User login
Possible link between dietary niacin intake and migraine prevalence
Key clinical point: Increased dietary niacin intake may have a beneficial effect on migraine outcomes in adults with inadequate niacin consumption and the effect seems to peak in patients with adequate niacin intake, with the threshold level being approximately 21.0 mg/day.
Major finding: The risk for migraine was lower among adults in the higher (18.4-26.2 mg/day: odds ratio [OR] 0.78; P = .004, and ≥26.3 mg/day: OR 0.74; P = .006) vs lower (≤12.3 mg/day) quartile of daily niacin intake, with the risk of developing migraine reducing by 2.5% with every 1 mg increase in daily dietary niacin consumption (OR 0.975; P = .011) in those with dietary niacin intake of <21 mg/day, but no such association was observed in those with dietary niacin intake of ≥21 mg/day.
Study details: This was a cross-sectional study including 10,246 participants aged ≥20 years, of whom 20.1% experienced migraine.
Disclosures: This study was supported by the National Natural Science Foundation of China. The authors declared no conflicts of interest.
Source: Liu H et al. Association between dietary niacin intake and migraine among american adults: National Health and Nutrition Examination Survey. Nutrients. 2022;14(15):3052 (Jul 25). Doi: 10.3390/nu14153052
Key clinical point: Increased dietary niacin intake may have a beneficial effect on migraine outcomes in adults with inadequate niacin consumption and the effect seems to peak in patients with adequate niacin intake, with the threshold level being approximately 21.0 mg/day.
Major finding: The risk for migraine was lower among adults in the higher (18.4-26.2 mg/day: odds ratio [OR] 0.78; P = .004, and ≥26.3 mg/day: OR 0.74; P = .006) vs lower (≤12.3 mg/day) quartile of daily niacin intake, with the risk of developing migraine reducing by 2.5% with every 1 mg increase in daily dietary niacin consumption (OR 0.975; P = .011) in those with dietary niacin intake of <21 mg/day, but no such association was observed in those with dietary niacin intake of ≥21 mg/day.
Study details: This was a cross-sectional study including 10,246 participants aged ≥20 years, of whom 20.1% experienced migraine.
Disclosures: This study was supported by the National Natural Science Foundation of China. The authors declared no conflicts of interest.
Source: Liu H et al. Association between dietary niacin intake and migraine among american adults: National Health and Nutrition Examination Survey. Nutrients. 2022;14(15):3052 (Jul 25). Doi: 10.3390/nu14153052
Key clinical point: Increased dietary niacin intake may have a beneficial effect on migraine outcomes in adults with inadequate niacin consumption and the effect seems to peak in patients with adequate niacin intake, with the threshold level being approximately 21.0 mg/day.
Major finding: The risk for migraine was lower among adults in the higher (18.4-26.2 mg/day: odds ratio [OR] 0.78; P = .004, and ≥26.3 mg/day: OR 0.74; P = .006) vs lower (≤12.3 mg/day) quartile of daily niacin intake, with the risk of developing migraine reducing by 2.5% with every 1 mg increase in daily dietary niacin consumption (OR 0.975; P = .011) in those with dietary niacin intake of <21 mg/day, but no such association was observed in those with dietary niacin intake of ≥21 mg/day.
Study details: This was a cross-sectional study including 10,246 participants aged ≥20 years, of whom 20.1% experienced migraine.
Disclosures: This study was supported by the National Natural Science Foundation of China. The authors declared no conflicts of interest.
Source: Liu H et al. Association between dietary niacin intake and migraine among american adults: National Health and Nutrition Examination Survey. Nutrients. 2022;14(15):3052 (Jul 25). Doi: 10.3390/nu14153052
Soy isoflavones improve migraine characteristics and CGRP levels in women with migraine
Key clinical point: Soy isoflavones significantly reduced the frequency and duration of migraine attacks, clinical indices, and calcitonin gene-related peptide (CGRP) levels and improved the quality of life in women with migraine.
Major finding: At 8 weeks, soy isoflavones vs placebo significantly reduced migraine frequency (mean change [MC] −2.36 vs −0.43; P < .001) and duration of attacks (MC −2.50 vs −0.02; P < .001), Migraine Headache Index score (MC −10.46 vs −1.47; P < .001), and CGRP levels (MC −12.18 vs −8.62 ng/L; P = .002) and significantly improved migraine-specific quality-of-life score (MC 16.76 vs 2.52; P < .001). No adverse effects were reported.
Study details: Findings are from a phase 3 trial including 88 adult women with migraine who had not reached menopausal/perimenopausal age and were randomly assigned to receive 50 mg/day soy isoflavones or placebo supplementation for 8 weeks.
Disclosures: This study was supported by Isfahan University of Medical Sciences, Iran. The authors declared no conflicts of interest.
Source: Babapour M et al. Effect of soy isoflavones supplementation on migraine characteristics, mental status and calcitonin gene-related peptide (CGRP) levels in women with migraine: results of randomised controlled trial. Nutr J. 2022;21:50 (Jul 30). Doi: 10.1186/s12937-022-00802-z
Key clinical point: Soy isoflavones significantly reduced the frequency and duration of migraine attacks, clinical indices, and calcitonin gene-related peptide (CGRP) levels and improved the quality of life in women with migraine.
Major finding: At 8 weeks, soy isoflavones vs placebo significantly reduced migraine frequency (mean change [MC] −2.36 vs −0.43; P < .001) and duration of attacks (MC −2.50 vs −0.02; P < .001), Migraine Headache Index score (MC −10.46 vs −1.47; P < .001), and CGRP levels (MC −12.18 vs −8.62 ng/L; P = .002) and significantly improved migraine-specific quality-of-life score (MC 16.76 vs 2.52; P < .001). No adverse effects were reported.
Study details: Findings are from a phase 3 trial including 88 adult women with migraine who had not reached menopausal/perimenopausal age and were randomly assigned to receive 50 mg/day soy isoflavones or placebo supplementation for 8 weeks.
Disclosures: This study was supported by Isfahan University of Medical Sciences, Iran. The authors declared no conflicts of interest.
Source: Babapour M et al. Effect of soy isoflavones supplementation on migraine characteristics, mental status and calcitonin gene-related peptide (CGRP) levels in women with migraine: results of randomised controlled trial. Nutr J. 2022;21:50 (Jul 30). Doi: 10.1186/s12937-022-00802-z
Key clinical point: Soy isoflavones significantly reduced the frequency and duration of migraine attacks, clinical indices, and calcitonin gene-related peptide (CGRP) levels and improved the quality of life in women with migraine.
Major finding: At 8 weeks, soy isoflavones vs placebo significantly reduced migraine frequency (mean change [MC] −2.36 vs −0.43; P < .001) and duration of attacks (MC −2.50 vs −0.02; P < .001), Migraine Headache Index score (MC −10.46 vs −1.47; P < .001), and CGRP levels (MC −12.18 vs −8.62 ng/L; P = .002) and significantly improved migraine-specific quality-of-life score (MC 16.76 vs 2.52; P < .001). No adverse effects were reported.
Study details: Findings are from a phase 3 trial including 88 adult women with migraine who had not reached menopausal/perimenopausal age and were randomly assigned to receive 50 mg/day soy isoflavones or placebo supplementation for 8 weeks.
Disclosures: This study was supported by Isfahan University of Medical Sciences, Iran. The authors declared no conflicts of interest.
Source: Babapour M et al. Effect of soy isoflavones supplementation on migraine characteristics, mental status and calcitonin gene-related peptide (CGRP) levels in women with migraine: results of randomised controlled trial. Nutr J. 2022;21:50 (Jul 30). Doi: 10.1186/s12937-022-00802-z
Diabetic retinopathy and migraine prevalence and incidence: What is the link?
Key clinical point: Patients with diabetes who were screened for diabetic retinopathy (DR) had a lower risk of having migraine; however, DR was not a protective marker of incident migraine.
Major finding: The prevalence of migraine was 17% lower in patients with vs without diabetes (odds ratio [OR] 0.83; 95% CI 0.81-0.85), with the risk being lower in patients with vs without DR (OR 0.69; 95% CI 0.65-0.72). The risk of developing migraine was significantly lower in patients with diabetes and DR level ranging between 1 and 4 compared with matched individuals without diabetes (hazard ratio [HR] 0.66; 95% CI 0.55-0.80), but the risk was independent of the presence of DR.
Study details: The data come from a cross-sectional study including patients with diabetes who attended DR screening (n = 205,970) and age- and sex-matched patients without diabetes (n = 1,003,170).
Disclosures: This study was funded by the The Velux Foundation, Denmark. The authors declared no competing interests.
Source: Vergmann AS et al. Investigation of the correlation between diabetic retinopathy and prevalent and incident migraine in a national cohort study. Sci Rep. 2022;12:12443 (Jul 20). Doi: 10.1038/s41598-022-16793-0
Key clinical point: Patients with diabetes who were screened for diabetic retinopathy (DR) had a lower risk of having migraine; however, DR was not a protective marker of incident migraine.
