User login
Fatigue and night sweats
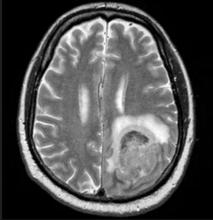
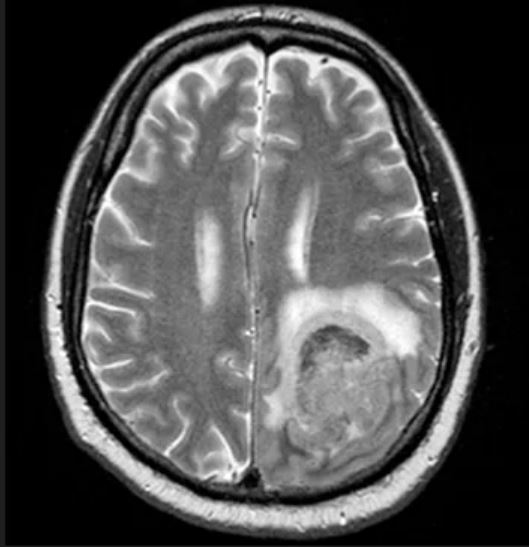
Given the patient's presentation of generalized lymphadenopathy, B symptoms, fatigue (probably from anemia), hepatosplenomegaly, immunophenotyping results of flow cell cytometry, and central nervous system (CNS) involvement, blastoid mantle cell lymphoma (MCL) is the most likely diagnosis. Although small lymphocytic lymphoma (SLL)/chronic lymphocytic leukemia (CLL) and diffuse large B-cell lymphoma (DLBCL) most often occur in men 60-70 years old with similar clinical findings, an initial presentation with a stage IV involvement is rare; moreover, SLL/CLL and DLBCL are typically CD23 positive. Pleomorphic MCL displays larger and more pleomorphic cells with irregular nuclei, prominent nucleoli, and pale cytoplasm, resembling DLBCL.
MCL is a rare type of mature B-cell lymphoma that was first described in 1992 and was recognized by World Health Organization in 2001. MCL represents 3%-10% of all non-Hodgkin lymphoma cases, with an incidence between 0.50 and 1.0 per 100,000 population. Men are more likely than women to present with MCL by a ratio of 3:1, with a median age at presentation of 67 years. Clinical presentation includes advanced disease with B symptoms (eg, night sweats, fever, weight loss), generalized lymphadenopathy, abdominal distention associated with hepatosplenomegaly, and fatigue. MCL usually affects the lymph nodes, with the spleen and bone marrow being significant sites of the disease. Stage IV disease is present in 70% of patients; the gastrointestinal tract, lung, pleura, and CNS are also frequently affected.
Besides classic MCL, several variants have been described that exhibit specific morphologic features, including small cell variant mimicking SLL marginal zone-like MCL (resembling marginal zone lymphoma), in situ mantle cell neoplasia (associated with indolent course), and two aggressive variants, including blastoid and pleomorphic MCL. These blastoid and pleomorphic variants are defined by cytomorphologic features; the criteria are somewhat subjective, but both are characterized by highly aggressive features and a dismal clinical course. In clinical cohorts, the frequency of these subsets varies widely but probably represents ∼10% of all cases.
Diagnosing MCL requires a multipronged approach. Lymph node biopsy and aspiration with immunophenotyping in MCL reveal monoclonal B cells expressing surface immunoglobulin, immunoglobulin M, or immunoglobulin D that are characteristically CD5+ and pan B-cell antigen positive (eg, CD19, CD20, CD22) but lack expression of CD10 and CD23 and overexpress cyclin D1. Bone marrow aspirate and biopsy are used more for staging than diagnosis. Blood studies commonly reveal anemia and cytopenias secondary to bone marrow infiltration (with 20%-40% of cases showing lymphocytosis > 4000 cells/μL), abnormal liver function tests, and elevated lactate dehydrogenase when tumor burden is high. The term "blastoid mantle cell lymphoma" describes a morphologic subgroup of lymphomas with blastic features that morphologically resemble the lymphoblasts found in lymphoblastic lymphoma/leukemia (roundish nuclei, a narrow rim of cytoplasm, and finely dispersed chromatin).
MCL is associated with a poor prognosis; patients generally experience disease progression after chemotherapy, even with initial treatment response rates ranging from 50% to 70%. The 5-year survival rate is about 50% in the overall population, 75% in persons younger than 50 years, and 36% in those aged 75 years or older. A poorer prognosis is also associated with the presence of the blastoid variant, commonly associated with TP53 mutations. Median survival can vary by as much as 5 years, depending on the expression of cyclin D1 and other proliferation signature genes.
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts.
Karl J. D'Silva, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Given the patient's presentation of generalized lymphadenopathy, B symptoms, fatigue (probably from anemia), hepatosplenomegaly, immunophenotyping results of flow cell cytometry, and central nervous system (CNS) involvement, blastoid mantle cell lymphoma (MCL) is the most likely diagnosis. Although small lymphocytic lymphoma (SLL)/chronic lymphocytic leukemia (CLL) and diffuse large B-cell lymphoma (DLBCL) most often occur in men 60-70 years old with similar clinical findings, an initial presentation with a stage IV involvement is rare; moreover, SLL/CLL and DLBCL are typically CD23 positive. Pleomorphic MCL displays larger and more pleomorphic cells with irregular nuclei, prominent nucleoli, and pale cytoplasm, resembling DLBCL.
MCL is a rare type of mature B-cell lymphoma that was first described in 1992 and was recognized by World Health Organization in 2001. MCL represents 3%-10% of all non-Hodgkin lymphoma cases, with an incidence between 0.50 and 1.0 per 100,000 population. Men are more likely than women to present with MCL by a ratio of 3:1, with a median age at presentation of 67 years. Clinical presentation includes advanced disease with B symptoms (eg, night sweats, fever, weight loss), generalized lymphadenopathy, abdominal distention associated with hepatosplenomegaly, and fatigue. MCL usually affects the lymph nodes, with the spleen and bone marrow being significant sites of the disease. Stage IV disease is present in 70% of patients; the gastrointestinal tract, lung, pleura, and CNS are also frequently affected.
