User login
Tofacitinib can be considered as a treatment option for PsA with enthesitis
Key clinical point: Compared with placebo, tofacitinib demonstrated greater improvement in enthesitis in patients with psoriatic arthritis (PsA), irrespective of enthesitis location and severity.
Major finding: Tofacitinib vs placebo led to greater changes in the Leeds Enthesitis Index (LEI) and Spondyloarthritis Research Consortium of Canada Enthesitis Index scores up to month 3, irrespective of baseline enthesitis locations and severities, with all improvements with tofacitinib being maintained and continued through month 6. Among patients with baseline LEI >0 whose enthesitis had resolved at month 1, relapse at month 3 was experienced by 26.3% and 15.6% vs 30.8% of patients treated with 5 mg tofacitinib and 10 mg tofacitinib vs placebo, respectively.
Study details: This post hoc analysis of pooled data from 2 phase 3 studies (OPAL Broaden and OPAL Beyond) and included 710 patients with PsA who received tofacitinib for 6 months or placebo for 3 months.
Disclosures: This study was sponsored by Pfizer Inc. Three authors declared being employees and shareholders of Pfizer Inc. Five authors declared receiving grants, research support, or consulting fees from or having ties with various sources, including Pfizer.
Source: Mease PJ et al. Efficacy of tofacitinib on enthesitis in patients with active psoriatic arthritis: Analysis of pooled data from two phase 3 studies. Arthritis Res Ther. 2023;25:153 (Aug 22). doi: 10.1186/s13075-023-03108-5
Key clinical point: Compared with placebo, tofacitinib demonstrated greater improvement in enthesitis in patients with psoriatic arthritis (PsA), irrespective of enthesitis location and severity.
Major finding: Tofacitinib vs placebo led to greater changes in the Leeds Enthesitis Index (LEI) and Spondyloarthritis Research Consortium of Canada Enthesitis Index scores up to month 3, irrespective of baseline enthesitis locations and severities, with all improvements with tofacitinib being maintained and continued through month 6. Among patients with baseline LEI >0 whose enthesitis had resolved at month 1, relapse at month 3 was experienced by 26.3% and 15.6% vs 30.8% of patients treated with 5 mg tofacitinib and 10 mg tofacitinib vs placebo, respectively.
Study details: This post hoc analysis of pooled data from 2 phase 3 studies (OPAL Broaden and OPAL Beyond) and included 710 patients with PsA who received tofacitinib for 6 months or placebo for 3 months.
Disclosures: This study was sponsored by Pfizer Inc. Three authors declared being employees and shareholders of Pfizer Inc. Five authors declared receiving grants, research support, or consulting fees from or having ties with various sources, including Pfizer.
Source: Mease PJ et al. Efficacy of tofacitinib on enthesitis in patients with active psoriatic arthritis: Analysis of pooled data from two phase 3 studies. Arthritis Res Ther. 2023;25:153 (Aug 22). doi: 10.1186/s13075-023-03108-5
Key clinical point: Compared with placebo, tofacitinib demonstrated greater improvement in enthesitis in patients with psoriatic arthritis (PsA), irrespective of enthesitis location and severity.
Major finding: Tofacitinib vs placebo led to greater changes in the Leeds Enthesitis Index (LEI) and Spondyloarthritis Research Consortium of Canada Enthesitis Index scores up to month 3, irrespective of baseline enthesitis locations and severities, with all improvements with tofacitinib being maintained and continued through month 6. Among patients with baseline LEI >0 whose enthesitis had resolved at month 1, relapse at month 3 was experienced by 26.3% and 15.6% vs 30.8% of patients treated with 5 mg tofacitinib and 10 mg tofacitinib vs placebo, respectively.
Study details: This post hoc analysis of pooled data from 2 phase 3 studies (OPAL Broaden and OPAL Beyond) and included 710 patients with PsA who received tofacitinib for 6 months or placebo for 3 months.
Disclosures: This study was sponsored by Pfizer Inc. Three authors declared being employees and shareholders of Pfizer Inc. Five authors declared receiving grants, research support, or consulting fees from or having ties with various sources, including Pfizer.
Source: Mease PJ et al. Efficacy of tofacitinib on enthesitis in patients with active psoriatic arthritis: Analysis of pooled data from two phase 3 studies. Arthritis Res Ther. 2023;25:153 (Aug 22). doi: 10.1186/s13075-023-03108-5
Secukinumab offers sustained improvement in synovitis and enthesitis in active PsA
Key clinical point: Patients with active psoriatic arthritis (PsA) who initiated secukinumab treatment had stable improvements in synovitis and sustained clinical improvements in enthesitis up to week 52.
Major finding: At week 12, secukinumab vs placebo led to significant improvements in power Doppler ultrasound (PDUS)-assessed synovitis (Global EULAR-OMERACT Synovitis Score: −9 vs −6; one-sided P = .004) and PDUS-assessed enthesitis (Spondyloarthritis Research Consortium of Canada enthesitis index score: −2.2 vs −1.6; one-sided P = .03), with the improvements being sustained up to week 52.
Study details: This 52-week, phase 3 ULTIMATE study included 166 patients with active PsA, who were naive to biologics and intolerant to conventional synthetic disease-modifying anti-rheumatic drugs and were randomly assigned to receive secukinumab or placebo.
Disclosures: This study was funded by Novartis Pharma AG, Basel, Switzerland. Three authors declared being employees or stockholders of Novartis. Several authors declared ties with various sources, including Novartis. Three authors declared no conflicts of interest.
Source: D’Agostino MA et al. Effects of secukinumab on synovitis and enthesitis in patients with psoriatic arthritis: 52-week clinical and ultrasound results from the randomised, double-blind ULTIMATE trial with open label extension. Semin Arthritis Rheum. 2023;63:152259 (Aug 19). doi: 10.1016/j.semarthrit.2023.152259
Key clinical point: Patients with active psoriatic arthritis (PsA) who initiated secukinumab treatment had stable improvements in synovitis and sustained clinical improvements in enthesitis up to week 52.
Major finding: At week 12, secukinumab vs placebo led to significant improvements in power Doppler ultrasound (PDUS)-assessed synovitis (Global EULAR-OMERACT Synovitis Score: −9 vs −6; one-sided P = .004) and PDUS-assessed enthesitis (Spondyloarthritis Research Consortium of Canada enthesitis index score: −2.2 vs −1.6; one-sided P = .03), with the improvements being sustained up to week 52.
Study details: This 52-week, phase 3 ULTIMATE study included 166 patients with active PsA, who were naive to biologics and intolerant to conventional synthetic disease-modifying anti-rheumatic drugs and were randomly assigned to receive secukinumab or placebo.
Disclosures: This study was funded by Novartis Pharma AG, Basel, Switzerland. Three authors declared being employees or stockholders of Novartis. Several authors declared ties with various sources, including Novartis. Three authors declared no conflicts of interest.
Source: D’Agostino MA et al. Effects of secukinumab on synovitis and enthesitis in patients with psoriatic arthritis: 52-week clinical and ultrasound results from the randomised, double-blind ULTIMATE trial with open label extension. Semin Arthritis Rheum. 2023;63:152259 (Aug 19). doi: 10.1016/j.semarthrit.2023.152259
Key clinical point: Patients with active psoriatic arthritis (PsA) who initiated secukinumab treatment had stable improvements in synovitis and sustained clinical improvements in enthesitis up to week 52.
Major finding: At week 12, secukinumab vs placebo led to significant improvements in power Doppler ultrasound (PDUS)-assessed synovitis (Global EULAR-OMERACT Synovitis Score: −9 vs −6; one-sided P = .004) and PDUS-assessed enthesitis (Spondyloarthritis Research Consortium of Canada enthesitis index score: −2.2 vs −1.6; one-sided P = .03), with the improvements being sustained up to week 52.
Study details: This 52-week, phase 3 ULTIMATE study included 166 patients with active PsA, who were naive to biologics and intolerant to conventional synthetic disease-modifying anti-rheumatic drugs and were randomly assigned to receive secukinumab or placebo.
Disclosures: This study was funded by Novartis Pharma AG, Basel, Switzerland. Three authors declared being employees or stockholders of Novartis. Several authors declared ties with various sources, including Novartis. Three authors declared no conflicts of interest.
Source: D’Agostino MA et al. Effects of secukinumab on synovitis and enthesitis in patients with psoriatic arthritis: 52-week clinical and ultrasound results from the randomised, double-blind ULTIMATE trial with open label extension. Semin Arthritis Rheum. 2023;63:152259 (Aug 19). doi: 10.1016/j.semarthrit.2023.152259
Real-world study confirms high retention rates and favorable safety of secukinumab in active PsA
Key clinical point: Secukinumab demonstrated sustained efficacy, high retention rates, and a consistent safety profile in patients with active psoriatic arthritis (PsA) who were followed for ≥ 2 years.
