User login
Laparoscopic anatomic hepatectomy achieves better follow-up outcomes than non-anatomical hepatectomy in HCC
Key clinical point: As laparoscopic anatomical hepatectomy (LAH) is associated with increased disease-free survival (DFS) and comparable long-term overall survival (OS) and postoperative complications compared with non-anatomical hepatectomy (LNAH), it is recommended over LNAH for selected patients with HCC.
Major finding: Patients who underwent LAH vs LNAH showed significantly higher 5-year DFS rate (33.9% vs 30.1%; P = .009) and comparable long-term OS (43.2% vs 35.2%; P = .054) and postoperative complication (8.9% vs 12.4%; P = .255) rates.
Study details: Findings are from a single-center, prospective randomized controlled trial including 385 adult patients with HCC (single tumor ≤10 cm in size) who were randomly assigned to undergo LAH (n = 192) or LNAH (n = 193).
Disclosures: The study was sponsored by the National Natural Science Foundation of China and Project of Chongqing Municipality. The authors reported no conflicts of interest.
Source: Liao K et al. Laparoscopic anatomical versus non-anatomical hepatectomy in the treatment of hepatocellular carcinoma: A randomised controlled trial. Int J Surg. 2022;102:106652 (May 4). Doi: 10.1016/j.ijsu.2022.106652
Key clinical point: As laparoscopic anatomical hepatectomy (LAH) is associated with increased disease-free survival (DFS) and comparable long-term overall survival (OS) and postoperative complications compared with non-anatomical hepatectomy (LNAH), it is recommended over LNAH for selected patients with HCC.
Major finding: Patients who underwent LAH vs LNAH showed significantly higher 5-year DFS rate (33.9% vs 30.1%; P = .009) and comparable long-term OS (43.2% vs 35.2%; P = .054) and postoperative complication (8.9% vs 12.4%; P = .255) rates.
Study details: Findings are from a single-center, prospective randomized controlled trial including 385 adult patients with HCC (single tumor ≤10 cm in size) who were randomly assigned to undergo LAH (n = 192) or LNAH (n = 193).
Disclosures: The study was sponsored by the National Natural Science Foundation of China and Project of Chongqing Municipality. The authors reported no conflicts of interest.
Source: Liao K et al. Laparoscopic anatomical versus non-anatomical hepatectomy in the treatment of hepatocellular carcinoma: A randomised controlled trial. Int J Surg. 2022;102:106652 (May 4). Doi: 10.1016/j.ijsu.2022.106652
Key clinical point: As laparoscopic anatomical hepatectomy (LAH) is associated with increased disease-free survival (DFS) and comparable long-term overall survival (OS) and postoperative complications compared with non-anatomical hepatectomy (LNAH), it is recommended over LNAH for selected patients with HCC.
Major finding: Patients who underwent LAH vs LNAH showed significantly higher 5-year DFS rate (33.9% vs 30.1%; P = .009) and comparable long-term OS (43.2% vs 35.2%; P = .054) and postoperative complication (8.9% vs 12.4%; P = .255) rates.
Study details: Findings are from a single-center, prospective randomized controlled trial including 385 adult patients with HCC (single tumor ≤10 cm in size) who were randomly assigned to undergo LAH (n = 192) or LNAH (n = 193).
Disclosures: The study was sponsored by the National Natural Science Foundation of China and Project of Chongqing Municipality. The authors reported no conflicts of interest.
Source: Liao K et al. Laparoscopic anatomical versus non-anatomical hepatectomy in the treatment of hepatocellular carcinoma: A randomised controlled trial. Int J Surg. 2022;102:106652 (May 4). Doi: 10.1016/j.ijsu.2022.106652
Does imaging surveillance intensity govern clinical outcomes in HCC?
Key clinical point: Compared with the standard ultrasonography (US)-based imaging surveillance for hepatocellular carcinoma (HCC), intensive surveillance using alternative computed tomography (CT)/magnetic resonance imaging (MRI) in addition to US may facilitate the diagnosis of very early-stage HCC without providing any survival advantage.
Major finding: Diagnosis of very early-stage HCC was better in the low- (adjusted odds ratio [aOR] 0.44; P = .034) and high- (aOR 0.40; P = .014) intensive surveillance groups than in the standard surveillance group. However, overall survival remained unaffected by the surveillance intensity (P > .05).
Study details: This was a retrospective cohort study including 529 patients with newly diagnosed HCC who were on regular surveillance and were monitored using only US (standard group; n = 62) or CT/MRI plus US (categorized into low-intensive group [n = 232] and high-intensive group [n = 235] based on the median percentage of CT/MRI investigations [cut-off, 27%]).
Disclosures: The study did not receive any funding. The authors disclosed no conflicts of interest.
Source: Hwang JA et al. Association between intensity of imaging surveillance and clinical outcomes in patients with hepatocellular carcinoma. Eur J Radiol. 2022;151:110328 (Apr 21). Doi: 10.1016/j.ejrad.2022.110328
Key clinical point: Compared with the standard ultrasonography (US)-based imaging surveillance for hepatocellular carcinoma (HCC), intensive surveillance using alternative computed tomography (CT)/magnetic resonance imaging (MRI) in addition to US may facilitate the diagnosis of very early-stage HCC without providing any survival advantage.
Major finding: Diagnosis of very early-stage HCC was better in the low- (adjusted odds ratio [aOR] 0.44; P = .034) and high- (aOR 0.40; P = .014) intensive surveillance groups than in the standard surveillance group. However, overall survival remained unaffected by the surveillance intensity (P > .05).
Study details: This was a retrospective cohort study including 529 patients with newly diagnosed HCC who were on regular surveillance and were monitored using only US (standard group; n = 62) or CT/MRI plus US (categorized into low-intensive group [n = 232] and high-intensive group [n = 235] based on the median percentage of CT/MRI investigations [cut-off, 27%]).
Disclosures: The study did not receive any funding. The authors disclosed no conflicts of interest.
Source: Hwang JA et al. Association between intensity of imaging surveillance and clinical outcomes in patients with hepatocellular carcinoma. Eur J Radiol. 2022;151:110328 (Apr 21). Doi: 10.1016/j.ejrad.2022.110328
Key clinical point: Compared with the standard ultrasonography (US)-based imaging surveillance for hepatocellular carcinoma (HCC), intensive surveillance using alternative computed tomography (CT)/magnetic resonance imaging (MRI) in addition to US may facilitate the diagnosis of very early-stage HCC without providing any survival advantage.
Major finding: Diagnosis of very early-stage HCC was better in the low- (adjusted odds ratio [aOR] 0.44; P = .034) and high- (aOR 0.40; P = .014) intensive surveillance groups than in the standard surveillance group. However, overall survival remained unaffected by the surveillance intensity (P > .05).
Study details: This was a retrospective cohort study including 529 patients with newly diagnosed HCC who were on regular surveillance and were monitored using only US (standard group; n = 62) or CT/MRI plus US (categorized into low-intensive group [n = 232] and high-intensive group [n = 235] based on the median percentage of CT/MRI investigations [cut-off, 27%]).
Disclosures: The study did not receive any funding. The authors disclosed no conflicts of interest.
Source: Hwang JA et al. Association between intensity of imaging surveillance and clinical outcomes in patients with hepatocellular carcinoma. Eur J Radiol. 2022;151:110328 (Apr 21). Doi: 10.1016/j.ejrad.2022.110328
Subsequent anticancer therapy after ICI treatment prolongs survival in HCC
Key clinical point: Compared with best supportive care (BSC), treatment with any type of anticancer therapy after immune checkpoint inhibitor (ICI) therapy discontinuation in hepatocellular carcinoma (HCC) is associated with a significant improvement in overall survival.
Major finding: After ICI therapy discontinuation, patients who received anticancer therapy vs. BSC showed a significantly improved median overall survival (12.2 vs 3.2 months; hazard ratio 0.4; P < .001).
Study details: This was a retrospective, multicenter study that included 420 patients with HCC who were treated with ICI followed by subsequent anticancer treatment (n = 163) or BSC (n = 152).
Disclosures: The study was supported by the Wellcome Trust Strategic Fund, UK. Some authors declared serving as advisors, consultants, or speakers for and receiving grants from various sources.
Source: Sharma R et al. Patterns and outcomes of subsequent therapy after immune checkpoint inhibitor discontinuation in HCC. Hepatol Commun. 2022 (Apr 28). Doi: 10.1002/hep4.1927
Key clinical point: Compared with best supportive care (BSC), treatment with any type of anticancer therapy after immune checkpoint inhibitor (ICI) therapy discontinuation in hepatocellular carcinoma (HCC) is associated with a significant improvement in overall survival.
Major finding: After ICI therapy discontinuation, patients who received anticancer therapy vs. BSC showed a significantly improved median overall survival (12.2 vs 3.2 months; hazard ratio 0.4; P < .001).
Study details: This was a retrospective, multicenter study that included 420 patients with HCC who were treated with ICI followed by subsequent anticancer treatment (n = 163) or BSC (n = 152).
Disclosures: The study was supported by the Wellcome Trust Strategic Fund, UK. Some authors declared serving as advisors, consultants, or speakers for and receiving grants from various sources.
Source: Sharma R et al. Patterns and outcomes of subsequent therapy after immune checkpoint inhibitor discontinuation in HCC. Hepatol Commun. 2022 (Apr 28). Doi: 10.1002/hep4.1927
Key clinical point: Compared with best supportive care (BSC), treatment with any type of anticancer therapy after immune checkpoint inhibitor (ICI) therapy discontinuation in hepatocellular carcinoma (HCC) is associated with a significant improvement in overall survival.
Major finding: After ICI therapy discontinuation, patients who received anticancer therapy vs. BSC showed a significantly improved median overall survival (12.2 vs 3.2 months; hazard ratio 0.4; P < .001).
Study details: This was a retrospective, multicenter study that included 420 patients with HCC who were treated with ICI followed by subsequent anticancer treatment (n = 163) or BSC (n = 152).
Disclosures: The study was supported by the Wellcome Trust Strategic Fund, UK. Some authors declared serving as advisors, consultants, or speakers for and receiving grants from various sources.
Source: Sharma R et al. Patterns and outcomes of subsequent therapy after immune checkpoint inhibitor discontinuation in HCC. Hepatol Commun. 2022 (Apr 28). Doi: 10.1002/hep4.1927
Pembrolizumab monotherapy shows promise for untreated advanced HCC in phase 2
Key clinical point: Pembrolizumab monotherapy showed favorable efficacy in systemic therapy-naive patients with advanced hepatocellular carcinoma (aHCC), with no new safety signals in addition to those observed for pembrolizumab in aHCC in a second-line setting.
Major finding: The objective response rate was 16% (95% CI 7%-29%), and the median duration of response was 16 months. The median progression-free and overall survival were 4 (95% CI 2-8) and 17 (95% CI 8-23) months, respectively. Only 16% of patients experienced grade ≥3 treatment-related adverse events.
Study details: Findings are from the phase 2 KEYNOTE-224 trial including 51 adult, systemic therapy-naive patients with aHCC who received 200 mg pembrolizumab intravenously every 3 weeks for up to 35 cycles.
Disclosures: The study was sponsored by Merck Sharp & Dohme Corp. (MSD), a subsidiary of Merck & Co., Inc., USA. Some authors reported being advisory board members, consultants, or advisors or receiving research grants, speaker honoraria, or travel and accommodation expenses from various sources, including MSD. The other authors are employees and stock owners of MSD.
Source: Verset G et al. Pembrolizumab monotherapy for previously untreated advanced hepatocellular carcinoma: Data from the open-label, phase II KEYNOTE-224 trial. Clin Cancer Res. 2022 (Apr 14). Doi: 10.1158/1078-0432.CCR-21-3807
Key clinical point: Pembrolizumab monotherapy showed favorable efficacy in systemic therapy-naive patients with advanced hepatocellular carcinoma (aHCC), with no new safety signals in addition to those observed for pembrolizumab in aHCC in a second-line setting.
Major finding: The objective response rate was 16% (95% CI 7%-29%), and the median duration of response was 16 months. The median progression-free and overall survival were 4 (95% CI 2-8) and 17 (95% CI 8-23) months, respectively. Only 16% of patients experienced grade ≥3 treatment-related adverse events.
Study details: Findings are from the phase 2 KEYNOTE-224 trial including 51 adult, systemic therapy-naive patients with aHCC who received 200 mg pembrolizumab intravenously every 3 weeks for up to 35 cycles.
Disclosures: The study was sponsored by Merck Sharp & Dohme Corp. (MSD), a subsidiary of Merck & Co., Inc., USA. Some authors reported being advisory board members, consultants, or advisors or receiving research grants, speaker honoraria, or travel and accommodation expenses from various sources, including MSD. The other authors are employees and stock owners of MSD.
Source: Verset G et al. Pembrolizumab monotherapy for previously untreated advanced hepatocellular carcinoma: Data from the open-label, phase II KEYNOTE-224 trial. Clin Cancer Res. 2022 (Apr 14). Doi: 10.1158/1078-0432.CCR-21-3807
Key clinical point: Pembrolizumab monotherapy showed favorable efficacy in systemic therapy-naive patients with advanced hepatocellular carcinoma (aHCC), with no new safety signals in addition to those observed for pembrolizumab in aHCC in a second-line setting.
Major finding: The objective response rate was 16% (95% CI 7%-29%), and the median duration of response was 16 months. The median progression-free and overall survival were 4 (95% CI 2-8) and 17 (95% CI 8-23) months, respectively. Only 16% of patients experienced grade ≥3 treatment-related adverse events.
Study details: Findings are from the phase 2 KEYNOTE-224 trial including 51 adult, systemic therapy-naive patients with aHCC who received 200 mg pembrolizumab intravenously every 3 weeks for up to 35 cycles.
Disclosures: The study was sponsored by Merck Sharp & Dohme Corp. (MSD), a subsidiary of Merck & Co., Inc., USA. Some authors reported being advisory board members, consultants, or advisors or receiving research grants, speaker honoraria, or travel and accommodation expenses from various sources, including MSD. The other authors are employees and stock owners of MSD.
Source: Verset G et al. Pembrolizumab monotherapy for previously untreated advanced hepatocellular carcinoma: Data from the open-label, phase II KEYNOTE-224 trial. Clin Cancer Res. 2022 (Apr 14). Doi: 10.1158/1078-0432.CCR-21-3807
Early-stage HCC: OLT offers better survival outcomes than ablative therapies in the elderly
Key clinical point: Compared with liver-directed ablative therapies, orthotopic liver transplantation (OLT) offers a survival advantage for elderly patients with early-stage hepatocellular carcinoma (HCC) and alpha-fetoprotein (AFP) levels <500 ng/mL.
Major finding: Multivariable analysis revealed a significant survival benefit of OLT compared with ablative therapy alone (adjusted hazard ratio [aHR] 0.31; P < .001), with OLT being associated with better survival even after adjusting for imbalanced factors after propensity matching (aHR 0.35; P < .001).
Study details: The data come from a retrospective review study that propensity score matched patients aged ≥70 years with stage I-II HCC and AFP levels of <500 ng/mL receiving OLT (n = 170) with those undergoing liver-directed ablative therapy (n = 170).
Disclosures: No source of funding or conflicts of interest was declared by the authors.
Source: Shah MB et al. Outcomes in elderly patients undergoing liver transplantation compared with liver-directed ablative therapy in early-stage hepatocellular carcinoma. J Am Coll Surg. 2022;234(5):892-899 (Apr 15). Doi: 10.1097/XCS.0000000000000135
Key clinical point: Compared with liver-directed ablative therapies, orthotopic liver transplantation (OLT) offers a survival advantage for elderly patients with early-stage hepatocellular carcinoma (HCC) and alpha-fetoprotein (AFP) levels <500 ng/mL.
Major finding: Multivariable analysis revealed a significant survival benefit of OLT compared with ablative therapy alone (adjusted hazard ratio [aHR] 0.31; P < .001), with OLT being associated with better survival even after adjusting for imbalanced factors after propensity matching (aHR 0.35; P < .001).
Study details: The data come from a retrospective review study that propensity score matched patients aged ≥70 years with stage I-II HCC and AFP levels of <500 ng/mL receiving OLT (n = 170) with those undergoing liver-directed ablative therapy (n = 170).
Disclosures: No source of funding or conflicts of interest was declared by the authors.
Source: Shah MB et al. Outcomes in elderly patients undergoing liver transplantation compared with liver-directed ablative therapy in early-stage hepatocellular carcinoma. J Am Coll Surg. 2022;234(5):892-899 (Apr 15). Doi: 10.1097/XCS.0000000000000135
Key clinical point: Compared with liver-directed ablative therapies, orthotopic liver transplantation (OLT) offers a survival advantage for elderly patients with early-stage hepatocellular carcinoma (HCC) and alpha-fetoprotein (AFP) levels <500 ng/mL.
Major finding: Multivariable analysis revealed a significant survival benefit of OLT compared with ablative therapy alone (adjusted hazard ratio [aHR] 0.31; P < .001), with OLT being associated with better survival even after adjusting for imbalanced factors after propensity matching (aHR 0.35; P < .001).
Study details: The data come from a retrospective review study that propensity score matched patients aged ≥70 years with stage I-II HCC and AFP levels of <500 ng/mL receiving OLT (n = 170) with those undergoing liver-directed ablative therapy (n = 170).
Disclosures: No source of funding or conflicts of interest was declared by the authors.
Source: Shah MB et al. Outcomes in elderly patients undergoing liver transplantation compared with liver-directed ablative therapy in early-stage hepatocellular carcinoma. J Am Coll Surg. 2022;234(5):892-899 (Apr 15). Doi: 10.1097/XCS.0000000000000135
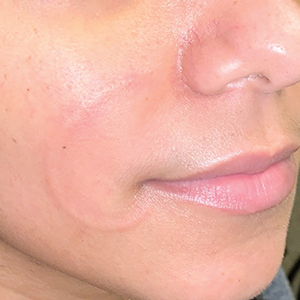
Recurrent Arciform Plaque on the Face
The Diagnosis: Lupus Erythematosus Tumidus
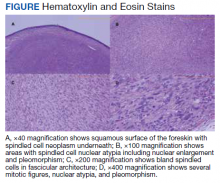
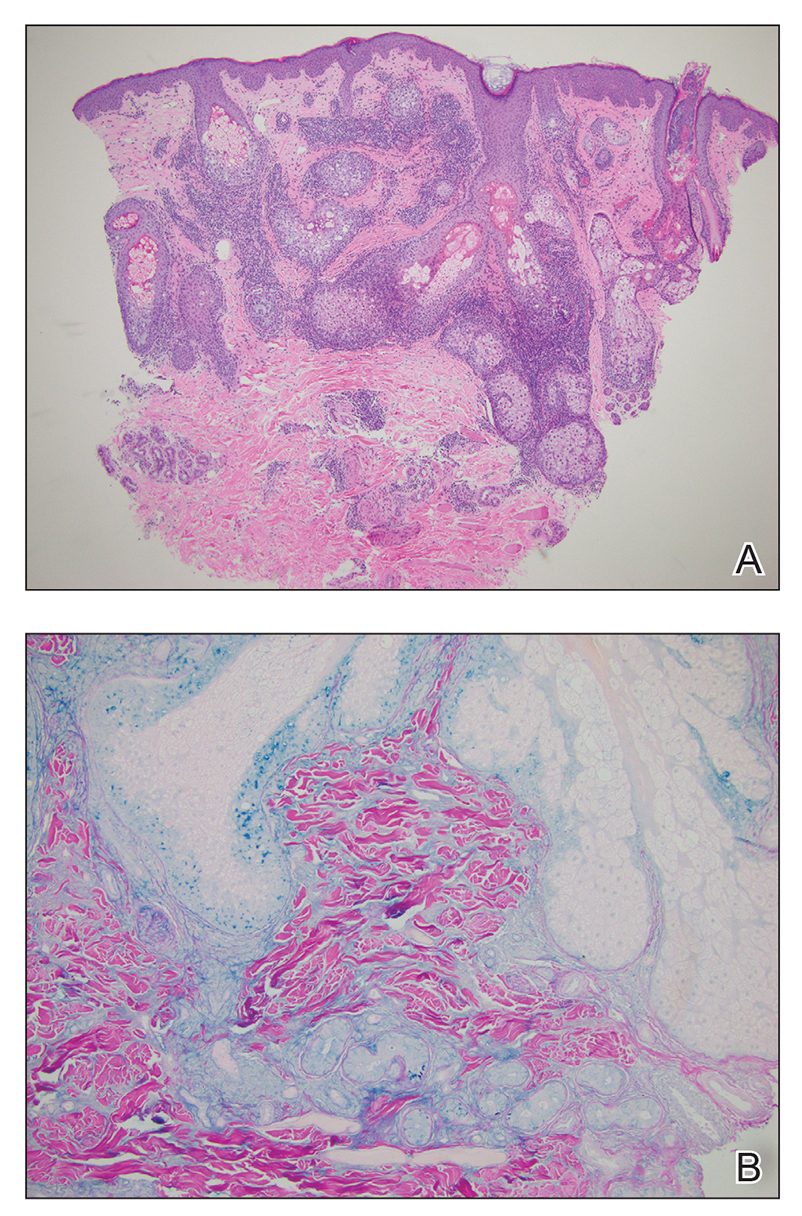
Histopathologic evaluation of a punch biopsy revealed focal dermal mucin deposition and CD123+ discrete clusters of plasmacytoid dendritic cells without interface changes (Figure 1), favoring a diagnosis of lupus erythematosus tumidus (LET) in our patient. There was no clinical improvement in symptoms when she previously was treated with topical antifungals or class III corticosteroid creams. Tacrolimus ointment 0.1% twice daily for 1 month did not result in substantial improvement in the appearance of the plaque, and it spontaneously resolved after 2 to 3 months. She declined treatment with hydroxychloroquine.
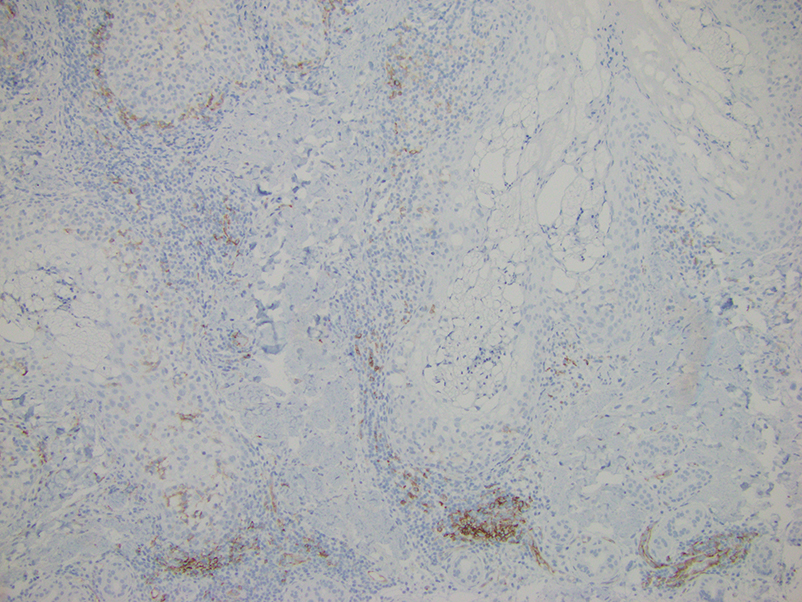
Lupus erythematosus tumidus is an uncommon subtype of chronic cutaneous lupus erythematosus with no distinct etiology. It is clinically characterized by edematous, urticarial, single or multiple plaques with a smooth surface affecting sun-exposed areas that can last for months to years.1 In contrast to other variations of chronic cutaneous lupus such as discoid lupus erythematosus, LET lesions lack surface papulosquamous features such as scaling, atrophy, and follicular plugging.1-4 Based solely on histologic findings, LET may be indistinguishable from reticular erythematous mucinosis and Jessner lymphocytic infiltration of the skin (JLIS) due to a similar lack of epidermal involvement and presence of a perivascular lymphocytic infiltrate (Figure 2).
The average age at disease onset is 36 years, nearly the same as that described in discoid lupus erythematosus.4 Lupus erythematosus tumidus has a favorable prognosis and commonly presents without other autoimmune signs, serologic abnormalities, or gender preference, with concomitant systemic lupus erythematosus sometimes reported.5
The absence of clinical and histological epidermal involvement are the most important clues to aid in the diagnosis. It has been postulated that JLIS could be an early cutaneous manifestation of LET.6 The differential diagnosis also may include erythema annulare centrifugum, granuloma annulare, and urticarial vasculitis. Lesions typically respond well to photoprotection, topical corticosteroids, and/or antimalarials. The addition of tacrolimus ointment 0.1% may result in complete regression without recurrence.7
Erythema annulare centrifugum is a reactive erythema that classically begins as a pink papule that gradually enlarges to form an annular erythematous plaque with a fine trailing scale that may recur.8 The histopathology of erythema annulare centrifugum shares features seen in LET, making the diagnosis difficult; however, secondary changes to the epidermis (eg, spongiosis, hyperkeratosis) may be seen. This condition has been associated with lymphoproliferative malignancies.8
Reticular erythematous mucinosis is clinically distinguished from LET, as it presents as reticular, rather than arciform, erythematous macules, papules, or plaques that may be asymptomatic or pruritic.9 Histopathology typically shows more superficial mucin deposition than in LET as well as superficial to mid-dermal perivascular and periadnexal lymphocytic infiltrates. Reticular erythematous mucinosis more frequently is reported in women in their 30s and 40s and has been associated with UV exposure and hormonal triggers, such as oral contraceptive medications and pregnancy.9
Granuloma annulare typically presents as asymptomatic, erythematous, annular plaques or papules in young women.10 There are several histologic subtypes that show focal collagen degeneration, inflammation with palisaded or interstitial histiocytes, and mucin deposition, regardless of clinical presentation. Granuloma annulare has been associated with systemic diseases including type 2 diabetes mellitus and thyroid disease. Localized granuloma annulare most commonly presents on the dorsal aspects of the hands or feet.10
We present a case of LET on the face. Although histologically similar to other dermatoses, LET often lacks epidermal involvement and presents on sun-exposed areas of the body. Jessner lymphocytic infiltration of the skin also should be considered in the differential, as there is an overlap of clinical and histopathological features; JLIS lacks mucin deposits.6 This case reinforces the importance of correlating clinical with histopathologic findings. Our patient was treated with tacrolimus ointment 0.1%, and the plaque eventually resolved in 2 to 3 months without recurrence. This condition should be included in the differential diagnosis of recurring annular plaques on sunexposed areas, particularly in middle-aged adults, even in the absence of systemic involvement.
- Liu E, Daze RP, Moon S. Tumid lupus erythematosus: a rare and distinctive variant of cutaneous lupus erythematosus masquerading as urticarial vasculitis. Cureus. 2020;12:E8305. doi:10.7759/cureus.8305
- Saleh D, Crane JS. Tumid lupus erythematosus. In: StatPearls. StatPearls Publishing; 2020.
- Verma P, Sharma S, Yadav P, et al. Tumid lupus erythematosus: an intriguing dermatopathological connotation treated successfully with topical tacrolimus and hydroxychloroquine combination. Indian J Dermatol. 2014;59:210. doi:10.4103/0019-5154.127716
- Kuhn A, Bein D, Bonsmann G. The 100th anniversary of lupus erythematosus tumidus. Autoimmun Rev. 2009;8:441-448. doi:10.1016/j. autrev.2008.12.010
- Jatwani K, Chugh K, Osholowu OS, et al. Tumid lupus erythematosus and systemic lupus erythematosus: a report on their rare coexistence. Cureus. 2020;12:E7545. doi:10.7759/cureus.7545
- Tomasini D, Mentzel T, Hantschke M, et al. Plasmacytoid dendritic cells: an overview of their presence and distribution in different inflammatory skin diseases, with special emphasis on Jessner’s lymphocytic infiltrate of the skin and cutaneous lupus erythematosus. J Cutan Pathol. 2010;37:1132-1139. doi:10.1111/j.1600-0560.2010.01587.x
- Patsinakidis N, Kautz O, Gibbs BF, et al. Lupus erythematosus tumidus: clinical perspectives. Clin Cosmet Investig Dermatol. 2019;12:707-719. doi:10.2147/CCID.S166723
- Mu EW, Sanchez M, Mir A, et al. Paraneoplastic erythema annulare centrifugum eruption (PEACE). Dermatol Online J. 2015;21:13030/ qt6053h29n.
