User login
Veterans Potentially Exposed to HIV, HCV at Georgia Hospital
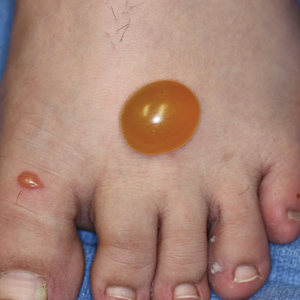
Testing is ongoing after more than 4,600 veterans who had received care at the Carl Vinson Veterans Affairs Medical Center in Dublin, Georgia, were alerted that they may have been exposed to HIV, hepatitis B, and hepatitis C. The exposure was due to improperly sterilized equipment. At least some of the patients have tested positive, but the facility has not indicated the number, the diseases, or whether the infections were the result of the exposure.
A mid-January internal review at the hospital found that not all steps were being followed in the procedures for sterilizing equipment between patients. Patients who had dentistry, endoscopy, urology, podiatry, optometry, or surgical procedures in 2021 may have been exposed to blood-borne pathogens.
In response, the VA sent teams from other hospitals to help, including a team from the Augusta Veterans Affairs Medical Center to reprocess all equipment and staff from VA facilities in Atlanta, South Carolina, and Alabama to provide personnel training. All staff at Carl Vinson Veterans Affairs Medical Center have since been retrained on all current guidelines.
The hospital says it’s still testing exposed veterans. Hospital spokesperson James Huckfeldt told a Macon-based newspaper, The Telegraph, that veterans with positive test results will undergo additional testing to determine whether the transmission is new or preexisting. “The findings from the additional testing will be used to accurately diagnose any impacted veterans and ensure that they receive appropriate medical treatment,” he said.
Manuel M. Davila, director of the hospital, sent letters to the patients at risk, alerting them to the exposure. “We sincerely apologize and accept responsibility for this mistake and are taking steps to prevent it from happening in the future,” Davilla wrote. “This event is unacceptable to us as well, and we want to work with you to correct the situation and ensure your safety and well-being. Because your safety is important to us and because we want to honor your trust in us, we want you to know that when concerns are raised over our processes or procedures, we take immediate steps to stop everything and make sure things are.”
Davilla reassured the veterans that “we are confident that the risk of infectious disease is very low.”
The Carl Vinson Medical Center has set up a communication center to answer questions for veterans: (478) 274-5400.
Testing is ongoing after more than 4,600 veterans who had received care at the Carl Vinson Veterans Affairs Medical Center in Dublin, Georgia, were alerted that they may have been exposed to HIV, hepatitis B, and hepatitis C. The exposure was due to improperly sterilized equipment. At least some of the patients have tested positive, but the facility has not indicated the number, the diseases, or whether the infections were the result of the exposure.
A mid-January internal review at the hospital found that not all steps were being followed in the procedures for sterilizing equipment between patients. Patients who had dentistry, endoscopy, urology, podiatry, optometry, or surgical procedures in 2021 may have been exposed to blood-borne pathogens.
In response, the VA sent teams from other hospitals to help, including a team from the Augusta Veterans Affairs Medical Center to reprocess all equipment and staff from VA facilities in Atlanta, South Carolina, and Alabama to provide personnel training. All staff at Carl Vinson Veterans Affairs Medical Center have since been retrained on all current guidelines.
The hospital says it’s still testing exposed veterans. Hospital spokesperson James Huckfeldt told a Macon-based newspaper, The Telegraph, that veterans with positive test results will undergo additional testing to determine whether the transmission is new or preexisting. “The findings from the additional testing will be used to accurately diagnose any impacted veterans and ensure that they receive appropriate medical treatment,” he said.
Manuel M. Davila, director of the hospital, sent letters to the patients at risk, alerting them to the exposure. “We sincerely apologize and accept responsibility for this mistake and are taking steps to prevent it from happening in the future,” Davilla wrote. “This event is unacceptable to us as well, and we want to work with you to correct the situation and ensure your safety and well-being. Because your safety is important to us and because we want to honor your trust in us, we want you to know that when concerns are raised over our processes or procedures, we take immediate steps to stop everything and make sure things are.”
Davilla reassured the veterans that “we are confident that the risk of infectious disease is very low.”
The Carl Vinson Medical Center has set up a communication center to answer questions for veterans: (478) 274-5400.
Testing is ongoing after more than 4,600 veterans who had received care at the Carl Vinson Veterans Affairs Medical Center in Dublin, Georgia, were alerted that they may have been exposed to HIV, hepatitis B, and hepatitis C. The exposure was due to improperly sterilized equipment. At least some of the patients have tested positive, but the facility has not indicated the number, the diseases, or whether the infections were the result of the exposure.
A mid-January internal review at the hospital found that not all steps were being followed in the procedures for sterilizing equipment between patients. Patients who had dentistry, endoscopy, urology, podiatry, optometry, or surgical procedures in 2021 may have been exposed to blood-borne pathogens.
In response, the VA sent teams from other hospitals to help, including a team from the Augusta Veterans Affairs Medical Center to reprocess all equipment and staff from VA facilities in Atlanta, South Carolina, and Alabama to provide personnel training. All staff at Carl Vinson Veterans Affairs Medical Center have since been retrained on all current guidelines.
The hospital says it’s still testing exposed veterans. Hospital spokesperson James Huckfeldt told a Macon-based newspaper, The Telegraph, that veterans with positive test results will undergo additional testing to determine whether the transmission is new or preexisting. “The findings from the additional testing will be used to accurately diagnose any impacted veterans and ensure that they receive appropriate medical treatment,” he said.
Manuel M. Davila, director of the hospital, sent letters to the patients at risk, alerting them to the exposure. “We sincerely apologize and accept responsibility for this mistake and are taking steps to prevent it from happening in the future,” Davilla wrote. “This event is unacceptable to us as well, and we want to work with you to correct the situation and ensure your safety and well-being. Because your safety is important to us and because we want to honor your trust in us, we want you to know that when concerns are raised over our processes or procedures, we take immediate steps to stop everything and make sure things are.”
Davilla reassured the veterans that “we are confident that the risk of infectious disease is very low.”
The Carl Vinson Medical Center has set up a communication center to answer questions for veterans: (478) 274-5400.
Infectious disease pop quiz: Clinical challenge #17 for the ObGyn
What are the best tests for identification of a patient with chronic hepatitis B infection?
Continue to the answer...
Patients with chronic hepatitis B infection typically test positive for the hepatitis B surface antigen (HBsAg) and for IgG antibody to the hepatitis B core antigen (HBcAg). In addition, they also may test positive for the hepatitis B e antigen (HBeAg), and their viral load can be quantified by polymerase chain reaction (PCR) when significant antigenemia is present. The presence of the e antigen indicates a high rate of viral replication and a corresponding high rate of infectivity.
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
What are the best tests for identification of a patient with chronic hepatitis B infection?
Continue to the answer...
Patients with chronic hepatitis B infection typically test positive for the hepatitis B surface antigen (HBsAg) and for IgG antibody to the hepatitis B core antigen (HBcAg). In addition, they also may test positive for the hepatitis B e antigen (HBeAg), and their viral load can be quantified by polymerase chain reaction (PCR) when significant antigenemia is present. The presence of the e antigen indicates a high rate of viral replication and a corresponding high rate of infectivity.
What are the best tests for identification of a patient with chronic hepatitis B infection?
Continue to the answer...
Patients with chronic hepatitis B infection typically test positive for the hepatitis B surface antigen (HBsAg) and for IgG antibody to the hepatitis B core antigen (HBcAg). In addition, they also may test positive for the hepatitis B e antigen (HBeAg), and their viral load can be quantified by polymerase chain reaction (PCR) when significant antigenemia is present. The presence of the e antigen indicates a high rate of viral replication and a corresponding high rate of infectivity.
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
The Impact of Prenatal Nutrition on the Development of Atopic Dermatitis in Infancy and Childhood
Atopic dermatitis (AD) is an inflammatory skin disease characterized by skin barrier disruption, skin inflammation, and pruritus.1 It is a common and often chronic skin condition associated with the development of food allergies, asthma, and allergic rhinitis, known as the atopic march.2 Atopic dermatitis is estimated to affect 10% to 25% of children, most with onset before 5 years of age, and up to 7% of adults worldwide.3 Most patients improve with time, but multiple disease trajectories are possible. Several studies have demonstrated that fewer than 4% of children develop the classic atopic march—AD followed by food allergies, asthma, and finally allergic rhinitis—with recent evidence pointing to a more complex heterogeneous progression of disease and allergic comorbidities often occurring together.4,5 The prevalence of AD has been increasing globally over the last 30 years,6 with a marked increase in developed countries.6,7 It is well accepted that AD is based on an interplay between genetic predisposition and environmental factors,8 but many suspect that the rapid rise in prevalence cannot be attributed to genetic factors alone.9 The precipitant triggers for AD remain an area of intense investigation, with ongoing debate between the “inside out” and “outside in” hypotheses; these revolve around whether abnormalities in the immune system trigger barrier dysfunction or barrier dysfunction triggers immune programming to atopy.8 Ongoing research related to genetic predisposition of AD has identified candidate genes implicated in both impaired skin barrier function and altered immune system pathways, further supporting that both theories may contribute to disease pathogenesis.
The increasing prevalence of AD, with increasing disease burden within socioeconomically advantaged countries, raises the possibility of early modifiable environmental factors that may contribute to the disease process.10 Many studies point to the influence of the 21st century lifestyle and Western diet as primary contributing factors.9,11 However, it is not clear how these factors may influence the development of allergic atopic disease. Several studies have suggested that nonheritable influences in utero can alter fetus immune function and influence the subsequent development of allergic disease.12,13 Although many studies have examined environmental factors contributing to the development of AD in infancy and childhood, less is understood about the influence of prenatal factors. Currently, in utero exposure to tobacco smoke, phthalates, and maternal distress have been potentially implicated in the development of AD.14,15 Several studies have examined the role of maternal diet and nutrition on the development of AD in offspring; however, formal recommendations and robust trial data are lacking. In this article, we examine the existing literature surrounding maternal diet on the development of AD in infancy and childhood.
Allergen Avoidance
Extrapolating from the food allergy literature, it was once suggested that allergen avoidance in early childhood had a protective effect on the subsequent development of allergies; however, more recent research has found that early exposure to common food allergens, such as peanuts or eggs, may actually reduce a child’s risk for developing these allergies later in life.16 Among infants at high risk for food allergy, sustained consumption of peanut products beginning in the first 11 months of life resulted in an 81% lower rate of peanut allergy at 60 months of age than the rate among children who avoided peanuts.17 Given the results that antigen avoidance during infancy/childhood does not protect against the development of allergies and may actually be counterproductive, it is not surprising that research studying antigen avoidance during pregnancy on the development of AD also has demonstrated limited efficacy. A systematic review of 5 trials on maternal dietary antigen avoidance (N=952) suggested no protective effects of avoiding antigenic foods during pregnancy on the development of AD in the first 18 months of life.18 Another meta-analysis evaluating 12 intervention trials looked at the effects of maternal allergenic food avoidance during pregnancy or lactation and found no reduced risk for subsequent development of allergic disease, including AD.19 The American Academy of Pediatrics 2019 consensus statement does not support maternal dietary restrictions in pregnancy for the prevention of atopic disease and makes note that the data remain limited, which complicates drawing any firm conclusions.20
Probiotic Supplementation
One of the most investigated dietary supplements for the prevention of atopic disease is probiotics, with possible benefits noted in both the prenatal and postnatal periods. Baquerizo Nole et al21 examined several studies looking at the various benefits of probiotics in AD, which included inhibition of the helper T cell (TH2) response, stimulation of the TH1 response, upregulation of regulatory T cells, acceleration of skin and mucosal barrier function, increase in intestinal microflora diversity, suppression of toxic fermentation products in the intestinal lumen from increased production of short-chain fatty acids, and inhibition of Staphylococcus aureus attachment on epidermal keratinocytes. It is unclear how this may affect infants prenatally; however, transfer of maternal intestinal microflora during delivery and shortly thereafter has demonstrated that probiotic strains remain detectable in the infant’s stool up to 6 months after delivery, even if the mother has discontinued use.22 A 2008 meta-analysis of 10 double-bind, randomized, controlled trials (N=1880) looking at the use of maternal prenatal and postnatal probiotic supplementation in the prevention of pediatric AD found a relative risk (RR) ratio of 0.69 (95% CI, 0.57-0.83) using a fixed effects model and RR ratio of 0.66 (95% CI, 0.49-0.89) using a random effects model. After exclusion of one study that evaluated the effect of postnatal probiotic supplementation only, the RR ratio decreased to 0.61 for both the fixed effects and random effects models.23 A systematic review by Panduru et al24 noted similar findings with a subgroup meta-analysis of 11 studies of prenatal supplementation followed by postnatal supplementation of probiotics, which demonstrated a protective effect on the development of AD (odds ratio [OR]=0.61, P<.001). Postnatal supplementation alone (4 studies) did not have the same association (OR=0.95, P<.82).24 A 2012 meta-analysis by Doege et al25 evaluated 7 randomized, double-blinded, placebo-controlled trials that assessed probiotic supplementation during pregnancy (without incorporation of postnatal supplementation) and found a significant risk reduction of 5.7% (P=.022) for AD in children aged 2 to 7 years. Interestingly, this was only significant for Lactobacillus and not for other bacterial strains, even if a mixture of strains included Lactobacillus. However, Panduru et al24 found both maternal Lactobacillus supplementation alone (8 studies) and in combination with Bifidobacterium (9 studies) was protective against AD development in children (OR=0.70, P=.004; OR=0.62, P<.001). A more recent 2015 meta-analysis of 17 studies (N=4755) evaluating the use of maternal probiotic supplementation in pregnancy and/or through the infant’s first 3 months of life found a significantly lower RR (0.78 [95% CI, 0.69-0.89], P=.0003) for the development of AD in infants treated with probiotics and found this risk to be even further decreased when a mixture of probiotics including both Lactobacillus and Bifidobacterium was used (RR=0.54 [95% CI, 0.43-0.68], P<.00001).26
Antioxidants
The Westernization of many developing countries’ diets—diets high in saturated fats, protein, sucrose, salt, and processed foods and low in fresh fruits and green vegetables—has led to a reduced intake of antioxidants and an increase in susceptibility to oxidative damage.27,28 One hypothesis suggests that a reduction in nutritional antioxidants and subsequent oxidative damage leads to airway inflammation that may contribute to an increased prevalence of asthma.27 In vitro data suggest that antioxidant deficiency may influence the differentiation of helper T cells to a TH2 phenotype, which can increase susceptibility to the development of asthma and allergies.29 Vitamin E specifically has been shown to inhibit IL-4 gene expression, which drives type 2 immunity and decreases expression of multiple genes that regulate epidermal barrier function, subsequently increasing susceptibility to allergic inflammation and AD.29,30 Regardless of the proposed mechanisms for antioxidant deficiency increasing susceptibility to allergic disease, studies evaluating the benefits of antioxidant intake during pregnancy in relation to AD have not been promising. Several studies have found no association between prenatal vitamin E intake and the risk for AD development in infants and children.31,32 Another study found a statistically significant inverse relationship between vitamin E intake in mothers with a history of atopy and the development of AD in their children at 2 years of age but not at 1 year of age (P-trend=.024).33 It has been suggested that varying vitamin E isoforms may contribute to the discrepant results previously discussed, with the γ-tocopherol isoform (found frequently in Westernized diets)34 as a driver of inflammation in murine models.35 West et al31 noted an association between vitamin C intake and development of “any allergic disease”—AD, IgE-mediated food allergy, or asthma—with a crude OR of 0.48 (95% CI, 0.25-0.93). However, the P-trend and adjusted OR were not statistically significant. The investigators found no association between maternal intake of beta-carotene, vitamin E, or zinc, but they did find copper supplementation to be protective on the development of AD at 1 year of age (P-trend=0.03). Interestingly, when the data for total antioxidant intake—vitamin C, vitamin E, zinc, beta-carotene, and copper from both diet and supplementation—were combined and analyzed, no statistically significant associations for any of the antioxidants were found.31 Another study of 763 Japanese mother-child pairs found a reduced risk for AD at 16 to 24 months of age with high maternal intake of beta-carotene but found no statistically significant exposure-response associations with other antioxidants, including alpha-carotene, vitamin C, or zinc from dietary intake alone.32 These results were substantiated by 2 meta-analyses evaluating a total of 93 combined intervention trials and cohorts where no association was found between vitamin or mineral intake during pregnancy and/or during infancy and the development of AD.19,36
Fatty Acids
Other dietary changes that are associated with an increased prevalence of atopic diseases in children include excess consumption of omega-6 (n-6) long-chain polyunsaturated fatty acids (LC-PUFA) and insufficient omega-3 (n-3) LC-PUFA consumption.37 Given prior evidence that allergic immune responses in infants may be primed before birth,38 researchers have questioned whether the anti-inflammatory properties of n-3 LC-PUFA when supplemented during pregnancy may have immunomodulatory effects on infants that could alter their predisposition to develop allergic disease, including AD.39 A systematic review and meta-analysis of randomized controlled trials found a statistically significant RR of 0.53 (95% CI, 0.35-0.81; P=.004) for the incidence of AD at 12 months of age with maternal supplementation of n-3 LC-PUFA.9 Another trial of 145 pregnant women randomized to supplementation with fish oil vs placebo starting at gestational week 25 and continuing through 3.5 months of breastfeeding found a reduced cumulative incidence of AD in the intervention group compared to controls at 2 years of age, with a statistically significant crude OR of 0.33 (95% CI, 0.11-0.97; P=.04).40 However, the adjusted OR was not statistically significant. In addition, they found that mothers and infants with higher proportions of docosahexaenoic acid and eicosapentaenoic acid in plasma phospholipids have been noted to have a lower prevalence of IgE-associated disease in a dose-dependent manner (P<.05 and P<.05, respectively).40 In another trial of 98 pregnant women randomized to fish oil supplementation or placebo from 20 weeks’ gestation to delivery found no difference in the frequency of AD but did note that infants in the exposure group had significantly less severe AD compared to controls (OR=0.09 [95% CI, 0.1-0.94]; P=.045).39 A prospective birth cohort study of 2641 children evaluated dietary composition during the last 4 weeks of pregnancy and found that consumption of foods rich in n-6 LC-PUFAs (eg, margarine, vegetable oil) increased the risk for developing AD, while foods rich in n-3 LC-PUFAs (eg, fish) decreased the risk for developing AD in offspring at 2 years of age. All P values for margarine, vegetable oil, and fish were statistically significant on logistic regression at P<.05.41 A longitudinal analysis of follow-up data from a randomized controlled trial looking at maternal prenatal n-3 LC-PUFA intake and the development of allergic disease (including AD) found no differences in the development of disease at 1-, 3-, or 6-year follow-up.42 Despite several studies demonstrating a possible benefit of omega-3 fatty acid intake on the development of AD in offspring, the longitudinal analysis by Best et al42 reminds us that long-term follow-up is critical in establishing benefit of any intervention given the heterogeneous and progressive nature of the atopic march and AD.
Specific Diets
Several studies have evaluated the role of dietary patterns and their influence on atopic disease. Studies evaluating dietary patterns or supplement intake can be challenging, as data often are derived from questionnaires with bias in response to families with higher socioeconomic status.9 Further, analysis of any one food group does not account for the potential interplay between nutrients.43 Studies should focus more on dietary patterns vs individual foods to assess true risk.43,44 Given these limitations, study results on diet should be carefully scrutinized; however, there are still some positive findings that deserve further investigation. Chatzi et al44 followed 460 children for 6.5 years and found a protective effect for the development of atopy in the offspring of women who had high adherence to the Mediterranean diet (OR 0.55 [95% CI, 0.31-0.97]). Another cohort study evaluating the effects of the Mediterranean diet and risk for AD in the first year of life in 2516 mother-child pairs from Spain and Greece found no statistically significant association with consumption of the Mediterranean diet and AD. The investigators also evaluated intake of fruits, nuts, vegetables, meats, processed meats, dairy products, and cereal and found no statistically significant protective benefit.45 Another systematic review of more than 90 observational studies identified no significant relationship between prenatal dietary exposures of fruits, vegetables, nuts, fat, fatty acids, eggs, cereal, milk, alcohol, tea, or coffee and risk for allergic disease in offspring, including AD.19
A Chinese prospective cohort study evaluated the dietary protein patterns of 713 mother-child pairs and the incidence of infant AD at 6 months of age.46 Dietary protein patterns were characterized as predominantly poultry, plant based, dairy and eggs, and red meat and fish. The investigators found a statistically significant reduced risk for AD in mothers who consumed plant-based or dairy and eggs protein patterns when compared to a poultry protein pattern with an adjusted OR of 0.572 (95% CI, 0.330-0.992) and 0.478 (95% CI, 0.274-0.837), respectively. This protective effect was not seen with the red meat and fish protein patterns.46 Similar results were seen in a 2020 Canadian study that evaluated the effects of a Western (fats, meats, processed foods, and starchy vegetables), balanced (diverse sources of animal proteins [especially fish], fruits, vegetables, nuts, and seeds), or plant-based (dairy, legumes, vegetables, whole grains, and an aversion to meats) diet in more than 2000 mother-infant pairs from 24 to 28 weeks’ gestation to 1 year of age. The investigators found a lower OR of AD in mothers who followed a mostly plant-based diet compared to other dietary patterns (OR 0.65 [95% CI, 0.55-0.76]; P<.001).10 Another prospective Japanese study looking at healthy (high intake of green and yellow vegetables, seaweed, mushrooms, white vegetables, pulses, potatoes, fish, sea products, fruit, and shellfish, and low intake of confectioneries and soft drinks), Western (high intake of vegetable oil, salt-containing seasonings, beef, pork, processed meat, eggs, chicken, and white vegetables, and low intake of fruit, soft drinks, and confectioneries), or Japanese (high intake of rice, miso soup, sea products, and fish, and low intake of bread, confectioneries, and dairy products) dietary patterns in 763 mother-child pairs found no association between diet during pregnancy and development of AD in offspring at 16 to 24 months.47 Unfortunately, a longitudinal data analysis has not been performed for this study.
Final Thoughts
Atopic dermatitis is a complex, progressive, and heterogeneous disease with both genetic and environmental influences. Studying the effects of diet on the development, progression, or severity of disease can be very difficult due to the heterogeneity of study designs, lack of long-term follow-up, and high potential for residual confounding. Studies evaluating dietary patterns or supplement intake can be equally challenging, as data often are derived from questionnaires with bias in response to families with higher socioeconomic status.9 Very few studies have looked specifically at maternal dietary composition and the development of AD alone (without inclusion of asthma or food allergy). Ultimately, the inconsistency of the data makes it difficult to draw conclusions and make formal recommendations for this vulnerable population. Additional evidence from well-powered trials with comparable methodology and objective outcome measures will be imperative to make formal recommendations. In addition, longitudinal follow-up will be essential to determine long-term benefit and influence on the atopic march.
- Nutten S. Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab. 2015;66(suppl 1):8-16.
- Kapoor R, Menon C, Hoffstad O, et al. The prevalence of atopic triad in children with physician-confirmed atopic dermatitis. J Am Acad Dermatol. 2008;58:68-73.
- Abuabara K, Magyari A, McCulloch CE, et al. Prevalence of atopic eczema among patients seen in primary care: data from the Health Improvement Network. Ann Intern Med. 2019;170:354-356.
- Belgrave DC, Granell R, Simpson A, et al. Developmental profiles of eczema, wheeze, and rhinitis: two population-based birth cohort studies. PLoS Medicine. 2014;11:E1001748.
- Aguilar D, Pinart M, Koppelman GH, et al. Computational analysis of multimorbidity between asthma, eczema and rhinitis. PloS One. 2017;12:E0179125.
- Deckers IA, McLean S, Linssen S, et al. Investigating international time trends in the incidence and prevalence of atopic eczema 1990-2010: a systematic review of epidemiological studies. PloS One. 2012;7:E39803.
- Williams H, Stewart A, von Mutius E, et al. Is eczema really on the increase worldwide? J Allergy Clin Immunol. 2008;121:947-954.
- Sullivan M, Silverberg NB. Current and emerging concepts in atopic dermatitis pathogenesis. Clin Dermatol. 2017;35:349-353.
- Best KP, Gold M, Kennedy D, et al. Omega-3 long-chain PUFA intake during pregnancy and allergic disease outcomes in the offspring: a systematic review and meta-analysis of observational studies and randomized controlled trials. Am J Clin Nutr. 2016;103:128-143.
- Zulyniak MA, de Souza RJ, Shaikh M, et al. Ethnic differences in maternal diet in pregnancy and infant eczema. PloS One. 2020;15:E0232170.
- Jena PK, Sheng L, Mcneil K, et al. Long-term Western diet intake leads to dysregulated bile acid signaling and dermatitis with Th2 and Th17 pathway features in mice. J Dermatol Sci. 2019;95:13-20.
- Grieger JA, Clifton VL, Tuck AR, et al. In utero programming of allergic susceptibility. Int Arch Allergy Immunol. 2016;169:80-92. doi:10.1159/000443961
- Khan TK, Palmer DJ, Prescott SL. In-utero exposures and the evolving epidemiology of paediatric allergy. Curr Opin Allergy Clin Immunol. 2015;15:402-408. doi:10.1097/ACI.0000000000000209
- Bauer SM. Atopic eczema: genetic associations and potential links to developmental exposures. Int J Toxicol. 2017;36:187-198.
- Shinohara M, Saito H, Matsumoto K. Different timings of prenatal or postnatal tobacco smoke exposure have different effects on the development of atopic eczema/dermatitis syndrome (AEDS) during infancy. J Allergy Clin Immunol. 2012;129:AB40.
- Lerodiakonou D, Garcia-Larsen V, Logan A, et al. Timing of allergenic food introduction to the infant diet and risk of allergic or autoimmune disease: a systematic review and meta-analysis. JAMA. 2016;316:1181-1192.
- Du Toit G, Roberts G, Sayre PH, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803-813.
- Kramer MS, Kakuma R. Maternal dietary antigen avoidance during pregnancy or lactation, or both, for preventing or treating atopic disease in the child. Evid Based Child Health. 2014;9:447-483.
- Garcia-Larsen V, Ierodiakonou D, Jarrold K, et al. Diet during pregnancy and infancy and risk of allergic or autoimmune disease: a systematic review and meta-analysis. PLoS Med. 2018;15:E1002507.
- Greer FR, Sicherer SH, Burks AW; Committee on Nutrition, Section on Allergy and Immunology. The effects of early nutritional interventions on the development of atopic disease in infants and children: the role of maternal dietary restriction, breastfeeding, timing of introduction of complementary foods, and hydrolyzed formulas. Pediatrics. 2019;143:e20190281.
- Baquerizo Nole KL, Yim E, Keri JE. Probiotics and prebiotics in dermatology. J Am Acad Dermatol. 2014;71:814-821.
- Schultz M, Göttl C, Young RJ, et al. Administration of oral probiotic bacteria to pregnant women causes temporary infantile colonization. J Pediatr Gastroenterol Nutr. 2004;38:293-297.
- Lee J, Seto D, Bielory L. Meta-analysis of clinical trials of probiotics for prevention and treatment of pediatric atopic dermatitis. J Allergy Clin Immunol. 2008;121:116-121.
- Panduru M, Panduru NM, Sa˘la˘va˘stru CM, et al. Probiotics and primary prevention of atopic dermatitis: a meta‐analysis of randomized controlled studies. J Eur Acad Dermatol Venereol. 2015;29:232-242.
- Doege K, Grajecki D, Zyriax BC, et al. Impact of maternal supplementation with probiotics during pregnancy on atopic eczema in childhood—a meta-analysis. Br J Nutr. 2012;107:1-6.
- Zuccotti G, Meneghin F, Aceti A, et al. Probiotics for prevention of atopic diseases in infants: systematic review and meta‐analysis. Allergy. 2015;70:1356-1371.
- Seaton A, Godden DJ, Brown K. Increase in asthma: a more toxic environment or a more susceptible population? Thorax. 1994;49:171-174.
- Manzel A, Muller DN, Hafler DA, et al. Role of “Western diet” in inflammatory autoimmune diseases. Curr Allergy Asthma Rep. 2014;14:1-8.
- Li-Weber M, Giasisi M, Trieber MK, et al. Vitamin E inhibits IL-4 gene expression in peripheral blood T cells. Eur J Immunol. 2002;32:2401-2408.
- Sehra S, Yao Y, Howell MD, et al. IL-4 regulates skin homeostasis and the predisposition toward allergic skin inflammation. J Immunol. 2010;184:3186-3190.
- West CE, Dunstan J, McCarthy S, et al. Associations between maternal antioxidant intakes in pregnancy and infant allergic outcomes. Nutrients. 2012;4:1747-1758.
- Miyake Y, Sasaki S, Tanaka K, et al. Consumption of vegetables, fruit, and antioxidants during pregnancy and wheeze and eczema in infants. Allergy. 2010;65:758-765.
- Martindale S, McNeill G, Devereux G, et al. Antioxidant intake in pregnancy in relation to wheeze and eczema in the first two years of life. Am J Respir Crit Care Med. 2005;171:121-128.
- Robison R, Kumar R. The effect of prenatal and postnatal dietary exposures on childhood development of atopic disease. Curr Opin Allergy Clin Immunol. 2010;10:139-144.
- Berdnikovs S, Abdala-Valencia H, McCary C, et al. Isoforms of vitamin E have opposing immunoregulatory functions during inflammation by regulating leukocyte recruitment. J Immunol. 2009;182:4395-4405.
- Beckhaus AA, Garcia‐Marcos L, Forno E, et al. Maternal nutrition during pregnancy and risk of asthma, wheeze, and atopic diseases during childhood: a systematic review and meta‐analysis. Allergy. 2015;70:1588-1604.
- Calder PC, Miles EA. Fatty acids and atopic disease. Pediatr Allergy Immunol. 2000;11(suppl 13):29-36.
- Prescott S, Macaubas C, Holt B, et al. Transplacental priming of the human immune system to environmental allergens: universal skewing of initial T-cell responses towards Th-2 cytokine profile. J Immunol. 1998;160:4730-4737.
- Dunstan JA, Mori TA, Barden A, et al. Fish oil supplementation in pregnancy modifies neonatal allergen-specific immune responses and clinical outcomes in infants at high risk of atopy: a randomized, controlled trial. J Allergy Clin Immunol. 2003;112:1178-1184.
- Furuhjelm C, Warstedt K, Fagerås M, et al. Allergic disease in infants up to 2 years of age in relation to plasma omega‐3 fatty acids and maternal fish oil supplementation in pregnancy and lactation. Pediatr Allergy Immunol. 2011;22:505-514.
- Sausenthaler S, Koletzko S, Schaaf B, et al; LISA Study Group. Maternal diet during pregnancy in relation to eczema and allergic sensitization in the offspring at 2 y of age. Am J Clin Nutr. 2007;85:530-537.
- Best KP, Sullivan TR, Palmer DJ, et al. Prenatal omega-3 LCPUFA and symptoms of allergic disease and sensitization throughout early childhood—a longitudinal analysis of long-term follow-up of a randomized controlled trial. World Allergy Organ J. 2018;11:10.
- Jacobs DR Jr, Steffen LM. Nutrients, foods, and dietary patterns as exposures in research: a framework for food synergy. Am J Clin Nutr. 2003;78:508-513.
- Chatzi L, Torrent M, Romieu I, et al. Mediterranean diet in pregnancy is protective for wheeze and atopy in childhood. Thorax. 2008;63:507-513.
- Chatzi L, Garcia R, Roumeliotaki T, et al. Mediterranean diet adherence during pregnancy and risk of wheeze and eczema in the first year of life: INMA (Spain) and RHEA (Greece) mother-child cohort studies. Br J Nutr. 2013;110:2058-2068.
- Zeng J, Wu W, Chen Y, et al. Maternal dietary protein patterns during pregnancy and the risk of infant eczema: a cohort study. Front Nutr. 2021;8:294.
- Miyake Y, Okubo H, Sasaki S, et al. Maternal dietary patterns during pregnancy and risk of wheeze and eczema in Japanese infants aged 16–24 months: the Osaka Maternal and Child Health Study. Pediatr Allergy Immunol. 2011;22:734-741.
Atopic dermatitis (AD) is an inflammatory skin disease characterized by skin barrier disruption, skin inflammation, and pruritus.1 It is a common and often chronic skin condition associated with the development of food allergies, asthma, and allergic rhinitis, known as the atopic march.2 Atopic dermatitis is estimated to affect 10% to 25% of children, most with onset before 5 years of age, and up to 7% of adults worldwide.3 Most patients improve with time, but multiple disease trajectories are possible. Several studies have demonstrated that fewer than 4% of children develop the classic atopic march—AD followed by food allergies, asthma, and finally allergic rhinitis—with recent evidence pointing to a more complex heterogeneous progression of disease and allergic comorbidities often occurring together.4,5 The prevalence of AD has been increasing globally over the last 30 years,6 with a marked increase in developed countries.6,7 It is well accepted that AD is based on an interplay between genetic predisposition and environmental factors,8 but many suspect that the rapid rise in prevalence cannot be attributed to genetic factors alone.9 The precipitant triggers for AD remain an area of intense investigation, with ongoing debate between the “inside out” and “outside in” hypotheses; these revolve around whether abnormalities in the immune system trigger barrier dysfunction or barrier dysfunction triggers immune programming to atopy.8 Ongoing research related to genetic predisposition of AD has identified candidate genes implicated in both impaired skin barrier function and altered immune system pathways, further supporting that both theories may contribute to disease pathogenesis.
The increasing prevalence of AD, with increasing disease burden within socioeconomically advantaged countries, raises the possibility of early modifiable environmental factors that may contribute to the disease process.10 Many studies point to the influence of the 21st century lifestyle and Western diet as primary contributing factors.9,11 However, it is not clear how these factors may influence the development of allergic atopic disease. Several studies have suggested that nonheritable influences in utero can alter fetus immune function and influence the subsequent development of allergic disease.12,13 Although many studies have examined environmental factors contributing to the development of AD in infancy and childhood, less is understood about the influence of prenatal factors. Currently, in utero exposure to tobacco smoke, phthalates, and maternal distress have been potentially implicated in the development of AD.14,15 Several studies have examined the role of maternal diet and nutrition on the development of AD in offspring; however, formal recommendations and robust trial data are lacking. In this article, we examine the existing literature surrounding maternal diet on the development of AD in infancy and childhood.
Allergen Avoidance
Extrapolating from the food allergy literature, it was once suggested that allergen avoidance in early childhood had a protective effect on the subsequent development of allergies; however, more recent research has found that early exposure to common food allergens, such as peanuts or eggs, may actually reduce a child’s risk for developing these allergies later in life.16 Among infants at high risk for food allergy, sustained consumption of peanut products beginning in the first 11 months of life resulted in an 81% lower rate of peanut allergy at 60 months of age than the rate among children who avoided peanuts.17 Given the results that antigen avoidance during infancy/childhood does not protect against the development of allergies and may actually be counterproductive, it is not surprising that research studying antigen avoidance during pregnancy on the development of AD also has demonstrated limited efficacy. A systematic review of 5 trials on maternal dietary antigen avoidance (N=952) suggested no protective effects of avoiding antigenic foods during pregnancy on the development of AD in the first 18 months of life.18 Another meta-analysis evaluating 12 intervention trials looked at the effects of maternal allergenic food avoidance during pregnancy or lactation and found no reduced risk for subsequent development of allergic disease, including AD.19 The American Academy of Pediatrics 2019 consensus statement does not support maternal dietary restrictions in pregnancy for the prevention of atopic disease and makes note that the data remain limited, which complicates drawing any firm conclusions.20
Probiotic Supplementation
One of the most investigated dietary supplements for the prevention of atopic disease is probiotics, with possible benefits noted in both the prenatal and postnatal periods. Baquerizo Nole et al21 examined several studies looking at the various benefits of probiotics in AD, which included inhibition of the helper T cell (TH2) response, stimulation of the TH1 response, upregulation of regulatory T cells, acceleration of skin and mucosal barrier function, increase in intestinal microflora diversity, suppression of toxic fermentation products in the intestinal lumen from increased production of short-chain fatty acids, and inhibition of Staphylococcus aureus attachment on epidermal keratinocytes. It is unclear how this may affect infants prenatally; however, transfer of maternal intestinal microflora during delivery and shortly thereafter has demonstrated that probiotic strains remain detectable in the infant’s stool up to 6 months after delivery, even if the mother has discontinued use.22 A 2008 meta-analysis of 10 double-bind, randomized, controlled trials (N=1880) looking at the use of maternal prenatal and postnatal probiotic supplementation in the prevention of pediatric AD found a relative risk (RR) ratio of 0.69 (95% CI, 0.57-0.83) using a fixed effects model and RR ratio of 0.66 (95% CI, 0.49-0.89) using a random effects model. After exclusion of one study that evaluated the effect of postnatal probiotic supplementation only, the RR ratio decreased to 0.61 for both the fixed effects and random effects models.23 A systematic review by Panduru et al24 noted similar findings with a subgroup meta-analysis of 11 studies of prenatal supplementation followed by postnatal supplementation of probiotics, which demonstrated a protective effect on the development of AD (odds ratio [OR]=0.61, P<.001). Postnatal supplementation alone (4 studies) did not have the same association (OR=0.95, P<.82).24 A 2012 meta-analysis by Doege et al25 evaluated 7 randomized, double-blinded, placebo-controlled trials that assessed probiotic supplementation during pregnancy (without incorporation of postnatal supplementation) and found a significant risk reduction of 5.7% (P=.022) for AD in children aged 2 to 7 years. Interestingly, this was only significant for Lactobacillus and not for other bacterial strains, even if a mixture of strains included Lactobacillus. However, Panduru et al24 found both maternal Lactobacillus supplementation alone (8 studies) and in combination with Bifidobacterium (9 studies) was protective against AD development in children (OR=0.70, P=.004; OR=0.62, P<.001). A more recent 2015 meta-analysis of 17 studies (N=4755) evaluating the use of maternal probiotic supplementation in pregnancy and/or through the infant’s first 3 months of life found a significantly lower RR (0.78 [95% CI, 0.69-0.89], P=.0003) for the development of AD in infants treated with probiotics and found this risk to be even further decreased when a mixture of probiotics including both Lactobacillus and Bifidobacterium was used (RR=0.54 [95% CI, 0.43-0.68], P<.00001).26
Antioxidants
The Westernization of many developing countries’ diets—diets high in saturated fats, protein, sucrose, salt, and processed foods and low in fresh fruits and green vegetables—has led to a reduced intake of antioxidants and an increase in susceptibility to oxidative damage.27,28 One hypothesis suggests that a reduction in nutritional antioxidants and subsequent oxidative damage leads to airway inflammation that may contribute to an increased prevalence of asthma.27 In vitro data suggest that antioxidant deficiency may influence the differentiation of helper T cells to a TH2 phenotype, which can increase susceptibility to the development of asthma and allergies.29 Vitamin E specifically has been shown to inhibit IL-4 gene expression, which drives type 2 immunity and decreases expression of multiple genes that regulate epidermal barrier function, subsequently increasing susceptibility to allergic inflammation and AD.29,30 Regardless of the proposed mechanisms for antioxidant deficiency increasing susceptibility to allergic disease, studies evaluating the benefits of antioxidant intake during pregnancy in relation to AD have not been promising. Several studies have found no association between prenatal vitamin E intake and the risk for AD development in infants and children.31,32 Another study found a statistically significant inverse relationship between vitamin E intake in mothers with a history of atopy and the development of AD in their children at 2 years of age but not at 1 year of age (P-trend=.024).33 It has been suggested that varying vitamin E isoforms may contribute to the discrepant results previously discussed, with the γ-tocopherol isoform (found frequently in Westernized diets)34 as a driver of inflammation in murine models.35 West et al31 noted an association between vitamin C intake and development of “any allergic disease”—AD, IgE-mediated food allergy, or asthma—with a crude OR of 0.48 (95% CI, 0.25-0.93). However, the P-trend and adjusted OR were not statistically significant. The investigators found no association between maternal intake of beta-carotene, vitamin E, or zinc, but they did find copper supplementation to be protective on the development of AD at 1 year of age (P-trend=0.03). Interestingly, when the data for total antioxidant intake—vitamin C, vitamin E, zinc, beta-carotene, and copper from both diet and supplementation—were combined and analyzed, no statistically significant associations for any of the antioxidants were found.31 Another study of 763 Japanese mother-child pairs found a reduced risk for AD at 16 to 24 months of age with high maternal intake of beta-carotene but found no statistically significant exposure-response associations with other antioxidants, including alpha-carotene, vitamin C, or zinc from dietary intake alone.32 These results were substantiated by 2 meta-analyses evaluating a total of 93 combined intervention trials and cohorts where no association was found between vitamin or mineral intake during pregnancy and/or during infancy and the development of AD.19,36
Fatty Acids
Other dietary changes that are associated with an increased prevalence of atopic diseases in children include excess consumption of omega-6 (n-6) long-chain polyunsaturated fatty acids (LC-PUFA) and insufficient omega-3 (n-3) LC-PUFA consumption.37 Given prior evidence that allergic immune responses in infants may be primed before birth,38 researchers have questioned whether the anti-inflammatory properties of n-3 LC-PUFA when supplemented during pregnancy may have immunomodulatory effects on infants that could alter their predisposition to develop allergic disease, including AD.39 A systematic review and meta-analysis of randomized controlled trials found a statistically significant RR of 0.53 (95% CI, 0.35-0.81; P=.004) for the incidence of AD at 12 months of age with maternal supplementation of n-3 LC-PUFA.9 Another trial of 145 pregnant women randomized to supplementation with fish oil vs placebo starting at gestational week 25 and continuing through 3.5 months of breastfeeding found a reduced cumulative incidence of AD in the intervention group compared to controls at 2 years of age, with a statistically significant crude OR of 0.33 (95% CI, 0.11-0.97; P=.04).40 However, the adjusted OR was not statistically significant. In addition, they found that mothers and infants with higher proportions of docosahexaenoic acid and eicosapentaenoic acid in plasma phospholipids have been noted to have a lower prevalence of IgE-associated disease in a dose-dependent manner (P<.05 and P<.05, respectively).40 In another trial of 98 pregnant women randomized to fish oil supplementation or placebo from 20 weeks’ gestation to delivery found no difference in the frequency of AD but did note that infants in the exposure group had significantly less severe AD compared to controls (OR=0.09 [95% CI, 0.1-0.94]; P=.045).39 A prospective birth cohort study of 2641 children evaluated dietary composition during the last 4 weeks of pregnancy and found that consumption of foods rich in n-6 LC-PUFAs (eg, margarine, vegetable oil) increased the risk for developing AD, while foods rich in n-3 LC-PUFAs (eg, fish) decreased the risk for developing AD in offspring at 2 years of age. All P values for margarine, vegetable oil, and fish were statistically significant on logistic regression at P<.05.41 A longitudinal analysis of follow-up data from a randomized controlled trial looking at maternal prenatal n-3 LC-PUFA intake and the development of allergic disease (including AD) found no differences in the development of disease at 1-, 3-, or 6-year follow-up.42 Despite several studies demonstrating a possible benefit of omega-3 fatty acid intake on the development of AD in offspring, the longitudinal analysis by Best et al42 reminds us that long-term follow-up is critical in establishing benefit of any intervention given the heterogeneous and progressive nature of the atopic march and AD.
Specific Diets
Several studies have evaluated the role of dietary patterns and their influence on atopic disease. Studies evaluating dietary patterns or supplement intake can be challenging, as data often are derived from questionnaires with bias in response to families with higher socioeconomic status.9 Further, analysis of any one food group does not account for the potential interplay between nutrients.43 Studies should focus more on dietary patterns vs individual foods to assess true risk.43,44 Given these limitations, study results on diet should be carefully scrutinized; however, there are still some positive findings that deserve further investigation. Chatzi et al44 followed 460 children for 6.5 years and found a protective effect for the development of atopy in the offspring of women who had high adherence to the Mediterranean diet (OR 0.55 [95% CI, 0.31-0.97]). Another cohort study evaluating the effects of the Mediterranean diet and risk for AD in the first year of life in 2516 mother-child pairs from Spain and Greece found no statistically significant association with consumption of the Mediterranean diet and AD. The investigators also evaluated intake of fruits, nuts, vegetables, meats, processed meats, dairy products, and cereal and found no statistically significant protective benefit.45 Another systematic review of more than 90 observational studies identified no significant relationship between prenatal dietary exposures of fruits, vegetables, nuts, fat, fatty acids, eggs, cereal, milk, alcohol, tea, or coffee and risk for allergic disease in offspring, including AD.19
A Chinese prospective cohort study evaluated the dietary protein patterns of 713 mother-child pairs and the incidence of infant AD at 6 months of age.46 Dietary protein patterns were characterized as predominantly poultry, plant based, dairy and eggs, and red meat and fish. The investigators found a statistically significant reduced risk for AD in mothers who consumed plant-based or dairy and eggs protein patterns when compared to a poultry protein pattern with an adjusted OR of 0.572 (95% CI, 0.330-0.992) and 0.478 (95% CI, 0.274-0.837), respectively. This protective effect was not seen with the red meat and fish protein patterns.46 Similar results were seen in a 2020 Canadian study that evaluated the effects of a Western (fats, meats, processed foods, and starchy vegetables), balanced (diverse sources of animal proteins [especially fish], fruits, vegetables, nuts, and seeds), or plant-based (dairy, legumes, vegetables, whole grains, and an aversion to meats) diet in more than 2000 mother-infant pairs from 24 to 28 weeks’ gestation to 1 year of age. The investigators found a lower OR of AD in mothers who followed a mostly plant-based diet compared to other dietary patterns (OR 0.65 [95% CI, 0.55-0.76]; P<.001).10 Another prospective Japanese study looking at healthy (high intake of green and yellow vegetables, seaweed, mushrooms, white vegetables, pulses, potatoes, fish, sea products, fruit, and shellfish, and low intake of confectioneries and soft drinks), Western (high intake of vegetable oil, salt-containing seasonings, beef, pork, processed meat, eggs, chicken, and white vegetables, and low intake of fruit, soft drinks, and confectioneries), or Japanese (high intake of rice, miso soup, sea products, and fish, and low intake of bread, confectioneries, and dairy products) dietary patterns in 763 mother-child pairs found no association between diet during pregnancy and development of AD in offspring at 16 to 24 months.47 Unfortunately, a longitudinal data analysis has not been performed for this study.
Final Thoughts
Atopic dermatitis is a complex, progressive, and heterogeneous disease with both genetic and environmental influences. Studying the effects of diet on the development, progression, or severity of disease can be very difficult due to the heterogeneity of study designs, lack of long-term follow-up, and high potential for residual confounding. Studies evaluating dietary patterns or supplement intake can be equally challenging, as data often are derived from questionnaires with bias in response to families with higher socioeconomic status.9 Very few studies have looked specifically at maternal dietary composition and the development of AD alone (without inclusion of asthma or food allergy). Ultimately, the inconsistency of the data makes it difficult to draw conclusions and make formal recommendations for this vulnerable population. Additional evidence from well-powered trials with comparable methodology and objective outcome measures will be imperative to make formal recommendations. In addition, longitudinal follow-up will be essential to determine long-term benefit and influence on the atopic march.
Atopic dermatitis (AD) is an inflammatory skin disease characterized by skin barrier disruption, skin inflammation, and pruritus.1 It is a common and often chronic skin condition associated with the development of food allergies, asthma, and allergic rhinitis, known as the atopic march.2 Atopic dermatitis is estimated to affect 10% to 25% of children, most with onset before 5 years of age, and up to 7% of adults worldwide.3 Most patients improve with time, but multiple disease trajectories are possible. Several studies have demonstrated that fewer than 4% of children develop the classic atopic march—AD followed by food allergies, asthma, and finally allergic rhinitis—with recent evidence pointing to a more complex heterogeneous progression of disease and allergic comorbidities often occurring together.4,5 The prevalence of AD has been increasing globally over the last 30 years,6 with a marked increase in developed countries.6,7 It is well accepted that AD is based on an interplay between genetic predisposition and environmental factors,8 but many suspect that the rapid rise in prevalence cannot be attributed to genetic factors alone.9 The precipitant triggers for AD remain an area of intense investigation, with ongoing debate between the “inside out” and “outside in” hypotheses; these revolve around whether abnormalities in the immune system trigger barrier dysfunction or barrier dysfunction triggers immune programming to atopy.8 Ongoing research related to genetic predisposition of AD has identified candidate genes implicated in both impaired skin barrier function and altered immune system pathways, further supporting that both theories may contribute to disease pathogenesis.
The increasing prevalence of AD, with increasing disease burden within socioeconomically advantaged countries, raises the possibility of early modifiable environmental factors that may contribute to the disease process.10 Many studies point to the influence of the 21st century lifestyle and Western diet as primary contributing factors.9,11 However, it is not clear how these factors may influence the development of allergic atopic disease. Several studies have suggested that nonheritable influences in utero can alter fetus immune function and influence the subsequent development of allergic disease.12,13 Although many studies have examined environmental factors contributing to the development of AD in infancy and childhood, less is understood about the influence of prenatal factors. Currently, in utero exposure to tobacco smoke, phthalates, and maternal distress have been potentially implicated in the development of AD.14,15 Several studies have examined the role of maternal diet and nutrition on the development of AD in offspring; however, formal recommendations and robust trial data are lacking. In this article, we examine the existing literature surrounding maternal diet on the development of AD in infancy and childhood.
Allergen Avoidance
Extrapolating from the food allergy literature, it was once suggested that allergen avoidance in early childhood had a protective effect on the subsequent development of allergies; however, more recent research has found that early exposure to common food allergens, such as peanuts or eggs, may actually reduce a child’s risk for developing these allergies later in life.16 Among infants at high risk for food allergy, sustained consumption of peanut products beginning in the first 11 months of life resulted in an 81% lower rate of peanut allergy at 60 months of age than the rate among children who avoided peanuts.17 Given the results that antigen avoidance during infancy/childhood does not protect against the development of allergies and may actually be counterproductive, it is not surprising that research studying antigen avoidance during pregnancy on the development of AD also has demonstrated limited efficacy. A systematic review of 5 trials on maternal dietary antigen avoidance (N=952) suggested no protective effects of avoiding antigenic foods during pregnancy on the development of AD in the first 18 months of life.18 Another meta-analysis evaluating 12 intervention trials looked at the effects of maternal allergenic food avoidance during pregnancy or lactation and found no reduced risk for subsequent development of allergic disease, including AD.19 The American Academy of Pediatrics 2019 consensus statement does not support maternal dietary restrictions in pregnancy for the prevention of atopic disease and makes note that the data remain limited, which complicates drawing any firm conclusions.20
Probiotic Supplementation
One of the most investigated dietary supplements for the prevention of atopic disease is probiotics, with possible benefits noted in both the prenatal and postnatal periods. Baquerizo Nole et al21 examined several studies looking at the various benefits of probiotics in AD, which included inhibition of the helper T cell (TH2) response, stimulation of the TH1 response, upregulation of regulatory T cells, acceleration of skin and mucosal barrier function, increase in intestinal microflora diversity, suppression of toxic fermentation products in the intestinal lumen from increased production of short-chain fatty acids, and inhibition of Staphylococcus aureus attachment on epidermal keratinocytes. It is unclear how this may affect infants prenatally; however, transfer of maternal intestinal microflora during delivery and shortly thereafter has demonstrated that probiotic strains remain detectable in the infant’s stool up to 6 months after delivery, even if the mother has discontinued use.22 A 2008 meta-analysis of 10 double-bind, randomized, controlled trials (N=1880) looking at the use of maternal prenatal and postnatal probiotic supplementation in the prevention of pediatric AD found a relative risk (RR) ratio of 0.69 (95% CI, 0.57-0.83) using a fixed effects model and RR ratio of 0.66 (95% CI, 0.49-0.89) using a random effects model. After exclusion of one study that evaluated the effect of postnatal probiotic supplementation only, the RR ratio decreased to 0.61 for both the fixed effects and random effects models.23 A systematic review by Panduru et al24 noted similar findings with a subgroup meta-analysis of 11 studies of prenatal supplementation followed by postnatal supplementation of probiotics, which demonstrated a protective effect on the development of AD (odds ratio [OR]=0.61, P<.001). Postnatal supplementation alone (4 studies) did not have the same association (OR=0.95, P<.82).24 A 2012 meta-analysis by Doege et al25 evaluated 7 randomized, double-blinded, placebo-controlled trials that assessed probiotic supplementation during pregnancy (without incorporation of postnatal supplementation) and found a significant risk reduction of 5.7% (P=.022) for AD in children aged 2 to 7 years. Interestingly, this was only significant for Lactobacillus and not for other bacterial strains, even if a mixture of strains included Lactobacillus. However, Panduru et al24 found both maternal Lactobacillus supplementation alone (8 studies) and in combination with Bifidobacterium (9 studies) was protective against AD development in children (OR=0.70, P=.004; OR=0.62, P<.001). A more recent 2015 meta-analysis of 17 studies (N=4755) evaluating the use of maternal probiotic supplementation in pregnancy and/or through the infant’s first 3 months of life found a significantly lower RR (0.78 [95% CI, 0.69-0.89], P=.0003) for the development of AD in infants treated with probiotics and found this risk to be even further decreased when a mixture of probiotics including both Lactobacillus and Bifidobacterium was used (RR=0.54 [95% CI, 0.43-0.68], P<.00001).26
Antioxidants
The Westernization of many developing countries’ diets—diets high in saturated fats, protein, sucrose, salt, and processed foods and low in fresh fruits and green vegetables—has led to a reduced intake of antioxidants and an increase in susceptibility to oxidative damage.27,28 One hypothesis suggests that a reduction in nutritional antioxidants and subsequent oxidative damage leads to airway inflammation that may contribute to an increased prevalence of asthma.27 In vitro data suggest that antioxidant deficiency may influence the differentiation of helper T cells to a TH2 phenotype, which can increase susceptibility to the development of asthma and allergies.29 Vitamin E specifically has been shown to inhibit IL-4 gene expression, which drives type 2 immunity and decreases expression of multiple genes that regulate epidermal barrier function, subsequently increasing susceptibility to allergic inflammation and AD.29,30 Regardless of the proposed mechanisms for antioxidant deficiency increasing susceptibility to allergic disease, studies evaluating the benefits of antioxidant intake during pregnancy in relation to AD have not been promising. Several studies have found no association between prenatal vitamin E intake and the risk for AD development in infants and children.31,32 Another study found a statistically significant inverse relationship between vitamin E intake in mothers with a history of atopy and the development of AD in their children at 2 years of age but not at 1 year of age (P-trend=.024).33 It has been suggested that varying vitamin E isoforms may contribute to the discrepant results previously discussed, with the γ-tocopherol isoform (found frequently in Westernized diets)34 as a driver of inflammation in murine models.35 West et al31 noted an association between vitamin C intake and development of “any allergic disease”—AD, IgE-mediated food allergy, or asthma—with a crude OR of 0.48 (95% CI, 0.25-0.93). However, the P-trend and adjusted OR were not statistically significant. The investigators found no association between maternal intake of beta-carotene, vitamin E, or zinc, but they did find copper supplementation to be protective on the development of AD at 1 year of age (P-trend=0.03). Interestingly, when the data for total antioxidant intake—vitamin C, vitamin E, zinc, beta-carotene, and copper from both diet and supplementation—were combined and analyzed, no statistically significant associations for any of the antioxidants were found.31 Another study of 763 Japanese mother-child pairs found a reduced risk for AD at 16 to 24 months of age with high maternal intake of beta-carotene but found no statistically significant exposure-response associations with other antioxidants, including alpha-carotene, vitamin C, or zinc from dietary intake alone.32 These results were substantiated by 2 meta-analyses evaluating a total of 93 combined intervention trials and cohorts where no association was found between vitamin or mineral intake during pregnancy and/or during infancy and the development of AD.19,36
Fatty Acids
Other dietary changes that are associated with an increased prevalence of atopic diseases in children include excess consumption of omega-6 (n-6) long-chain polyunsaturated fatty acids (LC-PUFA) and insufficient omega-3 (n-3) LC-PUFA consumption.37 Given prior evidence that allergic immune responses in infants may be primed before birth,38 researchers have questioned whether the anti-inflammatory properties of n-3 LC-PUFA when supplemented during pregnancy may have immunomodulatory effects on infants that could alter their predisposition to develop allergic disease, including AD.39 A systematic review and meta-analysis of randomized controlled trials found a statistically significant RR of 0.53 (95% CI, 0.35-0.81; P=.004) for the incidence of AD at 12 months of age with maternal supplementation of n-3 LC-PUFA.9 Another trial of 145 pregnant women randomized to supplementation with fish oil vs placebo starting at gestational week 25 and continuing through 3.5 months of breastfeeding found a reduced cumulative incidence of AD in the intervention group compared to controls at 2 years of age, with a statistically significant crude OR of 0.33 (95% CI, 0.11-0.97; P=.04).40 However, the adjusted OR was not statistically significant. In addition, they found that mothers and infants with higher proportions of docosahexaenoic acid and eicosapentaenoic acid in plasma phospholipids have been noted to have a lower prevalence of IgE-associated disease in a dose-dependent manner (P<.05 and P<.05, respectively).40 In another trial of 98 pregnant women randomized to fish oil supplementation or placebo from 20 weeks’ gestation to delivery found no difference in the frequency of AD but did note that infants in the exposure group had significantly less severe AD compared to controls (OR=0.09 [95% CI, 0.1-0.94]; P=.045).39 A prospective birth cohort study of 2641 children evaluated dietary composition during the last 4 weeks of pregnancy and found that consumption of foods rich in n-6 LC-PUFAs (eg, margarine, vegetable oil) increased the risk for developing AD, while foods rich in n-3 LC-PUFAs (eg, fish) decreased the risk for developing AD in offspring at 2 years of age. All P values for margarine, vegetable oil, and fish were statistically significant on logistic regression at P<.05.41 A longitudinal analysis of follow-up data from a randomized controlled trial looking at maternal prenatal n-3 LC-PUFA intake and the development of allergic disease (including AD) found no differences in the development of disease at 1-, 3-, or 6-year follow-up.42 Despite several studies demonstrating a possible benefit of omega-3 fatty acid intake on the development of AD in offspring, the longitudinal analysis by Best et al42 reminds us that long-term follow-up is critical in establishing benefit of any intervention given the heterogeneous and progressive nature of the atopic march and AD.
Specific Diets
Several studies have evaluated the role of dietary patterns and their influence on atopic disease. Studies evaluating dietary patterns or supplement intake can be challenging, as data often are derived from questionnaires with bias in response to families with higher socioeconomic status.9 Further, analysis of any one food group does not account for the potential interplay between nutrients.43 Studies should focus more on dietary patterns vs individual foods to assess true risk.43,44 Given these limitations, study results on diet should be carefully scrutinized; however, there are still some positive findings that deserve further investigation. Chatzi et al44 followed 460 children for 6.5 years and found a protective effect for the development of atopy in the offspring of women who had high adherence to the Mediterranean diet (OR 0.55 [95% CI, 0.31-0.97]). Another cohort study evaluating the effects of the Mediterranean diet and risk for AD in the first year of life in 2516 mother-child pairs from Spain and Greece found no statistically significant association with consumption of the Mediterranean diet and AD. The investigators also evaluated intake of fruits, nuts, vegetables, meats, processed meats, dairy products, and cereal and found no statistically significant protective benefit.45 Another systematic review of more than 90 observational studies identified no significant relationship between prenatal dietary exposures of fruits, vegetables, nuts, fat, fatty acids, eggs, cereal, milk, alcohol, tea, or coffee and risk for allergic disease in offspring, including AD.19
A Chinese prospective cohort study evaluated the dietary protein patterns of 713 mother-child pairs and the incidence of infant AD at 6 months of age.46 Dietary protein patterns were characterized as predominantly poultry, plant based, dairy and eggs, and red meat and fish. The investigators found a statistically significant reduced risk for AD in mothers who consumed plant-based or dairy and eggs protein patterns when compared to a poultry protein pattern with an adjusted OR of 0.572 (95% CI, 0.330-0.992) and 0.478 (95% CI, 0.274-0.837), respectively. This protective effect was not seen with the red meat and fish protein patterns.46 Similar results were seen in a 2020 Canadian study that evaluated the effects of a Western (fats, meats, processed foods, and starchy vegetables), balanced (diverse sources of animal proteins [especially fish], fruits, vegetables, nuts, and seeds), or plant-based (dairy, legumes, vegetables, whole grains, and an aversion to meats) diet in more than 2000 mother-infant pairs from 24 to 28 weeks’ gestation to 1 year of age. The investigators found a lower OR of AD in mothers who followed a mostly plant-based diet compared to other dietary patterns (OR 0.65 [95% CI, 0.55-0.76]; P<.001).10 Another prospective Japanese study looking at healthy (high intake of green and yellow vegetables, seaweed, mushrooms, white vegetables, pulses, potatoes, fish, sea products, fruit, and shellfish, and low intake of confectioneries and soft drinks), Western (high intake of vegetable oil, salt-containing seasonings, beef, pork, processed meat, eggs, chicken, and white vegetables, and low intake of fruit, soft drinks, and confectioneries), or Japanese (high intake of rice, miso soup, sea products, and fish, and low intake of bread, confectioneries, and dairy products) dietary patterns in 763 mother-child pairs found no association between diet during pregnancy and development of AD in offspring at 16 to 24 months.47 Unfortunately, a longitudinal data analysis has not been performed for this study.
Final Thoughts
Atopic dermatitis is a complex, progressive, and heterogeneous disease with both genetic and environmental influences. Studying the effects of diet on the development, progression, or severity of disease can be very difficult due to the heterogeneity of study designs, lack of long-term follow-up, and high potential for residual confounding. Studies evaluating dietary patterns or supplement intake can be equally challenging, as data often are derived from questionnaires with bias in response to families with higher socioeconomic status.9 Very few studies have looked specifically at maternal dietary composition and the development of AD alone (without inclusion of asthma or food allergy). Ultimately, the inconsistency of the data makes it difficult to draw conclusions and make formal recommendations for this vulnerable population. Additional evidence from well-powered trials with comparable methodology and objective outcome measures will be imperative to make formal recommendations. In addition, longitudinal follow-up will be essential to determine long-term benefit and influence on the atopic march.
- Nutten S. Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab. 2015;66(suppl 1):8-16.
- Kapoor R, Menon C, Hoffstad O, et al. The prevalence of atopic triad in children with physician-confirmed atopic dermatitis. J Am Acad Dermatol. 2008;58:68-73.
- Abuabara K, Magyari A, McCulloch CE, et al. Prevalence of atopic eczema among patients seen in primary care: data from the Health Improvement Network. Ann Intern Med. 2019;170:354-356.
- Belgrave DC, Granell R, Simpson A, et al. Developmental profiles of eczema, wheeze, and rhinitis: two population-based birth cohort studies. PLoS Medicine. 2014;11:E1001748.
- Aguilar D, Pinart M, Koppelman GH, et al. Computational analysis of multimorbidity between asthma, eczema and rhinitis. PloS One. 2017;12:E0179125.
- Deckers IA, McLean S, Linssen S, et al. Investigating international time trends in the incidence and prevalence of atopic eczema 1990-2010: a systematic review of epidemiological studies. PloS One. 2012;7:E39803.
- Williams H, Stewart A, von Mutius E, et al. Is eczema really on the increase worldwide? J Allergy Clin Immunol. 2008;121:947-954.
- Sullivan M, Silverberg NB. Current and emerging concepts in atopic dermatitis pathogenesis. Clin Dermatol. 2017;35:349-353.
- Best KP, Gold M, Kennedy D, et al. Omega-3 long-chain PUFA intake during pregnancy and allergic disease outcomes in the offspring: a systematic review and meta-analysis of observational studies and randomized controlled trials. Am J Clin Nutr. 2016;103:128-143.
- Zulyniak MA, de Souza RJ, Shaikh M, et al. Ethnic differences in maternal diet in pregnancy and infant eczema. PloS One. 2020;15:E0232170.
- Jena PK, Sheng L, Mcneil K, et al. Long-term Western diet intake leads to dysregulated bile acid signaling and dermatitis with Th2 and Th17 pathway features in mice. J Dermatol Sci. 2019;95:13-20.
- Grieger JA, Clifton VL, Tuck AR, et al. In utero programming of allergic susceptibility. Int Arch Allergy Immunol. 2016;169:80-92. doi:10.1159/000443961
- Khan TK, Palmer DJ, Prescott SL. In-utero exposures and the evolving epidemiology of paediatric allergy. Curr Opin Allergy Clin Immunol. 2015;15:402-408. doi:10.1097/ACI.0000000000000209
- Bauer SM. Atopic eczema: genetic associations and potential links to developmental exposures. Int J Toxicol. 2017;36:187-198.
- Shinohara M, Saito H, Matsumoto K. Different timings of prenatal or postnatal tobacco smoke exposure have different effects on the development of atopic eczema/dermatitis syndrome (AEDS) during infancy. J Allergy Clin Immunol. 2012;129:AB40.
- Lerodiakonou D, Garcia-Larsen V, Logan A, et al. Timing of allergenic food introduction to the infant diet and risk of allergic or autoimmune disease: a systematic review and meta-analysis. JAMA. 2016;316:1181-1192.
- Du Toit G, Roberts G, Sayre PH, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803-813.
- Kramer MS, Kakuma R. Maternal dietary antigen avoidance during pregnancy or lactation, or both, for preventing or treating atopic disease in the child. Evid Based Child Health. 2014;9:447-483.
- Garcia-Larsen V, Ierodiakonou D, Jarrold K, et al. Diet during pregnancy and infancy and risk of allergic or autoimmune disease: a systematic review and meta-analysis. PLoS Med. 2018;15:E1002507.
- Greer FR, Sicherer SH, Burks AW; Committee on Nutrition, Section on Allergy and Immunology. The effects of early nutritional interventions on the development of atopic disease in infants and children: the role of maternal dietary restriction, breastfeeding, timing of introduction of complementary foods, and hydrolyzed formulas. Pediatrics. 2019;143:e20190281.
- Baquerizo Nole KL, Yim E, Keri JE. Probiotics and prebiotics in dermatology. J Am Acad Dermatol. 2014;71:814-821.
- Schultz M, Göttl C, Young RJ, et al. Administration of oral probiotic bacteria to pregnant women causes temporary infantile colonization. J Pediatr Gastroenterol Nutr. 2004;38:293-297.
- Lee J, Seto D, Bielory L. Meta-analysis of clinical trials of probiotics for prevention and treatment of pediatric atopic dermatitis. J Allergy Clin Immunol. 2008;121:116-121.
- Panduru M, Panduru NM, Sa˘la˘va˘stru CM, et al. Probiotics and primary prevention of atopic dermatitis: a meta‐analysis of randomized controlled studies. J Eur Acad Dermatol Venereol. 2015;29:232-242.
- Doege K, Grajecki D, Zyriax BC, et al. Impact of maternal supplementation with probiotics during pregnancy on atopic eczema in childhood—a meta-analysis. Br J Nutr. 2012;107:1-6.
- Zuccotti G, Meneghin F, Aceti A, et al. Probiotics for prevention of atopic diseases in infants: systematic review and meta‐analysis. Allergy. 2015;70:1356-1371.
- Seaton A, Godden DJ, Brown K. Increase in asthma: a more toxic environment or a more susceptible population? Thorax. 1994;49:171-174.
- Manzel A, Muller DN, Hafler DA, et al. Role of “Western diet” in inflammatory autoimmune diseases. Curr Allergy Asthma Rep. 2014;14:1-8.
- Li-Weber M, Giasisi M, Trieber MK, et al. Vitamin E inhibits IL-4 gene expression in peripheral blood T cells. Eur J Immunol. 2002;32:2401-2408.
- Sehra S, Yao Y, Howell MD, et al. IL-4 regulates skin homeostasis and the predisposition toward allergic skin inflammation. J Immunol. 2010;184:3186-3190.
- West CE, Dunstan J, McCarthy S, et al. Associations between maternal antioxidant intakes in pregnancy and infant allergic outcomes. Nutrients. 2012;4:1747-1758.
- Miyake Y, Sasaki S, Tanaka K, et al. Consumption of vegetables, fruit, and antioxidants during pregnancy and wheeze and eczema in infants. Allergy. 2010;65:758-765.
- Martindale S, McNeill G, Devereux G, et al. Antioxidant intake in pregnancy in relation to wheeze and eczema in the first two years of life. Am J Respir Crit Care Med. 2005;171:121-128.
- Robison R, Kumar R. The effect of prenatal and postnatal dietary exposures on childhood development of atopic disease. Curr Opin Allergy Clin Immunol. 2010;10:139-144.
- Berdnikovs S, Abdala-Valencia H, McCary C, et al. Isoforms of vitamin E have opposing immunoregulatory functions during inflammation by regulating leukocyte recruitment. J Immunol. 2009;182:4395-4405.
- Beckhaus AA, Garcia‐Marcos L, Forno E, et al. Maternal nutrition during pregnancy and risk of asthma, wheeze, and atopic diseases during childhood: a systematic review and meta‐analysis. Allergy. 2015;70:1588-1604.
- Calder PC, Miles EA. Fatty acids and atopic disease. Pediatr Allergy Immunol. 2000;11(suppl 13):29-36.
- Prescott S, Macaubas C, Holt B, et al. Transplacental priming of the human immune system to environmental allergens: universal skewing of initial T-cell responses towards Th-2 cytokine profile. J Immunol. 1998;160:4730-4737.
- Dunstan JA, Mori TA, Barden A, et al. Fish oil supplementation in pregnancy modifies neonatal allergen-specific immune responses and clinical outcomes in infants at high risk of atopy: a randomized, controlled trial. J Allergy Clin Immunol. 2003;112:1178-1184.
- Furuhjelm C, Warstedt K, Fagerås M, et al. Allergic disease in infants up to 2 years of age in relation to plasma omega‐3 fatty acids and maternal fish oil supplementation in pregnancy and lactation. Pediatr Allergy Immunol. 2011;22:505-514.
- Sausenthaler S, Koletzko S, Schaaf B, et al; LISA Study Group. Maternal diet during pregnancy in relation to eczema and allergic sensitization in the offspring at 2 y of age. Am J Clin Nutr. 2007;85:530-537.
- Best KP, Sullivan TR, Palmer DJ, et al. Prenatal omega-3 LCPUFA and symptoms of allergic disease and sensitization throughout early childhood—a longitudinal analysis of long-term follow-up of a randomized controlled trial. World Allergy Organ J. 2018;11:10.
- Jacobs DR Jr, Steffen LM. Nutrients, foods, and dietary patterns as exposures in research: a framework for food synergy. Am J Clin Nutr. 2003;78:508-513.
- Chatzi L, Torrent M, Romieu I, et al. Mediterranean diet in pregnancy is protective for wheeze and atopy in childhood. Thorax. 2008;63:507-513.
- Chatzi L, Garcia R, Roumeliotaki T, et al. Mediterranean diet adherence during pregnancy and risk of wheeze and eczema in the first year of life: INMA (Spain) and RHEA (Greece) mother-child cohort studies. Br J Nutr. 2013;110:2058-2068.
- Zeng J, Wu W, Chen Y, et al. Maternal dietary protein patterns during pregnancy and the risk of infant eczema: a cohort study. Front Nutr. 2021;8:294.
- Miyake Y, Okubo H, Sasaki S, et al. Maternal dietary patterns during pregnancy and risk of wheeze and eczema in Japanese infants aged 16–24 months: the Osaka Maternal and Child Health Study. Pediatr Allergy Immunol. 2011;22:734-741.
- Nutten S. Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab. 2015;66(suppl 1):8-16.
- Kapoor R, Menon C, Hoffstad O, et al. The prevalence of atopic triad in children with physician-confirmed atopic dermatitis. J Am Acad Dermatol. 2008;58:68-73.
- Abuabara K, Magyari A, McCulloch CE, et al. Prevalence of atopic eczema among patients seen in primary care: data from the Health Improvement Network. Ann Intern Med. 2019;170:354-356.
- Belgrave DC, Granell R, Simpson A, et al. Developmental profiles of eczema, wheeze, and rhinitis: two population-based birth cohort studies. PLoS Medicine. 2014;11:E1001748.
- Aguilar D, Pinart M, Koppelman GH, et al. Computational analysis of multimorbidity between asthma, eczema and rhinitis. PloS One. 2017;12:E0179125.
- Deckers IA, McLean S, Linssen S, et al. Investigating international time trends in the incidence and prevalence of atopic eczema 1990-2010: a systematic review of epidemiological studies. PloS One. 2012;7:E39803.
- Williams H, Stewart A, von Mutius E, et al. Is eczema really on the increase worldwide? J Allergy Clin Immunol. 2008;121:947-954.
- Sullivan M, Silverberg NB. Current and emerging concepts in atopic dermatitis pathogenesis. Clin Dermatol. 2017;35:349-353.
- Best KP, Gold M, Kennedy D, et al. Omega-3 long-chain PUFA intake during pregnancy and allergic disease outcomes in the offspring: a systematic review and meta-analysis of observational studies and randomized controlled trials. Am J Clin Nutr. 2016;103:128-143.
- Zulyniak MA, de Souza RJ, Shaikh M, et al. Ethnic differences in maternal diet in pregnancy and infant eczema. PloS One. 2020;15:E0232170.
- Jena PK, Sheng L, Mcneil K, et al. Long-term Western diet intake leads to dysregulated bile acid signaling and dermatitis with Th2 and Th17 pathway features in mice. J Dermatol Sci. 2019;95:13-20.
- Grieger JA, Clifton VL, Tuck AR, et al. In utero programming of allergic susceptibility. Int Arch Allergy Immunol. 2016;169:80-92. doi:10.1159/000443961
- Khan TK, Palmer DJ, Prescott SL. In-utero exposures and the evolving epidemiology of paediatric allergy. Curr Opin Allergy Clin Immunol. 2015;15:402-408. doi:10.1097/ACI.0000000000000209
- Bauer SM. Atopic eczema: genetic associations and potential links to developmental exposures. Int J Toxicol. 2017;36:187-198.
- Shinohara M, Saito H, Matsumoto K. Different timings of prenatal or postnatal tobacco smoke exposure have different effects on the development of atopic eczema/dermatitis syndrome (AEDS) during infancy. J Allergy Clin Immunol. 2012;129:AB40.
- Lerodiakonou D, Garcia-Larsen V, Logan A, et al. Timing of allergenic food introduction to the infant diet and risk of allergic or autoimmune disease: a systematic review and meta-analysis. JAMA. 2016;316:1181-1192.
- Du Toit G, Roberts G, Sayre PH, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803-813.
- Kramer MS, Kakuma R. Maternal dietary antigen avoidance during pregnancy or lactation, or both, for preventing or treating atopic disease in the child. Evid Based Child Health. 2014;9:447-483.
- Garcia-Larsen V, Ierodiakonou D, Jarrold K, et al. Diet during pregnancy and infancy and risk of allergic or autoimmune disease: a systematic review and meta-analysis. PLoS Med. 2018;15:E1002507.
- Greer FR, Sicherer SH, Burks AW; Committee on Nutrition, Section on Allergy and Immunology. The effects of early nutritional interventions on the development of atopic disease in infants and children: the role of maternal dietary restriction, breastfeeding, timing of introduction of complementary foods, and hydrolyzed formulas. Pediatrics. 2019;143:e20190281.
- Baquerizo Nole KL, Yim E, Keri JE. Probiotics and prebiotics in dermatology. J Am Acad Dermatol. 2014;71:814-821.
- Schultz M, Göttl C, Young RJ, et al. Administration of oral probiotic bacteria to pregnant women causes temporary infantile colonization. J Pediatr Gastroenterol Nutr. 2004;38:293-297.
- Lee J, Seto D, Bielory L. Meta-analysis of clinical trials of probiotics for prevention and treatment of pediatric atopic dermatitis. J Allergy Clin Immunol. 2008;121:116-121.
- Panduru M, Panduru NM, Sa˘la˘va˘stru CM, et al. Probiotics and primary prevention of atopic dermatitis: a meta‐analysis of randomized controlled studies. J Eur Acad Dermatol Venereol. 2015;29:232-242.
- Doege K, Grajecki D, Zyriax BC, et al. Impact of maternal supplementation with probiotics during pregnancy on atopic eczema in childhood—a meta-analysis. Br J Nutr. 2012;107:1-6.
- Zuccotti G, Meneghin F, Aceti A, et al. Probiotics for prevention of atopic diseases in infants: systematic review and meta‐analysis. Allergy. 2015;70:1356-1371.
- Seaton A, Godden DJ, Brown K. Increase in asthma: a more toxic environment or a more susceptible population? Thorax. 1994;49:171-174.
- Manzel A, Muller DN, Hafler DA, et al. Role of “Western diet” in inflammatory autoimmune diseases. Curr Allergy Asthma Rep. 2014;14:1-8.
- Li-Weber M, Giasisi M, Trieber MK, et al. Vitamin E inhibits IL-4 gene expression in peripheral blood T cells. Eur J Immunol. 2002;32:2401-2408.
- Sehra S, Yao Y, Howell MD, et al. IL-4 regulates skin homeostasis and the predisposition toward allergic skin inflammation. J Immunol. 2010;184:3186-3190.
- West CE, Dunstan J, McCarthy S, et al. Associations between maternal antioxidant intakes in pregnancy and infant allergic outcomes. Nutrients. 2012;4:1747-1758.
- Miyake Y, Sasaki S, Tanaka K, et al. Consumption of vegetables, fruit, and antioxidants during pregnancy and wheeze and eczema in infants. Allergy. 2010;65:758-765.
- Martindale S, McNeill G, Devereux G, et al. Antioxidant intake in pregnancy in relation to wheeze and eczema in the first two years of life. Am J Respir Crit Care Med. 2005;171:121-128.
- Robison R, Kumar R. The effect of prenatal and postnatal dietary exposures on childhood development of atopic disease. Curr Opin Allergy Clin Immunol. 2010;10:139-144.
- Berdnikovs S, Abdala-Valencia H, McCary C, et al. Isoforms of vitamin E have opposing immunoregulatory functions during inflammation by regulating leukocyte recruitment. J Immunol. 2009;182:4395-4405.
- Beckhaus AA, Garcia‐Marcos L, Forno E, et al. Maternal nutrition during pregnancy and risk of asthma, wheeze, and atopic diseases during childhood: a systematic review and meta‐analysis. Allergy. 2015;70:1588-1604.
- Calder PC, Miles EA. Fatty acids and atopic disease. Pediatr Allergy Immunol. 2000;11(suppl 13):29-36.
- Prescott S, Macaubas C, Holt B, et al. Transplacental priming of the human immune system to environmental allergens: universal skewing of initial T-cell responses towards Th-2 cytokine profile. J Immunol. 1998;160:4730-4737.
- Dunstan JA, Mori TA, Barden A, et al. Fish oil supplementation in pregnancy modifies neonatal allergen-specific immune responses and clinical outcomes in infants at high risk of atopy: a randomized, controlled trial. J Allergy Clin Immunol. 2003;112:1178-1184.
- Furuhjelm C, Warstedt K, Fagerås M, et al. Allergic disease in infants up to 2 years of age in relation to plasma omega‐3 fatty acids and maternal fish oil supplementation in pregnancy and lactation. Pediatr Allergy Immunol. 2011;22:505-514.
- Sausenthaler S, Koletzko S, Schaaf B, et al; LISA Study Group. Maternal diet during pregnancy in relation to eczema and allergic sensitization in the offspring at 2 y of age. Am J Clin Nutr. 2007;85:530-537.
- Best KP, Sullivan TR, Palmer DJ, et al. Prenatal omega-3 LCPUFA and symptoms of allergic disease and sensitization throughout early childhood—a longitudinal analysis of long-term follow-up of a randomized controlled trial. World Allergy Organ J. 2018;11:10.
- Jacobs DR Jr, Steffen LM. Nutrients, foods, and dietary patterns as exposures in research: a framework for food synergy. Am J Clin Nutr. 2003;78:508-513.
- Chatzi L, Torrent M, Romieu I, et al. Mediterranean diet in pregnancy is protective for wheeze and atopy in childhood. Thorax. 2008;63:507-513.
- Chatzi L, Garcia R, Roumeliotaki T, et al. Mediterranean diet adherence during pregnancy and risk of wheeze and eczema in the first year of life: INMA (Spain) and RHEA (Greece) mother-child cohort studies. Br J Nutr. 2013;110:2058-2068.
- Zeng J, Wu W, Chen Y, et al. Maternal dietary protein patterns during pregnancy and the risk of infant eczema: a cohort study. Front Nutr. 2021;8:294.
- Miyake Y, Okubo H, Sasaki S, et al. Maternal dietary patterns during pregnancy and risk of wheeze and eczema in Japanese infants aged 16–24 months: the Osaka Maternal and Child Health Study. Pediatr Allergy Immunol. 2011;22:734-741.
Practice Points
- The prevalence of atopic dermatitis (AD) has been increasing globally, with a marked increase in developed countries.
- Maternal dietary restriction is not recommended in pregnancy for the prevention of atopic disease in infancy and childhood based on the existing literature.
- There is mixed evidence to support probiotic supplementation in the prenatal period.
- The recommendations supporting antioxidant and fatty acid supplementation as well as specific prenatal diets for the prevention of AD in infants and children are limited due to the heterogeneity of study designs.
What’s Eating You? Mosquitoes (Culicidae)
Incidence and Characteristics
Mosquitoes are insects categorized into the order of Diptera and family of Culicidae, and more than 3500 different species have been identified.1 In the United States, the most common genus of mosquitoes is Aedes, with other common genera including Culex, Anopheles, Culiseta, and Coquillettidia. Most bites are performed by female rather than male mosquitoes, as it serves to complete their life cycle (Figure 1).1

