgments—Sultan H. Qiblawi, MD, MBA; Eva Shelton, MD; and Christy T. Behnam, MD (all from Madison, Wisconsin), shared their insights and key takeaways from American Academy of Dermatology lecturers, which enriched the content of this article.
- Hills LS. Putting patients at ease with conversation. J Med Pract Manage. 2006;22:168-170.
- Barlow JO, Zalla MJ, Kyle A, et al. Treatment of basal cell carcinoma with curettage alone. J Am Acad Dermatol. 2006;54:1039-1045.
- Yakish K, Graham J, Hossler EW. Efficacy of curettage alone for invasive cutaneous squamous cell carcinoma: a retrospective cohort study. J Am Acad Dermatol. 2017;77:582-584.
- Shah NR. Commentary on: “surgical smoke—a health hazard in the operating theatre: a study to quantify exposure and a survey of the use of smoke extractor systems in UK plastic surgery units.”Ann Med Surg (Lond). 2012;1:23-24.
- Hills LS. Putting patients at ease with conversation. J Med Pract Manage. 2006;22:168-170.
- Barlow JO, Zalla MJ, Kyle A, et al. Treatment of basal cell carcinoma with curettage alone. J Am Acad Dermatol. 2006;54:1039-1045.
- Yakish K, Graham J, Hossler EW. Efficacy of curettage alone for invasive cutaneous squamous cell carcinoma: a retrospective cohort study. J Am Acad Dermatol. 2017;77:582-584.
- Shah NR. Commentary on: “surgical smoke—a health hazard in the operating theatre: a study to quantify exposure and a survey of the use of smoke extractor systems in UK plastic surgery units.”Ann Med Surg (Lond). 2012;1:23-24.
RESIDENT PEARLS
- By protecting yourself and ensuring your own longevity as a practicing physician, you will be better able to care for your patients over the long term. Focus on self-empowerment and positive psychology for a balanced life.
- Protect yourself from surgical plume by using smoke evacuators and ensuring proper room ventilation with HEPA (high-efficiency particulate absorbing) filters whenever possible. Stick to low-energy settings for electrocautery.
- During surgical procedures, maintain a neutral posture, keep your core and glutes engaged, and adjust the table height to reduce strain and improve precision.
Commentary: Aspirin, Childbirth, and Everolimus in BC, June 2024
The postpartum period represents a possibly vulnerable time window for development of new cancers with metastatic potential. Studies in young-onset breast cancer have shown a postpartum diagnosis up to 10 years after childbirth associated with adverse breast cancer survival outcomes.4 Women with germline BRCA1/2 pathogenic variants have a higher risk of developing breast cancer at a younger age compared to the general population.5 A prospective cohort study that included 903 women with germline BRCA1/2 mutations diagnosed with stage I-III breast cancer at age ≤ 45 years investigated whether time since childbirth and time since breast cancer diagnosis were associated with mortality in this population (
The mechanisms involved in development of endocrine therapy (ET) resistance are complex and may include changes in hormone signaling, alterations in growth factor signaling pathway components, and appearance of resistant clonal populations.6 Prior studies have shown efficacy with the mammalian target of rapamycin (mTOR) inhibitor everolimus in combination with various ET backbones. However, the sequencing of these combinations in current clinical practice has shifted in light of significant therapeutic advancements in this space.7 A retrospective observational study including 161 patients with advanced hormone receptor–positive (HR+)/ human epidermal growth factor receptor–2 negative (HER2-) breast cancer treated with everolimus plus ET (exemestane, fulvestrant, tamoxifen) reported outcomes on the real-world use of these regimens after progression on cyclin-dependent kinase (CDK) 4/6 inhibitor therapy (Sánchez-Bayona et al). At a median follow-up of 15 months, the estimated median progression-free survival (PFS) was 6.0 months (95% CI 5.3-7.8 months); PFS was longer among those with previous CDK4/6 inhibitor use lasting >18 months (8.7 months; 95% CI 6.6-11.3 months), patients without visceral disease (8.0 months; 95% CI 5.8-10.5 months), and those who were chemotherapy-naive in the advanced setting (7.2 months; 95% CI 5.9-8.4 months). These data support a role for everolimus plus ET as a treatment option post–CDK4/6 inhibitor treatment for selected patient populations, including those whose tumors lack targetable somatic mutations (such as PIK3CA and ESR1 mutations), and may provide meaningful clinical benefit in this setting.
Additional References
- Rothwell PM, Wilson M, Price JF, et al. Effect of daily aspirin on risk of cancer metastasis: A study of incident cancers during randomised controlled trials. Lancet. 2012;379:1591-601. doi: 10.1016/S0140-6736(12)60209-8 Source
- Okada S, Morimoto T, Ogawa H, et al, and the JPAD Trial Investigators. Effect of aspirin on cancer chemoprevention in Japanese patients with type 2 diabetes: 10-year observational follow-up of a randomized controlled trial. Diabetes Care. 2018;41:1757-1764. doi: 10.2337/dc18-0368 Source
- Burn J, Sheth H, Elliott F, et al, on behalf of the CAPP2 Investigators. Cancer prevention with aspirin in hereditary colorectal cancer (Lynch syndrome), 10-year follow-up and registry-based 20-year data in the CAPP2 study: A double-blind, randomised, placebo-controlled trial. Lancet. 2020;395:1855-1863. doi: 10.1016/S0140-6736(20)30366-4 Source
- Shao C, Yu Z, Xiao J, et al. Prognosis of pregnancy-associated breast cancer: A meta-analysis. BMC Cancer. 2020;20:746. doi: 10.1186/s12885-020-07248-8 Source
- Kuchenbaecker KB, Hopper JL, Barnes DR, et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA. 2017;317:2402-2416. doi: 10.1001/jama.2017.7112 Source
- Hanker AB, Sudhan DR, Arteaga CL. Overcoming endocrine resistance in breast cancer. Cancer Cell. 2020;37:496-513. doi: 10.1016/j.ccell.2020.03.009 Source
- Kornblum N, Zhao F, Manola J, et al. Randomized phase II trial of fulvestrant plus everolimus or placebo in postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer resistant to aromatase inhibitor therapy: Results of PrE0102. J Clin Oncol. 2018;36:1556-1563. doi: 10.1200/JCO.2017.76.9331 Source
The postpartum period represents a possibly vulnerable time window for development of new cancers with metastatic potential. Studies in young-onset breast cancer have shown a postpartum diagnosis up to 10 years after childbirth associated with adverse breast cancer survival outcomes.4 Women with germline BRCA1/2 pathogenic variants have a higher risk of developing breast cancer at a younger age compared to the general population.5 A prospective cohort study that included 903 women with germline BRCA1/2 mutations diagnosed with stage I-III breast cancer at age ≤ 45 years investigated whether time since childbirth and time since breast cancer diagnosis were associated with mortality in this population (
The mechanisms involved in development of endocrine therapy (ET) resistance are complex and may include changes in hormone signaling, alterations in growth factor signaling pathway components, and appearance of resistant clonal populations.6 Prior studies have shown efficacy with the mammalian target of rapamycin (mTOR) inhibitor everolimus in combination with various ET backbones. However, the sequencing of these combinations in current clinical practice has shifted in light of significant therapeutic advancements in this space.7 A retrospective observational study including 161 patients with advanced hormone receptor–positive (HR+)/ human epidermal growth factor receptor–2 negative (HER2-) breast cancer treated with everolimus plus ET (exemestane, fulvestrant, tamoxifen) reported outcomes on the real-world use of these regimens after progression on cyclin-dependent kinase (CDK) 4/6 inhibitor therapy (Sánchez-Bayona et al). At a median follow-up of 15 months, the estimated median progression-free survival (PFS) was 6.0 months (95% CI 5.3-7.8 months); PFS was longer among those with previous CDK4/6 inhibitor use lasting >18 months (8.7 months; 95% CI 6.6-11.3 months), patients without visceral disease (8.0 months; 95% CI 5.8-10.5 months), and those who were chemotherapy-naive in the advanced setting (7.2 months; 95% CI 5.9-8.4 months). These data support a role for everolimus plus ET as a treatment option post–CDK4/6 inhibitor treatment for selected patient populations, including those whose tumors lack targetable somatic mutations (such as PIK3CA and ESR1 mutations), and may provide meaningful clinical benefit in this setting.
