User login
Transient Skin Rippling in an Infant
The Diagnosis: Infantile Transient Smooth Muscle Contraction of the Skin
A diagnosis of infantile transient smooth muscle contraction of the skin (ITSMC) was made based on our patient’s clinical presentation and eliminating the diagnoses in the differential. No treatment ultimately was indicated, as episodes became less frequent over time.
The term infantile transient smooth muscle contraction of the skin was first proposed in 2013 by Torrelo et al,1 who described 9 newborns with episodic skin rippling occasionally associated with exposure to cold or friction. The authors postulated that ITSMC was the result of a transient contraction of the arrector pili smooth muscle fibers of the skin, secondary to autonomic immaturity, primitive reflexes, or smooth muscle hypersensitivity.1 Since this first description, ITSMC has remained a rarely reported and poorly understood phenomenon with rare identified cases in the literature.2,3 Clinical history and examination of infants with intermittent transient skin rippling help to distinguish ITSMC from other diagnoses without the need for biopsy, which is particularly undesirable in the pediatric population.
Congenital smooth muscle hamartoma is a benign proliferation of mature smooth muscle that also can arise from the arrector pili muscles.4 In contrast to ITSMC, a hamartoma does not clear; rather, it persists and grows proportionally with the child and is associated with overlying hyperpigmentation and hypertrichosis. The transient nature of ITSMC may be worrisome for mastocytoma; however, this condition presents as erythematous, yellow, red, or brown macules, papules, plaques, or nodules with a positive Darier sign.5 Although the differential diagnosis includes the shagreen patch characteristic of tuberous sclerosis, this irregular plaque typically is located on the lower back with overlying peau d’orange skin changes, and our patient lacked other features indicative of this condition.6 Becker nevus also remains a consideration in patients with rippled skin, but this entity typically becomes more notable at puberty and is associated with hyperpigmentation and hypertrichosis and is a type of smooth muscle hamartoma.4
Our case highlighted the unusual presentation of ITSMC, a condition that can easily go unrecognized, leading to unnecessary referrals and concern. Familiarity with this benign diagnosis is essential to inform prognosis and guide management.
- Torrelo A, Moreno S, Castro C, et al. Infantile transient smooth muscle contraction of the skin. J Am Acad Dermatol. 2013;69:498-500. doi:10.1016/j.jaad.2013.04.029
- Theodosiou G, Belfrage E, Berggård K, et al. Infantile transient smooth muscle contraction of the skin: a case report and literature review. Eur J Dermatol. 2021;31:260-261. doi:10.1684/ejd.2021.3996
- Topham C, Deacon DC, Bowen A, et al. More than goosebumps: a case of marked skin dimpling in an infant. Pediatr Dermatol. 2019;36:E71-E72. doi:10.1111/pde.13791
- Raboudi A, Litaiem N. Congenital smooth muscle hamartoma. StatPearls. StatPearls Publishing; 2022.
- Leung AKC, Lam JM, Leong KF. Childhood solitary cutaneous mastocytoma: clinical manifestations, diagnosis, evaluation, and management. Curr Pediatr Rev. 2019;15:42-46. doi:10.2174/1573396315666 181120163952
- Bongiorno MA, Nathan N, Oyerinde O, et al. Clinical characteristics of connective tissue nevi in tuberous sclerosis complex with special emphasis on shagreen patches. JAMA Dermatol. 2017;153:660-665. doi:10.1001/jamadermatol.2017.0298
The Diagnosis: Infantile Transient Smooth Muscle Contraction of the Skin
A diagnosis of infantile transient smooth muscle contraction of the skin (ITSMC) was made based on our patient’s clinical presentation and eliminating the diagnoses in the differential. No treatment ultimately was indicated, as episodes became less frequent over time.
The term infantile transient smooth muscle contraction of the skin was first proposed in 2013 by Torrelo et al,1 who described 9 newborns with episodic skin rippling occasionally associated with exposure to cold or friction. The authors postulated that ITSMC was the result of a transient contraction of the arrector pili smooth muscle fibers of the skin, secondary to autonomic immaturity, primitive reflexes, or smooth muscle hypersensitivity.1 Since this first description, ITSMC has remained a rarely reported and poorly understood phenomenon with rare identified cases in the literature.2,3 Clinical history and examination of infants with intermittent transient skin rippling help to distinguish ITSMC from other diagnoses without the need for biopsy, which is particularly undesirable in the pediatric population.
Congenital smooth muscle hamartoma is a benign proliferation of mature smooth muscle that also can arise from the arrector pili muscles.4 In contrast to ITSMC, a hamartoma does not clear; rather, it persists and grows proportionally with the child and is associated with overlying hyperpigmentation and hypertrichosis. The transient nature of ITSMC may be worrisome for mastocytoma; however, this condition presents as erythematous, yellow, red, or brown macules, papules, plaques, or nodules with a positive Darier sign.5 Although the differential diagnosis includes the shagreen patch characteristic of tuberous sclerosis, this irregular plaque typically is located on the lower back with overlying peau d’orange skin changes, and our patient lacked other features indicative of this condition.6 Becker nevus also remains a consideration in patients with rippled skin, but this entity typically becomes more notable at puberty and is associated with hyperpigmentation and hypertrichosis and is a type of smooth muscle hamartoma.4
Our case highlighted the unusual presentation of ITSMC, a condition that can easily go unrecognized, leading to unnecessary referrals and concern. Familiarity with this benign diagnosis is essential to inform prognosis and guide management.
The Diagnosis: Infantile Transient Smooth Muscle Contraction of the Skin
A diagnosis of infantile transient smooth muscle contraction of the skin (ITSMC) was made based on our patient’s clinical presentation and eliminating the diagnoses in the differential. No treatment ultimately was indicated, as episodes became less frequent over time.
The term infantile transient smooth muscle contraction of the skin was first proposed in 2013 by Torrelo et al,1 who described 9 newborns with episodic skin rippling occasionally associated with exposure to cold or friction. The authors postulated that ITSMC was the result of a transient contraction of the arrector pili smooth muscle fibers of the skin, secondary to autonomic immaturity, primitive reflexes, or smooth muscle hypersensitivity.1 Since this first description, ITSMC has remained a rarely reported and poorly understood phenomenon with rare identified cases in the literature.2,3 Clinical history and examination of infants with intermittent transient skin rippling help to distinguish ITSMC from other diagnoses without the need for biopsy, which is particularly undesirable in the pediatric population.
Congenital smooth muscle hamartoma is a benign proliferation of mature smooth muscle that also can arise from the arrector pili muscles.4 In contrast to ITSMC, a hamartoma does not clear; rather, it persists and grows proportionally with the child and is associated with overlying hyperpigmentation and hypertrichosis. The transient nature of ITSMC may be worrisome for mastocytoma; however, this condition presents as erythematous, yellow, red, or brown macules, papules, plaques, or nodules with a positive Darier sign.5 Although the differential diagnosis includes the shagreen patch characteristic of tuberous sclerosis, this irregular plaque typically is located on the lower back with overlying peau d’orange skin changes, and our patient lacked other features indicative of this condition.6 Becker nevus also remains a consideration in patients with rippled skin, but this entity typically becomes more notable at puberty and is associated with hyperpigmentation and hypertrichosis and is a type of smooth muscle hamartoma.4
Our case highlighted the unusual presentation of ITSMC, a condition that can easily go unrecognized, leading to unnecessary referrals and concern. Familiarity with this benign diagnosis is essential to inform prognosis and guide management.
- Torrelo A, Moreno S, Castro C, et al. Infantile transient smooth muscle contraction of the skin. J Am Acad Dermatol. 2013;69:498-500. doi:10.1016/j.jaad.2013.04.029
- Theodosiou G, Belfrage E, Berggård K, et al. Infantile transient smooth muscle contraction of the skin: a case report and literature review. Eur J Dermatol. 2021;31:260-261. doi:10.1684/ejd.2021.3996
- Topham C, Deacon DC, Bowen A, et al. More than goosebumps: a case of marked skin dimpling in an infant. Pediatr Dermatol. 2019;36:E71-E72. doi:10.1111/pde.13791
- Raboudi A, Litaiem N. Congenital smooth muscle hamartoma. StatPearls. StatPearls Publishing; 2022.
- Leung AKC, Lam JM, Leong KF. Childhood solitary cutaneous mastocytoma: clinical manifestations, diagnosis, evaluation, and management. Curr Pediatr Rev. 2019;15:42-46. doi:10.2174/1573396315666 181120163952
- Bongiorno MA, Nathan N, Oyerinde O, et al. Clinical characteristics of connective tissue nevi in tuberous sclerosis complex with special emphasis on shagreen patches. JAMA Dermatol. 2017;153:660-665. doi:10.1001/jamadermatol.2017.0298
- Torrelo A, Moreno S, Castro C, et al. Infantile transient smooth muscle contraction of the skin. J Am Acad Dermatol. 2013;69:498-500. doi:10.1016/j.jaad.2013.04.029
- Theodosiou G, Belfrage E, Berggård K, et al. Infantile transient smooth muscle contraction of the skin: a case report and literature review. Eur J Dermatol. 2021;31:260-261. doi:10.1684/ejd.2021.3996
- Topham C, Deacon DC, Bowen A, et al. More than goosebumps: a case of marked skin dimpling in an infant. Pediatr Dermatol. 2019;36:E71-E72. doi:10.1111/pde.13791
- Raboudi A, Litaiem N. Congenital smooth muscle hamartoma. StatPearls. StatPearls Publishing; 2022.
- Leung AKC, Lam JM, Leong KF. Childhood solitary cutaneous mastocytoma: clinical manifestations, diagnosis, evaluation, and management. Curr Pediatr Rev. 2019;15:42-46. doi:10.2174/1573396315666 181120163952
- Bongiorno MA, Nathan N, Oyerinde O, et al. Clinical characteristics of connective tissue nevi in tuberous sclerosis complex with special emphasis on shagreen patches. JAMA Dermatol. 2017;153:660-665. doi:10.1001/jamadermatol.2017.0298
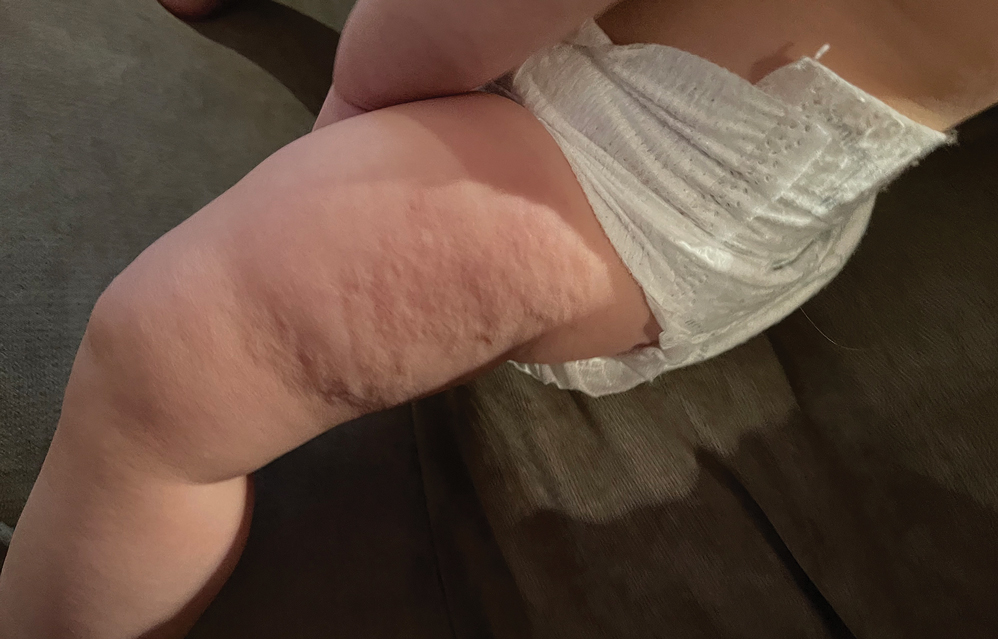
A healthy, full-term, 5-month-old infant boy presented to dermatology for evaluation of an intermittent, asymptomatic, rippled skin texture of the left thigh that resolved completely between flares. The parents noted fewer than 10 intermittent flares prior to the initial presentation at 5 months. Physical examination of the patient’s skin revealed no epidermal abnormalities, dermatographism, or subcutaneous nodules, and there was no positive Darier sign. A subsequent flare at 9 months of age occurred concurrently with fevers up to 39.4 °C (103 °F), and a corresponding photograph (quiz image) provided by the parents due to the intermittent and transient nature of the condition demonstrated an ill-defined, raised, rippled plaque on the left lateral thigh.
24-year-old woman • large joint arthralgias • history of type 1 diabetes, seizures, migraines • Dx?
THE CASE
A 24-year-old woman with a history of type 1 diabetes, seizure disorder, and migraines presented to a rural Federally Qualified Health Center (FQHC) with progressive and severe symmetric large joint arthralgias of several weeks’ duration. The patient’s existing medications included etonogestrel 68 mg subdermal implant, levetiracetam 1500 mg bid, insulin glargine 26 units subcutaneously nightly, and insulin lispro 20 units subcutaneously tid (before meals).
An examination revealed symmetrically edematous elbows, wrists, and fingers. Subsequent serologic analyses and a telemedicine consultation with a rheumatologist confirmed a diagnosis of rheumatoid arthritis (RA). The patient’s lab work was positive for antinuclear antibody titers (1:40), rheumatoid factor (513 IU/mL), and anticyclic citrullinated peptide antibodies (248 units/mL). Treatment was started with prednisone 60 mg PO daily, methotrexate 20 mg PO weekly, and hydroxychloroquine 400 mg PO daily. (The benefits of prednisone in treating this patient’s severe arthralgias outweighed concerns over its use in a patient with diabetes.)
After 2 months of receiving RA therapy, the patient underwent further work-up to assess its effectiveness
Upon receiving a diagnosis of active hepatitis C, the patient acknowledged that she’d had unprotected heterosexual intercourse and shared used insulin syringes with friends.
THE DIAGNOSIS
Consideration was given to a diagnosis of HCV arthropathy, which can present as an RA-like arthritis in HCV-infected individuals, in the differential diagnosis.1 A cohort study found HCV-associated arthropathy occurred in 6.8% of those with chronic HCV infection.2
However, the symmetrical involvement of shoulders and knees as the patient’s primary arthralgias, and a rheumatologic work-up showing the presence of anticyclic citrullinated peptide antibody levels, confirmed the diagnosis of RA with coexisting HCV.
DISCUSSION
Delivering interdisciplinary care in a rural area
Although evidence-based guidelines and online HCV Treatment Path programs guided the initial evaluation of potential treatments for this patient, her multiple comorbidities prompted us to seek out additional, interdisciplinary advice through a resource for underserved communities called Project Extension for Community Healthcare Outcomes (ECHO; see “What is Project ECHO?3,4”). The patient’s case was presented virtually, without identifying information, to a multidisciplinary HCV team. Two treatment options were suggested:
- sofosbuvir/velpatasvir (400 mg/100 mg) for 12 weeks or
- glecaprevir/pibrentasvir (100 mg/40 mg) for 8 weeks.
SIDEBAR
What is Project ECHO?
Project Extension for Community Healthcare Outcomes (ECHO) began as an avenue to connect hepatitis C virus (HCV) treatment experts to providers in underserved communities within New Mexico. Specialists can offer their clinical guidance to community clinicians without seeing the patient themselves.3 Project ECHO now has expanded to connect community clinicians across the United States and globally to specialists who treat other chronic conditions.4 More information about Project ECHO can be found at hsc.unm.edu/echo.
Both are evidence-based and recommended treatment options according to the HCV treatment guidelines issued jointly by the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America.5
In most patients with HCV, treatment is guided by a number of factors, including pill burden, access to care, duration of therapy, drug interactions, and patient-specific needs. After analyzing all aspects of this patient’s case, 2 major concerns guided our shared decision-making process on treatment.
The best treatment is what works for the patient
Owing to the patient’s multiple comorbidities and prescribed medications for chronic diseases, concerns about possible medication interactions with the HCV treatment options were a factor in her HCV treatment plan. Additionally, the patient had significant social determinants of health barriers that made continued treatment and follow-up challenging.
The potential interaction of HCV infection treatment with the patient’s current methotrexate therapy for her RA was a primary concern. To determine the risk for interactions, the team used the University of Liverpool HEP/HIV Drug Interactions Checker, which helps identify possible interactions with these disease-specific medication therapies.6
Both sofosbuvir/velpatasvir and glecaprevir/pibrentasvir have a potential interaction with methotrexate and are driven by a similar mechanism. Methotrexate is a substrate of the Breast Cancer Resistance Protein efflux transporter (BCRP), and the components of both sofosbuvir/velpatasvir and glecaprevir/pibrentasvir are inhibitors of BCRP.7 The inhibition of this efflux transporter can lead to an increased concentration of methotrexate, increasing the risk for methotrexate toxicity.7
Since no quantitative data exist regarding the degree of inhibition that these HCV drugs exert on BCRP, the team considered sofosbuvir/velpatasvir and glecaprevir/pibrentasvir to have equal risk with regard to potential for drug interactions.
The patient’s barriers to treatment were another area of concern that directed our therapy decision. The patient had multiple barriers, including poor access to health care because of transportation issues, multiple children requiring care, a variety of chronic diseases, and other life stressors. Shared decision-making ensured our patient’s autonomy in choosing a specific treatment.
The patient’s social situation and preference narrowed the team’s basis for medication choice primarily down to the duration of therapy: 8 weeks of glecaprevir/pibrentasvir vs 12 weeks of sofosbuvir/velpatasvir. The patient mentioned multiple transportation challenges for follow-up visits to the clinic and therefore wanted to utilize the shorter treatment duration. Follow-up is needed every 4 weeks, so the patient was able to go from 3 to 2 visits.
For problems, there are solutions. Following careful consideration of these patient-specific factors and preferences, the team decided to begin therapy with glecaprevir/pibrentasvir. The patient worked with an outreach specialist at the FQHC to coordinate care and complete paperwork for the Project ECHO consultation. The outreach specialist also assisted the patient in completing paperwork for the Patient Assistance Program for HCV treatment. Because the patient is being cared for at an FQHC, the clinic’s in-house pharmacy was able to utilize the 340B Federal Drug Pricing Program, which makes otherwise out-of-reach medicines affordable for patients such as ours.
Our patient has had no issues with treatment adherence, adverse effects, or follow-up appointments. The patient’s RA symptoms have improved significantly without any discernable worsening of her HCV infection.
THE TAKEAWAY
This case shines a light on the multiple challenges (clinical, geographic, and financial) that could have come between our patient and proper treatment—but ultimately, did not. The Project ECHO model of care remains a viable way to provide patients who live in rural and underserved communities and who have active HCV and other underlying chronic conditions with interdisciplinary care that can improve health outcomes.
1. Kemmer NM, Sherman KE. Hepatitis C-related arthropathy: diagnostic and treatment considerations. J Musculoskelet Med. 2010;27:351-354.
2. Ferucci ED, Choromanski TL, Varney DT, et al. Prevalence and correlates of hepatitis C virus-associated inflammatory arthritis in a population-based cohort. Semin Arthritis Rheum. 2017;47:445-450. doi: 10.1016/j.semarthrit.2017.04.004
3. Arora S, Kalishman S, Thornton K, et al. Expanding access to hepatitis C virus treatment--Extension for Community Healthcare Outcomes (ECHO) project: disruptive innovation in specialty care. Hepatology. 2010;52:1124-1133. doi: 10.1002/hep.23802
4. Blecker S, Paul MM, Jones S, et al. A Project ECHO and community health worker intervention for patients with diabetes. Am J Med. 2021;S0002-9343(21)00811-1. doi: 10.1016/j.amjmed.2021.12.002
5. AASLD-IDSA. Recommendations for testing, managing, and treating hepatitis C. Accessed June 16, 2023. www.hcvguidelines.org
6. HEP/HIV Drug Interactions Checker University of Liverpool. Interaction Report. Published 2022. Accessed June 26, 2023. www.hep-druginteractions.org/downloads/ajd45jg-4er5-67oy-ur43- 009ert.pdf?interaction_ids%5B%5D=88015&interaction_ids%5B%5D=91366
7. Hong J, Wright RC, Partovi N, et al. Review of clinically relevant drug interactions with next generation hepatitis C direct-acting antiviral agents. J Clin Transl Hepatol. 2020;8:322-335. doi: 10.14218/JCTH.2020.00034
THE CASE
A 24-year-old woman with a history of type 1 diabetes, seizure disorder, and migraines presented to a rural Federally Qualified Health Center (FQHC) with progressive and severe symmetric large joint arthralgias of several weeks’ duration. The patient’s existing medications included etonogestrel 68 mg subdermal implant, levetiracetam 1500 mg bid, insulin glargine 26 units subcutaneously nightly, and insulin lispro 20 units subcutaneously tid (before meals).
An examination revealed symmetrically edematous elbows, wrists, and fingers. Subsequent serologic analyses and a telemedicine consultation with a rheumatologist confirmed a diagnosis of rheumatoid arthritis (RA). The patient’s lab work was positive for antinuclear antibody titers (1:40), rheumatoid factor (513 IU/mL), and anticyclic citrullinated peptide antibodies (248 units/mL). Treatment was started with prednisone 60 mg PO daily, methotrexate 20 mg PO weekly, and hydroxychloroquine 400 mg PO daily. (The benefits of prednisone in treating this patient’s severe arthralgias outweighed concerns over its use in a patient with diabetes.)
After 2 months of receiving RA therapy, the patient underwent further work-up to assess its effectiveness
Upon receiving a diagnosis of active hepatitis C, the patient acknowledged that she’d had unprotected heterosexual intercourse and shared used insulin syringes with friends.
THE DIAGNOSIS
Consideration was given to a diagnosis of HCV arthropathy, which can present as an RA-like arthritis in HCV-infected individuals, in the differential diagnosis.1 A cohort study found HCV-associated arthropathy occurred in 6.8% of those with chronic HCV infection.2
However, the symmetrical involvement of shoulders and knees as the patient’s primary arthralgias, and a rheumatologic work-up showing the presence of anticyclic citrullinated peptide antibody levels, confirmed the diagnosis of RA with coexisting HCV.
DISCUSSION
Delivering interdisciplinary care in a rural area
Although evidence-based guidelines and online HCV Treatment Path programs guided the initial evaluation of potential treatments for this patient, her multiple comorbidities prompted us to seek out additional, interdisciplinary advice through a resource for underserved communities called Project Extension for Community Healthcare Outcomes (ECHO; see “What is Project ECHO?3,4”). The patient’s case was presented virtually, without identifying information, to a multidisciplinary HCV team. Two treatment options were suggested:
- sofosbuvir/velpatasvir (400 mg/100 mg) for 12 weeks or
- glecaprevir/pibrentasvir (100 mg/40 mg) for 8 weeks.
SIDEBAR
What is Project ECHO?
Project Extension for Community Healthcare Outcomes (ECHO) began as an avenue to connect hepatitis C virus (HCV) treatment experts to providers in underserved communities within New Mexico. Specialists can offer their clinical guidance to community clinicians without seeing the patient themselves.3 Project ECHO now has expanded to connect community clinicians across the United States and globally to specialists who treat other chronic conditions.4 More information about Project ECHO can be found at hsc.unm.edu/echo.
Both are evidence-based and recommended treatment options according to the HCV treatment guidelines issued jointly by the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America.5
In most patients with HCV, treatment is guided by a number of factors, including pill burden, access to care, duration of therapy, drug interactions, and patient-specific needs. After analyzing all aspects of this patient’s case, 2 major concerns guided our shared decision-making process on treatment.
The best treatment is what works for the patient
Owing to the patient’s multiple comorbidities and prescribed medications for chronic diseases, concerns about possible medication interactions with the HCV treatment options were a factor in her HCV treatment plan. Additionally, the patient had significant social determinants of health barriers that made continued treatment and follow-up challenging.
The potential interaction of HCV infection treatment with the patient’s current methotrexate therapy for her RA was a primary concern. To determine the risk for interactions, the team used the University of Liverpool HEP/HIV Drug Interactions Checker, which helps identify possible interactions with these disease-specific medication therapies.6
Both sofosbuvir/velpatasvir and glecaprevir/pibrentasvir have a potential interaction with methotrexate and are driven by a similar mechanism. Methotrexate is a substrate of the Breast Cancer Resistance Protein efflux transporter (BCRP), and the components of both sofosbuvir/velpatasvir and glecaprevir/pibrentasvir are inhibitors of BCRP.7 The inhibition of this efflux transporter can lead to an increased concentration of methotrexate, increasing the risk for methotrexate toxicity.7
Since no quantitative data exist regarding the degree of inhibition that these HCV drugs exert on BCRP, the team considered sofosbuvir/velpatasvir and glecaprevir/pibrentasvir to have equal risk with regard to potential for drug interactions.
The patient’s barriers to treatment were another area of concern that directed our therapy decision. The patient had multiple barriers, including poor access to health care because of transportation issues, multiple children requiring care, a variety of chronic diseases, and other life stressors. Shared decision-making ensured our patient’s autonomy in choosing a specific treatment.
The patient’s social situation and preference narrowed the team’s basis for medication choice primarily down to the duration of therapy: 8 weeks of glecaprevir/pibrentasvir vs 12 weeks of sofosbuvir/velpatasvir. The patient mentioned multiple transportation challenges for follow-up visits to the clinic and therefore wanted to utilize the shorter treatment duration. Follow-up is needed every 4 weeks, so the patient was able to go from 3 to 2 visits.
For problems, there are solutions. Following careful consideration of these patient-specific factors and preferences, the team decided to begin therapy with glecaprevir/pibrentasvir. The patient worked with an outreach specialist at the FQHC to coordinate care and complete paperwork for the Project ECHO consultation. The outreach specialist also assisted the patient in completing paperwork for the Patient Assistance Program for HCV treatment. Because the patient is being cared for at an FQHC, the clinic’s in-house pharmacy was able to utilize the 340B Federal Drug Pricing Program, which makes otherwise out-of-reach medicines affordable for patients such as ours.
Our patient has had no issues with treatment adherence, adverse effects, or follow-up appointments. The patient’s RA symptoms have improved significantly without any discernable worsening of her HCV infection.
THE TAKEAWAY
This case shines a light on the multiple challenges (clinical, geographic, and financial) that could have come between our patient and proper treatment—but ultimately, did not. The Project ECHO model of care remains a viable way to provide patients who live in rural and underserved communities and who have active HCV and other underlying chronic conditions with interdisciplinary care that can improve health outcomes.
THE CASE
A 24-year-old woman with a history of type 1 diabetes, seizure disorder, and migraines presented to a rural Federally Qualified Health Center (FQHC) with progressive and severe symmetric large joint arthralgias of several weeks’ duration. The patient’s existing medications included etonogestrel 68 mg subdermal implant, levetiracetam 1500 mg bid, insulin glargine 26 units subcutaneously nightly, and insulin lispro 20 units subcutaneously tid (before meals).
An examination revealed symmetrically edematous elbows, wrists, and fingers. Subsequent serologic analyses and a telemedicine consultation with a rheumatologist confirmed a diagnosis of rheumatoid arthritis (RA). The patient’s lab work was positive for antinuclear antibody titers (1:40), rheumatoid factor (513 IU/mL), and anticyclic citrullinated peptide antibodies (248 units/mL). Treatment was started with prednisone 60 mg PO daily, methotrexate 20 mg PO weekly, and hydroxychloroquine 400 mg PO daily. (The benefits of prednisone in treating this patient’s severe arthralgias outweighed concerns over its use in a patient with diabetes.)
After 2 months of receiving RA therapy, the patient underwent further work-up to assess its effectiveness
Upon receiving a diagnosis of active hepatitis C, the patient acknowledged that she’d had unprotected heterosexual intercourse and shared used insulin syringes with friends.
THE DIAGNOSIS
Consideration was given to a diagnosis of HCV arthropathy, which can present as an RA-like arthritis in HCV-infected individuals, in the differential diagnosis.1 A cohort study found HCV-associated arthropathy occurred in 6.8% of those with chronic HCV infection.2
However, the symmetrical involvement of shoulders and knees as the patient’s primary arthralgias, and a rheumatologic work-up showing the presence of anticyclic citrullinated peptide antibody levels, confirmed the diagnosis of RA with coexisting HCV.
DISCUSSION
Delivering interdisciplinary care in a rural area
Although evidence-based guidelines and online HCV Treatment Path programs guided the initial evaluation of potential treatments for this patient, her multiple comorbidities prompted us to seek out additional, interdisciplinary advice through a resource for underserved communities called Project Extension for Community Healthcare Outcomes (ECHO; see “What is Project ECHO?3,4”). The patient’s case was presented virtually, without identifying information, to a multidisciplinary HCV team. Two treatment options were suggested:
- sofosbuvir/velpatasvir (400 mg/100 mg) for 12 weeks or
- glecaprevir/pibrentasvir (100 mg/40 mg) for 8 weeks.
SIDEBAR
What is Project ECHO?
Project Extension for Community Healthcare Outcomes (ECHO) began as an avenue to connect hepatitis C virus (HCV) treatment experts to providers in underserved communities within New Mexico. Specialists can offer their clinical guidance to community clinicians without seeing the patient themselves.3 Project ECHO now has expanded to connect community clinicians across the United States and globally to specialists who treat other chronic conditions.4 More information about Project ECHO can be found at hsc.unm.edu/echo.
Both are evidence-based and recommended treatment options according to the HCV treatment guidelines issued jointly by the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America.5
In most patients with HCV, treatment is guided by a number of factors, including pill burden, access to care, duration of therapy, drug interactions, and patient-specific needs. After analyzing all aspects of this patient’s case, 2 major concerns guided our shared decision-making process on treatment.
The best treatment is what works for the patient
Owing to the patient’s multiple comorbidities and prescribed medications for chronic diseases, concerns about possible medication interactions with the HCV treatment options were a factor in her HCV treatment plan. Additionally, the patient had significant social determinants of health barriers that made continued treatment and follow-up challenging.
The potential interaction of HCV infection treatment with the patient’s current methotrexate therapy for her RA was a primary concern. To determine the risk for interactions, the team used the University of Liverpool HEP/HIV Drug Interactions Checker, which helps identify possible interactions with these disease-specific medication therapies.6
Both sofosbuvir/velpatasvir and glecaprevir/pibrentasvir have a potential interaction with methotrexate and are driven by a similar mechanism. Methotrexate is a substrate of the Breast Cancer Resistance Protein efflux transporter (BCRP), and the components of both sofosbuvir/velpatasvir and glecaprevir/pibrentasvir are inhibitors of BCRP.7 The inhibition of this efflux transporter can lead to an increased concentration of methotrexate, increasing the risk for methotrexate toxicity.7
Since no quantitative data exist regarding the degree of inhibition that these HCV drugs exert on BCRP, the team considered sofosbuvir/velpatasvir and glecaprevir/pibrentasvir to have equal risk with regard to potential for drug interactions.
The patient’s barriers to treatment were another area of concern that directed our therapy decision. The patient had multiple barriers, including poor access to health care because of transportation issues, multiple children requiring care, a variety of chronic diseases, and other life stressors. Shared decision-making ensured our patient’s autonomy in choosing a specific treatment.
The patient’s social situation and preference narrowed the team’s basis for medication choice primarily down to the duration of therapy: 8 weeks of glecaprevir/pibrentasvir vs 12 weeks of sofosbuvir/velpatasvir. The patient mentioned multiple transportation challenges for follow-up visits to the clinic and therefore wanted to utilize the shorter treatment duration. Follow-up is needed every 4 weeks, so the patient was able to go from 3 to 2 visits.
For problems, there are solutions. Following careful consideration of these patient-specific factors and preferences, the team decided to begin therapy with glecaprevir/pibrentasvir. The patient worked with an outreach specialist at the FQHC to coordinate care and complete paperwork for the Project ECHO consultation. The outreach specialist also assisted the patient in completing paperwork for the Patient Assistance Program for HCV treatment. Because the patient is being cared for at an FQHC, the clinic’s in-house pharmacy was able to utilize the 340B Federal Drug Pricing Program, which makes otherwise out-of-reach medicines affordable for patients such as ours.
Our patient has had no issues with treatment adherence, adverse effects, or follow-up appointments. The patient’s RA symptoms have improved significantly without any discernable worsening of her HCV infection.
THE TAKEAWAY
This case shines a light on the multiple challenges (clinical, geographic, and financial) that could have come between our patient and proper treatment—but ultimately, did not. The Project ECHO model of care remains a viable way to provide patients who live in rural and underserved communities and who have active HCV and other underlying chronic conditions with interdisciplinary care that can improve health outcomes.
1. Kemmer NM, Sherman KE. Hepatitis C-related arthropathy: diagnostic and treatment considerations. J Musculoskelet Med. 2010;27:351-354.
2. Ferucci ED, Choromanski TL, Varney DT, et al. Prevalence and correlates of hepatitis C virus-associated inflammatory arthritis in a population-based cohort. Semin Arthritis Rheum. 2017;47:445-450. doi: 10.1016/j.semarthrit.2017.04.004
3. Arora S, Kalishman S, Thornton K, et al. Expanding access to hepatitis C virus treatment--Extension for Community Healthcare Outcomes (ECHO) project: disruptive innovation in specialty care. Hepatology. 2010;52:1124-1133. doi: 10.1002/hep.23802
4. Blecker S, Paul MM, Jones S, et al. A Project ECHO and community health worker intervention for patients with diabetes. Am J Med. 2021;S0002-9343(21)00811-1. doi: 10.1016/j.amjmed.2021.12.002
5. AASLD-IDSA. Recommendations for testing, managing, and treating hepatitis C. Accessed June 16, 2023. www.hcvguidelines.org
6. HEP/HIV Drug Interactions Checker University of Liverpool. Interaction Report. Published 2022. Accessed June 26, 2023. www.hep-druginteractions.org/downloads/ajd45jg-4er5-67oy-ur43- 009ert.pdf?interaction_ids%5B%5D=88015&interaction_ids%5B%5D=91366
7. Hong J, Wright RC, Partovi N, et al. Review of clinically relevant drug interactions with next generation hepatitis C direct-acting antiviral agents. J Clin Transl Hepatol. 2020;8:322-335. doi: 10.14218/JCTH.2020.00034
1. Kemmer NM, Sherman KE. Hepatitis C-related arthropathy: diagnostic and treatment considerations. J Musculoskelet Med. 2010;27:351-354.
2. Ferucci ED, Choromanski TL, Varney DT, et al. Prevalence and correlates of hepatitis C virus-associated inflammatory arthritis in a population-based cohort. Semin Arthritis Rheum. 2017;47:445-450. doi: 10.1016/j.semarthrit.2017.04.004
3. Arora S, Kalishman S, Thornton K, et al. Expanding access to hepatitis C virus treatment--Extension for Community Healthcare Outcomes (ECHO) project: disruptive innovation in specialty care. Hepatology. 2010;52:1124-1133. doi: 10.1002/hep.23802
4. Blecker S, Paul MM, Jones S, et al. A Project ECHO and community health worker intervention for patients with diabetes. Am J Med. 2021;S0002-9343(21)00811-1. doi: 10.1016/j.amjmed.2021.12.002
5. AASLD-IDSA. Recommendations for testing, managing, and treating hepatitis C. Accessed June 16, 2023. www.hcvguidelines.org
6. HEP/HIV Drug Interactions Checker University of Liverpool. Interaction Report. Published 2022. Accessed June 26, 2023. www.hep-druginteractions.org/downloads/ajd45jg-4er5-67oy-ur43- 009ert.pdf?interaction_ids%5B%5D=88015&interaction_ids%5B%5D=91366
7. Hong J, Wright RC, Partovi N, et al. Review of clinically relevant drug interactions with next generation hepatitis C direct-acting antiviral agents. J Clin Transl Hepatol. 2020;8:322-335. doi: 10.14218/JCTH.2020.00034
► Large joint arthralgias
► History of type 1 diabetes, seizures, migraines
49-year-old woman • headache and neck pain radiating to ears and eyes • severe hypertension • Dx?
THE CASE
A 49-year-old woman was hospitalized with a headache and neck pain that radiated to her ears and eyes in the context of severe hypertension (270/150 mm Hg). Her medical history was significant for heterozygous factor V Leiden mutation, longstanding untreated hypertension, and multiple severe episodes of HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome during pregnancy.
After receiving antihypertensive treatment at a community hospital, her blood pressure gradually improved to 160/100 mm Hg with the addition of a third medication. However, on Day 3 of her stay, her systolic blood pressure rose to more than 200 mm Hg and was accompanied by somnolence, emesis, and paleness. She was transferred to a tertiary care center.
THE DIAGNOSIS
On admission, the patient had left-side hemiparesis and facial droop with dysarthria, resulting in a National Institutes of Health Stroke Scale (NIHSS) score of 7 (out of 42) and a Glasgow Coma Scale (GCS) score of 13 (out of 15). Noncontrast computed tomography (CT) and CT angiography of the head and neck were ordered and showed occlusion of both intracranial vertebral arteries. There were also signs of multifocal infarction in her occipital lobes, thus systemic recombinant human-tissue plasminogen activator (tPA) could not be administered.
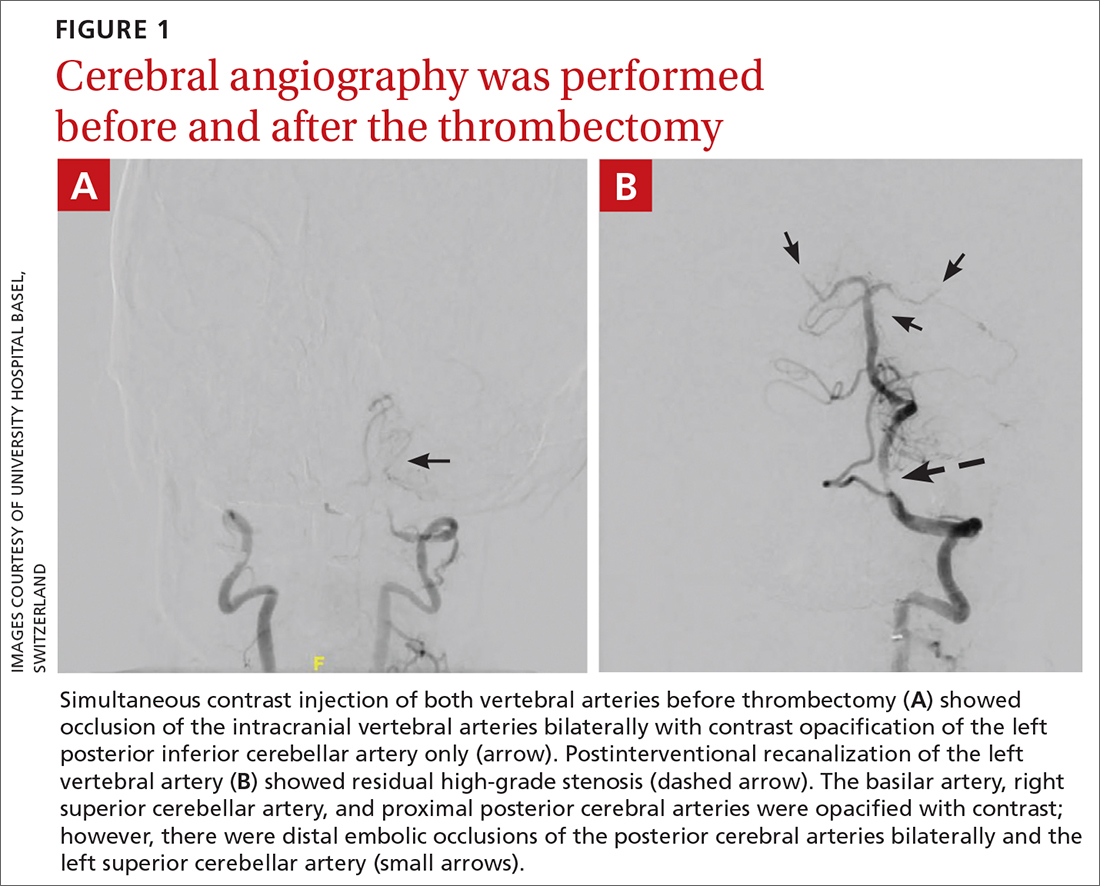
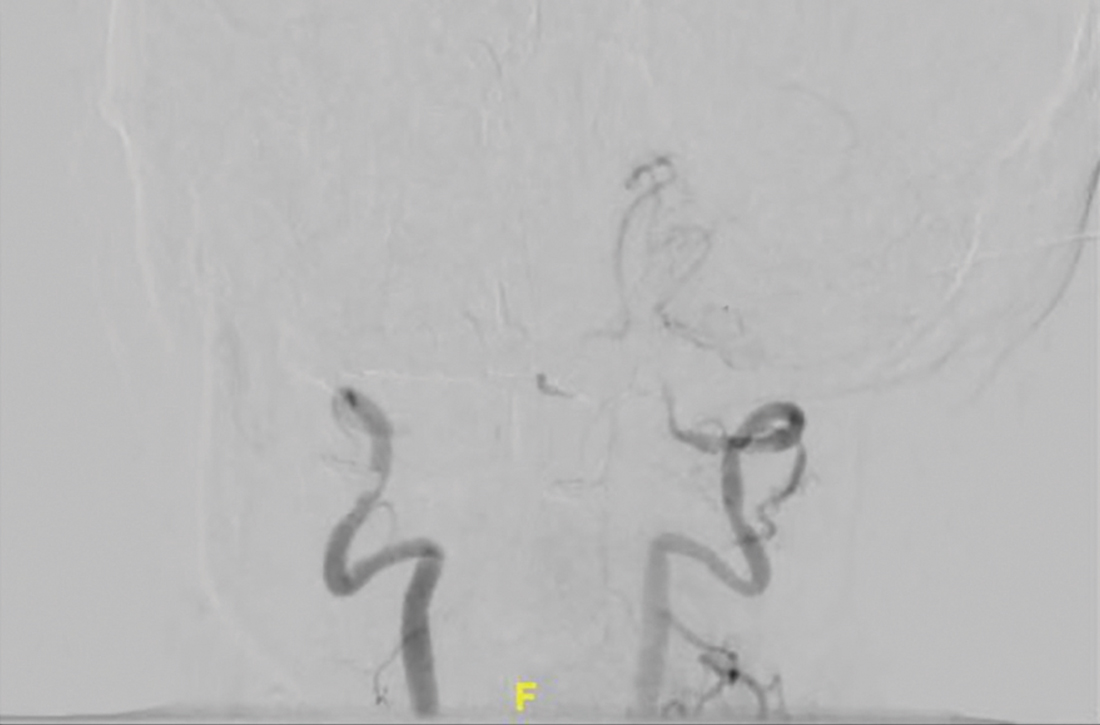
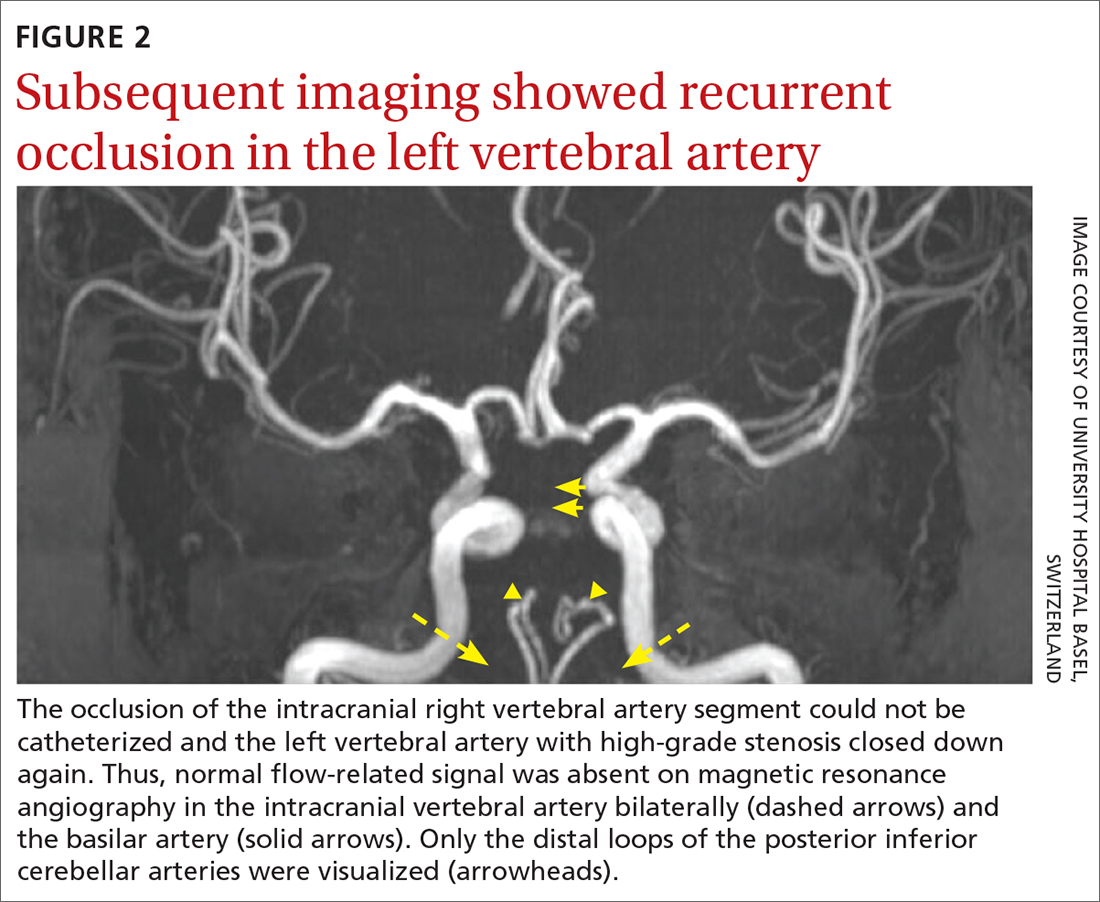
The patient was next taken to the angiography suite, where a digital subtraction angiography confirmed the presence of bilateral vertebral artery occlusions (FIGURE 1A). A thrombectomy was performed to open the left occluded segment, resulting in recanalization; however, a high-grade stenosis remained in the intracranial left vertebral artery (FIGURE 1B). The right vertebral artery had a severe extracranial origin stenosis, and balloon angioplasty was performed in order to reach the intracranial circulation; however, the occlusion of the intracranial right vertebral artery segment could not be catheterized. Subsequent magnetic resonance imaging (MRI) with a time-of-flight magnetic resonance angiography showed that the intracranial left vertebral artery with high-grade stenosis had closed down again; thus, there was occlusion of both intracranial vertebral arteries and absent flow signal in the basilar artery (FIGURE 2). There were scattered small acute strokes within the cerebellum, brainstem, and occipital lobes.
Unfortunately, within 48 hours, the patient’s NIHSS score increased from 7 to 29. She developed tetraplegia, was significantly less responsive (GCS score, 3/15), and required intubation and mechanical ventilation. Reopening the stenosis and keeping it open with a stent would be an aggressive procedure with poor odds for success and would require antithrombotic medications with the associated risk for intracranial hemorrhage in the setting of demarcated strokes. Thus, no further intervention was pursued.
Further standard stroke work-up (echocardiography, extracranial ultrasound of the cerebral circulation, and vasculitis screening) was unremarkable. In the intensive care unit, intravenous therapeutic heparin was initiated because of the potential prothrombotic effect of the factor V Leiden mutation but was subsequently switched to dual anti-aggregation therapy (aspirin 100 mg/d and clopidogrel 75 mg/d) as secondary stroke prevention given the final diagnosis of severe atherosclerosis. Nevertheless, the patient remained tetraplegic with a partial locked-in syndrome when she was discharged, after 2 weeks in the tertiary care center, to a rehabilitation center.
DISCUSSION
Posterior circulation strokes account for 20% to 25% of all ischemic strokes1,2 and are associated with infarction within the vertebrobasilar arterial system. Common etiologies of these infarctions include atherosclerosis (as seen in our patient), embolism, small-artery penetrating disease, and arterial dissection.2 Although the estimated overall mortality of these strokes is low (3.6% to 11%),2 basilar occlusion syndrome, in particular, is a life-threatening condition with a high mortality rate of 80% to 90%.3
Continue to: Diagnosis can be particularly challenging...
Diagnosis can be particularly challenging due to the anatomic variations of posterior arterial circulation, as well as the fluctuating nonfocal or multifocal symptoms.2 Specific symptoms include vertigo, ataxia, unilateral motor weakness, dysarthria, and oculomotor dysfunction. However, nonspecific symptoms such as headache, nausea, dizziness, hoarseness, falls, and Horner syndrome may be the only presenting signs of a posterior circulation stroke—as was the case with our patient.2 Her radiating neck pain could have been interpreted as a pointer to vertebral artery dissection within the context of severe hypertension.4 Unfortunately, the diagnosis was delayed and head imaging was obtained only after her mental status deteriorated.
Immediate neuroimaging is necessary to guide treatment in patients with suspected acute posterior circulation stroke,1,5,6 although it is not always definitive. While CT is pivotal in stroke work-up and may reliably exclude intracranial hemorrhage, its ability to detect acute posterior circulation ischemic strokes is limited given its poor visualization of the posterior fossa (as low as 16% sensitivity).5 Fortunately, CT angiography has a high sensitivity (nearing 100%) for large-vessel occlusion and high predictive values for dissection (65%-100% positive predictive value and 70%-98% negative predictive value).5,7 Diffusion-weighted MRI (when available in the emergency setting) has the highest sensitivity for detecting acute infarcts, although posterior circulation infarcts still can be missed (19% false-negative rate).5,8 Thus, correlative vessel imaging with magnetic resonance or CT angiography is very important, along with a high index of suspicion. In some instances, repeat MRI may be necessary to detect small strokes.
A patient-specific approach to management is key for individuals with suspected posterior circulation stroke.5 Because specific data for the appropriate management of posterior circulation ischemic stroke are lacking, current American Heart Association/American Stroke Association (AHA/ASA) guidelines apply to anterior and posterior circulation strokes.6 For eligible patients without multifocal disease, intravenous tPA is the first-line therapy and should be initiated according to guidelines within 4.5 hours of stroke onset9; it is important to note that these guidelines are based on studies that focused more on anterior circulation strokes than posterior circulation strokes.6,9-13 This can be done in combination with endovascular therapy, which consists of mechanical thrombectomy, intra-arterial thrombolysis, or a combination of revascularization techniques.3,5,6
Mechanical thrombectomy specifically has high proven recanalization rates for all target vessels.3-6 The latest AHA/ASA guidelines recommend mechanical thrombectomy be performed within 6 hours of stroke onset.6 However, there is emerging evidence that suggests this timeframe should be extended—even beyond 24 hours—given the poor prognosis of posterior circulation strokes.5,6,14 More data on the management of posterior circulation strokes are urgently needed to better understand which therapeutic approach is most efficient.
In patients such as ours, who have evidence of multifocal disease, treatment may be limited to endovascular therapy. Intracranial stenting of symptomatic lesions in particular has been controversial since the publication of the Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis trial, which found that aggressive medical management was superior to stenting in patients who recently had a transient ischemic attack or stroke attributed to stenosis.15 Although additional studies have been performed, there are no definitive data on the topic—and certainly no data in the emergency setting.16 Further challenges are raised in patients with bilateral disease, as was the case with this patient.
When our patient was admitted to the rehabilitation clinic, she had a GCS score of 10 to 11/15. After 9 months of rehabilitation, she was discharged home with a GCS score of 15/15 and persistent left-side hemiparesis.
THE TAKEAWAY
Posterior circulation stroke is a life-threatening disease that may manifest with a variety of symptoms and be difficult to identify on emergent imaging. Thus, a high degree of clinical suspicion and additional follow-up are paramount to ensure prompt diagnosis and a patient-tailored treatment strategy.
CORRESPONDENCE
Kristine A. Blackham, MD, Associate Professor, University Hospital Basel, Petersgraben 4, 4031 Basel, Switzerland; [email protected] Orcid no: 0000-0002-1620-1144 (Dr. Blackham); 0000-0002- 5225-5414 (Dr. Saleh)
1. Cloud GC, Markus HS. Diagnosis and management of vertebral artery stenosis. QJM. 2003;96:27-54. doi: 10.1093/qjmed/hcg003
2. Sparaco M, Ciolli L, Zini A. Posterior circulation ischaemic stroke–a review part I: anatomy, aetiology and clinical presentations. Neurol Sci. 2019;40:1995-2006. doi: 10.1007/s10072-019-03977-2
3. Lin DDM, Gailloud P, Beauchamp NJ, et al. Combined stent placement and thrombolysis in acute vertebrobasilar ischemic stroke. AJNR Am J Neuroradiol. 2003;24:1827-1833.
4. Pezzini A, Caso V, Zanferrari C, et al. Arterial hypertension as risk factor for spontaneous cervical artery dissection. A case-control study. J Neurol Neurosurg Psychiatry. 2006;77:95-97. doi:10.1136/jnnp.2005.063107
5. Merwick Á, Werring D. Posterior circulation ischaemic stroke. BMJ. 2014;348:g3175. doi: 10.1136/bmj.g3175
6. Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49:e46-e110. doi: 10.1161/STR.0000000000000158
7. Provenzale JM, Sarikaya B. Comparison of test performance characteristics of MRI, MR angiography, and CT angiography in the diagnosis of carotid and vertebral artery dissection: a review of the medical literature. AJR Am J Roentgenol. 2009;193:1167-1174. doi: 10.2214/AJR.08.1688
8. Husnoo Q. A case of missed diagnosis of posterior circulation stroke. Clin Med (Lond). 2019;19(suppl 2):63. doi: 10.7861/clinmedicine.19-2-s63
9. Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317-1329. doi: 10.1056/NEJMoa0804656
10. Schneider AM, Neuhaus AA, Hadley G, et al. Posterior circulation ischaemic stroke diagnosis and management. Clin Med (Lond). 2023;23:219-227. doi: 10.7861/clinmed.2022-0499
11. Dorňák T, Král M, Šaňák D, et al. Intravenous thrombolysis in posterior circulation stroke. Front Neurol. 2019;10:417. doi: 10.3389/fneur.2019.00417
12. van der Hoeven EJ, Schonewille WJ, Vos JA, et al. The Basilar Artery International Cooperation Study (BASICS): study protocol for a randomised controlled trial. Trials. 2013;14:200. doi: 10.1186/1745-6215-14-200
13. Nouh A, Remke J, Ruland S. Ischemic posterior circulation stroke: a review of anatomy, clinical presentations, diagnosis, and current management. Front Neurol. 2014;5:30. doi: 10.3389/fneur.2014.00030
14. Purrucker JC, Ringleb PA, Seker F, et al. Leaving the day behind: endovascular therapy beyond 24 h in acute stroke of the anterior and posterior circulation. Ther Adv Neurol Disord. 2022;15:17562864221101083. doi: 10.1177/17562864221101083
15. Chimowitz MI, Lynn MJ, Derdeyn CP, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011;365:993-1003. doi: 10.1056/NEJMoa1105335
16. Markus HS, Michel P. Treatment of posterior circulation stroke: acute management and secondary prevention. Int J Stroke. 2022;17:723-732. doi: 10.1177/17474930221107500
THE CASE
A 49-year-old woman was hospitalized with a headache and neck pain that radiated to her ears and eyes in the context of severe hypertension (270/150 mm Hg). Her medical history was significant for heterozygous factor V Leiden mutation, longstanding untreated hypertension, and multiple severe episodes of HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome during pregnancy.
After receiving antihypertensive treatment at a community hospital, her blood pressure gradually improved to 160/100 mm Hg with the addition of a third medication. However, on Day 3 of her stay, her systolic blood pressure rose to more than 200 mm Hg and was accompanied by somnolence, emesis, and paleness. She was transferred to a tertiary care center.
THE DIAGNOSIS
On admission, the patient had left-side hemiparesis and facial droop with dysarthria, resulting in a National Institutes of Health Stroke Scale (NIHSS) score of 7 (out of 42) and a Glasgow Coma Scale (GCS) score of 13 (out of 15). Noncontrast computed tomography (CT) and CT angiography of the head and neck were ordered and showed occlusion of both intracranial vertebral arteries. There were also signs of multifocal infarction in her occipital lobes, thus systemic recombinant human-tissue plasminogen activator (tPA) could not be administered.