- Ocanha-Xavier JP, Cola-Senra CO, Xavier-Junior JCC. Reticular erythematous mucinosis: literature review and case report of a 24-year-old patient with systemic erythematosus lupus. Lupus. 2021;30:325-335. doi:10.1177/0961203320965702 10. Keimig EL. Granuloma annulare. Dermatol Clin. 2015;33:315-329. doi:10.1016/j.det.2015.03.001
The Diagnosis: Lupus Erythematosus Tumidus
Histopathologic evaluation of a punch biopsy revealed focal dermal mucin deposition and CD123+ discrete clusters of plasmacytoid dendritic cells without interface changes (Figure 1), favoring a diagnosis of lupus erythematosus tumidus (LET) in our patient. There was no clinical improvement in symptoms when she previously was treated with topical antifungals or class III corticosteroid creams. Tacrolimus ointment 0.1% twice daily for 1 month did not result in substantial improvement in the appearance of the plaque, and it spontaneously resolved after 2 to 3 months. She declined treatment with hydroxychloroquine.

Lupus erythematosus tumidus is an uncommon subtype of chronic cutaneous lupus erythematosus with no distinct etiology. It is clinically characterized by edematous, urticarial, single or multiple plaques with a smooth surface affecting sun-exposed areas that can last for months to years.1 In contrast to other variations of chronic cutaneous lupus such as discoid lupus erythematosus, LET lesions lack surface papulosquamous features such as scaling, atrophy, and follicular plugging.1-4 Based solely on histologic findings, LET may be indistinguishable from reticular erythematous mucinosis and Jessner lymphocytic infiltration of the skin (JLIS) due to a similar lack of epidermal involvement and presence of a perivascular lymphocytic infiltrate (Figure 2).

The average age at disease onset is 36 years, nearly the same as that described in discoid lupus erythematosus.4 Lupus erythematosus tumidus has a favorable prognosis and commonly presents without other autoimmune signs, serologic abnormalities, or gender preference, with concomitant systemic lupus erythematosus sometimes reported.5
The absence of clinical and histological epidermal involvement are the most important clues to aid in the diagnosis. It has been postulated that JLIS could be an early cutaneous manifestation of LET.6 The differential diagnosis also may include erythema annulare centrifugum, granuloma annulare, and urticarial vasculitis. Lesions typically respond well to photoprotection, topical corticosteroids, and/or antimalarials. The addition of tacrolimus ointment 0.1% may result in complete regression without recurrence.7
Erythema annulare centrifugum is a reactive erythema that classically begins as a pink papule that gradually enlarges to form an annular erythematous plaque with a fine trailing scale that may recur.8 The histopathology of erythema annulare centrifugum shares features seen in LET, making the diagnosis difficult; however, secondary changes to the epidermis (eg, spongiosis, hyperkeratosis) may be seen. This condition has been associated with lymphoproliferative malignancies.8
Reticular erythematous mucinosis is clinically distinguished from LET, as it presents as reticular, rather than arciform, erythematous macules, papules, or plaques that may be asymptomatic or pruritic.9 Histopathology typically shows more superficial mucin deposition than in LET as well as superficial to mid-dermal perivascular and periadnexal lymphocytic infiltrates. Reticular erythematous mucinosis more frequently is reported in women in their 30s and 40s and has been associated with UV exposure and hormonal triggers, such as oral contraceptive medications and pregnancy.9
Granuloma annulare typically presents as asymptomatic, erythematous, annular plaques or papules in young women.10 There are several histologic subtypes that show focal collagen degeneration, inflammation with palisaded or interstitial histiocytes, and mucin deposition, regardless of clinical presentation. Granuloma annulare has been associated with systemic diseases including type 2 diabetes mellitus and thyroid disease. Localized granuloma annulare most commonly presents on the dorsal aspects of the hands or feet.10
We present a case of LET on the face. Although histologically similar to other dermatoses, LET often lacks epidermal involvement and presents on sun-exposed areas of the body. Jessner lymphocytic infiltration of the skin also should be considered in the differential, as there is an overlap of clinical and histopathological features; JLIS lacks mucin deposits.6 This case reinforces the importance of correlating clinical with histopathologic findings. Our patient was treated with tacrolimus ointment 0.1%, and the plaque eventually resolved in 2 to 3 months without recurrence. This condition should be included in the differential diagnosis of recurring annular plaques on sunexposed areas, particularly in middle-aged adults, even in the absence of systemic involvement.
The Diagnosis: Lupus Erythematosus Tumidus
Histopathologic evaluation of a punch biopsy revealed focal dermal mucin deposition and CD123+ discrete clusters of plasmacytoid dendritic cells without interface changes (Figure 1), favoring a diagnosis of lupus erythematosus tumidus (LET) in our patient. There was no clinical improvement in symptoms when she previously was treated with topical antifungals or class III corticosteroid creams. Tacrolimus ointment 0.1% twice daily for 1 month did not result in substantial improvement in the appearance of the plaque, and it spontaneously resolved after 2 to 3 months. She declined treatment with hydroxychloroquine.

Lupus erythematosus tumidus is an uncommon subtype of chronic cutaneous lupus erythematosus with no distinct etiology. It is clinically characterized by edematous, urticarial, single or multiple plaques with a smooth surface affecting sun-exposed areas that can last for months to years.1 In contrast to other variations of chronic cutaneous lupus such as discoid lupus erythematosus, LET lesions lack surface papulosquamous features such as scaling, atrophy, and follicular plugging.1-4 Based solely on histologic findings, LET may be indistinguishable from reticular erythematous mucinosis and Jessner lymphocytic infiltration of the skin (JLIS) due to a similar lack of epidermal involvement and presence of a perivascular lymphocytic infiltrate (Figure 2).

The average age at disease onset is 36 years, nearly the same as that described in discoid lupus erythematosus.4 Lupus erythematosus tumidus has a favorable prognosis and commonly presents without other autoimmune signs, serologic abnormalities, or gender preference, with concomitant systemic lupus erythematosus sometimes reported.5
The absence of clinical and histological epidermal involvement are the most important clues to aid in the diagnosis. It has been postulated that JLIS could be an early cutaneous manifestation of LET.6 The differential diagnosis also may include erythema annulare centrifugum, granuloma annulare, and urticarial vasculitis. Lesions typically respond well to photoprotection, topical corticosteroids, and/or antimalarials. The addition of tacrolimus ointment 0.1% may result in complete regression without recurrence.7
Erythema annulare centrifugum is a reactive erythema that classically begins as a pink papule that gradually enlarges to form an annular erythematous plaque with a fine trailing scale that may recur.8 The histopathology of erythema annulare centrifugum shares features seen in LET, making the diagnosis difficult; however, secondary changes to the epidermis (eg, spongiosis, hyperkeratosis) may be seen. This condition has been associated with lymphoproliferative malignancies.8
Reticular erythematous mucinosis is clinically distinguished from LET, as it presents as reticular, rather than arciform, erythematous macules, papules, or plaques that may be asymptomatic or pruritic.9 Histopathology typically shows more superficial mucin deposition than in LET as well as superficial to mid-dermal perivascular and periadnexal lymphocytic infiltrates. Reticular erythematous mucinosis more frequently is reported in women in their 30s and 40s and has been associated with UV exposure and hormonal triggers, such as oral contraceptive medications and pregnancy.9
Granuloma annulare typically presents as asymptomatic, erythematous, annular plaques or papules in young women.10 There are several histologic subtypes that show focal collagen degeneration, inflammation with palisaded or interstitial histiocytes, and mucin deposition, regardless of clinical presentation. Granuloma annulare has been associated with systemic diseases including type 2 diabetes mellitus and thyroid disease. Localized granuloma annulare most commonly presents on the dorsal aspects of the hands or feet.10
We present a case of LET on the face. Although histologically similar to other dermatoses, LET often lacks epidermal involvement and presents on sun-exposed areas of the body. Jessner lymphocytic infiltration of the skin also should be considered in the differential, as there is an overlap of clinical and histopathological features; JLIS lacks mucin deposits.6 This case reinforces the importance of correlating clinical with histopathologic findings. Our patient was treated with tacrolimus ointment 0.1%, and the plaque eventually resolved in 2 to 3 months without recurrence. This condition should be included in the differential diagnosis of recurring annular plaques on sunexposed areas, particularly in middle-aged adults, even in the absence of systemic involvement.
- Liu E, Daze RP, Moon S. Tumid lupus erythematosus: a rare and distinctive variant of cutaneous lupus erythematosus masquerading as urticarial vasculitis. Cureus. 2020;12:E8305. doi:10.7759/cureus.8305
- Saleh D, Crane JS. Tumid lupus erythematosus. In: StatPearls. StatPearls Publishing; 2020.
- Verma P, Sharma S, Yadav P, et al. Tumid lupus erythematosus: an intriguing dermatopathological connotation treated successfully with topical tacrolimus and hydroxychloroquine combination. Indian J Dermatol. 2014;59:210. doi:10.4103/0019-5154.127716
- Kuhn A, Bein D, Bonsmann G. The 100th anniversary of lupus erythematosus tumidus. Autoimmun Rev. 2009;8:441-448. doi:10.1016/j. autrev.2008.12.010
- Jatwani K, Chugh K, Osholowu OS, et al. Tumid lupus erythematosus and systemic lupus erythematosus: a report on their rare coexistence. Cureus. 2020;12:E7545. doi:10.7759/cureus.7545
- Tomasini D, Mentzel T, Hantschke M, et al. Plasmacytoid dendritic cells: an overview of their presence and distribution in different inflammatory skin diseases, with special emphasis on Jessner’s lymphocytic infiltrate of the skin and cutaneous lupus erythematosus. J Cutan Pathol. 2010;37:1132-1139. doi:10.1111/j.1600-0560.2010.01587.x
- Patsinakidis N, Kautz O, Gibbs BF, et al. Lupus erythematosus tumidus: clinical perspectives. Clin Cosmet Investig Dermatol. 2019;12:707-719. doi:10.2147/CCID.S166723
- Mu EW, Sanchez M, Mir A, et al. Paraneoplastic erythema annulare centrifugum eruption (PEACE). Dermatol Online J. 2015;21:13030/ qt6053h29n.
- Ocanha-Xavier JP, Cola-Senra CO, Xavier-Junior JCC. Reticular erythematous mucinosis: literature review and case report of a 24-year-old patient with systemic erythematosus lupus. Lupus. 2021;30:325-335. doi:10.1177/0961203320965702 10. Keimig EL. Granuloma annulare. Dermatol Clin. 2015;33:315-329. doi:10.1016/j.det.2015.03.001
- Liu E, Daze RP, Moon S. Tumid lupus erythematosus: a rare and distinctive variant of cutaneous lupus erythematosus masquerading as urticarial vasculitis. Cureus. 2020;12:E8305. doi:10.7759/cureus.8305
- Saleh D, Crane JS. Tumid lupus erythematosus. In: StatPearls. StatPearls Publishing; 2020.
- Verma P, Sharma S, Yadav P, et al. Tumid lupus erythematosus: an intriguing dermatopathological connotation treated successfully with topical tacrolimus and hydroxychloroquine combination. Indian J Dermatol. 2014;59:210. doi:10.4103/0019-5154.127716
- Kuhn A, Bein D, Bonsmann G. The 100th anniversary of lupus erythematosus tumidus. Autoimmun Rev. 2009;8:441-448. doi:10.1016/j. autrev.2008.12.010
- Jatwani K, Chugh K, Osholowu OS, et al. Tumid lupus erythematosus and systemic lupus erythematosus: a report on their rare coexistence. Cureus. 2020;12:E7545. doi:10.7759/cureus.7545
- Tomasini D, Mentzel T, Hantschke M, et al. Plasmacytoid dendritic cells: an overview of their presence and distribution in different inflammatory skin diseases, with special emphasis on Jessner’s lymphocytic infiltrate of the skin and cutaneous lupus erythematosus. J Cutan Pathol. 2010;37:1132-1139. doi:10.1111/j.1600-0560.2010.01587.x
- Patsinakidis N, Kautz O, Gibbs BF, et al. Lupus erythematosus tumidus: clinical perspectives. Clin Cosmet Investig Dermatol. 2019;12:707-719. doi:10.2147/CCID.S166723
- Mu EW, Sanchez M, Mir A, et al. Paraneoplastic erythema annulare centrifugum eruption (PEACE). Dermatol Online J. 2015;21:13030/ qt6053h29n.
- Ocanha-Xavier JP, Cola-Senra CO, Xavier-Junior JCC. Reticular erythematous mucinosis: literature review and case report of a 24-year-old patient with systemic erythematosus lupus. Lupus. 2021;30:325-335. doi:10.1177/0961203320965702 10. Keimig EL. Granuloma annulare. Dermatol Clin. 2015;33:315-329. doi:10.1016/j.det.2015.03.001
An otherwise healthy 31-year-old woman presented with a gradual growth of a semiannular, arciform, mildly pruritic plaque around the mouth of 10 years’ duration that recurred biannually, persisted for a few months, and spontaneously remitted without residual scarring. She denied joint pain, muscle aches, sores in the mouth, personal or family history of autoimmune diseases, or other remarkable review of systems. Physical examination revealed a welldefined, edematous, smooth, arciform plaque on the face with no mucous membrane involvement. Laboratory evaluation, including complete blood cell count, comprehensive metabolic panel, and antinuclear antibody titer, was unremarkable. A punch biopsy was obtained.

Ordering and Interpreting Precision Oncology Studies for Adults With Advanced Solid Tumors: A Primer
The ability to find and target specific biomarkers in the DNA of advanced cancers is rapidly changing options and outcomes for patients with locally advanced and metastatic solid tumors. This strategy is the basis for precision oncology, defined here as using predictive biomarkers from tumor and/or germline sequencing to guide therapies. This article focuses specifically on the use of DNA sequencing to find those biomarkers and provides guidance about which test is optimal in a specific situation, as well as interpretation of the results. We emphasize the identification of biomarkers that provide adult patients with advanced solid tumors access to therapies that would not be an option had sequencing not been performed and that have the potential for significant clinical benefit. The best approach is to have an expert team with experience in precision oncology to assist in the interpretation of results.
Which test?
Deciding what test of the array of assays available to use and which tissue to test can be overwhelming, and uncertainty may prevent oncology practitioners from ordering germline or somatic sequencing. For the purposes of this article, we will focus on DNA sequencing for inherited/germline alterations (including mutations, copy number changes, or fusions), which may inform treatment, or alterations that arise in the process of carcinogenesis and tumor evolution (somatic alterations in tumor DNA). This focus is not meant to exclude any specific test but to focus on DNA-based tests in patients with locally advanced or metastatic malignancy.
Germline Testing
Germline testing is the sequencing of inherited DNA in noncancerous cells to find alterations that may play a role in the development of cancers and are actionable in some cases. Germline alterations can inform therapeutic decisions, predict future cancer risk, and provide information that can help family members to better manage their risks of malignancy. Detailed discussions of the importance of germline testing to inform cancer surveillance, risk-reducing interventions, and the testing of relatives to determine who carries inherited alterations (cascade testing) is extremely important with several advantages and is covered in a number of excellent reviews elsewhere.1-3 Testing of germline DNA in patients with a metastatic malignancy can provide treatment options otherwise not available for patients, particularly for BRCA1/2 and Lynch syndrome–related cancers. Recent studies have shown that 10 to 15% of patients with advanced malignancies of many types have a pathogenic germline alteration.4,5
Germline DNA is usually acquired from peripheral blood, a buccal swab, or saliva collection and is therefore readily available. This is advantageous because it does not require a biopsy to identify relevant alterations. Germline testing is also less susceptible to the rare situations in which artifacts occur in formalin-fixed tissues and obscure relevant alterations.
The cost of germline testing varies, but most commercial vendor assays for germline testing are significantly less expensive than the cost of somatic testing. The disadvantages include the inability of germline testing to find any alterations that arise solely in tumor tissue and the smaller gene panels included in germline testing as compared to somatic testing panels. Other considerations relate to the inherited nature of pathogenic germline variants and its implications for family members that may affect the patient’s psychosocial health and potentially change the family dynamics.
Deciding who is appropriate for germline testing and when to perform the testing should be individualized to the patient’s wishes and disease status. Treatment planning may be less complicated if testing has been performed and germline status is known. In some cases urgent germline testing is indicated to inform pending procedures and/or surgical decisions for risk reduction, including more extensive tissue resection, such as the removal of additional organs or contralateral tissue. A minor point regarding germline testing is that the DNA of patients with hematologic malignancies may be difficult to sequence because of sample contamination by the circulating malignancy. For this reason, most laboratories will not accept peripheral blood or saliva samples for germline testing in patients with active hematologic malignancies; they often require DNA from another source such as fibroblasts from a skin biopsy or cells from a muscle biopsy. Germline testing is recommended for all patients with metastatic prostate cancer, as well as any patient with any stage of pancreatic cancer or ovarian cancer and patients with breast cancer diagnosed at age ≤ 45 years. More detailed criteria for who is appropriate for germline testing outside of these groups can be found in the appropriate National Comprehensive Cancer Network (NCCN) guidelines.6-8 In patients with some malignancies such as prostate and pancreatic cancer, approximately half of patients who have a BRCA-related cancer developed that malignancy because of a germline BRCA alteration.9-11 Testing germline DNA is therefore an easy way to quickly find almost half of all targetable alterations with a treatment approved by the US Food and Drug Administration (FDA) and at low cost, with the added benefit of providing critical information for families who may be unaware that members carry a relevant pathogenic germline alteration. In those families, cascade testing can provide surveillance and intervention strategies that can be lifesaving.
A related and particularly relevant question is when should a result found on a somatic testing panel prompt follow-up germline testing? Some institutions have algorithms in place to automate referral for germline testing based on specific genetic criteria.12 Excellent reviews are available that outline the following considerations in more detail.13 Typically, somatic testing results that would trigger follow-up germline testing would be truncating or deleterious or likely deleterious mutations per germline datasets in high-risk genes associated with highly penetrant autosomal dominant conditions (BRCA1, BRCA2, PALB2, MLH1, MSH2, and MSH6), selected moderate-risk genes (BRIP1, RAD51C, and RAD51D), and specific variants with a high probability of being germline because they are common germline founder mutations. Although the actionability and significance of specific genes remains a matter of some discussion, generally finding a somatic pathogenic sequencing result included in the 59-gene list of the American College of Medical Genetics and Genomics (ACMG) guidelines would be an indication for germline testing. Another indication for germline testing would be finding genes with germline mutations for which the NCCN has specific management guidelines, or the presence of alterations consistent with known founder mutations.14 When a patient’s tumor has microsatellite instability or is hypermutated (defined as > 10 mutations per megabase), a search for germline alterations is warranted given that about 15% of these patients with these tumors carry a Lynch syndrome gene.15 Genes that are commonly found as somatic alterations alone (eg, TP53 or APC) are generally not an indication for germline testing unless family history is compelling.
Although some clinicians use the variant allele fraction in the somatic sequencing report to decide whether to conduct germline testing, this approach is suboptimal, as allele fraction may be confounded by assay conditions and a high allele fraction may be found in pure tumors with loss of heterozygosity (LOH) of the other allele. There is also evidence that for a variety of reasons, somatic sequencing panels do not always detect germline alterations in somatic tissues.16 Reasons for this may include discordance between the genes being tested in the germline vs the somatic panel, technical differences such as interference of formalin-fixed paraffin-embedded (FFPE) artifact with detecting the germline variant, lack of expertise in germline variant interpretation among laboratories doing tumor-only sequencing, and, in rare cases, large deletions in tumor tissue masking a germline point mutation.
Variant Interpretation of Germline Testing
A general understanding of the terminology used for germline variant interpretation allows for the ordering health care practitioner (HCP) to provide the best quality care and an appreciation for the limitations of current molecular testing. Not all variants are associated with disease; the clinical significance of a genetic variant falls on a spectrum. The criteria for determining pathogenicity differ between molecular laboratories, but most are influenced by the standards and guidelines set forth by the ACMG.14 The clinical molecular laboratory determines variant classification, and a detailed discussion is beyond the scope of this primer. In brief, variant classification is based on evidence of varying strength in different categories including population data, computational and predictive data, functional data, segregation data, de novo data, allelic data, and information from various databases. The ACMG has proposed a 5-tiered classification system, by which most molecular laboratories adhere to in their genetic test reports (Table 1).14
Pathogenic and likely pathogenic variants are clinically actionable, whereas variants of uncertain significance (VUS) require additional data and/or functional studies before making clinical decisions. Depending on the clinical context and existing supporting evidence, it may be prudent to continue monitoring for worsening or new signs of disease in patients with one or more VUS while additional efforts are underway to understand the variant’s significance.
In some cases, variants are reclassified, which may alter the management and treatment of patients. Reclassification can occur with VUS, and in rare instances, can also occur with variants previously classified as pathogenic/likely pathogenic or benign/likely benign. In such a case, the reporting laboratory will typically make concerted efforts to alert the ordering HCP. However, variant reclassifications are not always communicated to the care team. Thus, it is important to periodically contact the molecular laboratory of interest to obtain updated test interpretations.
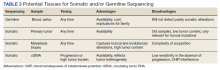
Somatic Testing
Testing of somatic (tumor) tissue is critical and is the approach most commonly taken in medical oncology (Table 2).
The advantages of primary tumor are that it is usually in hand as a diagnostic biopsy, acquisition is standard of care, and several targetable alterations are truncal, defined as driver mutations present at the time of tumor development. Also, the potential that the tumor arose in the background of a predisposing germline alteration can be suggested by sequencing primary tumor as discussed above. Moreover, sequencing the primary tumor can be done at any time unless the biopsy sample is considered too old or degraded (per specific platform requirements). The information gained can be used to anticipate additional treatment options that are relevant when patients experience disease progression. Disadvantages include the problem that primary specimens may be old or have limited tumor content, both of which increase the likelihood that sequencing will not be technically successful.
Alterations that are targetable and arise as a result of either treatment pressure or clonal evolution are considered evolutionary. If evolutionary alterations are the main focus for sequencing, then metastasis biopsy or ctDNA are better choices. The advantages of a metastasis biopsy are that tissue is contemporary, tumor content may be higher than in primary tumor, and both truncal and evolutionary alterations can be detected.
For specific tumors, continued analysis of evolving genomic alterations can play a critical role in management. In non–small cell lung cancer (NSCLC), somatic testing is conducted again at progression on repeat biopsies to evaluate for emerging resistance mutations. In epidermal growth factor receptor (EGFR)–mutated lung cancer, the resistance mutation, exon 20 p.T790M (point mutation), can present in patients after treatment with first- or second-generation EGFR tyrosine kinase inhibitors (TKI). Even in patients who are treated with the third-generation EGFR TKI osimertinib that can treat T790M-mutated lung cancer, multiple possible evolutionary mutations can occur at progression, including other EGFR mutations, MET/HER2 amplification, and BRAF V600E, to name a few.20 Resistance mechanisms develop due to treatment selection pressure and the molecular heterogeneity seen in lung cancer.
Disadvantages for metastatic biopsy include the inability to safely access a metastatic site, the time considerations for preauthorization and arrangement of biopsy, and a lower-than-average likelihood of successful sequencing from sites such as bone.21,22 In addition, there is some concern that a single metastatic site may not capture all relevant alterations for multiple reasons, including tumor heterogeneity.
Significant advances in the past decade have dramatically improved the ability to use ctDNA to guide therapy. Advantages include ease of acquisition as acquiring a sample requires only a blood draw, and the potential that the pool of ctDNA is a better reflection of the relevant biology as it potentially reflects all metastatic tissues. Disadvantages are that sequencing attempts may not be productive if the sample is acquired at a time when the tumor is either quiescent or tumor burden is so low that only limited amounts of DNA are being shed. Performing ctDNA analysis when a tumor is not progressing is less likely to be productive for a number of tumor types.23,24 Sequencing ctDNA is also more susceptible than sequencing tumor biopsies to detection of alterations that are not from the tumor of interest but from clonal hematopoiesis of indeterminate potential (CHIP) or other clonal hematopoietic disorders (see Confounders section below).
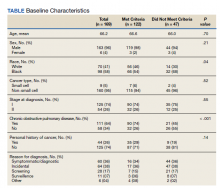
Selecting the Tissue
Deciding on the tissue to analyze is a critical part of the decision process (Table 3). If the primary tumor tissue is old the likelihood of productive sequencing is lower, although age alone is not the only consideration and the methods of fixation may be just as relevant.
For prostate cancer in particular, the ability to successfully sequence primary tumor tissue decreases as the amount of tumor decreases in low-volume biopsies such as prostate needle biopsies. Generally, if tumor content is < 10% of the biopsy specimen, then sequencing is less likely to be productive.25 Also, if the alteration of interest is not known to be truncal, then a relevant target might be missed by sequencing tissue that does not reflect current biology. Metastasis biopsy may be the most appropriate tissue, particularly if this specimen has already been acquired. As above, a metastasis biopsy may have a higher tumor content, and it should reflect relevant biology if it is recent. However, bone biopsies have a relatively low yield for successful sequencing, so a soft tissue lesion (eg, liver or lymph node metastasis) is generally preferred.
The inability to safely access tissue is often a consideration. Proximity to vital structures such as large blood vessels or the potential for significant morbidity in the event of a complication (liver or lung biopsies, particularly in patients on anticoagulation medications) may make the risk/benefit ratio too high. The inability to conduct somatic testing has been reported to often be due to inadequate tissue sampling.26 ctDNA is an attractive alternative but should typically be drawn when a tumor is progressing with a reasonable tumor burden that is more likely to be shedding DNA. Performing ctDNA analysis in patients without obvious radiographic metastasis or in patients whose tumor is under good control is unlikely to produce interpretable results.
Interpreting the Results
The intent of sequencing tumor tissue is to identify alterations that are biologically important and may provide a point of therapeutic leverage. However, deciding which alterations are relevant is not always straightforward. For example, any normal individual genome contains around 10,000 missense variants, hundreds of insertion/deletion variants, and dozens of protein-truncating variants. Distinguishing these alterations, which are part of the individual, from those that are tumor-specific and have functional significance can be difficult in the absence of paired sequencing of both normal and tissue samples.
Specific Alterations
Although most commercial vendors provide important information in sequencing reports to assist oncology HCPs in deciding which alterations are relevant, the reports are not always clear. In many cases the report will specifically indicate whether the alteration has been reported previously as pathogenic or benign. However, some platforms will report alterations that are not known to be drivers of tumor biology. It is critical to be aware that if variants are not reported as pathogenic, they should not be assumed to be pathogenic simply because they are included in the report. Alterations more likely to be drivers of relevant biology are those that change gene and protein structure and include frameshift (fs*), nonsense (denoted by sequence ending in “X” or “*”), or specific fusions or insertions/deletions (indel) that occur in important domains of the gene.
For some genes, only specific alterations are targetable and not all alterations have the same effect on protein function. Although overexpression of certain genes and proteins are actionable (eg, HER2), amplification of a gene does not necessarily indicate that it is targetable. In NSCLC, specific alterations convey sensitivity to targeted therapies. For example, in EGFR-mutated NSCLC, the sensitizing mutations to EGFR TKIs are exon19 deletions and exon 21 L858R point mutations (the most common mutations), as well as less common mutations found in exon 18-21. Exon 20 mutations, however, are not responsive to EGFR TKIs with a few exceptions.27 Patients who have tumors that do not harbor a sensitizing EGFR mutation should not be treated with an EGFR TKI. In a variety of solid tumors, gene fusions of the NTRK 1/2/3, act as oncogenic drivers. The chromosomal fusion events involving the carboxy-terminal kinase domain of TRK and upstream amino-terminal partners lead to overexpression of the chimeric proteins tropomyosin receptor kinase (TRK) A/B/C, resulting in constitutively active, ligand-independent downstream signaling. In patients with NTRK 1/2/3 gene fusions, larotrectinib and entrectinib, small molecule inhibitors to TRK, have shown antitumor activity.28,29 No alterations beyond these fusions are known to be targetable.