There are a variety of possible reactions to mosquito bites. Severe local reactions that are large (papules >30 mm in diameter) or are accompanied by systemic manifestations are referred to as hypersensitivity to mosquito bites (HMB).2 These hypersensitivity reactions vary according to multiple factors, including comorbid conditions, genetic predisposition, and geographic location. The majority of the world’s population will exhibit local reactions to mosquito bites at some point during life, with the median age of onset of the first bite at 2 years of age.3 In a study by Arias-Cruz et al,4 the incidence of patient-reported large local reactions was 2.5%. Hypersensitivity to mosquito bites, perhaps the most rare reaction, is more common among Asian and Central American children.5 The median age of diagnosis for HMB is 7 years, and most reactions occur during the first 2 decades of life.6,7
Clinical Presentation
Mosquitoes bite vertebrates in an attempt to feed and thus must locate the host’s blood vessels through a process known as probing, which often necessitates changing the bite site several times. Once the vessel is located and lacerated, the mosquito feeds either from the vessel directly or the hematoma around it. Not only does the bite cause trauma to the skin, but a cutaneous reaction also may occur in response to salivary gland secretions that concurrently are deposited in the host tissue.8 Mosquitoes’ salivary gland components are the primary cause of cutaneous reactions, as one study showed that bites from mosquitoes lacking salivary gland ducts were not associated with these reactions.9 Mosquito saliva contains a large number of compounds with biologic activities, including lysozymes, antibacterial glucosidases, anticoagulants, antiplatelet aggregating factors, and vasodilators, as well as a potentially large number of unknown allergenic proteins. As of 2016, 70 mosquito-derived allergens have been identified, but this number continues to grow.2 After a bite from a mosquito, these compounds may result in host sensitization over time, though interestingly, sensitization to mosquito bites from a species different from the original offender does not occur due to lack of cross-reactivity between species.1
Because mosquitoes reproduce by laying their eggs directly on or near water, people who live near bodies of water or wetlands are at the highest risk for mosquito bites. Patient factors that have been found to lead to increased rates of mosquito bites include lower microbial diversity on the skin, the presence of sweat or body odor, pregnancy, increased body temperature, type O blood, dark clothing, and perfumes.2 Exaggerated bite reactions are associated with Epstein-Barr virus (EBV) infection and hematologic malignancies.10
Immediate hypersensitivity is mediated by a specific IgE antibody and is characterized by erythema and a wheal at the bite site that peaks within minutes of the bite. In contrast, delayed hypersensitivity is lymphocyte mediated; occurs 24 hours after the bite; and causes an indurated, pruritic, and erythematous 2- to 10-mm papule that may blister.11 Although the evidence of immediate hypersensitivity disappears within hours, symptoms of delayed hypersensitivity may last days to weeks. Accompanying symptoms may include local swelling, pain, and warmth. The itch that often is experienced in conjunction with erythema and papule formation is elicited in 3 main ways: direct induction utilizing classic pruritic pathways, IgE-mediated hypersensitivity reaction to salivary components, and IgE-independent host immune response to salivary antigens. Papular urticaria is a common additional finding in children with mosquito bites.1 As an individual is repeatedly bitten, they may undergo 5 stages of sensitization: stage I (neither immediate nor delayed reaction), stage II (delayed reaction), stage III (immediate and delayed reaction), stage IV (immediate reaction), and stage V (neither immediate or delayed reaction).11
Although most mosquito bites cause common local reactions, patients rarely demonstrate systemic reactions that can be much more severe. Skeeter syndrome is a milder systemic response characterized by large local reactions (papules >30 mm in diameter) developing hours after a bite with accompanying fever.12 The reaction typically peaks over days to weeks.2 Although the reaction may resemble cellulitis clinically, a history of a preceding mosquito bite can help make the distinction.13
A more severe systemic reaction is HMB, which is characterized by intense local skin findings as well as generalized systemic symptoms. Initially, indurated, clear, or hemorrhagic bullae appear at the bite site (Figure 2). Later, there is progression to swelling, necrosis, and ulceration.10 Biopsies from the skin lesions associated with HMB reveal necrosis, interstitial and perivascular eosinophilic and lymphocytic infiltrates, and small vessels with fibrinoid necrosis.7 Systemically, high fever, general malaise, liver dysfunction, proteinuria, hematuria, hepatosplenomegaly, and lymph node enlargement may occur. Patients typically experience these severe symptoms each time they are bitten.10
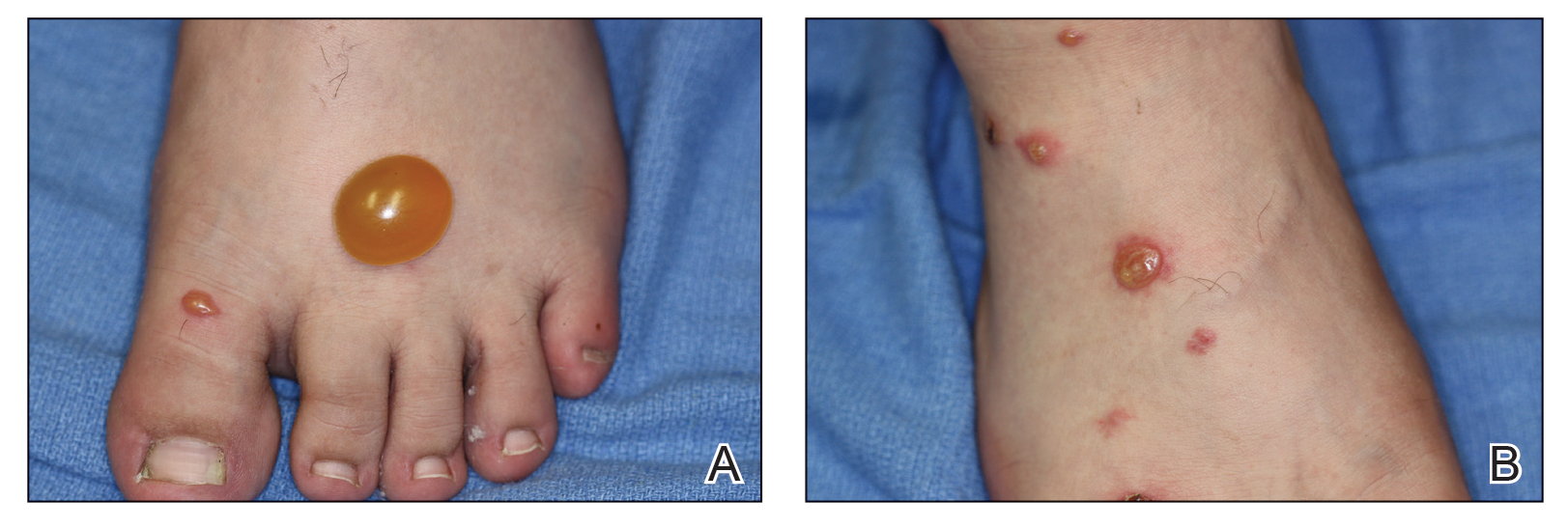
The mechanism of the HMB reaction is complex but has a close association with natural killer (NK) cell lymphoproliferative disorder and EBV infection (Figure 3). In fact, it is not uncommon for HMB patients to develop malignant lymphomas during their clinical course, even those unrelated to EBV.14 Epstein-Barr virus, one of the human herpesviruses, produces latent infection in NK cells. It is hypothesized that after a mosquito bite, EBV may be reactivated within these cells by induced expression of the viral lytic-cycle transactivator gene BamHI Z fragment leftward open reading frame 1, BZLF1.6 In response to mosquito salivary gland components, CD4+ T cells proliferate and induce expression of the EBV oncogene latent membrane protein 1, LMP1, on NK cells, which then infiltrate the bite site.15 These EBV-infected NK cells also overexpress the Fas ligand, thus contributing to organ and tissue damage.6 In addition to activating oncogene expression on NK cells, T cells also activate the basophils and mast cells carrying mosquito-specific IgE, both of which also add to the severe skin reaction of HMB.15 The particular triad of HMB, chronic active EBV infection, and NK cell lymphoproliferative disorder commonly is known as HMB-EBV-NK or HEN disease.1 Patients with HMB should be monitored for malignancy. The mortality of HMB is increased in patients in whom onset occurs when they are older than 9 years and with BZLF1 messenger RNA in skin lesions.6