The patient was next taken to the angiography suite, where a digital subtraction angiography confirmed the presence of bilateral vertebral artery occlusions (FIGURE 1A). A thrombectomy was performed to open the left occluded segment, resulting in recanalization; however, a high-grade stenosis remained in the intracranial left vertebral artery (FIGURE 1B). The right vertebral artery had a severe extracranial origin stenosis, and balloon angioplasty was performed in order to reach the intracranial circulation; however, the occlusion of the intracranial right vertebral artery segment could not be catheterized. Subsequent magnetic resonance imaging (MRI) with a time-of-flight magnetic resonance angiography showed that the intracranial left vertebral artery with high-grade stenosis had closed down again; thus, there was occlusion of both intracranial vertebral arteries and absent flow signal in the basilar artery (FIGURE 2). There were scattered small acute strokes within the cerebellum, brainstem, and occipital lobes.

Unfortunately, within 48 hours, the patient’s NIHSS score increased from 7 to 29. She developed tetraplegia, was significantly less responsive (GCS score, 3/15), and required intubation and mechanical ventilation. Reopening the stenosis and keeping it open with a stent would be an aggressive procedure with poor odds for success and would require antithrombotic medications with the associated risk for intracranial hemorrhage in the setting of demarcated strokes. Thus, no further intervention was pursued.
Further standard stroke work-up (echocardiography, extracranial ultrasound of the cerebral circulation, and vasculitis screening) was unremarkable. In the intensive care unit, intravenous therapeutic heparin was initiated because of the potential prothrombotic effect of the factor V Leiden mutation but was subsequently switched to dual anti-aggregation therapy (aspirin 100 mg/d and clopidogrel 75 mg/d) as secondary stroke prevention given the final diagnosis of severe atherosclerosis. Nevertheless, the patient remained tetraplegic with a partial locked-in syndrome when she was discharged, after 2 weeks in the tertiary care center, to a rehabilitation center.
DISCUSSION
Posterior circulation strokes account for 20% to 25% of all ischemic strokes1,2 and are associated with infarction within the vertebrobasilar arterial system. Common etiologies of these infarctions include atherosclerosis (as seen in our patient), embolism, small-artery penetrating disease, and arterial dissection.2 Although the estimated overall mortality of these strokes is low (3.6% to 11%),2 basilar occlusion syndrome, in particular, is a life-threatening condition with a high mortality rate of 80% to 90%.3
Continue to: Diagnosis can be particularly challenging...
Diagnosis can be particularly challenging due to the anatomic variations of posterior arterial circulation, as well as the fluctuating nonfocal or multifocal symptoms.2 Specific symptoms include vertigo, ataxia, unilateral motor weakness, dysarthria, and oculomotor dysfunction. However, nonspecific symptoms such as headache, nausea, dizziness, hoarseness, falls, and Horner syndrome may be the only presenting signs of a posterior circulation stroke—as was the case with our patient.2 Her radiating neck pain could have been interpreted as a pointer to vertebral artery dissection within the context of severe hypertension.4 Unfortunately, the diagnosis was delayed and head imaging was obtained only after her mental status deteriorated.
Immediate neuroimaging is necessary to guide treatment in patients with suspected acute posterior circulation stroke,1,5,6 although it is not always definitive. While CT is pivotal in stroke work-up and may reliably exclude intracranial hemorrhage, its ability to detect acute posterior circulation ischemic strokes is limited given its poor visualization of the posterior fossa (as low as 16% sensitivity).5 Fortunately, CT angiography has a high sensitivity (nearing 100%) for large-vessel occlusion and high predictive values for dissection (65%-100% positive predictive value and 70%-98% negative predictive value).5,7 Diffusion-weighted MRI (when available in the emergency setting) has the highest sensitivity for detecting acute infarcts, although posterior circulation infarcts still can be missed (19% false-negative rate).5,8 Thus, correlative vessel imaging with magnetic resonance or CT angiography is very important, along with a high index of suspicion. In some instances, repeat MRI may be necessary to detect small strokes.
A patient-specific approach to management is key for individuals with suspected posterior circulation stroke.5 Because specific data for the appropriate management of posterior circulation ischemic stroke are lacking, current American Heart Association/American Stroke Association (AHA/ASA) guidelines apply to anterior and posterior circulation strokes.6 For eligible patients without multifocal disease, intravenous tPA is the first-line therapy and should be initiated according to guidelines within 4.5 hours of stroke onset9; it is important to note that these guidelines are based on studies that focused more on anterior circulation strokes than posterior circulation strokes.6,9-13 This can be done in combination with endovascular therapy, which consists of mechanical thrombectomy, intra-arterial thrombolysis, or a combination of revascularization techniques.3,5,6
Mechanical thrombectomy specifically has high proven recanalization rates for all target vessels.3-6 The latest AHA/ASA guidelines recommend mechanical thrombectomy be performed within 6 hours of stroke onset.6 However, there is emerging evidence that suggests this timeframe should be extended—even beyond 24 hours—given the poor prognosis of posterior circulation strokes.5,6,14 More data on the management of posterior circulation strokes are urgently needed to better understand which therapeutic approach is most efficient.
In patients such as ours, who have evidence of multifocal disease, treatment may be limited to endovascular therapy. Intracranial stenting of symptomatic lesions in particular has been controversial since the publication of the Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis trial, which found that aggressive medical management was superior to stenting in patients who recently had a transient ischemic attack or stroke attributed to stenosis.15 Although additional studies have been performed, there are no definitive data on the topic—and certainly no data in the emergency setting.16 Further challenges are raised in patients with bilateral disease, as was the case with this patient.
When our patient was admitted to the rehabilitation clinic, she had a GCS score of 10 to 11/15. After 9 months of rehabilitation, she was discharged home with a GCS score of 15/15 and persistent left-side hemiparesis.
THE TAKEAWAY
Posterior circulation stroke is a life-threatening disease that may manifest with a variety of symptoms and be difficult to identify on emergent imaging. Thus, a high degree of clinical suspicion and additional follow-up are paramount to ensure prompt diagnosis and a patient-tailored treatment strategy.
CORRESPONDENCE
Kristine A. Blackham, MD, Associate Professor, University Hospital Basel, Petersgraben 4, 4031 Basel, Switzerland; [email protected] Orcid no: 0000-0002-1620-1144 (Dr. Blackham); 0000-0002- 5225-5414 (Dr. Saleh)
THE CASE
A 49-year-old woman was hospitalized with a headache and neck pain that radiated to her ears and eyes in the context of severe hypertension (270/150 mm Hg). Her medical history was significant for heterozygous factor V Leiden mutation, longstanding untreated hypertension, and multiple severe episodes of HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome during pregnancy.
After receiving antihypertensive treatment at a community hospital, her blood pressure gradually improved to 160/100 mm Hg with the addition of a third medication. However, on Day 3 of her stay, her systolic blood pressure rose to more than 200 mm Hg and was accompanied by somnolence, emesis, and paleness. She was transferred to a tertiary care center.
THE DIAGNOSIS
On admission, the patient had left-side hemiparesis and facial droop with dysarthria, resulting in a National Institutes of Health Stroke Scale (NIHSS) score of 7 (out of 42) and a Glasgow Coma Scale (GCS) score of 13 (out of 15). Noncontrast computed tomography (CT) and CT angiography of the head and neck were ordered and showed occlusion of both intracranial vertebral arteries. There were also signs of multifocal infarction in her occipital lobes, thus systemic recombinant human-tissue plasminogen activator (tPA) could not be administered.

The patient was next taken to the angiography suite, where a digital subtraction angiography confirmed the presence of bilateral vertebral artery occlusions (FIGURE 1A). A thrombectomy was performed to open the left occluded segment, resulting in recanalization; however, a high-grade stenosis remained in the intracranial left vertebral artery (FIGURE 1B). The right vertebral artery had a severe extracranial origin stenosis, and balloon angioplasty was performed in order to reach the intracranial circulation; however, the occlusion of the intracranial right vertebral artery segment could not be catheterized. Subsequent magnetic resonance imaging (MRI) with a time-of-flight magnetic resonance angiography showed that the intracranial left vertebral artery with high-grade stenosis had closed down again; thus, there was occlusion of both intracranial vertebral arteries and absent flow signal in the basilar artery (FIGURE 2). There were scattered small acute strokes within the cerebellum, brainstem, and occipital lobes.