Major finding: The prevalence of migraine was 17% lower in patients with vs without diabetes (odds ratio [OR] 0.83; 95% CI 0.81-0.85), with the risk being lower in patients with vs without DR (OR 0.69; 95% CI 0.65-0.72). The risk of developing migraine was significantly lower in patients with diabetes and DR level ranging between 1 and 4 compared with matched individuals without diabetes (hazard ratio [HR] 0.66; 95% CI 0.55-0.80), but the risk was independent of the presence of DR.
Study details: The data come from a cross-sectional study including patients with diabetes who attended DR screening (n = 205,970) and age- and sex-matched patients without diabetes (n = 1,003,170).
Disclosures: This study was funded by the The Velux Foundation, Denmark. The authors declared no competing interests.
Source: Vergmann AS et al. Investigation of the correlation between diabetic retinopathy and prevalent and incident migraine in a national cohort study. Sci Rep. 2022;12:12443 (Jul 20). Doi: 10.1038/s41598-022-16793-0
Key clinical point: Patients with diabetes who were screened for diabetic retinopathy (DR) had a lower risk of having migraine; however, DR was not a protective marker of incident migraine.
Major finding: The prevalence of migraine was 17% lower in patients with vs without diabetes (odds ratio [OR] 0.83; 95% CI 0.81-0.85), with the risk being lower in patients with vs without DR (OR 0.69; 95% CI 0.65-0.72). The risk of developing migraine was significantly lower in patients with diabetes and DR level ranging between 1 and 4 compared with matched individuals without diabetes (hazard ratio [HR] 0.66; 95% CI 0.55-0.80), but the risk was independent of the presence of DR.
Study details: The data come from a cross-sectional study including patients with diabetes who attended DR screening (n = 205,970) and age- and sex-matched patients without diabetes (n = 1,003,170).
Disclosures: This study was funded by the The Velux Foundation, Denmark. The authors declared no competing interests.
Source: Vergmann AS et al. Investigation of the correlation between diabetic retinopathy and prevalent and incident migraine in a national cohort study. Sci Rep. 2022;12:12443 (Jul 20). Doi: 10.1038/s41598-022-16793-0
Bariatric surgery improves symptoms, quality of life in chronic migraine
Key clinical point: Bariatric surgery significantly reduced the frequency of migraine attacks, headache severity, and improved the quality of life and disability in patients with chronic migraine and severe obesity.
Major finding: After a mean period of 7.5 ± 2.3 months, there was a significant reduction in the number of migraine attacks (20.9 to 8.3 days; P < .001), headache severity score (7.7 to 4.8; P < .001), Migraine-Specific Quality-of-Life score (44.6 to 26.8; P < .001), and Migraine Disability Assessment Scale score (64.4 to 25.5; P < .001) in patients with chronic migraine who underwent bariatric surgery.
Study details: Findings are from a prospective study including 60 patients with chronic migraine and severe obesity who were referred for bariatric surgery.
Disclosures: This study was supported by Isfahan University of Medical Sciences, Iran, and others. The authors declared no conflicts of interest.
Source: Etefagh HH et al. Bariatric surgery in migraine patients: CGRP level and weight loss. Obes Surg. 2022 (Aug 3). Doi: 10.1007/s11695-022-06218-2
Key clinical point: Bariatric surgery significantly reduced the frequency of migraine attacks, headache severity, and improved the quality of life and disability in patients with chronic migraine and severe obesity.
Major finding: After a mean period of 7.5 ± 2.3 months, there was a significant reduction in the number of migraine attacks (20.9 to 8.3 days; P < .001), headache severity score (7.7 to 4.8; P < .001), Migraine-Specific Quality-of-Life score (44.6 to 26.8; P < .001), and Migraine Disability Assessment Scale score (64.4 to 25.5; P < .001) in patients with chronic migraine who underwent bariatric surgery.
Study details: Findings are from a prospective study including 60 patients with chronic migraine and severe obesity who were referred for bariatric surgery.
Disclosures: This study was supported by Isfahan University of Medical Sciences, Iran, and others. The authors declared no conflicts of interest.
Source: Etefagh HH et al. Bariatric surgery in migraine patients: CGRP level and weight loss. Obes Surg. 2022 (Aug 3). Doi: 10.1007/s11695-022-06218-2
Key clinical point: Bariatric surgery significantly reduced the frequency of migraine attacks, headache severity, and improved the quality of life and disability in patients with chronic migraine and severe obesity.
Major finding: After a mean period of 7.5 ± 2.3 months, there was a significant reduction in the number of migraine attacks (20.9 to 8.3 days; P < .001), headache severity score (7.7 to 4.8; P < .001), Migraine-Specific Quality-of-Life score (44.6 to 26.8; P < .001), and Migraine Disability Assessment Scale score (64.4 to 25.5; P < .001) in patients with chronic migraine who underwent bariatric surgery.
Study details: Findings are from a prospective study including 60 patients with chronic migraine and severe obesity who were referred for bariatric surgery.
Disclosures: This study was supported by Isfahan University of Medical Sciences, Iran, and others. The authors declared no conflicts of interest.
Source: Etefagh HH et al. Bariatric surgery in migraine patients: CGRP level and weight loss. Obes Surg. 2022 (Aug 3). Doi: 10.1007/s11695-022-06218-2
Galcanezumab reduces total pain burden in treatment-resistant migraine
Key clinical point: Once-monthly 120 mg galcanezumab was more effective than placebo in reducing total pain burden (TPB) in patients with chronic or episodic migraine who previously did not benefit from 2-4 categories of migraine preventive medication.
Major finding: At 3 months, galcanezumab vs placebo led to a significantly higher overall percentage change in TPB in patients with chronic (mean difference [MD] −40.4%; P < .001) or episodic (MD −53.1%; P < .001) migraine and significant reductions in monthly number, duration, and severity of migraine headache days in the overall population (all P < .001).
Study details: Findings are from a post hoc analysis of a phase 3 trial, CONQUER, including 458 patients with chronic or episodic migraine who previously did not benefit from 2-4 categories of migraine preventive medication and were randomly assigned to receive galcanezumab or placebo.
Disclosures: This study was sponsored by Eli Lilly and Company. Four authors declared being current or former employees or stockholders of Eli Lilly. J Ailani reported ties with various sources, including Eli Lilly and Company.
Source: Ailani J et al. Effect of galcanezumab on total pain burden in patients who had previously not benefited from migraine preventive medication (CONQUER Trial): A post hoc analysis. Adv Ther. 2022 (Aug 5). Doi: 10.1007/s12325-022-02233-y
Key clinical point: Once-monthly 120 mg galcanezumab was more effective than placebo in reducing total pain burden (TPB) in patients with chronic or episodic migraine who previously did not benefit from 2-4 categories of migraine preventive medication.
Major finding: At 3 months, galcanezumab vs placebo led to a significantly higher overall percentage change in TPB in patients with chronic (mean difference [MD] −40.4%; P < .001) or episodic (MD −53.1%; P < .001) migraine and significant reductions in monthly number, duration, and severity of migraine headache days in the overall population (all P < .001).
Study details: Findings are from a post hoc analysis of a phase 3 trial, CONQUER, including 458 patients with chronic or episodic migraine who previously did not benefit from 2-4 categories of migraine preventive medication and were randomly assigned to receive galcanezumab or placebo.
Disclosures: This study was sponsored by Eli Lilly and Company. Four authors declared being current or former employees or stockholders of Eli Lilly. J Ailani reported ties with various sources, including Eli Lilly and Company.
Source: Ailani J et al. Effect of galcanezumab on total pain burden in patients who had previously not benefited from migraine preventive medication (CONQUER Trial): A post hoc analysis. Adv Ther. 2022 (Aug 5). Doi: 10.1007/s12325-022-02233-y
Key clinical point: Once-monthly 120 mg galcanezumab was more effective than placebo in reducing total pain burden (TPB) in patients with chronic or episodic migraine who previously did not benefit from 2-4 categories of migraine preventive medication.
Major finding: At 3 months, galcanezumab vs placebo led to a significantly higher overall percentage change in TPB in patients with chronic (mean difference [MD] −40.4%; P < .001) or episodic (MD −53.1%; P < .001) migraine and significant reductions in monthly number, duration, and severity of migraine headache days in the overall population (all P < .001).
Study details: Findings are from a post hoc analysis of a phase 3 trial, CONQUER, including 458 patients with chronic or episodic migraine who previously did not benefit from 2-4 categories of migraine preventive medication and were randomly assigned to receive galcanezumab or placebo.
Disclosures: This study was sponsored by Eli Lilly and Company. Four authors declared being current or former employees or stockholders of Eli Lilly. J Ailani reported ties with various sources, including Eli Lilly and Company.
Source: Ailani J et al. Effect of galcanezumab on total pain burden in patients who had previously not benefited from migraine preventive medication (CONQUER Trial): A post hoc analysis. Adv Ther. 2022 (Aug 5). Doi: 10.1007/s12325-022-02233-y
Galcanezumab effective and safe in episodic migraine
Key clinical point: A dose of 120 mg galcanezumab monthly was effective and well tolerated in patients with episodic migraine.
