User login
Drospirenone vs norethindrone progestin-only pills. Is there a clear winner?
Contraception and family planning have improved the health of all people by reducing maternal mortality, improving maternal and child health through birth spacing, supporting full education attainment, and advancing workforce participation.1 Contraception is cost-effective and should be supported by all health insurers. One economic study reported that depending on the contraceptive method utilized, up to $7 of health care costs were saved for each dollar spent on contraceptive services and supplies.2
Progestin-only pills (POPs) are an important contraceptive option for people in the following situations who3:
- have a contraindication to estrogen-containing contraceptives
- are actively breastfeeding
- are less than 21 days since birth
- have a preference to avoid estrogen.
POPs are contraindicated for women who have breast cancer, abnormal uterine bleeding, or active liver disease and for women who are pregnant. A history of bariatric surgery with a malabsorption procedure (Roux-en-Y and biliopancreatic diversion) and the use of antiepileptic medications that are strong enzyme inducers are additional situations where the risk of POP may outweigh the benefit.3 Alternative progestin-only options include the subdermal etonogestrel implant, depot medroxyprogesterone acetate, and levonorgestrel-releasing intrauterine devices. These 3 options provide superior contraceptive efficacy to POP.
As a contraceptive, norethindrone at a dose of 0.35 mg daily has two major flaws:
- it does not reliably inhibit ovulation
- it has a short half-life.
In clinical studies, norethindrone inhibits ovulation in approximately 50% of cycles.4,5 Because norethindrone at a dose of 0.35 mg does not reliably inhibit ovulation it relies on additional mechanisms for contraceptive efficacy, including thickening of the cervical mucus to block sperm entry into the upper reproductive tract, reduced fallopian tube motility, and thinning of the endometrium.6
Norethindrone POP is formulated in packs of 28 pills containing 0.35 mg intended for daily continuous administration and no medication-free intervals. One rationale for the low dose of 0.35 mg in norethindrone POP is that it approximates the lowest dose with contraceptive efficacy for breastfeeding women, which has the benefit of minimizing exposure of the baby to the medication. Estrogen-progestin birth control pills containing norethindrone as the progestin reliably inhibit ovulation and have a minimum of 1 mg of norethindrone in each hormone pill. A POP with 1 mg of norethindrone per pill would likely have greater contraceptive efficacy. When taken daily, norethindrone acetate 5 mg (Aygestin) suppresses ovarian estrogen production, ovulation, and often causes cessation of uterine bleeding.7 The short half-life of norethindrone (7.7 hours) further exacerbates the problem of an insufficient daily dose.6 The standard guidance is that norethindrone must be taken at the same time every day, a goal that is nearly impossible to achieve. If a dose of norethindrone is taken >3 hours late, backup contraception is recommended for 48 hours.6
Drospirenone is a chemical analogue of spironolactone. Drospirenone is a progestin that suppresses LH and FSH and has anti-androgenic and partial anti-mineralocorticoid effects.8 Drospirenone POP contains 4 mg of a nonmicronized formulation that is believed to provide a pharmacologically similar area under the curve in drug metabolism studies to the 3 mg of micronized drospirenone, present in drospirenone-containing estrogen-progestin contraceptives.8 It is provided in a pack of 28 pills with 24 drospirenone pills and 4 pills without hormone. Drospirenone has a long half-life of 30 to 34 hours.8 If ≥2 drospirenone pills are missed, backup contraception is recommended for 7 days.9 The contraceptive effectiveness of drospirenone POP is thought to be similar to estrogen-progestin pills.8 Theoretically, drospirenone, acting as an anti-mineralocorticoid, can cause hyperkalemia. People with renal and adrenal insufficiency are most vulnerable to this adverse effect and should not be prescribed drospirenone. Women taking drospirenone and a medication that strongly inhibits CYP3A4, an enzyme involved in drospirenone degradation—including ketoconazole, indinavir, boceprevir, and clarithromycin—may have increased circulating levels of drospirenone and be at an increased risk of hyperkalemia. The US Food and Drug Administration (FDA) suggests that clinicians consider monitoring potassium concentration in women taking drospirenone who are also prescribed a strong CYP3A4 inhibitor.9 In people with normal renal and adrenal function, drospirenone-induced hyperkalemia is not commonly observed.9
Drospirenone 4 mg has been reported to not affect the natural balance of pro- and anti-coagulation factors in women.10 Drospirenone 4 mg daily has been reported to cause a modest decrease in systolic (-8 mm Hg) and diastolic (-5 mm Hg) blood pressure for women with a baseline blood pressure ≥130 mm Hg. Drospirenone 4 mg daily did not change blood pressure measurement in women with a baseline systolic blood pressure <130 mm Hg.11 For women using drospirenone POP, circulating estradiol concentration is usually >30 pg/mL, with a mean concentration of 51 pg/mL.12,13 Drospirenone POP does not result in a significant change in body weight.14 Preliminary studies suggest that drospirenone is an effective contraceptive in women with a BMI >30 kg/m2.14,15 Drospirenone enters breast milk and the relative infant dose is reported to be 1.5%.9 In general, breastfeeding is considered reasonably safe when the relative infant dose of a medication is <10%.16
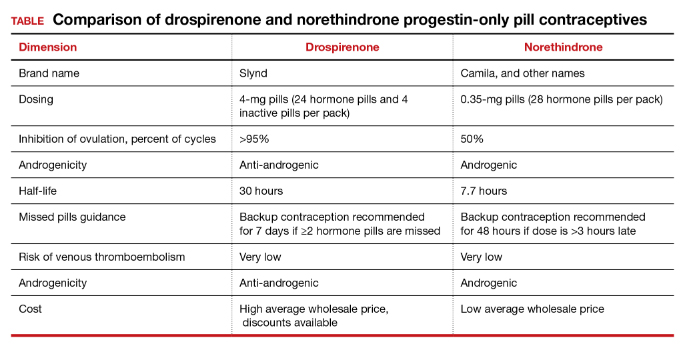
The most common adverse effect reported with both norethindrone and drospirenone POP is unscheduled uterine bleeding. With norethindrone POP about 50% of users have a relatively preserved monthly bleeding pattern and approximately 50% have bleeding between periods, spotting and/or prolonged bleeding.17,18 A similar frequency of unscheduled uterine bleeding has been reported with drospirenone POP.14,19 Unscheduled and bothersome uterine bleeding is a common reason people discontinue POP. For drospirenone POP, the FDA reports a Pearl Index of 4.9 Other studies report a Pearl Index of 0.73 (95% confidence interval [CI], 0.31 to 1.43) for drospirenone POP.14 For norethindrone POP, the FDA reports that in typical use about 5% of people using the contraceptive method would become pregnant.6 The TABLE provides a comparison of the key features of the two available POP contraceptives. My assessment is that drospirenone has superior contraceptive properties over norethindrone POP. However, a head-to-head clinical trial would be necessary to determine the relative contraceptive effectiveness of drospirenone versus norethindrone POP.
Maintaining contraception access
Access to contraception without a copayment is an important component of a comprehensive and equitable insurance program.20 The American College of Obstetricians and Gynecologists (ACOG) advocates that all people “should have unhindered and affordable access to all U.S. Food and Drug Administration-approved contraceptives.”21 ACOG also calls for the “full implementation of the Affordable Care Act requirement that new and revised private health insurance plans cover all U.S. Food and Drug Administration approved contraceptives without cost sharing, including nonequivalent options within one method category.” The National Women’s Law Center22 provides helpful resources to ensure access to legislated contraceptive benefits, including a phone script for speaking with an insurance benefits agent23 and a toolkit for advocating for your contraceptive choice.24 We need to ensure that people have unfettered access to all FDA-approved contraceptives because access to contraception is an important component of public health. Although drospirenone is more costly than norethindrone POP, drospirenone contraception should be available to all patients seeking POP contraception. ●
- Kavanaugh ML, Andreson RM. Contraception and beyond: the health benefits of services provided at family planning centers, NY. Guttmacher Institute. 2013. www.gutmacher.org/pubs/helth-benefits.pdf. Accessed January 13, 2022.
- Foster DG, Rostovtseva DP, Brindis CD, et al. Cost savings from the provision of specific methods of contraception in a publicly funded program. Am J Pub Health. 2009;99:446-451.
- Curtis M, Tepper NK, Jatlaoui TC, et al. U.S. Medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-103.
- Rice CF, Killick SR, Dieben T, et al. A comparison of the inhibition of ovulation achieved by desogestrel 75 µg and levonorgestrel 30 µg daily. Human Reprod. 1999;14:982-985.
- Milsom I, Korver T. Ovulation incidence with oral contraceptives: a literature review. J Fam Plann Reprod Health Care. 2008;34:237-246.
- OrthoMicronor [package insert]. OrthoMcNeil: Raritan, New Jersey. June 2008.
- Brown JB, Fotherby K, Loraine JA. The effect of norethisterone and its acetate on ovarian and pituitary function during the menstrual cycle. J Endocrinol. 1962;25:331-341.
- Romer T, Bitzer J, Egarter C, et al. Oral progestins in hormonal contraception: importance and future perspectives of a new progestin only-pill containing 4 mg drospirenone. Geburtsch Frauenheilk. 2021;81:1021-1030.
- Slynd [package insert]. Exeltis: Florham Park, New Jersey. May 2019.
- Regidor PA, Colli E, Schindlre AE. Drospirenone as estrogen-free pill and hemostasis: coagulatory study results comparing a novel 4 mg formulation in a 24+4 cycle with desogestrel 75 µg per day. Gynecol Endocrinol. 2016;32:749-751.
- Palacios S, Colli E, Regidor PA. Efficacy and cardiovascular safety of the new estrogen-free contraceptive pill containing 4 mg drospirenone alone in a 24/4 regime. BMC Womens Health. 2020;20:218.
- Hadji P, Colli E, Regidor PA. Bone health in estrogen-free contraception. Osteoporosis Int. 2019;30:2391-2400.
- Mitchell VE, Welling LM. Not all progestins are created equally: considering unique progestins individually in psychobehavioral research. Adapt Human Behav Physiol. 2020;6:381-412.
- Palacios S, Colli E, Regidor PA. Multicenter, phase III trials on the contraceptive efficacy, tolerability and safety of a new drospirenone-only pill. Acta Obstet Gynecol Scand. 2019;98:1549-1557.
- Archer DF, Ahrendt HJ, Drouin D. Drospirenone-only oral contraceptive: results from a multicenter noncomparative trial of efficacy, safety and tolerability. Contraception. 2015;92:439-444.
- Anderson PO, Sauberan JB. Modeling drug passage into human milk. Clin Pharmacol Ther. 2016;100:42-52. doi: 10.1002/cpt.377.
- Belsey EM. Vaginal bleeding patterns among women using one natural and eight hormonal methods of contraception. Contraception. 1988;38:181-206.
- Broome M, Fotherby K. Clinical experience with the progestin-only pill. Contraception. 1990;42:489-495.
- Apter D, Colli E, Gemzell-Danielsson K, et al. Multicenter, open-label trial to assess the safety and tolerability of drospirenone 4.0 mg over 6 cycles in female adolescents with a 7-cycle extension phase. Contraception. 2020;101:412.
- Birth control benefits. Healthcare.gov website. https://www.healthcare.gov/coverage/birth-control-benefits/. Accessed January 13, 2022.
- American College of Obstetricians and Gynecologists. Access to contraception. Committee Opinion No. 615. Obstet Gynecol. 2015;125:250-256.
- Health care and reproductive rights. National Women’s Law Center website. https://nwlc.org/issue/health-care. Accessed January 13, 2022.
- How to find out if your health plan covers birth control at no cost to you. National Women’s Law Center website. https://nwlc.org/sites/default/files/072014-insuranceflowchart_vupdated.pdf. Accessed January 13, 2022.
- Toolkit: Getting the coverage you deserve. National Women’s Law Center website. https://nwlc.org/sites/default/files/pdfs/final_nwlclogo_preventive servicestoolkit_9-25-13.pdf. Accessed January 13, 2022.
Contraception and family planning have improved the health of all people by reducing maternal mortality, improving maternal and child health through birth spacing, supporting full education attainment, and advancing workforce participation.1 Contraception is cost-effective and should be supported by all health insurers. One economic study reported that depending on the contraceptive method utilized, up to $7 of health care costs were saved for each dollar spent on contraceptive services and supplies.2
Progestin-only pills (POPs) are an important contraceptive option for people in the following situations who3:
- have a contraindication to estrogen-containing contraceptives
- are actively breastfeeding
- are less than 21 days since birth
- have a preference to avoid estrogen.
POPs are contraindicated for women who have breast cancer, abnormal uterine bleeding, or active liver disease and for women who are pregnant. A history of bariatric surgery with a malabsorption procedure (Roux-en-Y and biliopancreatic diversion) and the use of antiepileptic medications that are strong enzyme inducers are additional situations where the risk of POP may outweigh the benefit.3 Alternative progestin-only options include the subdermal etonogestrel implant, depot medroxyprogesterone acetate, and levonorgestrel-releasing intrauterine devices. These 3 options provide superior contraceptive efficacy to POP.
As a contraceptive, norethindrone at a dose of 0.35 mg daily has two major flaws:
- it does not reliably inhibit ovulation
- it has a short half-life.
In clinical studies, norethindrone inhibits ovulation in approximately 50% of cycles.4,5 Because norethindrone at a dose of 0.35 mg does not reliably inhibit ovulation it relies on additional mechanisms for contraceptive efficacy, including thickening of the cervical mucus to block sperm entry into the upper reproductive tract, reduced fallopian tube motility, and thinning of the endometrium.6
Norethindrone POP is formulated in packs of 28 pills containing 0.35 mg intended for daily continuous administration and no medication-free intervals. One rationale for the low dose of 0.35 mg in norethindrone POP is that it approximates the lowest dose with contraceptive efficacy for breastfeeding women, which has the benefit of minimizing exposure of the baby to the medication. Estrogen-progestin birth control pills containing norethindrone as the progestin reliably inhibit ovulation and have a minimum of 1 mg of norethindrone in each hormone pill. A POP with 1 mg of norethindrone per pill would likely have greater contraceptive efficacy. When taken daily, norethindrone acetate 5 mg (Aygestin) suppresses ovarian estrogen production, ovulation, and often causes cessation of uterine bleeding.7 The short half-life of norethindrone (7.7 hours) further exacerbates the problem of an insufficient daily dose.6 The standard guidance is that norethindrone must be taken at the same time every day, a goal that is nearly impossible to achieve. If a dose of norethindrone is taken >3 hours late, backup contraception is recommended for 48 hours.6
Drospirenone is a chemical analogue of spironolactone. Drospirenone is a progestin that suppresses LH and FSH and has anti-androgenic and partial anti-mineralocorticoid effects.8 Drospirenone POP contains 4 mg of a nonmicronized formulation that is believed to provide a pharmacologically similar area under the curve in drug metabolism studies to the 3 mg of micronized drospirenone, present in drospirenone-containing estrogen-progestin contraceptives.8 It is provided in a pack of 28 pills with 24 drospirenone pills and 4 pills without hormone. Drospirenone has a long half-life of 30 to 34 hours.8 If ≥2 drospirenone pills are missed, backup contraception is recommended for 7 days.9 The contraceptive effectiveness of drospirenone POP is thought to be similar to estrogen-progestin pills.8 Theoretically, drospirenone, acting as an anti-mineralocorticoid, can cause hyperkalemia. People with renal and adrenal insufficiency are most vulnerable to this adverse effect and should not be prescribed drospirenone. Women taking drospirenone and a medication that strongly inhibits CYP3A4, an enzyme involved in drospirenone degradation—including ketoconazole, indinavir, boceprevir, and clarithromycin—may have increased circulating levels of drospirenone and be at an increased risk of hyperkalemia. The US Food and Drug Administration (FDA) suggests that clinicians consider monitoring potassium concentration in women taking drospirenone who are also prescribed a strong CYP3A4 inhibitor.9 In people with normal renal and adrenal function, drospirenone-induced hyperkalemia is not commonly observed.9
Drospirenone 4 mg has been reported to not affect the natural balance of pro- and anti-coagulation factors in women.10 Drospirenone 4 mg daily has been reported to cause a modest decrease in systolic (-8 mm Hg) and diastolic (-5 mm Hg) blood pressure for women with a baseline blood pressure ≥130 mm Hg. Drospirenone 4 mg daily did not change blood pressure measurement in women with a baseline systolic blood pressure <130 mm Hg.11 For women using drospirenone POP, circulating estradiol concentration is usually >30 pg/mL, with a mean concentration of 51 pg/mL.12,13 Drospirenone POP does not result in a significant change in body weight.14 Preliminary studies suggest that drospirenone is an effective contraceptive in women with a BMI >30 kg/m2.14,15 Drospirenone enters breast milk and the relative infant dose is reported to be 1.5%.9 In general, breastfeeding is considered reasonably safe when the relative infant dose of a medication is <10%.16
The most common adverse effect reported with both norethindrone and drospirenone POP is unscheduled uterine bleeding. With norethindrone POP about 50% of users have a relatively preserved monthly bleeding pattern and approximately 50% have bleeding between periods, spotting and/or prolonged bleeding.17,18 A similar frequency of unscheduled uterine bleeding has been reported with drospirenone POP.14,19 Unscheduled and bothersome uterine bleeding is a common reason people discontinue POP. For drospirenone POP, the FDA reports a Pearl Index of 4.9 Other studies report a Pearl Index of 0.73 (95% confidence interval [CI], 0.31 to 1.43) for drospirenone POP.14 For norethindrone POP, the FDA reports that in typical use about 5% of people using the contraceptive method would become pregnant.6 The TABLE provides a comparison of the key features of the two available POP contraceptives. My assessment is that drospirenone has superior contraceptive properties over norethindrone POP. However, a head-to-head clinical trial would be necessary to determine the relative contraceptive effectiveness of drospirenone versus norethindrone POP.

Maintaining contraception access
Access to contraception without a copayment is an important component of a comprehensive and equitable insurance program.20 The American College of Obstetricians and Gynecologists (ACOG) advocates that all people “should have unhindered and affordable access to all U.S. Food and Drug Administration-approved contraceptives.”21 ACOG also calls for the “full implementation of the Affordable Care Act requirement that new and revised private health insurance plans cover all U.S. Food and Drug Administration approved contraceptives without cost sharing, including nonequivalent options within one method category.” The National Women’s Law Center22 provides helpful resources to ensure access to legislated contraceptive benefits, including a phone script for speaking with an insurance benefits agent23 and a toolkit for advocating for your contraceptive choice.24 We need to ensure that people have unfettered access to all FDA-approved contraceptives because access to contraception is an important component of public health. Although drospirenone is more costly than norethindrone POP, drospirenone contraception should be available to all patients seeking POP contraception. ●
Contraception and family planning have improved the health of all people by reducing maternal mortality, improving maternal and child health through birth spacing, supporting full education attainment, and advancing workforce participation.1 Contraception is cost-effective and should be supported by all health insurers. One economic study reported that depending on the contraceptive method utilized, up to $7 of health care costs were saved for each dollar spent on contraceptive services and supplies.2
Progestin-only pills (POPs) are an important contraceptive option for people in the following situations who3:
- have a contraindication to estrogen-containing contraceptives
- are actively breastfeeding
- are less than 21 days since birth
- have a preference to avoid estrogen.
POPs are contraindicated for women who have breast cancer, abnormal uterine bleeding, or active liver disease and for women who are pregnant. A history of bariatric surgery with a malabsorption procedure (Roux-en-Y and biliopancreatic diversion) and the use of antiepileptic medications that are strong enzyme inducers are additional situations where the risk of POP may outweigh the benefit.3 Alternative progestin-only options include the subdermal etonogestrel implant, depot medroxyprogesterone acetate, and levonorgestrel-releasing intrauterine devices. These 3 options provide superior contraceptive efficacy to POP.
As a contraceptive, norethindrone at a dose of 0.35 mg daily has two major flaws:
- it does not reliably inhibit ovulation
- it has a short half-life.
In clinical studies, norethindrone inhibits ovulation in approximately 50% of cycles.4,5 Because norethindrone at a dose of 0.35 mg does not reliably inhibit ovulation it relies on additional mechanisms for contraceptive efficacy, including thickening of the cervical mucus to block sperm entry into the upper reproductive tract, reduced fallopian tube motility, and thinning of the endometrium.6
Norethindrone POP is formulated in packs of 28 pills containing 0.35 mg intended for daily continuous administration and no medication-free intervals. One rationale for the low dose of 0.35 mg in norethindrone POP is that it approximates the lowest dose with contraceptive efficacy for breastfeeding women, which has the benefit of minimizing exposure of the baby to the medication. Estrogen-progestin birth control pills containing norethindrone as the progestin reliably inhibit ovulation and have a minimum of 1 mg of norethindrone in each hormone pill. A POP with 1 mg of norethindrone per pill would likely have greater contraceptive efficacy. When taken daily, norethindrone acetate 5 mg (Aygestin) suppresses ovarian estrogen production, ovulation, and often causes cessation of uterine bleeding.7 The short half-life of norethindrone (7.7 hours) further exacerbates the problem of an insufficient daily dose.6 The standard guidance is that norethindrone must be taken at the same time every day, a goal that is nearly impossible to achieve. If a dose of norethindrone is taken >3 hours late, backup contraception is recommended for 48 hours.6
Drospirenone is a chemical analogue of spironolactone. Drospirenone is a progestin that suppresses LH and FSH and has anti-androgenic and partial anti-mineralocorticoid effects.8 Drospirenone POP contains 4 mg of a nonmicronized formulation that is believed to provide a pharmacologically similar area under the curve in drug metabolism studies to the 3 mg of micronized drospirenone, present in drospirenone-containing estrogen-progestin contraceptives.8 It is provided in a pack of 28 pills with 24 drospirenone pills and 4 pills without hormone. Drospirenone has a long half-life of 30 to 34 hours.8 If ≥2 drospirenone pills are missed, backup contraception is recommended for 7 days.9 The contraceptive effectiveness of drospirenone POP is thought to be similar to estrogen-progestin pills.8 Theoretically, drospirenone, acting as an anti-mineralocorticoid, can cause hyperkalemia. People with renal and adrenal insufficiency are most vulnerable to this adverse effect and should not be prescribed drospirenone. Women taking drospirenone and a medication that strongly inhibits CYP3A4, an enzyme involved in drospirenone degradation—including ketoconazole, indinavir, boceprevir, and clarithromycin—may have increased circulating levels of drospirenone and be at an increased risk of hyperkalemia. The US Food and Drug Administration (FDA) suggests that clinicians consider monitoring potassium concentration in women taking drospirenone who are also prescribed a strong CYP3A4 inhibitor.9 In people with normal renal and adrenal function, drospirenone-induced hyperkalemia is not commonly observed.9
Drospirenone 4 mg has been reported to not affect the natural balance of pro- and anti-coagulation factors in women.10 Drospirenone 4 mg daily has been reported to cause a modest decrease in systolic (-8 mm Hg) and diastolic (-5 mm Hg) blood pressure for women with a baseline blood pressure ≥130 mm Hg. Drospirenone 4 mg daily did not change blood pressure measurement in women with a baseline systolic blood pressure <130 mm Hg.11 For women using drospirenone POP, circulating estradiol concentration is usually >30 pg/mL, with a mean concentration of 51 pg/mL.12,13 Drospirenone POP does not result in a significant change in body weight.14 Preliminary studies suggest that drospirenone is an effective contraceptive in women with a BMI >30 kg/m2.14,15 Drospirenone enters breast milk and the relative infant dose is reported to be 1.5%.9 In general, breastfeeding is considered reasonably safe when the relative infant dose of a medication is <10%.16
The most common adverse effect reported with both norethindrone and drospirenone POP is unscheduled uterine bleeding. With norethindrone POP about 50% of users have a relatively preserved monthly bleeding pattern and approximately 50% have bleeding between periods, spotting and/or prolonged bleeding.17,18 A similar frequency of unscheduled uterine bleeding has been reported with drospirenone POP.14,19 Unscheduled and bothersome uterine bleeding is a common reason people discontinue POP. For drospirenone POP, the FDA reports a Pearl Index of 4.9 Other studies report a Pearl Index of 0.73 (95% confidence interval [CI], 0.31 to 1.43) for drospirenone POP.14 For norethindrone POP, the FDA reports that in typical use about 5% of people using the contraceptive method would become pregnant.6 The TABLE provides a comparison of the key features of the two available POP contraceptives. My assessment is that drospirenone has superior contraceptive properties over norethindrone POP. However, a head-to-head clinical trial would be necessary to determine the relative contraceptive effectiveness of drospirenone versus norethindrone POP.

