User login
Cannabis and schizophrenia: A complex relationship
Approximately 1 in 200 individuals will be diagnosed with schizophrenia in their lifetime.1 DSM-5 criteria for the diagnosis of schizophrenia require the presence of ≥2 of 5 symptoms: delusions, hallucinations, disordered speech, grossly disorganized (or catatonic) behavior, and negative symptoms such as flat affect or avolition.2 Multiple studies have found increased rates of cannabis use among patients with schizophrenia. Because cognitive deficits are the chief predictor of clinical outcomes and quality of life in individuals with schizophrenia, the cognitive effects of cannabis use among these patients are of clinical significance.3 As legislation increasingly allows for the sale, possession, and consumption of cannabis, it is crucial to provide clinicians with evidence-based recommendations for treating patients who regularly use cannabis (approximately 8% of the adult population3). In this article, we analyze several peer-reviewed studies to investigate the impact of cannabis use on the onset and development of schizophrenia.
A look at substance-induced psychosis
Schizophrenia is associated with several structural brain changes, and some of these changes may be influenced by cannabis use (Box4). The biochemical etiology of schizophrenia is poorly understood but thought to involve dopamine, glutamate, serotonin, and gamma-aminobutyric acid. Certain positive symptoms, such as hallucinations, are uniquely human and difficult to study in animal models.5 Psychoactive substance use, especially cannabis, is frequently comorbid with schizophrenia. Additionally, certain individuals may be more predisposed to substance-induced psychosis than others based on genetic variation and underlying brain structure changes.4 Substance-induced psychosis is a psychotic state following the ingestion of a psychoactive substance or drug withdrawal lasting ≥48 hours.6 The psychoactive effects of cannabis have been associated with an exacerbation of existing schizophrenia symptoms.7 In 1998, Hall7 proposed 2 hypotheses to explain the relationship between cannabis and psychosis. The first was that heavy consumption of cannabis triggers a specific type of cannabis psychosis.7 The second was that cannabis use exacerbates existing schizophrenia, making the symptoms worse.7 Hall7 concluded that there was a complicated interaction among an individual’s vulnerability to their stressors, environment, and genetics.
Box
Schizophrenia is associated with several structural changes in the brain, including lateral ventriculomegaly, reduced prefrontal cortex volume, and generalized atrophy. These changes may precede illness and act as a risk marker.4 A multivariate regression analysis that compared patients with schizophrenia who were cannabis users vs patients with schizophrenia who were nonusers found that those with high-level cannabis use had relatively higher left and right lateral ventricle volume (r = 0.208, P = .13, and r = 0.226, P = .007, respectively) as well as increased third ventricle volume (r = 0.271, P = .001).4 These changes were dose-dependent and may lead to worse disease outcomes.4
Cannabis, COMT, and homocysteine
Great advances have been made in our ability to examine the association between genetics and metabolism. One example of this is the interaction between the catechol-O-methyltransferase (COMT) gene and the active component of cannabis, delta-9-tetrahydrocannabinol (THC). COMT codes for an enzyme that degrades cortical dopamine. The Val158Met polymorphism of this gene increases COMT activity, leading to increased dopamine catabolism, and thus decreased levels of extracellular dopamine, which induces an increase in mesolimbic dopaminergic activity, thereby increasing susceptibility to psychosis.3
In a study that genotyped 135 patients with schizophrenia, the Val158Met polymorphism was present in 29.63% of participants.3 Because THC can induce episodes of psychosis, individuals with this polymorphism may be at a higher risk of developing schizophrenia. Compared to Met carrier control participants with similar histories of cannabis consumption, those with the Val158Met polymorphism demonstrated markedly worse performance on tests of verbal fluency and processing speed.3 This is clinically significant because cognitive impairments are a major prognostic factor in schizophrenia, and identifying patients with this polymorphism could help personalize interventions for those who consume cannabis and are at risk of developing schizophrenia.
A study that evaluated 56 patients with first-episode schizophrenia found that having a history of cannabis abuse was associated with significantly higher levels of homocysteine as well as lower levels of high-density lipoprotein and vitamin B12.8 Homocysteine is an agonist at the glutamate binding site and a partial antagonist at the glycine co-agonist site in the N-methyl-
The C677T polymorphism in MTHFR may predict the risk of developing metabolic syndrome in patients taking second-generation antipsychotics.8 Elevations in homocysteine by as little as 5 μmol/L may increase schizophrenia risk by 70% compared to controls, possibly due to homocysteine initiating neuronal apoptosis, catalyzing dysfunction of the mitochondria, or increasing oxidative stress.8 There is a positive correlation between homocysteine levels and severity of negative symptoms (P = .006) and general psychopathology (P = .008) of schizophrenia when analyzed using the Positive and Negative Syndrome Scale.8 Negative symptoms such as blunted affect, apathy, anhedonia, and loss of motivation significantly impact the social and economic outcomes of patients diagnosed with schizophrenia.
Research paints a mixed picture
A Danish study analyzed the rates of conversion to schizophrenia or bipolar disorder (BD) among 6,788 individuals who received a diagnosis of substance-induced psychosis from 1994 to 2014.6 Ten comparison participants were selected for each case participant, matched on sex and year/month of birth. Participants were followed until the first occurrence of schizophrenia or BD, death, or emigration from Denmark. Substances implicated in the initial psychotic episode included cannabis, alcohol, opioids, sedatives, cocaine, amphetamines, hallucinogens, and combinations of substances.
Continue to: The overall conversion rate...
The overall conversion rate over 20 years was 32.2% (95% CI, 29.7 to 34.9), with 26.0% developing schizophrenia vs 8.4% developing BD.6 Of the substances involved, cannabis was the most common, implicated in 41.2% (95% CI, 36.6 to 46.2) of cases.6 One-half of male patients converted within 2.0 years and one-half of female patients converted within 4.4 years after a cannabis-induced psychosis.6
This study had several limitations. It could not account for any short-term psychotic symptoms experienced by the general population, especially after cannabis use. Such patients might not seek treatment. Thus, the results might not be generalizable to the general population. The study did not evaluate if conversion rates differed based on continued substance use following the psychosis episode, or the amount of each substance taken prior to the episode. Dose-dependence was not well elucidated, and this study only looked at patients from Denmark and did not account for socioeconomic status.6
Another Danish study looked at the influences of gender and cannabis use in the early course of the disease in 133 patients with schizophrenia.9 These researchers found that male gender was a significant predictor of earlier onset of dysfunction socially and in the workplace, as well as a higher risk of developing negative symptoms. However, compared to gender, cannabis use was a stronger predictor of age at first psychotic episode. For cannabis users, the median age of onset of negative symptoms was 23.7, compared to 38.4 for nonusers (P < .001).9
Cannabis use is significantly elevated among individuals with psychosis, with a 12-month prevalence of 29.2% compared to 4.0% among the general population of the United States.10 In a study that assessed 229 patients with a schizophrenia spectrum disorder during their first hospitalization and 6 months, 2 years, 4 years, and 10 years later, Foti et al10 found that the lifetime rate of cannabis use was 66.2%. Survival analysis found cannabis use doubled the risk of the onset of psychosis compared to nonusers of the same age (hazard ratio [HR] = 1.97; 95% CI, 1.48 to 2.62, P < .001), even after adjusting for socioeconomic status, age, and gender (HR = 1.34; 95% CI, 1.01 to 1.77, P < .05).10 Additionally, Foti et al10 found significant positive correlations between psychotic symptoms and cannabis use in patients with schizophrenia over the course of 10 years. An increase in symptoms was associated with a higher likelihood of cannabis use, and a decrease in symptoms was correlated with a lower likelihood of use (adjusted odds ratio = 1.64; 95% CI, 1.12 to 2.43, P < .0125).10
Ortiz-Medina et al11 conducted a meta-analysis of 22 studies of 15 cohorts from healthy populations and 12 other cohort follow-up studies that evaluated the onset of psychotic symptoms in individuals who used cannabis. Most studies found associations between cannabis use and the onset of symptoms of schizophrenia, and most determined cannabis was also a major risk factor for other psychotic disorders. Analyses of dose-dependence indicated that repeated cannabis use increased the risk of developing psychotic symptoms. This risk is increased when an individual starts using cannabis before age 15.11 Age seemed to be a stronger predictor of onset and outcome than sex, with no significant differences between men and women. One study in this review found that approximately 8% to 13% cases of schizophrenia may have been solely due to cannabis.11 The most significant limitation to the studies analyzed in this review is that retrospective studies utilize self-reported questionnaires.
Continue to: Other researchers have found...
Other researchers have found it would take a relatively high number of individuals to stop using cannabis to prevent 1 case of schizophrenia. In a study of data from England and Wales, Hickman et al12 evaluated the best available estimates of the incidence of schizophrenia, rates of heavy and light cannabis use, and risk that cannabis causes schizophrenia to determine the number needed to prevent (NNP) 1 case of schizophrenia. They estimated that it would require approximately 2,800 men age 20 to 24 (90% CI, 2,018 to 4,530) and 4,700 men age 35 to 39 (90% CI, 3,114 to 8,416) who heavily used cannabis to stop their consumption to prevent 1 case of schizophrenia.12 For women with heavy cannabis use, the mean NNP was 5,470 for women age 25 to 29 (90% CI, 3,640 to 9,839) and 10,870 for women age 35 to 39 (90% CI, 6,786 to 22,732).12 For light cannabis users, the NNP was 4 to 5 times higher than the NNP for heavy cannabis users. This suggests that clinical interventions aimed at preventing dependence on cannabis would be more effective than interventions aimed at eliminating cannabis use.
Medical cannabis and increased potency
In recent years, the use of medical cannabis, which is used to address adverse effects of chemotherapy as well as neuropathic pain, Parkinson’s disease, and epilepsy, has been increasing.13 However, there is a lack of well-conducted randomized clinical trials evaluating medical cannabis’ efficacy and safety. As medical cannabis continues to gain public acceptance and more states permit its legal use, patients and physicians should be fully informed of the known adverse effects, including impaired attention, learning, and motivation.13
Several studies have drawn attention to the dose-dependence of many of cannabis’ effects. Since at least the 1960s, the concentration of THC in cannabis has increased substantially, thus increasing its potency. Based on 66,747 samples across 8 studies, 1 meta-analysis estimated that THC concentrations in herbal cannabis increased by 0.29% (P < .001) each year between 1970 and 2017.14 Similarly, THC concentrations in cannabis resins were found to have increased by 0.57% (P = .017) each year between 1975 and 2017.14 Cannabis products with high concentrations of THC carry an increased risk of addiction and mental health disorders.14
Identifying those at highest risk
Despite ongoing research, scientific consensus on the relationship of cannabis to schizophrenia and psychosis has yet to be reached. The disparity between the relatively high prevalence of regular adult use of cannabis (8%7)and the low incidence of cannabis-induced psychosis suggests that cannabis use alone is unlikely to lead to episodes of psychosis in individuals who are not predisposed to such episodes. Sarrazin et al15 evaluated 170 patients with schizophrenia, 31 of whom had cannabis use disorder. They found no significant difference in lifetime symptom dimensions between groups, and proposed that cannabis-associated schizophrenia should not be categorized as a distinct clinical entity of schizophrenia with specific features.15
Policies that encourage follow-up of patients after episodes of drug-induced psychosis may mitigate the adverse social and economic effects of schizophrenia. Currently, these policies are not widely implemented in health care institutions, possibly because psychotic symptoms may fade after the drug’s effects have dissipated. Despite this, these patients are at high risk of developing schizophrenia and self-harm. New-onset schizophrenia should be promptly identified because delayed diagnosis is associated with worse prognosis.6 Additionally, identifying genetic susceptibilities to schizophrenia—such as the Val158Met polymorphisms—in individuals who use cannabis could help clinicians manage or slow the onset or progression of schizophrenia.3 Motivational interviewing strategies should be used to minimize or eliminate cannabis use in individuals with active schizophrenia or psychosis, thus preventing worse outcomes.
Bottom Line
Identifying susceptibilities to schizophrenia may guide interventions in patients who use cannabis. Several large studies have suggested that cannabis use may exacerbate symptoms and worsen the prognosis of schizophrenia. Motivational interviewing strategies aimed at minimizing cannabis use may improve outcomes in patients with schizophrenia.
Related Resources
- Khokhar JY, Dwiel LL, Henricks AM, et al. The link between schizophrenia and substance use disorder: a unifying hypothesis. Schizophr Res. 2018;194:78-85. doi:10.1016/j. schres.2017.04.016
- Otite ES, Solanky A, Doumas S. Adolescents, THC, and the risk of psychosis. Current Psychiatry. 2021;20(12):e1-e2. doi:10.12788/cp.0197
1. Simeone JC, Ward AJ, Rotella P, et al. An evaluation of variation in published estimates of schizophrenia prevalence from 1990-2013: a systematic literature review. BMC Psychiatry. 2015;15(1):193. doi:10.1186/s12888-015-0578-7
2. Tandon R, Gaebel W, Barch DM, et al. Definition and description of schizophrenia in the DSM-5. Schizophr Res. 2013;150(1):3-10. doi:10.1016/j.schres.2013.05.028
3. Bosia M, Buonocore M, Bechi M, et al. Schizophrenia, cannabis use and catechol-O-methyltransferase (COMT): modeling the interplay on cognition. Prog Neuropsychopharmacol Biol Psychiatry. 2019;92:363-368. doi:10.1016/j.pnpbp.2019.02.009
4. Welch KA, McIntosh AM, Job DE, et al. The impact of substance use on brain structure in people at high risk of developing schizophrenia. Schizophr Bull. 2011;37(5):1066-1076. doi:10.1093/schbul/sbq013
5. Winship IR, Dursun SM, Baker GB, et al. An overview of animal models related to schizophrenia. Can J Psychiatry. 2019;64(1):5-17. doi:10.1177/0706743718773728
6. Starzer MSK, Nordentoft M, Hjorthøj C. Rates and predictors of conversion to schizophrenia or bipolar disorder following substance-induced psychosis. Am J Psychiatry. 2018;175(4):343-350. doi:10.1176/appi.ajp.2017.17020223
7. Hall W. Cannabis use and psychosis. Drug Alcohol Rev. 1998;17(4):433-444. doi:10.1080/09595239800187271
8. Misiak B, Frydecka D, Slezak R, et al. Elevated homocysteine level in first-episode schizophrenia patients—the relevance of family history of schizophrenia and lifetime diagnosis of cannabis abuse. Metab Brain Dis. 2014;29(3):661-670. doi:10.1007/s11011-014-9534-3
9. Veen ND, Selten J, van der Tweel I, et al. Cannabis use and age at onset of schizophrenia. Am J Psychiatry. 2004;161(3):501-506. doi:10.1176/appi.ajp.161.3.501
10. Foti DJ, Kotov R, Guey LT, et al. Cannabis use and the course of schizophrenia: 10-year follow-up after first hospitalization. Am J Psychiatry. 2010;167(8):987-993. doi:10.1176/appi.ajp.2010.09020189
11. Ortiz-Medina MB, Perea M, Torales J, et al. Cannabis consumption and psychosis or schizophrenia development. Int J Soc Psychiatry. 2018;64(7):690-704. doi:10.1177/0020764018801690
12. Hickman M, Vickerman P, Macleod J, et al. If cannabis caused schizophrenia—how many cannabis users may need to be prevented in order to prevent one case of schizophrenia? England and Wales calculations. Addiction. 2009;104(11):1856-1861. doi:10.1111/j.1360-0443.2009.02736.x
13. Gupta S, Phalen T, Gupta S. Medical marijuana: do the benefits outweigh the risks? Current Psychiatry. 2018;17(1):34-41.
14. Freeman TP, Craft S, Wilson J, et al. Changes in delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) concentrations in cannabis over time: systematic review and meta-analysis. Addiction. 2021;116(5):1000-1010. doi:10.1111/add.15253
15. Sarrazin S, Louppe F, Doukhan R, et al. A clinical comparison of schizophrenia with and without pre-onset cannabis use disorder: a retrospective cohort study using categorical and dimensional approaches. Ann Gen Psychiatry. 2015;14:44. doi:10.1186/s12991-015-0083-x
Approximately 1 in 200 individuals will be diagnosed with schizophrenia in their lifetime.1 DSM-5 criteria for the diagnosis of schizophrenia require the presence of ≥2 of 5 symptoms: delusions, hallucinations, disordered speech, grossly disorganized (or catatonic) behavior, and negative symptoms such as flat affect or avolition.2 Multiple studies have found increased rates of cannabis use among patients with schizophrenia. Because cognitive deficits are the chief predictor of clinical outcomes and quality of life in individuals with schizophrenia, the cognitive effects of cannabis use among these patients are of clinical significance.3 As legislation increasingly allows for the sale, possession, and consumption of cannabis, it is crucial to provide clinicians with evidence-based recommendations for treating patients who regularly use cannabis (approximately 8% of the adult population3). In this article, we analyze several peer-reviewed studies to investigate the impact of cannabis use on the onset and development of schizophrenia.
A look at substance-induced psychosis
Schizophrenia is associated with several structural brain changes, and some of these changes may be influenced by cannabis use (Box4). The biochemical etiology of schizophrenia is poorly understood but thought to involve dopamine, glutamate, serotonin, and gamma-aminobutyric acid. Certain positive symptoms, such as hallucinations, are uniquely human and difficult to study in animal models.5 Psychoactive substance use, especially cannabis, is frequently comorbid with schizophrenia. Additionally, certain individuals may be more predisposed to substance-induced psychosis than others based on genetic variation and underlying brain structure changes.4 Substance-induced psychosis is a psychotic state following the ingestion of a psychoactive substance or drug withdrawal lasting ≥48 hours.6 The psychoactive effects of cannabis have been associated with an exacerbation of existing schizophrenia symptoms.7 In 1998, Hall7 proposed 2 hypotheses to explain the relationship between cannabis and psychosis. The first was that heavy consumption of cannabis triggers a specific type of cannabis psychosis.7 The second was that cannabis use exacerbates existing schizophrenia, making the symptoms worse.7 Hall7 concluded that there was a complicated interaction among an individual’s vulnerability to their stressors, environment, and genetics.
Box
Schizophrenia is associated with several structural changes in the brain, including lateral ventriculomegaly, reduced prefrontal cortex volume, and generalized atrophy. These changes may precede illness and act as a risk marker.4 A multivariate regression analysis that compared patients with schizophrenia who were cannabis users vs patients with schizophrenia who were nonusers found that those with high-level cannabis use had relatively higher left and right lateral ventricle volume (r = 0.208, P = .13, and r = 0.226, P = .007, respectively) as well as increased third ventricle volume (r = 0.271, P = .001).4 These changes were dose-dependent and may lead to worse disease outcomes.4
Cannabis, COMT, and homocysteine
Great advances have been made in our ability to examine the association between genetics and metabolism. One example of this is the interaction between the catechol-O-methyltransferase (COMT) gene and the active component of cannabis, delta-9-tetrahydrocannabinol (THC). COMT codes for an enzyme that degrades cortical dopamine. The Val158Met polymorphism of this gene increases COMT activity, leading to increased dopamine catabolism, and thus decreased levels of extracellular dopamine, which induces an increase in mesolimbic dopaminergic activity, thereby increasing susceptibility to psychosis.3
In a study that genotyped 135 patients with schizophrenia, the Val158Met polymorphism was present in 29.63% of participants.3 Because THC can induce episodes of psychosis, individuals with this polymorphism may be at a higher risk of developing schizophrenia. Compared to Met carrier control participants with similar histories of cannabis consumption, those with the Val158Met polymorphism demonstrated markedly worse performance on tests of verbal fluency and processing speed.3 This is clinically significant because cognitive impairments are a major prognostic factor in schizophrenia, and identifying patients with this polymorphism could help personalize interventions for those who consume cannabis and are at risk of developing schizophrenia.
A study that evaluated 56 patients with first-episode schizophrenia found that having a history of cannabis abuse was associated with significantly higher levels of homocysteine as well as lower levels of high-density lipoprotein and vitamin B12.8 Homocysteine is an agonist at the glutamate binding site and a partial antagonist at the glycine co-agonist site in the N-methyl-
The C677T polymorphism in MTHFR may predict the risk of developing metabolic syndrome in patients taking second-generation antipsychotics.8 Elevations in homocysteine by as little as 5 μmol/L may increase schizophrenia risk by 70% compared to controls, possibly due to homocysteine initiating neuronal apoptosis, catalyzing dysfunction of the mitochondria, or increasing oxidative stress.8 There is a positive correlation between homocysteine levels and severity of negative symptoms (P = .006) and general psychopathology (P = .008) of schizophrenia when analyzed using the Positive and Negative Syndrome Scale.8 Negative symptoms such as blunted affect, apathy, anhedonia, and loss of motivation significantly impact the social and economic outcomes of patients diagnosed with schizophrenia.
Research paints a mixed picture
A Danish study analyzed the rates of conversion to schizophrenia or bipolar disorder (BD) among 6,788 individuals who received a diagnosis of substance-induced psychosis from 1994 to 2014.6 Ten comparison participants were selected for each case participant, matched on sex and year/month of birth. Participants were followed until the first occurrence of schizophrenia or BD, death, or emigration from Denmark. Substances implicated in the initial psychotic episode included cannabis, alcohol, opioids, sedatives, cocaine, amphetamines, hallucinogens, and combinations of substances.
Continue to: The overall conversion rate...
The overall conversion rate over 20 years was 32.2% (95% CI, 29.7 to 34.9), with 26.0% developing schizophrenia vs 8.4% developing BD.6 Of the substances involved, cannabis was the most common, implicated in 41.2% (95% CI, 36.6 to 46.2) of cases.6 One-half of male patients converted within 2.0 years and one-half of female patients converted within 4.4 years after a cannabis-induced psychosis.6
This study had several limitations. It could not account for any short-term psychotic symptoms experienced by the general population, especially after cannabis use. Such patients might not seek treatment. Thus, the results might not be generalizable to the general population. The study did not evaluate if conversion rates differed based on continued substance use following the psychosis episode, or the amount of each substance taken prior to the episode. Dose-dependence was not well elucidated, and this study only looked at patients from Denmark and did not account for socioeconomic status.6
Another Danish study looked at the influences of gender and cannabis use in the early course of the disease in 133 patients with schizophrenia.9 These researchers found that male gender was a significant predictor of earlier onset of dysfunction socially and in the workplace, as well as a higher risk of developing negative symptoms. However, compared to gender, cannabis use was a stronger predictor of age at first psychotic episode. For cannabis users, the median age of onset of negative symptoms was 23.7, compared to 38.4 for nonusers (P < .001).9
Cannabis use is significantly elevated among individuals with psychosis, with a 12-month prevalence of 29.2% compared to 4.0% among the general population of the United States.10 In a study that assessed 229 patients with a schizophrenia spectrum disorder during their first hospitalization and 6 months, 2 years, 4 years, and 10 years later, Foti et al10 found that the lifetime rate of cannabis use was 66.2%. Survival analysis found cannabis use doubled the risk of the onset of psychosis compared to nonusers of the same age (hazard ratio [HR] = 1.97; 95% CI, 1.48 to 2.62, P < .001), even after adjusting for socioeconomic status, age, and gender (HR = 1.34; 95% CI, 1.01 to 1.77, P < .05).10 Additionally, Foti et al10 found significant positive correlations between psychotic symptoms and cannabis use in patients with schizophrenia over the course of 10 years. An increase in symptoms was associated with a higher likelihood of cannabis use, and a decrease in symptoms was correlated with a lower likelihood of use (adjusted odds ratio = 1.64; 95% CI, 1.12 to 2.43, P < .0125).10
Ortiz-Medina et al11 conducted a meta-analysis of 22 studies of 15 cohorts from healthy populations and 12 other cohort follow-up studies that evaluated the onset of psychotic symptoms in individuals who used cannabis. Most studies found associations between cannabis use and the onset of symptoms of schizophrenia, and most determined cannabis was also a major risk factor for other psychotic disorders. Analyses of dose-dependence indicated that repeated cannabis use increased the risk of developing psychotic symptoms. This risk is increased when an individual starts using cannabis before age 15.11 Age seemed to be a stronger predictor of onset and outcome than sex, with no significant differences between men and women. One study in this review found that approximately 8% to 13% cases of schizophrenia may have been solely due to cannabis.11 The most significant limitation to the studies analyzed in this review is that retrospective studies utilize self-reported questionnaires.
Continue to: Other researchers have found...
Other researchers have found it would take a relatively high number of individuals to stop using cannabis to prevent 1 case of schizophrenia. In a study of data from England and Wales, Hickman et al12 evaluated the best available estimates of the incidence of schizophrenia, rates of heavy and light cannabis use, and risk that cannabis causes schizophrenia to determine the number needed to prevent (NNP) 1 case of schizophrenia. They estimated that it would require approximately 2,800 men age 20 to 24 (90% CI, 2,018 to 4,530) and 4,700 men age 35 to 39 (90% CI, 3,114 to 8,416) who heavily used cannabis to stop their consumption to prevent 1 case of schizophrenia.12 For women with heavy cannabis use, the mean NNP was 5,470 for women age 25 to 29 (90% CI, 3,640 to 9,839) and 10,870 for women age 35 to 39 (90% CI, 6,786 to 22,732).12 For light cannabis users, the NNP was 4 to 5 times higher than the NNP for heavy cannabis users. This suggests that clinical interventions aimed at preventing dependence on cannabis would be more effective than interventions aimed at eliminating cannabis use.
Medical cannabis and increased potency
In recent years, the use of medical cannabis, which is used to address adverse effects of chemotherapy as well as neuropathic pain, Parkinson’s disease, and epilepsy, has been increasing.13 However, there is a lack of well-conducted randomized clinical trials evaluating medical cannabis’ efficacy and safety. As medical cannabis continues to gain public acceptance and more states permit its legal use, patients and physicians should be fully informed of the known adverse effects, including impaired attention, learning, and motivation.13
Several studies have drawn attention to the dose-dependence of many of cannabis’ effects. Since at least the 1960s, the concentration of THC in cannabis has increased substantially, thus increasing its potency. Based on 66,747 samples across 8 studies, 1 meta-analysis estimated that THC concentrations in herbal cannabis increased by 0.29% (P < .001) each year between 1970 and 2017.14 Similarly, THC concentrations in cannabis resins were found to have increased by 0.57% (P = .017) each year between 1975 and 2017.14 Cannabis products with high concentrations of THC carry an increased risk of addiction and mental health disorders.14
Identifying those at highest risk
Despite ongoing research, scientific consensus on the relationship of cannabis to schizophrenia and psychosis has yet to be reached. The disparity between the relatively high prevalence of regular adult use of cannabis (8%7)and the low incidence of cannabis-induced psychosis suggests that cannabis use alone is unlikely to lead to episodes of psychosis in individuals who are not predisposed to such episodes. Sarrazin et al15 evaluated 170 patients with schizophrenia, 31 of whom had cannabis use disorder. They found no significant difference in lifetime symptom dimensions between groups, and proposed that cannabis-associated schizophrenia should not be categorized as a distinct clinical entity of schizophrenia with specific features.15
Policies that encourage follow-up of patients after episodes of drug-induced psychosis may mitigate the adverse social and economic effects of schizophrenia. Currently, these policies are not widely implemented in health care institutions, possibly because psychotic symptoms may fade after the drug’s effects have dissipated. Despite this, these patients are at high risk of developing schizophrenia and self-harm. New-onset schizophrenia should be promptly identified because delayed diagnosis is associated with worse prognosis.6 Additionally, identifying genetic susceptibilities to schizophrenia—such as the Val158Met polymorphisms—in individuals who use cannabis could help clinicians manage or slow the onset or progression of schizophrenia.3 Motivational interviewing strategies should be used to minimize or eliminate cannabis use in individuals with active schizophrenia or psychosis, thus preventing worse outcomes.
Bottom Line
Identifying susceptibilities to schizophrenia may guide interventions in patients who use cannabis. Several large studies have suggested that cannabis use may exacerbate symptoms and worsen the prognosis of schizophrenia. Motivational interviewing strategies aimed at minimizing cannabis use may improve outcomes in patients with schizophrenia.
Related Resources
- Khokhar JY, Dwiel LL, Henricks AM, et al. The link between schizophrenia and substance use disorder: a unifying hypothesis. Schizophr Res. 2018;194:78-85. doi:10.1016/j. schres.2017.04.016
- Otite ES, Solanky A, Doumas S. Adolescents, THC, and the risk of psychosis. Current Psychiatry. 2021;20(12):e1-e2. doi:10.12788/cp.0197
Approximately 1 in 200 individuals will be diagnosed with schizophrenia in their lifetime.1 DSM-5 criteria for the diagnosis of schizophrenia require the presence of ≥2 of 5 symptoms: delusions, hallucinations, disordered speech, grossly disorganized (or catatonic) behavior, and negative symptoms such as flat affect or avolition.2 Multiple studies have found increased rates of cannabis use among patients with schizophrenia. Because cognitive deficits are the chief predictor of clinical outcomes and quality of life in individuals with schizophrenia, the cognitive effects of cannabis use among these patients are of clinical significance.3 As legislation increasingly allows for the sale, possession, and consumption of cannabis, it is crucial to provide clinicians with evidence-based recommendations for treating patients who regularly use cannabis (approximately 8% of the adult population3). In this article, we analyze several peer-reviewed studies to investigate the impact of cannabis use on the onset and development of schizophrenia.
A look at substance-induced psychosis
Schizophrenia is associated with several structural brain changes, and some of these changes may be influenced by cannabis use (Box4). The biochemical etiology of schizophrenia is poorly understood but thought to involve dopamine, glutamate, serotonin, and gamma-aminobutyric acid. Certain positive symptoms, such as hallucinations, are uniquely human and difficult to study in animal models.5 Psychoactive substance use, especially cannabis, is frequently comorbid with schizophrenia. Additionally, certain individuals may be more predisposed to substance-induced psychosis than others based on genetic variation and underlying brain structure changes.4 Substance-induced psychosis is a psychotic state following the ingestion of a psychoactive substance or drug withdrawal lasting ≥48 hours.6 The psychoactive effects of cannabis have been associated with an exacerbation of existing schizophrenia symptoms.7 In 1998, Hall7 proposed 2 hypotheses to explain the relationship between cannabis and psychosis. The first was that heavy consumption of cannabis triggers a specific type of cannabis psychosis.7 The second was that cannabis use exacerbates existing schizophrenia, making the symptoms worse.7 Hall7 concluded that there was a complicated interaction among an individual’s vulnerability to their stressors, environment, and genetics.
Box
Schizophrenia is associated with several structural changes in the brain, including lateral ventriculomegaly, reduced prefrontal cortex volume, and generalized atrophy. These changes may precede illness and act as a risk marker.4 A multivariate regression analysis that compared patients with schizophrenia who were cannabis users vs patients with schizophrenia who were nonusers found that those with high-level cannabis use had relatively higher left and right lateral ventricle volume (r = 0.208, P = .13, and r = 0.226, P = .007, respectively) as well as increased third ventricle volume (r = 0.271, P = .001).4 These changes were dose-dependent and may lead to worse disease outcomes.4
Cannabis, COMT, and homocysteine
Great advances have been made in our ability to examine the association between genetics and metabolism. One example of this is the interaction between the catechol-O-methyltransferase (COMT) gene and the active component of cannabis, delta-9-tetrahydrocannabinol (THC). COMT codes for an enzyme that degrades cortical dopamine. The Val158Met polymorphism of this gene increases COMT activity, leading to increased dopamine catabolism, and thus decreased levels of extracellular dopamine, which induces an increase in mesolimbic dopaminergic activity, thereby increasing susceptibility to psychosis.3
In a study that genotyped 135 patients with schizophrenia, the Val158Met polymorphism was present in 29.63% of participants.3 Because THC can induce episodes of psychosis, individuals with this polymorphism may be at a higher risk of developing schizophrenia. Compared to Met carrier control participants with similar histories of cannabis consumption, those with the Val158Met polymorphism demonstrated markedly worse performance on tests of verbal fluency and processing speed.3 This is clinically significant because cognitive impairments are a major prognostic factor in schizophrenia, and identifying patients with this polymorphism could help personalize interventions for those who consume cannabis and are at risk of developing schizophrenia.
A study that evaluated 56 patients with first-episode schizophrenia found that having a history of cannabis abuse was associated with significantly higher levels of homocysteine as well as lower levels of high-density lipoprotein and vitamin B12.8 Homocysteine is an agonist at the glutamate binding site and a partial antagonist at the glycine co-agonist site in the N-methyl-
The C677T polymorphism in MTHFR may predict the risk of developing metabolic syndrome in patients taking second-generation antipsychotics.8 Elevations in homocysteine by as little as 5 μmol/L may increase schizophrenia risk by 70% compared to controls, possibly due to homocysteine initiating neuronal apoptosis, catalyzing dysfunction of the mitochondria, or increasing oxidative stress.8 There is a positive correlation between homocysteine levels and severity of negative symptoms (P = .006) and general psychopathology (P = .008) of schizophrenia when analyzed using the Positive and Negative Syndrome Scale.8 Negative symptoms such as blunted affect, apathy, anhedonia, and loss of motivation significantly impact the social and economic outcomes of patients diagnosed with schizophrenia.
Research paints a mixed picture
A Danish study analyzed the rates of conversion to schizophrenia or bipolar disorder (BD) among 6,788 individuals who received a diagnosis of substance-induced psychosis from 1994 to 2014.6 Ten comparison participants were selected for each case participant, matched on sex and year/month of birth. Participants were followed until the first occurrence of schizophrenia or BD, death, or emigration from Denmark. Substances implicated in the initial psychotic episode included cannabis, alcohol, opioids, sedatives, cocaine, amphetamines, hallucinogens, and combinations of substances.
Continue to: The overall conversion rate...
The overall conversion rate over 20 years was 32.2% (95% CI, 29.7 to 34.9), with 26.0% developing schizophrenia vs 8.4% developing BD.6 Of the substances involved, cannabis was the most common, implicated in 41.2% (95% CI, 36.6 to 46.2) of cases.6 One-half of male patients converted within 2.0 years and one-half of female patients converted within 4.4 years after a cannabis-induced psychosis.6
This study had several limitations. It could not account for any short-term psychotic symptoms experienced by the general population, especially after cannabis use. Such patients might not seek treatment. Thus, the results might not be generalizable to the general population. The study did not evaluate if conversion rates differed based on continued substance use following the psychosis episode, or the amount of each substance taken prior to the episode. Dose-dependence was not well elucidated, and this study only looked at patients from Denmark and did not account for socioeconomic status.6
Another Danish study looked at the influences of gender and cannabis use in the early course of the disease in 133 patients with schizophrenia.9 These researchers found that male gender was a significant predictor of earlier onset of dysfunction socially and in the workplace, as well as a higher risk of developing negative symptoms. However, compared to gender, cannabis use was a stronger predictor of age at first psychotic episode. For cannabis users, the median age of onset of negative symptoms was 23.7, compared to 38.4 for nonusers (P < .001).9
Cannabis use is significantly elevated among individuals with psychosis, with a 12-month prevalence of 29.2% compared to 4.0% among the general population of the United States.10 In a study that assessed 229 patients with a schizophrenia spectrum disorder during their first hospitalization and 6 months, 2 years, 4 years, and 10 years later, Foti et al10 found that the lifetime rate of cannabis use was 66.2%. Survival analysis found cannabis use doubled the risk of the onset of psychosis compared to nonusers of the same age (hazard ratio [HR] = 1.97; 95% CI, 1.48 to 2.62, P < .001), even after adjusting for socioeconomic status, age, and gender (HR = 1.34; 95% CI, 1.01 to 1.77, P < .05).10 Additionally, Foti et al10 found significant positive correlations between psychotic symptoms and cannabis use in patients with schizophrenia over the course of 10 years. An increase in symptoms was associated with a higher likelihood of cannabis use, and a decrease in symptoms was correlated with a lower likelihood of use (adjusted odds ratio = 1.64; 95% CI, 1.12 to 2.43, P < .0125).10
Ortiz-Medina et al11 conducted a meta-analysis of 22 studies of 15 cohorts from healthy populations and 12 other cohort follow-up studies that evaluated the onset of psychotic symptoms in individuals who used cannabis. Most studies found associations between cannabis use and the onset of symptoms of schizophrenia, and most determined cannabis was also a major risk factor for other psychotic disorders. Analyses of dose-dependence indicated that repeated cannabis use increased the risk of developing psychotic symptoms. This risk is increased when an individual starts using cannabis before age 15.11 Age seemed to be a stronger predictor of onset and outcome than sex, with no significant differences between men and women. One study in this review found that approximately 8% to 13% cases of schizophrenia may have been solely due to cannabis.11 The most significant limitation to the studies analyzed in this review is that retrospective studies utilize self-reported questionnaires.
Continue to: Other researchers have found...
Other researchers have found it would take a relatively high number of individuals to stop using cannabis to prevent 1 case of schizophrenia. In a study of data from England and Wales, Hickman et al12 evaluated the best available estimates of the incidence of schizophrenia, rates of heavy and light cannabis use, and risk that cannabis causes schizophrenia to determine the number needed to prevent (NNP) 1 case of schizophrenia. They estimated that it would require approximately 2,800 men age 20 to 24 (90% CI, 2,018 to 4,530) and 4,700 men age 35 to 39 (90% CI, 3,114 to 8,416) who heavily used cannabis to stop their consumption to prevent 1 case of schizophrenia.12 For women with heavy cannabis use, the mean NNP was 5,470 for women age 25 to 29 (90% CI, 3,640 to 9,839) and 10,870 for women age 35 to 39 (90% CI, 6,786 to 22,732).12 For light cannabis users, the NNP was 4 to 5 times higher than the NNP for heavy cannabis users. This suggests that clinical interventions aimed at preventing dependence on cannabis would be more effective than interventions aimed at eliminating cannabis use.
Medical cannabis and increased potency
In recent years, the use of medical cannabis, which is used to address adverse effects of chemotherapy as well as neuropathic pain, Parkinson’s disease, and epilepsy, has been increasing.13 However, there is a lack of well-conducted randomized clinical trials evaluating medical cannabis’ efficacy and safety. As medical cannabis continues to gain public acceptance and more states permit its legal use, patients and physicians should be fully informed of the known adverse effects, including impaired attention, learning, and motivation.13
Several studies have drawn attention to the dose-dependence of many of cannabis’ effects. Since at least the 1960s, the concentration of THC in cannabis has increased substantially, thus increasing its potency. Based on 66,747 samples across 8 studies, 1 meta-analysis estimated that THC concentrations in herbal cannabis increased by 0.29% (P < .001) each year between 1970 and 2017.14 Similarly, THC concentrations in cannabis resins were found to have increased by 0.57% (P = .017) each year between 1975 and 2017.14 Cannabis products with high concentrations of THC carry an increased risk of addiction and mental health disorders.14
Identifying those at highest risk
Despite ongoing research, scientific consensus on the relationship of cannabis to schizophrenia and psychosis has yet to be reached. The disparity between the relatively high prevalence of regular adult use of cannabis (8%7)and the low incidence of cannabis-induced psychosis suggests that cannabis use alone is unlikely to lead to episodes of psychosis in individuals who are not predisposed to such episodes. Sarrazin et al15 evaluated 170 patients with schizophrenia, 31 of whom had cannabis use disorder. They found no significant difference in lifetime symptom dimensions between groups, and proposed that cannabis-associated schizophrenia should not be categorized as a distinct clinical entity of schizophrenia with specific features.15
Policies that encourage follow-up of patients after episodes of drug-induced psychosis may mitigate the adverse social and economic effects of schizophrenia. Currently, these policies are not widely implemented in health care institutions, possibly because psychotic symptoms may fade after the drug’s effects have dissipated. Despite this, these patients are at high risk of developing schizophrenia and self-harm. New-onset schizophrenia should be promptly identified because delayed diagnosis is associated with worse prognosis.6 Additionally, identifying genetic susceptibilities to schizophrenia—such as the Val158Met polymorphisms—in individuals who use cannabis could help clinicians manage or slow the onset or progression of schizophrenia.3 Motivational interviewing strategies should be used to minimize or eliminate cannabis use in individuals with active schizophrenia or psychosis, thus preventing worse outcomes.
Bottom Line
Identifying susceptibilities to schizophrenia may guide interventions in patients who use cannabis. Several large studies have suggested that cannabis use may exacerbate symptoms and worsen the prognosis of schizophrenia. Motivational interviewing strategies aimed at minimizing cannabis use may improve outcomes in patients with schizophrenia.
Related Resources
- Khokhar JY, Dwiel LL, Henricks AM, et al. The link between schizophrenia and substance use disorder: a unifying hypothesis. Schizophr Res. 2018;194:78-85. doi:10.1016/j. schres.2017.04.016
- Otite ES, Solanky A, Doumas S. Adolescents, THC, and the risk of psychosis. Current Psychiatry. 2021;20(12):e1-e2. doi:10.12788/cp.0197
1. Simeone JC, Ward AJ, Rotella P, et al. An evaluation of variation in published estimates of schizophrenia prevalence from 1990-2013: a systematic literature review. BMC Psychiatry. 2015;15(1):193. doi:10.1186/s12888-015-0578-7
2. Tandon R, Gaebel W, Barch DM, et al. Definition and description of schizophrenia in the DSM-5. Schizophr Res. 2013;150(1):3-10. doi:10.1016/j.schres.2013.05.028
3. Bosia M, Buonocore M, Bechi M, et al. Schizophrenia, cannabis use and catechol-O-methyltransferase (COMT): modeling the interplay on cognition. Prog Neuropsychopharmacol Biol Psychiatry. 2019;92:363-368. doi:10.1016/j.pnpbp.2019.02.009
4. Welch KA, McIntosh AM, Job DE, et al. The impact of substance use on brain structure in people at high risk of developing schizophrenia. Schizophr Bull. 2011;37(5):1066-1076. doi:10.1093/schbul/sbq013
5. Winship IR, Dursun SM, Baker GB, et al. An overview of animal models related to schizophrenia. Can J Psychiatry. 2019;64(1):5-17. doi:10.1177/0706743718773728
6. Starzer MSK, Nordentoft M, Hjorthøj C. Rates and predictors of conversion to schizophrenia or bipolar disorder following substance-induced psychosis. Am J Psychiatry. 2018;175(4):343-350. doi:10.1176/appi.ajp.2017.17020223
7. Hall W. Cannabis use and psychosis. Drug Alcohol Rev. 1998;17(4):433-444. doi:10.1080/09595239800187271
8. Misiak B, Frydecka D, Slezak R, et al. Elevated homocysteine level in first-episode schizophrenia patients—the relevance of family history of schizophrenia and lifetime diagnosis of cannabis abuse. Metab Brain Dis. 2014;29(3):661-670. doi:10.1007/s11011-014-9534-3
9. Veen ND, Selten J, van der Tweel I, et al. Cannabis use and age at onset of schizophrenia. Am J Psychiatry. 2004;161(3):501-506. doi:10.1176/appi.ajp.161.3.501
10. Foti DJ, Kotov R, Guey LT, et al. Cannabis use and the course of schizophrenia: 10-year follow-up after first hospitalization. Am J Psychiatry. 2010;167(8):987-993. doi:10.1176/appi.ajp.2010.09020189
11. Ortiz-Medina MB, Perea M, Torales J, et al. Cannabis consumption and psychosis or schizophrenia development. Int J Soc Psychiatry. 2018;64(7):690-704. doi:10.1177/0020764018801690
12. Hickman M, Vickerman P, Macleod J, et al. If cannabis caused schizophrenia—how many cannabis users may need to be prevented in order to prevent one case of schizophrenia? England and Wales calculations. Addiction. 2009;104(11):1856-1861. doi:10.1111/j.1360-0443.2009.02736.x
13. Gupta S, Phalen T, Gupta S. Medical marijuana: do the benefits outweigh the risks? Current Psychiatry. 2018;17(1):34-41.
14. Freeman TP, Craft S, Wilson J, et al. Changes in delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) concentrations in cannabis over time: systematic review and meta-analysis. Addiction. 2021;116(5):1000-1010. doi:10.1111/add.15253
15. Sarrazin S, Louppe F, Doukhan R, et al. A clinical comparison of schizophrenia with and without pre-onset cannabis use disorder: a retrospective cohort study using categorical and dimensional approaches. Ann Gen Psychiatry. 2015;14:44. doi:10.1186/s12991-015-0083-x
1. Simeone JC, Ward AJ, Rotella P, et al. An evaluation of variation in published estimates of schizophrenia prevalence from 1990-2013: a systematic literature review. BMC Psychiatry. 2015;15(1):193. doi:10.1186/s12888-015-0578-7
2. Tandon R, Gaebel W, Barch DM, et al. Definition and description of schizophrenia in the DSM-5. Schizophr Res. 2013;150(1):3-10. doi:10.1016/j.schres.2013.05.028
3. Bosia M, Buonocore M, Bechi M, et al. Schizophrenia, cannabis use and catechol-O-methyltransferase (COMT): modeling the interplay on cognition. Prog Neuropsychopharmacol Biol Psychiatry. 2019;92:363-368. doi:10.1016/j.pnpbp.2019.02.009
4. Welch KA, McIntosh AM, Job DE, et al. The impact of substance use on brain structure in people at high risk of developing schizophrenia. Schizophr Bull. 2011;37(5):1066-1076. doi:10.1093/schbul/sbq013
5. Winship IR, Dursun SM, Baker GB, et al. An overview of animal models related to schizophrenia. Can J Psychiatry. 2019;64(1):5-17. doi:10.1177/0706743718773728
6. Starzer MSK, Nordentoft M, Hjorthøj C. Rates and predictors of conversion to schizophrenia or bipolar disorder following substance-induced psychosis. Am J Psychiatry. 2018;175(4):343-350. doi:10.1176/appi.ajp.2017.17020223
7. Hall W. Cannabis use and psychosis. Drug Alcohol Rev. 1998;17(4):433-444. doi:10.1080/09595239800187271
8. Misiak B, Frydecka D, Slezak R, et al. Elevated homocysteine level in first-episode schizophrenia patients—the relevance of family history of schizophrenia and lifetime diagnosis of cannabis abuse. Metab Brain Dis. 2014;29(3):661-670. doi:10.1007/s11011-014-9534-3
9. Veen ND, Selten J, van der Tweel I, et al. Cannabis use and age at onset of schizophrenia. Am J Psychiatry. 2004;161(3):501-506. doi:10.1176/appi.ajp.161.3.501
10. Foti DJ, Kotov R, Guey LT, et al. Cannabis use and the course of schizophrenia: 10-year follow-up after first hospitalization. Am J Psychiatry. 2010;167(8):987-993. doi:10.1176/appi.ajp.2010.09020189
11. Ortiz-Medina MB, Perea M, Torales J, et al. Cannabis consumption and psychosis or schizophrenia development. Int J Soc Psychiatry. 2018;64(7):690-704. doi:10.1177/0020764018801690
12. Hickman M, Vickerman P, Macleod J, et al. If cannabis caused schizophrenia—how many cannabis users may need to be prevented in order to prevent one case of schizophrenia? England and Wales calculations. Addiction. 2009;104(11):1856-1861. doi:10.1111/j.1360-0443.2009.02736.x
13. Gupta S, Phalen T, Gupta S. Medical marijuana: do the benefits outweigh the risks? Current Psychiatry. 2018;17(1):34-41.
14. Freeman TP, Craft S, Wilson J, et al. Changes in delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) concentrations in cannabis over time: systematic review and meta-analysis. Addiction. 2021;116(5):1000-1010. doi:10.1111/add.15253
15. Sarrazin S, Louppe F, Doukhan R, et al. A clinical comparison of schizophrenia with and without pre-onset cannabis use disorder: a retrospective cohort study using categorical and dimensional approaches. Ann Gen Psychiatry. 2015;14:44. doi:10.1186/s12991-015-0083-x
Shakespeare and suicide
The medical knowledge that William Shakespeare possessed has awed scholars for centuries. Theories about the provenance of his knowledge abound (such as his son-in-law being a physician), and the inclusion of medical terms and ailments throughout his plays suggests a broad knowledge of disease and sickness. Scholars have noted how he sprinkles references to dermatologic, neurologic, orthopedic, and metabolic ailments throughout his plays, mentioning carbuncles, fistulas, corpulence, rhinophyma, scurvy, ague, enuresis, kyphosis, epilepsy, and parkinsonism.1 What seems to strike post-Enlightenment audiences—and what sets Shakespeare apart from many of his contemporaries—is his portrayal of “complex” characters, those with what we envision as rich interior worlds and with whom a modern audience can resonate. There is a reason psychiatrists such as Sigmund Freud have rushed back to Shakespeare and (sometimes anachronistically) found in his characters various psychiatric diagnoses such as depression, anxiety, paranoia, jealous delusions, and obsessive-compulsive disorder. Suicide and suicidal ideation are prevalent themes in some of Shakespeare’s most well-known characters.
A surprisingly common theme
The gravest outcome of a psychiatric illness is death by suicide, which occurs in 13 of Shakespeare’s characters.2 There are additional characters who exhibit suicidal ideation without a completed act. Shakespearean characters whose lives end in suicide are variably portrayed, dying by various means and circumstances. Hamlet (who dies at the hand of his foe, Laertes), famously soliloquizes the theme of suicide and the afterlife. He ponders “tak[ing] arms against a sea of troubles.” Ophelia dies ambiguously. Immediately after, her mother and brother recount her death in a brook—having had “too much of water” when her garments “heavy with their drink, | pull’d the poor wretch from her melodious lay | To muddy death.” The 2 clowns/gravediggers then debate whether Ophelia deserves a Christian burial and if her death should be considered a suicide: did the water drown her, or did she drown herself?3
Lady Macbeth’s suicide is offstage, punctuated by a “night-shriek.” Romeo drinks poison and dies “with a kiss.” Juliet quickly follows, making her body the sword’s sheath which “there rust, and let [her] die.” Othello stabs himself after requesting that his peers will “speak of me as I am.” One of King Lear’s daughters poisons her sister “and after [slays] herself.” Timon dies by his cave, “entomb’d upon the very hem o’ the sea.” In Antony and Cleopatra, after being told that Cleopatra has killed herself with Antony’s name on her lips, Antony begs to be stabbed and then stabs himself; he is not defeated by Caesar, but rather conquered by himself: “none but Antony | Should conquer Antony.” Cleopatra and her lady-in-waiting, Charmian, kill themselves with an asp. In Julius Caesar, Brutus runs upon his sword. Cassius begs for his own death, asking that “this good sword, | That ran through Caesar’s bowels, search this bosom.” Portia, it is reported, “swallowed fire.”
Shakespeare uses specific stylized language to portray characters in psychological anguish and suicidal states. Scholars have discussed how he uses certain stylistic language to highlight the anguish that happens during solitary, solipsistic moments of contemplation.4 Moments of anguish and suicidal ideation are marked by verbal repetition. An example of this repetition comes in Hamlet’s speech after he returns to the kingdom where his uncle has usurped his father, when he laments that he cannot end his own life. He says:
O, that this too too sullied flesh would melt,
Thaw, and resolve itself into a dew!
Or that the Everlasting had not fix’d
His canon ’gainst self-slaughter! O God, God,
How weary, stale, flat, and unprofitable
Seem to me all the uses of this world.
In these 6 lines, there are 2 instances of verbal repetition: “too too” and “God, God.” In this moment of solitude and despair, Hamlet’s speech fractures; his fractured speech reflects his fractured psyche. While Hamlet speaks of staleness and stagnation in the world, his words represent a sterile excess. No meaning is elicited by their repetition; there is no forward momentum to his speech. The words reflect the extent to which Hamlet is stuck and divided in this moment. Something similar happens in Macbeth’s “Tomorrow and tomorrow and tomorrow” speech. The words march on, and with each repetition they become increasingly hollow and brittle.
Why does this discussion of suicide in Shakespeare hold value for a contemporary psychiatrist? First, there is no single prototypical suicidal character in Shakespeare. His characters who are suicidal vary in their demographics and incentives for ending their lives. In this way, he provides a rich framework, one with which many people can engage. Second, this discussion fits into an existing paradigm for using art therapy (specifically Shakespeare) as a treatment modality for trauma.5 Programs such as DE-CRUIT have used the recitation of Shakespearean verse as a means of processing trauma in veterans.5 While Shakespeare does not mention a remedy for suicide in his plays, perhaps the text can serve as medicine. Third, the repetitive speech that Shakespeare uses in times of anguish could be a fairly accurate reflection of speech patterns in patients who are suicidal. Research that completed a spoken language analysis of patients who were suicidal has found “mechanical and repetitive phrasing” as a quality of these patients’ speech.6,7
For hundreds of years, critics have searched beyond the text for Shakespeare’s voice and opinion; what did he himself think of melancholy, despair, or suicide? We cannot know. We, as readers, are invited to explore a nuanced and multifaceted view of suicide, one that neither chides nor valorizes the act, and provides ambiguity rather than condemnation.
1. Paciaroni M, Bogousslavsky J. William Shakespeare’s neurology. Prog Brain Res. 2013;206:3-18.
2. Kirkland LR. To end itself by death: suicide in Shakespeare’s tragedies. South Med J. 1999;92(7):660-666.
3. Sale C. The “Amending Hand”: Hales v. Petit, Eyston v. Studd, and Equitable Action in Hamlet. In: Jordan C, Cunningham K, eds. The Law in Shakespeare. Early Modern Literature in History. Palgrave Macmillan; 2007:189-207. https://doi.org/10.1057/9780230626348_11
4. Langley E. Narcissism and Suicide in Shakespeare and His Contemporaries. Oxford University Press; 2009.
5. Ali A, Wolfert S, Lam I, et al. Intersecting modes of aesthetic distance and mimetic induction in therapeutic process: examining a drama-based treatment for military-related traumatic stress. Drama Therapy Review. 2018;4(2):153-165.
6. Belouali A, Gupta S, Sourirajan V, et al. Acoustic and language analysis of speech for suicidal ideation among US veterans. BioData Min. 2021;14(1):11. doi:10.1186/s13040-021-00245-y
7. Cummins N, Scherer S, Krajewski J, et al. A review of depression and suicide risk assessment using speech analysis. Speech Commun. 2015;71:10-49.
The medical knowledge that William Shakespeare possessed has awed scholars for centuries. Theories about the provenance of his knowledge abound (such as his son-in-law being a physician), and the inclusion of medical terms and ailments throughout his plays suggests a broad knowledge of disease and sickness. Scholars have noted how he sprinkles references to dermatologic, neurologic, orthopedic, and metabolic ailments throughout his plays, mentioning carbuncles, fistulas, corpulence, rhinophyma, scurvy, ague, enuresis, kyphosis, epilepsy, and parkinsonism.1 What seems to strike post-Enlightenment audiences—and what sets Shakespeare apart from many of his contemporaries—is his portrayal of “complex” characters, those with what we envision as rich interior worlds and with whom a modern audience can resonate. There is a reason psychiatrists such as Sigmund Freud have rushed back to Shakespeare and (sometimes anachronistically) found in his characters various psychiatric diagnoses such as depression, anxiety, paranoia, jealous delusions, and obsessive-compulsive disorder. Suicide and suicidal ideation are prevalent themes in some of Shakespeare’s most well-known characters.
A surprisingly common theme
The gravest outcome of a psychiatric illness is death by suicide, which occurs in 13 of Shakespeare’s characters.2 There are additional characters who exhibit suicidal ideation without a completed act. Shakespearean characters whose lives end in suicide are variably portrayed, dying by various means and circumstances. Hamlet (who dies at the hand of his foe, Laertes), famously soliloquizes the theme of suicide and the afterlife. He ponders “tak[ing] arms against a sea of troubles.” Ophelia dies ambiguously. Immediately after, her mother and brother recount her death in a brook—having had “too much of water” when her garments “heavy with their drink, | pull’d the poor wretch from her melodious lay | To muddy death.” The 2 clowns/gravediggers then debate whether Ophelia deserves a Christian burial and if her death should be considered a suicide: did the water drown her, or did she drown herself?3
Lady Macbeth’s suicide is offstage, punctuated by a “night-shriek.” Romeo drinks poison and dies “with a kiss.” Juliet quickly follows, making her body the sword’s sheath which “there rust, and let [her] die.” Othello stabs himself after requesting that his peers will “speak of me as I am.” One of King Lear’s daughters poisons her sister “and after [slays] herself.” Timon dies by his cave, “entomb’d upon the very hem o’ the sea.” In Antony and Cleopatra, after being told that Cleopatra has killed herself with Antony’s name on her lips, Antony begs to be stabbed and then stabs himself; he is not defeated by Caesar, but rather conquered by himself: “none but Antony | Should conquer Antony.” Cleopatra and her lady-in-waiting, Charmian, kill themselves with an asp. In Julius Caesar, Brutus runs upon his sword. Cassius begs for his own death, asking that “this good sword, | That ran through Caesar’s bowels, search this bosom.” Portia, it is reported, “swallowed fire.”
Shakespeare uses specific stylized language to portray characters in psychological anguish and suicidal states. Scholars have discussed how he uses certain stylistic language to highlight the anguish that happens during solitary, solipsistic moments of contemplation.4 Moments of anguish and suicidal ideation are marked by verbal repetition. An example of this repetition comes in Hamlet’s speech after he returns to the kingdom where his uncle has usurped his father, when he laments that he cannot end his own life. He says:
O, that this too too sullied flesh would melt,
Thaw, and resolve itself into a dew!
Or that the Everlasting had not fix’d
His canon ’gainst self-slaughter! O God, God,
How weary, stale, flat, and unprofitable
Seem to me all the uses of this world.
In these 6 lines, there are 2 instances of verbal repetition: “too too” and “God, God.” In this moment of solitude and despair, Hamlet’s speech fractures; his fractured speech reflects his fractured psyche. While Hamlet speaks of staleness and stagnation in the world, his words represent a sterile excess. No meaning is elicited by their repetition; there is no forward momentum to his speech. The words reflect the extent to which Hamlet is stuck and divided in this moment. Something similar happens in Macbeth’s “Tomorrow and tomorrow and tomorrow” speech. The words march on, and with each repetition they become increasingly hollow and brittle.
Why does this discussion of suicide in Shakespeare hold value for a contemporary psychiatrist? First, there is no single prototypical suicidal character in Shakespeare. His characters who are suicidal vary in their demographics and incentives for ending their lives. In this way, he provides a rich framework, one with which many people can engage. Second, this discussion fits into an existing paradigm for using art therapy (specifically Shakespeare) as a treatment modality for trauma.5 Programs such as DE-CRUIT have used the recitation of Shakespearean verse as a means of processing trauma in veterans.5 While Shakespeare does not mention a remedy for suicide in his plays, perhaps the text can serve as medicine. Third, the repetitive speech that Shakespeare uses in times of anguish could be a fairly accurate reflection of speech patterns in patients who are suicidal. Research that completed a spoken language analysis of patients who were suicidal has found “mechanical and repetitive phrasing” as a quality of these patients’ speech.6,7
For hundreds of years, critics have searched beyond the text for Shakespeare’s voice and opinion; what did he himself think of melancholy, despair, or suicide? We cannot know. We, as readers, are invited to explore a nuanced and multifaceted view of suicide, one that neither chides nor valorizes the act, and provides ambiguity rather than condemnation.
The medical knowledge that William Shakespeare possessed has awed scholars for centuries. Theories about the provenance of his knowledge abound (such as his son-in-law being a physician), and the inclusion of medical terms and ailments throughout his plays suggests a broad knowledge of disease and sickness. Scholars have noted how he sprinkles references to dermatologic, neurologic, orthopedic, and metabolic ailments throughout his plays, mentioning carbuncles, fistulas, corpulence, rhinophyma, scurvy, ague, enuresis, kyphosis, epilepsy, and parkinsonism.1 What seems to strike post-Enlightenment audiences—and what sets Shakespeare apart from many of his contemporaries—is his portrayal of “complex” characters, those with what we envision as rich interior worlds and with whom a modern audience can resonate. There is a reason psychiatrists such as Sigmund Freud have rushed back to Shakespeare and (sometimes anachronistically) found in his characters various psychiatric diagnoses such as depression, anxiety, paranoia, jealous delusions, and obsessive-compulsive disorder. Suicide and suicidal ideation are prevalent themes in some of Shakespeare’s most well-known characters.
A surprisingly common theme
The gravest outcome of a psychiatric illness is death by suicide, which occurs in 13 of Shakespeare’s characters.2 There are additional characters who exhibit suicidal ideation without a completed act. Shakespearean characters whose lives end in suicide are variably portrayed, dying by various means and circumstances. Hamlet (who dies at the hand of his foe, Laertes), famously soliloquizes the theme of suicide and the afterlife. He ponders “tak[ing] arms against a sea of troubles.” Ophelia dies ambiguously. Immediately after, her mother and brother recount her death in a brook—having had “too much of water” when her garments “heavy with their drink, | pull’d the poor wretch from her melodious lay | To muddy death.” The 2 clowns/gravediggers then debate whether Ophelia deserves a Christian burial and if her death should be considered a suicide: did the water drown her, or did she drown herself?3
Lady Macbeth’s suicide is offstage, punctuated by a “night-shriek.” Romeo drinks poison and dies “with a kiss.” Juliet quickly follows, making her body the sword’s sheath which “there rust, and let [her] die.” Othello stabs himself after requesting that his peers will “speak of me as I am.” One of King Lear’s daughters poisons her sister “and after [slays] herself.” Timon dies by his cave, “entomb’d upon the very hem o’ the sea.” In Antony and Cleopatra, after being told that Cleopatra has killed herself with Antony’s name on her lips, Antony begs to be stabbed and then stabs himself; he is not defeated by Caesar, but rather conquered by himself: “none but Antony | Should conquer Antony.” Cleopatra and her lady-in-waiting, Charmian, kill themselves with an asp. In Julius Caesar, Brutus runs upon his sword. Cassius begs for his own death, asking that “this good sword, | That ran through Caesar’s bowels, search this bosom.” Portia, it is reported, “swallowed fire.”
Shakespeare uses specific stylized language to portray characters in psychological anguish and suicidal states. Scholars have discussed how he uses certain stylistic language to highlight the anguish that happens during solitary, solipsistic moments of contemplation.4 Moments of anguish and suicidal ideation are marked by verbal repetition. An example of this repetition comes in Hamlet’s speech after he returns to the kingdom where his uncle has usurped his father, when he laments that he cannot end his own life. He says:
O, that this too too sullied flesh would melt,
Thaw, and resolve itself into a dew!
Or that the Everlasting had not fix’d
His canon ’gainst self-slaughter! O God, God,
How weary, stale, flat, and unprofitable
Seem to me all the uses of this world.
In these 6 lines, there are 2 instances of verbal repetition: “too too” and “God, God.” In this moment of solitude and despair, Hamlet’s speech fractures; his fractured speech reflects his fractured psyche. While Hamlet speaks of staleness and stagnation in the world, his words represent a sterile excess. No meaning is elicited by their repetition; there is no forward momentum to his speech. The words reflect the extent to which Hamlet is stuck and divided in this moment. Something similar happens in Macbeth’s “Tomorrow and tomorrow and tomorrow” speech. The words march on, and with each repetition they become increasingly hollow and brittle.
Why does this discussion of suicide in Shakespeare hold value for a contemporary psychiatrist? First, there is no single prototypical suicidal character in Shakespeare. His characters who are suicidal vary in their demographics and incentives for ending their lives. In this way, he provides a rich framework, one with which many people can engage. Second, this discussion fits into an existing paradigm for using art therapy (specifically Shakespeare) as a treatment modality for trauma.5 Programs such as DE-CRUIT have used the recitation of Shakespearean verse as a means of processing trauma in veterans.5 While Shakespeare does not mention a remedy for suicide in his plays, perhaps the text can serve as medicine. Third, the repetitive speech that Shakespeare uses in times of anguish could be a fairly accurate reflection of speech patterns in patients who are suicidal. Research that completed a spoken language analysis of patients who were suicidal has found “mechanical and repetitive phrasing” as a quality of these patients’ speech.6,7
For hundreds of years, critics have searched beyond the text for Shakespeare’s voice and opinion; what did he himself think of melancholy, despair, or suicide? We cannot know. We, as readers, are invited to explore a nuanced and multifaceted view of suicide, one that neither chides nor valorizes the act, and provides ambiguity rather than condemnation.
1. Paciaroni M, Bogousslavsky J. William Shakespeare’s neurology. Prog Brain Res. 2013;206:3-18.
2. Kirkland LR. To end itself by death: suicide in Shakespeare’s tragedies. South Med J. 1999;92(7):660-666.
3. Sale C. The “Amending Hand”: Hales v. Petit, Eyston v. Studd, and Equitable Action in Hamlet. In: Jordan C, Cunningham K, eds. The Law in Shakespeare. Early Modern Literature in History. Palgrave Macmillan; 2007:189-207. https://doi.org/10.1057/9780230626348_11
4. Langley E. Narcissism and Suicide in Shakespeare and His Contemporaries. Oxford University Press; 2009.
5. Ali A, Wolfert S, Lam I, et al. Intersecting modes of aesthetic distance and mimetic induction in therapeutic process: examining a drama-based treatment for military-related traumatic stress. Drama Therapy Review. 2018;4(2):153-165.
6. Belouali A, Gupta S, Sourirajan V, et al. Acoustic and language analysis of speech for suicidal ideation among US veterans. BioData Min. 2021;14(1):11. doi:10.1186/s13040-021-00245-y
7. Cummins N, Scherer S, Krajewski J, et al. A review of depression and suicide risk assessment using speech analysis. Speech Commun. 2015;71:10-49.
1. Paciaroni M, Bogousslavsky J. William Shakespeare’s neurology. Prog Brain Res. 2013;206:3-18.
2. Kirkland LR. To end itself by death: suicide in Shakespeare’s tragedies. South Med J. 1999;92(7):660-666.
3. Sale C. The “Amending Hand”: Hales v. Petit, Eyston v. Studd, and Equitable Action in Hamlet. In: Jordan C, Cunningham K, eds. The Law in Shakespeare. Early Modern Literature in History. Palgrave Macmillan; 2007:189-207. https://doi.org/10.1057/9780230626348_11
4. Langley E. Narcissism and Suicide in Shakespeare and His Contemporaries. Oxford University Press; 2009.
5. Ali A, Wolfert S, Lam I, et al. Intersecting modes of aesthetic distance and mimetic induction in therapeutic process: examining a drama-based treatment for military-related traumatic stress. Drama Therapy Review. 2018;4(2):153-165.
6. Belouali A, Gupta S, Sourirajan V, et al. Acoustic and language analysis of speech for suicidal ideation among US veterans. BioData Min. 2021;14(1):11. doi:10.1186/s13040-021-00245-y
7. Cummins N, Scherer S, Krajewski J, et al. A review of depression and suicide risk assessment using speech analysis. Speech Commun. 2015;71:10-49.
Valedictory
All that’s bright must fade,
The brightest still the fleetest;
All that’s sweet was made
But to be lost when sweetest.
Thomas Moore
I sometimes hold it half a sin
To put in words the grief I feel;
For words, like Nature, half reveal
And half conceal the Soul within.
Alfred, Lord Tennyson, In Memoriam
Dear Readers,
I have sad news to share with you. This is the last issue of
During my travels around the country over the past 2 decades, countless psychiatrists have told me that
As the saying goes: All good things eventually come to an end. I am so grateful to have had the opportunity to collaborate with a wonderful, highly competent editorial staff, as well as with outstanding colleagues who served on the editorial board all those years. A special shout-out to Jeff Bauer, the publishing staff editor, with whom I worked so closely. I very much appreciated all the authors and peer reviewers who contributed timely clinical articles month after month and made
This has been a unique journey for all of us who strived to transform
All that’s bright must fade,
The brightest still the fleetest;
All that’s sweet was made
But to be lost when sweetest.
Thomas Moore
I sometimes hold it half a sin
To put in words the grief I feel;
For words, like Nature, half reveal
And half conceal the Soul within.
Alfred, Lord Tennyson, In Memoriam
Dear Readers,
I have sad news to share with you. This is the last issue of
During my travels around the country over the past 2 decades, countless psychiatrists have told me that
As the saying goes: All good things eventually come to an end. I am so grateful to have had the opportunity to collaborate with a wonderful, highly competent editorial staff, as well as with outstanding colleagues who served on the editorial board all those years. A special shout-out to Jeff Bauer, the publishing staff editor, with whom I worked so closely. I very much appreciated all the authors and peer reviewers who contributed timely clinical articles month after month and made
This has been a unique journey for all of us who strived to transform
All that’s bright must fade,
The brightest still the fleetest;
All that’s sweet was made
But to be lost when sweetest.
Thomas Moore
I sometimes hold it half a sin
To put in words the grief I feel;
For words, like Nature, half reveal
And half conceal the Soul within.
Alfred, Lord Tennyson, In Memoriam
Dear Readers,
I have sad news to share with you. This is the last issue of
During my travels around the country over the past 2 decades, countless psychiatrists have told me that
As the saying goes: All good things eventually come to an end. I am so grateful to have had the opportunity to collaborate with a wonderful, highly competent editorial staff, as well as with outstanding colleagues who served on the editorial board all those years. A special shout-out to Jeff Bauer, the publishing staff editor, with whom I worked so closely. I very much appreciated all the authors and peer reviewers who contributed timely clinical articles month after month and made
This has been a unique journey for all of us who strived to transform
More on treating chronic insomnia
In “Treating chronic insomnia: An alternating medication strategy” (
Leslie Citrome, MD, MPH
Valhalla, New York
1. Rosenberg R, Citrome L, Drake CL. Advances in the treatment of chronic insomnia: a narrative review of new nonpharmacologic and pharmacologic therapies. Neuropsychiatr Dis Treat. 2021;17:2549-2566.
2. Citrome L. Dissecting clinical trials with ‘number needed to treat.’ Current Psychiatry. 2007;6(3):66-71.
3. Citrome L. Suvorexant for insomnia: a systematic review of the efficacy and safety profile for this newly approved hypnotic - what is the number needed to treat, number needed to harm and likelihood to be helped or harmed? Int J Clin Pract. 2014;68(12):1429-1441.
4. Citrome L, Juday TR, Frech F, et al. Lemborexant for the treatment of insomnia: direct and indirect comparisons with other hypnotics using number needed to treat, number needed to harm, and likelihood to be helped or harmed. J Clin Psychiatry. 2021;82:20m13795. doi:10.4088/JCP.20m13795
5. Citrome L, Juday TR, Lundwall C. Lemborexant and daridorexant for the treatment of insomnia: an indirect comparison using number needed to treat, number needed to harm, and likelihood to be helped or harmed. J Clin Psychiatry. 2023;84(6):23m14851. doi:10.4088/JCP.23m14851
In “Treating chronic insomnia: An alternating medication strategy” (
Leslie Citrome, MD, MPH
Valhalla, New York
In “Treating chronic insomnia: An alternating medication strategy” (
Leslie Citrome, MD, MPH
Valhalla, New York
1. Rosenberg R, Citrome L, Drake CL. Advances in the treatment of chronic insomnia: a narrative review of new nonpharmacologic and pharmacologic therapies. Neuropsychiatr Dis Treat. 2021;17:2549-2566.
2. Citrome L. Dissecting clinical trials with ‘number needed to treat.’ Current Psychiatry. 2007;6(3):66-71.
3. Citrome L. Suvorexant for insomnia: a systematic review of the efficacy and safety profile for this newly approved hypnotic - what is the number needed to treat, number needed to harm and likelihood to be helped or harmed? Int J Clin Pract. 2014;68(12):1429-1441.
4. Citrome L, Juday TR, Frech F, et al. Lemborexant for the treatment of insomnia: direct and indirect comparisons with other hypnotics using number needed to treat, number needed to harm, and likelihood to be helped or harmed. J Clin Psychiatry. 2021;82:20m13795. doi:10.4088/JCP.20m13795
5. Citrome L, Juday TR, Lundwall C. Lemborexant and daridorexant for the treatment of insomnia: an indirect comparison using number needed to treat, number needed to harm, and likelihood to be helped or harmed. J Clin Psychiatry. 2023;84(6):23m14851. doi:10.4088/JCP.23m14851
1. Rosenberg R, Citrome L, Drake CL. Advances in the treatment of chronic insomnia: a narrative review of new nonpharmacologic and pharmacologic therapies. Neuropsychiatr Dis Treat. 2021;17:2549-2566.
2. Citrome L. Dissecting clinical trials with ‘number needed to treat.’ Current Psychiatry. 2007;6(3):66-71.
3. Citrome L. Suvorexant for insomnia: a systematic review of the efficacy and safety profile for this newly approved hypnotic - what is the number needed to treat, number needed to harm and likelihood to be helped or harmed? Int J Clin Pract. 2014;68(12):1429-1441.
4. Citrome L, Juday TR, Frech F, et al. Lemborexant for the treatment of insomnia: direct and indirect comparisons with other hypnotics using number needed to treat, number needed to harm, and likelihood to be helped or harmed. J Clin Psychiatry. 2021;82:20m13795. doi:10.4088/JCP.20m13795
5. Citrome L, Juday TR, Lundwall C. Lemborexant and daridorexant for the treatment of insomnia: an indirect comparison using number needed to treat, number needed to harm, and likelihood to be helped or harmed. J Clin Psychiatry. 2023;84(6):23m14851. doi:10.4088/JCP.23m14851
Monoamine oxidase inhibitors and tricyclic antidepressants for MDD
Ms. B, age 45, has a history of major depressive disorder (MDD) and migraines. She is admitted after presenting with anhedonia, hopelessness, and hypersomnia. These symptoms have become more severe over the last few weeks. Ms. B describes a past suicide attempt via overdose on doxylamine for which she required treatment in the intensive care unit. The only activity she enjoys is her weekly girls’ night, during which she drinks a few glasses of wine. Ms. B’s current medications are dextromethorphan/bupropion 45/105 mg twice daily and aripiprazole 5 mg/d, which she has taken for 3 months. She states she has “been on every antidepressant there is.”
When clinicians review Ms. B’s medication history, it is clear she has had adequate trials of selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), intranasal esketamine, multiple augmentation strategies, and electroconvulsive therapy (ECT). Ms. B seeks an alternative medication to improve her depressive symptoms.
Treatment-resistant depression (TRD) is commonly defined as depression that has not responded to ≥2 adequate trials of an antidepressant.1 Some guidelines recommend monoamine oxidase inhibitors (MAOIs) and tricyclic antidepressants (TCAs) as second- or even third-line options for MDD,2 while others recommend reserving them for patients with insufficient responses to alternative treatment modalities.3,4 Although MAOIs and TCAs have been available since the 1950s, prescribing these medications has become less prevalent due to safety concerns, the availability of other pharmacologic options, and a lack of clinical training and comfort.5,6 Most research notes that MAOIs are superior for treating atypical depression while TCAs are more effective for melancholic depression.2-4 In a review of 20 studies, Thase et al7 found that 50% of TCA nonresponders benefited from an MAOI. In the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial, monotherapy with the MAOI tranylcypromine was associated with a lower remission rate than the TCA nortriptyline; many argue the dose of tranylcypromine was suboptimal, and few participants completed an adequate trial in the last level.8,9 A more recent study by Kim et al10 found MAOIs to be “generally more effective” than TCAs for TRD, particularly in patients with fewer antidepressant trials; however, this was a small retrospective exploratory trial. A network meta-analysis found both classes to be “competitive” with SSRIs based on efficacy and tolerability, which leads to the question of whether these medications should be considered earlier in therapy.11 Considering patient-specific factors and particular medication properties is an effective strategy when prescribing an MAOI or TCA.
Monoamine oxidase inhibitors
Four MAOIs are FDA-approved for treating MDD (Table 15,12-17): phenelzine, isocarboxazid, tranylcypromine, and selegiline. These medications irreversibly inhibit MAO, which exists as isomers A and B. MAO-A primarily metabolizes serotonin and norepinephrine, which is largely responsible for these medications’ antidepressant effects. Both isomers equally metabolize dopamine.5,12,18 It is best to avoid using MAOIs in patients with cerebrovascular disease, hepatic disease, or pheochromocytoma. Patients with active substance use disorders (particularly sympathomimetics and hallucinogens) are at an increased risk for hypertensive crises and serotonin syndrome, respectively. The most common adverse effects are orthostatic hypotension (despite more well-known concerns regarding hypertension), alterations in sleep patterns (insomnia or hypersomnia, depending on the agent), gastrointestinal issues, and anticholinergic adverse effects such as dry mouth and constipation.13,19-21
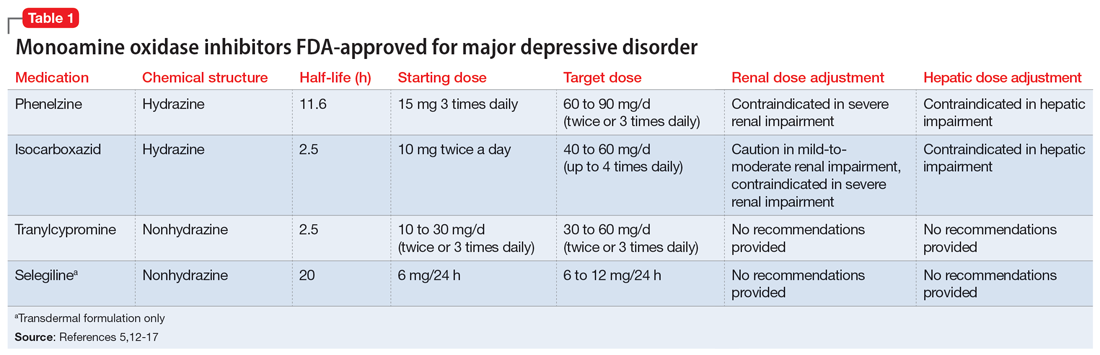
In one review and meta-analysis, phenelzine displayed the highest efficacy across all MAOIs.11 It likely requires high doses to achieve adequate MAO inhibition.11 A metabolite of phenelzine inhibits gamma-aminobutyric acid transaminase and may be helpful for patients with comorbid anxiety disorders or MDD with anxious distress.18,21 Additional considerations include phenelzine’s propensity for orthostasis (with rapid titrations and higher doses), sedation, weight gain, sexual dysfunction, and a rare adverse effect of vitamin B6 deficiency.5,13,14,20-22
Use of isocarboxazid in clinical practice is rare. Its adverse effects are similar to those of phenelzine but isocarboxazid is less studied. Tranylcypromine has a similar chemical structure to amphetamine. It can be stimulating at higher doses, potentially benefitting patients with comorbid attention-deficit/hyperactivity disorder (ADHD) or significant apathy.13,23 Selegiline’s distinct quality is its availability as a transdermal patch, which may be useful for patients who struggle to take oral medications. At low doses (6 mg/24 h), the selegiline transdermal patch allows patients to disregard a dietary tyramine restriction because it avoids first-pass metabolism. It inhibits both MAO isomers in the brain but is only selective for MAO-B once concentrations are distributed to the liver. Higher doses require a tyramine-restricted diet because there is still some MAO-A inhibition in the gut. Selegiline is also stimulating because it is converted to amphetamine and methamphetamine.5,12,13,17,19,24
Despite promising results from the use of MAOIs, physicians and patients may be reluctant to use these medications due to perceived limitations. One prominent barrier is the infamous “cheese reaction.” Tyramine, an amino acid found in certain food and beverages (Table 25,13-18,25-28), is broken down by MAO-A in the gut. When this enzyme is inhibited, higher concentrations of tyramine reach systemic circulation. Tyramine’s release of norepinephrine (which now cannot be broken down) can lead to a hypertensive crisis. Consequently, a tyramine-restricted diet is recommended for patients taking an MAOI. However, the common notion that cheese, wine, and beer must be avoided is false, because most of the dietary restrictions developed following the discovery of MAOIs are antiquated.5,12,25-28 Patients who take an MAOI only need to slightly adjust their diet, as outlined in Table 2.5,13-18,25-28 A reasonable serving size of most foods and beverages containing tyramine is unlikely to elicit this “pressor” response. Of the 4 MAOIs FDA-approved for MDD, tranylcypromine appears to be the most sensitive to tyramine.21 Transient postdose hypertension (regardless of tyramine) may occur after taking an MAOI.29 Encourage patients to monitor their blood pressure.
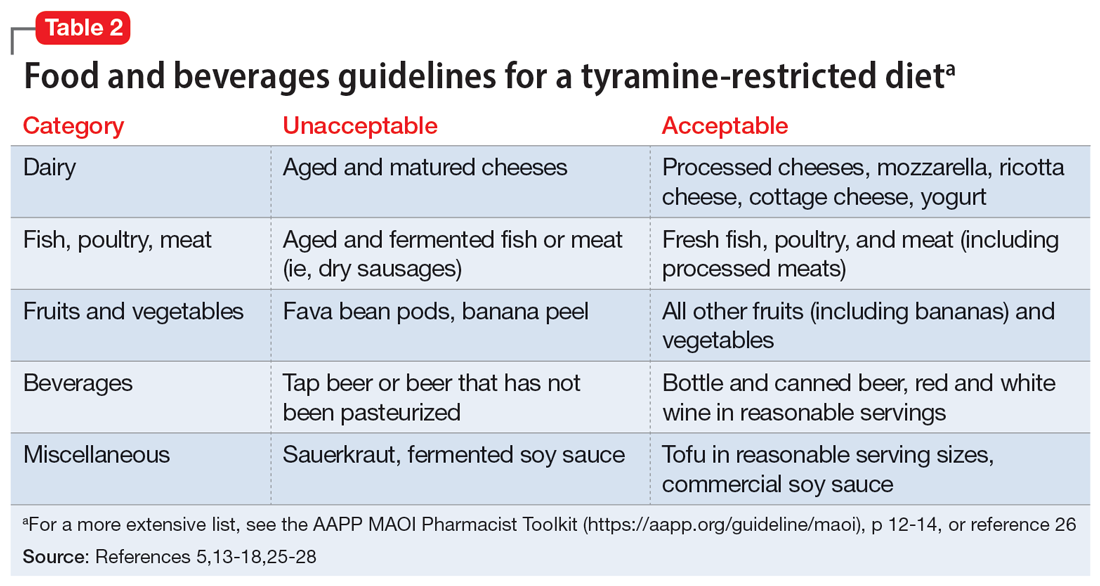
Continue to: Additional hurdles include...
Additional hurdles include the required washout period from serotonergic medications and interactions with sympathomimetics. MAOIs pose the highest risk of serotonin syndrome; however, this usually occurs if given concomitantly with other serotonergic agents. The standard recommendation is a 14-day washout period from SSRIs (5 weeks for fluoxetine and 3 weeks for vortioxetine), SNRIs, mirtazapine, and other antidepressants. It can be distressing for patients to be without medication during that period. Because some antidepressants have much shorter half-lives, waiting 5 half-lives (typically 5 to 7 days) for the discontinued medication to be excreted is feasible if patients are closely monitored.5,12,13,25,27,30 There are rare instances where a TCA may be combined with an MAOI (typically initiated within 1 to 2 days of each other), but never clomipramine or imipramine due to their potent serotonin reuptake inhibition.31 If switching to an alternative MAOI, waiting 7 to 14 days is recommended to allow adequate time for the inhibited enzyme to regenerate.14-17,32 Taking medications that increase dopamine and norepinephrine (eg, stimulants or oral over-the-counter decongestants) with an MAOI is typically not recommended due to the risk of hypertensive crisis.25,27 In severe TRD or comorbid ADHD, successful simultaneous use of methylphenidate or amphetamine—typically at low doses—with close blood pressure monitoring has been reported.33 There have also been positive cases of the use of modafinil in combination with an MAOI; however, this should be done with caution.34,35 Clinicians must use clinical judgment when considering a combination of medications that pose a higher risk.
Tricyclic antidepressants
TCAs work differently than MAOIs to increase monoamines. They inhibit presynaptic serotonin and norepinephrine transporters in the CNS to increase levels of these chemicals in the synaptic cleft. While all TCAs inhibit these transporters, they do so at varying levels (Table 336-51). Based on their chemical structure, TCAs can be categorized into secondary and tertiary amines. Tertiary amines are metabolized via demethylation into their derivatives (Table 336-51). Patients who have recently suffered a myocardial infarction (MI) should avoid tertiary amines. TCAs can reduce heart rate variability, which is already decreased after an MI, thus presenting the potential for cardiac arrhythmias. TCAs should also be avoided in patients with cardiac conduction abnormalities.38-46,52 Patients with a prior baseline cardiac conduction defect, such as a bundle branch block, are at higher risk for further cardiac abnormalities. In those with a preexisting first-degree heart block, TCAs can still be used, but electrocardiogram monitoring is recommended.52,53 TCAs have also been reported to decrease the seizure threshold.38-46 They can be used with caution in patients who have a history of epilepsy or head trauma, or with concomitant medications that lower the seizure threshold.38-46
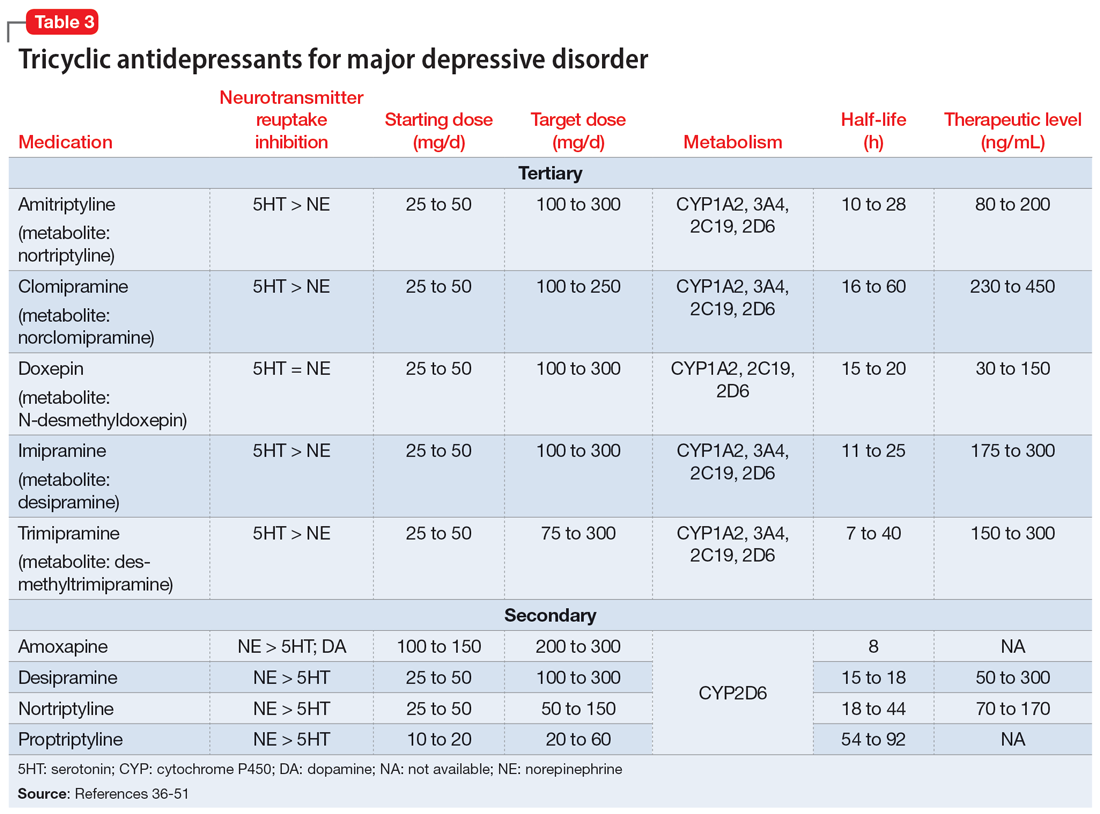
Overdose risk is a concern with TCAs because ingestion of 10 to 20 mg/kg can lead to significant toxicity.54 This is due to their blockage of voltage-gated sodium channels found in the CNS and heart, which contributes to overdose symptoms such as a widened QRS complex and seizures. Symptoms usually develop within 2 hours but may be delayed up to 6 hours.55 Patients with a history of overdose must be carefully assessed before initiating a TCA. Prescribing a limited supply of these medications may be valuable. The use of TCAs has often been limited due to their adverse effects, most of which are associated with their respective affinities for alpha 1, muscarinic 1, and histamine 1 receptors. Inhibition of the alpha 1 receptor is associated with hypotension, muscarinic 1 with anticholinergic adverse effects, and histamine 1 with sedation and weight gain. Tertiary amines have a higher affinity for these receptors compared to secondary amines, leading to a more significant adverse effect profile.36,50 Among TCAs, amitriptyline is the most likely to cause hypotension, whereas desipramine and nortriptyline are least likely. Amitriptyline and clomipramine are most likely to cause anticholinergic adverse effects, whereas desipramine and nortriptyline are the least likely. Amitriptyline, doxepin, and imipramine have the highest propensity for QTc prolongation.36
Beyond treating MDD, TCAs have shown benefits for treating other disease states (Table 438-46,49,56-61).These differing indications may help psychiatrists determine the best TCA to prescribe for a given patient. Amitriptyline is the most studied TCA for MDD; however, nortriptyline is typically preferred due to its favorable tolerability profile.4,62 Nortriptyline also has data supporting its use in ECT to prevent relapse.63 Amitriptyline and nortriptyline have shown benefits in patients with neuropathic pain and for migraine prophylaxis.56-60 Although frequently used for MDD, clomipramine is not FDA-approved for this indication, but is for obsessive-compulsive disorder.39 Doxepin is FDA-approved for insomnia at lower doses and for MDD at higher doses.40 Therefore, it may benefit patients with sleep difficulties secondary to depression. Desipramine has been used off-label to treat ADHD in children and has shown some benefits in adults.64-66 Protriptyline, trimipramine, and amoxapine are infrequently used in clinical practice.
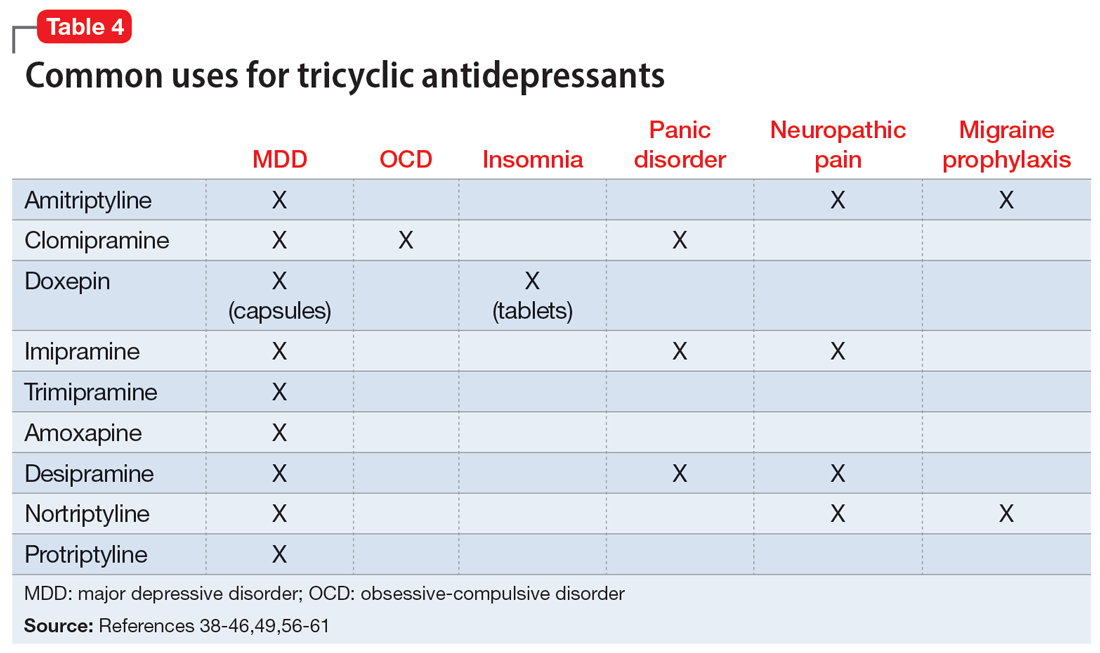
A unique feature of TCAs is the ability to monitor serum concentrations (Table 336-51).Guidelines recommend therapeutic drug monitoring (TDM) with amitriptyline, clomipramine, imipramine, and nortriptyline for routine use. TDM is still recommended for doxepin, desipramine, and trimipramine, but its utility is largely for treatment failure or resistance.37 These plasma levels can be altered based on coadministered medications (Table 538-46) and should be closely monitored. Physicians should obtain a trough level after at least 5 half-lives and before the next dose is due, and use TDM as indicated to optimize dosing.
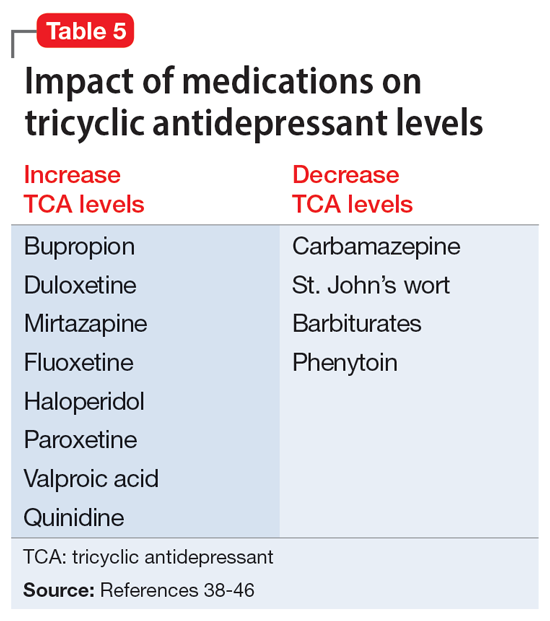
Continue to: CASE CONTINUED
CASE CONTINUED
Ms. B’s outpatient psychiatrist provides collateral information about her medical history and confirms her long-standing MDD with multiple medication trials, though she has never received an MAOI or TCA. Ms. B is adamant she does not want a medication-free period between treatments and refuses to adjust her diet, despite being educated on the few changes necessary. She has no contraindications for TCAs and may benefit from a TCA for her comorbid migraines. The care team expresses concern for TCA overdose to Ms. B and her family. Ms. B’s sister reassures the team they will have someone monitor and dispense her medications at home. They decide to discontinue her current psychiatric regimen, and Ms. B is started on nortriptyline 50 mg/d at night, with plans to titrate based on tolerability.
Related Resources
- Meyer JM. A concise guide to monoamine oxidase inhibitors. Current Psychiatry. 2017;16(12):14-16,18-23,47,A.
- Espejo GD. Treating major depressive disorder after limited response to an initial agent. Current Psychiatry. 2021;20(10):51-53. doi:10.12788/cp.0178
- American Association of Psychiatric Pharmacists (AAPP) MAOI Pharmacist Toolkit. https://aapp.org/guideline/maoi
Drug Brand Names
Amitriptyline • Elavil
Amphetamine • Adzenys, Dyanavel
Aripiprazole • Abilify
Clomipramine • Anafranil
Desipramine • Norpramin
Dextromethorphan/bupropion • Auvelity
Doxepin • Sinequan, Adapin
Esketamine • Spravato
Fluoxetine • Prozac
Imipramine • Tofranil
Isocarboxazid • Marplan
Methamphetamine • Desoxyn
Mirtazapine • Remeron
Modafinil • Provigil
Nortriptyline • Pamelor
Phenelzine • Nardil
Protriptyline • Vivactil
Selegiline • Emsam
Tranylcypromine • Parnate
Trimipramine • Surmontil
Vortioxetine • Trintellix
1. Gaynes BN, Lux L, Gartlehner G, et al. Defining treatment-resistant depression. Depress Anxiety. 2020;37(2):134-145. doi:10.1002/da.22968
2. Kennedy SH, Lam RW, McIntyre RS, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 3. Pharmacological treatments. Can J Psychiatry. 2016;61(9):540-560. doi:10.1177/0706743716659417
3. VA/DoD clinical practice guideline for the management of major depressive disorder. Veterans Health Administration and Department of Defense; 2016. https://www.healthquality.va.gov/guidelines/MH/mdd/VADoDMDDCPGFINAL82916.pdf
4. Gelenberg AJ, Freeman MP, Markowitz JC, et al. Practice guideline for the treatment of patients with major depressive disorder. Am J Psychiatry. 2010;167(Suppl 10):9-118.
5. Meyer JM. A concise guide to monoamine oxidase inhibitors. Current Psychiatry. 2017;16(12):14-16,18-23,47,A.
6. Taylor D. Selective serotonin reuptake inhibitors and tricyclic antidepressants in combination. Interactions and therapeutic uses. Br J Psychiatry. 1995;167(5):575-580. doi:10.1192/bjp.167.5.575
7. Thase ME, Trivedi MH, Rush AJ. MAOIs in the contemporary treatment of depression. Neuropsychopharmacology. 1995;12(3):185-219. doi:10.1016/0893-133X(94)00058-8
8. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905-1917. doi:10.1176/ajp.2006.163.11.1905
9. McGrath PJ, Stewart JW, Fava M, et al. Tranylcypromine versus venlafaxine plus mirtazapine following three failed antidepressant medication trials for depression: a STAR*D report. Am J Psychiatry. 2006;163(9):1531-1666. doi:10.1176/ajp.2006.163.9.1531
10. Kim T, Xu C, Amsterdam JD. Relative effectiveness of tricyclic antidepressant versus monoamine oxidase inhibitor monotherapy for treatment-resistant depression. J Affect Disord. 2019;250:199-203. doi:10.1016/j.jad.2019.03.028
11. Suchting R, Tirumalajaru V, Gareeb R, et al. Revisiting monoamine oxidase inhibitors for the treatment of depressive disorders: a systematic review and network meta-analysis. J Affect Disord. 2021;282:1153-1160. doi:10.1016/j.jad.2021.01.021
12. Stahl SM, Felker A. Monoamine oxidase inhibitors: a modern guide to an unrequited class of antidepressants. CNS Spectr. 2008;13(10):855-870. doi:10.1017/s1092852900016965
13. Chamberlain SR, Baldwin DS. Monoamine oxidase inhibitors (MAOIs) in psychiatric practice: how to use them safely and effectively. CNS Drugs. 2021;35(7):703-716. doi:10.1007/s40263-021-00832-x
14. Nardil [package insert]. New York, NY: Parke-Davis; 2009.
15. Marplan [package insert]. Parsippany, NJ: Validus Pharmaceuticals LLC; 2020.
16. Parnate [package insert]. Saint Michael, Barbados: Concordia Pharmaceuticals; 2015.
17. Emsam [package insert]. Morgantown, WV: Mylan Specialty LP; 2014.
18. Shulman KI, Herrmann N, Walker SE. Current place of monoamine oxidase inhibitors in the treatment of depression. CNS Drugs. 2013;27(10):789-797. doi:10.1007/s40263-013-0097-3
19. Sub Laban T, Saadabadi A. Monoamine oxidase inhibitors (MAOI). StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK539848/
20. Rabkin JG, Quitkin FM, McGrath P, et al. Adverse reactions to monoamine oxidase inhibitors. Part II. Treatment correlates and clinical management. J Clin Psychopharmacol. 1985;5(1):2-9.
21. Gillman PK. Advances pertaining to the pharmacology and interactions of irreversible nonselective monoamine oxidase inhibitors. J Clin Psychopharmacol. 2011;31(1):66-74. doi:10.1097/JCP.0b013e31820469ea
22. Sidhu G, Marwaha R. Phenelzine. StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK554508/
23. Frieling H, Bleich S. Tranylcypromine: new perspectives on an “old” drug. Eur Arch Psychiatry Clin Neurosci. 2006;256(5):268-273. doi:10.1007/s00406-006-0660-8
24. Goodnick PJ. Seligiline transdermal system in depression. Expert Opin Pharmacother. 2007;8(1):59-64. doi:10.1517/14656566.8.1.59
25. Edinoff AN, Swinford CR, Odisho AS, et al. Clinically relevant drug interactions with monoamine oxidase inhibitors. Health Psychol Res. 2022;10(4):39576. doi:10.52965/001c.39576
26. Gillman PK. A reassessment of the safety profile of monoamine oxidase inhibitors: elucidating tired old tyramine myths. J Neural Transm (Vienna). 2018;125(11):1707-1717. doi:10.1007/s00702-018-1932-y
27. Flockhart DA. Dietary restrictions and drug interactions with monoamine oxidase inhibitors: an update. J Clin Psychiatry. 2012;73 Suppl 1:17-24. doi:10.4088/JCP.11096su1c.03
28. McCabe-Sellers BJ, Staggs CG, Bogle ML. Tyramine in foods and monoamine oxidase inhibitor drugs: a crossroad where medicine, nutrition, pharmacy, and food industry converge. J Food Composit Anal. 2006;19:S58-S65. doi:10.1016/j.jfca.2005.12.008
29. Keck PE Jr, Vuckovic A, Pope HG Jr, et al. Acute cardiovascular response to monoamine oxidase inhibitors: a prospective assessment. J Clin Psychopharmacol. 1989;9(3):203-206.
30. Bodkin JA, Dunlop BW. Moving on with monoamine oxidase inhibitors. Focus (Am Psychiatr Publ). 2021;19(1):50-52. doi:10.1176/appi.focus.20200046
31. Amsterdam JD, Kim TT. Relative effectiveness of monoamine oxidase inhibitor and tricyclic antidepressant combination therapy for treatment-resistant depression. J Clin Psychopharmacol. 2019;39(6):649-652. doi:10.1097/JCP.0000000000001130
32. Keks N, Hope J, Keogh S. Switching and stopping antidepressants. Aust Prescr. 2016;39(3):76-83. doi:10.18773/austprescr.2016.039
33. Israel JA. Combining stimulants and monoamine oxidase inhibitors: a reexamination of the literature and a report of a new treatment combination. Prim Care Companion CNS Disord. 2015;17(6):10.4088/PCC.15br01836. doi:10.4088/PCC.15br01836
34. Clemons WE, Makela E, Young J. Concomitant use of modafinil and tranylcypromine in a patient with narcolepsy: a case report. Sleep Med. 2004;5(5):509-511. doi:10.1016/j.sleep.2004.06.006
35. Ashton AK. Modafinil augmentation of phenelzine for residual fatigue in dysthymia. Am J Psychiatry. 2004;161(9):1716-1717. doi:10.1176/appi.ajp.161.9.1716-a
36. O’Donnell JM, Bies RR, Shelton RC. Drug therapy of depression and anxiety disorders. In: Brunton LL, Hilal-Dandan R, Knollmann BC, eds. Goodman & Gilman’s: The Pharmacological Basis of Therapeutics. 13th ed. McGraw Hill; 2017. Accessed June 4, 2023. https://accessanesthesiology.mhmedical.com/content.aspx?bookid=2189§ionid=169518711
37. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-02):9-62. doi:10.1055/s-0043-116492
38. Amitriptyline hydrochloride [package insert]. East Brunswick, NJ: Unichem Pharmaceuticals (USA); 2021.
39. Clomipramine hydrochloride [package insert]. East Windsor, NJ: Aurobindo Pharma Limited; 2023.
40. Doxepin hydrochloride capsules, USP [package insert]. Bedminster, NJ: Alembic Pharmaceuticals Inc; 2021.
41. Imipramine hydrochloride tablet [package insert]. Fairfield, NJ: Leading Pharma LLC USA; 2022.
42. Trimipramine maleate [package insert]. Northvale, NJ: Elite Laboratories Inc; 2021.
43. Amoxapine [package insert]. Parsippany, NJ: Actavis Pharma Inc; 2015.
44. Desipramine hydrochloride tablets [package insert]. Bedminster, NJ: Alembic Pharmaceuticals Inc; 2023.
45. Nortriptyline hydrochloride capsules, USP [package insert]. Parsippany, NJ: Teva Pharmaceuticals Inc; 2021.
46. Protriptyline hydrochloride [package insert]. Bensalem, PA: Sigmapharm Laboratories, LLC; 2023.
47. Calvo B, García MJ, Pedraz JL, et al. Pharmacokinetics of amoxapine and its active metabolites. Int J Clin Pharmacol Ther Toxicol. 1985;23(4):180-185.
48. Ziegler VE, Biggs JT, Wylie LT, et al. Protriptyline kinetics. Clin Pharmacol Ther. 1978;23(5):580-584. doi:10.1002/cpt1978235580
49. Cleare A, Pariante CM, Young AH, et al. Evidence-based guidelines for treating depressive disorders with antidepressants: a revision of the 2008 British Association for Psychopharmacology guidelines. J Psychopharmacol. 2015;29(5):459-525. doi:10.1177/0269881115581093
50. Richelson E. Synaptic effects of antidepressants. J Clin Psychopharmacol. 1996;16(3 Suppl 2):1S-9S. doi:10.1097/00004714-199606002-00001
51. Vos CF, Aarnoutse RE, Op de Coul MJM, et al. Tricyclic antidepressants for major depressive disorder: a comprehensive evaluation of current practice in the Netherlands. BMC Psychiatry. 2021;21(1):481. doi:10.1186/s12888-021-03490-x
52. Alvarez W Jr, Pickworth KK. Safety of antidepressant drugs in the patient with cardiac disease: a review of the literature. Pharmacotherapy. 2003;23(6):754-771. doi:10.1592/phco.23.6.754.32185
53. Dietch JT, Fine M. The effect of nortriptyline in elderly patients with cardiac conduction disease. J Clin Psychiatry. 1990;51(2):65-67.
54. Valento M, Liebelt EL. Cyclic antidepressants. In: Nelson LS, Howland M, Lewin NA, et al, eds. Goldfrank’s Toxicologic Emergencies. 9th ed. McGraw Hill; 2011. Accessed June 10, 2023. https://accesspharmacy.mhmedical.com/content.aspx?bookid=2569§ionid=210274664
55. Woolf AD, Erdman AR, Nelson LS, et al. Tricyclic antidepressant poisoning: an evidence-based consensus guideline for out-of-hospital management. Clin Toxicol (Phila). 2007;45(3):203-233. doi:10.1080/15563650701226192
56. Baldwin DS, Anderson IM, Nutt DJ, et al. Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: a revision of the 2005 guidelines from the British Association for Psychopharmacology. J Psychopharmacol. 2014;28(5):403-439. doi:10.1177/0269881114525674
57. Moulin DE, Clark AJ, Gilron I, et al. Pharmacological management of chronic neuropathic pain - consensus statement and guidelines from the Canadian Pain Society. Pain Res Manag. 2007;12(1):13-21. doi:10.1155/2007/730785
58. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162-173. doi:10.1016/S1474-4422(14)70251-0
59. Saarto T, Wiffen PJ. Antidepressants for neuropathic pain. Cochrane Database Syst Rev. 2007;(4):CD005454. doi:10.1002/14651858.CD005454.pub2
60. Burch R. Antidepressants for preventive treatment of migraine. Curr Treat Options Neurol. 2019;21(4):18. doi:10.1007/s11940-019-0557-2
61. Ailani J, Burch RC, Robbins MS; Board of Directors of the American Headache Society. The American Headache Society consensus statement: update on integrating new migraine treatments into clinical practice. Headache. 2021;61(7):1021-1039. doi:10.1111/head.14153
62. Bauer M, Pfennig A, Severus E, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders, part 1: update 2013 on the acute and continuation treatment of unipolar depressive disorders. World J Biol Psychiatry. 2013;14(5):334-385. doi:10.3109/15622975.2013.804195
63. Jelovac A, Kolshus E, McLoughlin DM. Relapse following successful electroconvulsive therapy for major depression: a meta-analysis. Neuropsychopharmacology. 2013;38(12):2467-2474. doi:10.1038/npp.2013.149
64. Spencer T, Biederman J, Coffey B, et al. A double-blind comparison of desipramine and placebo in children and adolescents with chronic tic disorder and comorbid attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2002;59(7):649-656. doi:10.1001/archpsyc.59.7.649
65. Spencer T, Biederman J, Wilens T, et al. Pharmacotherapy of attention-deficit hyperactivity disorder across the life cycle. J Am Acad Child Adolesc Psychiatry. 1996;35(4):409-432. doi:10.1097/00004583-199604000-00008
66. Wilens TE, Biederman J, Prince J, et al. Six-week, double-blind, placebo-controlled study of desipramine for adult attention deficit hyperactivity disorder. Am J Psychiatry. 1996;153(9):1147-1153. doi:10.1176/ajp.153.9.1147
Ms. B, age 45, has a history of major depressive disorder (MDD) and migraines. She is admitted after presenting with anhedonia, hopelessness, and hypersomnia. These symptoms have become more severe over the last few weeks. Ms. B describes a past suicide attempt via overdose on doxylamine for which she required treatment in the intensive care unit. The only activity she enjoys is her weekly girls’ night, during which she drinks a few glasses of wine. Ms. B’s current medications are dextromethorphan/bupropion 45/105 mg twice daily and aripiprazole 5 mg/d, which she has taken for 3 months. She states she has “been on every antidepressant there is.”
When clinicians review Ms. B’s medication history, it is clear she has had adequate trials of selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), intranasal esketamine, multiple augmentation strategies, and electroconvulsive therapy (ECT). Ms. B seeks an alternative medication to improve her depressive symptoms.
Treatment-resistant depression (TRD) is commonly defined as depression that has not responded to ≥2 adequate trials of an antidepressant.1 Some guidelines recommend monoamine oxidase inhibitors (MAOIs) and tricyclic antidepressants (TCAs) as second- or even third-line options for MDD,2 while others recommend reserving them for patients with insufficient responses to alternative treatment modalities.3,4 Although MAOIs and TCAs have been available since the 1950s, prescribing these medications has become less prevalent due to safety concerns, the availability of other pharmacologic options, and a lack of clinical training and comfort.5,6 Most research notes that MAOIs are superior for treating atypical depression while TCAs are more effective for melancholic depression.2-4 In a review of 20 studies, Thase et al7 found that 50% of TCA nonresponders benefited from an MAOI. In the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial, monotherapy with the MAOI tranylcypromine was associated with a lower remission rate than the TCA nortriptyline; many argue the dose of tranylcypromine was suboptimal, and few participants completed an adequate trial in the last level.8,9 A more recent study by Kim et al10 found MAOIs to be “generally more effective” than TCAs for TRD, particularly in patients with fewer antidepressant trials; however, this was a small retrospective exploratory trial. A network meta-analysis found both classes to be “competitive” with SSRIs based on efficacy and tolerability, which leads to the question of whether these medications should be considered earlier in therapy.11 Considering patient-specific factors and particular medication properties is an effective strategy when prescribing an MAOI or TCA.
Monoamine oxidase inhibitors
Four MAOIs are FDA-approved for treating MDD (Table 15,12-17): phenelzine, isocarboxazid, tranylcypromine, and selegiline. These medications irreversibly inhibit MAO, which exists as isomers A and B. MAO-A primarily metabolizes serotonin and norepinephrine, which is largely responsible for these medications’ antidepressant effects. Both isomers equally metabolize dopamine.5,12,18 It is best to avoid using MAOIs in patients with cerebrovascular disease, hepatic disease, or pheochromocytoma. Patients with active substance use disorders (particularly sympathomimetics and hallucinogens) are at an increased risk for hypertensive crises and serotonin syndrome, respectively. The most common adverse effects are orthostatic hypotension (despite more well-known concerns regarding hypertension), alterations in sleep patterns (insomnia or hypersomnia, depending on the agent), gastrointestinal issues, and anticholinergic adverse effects such as dry mouth and constipation.13,19-21

