User login
Carbon nanoparticle suspension lymphography-guided distal gastrectomy improves lymph node detection rate
Key clinical point: Carbon nanoparticle suspension lymphography-guided distal gastrectomy improves postoperative lymph node detection rate in patients with gastric cancer undergoing gastrectomy.
Major finding: A higher mean number of lymph nodes were detected with vs without carbon nanoparticle suspension injection (CNSI; 59.6 vs 30.0; P < .001). A higher number of lymph nodes were detected in black- vs nonblack-stained stations (9.2 vs 3.5 lymph nodes per station; P < .001).
Study details: This was a retrospective cohort study including 156 propensity score-matched patients with clinical T1-T4 gastric cancer who underwent laparoscopic or robotic distal gastrectomy with or without (conventional group) CNSI between May 2019 and December 2020.
Disclosures: This study was supported by the University Research Project of Hebei Province and the Medical Research Project of Hebei Province, China. The authors declared no conflicts of interest.
Source: Tian Y et al. Assessment of carbon nanoparticle suspension lymphography–guided distal gastrectomy for gastric cancer. JAMA Netw Open. 2022;5(4):e227739 (Apr 18). Doi: 10.1001/jamanetworkopen.2022.7739
Key clinical point: Carbon nanoparticle suspension lymphography-guided distal gastrectomy improves postoperative lymph node detection rate in patients with gastric cancer undergoing gastrectomy.
Major finding: A higher mean number of lymph nodes were detected with vs without carbon nanoparticle suspension injection (CNSI; 59.6 vs 30.0; P < .001). A higher number of lymph nodes were detected in black- vs nonblack-stained stations (9.2 vs 3.5 lymph nodes per station; P < .001).
Study details: This was a retrospective cohort study including 156 propensity score-matched patients with clinical T1-T4 gastric cancer who underwent laparoscopic or robotic distal gastrectomy with or without (conventional group) CNSI between May 2019 and December 2020.
Disclosures: This study was supported by the University Research Project of Hebei Province and the Medical Research Project of Hebei Province, China. The authors declared no conflicts of interest.
Source: Tian Y et al. Assessment of carbon nanoparticle suspension lymphography–guided distal gastrectomy for gastric cancer. JAMA Netw Open. 2022;5(4):e227739 (Apr 18). Doi: 10.1001/jamanetworkopen.2022.7739
Key clinical point: Carbon nanoparticle suspension lymphography-guided distal gastrectomy improves postoperative lymph node detection rate in patients with gastric cancer undergoing gastrectomy.
Major finding: A higher mean number of lymph nodes were detected with vs without carbon nanoparticle suspension injection (CNSI; 59.6 vs 30.0; P < .001). A higher number of lymph nodes were detected in black- vs nonblack-stained stations (9.2 vs 3.5 lymph nodes per station; P < .001).
Study details: This was a retrospective cohort study including 156 propensity score-matched patients with clinical T1-T4 gastric cancer who underwent laparoscopic or robotic distal gastrectomy with or without (conventional group) CNSI between May 2019 and December 2020.
Disclosures: This study was supported by the University Research Project of Hebei Province and the Medical Research Project of Hebei Province, China. The authors declared no conflicts of interest.
Source: Tian Y et al. Assessment of carbon nanoparticle suspension lymphography–guided distal gastrectomy for gastric cancer. JAMA Netw Open. 2022;5(4):e227739 (Apr 18). Doi: 10.1001/jamanetworkopen.2022.7739
Gastric cancer: Perioperative prophylactic HIPEC shows benefit
Key clinical point: Prophylactic hyperthermic intraperitoneal chemotherapy (HIPEC) significantly decreased postoperative peritoneal recurrence and prolonged survival in patients with clinical T4 gastric cancer who underwent gastrectomy with lymphadenectomy.
Major finding: Prophylactic HIPEC vs no HIPEC significantly lowered the overall recurrence rate (34.3% vs 62.9%; P = .04) and postoperative peritoneal carcinomatosis (21.7% vs 57.1%; P = .03). HIPEC significantly improved the overall survival (OS) rate (71.4% vs 40.0%; P = .01). Patients in the HIPEC group had a significantly longer OS (adjusted hazard ratio [aHR] 0.37; P = .035) and disease-free survival (aHR 0.33; P = .017).
Study details: This was a retrospective study of 132 patients with clinical stage T4 gastric cancer who underwent gastrectomy plus D2 lymphadenectomy between 2014 and 2020. Thirty-five of the 132 patients received prophylactic HIPEC perioperatively.
Disclosures: No funding source was identified for this work. The authors declared no competing interests.
Source: Lee TY et al. Prophylactic hyperthermic intraperitoneal chemotherapy for patients with clinical T4 gastric cancer. Eur J Surg Oncol. 2022 (Apr 27). Doi: 10.1016/j.ejso.2022.04.018
Key clinical point: Prophylactic hyperthermic intraperitoneal chemotherapy (HIPEC) significantly decreased postoperative peritoneal recurrence and prolonged survival in patients with clinical T4 gastric cancer who underwent gastrectomy with lymphadenectomy.