Maintaining contraception access
Access to contraception without a copayment is an important component of a comprehensive and equitable insurance program.20 The American College of Obstetricians and Gynecologists (ACOG) advocates that all people “should have unhindered and affordable access to all U.S. Food and Drug Administration-approved contraceptives.”21 ACOG also calls for the “full implementation of the Affordable Care Act requirement that new and revised private health insurance plans cover all U.S. Food and Drug Administration approved contraceptives without cost sharing, including nonequivalent options within one method category.” The National Women’s Law Center22 provides helpful resources to ensure access to legislated contraceptive benefits, including a phone script for speaking with an insurance benefits agent23 and a toolkit for advocating for your contraceptive choice.24 We need to ensure that people have unfettered access to all FDA-approved contraceptives because access to contraception is an important component of public health. Although drospirenone is more costly than norethindrone POP, drospirenone contraception should be available to all patients seeking POP contraception. ●
- Kavanaugh ML, Andreson RM. Contraception and beyond: the health benefits of services provided at family planning centers, NY. Guttmacher Institute. 2013. www.gutmacher.org/pubs/helth-benefits.pdf. Accessed January 13, 2022.
- Foster DG, Rostovtseva DP, Brindis CD, et al. Cost savings from the provision of specific methods of contraception in a publicly funded program. Am J Pub Health. 2009;99:446-451.
- Curtis M, Tepper NK, Jatlaoui TC, et al. U.S. Medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-103.
- Rice CF, Killick SR, Dieben T, et al. A comparison of the inhibition of ovulation achieved by desogestrel 75 µg and levonorgestrel 30 µg daily. Human Reprod. 1999;14:982-985.
- Milsom I, Korver T. Ovulation incidence with oral contraceptives: a literature review. J Fam Plann Reprod Health Care. 2008;34:237-246.
- OrthoMicronor [package insert]. OrthoMcNeil: Raritan, New Jersey. June 2008.
- Brown JB, Fotherby K, Loraine JA. The effect of norethisterone and its acetate on ovarian and pituitary function during the menstrual cycle. J Endocrinol. 1962;25:331-341.
- Romer T, Bitzer J, Egarter C, et al. Oral progestins in hormonal contraception: importance and future perspectives of a new progestin only-pill containing 4 mg drospirenone. Geburtsch Frauenheilk. 2021;81:1021-1030.
- Slynd [package insert]. Exeltis: Florham Park, New Jersey. May 2019.
- Regidor PA, Colli E, Schindlre AE. Drospirenone as estrogen-free pill and hemostasis: coagulatory study results comparing a novel 4 mg formulation in a 24+4 cycle with desogestrel 75 µg per day. Gynecol Endocrinol. 2016;32:749-751.
- Palacios S, Colli E, Regidor PA. Efficacy and cardiovascular safety of the new estrogen-free contraceptive pill containing 4 mg drospirenone alone in a 24/4 regime. BMC Womens Health. 2020;20:218.
- Hadji P, Colli E, Regidor PA. Bone health in estrogen-free contraception. Osteoporosis Int. 2019;30:2391-2400.
- Mitchell VE, Welling LM. Not all progestins are created equally: considering unique progestins individually in psychobehavioral research. Adapt Human Behav Physiol. 2020;6:381-412.
- Palacios S, Colli E, Regidor PA. Multicenter, phase III trials on the contraceptive efficacy, tolerability and safety of a new drospirenone-only pill. Acta Obstet Gynecol Scand. 2019;98:1549-1557.
- Archer DF, Ahrendt HJ, Drouin D. Drospirenone-only oral contraceptive: results from a multicenter noncomparative trial of efficacy, safety and tolerability. Contraception. 2015;92:439-444.
- Anderson PO, Sauberan JB. Modeling drug passage into human milk. Clin Pharmacol Ther. 2016;100:42-52. doi: 10.1002/cpt.377.
- Belsey EM. Vaginal bleeding patterns among women using one natural and eight hormonal methods of contraception. Contraception. 1988;38:181-206.
- Broome M, Fotherby K. Clinical experience with the progestin-only pill. Contraception. 1990;42:489-495.
- Apter D, Colli E, Gemzell-Danielsson K, et al. Multicenter, open-label trial to assess the safety and tolerability of drospirenone 4.0 mg over 6 cycles in female adolescents with a 7-cycle extension phase. Contraception. 2020;101:412.
- Birth control benefits. Healthcare.gov website. https://www.healthcare.gov/coverage/birth-control-benefits/. Accessed January 13, 2022.
- American College of Obstetricians and Gynecologists. Access to contraception. Committee Opinion No. 615. Obstet Gynecol. 2015;125:250-256.
- Health care and reproductive rights. National Women’s Law Center website. https://nwlc.org/issue/health-care. Accessed January 13, 2022.
- How to find out if your health plan covers birth control at no cost to you. National Women’s Law Center website. https://nwlc.org/sites/default/files/072014-insuranceflowchart_vupdated.pdf. Accessed January 13, 2022.
- Toolkit: Getting the coverage you deserve. National Women’s Law Center website. https://nwlc.org/sites/default/files/pdfs/final_nwlclogo_preventive servicestoolkit_9-25-13.pdf. Accessed January 13, 2022.
- Kavanaugh ML, Andreson RM. Contraception and beyond: the health benefits of services provided at family planning centers, NY. Guttmacher Institute. 2013. www.gutmacher.org/pubs/helth-benefits.pdf. Accessed January 13, 2022.
- Foster DG, Rostovtseva DP, Brindis CD, et al. Cost savings from the provision of specific methods of contraception in a publicly funded program. Am J Pub Health. 2009;99:446-451.
- Curtis M, Tepper NK, Jatlaoui TC, et al. U.S. Medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-103.
- Rice CF, Killick SR, Dieben T, et al. A comparison of the inhibition of ovulation achieved by desogestrel 75 µg and levonorgestrel 30 µg daily. Human Reprod. 1999;14:982-985.
- Milsom I, Korver T. Ovulation incidence with oral contraceptives: a literature review. J Fam Plann Reprod Health Care. 2008;34:237-246.
- OrthoMicronor [package insert]. OrthoMcNeil: Raritan, New Jersey. June 2008.
- Brown JB, Fotherby K, Loraine JA. The effect of norethisterone and its acetate on ovarian and pituitary function during the menstrual cycle. J Endocrinol. 1962;25:331-341.
- Romer T, Bitzer J, Egarter C, et al. Oral progestins in hormonal contraception: importance and future perspectives of a new progestin only-pill containing 4 mg drospirenone. Geburtsch Frauenheilk. 2021;81:1021-1030.
- Slynd [package insert]. Exeltis: Florham Park, New Jersey. May 2019.
- Regidor PA, Colli E, Schindlre AE. Drospirenone as estrogen-free pill and hemostasis: coagulatory study results comparing a novel 4 mg formulation in a 24+4 cycle with desogestrel 75 µg per day. Gynecol Endocrinol. 2016;32:749-751.
- Palacios S, Colli E, Regidor PA. Efficacy and cardiovascular safety of the new estrogen-free contraceptive pill containing 4 mg drospirenone alone in a 24/4 regime. BMC Womens Health. 2020;20:218.
- Hadji P, Colli E, Regidor PA. Bone health in estrogen-free contraception. Osteoporosis Int. 2019;30:2391-2400.
- Mitchell VE, Welling LM. Not all progestins are created equally: considering unique progestins individually in psychobehavioral research. Adapt Human Behav Physiol. 2020;6:381-412.
- Palacios S, Colli E, Regidor PA. Multicenter, phase III trials on the contraceptive efficacy, tolerability and safety of a new drospirenone-only pill. Acta Obstet Gynecol Scand. 2019;98:1549-1557.
- Archer DF, Ahrendt HJ, Drouin D. Drospirenone-only oral contraceptive: results from a multicenter noncomparative trial of efficacy, safety and tolerability. Contraception. 2015;92:439-444.
- Anderson PO, Sauberan JB. Modeling drug passage into human milk. Clin Pharmacol Ther. 2016;100:42-52. doi: 10.1002/cpt.377.
- Belsey EM. Vaginal bleeding patterns among women using one natural and eight hormonal methods of contraception. Contraception. 1988;38:181-206.
- Broome M, Fotherby K. Clinical experience with the progestin-only pill. Contraception. 1990;42:489-495.
- Apter D, Colli E, Gemzell-Danielsson K, et al. Multicenter, open-label trial to assess the safety and tolerability of drospirenone 4.0 mg over 6 cycles in female adolescents with a 7-cycle extension phase. Contraception. 2020;101:412.
- Birth control benefits. Healthcare.gov website. https://www.healthcare.gov/coverage/birth-control-benefits/. Accessed January 13, 2022.
- American College of Obstetricians and Gynecologists. Access to contraception. Committee Opinion No. 615. Obstet Gynecol. 2015;125:250-256.
- Health care and reproductive rights. National Women’s Law Center website. https://nwlc.org/issue/health-care. Accessed January 13, 2022.
- How to find out if your health plan covers birth control at no cost to you. National Women’s Law Center website. https://nwlc.org/sites/default/files/072014-insuranceflowchart_vupdated.pdf. Accessed January 13, 2022.
- Toolkit: Getting the coverage you deserve. National Women’s Law Center website. https://nwlc.org/sites/default/files/pdfs/final_nwlclogo_preventive servicestoolkit_9-25-13.pdf. Accessed January 13, 2022.
Early Diagnosis and Management of Tardive Dyskinesia
Tardive dyskinesia (TD) is a delayed movement disorder resulting from treatment with dopamine receptor–blocking medications, Dr Karen Anderson of Georgetown University School of Medicine explains. TD is most commonly associated with long-term use of antipsychotic drugs.
TD is characterized by involuntary, jerking movements of the tongue, lips, face, trunk, extremities, or the whole body. Although its characteristic movements are sometimes viewed as cosmetic, TD can interfere with patients’ quality of life and add to the stigma of mental illness.
The sooner TD is diagnosed, the more likely it is for patients to achieve remission spontaneously and without treatment. To that end, patients who are prescribed antipsychotic medication should be evaluated at baseline and regularly thereafter using the Abnormal Involuntary Movement Scale (AIMS), which rates abnormal movements.
The optimal first step in TD management is to stop the dopamine receptor–blocking medication, but this option may not be possible in patients with chronic conditions, such as manic depression and schizophrenia. In these patients, dose reduction or switching to a newer antipsychotic may provide relief.
Two vesicular monoamine transporter 2 (VMAT2) inhibitors are approved to treat TD and have been found to not worsen patients’ underlying psychiatric condition. Dr Anderson cautions that patients treated with a VMAT2 inhibitor will require monitoring because side effects of these agents include suicidal ideation.
--
Karen Anderson, MD, Professor, Department of Psychiatry and Neurology, Georgetown University School of Medicine; Washington, DC
Karen Anderson, MD, has disclosed the following relevant financial relationships:
Received income in an amount equal to or greater than $250 from: Neurocrine; Teva
Tardive dyskinesia (TD) is a delayed movement disorder resulting from treatment with dopamine receptor–blocking medications, Dr Karen Anderson of Georgetown University School of Medicine explains. TD is most commonly associated with long-term use of antipsychotic drugs.
TD is characterized by involuntary, jerking movements of the tongue, lips, face, trunk, extremities, or the whole body. Although its characteristic movements are sometimes viewed as cosmetic, TD can interfere with patients’ quality of life and add to the stigma of mental illness.
The sooner TD is diagnosed, the more likely it is for patients to achieve remission spontaneously and without treatment. To that end, patients who are prescribed antipsychotic medication should be evaluated at baseline and regularly thereafter using the Abnormal Involuntary Movement Scale (AIMS), which rates abnormal movements.
The optimal first step in TD management is to stop the dopamine receptor–blocking medication, but this option may not be possible in patients with chronic conditions, such as manic depression and schizophrenia. In these patients, dose reduction or switching to a newer antipsychotic may provide relief.
Two vesicular monoamine transporter 2 (VMAT2) inhibitors are approved to treat TD and have been found to not worsen patients’ underlying psychiatric condition. Dr Anderson cautions that patients treated with a VMAT2 inhibitor will require monitoring because side effects of these agents include suicidal ideation.
--
Karen Anderson, MD, Professor, Department of Psychiatry and Neurology, Georgetown University School of Medicine; Washington, DC
Karen Anderson, MD, has disclosed the following relevant financial relationships:
Received income in an amount equal to or greater than $250 from: Neurocrine; Teva
Tardive dyskinesia (TD) is a delayed movement disorder resulting from treatment with dopamine receptor–blocking medications, Dr Karen Anderson of Georgetown University School of Medicine explains. TD is most commonly associated with long-term use of antipsychotic drugs.
TD is characterized by involuntary, jerking movements of the tongue, lips, face, trunk, extremities, or the whole body. Although its characteristic movements are sometimes viewed as cosmetic, TD can interfere with patients’ quality of life and add to the stigma of mental illness.
The sooner TD is diagnosed, the more likely it is for patients to achieve remission spontaneously and without treatment. To that end, patients who are prescribed antipsychotic medication should be evaluated at baseline and regularly thereafter using the Abnormal Involuntary Movement Scale (AIMS), which rates abnormal movements.
The optimal first step in TD management is to stop the dopamine receptor–blocking medication, but this option may not be possible in patients with chronic conditions, such as manic depression and schizophrenia. In these patients, dose reduction or switching to a newer antipsychotic may provide relief.
Two vesicular monoamine transporter 2 (VMAT2) inhibitors are approved to treat TD and have been found to not worsen patients’ underlying psychiatric condition. Dr Anderson cautions that patients treated with a VMAT2 inhibitor will require monitoring because side effects of these agents include suicidal ideation.
--
Karen Anderson, MD, Professor, Department of Psychiatry and Neurology, Georgetown University School of Medicine; Washington, DC
Karen Anderson, MD, has disclosed the following relevant financial relationships:
Received income in an amount equal to or greater than $250 from: Neurocrine; Teva