In one review and meta-analysis, phenelzine displayed the highest efficacy across all MAOIs.11 It likely requires high doses to achieve adequate MAO inhibition.11 A metabolite of phenelzine inhibits gamma-aminobutyric acid transaminase and may be helpful for patients with comorbid anxiety disorders or MDD with anxious distress.18,21 Additional considerations include phenelzine’s propensity for orthostasis (with rapid titrations and higher doses), sedation, weight gain, sexual dysfunction, and a rare adverse effect of vitamin B6 deficiency.5,13,14,20-22
Use of isocarboxazid in clinical practice is rare. Its adverse effects are similar to those of phenelzine but isocarboxazid is less studied. Tranylcypromine has a similar chemical structure to amphetamine. It can be stimulating at higher doses, potentially benefitting patients with comorbid attention-deficit/hyperactivity disorder (ADHD) or significant apathy.13,23 Selegiline’s distinct quality is its availability as a transdermal patch, which may be useful for patients who struggle to take oral medications. At low doses (6 mg/24 h), the selegiline transdermal patch allows patients to disregard a dietary tyramine restriction because it avoids first-pass metabolism. It inhibits both MAO isomers in the brain but is only selective for MAO-B once concentrations are distributed to the liver. Higher doses require a tyramine-restricted diet because there is still some MAO-A inhibition in the gut. Selegiline is also stimulating because it is converted to amphetamine and methamphetamine.5,12,13,17,19,24
Despite promising results from the use of MAOIs, physicians and patients may be reluctant to use these medications due to perceived limitations. One prominent barrier is the infamous “cheese reaction.” Tyramine, an amino acid found in certain food and beverages (Table 25,13-18,25-28), is broken down by MAO-A in the gut. When this enzyme is inhibited, higher concentrations of tyramine reach systemic circulation. Tyramine’s release of norepinephrine (which now cannot be broken down) can lead to a hypertensive crisis. Consequently, a tyramine-restricted diet is recommended for patients taking an MAOI. However, the common notion that cheese, wine, and beer must be avoided is false, because most of the dietary restrictions developed following the discovery of MAOIs are antiquated.5,12,25-28 Patients who take an MAOI only need to slightly adjust their diet, as outlined in Table 2.5,13-18,25-28 A reasonable serving size of most foods and beverages containing tyramine is unlikely to elicit this “pressor” response. Of the 4 MAOIs FDA-approved for MDD, tranylcypromine appears to be the most sensitive to tyramine.21 Transient postdose hypertension (regardless of tyramine) may occur after taking an MAOI.29 Encourage patients to monitor their blood pressure.