Major finding: Prophylactic HIPEC vs no HIPEC significantly lowered the overall recurrence rate (34.3% vs 62.9%; P = .04) and postoperative peritoneal carcinomatosis (21.7% vs 57.1%; P = .03). HIPEC significantly improved the overall survival (OS) rate (71.4% vs 40.0%; P = .01). Patients in the HIPEC group had a significantly longer OS (adjusted hazard ratio [aHR] 0.37; P = .035) and disease-free survival (aHR 0.33; P = .017).
Study details: This was a retrospective study of 132 patients with clinical stage T4 gastric cancer who underwent gastrectomy plus D2 lymphadenectomy between 2014 and 2020. Thirty-five of the 132 patients received prophylactic HIPEC perioperatively.
Disclosures: No funding source was identified for this work. The authors declared no competing interests.
Source: Lee TY et al. Prophylactic hyperthermic intraperitoneal chemotherapy for patients with clinical T4 gastric cancer. Eur J Surg Oncol. 2022 (Apr 27). Doi: 10.1016/j.ejso.2022.04.018
Key clinical point: Prophylactic hyperthermic intraperitoneal chemotherapy (HIPEC) significantly decreased postoperative peritoneal recurrence and prolonged survival in patients with clinical T4 gastric cancer who underwent gastrectomy with lymphadenectomy.
Major finding: Prophylactic HIPEC vs no HIPEC significantly lowered the overall recurrence rate (34.3% vs 62.9%; P = .04) and postoperative peritoneal carcinomatosis (21.7% vs 57.1%; P = .03). HIPEC significantly improved the overall survival (OS) rate (71.4% vs 40.0%; P = .01). Patients in the HIPEC group had a significantly longer OS (adjusted hazard ratio [aHR] 0.37; P = .035) and disease-free survival (aHR 0.33; P = .017).
Study details: This was a retrospective study of 132 patients with clinical stage T4 gastric cancer who underwent gastrectomy plus D2 lymphadenectomy between 2014 and 2020. Thirty-five of the 132 patients received prophylactic HIPEC perioperatively.
Disclosures: No funding source was identified for this work. The authors declared no competing interests.
Source: Lee TY et al. Prophylactic hyperthermic intraperitoneal chemotherapy for patients with clinical T4 gastric cancer. Eur J Surg Oncol. 2022 (Apr 27). Doi: 10.1016/j.ejso.2022.04.018
Gastric cancer: Preoperative body composition predicts complication risk
Key clinical point: The body composition variables were associated with major postoperative complications in patients with gastric cancer who received preoperative chemotherapy followed by gastrectomy.
Major finding: A high skeletal muscle (SM)-mass Z-score (difference in each patient’s standard deviation from the mean value) was associated with a lower risk for major postoperative complications (relative risk [RR] 0.47, P = .004). High visceral adipose tissue-radiation attenuation (VAT-RA; RR 2.82; P = .001) and subcutaneous adipose tissue-RA (SAT-RA; RR 1.95; P = .015) Z-scores were associated with an increased risk for major postoperative complications.
Study details: This was a side study of 112 patients with gastric cancer who received preoperative chemotherapy followed by gastrectomy in the LOGICA trial. The preoperative computed tomography scan was used to calculate the mass and RA for SM, VAT, and SAT.
Disclosures: This study was sponsored by the Netherlands Organization for Health Research and Development. The authors received consulting or advisory fees, travel or accommodations expenses, or grants outside this work.
Source: Tweed TTT et al. Body composition is a predictor for postoperative complications after gastrectomy for gastric cancer: A prospective side study of the LOGICA trial. J Gastrointest Surg. 2022 (Apr 29). Doi: 10.1007/s11605-022-05321-0
Key clinical point: The body composition variables were associated with major postoperative complications in patients with gastric cancer who received preoperative chemotherapy followed by gastrectomy.
Major finding: A high skeletal muscle (SM)-mass Z-score (difference in each patient’s standard deviation from the mean value) was associated with a lower risk for major postoperative complications (relative risk [RR] 0.47, P = .004). High visceral adipose tissue-radiation attenuation (VAT-RA; RR 2.82; P = .001) and subcutaneous adipose tissue-RA (SAT-RA; RR 1.95; P = .015) Z-scores were associated with an increased risk for major postoperative complications.
Study details: This was a side study of 112 patients with gastric cancer who received preoperative chemotherapy followed by gastrectomy in the LOGICA trial. The preoperative computed tomography scan was used to calculate the mass and RA for SM, VAT, and SAT.
Disclosures: This study was sponsored by the Netherlands Organization for Health Research and Development. The authors received consulting or advisory fees, travel or accommodations expenses, or grants outside this work.
Source: Tweed TTT et al. Body composition is a predictor for postoperative complications after gastrectomy for gastric cancer: A prospective side study of the LOGICA trial. J Gastrointest Surg. 2022 (Apr 29). Doi: 10.1007/s11605-022-05321-0
Key clinical point: The body composition variables were associated with major postoperative complications in patients with gastric cancer who received preoperative chemotherapy followed by gastrectomy.
Major finding: A high skeletal muscle (SM)-mass Z-score (difference in each patient’s standard deviation from the mean value) was associated with a lower risk for major postoperative complications (relative risk [RR] 0.47, P = .004). High visceral adipose tissue-radiation attenuation (VAT-RA; RR 2.82; P = .001) and subcutaneous adipose tissue-RA (SAT-RA; RR 1.95; P = .015) Z-scores were associated with an increased risk for major postoperative complications.