Unfortunately, within 48 hours, the patient’s NIHSS score increased from 7 to 29. She developed tetraplegia, was significantly less responsive (GCS score, 3/15), and required intubation and mechanical ventilation. Reopening the stenosis and keeping it open with a stent would be an aggressive procedure with poor odds for success and would require antithrombotic medications with the associated risk for intracranial hemorrhage in the setting of demarcated strokes. Thus, no further intervention was pursued.
Further standard stroke work-up (echocardiography, extracranial ultrasound of the cerebral circulation, and vasculitis screening) was unremarkable. In the intensive care unit, intravenous therapeutic heparin was initiated because of the potential prothrombotic effect of the factor V Leiden mutation but was subsequently switched to dual anti-aggregation therapy (aspirin 100 mg/d and clopidogrel 75 mg/d) as secondary stroke prevention given the final diagnosis of severe atherosclerosis. Nevertheless, the patient remained tetraplegic with a partial locked-in syndrome when she was discharged, after 2 weeks in the tertiary care center, to a rehabilitation center.
DISCUSSION
Posterior circulation strokes account for 20% to 25% of all ischemic strokes1,2 and are associated with infarction within the vertebrobasilar arterial system. Common etiologies of these infarctions include atherosclerosis (as seen in our patient), embolism, small-artery penetrating disease, and arterial dissection.2 Although the estimated overall mortality of these strokes is low (3.6% to 11%),2 basilar occlusion syndrome, in particular, is a life-threatening condition with a high mortality rate of 80% to 90%.3
Continue to: Diagnosis can be particularly challenging...
Diagnosis can be particularly challenging due to the anatomic variations of posterior arterial circulation, as well as the fluctuating nonfocal or multifocal symptoms.2 Specific symptoms include vertigo, ataxia, unilateral motor weakness, dysarthria, and oculomotor dysfunction. However, nonspecific symptoms such as headache, nausea, dizziness, hoarseness, falls, and Horner syndrome may be the only presenting signs of a posterior circulation stroke—as was the case with our patient.2 Her radiating neck pain could have been interpreted as a pointer to vertebral artery dissection within the context of severe hypertension.4 Unfortunately, the diagnosis was delayed and head imaging was obtained only after her mental status deteriorated.
Immediate neuroimaging is necessary to guide treatment in patients with suspected acute posterior circulation stroke,1,5,6 although it is not always definitive. While CT is pivotal in stroke work-up and may reliably exclude intracranial hemorrhage, its ability to detect acute posterior circulation ischemic strokes is limited given its poor visualization of the posterior fossa (as low as 16% sensitivity).5 Fortunately, CT angiography has a high sensitivity (nearing 100%) for large-vessel occlusion and high predictive values for dissection (65%-100% positive predictive value and 70%-98% negative predictive value).5,7 Diffusion-weighted MRI (when available in the emergency setting) has the highest sensitivity for detecting acute infarcts, although posterior circulation infarcts still can be missed (19% false-negative rate).5,8 Thus, correlative vessel imaging with magnetic resonance or CT angiography is very important, along with a high index of suspicion. In some instances, repeat MRI may be necessary to detect small strokes.
A patient-specific approach to management is key for individuals with suspected posterior circulation stroke.5 Because specific data for the appropriate management of posterior circulation ischemic stroke are lacking, current American Heart Association/American Stroke Association (AHA/ASA) guidelines apply to anterior and posterior circulation strokes.6 For eligible patients without multifocal disease, intravenous tPA is the first-line therapy and should be initiated according to guidelines within 4.5 hours of stroke onset9; it is important to note that these guidelines are based on studies that focused more on anterior circulation strokes than posterior circulation strokes.6,9-13 This can be done in combination with endovascular therapy, which consists of mechanical thrombectomy, intra-arterial thrombolysis, or a combination of revascularization techniques.3,5,6
Mechanical thrombectomy specifically has high proven recanalization rates for all target vessels.3-6 The latest AHA/ASA guidelines recommend mechanical thrombectomy be performed within 6 hours of stroke onset.6 However, there is emerging evidence that suggests this timeframe should be extended—even beyond 24 hours—given the poor prognosis of posterior circulation strokes.5,6,14 More data on the management of posterior circulation strokes are urgently needed to better understand which therapeutic approach is most efficient.
In patients such as ours, who have evidence of multifocal disease, treatment may be limited to endovascular therapy. Intracranial stenting of symptomatic lesions in particular has been controversial since the publication of the Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis trial, which found that aggressive medical management was superior to stenting in patients who recently had a transient ischemic attack or stroke attributed to stenosis.15 Although additional studies have been performed, there are no definitive data on the topic—and certainly no data in the emergency setting.16 Further challenges are raised in patients with bilateral disease, as was the case with this patient.
When our patient was admitted to the rehabilitation clinic, she had a GCS score of 10 to 11/15. After 9 months of rehabilitation, she was discharged home with a GCS score of 15/15 and persistent left-side hemiparesis.
THE TAKEAWAY
Posterior circulation stroke is a life-threatening disease that may manifest with a variety of symptoms and be difficult to identify on emergent imaging. Thus, a high degree of clinical suspicion and additional follow-up are paramount to ensure prompt diagnosis and a patient-tailored treatment strategy.
CORRESPONDENCE
Kristine A. Blackham, MD, Associate Professor, University Hospital Basel, Petersgraben 4, 4031 Basel, Switzerland; [email protected] Orcid no: 0000-0002-1620-1144 (Dr. Blackham); 0000-0002- 5225-5414 (Dr. Saleh)
1. Cloud GC, Markus HS. Diagnosis and management of vertebral artery stenosis. QJM. 2003;96:27-54. doi: 10.1093/qjmed/hcg003
2. Sparaco M, Ciolli L, Zini A. Posterior circulation ischaemic stroke–a review part I: anatomy, aetiology and clinical presentations. Neurol Sci. 2019;40:1995-2006. doi: 10.1007/s10072-019-03977-2
3. Lin DDM, Gailloud P, Beauchamp NJ, et al. Combined stent placement and thrombolysis in acute vertebrobasilar ischemic stroke. AJNR Am J Neuroradiol. 2003;24:1827-1833.
4. Pezzini A, Caso V, Zanferrari C, et al. Arterial hypertension as risk factor for spontaneous cervical artery dissection. A case-control study. J Neurol Neurosurg Psychiatry. 2006;77:95-97. doi:10.1136/jnnp.2005.063107
5. Merwick Á, Werring D. Posterior circulation ischaemic stroke. BMJ. 2014;348:g3175. doi: 10.1136/bmj.g3175
6. Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49:e46-e110. doi: 10.1161/STR.0000000000000158
7. Provenzale JM, Sarikaya B. Comparison of test performance characteristics of MRI, MR angiography, and CT angiography in the diagnosis of carotid and vertebral artery dissection: a review of the medical literature. AJR Am J Roentgenol. 2009;193:1167-1174. doi: 10.2214/AJR.08.1688
8. Husnoo Q. A case of missed diagnosis of posterior circulation stroke. Clin Med (Lond). 2019;19(suppl 2):63. doi: 10.7861/clinmedicine.19-2-s63
9. Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317-1329. doi: 10.1056/NEJMoa0804656
10. Schneider AM, Neuhaus AA, Hadley G, et al. Posterior circulation ischaemic stroke diagnosis and management. Clin Med (Lond). 2023;23:219-227. doi: 10.7861/clinmed.2022-0499
11. Dorňák T, Král M, Šaňák D, et al. Intravenous thrombolysis in posterior circulation stroke. Front Neurol. 2019;10:417. doi: 10.3389/fneur.2019.00417
12. van der Hoeven EJ, Schonewille WJ, Vos JA, et al. The Basilar Artery International Cooperation Study (BASICS): study protocol for a randomised controlled trial. Trials. 2013;14:200. doi: 10.1186/1745-6215-14-200
13. Nouh A, Remke J, Ruland S. Ischemic posterior circulation stroke: a review of anatomy, clinical presentations, diagnosis, and current management. Front Neurol. 2014;5:30. doi: 10.3389/fneur.2014.00030
14. Purrucker JC, Ringleb PA, Seker F, et al. Leaving the day behind: endovascular therapy beyond 24 h in acute stroke of the anterior and posterior circulation. Ther Adv Neurol Disord. 2022;15:17562864221101083. doi: 10.1177/17562864221101083
15. Chimowitz MI, Lynn MJ, Derdeyn CP, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011;365:993-1003. doi: 10.1056/NEJMoa1105335
16. Markus HS, Michel P. Treatment of posterior circulation stroke: acute management and secondary prevention. Int J Stroke. 2022;17:723-732. doi: 10.1177/17474930221107500
1. Cloud GC, Markus HS. Diagnosis and management of vertebral artery stenosis. QJM. 2003;96:27-54. doi: 10.1093/qjmed/hcg003
2. Sparaco M, Ciolli L, Zini A. Posterior circulation ischaemic stroke–a review part I: anatomy, aetiology and clinical presentations. Neurol Sci. 2019;40:1995-2006. doi: 10.1007/s10072-019-03977-2
3. Lin DDM, Gailloud P, Beauchamp NJ, et al. Combined stent placement and thrombolysis in acute vertebrobasilar ischemic stroke. AJNR Am J Neuroradiol. 2003;24:1827-1833.
4. Pezzini A, Caso V, Zanferrari C, et al. Arterial hypertension as risk factor for spontaneous cervical artery dissection. A case-control study. J Neurol Neurosurg Psychiatry. 2006;77:95-97. doi:10.1136/jnnp.2005.063107
5. Merwick Á, Werring D. Posterior circulation ischaemic stroke. BMJ. 2014;348:g3175. doi: 10.1136/bmj.g3175
6. Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49:e46-e110. doi: 10.1161/STR.0000000000000158
7. Provenzale JM, Sarikaya B. Comparison of test performance characteristics of MRI, MR angiography, and CT angiography in the diagnosis of carotid and vertebral artery dissection: a review of the medical literature. AJR Am J Roentgenol. 2009;193:1167-1174. doi: 10.2214/AJR.08.1688
8. Husnoo Q. A case of missed diagnosis of posterior circulation stroke. Clin Med (Lond). 2019;19(suppl 2):63. doi: 10.7861/clinmedicine.19-2-s63
9. Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317-1329. doi: 10.1056/NEJMoa0804656
10. Schneider AM, Neuhaus AA, Hadley G, et al. Posterior circulation ischaemic stroke diagnosis and management. Clin Med (Lond). 2023;23:219-227. doi: 10.7861/clinmed.2022-0499
11. Dorňák T, Král M, Šaňák D, et al. Intravenous thrombolysis in posterior circulation stroke. Front Neurol. 2019;10:417. doi: 10.3389/fneur.2019.00417
12. van der Hoeven EJ, Schonewille WJ, Vos JA, et al. The Basilar Artery International Cooperation Study (BASICS): study protocol for a randomised controlled trial. Trials. 2013;14:200. doi: 10.1186/1745-6215-14-200
13. Nouh A, Remke J, Ruland S. Ischemic posterior circulation stroke: a review of anatomy, clinical presentations, diagnosis, and current management. Front Neurol. 2014;5:30. doi: 10.3389/fneur.2014.00030
14. Purrucker JC, Ringleb PA, Seker F, et al. Leaving the day behind: endovascular therapy beyond 24 h in acute stroke of the anterior and posterior circulation. Ther Adv Neurol Disord. 2022;15:17562864221101083. doi: 10.1177/17562864221101083
15. Chimowitz MI, Lynn MJ, Derdeyn CP, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011;365:993-1003. doi: 10.1056/NEJMoa1105335
16. Markus HS, Michel P. Treatment of posterior circulation stroke: acute management and secondary prevention. Int J Stroke. 2022;17:723-732. doi: 10.1177/17474930221107500
► Headache and neck pain radiating to ears and eyes
► Severe hypertension
Should you treat prediabetes? It’s complicated
ILLUSTRATIVE CASE
A 51-year-old woman with a history of elevated cholesterol and a body mass index (BMI) of 31 presents to your clinic for a scheduled follow-up visit to review recent blood test results. Her A1C was elevated at 5.9%. She wants to know if she should start medication now.
Prediabetes is a high-risk state that confers increased risk for type 2 diabetes (T2D). It is identified by impaired fasting glucose (fasting plasma glucose [FPG], 100-125 mg/dL), impaired glucose tolerance (2-hour oral glucose tolerance test, 140-199 mg/dL), or an elevated A1C (between 5.7% and 6.4%).2
An estimated 96 million Americans—38% of the US adult population—have prediabetes, according to the Centers for Disease Control and Prevention.3 Family physicians frequently encounter this condition when screening for T2D in asymptomatic adults (ages 35 to 70 years) with overweight or obesity, as recommended by the US Preventive Services Task Force (grade “B”).4
To treat, or not? Studies have shown that interventions such as lifestyle modification and use of metformin by patients with prediabetes can decrease their risk for T2D.5,6 In the Diabetes Prevention Program (DPP) study, progression from prediabetes to T2D was reduced to 14% with lifestyle modification and 22% with metformin use, vs 29% with placebo.7
However, there is disagreement about whether to treat prediabetes, particularly with medication. Some argue that metformin is a safe, effective, and cost-saving treatment to prevent T2D and its associated health consequences.8 The current American Diabetes Association (ADA) guidelines suggest that metformin be considered in certain patients with prediabetes and high-risk factors, especially younger age, obesity or hyperglycemia, or a history of gestational diabetes.9 However, only an estimated 1% to 4% of adults with prediabetes are prescribed metformin.10
Others argue that treating a preclinical condition is not a patient-centered approach, especially since not all patients with prediabetes progress to T2D and the risk for development or progression of retinopathy and microalbuminuria is extremely low if A1C levels remain < 7.0%.11 By this standard, pharmacologic treatment should be initiated only if, or when, a patient develops T2D, with a focus on intensive lifestyle intervention for high-risk patients in the interim.11
Given the conflicting viewpoints, ongoing long-term studies on T2D prevention will help guide treatment decisions for patients with prediabetes. The study by Lee et al1 was the first to evaluate the effect of metformin or intensive lifestyle modification on all-cause and cause-specific mortality in patients at high risk for T2D.
Continue to: STUDY SUMMARY
STUDY SUMMARY
No mortality benefit from metformin or lifestyle modification
This secondary analysis evaluated mortality outcomes for patients at risk for T2D who were part of the DPP trial and then were followed long term in the Diabetes Prevention Program Outcomes Study (DPPOS).1 The initial DPP trial included 3234 adult patients at high risk for T2D (defined as having a BMI ≥ 24; an FPG of 95-125 mg/dL; and a 2-hour glucose level of 140-199 mg/dL). Participants were randomized into groups receiving either intensive lifestyle intervention (which focused on achieving ≥ 150 min/wk of exercise and ≥ 7% body weight loss), metformin 850 mg twice daily, or placebo twice daily; the latter 2 groups also received standard exercise and diet recommendations. Mean age was 51 years, mean BMI was 34, and 68% of participants were female.
At the conclusion of the initial 5-year trial, treatment was unmasked and 86% of the patients continued to be followed for long-term outcomes. Patients in the lifestyle group were offered semiannual lifestyle reinforcement, while the metformin group continued to receive the twice-daily 850-mg dose unless a contraindication developed. If FPG levels increased to ≥ 140 mg/dL in the DPP study, or A1C increased to ≥ 7% in the DPPOS, study metformin was discontinued and management of the patient’s diabetes was transferred to their health care provider. By the end of the DPPOS, 53% of patients in the lifestyle group and 55% in the metformin group had progressed to T2D, compared with 60% in the placebo group (P = 0.003).
After a median 21-year follow-up interval, the investigators collected data on cause of death for patients and evaluated hazard ratios (HRs) for overall and cause-specific mortality. In total, 14% of the participants died, with no statistically significant difference in rates between the 3 groups. Cancer (37%) was the leading cause of death in all groups, followed by cardiovascular disease (CVD; 29%).
Compared with the placebo group, patients taking metformin did not have a decreased rate of overall mortality (HR = 0.99; 95% CI, 0.79-1.25), mortality from cancer (HR = 1.04; 95% CI, 0.72-1.52), or mortality due to CVD (HR = 1.08; 95% CI, 0.70-1.66). Similarly, compared with the placebo group, lifestyle intervention did not decrease overall mortality (HR = 1.02; 95% CI, 0.81-1.28), mortality from cancer (HR = 1.07; 95% CI, 0.74-1.55), or mortality due to CVD (HR = 1.18; 95% CI, 0.77-1.81). Results were similar when adjusted for other factors, including out-of-study metformin use, T2D status and duration, BMI change, and other cardiovascular risk factors.
WHAT’S NEW
Long-term data clarifylimits to interventions’ utility
This study looked at long-term follow-up data on mortality outcomes for patients with prediabetes treated with metformin or lifestyle intervention. Although these interventions did support weight loss, reduce the incidence of T2D, and lower cardiovascular risk factors (eg, hypertension, dyslipidemia), the comorbidity benefits did not affect risk for all-cause or cause-specific mortality, which were similar between the treatment and placebo groups.
Continue to: CAVEATS
CAVEATS
Exclusion criteria, residual confounding may limit the findings
Patients with significant cardiovascular or renal disease were excluded, so results may not apply to patients with these comorbidities. Additionally, there was a high amount of “drop-in” use of metformin prescribed by physicians once patients developed T2D, which may not have been controlled for completely. And while the intensive lifestyle intervention group had specific goals, the metformin and placebo groups also were encouraged to follow standard diet and lifestyle recommendations—and during a bridge period, all participants were offered a modified group lifestyle intervention. However, multivariable adjustment did not change the study conclusion.
CHALLENGES TO IMPLEMENTATION
Physicians may be unwilling to change their current prescribing habits
Physicians may not be willing to change their practice of prescribing metformin in prediabetes based on a singular study (with residual confounding) that showed no long-term mortality differences between the study groups. However, there may be long-term morbidity differences of interest to patients that were not specifically evaluated in this study—such as quality-of-life benefits from weight loss that may outweigh the risks (eg, gastrointestinal adverse effects such as diarrhea, nausea, and abdominal pain) of metformin for some patients. Therefore, a discussion of the risks and benefits of treatment for prediabetes should be had with patients at high risk who would prefer a pharmacologic intervention.
1. Lee CG, Heckman-Stoddard B, et al; Diabetes Prevention Program Research Group. Effect of metformin and lifestyle interventions on mortality in the Diabetes Prevention Program and Diabetes Prevention Program Outcomes Study. Diabetes Care. 2021;44:2775-2782. doi: 10.2337/dc21-1046
2. American Diabetes Association. Understanding A1C: diagnosis. Accessed July 6, 2023. https://diabetes.org/diabetes/a1c/diagnosis
3. CDC. National diabetes statistics report. Reviewed June 29, 2022. Accessed January 23, 2023. www.cdc.gov/diabetes/data/statistics-report/index.html
4. USPSTF; Davidson KW, Barry MJ, Mangione CM, et al. Screening for prediabetes and type 2 diabetes: US Preventive Services Task Force recommendation statement. JAMA. 2021;326:736-743. doi: 10.1001/jama.2021.12531
5. Hostalek U, Campbell I. Metformin for diabetes prevention: update of the evidence base. Curr Med Res Opin. 2021;37:1705-1717. doi: 10.1080/03007995.2021.1955667
6. Aroda VR, Knowler WC, Crandall JP, et al; Diabetes Prevention Program Research Group. Metformin for diabetes prevention: insights gained from the Diabetes Prevention Program/Diabetes Prevention Program Outcomes Study. Diabetologia. 2017;60:1601-1611. doi: 10.1007/s00125-017-4361-9
7. Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403. doi: 10.1056/NEJMoa012512
8. Herman WH, Ratner RE. Metformin should be used to treat prediabetes in selected individuals. Diabetes Care. 2020;43:1988-1990. doi: 10.2337/dci20-0030
9. American Diabetes Association. 3. Prevention or delay of type 2 diabetes: standards of medical care in diabetes—2021. Diabetes Care. 2021;44(suppl 1):S34-S39. doi: 10.2337/dc21-S003
10. Tseng E, Yeh HC, Maruthur NM. Metformin use in prediabetes among US adults, 2005-2012. Diabetes Care. 2017;40:887-893. doi: 10.2337/dc16-1509
11. Davidson MB. Metformin should not be used to treat prediabetes. Diabetes Care. 2020;43:1983-1987. doi: 10.2337/dc19-2221
ILLUSTRATIVE CASE
A 51-year-old woman with a history of elevated cholesterol and a body mass index (BMI) of 31 presents to your clinic for a scheduled follow-up visit to review recent blood test results. Her A1C was elevated at 5.9%. She wants to know if she should start medication now.
Prediabetes is a high-risk state that confers increased risk for type 2 diabetes (T2D). It is identified by impaired fasting glucose (fasting plasma glucose [FPG], 100-125 mg/dL), impaired glucose tolerance (2-hour oral glucose tolerance test, 140-199 mg/dL), or an elevated A1C (between 5.7% and 6.4%).2
An estimated 96 million Americans—38% of the US adult population—have prediabetes, according to the Centers for Disease Control and Prevention.3 Family physicians frequently encounter this condition when screening for T2D in asymptomatic adults (ages 35 to 70 years) with overweight or obesity, as recommended by the US Preventive Services Task Force (grade “B”).4
To treat, or not? Studies have shown that interventions such as lifestyle modification and use of metformin by patients with prediabetes can decrease their risk for T2D.5,6 In the Diabetes Prevention Program (DPP) study, progression from prediabetes to T2D was reduced to 14% with lifestyle modification and 22% with metformin use, vs 29% with placebo.7
However, there is disagreement about whether to treat prediabetes, particularly with medication. Some argue that metformin is a safe, effective, and cost-saving treatment to prevent T2D and its associated health consequences.8 The current American Diabetes Association (ADA) guidelines suggest that metformin be considered in certain patients with prediabetes and high-risk factors, especially younger age, obesity or hyperglycemia, or a history of gestational diabetes.9 However, only an estimated 1% to 4% of adults with prediabetes are prescribed metformin.10
Others argue that treating a preclinical condition is not a patient-centered approach, especially since not all patients with prediabetes progress to T2D and the risk for development or progression of retinopathy and microalbuminuria is extremely low if A1C levels remain < 7.0%.11 By this standard, pharmacologic treatment should be initiated only if, or when, a patient develops T2D, with a focus on intensive lifestyle intervention for high-risk patients in the interim.11
Given the conflicting viewpoints, ongoing long-term studies on T2D prevention will help guide treatment decisions for patients with prediabetes. The study by Lee et al1 was the first to evaluate the effect of metformin or intensive lifestyle modification on all-cause and cause-specific mortality in patients at high risk for T2D.
Continue to: STUDY SUMMARY
STUDY SUMMARY
No mortality benefit from metformin or lifestyle modification
This secondary analysis evaluated mortality outcomes for patients at risk for T2D who were part of the DPP trial and then were followed long term in the Diabetes Prevention Program Outcomes Study (DPPOS).1 The initial DPP trial included 3234 adult patients at high risk for T2D (defined as having a BMI ≥ 24; an FPG of 95-125 mg/dL; and a 2-hour glucose level of 140-199 mg/dL). Participants were randomized into groups receiving either intensive lifestyle intervention (which focused on achieving ≥ 150 min/wk of exercise and ≥ 7% body weight loss), metformin 850 mg twice daily, or placebo twice daily; the latter 2 groups also received standard exercise and diet recommendations. Mean age was 51 years, mean BMI was 34, and 68% of participants were female.
At the conclusion of the initial 5-year trial, treatment was unmasked and 86% of the patients continued to be followed for long-term outcomes. Patients in the lifestyle group were offered semiannual lifestyle reinforcement, while the metformin group continued to receive the twice-daily 850-mg dose unless a contraindication developed. If FPG levels increased to ≥ 140 mg/dL in the DPP study, or A1C increased to ≥ 7% in the DPPOS, study metformin was discontinued and management of the patient’s diabetes was transferred to their health care provider. By the end of the DPPOS, 53% of patients in the lifestyle group and 55% in the metformin group had progressed to T2D, compared with 60% in the placebo group (P = 0.003).
After a median 21-year follow-up interval, the investigators collected data on cause of death for patients and evaluated hazard ratios (HRs) for overall and cause-specific mortality. In total, 14% of the participants died, with no statistically significant difference in rates between the 3 groups. Cancer (37%) was the leading cause of death in all groups, followed by cardiovascular disease (CVD; 29%).
Compared with the placebo group, patients taking metformin did not have a decreased rate of overall mortality (HR = 0.99; 95% CI, 0.79-1.25), mortality from cancer (HR = 1.04; 95% CI, 0.72-1.52), or mortality due to CVD (HR = 1.08; 95% CI, 0.70-1.66). Similarly, compared with the placebo group, lifestyle intervention did not decrease overall mortality (HR = 1.02; 95% CI, 0.81-1.28), mortality from cancer (HR = 1.07; 95% CI, 0.74-1.55), or mortality due to CVD (HR = 1.18; 95% CI, 0.77-1.81). Results were similar when adjusted for other factors, including out-of-study metformin use, T2D status and duration, BMI change, and other cardiovascular risk factors.
WHAT’S NEW
Long-term data clarifylimits to interventions’ utility
This study looked at long-term follow-up data on mortality outcomes for patients with prediabetes treated with metformin or lifestyle intervention. Although these interventions did support weight loss, reduce the incidence of T2D, and lower cardiovascular risk factors (eg, hypertension, dyslipidemia), the comorbidity benefits did not affect risk for all-cause or cause-specific mortality, which were similar between the treatment and placebo groups.
Continue to: CAVEATS
CAVEATS
Exclusion criteria, residual confounding may limit the findings
Patients with significant cardiovascular or renal disease were excluded, so results may not apply to patients with these comorbidities. Additionally, there was a high amount of “drop-in” use of metformin prescribed by physicians once patients developed T2D, which may not have been controlled for completely. And while the intensive lifestyle intervention group had specific goals, the metformin and placebo groups also were encouraged to follow standard diet and lifestyle recommendations—and during a bridge period, all participants were offered a modified group lifestyle intervention. However, multivariable adjustment did not change the study conclusion.
CHALLENGES TO IMPLEMENTATION
Physicians may be unwilling to change their current prescribing habits
Physicians may not be willing to change their practice of prescribing metformin in prediabetes based on a singular study (with residual confounding) that showed no long-term mortality differences between the study groups. However, there may be long-term morbidity differences of interest to patients that were not specifically evaluated in this study—such as quality-of-life benefits from weight loss that may outweigh the risks (eg, gastrointestinal adverse effects such as diarrhea, nausea, and abdominal pain) of metformin for some patients. Therefore, a discussion of the risks and benefits of treatment for prediabetes should be had with patients at high risk who would prefer a pharmacologic intervention.
ILLUSTRATIVE CASE
A 51-year-old woman with a history of elevated cholesterol and a body mass index (BMI) of 31 presents to your clinic for a scheduled follow-up visit to review recent blood test results. Her A1C was elevated at 5.9%. She wants to know if she should start medication now.
Prediabetes is a high-risk state that confers increased risk for type 2 diabetes (T2D). It is identified by impaired fasting glucose (fasting plasma glucose [FPG], 100-125 mg/dL), impaired glucose tolerance (2-hour oral glucose tolerance test, 140-199 mg/dL), or an elevated A1C (between 5.7% and 6.4%).2
An estimated 96 million Americans—38% of the US adult population—have prediabetes, according to the Centers for Disease Control and Prevention.3 Family physicians frequently encounter this condition when screening for T2D in asymptomatic adults (ages 35 to 70 years) with overweight or obesity, as recommended by the US Preventive Services Task Force (grade “B”).4
To treat, or not? Studies have shown that interventions such as lifestyle modification and use of metformin by patients with prediabetes can decrease their risk for T2D.5,6 In the Diabetes Prevention Program (DPP) study, progression from prediabetes to T2D was reduced to 14% with lifestyle modification and 22% with metformin use, vs 29% with placebo.7
However, there is disagreement about whether to treat prediabetes, particularly with medication. Some argue that metformin is a safe, effective, and cost-saving treatment to prevent T2D and its associated health consequences.8 The current American Diabetes Association (ADA) guidelines suggest that metformin be considered in certain patients with prediabetes and high-risk factors, especially younger age, obesity or hyperglycemia, or a history of gestational diabetes.9 However, only an estimated 1% to 4% of adults with prediabetes are prescribed metformin.10
Others argue that treating a preclinical condition is not a patient-centered approach, especially since not all patients with prediabetes progress to T2D and the risk for development or progression of retinopathy and microalbuminuria is extremely low if A1C levels remain < 7.0%.11 By this standard, pharmacologic treatment should be initiated only if, or when, a patient develops T2D, with a focus on intensive lifestyle intervention for high-risk patients in the interim.11
Given the conflicting viewpoints, ongoing long-term studies on T2D prevention will help guide treatment decisions for patients with prediabetes. The study by Lee et al1 was the first to evaluate the effect of metformin or intensive lifestyle modification on all-cause and cause-specific mortality in patients at high risk for T2D.
Continue to: STUDY SUMMARY
STUDY SUMMARY
No mortality benefit from metformin or lifestyle modification
This secondary analysis evaluated mortality outcomes for patients at risk for T2D who were part of the DPP trial and then were followed long term in the Diabetes Prevention Program Outcomes Study (DPPOS).1 The initial DPP trial included 3234 adult patients at high risk for T2D (defined as having a BMI ≥ 24; an FPG of 95-125 mg/dL; and a 2-hour glucose level of 140-199 mg/dL). Participants were randomized into groups receiving either intensive lifestyle intervention (which focused on achieving ≥ 150 min/wk of exercise and ≥ 7% body weight loss), metformin 850 mg twice daily, or placebo twice daily; the latter 2 groups also received standard exercise and diet recommendations. Mean age was 51 years, mean BMI was 34, and 68% of participants were female.
At the conclusion of the initial 5-year trial, treatment was unmasked and 86% of the patients continued to be followed for long-term outcomes. Patients in the lifestyle group were offered semiannual lifestyle reinforcement, while the metformin group continued to receive the twice-daily 850-mg dose unless a contraindication developed. If FPG levels increased to ≥ 140 mg/dL in the DPP study, or A1C increased to ≥ 7% in the DPPOS, study metformin was discontinued and management of the patient’s diabetes was transferred to their health care provider. By the end of the DPPOS, 53% of patients in the lifestyle group and 55% in the metformin group had progressed to T2D, compared with 60% in the placebo group (P = 0.003).
After a median 21-year follow-up interval, the investigators collected data on cause of death for patients and evaluated hazard ratios (HRs) for overall and cause-specific mortality. In total, 14% of the participants died, with no statistically significant difference in rates between the 3 groups. Cancer (37%) was the leading cause of death in all groups, followed by cardiovascular disease (CVD; 29%).
Compared with the placebo group, patients taking metformin did not have a decreased rate of overall mortality (HR = 0.99; 95% CI, 0.79-1.25), mortality from cancer (HR = 1.04; 95% CI, 0.72-1.52), or mortality due to CVD (HR = 1.08; 95% CI, 0.70-1.66). Similarly, compared with the placebo group, lifestyle intervention did not decrease overall mortality (HR = 1.02; 95% CI, 0.81-1.28), mortality from cancer (HR = 1.07; 95% CI, 0.74-1.55), or mortality due to CVD (HR = 1.18; 95% CI, 0.77-1.81). Results were similar when adjusted for other factors, including out-of-study metformin use, T2D status and duration, BMI change, and other cardiovascular risk factors.
WHAT’S NEW
Long-term data clarifylimits to interventions’ utility
This study looked at long-term follow-up data on mortality outcomes for patients with prediabetes treated with metformin or lifestyle intervention. Although these interventions did support weight loss, reduce the incidence of T2D, and lower cardiovascular risk factors (eg, hypertension, dyslipidemia), the comorbidity benefits did not affect risk for all-cause or cause-specific mortality, which were similar between the treatment and placebo groups.
Continue to: CAVEATS
CAVEATS
Exclusion criteria, residual confounding may limit the findings
Patients with significant cardiovascular or renal disease were excluded, so results may not apply to patients with these comorbidities. Additionally, there was a high amount of “drop-in” use of metformin prescribed by physicians once patients developed T2D, which may not have been controlled for completely. And while the intensive lifestyle intervention group had specific goals, the metformin and placebo groups also were encouraged to follow standard diet and lifestyle recommendations—and during a bridge period, all participants were offered a modified group lifestyle intervention. However, multivariable adjustment did not change the study conclusion.
CHALLENGES TO IMPLEMENTATION
Physicians may be unwilling to change their current prescribing habits
Physicians may not be willing to change their practice of prescribing metformin in prediabetes based on a singular study (with residual confounding) that showed no long-term mortality differences between the study groups. However, there may be long-term morbidity differences of interest to patients that were not specifically evaluated in this study—such as quality-of-life benefits from weight loss that may outweigh the risks (eg, gastrointestinal adverse effects such as diarrhea, nausea, and abdominal pain) of metformin for some patients. Therefore, a discussion of the risks and benefits of treatment for prediabetes should be had with patients at high risk who would prefer a pharmacologic intervention.
1. Lee CG, Heckman-Stoddard B, et al; Diabetes Prevention Program Research Group. Effect of metformin and lifestyle interventions on mortality in the Diabetes Prevention Program and Diabetes Prevention Program Outcomes Study. Diabetes Care. 2021;44:2775-2782. doi: 10.2337/dc21-1046
2. American Diabetes Association. Understanding A1C: diagnosis. Accessed July 6, 2023. https://diabetes.org/diabetes/a1c/diagnosis
3. CDC. National diabetes statistics report. Reviewed June 29, 2022. Accessed January 23, 2023. www.cdc.gov/diabetes/data/statistics-report/index.html
4. USPSTF; Davidson KW, Barry MJ, Mangione CM, et al. Screening for prediabetes and type 2 diabetes: US Preventive Services Task Force recommendation statement. JAMA. 2021;326:736-743. doi: 10.1001/jama.2021.12531
5. Hostalek U, Campbell I. Metformin for diabetes prevention: update of the evidence base. Curr Med Res Opin. 2021;37:1705-1717. doi: 10.1080/03007995.2021.1955667
6. Aroda VR, Knowler WC, Crandall JP, et al; Diabetes Prevention Program Research Group. Metformin for diabetes prevention: insights gained from the Diabetes Prevention Program/Diabetes Prevention Program Outcomes Study. Diabetologia. 2017;60:1601-1611. doi: 10.1007/s00125-017-4361-9
7. Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403. doi: 10.1056/NEJMoa012512
8. Herman WH, Ratner RE. Metformin should be used to treat prediabetes in selected individuals. Diabetes Care. 2020;43:1988-1990. doi: 10.2337/dci20-0030
9. American Diabetes Association. 3. Prevention or delay of type 2 diabetes: standards of medical care in diabetes—2021. Diabetes Care. 2021;44(suppl 1):S34-S39. doi: 10.2337/dc21-S003
10. Tseng E, Yeh HC, Maruthur NM. Metformin use in prediabetes among US adults, 2005-2012. Diabetes Care. 2017;40:887-893. doi: 10.2337/dc16-1509
11. Davidson MB. Metformin should not be used to treat prediabetes. Diabetes Care. 2020;43:1983-1987. doi: 10.2337/dc19-2221
1. Lee CG, Heckman-Stoddard B, et al; Diabetes Prevention Program Research Group. Effect of metformin and lifestyle interventions on mortality in the Diabetes Prevention Program and Diabetes Prevention Program Outcomes Study. Diabetes Care. 2021;44:2775-2782. doi: 10.2337/dc21-1046
2. American Diabetes Association. Understanding A1C: diagnosis. Accessed July 6, 2023. https://diabetes.org/diabetes/a1c/diagnosis
3. CDC. National diabetes statistics report. Reviewed June 29, 2022. Accessed January 23, 2023. www.cdc.gov/diabetes/data/statistics-report/index.html
4. USPSTF; Davidson KW, Barry MJ, Mangione CM, et al. Screening for prediabetes and type 2 diabetes: US Preventive Services Task Force recommendation statement. JAMA. 2021;326:736-743. doi: 10.1001/jama.2021.12531
5. Hostalek U, Campbell I. Metformin for diabetes prevention: update of the evidence base. Curr Med Res Opin. 2021;37:1705-1717. doi: 10.1080/03007995.2021.1955667
6. Aroda VR, Knowler WC, Crandall JP, et al; Diabetes Prevention Program Research Group. Metformin for diabetes prevention: insights gained from the Diabetes Prevention Program/Diabetes Prevention Program Outcomes Study. Diabetologia. 2017;60:1601-1611. doi: 10.1007/s00125-017-4361-9
7. Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403. doi: 10.1056/NEJMoa012512
8. Herman WH, Ratner RE. Metformin should be used to treat prediabetes in selected individuals. Diabetes Care. 2020;43:1988-1990. doi: 10.2337/dci20-0030
9. American Diabetes Association. 3. Prevention or delay of type 2 diabetes: standards of medical care in diabetes—2021. Diabetes Care. 2021;44(suppl 1):S34-S39. doi: 10.2337/dc21-S003
10. Tseng E, Yeh HC, Maruthur NM. Metformin use in prediabetes among US adults, 2005-2012. Diabetes Care. 2017;40:887-893. doi: 10.2337/dc16-1509
11. Davidson MB. Metformin should not be used to treat prediabetes. Diabetes Care. 2020;43:1983-1987. doi: 10.2337/dc19-2221
PRACTICE CHANGER
STRENGTH OF RECOMMENDATION
B: Based on a long-term follow-up of a randomized controlled trial.1
Lee CG, Heckman-Stoddard B, Dabelea D, et al; Diabetes Prevention Program Research Group. Effect of metformin and lifestyle interventions on mortality in the Diabetes Prevention Program and Diabetes Prevention Program Outcomes Study. Diabetes Care. 2021;44:2775-2782. doi: 10.2337/dc21-1046
A worthwhile tool in evaluating worrisome lesions
ABSTRACT
Background: We sought to examine whether electrical impedance spectroscopy (EIS), a diagnostic tool approved by the US Food and Drug Administration for the evaluation of pigmented skin lesions (PSLs), is beneficial to primary care providers (PCPs) by comparing the accuracy of PCPs’ management decisions for PSLs based on visual examination alone with those based on concurrent visual and EIS evaluation.
Methods: Physicians and nurse practitioners (NPs) participated in an anonymous online survey in which they viewed clinical images of PSLs and were asked to make 2 clinical decisions before and after being provided an EIS score that indicated the likelihood that the lesion was a melanoma. They were asked (1) if they would biopsy the lesion/refer the patient out and (2) what they expected the pathology results would show.
Results: Forty-four physicians and 17 NPs participated, making clinical decisions for 1354 presented lesions. Overall, with the addition of EIS to visual inspection of clinical images, the sensitivity of biopsy/referral decisions for melanomas and severely dysplastic nevi (SDN) increased from 69.2% to 90.0% (P < .001), while specificity increased from 44.0% to 72.6% (P < .001). Physicians and NPs, regardless of years of experience, each saw significant improvements in sensitivity, specificity, and diagnostic accuracy with the addition of EIS scores.
Conclusions: The incorporation of EIS data into clinical decision-making by PCPs significantly increased the sensitivity and specificity of biopsy/referral decisions for melanomas and SDN and overall diagnostic accuracy compared with visual inspection alone. The results of this study suggest that diagnostic accuracy for PSLs by PCPs may be improved with adjunctive use of EIS with visual inspection.
Primary care providers (PCPs) are often the first line of defense in detecting skin cancers. For patients with concerning skin lesions, PCPs may choose to perform a biopsy or facilitate access to specialty services (eg, Dermatology). Consequently, PCPs play a critical role in the timely detection of skin cancers, and it is paramount to employ continually improving detection methods, such as the application of technologic advances.1
Differentiating benign nevi from melanoma and severely dysplastic nevi (SDN), both of which warrant excision, poses a unique challenge to clinicians examining pigmented skin lesions (PSLs). PCPs often rely on visual inspection to differentiate benign skin lesions from malignant skin cancers. In some primary care practices, dermoscopy, which involves using a handheld device to evaluate lesions with polarized light and magnification, is used to improve melanoma detection. However, while visual inspection and dermoscopy are valid, effective techniques for the diagnosis of melanocytic lesions, in many instances they still can lead to missed cancers or unnecessary biopsies and specialty referrals. Adjunctive use of dermoscopy with visual inspection has been shown to increase the probability of skin cancer detection, but it fails to achieve a near-100% success rate.2 Furthermore, dermoscopy is heavily user-dependent, requiring significant training and experience for appropriate use.3
Another option is an electrical impedance spectroscopy (EIS) device (Nevisense, Scibase, Stockholm, Sweden), which has been approved by the US Food and Drug Administration (FDA) to assist in the detection of melanoma and differentiation from benign PSLs.4 EIS is a noninvasive, rapidly applied technology designed to accompany the visual examination of melanocytic lesions in office, with or without dermoscopy. Still relatively new, the technology is employed today by many dermatologists, increasing diagnostic accuracy for PSLs.5 The lightweight and portable instrument features a handheld probe, which is held against a lesion to obtain a reading. EIS uses a low-voltage electrode to apply a harmless electrical current to the skin at various frequencies.6 As benign and malignant tissues vary in cell shape, size, and composition, EIS distinguishes differential electrical resistance of the tissue to aid in diagnosis.7
Continue to: EIS provides high-sensitivity...
EIS provides high-sensitivity melanoma diagnosis vs histopathologic confirmation from biopsies, with 1 study showing a 96.6% sensitivity rating, detecting 256 of 265 melanomas.4 The EIS device, by measuring differences in electrical resistance between benign and cancerous cells, outputs a simple integer score ranging from 0 to 10 associated with the likelihood of the lesion being a melanoma.8 Based on data from the Nevisense pivotal trial,4 Nevisense reports that scores of 0 to 3 carry a negative predictive value of 99% for melanoma, whereas scores of 4 to 10 signify increasingly greater positive predictive values from 7% to 61%.
We aimed to assess whether EIS may be beneficial to PCPs by comparing the accuracy of clinical decision-making for PSLs based on visual examination alone with that based on concurrent visual and EIS evaluation.
METHODS
A questionnaire was distributed via email to 142 clinicians at clinics affiliated with either of 2 organizations delivering care to the New York City area through a network of community health centers: the Institute for Family Health (IFH) and the Community Healthcare Network (CHN). Of these recipients, 72 were affiliated with IFH across 27 community health centers and 70 were affiliated with CHN across 14 community health centers. Recipients were physicians and nurse practitioners (NPs) practicing at primary health care facilities.
Survey instrument. The first section of the survey instrument (APPENDIX) solicited demographic information and explained how to apply the EIS scores for diagnostic decision-making. The second featured images of 12 randomly selected, histologically confirmed, and EIS-evaluated PSLs from a previously published prospective blinded trial of 2416 lesions.4 The Institutional Review Board of the Icahn School of Medicine at Mount Sinai reviewed and approved the study and survey instrument.
Clinical images of these lesions, comprising 4 melanocytic nevi, 4 dysplastic nevi (including 3 mild-moderately dysplastic and 1 severely dysplastic nevus), and 4 melanomas
Continue to: Analysis
Analysis. A biopsy or referral rating of 4 or 5 was considered a decision to biopsy or refer (ie, a diagnostic decision consistent with melanoma or SDN warranting excision), whereas a selection of 1 to 3 was considered a decision not to biopsy or refer (ie, a diagnostic decision consistent with a benign PSL). The sensitivity and specificity of biopsy/referral decisions for melanomas and SDN, the proportion of missed melanomas and SDN, and the proportion of biopsy/referral decisions for benign lesions were separately determined for visual inspection alone and visual inspection with EIS score. Similarly, diagnostic accuracy was calculated for these clinical scenarios. These metrics were further stratified among different subsets of the respondent population. Differences in sensitivity, specificity, biopsy/referral decision proportions, and diagnostic accuracy were calculated using McNemar’s test for paired proportions.
RESULTS
Sixty-one respondents, comprising 44 physicians and 17 NPs, completed the survey, yielding a response rate of 43% (TABLE 1). In total, 1354 clinical decisions (677 based on visual inspection alone and 677 based on visual inspection plus EIS) were made. A biopsy/referral decision was made after assessing 416 of 677 cases (61%) with visual inspection alone and 360 of 677 cases (53%) when relying on visual inspection plus EIS. None of the respondents reported any prior experience with EIS.
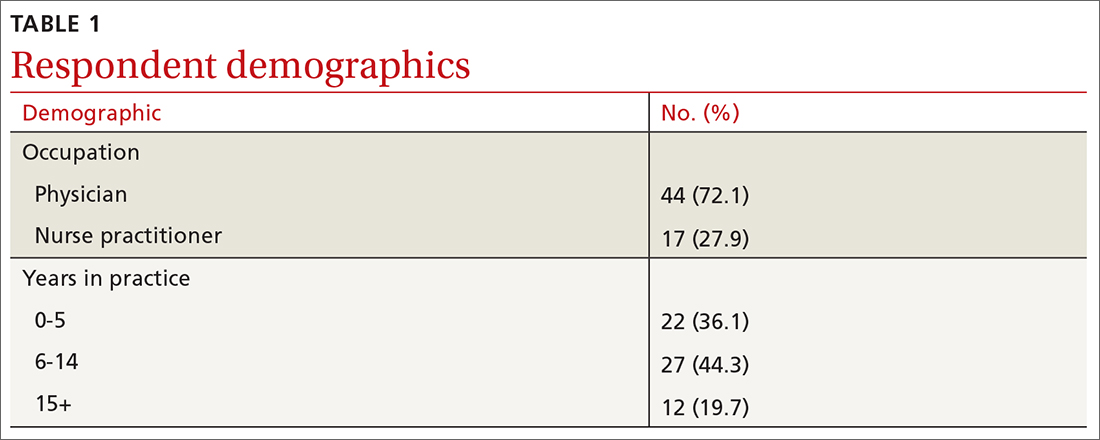
When incorporating EIS scores, respondents’ mean sensitivity for melanomas and SDN increased from 69.2% to 90.0% (P < .001) and specificity from 44.0% to 72.6% (P < .001; TABLE 2). At baseline, physicians demonstrated a sensitivity and specificity of 74.6% and 46.5%, respectively, while NPs demonstrated a sensitivity and specificity of 56.1% and 37.9%, respectively.
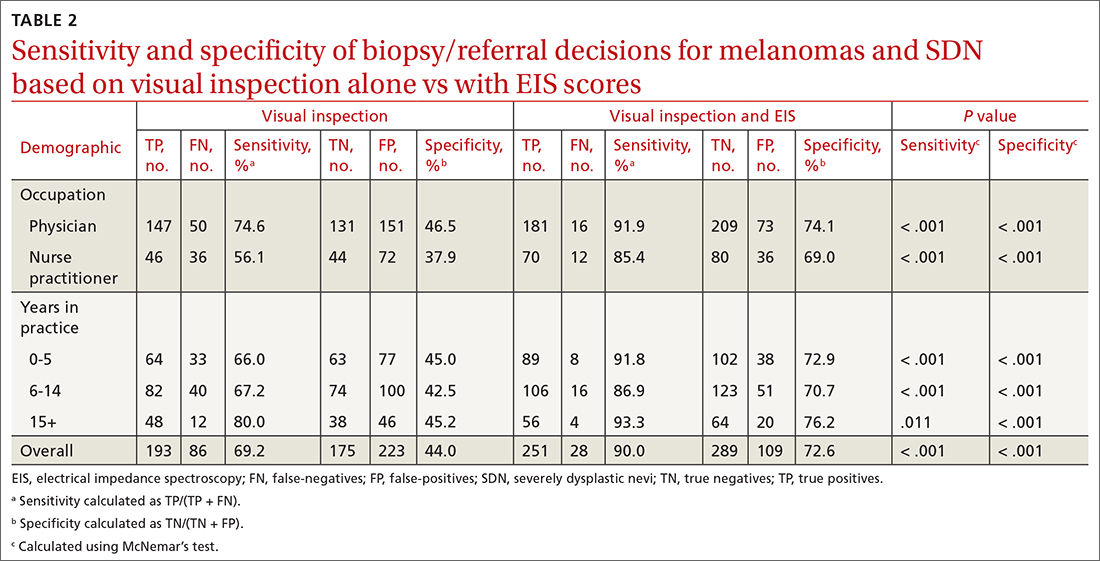
All respondent subgroups stratified by occupation and years of experience saw significant increases in both sensitivity and specificity upon the incorporation of EIS scores, with NPs seeing a greater increase in sensitivity (56.1% vs 85.4%; P < .001) and specificity (37.9% vs 69.0%; P < .001) than physicians (sensitivity: 74.6% vs 91.9%; P < .001; specificity: 46.5% vs 74.1%; P < .001). The only difference in diagnostic performance based on years of experience was a greater pre-EIS sensitivity by clinicians who had been in practice for ≥ 15 years, compared with those in practice for shorter periods (TABLE 2).
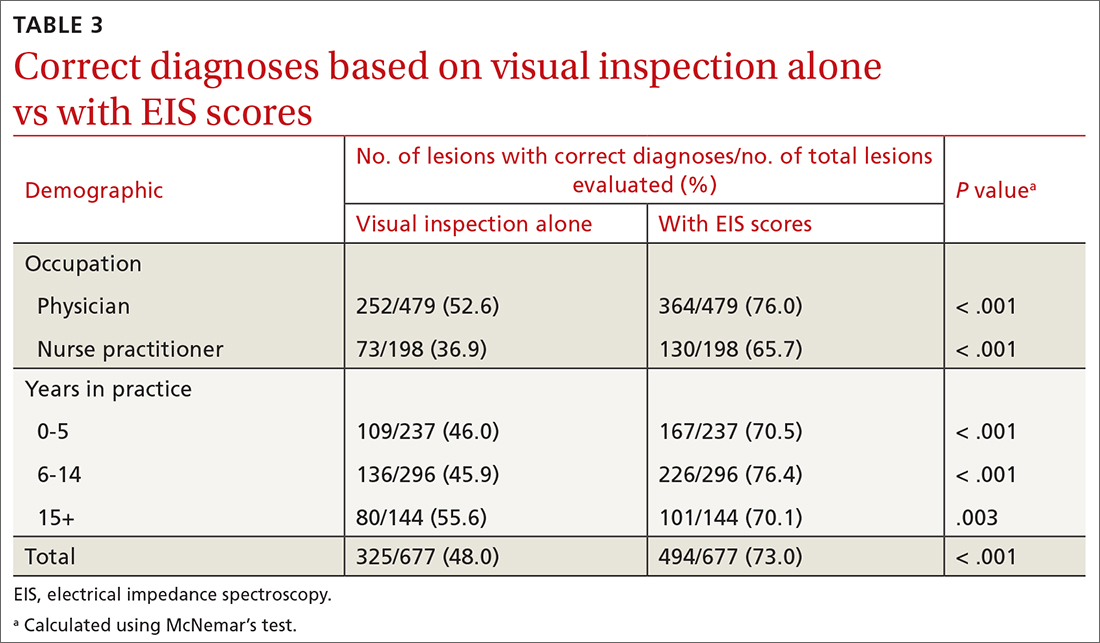
Diagnostic accuracy increased significantly from 48% when based on visual inspection alone to 73% with the addition of EIS scores (P < .001; TABLE 3). Physicians and NPs each significantly increased their diagnostic accuracy upon the incorporation of EIS, with NPs exhibiting the greatest increase (from 36.9% to 65.7%; P < .001). PCPs with 6 to 14 years of experience saw the greatest increase in diagnostic accuracy when adding EIS (45.9% vs 76.4%; P < .001). Overall, the addition of EIS scores resulted in 58 fewer missed melanomas and SDN and 114 fewer benign referrals or biopsies (TABLE 4).
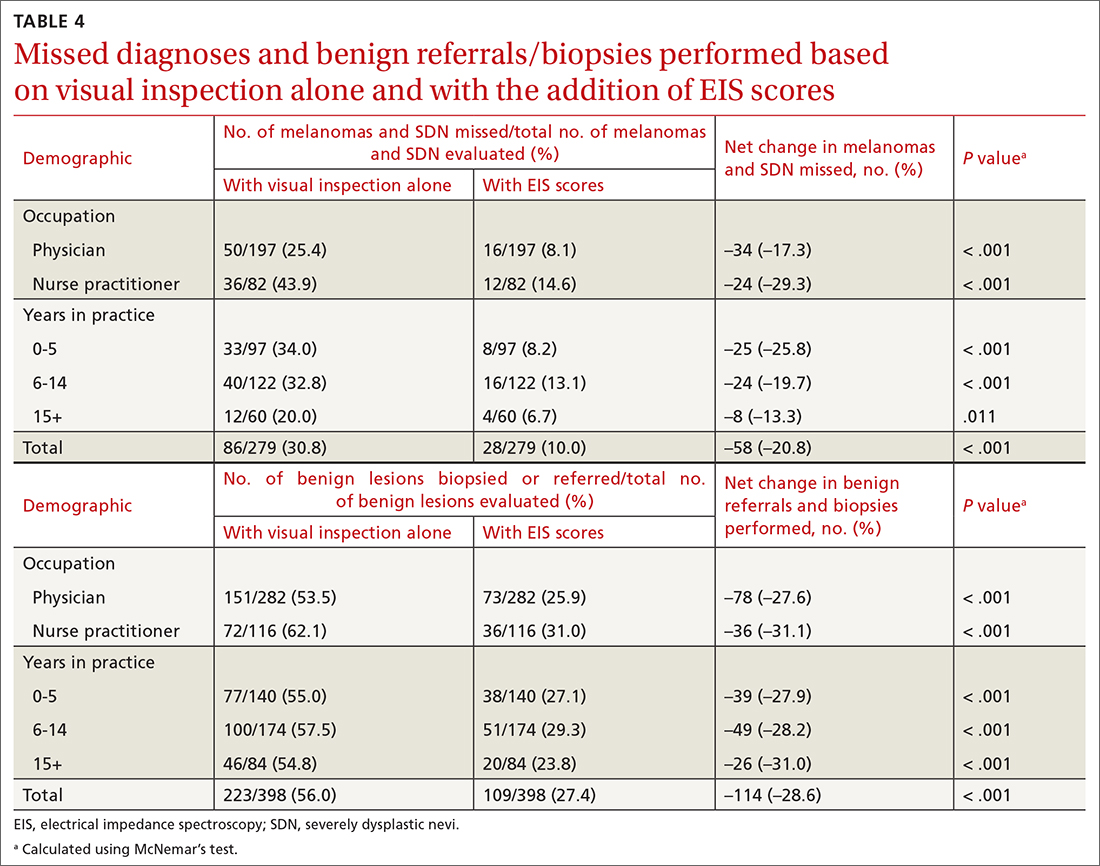
Continue to: DISCUSSION
DISCUSSION
Primary care evaluation plays a significant role in the diagnosis and management of PSLs, ultimately shaping outcomes for patients with melanoma. Improved accuracy of PSL classification could yield greater sensitivity for the diagnosis of melanomas and high-risk melanocytic lesions at earlier stages, while also reducing the number of unnecessary biopsies and referrals—leading to decreased patient morbidity and mortality and reduced health care spending.9
Diagnostic tools are valuable insofar as they can improve accuracy and positively impact clinical management and patient outcomes.10 In this case, increased sensitivity reduced missed melanoma diagnoses, while increased specificity avoided the additional costs and patient toll associated with a biopsy or referral for a benign lesion.
Dermoscopy has been shown to improve the sensitivity and specificity of PSL diagnosis compared with visual inspection alone; however, without substantial training and experience, accuracy with dermoscopy can be no better than examination with the naked eye.3,11,12 The dropout rates are high for training PCPs in its use, given that several months of training may be needed for competent use.13,14 To improve the clinical management of PSLs broadly in primary care, a need exists for easy-to-use adjunctive tools that increase diagnostic accuracy.15
In this study, with only a brief explanation of how to interpret EIS scores, clinicians without any prior experience using EIS demonstrated significantly improved accuracy in deciding appropriate management and classifying melanocytic lesions with the addition of EIS to visual inspection. These improvements, seen in clinicians of varying training and experience, suggest that the learning curve of EIS may not be as steep as that of dermoscopy.
The greater baseline sensitivity, specificity, and diagnostic accuracy of physicians’ clinical decision-making compared with NPs before the incorporation of EIS in the study may be a product of comparatively more extensive medical training. In addition, EIS yielded a greater benefit to NPs than to physicians, with greater increases in sensitivity and specificity noted. This suggests that the use of EIS is particularly advantageous to clinicians who are less proficient in assessing melanocytic lesions. Using visual inspection alone, more experienced respondents made biopsy/referral decisions with greater sensitivity but similar specificity to those with less experience. With the incorporation of EIS scores, the sensitivity and specificity of respondents’ clinical decision-making rose to comparable levels across all experience groups, providing further indication of EIS’s particular value to clinicians who are less proficient in PSL evaluation.
Continue to: This technology holds the potential...
This technology holds the potential to be seamlessly implemented into primary care practice, given that dermatology expertise training is not required to use the EIS device; this could allow for EIS measurement of lesions to be delegated to office staff (eg, nurses, medical assistants).16 Future studies are needed to assess EIS use among PCPs in a real-world setting, where factors such as its application on nonmelanocytic lesions (eg, seborrheic keratoses) and its pairing with patient historical data could produce varying results.
Limitations. While revealing, this study had its limitations. Respondents did not have access to additional pertinent clinical information, such as patients’ histories and risk factors. Clinical decisions in this survey were made based on digital images rather than in vivo examination. This may not represent a real-life evaluation; there is the potential for minimization of the true consequences of a missed melanoma or unnecessary biopsy in the minds of participants, and this does not factor in the operation of the actual EIS device. The Hawthorne effect may also have influenced PCPs’ diagnostic selections. Also, the limited sample size constitutes another limitation.
Of note, in this survey format, respondents rated their inclination to biopsy or refer each lesion from 1 to 5. For statistical analyses, lesions rated 1 to 3 were considered as not biopsied/referred and those rated 4 to 5 as biopsied/referred. The sensitivity and specificity values observed, for both visual examination and concurrent visual and EIS evaluation, are therefore based on this classification system of participants’ provided ratings. It is conceivable that differing sensitivity and specificity values might have been detected if clinicians were instead given a binary choice for referral/biopsy decisions.
CONCLUSIONS
Among PCPs tasked with evaluating melanocytic lesions, the incorporation of EIS data into clinical decision-making in this study significantly increased the sensitivity, specificity, and overall diagnostic accuracy of biopsy or referral decisions for melanomas and SDN compared with visual inspection alone. Overall, the results of this preliminary study suggest that diagnostic accuracy for PSLs by PCPs may be improved with the adjunctive use of EIS with visual inspection. This would ultimately improve patient care and reduce the morbidity and mortality of a melanoma diagnosis.
CORRESPONDENCE
Jonathan Ungar, MD, Kimberly and Eric J. Waldman Department of Dermatology, Icahn School of Medicine at Mount Sinai, 5 East 98th Street, 5th Floor, New York, NY 10029; [email protected]
1. Goetsch NJ, Hoehns JD, Sutherland JE, et al. Assessment of postgraduate skin lesion education among Iowa family physicians. SAGE Open Med. 2017;5:2050312117691392. doi: 10.1177/2050312117691392
2. Dinnes J, Deeks JJ, Chuchu N, et al. Dermoscopy, with and without visual inspection, for diagnosing melanoma in adults. Cochrane Database Syst Rev. 2018;12:CD011902. doi: 10.1002/14651858.CD011902.pub2
3. Jones OT, Jurascheck LC, van Melle MA, et al. Dermoscopy for melanoma detection and triage in primary care: a systematic review. BMJ Open. 2019;9:e027529. doi: 10.1136/bmjopen-2018-027529
4. Malvehy J, Hauschild A, Curiel-Lewandrowski C, et al. Clinical performance of the Nevisense system in cutaneous melanoma detection: an international, multicentre, prospective and blinded clinical trial on efficacy and safety. Br J Dermatol. 2014;171:1099-1107. doi: 10.1111/bjd.13121
5. Svoboda RM, Prado G, Mirsky RS, et al. Assessment of clinician accuracy for diagnosing melanoma on the basis of electrical impedance spectroscopy score plus morphology versus lesion morphology alone. J Am Acad Dermatol. 2019;80:285-287. doi: 10.1016/j.jaad.2018.08.048
6. Mohr P, Birgersson U, Berking C, et al. Electrical impedance spectroscopy as a potential adjunct diagnostic tool for cutaneous melanoma. Skin Res Technol. 2013;19:75-83. doi: 10.1111/srt.12008
7. Rocha L, Menzies SW, Lo S, et al. Analysis of an electrical impedance spectroscopy system in short-term digital dermoscopy imaging of melanocytic lesions. Br J Dermatol. 2017;177:1432-1438. doi: 10.1111/bjd.15595
8. Litchman GH, Teplitz RW, Marson JW, et al. Impact of electrical impedance spectroscopy on dermatologists’ number needed to biopsy metric and biopsy decisions for pigmented skin lesions. J Am Acad Dermatol. 2021;85:976-979. doi: 10.1016/j.jaad.2020.09.011
9. Greenwood-Lee J, Jewett L, Woodhouse L, et al. A categorisation of problems and solutions to improve patient referrals from primary to specialty care. BMC Health Serv Res. 2018;18:1-16. doi: 10.1186/s12913-018-3745-y
10. Bossuyt PM, Reitsma JB, Linnet K, et al. Beyond diagnostic accuracy: the clinical utility of diagnostic tests. Clin Chem. 2012;58:1636-1643. doi: 10.1373/clinchem.2012.182576
11. Argenziano G, Cerroni L, Zalaudek I , et al. Accuracy in melanoma detection: a 10-year multicenter survey. J Am Acad Dermatol. 2012;67:54-59. doi: 10.1016/j.jaad.2011.07.019
12. Menzies SW, Vestergaard ME, Macaskill P, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676. doi: 10.1111/j.1365-2133.2008.08713.x
13. Menzies SW, Emery J, Staples M, et al. Impact of dermoscopy and short-term sequential digital dermoscopy imaging for the management of pigmented lesions in primary care: a sequential intervention trial. Br J Dermatol. 2009;161:1270-1277. doi: 10.1111/j.1365-2133.2009.09374.x
14. Noor O, Nanda A, Rao BK. A dermoscopy survey to assess who is using it and why it is or is not being used. Int J Dermatol. 2009;48:951-952. doi: 10.1111/j.1365-4632.2009.04095.x
15. Weigl BH, Boyle DS, de los Santos T, et al. Simplicity of use: a critical feature for widespread adoption of diagnostic technologies in low-resource settings. Expert Rev Med Devices. 2009;6:461-464. doi: 10.1586/erd.09.31
16. Sarac E, Meiwes A, Eigentler T, et al. Diagnostic accuracy of electrical impedance spectroscopy in non-melanoma skin cancer. Acta Derm Venereol. 2020;100:adv00328. doi: 10.2340/00015555-3689
ABSTRACT
Background: We sought to examine whether electrical impedance spectroscopy (EIS), a diagnostic tool approved by the US Food and Drug Administration for the evaluation of pigmented skin lesions (PSLs), is beneficial to primary care providers (PCPs) by comparing the accuracy of PCPs’ management decisions for PSLs based on visual examination alone with those based on concurrent visual and EIS evaluation.
Methods: Physicians and nurse practitioners (NPs) participated in an anonymous online survey in which they viewed clinical images of PSLs and were asked to make 2 clinical decisions before and after being provided an EIS score that indicated the likelihood that the lesion was a melanoma. They were asked (1) if they would biopsy the lesion/refer the patient out and (2) what they expected the pathology results would show.
Results: Forty-four physicians and 17 NPs participated, making clinical decisions for 1354 presented lesions. Overall, with the addition of EIS to visual inspection of clinical images, the sensitivity of biopsy/referral decisions for melanomas and severely dysplastic nevi (SDN) increased from 69.2% to 90.0% (P < .001), while specificity increased from 44.0% to 72.6% (P < .001). Physicians and NPs, regardless of years of experience, each saw significant improvements in sensitivity, specificity, and diagnostic accuracy with the addition of EIS scores.
Conclusions: The incorporation of EIS data into clinical decision-making by PCPs significantly increased the sensitivity and specificity of biopsy/referral decisions for melanomas and SDN and overall diagnostic accuracy compared with visual inspection alone. The results of this study suggest that diagnostic accuracy for PSLs by PCPs may be improved with adjunctive use of EIS with visual inspection.
Primary care providers (PCPs) are often the first line of defense in detecting skin cancers. For patients with concerning skin lesions, PCPs may choose to perform a biopsy or facilitate access to specialty services (eg, Dermatology). Consequently, PCPs play a critical role in the timely detection of skin cancers, and it is paramount to employ continually improving detection methods, such as the application of technologic advances.1
Differentiating benign nevi from melanoma and severely dysplastic nevi (SDN), both of which warrant excision, poses a unique challenge to clinicians examining pigmented skin lesions (PSLs). PCPs often rely on visual inspection to differentiate benign skin lesions from malignant skin cancers. In some primary care practices, dermoscopy, which involves using a handheld device to evaluate lesions with polarized light and magnification, is used to improve melanoma detection. However, while visual inspection and dermoscopy are valid, effective techniques for the diagnosis of melanocytic lesions, in many instances they still can lead to missed cancers or unnecessary biopsies and specialty referrals. Adjunctive use of dermoscopy with visual inspection has been shown to increase the probability of skin cancer detection, but it fails to achieve a near-100% success rate.2 Furthermore, dermoscopy is heavily user-dependent, requiring significant training and experience for appropriate use.3
Another option is an electrical impedance spectroscopy (EIS) device (Nevisense, Scibase, Stockholm, Sweden), which has been approved by the US Food and Drug Administration (FDA) to assist in the detection of melanoma and differentiation from benign PSLs.4 EIS is a noninvasive, rapidly applied technology designed to accompany the visual examination of melanocytic lesions in office, with or without dermoscopy. Still relatively new, the technology is employed today by many dermatologists, increasing diagnostic accuracy for PSLs.5 The lightweight and portable instrument features a handheld probe, which is held against a lesion to obtain a reading. EIS uses a low-voltage electrode to apply a harmless electrical current to the skin at various frequencies.6 As benign and malignant tissues vary in cell shape, size, and composition, EIS distinguishes differential electrical resistance of the tissue to aid in diagnosis.7
Continue to: EIS provides high-sensitivity...
EIS provides high-sensitivity melanoma diagnosis vs histopathologic confirmation from biopsies, with 1 study showing a 96.6% sensitivity rating, detecting 256 of 265 melanomas.4 The EIS device, by measuring differences in electrical resistance between benign and cancerous cells, outputs a simple integer score ranging from 0 to 10 associated with the likelihood of the lesion being a melanoma.8 Based on data from the Nevisense pivotal trial,4 Nevisense reports that scores of 0 to 3 carry a negative predictive value of 99% for melanoma, whereas scores of 4 to 10 signify increasingly greater positive predictive values from 7% to 61%.
We aimed to assess whether EIS may be beneficial to PCPs by comparing the accuracy of clinical decision-making for PSLs based on visual examination alone with that based on concurrent visual and EIS evaluation.
METHODS
A questionnaire was distributed via email to 142 clinicians at clinics affiliated with either of 2 organizations delivering care to the New York City area through a network of community health centers: the Institute for Family Health (IFH) and the Community Healthcare Network (CHN). Of these recipients, 72 were affiliated with IFH across 27 community health centers and 70 were affiliated with CHN across 14 community health centers. Recipients were physicians and nurse practitioners (NPs) practicing at primary health care facilities.
Survey instrument. The first section of the survey instrument (APPENDIX) solicited demographic information and explained how to apply the EIS scores for diagnostic decision-making. The second featured images of 12 randomly selected, histologically confirmed, and EIS-evaluated PSLs from a previously published prospective blinded trial of 2416 lesions.4 The Institutional Review Board of the Icahn School of Medicine at Mount Sinai reviewed and approved the study and survey instrument.
Clinical images of these lesions, comprising 4 melanocytic nevi, 4 dysplastic nevi (including 3 mild-moderately dysplastic and 1 severely dysplastic nevus), and 4 melanomas
Continue to: Analysis
Analysis. A biopsy or referral rating of 4 or 5 was considered a decision to biopsy or refer (ie, a diagnostic decision consistent with melanoma or SDN warranting excision), whereas a selection of 1 to 3 was considered a decision not to biopsy or refer (ie, a diagnostic decision consistent with a benign PSL). The sensitivity and specificity of biopsy/referral decisions for melanomas and SDN, the proportion of missed melanomas and SDN, and the proportion of biopsy/referral decisions for benign lesions were separately determined for visual inspection alone and visual inspection with EIS score. Similarly, diagnostic accuracy was calculated for these clinical scenarios. These metrics were further stratified among different subsets of the respondent population. Differences in sensitivity, specificity, biopsy/referral decision proportions, and diagnostic accuracy were calculated using McNemar’s test for paired proportions.
RESULTS
Sixty-one respondents, comprising 44 physicians and 17 NPs, completed the survey, yielding a response rate of 43% (TABLE 1). In total, 1354 clinical decisions (677 based on visual inspection alone and 677 based on visual inspection plus EIS) were made. A biopsy/referral decision was made after assessing 416 of 677 cases (61%) with visual inspection alone and 360 of 677 cases (53%) when relying on visual inspection plus EIS. None of the respondents reported any prior experience with EIS.

