User login
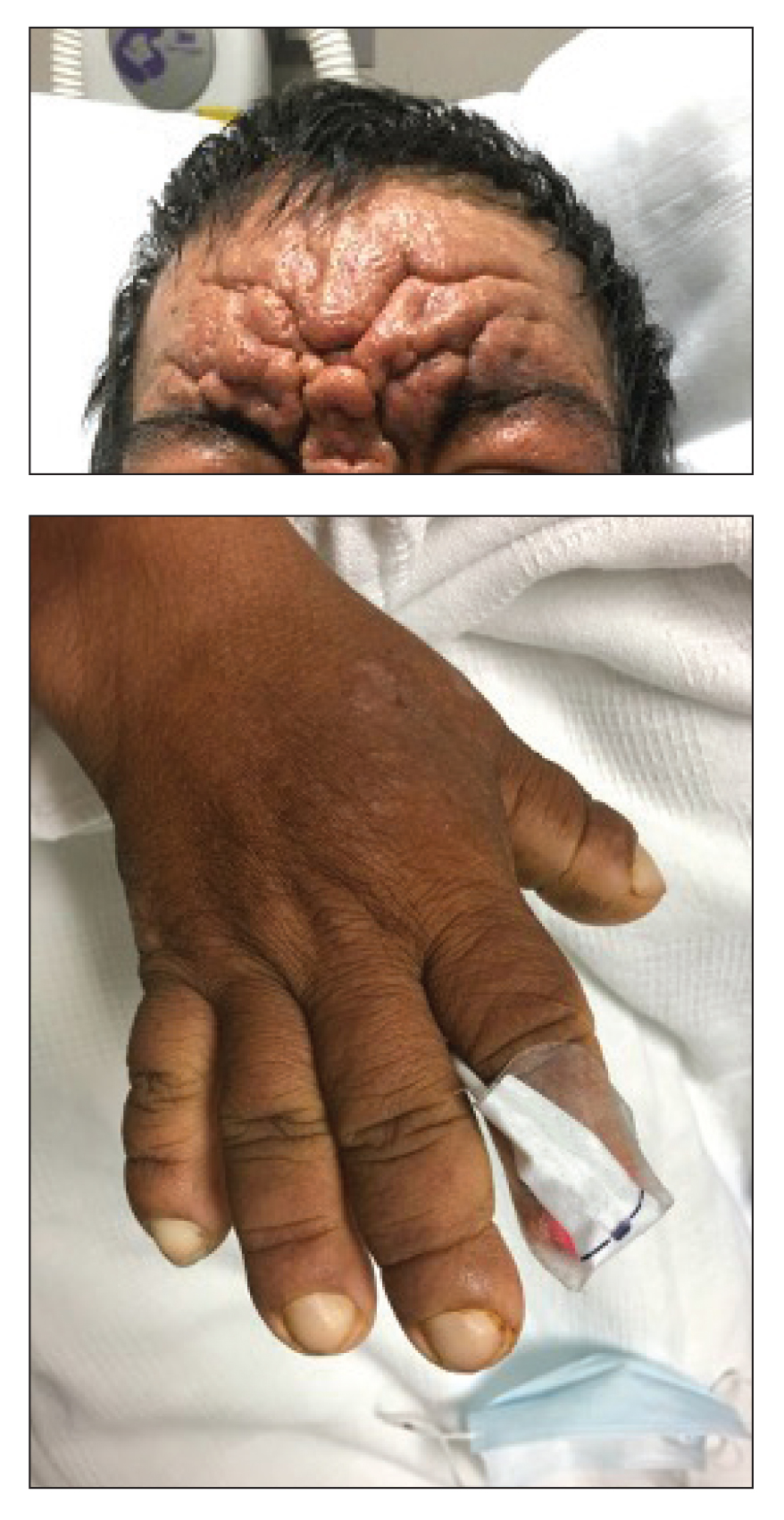
Exaggerated Facial Lines on the Forehead and Cheeks
The Diagnosis: Pachydermoperiostosis
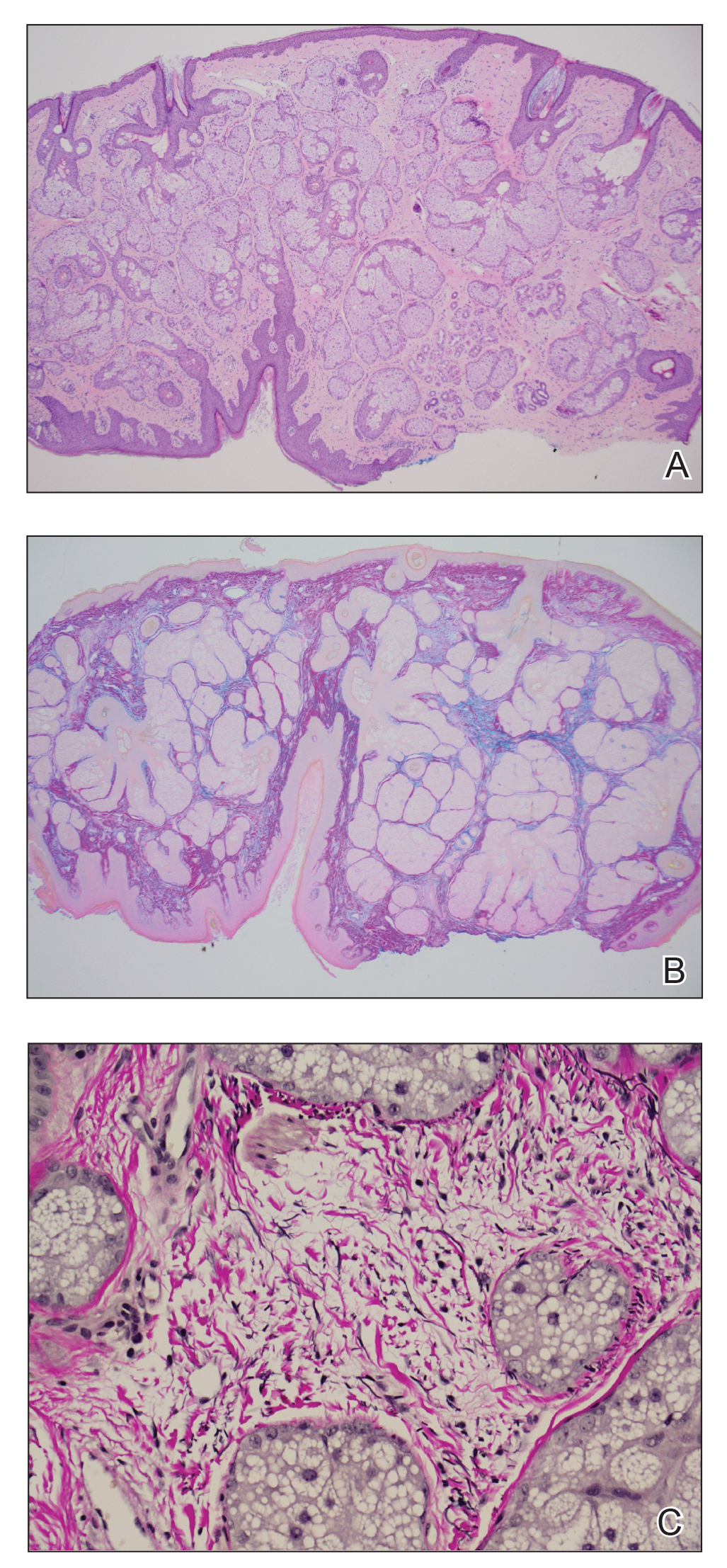
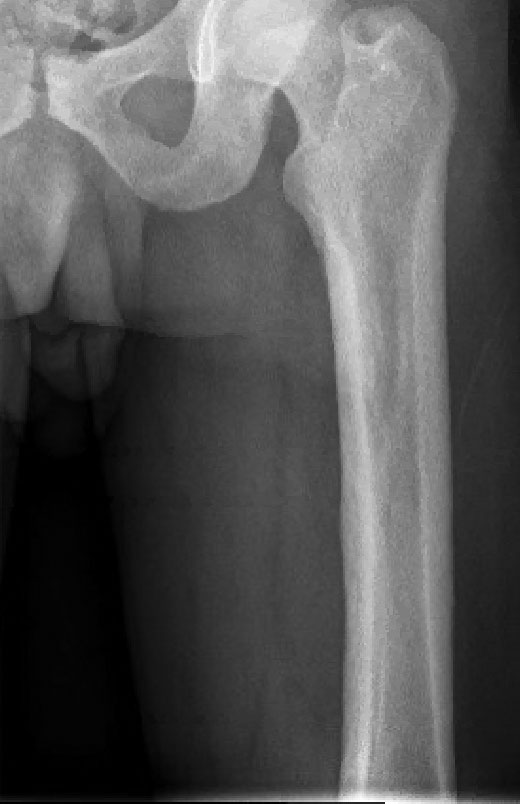
Histopathology of the forehead punch biopsy demonstrated sebaceous hyperplasia with an occupation rate of greater than 40%, increased mucin, elastic fiber degeneration, and fibrosis. Pachydermia is graded from 0 to 3 depending on the degree of these changes; our patient met criteria for grade 3 pachydermia (Figure 1). Radiography revealed diffuse cortical thickening of the long bones that was most marked in the left femur (Figure 2); however, no other findings were demonstrative of Paget disease.
Pachydermoperiostosis (PDP)(also known as Touraine-Solente-Golé syndrome or primary hypertrophic osteoarthropathy) is a rare genetic condition that affects both the dermatologic and skeletal systems. Clinical features of the disease include progressive thickening and furrowing of the skin on the scalp and face (known as pachydermia), digital clubbing, and periostosis. Other potential cutaneous features include seborrhea, acne, hyperhidrosis of the palms and soles, cutis verticis gyrata, eczema, and a burning sensation of the hands and feet. Myelofibrosis and gastrointestinal abnormalities also have been reported.1
The disease typically affects males (7:1 ratio); also, men typically display a more severe phenotype of the disease.2 It most commonly begins during puberty and follows a generally progressive course of 5 to 20 years before eventually stabilizing. Both autosomal-dominant with incomplete penetrance and recessive inheritance versions of PDP can occur. Prostaglandin E2 (PGE2) has been implicated in the pathogenesis of PDP; PGE2 is important in the inflammatory response and may evolve from disrupted protein degradation pathways.3 Sasaki et al4 additionally reported that the severity of pachydermia clinically and histologically appeared to correlate with the serum PGE2 levels in affected patients. Prostaglandin E2 causes a vasodilatory effect, perhaps explaining the clubbing observed in PDP, and also modifies the activity of osteoblasts and osteoclasts, causing the bone remodeling observed in the disease.4
In our patient, the initial differential diagnosis included PDP, as well as lepromatous leprosy, acromegaly, Paget disease of the bone, amyloidosis, scleromyxedema, and cutaneous T-cell lymphoma. However, the time course of the disease, lack of numerous symmetric thickened plaques and madarosis, and pathology argued against lepromatous leprosy. Acromegaly was ruled out due to lack of macroglossia as well as laboratory analysis within reference range including IGF-1 levels and thyroid function tests. Biopsy findings ultimately ruled out amyloidosis and cutaneous T-cell lymphoma. The bone scan revealed diffuse cortical thickening consistent with PDP, and there were no other radiologic findings suggestive of Paget disease. Pachydermoperiostosis is diagnosed using the Borochowitz criteria, which entails that 2 of the following 4 fulfillment criteria must be met: familial transmission, pachydermia, digital clubbing, and/or bony involvement with evidence of radiologic alterations or pain. Our patient met all 4 criteria. The clinical manifestations of PDP are variable with respect to skin and bone changes. The various clinical expressions include the complete form (ie, pachydermia, cutis verticis gyrata, periostosis), the incomplete form (ie, absence of cutis verticis gyrata), and forme fruste (ie, pachydermia with minimal or absent periostosis).5
Management for PDP involves surgical correction for cosmesis as well as for functional concerns if present. Symptoms secondary to periostosis should be managed with symptomatic treatment such as nonsteroidal antiinflammatory drugs. Patients managed with etoricoxib, a COX-2–selective nonsteroidal anti-inflammatory drug, have had normalized inflammatory markers that resulted in the lessening of forehead skin folds. Oral aescin has been shown to relieve joint pain due to its antiedematous effect.6 Our patient received treatment with nonsteroidal anti-inflammatory drugs for symptomatic management of the associated joint pain but unfortunately was lost to follow-up.
- Castori M, Sinibaldi L, Mingarelli R, et al. Pachydermoperiostosis: an update. Clin Genet. 2005;68:477-486.
- Reginato AJ, Shipachasse V, Guerrero R. Familial idiopathic hypertrophic osteoarthropathy and cranial suture defects in children. Skel Radiol. 1982;8:105-109.
- Coggins KG, Coffman TM, Koller BH. The Hippocratic finger points the blame at PGE2. Nat Genet. 2008;40:691-692.
- Sasaki T, Niizeki H, Shimizu A, et al. Identification of mutations in the prostaglandin transporter gene SLCO2A1 and its phenotype-genotype correlation in Japanese patients with pachydermoperiostosis. J Dermatol Sci. 2012;68:36-44.
- Bhaskaranand K, Shetty RR, Bhat AK. Pachydermoperiostosis: three case reports. J Orthop Surg (Hong Kong). 2001;9:61-66.
- Zhang H, Yang B. Successful treatment of pachydermoperiostosis patients with etoricoxib, aescin, and arthroscopic synovectomy: two case reports. Medicine (Baltimore). 2017;96:E8865.
The Diagnosis: Pachydermoperiostosis
Histopathology of the forehead punch biopsy demonstrated sebaceous hyperplasia with an occupation rate of greater than 40%, increased mucin, elastic fiber degeneration, and fibrosis. Pachydermia is graded from 0 to 3 depending on the degree of these changes; our patient met criteria for grade 3 pachydermia (Figure 1). Radiography revealed diffuse cortical thickening of the long bones that was most marked in the left femur (Figure 2); however, no other findings were demonstrative of Paget disease.

Pachydermoperiostosis (PDP)(also known as Touraine-Solente-Golé syndrome or primary hypertrophic osteoarthropathy) is a rare genetic condition that affects both the dermatologic and skeletal systems. Clinical features of the disease include progressive thickening and furrowing of the skin on the scalp and face (known as pachydermia), digital clubbing, and periostosis. Other potential cutaneous features include seborrhea, acne, hyperhidrosis of the palms and soles, cutis verticis gyrata, eczema, and a burning sensation of the hands and feet. Myelofibrosis and gastrointestinal abnormalities also have been reported.1

The disease typically affects males (7:1 ratio); also, men typically display a more severe phenotype of the disease.2 It most commonly begins during puberty and follows a generally progressive course of 5 to 20 years before eventually stabilizing. Both autosomal-dominant with incomplete penetrance and recessive inheritance versions of PDP can occur. Prostaglandin E2 (PGE2) has been implicated in the pathogenesis of PDP; PGE2 is important in the inflammatory response and may evolve from disrupted protein degradation pathways.3 Sasaki et al4 additionally reported that the severity of pachydermia clinically and histologically appeared to correlate with the serum PGE2 levels in affected patients. Prostaglandin E2 causes a vasodilatory effect, perhaps explaining the clubbing observed in PDP, and also modifies the activity of osteoblasts and osteoclasts, causing the bone remodeling observed in the disease.4
In our patient, the initial differential diagnosis included PDP, as well as lepromatous leprosy, acromegaly, Paget disease of the bone, amyloidosis, scleromyxedema, and cutaneous T-cell lymphoma. However, the time course of the disease, lack of numerous symmetric thickened plaques and madarosis, and pathology argued against lepromatous leprosy. Acromegaly was ruled out due to lack of macroglossia as well as laboratory analysis within reference range including IGF-1 levels and thyroid function tests. Biopsy findings ultimately ruled out amyloidosis and cutaneous T-cell lymphoma. The bone scan revealed diffuse cortical thickening consistent with PDP, and there were no other radiologic findings suggestive of Paget disease. Pachydermoperiostosis is diagnosed using the Borochowitz criteria, which entails that 2 of the following 4 fulfillment criteria must be met: familial transmission, pachydermia, digital clubbing, and/or bony involvement with evidence of radiologic alterations or pain. Our patient met all 4 criteria. The clinical manifestations of PDP are variable with respect to skin and bone changes. The various clinical expressions include the complete form (ie, pachydermia, cutis verticis gyrata, periostosis), the incomplete form (ie, absence of cutis verticis gyrata), and forme fruste (ie, pachydermia with minimal or absent periostosis).5
Management for PDP involves surgical correction for cosmesis as well as for functional concerns if present. Symptoms secondary to periostosis should be managed with symptomatic treatment such as nonsteroidal antiinflammatory drugs. Patients managed with etoricoxib, a COX-2–selective nonsteroidal anti-inflammatory drug, have had normalized inflammatory markers that resulted in the lessening of forehead skin folds. Oral aescin has been shown to relieve joint pain due to its antiedematous effect.6 Our patient received treatment with nonsteroidal anti-inflammatory drugs for symptomatic management of the associated joint pain but unfortunately was lost to follow-up.
The Diagnosis: Pachydermoperiostosis
Histopathology of the forehead punch biopsy demonstrated sebaceous hyperplasia with an occupation rate of greater than 40%, increased mucin, elastic fiber degeneration, and fibrosis. Pachydermia is graded from 0 to 3 depending on the degree of these changes; our patient met criteria for grade 3 pachydermia (Figure 1). Radiography revealed diffuse cortical thickening of the long bones that was most marked in the left femur (Figure 2); however, no other findings were demonstrative of Paget disease.

Pachydermoperiostosis (PDP)(also known as Touraine-Solente-Golé syndrome or primary hypertrophic osteoarthropathy) is a rare genetic condition that affects both the dermatologic and skeletal systems. Clinical features of the disease include progressive thickening and furrowing of the skin on the scalp and face (known as pachydermia), digital clubbing, and periostosis. Other potential cutaneous features include seborrhea, acne, hyperhidrosis of the palms and soles, cutis verticis gyrata, eczema, and a burning sensation of the hands and feet. Myelofibrosis and gastrointestinal abnormalities also have been reported.1

The disease typically affects males (7:1 ratio); also, men typically display a more severe phenotype of the disease.2 It most commonly begins during puberty and follows a generally progressive course of 5 to 20 years before eventually stabilizing. Both autosomal-dominant with incomplete penetrance and recessive inheritance versions of PDP can occur. Prostaglandin E2 (PGE2) has been implicated in the pathogenesis of PDP; PGE2 is important in the inflammatory response and may evolve from disrupted protein degradation pathways.3 Sasaki et al4 additionally reported that the severity of pachydermia clinically and histologically appeared to correlate with the serum PGE2 levels in affected patients. Prostaglandin E2 causes a vasodilatory effect, perhaps explaining the clubbing observed in PDP, and also modifies the activity of osteoblasts and osteoclasts, causing the bone remodeling observed in the disease.4
In our patient, the initial differential diagnosis included PDP, as well as lepromatous leprosy, acromegaly, Paget disease of the bone, amyloidosis, scleromyxedema, and cutaneous T-cell lymphoma. However, the time course of the disease, lack of numerous symmetric thickened plaques and madarosis, and pathology argued against lepromatous leprosy. Acromegaly was ruled out due to lack of macroglossia as well as laboratory analysis within reference range including IGF-1 levels and thyroid function tests. Biopsy findings ultimately ruled out amyloidosis and cutaneous T-cell lymphoma. The bone scan revealed diffuse cortical thickening consistent with PDP, and there were no other radiologic findings suggestive of Paget disease. Pachydermoperiostosis is diagnosed using the Borochowitz criteria, which entails that 2 of the following 4 fulfillment criteria must be met: familial transmission, pachydermia, digital clubbing, and/or bony involvement with evidence of radiologic alterations or pain. Our patient met all 4 criteria. The clinical manifestations of PDP are variable with respect to skin and bone changes. The various clinical expressions include the complete form (ie, pachydermia, cutis verticis gyrata, periostosis), the incomplete form (ie, absence of cutis verticis gyrata), and forme fruste (ie, pachydermia with minimal or absent periostosis).5
Management for PDP involves surgical correction for cosmesis as well as for functional concerns if present. Symptoms secondary to periostosis should be managed with symptomatic treatment such as nonsteroidal antiinflammatory drugs. Patients managed with etoricoxib, a COX-2–selective nonsteroidal anti-inflammatory drug, have had normalized inflammatory markers that resulted in the lessening of forehead skin folds. Oral aescin has been shown to relieve joint pain due to its antiedematous effect.6 Our patient received treatment with nonsteroidal anti-inflammatory drugs for symptomatic management of the associated joint pain but unfortunately was lost to follow-up.
- Castori M, Sinibaldi L, Mingarelli R, et al. Pachydermoperiostosis: an update. Clin Genet. 2005;68:477-486.
- Reginato AJ, Shipachasse V, Guerrero R. Familial idiopathic hypertrophic osteoarthropathy and cranial suture defects in children. Skel Radiol. 1982;8:105-109.
- Coggins KG, Coffman TM, Koller BH. The Hippocratic finger points the blame at PGE2. Nat Genet. 2008;40:691-692.
- Sasaki T, Niizeki H, Shimizu A, et al. Identification of mutations in the prostaglandin transporter gene SLCO2A1 and its phenotype-genotype correlation in Japanese patients with pachydermoperiostosis. J Dermatol Sci. 2012;68:36-44.
- Bhaskaranand K, Shetty RR, Bhat AK. Pachydermoperiostosis: three case reports. J Orthop Surg (Hong Kong). 2001;9:61-66.
- Zhang H, Yang B. Successful treatment of pachydermoperiostosis patients with etoricoxib, aescin, and arthroscopic synovectomy: two case reports. Medicine (Baltimore). 2017;96:E8865.
- Castori M, Sinibaldi L, Mingarelli R, et al. Pachydermoperiostosis: an update. Clin Genet. 2005;68:477-486.
- Reginato AJ, Shipachasse V, Guerrero R. Familial idiopathic hypertrophic osteoarthropathy and cranial suture defects in children. Skel Radiol. 1982;8:105-109.
- Coggins KG, Coffman TM, Koller BH. The Hippocratic finger points the blame at PGE2. Nat Genet. 2008;40:691-692.
- Sasaki T, Niizeki H, Shimizu A, et al. Identification of mutations in the prostaglandin transporter gene SLCO2A1 and its phenotype-genotype correlation in Japanese patients with pachydermoperiostosis. J Dermatol Sci. 2012;68:36-44.
- Bhaskaranand K, Shetty RR, Bhat AK. Pachydermoperiostosis: three case reports. J Orthop Surg (Hong Kong). 2001;9:61-66.
- Zhang H, Yang B. Successful treatment of pachydermoperiostosis patients with etoricoxib, aescin, and arthroscopic synovectomy: two case reports. Medicine (Baltimore). 2017;96:E8865.
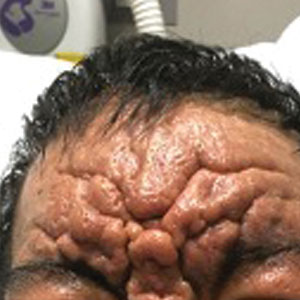
A 36-year-old man presented to the emergency department with an olecranon fracture after falling from a tree. The patient had a medical history of type 2 diabetes mellitus and a surgical history of facial cosmetic surgery. He underwent internal fixation with orthopedic surgery for the olecranon fracture, and dermatology subsequently was consulted due to diffuse skin changes on the face. He reported that these dermatologic changes began around 17 years of age and had progressed to the current presentation. He denied itching, burning, pain, or contact with armadillos. A family history revealed the patient’s brother also had a similar appearance. Physical examination revealed exaggerated facial lines on the forehead (top) and cheeks. Digital clubbing and skin thickening were noted on the hands (bottom) and feet; examination of the back revealed multiple hypopigmented patches. Observation of the scalp showed multiple symmetric ridges and grooves with sparse overlying hair consistent with cutis verticis gyrata. A punch biopsy of the forehead was obtained as well as bone radiography taken previously by the primary team.
Does PREDICT accurately estimate breast cancer survival?
The PREDICT score does not seem to be particularly accurate when it comes to estimating overall survival (OS) in patients with HER2-positive early breast cancer who are treated with modern chemotherapy and anti-HER2 targeted therapies. This is the conclusion of an international study published in the journal npj Breast Cancer. The work was supervised by Matteo Lambertini, MD, PhD, an oncologist at the IRCCS San Martino Polyclinic Hospital in Genoa, Italy.
As the authors explain, “PREDICT is a publicly available online tool that helps to predict the individual prognosis of patients with early breast cancer and to show the impact of adjuvant treatments administered after breast cancer surgery.” The tool uses traditional clinical-pathological factors. The authors also point out that the original version of this tool was validated in several datasets of patients with breast cancer. In 2011, it was updated to include HER2 status.
The investigators noted that, although the use of PREDICT is recommended to aid decision-making in the adjuvant setting, its prognostic role in patients with HER2-positive early breast cancer who are treated with modern chemotherapy and anti-HER2 therapies – even trastuzumab-based ones – remains unclear.
Therefore, they decided to analyze PREDICT’s prognostic performance using data extracted from the ALTTO trial, the largest adjuvant study ever conducted in the field of HER2-positive early breast cancer. That trial “represented a unique opportunity to investigate the reliability and prognostic performance of PREDICT in women with HER2-positive disease,” according to the investigators. They went on to specify that ALTTO evaluated adjuvant lapatinib plus trastuzumab vs. trastuzumab alone in 8,381 patients – 2,794 of whom were included in their own analysis.