Multifactorial Effects of Endometriosis as a Chronic Systemic Disease
Can you talk about your research thus far and what your overall lab work has shown regarding endometriosis as a chronic systemic disease?
Dr. Flores: Endometriosis has traditionally been characterized by its pelvic manifestation however, it is important to understand that it is profoundly more than a pelvic disease—it is a chronic, systemic disease with multifactorial effects throughout the body.
We and other groups have found increased expression of several inflammatory cytokines in women with endometriosis. Our lab has found that compared to women without endometriosis, women with endometriosis not only have certain inflammatory cytokines elevated but also have altered expression of microRNAs. MicroRNAs are small noncoding RNAs that bind to and modulate translation of mRNA. To help determine whether these miRNAs were involved in mediating increased expression of inflammatory cytokines in women with endometriosis, we then transfected these miRNAs into a macrophage cell line, and again found altered inflammatory cytokine expression. We and others have also found a role for stem cells (from bone marrow and other sources) in the pathogenesis of endometriosis. In addition, we have found that in endometriosis, women have a low body-mass index and altered metabolism, which is related to induction of induction of hepatic (anorexigenic) gene expression and microRNA-mediated changes in adipocyte (metabolic) gene expression. Furthermore, we have found altered gene expression in regions of the brain associated with anxiety and depression and altered pain sensitization. Taken together, this work helps provide support for the systemic nature of endometriosis.
How can your findings in this space help us in diagnosing clinically and ultimately avoid diagnostic delay?
Dr. Flores: It’s about understanding that endometriosis is not just a pelvic disease and understanding that endometriosis is leading to inflammation and altered expression of miRNAs which allows endometriosis to have long-range effects. For example, women with endometriosis commonly have anxiety and depression and low BMI. As mentioned earlier, we have found that in a murine model of endometriosis, there is altered gene expression in regions of the brain associated with anxiety and depression and altered metabolism in a murine model of endometriosis. Other groups have also found changes in brain volume in these same areas in women with endometriosis, and we have seen low BMI in women with endometriosis. In fact, a common misconception was that being thin was a risk factor for endometriosis, however we have found that the endometriosis itself, is causing women alteration in genes associated with metabolism.
With respect to the endometrium, in addition to being a pelvic pain disorder, we also see that women with endometriosis have a higher likelihood of having infertility. And we think that's in part because one, just like the lesions can be resistant to progesterone, the endometrium of these women can also be resistant to progesterone. Progesterone is necessary for decidualization/implantation. We have also seen that stem cells can be recruited and ultimately incorrectly incorporated into the endometrium, which may also contribute to infertility in women with endometriosis.
If we can understand this multifactorial nature of endometriosis, I think this will help us not only shift toward diagnosing endometriosis clinically, but also avoid diagnostic delay. If we can understand that endometriosis is not just a pelvic disorder, but that It can also involve altered mood, bowel/bladder symptoms, inflammation, altered metabolism and/or cause infertility, I think that will ultimately help us to diagnosing earlier.
In addition, we can also utilize pelvic pain symptomatology to help with diagnosis as well. We can ask about cyclic pelvic pain that's been getting progressively worse over the years, not responding to non-steroidal anti-inflammatory medications. Also, in understanding that endometriosis can affect other organs, asking about cyclic pain/symptoms in other areas, such as cyclical bowel or bladder symptoms.
Thinking about the fact that if you do have a patient like that, you're seeing that they have altered mood symptoms, or alterations in inflammatory markers. Maybe that will help us shift from a disease that was typically only considered to be diagnosed by surgery, by switching to a clinical diagnosis for endometriosis. Doing that will hopefully help avoid diagnostic delay.
If we understand that while we typically describe endometriosis as causing cyclic pain symptoms, sometimes because of the existing diagnostic delay, ultimately women can present with chronic pelvic pain. Thus, it's also important to ask patients presenting with chronic pelvic pain what the symptoms were like beforehand (i.e., was the pain cyclic and progressively worsening over the years/before it became chronic) doing so will also help in terms of diagnosing sooner.
Lastly, circulating miRNAs have been considered promising biomarker candidates because they are stable in circulation and have highly specific expression profiles. We have found that the combination of several miRNAs reliably distinguished endometriosis patients from controls, and a prospective, blinded study showed that the combination of several miRNAs could be used to accurately identify patients with endometriosis, with an area under the receiver operating characteristic curve of 0.93.
Roughly 11%, or more than 6.5 million, women in the United States between the ages of 15–44 years, may have endometriosis. Is this disease more common in any particular age range or ethnicity?
Dr. Flores: We’re actually actively investigating that right now. And I think what makes it challenging, especially with respect to the age range, is now we're -- I think in part because of so much more awareness and more research is being done looking at this disease as a chronic systemic disease-- we're now starting to see/diagnose adolescents with endometriosis.
I think as we start gathering more information about these individuals, we'll be able to better say if there is a particular age range. Right now, we usually say it's in the reproductive years, however for some women it may be later if they were not diagnosed earlier. Conversely, some who are hopefully reading this, and also who conduct research on endometriosis, may be able to diagnose someone earlier that may have been missed until they were in their 30s or 40s, for example.
With respect to ethnicity, I'm the task force leader for diversity, equity, and inclusion in research and recruitment. This is something that I'm actively starting to work on, as are other groups. I don't have the answer for that yet, but as we continue to collect more data, we will have more information on this.
What are some of the existing hormonal therapies you rely upon as well as the biomarkers in predicting response to treatment, and are there any new research or treatments on the horizon?
Dr. Flores: I'll first start by telling you a bit about our existing treatment regimens, and then how I decide who would benefit from a given one. First line has always been progestin-based therapy, either in the form of a combined oral contraceptive pill or as progesterone only pills. However, up to 1/3 of women fail progestin-based therapy—this is termed progesterone resistance.
When progestin-based therapies fail, we then rely on other agents that are focused more on estrogen deprivation because, while we don't know the complete etiology of endometriosis, we do know that it is estrogen-dependent. There are two classes— gonadotropin releasing hormone (GnRH) agonists and GnRH antagonists. The agonist binds to the GnRH receptors, and initially can cause a flare effect due to its agonist properties, initially stimulate release of estradiol, and ultimately the GnRH receptor becomes downregulated and estradiol is decreased to the menopausal range. As a result we routinely provided add-back therapy with norethindrone to help prevent hot flashes and ensure bone protection.
Within the past three years, there has been a new oral GnRH receptor antagonist approved for treating endometriosis. The medication is available as a once a day or twice a day dosing regimen. As this is a GnRH antagonist, upon binding to the GnRH receptor, it blocks receptor activity, thus avoiding the flare affect; essentially, within 24 hours, there is a decrease in estradiol production.
As two doses are available, you can tailor how much you dial down estrogen for a given patient. The low dose lowers estradiol to a range of 40 picograms while the high (twice a day) dosing lowers your estrogen to about 6 picograms. Also, although it was not studied originally in terms of giving add-back therapy for the higher dose, given the safety (and effectiveness) of add-back therapy with GnRH agonists we are using the same norethindrone add-back therapy for women who are taking the GnRH receptor antagonist.
The next question is, how do we decide which medication a given patient receives? To answer that, I will tell you a bit about my precision-based medicine research. As mentioned before, while progestin-based therapy is first-line, failure rates are high, and unfortunately, we previously have not been able to identify who will or will not respond to first-line therapy. As such, I decided to assess progesterone receptor expression in endometriotic lesions from women who had undergone surgery for endometriosis, and determine whether progesterone receptor expression levels in lesions could be used to predict response to progestin-based therapy. I found that in women that had high levels of the progesterone receptor, they responded completely to progestin-based therapy-- there was a 100% response rate to progestin-based therapy. This is in sharp contrast to women who had low PR expression, where there was only a 6% response rate to progestin-based therapy.
While this is great with respect to being able to predict who will or will not respond to first line therapy, the one limitation is that would mean that women have to undergo surgery in order to determine progesterone receptor status/response to progestin-based therapy. However, given that within two to five years following surgery, up to 50% of women will have recurrence of pain symptoms, where I see my test coming into play is postoperatively. This is because many times , women who had pain, or who were failing a given agent, are placed back on that same medical therapy they were failing after surgery. Usually that was a progestin. Therefore, instead of putting them on that same therapy that they were failing, we can use my test to place them on an alternative therapy (such as a GnRH analogue) that more specifically targets estradiol production.
In terms of future directions with respect to treatment, there is a microRNA that has been found to be low in women with endometriosis—miRNALet-7b. In a murine model of endometriosis, we have found that if we supplement with Let-7, there is decreased inflammation and decreased lesion size of endometriosis. We have also found that supplementing miRNA Let-7b in human endometriotic lesions results in decreased inflammation in cell culture.
That would be future directions in terms of focusing on microRNAs and seeing how we can manipulate those to essentially block inflammation and lesion growth. Furthermore, such treatment would be non-hormonal, which would be a novel therapeutic approach.
As-Sanie S, Harris RE, Napadow V, et al. Changes in regional gray matter volume in women with chronic pelvic pain: a voxel-based morphometry study. Pain. 2012;153(5):1006-1014.
Ballard K, Lowton K, Wright J. What's the delay? A qualitative study of women's experiences of reaching a diagnosis of endometriosis. Fertil Steril. 2006;86(5):1296-1301
Cosar E, Mamillapalli R, Ersoy GS, Cho S, Seifer B, Taylor HS. Serum microRNAs as diagnostic markers of endometriosis: a comprehensive array-based analysis. Fertil Steril. 2016;106(2):402-409.
Flores VA, Vanhie A, Dang T, Taylor HS. Progesterone Receptor Status Predicts Response to Progestin Therapy in Endometriosis. J Clin Endocrinol Metab. 2018 Dec 1;103(12):4561-4568
Goetz TG, Mamillapalli R, Taylor HS. Low Body Mass Index in Endometriosis Is Promoted by Hepatic Metabolic Gene Dysregulation in Mice. Biol Reprod. 2016;95(6):115.
Li T, et al. Endometriosis alters brain electrophysiology, gene expression and increases pain sensitization, anxiety, and depression in female mice. Biol Reprod. 2018;99(2):349-359.
Moustafa S, Burn M, Mamillapalli R, Nematian S, Flores V, Taylor HS. Accurate diagnosis of endometriosis using serum microRNAs. Am J Obstet Gynecol. 2020;223(4):557.e1-557.e11.
Nematian SE, et al. Systemic Inflammation Induced by microRNAs: Endometriosis-Derived Alterations in Circulating microRNA 125b-5p and Let-7b-5p Regulate Macrophage Cytokine Production. J Clin Endocrinol Metab. 2018;103(1):64-74.
Nnoaham KE, Hummelshoj L, Webster P, et al. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril. 2011;96(2):366-373.e8.
Rogers PA, D'Hooghe TM, Fazleabas A, et al. Defining future directions for endometriosis research: workshop report from the 2011 World Congress of Endometriosis In Montpellier, France. Reprod Sci. 2013;20(5):483-499.
Taylor HS, Kotlyar AM, Flores VA. Endometriosis is a chronic systemic disease: clinical challenges and novel innovations. Lancet. 2021 Feb 27
Zolbin MM, et al. Adipocyte alterations in endometriosis: reduced numbers of stem cells and microRNA induced alterations in adipocyte metabolic gene expression. Reprod Biol Endocrinol. 2019;17(1):36.
Can you talk about your research thus far and what your overall lab work has shown regarding endometriosis as a chronic systemic disease?
Dr. Flores: Endometriosis has traditionally been characterized by its pelvic manifestation however, it is important to understand that it is profoundly more than a pelvic disease—it is a chronic, systemic disease with multifactorial effects throughout the body.
We and other groups have found increased expression of several inflammatory cytokines in women with endometriosis. Our lab has found that compared to women without endometriosis, women with endometriosis not only have certain inflammatory cytokines elevated but also have altered expression of microRNAs. MicroRNAs are small noncoding RNAs that bind to and modulate translation of mRNA. To help determine whether these miRNAs were involved in mediating increased expression of inflammatory cytokines in women with endometriosis, we then transfected these miRNAs into a macrophage cell line, and again found altered inflammatory cytokine expression. We and others have also found a role for stem cells (from bone marrow and other sources) in the pathogenesis of endometriosis. In addition, we have found that in endometriosis, women have a low body-mass index and altered metabolism, which is related to induction of induction of hepatic (anorexigenic) gene expression and microRNA-mediated changes in adipocyte (metabolic) gene expression. Furthermore, we have found altered gene expression in regions of the brain associated with anxiety and depression and altered pain sensitization. Taken together, this work helps provide support for the systemic nature of endometriosis.
How can your findings in this space help us in diagnosing clinically and ultimately avoid diagnostic delay?
Dr. Flores: It’s about understanding that endometriosis is not just a pelvic disease and understanding that endometriosis is leading to inflammation and altered expression of miRNAs which allows endometriosis to have long-range effects. For example, women with endometriosis commonly have anxiety and depression and low BMI. As mentioned earlier, we have found that in a murine model of endometriosis, there is altered gene expression in regions of the brain associated with anxiety and depression and altered metabolism in a murine model of endometriosis. Other groups have also found changes in brain volume in these same areas in women with endometriosis, and we have seen low BMI in women with endometriosis. In fact, a common misconception was that being thin was a risk factor for endometriosis, however we have found that the endometriosis itself, is causing women alteration in genes associated with metabolism.
With respect to the endometrium, in addition to being a pelvic pain disorder, we also see that women with endometriosis have a higher likelihood of having infertility. And we think that's in part because one, just like the lesions can be resistant to progesterone, the endometrium of these women can also be resistant to progesterone. Progesterone is necessary for decidualization/implantation. We have also seen that stem cells can be recruited and ultimately incorrectly incorporated into the endometrium, which may also contribute to infertility in women with endometriosis.
If we can understand this multifactorial nature of endometriosis, I think this will help us not only shift toward diagnosing endometriosis clinically, but also avoid diagnostic delay. If we can understand that endometriosis is not just a pelvic disorder, but that It can also involve altered mood, bowel/bladder symptoms, inflammation, altered metabolism and/or cause infertility, I think that will ultimately help us to diagnosing earlier.
In addition, we can also utilize pelvic pain symptomatology to help with diagnosis as well. We can ask about cyclic pelvic pain that's been getting progressively worse over the years, not responding to non-steroidal anti-inflammatory medications. Also, in understanding that endometriosis can affect other organs, asking about cyclic pain/symptoms in other areas, such as cyclical bowel or bladder symptoms.
Thinking about the fact that if you do have a patient like that, you're seeing that they have altered mood symptoms, or alterations in inflammatory markers. Maybe that will help us shift from a disease that was typically only considered to be diagnosed by surgery, by switching to a clinical diagnosis for endometriosis. Doing that will hopefully help avoid diagnostic delay.
If we understand that while we typically describe endometriosis as causing cyclic pain symptoms, sometimes because of the existing diagnostic delay, ultimately women can present with chronic pelvic pain. Thus, it's also important to ask patients presenting with chronic pelvic pain what the symptoms were like beforehand (i.e., was the pain cyclic and progressively worsening over the years/before it became chronic) doing so will also help in terms of diagnosing sooner.
Lastly, circulating miRNAs have been considered promising biomarker candidates because they are stable in circulation and have highly specific expression profiles. We have found that the combination of several miRNAs reliably distinguished endometriosis patients from controls, and a prospective, blinded study showed that the combination of several miRNAs could be used to accurately identify patients with endometriosis, with an area under the receiver operating characteristic curve of 0.93.
Roughly 11%, or more than 6.5 million, women in the United States between the ages of 15–44 years, may have endometriosis. Is this disease more common in any particular age range or ethnicity?
Dr. Flores: We’re actually actively investigating that right now. And I think what makes it challenging, especially with respect to the age range, is now we're -- I think in part because of so much more awareness and more research is being done looking at this disease as a chronic systemic disease-- we're now starting to see/diagnose adolescents with endometriosis.
I think as we start gathering more information about these individuals, we'll be able to better say if there is a particular age range. Right now, we usually say it's in the reproductive years, however for some women it may be later if they were not diagnosed earlier. Conversely, some who are hopefully reading this, and also who conduct research on endometriosis, may be able to diagnose someone earlier that may have been missed until they were in their 30s or 40s, for example.
With respect to ethnicity, I'm the task force leader for diversity, equity, and inclusion in research and recruitment. This is something that I'm actively starting to work on, as are other groups. I don't have the answer for that yet, but as we continue to collect more data, we will have more information on this.
What are some of the existing hormonal therapies you rely upon as well as the biomarkers in predicting response to treatment, and are there any new research or treatments on the horizon?
Dr. Flores: I'll first start by telling you a bit about our existing treatment regimens, and then how I decide who would benefit from a given one. First line has always been progestin-based therapy, either in the form of a combined oral contraceptive pill or as progesterone only pills. However, up to 1/3 of women fail progestin-based therapy—this is termed progesterone resistance.
When progestin-based therapies fail, we then rely on other agents that are focused more on estrogen deprivation because, while we don't know the complete etiology of endometriosis, we do know that it is estrogen-dependent. There are two classes— gonadotropin releasing hormone (GnRH) agonists and GnRH antagonists. The agonist binds to the GnRH receptors, and initially can cause a flare effect due to its agonist properties, initially stimulate release of estradiol, and ultimately the GnRH receptor becomes downregulated and estradiol is decreased to the menopausal range. As a result we routinely provided add-back therapy with norethindrone to help prevent hot flashes and ensure bone protection.
Within the past three years, there has been a new oral GnRH receptor antagonist approved for treating endometriosis. The medication is available as a once a day or twice a day dosing regimen. As this is a GnRH antagonist, upon binding to the GnRH receptor, it blocks receptor activity, thus avoiding the flare affect; essentially, within 24 hours, there is a decrease in estradiol production.
As two doses are available, you can tailor how much you dial down estrogen for a given patient. The low dose lowers estradiol to a range of 40 picograms while the high (twice a day) dosing lowers your estrogen to about 6 picograms. Also, although it was not studied originally in terms of giving add-back therapy for the higher dose, given the safety (and effectiveness) of add-back therapy with GnRH agonists we are using the same norethindrone add-back therapy for women who are taking the GnRH receptor antagonist.
The next question is, how do we decide which medication a given patient receives? To answer that, I will tell you a bit about my precision-based medicine research. As mentioned before, while progestin-based therapy is first-line, failure rates are high, and unfortunately, we previously have not been able to identify who will or will not respond to first-line therapy. As such, I decided to assess progesterone receptor expression in endometriotic lesions from women who had undergone surgery for endometriosis, and determine whether progesterone receptor expression levels in lesions could be used to predict response to progestin-based therapy. I found that in women that had high levels of the progesterone receptor, they responded completely to progestin-based therapy-- there was a 100% response rate to progestin-based therapy. This is in sharp contrast to women who had low PR expression, where there was only a 6% response rate to progestin-based therapy.
While this is great with respect to being able to predict who will or will not respond to first line therapy, the one limitation is that would mean that women have to undergo surgery in order to determine progesterone receptor status/response to progestin-based therapy. However, given that within two to five years following surgery, up to 50% of women will have recurrence of pain symptoms, where I see my test coming into play is postoperatively. This is because many times , women who had pain, or who were failing a given agent, are placed back on that same medical therapy they were failing after surgery. Usually that was a progestin. Therefore, instead of putting them on that same therapy that they were failing, we can use my test to place them on an alternative therapy (such as a GnRH analogue) that more specifically targets estradiol production.
In terms of future directions with respect to treatment, there is a microRNA that has been found to be low in women with endometriosis—miRNALet-7b. In a murine model of endometriosis, we have found that if we supplement with Let-7, there is decreased inflammation and decreased lesion size of endometriosis. We have also found that supplementing miRNA Let-7b in human endometriotic lesions results in decreased inflammation in cell culture.
That would be future directions in terms of focusing on microRNAs and seeing how we can manipulate those to essentially block inflammation and lesion growth. Furthermore, such treatment would be non-hormonal, which would be a novel therapeutic approach.
Can you talk about your research thus far and what your overall lab work has shown regarding endometriosis as a chronic systemic disease?
Dr. Flores: Endometriosis has traditionally been characterized by its pelvic manifestation however, it is important to understand that it is profoundly more than a pelvic disease—it is a chronic, systemic disease with multifactorial effects throughout the body.
We and other groups have found increased expression of several inflammatory cytokines in women with endometriosis. Our lab has found that compared to women without endometriosis, women with endometriosis not only have certain inflammatory cytokines elevated but also have altered expression of microRNAs. MicroRNAs are small noncoding RNAs that bind to and modulate translation of mRNA. To help determine whether these miRNAs were involved in mediating increased expression of inflammatory cytokines in women with endometriosis, we then transfected these miRNAs into a macrophage cell line, and again found altered inflammatory cytokine expression. We and others have also found a role for stem cells (from bone marrow and other sources) in the pathogenesis of endometriosis. In addition, we have found that in endometriosis, women have a low body-mass index and altered metabolism, which is related to induction of induction of hepatic (anorexigenic) gene expression and microRNA-mediated changes in adipocyte (metabolic) gene expression. Furthermore, we have found altered gene expression in regions of the brain associated with anxiety and depression and altered pain sensitization. Taken together, this work helps provide support for the systemic nature of endometriosis.
How can your findings in this space help us in diagnosing clinically and ultimately avoid diagnostic delay?
Dr. Flores: It’s about understanding that endometriosis is not just a pelvic disease and understanding that endometriosis is leading to inflammation and altered expression of miRNAs which allows endometriosis to have long-range effects. For example, women with endometriosis commonly have anxiety and depression and low BMI. As mentioned earlier, we have found that in a murine model of endometriosis, there is altered gene expression in regions of the brain associated with anxiety and depression and altered metabolism in a murine model of endometriosis. Other groups have also found changes in brain volume in these same areas in women with endometriosis, and we have seen low BMI in women with endometriosis. In fact, a common misconception was that being thin was a risk factor for endometriosis, however we have found that the endometriosis itself, is causing women alteration in genes associated with metabolism.
With respect to the endometrium, in addition to being a pelvic pain disorder, we also see that women with endometriosis have a higher likelihood of having infertility. And we think that's in part because one, just like the lesions can be resistant to progesterone, the endometrium of these women can also be resistant to progesterone. Progesterone is necessary for decidualization/implantation. We have also seen that stem cells can be recruited and ultimately incorrectly incorporated into the endometrium, which may also contribute to infertility in women with endometriosis.
If we can understand this multifactorial nature of endometriosis, I think this will help us not only shift toward diagnosing endometriosis clinically, but also avoid diagnostic delay. If we can understand that endometriosis is not just a pelvic disorder, but that It can also involve altered mood, bowel/bladder symptoms, inflammation, altered metabolism and/or cause infertility, I think that will ultimately help us to diagnosing earlier.
In addition, we can also utilize pelvic pain symptomatology to help with diagnosis as well. We can ask about cyclic pelvic pain that's been getting progressively worse over the years, not responding to non-steroidal anti-inflammatory medications. Also, in understanding that endometriosis can affect other organs, asking about cyclic pain/symptoms in other areas, such as cyclical bowel or bladder symptoms.
Thinking about the fact that if you do have a patient like that, you're seeing that they have altered mood symptoms, or alterations in inflammatory markers. Maybe that will help us shift from a disease that was typically only considered to be diagnosed by surgery, by switching to a clinical diagnosis for endometriosis. Doing that will hopefully help avoid diagnostic delay.
If we understand that while we typically describe endometriosis as causing cyclic pain symptoms, sometimes because of the existing diagnostic delay, ultimately women can present with chronic pelvic pain. Thus, it's also important to ask patients presenting with chronic pelvic pain what the symptoms were like beforehand (i.e., was the pain cyclic and progressively worsening over the years/before it became chronic) doing so will also help in terms of diagnosing sooner.
Lastly, circulating miRNAs have been considered promising biomarker candidates because they are stable in circulation and have highly specific expression profiles. We have found that the combination of several miRNAs reliably distinguished endometriosis patients from controls, and a prospective, blinded study showed that the combination of several miRNAs could be used to accurately identify patients with endometriosis, with an area under the receiver operating characteristic curve of 0.93.
Roughly 11%, or more than 6.5 million, women in the United States between the ages of 15–44 years, may have endometriosis. Is this disease more common in any particular age range or ethnicity?
Dr. Flores: We’re actually actively investigating that right now. And I think what makes it challenging, especially with respect to the age range, is now we're -- I think in part because of so much more awareness and more research is being done looking at this disease as a chronic systemic disease-- we're now starting to see/diagnose adolescents with endometriosis.
I think as we start gathering more information about these individuals, we'll be able to better say if there is a particular age range. Right now, we usually say it's in the reproductive years, however for some women it may be later if they were not diagnosed earlier. Conversely, some who are hopefully reading this, and also who conduct research on endometriosis, may be able to diagnose someone earlier that may have been missed until they were in their 30s or 40s, for example.
With respect to ethnicity, I'm the task force leader for diversity, equity, and inclusion in research and recruitment. This is something that I'm actively starting to work on, as are other groups. I don't have the answer for that yet, but as we continue to collect more data, we will have more information on this.
What are some of the existing hormonal therapies you rely upon as well as the biomarkers in predicting response to treatment, and are there any new research or treatments on the horizon?
Dr. Flores: I'll first start by telling you a bit about our existing treatment regimens, and then how I decide who would benefit from a given one. First line has always been progestin-based therapy, either in the form of a combined oral contraceptive pill or as progesterone only pills. However, up to 1/3 of women fail progestin-based therapy—this is termed progesterone resistance.
When progestin-based therapies fail, we then rely on other agents that are focused more on estrogen deprivation because, while we don't know the complete etiology of endometriosis, we do know that it is estrogen-dependent. There are two classes— gonadotropin releasing hormone (GnRH) agonists and GnRH antagonists. The agonist binds to the GnRH receptors, and initially can cause a flare effect due to its agonist properties, initially stimulate release of estradiol, and ultimately the GnRH receptor becomes downregulated and estradiol is decreased to the menopausal range. As a result we routinely provided add-back therapy with norethindrone to help prevent hot flashes and ensure bone protection.
Within the past three years, there has been a new oral GnRH receptor antagonist approved for treating endometriosis. The medication is available as a once a day or twice a day dosing regimen. As this is a GnRH antagonist, upon binding to the GnRH receptor, it blocks receptor activity, thus avoiding the flare affect; essentially, within 24 hours, there is a decrease in estradiol production.
As two doses are available, you can tailor how much you dial down estrogen for a given patient. The low dose lowers estradiol to a range of 40 picograms while the high (twice a day) dosing lowers your estrogen to about 6 picograms. Also, although it was not studied originally in terms of giving add-back therapy for the higher dose, given the safety (and effectiveness) of add-back therapy with GnRH agonists we are using the same norethindrone add-back therapy for women who are taking the GnRH receptor antagonist.
The next question is, how do we decide which medication a given patient receives? To answer that, I will tell you a bit about my precision-based medicine research. As mentioned before, while progestin-based therapy is first-line, failure rates are high, and unfortunately, we previously have not been able to identify who will or will not respond to first-line therapy. As such, I decided to assess progesterone receptor expression in endometriotic lesions from women who had undergone surgery for endometriosis, and determine whether progesterone receptor expression levels in lesions could be used to predict response to progestin-based therapy. I found that in women that had high levels of the progesterone receptor, they responded completely to progestin-based therapy-- there was a 100% response rate to progestin-based therapy. This is in sharp contrast to women who had low PR expression, where there was only a 6% response rate to progestin-based therapy.
While this is great with respect to being able to predict who will or will not respond to first line therapy, the one limitation is that would mean that women have to undergo surgery in order to determine progesterone receptor status/response to progestin-based therapy. However, given that within two to five years following surgery, up to 50% of women will have recurrence of pain symptoms, where I see my test coming into play is postoperatively. This is because many times , women who had pain, or who were failing a given agent, are placed back on that same medical therapy they were failing after surgery. Usually that was a progestin. Therefore, instead of putting them on that same therapy that they were failing, we can use my test to place them on an alternative therapy (such as a GnRH analogue) that more specifically targets estradiol production.
In terms of future directions with respect to treatment, there is a microRNA that has been found to be low in women with endometriosis—miRNALet-7b. In a murine model of endometriosis, we have found that if we supplement with Let-7, there is decreased inflammation and decreased lesion size of endometriosis. We have also found that supplementing miRNA Let-7b in human endometriotic lesions results in decreased inflammation in cell culture.
That would be future directions in terms of focusing on microRNAs and seeing how we can manipulate those to essentially block inflammation and lesion growth. Furthermore, such treatment would be non-hormonal, which would be a novel therapeutic approach.
As-Sanie S, Harris RE, Napadow V, et al. Changes in regional gray matter volume in women with chronic pelvic pain: a voxel-based morphometry study. Pain. 2012;153(5):1006-1014.
Ballard K, Lowton K, Wright J. What's the delay? A qualitative study of women's experiences of reaching a diagnosis of endometriosis. Fertil Steril. 2006;86(5):1296-1301
Cosar E, Mamillapalli R, Ersoy GS, Cho S, Seifer B, Taylor HS. Serum microRNAs as diagnostic markers of endometriosis: a comprehensive array-based analysis. Fertil Steril. 2016;106(2):402-409.
Flores VA, Vanhie A, Dang T, Taylor HS. Progesterone Receptor Status Predicts Response to Progestin Therapy in Endometriosis. J Clin Endocrinol Metab. 2018 Dec 1;103(12):4561-4568
Goetz TG, Mamillapalli R, Taylor HS. Low Body Mass Index in Endometriosis Is Promoted by Hepatic Metabolic Gene Dysregulation in Mice. Biol Reprod. 2016;95(6):115.
Li T, et al. Endometriosis alters brain electrophysiology, gene expression and increases pain sensitization, anxiety, and depression in female mice. Biol Reprod. 2018;99(2):349-359.
Moustafa S, Burn M, Mamillapalli R, Nematian S, Flores V, Taylor HS. Accurate diagnosis of endometriosis using serum microRNAs. Am J Obstet Gynecol. 2020;223(4):557.e1-557.e11.
Nematian SE, et al. Systemic Inflammation Induced by microRNAs: Endometriosis-Derived Alterations in Circulating microRNA 125b-5p and Let-7b-5p Regulate Macrophage Cytokine Production. J Clin Endocrinol Metab. 2018;103(1):64-74.
Nnoaham KE, Hummelshoj L, Webster P, et al. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril. 2011;96(2):366-373.e8.
Rogers PA, D'Hooghe TM, Fazleabas A, et al. Defining future directions for endometriosis research: workshop report from the 2011 World Congress of Endometriosis In Montpellier, France. Reprod Sci. 2013;20(5):483-499.
Taylor HS, Kotlyar AM, Flores VA. Endometriosis is a chronic systemic disease: clinical challenges and novel innovations. Lancet. 2021 Feb 27
Zolbin MM, et al. Adipocyte alterations in endometriosis: reduced numbers of stem cells and microRNA induced alterations in adipocyte metabolic gene expression. Reprod Biol Endocrinol. 2019;17(1):36.
As-Sanie S, Harris RE, Napadow V, et al. Changes in regional gray matter volume in women with chronic pelvic pain: a voxel-based morphometry study. Pain. 2012;153(5):1006-1014.
Ballard K, Lowton K, Wright J. What's the delay? A qualitative study of women's experiences of reaching a diagnosis of endometriosis. Fertil Steril. 2006;86(5):1296-1301
Cosar E, Mamillapalli R, Ersoy GS, Cho S, Seifer B, Taylor HS. Serum microRNAs as diagnostic markers of endometriosis: a comprehensive array-based analysis. Fertil Steril. 2016;106(2):402-409.
Flores VA, Vanhie A, Dang T, Taylor HS. Progesterone Receptor Status Predicts Response to Progestin Therapy in Endometriosis. J Clin Endocrinol Metab. 2018 Dec 1;103(12):4561-4568
Goetz TG, Mamillapalli R, Taylor HS. Low Body Mass Index in Endometriosis Is Promoted by Hepatic Metabolic Gene Dysregulation in Mice. Biol Reprod. 2016;95(6):115.
Li T, et al. Endometriosis alters brain electrophysiology, gene expression and increases pain sensitization, anxiety, and depression in female mice. Biol Reprod. 2018;99(2):349-359.
Moustafa S, Burn M, Mamillapalli R, Nematian S, Flores V, Taylor HS. Accurate diagnosis of endometriosis using serum microRNAs. Am J Obstet Gynecol. 2020;223(4):557.e1-557.e11.
Nematian SE, et al. Systemic Inflammation Induced by microRNAs: Endometriosis-Derived Alterations in Circulating microRNA 125b-5p and Let-7b-5p Regulate Macrophage Cytokine Production. J Clin Endocrinol Metab. 2018;103(1):64-74.
Nnoaham KE, Hummelshoj L, Webster P, et al. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril. 2011;96(2):366-373.e8.
Rogers PA, D'Hooghe TM, Fazleabas A, et al. Defining future directions for endometriosis research: workshop report from the 2011 World Congress of Endometriosis In Montpellier, France. Reprod Sci. 2013;20(5):483-499.
Taylor HS, Kotlyar AM, Flores VA. Endometriosis is a chronic systemic disease: clinical challenges and novel innovations. Lancet. 2021 Feb 27
Zolbin MM, et al. Adipocyte alterations in endometriosis: reduced numbers of stem cells and microRNA induced alterations in adipocyte metabolic gene expression. Reprod Biol Endocrinol. 2019;17(1):36.
Teaching Evidence-Based Dermatology Using a Web-Based Journal Club: A Pilot Study and Survey
To the Editor:
With a steady increase in dermatology publications over recent decades, there is an expanding pool of evidence to address clinical questions.1 Residency training is the time when appraising the medical literature and practicing evidence-based medicine is most honed. Evidence-based medicine is an essential component of Practice-based Learning and Improvement, a required core competency of the Accreditation Council for Graduate Medical Education.2 Assimilation of new research evidence is traditionally taught through didactics and journal club discussions in residency.
However, at a time when the demand for information overwhelms safeguards that exist to evaluate its quality, it is more important than ever to be equipped with the proper tools to critically appraise novel literature. Beyond accepting a scientific article at face value, physicians must learn to ask targeted questions of the study design, results, and clinical relevance. These questions change based on the type of study, and organizations such as the Oxford Centre for Evidence-Based Medicine provide guidance through critical appraisal worksheets.3
To investigate the utility of using guided questions to evaluate the reliability, significance, and applicability of clinical evidence, we beta tested a novel web-based application in an academic dermatology setting to design and run a journal club for residents. Six dermatology residents participated in this institutional review board–approved study comprised of 3 phases: (1) independent article appraisal through the web-based application, (2) group discussion, and (3) anonymous postsurvey.
Using this platform, we uploaded a recent article into the interactive reader, which contained an integrated tool for appraisal based on specific questions. Because the article described the results of a randomized clinical trial, we used questions from the Centre for Evidence-Based Medicine’s Randomised Controlled Trials Critical Appraisal Worksheet, which has a series of questions to evaluate internal validity, results, and external validity and applicability.3
Residents used the platform to independently read the article, highlight areas of the text that corresponded to 8 critical appraisal questions, and answer yes or no to these questions. Based on residents’ answers, a final appraisal score (on a scale of 1% to 100%) was generated. Simultaneously, the attending dermatologist leading the journal club (C.W.) also completed the assignment to establish an expert score.
Scores from the residents’ independent appraisal ranged from 75% to 100% (mean, 85.4%). Upon discussing the article in a group setting, the residents established a consensus score of 75%. This consensus score matched the expert score, which suggested to us that both independently reviewing the article using guided questions and conducting a group debriefing were necessary to match the expert level of critical appraisal.
Of note, the residents’ average independent appraisal score was higher than both the consensus and expert scores, indicating that the residents evaluated the article less critically on their own. With more practice using this method, it is possible that the precision and accuracy of the residents’ critical appraisal of scientific articles will improve.
In the postsurvey, we asked residents about the critical appraisal of the medical literature. All residents agreed that evaluating the quality of evidence when reading a scientific article was somewhat important or very important to them; however, only 2 of 6 evaluated the quality of evidence all the time, and the other 4 did so half of the time or less than half of the time.
When critically appraising articles, 2 of 6 residents used specific rubrics half of the time; 4 of 6 less than half of the time. Most important, 5 of 6 residents agreed that the quality of evidence affected their management decisions more than half of the time or all of the time. Although it is clear that residents value evidence-based medicine and understand the importance of evaluating the quality of evidence, doing so currently might not be simple or practical.
An organized framework for appraising articles would streamline the process. Five of 6 residents agreed that the use of specific questions as a guide made it easier to appraise an article for the quality of its evidence. Four of 6 residents found that juxtaposing specific questions with the interactive reader was helpful; 5 of 6 agreed that they would use a web-based journal club platform if given the option.
Lastly, 5 of 6 residents agreed that if such a tool were available, a platform containing all major dermatology publications in an interactive reader format, along with relevant appraisal questions on the side, would be useful.
This pilot study augmented the typical journal club experience by emphasizing goal-directed reading and the importance of analyzing the quality of evidence. The combination of independent appraisal of an article using targeted questions and a group debrief led to better understanding of the evidence and its clinical applicability. The COVID-19 pandemic may be a better time than ever to explore innovative ways to teach evidence-based medicine in residency training.
- Mimouni D, Pavlovsky L, Akerman L, et al. Trends in dermatology publications over the past 15 years. Am J Clin Dermatol. 2010;11:55-58. doi:10.2165/11530190-000000000-00000.
- NEJM Knowledge+ Team. Exploring the ACGME Core Competencies: Practice-Based Learning and Improvement (part 2 of 7). Massachusetts Medical Society. NEJM Knowledge+ website. Published July 28, 2016. Accessed January 15, 2022. https://knowledgeplus.nejm.org/blog/practice-based-learning-and-improvement/
- University of Oxford. Critical appraisal tools. Centre for Evidence-Based Medicine website. Accessed January 2, 2022. www.cebm.ox.ac.uk/resources/ebm-tools/critical-appraisal-tools
To the Editor:
With a steady increase in dermatology publications over recent decades, there is an expanding pool of evidence to address clinical questions.1 Residency training is the time when appraising the medical literature and practicing evidence-based medicine is most honed. Evidence-based medicine is an essential component of Practice-based Learning and Improvement, a required core competency of the Accreditation Council for Graduate Medical Education.2 Assimilation of new research evidence is traditionally taught through didactics and journal club discussions in residency.
However, at a time when the demand for information overwhelms safeguards that exist to evaluate its quality, it is more important than ever to be equipped with the proper tools to critically appraise novel literature. Beyond accepting a scientific article at face value, physicians must learn to ask targeted questions of the study design, results, and clinical relevance. These questions change based on the type of study, and organizations such as the Oxford Centre for Evidence-Based Medicine provide guidance through critical appraisal worksheets.3
To investigate the utility of using guided questions to evaluate the reliability, significance, and applicability of clinical evidence, we beta tested a novel web-based application in an academic dermatology setting to design and run a journal club for residents. Six dermatology residents participated in this institutional review board–approved study comprised of 3 phases: (1) independent article appraisal through the web-based application, (2) group discussion, and (3) anonymous postsurvey.
Using this platform, we uploaded a recent article into the interactive reader, which contained an integrated tool for appraisal based on specific questions. Because the article described the results of a randomized clinical trial, we used questions from the Centre for Evidence-Based Medicine’s Randomised Controlled Trials Critical Appraisal Worksheet, which has a series of questions to evaluate internal validity, results, and external validity and applicability.3
Residents used the platform to independently read the article, highlight areas of the text that corresponded to 8 critical appraisal questions, and answer yes or no to these questions. Based on residents’ answers, a final appraisal score (on a scale of 1% to 100%) was generated. Simultaneously, the attending dermatologist leading the journal club (C.W.) also completed the assignment to establish an expert score.
Scores from the residents’ independent appraisal ranged from 75% to 100% (mean, 85.4%). Upon discussing the article in a group setting, the residents established a consensus score of 75%. This consensus score matched the expert score, which suggested to us that both independently reviewing the article using guided questions and conducting a group debriefing were necessary to match the expert level of critical appraisal.
Of note, the residents’ average independent appraisal score was higher than both the consensus and expert scores, indicating that the residents evaluated the article less critically on their own. With more practice using this method, it is possible that the precision and accuracy of the residents’ critical appraisal of scientific articles will improve.
In the postsurvey, we asked residents about the critical appraisal of the medical literature. All residents agreed that evaluating the quality of evidence when reading a scientific article was somewhat important or very important to them; however, only 2 of 6 evaluated the quality of evidence all the time, and the other 4 did so half of the time or less than half of the time.
When critically appraising articles, 2 of 6 residents used specific rubrics half of the time; 4 of 6 less than half of the time. Most important, 5 of 6 residents agreed that the quality of evidence affected their management decisions more than half of the time or all of the time. Although it is clear that residents value evidence-based medicine and understand the importance of evaluating the quality of evidence, doing so currently might not be simple or practical.
An organized framework for appraising articles would streamline the process. Five of 6 residents agreed that the use of specific questions as a guide made it easier to appraise an article for the quality of its evidence. Four of 6 residents found that juxtaposing specific questions with the interactive reader was helpful; 5 of 6 agreed that they would use a web-based journal club platform if given the option.
Lastly, 5 of 6 residents agreed that if such a tool were available, a platform containing all major dermatology publications in an interactive reader format, along with relevant appraisal questions on the side, would be useful.
This pilot study augmented the typical journal club experience by emphasizing goal-directed reading and the importance of analyzing the quality of evidence. The combination of independent appraisal of an article using targeted questions and a group debrief led to better understanding of the evidence and its clinical applicability. The COVID-19 pandemic may be a better time than ever to explore innovative ways to teach evidence-based medicine in residency training.
To the Editor:
With a steady increase in dermatology publications over recent decades, there is an expanding pool of evidence to address clinical questions.1 Residency training is the time when appraising the medical literature and practicing evidence-based medicine is most honed. Evidence-based medicine is an essential component of Practice-based Learning and Improvement, a required core competency of the Accreditation Council for Graduate Medical Education.2 Assimilation of new research evidence is traditionally taught through didactics and journal club discussions in residency.
However, at a time when the demand for information overwhelms safeguards that exist to evaluate its quality, it is more important than ever to be equipped with the proper tools to critically appraise novel literature. Beyond accepting a scientific article at face value, physicians must learn to ask targeted questions of the study design, results, and clinical relevance. These questions change based on the type of study, and organizations such as the Oxford Centre for Evidence-Based Medicine provide guidance through critical appraisal worksheets.3
To investigate the utility of using guided questions to evaluate the reliability, significance, and applicability of clinical evidence, we beta tested a novel web-based application in an academic dermatology setting to design and run a journal club for residents. Six dermatology residents participated in this institutional review board–approved study comprised of 3 phases: (1) independent article appraisal through the web-based application, (2) group discussion, and (3) anonymous postsurvey.
Using this platform, we uploaded a recent article into the interactive reader, which contained an integrated tool for appraisal based on specific questions. Because the article described the results of a randomized clinical trial, we used questions from the Centre for Evidence-Based Medicine’s Randomised Controlled Trials Critical Appraisal Worksheet, which has a series of questions to evaluate internal validity, results, and external validity and applicability.3
Residents used the platform to independently read the article, highlight areas of the text that corresponded to 8 critical appraisal questions, and answer yes or no to these questions. Based on residents’ answers, a final appraisal score (on a scale of 1% to 100%) was generated. Simultaneously, the attending dermatologist leading the journal club (C.W.) also completed the assignment to establish an expert score.
Scores from the residents’ independent appraisal ranged from 75% to 100% (mean, 85.4%). Upon discussing the article in a group setting, the residents established a consensus score of 75%. This consensus score matched the expert score, which suggested to us that both independently reviewing the article using guided questions and conducting a group debriefing were necessary to match the expert level of critical appraisal.
Of note, the residents’ average independent appraisal score was higher than both the consensus and expert scores, indicating that the residents evaluated the article less critically on their own. With more practice using this method, it is possible that the precision and accuracy of the residents’ critical appraisal of scientific articles will improve.
In the postsurvey, we asked residents about the critical appraisal of the medical literature. All residents agreed that evaluating the quality of evidence when reading a scientific article was somewhat important or very important to them; however, only 2 of 6 evaluated the quality of evidence all the time, and the other 4 did so half of the time or less than half of the time.
When critically appraising articles, 2 of 6 residents used specific rubrics half of the time; 4 of 6 less than half of the time. Most important, 5 of 6 residents agreed that the quality of evidence affected their management decisions more than half of the time or all of the time. Although it is clear that residents value evidence-based medicine and understand the importance of evaluating the quality of evidence, doing so currently might not be simple or practical.
An organized framework for appraising articles would streamline the process. Five of 6 residents agreed that the use of specific questions as a guide made it easier to appraise an article for the quality of its evidence. Four of 6 residents found that juxtaposing specific questions with the interactive reader was helpful; 5 of 6 agreed that they would use a web-based journal club platform if given the option.
Lastly, 5 of 6 residents agreed that if such a tool were available, a platform containing all major dermatology publications in an interactive reader format, along with relevant appraisal questions on the side, would be useful.
This pilot study augmented the typical journal club experience by emphasizing goal-directed reading and the importance of analyzing the quality of evidence. The combination of independent appraisal of an article using targeted questions and a group debrief led to better understanding of the evidence and its clinical applicability. The COVID-19 pandemic may be a better time than ever to explore innovative ways to teach evidence-based medicine in residency training.
- Mimouni D, Pavlovsky L, Akerman L, et al. Trends in dermatology publications over the past 15 years. Am J Clin Dermatol. 2010;11:55-58. doi:10.2165/11530190-000000000-00000.
- NEJM Knowledge+ Team. Exploring the ACGME Core Competencies: Practice-Based Learning and Improvement (part 2 of 7). Massachusetts Medical Society. NEJM Knowledge+ website. Published July 28, 2016. Accessed January 15, 2022. https://knowledgeplus.nejm.org/blog/practice-based-learning-and-improvement/
- University of Oxford. Critical appraisal tools. Centre for Evidence-Based Medicine website. Accessed January 2, 2022. www.cebm.ox.ac.uk/resources/ebm-tools/critical-appraisal-tools
- Mimouni D, Pavlovsky L, Akerman L, et al. Trends in dermatology publications over the past 15 years. Am J Clin Dermatol. 2010;11:55-58. doi:10.2165/11530190-000000000-00000.
- NEJM Knowledge+ Team. Exploring the ACGME Core Competencies: Practice-Based Learning and Improvement (part 2 of 7). Massachusetts Medical Society. NEJM Knowledge+ website. Published July 28, 2016. Accessed January 15, 2022. https://knowledgeplus.nejm.org/blog/practice-based-learning-and-improvement/
- University of Oxford. Critical appraisal tools. Centre for Evidence-Based Medicine website. Accessed January 2, 2022. www.cebm.ox.ac.uk/resources/ebm-tools/critical-appraisal-tools
Practice Points
- A novel web-based application was beta tested in an academic dermatology setting to design and run a journal club for residents.
- Goal-directed reading was emphasized by using guided questions to critically appraise literature based on reliability, significance, and applicability.
- The combination of independent appraisal of an article using targeted questions and a group debrief led to better understanding of the evidence and its clinical applicability.
Light Brown and Pink Macule on the Upper Arm
The Diagnosis: Desmoplastic Spitz Nevus
Desmoplastic Spitz nevus is a rare variant of Spitz nevus that commonly presents as a red to brown papule on the head, neck, or extremities. It is pertinent to review the histologic features of this neoplasm, as it can be confused with other more sinister entities such as spitzoid melanoma. Histologically, there is a dermal infiltrate of melanocytes containing eosinophilic cytoplasm and vesicular nuclei. Junctional involvement is rare, and there should be no pagetoid spread.1 This entity features abundant stromal fibrosis formed by dense collagen bundles, low cellular density, and polygonal-shaped melanocytes, which helps to differentiate it from melanoma.2,3 In a retrospective study comparing the characteristics of desmoplastic Spitz nevi with desmoplastic melanoma, desmoplastic Spitz nevi histologically were more symmetric and circumscribed with greater melanocytic maturation and adnexal structure involvement.3 Although this entity demonstrates maturation from the superficial to the deep dermis, it also may feature deep dermal vascular proliferation.4 S-100 and SRY-related HMG box 10, SOX-10, are noted to be positive in desmoplastic Spitz nevi, which can help to differentiate it from nonmelanocytic entities (Figure 1).
Although spitzoid lesions can be ambiguous and difficult even for experts to classify, spitzoid melanoma tends to have a high Breslow thickness, high cell density, marked atypia, and an increased nucleus to cytoplasm ratio.5 Additionally, desmoplastic melanoma was found to more often display “melanocytic junctional nests associated with discohesive cells, variations in size and shape of the nests, lentiginous melanocytic proliferation, actinic elastosis, pagetoid spread, dermal mitosis, perineural involvement and brisk inflammatory infiltrate.”3 Given the challenge of histologically separating desmoplastic Spitz nevi from melanoma, immunostaining can be useful. For example, Hilliard et al6 used a p16 antibody to differentiate desmoplastic Spitz nevi from desmoplastic melanoma, finding that most desmoplastic melanomas (81.8%; n=11) were negative for p16, whereas all desmoplastic Spitz nevi were at least moderately positive. However, another study re-evaluated the utility of p16 in desmoplastic melanoma and found that 72.7% (16/22) were at least focally reactive for the immunostain.7 Thus, caution must be exercised when using p16.
PReferentially expressed Antigen in MElanoma (PRAME) is a newer nuclear immunohistochemical marker that tends to be positive in melanomas and negative in nevi. Desmoplastic Spitz nevi would be expected to be negative for PRAME, while desmoplastic melanoma may be positive; however, this marker seems to be less effective in desmoplastic melanoma than in most other subtypes of the malignancy. In one study, only 35% (n=20) of desmoplastic melanomas were positive for PRAME.8 Likewise, another study showed that some benign Spitz nevi may diffusely express PRAME.9 As such, PRAME should be used prudently.
For cases in which immunohistochemistry is equivocal, molecular testing may aid in differentiating Spitz nevi from melanoma. For example, comparative genomic hybridization has revealed an increased copy number of chromosome 11p in approximately 20% of Spitz nevi cases10; this finding is not seen in melanoma. Mutation analyses of HRas proto-oncogene, GTPase, HRAS; B-Raf proto-oncogene, serine/threonine kinase, BRAF; and NRAS proto-oncogene, GTPase, NRAS, also have shown some promise in distinguishing spitzoid lesions from melanoma, but these analyses may be oversimplified.11 Fluorescence in situ hybridization (FISH) is another diagnostic modality that has been studied to differentiate benign nevi from melanoma. One study challenged the utility of FISH, reporting 7 of 15 desmoplastic melanomas tested positive compared to 0 of 15 sclerotic melanocytic nevi.12 Thus, negative FISH cannot reliably rule out melanoma. Ultimately, a combination of immunostains along with FISH or another genetic study would prove to be most effective in ruling out melanoma in difficult cases. Even then, a dermatopathologist may be faced with a degree of uncertainty.
Cellular blue nevi predominantly affect adults younger than 40 years and commonly are seen on the buttocks.13 This benign neoplasm demonstrates areas that are distinctly sclerotic as well as those that are cellular in nature.14 This entity demonstrates a well-circumscribed dermal growth pattern with 2 main populations of cells. The sclerotic portion of the cellular blue nevus mimics that of the blue nevus in that it is noted superficially with irregular margins. The cellular aspect of the nevus features spindle cells contained within well-circumscribed nodules (Figure 2). Stromal melanophages are not uncommon, and some can be observed adjacent to nerve fibers. Although this blue nevus variant displays features of the common blue nevus, its melanocytes track along adnexal and neurovascular structures similar to the deep penetrating nevus and the desmoplastic Spitz nevus. However, these melanocytes are variable in morphology and can appear on a spectrum spanning from pale and lightly pigmented to clear.15
The breast is the most common site of origin of tumor metastasis to the skin. These cutaneous metastases can vary in both their clinical and histological presentations. For example, cutaneous metastatic breast adenocarcinoma often can present clinically as pink-violaceous papules and plaques on the breast or on other parts of the body. Histologically, it can demonstrate a varying degree of patterns such as collagen infiltration by single cells, cords, tubules, and sheets of atypical cells (Figure 3) that can be observed together in areas of mucin or can form glandular structures.16 Metastatic breast carcinoma is noted to be positive for gross cystic disease fluid protein-15, estrogen receptor, and cytokeratin 7, which can help differentiate this entity from other tumors of glandular origin.16 Although rare, primary melanoma of the breast has been reported in the literature.17,18 These malignant melanocytic lesions easily could be differentiated from other breast tumors such as adenocarcinoma using immunohistochemical staining patterns.
Deep penetrating nevi most often are observed clinically as blue, brown, or black papules or nodules on the head or neck.19 Histologically, this lesion features a wedge-shaped infiltrate of deep dermal melanocytes with oval nuclei. It commonly extends to the reticular dermis or further into the subcutis (Figure 4).20,21 This neoplasm frequently tracks along adnexal and neurovascular structures, resulting in a plexiform appearance.22 The adnexal involvement of deep penetrating nevi is a shared feature with desmoplastic Spitz nevi. The presence of any number of melanophages is characteristic of this lesion.23 Lastly, there is a well-documented association between β-catenin mutations and deep penetrating nevi.24 Multicentric reticulohistiocytosis (MRH) is a rare form of non-Langerhans cell histiocytosis that has the pathognomonic clinical finding of pink-red papules (coral beading) with a predilection for acral surfaces. Histology of affected skin reveals a dermal infiltrate of ground glass as well as eosinophilic histiocytes that most often stain positive for CD68 and human alveolar macrophage 56 but negative for S-100 and CD1a (Figure 5).25 Although MRH is rare, negative staining for S-100 could serve as a useful diagnostic clue to differentiate it from other entities that are positive for S-100, such as the desmoplastic Spitz nevus. Arthritis mutilans is a potential complication of MRH, but a reported association with an underlying malignancy is seen in approximately 25% of cases.26 Thus, the cutaneous, rheumatologic, and oncologic implications of this disease help to distinguish it from other differential diagnoses that may be considered.
- Luzar B, Bastian BC, North JP, et al. Melanocytic nevi. In: Calonje E, Brenn T, Lazar AJ, et al, eds. McKee’s Pathology of the Skin. 5th ed. Elsevier; 2020:1275-1280.
- Busam KJ, Gerami P. Spitz nevi. In: Busam KJ, Gerami P, Scolyer RA, eds. Pathology of Melanocytic Tumors. Elsevier; 2019:37-60.
- Nojavan H, Cribier B, Mehregan DR. Desmoplastic Spitz nevus: a histopathological review and comparison with desmoplastic melanoma [in French]. Ann Dermatol Venereol. 2009;136:689-695.
- Tomizawa K. Desmoplastic Spitz nevus showing vascular proliferation more prominently in the deep portion. Am J Dermatopathol. 2002;24:184-185.
- Requena C, Botella R, Nagore E, et al. Characteristics of spitzoid melanoma and clues for differential diagnosis with Spitz nevus. Am J Dermatopathol. 2012;34:478-486.
- Hilliard NJ, Krahl D, Sellheyer K. p16 expression differentiates between desmoplastic Spitz nevus and desmoplastic melanoma. J Cutan Pathol. 2009;36:753-759.
- Blokhin E, Pulitzer M, Busam KJ. Immunohistochemical expression of p16 in desmoplastic melanoma. J Cutan Pathol. 2013;40:796-800.
- Lezcano C, Jungbluth AA, Nehal KS, et al. PRAME expression in melanocytic tumors. Am J Surg Pathol. 2018;42:1456-1465.
- Raghavan SS, Wang JY, Kwok S, et al. PRAME expression in melanocytic proliferations with intermediate histopathologic or spitzoid features. J Cutan Pathol. 2020;47:1123-1131.
- Bauer J, Bastian BC. DNA copy number changes in the diagnosis of melanocytic tumors [in German]. Pathologe. 2007;28:464-473.
- Luo S, Sepehr A, Tsao H. Spitz nevi and other spitzoid lesions part I. background and diagnoses. J Am Acad Dermatol. 2011;65:1073-1084.
- Gerami P, Beilfuss B, Haghighat Z, et al. Fluorescence in situ hybridization as an ancillary method for the distinction of desmoplastic melanomas from sclerosing melanocytic nevi. J Cutan Pathol. 2011;38:329-334.
- Zembowicz A. Blue nevi and related tumors. Clin Lab Med. 2017; 37:401-415.
- Rodriguez HA, Ackerman LV. Cellular blue nevus. clinicopathologic study of forty-five cases. Cancer. 1968;21:393-405.
- Phadke PA, Zembowicz A. Blue nevi and related tumors. Clin Lab Med. 2011;31:345-358.
- Ko CJ. Metastatic tumors and simulators. In: Elston DM, Ferringer T, eds. Dermatopathology. 3rd ed. Elsevier Limited; 2019:496-504.
- Drueppel D, Schultheis B, Solass W, et al. Primary malignant melanoma of the breast: case report and review of the literature. Anticancer Res. 2015;35:1709-1713.
- Kurul S, Tas¸ F, Büyükbabani N, et al. Different manifestations of malignant melanoma in the breast: a report of 12 cases and a review of the literature. Jpn J Clin Oncol. 2005;35:202-206.
- Strazzula L, Senna MM, Yasuda M, et al. The deep penetrating nevus. J Am Acad Dermatol. 2014;71:1234-1240.
- Mehregan DA, Mehregan AH. Deep penetrating nevus. Arch Dermatol. 1993;129:328-331.
- Robson A, Morley-Quante M, Hempel H, et al. Deep penetrating naevus: clinicopathological study of 31 cases with further delineation of histological features allowing distinction from other pigmented benign melanocytic lesions and melanoma. Histopathology. 2003;43:529-537.
- Luzar B, Calonje E. Deep penetrating nevus: a review. Arch Pathol Lab Med. 2011;135:321-326.
- Cooper PH. Deep penetrating (plexiform spindle cell) nevus. a frequent participant in combined nevus. J Cutan Pathol. 1992;19:172-180.
- de la Fouchardière A, Caillot C, Jacquemus J, et al. β-Catenin nuclear expression discriminates deep penetrating nevi from other cutaneous melanocytic tumors. Virchows Arch. 2019;474:539-550.
- Gorman JD, Danning C, Schumacher HR, et al. Multicentric reticulohistiocytosis: case report with immunohistochemical analysis and literature review. Arthritis Rheum. 2000;43:930-938.
- Selmi C, Greenspan A, Huntley A, et al. Multicentric reticulohistiocytosis: a critical review. Curr Rheumatol Rep. 2015;17:511.
The Diagnosis: Desmoplastic Spitz Nevus
Desmoplastic Spitz nevus is a rare variant of Spitz nevus that commonly presents as a red to brown papule on the head, neck, or extremities. It is pertinent to review the histologic features of this neoplasm, as it can be confused with other more sinister entities such as spitzoid melanoma. Histologically, there is a dermal infiltrate of melanocytes containing eosinophilic cytoplasm and vesicular nuclei. Junctional involvement is rare, and there should be no pagetoid spread.1 This entity features abundant stromal fibrosis formed by dense collagen bundles, low cellular density, and polygonal-shaped melanocytes, which helps to differentiate it from melanoma.2,3 In a retrospective study comparing the characteristics of desmoplastic Spitz nevi with desmoplastic melanoma, desmoplastic Spitz nevi histologically were more symmetric and circumscribed with greater melanocytic maturation and adnexal structure involvement.3 Although this entity demonstrates maturation from the superficial to the deep dermis, it also may feature deep dermal vascular proliferation.4 S-100 and SRY-related HMG box 10, SOX-10, are noted to be positive in desmoplastic Spitz nevi, which can help to differentiate it from nonmelanocytic entities (Figure 1).