Additional References
- Rothwell PM, Wilson M, Price JF, et al. Effect of daily aspirin on risk of cancer metastasis: A study of incident cancers during randomised controlled trials. Lancet. 2012;379:1591-601. doi: 10.1016/S0140-6736(12)60209-8 Source
- Okada S, Morimoto T, Ogawa H, et al, and the JPAD Trial Investigators. Effect of aspirin on cancer chemoprevention in Japanese patients with type 2 diabetes: 10-year observational follow-up of a randomized controlled trial. Diabetes Care. 2018;41:1757-1764. doi: 10.2337/dc18-0368 Source
- Burn J, Sheth H, Elliott F, et al, on behalf of the CAPP2 Investigators. Cancer prevention with aspirin in hereditary colorectal cancer (Lynch syndrome), 10-year follow-up and registry-based 20-year data in the CAPP2 study: A double-blind, randomised, placebo-controlled trial. Lancet. 2020;395:1855-1863. doi: 10.1016/S0140-6736(20)30366-4 Source
- Shao C, Yu Z, Xiao J, et al. Prognosis of pregnancy-associated breast cancer: A meta-analysis. BMC Cancer. 2020;20:746. doi: 10.1186/s12885-020-07248-8 Source
- Kuchenbaecker KB, Hopper JL, Barnes DR, et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA. 2017;317:2402-2416. doi: 10.1001/jama.2017.7112 Source
- Hanker AB, Sudhan DR, Arteaga CL. Overcoming endocrine resistance in breast cancer. Cancer Cell. 2020;37:496-513. doi: 10.1016/j.ccell.2020.03.009 Source
- Kornblum N, Zhao F, Manola J, et al. Randomized phase II trial of fulvestrant plus everolimus or placebo in postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer resistant to aromatase inhibitor therapy: Results of PrE0102. J Clin Oncol. 2018;36:1556-1563. doi: 10.1200/JCO.2017.76.9331 Source
The postpartum period represents a possibly vulnerable time window for development of new cancers with metastatic potential. Studies in young-onset breast cancer have shown a postpartum diagnosis up to 10 years after childbirth associated with adverse breast cancer survival outcomes.4 Women with germline BRCA1/2 pathogenic variants have a higher risk of developing breast cancer at a younger age compared to the general population.5 A prospective cohort study that included 903 women with germline BRCA1/2 mutations diagnosed with stage I-III breast cancer at age ≤ 45 years investigated whether time since childbirth and time since breast cancer diagnosis were associated with mortality in this population (
The mechanisms involved in development of endocrine therapy (ET) resistance are complex and may include changes in hormone signaling, alterations in growth factor signaling pathway components, and appearance of resistant clonal populations.6 Prior studies have shown efficacy with the mammalian target of rapamycin (mTOR) inhibitor everolimus in combination with various ET backbones. However, the sequencing of these combinations in current clinical practice has shifted in light of significant therapeutic advancements in this space.7 A retrospective observational study including 161 patients with advanced hormone receptor–positive (HR+)/ human epidermal growth factor receptor–2 negative (HER2-) breast cancer treated with everolimus plus ET (exemestane, fulvestrant, tamoxifen) reported outcomes on the real-world use of these regimens after progression on cyclin-dependent kinase (CDK) 4/6 inhibitor therapy (Sánchez-Bayona et al). At a median follow-up of 15 months, the estimated median progression-free survival (PFS) was 6.0 months (95% CI 5.3-7.8 months); PFS was longer among those with previous CDK4/6 inhibitor use lasting >18 months (8.7 months; 95% CI 6.6-11.3 months), patients without visceral disease (8.0 months; 95% CI 5.8-10.5 months), and those who were chemotherapy-naive in the advanced setting (7.2 months; 95% CI 5.9-8.4 months). These data support a role for everolimus plus ET as a treatment option post–CDK4/6 inhibitor treatment for selected patient populations, including those whose tumors lack targetable somatic mutations (such as PIK3CA and ESR1 mutations), and may provide meaningful clinical benefit in this setting.
Additional References
- Rothwell PM, Wilson M, Price JF, et al. Effect of daily aspirin on risk of cancer metastasis: A study of incident cancers during randomised controlled trials. Lancet. 2012;379:1591-601. doi: 10.1016/S0140-6736(12)60209-8 Source
- Okada S, Morimoto T, Ogawa H, et al, and the JPAD Trial Investigators. Effect of aspirin on cancer chemoprevention in Japanese patients with type 2 diabetes: 10-year observational follow-up of a randomized controlled trial. Diabetes Care. 2018;41:1757-1764. doi: 10.2337/dc18-0368 Source
- Burn J, Sheth H, Elliott F, et al, on behalf of the CAPP2 Investigators. Cancer prevention with aspirin in hereditary colorectal cancer (Lynch syndrome), 10-year follow-up and registry-based 20-year data in the CAPP2 study: A double-blind, randomised, placebo-controlled trial. Lancet. 2020;395:1855-1863. doi: 10.1016/S0140-6736(20)30366-4 Source
- Shao C, Yu Z, Xiao J, et al. Prognosis of pregnancy-associated breast cancer: A meta-analysis. BMC Cancer. 2020;20:746. doi: 10.1186/s12885-020-07248-8 Source
- Kuchenbaecker KB, Hopper JL, Barnes DR, et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA. 2017;317:2402-2416. doi: 10.1001/jama.2017.7112 Source
- Hanker AB, Sudhan DR, Arteaga CL. Overcoming endocrine resistance in breast cancer. Cancer Cell. 2020;37:496-513. doi: 10.1016/j.ccell.2020.03.009 Source
- Kornblum N, Zhao F, Manola J, et al. Randomized phase II trial of fulvestrant plus everolimus or placebo in postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer resistant to aromatase inhibitor therapy: Results of PrE0102. J Clin Oncol. 2018;36:1556-1563. doi: 10.1200/JCO.2017.76.9331 Source
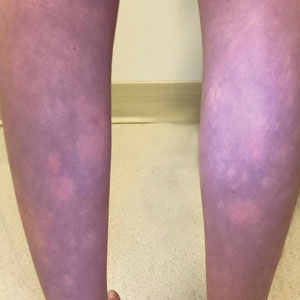
Transient Symmetric Blanching Macules on a Background of Reticulate Erythema
The Diagnosis: BASCULE Syndrome
The patient had previously been thought to have livedo reticularis by primary care. Repeat antinuclear antibody (ANA) testing was positive (1:1280 homogeneous [reflexive titers all negative]). However, upon dermatologic evaluation, the manifestation of the rash in addition to onset occurring with postural changes challenged the livedo reticularis diagnosis. Extensive research and consultation with dermatologic colleagues led to the diagnosis of the rare entity BASCULE syndrome. BASCULE (Bier anemic spots, cyanosis, and urticarialike eruption) syndrome was described by Bessis et al1 in 2016. It is a rare condition but may be underreported.2 It is a benign pediatric disorder in the vascular acrosyndrome family that is characterized by underlying vasomotor dysfunction in distal regions of the body. Raynaud phenomenon is a widely known member of this family. As seen in our patient, it typically presents on the distal legs and feet with numerous irregular hypopigmented macules on a cyanotic background. Red-orange papules may appear on the hypopigmented macules and often are pruritic. Lesions on the distal upper extremities are less common, and a case involving the trunk has been reported.3 Onset generally begins within a couple of minutes of standing or mechanical compression of the lower legs, with full reversal of symptoms occurring within minutes of laying down or walking. Commonly reported associated symptoms include tenderness, pruritus, edema, and pain; however, the cutaneous lesions may be asymptomatic. The condition tends to affect adolescents, as seen in our patient; however, there have been reports in infants as young as 3 months to adults aged 19 years.2
The pathophysiology behind BASCULE syndrome remains unclear but is believed to be centered around the role of physiologic venous stasis that occurs when standing. The hypoxia secondary to stasis is thought to induce amplified vasoconstriction of arterioles. These responses are further exaggerated due to absence of venoarteriolar reflexes in dermal ascending arterioles, leading to Bier spots.2 The role of mast cells and eosinophils remains unclear. It is a clinical diagnosis without clear histologic findings; therefore, biopsy was not pursued in our patient.