What the analysis found was that, overall, PREDICT underestimated 5-year OS by 6.7%. The observed 5-year OS was 94.7%, and the predicted 5-year OS was 88.0%.
The highest absolute differences were observed for patients with hormone receptor–negative disease, nodal involvement, and large tumor size (13.0%, 15.8%, and 15.3%, respectively),” they wrote. Furthermore, they reported that “the suboptimal performance of this prognostic tool was observed irrespective of type of anti-HER2 treatment, type of chemotherapy regimen, age of the patients at the time of diagnosis, central hormone receptor status, pathological nodal status, and pathological tumor size.”
To potentially explain the reasons for the underestimation of patients’ OS, the authors questioned whether the population used to validate PREDICT accurately mirrored the real-world population of patients with HER2-positive disease treated in the modern era with effective chemotherapy and anti-HER2 targeted therapies. “Moreover, the current standard of care for early breast cancer is even superior to the treatment received by many patients in the ALTTO study. … As such, the discordance between OS estimated by PREDICT and the current real-world OS is expected to be even higher. Therefore,” the researchers concluded, “our results suggest that the current version of PREDICT should be used with caution for prognostication in HER2-positive early breast cancer patients treated in the modern era with effective chemotherapy and anti-HER2 targeted therapies.”
A version of this article first appeared on Medscape.com. This article was translated from Univadis Italy.
The PREDICT score does not seem to be particularly accurate when it comes to estimating overall survival (OS) in patients with HER2-positive early breast cancer who are treated with modern chemotherapy and anti-HER2 targeted therapies. This is the conclusion of an international study published in the journal npj Breast Cancer. The work was supervised by Matteo Lambertini, MD, PhD, an oncologist at the IRCCS San Martino Polyclinic Hospital in Genoa, Italy.
As the authors explain, “PREDICT is a publicly available online tool that helps to predict the individual prognosis of patients with early breast cancer and to show the impact of adjuvant treatments administered after breast cancer surgery.” The tool uses traditional clinical-pathological factors. The authors also point out that the original version of this tool was validated in several datasets of patients with breast cancer. In 2011, it was updated to include HER2 status.
The investigators noted that, although the use of PREDICT is recommended to aid decision-making in the adjuvant setting, its prognostic role in patients with HER2-positive early breast cancer who are treated with modern chemotherapy and anti-HER2 therapies – even trastuzumab-based ones – remains unclear.
Therefore, they decided to analyze PREDICT’s prognostic performance using data extracted from the ALTTO trial, the largest adjuvant study ever conducted in the field of HER2-positive early breast cancer. That trial “represented a unique opportunity to investigate the reliability and prognostic performance of PREDICT in women with HER2-positive disease,” according to the investigators. They went on to specify that ALTTO evaluated adjuvant lapatinib plus trastuzumab vs. trastuzumab alone in 8,381 patients – 2,794 of whom were included in their own analysis.
What the analysis found was that, overall, PREDICT underestimated 5-year OS by 6.7%. The observed 5-year OS was 94.7%, and the predicted 5-year OS was 88.0%.
The highest absolute differences were observed for patients with hormone receptor–negative disease, nodal involvement, and large tumor size (13.0%, 15.8%, and 15.3%, respectively),” they wrote. Furthermore, they reported that “the suboptimal performance of this prognostic tool was observed irrespective of type of anti-HER2 treatment, type of chemotherapy regimen, age of the patients at the time of diagnosis, central hormone receptor status, pathological nodal status, and pathological tumor size.”
To potentially explain the reasons for the underestimation of patients’ OS, the authors questioned whether the population used to validate PREDICT accurately mirrored the real-world population of patients with HER2-positive disease treated in the modern era with effective chemotherapy and anti-HER2 targeted therapies. “Moreover, the current standard of care for early breast cancer is even superior to the treatment received by many patients in the ALTTO study. … As such, the discordance between OS estimated by PREDICT and the current real-world OS is expected to be even higher. Therefore,” the researchers concluded, “our results suggest that the current version of PREDICT should be used with caution for prognostication in HER2-positive early breast cancer patients treated in the modern era with effective chemotherapy and anti-HER2 targeted therapies.”
A version of this article first appeared on Medscape.com. This article was translated from Univadis Italy.
The PREDICT score does not seem to be particularly accurate when it comes to estimating overall survival (OS) in patients with HER2-positive early breast cancer who are treated with modern chemotherapy and anti-HER2 targeted therapies. This is the conclusion of an international study published in the journal npj Breast Cancer. The work was supervised by Matteo Lambertini, MD, PhD, an oncologist at the IRCCS San Martino Polyclinic Hospital in Genoa, Italy.
As the authors explain, “PREDICT is a publicly available online tool that helps to predict the individual prognosis of patients with early breast cancer and to show the impact of adjuvant treatments administered after breast cancer surgery.” The tool uses traditional clinical-pathological factors. The authors also point out that the original version of this tool was validated in several datasets of patients with breast cancer. In 2011, it was updated to include HER2 status.
The investigators noted that, although the use of PREDICT is recommended to aid decision-making in the adjuvant setting, its prognostic role in patients with HER2-positive early breast cancer who are treated with modern chemotherapy and anti-HER2 therapies – even trastuzumab-based ones – remains unclear.
Therefore, they decided to analyze PREDICT’s prognostic performance using data extracted from the ALTTO trial, the largest adjuvant study ever conducted in the field of HER2-positive early breast cancer. That trial “represented a unique opportunity to investigate the reliability and prognostic performance of PREDICT in women with HER2-positive disease,” according to the investigators. They went on to specify that ALTTO evaluated adjuvant lapatinib plus trastuzumab vs. trastuzumab alone in 8,381 patients – 2,794 of whom were included in their own analysis.
What the analysis found was that, overall, PREDICT underestimated 5-year OS by 6.7%. The observed 5-year OS was 94.7%, and the predicted 5-year OS was 88.0%.
The highest absolute differences were observed for patients with hormone receptor–negative disease, nodal involvement, and large tumor size (13.0%, 15.8%, and 15.3%, respectively),” they wrote. Furthermore, they reported that “the suboptimal performance of this prognostic tool was observed irrespective of type of anti-HER2 treatment, type of chemotherapy regimen, age of the patients at the time of diagnosis, central hormone receptor status, pathological nodal status, and pathological tumor size.”
To potentially explain the reasons for the underestimation of patients’ OS, the authors questioned whether the population used to validate PREDICT accurately mirrored the real-world population of patients with HER2-positive disease treated in the modern era with effective chemotherapy and anti-HER2 targeted therapies. “Moreover, the current standard of care for early breast cancer is even superior to the treatment received by many patients in the ALTTO study. … As such, the discordance between OS estimated by PREDICT and the current real-world OS is expected to be even higher. Therefore,” the researchers concluded, “our results suggest that the current version of PREDICT should be used with caution for prognostication in HER2-positive early breast cancer patients treated in the modern era with effective chemotherapy and anti-HER2 targeted therapies.”
A version of this article first appeared on Medscape.com. This article was translated from Univadis Italy.
FROM NPJ BREAST CANCER
Ulcerative Colitis Medications
Safety Profile of Mutant EGFR-TK Inhibitors in Advanced Non–Small Cell Lung Cancer: A Meta-analysis
Lung cancer has been the leading cause of cancer-related mortality for decades. It is also predicted to remain as the leading cause of cancer-related mortality through 2030.1 Platinum-based chemotherapy, including carboplatin and paclitaxel, was introduced 3 decades ago and revolutionized the management of advanced non–small cell lung cancer (NSCLC). A more recent advancement has been mutant epidermal growth factor receptor–tyrosine kinase (EGFR-TK) inhibitors.1 EGFR is a transmembrane protein that functions by transducing essential growth factor signaling from the extracellular milieu to the cell. As 60% of the advanced NSCLC expresses this receptor, blocking the mutant EGFR receptor was a groundbreaking development in the management of advanced NSCLC.2 Development of mutant EGFR-TK inhibitors has revolutionized the management of advanced NSCLC. This study was conducted to determine the safety profile of mutant EGFR-TK inhibitors in the management of advanced NSCLC.
Methods
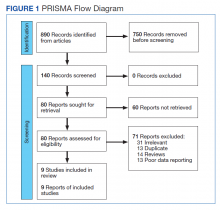
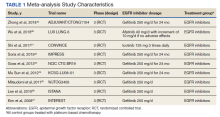
This meta-analysis was conducted according to Cochrane Collaboration guidelines and reported as per Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The findings are summarized in the PRISMA flow diagram (Figure 1). Two authors (MZ and MM) performed a systematic literature search using databases such as MEDLINE (via PubMed), Embase, and Cochrane Library using the medical search terms and their respective entry words with the following search strategy: safety, “mutant EGFR-TK inhibitors,” advanced, “non–small cell,” “lung cancer,” “adverse effect,” and literature. Additionally, unpublished trials were identified from clinicaltrials.gov, and references of all pertinent articles were also scrutinized to ensure the inclusion of all relevant studies. The search was completed on June 1, 2021, and we only included studies available in English. Two authors (MM and MZ) independently screened the search results in a 2-step process based on predetermined inclusion/exclusion criteria. First, 890 articles were evaluated for relevance on title and abstract level, followed by full-text screening of the final list of 140 articles. Any disagreements were resolved by discussion or third-party review, and a total of 9 articles were included in the study.
The following eligibility criteria were used: original articles reporting adverse effects (AEs) of mutant EGFR-TK inhibitors in patients with advanced NSCLC compared with control groups receiving platinum-based chemotherapy. All the patients included in the study had an EGFR mutation but randomly assigned to either treatment or control group. All articles with subjective data on mutant EGFR-TK inhibitors AEs in patients with advanced NSCLC compared with control groups receiving platinum-based chemotherapy were included in the analysis. Only 9 articles qualified the aforementioned selection criteria for eligibility. All qualifying studies were nationwide inpatient or pooled clinical trials data. The reasons for exclusion of the other 71 articles were irrelevant (n = 31), duplicate (n = 13), reviews (n = 14), and poor data reporting (n = 12). Out of the 9 included studies, 9 studies showed correlation of AEs, including rash, diarrhea, nausea, and fatigue. Seven studies showed correlation of AEs including neutropenia, anorexia, and vomiting. Six studies showed correlation of anemia, cough, and stomatitis. Five studies showed correlation of elevated aspartate aminotransferase (AST), alanine aminotransferase (ALT), and leucopenia. Four studies showed correlation of fever between mutant EGFR-TK inhibitors and platinum-based chemotherapy.
The primary endpoints were reported AEs including rash, diarrhea, elevated ALT, elevated AST, stomatitis, nausea, leucopenia, fatigue, neutropenia, anorexia, anemia, cough, vomiting, and fever, respectively. Data on baseline characteristics and clinical outcomes were then extracted, and summary tables were created. Summary estimates of the clinical endpoints were then calculated with risk ratio (RR) and 95% confidence intervals (CIs) using the random-effects model. Heterogeneity between studies was examined with the Cochran Q I2 statistic which can be defined as low (25% to 50%), moderate (50% to 75%), or high (> 75%). Statistical analysis was performed using Comprehensive Meta-Analysis Software CMA Version 3.0.
Results
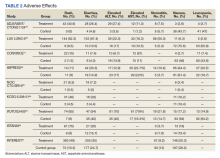
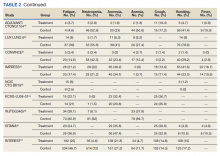
A total of 9 studies including 3415 patients (1775 in EGFR-TK inhibitor treatment group while 1640 patients in platinum-based chemotherapy control group) were included in the study. All 9 studies were phase III randomized control clinical trials conducted to compare the safety profile of mutant EGFR-TK inhibitors in patients with advanced NSCLC. Mean age was 61 years in both treatment and control groups. Further details on study and participant characteristics and safety profile including AEs are summarized in Tables 1 and 2. No evidence of publication bias was found.
Rash developed in 45.8% of patients in the treatment group receiving mutant EGFR-TK inhibitors vs only 5.6% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 7.38 with the 95% CI noted, which was statistically significant, confirming higher rash event rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 2).
Diarrhea occurred in 33.6% of patients in the mutant EGFR-TK inhibitors treatment group vs 13.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 2.63 and 95% CI was noted, which was statistically significant, confirming higher diarrheal rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 3).
Elevated ALT levels developed in 27.9% of patients in the treatment group receiving mutant EGFR-TK inhibitors compared with 15.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.37 and 95% CI was noted, which was statistically significant, confirming higher ALT levels in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 4).
Elevated AST levels occurred in 40.7% of patients in the mutant EGFR-TK inhibitors treatment group vs 12.8% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.77 and 95% CI was noted, which was statistically significant, confirming elevated AST levels in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 5).
Stomatitis developed in 17.2% of patients in the treatment group receiving mutant EGFR-TK inhibitors compared with 7.9% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.53 and 95% CI was noted, which was statistically significant, confirming higher stomatitis event rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 6).
Nausea occurred in 16.5% of patients in the mutant EGFR-TK inhibitors group vs 42.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.37 and 95% CI was noted, which was statistically significant, confirming higher nausea rates in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 7).
Leucopenia developed in 9.7% of patients in the mutant EGFR-TK inhibitors group compared with 51.3% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.18 and 95% CI was noted, which was statistically significant, confirming higher leucopenia incidence in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 8).
Fatigue was reported in 17% of patients in the mutant EGFR-TK inhibitors group compared with 29.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.59 and 95% CI was noted, which was statistically significant, confirming higher fatigue rates in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 9).
Neutropenia developed in 6.1% of patients in the mutant EGFR-TK inhibitors group vs 48.2% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.11 and 95% CI was noted, which was statistically significant, confirming higher neutropenia rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 10).
Anorexia developed in 21.3% of patients in the mutant EGFR-TK inhibitors group vs 31.4% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.44 and 95% CI was noted, which was statistically significant, confirming higher anorexia rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 11).
Anemia occurred in 8.7% of patients in the mutant EGFR-TK inhibitors group compared with 32.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.24 and 95% CI was noted, which was statistically significant, confirming higher anorexia rates in patients receiving platinum-based chemotherapy compared with treatment for their advanced NSCLC (Figure 12).
Cough was reported in 17.8% of patients in the mutant EGFR-TK inhibitors group compared with 18.9% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.99 and 95% CI was noted, which was statistically significant, confirming slightly higher cough rates in patients receiving platinum-based chemotherapy compared with treatment for their advanced NSCLC (Figure 13).
Vomiting developed in 11% of patients in the mutant EGFR-TK inhibitors group vs 30.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.35 and 95% CI was noted, which was statistically significant, confirming higher vomiting rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 14).
Fever occurred in 5.6% of patients in the mutant EGFR-TK inhibitors group compared with 30.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.41 and 95% CI was noted, which was statistically significant, confirming higher fever rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 15).
Discussion
Despite the advancement in the treatment of metastatic NSCLC, lung cancer stays as most common cause of cancer-related death in North America and European countries, as patients usually have an advanced disease at the time of diagnosis.3 In the past, platinum-based chemotherapy remained the standard of care for most of the patients affected with advanced NSCLC, but the higher recurrence rate and increase in frequency and intensity of AEs with platinum-based chemotherapy led to the development of targeted therapy for NSCLC, one of which includes
Smoking is the most common reversible risk factor associated with lung cancer. The EURTAC trial was the first perspective study in this regard, which compared safety and efficacy of mutant EGFR-TK inhibitors with platinum-based chemotherapy. Results analyzed in this study were in favor of mutant EGFR-TK inhibitors except in the group of former smokers.5 On the contrary, the OPTIMAL trial showed results in favor of mutant EGFR-TK inhibitors both in active and former smokers; this trial also confirmed the efficacy of mutant EGFR-TK inhibitors in European and Asian populations, confirming the rationale for routine testing of EGFR mutation in all the patients being diagnosed with advanced NSCLC.6 Similarly, osimertinib is one of the most recent mutant EGFR-TK inhibitors developed for the treatment of advanced NSCLC in patients with EGFR-positive receptors.
According to the FLAURA trial, patients receiving osimertinib showed significantly longer progression-free survival compared with platinum-based chemotherapy and early mutant EGFR-TK inhibitors. Median progression-free survival was noted to be 18.9 months, which showed 54% lower risk of disease progression in the treatment group receiving osimertinib.7 The ARCHER study emphasized a significant improvement in overall survival as well as progression-free survival among a patient population receiving dacomitinib compared with platinum-based chemotherapy.8,9
Being a potent targeted therapy, mutant EGFR-TK inhibitors do come with some AEs including diarrhea, which was seen in 33.6% of the patients receiving mutant EGFR-TK inhibitors in our study vs 53% in the chemotherapy group, as was observed in the study conducted by Pless and colleagues.10 Similarly, only 16.5% of patients receiving mutant EGFR-TK inhibitors developed nausea compared with 66% being observed in patients receiving chemotherapy. Correspondingly, only a small fraction of patients (9.7%) receiving mutant EGFR-TK inhibitors developed leucopenia, which was 10 times less reported in mutant EGFR-TK inhibitors compared with patients receiving chemotherapy having a percentage of 100%. A similar trend was reported for neutropenia and anemia in mutant EGFR-TK inhibitors with an incidence of 6.1% and 8.7%, compared with the platinum-based chemotherapy group in which the incidence was found to be 80% and 100%, respectively. It was concluded that platinum-based chemotherapy had played a vital role in the treatment of advanced NSCLC but at an expense of serious and severe AEs which led to discontinuation or withdrawal of treatment, leading to relapse and recurrence of lung cancer.10,11
Zhong and colleagues conducted a phase 2 randomized clinical trial comparing mutant EGFR-TK inhibitors with platinum-based chemotherapy. They concluded that in patients receiving platinum-based chemotherapy, incidence of rash, vomiting, anorexia, neutropenia, and nausea were 29.4%, 47%, 41.2%, 55.8%, and 32.4% compared with 45.8%, 11%, 21.3%, 6.1%, and 16.5%, respectively, reported in patients receiving mutant EGFR-TK inhibitors for their advanced NSCLC.12
Another study was conducted in 2019 by Noronha and colleagues to determine the impact of platinum-based chemotherapy combined with gefitinib on patients with advanced NSCLC.13 They concluded that 70% of the patients receiving combination treatment developed rash, which was significantly higher compared with 45.8% patients receiving the mutant EGFR-TK inhibitors alone in our study. Also, 56% of patients receiving combination therapy developed diarrhea vs 33.6% of patients receiving mutant EGFR-TK inhibitors only. Similarly, 96% of patients in the combination therapy group developed some degree of anemia compared with only 8.7% patients in the mutant EGFR-TK inhibitors group included in our study. In the same way, neutropenia was observed in 55% of patients receiving combination therapy vs 6.1% in patients receiving mutant EGFR-TK inhibitors solely. They concluded that mutant EGFR-TK inhibitors when combined with platinum-based chemotherapy increase the incidence of AEs of chemotherapy by many folds.13,14
Kato and colleagues conducted a study to determine the impact on AEs when erlotinib was combined with anti–vascular endothelial growth factor (VEGF) inhibitors like bevacizumab, they stated that 98.7% of patient in combination therapy developed rash, the incidence of which was only 45.8% in patients receiving mutant EGFR-TK inhibitors as was observed in our study. Similar trends were noticed with other AEs, including diarrhea, fatigue, nausea, and elevated liver enzymes.15
With the latest advancements in the management of advanced NSCLC, nivolumab, a programmed death ligand 1 (PD-L1) inhibitor, was developed and either used as monotherapy in patients with PD-L1 expression or was combined with platinum-based chemotherapy regardless of PD-L1 expression.16,17 Patients expressing lower PD-L1 levels were not omitted from receiving nivolumab as no significant difference was noted in progression-free span and overall survival in patients receiving nivolumab irrespective of PD-L1 levels.15 Rash developed in 17% of patients after receiving nivolumab vs 45.8% patients being observed in our study. A similar trend was observed with diarrhea as only 17% of the population receiving nivolumab developed diarrhea compared with 33.6% of the population receiving mutant EGFR-TK inhibitors in our study. Likewise, only 9.9% of the patients receiving nivolumab developed nausea as an AE compared with 16.5% being observed in mutant EGFR-TK inhibitors in our study. Also, fatigue was observed in 14.4% of the population receiving nivolumab vs 17% observed in patients receiving mutant EGFR-TK inhibitors as was noticed in our study.7,8
Rizvi and colleagues conducted a study on the role of nivolumab when combined with platinum-based chemotherapy in patients with advanced NSCLC and reported that 40% of patients included in the study developed rash compared with 45.8% reported in mutant EGFR-TK inhibitors in our study. Similarly, only 13% of patients in the nivolumab group developed diarrhea vs 33.6% cases reported in the mutant EGFR-TK inhibitors group included in our study. Also, 7% of patients in the nivolumab group developed elevated ALT levels vs 27.9% of patients receiving mutant EGFR-TK inhibitors included in our study, concluding that addition of immune checkpoint inhibitors like nivolumab to platinum-based chemotherapy does not increase the frequency of AEs.18
Conclusions
Our study focused on the safety profile of mutant EGFR-TK inhibitors vs platinum-based chemotherapy in the treatment of advanced NSCLC. Mutant EGFR-TK inhibitors are safer than platinum-based chemotherapy when compared for nausea, leucopenia, fatigue, neutropenia, anorexia, anemia, cough, vomiting, and fever. On the other end, mutant EGFR-TK inhibitors cause slightly higher AEs, including rash, diarrhea, elevated AST and ALT levels, and stomatitis. However, considering that the development of mutant EGFR-TK inhibitors laid a foundation of targeted therapy, we recommend continuing using mutant EGFR-TK inhibitors in patients with advanced NSCLC especially in patients having mutant EGFR receptors. AEs caused by mutant EGFR-TK inhibitors are significant but are usually tolerable and can be avoided by reducing the dosage of it with each cycle or by skipping or delaying the dose until the patient is symptomatic.
1. Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913-2921. doi:10.1158/0008-5472.CAN-14-0155
2. da Cunha Santos G, Shepherd FA, Tsao MS. EGFR mutations and lung cancer. Annu Rev Pathol. 2011;6:49-69. doi:10.1146/annurev-pathol-011110-130206
3. Sgambato A, Casaluce F, Maione P, et al. The role of EGFR tyrosine kinase inhibitors in the first-line treatment of advanced non small cell lung cancer patients harboring EGFR mutation. Curr Med Chem. 2012;19(20):3337-3352. doi:10.2174/092986712801215973
4. Rossi A, Di Maio M. Platinum-based chemotherapy in advanced non–small-cell lung cancer: optimal number of treatment cycles. Expert Rev Anticancer Ther. 2016;16(6):653-660. doi:10.1586/14737140.2016.1170596
5. Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non–small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239-246. doi:10.1016/S1470-2045(11)70393-X
6. Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non–small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735-742. doi:10.1016/S1470-2045(11)70184-X
7. Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non–small-cell lung cancer. N Engl J Med. 2018;378(2):113-125. doi:10.1056/NEJMoa1713137
8. Mok TS, Cheng Y, Zhou X, et al. Improvement in overall survival in a randomized study that compared dacomitinib with gefitinib in patients with advanced non–small-cell lung cancer and EGFR-activating mutations. J Clin Oncol. 2018;36(22):2244-2250. doi:10.1200/JCO.2018.78.7994
9. Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947-957. doi:10.1056/NEJMoa0810699
10. Pless M, Stupp R, Ris HB, et al. Induction chemoradiation in stage IIIA/N2 non–small-cell lung cancer: a phase 3 randomised trial. Lancet. 2015;386(9998):1049-1056. doi:10.1016/S0140-6736(15)60294-X
11. Albain KS, Rusch VW, Crowley JJ, et al. Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stages IIIA (N2) and IIIB non–small-cell lung cancer: mature results of Southwest Oncology Group phase II study 8805. J Clin Oncol. 1995;13(8):1880-1892. doi:10.1200/JCO.1995.13.8.1880
12. Zhong WZ, Chen KN, Chen C, et al. Erlotinib versus gemcitabine plus cisplatin as neoadjuvant treatment of Stage IIIA-N2 EGFR-mutant non–small-cell lung cancer (EMERGING-CTONG 1103): a randomized phase II study. J Clin Oncol. 2019;37(25):2235-2245. doi:10.1200/JCO.19.00075
13. Noronha V, Patil VM, Joshi A, et al. Gefitinib versus gefitinib plus pemetrexed and carboplatin chemotherapy in EGFR-mutated lung cancer. J Clin Oncol. 2020;38(2):124-136. doi:10.1200/JCO.19.01154
14. Noronha V, Prabhash K, Thavamani A, et al. EGFR mutations in Indian lung cancer patients: clinical correlation and outcome to EGFR targeted therapy. PLoS One. 2013;8(4):e61561. Published 2013 Apr 19. doi:10.1371/journal.pone.0061561
15. Kato T, Seto T, Nishio M, et al. Erlotinib plus bevacizumab phase ll study in patients with advanced non–small-cell lung cancer (JO25567): updated safety results. Drug Saf. 2018;41(2):229-237. doi:10.1007/s40264-017-0596-0
16. Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non–small-cell lung cancer. N Engl J Med. 2019;381(21):2020-2031. doi:10.1056/NEJMoa1910231
17. Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378(22):2093-2104. doi:10.1056/NEJMoa1801946
18. Rizvi NA, Hellmann MD, Brahmer JR, et al. Nivolumab in combination with platinum-based doublet chemotherapy for first-line treatment of advanced non–small-cell lung cancer. J Clin Oncol. 2016;34(25):2969-2979. doi:10.1200/JCO.2016.66.9861
19. Zhong WZ, Wang Q, Mao WM, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC: final overall survival analysis of CTONG1104 Phase III Trial. J Clin Oncol. 2021;39(7):713-722. doi:10.1200/JCO.20.01820
20. Yang JC, Sequist LV, Geater SL, et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol. 2015;16(7):830-838. doi:10.1016/S1470-2045(15)00026-1
21. Shi YK, Wang L, Han BH, et al. First-line icotinib versus cisplatin/pemetrexed plus pemetrexed maintenance therapy for patients with advanced EGFR mutation-positive lung adenocarcinoma (CONVINCE): a phase 3, open-label, randomized study. Ann Oncol. 2017;28(10):2443-2450. doi:10.1093/annonc/mdx359
22. Soria JC, Wu YL, Nakagawa K, et al. Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on first-line gefitinib (IMPRESS): a phase 3 randomized trial. Lancet Oncol. 2015;16(8):990-998 doi:10.1016/S1470-2045(15)00121-7
23. Goss GD, O’Callaghan C, Lorimer I, et al. Gefitinib versus placebo in completely resected non-small-cell lung cancer: results of the NCIC CTG BR19 study. J Clin Oncol. 2013;31(27):3320-3326. doi:10.1200/JCO.2013.51.1816
24. Sun JM, Lee KH, Kim SW, et al. Gefitinib versus pemetrexed as second-line treatment in patients with non-small cell lung cancer previously treated with platinum-based chemotherapy (KCSG-LU08-01): an open-label, phase 3 trial. Cancer. 2012;118(24):6234-6242. doi:10.1200/JCO.2013.51.1816
25. Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomized phase 3 trial. Lancet Oncol. 2010;11(2):121-128. doi:10.1016/S1470-2045(09)70364-X
26. Lee DH, Park K, Kim JH, Lee JS, et al. Randomized phase III trial of gefitinib versus docetaxel in non-small cell lung cancer patients who have previously received platinum-based chemotherapy. Clin Cancer Res. 2010;16(4):1307-1314. doi:10.1158/1078-0432.CCR-09-1903
27. Kim ES, Hirsh V, Mok T, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomized phase III trial. Lancet. 2008;22;372(9652):1809-1818. doi:10.1016/S0140-6736(08)61758-4
Lung cancer has been the leading cause of cancer-related mortality for decades. It is also predicted to remain as the leading cause of cancer-related mortality through 2030.1 Platinum-based chemotherapy, including carboplatin and paclitaxel, was introduced 3 decades ago and revolutionized the management of advanced non–small cell lung cancer (NSCLC). A more recent advancement has been mutant epidermal growth factor receptor–tyrosine kinase (EGFR-TK) inhibitors.1 EGFR is a transmembrane protein that functions by transducing essential growth factor signaling from the extracellular milieu to the cell. As 60% of the advanced NSCLC expresses this receptor, blocking the mutant EGFR receptor was a groundbreaking development in the management of advanced NSCLC.2 Development of mutant EGFR-TK inhibitors has revolutionized the management of advanced NSCLC. This study was conducted to determine the safety profile of mutant EGFR-TK inhibitors in the management of advanced NSCLC.
Methods
This meta-analysis was conducted according to Cochrane Collaboration guidelines and reported as per Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The findings are summarized in the PRISMA flow diagram (Figure 1). Two authors (MZ and MM) performed a systematic literature search using databases such as MEDLINE (via PubMed), Embase, and Cochrane Library using the medical search terms and their respective entry words with the following search strategy: safety, “mutant EGFR-TK inhibitors,” advanced, “non–small cell,” “lung cancer,” “adverse effect,” and literature. Additionally, unpublished trials were identified from clinicaltrials.gov, and references of all pertinent articles were also scrutinized to ensure the inclusion of all relevant studies. The search was completed on June 1, 2021, and we only included studies available in English. Two authors (MM and MZ) independently screened the search results in a 2-step process based on predetermined inclusion/exclusion criteria. First, 890 articles were evaluated for relevance on title and abstract level, followed by full-text screening of the final list of 140 articles. Any disagreements were resolved by discussion or third-party review, and a total of 9 articles were included in the study.
The following eligibility criteria were used: original articles reporting adverse effects (AEs) of mutant EGFR-TK inhibitors in patients with advanced NSCLC compared with control groups receiving platinum-based chemotherapy. All the patients included in the study had an EGFR mutation but randomly assigned to either treatment or control group. All articles with subjective data on mutant EGFR-TK inhibitors AEs in patients with advanced NSCLC compared with control groups receiving platinum-based chemotherapy were included in the analysis. Only 9 articles qualified the aforementioned selection criteria for eligibility. All qualifying studies were nationwide inpatient or pooled clinical trials data. The reasons for exclusion of the other 71 articles were irrelevant (n = 31), duplicate (n = 13), reviews (n = 14), and poor data reporting (n = 12). Out of the 9 included studies, 9 studies showed correlation of AEs, including rash, diarrhea, nausea, and fatigue. Seven studies showed correlation of AEs including neutropenia, anorexia, and vomiting. Six studies showed correlation of anemia, cough, and stomatitis. Five studies showed correlation of elevated aspartate aminotransferase (AST), alanine aminotransferase (ALT), and leucopenia. Four studies showed correlation of fever between mutant EGFR-TK inhibitors and platinum-based chemotherapy.
The primary endpoints were reported AEs including rash, diarrhea, elevated ALT, elevated AST, stomatitis, nausea, leucopenia, fatigue, neutropenia, anorexia, anemia, cough, vomiting, and fever, respectively. Data on baseline characteristics and clinical outcomes were then extracted, and summary tables were created. Summary estimates of the clinical endpoints were then calculated with risk ratio (RR) and 95% confidence intervals (CIs) using the random-effects model. Heterogeneity between studies was examined with the Cochran Q I2 statistic which can be defined as low (25% to 50%), moderate (50% to 75%), or high (> 75%). Statistical analysis was performed using Comprehensive Meta-Analysis Software CMA Version 3.0.
Results
A total of 9 studies including 3415 patients (1775 in EGFR-TK inhibitor treatment group while 1640 patients in platinum-based chemotherapy control group) were included in the study. All 9 studies were phase III randomized control clinical trials conducted to compare the safety profile of mutant EGFR-TK inhibitors in patients with advanced NSCLC. Mean age was 61 years in both treatment and control groups. Further details on study and participant characteristics and safety profile including AEs are summarized in Tables 1 and 2. No evidence of publication bias was found.
Rash developed in 45.8% of patients in the treatment group receiving mutant EGFR-TK inhibitors vs only 5.6% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 7.38 with the 95% CI noted, which was statistically significant, confirming higher rash event rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 2).
Diarrhea occurred in 33.6% of patients in the mutant EGFR-TK inhibitors treatment group vs 13.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 2.63 and 95% CI was noted, which was statistically significant, confirming higher diarrheal rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 3).
Elevated ALT levels developed in 27.9% of patients in the treatment group receiving mutant EGFR-TK inhibitors compared with 15.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.37 and 95% CI was noted, which was statistically significant, confirming higher ALT levels in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 4).
Elevated AST levels occurred in 40.7% of patients in the mutant EGFR-TK inhibitors treatment group vs 12.8% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.77 and 95% CI was noted, which was statistically significant, confirming elevated AST levels in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 5).
Stomatitis developed in 17.2% of patients in the treatment group receiving mutant EGFR-TK inhibitors compared with 7.9% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.53 and 95% CI was noted, which was statistically significant, confirming higher stomatitis event rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 6).
Nausea occurred in 16.5% of patients in the mutant EGFR-TK inhibitors group vs 42.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.37 and 95% CI was noted, which was statistically significant, confirming higher nausea rates in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 7).
Leucopenia developed in 9.7% of patients in the mutant EGFR-TK inhibitors group compared with 51.3% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.18 and 95% CI was noted, which was statistically significant, confirming higher leucopenia incidence in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 8).
Fatigue was reported in 17% of patients in the mutant EGFR-TK inhibitors group compared with 29.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.59 and 95% CI was noted, which was statistically significant, confirming higher fatigue rates in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 9).
Neutropenia developed in 6.1% of patients in the mutant EGFR-TK inhibitors group vs 48.2% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.11 and 95% CI was noted, which was statistically significant, confirming higher neutropenia rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 10).
Anorexia developed in 21.3% of patients in the mutant EGFR-TK inhibitors group vs 31.4% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.44 and 95% CI was noted, which was statistically significant, confirming higher anorexia rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 11).
Anemia occurred in 8.7% of patients in the mutant EGFR-TK inhibitors group compared with 32.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.24 and 95% CI was noted, which was statistically significant, confirming higher anorexia rates in patients receiving platinum-based chemotherapy compared with treatment for their advanced NSCLC (Figure 12).
Cough was reported in 17.8% of patients in the mutant EGFR-TK inhibitors group compared with 18.9% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.99 and 95% CI was noted, which was statistically significant, confirming slightly higher cough rates in patients receiving platinum-based chemotherapy compared with treatment for their advanced NSCLC (Figure 13).
Vomiting developed in 11% of patients in the mutant EGFR-TK inhibitors group vs 30.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.35 and 95% CI was noted, which was statistically significant, confirming higher vomiting rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 14).
Fever occurred in 5.6% of patients in the mutant EGFR-TK inhibitors group compared with 30.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.41 and 95% CI was noted, which was statistically significant, confirming higher fever rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 15).
Discussion
Despite the advancement in the treatment of metastatic NSCLC, lung cancer stays as most common cause of cancer-related death in North America and European countries, as patients usually have an advanced disease at the time of diagnosis.3 In the past, platinum-based chemotherapy remained the standard of care for most of the patients affected with advanced NSCLC, but the higher recurrence rate and increase in frequency and intensity of AEs with platinum-based chemotherapy led to the development of targeted therapy for NSCLC, one of which includes
Smoking is the most common reversible risk factor associated with lung cancer. The EURTAC trial was the first perspective study in this regard, which compared safety and efficacy of mutant EGFR-TK inhibitors with platinum-based chemotherapy. Results analyzed in this study were in favor of mutant EGFR-TK inhibitors except in the group of former smokers.5 On the contrary, the OPTIMAL trial showed results in favor of mutant EGFR-TK inhibitors both in active and former smokers; this trial also confirmed the efficacy of mutant EGFR-TK inhibitors in European and Asian populations, confirming the rationale for routine testing of EGFR mutation in all the patients being diagnosed with advanced NSCLC.6 Similarly, osimertinib is one of the most recent mutant EGFR-TK inhibitors developed for the treatment of advanced NSCLC in patients with EGFR-positive receptors.
According to the FLAURA trial, patients receiving osimertinib showed significantly longer progression-free survival compared with platinum-based chemotherapy and early mutant EGFR-TK inhibitors. Median progression-free survival was noted to be 18.9 months, which showed 54% lower risk of disease progression in the treatment group receiving osimertinib.7 The ARCHER study emphasized a significant improvement in overall survival as well as progression-free survival among a patient population receiving dacomitinib compared with platinum-based chemotherapy.8,9
Being a potent targeted therapy, mutant EGFR-TK inhibitors do come with some AEs including diarrhea, which was seen in 33.6% of the patients receiving mutant EGFR-TK inhibitors in our study vs 53% in the chemotherapy group, as was observed in the study conducted by Pless and colleagues.10 Similarly, only 16.5% of patients receiving mutant EGFR-TK inhibitors developed nausea compared with 66% being observed in patients receiving chemotherapy. Correspondingly, only a small fraction of patients (9.7%) receiving mutant EGFR-TK inhibitors developed leucopenia, which was 10 times less reported in mutant EGFR-TK inhibitors compared with patients receiving chemotherapy having a percentage of 100%. A similar trend was reported for neutropenia and anemia in mutant EGFR-TK inhibitors with an incidence of 6.1% and 8.7%, compared with the platinum-based chemotherapy group in which the incidence was found to be 80% and 100%, respectively. It was concluded that platinum-based chemotherapy had played a vital role in the treatment of advanced NSCLC but at an expense of serious and severe AEs which led to discontinuation or withdrawal of treatment, leading to relapse and recurrence of lung cancer.10,11
Zhong and colleagues conducted a phase 2 randomized clinical trial comparing mutant EGFR-TK inhibitors with platinum-based chemotherapy. They concluded that in patients receiving platinum-based chemotherapy, incidence of rash, vomiting, anorexia, neutropenia, and nausea were 29.4%, 47%, 41.2%, 55.8%, and 32.4% compared with 45.8%, 11%, 21.3%, 6.1%, and 16.5%, respectively, reported in patients receiving mutant EGFR-TK inhibitors for their advanced NSCLC.12
Another study was conducted in 2019 by Noronha and colleagues to determine the impact of platinum-based chemotherapy combined with gefitinib on patients with advanced NSCLC.13 They concluded that 70% of the patients receiving combination treatment developed rash, which was significantly higher compared with 45.8% patients receiving the mutant EGFR-TK inhibitors alone in our study. Also, 56% of patients receiving combination therapy developed diarrhea vs 33.6% of patients receiving mutant EGFR-TK inhibitors only. Similarly, 96% of patients in the combination therapy group developed some degree of anemia compared with only 8.7% patients in the mutant EGFR-TK inhibitors group included in our study. In the same way, neutropenia was observed in 55% of patients receiving combination therapy vs 6.1% in patients receiving mutant EGFR-TK inhibitors solely. They concluded that mutant EGFR-TK inhibitors when combined with platinum-based chemotherapy increase the incidence of AEs of chemotherapy by many folds.13,14
Kato and colleagues conducted a study to determine the impact on AEs when erlotinib was combined with anti–vascular endothelial growth factor (VEGF) inhibitors like bevacizumab, they stated that 98.7% of patient in combination therapy developed rash, the incidence of which was only 45.8% in patients receiving mutant EGFR-TK inhibitors as was observed in our study. Similar trends were noticed with other AEs, including diarrhea, fatigue, nausea, and elevated liver enzymes.15
With the latest advancements in the management of advanced NSCLC, nivolumab, a programmed death ligand 1 (PD-L1) inhibitor, was developed and either used as monotherapy in patients with PD-L1 expression or was combined with platinum-based chemotherapy regardless of PD-L1 expression.16,17 Patients expressing lower PD-L1 levels were not omitted from receiving nivolumab as no significant difference was noted in progression-free span and overall survival in patients receiving nivolumab irrespective of PD-L1 levels.15 Rash developed in 17% of patients after receiving nivolumab vs 45.8% patients being observed in our study. A similar trend was observed with diarrhea as only 17% of the population receiving nivolumab developed diarrhea compared with 33.6% of the population receiving mutant EGFR-TK inhibitors in our study. Likewise, only 9.9% of the patients receiving nivolumab developed nausea as an AE compared with 16.5% being observed in mutant EGFR-TK inhibitors in our study. Also, fatigue was observed in 14.4% of the population receiving nivolumab vs 17% observed in patients receiving mutant EGFR-TK inhibitors as was noticed in our study.7,8
Rizvi and colleagues conducted a study on the role of nivolumab when combined with platinum-based chemotherapy in patients with advanced NSCLC and reported that 40% of patients included in the study developed rash compared with 45.8% reported in mutant EGFR-TK inhibitors in our study. Similarly, only 13% of patients in the nivolumab group developed diarrhea vs 33.6% cases reported in the mutant EGFR-TK inhibitors group included in our study. Also, 7% of patients in the nivolumab group developed elevated ALT levels vs 27.9% of patients receiving mutant EGFR-TK inhibitors included in our study, concluding that addition of immune checkpoint inhibitors like nivolumab to platinum-based chemotherapy does not increase the frequency of AEs.18
Conclusions
Our study focused on the safety profile of mutant EGFR-TK inhibitors vs platinum-based chemotherapy in the treatment of advanced NSCLC. Mutant EGFR-TK inhibitors are safer than platinum-based chemotherapy when compared for nausea, leucopenia, fatigue, neutropenia, anorexia, anemia, cough, vomiting, and fever. On the other end, mutant EGFR-TK inhibitors cause slightly higher AEs, including rash, diarrhea, elevated AST and ALT levels, and stomatitis. However, considering that the development of mutant EGFR-TK inhibitors laid a foundation of targeted therapy, we recommend continuing using mutant EGFR-TK inhibitors in patients with advanced NSCLC especially in patients having mutant EGFR receptors. AEs caused by mutant EGFR-TK inhibitors are significant but are usually tolerable and can be avoided by reducing the dosage of it with each cycle or by skipping or delaying the dose until the patient is symptomatic.
Lung cancer has been the leading cause of cancer-related mortality for decades. It is also predicted to remain as the leading cause of cancer-related mortality through 2030.1 Platinum-based chemotherapy, including carboplatin and paclitaxel, was introduced 3 decades ago and revolutionized the management of advanced non–small cell lung cancer (NSCLC). A more recent advancement has been mutant epidermal growth factor receptor–tyrosine kinase (EGFR-TK) inhibitors.1 EGFR is a transmembrane protein that functions by transducing essential growth factor signaling from the extracellular milieu to the cell. As 60% of the advanced NSCLC expresses this receptor, blocking the mutant EGFR receptor was a groundbreaking development in the management of advanced NSCLC.2 Development of mutant EGFR-TK inhibitors has revolutionized the management of advanced NSCLC. This study was conducted to determine the safety profile of mutant EGFR-TK inhibitors in the management of advanced NSCLC.
Methods
This meta-analysis was conducted according to Cochrane Collaboration guidelines and reported as per Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The findings are summarized in the PRISMA flow diagram (Figure 1). Two authors (MZ and MM) performed a systematic literature search using databases such as MEDLINE (via PubMed), Embase, and Cochrane Library using the medical search terms and their respective entry words with the following search strategy: safety, “mutant EGFR-TK inhibitors,” advanced, “non–small cell,” “lung cancer,” “adverse effect,” and literature. Additionally, unpublished trials were identified from clinicaltrials.gov, and references of all pertinent articles were also scrutinized to ensure the inclusion of all relevant studies. The search was completed on June 1, 2021, and we only included studies available in English. Two authors (MM and MZ) independently screened the search results in a 2-step process based on predetermined inclusion/exclusion criteria. First, 890 articles were evaluated for relevance on title and abstract level, followed by full-text screening of the final list of 140 articles. Any disagreements were resolved by discussion or third-party review, and a total of 9 articles were included in the study.
The following eligibility criteria were used: original articles reporting adverse effects (AEs) of mutant EGFR-TK inhibitors in patients with advanced NSCLC compared with control groups receiving platinum-based chemotherapy. All the patients included in the study had an EGFR mutation but randomly assigned to either treatment or control group. All articles with subjective data on mutant EGFR-TK inhibitors AEs in patients with advanced NSCLC compared with control groups receiving platinum-based chemotherapy were included in the analysis. Only 9 articles qualified the aforementioned selection criteria for eligibility. All qualifying studies were nationwide inpatient or pooled clinical trials data. The reasons for exclusion of the other 71 articles were irrelevant (n = 31), duplicate (n = 13), reviews (n = 14), and poor data reporting (n = 12). Out of the 9 included studies, 9 studies showed correlation of AEs, including rash, diarrhea, nausea, and fatigue. Seven studies showed correlation of AEs including neutropenia, anorexia, and vomiting. Six studies showed correlation of anemia, cough, and stomatitis. Five studies showed correlation of elevated aspartate aminotransferase (AST), alanine aminotransferase (ALT), and leucopenia. Four studies showed correlation of fever between mutant EGFR-TK inhibitors and platinum-based chemotherapy.
The primary endpoints were reported AEs including rash, diarrhea, elevated ALT, elevated AST, stomatitis, nausea, leucopenia, fatigue, neutropenia, anorexia, anemia, cough, vomiting, and fever, respectively. Data on baseline characteristics and clinical outcomes were then extracted, and summary tables were created. Summary estimates of the clinical endpoints were then calculated with risk ratio (RR) and 95% confidence intervals (CIs) using the random-effects model. Heterogeneity between studies was examined with the Cochran Q I2 statistic which can be defined as low (25% to 50%), moderate (50% to 75%), or high (> 75%). Statistical analysis was performed using Comprehensive Meta-Analysis Software CMA Version 3.0.
Results
A total of 9 studies including 3415 patients (1775 in EGFR-TK inhibitor treatment group while 1640 patients in platinum-based chemotherapy control group) were included in the study. All 9 studies were phase III randomized control clinical trials conducted to compare the safety profile of mutant EGFR-TK inhibitors in patients with advanced NSCLC. Mean age was 61 years in both treatment and control groups. Further details on study and participant characteristics and safety profile including AEs are summarized in Tables 1 and 2. No evidence of publication bias was found.
Rash developed in 45.8% of patients in the treatment group receiving mutant EGFR-TK inhibitors vs only 5.6% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 7.38 with the 95% CI noted, which was statistically significant, confirming higher rash event rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 2).
Diarrhea occurred in 33.6% of patients in the mutant EGFR-TK inhibitors treatment group vs 13.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 2.63 and 95% CI was noted, which was statistically significant, confirming higher diarrheal rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 3).
Elevated ALT levels developed in 27.9% of patients in the treatment group receiving mutant EGFR-TK inhibitors compared with 15.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.37 and 95% CI was noted, which was statistically significant, confirming higher ALT levels in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 4).
Elevated AST levels occurred in 40.7% of patients in the mutant EGFR-TK inhibitors treatment group vs 12.8% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.77 and 95% CI was noted, which was statistically significant, confirming elevated AST levels in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 5).
Stomatitis developed in 17.2% of patients in the treatment group receiving mutant EGFR-TK inhibitors compared with 7.9% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.53 and 95% CI was noted, which was statistically significant, confirming higher stomatitis event rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 6).
Nausea occurred in 16.5% of patients in the mutant EGFR-TK inhibitors group vs 42.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.37 and 95% CI was noted, which was statistically significant, confirming higher nausea rates in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 7).
Leucopenia developed in 9.7% of patients in the mutant EGFR-TK inhibitors group compared with 51.3% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.18 and 95% CI was noted, which was statistically significant, confirming higher leucopenia incidence in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 8).
Fatigue was reported in 17% of patients in the mutant EGFR-TK inhibitors group compared with 29.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.59 and 95% CI was noted, which was statistically significant, confirming higher fatigue rates in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 9).
Neutropenia developed in 6.1% of patients in the mutant EGFR-TK inhibitors group vs 48.2% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.11 and 95% CI was noted, which was statistically significant, confirming higher neutropenia rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 10).
Anorexia developed in 21.3% of patients in the mutant EGFR-TK inhibitors group vs 31.4% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.44 and 95% CI was noted, which was statistically significant, confirming higher anorexia rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 11).
Anemia occurred in 8.7% of patients in the mutant EGFR-TK inhibitors group compared with 32.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.24 and 95% CI was noted, which was statistically significant, confirming higher anorexia rates in patients receiving platinum-based chemotherapy compared with treatment for their advanced NSCLC (Figure 12).
Cough was reported in 17.8% of patients in the mutant EGFR-TK inhibitors group compared with 18.9% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.99 and 95% CI was noted, which was statistically significant, confirming slightly higher cough rates in patients receiving platinum-based chemotherapy compared with treatment for their advanced NSCLC (Figure 13).
Vomiting developed in 11% of patients in the mutant EGFR-TK inhibitors group vs 30.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.35 and 95% CI was noted, which was statistically significant, confirming higher vomiting rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 14).
Fever occurred in 5.6% of patients in the mutant EGFR-TK inhibitors group compared with 30.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.41 and 95% CI was noted, which was statistically significant, confirming higher fever rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 15).
Discussion
Despite the advancement in the treatment of metastatic NSCLC, lung cancer stays as most common cause of cancer-related death in North America and European countries, as patients usually have an advanced disease at the time of diagnosis.3 In the past, platinum-based chemotherapy remained the standard of care for most of the patients affected with advanced NSCLC, but the higher recurrence rate and increase in frequency and intensity of AEs with platinum-based chemotherapy led to the development of targeted therapy for NSCLC, one of which includes
Smoking is the most common reversible risk factor associated with lung cancer. The EURTAC trial was the first perspective study in this regard, which compared safety and efficacy of mutant EGFR-TK inhibitors with platinum-based chemotherapy. Results analyzed in this study were in favor of mutant EGFR-TK inhibitors except in the group of former smokers.5 On the contrary, the OPTIMAL trial showed results in favor of mutant EGFR-TK inhibitors both in active and former smokers; this trial also confirmed the efficacy of mutant EGFR-TK inhibitors in European and Asian populations, confirming the rationale for routine testing of EGFR mutation in all the patients being diagnosed with advanced NSCLC.6 Similarly, osimertinib is one of the most recent mutant EGFR-TK inhibitors developed for the treatment of advanced NSCLC in patients with EGFR-positive receptors.