Although spitzoid lesions can be ambiguous and difficult even for experts to classify, spitzoid melanoma tends to have a high Breslow thickness, high cell density, marked atypia, and an increased nucleus to cytoplasm ratio.5 Additionally, desmoplastic melanoma was found to more often display “melanocytic junctional nests associated with discohesive cells, variations in size and shape of the nests, lentiginous melanocytic proliferation, actinic elastosis, pagetoid spread, dermal mitosis, perineural involvement and brisk inflammatory infiltrate.”3 Given the challenge of histologically separating desmoplastic Spitz nevi from melanoma, immunostaining can be useful. For example, Hilliard et al6 used a p16 antibody to differentiate desmoplastic Spitz nevi from desmoplastic melanoma, finding that most desmoplastic melanomas (81.8%; n=11) were negative for p16, whereas all desmoplastic Spitz nevi were at least moderately positive. However, another study re-evaluated the utility of p16 in desmoplastic melanoma and found that 72.7% (16/22) were at least focally reactive for the immunostain.7 Thus, caution must be exercised when using p16.
PReferentially expressed Antigen in MElanoma (PRAME) is a newer nuclear immunohistochemical marker that tends to be positive in melanomas and negative in nevi. Desmoplastic Spitz nevi would be expected to be negative for PRAME, while desmoplastic melanoma may be positive; however, this marker seems to be less effective in desmoplastic melanoma than in most other subtypes of the malignancy. In one study, only 35% (n=20) of desmoplastic melanomas were positive for PRAME.8 Likewise, another study showed that some benign Spitz nevi may diffusely express PRAME.9 As such, PRAME should be used prudently.
For cases in which immunohistochemistry is equivocal, molecular testing may aid in differentiating Spitz nevi from melanoma. For example, comparative genomic hybridization has revealed an increased copy number of chromosome 11p in approximately 20% of Spitz nevi cases10; this finding is not seen in melanoma. Mutation analyses of HRas proto-oncogene, GTPase, HRAS; B-Raf proto-oncogene, serine/threonine kinase, BRAF; and NRAS proto-oncogene, GTPase, NRAS, also have shown some promise in distinguishing spitzoid lesions from melanoma, but these analyses may be oversimplified.11 Fluorescence in situ hybridization (FISH) is another diagnostic modality that has been studied to differentiate benign nevi from melanoma. One study challenged the utility of FISH, reporting 7 of 15 desmoplastic melanomas tested positive compared to 0 of 15 sclerotic melanocytic nevi.12 Thus, negative FISH cannot reliably rule out melanoma. Ultimately, a combination of immunostains along with FISH or another genetic study would prove to be most effective in ruling out melanoma in difficult cases. Even then, a dermatopathologist may be faced with a degree of uncertainty.
Cellular blue nevi predominantly affect adults younger than 40 years and commonly are seen on the buttocks.13 This benign neoplasm demonstrates areas that are distinctly sclerotic as well as those that are cellular in nature.14 This entity demonstrates a well-circumscribed dermal growth pattern with 2 main populations of cells. The sclerotic portion of the cellular blue nevus mimics that of the blue nevus in that it is noted superficially with irregular margins. The cellular aspect of the nevus features spindle cells contained within well-circumscribed nodules (Figure 2). Stromal melanophages are not uncommon, and some can be observed adjacent to nerve fibers. Although this blue nevus variant displays features of the common blue nevus, its melanocytes track along adnexal and neurovascular structures similar to the deep penetrating nevus and the desmoplastic Spitz nevus. However, these melanocytes are variable in morphology and can appear on a spectrum spanning from pale and lightly pigmented to clear.15

The breast is the most common site of origin of tumor metastasis to the skin. These cutaneous metastases can vary in both their clinical and histological presentations. For example, cutaneous metastatic breast adenocarcinoma often can present clinically as pink-violaceous papules and plaques on the breast or on other parts of the body. Histologically, it can demonstrate a varying degree of patterns such as collagen infiltration by single cells, cords, tubules, and sheets of atypical cells (Figure 3) that can be observed together in areas of mucin or can form glandular structures.16 Metastatic breast carcinoma is noted to be positive for gross cystic disease fluid protein-15, estrogen receptor, and cytokeratin 7, which can help differentiate this entity from other tumors of glandular origin.16 Although rare, primary melanoma of the breast has been reported in the literature.17,18 These malignant melanocytic lesions easily could be differentiated from other breast tumors such as adenocarcinoma using immunohistochemical staining patterns.

Deep penetrating nevi most often are observed clinically as blue, brown, or black papules or nodules on the head or neck.19 Histologically, this lesion features a wedge-shaped infiltrate of deep dermal melanocytes with oval nuclei. It commonly extends to the reticular dermis or further into the subcutis (Figure 4).20,21 This neoplasm frequently tracks along adnexal and neurovascular structures, resulting in a plexiform appearance.22 The adnexal involvement of deep penetrating nevi is a shared feature with desmoplastic Spitz nevi. The presence of any number of melanophages is characteristic of this lesion.23 Lastly, there is a well-documented association between β-catenin mutations and deep penetrating nevi.24 Multicentric reticulohistiocytosis (MRH) is a rare form of non-Langerhans cell histiocytosis that has the pathognomonic clinical finding of pink-red papules (coral beading) with a predilection for acral surfaces. Histology of affected skin reveals a dermal infiltrate of ground glass as well as eosinophilic histiocytes that most often stain positive for CD68 and human alveolar macrophage 56 but negative for S-100 and CD1a (Figure 5).25 Although MRH is rare, negative staining for S-100 could serve as a useful diagnostic clue to differentiate it from other entities that are positive for S-100, such as the desmoplastic Spitz nevus. Arthritis mutilans is a potential complication of MRH, but a reported association with an underlying malignancy is seen in approximately 25% of cases.26 Thus, the cutaneous, rheumatologic, and oncologic implications of this disease help to distinguish it from other differential diagnoses that may be considered.


The Diagnosis: Desmoplastic Spitz Nevus
Desmoplastic Spitz nevus is a rare variant of Spitz nevus that commonly presents as a red to brown papule on the head, neck, or extremities. It is pertinent to review the histologic features of this neoplasm, as it can be confused with other more sinister entities such as spitzoid melanoma. Histologically, there is a dermal infiltrate of melanocytes containing eosinophilic cytoplasm and vesicular nuclei. Junctional involvement is rare, and there should be no pagetoid spread.1 This entity features abundant stromal fibrosis formed by dense collagen bundles, low cellular density, and polygonal-shaped melanocytes, which helps to differentiate it from melanoma.2,3 In a retrospective study comparing the characteristics of desmoplastic Spitz nevi with desmoplastic melanoma, desmoplastic Spitz nevi histologically were more symmetric and circumscribed with greater melanocytic maturation and adnexal structure involvement.3 Although this entity demonstrates maturation from the superficial to the deep dermis, it also may feature deep dermal vascular proliferation.4 S-100 and SRY-related HMG box 10, SOX-10, are noted to be positive in desmoplastic Spitz nevi, which can help to differentiate it from nonmelanocytic entities (Figure 1).

Although spitzoid lesions can be ambiguous and difficult even for experts to classify, spitzoid melanoma tends to have a high Breslow thickness, high cell density, marked atypia, and an increased nucleus to cytoplasm ratio.5 Additionally, desmoplastic melanoma was found to more often display “melanocytic junctional nests associated with discohesive cells, variations in size and shape of the nests, lentiginous melanocytic proliferation, actinic elastosis, pagetoid spread, dermal mitosis, perineural involvement and brisk inflammatory infiltrate.”3 Given the challenge of histologically separating desmoplastic Spitz nevi from melanoma, immunostaining can be useful. For example, Hilliard et al6 used a p16 antibody to differentiate desmoplastic Spitz nevi from desmoplastic melanoma, finding that most desmoplastic melanomas (81.8%; n=11) were negative for p16, whereas all desmoplastic Spitz nevi were at least moderately positive. However, another study re-evaluated the utility of p16 in desmoplastic melanoma and found that 72.7% (16/22) were at least focally reactive for the immunostain.7 Thus, caution must be exercised when using p16.
PReferentially expressed Antigen in MElanoma (PRAME) is a newer nuclear immunohistochemical marker that tends to be positive in melanomas and negative in nevi. Desmoplastic Spitz nevi would be expected to be negative for PRAME, while desmoplastic melanoma may be positive; however, this marker seems to be less effective in desmoplastic melanoma than in most other subtypes of the malignancy. In one study, only 35% (n=20) of desmoplastic melanomas were positive for PRAME.8 Likewise, another study showed that some benign Spitz nevi may diffusely express PRAME.9 As such, PRAME should be used prudently.
For cases in which immunohistochemistry is equivocal, molecular testing may aid in differentiating Spitz nevi from melanoma. For example, comparative genomic hybridization has revealed an increased copy number of chromosome 11p in approximately 20% of Spitz nevi cases10; this finding is not seen in melanoma. Mutation analyses of HRas proto-oncogene, GTPase, HRAS; B-Raf proto-oncogene, serine/threonine kinase, BRAF; and NRAS proto-oncogene, GTPase, NRAS, also have shown some promise in distinguishing spitzoid lesions from melanoma, but these analyses may be oversimplified.11 Fluorescence in situ hybridization (FISH) is another diagnostic modality that has been studied to differentiate benign nevi from melanoma. One study challenged the utility of FISH, reporting 7 of 15 desmoplastic melanomas tested positive compared to 0 of 15 sclerotic melanocytic nevi.12 Thus, negative FISH cannot reliably rule out melanoma. Ultimately, a combination of immunostains along with FISH or another genetic study would prove to be most effective in ruling out melanoma in difficult cases. Even then, a dermatopathologist may be faced with a degree of uncertainty.
Cellular blue nevi predominantly affect adults younger than 40 years and commonly are seen on the buttocks.13 This benign neoplasm demonstrates areas that are distinctly sclerotic as well as those that are cellular in nature.14 This entity demonstrates a well-circumscribed dermal growth pattern with 2 main populations of cells. The sclerotic portion of the cellular blue nevus mimics that of the blue nevus in that it is noted superficially with irregular margins. The cellular aspect of the nevus features spindle cells contained within well-circumscribed nodules (Figure 2). Stromal melanophages are not uncommon, and some can be observed adjacent to nerve fibers. Although this blue nevus variant displays features of the common blue nevus, its melanocytes track along adnexal and neurovascular structures similar to the deep penetrating nevus and the desmoplastic Spitz nevus. However, these melanocytes are variable in morphology and can appear on a spectrum spanning from pale and lightly pigmented to clear.15

The breast is the most common site of origin of tumor metastasis to the skin. These cutaneous metastases can vary in both their clinical and histological presentations. For example, cutaneous metastatic breast adenocarcinoma often can present clinically as pink-violaceous papules and plaques on the breast or on other parts of the body. Histologically, it can demonstrate a varying degree of patterns such as collagen infiltration by single cells, cords, tubules, and sheets of atypical cells (Figure 3) that can be observed together in areas of mucin or can form glandular structures.16 Metastatic breast carcinoma is noted to be positive for gross cystic disease fluid protein-15, estrogen receptor, and cytokeratin 7, which can help differentiate this entity from other tumors of glandular origin.16 Although rare, primary melanoma of the breast has been reported in the literature.17,18 These malignant melanocytic lesions easily could be differentiated from other breast tumors such as adenocarcinoma using immunohistochemical staining patterns.

Deep penetrating nevi most often are observed clinically as blue, brown, or black papules or nodules on the head or neck.19 Histologically, this lesion features a wedge-shaped infiltrate of deep dermal melanocytes with oval nuclei. It commonly extends to the reticular dermis or further into the subcutis (Figure 4).20,21 This neoplasm frequently tracks along adnexal and neurovascular structures, resulting in a plexiform appearance.22 The adnexal involvement of deep penetrating nevi is a shared feature with desmoplastic Spitz nevi. The presence of any number of melanophages is characteristic of this lesion.23 Lastly, there is a well-documented association between β-catenin mutations and deep penetrating nevi.24 Multicentric reticulohistiocytosis (MRH) is a rare form of non-Langerhans cell histiocytosis that has the pathognomonic clinical finding of pink-red papules (coral beading) with a predilection for acral surfaces. Histology of affected skin reveals a dermal infiltrate of ground glass as well as eosinophilic histiocytes that most often stain positive for CD68 and human alveolar macrophage 56 but negative for S-100 and CD1a (Figure 5).25 Although MRH is rare, negative staining for S-100 could serve as a useful diagnostic clue to differentiate it from other entities that are positive for S-100, such as the desmoplastic Spitz nevus. Arthritis mutilans is a potential complication of MRH, but a reported association with an underlying malignancy is seen in approximately 25% of cases.26 Thus, the cutaneous, rheumatologic, and oncologic implications of this disease help to distinguish it from other differential diagnoses that may be considered.