Although BASCULE syndrome is a benign entity, it is imperative that it be recognized to avoid a time consuming, expensive, and anxiety-producing diagnostic workup, as occurred in our patient. Although not a manifestation of systemic disease, BASCULE syndrome may be associated with orthostatic hypotension in up to 20% of cases.2,4 Therefore, these patients should undergo orthostatic testing, including the tilt table test. In our patient, these manifestations were not appreciated.
There are no current guidelines for effective treatment of BASCULE syndrome. Given the possible role of mast cells in the condition, H1 antihistamines are proposed as first-line treatment. Desloratadine (10 mg/d for 7 days) has been found to be associated with improvement of pruritus. However, a recent literature review found little evidence to support the use of H1 antihistamines for resolution of other symptoms.2
The differential diagnosis includes livedo reticularis, Bier spots, Sneddon syndrome, and urticarial vasculitis. Livedo reticularis presents as distinct, netlike, blue-erythematousviolaceous discoloration, which differs from the distinct orange-red macules in BASCULE syndrome.5 In addition to distinct variances in dermatologic presentation, livedo reticularis typically is associated with cold exposure as a causative agent, with cold avoidance as the treatment for this benign and often transient condition.6 This phenomenon was not appreciated in our patient. Livedo reticularis commonly occurs with antiphospholipid syndrome.5 This association in combination with our patient's positive ANA findings and her mother's history of miscarriages resulted in the misdiagnosis as livedo reticularis.
Bier spots manifest as white macules with surrounding erythema and typically present in young adults. When first described in the literature, it was debated if BASCULE syndrome was simply another manifestation of Bier spots or postural orthostatic intolerance,4 as there was a large consensus that postural orthostatic intolerance was associated with BASCULE syndrome, with the majority of patients not meeting criteria for the condition. Heymann4 addressed the differences in BASCULE manifestations vs typical Bier spots. The author extended the syndrome to include cyanosis, an urticarialike eruption of red-orange macules with central papules located centrally, pruritus, tenderness, and partial or diffuse edema, in addition to Bier spots.4
Sneddon syndrome is a rare progressive disorder that affects small- to medium-sized blood vessels resulting in multiple episodes of ischemia in the brain. Skin manifestations of these repeated strokes are similar to livedo reticularis, typically manifesting as livedo racemosa—irregular reticular patterns of skin mottling with reddish-blue hues.6 However, Sneddon syndrome is more generalized and widespread and differs from BASCULE syndrome in shape and histologic findings. Our patient presented with findings on the legs, which is more characteristic of livedo reticularis vs livedo racemosa. Our patient experienced resolution upon laying down and sitting, and Sneddon syndrome persists beyond postural changes. Furthermore, patients with Sneddon syndrome present with neurologic symptoms such as prodromal headaches.6
Urticarial vasculitis was ruled out in our patient because of the duration of symptoms as well as the spatial changes. Urticarial vasculitis is a rare skin condition characterized by chronic recurring urticarial lesions that may persist for more than a day. This condition typically presents in middle-aged women and rarely in children. Urticarial vasculitis is thought to be immune-complex mediated, but its cause is largely unknown. It is a common manifestation of underlying conditions such as systemic lupus erythematosus.6 Our patient had a positive ANA and possible autoimmune history from her mother; however, urticarial vasculitis does not present transiently on the legs or in the rash pattern appreciated in our patient.
- Bessis D, Jeziorski E, Rigau V, et al. Bier anaemic spots, cyanosis with urticaria-like eruption (BASCULE) syndrome: a new entity? Br J Dermatol. 2016;175:218-220. doi:10.1111/bjd.14589
- Baurens N, Briand C, Giovannini-Chami L, et al. Case report, practices survey and literature review of an under-recognized pediatric vascular disorder: the BASCULE syndrome. Front Pediatr. 2022;10:849914. doi:10.3389/fped.2022.849914
- Jiménez-Gallo D, Collantes-Rodríguez C, Ossorio-García L, et al. Bier anaemic spots, cyanosis with urticaria-like eruption (BASCULE) syndrome on trunk and upper limbs. Pediatr Dermatol. 2018;35:E313-E315. doi:10.1111/pde.13558
- Heymann WR. BASCULE syndrome: is something brewing with Bier spots? Dermatology World Insights and Inquiries. September 7, 2022. https://www.aad.org/dw/dw-insights-and-inquiries/archive/2022/bascule-syndrome
- Sajjan VV, Lunge S, Swamy MB, et al. Livedo reticularis: a review of the literature. Indian Dermatol Online J. 2015;6:315-321. doi:10.4103/2229-5178.164493
- Gu SL, Jorizzo JL. Urticarial vasculitis. Int J Womens Dermatol. 2021;7:290-297. doi:10.1016/j.ijwd.2021.01.021
The Diagnosis: BASCULE Syndrome
The patient had previously been thought to have livedo reticularis by primary care. Repeat antinuclear antibody (ANA) testing was positive (1:1280 homogeneous [reflexive titers all negative]). However, upon dermatologic evaluation, the manifestation of the rash in addition to onset occurring with postural changes challenged the livedo reticularis diagnosis. Extensive research and consultation with dermatologic colleagues led to the diagnosis of the rare entity BASCULE syndrome. BASCULE (Bier anemic spots, cyanosis, and urticarialike eruption) syndrome was described by Bessis et al1 in 2016. It is a rare condition but may be underreported.2 It is a benign pediatric disorder in the vascular acrosyndrome family that is characterized by underlying vasomotor dysfunction in distal regions of the body. Raynaud phenomenon is a widely known member of this family. As seen in our patient, it typically presents on the distal legs and feet with numerous irregular hypopigmented macules on a cyanotic background. Red-orange papules may appear on the hypopigmented macules and often are pruritic. Lesions on the distal upper extremities are less common, and a case involving the trunk has been reported.3 Onset generally begins within a couple of minutes of standing or mechanical compression of the lower legs, with full reversal of symptoms occurring within minutes of laying down or walking. Commonly reported associated symptoms include tenderness, pruritus, edema, and pain; however, the cutaneous lesions may be asymptomatic. The condition tends to affect adolescents, as seen in our patient; however, there have been reports in infants as young as 3 months to adults aged 19 years.2
The pathophysiology behind BASCULE syndrome remains unclear but is believed to be centered around the role of physiologic venous stasis that occurs when standing. The hypoxia secondary to stasis is thought to induce amplified vasoconstriction of arterioles. These responses are further exaggerated due to absence of venoarteriolar reflexes in dermal ascending arterioles, leading to Bier spots.2 The role of mast cells and eosinophils remains unclear. It is a clinical diagnosis without clear histologic findings; therefore, biopsy was not pursued in our patient.
Although BASCULE syndrome is a benign entity, it is imperative that it be recognized to avoid a time consuming, expensive, and anxiety-producing diagnostic workup, as occurred in our patient. Although not a manifestation of systemic disease, BASCULE syndrome may be associated with orthostatic hypotension in up to 20% of cases.2,4 Therefore, these patients should undergo orthostatic testing, including the tilt table test. In our patient, these manifestations were not appreciated.