Major finding: The treatment retention rate with secukinumab was 78.2% in PsA. The mean swollen joint counts (enrolment vs 2 years: 4.5 vs 3.6) and tender joint counts (enrolment vs 2 years: 12.8 vs 9.2) remained stable over 2 years of treatment. Serious adverse events occurred in 13.6% of patients, but no deaths related to treatment-emergent adverse events were reported.
Study details: Findings are from an interim analysis of the ongoing SERENA study including patients with active PsA (n = 81) or radiographic axial spondyloarthritis (n = 108) who had received secukinumab for ≥16 weeks prior to enrolment.
Disclosures: This study was supported by Novartis Pharma AG. Two authors declared being employees of Novartis Pharmaceuticals U.K. Ltd. Three authors declared ties with various sources, including Novartis.
Source: Gaffney K et al. Real-world evidence for secukinumab in UK patients with psoriatic arthritis or radiographic axial spondyloarthritis: Interim 2-year analysis from SERENA. Rheumatol Adv Pract. 2023;7(3):rkad055 (Aug 21). doi: 10.1093/rap/rkad055
Key clinical point: Secukinumab demonstrated sustained efficacy, high retention rates, and a consistent safety profile in patients with active psoriatic arthritis (PsA) who were followed for ≥ 2 years.
Major finding: The treatment retention rate with secukinumab was 78.2% in PsA. The mean swollen joint counts (enrolment vs 2 years: 4.5 vs 3.6) and tender joint counts (enrolment vs 2 years: 12.8 vs 9.2) remained stable over 2 years of treatment. Serious adverse events occurred in 13.6% of patients, but no deaths related to treatment-emergent adverse events were reported.
Study details: Findings are from an interim analysis of the ongoing SERENA study including patients with active PsA (n = 81) or radiographic axial spondyloarthritis (n = 108) who had received secukinumab for ≥16 weeks prior to enrolment.
Disclosures: This study was supported by Novartis Pharma AG. Two authors declared being employees of Novartis Pharmaceuticals U.K. Ltd. Three authors declared ties with various sources, including Novartis.
Source: Gaffney K et al. Real-world evidence for secukinumab in UK patients with psoriatic arthritis or radiographic axial spondyloarthritis: Interim 2-year analysis from SERENA. Rheumatol Adv Pract. 2023;7(3):rkad055 (Aug 21). doi: 10.1093/rap/rkad055
Key clinical point: Secukinumab demonstrated sustained efficacy, high retention rates, and a consistent safety profile in patients with active psoriatic arthritis (PsA) who were followed for ≥ 2 years.
Major finding: The treatment retention rate with secukinumab was 78.2% in PsA. The mean swollen joint counts (enrolment vs 2 years: 4.5 vs 3.6) and tender joint counts (enrolment vs 2 years: 12.8 vs 9.2) remained stable over 2 years of treatment. Serious adverse events occurred in 13.6% of patients, but no deaths related to treatment-emergent adverse events were reported.
Study details: Findings are from an interim analysis of the ongoing SERENA study including patients with active PsA (n = 81) or radiographic axial spondyloarthritis (n = 108) who had received secukinumab for ≥16 weeks prior to enrolment.
Disclosures: This study was supported by Novartis Pharma AG. Two authors declared being employees of Novartis Pharmaceuticals U.K. Ltd. Three authors declared ties with various sources, including Novartis.
Source: Gaffney K et al. Real-world evidence for secukinumab in UK patients with psoriatic arthritis or radiographic axial spondyloarthritis: Interim 2-year analysis from SERENA. Rheumatol Adv Pract. 2023;7(3):rkad055 (Aug 21). doi: 10.1093/rap/rkad055
Persistence and multidomain effectiveness of guselkumab in active PsA
Key clinical point: In a real-world population of patients with treatment-resistant, long-standing active psoriatic arthritis (PsA), nearly 80% persisted with guselkumab treatment for 6 months and showed improvements in peripheral joint and skin symptoms of PsA.
Major finding: Overall, 78.9% of patients who initiated guselkumab had persistent use at the 6-month follow-up. The mean scores for clinical Disease Activity in PsA (mean change [Δ] −5.4), overall joint+skin activity (Δ −19.0), patient-reported pain (Δ −9.1), and percentage of skin with psoriasis (Δ −5.1%) improved significantly in patients receiving guselkumab for 6 months (all P < .001).
Study details: This study evaluated 114 patients with active PsA from the CorEvitas PsA/Spondyloarthritis Registry who initiated guselkumab.
Disclosures: This study was sponsored by CorEvitas, LLC, and the analysis was funded by Janssen Scientific Affairs, LLC. Six authors declared employment with CorEvitas, LLC, or Janssen Scientific Affairs, LLC, or owned stock or stock options in Johnson & Johnson or others. Several authors declared ties with various sources, including Janssen and CorEvitas.
Source: Mease PJ et al. Six-month persistence and multi-domain effectiveness of guselkumab in adults with psoriatic arthritis: Real-world data from the CorEvitas psoriatic arthritis/spondyloarthritis registry. Rheumatol Ther. 2023 (Aug 19). doi: 10.1007/s40744-023-00582-w
Key clinical point: In a real-world population of patients with treatment-resistant, long-standing active psoriatic arthritis (PsA), nearly 80% persisted with guselkumab treatment for 6 months and showed improvements in peripheral joint and skin symptoms of PsA.
Major finding: Overall, 78.9% of patients who initiated guselkumab had persistent use at the 6-month follow-up. The mean scores for clinical Disease Activity in PsA (mean change [Δ] −5.4), overall joint+skin activity (Δ −19.0), patient-reported pain (Δ −9.1), and percentage of skin with psoriasis (Δ −5.1%) improved significantly in patients receiving guselkumab for 6 months (all P < .001).
Study details: This study evaluated 114 patients with active PsA from the CorEvitas PsA/Spondyloarthritis Registry who initiated guselkumab.
Disclosures: This study was sponsored by CorEvitas, LLC, and the analysis was funded by Janssen Scientific Affairs, LLC. Six authors declared employment with CorEvitas, LLC, or Janssen Scientific Affairs, LLC, or owned stock or stock options in Johnson & Johnson or others. Several authors declared ties with various sources, including Janssen and CorEvitas.
Source: Mease PJ et al. Six-month persistence and multi-domain effectiveness of guselkumab in adults with psoriatic arthritis: Real-world data from the CorEvitas psoriatic arthritis/spondyloarthritis registry. Rheumatol Ther. 2023 (Aug 19). doi: 10.1007/s40744-023-00582-w
Key clinical point: In a real-world population of patients with treatment-resistant, long-standing active psoriatic arthritis (PsA), nearly 80% persisted with guselkumab treatment for 6 months and showed improvements in peripheral joint and skin symptoms of PsA.
Major finding: Overall, 78.9% of patients who initiated guselkumab had persistent use at the 6-month follow-up. The mean scores for clinical Disease Activity in PsA (mean change [Δ] −5.4), overall joint+skin activity (Δ −19.0), patient-reported pain (Δ −9.1), and percentage of skin with psoriasis (Δ −5.1%) improved significantly in patients receiving guselkumab for 6 months (all P < .001).
Study details: This study evaluated 114 patients with active PsA from the CorEvitas PsA/Spondyloarthritis Registry who initiated guselkumab.
Disclosures: This study was sponsored by CorEvitas, LLC, and the analysis was funded by Janssen Scientific Affairs, LLC. Six authors declared employment with CorEvitas, LLC, or Janssen Scientific Affairs, LLC, or owned stock or stock options in Johnson & Johnson or others. Several authors declared ties with various sources, including Janssen and CorEvitas.
Source: Mease PJ et al. Six-month persistence and multi-domain effectiveness of guselkumab in adults with psoriatic arthritis: Real-world data from the CorEvitas psoriatic arthritis/spondyloarthritis registry. Rheumatol Ther. 2023 (Aug 19). doi: 10.1007/s40744-023-00582-w
Ixekizumab improves axial symptoms in PsA
Key clinical point: Ixekizumab was more effective than placebo in improving axial symptoms, such as back pain and morning stiffness, in patients with active psoriatic arthritis (PsA) presenting with axial manifestations.
Major finding: At weeks 16 and 24, ixekizumab vs placebo led to greater improvements in axial manifestations, such as back pain and morning stiffness (P < .001), as indicated by Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) scores, with a significantly higher proportion of patients achieving a 50% improvement in BASDAI scores (P < .001). All improvements with ixekizumab were sustained through week 52.