Major finding: The reduction in mean monthly migraine headache days (MMHD) over 3 months was significantly higher with galcanezumab vs placebo (least squares mean change −3.81 vs −1.99 days; P < .0001), with a higher proportion of patients receiving galcanezumab vs placebo achieving ≥50%, ≥75%, and 100% reductions in MMHD (all P < .0001). The occurrence of serious adverse events was low, with none leading to treatment discontinuation.
Study details: Findings are from the phase 3, PERSIST trial including 520 patients with episodic migraine who were randomly assigned to receive monthly 120 mg galcanezumab or placebo.
Disclosures: This study was funded by Eli Lilly and Company. J Zhuang reported being a full-time employee, and 8 authors reported receiving clinical research fees from Eli Lilly. S Yu reported serving as an associate editor for the Journal of Headache and Pain.
Source: Hu B et al. Galcanezumab in episodic migraine: The phase 3, randomized, double-blind, placebo-controlled PERSIST study. J Headache Pain. 2022;23:90 (Jul 28). Doi: 10.1186/s10194-022-01458-0
Key clinical point: A dose of 120 mg galcanezumab monthly was effective and well tolerated in patients with episodic migraine.
Major finding: The reduction in mean monthly migraine headache days (MMHD) over 3 months was significantly higher with galcanezumab vs placebo (least squares mean change −3.81 vs −1.99 days; P < .0001), with a higher proportion of patients receiving galcanezumab vs placebo achieving ≥50%, ≥75%, and 100% reductions in MMHD (all P < .0001). The occurrence of serious adverse events was low, with none leading to treatment discontinuation.
Study details: Findings are from the phase 3, PERSIST trial including 520 patients with episodic migraine who were randomly assigned to receive monthly 120 mg galcanezumab or placebo.
Disclosures: This study was funded by Eli Lilly and Company. J Zhuang reported being a full-time employee, and 8 authors reported receiving clinical research fees from Eli Lilly. S Yu reported serving as an associate editor for the Journal of Headache and Pain.
Source: Hu B et al. Galcanezumab in episodic migraine: The phase 3, randomized, double-blind, placebo-controlled PERSIST study. J Headache Pain. 2022;23:90 (Jul 28). Doi: 10.1186/s10194-022-01458-0
Key clinical point: A dose of 120 mg galcanezumab monthly was effective and well tolerated in patients with episodic migraine.
Major finding: The reduction in mean monthly migraine headache days (MMHD) over 3 months was significantly higher with galcanezumab vs placebo (least squares mean change −3.81 vs −1.99 days; P < .0001), with a higher proportion of patients receiving galcanezumab vs placebo achieving ≥50%, ≥75%, and 100% reductions in MMHD (all P < .0001). The occurrence of serious adverse events was low, with none leading to treatment discontinuation.
Study details: Findings are from the phase 3, PERSIST trial including 520 patients with episodic migraine who were randomly assigned to receive monthly 120 mg galcanezumab or placebo.
Disclosures: This study was funded by Eli Lilly and Company. J Zhuang reported being a full-time employee, and 8 authors reported receiving clinical research fees from Eli Lilly. S Yu reported serving as an associate editor for the Journal of Headache and Pain.
Source: Hu B et al. Galcanezumab in episodic migraine: The phase 3, randomized, double-blind, placebo-controlled PERSIST study. J Headache Pain. 2022;23:90 (Jul 28). Doi: 10.1186/s10194-022-01458-0
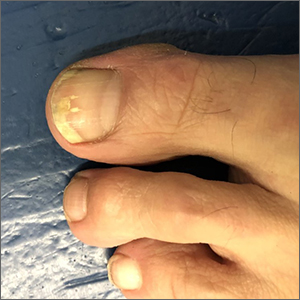
Toenail trauma
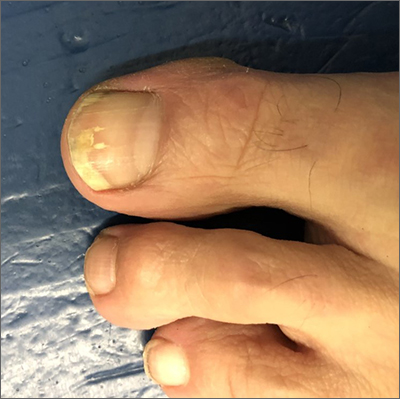
The patient’s initial injury was probably a subungual hematoma, which can take 12 to 18 months to resolve (the time it takes for a new toenail to grow). However, the precipitating trauma likely created an opportunity for fungal elements to invade the nail plate, resulting in the current complaint of superficial onychomycosis.
Onychomycosis is a frequently seen condition with increasing prevalence in older patients. It has several clinical presentations: Superficial onychomycosis manifests with chalky white changes on the surface of the nail. Distal subungual onychomycosis develops at the distal aspect of the nail with thickening and subungual debris. Proximal subungual onychomycosis occurs in the proximal aspect of the nail.
Although often asymptomatic, onychomycosis can cause thickening of the nails and development of subsequent deformity or pincer nails (which painfully “pinch” the underlying skin). It is especially concerning in patients with diabetes or peripheral neuropathy, in whom the abnormal thickness and shape of the nails can lead to microtrauma at the proximal and lateral attachments of the nail. These patients have an increased risk of secondary infection, possible complications, and even, for some, amputation.
If the patient is asymptomatic, and does not have diabetes, neuropathy, or other risk factors, treatment is not required. For those who would benefit from treatment, it is usually safe and inexpensive with the current generation of oral antifungal medications.
Some recommend confirmatory testing before treatment intiation,1 but the low adverse effect profile of terbinafine and its current cost below $10/month2 make empiric treatment safe and cost effective in most cases.3 If needed, and with access to microscopy, a potassium hydroxide (KOH) prep can be performed on scrapings from the affected portions of the nail. If that is not available, scrapings or clippings can be sent to the lab for KOH and periodic acid-Schiff staining.
The US Food and Drug Administration previously recommended follow-up liver enzyme tests if terbinafine is used for more than 6 weeks. (Fingernails require only 6 weeks of treatment, but toenails grow more slowly and require 12 weeks of treatment.) However, research has demonstrated that hepatotoxicity risk is extremely low and transaminase elevations are rare.4 In the rare cases that liver dysfunction has occurred, patients developed symptoms of jaundice, malaise, dark urine, or pruritis.4
This patient was counseled regarding the fungal nature of onychomycosis and the general safety of a 90-day course of oral terbinafine 250 mg/d—provided he did not have underlying liver or kidney disease or leukopenia. He reported that he had not had any blood work performed in the past year but was due for his annual wellness evaluation, at which he would discuss his overall health with his primary care provider, obtain baseline blood testing, and determine whether to proceed with treatment. He was advised that if, after starting treatment, he developed any symptoms of jaundice, dark urine, or other difficulties, he should report them to his care team.
Photo courtesy of Daniel Stulberg, MD. Text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
- Frazier WT, Santiago-Delgado ZM, Stupka KC 2nd. Onychomycosis: rapid evidence review. Am Fam Physician. 2021;104:359-367.
- Terbinafine. GoodRx. Accessed August 9, 2022. https://www.goodrx.com/terbinafine
- Mikailov A, Cohen J, Joyce C, et al. Cost-effectiveness of confirmatory testing before treatment of onychomycosis. JAMA Dermatol. 2016;152:276-281. doi: 10.1001/jamadermatol.2015.4190
- Sun CW, Hsu S. Terbinafine: safety profile and monitoring in treatment of dermatophyte infections. Dermatol Ther. 2019;32:e13111. doi: 10.1111/dth.13111

The patient’s initial injury was probably a subungual hematoma, which can take 12 to 18 months to resolve (the time it takes for a new toenail to grow). However, the precipitating trauma likely created an opportunity for fungal elements to invade the nail plate, resulting in the current complaint of superficial onychomycosis.
Onychomycosis is a frequently seen condition with increasing prevalence in older patients. It has several clinical presentations: Superficial onychomycosis manifests with chalky white changes on the surface of the nail. Distal subungual onychomycosis develops at the distal aspect of the nail with thickening and subungual debris. Proximal subungual onychomycosis occurs in the proximal aspect of the nail.
Although often asymptomatic, onychomycosis can cause thickening of the nails and development of subsequent deformity or pincer nails (which painfully “pinch” the underlying skin). It is especially concerning in patients with diabetes or peripheral neuropathy, in whom the abnormal thickness and shape of the nails can lead to microtrauma at the proximal and lateral attachments of the nail. These patients have an increased risk of secondary infection, possible complications, and even, for some, amputation.
If the patient is asymptomatic, and does not have diabetes, neuropathy, or other risk factors, treatment is not required. For those who would benefit from treatment, it is usually safe and inexpensive with the current generation of oral antifungal medications.
Some recommend confirmatory testing before treatment intiation,1 but the low adverse effect profile of terbinafine and its current cost below $10/month2 make empiric treatment safe and cost effective in most cases.3 If needed, and with access to microscopy, a potassium hydroxide (KOH) prep can be performed on scrapings from the affected portions of the nail. If that is not available, scrapings or clippings can be sent to the lab for KOH and periodic acid-Schiff staining.