There are no current guidelines for effective treatment of BASCULE syndrome. Given the possible role of mast cells in the condition, H1 antihistamines are proposed as first-line treatment. Desloratadine (10 mg/d for 7 days) has been found to be associated with improvement of pruritus. However, a recent literature review found little evidence to support the use of H1 antihistamines for resolution of other symptoms.2
The differential diagnosis includes livedo reticularis, Bier spots, Sneddon syndrome, and urticarial vasculitis. Livedo reticularis presents as distinct, netlike, blue-erythematousviolaceous discoloration, which differs from the distinct orange-red macules in BASCULE syndrome.5 In addition to distinct variances in dermatologic presentation, livedo reticularis typically is associated with cold exposure as a causative agent, with cold avoidance as the treatment for this benign and often transient condition.6 This phenomenon was not appreciated in our patient. Livedo reticularis commonly occurs with antiphospholipid syndrome.5 This association in combination with our patient's positive ANA findings and her mother's history of miscarriages resulted in the misdiagnosis as livedo reticularis.
Bier spots manifest as white macules with surrounding erythema and typically present in young adults. When first described in the literature, it was debated if BASCULE syndrome was simply another manifestation of Bier spots or postural orthostatic intolerance,4 as there was a large consensus that postural orthostatic intolerance was associated with BASCULE syndrome, with the majority of patients not meeting criteria for the condition. Heymann4 addressed the differences in BASCULE manifestations vs typical Bier spots. The author extended the syndrome to include cyanosis, an urticarialike eruption of red-orange macules with central papules located centrally, pruritus, tenderness, and partial or diffuse edema, in addition to Bier spots.4
Sneddon syndrome is a rare progressive disorder that affects small- to medium-sized blood vessels resulting in multiple episodes of ischemia in the brain. Skin manifestations of these repeated strokes are similar to livedo reticularis, typically manifesting as livedo racemosa—irregular reticular patterns of skin mottling with reddish-blue hues.6 However, Sneddon syndrome is more generalized and widespread and differs from BASCULE syndrome in shape and histologic findings. Our patient presented with findings on the legs, which is more characteristic of livedo reticularis vs livedo racemosa. Our patient experienced resolution upon laying down and sitting, and Sneddon syndrome persists beyond postural changes. Furthermore, patients with Sneddon syndrome present with neurologic symptoms such as prodromal headaches.6
Urticarial vasculitis was ruled out in our patient because of the duration of symptoms as well as the spatial changes. Urticarial vasculitis is a rare skin condition characterized by chronic recurring urticarial lesions that may persist for more than a day. This condition typically presents in middle-aged women and rarely in children. Urticarial vasculitis is thought to be immune-complex mediated, but its cause is largely unknown. It is a common manifestation of underlying conditions such as systemic lupus erythematosus.6 Our patient had a positive ANA and possible autoimmune history from her mother; however, urticarial vasculitis does not present transiently on the legs or in the rash pattern appreciated in our patient.
The Diagnosis: BASCULE Syndrome
The patient had previously been thought to have livedo reticularis by primary care. Repeat antinuclear antibody (ANA) testing was positive (1:1280 homogeneous [reflexive titers all negative]). However, upon dermatologic evaluation, the manifestation of the rash in addition to onset occurring with postural changes challenged the livedo reticularis diagnosis. Extensive research and consultation with dermatologic colleagues led to the diagnosis of the rare entity BASCULE syndrome. BASCULE (Bier anemic spots, cyanosis, and urticarialike eruption) syndrome was described by Bessis et al1 in 2016. It is a rare condition but may be underreported.2 It is a benign pediatric disorder in the vascular acrosyndrome family that is characterized by underlying vasomotor dysfunction in distal regions of the body. Raynaud phenomenon is a widely known member of this family. As seen in our patient, it typically presents on the distal legs and feet with numerous irregular hypopigmented macules on a cyanotic background. Red-orange papules may appear on the hypopigmented macules and often are pruritic. Lesions on the distal upper extremities are less common, and a case involving the trunk has been reported.3 Onset generally begins within a couple of minutes of standing or mechanical compression of the lower legs, with full reversal of symptoms occurring within minutes of laying down or walking. Commonly reported associated symptoms include tenderness, pruritus, edema, and pain; however, the cutaneous lesions may be asymptomatic. The condition tends to affect adolescents, as seen in our patient; however, there have been reports in infants as young as 3 months to adults aged 19 years.2
The pathophysiology behind BASCULE syndrome remains unclear but is believed to be centered around the role of physiologic venous stasis that occurs when standing. The hypoxia secondary to stasis is thought to induce amplified vasoconstriction of arterioles. These responses are further exaggerated due to absence of venoarteriolar reflexes in dermal ascending arterioles, leading to Bier spots.2 The role of mast cells and eosinophils remains unclear. It is a clinical diagnosis without clear histologic findings; therefore, biopsy was not pursued in our patient.
Although BASCULE syndrome is a benign entity, it is imperative that it be recognized to avoid a time consuming, expensive, and anxiety-producing diagnostic workup, as occurred in our patient. Although not a manifestation of systemic disease, BASCULE syndrome may be associated with orthostatic hypotension in up to 20% of cases.2,4 Therefore, these patients should undergo orthostatic testing, including the tilt table test. In our patient, these manifestations were not appreciated.
There are no current guidelines for effective treatment of BASCULE syndrome. Given the possible role of mast cells in the condition, H1 antihistamines are proposed as first-line treatment. Desloratadine (10 mg/d for 7 days) has been found to be associated with improvement of pruritus. However, a recent literature review found little evidence to support the use of H1 antihistamines for resolution of other symptoms.2
The differential diagnosis includes livedo reticularis, Bier spots, Sneddon syndrome, and urticarial vasculitis. Livedo reticularis presents as distinct, netlike, blue-erythematousviolaceous discoloration, which differs from the distinct orange-red macules in BASCULE syndrome.5 In addition to distinct variances in dermatologic presentation, livedo reticularis typically is associated with cold exposure as a causative agent, with cold avoidance as the treatment for this benign and often transient condition.6 This phenomenon was not appreciated in our patient. Livedo reticularis commonly occurs with antiphospholipid syndrome.5 This association in combination with our patient's positive ANA findings and her mother's history of miscarriages resulted in the misdiagnosis as livedo reticularis.
Bier spots manifest as white macules with surrounding erythema and typically present in young adults. When first described in the literature, it was debated if BASCULE syndrome was simply another manifestation of Bier spots or postural orthostatic intolerance,4 as there was a large consensus that postural orthostatic intolerance was associated with BASCULE syndrome, with the majority of patients not meeting criteria for the condition. Heymann4 addressed the differences in BASCULE manifestations vs typical Bier spots. The author extended the syndrome to include cyanosis, an urticarialike eruption of red-orange macules with central papules located centrally, pruritus, tenderness, and partial or diffuse edema, in addition to Bier spots.4
Sneddon syndrome is a rare progressive disorder that affects small- to medium-sized blood vessels resulting in multiple episodes of ischemia in the brain. Skin manifestations of these repeated strokes are similar to livedo reticularis, typically manifesting as livedo racemosa—irregular reticular patterns of skin mottling with reddish-blue hues.6 However, Sneddon syndrome is more generalized and widespread and differs from BASCULE syndrome in shape and histologic findings. Our patient presented with findings on the legs, which is more characteristic of livedo reticularis vs livedo racemosa. Our patient experienced resolution upon laying down and sitting, and Sneddon syndrome persists beyond postural changes. Furthermore, patients with Sneddon syndrome present with neurologic symptoms such as prodromal headaches.6
Urticarial vasculitis was ruled out in our patient because of the duration of symptoms as well as the spatial changes. Urticarial vasculitis is a rare skin condition characterized by chronic recurring urticarial lesions that may persist for more than a day. This condition typically presents in middle-aged women and rarely in children. Urticarial vasculitis is thought to be immune-complex mediated, but its cause is largely unknown. It is a common manifestation of underlying conditions such as systemic lupus erythematosus.6 Our patient had a positive ANA and possible autoimmune history from her mother; however, urticarial vasculitis does not present transiently on the legs or in the rash pattern appreciated in our patient.