Study details: This post hoc analysis of pooled data from two phase 3 studies included biologic-naive and tumor necrosis factor inhibitor-experienced patients with active PsA and axial manifestations (n = 313) who were randomly assigned to receive either ixekizumab or placebo.
Disclosures: The studies described in this post hoc analysis were sponsored by Eli Lilly and Company. Four authors declared being employees and shareholders of Eli Lilly and Company. Several authors declared having ties with various sources, including Eli Lilly and Company.
Source: Deodhar A et al. The effect of ixekizumab on axial manifestations in patients with psoriatic arthritis from two phase III clinical trials: SPIRIT-P1 and SPIRIT-P2. Ther Adv Musculoskelet Dis. 2023 (Aug 24). doi: 10.1177/1759720X231189005
Key clinical point: Ixekizumab was more effective than placebo in improving axial symptoms, such as back pain and morning stiffness, in patients with active psoriatic arthritis (PsA) presenting with axial manifestations.
Major finding: At weeks 16 and 24, ixekizumab vs placebo led to greater improvements in axial manifestations, such as back pain and morning stiffness (P < .001), as indicated by Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) scores, with a significantly higher proportion of patients achieving a 50% improvement in BASDAI scores (P < .001). All improvements with ixekizumab were sustained through week 52.
Study details: This post hoc analysis of pooled data from two phase 3 studies included biologic-naive and tumor necrosis factor inhibitor-experienced patients with active PsA and axial manifestations (n = 313) who were randomly assigned to receive either ixekizumab or placebo.
Disclosures: The studies described in this post hoc analysis were sponsored by Eli Lilly and Company. Four authors declared being employees and shareholders of Eli Lilly and Company. Several authors declared having ties with various sources, including Eli Lilly and Company.
Source: Deodhar A et al. The effect of ixekizumab on axial manifestations in patients with psoriatic arthritis from two phase III clinical trials: SPIRIT-P1 and SPIRIT-P2. Ther Adv Musculoskelet Dis. 2023 (Aug 24). doi: 10.1177/1759720X231189005
Key clinical point: Ixekizumab was more effective than placebo in improving axial symptoms, such as back pain and morning stiffness, in patients with active psoriatic arthritis (PsA) presenting with axial manifestations.
Major finding: At weeks 16 and 24, ixekizumab vs placebo led to greater improvements in axial manifestations, such as back pain and morning stiffness (P < .001), as indicated by Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) scores, with a significantly higher proportion of patients achieving a 50% improvement in BASDAI scores (P < .001). All improvements with ixekizumab were sustained through week 52.
Study details: This post hoc analysis of pooled data from two phase 3 studies included biologic-naive and tumor necrosis factor inhibitor-experienced patients with active PsA and axial manifestations (n = 313) who were randomly assigned to receive either ixekizumab or placebo.
Disclosures: The studies described in this post hoc analysis were sponsored by Eli Lilly and Company. Four authors declared being employees and shareholders of Eli Lilly and Company. Several authors declared having ties with various sources, including Eli Lilly and Company.
Source: Deodhar A et al. The effect of ixekizumab on axial manifestations in patients with psoriatic arthritis from two phase III clinical trials: SPIRIT-P1 and SPIRIT-P2. Ther Adv Musculoskelet Dis. 2023 (Aug 24). doi: 10.1177/1759720X231189005
Acitretin use poses no additional risk for PsA compared with DMARD in patients with psoriasis
Key clinical point: The risk for psoriatic arthritis (PsA) was not higher among patients with psoriasis treated with acitretin vs disease-modifying antirheumatic drugs (DMARD), irrespective of the use of non-steroidal anti-inflammatory drugs (NSAID).
Major finding: The 5-year cumulative incidence rate for PsA was lower in the acitretin vs DMARD cohort (7.52% vs 9.93%; P = .005), with the incidence rates of PsA being markedly lower in the subgroup of patients receiving NSAID in the acitretin vs DMARD cohort (14.31% vs 23.83%; P = .008). Acitretin therapy showed no association with PsA development (hazard ratio 0.84; 95% CI 0.66-1.07).
Study details: Findings are from a retrospective cohort study including patients with psoriasis and without PsA who received either acitretin (n = 1948) or DMARD (n = 1948) for ≥ 30 days within a year.
Disclosures: This study was supported in part by the Taichung Veterans General Hospital. The authors declared no conflicts of interest.
Source: Lin TL et al. Psoriatic arthritis risk in psoriasis patients prescribed acitretin versus disease-modifying antirheumatic drugs: A nationwide cohort study. Rheumatology (Oxford). 2023 (Sep 1). doi: 10.1093/rheumatology/kead446
Key clinical point: The risk for psoriatic arthritis (PsA) was not higher among patients with psoriasis treated with acitretin vs disease-modifying antirheumatic drugs (DMARD), irrespective of the use of non-steroidal anti-inflammatory drugs (NSAID).
Major finding: The 5-year cumulative incidence rate for PsA was lower in the acitretin vs DMARD cohort (7.52% vs 9.93%; P = .005), with the incidence rates of PsA being markedly lower in the subgroup of patients receiving NSAID in the acitretin vs DMARD cohort (14.31% vs 23.83%; P = .008). Acitretin therapy showed no association with PsA development (hazard ratio 0.84; 95% CI 0.66-1.07).
Study details: Findings are from a retrospective cohort study including patients with psoriasis and without PsA who received either acitretin (n = 1948) or DMARD (n = 1948) for ≥ 30 days within a year.
Disclosures: This study was supported in part by the Taichung Veterans General Hospital. The authors declared no conflicts of interest.
Source: Lin TL et al. Psoriatic arthritis risk in psoriasis patients prescribed acitretin versus disease-modifying antirheumatic drugs: A nationwide cohort study. Rheumatology (Oxford). 2023 (Sep 1). doi: 10.1093/rheumatology/kead446
Key clinical point: The risk for psoriatic arthritis (PsA) was not higher among patients with psoriasis treated with acitretin vs disease-modifying antirheumatic drugs (DMARD), irrespective of the use of non-steroidal anti-inflammatory drugs (NSAID).
Major finding: The 5-year cumulative incidence rate for PsA was lower in the acitretin vs DMARD cohort (7.52% vs 9.93%; P = .005), with the incidence rates of PsA being markedly lower in the subgroup of patients receiving NSAID in the acitretin vs DMARD cohort (14.31% vs 23.83%; P = .008). Acitretin therapy showed no association with PsA development (hazard ratio 0.84; 95% CI 0.66-1.07).
Study details: Findings are from a retrospective cohort study including patients with psoriasis and without PsA who received either acitretin (n = 1948) or DMARD (n = 1948) for ≥ 30 days within a year.
Disclosures: This study was supported in part by the Taichung Veterans General Hospital. The authors declared no conflicts of interest.
Source: Lin TL et al. Psoriatic arthritis risk in psoriasis patients prescribed acitretin versus disease-modifying antirheumatic drugs: A nationwide cohort study. Rheumatology (Oxford). 2023 (Sep 1). doi: 10.1093/rheumatology/kead446
Failure of first-line IL-17A inhibitor should not deter treatment with second-line IL-17A
Key clinical point: Patients with psoriatic arthritis (PsA) showed similar adherence to secukinumab and ixekizumab as first-line or second-line interleukin (IL)-17A inhibitors, which indicates that the failure of a first-line IL-17A inhibitor therapy should not deter treatment with a second-line IL-17A inhibitors.
Major finding: Similar adherence to treatment was observed between first-line and second-line IL-17A inhibitor switchers and between second-line secukinumab and second-line ixekizumab switchers. Withdrawal reasons were similar for both first-line and second-line switchers when considering adverse events (14% for both); however, withdrawal due to failure of therapy was higher for the first-line vs second-line switchers (34% vs 18%).
Study details: Findings are from a population-based cohort study including patients with PsA who underwent prior treatment with ≥ 1 tumor necrosis factor inhibitor and switched to either first-line (n = 534) or second-line (n = 102) IL-17A inhibitors (ixekizumab or secukinumab).
Disclosures: This study was funded by the Oak Foundation. Five authors declared having ties with various sources, and three authors declared no conflicts of interest.
Source: Hansen RL et al. Adherence to therapy of ixekizumab and secukinumab in psoriatic arthritis patients using first- or second-line IL-17A inhibitor treatment: A Danish population-based cohort study. Rheumatology (Oxford). 2023 (Aug 30). doi: 10.1093/rheumatology/kead434
Key clinical point: Patients with psoriatic arthritis (PsA) showed similar adherence to secukinumab and ixekizumab as first-line or second-line interleukin (IL)-17A inhibitors, which indicates that the failure of a first-line IL-17A inhibitor therapy should not deter treatment with a second-line IL-17A inhibitors.