According to the FLAURA trial, patients receiving osimertinib showed significantly longer progression-free survival compared with platinum-based chemotherapy and early mutant EGFR-TK inhibitors. Median progression-free survival was noted to be 18.9 months, which showed 54% lower risk of disease progression in the treatment group receiving osimertinib.7 The ARCHER study emphasized a significant improvement in overall survival as well as progression-free survival among a patient population receiving dacomitinib compared with platinum-based chemotherapy.8,9
Being a potent targeted therapy, mutant EGFR-TK inhibitors do come with some AEs including diarrhea, which was seen in 33.6% of the patients receiving mutant EGFR-TK inhibitors in our study vs 53% in the chemotherapy group, as was observed in the study conducted by Pless and colleagues.10 Similarly, only 16.5% of patients receiving mutant EGFR-TK inhibitors developed nausea compared with 66% being observed in patients receiving chemotherapy. Correspondingly, only a small fraction of patients (9.7%) receiving mutant EGFR-TK inhibitors developed leucopenia, which was 10 times less reported in mutant EGFR-TK inhibitors compared with patients receiving chemotherapy having a percentage of 100%. A similar trend was reported for neutropenia and anemia in mutant EGFR-TK inhibitors with an incidence of 6.1% and 8.7%, compared with the platinum-based chemotherapy group in which the incidence was found to be 80% and 100%, respectively. It was concluded that platinum-based chemotherapy had played a vital role in the treatment of advanced NSCLC but at an expense of serious and severe AEs which led to discontinuation or withdrawal of treatment, leading to relapse and recurrence of lung cancer.10,11
Zhong and colleagues conducted a phase 2 randomized clinical trial comparing mutant EGFR-TK inhibitors with platinum-based chemotherapy. They concluded that in patients receiving platinum-based chemotherapy, incidence of rash, vomiting, anorexia, neutropenia, and nausea were 29.4%, 47%, 41.2%, 55.8%, and 32.4% compared with 45.8%, 11%, 21.3%, 6.1%, and 16.5%, respectively, reported in patients receiving mutant EGFR-TK inhibitors for their advanced NSCLC.12
Another study was conducted in 2019 by Noronha and colleagues to determine the impact of platinum-based chemotherapy combined with gefitinib on patients with advanced NSCLC.13 They concluded that 70% of the patients receiving combination treatment developed rash, which was significantly higher compared with 45.8% patients receiving the mutant EGFR-TK inhibitors alone in our study. Also, 56% of patients receiving combination therapy developed diarrhea vs 33.6% of patients receiving mutant EGFR-TK inhibitors only. Similarly, 96% of patients in the combination therapy group developed some degree of anemia compared with only 8.7% patients in the mutant EGFR-TK inhibitors group included in our study. In the same way, neutropenia was observed in 55% of patients receiving combination therapy vs 6.1% in patients receiving mutant EGFR-TK inhibitors solely. They concluded that mutant EGFR-TK inhibitors when combined with platinum-based chemotherapy increase the incidence of AEs of chemotherapy by many folds.13,14
Kato and colleagues conducted a study to determine the impact on AEs when erlotinib was combined with anti–vascular endothelial growth factor (VEGF) inhibitors like bevacizumab, they stated that 98.7% of patient in combination therapy developed rash, the incidence of which was only 45.8% in patients receiving mutant EGFR-TK inhibitors as was observed in our study. Similar trends were noticed with other AEs, including diarrhea, fatigue, nausea, and elevated liver enzymes.15
With the latest advancements in the management of advanced NSCLC, nivolumab, a programmed death ligand 1 (PD-L1) inhibitor, was developed and either used as monotherapy in patients with PD-L1 expression or was combined with platinum-based chemotherapy regardless of PD-L1 expression.16,17 Patients expressing lower PD-L1 levels were not omitted from receiving nivolumab as no significant difference was noted in progression-free span and overall survival in patients receiving nivolumab irrespective of PD-L1 levels.15 Rash developed in 17% of patients after receiving nivolumab vs 45.8% patients being observed in our study. A similar trend was observed with diarrhea as only 17% of the population receiving nivolumab developed diarrhea compared with 33.6% of the population receiving mutant EGFR-TK inhibitors in our study. Likewise, only 9.9% of the patients receiving nivolumab developed nausea as an AE compared with 16.5% being observed in mutant EGFR-TK inhibitors in our study. Also, fatigue was observed in 14.4% of the population receiving nivolumab vs 17% observed in patients receiving mutant EGFR-TK inhibitors as was noticed in our study.7,8
Rizvi and colleagues conducted a study on the role of nivolumab when combined with platinum-based chemotherapy in patients with advanced NSCLC and reported that 40% of patients included in the study developed rash compared with 45.8% reported in mutant EGFR-TK inhibitors in our study. Similarly, only 13% of patients in the nivolumab group developed diarrhea vs 33.6% cases reported in the mutant EGFR-TK inhibitors group included in our study. Also, 7% of patients in the nivolumab group developed elevated ALT levels vs 27.9% of patients receiving mutant EGFR-TK inhibitors included in our study, concluding that addition of immune checkpoint inhibitors like nivolumab to platinum-based chemotherapy does not increase the frequency of AEs.18
Conclusions
Our study focused on the safety profile of mutant EGFR-TK inhibitors vs platinum-based chemotherapy in the treatment of advanced NSCLC. Mutant EGFR-TK inhibitors are safer than platinum-based chemotherapy when compared for nausea, leucopenia, fatigue, neutropenia, anorexia, anemia, cough, vomiting, and fever. On the other end, mutant EGFR-TK inhibitors cause slightly higher AEs, including rash, diarrhea, elevated AST and ALT levels, and stomatitis. However, considering that the development of mutant EGFR-TK inhibitors laid a foundation of targeted therapy, we recommend continuing using mutant EGFR-TK inhibitors in patients with advanced NSCLC especially in patients having mutant EGFR receptors. AEs caused by mutant EGFR-TK inhibitors are significant but are usually tolerable and can be avoided by reducing the dosage of it with each cycle or by skipping or delaying the dose until the patient is symptomatic.
1. Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913-2921. doi:10.1158/0008-5472.CAN-14-0155
2. da Cunha Santos G, Shepherd FA, Tsao MS. EGFR mutations and lung cancer. Annu Rev Pathol. 2011;6:49-69. doi:10.1146/annurev-pathol-011110-130206
3. Sgambato A, Casaluce F, Maione P, et al. The role of EGFR tyrosine kinase inhibitors in the first-line treatment of advanced non small cell lung cancer patients harboring EGFR mutation. Curr Med Chem. 2012;19(20):3337-3352. doi:10.2174/092986712801215973
4. Rossi A, Di Maio M. Platinum-based chemotherapy in advanced non–small-cell lung cancer: optimal number of treatment cycles. Expert Rev Anticancer Ther. 2016;16(6):653-660. doi:10.1586/14737140.2016.1170596
5. Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non–small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239-246. doi:10.1016/S1470-2045(11)70393-X
6. Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non–small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735-742. doi:10.1016/S1470-2045(11)70184-X
7. Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non–small-cell lung cancer. N Engl J Med. 2018;378(2):113-125. doi:10.1056/NEJMoa1713137
8. Mok TS, Cheng Y, Zhou X, et al. Improvement in overall survival in a randomized study that compared dacomitinib with gefitinib in patients with advanced non–small-cell lung cancer and EGFR-activating mutations. J Clin Oncol. 2018;36(22):2244-2250. doi:10.1200/JCO.2018.78.7994
9. Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947-957. doi:10.1056/NEJMoa0810699
10. Pless M, Stupp R, Ris HB, et al. Induction chemoradiation in stage IIIA/N2 non–small-cell lung cancer: a phase 3 randomised trial. Lancet. 2015;386(9998):1049-1056. doi:10.1016/S0140-6736(15)60294-X
11. Albain KS, Rusch VW, Crowley JJ, et al. Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stages IIIA (N2) and IIIB non–small-cell lung cancer: mature results of Southwest Oncology Group phase II study 8805. J Clin Oncol. 1995;13(8):1880-1892. doi:10.1200/JCO.1995.13.8.1880
12. Zhong WZ, Chen KN, Chen C, et al. Erlotinib versus gemcitabine plus cisplatin as neoadjuvant treatment of Stage IIIA-N2 EGFR-mutant non–small-cell lung cancer (EMERGING-CTONG 1103): a randomized phase II study. J Clin Oncol. 2019;37(25):2235-2245. doi:10.1200/JCO.19.00075
13. Noronha V, Patil VM, Joshi A, et al. Gefitinib versus gefitinib plus pemetrexed and carboplatin chemotherapy in EGFR-mutated lung cancer. J Clin Oncol. 2020;38(2):124-136. doi:10.1200/JCO.19.01154
14. Noronha V, Prabhash K, Thavamani A, et al. EGFR mutations in Indian lung cancer patients: clinical correlation and outcome to EGFR targeted therapy. PLoS One. 2013;8(4):e61561. Published 2013 Apr 19. doi:10.1371/journal.pone.0061561
15. Kato T, Seto T, Nishio M, et al. Erlotinib plus bevacizumab phase ll study in patients with advanced non–small-cell lung cancer (JO25567): updated safety results. Drug Saf. 2018;41(2):229-237. doi:10.1007/s40264-017-0596-0
16. Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non–small-cell lung cancer. N Engl J Med. 2019;381(21):2020-2031. doi:10.1056/NEJMoa1910231
17. Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378(22):2093-2104. doi:10.1056/NEJMoa1801946
18. Rizvi NA, Hellmann MD, Brahmer JR, et al. Nivolumab in combination with platinum-based doublet chemotherapy for first-line treatment of advanced non–small-cell lung cancer. J Clin Oncol. 2016;34(25):2969-2979. doi:10.1200/JCO.2016.66.9861
19. Zhong WZ, Wang Q, Mao WM, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC: final overall survival analysis of CTONG1104 Phase III Trial. J Clin Oncol. 2021;39(7):713-722. doi:10.1200/JCO.20.01820
20. Yang JC, Sequist LV, Geater SL, et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol. 2015;16(7):830-838. doi:10.1016/S1470-2045(15)00026-1
21. Shi YK, Wang L, Han BH, et al. First-line icotinib versus cisplatin/pemetrexed plus pemetrexed maintenance therapy for patients with advanced EGFR mutation-positive lung adenocarcinoma (CONVINCE): a phase 3, open-label, randomized study. Ann Oncol. 2017;28(10):2443-2450. doi:10.1093/annonc/mdx359
22. Soria JC, Wu YL, Nakagawa K, et al. Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on first-line gefitinib (IMPRESS): a phase 3 randomized trial. Lancet Oncol. 2015;16(8):990-998 doi:10.1016/S1470-2045(15)00121-7
23. Goss GD, O’Callaghan C, Lorimer I, et al. Gefitinib versus placebo in completely resected non-small-cell lung cancer: results of the NCIC CTG BR19 study. J Clin Oncol. 2013;31(27):3320-3326. doi:10.1200/JCO.2013.51.1816
24. Sun JM, Lee KH, Kim SW, et al. Gefitinib versus pemetrexed as second-line treatment in patients with non-small cell lung cancer previously treated with platinum-based chemotherapy (KCSG-LU08-01): an open-label, phase 3 trial. Cancer. 2012;118(24):6234-6242. doi:10.1200/JCO.2013.51.1816
25. Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomized phase 3 trial. Lancet Oncol. 2010;11(2):121-128. doi:10.1016/S1470-2045(09)70364-X
26. Lee DH, Park K, Kim JH, Lee JS, et al. Randomized phase III trial of gefitinib versus docetaxel in non-small cell lung cancer patients who have previously received platinum-based chemotherapy. Clin Cancer Res. 2010;16(4):1307-1314. doi:10.1158/1078-0432.CCR-09-1903
27. Kim ES, Hirsh V, Mok T, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomized phase III trial. Lancet. 2008;22;372(9652):1809-1818. doi:10.1016/S0140-6736(08)61758-4
1. Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913-2921. doi:10.1158/0008-5472.CAN-14-0155
2. da Cunha Santos G, Shepherd FA, Tsao MS. EGFR mutations and lung cancer. Annu Rev Pathol. 2011;6:49-69. doi:10.1146/annurev-pathol-011110-130206
3. Sgambato A, Casaluce F, Maione P, et al. The role of EGFR tyrosine kinase inhibitors in the first-line treatment of advanced non small cell lung cancer patients harboring EGFR mutation. Curr Med Chem. 2012;19(20):3337-3352. doi:10.2174/092986712801215973
4. Rossi A, Di Maio M. Platinum-based chemotherapy in advanced non–small-cell lung cancer: optimal number of treatment cycles. Expert Rev Anticancer Ther. 2016;16(6):653-660. doi:10.1586/14737140.2016.1170596
5. Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non–small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239-246. doi:10.1016/S1470-2045(11)70393-X
6. Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non–small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735-742. doi:10.1016/S1470-2045(11)70184-X
7. Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non–small-cell lung cancer. N Engl J Med. 2018;378(2):113-125. doi:10.1056/NEJMoa1713137
8. Mok TS, Cheng Y, Zhou X, et al. Improvement in overall survival in a randomized study that compared dacomitinib with gefitinib in patients with advanced non–small-cell lung cancer and EGFR-activating mutations. J Clin Oncol. 2018;36(22):2244-2250. doi:10.1200/JCO.2018.78.7994
9. Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947-957. doi:10.1056/NEJMoa0810699
10. Pless M, Stupp R, Ris HB, et al. Induction chemoradiation in stage IIIA/N2 non–small-cell lung cancer: a phase 3 randomised trial. Lancet. 2015;386(9998):1049-1056. doi:10.1016/S0140-6736(15)60294-X
11. Albain KS, Rusch VW, Crowley JJ, et al. Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stages IIIA (N2) and IIIB non–small-cell lung cancer: mature results of Southwest Oncology Group phase II study 8805. J Clin Oncol. 1995;13(8):1880-1892. doi:10.1200/JCO.1995.13.8.1880
12. Zhong WZ, Chen KN, Chen C, et al. Erlotinib versus gemcitabine plus cisplatin as neoadjuvant treatment of Stage IIIA-N2 EGFR-mutant non–small-cell lung cancer (EMERGING-CTONG 1103): a randomized phase II study. J Clin Oncol. 2019;37(25):2235-2245. doi:10.1200/JCO.19.00075
13. Noronha V, Patil VM, Joshi A, et al. Gefitinib versus gefitinib plus pemetrexed and carboplatin chemotherapy in EGFR-mutated lung cancer. J Clin Oncol. 2020;38(2):124-136. doi:10.1200/JCO.19.01154
14. Noronha V, Prabhash K, Thavamani A, et al. EGFR mutations in Indian lung cancer patients: clinical correlation and outcome to EGFR targeted therapy. PLoS One. 2013;8(4):e61561. Published 2013 Apr 19. doi:10.1371/journal.pone.0061561
15. Kato T, Seto T, Nishio M, et al. Erlotinib plus bevacizumab phase ll study in patients with advanced non–small-cell lung cancer (JO25567): updated safety results. Drug Saf. 2018;41(2):229-237. doi:10.1007/s40264-017-0596-0
16. Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non–small-cell lung cancer. N Engl J Med. 2019;381(21):2020-2031. doi:10.1056/NEJMoa1910231
17. Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378(22):2093-2104. doi:10.1056/NEJMoa1801946
18. Rizvi NA, Hellmann MD, Brahmer JR, et al. Nivolumab in combination with platinum-based doublet chemotherapy for first-line treatment of advanced non–small-cell lung cancer. J Clin Oncol. 2016;34(25):2969-2979. doi:10.1200/JCO.2016.66.9861
19. Zhong WZ, Wang Q, Mao WM, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC: final overall survival analysis of CTONG1104 Phase III Trial. J Clin Oncol. 2021;39(7):713-722. doi:10.1200/JCO.20.01820
20. Yang JC, Sequist LV, Geater SL, et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol. 2015;16(7):830-838. doi:10.1016/S1470-2045(15)00026-1
21. Shi YK, Wang L, Han BH, et al. First-line icotinib versus cisplatin/pemetrexed plus pemetrexed maintenance therapy for patients with advanced EGFR mutation-positive lung adenocarcinoma (CONVINCE): a phase 3, open-label, randomized study. Ann Oncol. 2017;28(10):2443-2450. doi:10.1093/annonc/mdx359
22. Soria JC, Wu YL, Nakagawa K, et al. Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on first-line gefitinib (IMPRESS): a phase 3 randomized trial. Lancet Oncol. 2015;16(8):990-998 doi:10.1016/S1470-2045(15)00121-7
23. Goss GD, O’Callaghan C, Lorimer I, et al. Gefitinib versus placebo in completely resected non-small-cell lung cancer: results of the NCIC CTG BR19 study. J Clin Oncol. 2013;31(27):3320-3326. doi:10.1200/JCO.2013.51.1816
24. Sun JM, Lee KH, Kim SW, et al. Gefitinib versus pemetrexed as second-line treatment in patients with non-small cell lung cancer previously treated with platinum-based chemotherapy (KCSG-LU08-01): an open-label, phase 3 trial. Cancer. 2012;118(24):6234-6242. doi:10.1200/JCO.2013.51.1816
25. Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomized phase 3 trial. Lancet Oncol. 2010;11(2):121-128. doi:10.1016/S1470-2045(09)70364-X
26. Lee DH, Park K, Kim JH, Lee JS, et al. Randomized phase III trial of gefitinib versus docetaxel in non-small cell lung cancer patients who have previously received platinum-based chemotherapy. Clin Cancer Res. 2010;16(4):1307-1314. doi:10.1158/1078-0432.CCR-09-1903
27. Kim ES, Hirsh V, Mok T, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomized phase III trial. Lancet. 2008;22;372(9652):1809-1818. doi:10.1016/S0140-6736(08)61758-4
Genomic Assays in HR-Positive/HER2-Negative Breast Cancer
As both a clinician and investigator in the breast cancer space, how would you describe our current understanding of genomic assays in hormone receptor (HR)-positive/human epidermal growth factor receptor 2 (HER2)-negative disease?
Dr. Kalinsky: There are a number of commercially-available assays for patients with early-stage HR-positive/HER2-negative breast cancer. We have seen the results of 3 large randomized phase 3 trials that have demonstrated and helped establish the clinical utility of assays, including the oncotype DX 21-gene recurrence score. This was evaluated in patients with node-negative breast cancer in the TAILORx trial and in patients with 1 to 3 nodes involved in the RxPONDER trial.
We also have data with the 70-gene MammaPrint assay from the MINDACT study, looking for patients who had a discordance between clinical and genomic risk. MINDACT has been published and was updated recently with data for 8-year distant metastasis-free survival. It is these studies that have helped establish what we do in the clinic and when we consider offering genomic assays in patients with this subtype of breast cancer.
How are you working to bring these genomic assays into practice?
Dr. Kalinsky: In 2020, we reported the initial results from the RxPonder study demonstrating that for patients with HR-positive HER2-negative breast cancer with 1 to 3 nodes involved, two-thirds of the patients were postmenopausal. For patients who had a recurrence score of 25 or less, we did not identify a subgroup of patients who benefited from chemotherapy.
For the premenopausal women, which was one-third of the patients, we saw that all those patients benefited from the addition of chemotherapy if the recurrence score was 25 or less. We did a number of subgroup analyses, which we reported on at the San Antonio Breast Cancer Symposium in 2021.
Several analyses are ongoing. These include some subgroup analyses looking at quality of life as well as a collection of circulating markers. In addition, there is ongoing biomarker work looking at tumor tissue to see if there are differences between the biology of premenopausal versus postmenopausal women.
What value does genomic testing bring to the treatment of HR-positive/HER2-negative breast cancer?
Dr. Kalinsky: These assays have achieved clinical utility, and this has been reflected in the recent update to the ASCO Guidelines for genomic assays. We have also learned that it is not just the assay by itself, but also the clinical features of a patient that help determine risk. In other words, it’s not just dependent on the score, but also involves the context of other important clinical features, including patient and tumor characteristics such as tumor size, patient age, and tumor grade. All of these add value and help us assess a patient’s individualized risk.
Is there a specific profile or qualifications candidates must meet for genomic testing to be done?
Dr. Kalinsky: We offer genomic tests for patients with HR-positive/HER2-negative breast cancer who are node-negative or have 1 to 3 nodes involved. There are other commercially-available tests such as the Breast Cancer Index, which assesses risk of recurrence in years 5 to 10 and looks at whether there is potential utility for continuing anti-estrogen therapy. That assay provides both prognostic and predictive information.
Is there any additional insight on genomic assays in HR-positive/HER2-negative breast cancer you would like to share?
Dr. Kalinsky: We’ve been talking about tumor-based assays. However, the question is, what’s going to be the role for circulating markers, such as circulating tumor DNA (ctDNA) or circulating tumor cells? There is a lot of information that we’re hoping to understand, not just regarding the prognostic significance but also the predictive utility. If you have a patient with a subtype of breast cancer and we know this subgroup can be at risk for late recurrence, if you identify said marker and you switch the therapy, do you see clearance of ctDNA? Does that lead to an improvement in outcome? That is an important question that is going to be answered in current and future trials.
As both a clinician and investigator in the breast cancer space, how would you describe our current understanding of genomic assays in hormone receptor (HR)-positive/human epidermal growth factor receptor 2 (HER2)-negative disease?
Dr. Kalinsky: There are a number of commercially-available assays for patients with early-stage HR-positive/HER2-negative breast cancer. We have seen the results of 3 large randomized phase 3 trials that have demonstrated and helped establish the clinical utility of assays, including the oncotype DX 21-gene recurrence score. This was evaluated in patients with node-negative breast cancer in the TAILORx trial and in patients with 1 to 3 nodes involved in the RxPONDER trial.
We also have data with the 70-gene MammaPrint assay from the MINDACT study, looking for patients who had a discordance between clinical and genomic risk. MINDACT has been published and was updated recently with data for 8-year distant metastasis-free survival. It is these studies that have helped establish what we do in the clinic and when we consider offering genomic assays in patients with this subtype of breast cancer.
How are you working to bring these genomic assays into practice?
Dr. Kalinsky: In 2020, we reported the initial results from the RxPonder study demonstrating that for patients with HR-positive HER2-negative breast cancer with 1 to 3 nodes involved, two-thirds of the patients were postmenopausal. For patients who had a recurrence score of 25 or less, we did not identify a subgroup of patients who benefited from chemotherapy.
For the premenopausal women, which was one-third of the patients, we saw that all those patients benefited from the addition of chemotherapy if the recurrence score was 25 or less. We did a number of subgroup analyses, which we reported on at the San Antonio Breast Cancer Symposium in 2021.
Several analyses are ongoing. These include some subgroup analyses looking at quality of life as well as a collection of circulating markers. In addition, there is ongoing biomarker work looking at tumor tissue to see if there are differences between the biology of premenopausal versus postmenopausal women.
What value does genomic testing bring to the treatment of HR-positive/HER2-negative breast cancer?
Dr. Kalinsky: These assays have achieved clinical utility, and this has been reflected in the recent update to the ASCO Guidelines for genomic assays. We have also learned that it is not just the assay by itself, but also the clinical features of a patient that help determine risk. In other words, it’s not just dependent on the score, but also involves the context of other important clinical features, including patient and tumor characteristics such as tumor size, patient age, and tumor grade. All of these add value and help us assess a patient’s individualized risk.
Is there a specific profile or qualifications candidates must meet for genomic testing to be done?
Dr. Kalinsky: We offer genomic tests for patients with HR-positive/HER2-negative breast cancer who are node-negative or have 1 to 3 nodes involved. There are other commercially-available tests such as the Breast Cancer Index, which assesses risk of recurrence in years 5 to 10 and looks at whether there is potential utility for continuing anti-estrogen therapy. That assay provides both prognostic and predictive information.
Is there any additional insight on genomic assays in HR-positive/HER2-negative breast cancer you would like to share?
Dr. Kalinsky: We’ve been talking about tumor-based assays. However, the question is, what’s going to be the role for circulating markers, such as circulating tumor DNA (ctDNA) or circulating tumor cells? There is a lot of information that we’re hoping to understand, not just regarding the prognostic significance but also the predictive utility. If you have a patient with a subtype of breast cancer and we know this subgroup can be at risk for late recurrence, if you identify said marker and you switch the therapy, do you see clearance of ctDNA? Does that lead to an improvement in outcome? That is an important question that is going to be answered in current and future trials.
As both a clinician and investigator in the breast cancer space, how would you describe our current understanding of genomic assays in hormone receptor (HR)-positive/human epidermal growth factor receptor 2 (HER2)-negative disease?
Dr. Kalinsky: There are a number of commercially-available assays for patients with early-stage HR-positive/HER2-negative breast cancer. We have seen the results of 3 large randomized phase 3 trials that have demonstrated and helped establish the clinical utility of assays, including the oncotype DX 21-gene recurrence score. This was evaluated in patients with node-negative breast cancer in the TAILORx trial and in patients with 1 to 3 nodes involved in the RxPONDER trial.
We also have data with the 70-gene MammaPrint assay from the MINDACT study, looking for patients who had a discordance between clinical and genomic risk. MINDACT has been published and was updated recently with data for 8-year distant metastasis-free survival. It is these studies that have helped establish what we do in the clinic and when we consider offering genomic assays in patients with this subtype of breast cancer.
How are you working to bring these genomic assays into practice?
Dr. Kalinsky: In 2020, we reported the initial results from the RxPonder study demonstrating that for patients with HR-positive HER2-negative breast cancer with 1 to 3 nodes involved, two-thirds of the patients were postmenopausal. For patients who had a recurrence score of 25 or less, we did not identify a subgroup of patients who benefited from chemotherapy.