- Bessis D, Jeziorski E, Rigau V, et al. Bier anaemic spots, cyanosis with urticaria-like eruption (BASCULE) syndrome: a new entity? Br J Dermatol. 2016;175:218-220. doi:10.1111/bjd.14589
- Baurens N, Briand C, Giovannini-Chami L, et al. Case report, practices survey and literature review of an under-recognized pediatric vascular disorder: the BASCULE syndrome. Front Pediatr. 2022;10:849914. doi:10.3389/fped.2022.849914
- Jiménez-Gallo D, Collantes-Rodríguez C, Ossorio-García L, et al. Bier anaemic spots, cyanosis with urticaria-like eruption (BASCULE) syndrome on trunk and upper limbs. Pediatr Dermatol. 2018;35:E313-E315. doi:10.1111/pde.13558
- Heymann WR. BASCULE syndrome: is something brewing with Bier spots? Dermatology World Insights and Inquiries. September 7, 2022. https://www.aad.org/dw/dw-insights-and-inquiries/archive/2022/bascule-syndrome
- Sajjan VV, Lunge S, Swamy MB, et al. Livedo reticularis: a review of the literature. Indian Dermatol Online J. 2015;6:315-321. doi:10.4103/2229-5178.164493
- Gu SL, Jorizzo JL. Urticarial vasculitis. Int J Womens Dermatol. 2021;7:290-297. doi:10.1016/j.ijwd.2021.01.021
- Bessis D, Jeziorski E, Rigau V, et al. Bier anaemic spots, cyanosis with urticaria-like eruption (BASCULE) syndrome: a new entity? Br J Dermatol. 2016;175:218-220. doi:10.1111/bjd.14589
- Baurens N, Briand C, Giovannini-Chami L, et al. Case report, practices survey and literature review of an under-recognized pediatric vascular disorder: the BASCULE syndrome. Front Pediatr. 2022;10:849914. doi:10.3389/fped.2022.849914
- Jiménez-Gallo D, Collantes-Rodríguez C, Ossorio-García L, et al. Bier anaemic spots, cyanosis with urticaria-like eruption (BASCULE) syndrome on trunk and upper limbs. Pediatr Dermatol. 2018;35:E313-E315. doi:10.1111/pde.13558
- Heymann WR. BASCULE syndrome: is something brewing with Bier spots? Dermatology World Insights and Inquiries. September 7, 2022. https://www.aad.org/dw/dw-insights-and-inquiries/archive/2022/bascule-syndrome
- Sajjan VV, Lunge S, Swamy MB, et al. Livedo reticularis: a review of the literature. Indian Dermatol Online J. 2015;6:315-321. doi:10.4103/2229-5178.164493
- Gu SL, Jorizzo JL. Urticarial vasculitis. Int J Womens Dermatol. 2021;7:290-297. doi:10.1016/j.ijwd.2021.01.021
An 11-year-old girl was referred to the dermatology clinic for evaluation of a rash on the legs and feet of 1 year’s duration. The rash appeared every time she was standing for longer than 10 to 15 minutes and resolved when sitting or laying down. After the initial onset, the rash did not spread to other body areas but became more prominent in appearance. The patient endorsed intense pruritus associated with the rash. A review of systems was negative for fever, headaches, history of blood clots, and joint pain. She did not have any known medical conditions or take any medications. The patient’s mother reported that the patient experienced episodes of leg numbness while sitting in vehicles from 6 to 10 years of age. There was no family history of rheumatologic, hematologic, or cardiac conditions. The patient’s mother had experienced 2 miscarriages but denied any other obstetric complications. The patient had 1 sibling who was unaffected. Physical examination revealed reticulate erythema on the calves with scattered regions of blanching and evanescent pink macules as well as dermatographism.
One month prior to presenting to dermatology, the patient was evaluated by rheumatology, endocrinology, and hematology. Laboratory workup completed at age 3 years included antinuclear antibody, anticardiolipin antibody, and antithrombin III activity; factor V Leiden; cryoglobulins; quantitation (human chorionic gonadotropin); proteins S and C activity; antineutrophil cytoplasmic antibody screen; thyroid studies; prothrombin time; and partial thromboplastin time. All laboratory results were within reference range.
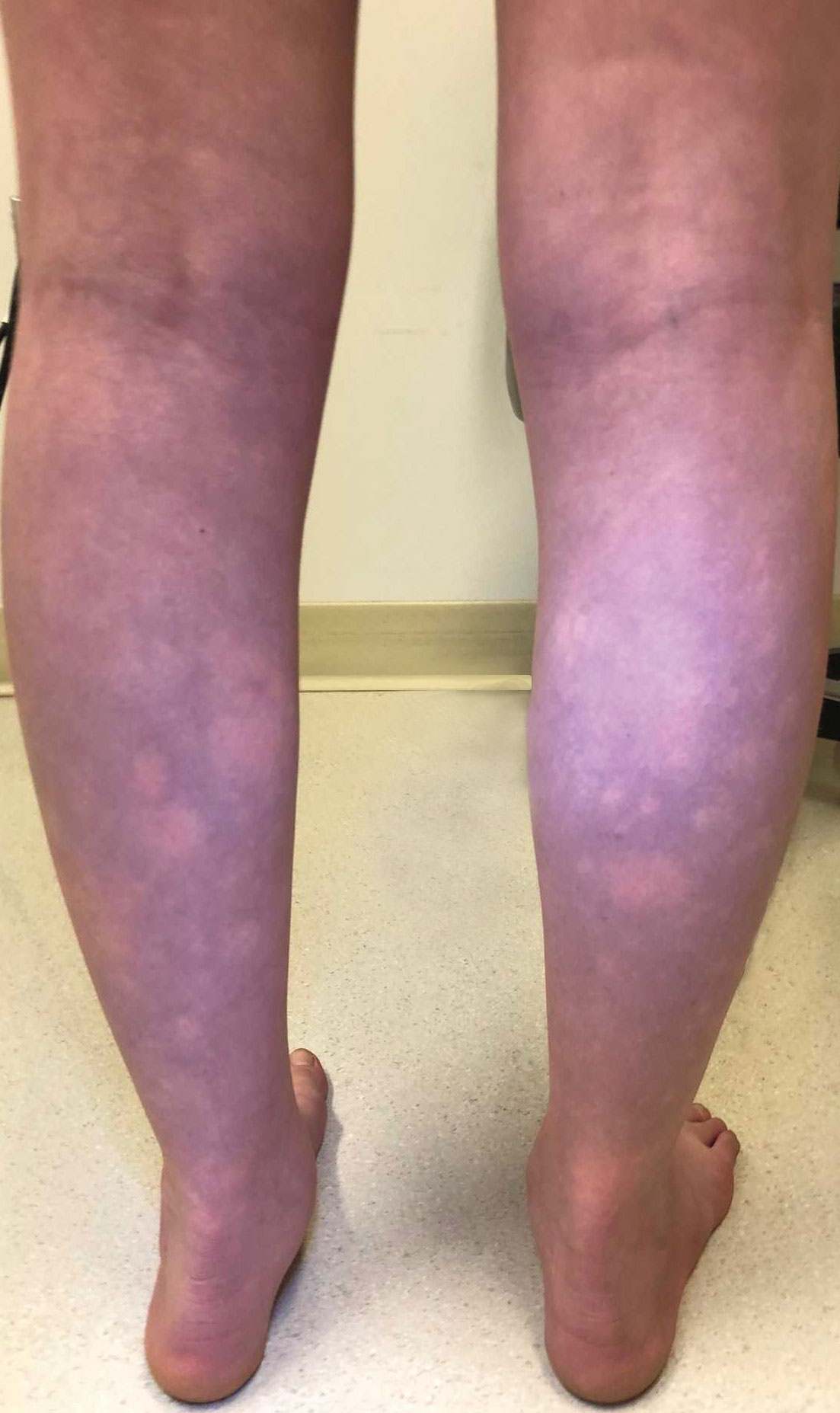
Diphenhydramine and Prochlorperazine Combo Not Associated With Migraine Treatment Failure
Key clinical point: The odds of treatment failure were not increased, and no extrapyramidal adverse events were reported in pediatric patients with migraine when diphenhydramine was coadministered with prochlorperazine in an emergency department (ED) setting.