Continue to: Additional hurdles include...
Additional hurdles include the required washout period from serotonergic medications and interactions with sympathomimetics. MAOIs pose the highest risk of serotonin syndrome; however, this usually occurs if given concomitantly with other serotonergic agents. The standard recommendation is a 14-day washout period from SSRIs (5 weeks for fluoxetine and 3 weeks for vortioxetine), SNRIs, mirtazapine, and other antidepressants. It can be distressing for patients to be without medication during that period. Because some antidepressants have much shorter half-lives, waiting 5 half-lives (typically 5 to 7 days) for the discontinued medication to be excreted is feasible if patients are closely monitored.5,12,13,25,27,30 There are rare instances where a TCA may be combined with an MAOI (typically initiated within 1 to 2 days of each other), but never clomipramine or imipramine due to their potent serotonin reuptake inhibition.31 If switching to an alternative MAOI, waiting 7 to 14 days is recommended to allow adequate time for the inhibited enzyme to regenerate.14-17,32 Taking medications that increase dopamine and norepinephrine (eg, stimulants or oral over-the-counter decongestants) with an MAOI is typically not recommended due to the risk of hypertensive crisis.25,27 In severe TRD or comorbid ADHD, successful simultaneous use of methylphenidate or amphetamine—typically at low doses—with close blood pressure monitoring has been reported.33 There have also been positive cases of the use of modafinil in combination with an MAOI; however, this should be done with caution.34,35 Clinicians must use clinical judgment when considering a combination of medications that pose a higher risk.
Tricyclic antidepressants
TCAs work differently than MAOIs to increase monoamines. They inhibit presynaptic serotonin and norepinephrine transporters in the CNS to increase levels of these chemicals in the synaptic cleft. While all TCAs inhibit these transporters, they do so at varying levels (Table 336-51). Based on their chemical structure, TCAs can be categorized into secondary and tertiary amines. Tertiary amines are metabolized via demethylation into their derivatives (Table 336-51). Patients who have recently suffered a myocardial infarction (MI) should avoid tertiary amines. TCAs can reduce heart rate variability, which is already decreased after an MI, thus presenting the potential for cardiac arrhythmias. TCAs should also be avoided in patients with cardiac conduction abnormalities.38-46,52 Patients with a prior baseline cardiac conduction defect, such as a bundle branch block, are at higher risk for further cardiac abnormalities. In those with a preexisting first-degree heart block, TCAs can still be used, but electrocardiogram monitoring is recommended.52,53 TCAs have also been reported to decrease the seizure threshold.38-46 They can be used with caution in patients who have a history of epilepsy or head trauma, or with concomitant medications that lower the seizure threshold.38-46