Besides classic MCL, several variants have been described that exhibit specific morphologic features, including small cell variant mimicking SLL marginal zone-like MCL (resembling marginal zone lymphoma), in situ mantle cell neoplasia (associated with indolent course), and two aggressive variants, including blastoid and pleomorphic MCL. These blastoid and pleomorphic variants are defined by cytomorphologic features; the criteria are somewhat subjective, but both are characterized by highly aggressive features and a dismal clinical course. In clinical cohorts, the frequency of these subsets varies widely but probably represents ∼10% of all cases.
Diagnosing MCL requires a multipronged approach. Lymph node biopsy and aspiration with immunophenotyping in MCL reveal monoclonal B cells expressing surface immunoglobulin, immunoglobulin M, or immunoglobulin D that are characteristically CD5+ and pan B-cell antigen positive (eg, CD19, CD20, CD22) but lack expression of CD10 and CD23 and overexpress cyclin D1. Bone marrow aspirate and biopsy are used more for staging than diagnosis. Blood studies commonly reveal anemia and cytopenias secondary to bone marrow infiltration (with 20%-40% of cases showing lymphocytosis > 4000 cells/μL), abnormal liver function tests, and elevated lactate dehydrogenase when tumor burden is high. The term "blastoid mantle cell lymphoma" describes a morphologic subgroup of lymphomas with blastic features that morphologically resemble the lymphoblasts found in lymphoblastic lymphoma/leukemia (roundish nuclei, a narrow rim of cytoplasm, and finely dispersed chromatin).
MCL is associated with a poor prognosis; patients generally experience disease progression after chemotherapy, even with initial treatment response rates ranging from 50% to 70%. The 5-year survival rate is about 50% in the overall population, 75% in persons younger than 50 years, and 36% in those aged 75 years or older. A poorer prognosis is also associated with the presence of the blastoid variant, commonly associated with TP53 mutations. Median survival can vary by as much as 5 years, depending on the expression of cyclin D1 and other proliferation signature genes.
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts.
Karl J. D'Silva, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Given the patient's presentation of generalized lymphadenopathy, B symptoms, fatigue (probably from anemia), hepatosplenomegaly, immunophenotyping results of flow cell cytometry, and central nervous system (CNS) involvement, blastoid mantle cell lymphoma (MCL) is the most likely diagnosis. Although small lymphocytic lymphoma (SLL)/chronic lymphocytic leukemia (CLL) and diffuse large B-cell lymphoma (DLBCL) most often occur in men 60-70 years old with similar clinical findings, an initial presentation with a stage IV involvement is rare; moreover, SLL/CLL and DLBCL are typically CD23 positive. Pleomorphic MCL displays larger and more pleomorphic cells with irregular nuclei, prominent nucleoli, and pale cytoplasm, resembling DLBCL.
MCL is a rare type of mature B-cell lymphoma that was first described in 1992 and was recognized by World Health Organization in 2001. MCL represents 3%-10% of all non-Hodgkin lymphoma cases, with an incidence between 0.50 and 1.0 per 100,000 population. Men are more likely than women to present with MCL by a ratio of 3:1, with a median age at presentation of 67 years. Clinical presentation includes advanced disease with B symptoms (eg, night sweats, fever, weight loss), generalized lymphadenopathy, abdominal distention associated with hepatosplenomegaly, and fatigue. MCL usually affects the lymph nodes, with the spleen and bone marrow being significant sites of the disease. Stage IV disease is present in 70% of patients; the gastrointestinal tract, lung, pleura, and CNS are also frequently affected.
Besides classic MCL, several variants have been described that exhibit specific morphologic features, including small cell variant mimicking SLL marginal zone-like MCL (resembling marginal zone lymphoma), in situ mantle cell neoplasia (associated with indolent course), and two aggressive variants, including blastoid and pleomorphic MCL. These blastoid and pleomorphic variants are defined by cytomorphologic features; the criteria are somewhat subjective, but both are characterized by highly aggressive features and a dismal clinical course. In clinical cohorts, the frequency of these subsets varies widely but probably represents ∼10% of all cases.
Diagnosing MCL requires a multipronged approach. Lymph node biopsy and aspiration with immunophenotyping in MCL reveal monoclonal B cells expressing surface immunoglobulin, immunoglobulin M, or immunoglobulin D that are characteristically CD5+ and pan B-cell antigen positive (eg, CD19, CD20, CD22) but lack expression of CD10 and CD23 and overexpress cyclin D1. Bone marrow aspirate and biopsy are used more for staging than diagnosis. Blood studies commonly reveal anemia and cytopenias secondary to bone marrow infiltration (with 20%-40% of cases showing lymphocytosis > 4000 cells/μL), abnormal liver function tests, and elevated lactate dehydrogenase when tumor burden is high. The term "blastoid mantle cell lymphoma" describes a morphologic subgroup of lymphomas with blastic features that morphologically resemble the lymphoblasts found in lymphoblastic lymphoma/leukemia (roundish nuclei, a narrow rim of cytoplasm, and finely dispersed chromatin).
MCL is associated with a poor prognosis; patients generally experience disease progression after chemotherapy, even with initial treatment response rates ranging from 50% to 70%. The 5-year survival rate is about 50% in the overall population, 75% in persons younger than 50 years, and 36% in those aged 75 years or older. A poorer prognosis is also associated with the presence of the blastoid variant, commonly associated with TP53 mutations. Median survival can vary by as much as 5 years, depending on the expression of cyclin D1 and other proliferation signature genes.
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts.