When incorporating EIS scores, respondents’ mean sensitivity for melanomas and SDN increased from 69.2% to 90.0% (P < .001) and specificity from 44.0% to 72.6% (P < .001; TABLE 2). At baseline, physicians demonstrated a sensitivity and specificity of 74.6% and 46.5%, respectively, while NPs demonstrated a sensitivity and specificity of 56.1% and 37.9%, respectively.

All respondent subgroups stratified by occupation and years of experience saw significant increases in both sensitivity and specificity upon the incorporation of EIS scores, with NPs seeing a greater increase in sensitivity (56.1% vs 85.4%; P < .001) and specificity (37.9% vs 69.0%; P < .001) than physicians (sensitivity: 74.6% vs 91.9%; P < .001; specificity: 46.5% vs 74.1%; P < .001). The only difference in diagnostic performance based on years of experience was a greater pre-EIS sensitivity by clinicians who had been in practice for ≥ 15 years, compared with those in practice for shorter periods (TABLE 2).

Diagnostic accuracy increased significantly from 48% when based on visual inspection alone to 73% with the addition of EIS scores (P < .001; TABLE 3). Physicians and NPs each significantly increased their diagnostic accuracy upon the incorporation of EIS, with NPs exhibiting the greatest increase (from 36.9% to 65.7%; P < .001). PCPs with 6 to 14 years of experience saw the greatest increase in diagnostic accuracy when adding EIS (45.9% vs 76.4%; P < .001). Overall, the addition of EIS scores resulted in 58 fewer missed melanomas and SDN and 114 fewer benign referrals or biopsies (TABLE 4).

Continue to: DISCUSSION
DISCUSSION
Primary care evaluation plays a significant role in the diagnosis and management of PSLs, ultimately shaping outcomes for patients with melanoma. Improved accuracy of PSL classification could yield greater sensitivity for the diagnosis of melanomas and high-risk melanocytic lesions at earlier stages, while also reducing the number of unnecessary biopsies and referrals—leading to decreased patient morbidity and mortality and reduced health care spending.9
Diagnostic tools are valuable insofar as they can improve accuracy and positively impact clinical management and patient outcomes.10 In this case, increased sensitivity reduced missed melanoma diagnoses, while increased specificity avoided the additional costs and patient toll associated with a biopsy or referral for a benign lesion.
Dermoscopy has been shown to improve the sensitivity and specificity of PSL diagnosis compared with visual inspection alone; however, without substantial training and experience, accuracy with dermoscopy can be no better than examination with the naked eye.3,11,12 The dropout rates are high for training PCPs in its use, given that several months of training may be needed for competent use.13,14 To improve the clinical management of PSLs broadly in primary care, a need exists for easy-to-use adjunctive tools that increase diagnostic accuracy.15
In this study, with only a brief explanation of how to interpret EIS scores, clinicians without any prior experience using EIS demonstrated significantly improved accuracy in deciding appropriate management and classifying melanocytic lesions with the addition of EIS to visual inspection. These improvements, seen in clinicians of varying training and experience, suggest that the learning curve of EIS may not be as steep as that of dermoscopy.
The greater baseline sensitivity, specificity, and diagnostic accuracy of physicians’ clinical decision-making compared with NPs before the incorporation of EIS in the study may be a product of comparatively more extensive medical training. In addition, EIS yielded a greater benefit to NPs than to physicians, with greater increases in sensitivity and specificity noted. This suggests that the use of EIS is particularly advantageous to clinicians who are less proficient in assessing melanocytic lesions. Using visual inspection alone, more experienced respondents made biopsy/referral decisions with greater sensitivity but similar specificity to those with less experience. With the incorporation of EIS scores, the sensitivity and specificity of respondents’ clinical decision-making rose to comparable levels across all experience groups, providing further indication of EIS’s particular value to clinicians who are less proficient in PSL evaluation.
Continue to: This technology holds the potential...
This technology holds the potential to be seamlessly implemented into primary care practice, given that dermatology expertise training is not required to use the EIS device; this could allow for EIS measurement of lesions to be delegated to office staff (eg, nurses, medical assistants).16 Future studies are needed to assess EIS use among PCPs in a real-world setting, where factors such as its application on nonmelanocytic lesions (eg, seborrheic keratoses) and its pairing with patient historical data could produce varying results.
Limitations. While revealing, this study had its limitations. Respondents did not have access to additional pertinent clinical information, such as patients’ histories and risk factors. Clinical decisions in this survey were made based on digital images rather than in vivo examination. This may not represent a real-life evaluation; there is the potential for minimization of the true consequences of a missed melanoma or unnecessary biopsy in the minds of participants, and this does not factor in the operation of the actual EIS device. The Hawthorne effect may also have influenced PCPs’ diagnostic selections. Also, the limited sample size constitutes another limitation.
Of note, in this survey format, respondents rated their inclination to biopsy or refer each lesion from 1 to 5. For statistical analyses, lesions rated 1 to 3 were considered as not biopsied/referred and those rated 4 to 5 as biopsied/referred. The sensitivity and specificity values observed, for both visual examination and concurrent visual and EIS evaluation, are therefore based on this classification system of participants’ provided ratings. It is conceivable that differing sensitivity and specificity values might have been detected if clinicians were instead given a binary choice for referral/biopsy decisions.
CONCLUSIONS
Among PCPs tasked with evaluating melanocytic lesions, the incorporation of EIS data into clinical decision-making in this study significantly increased the sensitivity, specificity, and overall diagnostic accuracy of biopsy or referral decisions for melanomas and SDN compared with visual inspection alone. Overall, the results of this preliminary study suggest that diagnostic accuracy for PSLs by PCPs may be improved with the adjunctive use of EIS with visual inspection. This would ultimately improve patient care and reduce the morbidity and mortality of a melanoma diagnosis.
CORRESPONDENCE
Jonathan Ungar, MD, Kimberly and Eric J. Waldman Department of Dermatology, Icahn School of Medicine at Mount Sinai, 5 East 98th Street, 5th Floor, New York, NY 10029; [email protected]
ABSTRACT
Background: We sought to examine whether electrical impedance spectroscopy (EIS), a diagnostic tool approved by the US Food and Drug Administration for the evaluation of pigmented skin lesions (PSLs), is beneficial to primary care providers (PCPs) by comparing the accuracy of PCPs’ management decisions for PSLs based on visual examination alone with those based on concurrent visual and EIS evaluation.
Methods: Physicians and nurse practitioners (NPs) participated in an anonymous online survey in which they viewed clinical images of PSLs and were asked to make 2 clinical decisions before and after being provided an EIS score that indicated the likelihood that the lesion was a melanoma. They were asked (1) if they would biopsy the lesion/refer the patient out and (2) what they expected the pathology results would show.
Results: Forty-four physicians and 17 NPs participated, making clinical decisions for 1354 presented lesions. Overall, with the addition of EIS to visual inspection of clinical images, the sensitivity of biopsy/referral decisions for melanomas and severely dysplastic nevi (SDN) increased from 69.2% to 90.0% (P < .001), while specificity increased from 44.0% to 72.6% (P < .001). Physicians and NPs, regardless of years of experience, each saw significant improvements in sensitivity, specificity, and diagnostic accuracy with the addition of EIS scores.
Conclusions: The incorporation of EIS data into clinical decision-making by PCPs significantly increased the sensitivity and specificity of biopsy/referral decisions for melanomas and SDN and overall diagnostic accuracy compared with visual inspection alone. The results of this study suggest that diagnostic accuracy for PSLs by PCPs may be improved with adjunctive use of EIS with visual inspection.
Primary care providers (PCPs) are often the first line of defense in detecting skin cancers. For patients with concerning skin lesions, PCPs may choose to perform a biopsy or facilitate access to specialty services (eg, Dermatology). Consequently, PCPs play a critical role in the timely detection of skin cancers, and it is paramount to employ continually improving detection methods, such as the application of technologic advances.1
Differentiating benign nevi from melanoma and severely dysplastic nevi (SDN), both of which warrant excision, poses a unique challenge to clinicians examining pigmented skin lesions (PSLs). PCPs often rely on visual inspection to differentiate benign skin lesions from malignant skin cancers. In some primary care practices, dermoscopy, which involves using a handheld device to evaluate lesions with polarized light and magnification, is used to improve melanoma detection. However, while visual inspection and dermoscopy are valid, effective techniques for the diagnosis of melanocytic lesions, in many instances they still can lead to missed cancers or unnecessary biopsies and specialty referrals. Adjunctive use of dermoscopy with visual inspection has been shown to increase the probability of skin cancer detection, but it fails to achieve a near-100% success rate.2 Furthermore, dermoscopy is heavily user-dependent, requiring significant training and experience for appropriate use.3
Another option is an electrical impedance spectroscopy (EIS) device (Nevisense, Scibase, Stockholm, Sweden), which has been approved by the US Food and Drug Administration (FDA) to assist in the detection of melanoma and differentiation from benign PSLs.4 EIS is a noninvasive, rapidly applied technology designed to accompany the visual examination of melanocytic lesions in office, with or without dermoscopy. Still relatively new, the technology is employed today by many dermatologists, increasing diagnostic accuracy for PSLs.5 The lightweight and portable instrument features a handheld probe, which is held against a lesion to obtain a reading. EIS uses a low-voltage electrode to apply a harmless electrical current to the skin at various frequencies.6 As benign and malignant tissues vary in cell shape, size, and composition, EIS distinguishes differential electrical resistance of the tissue to aid in diagnosis.7
Continue to: EIS provides high-sensitivity...
EIS provides high-sensitivity melanoma diagnosis vs histopathologic confirmation from biopsies, with 1 study showing a 96.6% sensitivity rating, detecting 256 of 265 melanomas.4 The EIS device, by measuring differences in electrical resistance between benign and cancerous cells, outputs a simple integer score ranging from 0 to 10 associated with the likelihood of the lesion being a melanoma.8 Based on data from the Nevisense pivotal trial,4 Nevisense reports that scores of 0 to 3 carry a negative predictive value of 99% for melanoma, whereas scores of 4 to 10 signify increasingly greater positive predictive values from 7% to 61%.
We aimed to assess whether EIS may be beneficial to PCPs by comparing the accuracy of clinical decision-making for PSLs based on visual examination alone with that based on concurrent visual and EIS evaluation.
METHODS
A questionnaire was distributed via email to 142 clinicians at clinics affiliated with either of 2 organizations delivering care to the New York City area through a network of community health centers: the Institute for Family Health (IFH) and the Community Healthcare Network (CHN). Of these recipients, 72 were affiliated with IFH across 27 community health centers and 70 were affiliated with CHN across 14 community health centers. Recipients were physicians and nurse practitioners (NPs) practicing at primary health care facilities.
Survey instrument. The first section of the survey instrument (APPENDIX) solicited demographic information and explained how to apply the EIS scores for diagnostic decision-making. The second featured images of 12 randomly selected, histologically confirmed, and EIS-evaluated PSLs from a previously published prospective blinded trial of 2416 lesions.4 The Institutional Review Board of the Icahn School of Medicine at Mount Sinai reviewed and approved the study and survey instrument.
Clinical images of these lesions, comprising 4 melanocytic nevi, 4 dysplastic nevi (including 3 mild-moderately dysplastic and 1 severely dysplastic nevus), and 4 melanomas
Continue to: Analysis
Analysis. A biopsy or referral rating of 4 or 5 was considered a decision to biopsy or refer (ie, a diagnostic decision consistent with melanoma or SDN warranting excision), whereas a selection of 1 to 3 was considered a decision not to biopsy or refer (ie, a diagnostic decision consistent with a benign PSL). The sensitivity and specificity of biopsy/referral decisions for melanomas and SDN, the proportion of missed melanomas and SDN, and the proportion of biopsy/referral decisions for benign lesions were separately determined for visual inspection alone and visual inspection with EIS score. Similarly, diagnostic accuracy was calculated for these clinical scenarios. These metrics were further stratified among different subsets of the respondent population. Differences in sensitivity, specificity, biopsy/referral decision proportions, and diagnostic accuracy were calculated using McNemar’s test for paired proportions.
RESULTS
Sixty-one respondents, comprising 44 physicians and 17 NPs, completed the survey, yielding a response rate of 43% (TABLE 1). In total, 1354 clinical decisions (677 based on visual inspection alone and 677 based on visual inspection plus EIS) were made. A biopsy/referral decision was made after assessing 416 of 677 cases (61%) with visual inspection alone and 360 of 677 cases (53%) when relying on visual inspection plus EIS. None of the respondents reported any prior experience with EIS.

When incorporating EIS scores, respondents’ mean sensitivity for melanomas and SDN increased from 69.2% to 90.0% (P < .001) and specificity from 44.0% to 72.6% (P < .001; TABLE 2). At baseline, physicians demonstrated a sensitivity and specificity of 74.6% and 46.5%, respectively, while NPs demonstrated a sensitivity and specificity of 56.1% and 37.9%, respectively.

All respondent subgroups stratified by occupation and years of experience saw significant increases in both sensitivity and specificity upon the incorporation of EIS scores, with NPs seeing a greater increase in sensitivity (56.1% vs 85.4%; P < .001) and specificity (37.9% vs 69.0%; P < .001) than physicians (sensitivity: 74.6% vs 91.9%; P < .001; specificity: 46.5% vs 74.1%; P < .001). The only difference in diagnostic performance based on years of experience was a greater pre-EIS sensitivity by clinicians who had been in practice for ≥ 15 years, compared with those in practice for shorter periods (TABLE 2).

Diagnostic accuracy increased significantly from 48% when based on visual inspection alone to 73% with the addition of EIS scores (P < .001; TABLE 3). Physicians and NPs each significantly increased their diagnostic accuracy upon the incorporation of EIS, with NPs exhibiting the greatest increase (from 36.9% to 65.7%; P < .001). PCPs with 6 to 14 years of experience saw the greatest increase in diagnostic accuracy when adding EIS (45.9% vs 76.4%; P < .001). Overall, the addition of EIS scores resulted in 58 fewer missed melanomas and SDN and 114 fewer benign referrals or biopsies (TABLE 4).