Other rare reactions to mosquito bites include Wells syndrome, anaphylaxis, and superficial lymphangitis. Wells syndrome (also known as eosinophilic cellulitis) is characterized by erythematous or violaceous plaques and pruritic blisters. Although its etiology has not been defined, it is thought to be evoked or exacerbated by insect bites, with CD4+ T cells playing a primary role.1 Anaphylaxis (angioedema, urticaria, and wheezing) rarely may occur due to mosquito salivary gland components but typically is caused by other stinging insects. Superficial lymphangitis, often misdiagnosed as an infection of the lymphatic system, presents within minutes as nontender pink streaks originating from the bite site. A biopsy with eosinophil and mast cell infiltrates consistent with an allergic-type reaction confirms the absence of infection. Patients respond well to glucocorticoid treatment.
Mosquitoes are vectors for many blood-borne diseases, including dengue hemorrhagic fever, malaria, Chikungunya virus, La Crosse encephalitis, St. Louis encephalitis, West Nile virus, and yellow fever.16 Additionally, scratching the bites may lead to superinfection and scarring.1
Prevention and Treatment
Patients with known mosquito sensitivity should avoid areas of stagnant water and utilize preventative measures such as wearing protective clothing and using mosquito repellent containing DEET (N,N-diethyl-meta-toluamide), IR3535 (ethyl butylacetylaminopropionate), picaridin, or 2-undecanone (methyl nonyl ketone or IBI-246) when outdoors. Essential oils such as lemon, eucalyptus, citronella, and garlic are somewhat effective.1 Additionally, prophylactic dosing of antihistamines may prevent milder reactions.
Although often supportive, treatment and management of mosquito bites depends on the extent of the reaction. For common local reactions, symptomatic management with topical anesthetics, calamine lotion, or corticosteroid creams is appropriate. If superinfection from scratching is a concern, antibiotics may be appropriate.
Management of more severe and systemic reactions such as HMB also is supportive, and the addition of oral corticosteroids to decrease inflammation is required.7 Severe HMB also has been treated with immunosuppressive and anticancer drugs, though the efficacy is limited. Venom immunotherapy is a preventative option for patients with mosquito-specific IgE antibodies, and hematopoietic stem cell transplant may be required in patients with HMB.14,16
Conclusion
Mosquito allergens can cause a variety of reactions, ranging from those limited to the skin to those characterized by severe systemic effects. Although common local reactions can be symptomatically treated with topical medication, more severe reactions such as HMB require more involved clinical management. Hypersensitivity to mosquito bites is an important condition to recognize, as it is related to multiple organ impairment as well as later development of malignancy. Patients should be closely monitored during the entire clinical course and in the years following.
- Fostini AC, Golpanian RS, Rosen JD, et al. Beat the bite: pathophysiology and management of itch in mosquito bites. Itch. 2019;4:1.
- Engler RJ, Crisp HC, Freeman T, et al. Mosquito hypersensitivity: clinical updates. In: Freeman TM, Tracy JM, eds. Stinging Insect Allergy: A Clinician’s Guide. Springer; 2017:203-230.
- Manuyakorn W, Itsaradisaikul S, Benjaponpitak S, et al. Mosquito allergy in children: clinical features and limitation of commercially-available diagnostic tests. Asian Pac J Allergy Immunol. 2017;35:186-190.
- Arias-Cruz A, Avitia-Valenzuela E, González-Díaz SN, et al. Epidemiology of mosquito bite allergy in the Centre of Allergy and Clinical Immunology of Monterrey, Mexico. J Allergy Clin Immunol. 2006;117:S128.
- Jiang S, Manandhar U, Zheng KP, et al. A case of nodal marginal zone lymphoma with hypersensitivity to mosquito bites as initial symptom. J Cutan Pathol. 2019;46:769-774.
- Kyriakidis I, Vasileiou E, Karastrati S, et al. Primary EBV infection and hypersensitivity to mosquito bites: a case report. Virol Sin. 2016;31:517-520.
- Chiu TM, Lin YM, Wang SC, et al. Hypersensitivity to mosquito bites as the primary clinical manifestation of an Epstein-Barr virus infection. J Microbiol Immunol Infect. 2016;49:613-616.
- Henrique MO, Neto LS, Assis JB, et al. Evaluation of inflammatory skin infiltrate following Aedes aegypti bites in sensitized and non-sensitized mice reveals saliva-dependent and immune-dependent phenotypes. Immunology. 2019;158:47-59.
- Hudson A, Bowman L, Orr CWM. Effects of absence of saliva on blood feeding by mosquitoes. Science. 1960;131:1730-1731.
- Tatsuno K, Fujiyama T, Matsuoka H, et al. Clinical categories of exaggerated skin reactions to mosquito bites and their pathophysiology. J Dermatol Sci. 2016;82:145-152.
- Oka K, Ohtaki N, Igawa K, et al. Study on the correlation between age and changes in mosquito bite response. J Dermatol. 2018;45:1471-1474.
- Ferdman RM. Superficial allergic lymphangitis with a cutaneous recall reaction to a mosquito bite. Ann Allergy Asthma Immunol. 2019;123:521-522.
- Crisp HS, Johnson KS. Mosquito allergy. Ann Allergy Asthma Immunol. 2013;110:65-69.
- Washio K, Oka T, Abdalkader L, et al. Gene expression analysis of hypersensitivity to mosquito bite, chronic active EBV infection and NK/T-lymphoma/leukemia. Leuk Lymphoma. 2017;58:2683-2694.
- Sakakibara Y, Wada T, Muraoka M, et al. Basophil activation by mosquito extracts in patients with hypersensitivity to mosquito bites. Cancer Sci. 2015;106:965-971.
- Lee H, Halvorsen S, Mackey R, et al. Insect allergy. Prim Care. 2016;43:417-431.
Incidence and Characteristics
Mosquitoes are insects categorized into the order of Diptera and family of Culicidae, and more than 3500 different species have been identified.1 In the United States, the most common genus of mosquitoes is Aedes, with other common genera including Culex, Anopheles, Culiseta, and Coquillettidia. Most bites are performed by female rather than male mosquitoes, as it serves to complete their life cycle (Figure 1).1