Major finding: The administration of diphenhydramine plus prochlorperazine vs prochlorperazine alone was not associated with increased odds of additional migraine therapy (P = .347), hospitalization rates (P = .425), and 72-hour return visit rates (P = .271). None of the patients in the diphenhydramine plus prochlorperazine group experienced extrapyramidal adverse events, while 2.4% of patients in prochlorperazine group experienced extrapyramidal adverse events.
Study details: Findings are from a retrospective cohort study that included 1683 pediatric patients with migraine presenting to the ED who received diphenhydramine plus prochlorperazine (n = 1215) or prochlorperazine only (n = 468).
Disclosures: This study did not disclose any funding source. The authors declared no conflicts of interest.
Source: Naeem S, Lozano JM, Ruiz Castaneda AM, Lowe D. Diphenhydramine and migraine treatment failure in pediatric patients receiving prochlorperazine. Pediatr Emerg Care. 2024 (May 9). doi: 10.1097/PEC.0000000000003202 Source
Key clinical point: The odds of treatment failure were not increased, and no extrapyramidal adverse events were reported in pediatric patients with migraine when diphenhydramine was coadministered with prochlorperazine in an emergency department (ED) setting.
Major finding: The administration of diphenhydramine plus prochlorperazine vs prochlorperazine alone was not associated with increased odds of additional migraine therapy (P = .347), hospitalization rates (P = .425), and 72-hour return visit rates (P = .271). None of the patients in the diphenhydramine plus prochlorperazine group experienced extrapyramidal adverse events, while 2.4% of patients in prochlorperazine group experienced extrapyramidal adverse events.
Study details: Findings are from a retrospective cohort study that included 1683 pediatric patients with migraine presenting to the ED who received diphenhydramine plus prochlorperazine (n = 1215) or prochlorperazine only (n = 468).
Disclosures: This study did not disclose any funding source. The authors declared no conflicts of interest.
Source: Naeem S, Lozano JM, Ruiz Castaneda AM, Lowe D. Diphenhydramine and migraine treatment failure in pediatric patients receiving prochlorperazine. Pediatr Emerg Care. 2024 (May 9). doi: 10.1097/PEC.0000000000003202 Source
Key clinical point: The odds of treatment failure were not increased, and no extrapyramidal adverse events were reported in pediatric patients with migraine when diphenhydramine was coadministered with prochlorperazine in an emergency department (ED) setting.
Major finding: The administration of diphenhydramine plus prochlorperazine vs prochlorperazine alone was not associated with increased odds of additional migraine therapy (P = .347), hospitalization rates (P = .425), and 72-hour return visit rates (P = .271). None of the patients in the diphenhydramine plus prochlorperazine group experienced extrapyramidal adverse events, while 2.4% of patients in prochlorperazine group experienced extrapyramidal adverse events.
Study details: Findings are from a retrospective cohort study that included 1683 pediatric patients with migraine presenting to the ED who received diphenhydramine plus prochlorperazine (n = 1215) or prochlorperazine only (n = 468).
Disclosures: This study did not disclose any funding source. The authors declared no conflicts of interest.
Source: Naeem S, Lozano JM, Ruiz Castaneda AM, Lowe D. Diphenhydramine and migraine treatment failure in pediatric patients receiving prochlorperazine. Pediatr Emerg Care. 2024 (May 9). doi: 10.1097/PEC.0000000000003202 Source
Amitriptyline May Be a Better Treatment Choice Than Cinnarizine for Pediatric Migraine
Key clinical point: Both cinnarizine and amitriptyline effectively improved migraine symptoms in children and adolescents with migraine, but amitriptyline was a more preferable treatment option since it reduced headache frequency and duration more effectively than cinnarizine.
Major finding: Amitriptyline was more effective than cinnarizine in reducing headache frequency at 4 weeks (mean difference [MD] −8.81 attacks/months; P = .004) and headache duration at 4 (MD −123.0 minutes; P = .017), 8 (MD −110.3 minutes; P = .033), and 12 (MD −123.3 minutes; P = .018) weeks. However, there were no significant differences in headache severity and migraine-related disability between the groups at 4, 8, and 12 weeks (all P > .005).
Study details: Findings are from a randomized, double-blind controlled trial including 43 children with migraine (age 4-17 years) who were randomly assigned to receive cinnarizine (n = 22) and amitriptyline (n = 21).
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Olfat M, Hosseinpour S, Masoumi S, et al. A comparative study on prophylactic efficacy of cinnarizine and amitriptyline in childhood migraine: A randomized double-blind clinical trial. Cephalalgia. 2024 (Apr 20). doi: 10.1177/03331024241230963 Source
Key clinical point: Both cinnarizine and amitriptyline effectively improved migraine symptoms in children and adolescents with migraine, but amitriptyline was a more preferable treatment option since it reduced headache frequency and duration more effectively than cinnarizine.
Major finding: Amitriptyline was more effective than cinnarizine in reducing headache frequency at 4 weeks (mean difference [MD] −8.81 attacks/months; P = .004) and headache duration at 4 (MD −123.0 minutes; P = .017), 8 (MD −110.3 minutes; P = .033), and 12 (MD −123.3 minutes; P = .018) weeks. However, there were no significant differences in headache severity and migraine-related disability between the groups at 4, 8, and 12 weeks (all P > .005).
Study details: Findings are from a randomized, double-blind controlled trial including 43 children with migraine (age 4-17 years) who were randomly assigned to receive cinnarizine (n = 22) and amitriptyline (n = 21).
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Olfat M, Hosseinpour S, Masoumi S, et al. A comparative study on prophylactic efficacy of cinnarizine and amitriptyline in childhood migraine: A randomized double-blind clinical trial. Cephalalgia. 2024 (Apr 20). doi: 10.1177/03331024241230963 Source
Key clinical point: Both cinnarizine and amitriptyline effectively improved migraine symptoms in children and adolescents with migraine, but amitriptyline was a more preferable treatment option since it reduced headache frequency and duration more effectively than cinnarizine.
Major finding: Amitriptyline was more effective than cinnarizine in reducing headache frequency at 4 weeks (mean difference [MD] −8.81 attacks/months; P = .004) and headache duration at 4 (MD −123.0 minutes; P = .017), 8 (MD −110.3 minutes; P = .033), and 12 (MD −123.3 minutes; P = .018) weeks. However, there were no significant differences in headache severity and migraine-related disability between the groups at 4, 8, and 12 weeks (all P > .005).
Study details: Findings are from a randomized, double-blind controlled trial including 43 children with migraine (age 4-17 years) who were randomly assigned to receive cinnarizine (n = 22) and amitriptyline (n = 21).
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Olfat M, Hosseinpour S, Masoumi S, et al. A comparative study on prophylactic efficacy of cinnarizine and amitriptyline in childhood migraine: A randomized double-blind clinical trial. Cephalalgia. 2024 (Apr 20). doi: 10.1177/03331024241230963 Source
Severe Headache or Migraine Raises Risk for Erectile Dysfunction
Key clinical point: This cross-sectional study demonstrated a significant association between severe headache or migraine and erectile dysfunction (ED) in adult men in the US; however, the results should be interpreted carefully as it did not investigate the effects of depression and anxiety on ED.
Major finding: Presence vs absence of severe headache or migraine was associated with 51% increased risk of ED (adjusted odd ratio 1.51; P = .0036). Age of 40-60 years (P = 0.0292), body mass index < 25 kg/m2 (P = .0406) or ≥30 kg/m2 (P = .0222), metabolic disorders, such as hypertension (P = .0029), diabetes mellitus (P < .001), and hyperlipidemia (P = .0281), were significant risk factors for ED in those with severe headache or migraine.
Study details: This cross-sectional study included 3117 adult men with (n = 582) and without (n = 2535) history of ED from the US National Health and Nutrition Examination Survey (2001-2 and 2003-4), of whom 16.85% had severe headache or migraine.
Disclosures: This study was funded by National Natural Science Foundation of China. The authors declared no competing interests.