Karl J. D'Silva, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 65-year-old man presents to the oncology clinic with a 6-week history of fatigue, night sweats, and unintentional weight loss of 15 lb. He reports occasional fevers and generalized discomfort in his abdomen and has recently been experiencing painful headaches that are not relieved with nonsteroidal anti-inflammatory drugs. His medical history is otherwise unremarkable except for mild hypertension, for which he takes medication. His family history is unremarkable.
Physical examination reveals palpable lymph nodes in the neck, axilla, and inguinal regions; the spleen is palpable 3 cm below the left costal margin. A complete blood count shows anemia (hemoglobin level, 9.1g/dL) thrombocytopenia (platelet count, 90,000 cells/μL), and lymphocytosis (total leukocyte count, 5000 cells/μL); peripheral blood smear shows small, monomorphic lymphoid cells with oval-shaped nuclei and high nuclear-to-cytoplasmic ratio. Flow cytometry of lymph node biopsy is CD5-positive and pan B-cell antigen positive (eg, CD19, CD20, and CD22) but lacks expression of CD10 and CD23. A T2-weighted MRI is ordered.
No association between atopic dermatitis and non-alcoholic fatty liver disease
Key clinical point: Comparable prevalence rates of non-alcoholic fatty liver disease (NAFLD) in patients with moderate-to-severe atopic dermatitis (AD) and those with in situ melanoma suggest that AD is not a risk factor for NAFLD.
Major finding: The prevalence rate of NAFLD was similar in patients with AD (24.1%) and those with in situ melanoma (23.2%), but it was significantly higher in patients with moderate-to-severe chronic plaque psoriasis (49.8%) compared with the other two groups (both P < .01). AD was not independently associated with NAFLD (adjusted odds ratio 1.02; 95% CI 0.78-1.26).
Study details: Findings are from a retrospective cross-sectional study including adult patients with moderate-to-severe AD (n = 144), moderate-to-severe chronic plaque psoriasis (n = 466), or in situ melanoma (n = 99).
Disclosures: This study was funded by European Union-Next Generation EU-NRRP M6C2-Investment 2.1 Enhancement and Strengthening of Biomedical Research in the National Health Service. The authors declared no conflicts of interest.
Source: Maurelli M et al. Prevalence of non-alcoholic fatty liver disease in adult individuals with moderate-to-severe atopic dermatitis. J Clin Med. 2023;12(18):6057 (Sep 19). doi: 10.3390/jcm12186057
Key clinical point: Comparable prevalence rates of non-alcoholic fatty liver disease (NAFLD) in patients with moderate-to-severe atopic dermatitis (AD) and those with in situ melanoma suggest that AD is not a risk factor for NAFLD.
Major finding: The prevalence rate of NAFLD was similar in patients with AD (24.1%) and those with in situ melanoma (23.2%), but it was significantly higher in patients with moderate-to-severe chronic plaque psoriasis (49.8%) compared with the other two groups (both P < .01). AD was not independently associated with NAFLD (adjusted odds ratio 1.02; 95% CI 0.78-1.26).
Study details: Findings are from a retrospective cross-sectional study including adult patients with moderate-to-severe AD (n = 144), moderate-to-severe chronic plaque psoriasis (n = 466), or in situ melanoma (n = 99).
Disclosures: This study was funded by European Union-Next Generation EU-NRRP M6C2-Investment 2.1 Enhancement and Strengthening of Biomedical Research in the National Health Service. The authors declared no conflicts of interest.
Source: Maurelli M et al. Prevalence of non-alcoholic fatty liver disease in adult individuals with moderate-to-severe atopic dermatitis. J Clin Med. 2023;12(18):6057 (Sep 19). doi: 10.3390/jcm12186057
Key clinical point: Comparable prevalence rates of non-alcoholic fatty liver disease (NAFLD) in patients with moderate-to-severe atopic dermatitis (AD) and those with in situ melanoma suggest that AD is not a risk factor for NAFLD.
Major finding: The prevalence rate of NAFLD was similar in patients with AD (24.1%) and those with in situ melanoma (23.2%), but it was significantly higher in patients with moderate-to-severe chronic plaque psoriasis (49.8%) compared with the other two groups (both P < .01). AD was not independently associated with NAFLD (adjusted odds ratio 1.02; 95% CI 0.78-1.26).
Study details: Findings are from a retrospective cross-sectional study including adult patients with moderate-to-severe AD (n = 144), moderate-to-severe chronic plaque psoriasis (n = 466), or in situ melanoma (n = 99).
Disclosures: This study was funded by European Union-Next Generation EU-NRRP M6C2-Investment 2.1 Enhancement and Strengthening of Biomedical Research in the National Health Service. The authors declared no conflicts of interest.
Source: Maurelli M et al. Prevalence of non-alcoholic fatty liver disease in adult individuals with moderate-to-severe atopic dermatitis. J Clin Med. 2023;12(18):6057 (Sep 19). doi: 10.3390/jcm12186057
Dupilumab shows long-term safety and efficacy in severe pediatric atopic dermatitis
Key clinical point: Long-term dupilumab treatment provides sustained clinical benefits and acceptable safety in children age 6-11 years with uncontrolled severe atopic dermatitis (AD).
Major finding: By week 52, 41% of patients achieved an Investigator’s Global Assessment score of 0 or 1, and 82% of patients achieved ≥75% improvement in the Eczema Area and Severity Index scores compared with the LIBERTY AD PEDS baseline. Treatment-emergent adverse events were mostly of mild or moderate severity.
Study details: This analysis of data from the LIBERTY AD PED-OLE study included 321 children (age 6-11 years) with severe AD who previously participated in LIBERTY AD PEDS and received 300 mg dupilumab every 4 weeks or an up-titrated weight-tiered dose of 200 or 300 mg dupilumab every 2 weeks.