Study details: This was a side study of 112 patients with gastric cancer who received preoperative chemotherapy followed by gastrectomy in the LOGICA trial. The preoperative computed tomography scan was used to calculate the mass and RA for SM, VAT, and SAT.
Disclosures: This study was sponsored by the Netherlands Organization for Health Research and Development. The authors received consulting or advisory fees, travel or accommodations expenses, or grants outside this work.
Source: Tweed TTT et al. Body composition is a predictor for postoperative complications after gastrectomy for gastric cancer: A prospective side study of the LOGICA trial. J Gastrointest Surg. 2022 (Apr 29). Doi: 10.1007/s11605-022-05321-0
Gastric cancer: First-degree relatives show high prevalence of precancer lesions
Key clinical point: First-degree relatives of patients with gastric cancer show a high prevalence of preneoplastic lesions (PNL).
Major finding: The prevalence of PNL, atrophic gastritis, and intestinal metaplasia in first-degree relatives of gastric cancer patients were 86.4%, 82.7%, and 54.5%, respectively. The incidence of PNL was not significantly associated with sex (odds ratio [OR] 3.10; 95% confidence interval [CI] 1.00-9.64), age (OR 0.74; 95% CI, 0.26-2.14), and Helicobacter pylorii infection (OR 0.58; 95% CI 0.12-2.77). The advanced stages of Operative Link on Gastritis Assessment and Operative Link on Gastritis/Intestinal-Metaplasia Assessment were verified in 18.0% and 16.3% of the first-degree relatives, respectively.
Study details: This was a cross-sectional study including 110 first-degree relatives of patients with gastric cancer.
Disclosures: This study was partially supported by CONICYT, Chile. The authors declared no conflicts of interest.
Source: Sotelo S et al. Prevalence of gastric preneoplastic lesions in first-degree relatives of patients with gastric cancer: A cross-sectional study. J Gastrointest Cancer. 2022 (Apr 30). Doi: 10.1007/s12029-022-00827-x
Key clinical point: First-degree relatives of patients with gastric cancer show a high prevalence of preneoplastic lesions (PNL).
Major finding: The prevalence of PNL, atrophic gastritis, and intestinal metaplasia in first-degree relatives of gastric cancer patients were 86.4%, 82.7%, and 54.5%, respectively. The incidence of PNL was not significantly associated with sex (odds ratio [OR] 3.10; 95% confidence interval [CI] 1.00-9.64), age (OR 0.74; 95% CI, 0.26-2.14), and Helicobacter pylorii infection (OR 0.58; 95% CI 0.12-2.77). The advanced stages of Operative Link on Gastritis Assessment and Operative Link on Gastritis/Intestinal-Metaplasia Assessment were verified in 18.0% and 16.3% of the first-degree relatives, respectively.
Study details: This was a cross-sectional study including 110 first-degree relatives of patients with gastric cancer.
Disclosures: This study was partially supported by CONICYT, Chile. The authors declared no conflicts of interest.
Source: Sotelo S et al. Prevalence of gastric preneoplastic lesions in first-degree relatives of patients with gastric cancer: A cross-sectional study. J Gastrointest Cancer. 2022 (Apr 30). Doi: 10.1007/s12029-022-00827-x
Key clinical point: First-degree relatives of patients with gastric cancer show a high prevalence of preneoplastic lesions (PNL).
Major finding: The prevalence of PNL, atrophic gastritis, and intestinal metaplasia in first-degree relatives of gastric cancer patients were 86.4%, 82.7%, and 54.5%, respectively. The incidence of PNL was not significantly associated with sex (odds ratio [OR] 3.10; 95% confidence interval [CI] 1.00-9.64), age (OR 0.74; 95% CI, 0.26-2.14), and Helicobacter pylorii infection (OR 0.58; 95% CI 0.12-2.77). The advanced stages of Operative Link on Gastritis Assessment and Operative Link on Gastritis/Intestinal-Metaplasia Assessment were verified in 18.0% and 16.3% of the first-degree relatives, respectively.
Study details: This was a cross-sectional study including 110 first-degree relatives of patients with gastric cancer.
Disclosures: This study was partially supported by CONICYT, Chile. The authors declared no conflicts of interest.
Source: Sotelo S et al. Prevalence of gastric preneoplastic lesions in first-degree relatives of patients with gastric cancer: A cross-sectional study. J Gastrointest Cancer. 2022 (Apr 30). Doi: 10.1007/s12029-022-00827-x
Gastric cancer: Oxaliplatin and cisplatin confer similar survival in elderly patients
Key clinical point: The oxaliplatin- vs cisplatin-based regimen does not show significant difference in survival benefits in elderly patients with advanced gastric cancer.
Major finding: The overall survival was not significantly different between the oxaliplatin and cisplatin groups (hazard ratio 1.13; P = .70). A significantly lower number of patients received granulocyte colony-stimulating factor in the oxaliplatin vs cisplatin group (2.3% vs 22.7%; P = .01).
Study details: This was a propensity score-matched analysis of 242 patients aged ≥70 years with advanced gastric cancer who received oxaliplatin- or cisplatin-based treatment regimen.