Overdose risk is a concern with TCAs because ingestion of 10 to 20 mg/kg can lead to significant toxicity.54 This is due to their blockage of voltage-gated sodium channels found in the CNS and heart, which contributes to overdose symptoms such as a widened QRS complex and seizures. Symptoms usually develop within 2 hours but may be delayed up to 6 hours.55 Patients with a history of overdose must be carefully assessed before initiating a TCA. Prescribing a limited supply of these medications may be valuable. The use of TCAs has often been limited due to their adverse effects, most of which are associated with their respective affinities for alpha 1, muscarinic 1, and histamine 1 receptors. Inhibition of the alpha 1 receptor is associated with hypotension, muscarinic 1 with anticholinergic adverse effects, and histamine 1 with sedation and weight gain. Tertiary amines have a higher affinity for these receptors compared to secondary amines, leading to a more significant adverse effect profile.36,50 Among TCAs, amitriptyline is the most likely to cause hypotension, whereas desipramine and nortriptyline are least likely. Amitriptyline and clomipramine are most likely to cause anticholinergic adverse effects, whereas desipramine and nortriptyline are the least likely. Amitriptyline, doxepin, and imipramine have the highest propensity for QTc prolongation.36
Beyond treating MDD, TCAs have shown benefits for treating other disease states (Table 438-46,49,56-61).These differing indications may help psychiatrists determine the best TCA to prescribe for a given patient. Amitriptyline is the most studied TCA for MDD; however, nortriptyline is typically preferred due to its favorable tolerability profile.4,62 Nortriptyline also has data supporting its use in ECT to prevent relapse.63 Amitriptyline and nortriptyline have shown benefits in patients with neuropathic pain and for migraine prophylaxis.56-60 Although frequently used for MDD, clomipramine is not FDA-approved for this indication, but is for obsessive-compulsive disorder.39 Doxepin is FDA-approved for insomnia at lower doses and for MDD at higher doses.40 Therefore, it may benefit patients with sleep difficulties secondary to depression. Desipramine has been used off-label to treat ADHD in children and has shown some benefits in adults.64-66 Protriptyline, trimipramine, and amoxapine are infrequently used in clinical practice.