Disclosures: This study was sponsored by Sanofi and Regeneron Pharmaceuticals, Inc. Seven authors declared being employees of or holding stocks or stock options in Sanofi or Regeneron. The other authors declared ties with various sources, including Sanofi and Regeneron.
Source: Cork MJ et al. Dupilumab safety and efficacy in a phase III open-label extension trial in children 6-11 years of age with severe atopic dermatitis. Dermatol Ther (Heidelb). 2023 (Sep 26). doi: 10.1007/s13555-023-01016-9
Key clinical point: Long-term dupilumab treatment provides sustained clinical benefits and acceptable safety in children age 6-11 years with uncontrolled severe atopic dermatitis (AD).
Major finding: By week 52, 41% of patients achieved an Investigator’s Global Assessment score of 0 or 1, and 82% of patients achieved ≥75% improvement in the Eczema Area and Severity Index scores compared with the LIBERTY AD PEDS baseline. Treatment-emergent adverse events were mostly of mild or moderate severity.
Study details: This analysis of data from the LIBERTY AD PED-OLE study included 321 children (age 6-11 years) with severe AD who previously participated in LIBERTY AD PEDS and received 300 mg dupilumab every 4 weeks or an up-titrated weight-tiered dose of 200 or 300 mg dupilumab every 2 weeks.
Disclosures: This study was sponsored by Sanofi and Regeneron Pharmaceuticals, Inc. Seven authors declared being employees of or holding stocks or stock options in Sanofi or Regeneron. The other authors declared ties with various sources, including Sanofi and Regeneron.
Source: Cork MJ et al. Dupilumab safety and efficacy in a phase III open-label extension trial in children 6-11 years of age with severe atopic dermatitis. Dermatol Ther (Heidelb). 2023 (Sep 26). doi: 10.1007/s13555-023-01016-9
Key clinical point: Long-term dupilumab treatment provides sustained clinical benefits and acceptable safety in children age 6-11 years with uncontrolled severe atopic dermatitis (AD).
Major finding: By week 52, 41% of patients achieved an Investigator’s Global Assessment score of 0 or 1, and 82% of patients achieved ≥75% improvement in the Eczema Area and Severity Index scores compared with the LIBERTY AD PEDS baseline. Treatment-emergent adverse events were mostly of mild or moderate severity.
Study details: This analysis of data from the LIBERTY AD PED-OLE study included 321 children (age 6-11 years) with severe AD who previously participated in LIBERTY AD PEDS and received 300 mg dupilumab every 4 weeks or an up-titrated weight-tiered dose of 200 or 300 mg dupilumab every 2 weeks.
Disclosures: This study was sponsored by Sanofi and Regeneron Pharmaceuticals, Inc. Seven authors declared being employees of or holding stocks or stock options in Sanofi or Regeneron. The other authors declared ties with various sources, including Sanofi and Regeneron.
Source: Cork MJ et al. Dupilumab safety and efficacy in a phase III open-label extension trial in children 6-11 years of age with severe atopic dermatitis. Dermatol Ther (Heidelb). 2023 (Sep 26). doi: 10.1007/s13555-023-01016-9
Tralokinumab improves clinical responses at week 16 in moderate-to-severe AD despite IGA 0/1 nonachievement
Key clinical point: Tralokinumab led to clinically meaningful responses in adults with moderate-to-severe atopic dermatitis (AD) who failed to achieve an Investigator’s Global Assessment (IGA) score of 0 or 1 at 16 weeks without rescue medication.
Major finding: At week 16, a significantly greater proportion of patients receiving tralokinumab vs placebo achieved ≥ 50% improvement in the Eczema Area and Severity Index scores (33.0% vs 13.0%; P < .0001) and ≥ 3-point improvement in the itch Numerical Rating Scale scores (22.6% vs 9.4%; P < .0001).
Study details: This post hoc analysis of data from ECZTRA 1 and 2 trials included adults with moderate-to-severe AD who were randomized to receive tralokinumab (n = 966) or placebo (n = 362) and failed to achieve an IGA score of 0 or 1 at week 16 without rescue medication.
Disclosures: ECZTRA 1 and 2 were sponsored by LEO Pharma A/S, Denmark. Several authors declared ties with LEO Pharma, among others. T Mark declared being an employee and stockholder of LEO Pharma A/S.
Source: Simpson EL et al. Tralokinumab provides clinically meaningful responses at week 16 in adults with moderate-to-severe atopic dermatitis who do not achieve IGA 0/1. Am J Clin Dermatol. 2023 (Oct 7). doi: 10.1007/s40257-023-00817-0
Key clinical point: Tralokinumab led to clinically meaningful responses in adults with moderate-to-severe atopic dermatitis (AD) who failed to achieve an Investigator’s Global Assessment (IGA) score of 0 or 1 at 16 weeks without rescue medication.
Major finding: At week 16, a significantly greater proportion of patients receiving tralokinumab vs placebo achieved ≥ 50% improvement in the Eczema Area and Severity Index scores (33.0% vs 13.0%; P < .0001) and ≥ 3-point improvement in the itch Numerical Rating Scale scores (22.6% vs 9.4%; P < .0001).
Study details: This post hoc analysis of data from ECZTRA 1 and 2 trials included adults with moderate-to-severe AD who were randomized to receive tralokinumab (n = 966) or placebo (n = 362) and failed to achieve an IGA score of 0 or 1 at week 16 without rescue medication.
Disclosures: ECZTRA 1 and 2 were sponsored by LEO Pharma A/S, Denmark. Several authors declared ties with LEO Pharma, among others. T Mark declared being an employee and stockholder of LEO Pharma A/S.
Source: Simpson EL et al. Tralokinumab provides clinically meaningful responses at week 16 in adults with moderate-to-severe atopic dermatitis who do not achieve IGA 0/1. Am J Clin Dermatol. 2023 (Oct 7). doi: 10.1007/s40257-023-00817-0
Key clinical point: Tralokinumab led to clinically meaningful responses in adults with moderate-to-severe atopic dermatitis (AD) who failed to achieve an Investigator’s Global Assessment (IGA) score of 0 or 1 at 16 weeks without rescue medication.