There are a variety of possible reactions to mosquito bites. Severe local reactions that are large (papules >30 mm in diameter) or are accompanied by systemic manifestations are referred to as hypersensitivity to mosquito bites (HMB).2 These hypersensitivity reactions vary according to multiple factors, including comorbid conditions, genetic predisposition, and geographic location. The majority of the world’s population will exhibit local reactions to mosquito bites at some point during life, with the median age of onset of the first bite at 2 years of age.3 In a study by Arias-Cruz et al,4 the incidence of patient-reported large local reactions was 2.5%. Hypersensitivity to mosquito bites, perhaps the most rare reaction, is more common among Asian and Central American children.5 The median age of diagnosis for HMB is 7 years, and most reactions occur during the first 2 decades of life.6,7
Clinical Presentation
Mosquitoes bite vertebrates in an attempt to feed and thus must locate the host’s blood vessels through a process known as probing, which often necessitates changing the bite site several times. Once the vessel is located and lacerated, the mosquito feeds either from the vessel directly or the hematoma around it. Not only does the bite cause trauma to the skin, but a cutaneous reaction also may occur in response to salivary gland secretions that concurrently are deposited in the host tissue.8 Mosquitoes’ salivary gland components are the primary cause of cutaneous reactions, as one study showed that bites from mosquitoes lacking salivary gland ducts were not associated with these reactions.9 Mosquito saliva contains a large number of compounds with biologic activities, including lysozymes, antibacterial glucosidases, anticoagulants, antiplatelet aggregating factors, and vasodilators, as well as a potentially large number of unknown allergenic proteins. As of 2016, 70 mosquito-derived allergens have been identified, but this number continues to grow.2 After a bite from a mosquito, these compounds may result in host sensitization over time, though interestingly, sensitization to mosquito bites from a species different from the original offender does not occur due to lack of cross-reactivity between species.1
Because mosquitoes reproduce by laying their eggs directly on or near water, people who live near bodies of water or wetlands are at the highest risk for mosquito bites. Patient factors that have been found to lead to increased rates of mosquito bites include lower microbial diversity on the skin, the presence of sweat or body odor, pregnancy, increased body temperature, type O blood, dark clothing, and perfumes.2 Exaggerated bite reactions are associated with Epstein-Barr virus (EBV) infection and hematologic malignancies.10
Immediate hypersensitivity is mediated by a specific IgE antibody and is characterized by erythema and a wheal at the bite site that peaks within minutes of the bite. In contrast, delayed hypersensitivity is lymphocyte mediated; occurs 24 hours after the bite; and causes an indurated, pruritic, and erythematous 2- to 10-mm papule that may blister.11 Although the evidence of immediate hypersensitivity disappears within hours, symptoms of delayed hypersensitivity may last days to weeks. Accompanying symptoms may include local swelling, pain, and warmth. The itch that often is experienced in conjunction with erythema and papule formation is elicited in 3 main ways: direct induction utilizing classic pruritic pathways, IgE-mediated hypersensitivity reaction to salivary components, and IgE-independent host immune response to salivary antigens. Papular urticaria is a common additional finding in children with mosquito bites.1 As an individual is repeatedly bitten, they may undergo 5 stages of sensitization: stage I (neither immediate nor delayed reaction), stage II (delayed reaction), stage III (immediate and delayed reaction), stage IV (immediate reaction), and stage V (neither immediate or delayed reaction).11
Although most mosquito bites cause common local reactions, patients rarely demonstrate systemic reactions that can be much more severe. Skeeter syndrome is a milder systemic response characterized by large local reactions (papules >30 mm in diameter) developing hours after a bite with accompanying fever.12 The reaction typically peaks over days to weeks.2 Although the reaction may resemble cellulitis clinically, a history of a preceding mosquito bite can help make the distinction.13
A more severe systemic reaction is HMB, which is characterized by intense local skin findings as well as generalized systemic symptoms. Initially, indurated, clear, or hemorrhagic bullae appear at the bite site (Figure 2). Later, there is progression to swelling, necrosis, and ulceration.10 Biopsies from the skin lesions associated with HMB reveal necrosis, interstitial and perivascular eosinophilic and lymphocytic infiltrates, and small vessels with fibrinoid necrosis.7 Systemically, high fever, general malaise, liver dysfunction, proteinuria, hematuria, hepatosplenomegaly, and lymph node enlargement may occur. Patients typically experience these severe symptoms each time they are bitten.10

The mechanism of the HMB reaction is complex but has a close association with natural killer (NK) cell lymphoproliferative disorder and EBV infection (Figure 3). In fact, it is not uncommon for HMB patients to develop malignant lymphomas during their clinical course, even those unrelated to EBV.14 Epstein-Barr virus, one of the human herpesviruses, produces latent infection in NK cells. It is hypothesized that after a mosquito bite, EBV may be reactivated within these cells by induced expression of the viral lytic-cycle transactivator gene BamHI Z fragment leftward open reading frame 1, BZLF1.6 In response to mosquito salivary gland components, CD4+ T cells proliferate and induce expression of the EBV oncogene latent membrane protein 1, LMP1, on NK cells, which then infiltrate the bite site.15 These EBV-infected NK cells also overexpress the Fas ligand, thus contributing to organ and tissue damage.6 In addition to activating oncogene expression on NK cells, T cells also activate the basophils and mast cells carrying mosquito-specific IgE, both of which also add to the severe skin reaction of HMB.15 The particular triad of HMB, chronic active EBV infection, and NK cell lymphoproliferative disorder commonly is known as HMB-EBV-NK or HEN disease.1 Patients with HMB should be monitored for malignancy. The mortality of HMB is increased in patients in whom onset occurs when they are older than 9 years and with BZLF1 messenger RNA in skin lesions.6

Other rare reactions to mosquito bites include Wells syndrome, anaphylaxis, and superficial lymphangitis. Wells syndrome (also known as eosinophilic cellulitis) is characterized by erythematous or violaceous plaques and pruritic blisters. Although its etiology has not been defined, it is thought to be evoked or exacerbated by insect bites, with CD4+ T cells playing a primary role.1 Anaphylaxis (angioedema, urticaria, and wheezing) rarely may occur due to mosquito salivary gland components but typically is caused by other stinging insects. Superficial lymphangitis, often misdiagnosed as an infection of the lymphatic system, presents within minutes as nontender pink streaks originating from the bite site. A biopsy with eosinophil and mast cell infiltrates consistent with an allergic-type reaction confirms the absence of infection. Patients respond well to glucocorticoid treatment.
Mosquitoes are vectors for many blood-borne diseases, including dengue hemorrhagic fever, malaria, Chikungunya virus, La Crosse encephalitis, St. Louis encephalitis, West Nile virus, and yellow fever.16 Additionally, scratching the bites may lead to superinfection and scarring.1
Prevention and Treatment
Patients with known mosquito sensitivity should avoid areas of stagnant water and utilize preventative measures such as wearing protective clothing and using mosquito repellent containing DEET (N,N-diethyl-meta-toluamide), IR3535 (ethyl butylacetylaminopropionate), picaridin, or 2-undecanone (methyl nonyl ketone or IBI-246) when outdoors. Essential oils such as lemon, eucalyptus, citronella, and garlic are somewhat effective.1 Additionally, prophylactic dosing of antihistamines may prevent milder reactions.
Although often supportive, treatment and management of mosquito bites depends on the extent of the reaction. For common local reactions, symptomatic management with topical anesthetics, calamine lotion, or corticosteroid creams is appropriate. If superinfection from scratching is a concern, antibiotics may be appropriate.
Management of more severe and systemic reactions such as HMB also is supportive, and the addition of oral corticosteroids to decrease inflammation is required.7 Severe HMB also has been treated with immunosuppressive and anticancer drugs, though the efficacy is limited. Venom immunotherapy is a preventative option for patients with mosquito-specific IgE antibodies, and hematopoietic stem cell transplant may be required in patients with HMB.14,16
Conclusion
Mosquito allergens can cause a variety of reactions, ranging from those limited to the skin to those characterized by severe systemic effects. Although common local reactions can be symptomatically treated with topical medication, more severe reactions such as HMB require more involved clinical management. Hypersensitivity to mosquito bites is an important condition to recognize, as it is related to multiple organ impairment as well as later development of malignancy. Patients should be closely monitored during the entire clinical course and in the years following.
Incidence and Characteristics
Mosquitoes are insects categorized into the order of Diptera and family of Culicidae, and more than 3500 different species have been identified.1 In the United States, the most common genus of mosquitoes is Aedes, with other common genera including Culex, Anopheles, Culiseta, and Coquillettidia. Most bites are performed by female rather than male mosquitoes, as it serves to complete their life cycle (Figure 1).1

There are a variety of possible reactions to mosquito bites. Severe local reactions that are large (papules >30 mm in diameter) or are accompanied by systemic manifestations are referred to as hypersensitivity to mosquito bites (HMB).2 These hypersensitivity reactions vary according to multiple factors, including comorbid conditions, genetic predisposition, and geographic location. The majority of the world’s population will exhibit local reactions to mosquito bites at some point during life, with the median age of onset of the first bite at 2 years of age.3 In a study by Arias-Cruz et al,4 the incidence of patient-reported large local reactions was 2.5%. Hypersensitivity to mosquito bites, perhaps the most rare reaction, is more common among Asian and Central American children.5 The median age of diagnosis for HMB is 7 years, and most reactions occur during the first 2 decades of life.6,7
Clinical Presentation
Mosquitoes bite vertebrates in an attempt to feed and thus must locate the host’s blood vessels through a process known as probing, which often necessitates changing the bite site several times. Once the vessel is located and lacerated, the mosquito feeds either from the vessel directly or the hematoma around it. Not only does the bite cause trauma to the skin, but a cutaneous reaction also may occur in response to salivary gland secretions that concurrently are deposited in the host tissue.8 Mosquitoes’ salivary gland components are the primary cause of cutaneous reactions, as one study showed that bites from mosquitoes lacking salivary gland ducts were not associated with these reactions.9 Mosquito saliva contains a large number of compounds with biologic activities, including lysozymes, antibacterial glucosidases, anticoagulants, antiplatelet aggregating factors, and vasodilators, as well as a potentially large number of unknown allergenic proteins. As of 2016, 70 mosquito-derived allergens have been identified, but this number continues to grow.2 After a bite from a mosquito, these compounds may result in host sensitization over time, though interestingly, sensitization to mosquito bites from a species different from the original offender does not occur due to lack of cross-reactivity between species.1
Because mosquitoes reproduce by laying their eggs directly on or near water, people who live near bodies of water or wetlands are at the highest risk for mosquito bites. Patient factors that have been found to lead to increased rates of mosquito bites include lower microbial diversity on the skin, the presence of sweat or body odor, pregnancy, increased body temperature, type O blood, dark clothing, and perfumes.2 Exaggerated bite reactions are associated with Epstein-Barr virus (EBV) infection and hematologic malignancies.10
Immediate hypersensitivity is mediated by a specific IgE antibody and is characterized by erythema and a wheal at the bite site that peaks within minutes of the bite. In contrast, delayed hypersensitivity is lymphocyte mediated; occurs 24 hours after the bite; and causes an indurated, pruritic, and erythematous 2- to 10-mm papule that may blister.11 Although the evidence of immediate hypersensitivity disappears within hours, symptoms of delayed hypersensitivity may last days to weeks. Accompanying symptoms may include local swelling, pain, and warmth. The itch that often is experienced in conjunction with erythema and papule formation is elicited in 3 main ways: direct induction utilizing classic pruritic pathways, IgE-mediated hypersensitivity reaction to salivary components, and IgE-independent host immune response to salivary antigens. Papular urticaria is a common additional finding in children with mosquito bites.1 As an individual is repeatedly bitten, they may undergo 5 stages of sensitization: stage I (neither immediate nor delayed reaction), stage II (delayed reaction), stage III (immediate and delayed reaction), stage IV (immediate reaction), and stage V (neither immediate or delayed reaction).11
Although most mosquito bites cause common local reactions, patients rarely demonstrate systemic reactions that can be much more severe. Skeeter syndrome is a milder systemic response characterized by large local reactions (papules >30 mm in diameter) developing hours after a bite with accompanying fever.12 The reaction typically peaks over days to weeks.2 Although the reaction may resemble cellulitis clinically, a history of a preceding mosquito bite can help make the distinction.13
A more severe systemic reaction is HMB, which is characterized by intense local skin findings as well as generalized systemic symptoms. Initially, indurated, clear, or hemorrhagic bullae appear at the bite site (Figure 2). Later, there is progression to swelling, necrosis, and ulceration.10 Biopsies from the skin lesions associated with HMB reveal necrosis, interstitial and perivascular eosinophilic and lymphocytic infiltrates, and small vessels with fibrinoid necrosis.7 Systemically, high fever, general malaise, liver dysfunction, proteinuria, hematuria, hepatosplenomegaly, and lymph node enlargement may occur. Patients typically experience these severe symptoms each time they are bitten.10

The mechanism of the HMB reaction is complex but has a close association with natural killer (NK) cell lymphoproliferative disorder and EBV infection (Figure 3). In fact, it is not uncommon for HMB patients to develop malignant lymphomas during their clinical course, even those unrelated to EBV.14 Epstein-Barr virus, one of the human herpesviruses, produces latent infection in NK cells. It is hypothesized that after a mosquito bite, EBV may be reactivated within these cells by induced expression of the viral lytic-cycle transactivator gene BamHI Z fragment leftward open reading frame 1, BZLF1.6 In response to mosquito salivary gland components, CD4+ T cells proliferate and induce expression of the EBV oncogene latent membrane protein 1, LMP1, on NK cells, which then infiltrate the bite site.15 These EBV-infected NK cells also overexpress the Fas ligand, thus contributing to organ and tissue damage.6 In addition to activating oncogene expression on NK cells, T cells also activate the basophils and mast cells carrying mosquito-specific IgE, both of which also add to the severe skin reaction of HMB.15 The particular triad of HMB, chronic active EBV infection, and NK cell lymphoproliferative disorder commonly is known as HMB-EBV-NK or HEN disease.1 Patients with HMB should be monitored for malignancy. The mortality of HMB is increased in patients in whom onset occurs when they are older than 9 years and with BZLF1 messenger RNA in skin lesions.6