Major finding: Similar adherence to treatment was observed between first-line and second-line IL-17A inhibitor switchers and between second-line secukinumab and second-line ixekizumab switchers. Withdrawal reasons were similar for both first-line and second-line switchers when considering adverse events (14% for both); however, withdrawal due to failure of therapy was higher for the first-line vs second-line switchers (34% vs 18%).
Study details: Findings are from a population-based cohort study including patients with PsA who underwent prior treatment with ≥ 1 tumor necrosis factor inhibitor and switched to either first-line (n = 534) or second-line (n = 102) IL-17A inhibitors (ixekizumab or secukinumab).
Disclosures: This study was funded by the Oak Foundation. Five authors declared having ties with various sources, and three authors declared no conflicts of interest.
Source: Hansen RL et al. Adherence to therapy of ixekizumab and secukinumab in psoriatic arthritis patients using first- or second-line IL-17A inhibitor treatment: A Danish population-based cohort study. Rheumatology (Oxford). 2023 (Aug 30). doi: 10.1093/rheumatology/kead434
Key clinical point: Patients with psoriatic arthritis (PsA) showed similar adherence to secukinumab and ixekizumab as first-line or second-line interleukin (IL)-17A inhibitors, which indicates that the failure of a first-line IL-17A inhibitor therapy should not deter treatment with a second-line IL-17A inhibitors.
Major finding: Similar adherence to treatment was observed between first-line and second-line IL-17A inhibitor switchers and between second-line secukinumab and second-line ixekizumab switchers. Withdrawal reasons were similar for both first-line and second-line switchers when considering adverse events (14% for both); however, withdrawal due to failure of therapy was higher for the first-line vs second-line switchers (34% vs 18%).
Study details: Findings are from a population-based cohort study including patients with PsA who underwent prior treatment with ≥ 1 tumor necrosis factor inhibitor and switched to either first-line (n = 534) or second-line (n = 102) IL-17A inhibitors (ixekizumab or secukinumab).
Disclosures: This study was funded by the Oak Foundation. Five authors declared having ties with various sources, and three authors declared no conflicts of interest.
Source: Hansen RL et al. Adherence to therapy of ixekizumab and secukinumab in psoriatic arthritis patients using first- or second-line IL-17A inhibitor treatment: A Danish population-based cohort study. Rheumatology (Oxford). 2023 (Aug 30). doi: 10.1093/rheumatology/kead434
Biological DMARD equally effective in PsA patients with low or high joint counts
Key clinical point: Biological disease-modifying antirheumatic drugs (bDMARD) showed comparable efficacy in patients with psoriatic arthritis (PsA) with either low joint count (LJC) or high joint count (HJC), which highlights the feasibility of treatment escalation to bDMARD in symptomatic patients with LJC as well as in those with HJC.
Major finding: Patients with LJC (<3 tender or swollen joints) vs HJC (≥3 tender or swollen joints) showed similar treatment efficacy in terms of bDMARD retention (adjusted hazard ratio [aHR] 1.09; P = .63). Treatment discontinuation was unaffected by swollen or tender joint count status (aHR 1.12; P = .47).
Study details: Findings are from an observational cohort study that included 387 patients with PsA who had either LJC (n = 197) or HJC (n = 190) and received bDMARD.
Disclosures: This study was supported by Celgene International Sarl, Boudry, Switzerland. Five authors declared ties with various sources, whereas the other authors declared no conflicts of interest.
Source: Möller B et al for the Swiss Clinical Quality Management (SCQM) physicians, researchers and patients. Biological disease-modifying anti-rheumatic drugs are equally effective in psoriatic arthritis patients with low and high joint counts. Rheumatology (Oxford). 2023 (Sep 7). doi: 10.1093/rheumatology/kead455
Key clinical point: Biological disease-modifying antirheumatic drugs (bDMARD) showed comparable efficacy in patients with psoriatic arthritis (PsA) with either low joint count (LJC) or high joint count (HJC), which highlights the feasibility of treatment escalation to bDMARD in symptomatic patients with LJC as well as in those with HJC.
Major finding: Patients with LJC (<3 tender or swollen joints) vs HJC (≥3 tender or swollen joints) showed similar treatment efficacy in terms of bDMARD retention (adjusted hazard ratio [aHR] 1.09; P = .63). Treatment discontinuation was unaffected by swollen or tender joint count status (aHR 1.12; P = .47).
Study details: Findings are from an observational cohort study that included 387 patients with PsA who had either LJC (n = 197) or HJC (n = 190) and received bDMARD.
Disclosures: This study was supported by Celgene International Sarl, Boudry, Switzerland. Five authors declared ties with various sources, whereas the other authors declared no conflicts of interest.
Source: Möller B et al for the Swiss Clinical Quality Management (SCQM) physicians, researchers and patients. Biological disease-modifying anti-rheumatic drugs are equally effective in psoriatic arthritis patients with low and high joint counts. Rheumatology (Oxford). 2023 (Sep 7). doi: 10.1093/rheumatology/kead455
Key clinical point: Biological disease-modifying antirheumatic drugs (bDMARD) showed comparable efficacy in patients with psoriatic arthritis (PsA) with either low joint count (LJC) or high joint count (HJC), which highlights the feasibility of treatment escalation to bDMARD in symptomatic patients with LJC as well as in those with HJC.
Major finding: Patients with LJC (<3 tender or swollen joints) vs HJC (≥3 tender or swollen joints) showed similar treatment efficacy in terms of bDMARD retention (adjusted hazard ratio [aHR] 1.09; P = .63). Treatment discontinuation was unaffected by swollen or tender joint count status (aHR 1.12; P = .47).
Study details: Findings are from an observational cohort study that included 387 patients with PsA who had either LJC (n = 197) or HJC (n = 190) and received bDMARD.
Disclosures: This study was supported by Celgene International Sarl, Boudry, Switzerland. Five authors declared ties with various sources, whereas the other authors declared no conflicts of interest.
Source: Möller B et al for the Swiss Clinical Quality Management (SCQM) physicians, researchers and patients. Biological disease-modifying anti-rheumatic drugs are equally effective in psoriatic arthritis patients with low and high joint counts. Rheumatology (Oxford). 2023 (Sep 7). doi: 10.1093/rheumatology/kead455
Guselkumab improves effector cytokine levels in PsA patients with inadequate response to TNFi
Key clinical point: Guselkumab led to early reduction of inflammatory cytokines, which was sustained through 48 weeks, with subsequent improvements in clinical response in patients with active psoriatic arthritis (PsA) and an inadequate response to tumor necrosis factor inhibitors (TNFi-IR).
Major finding: Serum levels of interleukin (IL)-17A, IL-17F, IL-22, and serum amyloid A reduced significantly by week 4 and were sustained through week 48 in the guselkumab (P < .05) vs placebo group when compared with control individuals without PsA. Patients who achieved a clinical response to guselkumab at week 24 showed significant reduction in IL-6 levels at week 4 compared with non-responders (P < .05).
Study details: Findings are from the phase 3b COSMOS study including patients with active PsA and TNFi-IR who were randomly assigned to receive either guselkumab (n = 189) or placebo (n = 96) and compared with matched control individuals.
Disclosures: This study was sponsored by Janssen Research & Development (R&D), LLC, USA. Seven authors declared being employees of Janssen R&D, LLC, or others or owning stocks or stock options in Johnson & Johnson. Several authors declared ties with various sources, including Janssen.
Source: Schett G et al. Effect of guselkumab on serum biomarkers in patients with active psoriatic arthritis and inadequate response to tumor necrosis factor inhibitors: Results from the COSMOS phase 3b study. Arthritis Res Ther. 2023;25:150 (Aug 16, corrected Sep 15). doi: 10.1186/s13075-023-03125-4
Key clinical point: Guselkumab led to early reduction of inflammatory cytokines, which was sustained through 48 weeks, with subsequent improvements in clinical response in patients with active psoriatic arthritis (PsA) and an inadequate response to tumor necrosis factor inhibitors (TNFi-IR).
Major finding: Serum levels of interleukin (IL)-17A, IL-17F, IL-22, and serum amyloid A reduced significantly by week 4 and were sustained through week 48 in the guselkumab (P < .05) vs placebo group when compared with control individuals without PsA. Patients who achieved a clinical response to guselkumab at week 24 showed significant reduction in IL-6 levels at week 4 compared with non-responders (P < .05).
Study details: Findings are from the phase 3b COSMOS study including patients with active PsA and TNFi-IR who were randomly assigned to receive either guselkumab (n = 189) or placebo (n = 96) and compared with matched control individuals.
Disclosures: This study was sponsored by Janssen Research & Development (R&D), LLC, USA. Seven authors declared being employees of Janssen R&D, LLC, or others or owning stocks or stock options in Johnson & Johnson. Several authors declared ties with various sources, including Janssen.