Major finding: At week 16, a significantly greater proportion of patients receiving tralokinumab vs placebo achieved ≥ 50% improvement in the Eczema Area and Severity Index scores (33.0% vs 13.0%; P < .0001) and ≥ 3-point improvement in the itch Numerical Rating Scale scores (22.6% vs 9.4%; P < .0001).
Study details: This post hoc analysis of data from ECZTRA 1 and 2 trials included adults with moderate-to-severe AD who were randomized to receive tralokinumab (n = 966) or placebo (n = 362) and failed to achieve an IGA score of 0 or 1 at week 16 without rescue medication.
Disclosures: ECZTRA 1 and 2 were sponsored by LEO Pharma A/S, Denmark. Several authors declared ties with LEO Pharma, among others. T Mark declared being an employee and stockholder of LEO Pharma A/S.
Source: Simpson EL et al. Tralokinumab provides clinically meaningful responses at week 16 in adults with moderate-to-severe atopic dermatitis who do not achieve IGA 0/1. Am J Clin Dermatol. 2023 (Oct 7). doi: 10.1007/s40257-023-00817-0
Methotrexate reduces epidermal hyperplasia and alters cutaneous IL-31 and IL-31RA expression in AD
Key clinical point: Methotrexate treatment decreased epidermal hyperplasia and altered the cutaneous expression of pruritus-related inflammatory cytokines and receptors, including interleukin-31 (IL-31) and IL-31 alpha receptor subunits (RA), in adults with moderate-to-severe refractory atopic dermatitis (AD).
Major finding: At 24 weeks of treatment, methotrexate led to a significant increase in IL-31RA expression in the epidermis (P = .016), decrease in IL-31 gene expression in lesional skin (P = .019), and reduction in the mean epidermal thickness (P = .021).
Study details: Findings are from a prospective cohort study including 12 adults with moderate-to-severe refractory AD who orally received 15 mg methotrexate per week and were matched with 10 control individuals without AD.
Disclosures: This study was supported by Fundo de Apoio à Dermatologia de São Paulo, Brazil. The authors declared no conflicts of interest.
Source: Samorano LP et al. Methotrexate for refractory adult atopic dermatitis leads to alterations in cutaneous IL-31 and IL-31RA expression. An Bras Dermatol. 2023 (Sep 18). doi: 10.1016/j.abd.2023.01.002
Key clinical point: Methotrexate treatment decreased epidermal hyperplasia and altered the cutaneous expression of pruritus-related inflammatory cytokines and receptors, including interleukin-31 (IL-31) and IL-31 alpha receptor subunits (RA), in adults with moderate-to-severe refractory atopic dermatitis (AD).
Major finding: At 24 weeks of treatment, methotrexate led to a significant increase in IL-31RA expression in the epidermis (P = .016), decrease in IL-31 gene expression in lesional skin (P = .019), and reduction in the mean epidermal thickness (P = .021).
Study details: Findings are from a prospective cohort study including 12 adults with moderate-to-severe refractory AD who orally received 15 mg methotrexate per week and were matched with 10 control individuals without AD.
Disclosures: This study was supported by Fundo de Apoio à Dermatologia de São Paulo, Brazil. The authors declared no conflicts of interest.
Source: Samorano LP et al. Methotrexate for refractory adult atopic dermatitis leads to alterations in cutaneous IL-31 and IL-31RA expression. An Bras Dermatol. 2023 (Sep 18). doi: 10.1016/j.abd.2023.01.002
Key clinical point: Methotrexate treatment decreased epidermal hyperplasia and altered the cutaneous expression of pruritus-related inflammatory cytokines and receptors, including interleukin-31 (IL-31) and IL-31 alpha receptor subunits (RA), in adults with moderate-to-severe refractory atopic dermatitis (AD).
Major finding: At 24 weeks of treatment, methotrexate led to a significant increase in IL-31RA expression in the epidermis (P = .016), decrease in IL-31 gene expression in lesional skin (P = .019), and reduction in the mean epidermal thickness (P = .021).
Study details: Findings are from a prospective cohort study including 12 adults with moderate-to-severe refractory AD who orally received 15 mg methotrexate per week and were matched with 10 control individuals without AD.
Disclosures: This study was supported by Fundo de Apoio à Dermatologia de São Paulo, Brazil. The authors declared no conflicts of interest.
Source: Samorano LP et al. Methotrexate for refractory adult atopic dermatitis leads to alterations in cutaneous IL-31 and IL-31RA expression. An Bras Dermatol. 2023 (Sep 18). doi: 10.1016/j.abd.2023.01.002
Tacrolimus tops hydrocortisone in pediatric atopic dermatitis treatment
Key clinical point: Topical treatment with tacrolimus vs hydrocortisone was more efficacious in reducing inflammatory marker levels and had a better safety profile in children with atopic dermatitis (AD).
Major finding: After 3 weeks, patients receiving tacrolimus vs hydrocortisone showed a significantly greater decrease in the mean serum levels of IL-10 (P = .05), IL-17 (P = .021), and IL-23 (P = .03), lower rates of skin atrophy (P < .05), and higher but manageable incidences of mild-to-moderate transient stinging and erythema (P < .05).
Study details: Findings are from a prospective randomized clinical trial including 200 children with AD (age 2-16 years) who were randomly assigned in a 1:1 ratio to receive either 0.03% topical tacrolimus ointment or 1% hydrocortisone cream twice daily.
Disclosures: This study did not disclose any funding source. The authors declared no conflicts of interest.