Other rare reactions to mosquito bites include Wells syndrome, anaphylaxis, and superficial lymphangitis. Wells syndrome (also known as eosinophilic cellulitis) is characterized by erythematous or violaceous plaques and pruritic blisters. Although its etiology has not been defined, it is thought to be evoked or exacerbated by insect bites, with CD4+ T cells playing a primary role.1 Anaphylaxis (angioedema, urticaria, and wheezing) rarely may occur due to mosquito salivary gland components but typically is caused by other stinging insects. Superficial lymphangitis, often misdiagnosed as an infection of the lymphatic system, presents within minutes as nontender pink streaks originating from the bite site. A biopsy with eosinophil and mast cell infiltrates consistent with an allergic-type reaction confirms the absence of infection. Patients respond well to glucocorticoid treatment.
Mosquitoes are vectors for many blood-borne diseases, including dengue hemorrhagic fever, malaria, Chikungunya virus, La Crosse encephalitis, St. Louis encephalitis, West Nile virus, and yellow fever.16 Additionally, scratching the bites may lead to superinfection and scarring.1
Prevention and Treatment
Patients with known mosquito sensitivity should avoid areas of stagnant water and utilize preventative measures such as wearing protective clothing and using mosquito repellent containing DEET (N,N-diethyl-meta-toluamide), IR3535 (ethyl butylacetylaminopropionate), picaridin, or 2-undecanone (methyl nonyl ketone or IBI-246) when outdoors. Essential oils such as lemon, eucalyptus, citronella, and garlic are somewhat effective.1 Additionally, prophylactic dosing of antihistamines may prevent milder reactions.
Although often supportive, treatment and management of mosquito bites depends on the extent of the reaction. For common local reactions, symptomatic management with topical anesthetics, calamine lotion, or corticosteroid creams is appropriate. If superinfection from scratching is a concern, antibiotics may be appropriate.
Management of more severe and systemic reactions such as HMB also is supportive, and the addition of oral corticosteroids to decrease inflammation is required.7 Severe HMB also has been treated with immunosuppressive and anticancer drugs, though the efficacy is limited. Venom immunotherapy is a preventative option for patients with mosquito-specific IgE antibodies, and hematopoietic stem cell transplant may be required in patients with HMB.14,16
Conclusion
Mosquito allergens can cause a variety of reactions, ranging from those limited to the skin to those characterized by severe systemic effects. Although common local reactions can be symptomatically treated with topical medication, more severe reactions such as HMB require more involved clinical management. Hypersensitivity to mosquito bites is an important condition to recognize, as it is related to multiple organ impairment as well as later development of malignancy. Patients should be closely monitored during the entire clinical course and in the years following.
- Fostini AC, Golpanian RS, Rosen JD, et al. Beat the bite: pathophysiology and management of itch in mosquito bites. Itch. 2019;4:1.
- Engler RJ, Crisp HC, Freeman T, et al. Mosquito hypersensitivity: clinical updates. In: Freeman TM, Tracy JM, eds. Stinging Insect Allergy: A Clinician’s Guide. Springer; 2017:203-230.
- Manuyakorn W, Itsaradisaikul S, Benjaponpitak S, et al. Mosquito allergy in children: clinical features and limitation of commercially-available diagnostic tests. Asian Pac J Allergy Immunol. 2017;35:186-190.
- Arias-Cruz A, Avitia-Valenzuela E, González-Díaz SN, et al. Epidemiology of mosquito bite allergy in the Centre of Allergy and Clinical Immunology of Monterrey, Mexico. J Allergy Clin Immunol. 2006;117:S128.
- Jiang S, Manandhar U, Zheng KP, et al. A case of nodal marginal zone lymphoma with hypersensitivity to mosquito bites as initial symptom. J Cutan Pathol. 2019;46:769-774.
- Kyriakidis I, Vasileiou E, Karastrati S, et al. Primary EBV infection and hypersensitivity to mosquito bites: a case report. Virol Sin. 2016;31:517-520.
- Chiu TM, Lin YM, Wang SC, et al. Hypersensitivity to mosquito bites as the primary clinical manifestation of an Epstein-Barr virus infection. J Microbiol Immunol Infect. 2016;49:613-616.
- Henrique MO, Neto LS, Assis JB, et al. Evaluation of inflammatory skin infiltrate following Aedes aegypti bites in sensitized and non-sensitized mice reveals saliva-dependent and immune-dependent phenotypes. Immunology. 2019;158:47-59.
- Hudson A, Bowman L, Orr CWM. Effects of absence of saliva on blood feeding by mosquitoes. Science. 1960;131:1730-1731.
- Tatsuno K, Fujiyama T, Matsuoka H, et al. Clinical categories of exaggerated skin reactions to mosquito bites and their pathophysiology. J Dermatol Sci. 2016;82:145-152.
- Oka K, Ohtaki N, Igawa K, et al. Study on the correlation between age and changes in mosquito bite response. J Dermatol. 2018;45:1471-1474.
- Ferdman RM. Superficial allergic lymphangitis with a cutaneous recall reaction to a mosquito bite. Ann Allergy Asthma Immunol. 2019;123:521-522.
- Crisp HS, Johnson KS. Mosquito allergy. Ann Allergy Asthma Immunol. 2013;110:65-69.
- Washio K, Oka T, Abdalkader L, et al. Gene expression analysis of hypersensitivity to mosquito bite, chronic active EBV infection and NK/T-lymphoma/leukemia. Leuk Lymphoma. 2017;58:2683-2694.
- Sakakibara Y, Wada T, Muraoka M, et al. Basophil activation by mosquito extracts in patients with hypersensitivity to mosquito bites. Cancer Sci. 2015;106:965-971.
- Lee H, Halvorsen S, Mackey R, et al. Insect allergy. Prim Care. 2016;43:417-431.
- Fostini AC, Golpanian RS, Rosen JD, et al. Beat the bite: pathophysiology and management of itch in mosquito bites. Itch. 2019;4:1.
- Engler RJ, Crisp HC, Freeman T, et al. Mosquito hypersensitivity: clinical updates. In: Freeman TM, Tracy JM, eds. Stinging Insect Allergy: A Clinician’s Guide. Springer; 2017:203-230.
- Manuyakorn W, Itsaradisaikul S, Benjaponpitak S, et al. Mosquito allergy in children: clinical features and limitation of commercially-available diagnostic tests. Asian Pac J Allergy Immunol. 2017;35:186-190.
- Arias-Cruz A, Avitia-Valenzuela E, González-Díaz SN, et al. Epidemiology of mosquito bite allergy in the Centre of Allergy and Clinical Immunology of Monterrey, Mexico. J Allergy Clin Immunol. 2006;117:S128.
- Jiang S, Manandhar U, Zheng KP, et al. A case of nodal marginal zone lymphoma with hypersensitivity to mosquito bites as initial symptom. J Cutan Pathol. 2019;46:769-774.
- Kyriakidis I, Vasileiou E, Karastrati S, et al. Primary EBV infection and hypersensitivity to mosquito bites: a case report. Virol Sin. 2016;31:517-520.
- Chiu TM, Lin YM, Wang SC, et al. Hypersensitivity to mosquito bites as the primary clinical manifestation of an Epstein-Barr virus infection. J Microbiol Immunol Infect. 2016;49:613-616.
- Henrique MO, Neto LS, Assis JB, et al. Evaluation of inflammatory skin infiltrate following Aedes aegypti bites in sensitized and non-sensitized mice reveals saliva-dependent and immune-dependent phenotypes. Immunology. 2019;158:47-59.
- Hudson A, Bowman L, Orr CWM. Effects of absence of saliva on blood feeding by mosquitoes. Science. 1960;131:1730-1731.
- Tatsuno K, Fujiyama T, Matsuoka H, et al. Clinical categories of exaggerated skin reactions to mosquito bites and their pathophysiology. J Dermatol Sci. 2016;82:145-152.
- Oka K, Ohtaki N, Igawa K, et al. Study on the correlation between age and changes in mosquito bite response. J Dermatol. 2018;45:1471-1474.
- Ferdman RM. Superficial allergic lymphangitis with a cutaneous recall reaction to a mosquito bite. Ann Allergy Asthma Immunol. 2019;123:521-522.
- Crisp HS, Johnson KS. Mosquito allergy. Ann Allergy Asthma Immunol. 2013;110:65-69.
- Washio K, Oka T, Abdalkader L, et al. Gene expression analysis of hypersensitivity to mosquito bite, chronic active EBV infection and NK/T-lymphoma/leukemia. Leuk Lymphoma. 2017;58:2683-2694.
- Sakakibara Y, Wada T, Muraoka M, et al. Basophil activation by mosquito extracts in patients with hypersensitivity to mosquito bites. Cancer Sci. 2015;106:965-971.
- Lee H, Halvorsen S, Mackey R, et al. Insect allergy. Prim Care. 2016;43:417-431.
Practice Points
- Common local reactions to mosquito bites include immediate and delayed hypersensitivity reactions. With repeated exposure, reactions can increase in severity.
- Hypersensitivity to mosquito bites is a severe systemic reaction to mosquito salivary gland components characterized by bullous necrotic skin lesions associated with systemic manifestations such as high fever, malaise, liver dysfunction, proteinuria, hematuria, hepatosplenomegaly, and lymph node enlargement.
- Hypersensitivity to mosquito bites is closely associated with chronic Epstein-Barr virus infection and lymphoproliferative disorders.
Dermatologic Management of Hidradenitis Suppurativa and Impact on Pregnancy and Breastfeeding
Hidradenitis suppurativa (HS) is a chronic inflammatory skin disease associated with hyperandrogenism and is caused by occlusion or rupture of follicular units and inflammation of the apocrine glands.1-3 The disease most commonly affects women (female to male ratio of 3:1) of childbearing age.1,2,4,5 Body areas affected include the axillae and groin, and less commonly the perineum; perianal region; and skin folds, such as gluteal, inframammary, and infraumbilical folds.1,2 Symptoms manifest as painful subcutaneous nodules with possible accompanying purulent drainage, sinus tracts, and/or dermal contractures. Although the pathophysiology is unclear, androgens affect the course of HS during pregnancy by stimulating the affected glands and altering cytokines.1,2,6
During pregnancy, maternal immune function switches from cell-mediated T helper cell (TH1) to humoral TH2 cytokine production. The activity of sebaceous and eccrine glands increases while the activity of apocrine glands decreases, thus changing the inflammatory course of HS during pregnancy.3 Approximately 20% of women with HS experience improvement of symptoms during pregnancy, while the remainder either experience no relief or deterioration of symptoms.1 Improvement in symptoms during pregnancy was found to occur more frequently in those who had worsening symptoms during menses owing to the possible hormonal effect estrogen has on inhibiting TH1 and TH17 proinflammatory cytokines, which promotes an immunosuppressive environment.4
Lactation and breastfeeding abilities may be hindered if a woman has HS affecting the apocrine glands of breast tissue and a symptom flare in the postpartum period. If HS causes notable inflammation in the nipple-areolar complex during pregnancy, the patient may experience difficulties with lactation and milk fistula formation, leading to inability to breastfeed.2 Another reason why mothers with HS may not be able to breastfeed is that the medications required to treat the disease are unsafe if passed to the infant via breast milk. In addition, the teratogenic effects of HS medications may necessitate therapy adjustments in pregnancy.1 Here, we provide a brief overview of the medical management considerations of HS in the setting of pregnancy and the impact on breastfeeding.
MEDICAL MANAGEMENT AND DRUG SAFETY
Dermatologists prescribe a myriad of topical and systemic medications to ameliorate symptoms of HS. Therapy regimens often are multimodal and include antibiotics, biologics, and immunosuppressants.1,3
Antibiotics
First-line antibiotics include clindamycin, metronidazole, tetracyclines, erythromycin, rifampin, dapsone, and fluoroquinolones. Topical clindamycin 1%, metronidazole 0.75%, and erythromycin 2% are used for open or active HS lesions and are all safe to use in pregnancy since there is minimal systemic absorption and minimal excretion into breast milk.1 Topical antimicrobial washes such as benzoyl peroxide and chlorhexidine often are used in combination with systemic medications to treat HS. These washes are safe during pregnancy and lactation, as they have minimal systemic absorption.7
Of these first-line antibiotics, only tetracyclines are contraindicated during pregnancy and lactation, as they are deemed to be in category D by the US Food and Drug Administration (FDA).1 Aside from tetracyclines, these antibiotics do not cause birth defects and are safe for nursing infants.1,8 Systemic clindamycin is safe during pregnancy and breastfeeding. Systemic metronidazole also is safe for use in pregnant patients but needs to be discontinued 12 to 24 hours prior to breastfeeding, which often prohibits appropriate dosing.1
Systemic Erythromycin—There are several forms of systemic erythromycin, including erythromycin base, erythromycin estolate, erythromycin ethylsuccinate (EES), and erythromycin stearate. Erythromycin estolate is contraindicated in pregnancy because it is associated with reversible maternal hepatoxicity and jaundice.9-11 Erythromycin ethylsuccinate is the preferred form for pregnant patients. Providers should exercise caution when prescribing EES to lactating mothers, as small amounts are still secreted through breast milk.11 Some studies have shown an increased risk for development of infantile hypertrophic pyloric stenosis with systemic erythromycin use, especially if a neonate is exposed in the first 14 days of life. Thus, we recommend withholding EES for 2 weeks after delivery if the patient is breastfeeding. A follow-up study did not find any association between erythromycin and infantile hypertrophic pyloric stenosis; however, the American Academy of Pediatrics still recommends short-term use only of erythromycin if it is to be used in the systemic form.8
Rifampin—Rifampin is excreted into breast milk but without adverse effects to the infant. Rifampin also is safe in pregnancy but should be used on a case-by-case basis in pregnant or nursing women because it is a cytochrome P450 inducer.
Dapsone—Dapsone has no increased risk for congenital anomalies. However, it is associated with hemolytic anemia and neonatal hyperbilirubinemia, especially in patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency.12 Newborns exposed to dapsone are at an increased risk for methemoglobinemia owing to increased sensitivity of fetal erythrocytes to oxidizing agents.13 If dapsone use is necessary, stopping dapsone treatment in the last month of gestation is recommended to minimize risk for kernicterus.9 Dapsone can be found in high concentrations in breast milk at 14.3% of the maternal dose. It is still safe to use during breastfeeding, but there is a risk of the infant developing hyperbilirubinemia/G6PD deficiency.1,8 Thus, physicians may consider performing a G6PD screen on infants to determine if breastfeeding is safe.12
Fluoroquinolones—Quinolones are not contraindicated during pregnancy, but they can damage fetal cartilage and thus should be reserved for use in complicated infections when the benefits outweigh the risks.12 Quinolones are believed to increase risk for arthropathy but are safe for use in lactation. When quinolones are digested with milk, exposure decreases below pediatric doses because of the ionized property of calcium in milk.8
Tumor Necrosis Factor α Inhibitors—The safety of anti–tumor necrosis factor (TNF) α biologics in pregnancy is less certain when compared with antibiotics.1 Anti–TNF-α inhibitors such as etanercept, adalimumab, and infliximab are all labeled as FDA category B, meaning there are no well-controlled human studies of the drugs.9 There are limited data that support safe use of TNF-α inhibitors prior to the third trimester before maternal IgG antibodies are transferred to the fetus via the placenta.1,13 Anti–TNF-α inhibitors may be safe when breastfeeding because the drugs have large molecular weights that prevent them from entering breast milk in large amounts. Absorption also is limited due to the infant’s digestive acids and enzymes breaking down the protein structure of the medication.8 Overall, TNF-α inhibitor use is still controversial and only used if the benefits outweigh the risks during pregnancy or if there is no alternative treatment.1,3,9
Ustekinumab and Anakinra—Ustekinumab (an IL-12/IL-23 inhibitor) and anakinra (an IL-1α and IL-1β inhibitor) also are FDA category B drugs and have limited data supporting their use as HS treatment in pregnancy. Anakinra may have evidence of compatibility with breastfeeding, as endogenous IL-1α inhibitor is found in colostrum and mature breast milk.1
Immunosuppressants
Immunosuppressants that are used to treat HS include corticosteroids and cyclosporine.
Corticosteroids—Topical corticosteroids can be used safely in lactation if they are not applied directly to the nipple or any area that makes direct contact with the infant’s mouth. Intralesional corticosteroid injections are safe for use during both pregnancy and breastfeeding to decrease inflammation of acutely flaring lesions and can be considered first-line treatment.1 Oral glucocorticoids also can be safely used for acute flares during pregnancy; however, prolonged use is associated with pregnancy complications such as preeclampsia, eclampsia, premature delivery, and gestational diabetes.12 There also is a small risk of oral cleft deformity in the infant; thus, potent corticosteroids are recommended in short durations during pregnancy, and there are no adverse effects if the maternal dose is less than 10 mg daily.8,12 Systemic steroids are safe to use with breastfeeding, but patients should be advised to wait 4 hours after ingesting medication before breastfeeding.1,8
Cyclosporine—Topical and oral calcineurin inhibitors such as cyclosporine have low risk for transmission into breast milk; however, potential effects of exposure through breast milk are unknown. For that reason, manufacturers state that cyclosporine use is contraindicated during lactation.8 If cyclosporine is to be used by a breastfeeding woman, monitoring cyclosporine concentrations in the infant is suggested to ensure that the exposure is less than 5% to 10% of the therapeutic dose.13 The use of cyclosporine has been extensively studied in pregnant transplant patients and is considered relatively safe for use in pregnancy.14 Cyclosporine is lipid soluble and thus is quickly metabolized and spread throughout the body; it can easily cross the placenta.9,13 Blood concentration in the fetus is 30% to 64% that of the maternal circulation. However, cyclosporine is only toxic to the fetus at maternally toxic doses, which can result in low birth weight and increased prenatal and postnatal mortality.13
Isotretinoin, Oral Contraceptive Pills, and Spironolactone
Isotretinoin and hormonal treatments such as oral contraceptive pills and spironolactone (an androgen receptor blocker) commonly are used to treat HS, but all are contraindicated in pregnancy and lactation. Isotretinoin is a well-established teratogen, but adverse effects on nursing babies have not been described. However, the manufacturer of isotretinoin advises against its use in lactation. Oral contraceptive pill use in early pregnancy is associated with increased risk for Down syndrome. Oral contraceptive pill use also is contraindicated in lactation for 2 reasons: decreased milk production and risk for fetal feminization. Antiandrogenic agents such as spironolactone have been shown to be associated with hypospadias and feminization of the male fetus.7
COMMENT
Women with HS usually require ongoing medical treatment during pregnancy and immediately postpartum; thus, it is important that treatments are proven to be safe for use in this specific population. Current management guidelines are not entirely suitable for pregnant and breastfeeding women given that many HS drugs have teratogenic effects and/or can be excreted into breast milk.1 Several treatments have uncertain safety profiles in pregnancy and breastfeeding, which calls for dermatologists to change or create new regimens for their patients. Close management also is necessary to prevent excess inflammation of breast tissue and milk fistula formation, which would hinder normal breastfeeding.
The eTable lists medications used to treat HS. The FDA category is listed next to each drug. However, it should be noted that these FDA letter categories were replaced with the Pregnancy and Lactation Labeling Rule in 2015. The letter ratings were deemed overly simplistic and replaced with narrative-based labeling that provides more detailed adverse effects and clinical considerations.9
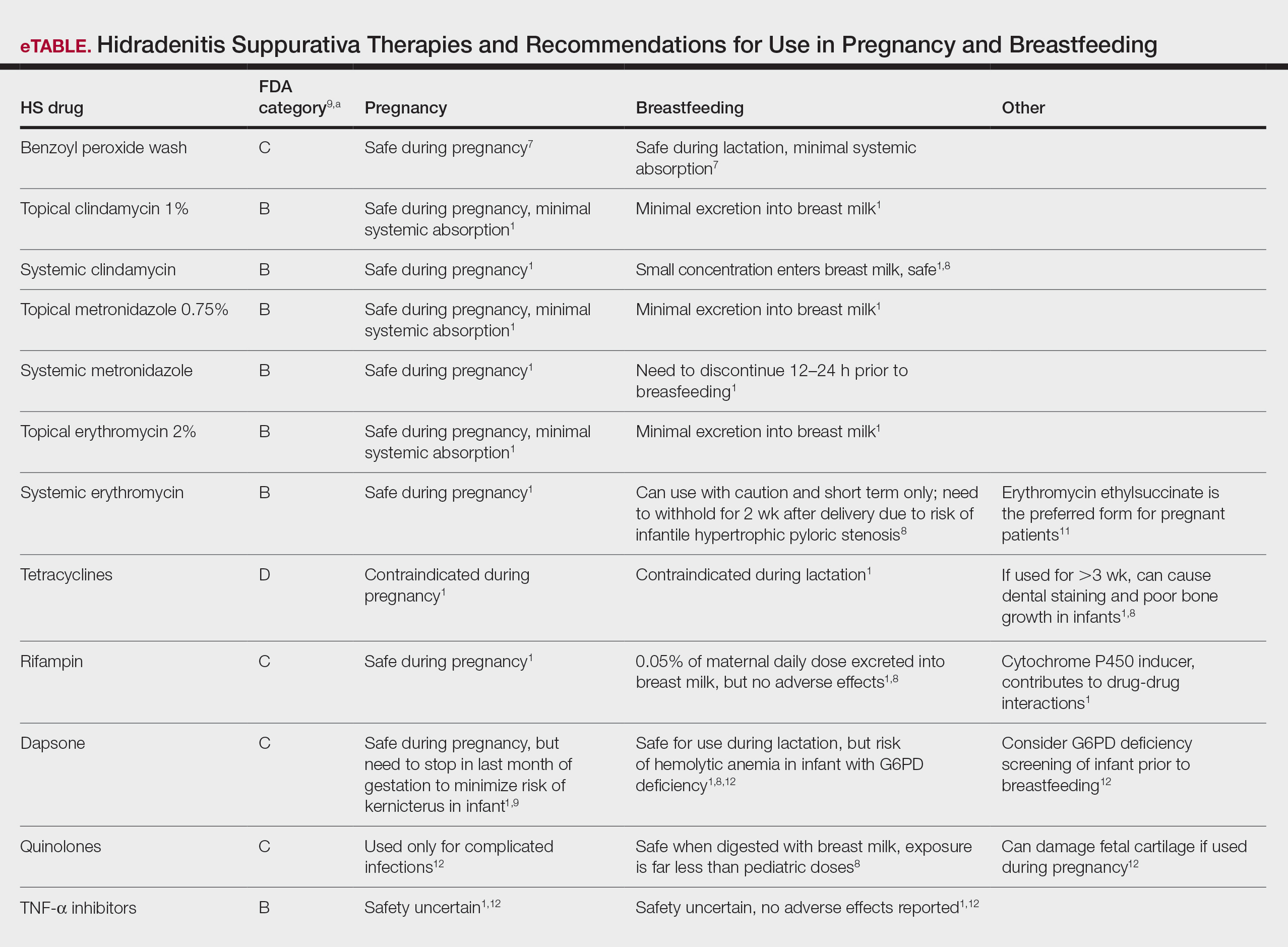
Risk Factors of HS—Predisposing risk factors for HS flares that are controllable include obesity and smoking.2 Pregnancy weight gain may cause increased skin maceration at intertriginous sites, which can contribute to worsening HS symptoms.1,5 Adipocytes play a role in HS exacerbation by promoting secretion of TNF-α, leading to increased inflammation.5 Dermatologists can help prevent postpartum HS flares by monitoring weight gain during pregnancy, encouraging smoking cessation, and promoting weight and nutrition goals as set by an obstetrician.1 In addition to medications, management of HS should include emotional support and education on wearing loose-fitting clothing to avoid irritation of the affected areas.3 An emphasis on dermatologist counseling for all patients with HS, even for those with milder disease, can reduce exacerbations during pregnancy.5
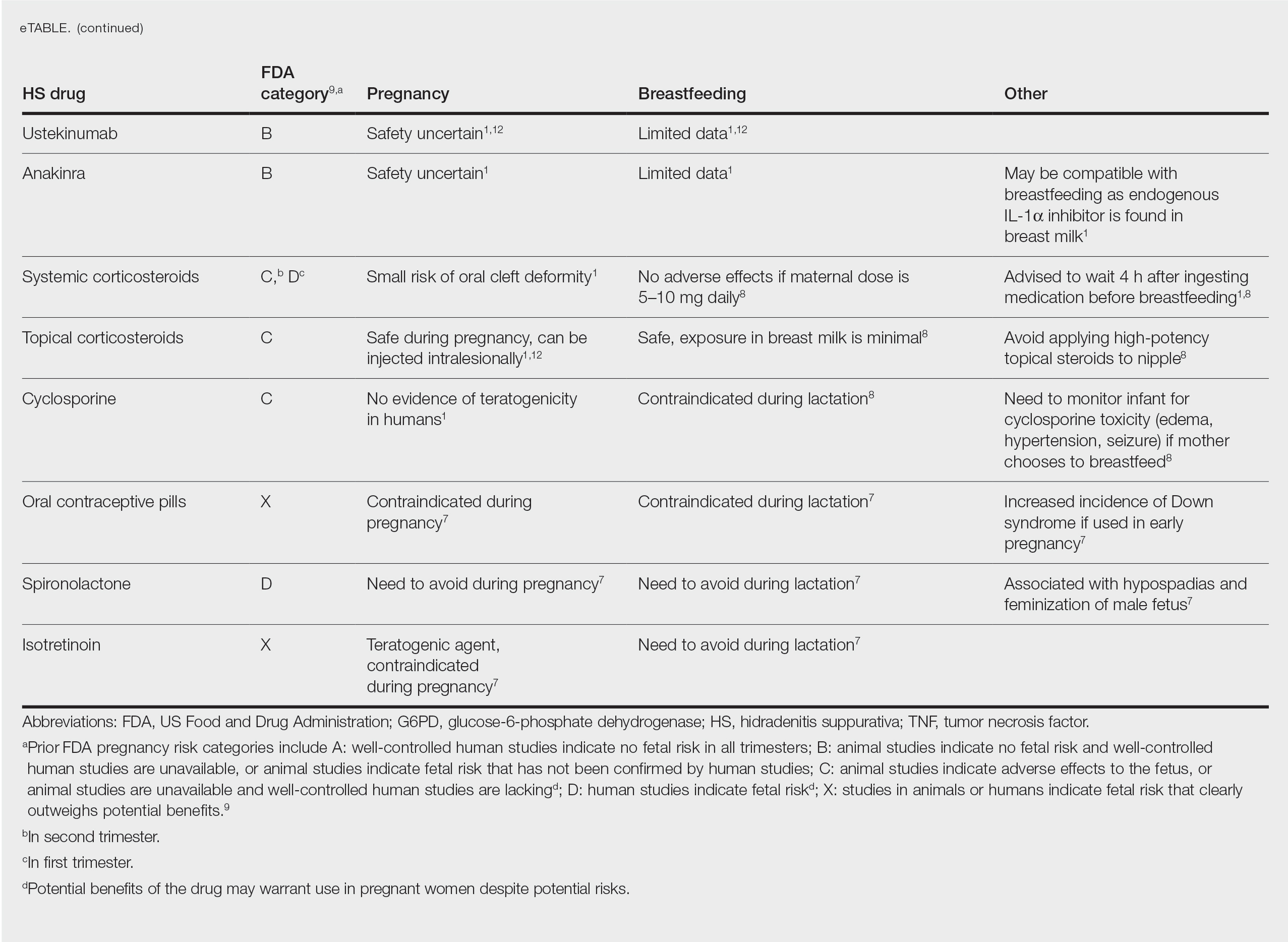
CONCLUSION
The selection of dermatologic drugs for the treatment of HS in the setting of pregnancy involves complex decision-making. Dermatologists need more guidelines and proven safety data in human trials, especially regarding use of biologics and immunosuppressants to better treat HS in pregnancy. With more data, they can create more evidence-based treatment regimens to help prevent postpartum exacerbations of HS. Thus, patients can breastfeed their infants comfortably and without any risks of impaired child development. In the meantime, dermatologists can continue to work together with obstetricians and psychiatrists to decrease disease flares through counseling patients on nutrition and weight gain and providing emotional support.
- Perng P, Zampella JG, Okoye GA. Management of hidradenitis suppurativa in pregnancy. J Am Acad Dermatol. 2017;76:979-989. doi:10.1016/j.jaad.2016.10.032
- Samuel S, Tremelling A, Murray M. Presentation and surgical management of hidradenitis suppurativa of the breast during pregnancy: a case report. Int J Surg Case Rep. 2018;51:21-24. doi:10.1016/j.ijscr.2018.08.013
- Yang CS, Teeple M, Muglia J, et al. Inflammatory and glandular skin disease in pregnancy. Clin Dermatol. 2016;34:335-343. doi:10.1016/j.clindermatol.2016.02.005
- Vossen AR, van Straalen KR, Prens EP, et al. Menses and pregnancy affect symptoms in hidradenitis suppurativa: a cross-sectional study. J Am Acad Dermatol. 2017;76:155-156. doi:10.1016/j.jaad.2016.07.024
- Lyons AB, Peacock A, McKenzie SA, et al. Evaluation of hidradenitis suppurativa disease course during pregnancy and postpartum. JAMA Dermatol. 2020;156:681-685. doi:10.1001/jamadermatol.2020.0777
- Riis PT, Ring HC, Themstrup L, et al. The role of androgens and estrogens in hidradenitis suppurativa—a systematic review. Acta Dermatovenerol Croat. 2016;24:239-249.
- Kong YL, Tey HL. Treatment of acne vulgaris during pregnancy and lactation. Drugs. 2013;73:779-787. doi:10.1007/s40265-013-0060-0
- Butler DC, Heller MM, Murase JE. Safety of dermatologic medications in pregnancy and lactation: part II. lactation. J Am Acad Dermatol. 2014;70:417:E1-E10. doi:10.1016/j.jaad.2013.09.009
- Wilmer E, Chai S, Kroumpouzos G. Drug safety: pregnancy rating classifications and controversies. Clin Dermatol. 2016;34:401-409. doi:10.1016/j.clindermatol.2016.02.013
- Inman WH, Rawson NS. Erythromycin estolate and jaundice. Br Med J (Clin Res Ed). 1983;286:1954-1955. doi:10.1136/bmj.286.6382.1954
- Workowski KA, Berman SM. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep. 2006;55(RR-11):1-94.
- Murase JE, Heller MM, Butler DC. Safety of dermatologic medications in pregnancy and lactation: part I. pregnancy. J Am Acad Dermatol. 2014;70:401.e1-14; quiz 415. doi:10.1016/j.jaad.2013.09.010
- Brown SM, Aljefri K, Waas R, et al. Systemic medications used in treatment of common dermatological conditions: safety profile with respect to pregnancy, breast feeding and content in seminal fluid. J Dermatolog Treat. 2019;30:2-18. doi:10.1080/09546634.2016.1202402
- Kamarajah SK, Arntdz K, Bundred J, et al. Outcomes of pregnancy in recipients of liver transplants. Clin Gastroenterol Hepatol. 2019;17:1398-1404.e1. doi:10.1016/j.cgh.2018.11.055
Hidradenitis suppurativa (HS) is a chronic inflammatory skin disease associated with hyperandrogenism and is caused by occlusion or rupture of follicular units and inflammation of the apocrine glands.1-3 The disease most commonly affects women (female to male ratio of 3:1) of childbearing age.1,2,4,5 Body areas affected include the axillae and groin, and less commonly the perineum; perianal region; and skin folds, such as gluteal, inframammary, and infraumbilical folds.1,2 Symptoms manifest as painful subcutaneous nodules with possible accompanying purulent drainage, sinus tracts, and/or dermal contractures. Although the pathophysiology is unclear, androgens affect the course of HS during pregnancy by stimulating the affected glands and altering cytokines.1,2,6
During pregnancy, maternal immune function switches from cell-mediated T helper cell (TH1) to humoral TH2 cytokine production. The activity of sebaceous and eccrine glands increases while the activity of apocrine glands decreases, thus changing the inflammatory course of HS during pregnancy.3 Approximately 20% of women with HS experience improvement of symptoms during pregnancy, while the remainder either experience no relief or deterioration of symptoms.1 Improvement in symptoms during pregnancy was found to occur more frequently in those who had worsening symptoms during menses owing to the possible hormonal effect estrogen has on inhibiting TH1 and TH17 proinflammatory cytokines, which promotes an immunosuppressive environment.4
Lactation and breastfeeding abilities may be hindered if a woman has HS affecting the apocrine glands of breast tissue and a symptom flare in the postpartum period. If HS causes notable inflammation in the nipple-areolar complex during pregnancy, the patient may experience difficulties with lactation and milk fistula formation, leading to inability to breastfeed.2 Another reason why mothers with HS may not be able to breastfeed is that the medications required to treat the disease are unsafe if passed to the infant via breast milk. In addition, the teratogenic effects of HS medications may necessitate therapy adjustments in pregnancy.1 Here, we provide a brief overview of the medical management considerations of HS in the setting of pregnancy and the impact on breastfeeding.
MEDICAL MANAGEMENT AND DRUG SAFETY
Dermatologists prescribe a myriad of topical and systemic medications to ameliorate symptoms of HS. Therapy regimens often are multimodal and include antibiotics, biologics, and immunosuppressants.1,3
Antibiotics
First-line antibiotics include clindamycin, metronidazole, tetracyclines, erythromycin, rifampin, dapsone, and fluoroquinolones. Topical clindamycin 1%, metronidazole 0.75%, and erythromycin 2% are used for open or active HS lesions and are all safe to use in pregnancy since there is minimal systemic absorption and minimal excretion into breast milk.1 Topical antimicrobial washes such as benzoyl peroxide and chlorhexidine often are used in combination with systemic medications to treat HS. These washes are safe during pregnancy and lactation, as they have minimal systemic absorption.7
Of these first-line antibiotics, only tetracyclines are contraindicated during pregnancy and lactation, as they are deemed to be in category D by the US Food and Drug Administration (FDA).1 Aside from tetracyclines, these antibiotics do not cause birth defects and are safe for nursing infants.1,8 Systemic clindamycin is safe during pregnancy and breastfeeding. Systemic metronidazole also is safe for use in pregnant patients but needs to be discontinued 12 to 24 hours prior to breastfeeding, which often prohibits appropriate dosing.1
Systemic Erythromycin—There are several forms of systemic erythromycin, including erythromycin base, erythromycin estolate, erythromycin ethylsuccinate (EES), and erythromycin stearate. Erythromycin estolate is contraindicated in pregnancy because it is associated with reversible maternal hepatoxicity and jaundice.9-11 Erythromycin ethylsuccinate is the preferred form for pregnant patients. Providers should exercise caution when prescribing EES to lactating mothers, as small amounts are still secreted through breast milk.11 Some studies have shown an increased risk for development of infantile hypertrophic pyloric stenosis with systemic erythromycin use, especially if a neonate is exposed in the first 14 days of life. Thus, we recommend withholding EES for 2 weeks after delivery if the patient is breastfeeding. A follow-up study did not find any association between erythromycin and infantile hypertrophic pyloric stenosis; however, the American Academy of Pediatrics still recommends short-term use only of erythromycin if it is to be used in the systemic form.8
Rifampin—Rifampin is excreted into breast milk but without adverse effects to the infant. Rifampin also is safe in pregnancy but should be used on a case-by-case basis in pregnant or nursing women because it is a cytochrome P450 inducer.
Dapsone—Dapsone has no increased risk for congenital anomalies. However, it is associated with hemolytic anemia and neonatal hyperbilirubinemia, especially in patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency.12 Newborns exposed to dapsone are at an increased risk for methemoglobinemia owing to increased sensitivity of fetal erythrocytes to oxidizing agents.13 If dapsone use is necessary, stopping dapsone treatment in the last month of gestation is recommended to minimize risk for kernicterus.9 Dapsone can be found in high concentrations in breast milk at 14.3% of the maternal dose. It is still safe to use during breastfeeding, but there is a risk of the infant developing hyperbilirubinemia/G6PD deficiency.1,8 Thus, physicians may consider performing a G6PD screen on infants to determine if breastfeeding is safe.12
Fluoroquinolones—Quinolones are not contraindicated during pregnancy, but they can damage fetal cartilage and thus should be reserved for use in complicated infections when the benefits outweigh the risks.12 Quinolones are believed to increase risk for arthropathy but are safe for use in lactation. When quinolones are digested with milk, exposure decreases below pediatric doses because of the ionized property of calcium in milk.8
Tumor Necrosis Factor α Inhibitors—The safety of anti–tumor necrosis factor (TNF) α biologics in pregnancy is less certain when compared with antibiotics.1 Anti–TNF-α inhibitors such as etanercept, adalimumab, and infliximab are all labeled as FDA category B, meaning there are no well-controlled human studies of the drugs.9 There are limited data that support safe use of TNF-α inhibitors prior to the third trimester before maternal IgG antibodies are transferred to the fetus via the placenta.1,13 Anti–TNF-α inhibitors may be safe when breastfeeding because the drugs have large molecular weights that prevent them from entering breast milk in large amounts. Absorption also is limited due to the infant’s digestive acids and enzymes breaking down the protein structure of the medication.8 Overall, TNF-α inhibitor use is still controversial and only used if the benefits outweigh the risks during pregnancy or if there is no alternative treatment.1,3,9
Ustekinumab and Anakinra—Ustekinumab (an IL-12/IL-23 inhibitor) and anakinra (an IL-1α and IL-1β inhibitor) also are FDA category B drugs and have limited data supporting their use as HS treatment in pregnancy. Anakinra may have evidence of compatibility with breastfeeding, as endogenous IL-1α inhibitor is found in colostrum and mature breast milk.1
Immunosuppressants
Immunosuppressants that are used to treat HS include corticosteroids and cyclosporine.
Corticosteroids—Topical corticosteroids can be used safely in lactation if they are not applied directly to the nipple or any area that makes direct contact with the infant’s mouth. Intralesional corticosteroid injections are safe for use during both pregnancy and breastfeeding to decrease inflammation of acutely flaring lesions and can be considered first-line treatment.1 Oral glucocorticoids also can be safely used for acute flares during pregnancy; however, prolonged use is associated with pregnancy complications such as preeclampsia, eclampsia, premature delivery, and gestational diabetes.12 There also is a small risk of oral cleft deformity in the infant; thus, potent corticosteroids are recommended in short durations during pregnancy, and there are no adverse effects if the maternal dose is less than 10 mg daily.8,12 Systemic steroids are safe to use with breastfeeding, but patients should be advised to wait 4 hours after ingesting medication before breastfeeding.1,8
Cyclosporine—Topical and oral calcineurin inhibitors such as cyclosporine have low risk for transmission into breast milk; however, potential effects of exposure through breast milk are unknown. For that reason, manufacturers state that cyclosporine use is contraindicated during lactation.8 If cyclosporine is to be used by a breastfeeding woman, monitoring cyclosporine concentrations in the infant is suggested to ensure that the exposure is less than 5% to 10% of the therapeutic dose.13 The use of cyclosporine has been extensively studied in pregnant transplant patients and is considered relatively safe for use in pregnancy.14 Cyclosporine is lipid soluble and thus is quickly metabolized and spread throughout the body; it can easily cross the placenta.9,13 Blood concentration in the fetus is 30% to 64% that of the maternal circulation. However, cyclosporine is only toxic to the fetus at maternally toxic doses, which can result in low birth weight and increased prenatal and postnatal mortality.13
Isotretinoin, Oral Contraceptive Pills, and Spironolactone
Isotretinoin and hormonal treatments such as oral contraceptive pills and spironolactone (an androgen receptor blocker) commonly are used to treat HS, but all are contraindicated in pregnancy and lactation. Isotretinoin is a well-established teratogen, but adverse effects on nursing babies have not been described. However, the manufacturer of isotretinoin advises against its use in lactation. Oral contraceptive pill use in early pregnancy is associated with increased risk for Down syndrome. Oral contraceptive pill use also is contraindicated in lactation for 2 reasons: decreased milk production and risk for fetal feminization. Antiandrogenic agents such as spironolactone have been shown to be associated with hypospadias and feminization of the male fetus.7
COMMENT
Women with HS usually require ongoing medical treatment during pregnancy and immediately postpartum; thus, it is important that treatments are proven to be safe for use in this specific population. Current management guidelines are not entirely suitable for pregnant and breastfeeding women given that many HS drugs have teratogenic effects and/or can be excreted into breast milk.1 Several treatments have uncertain safety profiles in pregnancy and breastfeeding, which calls for dermatologists to change or create new regimens for their patients. Close management also is necessary to prevent excess inflammation of breast tissue and milk fistula formation, which would hinder normal breastfeeding.
The eTable lists medications used to treat HS. The FDA category is listed next to each drug. However, it should be noted that these FDA letter categories were replaced with the Pregnancy and Lactation Labeling Rule in 2015. The letter ratings were deemed overly simplistic and replaced with narrative-based labeling that provides more detailed adverse effects and clinical considerations.9