The US Food and Drug Administration previously recommended follow-up liver enzyme tests if terbinafine is used for more than 6 weeks. (Fingernails require only 6 weeks of treatment, but toenails grow more slowly and require 12 weeks of treatment.) However, research has demonstrated that hepatotoxicity risk is extremely low and transaminase elevations are rare.4 In the rare cases that liver dysfunction has occurred, patients developed symptoms of jaundice, malaise, dark urine, or pruritis.4
This patient was counseled regarding the fungal nature of onychomycosis and the general safety of a 90-day course of oral terbinafine 250 mg/d—provided he did not have underlying liver or kidney disease or leukopenia. He reported that he had not had any blood work performed in the past year but was due for his annual wellness evaluation, at which he would discuss his overall health with his primary care provider, obtain baseline blood testing, and determine whether to proceed with treatment. He was advised that if, after starting treatment, he developed any symptoms of jaundice, dark urine, or other difficulties, he should report them to his care team.
Photo courtesy of Daniel Stulberg, MD. Text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

The patient’s initial injury was probably a subungual hematoma, which can take 12 to 18 months to resolve (the time it takes for a new toenail to grow). However, the precipitating trauma likely created an opportunity for fungal elements to invade the nail plate, resulting in the current complaint of superficial onychomycosis.
Onychomycosis is a frequently seen condition with increasing prevalence in older patients. It has several clinical presentations: Superficial onychomycosis manifests with chalky white changes on the surface of the nail. Distal subungual onychomycosis develops at the distal aspect of the nail with thickening and subungual debris. Proximal subungual onychomycosis occurs in the proximal aspect of the nail.
Although often asymptomatic, onychomycosis can cause thickening of the nails and development of subsequent deformity or pincer nails (which painfully “pinch” the underlying skin). It is especially concerning in patients with diabetes or peripheral neuropathy, in whom the abnormal thickness and shape of the nails can lead to microtrauma at the proximal and lateral attachments of the nail. These patients have an increased risk of secondary infection, possible complications, and even, for some, amputation.
If the patient is asymptomatic, and does not have diabetes, neuropathy, or other risk factors, treatment is not required. For those who would benefit from treatment, it is usually safe and inexpensive with the current generation of oral antifungal medications.
Some recommend confirmatory testing before treatment intiation,1 but the low adverse effect profile of terbinafine and its current cost below $10/month2 make empiric treatment safe and cost effective in most cases.3 If needed, and with access to microscopy, a potassium hydroxide (KOH) prep can be performed on scrapings from the affected portions of the nail. If that is not available, scrapings or clippings can be sent to the lab for KOH and periodic acid-Schiff staining.
The US Food and Drug Administration previously recommended follow-up liver enzyme tests if terbinafine is used for more than 6 weeks. (Fingernails require only 6 weeks of treatment, but toenails grow more slowly and require 12 weeks of treatment.) However, research has demonstrated that hepatotoxicity risk is extremely low and transaminase elevations are rare.4 In the rare cases that liver dysfunction has occurred, patients developed symptoms of jaundice, malaise, dark urine, or pruritis.4
This patient was counseled regarding the fungal nature of onychomycosis and the general safety of a 90-day course of oral terbinafine 250 mg/d—provided he did not have underlying liver or kidney disease or leukopenia. He reported that he had not had any blood work performed in the past year but was due for his annual wellness evaluation, at which he would discuss his overall health with his primary care provider, obtain baseline blood testing, and determine whether to proceed with treatment. He was advised that if, after starting treatment, he developed any symptoms of jaundice, dark urine, or other difficulties, he should report them to his care team.
Photo courtesy of Daniel Stulberg, MD. Text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
- Frazier WT, Santiago-Delgado ZM, Stupka KC 2nd. Onychomycosis: rapid evidence review. Am Fam Physician. 2021;104:359-367.
- Terbinafine. GoodRx. Accessed August 9, 2022. https://www.goodrx.com/terbinafine
- Mikailov A, Cohen J, Joyce C, et al. Cost-effectiveness of confirmatory testing before treatment of onychomycosis. JAMA Dermatol. 2016;152:276-281. doi: 10.1001/jamadermatol.2015.4190
- Sun CW, Hsu S. Terbinafine: safety profile and monitoring in treatment of dermatophyte infections. Dermatol Ther. 2019;32:e13111. doi: 10.1111/dth.13111
- Frazier WT, Santiago-Delgado ZM, Stupka KC 2nd. Onychomycosis: rapid evidence review. Am Fam Physician. 2021;104:359-367.
- Terbinafine. GoodRx. Accessed August 9, 2022. https://www.goodrx.com/terbinafine
- Mikailov A, Cohen J, Joyce C, et al. Cost-effectiveness of confirmatory testing before treatment of onychomycosis. JAMA Dermatol. 2016;152:276-281. doi: 10.1001/jamadermatol.2015.4190
- Sun CW, Hsu S. Terbinafine: safety profile and monitoring in treatment of dermatophyte infections. Dermatol Ther. 2019;32:e13111. doi: 10.1111/dth.13111
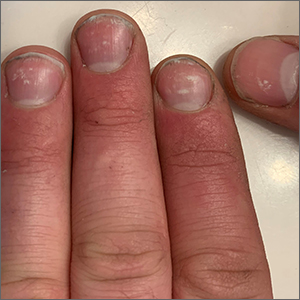
Spotted white fingernails
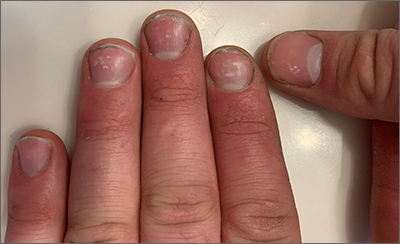
White nail changes are broadly called leukonychia: “leuko” meaning white and “nychia” referring to the nail. Scattered or single asymptomatic cloudy white nail lesions occurring without other associated skin or nail disorders are more specifically called punctate leukonychia.
Punctate leukonychia is theorized to be caused by trauma at the proximal nail matrix, affecting the developing nail.1 The trauma may result from aggressive nail care practices or damage to the cuticle. In many cases, there is no history of known trauma. For this patient with multiple lesions, who performed manual work, multiple small traumas may have induced the punctate leukonychia.
Other causes of leukonychia include superficial onychomycosis (in which discoloration may be whiter than the usual yellow-brown), renal disease, and arsenic toxicity.1 Arsenic toxicity causes transverse leukonychia in a band-like fashion, since it is a systemic insult to the growing nails. Longitudinal leukonychia is due to a more localized insult to the nail matrix, causing the white lines to grow out with the nail along the axis of the digit. Other than avoiding trauma, there is no treatment needed or recommended for punctate leukonychia.
The patient was counseled on the benign nature of his punctate leukonychia and assured that no treatment was necessary.
Photo courtesy of Daniel Stulberg, MD. Text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Iorizzo M, Starace M, Pasch MC. Leukonychia: what can white nails tell us? Am J Clin Dermatol. 2022;23:177-193. doi: 10.1007/s40257-022-00671-6

White nail changes are broadly called leukonychia: “leuko” meaning white and “nychia” referring to the nail. Scattered or single asymptomatic cloudy white nail lesions occurring without other associated skin or nail disorders are more specifically called punctate leukonychia.
Punctate leukonychia is theorized to be caused by trauma at the proximal nail matrix, affecting the developing nail.1 The trauma may result from aggressive nail care practices or damage to the cuticle. In many cases, there is no history of known trauma. For this patient with multiple lesions, who performed manual work, multiple small traumas may have induced the punctate leukonychia.
Other causes of leukonychia include superficial onychomycosis (in which discoloration may be whiter than the usual yellow-brown), renal disease, and arsenic toxicity.1 Arsenic toxicity causes transverse leukonychia in a band-like fashion, since it is a systemic insult to the growing nails. Longitudinal leukonychia is due to a more localized insult to the nail matrix, causing the white lines to grow out with the nail along the axis of the digit. Other than avoiding trauma, there is no treatment needed or recommended for punctate leukonychia.
The patient was counseled on the benign nature of his punctate leukonychia and assured that no treatment was necessary.
Photo courtesy of Daniel Stulberg, MD. Text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

White nail changes are broadly called leukonychia: “leuko” meaning white and “nychia” referring to the nail. Scattered or single asymptomatic cloudy white nail lesions occurring without other associated skin or nail disorders are more specifically called punctate leukonychia.
Punctate leukonychia is theorized to be caused by trauma at the proximal nail matrix, affecting the developing nail.1 The trauma may result from aggressive nail care practices or damage to the cuticle. In many cases, there is no history of known trauma. For this patient with multiple lesions, who performed manual work, multiple small traumas may have induced the punctate leukonychia.
Other causes of leukonychia include superficial onychomycosis (in which discoloration may be whiter than the usual yellow-brown), renal disease, and arsenic toxicity.1 Arsenic toxicity causes transverse leukonychia in a band-like fashion, since it is a systemic insult to the growing nails. Longitudinal leukonychia is due to a more localized insult to the nail matrix, causing the white lines to grow out with the nail along the axis of the digit. Other than avoiding trauma, there is no treatment needed or recommended for punctate leukonychia.
The patient was counseled on the benign nature of his punctate leukonychia and assured that no treatment was necessary.