Source: Schett G et al. Effect of guselkumab on serum biomarkers in patients with active psoriatic arthritis and inadequate response to tumor necrosis factor inhibitors: Results from the COSMOS phase 3b study. Arthritis Res Ther. 2023;25:150 (Aug 16, corrected Sep 15). doi: 10.1186/s13075-023-03125-4
Key clinical point: Guselkumab led to early reduction of inflammatory cytokines, which was sustained through 48 weeks, with subsequent improvements in clinical response in patients with active psoriatic arthritis (PsA) and an inadequate response to tumor necrosis factor inhibitors (TNFi-IR).
Major finding: Serum levels of interleukin (IL)-17A, IL-17F, IL-22, and serum amyloid A reduced significantly by week 4 and were sustained through week 48 in the guselkumab (P < .05) vs placebo group when compared with control individuals without PsA. Patients who achieved a clinical response to guselkumab at week 24 showed significant reduction in IL-6 levels at week 4 compared with non-responders (P < .05).
Study details: Findings are from the phase 3b COSMOS study including patients with active PsA and TNFi-IR who were randomly assigned to receive either guselkumab (n = 189) or placebo (n = 96) and compared with matched control individuals.
Disclosures: This study was sponsored by Janssen Research & Development (R&D), LLC, USA. Seven authors declared being employees of Janssen R&D, LLC, or others or owning stocks or stock options in Johnson & Johnson. Several authors declared ties with various sources, including Janssen.
Source: Schett G et al. Effect of guselkumab on serum biomarkers in patients with active psoriatic arthritis and inadequate response to tumor necrosis factor inhibitors: Results from the COSMOS phase 3b study. Arthritis Res Ther. 2023;25:150 (Aug 16, corrected Sep 15). doi: 10.1186/s13075-023-03125-4
Cat Scratch Disease Presenting With Concurrent Pityriasis Rosea in a 10-Year-Old Girl
To the Editor:
Cat scratch disease (CSD) is caused by Bartonella henselae and Bartonella clarridgeiae bacteria transferred from cats to humans that results in an inflamed inoculation site and tender lymphadenopathy. Pityriasis rosea (PR) and PR-like eruptions are self-limited, acute exanthems that have been associated with infections, vaccinations, and medications. We report a case of PR occurring in a 10-year-old girl with CSD, which may suggest an association between the 2 diseases.

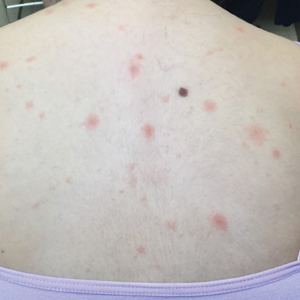
A 10-year-old girl who was otherwise healthy presented in the winter with a rash of 5 days’ duration. Fourteen days prior to the rash, the patient reported being scratched by a new kitten and noted a pinpoint “puncture” on the left forearm that developed into a red papule over the following week. Seven days after the cat scratch, the patient experienced pain and swelling in the left axilla. Approximately 1 week after the onset of lymphadenopathy, the patient developed an asymptomatic rash that started with a large spot on the left chest, followed by smaller spots appearing over the next 2 days and spreading to the rest of the trunk. Four days after the rash onset, the patient experienced a mild headache, low-grade subjective fever, and chills. She denied any recent travel, bug bites, sore throat, and diarrhea. She was up-to-date on all vaccinations and had not received any vaccines preceding the symptoms. Physical examination revealed a 2-cm pink, scaly, thin plaque with a collarette of scale on the left upper chest (herald patch), along with multiple thin pink papules and small plaques with central scale on the trunk (Figure 1). A pustule with adjacent linear erosion was present on the left ventral forearm (Figure 2). The patient had a tender subcutaneous nodule in the left axilla as well as bilateral anterior and posterior cervical-chain subcutaneous tender nodules. There was no involvement of the palms, soles, or mucosae.

The patient was empirically treated for CSD with azithromycin (200 mg/5 mL), 404 mg on day 1 followed by 202 mg daily for 4 days. The rash was treated with hydrocortisone cream 2.5% twice daily for 2 weeks. A wound culture of the pustule on the left forearm was negative for neutrophils and organisms. Antibody serologies obtained 4 weeks after presentation were notable for an elevated B henselae IgG titer of 1:640, confirming the diagnosis of CSD. Following treatment with azithromycin and hydrocortisone, all of the patient’s symptoms resolved after 1 to 2 weeks.
Cat scratch disease is a zoonotic infection caused by the bacteria B henselae and the more recently described pathogen B clarridgeiae. Cat fleas spread these bacteria among cats, which subsequently inoculate the bacteria into humans through bites and scratches. The incidence of CSD in the United States is estimated to be 4.5 to 9.3 per 100,000 individuals in the outpatient setting and 0.19 to 0.86 per 100,000 individuals in the inpatient setting.1 Geographic variance can occur based on flea populations, resulting in higher incidence in warm humid climates and lower incidence in mountainous arid climates. The incidence of CSD in the pediatric population is highest in children aged 5 to 9 years. A national representative survey (N=3011) from 2017 revealed that 37.2% of primary care providers had diagnosed CSD in the prior year.1
Classic CSD presents as an erythematous papule at the inoculation site lasting days to weeks, with progression to tender lymphadenopathy lasting weeks to months. Fever, malaise, and chills also can be seen. Atypical CSD occurs in up to 24% of cases in immunocompetent patients.1 Atypical and systemic presentations are varied and can include fever of unknown origin, neuroretinitis, uveitis, retinal vessel occlusion, encephalitis, hepatosplenic lesions, Parinaud oculoglandular syndrome, osteomyelitis, and endocarditis.1,2 Atypical dermatologic presentations of CSD include maculopapular rash in 7% of cases and erythema nodosum in 2.5% of cases, as well as rare reports of cutaneous vasculitis, urticaria, immune thrombocytopenic purpura, and papuloedematous eruption.3 Treatment guidelines for CSD vary widely depending on the clinical presentation as well as the immunocompetence of the infected individual. Our patient had limited regional lymphadenopathy with no signs of dissemination or neurologic involvement and was successfully treated with a 5-day course of oral azithromycin (weight based, 10 mg/kg). More extensive disease such as hepatosplenic or neurologic CSD may require multiple antibiotics for up to 6 weeks. Alternative or additional antibiotics used for CSD include rifampin, trimethoprim-sulfamethoxazole, ciprofloxacin, doxycycline, gentamicin, and clarithromycin. Opinions vary as to whether all patients or just those with complicated infections warrant antibiotic therapy.4-6
Pityriasis rosea is a self-limited acute exanthematous disease that is classically associated with a systemic reactivation of human herpesvirus (HHV) 6 and/or HHV-7. The incidence of PR is estimated to be 480 per 100,000 dermatologic patients. It is slightly more common in females and occurs most often in patients aged 10 to 35 years.7 Clinically, PR appears with the abrupt onset of a single erythematous scaly patch (termed the herald patch), followed by a secondary eruption of smaller erythematous scaly macules and patches along the trunk’s cleavage lines. The secondary eruption on the back is sometimes termed a Christmas or fir tree pattern.7,8
In addition to the classic presentation of PR, there have been reports of numerous atypical clinical presentations. The herald patch, which classically presents on the trunk, also has been reported to present on the extremities; PR of the extremities is defined by lesions that appear as large scaly plaques on the extremities only. Inverse PR presents with lesions occurring in flexural areas and acral surfaces but not on the trunk. There also is an acral PR variant in which lesions appear only on the palms, wrists, and soles. Purpuric or hemorrhagic PR has been described and presents with purpura and petechiae with or without collarettes of scale in diffuse locations, including the palate. Oral PR presents more commonly in patients of color as erosions, ulcers, hemorrhagic lesions, bullae, or geographic tongue. Erythema multiforme–like PR appears with targetoid lesions on the trunk, face, neck, and arms without a history of herpes simplex virus infection. A large pear-shaped herald patch has been reported and characterizes the gigantea PR of Darier variant. Irritated PR occurs with typical PR findings, but afflicted patients report severe pain and burning with diaphoresis. Relapsing PR can occur within 1 year of a prior episode of PR and presents without a herald patch. Persistent PR is defined by PR lasting more than 3 months, and most reported cases have included oral lesions. Finally, other PR variants that have been described include urticarial, papular, follicular, vesicular, and hypopigmented types.7-9
Furthermore, there have been reports of multiple atypical presentations occurring simultaneously in the same patient.10 Although PR classically has been associated with HHV-6 and/or HHV-7 reactivation, it has been reported with a few other clinical situations and conditions. Pityriasislike eruption specifically refers to an exanthem secondary to drugs or vaccination that resembles PR but shows clinical differences, including diffuse and confluent dusky-red macules and/or plaques with or without desquamation on the trunk, extremities, and face. Drugs that have been implicated as triggers include ACE inhibitors, gold, isotretinoin, nonsteroidal anti-inflammatory agents, omeprazole, terbinafine, and tyrosine kinase inhibitors. Smallpox, tuberculosis, poliomyelitis, influenza, diphtheria, tetanus, hepatitis B virus, pneumococcus, papillomavirus, yellow fever, and pertussis vaccinations also have been associated with PR.7,11,12 Additionally, PR has been reported to occur with active systemic infections, specifically H1N1 influenza, though it is rare.13 Because of its self-limited course, treatment of PR most often involves only reassurance. Topical corticosteroids may be appropriate for pruritus.7,8
Pediatric health care providers including dermatologists should be familiar with both CSD and PR because they are common diseases that more often are encountered in the pediatric population. We present a unique case of CSD presenting with concurrent PR, which highlights a potential new etiology for PR and a rare cutaneous manifestation of CSD. Further investigation into a possible relationship between CSD and PR may be warranted. Patients with any signs and symptoms of fever, tender lymphadenopathy, worsening rash, or exposure to cats warrant a thorough history and physical examination to ensure that neither entity is overlooked.