- Luzar B, Bastian BC, North JP, et al. Melanocytic nevi. In: Calonje E, Brenn T, Lazar AJ, et al, eds. McKee’s Pathology of the Skin. 5th ed. Elsevier; 2020:1275-1280.
- Busam KJ, Gerami P. Spitz nevi. In: Busam KJ, Gerami P, Scolyer RA, eds. Pathology of Melanocytic Tumors. Elsevier; 2019:37-60.
- Nojavan H, Cribier B, Mehregan DR. Desmoplastic Spitz nevus: a histopathological review and comparison with desmoplastic melanoma [in French]. Ann Dermatol Venereol. 2009;136:689-695.
- Tomizawa K. Desmoplastic Spitz nevus showing vascular proliferation more prominently in the deep portion. Am J Dermatopathol. 2002;24:184-185.
- Requena C, Botella R, Nagore E, et al. Characteristics of spitzoid melanoma and clues for differential diagnosis with Spitz nevus. Am J Dermatopathol. 2012;34:478-486.
- Hilliard NJ, Krahl D, Sellheyer K. p16 expression differentiates between desmoplastic Spitz nevus and desmoplastic melanoma. J Cutan Pathol. 2009;36:753-759.
- Blokhin E, Pulitzer M, Busam KJ. Immunohistochemical expression of p16 in desmoplastic melanoma. J Cutan Pathol. 2013;40:796-800.
- Lezcano C, Jungbluth AA, Nehal KS, et al. PRAME expression in melanocytic tumors. Am J Surg Pathol. 2018;42:1456-1465.
- Raghavan SS, Wang JY, Kwok S, et al. PRAME expression in melanocytic proliferations with intermediate histopathologic or spitzoid features. J Cutan Pathol. 2020;47:1123-1131.
- Bauer J, Bastian BC. DNA copy number changes in the diagnosis of melanocytic tumors [in German]. Pathologe. 2007;28:464-473.
- Luo S, Sepehr A, Tsao H. Spitz nevi and other spitzoid lesions part I. background and diagnoses. J Am Acad Dermatol. 2011;65:1073-1084.
- Gerami P, Beilfuss B, Haghighat Z, et al. Fluorescence in situ hybridization as an ancillary method for the distinction of desmoplastic melanomas from sclerosing melanocytic nevi. J Cutan Pathol. 2011;38:329-334.
- Zembowicz A. Blue nevi and related tumors. Clin Lab Med. 2017; 37:401-415.
- Rodriguez HA, Ackerman LV. Cellular blue nevus. clinicopathologic study of forty-five cases. Cancer. 1968;21:393-405.
- Phadke PA, Zembowicz A. Blue nevi and related tumors. Clin Lab Med. 2011;31:345-358.
- Ko CJ. Metastatic tumors and simulators. In: Elston DM, Ferringer T, eds. Dermatopathology. 3rd ed. Elsevier Limited; 2019:496-504.
- Drueppel D, Schultheis B, Solass W, et al. Primary malignant melanoma of the breast: case report and review of the literature. Anticancer Res. 2015;35:1709-1713.
- Kurul S, Tas¸ F, Büyükbabani N, et al. Different manifestations of malignant melanoma in the breast: a report of 12 cases and a review of the literature. Jpn J Clin Oncol. 2005;35:202-206.
- Strazzula L, Senna MM, Yasuda M, et al. The deep penetrating nevus. J Am Acad Dermatol. 2014;71:1234-1240.
- Mehregan DA, Mehregan AH. Deep penetrating nevus. Arch Dermatol. 1993;129:328-331.
- Robson A, Morley-Quante M, Hempel H, et al. Deep penetrating naevus: clinicopathological study of 31 cases with further delineation of histological features allowing distinction from other pigmented benign melanocytic lesions and melanoma. Histopathology. 2003;43:529-537.
- Luzar B, Calonje E. Deep penetrating nevus: a review. Arch Pathol Lab Med. 2011;135:321-326.
- Cooper PH. Deep penetrating (plexiform spindle cell) nevus. a frequent participant in combined nevus. J Cutan Pathol. 1992;19:172-180.
- de la Fouchardière A, Caillot C, Jacquemus J, et al. β-Catenin nuclear expression discriminates deep penetrating nevi from other cutaneous melanocytic tumors. Virchows Arch. 2019;474:539-550.
- Gorman JD, Danning C, Schumacher HR, et al. Multicentric reticulohistiocytosis: case report with immunohistochemical analysis and literature review. Arthritis Rheum. 2000;43:930-938.
- Selmi C, Greenspan A, Huntley A, et al. Multicentric reticulohistiocytosis: a critical review. Curr Rheumatol Rep. 2015;17:511.
- Luzar B, Bastian BC, North JP, et al. Melanocytic nevi. In: Calonje E, Brenn T, Lazar AJ, et al, eds. McKee’s Pathology of the Skin. 5th ed. Elsevier; 2020:1275-1280.
- Busam KJ, Gerami P. Spitz nevi. In: Busam KJ, Gerami P, Scolyer RA, eds. Pathology of Melanocytic Tumors. Elsevier; 2019:37-60.
- Nojavan H, Cribier B, Mehregan DR. Desmoplastic Spitz nevus: a histopathological review and comparison with desmoplastic melanoma [in French]. Ann Dermatol Venereol. 2009;136:689-695.
- Tomizawa K. Desmoplastic Spitz nevus showing vascular proliferation more prominently in the deep portion. Am J Dermatopathol. 2002;24:184-185.
- Requena C, Botella R, Nagore E, et al. Characteristics of spitzoid melanoma and clues for differential diagnosis with Spitz nevus. Am J Dermatopathol. 2012;34:478-486.
- Hilliard NJ, Krahl D, Sellheyer K. p16 expression differentiates between desmoplastic Spitz nevus and desmoplastic melanoma. J Cutan Pathol. 2009;36:753-759.
- Blokhin E, Pulitzer M, Busam KJ. Immunohistochemical expression of p16 in desmoplastic melanoma. J Cutan Pathol. 2013;40:796-800.
- Lezcano C, Jungbluth AA, Nehal KS, et al. PRAME expression in melanocytic tumors. Am J Surg Pathol. 2018;42:1456-1465.
- Raghavan SS, Wang JY, Kwok S, et al. PRAME expression in melanocytic proliferations with intermediate histopathologic or spitzoid features. J Cutan Pathol. 2020;47:1123-1131.
- Bauer J, Bastian BC. DNA copy number changes in the diagnosis of melanocytic tumors [in German]. Pathologe. 2007;28:464-473.
- Luo S, Sepehr A, Tsao H. Spitz nevi and other spitzoid lesions part I. background and diagnoses. J Am Acad Dermatol. 2011;65:1073-1084.
- Gerami P, Beilfuss B, Haghighat Z, et al. Fluorescence in situ hybridization as an ancillary method for the distinction of desmoplastic melanomas from sclerosing melanocytic nevi. J Cutan Pathol. 2011;38:329-334.
- Zembowicz A. Blue nevi and related tumors. Clin Lab Med. 2017; 37:401-415.
- Rodriguez HA, Ackerman LV. Cellular blue nevus. clinicopathologic study of forty-five cases. Cancer. 1968;21:393-405.
- Phadke PA, Zembowicz A. Blue nevi and related tumors. Clin Lab Med. 2011;31:345-358.
- Ko CJ. Metastatic tumors and simulators. In: Elston DM, Ferringer T, eds. Dermatopathology. 3rd ed. Elsevier Limited; 2019:496-504.
- Drueppel D, Schultheis B, Solass W, et al. Primary malignant melanoma of the breast: case report and review of the literature. Anticancer Res. 2015;35:1709-1713.
- Kurul S, Tas¸ F, Büyükbabani N, et al. Different manifestations of malignant melanoma in the breast: a report of 12 cases and a review of the literature. Jpn J Clin Oncol. 2005;35:202-206.
- Strazzula L, Senna MM, Yasuda M, et al. The deep penetrating nevus. J Am Acad Dermatol. 2014;71:1234-1240.
- Mehregan DA, Mehregan AH. Deep penetrating nevus. Arch Dermatol. 1993;129:328-331.
- Robson A, Morley-Quante M, Hempel H, et al. Deep penetrating naevus: clinicopathological study of 31 cases with further delineation of histological features allowing distinction from other pigmented benign melanocytic lesions and melanoma. Histopathology. 2003;43:529-537.
- Luzar B, Calonje E. Deep penetrating nevus: a review. Arch Pathol Lab Med. 2011;135:321-326.
- Cooper PH. Deep penetrating (plexiform spindle cell) nevus. a frequent participant in combined nevus. J Cutan Pathol. 1992;19:172-180.
- de la Fouchardière A, Caillot C, Jacquemus J, et al. β-Catenin nuclear expression discriminates deep penetrating nevi from other cutaneous melanocytic tumors. Virchows Arch. 2019;474:539-550.
- Gorman JD, Danning C, Schumacher HR, et al. Multicentric reticulohistiocytosis: case report with immunohistochemical analysis and literature review. Arthritis Rheum. 2000;43:930-938.
- Selmi C, Greenspan A, Huntley A, et al. Multicentric reticulohistiocytosis: a critical review. Curr Rheumatol Rep. 2015;17:511.
A 37-year-old woman with a history of fibrocystic breast disease and a family history of breast cancer presented with a light brown macule on the right upper arm of 10 years’ duration. The patient first noticed this macule 10 years prior; however, within the last 4 months she noticed a small amount of homogenous darkening and occasional pruritus. Physical examination revealed a 4.0-mm, light brown and pink macule on the right upper arm. Dermoscopy showed a homogenous pigment network with reticular lines and branched streaks centrally. No crystalline structures, milky red globules, or pseudopods were appreciated. A tangential shave biopsy was obtained and submitted for hematoxylin and eosin staining.
The Final Rule for 2022: What’s New and How Changes in the Medicare Physician Fee Schedule and Quality Payment Program Affect Dermatologists
On November 2, 2021, the Centers for Medicare & Medicaid Services (CMS) released its final rule for the 2022 Medicare Physician Fee Schedule (PFS) and the Quality Payment Program (QPP).1,2 These guidelines contain updates that will remarkably impact the field of medicine—and dermatology in particular—in 2022. This article will walk you through some of the updates most relevant to dermatology and how they may affect your practice.
Process for the Final Rule
The CMS releases an annual rule for the PFS and QPP. The interim rule generally is released over the summer with preliminary guidelines for the upcoming payment year. There is then a period of open comment where those affected by these changes, including physicians and medical associations, can submit comments to support what has been proposed or advocate for any changes. This input is then reviewed, and a final rule generally is published in the fall.
For this calendar year, the interim 2022 rule was released on July 13, 2021,3 and included many of guidelines that will be discussed in more detail in this article. Many associations that represent medicine overall and specifically dermatology, including the American Medical Association and the American Academy of Dermatology, submitted comments in response to these proposals.4,5
PFS Conversion Factor
The PFS conversion factor is updated annually to ensure budget neutrality in the setting of changes in relative value units. For 2022, the PFS conversion factor is $34.6062, representing a reduction of approximately $0.29 from the 2021 PFS conversion factor of $34.8931.6 This reduction does not take into account other payment adjustments due to legislative changes.
In combination, these changes previously were estimated to represent an overall payment cut of 10% or higher for dermatology, with those practitioners doing more procedural work or dermatopathology likely being impacted more heavily. However, with the passing of the Protecting Medicare and American Farmers from Sequester Cuts Act, it is estimated that the reductions in payment to dermatology will begin at 0.75% and reach 2.75% in the second half of the year with the phased-in reinstatement of the Medicare sequester.4,5,7
Clinical Labor Pricing Updates
Starting in 2022, the CMS will utilize updated wage rates from the US Bureau of Labor Statistics to revise clinical labor costs over a 4-year period. Clinical labor rates are important, as they are used to calculate practice expense within the PFS. These clinical labor rates were last updated in 2002.8 Median wage data, as opposed to mean data, from the US Bureau of Labor Statistics will be utilized to calculate the updated clinical labor rates.
A multiyear implementation plan was put into place by CMS due to multiple concerns, including that current wage rates are inadequate and may not reflect current labor rate information. Additionally, comments on this proposal voiced concern that updating the supply and equipment pricing without updating the clinical labor pricing could create distortions in the allocation of direct practice expense, which also factored into the implementation of a multiyear plan.8
It is anticipated that specialties that rely primarily on clinical labor will receive the largest increases in these rates and that specialties that rely primarily on supply or equipment items are anticipated to receive the largest reductions relative to other specialties. Dermatology is estimated to have a 0% change during the year 1 transition period; however, it will have an estimated 1% reduction in clinical labor pricing overall once the updates are completed.1 Pathology also is estimated to have a similar overall decrease during this transition period.
Evaluation and Management Visits
The biggest update in this area primarily is related to refining policies for split (shared) evaluation and management (E/M) visits and teaching physician activities. Split E/M visits are defined by the CMS as visits provided in the facility setting by a physician and nonphysician practitioner in the same group, with the visit billed by whomever provides the substantive portion of the visit. For 2022, the term substantive portion will be defined by the CMS as history, physical examination, medical decision-making, or more than half of the total time; for 2023, it will be defined as more than half of the total time spent.3 A split visit also can apply to an E/M visit provided in part by both a teaching physician and resident. Split visits can be reported for new or established patients. For proper reimbursement, the 2 practitioners who performed the services must be documented in the medical record, and the practitioner who provided the substantive portion must sign and date the encounter in the medical record. Additionally, the CMS has indicated the modifier FS must be included on the claim to indicate the split visit.9
For dermatologists who act as teaching physicians, it is important to note that many of the existing CMS policies for billing E/M services are still in place, specifically that if a resident participates in a service in a teaching setting, the teaching physician can bill for the service only if they are present for the key or critical portion of the service. A primary care exception does exist, in which teaching physicians at certain teaching hospital primary care centers can bill for some services performed independently by a resident without the physical presence of the teaching physician; however, this often is not applicable within dermatology.
With updated outpatient E/M guidelines, if time is being selected to bill, only the time that the teaching physician was present can be included to determine the overall E/M level.
Billing for Physician Assistant Services
Currently Medicare can only make payments to the employer or independent contractor of a physician assistant (PA); however, starting January 1, 2022, the CMS has authorized Medicare to make direct payments to PAs for qualifying professional services, in the same manner that nurse practitioners can currently bill. This also will allow PAs to incorporate as a group and bill Medicare for PA services. This stems from a congressional mandate within the Consolidated Appropriations Act of 2021.8 As a result, in states where PAs can practice independently, they can opt out of physician-led care teams and furnish services independently, including dermatologic services.
QPP Updates
Several changes were made to the Merit-Based Incentive Payment System (MIPS). Some of these changes include:
- Increase the MIPS performance threshold to 75 points from 60 points.
- Set the performance threshold at 89 points.
- Reduce the quality performance category weight from 40% to 30% of the final MIPS score.
- Increase the cost performance category weight from 20% to 30% of the final MIPS score.
- The extreme and uncontrollable circumstances application also has been extended to the end of 2022, allowing those remarkably impacted by the COVID-19 public health emergency to request for reweighting on any or all MIPS performance categories.
Cost Measures and MIPS Value Pathways
The melanoma resection cost measure will be implemented in 2022, representing the first dermatology cost measure, which will include the cost to Medicare over a 1-year period for all patient care for the excision of a melanoma. Although cost measures will be part of the MIPS value pathways (MVPs) reporting, dermatology currently is not part of the MVP; however, with the CMS moving forward with an initial set of MVPs that physicians can voluntarily report on in 2023, there is a possibility that dermatology will be asked to be part of the program in the future.10
Final Thoughts
There are many upcoming changes as part of the 2022 final rule, including to the conversion factor, E/M split visits, PA billing, and the QPP. Advocacy in these areas to the CMS and lawmakers, either directly or through dermatologic and other medical societies, is critical to help influence eventual recommendations.
- Medicare Program; CY 2022 payment policies under the Physician Fee Schedule and other changes to part B payment policies; Medicare Shared Savings Program requirements; provider enrollment regulation updates; and provider and supplier prepayment and post-payment medical review requirements. Fed Regist. 2021;86:64996-66031. To be codified at 42 CFR §403, §405, §410, §411, §414, §415, §423, §424, and §425. https://www.federalregister.gov/documents/2021/11/19/2021-23972/medicare-program-cy-2022-payment-policies-under-the-physician-fee-schedule-and-other-changes-to-part
- Centers for Medicare & Medicaid Services. CMS physician payment rule promotes greater access to telehealth services, diabetes prevention programs. Published November 2, 2021. Accessed January 10, 2022. https://www.cms.gov/newsroom/press-releases/cms-physician-payment-rule-promotes-greater-access-telehealth-services-diabetes-prevention-programs
- Centers for Medicare & Medicaid Services. Calendar year (CY) 2022 Medicare Physician Fee Schedule proposed rule. Published July 13, 2021. Accessed January 10, 2022. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2022-medicare-physician-fee-schedule-proposed-rule
- American Academy of Dermatology. Dermatology World Weekly. October 27, 2021. Accessed January 20, 2022. https://www.aad.org/dw/weekly
- O’Reilly KB. 2022 Medicare pay schedule confirms Congress needs to act. American Medical Association website. Published November 10, 2021. Accessed January 10, 2021. https://www.ama-assn.org/practice-management/medicare-medicaid/2022-medicare-pay-schedule-confirms-congress-needs-act
- History of Medicare conversion factors. American Medical Association website. Accessed January 19, 2022. https://www.ama-assn.org/system/files/2021-01/cf-history.pdf
- American Academy of Dermatology. Dermatology World Weekly. December 15, 2021. Accessed January 20, 2022. https://www.aad.org/dw/weekly
- American Medical Association. CY 2022 Medicare Physician Fee Schedule (PFS) and Quality Payment Program (QPP) final rule summary. Accessed January 10, 2021. https://www.ama-assn.org/system/files/2022-pfs-qpp-final-rule.pdf
- Centers for Medicare & Medicaid Services. January 2022 alpha-numeric HCPCS file. Updated December 20, 2021. Accessed January 20, 2022. https://www.cms.gov/Medicare/Coding/HCPCSReleaseCodeSets/HCPCS-Quarterly-Update
- CMS finalizes Medicare payments for 2022. American Academy of Dermatology website. NEED PUB DATE. Accessed January 20, 2022. https://www.aad.org/member/practice/mips/fee-schedule/2022-fee-schedule-final
On November 2, 2021, the Centers for Medicare & Medicaid Services (CMS) released its final rule for the 2022 Medicare Physician Fee Schedule (PFS) and the Quality Payment Program (QPP).1,2 These guidelines contain updates that will remarkably impact the field of medicine—and dermatology in particular—in 2022. This article will walk you through some of the updates most relevant to dermatology and how they may affect your practice.
Process for the Final Rule
The CMS releases an annual rule for the PFS and QPP. The interim rule generally is released over the summer with preliminary guidelines for the upcoming payment year. There is then a period of open comment where those affected by these changes, including physicians and medical associations, can submit comments to support what has been proposed or advocate for any changes. This input is then reviewed, and a final rule generally is published in the fall.
For this calendar year, the interim 2022 rule was released on July 13, 2021,3 and included many of guidelines that will be discussed in more detail in this article. Many associations that represent medicine overall and specifically dermatology, including the American Medical Association and the American Academy of Dermatology, submitted comments in response to these proposals.4,5
PFS Conversion Factor
The PFS conversion factor is updated annually to ensure budget neutrality in the setting of changes in relative value units. For 2022, the PFS conversion factor is $34.6062, representing a reduction of approximately $0.29 from the 2021 PFS conversion factor of $34.8931.6 This reduction does not take into account other payment adjustments due to legislative changes.
In combination, these changes previously were estimated to represent an overall payment cut of 10% or higher for dermatology, with those practitioners doing more procedural work or dermatopathology likely being impacted more heavily. However, with the passing of the Protecting Medicare and American Farmers from Sequester Cuts Act, it is estimated that the reductions in payment to dermatology will begin at 0.75% and reach 2.75% in the second half of the year with the phased-in reinstatement of the Medicare sequester.4,5,7
Clinical Labor Pricing Updates
Starting in 2022, the CMS will utilize updated wage rates from the US Bureau of Labor Statistics to revise clinical labor costs over a 4-year period. Clinical labor rates are important, as they are used to calculate practice expense within the PFS. These clinical labor rates were last updated in 2002.8 Median wage data, as opposed to mean data, from the US Bureau of Labor Statistics will be utilized to calculate the updated clinical labor rates.
A multiyear implementation plan was put into place by CMS due to multiple concerns, including that current wage rates are inadequate and may not reflect current labor rate information. Additionally, comments on this proposal voiced concern that updating the supply and equipment pricing without updating the clinical labor pricing could create distortions in the allocation of direct practice expense, which also factored into the implementation of a multiyear plan.8
It is anticipated that specialties that rely primarily on clinical labor will receive the largest increases in these rates and that specialties that rely primarily on supply or equipment items are anticipated to receive the largest reductions relative to other specialties. Dermatology is estimated to have a 0% change during the year 1 transition period; however, it will have an estimated 1% reduction in clinical labor pricing overall once the updates are completed.1 Pathology also is estimated to have a similar overall decrease during this transition period.
Evaluation and Management Visits
The biggest update in this area primarily is related to refining policies for split (shared) evaluation and management (E/M) visits and teaching physician activities. Split E/M visits are defined by the CMS as visits provided in the facility setting by a physician and nonphysician practitioner in the same group, with the visit billed by whomever provides the substantive portion of the visit. For 2022, the term substantive portion will be defined by the CMS as history, physical examination, medical decision-making, or more than half of the total time; for 2023, it will be defined as more than half of the total time spent.3 A split visit also can apply to an E/M visit provided in part by both a teaching physician and resident. Split visits can be reported for new or established patients. For proper reimbursement, the 2 practitioners who performed the services must be documented in the medical record, and the practitioner who provided the substantive portion must sign and date the encounter in the medical record. Additionally, the CMS has indicated the modifier FS must be included on the claim to indicate the split visit.9
For dermatologists who act as teaching physicians, it is important to note that many of the existing CMS policies for billing E/M services are still in place, specifically that if a resident participates in a service in a teaching setting, the teaching physician can bill for the service only if they are present for the key or critical portion of the service. A primary care exception does exist, in which teaching physicians at certain teaching hospital primary care centers can bill for some services performed independently by a resident without the physical presence of the teaching physician; however, this often is not applicable within dermatology.
With updated outpatient E/M guidelines, if time is being selected to bill, only the time that the teaching physician was present can be included to determine the overall E/M level.
Billing for Physician Assistant Services
Currently Medicare can only make payments to the employer or independent contractor of a physician assistant (PA); however, starting January 1, 2022, the CMS has authorized Medicare to make direct payments to PAs for qualifying professional services, in the same manner that nurse practitioners can currently bill. This also will allow PAs to incorporate as a group and bill Medicare for PA services. This stems from a congressional mandate within the Consolidated Appropriations Act of 2021.8 As a result, in states where PAs can practice independently, they can opt out of physician-led care teams and furnish services independently, including dermatologic services.
QPP Updates
Several changes were made to the Merit-Based Incentive Payment System (MIPS). Some of these changes include:
- Increase the MIPS performance threshold to 75 points from 60 points.
- Set the performance threshold at 89 points.
- Reduce the quality performance category weight from 40% to 30% of the final MIPS score.
- Increase the cost performance category weight from 20% to 30% of the final MIPS score.
- The extreme and uncontrollable circumstances application also has been extended to the end of 2022, allowing those remarkably impacted by the COVID-19 public health emergency to request for reweighting on any or all MIPS performance categories.
Cost Measures and MIPS Value Pathways
The melanoma resection cost measure will be implemented in 2022, representing the first dermatology cost measure, which will include the cost to Medicare over a 1-year period for all patient care for the excision of a melanoma. Although cost measures will be part of the MIPS value pathways (MVPs) reporting, dermatology currently is not part of the MVP; however, with the CMS moving forward with an initial set of MVPs that physicians can voluntarily report on in 2023, there is a possibility that dermatology will be asked to be part of the program in the future.10
Final Thoughts
There are many upcoming changes as part of the 2022 final rule, including to the conversion factor, E/M split visits, PA billing, and the QPP. Advocacy in these areas to the CMS and lawmakers, either directly or through dermatologic and other medical societies, is critical to help influence eventual recommendations.
On November 2, 2021, the Centers for Medicare & Medicaid Services (CMS) released its final rule for the 2022 Medicare Physician Fee Schedule (PFS) and the Quality Payment Program (QPP).1,2 These guidelines contain updates that will remarkably impact the field of medicine—and dermatology in particular—in 2022. This article will walk you through some of the updates most relevant to dermatology and how they may affect your practice.
Process for the Final Rule
The CMS releases an annual rule for the PFS and QPP. The interim rule generally is released over the summer with preliminary guidelines for the upcoming payment year. There is then a period of open comment where those affected by these changes, including physicians and medical associations, can submit comments to support what has been proposed or advocate for any changes. This input is then reviewed, and a final rule generally is published in the fall.
For this calendar year, the interim 2022 rule was released on July 13, 2021,3 and included many of guidelines that will be discussed in more detail in this article. Many associations that represent medicine overall and specifically dermatology, including the American Medical Association and the American Academy of Dermatology, submitted comments in response to these proposals.4,5
PFS Conversion Factor
The PFS conversion factor is updated annually to ensure budget neutrality in the setting of changes in relative value units. For 2022, the PFS conversion factor is $34.6062, representing a reduction of approximately $0.29 from the 2021 PFS conversion factor of $34.8931.6 This reduction does not take into account other payment adjustments due to legislative changes.
In combination, these changes previously were estimated to represent an overall payment cut of 10% or higher for dermatology, with those practitioners doing more procedural work or dermatopathology likely being impacted more heavily. However, with the passing of the Protecting Medicare and American Farmers from Sequester Cuts Act, it is estimated that the reductions in payment to dermatology will begin at 0.75% and reach 2.75% in the second half of the year with the phased-in reinstatement of the Medicare sequester.4,5,7
Clinical Labor Pricing Updates
Starting in 2022, the CMS will utilize updated wage rates from the US Bureau of Labor Statistics to revise clinical labor costs over a 4-year period. Clinical labor rates are important, as they are used to calculate practice expense within the PFS. These clinical labor rates were last updated in 2002.8 Median wage data, as opposed to mean data, from the US Bureau of Labor Statistics will be utilized to calculate the updated clinical labor rates.
A multiyear implementation plan was put into place by CMS due to multiple concerns, including that current wage rates are inadequate and may not reflect current labor rate information. Additionally, comments on this proposal voiced concern that updating the supply and equipment pricing without updating the clinical labor pricing could create distortions in the allocation of direct practice expense, which also factored into the implementation of a multiyear plan.8
It is anticipated that specialties that rely primarily on clinical labor will receive the largest increases in these rates and that specialties that rely primarily on supply or equipment items are anticipated to receive the largest reductions relative to other specialties. Dermatology is estimated to have a 0% change during the year 1 transition period; however, it will have an estimated 1% reduction in clinical labor pricing overall once the updates are completed.1 Pathology also is estimated to have a similar overall decrease during this transition period.
Evaluation and Management Visits
The biggest update in this area primarily is related to refining policies for split (shared) evaluation and management (E/M) visits and teaching physician activities. Split E/M visits are defined by the CMS as visits provided in the facility setting by a physician and nonphysician practitioner in the same group, with the visit billed by whomever provides the substantive portion of the visit. For 2022, the term substantive portion will be defined by the CMS as history, physical examination, medical decision-making, or more than half of the total time; for 2023, it will be defined as more than half of the total time spent.3 A split visit also can apply to an E/M visit provided in part by both a teaching physician and resident. Split visits can be reported for new or established patients. For proper reimbursement, the 2 practitioners who performed the services must be documented in the medical record, and the practitioner who provided the substantive portion must sign and date the encounter in the medical record. Additionally, the CMS has indicated the modifier FS must be included on the claim to indicate the split visit.9
For dermatologists who act as teaching physicians, it is important to note that many of the existing CMS policies for billing E/M services are still in place, specifically that if a resident participates in a service in a teaching setting, the teaching physician can bill for the service only if they are present for the key or critical portion of the service. A primary care exception does exist, in which teaching physicians at certain teaching hospital primary care centers can bill for some services performed independently by a resident without the physical presence of the teaching physician; however, this often is not applicable within dermatology.
With updated outpatient E/M guidelines, if time is being selected to bill, only the time that the teaching physician was present can be included to determine the overall E/M level.
Billing for Physician Assistant Services
Currently Medicare can only make payments to the employer or independent contractor of a physician assistant (PA); however, starting January 1, 2022, the CMS has authorized Medicare to make direct payments to PAs for qualifying professional services, in the same manner that nurse practitioners can currently bill. This also will allow PAs to incorporate as a group and bill Medicare for PA services. This stems from a congressional mandate within the Consolidated Appropriations Act of 2021.8 As a result, in states where PAs can practice independently, they can opt out of physician-led care teams and furnish services independently, including dermatologic services.
QPP Updates
Several changes were made to the Merit-Based Incentive Payment System (MIPS). Some of these changes include:
- Increase the MIPS performance threshold to 75 points from 60 points.
- Set the performance threshold at 89 points.
- Reduce the quality performance category weight from 40% to 30% of the final MIPS score.
- Increase the cost performance category weight from 20% to 30% of the final MIPS score.
- The extreme and uncontrollable circumstances application also has been extended to the end of 2022, allowing those remarkably impacted by the COVID-19 public health emergency to request for reweighting on any or all MIPS performance categories.
Cost Measures and MIPS Value Pathways
The melanoma resection cost measure will be implemented in 2022, representing the first dermatology cost measure, which will include the cost to Medicare over a 1-year period for all patient care for the excision of a melanoma. Although cost measures will be part of the MIPS value pathways (MVPs) reporting, dermatology currently is not part of the MVP; however, with the CMS moving forward with an initial set of MVPs that physicians can voluntarily report on in 2023, there is a possibility that dermatology will be asked to be part of the program in the future.10
Final Thoughts
There are many upcoming changes as part of the 2022 final rule, including to the conversion factor, E/M split visits, PA billing, and the QPP. Advocacy in these areas to the CMS and lawmakers, either directly or through dermatologic and other medical societies, is critical to help influence eventual recommendations.
- Medicare Program; CY 2022 payment policies under the Physician Fee Schedule and other changes to part B payment policies; Medicare Shared Savings Program requirements; provider enrollment regulation updates; and provider and supplier prepayment and post-payment medical review requirements. Fed Regist. 2021;86:64996-66031. To be codified at 42 CFR §403, §405, §410, §411, §414, §415, §423, §424, and §425. https://www.federalregister.gov/documents/2021/11/19/2021-23972/medicare-program-cy-2022-payment-policies-under-the-physician-fee-schedule-and-other-changes-to-part
- Centers for Medicare & Medicaid Services. CMS physician payment rule promotes greater access to telehealth services, diabetes prevention programs. Published November 2, 2021. Accessed January 10, 2022. https://www.cms.gov/newsroom/press-releases/cms-physician-payment-rule-promotes-greater-access-telehealth-services-diabetes-prevention-programs
- Centers for Medicare & Medicaid Services. Calendar year (CY) 2022 Medicare Physician Fee Schedule proposed rule. Published July 13, 2021. Accessed January 10, 2022. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2022-medicare-physician-fee-schedule-proposed-rule
- American Academy of Dermatology. Dermatology World Weekly. October 27, 2021. Accessed January 20, 2022. https://www.aad.org/dw/weekly
- O’Reilly KB. 2022 Medicare pay schedule confirms Congress needs to act. American Medical Association website. Published November 10, 2021. Accessed January 10, 2021. https://www.ama-assn.org/practice-management/medicare-medicaid/2022-medicare-pay-schedule-confirms-congress-needs-act
- History of Medicare conversion factors. American Medical Association website. Accessed January 19, 2022. https://www.ama-assn.org/system/files/2021-01/cf-history.pdf
- American Academy of Dermatology. Dermatology World Weekly. December 15, 2021. Accessed January 20, 2022. https://www.aad.org/dw/weekly
- American Medical Association. CY 2022 Medicare Physician Fee Schedule (PFS) and Quality Payment Program (QPP) final rule summary. Accessed January 10, 2021. https://www.ama-assn.org/system/files/2022-pfs-qpp-final-rule.pdf
- Centers for Medicare & Medicaid Services. January 2022 alpha-numeric HCPCS file. Updated December 20, 2021. Accessed January 20, 2022. https://www.cms.gov/Medicare/Coding/HCPCSReleaseCodeSets/HCPCS-Quarterly-Update
- CMS finalizes Medicare payments for 2022. American Academy of Dermatology website. NEED PUB DATE. Accessed January 20, 2022. https://www.aad.org/member/practice/mips/fee-schedule/2022-fee-schedule-final
- Medicare Program; CY 2022 payment policies under the Physician Fee Schedule and other changes to part B payment policies; Medicare Shared Savings Program requirements; provider enrollment regulation updates; and provider and supplier prepayment and post-payment medical review requirements. Fed Regist. 2021;86:64996-66031. To be codified at 42 CFR §403, §405, §410, §411, §414, §415, §423, §424, and §425. https://www.federalregister.gov/documents/2021/11/19/2021-23972/medicare-program-cy-2022-payment-policies-under-the-physician-fee-schedule-and-other-changes-to-part
- Centers for Medicare & Medicaid Services. CMS physician payment rule promotes greater access to telehealth services, diabetes prevention programs. Published November 2, 2021. Accessed January 10, 2022. https://www.cms.gov/newsroom/press-releases/cms-physician-payment-rule-promotes-greater-access-telehealth-services-diabetes-prevention-programs
- Centers for Medicare & Medicaid Services. Calendar year (CY) 2022 Medicare Physician Fee Schedule proposed rule. Published July 13, 2021. Accessed January 10, 2022. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2022-medicare-physician-fee-schedule-proposed-rule
- American Academy of Dermatology. Dermatology World Weekly. October 27, 2021. Accessed January 20, 2022. https://www.aad.org/dw/weekly
- O’Reilly KB. 2022 Medicare pay schedule confirms Congress needs to act. American Medical Association website. Published November 10, 2021. Accessed January 10, 2021. https://www.ama-assn.org/practice-management/medicare-medicaid/2022-medicare-pay-schedule-confirms-congress-needs-act
- History of Medicare conversion factors. American Medical Association website. Accessed January 19, 2022. https://www.ama-assn.org/system/files/2021-01/cf-history.pdf
- American Academy of Dermatology. Dermatology World Weekly. December 15, 2021. Accessed January 20, 2022. https://www.aad.org/dw/weekly
- American Medical Association. CY 2022 Medicare Physician Fee Schedule (PFS) and Quality Payment Program (QPP) final rule summary. Accessed January 10, 2021. https://www.ama-assn.org/system/files/2022-pfs-qpp-final-rule.pdf
- Centers for Medicare & Medicaid Services. January 2022 alpha-numeric HCPCS file. Updated December 20, 2021. Accessed January 20, 2022. https://www.cms.gov/Medicare/Coding/HCPCSReleaseCodeSets/HCPCS-Quarterly-Update
- CMS finalizes Medicare payments for 2022. American Academy of Dermatology website. NEED PUB DATE. Accessed January 20, 2022. https://www.aad.org/member/practice/mips/fee-schedule/2022-fee-schedule-final
Practice Points
- The Centers for Medicare & Medicaid Services (CMS) 2022 final rule contains multiple updates affecting the practice of dermatology.
- Adjustments to the conversion factor and legislative-level actions have led to changes in reimbursement for many procedures within dermatology and beyond.
- Other notable updates include refining the definition of split evaluation and management visits, clinical labor pricing, and billing for physician assistant services.
- Changes in the Merit-Based Incentive Payment System (MIPS), cost measures, and MIPS value pathways also will impact many dermatology practices.
Oral Isotretinoin for Acne in the US Military: How Accelerated Courses and Teledermatology Can Minimize the Duty-Limiting Impacts of Treatment
Acne vulgaris is an extremely common dermatologic disease affecting 40 to 50 million individuals in the United States each year, with a prevalence of 85% in adolescents and young adults aged 12 to 24 years. For some patients, the disease may persist well into adulthood, affecting 8% of adults aged 25 and 34 years.1 Acne negatively impacts patients’ quality of life and productivity, with an estimated direct and indirect cost of over $3 billion per year.2
Oral isotretinoin, a vitamin A derivative, is approved by the US Food and Drug Administration for the treatment of severe nodulocystic acne. Isotretinoin reduces the size and secretions of sebaceous glands, inhibits growth and resulting inflammation of Cutibacterium acnes, and normalizes the differentiation of follicular keratinocytes, resulting in permanent changes in the pathogenesis of acne that may lead to remission.3 The use of oral isotretinoin in the active-duty US Military population may cause service members to be nondeployable or limit their ability to function in special roles (eg, pilot, submariner).4 Treatment regimens that minimize the course duration of isotretinoin and reduce the risk for relapse that requires a retrial of isotretinoin may, in turn, increase a service member’s readiness, deployment availability, and ability to perform unique occupational roles.
Additionally, teledermatology has been increasingly utilized to maintain treatment continuity for patients on isotretinoin during the COVID-19 pandemic.5 Application of this technology in the military also may be used to facilitate timely isotretinoin treatment regimens in active-duty service members to minimize course duration and increase readiness.
In this article, we discuss an accelerated course of oral isotretinoin as a safe and effective option for military service members bound by duty restrictions and operational timelines and explore the role of teledermatology for the treatment of acne in military service members.
Isotretinoin for Acne
Isotretinoin typically is initiated at a dosage of 0.5 mg/kg daily, increasing to 1 mg/kg daily with a goal cumulative dose between 120 and 150 mg/kg. Relapse may occur after completing a treatment course and is associated with cumulative dosing less than 120 mg/kg.6 The average duration of acne treatment with oral isotretinoin is approximately 6 months.7 At therapeutic doses, nearly all patients experience side effects, most commonly dryness and desquamation of the skin and mucous membranes, as well as possible involvement of the lips, eyes, and nose. Notable extracutaneous side effects include headache, visual disturbances at night, idiopathic intracranial hypertension, and myalgia. Serum cholesterol, triglycerides, and transaminases may be increased in patients taking isotretinoin, which requires routine monitoring using serum lipid profiles and liver function studies. A potential association between isotretinoin and inflammatory bowel disease and changes in mood have been reported, but current data do not suggest an evidence-based link.6,8 Isotretinoin is a potent teratogen, and in the United States, all patients are required to enroll in iPLEDGE, a US Food and Drug Administration–approved pregnancy prevention program that monitors prescribing and dispensing of the medication. For patients who can become pregnant, iPLEDGE requires use of 2 forms of contraception as well as monthly pregnancy tests prior to dispensing the medication.
Acne in Military Service Members
Acne is exceedingly common in the active-duty military population. In 2018, more than 40% of soldiers, sailors, airmen, and marines were 25 years or younger, and 75% of all US service members were 35 years or younger, corresponding to acne peak incidences.1,9 Management of acne in this population requires unique treatment considerations due to distinctive occupational requirements of and hazards faced by military personnel. Use of personal protective equipment, including gas masks, safety restraints, parachute rigging, and flak jackets, may be limiting in individuals with moderate to severe acne.10 For example, severe nodulocystic acne on the chin and jawline can interfere with proper wear of the chin strap on a Kevlar helmet. The severity of acne often necessitates the use of oral isotretinoin therapy, which is considered disqualifying for many special military assignments, including submarine duty, nuclear field duty, and diving duty.11 In military aviation communities, oral isotretinoin requires grounding for the duration of therapy plus 3 months after cessation. Slit-lamp examination, triglycerides, and transaminase levels must be normal prior to returning to unrestricted duty.12 Furthermore, use of oral isotretinoin may limit overseas assignments or deployment eligibility.4
The high prevalence of acne and the operationally limiting consequences of isotretinoin therapy present a unique challenge for dermatologists treating military personnel. The average duration of isotretinoin treatment is approximately 6 months,7 which represents a considerable amount of time during an average 4-year enlistment contract. Therapeutic treatment strategies that (1) reduce the duration of oral isotretinoin therapy, (2) reduce the risk for relapse, and (3) increase medication compliance can reduce the operational impact of this acne treatment. Such treatment strategies are discussed below.
High-Dose Isotretinoin
An optimal isotretinoin dosing regimen would achieve swift resolution of acne lesions and reduce the overall relapse rate requiring retrial of isotretinoin, thereby minimizing the operational- and duty-limiting impacts of the medication. Cyrulnik et al13 studied treatment outcomes of high-dose isotretinoin for acne vulgaris using a mean dosage of 1.6 mg/kg daily with an average cumulative dosage of 290 mg/kg. They demonstrated 100% clearance of lesions over 6 months, with a 12.5% relapse rate at 3 years. Aside from an increased rate of elevated transaminases, incidence of adverse effects and laboratory abnormalities were not significantly increased compared to conventional dosing regimens.13 The goal cumulative dosing of 120 to 150 mg/kg can be achieved 1 to 2 months earlier using a dosage of 1.6 mg/kg daily vs a conventional dosage of 1 mg/kg daily.
It has been hypothesized that higher cumulative doses of oral isotretinoin reduce the risk for relapse of acne and retrial of oral isotretinoin.14 Blasiak et al15 studied relapse and retrial of oral isotretinoin in acne patients who received cumulative dosing higher or lower than 220 mg/kg. A clinically but not statistically significant reduced relapse rate was observed in the cohort that received cumulative dosing higher than 220 mg/kg. No statistically significant difference in rates of adverse advents was observed aside from an increase in retinoid dermatitis in the cohort that received cumulative dosing higher than 220 mg/kg. Higher but not statistically significant rates of adverse events were seen in the group that received dosing higher than 220 mg/kg.15 Cumulative doses of oral isotretinoin higher than the 120 to 150 mg/kg range may decrease the risk for acne relapse and the need for an additional course of oral isotretinoin, which would reduce a service member’s total time away from deployment and full duty.
Relapse requiring a retrial of oral isotretinoin not only increases the operational cost of acne treatment but also considerably increases the monetary cost to the health care system. In a cost-analysis model, cumulative doses of oral isotretinoin higher than 230 mg/kg have a decreased overall cost compared to traditional cumulative dosing of less than 150 mg/kg due to the cost of relapse.16
Limitations of high daily and cumulative dosing regimens of oral isotretinoin are chiefly the dose-dependent rate of adverse effects. Low-dose regimens are associated with a reduced risk of isotretinoin-related side effects.6,17 Acute acne flares may be seen following initial administration of oral isotretinoin and are aggravated by increases in dosage.18 Isotretinoin-induced acne fulminans is a rare but devastating complication observed with high initial doses of oral isotretinoin in patients with severe acne.19 The risks and benefits of high daily and cumulatively dosed isotretinoin must be carefully considered in patients with severe acne.
Teledermatology: A Force for Readiness
The COVID-19 pandemic drastically changed the dermatology practice landscape with recommendations to cancel all elective outpatient visits in favor of teledermatology encounters.20 This decreased access to care, which resulted in an increase in drug interruption for dermatology patients, including patients on oral isotretinoin.21 Teledermatology has been increasingly utilized to maintain continuity of care for the management of patients taking isotretinoin.5 Routine utilization of teledermatology evaluation in military practices could expedite care, decrease patient travel time, and allow for in-clinic visits to be utilized for higher-acuity concerns.22
The use of teledermatology for uncomplicated oral isotretinoin management has the potential to increase medication compliance and decrease the amount of travel time for active-duty service members; for example, consider a military dermatology practice based in San Diego, California, that accepts referrals from military bases 3 hours away by car. After an initial consultation for consideration and initiation of oral isotretinoin, teledermatology appointments can save the active-duty service member 3 hours of travel time for each follow-up visit per month. This ultimately increases operational productivity, reduces barriers to accessing care, and improves patient satisfaction.23
Although military personnel usually are located at duty stations for 2 to 4 years, training exercises and military vocational schools often temporarily take personnel away from their home station. These temporary-duty assignments have the potential to interrupt medical follow-up appointments and may cause delays in treatment for individuals who miss monthly isotretinoin visits. When deemed appropriate by the prescribing dermatologist, teledermatology allows for increased continuity of care for active-duty service members and maintenance of a therapeutic isotretinoin course despite temporary geographic displacement.
By facilitating regular follow-up appointments, teledermatology can minimize the amount of time an active-duty service member is on a course of oral isotretinoin, thereby reducing the operational and duty-limiting implications of the medication.
Final Thoughts
Acne is a common dermatologic concern within the active-duty military population. Oral isotretinoin is indicated for treatment-resistant moderate or severe acne; however, it limits the ability of service members to deploy and is disqualifying for special military assignments. High daily- and cumulative-dose isotretinoin treatment strategies can reduce the duration of therapy and may be associated with a decrease in acne relapse and the need for retrial. Teledermatology can increase access to care and facilitate the completion of oral isotretinoin courses in a timely manner. These treatment strategies may help mitigate the duty-limiting impact of oral isotretinoin therapy in military service members.
- White GM. Recent findings in the epidemiologic evidence, classification, and subtypes of acne vulgaris. J Am Acad Dermatol. 1998;39:S34-S37. doi:10.1016/s0190-9622(98)70442-6
- Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55:490-500. doi:10.1016/j.jaad.2006.05.048
- James WD. Clinical practice. acne. N Engl J Med. 2005;352:1463-1472. doi:10.1056/NEJMcp033487
- Burke KR, Larrymore DC, Cho SH. Treatment consideration for US military members with skin disease. Cutis. 2019;103:329-332.
- Rosamilia LL. Isotretinoin meets COVID-19: revisiting a fragmented paradigm. Cutis. 2021;108:8-12. doi:10.12788/cutis.0299
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e33. doi:10.1016/j.jaad.2015.12.037
- Huang KE, Carstensen SE, Feldman SR. The duration of acne treatment. J Drugs Dermatol. 2014;13:655-656.
- Bettoli V, Guerra-Tapia A, Herane MI, et al. Challenges and solutions in oral isotretinoin in acne: reflections on 35 years of experience. Clin Cosmet Investig Dermatol. 2019;12:943-951. doi:10.2147/CCID.S234231
- US Department of Defense. 2018 demographics report: profile of the military community. Accessed January 18, 2022. https://download.militaryonesource.mil/12038/MOS/Reports/2018-demographics-report.pdf
- Brahe C, Peters K. Fighting acne for the fighting forces. Cutis. 2020;106:18-20, 22. doi:10.12788/cutis.0057
- US Department of the Navy. Change 167. manual of the medical department. Published February 15, 2019. Accessed January 18, 2022. https://www.med.navy.mil/Portals/62/Documents/BUMED/Directives/MANMED/Chapter%2015%20Medical%20Examinations%20(incorporates%20Changes%20126_135-138_140_145_150-152_154-156_160_164-167).pdf?ver=Rj7AoH54dNAX5uS3F1JUfw%3d%3d
- US Department of the Navy. US Navy aeromedical reference and waiver guide. Published August 11, 2021. Accessed January 18, 2022. https://www.med.navy.mil/Portals/62/Documents/NMFSC/NMOTC/NAMI/ARWG/Waiver%20Guide/ARWG%20COMPLETE_210811.pdf?ver=_pLPzFrtl8E2swFESnN4rA%3d%3d
- Cyrulnik AA, Viola KV, Gewirtzman AJ, et al. High-dose isotretinoin in acne vulgaris: improved treatment outcomes and quality of life. Int J Dermatol. 2012;51:1123-1130. doi:10.1111/j.1365-4632.2011.05409.x
- Coloe J, Du H, Morrell DS. Could higher doses of isotretinoin reduce the frequency of treatment failure in patients with acne? J Am Acad Dermatol. 2011;65:422-423. doi:10.1016/j.jaad.2010.06.025
- Blasiak RC, Stamey CR, Burkhart CN, et al. High-dose isotretinoin treatment and the rate of retrial, relapse, and adverse effects in patients with acne vulgaris. JAMA Dermatol. 2013;149:1392-1398. doi:10.1001/jamadermatol.2013.6746
- Zeitany AE, Bowers EV, Morrell DS. High-dose isotretinoin has lower impact on wallets: a cost analysis of dosing approaches. J Am Acad Dermatol. 2016;74:174-176. doi:10.1016/j.jaad.2015.08.012
- Amichai B, Shemer A, Grunwald MH. Low-dose isotretinoin in the treatment of acne vulgaris. J Am Acad Dermatol. 2006;54:644-666. doi:10.1016/j.jaad.2005.11.1061
- Borghi A, Mantovani L, Minghetti S, et al. Acute acne flare following isotretinoin administration: potential protective role of low starting dose. Dermatology. 2009;218:178-180. doi:10.1159/000182270
- Greywal T, Zaenglein AL, Baldwin HE, et al. Evidence-based recommendations for the management of acne fulminans and its variants. J Am Acad Dermatol. 2017;77:109-117. doi:10.1016/j.jaad.2016.11.028
- Kwatra SG, Sweren RJ, Grossberg AL. Dermatology practices as vectors for COVID-19 transmission: a call for immediate cessation of nonemergent dermatology visits. J Am Acad Dermatol. 2020;82:E179-E180. doi:10.1016/j.jaad.2020.03.037
- Alshiyab DM, Al-Qarqaz FA, Muhaidat JM. Impact of COVID-19 pandemic on the continuity of care for dermatologic patients on systemic therapy during the period of strict lockdown. Ann Med Surg (Lond). 2020;60:571-574. doi:10.1016/j.amsu.2020.11.056
- Hwang J, Kakimoto C. Teledermatology in the US military: a historic foundation for current and future applications. Cutis. 2018;101:335,337,345.
- Ruggiero A, Megna M, Annunziata MC, et al. Teledermatology for acne during COVID-19: high patients’ satisfaction in spite of the emergency. J Eur Acad Dermatol Venereol. 2020;34:E662-E663. doi:10.1111/jdv.16746
Acne vulgaris is an extremely common dermatologic disease affecting 40 to 50 million individuals in the United States each year, with a prevalence of 85% in adolescents and young adults aged 12 to 24 years. For some patients, the disease may persist well into adulthood, affecting 8% of adults aged 25 and 34 years.1 Acne negatively impacts patients’ quality of life and productivity, with an estimated direct and indirect cost of over $3 billion per year.2
Oral isotretinoin, a vitamin A derivative, is approved by the US Food and Drug Administration for the treatment of severe nodulocystic acne. Isotretinoin reduces the size and secretions of sebaceous glands, inhibits growth and resulting inflammation of Cutibacterium acnes, and normalizes the differentiation of follicular keratinocytes, resulting in permanent changes in the pathogenesis of acne that may lead to remission.3 The use of oral isotretinoin in the active-duty US Military population may cause service members to be nondeployable or limit their ability to function in special roles (eg, pilot, submariner).4 Treatment regimens that minimize the course duration of isotretinoin and reduce the risk for relapse that requires a retrial of isotretinoin may, in turn, increase a service member’s readiness, deployment availability, and ability to perform unique occupational roles.
Additionally, teledermatology has been increasingly utilized to maintain treatment continuity for patients on isotretinoin during the COVID-19 pandemic.5 Application of this technology in the military also may be used to facilitate timely isotretinoin treatment regimens in active-duty service members to minimize course duration and increase readiness.
In this article, we discuss an accelerated course of oral isotretinoin as a safe and effective option for military service members bound by duty restrictions and operational timelines and explore the role of teledermatology for the treatment of acne in military service members.
Isotretinoin for Acne
Isotretinoin typically is initiated at a dosage of 0.5 mg/kg daily, increasing to 1 mg/kg daily with a goal cumulative dose between 120 and 150 mg/kg. Relapse may occur after completing a treatment course and is associated with cumulative dosing less than 120 mg/kg.6 The average duration of acne treatment with oral isotretinoin is approximately 6 months.7 At therapeutic doses, nearly all patients experience side effects, most commonly dryness and desquamation of the skin and mucous membranes, as well as possible involvement of the lips, eyes, and nose. Notable extracutaneous side effects include headache, visual disturbances at night, idiopathic intracranial hypertension, and myalgia. Serum cholesterol, triglycerides, and transaminases may be increased in patients taking isotretinoin, which requires routine monitoring using serum lipid profiles and liver function studies. A potential association between isotretinoin and inflammatory bowel disease and changes in mood have been reported, but current data do not suggest an evidence-based link.6,8 Isotretinoin is a potent teratogen, and in the United States, all patients are required to enroll in iPLEDGE, a US Food and Drug Administration–approved pregnancy prevention program that monitors prescribing and dispensing of the medication. For patients who can become pregnant, iPLEDGE requires use of 2 forms of contraception as well as monthly pregnancy tests prior to dispensing the medication.
Acne in Military Service Members
Acne is exceedingly common in the active-duty military population. In 2018, more than 40% of soldiers, sailors, airmen, and marines were 25 years or younger, and 75% of all US service members were 35 years or younger, corresponding to acne peak incidences.1,9 Management of acne in this population requires unique treatment considerations due to distinctive occupational requirements of and hazards faced by military personnel. Use of personal protective equipment, including gas masks, safety restraints, parachute rigging, and flak jackets, may be limiting in individuals with moderate to severe acne.10 For example, severe nodulocystic acne on the chin and jawline can interfere with proper wear of the chin strap on a Kevlar helmet. The severity of acne often necessitates the use of oral isotretinoin therapy, which is considered disqualifying for many special military assignments, including submarine duty, nuclear field duty, and diving duty.11 In military aviation communities, oral isotretinoin requires grounding for the duration of therapy plus 3 months after cessation. Slit-lamp examination, triglycerides, and transaminase levels must be normal prior to returning to unrestricted duty.12 Furthermore, use of oral isotretinoin may limit overseas assignments or deployment eligibility.4
The high prevalence of acne and the operationally limiting consequences of isotretinoin therapy present a unique challenge for dermatologists treating military personnel. The average duration of isotretinoin treatment is approximately 6 months,7 which represents a considerable amount of time during an average 4-year enlistment contract. Therapeutic treatment strategies that (1) reduce the duration of oral isotretinoin therapy, (2) reduce the risk for relapse, and (3) increase medication compliance can reduce the operational impact of this acne treatment. Such treatment strategies are discussed below.
High-Dose Isotretinoin
An optimal isotretinoin dosing regimen would achieve swift resolution of acne lesions and reduce the overall relapse rate requiring retrial of isotretinoin, thereby minimizing the operational- and duty-limiting impacts of the medication. Cyrulnik et al13 studied treatment outcomes of high-dose isotretinoin for acne vulgaris using a mean dosage of 1.6 mg/kg daily with an average cumulative dosage of 290 mg/kg. They demonstrated 100% clearance of lesions over 6 months, with a 12.5% relapse rate at 3 years. Aside from an increased rate of elevated transaminases, incidence of adverse effects and laboratory abnormalities were not significantly increased compared to conventional dosing regimens.13 The goal cumulative dosing of 120 to 150 mg/kg can be achieved 1 to 2 months earlier using a dosage of 1.6 mg/kg daily vs a conventional dosage of 1 mg/kg daily.
It has been hypothesized that higher cumulative doses of oral isotretinoin reduce the risk for relapse of acne and retrial of oral isotretinoin.14 Blasiak et al15 studied relapse and retrial of oral isotretinoin in acne patients who received cumulative dosing higher or lower than 220 mg/kg. A clinically but not statistically significant reduced relapse rate was observed in the cohort that received cumulative dosing higher than 220 mg/kg. No statistically significant difference in rates of adverse advents was observed aside from an increase in retinoid dermatitis in the cohort that received cumulative dosing higher than 220 mg/kg. Higher but not statistically significant rates of adverse events were seen in the group that received dosing higher than 220 mg/kg.15 Cumulative doses of oral isotretinoin higher than the 120 to 150 mg/kg range may decrease the risk for acne relapse and the need for an additional course of oral isotretinoin, which would reduce a service member’s total time away from deployment and full duty.
Relapse requiring a retrial of oral isotretinoin not only increases the operational cost of acne treatment but also considerably increases the monetary cost to the health care system. In a cost-analysis model, cumulative doses of oral isotretinoin higher than 230 mg/kg have a decreased overall cost compared to traditional cumulative dosing of less than 150 mg/kg due to the cost of relapse.16
Limitations of high daily and cumulative dosing regimens of oral isotretinoin are chiefly the dose-dependent rate of adverse effects. Low-dose regimens are associated with a reduced risk of isotretinoin-related side effects.6,17 Acute acne flares may be seen following initial administration of oral isotretinoin and are aggravated by increases in dosage.18 Isotretinoin-induced acne fulminans is a rare but devastating complication observed with high initial doses of oral isotretinoin in patients with severe acne.19 The risks and benefits of high daily and cumulatively dosed isotretinoin must be carefully considered in patients with severe acne.
Teledermatology: A Force for Readiness
The COVID-19 pandemic drastically changed the dermatology practice landscape with recommendations to cancel all elective outpatient visits in favor of teledermatology encounters.20 This decreased access to care, which resulted in an increase in drug interruption for dermatology patients, including patients on oral isotretinoin.21 Teledermatology has been increasingly utilized to maintain continuity of care for the management of patients taking isotretinoin.5 Routine utilization of teledermatology evaluation in military practices could expedite care, decrease patient travel time, and allow for in-clinic visits to be utilized for higher-acuity concerns.22
The use of teledermatology for uncomplicated oral isotretinoin management has the potential to increase medication compliance and decrease the amount of travel time for active-duty service members; for example, consider a military dermatology practice based in San Diego, California, that accepts referrals from military bases 3 hours away by car. After an initial consultation for consideration and initiation of oral isotretinoin, teledermatology appointments can save the active-duty service member 3 hours of travel time for each follow-up visit per month. This ultimately increases operational productivity, reduces barriers to accessing care, and improves patient satisfaction.23
Although military personnel usually are located at duty stations for 2 to 4 years, training exercises and military vocational schools often temporarily take personnel away from their home station. These temporary-duty assignments have the potential to interrupt medical follow-up appointments and may cause delays in treatment for individuals who miss monthly isotretinoin visits. When deemed appropriate by the prescribing dermatologist, teledermatology allows for increased continuity of care for active-duty service members and maintenance of a therapeutic isotretinoin course despite temporary geographic displacement.
By facilitating regular follow-up appointments, teledermatology can minimize the amount of time an active-duty service member is on a course of oral isotretinoin, thereby reducing the operational and duty-limiting implications of the medication.
Final Thoughts
Acne is a common dermatologic concern within the active-duty military population. Oral isotretinoin is indicated for treatment-resistant moderate or severe acne; however, it limits the ability of service members to deploy and is disqualifying for special military assignments. High daily- and cumulative-dose isotretinoin treatment strategies can reduce the duration of therapy and may be associated with a decrease in acne relapse and the need for retrial. Teledermatology can increase access to care and facilitate the completion of oral isotretinoin courses in a timely manner. These treatment strategies may help mitigate the duty-limiting impact of oral isotretinoin therapy in military service members.
Acne vulgaris is an extremely common dermatologic disease affecting 40 to 50 million individuals in the United States each year, with a prevalence of 85% in adolescents and young adults aged 12 to 24 years. For some patients, the disease may persist well into adulthood, affecting 8% of adults aged 25 and 34 years.1 Acne negatively impacts patients’ quality of life and productivity, with an estimated direct and indirect cost of over $3 billion per year.2
Oral isotretinoin, a vitamin A derivative, is approved by the US Food and Drug Administration for the treatment of severe nodulocystic acne. Isotretinoin reduces the size and secretions of sebaceous glands, inhibits growth and resulting inflammation of Cutibacterium acnes, and normalizes the differentiation of follicular keratinocytes, resulting in permanent changes in the pathogenesis of acne that may lead to remission.3 The use of oral isotretinoin in the active-duty US Military population may cause service members to be nondeployable or limit their ability to function in special roles (eg, pilot, submariner).4 Treatment regimens that minimize the course duration of isotretinoin and reduce the risk for relapse that requires a retrial of isotretinoin may, in turn, increase a service member’s readiness, deployment availability, and ability to perform unique occupational roles.
Additionally, teledermatology has been increasingly utilized to maintain treatment continuity for patients on isotretinoin during the COVID-19 pandemic.5 Application of this technology in the military also may be used to facilitate timely isotretinoin treatment regimens in active-duty service members to minimize course duration and increase readiness.
In this article, we discuss an accelerated course of oral isotretinoin as a safe and effective option for military service members bound by duty restrictions and operational timelines and explore the role of teledermatology for the treatment of acne in military service members.
Isotretinoin for Acne
Isotretinoin typically is initiated at a dosage of 0.5 mg/kg daily, increasing to 1 mg/kg daily with a goal cumulative dose between 120 and 150 mg/kg. Relapse may occur after completing a treatment course and is associated with cumulative dosing less than 120 mg/kg.6 The average duration of acne treatment with oral isotretinoin is approximately 6 months.7 At therapeutic doses, nearly all patients experience side effects, most commonly dryness and desquamation of the skin and mucous membranes, as well as possible involvement of the lips, eyes, and nose. Notable extracutaneous side effects include headache, visual disturbances at night, idiopathic intracranial hypertension, and myalgia. Serum cholesterol, triglycerides, and transaminases may be increased in patients taking isotretinoin, which requires routine monitoring using serum lipid profiles and liver function studies. A potential association between isotretinoin and inflammatory bowel disease and changes in mood have been reported, but current data do not suggest an evidence-based link.6,8 Isotretinoin is a potent teratogen, and in the United States, all patients are required to enroll in iPLEDGE, a US Food and Drug Administration–approved pregnancy prevention program that monitors prescribing and dispensing of the medication. For patients who can become pregnant, iPLEDGE requires use of 2 forms of contraception as well as monthly pregnancy tests prior to dispensing the medication.
Acne in Military Service Members
Acne is exceedingly common in the active-duty military population. In 2018, more than 40% of soldiers, sailors, airmen, and marines were 25 years or younger, and 75% of all US service members were 35 years or younger, corresponding to acne peak incidences.1,9 Management of acne in this population requires unique treatment considerations due to distinctive occupational requirements of and hazards faced by military personnel. Use of personal protective equipment, including gas masks, safety restraints, parachute rigging, and flak jackets, may be limiting in individuals with moderate to severe acne.10 For example, severe nodulocystic acne on the chin and jawline can interfere with proper wear of the chin strap on a Kevlar helmet. The severity of acne often necessitates the use of oral isotretinoin therapy, which is considered disqualifying for many special military assignments, including submarine duty, nuclear field duty, and diving duty.11 In military aviation communities, oral isotretinoin requires grounding for the duration of therapy plus 3 months after cessation. Slit-lamp examination, triglycerides, and transaminase levels must be normal prior to returning to unrestricted duty.12 Furthermore, use of oral isotretinoin may limit overseas assignments or deployment eligibility.4
The high prevalence of acne and the operationally limiting consequences of isotretinoin therapy present a unique challenge for dermatologists treating military personnel. The average duration of isotretinoin treatment is approximately 6 months,7 which represents a considerable amount of time during an average 4-year enlistment contract. Therapeutic treatment strategies that (1) reduce the duration of oral isotretinoin therapy, (2) reduce the risk for relapse, and (3) increase medication compliance can reduce the operational impact of this acne treatment. Such treatment strategies are discussed below.
High-Dose Isotretinoin
An optimal isotretinoin dosing regimen would achieve swift resolution of acne lesions and reduce the overall relapse rate requiring retrial of isotretinoin, thereby minimizing the operational- and duty-limiting impacts of the medication. Cyrulnik et al13 studied treatment outcomes of high-dose isotretinoin for acne vulgaris using a mean dosage of 1.6 mg/kg daily with an average cumulative dosage of 290 mg/kg. They demonstrated 100% clearance of lesions over 6 months, with a 12.5% relapse rate at 3 years. Aside from an increased rate of elevated transaminases, incidence of adverse effects and laboratory abnormalities were not significantly increased compared to conventional dosing regimens.13 The goal cumulative dosing of 120 to 150 mg/kg can be achieved 1 to 2 months earlier using a dosage of 1.6 mg/kg daily vs a conventional dosage of 1 mg/kg daily.
It has been hypothesized that higher cumulative doses of oral isotretinoin reduce the risk for relapse of acne and retrial of oral isotretinoin.14 Blasiak et al15 studied relapse and retrial of oral isotretinoin in acne patients who received cumulative dosing higher or lower than 220 mg/kg. A clinically but not statistically significant reduced relapse rate was observed in the cohort that received cumulative dosing higher than 220 mg/kg. No statistically significant difference in rates of adverse advents was observed aside from an increase in retinoid dermatitis in the cohort that received cumulative dosing higher than 220 mg/kg. Higher but not statistically significant rates of adverse events were seen in the group that received dosing higher than 220 mg/kg.15 Cumulative doses of oral isotretinoin higher than the 120 to 150 mg/kg range may decrease the risk for acne relapse and the need for an additional course of oral isotretinoin, which would reduce a service member’s total time away from deployment and full duty.
Relapse requiring a retrial of oral isotretinoin not only increases the operational cost of acne treatment but also considerably increases the monetary cost to the health care system. In a cost-analysis model, cumulative doses of oral isotretinoin higher than 230 mg/kg have a decreased overall cost compared to traditional cumulative dosing of less than 150 mg/kg due to the cost of relapse.16
Limitations of high daily and cumulative dosing regimens of oral isotretinoin are chiefly the dose-dependent rate of adverse effects. Low-dose regimens are associated with a reduced risk of isotretinoin-related side effects.6,17 Acute acne flares may be seen following initial administration of oral isotretinoin and are aggravated by increases in dosage.18 Isotretinoin-induced acne fulminans is a rare but devastating complication observed with high initial doses of oral isotretinoin in patients with severe acne.19 The risks and benefits of high daily and cumulatively dosed isotretinoin must be carefully considered in patients with severe acne.
Teledermatology: A Force for Readiness
The COVID-19 pandemic drastically changed the dermatology practice landscape with recommendations to cancel all elective outpatient visits in favor of teledermatology encounters.20 This decreased access to care, which resulted in an increase in drug interruption for dermatology patients, including patients on oral isotretinoin.21 Teledermatology has been increasingly utilized to maintain continuity of care for the management of patients taking isotretinoin.5 Routine utilization of teledermatology evaluation in military practices could expedite care, decrease patient travel time, and allow for in-clinic visits to be utilized for higher-acuity concerns.22
The use of teledermatology for uncomplicated oral isotretinoin management has the potential to increase medication compliance and decrease the amount of travel time for active-duty service members; for example, consider a military dermatology practice based in San Diego, California, that accepts referrals from military bases 3 hours away by car. After an initial consultation for consideration and initiation of oral isotretinoin, teledermatology appointments can save the active-duty service member 3 hours of travel time for each follow-up visit per month. This ultimately increases operational productivity, reduces barriers to accessing care, and improves patient satisfaction.23
Although military personnel usually are located at duty stations for 2 to 4 years, training exercises and military vocational schools often temporarily take personnel away from their home station. These temporary-duty assignments have the potential to interrupt medical follow-up appointments and may cause delays in treatment for individuals who miss monthly isotretinoin visits. When deemed appropriate by the prescribing dermatologist, teledermatology allows for increased continuity of care for active-duty service members and maintenance of a therapeutic isotretinoin course despite temporary geographic displacement.
By facilitating regular follow-up appointments, teledermatology can minimize the amount of time an active-duty service member is on a course of oral isotretinoin, thereby reducing the operational and duty-limiting implications of the medication.
Final Thoughts
Acne is a common dermatologic concern within the active-duty military population. Oral isotretinoin is indicated for treatment-resistant moderate or severe acne; however, it limits the ability of service members to deploy and is disqualifying for special military assignments. High daily- and cumulative-dose isotretinoin treatment strategies can reduce the duration of therapy and may be associated with a decrease in acne relapse and the need for retrial. Teledermatology can increase access to care and facilitate the completion of oral isotretinoin courses in a timely manner. These treatment strategies may help mitigate the duty-limiting impact of oral isotretinoin therapy in military service members.
- White GM. Recent findings in the epidemiologic evidence, classification, and subtypes of acne vulgaris. J Am Acad Dermatol. 1998;39:S34-S37. doi:10.1016/s0190-9622(98)70442-6
- Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55:490-500. doi:10.1016/j.jaad.2006.05.048
- James WD. Clinical practice. acne. N Engl J Med. 2005;352:1463-1472. doi:10.1056/NEJMcp033487
- Burke KR, Larrymore DC, Cho SH. Treatment consideration for US military members with skin disease. Cutis. 2019;103:329-332.
- Rosamilia LL. Isotretinoin meets COVID-19: revisiting a fragmented paradigm. Cutis. 2021;108:8-12. doi:10.12788/cutis.0299
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e33. doi:10.1016/j.jaad.2015.12.037
- Huang KE, Carstensen SE, Feldman SR. The duration of acne treatment. J Drugs Dermatol. 2014;13:655-656.
- Bettoli V, Guerra-Tapia A, Herane MI, et al. Challenges and solutions in oral isotretinoin in acne: reflections on 35 years of experience. Clin Cosmet Investig Dermatol. 2019;12:943-951. doi:10.2147/CCID.S234231
- US Department of Defense. 2018 demographics report: profile of the military community. Accessed January 18, 2022. https://download.militaryonesource.mil/12038/MOS/Reports/2018-demographics-report.pdf
- Brahe C, Peters K. Fighting acne for the fighting forces. Cutis. 2020;106:18-20, 22. doi:10.12788/cutis.0057
- US Department of the Navy. Change 167. manual of the medical department. Published February 15, 2019. Accessed January 18, 2022. https://www.med.navy.mil/Portals/62/Documents/BUMED/Directives/MANMED/Chapter%2015%20Medical%20Examinations%20(incorporates%20Changes%20126_135-138_140_145_150-152_154-156_160_164-167).pdf?ver=Rj7AoH54dNAX5uS3F1JUfw%3d%3d
- US Department of the Navy. US Navy aeromedical reference and waiver guide. Published August 11, 2021. Accessed January 18, 2022. https://www.med.navy.mil/Portals/62/Documents/NMFSC/NMOTC/NAMI/ARWG/Waiver%20Guide/ARWG%20COMPLETE_210811.pdf?ver=_pLPzFrtl8E2swFESnN4rA%3d%3d
- Cyrulnik AA, Viola KV, Gewirtzman AJ, et al. High-dose isotretinoin in acne vulgaris: improved treatment outcomes and quality of life. Int J Dermatol. 2012;51:1123-1130. doi:10.1111/j.1365-4632.2011.05409.x
- Coloe J, Du H, Morrell DS. Could higher doses of isotretinoin reduce the frequency of treatment failure in patients with acne? J Am Acad Dermatol. 2011;65:422-423. doi:10.1016/j.jaad.2010.06.025
- Blasiak RC, Stamey CR, Burkhart CN, et al. High-dose isotretinoin treatment and the rate of retrial, relapse, and adverse effects in patients with acne vulgaris. JAMA Dermatol. 2013;149:1392-1398. doi:10.1001/jamadermatol.2013.6746
- Zeitany AE, Bowers EV, Morrell DS. High-dose isotretinoin has lower impact on wallets: a cost analysis of dosing approaches. J Am Acad Dermatol. 2016;74:174-176. doi:10.1016/j.jaad.2015.08.012
- Amichai B, Shemer A, Grunwald MH. Low-dose isotretinoin in the treatment of acne vulgaris. J Am Acad Dermatol. 2006;54:644-666. doi:10.1016/j.jaad.2005.11.1061
- Borghi A, Mantovani L, Minghetti S, et al. Acute acne flare following isotretinoin administration: potential protective role of low starting dose. Dermatology. 2009;218:178-180. doi:10.1159/000182270
- Greywal T, Zaenglein AL, Baldwin HE, et al. Evidence-based recommendations for the management of acne fulminans and its variants. J Am Acad Dermatol. 2017;77:109-117. doi:10.1016/j.jaad.2016.11.028
- Kwatra SG, Sweren RJ, Grossberg AL. Dermatology practices as vectors for COVID-19 transmission: a call for immediate cessation of nonemergent dermatology visits. J Am Acad Dermatol. 2020;82:E179-E180. doi:10.1016/j.jaad.2020.03.037
- Alshiyab DM, Al-Qarqaz FA, Muhaidat JM. Impact of COVID-19 pandemic on the continuity of care for dermatologic patients on systemic therapy during the period of strict lockdown. Ann Med Surg (Lond). 2020;60:571-574. doi:10.1016/j.amsu.2020.11.056
- Hwang J, Kakimoto C. Teledermatology in the US military: a historic foundation for current and future applications. Cutis. 2018;101:335,337,345.
- Ruggiero A, Megna M, Annunziata MC, et al. Teledermatology for acne during COVID-19: high patients’ satisfaction in spite of the emergency. J Eur Acad Dermatol Venereol. 2020;34:E662-E663. doi:10.1111/jdv.16746
- White GM. Recent findings in the epidemiologic evidence, classification, and subtypes of acne vulgaris. J Am Acad Dermatol. 1998;39:S34-S37. doi:10.1016/s0190-9622(98)70442-6
- Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55:490-500. doi:10.1016/j.jaad.2006.05.048
- James WD. Clinical practice. acne. N Engl J Med. 2005;352:1463-1472. doi:10.1056/NEJMcp033487
- Burke KR, Larrymore DC, Cho SH. Treatment consideration for US military members with skin disease. Cutis. 2019;103:329-332.
- Rosamilia LL. Isotretinoin meets COVID-19: revisiting a fragmented paradigm. Cutis. 2021;108:8-12. doi:10.12788/cutis.0299
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e33. doi:10.1016/j.jaad.2015.12.037
- Huang KE, Carstensen SE, Feldman SR. The duration of acne treatment. J Drugs Dermatol. 2014;13:655-656.
- Bettoli V, Guerra-Tapia A, Herane MI, et al. Challenges and solutions in oral isotretinoin in acne: reflections on 35 years of experience. Clin Cosmet Investig Dermatol. 2019;12:943-951. doi:10.2147/CCID.S234231
- US Department of Defense. 2018 demographics report: profile of the military community. Accessed January 18, 2022. https://download.militaryonesource.mil/12038/MOS/Reports/2018-demographics-report.pdf
- Brahe C, Peters K. Fighting acne for the fighting forces. Cutis. 2020;106:18-20, 22. doi:10.12788/cutis.0057
- US Department of the Navy. Change 167. manual of the medical department. Published February 15, 2019. Accessed January 18, 2022. https://www.med.navy.mil/Portals/62/Documents/BUMED/Directives/MANMED/Chapter%2015%20Medical%20Examinations%20(incorporates%20Changes%20126_135-138_140_145_150-152_154-156_160_164-167).pdf?ver=Rj7AoH54dNAX5uS3F1JUfw%3d%3d
- US Department of the Navy. US Navy aeromedical reference and waiver guide. Published August 11, 2021. Accessed January 18, 2022. https://www.med.navy.mil/Portals/62/Documents/NMFSC/NMOTC/NAMI/ARWG/Waiver%20Guide/ARWG%20COMPLETE_210811.pdf?ver=_pLPzFrtl8E2swFESnN4rA%3d%3d
- Cyrulnik AA, Viola KV, Gewirtzman AJ, et al. High-dose isotretinoin in acne vulgaris: improved treatment outcomes and quality of life. Int J Dermatol. 2012;51:1123-1130. doi:10.1111/j.1365-4632.2011.05409.x
- Coloe J, Du H, Morrell DS. Could higher doses of isotretinoin reduce the frequency of treatment failure in patients with acne? J Am Acad Dermatol. 2011;65:422-423. doi:10.1016/j.jaad.2010.06.025
- Blasiak RC, Stamey CR, Burkhart CN, et al. High-dose isotretinoin treatment and the rate of retrial, relapse, and adverse effects in patients with acne vulgaris. JAMA Dermatol. 2013;149:1392-1398. doi:10.1001/jamadermatol.2013.6746
- Zeitany AE, Bowers EV, Morrell DS. High-dose isotretinoin has lower impact on wallets: a cost analysis of dosing approaches. J Am Acad Dermatol. 2016;74:174-176. doi:10.1016/j.jaad.2015.08.012
- Amichai B, Shemer A, Grunwald MH. Low-dose isotretinoin in the treatment of acne vulgaris. J Am Acad Dermatol. 2006;54:644-666. doi:10.1016/j.jaad.2005.11.1061
- Borghi A, Mantovani L, Minghetti S, et al. Acute acne flare following isotretinoin administration: potential protective role of low starting dose. Dermatology. 2009;218:178-180. doi:10.1159/000182270
- Greywal T, Zaenglein AL, Baldwin HE, et al. Evidence-based recommendations for the management of acne fulminans and its variants. J Am Acad Dermatol. 2017;77:109-117. doi:10.1016/j.jaad.2016.11.028
- Kwatra SG, Sweren RJ, Grossberg AL. Dermatology practices as vectors for COVID-19 transmission: a call for immediate cessation of nonemergent dermatology visits. J Am Acad Dermatol. 2020;82:E179-E180. doi:10.1016/j.jaad.2020.03.037
- Alshiyab DM, Al-Qarqaz FA, Muhaidat JM. Impact of COVID-19 pandemic on the continuity of care for dermatologic patients on systemic therapy during the period of strict lockdown. Ann Med Surg (Lond). 2020;60:571-574. doi:10.1016/j.amsu.2020.11.056
- Hwang J, Kakimoto C. Teledermatology in the US military: a historic foundation for current and future applications. Cutis. 2018;101:335,337,345.
- Ruggiero A, Megna M, Annunziata MC, et al. Teledermatology for acne during COVID-19: high patients’ satisfaction in spite of the emergency. J Eur Acad Dermatol Venereol. 2020;34:E662-E663. doi:10.1111/jdv.16746
Practice Points
- Acne is a common skin disease with a high prevalence in the active-duty US Military population.
- Oral isotretinoin is a commonly utilized acne medication that can limit the ability for military service members to deploy and is considered disqualifying for some special duty assignments.
- High daily- and cumulative-dose oral isotretinoin therapy as well as teledermatology can minimize the duty-limiting impact of isotretinoin therapy for military service members.
Disseminated Erythematous-Violet Edematous Plaques and Necrotic Nodules
The Diagnosis: Histiocytoid Sweet Syndrome
The patient was admitted for clinical study and treatment monitoring. During the first 72 hours of admittance, the lesions and general malaise further developed along with C-reactive protein elevation (126 mg/L). Administration of intravenous prednisone at a dosage of 1 mg/kg daily was accompanied by substantial improvement after 1 week of treatment, with subsequent follow-up and outpatient monitoring. An underlying neoplasia was ruled out after review of medical history, physical examination, complete blood cell count, chest radiography, abdominal ultrasonography, colonoscopy, and bone marrow aspiration.