Disclosures: This study was supported by the Ministry of Education, Culture, Sports, Science, and Technology, Japan. The authors declared no competing interests.
Source: Chinen T et al. Oxaliplatin- versus cisplatin-based regimens for elderly individuals with advanced gastric cancer: a retrospective cohort study. BMC Cancer. 2022;22:460 (Apr 26). Doi: 10.1186/s12885-022-09581-6
Key clinical point: The oxaliplatin- vs cisplatin-based regimen does not show significant difference in survival benefits in elderly patients with advanced gastric cancer.
Major finding: The overall survival was not significantly different between the oxaliplatin and cisplatin groups (hazard ratio 1.13; P = .70). A significantly lower number of patients received granulocyte colony-stimulating factor in the oxaliplatin vs cisplatin group (2.3% vs 22.7%; P = .01).
Study details: This was a propensity score-matched analysis of 242 patients aged ≥70 years with advanced gastric cancer who received oxaliplatin- or cisplatin-based treatment regimen.
Disclosures: This study was supported by the Ministry of Education, Culture, Sports, Science, and Technology, Japan. The authors declared no competing interests.
Source: Chinen T et al. Oxaliplatin- versus cisplatin-based regimens for elderly individuals with advanced gastric cancer: a retrospective cohort study. BMC Cancer. 2022;22:460 (Apr 26). Doi: 10.1186/s12885-022-09581-6
Key clinical point: The oxaliplatin- vs cisplatin-based regimen does not show significant difference in survival benefits in elderly patients with advanced gastric cancer.
Major finding: The overall survival was not significantly different between the oxaliplatin and cisplatin groups (hazard ratio 1.13; P = .70). A significantly lower number of patients received granulocyte colony-stimulating factor in the oxaliplatin vs cisplatin group (2.3% vs 22.7%; P = .01).
Study details: This was a propensity score-matched analysis of 242 patients aged ≥70 years with advanced gastric cancer who received oxaliplatin- or cisplatin-based treatment regimen.
Disclosures: This study was supported by the Ministry of Education, Culture, Sports, Science, and Technology, Japan. The authors declared no competing interests.
Source: Chinen T et al. Oxaliplatin- versus cisplatin-based regimens for elderly individuals with advanced gastric cancer: a retrospective cohort study. BMC Cancer. 2022;22:460 (Apr 26). Doi: 10.1186/s12885-022-09581-6
Serum pepsinogen is associated with gastric cancer risk
Key clinical point: Baseline serum pepsinogen levels are associated with a significant risk for gastric cancer, particularly the noncardia type.
Major finding: A higher proportion of patients with gastric cancer vs matched controls had a positive baseline serum pepsinogen status (31.4% vs 5.5%; P < .001). A positive serum pepsinogen status was associated with an increased risk for gastric cancer (adjusted odds ratio [aOR] 10.6; 95% CI 4.3-26.2). In subgroup analysis, a positive pepsinogen status was associated with a higher risk for noncardia gastric cancer (aOR 14.3; 95% CI 4.8-42.0).
Study details: This was a nested case-control study using the Prostate, Lung, Colorectal, and Ovarian Cancer Screening trial data of 105 participants who developed gastric cancer and 209 matched control individuals.
Disclosures: This study was sponsored by the Society for Surgery of the Alimentary Tract Health Care Disparities Research Award and National Institutes of Health-National Center for Advancing Translational Sciences grant. The authors declared no competing interests.
Source: In H et al. Serum pepsinogen as a biomarker for gastric cancer in the United States: A nested case-control study using the PLCO Cancer Screening Trial Data. Cancer Epidemiol Biomarkers Prev. 2022 (May 9). Doi: 10.1158/1055-9965.EPI-21-1328
Key clinical point: Baseline serum pepsinogen levels are associated with a significant risk for gastric cancer, particularly the noncardia type.
Major finding: A higher proportion of patients with gastric cancer vs matched controls had a positive baseline serum pepsinogen status (31.4% vs 5.5%; P < .001). A positive serum pepsinogen status was associated with an increased risk for gastric cancer (adjusted odds ratio [aOR] 10.6; 95% CI 4.3-26.2). In subgroup analysis, a positive pepsinogen status was associated with a higher risk for noncardia gastric cancer (aOR 14.3; 95% CI 4.8-42.0).
Study details: This was a nested case-control study using the Prostate, Lung, Colorectal, and Ovarian Cancer Screening trial data of 105 participants who developed gastric cancer and 209 matched control individuals.
Disclosures: This study was sponsored by the Society for Surgery of the Alimentary Tract Health Care Disparities Research Award and National Institutes of Health-National Center for Advancing Translational Sciences grant. The authors declared no competing interests.
Source: In H et al. Serum pepsinogen as a biomarker for gastric cancer in the United States: A nested case-control study using the PLCO Cancer Screening Trial Data. Cancer Epidemiol Biomarkers Prev. 2022 (May 9). Doi: 10.1158/1055-9965.EPI-21-1328
Key clinical point: Baseline serum pepsinogen levels are associated with a significant risk for gastric cancer, particularly the noncardia type.