For the premenopausal women, which was one-third of the patients, we saw that all those patients benefited from the addition of chemotherapy if the recurrence score was 25 or less. We did a number of subgroup analyses, which we reported on at the San Antonio Breast Cancer Symposium in 2021.
Several analyses are ongoing. These include some subgroup analyses looking at quality of life as well as a collection of circulating markers. In addition, there is ongoing biomarker work looking at tumor tissue to see if there are differences between the biology of premenopausal versus postmenopausal women.
What value does genomic testing bring to the treatment of HR-positive/HER2-negative breast cancer?
Dr. Kalinsky: These assays have achieved clinical utility, and this has been reflected in the recent update to the ASCO Guidelines for genomic assays. We have also learned that it is not just the assay by itself, but also the clinical features of a patient that help determine risk. In other words, it’s not just dependent on the score, but also involves the context of other important clinical features, including patient and tumor characteristics such as tumor size, patient age, and tumor grade. All of these add value and help us assess a patient’s individualized risk.
Is there a specific profile or qualifications candidates must meet for genomic testing to be done?
Dr. Kalinsky: We offer genomic tests for patients with HR-positive/HER2-negative breast cancer who are node-negative or have 1 to 3 nodes involved. There are other commercially-available tests such as the Breast Cancer Index, which assesses risk of recurrence in years 5 to 10 and looks at whether there is potential utility for continuing anti-estrogen therapy. That assay provides both prognostic and predictive information.
Is there any additional insight on genomic assays in HR-positive/HER2-negative breast cancer you would like to share?
Dr. Kalinsky: We’ve been talking about tumor-based assays. However, the question is, what’s going to be the role for circulating markers, such as circulating tumor DNA (ctDNA) or circulating tumor cells? There is a lot of information that we’re hoping to understand, not just regarding the prognostic significance but also the predictive utility. If you have a patient with a subtype of breast cancer and we know this subgroup can be at risk for late recurrence, if you identify said marker and you switch the therapy, do you see clearance of ctDNA? Does that lead to an improvement in outcome? That is an important question that is going to be answered in current and future trials.
The Effect of Race on Outcomes in Veterans With Hepatocellular Carcinoma at a Single Center
Hepatocellular carcinoma (HCC) is the sixth most common and third most deadly malignancy worldwide, carrying a mean survival rate without treatment of 6 to 20 months depending on stage.1 Fifty-seven percent of patients with liver cancer are diagnosed with regional or distant metastatic disease that carries 5-year relative survival rates of 10.7% and 3.1%, respectively.2 HCC arises most commonly from liver cirrhosis due to chronic hepatocyte injury, which may be mediated by viral hepatitis, alcoholism, and metabolic disease. Other less common causes include autoimmune disease, exposure to environmental hazards, and certain genetic diseases, such as α-1 antitrypsin deficiency and Wilson disease.
Multiple staging systems for HCC exist that incorporate some variation of the following features: size and invasion of the tumor, distant metastases, and liver function. Stage-directed treatments for HCC include ablation, embolization, resection, transplant, and systemic therapy, such as tyrosine kinase inhibitors, immunotherapies, and monoclonal antibodies. In addition to tumor/node/metastasis (TNM) staging, α-fetoprotein (AFP) is a diagnostic marker with prognostic value in HCC with higher levels correlating to higher tumor burden and a worse prognosis. With treatment, the 5-year survival rate for early stage HCC ranges from 60% to 80% but decreases significantly with higher stages.1 HCC screening in at-risk populations has accounted for > 40% of diagnoses since the practice became widely adopted, and earlier recognition has led to an improvement in survival even when adjusting for lead time bias.3
Systemic therapy for advanced disease continues to improve. Sorafenib remained the standard first-line systemic therapy since it was introduced in 2008.4 First-line therapy improved with immunotherapies. The phase 3 IMBrave150 trial comparing atezolizumab plus bevacizumab to sorafenib showed a median overall survival (OS) > 19 months with 7.7% of patients achieving a complete response.5 HIMALAYA, another phase 3 trial set for publication later this year, also reported promising results when a priming dose of the CTLA-4 inhibitor tremelimumab followed by durvalumab was compared with sorafenib.6
There has been a rise in incidence of HCC in the United States across all races and ethnicities, though Black, Hispanic, and Asian patients remain disproportionately affected. Subsequently, identifying causative biologic, socioeconomic, and cultural factors, as well as implicit bias in health care continues to be a topic of great interest.7-9 Using Surveillance, Epidemiology, and End Results (SEER) data, a number of large studies have found that Black patients with HCC were more likely to present with an advanced stage, less likely to receive curative intent treatment, and had significantly reduced survival compared with that of White patients.1,7-9 An analysis of 1117 patients by Rich and colleagues noted a 34% increased risk of death for Black patients with HCC compared with that of White patients, and other studies have shown about a 50% reduction in rate of liver transplantation for Black patients.10-12 Our study aimed to investigate potential disparities in incidence, etiology, AFP level at diagnosis, and outcomes of HCC in Black and White veterans managed at the Memphis Veterans Affairs Medical Center (VAMC) in Tennessee.
Methods
A single center retrospective chart review was conducted at the Memphis VAMC using the Computerized Patient Record System (CPRS) and the International Statistical Classification of Diseases, Tenth Revision (ICD-10) code C22.0 for HCC. Initial results were manually refined by prespecified criteria. Patients were included if they were diagnosed with HCC and received HCC treatment at the Memphis VAMC. Patients were excluded if HCC was not diagnosed histologically or clinically by imaging characteristics and AFP level, if the patient’s primary treatment was not provided at the Memphis VAMC, if they were lost to follow-up, or if race was not specified as either Black or White.
The following patient variables were examined: age, sex, comorbidities (alcohol or substance use disorder, cirrhosis, HIV), tumor stage, AFP, method of diagnosis, first-line treatments, systemic treatment, surgical options offered, and mortality. Staging was based on the American Joint Committee on Cancer TNM staging for HCC.13 Surgical options were recorded as resection or transplant. Patients who were offered treatment but lost to follow-up were excluded from the analysis.
Data Analysis
Our primary endpoint was identifying differences in OS among Memphis VAMC patients with HCC related to race. Kaplan-Meier analysis was used to investigate differences in OS and cumulative hazard ratio (HR) for death. Cox regression multivariate analysis further evaluated discrepancies among investigated patient variables, including age, race, alcohol, tobacco, or illicit drug use, HIV coinfection, and cirrhosis. Treatment factors were further defined by first-line treatment, systemic therapy, surgical resection, and transplant. χ2 analysis was used to investigate differences in treatment modalities.
Results
We identified 227 veterans, 95 Black and 132 White, between 2009 and 2021 meeting criteria for primary HCC treated at the Memphis VAMC. This study did not show a significant difference in OS between White and Black veterans (P = .24). Kaplan-Meier assessment showed OS was 1247 days (41 months) for Black veterans compared with 1032 days (34 months) for White veterans (Figure; Table 1).
Additionally, no significant difference was found between veterans for age or stage at diagnosis when stratified by race. The mean age of diagnosis for both groups was 65 years (P = .09). The mean TNM staging was 1.7 for White veterans vs 1.8 for Black veterans (P = .57). There was a significant increase in the AFP level at diagnosis for Black veterans (P = .001) (Table 2).
The most common initial treatment for both groups was transarterial chemoembolization and radiofrequency ablation with 68% of White and 64% of Black veterans receiving this therapy. There was no significant difference between who received systemic therapy.
However, we found significant differences by race for some forms of treatment. In our analysis, significant differences existed between those who did not receive any form of treatment as well as who received surgical resection and transplant. Among Black veterans, 11.6% received no treatment vs 6.1% for White veterans (P = .001). Only 2.1% of Black veterans underwent surgical resection vs 8.3% of White veterans (P = .046). Similarly, 13 (9.8%) White veterans vs 3 (3.2%) Black veterans received orthotopic liver transplantation (P = .052) in our cohort (eAppendix available at doi:10.12788/fp.0304). We found no differences in patient characteristics affecting OS, including alcohol use, tobacco use, illicit drug use, HIV coinfection, or liver cirrhosis (Table 3).
Discussion
In this retrospective analysis, Black veterans with HCC did not experience a statistically significant decrease in OS compared with that of White veterans despite some differences in therapy offered. Other studies have found that surgery was less frequently recommended to Black patients across multiple cancer types, and in most cases this carried a negative impact on OS.8,10,11,14,15 A number of other studies have demonstrated a greater percentage of Black patients receiving no treatment, although these studies are often based on SEER data, which captures only cancer-directed surgery and no other methods of treatment. Inequities in patient factors like insurance and socioeconomic status as well as willingness to receive certain treatments are often cited as major influences in health care disparities, but systemic and clinician factors like hospital volume, clinician expertise, specialist availability, and implicit racial bias all affect outcomes.16 One benefit of our study was that CPRS provided a centralized recording of all treatments received. Interestingly, the treatment discrepancy in our study was not attributable to a statistically significant difference in tumor stage at presentation. There should be no misconception that US Department of Veterans Affairs patients are less affected by socioeconomic inequities, though still this suggests clinician and systemic factors were significant drivers behind our findings.
This study did not intend to determine differences in incidence of HCC by race, although many studies have shown an age-adjusted incidence of HCC among Black and Hispanic patients up to twice that of White patients.1,8-10 Notably, the rate of orthotopic liver transplantation in this study was low regardless of race compared with that of other larger studies of patients with HCC.12,15 Discrepancies in HCC care among White and Black patients have been suggested to stem from a variety of influences, including access to early diagnosis and treatment of hepatitis C virus, comorbid conditions, as well as complex socioeconomic factors. It also has been shown that oncologists’ implicit racial bias has a negative impact on patients’ perceived quality of communication, their confidence in the recommended treatment, and the understood difficulty of the treatment by the patient and should be considered as a contributor to health disparities.17,18
Studies evaluating survival in HCC using SEER data generally stratify disease by localized, regional, or distant metastasis. For our study, TNM staging provided a more accurate assessment of the disease and reduced the chances that broader staging definitions could obscure differences in treatment choices. Future studies could be improved by stratifying patients by variables impacting treatment choice, such as Child-Pugh score or Barcelona Clinic Liver Cancer staging. Our study demonstrated a statistically significant difference in AFP level between White and Black veterans. This has been observed in prior studies as well, and while no specific cause has been identified, it suggests differences in tumor biologic features across different races. In addition, we found that an elevated AFP level at the time of diagnosis (defined as > 400) correlates with a worsened OS (HR, 1.36; P = .01).
Limitations
This study has several limitations, notably the number of veterans eligible for analysis at a single institution. A larger cohort would be needed to evaluate for statistically significant differences in outcomes by race. Additionally, our study did not account for therapy that was offered to but not pursued by the patient, and this would be useful to determine whether patient or practitioner factors were the more significant influence on the type of therapy received.
Conclusions
This study demonstrated a statistically significant difference in the rate of resection and liver transplantation between White and Black veterans at a single institution, although no difference in OS was observed. This discrepancy was not explained by differences in tumor staging. Additional, larger studies will be useful in clarifying the biologic, cultural, and socioeconomic drivers in HCC treatment and mortality.
Acknowledgments
The authors thank Lorri Reaves, Memphis Veterans Affairs Medical Center, Department of Hepatology.
1. Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27(9):1485-1491. doi:10.1200/JCO.2008.20.7753
2. Howlader N, Noone AM, Krapcho M, et al (eds). SEER Cancer Statistics Review, 1975-2012, National Cancer Institute. Accessed July 8, 2022. https://seer.cancer.gov/archive/csr/1975_2012/results_merged/sect_14_liver_bile.pdf#page=8
3. Singal AG, Mittal S, Yerokun OA, et al. Hepatocellular carcinoma screening associated with early tumor detection and improved survival among patients with cirrhosis in the US. Am J Med. 2017;130(9):1099-1106.e1. doi:10.1016/j.amjmed.2017.01.021
4. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378-390. doi:10.1056/NEJMoa0708857
5. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894-1905. doi:10.1056/NEJMoa1915745
6. Abou-Alfa GK, Chan SL, Kudo M, et al. Phase 3 randomized, open-label, multicenter study of tremelimumab (T) and durvalumab (D) as first-line therapy in patients (pts) with unresectable hepatocellular carcinoma (uHCC): HIMALAYA. J Clin Oncol. 2022;40(suppl 4):379. doi:10.1200/JCO.2022.40.4_suppl.379
7. Franco RA, Fan Y, Jarosek S, Bae S, Galbraith J. Racial and geographic disparities in hepatocellular carcinoma outcomes. Am J Prev Med. 2018;55(5)(suppl 1):S40-S48. doi:10.1016/j.amepre.2018.05.030
8. Ha J, Yan M, Aguilar M, et al. Race/ethnicity-specific disparities in hepatocellular carcinoma stage at diagnosis and its impact on receipt of curative therapies. J Clin Gastroenterol. 2016;50(5):423-430. doi:10.1097/MCG.0000000000000448
9. Wong R, Corley DA. Racial and ethnic variations in hepatocellular carcinoma incidence within the United States. Am J Med. 2008;121(6):525-531. doi:10.1016/j.amjmed.2008.03.005
10. Rich NE, Hester C, Odewole M, et al. Racial and ethnic differences in presentation and outcomes of hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2019;17(3):551-559.e1. doi:10.1016/j.cgh.2018.05.039
11. Peters NA, Javed AA, He J, Wolfgang CL, Weiss MJ. Association of socioeconomics, surgical therapy, and survival of early stage hepatocellular carcinoma. J Surg Res. 2017;210:253-260. doi:10.1016/j.jss.2016.11.042
12. Wong RJ, Devaki P, Nguyen L, Cheung R, Nguyen MH. Ethnic disparities and liver transplantation rates in hepatocellular carcinoma patients in the recent era: results from the Surveillance, Epidemiology, and End Results registry. Liver Transpl. 2014;20(5):528-535. doi:10.1002/lt.23820
13. Minagawa M, Ikai I, Matsuyama Y, Yamaoka Y, Makuuchi M. Staging of hepatocellular carcinoma: assessment of the Japanese TNM and AJCC/UICC TNM systems in a cohort of 13,772 patients in Japan. Ann Surg. 2007;245(6):909-922. doi:10.1097/01.sla.0000254368.65878.da.
14. Harrison LE, Reichman T, Koneru B, et al. Racial discrepancies in the outcome of patients with hepatocellular carcinoma. Arch Surg. 2004;139(9):992-996. doi:10.1001/archsurg.139.9.992
15. Sloane D, Chen H, Howell C. Racial disparity in primary hepatocellular carcinoma: tumor stage at presentation, surgical treatment and survival. J Natl Med Assoc. 2006;98(12):1934-1939.
16. Haider AH, Scott VK, Rehman KA, et al. Racial disparities in surgical care and outcomes in the United States: a comprehensive review of patient, provider, and systemic factors. J Am Coll Surg. 2013;216(3):482-92.e12. doi:10.1016/j.jamcollsurg.2012.11.014
17. Cooper LA, Roter DL, Carson KA, et al. The associations of clinicians’ implicit attitudes about race with medical visit communication and patient ratings of interpersonal care. Am J Public Health. 2012;102(5):979-987. doi:10.2105/AJPH.2011.300558
18. Penner LA, Dovidio JF, Gonzalez R, et al. The effects of oncologist implicit racial bias in racially discordant oncology interactions. J Clin Oncol. 2016;34(24):2874-2880. doi:10.1200/JCO.2015.66.3658
Hepatocellular carcinoma (HCC) is the sixth most common and third most deadly malignancy worldwide, carrying a mean survival rate without treatment of 6 to 20 months depending on stage.1 Fifty-seven percent of patients with liver cancer are diagnosed with regional or distant metastatic disease that carries 5-year relative survival rates of 10.7% and 3.1%, respectively.2 HCC arises most commonly from liver cirrhosis due to chronic hepatocyte injury, which may be mediated by viral hepatitis, alcoholism, and metabolic disease. Other less common causes include autoimmune disease, exposure to environmental hazards, and certain genetic diseases, such as α-1 antitrypsin deficiency and Wilson disease.
Multiple staging systems for HCC exist that incorporate some variation of the following features: size and invasion of the tumor, distant metastases, and liver function. Stage-directed treatments for HCC include ablation, embolization, resection, transplant, and systemic therapy, such as tyrosine kinase inhibitors, immunotherapies, and monoclonal antibodies. In addition to tumor/node/metastasis (TNM) staging, α-fetoprotein (AFP) is a diagnostic marker with prognostic value in HCC with higher levels correlating to higher tumor burden and a worse prognosis. With treatment, the 5-year survival rate for early stage HCC ranges from 60% to 80% but decreases significantly with higher stages.1 HCC screening in at-risk populations has accounted for > 40% of diagnoses since the practice became widely adopted, and earlier recognition has led to an improvement in survival even when adjusting for lead time bias.3
Systemic therapy for advanced disease continues to improve. Sorafenib remained the standard first-line systemic therapy since it was introduced in 2008.4 First-line therapy improved with immunotherapies. The phase 3 IMBrave150 trial comparing atezolizumab plus bevacizumab to sorafenib showed a median overall survival (OS) > 19 months with 7.7% of patients achieving a complete response.5 HIMALAYA, another phase 3 trial set for publication later this year, also reported promising results when a priming dose of the CTLA-4 inhibitor tremelimumab followed by durvalumab was compared with sorafenib.6
There has been a rise in incidence of HCC in the United States across all races and ethnicities, though Black, Hispanic, and Asian patients remain disproportionately affected. Subsequently, identifying causative biologic, socioeconomic, and cultural factors, as well as implicit bias in health care continues to be a topic of great interest.7-9 Using Surveillance, Epidemiology, and End Results (SEER) data, a number of large studies have found that Black patients with HCC were more likely to present with an advanced stage, less likely to receive curative intent treatment, and had significantly reduced survival compared with that of White patients.1,7-9 An analysis of 1117 patients by Rich and colleagues noted a 34% increased risk of death for Black patients with HCC compared with that of White patients, and other studies have shown about a 50% reduction in rate of liver transplantation for Black patients.10-12 Our study aimed to investigate potential disparities in incidence, etiology, AFP level at diagnosis, and outcomes of HCC in Black and White veterans managed at the Memphis Veterans Affairs Medical Center (VAMC) in Tennessee.
Methods
A single center retrospective chart review was conducted at the Memphis VAMC using the Computerized Patient Record System (CPRS) and the International Statistical Classification of Diseases, Tenth Revision (ICD-10) code C22.0 for HCC. Initial results were manually refined by prespecified criteria. Patients were included if they were diagnosed with HCC and received HCC treatment at the Memphis VAMC. Patients were excluded if HCC was not diagnosed histologically or clinically by imaging characteristics and AFP level, if the patient’s primary treatment was not provided at the Memphis VAMC, if they were lost to follow-up, or if race was not specified as either Black or White.
The following patient variables were examined: age, sex, comorbidities (alcohol or substance use disorder, cirrhosis, HIV), tumor stage, AFP, method of diagnosis, first-line treatments, systemic treatment, surgical options offered, and mortality. Staging was based on the American Joint Committee on Cancer TNM staging for HCC.13 Surgical options were recorded as resection or transplant. Patients who were offered treatment but lost to follow-up were excluded from the analysis.
Data Analysis
Our primary endpoint was identifying differences in OS among Memphis VAMC patients with HCC related to race. Kaplan-Meier analysis was used to investigate differences in OS and cumulative hazard ratio (HR) for death. Cox regression multivariate analysis further evaluated discrepancies among investigated patient variables, including age, race, alcohol, tobacco, or illicit drug use, HIV coinfection, and cirrhosis. Treatment factors were further defined by first-line treatment, systemic therapy, surgical resection, and transplant. χ2 analysis was used to investigate differences in treatment modalities.
Results
We identified 227 veterans, 95 Black and 132 White, between 2009 and 2021 meeting criteria for primary HCC treated at the Memphis VAMC. This study did not show a significant difference in OS between White and Black veterans (P = .24). Kaplan-Meier assessment showed OS was 1247 days (41 months) for Black veterans compared with 1032 days (34 months) for White veterans (Figure; Table 1).
Additionally, no significant difference was found between veterans for age or stage at diagnosis when stratified by race. The mean age of diagnosis for both groups was 65 years (P = .09). The mean TNM staging was 1.7 for White veterans vs 1.8 for Black veterans (P = .57). There was a significant increase in the AFP level at diagnosis for Black veterans (P = .001) (Table 2).
The most common initial treatment for both groups was transarterial chemoembolization and radiofrequency ablation with 68% of White and 64% of Black veterans receiving this therapy. There was no significant difference between who received systemic therapy.
However, we found significant differences by race for some forms of treatment. In our analysis, significant differences existed between those who did not receive any form of treatment as well as who received surgical resection and transplant. Among Black veterans, 11.6% received no treatment vs 6.1% for White veterans (P = .001). Only 2.1% of Black veterans underwent surgical resection vs 8.3% of White veterans (P = .046). Similarly, 13 (9.8%) White veterans vs 3 (3.2%) Black veterans received orthotopic liver transplantation (P = .052) in our cohort (eAppendix available at doi:10.12788/fp.0304). We found no differences in patient characteristics affecting OS, including alcohol use, tobacco use, illicit drug use, HIV coinfection, or liver cirrhosis (Table 3).
Discussion
In this retrospective analysis, Black veterans with HCC did not experience a statistically significant decrease in OS compared with that of White veterans despite some differences in therapy offered. Other studies have found that surgery was less frequently recommended to Black patients across multiple cancer types, and in most cases this carried a negative impact on OS.8,10,11,14,15 A number of other studies have demonstrated a greater percentage of Black patients receiving no treatment, although these studies are often based on SEER data, which captures only cancer-directed surgery and no other methods of treatment. Inequities in patient factors like insurance and socioeconomic status as well as willingness to receive certain treatments are often cited as major influences in health care disparities, but systemic and clinician factors like hospital volume, clinician expertise, specialist availability, and implicit racial bias all affect outcomes.16 One benefit of our study was that CPRS provided a centralized recording of all treatments received. Interestingly, the treatment discrepancy in our study was not attributable to a statistically significant difference in tumor stage at presentation. There should be no misconception that US Department of Veterans Affairs patients are less affected by socioeconomic inequities, though still this suggests clinician and systemic factors were significant drivers behind our findings.
This study did not intend to determine differences in incidence of HCC by race, although many studies have shown an age-adjusted incidence of HCC among Black and Hispanic patients up to twice that of White patients.1,8-10 Notably, the rate of orthotopic liver transplantation in this study was low regardless of race compared with that of other larger studies of patients with HCC.12,15 Discrepancies in HCC care among White and Black patients have been suggested to stem from a variety of influences, including access to early diagnosis and treatment of hepatitis C virus, comorbid conditions, as well as complex socioeconomic factors. It also has been shown that oncologists’ implicit racial bias has a negative impact on patients’ perceived quality of communication, their confidence in the recommended treatment, and the understood difficulty of the treatment by the patient and should be considered as a contributor to health disparities.17,18
Studies evaluating survival in HCC using SEER data generally stratify disease by localized, regional, or distant metastasis. For our study, TNM staging provided a more accurate assessment of the disease and reduced the chances that broader staging definitions could obscure differences in treatment choices. Future studies could be improved by stratifying patients by variables impacting treatment choice, such as Child-Pugh score or Barcelona Clinic Liver Cancer staging. Our study demonstrated a statistically significant difference in AFP level between White and Black veterans. This has been observed in prior studies as well, and while no specific cause has been identified, it suggests differences in tumor biologic features across different races. In addition, we found that an elevated AFP level at the time of diagnosis (defined as > 400) correlates with a worsened OS (HR, 1.36; P = .01).
Limitations
This study has several limitations, notably the number of veterans eligible for analysis at a single institution. A larger cohort would be needed to evaluate for statistically significant differences in outcomes by race. Additionally, our study did not account for therapy that was offered to but not pursued by the patient, and this would be useful to determine whether patient or practitioner factors were the more significant influence on the type of therapy received.
Conclusions
This study demonstrated a statistically significant difference in the rate of resection and liver transplantation between White and Black veterans at a single institution, although no difference in OS was observed. This discrepancy was not explained by differences in tumor staging. Additional, larger studies will be useful in clarifying the biologic, cultural, and socioeconomic drivers in HCC treatment and mortality.
Acknowledgments
The authors thank Lorri Reaves, Memphis Veterans Affairs Medical Center, Department of Hepatology.
Hepatocellular carcinoma (HCC) is the sixth most common and third most deadly malignancy worldwide, carrying a mean survival rate without treatment of 6 to 20 months depending on stage.1 Fifty-seven percent of patients with liver cancer are diagnosed with regional or distant metastatic disease that carries 5-year relative survival rates of 10.7% and 3.1%, respectively.2 HCC arises most commonly from liver cirrhosis due to chronic hepatocyte injury, which may be mediated by viral hepatitis, alcoholism, and metabolic disease. Other less common causes include autoimmune disease, exposure to environmental hazards, and certain genetic diseases, such as α-1 antitrypsin deficiency and Wilson disease.