A unique feature of TCAs is the ability to monitor serum concentrations (Table 336-51).Guidelines recommend therapeutic drug monitoring (TDM) with amitriptyline, clomipramine, imipramine, and nortriptyline for routine use. TDM is still recommended for doxepin, desipramine, and trimipramine, but its utility is largely for treatment failure or resistance.37 These plasma levels can be altered based on coadministered medications (Table 538-46) and should be closely monitored. Physicians should obtain a trough level after at least 5 half-lives and before the next dose is due, and use TDM as indicated to optimize dosing.

Continue to: CASE CONTINUED
CASE CONTINUED
Ms. B’s outpatient psychiatrist provides collateral information about her medical history and confirms her long-standing MDD with multiple medication trials, though she has never received an MAOI or TCA. Ms. B is adamant she does not want a medication-free period between treatments and refuses to adjust her diet, despite being educated on the few changes necessary. She has no contraindications for TCAs and may benefit from a TCA for her comorbid migraines. The care team expresses concern for TCA overdose to Ms. B and her family. Ms. B’s sister reassures the team they will have someone monitor and dispense her medications at home. They decide to discontinue her current psychiatric regimen, and Ms. B is started on nortriptyline 50 mg/d at night, with plans to titrate based on tolerability.
Related Resources
- Meyer JM. A concise guide to monoamine oxidase inhibitors. Current Psychiatry. 2017;16(12):14-16,18-23,47,A.
- Espejo GD. Treating major depressive disorder after limited response to an initial agent. Current Psychiatry. 2021;20(10):51-53. doi:10.12788/cp.0178
- American Association of Psychiatric Pharmacists (AAPP) MAOI Pharmacist Toolkit. https://aapp.org/guideline/maoi
Drug Brand Names
Amitriptyline • Elavil
Amphetamine • Adzenys, Dyanavel
Aripiprazole • Abilify
Clomipramine • Anafranil
Desipramine • Norpramin
Dextromethorphan/bupropion • Auvelity
Doxepin • Sinequan, Adapin
Esketamine • Spravato
Fluoxetine • Prozac
Imipramine • Tofranil
Isocarboxazid • Marplan
Methamphetamine • Desoxyn
Mirtazapine • Remeron
Modafinil • Provigil
Nortriptyline • Pamelor
Phenelzine • Nardil
Protriptyline • Vivactil
Selegiline • Emsam
Tranylcypromine • Parnate
Trimipramine • Surmontil
Vortioxetine • Trintellix
Ms. B, age 45, has a history of major depressive disorder (MDD) and migraines. She is admitted after presenting with anhedonia, hopelessness, and hypersomnia. These symptoms have become more severe over the last few weeks. Ms. B describes a past suicide attempt via overdose on doxylamine for which she required treatment in the intensive care unit. The only activity she enjoys is her weekly girls’ night, during which she drinks a few glasses of wine. Ms. B’s current medications are dextromethorphan/bupropion 45/105 mg twice daily and aripiprazole 5 mg/d, which she has taken for 3 months. She states she has “been on every antidepressant there is.”
When clinicians review Ms. B’s medication history, it is clear she has had adequate trials of selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), intranasal esketamine, multiple augmentation strategies, and electroconvulsive therapy (ECT). Ms. B seeks an alternative medication to improve her depressive symptoms.
Treatment-resistant depression (TRD) is commonly defined as depression that has not responded to ≥2 adequate trials of an antidepressant.1 Some guidelines recommend monoamine oxidase inhibitors (MAOIs) and tricyclic antidepressants (TCAs) as second- or even third-line options for MDD,2 while others recommend reserving them for patients with insufficient responses to alternative treatment modalities.3,4 Although MAOIs and TCAs have been available since the 1950s, prescribing these medications has become less prevalent due to safety concerns, the availability of other pharmacologic options, and a lack of clinical training and comfort.5,6 Most research notes that MAOIs are superior for treating atypical depression while TCAs are more effective for melancholic depression.2-4 In a review of 20 studies, Thase et al7 found that 50% of TCA nonresponders benefited from an MAOI. In the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial, monotherapy with the MAOI tranylcypromine was associated with a lower remission rate than the TCA nortriptyline; many argue the dose of tranylcypromine was suboptimal, and few participants completed an adequate trial in the last level.8,9 A more recent study by Kim et al10 found MAOIs to be “generally more effective” than TCAs for TRD, particularly in patients with fewer antidepressant trials; however, this was a small retrospective exploratory trial. A network meta-analysis found both classes to be “competitive” with SSRIs based on efficacy and tolerability, which leads to the question of whether these medications should be considered earlier in therapy.11 Considering patient-specific factors and particular medication properties is an effective strategy when prescribing an MAOI or TCA.
Monoamine oxidase inhibitors
Four MAOIs are FDA-approved for treating MDD (Table 15,12-17): phenelzine, isocarboxazid, tranylcypromine, and selegiline. These medications irreversibly inhibit MAO, which exists as isomers A and B. MAO-A primarily metabolizes serotonin and norepinephrine, which is largely responsible for these medications’ antidepressant effects. Both isomers equally metabolize dopamine.5,12,18 It is best to avoid using MAOIs in patients with cerebrovascular disease, hepatic disease, or pheochromocytoma. Patients with active substance use disorders (particularly sympathomimetics and hallucinogens) are at an increased risk for hypertensive crises and serotonin syndrome, respectively. The most common adverse effects are orthostatic hypotension (despite more well-known concerns regarding hypertension), alterations in sleep patterns (insomnia or hypersomnia, depending on the agent), gastrointestinal issues, and anticholinergic adverse effects such as dry mouth and constipation.13,19-21

In one review and meta-analysis, phenelzine displayed the highest efficacy across all MAOIs.11 It likely requires high doses to achieve adequate MAO inhibition.11 A metabolite of phenelzine inhibits gamma-aminobutyric acid transaminase and may be helpful for patients with comorbid anxiety disorders or MDD with anxious distress.18,21 Additional considerations include phenelzine’s propensity for orthostasis (with rapid titrations and higher doses), sedation, weight gain, sexual dysfunction, and a rare adverse effect of vitamin B6 deficiency.5,13,14,20-22
Use of isocarboxazid in clinical practice is rare. Its adverse effects are similar to those of phenelzine but isocarboxazid is less studied. Tranylcypromine has a similar chemical structure to amphetamine. It can be stimulating at higher doses, potentially benefitting patients with comorbid attention-deficit/hyperactivity disorder (ADHD) or significant apathy.13,23 Selegiline’s distinct quality is its availability as a transdermal patch, which may be useful for patients who struggle to take oral medications. At low doses (6 mg/24 h), the selegiline transdermal patch allows patients to disregard a dietary tyramine restriction because it avoids first-pass metabolism. It inhibits both MAO isomers in the brain but is only selective for MAO-B once concentrations are distributed to the liver. Higher doses require a tyramine-restricted diet because there is still some MAO-A inhibition in the gut. Selegiline is also stimulating because it is converted to amphetamine and methamphetamine.5,12,13,17,19,24
Despite promising results from the use of MAOIs, physicians and patients may be reluctant to use these medications due to perceived limitations. One prominent barrier is the infamous “cheese reaction.” Tyramine, an amino acid found in certain food and beverages (Table 25,13-18,25-28), is broken down by MAO-A in the gut. When this enzyme is inhibited, higher concentrations of tyramine reach systemic circulation. Tyramine’s release of norepinephrine (which now cannot be broken down) can lead to a hypertensive crisis. Consequently, a tyramine-restricted diet is recommended for patients taking an MAOI. However, the common notion that cheese, wine, and beer must be avoided is false, because most of the dietary restrictions developed following the discovery of MAOIs are antiquated.5,12,25-28 Patients who take an MAOI only need to slightly adjust their diet, as outlined in Table 2.5,13-18,25-28 A reasonable serving size of most foods and beverages containing tyramine is unlikely to elicit this “pressor” response. Of the 4 MAOIs FDA-approved for MDD, tranylcypromine appears to be the most sensitive to tyramine.21 Transient postdose hypertension (regardless of tyramine) may occur after taking an MAOI.29 Encourage patients to monitor their blood pressure.

Continue to: Additional hurdles include...
Additional hurdles include the required washout period from serotonergic medications and interactions with sympathomimetics. MAOIs pose the highest risk of serotonin syndrome; however, this usually occurs if given concomitantly with other serotonergic agents. The standard recommendation is a 14-day washout period from SSRIs (5 weeks for fluoxetine and 3 weeks for vortioxetine), SNRIs, mirtazapine, and other antidepressants. It can be distressing for patients to be without medication during that period. Because some antidepressants have much shorter half-lives, waiting 5 half-lives (typically 5 to 7 days) for the discontinued medication to be excreted is feasible if patients are closely monitored.5,12,13,25,27,30 There are rare instances where a TCA may be combined with an MAOI (typically initiated within 1 to 2 days of each other), but never clomipramine or imipramine due to their potent serotonin reuptake inhibition.31 If switching to an alternative MAOI, waiting 7 to 14 days is recommended to allow adequate time for the inhibited enzyme to regenerate.14-17,32 Taking medications that increase dopamine and norepinephrine (eg, stimulants or oral over-the-counter decongestants) with an MAOI is typically not recommended due to the risk of hypertensive crisis.25,27 In severe TRD or comorbid ADHD, successful simultaneous use of methylphenidate or amphetamine—typically at low doses—with close blood pressure monitoring has been reported.33 There have also been positive cases of the use of modafinil in combination with an MAOI; however, this should be done with caution.34,35 Clinicians must use clinical judgment when considering a combination of medications that pose a higher risk.
Tricyclic antidepressants
TCAs work differently than MAOIs to increase monoamines. They inhibit presynaptic serotonin and norepinephrine transporters in the CNS to increase levels of these chemicals in the synaptic cleft. While all TCAs inhibit these transporters, they do so at varying levels (Table 336-51). Based on their chemical structure, TCAs can be categorized into secondary and tertiary amines. Tertiary amines are metabolized via demethylation into their derivatives (Table 336-51). Patients who have recently suffered a myocardial infarction (MI) should avoid tertiary amines. TCAs can reduce heart rate variability, which is already decreased after an MI, thus presenting the potential for cardiac arrhythmias. TCAs should also be avoided in patients with cardiac conduction abnormalities.38-46,52 Patients with a prior baseline cardiac conduction defect, such as a bundle branch block, are at higher risk for further cardiac abnormalities. In those with a preexisting first-degree heart block, TCAs can still be used, but electrocardiogram monitoring is recommended.52,53 TCAs have also been reported to decrease the seizure threshold.38-46 They can be used with caution in patients who have a history of epilepsy or head trauma, or with concomitant medications that lower the seizure threshold.38-46

Overdose risk is a concern with TCAs because ingestion of 10 to 20 mg/kg can lead to significant toxicity.54 This is due to their blockage of voltage-gated sodium channels found in the CNS and heart, which contributes to overdose symptoms such as a widened QRS complex and seizures. Symptoms usually develop within 2 hours but may be delayed up to 6 hours.55 Patients with a history of overdose must be carefully assessed before initiating a TCA. Prescribing a limited supply of these medications may be valuable. The use of TCAs has often been limited due to their adverse effects, most of which are associated with their respective affinities for alpha 1, muscarinic 1, and histamine 1 receptors. Inhibition of the alpha 1 receptor is associated with hypotension, muscarinic 1 with anticholinergic adverse effects, and histamine 1 with sedation and weight gain. Tertiary amines have a higher affinity for these receptors compared to secondary amines, leading to a more significant adverse effect profile.36,50 Among TCAs, amitriptyline is the most likely to cause hypotension, whereas desipramine and nortriptyline are least likely. Amitriptyline and clomipramine are most likely to cause anticholinergic adverse effects, whereas desipramine and nortriptyline are the least likely. Amitriptyline, doxepin, and imipramine have the highest propensity for QTc prolongation.36
Beyond treating MDD, TCAs have shown benefits for treating other disease states (Table 438-46,49,56-61).These differing indications may help psychiatrists determine the best TCA to prescribe for a given patient. Amitriptyline is the most studied TCA for MDD; however, nortriptyline is typically preferred due to its favorable tolerability profile.4,62 Nortriptyline also has data supporting its use in ECT to prevent relapse.63 Amitriptyline and nortriptyline have shown benefits in patients with neuropathic pain and for migraine prophylaxis.56-60 Although frequently used for MDD, clomipramine is not FDA-approved for this indication, but is for obsessive-compulsive disorder.39 Doxepin is FDA-approved for insomnia at lower doses and for MDD at higher doses.40 Therefore, it may benefit patients with sleep difficulties secondary to depression. Desipramine has been used off-label to treat ADHD in children and has shown some benefits in adults.64-66 Protriptyline, trimipramine, and amoxapine are infrequently used in clinical practice.

A unique feature of TCAs is the ability to monitor serum concentrations (Table 336-51).Guidelines recommend therapeutic drug monitoring (TDM) with amitriptyline, clomipramine, imipramine, and nortriptyline for routine use. TDM is still recommended for doxepin, desipramine, and trimipramine, but its utility is largely for treatment failure or resistance.37 These plasma levels can be altered based on coadministered medications (Table 538-46) and should be closely monitored. Physicians should obtain a trough level after at least 5 half-lives and before the next dose is due, and use TDM as indicated to optimize dosing.