A 4-mm skin biopsy was performed from a lesion on the neck (Figure 1). Histology revealed a dermis with prominent edema alongside superficial, deep, and periadnexal perivascular inflammatory infiltrates, as well as predominant lymphocytes and cells with a histiocytoid profile (Figure 2). These findings were accompanied by isolated neutrophil foci. The absence of leukocytoclastic vasculitis was noted. Immunohistochemistry demonstrated that the histiocyte population was positive for myeloperoxidase and CD68, which categorized them as immature cells of myeloid origin (Figure 3). Clinical and histopathologic findings led to a definitive diagnosis of histiocytoid Sweet syndrome (SS). Sweet syndrome consists of a neutrophilic dermatosis profile. Clinically, it manifests as a sudden onset of painful nodules and plaques accompanied by fever, malaise, and leukocytosis.
Histiocytoid SS is a rare histologic variant of SS initially described by Requena et al1 in 2005. In histiocytoid SS, the main inflammatory infiltrates are promyelocytes and myelocytes.2 Immunohistochemistry shows positivity for myeloperoxidase, CD15, CD43, CD45, CD68, MAC-386, and HAM56.1 The diagnosis is determined by exclusion after adequate clinical and histopathologic correlation, which also should exclude other diagnoses such as leukemia cutis and interstitial granulomatous dermatitis.3 Histiocytoid SS may be related to an increased risk for underlying malignancy. Haber et al4 performed a systematic review in which they concluded that approximately 40% of patients newly diagnosed with histiocytoid SS subsequently were diagnosed or already were diagnosed with a hematologic or solid cancer vs 21% in the classical neutrophilic infiltrate of SS (NSS). Histiocytoid SS more commonly was associated with myelodysplastic syndrome (46% vs 2.5% in NSS) and hematologic malignancies (42.5% vs 25% in SS).
The initial differential diagnoses include inflammatory dermatoses, infections, neoplasms, and systemic diseases. In exudative erythema multiforme, early lesions are composed of typical target lesions with mucosal involvement in 25% to 60% of patients.5 Erythema elevatum diutinum is a chronic dermatosis characterized by asymptomatic papules and red-violet nodules. The most characteristic histologic finding is leukocytoclastic vasculitis.6 The absence of vasculitis is part of the major diagnostic criteria for SS.7 Wells syndrome is associated with general malaise, and edematous and erythematous-violet plaques or nodules appear on the limbs; however, it frequently is associated with eosinophilia in peripheral blood, and histology shows that the main cell population of the inflammatory infiltrate also is eosinophilic.8 Painful, superficial, and erosive blisters appear preferentially on the face and backs of the arms in bullous pyoderma gangrenosum. It usually is not associated with the typical systemic manifestations of SS (ie, fever, arthralgia, damage to target organs). On histopathology, the neutrophilic infiltrate is accompanied by subepidermal vesicles.9
Histiocytoid SS responds dramatically to corticosteroids. Other first-line treatments that avoid use of corticosteroids are colchicine, dapsone, and potassium iodide. Multiple treatments were attempted in our patient, including corticosteroids, methotrexate, dapsone, colchicine, and anakinra. Despite patients responding well to treatment, a possible underlying neoplasm, most frequently of hematologic origin, must be excluded.10
- Requena L, Kutzner H, Palmedo G, et al. Histiocytoid Sweet syndrome: a dermal infiltration of immature neutrophilic granulocytes. Arch Dermatol. 2005;141:834-842. doi:10.1001/archderm.141.7.834
- Alegría-Landa V, Rodríguez-Pinilla SM, Santos-Briz A, et al. Clinicopathologic, immunohistochemical, and molecular features of histiocytoid Sweet syndrome. JAMA Dermatol. 2017;153:651-659. doi:10.1001/jamadermatol.2016.6092
- Llamas-Velasco M, Concha-Garzón MJ, Fraga J, et al. Histiocytoid Sweet syndrome related to bortezomib: a mimicker of cutaneous infiltration by myeloma. Indian J Dermatol Venereol Leprol. 2015; 81:305-306. doi:10.4103/0378-6323.152743
- Haber R, Feghali J, El Gemayel M. Risk of malignancy in histiocytoid Sweet syndrome: a systematic review and reappraisal [published online February 21, 2020]. J Am Acad Dermatol. 2020;83:661-663. doi:10.1016/j.jaad.2020.02.048
- Sokumbi O, Wetter DA. Clinical features, diagnosis, and treatment of erythema multiforme: a review for the practicing dermatologist. Int J Dermatol. 2012;51:889-902. doi:10.1111/j.1365-4632.2011.05348.x
- Newburger J, Schmieder GJ. Erythema elevatum diutinum. StatPearls. StatPearls Publishing; 2021. http://www.ncbi.nlm.nih.gov /books/NBK448069/
- Su WP, Liu HN. Diagnostic criteria for Sweet’s syndrome. Cutis. 1986;37:167-174.
- Weins AB, Biedermann T, Weiss T, et al. Wells syndrome. J Dtsch Dermatol Ges. 2016;14:989-993. doi:10.1111/ddg.13132
- Powell FC, Su WP, Perry HO. Pyoderma gangrenosum: classification and management. J Am Acad Dermatol. 1996;34:395-409; quiz 410-412. doi:10.1016/s0190-9622(96)90428-4
- Villarreal-Villarreal CD, Ocampo-Candiani J, Villarreal-Martínez A. Sweet syndrome: a review and update. Actas Dermosifiliogr. 2016;107:369-378. doi:10.1016/j.ad.2015.12.001
The Diagnosis: Histiocytoid Sweet Syndrome
The patient was admitted for clinical study and treatment monitoring. During the first 72 hours of admittance, the lesions and general malaise further developed along with C-reactive protein elevation (126 mg/L). Administration of intravenous prednisone at a dosage of 1 mg/kg daily was accompanied by substantial improvement after 1 week of treatment, with subsequent follow-up and outpatient monitoring. An underlying neoplasia was ruled out after review of medical history, physical examination, complete blood cell count, chest radiography, abdominal ultrasonography, colonoscopy, and bone marrow aspiration.