Source: Wu X, Zhang Y, Liu G, et al. Association between severe headache or migraine and erectile dysfunction in American adults: A cross-sectional of data study from the NHANES. Int J Impot Res. 2024 (Apr 12). doi: 10.1038/s41443-024-00867-w Source
Key clinical point: This cross-sectional study demonstrated a significant association between severe headache or migraine and erectile dysfunction (ED) in adult men in the US; however, the results should be interpreted carefully as it did not investigate the effects of depression and anxiety on ED.
Major finding: Presence vs absence of severe headache or migraine was associated with 51% increased risk of ED (adjusted odd ratio 1.51; P = .0036). Age of 40-60 years (P = 0.0292), body mass index < 25 kg/m2 (P = .0406) or ≥30 kg/m2 (P = .0222), metabolic disorders, such as hypertension (P = .0029), diabetes mellitus (P < .001), and hyperlipidemia (P = .0281), were significant risk factors for ED in those with severe headache or migraine.
Study details: This cross-sectional study included 3117 adult men with (n = 582) and without (n = 2535) history of ED from the US National Health and Nutrition Examination Survey (2001-2 and 2003-4), of whom 16.85% had severe headache or migraine.
Disclosures: This study was funded by National Natural Science Foundation of China. The authors declared no competing interests.
Source: Wu X, Zhang Y, Liu G, et al. Association between severe headache or migraine and erectile dysfunction in American adults: A cross-sectional of data study from the NHANES. Int J Impot Res. 2024 (Apr 12). doi: 10.1038/s41443-024-00867-w Source
Key clinical point: This cross-sectional study demonstrated a significant association between severe headache or migraine and erectile dysfunction (ED) in adult men in the US; however, the results should be interpreted carefully as it did not investigate the effects of depression and anxiety on ED.
Major finding: Presence vs absence of severe headache or migraine was associated with 51% increased risk of ED (adjusted odd ratio 1.51; P = .0036). Age of 40-60 years (P = 0.0292), body mass index < 25 kg/m2 (P = .0406) or ≥30 kg/m2 (P = .0222), metabolic disorders, such as hypertension (P = .0029), diabetes mellitus (P < .001), and hyperlipidemia (P = .0281), were significant risk factors for ED in those with severe headache or migraine.
Study details: This cross-sectional study included 3117 adult men with (n = 582) and without (n = 2535) history of ED from the US National Health and Nutrition Examination Survey (2001-2 and 2003-4), of whom 16.85% had severe headache or migraine.
Disclosures: This study was funded by National Natural Science Foundation of China. The authors declared no competing interests.
Source: Wu X, Zhang Y, Liu G, et al. Association between severe headache or migraine and erectile dysfunction in American adults: A cross-sectional of data study from the NHANES. Int J Impot Res. 2024 (Apr 12). doi: 10.1038/s41443-024-00867-w Source
Study Shows Reciprocal Causal Association Between Migraine and Venous Thromboembolism
Key clinical point: Presence of migraine poses a strong risk for incident venous thromboembolism (VTE), whereas VTE is modest risk factor for the onset of migraine.
Major finding: The risk of developing VTE was significantly higher in patients with vs without migraine (odds ratio [OR] 96.155; P = .004). Conversely, the risk for migraine was modestly higher in patients with vs without VTE (OR 1.002; P = .016).
Study details: This two-sample bidirectional Mendelian randomization study evaluated the causal association between migraine and VTE using single-nucleotide polymorphisms as instrumental variables obtained from large-scale Genome-Wide Association Studies public databases (IEU Open GWAS project, FinnGen).
Disclosures: The study did not disclose any funding. The authors declared no conflicts of interest.
Source: Wang Y, Hu X, Wang X, et al. Exploring the two-way link between migraines and venous thromboembolism: A bidirectional two-sample Mendelian randomization study. Thromb Haemost. 2024 (Apr 24). doi: 10.1055/a-2313-0311 Source
Key clinical point: Presence of migraine poses a strong risk for incident venous thromboembolism (VTE), whereas VTE is modest risk factor for the onset of migraine.
Major finding: The risk of developing VTE was significantly higher in patients with vs without migraine (odds ratio [OR] 96.155; P = .004). Conversely, the risk for migraine was modestly higher in patients with vs without VTE (OR 1.002; P = .016).
Study details: This two-sample bidirectional Mendelian randomization study evaluated the causal association between migraine and VTE using single-nucleotide polymorphisms as instrumental variables obtained from large-scale Genome-Wide Association Studies public databases (IEU Open GWAS project, FinnGen).
Disclosures: The study did not disclose any funding. The authors declared no conflicts of interest.
Source: Wang Y, Hu X, Wang X, et al. Exploring the two-way link between migraines and venous thromboembolism: A bidirectional two-sample Mendelian randomization study. Thromb Haemost. 2024 (Apr 24). doi: 10.1055/a-2313-0311 Source
Key clinical point: Presence of migraine poses a strong risk for incident venous thromboembolism (VTE), whereas VTE is modest risk factor for the onset of migraine.
Major finding: The risk of developing VTE was significantly higher in patients with vs without migraine (odds ratio [OR] 96.155; P = .004). Conversely, the risk for migraine was modestly higher in patients with vs without VTE (OR 1.002; P = .016).
Study details: This two-sample bidirectional Mendelian randomization study evaluated the causal association between migraine and VTE using single-nucleotide polymorphisms as instrumental variables obtained from large-scale Genome-Wide Association Studies public databases (IEU Open GWAS project, FinnGen).
Disclosures: The study did not disclose any funding. The authors declared no conflicts of interest.
Source: Wang Y, Hu X, Wang X, et al. Exploring the two-way link between migraines and venous thromboembolism: A bidirectional two-sample Mendelian randomization study. Thromb Haemost. 2024 (Apr 24). doi: 10.1055/a-2313-0311 Source
Meta-analysis Shows Inverse Correlation Between PACAP and Migraine Duration
Key clinical point: Very low-quality evidence showed that serum pituitary adenylates cyclase–activating polypeptide (PACAP) levels were lower in adults with a longer history of migraine.
Major finding: Serum levels of PACAP were inversely associated with history of migraine duration in adults with migraine (summary r −0.35; P < .01). It was also seen that serum PACAP levels were higher during the ictal vs interictal period in both adults and children with migraine (standardized mean difference 0.41; 95% CI 0.17-0.66).
Study details: Findings are from a meta-analysis of eight observational studies including 674 patients with migraine and 371 control individuals without migraine.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Zhu G, Wang M, Kong F. Blood serum levels of PACAP and migraine onset: A systematic review and meta-analysis of observational studies. Headache. 2024 (Apr 24). doi: 10.1111/head.14711 Source
Key clinical point: Very low-quality evidence showed that serum pituitary adenylates cyclase–activating polypeptide (PACAP) levels were lower in adults with a longer history of migraine.
Major finding: Serum levels of PACAP were inversely associated with history of migraine duration in adults with migraine (summary r −0.35; P < .01). It was also seen that serum PACAP levels were higher during the ictal vs interictal period in both adults and children with migraine (standardized mean difference 0.41; 95% CI 0.17-0.66).
Study details: Findings are from a meta-analysis of eight observational studies including 674 patients with migraine and 371 control individuals without migraine.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Zhu G, Wang M, Kong F. Blood serum levels of PACAP and migraine onset: A systematic review and meta-analysis of observational studies. Headache. 2024 (Apr 24). doi: 10.1111/head.14711 Source
Key clinical point: Very low-quality evidence showed that serum pituitary adenylates cyclase–activating polypeptide (PACAP) levels were lower in adults with a longer history of migraine.
Major finding: Serum levels of PACAP were inversely associated with history of migraine duration in adults with migraine (summary r −0.35; P < .01). It was also seen that serum PACAP levels were higher during the ictal vs interictal period in both adults and children with migraine (standardized mean difference 0.41; 95% CI 0.17-0.66).