Multiple staging systems for HCC exist that incorporate some variation of the following features: size and invasion of the tumor, distant metastases, and liver function. Stage-directed treatments for HCC include ablation, embolization, resection, transplant, and systemic therapy, such as tyrosine kinase inhibitors, immunotherapies, and monoclonal antibodies. In addition to tumor/node/metastasis (TNM) staging, α-fetoprotein (AFP) is a diagnostic marker with prognostic value in HCC with higher levels correlating to higher tumor burden and a worse prognosis. With treatment, the 5-year survival rate for early stage HCC ranges from 60% to 80% but decreases significantly with higher stages.1 HCC screening in at-risk populations has accounted for > 40% of diagnoses since the practice became widely adopted, and earlier recognition has led to an improvement in survival even when adjusting for lead time bias.3
Systemic therapy for advanced disease continues to improve. Sorafenib remained the standard first-line systemic therapy since it was introduced in 2008.4 First-line therapy improved with immunotherapies. The phase 3 IMBrave150 trial comparing atezolizumab plus bevacizumab to sorafenib showed a median overall survival (OS) > 19 months with 7.7% of patients achieving a complete response.5 HIMALAYA, another phase 3 trial set for publication later this year, also reported promising results when a priming dose of the CTLA-4 inhibitor tremelimumab followed by durvalumab was compared with sorafenib.6
There has been a rise in incidence of HCC in the United States across all races and ethnicities, though Black, Hispanic, and Asian patients remain disproportionately affected. Subsequently, identifying causative biologic, socioeconomic, and cultural factors, as well as implicit bias in health care continues to be a topic of great interest.7-9 Using Surveillance, Epidemiology, and End Results (SEER) data, a number of large studies have found that Black patients with HCC were more likely to present with an advanced stage, less likely to receive curative intent treatment, and had significantly reduced survival compared with that of White patients.1,7-9 An analysis of 1117 patients by Rich and colleagues noted a 34% increased risk of death for Black patients with HCC compared with that of White patients, and other studies have shown about a 50% reduction in rate of liver transplantation for Black patients.10-12 Our study aimed to investigate potential disparities in incidence, etiology, AFP level at diagnosis, and outcomes of HCC in Black and White veterans managed at the Memphis Veterans Affairs Medical Center (VAMC) in Tennessee.
Methods
A single center retrospective chart review was conducted at the Memphis VAMC using the Computerized Patient Record System (CPRS) and the International Statistical Classification of Diseases, Tenth Revision (ICD-10) code C22.0 for HCC. Initial results were manually refined by prespecified criteria. Patients were included if they were diagnosed with HCC and received HCC treatment at the Memphis VAMC. Patients were excluded if HCC was not diagnosed histologically or clinically by imaging characteristics and AFP level, if the patient’s primary treatment was not provided at the Memphis VAMC, if they were lost to follow-up, or if race was not specified as either Black or White.
The following patient variables were examined: age, sex, comorbidities (alcohol or substance use disorder, cirrhosis, HIV), tumor stage, AFP, method of diagnosis, first-line treatments, systemic treatment, surgical options offered, and mortality. Staging was based on the American Joint Committee on Cancer TNM staging for HCC.13 Surgical options were recorded as resection or transplant. Patients who were offered treatment but lost to follow-up were excluded from the analysis.
Data Analysis
Our primary endpoint was identifying differences in OS among Memphis VAMC patients with HCC related to race. Kaplan-Meier analysis was used to investigate differences in OS and cumulative hazard ratio (HR) for death. Cox regression multivariate analysis further evaluated discrepancies among investigated patient variables, including age, race, alcohol, tobacco, or illicit drug use, HIV coinfection, and cirrhosis. Treatment factors were further defined by first-line treatment, systemic therapy, surgical resection, and transplant. χ2 analysis was used to investigate differences in treatment modalities.
Results
We identified 227 veterans, 95 Black and 132 White, between 2009 and 2021 meeting criteria for primary HCC treated at the Memphis VAMC. This study did not show a significant difference in OS between White and Black veterans (P = .24). Kaplan-Meier assessment showed OS was 1247 days (41 months) for Black veterans compared with 1032 days (34 months) for White veterans (Figure; Table 1).
Additionally, no significant difference was found between veterans for age or stage at diagnosis when stratified by race. The mean age of diagnosis for both groups was 65 years (P = .09). The mean TNM staging was 1.7 for White veterans vs 1.8 for Black veterans (P = .57). There was a significant increase in the AFP level at diagnosis for Black veterans (P = .001) (Table 2).
The most common initial treatment for both groups was transarterial chemoembolization and radiofrequency ablation with 68% of White and 64% of Black veterans receiving this therapy. There was no significant difference between who received systemic therapy.
However, we found significant differences by race for some forms of treatment. In our analysis, significant differences existed between those who did not receive any form of treatment as well as who received surgical resection and transplant. Among Black veterans, 11.6% received no treatment vs 6.1% for White veterans (P = .001). Only 2.1% of Black veterans underwent surgical resection vs 8.3% of White veterans (P = .046). Similarly, 13 (9.8%) White veterans vs 3 (3.2%) Black veterans received orthotopic liver transplantation (P = .052) in our cohort (eAppendix available at doi:10.12788/fp.0304). We found no differences in patient characteristics affecting OS, including alcohol use, tobacco use, illicit drug use, HIV coinfection, or liver cirrhosis (Table 3).
Discussion
In this retrospective analysis, Black veterans with HCC did not experience a statistically significant decrease in OS compared with that of White veterans despite some differences in therapy offered. Other studies have found that surgery was less frequently recommended to Black patients across multiple cancer types, and in most cases this carried a negative impact on OS.8,10,11,14,15 A number of other studies have demonstrated a greater percentage of Black patients receiving no treatment, although these studies are often based on SEER data, which captures only cancer-directed surgery and no other methods of treatment. Inequities in patient factors like insurance and socioeconomic status as well as willingness to receive certain treatments are often cited as major influences in health care disparities, but systemic and clinician factors like hospital volume, clinician expertise, specialist availability, and implicit racial bias all affect outcomes.16 One benefit of our study was that CPRS provided a centralized recording of all treatments received. Interestingly, the treatment discrepancy in our study was not attributable to a statistically significant difference in tumor stage at presentation. There should be no misconception that US Department of Veterans Affairs patients are less affected by socioeconomic inequities, though still this suggests clinician and systemic factors were significant drivers behind our findings.
This study did not intend to determine differences in incidence of HCC by race, although many studies have shown an age-adjusted incidence of HCC among Black and Hispanic patients up to twice that of White patients.1,8-10 Notably, the rate of orthotopic liver transplantation in this study was low regardless of race compared with that of other larger studies of patients with HCC.12,15 Discrepancies in HCC care among White and Black patients have been suggested to stem from a variety of influences, including access to early diagnosis and treatment of hepatitis C virus, comorbid conditions, as well as complex socioeconomic factors. It also has been shown that oncologists’ implicit racial bias has a negative impact on patients’ perceived quality of communication, their confidence in the recommended treatment, and the understood difficulty of the treatment by the patient and should be considered as a contributor to health disparities.17,18
Studies evaluating survival in HCC using SEER data generally stratify disease by localized, regional, or distant metastasis. For our study, TNM staging provided a more accurate assessment of the disease and reduced the chances that broader staging definitions could obscure differences in treatment choices. Future studies could be improved by stratifying patients by variables impacting treatment choice, such as Child-Pugh score or Barcelona Clinic Liver Cancer staging. Our study demonstrated a statistically significant difference in AFP level between White and Black veterans. This has been observed in prior studies as well, and while no specific cause has been identified, it suggests differences in tumor biologic features across different races. In addition, we found that an elevated AFP level at the time of diagnosis (defined as > 400) correlates with a worsened OS (HR, 1.36; P = .01).
Limitations
This study has several limitations, notably the number of veterans eligible for analysis at a single institution. A larger cohort would be needed to evaluate for statistically significant differences in outcomes by race. Additionally, our study did not account for therapy that was offered to but not pursued by the patient, and this would be useful to determine whether patient or practitioner factors were the more significant influence on the type of therapy received.
Conclusions
This study demonstrated a statistically significant difference in the rate of resection and liver transplantation between White and Black veterans at a single institution, although no difference in OS was observed. This discrepancy was not explained by differences in tumor staging. Additional, larger studies will be useful in clarifying the biologic, cultural, and socioeconomic drivers in HCC treatment and mortality.
Acknowledgments
The authors thank Lorri Reaves, Memphis Veterans Affairs Medical Center, Department of Hepatology.
1. Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27(9):1485-1491. doi:10.1200/JCO.2008.20.7753
2. Howlader N, Noone AM, Krapcho M, et al (eds). SEER Cancer Statistics Review, 1975-2012, National Cancer Institute. Accessed July 8, 2022. https://seer.cancer.gov/archive/csr/1975_2012/results_merged/sect_14_liver_bile.pdf#page=8
3. Singal AG, Mittal S, Yerokun OA, et al. Hepatocellular carcinoma screening associated with early tumor detection and improved survival among patients with cirrhosis in the US. Am J Med. 2017;130(9):1099-1106.e1. doi:10.1016/j.amjmed.2017.01.021
4. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378-390. doi:10.1056/NEJMoa0708857
5. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894-1905. doi:10.1056/NEJMoa1915745
6. Abou-Alfa GK, Chan SL, Kudo M, et al. Phase 3 randomized, open-label, multicenter study of tremelimumab (T) and durvalumab (D) as first-line therapy in patients (pts) with unresectable hepatocellular carcinoma (uHCC): HIMALAYA. J Clin Oncol. 2022;40(suppl 4):379. doi:10.1200/JCO.2022.40.4_suppl.379
7. Franco RA, Fan Y, Jarosek S, Bae S, Galbraith J. Racial and geographic disparities in hepatocellular carcinoma outcomes. Am J Prev Med. 2018;55(5)(suppl 1):S40-S48. doi:10.1016/j.amepre.2018.05.030
8. Ha J, Yan M, Aguilar M, et al. Race/ethnicity-specific disparities in hepatocellular carcinoma stage at diagnosis and its impact on receipt of curative therapies. J Clin Gastroenterol. 2016;50(5):423-430. doi:10.1097/MCG.0000000000000448
9. Wong R, Corley DA. Racial and ethnic variations in hepatocellular carcinoma incidence within the United States. Am J Med. 2008;121(6):525-531. doi:10.1016/j.amjmed.2008.03.005
10. Rich NE, Hester C, Odewole M, et al. Racial and ethnic differences in presentation and outcomes of hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2019;17(3):551-559.e1. doi:10.1016/j.cgh.2018.05.039
11. Peters NA, Javed AA, He J, Wolfgang CL, Weiss MJ. Association of socioeconomics, surgical therapy, and survival of early stage hepatocellular carcinoma. J Surg Res. 2017;210:253-260. doi:10.1016/j.jss.2016.11.042
12. Wong RJ, Devaki P, Nguyen L, Cheung R, Nguyen MH. Ethnic disparities and liver transplantation rates in hepatocellular carcinoma patients in the recent era: results from the Surveillance, Epidemiology, and End Results registry. Liver Transpl. 2014;20(5):528-535. doi:10.1002/lt.23820
13. Minagawa M, Ikai I, Matsuyama Y, Yamaoka Y, Makuuchi M. Staging of hepatocellular carcinoma: assessment of the Japanese TNM and AJCC/UICC TNM systems in a cohort of 13,772 patients in Japan. Ann Surg. 2007;245(6):909-922. doi:10.1097/01.sla.0000254368.65878.da.
14. Harrison LE, Reichman T, Koneru B, et al. Racial discrepancies in the outcome of patients with hepatocellular carcinoma. Arch Surg. 2004;139(9):992-996. doi:10.1001/archsurg.139.9.992
15. Sloane D, Chen H, Howell C. Racial disparity in primary hepatocellular carcinoma: tumor stage at presentation, surgical treatment and survival. J Natl Med Assoc. 2006;98(12):1934-1939.
16. Haider AH, Scott VK, Rehman KA, et al. Racial disparities in surgical care and outcomes in the United States: a comprehensive review of patient, provider, and systemic factors. J Am Coll Surg. 2013;216(3):482-92.e12. doi:10.1016/j.jamcollsurg.2012.11.014
17. Cooper LA, Roter DL, Carson KA, et al. The associations of clinicians’ implicit attitudes about race with medical visit communication and patient ratings of interpersonal care. Am J Public Health. 2012;102(5):979-987. doi:10.2105/AJPH.2011.300558
18. Penner LA, Dovidio JF, Gonzalez R, et al. The effects of oncologist implicit racial bias in racially discordant oncology interactions. J Clin Oncol. 2016;34(24):2874-2880. doi:10.1200/JCO.2015.66.3658
1. Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27(9):1485-1491. doi:10.1200/JCO.2008.20.7753
2. Howlader N, Noone AM, Krapcho M, et al (eds). SEER Cancer Statistics Review, 1975-2012, National Cancer Institute. Accessed July 8, 2022. https://seer.cancer.gov/archive/csr/1975_2012/results_merged/sect_14_liver_bile.pdf#page=8
3. Singal AG, Mittal S, Yerokun OA, et al. Hepatocellular carcinoma screening associated with early tumor detection and improved survival among patients with cirrhosis in the US. Am J Med. 2017;130(9):1099-1106.e1. doi:10.1016/j.amjmed.2017.01.021
4. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378-390. doi:10.1056/NEJMoa0708857
5. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894-1905. doi:10.1056/NEJMoa1915745
6. Abou-Alfa GK, Chan SL, Kudo M, et al. Phase 3 randomized, open-label, multicenter study of tremelimumab (T) and durvalumab (D) as first-line therapy in patients (pts) with unresectable hepatocellular carcinoma (uHCC): HIMALAYA. J Clin Oncol. 2022;40(suppl 4):379. doi:10.1200/JCO.2022.40.4_suppl.379
7. Franco RA, Fan Y, Jarosek S, Bae S, Galbraith J. Racial and geographic disparities in hepatocellular carcinoma outcomes. Am J Prev Med. 2018;55(5)(suppl 1):S40-S48. doi:10.1016/j.amepre.2018.05.030
8. Ha J, Yan M, Aguilar M, et al. Race/ethnicity-specific disparities in hepatocellular carcinoma stage at diagnosis and its impact on receipt of curative therapies. J Clin Gastroenterol. 2016;50(5):423-430. doi:10.1097/MCG.0000000000000448
9. Wong R, Corley DA. Racial and ethnic variations in hepatocellular carcinoma incidence within the United States. Am J Med. 2008;121(6):525-531. doi:10.1016/j.amjmed.2008.03.005
10. Rich NE, Hester C, Odewole M, et al. Racial and ethnic differences in presentation and outcomes of hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2019;17(3):551-559.e1. doi:10.1016/j.cgh.2018.05.039
11. Peters NA, Javed AA, He J, Wolfgang CL, Weiss MJ. Association of socioeconomics, surgical therapy, and survival of early stage hepatocellular carcinoma. J Surg Res. 2017;210:253-260. doi:10.1016/j.jss.2016.11.042
12. Wong RJ, Devaki P, Nguyen L, Cheung R, Nguyen MH. Ethnic disparities and liver transplantation rates in hepatocellular carcinoma patients in the recent era: results from the Surveillance, Epidemiology, and End Results registry. Liver Transpl. 2014;20(5):528-535. doi:10.1002/lt.23820
13. Minagawa M, Ikai I, Matsuyama Y, Yamaoka Y, Makuuchi M. Staging of hepatocellular carcinoma: assessment of the Japanese TNM and AJCC/UICC TNM systems in a cohort of 13,772 patients in Japan. Ann Surg. 2007;245(6):909-922. doi:10.1097/01.sla.0000254368.65878.da.
14. Harrison LE, Reichman T, Koneru B, et al. Racial discrepancies in the outcome of patients with hepatocellular carcinoma. Arch Surg. 2004;139(9):992-996. doi:10.1001/archsurg.139.9.992
15. Sloane D, Chen H, Howell C. Racial disparity in primary hepatocellular carcinoma: tumor stage at presentation, surgical treatment and survival. J Natl Med Assoc. 2006;98(12):1934-1939.
16. Haider AH, Scott VK, Rehman KA, et al. Racial disparities in surgical care and outcomes in the United States: a comprehensive review of patient, provider, and systemic factors. J Am Coll Surg. 2013;216(3):482-92.e12. doi:10.1016/j.jamcollsurg.2012.11.014
17. Cooper LA, Roter DL, Carson KA, et al. The associations of clinicians’ implicit attitudes about race with medical visit communication and patient ratings of interpersonal care. Am J Public Health. 2012;102(5):979-987. doi:10.2105/AJPH.2011.300558
18. Penner LA, Dovidio JF, Gonzalez R, et al. The effects of oncologist implicit racial bias in racially discordant oncology interactions. J Clin Oncol. 2016;34(24):2874-2880. doi:10.1200/JCO.2015.66.3658
After ‘a Lot of Doors Shut in Our Face,’ Crusading Couple Celebrate Passage of Burn Pit Bill
The battle was just beginning for Le Roy Torres and his wife, Rosie, when the Army captain returned to Texas in 2008, already starting to suffer from the toxic substances he’d inhaled from the 10-acre burn pit at Camp Anaconda in Balad, Iraq.
Along the way, Le Roy would lose the job he loved as a Texas state trooper and take his fight all the way to a Supreme Court victory. He would be rushed to the emergency room hundreds of times, be denied health benefits by the Department of Veterans Affairs for years, attempt suicide, and seek experimental cures for the damage done to his lungs and brain.
Amid all that, Le Roy and Rosie founded an organization to help others and push Congress to fix the laws that allowed the suffering of veterans to go on, and ultimately enlist people like comedian and activist Jon Stewart, who helped them win a dramatic showdown in the Senate last week.
Their struggle will never really be over. But the Torreses’ campaign to make sure no other veterans experience what they had to ends Aug. 10, when they are set to join President Joe Biden as he signs a law to guarantee that 3.5 million American warriors exposed to similar hazards can get care.
“I mean, to think that 13 years ago we were walking the halls [of Congress] — it’s really emotional,” Rosie said recently, halting to collect herself and wipe back tears, “because I think of all the people that died along the way.”
The bill provides a new entitlement program for veterans who served in a combat zone in the past 32 years. If they are diagnosed with any of 23 conditions identified in the legislation — ranging from specific cancers to breathing ailments — they would be deemed automatically eligible for health coverage. The Congressional Budget Office estimated the new benefits would cost $280 billion over the next 10 years.
Most veterans — nearly 80% — who start experiencing symptoms after leaving the service get denied what’s known as a service connection when they seek help from the VA. The system has been designed to disbelieve them, the veterans complain. They must prove their breathing problems or cancers came from the toxic trash smoke they breathed overseas, which is extremely difficult.
When Le Roy returned home from Balad Air Base — the second-largest U.S. post in Iraq and where the military incinerated tons of debris daily, including plastic, ammunition, and medical waste — he was already sick. He was rushed to the hospital a few weeks later with a severe respiratory infection.
He had expected to keep working as a state trooper, but by 2010 it was clear he couldn’t perform all the duties because of his illness. When he asked for a different job with the Texas Department of Public Safety, he was denied. He was told he had to resign if he wanted to apply for medical retirement. The retirement request was then rejected. So he sued and eventually took the case to the Supreme Court, which in June ruled that states were not immune from such lawsuits by service members.
In those early years, the military and VA doctors couldn’t say what caused his breathing problems and splitting headaches. As with other victims of toxic exposure, diagnoses proved to be difficult. Some doctors suggested the problems weren’t real — a pronouncement often encountered by other vets whose claims are denied.
Like so many others, Rosie turned to the internet for information she couldn’t get from the VA, where she had worked for 23 years. She discovered a Facebook group that she would use as the basis for a new advocacy group, Burn Pits 360.
Le Roy was ultimately diagnosed with constrictive bronchiolitis, fibrosis of the lungs, and toxic encephalopathy. He eventually got his benefits in early 2013. By then, the family was deep in debt.
For years he lived with the reality that the military he had served for 23 years refused to answer his needs, and the police force he loved didn’t seem to care.
“It’s something that we have now learned is known as moral injury and compound loss,” Rosie said.
As a man, he began to wonder how he could provide for his family, if he was any use to anyone, she added. “So then that led to him attempting to take his life.”
It also led the couple and parents of three to beseech Congress to fix the problems. They started walking the halls in the Capitol. Success there was not any easier.
“We came to Capitol Hill and just handed out information we had printed about burn pit exposure,” Le Roy said at his last visit to the Hill in June, an oxygen tube strung under his nose.
“There were a lot of doors shut in our face,” Rosie said.
While making little progress in Congress, they built Burn Pits 360 into an advocacy group and a clearinghouse to help other veterans similarly frustrated by a system that seemed to be failing them.
The breakthrough for Rosie began when she saw Stewart and 9/11 survivors’ advocate John Feal winning a similar battle to make Congress fully fund health and compensation programs for responders of the Sept. 11, 2001, terror attacks. She recalls reading up on the toxic substances in the dust and smoke that spewed from the collapsed twin towers and discovering they were remarkably similar to the poisons inhaled by troops near the waste fires that were also set ablaze with jet fuel.
She called Feal. Feal called Stewart, and by February 2019 the four of them were meeting on Capitol Hill with lawmakers, including Sen. Kirsten Gillibrand (D-N.Y.), one of the authors of the 9/11 legislation.
The key, they decided in those first meetings, was to remove the obstacles for the most common illnesses and eliminate the burden of proof on ill former soldiers. Gillibrand’s office wrote that bill, along with Rep. Raul Ruiz (D-Calif.), who championed it in the House.
Related Links
- Senate GOP Puts Up Roadblocks to Bipartisan House Bill for Veterans’ Burn Pit Care
- Doctors Found Jet Fuel in Veteran’s Lungs. He Can’t Get Full Benefits.
- Role Reversal: Covid Increases Ranks of Child Caregivers
Ultimately, that bill became the heart of the measure that passed, known as the PACT Act and named for a soldier who died from cancer linked to his service.
“Our bill was the first federal presumption for burn pits coverage ever. And that was all because of Rosie and Le Roy,” said Gillibrand.
But just as with the 9/11 legislation, many in Congress weren’t that interested.
“It’s about money, and nobody likes to spend money,” Gillibrand said. “Congress never wants to accept the fact that treating these veterans and addressing their health care is the cost of war.”
Weeks ago, the bill appeared ready to glide through. It passed both the House and Senate but needed another vote to fix a technical legislative issue. Then on July 27, Sen. Pat Toomey (R-Pa.), who opposed the measure, unexpectedly persuaded 25 of his Republican colleagues who had supported the bill to vote against it, claiming that because the bill made the spending mandatory — not subject to the annual whims of Congress — Democrats would spend $400 billion elsewhere in the budget. Democrats countered that the money Toomey cited is already being spent and, regardless of how it’s categorized, it’s still up to Congress to appropriate it.
Rosie and veterans who had come to the Capitol that day to celebrate instead had to dig in one more time, with Stewart bringing the high-wattage attention that led the Republicans to reconsider. On Aug. 2, most Republicans decided to agree with the Democrats, and the bill passed 86 to 11.
Rosie said it never would have happened without Feal and Stewart. Stewart said it was all about Rosie, bringing together veterans in a way that Congress couldn’t ignore.
“She’s the reason I’m doing it, her and Le Roy,” Stewart said, standing outside the Capitol with Rosie the day before the vote.
Stewart, the Torreses, and untold other veterans tempered their joy with the warning that it will be a hard journey making the new program work with a VA that already has a massive backlog. The legislation has provisions to create facilities and bring in private doctors, but some vets remain dubious.
Iraq War veteran Brian Alvarado of Long Beach, California, was diagnosed with neck and throat cancer soon after returning from Iraq in 2006. He had been assigned to patrol one of the many burn pits. He eats and breathes through tubes and struggles to keep weight on. Radiation and a tracheostomy have left his voice almost inaudible.
“You can pass laws, but it all boils down to the VA. How are they going to implement the changes? The claims, the compensation, the treatment,” he asked in June. “And how long will it take?”
For the time being, though, Rosie said that even more than a visit to the White House, she was looking forward to going back to Texas and her family.
“You know, I lost 13 years away from my children, with trips to the hospital, coming to D.C.,” she said. “It means I can go home.”
Le Roy and Rosie can also reflect that as painful as this path has been, 3.5 million veterans are guaranteed a backstop because of this law, and thousands of veterans and active-duty service members who work for state and local governments now have recourse if they are fired after being injured at war.
“It is good to know that so many people will be helped,” Le Roy said from his home in Robstown, Texas. “It does help.”
KHN reporter Heidi de Marco contributed to this article.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
The battle was just beginning for Le Roy Torres and his wife, Rosie, when the Army captain returned to Texas in 2008, already starting to suffer from the toxic substances he’d inhaled from the 10-acre burn pit at Camp Anaconda in Balad, Iraq.
Along the way, Le Roy would lose the job he loved as a Texas state trooper and take his fight all the way to a Supreme Court victory. He would be rushed to the emergency room hundreds of times, be denied health benefits by the Department of Veterans Affairs for years, attempt suicide, and seek experimental cures for the damage done to his lungs and brain.
Amid all that, Le Roy and Rosie founded an organization to help others and push Congress to fix the laws that allowed the suffering of veterans to go on, and ultimately enlist people like comedian and activist Jon Stewart, who helped them win a dramatic showdown in the Senate last week.
Their struggle will never really be over. But the Torreses’ campaign to make sure no other veterans experience what they had to ends Aug. 10, when they are set to join President Joe Biden as he signs a law to guarantee that 3.5 million American warriors exposed to similar hazards can get care.