Continue to: DISCUSSION
DISCUSSION
Primary care evaluation plays a significant role in the diagnosis and management of PSLs, ultimately shaping outcomes for patients with melanoma. Improved accuracy of PSL classification could yield greater sensitivity for the diagnosis of melanomas and high-risk melanocytic lesions at earlier stages, while also reducing the number of unnecessary biopsies and referrals—leading to decreased patient morbidity and mortality and reduced health care spending.9
Diagnostic tools are valuable insofar as they can improve accuracy and positively impact clinical management and patient outcomes.10 In this case, increased sensitivity reduced missed melanoma diagnoses, while increased specificity avoided the additional costs and patient toll associated with a biopsy or referral for a benign lesion.
Dermoscopy has been shown to improve the sensitivity and specificity of PSL diagnosis compared with visual inspection alone; however, without substantial training and experience, accuracy with dermoscopy can be no better than examination with the naked eye.3,11,12 The dropout rates are high for training PCPs in its use, given that several months of training may be needed for competent use.13,14 To improve the clinical management of PSLs broadly in primary care, a need exists for easy-to-use adjunctive tools that increase diagnostic accuracy.15
In this study, with only a brief explanation of how to interpret EIS scores, clinicians without any prior experience using EIS demonstrated significantly improved accuracy in deciding appropriate management and classifying melanocytic lesions with the addition of EIS to visual inspection. These improvements, seen in clinicians of varying training and experience, suggest that the learning curve of EIS may not be as steep as that of dermoscopy.
The greater baseline sensitivity, specificity, and diagnostic accuracy of physicians’ clinical decision-making compared with NPs before the incorporation of EIS in the study may be a product of comparatively more extensive medical training. In addition, EIS yielded a greater benefit to NPs than to physicians, with greater increases in sensitivity and specificity noted. This suggests that the use of EIS is particularly advantageous to clinicians who are less proficient in assessing melanocytic lesions. Using visual inspection alone, more experienced respondents made biopsy/referral decisions with greater sensitivity but similar specificity to those with less experience. With the incorporation of EIS scores, the sensitivity and specificity of respondents’ clinical decision-making rose to comparable levels across all experience groups, providing further indication of EIS’s particular value to clinicians who are less proficient in PSL evaluation.
Continue to: This technology holds the potential...
This technology holds the potential to be seamlessly implemented into primary care practice, given that dermatology expertise training is not required to use the EIS device; this could allow for EIS measurement of lesions to be delegated to office staff (eg, nurses, medical assistants).16 Future studies are needed to assess EIS use among PCPs in a real-world setting, where factors such as its application on nonmelanocytic lesions (eg, seborrheic keratoses) and its pairing with patient historical data could produce varying results.
Limitations. While revealing, this study had its limitations. Respondents did not have access to additional pertinent clinical information, such as patients’ histories and risk factors. Clinical decisions in this survey were made based on digital images rather than in vivo examination. This may not represent a real-life evaluation; there is the potential for minimization of the true consequences of a missed melanoma or unnecessary biopsy in the minds of participants, and this does not factor in the operation of the actual EIS device. The Hawthorne effect may also have influenced PCPs’ diagnostic selections. Also, the limited sample size constitutes another limitation.
Of note, in this survey format, respondents rated their inclination to biopsy or refer each lesion from 1 to 5. For statistical analyses, lesions rated 1 to 3 were considered as not biopsied/referred and those rated 4 to 5 as biopsied/referred. The sensitivity and specificity values observed, for both visual examination and concurrent visual and EIS evaluation, are therefore based on this classification system of participants’ provided ratings. It is conceivable that differing sensitivity and specificity values might have been detected if clinicians were instead given a binary choice for referral/biopsy decisions.
CONCLUSIONS
Among PCPs tasked with evaluating melanocytic lesions, the incorporation of EIS data into clinical decision-making in this study significantly increased the sensitivity, specificity, and overall diagnostic accuracy of biopsy or referral decisions for melanomas and SDN compared with visual inspection alone. Overall, the results of this preliminary study suggest that diagnostic accuracy for PSLs by PCPs may be improved with the adjunctive use of EIS with visual inspection. This would ultimately improve patient care and reduce the morbidity and mortality of a melanoma diagnosis.
CORRESPONDENCE
Jonathan Ungar, MD, Kimberly and Eric J. Waldman Department of Dermatology, Icahn School of Medicine at Mount Sinai, 5 East 98th Street, 5th Floor, New York, NY 10029; [email protected]
1. Goetsch NJ, Hoehns JD, Sutherland JE, et al. Assessment of postgraduate skin lesion education among Iowa family physicians. SAGE Open Med. 2017;5:2050312117691392. doi: 10.1177/2050312117691392
2. Dinnes J, Deeks JJ, Chuchu N, et al. Dermoscopy, with and without visual inspection, for diagnosing melanoma in adults. Cochrane Database Syst Rev. 2018;12:CD011902. doi: 10.1002/14651858.CD011902.pub2
3. Jones OT, Jurascheck LC, van Melle MA, et al. Dermoscopy for melanoma detection and triage in primary care: a systematic review. BMJ Open. 2019;9:e027529. doi: 10.1136/bmjopen-2018-027529
4. Malvehy J, Hauschild A, Curiel-Lewandrowski C, et al. Clinical performance of the Nevisense system in cutaneous melanoma detection: an international, multicentre, prospective and blinded clinical trial on efficacy and safety. Br J Dermatol. 2014;171:1099-1107. doi: 10.1111/bjd.13121
5. Svoboda RM, Prado G, Mirsky RS, et al. Assessment of clinician accuracy for diagnosing melanoma on the basis of electrical impedance spectroscopy score plus morphology versus lesion morphology alone. J Am Acad Dermatol. 2019;80:285-287. doi: 10.1016/j.jaad.2018.08.048
6. Mohr P, Birgersson U, Berking C, et al. Electrical impedance spectroscopy as a potential adjunct diagnostic tool for cutaneous melanoma. Skin Res Technol. 2013;19:75-83. doi: 10.1111/srt.12008
7. Rocha L, Menzies SW, Lo S, et al. Analysis of an electrical impedance spectroscopy system in short-term digital dermoscopy imaging of melanocytic lesions. Br J Dermatol. 2017;177:1432-1438. doi: 10.1111/bjd.15595
8. Litchman GH, Teplitz RW, Marson JW, et al. Impact of electrical impedance spectroscopy on dermatologists’ number needed to biopsy metric and biopsy decisions for pigmented skin lesions. J Am Acad Dermatol. 2021;85:976-979. doi: 10.1016/j.jaad.2020.09.011
9. Greenwood-Lee J, Jewett L, Woodhouse L, et al. A categorisation of problems and solutions to improve patient referrals from primary to specialty care. BMC Health Serv Res. 2018;18:1-16. doi: 10.1186/s12913-018-3745-y
10. Bossuyt PM, Reitsma JB, Linnet K, et al. Beyond diagnostic accuracy: the clinical utility of diagnostic tests. Clin Chem. 2012;58:1636-1643. doi: 10.1373/clinchem.2012.182576
11. Argenziano G, Cerroni L, Zalaudek I , et al. Accuracy in melanoma detection: a 10-year multicenter survey. J Am Acad Dermatol. 2012;67:54-59. doi: 10.1016/j.jaad.2011.07.019
12. Menzies SW, Vestergaard ME, Macaskill P, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676. doi: 10.1111/j.1365-2133.2008.08713.x
13. Menzies SW, Emery J, Staples M, et al. Impact of dermoscopy and short-term sequential digital dermoscopy imaging for the management of pigmented lesions in primary care: a sequential intervention trial. Br J Dermatol. 2009;161:1270-1277. doi: 10.1111/j.1365-2133.2009.09374.x
14. Noor O, Nanda A, Rao BK. A dermoscopy survey to assess who is using it and why it is or is not being used. Int J Dermatol. 2009;48:951-952. doi: 10.1111/j.1365-4632.2009.04095.x
15. Weigl BH, Boyle DS, de los Santos T, et al. Simplicity of use: a critical feature for widespread adoption of diagnostic technologies in low-resource settings. Expert Rev Med Devices. 2009;6:461-464. doi: 10.1586/erd.09.31
16. Sarac E, Meiwes A, Eigentler T, et al. Diagnostic accuracy of electrical impedance spectroscopy in non-melanoma skin cancer. Acta Derm Venereol. 2020;100:adv00328. doi: 10.2340/00015555-3689
1. Goetsch NJ, Hoehns JD, Sutherland JE, et al. Assessment of postgraduate skin lesion education among Iowa family physicians. SAGE Open Med. 2017;5:2050312117691392. doi: 10.1177/2050312117691392
2. Dinnes J, Deeks JJ, Chuchu N, et al. Dermoscopy, with and without visual inspection, for diagnosing melanoma in adults. Cochrane Database Syst Rev. 2018;12:CD011902. doi: 10.1002/14651858.CD011902.pub2
3. Jones OT, Jurascheck LC, van Melle MA, et al. Dermoscopy for melanoma detection and triage in primary care: a systematic review. BMJ Open. 2019;9:e027529. doi: 10.1136/bmjopen-2018-027529
4. Malvehy J, Hauschild A, Curiel-Lewandrowski C, et al. Clinical performance of the Nevisense system in cutaneous melanoma detection: an international, multicentre, prospective and blinded clinical trial on efficacy and safety. Br J Dermatol. 2014;171:1099-1107. doi: 10.1111/bjd.13121
5. Svoboda RM, Prado G, Mirsky RS, et al. Assessment of clinician accuracy for diagnosing melanoma on the basis of electrical impedance spectroscopy score plus morphology versus lesion morphology alone. J Am Acad Dermatol. 2019;80:285-287. doi: 10.1016/j.jaad.2018.08.048
6. Mohr P, Birgersson U, Berking C, et al. Electrical impedance spectroscopy as a potential adjunct diagnostic tool for cutaneous melanoma. Skin Res Technol. 2013;19:75-83. doi: 10.1111/srt.12008
7. Rocha L, Menzies SW, Lo S, et al. Analysis of an electrical impedance spectroscopy system in short-term digital dermoscopy imaging of melanocytic lesions. Br J Dermatol. 2017;177:1432-1438. doi: 10.1111/bjd.15595
8. Litchman GH, Teplitz RW, Marson JW, et al. Impact of electrical impedance spectroscopy on dermatologists’ number needed to biopsy metric and biopsy decisions for pigmented skin lesions. J Am Acad Dermatol. 2021;85:976-979. doi: 10.1016/j.jaad.2020.09.011
9. Greenwood-Lee J, Jewett L, Woodhouse L, et al. A categorisation of problems and solutions to improve patient referrals from primary to specialty care. BMC Health Serv Res. 2018;18:1-16. doi: 10.1186/s12913-018-3745-y
10. Bossuyt PM, Reitsma JB, Linnet K, et al. Beyond diagnostic accuracy: the clinical utility of diagnostic tests. Clin Chem. 2012;58:1636-1643. doi: 10.1373/clinchem.2012.182576
11. Argenziano G, Cerroni L, Zalaudek I , et al. Accuracy in melanoma detection: a 10-year multicenter survey. J Am Acad Dermatol. 2012;67:54-59. doi: 10.1016/j.jaad.2011.07.019
12. Menzies SW, Vestergaard ME, Macaskill P, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676. doi: 10.1111/j.1365-2133.2008.08713.x
13. Menzies SW, Emery J, Staples M, et al. Impact of dermoscopy and short-term sequential digital dermoscopy imaging for the management of pigmented lesions in primary care: a sequential intervention trial. Br J Dermatol. 2009;161:1270-1277. doi: 10.1111/j.1365-2133.2009.09374.x
14. Noor O, Nanda A, Rao BK. A dermoscopy survey to assess who is using it and why it is or is not being used. Int J Dermatol. 2009;48:951-952. doi: 10.1111/j.1365-4632.2009.04095.x
15. Weigl BH, Boyle DS, de los Santos T, et al. Simplicity of use: a critical feature for widespread adoption of diagnostic technologies in low-resource settings. Expert Rev Med Devices. 2009;6:461-464. doi: 10.1586/erd.09.31
16. Sarac E, Meiwes A, Eigentler T, et al. Diagnostic accuracy of electrical impedance spectroscopy in non-melanoma skin cancer. Acta Derm Venereol. 2020;100:adv00328. doi: 10.2340/00015555-3689
Tools—and rules—to support behavior change
Changing behavior is hard. And at nearly every clinical encounter, we counsel/encourage/remind/help (choose a verb) our patients to make a change—to do something hard. We tell them they need to increase their physical activity, get more sleep, or alter their eating habits. We know that if they make the needed changes, they can improve their health and possibly lengthen their lives. But we also know (from the systematic reviews the US Preventive Services Task Force [USPSTF] uses to make its recommendations) that brief counseling in our offices is largely ineffective unless we connect patients to resources to support the recommended change.
As examples, the USPSTF currently recommends the following (both grade “B”):
- offer or refer adults with cardiovascular disease risk factors to behavioral counseling interventions to promote a healthy diet and physical activity.1
- offer or refer adults with a body mass index of 30 or higher to intensive, multicomponent behavioral interventions.2
To support our patients when making recommendations such as these, we might refer them to a dietitian for intensive counseling and meal-planning guidance. The American Diabetes Association says that patients seeking to manage their diabetes and prediabetes “can start by working with a registered dietitian nutritionist … to make an eating plan that works for [them].”3 However, this kind of resource is unavailable to many of our patients.
So what else can we do?
We can help patients decide what to buy in the grocery aisle. Nutrition labels are useful, but they are limited by their complexity and requisite level of health literacy.4 Even the concept of “calories” is not so intuitive. This challenge with interpreting calories led me (in some of my prior work) to explore a potentially more useful approach: conveying calorie information as physical activity equivalents.5
In this issue of The Journal of Family Practice, Dong and colleagues present their findings on whether a simple equation (the Altman Rule) that uses information on nutrition labels may be a reasonable proxy for an even more difficult concept—glycemic load.6 The idea is that consumers (eg, patients with diabetes) can use this rule to help them in their decision-making at the grocery store (or the convenience store or gas station, for that matter, where the high-glycemic-load carbohydrates may be even more tempting). The 2-step rule is tech-free and can be applied in a few seconds. Their research demonstrated that the rule is a reasonable proxy for glycemic load for packaged carbohydrates (eg, chips, cereals, crackers, granola bars). Caveats acknowledged, foods that meet the rule are likely to be healthier choices.
Looking ahead, I would like to see whether counseling patients about the Altman Rule leads to their use of it, and how that translates into healthier eating, lower A1C, and ideally better health. For now, the Altman Rule is worth learning about. It may serve as another tool that you can use to support your patients when you ask them to do the hard work of making healthier food choices.
1. US Preventive Services Task Force. Behavioral counseling interventions to promote a healthy diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: US Preventive Services Task Force recommendation statement. JAMA. 2020;324:2069-2075. doi: 10.1001/jama.2020.21749
2. US Preventive Services Task Force. Behavioral weight loss interventions to prevent obesity-related morbidity and mortality in adults: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:1163-1171. doi: 10.1001/jama.2018.13022
3. American Diabetes Association. Eating right doesn’t have to be boring. Accessed August 23, 2023. diabetes.org/healthy-living/recipes-nutrition
4. Weiss BD, Mays MZ, Martz W, et al. Quick assessment of literacy in primary care: the newest vital sign. Ann Fam Med. 2005;3:514-522. doi: 10.1370/afm.405
5. Viera AJ, Gizlice Z, Tuttle L, et al. Effect of calories-only vs physical activity calorie expenditure labeling on lunch calories purchased in worksite cafeterias. BMC Public Health. 2019;19:107. doi: 10.1186/s12889-019-6433-x
6. Dong KR, Eustis S, Hawkins K, et al. Is the Altman Rule a proxy for glycemic load? J Fam Pract. 2023;72:286-291. doi: 10.12788/jfp.0656
Changing behavior is hard. And at nearly every clinical encounter, we counsel/encourage/remind/help (choose a verb) our patients to make a change—to do something hard. We tell them they need to increase their physical activity, get more sleep, or alter their eating habits. We know that if they make the needed changes, they can improve their health and possibly lengthen their lives. But we also know (from the systematic reviews the US Preventive Services Task Force [USPSTF] uses to make its recommendations) that brief counseling in our offices is largely ineffective unless we connect patients to resources to support the recommended change.
As examples, the USPSTF currently recommends the following (both grade “B”):
- offer or refer adults with cardiovascular disease risk factors to behavioral counseling interventions to promote a healthy diet and physical activity.1
- offer or refer adults with a body mass index of 30 or higher to intensive, multicomponent behavioral interventions.2
To support our patients when making recommendations such as these, we might refer them to a dietitian for intensive counseling and meal-planning guidance. The American Diabetes Association says that patients seeking to manage their diabetes and prediabetes “can start by working with a registered dietitian nutritionist … to make an eating plan that works for [them].”3 However, this kind of resource is unavailable to many of our patients.
So what else can we do?
We can help patients decide what to buy in the grocery aisle. Nutrition labels are useful, but they are limited by their complexity and requisite level of health literacy.4 Even the concept of “calories” is not so intuitive. This challenge with interpreting calories led me (in some of my prior work) to explore a potentially more useful approach: conveying calorie information as physical activity equivalents.5
In this issue of The Journal of Family Practice, Dong and colleagues present their findings on whether a simple equation (the Altman Rule) that uses information on nutrition labels may be a reasonable proxy for an even more difficult concept—glycemic load.6 The idea is that consumers (eg, patients with diabetes) can use this rule to help them in their decision-making at the grocery store (or the convenience store or gas station, for that matter, where the high-glycemic-load carbohydrates may be even more tempting). The 2-step rule is tech-free and can be applied in a few seconds. Their research demonstrated that the rule is a reasonable proxy for glycemic load for packaged carbohydrates (eg, chips, cereals, crackers, granola bars). Caveats acknowledged, foods that meet the rule are likely to be healthier choices.
Looking ahead, I would like to see whether counseling patients about the Altman Rule leads to their use of it, and how that translates into healthier eating, lower A1C, and ideally better health. For now, the Altman Rule is worth learning about. It may serve as another tool that you can use to support your patients when you ask them to do the hard work of making healthier food choices.
Changing behavior is hard. And at nearly every clinical encounter, we counsel/encourage/remind/help (choose a verb) our patients to make a change—to do something hard. We tell them they need to increase their physical activity, get more sleep, or alter their eating habits. We know that if they make the needed changes, they can improve their health and possibly lengthen their lives. But we also know (from the systematic reviews the US Preventive Services Task Force [USPSTF] uses to make its recommendations) that brief counseling in our offices is largely ineffective unless we connect patients to resources to support the recommended change.
As examples, the USPSTF currently recommends the following (both grade “B”):
- offer or refer adults with cardiovascular disease risk factors to behavioral counseling interventions to promote a healthy diet and physical activity.1
- offer or refer adults with a body mass index of 30 or higher to intensive, multicomponent behavioral interventions.2
To support our patients when making recommendations such as these, we might refer them to a dietitian for intensive counseling and meal-planning guidance. The American Diabetes Association says that patients seeking to manage their diabetes and prediabetes “can start by working with a registered dietitian nutritionist … to make an eating plan that works for [them].”3 However, this kind of resource is unavailable to many of our patients.
So what else can we do?
We can help patients decide what to buy in the grocery aisle. Nutrition labels are useful, but they are limited by their complexity and requisite level of health literacy.4 Even the concept of “calories” is not so intuitive. This challenge with interpreting calories led me (in some of my prior work) to explore a potentially more useful approach: conveying calorie information as physical activity equivalents.5
In this issue of The Journal of Family Practice, Dong and colleagues present their findings on whether a simple equation (the Altman Rule) that uses information on nutrition labels may be a reasonable proxy for an even more difficult concept—glycemic load.6 The idea is that consumers (eg, patients with diabetes) can use this rule to help them in their decision-making at the grocery store (or the convenience store or gas station, for that matter, where the high-glycemic-load carbohydrates may be even more tempting). The 2-step rule is tech-free and can be applied in a few seconds. Their research demonstrated that the rule is a reasonable proxy for glycemic load for packaged carbohydrates (eg, chips, cereals, crackers, granola bars). Caveats acknowledged, foods that meet the rule are likely to be healthier choices.
Looking ahead, I would like to see whether counseling patients about the Altman Rule leads to their use of it, and how that translates into healthier eating, lower A1C, and ideally better health. For now, the Altman Rule is worth learning about. It may serve as another tool that you can use to support your patients when you ask them to do the hard work of making healthier food choices.
1. US Preventive Services Task Force. Behavioral counseling interventions to promote a healthy diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: US Preventive Services Task Force recommendation statement. JAMA. 2020;324:2069-2075. doi: 10.1001/jama.2020.21749
2. US Preventive Services Task Force. Behavioral weight loss interventions to prevent obesity-related morbidity and mortality in adults: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:1163-1171. doi: 10.1001/jama.2018.13022
3. American Diabetes Association. Eating right doesn’t have to be boring. Accessed August 23, 2023. diabetes.org/healthy-living/recipes-nutrition
4. Weiss BD, Mays MZ, Martz W, et al. Quick assessment of literacy in primary care: the newest vital sign. Ann Fam Med. 2005;3:514-522. doi: 10.1370/afm.405
5. Viera AJ, Gizlice Z, Tuttle L, et al. Effect of calories-only vs physical activity calorie expenditure labeling on lunch calories purchased in worksite cafeterias. BMC Public Health. 2019;19:107. doi: 10.1186/s12889-019-6433-x
6. Dong KR, Eustis S, Hawkins K, et al. Is the Altman Rule a proxy for glycemic load? J Fam Pract. 2023;72:286-291. doi: 10.12788/jfp.0656
1. US Preventive Services Task Force. Behavioral counseling interventions to promote a healthy diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: US Preventive Services Task Force recommendation statement. JAMA. 2020;324:2069-2075. doi: 10.1001/jama.2020.21749
2. US Preventive Services Task Force. Behavioral weight loss interventions to prevent obesity-related morbidity and mortality in adults: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:1163-1171. doi: 10.1001/jama.2018.13022
3. American Diabetes Association. Eating right doesn’t have to be boring. Accessed August 23, 2023. diabetes.org/healthy-living/recipes-nutrition
4. Weiss BD, Mays MZ, Martz W, et al. Quick assessment of literacy in primary care: the newest vital sign. Ann Fam Med. 2005;3:514-522. doi: 10.1370/afm.405
5. Viera AJ, Gizlice Z, Tuttle L, et al. Effect of calories-only vs physical activity calorie expenditure labeling on lunch calories purchased in worksite cafeterias. BMC Public Health. 2019;19:107. doi: 10.1186/s12889-019-6433-x
6. Dong KR, Eustis S, Hawkins K, et al. Is the Altman Rule a proxy for glycemic load? J Fam Pract. 2023;72:286-291. doi: 10.12788/jfp.0656
Persistent ‘postherpetic neuralgia’ and well-demarcated plaque
A 75-YEAR-OLD MAN presented to the dermatology clinic for evaluation of localized, persistent burning pain and discomfort attributed to shingles and postherpetic neuralgia. He had received a diagnosis of shingles on his left upper back about 3 years prior to this presentation.
In the ensuing years, the patient had been evaluated and treated by his primary care physician, a pain management team, and a neurologist. These clinicians treated the symptoms as postherpetic neuralgia, with no consensus explanation for the skin findings. The patient reported that his symptoms were unresponsive to trials of gabapentin 800 mg tid, duloxetine 60 mg PO qd, and acetaminophen 1 to 3 g/d PO. He also had undergone several rounds of acupuncture, thoracic and cervical spine steroid injections, and epidurals, without resolution of symptoms. The patient believed the only treatment that helped was a lidocaine 4% patch, which he had used nearly every day for the previous 3 years.
Physical exam by the dermatologist revealed a lidocaine patch applied to the patient’s left upper back. Upon its removal, skin examination showed a well-demarcated, erythematous, hyperpigmented, lichenified plaque with excoriations and erosions where the patch had been (FIGURE).
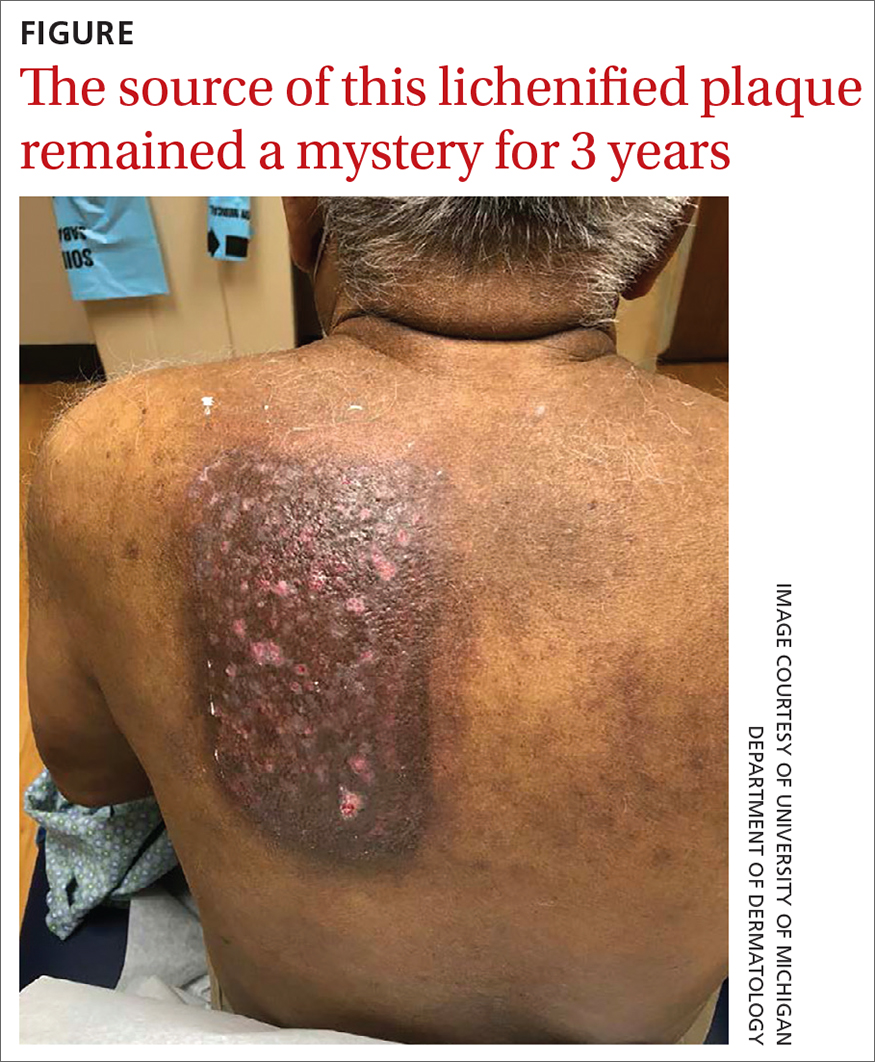
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Contact dermatitis
The patient’s history and skin exam provided enough information to diagnose contact dermatitis. The pruritus, burning, and pain the patient had experienced were due to continuous application of the lidocaine patch to the area rather than postherpetic neuralgia.
There are 2 types of contact dermatitis: irritant and allergic. Irritant contact dermatitis is an inflammatory reaction caused directly by a substance, while allergic contact dermatitis is a delayed hypersensitivity reaction to specific allergens.1 While data to elucidate the incidence and prevalence of allergic contact dermatitis are unknown, common causes include latex, dyes, oils, resins, and compounds in textiles, rubber, cosmetics, and other products used in daily life.1
Allergic contact dermatitis due to lidocaine is becoming more prevalent with increased use and availability of over-the-counter products.2 A retrospective chart review of 1819 patch-tested patients from the University of British Columbia Contact Dermatitis Clinic showed a significant proportion of patients (2.4%) were found to have
The differential varies by area affected
The differential diagnosis for contact dermatitis varies by area affected and the distribution of rash. Atopic dermatitis, lichen planus, and psoriasis are a few dermatologic conditions to consider in the differential diagnosis. They can look similar to contact dermatitis, but the patient’s history can help to discern the most likely diagnosis.1
Atopic dermatitis is a complex dysfunction of the skin barrier and immune factors that often begins in childhood and persists in some patients throughout their lifetime. Atopic dermatitis is associated with other forms of atopy including asthma, allergic rhinitis, and food and contact allergies. Atopic dermatitis in the absence of contact allergies may manifest with chronic, diffuse, scaly patches with poorly defined borders. The patches appear in a symmetrical distribution and favor the flexural surfaces, such as the antecubital fossa, wrists, and neck.
Continue to: Lichen planus
Lichen planus most often manifests in the fourth through sixth decade of life as flat-topped itchy pink-to-purple polygonal papules to plaques. Lesions range from 2 to 10 mm and favor the volar wrists, shins, and lower back, although they may be widespread. Oral lesions manifesting as ulcers or white lacy patches in the buccal mucosa are common and may be a clue to the diagnosis. Unlike more generalized contact dermatitis, lichen planus lesions are discrete.
Psoriasis manifests as well-demarcated scaly plaques distributed symmetrically over extensor surfaces. The plaques commonly are found on the elbows, knees, and scalp. When psoriasis manifests in a very limited form (as just a single plaque or limited number of plaques), it can be hard to confidently exclude other etiologies. In these circumstances, look for psoriasis signs in more unique locations (eg, pitting in the nails or plaques on the scalp or in the gluteal cleft). Adding those findings to an otherwise solitary plaque significantly adds to diagnostic certainty.
Diagnosis entails getting the shape of things
Diagnosis is based on history of exposure to irritating or allergic substances, as well as a clinical exam. Skin examination of contact dermatitis can vary based on how long it has been present: Acute manifestations include erythema, oozing, scale, vesicles, and bullae, while chronic contact dermatitis tends to demonstrate lichenification and scale.1
Distinctive findings. The most distinctive physical exam findings in patients with contact dermatitis are often shape and distribution of the rash, which reflect points of contact with the offending agent. This clue helped to elucidate the diagnosis in our patient: his rash was perfectly demarcated within the precise area where the patch was applied daily.
Irritant vs allergic. Patch testing can be performed to differentiate irritant vs allergic contact dermatitis.1 Irritant contact dermatitis usually is apparent when removing a patch and will resolve over a day, whereas allergic contact dermatitis forms over time and the skin rash is most prominent several days after the patch has been removed.1
Continue to: Treatment
Treatment: First, stop the offense
Treatment of both variants of contact dermatitis includes avoidance of the causative substance and symptomatic treatment with topical steroids, antihistamines, and possibly oral steroids depending on the severity.1
For our patient, a viral swab was taken and submitted for varicella zoster virus polymerase chain reaction testing to rule out persistent herpes zoster infection; the result was negative. The patient was counseled to discontinue use of the lidocaine patch.
Given the severity and protracted duration of the patient’s symptoms, he also was started on high-potency topical steroids (clobetasol 0.05% ointment to be applied twice daily under occlusion for 2 months), a 4-week prednisone taper (60 mg × 1 week, 40 mg × 1 week, 20 mg × 1 week, 10 mg × 1 week, then stop), and hydroxyzine (25 mg nightly as needed for pruritus). The patient’s rash and symptoms improved dramatically within the first few doses of prednisone and completely cleared by Week 4 of the prednisone taper. At his follow-up appointment 1 month after completing the prednisone taper, he stated that the pain on his back had resolved
1. Li Y, Li L. Contact dermatitis: classifications and management. Clin Rev Allergy Immunol. 2021;61:245-281. doi: 10.1007/s12016-021-08875-0
2. Cline AE, Turrentine JE. Compounded topical analgesics for chronic pain. Dermatitis. 2016;27:263-271. doi: 10.1097/DER.0000000000000216
3. To D, Kossintseva I, de Gannes G. Lidocaine contact allergy is becoming more prevalent. Dermatol Surg. 2014;40:1367-1372. doi: 10.1097/DSS.0000000000000190
A 75-YEAR-OLD MAN presented to the dermatology clinic for evaluation of localized, persistent burning pain and discomfort attributed to shingles and postherpetic neuralgia. He had received a diagnosis of shingles on his left upper back about 3 years prior to this presentation.
In the ensuing years, the patient had been evaluated and treated by his primary care physician, a pain management team, and a neurologist. These clinicians treated the symptoms as postherpetic neuralgia, with no consensus explanation for the skin findings. The patient reported that his symptoms were unresponsive to trials of gabapentin 800 mg tid, duloxetine 60 mg PO qd, and acetaminophen 1 to 3 g/d PO. He also had undergone several rounds of acupuncture, thoracic and cervical spine steroid injections, and epidurals, without resolution of symptoms. The patient believed the only treatment that helped was a lidocaine 4% patch, which he had used nearly every day for the previous 3 years.
Physical exam by the dermatologist revealed a lidocaine patch applied to the patient’s left upper back. Upon its removal, skin examination showed a well-demarcated, erythematous, hyperpigmented, lichenified plaque with excoriations and erosions where the patch had been (FIGURE).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Contact dermatitis
The patient’s history and skin exam provided enough information to diagnose contact dermatitis. The pruritus, burning, and pain the patient had experienced were due to continuous application of the lidocaine patch to the area rather than postherpetic neuralgia.
There are 2 types of contact dermatitis: irritant and allergic. Irritant contact dermatitis is an inflammatory reaction caused directly by a substance, while allergic contact dermatitis is a delayed hypersensitivity reaction to specific allergens.1 While data to elucidate the incidence and prevalence of allergic contact dermatitis are unknown, common causes include latex, dyes, oils, resins, and compounds in textiles, rubber, cosmetics, and other products used in daily life.1
Allergic contact dermatitis due to lidocaine is becoming more prevalent with increased use and availability of over-the-counter products.2 A retrospective chart review of 1819 patch-tested patients from the University of British Columbia Contact Dermatitis Clinic showed a significant proportion of patients (2.4%) were found to have
The differential varies by area affected
The differential diagnosis for contact dermatitis varies by area affected and the distribution of rash. Atopic dermatitis, lichen planus, and psoriasis are a few dermatologic conditions to consider in the differential diagnosis. They can look similar to contact dermatitis, but the patient’s history can help to discern the most likely diagnosis.1
Atopic dermatitis is a complex dysfunction of the skin barrier and immune factors that often begins in childhood and persists in some patients throughout their lifetime. Atopic dermatitis is associated with other forms of atopy including asthma, allergic rhinitis, and food and contact allergies. Atopic dermatitis in the absence of contact allergies may manifest with chronic, diffuse, scaly patches with poorly defined borders. The patches appear in a symmetrical distribution and favor the flexural surfaces, such as the antecubital fossa, wrists, and neck.
Continue to: Lichen planus
Lichen planus most often manifests in the fourth through sixth decade of life as flat-topped itchy pink-to-purple polygonal papules to plaques. Lesions range from 2 to 10 mm and favor the volar wrists, shins, and lower back, although they may be widespread. Oral lesions manifesting as ulcers or white lacy patches in the buccal mucosa are common and may be a clue to the diagnosis. Unlike more generalized contact dermatitis, lichen planus lesions are discrete.
Psoriasis manifests as well-demarcated scaly plaques distributed symmetrically over extensor surfaces. The plaques commonly are found on the elbows, knees, and scalp. When psoriasis manifests in a very limited form (as just a single plaque or limited number of plaques), it can be hard to confidently exclude other etiologies. In these circumstances, look for psoriasis signs in more unique locations (eg, pitting in the nails or plaques on the scalp or in the gluteal cleft). Adding those findings to an otherwise solitary plaque significantly adds to diagnostic certainty.
Diagnosis entails getting the shape of things
Diagnosis is based on history of exposure to irritating or allergic substances, as well as a clinical exam. Skin examination of contact dermatitis can vary based on how long it has been present: Acute manifestations include erythema, oozing, scale, vesicles, and bullae, while chronic contact dermatitis tends to demonstrate lichenification and scale.1
Distinctive findings. The most distinctive physical exam findings in patients with contact dermatitis are often shape and distribution of the rash, which reflect points of contact with the offending agent. This clue helped to elucidate the diagnosis in our patient: his rash was perfectly demarcated within the precise area where the patch was applied daily.
Irritant vs allergic. Patch testing can be performed to differentiate irritant vs allergic contact dermatitis.1 Irritant contact dermatitis usually is apparent when removing a patch and will resolve over a day, whereas allergic contact dermatitis forms over time and the skin rash is most prominent several days after the patch has been removed.1
Continue to: Treatment
Treatment: First, stop the offense
Treatment of both variants of contact dermatitis includes avoidance of the causative substance and symptomatic treatment with topical steroids, antihistamines, and possibly oral steroids depending on the severity.1
For our patient, a viral swab was taken and submitted for varicella zoster virus polymerase chain reaction testing to rule out persistent herpes zoster infection; the result was negative. The patient was counseled to discontinue use of the lidocaine patch.
Given the severity and protracted duration of the patient’s symptoms, he also was started on high-potency topical steroids (clobetasol 0.05% ointment to be applied twice daily under occlusion for 2 months), a 4-week prednisone taper (60 mg × 1 week, 40 mg × 1 week, 20 mg × 1 week, 10 mg × 1 week, then stop), and hydroxyzine (25 mg nightly as needed for pruritus). The patient’s rash and symptoms improved dramatically within the first few doses of prednisone and completely cleared by Week 4 of the prednisone taper. At his follow-up appointment 1 month after completing the prednisone taper, he stated that the pain on his back had resolved
A 75-YEAR-OLD MAN presented to the dermatology clinic for evaluation of localized, persistent burning pain and discomfort attributed to shingles and postherpetic neuralgia. He had received a diagnosis of shingles on his left upper back about 3 years prior to this presentation.
In the ensuing years, the patient had been evaluated and treated by his primary care physician, a pain management team, and a neurologist. These clinicians treated the symptoms as postherpetic neuralgia, with no consensus explanation for the skin findings. The patient reported that his symptoms were unresponsive to trials of gabapentin 800 mg tid, duloxetine 60 mg PO qd, and acetaminophen 1 to 3 g/d PO. He also had undergone several rounds of acupuncture, thoracic and cervical spine steroid injections, and epidurals, without resolution of symptoms. The patient believed the only treatment that helped was a lidocaine 4% patch, which he had used nearly every day for the previous 3 years.
Physical exam by the dermatologist revealed a lidocaine patch applied to the patient’s left upper back. Upon its removal, skin examination showed a well-demarcated, erythematous, hyperpigmented, lichenified plaque with excoriations and erosions where the patch had been (FIGURE).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Contact dermatitis
The patient’s history and skin exam provided enough information to diagnose contact dermatitis. The pruritus, burning, and pain the patient had experienced were due to continuous application of the lidocaine patch to the area rather than postherpetic neuralgia.
There are 2 types of contact dermatitis: irritant and allergic. Irritant contact dermatitis is an inflammatory reaction caused directly by a substance, while allergic contact dermatitis is a delayed hypersensitivity reaction to specific allergens.1 While data to elucidate the incidence and prevalence of allergic contact dermatitis are unknown, common causes include latex, dyes, oils, resins, and compounds in textiles, rubber, cosmetics, and other products used in daily life.1
Allergic contact dermatitis due to lidocaine is becoming more prevalent with increased use and availability of over-the-counter products.2 A retrospective chart review of 1819 patch-tested patients from the University of British Columbia Contact Dermatitis Clinic showed a significant proportion of patients (2.4%) were found to have
The differential varies by area affected
The differential diagnosis for contact dermatitis varies by area affected and the distribution of rash. Atopic dermatitis, lichen planus, and psoriasis are a few dermatologic conditions to consider in the differential diagnosis. They can look similar to contact dermatitis, but the patient’s history can help to discern the most likely diagnosis.1
Atopic dermatitis is a complex dysfunction of the skin barrier and immune factors that often begins in childhood and persists in some patients throughout their lifetime. Atopic dermatitis is associated with other forms of atopy including asthma, allergic rhinitis, and food and contact allergies. Atopic dermatitis in the absence of contact allergies may manifest with chronic, diffuse, scaly patches with poorly defined borders. The patches appear in a symmetrical distribution and favor the flexural surfaces, such as the antecubital fossa, wrists, and neck.
Continue to: Lichen planus
Lichen planus most often manifests in the fourth through sixth decade of life as flat-topped itchy pink-to-purple polygonal papules to plaques. Lesions range from 2 to 10 mm and favor the volar wrists, shins, and lower back, although they may be widespread. Oral lesions manifesting as ulcers or white lacy patches in the buccal mucosa are common and may be a clue to the diagnosis. Unlike more generalized contact dermatitis, lichen planus lesions are discrete.
Psoriasis manifests as well-demarcated scaly plaques distributed symmetrically over extensor surfaces. The plaques commonly are found on the elbows, knees, and scalp. When psoriasis manifests in a very limited form (as just a single plaque or limited number of plaques), it can be hard to confidently exclude other etiologies. In these circumstances, look for psoriasis signs in more unique locations (eg, pitting in the nails or plaques on the scalp or in the gluteal cleft). Adding those findings to an otherwise solitary plaque significantly adds to diagnostic certainty.
Diagnosis entails getting the shape of things
Diagnosis is based on history of exposure to irritating or allergic substances, as well as a clinical exam. Skin examination of contact dermatitis can vary based on how long it has been present: Acute manifestations include erythema, oozing, scale, vesicles, and bullae, while chronic contact dermatitis tends to demonstrate lichenification and scale.1
Distinctive findings. The most distinctive physical exam findings in patients with contact dermatitis are often shape and distribution of the rash, which reflect points of contact with the offending agent. This clue helped to elucidate the diagnosis in our patient: his rash was perfectly demarcated within the precise area where the patch was applied daily.
Irritant vs allergic. Patch testing can be performed to differentiate irritant vs allergic contact dermatitis.1 Irritant contact dermatitis usually is apparent when removing a patch and will resolve over a day, whereas allergic contact dermatitis forms over time and the skin rash is most prominent several days after the patch has been removed.1
Continue to: Treatment
Treatment: First, stop the offense
Treatment of both variants of contact dermatitis includes avoidance of the causative substance and symptomatic treatment with topical steroids, antihistamines, and possibly oral steroids depending on the severity.1
For our patient, a viral swab was taken and submitted for varicella zoster virus polymerase chain reaction testing to rule out persistent herpes zoster infection; the result was negative. The patient was counseled to discontinue use of the lidocaine patch.
Given the severity and protracted duration of the patient’s symptoms, he also was started on high-potency topical steroids (clobetasol 0.05% ointment to be applied twice daily under occlusion for 2 months), a 4-week prednisone taper (60 mg × 1 week, 40 mg × 1 week, 20 mg × 1 week, 10 mg × 1 week, then stop), and hydroxyzine (25 mg nightly as needed for pruritus). The patient’s rash and symptoms improved dramatically within the first few doses of prednisone and completely cleared by Week 4 of the prednisone taper. At his follow-up appointment 1 month after completing the prednisone taper, he stated that the pain on his back had resolved
1. Li Y, Li L. Contact dermatitis: classifications and management. Clin Rev Allergy Immunol. 2021;61:245-281. doi: 10.1007/s12016-021-08875-0
2. Cline AE, Turrentine JE. Compounded topical analgesics for chronic pain. Dermatitis. 2016;27:263-271. doi: 10.1097/DER.0000000000000216
3. To D, Kossintseva I, de Gannes G. Lidocaine contact allergy is becoming more prevalent. Dermatol Surg. 2014;40:1367-1372. doi: 10.1097/DSS.0000000000000190
1. Li Y, Li L. Contact dermatitis: classifications and management. Clin Rev Allergy Immunol. 2021;61:245-281. doi: 10.1007/s12016-021-08875-0
2. Cline AE, Turrentine JE. Compounded topical analgesics for chronic pain. Dermatitis. 2016;27:263-271. doi: 10.1097/DER.0000000000000216
3. To D, Kossintseva I, de Gannes G. Lidocaine contact allergy is becoming more prevalent. Dermatol Surg. 2014;40:1367-1372. doi: 10.1097/DSS.0000000000000190
Is low-dose naltrexone effective in chronic pain management?
Evidence summary
Naltrexone is comparable to amitriptyline for diabetic neuropathy pain
A 2021 randomized, double-blind, active-comparator, crossover clinical trial conducted in India examined the efficacy of low-dose naltrexone vs standard-of-care amitriptyline in patients (N = 67) with painful diabetic neuropathy. Participants were adults (ages 18 to 75 years) with painful diabetic neuropathy who had been on a stable dose of nonopioid pain medication for at least 1 month.1
Patients were randomly assigned to start receiving naltrexone 2 mg (n = 33) or amitriptyline 10 mg (n = 34). They received their starting medication for 6 weeks (with follow-up every 2 weeks), then completed a 2-week washout period, and then switched to the other study medication for 6 weeks (same follow-up schedule). If patients reported < 20% pain reduction on the Visual Analog Scale (VAS; 0-100 scoring system with 0 = no pain and 100 = worst pain) at a follow-up visit, their medication dose was titrated up, to a maximum of 4 mg of naltrexone or 25 to 50 mg of amitriptyline.1
The primary outcome of interest was the mean change in VAS pain score following 6 weeks of treatment. There was no statistically different change from baseline VAS pain score between the amitriptyline and naltrexone groups (mean difference [MD] = 1.6; 95% CI, –0.9 to 4.2; P = 0.21). These findings were consistent across the secondary endpoints (Likert 5-point pain scale and McGill Pain Questionnaire scores). There was no statistically significant difference in Hamilton Depression Rating Scale scores (13 in the naltrexone group vs 11 in the amitriptyline group; P = .81), no reports of decreased sleep quality in either group, and no significant difference in Patients’ Global Impression of Change scores at 6-week evaluation.1
The naltrexone cohort experienced 8 adverse events (most commonly, mild diarrhea), while the amitriptyline cohort experienced 52 adverse events (most commonly, somnolence) (P < .001). The limitations of the study include the lack of a placebo arm and a relatively small sample size.1
Greater reduction in pain scores with naltrexone
A 2022 retrospective cohort study evaluated the effectiveness of naltrexone for patients treated at a single outpatient integrative pain management practice in Alaska between 2014 and 2019. The exposure group (n = 36) included patients who had completed at least a 2-month continuous regimen of oral naltrexone 4.5 mg. Controls (n = 42) were selected from the remaining practice population receiving standard care and were primarily matched by diagnosis code, followed by gender, then age +/– 5 years. Patients were divided into subgroups for inflammatory and neuropathic pain.2
The primary outcome measured was the mean change in VAS score or numeric rating score (NRS; both used a 1-10 rating system), which was assessed during a patient’s appointment from initiation of treatment to the most recent visit or at the termination of therapy (intervention interquartile range, 12-14 months). There was no statistically significant difference in VAS/NRS between the low-dose naltrexone and control groups at baseline (6.09 vs 6.38; P = .454). The low-dose naltrexone group experienced a greater reduction in VAS/NRS pain scores compared to the control group (–37.8% vs –4.3%; P < .001).2
Compared with control patients in each group, patients in the inflammatory pain subgroup and the neuropathic pain subgroup who received low-dose naltrexone reported reductions in pain scores of 32% (P < .001) and 44% (P = .048), respectively. There was no statistically significant difference in mean change in VAS/NRS scores between the inflammatory and neuropathic subgroups (P = .763). A multivariate linear regression analysis did not identify significant variables other than low-dose naltrexone that correlated with pain improvement. The number needed to treat to observe a ≥ 50% reduction in pain scores was 3.2.2
Continue to: Limitations for this study...
Limitations for this study include its small sample size and open-label design.2
Low-dose naltrexone is effective for fibromyalgia pain
A 2020 single-blind prospective dose-response study utilized the up-and-down method to identify effective naltrexone dose for patients in a Danish university hospital pain clinic. Patients were White women ages 18 to 60 years (N = 25) who had a diagnosis of fibromyalgia unresponsive to traditional pharmacologic treatment. All patients received treatment with low-dose naltrexone (ranging from 0.75 mg to 6.0 mg) but were blinded to dose.3
Patients were evaluated for improvement in fibromyalgia symptoms using the Patient Global Impression of Improvement (PGI-I) scale—which ranges from 1 (very much improved) to 7 (very much worse), with 4 being “no change”—at baseline and after 2 to 3 weeks of treatment with low-dose naltrexone. A patient was considered a responder if they scored 1 to 3 on the follow-up PGI-I scale or if they experienced a > 30% pain reduction on the VAS. If a patient did not respond to their dose, the next patient began treatment at a dose 0.75 mg higher than the previous patient’s ending dose. If a patient did respond to low-dose naltrexone treatment, the next patient’s starting dose was 0.75 mg less than the previous patient’s. Eleven of 25 patients were considered responders.3
The primary outcomes were effective dose for 50% of fibromyalgia patients (3.88 mg; 95% CI, 3.39-4.35) and effective dose for 95% of fibromyalgia patients (5.4 mg; 95% CI, 4.66-6.13). Secondary outcomes were fibromyalgia symptoms as evaluated on the Fibromyalgia Impact Questionnaire Revised. Five of the 11 responders reported a > 30% improvement in tenderness and 8 of the 11 responders reported a > 30% decrease in waking unrefreshed.3
Limitations of the study include the short time period of treatment before response was assessed and the decision to use low test doses, which may have hindered detection of effective doses > 6 mg in fibromyalgia.3
Editor’s takeaway
Low-dose naltrexone, a less-often-used form of pain management, is a welcome option. Studies show some effectiveness in a variety of pain conditions with few adverse effects. The small number of studies, the small sample sizes, and the limited follow-up duration should encourage more investigation into how to best use this intervention.
1. Srinivasan A, Dutta P, Bansal D, et al. Efficacy and safety of low-dose naltrexone in painful diabetic neuropathy: a randomized, double-blind, active-control, crossover clinical trial. J Diabetes. 2021;13:770-778. doi: 10.1111/1753-0407.13202
2. Martin SJ, McAnally HB, Okediji P, et al. Low-dose naltrexone, an opioid-receptor antagonist, is a broad-spectrum analgesic: a retrospective cohort study. Pain Management. 2022;12:699-709. doi: 10.2217/pmt-2021-0122
3. Bruun-Plesner K, Blichfeldt-Eckhardt MR, Vaegter HB, et al. Low-dose naltrexone for the treatment of fibromyalgia: investigation of dose-response relationships. Pain Med. 2020;21:2253-2261. doi: 10.1093/pm/pnaa001
Evidence summary
Naltrexone is comparable to amitriptyline for diabetic neuropathy pain
A 2021 randomized, double-blind, active-comparator, crossover clinical trial conducted in India examined the efficacy of low-dose naltrexone vs standard-of-care amitriptyline in patients (N = 67) with painful diabetic neuropathy. Participants were adults (ages 18 to 75 years) with painful diabetic neuropathy who had been on a stable dose of nonopioid pain medication for at least 1 month.1
Patients were randomly assigned to start receiving naltrexone 2 mg (n = 33) or amitriptyline 10 mg (n = 34). They received their starting medication for 6 weeks (with follow-up every 2 weeks), then completed a 2-week washout period, and then switched to the other study medication for 6 weeks (same follow-up schedule). If patients reported < 20% pain reduction on the Visual Analog Scale (VAS; 0-100 scoring system with 0 = no pain and 100 = worst pain) at a follow-up visit, their medication dose was titrated up, to a maximum of 4 mg of naltrexone or 25 to 50 mg of amitriptyline.1
The primary outcome of interest was the mean change in VAS pain score following 6 weeks of treatment. There was no statistically different change from baseline VAS pain score between the amitriptyline and naltrexone groups (mean difference [MD] = 1.6; 95% CI, –0.9 to 4.2; P = 0.21). These findings were consistent across the secondary endpoints (Likert 5-point pain scale and McGill Pain Questionnaire scores). There was no statistically significant difference in Hamilton Depression Rating Scale scores (13 in the naltrexone group vs 11 in the amitriptyline group; P = .81), no reports of decreased sleep quality in either group, and no significant difference in Patients’ Global Impression of Change scores at 6-week evaluation.1
The naltrexone cohort experienced 8 adverse events (most commonly, mild diarrhea), while the amitriptyline cohort experienced 52 adverse events (most commonly, somnolence) (P < .001). The limitations of the study include the lack of a placebo arm and a relatively small sample size.1
Greater reduction in pain scores with naltrexone
A 2022 retrospective cohort study evaluated the effectiveness of naltrexone for patients treated at a single outpatient integrative pain management practice in Alaska between 2014 and 2019. The exposure group (n = 36) included patients who had completed at least a 2-month continuous regimen of oral naltrexone 4.5 mg. Controls (n = 42) were selected from the remaining practice population receiving standard care and were primarily matched by diagnosis code, followed by gender, then age +/– 5 years. Patients were divided into subgroups for inflammatory and neuropathic pain.2