Major finding: A higher proportion of patients with gastric cancer vs matched controls had a positive baseline serum pepsinogen status (31.4% vs 5.5%; P < .001). A positive serum pepsinogen status was associated with an increased risk for gastric cancer (adjusted odds ratio [aOR] 10.6; 95% CI 4.3-26.2). In subgroup analysis, a positive pepsinogen status was associated with a higher risk for noncardia gastric cancer (aOR 14.3; 95% CI 4.8-42.0).
Study details: This was a nested case-control study using the Prostate, Lung, Colorectal, and Ovarian Cancer Screening trial data of 105 participants who developed gastric cancer and 209 matched control individuals.
Disclosures: This study was sponsored by the Society for Surgery of the Alimentary Tract Health Care Disparities Research Award and National Institutes of Health-National Center for Advancing Translational Sciences grant. The authors declared no competing interests.
Source: In H et al. Serum pepsinogen as a biomarker for gastric cancer in the United States: A nested case-control study using the PLCO Cancer Screening Trial Data. Cancer Epidemiol Biomarkers Prev. 2022 (May 9). Doi: 10.1158/1055-9965.EPI-21-1328
Advanced gastric cancer: Taxane-based chemotherapy regimen improves outcomes
Key clinical point: Taxanes plus basic chemotherapy vs basic chemotherapy alone improves oncologic outcomes in treatment-naive patients with advanced gastric cancer.
Major finding: Basic chemotherapy with vs without taxanes significantly improved progression-free survival (hazard ratio [HR] 0.73; P = .001), overall survival (HR 0.80; P = .003), objective response rate (risk ratio [RR] 1.34; P = .0001), and disease control rate (RR 1.20; P = .001). Patients who received taxanes had a significantly higher risk for neutropenia (RR 3.54; P = .0003), leucopenia (RR 24.99; P = .03), and diarrhea (RR 4.41; P < .00001).
Study details: A meta-analysis of six randomized controlled trials including 2263 patients with advanced gastric cancer who received first-line chemotherapy.
Disclosures: This study was supported by Beijing Natural Science Foundation of China. The authors declared no conflicts of interest.
Source: Ma X et al. Efficacy and safety of combination chemotherapy regimens containing taxanes for first-line treatment in advanced gastric cancer. Clin Exp Med. 2022 (Apr 16). Doi: 10.1007/s10238-022-00824-1
Key clinical point: Taxanes plus basic chemotherapy vs basic chemotherapy alone improves oncologic outcomes in treatment-naive patients with advanced gastric cancer.
Major finding: Basic chemotherapy with vs without taxanes significantly improved progression-free survival (hazard ratio [HR] 0.73; P = .001), overall survival (HR 0.80; P = .003), objective response rate (risk ratio [RR] 1.34; P = .0001), and disease control rate (RR 1.20; P = .001). Patients who received taxanes had a significantly higher risk for neutropenia (RR 3.54; P = .0003), leucopenia (RR 24.99; P = .03), and diarrhea (RR 4.41; P < .00001).
Study details: A meta-analysis of six randomized controlled trials including 2263 patients with advanced gastric cancer who received first-line chemotherapy.
Disclosures: This study was supported by Beijing Natural Science Foundation of China. The authors declared no conflicts of interest.
Source: Ma X et al. Efficacy and safety of combination chemotherapy regimens containing taxanes for first-line treatment in advanced gastric cancer. Clin Exp Med. 2022 (Apr 16). Doi: 10.1007/s10238-022-00824-1
Key clinical point: Taxanes plus basic chemotherapy vs basic chemotherapy alone improves oncologic outcomes in treatment-naive patients with advanced gastric cancer.
Major finding: Basic chemotherapy with vs without taxanes significantly improved progression-free survival (hazard ratio [HR] 0.73; P = .001), overall survival (HR 0.80; P = .003), objective response rate (risk ratio [RR] 1.34; P = .0001), and disease control rate (RR 1.20; P = .001). Patients who received taxanes had a significantly higher risk for neutropenia (RR 3.54; P = .0003), leucopenia (RR 24.99; P = .03), and diarrhea (RR 4.41; P < .00001).
Study details: A meta-analysis of six randomized controlled trials including 2263 patients with advanced gastric cancer who received first-line chemotherapy.
Disclosures: This study was supported by Beijing Natural Science Foundation of China. The authors declared no conflicts of interest.
Source: Ma X et al. Efficacy and safety of combination chemotherapy regimens containing taxanes for first-line treatment in advanced gastric cancer. Clin Exp Med. 2022 (Apr 16). Doi: 10.1007/s10238-022-00824-1
Persistent dry cough
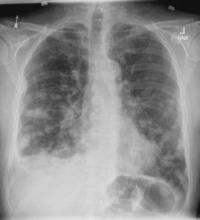
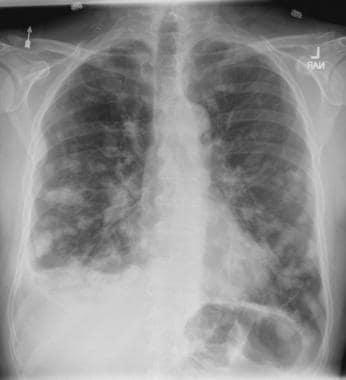
On the basis of the patient's presentation, history, and imaging results, the likely diagnosis is metastatic small cell lung cancer (SCLC). Most patients with SCLC present with hematogenous metastases; only about one third present with limited disease confined to the chest that is amenable to multimodal therapy. Patients with SCLC often present with symptoms of widespread metastases, including weight loss, bone pain, and neurologic compromise. It is uncommon for patients to present with a solitary peripheral nodule. In earlier stages, the differential diagnosis of SCLC spans other neuroendocrine lung tumors and NSCLC, in particular, basaloid carcinoma, extrapulmonary small cell tumors, and lymphoma.