Study details: Findings are from a meta-analysis of eight observational studies including 674 patients with migraine and 371 control individuals without migraine.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Zhu G, Wang M, Kong F. Blood serum levels of PACAP and migraine onset: A systematic review and meta-analysis of observational studies. Headache. 2024 (Apr 24). doi: 10.1111/head.14711 Source
Rimegepant Effective and Well Tolerated for Acute Migraine
Key clinical point: Rimegepant orally disintegrating tablet (ODT) offers pain relief within 2 hours of treatment in adults with migraine, with a tolerable safety profile.
Major finding: At 2 hours post dose, rimegepant was more effective than placebo in providing freedom from pain (risk difference 7.6; P = .0004) and the most bothersome symptom (risk difference 16.2; P < .0001). The overall rates of treatment-emergent adverse events were comparable between the rimegepant and placebo groups (15.2% and 16.4%, respectively).
Study details: This subgroup analysis of a double-blind, randomized, placebo-controlled phase 3 clinical trial included 1075 patients with acute migraine with or without aura (age ≥ 18 years) who were randomly assigned to receive either 75 mg rimegepant ODT (n = 538) or placebo (n = 537).
Disclosures: This study was funded by BioShin, a wholly owned subsidiary of Biohaven Pharmaceuticals, which was acquired by Pfizer. Pfizer provided writing support. Five authors declared being employees or stock owners of Biohaven, BioShin, or Pfizer. The remaining authors declared no conflicts of interest.
Source: Yu S, Guo A, Wang Z, et al. Rimegepant orally disintegrating tablet 75 mg for acute treatment of migraine in adults from China: A subgroup analysis of a double-blind, randomized, placebo-controlled, phase 3 clinical trial. J Headache Pain. 2024;25:57 (Apr 16). Source
Key clinical point: Rimegepant orally disintegrating tablet (ODT) offers pain relief within 2 hours of treatment in adults with migraine, with a tolerable safety profile.
Major finding: At 2 hours post dose, rimegepant was more effective than placebo in providing freedom from pain (risk difference 7.6; P = .0004) and the most bothersome symptom (risk difference 16.2; P < .0001). The overall rates of treatment-emergent adverse events were comparable between the rimegepant and placebo groups (15.2% and 16.4%, respectively).
Study details: This subgroup analysis of a double-blind, randomized, placebo-controlled phase 3 clinical trial included 1075 patients with acute migraine with or without aura (age ≥ 18 years) who were randomly assigned to receive either 75 mg rimegepant ODT (n = 538) or placebo (n = 537).
Disclosures: This study was funded by BioShin, a wholly owned subsidiary of Biohaven Pharmaceuticals, which was acquired by Pfizer. Pfizer provided writing support. Five authors declared being employees or stock owners of Biohaven, BioShin, or Pfizer. The remaining authors declared no conflicts of interest.
Source: Yu S, Guo A, Wang Z, et al. Rimegepant orally disintegrating tablet 75 mg for acute treatment of migraine in adults from China: A subgroup analysis of a double-blind, randomized, placebo-controlled, phase 3 clinical trial. J Headache Pain. 2024;25:57 (Apr 16). Source
Key clinical point: Rimegepant orally disintegrating tablet (ODT) offers pain relief within 2 hours of treatment in adults with migraine, with a tolerable safety profile.
Major finding: At 2 hours post dose, rimegepant was more effective than placebo in providing freedom from pain (risk difference 7.6; P = .0004) and the most bothersome symptom (risk difference 16.2; P < .0001). The overall rates of treatment-emergent adverse events were comparable between the rimegepant and placebo groups (15.2% and 16.4%, respectively).
Study details: This subgroup analysis of a double-blind, randomized, placebo-controlled phase 3 clinical trial included 1075 patients with acute migraine with or without aura (age ≥ 18 years) who were randomly assigned to receive either 75 mg rimegepant ODT (n = 538) or placebo (n = 537).
Disclosures: This study was funded by BioShin, a wholly owned subsidiary of Biohaven Pharmaceuticals, which was acquired by Pfizer. Pfizer provided writing support. Five authors declared being employees or stock owners of Biohaven, BioShin, or Pfizer. The remaining authors declared no conflicts of interest.
Source: Yu S, Guo A, Wang Z, et al. Rimegepant orally disintegrating tablet 75 mg for acute treatment of migraine in adults from China: A subgroup analysis of a double-blind, randomized, placebo-controlled, phase 3 clinical trial. J Headache Pain. 2024;25:57 (Apr 16). Source
Meta-analysis Compares Effectiveness of Parenteral Agents for Migraine Pain in ED
Key clinical point: Combination therapy with two parenteral agents or monotherapy with either neuroleptics or metoclopramide can be considered as a first-line treatment option for the management of acute migraine pain in the emergency department (ED) settings.
Major finding: Combination therapy of two parenteral agents vs placebo was an effective treatment option in reducing pain intensity scores (mean difference −3.36; 95% CI −4.64 to −2.08) and increasing the rate of achievement of pain relief (risk ratio 2.83; 95% CI 1.74-4.61). Monotherapy with neuroleptics and metoclopramide also provided pain relief and helped patients achieve pain-free status prior to discharge from the ED but increased the risk for adverse events, especially akathisia.
Study details: This meta-analysis of 97 randomized controlled trials evaluated the effectiveness of various parenteral agents for pain relief in patients with acute migraine presenting to the ED.
Disclosures: This study was funded by the Emergency Medicine Research Group, Canada. The authors declared no conflicts of interest.
Source: Kirkland SW, Visser L, Meyer J, et al. The effectiveness of parenteral agents for pain reduction in patients with migraine presenting to emergency settings: A systematic review and network analysis. Headache. 2024;64(4):424-447. doi: 10.1111/head.14704 Source
Key clinical point: Combination therapy with two parenteral agents or monotherapy with either neuroleptics or metoclopramide can be considered as a first-line treatment option for the management of acute migraine pain in the emergency department (ED) settings.
Major finding: Combination therapy of two parenteral agents vs placebo was an effective treatment option in reducing pain intensity scores (mean difference −3.36; 95% CI −4.64 to −2.08) and increasing the rate of achievement of pain relief (risk ratio 2.83; 95% CI 1.74-4.61). Monotherapy with neuroleptics and metoclopramide also provided pain relief and helped patients achieve pain-free status prior to discharge from the ED but increased the risk for adverse events, especially akathisia.
Study details: This meta-analysis of 97 randomized controlled trials evaluated the effectiveness of various parenteral agents for pain relief in patients with acute migraine presenting to the ED.
Disclosures: This study was funded by the Emergency Medicine Research Group, Canada. The authors declared no conflicts of interest.
Source: Kirkland SW, Visser L, Meyer J, et al. The effectiveness of parenteral agents for pain reduction in patients with migraine presenting to emergency settings: A systematic review and network analysis. Headache. 2024;64(4):424-447. doi: 10.1111/head.14704 Source
Key clinical point: Combination therapy with two parenteral agents or monotherapy with either neuroleptics or metoclopramide can be considered as a first-line treatment option for the management of acute migraine pain in the emergency department (ED) settings.
Major finding: Combination therapy of two parenteral agents vs placebo was an effective treatment option in reducing pain intensity scores (mean difference −3.36; 95% CI −4.64 to −2.08) and increasing the rate of achievement of pain relief (risk ratio 2.83; 95% CI 1.74-4.61). Monotherapy with neuroleptics and metoclopramide also provided pain relief and helped patients achieve pain-free status prior to discharge from the ED but increased the risk for adverse events, especially akathisia.
Study details: This meta-analysis of 97 randomized controlled trials evaluated the effectiveness of various parenteral agents for pain relief in patients with acute migraine presenting to the ED.
Disclosures: This study was funded by the Emergency Medicine Research Group, Canada. The authors declared no conflicts of interest.
Source: Kirkland SW, Visser L, Meyer J, et al. The effectiveness of parenteral agents for pain reduction in patients with migraine presenting to emergency settings: A systematic review and network analysis. Headache. 2024;64(4):424-447. doi: 10.1111/head.14704 Source