- Nelson CA, Moore AR, Perea AE, et al. Cat scratch disease: U.S. clinicians’ experience and knowledge [published online July 14, 2017]. Zoonoses Public Health. 2018;65:67-73. doi:10.1111/zph.12368
- Habot-Wilner Z, Trivizki O, Goldstein M, et al. Cat-scratch disease: ocular manifestations and treatment outcome. Acta Ophthalmol. 2018;96:E524-E532. doi:10.1111/aos.13684
- Schattner A, Uliel L, Dubin I. The cat did it: erythema nodosum and additional atypical presentations of Bartonella henselae infection in immunocompetent hosts [published online February 16, 2018]. BMJ Case Rep. doi:10.1136/bcr-2017-222511
- Shorbatli L, Koranyi K, Nahata M. Effectiveness of antibiotic therapy in pediatric patients with cat scratch disease. Int J Clin Pharm. 2018;40:1458-1461. doi: 10.1007/s11096-018-0746-1
- Bass JW, Freitas BC, Freitas AD, et al. Prospective randomized double blind placebo-controlled evaluation of azithromycin for treatment of cat-scratch disease. Pediatr Infect Dis J. 1998;17:447-452. doi:10.1097/00006454-199806000-00002
- Spach DH, Kaplan SL. Treatment of cat scratch disease. UpToDate. Updated December 9, 2021. Accessed September 12, 2023. https://www.uptodate.com/contents/treatment-of-cat-scratch-disease
- Drago F, Ciccarese G, Rebora A, et al. Pityriasis rosea: a comprehensive classification. Dermatology. 2016;232:431-437. doi:10.1159/000445375
- Urbina F, Das A, Sudy E. Clinical variants of pityriasis rosea. World J Clin Cases. 2017;5:203-211. doi:10.12998/wjcc.v5.i6.203
- Alzahrani NA, Al Jasser MI. Geographic tonguelike presentation in a child with pityriasis rosea: case report and review of oral manifestations of pityriasis rosea. Pediatr Dermatol. 2018;35:E124-E127. doi:10.1111/pde.13417
- Sinha S, Sardana K, Garg V. Coexistence of two atypical variants of pityriasis rosea: a case report and review of literature. Pediatr Dermatol. 2012;29:538-540. doi:10.1111/j.1525-1470.2011.01549.x
- Drago F, Ciccarese G, Parodi A. Pityriasis rosea and pityriasis rosea-like eruptions: how to distinguish them? JAAD Case Rep. 2018;4:800-801. doi:10.1016/j.jdcr.2018.04.002
- Drago F, Ciccarese G, Javor S, et al. Vaccine-induced pityriasis rosea and pityriasis rosea-like eruptions: a review of the literature. J Eur Acad Dermatol Venereol. 2016;30:544-545. doi:10.1111/jdv.12942
- Mubki TF, Bin Dayel SA, Kadry R. A case of pityriasis rosea concurrent with the novel influenza A (H1N1) infection. Pediatr Dermatol. 2011;28:341-342. doi:10.1111/j.1525-1470.2010.01090.x
To the Editor:
Cat scratch disease (CSD) is caused by Bartonella henselae and Bartonella clarridgeiae bacteria transferred from cats to humans that results in an inflamed inoculation site and tender lymphadenopathy. Pityriasis rosea (PR) and PR-like eruptions are self-limited, acute exanthems that have been associated with infections, vaccinations, and medications. We report a case of PR occurring in a 10-year-old girl with CSD, which may suggest an association between the 2 diseases.

A 10-year-old girl who was otherwise healthy presented in the winter with a rash of 5 days’ duration. Fourteen days prior to the rash, the patient reported being scratched by a new kitten and noted a pinpoint “puncture” on the left forearm that developed into a red papule over the following week. Seven days after the cat scratch, the patient experienced pain and swelling in the left axilla. Approximately 1 week after the onset of lymphadenopathy, the patient developed an asymptomatic rash that started with a large spot on the left chest, followed by smaller spots appearing over the next 2 days and spreading to the rest of the trunk. Four days after the rash onset, the patient experienced a mild headache, low-grade subjective fever, and chills. She denied any recent travel, bug bites, sore throat, and diarrhea. She was up-to-date on all vaccinations and had not received any vaccines preceding the symptoms. Physical examination revealed a 2-cm pink, scaly, thin plaque with a collarette of scale on the left upper chest (herald patch), along with multiple thin pink papules and small plaques with central scale on the trunk (Figure 1). A pustule with adjacent linear erosion was present on the left ventral forearm (Figure 2). The patient had a tender subcutaneous nodule in the left axilla as well as bilateral anterior and posterior cervical-chain subcutaneous tender nodules. There was no involvement of the palms, soles, or mucosae.

The patient was empirically treated for CSD with azithromycin (200 mg/5 mL), 404 mg on day 1 followed by 202 mg daily for 4 days. The rash was treated with hydrocortisone cream 2.5% twice daily for 2 weeks. A wound culture of the pustule on the left forearm was negative for neutrophils and organisms. Antibody serologies obtained 4 weeks after presentation were notable for an elevated B henselae IgG titer of 1:640, confirming the diagnosis of CSD. Following treatment with azithromycin and hydrocortisone, all of the patient’s symptoms resolved after 1 to 2 weeks.