Continue to: CASE CONTINUED
CASE CONTINUED
Ms. B’s outpatient psychiatrist provides collateral information about her medical history and confirms her long-standing MDD with multiple medication trials, though she has never received an MAOI or TCA. Ms. B is adamant she does not want a medication-free period between treatments and refuses to adjust her diet, despite being educated on the few changes necessary. She has no contraindications for TCAs and may benefit from a TCA for her comorbid migraines. The care team expresses concern for TCA overdose to Ms. B and her family. Ms. B’s sister reassures the team they will have someone monitor and dispense her medications at home. They decide to discontinue her current psychiatric regimen, and Ms. B is started on nortriptyline 50 mg/d at night, with plans to titrate based on tolerability.
Related Resources
- Meyer JM. A concise guide to monoamine oxidase inhibitors. Current Psychiatry. 2017;16(12):14-16,18-23,47,A.
- Espejo GD. Treating major depressive disorder after limited response to an initial agent. Current Psychiatry. 2021;20(10):51-53. doi:10.12788/cp.0178
- American Association of Psychiatric Pharmacists (AAPP) MAOI Pharmacist Toolkit. https://aapp.org/guideline/maoi
Drug Brand Names
Amitriptyline • Elavil
Amphetamine • Adzenys, Dyanavel
Aripiprazole • Abilify
Clomipramine • Anafranil
Desipramine • Norpramin
Dextromethorphan/bupropion • Auvelity
Doxepin • Sinequan, Adapin
Esketamine • Spravato
Fluoxetine • Prozac
Imipramine • Tofranil
Isocarboxazid • Marplan
Methamphetamine • Desoxyn
Mirtazapine • Remeron
Modafinil • Provigil
Nortriptyline • Pamelor
Phenelzine • Nardil
Protriptyline • Vivactil
Selegiline • Emsam
Tranylcypromine • Parnate
Trimipramine • Surmontil
Vortioxetine • Trintellix
1. Gaynes BN, Lux L, Gartlehner G, et al. Defining treatment-resistant depression. Depress Anxiety. 2020;37(2):134-145. doi:10.1002/da.22968
2. Kennedy SH, Lam RW, McIntyre RS, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 3. Pharmacological treatments. Can J Psychiatry. 2016;61(9):540-560. doi:10.1177/0706743716659417
3. VA/DoD clinical practice guideline for the management of major depressive disorder. Veterans Health Administration and Department of Defense; 2016. https://www.healthquality.va.gov/guidelines/MH/mdd/VADoDMDDCPGFINAL82916.pdf
4. Gelenberg AJ, Freeman MP, Markowitz JC, et al. Practice guideline for the treatment of patients with major depressive disorder. Am J Psychiatry. 2010;167(Suppl 10):9-118.
5. Meyer JM. A concise guide to monoamine oxidase inhibitors. Current Psychiatry. 2017;16(12):14-16,18-23,47,A.
6. Taylor D. Selective serotonin reuptake inhibitors and tricyclic antidepressants in combination. Interactions and therapeutic uses. Br J Psychiatry. 1995;167(5):575-580. doi:10.1192/bjp.167.5.575
7. Thase ME, Trivedi MH, Rush AJ. MAOIs in the contemporary treatment of depression. Neuropsychopharmacology. 1995;12(3):185-219. doi:10.1016/0893-133X(94)00058-8
8. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905-1917. doi:10.1176/ajp.2006.163.11.1905
9. McGrath PJ, Stewart JW, Fava M, et al. Tranylcypromine versus venlafaxine plus mirtazapine following three failed antidepressant medication trials for depression: a STAR*D report. Am J Psychiatry. 2006;163(9):1531-1666. doi:10.1176/ajp.2006.163.9.1531
10. Kim T, Xu C, Amsterdam JD. Relative effectiveness of tricyclic antidepressant versus monoamine oxidase inhibitor monotherapy for treatment-resistant depression. J Affect Disord. 2019;250:199-203. doi:10.1016/j.jad.2019.03.028
11. Suchting R, Tirumalajaru V, Gareeb R, et al. Revisiting monoamine oxidase inhibitors for the treatment of depressive disorders: a systematic review and network meta-analysis. J Affect Disord. 2021;282:1153-1160. doi:10.1016/j.jad.2021.01.021
12. Stahl SM, Felker A. Monoamine oxidase inhibitors: a modern guide to an unrequited class of antidepressants. CNS Spectr. 2008;13(10):855-870. doi:10.1017/s1092852900016965
13. Chamberlain SR, Baldwin DS. Monoamine oxidase inhibitors (MAOIs) in psychiatric practice: how to use them safely and effectively. CNS Drugs. 2021;35(7):703-716. doi:10.1007/s40263-021-00832-x
14. Nardil [package insert]. New York, NY: Parke-Davis; 2009.
15. Marplan [package insert]. Parsippany, NJ: Validus Pharmaceuticals LLC; 2020.
16. Parnate [package insert]. Saint Michael, Barbados: Concordia Pharmaceuticals; 2015.
17. Emsam [package insert]. Morgantown, WV: Mylan Specialty LP; 2014.
18. Shulman KI, Herrmann N, Walker SE. Current place of monoamine oxidase inhibitors in the treatment of depression. CNS Drugs. 2013;27(10):789-797. doi:10.1007/s40263-013-0097-3
19. Sub Laban T, Saadabadi A. Monoamine oxidase inhibitors (MAOI). StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK539848/
20. Rabkin JG, Quitkin FM, McGrath P, et al. Adverse reactions to monoamine oxidase inhibitors. Part II. Treatment correlates and clinical management. J Clin Psychopharmacol. 1985;5(1):2-9.
21. Gillman PK. Advances pertaining to the pharmacology and interactions of irreversible nonselective monoamine oxidase inhibitors. J Clin Psychopharmacol. 2011;31(1):66-74. doi:10.1097/JCP.0b013e31820469ea
22. Sidhu G, Marwaha R. Phenelzine. StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK554508/
23. Frieling H, Bleich S. Tranylcypromine: new perspectives on an “old” drug. Eur Arch Psychiatry Clin Neurosci. 2006;256(5):268-273. doi:10.1007/s00406-006-0660-8
24. Goodnick PJ. Seligiline transdermal system in depression. Expert Opin Pharmacother. 2007;8(1):59-64. doi:10.1517/14656566.8.1.59
25. Edinoff AN, Swinford CR, Odisho AS, et al. Clinically relevant drug interactions with monoamine oxidase inhibitors. Health Psychol Res. 2022;10(4):39576. doi:10.52965/001c.39576
26. Gillman PK. A reassessment of the safety profile of monoamine oxidase inhibitors: elucidating tired old tyramine myths. J Neural Transm (Vienna). 2018;125(11):1707-1717. doi:10.1007/s00702-018-1932-y
27. Flockhart DA. Dietary restrictions and drug interactions with monoamine oxidase inhibitors: an update. J Clin Psychiatry. 2012;73 Suppl 1:17-24. doi:10.4088/JCP.11096su1c.03
28. McCabe-Sellers BJ, Staggs CG, Bogle ML. Tyramine in foods and monoamine oxidase inhibitor drugs: a crossroad where medicine, nutrition, pharmacy, and food industry converge. J Food Composit Anal. 2006;19:S58-S65. doi:10.1016/j.jfca.2005.12.008
29. Keck PE Jr, Vuckovic A, Pope HG Jr, et al. Acute cardiovascular response to monoamine oxidase inhibitors: a prospective assessment. J Clin Psychopharmacol. 1989;9(3):203-206.
30. Bodkin JA, Dunlop BW. Moving on with monoamine oxidase inhibitors. Focus (Am Psychiatr Publ). 2021;19(1):50-52. doi:10.1176/appi.focus.20200046
31. Amsterdam JD, Kim TT. Relative effectiveness of monoamine oxidase inhibitor and tricyclic antidepressant combination therapy for treatment-resistant depression. J Clin Psychopharmacol. 2019;39(6):649-652. doi:10.1097/JCP.0000000000001130
32. Keks N, Hope J, Keogh S. Switching and stopping antidepressants. Aust Prescr. 2016;39(3):76-83. doi:10.18773/austprescr.2016.039
33. Israel JA. Combining stimulants and monoamine oxidase inhibitors: a reexamination of the literature and a report of a new treatment combination. Prim Care Companion CNS Disord. 2015;17(6):10.4088/PCC.15br01836. doi:10.4088/PCC.15br01836
34. Clemons WE, Makela E, Young J. Concomitant use of modafinil and tranylcypromine in a patient with narcolepsy: a case report. Sleep Med. 2004;5(5):509-511. doi:10.1016/j.sleep.2004.06.006
35. Ashton AK. Modafinil augmentation of phenelzine for residual fatigue in dysthymia. Am J Psychiatry. 2004;161(9):1716-1717. doi:10.1176/appi.ajp.161.9.1716-a
36. O’Donnell JM, Bies RR, Shelton RC. Drug therapy of depression and anxiety disorders. In: Brunton LL, Hilal-Dandan R, Knollmann BC, eds. Goodman & Gilman’s: The Pharmacological Basis of Therapeutics. 13th ed. McGraw Hill; 2017. Accessed June 4, 2023. https://accessanesthesiology.mhmedical.com/content.aspx?bookid=2189§ionid=169518711
37. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-02):9-62. doi:10.1055/s-0043-116492
38. Amitriptyline hydrochloride [package insert]. East Brunswick, NJ: Unichem Pharmaceuticals (USA); 2021.
39. Clomipramine hydrochloride [package insert]. East Windsor, NJ: Aurobindo Pharma Limited; 2023.
40. Doxepin hydrochloride capsules, USP [package insert]. Bedminster, NJ: Alembic Pharmaceuticals Inc; 2021.
41. Imipramine hydrochloride tablet [package insert]. Fairfield, NJ: Leading Pharma LLC USA; 2022.
42. Trimipramine maleate [package insert]. Northvale, NJ: Elite Laboratories Inc; 2021.
43. Amoxapine [package insert]. Parsippany, NJ: Actavis Pharma Inc; 2015.
44. Desipramine hydrochloride tablets [package insert]. Bedminster, NJ: Alembic Pharmaceuticals Inc; 2023.
45. Nortriptyline hydrochloride capsules, USP [package insert]. Parsippany, NJ: Teva Pharmaceuticals Inc; 2021.
46. Protriptyline hydrochloride [package insert]. Bensalem, PA: Sigmapharm Laboratories, LLC; 2023.
47. Calvo B, García MJ, Pedraz JL, et al. Pharmacokinetics of amoxapine and its active metabolites. Int J Clin Pharmacol Ther Toxicol. 1985;23(4):180-185.
48. Ziegler VE, Biggs JT, Wylie LT, et al. Protriptyline kinetics. Clin Pharmacol Ther. 1978;23(5):580-584. doi:10.1002/cpt1978235580
49. Cleare A, Pariante CM, Young AH, et al. Evidence-based guidelines for treating depressive disorders with antidepressants: a revision of the 2008 British Association for Psychopharmacology guidelines. J Psychopharmacol. 2015;29(5):459-525. doi:10.1177/0269881115581093
50. Richelson E. Synaptic effects of antidepressants. J Clin Psychopharmacol. 1996;16(3 Suppl 2):1S-9S. doi:10.1097/00004714-199606002-00001
51. Vos CF, Aarnoutse RE, Op de Coul MJM, et al. Tricyclic antidepressants for major depressive disorder: a comprehensive evaluation of current practice in the Netherlands. BMC Psychiatry. 2021;21(1):481. doi:10.1186/s12888-021-03490-x
52. Alvarez W Jr, Pickworth KK. Safety of antidepressant drugs in the patient with cardiac disease: a review of the literature. Pharmacotherapy. 2003;23(6):754-771. doi:10.1592/phco.23.6.754.32185
53. Dietch JT, Fine M. The effect of nortriptyline in elderly patients with cardiac conduction disease. J Clin Psychiatry. 1990;51(2):65-67.
54. Valento M, Liebelt EL. Cyclic antidepressants. In: Nelson LS, Howland M, Lewin NA, et al, eds. Goldfrank’s Toxicologic Emergencies. 9th ed. McGraw Hill; 2011. Accessed June 10, 2023. https://accesspharmacy.mhmedical.com/content.aspx?bookid=2569§ionid=210274664
55. Woolf AD, Erdman AR, Nelson LS, et al. Tricyclic antidepressant poisoning: an evidence-based consensus guideline for out-of-hospital management. Clin Toxicol (Phila). 2007;45(3):203-233. doi:10.1080/15563650701226192
56. Baldwin DS, Anderson IM, Nutt DJ, et al. Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: a revision of the 2005 guidelines from the British Association for Psychopharmacology. J Psychopharmacol. 2014;28(5):403-439. doi:10.1177/0269881114525674
57. Moulin DE, Clark AJ, Gilron I, et al. Pharmacological management of chronic neuropathic pain - consensus statement and guidelines from the Canadian Pain Society. Pain Res Manag. 2007;12(1):13-21. doi:10.1155/2007/730785
58. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162-173. doi:10.1016/S1474-4422(14)70251-0
59. Saarto T, Wiffen PJ. Antidepressants for neuropathic pain. Cochrane Database Syst Rev. 2007;(4):CD005454. doi:10.1002/14651858.CD005454.pub2
60. Burch R. Antidepressants for preventive treatment of migraine. Curr Treat Options Neurol. 2019;21(4):18. doi:10.1007/s11940-019-0557-2
61. Ailani J, Burch RC, Robbins MS; Board of Directors of the American Headache Society. The American Headache Society consensus statement: update on integrating new migraine treatments into clinical practice. Headache. 2021;61(7):1021-1039. doi:10.1111/head.14153
62. Bauer M, Pfennig A, Severus E, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders, part 1: update 2013 on the acute and continuation treatment of unipolar depressive disorders. World J Biol Psychiatry. 2013;14(5):334-385. doi:10.3109/15622975.2013.804195
63. Jelovac A, Kolshus E, McLoughlin DM. Relapse following successful electroconvulsive therapy for major depression: a meta-analysis. Neuropsychopharmacology. 2013;38(12):2467-2474. doi:10.1038/npp.2013.149
64. Spencer T, Biederman J, Coffey B, et al. A double-blind comparison of desipramine and placebo in children and adolescents with chronic tic disorder and comorbid attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2002;59(7):649-656. doi:10.1001/archpsyc.59.7.649
65. Spencer T, Biederman J, Wilens T, et al. Pharmacotherapy of attention-deficit hyperactivity disorder across the life cycle. J Am Acad Child Adolesc Psychiatry. 1996;35(4):409-432. doi:10.1097/00004583-199604000-00008
66. Wilens TE, Biederman J, Prince J, et al. Six-week, double-blind, placebo-controlled study of desipramine for adult attention deficit hyperactivity disorder. Am J Psychiatry. 1996;153(9):1147-1153. doi:10.1176/ajp.153.9.1147
1. Gaynes BN, Lux L, Gartlehner G, et al. Defining treatment-resistant depression. Depress Anxiety. 2020;37(2):134-145. doi:10.1002/da.22968
2. Kennedy SH, Lam RW, McIntyre RS, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 3. Pharmacological treatments. Can J Psychiatry. 2016;61(9):540-560. doi:10.1177/0706743716659417
3. VA/DoD clinical practice guideline for the management of major depressive disorder. Veterans Health Administration and Department of Defense; 2016. https://www.healthquality.va.gov/guidelines/MH/mdd/VADoDMDDCPGFINAL82916.pdf
4. Gelenberg AJ, Freeman MP, Markowitz JC, et al. Practice guideline for the treatment of patients with major depressive disorder. Am J Psychiatry. 2010;167(Suppl 10):9-118.
5. Meyer JM. A concise guide to monoamine oxidase inhibitors. Current Psychiatry. 2017;16(12):14-16,18-23,47,A.
6. Taylor D. Selective serotonin reuptake inhibitors and tricyclic antidepressants in combination. Interactions and therapeutic uses. Br J Psychiatry. 1995;167(5):575-580. doi:10.1192/bjp.167.5.575
7. Thase ME, Trivedi MH, Rush AJ. MAOIs in the contemporary treatment of depression. Neuropsychopharmacology. 1995;12(3):185-219. doi:10.1016/0893-133X(94)00058-8
8. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905-1917. doi:10.1176/ajp.2006.163.11.1905
9. McGrath PJ, Stewart JW, Fava M, et al. Tranylcypromine versus venlafaxine plus mirtazapine following three failed antidepressant medication trials for depression: a STAR*D report. Am J Psychiatry. 2006;163(9):1531-1666. doi:10.1176/ajp.2006.163.9.1531
10. Kim T, Xu C, Amsterdam JD. Relative effectiveness of tricyclic antidepressant versus monoamine oxidase inhibitor monotherapy for treatment-resistant depression. J Affect Disord. 2019;250:199-203. doi:10.1016/j.jad.2019.03.028
11. Suchting R, Tirumalajaru V, Gareeb R, et al. Revisiting monoamine oxidase inhibitors for the treatment of depressive disorders: a systematic review and network meta-analysis. J Affect Disord. 2021;282:1153-1160. doi:10.1016/j.jad.2021.01.021
12. Stahl SM, Felker A. Monoamine oxidase inhibitors: a modern guide to an unrequited class of antidepressants. CNS Spectr. 2008;13(10):855-870. doi:10.1017/s1092852900016965
13. Chamberlain SR, Baldwin DS. Monoamine oxidase inhibitors (MAOIs) in psychiatric practice: how to use them safely and effectively. CNS Drugs. 2021;35(7):703-716. doi:10.1007/s40263-021-00832-x
14. Nardil [package insert]. New York, NY: Parke-Davis; 2009.
15. Marplan [package insert]. Parsippany, NJ: Validus Pharmaceuticals LLC; 2020.
16. Parnate [package insert]. Saint Michael, Barbados: Concordia Pharmaceuticals; 2015.
17. Emsam [package insert]. Morgantown, WV: Mylan Specialty LP; 2014.
18. Shulman KI, Herrmann N, Walker SE. Current place of monoamine oxidase inhibitors in the treatment of depression. CNS Drugs. 2013;27(10):789-797. doi:10.1007/s40263-013-0097-3
19. Sub Laban T, Saadabadi A. Monoamine oxidase inhibitors (MAOI). StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK539848/
20. Rabkin JG, Quitkin FM, McGrath P, et al. Adverse reactions to monoamine oxidase inhibitors. Part II. Treatment correlates and clinical management. J Clin Psychopharmacol. 1985;5(1):2-9.
21. Gillman PK. Advances pertaining to the pharmacology and interactions of irreversible nonselective monoamine oxidase inhibitors. J Clin Psychopharmacol. 2011;31(1):66-74. doi:10.1097/JCP.0b013e31820469ea
22. Sidhu G, Marwaha R. Phenelzine. StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK554508/
23. Frieling H, Bleich S. Tranylcypromine: new perspectives on an “old” drug. Eur Arch Psychiatry Clin Neurosci. 2006;256(5):268-273. doi:10.1007/s00406-006-0660-8
24. Goodnick PJ. Seligiline transdermal system in depression. Expert Opin Pharmacother. 2007;8(1):59-64. doi:10.1517/14656566.8.1.59
25. Edinoff AN, Swinford CR, Odisho AS, et al. Clinically relevant drug interactions with monoamine oxidase inhibitors. Health Psychol Res. 2022;10(4):39576. doi:10.52965/001c.39576
26. Gillman PK. A reassessment of the safety profile of monoamine oxidase inhibitors: elucidating tired old tyramine myths. J Neural Transm (Vienna). 2018;125(11):1707-1717. doi:10.1007/s00702-018-1932-y
27. Flockhart DA. Dietary restrictions and drug interactions with monoamine oxidase inhibitors: an update. J Clin Psychiatry. 2012;73 Suppl 1:17-24. doi:10.4088/JCP.11096su1c.03
28. McCabe-Sellers BJ, Staggs CG, Bogle ML. Tyramine in foods and monoamine oxidase inhibitor drugs: a crossroad where medicine, nutrition, pharmacy, and food industry converge. J Food Composit Anal. 2006;19:S58-S65. doi:10.1016/j.jfca.2005.12.008
29. Keck PE Jr, Vuckovic A, Pope HG Jr, et al. Acute cardiovascular response to monoamine oxidase inhibitors: a prospective assessment. J Clin Psychopharmacol. 1989;9(3):203-206.
30. Bodkin JA, Dunlop BW. Moving on with monoamine oxidase inhibitors. Focus (Am Psychiatr Publ). 2021;19(1):50-52. doi:10.1176/appi.focus.20200046
31. Amsterdam JD, Kim TT. Relative effectiveness of monoamine oxidase inhibitor and tricyclic antidepressant combination therapy for treatment-resistant depression. J Clin Psychopharmacol. 2019;39(6):649-652. doi:10.1097/JCP.0000000000001130
32. Keks N, Hope J, Keogh S. Switching and stopping antidepressants. Aust Prescr. 2016;39(3):76-83. doi:10.18773/austprescr.2016.039
33. Israel JA. Combining stimulants and monoamine oxidase inhibitors: a reexamination of the literature and a report of a new treatment combination. Prim Care Companion CNS Disord. 2015;17(6):10.4088/PCC.15br01836. doi:10.4088/PCC.15br01836
34. Clemons WE, Makela E, Young J. Concomitant use of modafinil and tranylcypromine in a patient with narcolepsy: a case report. Sleep Med. 2004;5(5):509-511. doi:10.1016/j.sleep.2004.06.006
35. Ashton AK. Modafinil augmentation of phenelzine for residual fatigue in dysthymia. Am J Psychiatry. 2004;161(9):1716-1717. doi:10.1176/appi.ajp.161.9.1716-a
36. O’Donnell JM, Bies RR, Shelton RC. Drug therapy of depression and anxiety disorders. In: Brunton LL, Hilal-Dandan R, Knollmann BC, eds. Goodman & Gilman’s: The Pharmacological Basis of Therapeutics. 13th ed. McGraw Hill; 2017. Accessed June 4, 2023. https://accessanesthesiology.mhmedical.com/content.aspx?bookid=2189§ionid=169518711
37. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-02):9-62. doi:10.1055/s-0043-116492
38. Amitriptyline hydrochloride [package insert]. East Brunswick, NJ: Unichem Pharmaceuticals (USA); 2021.
39. Clomipramine hydrochloride [package insert]. East Windsor, NJ: Aurobindo Pharma Limited; 2023.
40. Doxepin hydrochloride capsules, USP [package insert]. Bedminster, NJ: Alembic Pharmaceuticals Inc; 2021.
41. Imipramine hydrochloride tablet [package insert]. Fairfield, NJ: Leading Pharma LLC USA; 2022.
42. Trimipramine maleate [package insert]. Northvale, NJ: Elite Laboratories Inc; 2021.
43. Amoxapine [package insert]. Parsippany, NJ: Actavis Pharma Inc; 2015.
44. Desipramine hydrochloride tablets [package insert]. Bedminster, NJ: Alembic Pharmaceuticals Inc; 2023.
45. Nortriptyline hydrochloride capsules, USP [package insert]. Parsippany, NJ: Teva Pharmaceuticals Inc; 2021.
46. Protriptyline hydrochloride [package insert]. Bensalem, PA: Sigmapharm Laboratories, LLC; 2023.
47. Calvo B, García MJ, Pedraz JL, et al. Pharmacokinetics of amoxapine and its active metabolites. Int J Clin Pharmacol Ther Toxicol. 1985;23(4):180-185.
48. Ziegler VE, Biggs JT, Wylie LT, et al. Protriptyline kinetics. Clin Pharmacol Ther. 1978;23(5):580-584. doi:10.1002/cpt1978235580
49. Cleare A, Pariante CM, Young AH, et al. Evidence-based guidelines for treating depressive disorders with antidepressants: a revision of the 2008 British Association for Psychopharmacology guidelines. J Psychopharmacol. 2015;29(5):459-525. doi:10.1177/0269881115581093
50. Richelson E. Synaptic effects of antidepressants. J Clin Psychopharmacol. 1996;16(3 Suppl 2):1S-9S. doi:10.1097/00004714-199606002-00001
51. Vos CF, Aarnoutse RE, Op de Coul MJM, et al. Tricyclic antidepressants for major depressive disorder: a comprehensive evaluation of current practice in the Netherlands. BMC Psychiatry. 2021;21(1):481. doi:10.1186/s12888-021-03490-x
52. Alvarez W Jr, Pickworth KK. Safety of antidepressant drugs in the patient with cardiac disease: a review of the literature. Pharmacotherapy. 2003;23(6):754-771. doi:10.1592/phco.23.6.754.32185
53. Dietch JT, Fine M. The effect of nortriptyline in elderly patients with cardiac conduction disease. J Clin Psychiatry. 1990;51(2):65-67.
54. Valento M, Liebelt EL. Cyclic antidepressants. In: Nelson LS, Howland M, Lewin NA, et al, eds. Goldfrank’s Toxicologic Emergencies. 9th ed. McGraw Hill; 2011. Accessed June 10, 2023. https://accesspharmacy.mhmedical.com/content.aspx?bookid=2569§ionid=210274664
55. Woolf AD, Erdman AR, Nelson LS, et al. Tricyclic antidepressant poisoning: an evidence-based consensus guideline for out-of-hospital management. Clin Toxicol (Phila). 2007;45(3):203-233. doi:10.1080/15563650701226192
56. Baldwin DS, Anderson IM, Nutt DJ, et al. Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: a revision of the 2005 guidelines from the British Association for Psychopharmacology. J Psychopharmacol. 2014;28(5):403-439. doi:10.1177/0269881114525674
57. Moulin DE, Clark AJ, Gilron I, et al. Pharmacological management of chronic neuropathic pain - consensus statement and guidelines from the Canadian Pain Society. Pain Res Manag. 2007;12(1):13-21. doi:10.1155/2007/730785
58. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162-173. doi:10.1016/S1474-4422(14)70251-0
59. Saarto T, Wiffen PJ. Antidepressants for neuropathic pain. Cochrane Database Syst Rev. 2007;(4):CD005454. doi:10.1002/14651858.CD005454.pub2
60. Burch R. Antidepressants for preventive treatment of migraine. Curr Treat Options Neurol. 2019;21(4):18. doi:10.1007/s11940-019-0557-2
61. Ailani J, Burch RC, Robbins MS; Board of Directors of the American Headache Society. The American Headache Society consensus statement: update on integrating new migraine treatments into clinical practice. Headache. 2021;61(7):1021-1039. doi:10.1111/head.14153
62. Bauer M, Pfennig A, Severus E, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders, part 1: update 2013 on the acute and continuation treatment of unipolar depressive disorders. World J Biol Psychiatry. 2013;14(5):334-385. doi:10.3109/15622975.2013.804195
63. Jelovac A, Kolshus E, McLoughlin DM. Relapse following successful electroconvulsive therapy for major depression: a meta-analysis. Neuropsychopharmacology. 2013;38(12):2467-2474. doi:10.1038/npp.2013.149
64. Spencer T, Biederman J, Coffey B, et al. A double-blind comparison of desipramine and placebo in children and adolescents with chronic tic disorder and comorbid attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2002;59(7):649-656. doi:10.1001/archpsyc.59.7.649
65. Spencer T, Biederman J, Wilens T, et al. Pharmacotherapy of attention-deficit hyperactivity disorder across the life cycle. J Am Acad Child Adolesc Psychiatry. 1996;35(4):409-432. doi:10.1097/00004583-199604000-00008
66. Wilens TE, Biederman J, Prince J, et al. Six-week, double-blind, placebo-controlled study of desipramine for adult attention deficit hyperactivity disorder. Am J Psychiatry. 1996;153(9):1147-1153. doi:10.1176/ajp.153.9.1147
Symptoms of psychosis and OCD in a patient with postpartum depression
CASE Thoughts of harming baby
Ms. A, age 37, is G4P2, 4 months postpartum, and breastfeeding. She has major depressive disorder (MDD) with peripartum onset, posttraumatic stress disorder, and mild intellectual disability. For years she has been stable on fluoxetine 40 mg/d and prazosin 2 mg/d. Despite recent titration of her medications, at her most recent outpatient appointment Ms. A reports having a depressed mood with frequent crying, insomnia, a lack of desire to bond with her baby, and feelings of shame. She also says she has had auditory hallucinations and thoughts of harming her baby. Ms. A’s outpatient physician makes an urgent request for her to be evaluated at the psychiatric emergency department (ED).
HISTORY Depression and possible auditory hallucinations
Ms. A developed MDD following the birth of her first child, for which her care team initiated fluoxetine at 20 mg/d and titrated it to 40 mg/d,which was effective. At that time, her outpatient physician documented potential psychotic features, including vague descriptions of derogatory auditory hallucinations. However, it was unclear if these auditory hallucinations were more representative of a distressing inner monologue without the quality of an external voice. The team determined that Ms. A was not at acute risk for harm to herself or her baby and was appropriate for outpatient care. Because the nature of these possible auditory hallucinations was mild, nondistressing, and nonthreatening, the treatment team did not initiate an antipsychotic and Ms. A was not hospitalized. She has no history of hypomanic/manic episodes and has never met criteria for a psychotic disorder.
EVALUATION Distressing thoughts and discontinued medications
During the evaluation by psychiatric emergency services, Ms. A reports that 2 weeks after giving birth she experienced a worsening of her depressive symptoms. She says she began hearing voices telling her to harm herself and her baby and describes frequent distressing thoughts, such as stabbing her baby with a knife and running over her baby with a car. Ms. A says she repeatedly wakes up at night to check on her baby’s breathing, overfeeds her baby due to a fear of inadequate nutrition, and notes intermittent feelings of confusion. Afraid of being alone with her infant, Ms. A asks her partner and mother to move in with her. Additionally, she says 2 weeks ago she discontinued all her medications at the suggestion of her partner, who recommended herbal supplements. Ms. A’s initial routine laboratory results are unremarkable and her urine drug screen is negative for all substances.
[polldaddy:13041928]
The authors’ observations
Approximately 85% of birthing parents experience some form of postpartum mood disturbance; 10% to 15% develop more significant symptoms of anxiety or depression.3 The etiology of postpartum illness is multifactorial, and includes psychiatric personal/family history, insomnia, acute and chronic psychosocial stressors, and rapid hormone fluctuations.1 As a result, the postpartum period represents a vulnerable time for birthing parents, particularly those with previously established psychiatric illness.
Ms. A’s initial presentation was concerning for a possible diagnosis of postpartum psychosis vs obsessive-compulsive disorder (OCD) with postpartum onset; other differential diagnoses included MDD with peripartum onset and psychotic features (Table1-6). Ms. A’s subjective clinical history was significant for critical pertinent findings of both OCD with postpartum onset (ie, egodystonic intrusive thoughts, checking behaviors, feelings of shame, and seeking reassurance) and postpartum psychosis (ie, command auditory hallucinations and waxing/waning confusion), which added to diagnostic complexity.
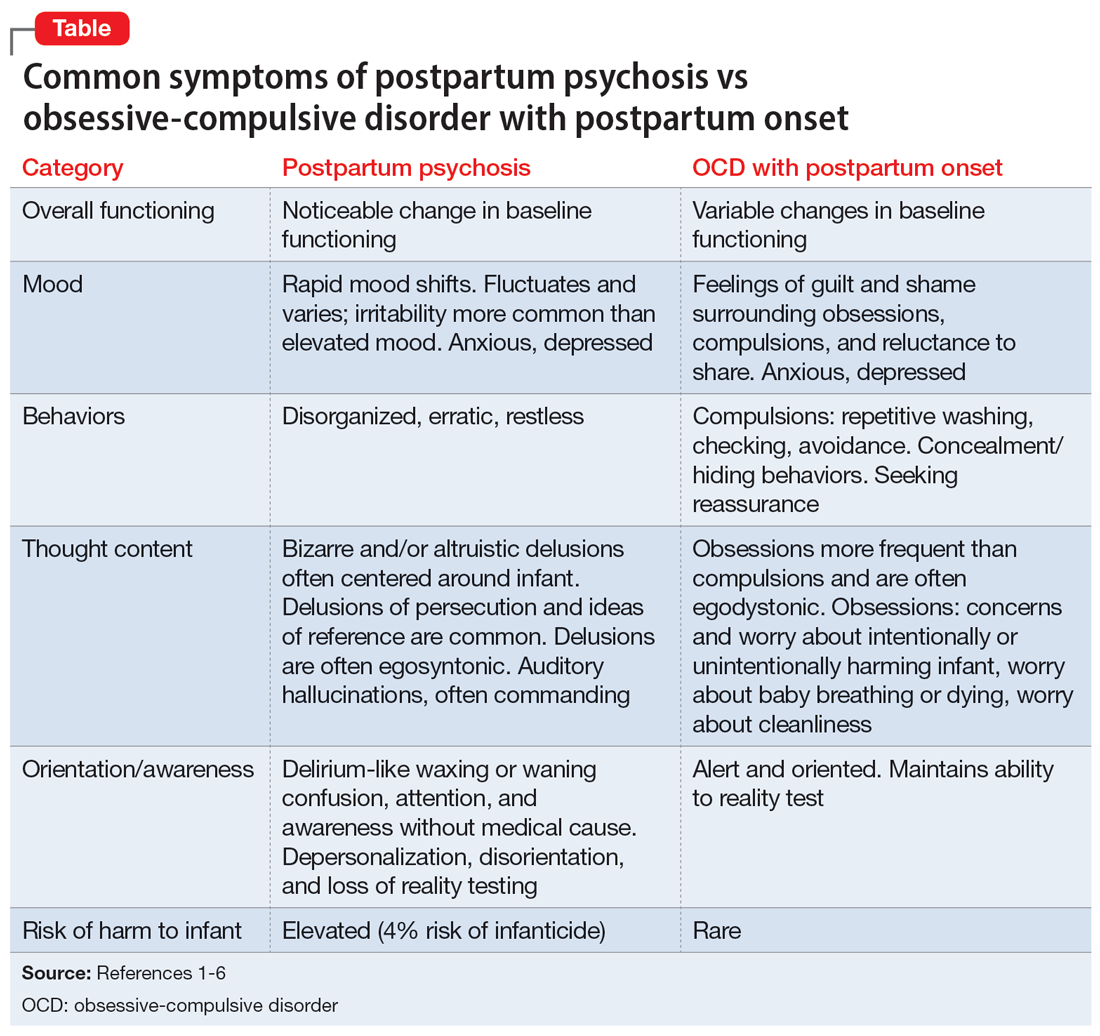
Although postpartum psychosis is rare (1 to 2 cases per 1,000 women),5 it is considered a psychiatric emergency because it has significant potential for infanticide, morbidity, and mortality. Most symptoms develop within the first 2 weeks of the postpartum period.2 There are many risk factors for the development of postpartum psychosis; however, in first-time pregnancies, a previous diagnosis of BD I is the single most important risk factor.1 Approximately 20% to 30% of women with BD experience postpartum psychosis.4
For many patients (approximately 56.7%, according to 1 meta-analysis7), postpartum psychosis denotes an episode of BD, representing a more severe form of illness with increased risk of recurrence. Most manic or mixed mood episodes reoccur within the first year removed from the perinatal period. In contrast, for some patients (approximately 43.5% according to the same meta-analysis), the episode denotes “isolated postpartum psychosis.”7 Isolated postpartum psychosis is a psychotic episode that occurs only in the postpartum period with no recurrence of psychosis or recurrence of psychosis exclusive to postpartum periods. If treated, this type of postpartum psychosis has a more favorable prognosis than postpartum psychosis in a patient with BD.7 As such, a BD diagnosis should not be established at the onset of a patient’s first postpartum psychosis presentation. Regardless of type, all presentations of postpartum psychosis are considered a psychiatry emergency.
Continue to: The prevalence of OCD...
The prevalence of OCD with postpartum onset varies. One study estimated it occurs in 2.43% of cases.4 However, the true prevalence is likely underreported due to feelings of guilt or shame associated with intrusive thoughts, and fear of stigmatization and separation from the baby. Approximately 70.6% of women experiencing OCD with postpartum onset have a comorbid depressive disorder.4
Ms. A’s presentation to the psychiatric ED carried with it diagnostic complexity and uncertainty. Her initial presentation was concerning for elements of both postpartum psychosis and OCD with postpartum onset. After her evaluation in the psychiatric ED, there remained a lack of clear and convincing evidence for a diagnosis of OCD with postpartum onset, which eliminated the possibility of discharging Ms. A with robust safety planning and reinitiation of a selective serotonin reuptake inhibitor.
Additionally, because auditory hallucinations are atypical in OCD, the treatment team remained concerned for a diagnosis of postpartum psychosis, which would warrant hospitalization. With assistance from the institution’s reproductive psychiatrists, the treatment team discussed the importance of inpatient hospitalization for risk mitigation, close observation, and thorough evaluation for greater diagnostic clarity and certainty.
TREATMENT Involuntary hospitalization
The treatment team counsels Ms. A and her partner on her differential diagnoses, including the elevated acute risk of harm to herself and her baby if she has postpartum psychosis, as well as the need for continued observation and evaluation. When alone with a clinician, Ms. A says she understands and agrees to voluntary hospitalization. However, following a subsequent risk-benefit discussion with her partner, they both grew increasingly concerned about her separation from the baby and reinitiating her medications. Amid these concerns, the treatment team notices that Ms. A attempts to minimize her symptoms. Ms. A changes her mind and no longer consents to hospitalization. She is placed on a psychiatric hold for involuntary hospitalization on the psychiatric inpatient unit.
On the inpatient unit, the inpatient clinicians and a reproductive psychiatrist continue to evaluate Ms. A. Though her diagnosis remains unclear, Ms. A agrees to start a trial of quetiapine 100 mg/d titrated to 150 mg/d to manage her potential postpartum psychosis, depressed mood, insomnia (off-label), anxiety (off-label), and OCD (off-label). Lithium is deferred because Ms. A is breastfeeding.
[polldaddy:13041932]
Continue to: The authors' observations
The authors’ observations
Due to an elevated acute risk of suicide and infanticide, postpartum psychosis represents a psychiatric emergency and often requires hospitalization. The Figure outlines steps in evaluating a patient with concerns for postpartum psychosis in a psychiatric emergency service setting. Due to the waxing and waning nature of symptoms, patients may appear psychiatrically stable at any time but remain at an overall elevated acute risk of harm to self and/or their baby.
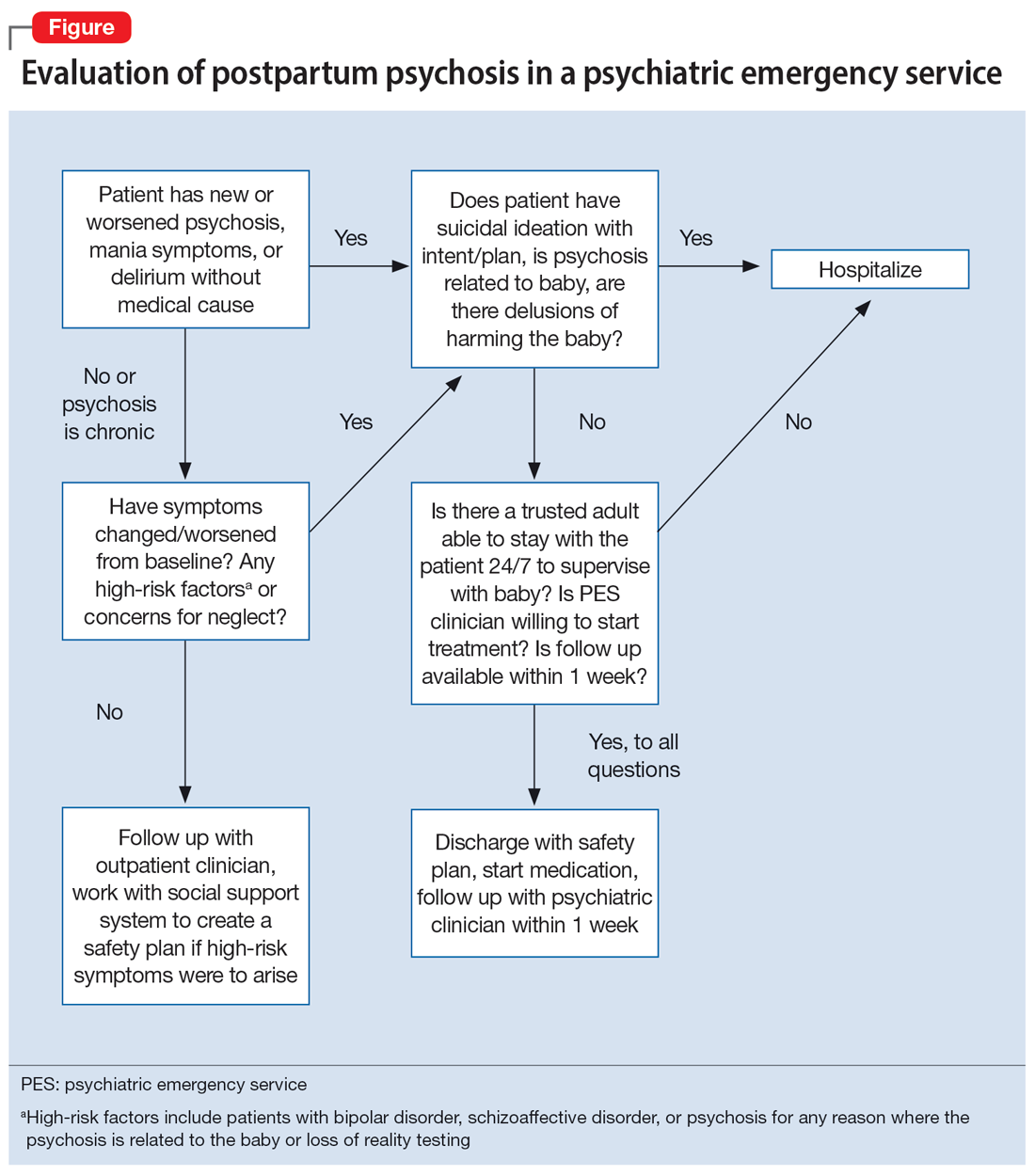
If a patient is being considered for discharge based on yes answers to all questions in Step 2 of the Figure, the emergency psychiatric clinician must initiate appropriate psychotropic medications and complete robust safety planning with the patient and a trusted adult who will provide direct supervision. Safety planning may include (but is not limited to) strict return precautions, education on concerning symptoms and behaviors, psychotropic education and agreement of compliance, and detailed instructions on outpatient follow-up within 1 week. Ideally—and as was the case for Ms. A—a reproductive psychiatrist should be consulted in the emergency setting for shared decision-making on admission vs discharge, medication management, and outpatient follow-up considerations.
Because postpartum psychosis carries significant risks and hospitalization generally results in separating the patient from their baby, initiating psychotropics should not be delayed. Clinicians must consider the patient’s psychiatric history, allergies, and breastfeeding status.
Based on current evidence, first-line treatment for postpartum psychosis includes a mood stabilizer, an antipsychotic, and possibly a benzodiazepine.6 Thus, an appropriate initial treatment regimen would be a benzodiazepine (particularly lorazepam due to its relatively shorter half-life) and an antipsychotic (eg, haloperidol, olanzapine, or quetiapine) for acute psychosis, plus lithium for mood stabilization.1,5
If the postpartum psychosis represents an episode of BD, use of a long-term mood stabilizer may be required. In contrast, for isolated postpartum psychosis, clinicians may consider initiating psychotropics only in the immediate postpartum period, with an eventual slow taper. In future pregnancies, psychotropics may be reintroduced postpartum, which will avoid peripartum fetal exposure.8 If the patient is breastfeeding, lithium may be deferred in an acute care setting. For patients with evidence of catatonia, severe suicidality, refusal of oral intake with compromised nutrition, severe agitation, or treatment resistance, electroconvulsive therapy remains a safe and effective treatment option.6 Additionally, the safety of continued breastfeeding in acute psychosis must be considered, with the potential for recommending discontinuation, which would decrease sleep disruptions at night and increase the ability of others to feed the baby. Comprehensive care requires nonpharmacologic interventions, including psychoeducation for the patient and their family, individual psychotherapy, and expansion of psychosocial supports.
Continue to: Patients who have experienced...
Patients who have experienced an episode of postpartum psychosis are predisposed to another episode in future pregnancies.1 Current research recommends prophylaxis of recurrence with lithium monotherapy.1,2,5,6 Similar to other psychotropics in reproductive psychiatry, maintenance therapy on lithium requires a thorough “risk vs risk” discussion with the patient. The risk of lithium use while pregnant and/or breastfeeding must be weighed against the risks associated with postpartum psychosis (ie, infanticide, suicide, poor peripartum care, or poor infant bonding).
OUTCOME Improved mood
After 7 days of inpatient treatment with quetiapine, Ms. A demonstrates improvement in the targeted depressive symptoms (including improved motivation/energy and insomnia, decreased feelings of guilt, and denial of ongoing suicidal ideation). Additionally, the thoughts of harming her baby are less frequent, and command auditory hallucinations resolve. Upon discharge, Ms. A and her partner meet with inpatient clinicians for continued counseling, safety planning, and plans for outpatient follow-up with the institution’s reproductive psychiatrist.
The authors’ observations
Many aspects of Ms. A’s initial presentation in the psychiatric ED were challenging. Given the presence of symptoms of both psychosis and OCD, a diagnosis was difficult to ascertain in the emergency setting. Since command auditory hallucinations are atypical in patients with postpartum OCD, the treatment team maintained high suspicion for postpartum psychosis, which represented an emergency requiring inpatient care.
Hospitalization separated Ms. A from her baby, for whom she was the primary caregiver. Additional considerations for inpatient admission and psychotropic initiation were necessary, because Ms. A was breastfeeding. Although Ms. A’s partner was able to provide full-time childcare, the patient ultimately did not agree to hospitalization and required an emergency hold for involuntary admission, which was an additional barrier to care. Furthermore, her partner held unfavorable beliefs regarding psychotropic medications and Ms. A’s need for hospital admission, which required ongoing patient and partner education in the emergency, inpatient, and outpatient settings. Moreover, if Ms. A’s symptoms were ultimately attributable to postpartum OCD, the patient’s involuntary hospitalization might have increased the risk of stigmatization of mental illness and treatment with psychotropics.
Bottom Line
The peripartum period is a vulnerable time for patients, particularly those with previously diagnosed psychiatric illnesses. Postpartum psychosis is the most severe form of postpartum psychiatric illness and often represents an episode of bipolar disorder. Due to an elevated acute risk of suicide and infanticide, postpartum psychosis is a psychiatric emergency and warrants inpatient hospitalization for immediate intervention.
Related Resources
- Sharma V. Does your patient have postpartum OCD? Current Psychiatry. 2019;18(5):9-10.
- Hatters Friedman S, Prakash C, Nagel-Yang S. Postpartum psychosis: protecting mother and infant. Current Psychiatry. 2019;18(4):12-21.
Drug Brand Names
Fluoxetine • Prozac
Haloperidol • Haldol
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Olanzapine • Zyprexa
Prazosin • Minipress
Quetiapine • Seroquel
Sertraline • Zoloft
Valproic acid • Depakene
1. Raza SK, Raza S. Postpartum Psychosis. StatPearls Publishing; 2023. Updated June 26, 2023. https://www.ncbi.nlm.nih.gov/books/NBK544304/
2. MGH Center for Women’s Mental Health. What Is Postpartum Psychosis: This Is What You Need to Know. MGH Center for Women’s Mental Health. Published November 15, 2019. Accessed June 22, 2023. https://womensmentalhealth.org/posts/postpartum-psychosis-ten-things-need-know-2/
3. MGH Center for Women’s Mental Health. Postpartum Psychiatric Disorders. MGH Center for Women’s Mental Health. Accessed October 7, 2023. https://womensmentalhealth.org/specialty-clinics-2/postpartum-psychiatric-disorders-2/
4. Sharma V, Sommerdyk C. Obsessive-compulsive disorder in the postpartum period: diagnosis, differential diagnosis and management. Womens Health (Lond). 2015;11(4):543-552. doi:10.2217/whe.15.20
5. Osborne LM. Recognizing and managing postpartum psychosis: a clinical guide for obstetric providers. Obstet Gynecol Clin North Am. 2018;45(3):455-468. doi:10.1016/j.ogc.2018.04.005
6. Hutner LA, Catapano LA, Nagle-Yang SM, et al, eds. Textbook of Women’s Reproductive Mental Health. American Psychiatric Association; 2022.
7. Gilden J, Kamperman AM, Munk-Olsen T, et al. Long-term outcomes of postpartum psychosis: a systematic review and meta-analysis. J Clin Psychiatry. 2020;81(2):19r12906. doi:10.4088/JCP.19r12906
8. Bergink V, Boyce P, Munk-Olsen T. Postpartum psychosis: a valuable misnomer. Aust N Z J Psychiatry. 2015;49(2):102-103. doi:10.1177/0004867414564698
CASE Thoughts of harming baby
Ms. A, age 37, is G4P2, 4 months postpartum, and breastfeeding. She has major depressive disorder (MDD) with peripartum onset, posttraumatic stress disorder, and mild intellectual disability. For years she has been stable on fluoxetine 40 mg/d and prazosin 2 mg/d. Despite recent titration of her medications, at her most recent outpatient appointment Ms. A reports having a depressed mood with frequent crying, insomnia, a lack of desire to bond with her baby, and feelings of shame. She also says she has had auditory hallucinations and thoughts of harming her baby. Ms. A’s outpatient physician makes an urgent request for her to be evaluated at the psychiatric emergency department (ED).
HISTORY Depression and possible auditory hallucinations
Ms. A developed MDD following the birth of her first child, for which her care team initiated fluoxetine at 20 mg/d and titrated it to 40 mg/d,which was effective. At that time, her outpatient physician documented potential psychotic features, including vague descriptions of derogatory auditory hallucinations. However, it was unclear if these auditory hallucinations were more representative of a distressing inner monologue without the quality of an external voice. The team determined that Ms. A was not at acute risk for harm to herself or her baby and was appropriate for outpatient care. Because the nature of these possible auditory hallucinations was mild, nondistressing, and nonthreatening, the treatment team did not initiate an antipsychotic and Ms. A was not hospitalized. She has no history of hypomanic/manic episodes and has never met criteria for a psychotic disorder.
EVALUATION Distressing thoughts and discontinued medications
During the evaluation by psychiatric emergency services, Ms. A reports that 2 weeks after giving birth she experienced a worsening of her depressive symptoms. She says she began hearing voices telling her to harm herself and her baby and describes frequent distressing thoughts, such as stabbing her baby with a knife and running over her baby with a car. Ms. A says she repeatedly wakes up at night to check on her baby’s breathing, overfeeds her baby due to a fear of inadequate nutrition, and notes intermittent feelings of confusion. Afraid of being alone with her infant, Ms. A asks her partner and mother to move in with her. Additionally, she says 2 weeks ago she discontinued all her medications at the suggestion of her partner, who recommended herbal supplements. Ms. A’s initial routine laboratory results are unremarkable and her urine drug screen is negative for all substances.
[polldaddy:13041928]
The authors’ observations
Approximately 85% of birthing parents experience some form of postpartum mood disturbance; 10% to 15% develop more significant symptoms of anxiety or depression.3 The etiology of postpartum illness is multifactorial, and includes psychiatric personal/family history, insomnia, acute and chronic psychosocial stressors, and rapid hormone fluctuations.1 As a result, the postpartum period represents a vulnerable time for birthing parents, particularly those with previously established psychiatric illness.
Ms. A’s initial presentation was concerning for a possible diagnosis of postpartum psychosis vs obsessive-compulsive disorder (OCD) with postpartum onset; other differential diagnoses included MDD with peripartum onset and psychotic features (Table1-6). Ms. A’s subjective clinical history was significant for critical pertinent findings of both OCD with postpartum onset (ie, egodystonic intrusive thoughts, checking behaviors, feelings of shame, and seeking reassurance) and postpartum psychosis (ie, command auditory hallucinations and waxing/waning confusion), which added to diagnostic complexity.