Allele Fraction
Knowing the fraction (or proportion) of the alteration of interest in the sequenced tissue relative to the estimated tumor content can assist in decision making. Not all platforms will provide this information, which is referred to as mutation allele fraction (MAF) or variant allele fraction (VAF), but sometimes will provide it on request. Platforms will usually provide an estimate of the percent tumor in the tissue being sampled if it is from a biopsy. If the MAF is around 50% in the sequenced tissue (including ctDNA), then there is a reasonable chance that it is a germline variant. However, there are nuances as germline alterations in some genes, such as BRCA1/2, can be accompanied by loss of the other allele of the gene (LOH). In that case, if most of the circulating DNA is from tumor, then the MAF can be > 50%.
If there are 2 alterations of the same gene with MAF percentages that are each half of the total percent tumor, there is a high likelihood of biallelic alteration. These sorts of paired alterations or one mutation with apparent LOH or copy loss would again indicate a high likelihood that the alteration is in fact pathogenic and a relevant driver. Not all pathogenic alterations have to be biallelic to be driver mutations but in BRCA1/2, or mismatch repair deficiency genes, the presence of biallelic alterations increases the likelihood of their being pathogenic.
Tumors that are hypermutated—containing sometimes hundreds of mutations per megabyte of DNA—can be particularly complicated to interpret, because the likelihood increases that many of the alterations are a function of the hypermutation and not a driver mutation. This is particularly important when there are concurrent mutations in mismatch repair genes and genes, such as BRCA1/2. If the tumor is
Confounders
In some situations, interpretation can be particularly challenging. For example, several alterations for which there are FDA on-label indications (such as ATM or BRCA2) can be detected in ctDNA that may not be due to the tumor but to CHIP. CHIP represents hematopoietic clones that are dysplastic as a result of exposure to DNA-damaging agents (eg, platinum chemotherapy) or as a result of aging and arise when mutations in hematopoietic stem cells provide a competitive advantage.31 The most common CHIP clones that can be detected are DNMT3A, ASXL1, or TET2; because these alterations are not targetable, their importance lies primarily in whether patients have evidence of hematologic abnormality, which might represent an evolving hematopoietic disorder. Because CHIP alterations can overlap with somatic alterations for which FDA-approved drugs exist, such as ATM or CHEK2 (olaparib for prostate cancer) and BRCA2 (poly-ADP-ribose polymerase inhibitors in a range of indications) there is concern that CHIP might result in patient harm from inappropriate treatment of CHIP rather than the tumor, with no likelihood that the treatment would affect the tumor, causing treatment delays.32 General considerations for deciding whether an alteration represents CHIP include excluding alteration in which the VAF is < 1% and when the VAF in the alteration of interest is < 20% of the estimated tumor fraction in the sample. Exceptions to this are found in patients with true myelodysplasia or chronic lymphocytic leukemia, in whom the VAF can be well over 50% because of circulating tumor burden. The only way to be certain that an alteration detected on ctDNA reflects tumor rather than CHIP is to utilize an assay with matched tumor-normal sequencing.
Resources for Assistance
For oncology HCPs, perhaps the best resource to help in selecting and interpreting the appropriate testing is through a dedicated molecular oncology tumor board and subject matter experts who contribute to those tumor boards. In the US Department of Veterans Affairs, the national precision oncology program and its affiliated clinical services, such as the option to order a national consultation and molecular tumor board education, are easily accessible to all HCPs (www.cancer.va.gov). Many commercial vendors provide support to assist with questions of interpretation and to inform clinical decision-making. Other resources that can assist with deciding whether an alteration is pathogenic include extensive curated databases such as ClinVar (www.ncbi.nlm.nih.gov/clinvar) and the Human Genetic Mutation Database (www.hgmd.cf.ac.uk/ac/index.php) for germline alterations or COSMIC (cancer.sanger.ac.uk/cosmic) for somatic alterations. OncoKB (www.oncokb.org) is a resource for assistance in defining levels of evidence for the use of agents to target specific alterations and to assist in assigning pathogenicity to specific alterations. Additional educational resources for training in genomics and genetics are also included in the Appendix.
The rapid growth in technology and ability to enhance understanding of relevant tumor biology continues to improve the therapeutic landscape for men and women dealing with malignancy and our ability to find targetable genetic alterations with the potential for meaningful clinical benefit.
Acknowledgments
Dedicated to Neil Spector.
1. Domchek SM, Mardis E, Carlisle JW, Owonikoko TK. Integrating genetic and genomic testing into oncology practice. Am Soc Clin Oncol Educ Book. 2020;40:e259-e263. doi:10.1200/EDBK_280607
2. Stoffel EM, Carethers JM. Current approaches to germline cancer genetic testing. Annu Rev Med. 2020;71:85-102. doi:10.1146/annurev-med-052318-101009
3. Lappalainen T, Scott AJ, Brandt M, Hall IM. Genomic analysis in the age of human genome sequencing. Cell. 2019;177(1):70-84. doi:10.1016/j.cell.2019.02.032
4. Samadder NJ, Riegert-Johnson D, Boardman L, et al. Comparison of universal genetic testing vs guideline-directed targeted testing for patients with hereditary cancer syndrome. JAMA Oncol. 2021;7(2):230-237. doi:10.1001/jamaoncol.2020.6252
5. Schneider BP, Stout L, Philips S, et al. Implications of incidental germline findings identified in the context of clinical whole exome sequencing for guiding cancer therapy. JCO Precis Oncol. 2020;4:1109-1121. doi:10.1200/PO.19.00354
6. National Comprehensive Cancer Network. Pancreatic cancer (Version 1.2022). Updated February 24, 2022. Accessed April 13, 2022. https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf
7. National Comprehensive Cancer Network. Prostate cancer (Version 3.2022). Updated January 10, 2022. Accessed April 13, 2022. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf
8. National Comprehensive Cancer Network. Genetic/familial high-risk assessment: breast, ovarian, and pancreatic (Version 2.2022). Updated March 9, 2022. Accessed April 13, 2022. https://www.nccn.org/professionals/physician_gls/pdf/genetics_bop.pdf
9. Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161(5):1215-1228. doi:10.1016/j.cell.2015.05.001
10. Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;375(5):443-453. doi:10.1056/NEJMoa1603144
11. Cancer Genome Atlas Research Network. Integrated genomic characterization of pancreatic ductal adenocarcinoma. Cancer Cell. 2017;32(2):185-203.e13. doi:10.1016/j.ccell.2017.07.007
12. Clark DF, Maxwell KN, Powers J, et al. Identification and confirmation of potentially actionable germline mutations in tumor-only genomic sequencing. JCO Precis Oncol. 2019;3:PO.19.00076. doi:10.1200/PO.19.00076
13. DeLeonardis K, Hogan L, Cannistra SA, Rangachari D, Tung N. When should tumor genomic profiling prompt consideration of germline testing? J Oncol Pract. 2019;15(9):465-473. doi:10.1200/JOP.19.00201
14. Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405-424. doi:10.1038/gim.2015.30
15. Latham A, Srinivasan P, Kemel Y, et al. Microsatellite instability is associated with the presence of Lynch syndrome pan-cancer. J Clin Oncol. 2019;37(4):286-295. doi:10.1200/JCO.18.00283
16. Lincoln SE, Nussbaum RL, Kurian AW, et al. Yield and utility of germline testing following tumor sequencing in patients with cancer. JAMA Netw Open. 2020;3(10):e2019452. doi:10.1001/jamanetworkopen.2020.19452
17. National Comprehensive Cancer Network. Non-small cell lung cancer (Version: 3.2022). Updated March 16, 2022. Accessed April 13, 2022. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
18. National Comprehensive Cancer Network. Colon cancer (Version 1.2022). February 25, 2022. Accessed April 13, 2022. https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf
19. National Comprehensive Cancer Network. Melanoma: cutaneous (Version 3.2022). April 11, 2022. Accessed April 13, 2022. https://www.nccn.org/professionals/physician_gls/pdf/cutaneous_melanoma.pdf
20. Leonetti A, Sharma S, Minari R, Perego P, Giovannetti E, Tiseo M. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br J Cancer. 2019;121(9):725-737. doi:10.1038/s41416-019-0573-8
21. Zheng G, Lin MT, Lokhandwala PM, et al. Clinical mutational profiling of bone metastases of lung and colon carcinoma and malignant melanoma using next-generation sequencing. Cancer Cytopathol. 2016;124(10):744-753. doi:10.1002/cncy.21743
22. Spritzer CE, Afonso PD, Vinson EN, et al. Bone marrow biopsy: RNA isolation with expression profiling in men with metastatic castration-resistant prostate cancer—factors affecting diagnostic success. Radiology. 2013;269(3):816-823. doi:10.1148/radiol.13121782
23. Schweizer MT, Gulati R, Beightol M, et al. Clinical determinants for successful circulating tumor DNA analysis in prostate cancer. Prostate. 2019;79(7):701-708. doi:10.1002/pros.23778
24. Bettegowda C, Sausen M, Leary RJ, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med. 2014;6(224):224ra224. doi:10.1126/scitranslmed.3007094
25. Pritchard CC, Salipante SJ, Koehler K, et al. Validation and implementation of targeted capture and sequencing for the detection of actionable mutation, copy number variation, and gene rearrangement in clinical cancer specimens. J Mol Diagn. 2014;16(1):56-67. doi:10.1016/j.jmoldx.2013.08.004
26. Gutierrez ME, Choi K, Lanman RB, et al. Genomic profiling of advanced non-small cell lung cancer in community settings: gaps and opportunities. Clin Lung Cancer. 2017;18(6):651-659. doi:10.1016/j.cllc.2017.04.004
27. Malapelle U, Pilotto S, Passiglia F, et al. Dealing with NSCLC EGFR mutation testing and treatment: a comprehensive review with an Italian real-world perspective. Crit Rev Oncol Hematol. 2021;160:103300. doi:10.1016/j.critrevonc.2021.103300
28. Drilon A, Laetsch TW, Kummar S, et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med. 2018;378(8):731-739. doi:10.1056/NEJMoa1714448
29. Doebele RC, Drilon A, Paz-Ares L, et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1-2 trials. Lancet Oncol. 2020;21(2):271-282. doi:10.1016/S1470-2045(19)30691-6
30. Jonsson P, Bandlamudi C, Cheng ML, et al. Tumour lineage shapes BRCA-mediated phenotypes. Nature. 2019;571(7766):576-579. doi:10.1038/s41586-019-1382-1
31. Steensma DP. Clinical consequences of clonal hematopoiesis of indeterminate potential. Hematology Am Soc Hematol Educ Program. 2018;2018(1):264-269. doi:10.1182/asheducation-2018.1.264
32. Jensen K, Konnick EQ, Schweizer MT, et al. Association of clonal hematopoiesis in DNA repair genes with prostate cancer plasma cell-free DNA testing interference. JAMA Oncol. 2021;7(1):107-110. doi:10.1001/jamaoncol.2020.5161
The ability to find and target specific biomarkers in the DNA of advanced cancers is rapidly changing options and outcomes for patients with locally advanced and metastatic solid tumors. This strategy is the basis for precision oncology, defined here as using predictive biomarkers from tumor and/or germline sequencing to guide therapies. This article focuses specifically on the use of DNA sequencing to find those biomarkers and provides guidance about which test is optimal in a specific situation, as well as interpretation of the results. We emphasize the identification of biomarkers that provide adult patients with advanced solid tumors access to therapies that would not be an option had sequencing not been performed and that have the potential for significant clinical benefit. The best approach is to have an expert team with experience in precision oncology to assist in the interpretation of results.
Which test?
Deciding what test of the array of assays available to use and which tissue to test can be overwhelming, and uncertainty may prevent oncology practitioners from ordering germline or somatic sequencing. For the purposes of this article, we will focus on DNA sequencing for inherited/germline alterations (including mutations, copy number changes, or fusions), which may inform treatment, or alterations that arise in the process of carcinogenesis and tumor evolution (somatic alterations in tumor DNA). This focus is not meant to exclude any specific test but to focus on DNA-based tests in patients with locally advanced or metastatic malignancy.
Germline Testing
Germline testing is the sequencing of inherited DNA in noncancerous cells to find alterations that may play a role in the development of cancers and are actionable in some cases. Germline alterations can inform therapeutic decisions, predict future cancer risk, and provide information that can help family members to better manage their risks of malignancy. Detailed discussions of the importance of germline testing to inform cancer surveillance, risk-reducing interventions, and the testing of relatives to determine who carries inherited alterations (cascade testing) is extremely important with several advantages and is covered in a number of excellent reviews elsewhere.1-3 Testing of germline DNA in patients with a metastatic malignancy can provide treatment options otherwise not available for patients, particularly for BRCA1/2 and Lynch syndrome–related cancers. Recent studies have shown that 10 to 15% of patients with advanced malignancies of many types have a pathogenic germline alteration.4,5
Germline DNA is usually acquired from peripheral blood, a buccal swab, or saliva collection and is therefore readily available. This is advantageous because it does not require a biopsy to identify relevant alterations. Germline testing is also less susceptible to the rare situations in which artifacts occur in formalin-fixed tissues and obscure relevant alterations.
The cost of germline testing varies, but most commercial vendor assays for germline testing are significantly less expensive than the cost of somatic testing. The disadvantages include the inability of germline testing to find any alterations that arise solely in tumor tissue and the smaller gene panels included in germline testing as compared to somatic testing panels. Other considerations relate to the inherited nature of pathogenic germline variants and its implications for family members that may affect the patient’s psychosocial health and potentially change the family dynamics.
Deciding who is appropriate for germline testing and when to perform the testing should be individualized to the patient’s wishes and disease status. Treatment planning may be less complicated if testing has been performed and germline status is known. In some cases urgent germline testing is indicated to inform pending procedures and/or surgical decisions for risk reduction, including more extensive tissue resection, such as the removal of additional organs or contralateral tissue. A minor point regarding germline testing is that the DNA of patients with hematologic malignancies may be difficult to sequence because of sample contamination by the circulating malignancy. For this reason, most laboratories will not accept peripheral blood or saliva samples for germline testing in patients with active hematologic malignancies; they often require DNA from another source such as fibroblasts from a skin biopsy or cells from a muscle biopsy. Germline testing is recommended for all patients with metastatic prostate cancer, as well as any patient with any stage of pancreatic cancer or ovarian cancer and patients with breast cancer diagnosed at age ≤ 45 years. More detailed criteria for who is appropriate for germline testing outside of these groups can be found in the appropriate National Comprehensive Cancer Network (NCCN) guidelines.6-8 In patients with some malignancies such as prostate and pancreatic cancer, approximately half of patients who have a BRCA-related cancer developed that malignancy because of a germline BRCA alteration.9-11 Testing germline DNA is therefore an easy way to quickly find almost half of all targetable alterations with a treatment approved by the US Food and Drug Administration (FDA) and at low cost, with the added benefit of providing critical information for families who may be unaware that members carry a relevant pathogenic germline alteration. In those families, cascade testing can provide surveillance and intervention strategies that can be lifesaving.
A related and particularly relevant question is when should a result found on a somatic testing panel prompt follow-up germline testing? Some institutions have algorithms in place to automate referral for germline testing based on specific genetic criteria.12 Excellent reviews are available that outline the following considerations in more detail.13 Typically, somatic testing results that would trigger follow-up germline testing would be truncating or deleterious or likely deleterious mutations per germline datasets in high-risk genes associated with highly penetrant autosomal dominant conditions (BRCA1, BRCA2, PALB2, MLH1, MSH2, and MSH6), selected moderate-risk genes (BRIP1, RAD51C, and RAD51D), and specific variants with a high probability of being germline because they are common germline founder mutations. Although the actionability and significance of specific genes remains a matter of some discussion, generally finding a somatic pathogenic sequencing result included in the 59-gene list of the American College of Medical Genetics and Genomics (ACMG) guidelines would be an indication for germline testing. Another indication for germline testing would be finding genes with germline mutations for which the NCCN has specific management guidelines, or the presence of alterations consistent with known founder mutations.14 When a patient’s tumor has microsatellite instability or is hypermutated (defined as > 10 mutations per megabase), a search for germline alterations is warranted given that about 15% of these patients with these tumors carry a Lynch syndrome gene.15 Genes that are commonly found as somatic alterations alone (eg, TP53 or APC) are generally not an indication for germline testing unless family history is compelling.
Although some clinicians use the variant allele fraction in the somatic sequencing report to decide whether to conduct germline testing, this approach is suboptimal, as allele fraction may be confounded by assay conditions and a high allele fraction may be found in pure tumors with loss of heterozygosity (LOH) of the other allele. There is also evidence that for a variety of reasons, somatic sequencing panels do not always detect germline alterations in somatic tissues.16 Reasons for this may include discordance between the genes being tested in the germline vs the somatic panel, technical differences such as interference of formalin-fixed paraffin-embedded (FFPE) artifact with detecting the germline variant, lack of expertise in germline variant interpretation among laboratories doing tumor-only sequencing, and, in rare cases, large deletions in tumor tissue masking a germline point mutation.
Variant Interpretation of Germline Testing
A general understanding of the terminology used for germline variant interpretation allows for the ordering health care practitioner (HCP) to provide the best quality care and an appreciation for the limitations of current molecular testing. Not all variants are associated with disease; the clinical significance of a genetic variant falls on a spectrum. The criteria for determining pathogenicity differ between molecular laboratories, but most are influenced by the standards and guidelines set forth by the ACMG.14 The clinical molecular laboratory determines variant classification, and a detailed discussion is beyond the scope of this primer. In brief, variant classification is based on evidence of varying strength in different categories including population data, computational and predictive data, functional data, segregation data, de novo data, allelic data, and information from various databases. The ACMG has proposed a 5-tiered classification system, by which most molecular laboratories adhere to in their genetic test reports (Table 1).14
Pathogenic and likely pathogenic variants are clinically actionable, whereas variants of uncertain significance (VUS) require additional data and/or functional studies before making clinical decisions. Depending on the clinical context and existing supporting evidence, it may be prudent to continue monitoring for worsening or new signs of disease in patients with one or more VUS while additional efforts are underway to understand the variant’s significance.
In some cases, variants are reclassified, which may alter the management and treatment of patients. Reclassification can occur with VUS, and in rare instances, can also occur with variants previously classified as pathogenic/likely pathogenic or benign/likely benign. In such a case, the reporting laboratory will typically make concerted efforts to alert the ordering HCP. However, variant reclassifications are not always communicated to the care team. Thus, it is important to periodically contact the molecular laboratory of interest to obtain updated test interpretations.
Somatic Testing
Testing of somatic (tumor) tissue is critical and is the approach most commonly taken in medical oncology (Table 2).
The advantages of primary tumor are that it is usually in hand as a diagnostic biopsy, acquisition is standard of care, and several targetable alterations are truncal, defined as driver mutations present at the time of tumor development. Also, the potential that the tumor arose in the background of a predisposing germline alteration can be suggested by sequencing primary tumor as discussed above. Moreover, sequencing the primary tumor can be done at any time unless the biopsy sample is considered too old or degraded (per specific platform requirements). The information gained can be used to anticipate additional treatment options that are relevant when patients experience disease progression. Disadvantages include the problem that primary specimens may be old or have limited tumor content, both of which increase the likelihood that sequencing will not be technically successful.
Alterations that are targetable and arise as a result of either treatment pressure or clonal evolution are considered evolutionary. If evolutionary alterations are the main focus for sequencing, then metastasis biopsy or ctDNA are better choices. The advantages of a metastasis biopsy are that tissue is contemporary, tumor content may be higher than in primary tumor, and both truncal and evolutionary alterations can be detected.
For specific tumors, continued analysis of evolving genomic alterations can play a critical role in management. In non–small cell lung cancer (NSCLC), somatic testing is conducted again at progression on repeat biopsies to evaluate for emerging resistance mutations. In epidermal growth factor receptor (EGFR)–mutated lung cancer, the resistance mutation, exon 20 p.T790M (point mutation), can present in patients after treatment with first- or second-generation EGFR tyrosine kinase inhibitors (TKI). Even in patients who are treated with the third-generation EGFR TKI osimertinib that can treat T790M-mutated lung cancer, multiple possible evolutionary mutations can occur at progression, including other EGFR mutations, MET/HER2 amplification, and BRAF V600E, to name a few.20 Resistance mechanisms develop due to treatment selection pressure and the molecular heterogeneity seen in lung cancer.
Disadvantages for metastatic biopsy include the inability to safely access a metastatic site, the time considerations for preauthorization and arrangement of biopsy, and a lower-than-average likelihood of successful sequencing from sites such as bone.21,22 In addition, there is some concern that a single metastatic site may not capture all relevant alterations for multiple reasons, including tumor heterogeneity.
Significant advances in the past decade have dramatically improved the ability to use ctDNA to guide therapy. Advantages include ease of acquisition as acquiring a sample requires only a blood draw, and the potential that the pool of ctDNA is a better reflection of the relevant biology as it potentially reflects all metastatic tissues. Disadvantages are that sequencing attempts may not be productive if the sample is acquired at a time when the tumor is either quiescent or tumor burden is so low that only limited amounts of DNA are being shed. Performing ctDNA analysis when a tumor is not progressing is less likely to be productive for a number of tumor types.23,24 Sequencing ctDNA is also more susceptible than sequencing tumor biopsies to detection of alterations that are not from the tumor of interest but from clonal hematopoiesis of indeterminate potential (CHIP) or other clonal hematopoietic disorders (see Confounders section below).
Selecting the Tissue
Deciding on the tissue to analyze is a critical part of the decision process (Table 3). If the primary tumor tissue is old the likelihood of productive sequencing is lower, although age alone is not the only consideration and the methods of fixation may be just as relevant.
For prostate cancer in particular, the ability to successfully sequence primary tumor tissue decreases as the amount of tumor decreases in low-volume biopsies such as prostate needle biopsies. Generally, if tumor content is < 10% of the biopsy specimen, then sequencing is less likely to be productive.25 Also, if the alteration of interest is not known to be truncal, then a relevant target might be missed by sequencing tissue that does not reflect current biology. Metastasis biopsy may be the most appropriate tissue, particularly if this specimen has already been acquired. As above, a metastasis biopsy may have a higher tumor content, and it should reflect relevant biology if it is recent. However, bone biopsies have a relatively low yield for successful sequencing, so a soft tissue lesion (eg, liver or lymph node metastasis) is generally preferred.
The inability to safely access tissue is often a consideration. Proximity to vital structures such as large blood vessels or the potential for significant morbidity in the event of a complication (liver or lung biopsies, particularly in patients on anticoagulation medications) may make the risk/benefit ratio too high. The inability to conduct somatic testing has been reported to often be due to inadequate tissue sampling.26 ctDNA is an attractive alternative but should typically be drawn when a tumor is progressing with a reasonable tumor burden that is more likely to be shedding DNA. Performing ctDNA analysis in patients without obvious radiographic metastasis or in patients whose tumor is under good control is unlikely to produce interpretable results.
Interpreting the Results
The intent of sequencing tumor tissue is to identify alterations that are biologically important and may provide a point of therapeutic leverage. However, deciding which alterations are relevant is not always straightforward. For example, any normal individual genome contains around 10,000 missense variants, hundreds of insertion/deletion variants, and dozens of protein-truncating variants. Distinguishing these alterations, which are part of the individual, from those that are tumor-specific and have functional significance can be difficult in the absence of paired sequencing of both normal and tissue samples.
Specific Alterations
Although most commercial vendors provide important information in sequencing reports to assist oncology HCPs in deciding which alterations are relevant, the reports are not always clear. In many cases the report will specifically indicate whether the alteration has been reported previously as pathogenic or benign. However, some platforms will report alterations that are not known to be drivers of tumor biology. It is critical to be aware that if variants are not reported as pathogenic, they should not be assumed to be pathogenic simply because they are included in the report. Alterations more likely to be drivers of relevant biology are those that change gene and protein structure and include frameshift (fs*), nonsense (denoted by sequence ending in “X” or “*”), or specific fusions or insertions/deletions (indel) that occur in important domains of the gene.
For some genes, only specific alterations are targetable and not all alterations have the same effect on protein function. Although overexpression of certain genes and proteins are actionable (eg, HER2), amplification of a gene does not necessarily indicate that it is targetable. In NSCLC, specific alterations convey sensitivity to targeted therapies. For example, in EGFR-mutated NSCLC, the sensitizing mutations to EGFR TKIs are exon19 deletions and exon 21 L858R point mutations (the most common mutations), as well as less common mutations found in exon 18-21. Exon 20 mutations, however, are not responsive to EGFR TKIs with a few exceptions.27 Patients who have tumors that do not harbor a sensitizing EGFR mutation should not be treated with an EGFR TKI. In a variety of solid tumors, gene fusions of the NTRK 1/2/3, act as oncogenic drivers. The chromosomal fusion events involving the carboxy-terminal kinase domain of TRK and upstream amino-terminal partners lead to overexpression of the chimeric proteins tropomyosin receptor kinase (TRK) A/B/C, resulting in constitutively active, ligand-independent downstream signaling. In patients with NTRK 1/2/3 gene fusions, larotrectinib and entrectinib, small molecule inhibitors to TRK, have shown antitumor activity.28,29 No alterations beyond these fusions are known to be targetable.
Allele Fraction
Knowing the fraction (or proportion) of the alteration of interest in the sequenced tissue relative to the estimated tumor content can assist in decision making. Not all platforms will provide this information, which is referred to as mutation allele fraction (MAF) or variant allele fraction (VAF), but sometimes will provide it on request. Platforms will usually provide an estimate of the percent tumor in the tissue being sampled if it is from a biopsy. If the MAF is around 50% in the sequenced tissue (including ctDNA), then there is a reasonable chance that it is a germline variant. However, there are nuances as germline alterations in some genes, such as BRCA1/2, can be accompanied by loss of the other allele of the gene (LOH). In that case, if most of the circulating DNA is from tumor, then the MAF can be > 50%.
If there are 2 alterations of the same gene with MAF percentages that are each half of the total percent tumor, there is a high likelihood of biallelic alteration. These sorts of paired alterations or one mutation with apparent LOH or copy loss would again indicate a high likelihood that the alteration is in fact pathogenic and a relevant driver. Not all pathogenic alterations have to be biallelic to be driver mutations but in BRCA1/2, or mismatch repair deficiency genes, the presence of biallelic alterations increases the likelihood of their being pathogenic.
Tumors that are hypermutated—containing sometimes hundreds of mutations per megabyte of DNA—can be particularly complicated to interpret, because the likelihood increases that many of the alterations are a function of the hypermutation and not a driver mutation. This is particularly important when there are concurrent mutations in mismatch repair genes and genes, such as BRCA1/2. If the tumor is
Confounders
In some situations, interpretation can be particularly challenging. For example, several alterations for which there are FDA on-label indications (such as ATM or BRCA2) can be detected in ctDNA that may not be due to the tumor but to CHIP. CHIP represents hematopoietic clones that are dysplastic as a result of exposure to DNA-damaging agents (eg, platinum chemotherapy) or as a result of aging and arise when mutations in hematopoietic stem cells provide a competitive advantage.31 The most common CHIP clones that can be detected are DNMT3A, ASXL1, or TET2; because these alterations are not targetable, their importance lies primarily in whether patients have evidence of hematologic abnormality, which might represent an evolving hematopoietic disorder. Because CHIP alterations can overlap with somatic alterations for which FDA-approved drugs exist, such as ATM or CHEK2 (olaparib for prostate cancer) and BRCA2 (poly-ADP-ribose polymerase inhibitors in a range of indications) there is concern that CHIP might result in patient harm from inappropriate treatment of CHIP rather than the tumor, with no likelihood that the treatment would affect the tumor, causing treatment delays.32 General considerations for deciding whether an alteration represents CHIP include excluding alteration in which the VAF is < 1% and when the VAF in the alteration of interest is < 20% of the estimated tumor fraction in the sample. Exceptions to this are found in patients with true myelodysplasia or chronic lymphocytic leukemia, in whom the VAF can be well over 50% because of circulating tumor burden. The only way to be certain that an alteration detected on ctDNA reflects tumor rather than CHIP is to utilize an assay with matched tumor-normal sequencing.