Because the concentration of circulating tumor cells in SCLC is among the highest of any solid tumor, SCLC is characterized by a rapid doubling time, high growth fraction, and early development of widespread metastases. It is likely for this reason that CT screening does not seem effective in detecting early-stage SCLC. Common sites of SCLC metastasis are the contralateral lung, the brain, liver, adrenal glands, and bone. Most cases of SCLC are caused by smoking.
Metastatic spread is often evident on radiologic exam, sometimes showing pleural and pericardial effusions. In general, workup for SCLC includes imaging (contrast-enhanced CT or F-FDG PET–CT of the chest, abdomen, and pelvis and brain MRI with contrast), blood tests (cell count, liver and kidney function, and lactate dehydrogenase), and ECG. Biopsies are generally procured by bronchoscopy with or without endobronchial ultrasonography; if accessible, a biopsy of a distal metastatic site may be obtained. Diagnosis of SCLC is confirmed by histopathologic examination via cytology.
Patients with extensive-stage SCLC are typically treated with systemic chemotherapy with or without immunotherapy. In the early stages, SCLC is very responsive to cytotoxic therapies, with response rates over 60% even in patients with metastatic disease. Until recently, the only second-line therapy for recurrent metastatic SCLC was the topoisomerase I inhibitor topotecan. However, lurbinectedin was granted accelerated approval for second-line therapy after demonstrating a 35% response rate in a single-arm phase 2 study of 105 patients. In addition, the anti–programmed cell death protein 1 monoclonal antibodies nivolumab and pembrolizumab were granted accelerated approval for third-line use. Finally, the National Comprehensive Cancer Network guidelines note that participation in clinical trials should be strongly encouraged for all patients with SCLC.
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts.
Karl J. D'Silva, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
On the basis of the patient's presentation, history, and imaging results, the likely diagnosis is metastatic small cell lung cancer (SCLC). Most patients with SCLC present with hematogenous metastases; only about one third present with limited disease confined to the chest that is amenable to multimodal therapy. Patients with SCLC often present with symptoms of widespread metastases, including weight loss, bone pain, and neurologic compromise. It is uncommon for patients to present with a solitary peripheral nodule. In earlier stages, the differential diagnosis of SCLC spans other neuroendocrine lung tumors and NSCLC, in particular, basaloid carcinoma, extrapulmonary small cell tumors, and lymphoma.
Because the concentration of circulating tumor cells in SCLC is among the highest of any solid tumor, SCLC is characterized by a rapid doubling time, high growth fraction, and early development of widespread metastases. It is likely for this reason that CT screening does not seem effective in detecting early-stage SCLC. Common sites of SCLC metastasis are the contralateral lung, the brain, liver, adrenal glands, and bone. Most cases of SCLC are caused by smoking.
Metastatic spread is often evident on radiologic exam, sometimes showing pleural and pericardial effusions. In general, workup for SCLC includes imaging (contrast-enhanced CT or F-FDG PET–CT of the chest, abdomen, and pelvis and brain MRI with contrast), blood tests (cell count, liver and kidney function, and lactate dehydrogenase), and ECG. Biopsies are generally procured by bronchoscopy with or without endobronchial ultrasonography; if accessible, a biopsy of a distal metastatic site may be obtained. Diagnosis of SCLC is confirmed by histopathologic examination via cytology.
Patients with extensive-stage SCLC are typically treated with systemic chemotherapy with or without immunotherapy. In the early stages, SCLC is very responsive to cytotoxic therapies, with response rates over 60% even in patients with metastatic disease. Until recently, the only second-line therapy for recurrent metastatic SCLC was the topoisomerase I inhibitor topotecan. However, lurbinectedin was granted accelerated approval for second-line therapy after demonstrating a 35% response rate in a single-arm phase 2 study of 105 patients. In addition, the anti–programmed cell death protein 1 monoclonal antibodies nivolumab and pembrolizumab were granted accelerated approval for third-line use. Finally, the National Comprehensive Cancer Network guidelines note that participation in clinical trials should be strongly encouraged for all patients with SCLC.
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts.
Karl J. D'Silva, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
On the basis of the patient's presentation, history, and imaging results, the likely diagnosis is metastatic small cell lung cancer (SCLC). Most patients with SCLC present with hematogenous metastases; only about one third present with limited disease confined to the chest that is amenable to multimodal therapy. Patients with SCLC often present with symptoms of widespread metastases, including weight loss, bone pain, and neurologic compromise. It is uncommon for patients to present with a solitary peripheral nodule. In earlier stages, the differential diagnosis of SCLC spans other neuroendocrine lung tumors and NSCLC, in particular, basaloid carcinoma, extrapulmonary small cell tumors, and lymphoma.