The primary outcome measured was the mean change in VAS score or numeric rating score (NRS; both used a 1-10 rating system), which was assessed during a patient’s appointment from initiation of treatment to the most recent visit or at the termination of therapy (intervention interquartile range, 12-14 months). There was no statistically significant difference in VAS/NRS between the low-dose naltrexone and control groups at baseline (6.09 vs 6.38; P = .454). The low-dose naltrexone group experienced a greater reduction in VAS/NRS pain scores compared to the control group (–37.8% vs –4.3%; P < .001).2
Compared with control patients in each group, patients in the inflammatory pain subgroup and the neuropathic pain subgroup who received low-dose naltrexone reported reductions in pain scores of 32% (P < .001) and 44% (P = .048), respectively. There was no statistically significant difference in mean change in VAS/NRS scores between the inflammatory and neuropathic subgroups (P = .763). A multivariate linear regression analysis did not identify significant variables other than low-dose naltrexone that correlated with pain improvement. The number needed to treat to observe a ≥ 50% reduction in pain scores was 3.2.2
Continue to: Limitations for this study...
Limitations for this study include its small sample size and open-label design.2
Low-dose naltrexone is effective for fibromyalgia pain
A 2020 single-blind prospective dose-response study utilized the up-and-down method to identify effective naltrexone dose for patients in a Danish university hospital pain clinic. Patients were White women ages 18 to 60 years (N = 25) who had a diagnosis of fibromyalgia unresponsive to traditional pharmacologic treatment. All patients received treatment with low-dose naltrexone (ranging from 0.75 mg to 6.0 mg) but were blinded to dose.3
Patients were evaluated for improvement in fibromyalgia symptoms using the Patient Global Impression of Improvement (PGI-I) scale—which ranges from 1 (very much improved) to 7 (very much worse), with 4 being “no change”—at baseline and after 2 to 3 weeks of treatment with low-dose naltrexone. A patient was considered a responder if they scored 1 to 3 on the follow-up PGI-I scale or if they experienced a > 30% pain reduction on the VAS. If a patient did not respond to their dose, the next patient began treatment at a dose 0.75 mg higher than the previous patient’s ending dose. If a patient did respond to low-dose naltrexone treatment, the next patient’s starting dose was 0.75 mg less than the previous patient’s. Eleven of 25 patients were considered responders.3
The primary outcomes were effective dose for 50% of fibromyalgia patients (3.88 mg; 95% CI, 3.39-4.35) and effective dose for 95% of fibromyalgia patients (5.4 mg; 95% CI, 4.66-6.13). Secondary outcomes were fibromyalgia symptoms as evaluated on the Fibromyalgia Impact Questionnaire Revised. Five of the 11 responders reported a > 30% improvement in tenderness and 8 of the 11 responders reported a > 30% decrease in waking unrefreshed.3
Limitations of the study include the short time period of treatment before response was assessed and the decision to use low test doses, which may have hindered detection of effective doses > 6 mg in fibromyalgia.3
Editor’s takeaway
Low-dose naltrexone, a less-often-used form of pain management, is a welcome option. Studies show some effectiveness in a variety of pain conditions with few adverse effects. The small number of studies, the small sample sizes, and the limited follow-up duration should encourage more investigation into how to best use this intervention.
Evidence summary
Naltrexone is comparable to amitriptyline for diabetic neuropathy pain
A 2021 randomized, double-blind, active-comparator, crossover clinical trial conducted in India examined the efficacy of low-dose naltrexone vs standard-of-care amitriptyline in patients (N = 67) with painful diabetic neuropathy. Participants were adults (ages 18 to 75 years) with painful diabetic neuropathy who had been on a stable dose of nonopioid pain medication for at least 1 month.1
Patients were randomly assigned to start receiving naltrexone 2 mg (n = 33) or amitriptyline 10 mg (n = 34). They received their starting medication for 6 weeks (with follow-up every 2 weeks), then completed a 2-week washout period, and then switched to the other study medication for 6 weeks (same follow-up schedule). If patients reported < 20% pain reduction on the Visual Analog Scale (VAS; 0-100 scoring system with 0 = no pain and 100 = worst pain) at a follow-up visit, their medication dose was titrated up, to a maximum of 4 mg of naltrexone or 25 to 50 mg of amitriptyline.1
The primary outcome of interest was the mean change in VAS pain score following 6 weeks of treatment. There was no statistically different change from baseline VAS pain score between the amitriptyline and naltrexone groups (mean difference [MD] = 1.6; 95% CI, –0.9 to 4.2; P = 0.21). These findings were consistent across the secondary endpoints (Likert 5-point pain scale and McGill Pain Questionnaire scores). There was no statistically significant difference in Hamilton Depression Rating Scale scores (13 in the naltrexone group vs 11 in the amitriptyline group; P = .81), no reports of decreased sleep quality in either group, and no significant difference in Patients’ Global Impression of Change scores at 6-week evaluation.1
The naltrexone cohort experienced 8 adverse events (most commonly, mild diarrhea), while the amitriptyline cohort experienced 52 adverse events (most commonly, somnolence) (P < .001). The limitations of the study include the lack of a placebo arm and a relatively small sample size.1
Greater reduction in pain scores with naltrexone
A 2022 retrospective cohort study evaluated the effectiveness of naltrexone for patients treated at a single outpatient integrative pain management practice in Alaska between 2014 and 2019. The exposure group (n = 36) included patients who had completed at least a 2-month continuous regimen of oral naltrexone 4.5 mg. Controls (n = 42) were selected from the remaining practice population receiving standard care and were primarily matched by diagnosis code, followed by gender, then age +/– 5 years. Patients were divided into subgroups for inflammatory and neuropathic pain.2
The primary outcome measured was the mean change in VAS score or numeric rating score (NRS; both used a 1-10 rating system), which was assessed during a patient’s appointment from initiation of treatment to the most recent visit or at the termination of therapy (intervention interquartile range, 12-14 months). There was no statistically significant difference in VAS/NRS between the low-dose naltrexone and control groups at baseline (6.09 vs 6.38; P = .454). The low-dose naltrexone group experienced a greater reduction in VAS/NRS pain scores compared to the control group (–37.8% vs –4.3%; P < .001).2
Compared with control patients in each group, patients in the inflammatory pain subgroup and the neuropathic pain subgroup who received low-dose naltrexone reported reductions in pain scores of 32% (P < .001) and 44% (P = .048), respectively. There was no statistically significant difference in mean change in VAS/NRS scores between the inflammatory and neuropathic subgroups (P = .763). A multivariate linear regression analysis did not identify significant variables other than low-dose naltrexone that correlated with pain improvement. The number needed to treat to observe a ≥ 50% reduction in pain scores was 3.2.2
Continue to: Limitations for this study...
Limitations for this study include its small sample size and open-label design.2
Low-dose naltrexone is effective for fibromyalgia pain
A 2020 single-blind prospective dose-response study utilized the up-and-down method to identify effective naltrexone dose for patients in a Danish university hospital pain clinic. Patients were White women ages 18 to 60 years (N = 25) who had a diagnosis of fibromyalgia unresponsive to traditional pharmacologic treatment. All patients received treatment with low-dose naltrexone (ranging from 0.75 mg to 6.0 mg) but were blinded to dose.3
Patients were evaluated for improvement in fibromyalgia symptoms using the Patient Global Impression of Improvement (PGI-I) scale—which ranges from 1 (very much improved) to 7 (very much worse), with 4 being “no change”—at baseline and after 2 to 3 weeks of treatment with low-dose naltrexone. A patient was considered a responder if they scored 1 to 3 on the follow-up PGI-I scale or if they experienced a > 30% pain reduction on the VAS. If a patient did not respond to their dose, the next patient began treatment at a dose 0.75 mg higher than the previous patient’s ending dose. If a patient did respond to low-dose naltrexone treatment, the next patient’s starting dose was 0.75 mg less than the previous patient’s. Eleven of 25 patients were considered responders.3
The primary outcomes were effective dose for 50% of fibromyalgia patients (3.88 mg; 95% CI, 3.39-4.35) and effective dose for 95% of fibromyalgia patients (5.4 mg; 95% CI, 4.66-6.13). Secondary outcomes were fibromyalgia symptoms as evaluated on the Fibromyalgia Impact Questionnaire Revised. Five of the 11 responders reported a > 30% improvement in tenderness and 8 of the 11 responders reported a > 30% decrease in waking unrefreshed.3
Limitations of the study include the short time period of treatment before response was assessed and the decision to use low test doses, which may have hindered detection of effective doses > 6 mg in fibromyalgia.3
Editor’s takeaway
Low-dose naltrexone, a less-often-used form of pain management, is a welcome option. Studies show some effectiveness in a variety of pain conditions with few adverse effects. The small number of studies, the small sample sizes, and the limited follow-up duration should encourage more investigation into how to best use this intervention.
1. Srinivasan A, Dutta P, Bansal D, et al. Efficacy and safety of low-dose naltrexone in painful diabetic neuropathy: a randomized, double-blind, active-control, crossover clinical trial. J Diabetes. 2021;13:770-778. doi: 10.1111/1753-0407.13202
2. Martin SJ, McAnally HB, Okediji P, et al. Low-dose naltrexone, an opioid-receptor antagonist, is a broad-spectrum analgesic: a retrospective cohort study. Pain Management. 2022;12:699-709. doi: 10.2217/pmt-2021-0122
3. Bruun-Plesner K, Blichfeldt-Eckhardt MR, Vaegter HB, et al. Low-dose naltrexone for the treatment of fibromyalgia: investigation of dose-response relationships. Pain Med. 2020;21:2253-2261. doi: 10.1093/pm/pnaa001
1. Srinivasan A, Dutta P, Bansal D, et al. Efficacy and safety of low-dose naltrexone in painful diabetic neuropathy: a randomized, double-blind, active-control, crossover clinical trial. J Diabetes. 2021;13:770-778. doi: 10.1111/1753-0407.13202
2. Martin SJ, McAnally HB, Okediji P, et al. Low-dose naltrexone, an opioid-receptor antagonist, is a broad-spectrum analgesic: a retrospective cohort study. Pain Management. 2022;12:699-709. doi: 10.2217/pmt-2021-0122
3. Bruun-Plesner K, Blichfeldt-Eckhardt MR, Vaegter HB, et al. Low-dose naltrexone for the treatment of fibromyalgia: investigation of dose-response relationships. Pain Med. 2020;21:2253-2261. doi: 10.1093/pm/pnaa001
EVIDENCE-BASED ANSWER:
YES. Low-dose naltrexone is as effective as amitriptyline in the treatment of painful diabetic neuropathy and has a superior safety profile (strength of recommendation [SOR], B; single randomized controlled trial [RCT]).
Low-dose naltrexone significantly reduced pain by 32% in inflammatory conditions and 44% in neuropathic conditions (SOR, B; single retrospective cohort study).
Doses as low as 5.4 mg were found to reduce pain in 95% of patients with fibromyalgia (SOR, B; single prospective dose-response study).
School avoidance: How to help when a child refuses to go
THE CASE
Juana*, a 10-year-old who identifies as a cisgender, Hispanic female, was referred to our integrated behavioral health program by her primary care physician. Her mother was concerned because Juana had been refusing to attend school due to complaints of gastrointestinal upset. This concern began when Juana was in first grade but had increased in severity over the past few months.
Upon further questioning, the patient reported that she initially did not want to attend school due to academic difficulties and bullying. However, since COVID-19, her fears of attending school had significantly worsened. Juana’s mother’s primary language was Spanish and she had limited English proficiency; she reported difficulty communicating with school personnel about Juana’s poor attendance.
Juana had recently had a complete medical work-up for her gastrointestinal concerns, with negative results. Since the negative work-up, Juana’s mother had told her daughter that she would be punished if she didn’t go to school.
●
* The patient’s name has been changed to protect her identity.
School avoidance, also referred to as school refusal, is a symptom of an emotional condition that manifests as a child refusing to go to school or having difficulty going to school or remaining in the classroom for the entire day. School avoidance is not a clinical diagnosis but often is related to an underlying disorder.1
School avoidance is common, affecting 5% to 28% of youth sometime in their school career.2 Available data are not specific to school avoidance but focus on chronic absenteeism (missing ≥ 15 days per school year). Rates of chronic absenteeism are high in elementary and middle school (about 14% each) and tend to increase in high school (about 21%).3 Students with disabilities are 1.5 times more likely to be chronically absent than students without disabilities.3 Compared to White students, American Indian and Pacific Islander students are > 50% more likely, Black students 40% more likely, and Hispanic students 17% more likely to miss ≥ 3 weeks of school.3 Rates of chronic absenteeism are similar (about 16%) for males and females.3
Absenteeism can have immediate and long-term negative effects.4 School attendance issues are correlated to negative life outcomes, such as delinquency, teen pregnancy, substance use, and poor academic achievement.5 According to the US Department of Education, individuals who chronically miss school are less likely to achieve educational milestones (particularly in younger years) and may be more likely to drop out of school.3
What school avoidance is (and what it isn’t)
It is important to distinguish school avoidance from truancy. Truancy often is associated with antisocial behavior such as lying and stealing, while school avoidance occurs in the absence of significant antisocial disorders.6 With truancy, the absence usually is hidden from the parent. In contrast, with school avoidance, the parents usually know where their child is; the child often spends the day secluded in their bedroom. Students who engage in truancy do not demonstrate excessive anxiety about attending school but may have decreased interest in schoolwork and academic performance.6 With school avoidance, the child exhibits severe emotional distress about attending school but is willing to complete schoolwork at home.
Why children may avoid school
School avoidance is a biopsychosocial condition with a multitude of underlying causes.4 It is associated most commonly with anxiety disorders and neurodevelopmental disorders, including but not limited to learning disabilities and attention-deficit/hyperactivity disorder.1 Depressive disorders also have been associated with school avoidance.7 Social concerns related to changes with school personnel or classes, academic challenges, bullying, health emergencies, and family stressors also can result in symptoms of school avoidance.1
Continue to: A child seeking to avoid...
A child seeking to avoid school may be motivated by potential negative and/or positive effects of doing so. Kearney and Silverman8 identified 4 primary functions of school refusal behaviors:
- avoiding stimuli at school that lend to negative affect (depression, anxiety)
- escaping the social interactions and/or situations for evaluation that occur at school
- gaining more attention from caregivers, and
- obtaining tangible rewards or benefits outside the school environment.
How school avoidance manifests
School avoidance has attributes of internalizing (depression, anxiety, somatic complaints) and externalizing (aggression, tantrums, running away, clinginess) behaviors. It can cause distress for the student, parents and caregivers, and school personnel.
The avoidance may manifest with behaviors such as crying, hiding, emotional outbursts, and refusing to move prior to the start of the school day. Additionally, the child may beg their parents not to make them go to school or, when at school, they may leave the classroom to go to a safe place such as the nurse’s or counselor’s office.
The avoidance may occur abruptly, such as after a break in the school schedule or a change of school. Or it may be the final result of the student’s gradual inability to cope with the underlying issue.
How to assess for school avoidance
Due to the multifactorial nature of this presenting concern, a comprehensive evaluation is recommended when school avoidance is reported.4 Often the child will present with physical symptoms, such as abdominal pain, nausea, vomiting, diarrhea, headaches, shortness of breath, dizziness, chest pain, and palpitations. A thorough medical examination should be performed to rule out a physiological cause. The medical visit should include clinical interviews with the patient and family members or guardians.
Continue to: To identify school avoidance...
To identify school avoidance in pediatric and adolescent populations, medical history and physical examination—along with social history to better understand familial, social, and academic concerns—should be a regular part of the medical encounter. The School Refusal Assessment Scale-Revised (SRAS-R) for both parents and their children was developed to assess for school avoidance and can be utilized within the primary care setting. Additional psychiatric history for both the family and patient may be beneficial, due to associations between parental mental health concerns and school avoidance in their children.9,10
Assessment for an underlying mental health condition, such as an anxiety or depressive disorder, should be completed when a patient presents with school avoidance.4 More than one-third of children with behavioral problems, such as school avoidance, have been diagnosed with anxiety.11 The 2020 National Survey of Children’s Health found that 7.8% of children and adolescents ages 3 to 17 years had a current anxiety disorder, leading the US Preventive Services Task Force to recommend screening for anxiety in children and adolescents ages 8 to 18 years.12,13 Furthermore, if academic achievement is of concern, then consideration of further assessment for neurodevelopmental disorders is warranted.1
Treatment is multimodal and multidisciplinary
Treatment for school avoidance is often multimodal and may involve interdisciplinary, team-based care including the medical provider, school system (eg, Child Study Team), family, and mental health care provider.1,4
Cognitive behavioral therapy (CBT) is the most-studied intervention for school avoidance, with behavioral, exposure-based interventions often central to therapeutic gains in treatment.1,14,15 The goals of treatment are to increase school attendance while decreasing emotional distress through various strategies, including exposure-based interventions, contingency management with parents and school staff, relaxation training, and/or social skills training.14,16 Collaborative involvement between the medical provider and the school system is key to successful treatment.
Medication may be considered alone or in combination with CBT when comorbid mental health conditions have been identified. Selective serotonin reuptake inhibitors (SSRIs)—including fluoxetine, sertraline, and escitalopram—are considered first-line treatment for anxiety in children and adolescents.17 Serotonin-norepinephrine reuptake inhibitors (SNRIs), such as duloxetine and venlafaxine, also have been shown to be effective. Duloxetine is the only medication approved by the US Food and Drug Administration (FDA) for treatment of generalized anxiety disorder in children ages 7 years and older.17
Continue to: SSRIs and SNRIs have a boxed warning...
SSRIs and SNRIs have a boxed warning from the FDA for increased suicidal thoughts and behaviors in children and adolescents. Although this risk is rare, it should be discussed with the patient and parent/guardian in order to obtain informed consent prior to treatment initiation.
Medication should be started at the lowest possible dose and increased gradually. Patients should remain on the medication for 6 to 12 months after symptom resolution and should be tapered during a nonstressful time, such as the summer break.
THE CASE
Based on the concerns of continued school refusal after negative gastrointestinal work-up, Juana’s physician screened her for anxiety and conducted a clinical interview to better understand any psychosocial concerns. Juana’s score of 10 on the General Anxiety Disorder-7 scale indicated moderate anxiety. She reported symptoms consistent with social anxiety disorder contributing to school avoidance.
The physician consulted with the clinic’s behavioral health consultant (BHC) to confirm the multimodal treatment plan, which was then discussed with Juana and her mother. The physician discussed medication options (SSRIs) and provided documentation (in both English and Spanish) from the visit to Juana’s mother so she could initiate a school-based intervention with the Child Study Team at Juana’s school. A plan for CBT—including a collaborative contingency management plan between the patient and her parent (eg, a reward chart for attending school) and exposure interventions (eg, a graduated plan to participate in school-based activities with the end goal to resume full school attendance)—was developed with the BHC. Biweekly follow-up appointments were scheduled with the BHC and monthly appointments were scheduled with the physician to reinforce the interventions.
CORRESPONDENCE
Meredith L. C. Williamson, PhD, 2900 East 29th Street, Suite 100, Bryan, TX 77840; [email protected]
1. School Avoidance Alliance. School avoidance facts. Published September 16, 2021. Accessed July 27, 2023. https://schoolavoidance.org/school-avoidance-facts/
2. Kearney CA. School Refusal Behavior in Youth: A Functional Approach to Assessment and Treatment. American Psychological Association; 2001.
3. US Department of Education. Chronic absenteeism in the nation’s schools: a hidden educational crisis. Updated January 2019. Accessed August 3, 2023. www2.ed.gov/datastory/chronicabsenteeism.html
4. Allen CW, Diamond-Myrsten S, Rollins LK. School absenteeism in children and adolescents. Am Fam Physician. 2018;98:738-744.
5. Gonzálvez C, Díaz-Herrero Á, Vicent M, et al. School refusal behavior: latent class analysis approach and its relationship with psychopathological symptoms. Curr Psychology. 2022;41:2078-2088. doi: 10.1007/s12144-020-00711-6
6. Fremont WP. School refusal in children and adolescents. Am Fam Physician. 2003;68:1555-1560.
7. McShane G, Walter G, Rey JM. Characteristics of adolescents with school refusal. Aust N Z J Psychiatry. 2001;35:822-826. doi: 10.1046/j.1440-1614.2001.00955.x
8. Kearney CA, Silverman WK. The evolution and reconciliation of taxonomic strategies for school refusal behavior. Clin Psychology Sci Pract. 1996;3:339-354. doi: 10.1111/j.1468-2850.1996.tb00087.x
9. Kearney CA, Albano AM. School Refusal Assessment Scale-Revised C. Oxford University Press; 2007.
10. Heyne D. School refusal. In: Fisher JE, O’Donohue WT (eds). Practitioner’s Guide to Evidence-based Psychotherapy. Springer Science + Business Media. 2006;600-619. doi: 10.1007/978-0-387-28370-8_60
11. Ghandour RM, Sherman LJ, Vladutiu CJ, et al. Prevalence and treatment of depression, anxiety, and conduct problems in US children. J Pediatrics. 2019;206:256-267.e3. doi: 10.1016/j.jpeds.2018.09.021
12. US Census Bureau. 2020 National Survey of Children’s Health: Topical Frequencies. Published June 2, 2021. Accessed August 4, 2023. www2.census.gov/programs-surveys/nsch/technical-documentation/codebook/NSCH_2020_Topical_Frequencies.pdf
13. USPSTF. Anxiety in children and adolescents: screening. Final Recommendation Statement. Published October 11, 2022. Accessed August 4, 2023. www.uspreventiveservicestaskforce.org/uspstf/recommendation/screening-anxiety-children-adolescents
14. Maynard BR, Brendel KE, Bulanda JJ, et al. Psychosocial interventions for school refusal with primary and secondary school students: a systematic review. Campbell Systematic Rev. 2015;11:1-76. doi: 10.4073/csr.2015.12
15. Kearney CA, Albano AM. When Children Refuse School: Parent Workbook. 3rd ed. Oxford University Press; 2018. doi: 10.1093/med-psych/9780190604080.001.0001
16. Heyne DA, Sauter FM. School refusal. In: Essau CA, Ollendick TH. The Wiley-Blackwell Handbook of the Treatment of Childhood and Adolescent Anxiety. Wiley Blackwell; 2013:471-517.
17. Kowalchuk A, Gonzalez SJ, Zoorob RJ. Anxiety disorders in children and adolescents. Am Fam Physician. 2022;106:657-664.
THE CASE
Juana*, a 10-year-old who identifies as a cisgender, Hispanic female, was referred to our integrated behavioral health program by her primary care physician. Her mother was concerned because Juana had been refusing to attend school due to complaints of gastrointestinal upset. This concern began when Juana was in first grade but had increased in severity over the past few months.
Upon further questioning, the patient reported that she initially did not want to attend school due to academic difficulties and bullying. However, since COVID-19, her fears of attending school had significantly worsened. Juana’s mother’s primary language was Spanish and she had limited English proficiency; she reported difficulty communicating with school personnel about Juana’s poor attendance.
Juana had recently had a complete medical work-up for her gastrointestinal concerns, with negative results. Since the negative work-up, Juana’s mother had told her daughter that she would be punished if she didn’t go to school.
●
* The patient’s name has been changed to protect her identity.
School avoidance, also referred to as school refusal, is a symptom of an emotional condition that manifests as a child refusing to go to school or having difficulty going to school or remaining in the classroom for the entire day. School avoidance is not a clinical diagnosis but often is related to an underlying disorder.1
School avoidance is common, affecting 5% to 28% of youth sometime in their school career.2 Available data are not specific to school avoidance but focus on chronic absenteeism (missing ≥ 15 days per school year). Rates of chronic absenteeism are high in elementary and middle school (about 14% each) and tend to increase in high school (about 21%).3 Students with disabilities are 1.5 times more likely to be chronically absent than students without disabilities.3 Compared to White students, American Indian and Pacific Islander students are > 50% more likely, Black students 40% more likely, and Hispanic students 17% more likely to miss ≥ 3 weeks of school.3 Rates of chronic absenteeism are similar (about 16%) for males and females.3
Absenteeism can have immediate and long-term negative effects.4 School attendance issues are correlated to negative life outcomes, such as delinquency, teen pregnancy, substance use, and poor academic achievement.5 According to the US Department of Education, individuals who chronically miss school are less likely to achieve educational milestones (particularly in younger years) and may be more likely to drop out of school.3
What school avoidance is (and what it isn’t)
It is important to distinguish school avoidance from truancy. Truancy often is associated with antisocial behavior such as lying and stealing, while school avoidance occurs in the absence of significant antisocial disorders.6 With truancy, the absence usually is hidden from the parent. In contrast, with school avoidance, the parents usually know where their child is; the child often spends the day secluded in their bedroom. Students who engage in truancy do not demonstrate excessive anxiety about attending school but may have decreased interest in schoolwork and academic performance.6 With school avoidance, the child exhibits severe emotional distress about attending school but is willing to complete schoolwork at home.
Why children may avoid school
School avoidance is a biopsychosocial condition with a multitude of underlying causes.4 It is associated most commonly with anxiety disorders and neurodevelopmental disorders, including but not limited to learning disabilities and attention-deficit/hyperactivity disorder.1 Depressive disorders also have been associated with school avoidance.7 Social concerns related to changes with school personnel or classes, academic challenges, bullying, health emergencies, and family stressors also can result in symptoms of school avoidance.1
Continue to: A child seeking to avoid...
A child seeking to avoid school may be motivated by potential negative and/or positive effects of doing so. Kearney and Silverman8 identified 4 primary functions of school refusal behaviors:
- avoiding stimuli at school that lend to negative affect (depression, anxiety)
- escaping the social interactions and/or situations for evaluation that occur at school
- gaining more attention from caregivers, and
- obtaining tangible rewards or benefits outside the school environment.
How school avoidance manifests
School avoidance has attributes of internalizing (depression, anxiety, somatic complaints) and externalizing (aggression, tantrums, running away, clinginess) behaviors. It can cause distress for the student, parents and caregivers, and school personnel.
The avoidance may manifest with behaviors such as crying, hiding, emotional outbursts, and refusing to move prior to the start of the school day. Additionally, the child may beg their parents not to make them go to school or, when at school, they may leave the classroom to go to a safe place such as the nurse’s or counselor’s office.
The avoidance may occur abruptly, such as after a break in the school schedule or a change of school. Or it may be the final result of the student’s gradual inability to cope with the underlying issue.
How to assess for school avoidance
Due to the multifactorial nature of this presenting concern, a comprehensive evaluation is recommended when school avoidance is reported.4 Often the child will present with physical symptoms, such as abdominal pain, nausea, vomiting, diarrhea, headaches, shortness of breath, dizziness, chest pain, and palpitations. A thorough medical examination should be performed to rule out a physiological cause. The medical visit should include clinical interviews with the patient and family members or guardians.
Continue to: To identify school avoidance...
To identify school avoidance in pediatric and adolescent populations, medical history and physical examination—along with social history to better understand familial, social, and academic concerns—should be a regular part of the medical encounter. The School Refusal Assessment Scale-Revised (SRAS-R) for both parents and their children was developed to assess for school avoidance and can be utilized within the primary care setting. Additional psychiatric history for both the family and patient may be beneficial, due to associations between parental mental health concerns and school avoidance in their children.9,10
Assessment for an underlying mental health condition, such as an anxiety or depressive disorder, should be completed when a patient presents with school avoidance.4 More than one-third of children with behavioral problems, such as school avoidance, have been diagnosed with anxiety.11 The 2020 National Survey of Children’s Health found that 7.8% of children and adolescents ages 3 to 17 years had a current anxiety disorder, leading the US Preventive Services Task Force to recommend screening for anxiety in children and adolescents ages 8 to 18 years.12,13 Furthermore, if academic achievement is of concern, then consideration of further assessment for neurodevelopmental disorders is warranted.1
Treatment is multimodal and multidisciplinary
Treatment for school avoidance is often multimodal and may involve interdisciplinary, team-based care including the medical provider, school system (eg, Child Study Team), family, and mental health care provider.1,4
Cognitive behavioral therapy (CBT) is the most-studied intervention for school avoidance, with behavioral, exposure-based interventions often central to therapeutic gains in treatment.1,14,15 The goals of treatment are to increase school attendance while decreasing emotional distress through various strategies, including exposure-based interventions, contingency management with parents and school staff, relaxation training, and/or social skills training.14,16 Collaborative involvement between the medical provider and the school system is key to successful treatment.
Medication may be considered alone or in combination with CBT when comorbid mental health conditions have been identified. Selective serotonin reuptake inhibitors (SSRIs)—including fluoxetine, sertraline, and escitalopram—are considered first-line treatment for anxiety in children and adolescents.17 Serotonin-norepinephrine reuptake inhibitors (SNRIs), such as duloxetine and venlafaxine, also have been shown to be effective. Duloxetine is the only medication approved by the US Food and Drug Administration (FDA) for treatment of generalized anxiety disorder in children ages 7 years and older.17
Continue to: SSRIs and SNRIs have a boxed warning...
SSRIs and SNRIs have a boxed warning from the FDA for increased suicidal thoughts and behaviors in children and adolescents. Although this risk is rare, it should be discussed with the patient and parent/guardian in order to obtain informed consent prior to treatment initiation.
Medication should be started at the lowest possible dose and increased gradually. Patients should remain on the medication for 6 to 12 months after symptom resolution and should be tapered during a nonstressful time, such as the summer break.
THE CASE
Based on the concerns of continued school refusal after negative gastrointestinal work-up, Juana’s physician screened her for anxiety and conducted a clinical interview to better understand any psychosocial concerns. Juana’s score of 10 on the General Anxiety Disorder-7 scale indicated moderate anxiety. She reported symptoms consistent with social anxiety disorder contributing to school avoidance.
The physician consulted with the clinic’s behavioral health consultant (BHC) to confirm the multimodal treatment plan, which was then discussed with Juana and her mother. The physician discussed medication options (SSRIs) and provided documentation (in both English and Spanish) from the visit to Juana’s mother so she could initiate a school-based intervention with the Child Study Team at Juana’s school. A plan for CBT—including a collaborative contingency management plan between the patient and her parent (eg, a reward chart for attending school) and exposure interventions (eg, a graduated plan to participate in school-based activities with the end goal to resume full school attendance)—was developed with the BHC. Biweekly follow-up appointments were scheduled with the BHC and monthly appointments were scheduled with the physician to reinforce the interventions.
CORRESPONDENCE
Meredith L. C. Williamson, PhD, 2900 East 29th Street, Suite 100, Bryan, TX 77840; [email protected]
THE CASE
Juana*, a 10-year-old who identifies as a cisgender, Hispanic female, was referred to our integrated behavioral health program by her primary care physician. Her mother was concerned because Juana had been refusing to attend school due to complaints of gastrointestinal upset. This concern began when Juana was in first grade but had increased in severity over the past few months.
Upon further questioning, the patient reported that she initially did not want to attend school due to academic difficulties and bullying. However, since COVID-19, her fears of attending school had significantly worsened. Juana’s mother’s primary language was Spanish and she had limited English proficiency; she reported difficulty communicating with school personnel about Juana’s poor attendance.
Juana had recently had a complete medical work-up for her gastrointestinal concerns, with negative results. Since the negative work-up, Juana’s mother had told her daughter that she would be punished if she didn’t go to school.
●
* The patient’s name has been changed to protect her identity.
School avoidance, also referred to as school refusal, is a symptom of an emotional condition that manifests as a child refusing to go to school or having difficulty going to school or remaining in the classroom for the entire day. School avoidance is not a clinical diagnosis but often is related to an underlying disorder.1
School avoidance is common, affecting 5% to 28% of youth sometime in their school career.2 Available data are not specific to school avoidance but focus on chronic absenteeism (missing ≥ 15 days per school year). Rates of chronic absenteeism are high in elementary and middle school (about 14% each) and tend to increase in high school (about 21%).3 Students with disabilities are 1.5 times more likely to be chronically absent than students without disabilities.3 Compared to White students, American Indian and Pacific Islander students are > 50% more likely, Black students 40% more likely, and Hispanic students 17% more likely to miss ≥ 3 weeks of school.3 Rates of chronic absenteeism are similar (about 16%) for males and females.3
Absenteeism can have immediate and long-term negative effects.4 School attendance issues are correlated to negative life outcomes, such as delinquency, teen pregnancy, substance use, and poor academic achievement.5 According to the US Department of Education, individuals who chronically miss school are less likely to achieve educational milestones (particularly in younger years) and may be more likely to drop out of school.3
What school avoidance is (and what it isn’t)
It is important to distinguish school avoidance from truancy. Truancy often is associated with antisocial behavior such as lying and stealing, while school avoidance occurs in the absence of significant antisocial disorders.6 With truancy, the absence usually is hidden from the parent. In contrast, with school avoidance, the parents usually know where their child is; the child often spends the day secluded in their bedroom. Students who engage in truancy do not demonstrate excessive anxiety about attending school but may have decreased interest in schoolwork and academic performance.6 With school avoidance, the child exhibits severe emotional distress about attending school but is willing to complete schoolwork at home.
Why children may avoid school
School avoidance is a biopsychosocial condition with a multitude of underlying causes.4 It is associated most commonly with anxiety disorders and neurodevelopmental disorders, including but not limited to learning disabilities and attention-deficit/hyperactivity disorder.1 Depressive disorders also have been associated with school avoidance.7 Social concerns related to changes with school personnel or classes, academic challenges, bullying, health emergencies, and family stressors also can result in symptoms of school avoidance.1
Continue to: A child seeking to avoid...
A child seeking to avoid school may be motivated by potential negative and/or positive effects of doing so. Kearney and Silverman8 identified 4 primary functions of school refusal behaviors:
- avoiding stimuli at school that lend to negative affect (depression, anxiety)
- escaping the social interactions and/or situations for evaluation that occur at school
- gaining more attention from caregivers, and
- obtaining tangible rewards or benefits outside the school environment.
How school avoidance manifests
School avoidance has attributes of internalizing (depression, anxiety, somatic complaints) and externalizing (aggression, tantrums, running away, clinginess) behaviors. It can cause distress for the student, parents and caregivers, and school personnel.
The avoidance may manifest with behaviors such as crying, hiding, emotional outbursts, and refusing to move prior to the start of the school day. Additionally, the child may beg their parents not to make them go to school or, when at school, they may leave the classroom to go to a safe place such as the nurse’s or counselor’s office.
The avoidance may occur abruptly, such as after a break in the school schedule or a change of school. Or it may be the final result of the student’s gradual inability to cope with the underlying issue.
How to assess for school avoidance
Due to the multifactorial nature of this presenting concern, a comprehensive evaluation is recommended when school avoidance is reported.4 Often the child will present with physical symptoms, such as abdominal pain, nausea, vomiting, diarrhea, headaches, shortness of breath, dizziness, chest pain, and palpitations. A thorough medical examination should be performed to rule out a physiological cause. The medical visit should include clinical interviews with the patient and family members or guardians.
Continue to: To identify school avoidance...
To identify school avoidance in pediatric and adolescent populations, medical history and physical examination—along with social history to better understand familial, social, and academic concerns—should be a regular part of the medical encounter. The School Refusal Assessment Scale-Revised (SRAS-R) for both parents and their children was developed to assess for school avoidance and can be utilized within the primary care setting. Additional psychiatric history for both the family and patient may be beneficial, due to associations between parental mental health concerns and school avoidance in their children.9,10
Assessment for an underlying mental health condition, such as an anxiety or depressive disorder, should be completed when a patient presents with school avoidance.4 More than one-third of children with behavioral problems, such as school avoidance, have been diagnosed with anxiety.11 The 2020 National Survey of Children’s Health found that 7.8% of children and adolescents ages 3 to 17 years had a current anxiety disorder, leading the US Preventive Services Task Force to recommend screening for anxiety in children and adolescents ages 8 to 18 years.12,13 Furthermore, if academic achievement is of concern, then consideration of further assessment for neurodevelopmental disorders is warranted.1
Treatment is multimodal and multidisciplinary
Treatment for school avoidance is often multimodal and may involve interdisciplinary, team-based care including the medical provider, school system (eg, Child Study Team), family, and mental health care provider.1,4
Cognitive behavioral therapy (CBT) is the most-studied intervention for school avoidance, with behavioral, exposure-based interventions often central to therapeutic gains in treatment.1,14,15 The goals of treatment are to increase school attendance while decreasing emotional distress through various strategies, including exposure-based interventions, contingency management with parents and school staff, relaxation training, and/or social skills training.14,16 Collaborative involvement between the medical provider and the school system is key to successful treatment.
Medication may be considered alone or in combination with CBT when comorbid mental health conditions have been identified. Selective serotonin reuptake inhibitors (SSRIs)—including fluoxetine, sertraline, and escitalopram—are considered first-line treatment for anxiety in children and adolescents.17 Serotonin-norepinephrine reuptake inhibitors (SNRIs), such as duloxetine and venlafaxine, also have been shown to be effective. Duloxetine is the only medication approved by the US Food and Drug Administration (FDA) for treatment of generalized anxiety disorder in children ages 7 years and older.17
Continue to: SSRIs and SNRIs have a boxed warning...
SSRIs and SNRIs have a boxed warning from the FDA for increased suicidal thoughts and behaviors in children and adolescents. Although this risk is rare, it should be discussed with the patient and parent/guardian in order to obtain informed consent prior to treatment initiation.
Medication should be started at the lowest possible dose and increased gradually. Patients should remain on the medication for 6 to 12 months after symptom resolution and should be tapered during a nonstressful time, such as the summer break.
THE CASE
Based on the concerns of continued school refusal after negative gastrointestinal work-up, Juana’s physician screened her for anxiety and conducted a clinical interview to better understand any psychosocial concerns. Juana’s score of 10 on the General Anxiety Disorder-7 scale indicated moderate anxiety. She reported symptoms consistent with social anxiety disorder contributing to school avoidance.
The physician consulted with the clinic’s behavioral health consultant (BHC) to confirm the multimodal treatment plan, which was then discussed with Juana and her mother. The physician discussed medication options (SSRIs) and provided documentation (in both English and Spanish) from the visit to Juana’s mother so she could initiate a school-based intervention with the Child Study Team at Juana’s school. A plan for CBT—including a collaborative contingency management plan between the patient and her parent (eg, a reward chart for attending school) and exposure interventions (eg, a graduated plan to participate in school-based activities with the end goal to resume full school attendance)—was developed with the BHC. Biweekly follow-up appointments were scheduled with the BHC and monthly appointments were scheduled with the physician to reinforce the interventions.
CORRESPONDENCE
Meredith L. C. Williamson, PhD, 2900 East 29th Street, Suite 100, Bryan, TX 77840; [email protected]
1. School Avoidance Alliance. School avoidance facts. Published September 16, 2021. Accessed July 27, 2023. https://schoolavoidance.org/school-avoidance-facts/
2. Kearney CA. School Refusal Behavior in Youth: A Functional Approach to Assessment and Treatment. American Psychological Association; 2001.
3. US Department of Education. Chronic absenteeism in the nation’s schools: a hidden educational crisis. Updated January 2019. Accessed August 3, 2023. www2.ed.gov/datastory/chronicabsenteeism.html
4. Allen CW, Diamond-Myrsten S, Rollins LK. School absenteeism in children and adolescents. Am Fam Physician. 2018;98:738-744.
5. Gonzálvez C, Díaz-Herrero Á, Vicent M, et al. School refusal behavior: latent class analysis approach and its relationship with psychopathological symptoms. Curr Psychology. 2022;41:2078-2088. doi: 10.1007/s12144-020-00711-6
6. Fremont WP. School refusal in children and adolescents. Am Fam Physician. 2003;68:1555-1560.
7. McShane G, Walter G, Rey JM. Characteristics of adolescents with school refusal. Aust N Z J Psychiatry. 2001;35:822-826. doi: 10.1046/j.1440-1614.2001.00955.x
8. Kearney CA, Silverman WK. The evolution and reconciliation of taxonomic strategies for school refusal behavior. Clin Psychology Sci Pract. 1996;3:339-354. doi: 10.1111/j.1468-2850.1996.tb00087.x
9. Kearney CA, Albano AM. School Refusal Assessment Scale-Revised C. Oxford University Press; 2007.
10. Heyne D. School refusal. In: Fisher JE, O’Donohue WT (eds). Practitioner’s Guide to Evidence-based Psychotherapy. Springer Science + Business Media. 2006;600-619. doi: 10.1007/978-0-387-28370-8_60
11. Ghandour RM, Sherman LJ, Vladutiu CJ, et al. Prevalence and treatment of depression, anxiety, and conduct problems in US children. J Pediatrics. 2019;206:256-267.e3. doi: 10.1016/j.jpeds.2018.09.021
12. US Census Bureau. 2020 National Survey of Children’s Health: Topical Frequencies. Published June 2, 2021. Accessed August 4, 2023. www2.census.gov/programs-surveys/nsch/technical-documentation/codebook/NSCH_2020_Topical_Frequencies.pdf
13. USPSTF. Anxiety in children and adolescents: screening. Final Recommendation Statement. Published October 11, 2022. Accessed August 4, 2023. www.uspreventiveservicestaskforce.org/uspstf/recommendation/screening-anxiety-children-adolescents
14. Maynard BR, Brendel KE, Bulanda JJ, et al. Psychosocial interventions for school refusal with primary and secondary school students: a systematic review. Campbell Systematic Rev. 2015;11:1-76. doi: 10.4073/csr.2015.12
15. Kearney CA, Albano AM. When Children Refuse School: Parent Workbook. 3rd ed. Oxford University Press; 2018. doi: 10.1093/med-psych/9780190604080.001.0001
16. Heyne DA, Sauter FM. School refusal. In: Essau CA, Ollendick TH. The Wiley-Blackwell Handbook of the Treatment of Childhood and Adolescent Anxiety. Wiley Blackwell; 2013:471-517.
17. Kowalchuk A, Gonzalez SJ, Zoorob RJ. Anxiety disorders in children and adolescents. Am Fam Physician. 2022;106:657-664.
1. School Avoidance Alliance. School avoidance facts. Published September 16, 2021. Accessed July 27, 2023. https://schoolavoidance.org/school-avoidance-facts/
2. Kearney CA. School Refusal Behavior in Youth: A Functional Approach to Assessment and Treatment. American Psychological Association; 2001.
3. US Department of Education. Chronic absenteeism in the nation’s schools: a hidden educational crisis. Updated January 2019. Accessed August 3, 2023. www2.ed.gov/datastory/chronicabsenteeism.html
4. Allen CW, Diamond-Myrsten S, Rollins LK. School absenteeism in children and adolescents. Am Fam Physician. 2018;98:738-744.
5. Gonzálvez C, Díaz-Herrero Á, Vicent M, et al. School refusal behavior: latent class analysis approach and its relationship with psychopathological symptoms. Curr Psychology. 2022;41:2078-2088. doi: 10.1007/s12144-020-00711-6
6. Fremont WP. School refusal in children and adolescents. Am Fam Physician. 2003;68:1555-1560.
7. McShane G, Walter G, Rey JM. Characteristics of adolescents with school refusal. Aust N Z J Psychiatry. 2001;35:822-826. doi: 10.1046/j.1440-1614.2001.00955.x
8. Kearney CA, Silverman WK. The evolution and reconciliation of taxonomic strategies for school refusal behavior. Clin Psychology Sci Pract. 1996;3:339-354. doi: 10.1111/j.1468-2850.1996.tb00087.x
9. Kearney CA, Albano AM. School Refusal Assessment Scale-Revised C. Oxford University Press; 2007.
10. Heyne D. School refusal. In: Fisher JE, O’Donohue WT (eds). Practitioner’s Guide to Evidence-based Psychotherapy. Springer Science + Business Media. 2006;600-619. doi: 10.1007/978-0-387-28370-8_60
11. Ghandour RM, Sherman LJ, Vladutiu CJ, et al. Prevalence and treatment of depression, anxiety, and conduct problems in US children. J Pediatrics. 2019;206:256-267.e3. doi: 10.1016/j.jpeds.2018.09.021
12. US Census Bureau. 2020 National Survey of Children’s Health: Topical Frequencies. Published June 2, 2021. Accessed August 4, 2023. www2.census.gov/programs-surveys/nsch/technical-documentation/codebook/NSCH_2020_Topical_Frequencies.pdf
13. USPSTF. Anxiety in children and adolescents: screening. Final Recommendation Statement. Published October 11, 2022. Accessed August 4, 2023. www.uspreventiveservicestaskforce.org/uspstf/recommendation/screening-anxiety-children-adolescents
14. Maynard BR, Brendel KE, Bulanda JJ, et al. Psychosocial interventions for school refusal with primary and secondary school students: a systematic review. Campbell Systematic Rev. 2015;11:1-76. doi: 10.4073/csr.2015.12
15. Kearney CA, Albano AM. When Children Refuse School: Parent Workbook. 3rd ed. Oxford University Press; 2018. doi: 10.1093/med-psych/9780190604080.001.0001
16. Heyne DA, Sauter FM. School refusal. In: Essau CA, Ollendick TH. The Wiley-Blackwell Handbook of the Treatment of Childhood and Adolescent Anxiety. Wiley Blackwell; 2013:471-517.
17. Kowalchuk A, Gonzalez SJ, Zoorob RJ. Anxiety disorders in children and adolescents. Am Fam Physician. 2022;106:657-664.
Is the Altman Rule a proxy for glycemic load?
ABSTRACT
Background: The Altman Rule, a simple tool for consumers seeking to make healthier packaged food choices at the point of sale, applies to packaged carbohydrates. According to the Altman Rule, a food is a healthier option if it has at least 3 g of fiber per serving and the grams of fiber plus the grams of protein exceed the grams of sugar per serving. This study sought to evaluate whether the Altman Rule is a valid proxy for glycemic load (GL).
Methods: We compared the binary outcome of whether a food item meets the Altman Rule with the GL of all foods categorized as cereals, chips, crackers, and granola bars in the Nutrition Data System for Research Database (University of Minnesota, Version 2010). We examined the percentage of foods in low-, medium-, and high-GL categories that met the Altman Rule.
Results: There were 1235 foods (342 cereals, 305 chips, 379 crackers, and 209 granola bars) in this analysis. There was a significant relationship between the GL of foods and the Altman Rule (P < .001) in that most low-GL (68%), almost half of medium-GL (48%), and very few high-GL (7%) foods met the criteria of the rule.
Conclusions: The Altman Rule is a reasonable proxy for GL and can be a useful and accessible tool for consumers interested in buying healthier packaged carbohydrate foods.
Nutrition can be complicated for consumers interested in making healthier choices at the grocery store. Consumers may have difficulty identifying more nutritious options, especially when food labels are adorned with claims such as “Good Source of Fiber” or “Heart Healthy.”1 In addition, when reading food labels, consumers may find it difficult to decipher which data to prioritize when carbohydrates, total sugars, added sugars, total dietary fiber, soluble fiber, and insoluble fiber are all listed.
The concept of glycemic load (GL) is an important consideration, especially for people with diabetes. GL approximates the blood sugar response to different foods. A food with a high GL is digested quickly, and its carbohydrates are taken into the bloodstream rapidly. This leads to a spike and subsequent drop in blood sugars, which can cause symptoms of hyperglycemia and hypoglycemia in a person with diabetes.2,3 Despite its usefulness, GL may be too complicated for a consumer to understand, and it does not appear anywhere on the food label. Since GL is calculated using pooled blood sugar response from individuals after the ingestion of the particular food, estimation of the GL is not intuitable.4
Point-of-sale tools. People seeking to lose weight, control diabetes, improve dyslipidemia and/or blood pressure, and/or decrease their risk for heart disease may benefit from point-of-sale tools such as the Altman Rule, which simplifies and encourages the selection of more nutritious foods.1 Other tools—such as Guiding Stars (https://guidingstars.com), NuVal (www.nuval.com), and different variations of traffic lights—have been created to help consumers make more informed and healthier food choices.5-8 However, Guiding Stars and NuVal are based on complicated algorithms that are not entirely transparent and not accessible to the average consumer.6,7 Evaluations of these nutrition tools indicate that consumers tend to underrate the healthiness of some foods, such as raw almonds and salmon, and overrate the healthiness of others, such as fruit punch and diet soda, when using traffic light systems.6 Furthermore, these nutrition tools are not available in many supermarkets. Previous research suggests that the use of point-of-sale nutrition apps decreases with the time and effort involved in using an app.9
Continue to: The Altman Rule
The Altman Rule was developed by a family physician (author WA) to provide a more accessible tool for people interested in choosing healthier prepackaged carbohydrate foods while shopping. Since the user does not need to have a smartphone, and they are not required to download or understand an app for each purchase, the Altman Rule may be more usable compared with more complicated alternatives.