Resources for Assistance
For oncology HCPs, perhaps the best resource to help in selecting and interpreting the appropriate testing is through a dedicated molecular oncology tumor board and subject matter experts who contribute to those tumor boards. In the US Department of Veterans Affairs, the national precision oncology program and its affiliated clinical services, such as the option to order a national consultation and molecular tumor board education, are easily accessible to all HCPs (www.cancer.va.gov). Many commercial vendors provide support to assist with questions of interpretation and to inform clinical decision-making. Other resources that can assist with deciding whether an alteration is pathogenic include extensive curated databases such as ClinVar (www.ncbi.nlm.nih.gov/clinvar) and the Human Genetic Mutation Database (www.hgmd.cf.ac.uk/ac/index.php) for germline alterations or COSMIC (cancer.sanger.ac.uk/cosmic) for somatic alterations. OncoKB (www.oncokb.org) is a resource for assistance in defining levels of evidence for the use of agents to target specific alterations and to assist in assigning pathogenicity to specific alterations. Additional educational resources for training in genomics and genetics are also included in the Appendix.
The rapid growth in technology and ability to enhance understanding of relevant tumor biology continues to improve the therapeutic landscape for men and women dealing with malignancy and our ability to find targetable genetic alterations with the potential for meaningful clinical benefit.
Acknowledgments
Dedicated to Neil Spector.
The ability to find and target specific biomarkers in the DNA of advanced cancers is rapidly changing options and outcomes for patients with locally advanced and metastatic solid tumors. This strategy is the basis for precision oncology, defined here as using predictive biomarkers from tumor and/or germline sequencing to guide therapies. This article focuses specifically on the use of DNA sequencing to find those biomarkers and provides guidance about which test is optimal in a specific situation, as well as interpretation of the results. We emphasize the identification of biomarkers that provide adult patients with advanced solid tumors access to therapies that would not be an option had sequencing not been performed and that have the potential for significant clinical benefit. The best approach is to have an expert team with experience in precision oncology to assist in the interpretation of results.
Which test?
Deciding what test of the array of assays available to use and which tissue to test can be overwhelming, and uncertainty may prevent oncology practitioners from ordering germline or somatic sequencing. For the purposes of this article, we will focus on DNA sequencing for inherited/germline alterations (including mutations, copy number changes, or fusions), which may inform treatment, or alterations that arise in the process of carcinogenesis and tumor evolution (somatic alterations in tumor DNA). This focus is not meant to exclude any specific test but to focus on DNA-based tests in patients with locally advanced or metastatic malignancy.
Germline Testing
Germline testing is the sequencing of inherited DNA in noncancerous cells to find alterations that may play a role in the development of cancers and are actionable in some cases. Germline alterations can inform therapeutic decisions, predict future cancer risk, and provide information that can help family members to better manage their risks of malignancy. Detailed discussions of the importance of germline testing to inform cancer surveillance, risk-reducing interventions, and the testing of relatives to determine who carries inherited alterations (cascade testing) is extremely important with several advantages and is covered in a number of excellent reviews elsewhere.1-3 Testing of germline DNA in patients with a metastatic malignancy can provide treatment options otherwise not available for patients, particularly for BRCA1/2 and Lynch syndrome–related cancers. Recent studies have shown that 10 to 15% of patients with advanced malignancies of many types have a pathogenic germline alteration.4,5
Germline DNA is usually acquired from peripheral blood, a buccal swab, or saliva collection and is therefore readily available. This is advantageous because it does not require a biopsy to identify relevant alterations. Germline testing is also less susceptible to the rare situations in which artifacts occur in formalin-fixed tissues and obscure relevant alterations.
The cost of germline testing varies, but most commercial vendor assays for germline testing are significantly less expensive than the cost of somatic testing. The disadvantages include the inability of germline testing to find any alterations that arise solely in tumor tissue and the smaller gene panels included in germline testing as compared to somatic testing panels. Other considerations relate to the inherited nature of pathogenic germline variants and its implications for family members that may affect the patient’s psychosocial health and potentially change the family dynamics.
Deciding who is appropriate for germline testing and when to perform the testing should be individualized to the patient’s wishes and disease status. Treatment planning may be less complicated if testing has been performed and germline status is known. In some cases urgent germline testing is indicated to inform pending procedures and/or surgical decisions for risk reduction, including more extensive tissue resection, such as the removal of additional organs or contralateral tissue. A minor point regarding germline testing is that the DNA of patients with hematologic malignancies may be difficult to sequence because of sample contamination by the circulating malignancy. For this reason, most laboratories will not accept peripheral blood or saliva samples for germline testing in patients with active hematologic malignancies; they often require DNA from another source such as fibroblasts from a skin biopsy or cells from a muscle biopsy. Germline testing is recommended for all patients with metastatic prostate cancer, as well as any patient with any stage of pancreatic cancer or ovarian cancer and patients with breast cancer diagnosed at age ≤ 45 years. More detailed criteria for who is appropriate for germline testing outside of these groups can be found in the appropriate National Comprehensive Cancer Network (NCCN) guidelines.6-8 In patients with some malignancies such as prostate and pancreatic cancer, approximately half of patients who have a BRCA-related cancer developed that malignancy because of a germline BRCA alteration.9-11 Testing germline DNA is therefore an easy way to quickly find almost half of all targetable alterations with a treatment approved by the US Food and Drug Administration (FDA) and at low cost, with the added benefit of providing critical information for families who may be unaware that members carry a relevant pathogenic germline alteration. In those families, cascade testing can provide surveillance and intervention strategies that can be lifesaving.
A related and particularly relevant question is when should a result found on a somatic testing panel prompt follow-up germline testing? Some institutions have algorithms in place to automate referral for germline testing based on specific genetic criteria.12 Excellent reviews are available that outline the following considerations in more detail.13 Typically, somatic testing results that would trigger follow-up germline testing would be truncating or deleterious or likely deleterious mutations per germline datasets in high-risk genes associated with highly penetrant autosomal dominant conditions (BRCA1, BRCA2, PALB2, MLH1, MSH2, and MSH6), selected moderate-risk genes (BRIP1, RAD51C, and RAD51D), and specific variants with a high probability of being germline because they are common germline founder mutations. Although the actionability and significance of specific genes remains a matter of some discussion, generally finding a somatic pathogenic sequencing result included in the 59-gene list of the American College of Medical Genetics and Genomics (ACMG) guidelines would be an indication for germline testing. Another indication for germline testing would be finding genes with germline mutations for which the NCCN has specific management guidelines, or the presence of alterations consistent with known founder mutations.14 When a patient’s tumor has microsatellite instability or is hypermutated (defined as > 10 mutations per megabase), a search for germline alterations is warranted given that about 15% of these patients with these tumors carry a Lynch syndrome gene.15 Genes that are commonly found as somatic alterations alone (eg, TP53 or APC) are generally not an indication for germline testing unless family history is compelling.
Although some clinicians use the variant allele fraction in the somatic sequencing report to decide whether to conduct germline testing, this approach is suboptimal, as allele fraction may be confounded by assay conditions and a high allele fraction may be found in pure tumors with loss of heterozygosity (LOH) of the other allele. There is also evidence that for a variety of reasons, somatic sequencing panels do not always detect germline alterations in somatic tissues.16 Reasons for this may include discordance between the genes being tested in the germline vs the somatic panel, technical differences such as interference of formalin-fixed paraffin-embedded (FFPE) artifact with detecting the germline variant, lack of expertise in germline variant interpretation among laboratories doing tumor-only sequencing, and, in rare cases, large deletions in tumor tissue masking a germline point mutation.
Variant Interpretation of Germline Testing
A general understanding of the terminology used for germline variant interpretation allows for the ordering health care practitioner (HCP) to provide the best quality care and an appreciation for the limitations of current molecular testing. Not all variants are associated with disease; the clinical significance of a genetic variant falls on a spectrum. The criteria for determining pathogenicity differ between molecular laboratories, but most are influenced by the standards and guidelines set forth by the ACMG.14 The clinical molecular laboratory determines variant classification, and a detailed discussion is beyond the scope of this primer. In brief, variant classification is based on evidence of varying strength in different categories including population data, computational and predictive data, functional data, segregation data, de novo data, allelic data, and information from various databases. The ACMG has proposed a 5-tiered classification system, by which most molecular laboratories adhere to in their genetic test reports (Table 1).14
Pathogenic and likely pathogenic variants are clinically actionable, whereas variants of uncertain significance (VUS) require additional data and/or functional studies before making clinical decisions. Depending on the clinical context and existing supporting evidence, it may be prudent to continue monitoring for worsening or new signs of disease in patients with one or more VUS while additional efforts are underway to understand the variant’s significance.
In some cases, variants are reclassified, which may alter the management and treatment of patients. Reclassification can occur with VUS, and in rare instances, can also occur with variants previously classified as pathogenic/likely pathogenic or benign/likely benign. In such a case, the reporting laboratory will typically make concerted efforts to alert the ordering HCP. However, variant reclassifications are not always communicated to the care team. Thus, it is important to periodically contact the molecular laboratory of interest to obtain updated test interpretations.
Somatic Testing
Testing of somatic (tumor) tissue is critical and is the approach most commonly taken in medical oncology (Table 2).
The advantages of primary tumor are that it is usually in hand as a diagnostic biopsy, acquisition is standard of care, and several targetable alterations are truncal, defined as driver mutations present at the time of tumor development. Also, the potential that the tumor arose in the background of a predisposing germline alteration can be suggested by sequencing primary tumor as discussed above. Moreover, sequencing the primary tumor can be done at any time unless the biopsy sample is considered too old or degraded (per specific platform requirements). The information gained can be used to anticipate additional treatment options that are relevant when patients experience disease progression. Disadvantages include the problem that primary specimens may be old or have limited tumor content, both of which increase the likelihood that sequencing will not be technically successful.
Alterations that are targetable and arise as a result of either treatment pressure or clonal evolution are considered evolutionary. If evolutionary alterations are the main focus for sequencing, then metastasis biopsy or ctDNA are better choices. The advantages of a metastasis biopsy are that tissue is contemporary, tumor content may be higher than in primary tumor, and both truncal and evolutionary alterations can be detected.
For specific tumors, continued analysis of evolving genomic alterations can play a critical role in management. In non–small cell lung cancer (NSCLC), somatic testing is conducted again at progression on repeat biopsies to evaluate for emerging resistance mutations. In epidermal growth factor receptor (EGFR)–mutated lung cancer, the resistance mutation, exon 20 p.T790M (point mutation), can present in patients after treatment with first- or second-generation EGFR tyrosine kinase inhibitors (TKI). Even in patients who are treated with the third-generation EGFR TKI osimertinib that can treat T790M-mutated lung cancer, multiple possible evolutionary mutations can occur at progression, including other EGFR mutations, MET/HER2 amplification, and BRAF V600E, to name a few.20 Resistance mechanisms develop due to treatment selection pressure and the molecular heterogeneity seen in lung cancer.
Disadvantages for metastatic biopsy include the inability to safely access a metastatic site, the time considerations for preauthorization and arrangement of biopsy, and a lower-than-average likelihood of successful sequencing from sites such as bone.21,22 In addition, there is some concern that a single metastatic site may not capture all relevant alterations for multiple reasons, including tumor heterogeneity.
Significant advances in the past decade have dramatically improved the ability to use ctDNA to guide therapy. Advantages include ease of acquisition as acquiring a sample requires only a blood draw, and the potential that the pool of ctDNA is a better reflection of the relevant biology as it potentially reflects all metastatic tissues. Disadvantages are that sequencing attempts may not be productive if the sample is acquired at a time when the tumor is either quiescent or tumor burden is so low that only limited amounts of DNA are being shed. Performing ctDNA analysis when a tumor is not progressing is less likely to be productive for a number of tumor types.23,24 Sequencing ctDNA is also more susceptible than sequencing tumor biopsies to detection of alterations that are not from the tumor of interest but from clonal hematopoiesis of indeterminate potential (CHIP) or other clonal hematopoietic disorders (see Confounders section below).
Selecting the Tissue
Deciding on the tissue to analyze is a critical part of the decision process (Table 3). If the primary tumor tissue is old the likelihood of productive sequencing is lower, although age alone is not the only consideration and the methods of fixation may be just as relevant.
For prostate cancer in particular, the ability to successfully sequence primary tumor tissue decreases as the amount of tumor decreases in low-volume biopsies such as prostate needle biopsies. Generally, if tumor content is < 10% of the biopsy specimen, then sequencing is less likely to be productive.25 Also, if the alteration of interest is not known to be truncal, then a relevant target might be missed by sequencing tissue that does not reflect current biology. Metastasis biopsy may be the most appropriate tissue, particularly if this specimen has already been acquired. As above, a metastasis biopsy may have a higher tumor content, and it should reflect relevant biology if it is recent. However, bone biopsies have a relatively low yield for successful sequencing, so a soft tissue lesion (eg, liver or lymph node metastasis) is generally preferred.
The inability to safely access tissue is often a consideration. Proximity to vital structures such as large blood vessels or the potential for significant morbidity in the event of a complication (liver or lung biopsies, particularly in patients on anticoagulation medications) may make the risk/benefit ratio too high. The inability to conduct somatic testing has been reported to often be due to inadequate tissue sampling.26 ctDNA is an attractive alternative but should typically be drawn when a tumor is progressing with a reasonable tumor burden that is more likely to be shedding DNA. Performing ctDNA analysis in patients without obvious radiographic metastasis or in patients whose tumor is under good control is unlikely to produce interpretable results.
Interpreting the Results
The intent of sequencing tumor tissue is to identify alterations that are biologically important and may provide a point of therapeutic leverage. However, deciding which alterations are relevant is not always straightforward. For example, any normal individual genome contains around 10,000 missense variants, hundreds of insertion/deletion variants, and dozens of protein-truncating variants. Distinguishing these alterations, which are part of the individual, from those that are tumor-specific and have functional significance can be difficult in the absence of paired sequencing of both normal and tissue samples.
Specific Alterations
Although most commercial vendors provide important information in sequencing reports to assist oncology HCPs in deciding which alterations are relevant, the reports are not always clear. In many cases the report will specifically indicate whether the alteration has been reported previously as pathogenic or benign. However, some platforms will report alterations that are not known to be drivers of tumor biology. It is critical to be aware that if variants are not reported as pathogenic, they should not be assumed to be pathogenic simply because they are included in the report. Alterations more likely to be drivers of relevant biology are those that change gene and protein structure and include frameshift (fs*), nonsense (denoted by sequence ending in “X” or “*”), or specific fusions or insertions/deletions (indel) that occur in important domains of the gene.
For some genes, only specific alterations are targetable and not all alterations have the same effect on protein function. Although overexpression of certain genes and proteins are actionable (eg, HER2), amplification of a gene does not necessarily indicate that it is targetable. In NSCLC, specific alterations convey sensitivity to targeted therapies. For example, in EGFR-mutated NSCLC, the sensitizing mutations to EGFR TKIs are exon19 deletions and exon 21 L858R point mutations (the most common mutations), as well as less common mutations found in exon 18-21. Exon 20 mutations, however, are not responsive to EGFR TKIs with a few exceptions.27 Patients who have tumors that do not harbor a sensitizing EGFR mutation should not be treated with an EGFR TKI. In a variety of solid tumors, gene fusions of the NTRK 1/2/3, act as oncogenic drivers. The chromosomal fusion events involving the carboxy-terminal kinase domain of TRK and upstream amino-terminal partners lead to overexpression of the chimeric proteins tropomyosin receptor kinase (TRK) A/B/C, resulting in constitutively active, ligand-independent downstream signaling. In patients with NTRK 1/2/3 gene fusions, larotrectinib and entrectinib, small molecule inhibitors to TRK, have shown antitumor activity.28,29 No alterations beyond these fusions are known to be targetable.
Allele Fraction
Knowing the fraction (or proportion) of the alteration of interest in the sequenced tissue relative to the estimated tumor content can assist in decision making. Not all platforms will provide this information, which is referred to as mutation allele fraction (MAF) or variant allele fraction (VAF), but sometimes will provide it on request. Platforms will usually provide an estimate of the percent tumor in the tissue being sampled if it is from a biopsy. If the MAF is around 50% in the sequenced tissue (including ctDNA), then there is a reasonable chance that it is a germline variant. However, there are nuances as germline alterations in some genes, such as BRCA1/2, can be accompanied by loss of the other allele of the gene (LOH). In that case, if most of the circulating DNA is from tumor, then the MAF can be > 50%.
If there are 2 alterations of the same gene with MAF percentages that are each half of the total percent tumor, there is a high likelihood of biallelic alteration. These sorts of paired alterations or one mutation with apparent LOH or copy loss would again indicate a high likelihood that the alteration is in fact pathogenic and a relevant driver. Not all pathogenic alterations have to be biallelic to be driver mutations but in BRCA1/2, or mismatch repair deficiency genes, the presence of biallelic alterations increases the likelihood of their being pathogenic.
Tumors that are hypermutated—containing sometimes hundreds of mutations per megabyte of DNA—can be particularly complicated to interpret, because the likelihood increases that many of the alterations are a function of the hypermutation and not a driver mutation. This is particularly important when there are concurrent mutations in mismatch repair genes and genes, such as BRCA1/2. If the tumor is
Confounders
In some situations, interpretation can be particularly challenging. For example, several alterations for which there are FDA on-label indications (such as ATM or BRCA2) can be detected in ctDNA that may not be due to the tumor but to CHIP. CHIP represents hematopoietic clones that are dysplastic as a result of exposure to DNA-damaging agents (eg, platinum chemotherapy) or as a result of aging and arise when mutations in hematopoietic stem cells provide a competitive advantage.31 The most common CHIP clones that can be detected are DNMT3A, ASXL1, or TET2; because these alterations are not targetable, their importance lies primarily in whether patients have evidence of hematologic abnormality, which might represent an evolving hematopoietic disorder. Because CHIP alterations can overlap with somatic alterations for which FDA-approved drugs exist, such as ATM or CHEK2 (olaparib for prostate cancer) and BRCA2 (poly-ADP-ribose polymerase inhibitors in a range of indications) there is concern that CHIP might result in patient harm from inappropriate treatment of CHIP rather than the tumor, with no likelihood that the treatment would affect the tumor, causing treatment delays.32 General considerations for deciding whether an alteration represents CHIP include excluding alteration in which the VAF is < 1% and when the VAF in the alteration of interest is < 20% of the estimated tumor fraction in the sample. Exceptions to this are found in patients with true myelodysplasia or chronic lymphocytic leukemia, in whom the VAF can be well over 50% because of circulating tumor burden. The only way to be certain that an alteration detected on ctDNA reflects tumor rather than CHIP is to utilize an assay with matched tumor-normal sequencing.
Resources for Assistance
For oncology HCPs, perhaps the best resource to help in selecting and interpreting the appropriate testing is through a dedicated molecular oncology tumor board and subject matter experts who contribute to those tumor boards. In the US Department of Veterans Affairs, the national precision oncology program and its affiliated clinical services, such as the option to order a national consultation and molecular tumor board education, are easily accessible to all HCPs (www.cancer.va.gov). Many commercial vendors provide support to assist with questions of interpretation and to inform clinical decision-making. Other resources that can assist with deciding whether an alteration is pathogenic include extensive curated databases such as ClinVar (www.ncbi.nlm.nih.gov/clinvar) and the Human Genetic Mutation Database (www.hgmd.cf.ac.uk/ac/index.php) for germline alterations or COSMIC (cancer.sanger.ac.uk/cosmic) for somatic alterations. OncoKB (www.oncokb.org) is a resource for assistance in defining levels of evidence for the use of agents to target specific alterations and to assist in assigning pathogenicity to specific alterations. Additional educational resources for training in genomics and genetics are also included in the Appendix.
The rapid growth in technology and ability to enhance understanding of relevant tumor biology continues to improve the therapeutic landscape for men and women dealing with malignancy and our ability to find targetable genetic alterations with the potential for meaningful clinical benefit.
Acknowledgments
Dedicated to Neil Spector.
1. Domchek SM, Mardis E, Carlisle JW, Owonikoko TK. Integrating genetic and genomic testing into oncology practice. Am Soc Clin Oncol Educ Book. 2020;40:e259-e263. doi:10.1200/EDBK_280607
2. Stoffel EM, Carethers JM. Current approaches to germline cancer genetic testing. Annu Rev Med. 2020;71:85-102. doi:10.1146/annurev-med-052318-101009
3. Lappalainen T, Scott AJ, Brandt M, Hall IM. Genomic analysis in the age of human genome sequencing. Cell. 2019;177(1):70-84. doi:10.1016/j.cell.2019.02.032
4. Samadder NJ, Riegert-Johnson D, Boardman L, et al. Comparison of universal genetic testing vs guideline-directed targeted testing for patients with hereditary cancer syndrome. JAMA Oncol. 2021;7(2):230-237. doi:10.1001/jamaoncol.2020.6252
5. Schneider BP, Stout L, Philips S, et al. Implications of incidental germline findings identified in the context of clinical whole exome sequencing for guiding cancer therapy. JCO Precis Oncol. 2020;4:1109-1121. doi:10.1200/PO.19.00354
6. National Comprehensive Cancer Network. Pancreatic cancer (Version 1.2022). Updated February 24, 2022. Accessed April 13, 2022. https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf
7. National Comprehensive Cancer Network. Prostate cancer (Version 3.2022). Updated January 10, 2022. Accessed April 13, 2022. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf
8. National Comprehensive Cancer Network. Genetic/familial high-risk assessment: breast, ovarian, and pancreatic (Version 2.2022). Updated March 9, 2022. Accessed April 13, 2022. https://www.nccn.org/professionals/physician_gls/pdf/genetics_bop.pdf
9. Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161(5):1215-1228. doi:10.1016/j.cell.2015.05.001
10. Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;375(5):443-453. doi:10.1056/NEJMoa1603144
11. Cancer Genome Atlas Research Network. Integrated genomic characterization of pancreatic ductal adenocarcinoma. Cancer Cell. 2017;32(2):185-203.e13. doi:10.1016/j.ccell.2017.07.007
12. Clark DF, Maxwell KN, Powers J, et al. Identification and confirmation of potentially actionable germline mutations in tumor-only genomic sequencing. JCO Precis Oncol. 2019;3:PO.19.00076. doi:10.1200/PO.19.00076
13. DeLeonardis K, Hogan L, Cannistra SA, Rangachari D, Tung N. When should tumor genomic profiling prompt consideration of germline testing? J Oncol Pract. 2019;15(9):465-473. doi:10.1200/JOP.19.00201
14. Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405-424. doi:10.1038/gim.2015.30
15. Latham A, Srinivasan P, Kemel Y, et al. Microsatellite instability is associated with the presence of Lynch syndrome pan-cancer. J Clin Oncol. 2019;37(4):286-295. doi:10.1200/JCO.18.00283
16. Lincoln SE, Nussbaum RL, Kurian AW, et al. Yield and utility of germline testing following tumor sequencing in patients with cancer. JAMA Netw Open. 2020;3(10):e2019452. doi:10.1001/jamanetworkopen.2020.19452
17. National Comprehensive Cancer Network. Non-small cell lung cancer (Version: 3.2022). Updated March 16, 2022. Accessed April 13, 2022. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
18. National Comprehensive Cancer Network. Colon cancer (Version 1.2022). February 25, 2022. Accessed April 13, 2022. https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf
19. National Comprehensive Cancer Network. Melanoma: cutaneous (Version 3.2022). April 11, 2022. Accessed April 13, 2022. https://www.nccn.org/professionals/physician_gls/pdf/cutaneous_melanoma.pdf
20. Leonetti A, Sharma S, Minari R, Perego P, Giovannetti E, Tiseo M. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br J Cancer. 2019;121(9):725-737. doi:10.1038/s41416-019-0573-8
21. Zheng G, Lin MT, Lokhandwala PM, et al. Clinical mutational profiling of bone metastases of lung and colon carcinoma and malignant melanoma using next-generation sequencing. Cancer Cytopathol. 2016;124(10):744-753. doi:10.1002/cncy.21743
22. Spritzer CE, Afonso PD, Vinson EN, et al. Bone marrow biopsy: RNA isolation with expression profiling in men with metastatic castration-resistant prostate cancer—factors affecting diagnostic success. Radiology. 2013;269(3):816-823. doi:10.1148/radiol.13121782
23. Schweizer MT, Gulati R, Beightol M, et al. Clinical determinants for successful circulating tumor DNA analysis in prostate cancer. Prostate. 2019;79(7):701-708. doi:10.1002/pros.23778
24. Bettegowda C, Sausen M, Leary RJ, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med. 2014;6(224):224ra224. doi:10.1126/scitranslmed.3007094
25. Pritchard CC, Salipante SJ, Koehler K, et al. Validation and implementation of targeted capture and sequencing for the detection of actionable mutation, copy number variation, and gene rearrangement in clinical cancer specimens. J Mol Diagn. 2014;16(1):56-67. doi:10.1016/j.jmoldx.2013.08.004
26. Gutierrez ME, Choi K, Lanman RB, et al. Genomic profiling of advanced non-small cell lung cancer in community settings: gaps and opportunities. Clin Lung Cancer. 2017;18(6):651-659. doi:10.1016/j.cllc.2017.04.004
27. Malapelle U, Pilotto S, Passiglia F, et al. Dealing with NSCLC EGFR mutation testing and treatment: a comprehensive review with an Italian real-world perspective. Crit Rev Oncol Hematol. 2021;160:103300. doi:10.1016/j.critrevonc.2021.103300
28. Drilon A, Laetsch TW, Kummar S, et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med. 2018;378(8):731-739. doi:10.1056/NEJMoa1714448
29. Doebele RC, Drilon A, Paz-Ares L, et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1-2 trials. Lancet Oncol. 2020;21(2):271-282. doi:10.1016/S1470-2045(19)30691-6
30. Jonsson P, Bandlamudi C, Cheng ML, et al. Tumour lineage shapes BRCA-mediated phenotypes. Nature. 2019;571(7766):576-579. doi:10.1038/s41586-019-1382-1
31. Steensma DP. Clinical consequences of clonal hematopoiesis of indeterminate potential. Hematology Am Soc Hematol Educ Program. 2018;2018(1):264-269. doi:10.1182/asheducation-2018.1.264
32. Jensen K, Konnick EQ, Schweizer MT, et al. Association of clonal hematopoiesis in DNA repair genes with prostate cancer plasma cell-free DNA testing interference. JAMA Oncol. 2021;7(1):107-110. doi:10.1001/jamaoncol.2020.5161
1. Domchek SM, Mardis E, Carlisle JW, Owonikoko TK. Integrating genetic and genomic testing into oncology practice. Am Soc Clin Oncol Educ Book. 2020;40:e259-e263. doi:10.1200/EDBK_280607
2. Stoffel EM, Carethers JM. Current approaches to germline cancer genetic testing. Annu Rev Med. 2020;71:85-102. doi:10.1146/annurev-med-052318-101009
3. Lappalainen T, Scott AJ, Brandt M, Hall IM. Genomic analysis in the age of human genome sequencing. Cell. 2019;177(1):70-84. doi:10.1016/j.cell.2019.02.032
4. Samadder NJ, Riegert-Johnson D, Boardman L, et al. Comparison of universal genetic testing vs guideline-directed targeted testing for patients with hereditary cancer syndrome. JAMA Oncol. 2021;7(2):230-237. doi:10.1001/jamaoncol.2020.6252
5. Schneider BP, Stout L, Philips S, et al. Implications of incidental germline findings identified in the context of clinical whole exome sequencing for guiding cancer therapy. JCO Precis Oncol. 2020;4:1109-1121. doi:10.1200/PO.19.00354
6. National Comprehensive Cancer Network. Pancreatic cancer (Version 1.2022). Updated February 24, 2022. Accessed April 13, 2022. https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf
7. National Comprehensive Cancer Network. Prostate cancer (Version 3.2022). Updated January 10, 2022. Accessed April 13, 2022. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf
8. National Comprehensive Cancer Network. Genetic/familial high-risk assessment: breast, ovarian, and pancreatic (Version 2.2022). Updated March 9, 2022. Accessed April 13, 2022. https://www.nccn.org/professionals/physician_gls/pdf/genetics_bop.pdf
9. Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161(5):1215-1228. doi:10.1016/j.cell.2015.05.001
10. Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;375(5):443-453. doi:10.1056/NEJMoa1603144
11. Cancer Genome Atlas Research Network. Integrated genomic characterization of pancreatic ductal adenocarcinoma. Cancer Cell. 2017;32(2):185-203.e13. doi:10.1016/j.ccell.2017.07.007
12. Clark DF, Maxwell KN, Powers J, et al. Identification and confirmation of potentially actionable germline mutations in tumor-only genomic sequencing. JCO Precis Oncol. 2019;3:PO.19.00076. doi:10.1200/PO.19.00076
13. DeLeonardis K, Hogan L, Cannistra SA, Rangachari D, Tung N. When should tumor genomic profiling prompt consideration of germline testing? J Oncol Pract. 2019;15(9):465-473. doi:10.1200/JOP.19.00201
14. Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405-424. doi:10.1038/gim.2015.30
15. Latham A, Srinivasan P, Kemel Y, et al. Microsatellite instability is associated with the presence of Lynch syndrome pan-cancer. J Clin Oncol. 2019;37(4):286-295. doi:10.1200/JCO.18.00283
16. Lincoln SE, Nussbaum RL, Kurian AW, et al. Yield and utility of germline testing following tumor sequencing in patients with cancer. JAMA Netw Open. 2020;3(10):e2019452. doi:10.1001/jamanetworkopen.2020.19452
17. National Comprehensive Cancer Network. Non-small cell lung cancer (Version: 3.2022). Updated March 16, 2022. Accessed April 13, 2022. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
18. National Comprehensive Cancer Network. Colon cancer (Version 1.2022). February 25, 2022. Accessed April 13, 2022. https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf
19. National Comprehensive Cancer Network. Melanoma: cutaneous (Version 3.2022). April 11, 2022. Accessed April 13, 2022. https://www.nccn.org/professionals/physician_gls/pdf/cutaneous_melanoma.pdf
20. Leonetti A, Sharma S, Minari R, Perego P, Giovannetti E, Tiseo M. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br J Cancer. 2019;121(9):725-737. doi:10.1038/s41416-019-0573-8
21. Zheng G, Lin MT, Lokhandwala PM, et al. Clinical mutational profiling of bone metastases of lung and colon carcinoma and malignant melanoma using next-generation sequencing. Cancer Cytopathol. 2016;124(10):744-753. doi:10.1002/cncy.21743
22. Spritzer CE, Afonso PD, Vinson EN, et al. Bone marrow biopsy: RNA isolation with expression profiling in men with metastatic castration-resistant prostate cancer—factors affecting diagnostic success. Radiology. 2013;269(3):816-823. doi:10.1148/radiol.13121782
23. Schweizer MT, Gulati R, Beightol M, et al. Clinical determinants for successful circulating tumor DNA analysis in prostate cancer. Prostate. 2019;79(7):701-708. doi:10.1002/pros.23778
24. Bettegowda C, Sausen M, Leary RJ, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med. 2014;6(224):224ra224. doi:10.1126/scitranslmed.3007094
25. Pritchard CC, Salipante SJ, Koehler K, et al. Validation and implementation of targeted capture and sequencing for the detection of actionable mutation, copy number variation, and gene rearrangement in clinical cancer specimens. J Mol Diagn. 2014;16(1):56-67. doi:10.1016/j.jmoldx.2013.08.004
26. Gutierrez ME, Choi K, Lanman RB, et al. Genomic profiling of advanced non-small cell lung cancer in community settings: gaps and opportunities. Clin Lung Cancer. 2017;18(6):651-659. doi:10.1016/j.cllc.2017.04.004
27. Malapelle U, Pilotto S, Passiglia F, et al. Dealing with NSCLC EGFR mutation testing and treatment: a comprehensive review with an Italian real-world perspective. Crit Rev Oncol Hematol. 2021;160:103300. doi:10.1016/j.critrevonc.2021.103300
28. Drilon A, Laetsch TW, Kummar S, et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med. 2018;378(8):731-739. doi:10.1056/NEJMoa1714448
29. Doebele RC, Drilon A, Paz-Ares L, et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1-2 trials. Lancet Oncol. 2020;21(2):271-282. doi:10.1016/S1470-2045(19)30691-6
30. Jonsson P, Bandlamudi C, Cheng ML, et al. Tumour lineage shapes BRCA-mediated phenotypes. Nature. 2019;571(7766):576-579. doi:10.1038/s41586-019-1382-1
31. Steensma DP. Clinical consequences of clonal hematopoiesis of indeterminate potential. Hematology Am Soc Hematol Educ Program. 2018;2018(1):264-269. doi:10.1182/asheducation-2018.1.264
32. Jensen K, Konnick EQ, Schweizer MT, et al. Association of clonal hematopoiesis in DNA repair genes with prostate cancer plasma cell-free DNA testing interference. JAMA Oncol. 2021;7(1):107-110. doi:10.1001/jamaoncol.2020.5161
Applicability of the USPSTF Lung Cancer Screening Guidelines in a Predominantly Black Veteran Population
Lung cancer is the leading cause of cancer death in the United States.1 The 2011 National Lung Screening Trial (NLST) demonstrated that low-dose computed tomography (LDCT) screening provided a 20% relative reduction in lung cancer–specific mortality.2 Based on these findings, the United States Preventive Services Task Force (USPSTF) published lung cancer screening guidelines in 2013 recommending an annual LDCT of the thorax in patients aged 55 to 80 years with a 30 pack-year smoking history and who currently smoke or quit within the past 15 years.