Photo courtesy of Daniel Stulberg, MD. Text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Iorizzo M, Starace M, Pasch MC. Leukonychia: what can white nails tell us? Am J Clin Dermatol. 2022;23:177-193. doi: 10.1007/s40257-022-00671-6
1. Iorizzo M, Starace M, Pasch MC. Leukonychia: what can white nails tell us? Am J Clin Dermatol. 2022;23:177-193. doi: 10.1007/s40257-022-00671-6
Sun Protection Factor Testing: A Call for an In Vitro Method
The sun protection factor (SPF) value indicates to consumers the level of protection that a given sunscreen formulation provides against erythemally effective UV radiation (UVR). 1 In vivo SPF testing, the gold standard for determining SPF, yields highly variable results and can harm human test participants. 2 In vitro SPF testing methodologies have been under development for years but none have (yet) replaced the in vivo test required by national and international regulatory agencies.
Recent European studies have shown strong data to support a highly standardized in vitro method,1 now under development by the International Organization for Standardization (ISO)—potentially to serve as a new SPF determination standard.1,3 Academia and industry should follow this example and actively take steps to develop and validate a suitable replacement for in vivo SPF testing.
In Vivo SPF Testing
The in vivo SPF test involves comparing doses of UVR necessary to induce erythema in human participants with and without sunscreen applied.2 Although this method has long been the standard for SPF determination, it is associated with the following major disadvantages:
- Cost: The in vivo test is expensive.
- Variability: Results of the test are subject to high interlaboratory variability due to the inherent subjectivity of identifying erythema, the variable skin types of human participants, and other laboratory-dependent factors.2 A study found that the average coefficient of variation for SPF values obtained from 3 or 4 laboratories to be 20%—with values exceeding 50% in some cases. With that level of variability, the same sunscreen may be labeled SPF 30, SPF 50, or SPF 50+, thereby posing a health risk to consumers who rely on the accuracy of such claims. In fact, Miksa et al2 concluded that “the largest obstacle to a reliable SPF assessment for consumer health is the in vivo SPF test itself.”
- Ethical concerns: Human participants are intentionally exposed to harmful UVR until sunburn is achieved. For that reason, there have been calls to abandon the practice of in vivo testing.1
Alternatives to In Vivo SPF Testing
There has been international interest in developing in silico and in vitro alternatives to the in vivo SPF test. These options are attractive because they are relatively inexpensive; avoid exposing human participants to harmful UVR; and have the potential to be more accurate and more reproducible than in vivo tests.
In Vitro Protocols—Many such in vitro tests exist; all generally involve applying a layer of sunscreen to an artificial substrate, exposing it to UVR from a solar simulator, and measuring the UVR transmittance through the product and film by spectrophotometry.1 Prior shortcomings of this method have included suboptimal reproducibility, lack of data on substrate and product properties, and lack of demonstrated equivalency to in vivo SPF testing.4
In Silico Protocols—These tests use data on the UV spectra of sunscreen filters, physical characteristics of sunscreen films on skin, and the unique photoinstability of filters to calculate expected UVR transmittance and SPF of sunscreens based on their ingredients.5 Reports have shown high correlation with in vivo values. Results are not subject to random error; reproducibility is theoretically perfect.5
Regulatory Agencies and In Vitro Testing
In the United States, sunscreens are regulated as over-the-counter drugs. In vivo testing is the only US Food and Drug Administration (FDA)–approved method for determining SPF for labeling purposes.1 In a 2007 Proposed Rule and a 2011 Final Rule, the FDA stated that in vitro SPF tests were an inadequate alternative to in vivo tests because of their shortcomings.4,6
Acknowledging the potential benefits of in vitro testing, the FDA wrote that it would consider in vitro alternatives if equivalency to the in vivo test could be proved.6 The agency has not published an official stance on in vitro SPF testing since those statements in 2007 and 2011. Of note, the FDA deems in vitro testing sufficient for making claims of broad-spectrum coverage.4
In contrast to the regulatory scenario in the United States, Europe regulates sunscreens as cosmetics, and the European Union (EU) has banned animal testing of cosmetics,7 which poses a problem for the development of new sunscreens. It is not surprising, therefore, that in 2006 the European Commission (the executive arm of the EU) published a mandate that in vitro SPF testing methods be actively developed due to ethical concerns associated with in vivo methods.8 In 2017, the International Organization for Standardization released specific validation criteria for proposed in vitro tests to facilitate the eventual approval of such methods.1
Progress of In Vitro Methods
In recent years, advances in in vitro SPF testing methods have addressed shortcomings noted previously by the FDA, which has led to notably improved reproducibility of results and correlation with in vivo values, in large part due to strict standardization of protocols,1 such as tight temperature control of samples, a multisubstrate approach, robotic product application to ensure even distribution, and pre-irradiation of sunscreen samples.
With these improvements, a 2018 study demonstrated an in vitro SPF testing methodology that exceeded published ISO validation criteria for emulsion-type products.1 This method was found to have low interlaboratory variability and high correlation with in vivo SPF values (Pearson r=0.88). Importantly, the authors noted that the consistency and reliability of in vitro SPF testing requires broad institution of a single unified method.1
The method described in the 2018 study1 has been accepted by the ISO Technical Committee and is undergoing further development3
Final Thoughts and Future Steps
Recent data confirm the potential viability of in vitro testing as a primary method of determining SPF values.1 Although ISO has moved forward with development of this method, the FDA has been quiet on in vitro SPF testing since 2011.4 The agency has, however, acknowledged the disadvantages of in vivo broad-spectrum testing, including exposure of human participants to harmful UVR and poor interlaboratory reproducibility.6
Given the technical developments and substantial potential benefits of in vitro testing, we believe that it is time for the FDA to revisit this matter. We propose that the FDA take 2 steps toward in vitro testing. First, publish specific validation criteria that would be deemed necessary for approval of such a test, similar to what ISO published in 2017. Second, thoroughly assess new data supporting the viability of available in vitro testing to determine if the FDA’s stated position that in vitro testing is inadequate remains true.
Although these 2 steps will be important to the process, adoption of an in vitro standard will require more than statements from the FDA. Additional funding should be allocated to researchers who are studying in vitro methodologies, and companies that profit from the multibillion-dollar sunscreen industry should be encouraged to invest in the development of more accurate and more ethical alternatives to in vivo SPF testing.
In vitro SPF testing is inexpensive, avoids the moral quandary of intentionally sunburning human participants, and is more reliable than in vivo testing. It is time for the FDA to facilitate the efforts of academia and industry in taking concrete steps toward approval of an in vitro alternative to in vivo SPF testing.
- Pissavini M, Tricaud C, Wiener G, et al. Validation of an in vitro sun protection factor (SPF) method in blinded ring-testing. Int J Cosmet Sci. 2018;40:263-268. doi:10.1111/ics.12459
- Miksa S, Lutz D, Guy C, et al. Sunscreen sun protection factor claim based on in vivo interlaboratory variability. Int J Cosmet Sci. 2016;38:541-549. doi:10.1111/ics.12333
- ISO/CD 23675: Cosmetics—sun protection test methods—in vitro determination of sun protection factor. International Organization for Standardization (ISO). July 25, 2020. Accessed May 17, 2022. https://www.iso.org/standard/76616.html
- US Food and Drug Administration. Labeling and effectiveness testing; sunscreen drug products for over-the-counter human use. Fed Regist. 2011;76(117):35620-35665. Accessed August 9, 2022. https://www.govinfo.gov/content/pkg/FR-2011-06-17/pdf/2011-14766.pdf
- Herzog B, Osterwalder U. Simulation of sunscreen performance. Pure Appl Chem. 2015;87:937-951. doi:10.1515/pac-2015-0401
- US Food and Drug Administration. Sunscreen drug products for over-the-counter human use; proposed amendment of final monograph. Fed Regist. 2007;72(165):49070-49122. Published August 27, 2007. Accessed August 9, 2022. https://www.govinfo.gov/content/pkg/FR-2007-08-27/pdf/07-4131.pdf
- Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products. November 30, 2009. Accessed August 10, 2022. https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:02009R1223-20190813
- European Commission Recommendation 2006/647/EC. Published September 22, 2006. Accessed August 10, 2022. http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32006H0647
The sun protection factor (SPF) value indicates to consumers the level of protection that a given sunscreen formulation provides against erythemally effective UV radiation (UVR). 1 In vivo SPF testing, the gold standard for determining SPF, yields highly variable results and can harm human test participants. 2 In vitro SPF testing methodologies have been under development for years but none have (yet) replaced the in vivo test required by national and international regulatory agencies.
Recent European studies have shown strong data to support a highly standardized in vitro method,1 now under development by the International Organization for Standardization (ISO)—potentially to serve as a new SPF determination standard.1,3 Academia and industry should follow this example and actively take steps to develop and validate a suitable replacement for in vivo SPF testing.
In Vivo SPF Testing
The in vivo SPF test involves comparing doses of UVR necessary to induce erythema in human participants with and without sunscreen applied.2 Although this method has long been the standard for SPF determination, it is associated with the following major disadvantages:
- Cost: The in vivo test is expensive.