Source: Mohamed AA et al. A comparative randomized clinical trial evaluating the efficacy and safety of tacrolimus versus hydrocortisone as a topical treatment of atopic dermatitis in children. Front Pharmacol. 2023;14:1202325 (Sep 20). doi: 10.3389/fphar.2023.1202325
Key clinical point: Topical treatment with tacrolimus vs hydrocortisone was more efficacious in reducing inflammatory marker levels and had a better safety profile in children with atopic dermatitis (AD).
Major finding: After 3 weeks, patients receiving tacrolimus vs hydrocortisone showed a significantly greater decrease in the mean serum levels of IL-10 (P = .05), IL-17 (P = .021), and IL-23 (P = .03), lower rates of skin atrophy (P < .05), and higher but manageable incidences of mild-to-moderate transient stinging and erythema (P < .05).
Study details: Findings are from a prospective randomized clinical trial including 200 children with AD (age 2-16 years) who were randomly assigned in a 1:1 ratio to receive either 0.03% topical tacrolimus ointment or 1% hydrocortisone cream twice daily.
Disclosures: This study did not disclose any funding source. The authors declared no conflicts of interest.
Source: Mohamed AA et al. A comparative randomized clinical trial evaluating the efficacy and safety of tacrolimus versus hydrocortisone as a topical treatment of atopic dermatitis in children. Front Pharmacol. 2023;14:1202325 (Sep 20). doi: 10.3389/fphar.2023.1202325
Key clinical point: Topical treatment with tacrolimus vs hydrocortisone was more efficacious in reducing inflammatory marker levels and had a better safety profile in children with atopic dermatitis (AD).
Major finding: After 3 weeks, patients receiving tacrolimus vs hydrocortisone showed a significantly greater decrease in the mean serum levels of IL-10 (P = .05), IL-17 (P = .021), and IL-23 (P = .03), lower rates of skin atrophy (P < .05), and higher but manageable incidences of mild-to-moderate transient stinging and erythema (P < .05).
Study details: Findings are from a prospective randomized clinical trial including 200 children with AD (age 2-16 years) who were randomly assigned in a 1:1 ratio to receive either 0.03% topical tacrolimus ointment or 1% hydrocortisone cream twice daily.
Disclosures: This study did not disclose any funding source. The authors declared no conflicts of interest.
Source: Mohamed AA et al. A comparative randomized clinical trial evaluating the efficacy and safety of tacrolimus versus hydrocortisone as a topical treatment of atopic dermatitis in children. Front Pharmacol. 2023;14:1202325 (Sep 20). doi: 10.3389/fphar.2023.1202325
Atopic dermatitis affects outcomes in occupational contact dermatitis
Key clinical point: Atopic dermatitis (AD) negatively affects the prognosis, quality of life (QoL), and work life in young workers with occupational contact dermatitis (OCD).
Major finding: The prevalence of previously diagnosed AD was 41.8%. A higher proportion of workers with vs without AD experienced eczema during the last 3 months of response submission (adjusted odds ratio [aOR] 1.7; P < .001) and reported that OCD had negatively affected their choice of jobs and occupations (aOR 1.4; P < .001). Workers with vs without AD had significantly higher mean scores in the emotions (P < .01) and symptoms (P < .001) subscales of the Skindex-29 assessment of QoL.
Study details: Findings are from a retrospective questionnaire-based study including 2392 workers age < 35 years with OCD who answered a question about being previously diagnosed with AD.
Disclosures: This study was funded by the Danish Working Environment Research Fund. The authors declared no conflicts of interest.
Source: Dietz JB et al. Impact of atopic dermatitis on occupational contact dermatitis among young people: A retrospective cohort study. Contact Dermatitis. 2023 (Sep 26). doi: 10.1111/cod.14426
Key clinical point: Atopic dermatitis (AD) negatively affects the prognosis, quality of life (QoL), and work life in young workers with occupational contact dermatitis (OCD).
Major finding: The prevalence of previously diagnosed AD was 41.8%. A higher proportion of workers with vs without AD experienced eczema during the last 3 months of response submission (adjusted odds ratio [aOR] 1.7; P < .001) and reported that OCD had negatively affected their choice of jobs and occupations (aOR 1.4; P < .001). Workers with vs without AD had significantly higher mean scores in the emotions (P < .01) and symptoms (P < .001) subscales of the Skindex-29 assessment of QoL.
Study details: Findings are from a retrospective questionnaire-based study including 2392 workers age < 35 years with OCD who answered a question about being previously diagnosed with AD.
Disclosures: This study was funded by the Danish Working Environment Research Fund. The authors declared no conflicts of interest.
Source: Dietz JB et al. Impact of atopic dermatitis on occupational contact dermatitis among young people: A retrospective cohort study. Contact Dermatitis. 2023 (Sep 26). doi: 10.1111/cod.14426
Key clinical point: Atopic dermatitis (AD) negatively affects the prognosis, quality of life (QoL), and work life in young workers with occupational contact dermatitis (OCD).
Major finding: The prevalence of previously diagnosed AD was 41.8%. A higher proportion of workers with vs without AD experienced eczema during the last 3 months of response submission (adjusted odds ratio [aOR] 1.7; P < .001) and reported that OCD had negatively affected their choice of jobs and occupations (aOR 1.4; P < .001). Workers with vs without AD had significantly higher mean scores in the emotions (P < .01) and symptoms (P < .001) subscales of the Skindex-29 assessment of QoL.
Study details: Findings are from a retrospective questionnaire-based study including 2392 workers age < 35 years with OCD who answered a question about being previously diagnosed with AD.
Disclosures: This study was funded by the Danish Working Environment Research Fund. The authors declared no conflicts of interest.
Source: Dietz JB et al. Impact of atopic dermatitis on occupational contact dermatitis among young people: A retrospective cohort study. Contact Dermatitis. 2023 (Sep 26). doi: 10.1111/cod.14426
Xyloglucan-pea protein a possible steroid-sparing alternative for treating pediatric AD
Key clinical point: Xyloglucan and pea protein (XG-PP)-based topical treatment shows safety and efficacy outcomes comparable with those of hydrocortisone in pediatric patients with atopic dermatitis (AD).