A 4-mm skin biopsy was performed from a lesion on the neck (Figure 1). Histology revealed a dermis with prominent edema alongside superficial, deep, and periadnexal perivascular inflammatory infiltrates, as well as predominant lymphocytes and cells with a histiocytoid profile (Figure 2). These findings were accompanied by isolated neutrophil foci. The absence of leukocytoclastic vasculitis was noted. Immunohistochemistry demonstrated that the histiocyte population was positive for myeloperoxidase and CD68, which categorized them as immature cells of myeloid origin (Figure 3). Clinical and histopathologic findings led to a definitive diagnosis of histiocytoid Sweet syndrome (SS). Sweet syndrome consists of a neutrophilic dermatosis profile. Clinically, it manifests as a sudden onset of painful nodules and plaques accompanied by fever, malaise, and leukocytosis.

Histiocytoid SS is a rare histologic variant of SS initially described by Requena et al1 in 2005. In histiocytoid SS, the main inflammatory infiltrates are promyelocytes and myelocytes.2 Immunohistochemistry shows positivity for myeloperoxidase, CD15, CD43, CD45, CD68, MAC-386, and HAM56.1 The diagnosis is determined by exclusion after adequate clinical and histopathologic correlation, which also should exclude other diagnoses such as leukemia cutis and interstitial granulomatous dermatitis.3 Histiocytoid SS may be related to an increased risk for underlying malignancy. Haber et al4 performed a systematic review in which they concluded that approximately 40% of patients newly diagnosed with histiocytoid SS subsequently were diagnosed or already were diagnosed with a hematologic or solid cancer vs 21% in the classical neutrophilic infiltrate of SS (NSS). Histiocytoid SS more commonly was associated with myelodysplastic syndrome (46% vs 2.5% in NSS) and hematologic malignancies (42.5% vs 25% in SS).

The initial differential diagnoses include inflammatory dermatoses, infections, neoplasms, and systemic diseases. In exudative erythema multiforme, early lesions are composed of typical target lesions with mucosal involvement in 25% to 60% of patients.5 Erythema elevatum diutinum is a chronic dermatosis characterized by asymptomatic papules and red-violet nodules. The most characteristic histologic finding is leukocytoclastic vasculitis.6 The absence of vasculitis is part of the major diagnostic criteria for SS.7 Wells syndrome is associated with general malaise, and edematous and erythematous-violet plaques or nodules appear on the limbs; however, it frequently is associated with eosinophilia in peripheral blood, and histology shows that the main cell population of the inflammatory infiltrate also is eosinophilic.8 Painful, superficial, and erosive blisters appear preferentially on the face and backs of the arms in bullous pyoderma gangrenosum. It usually is not associated with the typical systemic manifestations of SS (ie, fever, arthralgia, damage to target organs). On histopathology, the neutrophilic infiltrate is accompanied by subepidermal vesicles.9
Histiocytoid SS responds dramatically to corticosteroids. Other first-line treatments that avoid use of corticosteroids are colchicine, dapsone, and potassium iodide. Multiple treatments were attempted in our patient, including corticosteroids, methotrexate, dapsone, colchicine, and anakinra. Despite patients responding well to treatment, a possible underlying neoplasm, most frequently of hematologic origin, must be excluded.10
The Diagnosis: Histiocytoid Sweet Syndrome
The patient was admitted for clinical study and treatment monitoring. During the first 72 hours of admittance, the lesions and general malaise further developed along with C-reactive protein elevation (126 mg/L). Administration of intravenous prednisone at a dosage of 1 mg/kg daily was accompanied by substantial improvement after 1 week of treatment, with subsequent follow-up and outpatient monitoring. An underlying neoplasia was ruled out after review of medical history, physical examination, complete blood cell count, chest radiography, abdominal ultrasonography, colonoscopy, and bone marrow aspiration.

A 4-mm skin biopsy was performed from a lesion on the neck (Figure 1). Histology revealed a dermis with prominent edema alongside superficial, deep, and periadnexal perivascular inflammatory infiltrates, as well as predominant lymphocytes and cells with a histiocytoid profile (Figure 2). These findings were accompanied by isolated neutrophil foci. The absence of leukocytoclastic vasculitis was noted. Immunohistochemistry demonstrated that the histiocyte population was positive for myeloperoxidase and CD68, which categorized them as immature cells of myeloid origin (Figure 3). Clinical and histopathologic findings led to a definitive diagnosis of histiocytoid Sweet syndrome (SS). Sweet syndrome consists of a neutrophilic dermatosis profile. Clinically, it manifests as a sudden onset of painful nodules and plaques accompanied by fever, malaise, and leukocytosis.

Histiocytoid SS is a rare histologic variant of SS initially described by Requena et al1 in 2005. In histiocytoid SS, the main inflammatory infiltrates are promyelocytes and myelocytes.2 Immunohistochemistry shows positivity for myeloperoxidase, CD15, CD43, CD45, CD68, MAC-386, and HAM56.1 The diagnosis is determined by exclusion after adequate clinical and histopathologic correlation, which also should exclude other diagnoses such as leukemia cutis and interstitial granulomatous dermatitis.3 Histiocytoid SS may be related to an increased risk for underlying malignancy. Haber et al4 performed a systematic review in which they concluded that approximately 40% of patients newly diagnosed with histiocytoid SS subsequently were diagnosed or already were diagnosed with a hematologic or solid cancer vs 21% in the classical neutrophilic infiltrate of SS (NSS). Histiocytoid SS more commonly was associated with myelodysplastic syndrome (46% vs 2.5% in NSS) and hematologic malignancies (42.5% vs 25% in SS).