Although postpartum psychosis is rare (1 to 2 cases per 1,000 women),5 it is considered a psychiatric emergency because it has significant potential for infanticide, morbidity, and mortality. Most symptoms develop within the first 2 weeks of the postpartum period.2 There are many risk factors for the development of postpartum psychosis; however, in first-time pregnancies, a previous diagnosis of BD I is the single most important risk factor.1 Approximately 20% to 30% of women with BD experience postpartum psychosis.4
For many patients (approximately 56.7%, according to 1 meta-analysis7), postpartum psychosis denotes an episode of BD, representing a more severe form of illness with increased risk of recurrence. Most manic or mixed mood episodes reoccur within the first year removed from the perinatal period. In contrast, for some patients (approximately 43.5% according to the same meta-analysis), the episode denotes “isolated postpartum psychosis.”7 Isolated postpartum psychosis is a psychotic episode that occurs only in the postpartum period with no recurrence of psychosis or recurrence of psychosis exclusive to postpartum periods. If treated, this type of postpartum psychosis has a more favorable prognosis than postpartum psychosis in a patient with BD.7 As such, a BD diagnosis should not be established at the onset of a patient’s first postpartum psychosis presentation. Regardless of type, all presentations of postpartum psychosis are considered a psychiatry emergency.
Continue to: The prevalence of OCD...
The prevalence of OCD with postpartum onset varies. One study estimated it occurs in 2.43% of cases.4 However, the true prevalence is likely underreported due to feelings of guilt or shame associated with intrusive thoughts, and fear of stigmatization and separation from the baby. Approximately 70.6% of women experiencing OCD with postpartum onset have a comorbid depressive disorder.4
Ms. A’s presentation to the psychiatric ED carried with it diagnostic complexity and uncertainty. Her initial presentation was concerning for elements of both postpartum psychosis and OCD with postpartum onset. After her evaluation in the psychiatric ED, there remained a lack of clear and convincing evidence for a diagnosis of OCD with postpartum onset, which eliminated the possibility of discharging Ms. A with robust safety planning and reinitiation of a selective serotonin reuptake inhibitor.
Additionally, because auditory hallucinations are atypical in OCD, the treatment team remained concerned for a diagnosis of postpartum psychosis, which would warrant hospitalization. With assistance from the institution’s reproductive psychiatrists, the treatment team discussed the importance of inpatient hospitalization for risk mitigation, close observation, and thorough evaluation for greater diagnostic clarity and certainty.
TREATMENT Involuntary hospitalization
The treatment team counsels Ms. A and her partner on her differential diagnoses, including the elevated acute risk of harm to herself and her baby if she has postpartum psychosis, as well as the need for continued observation and evaluation. When alone with a clinician, Ms. A says she understands and agrees to voluntary hospitalization. However, following a subsequent risk-benefit discussion with her partner, they both grew increasingly concerned about her separation from the baby and reinitiating her medications. Amid these concerns, the treatment team notices that Ms. A attempts to minimize her symptoms. Ms. A changes her mind and no longer consents to hospitalization. She is placed on a psychiatric hold for involuntary hospitalization on the psychiatric inpatient unit.
On the inpatient unit, the inpatient clinicians and a reproductive psychiatrist continue to evaluate Ms. A. Though her diagnosis remains unclear, Ms. A agrees to start a trial of quetiapine 100 mg/d titrated to 150 mg/d to manage her potential postpartum psychosis, depressed mood, insomnia (off-label), anxiety (off-label), and OCD (off-label). Lithium is deferred because Ms. A is breastfeeding.
[polldaddy:13041932]
Continue to: The authors' observations
The authors’ observations
Due to an elevated acute risk of suicide and infanticide, postpartum psychosis represents a psychiatric emergency and often requires hospitalization. The Figure outlines steps in evaluating a patient with concerns for postpartum psychosis in a psychiatric emergency service setting. Due to the waxing and waning nature of symptoms, patients may appear psychiatrically stable at any time but remain at an overall elevated acute risk of harm to self and/or their baby.

If a patient is being considered for discharge based on yes answers to all questions in Step 2 of the Figure, the emergency psychiatric clinician must initiate appropriate psychotropic medications and complete robust safety planning with the patient and a trusted adult who will provide direct supervision. Safety planning may include (but is not limited to) strict return precautions, education on concerning symptoms and behaviors, psychotropic education and agreement of compliance, and detailed instructions on outpatient follow-up within 1 week. Ideally—and as was the case for Ms. A—a reproductive psychiatrist should be consulted in the emergency setting for shared decision-making on admission vs discharge, medication management, and outpatient follow-up considerations.
Because postpartum psychosis carries significant risks and hospitalization generally results in separating the patient from their baby, initiating psychotropics should not be delayed. Clinicians must consider the patient’s psychiatric history, allergies, and breastfeeding status.
Based on current evidence, first-line treatment for postpartum psychosis includes a mood stabilizer, an antipsychotic, and possibly a benzodiazepine.6 Thus, an appropriate initial treatment regimen would be a benzodiazepine (particularly lorazepam due to its relatively shorter half-life) and an antipsychotic (eg, haloperidol, olanzapine, or quetiapine) for acute psychosis, plus lithium for mood stabilization.1,5
If the postpartum psychosis represents an episode of BD, use of a long-term mood stabilizer may be required. In contrast, for isolated postpartum psychosis, clinicians may consider initiating psychotropics only in the immediate postpartum period, with an eventual slow taper. In future pregnancies, psychotropics may be reintroduced postpartum, which will avoid peripartum fetal exposure.8 If the patient is breastfeeding, lithium may be deferred in an acute care setting. For patients with evidence of catatonia, severe suicidality, refusal of oral intake with compromised nutrition, severe agitation, or treatment resistance, electroconvulsive therapy remains a safe and effective treatment option.6 Additionally, the safety of continued breastfeeding in acute psychosis must be considered, with the potential for recommending discontinuation, which would decrease sleep disruptions at night and increase the ability of others to feed the baby. Comprehensive care requires nonpharmacologic interventions, including psychoeducation for the patient and their family, individual psychotherapy, and expansion of psychosocial supports.
Continue to: Patients who have experienced...
Patients who have experienced an episode of postpartum psychosis are predisposed to another episode in future pregnancies.1 Current research recommends prophylaxis of recurrence with lithium monotherapy.1,2,5,6 Similar to other psychotropics in reproductive psychiatry, maintenance therapy on lithium requires a thorough “risk vs risk” discussion with the patient. The risk of lithium use while pregnant and/or breastfeeding must be weighed against the risks associated with postpartum psychosis (ie, infanticide, suicide, poor peripartum care, or poor infant bonding).
OUTCOME Improved mood
After 7 days of inpatient treatment with quetiapine, Ms. A demonstrates improvement in the targeted depressive symptoms (including improved motivation/energy and insomnia, decreased feelings of guilt, and denial of ongoing suicidal ideation). Additionally, the thoughts of harming her baby are less frequent, and command auditory hallucinations resolve. Upon discharge, Ms. A and her partner meet with inpatient clinicians for continued counseling, safety planning, and plans for outpatient follow-up with the institution’s reproductive psychiatrist.
The authors’ observations
Many aspects of Ms. A’s initial presentation in the psychiatric ED were challenging. Given the presence of symptoms of both psychosis and OCD, a diagnosis was difficult to ascertain in the emergency setting. Since command auditory hallucinations are atypical in patients with postpartum OCD, the treatment team maintained high suspicion for postpartum psychosis, which represented an emergency requiring inpatient care.
Hospitalization separated Ms. A from her baby, for whom she was the primary caregiver. Additional considerations for inpatient admission and psychotropic initiation were necessary, because Ms. A was breastfeeding. Although Ms. A’s partner was able to provide full-time childcare, the patient ultimately did not agree to hospitalization and required an emergency hold for involuntary admission, which was an additional barrier to care. Furthermore, her partner held unfavorable beliefs regarding psychotropic medications and Ms. A’s need for hospital admission, which required ongoing patient and partner education in the emergency, inpatient, and outpatient settings. Moreover, if Ms. A’s symptoms were ultimately attributable to postpartum OCD, the patient’s involuntary hospitalization might have increased the risk of stigmatization of mental illness and treatment with psychotropics.
Bottom Line
The peripartum period is a vulnerable time for patients, particularly those with previously diagnosed psychiatric illnesses. Postpartum psychosis is the most severe form of postpartum psychiatric illness and often represents an episode of bipolar disorder. Due to an elevated acute risk of suicide and infanticide, postpartum psychosis is a psychiatric emergency and warrants inpatient hospitalization for immediate intervention.
Related Resources
- Sharma V. Does your patient have postpartum OCD? Current Psychiatry. 2019;18(5):9-10.
- Hatters Friedman S, Prakash C, Nagel-Yang S. Postpartum psychosis: protecting mother and infant. Current Psychiatry. 2019;18(4):12-21.
Drug Brand Names
Fluoxetine • Prozac
Haloperidol • Haldol
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Olanzapine • Zyprexa
Prazosin • Minipress
Quetiapine • Seroquel
Sertraline • Zoloft
Valproic acid • Depakene
CASE Thoughts of harming baby
Ms. A, age 37, is G4P2, 4 months postpartum, and breastfeeding. She has major depressive disorder (MDD) with peripartum onset, posttraumatic stress disorder, and mild intellectual disability. For years she has been stable on fluoxetine 40 mg/d and prazosin 2 mg/d. Despite recent titration of her medications, at her most recent outpatient appointment Ms. A reports having a depressed mood with frequent crying, insomnia, a lack of desire to bond with her baby, and feelings of shame. She also says she has had auditory hallucinations and thoughts of harming her baby. Ms. A’s outpatient physician makes an urgent request for her to be evaluated at the psychiatric emergency department (ED).
HISTORY Depression and possible auditory hallucinations
Ms. A developed MDD following the birth of her first child, for which her care team initiated fluoxetine at 20 mg/d and titrated it to 40 mg/d,which was effective. At that time, her outpatient physician documented potential psychotic features, including vague descriptions of derogatory auditory hallucinations. However, it was unclear if these auditory hallucinations were more representative of a distressing inner monologue without the quality of an external voice. The team determined that Ms. A was not at acute risk for harm to herself or her baby and was appropriate for outpatient care. Because the nature of these possible auditory hallucinations was mild, nondistressing, and nonthreatening, the treatment team did not initiate an antipsychotic and Ms. A was not hospitalized. She has no history of hypomanic/manic episodes and has never met criteria for a psychotic disorder.
EVALUATION Distressing thoughts and discontinued medications
During the evaluation by psychiatric emergency services, Ms. A reports that 2 weeks after giving birth she experienced a worsening of her depressive symptoms. She says she began hearing voices telling her to harm herself and her baby and describes frequent distressing thoughts, such as stabbing her baby with a knife and running over her baby with a car. Ms. A says she repeatedly wakes up at night to check on her baby’s breathing, overfeeds her baby due to a fear of inadequate nutrition, and notes intermittent feelings of confusion. Afraid of being alone with her infant, Ms. A asks her partner and mother to move in with her. Additionally, she says 2 weeks ago she discontinued all her medications at the suggestion of her partner, who recommended herbal supplements. Ms. A’s initial routine laboratory results are unremarkable and her urine drug screen is negative for all substances.
[polldaddy:13041928]
The authors’ observations
Approximately 85% of birthing parents experience some form of postpartum mood disturbance; 10% to 15% develop more significant symptoms of anxiety or depression.3 The etiology of postpartum illness is multifactorial, and includes psychiatric personal/family history, insomnia, acute and chronic psychosocial stressors, and rapid hormone fluctuations.1 As a result, the postpartum period represents a vulnerable time for birthing parents, particularly those with previously established psychiatric illness.
Ms. A’s initial presentation was concerning for a possible diagnosis of postpartum psychosis vs obsessive-compulsive disorder (OCD) with postpartum onset; other differential diagnoses included MDD with peripartum onset and psychotic features (Table1-6). Ms. A’s subjective clinical history was significant for critical pertinent findings of both OCD with postpartum onset (ie, egodystonic intrusive thoughts, checking behaviors, feelings of shame, and seeking reassurance) and postpartum psychosis (ie, command auditory hallucinations and waxing/waning confusion), which added to diagnostic complexity.

Although postpartum psychosis is rare (1 to 2 cases per 1,000 women),5 it is considered a psychiatric emergency because it has significant potential for infanticide, morbidity, and mortality. Most symptoms develop within the first 2 weeks of the postpartum period.2 There are many risk factors for the development of postpartum psychosis; however, in first-time pregnancies, a previous diagnosis of BD I is the single most important risk factor.1 Approximately 20% to 30% of women with BD experience postpartum psychosis.4
For many patients (approximately 56.7%, according to 1 meta-analysis7), postpartum psychosis denotes an episode of BD, representing a more severe form of illness with increased risk of recurrence. Most manic or mixed mood episodes reoccur within the first year removed from the perinatal period. In contrast, for some patients (approximately 43.5% according to the same meta-analysis), the episode denotes “isolated postpartum psychosis.”7 Isolated postpartum psychosis is a psychotic episode that occurs only in the postpartum period with no recurrence of psychosis or recurrence of psychosis exclusive to postpartum periods. If treated, this type of postpartum psychosis has a more favorable prognosis than postpartum psychosis in a patient with BD.7 As such, a BD diagnosis should not be established at the onset of a patient’s first postpartum psychosis presentation. Regardless of type, all presentations of postpartum psychosis are considered a psychiatry emergency.
Continue to: The prevalence of OCD...
The prevalence of OCD with postpartum onset varies. One study estimated it occurs in 2.43% of cases.4 However, the true prevalence is likely underreported due to feelings of guilt or shame associated with intrusive thoughts, and fear of stigmatization and separation from the baby. Approximately 70.6% of women experiencing OCD with postpartum onset have a comorbid depressive disorder.4
Ms. A’s presentation to the psychiatric ED carried with it diagnostic complexity and uncertainty. Her initial presentation was concerning for elements of both postpartum psychosis and OCD with postpartum onset. After her evaluation in the psychiatric ED, there remained a lack of clear and convincing evidence for a diagnosis of OCD with postpartum onset, which eliminated the possibility of discharging Ms. A with robust safety planning and reinitiation of a selective serotonin reuptake inhibitor.
Additionally, because auditory hallucinations are atypical in OCD, the treatment team remained concerned for a diagnosis of postpartum psychosis, which would warrant hospitalization. With assistance from the institution’s reproductive psychiatrists, the treatment team discussed the importance of inpatient hospitalization for risk mitigation, close observation, and thorough evaluation for greater diagnostic clarity and certainty.
TREATMENT Involuntary hospitalization
The treatment team counsels Ms. A and her partner on her differential diagnoses, including the elevated acute risk of harm to herself and her baby if she has postpartum psychosis, as well as the need for continued observation and evaluation. When alone with a clinician, Ms. A says she understands and agrees to voluntary hospitalization. However, following a subsequent risk-benefit discussion with her partner, they both grew increasingly concerned about her separation from the baby and reinitiating her medications. Amid these concerns, the treatment team notices that Ms. A attempts to minimize her symptoms. Ms. A changes her mind and no longer consents to hospitalization. She is placed on a psychiatric hold for involuntary hospitalization on the psychiatric inpatient unit.
On the inpatient unit, the inpatient clinicians and a reproductive psychiatrist continue to evaluate Ms. A. Though her diagnosis remains unclear, Ms. A agrees to start a trial of quetiapine 100 mg/d titrated to 150 mg/d to manage her potential postpartum psychosis, depressed mood, insomnia (off-label), anxiety (off-label), and OCD (off-label). Lithium is deferred because Ms. A is breastfeeding.
[polldaddy:13041932]
Continue to: The authors' observations
The authors’ observations
Due to an elevated acute risk of suicide and infanticide, postpartum psychosis represents a psychiatric emergency and often requires hospitalization. The Figure outlines steps in evaluating a patient with concerns for postpartum psychosis in a psychiatric emergency service setting. Due to the waxing and waning nature of symptoms, patients may appear psychiatrically stable at any time but remain at an overall elevated acute risk of harm to self and/or their baby.