Major finding: At 8 and 15 days of treatment, both XG-PP and hydrocortisone led to significant decreases in the AD Severity Index (ADSI) score (all P = .00001). Both treatment arms showed similar decrease in ADSI scores at 8 (P = .91) and 15 (P = .92) days. No adverse events were reported in the XG-PP treatment arm.
Study details: Findings are from a prospective multicenter study including 42 pediatric patients with mild-to-moderate AD (age 6 months-12 years) who were randomly assigned to receive either topical XG-PP-based cream or hydrocortisone twice daily for 14 consecutive days.
Disclosures: This study was sponsored by Novintethical Pharma SA. The authors declared no conflicts of interest.
Source: Sowlati M et al. Efficacy and tolerability of a novel topical treatment containing pea protein and xyloglucan in the management of atopic dermatitis in children: A prospective, multicenter clinical study. Dermatol Ther (Heidelb). 2023 (Sep 23). doi: 10.1007/s13555-023-01035-6
Key clinical point: Xyloglucan and pea protein (XG-PP)-based topical treatment shows safety and efficacy outcomes comparable with those of hydrocortisone in pediatric patients with atopic dermatitis (AD).
Major finding: At 8 and 15 days of treatment, both XG-PP and hydrocortisone led to significant decreases in the AD Severity Index (ADSI) score (all P = .00001). Both treatment arms showed similar decrease in ADSI scores at 8 (P = .91) and 15 (P = .92) days. No adverse events were reported in the XG-PP treatment arm.
Study details: Findings are from a prospective multicenter study including 42 pediatric patients with mild-to-moderate AD (age 6 months-12 years) who were randomly assigned to receive either topical XG-PP-based cream or hydrocortisone twice daily for 14 consecutive days.
Disclosures: This study was sponsored by Novintethical Pharma SA. The authors declared no conflicts of interest.
Source: Sowlati M et al. Efficacy and tolerability of a novel topical treatment containing pea protein and xyloglucan in the management of atopic dermatitis in children: A prospective, multicenter clinical study. Dermatol Ther (Heidelb). 2023 (Sep 23). doi: 10.1007/s13555-023-01035-6
Key clinical point: Xyloglucan and pea protein (XG-PP)-based topical treatment shows safety and efficacy outcomes comparable with those of hydrocortisone in pediatric patients with atopic dermatitis (AD).
Major finding: At 8 and 15 days of treatment, both XG-PP and hydrocortisone led to significant decreases in the AD Severity Index (ADSI) score (all P = .00001). Both treatment arms showed similar decrease in ADSI scores at 8 (P = .91) and 15 (P = .92) days. No adverse events were reported in the XG-PP treatment arm.
Study details: Findings are from a prospective multicenter study including 42 pediatric patients with mild-to-moderate AD (age 6 months-12 years) who were randomly assigned to receive either topical XG-PP-based cream or hydrocortisone twice daily for 14 consecutive days.
Disclosures: This study was sponsored by Novintethical Pharma SA. The authors declared no conflicts of interest.
Source: Sowlati M et al. Efficacy and tolerability of a novel topical treatment containing pea protein and xyloglucan in the management of atopic dermatitis in children: A prospective, multicenter clinical study. Dermatol Ther (Heidelb). 2023 (Sep 23). doi: 10.1007/s13555-023-01035-6
Increased risk for neuropsychiatric disorders in adults with AD
Key clinical point: Patients with atopic dermatitis (AD) have an increased risk for multiple neuropsychiatric conditions; however, the risk profiles for specific neuropsychiatric conditions differ with AD severity.
Major finding: Adults with AD (of any severity level) vs without AD had a higher risk for anxiety (adjusted hazard ratio [aHR] 1.14, 95% CI 1.13-1.15), depression (aHR 1.14, 95% CI 1.13-1.15), and obsessive-compulsive disorder (aHR 1.48, 95% CI 1.38-1.58); the risk for autism increased in patients with mild (aHR 1.55; 95% CI 1.26-1.89) and moderate (aHR 1.40; 95% CI 1.07-1.83) AD and that for attention-deficit/hyperactivity disorder increased in those with mild AD (aHR 1.27; 95% CI 1.03-1.55].
Study details: This population-based cohort study included 625,083 adults with AD who were matched with 2,678,888 control adults without AD.
Disclosures: This study was supported by a contract from Pfizer, Inc. Some authors declared receiving research or fellowship funding or consultation honoraria from various sources, including Pfizer. AR Lemeshow declared being an employee of Pfizer.
Source: Wan J et al. Neuropsychiatric disorders in adults with atopic dermatitis: A population-based cohort study. J Eur Acad Dermatol Venereol. 2023 (Sep 20). doi: 10.1111/jdv.19518
Key clinical point: Patients with atopic dermatitis (AD) have an increased risk for multiple neuropsychiatric conditions; however, the risk profiles for specific neuropsychiatric conditions differ with AD severity.
Major finding: Adults with AD (of any severity level) vs without AD had a higher risk for anxiety (adjusted hazard ratio [aHR] 1.14, 95% CI 1.13-1.15), depression (aHR 1.14, 95% CI 1.13-1.15), and obsessive-compulsive disorder (aHR 1.48, 95% CI 1.38-1.58); the risk for autism increased in patients with mild (aHR 1.55; 95% CI 1.26-1.89) and moderate (aHR 1.40; 95% CI 1.07-1.83) AD and that for attention-deficit/hyperactivity disorder increased in those with mild AD (aHR 1.27; 95% CI 1.03-1.55].
Study details: This population-based cohort study included 625,083 adults with AD who were matched with 2,678,888 control adults without AD.