The initial differential diagnoses include inflammatory dermatoses, infections, neoplasms, and systemic diseases. In exudative erythema multiforme, early lesions are composed of typical target lesions with mucosal involvement in 25% to 60% of patients.5 Erythema elevatum diutinum is a chronic dermatosis characterized by asymptomatic papules and red-violet nodules. The most characteristic histologic finding is leukocytoclastic vasculitis.6 The absence of vasculitis is part of the major diagnostic criteria for SS.7 Wells syndrome is associated with general malaise, and edematous and erythematous-violet plaques or nodules appear on the limbs; however, it frequently is associated with eosinophilia in peripheral blood, and histology shows that the main cell population of the inflammatory infiltrate also is eosinophilic.8 Painful, superficial, and erosive blisters appear preferentially on the face and backs of the arms in bullous pyoderma gangrenosum. It usually is not associated with the typical systemic manifestations of SS (ie, fever, arthralgia, damage to target organs). On histopathology, the neutrophilic infiltrate is accompanied by subepidermal vesicles.9
Histiocytoid SS responds dramatically to corticosteroids. Other first-line treatments that avoid use of corticosteroids are colchicine, dapsone, and potassium iodide. Multiple treatments were attempted in our patient, including corticosteroids, methotrexate, dapsone, colchicine, and anakinra. Despite patients responding well to treatment, a possible underlying neoplasm, most frequently of hematologic origin, must be excluded.10
- Requena L, Kutzner H, Palmedo G, et al. Histiocytoid Sweet syndrome: a dermal infiltration of immature neutrophilic granulocytes. Arch Dermatol. 2005;141:834-842. doi:10.1001/archderm.141.7.834
- Alegría-Landa V, Rodríguez-Pinilla SM, Santos-Briz A, et al. Clinicopathologic, immunohistochemical, and molecular features of histiocytoid Sweet syndrome. JAMA Dermatol. 2017;153:651-659. doi:10.1001/jamadermatol.2016.6092
- Llamas-Velasco M, Concha-Garzón MJ, Fraga J, et al. Histiocytoid Sweet syndrome related to bortezomib: a mimicker of cutaneous infiltration by myeloma. Indian J Dermatol Venereol Leprol. 2015; 81:305-306. doi:10.4103/0378-6323.152743
- Haber R, Feghali J, El Gemayel M. Risk of malignancy in histiocytoid Sweet syndrome: a systematic review and reappraisal [published online February 21, 2020]. J Am Acad Dermatol. 2020;83:661-663. doi:10.1016/j.jaad.2020.02.048
- Sokumbi O, Wetter DA. Clinical features, diagnosis, and treatment of erythema multiforme: a review for the practicing dermatologist. Int J Dermatol. 2012;51:889-902. doi:10.1111/j.1365-4632.2011.05348.x
- Newburger J, Schmieder GJ. Erythema elevatum diutinum. StatPearls. StatPearls Publishing; 2021. http://www.ncbi.nlm.nih.gov /books/NBK448069/
- Su WP, Liu HN. Diagnostic criteria for Sweet’s syndrome. Cutis. 1986;37:167-174.
- Weins AB, Biedermann T, Weiss T, et al. Wells syndrome. J Dtsch Dermatol Ges. 2016;14:989-993. doi:10.1111/ddg.13132
- Powell FC, Su WP, Perry HO. Pyoderma gangrenosum: classification and management. J Am Acad Dermatol. 1996;34:395-409; quiz 410-412. doi:10.1016/s0190-9622(96)90428-4
- Villarreal-Villarreal CD, Ocampo-Candiani J, Villarreal-Martínez A. Sweet syndrome: a review and update. Actas Dermosifiliogr. 2016;107:369-378. doi:10.1016/j.ad.2015.12.001
- Requena L, Kutzner H, Palmedo G, et al. Histiocytoid Sweet syndrome: a dermal infiltration of immature neutrophilic granulocytes. Arch Dermatol. 2005;141:834-842. doi:10.1001/archderm.141.7.834
- Alegría-Landa V, Rodríguez-Pinilla SM, Santos-Briz A, et al. Clinicopathologic, immunohistochemical, and molecular features of histiocytoid Sweet syndrome. JAMA Dermatol. 2017;153:651-659. doi:10.1001/jamadermatol.2016.6092
- Llamas-Velasco M, Concha-Garzón MJ, Fraga J, et al. Histiocytoid Sweet syndrome related to bortezomib: a mimicker of cutaneous infiltration by myeloma. Indian J Dermatol Venereol Leprol. 2015; 81:305-306. doi:10.4103/0378-6323.152743
- Haber R, Feghali J, El Gemayel M. Risk of malignancy in histiocytoid Sweet syndrome: a systematic review and reappraisal [published online February 21, 2020]. J Am Acad Dermatol. 2020;83:661-663. doi:10.1016/j.jaad.2020.02.048
- Sokumbi O, Wetter DA. Clinical features, diagnosis, and treatment of erythema multiforme: a review for the practicing dermatologist. Int J Dermatol. 2012;51:889-902. doi:10.1111/j.1365-4632.2011.05348.x
- Newburger J, Schmieder GJ. Erythema elevatum diutinum. StatPearls. StatPearls Publishing; 2021. http://www.ncbi.nlm.nih.gov /books/NBK448069/
- Su WP, Liu HN. Diagnostic criteria for Sweet’s syndrome. Cutis. 1986;37:167-174.
- Weins AB, Biedermann T, Weiss T, et al. Wells syndrome. J Dtsch Dermatol Ges. 2016;14:989-993. doi:10.1111/ddg.13132
- Powell FC, Su WP, Perry HO. Pyoderma gangrenosum: classification and management. J Am Acad Dermatol. 1996;34:395-409; quiz 410-412. doi:10.1016/s0190-9622(96)90428-4
- Villarreal-Villarreal CD, Ocampo-Candiani J, Villarreal-Martínez A. Sweet syndrome: a review and update. Actas Dermosifiliogr. 2016;107:369-378. doi:10.1016/j.ad.2015.12.001
A 53-year-old man presented to the emergency department with a fever and painful skin lesions of 2 days’ duration. He reported a medical history of an upper respiratory infection 4 weeks prior. Physical examination was notable for erythematous-violet edematous papules, necrotic lesions, and pseudovesicles located on the face (top), head, neck, arms, and legs (bottom). Hemorrhagic splinters were evidenced in multiple nail sections. Urgent blood work revealed microcytic anemia (hemoglobin, 12.6 g/dL [reference range, 14.0–17.5 g/dL]) and elevated C-reactive protein (58 mg/L [reference range, 0.0–5.0 mg/L]).
Community Care Program Lacks Essential Data for Health Care Decisions
In 2014, amidst stories of delays at Veterans Health Administration facilities, Congress established the Veterans Choice Program, which expanded access to private sector health care practitioners. When the program expired in 2018, lawmakers replaced it with the Veterans Community Care Program (VCCP) as part of the US Department of Veterans Affairs (VA) Maintaining Internal Systems and Strengthening Integrated Outside Networks Act (38 USC § 1703 MISSION Act). Since then, the VCCP has grown exponentially; 34% of current veteran health care visits are with private clinicians.
Along with broader private sector access, the MISSION Act also mandated the creation of quality-of-care standards for both VA and VCCP, and stipulated that data be compiled and made available to “provide covered veterans relevant comparative information to make informed decisions regarding their health care.” Two-and-a-half years later, data about the quality of VCCP care remains largely unknown.
Access to Care Website
In the lead up to the MISSION Act, the VA launched its Access to Care website, an online tool that publishes institutional performance data on key metrics so that veterans can make “more informed choices about where, when, and how they receive their health care.” Following the bill’s passage, the VA added a MISSION Act Quality Standards section, which includes results of 27 conventional quality measures for every VA facility. These scores are posted alongside data of regional facilities.
This trailblazing tool is exceedingly comprehensive. Yet, multiple website gaps compromise its utility for veterans deliberating whether to obtain VCCP care, including:
- Data isn’t about VCCP care. The hospitals are selected because they are local, not whether they participate in VCCP. Further, it appears that aggregate scores include non-VCCP facilities.
- Missing conditions/treatments. While the website contains quality scores for an ample range of procedures, it lacks information for many conditions that disproportionately affect veterans. A veteran with posttraumatic stress disorder (PTSD) or traumatic brain injury (TBI), for example, has no data to check.
- Skewed comparison population. Private sector practitioners primarily treat nonveteran patients, a population that is, on average, healthier and of higher socioeconomic status when compared with VA patients. Outcomes differ, for example, when patients have coexisting mental illness or homelessness. For VCCP scores to be beneficial for comparisons, they should derive from treated veterans or be accurately risk-adjusted.
- Tangential measures. The Institute of Medicine defined health care quality as “improvement of outcomes.” Patients considering health care options benefit from information about treatment effectiveness and symptom reduction. But because obtaining that quality data is labor intensive, proxy measures are substituted. For example, the measure advising smokers to quit is the closest the website comes to reporting on the quality of mental health care.
High-Performers
The VA initiated a second means to inform veterans about the quality of furnished care. Specifically, they guided third-party administrators (TPAs)—TriWest Healthcare Alliance and Optum—in creating algorithms designating that VCCP individual clinicians, practice groups, and hospitals can be deemed high performing providers (HPPs). The algorithms are calculated using a mix of Healthcare Effectiveness Data and Information Set (HEDIS), Physician Quality Reporting System (PQRS), and Blue Health Intelligence (BHI) primary and specialty care measures. The designations are intended to be accessible to local VA community care schedulers to connect veterans with HPPs.
Many aspects of the HPP system are not yet public, including the measures that comprise the algorithms and when the designations will become operational. From what is publicly discoverable about HPP designations, there are crucial gaps like those on the Access to Care website. Behavioral and mental health conditions, for instance, are intentionally excluded in HPP monitoring. HPP algorithms draw from care provided to the general population; an HPP’s patient panel may contain no veterans (with their common comorbidities) at all. Most limiting, there’s no expectation that VCCP clinicians be high performing. Of the 1.2 million program clinicians treating veterans as of November 2020, only a nominal 13.4% were HPP.
After studying the HPP system, VA Partnered Evidence-based Policy Resource Center acknowledged that “it remains unclear whether the quality metrics and referral system result in higher quality of care for VA patients or whether the program improves veteran health.”
Quality of VCCP Mental Health Treatment
The MISSION Act mandated the VA to “establish standards and requirements for the provision of care by non-VA health care practitioners in clinical areas for which the Department of Veterans Affairs has special expertise, including PTSD, military sexual trauma-related conditions (MST), and TBI.” This requirement arose from a recognition that mental health care provided in the private sector pales in comparison to the VA’s rigorous evidence-based training, consultation, case review and care delivery. For example, over 8500 VA clinicians have received training in evidence-based cognitive processing therapy and/or prolonged exposure therapy for PTSD.
The MISSION Act also mandated that VCCP providers must “fulfill training requirements established by the Secretary on how to deliver evidence-based treatments in the clinical areas for which the Department of Veterans Affairs has special expertise” before furnishing care pursuant to a contract with the VA. However, the VA elected to disregard the directive, and left it up to VCCP clinician’s discretion whether to obtain training or proficiency.
Two bills introduced in Congress in 2021 aim to uphold these vital mandates for the VCCP program. The Veterans’ Culturally Competent Care Act requires VCCP mental health practitioners to take courses on the evaluation and management of suicide, PTSD, TBI, and MST. The Lethal Means Safety Training Act aligns VCCP clinicians suicide prevention training with existing VA standards.
Recommendations to Assure the Quality of VCCP Care
With review and revision of VCCP quality standards now underway, the following remedial actions are recommended:
- VCCP metrics must be compiled using data on veterans’ care, not the general population, and be published on the Access to Care website. This indispensable information is published on the website for VA care but not for VCCP. Unless VCCP is required to track their veterans, apples-to-apples comparisons of quality of care will remain difficult to attain. Supplemental research that directly contrasts quality of VA to VCCP care should be posted. For example, a 2021 study of enrolled veterans brought by ambulance to VA or community emergency rooms found that all 170 VA medical centers had lower comparative death rates.
- VCCP providers should be held to the same quality standards as those applied to VA clinicians. In a 2020 critical issue update on implementation of the MISSION Act, major veterans service organizations (VSOs) recommended that competency, training, and quality standards for non-VA community clinicians must be equivalent to benchmarks expected of VA clinicians. That includes credentials, initial and follow-up training, diagnostic screening, care-delivery, and documentation standards. Enacting the Veterans’ Culturally Competent Care Act and the Lethal Means Safety Training Act would begin to meet the MISSION Act’s clear statutory language.
- The VA and VCCP should add quality information about major diagnostic categories. This will allow veterans to make informed decisions about their personal condition. For most health diagnoses, there is no searchable listing by disorder.
- Quality assessments should be realigned to focus on outcome measures. For prospective patients, outcome results provide the most meaningful basis for comparing and selecting clinicians. Proxy measures may have little bearing on whether veterans receive effective care. (As Albert Einstein’s famously observed, “Not everything that can be counted counts.”). Also, the specific measures used for a clinician’s HPP designation should be delineated.
- The VA must enforce the MISSION Act’s instruction to renew or cancel contracts based on demonstrated quality of care. As VSOs emphasized, “if the private sector is unwilling or unable to match the VA’s access and quality standards, the VA must consider whether it needs to find new community partners.”
Seventeen billion dollars is spent yearly on purchased health care whose quality remains indeterminate. Ironclad commitments are needed from Congress and the VA to ensure that the effectiveness of, and standards for, veterans care options in the private sector match that in the VA.
In 2014, amidst stories of delays at Veterans Health Administration facilities, Congress established the Veterans Choice Program, which expanded access to private sector health care practitioners. When the program expired in 2018, lawmakers replaced it with the Veterans Community Care Program (VCCP) as part of the US Department of Veterans Affairs (VA) Maintaining Internal Systems and Strengthening Integrated Outside Networks Act (38 USC § 1703 MISSION Act). Since then, the VCCP has grown exponentially; 34% of current veteran health care visits are with private clinicians.
Along with broader private sector access, the MISSION Act also mandated the creation of quality-of-care standards for both VA and VCCP, and stipulated that data be compiled and made available to “provide covered veterans relevant comparative information to make informed decisions regarding their health care.” Two-and-a-half years later, data about the quality of VCCP care remains largely unknown.
Access to Care Website
In the lead up to the MISSION Act, the VA launched its Access to Care website, an online tool that publishes institutional performance data on key metrics so that veterans can make “more informed choices about where, when, and how they receive their health care.” Following the bill’s passage, the VA added a MISSION Act Quality Standards section, which includes results of 27 conventional quality measures for every VA facility. These scores are posted alongside data of regional facilities.
This trailblazing tool is exceedingly comprehensive. Yet, multiple website gaps compromise its utility for veterans deliberating whether to obtain VCCP care, including:
- Data isn’t about VCCP care. The hospitals are selected because they are local, not whether they participate in VCCP. Further, it appears that aggregate scores include non-VCCP facilities.
- Missing conditions/treatments. While the website contains quality scores for an ample range of procedures, it lacks information for many conditions that disproportionately affect veterans. A veteran with posttraumatic stress disorder (PTSD) or traumatic brain injury (TBI), for example, has no data to check.
- Skewed comparison population. Private sector practitioners primarily treat nonveteran patients, a population that is, on average, healthier and of higher socioeconomic status when compared with VA patients. Outcomes differ, for example, when patients have coexisting mental illness or homelessness. For VCCP scores to be beneficial for comparisons, they should derive from treated veterans or be accurately risk-adjusted.
- Tangential measures. The Institute of Medicine defined health care quality as “improvement of outcomes.” Patients considering health care options benefit from information about treatment effectiveness and symptom reduction. But because obtaining that quality data is labor intensive, proxy measures are substituted. For example, the measure advising smokers to quit is the closest the website comes to reporting on the quality of mental health care.
High-Performers
The VA initiated a second means to inform veterans about the quality of furnished care. Specifically, they guided third-party administrators (TPAs)—TriWest Healthcare Alliance and Optum—in creating algorithms designating that VCCP individual clinicians, practice groups, and hospitals can be deemed high performing providers (HPPs). The algorithms are calculated using a mix of Healthcare Effectiveness Data and Information Set (HEDIS), Physician Quality Reporting System (PQRS), and Blue Health Intelligence (BHI) primary and specialty care measures. The designations are intended to be accessible to local VA community care schedulers to connect veterans with HPPs.
Many aspects of the HPP system are not yet public, including the measures that comprise the algorithms and when the designations will become operational. From what is publicly discoverable about HPP designations, there are crucial gaps like those on the Access to Care website. Behavioral and mental health conditions, for instance, are intentionally excluded in HPP monitoring. HPP algorithms draw from care provided to the general population; an HPP’s patient panel may contain no veterans (with their common comorbidities) at all. Most limiting, there’s no expectation that VCCP clinicians be high performing. Of the 1.2 million program clinicians treating veterans as of November 2020, only a nominal 13.4% were HPP.
After studying the HPP system, VA Partnered Evidence-based Policy Resource Center acknowledged that “it remains unclear whether the quality metrics and referral system result in higher quality of care for VA patients or whether the program improves veteran health.”
Quality of VCCP Mental Health Treatment
The MISSION Act mandated the VA to “establish standards and requirements for the provision of care by non-VA health care practitioners in clinical areas for which the Department of Veterans Affairs has special expertise, including PTSD, military sexual trauma-related conditions (MST), and TBI.” This requirement arose from a recognition that mental health care provided in the private sector pales in comparison to the VA’s rigorous evidence-based training, consultation, case review and care delivery. For example, over 8500 VA clinicians have received training in evidence-based cognitive processing therapy and/or prolonged exposure therapy for PTSD.
The MISSION Act also mandated that VCCP providers must “fulfill training requirements established by the Secretary on how to deliver evidence-based treatments in the clinical areas for which the Department of Veterans Affairs has special expertise” before furnishing care pursuant to a contract with the VA. However, the VA elected to disregard the directive, and left it up to VCCP clinician’s discretion whether to obtain training or proficiency.
Two bills introduced in Congress in 2021 aim to uphold these vital mandates for the VCCP program. The Veterans’ Culturally Competent Care Act requires VCCP mental health practitioners to take courses on the evaluation and management of suicide, PTSD, TBI, and MST. The Lethal Means Safety Training Act aligns VCCP clinicians suicide prevention training with existing VA standards.
Recommendations to Assure the Quality of VCCP Care
With review and revision of VCCP quality standards now underway, the following remedial actions are recommended:
- VCCP metrics must be compiled using data on veterans’ care, not the general population, and be published on the Access to Care website. This indispensable information is published on the website for VA care but not for VCCP. Unless VCCP is required to track their veterans, apples-to-apples comparisons of quality of care will remain difficult to attain. Supplemental research that directly contrasts quality of VA to VCCP care should be posted. For example, a 2021 study of enrolled veterans brought by ambulance to VA or community emergency rooms found that all 170 VA medical centers had lower comparative death rates.
- VCCP providers should be held to the same quality standards as those applied to VA clinicians. In a 2020 critical issue update on implementation of the MISSION Act, major veterans service organizations (VSOs) recommended that competency, training, and quality standards for non-VA community clinicians must be equivalent to benchmarks expected of VA clinicians. That includes credentials, initial and follow-up training, diagnostic screening, care-delivery, and documentation standards. Enacting the Veterans’ Culturally Competent Care Act and the Lethal Means Safety Training Act would begin to meet the MISSION Act’s clear statutory language.
- The VA and VCCP should add quality information about major diagnostic categories. This will allow veterans to make informed decisions about their personal condition. For most health diagnoses, there is no searchable listing by disorder.
- Quality assessments should be realigned to focus on outcome measures. For prospective patients, outcome results provide the most meaningful basis for comparing and selecting clinicians. Proxy measures may have little bearing on whether veterans receive effective care. (As Albert Einstein’s famously observed, “Not everything that can be counted counts.”). Also, the specific measures used for a clinician’s HPP designation should be delineated.
- The VA must enforce the MISSION Act’s instruction to renew or cancel contracts based on demonstrated quality of care. As VSOs emphasized, “if the private sector is unwilling or unable to match the VA’s access and quality standards, the VA must consider whether it needs to find new community partners.”
Seventeen billion dollars is spent yearly on purchased health care whose quality remains indeterminate. Ironclad commitments are needed from Congress and the VA to ensure that the effectiveness of, and standards for, veterans care options in the private sector match that in the VA.
In 2014, amidst stories of delays at Veterans Health Administration facilities, Congress established the Veterans Choice Program, which expanded access to private sector health care practitioners. When the program expired in 2018, lawmakers replaced it with the Veterans Community Care Program (VCCP) as part of the US Department of Veterans Affairs (VA) Maintaining Internal Systems and Strengthening Integrated Outside Networks Act (38 USC § 1703 MISSION Act). Since then, the VCCP has grown exponentially; 34% of current veteran health care visits are with private clinicians.
Along with broader private sector access, the MISSION Act also mandated the creation of quality-of-care standards for both VA and VCCP, and stipulated that data be compiled and made available to “provide covered veterans relevant comparative information to make informed decisions regarding their health care.” Two-and-a-half years later, data about the quality of VCCP care remains largely unknown.
Access to Care Website
In the lead up to the MISSION Act, the VA launched its Access to Care website, an online tool that publishes institutional performance data on key metrics so that veterans can make “more informed choices about where, when, and how they receive their health care.” Following the bill’s passage, the VA added a MISSION Act Quality Standards section, which includes results of 27 conventional quality measures for every VA facility. These scores are posted alongside data of regional facilities.
This trailblazing tool is exceedingly comprehensive. Yet, multiple website gaps compromise its utility for veterans deliberating whether to obtain VCCP care, including:
- Data isn’t about VCCP care. The hospitals are selected because they are local, not whether they participate in VCCP. Further, it appears that aggregate scores include non-VCCP facilities.
- Missing conditions/treatments. While the website contains quality scores for an ample range of procedures, it lacks information for many conditions that disproportionately affect veterans. A veteran with posttraumatic stress disorder (PTSD) or traumatic brain injury (TBI), for example, has no data to check.
- Skewed comparison population. Private sector practitioners primarily treat nonveteran patients, a population that is, on average, healthier and of higher socioeconomic status when compared with VA patients. Outcomes differ, for example, when patients have coexisting mental illness or homelessness. For VCCP scores to be beneficial for comparisons, they should derive from treated veterans or be accurately risk-adjusted.
- Tangential measures. The Institute of Medicine defined health care quality as “improvement of outcomes.” Patients considering health care options benefit from information about treatment effectiveness and symptom reduction. But because obtaining that quality data is labor intensive, proxy measures are substituted. For example, the measure advising smokers to quit is the closest the website comes to reporting on the quality of mental health care.
High-Performers
The VA initiated a second means to inform veterans about the quality of furnished care. Specifically, they guided third-party administrators (TPAs)—TriWest Healthcare Alliance and Optum—in creating algorithms designating that VCCP individual clinicians, practice groups, and hospitals can be deemed high performing providers (HPPs). The algorithms are calculated using a mix of Healthcare Effectiveness Data and Information Set (HEDIS), Physician Quality Reporting System (PQRS), and Blue Health Intelligence (BHI) primary and specialty care measures. The designations are intended to be accessible to local VA community care schedulers to connect veterans with HPPs.
Many aspects of the HPP system are not yet public, including the measures that comprise the algorithms and when the designations will become operational. From what is publicly discoverable about HPP designations, there are crucial gaps like those on the Access to Care website. Behavioral and mental health conditions, for instance, are intentionally excluded in HPP monitoring. HPP algorithms draw from care provided to the general population; an HPP’s patient panel may contain no veterans (with their common comorbidities) at all. Most limiting, there’s no expectation that VCCP clinicians be high performing. Of the 1.2 million program clinicians treating veterans as of November 2020, only a nominal 13.4% were HPP.
After studying the HPP system, VA Partnered Evidence-based Policy Resource Center acknowledged that “it remains unclear whether the quality metrics and referral system result in higher quality of care for VA patients or whether the program improves veteran health.”
Quality of VCCP Mental Health Treatment
The MISSION Act mandated the VA to “establish standards and requirements for the provision of care by non-VA health care practitioners in clinical areas for which the Department of Veterans Affairs has special expertise, including PTSD, military sexual trauma-related conditions (MST), and TBI.” This requirement arose from a recognition that mental health care provided in the private sector pales in comparison to the VA’s rigorous evidence-based training, consultation, case review and care delivery. For example, over 8500 VA clinicians have received training in evidence-based cognitive processing therapy and/or prolonged exposure therapy for PTSD.
The MISSION Act also mandated that VCCP providers must “fulfill training requirements established by the Secretary on how to deliver evidence-based treatments in the clinical areas for which the Department of Veterans Affairs has special expertise” before furnishing care pursuant to a contract with the VA. However, the VA elected to disregard the directive, and left it up to VCCP clinician’s discretion whether to obtain training or proficiency.
Two bills introduced in Congress in 2021 aim to uphold these vital mandates for the VCCP program. The Veterans’ Culturally Competent Care Act requires VCCP mental health practitioners to take courses on the evaluation and management of suicide, PTSD, TBI, and MST. The Lethal Means Safety Training Act aligns VCCP clinicians suicide prevention training with existing VA standards.
Recommendations to Assure the Quality of VCCP Care
With review and revision of VCCP quality standards now underway, the following remedial actions are recommended:
- VCCP metrics must be compiled using data on veterans’ care, not the general population, and be published on the Access to Care website. This indispensable information is published on the website for VA care but not for VCCP. Unless VCCP is required to track their veterans, apples-to-apples comparisons of quality of care will remain difficult to attain. Supplemental research that directly contrasts quality of VA to VCCP care should be posted. For example, a 2021 study of enrolled veterans brought by ambulance to VA or community emergency rooms found that all 170 VA medical centers had lower comparative death rates.
- VCCP providers should be held to the same quality standards as those applied to VA clinicians. In a 2020 critical issue update on implementation of the MISSION Act, major veterans service organizations (VSOs) recommended that competency, training, and quality standards for non-VA community clinicians must be equivalent to benchmarks expected of VA clinicians. That includes credentials, initial and follow-up training, diagnostic screening, care-delivery, and documentation standards. Enacting the Veterans’ Culturally Competent Care Act and the Lethal Means Safety Training Act would begin to meet the MISSION Act’s clear statutory language.
- The VA and VCCP should add quality information about major diagnostic categories. This will allow veterans to make informed decisions about their personal condition. For most health diagnoses, there is no searchable listing by disorder.
- Quality assessments should be realigned to focus on outcome measures. For prospective patients, outcome results provide the most meaningful basis for comparing and selecting clinicians. Proxy measures may have little bearing on whether veterans receive effective care. (As Albert Einstein’s famously observed, “Not everything that can be counted counts.”). Also, the specific measures used for a clinician’s HPP designation should be delineated.
- The VA must enforce the MISSION Act’s instruction to renew or cancel contracts based on demonstrated quality of care. As VSOs emphasized, “if the private sector is unwilling or unable to match the VA’s access and quality standards, the VA must consider whether it needs to find new community partners.”
Seventeen billion dollars is spent yearly on purchased health care whose quality remains indeterminate. Ironclad commitments are needed from Congress and the VA to ensure that the effectiveness of, and standards for, veterans care options in the private sector match that in the VA.
Indurated Violaceous Lesions on the Face, Trunk, and Legs
The Diagnosis: Kaposi Sarcoma
A punch biopsy of a lesion on the right side of the back revealed a diffuse, poorly circumscribed, spindle cell neoplasm of the papillary and reticular dermis with associated vascular and pseudovascular spaces distended by erythrocytes (Figure 1). Immunostaining was positive for human herpesvirus 8 (HHV-8)(Figure 2), ETS-related gene, CD31, and CD34 and negative for pan cytokeratin, confirming the diagnosis of Kaposi sarcoma (KS). Bacterial, fungal, and mycobacterial tissue cultures were negative. The patient was tested for HIV and referred to infectious disease and oncology. He subsequently was found to have HIV with a viral load greater than 1 million copies. He was started on antiretroviral therapy and Pneumocystis jirovecii pneumonia prophylaxis. Computed tomography of the chest, abdomen, and pelvis showed bilateral, multifocal, perihilar, flame-shaped consolidations suggestive of KS. The patient later disclosed having an intermittent dry cough of more than a year’s duration with occasional bright red blood per rectum after bowel movements. After workup, the patient was found to have cytomegalovirus esophagitis/gastritis and candidal esophagitis that were treated with valganciclovir and fluconazole, respectively.
Kaposi sarcoma is an angioproliferative, AIDSdefining disease associated with HHV-8. There are 4 types of KS as defined by the populations they affect. AIDS-associated KS occurs in individuals with HIV, as seen in our patient. It often is accompanied by extensive mucocutaneous and visceral lesions, as well as systemic symptoms such as fever, weight loss, and diarrhea.1 Classic KS is a variant that presents in older men of Mediterranean, Eastern European, and South American descent. Cutaneous lesions typically are distributed on the lower extremities.2,3 Endemic (African) KS is seen in HIV-negative children and young adults in equatorial Africa. It most commonly affects the lower extremities or lymph nodes and usually follows a more aggressive course.2 Lastly, iatrogenic KS is associated with immunosuppressive medications or conditions, such as organ transplantation, chemotherapy, and rheumatologic disorders.3,4
Kaposi sarcoma commonly presents as violaceous or dark red macules, patches, papules, plaques, and nodules on various parts of the body (Figure 3). Lesions typically begin as macules and progress into plaques or nodules. Our patient presented as a deceptively healthy young man with lesions at various stages of development. In addition to the skin and oral mucosa, the lungs, lymph nodes, and gastrointestinal tract commonly are involved in AIDS-associated KS.5 Patients may experience symptoms of internal involvement, including bleeding, hematochezia, odynophagia, or dyspnea.
The differential diagnosis includes conditions that can mimic KS, including bacillary angiomatosis, angioinvasive fungal disease, sarcoid, and other malignancies. A skin biopsy is the gold standard for definitive diagnosis of KS. Histopathology shows a vascular proliferation in the dermis and spindle cell proliferation.6 Kaposi sarcoma stains positively for factor VIII–related antigen, CD31, and CD34.2 Additionally, staining for HHV-8 gene products, such as latency-associated nuclear antigen 1, is helpful in differentiating KS from other conditions.7
In HIV-associated KS, the mainstay of treatment is initiation of highly active antiretroviral therapy. Typically, as the CD4 count rises with treatment, the tumor burden classic KS, effective treatment options include recurrent cryotherapy or intralesional chemotherapeutics, such as vincristine, for localized lesions; for widespread disease, pegylated liposomal doxorubicin or radiation have been found to be effective options. Lastly, for patients with iatrogenic KS, reducing immunosuppressive medications is a reasonable first step in management. If this does not yield adequate improvement, transitioning from calcineurin inhibitors (eg, cyclosporine) to proliferation signal inhibitors (eg, sirolimus) may lead to resolution.7
- Friedman-Kien AE, Saltzman BR. Clinical manifestations of classical, endemic African, and epidemic AIDS-associated Kaposi’s sarcoma. J Am Acad Dermatol. 1990;22:1237-1250.
- Radu O, Pantanowitz L. Kaposi sarcoma. Arch Pathol Lab Med. 2013;137:289-294.
- Vangipuram R, Tyring SK. Epidemiology of Kaposi sarcoma: review and description of the nonepidemic variant. Int J Dermatol. 2019;58:538-542.
- Klepp O, Dahl O, Stenwig JT. Association of Kaposi’s sarcoma and prior immunosuppressive therapy. a 5‐year material of Kaposi’s sarcoma in Norway. Cancer. 1978;42:2626-2630.
- Lemlich G, Schwam L, Lebwohl M. Kaposi’s sarcoma and acquired immunodeficiency syndrome: postmortem findings in twenty-four cases. J Am Acad Dermatol. 1987;16:319-325.
- Kaposi sarcoma. Nat Rev Dis Primers. 2019;5:10.
- Curtiss P, Strazzulla LC, Friedman-Kien AE. An update on Kaposi’s sarcoma: epidemiology, pathogenesis and treatment. Dermatol Ther. 2016;6:465-470.
The Diagnosis: Kaposi Sarcoma
A punch biopsy of a lesion on the right side of the back revealed a diffuse, poorly circumscribed, spindle cell neoplasm of the papillary and reticular dermis with associated vascular and pseudovascular spaces distended by erythrocytes (Figure 1). Immunostaining was positive for human herpesvirus 8 (HHV-8)(Figure 2), ETS-related gene, CD31, and CD34 and negative for pan cytokeratin, confirming the diagnosis of Kaposi sarcoma (KS). Bacterial, fungal, and mycobacterial tissue cultures were negative. The patient was tested for HIV and referred to infectious disease and oncology. He subsequently was found to have HIV with a viral load greater than 1 million copies. He was started on antiretroviral therapy and Pneumocystis jirovecii pneumonia prophylaxis. Computed tomography of the chest, abdomen, and pelvis showed bilateral, multifocal, perihilar, flame-shaped consolidations suggestive of KS. The patient later disclosed having an intermittent dry cough of more than a year’s duration with occasional bright red blood per rectum after bowel movements. After workup, the patient was found to have cytomegalovirus esophagitis/gastritis and candidal esophagitis that were treated with valganciclovir and fluconazole, respectively.

Kaposi sarcoma is an angioproliferative, AIDSdefining disease associated with HHV-8. There are 4 types of KS as defined by the populations they affect. AIDS-associated KS occurs in individuals with HIV, as seen in our patient. It often is accompanied by extensive mucocutaneous and visceral lesions, as well as systemic symptoms such as fever, weight loss, and diarrhea.1 Classic KS is a variant that presents in older men of Mediterranean, Eastern European, and South American descent. Cutaneous lesions typically are distributed on the lower extremities.2,3 Endemic (African) KS is seen in HIV-negative children and young adults in equatorial Africa. It most commonly affects the lower extremities or lymph nodes and usually follows a more aggressive course.2 Lastly, iatrogenic KS is associated with immunosuppressive medications or conditions, such as organ transplantation, chemotherapy, and rheumatologic disorders.3,4

Kaposi sarcoma commonly presents as violaceous or dark red macules, patches, papules, plaques, and nodules on various parts of the body (Figure 3). Lesions typically begin as macules and progress into plaques or nodules. Our patient presented as a deceptively healthy young man with lesions at various stages of development. In addition to the skin and oral mucosa, the lungs, lymph nodes, and gastrointestinal tract commonly are involved in AIDS-associated KS.5 Patients may experience symptoms of internal involvement, including bleeding, hematochezia, odynophagia, or dyspnea.

The differential diagnosis includes conditions that can mimic KS, including bacillary angiomatosis, angioinvasive fungal disease, sarcoid, and other malignancies. A skin biopsy is the gold standard for definitive diagnosis of KS. Histopathology shows a vascular proliferation in the dermis and spindle cell proliferation.6 Kaposi sarcoma stains positively for factor VIII–related antigen, CD31, and CD34.2 Additionally, staining for HHV-8 gene products, such as latency-associated nuclear antigen 1, is helpful in differentiating KS from other conditions.7
In HIV-associated KS, the mainstay of treatment is initiation of highly active antiretroviral therapy. Typically, as the CD4 count rises with treatment, the tumor burden classic KS, effective treatment options include recurrent cryotherapy or intralesional chemotherapeutics, such as vincristine, for localized lesions; for widespread disease, pegylated liposomal doxorubicin or radiation have been found to be effective options. Lastly, for patients with iatrogenic KS, reducing immunosuppressive medications is a reasonable first step in management. If this does not yield adequate improvement, transitioning from calcineurin inhibitors (eg, cyclosporine) to proliferation signal inhibitors (eg, sirolimus) may lead to resolution.7
The Diagnosis: Kaposi Sarcoma
A punch biopsy of a lesion on the right side of the back revealed a diffuse, poorly circumscribed, spindle cell neoplasm of the papillary and reticular dermis with associated vascular and pseudovascular spaces distended by erythrocytes (Figure 1). Immunostaining was positive for human herpesvirus 8 (HHV-8)(Figure 2), ETS-related gene, CD31, and CD34 and negative for pan cytokeratin, confirming the diagnosis of Kaposi sarcoma (KS). Bacterial, fungal, and mycobacterial tissue cultures were negative. The patient was tested for HIV and referred to infectious disease and oncology. He subsequently was found to have HIV with a viral load greater than 1 million copies. He was started on antiretroviral therapy and Pneumocystis jirovecii pneumonia prophylaxis. Computed tomography of the chest, abdomen, and pelvis showed bilateral, multifocal, perihilar, flame-shaped consolidations suggestive of KS. The patient later disclosed having an intermittent dry cough of more than a year’s duration with occasional bright red blood per rectum after bowel movements. After workup, the patient was found to have cytomegalovirus esophagitis/gastritis and candidal esophagitis that were treated with valganciclovir and fluconazole, respectively.

Kaposi sarcoma is an angioproliferative, AIDSdefining disease associated with HHV-8. There are 4 types of KS as defined by the populations they affect. AIDS-associated KS occurs in individuals with HIV, as seen in our patient. It often is accompanied by extensive mucocutaneous and visceral lesions, as well as systemic symptoms such as fever, weight loss, and diarrhea.1 Classic KS is a variant that presents in older men of Mediterranean, Eastern European, and South American descent. Cutaneous lesions typically are distributed on the lower extremities.2,3 Endemic (African) KS is seen in HIV-negative children and young adults in equatorial Africa. It most commonly affects the lower extremities or lymph nodes and usually follows a more aggressive course.2 Lastly, iatrogenic KS is associated with immunosuppressive medications or conditions, such as organ transplantation, chemotherapy, and rheumatologic disorders.3,4

Kaposi sarcoma commonly presents as violaceous or dark red macules, patches, papules, plaques, and nodules on various parts of the body (Figure 3). Lesions typically begin as macules and progress into plaques or nodules. Our patient presented as a deceptively healthy young man with lesions at various stages of development. In addition to the skin and oral mucosa, the lungs, lymph nodes, and gastrointestinal tract commonly are involved in AIDS-associated KS.5 Patients may experience symptoms of internal involvement, including bleeding, hematochezia, odynophagia, or dyspnea.

The differential diagnosis includes conditions that can mimic KS, including bacillary angiomatosis, angioinvasive fungal disease, sarcoid, and other malignancies. A skin biopsy is the gold standard for definitive diagnosis of KS. Histopathology shows a vascular proliferation in the dermis and spindle cell proliferation.6 Kaposi sarcoma stains positively for factor VIII–related antigen, CD31, and CD34.2 Additionally, staining for HHV-8 gene products, such as latency-associated nuclear antigen 1, is helpful in differentiating KS from other conditions.7
In HIV-associated KS, the mainstay of treatment is initiation of highly active antiretroviral therapy. Typically, as the CD4 count rises with treatment, the tumor burden classic KS, effective treatment options include recurrent cryotherapy or intralesional chemotherapeutics, such as vincristine, for localized lesions; for widespread disease, pegylated liposomal doxorubicin or radiation have been found to be effective options. Lastly, for patients with iatrogenic KS, reducing immunosuppressive medications is a reasonable first step in management. If this does not yield adequate improvement, transitioning from calcineurin inhibitors (eg, cyclosporine) to proliferation signal inhibitors (eg, sirolimus) may lead to resolution.7
- Friedman-Kien AE, Saltzman BR. Clinical manifestations of classical, endemic African, and epidemic AIDS-associated Kaposi’s sarcoma. J Am Acad Dermatol. 1990;22:1237-1250.
- Radu O, Pantanowitz L. Kaposi sarcoma. Arch Pathol Lab Med. 2013;137:289-294.
- Vangipuram R, Tyring SK. Epidemiology of Kaposi sarcoma: review and description of the nonepidemic variant. Int J Dermatol. 2019;58:538-542.
- Klepp O, Dahl O, Stenwig JT. Association of Kaposi’s sarcoma and prior immunosuppressive therapy. a 5‐year material of Kaposi’s sarcoma in Norway. Cancer. 1978;42:2626-2630.
- Lemlich G, Schwam L, Lebwohl M. Kaposi’s sarcoma and acquired immunodeficiency syndrome: postmortem findings in twenty-four cases. J Am Acad Dermatol. 1987;16:319-325.
- Kaposi sarcoma. Nat Rev Dis Primers. 2019;5:10.
- Curtiss P, Strazzulla LC, Friedman-Kien AE. An update on Kaposi’s sarcoma: epidemiology, pathogenesis and treatment. Dermatol Ther. 2016;6:465-470.
- Friedman-Kien AE, Saltzman BR. Clinical manifestations of classical, endemic African, and epidemic AIDS-associated Kaposi’s sarcoma. J Am Acad Dermatol. 1990;22:1237-1250.
- Radu O, Pantanowitz L. Kaposi sarcoma. Arch Pathol Lab Med. 2013;137:289-294.
- Vangipuram R, Tyring SK. Epidemiology of Kaposi sarcoma: review and description of the nonepidemic variant. Int J Dermatol. 2019;58:538-542.
- Klepp O, Dahl O, Stenwig JT. Association of Kaposi’s sarcoma and prior immunosuppressive therapy. a 5‐year material of Kaposi’s sarcoma in Norway. Cancer. 1978;42:2626-2630.
- Lemlich G, Schwam L, Lebwohl M. Kaposi’s sarcoma and acquired immunodeficiency syndrome: postmortem findings in twenty-four cases. J Am Acad Dermatol. 1987;16:319-325.
- Kaposi sarcoma. Nat Rev Dis Primers. 2019;5:10.
- Curtiss P, Strazzulla LC, Friedman-Kien AE. An update on Kaposi’s sarcoma: epidemiology, pathogenesis and treatment. Dermatol Ther. 2016;6:465-470.
A 25-year-old man with no notable medical history presented to the dermatology clinic with growing selfdescribed cysts on the face, trunk, and legs of 6 months’ duration. The lesions started as bruiselike discolorations and progressed to become firm nodules and inflamed masses. Some were minimally itchy and sensitive to touch, but there was no history of bleeding or drainage. The patient denied any new or recent environmental or animal exposures, use of illicit drugs, or travel correlating with the rash onset. He denied any prior treatments. He reported being in his normal state of health and was not taking any medications. Physical examination revealed indurated, violaceous, purpuric subcutaneous nodules, plaques, and masses on the forehead, cheek (top), jaw, flank, axillae (bottom), and back.