- Variability: Results of the test are subject to high interlaboratory variability due to the inherent subjectivity of identifying erythema, the variable skin types of human participants, and other laboratory-dependent factors.2 A study found that the average coefficient of variation for SPF values obtained from 3 or 4 laboratories to be 20%—with values exceeding 50% in some cases. With that level of variability, the same sunscreen may be labeled SPF 30, SPF 50, or SPF 50+, thereby posing a health risk to consumers who rely on the accuracy of such claims. In fact, Miksa et al2 concluded that “the largest obstacle to a reliable SPF assessment for consumer health is the in vivo SPF test itself.”
- Ethical concerns: Human participants are intentionally exposed to harmful UVR until sunburn is achieved. For that reason, there have been calls to abandon the practice of in vivo testing.1
Alternatives to In Vivo SPF Testing
There has been international interest in developing in silico and in vitro alternatives to the in vivo SPF test. These options are attractive because they are relatively inexpensive; avoid exposing human participants to harmful UVR; and have the potential to be more accurate and more reproducible than in vivo tests.
In Vitro Protocols—Many such in vitro tests exist; all generally involve applying a layer of sunscreen to an artificial substrate, exposing it to UVR from a solar simulator, and measuring the UVR transmittance through the product and film by spectrophotometry.1 Prior shortcomings of this method have included suboptimal reproducibility, lack of data on substrate and product properties, and lack of demonstrated equivalency to in vivo SPF testing.4
In Silico Protocols—These tests use data on the UV spectra of sunscreen filters, physical characteristics of sunscreen films on skin, and the unique photoinstability of filters to calculate expected UVR transmittance and SPF of sunscreens based on their ingredients.5 Reports have shown high correlation with in vivo values. Results are not subject to random error; reproducibility is theoretically perfect.5
Regulatory Agencies and In Vitro Testing
In the United States, sunscreens are regulated as over-the-counter drugs. In vivo testing is the only US Food and Drug Administration (FDA)–approved method for determining SPF for labeling purposes.1 In a 2007 Proposed Rule and a 2011 Final Rule, the FDA stated that in vitro SPF tests were an inadequate alternative to in vivo tests because of their shortcomings.4,6
Acknowledging the potential benefits of in vitro testing, the FDA wrote that it would consider in vitro alternatives if equivalency to the in vivo test could be proved.6 The agency has not published an official stance on in vitro SPF testing since those statements in 2007 and 2011. Of note, the FDA deems in vitro testing sufficient for making claims of broad-spectrum coverage.4
In contrast to the regulatory scenario in the United States, Europe regulates sunscreens as cosmetics, and the European Union (EU) has banned animal testing of cosmetics,7 which poses a problem for the development of new sunscreens. It is not surprising, therefore, that in 2006 the European Commission (the executive arm of the EU) published a mandate that in vitro SPF testing methods be actively developed due to ethical concerns associated with in vivo methods.8 In 2017, the International Organization for Standardization released specific validation criteria for proposed in vitro tests to facilitate the eventual approval of such methods.1
Progress of In Vitro Methods
In recent years, advances in in vitro SPF testing methods have addressed shortcomings noted previously by the FDA, which has led to notably improved reproducibility of results and correlation with in vivo values, in large part due to strict standardization of protocols,1 such as tight temperature control of samples, a multisubstrate approach, robotic product application to ensure even distribution, and pre-irradiation of sunscreen samples.
With these improvements, a 2018 study demonstrated an in vitro SPF testing methodology that exceeded published ISO validation criteria for emulsion-type products.1 This method was found to have low interlaboratory variability and high correlation with in vivo SPF values (Pearson r=0.88). Importantly, the authors noted that the consistency and reliability of in vitro SPF testing requires broad institution of a single unified method.1
The method described in the 2018 study1 has been accepted by the ISO Technical Committee and is undergoing further development3
Final Thoughts and Future Steps
Recent data confirm the potential viability of in vitro testing as a primary method of determining SPF values.1 Although ISO has moved forward with development of this method, the FDA has been quiet on in vitro SPF testing since 2011.4 The agency has, however, acknowledged the disadvantages of in vivo broad-spectrum testing, including exposure of human participants to harmful UVR and poor interlaboratory reproducibility.6
Given the technical developments and substantial potential benefits of in vitro testing, we believe that it is time for the FDA to revisit this matter. We propose that the FDA take 2 steps toward in vitro testing. First, publish specific validation criteria that would be deemed necessary for approval of such a test, similar to what ISO published in 2017. Second, thoroughly assess new data supporting the viability of available in vitro testing to determine if the FDA’s stated position that in vitro testing is inadequate remains true.
Although these 2 steps will be important to the process, adoption of an in vitro standard will require more than statements from the FDA. Additional funding should be allocated to researchers who are studying in vitro methodologies, and companies that profit from the multibillion-dollar sunscreen industry should be encouraged to invest in the development of more accurate and more ethical alternatives to in vivo SPF testing.
In vitro SPF testing is inexpensive, avoids the moral quandary of intentionally sunburning human participants, and is more reliable than in vivo testing. It is time for the FDA to facilitate the efforts of academia and industry in taking concrete steps toward approval of an in vitro alternative to in vivo SPF testing.
The sun protection factor (SPF) value indicates to consumers the level of protection that a given sunscreen formulation provides against erythemally effective UV radiation (UVR). 1 In vivo SPF testing, the gold standard for determining SPF, yields highly variable results and can harm human test participants. 2 In vitro SPF testing methodologies have been under development for years but none have (yet) replaced the in vivo test required by national and international regulatory agencies.
Recent European studies have shown strong data to support a highly standardized in vitro method,1 now under development by the International Organization for Standardization (ISO)—potentially to serve as a new SPF determination standard.1,3 Academia and industry should follow this example and actively take steps to develop and validate a suitable replacement for in vivo SPF testing.
In Vivo SPF Testing
The in vivo SPF test involves comparing doses of UVR necessary to induce erythema in human participants with and without sunscreen applied.2 Although this method has long been the standard for SPF determination, it is associated with the following major disadvantages:
- Cost: The in vivo test is expensive.
- Variability: Results of the test are subject to high interlaboratory variability due to the inherent subjectivity of identifying erythema, the variable skin types of human participants, and other laboratory-dependent factors.2 A study found that the average coefficient of variation for SPF values obtained from 3 or 4 laboratories to be 20%—with values exceeding 50% in some cases. With that level of variability, the same sunscreen may be labeled SPF 30, SPF 50, or SPF 50+, thereby posing a health risk to consumers who rely on the accuracy of such claims. In fact, Miksa et al2 concluded that “the largest obstacle to a reliable SPF assessment for consumer health is the in vivo SPF test itself.”
- Ethical concerns: Human participants are intentionally exposed to harmful UVR until sunburn is achieved. For that reason, there have been calls to abandon the practice of in vivo testing.1
Alternatives to In Vivo SPF Testing
There has been international interest in developing in silico and in vitro alternatives to the in vivo SPF test. These options are attractive because they are relatively inexpensive; avoid exposing human participants to harmful UVR; and have the potential to be more accurate and more reproducible than in vivo tests.
In Vitro Protocols—Many such in vitro tests exist; all generally involve applying a layer of sunscreen to an artificial substrate, exposing it to UVR from a solar simulator, and measuring the UVR transmittance through the product and film by spectrophotometry.1 Prior shortcomings of this method have included suboptimal reproducibility, lack of data on substrate and product properties, and lack of demonstrated equivalency to in vivo SPF testing.4
In Silico Protocols—These tests use data on the UV spectra of sunscreen filters, physical characteristics of sunscreen films on skin, and the unique photoinstability of filters to calculate expected UVR transmittance and SPF of sunscreens based on their ingredients.5 Reports have shown high correlation with in vivo values. Results are not subject to random error; reproducibility is theoretically perfect.5
Regulatory Agencies and In Vitro Testing
In the United States, sunscreens are regulated as over-the-counter drugs. In vivo testing is the only US Food and Drug Administration (FDA)–approved method for determining SPF for labeling purposes.1 In a 2007 Proposed Rule and a 2011 Final Rule, the FDA stated that in vitro SPF tests were an inadequate alternative to in vivo tests because of their shortcomings.4,6
Acknowledging the potential benefits of in vitro testing, the FDA wrote that it would consider in vitro alternatives if equivalency to the in vivo test could be proved.6 The agency has not published an official stance on in vitro SPF testing since those statements in 2007 and 2011. Of note, the FDA deems in vitro testing sufficient for making claims of broad-spectrum coverage.4
In contrast to the regulatory scenario in the United States, Europe regulates sunscreens as cosmetics, and the European Union (EU) has banned animal testing of cosmetics,7 which poses a problem for the development of new sunscreens. It is not surprising, therefore, that in 2006 the European Commission (the executive arm of the EU) published a mandate that in vitro SPF testing methods be actively developed due to ethical concerns associated with in vivo methods.8 In 2017, the International Organization for Standardization released specific validation criteria for proposed in vitro tests to facilitate the eventual approval of such methods.1
Progress of In Vitro Methods
In recent years, advances in in vitro SPF testing methods have addressed shortcomings noted previously by the FDA, which has led to notably improved reproducibility of results and correlation with in vivo values, in large part due to strict standardization of protocols,1 such as tight temperature control of samples, a multisubstrate approach, robotic product application to ensure even distribution, and pre-irradiation of sunscreen samples.