Disclosures: This study was supported by a contract from Pfizer, Inc. Some authors declared receiving research or fellowship funding or consultation honoraria from various sources, including Pfizer. AR Lemeshow declared being an employee of Pfizer.
Source: Wan J et al. Neuropsychiatric disorders in adults with atopic dermatitis: A population-based cohort study. J Eur Acad Dermatol Venereol. 2023 (Sep 20). doi: 10.1111/jdv.19518
Key clinical point: Patients with atopic dermatitis (AD) have an increased risk for multiple neuropsychiatric conditions; however, the risk profiles for specific neuropsychiatric conditions differ with AD severity.
Major finding: Adults with AD (of any severity level) vs without AD had a higher risk for anxiety (adjusted hazard ratio [aHR] 1.14, 95% CI 1.13-1.15), depression (aHR 1.14, 95% CI 1.13-1.15), and obsessive-compulsive disorder (aHR 1.48, 95% CI 1.38-1.58); the risk for autism increased in patients with mild (aHR 1.55; 95% CI 1.26-1.89) and moderate (aHR 1.40; 95% CI 1.07-1.83) AD and that for attention-deficit/hyperactivity disorder increased in those with mild AD (aHR 1.27; 95% CI 1.03-1.55].
Study details: This population-based cohort study included 625,083 adults with AD who were matched with 2,678,888 control adults without AD.
Disclosures: This study was supported by a contract from Pfizer, Inc. Some authors declared receiving research or fellowship funding or consultation honoraria from various sources, including Pfizer. AR Lemeshow declared being an employee of Pfizer.
Source: Wan J et al. Neuropsychiatric disorders in adults with atopic dermatitis: A population-based cohort study. J Eur Acad Dermatol Venereol. 2023 (Sep 20). doi: 10.1111/jdv.19518
Methotrexate is a safe and efficacious alternative to ciclosporin in children with severe AD
Key clinical point: Both ciclosporin and methotrexate were effective against severe atopic dermatitis (AD) in children, but ciclosporin resulted in a more rapid response whereas methotrexate led to more sustained disease control even after treatment discontinuation.
Major finding: At 12 weeks, a significantly higher proportion of patients achieved 50% improvement in Objective Severity Scoring of Atopic Dermatitis scores (o-SCORAD-50) with ciclosporin vs methotrexate (P = .012). However, at 60 weeks, the proportion of patients who achieved o-SCORAD-50 was higher with methotrexate vs ciclosporin (P = .022). Adverse event rates were comparable in both groups.
Study details: The TREatment of severe Atopic Eczema Trial included 103 children with severe AD (age 2-16 years) who were unresponsive to topical treatments and were randomly assigned to receive ciclosporin or methotrexate.
Disclosures: This study was funded by the UK Medical Research Council/National Institute for Health Research (NIHR). Some authors, including the lead author, declared receiving consulting fees, advisory fees, or research funding from various sources, including UK NIHR.
Source: Flohr C et al and the TREAT Trial Investigators. Efficacy and safety of ciclosporin versus methotrexate in the treatment of severe atopic dermatitis in children and young people (TREAT): A multicentre, parallel group, assessor-blinded clinical trial. Br J Dermatol. 2023 (Sep 19). doi: 10.1093/bjd/ljad281
Key clinical point: Both ciclosporin and methotrexate were effective against severe atopic dermatitis (AD) in children, but ciclosporin resulted in a more rapid response whereas methotrexate led to more sustained disease control even after treatment discontinuation.
Major finding: At 12 weeks, a significantly higher proportion of patients achieved 50% improvement in Objective Severity Scoring of Atopic Dermatitis scores (o-SCORAD-50) with ciclosporin vs methotrexate (P = .012). However, at 60 weeks, the proportion of patients who achieved o-SCORAD-50 was higher with methotrexate vs ciclosporin (P = .022). Adverse event rates were comparable in both groups.
Study details: The TREatment of severe Atopic Eczema Trial included 103 children with severe AD (age 2-16 years) who were unresponsive to topical treatments and were randomly assigned to receive ciclosporin or methotrexate.
Disclosures: This study was funded by the UK Medical Research Council/National Institute for Health Research (NIHR). Some authors, including the lead author, declared receiving consulting fees, advisory fees, or research funding from various sources, including UK NIHR.
Source: Flohr C et al and the TREAT Trial Investigators. Efficacy and safety of ciclosporin versus methotrexate in the treatment of severe atopic dermatitis in children and young people (TREAT): A multicentre, parallel group, assessor-blinded clinical trial. Br J Dermatol. 2023 (Sep 19). doi: 10.1093/bjd/ljad281
Key clinical point: Both ciclosporin and methotrexate were effective against severe atopic dermatitis (AD) in children, but ciclosporin resulted in a more rapid response whereas methotrexate led to more sustained disease control even after treatment discontinuation.
Major finding: At 12 weeks, a significantly higher proportion of patients achieved 50% improvement in Objective Severity Scoring of Atopic Dermatitis scores (o-SCORAD-50) with ciclosporin vs methotrexate (P = .012). However, at 60 weeks, the proportion of patients who achieved o-SCORAD-50 was higher with methotrexate vs ciclosporin (P = .022). Adverse event rates were comparable in both groups.
Study details: The TREatment of severe Atopic Eczema Trial included 103 children with severe AD (age 2-16 years) who were unresponsive to topical treatments and were randomly assigned to receive ciclosporin or methotrexate.
Disclosures: This study was funded by the UK Medical Research Council/National Institute for Health Research (NIHR). Some authors, including the lead author, declared receiving consulting fees, advisory fees, or research funding from various sources, including UK NIHR.
Source: Flohr C et al and the TREAT Trial Investigators. Efficacy and safety of ciclosporin versus methotrexate in the treatment of severe atopic dermatitis in children and young people (TREAT): A multicentre, parallel group, assessor-blinded clinical trial. Br J Dermatol. 2023 (Sep 19). doi: 10.1093/bjd/ljad281