Cat scratch disease is a zoonotic infection caused by the bacteria B henselae and the more recently described pathogen B clarridgeiae. Cat fleas spread these bacteria among cats, which subsequently inoculate the bacteria into humans through bites and scratches. The incidence of CSD in the United States is estimated to be 4.5 to 9.3 per 100,000 individuals in the outpatient setting and 0.19 to 0.86 per 100,000 individuals in the inpatient setting.1 Geographic variance can occur based on flea populations, resulting in higher incidence in warm humid climates and lower incidence in mountainous arid climates. The incidence of CSD in the pediatric population is highest in children aged 5 to 9 years. A national representative survey (N=3011) from 2017 revealed that 37.2% of primary care providers had diagnosed CSD in the prior year.1
Classic CSD presents as an erythematous papule at the inoculation site lasting days to weeks, with progression to tender lymphadenopathy lasting weeks to months. Fever, malaise, and chills also can be seen. Atypical CSD occurs in up to 24% of cases in immunocompetent patients.1 Atypical and systemic presentations are varied and can include fever of unknown origin, neuroretinitis, uveitis, retinal vessel occlusion, encephalitis, hepatosplenic lesions, Parinaud oculoglandular syndrome, osteomyelitis, and endocarditis.1,2 Atypical dermatologic presentations of CSD include maculopapular rash in 7% of cases and erythema nodosum in 2.5% of cases, as well as rare reports of cutaneous vasculitis, urticaria, immune thrombocytopenic purpura, and papuloedematous eruption.3 Treatment guidelines for CSD vary widely depending on the clinical presentation as well as the immunocompetence of the infected individual. Our patient had limited regional lymphadenopathy with no signs of dissemination or neurologic involvement and was successfully treated with a 5-day course of oral azithromycin (weight based, 10 mg/kg). More extensive disease such as hepatosplenic or neurologic CSD may require multiple antibiotics for up to 6 weeks. Alternative or additional antibiotics used for CSD include rifampin, trimethoprim-sulfamethoxazole, ciprofloxacin, doxycycline, gentamicin, and clarithromycin. Opinions vary as to whether all patients or just those with complicated infections warrant antibiotic therapy.4-6
Pityriasis rosea is a self-limited acute exanthematous disease that is classically associated with a systemic reactivation of human herpesvirus (HHV) 6 and/or HHV-7. The incidence of PR is estimated to be 480 per 100,000 dermatologic patients. It is slightly more common in females and occurs most often in patients aged 10 to 35 years.7 Clinically, PR appears with the abrupt onset of a single erythematous scaly patch (termed the herald patch), followed by a secondary eruption of smaller erythematous scaly macules and patches along the trunk’s cleavage lines. The secondary eruption on the back is sometimes termed a Christmas or fir tree pattern.7,8
In addition to the classic presentation of PR, there have been reports of numerous atypical clinical presentations. The herald patch, which classically presents on the trunk, also has been reported to present on the extremities; PR of the extremities is defined by lesions that appear as large scaly plaques on the extremities only. Inverse PR presents with lesions occurring in flexural areas and acral surfaces but not on the trunk. There also is an acral PR variant in which lesions appear only on the palms, wrists, and soles. Purpuric or hemorrhagic PR has been described and presents with purpura and petechiae with or without collarettes of scale in diffuse locations, including the palate. Oral PR presents more commonly in patients of color as erosions, ulcers, hemorrhagic lesions, bullae, or geographic tongue. Erythema multiforme–like PR appears with targetoid lesions on the trunk, face, neck, and arms without a history of herpes simplex virus infection. A large pear-shaped herald patch has been reported and characterizes the gigantea PR of Darier variant. Irritated PR occurs with typical PR findings, but afflicted patients report severe pain and burning with diaphoresis. Relapsing PR can occur within 1 year of a prior episode of PR and presents without a herald patch. Persistent PR is defined by PR lasting more than 3 months, and most reported cases have included oral lesions. Finally, other PR variants that have been described include urticarial, papular, follicular, vesicular, and hypopigmented types.7-9
Furthermore, there have been reports of multiple atypical presentations occurring simultaneously in the same patient.10 Although PR classically has been associated with HHV-6 and/or HHV-7 reactivation, it has been reported with a few other clinical situations and conditions. Pityriasislike eruption specifically refers to an exanthem secondary to drugs or vaccination that resembles PR but shows clinical differences, including diffuse and confluent dusky-red macules and/or plaques with or without desquamation on the trunk, extremities, and face. Drugs that have been implicated as triggers include ACE inhibitors, gold, isotretinoin, nonsteroidal anti-inflammatory agents, omeprazole, terbinafine, and tyrosine kinase inhibitors. Smallpox, tuberculosis, poliomyelitis, influenza, diphtheria, tetanus, hepatitis B virus, pneumococcus, papillomavirus, yellow fever, and pertussis vaccinations also have been associated with PR.7,11,12 Additionally, PR has been reported to occur with active systemic infections, specifically H1N1 influenza, though it is rare.13 Because of its self-limited course, treatment of PR most often involves only reassurance. Topical corticosteroids may be appropriate for pruritus.7,8
Pediatric health care providers including dermatologists should be familiar with both CSD and PR because they are common diseases that more often are encountered in the pediatric population. We present a unique case of CSD presenting with concurrent PR, which highlights a potential new etiology for PR and a rare cutaneous manifestation of CSD. Further investigation into a possible relationship between CSD and PR may be warranted. Patients with any signs and symptoms of fever, tender lymphadenopathy, worsening rash, or exposure to cats warrant a thorough history and physical examination to ensure that neither entity is overlooked.
To the Editor:
Cat scratch disease (CSD) is caused by Bartonella henselae and Bartonella clarridgeiae bacteria transferred from cats to humans that results in an inflamed inoculation site and tender lymphadenopathy. Pityriasis rosea (PR) and PR-like eruptions are self-limited, acute exanthems that have been associated with infections, vaccinations, and medications. We report a case of PR occurring in a 10-year-old girl with CSD, which may suggest an association between the 2 diseases.

A 10-year-old girl who was otherwise healthy presented in the winter with a rash of 5 days’ duration. Fourteen days prior to the rash, the patient reported being scratched by a new kitten and noted a pinpoint “puncture” on the left forearm that developed into a red papule over the following week. Seven days after the cat scratch, the patient experienced pain and swelling in the left axilla. Approximately 1 week after the onset of lymphadenopathy, the patient developed an asymptomatic rash that started with a large spot on the left chest, followed by smaller spots appearing over the next 2 days and spreading to the rest of the trunk. Four days after the rash onset, the patient experienced a mild headache, low-grade subjective fever, and chills. She denied any recent travel, bug bites, sore throat, and diarrhea. She was up-to-date on all vaccinations and had not received any vaccines preceding the symptoms. Physical examination revealed a 2-cm pink, scaly, thin plaque with a collarette of scale on the left upper chest (herald patch), along with multiple thin pink papules and small plaques with central scale on the trunk (Figure 1). A pustule with adjacent linear erosion was present on the left ventral forearm (Figure 2). The patient had a tender subcutaneous nodule in the left axilla as well as bilateral anterior and posterior cervical-chain subcutaneous tender nodules. There was no involvement of the palms, soles, or mucosae.

The patient was empirically treated for CSD with azithromycin (200 mg/5 mL), 404 mg on day 1 followed by 202 mg daily for 4 days. The rash was treated with hydrocortisone cream 2.5% twice daily for 2 weeks. A wound culture of the pustule on the left forearm was negative for neutrophils and organisms. Antibody serologies obtained 4 weeks after presentation were notable for an elevated B henselae IgG titer of 1:640, confirming the diagnosis of CSD. Following treatment with azithromycin and hydrocortisone, all of the patient’s symptoms resolved after 1 to 2 weeks.
Cat scratch disease is a zoonotic infection caused by the bacteria B henselae and the more recently described pathogen B clarridgeiae. Cat fleas spread these bacteria among cats, which subsequently inoculate the bacteria into humans through bites and scratches. The incidence of CSD in the United States is estimated to be 4.5 to 9.3 per 100,000 individuals in the outpatient setting and 0.19 to 0.86 per 100,000 individuals in the inpatient setting.1 Geographic variance can occur based on flea populations, resulting in higher incidence in warm humid climates and lower incidence in mountainous arid climates. The incidence of CSD in the pediatric population is highest in children aged 5 to 9 years. A national representative survey (N=3011) from 2017 revealed that 37.2% of primary care providers had diagnosed CSD in the prior year.1
Classic CSD presents as an erythematous papule at the inoculation site lasting days to weeks, with progression to tender lymphadenopathy lasting weeks to months. Fever, malaise, and chills also can be seen. Atypical CSD occurs in up to 24% of cases in immunocompetent patients.1 Atypical and systemic presentations are varied and can include fever of unknown origin, neuroretinitis, uveitis, retinal vessel occlusion, encephalitis, hepatosplenic lesions, Parinaud oculoglandular syndrome, osteomyelitis, and endocarditis.1,2 Atypical dermatologic presentations of CSD include maculopapular rash in 7% of cases and erythema nodosum in 2.5% of cases, as well as rare reports of cutaneous vasculitis, urticaria, immune thrombocytopenic purpura, and papuloedematous eruption.3 Treatment guidelines for CSD vary widely depending on the clinical presentation as well as the immunocompetence of the infected individual. Our patient had limited regional lymphadenopathy with no signs of dissemination or neurologic involvement and was successfully treated with a 5-day course of oral azithromycin (weight based, 10 mg/kg). More extensive disease such as hepatosplenic or neurologic CSD may require multiple antibiotics for up to 6 weeks. Alternative or additional antibiotics used for CSD include rifampin, trimethoprim-sulfamethoxazole, ciprofloxacin, doxycycline, gentamicin, and clarithromycin. Opinions vary as to whether all patients or just those with complicated infections warrant antibiotic therapy.4-6
Pityriasis rosea is a self-limited acute exanthematous disease that is classically associated with a systemic reactivation of human herpesvirus (HHV) 6 and/or HHV-7. The incidence of PR is estimated to be 480 per 100,000 dermatologic patients. It is slightly more common in females and occurs most often in patients aged 10 to 35 years.7 Clinically, PR appears with the abrupt onset of a single erythematous scaly patch (termed the herald patch), followed by a secondary eruption of smaller erythematous scaly macules and patches along the trunk’s cleavage lines. The secondary eruption on the back is sometimes termed a Christmas or fir tree pattern.7,8
In addition to the classic presentation of PR, there have been reports of numerous atypical clinical presentations. The herald patch, which classically presents on the trunk, also has been reported to present on the extremities; PR of the extremities is defined by lesions that appear as large scaly plaques on the extremities only. Inverse PR presents with lesions occurring in flexural areas and acral surfaces but not on the trunk. There also is an acral PR variant in which lesions appear only on the palms, wrists, and soles. Purpuric or hemorrhagic PR has been described and presents with purpura and petechiae with or without collarettes of scale in diffuse locations, including the palate. Oral PR presents more commonly in patients of color as erosions, ulcers, hemorrhagic lesions, bullae, or geographic tongue. Erythema multiforme–like PR appears with targetoid lesions on the trunk, face, neck, and arms without a history of herpes simplex virus infection. A large pear-shaped herald patch has been reported and characterizes the gigantea PR of Darier variant. Irritated PR occurs with typical PR findings, but afflicted patients report severe pain and burning with diaphoresis. Relapsing PR can occur within 1 year of a prior episode of PR and presents without a herald patch. Persistent PR is defined by PR lasting more than 3 months, and most reported cases have included oral lesions. Finally, other PR variants that have been described include urticarial, papular, follicular, vesicular, and hypopigmented types.7-9
Furthermore, there have been reports of multiple atypical presentations occurring simultaneously in the same patient.10 Although PR classically has been associated with HHV-6 and/or HHV-7 reactivation, it has been reported with a few other clinical situations and conditions. Pityriasislike eruption specifically refers to an exanthem secondary to drugs or vaccination that resembles PR but shows clinical differences, including diffuse and confluent dusky-red macules and/or plaques with or without desquamation on the trunk, extremities, and face. Drugs that have been implicated as triggers include ACE inhibitors, gold, isotretinoin, nonsteroidal anti-inflammatory agents, omeprazole, terbinafine, and tyrosine kinase inhibitors. Smallpox, tuberculosis, poliomyelitis, influenza, diphtheria, tetanus, hepatitis B virus, pneumococcus, papillomavirus, yellow fever, and pertussis vaccinations also have been associated with PR.7,11,12 Additionally, PR has been reported to occur with active systemic infections, specifically H1N1 influenza, though it is rare.13 Because of its self-limited course, treatment of PR most often involves only reassurance. Topical corticosteroids may be appropriate for pruritus.7,8
Pediatric health care providers including dermatologists should be familiar with both CSD and PR because they are common diseases that more often are encountered in the pediatric population. We present a unique case of CSD presenting with concurrent PR, which highlights a potential new etiology for PR and a rare cutaneous manifestation of CSD. Further investigation into a possible relationship between CSD and PR may be warranted. Patients with any signs and symptoms of fever, tender lymphadenopathy, worsening rash, or exposure to cats warrant a thorough history and physical examination to ensure that neither entity is overlooked.