Risk Factors of HS—Predisposing risk factors for HS flares that are controllable include obesity and smoking.2 Pregnancy weight gain may cause increased skin maceration at intertriginous sites, which can contribute to worsening HS symptoms.1,5 Adipocytes play a role in HS exacerbation by promoting secretion of TNF-α, leading to increased inflammation.5 Dermatologists can help prevent postpartum HS flares by monitoring weight gain during pregnancy, encouraging smoking cessation, and promoting weight and nutrition goals as set by an obstetrician.1 In addition to medications, management of HS should include emotional support and education on wearing loose-fitting clothing to avoid irritation of the affected areas.3 An emphasis on dermatologist counseling for all patients with HS, even for those with milder disease, can reduce exacerbations during pregnancy.5

CONCLUSION
The selection of dermatologic drugs for the treatment of HS in the setting of pregnancy involves complex decision-making. Dermatologists need more guidelines and proven safety data in human trials, especially regarding use of biologics and immunosuppressants to better treat HS in pregnancy. With more data, they can create more evidence-based treatment regimens to help prevent postpartum exacerbations of HS. Thus, patients can breastfeed their infants comfortably and without any risks of impaired child development. In the meantime, dermatologists can continue to work together with obstetricians and psychiatrists to decrease disease flares through counseling patients on nutrition and weight gain and providing emotional support.
Hidradenitis suppurativa (HS) is a chronic inflammatory skin disease associated with hyperandrogenism and is caused by occlusion or rupture of follicular units and inflammation of the apocrine glands.1-3 The disease most commonly affects women (female to male ratio of 3:1) of childbearing age.1,2,4,5 Body areas affected include the axillae and groin, and less commonly the perineum; perianal region; and skin folds, such as gluteal, inframammary, and infraumbilical folds.1,2 Symptoms manifest as painful subcutaneous nodules with possible accompanying purulent drainage, sinus tracts, and/or dermal contractures. Although the pathophysiology is unclear, androgens affect the course of HS during pregnancy by stimulating the affected glands and altering cytokines.1,2,6
During pregnancy, maternal immune function switches from cell-mediated T helper cell (TH1) to humoral TH2 cytokine production. The activity of sebaceous and eccrine glands increases while the activity of apocrine glands decreases, thus changing the inflammatory course of HS during pregnancy.3 Approximately 20% of women with HS experience improvement of symptoms during pregnancy, while the remainder either experience no relief or deterioration of symptoms.1 Improvement in symptoms during pregnancy was found to occur more frequently in those who had worsening symptoms during menses owing to the possible hormonal effect estrogen has on inhibiting TH1 and TH17 proinflammatory cytokines, which promotes an immunosuppressive environment.4
Lactation and breastfeeding abilities may be hindered if a woman has HS affecting the apocrine glands of breast tissue and a symptom flare in the postpartum period. If HS causes notable inflammation in the nipple-areolar complex during pregnancy, the patient may experience difficulties with lactation and milk fistula formation, leading to inability to breastfeed.2 Another reason why mothers with HS may not be able to breastfeed is that the medications required to treat the disease are unsafe if passed to the infant via breast milk. In addition, the teratogenic effects of HS medications may necessitate therapy adjustments in pregnancy.1 Here, we provide a brief overview of the medical management considerations of HS in the setting of pregnancy and the impact on breastfeeding.
MEDICAL MANAGEMENT AND DRUG SAFETY
Dermatologists prescribe a myriad of topical and systemic medications to ameliorate symptoms of HS. Therapy regimens often are multimodal and include antibiotics, biologics, and immunosuppressants.1,3
Antibiotics
First-line antibiotics include clindamycin, metronidazole, tetracyclines, erythromycin, rifampin, dapsone, and fluoroquinolones. Topical clindamycin 1%, metronidazole 0.75%, and erythromycin 2% are used for open or active HS lesions and are all safe to use in pregnancy since there is minimal systemic absorption and minimal excretion into breast milk.1 Topical antimicrobial washes such as benzoyl peroxide and chlorhexidine often are used in combination with systemic medications to treat HS. These washes are safe during pregnancy and lactation, as they have minimal systemic absorption.7
Of these first-line antibiotics, only tetracyclines are contraindicated during pregnancy and lactation, as they are deemed to be in category D by the US Food and Drug Administration (FDA).1 Aside from tetracyclines, these antibiotics do not cause birth defects and are safe for nursing infants.1,8 Systemic clindamycin is safe during pregnancy and breastfeeding. Systemic metronidazole also is safe for use in pregnant patients but needs to be discontinued 12 to 24 hours prior to breastfeeding, which often prohibits appropriate dosing.1
Systemic Erythromycin—There are several forms of systemic erythromycin, including erythromycin base, erythromycin estolate, erythromycin ethylsuccinate (EES), and erythromycin stearate. Erythromycin estolate is contraindicated in pregnancy because it is associated with reversible maternal hepatoxicity and jaundice.9-11 Erythromycin ethylsuccinate is the preferred form for pregnant patients. Providers should exercise caution when prescribing EES to lactating mothers, as small amounts are still secreted through breast milk.11 Some studies have shown an increased risk for development of infantile hypertrophic pyloric stenosis with systemic erythromycin use, especially if a neonate is exposed in the first 14 days of life. Thus, we recommend withholding EES for 2 weeks after delivery if the patient is breastfeeding. A follow-up study did not find any association between erythromycin and infantile hypertrophic pyloric stenosis; however, the American Academy of Pediatrics still recommends short-term use only of erythromycin if it is to be used in the systemic form.8
Rifampin—Rifampin is excreted into breast milk but without adverse effects to the infant. Rifampin also is safe in pregnancy but should be used on a case-by-case basis in pregnant or nursing women because it is a cytochrome P450 inducer.
Dapsone—Dapsone has no increased risk for congenital anomalies. However, it is associated with hemolytic anemia and neonatal hyperbilirubinemia, especially in patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency.12 Newborns exposed to dapsone are at an increased risk for methemoglobinemia owing to increased sensitivity of fetal erythrocytes to oxidizing agents.13 If dapsone use is necessary, stopping dapsone treatment in the last month of gestation is recommended to minimize risk for kernicterus.9 Dapsone can be found in high concentrations in breast milk at 14.3% of the maternal dose. It is still safe to use during breastfeeding, but there is a risk of the infant developing hyperbilirubinemia/G6PD deficiency.1,8 Thus, physicians may consider performing a G6PD screen on infants to determine if breastfeeding is safe.12
Fluoroquinolones—Quinolones are not contraindicated during pregnancy, but they can damage fetal cartilage and thus should be reserved for use in complicated infections when the benefits outweigh the risks.12 Quinolones are believed to increase risk for arthropathy but are safe for use in lactation. When quinolones are digested with milk, exposure decreases below pediatric doses because of the ionized property of calcium in milk.8
Tumor Necrosis Factor α Inhibitors—The safety of anti–tumor necrosis factor (TNF) α biologics in pregnancy is less certain when compared with antibiotics.1 Anti–TNF-α inhibitors such as etanercept, adalimumab, and infliximab are all labeled as FDA category B, meaning there are no well-controlled human studies of the drugs.9 There are limited data that support safe use of TNF-α inhibitors prior to the third trimester before maternal IgG antibodies are transferred to the fetus via the placenta.1,13 Anti–TNF-α inhibitors may be safe when breastfeeding because the drugs have large molecular weights that prevent them from entering breast milk in large amounts. Absorption also is limited due to the infant’s digestive acids and enzymes breaking down the protein structure of the medication.8 Overall, TNF-α inhibitor use is still controversial and only used if the benefits outweigh the risks during pregnancy or if there is no alternative treatment.1,3,9
Ustekinumab and Anakinra—Ustekinumab (an IL-12/IL-23 inhibitor) and anakinra (an IL-1α and IL-1β inhibitor) also are FDA category B drugs and have limited data supporting their use as HS treatment in pregnancy. Anakinra may have evidence of compatibility with breastfeeding, as endogenous IL-1α inhibitor is found in colostrum and mature breast milk.1
Immunosuppressants
Immunosuppressants that are used to treat HS include corticosteroids and cyclosporine.
Corticosteroids—Topical corticosteroids can be used safely in lactation if they are not applied directly to the nipple or any area that makes direct contact with the infant’s mouth. Intralesional corticosteroid injections are safe for use during both pregnancy and breastfeeding to decrease inflammation of acutely flaring lesions and can be considered first-line treatment.1 Oral glucocorticoids also can be safely used for acute flares during pregnancy; however, prolonged use is associated with pregnancy complications such as preeclampsia, eclampsia, premature delivery, and gestational diabetes.12 There also is a small risk of oral cleft deformity in the infant; thus, potent corticosteroids are recommended in short durations during pregnancy, and there are no adverse effects if the maternal dose is less than 10 mg daily.8,12 Systemic steroids are safe to use with breastfeeding, but patients should be advised to wait 4 hours after ingesting medication before breastfeeding.1,8
Cyclosporine—Topical and oral calcineurin inhibitors such as cyclosporine have low risk for transmission into breast milk; however, potential effects of exposure through breast milk are unknown. For that reason, manufacturers state that cyclosporine use is contraindicated during lactation.8 If cyclosporine is to be used by a breastfeeding woman, monitoring cyclosporine concentrations in the infant is suggested to ensure that the exposure is less than 5% to 10% of the therapeutic dose.13 The use of cyclosporine has been extensively studied in pregnant transplant patients and is considered relatively safe for use in pregnancy.14 Cyclosporine is lipid soluble and thus is quickly metabolized and spread throughout the body; it can easily cross the placenta.9,13 Blood concentration in the fetus is 30% to 64% that of the maternal circulation. However, cyclosporine is only toxic to the fetus at maternally toxic doses, which can result in low birth weight and increased prenatal and postnatal mortality.13
Isotretinoin, Oral Contraceptive Pills, and Spironolactone
Isotretinoin and hormonal treatments such as oral contraceptive pills and spironolactone (an androgen receptor blocker) commonly are used to treat HS, but all are contraindicated in pregnancy and lactation. Isotretinoin is a well-established teratogen, but adverse effects on nursing babies have not been described. However, the manufacturer of isotretinoin advises against its use in lactation. Oral contraceptive pill use in early pregnancy is associated with increased risk for Down syndrome. Oral contraceptive pill use also is contraindicated in lactation for 2 reasons: decreased milk production and risk for fetal feminization. Antiandrogenic agents such as spironolactone have been shown to be associated with hypospadias and feminization of the male fetus.7
COMMENT
Women with HS usually require ongoing medical treatment during pregnancy and immediately postpartum; thus, it is important that treatments are proven to be safe for use in this specific population. Current management guidelines are not entirely suitable for pregnant and breastfeeding women given that many HS drugs have teratogenic effects and/or can be excreted into breast milk.1 Several treatments have uncertain safety profiles in pregnancy and breastfeeding, which calls for dermatologists to change or create new regimens for their patients. Close management also is necessary to prevent excess inflammation of breast tissue and milk fistula formation, which would hinder normal breastfeeding.
The eTable lists medications used to treat HS. The FDA category is listed next to each drug. However, it should be noted that these FDA letter categories were replaced with the Pregnancy and Lactation Labeling Rule in 2015. The letter ratings were deemed overly simplistic and replaced with narrative-based labeling that provides more detailed adverse effects and clinical considerations.9

Risk Factors of HS—Predisposing risk factors for HS flares that are controllable include obesity and smoking.2 Pregnancy weight gain may cause increased skin maceration at intertriginous sites, which can contribute to worsening HS symptoms.1,5 Adipocytes play a role in HS exacerbation by promoting secretion of TNF-α, leading to increased inflammation.5 Dermatologists can help prevent postpartum HS flares by monitoring weight gain during pregnancy, encouraging smoking cessation, and promoting weight and nutrition goals as set by an obstetrician.1 In addition to medications, management of HS should include emotional support and education on wearing loose-fitting clothing to avoid irritation of the affected areas.3 An emphasis on dermatologist counseling for all patients with HS, even for those with milder disease, can reduce exacerbations during pregnancy.5

CONCLUSION
The selection of dermatologic drugs for the treatment of HS in the setting of pregnancy involves complex decision-making. Dermatologists need more guidelines and proven safety data in human trials, especially regarding use of biologics and immunosuppressants to better treat HS in pregnancy. With more data, they can create more evidence-based treatment regimens to help prevent postpartum exacerbations of HS. Thus, patients can breastfeed their infants comfortably and without any risks of impaired child development. In the meantime, dermatologists can continue to work together with obstetricians and psychiatrists to decrease disease flares through counseling patients on nutrition and weight gain and providing emotional support.
- Perng P, Zampella JG, Okoye GA. Management of hidradenitis suppurativa in pregnancy. J Am Acad Dermatol. 2017;76:979-989. doi:10.1016/j.jaad.2016.10.032
- Samuel S, Tremelling A, Murray M. Presentation and surgical management of hidradenitis suppurativa of the breast during pregnancy: a case report. Int J Surg Case Rep. 2018;51:21-24. doi:10.1016/j.ijscr.2018.08.013
- Yang CS, Teeple M, Muglia J, et al. Inflammatory and glandular skin disease in pregnancy. Clin Dermatol. 2016;34:335-343. doi:10.1016/j.clindermatol.2016.02.005
- Vossen AR, van Straalen KR, Prens EP, et al. Menses and pregnancy affect symptoms in hidradenitis suppurativa: a cross-sectional study. J Am Acad Dermatol. 2017;76:155-156. doi:10.1016/j.jaad.2016.07.024
- Lyons AB, Peacock A, McKenzie SA, et al. Evaluation of hidradenitis suppurativa disease course during pregnancy and postpartum. JAMA Dermatol. 2020;156:681-685. doi:10.1001/jamadermatol.2020.0777
- Riis PT, Ring HC, Themstrup L, et al. The role of androgens and estrogens in hidradenitis suppurativa—a systematic review. Acta Dermatovenerol Croat. 2016;24:239-249.
- Kong YL, Tey HL. Treatment of acne vulgaris during pregnancy and lactation. Drugs. 2013;73:779-787. doi:10.1007/s40265-013-0060-0
- Butler DC, Heller MM, Murase JE. Safety of dermatologic medications in pregnancy and lactation: part II. lactation. J Am Acad Dermatol. 2014;70:417:E1-E10. doi:10.1016/j.jaad.2013.09.009
- Wilmer E, Chai S, Kroumpouzos G. Drug safety: pregnancy rating classifications and controversies. Clin Dermatol. 2016;34:401-409. doi:10.1016/j.clindermatol.2016.02.013
- Inman WH, Rawson NS. Erythromycin estolate and jaundice. Br Med J (Clin Res Ed). 1983;286:1954-1955. doi:10.1136/bmj.286.6382.1954
- Workowski KA, Berman SM. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep. 2006;55(RR-11):1-94.
- Murase JE, Heller MM, Butler DC. Safety of dermatologic medications in pregnancy and lactation: part I. pregnancy. J Am Acad Dermatol. 2014;70:401.e1-14; quiz 415. doi:10.1016/j.jaad.2013.09.010
- Brown SM, Aljefri K, Waas R, et al. Systemic medications used in treatment of common dermatological conditions: safety profile with respect to pregnancy, breast feeding and content in seminal fluid. J Dermatolog Treat. 2019;30:2-18. doi:10.1080/09546634.2016.1202402
- Kamarajah SK, Arntdz K, Bundred J, et al. Outcomes of pregnancy in recipients of liver transplants. Clin Gastroenterol Hepatol. 2019;17:1398-1404.e1. doi:10.1016/j.cgh.2018.11.055
- Perng P, Zampella JG, Okoye GA. Management of hidradenitis suppurativa in pregnancy. J Am Acad Dermatol. 2017;76:979-989. doi:10.1016/j.jaad.2016.10.032
- Samuel S, Tremelling A, Murray M. Presentation and surgical management of hidradenitis suppurativa of the breast during pregnancy: a case report. Int J Surg Case Rep. 2018;51:21-24. doi:10.1016/j.ijscr.2018.08.013
- Yang CS, Teeple M, Muglia J, et al. Inflammatory and glandular skin disease in pregnancy. Clin Dermatol. 2016;34:335-343. doi:10.1016/j.clindermatol.2016.02.005
- Vossen AR, van Straalen KR, Prens EP, et al. Menses and pregnancy affect symptoms in hidradenitis suppurativa: a cross-sectional study. J Am Acad Dermatol. 2017;76:155-156. doi:10.1016/j.jaad.2016.07.024
- Lyons AB, Peacock A, McKenzie SA, et al. Evaluation of hidradenitis suppurativa disease course during pregnancy and postpartum. JAMA Dermatol. 2020;156:681-685. doi:10.1001/jamadermatol.2020.0777
- Riis PT, Ring HC, Themstrup L, et al. The role of androgens and estrogens in hidradenitis suppurativa—a systematic review. Acta Dermatovenerol Croat. 2016;24:239-249.
- Kong YL, Tey HL. Treatment of acne vulgaris during pregnancy and lactation. Drugs. 2013;73:779-787. doi:10.1007/s40265-013-0060-0
- Butler DC, Heller MM, Murase JE. Safety of dermatologic medications in pregnancy and lactation: part II. lactation. J Am Acad Dermatol. 2014;70:417:E1-E10. doi:10.1016/j.jaad.2013.09.009
- Wilmer E, Chai S, Kroumpouzos G. Drug safety: pregnancy rating classifications and controversies. Clin Dermatol. 2016;34:401-409. doi:10.1016/j.clindermatol.2016.02.013
- Inman WH, Rawson NS. Erythromycin estolate and jaundice. Br Med J (Clin Res Ed). 1983;286:1954-1955. doi:10.1136/bmj.286.6382.1954
- Workowski KA, Berman SM. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep. 2006;55(RR-11):1-94.
- Murase JE, Heller MM, Butler DC. Safety of dermatologic medications in pregnancy and lactation: part I. pregnancy. J Am Acad Dermatol. 2014;70:401.e1-14; quiz 415. doi:10.1016/j.jaad.2013.09.010
- Brown SM, Aljefri K, Waas R, et al. Systemic medications used in treatment of common dermatological conditions: safety profile with respect to pregnancy, breast feeding and content in seminal fluid. J Dermatolog Treat. 2019;30:2-18. doi:10.1080/09546634.2016.1202402
- Kamarajah SK, Arntdz K, Bundred J, et al. Outcomes of pregnancy in recipients of liver transplants. Clin Gastroenterol Hepatol. 2019;17:1398-1404.e1. doi:10.1016/j.cgh.2018.11.055
Practice Points
- Some medications used to treat hidradenitis suppurativa (HS) may have teratogenic effects and be contraindicated during breastfeeding.
- We summarize what treatments are proven to be safe in pregnancy and breastfeeding and highlight the need for more guidelines and safety data for dermatologists to manage their pregnant patients with HS.
Reactivation of a BCG Vaccination Scar Following the First Dose of the Moderna COVID-19 Vaccine
The COVID-19 pandemic has resulted in notable morbidity and mortality worldwide. In December 2020, the US Food and Drug Administration issued an Emergency Use Authorization for 2 messenger RNA (mRNA) vaccines—produced by Pfizer-BioNTech and Moderna—for the prevention of COVID-19. Phase 3 trials of the vaccine developed by Moderna showed 94.1% efficacy at preventing COVID-19 after 2 doses.1
Common cutaneous adverse effects of the Moderna COVID-19 Vaccine include injection-site reactions, such as pain, induration, and erythema. Less frequently reported dermatologic adverse effects include diffuse bullous rash and hypersensitivity reactions.1 We report a case of reactivation of a BCG vaccination scar after the first dose of the Moderna COVID-19 Vaccine.
Case Report
A 48-year-old Asian man who was otherwise healthy presented with erythema, induration, and mild pruritus on the deltoid muscle of the left arm, near the scar from an earlier BCG vaccine, which he received at approximately 5 years of age when living in Taiwan. The patient received the first dose of the Moderna COVID-19 Vaccine approximately 5 to 7 cm distant from the BCG vaccination scar. One to 2 days after inoculation, the patient endorsed tenderness at the site of COVID-19 vaccination but denied systemic symptoms. He had never been given a diagnosis of COVID-19. His SARS-CoV-2 antibody status was unknown.
Eight days later, the patient noticed a well-defined, erythematous, indurated plaque with mild itchiness overlying and around the BCG vaccination scar that did not involve the COVID-19 vaccination site. The following day, the redness and induration became worse (Figure).
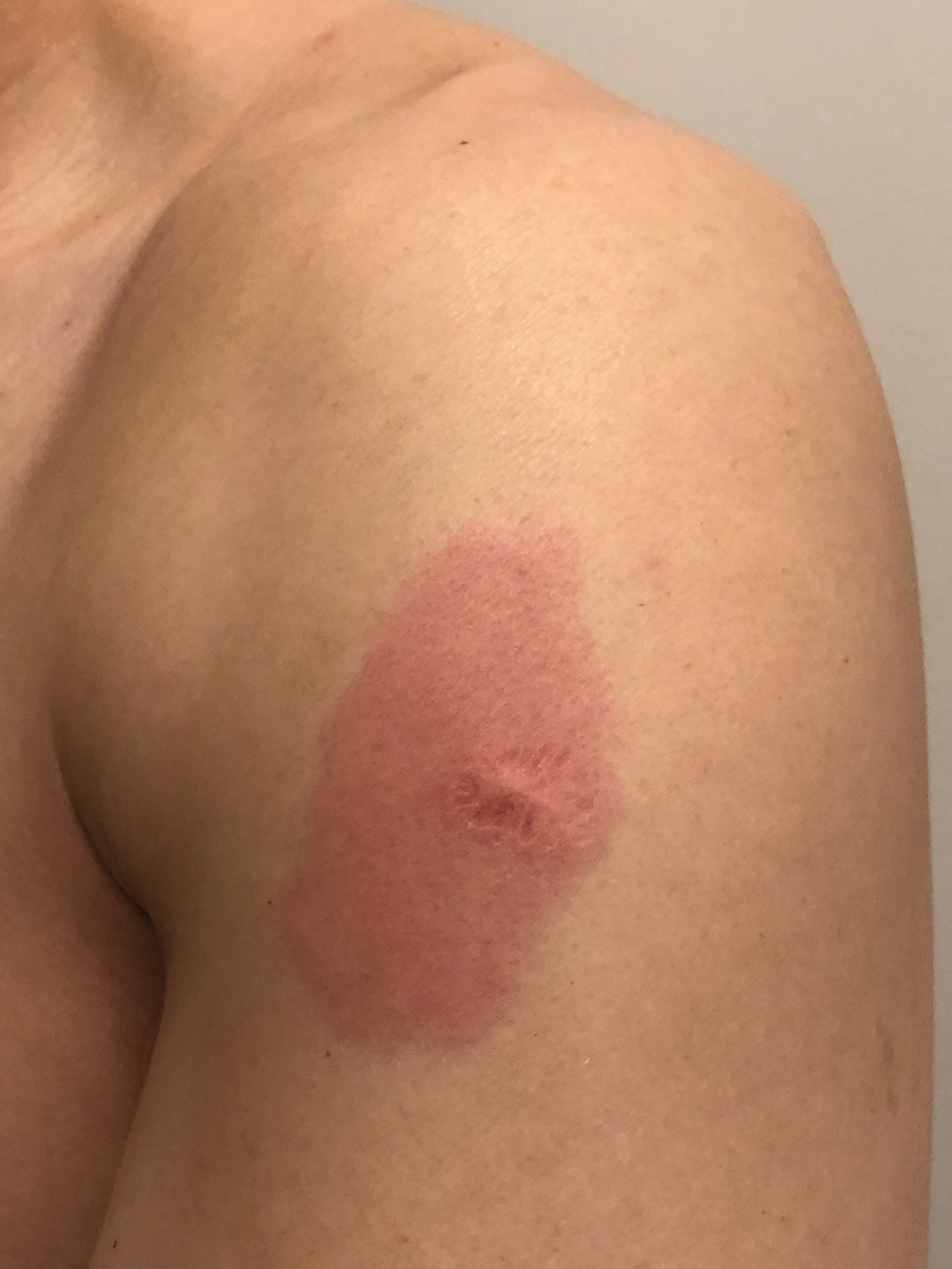
The patient was otherwise well. Vital signs were normal; there was no lymphadenopathy. The rash resolved without treatment over the next 4 days.
Comment
The BCG vaccine is an intradermal live attenuated virus vaccine used to prevent certain forms of tuberculosis and potentially other Mycobacterium infections. Although the vaccine is not routinely administered in the United States, it is part of the vaccination schedule in most countries, administered most often to newborns and infants. Administration of the BCG vaccine commonly results in mild localized erythema, swelling, and pain at the injection site. Most inoculated patients also develop an ulcer that heals with the characteristic BCG vaccination scar.2,3
There is evidence that the BCG vaccine can enhance the innate immune system response and might decrease the rate of infection by unrelated pathogens, including viruses.4 Several epidemiologic studies have suggested that the BCG vaccine might offer some protection against COVID-19, possibly due to a resemblance of the amino acid sequences of BCG and SARS-CoV-2, which might provoke cross-reactive T cells.5,6 Further studies are underway to determine whether the BCG vaccine is truly protective against COVID-19.
BCG vaccination scar reactivation presents as redness, swelling, or ulceration at the BCG injection site months to years after inoculation. Although erythema and induration of the BCG scar are not included in the diagnostic criteria of Kawasaki disease, likely due to variable vaccine requirements in different countries, these findings are largely recognized as specific for Kawasaki disease and present in approximately half of affected patients who received the BCG vaccine.2
Heat Shock Proteins—Heat shock proteins (HSPs) are produced by cells in response to stressors. The proposed mechanism of BCG vaccination scar reactivation is a cross-reaction between human homologue HSP 63 and Mycobacterium HSP 65, leading to hyperactivity of the immune system against BCG.7 There also are reports of reactivation of a BCG vaccination scar from measles infection and influenza vaccination.2,8,9 Most prior reports of BCG vaccination scar reactivation have been in pediatric patients; our patient is an adult who received the BCG vaccine more than 40 years ago.
Mechanism of Reactivation—The mechanism of BCG vaccination scar reactivation in our patient, who received the Moderna COVID-19 Vaccine, is unclear. Possible mechanisms include (1) release of HSP mediated by the COVID-19 vaccine, leading to an immune response at the BCG vaccine scar, or (2) another immune-mediated cross-reaction between BCG and the Moderna COVID-19 Vaccine mRNA nanoparticle or encoded spike protein antigen. It has been hypothesized that the BCG vaccine might offer some protection against COVID-19; this remains uncertain and is under further investigation.10 A recent retrospective cohort study showed that a BCG vaccination booster may decrease COVID-19 infection rates in higher-risk populations.11
Conclusion
We present a case of BCG vaccine scar reactivation occurring after a dose of the Moderna COVID-19 Vaccine, a likely underreported, self-limiting, cutaneous adverse effect of this mRNA vaccine.
- Baden LR, El Sahly HM, Essink B, et al; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2020;384:403-416. doi:10.1056/NEJMoa2035389
- Muthuvelu S, Lim KS, Huang L-Y, et al. Measles infection causing bacillus Calmette-Guérin reactivation: a case report. BMC Pediatr. 2019;19:251. doi:10.1186/s12887-019-1635-z
- Fatima S, Kumari A, Das G, et al. Tuberculosis vaccine: a journey from BCG to present. Life Sci. 2020;252:117594. doi:10.1016/j.lfs.2020.117594
- O’Neill LAJ, Netea MG. BCG-induced trained immunity: can it offer protection against COVID-19? Nat Rev Immunol. 2020;20:335-337. doi:10.1038/s41577-020-0337-y
- Brooks NA, Puri A, Garg S, et al. The association of coronavirus disease-19 mortality and prior bacille Calmette-Guérin vaccination: a robust ecological analysis using unsupervised machine learning. Sci Rep. 2021;11:774. doi:10.1038/s41598-020-80787-z
- Tomita Y, Sato R, Ikeda T, et al. BCG vaccine may generate cross-reactive T-cells against SARS-CoV-2: in silico analyses and a hypothesis. Vaccine. 2020;38:6352-6356. doi:10.1016/j.vaccine.2020.08.045
- Lim KYY, Chua MC, Tan NWH, et al. Reactivation of BCG inoculation site in a child with febrile exanthema of 3 days duration: an early indicator of incomplete Kawasaki disease. BMJ Case Rep. 2020;13:E239648. doi:10.1136/bcr-2020-239648
- Kondo M, Goto H, Yamamoto S. First case of redness and erosion at bacillus Calmette-Guérin inoculation site after vaccination against influenza. J Dermatol. 2016;43:1229-1231. doi:10.1111/1346-8138.13365
- Chavarri-Guerra Y, Soto-Pérez-de-Celis E. Erythema at the bacillus Calmette-Guerin scar after influenza vaccination. Rev Soc Bras Med Trop. 2019;53:E20190390. doi:10.1590/0037-8682-0390-2019
- Fu W, Ho P-C, Liu C-L, et al. Reconcile the debate over protective effects of BCG vaccine against COVID-19. Sci Rep. 2021;11:8356. doi:10.1038/s41598-021-87731-9
- Amirlak L, Haddad R, Hardy JD, et al. Effectiveness of booster BCG vaccination in preventing COVID-19 infection. Hum Vaccin Immunother. 2021;17:3913-3915. doi:10.1080/21645515.2021.1956228
The COVID-19 pandemic has resulted in notable morbidity and mortality worldwide. In December 2020, the US Food and Drug Administration issued an Emergency Use Authorization for 2 messenger RNA (mRNA) vaccines—produced by Pfizer-BioNTech and Moderna—for the prevention of COVID-19. Phase 3 trials of the vaccine developed by Moderna showed 94.1% efficacy at preventing COVID-19 after 2 doses.1
Common cutaneous adverse effects of the Moderna COVID-19 Vaccine include injection-site reactions, such as pain, induration, and erythema. Less frequently reported dermatologic adverse effects include diffuse bullous rash and hypersensitivity reactions.1 We report a case of reactivation of a BCG vaccination scar after the first dose of the Moderna COVID-19 Vaccine.
Case Report
A 48-year-old Asian man who was otherwise healthy presented with erythema, induration, and mild pruritus on the deltoid muscle of the left arm, near the scar from an earlier BCG vaccine, which he received at approximately 5 years of age when living in Taiwan. The patient received the first dose of the Moderna COVID-19 Vaccine approximately 5 to 7 cm distant from the BCG vaccination scar. One to 2 days after inoculation, the patient endorsed tenderness at the site of COVID-19 vaccination but denied systemic symptoms. He had never been given a diagnosis of COVID-19. His SARS-CoV-2 antibody status was unknown.
Eight days later, the patient noticed a well-defined, erythematous, indurated plaque with mild itchiness overlying and around the BCG vaccination scar that did not involve the COVID-19 vaccination site. The following day, the redness and induration became worse (Figure).