In 2021, the USPSTF updated its recommendations by reducing the qualifications for annual screening to a 20 pack-year smoking history.3 The updated guidelines recognized the increased risk of lung cancer for Black individuals.4,5 Evidence suggests the 2013 screening criteria was too conservative for this population.6,7
Similarly, US Department of Veteran Affairs (VA) patients are a population at higher risk for lung cancer due to a male predominance, presence of comorbidities, exposure to carcinogenic agents, and possibly a higher prevalence of tobacco smoking.8 This study sought to examine the applicability of the USPSTF guidelines in a VA health care system with a predominantly Black population.
Methods
A retrospective chart review of adult patients who were diagnosed and treated with early-stage small cell or non–small cell lung cancer (stage I or II) was performed within the Southeast Louisiana Veterans Health Care System (SLVHCS) in New Orleans. The review used data from the VA Cancer Registry from January 1, 2005, through December 31, 2017. Patients were grouped by whether they met 2013 USPSTF screening criteria at time of diagnosis vs those that did not. Data collected included type and stage of lung cancer at time of diagnosis, context of diagnosis (incidental, screening, symptomatic), diagnostic method, smoking history, and presence of chronic obstructive pulmonary disease (COPD). Patients without a clear smoking history documented in the health record were excluded.
Statistical analyses were performed with GraphPad Prism 8.0. Student t test and Fischer exact test were performed for most of the statistical analyses, with differences between groups noted to be statistically significant at a P < .05.
Results
A total of 182 patient charts were reviewed and 13 patients were excluded for missing information related to the USPSTF screening criteria. Of the 169 patients included, 122 (72%) met USPSTF screening criteria while 47 (28%) patients did not. The reasons for not meeting screening criteria were 14 patients were too young at and 9 patients were too old at time of diagnosis, 7 had a < 20 pack-year smoking history, 7 patients had quit > 15 years previously, and 12 patients met multiple exclusion criteria. The study population was 96% male and there was an overall predominance of Black patients (58%) within the sample (Table).
There was a significantly higher proportion of Black patients in the group that did not meet screening criteria compared with the group that met screening criteria (68% vs 54%, P = .04). Cancer type and stage at diagnosis were similar in both patient populations. There was a statistically significant difference in COPD diagnosis between the groups, with a larger proportion of COPD patients in the met screening criteria group (74% vs 45%, P < .001). The mean smoking history was 61.4 pack-years in the met criteria group and 43.3 pack-years in the did not meet criteria group.
Five additional patients in the group that did not meet the 2013 USPSTF screening criteria would have met criteria if the 2021 USPSTF guidelines were applied. All 5 were Black patients. Using the 2021 guidelines, Black patients would have made up 56% of the patients who met screening criteria and 54% of the patients who did not meet screening criteria at time of diagnosis.
Discussion
This study sought to determine the hypothetical effectiveness of national lung cancer screening guidelines in detecting early-stage lung cancer for a high-risk population. Patients diagnosed with early-stage lung cancer were selected as these patients have improved outcomes with treatment, and thus would theoretically benefit from early detection through screening. As expected, the study population had a majority of Black veterans (58%), with a higher proportion of Black patients in the did not meet screening criteria group compared with the met screening criteria group (68% vs 54%, P = .04). This difference highlights the concern that Black individuals were being underscreened with the 2013 USPSTF guidelines.7 This is not all surprising as the NLST, from which the initial screening guidelines were based, included a majority White population with only 4.4% of their population being Black.2 The USPSTF also cites the NELSON trial as evidence to support annual lung cancer screening, a trial that was performed in the Netherlands with a very different population compared with that of southeast Louisiana.9
Given concern that the old criteria were underscreening certain populations, the updated 2021 USPSTF guidelines sought to expand the screening population. In this study, the implementation of these new guidelines resulted in more Black patients meeting screening criteria.
Racial and ethnic disparities in health care in the US are no secret, as Black individuals consistently have increased disease and death rates, higher rates of unemployment, and decreased access to preventive medical care compared to White individuals.10 Despite the updated USPSTF guidelines, additional modifications to the screening criteria could improve the ability to identify high-risk patients. A modified model using data from the Prostate, Lung, Colorectal, and Ovarian Screening Trial (PLCO) incorporating COPD history, race and ethnicity, and personal history of cancer increased the sensitivity for high-risk Black ever-smokers.11 Additional models and analyses also support the utility of incorporating race and ethnicity in lung cancer screening criteria.7,12 Using race and ethnicity to guide screening criteria for cancer is not unheard of; in 2017, the US Multi-Society Task Force recommended that Black individuals start colon cancer screening at age 45 years rather than the typical age of 50 years, before updating the guidelines again in 2021 to recommend that all adults start at age 45 years.13,14
Limitations
This study had the inherent weakness of being a retrospective study at a single institution. Additionally, the 7th edition of the International Association for the Study of Lung Cancer was published in 2010, during the 2005 to 2017 time frame from which our data was collected, leading to possible inconsistencies in staging between patients before and after 2010.15 However, these changes in staging are unlikely to significantly impact the results for in this study, since the vast majority of the patients diagnosed with lung cancer stage I or II before 2010 would still be in the those 2 stages in the 2010 edition. Finally, specific to our patient population, it was often difficult to ascertain an accurate smoking history for each patient, especially in the early years of the data set, likely due to the disruption of care caused by Hurricane Katrina.
Conclusions
In this retrospective study performed at the SLVHCS in New Orleans, a significantly higher proportion of Black patients compared with White patients with early-stage lung cancer did not meet the 2013 USPSTF lung cancer screening guidelines at time of diagnosis, highlighting the concern that this population was being underscreened. These findings demonstrate the challenges and failures of applying national guidelines to a unique, high-risk population. An individualized, risk-based screening model incorporating race and ethnicity could be more effective at diagnosing early-stage lung cancer and requires more investigation. Centralized lung cancer screening programs within the VA system could also be beneficial for early detection and treatment, as well as provide insight into the increased risk within the veteran population.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7-30. doi:10.3322/caac.21590
2. National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409. doi:10.1056/NEJMoa110287
3. US Preventive Services Task Force, Krist AH, Davidson KW, et al. Screening for lung cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325(10):962-970. doi:10.1001/jama.2021.1117
4. Jonas DE, Reuland DS, Reddy SM, et al. Screening for lung cancer with low-dose computed tomography: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;325(10):971-987. doi:10.1001/jama.2021.0377
5. Haiman CA, Stram DO, Wilkens LR, et al. Ethnic and racial differences in the smoking-related risk of lung cancer. N Engl J Med. 2006;354(4):333-342. doi:10.1056/NEJMoa033250
6. DeSantis CE, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Cancer statistics for African Americans, 2019. CA Cancer J Clin. 2019;69(3):211-233. doi:10.3322/caac.21555
7. Aldrich MC, Mercaldo SF, Sandler KL, Blot WJ, Grogan EL, Blume JD. Evaluation of USPSTF Lung Cancer Screening Guidelines among African American adult smokers. JAMA Oncol. 2019;5(9):1318-1324. doi:10.1001/jamaoncol.2019.1402
8. Brown DW. Smoking prevalence among US veterans. J Gen Intern Med. 2010;25(2):147-149. doi:10.1007/s11606-009-1160-0
9. de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020;382(6):503-513. doi:10.1056/NEJMoa1911793
10. Williams DR, Rucker TD. Understanding and addressing racial disparities in health care. Health Care Financ Rev. 2000;21(4):75-90.
11. Pasquinelli MM, Tammemägi MC, Kovitz KL, et al. Risk prediction model versus United States Preventive Services Task Force lung cancer screening eligibility criteria: reducing race disparities. J Thorac Oncol. 2020;15(11):1738-1747. doi:10.1016/j.jtho.2020.08.006
12. Ten Haaf K, Bastani M, Cao P, et al. A comparative modeling analysis of risk-based lung cancer screening strategies. J Natl Cancer Inst. 2020;112(5):466-479. doi:10.1093/jnci/djz164
13. Rex DK, Boland CR, Dominitz JA, et al. Colorectal cancer screening: recommendations for physicians and patients from the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2017;153(1):307-323. doi:10.1053/j.gastro.2017.05.013
14. US Preventive Services Task Force, Davidson KW, Barry MJ, et al. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325(19):1965-1977. doi:10.1001/jama.2021.6238
15. Mirsadraee S, Oswal D, Alizadeh Y, Caulo A, van Beek E Jr. The 7th lung cancer TNM classification and staging system: review of the changes and implications. World J Radiol. 2012;4(4):128-134. doi:10.4329/wjr.v4.i4.128
Lung cancer is the leading cause of cancer death in the United States.1 The 2011 National Lung Screening Trial (NLST) demonstrated that low-dose computed tomography (LDCT) screening provided a 20% relative reduction in lung cancer–specific mortality.2 Based on these findings, the United States Preventive Services Task Force (USPSTF) published lung cancer screening guidelines in 2013 recommending an annual LDCT of the thorax in patients aged 55 to 80 years with a 30 pack-year smoking history and who currently smoke or quit within the past 15 years.
In 2021, the USPSTF updated its recommendations by reducing the qualifications for annual screening to a 20 pack-year smoking history.3 The updated guidelines recognized the increased risk of lung cancer for Black individuals.4,5 Evidence suggests the 2013 screening criteria was too conservative for this population.6,7
Similarly, US Department of Veteran Affairs (VA) patients are a population at higher risk for lung cancer due to a male predominance, presence of comorbidities, exposure to carcinogenic agents, and possibly a higher prevalence of tobacco smoking.8 This study sought to examine the applicability of the USPSTF guidelines in a VA health care system with a predominantly Black population.
Methods
A retrospective chart review of adult patients who were diagnosed and treated with early-stage small cell or non–small cell lung cancer (stage I or II) was performed within the Southeast Louisiana Veterans Health Care System (SLVHCS) in New Orleans. The review used data from the VA Cancer Registry from January 1, 2005, through December 31, 2017. Patients were grouped by whether they met 2013 USPSTF screening criteria at time of diagnosis vs those that did not. Data collected included type and stage of lung cancer at time of diagnosis, context of diagnosis (incidental, screening, symptomatic), diagnostic method, smoking history, and presence of chronic obstructive pulmonary disease (COPD). Patients without a clear smoking history documented in the health record were excluded.
Statistical analyses were performed with GraphPad Prism 8.0. Student t test and Fischer exact test were performed for most of the statistical analyses, with differences between groups noted to be statistically significant at a P < .05.
Results
A total of 182 patient charts were reviewed and 13 patients were excluded for missing information related to the USPSTF screening criteria. Of the 169 patients included, 122 (72%) met USPSTF screening criteria while 47 (28%) patients did not. The reasons for not meeting screening criteria were 14 patients were too young at and 9 patients were too old at time of diagnosis, 7 had a < 20 pack-year smoking history, 7 patients had quit > 15 years previously, and 12 patients met multiple exclusion criteria. The study population was 96% male and there was an overall predominance of Black patients (58%) within the sample (Table).
There was a significantly higher proportion of Black patients in the group that did not meet screening criteria compared with the group that met screening criteria (68% vs 54%, P = .04). Cancer type and stage at diagnosis were similar in both patient populations. There was a statistically significant difference in COPD diagnosis between the groups, with a larger proportion of COPD patients in the met screening criteria group (74% vs 45%, P < .001). The mean smoking history was 61.4 pack-years in the met criteria group and 43.3 pack-years in the did not meet criteria group.
Five additional patients in the group that did not meet the 2013 USPSTF screening criteria would have met criteria if the 2021 USPSTF guidelines were applied. All 5 were Black patients. Using the 2021 guidelines, Black patients would have made up 56% of the patients who met screening criteria and 54% of the patients who did not meet screening criteria at time of diagnosis.
Discussion
This study sought to determine the hypothetical effectiveness of national lung cancer screening guidelines in detecting early-stage lung cancer for a high-risk population. Patients diagnosed with early-stage lung cancer were selected as these patients have improved outcomes with treatment, and thus would theoretically benefit from early detection through screening. As expected, the study population had a majority of Black veterans (58%), with a higher proportion of Black patients in the did not meet screening criteria group compared with the met screening criteria group (68% vs 54%, P = .04). This difference highlights the concern that Black individuals were being underscreened with the 2013 USPSTF guidelines.7 This is not all surprising as the NLST, from which the initial screening guidelines were based, included a majority White population with only 4.4% of their population being Black.2 The USPSTF also cites the NELSON trial as evidence to support annual lung cancer screening, a trial that was performed in the Netherlands with a very different population compared with that of southeast Louisiana.9
Given concern that the old criteria were underscreening certain populations, the updated 2021 USPSTF guidelines sought to expand the screening population. In this study, the implementation of these new guidelines resulted in more Black patients meeting screening criteria.
Racial and ethnic disparities in health care in the US are no secret, as Black individuals consistently have increased disease and death rates, higher rates of unemployment, and decreased access to preventive medical care compared to White individuals.10 Despite the updated USPSTF guidelines, additional modifications to the screening criteria could improve the ability to identify high-risk patients. A modified model using data from the Prostate, Lung, Colorectal, and Ovarian Screening Trial (PLCO) incorporating COPD history, race and ethnicity, and personal history of cancer increased the sensitivity for high-risk Black ever-smokers.11 Additional models and analyses also support the utility of incorporating race and ethnicity in lung cancer screening criteria.7,12 Using race and ethnicity to guide screening criteria for cancer is not unheard of; in 2017, the US Multi-Society Task Force recommended that Black individuals start colon cancer screening at age 45 years rather than the typical age of 50 years, before updating the guidelines again in 2021 to recommend that all adults start at age 45 years.13,14
Limitations
This study had the inherent weakness of being a retrospective study at a single institution. Additionally, the 7th edition of the International Association for the Study of Lung Cancer was published in 2010, during the 2005 to 2017 time frame from which our data was collected, leading to possible inconsistencies in staging between patients before and after 2010.15 However, these changes in staging are unlikely to significantly impact the results for in this study, since the vast majority of the patients diagnosed with lung cancer stage I or II before 2010 would still be in the those 2 stages in the 2010 edition. Finally, specific to our patient population, it was often difficult to ascertain an accurate smoking history for each patient, especially in the early years of the data set, likely due to the disruption of care caused by Hurricane Katrina.
Conclusions
In this retrospective study performed at the SLVHCS in New Orleans, a significantly higher proportion of Black patients compared with White patients with early-stage lung cancer did not meet the 2013 USPSTF lung cancer screening guidelines at time of diagnosis, highlighting the concern that this population was being underscreened. These findings demonstrate the challenges and failures of applying national guidelines to a unique, high-risk population. An individualized, risk-based screening model incorporating race and ethnicity could be more effective at diagnosing early-stage lung cancer and requires more investigation. Centralized lung cancer screening programs within the VA system could also be beneficial for early detection and treatment, as well as provide insight into the increased risk within the veteran population.
Lung cancer is the leading cause of cancer death in the United States.1 The 2011 National Lung Screening Trial (NLST) demonstrated that low-dose computed tomography (LDCT) screening provided a 20% relative reduction in lung cancer–specific mortality.2 Based on these findings, the United States Preventive Services Task Force (USPSTF) published lung cancer screening guidelines in 2013 recommending an annual LDCT of the thorax in patients aged 55 to 80 years with a 30 pack-year smoking history and who currently smoke or quit within the past 15 years.
In 2021, the USPSTF updated its recommendations by reducing the qualifications for annual screening to a 20 pack-year smoking history.3 The updated guidelines recognized the increased risk of lung cancer for Black individuals.4,5 Evidence suggests the 2013 screening criteria was too conservative for this population.6,7
Similarly, US Department of Veteran Affairs (VA) patients are a population at higher risk for lung cancer due to a male predominance, presence of comorbidities, exposure to carcinogenic agents, and possibly a higher prevalence of tobacco smoking.8 This study sought to examine the applicability of the USPSTF guidelines in a VA health care system with a predominantly Black population.
Methods
A retrospective chart review of adult patients who were diagnosed and treated with early-stage small cell or non–small cell lung cancer (stage I or II) was performed within the Southeast Louisiana Veterans Health Care System (SLVHCS) in New Orleans. The review used data from the VA Cancer Registry from January 1, 2005, through December 31, 2017. Patients were grouped by whether they met 2013 USPSTF screening criteria at time of diagnosis vs those that did not. Data collected included type and stage of lung cancer at time of diagnosis, context of diagnosis (incidental, screening, symptomatic), diagnostic method, smoking history, and presence of chronic obstructive pulmonary disease (COPD). Patients without a clear smoking history documented in the health record were excluded.
Statistical analyses were performed with GraphPad Prism 8.0. Student t test and Fischer exact test were performed for most of the statistical analyses, with differences between groups noted to be statistically significant at a P < .05.
Results
A total of 182 patient charts were reviewed and 13 patients were excluded for missing information related to the USPSTF screening criteria. Of the 169 patients included, 122 (72%) met USPSTF screening criteria while 47 (28%) patients did not. The reasons for not meeting screening criteria were 14 patients were too young at and 9 patients were too old at time of diagnosis, 7 had a < 20 pack-year smoking history, 7 patients had quit > 15 years previously, and 12 patients met multiple exclusion criteria. The study population was 96% male and there was an overall predominance of Black patients (58%) within the sample (Table).
There was a significantly higher proportion of Black patients in the group that did not meet screening criteria compared with the group that met screening criteria (68% vs 54%, P = .04). Cancer type and stage at diagnosis were similar in both patient populations. There was a statistically significant difference in COPD diagnosis between the groups, with a larger proportion of COPD patients in the met screening criteria group (74% vs 45%, P < .001). The mean smoking history was 61.4 pack-years in the met criteria group and 43.3 pack-years in the did not meet criteria group.
Five additional patients in the group that did not meet the 2013 USPSTF screening criteria would have met criteria if the 2021 USPSTF guidelines were applied. All 5 were Black patients. Using the 2021 guidelines, Black patients would have made up 56% of the patients who met screening criteria and 54% of the patients who did not meet screening criteria at time of diagnosis.
Discussion
This study sought to determine the hypothetical effectiveness of national lung cancer screening guidelines in detecting early-stage lung cancer for a high-risk population. Patients diagnosed with early-stage lung cancer were selected as these patients have improved outcomes with treatment, and thus would theoretically benefit from early detection through screening. As expected, the study population had a majority of Black veterans (58%), with a higher proportion of Black patients in the did not meet screening criteria group compared with the met screening criteria group (68% vs 54%, P = .04). This difference highlights the concern that Black individuals were being underscreened with the 2013 USPSTF guidelines.7 This is not all surprising as the NLST, from which the initial screening guidelines were based, included a majority White population with only 4.4% of their population being Black.2 The USPSTF also cites the NELSON trial as evidence to support annual lung cancer screening, a trial that was performed in the Netherlands with a very different population compared with that of southeast Louisiana.9
Given concern that the old criteria were underscreening certain populations, the updated 2021 USPSTF guidelines sought to expand the screening population. In this study, the implementation of these new guidelines resulted in more Black patients meeting screening criteria.
Racial and ethnic disparities in health care in the US are no secret, as Black individuals consistently have increased disease and death rates, higher rates of unemployment, and decreased access to preventive medical care compared to White individuals.10 Despite the updated USPSTF guidelines, additional modifications to the screening criteria could improve the ability to identify high-risk patients. A modified model using data from the Prostate, Lung, Colorectal, and Ovarian Screening Trial (PLCO) incorporating COPD history, race and ethnicity, and personal history of cancer increased the sensitivity for high-risk Black ever-smokers.11 Additional models and analyses also support the utility of incorporating race and ethnicity in lung cancer screening criteria.7,12 Using race and ethnicity to guide screening criteria for cancer is not unheard of; in 2017, the US Multi-Society Task Force recommended that Black individuals start colon cancer screening at age 45 years rather than the typical age of 50 years, before updating the guidelines again in 2021 to recommend that all adults start at age 45 years.13,14
Limitations
This study had the inherent weakness of being a retrospective study at a single institution. Additionally, the 7th edition of the International Association for the Study of Lung Cancer was published in 2010, during the 2005 to 2017 time frame from which our data was collected, leading to possible inconsistencies in staging between patients before and after 2010.15 However, these changes in staging are unlikely to significantly impact the results for in this study, since the vast majority of the patients diagnosed with lung cancer stage I or II before 2010 would still be in the those 2 stages in the 2010 edition. Finally, specific to our patient population, it was often difficult to ascertain an accurate smoking history for each patient, especially in the early years of the data set, likely due to the disruption of care caused by Hurricane Katrina.
Conclusions
In this retrospective study performed at the SLVHCS in New Orleans, a significantly higher proportion of Black patients compared with White patients with early-stage lung cancer did not meet the 2013 USPSTF lung cancer screening guidelines at time of diagnosis, highlighting the concern that this population was being underscreened. These findings demonstrate the challenges and failures of applying national guidelines to a unique, high-risk population. An individualized, risk-based screening model incorporating race and ethnicity could be more effective at diagnosing early-stage lung cancer and requires more investigation. Centralized lung cancer screening programs within the VA system could also be beneficial for early detection and treatment, as well as provide insight into the increased risk within the veteran population.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7-30. doi:10.3322/caac.21590
2. National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409. doi:10.1056/NEJMoa110287
3. US Preventive Services Task Force, Krist AH, Davidson KW, et al. Screening for lung cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325(10):962-970. doi:10.1001/jama.2021.1117
4. Jonas DE, Reuland DS, Reddy SM, et al. Screening for lung cancer with low-dose computed tomography: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;325(10):971-987. doi:10.1001/jama.2021.0377
5. Haiman CA, Stram DO, Wilkens LR, et al. Ethnic and racial differences in the smoking-related risk of lung cancer. N Engl J Med. 2006;354(4):333-342. doi:10.1056/NEJMoa033250
6. DeSantis CE, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Cancer statistics for African Americans, 2019. CA Cancer J Clin. 2019;69(3):211-233. doi:10.3322/caac.21555
7. Aldrich MC, Mercaldo SF, Sandler KL, Blot WJ, Grogan EL, Blume JD. Evaluation of USPSTF Lung Cancer Screening Guidelines among African American adult smokers. JAMA Oncol. 2019;5(9):1318-1324. doi:10.1001/jamaoncol.2019.1402
8. Brown DW. Smoking prevalence among US veterans. J Gen Intern Med. 2010;25(2):147-149. doi:10.1007/s11606-009-1160-0
9. de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020;382(6):503-513. doi:10.1056/NEJMoa1911793
10. Williams DR, Rucker TD. Understanding and addressing racial disparities in health care. Health Care Financ Rev. 2000;21(4):75-90.