The Altman Rule can be used with nutrition labels that feature serving information and calories in enlarged and bold type, in compliance with the most recent US Food and Drug Administration (FDA) guideline from 2016. Many foods with high fiber also have high amounts of sugar, so the criteria of the Altman Rule includes a 2-step process requiring (1) a minimum of 3 g of total dietary fiber per serving and (2) the sum of the grams of fiber plus the grams of protein per serving to be greater than the total grams of sugar (not grams of added sugar or grams of carbohydrate) per serving (FIGURE 1A). Unlike the relatively complicated formula related to GL, this 2-part rule can be applied in seconds while shopping (FIGURE 1B).
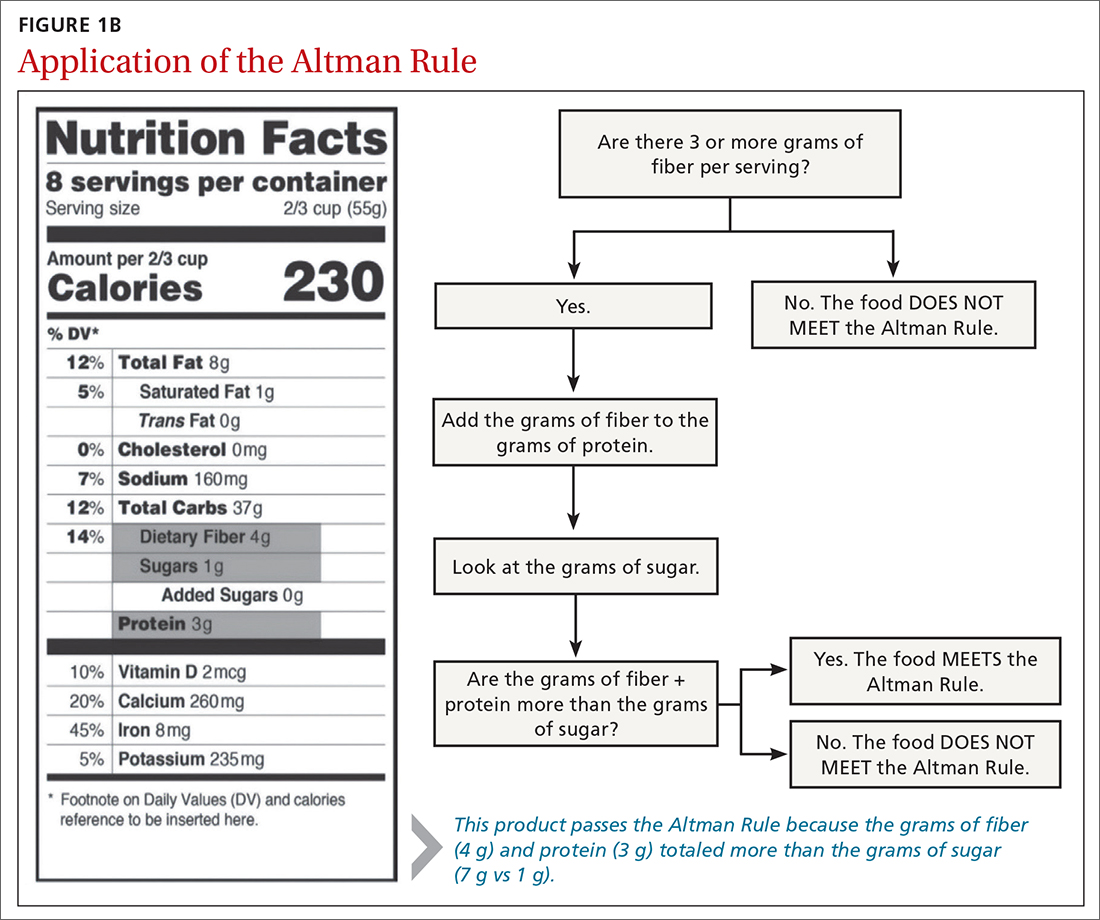
The rule is intended only to be used for packaged carbohydrate products, such as bread, muffins, bagels, pasta, rice, oatmeal, cereals, snack bars, chips, and crackers. It does not apply to whole foods, such as meat, dairy, fruits, or vegetables. These foods are excluded to prevent any consumer confusion related to the nutritional content of whole foods (eg, an apple may have more sugar than fiber and protein combined, but it is still a nutritious option).
This study aimed to determine if the Altman Rule is a reasonable proxy for the more complicated concept of GL. We calculated the relationship between the GL of commercially available packaged carbohydrate foods and whether those foods met the Altman Rule.
METHODS
The Altman Rule was tested by comparing the binary outcome of the rule (meets/does not meet) with data on all foods categorized as cereals, chips, crackers, and granola bars in the Nutrition Data System for Research (NDSR) Database (University of Minnesota, Version 2010).
Continue to: To account for differences...
To account for differences in serving size, we used the standard of 50 g for each product as 1 serving. We used 50 g (about 1.7 oz) to help compare the different foods and between foods within the same group. Additionally, 50 g is close to 1 serving for most foods in these groups; it is about the size of a typical granola bar, three-quarters to 2 cups of cereal, 10 to 12 crackers, and 15 to 25 chips. We determined the GL for each product by multiplying the number of available carbohydrates (total carbohydrate – dietary fiber) by the product’s glycemic index/100. In general, GL is categorized as low (≤ 10), medium (11-19), or high (≥ 20).
We applied the Altman Rule to categorize each product as meeting or not meeting the rule. We compared the proportion of foods meeting the Altman Rule, stratified by GL and by specific foods, and used chi-square to determine if differences were statistically significant. These data were collected and analyzed in the summer of 2019.
RESULTS
There were 1235 foods (342 breakfast cereals, 305 chips, 379 crackers, and 209 granola bars) used for this analysis. There is a significant relationship between the GL of foods and the Altman Rule in that most low-GL (68%), almost half of medium-GL (48%), and only a few high-GL foods (7%) met the rule (P < .001) (TABLE 1). There was also a significant relationship between “meeting the Altman Rule” and GL within each food type (P < .001) (TABLE 2).
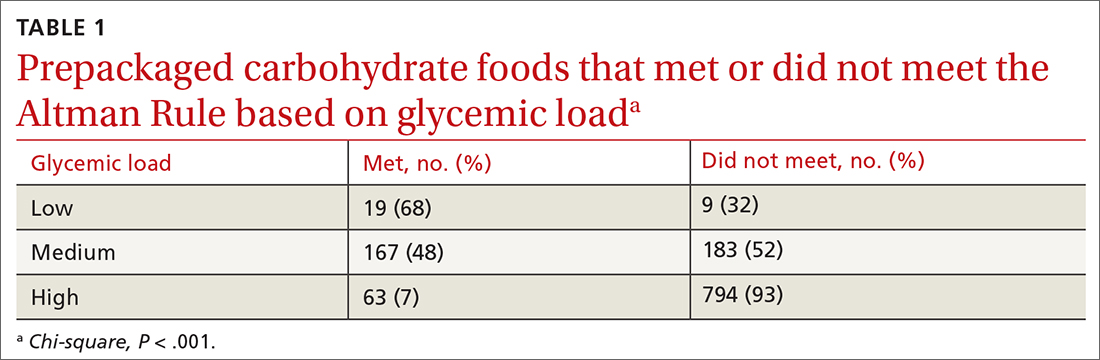
The medium-GL foods were the second largest category of foods we calculated; thus we further broke them into binary categories of
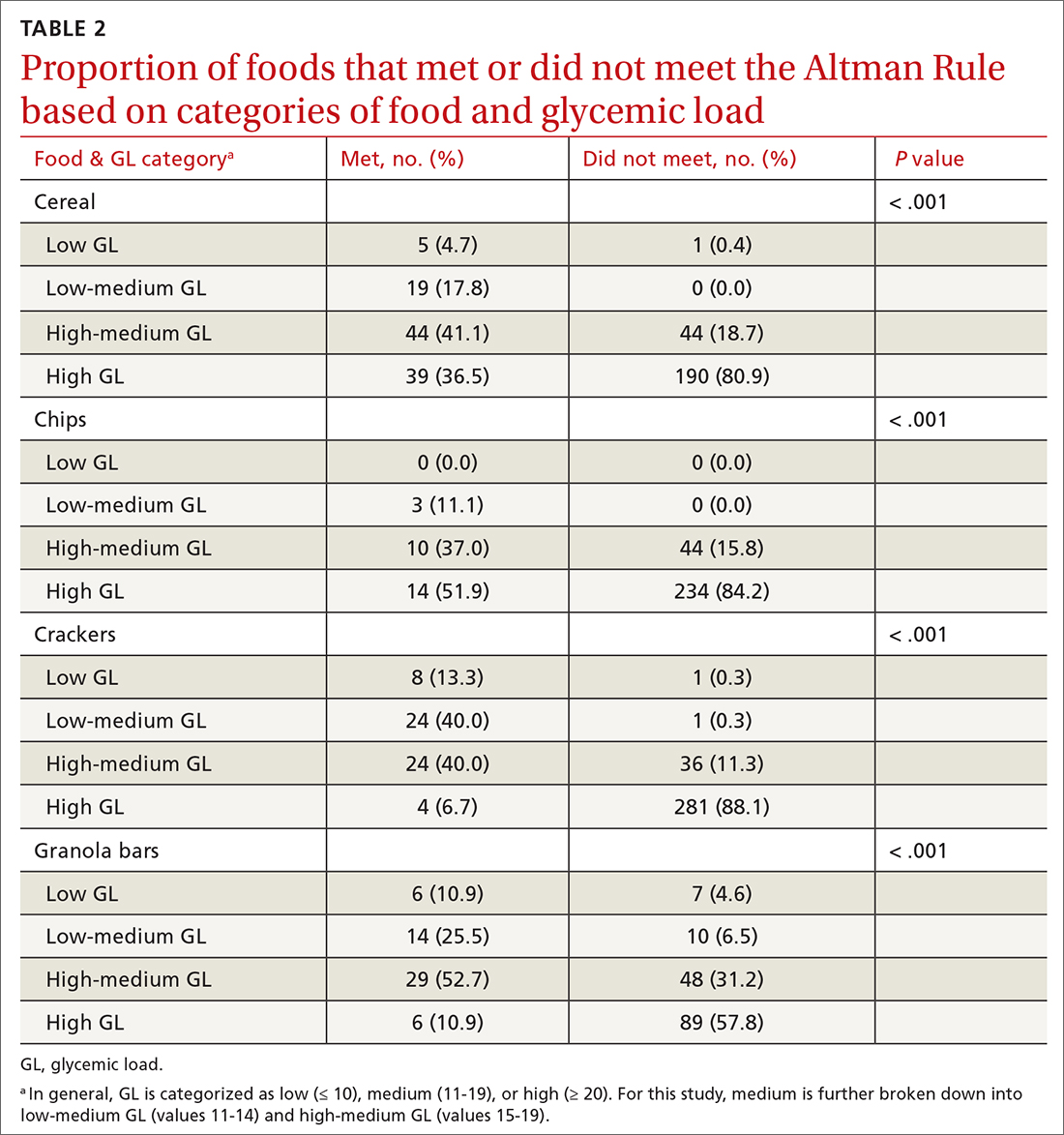
Foods that met the rule were more likely to be low GL and foods that did not pass the rule were more likely high GL. Within the medium-GL category, foods that met the rule were more likely to be low-medium GL.
Continue to: The findings within food categories...
The findings within food categories showed that very few cereals, chips, crackers, and granola bars were low GL. For every food category, except granola bars, far more low-GL foods met the Altman Rule than those that did not. At the same time, very few high-GL foods met the Altman Rule. The category with the most individual high-GL food items meeting the Altman Rule was cereal. This was also the subcategory with the largest percentage of high-GL food items meeting the Altman Rule. Thirty-nine cereals that were high GL met the rule, but more than 4 times as many high-GL cereals did not (n = 190).
DISCUSSION
Marketing and nutrition messaging create consumer confusion that makes it challenging to identify packaged food items that are more nutrient dense. The Altman Rule simplifies food choices that have become unnecessarily complex. Our findings suggest this 2-step rule is a reasonable proxy for the more complicated and less accessible GL for packaged carbohydrates, such as cereals, chips, crackers, and snack bars. Foods that meet the rule are likely low or low-medium GL and thus are foods that are likely to be healthier choices.
Of note, only 9% of chips (n = 27) passed the Altman Rule, likely due to their low dietary fiber content, which was typical of chips. If a food item does not have at least 3 grams of total dietary fiber per serving, it does not pass the Altman Rule, regardless of how much protein or sugar is in the product. This may be considered a strength or a weakness of the Altman Rule. Few nutrition-dense foods are low in fiber, but some foods could be nutritious but do not meet the Altman Rule due to having < 3 g of fiber.
With the high prevalence of chronic diseases such as hypertension, diabetes, hyperlipidemia, and cardiovascular disease, it is essential to help consumers prevent chronic disease altogether or manage their chronic disease by providing tools to identify healthier food choices. The tool also has a place in clinical medicine for use by physicians and other health care professionals. Research shows that physicians find both time and lack of knowledge/resources to be a barrier to providing nutritional counseling to patients.10 Since the Altman Rule can be shared and explained with very little time and without extensive nutritional knowledge, it meets these needs.
Limitations
Glycemic load. We acknowledge that the Altman Rule is not foolproof and that assessing this rule based on GL has some limitations. GL is not a perfect or comprehensive way to measure the nutritional value of a food. For example, fruits such as watermelon and grapes are nutritionally dense. However, they contain high amounts of natural sugars—and as such, their GL is relatively high, which could lead a consumer to perceive them as unhealthy. Nevertheless, GL is both a useful and accepted tool and a reasonable way to assess the validity of the rule, specifically when assessing packaged carbohydrates. The simplicity of the Altman Rule and its relationship with GL makes it such that consumers are more likely to make a healthier food choice using it.9
Continue to: Specificity and sensitivity
Specificity and sensitivity. There are other limitations to the Altman Rule, given that a small number of high-GL foods meet the rule. For example, some granola bars had high dietary protein, which offset a high sugar content just enough to pass the rule despite a higher GL. As such, concluding that a snack bar is a healthier choice because it meets the Altman Rule when it has high amounts of sugar may not be appropriate. This limitation could be considered a lack of specificity (the rule includes food it ought not to include). Another limitation to consider would be a lack of sensitivity, given that only 68% of low-GL foods passed the Altman Rule. Since GL is associated with carbohydrate content, foods with a low carbohydrate count often have little to no fiber and thus would fall into the category of foods that did not meet the Altman Rule but had low GL. In this case, however, the low amount of fiber may render the Altman Rule a better indicator of a healthier food choice than the GL.
Hidden sugars. Foods with sugar alcohols and artificial sweeteners may be as deleterious as caloric alternatives while not being accounted for when reporting the grams of sugar per serving on the nutrition label.7 This may represent an exception to the Altman Rule, as foods that are not healthier choices may pass the rule because the sugar content on the nutrition label is, in a sense, artificially lowered. Future research may investigate the hypothesis that these foods are nutritionally inferior despite meeting the Altman Rule.
The sample. Our study also was limited to working only with foods that were included in the NDSR database up to 2010. This limitation is mitigated by the fact that the sample size was large (> 1000 packaged food items were included in our analyses). The study also could be limited by the food categories that were analyzed; food categories such as bread, rice, pasta, and bagels were not included.
The objective of this research was to investigate the relationship between GL and the Altman Rule, rather than to conduct an exhaustive analysis of the Altman Rule for every possible food category. Studying the relationship between the Altman Rule and GL in other categories of food is an objective for future research. The data so far support a relationship between these entities. The likelihood of the nutrition facts of foods changing without the GL changing (or vice versa) is very low. As such, the Altman Rule still seems to be a reasonable proxy of GL.
CONCLUSIONS
Research indicates that point-of-sale tools, such as Guiding Stars, NuVal, and other stoplight tools, can successfully alter consumers’ behaviors.9 These tools can be helpful but are not available in many supermarkets. Despite the limitations, the Altman Rule is a useful decision aid that is accessible to all consumers no matter where they live or shop and is easy to use and remember.
The Altman rule can be used in clinical practice by health care professionals, such as physicians, nurse practitioners, physician assistants, dietitians, and health coaches. It also has the potential to be used in commercial settings, such as grocery stores, to help consumers easily identify healthier convenience foods. This has public health implications, as the rule can both empower consumers and potentially incentivize food manufacturers to upgrade their products nutritionally.
Additional research would be useful to evaluate consumers’ preferences and perceptions about how user-friendly the Altman Rule is at the point of sale with packaged carbohydrate foods. This would help to further understand how the use of information on food packaging can motivate healthier decisions—thereby helping to alleviate the burden of chronic disease.
CORRESPONDENCE
Kimberly R. Dong, DrPH, MS, RDN, Tufts University School of Medicine, Department of Public Health and Community Medicine, 136 Harrison Avenue, MV Building, Boston, MA 02111; [email protected]
1. Hersey JC, Wohlgenant KC, Arsenault JE, et al. Effects of front-of-package and shelf nutrition labeling systems on consumers. Nutr Rev. 2013;71:1-14. doi: 10.1111/nure.12000
2. Jenkins DJA, Dehghan M, Mente A, et al. Glycemic index, glycemic load, and cardiovascular disease and mortality. N Engl J Med. 2021;384:1312-1322. doi: 10.1056/NEJMoa2007123
3. Brand-Miller J, Hayne S, Petocz P, et al. Low–glycemic index diets in the management of diabetes. Diabetes Care. 2003;26:2261-2267. doi: 10.2337/diacare.26.8.2261
4. Matthan NR, Ausman LM, Meng H, et al. Estimating the reliability of glycemic index values and potential sources of methodological and biological variability. Am J Clin Nutr. 2016;104:1004-1013. doi: 10.3945/ajcn.116.137208
5. Sonnenberg L, Gelsomin E, Levy DE, et al. A traffic light food labeling intervention increases consumer awareness of health and healthy choices at the point-of-purchase. Prev Med. 2013;57:253-257. doi: 10.1016/j.ypmed.2013.07.001
6. Savoie N, Barlow K, Harvey KL, et al. Consumer perceptions of front-of-package labelling systems and healthiness of foods. Can J Public Health. 2013;104:e359-e363. doi: 10.17269/cjph.104.4027
7. Fischer LM, Sutherland LA, Kaley LA, et al. Development and implementation of the Guiding Stars nutrition guidance program. Am J Health Promot. 2011;26:e55-e63. doi: 10.4278/ajhp.100709-QUAL-238
8. Maubach N, Hoek J, Mather D. Interpretive front-of-pack nutrition labels. Comparing competing recommendations. Appetite. 2014;82:67-77. doi: 10.1016/j.appet.2014.07.006
9. Chan J, McMahon E, Brimblecombe J. Point‐of‐sale nutrition information interventions in food retail stores to promote healthier food purchase and intake: a systematic review. Obes Rev. 2021;22. doi: 10.1111/obr.13311
10. Mathioudakis N, Bashura H, Boyér L, et al. Development, implementation, and evaluation of a physician-targeted inpatient glycemic management curriculum. J Med Educ Curric Dev. 2019;6:238212051986134. doi: 10.1177/2382120519861342
ABSTRACT
Background: The Altman Rule, a simple tool for consumers seeking to make healthier packaged food choices at the point of sale, applies to packaged carbohydrates. According to the Altman Rule, a food is a healthier option if it has at least 3 g of fiber per serving and the grams of fiber plus the grams of protein exceed the grams of sugar per serving. This study sought to evaluate whether the Altman Rule is a valid proxy for glycemic load (GL).
Methods: We compared the binary outcome of whether a food item meets the Altman Rule with the GL of all foods categorized as cereals, chips, crackers, and granola bars in the Nutrition Data System for Research Database (University of Minnesota, Version 2010). We examined the percentage of foods in low-, medium-, and high-GL categories that met the Altman Rule.
Results: There were 1235 foods (342 cereals, 305 chips, 379 crackers, and 209 granola bars) in this analysis. There was a significant relationship between the GL of foods and the Altman Rule (P < .001) in that most low-GL (68%), almost half of medium-GL (48%), and very few high-GL (7%) foods met the criteria of the rule.
Conclusions: The Altman Rule is a reasonable proxy for GL and can be a useful and accessible tool for consumers interested in buying healthier packaged carbohydrate foods.
Nutrition can be complicated for consumers interested in making healthier choices at the grocery store. Consumers may have difficulty identifying more nutritious options, especially when food labels are adorned with claims such as “Good Source of Fiber” or “Heart Healthy.”1 In addition, when reading food labels, consumers may find it difficult to decipher which data to prioritize when carbohydrates, total sugars, added sugars, total dietary fiber, soluble fiber, and insoluble fiber are all listed.
The concept of glycemic load (GL) is an important consideration, especially for people with diabetes. GL approximates the blood sugar response to different foods. A food with a high GL is digested quickly, and its carbohydrates are taken into the bloodstream rapidly. This leads to a spike and subsequent drop in blood sugars, which can cause symptoms of hyperglycemia and hypoglycemia in a person with diabetes.2,3 Despite its usefulness, GL may be too complicated for a consumer to understand, and it does not appear anywhere on the food label. Since GL is calculated using pooled blood sugar response from individuals after the ingestion of the particular food, estimation of the GL is not intuitable.4
Point-of-sale tools. People seeking to lose weight, control diabetes, improve dyslipidemia and/or blood pressure, and/or decrease their risk for heart disease may benefit from point-of-sale tools such as the Altman Rule, which simplifies and encourages the selection of more nutritious foods.1 Other tools—such as Guiding Stars (https://guidingstars.com), NuVal (www.nuval.com), and different variations of traffic lights—have been created to help consumers make more informed and healthier food choices.5-8 However, Guiding Stars and NuVal are based on complicated algorithms that are not entirely transparent and not accessible to the average consumer.6,7 Evaluations of these nutrition tools indicate that consumers tend to underrate the healthiness of some foods, such as raw almonds and salmon, and overrate the healthiness of others, such as fruit punch and diet soda, when using traffic light systems.6 Furthermore, these nutrition tools are not available in many supermarkets. Previous research suggests that the use of point-of-sale nutrition apps decreases with the time and effort involved in using an app.9
Continue to: The Altman Rule
The Altman Rule was developed by a family physician (author WA) to provide a more accessible tool for people interested in choosing healthier prepackaged carbohydrate foods while shopping. Since the user does not need to have a smartphone, and they are not required to download or understand an app for each purchase, the Altman Rule may be more usable compared with more complicated alternatives.