The patient was otherwise well. Vital signs were normal; there was no lymphadenopathy. The rash resolved without treatment over the next 4 days.
Comment
The BCG vaccine is an intradermal live attenuated virus vaccine used to prevent certain forms of tuberculosis and potentially other Mycobacterium infections. Although the vaccine is not routinely administered in the United States, it is part of the vaccination schedule in most countries, administered most often to newborns and infants. Administration of the BCG vaccine commonly results in mild localized erythema, swelling, and pain at the injection site. Most inoculated patients also develop an ulcer that heals with the characteristic BCG vaccination scar.2,3
There is evidence that the BCG vaccine can enhance the innate immune system response and might decrease the rate of infection by unrelated pathogens, including viruses.4 Several epidemiologic studies have suggested that the BCG vaccine might offer some protection against COVID-19, possibly due to a resemblance of the amino acid sequences of BCG and SARS-CoV-2, which might provoke cross-reactive T cells.5,6 Further studies are underway to determine whether the BCG vaccine is truly protective against COVID-19.
BCG vaccination scar reactivation presents as redness, swelling, or ulceration at the BCG injection site months to years after inoculation. Although erythema and induration of the BCG scar are not included in the diagnostic criteria of Kawasaki disease, likely due to variable vaccine requirements in different countries, these findings are largely recognized as specific for Kawasaki disease and present in approximately half of affected patients who received the BCG vaccine.2
Heat Shock Proteins—Heat shock proteins (HSPs) are produced by cells in response to stressors. The proposed mechanism of BCG vaccination scar reactivation is a cross-reaction between human homologue HSP 63 and Mycobacterium HSP 65, leading to hyperactivity of the immune system against BCG.7 There also are reports of reactivation of a BCG vaccination scar from measles infection and influenza vaccination.2,8,9 Most prior reports of BCG vaccination scar reactivation have been in pediatric patients; our patient is an adult who received the BCG vaccine more than 40 years ago.
Mechanism of Reactivation—The mechanism of BCG vaccination scar reactivation in our patient, who received the Moderna COVID-19 Vaccine, is unclear. Possible mechanisms include (1) release of HSP mediated by the COVID-19 vaccine, leading to an immune response at the BCG vaccine scar, or (2) another immune-mediated cross-reaction between BCG and the Moderna COVID-19 Vaccine mRNA nanoparticle or encoded spike protein antigen. It has been hypothesized that the BCG vaccine might offer some protection against COVID-19; this remains uncertain and is under further investigation.10 A recent retrospective cohort study showed that a BCG vaccination booster may decrease COVID-19 infection rates in higher-risk populations.11
Conclusion
We present a case of BCG vaccine scar reactivation occurring after a dose of the Moderna COVID-19 Vaccine, a likely underreported, self-limiting, cutaneous adverse effect of this mRNA vaccine.
The COVID-19 pandemic has resulted in notable morbidity and mortality worldwide. In December 2020, the US Food and Drug Administration issued an Emergency Use Authorization for 2 messenger RNA (mRNA) vaccines—produced by Pfizer-BioNTech and Moderna—for the prevention of COVID-19. Phase 3 trials of the vaccine developed by Moderna showed 94.1% efficacy at preventing COVID-19 after 2 doses.1
Common cutaneous adverse effects of the Moderna COVID-19 Vaccine include injection-site reactions, such as pain, induration, and erythema. Less frequently reported dermatologic adverse effects include diffuse bullous rash and hypersensitivity reactions.1 We report a case of reactivation of a BCG vaccination scar after the first dose of the Moderna COVID-19 Vaccine.
Case Report
A 48-year-old Asian man who was otherwise healthy presented with erythema, induration, and mild pruritus on the deltoid muscle of the left arm, near the scar from an earlier BCG vaccine, which he received at approximately 5 years of age when living in Taiwan. The patient received the first dose of the Moderna COVID-19 Vaccine approximately 5 to 7 cm distant from the BCG vaccination scar. One to 2 days after inoculation, the patient endorsed tenderness at the site of COVID-19 vaccination but denied systemic symptoms. He had never been given a diagnosis of COVID-19. His SARS-CoV-2 antibody status was unknown.
Eight days later, the patient noticed a well-defined, erythematous, indurated plaque with mild itchiness overlying and around the BCG vaccination scar that did not involve the COVID-19 vaccination site. The following day, the redness and induration became worse (Figure).