11. Pasquinelli MM, Tammemägi MC, Kovitz KL, et al. Risk prediction model versus United States Preventive Services Task Force lung cancer screening eligibility criteria: reducing race disparities. J Thorac Oncol. 2020;15(11):1738-1747. doi:10.1016/j.jtho.2020.08.006
12. Ten Haaf K, Bastani M, Cao P, et al. A comparative modeling analysis of risk-based lung cancer screening strategies. J Natl Cancer Inst. 2020;112(5):466-479. doi:10.1093/jnci/djz164
13. Rex DK, Boland CR, Dominitz JA, et al. Colorectal cancer screening: recommendations for physicians and patients from the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2017;153(1):307-323. doi:10.1053/j.gastro.2017.05.013
14. US Preventive Services Task Force, Davidson KW, Barry MJ, et al. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325(19):1965-1977. doi:10.1001/jama.2021.6238
15. Mirsadraee S, Oswal D, Alizadeh Y, Caulo A, van Beek E Jr. The 7th lung cancer TNM classification and staging system: review of the changes and implications. World J Radiol. 2012;4(4):128-134. doi:10.4329/wjr.v4.i4.128
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7-30. doi:10.3322/caac.21590
2. National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409. doi:10.1056/NEJMoa110287
3. US Preventive Services Task Force, Krist AH, Davidson KW, et al. Screening for lung cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325(10):962-970. doi:10.1001/jama.2021.1117
4. Jonas DE, Reuland DS, Reddy SM, et al. Screening for lung cancer with low-dose computed tomography: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;325(10):971-987. doi:10.1001/jama.2021.0377
5. Haiman CA, Stram DO, Wilkens LR, et al. Ethnic and racial differences in the smoking-related risk of lung cancer. N Engl J Med. 2006;354(4):333-342. doi:10.1056/NEJMoa033250
6. DeSantis CE, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Cancer statistics for African Americans, 2019. CA Cancer J Clin. 2019;69(3):211-233. doi:10.3322/caac.21555
7. Aldrich MC, Mercaldo SF, Sandler KL, Blot WJ, Grogan EL, Blume JD. Evaluation of USPSTF Lung Cancer Screening Guidelines among African American adult smokers. JAMA Oncol. 2019;5(9):1318-1324. doi:10.1001/jamaoncol.2019.1402
8. Brown DW. Smoking prevalence among US veterans. J Gen Intern Med. 2010;25(2):147-149. doi:10.1007/s11606-009-1160-0
9. de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020;382(6):503-513. doi:10.1056/NEJMoa1911793
10. Williams DR, Rucker TD. Understanding and addressing racial disparities in health care. Health Care Financ Rev. 2000;21(4):75-90.
11. Pasquinelli MM, Tammemägi MC, Kovitz KL, et al. Risk prediction model versus United States Preventive Services Task Force lung cancer screening eligibility criteria: reducing race disparities. J Thorac Oncol. 2020;15(11):1738-1747. doi:10.1016/j.jtho.2020.08.006
12. Ten Haaf K, Bastani M, Cao P, et al. A comparative modeling analysis of risk-based lung cancer screening strategies. J Natl Cancer Inst. 2020;112(5):466-479. doi:10.1093/jnci/djz164
13. Rex DK, Boland CR, Dominitz JA, et al. Colorectal cancer screening: recommendations for physicians and patients from the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2017;153(1):307-323. doi:10.1053/j.gastro.2017.05.013
14. US Preventive Services Task Force, Davidson KW, Barry MJ, et al. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325(19):1965-1977. doi:10.1001/jama.2021.6238
15. Mirsadraee S, Oswal D, Alizadeh Y, Caulo A, van Beek E Jr. The 7th lung cancer TNM classification and staging system: review of the changes and implications. World J Radiol. 2012;4(4):128-134. doi:10.4329/wjr.v4.i4.128
Leiomyosarcoma of the Penis: A Case Report and Re-Appraisal
Penile cancer is rare with a worldwide incidence of 0.8 cases per 100,000 men.1 The most common type is squamous cell carcinoma (SCC) followed by soft tissue sarcoma (STS) and Kaposi sarcoma.2 Leiomyosarcoma (LMS) is the second most common STS subtype at this location.3 Approximately 50 cases of penile LMS have been reported in the English literature, most as isolated case reports while Fetsch and colleagues reported 14 cases from a single institute.4 We present a case of penile LMS with a review of 31 cases. We also describe presentation, treatment options, and recurrence pattern of this rare malignancy.
Case Presentation
A patient aged 70 years presented to the urology clinic with 1-year history of a slowly enlarging penile mass associated with phimosis. He reported no pain, dysuria, or hesitancy. On examination a 2 × 2-cm smooth, mobile, nonulcerating mass was seen on the tip of his left glans without inguinal lymphadenopathy. He underwent circumcision and excision biopsy that revealed an encapsulated tan-white mass measuring 3 × 2.2 × 1.5 cm under the surface of the foreskin. Histology showed a spindle cell tumor with areas of increased cellularity, prominent atypia, and pleomorphism, focal necrosis, and scattered mitoses, including atypical forms. The tumor stained positive for smooth muscle actin and desmin. Ki-67 staining showed foci with a very high proliferation index (Figure). Resection margins were negative. Final Fédération Nationale des Centres de Lutte Contre Le Cancer score was grade 2 (differentiation, 1; mitotic, 3; necrosis, 1). Computed tomography of the chest, abdomen, and pelvis did not show evidence of metastasis. The tumor was classified as superficial, stage IIA (pT1cN0cM0). Local excision with negative margins was deemed adequate treatment.
Discussion
Penile LMS is rare and arises from smooth muscles, which in the penis can be from dartos fascia, erector pili in the skin covering the shaft, or from tunica media of the superficial vessels and cavernosa.5 It commonly presents as a nodule or ulcer that might be accompanied by paraphimosis, phimosis, erectile dysfunction, and lower urinary tract symptoms depending on the extent of local tissue involvement. In our review of 31 cases, the age at presentation ranged from 38 to 85 years, with 1 case report of LMS in a 6-year-old. The highest incidence was in the 6th decade. Tumor behavior can be indolent or aggressive. Most patients in our review had asymptomatic, slow-growing lesions for 6 to 24 months before presentation—including our patient—while others had an aggressive tumor with symptoms for a few weeks followed by rapid metastatic spread.6,7
Histology and Staging
Diagnosis requires biopsy followed by histologic examination and immunohistochemistry of the lesion. Typically, LMS shows fascicles of spindle cells with varying degrees of nuclear atypia, pleomorphisms, and necrotic regions. Mitotic rate is variable and usually > 5 per high power field. Cells stain positive for smooth muscle actin, desmin, and h-caldesmon.8 TNM (tumor, nodes, metastasis) stage is determined by the American Joint Committee on Cancer guidelines for STS.
Pratt and colleagues were the first to categorize penile LMS as superficial or deep.9 The former includes all lesions superficial to tunica albuginea while the latter run deep to this layer. Anatomical distinction is an important factor in tumor behavior, treatment selection, and prognosis. In our review, we found 14 cases of superficial and 17 cases of deep LMS.
Treatment
There are no established guidelines on optimum treatment of penile LMS. However, we can extrapolate principles from current guidelines on penile cancer, cutaneous leiomyosarcoma, and limb sarcomas. At present, the first-line treatment for superficial penile LMS is wide local excision to achieve negative margins. Circumcision alone might be sufficient for tumors of the distal prepuce, as in our case.10 Radical resection generally is not required for these early-stage tumors. In our review, no patient in this category developed recurrence or metastasis regardless of initial surgery type (Table 1).6,11,12
For deep lesions, partial—if functional penile stump and negative margins can be achieved—or total penectomy is required.10 In our review, more conservative approaches to deep tumors were associated with local recurrences.7,13,14 Lymphatic spread is rare for LMS. Additionally, involvement of local lymph nodes usually coincides with distant spread. Inguinal lymph node dissection is not indicated if initial negative surgical margins are achieved.
For STS at other sites in the body, radiation therapy is recommended postoperatively for high-grade lesions, which can be extrapolated to penile LMS as well. The benefit of preoperative radiation therapy is less certain. In limb sarcomas, radiation is associated with better local control for large-sized tumors and is used for patients with initial unresectable tumors.15 Similar recommendation could be extended to penile LMS with local spread to inguinal lymph nodes, scrotum, or abdominal wall. In our review, postoperative radiation therapy was used in 3 patients with deep tumors.16-18 Of these, short-term relapse occurred in 1 patient.
Chemotherapy for LMS remains controversial. The tumor generally is resistant to chemotherapy and systemic therapy, if employed, is for palliative purpose. The most promising results for adjuvant chemotherapy for resectable STS is seen in limb and uterine sarcomas with high-grade, metastatic, or relapsed tumors but improvement in overall survival has been marginal.19,20Single and multidrug regimens based on doxorubicin, ifosfamide, and gemcitabine have been studied with results showing no efficacy or a slight benefit.8,21 Immunotherapy and targeted therapy for penile STS have not been studied. In our review, postoperative chemotherapy was used for 2 patients with deep tumors and 1 patient with a superficial tumor while preoperative chemotherapy was used for 1 patient.16,18,22 Short-term relapse was seen in 2 of 4 of these patients (Table 2).
Metastatic Disease
LMS tends to metastasize hematogenously and lymphatic spread is uncommon. In our review, 7 patients developed metastasis. These patients had deep tumors at presentation with tumor size > 3 cm. Five of 7 patients had involvement of corpora cavernosa at presentation. The lung was the most common site of metastasis, followed by local extension to lower abdominal wall and scrotum. Of the 7 patients, 3 were treated with initial limited excision or partial penectomy and then experienced local recurrence or distant metastasis.7,13,14,23 This supports the use of radical surgery in large, deep tumors. In an additional 4 cases, metastasis occurred despite initial treatment with total penectomy and use of adjuvant chemoradiation therapy.
In most cases penile LMS is a de novo tumor, however, on occasion it could be accompanied by another epithelial malignancy. Similarly, penile LMS might be a site of recurrence for a primary LMS at another site, as seen in 3 of the reviewed cases. In the first, a patient presented with a nodule on the glans suspicious for SCC, second with synchronous SCC and LMS, and a third case where a patient presented with penile LMS 9 years after being treated for similar tumor in the epididymis.17,24,25
Prognosis
Penile LMS prognosis is difficult to ascertain because reported cases are rare. In our review, the longest documented disease-free survival was 3.5 years for a patient with superficial LMS treated with local excision.26 In cases of distant metastasis, average survival was 4.6 months, while the longest survival since initial presentation and last documented local recurrence was 16 years.14 Five-year survival has not been reported.
Conclusions
LMS of the penis is a rare and potentially aggressive malignancy. It can be classified as superficial or deep based on tumor relation to the tunica albuginea. Deep tumors, those > 3 cm, high-grade lesions, and tumors with involvement of corpora cavernosa, tend to spread locally, metastasize to distant areas, and require more radical surgery with or without postoperative radiation therapy. In comparison, superficial lesions can be treated with local excision only. Both superficial and deep tumors require close follow-up.
1. Montes Cardona CE, García-Perdomo HA. Incidence of penile cancer worldwide: systematic review and meta-analysis. Rev Panam Salud Publica. 2017;41:e117. Published 2017 Nov 30. doi:10.26633/RPSP.2017.117
2. Volker HU, Zettl A, Haralambieva E, et al. Leiomyosarcoma of the larynx as a local relapse of squamous cell carcinoma—report of an unusual case. Head Neck. 2010;32(5):679-683. doi:10.1002/hed.21127
3. Wollina U, Steinbach F, Verma S, et al. Penile tumours: a review. J Eur Acad Dermatol Venereol. 2014;28(10):1267-1276. doi:10.1111/jdv.12491
4. Fetsch JF, Davis CJ Jr, Miettinen M, Sesterhenn IA. Leiomyosarcoma of the penis: a clinicopathologic study of 14 cases with review of the literature and discussion of the differential diagnosis. Am J Surg Pathol. 2004;28(1):115-125. doi:10.1097/00000478-200401000-00014
5. Sundersingh S, Majhi U, Narayanaswamy K, Balasubramanian S. Primary leiomyosarcoma of the penis. Indian J Pathol Microbiol. 2009;52(3):447-448. doi:10.4103/0377-4929.55028
6. Mendis D, Bott SR, Davies JH. Subcutaneous leiomyosarcoma of the frenulum. Scientific World J. 2005;5:571-575. doi:10.1100/tsw.2005.76
7. Elem B, Nieslanik J. Leiomyosarcoma of the penis. Br J Urol. 1979;51(1):46. doi:10.1111/j.1464-410x.1979.tb04244.x
8. Serrano C, George S. Leiomyosarcoma. Hematol Oncol Clin North Am. 2013;27(5):957-974. doi:10.1016/j.hoc.2013.07.002
9. Pratt RM, Ross RT. Leiomyosarcoma of the penis. A report of a case. Br J Surg. 1969;56(11):870-872. doi:10.1002/bjs.1800561122
10. National Comprehensive Cancer Network. Penile cancer. NCCN evidence blocks. Version 2.2022 Updated January 26, 2022. Accessed March 16, 2022. https://www.nccn.org/professionals/physician_gls/pdf/penile_blocks.pdf
11. Ashley DJ, Edwards EC. Sarcoma of the penis; leiomyosarcoma of the penis: report of a case with a review of the literature on sarcoma of the penis. Br J Surg. 1957;45(190):170-179. doi:10.1002/bjs.18004519011
12. Pow-Sang MR, Orihuela E. Leiomyosarcoma of the penis. J Urol. 1994;151(6):1643-1645. doi:10.1016/s0022-5347(17)35328-413. Isa SS, Almaraz R, Magovern J. Leiomyosarcoma of the penis. Case report and review of the literature. Cancer. 1984;54(5):939-942. doi:10.1002/1097-0142(19840901)54:5<939::aid-cncr2820540533>3.0.co;2-y
14. Hutcheson JB, Wittaker WW, Fronstin MH. Leiomyosarcoma of the penis: case report and review of literature. J Urol. 1969;101(6):874-875. doi:10.1016/s0022-5347(17)62446-7
15. Grimer R, Judson I, Peake D, et al. Guidelines for the management of soft tissue sarcomas. Sarcoma. 2010;2010:506182. doi:10.1155/2010/506182
16. McDonald MW, O’Connell JR, Manning JT, Benjamin RS. Leiomyosarcoma of the penis. J Urol. 1983;130(4):788-789. doi:10.1016/s0022-5347(17)51464-0
17. Planz B, Brunner K, Kalem T, Schlick RW, Kind M. Primary leiomyosarcoma of the epididymis and late recurrence on the penis. J Urol. 1998;159(2):508. doi:10.1016/s0022-5347(01)63966-1
18. Smart RH. Leiomyosarcoma of the penis. J Urol. 1984;132(2):356-357. doi:10.1016/s0022-5347(17)49624-8
19. Patrikidou A, Domont J, Cioffi A, Le Cesne A. Treating soft tissue sarcomas with adjuvant chemotherapy. Curr Treat Options Oncol. 2011;12(1):21-31. doi:10.1007/s11864-011-0145-5
20. Italiano A, Delva F, Mathoulin-Pelissier S, et al. Effect of adjuvant chemotherapy on survival in FNCLCC grade 3 soft tissue sarcomas: a multivariate analysis of the French Sarcoma Group Database. Ann Oncol. 2010;21(12):2436-2441. doi:10.1093/annonc/mdq238
21. Pervaiz N, Colterjohn N, Farrokhyar F, Tozer R, Figueredo A, Ghert M. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer. 2008;113(3):573-581. doi:10.1002/cncr.23592
22. Lacarrière E, Galliot I, Gobet F, Sibert L. Leiomyosarcoma of the corpus cavernosum mimicking a Peyronie’s plaque. Urology. 2012;79(4):e53-e54. doi:10.1016/j.urology.2011.07.1410
23. Hamal PB. Leiomyosarcoma of penis—case report and review of the literature. Br J Urol. 1975;47(3):319-324. doi:10.1111/j.1464-410x.1975.tb03974.x
24. Greenwood N, Fox H, Edwards EC. Leiomyosarcoma of the penis. Cancer. 1972;29(2):481-483. doi:10.1002/1097-0142(197202)29:2<481::aid -cncr2820290237>3.0.co;2-q
25. Koizumi H, Nagano K, Kosaka S. A case of penile tumor: combination of leiomyosarcoma and squamous cell carcinoma. Hinyokika Kiyo. 1987;33(9):1489-1491.
26. Romero Gonzalez EJ, Marenco Jimenez JL, Mayorga Pineda MP, Martínez Morán A, Castiñeiras Fernández J. Leiomyosarcoma of the penis, an exceptional entity. Urol Case Rep. 2015;3(3):63-64. doi:10.1016/j.eucr.2014.12.007
Penile cancer is rare with a worldwide incidence of 0.8 cases per 100,000 men.1 The most common type is squamous cell carcinoma (SCC) followed by soft tissue sarcoma (STS) and Kaposi sarcoma.2 Leiomyosarcoma (LMS) is the second most common STS subtype at this location.3 Approximately 50 cases of penile LMS have been reported in the English literature, most as isolated case reports while Fetsch and colleagues reported 14 cases from a single institute.4 We present a case of penile LMS with a review of 31 cases. We also describe presentation, treatment options, and recurrence pattern of this rare malignancy.
Case Presentation
A patient aged 70 years presented to the urology clinic with 1-year history of a slowly enlarging penile mass associated with phimosis. He reported no pain, dysuria, or hesitancy. On examination a 2 × 2-cm smooth, mobile, nonulcerating mass was seen on the tip of his left glans without inguinal lymphadenopathy. He underwent circumcision and excision biopsy that revealed an encapsulated tan-white mass measuring 3 × 2.2 × 1.5 cm under the surface of the foreskin. Histology showed a spindle cell tumor with areas of increased cellularity, prominent atypia, and pleomorphism, focal necrosis, and scattered mitoses, including atypical forms. The tumor stained positive for smooth muscle actin and desmin. Ki-67 staining showed foci with a very high proliferation index (Figure). Resection margins were negative. Final Fédération Nationale des Centres de Lutte Contre Le Cancer score was grade 2 (differentiation, 1; mitotic, 3; necrosis, 1). Computed tomography of the chest, abdomen, and pelvis did not show evidence of metastasis. The tumor was classified as superficial, stage IIA (pT1cN0cM0). Local excision with negative margins was deemed adequate treatment.
Discussion
Penile LMS is rare and arises from smooth muscles, which in the penis can be from dartos fascia, erector pili in the skin covering the shaft, or from tunica media of the superficial vessels and cavernosa.5 It commonly presents as a nodule or ulcer that might be accompanied by paraphimosis, phimosis, erectile dysfunction, and lower urinary tract symptoms depending on the extent of local tissue involvement. In our review of 31 cases, the age at presentation ranged from 38 to 85 years, with 1 case report of LMS in a 6-year-old. The highest incidence was in the 6th decade. Tumor behavior can be indolent or aggressive. Most patients in our review had asymptomatic, slow-growing lesions for 6 to 24 months before presentation—including our patient—while others had an aggressive tumor with symptoms for a few weeks followed by rapid metastatic spread.6,7
Histology and Staging
Diagnosis requires biopsy followed by histologic examination and immunohistochemistry of the lesion. Typically, LMS shows fascicles of spindle cells with varying degrees of nuclear atypia, pleomorphisms, and necrotic regions. Mitotic rate is variable and usually > 5 per high power field. Cells stain positive for smooth muscle actin, desmin, and h-caldesmon.8 TNM (tumor, nodes, metastasis) stage is determined by the American Joint Committee on Cancer guidelines for STS.
Pratt and colleagues were the first to categorize penile LMS as superficial or deep.9 The former includes all lesions superficial to tunica albuginea while the latter run deep to this layer. Anatomical distinction is an important factor in tumor behavior, treatment selection, and prognosis. In our review, we found 14 cases of superficial and 17 cases of deep LMS.
Treatment
There are no established guidelines on optimum treatment of penile LMS. However, we can extrapolate principles from current guidelines on penile cancer, cutaneous leiomyosarcoma, and limb sarcomas. At present, the first-line treatment for superficial penile LMS is wide local excision to achieve negative margins. Circumcision alone might be sufficient for tumors of the distal prepuce, as in our case.10 Radical resection generally is not required for these early-stage tumors. In our review, no patient in this category developed recurrence or metastasis regardless of initial surgery type (Table 1).6,11,12
For deep lesions, partial—if functional penile stump and negative margins can be achieved—or total penectomy is required.10 In our review, more conservative approaches to deep tumors were associated with local recurrences.7,13,14 Lymphatic spread is rare for LMS. Additionally, involvement of local lymph nodes usually coincides with distant spread. Inguinal lymph node dissection is not indicated if initial negative surgical margins are achieved.
For STS at other sites in the body, radiation therapy is recommended postoperatively for high-grade lesions, which can be extrapolated to penile LMS as well. The benefit of preoperative radiation therapy is less certain. In limb sarcomas, radiation is associated with better local control for large-sized tumors and is used for patients with initial unresectable tumors.15 Similar recommendation could be extended to penile LMS with local spread to inguinal lymph nodes, scrotum, or abdominal wall. In our review, postoperative radiation therapy was used in 3 patients with deep tumors.16-18 Of these, short-term relapse occurred in 1 patient.
Chemotherapy for LMS remains controversial. The tumor generally is resistant to chemotherapy and systemic therapy, if employed, is for palliative purpose. The most promising results for adjuvant chemotherapy for resectable STS is seen in limb and uterine sarcomas with high-grade, metastatic, or relapsed tumors but improvement in overall survival has been marginal.19,20Single and multidrug regimens based on doxorubicin, ifosfamide, and gemcitabine have been studied with results showing no efficacy or a slight benefit.8,21 Immunotherapy and targeted therapy for penile STS have not been studied. In our review, postoperative chemotherapy was used for 2 patients with deep tumors and 1 patient with a superficial tumor while preoperative chemotherapy was used for 1 patient.16,18,22 Short-term relapse was seen in 2 of 4 of these patients (Table 2).
Metastatic Disease
LMS tends to metastasize hematogenously and lymphatic spread is uncommon. In our review, 7 patients developed metastasis. These patients had deep tumors at presentation with tumor size > 3 cm. Five of 7 patients had involvement of corpora cavernosa at presentation. The lung was the most common site of metastasis, followed by local extension to lower abdominal wall and scrotum. Of the 7 patients, 3 were treated with initial limited excision or partial penectomy and then experienced local recurrence or distant metastasis.7,13,14,23 This supports the use of radical surgery in large, deep tumors. In an additional 4 cases, metastasis occurred despite initial treatment with total penectomy and use of adjuvant chemoradiation therapy.
In most cases penile LMS is a de novo tumor, however, on occasion it could be accompanied by another epithelial malignancy. Similarly, penile LMS might be a site of recurrence for a primary LMS at another site, as seen in 3 of the reviewed cases. In the first, a patient presented with a nodule on the glans suspicious for SCC, second with synchronous SCC and LMS, and a third case where a patient presented with penile LMS 9 years after being treated for similar tumor in the epididymis.17,24,25
Prognosis
Penile LMS prognosis is difficult to ascertain because reported cases are rare. In our review, the longest documented disease-free survival was 3.5 years for a patient with superficial LMS treated with local excision.26 In cases of distant metastasis, average survival was 4.6 months, while the longest survival since initial presentation and last documented local recurrence was 16 years.14 Five-year survival has not been reported.
Conclusions
LMS of the penis is a rare and potentially aggressive malignancy. It can be classified as superficial or deep based on tumor relation to the tunica albuginea. Deep tumors, those > 3 cm, high-grade lesions, and tumors with involvement of corpora cavernosa, tend to spread locally, metastasize to distant areas, and require more radical surgery with or without postoperative radiation therapy. In comparison, superficial lesions can be treated with local excision only. Both superficial and deep tumors require close follow-up.
Penile cancer is rare with a worldwide incidence of 0.8 cases per 100,000 men.1 The most common type is squamous cell carcinoma (SCC) followed by soft tissue sarcoma (STS) and Kaposi sarcoma.2 Leiomyosarcoma (LMS) is the second most common STS subtype at this location.3 Approximately 50 cases of penile LMS have been reported in the English literature, most as isolated case reports while Fetsch and colleagues reported 14 cases from a single institute.4 We present a case of penile LMS with a review of 31 cases. We also describe presentation, treatment options, and recurrence pattern of this rare malignancy.
Case Presentation
A patient aged 70 years presented to the urology clinic with 1-year history of a slowly enlarging penile mass associated with phimosis. He reported no pain, dysuria, or hesitancy. On examination a 2 × 2-cm smooth, mobile, nonulcerating mass was seen on the tip of his left glans without inguinal lymphadenopathy. He underwent circumcision and excision biopsy that revealed an encapsulated tan-white mass measuring 3 × 2.2 × 1.5 cm under the surface of the foreskin. Histology showed a spindle cell tumor with areas of increased cellularity, prominent atypia, and pleomorphism, focal necrosis, and scattered mitoses, including atypical forms. The tumor stained positive for smooth muscle actin and desmin. Ki-67 staining showed foci with a very high proliferation index (Figure). Resection margins were negative. Final Fédération Nationale des Centres de Lutte Contre Le Cancer score was grade 2 (differentiation, 1; mitotic, 3; necrosis, 1). Computed tomography of the chest, abdomen, and pelvis did not show evidence of metastasis. The tumor was classified as superficial, stage IIA (pT1cN0cM0). Local excision with negative margins was deemed adequate treatment.
Discussion
Penile LMS is rare and arises from smooth muscles, which in the penis can be from dartos fascia, erector pili in the skin covering the shaft, or from tunica media of the superficial vessels and cavernosa.5 It commonly presents as a nodule or ulcer that might be accompanied by paraphimosis, phimosis, erectile dysfunction, and lower urinary tract symptoms depending on the extent of local tissue involvement. In our review of 31 cases, the age at presentation ranged from 38 to 85 years, with 1 case report of LMS in a 6-year-old. The highest incidence was in the 6th decade. Tumor behavior can be indolent or aggressive. Most patients in our review had asymptomatic, slow-growing lesions for 6 to 24 months before presentation—including our patient—while others had an aggressive tumor with symptoms for a few weeks followed by rapid metastatic spread.6,7
Histology and Staging
Diagnosis requires biopsy followed by histologic examination and immunohistochemistry of the lesion. Typically, LMS shows fascicles of spindle cells with varying degrees of nuclear atypia, pleomorphisms, and necrotic regions. Mitotic rate is variable and usually > 5 per high power field. Cells stain positive for smooth muscle actin, desmin, and h-caldesmon.8 TNM (tumor, nodes, metastasis) stage is determined by the American Joint Committee on Cancer guidelines for STS.
Pratt and colleagues were the first to categorize penile LMS as superficial or deep.9 The former includes all lesions superficial to tunica albuginea while the latter run deep to this layer. Anatomical distinction is an important factor in tumor behavior, treatment selection, and prognosis. In our review, we found 14 cases of superficial and 17 cases of deep LMS.