“I mean, to think that 13 years ago we were walking the halls [of Congress] — it’s really emotional,” Rosie said recently, halting to collect herself and wipe back tears, “because I think of all the people that died along the way.”
The bill provides a new entitlement program for veterans who served in a combat zone in the past 32 years. If they are diagnosed with any of 23 conditions identified in the legislation — ranging from specific cancers to breathing ailments — they would be deemed automatically eligible for health coverage. The Congressional Budget Office estimated the new benefits would cost $280 billion over the next 10 years.
Most veterans — nearly 80% — who start experiencing symptoms after leaving the service get denied what’s known as a service connection when they seek help from the VA. The system has been designed to disbelieve them, the veterans complain. They must prove their breathing problems or cancers came from the toxic trash smoke they breathed overseas, which is extremely difficult.
When Le Roy returned home from Balad Air Base — the second-largest U.S. post in Iraq and where the military incinerated tons of debris daily, including plastic, ammunition, and medical waste — he was already sick. He was rushed to the hospital a few weeks later with a severe respiratory infection.
He had expected to keep working as a state trooper, but by 2010 it was clear he couldn’t perform all the duties because of his illness. When he asked for a different job with the Texas Department of Public Safety, he was denied. He was told he had to resign if he wanted to apply for medical retirement. The retirement request was then rejected. So he sued and eventually took the case to the Supreme Court, which in June ruled that states were not immune from such lawsuits by service members.
In those early years, the military and VA doctors couldn’t say what caused his breathing problems and splitting headaches. As with other victims of toxic exposure, diagnoses proved to be difficult. Some doctors suggested the problems weren’t real — a pronouncement often encountered by other vets whose claims are denied.
Like so many others, Rosie turned to the internet for information she couldn’t get from the VA, where she had worked for 23 years. She discovered a Facebook group that she would use as the basis for a new advocacy group, Burn Pits 360.
Le Roy was ultimately diagnosed with constrictive bronchiolitis, fibrosis of the lungs, and toxic encephalopathy. He eventually got his benefits in early 2013. By then, the family was deep in debt.
For years he lived with the reality that the military he had served for 23 years refused to answer his needs, and the police force he loved didn’t seem to care.
“It’s something that we have now learned is known as moral injury and compound loss,” Rosie said.
As a man, he began to wonder how he could provide for his family, if he was any use to anyone, she added. “So then that led to him attempting to take his life.”
It also led the couple and parents of three to beseech Congress to fix the problems. They started walking the halls in the Capitol. Success there was not any easier.
“We came to Capitol Hill and just handed out information we had printed about burn pit exposure,” Le Roy said at his last visit to the Hill in June, an oxygen tube strung under his nose.
“There were a lot of doors shut in our face,” Rosie said.
While making little progress in Congress, they built Burn Pits 360 into an advocacy group and a clearinghouse to help other veterans similarly frustrated by a system that seemed to be failing them.
The breakthrough for Rosie began when she saw Stewart and 9/11 survivors’ advocate John Feal winning a similar battle to make Congress fully fund health and compensation programs for responders of the Sept. 11, 2001, terror attacks. She recalls reading up on the toxic substances in the dust and smoke that spewed from the collapsed twin towers and discovering they were remarkably similar to the poisons inhaled by troops near the waste fires that were also set ablaze with jet fuel.
She called Feal. Feal called Stewart, and by February 2019 the four of them were meeting on Capitol Hill with lawmakers, including Sen. Kirsten Gillibrand (D-N.Y.), one of the authors of the 9/11 legislation.
The key, they decided in those first meetings, was to remove the obstacles for the most common illnesses and eliminate the burden of proof on ill former soldiers. Gillibrand’s office wrote that bill, along with Rep. Raul Ruiz (D-Calif.), who championed it in the House.
Related Links
- Senate GOP Puts Up Roadblocks to Bipartisan House Bill for Veterans’ Burn Pit Care
- Doctors Found Jet Fuel in Veteran’s Lungs. He Can’t Get Full Benefits.
- Role Reversal: Covid Increases Ranks of Child Caregivers
Ultimately, that bill became the heart of the measure that passed, known as the PACT Act and named for a soldier who died from cancer linked to his service.
“Our bill was the first federal presumption for burn pits coverage ever. And that was all because of Rosie and Le Roy,” said Gillibrand.
But just as with the 9/11 legislation, many in Congress weren’t that interested.
“It’s about money, and nobody likes to spend money,” Gillibrand said. “Congress never wants to accept the fact that treating these veterans and addressing their health care is the cost of war.”
Weeks ago, the bill appeared ready to glide through. It passed both the House and Senate but needed another vote to fix a technical legislative issue. Then on July 27, Sen. Pat Toomey (R-Pa.), who opposed the measure, unexpectedly persuaded 25 of his Republican colleagues who had supported the bill to vote against it, claiming that because the bill made the spending mandatory — not subject to the annual whims of Congress — Democrats would spend $400 billion elsewhere in the budget. Democrats countered that the money Toomey cited is already being spent and, regardless of how it’s categorized, it’s still up to Congress to appropriate it.
Rosie and veterans who had come to the Capitol that day to celebrate instead had to dig in one more time, with Stewart bringing the high-wattage attention that led the Republicans to reconsider. On Aug. 2, most Republicans decided to agree with the Democrats, and the bill passed 86 to 11.
Rosie said it never would have happened without Feal and Stewart. Stewart said it was all about Rosie, bringing together veterans in a way that Congress couldn’t ignore.
“She’s the reason I’m doing it, her and Le Roy,” Stewart said, standing outside the Capitol with Rosie the day before the vote.
Stewart, the Torreses, and untold other veterans tempered their joy with the warning that it will be a hard journey making the new program work with a VA that already has a massive backlog. The legislation has provisions to create facilities and bring in private doctors, but some vets remain dubious.
Iraq War veteran Brian Alvarado of Long Beach, California, was diagnosed with neck and throat cancer soon after returning from Iraq in 2006. He had been assigned to patrol one of the many burn pits. He eats and breathes through tubes and struggles to keep weight on. Radiation and a tracheostomy have left his voice almost inaudible.
“You can pass laws, but it all boils down to the VA. How are they going to implement the changes? The claims, the compensation, the treatment,” he asked in June. “And how long will it take?”
For the time being, though, Rosie said that even more than a visit to the White House, she was looking forward to going back to Texas and her family.
“You know, I lost 13 years away from my children, with trips to the hospital, coming to D.C.,” she said. “It means I can go home.”
Le Roy and Rosie can also reflect that as painful as this path has been, 3.5 million veterans are guaranteed a backstop because of this law, and thousands of veterans and active-duty service members who work for state and local governments now have recourse if they are fired after being injured at war.
“It is good to know that so many people will be helped,” Le Roy said from his home in Robstown, Texas. “It does help.”
KHN reporter Heidi de Marco contributed to this article.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
The battle was just beginning for Le Roy Torres and his wife, Rosie, when the Army captain returned to Texas in 2008, already starting to suffer from the toxic substances he’d inhaled from the 10-acre burn pit at Camp Anaconda in Balad, Iraq.
Along the way, Le Roy would lose the job he loved as a Texas state trooper and take his fight all the way to a Supreme Court victory. He would be rushed to the emergency room hundreds of times, be denied health benefits by the Department of Veterans Affairs for years, attempt suicide, and seek experimental cures for the damage done to his lungs and brain.
Amid all that, Le Roy and Rosie founded an organization to help others and push Congress to fix the laws that allowed the suffering of veterans to go on, and ultimately enlist people like comedian and activist Jon Stewart, who helped them win a dramatic showdown in the Senate last week.
Their struggle will never really be over. But the Torreses’ campaign to make sure no other veterans experience what they had to ends Aug. 10, when they are set to join President Joe Biden as he signs a law to guarantee that 3.5 million American warriors exposed to similar hazards can get care.
“I mean, to think that 13 years ago we were walking the halls [of Congress] — it’s really emotional,” Rosie said recently, halting to collect herself and wipe back tears, “because I think of all the people that died along the way.”
The bill provides a new entitlement program for veterans who served in a combat zone in the past 32 years. If they are diagnosed with any of 23 conditions identified in the legislation — ranging from specific cancers to breathing ailments — they would be deemed automatically eligible for health coverage. The Congressional Budget Office estimated the new benefits would cost $280 billion over the next 10 years.
Most veterans — nearly 80% — who start experiencing symptoms after leaving the service get denied what’s known as a service connection when they seek help from the VA. The system has been designed to disbelieve them, the veterans complain. They must prove their breathing problems or cancers came from the toxic trash smoke they breathed overseas, which is extremely difficult.
When Le Roy returned home from Balad Air Base — the second-largest U.S. post in Iraq and where the military incinerated tons of debris daily, including plastic, ammunition, and medical waste — he was already sick. He was rushed to the hospital a few weeks later with a severe respiratory infection.
He had expected to keep working as a state trooper, but by 2010 it was clear he couldn’t perform all the duties because of his illness. When he asked for a different job with the Texas Department of Public Safety, he was denied. He was told he had to resign if he wanted to apply for medical retirement. The retirement request was then rejected. So he sued and eventually took the case to the Supreme Court, which in June ruled that states were not immune from such lawsuits by service members.
In those early years, the military and VA doctors couldn’t say what caused his breathing problems and splitting headaches. As with other victims of toxic exposure, diagnoses proved to be difficult. Some doctors suggested the problems weren’t real — a pronouncement often encountered by other vets whose claims are denied.
Like so many others, Rosie turned to the internet for information she couldn’t get from the VA, where she had worked for 23 years. She discovered a Facebook group that she would use as the basis for a new advocacy group, Burn Pits 360.
Le Roy was ultimately diagnosed with constrictive bronchiolitis, fibrosis of the lungs, and toxic encephalopathy. He eventually got his benefits in early 2013. By then, the family was deep in debt.
For years he lived with the reality that the military he had served for 23 years refused to answer his needs, and the police force he loved didn’t seem to care.
“It’s something that we have now learned is known as moral injury and compound loss,” Rosie said.
As a man, he began to wonder how he could provide for his family, if he was any use to anyone, she added. “So then that led to him attempting to take his life.”
It also led the couple and parents of three to beseech Congress to fix the problems. They started walking the halls in the Capitol. Success there was not any easier.
“We came to Capitol Hill and just handed out information we had printed about burn pit exposure,” Le Roy said at his last visit to the Hill in June, an oxygen tube strung under his nose.
“There were a lot of doors shut in our face,” Rosie said.
While making little progress in Congress, they built Burn Pits 360 into an advocacy group and a clearinghouse to help other veterans similarly frustrated by a system that seemed to be failing them.
The breakthrough for Rosie began when she saw Stewart and 9/11 survivors’ advocate John Feal winning a similar battle to make Congress fully fund health and compensation programs for responders of the Sept. 11, 2001, terror attacks. She recalls reading up on the toxic substances in the dust and smoke that spewed from the collapsed twin towers and discovering they were remarkably similar to the poisons inhaled by troops near the waste fires that were also set ablaze with jet fuel.
She called Feal. Feal called Stewart, and by February 2019 the four of them were meeting on Capitol Hill with lawmakers, including Sen. Kirsten Gillibrand (D-N.Y.), one of the authors of the 9/11 legislation.
The key, they decided in those first meetings, was to remove the obstacles for the most common illnesses and eliminate the burden of proof on ill former soldiers. Gillibrand’s office wrote that bill, along with Rep. Raul Ruiz (D-Calif.), who championed it in the House.
Related Links
- Senate GOP Puts Up Roadblocks to Bipartisan House Bill for Veterans’ Burn Pit Care
- Doctors Found Jet Fuel in Veteran’s Lungs. He Can’t Get Full Benefits.
- Role Reversal: Covid Increases Ranks of Child Caregivers
Ultimately, that bill became the heart of the measure that passed, known as the PACT Act and named for a soldier who died from cancer linked to his service.
“Our bill was the first federal presumption for burn pits coverage ever. And that was all because of Rosie and Le Roy,” said Gillibrand.
But just as with the 9/11 legislation, many in Congress weren’t that interested.
“It’s about money, and nobody likes to spend money,” Gillibrand said. “Congress never wants to accept the fact that treating these veterans and addressing their health care is the cost of war.”
Weeks ago, the bill appeared ready to glide through. It passed both the House and Senate but needed another vote to fix a technical legislative issue. Then on July 27, Sen. Pat Toomey (R-Pa.), who opposed the measure, unexpectedly persuaded 25 of his Republican colleagues who had supported the bill to vote against it, claiming that because the bill made the spending mandatory — not subject to the annual whims of Congress — Democrats would spend $400 billion elsewhere in the budget. Democrats countered that the money Toomey cited is already being spent and, regardless of how it’s categorized, it’s still up to Congress to appropriate it.
Rosie and veterans who had come to the Capitol that day to celebrate instead had to dig in one more time, with Stewart bringing the high-wattage attention that led the Republicans to reconsider. On Aug. 2, most Republicans decided to agree with the Democrats, and the bill passed 86 to 11.
Rosie said it never would have happened without Feal and Stewart. Stewart said it was all about Rosie, bringing together veterans in a way that Congress couldn’t ignore.
“She’s the reason I’m doing it, her and Le Roy,” Stewart said, standing outside the Capitol with Rosie the day before the vote.
Stewart, the Torreses, and untold other veterans tempered their joy with the warning that it will be a hard journey making the new program work with a VA that already has a massive backlog. The legislation has provisions to create facilities and bring in private doctors, but some vets remain dubious.
Iraq War veteran Brian Alvarado of Long Beach, California, was diagnosed with neck and throat cancer soon after returning from Iraq in 2006. He had been assigned to patrol one of the many burn pits. He eats and breathes through tubes and struggles to keep weight on. Radiation and a tracheostomy have left his voice almost inaudible.
“You can pass laws, but it all boils down to the VA. How are they going to implement the changes? The claims, the compensation, the treatment,” he asked in June. “And how long will it take?”
For the time being, though, Rosie said that even more than a visit to the White House, she was looking forward to going back to Texas and her family.
“You know, I lost 13 years away from my children, with trips to the hospital, coming to D.C.,” she said. “It means I can go home.”
Le Roy and Rosie can also reflect that as painful as this path has been, 3.5 million veterans are guaranteed a backstop because of this law, and thousands of veterans and active-duty service members who work for state and local governments now have recourse if they are fired after being injured at war.
“It is good to know that so many people will be helped,” Le Roy said from his home in Robstown, Texas. “It does help.”
KHN reporter Heidi de Marco contributed to this article.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Commentary: Comparing Migraine Treatments, August 2022
Migraine is a unique neurologic condition, in that a person can't prove they have it and there are few objective tools neurologists have to guide their diagnostic process. The recognition of the role of the vasoactive peptide calcitonin gene-related peptide (CGRP) in the 1990s changed the way many researchers and clinicians conceptualized migraine. Subsequent studies have used CGRP as a human model for migraine, and most recently pituitary adenylate cyclase–activating polypeptide 38 (PACAP-38) has also been recognized for its important role in migraine propagation. All of the existing data have been in adults, and no studies until now have specifically investigated the presence of these peptides in children with migraine.
Pediatric migraine is unique in a number of ways. Children with migraine present less unilaterally, the duration of their attacks is typically shorter, and the associated symptoms can often be more prominent than the headache pain during an attack. There are unique pediatric migraine subtypes that are exceptionally rare in adults, such as periodic paralysis attacks and abdominal migraine. For this reason, it is not entirely clear whether the same biomarkers of disease in adults would also be present in the pediatric population.
In the study by Liu and colleagues, the investigators enrolled 76 pediatric patients with migraine (the diagnosis was confirmed by at least two neurologists). Patients were excluded if there was any analgesic medication use over the past 2 months; if there was concern for secondary headache; or any underlying mood disorders, congenital disease, or other major medical conditions. An additional 77 controls were matched for age and sex. Blood was collected from all participants after an 8-hour fast to avoid collecting after potentially ingesting a food trigger. Blood samples were obtained during an ictal period (within 8 hours of a migraine attack) as well as interictally (not taken if the participant had a migraine attack within the past 24 hours).
The plasma CGRP and PACAP-38 levels were significantly higher in pediatric patients with migraine than in those without a migraine history, in both the ictal state and the interictal state. Among patients with migraine, there was a nonsignificant trend toward a higher CGRP level in the ictal phase, and no difference in these phases with PACAP-38. There was no difference in the CGRP or PACAP-38 levels between participants with and those without aura. When different aura groups were compared (with the participants separated on the basis of a history of motor vs vision vs sensory aura), no difference was seen among the different aura groups. Binary logistic regression testing and analysis of variance also showed that CGRP and PACAP-38 are independent risk factors for pediatric migraine, and specific levels of each were associated with an 11 and a 13 times increased risk, respectively.
Biomarker testing is still not clinically performed for migraine either in adults or children. This is primarily due to cost and the fact that most commercially available laboratories do not currently offer these tests. The results above do shed additional light on migraine pathogenesis and indicate that the phenotypic differences seen in pediatric migraine are less likely related to differences in brain function in children.
Levetiracetam is a commonly used antiepileptic medication. Prior studies have investigated the use of this medication for migraine, both acutely and preventively. Other antiepileptic medications have been shown to be very effective for both of these indications. Topiramate and valproic acid are both commonly used for migraine: topiramate primarily preventively and valproic acid both for prevention and, commonly in its intravenous form, for acute treatment. Levetiracetam is currently not commonly used for migraine, although some institutions will use the intravenous formulation for severe refractory status migrainosus.
Evers and colleagues investigated the open label use of levetiracetam for migraine prevention at a dose of 1000 mg twice daily in a small population of 50 persons. The study participants were started at a dose of 500 mg twice daily for 4 weeks, then increased to 1000 mg twice daily for a total of 12 weeks. The primary endpoint was migraine attack frequency during the last 4 weeks of treatment.
A 50% reduction in headache frequency was seen in 46% of the enrolled participants. The most common reported side effects were sedation, nausea, and weight gain, as well as cognitive change (five patients dropped out of the study owing to intolerance of the treatment). A post hoc comparison between the patients with and without response to levetiracetam revealed that those who responded were those with a less refractory history — they had tried fewer medications and were using fewer acute medications as well.
The antiepileptic class of preventive migraine medications is notorious for issues with tolerance. Among the antiepileptic medications, levetiracetam is commonly used but also commonly stopped owing to mood and cognitive complaints. Although the researchers here do show early evidence for a moderate amount of efficacy for treating migraine, the fact that there are now more migraine-specific preventive medications that are better tolerated and overall more efficacious make choosing levetiracetam for prevention less necessary.
Now that there are multiple classes of migraine-specific acute medications, the outstanding question remains: What are the potential benefits and drawbacks for the use of triptans compared with the oral CGRP receptor antagonists (gepants)? Most obviously, triptan medications are contraindicated in patients with significant vascular risk factors; however, what is not known is whether some of the other adverse events associated with triptans are more or less prominent with gepant use. Lee and colleagues conducted a meta-analysis of 15 studies to review this data.
A previous meta-analysis demonstrated that oral CGRP receptor antagonists are more effective than placebo, but less effective than triptans against acute migraine. The most common intolerances for gepants are nausea, somnolence, and dry mouth, but the safety and tolerability of gepants have not been compared with that of triptans. These authors pooled the data on five gepant medications (BI44370TA, MK-3207, rimegepant, telcagepant, and ubrogepant). The primary outcome was incidence of treatment-related adverse events and the secondary outcome was the incidence of the specific intolerances of diarrhea, dizziness, dry mouth, fatigue, nausea, paresthesia, somnolence, upper abdominal pain, and vomiting.
Compared with placebo, the relative risk for any adverse event was found to be low, at 1.15, and the relative risk for treatment-related adverse events was only slightly higher, at 1.18. Gepants were found to be significantly more associated with an increased risk for fatigue, nausea, and somnolence vs placebo. Compared with triptans, the CGRP antagonists were associated with significantly less treatment-related adverse events as well as any adverse event. There was no significant difference in the incidence of diarrhea, nausea, and vomiting between the two groups.
This study helps elucidate some of the differences between the two classes of migraine-specific acute medications. As noted above, a prior meta-analysis did reveal some benefits with the triptan class, specifically better effectiveness. When choosing a better-tolerated medication for your patients, you may want to consider a gepant; when considering a stronger or more potent option, you might stick with a triptan.
Migraine is a unique neurologic condition, in that a person can't prove they have it and there are few objective tools neurologists have to guide their diagnostic process. The recognition of the role of the vasoactive peptide calcitonin gene-related peptide (CGRP) in the 1990s changed the way many researchers and clinicians conceptualized migraine. Subsequent studies have used CGRP as a human model for migraine, and most recently pituitary adenylate cyclase–activating polypeptide 38 (PACAP-38) has also been recognized for its important role in migraine propagation. All of the existing data have been in adults, and no studies until now have specifically investigated the presence of these peptides in children with migraine.
Pediatric migraine is unique in a number of ways. Children with migraine present less unilaterally, the duration of their attacks is typically shorter, and the associated symptoms can often be more prominent than the headache pain during an attack. There are unique pediatric migraine subtypes that are exceptionally rare in adults, such as periodic paralysis attacks and abdominal migraine. For this reason, it is not entirely clear whether the same biomarkers of disease in adults would also be present in the pediatric population.
In the study by Liu and colleagues, the investigators enrolled 76 pediatric patients with migraine (the diagnosis was confirmed by at least two neurologists). Patients were excluded if there was any analgesic medication use over the past 2 months; if there was concern for secondary headache; or any underlying mood disorders, congenital disease, or other major medical conditions. An additional 77 controls were matched for age and sex. Blood was collected from all participants after an 8-hour fast to avoid collecting after potentially ingesting a food trigger. Blood samples were obtained during an ictal period (within 8 hours of a migraine attack) as well as interictally (not taken if the participant had a migraine attack within the past 24 hours).
The plasma CGRP and PACAP-38 levels were significantly higher in pediatric patients with migraine than in those without a migraine history, in both the ictal state and the interictal state. Among patients with migraine, there was a nonsignificant trend toward a higher CGRP level in the ictal phase, and no difference in these phases with PACAP-38. There was no difference in the CGRP or PACAP-38 levels between participants with and those without aura. When different aura groups were compared (with the participants separated on the basis of a history of motor vs vision vs sensory aura), no difference was seen among the different aura groups. Binary logistic regression testing and analysis of variance also showed that CGRP and PACAP-38 are independent risk factors for pediatric migraine, and specific levels of each were associated with an 11 and a 13 times increased risk, respectively.
Biomarker testing is still not clinically performed for migraine either in adults or children. This is primarily due to cost and the fact that most commercially available laboratories do not currently offer these tests. The results above do shed additional light on migraine pathogenesis and indicate that the phenotypic differences seen in pediatric migraine are less likely related to differences in brain function in children.
Levetiracetam is a commonly used antiepileptic medication. Prior studies have investigated the use of this medication for migraine, both acutely and preventively. Other antiepileptic medications have been shown to be very effective for both of these indications. Topiramate and valproic acid are both commonly used for migraine: topiramate primarily preventively and valproic acid both for prevention and, commonly in its intravenous form, for acute treatment. Levetiracetam is currently not commonly used for migraine, although some institutions will use the intravenous formulation for severe refractory status migrainosus.
Evers and colleagues investigated the open label use of levetiracetam for migraine prevention at a dose of 1000 mg twice daily in a small population of 50 persons. The study participants were started at a dose of 500 mg twice daily for 4 weeks, then increased to 1000 mg twice daily for a total of 12 weeks. The primary endpoint was migraine attack frequency during the last 4 weeks of treatment.
A 50% reduction in headache frequency was seen in 46% of the enrolled participants. The most common reported side effects were sedation, nausea, and weight gain, as well as cognitive change (five patients dropped out of the study owing to intolerance of the treatment). A post hoc comparison between the patients with and without response to levetiracetam revealed that those who responded were those with a less refractory history — they had tried fewer medications and were using fewer acute medications as well.
The antiepileptic class of preventive migraine medications is notorious for issues with tolerance. Among the antiepileptic medications, levetiracetam is commonly used but also commonly stopped owing to mood and cognitive complaints. Although the researchers here do show early evidence for a moderate amount of efficacy for treating migraine, the fact that there are now more migraine-specific preventive medications that are better tolerated and overall more efficacious make choosing levetiracetam for prevention less necessary.
Now that there are multiple classes of migraine-specific acute medications, the outstanding question remains: What are the potential benefits and drawbacks for the use of triptans compared with the oral CGRP receptor antagonists (gepants)? Most obviously, triptan medications are contraindicated in patients with significant vascular risk factors; however, what is not known is whether some of the other adverse events associated with triptans are more or less prominent with gepant use. Lee and colleagues conducted a meta-analysis of 15 studies to review this data.
A previous meta-analysis demonstrated that oral CGRP receptor antagonists are more effective than placebo, but less effective than triptans against acute migraine. The most common intolerances for gepants are nausea, somnolence, and dry mouth, but the safety and tolerability of gepants have not been compared with that of triptans. These authors pooled the data on five gepant medications (BI44370TA, MK-3207, rimegepant, telcagepant, and ubrogepant). The primary outcome was incidence of treatment-related adverse events and the secondary outcome was the incidence of the specific intolerances of diarrhea, dizziness, dry mouth, fatigue, nausea, paresthesia, somnolence, upper abdominal pain, and vomiting.