Because the concentration of circulating tumor cells in SCLC is among the highest of any solid tumor, SCLC is characterized by a rapid doubling time, high growth fraction, and early development of widespread metastases. It is likely for this reason that CT screening does not seem effective in detecting early-stage SCLC. Common sites of SCLC metastasis are the contralateral lung, the brain, liver, adrenal glands, and bone. Most cases of SCLC are caused by smoking.
Metastatic spread is often evident on radiologic exam, sometimes showing pleural and pericardial effusions. In general, workup for SCLC includes imaging (contrast-enhanced CT or F-FDG PET–CT of the chest, abdomen, and pelvis and brain MRI with contrast), blood tests (cell count, liver and kidney function, and lactate dehydrogenase), and ECG. Biopsies are generally procured by bronchoscopy with or without endobronchial ultrasonography; if accessible, a biopsy of a distal metastatic site may be obtained. Diagnosis of SCLC is confirmed by histopathologic examination via cytology.
Patients with extensive-stage SCLC are typically treated with systemic chemotherapy with or without immunotherapy. In the early stages, SCLC is very responsive to cytotoxic therapies, with response rates over 60% even in patients with metastatic disease. Until recently, the only second-line therapy for recurrent metastatic SCLC was the topoisomerase I inhibitor topotecan. However, lurbinectedin was granted accelerated approval for second-line therapy after demonstrating a 35% response rate in a single-arm phase 2 study of 105 patients. In addition, the anti–programmed cell death protein 1 monoclonal antibodies nivolumab and pembrolizumab were granted accelerated approval for third-line use. Finally, the National Comprehensive Cancer Network guidelines note that participation in clinical trials should be strongly encouraged for all patients with SCLC.
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts.
Karl J. D'Silva, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 58-year-old man presents with a persistent dry cough that has developed over the past 8 weeks. He has lost about 8-10 lb in under 3 months. Height is 5 ft 10 in and weight is 172 lb (BMI 24.7). Although he quit smoking about 15 years ago, his wife still smokes. He has been screened twice for non–small cell lung cancer (NSCLC), most recently a year and a half ago. Chest radiograph shows multiple pulmonary nodules of varying sizes and a small right basal effusion.
Advances In Neurologic Care
- Pharmacist Impact on Access to Care in an Epilepsy Clinic
- MRI Protocols for Veterans With Multiple Sclerosis
- Neuroimaging in the Era of Artificial Intelligence
- Autonomic Dysfunction in CADASIL Syndrome
- Pharmacist Impact on Access to Care in an Epilepsy Clinic
- MRI Protocols for Veterans With Multiple Sclerosis
- Neuroimaging in the Era of Artificial Intelligence
- Autonomic Dysfunction in CADASIL Syndrome
- Pharmacist Impact on Access to Care in an Epilepsy Clinic
- MRI Protocols for Veterans With Multiple Sclerosis
- Neuroimaging in the Era of Artificial Intelligence
- Autonomic Dysfunction in CADASIL Syndrome
Man with distal flexion deformities
On the basis of history and presentation, this patient's psoriatic disease has probably evolved to psoriatic arthritis mutilans (PAM). PAM is considered the most severe form of psoriatic arthritis (PsA), causing joint destruction and functional disability. It is estimated to affect about 5% of patients with PsA, with an equal sex distribution. Psoriatic nail dystrophy, a hallmark of PsA, appears to be a clinical biomarker of PAM development. Patients with PAM are generally younger at diagnosis than those with less severe forms of disease. Disease-modifying antirheumatic drugs and anti-TNF therapy do not appear to prevent the development of PAM, as evidenced by the present case.
In general, clinical presentation of PsA is heterogeneous and can be similar to that of other rheumatic diseases such as rheumatoid arthritis or osteoarthritis, complicating the differential diagnosis. The Classification Criteria for Psoriatic Arthritis (CASPAR) are considered the most sensitive diagnostic criteria, encompassing evidence of psoriasis; nail dystrophy; lab findings of typical autoantibodies (negative rheumatoid factor); and phenomena that are characteristic of PsA, like dactylitis.
Workup for PAM often includes radiography, ultrasound, and MRI or CT. With no established consensus, classification systems for the condition vary clinically and radiographically. Radiographic features suggestive of PAM include osteolysis or extended bone resorption; pencil-in-cup changes; joint subluxation; and, less often, ankylosis. Osteolysis has been defined as bone resorption with more than 50% loss of joint surface on both sides of the joint. Clinically, dissolution of the joint causes redundant, overlying skin with a telescoping motion of the digit. Other clinical features of PAM include digital shortening and flail joints. Of note, involvement of one small joint in the hands or feet is diagnostic of PAM.
In the setting of PsA, multiple genetic factors have been described, including presence of HLA-B27 and HLA-DRB1, but none are considered defining factors for the disease. A recent population-based study shows that presence of HLA-B27 was significantly increased among patients with PAM (45%) compared with patients with less severe PsA (13%) and healthy controls (13%).