Treatment
There are no established guidelines on optimum treatment of penile LMS. However, we can extrapolate principles from current guidelines on penile cancer, cutaneous leiomyosarcoma, and limb sarcomas. At present, the first-line treatment for superficial penile LMS is wide local excision to achieve negative margins. Circumcision alone might be sufficient for tumors of the distal prepuce, as in our case.10 Radical resection generally is not required for these early-stage tumors. In our review, no patient in this category developed recurrence or metastasis regardless of initial surgery type (Table 1).6,11,12
For deep lesions, partial—if functional penile stump and negative margins can be achieved—or total penectomy is required.10 In our review, more conservative approaches to deep tumors were associated with local recurrences.7,13,14 Lymphatic spread is rare for LMS. Additionally, involvement of local lymph nodes usually coincides with distant spread. Inguinal lymph node dissection is not indicated if initial negative surgical margins are achieved.
For STS at other sites in the body, radiation therapy is recommended postoperatively for high-grade lesions, which can be extrapolated to penile LMS as well. The benefit of preoperative radiation therapy is less certain. In limb sarcomas, radiation is associated with better local control for large-sized tumors and is used for patients with initial unresectable tumors.15 Similar recommendation could be extended to penile LMS with local spread to inguinal lymph nodes, scrotum, or abdominal wall. In our review, postoperative radiation therapy was used in 3 patients with deep tumors.16-18 Of these, short-term relapse occurred in 1 patient.
Chemotherapy for LMS remains controversial. The tumor generally is resistant to chemotherapy and systemic therapy, if employed, is for palliative purpose. The most promising results for adjuvant chemotherapy for resectable STS is seen in limb and uterine sarcomas with high-grade, metastatic, or relapsed tumors but improvement in overall survival has been marginal.19,20Single and multidrug regimens based on doxorubicin, ifosfamide, and gemcitabine have been studied with results showing no efficacy or a slight benefit.8,21 Immunotherapy and targeted therapy for penile STS have not been studied. In our review, postoperative chemotherapy was used for 2 patients with deep tumors and 1 patient with a superficial tumor while preoperative chemotherapy was used for 1 patient.16,18,22 Short-term relapse was seen in 2 of 4 of these patients (Table 2).
Metastatic Disease
LMS tends to metastasize hematogenously and lymphatic spread is uncommon. In our review, 7 patients developed metastasis. These patients had deep tumors at presentation with tumor size > 3 cm. Five of 7 patients had involvement of corpora cavernosa at presentation. The lung was the most common site of metastasis, followed by local extension to lower abdominal wall and scrotum. Of the 7 patients, 3 were treated with initial limited excision or partial penectomy and then experienced local recurrence or distant metastasis.7,13,14,23 This supports the use of radical surgery in large, deep tumors. In an additional 4 cases, metastasis occurred despite initial treatment with total penectomy and use of adjuvant chemoradiation therapy.
In most cases penile LMS is a de novo tumor, however, on occasion it could be accompanied by another epithelial malignancy. Similarly, penile LMS might be a site of recurrence for a primary LMS at another site, as seen in 3 of the reviewed cases. In the first, a patient presented with a nodule on the glans suspicious for SCC, second with synchronous SCC and LMS, and a third case where a patient presented with penile LMS 9 years after being treated for similar tumor in the epididymis.17,24,25
Prognosis
Penile LMS prognosis is difficult to ascertain because reported cases are rare. In our review, the longest documented disease-free survival was 3.5 years for a patient with superficial LMS treated with local excision.26 In cases of distant metastasis, average survival was 4.6 months, while the longest survival since initial presentation and last documented local recurrence was 16 years.14 Five-year survival has not been reported.
Conclusions
LMS of the penis is a rare and potentially aggressive malignancy. It can be classified as superficial or deep based on tumor relation to the tunica albuginea. Deep tumors, those > 3 cm, high-grade lesions, and tumors with involvement of corpora cavernosa, tend to spread locally, metastasize to distant areas, and require more radical surgery with or without postoperative radiation therapy. In comparison, superficial lesions can be treated with local excision only. Both superficial and deep tumors require close follow-up.
1. Montes Cardona CE, García-Perdomo HA. Incidence of penile cancer worldwide: systematic review and meta-analysis. Rev Panam Salud Publica. 2017;41:e117. Published 2017 Nov 30. doi:10.26633/RPSP.2017.117
2. Volker HU, Zettl A, Haralambieva E, et al. Leiomyosarcoma of the larynx as a local relapse of squamous cell carcinoma—report of an unusual case. Head Neck. 2010;32(5):679-683. doi:10.1002/hed.21127
3. Wollina U, Steinbach F, Verma S, et al. Penile tumours: a review. J Eur Acad Dermatol Venereol. 2014;28(10):1267-1276. doi:10.1111/jdv.12491
4. Fetsch JF, Davis CJ Jr, Miettinen M, Sesterhenn IA. Leiomyosarcoma of the penis: a clinicopathologic study of 14 cases with review of the literature and discussion of the differential diagnosis. Am J Surg Pathol. 2004;28(1):115-125. doi:10.1097/00000478-200401000-00014
5. Sundersingh S, Majhi U, Narayanaswamy K, Balasubramanian S. Primary leiomyosarcoma of the penis. Indian J Pathol Microbiol. 2009;52(3):447-448. doi:10.4103/0377-4929.55028
6. Mendis D, Bott SR, Davies JH. Subcutaneous leiomyosarcoma of the frenulum. Scientific World J. 2005;5:571-575. doi:10.1100/tsw.2005.76
7. Elem B, Nieslanik J. Leiomyosarcoma of the penis. Br J Urol. 1979;51(1):46. doi:10.1111/j.1464-410x.1979.tb04244.x
8. Serrano C, George S. Leiomyosarcoma. Hematol Oncol Clin North Am. 2013;27(5):957-974. doi:10.1016/j.hoc.2013.07.002
9. Pratt RM, Ross RT. Leiomyosarcoma of the penis. A report of a case. Br J Surg. 1969;56(11):870-872. doi:10.1002/bjs.1800561122
10. National Comprehensive Cancer Network. Penile cancer. NCCN evidence blocks. Version 2.2022 Updated January 26, 2022. Accessed March 16, 2022. https://www.nccn.org/professionals/physician_gls/pdf/penile_blocks.pdf
11. Ashley DJ, Edwards EC. Sarcoma of the penis; leiomyosarcoma of the penis: report of a case with a review of the literature on sarcoma of the penis. Br J Surg. 1957;45(190):170-179. doi:10.1002/bjs.18004519011
12. Pow-Sang MR, Orihuela E. Leiomyosarcoma of the penis. J Urol. 1994;151(6):1643-1645. doi:10.1016/s0022-5347(17)35328-413. Isa SS, Almaraz R, Magovern J. Leiomyosarcoma of the penis. Case report and review of the literature. Cancer. 1984;54(5):939-942. doi:10.1002/1097-0142(19840901)54:5<939::aid-cncr2820540533>3.0.co;2-y
14. Hutcheson JB, Wittaker WW, Fronstin MH. Leiomyosarcoma of the penis: case report and review of literature. J Urol. 1969;101(6):874-875. doi:10.1016/s0022-5347(17)62446-7
15. Grimer R, Judson I, Peake D, et al. Guidelines for the management of soft tissue sarcomas. Sarcoma. 2010;2010:506182. doi:10.1155/2010/506182
16. McDonald MW, O’Connell JR, Manning JT, Benjamin RS. Leiomyosarcoma of the penis. J Urol. 1983;130(4):788-789. doi:10.1016/s0022-5347(17)51464-0
17. Planz B, Brunner K, Kalem T, Schlick RW, Kind M. Primary leiomyosarcoma of the epididymis and late recurrence on the penis. J Urol. 1998;159(2):508. doi:10.1016/s0022-5347(01)63966-1
18. Smart RH. Leiomyosarcoma of the penis. J Urol. 1984;132(2):356-357. doi:10.1016/s0022-5347(17)49624-8
19. Patrikidou A, Domont J, Cioffi A, Le Cesne A. Treating soft tissue sarcomas with adjuvant chemotherapy. Curr Treat Options Oncol. 2011;12(1):21-31. doi:10.1007/s11864-011-0145-5
20. Italiano A, Delva F, Mathoulin-Pelissier S, et al. Effect of adjuvant chemotherapy on survival in FNCLCC grade 3 soft tissue sarcomas: a multivariate analysis of the French Sarcoma Group Database. Ann Oncol. 2010;21(12):2436-2441. doi:10.1093/annonc/mdq238
21. Pervaiz N, Colterjohn N, Farrokhyar F, Tozer R, Figueredo A, Ghert M. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer. 2008;113(3):573-581. doi:10.1002/cncr.23592
22. Lacarrière E, Galliot I, Gobet F, Sibert L. Leiomyosarcoma of the corpus cavernosum mimicking a Peyronie’s plaque. Urology. 2012;79(4):e53-e54. doi:10.1016/j.urology.2011.07.1410
23. Hamal PB. Leiomyosarcoma of penis—case report and review of the literature. Br J Urol. 1975;47(3):319-324. doi:10.1111/j.1464-410x.1975.tb03974.x
24. Greenwood N, Fox H, Edwards EC. Leiomyosarcoma of the penis. Cancer. 1972;29(2):481-483. doi:10.1002/1097-0142(197202)29:2<481::aid -cncr2820290237>3.0.co;2-q
25. Koizumi H, Nagano K, Kosaka S. A case of penile tumor: combination of leiomyosarcoma and squamous cell carcinoma. Hinyokika Kiyo. 1987;33(9):1489-1491.
26. Romero Gonzalez EJ, Marenco Jimenez JL, Mayorga Pineda MP, Martínez Morán A, Castiñeiras Fernández J. Leiomyosarcoma of the penis, an exceptional entity. Urol Case Rep. 2015;3(3):63-64. doi:10.1016/j.eucr.2014.12.007
1. Montes Cardona CE, García-Perdomo HA. Incidence of penile cancer worldwide: systematic review and meta-analysis. Rev Panam Salud Publica. 2017;41:e117. Published 2017 Nov 30. doi:10.26633/RPSP.2017.117
2. Volker HU, Zettl A, Haralambieva E, et al. Leiomyosarcoma of the larynx as a local relapse of squamous cell carcinoma—report of an unusual case. Head Neck. 2010;32(5):679-683. doi:10.1002/hed.21127
3. Wollina U, Steinbach F, Verma S, et al. Penile tumours: a review. J Eur Acad Dermatol Venereol. 2014;28(10):1267-1276. doi:10.1111/jdv.12491
4. Fetsch JF, Davis CJ Jr, Miettinen M, Sesterhenn IA. Leiomyosarcoma of the penis: a clinicopathologic study of 14 cases with review of the literature and discussion of the differential diagnosis. Am J Surg Pathol. 2004;28(1):115-125. doi:10.1097/00000478-200401000-00014
5. Sundersingh S, Majhi U, Narayanaswamy K, Balasubramanian S. Primary leiomyosarcoma of the penis. Indian J Pathol Microbiol. 2009;52(3):447-448. doi:10.4103/0377-4929.55028
6. Mendis D, Bott SR, Davies JH. Subcutaneous leiomyosarcoma of the frenulum. Scientific World J. 2005;5:571-575. doi:10.1100/tsw.2005.76
7. Elem B, Nieslanik J. Leiomyosarcoma of the penis. Br J Urol. 1979;51(1):46. doi:10.1111/j.1464-410x.1979.tb04244.x
8. Serrano C, George S. Leiomyosarcoma. Hematol Oncol Clin North Am. 2013;27(5):957-974. doi:10.1016/j.hoc.2013.07.002
9. Pratt RM, Ross RT. Leiomyosarcoma of the penis. A report of a case. Br J Surg. 1969;56(11):870-872. doi:10.1002/bjs.1800561122
10. National Comprehensive Cancer Network. Penile cancer. NCCN evidence blocks. Version 2.2022 Updated January 26, 2022. Accessed March 16, 2022. https://www.nccn.org/professionals/physician_gls/pdf/penile_blocks.pdf
11. Ashley DJ, Edwards EC. Sarcoma of the penis; leiomyosarcoma of the penis: report of a case with a review of the literature on sarcoma of the penis. Br J Surg. 1957;45(190):170-179. doi:10.1002/bjs.18004519011
12. Pow-Sang MR, Orihuela E. Leiomyosarcoma of the penis. J Urol. 1994;151(6):1643-1645. doi:10.1016/s0022-5347(17)35328-413. Isa SS, Almaraz R, Magovern J. Leiomyosarcoma of the penis. Case report and review of the literature. Cancer. 1984;54(5):939-942. doi:10.1002/1097-0142(19840901)54:5<939::aid-cncr2820540533>3.0.co;2-y
14. Hutcheson JB, Wittaker WW, Fronstin MH. Leiomyosarcoma of the penis: case report and review of literature. J Urol. 1969;101(6):874-875. doi:10.1016/s0022-5347(17)62446-7
15. Grimer R, Judson I, Peake D, et al. Guidelines for the management of soft tissue sarcomas. Sarcoma. 2010;2010:506182. doi:10.1155/2010/506182
16. McDonald MW, O’Connell JR, Manning JT, Benjamin RS. Leiomyosarcoma of the penis. J Urol. 1983;130(4):788-789. doi:10.1016/s0022-5347(17)51464-0
17. Planz B, Brunner K, Kalem T, Schlick RW, Kind M. Primary leiomyosarcoma of the epididymis and late recurrence on the penis. J Urol. 1998;159(2):508. doi:10.1016/s0022-5347(01)63966-1
18. Smart RH. Leiomyosarcoma of the penis. J Urol. 1984;132(2):356-357. doi:10.1016/s0022-5347(17)49624-8
19. Patrikidou A, Domont J, Cioffi A, Le Cesne A. Treating soft tissue sarcomas with adjuvant chemotherapy. Curr Treat Options Oncol. 2011;12(1):21-31. doi:10.1007/s11864-011-0145-5
20. Italiano A, Delva F, Mathoulin-Pelissier S, et al. Effect of adjuvant chemotherapy on survival in FNCLCC grade 3 soft tissue sarcomas: a multivariate analysis of the French Sarcoma Group Database. Ann Oncol. 2010;21(12):2436-2441. doi:10.1093/annonc/mdq238
21. Pervaiz N, Colterjohn N, Farrokhyar F, Tozer R, Figueredo A, Ghert M. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer. 2008;113(3):573-581. doi:10.1002/cncr.23592
22. Lacarrière E, Galliot I, Gobet F, Sibert L. Leiomyosarcoma of the corpus cavernosum mimicking a Peyronie’s plaque. Urology. 2012;79(4):e53-e54. doi:10.1016/j.urology.2011.07.1410
23. Hamal PB. Leiomyosarcoma of penis—case report and review of the literature. Br J Urol. 1975;47(3):319-324. doi:10.1111/j.1464-410x.1975.tb03974.x
24. Greenwood N, Fox H, Edwards EC. Leiomyosarcoma of the penis. Cancer. 1972;29(2):481-483. doi:10.1002/1097-0142(197202)29:2<481::aid -cncr2820290237>3.0.co;2-q
25. Koizumi H, Nagano K, Kosaka S. A case of penile tumor: combination of leiomyosarcoma and squamous cell carcinoma. Hinyokika Kiyo. 1987;33(9):1489-1491.
26. Romero Gonzalez EJ, Marenco Jimenez JL, Mayorga Pineda MP, Martínez Morán A, Castiñeiras Fernández J. Leiomyosarcoma of the penis, an exceptional entity. Urol Case Rep. 2015;3(3):63-64. doi:10.1016/j.eucr.2014.12.007
Psychosocial Barriers and Their Impact on Hepatocellular Carcinoma Care in US Veterans: Tumor Board Model of Care
Hepatocellular carcinoma (HCC) remains a major global health problem and is the third leading cause of cancer-related mortality worldwide.1 Management of HCC is complex; as it largely occurs in the background of chronic liver disease, its management must simultaneously address challenges related to the patient’s tumor burden, as well as their underlying liver dysfunction and performance status. HCC is universally fatal without treatment, with a 5-year survival < 10%.2 However, if detected early HCC is potentially curable, with treatments such as hepatic resection, ablation, and/or liver transplantation, which are associated with 5-year survival rates as high as 70%.2 HCC-specific palliative treatments, including intra-arterial therapies (eg, trans-arterial chemoembolization, radioembolization) and systemic chemotherapy, have also been shown to prolong survival in patients with advanced HCC. Therefore, a key driver of patient survival is receipt of HCC-specific therapy.
There is rising incidence and mortality related to HCC in the US veteran population, largely attributed to acquisition of chronic hepatitis C virus (HCV) infection decades prior.3 There is also a high prevalence of psychosocial barriers in this population, such as low socioeconomic status, homelessness, alcohol and substance use disorders, and psychiatric disorders which can negatively influence receipt of medical treatment, including cancer care.4,5 Given the complexity of managing HCC, as well as the plethora of potential treatment options available, it is widely accepted that a multidisciplinary team approach, such as the multidisciplinary tumor board (MDTB) provides optimal care to patients with HCC.2,6 The aim of the present study was to identify in a population of veterans diagnosed with HCC the prevalence of psychosocial barriers to care and assess their impact and the role of an MDTB on receipt of HCC-specific care.
Methods
In June 2007, a joint institutional MDTB was established for patients with primary liver tumors receiving care at the William S. Middleton Memorial Veterans’ Hospital (WSMMVH) in Madison, Wisconsin. As we have described elsewhere, individual cases with their corresponding imaging studies were reviewed at a weekly conference attended by transplant hepatologists, medical oncologists, hepatobiliary and transplant surgeons, pathologists, diagnostic and interventional radiologists, and nurse coordinators.6 Potential therapies offered included surgical resection, liver transplantation (LT), thermal ablation, intra-arterial therapies (chemo and/or radioembolization), systemic chemotherapy, stereotactic radiation, and best supportive care. Decisions regarding the appropriate treatment modality were made based on patient factors, review of their cross-sectional imaging studies and/or histopathology, and context of their underlying liver dysfunction. The tumor board discussion was summarized in meeting minutes as well as tumor board encounters recorded in each patient’s health record. Although patients with benign tumors were presented at the MDTB, only patients with a diagnosis of HCC were included in this study.
A database analysis was conducted of all veteran patients with HCC managed through the WSMMVH MDTB, since its inception up to December 31, 2016, with follow-up until December 31, 2018. Data for analysis included demographics, laboratory parameters at time of diagnosis and treatment, imaging findings, histopathology and/or surgical pathology, treatment rendered, and follow-up information. The primary outcome measured in this study included receipt of any therapy and secondarily, patient survival.
Discrete variables were analyzed with χ2 statistics or Fisher exact test and continuous variables with the student t test. Multivariable analyses were carried out with logistic regression. Variables with a P < .05 were considered statistically significant. Analyses were carried out using IBM SPSS v24.0.
As a quality-improvement initiative for the care and management of veterans with HCC, this study was determined to be exempt from review by the WSMMVH and University of Wisconsin School of Medicine and Public Health Institutional Review Board.
Results
From January 1, 2007, through December 31, 2016, 149 patients with HCC were managed through the MDTB. Baseline demographic data, Model for End-stage Liver Disease (MELD) score and Child-Turcotte-Pugh class, and baseline HCC characteristics of the cohort are shown in Tables 1 and 2.
There was a high prevalence of psychosocial barriers in our study cohort, including alcohol or substance use disorder, mental illness diagnosis, and low socioeconomic status (Table 3). The mean distance traveled to WSMMVH for HCC-specific care was 206 km. Fifty patients in the cohort utilized travel assistance and 33 patients accessed lodging assistance.
HCC Treatments
There was a high rate of receipt of treatment in our study cohort with 127 (85%) patients receiving at least one HCC-specific therapy. Care was individualized and coordinated through our institutional MDTB, with both curative and palliative treatment modalities utilized (Table 4).
Curative treatment, which includes LT, ablation, or resection, was offered to 78 (52%) patients who were within T2 stage. Of these 78 patients who were potential candidates for LT as a curative treatment for HCC, 31 were not deemed suitable transplant candidates. Psychosocial barriers precluded consideration for LT in 7 of the 31 patients due to active substance use, homelessness in 1 patient, and severe mental illness in 3 patients. Medical comorbidities, advanced patient age, and patient preference accounted for the remainder.
In a univariate analysis of the cohort of 149 patients, factors that decreased the likelihood of receipt of curative HCC therapy included T2 stage or higher at diagnosis and a diagnosis of depression, whereas provision for lodging was associated with increased likelihood of receiving HCC-specific care (Table 5). Other factors that influenced receipt of any treatment included patient’s MELD score, total bilirubin, and serum α-fetoprotein, a surrogate marker for tumor stage. In the multivariable analysis, predictors of receiving curative therapy included absence of substance use, within T2 stage of tumor, and Child-Turcotte-Pugh class A cirrhosis. The presence of psychosocial barriers apart from substance use did not predict a lower chance of receiving curative HCC therapy (including homelessness, distance traveled to center, mental health disorder, and low income).
Median survival was 727 (95% CI, 488-966) days from diagnosis. Survival from HCC diagnosis in study cohort was 72% at 1 year, 50% at 2 years, 39% at 3 years, and 36% at 5 years. Death occurred in 71 (48%) patients; HCC accounted for death in 52 (73%) patients, complications of end-stage liver disease in 13 (18%) patients, and other causes for the remainder of patients.
Discussion
Increases in prevalence and mortality related to cirrhosis and HCC have been reported among the US veteran population.3 This is in large part attributable to the burden of chronic HCV infection in this population. As mirrored in the US population in general, we may be at a turning point regarding the gradual increase in prevalence in HCC.7 The prevalence of cirrhosis and viral-related HCC related to HCV infection will decline with availability of effective antiviral therapy. Alcoholic liver disease remains a main etiological factor for development of cirrhosis and HCC. Nonalcoholic fatty liver disease is becoming a more prevalent cause for development of cirrhosis, indication for liver transplantation, and development of HCC, and indeed may lead to HCC even in the absence of cirrhosis.8
HCC remains a challenging clinical problem.2 As the vast majority of cases arise in the context of cirrhosis, management of HCC not only must address the cancer stage at diagnosis, but also the patient’s underlying liver dysfunction and performance status. Receipt of HCC-specific therapy is a key driver of patient outcome, with curative therapies available for those diagnosed with early-stage disease. We and others have shown that a multidisciplinary approach to coordinate, individualize, and optimize care for these complex patients can improve the rate of treatment utilization, reduce treatment delays, and improve patient survival.6,9,10
Patient psychosocial barriers, such as low socioeconomic status, homelessness, alcohol and substance use, and psychiatric disorders, are more prevalent among the veteran population and have the potential to negatively influence successful health care delivery. One retrospective study of 100 veterans at a US Department of Veterans Affairs (VA) medical center treated for HCC from 2009 to 2014 showed a majority of the patients lived on a meager income, a high prevalence of homelessness, substance use history in 96% of their cohort, and psychiatric illness in 65% of patients.11 Other studies have documented similar findings in the veteran population, with alcohol, substance use, as well as other uncontrolled comorbidities as barriers to providing care, such as antiviral therapy for chronic HCV infection.12
Herein, we present a cohort of veterans with HCC managed through our MDTB from 2007 to 2016, for whom chronic HCV infection and/or alcoholic liver disease were the main causes of cirrhosis. Our cohort had a high burden of alcohol and substance use disorders while other psychiatric illnesses were also common. Our cohort includes patients who were poor, and even some veterans who lacked a stable home. This profile of poverty and social deprivation among veterans is matched in national data.13-15 Using a tumor board model of nurse navigation and multidisciplinary care, we were able to provide travel and lodging assistance to 50 (34%) and 33 (22%) patients, respectively, in order to facilitate their care.
Our data demonstrate that the impact of psychosocial barriers on our capacity to deliver care varies with the nature of the treatment under consideration: curative vs cancer control. For example, active substance use disorder, homelessness, and severe established mental illness were often considered insurmountable when the treatment in question was LT. Nevertheless, despite the high prevalence in our study group of barriers, such as lack of transport while living far from a VA medical center, or alcohol use disorder, a curative treatment with either LT, tumor ablation, or resection could be offered to over half of our cohort. When noncurative therapies are included, most patients (85%) received HCC-specific care, with good relative survival.
Our reported high receipt of HCC-specific care and patient survival is in contrast to previously reported low rates of HCC-specific care in in a national survey of management of 1296 veteran patients infected with HCV who developed HCC from 1998 to 2006. In this population, HCC-specific treatment was provided to 34%.16 However our data are consistent with our previously published data of patients with HCC managed through an institutional MDTB.6 Indeed, as shown by a univariate analysis in our present study, individualizing care to address modifiable patient barriers, such as providing provisions for lodging if needed, was associated with an increased likelihood of receiving HCC-specific care. On the other hand, advanced tumor stage (> T2) at diagnosis and a diagnosis of depression, which was the most common psychiatric diagnosis in our cohort, were both associated with decreased likelihood of receiving HCC-specific care. Clinical factors such as MELD score, total bilirubin, and serum AFP all affected the likelihood of providing HCC-specific care. In a multivariate analysis, factors that predicted ability to receive curative therapy included absence of substance use, T2 stage of tumor, and Child-Turcotte-Pugh class A cirrhosis. This is expected as patients with HCC within T2 stage (or Milan criteria) with compensated cirrhosis are most likely to receive curative therapies, such as resection, ablation, or LT.2
Conclusions
Our study demonstrates a high burden of psychosocial challenges in veterans with HCC. These accounted for a significant barrier to receive HCC-specific care. Despite the presence of these patient barriers, high rates of HCC-specific treatment are attainable through individualization and coordination of patient care in the context of a MDTB model with nurse navigation. Provision of targeted social support to ameliorate these modifiable factors improves patient outcomes.
1. McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of hepatocellular carcinoma. Hepatology. 2021;73(suppl 1):4-13. doi:10.1002/hep.31288.
2. Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723-750. doi:10.1002/hep.29913
3. Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001-2013. Gastroenterology. 2015;149(6):1471-e18. doi:10.1053/j.gastro.2015.07.056
4. Kazis LE, Miller DR, Clark J, et al. Health-related quality of life in patients served by the Department of Veterans Affairs: results from the Veterans Health Study. Arch Intern Med. 1998;158(6):626-632. doi:10.1001/archinte.158.6.626
5. Slind LM, Keating TM, Fisher AG, Rose TG. A patient navigation model for veterans traveling for cancer care. Fed Pract. 2016;33(suppl 1):40S-45S.
6. Agarwal PD, Phillips P, Hillman L, et al. Multidisciplinary management of hepatocellular carcinoma improves access to therapy and patient survival. J Clin Gastroenterol. 2017;51(9):845-849. doi:10.1097/MCG.0000000000000825
7. White DL, Thrift AP, Kanwal F, Davila J, El-Serag HB. Incidence of hepatocellular carcinoma in all 50 United States, From 2000 Through 2012. Gastroenterology. 2017;152(4):812-820.e5. doi:10.1053/j.gastro.2016.11.020
8. Kanwal F, Kramer JR, Mapakshi S, et al. Risk of hepatocellular cancer in patients with non-alcoholic fatty liver disease. Gastroenterology. 2018;155(6):1828-1837.e2. doi:10.1053/j.gastro.2018.08.024
9. Yopp AC, Mansour JC, Beg MS, et al. Establishment of a multidisciplinary hepatocellular carcinoma clinic is associated with improved clinical outcome. Ann Surg Oncol. 2014;21(4):1287-1295. doi:10.1245/s10434-013-3413-8
10. Chang TT, Sawhney R, Monto A, et al. Implementation of a multidisciplinary treatment team for hepatocellular cancer at a Veterans Affairs Medical Center improves survival. HPB (Oxford). 2008;10(6):405-411. doi:10.1080/13651820802356572
11. Hwa KJ, Dua MM, Wren SM, Visser BC. Missing the obvious: psychosocial obstacles in veterans with hepatocellular carcinoma. HPB (Oxford). 2015;17(12):1124-1129. doi:10.1111/hpb.12508
12. Taylor J, Carr-Lopez S, Robinson A, et al. Determinants of treatment eligibility in veterans with hepatitis C viral infection. Clin Ther. 2017;39(1):130-137. doi:10.1016/j.clinthera.2016.11.019
13. Fargo J, Metraux S, Byrne T, et al. Prevalence and risk of homelessness among US veterans. Prev Chronic Dis. 2012;9:E45.