Compared with placebo, the relative risk for any adverse event was found to be low, at 1.15, and the relative risk for treatment-related adverse events was only slightly higher, at 1.18. Gepants were found to be significantly more associated with an increased risk for fatigue, nausea, and somnolence vs placebo. Compared with triptans, the CGRP antagonists were associated with significantly less treatment-related adverse events as well as any adverse event. There was no significant difference in the incidence of diarrhea, nausea, and vomiting between the two groups.
This study helps elucidate some of the differences between the two classes of migraine-specific acute medications. As noted above, a prior meta-analysis did reveal some benefits with the triptan class, specifically better effectiveness. When choosing a better-tolerated medication for your patients, you may want to consider a gepant; when considering a stronger or more potent option, you might stick with a triptan.
Migraine is a unique neurologic condition, in that a person can't prove they have it and there are few objective tools neurologists have to guide their diagnostic process. The recognition of the role of the vasoactive peptide calcitonin gene-related peptide (CGRP) in the 1990s changed the way many researchers and clinicians conceptualized migraine. Subsequent studies have used CGRP as a human model for migraine, and most recently pituitary adenylate cyclase–activating polypeptide 38 (PACAP-38) has also been recognized for its important role in migraine propagation. All of the existing data have been in adults, and no studies until now have specifically investigated the presence of these peptides in children with migraine.
Pediatric migraine is unique in a number of ways. Children with migraine present less unilaterally, the duration of their attacks is typically shorter, and the associated symptoms can often be more prominent than the headache pain during an attack. There are unique pediatric migraine subtypes that are exceptionally rare in adults, such as periodic paralysis attacks and abdominal migraine. For this reason, it is not entirely clear whether the same biomarkers of disease in adults would also be present in the pediatric population.
In the study by Liu and colleagues, the investigators enrolled 76 pediatric patients with migraine (the diagnosis was confirmed by at least two neurologists). Patients were excluded if there was any analgesic medication use over the past 2 months; if there was concern for secondary headache; or any underlying mood disorders, congenital disease, or other major medical conditions. An additional 77 controls were matched for age and sex. Blood was collected from all participants after an 8-hour fast to avoid collecting after potentially ingesting a food trigger. Blood samples were obtained during an ictal period (within 8 hours of a migraine attack) as well as interictally (not taken if the participant had a migraine attack within the past 24 hours).
The plasma CGRP and PACAP-38 levels were significantly higher in pediatric patients with migraine than in those without a migraine history, in both the ictal state and the interictal state. Among patients with migraine, there was a nonsignificant trend toward a higher CGRP level in the ictal phase, and no difference in these phases with PACAP-38. There was no difference in the CGRP or PACAP-38 levels between participants with and those without aura. When different aura groups were compared (with the participants separated on the basis of a history of motor vs vision vs sensory aura), no difference was seen among the different aura groups. Binary logistic regression testing and analysis of variance also showed that CGRP and PACAP-38 are independent risk factors for pediatric migraine, and specific levels of each were associated with an 11 and a 13 times increased risk, respectively.
Biomarker testing is still not clinically performed for migraine either in adults or children. This is primarily due to cost and the fact that most commercially available laboratories do not currently offer these tests. The results above do shed additional light on migraine pathogenesis and indicate that the phenotypic differences seen in pediatric migraine are less likely related to differences in brain function in children.
Levetiracetam is a commonly used antiepileptic medication. Prior studies have investigated the use of this medication for migraine, both acutely and preventively. Other antiepileptic medications have been shown to be very effective for both of these indications. Topiramate and valproic acid are both commonly used for migraine: topiramate primarily preventively and valproic acid both for prevention and, commonly in its intravenous form, for acute treatment. Levetiracetam is currently not commonly used for migraine, although some institutions will use the intravenous formulation for severe refractory status migrainosus.
Evers and colleagues investigated the open label use of levetiracetam for migraine prevention at a dose of 1000 mg twice daily in a small population of 50 persons. The study participants were started at a dose of 500 mg twice daily for 4 weeks, then increased to 1000 mg twice daily for a total of 12 weeks. The primary endpoint was migraine attack frequency during the last 4 weeks of treatment.
A 50% reduction in headache frequency was seen in 46% of the enrolled participants. The most common reported side effects were sedation, nausea, and weight gain, as well as cognitive change (five patients dropped out of the study owing to intolerance of the treatment). A post hoc comparison between the patients with and without response to levetiracetam revealed that those who responded were those with a less refractory history — they had tried fewer medications and were using fewer acute medications as well.
The antiepileptic class of preventive migraine medications is notorious for issues with tolerance. Among the antiepileptic medications, levetiracetam is commonly used but also commonly stopped owing to mood and cognitive complaints. Although the researchers here do show early evidence for a moderate amount of efficacy for treating migraine, the fact that there are now more migraine-specific preventive medications that are better tolerated and overall more efficacious make choosing levetiracetam for prevention less necessary.
Now that there are multiple classes of migraine-specific acute medications, the outstanding question remains: What are the potential benefits and drawbacks for the use of triptans compared with the oral CGRP receptor antagonists (gepants)? Most obviously, triptan medications are contraindicated in patients with significant vascular risk factors; however, what is not known is whether some of the other adverse events associated with triptans are more or less prominent with gepant use. Lee and colleagues conducted a meta-analysis of 15 studies to review this data.
A previous meta-analysis demonstrated that oral CGRP receptor antagonists are more effective than placebo, but less effective than triptans against acute migraine. The most common intolerances for gepants are nausea, somnolence, and dry mouth, but the safety and tolerability of gepants have not been compared with that of triptans. These authors pooled the data on five gepant medications (BI44370TA, MK-3207, rimegepant, telcagepant, and ubrogepant). The primary outcome was incidence of treatment-related adverse events and the secondary outcome was the incidence of the specific intolerances of diarrhea, dizziness, dry mouth, fatigue, nausea, paresthesia, somnolence, upper abdominal pain, and vomiting.
Compared with placebo, the relative risk for any adverse event was found to be low, at 1.15, and the relative risk for treatment-related adverse events was only slightly higher, at 1.18. Gepants were found to be significantly more associated with an increased risk for fatigue, nausea, and somnolence vs placebo. Compared with triptans, the CGRP antagonists were associated with significantly less treatment-related adverse events as well as any adverse event. There was no significant difference in the incidence of diarrhea, nausea, and vomiting between the two groups.
This study helps elucidate some of the differences between the two classes of migraine-specific acute medications. As noted above, a prior meta-analysis did reveal some benefits with the triptan class, specifically better effectiveness. When choosing a better-tolerated medication for your patients, you may want to consider a gepant; when considering a stronger or more potent option, you might stick with a triptan.
Commentary: Concomitant Therapies May Affect NSCLC Survival, August 2022
A Danish population-based cohort study by Ehrenstein and colleagues involved 21,282 patients with non–small-cell lung cancer (NSCLC), 8758 of whom received a diagnosis at stage I-IIIA. Of those, 4071 (46%) were tested for epidermal growth factor receptor (EGFR) mutations at diagnosis. Median overall survival (OS) was 5.7 years among patients with EGFR mutation–positive status (n = 361) and 4.4 years among patients with EGFR mutation–negative status (n = 3710). EGFR mutation–positive status was associated with lower all-cause mortality in all subgroups. This is not surprising, because EGFR-mutated lung cancers are associated with never or light smoking history and the patients tend to be younger and have fewer medical comorbidities. Nevertheless, the lower risk for all-cause mortality was consistent across all subgroups (stage at diagnosis, age, sex, comorbidity, and surgery receipt), with hazard ratios (HR) ranging from 0.48 to 0.83. In addition, targeted therapies, such as osimertinib, improved OS and progression-free survival (PFS) in the metastatic setting as first-line treatment. Now that the ADAURA study has demonstrated a substantial disease-free survival benefit with osimertinib in the adjuvant setting in early-stage EGFR-mutated NSCLC (with final OS results still to come) it will be interesting to see whether this magnitude of difference in OS grows over time.
Wang and colleagues conducted a large meta-analysis of 10 retrospective studies and one prospective study including a total of 5892 patients with NSCLC who were receiving programmed cell death protein 1 (PD-1)/ programmed death-ligand 1 (PD-L1) inhibitors with the concomitant use of gastric acid suppressants (GAS). Use of PD-1/PD-L1 inhibitors with vs without GAS worsened PFS by 32% (HR 1.32; P < .001) and OS by 36% (HR 1.36; P < .001). The GAS in these studies were predominantly proton-pump inhibitors (PPI). There is still much to learn about medications that may influence outcomes to immune checkpoint blockade, such as steroids, antibiotics, GAS, and others. We are also learning that the microbiome probably plays an important role in contributing to activity of PD-1/PDL-1 antibodies and PPI may modify the microbiome. More research is needed, but it is reasonable to try and switch patients receiving PD-1/PDL-1 inhibitors from PPI to other GAS, if clinically appropriate.
Nazha and colleagues performed a SEER-Medicare database analysis evaluating 367,750 patients with lung cancer. A total of 11,061 patients had an initial prostate cancer diagnosis and subsequent lung cancer diagnosis, 3017 had an initial lung cancer diagnosis and subsequent prostate cancer diagnosis, and the remaining patients had an isolated lung cancer diagnosis. Patients who received androgen deprivation therapy (ADT) for a previously diagnosed prostate cancer showed improved survival after lung cancer diagnosis (adjusted HR for death 0.88; P = .02) and a shorter latency period to the diagnosis of lung cancer (40 vs 47 months; P < .001) compared with those who did not receive ADT. This finding applied mainly to White patients and may not apply to Black patients because there was an underrepresentation of Black patients in the study. The association of ADT for prostate cancer improving clinical outcomes in patients subsequently diagnosed with lung cancer is intriguing. There is known crosstalk between receptor kinase signaling and androgen receptor signaling that may biologically explain the findings in this study. This theoretically could apply more to certain molecular subtypes of lung cancer, such as EGFR-mutated lung cancer. However, further studies are needed to confirm this because confounding factors and immortal time bias (where patients receiving ADT may be more likely to have more frequent interactions in the healthcare system and thus receive an earlier lung cancer diagnosis) may in part explain the findings in this retrospective analysis. More research is needed to determine whether patients with prostate cancer receiving ADT had improved survival compared with those who did not receive ADT.
A Danish population-based cohort study by Ehrenstein and colleagues involved 21,282 patients with non–small-cell lung cancer (NSCLC), 8758 of whom received a diagnosis at stage I-IIIA. Of those, 4071 (46%) were tested for epidermal growth factor receptor (EGFR) mutations at diagnosis. Median overall survival (OS) was 5.7 years among patients with EGFR mutation–positive status (n = 361) and 4.4 years among patients with EGFR mutation–negative status (n = 3710). EGFR mutation–positive status was associated with lower all-cause mortality in all subgroups. This is not surprising, because EGFR-mutated lung cancers are associated with never or light smoking history and the patients tend to be younger and have fewer medical comorbidities. Nevertheless, the lower risk for all-cause mortality was consistent across all subgroups (stage at diagnosis, age, sex, comorbidity, and surgery receipt), with hazard ratios (HR) ranging from 0.48 to 0.83. In addition, targeted therapies, such as osimertinib, improved OS and progression-free survival (PFS) in the metastatic setting as first-line treatment. Now that the ADAURA study has demonstrated a substantial disease-free survival benefit with osimertinib in the adjuvant setting in early-stage EGFR-mutated NSCLC (with final OS results still to come) it will be interesting to see whether this magnitude of difference in OS grows over time.
Wang and colleagues conducted a large meta-analysis of 10 retrospective studies and one prospective study including a total of 5892 patients with NSCLC who were receiving programmed cell death protein 1 (PD-1)/ programmed death-ligand 1 (PD-L1) inhibitors with the concomitant use of gastric acid suppressants (GAS). Use of PD-1/PD-L1 inhibitors with vs without GAS worsened PFS by 32% (HR 1.32; P < .001) and OS by 36% (HR 1.36; P < .001). The GAS in these studies were predominantly proton-pump inhibitors (PPI). There is still much to learn about medications that may influence outcomes to immune checkpoint blockade, such as steroids, antibiotics, GAS, and others. We are also learning that the microbiome probably plays an important role in contributing to activity of PD-1/PDL-1 antibodies and PPI may modify the microbiome. More research is needed, but it is reasonable to try and switch patients receiving PD-1/PDL-1 inhibitors from PPI to other GAS, if clinically appropriate.
Nazha and colleagues performed a SEER-Medicare database analysis evaluating 367,750 patients with lung cancer. A total of 11,061 patients had an initial prostate cancer diagnosis and subsequent lung cancer diagnosis, 3017 had an initial lung cancer diagnosis and subsequent prostate cancer diagnosis, and the remaining patients had an isolated lung cancer diagnosis. Patients who received androgen deprivation therapy (ADT) for a previously diagnosed prostate cancer showed improved survival after lung cancer diagnosis (adjusted HR for death 0.88; P = .02) and a shorter latency period to the diagnosis of lung cancer (40 vs 47 months; P < .001) compared with those who did not receive ADT. This finding applied mainly to White patients and may not apply to Black patients because there was an underrepresentation of Black patients in the study. The association of ADT for prostate cancer improving clinical outcomes in patients subsequently diagnosed with lung cancer is intriguing. There is known crosstalk between receptor kinase signaling and androgen receptor signaling that may biologically explain the findings in this study. This theoretically could apply more to certain molecular subtypes of lung cancer, such as EGFR-mutated lung cancer. However, further studies are needed to confirm this because confounding factors and immortal time bias (where patients receiving ADT may be more likely to have more frequent interactions in the healthcare system and thus receive an earlier lung cancer diagnosis) may in part explain the findings in this retrospective analysis. More research is needed to determine whether patients with prostate cancer receiving ADT had improved survival compared with those who did not receive ADT.
A Danish population-based cohort study by Ehrenstein and colleagues involved 21,282 patients with non–small-cell lung cancer (NSCLC), 8758 of whom received a diagnosis at stage I-IIIA. Of those, 4071 (46%) were tested for epidermal growth factor receptor (EGFR) mutations at diagnosis. Median overall survival (OS) was 5.7 years among patients with EGFR mutation–positive status (n = 361) and 4.4 years among patients with EGFR mutation–negative status (n = 3710). EGFR mutation–positive status was associated with lower all-cause mortality in all subgroups. This is not surprising, because EGFR-mutated lung cancers are associated with never or light smoking history and the patients tend to be younger and have fewer medical comorbidities. Nevertheless, the lower risk for all-cause mortality was consistent across all subgroups (stage at diagnosis, age, sex, comorbidity, and surgery receipt), with hazard ratios (HR) ranging from 0.48 to 0.83. In addition, targeted therapies, such as osimertinib, improved OS and progression-free survival (PFS) in the metastatic setting as first-line treatment. Now that the ADAURA study has demonstrated a substantial disease-free survival benefit with osimertinib in the adjuvant setting in early-stage EGFR-mutated NSCLC (with final OS results still to come) it will be interesting to see whether this magnitude of difference in OS grows over time.
Wang and colleagues conducted a large meta-analysis of 10 retrospective studies and one prospective study including a total of 5892 patients with NSCLC who were receiving programmed cell death protein 1 (PD-1)/ programmed death-ligand 1 (PD-L1) inhibitors with the concomitant use of gastric acid suppressants (GAS). Use of PD-1/PD-L1 inhibitors with vs without GAS worsened PFS by 32% (HR 1.32; P < .001) and OS by 36% (HR 1.36; P < .001). The GAS in these studies were predominantly proton-pump inhibitors (PPI). There is still much to learn about medications that may influence outcomes to immune checkpoint blockade, such as steroids, antibiotics, GAS, and others. We are also learning that the microbiome probably plays an important role in contributing to activity of PD-1/PDL-1 antibodies and PPI may modify the microbiome. More research is needed, but it is reasonable to try and switch patients receiving PD-1/PDL-1 inhibitors from PPI to other GAS, if clinically appropriate.
Nazha and colleagues performed a SEER-Medicare database analysis evaluating 367,750 patients with lung cancer. A total of 11,061 patients had an initial prostate cancer diagnosis and subsequent lung cancer diagnosis, 3017 had an initial lung cancer diagnosis and subsequent prostate cancer diagnosis, and the remaining patients had an isolated lung cancer diagnosis. Patients who received androgen deprivation therapy (ADT) for a previously diagnosed prostate cancer showed improved survival after lung cancer diagnosis (adjusted HR for death 0.88; P = .02) and a shorter latency period to the diagnosis of lung cancer (40 vs 47 months; P < .001) compared with those who did not receive ADT. This finding applied mainly to White patients and may not apply to Black patients because there was an underrepresentation of Black patients in the study. The association of ADT for prostate cancer improving clinical outcomes in patients subsequently diagnosed with lung cancer is intriguing. There is known crosstalk between receptor kinase signaling and androgen receptor signaling that may biologically explain the findings in this study. This theoretically could apply more to certain molecular subtypes of lung cancer, such as EGFR-mutated lung cancer. However, further studies are needed to confirm this because confounding factors and immortal time bias (where patients receiving ADT may be more likely to have more frequent interactions in the healthcare system and thus receive an earlier lung cancer diagnosis) may in part explain the findings in this retrospective analysis. More research is needed to determine whether patients with prostate cancer receiving ADT had improved survival compared with those who did not receive ADT.
Federal Health Care Data Trends 2022: Military Sexual Trauma
- Hard truths and the duty to change: recommendations from the Independent Review Commission on Sexual Assault in the Military. Published July 1, 2022. Accessed May 20, 2022. https://media.defense.gov/2021/Jul/02/2002755437/-1/-1/0/IRC-FULL-REPORT-FINAL-1923-7-1-2.PDF
- Gross GM, Ronzitti S, Combellick JL, et al. Sex differences in military sexual trauma and severe self-directed violence. Am J Prev Med. 2020;58(5):675-682. http://doi.org/10.1016/j.amepre.2019.12.006
- Nitcher B, Holliday R, Monteith LL, et al. Military sexual trauma in the United States: results from a population-based study. J Affect Disord. 2022;306:19-27. http://doi.org/10.1016/j.jad.2022.03.016
- Lofgreen AM, Carroll KK, Dugan SA, Karnik NS. An overview of sexual trauma in the US Military. Focus (Am Psychiatr Publ). 2017;15(4):411-419. http://doi.org/10.1176/appi.focus.20170024
- Schuyler AC, Klemmer C, Mamey MR, et al. Experiences of sexual harassment, stalking, and sexual assault during military service among LGBT and non-LGBT service members. J Trauma Stress. 2020;33(3):257-266. http://doi.org/10.1002/jts.22506
- Department of Defense annual report on sexual assault in the military: fiscal year 2020. May 15, 2021. Accessed March 24, 2022. https://www.sapr.mil/sites/default/files/DOD_Annual_Report_on_Sexual_Assault_in_the_Military_FY2020.pdf
- Department of Defense annual report on sexual assault in the military: fiscal year 2020. Appendix B: statistical data on sexual assault. May 15, 2021. Accessed March 24, 2022. https://www.sapr.mil/sites/default/files/Appendix_B_Statistical_Data_On_Sexual_Assault_FY2020.pdf
- Department of Defense annual report on sexual assault in the military: fiscal year 2020. Appendix C: metrics and non-metrics on sexual assault. May 15, 2021. Accessed March 24, 2022. https://www.sapr.mil/sites/default/files/Appendix_C_Metrics_and_NonMetrics_on_Sexual_Assault_FY2020.pdf
- Barth SK, Kimerling RE, Pavao J, et al. Military sexual trauma among recent veterans: correlates of sexual assault and sexual harassment. Am J Prev Med. 2016;50(1):77-86. http://doi.org/10.1016/j.amepre.2015.06.012
- Military sexual trauma. US Department of Veterans Affairs. Updated March 11, 2022. Accessed March 24, 2022. https://www.mentalhealth.va.gov/msthome/index.asp
- Hahn CK, Turchik J, Kimerling R. A latent class analysis of mental health beliefs related to military sexual trauma. J Trauma Stress. 2021;34(2):394-404. http://doi.org/10.1002/jts.22585
- Hard truths and the duty to change: recommendations from the Independent Review Commission on Sexual Assault in the Military. Published July 1, 2022. Accessed May 20, 2022. https://media.defense.gov/2021/Jul/02/2002755437/-1/-1/0/IRC-FULL-REPORT-FINAL-1923-7-1-2.PDF
- Gross GM, Ronzitti S, Combellick JL, et al. Sex differences in military sexual trauma and severe self-directed violence. Am J Prev Med. 2020;58(5):675-682. http://doi.org/10.1016/j.amepre.2019.12.006
- Nitcher B, Holliday R, Monteith LL, et al. Military sexual trauma in the United States: results from a population-based study. J Affect Disord. 2022;306:19-27. http://doi.org/10.1016/j.jad.2022.03.016
- Lofgreen AM, Carroll KK, Dugan SA, Karnik NS. An overview of sexual trauma in the US Military. Focus (Am Psychiatr Publ). 2017;15(4):411-419. http://doi.org/10.1176/appi.focus.20170024
- Schuyler AC, Klemmer C, Mamey MR, et al. Experiences of sexual harassment, stalking, and sexual assault during military service among LGBT and non-LGBT service members. J Trauma Stress. 2020;33(3):257-266. http://doi.org/10.1002/jts.22506
- Department of Defense annual report on sexual assault in the military: fiscal year 2020. May 15, 2021. Accessed March 24, 2022. https://www.sapr.mil/sites/default/files/DOD_Annual_Report_on_Sexual_Assault_in_the_Military_FY2020.pdf
- Department of Defense annual report on sexual assault in the military: fiscal year 2020. Appendix B: statistical data on sexual assault. May 15, 2021. Accessed March 24, 2022. https://www.sapr.mil/sites/default/files/Appendix_B_Statistical_Data_On_Sexual_Assault_FY2020.pdf
- Department of Defense annual report on sexual assault in the military: fiscal year 2020. Appendix C: metrics and non-metrics on sexual assault. May 15, 2021. Accessed March 24, 2022. https://www.sapr.mil/sites/default/files/Appendix_C_Metrics_and_NonMetrics_on_Sexual_Assault_FY2020.pdf
- Barth SK, Kimerling RE, Pavao J, et al. Military sexual trauma among recent veterans: correlates of sexual assault and sexual harassment. Am J Prev Med. 2016;50(1):77-86. http://doi.org/10.1016/j.amepre.2015.06.012
- Military sexual trauma. US Department of Veterans Affairs. Updated March 11, 2022. Accessed March 24, 2022. https://www.mentalhealth.va.gov/msthome/index.asp
- Hahn CK, Turchik J, Kimerling R. A latent class analysis of mental health beliefs related to military sexual trauma. J Trauma Stress. 2021;34(2):394-404. http://doi.org/10.1002/jts.22585
- Hard truths and the duty to change: recommendations from the Independent Review Commission on Sexual Assault in the Military. Published July 1, 2022. Accessed May 20, 2022. https://media.defense.gov/2021/Jul/02/2002755437/-1/-1/0/IRC-FULL-REPORT-FINAL-1923-7-1-2.PDF
- Gross GM, Ronzitti S, Combellick JL, et al. Sex differences in military sexual trauma and severe self-directed violence. Am J Prev Med. 2020;58(5):675-682. http://doi.org/10.1016/j.amepre.2019.12.006
- Nitcher B, Holliday R, Monteith LL, et al. Military sexual trauma in the United States: results from a population-based study. J Affect Disord. 2022;306:19-27. http://doi.org/10.1016/j.jad.2022.03.016
- Lofgreen AM, Carroll KK, Dugan SA, Karnik NS. An overview of sexual trauma in the US Military. Focus (Am Psychiatr Publ). 2017;15(4):411-419. http://doi.org/10.1176/appi.focus.20170024
- Schuyler AC, Klemmer C, Mamey MR, et al. Experiences of sexual harassment, stalking, and sexual assault during military service among LGBT and non-LGBT service members. J Trauma Stress. 2020;33(3):257-266. http://doi.org/10.1002/jts.22506
- Department of Defense annual report on sexual assault in the military: fiscal year 2020. May 15, 2021. Accessed March 24, 2022. https://www.sapr.mil/sites/default/files/DOD_Annual_Report_on_Sexual_Assault_in_the_Military_FY2020.pdf
- Department of Defense annual report on sexual assault in the military: fiscal year 2020. Appendix B: statistical data on sexual assault. May 15, 2021. Accessed March 24, 2022. https://www.sapr.mil/sites/default/files/Appendix_B_Statistical_Data_On_Sexual_Assault_FY2020.pdf
- Department of Defense annual report on sexual assault in the military: fiscal year 2020. Appendix C: metrics and non-metrics on sexual assault. May 15, 2021. Accessed March 24, 2022. https://www.sapr.mil/sites/default/files/Appendix_C_Metrics_and_NonMetrics_on_Sexual_Assault_FY2020.pdf
- Barth SK, Kimerling RE, Pavao J, et al. Military sexual trauma among recent veterans: correlates of sexual assault and sexual harassment. Am J Prev Med. 2016;50(1):77-86. http://doi.org/10.1016/j.amepre.2015.06.012
- Military sexual trauma. US Department of Veterans Affairs. Updated March 11, 2022. Accessed March 24, 2022. https://www.mentalhealth.va.gov/msthome/index.asp
- Hahn CK, Turchik J, Kimerling R. A latent class analysis of mental health beliefs related to military sexual trauma. J Trauma Stress. 2021;34(2):394-404. http://doi.org/10.1002/jts.22585