The patient was otherwise well. Vital signs were normal; there was no lymphadenopathy. The rash resolved without treatment over the next 4 days.
Comment
The BCG vaccine is an intradermal live attenuated virus vaccine used to prevent certain forms of tuberculosis and potentially other Mycobacterium infections. Although the vaccine is not routinely administered in the United States, it is part of the vaccination schedule in most countries, administered most often to newborns and infants. Administration of the BCG vaccine commonly results in mild localized erythema, swelling, and pain at the injection site. Most inoculated patients also develop an ulcer that heals with the characteristic BCG vaccination scar.2,3
There is evidence that the BCG vaccine can enhance the innate immune system response and might decrease the rate of infection by unrelated pathogens, including viruses.4 Several epidemiologic studies have suggested that the BCG vaccine might offer some protection against COVID-19, possibly due to a resemblance of the amino acid sequences of BCG and SARS-CoV-2, which might provoke cross-reactive T cells.5,6 Further studies are underway to determine whether the BCG vaccine is truly protective against COVID-19.
BCG vaccination scar reactivation presents as redness, swelling, or ulceration at the BCG injection site months to years after inoculation. Although erythema and induration of the BCG scar are not included in the diagnostic criteria of Kawasaki disease, likely due to variable vaccine requirements in different countries, these findings are largely recognized as specific for Kawasaki disease and present in approximately half of affected patients who received the BCG vaccine.2
Heat Shock Proteins—Heat shock proteins (HSPs) are produced by cells in response to stressors. The proposed mechanism of BCG vaccination scar reactivation is a cross-reaction between human homologue HSP 63 and Mycobacterium HSP 65, leading to hyperactivity of the immune system against BCG.7 There also are reports of reactivation of a BCG vaccination scar from measles infection and influenza vaccination.2,8,9 Most prior reports of BCG vaccination scar reactivation have been in pediatric patients; our patient is an adult who received the BCG vaccine more than 40 years ago.
Mechanism of Reactivation—The mechanism of BCG vaccination scar reactivation in our patient, who received the Moderna COVID-19 Vaccine, is unclear. Possible mechanisms include (1) release of HSP mediated by the COVID-19 vaccine, leading to an immune response at the BCG vaccine scar, or (2) another immune-mediated cross-reaction between BCG and the Moderna COVID-19 Vaccine mRNA nanoparticle or encoded spike protein antigen. It has been hypothesized that the BCG vaccine might offer some protection against COVID-19; this remains uncertain and is under further investigation.10 A recent retrospective cohort study showed that a BCG vaccination booster may decrease COVID-19 infection rates in higher-risk populations.11
Conclusion
We present a case of BCG vaccine scar reactivation occurring after a dose of the Moderna COVID-19 Vaccine, a likely underreported, self-limiting, cutaneous adverse effect of this mRNA vaccine.
- Baden LR, El Sahly HM, Essink B, et al; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2020;384:403-416. doi:10.1056/NEJMoa2035389
- Muthuvelu S, Lim KS, Huang L-Y, et al. Measles infection causing bacillus Calmette-Guérin reactivation: a case report. BMC Pediatr. 2019;19:251. doi:10.1186/s12887-019-1635-z
- Fatima S, Kumari A, Das G, et al. Tuberculosis vaccine: a journey from BCG to present. Life Sci. 2020;252:117594. doi:10.1016/j.lfs.2020.117594
- O’Neill LAJ, Netea MG. BCG-induced trained immunity: can it offer protection against COVID-19? Nat Rev Immunol. 2020;20:335-337. doi:10.1038/s41577-020-0337-y
- Brooks NA, Puri A, Garg S, et al. The association of coronavirus disease-19 mortality and prior bacille Calmette-Guérin vaccination: a robust ecological analysis using unsupervised machine learning. Sci Rep. 2021;11:774. doi:10.1038/s41598-020-80787-z
- Tomita Y, Sato R, Ikeda T, et al. BCG vaccine may generate cross-reactive T-cells against SARS-CoV-2: in silico analyses and a hypothesis. Vaccine. 2020;38:6352-6356. doi:10.1016/j.vaccine.2020.08.045
- Lim KYY, Chua MC, Tan NWH, et al. Reactivation of BCG inoculation site in a child with febrile exanthema of 3 days duration: an early indicator of incomplete Kawasaki disease. BMJ Case Rep. 2020;13:E239648. doi:10.1136/bcr-2020-239648
- Kondo M, Goto H, Yamamoto S. First case of redness and erosion at bacillus Calmette-Guérin inoculation site after vaccination against influenza. J Dermatol. 2016;43:1229-1231. doi:10.1111/1346-8138.13365
- Chavarri-Guerra Y, Soto-Pérez-de-Celis E. Erythema at the bacillus Calmette-Guerin scar after influenza vaccination. Rev Soc Bras Med Trop. 2019;53:E20190390. doi:10.1590/0037-8682-0390-2019
- Fu W, Ho P-C, Liu C-L, et al. Reconcile the debate over protective effects of BCG vaccine against COVID-19. Sci Rep. 2021;11:8356. doi:10.1038/s41598-021-87731-9
- Amirlak L, Haddad R, Hardy JD, et al. Effectiveness of booster BCG vaccination in preventing COVID-19 infection. Hum Vaccin Immunother. 2021;17:3913-3915. doi:10.1080/21645515.2021.1956228
- Baden LR, El Sahly HM, Essink B, et al; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2020;384:403-416. doi:10.1056/NEJMoa2035389
- Muthuvelu S, Lim KS, Huang L-Y, et al. Measles infection causing bacillus Calmette-Guérin reactivation: a case report. BMC Pediatr. 2019;19:251. doi:10.1186/s12887-019-1635-z
- Fatima S, Kumari A, Das G, et al. Tuberculosis vaccine: a journey from BCG to present. Life Sci. 2020;252:117594. doi:10.1016/j.lfs.2020.117594
- O’Neill LAJ, Netea MG. BCG-induced trained immunity: can it offer protection against COVID-19? Nat Rev Immunol. 2020;20:335-337. doi:10.1038/s41577-020-0337-y
- Brooks NA, Puri A, Garg S, et al. The association of coronavirus disease-19 mortality and prior bacille Calmette-Guérin vaccination: a robust ecological analysis using unsupervised machine learning. Sci Rep. 2021;11:774. doi:10.1038/s41598-020-80787-z
- Tomita Y, Sato R, Ikeda T, et al. BCG vaccine may generate cross-reactive T-cells against SARS-CoV-2: in silico analyses and a hypothesis. Vaccine. 2020;38:6352-6356. doi:10.1016/j.vaccine.2020.08.045
- Lim KYY, Chua MC, Tan NWH, et al. Reactivation of BCG inoculation site in a child with febrile exanthema of 3 days duration: an early indicator of incomplete Kawasaki disease. BMJ Case Rep. 2020;13:E239648. doi:10.1136/bcr-2020-239648
- Kondo M, Goto H, Yamamoto S. First case of redness and erosion at bacillus Calmette-Guérin inoculation site after vaccination against influenza. J Dermatol. 2016;43:1229-1231. doi:10.1111/1346-8138.13365
- Chavarri-Guerra Y, Soto-Pérez-de-Celis E. Erythema at the bacillus Calmette-Guerin scar after influenza vaccination. Rev Soc Bras Med Trop. 2019;53:E20190390. doi:10.1590/0037-8682-0390-2019
- Fu W, Ho P-C, Liu C-L, et al. Reconcile the debate over protective effects of BCG vaccine against COVID-19. Sci Rep. 2021;11:8356. doi:10.1038/s41598-021-87731-9
- Amirlak L, Haddad R, Hardy JD, et al. Effectiveness of booster BCG vaccination in preventing COVID-19 infection. Hum Vaccin Immunother. 2021;17:3913-3915. doi:10.1080/21645515.2021.1956228
Practice Points
- BCG vaccination scar reactivation is a potential benign, self-limited reaction in patients who receive the Moderna COVID-19 Vaccine.
- Symptoms of BCG vaccination scar reactivation, which is seen more commonly in children with Kawasaki disease, include redness, swelling, and ulceration.
Drug Overdose Suicide Rates: Down, But Also Up
Who is most at risk of suicide by drug overdose? Has that changed in recent years? Researchers at the National Institute on Drug Abuse analyzed data from 2001 to 2019 from the Centers for Disease Control and Prevention’s National Vital Statistics System to find out.
On the whole, they say, intentional overdose deaths have declined. But suicide rates increased in certain subgroups: young adults (aged 15-24 years), older adults (aged 75-84 years), and non-Hispanic Black women. Rates among women were “consistently higher” than those of men. The highest rates were observed in women aged 45 to 64 years.
Monday was the worst day, and the weekends had the lowest rates. The researchers say social factors, such as more social interactions on the weekend and reluctance about starting the workweek, could be factors.
Seasonally, the numbers ran true to the pattern seen in previous studies: The lowest rates occurred in December and highest in late spring and summer. Perhaps the “collective optimism” of the holiday season and social interactions exert protective effects against suicidality, the researchers suggest.
Factors also may include biological changes. In this study, the researchers found a positive linear relationship between daylength, which varies by latitude, and intentional overdose deaths for both sexes. Daylength is associated with mu opioid receptor (MOR) availability that might underlie seasonal variations in mood, they posit. MORs are the main target of opioid drugs; the researchers cite a study that found altered MOR expression in postmortem brains of suicide victims.
They note some limitations of their study, one being that, in 2019, 5% of overdose deaths had undetermined intent. Improving classifications of overdose deaths is needed, they say.
Moreover, the trends might have changed during the pandemic, as provisional mortality data indicate decreases in deaths by suicides, but also an approximate 30% increase in overall overdose deaths.
“This research underscores the importance of external support structures and environmental factors in determining a person’s suicide risk,” said Emily B. Einstein, PhD, chief of the National Institute on Drug Abuse’s Science Policy Branch and an author on the study. “The risk of intentional overdoses, and suicide risk in general, is not static. This is crucial for clinicians to keep in mind, as they may need to assess patients’ suicide risk frequently rather than at one point in time. It is also important for friends and family members of people who may be at an increased risk of suicide, and for those people themselves, so that they can be aware of the greatest periods of risk and seek help when needed.”
Sources: https://www.nih.gov/news-events/news-releases/suicides-drug-overdose-increased-among-young-people-elderly-people-black-women-despite-overall-downward-tren
Han B, Compton WM, Einstein EB, et al. Intentional drug overdose deaths in the United States. Am J Psychiatry. doi:10.1176/appi.ajp.2021.21060604
Who is most at risk of suicide by drug overdose? Has that changed in recent years? Researchers at the National Institute on Drug Abuse analyzed data from 2001 to 2019 from the Centers for Disease Control and Prevention’s National Vital Statistics System to find out.
On the whole, they say, intentional overdose deaths have declined. But suicide rates increased in certain subgroups: young adults (aged 15-24 years), older adults (aged 75-84 years), and non-Hispanic Black women. Rates among women were “consistently higher” than those of men. The highest rates were observed in women aged 45 to 64 years.
Monday was the worst day, and the weekends had the lowest rates. The researchers say social factors, such as more social interactions on the weekend and reluctance about starting the workweek, could be factors.
Seasonally, the numbers ran true to the pattern seen in previous studies: The lowest rates occurred in December and highest in late spring and summer. Perhaps the “collective optimism” of the holiday season and social interactions exert protective effects against suicidality, the researchers suggest.
Factors also may include biological changes. In this study, the researchers found a positive linear relationship between daylength, which varies by latitude, and intentional overdose deaths for both sexes. Daylength is associated with mu opioid receptor (MOR) availability that might underlie seasonal variations in mood, they posit. MORs are the main target of opioid drugs; the researchers cite a study that found altered MOR expression in postmortem brains of suicide victims.
They note some limitations of their study, one being that, in 2019, 5% of overdose deaths had undetermined intent. Improving classifications of overdose deaths is needed, they say.
Moreover, the trends might have changed during the pandemic, as provisional mortality data indicate decreases in deaths by suicides, but also an approximate 30% increase in overall overdose deaths.
“This research underscores the importance of external support structures and environmental factors in determining a person’s suicide risk,” said Emily B. Einstein, PhD, chief of the National Institute on Drug Abuse’s Science Policy Branch and an author on the study. “The risk of intentional overdoses, and suicide risk in general, is not static. This is crucial for clinicians to keep in mind, as they may need to assess patients’ suicide risk frequently rather than at one point in time. It is also important for friends and family members of people who may be at an increased risk of suicide, and for those people themselves, so that they can be aware of the greatest periods of risk and seek help when needed.”
Sources: https://www.nih.gov/news-events/news-releases/suicides-drug-overdose-increased-among-young-people-elderly-people-black-women-despite-overall-downward-tren
Han B, Compton WM, Einstein EB, et al. Intentional drug overdose deaths in the United States. Am J Psychiatry. doi:10.1176/appi.ajp.2021.21060604
Who is most at risk of suicide by drug overdose? Has that changed in recent years? Researchers at the National Institute on Drug Abuse analyzed data from 2001 to 2019 from the Centers for Disease Control and Prevention’s National Vital Statistics System to find out.
On the whole, they say, intentional overdose deaths have declined. But suicide rates increased in certain subgroups: young adults (aged 15-24 years), older adults (aged 75-84 years), and non-Hispanic Black women. Rates among women were “consistently higher” than those of men. The highest rates were observed in women aged 45 to 64 years.
Monday was the worst day, and the weekends had the lowest rates. The researchers say social factors, such as more social interactions on the weekend and reluctance about starting the workweek, could be factors.
Seasonally, the numbers ran true to the pattern seen in previous studies: The lowest rates occurred in December and highest in late spring and summer. Perhaps the “collective optimism” of the holiday season and social interactions exert protective effects against suicidality, the researchers suggest.
Factors also may include biological changes. In this study, the researchers found a positive linear relationship between daylength, which varies by latitude, and intentional overdose deaths for both sexes. Daylength is associated with mu opioid receptor (MOR) availability that might underlie seasonal variations in mood, they posit. MORs are the main target of opioid drugs; the researchers cite a study that found altered MOR expression in postmortem brains of suicide victims.
They note some limitations of their study, one being that, in 2019, 5% of overdose deaths had undetermined intent. Improving classifications of overdose deaths is needed, they say.
Moreover, the trends might have changed during the pandemic, as provisional mortality data indicate decreases in deaths by suicides, but also an approximate 30% increase in overall overdose deaths.
“This research underscores the importance of external support structures and environmental factors in determining a person’s suicide risk,” said Emily B. Einstein, PhD, chief of the National Institute on Drug Abuse’s Science Policy Branch and an author on the study. “The risk of intentional overdoses, and suicide risk in general, is not static. This is crucial for clinicians to keep in mind, as they may need to assess patients’ suicide risk frequently rather than at one point in time. It is also important for friends and family members of people who may be at an increased risk of suicide, and for those people themselves, so that they can be aware of the greatest periods of risk and seek help when needed.”
Sources: https://www.nih.gov/news-events/news-releases/suicides-drug-overdose-increased-among-young-people-elderly-people-black-women-despite-overall-downward-tren
Han B, Compton WM, Einstein EB, et al. Intentional drug overdose deaths in the United States. Am J Psychiatry. doi:10.1176/appi.ajp.2021.21060604
15th Report on Carcinogens Adds to Its List
From environmental tobacco smoke to ultraviolet (UV) radiation, diesel exhaust particulates, lead, and now, chronic infection with Helicobacter pylori (H pylori)—the Report on Carcinogens has regularly updated the list of substances known or “reasonably anticipated” to cause cancer.
The 15th report, which is prepared by the National Toxicology Program (NTP) for the Department of Health and Human Services, has 8 new entries, bringing the number of human carcinogens (eg, metals, pesticides, and drugs) on the list to 256. (The first report, released in 1980, listed 26.) In addition to H pylori infection, this edition adds the flame-retardant chemical antimony trioxide, and 6 haloacetic acids found as water disinfection byproducts.
In 1971, then President Nixon declared “war on cancer” (the second leading cause of death in the US) and signed the National Cancer Act. In 1978, Congress ordered the Report on Carcinogens, to educate the public and health professionals on potential environmental carcinogenic hazards.
Perhaps disheartening to know that even with 256 entries, the list probably understates the number of carcinogens humans and other creatures are exposed to. But things can change with time. Each list goes through a rigorous round of reviews. Sometimes substances are “delisted” after, for instance, litigation or new research. Saccharin, for example, was removed from the ninth edition. It was listed as “reasonably anticipated” in 1981, based on “sufficient evidence of carcinogenicity in experimental animals.” It was removed, however, after extensive review of decades of saccharin use determined that the data were not sufficient to meet current criteria. Further research had revealed, also, that the observed bladder tumors in rats arose from a mechanism not relevant to humans.
Other entries, such as the controversial listing of the cancer drug tamoxifen, walk a fine line between risk and benefit. Tamoxifen, first listed in the ninth report (and still in the 15th report), was included because studies revealed that it could increase the risk of uterine cancer in women. But there also was conclusive evidence that it may prevent or delay breast cancer in women who are at high risk.
Ultimately, the report’s authors make it clear that it is for informative value and guidance, not necessarily a dictate. As one report put it: “Personal decisions concerning voluntary exposures to carcinogenic agents need to be based on additional information that is beyond the scope” of the report.
“As the identification of carcinogens is a key step in cancer prevention,” said Rick Woychik, PhD, director of the National Institute of Environmental Health Sciences and NTP, “publication of the report represents an important government activity towards improving public health.”
From environmental tobacco smoke to ultraviolet (UV) radiation, diesel exhaust particulates, lead, and now, chronic infection with Helicobacter pylori (H pylori)—the Report on Carcinogens has regularly updated the list of substances known or “reasonably anticipated” to cause cancer.
The 15th report, which is prepared by the National Toxicology Program (NTP) for the Department of Health and Human Services, has 8 new entries, bringing the number of human carcinogens (eg, metals, pesticides, and drugs) on the list to 256. (The first report, released in 1980, listed 26.) In addition to H pylori infection, this edition adds the flame-retardant chemical antimony trioxide, and 6 haloacetic acids found as water disinfection byproducts.
In 1971, then President Nixon declared “war on cancer” (the second leading cause of death in the US) and signed the National Cancer Act. In 1978, Congress ordered the Report on Carcinogens, to educate the public and health professionals on potential environmental carcinogenic hazards.
Perhaps disheartening to know that even with 256 entries, the list probably understates the number of carcinogens humans and other creatures are exposed to. But things can change with time. Each list goes through a rigorous round of reviews. Sometimes substances are “delisted” after, for instance, litigation or new research. Saccharin, for example, was removed from the ninth edition. It was listed as “reasonably anticipated” in 1981, based on “sufficient evidence of carcinogenicity in experimental animals.” It was removed, however, after extensive review of decades of saccharin use determined that the data were not sufficient to meet current criteria. Further research had revealed, also, that the observed bladder tumors in rats arose from a mechanism not relevant to humans.
Other entries, such as the controversial listing of the cancer drug tamoxifen, walk a fine line between risk and benefit. Tamoxifen, first listed in the ninth report (and still in the 15th report), was included because studies revealed that it could increase the risk of uterine cancer in women. But there also was conclusive evidence that it may prevent or delay breast cancer in women who are at high risk.
Ultimately, the report’s authors make it clear that it is for informative value and guidance, not necessarily a dictate. As one report put it: “Personal decisions concerning voluntary exposures to carcinogenic agents need to be based on additional information that is beyond the scope” of the report.
“As the identification of carcinogens is a key step in cancer prevention,” said Rick Woychik, PhD, director of the National Institute of Environmental Health Sciences and NTP, “publication of the report represents an important government activity towards improving public health.”
From environmental tobacco smoke to ultraviolet (UV) radiation, diesel exhaust particulates, lead, and now, chronic infection with Helicobacter pylori (H pylori)—the Report on Carcinogens has regularly updated the list of substances known or “reasonably anticipated” to cause cancer.
The 15th report, which is prepared by the National Toxicology Program (NTP) for the Department of Health and Human Services, has 8 new entries, bringing the number of human carcinogens (eg, metals, pesticides, and drugs) on the list to 256. (The first report, released in 1980, listed 26.) In addition to H pylori infection, this edition adds the flame-retardant chemical antimony trioxide, and 6 haloacetic acids found as water disinfection byproducts.
In 1971, then President Nixon declared “war on cancer” (the second leading cause of death in the US) and signed the National Cancer Act. In 1978, Congress ordered the Report on Carcinogens, to educate the public and health professionals on potential environmental carcinogenic hazards.
Perhaps disheartening to know that even with 256 entries, the list probably understates the number of carcinogens humans and other creatures are exposed to. But things can change with time. Each list goes through a rigorous round of reviews. Sometimes substances are “delisted” after, for instance, litigation or new research. Saccharin, for example, was removed from the ninth edition. It was listed as “reasonably anticipated” in 1981, based on “sufficient evidence of carcinogenicity in experimental animals.” It was removed, however, after extensive review of decades of saccharin use determined that the data were not sufficient to meet current criteria. Further research had revealed, also, that the observed bladder tumors in rats arose from a mechanism not relevant to humans.
Other entries, such as the controversial listing of the cancer drug tamoxifen, walk a fine line between risk and benefit. Tamoxifen, first listed in the ninth report (and still in the 15th report), was included because studies revealed that it could increase the risk of uterine cancer in women. But there also was conclusive evidence that it may prevent or delay breast cancer in women who are at high risk.
Ultimately, the report’s authors make it clear that it is for informative value and guidance, not necessarily a dictate. As one report put it: “Personal decisions concerning voluntary exposures to carcinogenic agents need to be based on additional information that is beyond the scope” of the report.
“As the identification of carcinogens is a key step in cancer prevention,” said Rick Woychik, PhD, director of the National Institute of Environmental Health Sciences and NTP, “publication of the report represents an important government activity towards improving public health.”
Let’s be more careful about the data—and commentary—we publish
In a recent letter to the editor, “25-hydroxyvitamin D concentration is key to analyzing vitamin D’s effects” (J Fam Pract. 2021;70:472), Dr. Grant links vitamin D supplementation with important health outcomes. He concludes that the positivity rate of SARS-CoV-2 was only 5.9% in people with higher concentrations of 25(OH)D vs 12.5% in those with lower concentrations. This is a flawed conclusion on the face of it, because the great confabulatory factor is behavior. Is it possible that those more likely to take supplemental vitamin D do so as a result of overall healthier lifestyles and choices (eg, vaccinations)? As health care representatives, we must be very careful about the data we publish and the commentary we attach to it, lest we advertise inadvertent follies. I see so much of that in our “peer-reviewed literature.”
I came to medicine as a chemist, and the rigors of peer review impressed upon the hard (fundamental) sciences are markedly different from those we “claim” adherence to in medicine. I find that some of the medical literature and study designs fall short of what would pass muster in the fundamental science industry. That is a shame! Such statements, as discussed here, have to be served for public consumption, and even to our colleagues, with a generous helping of skepticism and qualification.
RA Segal, MD, MPH
Gainesville, FL
In a recent letter to the editor, “25-hydroxyvitamin D concentration is key to analyzing vitamin D’s effects” (J Fam Pract. 2021;70:472), Dr. Grant links vitamin D supplementation with important health outcomes. He concludes that the positivity rate of SARS-CoV-2 was only 5.9% in people with higher concentrations of 25(OH)D vs 12.5% in those with lower concentrations. This is a flawed conclusion on the face of it, because the great confabulatory factor is behavior. Is it possible that those more likely to take supplemental vitamin D do so as a result of overall healthier lifestyles and choices (eg, vaccinations)? As health care representatives, we must be very careful about the data we publish and the commentary we attach to it, lest we advertise inadvertent follies. I see so much of that in our “peer-reviewed literature.”
I came to medicine as a chemist, and the rigors of peer review impressed upon the hard (fundamental) sciences are markedly different from those we “claim” adherence to in medicine. I find that some of the medical literature and study designs fall short of what would pass muster in the fundamental science industry. That is a shame! Such statements, as discussed here, have to be served for public consumption, and even to our colleagues, with a generous helping of skepticism and qualification.
RA Segal, MD, MPH
Gainesville, FL
In a recent letter to the editor, “25-hydroxyvitamin D concentration is key to analyzing vitamin D’s effects” (J Fam Pract. 2021;70:472), Dr. Grant links vitamin D supplementation with important health outcomes. He concludes that the positivity rate of SARS-CoV-2 was only 5.9% in people with higher concentrations of 25(OH)D vs 12.5% in those with lower concentrations. This is a flawed conclusion on the face of it, because the great confabulatory factor is behavior. Is it possible that those more likely to take supplemental vitamin D do so as a result of overall healthier lifestyles and choices (eg, vaccinations)? As health care representatives, we must be very careful about the data we publish and the commentary we attach to it, lest we advertise inadvertent follies. I see so much of that in our “peer-reviewed literature.”
I came to medicine as a chemist, and the rigors of peer review impressed upon the hard (fundamental) sciences are markedly different from those we “claim” adherence to in medicine. I find that some of the medical literature and study designs fall short of what would pass muster in the fundamental science industry. That is a shame! Such statements, as discussed here, have to be served for public consumption, and even to our colleagues, with a generous helping of skepticism and qualification.
RA Segal, MD, MPH
Gainesville, FL
Innumerable pulmonary nodules
A 56-year-old woman with a history of a thyroid goiter following a partial thyroidectomy presented to the emergency department with shortness of breath, progressive weakness, and fatigue. She also reported a poor appetite and unintentional weight loss of approximately 40 lbs over the past 2 months but had not sought medical care. She denied having a cough, chest pain, hemoptysis, hematemesis, fevers, chills, or night sweats.
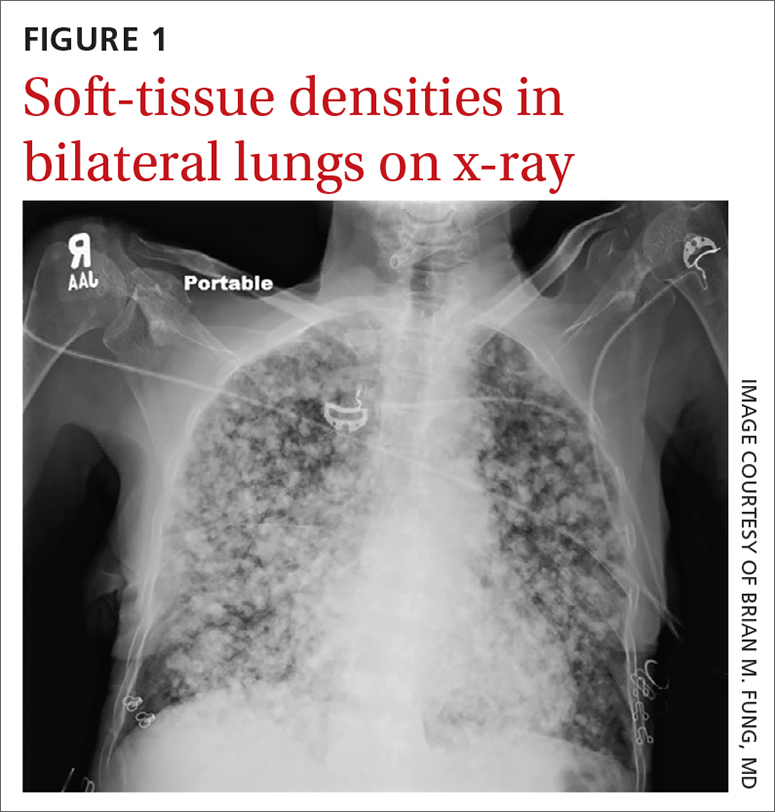
Physical examination revealed a cachectic woman with tachycardia and tachypnea, along with diffuse coarse rales throughout both lungs. The patient’s initial heart rate was 101 beats/min; respiratory rate, 25 breaths/min; and oxygen saturation, 74% on room air. The results of a complete blood count and comprehensive metabolic panel were within normal limits. A chest radiograph was performed, showing innumerable subcentimeter- and pericentimeter-sized soft-tissue densities in both lungs (FIGURE 1). Computed tomography (CT) of the chest was subsequently obtained to better characterize the nodules (FIGURES 2A and 2B). The CT revealed innumerable noncalcified pulmonary nodules as well as multiple hilar masses suggestive of malignancy; however, infectious etiologies could not be ruled out.
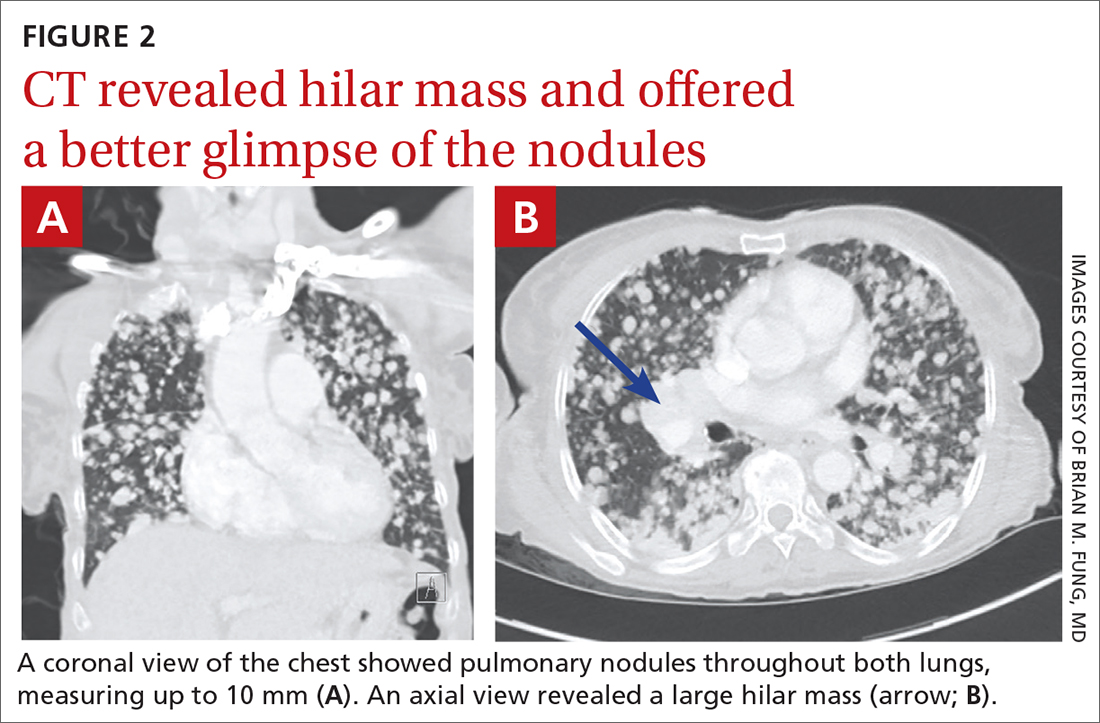
As the patient was visiting from a tuberculosis-endemic area, she was empirically started on rifampin, isoniazid, pyrazinamide, and ethambutol (RIPE) therapy for treatment of Mycobacterium tuberculosis infection. Subsequently, polymerase chain reaction (PCR) testing and acid-fast bacillus sputum smear came back negative for M tuberculosis and RIPE therapy was stopped. Bacterial culture and fungal screens for Pneumocystis jirovecii, Coccidioides, Cryptococcus, and Histoplasma capsulatum were negative.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Metastatic follicular thyroid carcinoma
A biopsy of one of the lung nodules was performed and showed strong positive staining for thyroglobulin and thyroid transcription factor-1, establishing a diagnosis of metastatic follicular thyroid carcinoma. Further imaging revealed additional metastases to the brain, liver, adrenal glands, and spine.
Follicular thyroid carcinoma is the second most common histologic type of thyroid cancer, comprising 10% to 15% of thyroid cancer cases.1 Distant metastases are not uncommon in this histologic type; about 11% of patients have distant metastases at presentation.2 Compared with papillary thyroid cancer, which spreads via the lymphatics, the follicular type can metastasize via vascular invasion, thus leading to its “aggressiveness” and frequent occurrence of metastasis outside the neck (most commonly to the lung).1 For this reason, an early diagnosis is important.3 In this case, the patient revealed that she hadn’t sought medical care at the onset of her symptoms due to financial limitations and limited access to medical providers; this likely contributed to the patient’s diagnosis at a late stage of disease.
Infectious and septic diseases are part of the differential Dx
The differential diagnosis for diffuse pulmonary nodules includes various infections, silicosis, coal worker’s pneumoconiosis, septic pulmonary emboli, and pulmonary sarcoidosis. All of these diagnoses can cause constitutional, as well as pulmonary, symptoms.
Infections, such as pulmonary tuberculosis, pulmonary coccidioidomycosis, or other cavitating infections, can be ruled out with specific testing for the organism of concern and/or culture.
Silicosis and coal worker’s pneumoconiosis generally cause lesions in the upper lobes and require a significant occupational exposure to silica or coal, respectively.
Continue to: Septic pulmonary emboli
Septic pulmonary emboli are usually seen in the context of sepsis, and there are positive blood cultures at the time of imaging.
Pulmonary sarcoidosis typically manifests with hilar and mediastinal lymphadenopathy and is often accompanied by other organ involvement.
There are options for treatment if diagnosis is made early
Thyroidectomy, radioiodine treatment, and thyroid-stimulating hormone suppressive therapy are the most commonly used therapies for treatment of follicular thyroid carcinoma.4 If follicular thyroid carcinoma is caught early (with only local involvement), 5-year relative survival rates are near 100%.5 However, outcomes are generally poor in patients who are of older age and those who have macronodular lung metastases or multiple bone metastases.6
Our patient was started on levothyroxine. Radioiodine and targeted drug therapy with a tyrosine kinase inhibitor were attempted, but the patient was unable to tolerate them due to her poor functional status. She was thus transitioned to hospice care.
1. Aschebrook-Kilfoy B, Grogan RH, Ward MH, et al. Follicular thyroid cancer incidence patterns in the United States, 1980-2009. Thyroid. 2013;23:1015-1021. doi: 10.1089/thy.2012.0356
2. Shaha AR, Shah JP, Loree TR. Differentiated thyroid cancer presenting initially with distant metastasis. Am J Surg. 1997;174:474-476. doi: 10.1016/s0002-9610(97)00158-x
3. Lim IIP, Hochman T, Blumberg SN, et al. Disparities in the initial presentation of differentiated thyroid cancer in a large public hospital and adjoining university teaching hospital. Thyroid. 2012;22:269-274. doi: 10.1089/thy.2010.0385
4. Grani G, Lamartina L, Durante C, et al. Follicular thyroid cancer and Hürthle cell carcinoma: challenges in diagnosis, treatment, and clinical management. Lancet Diabetes Endocrinol. 2018;6:500-514. doi: 10.1016/S2213-8587(17)30325-X
5. Thyroid cancer survival rates, by type and stage. American Cancer Society. Updated January 25, 2021. Accessed February 1, 2022. www.cancer.org/cancer/thyroid-cancer/detection-diagnosis-staging/survival-rates.html
6. Durante C, Haddy N, Baudin E, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. 2006;91:2892-2899. doi: 10.1210/jc.2005-2838
A 56-year-old woman with a history of a thyroid goiter following a partial thyroidectomy presented to the emergency department with shortness of breath, progressive weakness, and fatigue. She also reported a poor appetite and unintentional weight loss of approximately 40 lbs over the past 2 months but had not sought medical care. She denied having a cough, chest pain, hemoptysis, hematemesis, fevers, chills, or night sweats.

Physical examination revealed a cachectic woman with tachycardia and tachypnea, along with diffuse coarse rales throughout both lungs. The patient’s initial heart rate was 101 beats/min; respiratory rate, 25 breaths/min; and oxygen saturation, 74% on room air. The results of a complete blood count and comprehensive metabolic panel were within normal limits. A chest radiograph was performed, showing innumerable subcentimeter- and pericentimeter-sized soft-tissue densities in both lungs (FIGURE 1). Computed tomography (CT) of the chest was subsequently obtained to better characterize the nodules (FIGURES 2A and 2B). The CT revealed innumerable noncalcified pulmonary nodules as well as multiple hilar masses suggestive of malignancy; however, infectious etiologies could not be ruled out.

As the patient was visiting from a tuberculosis-endemic area, she was empirically started on rifampin, isoniazid, pyrazinamide, and ethambutol (RIPE) therapy for treatment of Mycobacterium tuberculosis infection. Subsequently, polymerase chain reaction (PCR) testing and acid-fast bacillus sputum smear came back negative for M tuberculosis and RIPE therapy was stopped. Bacterial culture and fungal screens for Pneumocystis jirovecii, Coccidioides, Cryptococcus, and Histoplasma capsulatum were negative.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Metastatic follicular thyroid carcinoma
A biopsy of one of the lung nodules was performed and showed strong positive staining for thyroglobulin and thyroid transcription factor-1, establishing a diagnosis of metastatic follicular thyroid carcinoma. Further imaging revealed additional metastases to the brain, liver, adrenal glands, and spine.
Follicular thyroid carcinoma is the second most common histologic type of thyroid cancer, comprising 10% to 15% of thyroid cancer cases.1 Distant metastases are not uncommon in this histologic type; about 11% of patients have distant metastases at presentation.2 Compared with papillary thyroid cancer, which spreads via the lymphatics, the follicular type can metastasize via vascular invasion, thus leading to its “aggressiveness” and frequent occurrence of metastasis outside the neck (most commonly to the lung).1 For this reason, an early diagnosis is important.3 In this case, the patient revealed that she hadn’t sought medical care at the onset of her symptoms due to financial limitations and limited access to medical providers; this likely contributed to the patient’s diagnosis at a late stage of disease.
Infectious and septic diseases are part of the differential Dx
The differential diagnosis for diffuse pulmonary nodules includes various infections, silicosis, coal worker’s pneumoconiosis, septic pulmonary emboli, and pulmonary sarcoidosis. All of these diagnoses can cause constitutional, as well as pulmonary, symptoms.
Infections, such as pulmonary tuberculosis, pulmonary coccidioidomycosis, or other cavitating infections, can be ruled out with specific testing for the organism of concern and/or culture.
Silicosis and coal worker’s pneumoconiosis generally cause lesions in the upper lobes and require a significant occupational exposure to silica or coal, respectively.
Continue to: Septic pulmonary emboli
Septic pulmonary emboli are usually seen in the context of sepsis, and there are positive blood cultures at the time of imaging.
Pulmonary sarcoidosis typically manifests with hilar and mediastinal lymphadenopathy and is often accompanied by other organ involvement.
There are options for treatment if diagnosis is made early
Thyroidectomy, radioiodine treatment, and thyroid-stimulating hormone suppressive therapy are the most commonly used therapies for treatment of follicular thyroid carcinoma.4 If follicular thyroid carcinoma is caught early (with only local involvement), 5-year relative survival rates are near 100%.5 However, outcomes are generally poor in patients who are of older age and those who have macronodular lung metastases or multiple bone metastases.6
Our patient was started on levothyroxine. Radioiodine and targeted drug therapy with a tyrosine kinase inhibitor were attempted, but the patient was unable to tolerate them due to her poor functional status. She was thus transitioned to hospice care.
A 56-year-old woman with a history of a thyroid goiter following a partial thyroidectomy presented to the emergency department with shortness of breath, progressive weakness, and fatigue. She also reported a poor appetite and unintentional weight loss of approximately 40 lbs over the past 2 months but had not sought medical care. She denied having a cough, chest pain, hemoptysis, hematemesis, fevers, chills, or night sweats.

Physical examination revealed a cachectic woman with tachycardia and tachypnea, along with diffuse coarse rales throughout both lungs. The patient’s initial heart rate was 101 beats/min; respiratory rate, 25 breaths/min; and oxygen saturation, 74% on room air. The results of a complete blood count and comprehensive metabolic panel were within normal limits. A chest radiograph was performed, showing innumerable subcentimeter- and pericentimeter-sized soft-tissue densities in both lungs (FIGURE 1). Computed tomography (CT) of the chest was subsequently obtained to better characterize the nodules (FIGURES 2A and 2B). The CT revealed innumerable noncalcified pulmonary nodules as well as multiple hilar masses suggestive of malignancy; however, infectious etiologies could not be ruled out.

As the patient was visiting from a tuberculosis-endemic area, she was empirically started on rifampin, isoniazid, pyrazinamide, and ethambutol (RIPE) therapy for treatment of Mycobacterium tuberculosis infection. Subsequently, polymerase chain reaction (PCR) testing and acid-fast bacillus sputum smear came back negative for M tuberculosis and RIPE therapy was stopped. Bacterial culture and fungal screens for Pneumocystis jirovecii, Coccidioides, Cryptococcus, and Histoplasma capsulatum were negative.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Metastatic follicular thyroid carcinoma
A biopsy of one of the lung nodules was performed and showed strong positive staining for thyroglobulin and thyroid transcription factor-1, establishing a diagnosis of metastatic follicular thyroid carcinoma. Further imaging revealed additional metastases to the brain, liver, adrenal glands, and spine.
Follicular thyroid carcinoma is the second most common histologic type of thyroid cancer, comprising 10% to 15% of thyroid cancer cases.1 Distant metastases are not uncommon in this histologic type; about 11% of patients have distant metastases at presentation.2 Compared with papillary thyroid cancer, which spreads via the lymphatics, the follicular type can metastasize via vascular invasion, thus leading to its “aggressiveness” and frequent occurrence of metastasis outside the neck (most commonly to the lung).1 For this reason, an early diagnosis is important.3 In this case, the patient revealed that she hadn’t sought medical care at the onset of her symptoms due to financial limitations and limited access to medical providers; this likely contributed to the patient’s diagnosis at a late stage of disease.
Infectious and septic diseases are part of the differential Dx
The differential diagnosis for diffuse pulmonary nodules includes various infections, silicosis, coal worker’s pneumoconiosis, septic pulmonary emboli, and pulmonary sarcoidosis. All of these diagnoses can cause constitutional, as well as pulmonary, symptoms.
Infections, such as pulmonary tuberculosis, pulmonary coccidioidomycosis, or other cavitating infections, can be ruled out with specific testing for the organism of concern and/or culture.
Silicosis and coal worker’s pneumoconiosis generally cause lesions in the upper lobes and require a significant occupational exposure to silica or coal, respectively.
Continue to: Septic pulmonary emboli
Septic pulmonary emboli are usually seen in the context of sepsis, and there are positive blood cultures at the time of imaging.
Pulmonary sarcoidosis typically manifests with hilar and mediastinal lymphadenopathy and is often accompanied by other organ involvement.
There are options for treatment if diagnosis is made early
Thyroidectomy, radioiodine treatment, and thyroid-stimulating hormone suppressive therapy are the most commonly used therapies for treatment of follicular thyroid carcinoma.4 If follicular thyroid carcinoma is caught early (with only local involvement), 5-year relative survival rates are near 100%.5 However, outcomes are generally poor in patients who are of older age and those who have macronodular lung metastases or multiple bone metastases.6
Our patient was started on levothyroxine. Radioiodine and targeted drug therapy with a tyrosine kinase inhibitor were attempted, but the patient was unable to tolerate them due to her poor functional status. She was thus transitioned to hospice care.
1. Aschebrook-Kilfoy B, Grogan RH, Ward MH, et al. Follicular thyroid cancer incidence patterns in the United States, 1980-2009. Thyroid. 2013;23:1015-1021. doi: 10.1089/thy.2012.0356
2. Shaha AR, Shah JP, Loree TR. Differentiated thyroid cancer presenting initially with distant metastasis. Am J Surg. 1997;174:474-476. doi: 10.1016/s0002-9610(97)00158-x
3. Lim IIP, Hochman T, Blumberg SN, et al. Disparities in the initial presentation of differentiated thyroid cancer in a large public hospital and adjoining university teaching hospital. Thyroid. 2012;22:269-274. doi: 10.1089/thy.2010.0385
4. Grani G, Lamartina L, Durante C, et al. Follicular thyroid cancer and Hürthle cell carcinoma: challenges in diagnosis, treatment, and clinical management. Lancet Diabetes Endocrinol. 2018;6:500-514. doi: 10.1016/S2213-8587(17)30325-X
5. Thyroid cancer survival rates, by type and stage. American Cancer Society. Updated January 25, 2021. Accessed February 1, 2022. www.cancer.org/cancer/thyroid-cancer/detection-diagnosis-staging/survival-rates.html
6. Durante C, Haddy N, Baudin E, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. 2006;91:2892-2899. doi: 10.1210/jc.2005-2838
1. Aschebrook-Kilfoy B, Grogan RH, Ward MH, et al. Follicular thyroid cancer incidence patterns in the United States, 1980-2009. Thyroid. 2013;23:1015-1021. doi: 10.1089/thy.2012.0356
2. Shaha AR, Shah JP, Loree TR. Differentiated thyroid cancer presenting initially with distant metastasis. Am J Surg. 1997;174:474-476. doi: 10.1016/s0002-9610(97)00158-x
3. Lim IIP, Hochman T, Blumberg SN, et al. Disparities in the initial presentation of differentiated thyroid cancer in a large public hospital and adjoining university teaching hospital. Thyroid. 2012;22:269-274. doi: 10.1089/thy.2010.0385
4. Grani G, Lamartina L, Durante C, et al. Follicular thyroid cancer and Hürthle cell carcinoma: challenges in diagnosis, treatment, and clinical management. Lancet Diabetes Endocrinol. 2018;6:500-514. doi: 10.1016/S2213-8587(17)30325-X
5. Thyroid cancer survival rates, by type and stage. American Cancer Society. Updated January 25, 2021. Accessed February 1, 2022. www.cancer.org/cancer/thyroid-cancer/detection-diagnosis-staging/survival-rates.html
6. Durante C, Haddy N, Baudin E, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. 2006;91:2892-2899. doi: 10.1210/jc.2005-2838