14. Tsai J, Rosenheck RA. Risk factors for homelessness among US veterans. Epidemiol Rev. 2015;37:177-195. doi:10.1093/epirev/mxu004
15. Tsai J, Link B, Rosenheck RA, Pietrzak RH. Homelessness among a nationally representative sample of US veterans: prevalence, service utilization, and correlates. Soc Psychiatry Psychiatr Epidemiol. 2016;51(6):907-916. doi:10.1007/s00127-016-1210-y
16. Davila JA, Kramer JR, Duan Z, et al. Referral and receipt of treatment for hepatocellular carcinoma in United States veterans: effect of patient and nonpatient factors. Hepatology. 2013;57(5):1858-1868. doi:10.1002/hep.26287
Hepatocellular carcinoma (HCC) remains a major global health problem and is the third leading cause of cancer-related mortality worldwide.1 Management of HCC is complex; as it largely occurs in the background of chronic liver disease, its management must simultaneously address challenges related to the patient’s tumor burden, as well as their underlying liver dysfunction and performance status. HCC is universally fatal without treatment, with a 5-year survival < 10%.2 However, if detected early HCC is potentially curable, with treatments such as hepatic resection, ablation, and/or liver transplantation, which are associated with 5-year survival rates as high as 70%.2 HCC-specific palliative treatments, including intra-arterial therapies (eg, trans-arterial chemoembolization, radioembolization) and systemic chemotherapy, have also been shown to prolong survival in patients with advanced HCC. Therefore, a key driver of patient survival is receipt of HCC-specific therapy.
There is rising incidence and mortality related to HCC in the US veteran population, largely attributed to acquisition of chronic hepatitis C virus (HCV) infection decades prior.3 There is also a high prevalence of psychosocial barriers in this population, such as low socioeconomic status, homelessness, alcohol and substance use disorders, and psychiatric disorders which can negatively influence receipt of medical treatment, including cancer care.4,5 Given the complexity of managing HCC, as well as the plethora of potential treatment options available, it is widely accepted that a multidisciplinary team approach, such as the multidisciplinary tumor board (MDTB) provides optimal care to patients with HCC.2,6 The aim of the present study was to identify in a population of veterans diagnosed with HCC the prevalence of psychosocial barriers to care and assess their impact and the role of an MDTB on receipt of HCC-specific care.
Methods
In June 2007, a joint institutional MDTB was established for patients with primary liver tumors receiving care at the William S. Middleton Memorial Veterans’ Hospital (WSMMVH) in Madison, Wisconsin. As we have described elsewhere, individual cases with their corresponding imaging studies were reviewed at a weekly conference attended by transplant hepatologists, medical oncologists, hepatobiliary and transplant surgeons, pathologists, diagnostic and interventional radiologists, and nurse coordinators.6 Potential therapies offered included surgical resection, liver transplantation (LT), thermal ablation, intra-arterial therapies (chemo and/or radioembolization), systemic chemotherapy, stereotactic radiation, and best supportive care. Decisions regarding the appropriate treatment modality were made based on patient factors, review of their cross-sectional imaging studies and/or histopathology, and context of their underlying liver dysfunction. The tumor board discussion was summarized in meeting minutes as well as tumor board encounters recorded in each patient’s health record. Although patients with benign tumors were presented at the MDTB, only patients with a diagnosis of HCC were included in this study.
A database analysis was conducted of all veteran patients with HCC managed through the WSMMVH MDTB, since its inception up to December 31, 2016, with follow-up until December 31, 2018. Data for analysis included demographics, laboratory parameters at time of diagnosis and treatment, imaging findings, histopathology and/or surgical pathology, treatment rendered, and follow-up information. The primary outcome measured in this study included receipt of any therapy and secondarily, patient survival.
Discrete variables were analyzed with χ2 statistics or Fisher exact test and continuous variables with the student t test. Multivariable analyses were carried out with logistic regression. Variables with a P < .05 were considered statistically significant. Analyses were carried out using IBM SPSS v24.0.
As a quality-improvement initiative for the care and management of veterans with HCC, this study was determined to be exempt from review by the WSMMVH and University of Wisconsin School of Medicine and Public Health Institutional Review Board.
Results
From January 1, 2007, through December 31, 2016, 149 patients with HCC were managed through the MDTB. Baseline demographic data, Model for End-stage Liver Disease (MELD) score and Child-Turcotte-Pugh class, and baseline HCC characteristics of the cohort are shown in Tables 1 and 2.
There was a high prevalence of psychosocial barriers in our study cohort, including alcohol or substance use disorder, mental illness diagnosis, and low socioeconomic status (Table 3). The mean distance traveled to WSMMVH for HCC-specific care was 206 km. Fifty patients in the cohort utilized travel assistance and 33 patients accessed lodging assistance.
HCC Treatments
There was a high rate of receipt of treatment in our study cohort with 127 (85%) patients receiving at least one HCC-specific therapy. Care was individualized and coordinated through our institutional MDTB, with both curative and palliative treatment modalities utilized (Table 4).
Curative treatment, which includes LT, ablation, or resection, was offered to 78 (52%) patients who were within T2 stage. Of these 78 patients who were potential candidates for LT as a curative treatment for HCC, 31 were not deemed suitable transplant candidates. Psychosocial barriers precluded consideration for LT in 7 of the 31 patients due to active substance use, homelessness in 1 patient, and severe mental illness in 3 patients. Medical comorbidities, advanced patient age, and patient preference accounted for the remainder.
In a univariate analysis of the cohort of 149 patients, factors that decreased the likelihood of receipt of curative HCC therapy included T2 stage or higher at diagnosis and a diagnosis of depression, whereas provision for lodging was associated with increased likelihood of receiving HCC-specific care (Table 5). Other factors that influenced receipt of any treatment included patient’s MELD score, total bilirubin, and serum α-fetoprotein, a surrogate marker for tumor stage. In the multivariable analysis, predictors of receiving curative therapy included absence of substance use, within T2 stage of tumor, and Child-Turcotte-Pugh class A cirrhosis. The presence of psychosocial barriers apart from substance use did not predict a lower chance of receiving curative HCC therapy (including homelessness, distance traveled to center, mental health disorder, and low income).
Median survival was 727 (95% CI, 488-966) days from diagnosis. Survival from HCC diagnosis in study cohort was 72% at 1 year, 50% at 2 years, 39% at 3 years, and 36% at 5 years. Death occurred in 71 (48%) patients; HCC accounted for death in 52 (73%) patients, complications of end-stage liver disease in 13 (18%) patients, and other causes for the remainder of patients.
Discussion
Increases in prevalence and mortality related to cirrhosis and HCC have been reported among the US veteran population.3 This is in large part attributable to the burden of chronic HCV infection in this population. As mirrored in the US population in general, we may be at a turning point regarding the gradual increase in prevalence in HCC.7 The prevalence of cirrhosis and viral-related HCC related to HCV infection will decline with availability of effective antiviral therapy. Alcoholic liver disease remains a main etiological factor for development of cirrhosis and HCC. Nonalcoholic fatty liver disease is becoming a more prevalent cause for development of cirrhosis, indication for liver transplantation, and development of HCC, and indeed may lead to HCC even in the absence of cirrhosis.8
HCC remains a challenging clinical problem.2 As the vast majority of cases arise in the context of cirrhosis, management of HCC not only must address the cancer stage at diagnosis, but also the patient’s underlying liver dysfunction and performance status. Receipt of HCC-specific therapy is a key driver of patient outcome, with curative therapies available for those diagnosed with early-stage disease. We and others have shown that a multidisciplinary approach to coordinate, individualize, and optimize care for these complex patients can improve the rate of treatment utilization, reduce treatment delays, and improve patient survival.6,9,10
Patient psychosocial barriers, such as low socioeconomic status, homelessness, alcohol and substance use, and psychiatric disorders, are more prevalent among the veteran population and have the potential to negatively influence successful health care delivery. One retrospective study of 100 veterans at a US Department of Veterans Affairs (VA) medical center treated for HCC from 2009 to 2014 showed a majority of the patients lived on a meager income, a high prevalence of homelessness, substance use history in 96% of their cohort, and psychiatric illness in 65% of patients.11 Other studies have documented similar findings in the veteran population, with alcohol, substance use, as well as other uncontrolled comorbidities as barriers to providing care, such as antiviral therapy for chronic HCV infection.12
Herein, we present a cohort of veterans with HCC managed through our MDTB from 2007 to 2016, for whom chronic HCV infection and/or alcoholic liver disease were the main causes of cirrhosis. Our cohort had a high burden of alcohol and substance use disorders while other psychiatric illnesses were also common. Our cohort includes patients who were poor, and even some veterans who lacked a stable home. This profile of poverty and social deprivation among veterans is matched in national data.13-15 Using a tumor board model of nurse navigation and multidisciplinary care, we were able to provide travel and lodging assistance to 50 (34%) and 33 (22%) patients, respectively, in order to facilitate their care.
Our data demonstrate that the impact of psychosocial barriers on our capacity to deliver care varies with the nature of the treatment under consideration: curative vs cancer control. For example, active substance use disorder, homelessness, and severe established mental illness were often considered insurmountable when the treatment in question was LT. Nevertheless, despite the high prevalence in our study group of barriers, such as lack of transport while living far from a VA medical center, or alcohol use disorder, a curative treatment with either LT, tumor ablation, or resection could be offered to over half of our cohort. When noncurative therapies are included, most patients (85%) received HCC-specific care, with good relative survival.
Our reported high receipt of HCC-specific care and patient survival is in contrast to previously reported low rates of HCC-specific care in in a national survey of management of 1296 veteran patients infected with HCV who developed HCC from 1998 to 2006. In this population, HCC-specific treatment was provided to 34%.16 However our data are consistent with our previously published data of patients with HCC managed through an institutional MDTB.6 Indeed, as shown by a univariate analysis in our present study, individualizing care to address modifiable patient barriers, such as providing provisions for lodging if needed, was associated with an increased likelihood of receiving HCC-specific care. On the other hand, advanced tumor stage (> T2) at diagnosis and a diagnosis of depression, which was the most common psychiatric diagnosis in our cohort, were both associated with decreased likelihood of receiving HCC-specific care. Clinical factors such as MELD score, total bilirubin, and serum AFP all affected the likelihood of providing HCC-specific care. In a multivariate analysis, factors that predicted ability to receive curative therapy included absence of substance use, T2 stage of tumor, and Child-Turcotte-Pugh class A cirrhosis. This is expected as patients with HCC within T2 stage (or Milan criteria) with compensated cirrhosis are most likely to receive curative therapies, such as resection, ablation, or LT.2
Conclusions
Our study demonstrates a high burden of psychosocial challenges in veterans with HCC. These accounted for a significant barrier to receive HCC-specific care. Despite the presence of these patient barriers, high rates of HCC-specific treatment are attainable through individualization and coordination of patient care in the context of a MDTB model with nurse navigation. Provision of targeted social support to ameliorate these modifiable factors improves patient outcomes.
Hepatocellular carcinoma (HCC) remains a major global health problem and is the third leading cause of cancer-related mortality worldwide.1 Management of HCC is complex; as it largely occurs in the background of chronic liver disease, its management must simultaneously address challenges related to the patient’s tumor burden, as well as their underlying liver dysfunction and performance status. HCC is universally fatal without treatment, with a 5-year survival < 10%.2 However, if detected early HCC is potentially curable, with treatments such as hepatic resection, ablation, and/or liver transplantation, which are associated with 5-year survival rates as high as 70%.2 HCC-specific palliative treatments, including intra-arterial therapies (eg, trans-arterial chemoembolization, radioembolization) and systemic chemotherapy, have also been shown to prolong survival in patients with advanced HCC. Therefore, a key driver of patient survival is receipt of HCC-specific therapy.
There is rising incidence and mortality related to HCC in the US veteran population, largely attributed to acquisition of chronic hepatitis C virus (HCV) infection decades prior.3 There is also a high prevalence of psychosocial barriers in this population, such as low socioeconomic status, homelessness, alcohol and substance use disorders, and psychiatric disorders which can negatively influence receipt of medical treatment, including cancer care.4,5 Given the complexity of managing HCC, as well as the plethora of potential treatment options available, it is widely accepted that a multidisciplinary team approach, such as the multidisciplinary tumor board (MDTB) provides optimal care to patients with HCC.2,6 The aim of the present study was to identify in a population of veterans diagnosed with HCC the prevalence of psychosocial barriers to care and assess their impact and the role of an MDTB on receipt of HCC-specific care.
Methods
In June 2007, a joint institutional MDTB was established for patients with primary liver tumors receiving care at the William S. Middleton Memorial Veterans’ Hospital (WSMMVH) in Madison, Wisconsin. As we have described elsewhere, individual cases with their corresponding imaging studies were reviewed at a weekly conference attended by transplant hepatologists, medical oncologists, hepatobiliary and transplant surgeons, pathologists, diagnostic and interventional radiologists, and nurse coordinators.6 Potential therapies offered included surgical resection, liver transplantation (LT), thermal ablation, intra-arterial therapies (chemo and/or radioembolization), systemic chemotherapy, stereotactic radiation, and best supportive care. Decisions regarding the appropriate treatment modality were made based on patient factors, review of their cross-sectional imaging studies and/or histopathology, and context of their underlying liver dysfunction. The tumor board discussion was summarized in meeting minutes as well as tumor board encounters recorded in each patient’s health record. Although patients with benign tumors were presented at the MDTB, only patients with a diagnosis of HCC were included in this study.
A database analysis was conducted of all veteran patients with HCC managed through the WSMMVH MDTB, since its inception up to December 31, 2016, with follow-up until December 31, 2018. Data for analysis included demographics, laboratory parameters at time of diagnosis and treatment, imaging findings, histopathology and/or surgical pathology, treatment rendered, and follow-up information. The primary outcome measured in this study included receipt of any therapy and secondarily, patient survival.
Discrete variables were analyzed with χ2 statistics or Fisher exact test and continuous variables with the student t test. Multivariable analyses were carried out with logistic regression. Variables with a P < .05 were considered statistically significant. Analyses were carried out using IBM SPSS v24.0.
As a quality-improvement initiative for the care and management of veterans with HCC, this study was determined to be exempt from review by the WSMMVH and University of Wisconsin School of Medicine and Public Health Institutional Review Board.
Results
From January 1, 2007, through December 31, 2016, 149 patients with HCC were managed through the MDTB. Baseline demographic data, Model for End-stage Liver Disease (MELD) score and Child-Turcotte-Pugh class, and baseline HCC characteristics of the cohort are shown in Tables 1 and 2.
There was a high prevalence of psychosocial barriers in our study cohort, including alcohol or substance use disorder, mental illness diagnosis, and low socioeconomic status (Table 3). The mean distance traveled to WSMMVH for HCC-specific care was 206 km. Fifty patients in the cohort utilized travel assistance and 33 patients accessed lodging assistance.
HCC Treatments
There was a high rate of receipt of treatment in our study cohort with 127 (85%) patients receiving at least one HCC-specific therapy. Care was individualized and coordinated through our institutional MDTB, with both curative and palliative treatment modalities utilized (Table 4).
Curative treatment, which includes LT, ablation, or resection, was offered to 78 (52%) patients who were within T2 stage. Of these 78 patients who were potential candidates for LT as a curative treatment for HCC, 31 were not deemed suitable transplant candidates. Psychosocial barriers precluded consideration for LT in 7 of the 31 patients due to active substance use, homelessness in 1 patient, and severe mental illness in 3 patients. Medical comorbidities, advanced patient age, and patient preference accounted for the remainder.
In a univariate analysis of the cohort of 149 patients, factors that decreased the likelihood of receipt of curative HCC therapy included T2 stage or higher at diagnosis and a diagnosis of depression, whereas provision for lodging was associated with increased likelihood of receiving HCC-specific care (Table 5). Other factors that influenced receipt of any treatment included patient’s MELD score, total bilirubin, and serum α-fetoprotein, a surrogate marker for tumor stage. In the multivariable analysis, predictors of receiving curative therapy included absence of substance use, within T2 stage of tumor, and Child-Turcotte-Pugh class A cirrhosis. The presence of psychosocial barriers apart from substance use did not predict a lower chance of receiving curative HCC therapy (including homelessness, distance traveled to center, mental health disorder, and low income).
Median survival was 727 (95% CI, 488-966) days from diagnosis. Survival from HCC diagnosis in study cohort was 72% at 1 year, 50% at 2 years, 39% at 3 years, and 36% at 5 years. Death occurred in 71 (48%) patients; HCC accounted for death in 52 (73%) patients, complications of end-stage liver disease in 13 (18%) patients, and other causes for the remainder of patients.
Discussion
Increases in prevalence and mortality related to cirrhosis and HCC have been reported among the US veteran population.3 This is in large part attributable to the burden of chronic HCV infection in this population. As mirrored in the US population in general, we may be at a turning point regarding the gradual increase in prevalence in HCC.7 The prevalence of cirrhosis and viral-related HCC related to HCV infection will decline with availability of effective antiviral therapy. Alcoholic liver disease remains a main etiological factor for development of cirrhosis and HCC. Nonalcoholic fatty liver disease is becoming a more prevalent cause for development of cirrhosis, indication for liver transplantation, and development of HCC, and indeed may lead to HCC even in the absence of cirrhosis.8
HCC remains a challenging clinical problem.2 As the vast majority of cases arise in the context of cirrhosis, management of HCC not only must address the cancer stage at diagnosis, but also the patient’s underlying liver dysfunction and performance status. Receipt of HCC-specific therapy is a key driver of patient outcome, with curative therapies available for those diagnosed with early-stage disease. We and others have shown that a multidisciplinary approach to coordinate, individualize, and optimize care for these complex patients can improve the rate of treatment utilization, reduce treatment delays, and improve patient survival.6,9,10
Patient psychosocial barriers, such as low socioeconomic status, homelessness, alcohol and substance use, and psychiatric disorders, are more prevalent among the veteran population and have the potential to negatively influence successful health care delivery. One retrospective study of 100 veterans at a US Department of Veterans Affairs (VA) medical center treated for HCC from 2009 to 2014 showed a majority of the patients lived on a meager income, a high prevalence of homelessness, substance use history in 96% of their cohort, and psychiatric illness in 65% of patients.11 Other studies have documented similar findings in the veteran population, with alcohol, substance use, as well as other uncontrolled comorbidities as barriers to providing care, such as antiviral therapy for chronic HCV infection.12
Herein, we present a cohort of veterans with HCC managed through our MDTB from 2007 to 2016, for whom chronic HCV infection and/or alcoholic liver disease were the main causes of cirrhosis. Our cohort had a high burden of alcohol and substance use disorders while other psychiatric illnesses were also common. Our cohort includes patients who were poor, and even some veterans who lacked a stable home. This profile of poverty and social deprivation among veterans is matched in national data.13-15 Using a tumor board model of nurse navigation and multidisciplinary care, we were able to provide travel and lodging assistance to 50 (34%) and 33 (22%) patients, respectively, in order to facilitate their care.
Our data demonstrate that the impact of psychosocial barriers on our capacity to deliver care varies with the nature of the treatment under consideration: curative vs cancer control. For example, active substance use disorder, homelessness, and severe established mental illness were often considered insurmountable when the treatment in question was LT. Nevertheless, despite the high prevalence in our study group of barriers, such as lack of transport while living far from a VA medical center, or alcohol use disorder, a curative treatment with either LT, tumor ablation, or resection could be offered to over half of our cohort. When noncurative therapies are included, most patients (85%) received HCC-specific care, with good relative survival.
Our reported high receipt of HCC-specific care and patient survival is in contrast to previously reported low rates of HCC-specific care in in a national survey of management of 1296 veteran patients infected with HCV who developed HCC from 1998 to 2006. In this population, HCC-specific treatment was provided to 34%.16 However our data are consistent with our previously published data of patients with HCC managed through an institutional MDTB.6 Indeed, as shown by a univariate analysis in our present study, individualizing care to address modifiable patient barriers, such as providing provisions for lodging if needed, was associated with an increased likelihood of receiving HCC-specific care. On the other hand, advanced tumor stage (> T2) at diagnosis and a diagnosis of depression, which was the most common psychiatric diagnosis in our cohort, were both associated with decreased likelihood of receiving HCC-specific care. Clinical factors such as MELD score, total bilirubin, and serum AFP all affected the likelihood of providing HCC-specific care. In a multivariate analysis, factors that predicted ability to receive curative therapy included absence of substance use, T2 stage of tumor, and Child-Turcotte-Pugh class A cirrhosis. This is expected as patients with HCC within T2 stage (or Milan criteria) with compensated cirrhosis are most likely to receive curative therapies, such as resection, ablation, or LT.2
Conclusions
Our study demonstrates a high burden of psychosocial challenges in veterans with HCC. These accounted for a significant barrier to receive HCC-specific care. Despite the presence of these patient barriers, high rates of HCC-specific treatment are attainable through individualization and coordination of patient care in the context of a MDTB model with nurse navigation. Provision of targeted social support to ameliorate these modifiable factors improves patient outcomes.
1. McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of hepatocellular carcinoma. Hepatology. 2021;73(suppl 1):4-13. doi:10.1002/hep.31288.
2. Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723-750. doi:10.1002/hep.29913
3. Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001-2013. Gastroenterology. 2015;149(6):1471-e18. doi:10.1053/j.gastro.2015.07.056
4. Kazis LE, Miller DR, Clark J, et al. Health-related quality of life in patients served by the Department of Veterans Affairs: results from the Veterans Health Study. Arch Intern Med. 1998;158(6):626-632. doi:10.1001/archinte.158.6.626
5. Slind LM, Keating TM, Fisher AG, Rose TG. A patient navigation model for veterans traveling for cancer care. Fed Pract. 2016;33(suppl 1):40S-45S.
6. Agarwal PD, Phillips P, Hillman L, et al. Multidisciplinary management of hepatocellular carcinoma improves access to therapy and patient survival. J Clin Gastroenterol. 2017;51(9):845-849. doi:10.1097/MCG.0000000000000825
7. White DL, Thrift AP, Kanwal F, Davila J, El-Serag HB. Incidence of hepatocellular carcinoma in all 50 United States, From 2000 Through 2012. Gastroenterology. 2017;152(4):812-820.e5. doi:10.1053/j.gastro.2016.11.020
8. Kanwal F, Kramer JR, Mapakshi S, et al. Risk of hepatocellular cancer in patients with non-alcoholic fatty liver disease. Gastroenterology. 2018;155(6):1828-1837.e2. doi:10.1053/j.gastro.2018.08.024
9. Yopp AC, Mansour JC, Beg MS, et al. Establishment of a multidisciplinary hepatocellular carcinoma clinic is associated with improved clinical outcome. Ann Surg Oncol. 2014;21(4):1287-1295. doi:10.1245/s10434-013-3413-8
10. Chang TT, Sawhney R, Monto A, et al. Implementation of a multidisciplinary treatment team for hepatocellular cancer at a Veterans Affairs Medical Center improves survival. HPB (Oxford). 2008;10(6):405-411. doi:10.1080/13651820802356572
11. Hwa KJ, Dua MM, Wren SM, Visser BC. Missing the obvious: psychosocial obstacles in veterans with hepatocellular carcinoma. HPB (Oxford). 2015;17(12):1124-1129. doi:10.1111/hpb.12508
12. Taylor J, Carr-Lopez S, Robinson A, et al. Determinants of treatment eligibility in veterans with hepatitis C viral infection. Clin Ther. 2017;39(1):130-137. doi:10.1016/j.clinthera.2016.11.019
13. Fargo J, Metraux S, Byrne T, et al. Prevalence and risk of homelessness among US veterans. Prev Chronic Dis. 2012;9:E45.
14. Tsai J, Rosenheck RA. Risk factors for homelessness among US veterans. Epidemiol Rev. 2015;37:177-195. doi:10.1093/epirev/mxu004
15. Tsai J, Link B, Rosenheck RA, Pietrzak RH. Homelessness among a nationally representative sample of US veterans: prevalence, service utilization, and correlates. Soc Psychiatry Psychiatr Epidemiol. 2016;51(6):907-916. doi:10.1007/s00127-016-1210-y
16. Davila JA, Kramer JR, Duan Z, et al. Referral and receipt of treatment for hepatocellular carcinoma in United States veterans: effect of patient and nonpatient factors. Hepatology. 2013;57(5):1858-1868. doi:10.1002/hep.26287
1. McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of hepatocellular carcinoma. Hepatology. 2021;73(suppl 1):4-13. doi:10.1002/hep.31288.
2. Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723-750. doi:10.1002/hep.29913
3. Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001-2013. Gastroenterology. 2015;149(6):1471-e18. doi:10.1053/j.gastro.2015.07.056
4. Kazis LE, Miller DR, Clark J, et al. Health-related quality of life in patients served by the Department of Veterans Affairs: results from the Veterans Health Study. Arch Intern Med. 1998;158(6):626-632. doi:10.1001/archinte.158.6.626
5. Slind LM, Keating TM, Fisher AG, Rose TG. A patient navigation model for veterans traveling for cancer care. Fed Pract. 2016;33(suppl 1):40S-45S.
6. Agarwal PD, Phillips P, Hillman L, et al. Multidisciplinary management of hepatocellular carcinoma improves access to therapy and patient survival. J Clin Gastroenterol. 2017;51(9):845-849. doi:10.1097/MCG.0000000000000825
7. White DL, Thrift AP, Kanwal F, Davila J, El-Serag HB. Incidence of hepatocellular carcinoma in all 50 United States, From 2000 Through 2012. Gastroenterology. 2017;152(4):812-820.e5. doi:10.1053/j.gastro.2016.11.020
8. Kanwal F, Kramer JR, Mapakshi S, et al. Risk of hepatocellular cancer in patients with non-alcoholic fatty liver disease. Gastroenterology. 2018;155(6):1828-1837.e2. doi:10.1053/j.gastro.2018.08.024
9. Yopp AC, Mansour JC, Beg MS, et al. Establishment of a multidisciplinary hepatocellular carcinoma clinic is associated with improved clinical outcome. Ann Surg Oncol. 2014;21(4):1287-1295. doi:10.1245/s10434-013-3413-8
10. Chang TT, Sawhney R, Monto A, et al. Implementation of a multidisciplinary treatment team for hepatocellular cancer at a Veterans Affairs Medical Center improves survival. HPB (Oxford). 2008;10(6):405-411. doi:10.1080/13651820802356572
11. Hwa KJ, Dua MM, Wren SM, Visser BC. Missing the obvious: psychosocial obstacles in veterans with hepatocellular carcinoma. HPB (Oxford). 2015;17(12):1124-1129. doi:10.1111/hpb.12508
12. Taylor J, Carr-Lopez S, Robinson A, et al. Determinants of treatment eligibility in veterans with hepatitis C viral infection. Clin Ther. 2017;39(1):130-137. doi:10.1016/j.clinthera.2016.11.019
13. Fargo J, Metraux S, Byrne T, et al. Prevalence and risk of homelessness among US veterans. Prev Chronic Dis. 2012;9:E45.
14. Tsai J, Rosenheck RA. Risk factors for homelessness among US veterans. Epidemiol Rev. 2015;37:177-195. doi:10.1093/epirev/mxu004
15. Tsai J, Link B, Rosenheck RA, Pietrzak RH. Homelessness among a nationally representative sample of US veterans: prevalence, service utilization, and correlates. Soc Psychiatry Psychiatr Epidemiol. 2016;51(6):907-916. doi:10.1007/s00127-016-1210-y
16. Davila JA, Kramer JR, Duan Z, et al. Referral and receipt of treatment for hepatocellular carcinoma in United States veterans: effect of patient and nonpatient factors. Hepatology. 2013;57(5):1858-1868. doi:10.1002/hep.26287