According to the American College of Rheumatology guidelines, first-line therapy in adult patients who have active PsA and are treatment-naive is a TNFi biologic agent. For the patient in this case, who has active PsA despite treatment with TNFi biologic monotherapy, switching to a different TNFi biologic may be appropriate; however, switching to an interleukin-17 inhibitor may also be considered because this patient has severe disease. Data on the comparative efficacy of different biological agents for treatment of PAM are not yet available.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
On the basis of history and presentation, this patient's psoriatic disease has probably evolved to psoriatic arthritis mutilans (PAM). PAM is considered the most severe form of psoriatic arthritis (PsA), causing joint destruction and functional disability. It is estimated to affect about 5% of patients with PsA, with an equal sex distribution. Psoriatic nail dystrophy, a hallmark of PsA, appears to be a clinical biomarker of PAM development. Patients with PAM are generally younger at diagnosis than those with less severe forms of disease. Disease-modifying antirheumatic drugs and anti-TNF therapy do not appear to prevent the development of PAM, as evidenced by the present case.
In general, clinical presentation of PsA is heterogeneous and can be similar to that of other rheumatic diseases such as rheumatoid arthritis or osteoarthritis, complicating the differential diagnosis. The Classification Criteria for Psoriatic Arthritis (CASPAR) are considered the most sensitive diagnostic criteria, encompassing evidence of psoriasis; nail dystrophy; lab findings of typical autoantibodies (negative rheumatoid factor); and phenomena that are characteristic of PsA, like dactylitis.
Workup for PAM often includes radiography, ultrasound, and MRI or CT. With no established consensus, classification systems for the condition vary clinically and radiographically. Radiographic features suggestive of PAM include osteolysis or extended bone resorption; pencil-in-cup changes; joint subluxation; and, less often, ankylosis. Osteolysis has been defined as bone resorption with more than 50% loss of joint surface on both sides of the joint. Clinically, dissolution of the joint causes redundant, overlying skin with a telescoping motion of the digit. Other clinical features of PAM include digital shortening and flail joints. Of note, involvement of one small joint in the hands or feet is diagnostic of PAM.
In the setting of PsA, multiple genetic factors have been described, including presence of HLA-B27 and HLA-DRB1, but none are considered defining factors for the disease. A recent population-based study shows that presence of HLA-B27 was significantly increased among patients with PAM (45%) compared with patients with less severe PsA (13%) and healthy controls (13%).
According to the American College of Rheumatology guidelines, first-line therapy in adult patients who have active PsA and are treatment-naive is a TNFi biologic agent. For the patient in this case, who has active PsA despite treatment with TNFi biologic monotherapy, switching to a different TNFi biologic may be appropriate; however, switching to an interleukin-17 inhibitor may also be considered because this patient has severe disease. Data on the comparative efficacy of different biological agents for treatment of PAM are not yet available.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
On the basis of history and presentation, this patient's psoriatic disease has probably evolved to psoriatic arthritis mutilans (PAM). PAM is considered the most severe form of psoriatic arthritis (PsA), causing joint destruction and functional disability. It is estimated to affect about 5% of patients with PsA, with an equal sex distribution. Psoriatic nail dystrophy, a hallmark of PsA, appears to be a clinical biomarker of PAM development. Patients with PAM are generally younger at diagnosis than those with less severe forms of disease. Disease-modifying antirheumatic drugs and anti-TNF therapy do not appear to prevent the development of PAM, as evidenced by the present case.
In general, clinical presentation of PsA is heterogeneous and can be similar to that of other rheumatic diseases such as rheumatoid arthritis or osteoarthritis, complicating the differential diagnosis. The Classification Criteria for Psoriatic Arthritis (CASPAR) are considered the most sensitive diagnostic criteria, encompassing evidence of psoriasis; nail dystrophy; lab findings of typical autoantibodies (negative rheumatoid factor); and phenomena that are characteristic of PsA, like dactylitis.
Workup for PAM often includes radiography, ultrasound, and MRI or CT. With no established consensus, classification systems for the condition vary clinically and radiographically. Radiographic features suggestive of PAM include osteolysis or extended bone resorption; pencil-in-cup changes; joint subluxation; and, less often, ankylosis. Osteolysis has been defined as bone resorption with more than 50% loss of joint surface on both sides of the joint. Clinically, dissolution of the joint causes redundant, overlying skin with a telescoping motion of the digit. Other clinical features of PAM include digital shortening and flail joints. Of note, involvement of one small joint in the hands or feet is diagnostic of PAM.
In the setting of PsA, multiple genetic factors have been described, including presence of HLA-B27 and HLA-DRB1, but none are considered defining factors for the disease. A recent population-based study shows that presence of HLA-B27 was significantly increased among patients with PAM (45%) compared with patients with less severe PsA (13%) and healthy controls (13%).
According to the American College of Rheumatology guidelines, first-line therapy in adult patients who have active PsA and are treatment-naive is a TNFi biologic agent. For the patient in this case, who has active PsA despite treatment with TNFi biologic monotherapy, switching to a different TNFi biologic may be appropriate; however, switching to an interleukin-17 inhibitor may also be considered because this patient has severe disease. Data on the comparative efficacy of different biological agents for treatment of PAM are not yet available.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 43-year-old man presents with distal flexion deformities and telescoping of the digits. The patient was diagnosed with psoriasis at age 31 and he has several immediate family members who previously received the same diagnosis. He has been treated intermittently with tumor necrosis factor inhibitor (TNFi) biologic monotherapy but admits to nonadherence when disease activity seems to quiet down. Radiography shows osteolysis and dissolution of the joint.