- Nelson CA, Moore AR, Perea AE, et al. Cat scratch disease: U.S. clinicians’ experience and knowledge [published online July 14, 2017]. Zoonoses Public Health. 2018;65:67-73. doi:10.1111/zph.12368
- Habot-Wilner Z, Trivizki O, Goldstein M, et al. Cat-scratch disease: ocular manifestations and treatment outcome. Acta Ophthalmol. 2018;96:E524-E532. doi:10.1111/aos.13684
- Schattner A, Uliel L, Dubin I. The cat did it: erythema nodosum and additional atypical presentations of Bartonella henselae infection in immunocompetent hosts [published online February 16, 2018]. BMJ Case Rep. doi:10.1136/bcr-2017-222511
- Shorbatli L, Koranyi K, Nahata M. Effectiveness of antibiotic therapy in pediatric patients with cat scratch disease. Int J Clin Pharm. 2018;40:1458-1461. doi: 10.1007/s11096-018-0746-1
- Bass JW, Freitas BC, Freitas AD, et al. Prospective randomized double blind placebo-controlled evaluation of azithromycin for treatment of cat-scratch disease. Pediatr Infect Dis J. 1998;17:447-452. doi:10.1097/00006454-199806000-00002
- Spach DH, Kaplan SL. Treatment of cat scratch disease. UpToDate. Updated December 9, 2021. Accessed September 12, 2023. https://www.uptodate.com/contents/treatment-of-cat-scratch-disease
- Drago F, Ciccarese G, Rebora A, et al. Pityriasis rosea: a comprehensive classification. Dermatology. 2016;232:431-437. doi:10.1159/000445375
- Urbina F, Das A, Sudy E. Clinical variants of pityriasis rosea. World J Clin Cases. 2017;5:203-211. doi:10.12998/wjcc.v5.i6.203
- Alzahrani NA, Al Jasser MI. Geographic tonguelike presentation in a child with pityriasis rosea: case report and review of oral manifestations of pityriasis rosea. Pediatr Dermatol. 2018;35:E124-E127. doi:10.1111/pde.13417
- Sinha S, Sardana K, Garg V. Coexistence of two atypical variants of pityriasis rosea: a case report and review of literature. Pediatr Dermatol. 2012;29:538-540. doi:10.1111/j.1525-1470.2011.01549.x
- Drago F, Ciccarese G, Parodi A. Pityriasis rosea and pityriasis rosea-like eruptions: how to distinguish them? JAAD Case Rep. 2018;4:800-801. doi:10.1016/j.jdcr.2018.04.002
- Drago F, Ciccarese G, Javor S, et al. Vaccine-induced pityriasis rosea and pityriasis rosea-like eruptions: a review of the literature. J Eur Acad Dermatol Venereol. 2016;30:544-545. doi:10.1111/jdv.12942
- Mubki TF, Bin Dayel SA, Kadry R. A case of pityriasis rosea concurrent with the novel influenza A (H1N1) infection. Pediatr Dermatol. 2011;28:341-342. doi:10.1111/j.1525-1470.2010.01090.x
- Nelson CA, Moore AR, Perea AE, et al. Cat scratch disease: U.S. clinicians’ experience and knowledge [published online July 14, 2017]. Zoonoses Public Health. 2018;65:67-73. doi:10.1111/zph.12368
- Habot-Wilner Z, Trivizki O, Goldstein M, et al. Cat-scratch disease: ocular manifestations and treatment outcome. Acta Ophthalmol. 2018;96:E524-E532. doi:10.1111/aos.13684
- Schattner A, Uliel L, Dubin I. The cat did it: erythema nodosum and additional atypical presentations of Bartonella henselae infection in immunocompetent hosts [published online February 16, 2018]. BMJ Case Rep. doi:10.1136/bcr-2017-222511
- Shorbatli L, Koranyi K, Nahata M. Effectiveness of antibiotic therapy in pediatric patients with cat scratch disease. Int J Clin Pharm. 2018;40:1458-1461. doi: 10.1007/s11096-018-0746-1
- Bass JW, Freitas BC, Freitas AD, et al. Prospective randomized double blind placebo-controlled evaluation of azithromycin for treatment of cat-scratch disease. Pediatr Infect Dis J. 1998;17:447-452. doi:10.1097/00006454-199806000-00002
- Spach DH, Kaplan SL. Treatment of cat scratch disease. UpToDate. Updated December 9, 2021. Accessed September 12, 2023. https://www.uptodate.com/contents/treatment-of-cat-scratch-disease
- Drago F, Ciccarese G, Rebora A, et al. Pityriasis rosea: a comprehensive classification. Dermatology. 2016;232:431-437. doi:10.1159/000445375
- Urbina F, Das A, Sudy E. Clinical variants of pityriasis rosea. World J Clin Cases. 2017;5:203-211. doi:10.12998/wjcc.v5.i6.203
- Alzahrani NA, Al Jasser MI. Geographic tonguelike presentation in a child with pityriasis rosea: case report and review of oral manifestations of pityriasis rosea. Pediatr Dermatol. 2018;35:E124-E127. doi:10.1111/pde.13417
- Sinha S, Sardana K, Garg V. Coexistence of two atypical variants of pityriasis rosea: a case report and review of literature. Pediatr Dermatol. 2012;29:538-540. doi:10.1111/j.1525-1470.2011.01549.x
- Drago F, Ciccarese G, Parodi A. Pityriasis rosea and pityriasis rosea-like eruptions: how to distinguish them? JAAD Case Rep. 2018;4:800-801. doi:10.1016/j.jdcr.2018.04.002
- Drago F, Ciccarese G, Javor S, et al. Vaccine-induced pityriasis rosea and pityriasis rosea-like eruptions: a review of the literature. J Eur Acad Dermatol Venereol. 2016;30:544-545. doi:10.1111/jdv.12942
- Mubki TF, Bin Dayel SA, Kadry R. A case of pityriasis rosea concurrent with the novel influenza A (H1N1) infection. Pediatr Dermatol. 2011;28:341-342. doi:10.1111/j.1525-1470.2010.01090.x
Practice Points
- Dermatologists should familiarize themselves with the physical examination findings of cat scratch disease.
- There are numerous clinical variants and triggers of pityriasis rosea (PR).
- There may be a new infectious trigger for PR, and exposure to cats prior to a classic PR eruption should raise one’s suspicion as a possible cause.