With these improvements, a 2018 study demonstrated an in vitro SPF testing methodology that exceeded published ISO validation criteria for emulsion-type products.1 This method was found to have low interlaboratory variability and high correlation with in vivo SPF values (Pearson r=0.88). Importantly, the authors noted that the consistency and reliability of in vitro SPF testing requires broad institution of a single unified method.1
The method described in the 2018 study1 has been accepted by the ISO Technical Committee and is undergoing further development3
Final Thoughts and Future Steps
Recent data confirm the potential viability of in vitro testing as a primary method of determining SPF values.1 Although ISO has moved forward with development of this method, the FDA has been quiet on in vitro SPF testing since 2011.4 The agency has, however, acknowledged the disadvantages of in vivo broad-spectrum testing, including exposure of human participants to harmful UVR and poor interlaboratory reproducibility.6
Given the technical developments and substantial potential benefits of in vitro testing, we believe that it is time for the FDA to revisit this matter. We propose that the FDA take 2 steps toward in vitro testing. First, publish specific validation criteria that would be deemed necessary for approval of such a test, similar to what ISO published in 2017. Second, thoroughly assess new data supporting the viability of available in vitro testing to determine if the FDA’s stated position that in vitro testing is inadequate remains true.
Although these 2 steps will be important to the process, adoption of an in vitro standard will require more than statements from the FDA. Additional funding should be allocated to researchers who are studying in vitro methodologies, and companies that profit from the multibillion-dollar sunscreen industry should be encouraged to invest in the development of more accurate and more ethical alternatives to in vivo SPF testing.
In vitro SPF testing is inexpensive, avoids the moral quandary of intentionally sunburning human participants, and is more reliable than in vivo testing. It is time for the FDA to facilitate the efforts of academia and industry in taking concrete steps toward approval of an in vitro alternative to in vivo SPF testing.
- Pissavini M, Tricaud C, Wiener G, et al. Validation of an in vitro sun protection factor (SPF) method in blinded ring-testing. Int J Cosmet Sci. 2018;40:263-268. doi:10.1111/ics.12459
- Miksa S, Lutz D, Guy C, et al. Sunscreen sun protection factor claim based on in vivo interlaboratory variability. Int J Cosmet Sci. 2016;38:541-549. doi:10.1111/ics.12333
- ISO/CD 23675: Cosmetics—sun protection test methods—in vitro determination of sun protection factor. International Organization for Standardization (ISO). July 25, 2020. Accessed May 17, 2022. https://www.iso.org/standard/76616.html
- US Food and Drug Administration. Labeling and effectiveness testing; sunscreen drug products for over-the-counter human use. Fed Regist. 2011;76(117):35620-35665. Accessed August 9, 2022. https://www.govinfo.gov/content/pkg/FR-2011-06-17/pdf/2011-14766.pdf
- Herzog B, Osterwalder U. Simulation of sunscreen performance. Pure Appl Chem. 2015;87:937-951. doi:10.1515/pac-2015-0401
- US Food and Drug Administration. Sunscreen drug products for over-the-counter human use; proposed amendment of final monograph. Fed Regist. 2007;72(165):49070-49122. Published August 27, 2007. Accessed August 9, 2022. https://www.govinfo.gov/content/pkg/FR-2007-08-27/pdf/07-4131.pdf
- Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products. November 30, 2009. Accessed August 10, 2022. https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:02009R1223-20190813
- European Commission Recommendation 2006/647/EC. Published September 22, 2006. Accessed August 10, 2022. http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32006H0647
- Pissavini M, Tricaud C, Wiener G, et al. Validation of an in vitro sun protection factor (SPF) method in blinded ring-testing. Int J Cosmet Sci. 2018;40:263-268. doi:10.1111/ics.12459
- Miksa S, Lutz D, Guy C, et al. Sunscreen sun protection factor claim based on in vivo interlaboratory variability. Int J Cosmet Sci. 2016;38:541-549. doi:10.1111/ics.12333
- ISO/CD 23675: Cosmetics—sun protection test methods—in vitro determination of sun protection factor. International Organization for Standardization (ISO). July 25, 2020. Accessed May 17, 2022. https://www.iso.org/standard/76616.html
- US Food and Drug Administration. Labeling and effectiveness testing; sunscreen drug products for over-the-counter human use. Fed Regist. 2011;76(117):35620-35665. Accessed August 9, 2022. https://www.govinfo.gov/content/pkg/FR-2011-06-17/pdf/2011-14766.pdf
- Herzog B, Osterwalder U. Simulation of sunscreen performance. Pure Appl Chem. 2015;87:937-951. doi:10.1515/pac-2015-0401
- US Food and Drug Administration. Sunscreen drug products for over-the-counter human use; proposed amendment of final monograph. Fed Regist. 2007;72(165):49070-49122. Published August 27, 2007. Accessed August 9, 2022. https://www.govinfo.gov/content/pkg/FR-2007-08-27/pdf/07-4131.pdf
- Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products. November 30, 2009. Accessed August 10, 2022. https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:02009R1223-20190813
- European Commission Recommendation 2006/647/EC. Published September 22, 2006. Accessed August 10, 2022. http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32006H0647
Practice Points
- The methodology for determining sun protection factor (SPF) that currently is accepted by the US Food and Drug Administration is an expensive and imprecise in vivo test that exposes human participants to harmful UV radiation.
- In vitro tests for determining SPF may be viable alternatives to the current in vivo gold standard.
- Researchers and the sunscreen industry should actively develop these in vitro methodologies to adopt a more accurate and less harmful test for SPF.
Intralesional Human Papillomavirus Vaccine Therapy for Recalcitrant Plantar Wart Triggers Gout Flare
To the Editor:
There is increasing evidence supporting the use of the human papillomavirus (HPV) vaccine in the treatment of recalcitrant common warts.1 We describe a potential complication associated with HPV vaccine treatment of warts that would be of interest to dermatologists.
A 70-year-old woman presented with a plantar wart measuring 6 mm in diameter at the base of the right hallux of 5 years’ duration. Prior failed therapies for wart removal included multiple paring treatments, cryotherapy, and topical salicylic acid 40% to 60%. The patient had no notable comorbidities; no history of gout; and no known risk factors for gout, such as hypertension, renal insufficiency, diuretic use, obesity, family history, or trauma.
Prior reports cited effective treatment of recalcitrant warts with recombinant HPV vaccines, both intralesionally1 and intramuscularly.2,3 With this knowledge in mind, we administered an intralesional injection with 0.1-mL recombinant HPV 9-valent vaccine to the patient’s plantar wart. Gradual erythema and swelling of the right first metatarsophalangeal joint developed over the next 7 days. Synovial fluid analysis demonstrated negatively birefringent crystals. The patient commenced treatment with colchicine and indomethacin and improved over the next 5 days. The wart resolved 3 months later and required no further treatment.
Prophylactic quadrivalent HPV vaccines have shown efficacy in treating HPV-associated precancerous and cancerous lesions.4 Case reports have suggested that HPV vaccines may be an effective treatment option for recalcitrant warts,1-3,5 especially in cases that do not respond to traditional treatment. It is possible that the mechanism of wart treatment involves overlap in the antigenic epitopes of the HPV types targeted by the vaccine vs the HPV types responsible for causing warts.2 Papillomaviruslike particles, based on the L1 capsid protein, can induce a specific CD8+ activation signal, leading to a vaccine-induced cytotoxic T-cell response that targets the wart cells with HPV-like antigens.6 The HPV vaccine contains aluminium, which has been shown to activate NLRP3 inflammasome,5 which may trigger gout by increasing monosodium urate crystal deposition via IL-1β production.7 This may lead to an increased risk for gout flares, an adverse effect of the HPV vaccine. This finding is supported by other studies of aluminium-containing vaccines that show an association with gout.6 It is noted that these vaccines are mostly delivered intramuscularly or subcutaneously in some cases.
We reported a case of gout triggered by intralesional HPV vaccine treatment of warts. It is unclear whether the gout was induced by the vaccine itself or whether it was due to trauma caused by the intralesional injection near the joint space. Based on our findings, we recommend that patients receiving intralesional injections for wart treatment be advised of this potential adverse effect, especially if they have risk factors for gout or have a history of gout.
- Nofal A, Marei A, Ibrahim AM et al. Intralesional versus intramuscular bivalent human papillomavirus vaccine in the treatment of recalcitrant common warts. J Am Acad Dermatol. 2020;82:94-100.
- Venugopal SS, Murrell DF. Recalcitrant cutaneous warts treated with recombinant quadrivalent human papillomavirus vaccine (types 6, 11, 16, and 18) in a developmentally delayed, 31-year-old white man. Arch Dermatol. 2010;146:475-477.
- Daniel BS, Murrell DF. Complete resolution of chronic multiple verruca vulgaris treated with quadrivalent human papillomavirus vaccine. JAMA Dermatol. 2013;149:370-372.