The Altman Rule can be used with nutrition labels that feature serving information and calories in enlarged and bold type, in compliance with the most recent US Food and Drug Administration (FDA) guideline from 2016. Many foods with high fiber also have high amounts of sugar, so the criteria of the Altman Rule includes a 2-step process requiring (1) a minimum of 3 g of total dietary fiber per serving and (2) the sum of the grams of fiber plus the grams of protein per serving to be greater than the total grams of sugar (not grams of added sugar or grams of carbohydrate) per serving (FIGURE 1A). Unlike the relatively complicated formula related to GL, this 2-part rule can be applied in seconds while shopping (FIGURE 1B).

The rule is intended only to be used for packaged carbohydrate products, such as bread, muffins, bagels, pasta, rice, oatmeal, cereals, snack bars, chips, and crackers. It does not apply to whole foods, such as meat, dairy, fruits, or vegetables. These foods are excluded to prevent any consumer confusion related to the nutritional content of whole foods (eg, an apple may have more sugar than fiber and protein combined, but it is still a nutritious option).
This study aimed to determine if the Altman Rule is a reasonable proxy for the more complicated concept of GL. We calculated the relationship between the GL of commercially available packaged carbohydrate foods and whether those foods met the Altman Rule.
METHODS
The Altman Rule was tested by comparing the binary outcome of the rule (meets/does not meet) with data on all foods categorized as cereals, chips, crackers, and granola bars in the Nutrition Data System for Research (NDSR) Database (University of Minnesota, Version 2010).
Continue to: To account for differences...
To account for differences in serving size, we used the standard of 50 g for each product as 1 serving. We used 50 g (about 1.7 oz) to help compare the different foods and between foods within the same group. Additionally, 50 g is close to 1 serving for most foods in these groups; it is about the size of a typical granola bar, three-quarters to 2 cups of cereal, 10 to 12 crackers, and 15 to 25 chips. We determined the GL for each product by multiplying the number of available carbohydrates (total carbohydrate – dietary fiber) by the product’s glycemic index/100. In general, GL is categorized as low (≤ 10), medium (11-19), or high (≥ 20).
We applied the Altman Rule to categorize each product as meeting or not meeting the rule. We compared the proportion of foods meeting the Altman Rule, stratified by GL and by specific foods, and used chi-square to determine if differences were statistically significant. These data were collected and analyzed in the summer of 2019.
RESULTS
There were 1235 foods (342 breakfast cereals, 305 chips, 379 crackers, and 209 granola bars) used for this analysis. There is a significant relationship between the GL of foods and the Altman Rule in that most low-GL (68%), almost half of medium-GL (48%), and only a few high-GL foods (7%) met the rule (P < .001) (TABLE 1). There was also a significant relationship between “meeting the Altman Rule” and GL within each food type (P < .001) (TABLE 2).

The medium-GL foods were the second largest category of foods we calculated; thus we further broke them into binary categories of

Foods that met the rule were more likely to be low GL and foods that did not pass the rule were more likely high GL. Within the medium-GL category, foods that met the rule were more likely to be low-medium GL.
Continue to: The findings within food categories...
The findings within food categories showed that very few cereals, chips, crackers, and granola bars were low GL. For every food category, except granola bars, far more low-GL foods met the Altman Rule than those that did not. At the same time, very few high-GL foods met the Altman Rule. The category with the most individual high-GL food items meeting the Altman Rule was cereal. This was also the subcategory with the largest percentage of high-GL food items meeting the Altman Rule. Thirty-nine cereals that were high GL met the rule, but more than 4 times as many high-GL cereals did not (n = 190).
DISCUSSION
Marketing and nutrition messaging create consumer confusion that makes it challenging to identify packaged food items that are more nutrient dense. The Altman Rule simplifies food choices that have become unnecessarily complex. Our findings suggest this 2-step rule is a reasonable proxy for the more complicated and less accessible GL for packaged carbohydrates, such as cereals, chips, crackers, and snack bars. Foods that meet the rule are likely low or low-medium GL and thus are foods that are likely to be healthier choices.
Of note, only 9% of chips (n = 27) passed the Altman Rule, likely due to their low dietary fiber content, which was typical of chips. If a food item does not have at least 3 grams of total dietary fiber per serving, it does not pass the Altman Rule, regardless of how much protein or sugar is in the product. This may be considered a strength or a weakness of the Altman Rule. Few nutrition-dense foods are low in fiber, but some foods could be nutritious but do not meet the Altman Rule due to having < 3 g of fiber.
With the high prevalence of chronic diseases such as hypertension, diabetes, hyperlipidemia, and cardiovascular disease, it is essential to help consumers prevent chronic disease altogether or manage their chronic disease by providing tools to identify healthier food choices. The tool also has a place in clinical medicine for use by physicians and other health care professionals. Research shows that physicians find both time and lack of knowledge/resources to be a barrier to providing nutritional counseling to patients.10 Since the Altman Rule can be shared and explained with very little time and without extensive nutritional knowledge, it meets these needs.
Limitations
Glycemic load. We acknowledge that the Altman Rule is not foolproof and that assessing this rule based on GL has some limitations. GL is not a perfect or comprehensive way to measure the nutritional value of a food. For example, fruits such as watermelon and grapes are nutritionally dense. However, they contain high amounts of natural sugars—and as such, their GL is relatively high, which could lead a consumer to perceive them as unhealthy. Nevertheless, GL is both a useful and accepted tool and a reasonable way to assess the validity of the rule, specifically when assessing packaged carbohydrates. The simplicity of the Altman Rule and its relationship with GL makes it such that consumers are more likely to make a healthier food choice using it.9
Continue to: Specificity and sensitivity
Specificity and sensitivity. There are other limitations to the Altman Rule, given that a small number of high-GL foods meet the rule. For example, some granola bars had high dietary protein, which offset a high sugar content just enough to pass the rule despite a higher GL. As such, concluding that a snack bar is a healthier choice because it meets the Altman Rule when it has high amounts of sugar may not be appropriate. This limitation could be considered a lack of specificity (the rule includes food it ought not to include). Another limitation to consider would be a lack of sensitivity, given that only 68% of low-GL foods passed the Altman Rule. Since GL is associated with carbohydrate content, foods with a low carbohydrate count often have little to no fiber and thus would fall into the category of foods that did not meet the Altman Rule but had low GL. In this case, however, the low amount of fiber may render the Altman Rule a better indicator of a healthier food choice than the GL.
Hidden sugars. Foods with sugar alcohols and artificial sweeteners may be as deleterious as caloric alternatives while not being accounted for when reporting the grams of sugar per serving on the nutrition label.7 This may represent an exception to the Altman Rule, as foods that are not healthier choices may pass the rule because the sugar content on the nutrition label is, in a sense, artificially lowered. Future research may investigate the hypothesis that these foods are nutritionally inferior despite meeting the Altman Rule.
The sample. Our study also was limited to working only with foods that were included in the NDSR database up to 2010. This limitation is mitigated by the fact that the sample size was large (> 1000 packaged food items were included in our analyses). The study also could be limited by the food categories that were analyzed; food categories such as bread, rice, pasta, and bagels were not included.
The objective of this research was to investigate the relationship between GL and the Altman Rule, rather than to conduct an exhaustive analysis of the Altman Rule for every possible food category. Studying the relationship between the Altman Rule and GL in other categories of food is an objective for future research. The data so far support a relationship between these entities. The likelihood of the nutrition facts of foods changing without the GL changing (or vice versa) is very low. As such, the Altman Rule still seems to be a reasonable proxy of GL.
CONCLUSIONS
Research indicates that point-of-sale tools, such as Guiding Stars, NuVal, and other stoplight tools, can successfully alter consumers’ behaviors.9 These tools can be helpful but are not available in many supermarkets. Despite the limitations, the Altman Rule is a useful decision aid that is accessible to all consumers no matter where they live or shop and is easy to use and remember.
The Altman rule can be used in clinical practice by health care professionals, such as physicians, nurse practitioners, physician assistants, dietitians, and health coaches. It also has the potential to be used in commercial settings, such as grocery stores, to help consumers easily identify healthier convenience foods. This has public health implications, as the rule can both empower consumers and potentially incentivize food manufacturers to upgrade their products nutritionally.
Additional research would be useful to evaluate consumers’ preferences and perceptions about how user-friendly the Altman Rule is at the point of sale with packaged carbohydrate foods. This would help to further understand how the use of information on food packaging can motivate healthier decisions—thereby helping to alleviate the burden of chronic disease.
CORRESPONDENCE
Kimberly R. Dong, DrPH, MS, RDN, Tufts University School of Medicine, Department of Public Health and Community Medicine, 136 Harrison Avenue, MV Building, Boston, MA 02111; [email protected]
ABSTRACT
Background: The Altman Rule, a simple tool for consumers seeking to make healthier packaged food choices at the point of sale, applies to packaged carbohydrates. According to the Altman Rule, a food is a healthier option if it has at least 3 g of fiber per serving and the grams of fiber plus the grams of protein exceed the grams of sugar per serving. This study sought to evaluate whether the Altman Rule is a valid proxy for glycemic load (GL).
Methods: We compared the binary outcome of whether a food item meets the Altman Rule with the GL of all foods categorized as cereals, chips, crackers, and granola bars in the Nutrition Data System for Research Database (University of Minnesota, Version 2010). We examined the percentage of foods in low-, medium-, and high-GL categories that met the Altman Rule.
Results: There were 1235 foods (342 cereals, 305 chips, 379 crackers, and 209 granola bars) in this analysis. There was a significant relationship between the GL of foods and the Altman Rule (P < .001) in that most low-GL (68%), almost half of medium-GL (48%), and very few high-GL (7%) foods met the criteria of the rule.
Conclusions: The Altman Rule is a reasonable proxy for GL and can be a useful and accessible tool for consumers interested in buying healthier packaged carbohydrate foods.
Nutrition can be complicated for consumers interested in making healthier choices at the grocery store. Consumers may have difficulty identifying more nutritious options, especially when food labels are adorned with claims such as “Good Source of Fiber” or “Heart Healthy.”1 In addition, when reading food labels, consumers may find it difficult to decipher which data to prioritize when carbohydrates, total sugars, added sugars, total dietary fiber, soluble fiber, and insoluble fiber are all listed.
The concept of glycemic load (GL) is an important consideration, especially for people with diabetes. GL approximates the blood sugar response to different foods. A food with a high GL is digested quickly, and its carbohydrates are taken into the bloodstream rapidly. This leads to a spike and subsequent drop in blood sugars, which can cause symptoms of hyperglycemia and hypoglycemia in a person with diabetes.2,3 Despite its usefulness, GL may be too complicated for a consumer to understand, and it does not appear anywhere on the food label. Since GL is calculated using pooled blood sugar response from individuals after the ingestion of the particular food, estimation of the GL is not intuitable.4
Point-of-sale tools. People seeking to lose weight, control diabetes, improve dyslipidemia and/or blood pressure, and/or decrease their risk for heart disease may benefit from point-of-sale tools such as the Altman Rule, which simplifies and encourages the selection of more nutritious foods.1 Other tools—such as Guiding Stars (https://guidingstars.com), NuVal (www.nuval.com), and different variations of traffic lights—have been created to help consumers make more informed and healthier food choices.5-8 However, Guiding Stars and NuVal are based on complicated algorithms that are not entirely transparent and not accessible to the average consumer.6,7 Evaluations of these nutrition tools indicate that consumers tend to underrate the healthiness of some foods, such as raw almonds and salmon, and overrate the healthiness of others, such as fruit punch and diet soda, when using traffic light systems.6 Furthermore, these nutrition tools are not available in many supermarkets. Previous research suggests that the use of point-of-sale nutrition apps decreases with the time and effort involved in using an app.9
Continue to: The Altman Rule
The Altman Rule was developed by a family physician (author WA) to provide a more accessible tool for people interested in choosing healthier prepackaged carbohydrate foods while shopping. Since the user does not need to have a smartphone, and they are not required to download or understand an app for each purchase, the Altman Rule may be more usable compared with more complicated alternatives.

The Altman Rule can be used with nutrition labels that feature serving information and calories in enlarged and bold type, in compliance with the most recent US Food and Drug Administration (FDA) guideline from 2016. Many foods with high fiber also have high amounts of sugar, so the criteria of the Altman Rule includes a 2-step process requiring (1) a minimum of 3 g of total dietary fiber per serving and (2) the sum of the grams of fiber plus the grams of protein per serving to be greater than the total grams of sugar (not grams of added sugar or grams of carbohydrate) per serving (FIGURE 1A). Unlike the relatively complicated formula related to GL, this 2-part rule can be applied in seconds while shopping (FIGURE 1B).

The rule is intended only to be used for packaged carbohydrate products, such as bread, muffins, bagels, pasta, rice, oatmeal, cereals, snack bars, chips, and crackers. It does not apply to whole foods, such as meat, dairy, fruits, or vegetables. These foods are excluded to prevent any consumer confusion related to the nutritional content of whole foods (eg, an apple may have more sugar than fiber and protein combined, but it is still a nutritious option).
This study aimed to determine if the Altman Rule is a reasonable proxy for the more complicated concept of GL. We calculated the relationship between the GL of commercially available packaged carbohydrate foods and whether those foods met the Altman Rule.
METHODS
The Altman Rule was tested by comparing the binary outcome of the rule (meets/does not meet) with data on all foods categorized as cereals, chips, crackers, and granola bars in the Nutrition Data System for Research (NDSR) Database (University of Minnesota, Version 2010).
Continue to: To account for differences...
To account for differences in serving size, we used the standard of 50 g for each product as 1 serving. We used 50 g (about 1.7 oz) to help compare the different foods and between foods within the same group. Additionally, 50 g is close to 1 serving for most foods in these groups; it is about the size of a typical granola bar, three-quarters to 2 cups of cereal, 10 to 12 crackers, and 15 to 25 chips. We determined the GL for each product by multiplying the number of available carbohydrates (total carbohydrate – dietary fiber) by the product’s glycemic index/100. In general, GL is categorized as low (≤ 10), medium (11-19), or high (≥ 20).
We applied the Altman Rule to categorize each product as meeting or not meeting the rule. We compared the proportion of foods meeting the Altman Rule, stratified by GL and by specific foods, and used chi-square to determine if differences were statistically significant. These data were collected and analyzed in the summer of 2019.
RESULTS
There were 1235 foods (342 breakfast cereals, 305 chips, 379 crackers, and 209 granola bars) used for this analysis. There is a significant relationship between the GL of foods and the Altman Rule in that most low-GL (68%), almost half of medium-GL (48%), and only a few high-GL foods (7%) met the rule (P < .001) (TABLE 1). There was also a significant relationship between “meeting the Altman Rule” and GL within each food type (P < .001) (TABLE 2).

The medium-GL foods were the second largest category of foods we calculated; thus we further broke them into binary categories of

Foods that met the rule were more likely to be low GL and foods that did not pass the rule were more likely high GL. Within the medium-GL category, foods that met the rule were more likely to be low-medium GL.
Continue to: The findings within food categories...
The findings within food categories showed that very few cereals, chips, crackers, and granola bars were low GL. For every food category, except granola bars, far more low-GL foods met the Altman Rule than those that did not. At the same time, very few high-GL foods met the Altman Rule. The category with the most individual high-GL food items meeting the Altman Rule was cereal. This was also the subcategory with the largest percentage of high-GL food items meeting the Altman Rule. Thirty-nine cereals that were high GL met the rule, but more than 4 times as many high-GL cereals did not (n = 190).
DISCUSSION
Marketing and nutrition messaging create consumer confusion that makes it challenging to identify packaged food items that are more nutrient dense. The Altman Rule simplifies food choices that have become unnecessarily complex. Our findings suggest this 2-step rule is a reasonable proxy for the more complicated and less accessible GL for packaged carbohydrates, such as cereals, chips, crackers, and snack bars. Foods that meet the rule are likely low or low-medium GL and thus are foods that are likely to be healthier choices.
Of note, only 9% of chips (n = 27) passed the Altman Rule, likely due to their low dietary fiber content, which was typical of chips. If a food item does not have at least 3 grams of total dietary fiber per serving, it does not pass the Altman Rule, regardless of how much protein or sugar is in the product. This may be considered a strength or a weakness of the Altman Rule. Few nutrition-dense foods are low in fiber, but some foods could be nutritious but do not meet the Altman Rule due to having < 3 g of fiber.
With the high prevalence of chronic diseases such as hypertension, diabetes, hyperlipidemia, and cardiovascular disease, it is essential to help consumers prevent chronic disease altogether or manage their chronic disease by providing tools to identify healthier food choices. The tool also has a place in clinical medicine for use by physicians and other health care professionals. Research shows that physicians find both time and lack of knowledge/resources to be a barrier to providing nutritional counseling to patients.10 Since the Altman Rule can be shared and explained with very little time and without extensive nutritional knowledge, it meets these needs.
Limitations
Glycemic load. We acknowledge that the Altman Rule is not foolproof and that assessing this rule based on GL has some limitations. GL is not a perfect or comprehensive way to measure the nutritional value of a food. For example, fruits such as watermelon and grapes are nutritionally dense. However, they contain high amounts of natural sugars—and as such, their GL is relatively high, which could lead a consumer to perceive them as unhealthy. Nevertheless, GL is both a useful and accepted tool and a reasonable way to assess the validity of the rule, specifically when assessing packaged carbohydrates. The simplicity of the Altman Rule and its relationship with GL makes it such that consumers are more likely to make a healthier food choice using it.9
Continue to: Specificity and sensitivity
Specificity and sensitivity. There are other limitations to the Altman Rule, given that a small number of high-GL foods meet the rule. For example, some granola bars had high dietary protein, which offset a high sugar content just enough to pass the rule despite a higher GL. As such, concluding that a snack bar is a healthier choice because it meets the Altman Rule when it has high amounts of sugar may not be appropriate. This limitation could be considered a lack of specificity (the rule includes food it ought not to include). Another limitation to consider would be a lack of sensitivity, given that only 68% of low-GL foods passed the Altman Rule. Since GL is associated with carbohydrate content, foods with a low carbohydrate count often have little to no fiber and thus would fall into the category of foods that did not meet the Altman Rule but had low GL. In this case, however, the low amount of fiber may render the Altman Rule a better indicator of a healthier food choice than the GL.
Hidden sugars. Foods with sugar alcohols and artificial sweeteners may be as deleterious as caloric alternatives while not being accounted for when reporting the grams of sugar per serving on the nutrition label.7 This may represent an exception to the Altman Rule, as foods that are not healthier choices may pass the rule because the sugar content on the nutrition label is, in a sense, artificially lowered. Future research may investigate the hypothesis that these foods are nutritionally inferior despite meeting the Altman Rule.
The sample. Our study also was limited to working only with foods that were included in the NDSR database up to 2010. This limitation is mitigated by the fact that the sample size was large (> 1000 packaged food items were included in our analyses). The study also could be limited by the food categories that were analyzed; food categories such as bread, rice, pasta, and bagels were not included.
The objective of this research was to investigate the relationship between GL and the Altman Rule, rather than to conduct an exhaustive analysis of the Altman Rule for every possible food category. Studying the relationship between the Altman Rule and GL in other categories of food is an objective for future research. The data so far support a relationship between these entities. The likelihood of the nutrition facts of foods changing without the GL changing (or vice versa) is very low. As such, the Altman Rule still seems to be a reasonable proxy of GL.
CONCLUSIONS
Research indicates that point-of-sale tools, such as Guiding Stars, NuVal, and other stoplight tools, can successfully alter consumers’ behaviors.9 These tools can be helpful but are not available in many supermarkets. Despite the limitations, the Altman Rule is a useful decision aid that is accessible to all consumers no matter where they live or shop and is easy to use and remember.
The Altman rule can be used in clinical practice by health care professionals, such as physicians, nurse practitioners, physician assistants, dietitians, and health coaches. It also has the potential to be used in commercial settings, such as grocery stores, to help consumers easily identify healthier convenience foods. This has public health implications, as the rule can both empower consumers and potentially incentivize food manufacturers to upgrade their products nutritionally.
Additional research would be useful to evaluate consumers’ preferences and perceptions about how user-friendly the Altman Rule is at the point of sale with packaged carbohydrate foods. This would help to further understand how the use of information on food packaging can motivate healthier decisions—thereby helping to alleviate the burden of chronic disease.
CORRESPONDENCE
Kimberly R. Dong, DrPH, MS, RDN, Tufts University School of Medicine, Department of Public Health and Community Medicine, 136 Harrison Avenue, MV Building, Boston, MA 02111; [email protected]
1. Hersey JC, Wohlgenant KC, Arsenault JE, et al. Effects of front-of-package and shelf nutrition labeling systems on consumers. Nutr Rev. 2013;71:1-14. doi: 10.1111/nure.12000
2. Jenkins DJA, Dehghan M, Mente A, et al. Glycemic index, glycemic load, and cardiovascular disease and mortality. N Engl J Med. 2021;384:1312-1322. doi: 10.1056/NEJMoa2007123
3. Brand-Miller J, Hayne S, Petocz P, et al. Low–glycemic index diets in the management of diabetes. Diabetes Care. 2003;26:2261-2267. doi: 10.2337/diacare.26.8.2261
4. Matthan NR, Ausman LM, Meng H, et al. Estimating the reliability of glycemic index values and potential sources of methodological and biological variability. Am J Clin Nutr. 2016;104:1004-1013. doi: 10.3945/ajcn.116.137208
5. Sonnenberg L, Gelsomin E, Levy DE, et al. A traffic light food labeling intervention increases consumer awareness of health and healthy choices at the point-of-purchase. Prev Med. 2013;57:253-257. doi: 10.1016/j.ypmed.2013.07.001
6. Savoie N, Barlow K, Harvey KL, et al. Consumer perceptions of front-of-package labelling systems and healthiness of foods. Can J Public Health. 2013;104:e359-e363. doi: 10.17269/cjph.104.4027
7. Fischer LM, Sutherland LA, Kaley LA, et al. Development and implementation of the Guiding Stars nutrition guidance program. Am J Health Promot. 2011;26:e55-e63. doi: 10.4278/ajhp.100709-QUAL-238
8. Maubach N, Hoek J, Mather D. Interpretive front-of-pack nutrition labels. Comparing competing recommendations. Appetite. 2014;82:67-77. doi: 10.1016/j.appet.2014.07.006
9. Chan J, McMahon E, Brimblecombe J. Point‐of‐sale nutrition information interventions in food retail stores to promote healthier food purchase and intake: a systematic review. Obes Rev. 2021;22. doi: 10.1111/obr.13311
10. Mathioudakis N, Bashura H, Boyér L, et al. Development, implementation, and evaluation of a physician-targeted inpatient glycemic management curriculum. J Med Educ Curric Dev. 2019;6:238212051986134. doi: 10.1177/2382120519861342
1. Hersey JC, Wohlgenant KC, Arsenault JE, et al. Effects of front-of-package and shelf nutrition labeling systems on consumers. Nutr Rev. 2013;71:1-14. doi: 10.1111/nure.12000
2. Jenkins DJA, Dehghan M, Mente A, et al. Glycemic index, glycemic load, and cardiovascular disease and mortality. N Engl J Med. 2021;384:1312-1322. doi: 10.1056/NEJMoa2007123
3. Brand-Miller J, Hayne S, Petocz P, et al. Low–glycemic index diets in the management of diabetes. Diabetes Care. 2003;26:2261-2267. doi: 10.2337/diacare.26.8.2261
4. Matthan NR, Ausman LM, Meng H, et al. Estimating the reliability of glycemic index values and potential sources of methodological and biological variability. Am J Clin Nutr. 2016;104:1004-1013. doi: 10.3945/ajcn.116.137208
5. Sonnenberg L, Gelsomin E, Levy DE, et al. A traffic light food labeling intervention increases consumer awareness of health and healthy choices at the point-of-purchase. Prev Med. 2013;57:253-257. doi: 10.1016/j.ypmed.2013.07.001
6. Savoie N, Barlow K, Harvey KL, et al. Consumer perceptions of front-of-package labelling systems and healthiness of foods. Can J Public Health. 2013;104:e359-e363. doi: 10.17269/cjph.104.4027
7. Fischer LM, Sutherland LA, Kaley LA, et al. Development and implementation of the Guiding Stars nutrition guidance program. Am J Health Promot. 2011;26:e55-e63. doi: 10.4278/ajhp.100709-QUAL-238
8. Maubach N, Hoek J, Mather D. Interpretive front-of-pack nutrition labels. Comparing competing recommendations. Appetite. 2014;82:67-77. doi: 10.1016/j.appet.2014.07.006
9. Chan J, McMahon E, Brimblecombe J. Point‐of‐sale nutrition information interventions in food retail stores to promote healthier food purchase and intake: a systematic review. Obes Rev. 2021;22. doi: 10.1111/obr.13311
10. Mathioudakis N, Bashura H, Boyér L, et al. Development, implementation, and evaluation of a physician-targeted inpatient glycemic management curriculum. J Med Educ Curric Dev. 2019;6:238212051986134. doi: 10.1177/2382120519861342