- Kenter GG, Welters MJ, Valentijn AR, et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med. 2009;361:1838-1847.
- Eisenbarth SC, Colegio OR, O’Connor W, et al. Crucial role for the NALP3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122-1166.
- Bellone S, El-Sahwi K, Cocco E, et al. Human papillomavirus type 16 (HPV-16) virus-like particle L1-specific CD8+ cytotoxic T lymphocytes (CTLs) are equally effective as E7-specific CD8+ CTLs in killing autologous HPV-16-positive tumor cells in cervical cancer patients: implications for L1 dendritic cell-based therapeutic vaccines. J Virol. 2009;83:6779-6789.
- Yokose C, McCormick N, Chen C, et al. Risk of gout flares after vaccination: a prospective case cross-over study. Ann Rheum Dis. 2019;78:1601-1604.
To the Editor:
There is increasing evidence supporting the use of the human papillomavirus (HPV) vaccine in the treatment of recalcitrant common warts.1 We describe a potential complication associated with HPV vaccine treatment of warts that would be of interest to dermatologists.
A 70-year-old woman presented with a plantar wart measuring 6 mm in diameter at the base of the right hallux of 5 years’ duration. Prior failed therapies for wart removal included multiple paring treatments, cryotherapy, and topical salicylic acid 40% to 60%. The patient had no notable comorbidities; no history of gout; and no known risk factors for gout, such as hypertension, renal insufficiency, diuretic use, obesity, family history, or trauma.
Prior reports cited effective treatment of recalcitrant warts with recombinant HPV vaccines, both intralesionally1 and intramuscularly.2,3 With this knowledge in mind, we administered an intralesional injection with 0.1-mL recombinant HPV 9-valent vaccine to the patient’s plantar wart. Gradual erythema and swelling of the right first metatarsophalangeal joint developed over the next 7 days. Synovial fluid analysis demonstrated negatively birefringent crystals. The patient commenced treatment with colchicine and indomethacin and improved over the next 5 days. The wart resolved 3 months later and required no further treatment.
Prophylactic quadrivalent HPV vaccines have shown efficacy in treating HPV-associated precancerous and cancerous lesions.4 Case reports have suggested that HPV vaccines may be an effective treatment option for recalcitrant warts,1-3,5 especially in cases that do not respond to traditional treatment. It is possible that the mechanism of wart treatment involves overlap in the antigenic epitopes of the HPV types targeted by the vaccine vs the HPV types responsible for causing warts.2 Papillomaviruslike particles, based on the L1 capsid protein, can induce a specific CD8+ activation signal, leading to a vaccine-induced cytotoxic T-cell response that targets the wart cells with HPV-like antigens.6 The HPV vaccine contains aluminium, which has been shown to activate NLRP3 inflammasome,5 which may trigger gout by increasing monosodium urate crystal deposition via IL-1β production.7 This may lead to an increased risk for gout flares, an adverse effect of the HPV vaccine. This finding is supported by other studies of aluminium-containing vaccines that show an association with gout.6 It is noted that these vaccines are mostly delivered intramuscularly or subcutaneously in some cases.
We reported a case of gout triggered by intralesional HPV vaccine treatment of warts. It is unclear whether the gout was induced by the vaccine itself or whether it was due to trauma caused by the intralesional injection near the joint space. Based on our findings, we recommend that patients receiving intralesional injections for wart treatment be advised of this potential adverse effect, especially if they have risk factors for gout or have a history of gout.
To the Editor:
There is increasing evidence supporting the use of the human papillomavirus (HPV) vaccine in the treatment of recalcitrant common warts.1 We describe a potential complication associated with HPV vaccine treatment of warts that would be of interest to dermatologists.
A 70-year-old woman presented with a plantar wart measuring 6 mm in diameter at the base of the right hallux of 5 years’ duration. Prior failed therapies for wart removal included multiple paring treatments, cryotherapy, and topical salicylic acid 40% to 60%. The patient had no notable comorbidities; no history of gout; and no known risk factors for gout, such as hypertension, renal insufficiency, diuretic use, obesity, family history, or trauma.
Prior reports cited effective treatment of recalcitrant warts with recombinant HPV vaccines, both intralesionally1 and intramuscularly.2,3 With this knowledge in mind, we administered an intralesional injection with 0.1-mL recombinant HPV 9-valent vaccine to the patient’s plantar wart. Gradual erythema and swelling of the right first metatarsophalangeal joint developed over the next 7 days. Synovial fluid analysis demonstrated negatively birefringent crystals. The patient commenced treatment with colchicine and indomethacin and improved over the next 5 days. The wart resolved 3 months later and required no further treatment.
Prophylactic quadrivalent HPV vaccines have shown efficacy in treating HPV-associated precancerous and cancerous lesions.4 Case reports have suggested that HPV vaccines may be an effective treatment option for recalcitrant warts,1-3,5 especially in cases that do not respond to traditional treatment. It is possible that the mechanism of wart treatment involves overlap in the antigenic epitopes of the HPV types targeted by the vaccine vs the HPV types responsible for causing warts.2 Papillomaviruslike particles, based on the L1 capsid protein, can induce a specific CD8+ activation signal, leading to a vaccine-induced cytotoxic T-cell response that targets the wart cells with HPV-like antigens.6 The HPV vaccine contains aluminium, which has been shown to activate NLRP3 inflammasome,5 which may trigger gout by increasing monosodium urate crystal deposition via IL-1β production.7 This may lead to an increased risk for gout flares, an adverse effect of the HPV vaccine. This finding is supported by other studies of aluminium-containing vaccines that show an association with gout.6 It is noted that these vaccines are mostly delivered intramuscularly or subcutaneously in some cases.
We reported a case of gout triggered by intralesional HPV vaccine treatment of warts. It is unclear whether the gout was induced by the vaccine itself or whether it was due to trauma caused by the intralesional injection near the joint space. Based on our findings, we recommend that patients receiving intralesional injections for wart treatment be advised of this potential adverse effect, especially if they have risk factors for gout or have a history of gout.
- Nofal A, Marei A, Ibrahim AM et al. Intralesional versus intramuscular bivalent human papillomavirus vaccine in the treatment of recalcitrant common warts. J Am Acad Dermatol. 2020;82:94-100.
- Venugopal SS, Murrell DF. Recalcitrant cutaneous warts treated with recombinant quadrivalent human papillomavirus vaccine (types 6, 11, 16, and 18) in a developmentally delayed, 31-year-old white man. Arch Dermatol. 2010;146:475-477.
- Daniel BS, Murrell DF. Complete resolution of chronic multiple verruca vulgaris treated with quadrivalent human papillomavirus vaccine. JAMA Dermatol. 2013;149:370-372.
- Kenter GG, Welters MJ, Valentijn AR, et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med. 2009;361:1838-1847.
- Eisenbarth SC, Colegio OR, O’Connor W, et al. Crucial role for the NALP3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122-1166.
- Bellone S, El-Sahwi K, Cocco E, et al. Human papillomavirus type 16 (HPV-16) virus-like particle L1-specific CD8+ cytotoxic T lymphocytes (CTLs) are equally effective as E7-specific CD8+ CTLs in killing autologous HPV-16-positive tumor cells in cervical cancer patients: implications for L1 dendritic cell-based therapeutic vaccines. J Virol. 2009;83:6779-6789.
- Yokose C, McCormick N, Chen C, et al. Risk of gout flares after vaccination: a prospective case cross-over study. Ann Rheum Dis. 2019;78:1601-1604.
- Nofal A, Marei A, Ibrahim AM et al. Intralesional versus intramuscular bivalent human papillomavirus vaccine in the treatment of recalcitrant common warts. J Am Acad Dermatol. 2020;82:94-100.
- Venugopal SS, Murrell DF. Recalcitrant cutaneous warts treated with recombinant quadrivalent human papillomavirus vaccine (types 6, 11, 16, and 18) in a developmentally delayed, 31-year-old white man. Arch Dermatol. 2010;146:475-477.
- Daniel BS, Murrell DF. Complete resolution of chronic multiple verruca vulgaris treated with quadrivalent human papillomavirus vaccine. JAMA Dermatol. 2013;149:370-372.
- Kenter GG, Welters MJ, Valentijn AR, et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med. 2009;361:1838-1847.
- Eisenbarth SC, Colegio OR, O’Connor W, et al. Crucial role for the NALP3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122-1166.
- Bellone S, El-Sahwi K, Cocco E, et al. Human papillomavirus type 16 (HPV-16) virus-like particle L1-specific CD8+ cytotoxic T lymphocytes (CTLs) are equally effective as E7-specific CD8+ CTLs in killing autologous HPV-16-positive tumor cells in cervical cancer patients: implications for L1 dendritic cell-based therapeutic vaccines. J Virol. 2009;83:6779-6789.
- Yokose C, McCormick N, Chen C, et al. Risk of gout flares after vaccination: a prospective case cross-over study. Ann Rheum Dis. 2019;78:1601-1604.
Practice Points
- Human papillomavirus (HPV) vaccines are increasingly used for recalcitrant warts.
- We describe an unreported adverse effect of gout flare following HPV vaccine treatment of plantar wart.