If a patient is being considered for discharge based on yes answers to all questions in Step 2 of the Figure, the emergency psychiatric clinician must initiate appropriate psychotropic medications and complete robust safety planning with the patient and a trusted adult who will provide direct supervision. Safety planning may include (but is not limited to) strict return precautions, education on concerning symptoms and behaviors, psychotropic education and agreement of compliance, and detailed instructions on outpatient follow-up within 1 week. Ideally—and as was the case for Ms. A—a reproductive psychiatrist should be consulted in the emergency setting for shared decision-making on admission vs discharge, medication management, and outpatient follow-up considerations.
Because postpartum psychosis carries significant risks and hospitalization generally results in separating the patient from their baby, initiating psychotropics should not be delayed. Clinicians must consider the patient’s psychiatric history, allergies, and breastfeeding status.
Based on current evidence, first-line treatment for postpartum psychosis includes a mood stabilizer, an antipsychotic, and possibly a benzodiazepine.6 Thus, an appropriate initial treatment regimen would be a benzodiazepine (particularly lorazepam due to its relatively shorter half-life) and an antipsychotic (eg, haloperidol, olanzapine, or quetiapine) for acute psychosis, plus lithium for mood stabilization.1,5
If the postpartum psychosis represents an episode of BD, use of a long-term mood stabilizer may be required. In contrast, for isolated postpartum psychosis, clinicians may consider initiating psychotropics only in the immediate postpartum period, with an eventual slow taper. In future pregnancies, psychotropics may be reintroduced postpartum, which will avoid peripartum fetal exposure.8 If the patient is breastfeeding, lithium may be deferred in an acute care setting. For patients with evidence of catatonia, severe suicidality, refusal of oral intake with compromised nutrition, severe agitation, or treatment resistance, electroconvulsive therapy remains a safe and effective treatment option.6 Additionally, the safety of continued breastfeeding in acute psychosis must be considered, with the potential for recommending discontinuation, which would decrease sleep disruptions at night and increase the ability of others to feed the baby. Comprehensive care requires nonpharmacologic interventions, including psychoeducation for the patient and their family, individual psychotherapy, and expansion of psychosocial supports.
Continue to: Patients who have experienced...
Patients who have experienced an episode of postpartum psychosis are predisposed to another episode in future pregnancies.1 Current research recommends prophylaxis of recurrence with lithium monotherapy.1,2,5,6 Similar to other psychotropics in reproductive psychiatry, maintenance therapy on lithium requires a thorough “risk vs risk” discussion with the patient. The risk of lithium use while pregnant and/or breastfeeding must be weighed against the risks associated with postpartum psychosis (ie, infanticide, suicide, poor peripartum care, or poor infant bonding).
OUTCOME Improved mood
After 7 days of inpatient treatment with quetiapine, Ms. A demonstrates improvement in the targeted depressive symptoms (including improved motivation/energy and insomnia, decreased feelings of guilt, and denial of ongoing suicidal ideation). Additionally, the thoughts of harming her baby are less frequent, and command auditory hallucinations resolve. Upon discharge, Ms. A and her partner meet with inpatient clinicians for continued counseling, safety planning, and plans for outpatient follow-up with the institution’s reproductive psychiatrist.
The authors’ observations
Many aspects of Ms. A’s initial presentation in the psychiatric ED were challenging. Given the presence of symptoms of both psychosis and OCD, a diagnosis was difficult to ascertain in the emergency setting. Since command auditory hallucinations are atypical in patients with postpartum OCD, the treatment team maintained high suspicion for postpartum psychosis, which represented an emergency requiring inpatient care.
Hospitalization separated Ms. A from her baby, for whom she was the primary caregiver. Additional considerations for inpatient admission and psychotropic initiation were necessary, because Ms. A was breastfeeding. Although Ms. A’s partner was able to provide full-time childcare, the patient ultimately did not agree to hospitalization and required an emergency hold for involuntary admission, which was an additional barrier to care. Furthermore, her partner held unfavorable beliefs regarding psychotropic medications and Ms. A’s need for hospital admission, which required ongoing patient and partner education in the emergency, inpatient, and outpatient settings. Moreover, if Ms. A’s symptoms were ultimately attributable to postpartum OCD, the patient’s involuntary hospitalization might have increased the risk of stigmatization of mental illness and treatment with psychotropics.
Bottom Line
The peripartum period is a vulnerable time for patients, particularly those with previously diagnosed psychiatric illnesses. Postpartum psychosis is the most severe form of postpartum psychiatric illness and often represents an episode of bipolar disorder. Due to an elevated acute risk of suicide and infanticide, postpartum psychosis is a psychiatric emergency and warrants inpatient hospitalization for immediate intervention.
Related Resources
- Sharma V. Does your patient have postpartum OCD? Current Psychiatry. 2019;18(5):9-10.
- Hatters Friedman S, Prakash C, Nagel-Yang S. Postpartum psychosis: protecting mother and infant. Current Psychiatry. 2019;18(4):12-21.
Drug Brand Names
Fluoxetine • Prozac
Haloperidol • Haldol
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Olanzapine • Zyprexa
Prazosin • Minipress
Quetiapine • Seroquel
Sertraline • Zoloft
Valproic acid • Depakene
1. Raza SK, Raza S. Postpartum Psychosis. StatPearls Publishing; 2023. Updated June 26, 2023. https://www.ncbi.nlm.nih.gov/books/NBK544304/
2. MGH Center for Women’s Mental Health. What Is Postpartum Psychosis: This Is What You Need to Know. MGH Center for Women’s Mental Health. Published November 15, 2019. Accessed June 22, 2023. https://womensmentalhealth.org/posts/postpartum-psychosis-ten-things-need-know-2/
3. MGH Center for Women’s Mental Health. Postpartum Psychiatric Disorders. MGH Center for Women’s Mental Health. Accessed October 7, 2023. https://womensmentalhealth.org/specialty-clinics-2/postpartum-psychiatric-disorders-2/
4. Sharma V, Sommerdyk C. Obsessive-compulsive disorder in the postpartum period: diagnosis, differential diagnosis and management. Womens Health (Lond). 2015;11(4):543-552. doi:10.2217/whe.15.20
5. Osborne LM. Recognizing and managing postpartum psychosis: a clinical guide for obstetric providers. Obstet Gynecol Clin North Am. 2018;45(3):455-468. doi:10.1016/j.ogc.2018.04.005
6. Hutner LA, Catapano LA, Nagle-Yang SM, et al, eds. Textbook of Women’s Reproductive Mental Health. American Psychiatric Association; 2022.
7. Gilden J, Kamperman AM, Munk-Olsen T, et al. Long-term outcomes of postpartum psychosis: a systematic review and meta-analysis. J Clin Psychiatry. 2020;81(2):19r12906. doi:10.4088/JCP.19r12906
8. Bergink V, Boyce P, Munk-Olsen T. Postpartum psychosis: a valuable misnomer. Aust N Z J Psychiatry. 2015;49(2):102-103. doi:10.1177/0004867414564698
1. Raza SK, Raza S. Postpartum Psychosis. StatPearls Publishing; 2023. Updated June 26, 2023. https://www.ncbi.nlm.nih.gov/books/NBK544304/
2. MGH Center for Women’s Mental Health. What Is Postpartum Psychosis: This Is What You Need to Know. MGH Center for Women’s Mental Health. Published November 15, 2019. Accessed June 22, 2023. https://womensmentalhealth.org/posts/postpartum-psychosis-ten-things-need-know-2/
3. MGH Center for Women’s Mental Health. Postpartum Psychiatric Disorders. MGH Center for Women’s Mental Health. Accessed October 7, 2023. https://womensmentalhealth.org/specialty-clinics-2/postpartum-psychiatric-disorders-2/
4. Sharma V, Sommerdyk C. Obsessive-compulsive disorder in the postpartum period: diagnosis, differential diagnosis and management. Womens Health (Lond). 2015;11(4):543-552. doi:10.2217/whe.15.20
5. Osborne LM. Recognizing and managing postpartum psychosis: a clinical guide for obstetric providers. Obstet Gynecol Clin North Am. 2018;45(3):455-468. doi:10.1016/j.ogc.2018.04.005
6. Hutner LA, Catapano LA, Nagle-Yang SM, et al, eds. Textbook of Women’s Reproductive Mental Health. American Psychiatric Association; 2022.
7. Gilden J, Kamperman AM, Munk-Olsen T, et al. Long-term outcomes of postpartum psychosis: a systematic review and meta-analysis. J Clin Psychiatry. 2020;81(2):19r12906. doi:10.4088/JCP.19r12906
8. Bergink V, Boyce P, Munk-Olsen T. Postpartum psychosis: a valuable misnomer. Aust N Z J Psychiatry. 2015;49(2):102-103. doi:10.1177/0004867414564698
Adult ADHD: Tips for an accurate diagnosis
With the diagnosis of attention-deficit/hyperactivity disorder (ADHD) on the rise1 and a surge in prescriptions to treat the disorder leading to stimulant shortages,2 ensuring that patients are appropriately evaluated for ADHD is more critical than ever. ADHD is a clinical diagnosis that can be established by clinical interview, although the results of neuropsychological testing and collateral information from family members are helpful. Assessing adults for ADHD can be challenging when they appear to want to convince the clinician that they have the disorder. In this article, I provide tips to help you accurately diagnose ADHD in adult patients.
Use an ADHD symptom scale
An ADHD symptom checklist, such as the Adult ADHD Self-Report Scale, is an effective tool to establish the presence of ADHD symptoms. A patient can complete this self-assessment tool before their visit, and you can use the results as a springboard to ask them about ADHD symptoms. It is important to elicit specific examples of the ADHD symptoms the patient reports, and to understand how these symptoms affect their functioning and quality of life.
Review the prescription drug monitoring program
Review your state’s prescription drug monitoring program to explore the patient’s prior and current prescriptions of stimulants and other controlled substances. Discern if, when, and by whom a patient was previously treated for ADHD, and rule out the rare possibility that the patient has obtained multiple prescriptions for controlled substances from multiple clinicians, which suggests the patient may have a substance use disorder.
Begin the assessment at your initial contact with the patient
How patients present on an initial screening call or how they compose emails can reveal clues about their level of organization and overall executive functioning. The way patients complete intake forms (eg, using a concise vs a meandering writing style) as well as their punctuality when presenting to appointments can also be telling.
Conduct a mental status examination
Patients can have difficulty focusing and completing tasks for reasons other than having ADHD. A mental status examination can sometimes provide objective clues that an individual has ADHD. A digressive thought process, visible physical restlessness, and instances of a patient interrupting the evaluator are suggestive of ADHD, although all these symptoms can be present in other conditions (eg, mania). However, signs of ADHD in the mental status examination do not confirm an ADHD diagnosis, nor does their absence rule it out.
Maintain an appropriate diagnostic threshold
Per DSM-5, an ADHD diagnosis requires that the symptoms cause a significant impairment in functioning.3 It is up to the clinician to determine if this threshold is met. It is imperative to thoughtfully consider this because stimulants are first-line treatment for ADHD and are commonly misused. Psychiatrists are usually motivated to please their patients in order to maintain them as patients and develop a positive therapeutic relationship, which improves outcomes.4 However, it is important to demonstrate integrity, provide an accurate diagnosis, and not be unduly swayed by a patient’s wish to receive an ADHD diagnosis. If you sense that a prospective patient is hoping they will receive an ADHD diagnosis and be prescribed a stimulant, it may be prudent to emphasize that the patient will be assessed for multiple mental health conditions, including ADHD, and that treatment will depend on the outcome of the evaluation.
1. Chung W, Jiang SF, Paksarian D, et al. Trends in the prevalence and incidence of attention-deficit/hyperactivity disorder among adults and children of different racial and ethnic groups. JAMA Netw Open. 2019;2(11):e1914344. doi:10.1001/jamanetworkopen.2019.14344
2. Danielson ML, Bohm MK, Newsome K, et al. Trends in stimulant prescription fills among commercially insured children and adults - United States, 2016-2021. MMWR Morb Mortal Wkly Rep. 2023;72(13):327-332. doi:10.15585/mmwr.mm7213a1
3. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013:59-63.
4. Totura CMW, Fields SA, Karver MS. The role of the therapeutic relationship in psychopharmacological treatment outcomes: a meta-analytic review. Pyschiatr Serv. 2018;69(1):41-47. doi:10.1176/appi.ps.201700114
With the diagnosis of attention-deficit/hyperactivity disorder (ADHD) on the rise1 and a surge in prescriptions to treat the disorder leading to stimulant shortages,2 ensuring that patients are appropriately evaluated for ADHD is more critical than ever. ADHD is a clinical diagnosis that can be established by clinical interview, although the results of neuropsychological testing and collateral information from family members are helpful. Assessing adults for ADHD can be challenging when they appear to want to convince the clinician that they have the disorder. In this article, I provide tips to help you accurately diagnose ADHD in adult patients.
Use an ADHD symptom scale
An ADHD symptom checklist, such as the Adult ADHD Self-Report Scale, is an effective tool to establish the presence of ADHD symptoms. A patient can complete this self-assessment tool before their visit, and you can use the results as a springboard to ask them about ADHD symptoms. It is important to elicit specific examples of the ADHD symptoms the patient reports, and to understand how these symptoms affect their functioning and quality of life.
Review the prescription drug monitoring program
Review your state’s prescription drug monitoring program to explore the patient’s prior and current prescriptions of stimulants and other controlled substances. Discern if, when, and by whom a patient was previously treated for ADHD, and rule out the rare possibility that the patient has obtained multiple prescriptions for controlled substances from multiple clinicians, which suggests the patient may have a substance use disorder.
Begin the assessment at your initial contact with the patient
How patients present on an initial screening call or how they compose emails can reveal clues about their level of organization and overall executive functioning. The way patients complete intake forms (eg, using a concise vs a meandering writing style) as well as their punctuality when presenting to appointments can also be telling.
Conduct a mental status examination
Patients can have difficulty focusing and completing tasks for reasons other than having ADHD. A mental status examination can sometimes provide objective clues that an individual has ADHD. A digressive thought process, visible physical restlessness, and instances of a patient interrupting the evaluator are suggestive of ADHD, although all these symptoms can be present in other conditions (eg, mania). However, signs of ADHD in the mental status examination do not confirm an ADHD diagnosis, nor does their absence rule it out.
Maintain an appropriate diagnostic threshold
Per DSM-5, an ADHD diagnosis requires that the symptoms cause a significant impairment in functioning.3 It is up to the clinician to determine if this threshold is met. It is imperative to thoughtfully consider this because stimulants are first-line treatment for ADHD and are commonly misused. Psychiatrists are usually motivated to please their patients in order to maintain them as patients and develop a positive therapeutic relationship, which improves outcomes.4 However, it is important to demonstrate integrity, provide an accurate diagnosis, and not be unduly swayed by a patient’s wish to receive an ADHD diagnosis. If you sense that a prospective patient is hoping they will receive an ADHD diagnosis and be prescribed a stimulant, it may be prudent to emphasize that the patient will be assessed for multiple mental health conditions, including ADHD, and that treatment will depend on the outcome of the evaluation.
With the diagnosis of attention-deficit/hyperactivity disorder (ADHD) on the rise1 and a surge in prescriptions to treat the disorder leading to stimulant shortages,2 ensuring that patients are appropriately evaluated for ADHD is more critical than ever. ADHD is a clinical diagnosis that can be established by clinical interview, although the results of neuropsychological testing and collateral information from family members are helpful. Assessing adults for ADHD can be challenging when they appear to want to convince the clinician that they have the disorder. In this article, I provide tips to help you accurately diagnose ADHD in adult patients.
Use an ADHD symptom scale
An ADHD symptom checklist, such as the Adult ADHD Self-Report Scale, is an effective tool to establish the presence of ADHD symptoms. A patient can complete this self-assessment tool before their visit, and you can use the results as a springboard to ask them about ADHD symptoms. It is important to elicit specific examples of the ADHD symptoms the patient reports, and to understand how these symptoms affect their functioning and quality of life.
Review the prescription drug monitoring program
Review your state’s prescription drug monitoring program to explore the patient’s prior and current prescriptions of stimulants and other controlled substances. Discern if, when, and by whom a patient was previously treated for ADHD, and rule out the rare possibility that the patient has obtained multiple prescriptions for controlled substances from multiple clinicians, which suggests the patient may have a substance use disorder.
Begin the assessment at your initial contact with the patient
How patients present on an initial screening call or how they compose emails can reveal clues about their level of organization and overall executive functioning. The way patients complete intake forms (eg, using a concise vs a meandering writing style) as well as their punctuality when presenting to appointments can also be telling.
Conduct a mental status examination
Patients can have difficulty focusing and completing tasks for reasons other than having ADHD. A mental status examination can sometimes provide objective clues that an individual has ADHD. A digressive thought process, visible physical restlessness, and instances of a patient interrupting the evaluator are suggestive of ADHD, although all these symptoms can be present in other conditions (eg, mania). However, signs of ADHD in the mental status examination do not confirm an ADHD diagnosis, nor does their absence rule it out.
Maintain an appropriate diagnostic threshold
Per DSM-5, an ADHD diagnosis requires that the symptoms cause a significant impairment in functioning.3 It is up to the clinician to determine if this threshold is met. It is imperative to thoughtfully consider this because stimulants are first-line treatment for ADHD and are commonly misused. Psychiatrists are usually motivated to please their patients in order to maintain them as patients and develop a positive therapeutic relationship, which improves outcomes.4 However, it is important to demonstrate integrity, provide an accurate diagnosis, and not be unduly swayed by a patient’s wish to receive an ADHD diagnosis. If you sense that a prospective patient is hoping they will receive an ADHD diagnosis and be prescribed a stimulant, it may be prudent to emphasize that the patient will be assessed for multiple mental health conditions, including ADHD, and that treatment will depend on the outcome of the evaluation.
1. Chung W, Jiang SF, Paksarian D, et al. Trends in the prevalence and incidence of attention-deficit/hyperactivity disorder among adults and children of different racial and ethnic groups. JAMA Netw Open. 2019;2(11):e1914344. doi:10.1001/jamanetworkopen.2019.14344
2. Danielson ML, Bohm MK, Newsome K, et al. Trends in stimulant prescription fills among commercially insured children and adults - United States, 2016-2021. MMWR Morb Mortal Wkly Rep. 2023;72(13):327-332. doi:10.15585/mmwr.mm7213a1
3. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013:59-63.
4. Totura CMW, Fields SA, Karver MS. The role of the therapeutic relationship in psychopharmacological treatment outcomes: a meta-analytic review. Pyschiatr Serv. 2018;69(1):41-47. doi:10.1176/appi.ps.201700114
1. Chung W, Jiang SF, Paksarian D, et al. Trends in the prevalence and incidence of attention-deficit/hyperactivity disorder among adults and children of different racial and ethnic groups. JAMA Netw Open. 2019;2(11):e1914344. doi:10.1001/jamanetworkopen.2019.14344
2. Danielson ML, Bohm MK, Newsome K, et al. Trends in stimulant prescription fills among commercially insured children and adults - United States, 2016-2021. MMWR Morb Mortal Wkly Rep. 2023;72(13):327-332. doi:10.15585/mmwr.mm7213a1
3. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013:59-63.
4. Totura CMW, Fields SA, Karver MS. The role of the therapeutic relationship in psychopharmacological treatment outcomes: a meta-analytic review. Pyschiatr Serv. 2018;69(1):41-47. doi:10.1176/appi.ps.201700114
Childbirth-related PTSD: How it differs and who’s at risk
Childbirth-related posttraumatic stress disorder (CB-PTSD) is a form of PTSD that can develop related to trauma surrounding the events of giving birth. It affects approximately 5% of women after any birth, which is similar to the rate of PTSD after experiencing a natural disaster.1 Up to 17% of women may have posttraumatic symptoms in the postpartum period.1 Despite the high prevalence of CB-PTSD, many psychiatric clinicians have not incorporated screening for and management of CB-PTSD into their practice.
This is partly because childbirth has been conceptualized as a “stressful but positive life event.”2 Historically, childbirth was not recognized as a traumatic event; for example, in DSM-III-R, the criteria for trauma in PTSD required an event outside the range of usual human experience, and childbirth was implicitly excluded as being too common to be traumatic. In the past decade, this clinical phenomenon has been more formally recognized and studied.2

CB-PTSD presents with symptoms similar to those of other forms of PTSD, with some nuances, as outlined in Table 1.3 Avoidance can be the predominant symptom; this can affect mothers’ engagement in postnatal care and is a major risk factor for postpartum depression.3
Many risk factors in the peripartum period can impact the development of CB-PTSD (Table 23). The most significant risk factor is whether the patient views the delivery of their baby as a subjectively negative experience, regardless of the presence or lack of peripartum complications.1 However, parents of infants who require treatment in a neonatal intensive care unit and women who require emergency medical treatment following delivery are at higher risk.
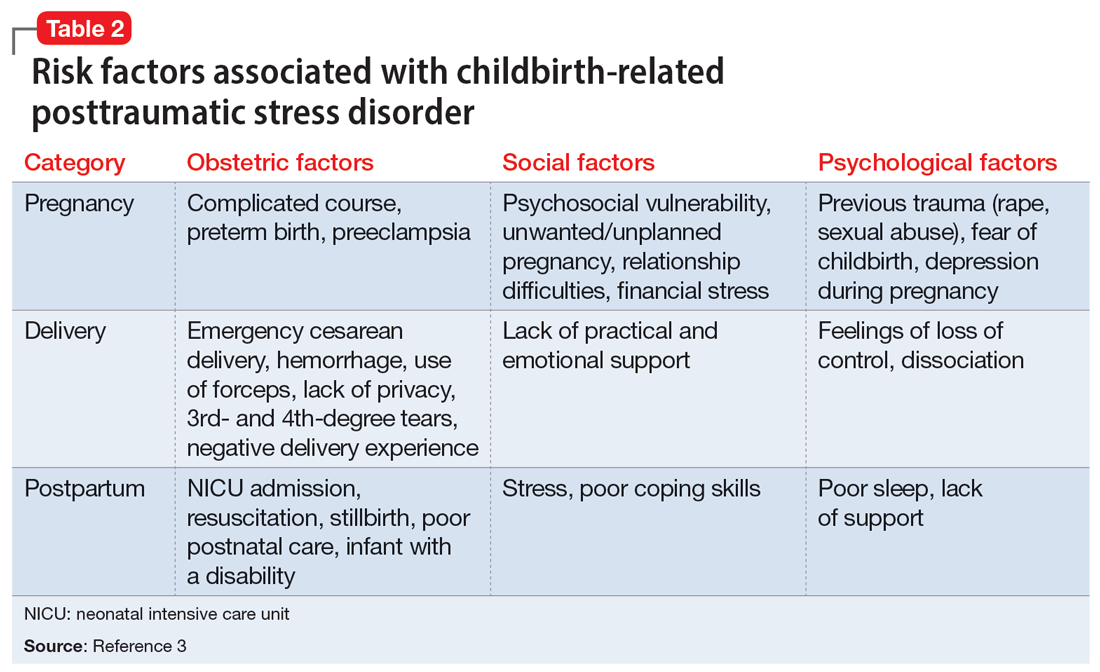
Screening and treatment
Ideally, every woman should be screened for CB-PTSD by their psychiatrist or obstetrician during a postpartum visit at least 1 month after delivery. In particular, high-risk populations and women with subjectively negative birth experiences should be screened, as well as women with postpartum depression that may have been precipitated or perpetuated by a traumatic experience. The City Birth Trauma Scale is a free 31-item self-report scale that can be used for such screening. It addresses both general and birth-related symptoms and is validated in multiple languages.4
Selective serotonin reuptake inhibitors and prazosin may be helpful for symptomatic treatment of CB-PTSD. Ongoing research studying the efficacy of cognitive-behavioral therapy and eye movement desensitization and reprocessing for CB-PTSD has yielded promising results but is limited in its generalizability.
Many women who develop CB-PTSD choose to get pregnant again. Psychiatrists can apply the principles of trauma-informed care and collaborate with obstetric and pediatric physicians to reduce the risk of retraumatization. It is critical to identify at-risk women and educate and prepare them for their next delivery experience. By focusing on communication, informed consent, and emotional support, we can do our best to prevent the recurrence of CB-PTSD.
1. Dekel S, Stuebe C, Dishy G. Childbirth induced posttraumatic stress syndrome: a systematic review of prevalence and risk factors. Front Psych. 2017;8:560. doi:10.3389/fpsyg.2017.00560
2. Horesh D, Garthus-Niegel S, Horsch A. Childbirth-related PTSD: is it a unique post-traumatic disorder? J Reprod Infant Psych. 2021;39(3):221-224. doi:10.1080/02646838.2021.1930739
3. Kranenburg L, Lambregtse-van den Berg M, Stramrood C. Traumatic childbirth experience and childbirth-related post-traumatic stress disorder (PTSD): a contemporary overview. Int J Environ Res Public Health. 2023;20(4):2775. doi:10.3390/ijerph20042775
4. Ayers S, Wright DB, Thornton A. Development of a measure of postpartum PTSD: The City Birth Trauma Scale. Front Psychiatry. 2018;9:409. doi:10.3389/fpsyt.2018.00409
Childbirth-related posttraumatic stress disorder (CB-PTSD) is a form of PTSD that can develop related to trauma surrounding the events of giving birth. It affects approximately 5% of women after any birth, which is similar to the rate of PTSD after experiencing a natural disaster.1 Up to 17% of women may have posttraumatic symptoms in the postpartum period.1 Despite the high prevalence of CB-PTSD, many psychiatric clinicians have not incorporated screening for and management of CB-PTSD into their practice.
This is partly because childbirth has been conceptualized as a “stressful but positive life event.”2 Historically, childbirth was not recognized as a traumatic event; for example, in DSM-III-R, the criteria for trauma in PTSD required an event outside the range of usual human experience, and childbirth was implicitly excluded as being too common to be traumatic. In the past decade, this clinical phenomenon has been more formally recognized and studied.2

CB-PTSD presents with symptoms similar to those of other forms of PTSD, with some nuances, as outlined in Table 1.3 Avoidance can be the predominant symptom; this can affect mothers’ engagement in postnatal care and is a major risk factor for postpartum depression.3
Many risk factors in the peripartum period can impact the development of CB-PTSD (Table 23). The most significant risk factor is whether the patient views the delivery of their baby as a subjectively negative experience, regardless of the presence or lack of peripartum complications.1 However, parents of infants who require treatment in a neonatal intensive care unit and women who require emergency medical treatment following delivery are at higher risk.

Screening and treatment
Ideally, every woman should be screened for CB-PTSD by their psychiatrist or obstetrician during a postpartum visit at least 1 month after delivery. In particular, high-risk populations and women with subjectively negative birth experiences should be screened, as well as women with postpartum depression that may have been precipitated or perpetuated by a traumatic experience. The City Birth Trauma Scale is a free 31-item self-report scale that can be used for such screening. It addresses both general and birth-related symptoms and is validated in multiple languages.4
Selective serotonin reuptake inhibitors and prazosin may be helpful for symptomatic treatment of CB-PTSD. Ongoing research studying the efficacy of cognitive-behavioral therapy and eye movement desensitization and reprocessing for CB-PTSD has yielded promising results but is limited in its generalizability.
Many women who develop CB-PTSD choose to get pregnant again. Psychiatrists can apply the principles of trauma-informed care and collaborate with obstetric and pediatric physicians to reduce the risk of retraumatization. It is critical to identify at-risk women and educate and prepare them for their next delivery experience. By focusing on communication, informed consent, and emotional support, we can do our best to prevent the recurrence of CB-PTSD.
Childbirth-related posttraumatic stress disorder (CB-PTSD) is a form of PTSD that can develop related to trauma surrounding the events of giving birth. It affects approximately 5% of women after any birth, which is similar to the rate of PTSD after experiencing a natural disaster.1 Up to 17% of women may have posttraumatic symptoms in the postpartum period.1 Despite the high prevalence of CB-PTSD, many psychiatric clinicians have not incorporated screening for and management of CB-PTSD into their practice.
This is partly because childbirth has been conceptualized as a “stressful but positive life event.”2 Historically, childbirth was not recognized as a traumatic event; for example, in DSM-III-R, the criteria for trauma in PTSD required an event outside the range of usual human experience, and childbirth was implicitly excluded as being too common to be traumatic. In the past decade, this clinical phenomenon has been more formally recognized and studied.2

CB-PTSD presents with symptoms similar to those of other forms of PTSD, with some nuances, as outlined in Table 1.3 Avoidance can be the predominant symptom; this can affect mothers’ engagement in postnatal care and is a major risk factor for postpartum depression.3
Many risk factors in the peripartum period can impact the development of CB-PTSD (Table 23). The most significant risk factor is whether the patient views the delivery of their baby as a subjectively negative experience, regardless of the presence or lack of peripartum complications.1 However, parents of infants who require treatment in a neonatal intensive care unit and women who require emergency medical treatment following delivery are at higher risk.

Screening and treatment
Ideally, every woman should be screened for CB-PTSD by their psychiatrist or obstetrician during a postpartum visit at least 1 month after delivery. In particular, high-risk populations and women with subjectively negative birth experiences should be screened, as well as women with postpartum depression that may have been precipitated or perpetuated by a traumatic experience. The City Birth Trauma Scale is a free 31-item self-report scale that can be used for such screening. It addresses both general and birth-related symptoms and is validated in multiple languages.4
Selective serotonin reuptake inhibitors and prazosin may be helpful for symptomatic treatment of CB-PTSD. Ongoing research studying the efficacy of cognitive-behavioral therapy and eye movement desensitization and reprocessing for CB-PTSD has yielded promising results but is limited in its generalizability.
Many women who develop CB-PTSD choose to get pregnant again. Psychiatrists can apply the principles of trauma-informed care and collaborate with obstetric and pediatric physicians to reduce the risk of retraumatization. It is critical to identify at-risk women and educate and prepare them for their next delivery experience. By focusing on communication, informed consent, and emotional support, we can do our best to prevent the recurrence of CB-PTSD.
1. Dekel S, Stuebe C, Dishy G. Childbirth induced posttraumatic stress syndrome: a systematic review of prevalence and risk factors. Front Psych. 2017;8:560. doi:10.3389/fpsyg.2017.00560
2. Horesh D, Garthus-Niegel S, Horsch A. Childbirth-related PTSD: is it a unique post-traumatic disorder? J Reprod Infant Psych. 2021;39(3):221-224. doi:10.1080/02646838.2021.1930739
3. Kranenburg L, Lambregtse-van den Berg M, Stramrood C. Traumatic childbirth experience and childbirth-related post-traumatic stress disorder (PTSD): a contemporary overview. Int J Environ Res Public Health. 2023;20(4):2775. doi:10.3390/ijerph20042775
4. Ayers S, Wright DB, Thornton A. Development of a measure of postpartum PTSD: The City Birth Trauma Scale. Front Psychiatry. 2018;9:409. doi:10.3389/fpsyt.2018.00409
1. Dekel S, Stuebe C, Dishy G. Childbirth induced posttraumatic stress syndrome: a systematic review of prevalence and risk factors. Front Psych. 2017;8:560. doi:10.3389/fpsyg.2017.00560
2. Horesh D, Garthus-Niegel S, Horsch A. Childbirth-related PTSD: is it a unique post-traumatic disorder? J Reprod Infant Psych. 2021;39(3):221-224. doi:10.1080/02646838.2021.1930739
3. Kranenburg L, Lambregtse-van den Berg M, Stramrood C. Traumatic childbirth experience and childbirth-related post-traumatic stress disorder (PTSD): a contemporary overview. Int J Environ Res Public Health. 2023;20(4):2775. doi:10.3390/ijerph20042775
4. Ayers S, Wright DB, Thornton A. Development of a measure of postpartum PTSD: The City Birth Trauma Scale. Front Psychiatry. 2018;9:409. doi:10.3389/fpsyt.2018.00409
Perinatal psychiatric screening: What to ask
Perinatal psychiatry focuses on the evaluation, diagnosis, and treatment of mental health disorders during the preconception, pregnancy, and postpartum periods. Mood disorders, anxiety disorders, and posttraumatic stress disorder are the most common mental health conditions that arise during the perinatal period.1 Mediating factors include hormone fluctuations, sleep deprivation, trauma exposure, financial stress, having a history of psychiatric illness, and factors related to newborn care.
During the perinatal period, a comprehensive psychiatric interview is crucial. Effective screening and identification of maternal mental health conditions necessitate more than merely checking off boxes on a questionnaire. It requires compassionate, informed, and individualized conversations between physicians and their patients.
In addition to asking about pertinent positive and negative psychiatric symptoms, the following screening questions could be asked during a structured interview to identify perinatal issues during pregnancy and the postpartum period.
During pregnancy
- How do you feel about your pregnancy?
- Was this pregnancy planned or unplanned, desired or not?
- Was fertility treatment needed or used?
- Did you think about stopping the pregnancy? If so, was your decision influenced by laws that restrict abortion in your state?
- Do you feel connected to the fetus?
- Do you have a room or crib at home for the baby? A car seat? Clothing? Baby supplies?
- Are you planning on breastfeeding?
- Do you have thoughts on future desired fertility and/or contraception?
- Who is your support system at home?
- How is your relationship with the baby’s father?
- How is the baby’s father’s mental well-being?
- Have you been subject to any abuse, intimate partner violence, or neglect?
- Are your other children being taken care of properly? What is the plan for them during delivery days at the hospital?
During the postpartum period
- Was your baby born prematurely?
- Did you have a vaginal or cesarean delivery?
- Did you encounter any delivery complications?
- Did you see the baby after the delivery?
- Do you feel connected to or able to bond with the baby?
- Do you have access to maternity leave from work?
- Have you had scary or upsetting thoughts about hurting your baby?
- Do you have any concerns about your treatment plan, such as medication use?
- In case of an emergency, are you aware of perinatal psychiatry resources in your area or the national maternal mental health hotline (833-852-6262)?
The American College of Obstetricians and Gynecologists clinical practice guidelines recommend that clinicians conduct depression and anxiety screening at least once during the perinatal period by using a standardized, validated tool.2 Psychiatry residents should receive adequate guidance and education about perinatal psychiatric evaluation, risk assessment, and treatment counseling. Early detection of mental health symptoms allows for early referral, close surveillance during episodes of vulnerability, and better access to mental health care during the perinatal period.
1. Howard LM, Khalifeh H. Perinatal mental health: a review of progress and challenges. World Psychiatry. 2020;19(3):313-327. doi:10.1002/wps.20769
2. American College of Obstetricians and Gynecologists. Screening and diagnosis of mental health conditions during pregnancy and postpartum. Clinical Practice Guideline Number 4. June 2023. Accessed November 3, 2023. https://www.acog.org/clinical/clinical-guidance/clinical-practice-guideline/articles/2023/06/screening-and-diagnosis-of-mental-health-conditions-during-pregnancy-and-postpartum
Perinatal psychiatry focuses on the evaluation, diagnosis, and treatment of mental health disorders during the preconception, pregnancy, and postpartum periods. Mood disorders, anxiety disorders, and posttraumatic stress disorder are the most common mental health conditions that arise during the perinatal period.1 Mediating factors include hormone fluctuations, sleep deprivation, trauma exposure, financial stress, having a history of psychiatric illness, and factors related to newborn care.
During the perinatal period, a comprehensive psychiatric interview is crucial. Effective screening and identification of maternal mental health conditions necessitate more than merely checking off boxes on a questionnaire. It requires compassionate, informed, and individualized conversations between physicians and their patients.
In addition to asking about pertinent positive and negative psychiatric symptoms, the following screening questions could be asked during a structured interview to identify perinatal issues during pregnancy and the postpartum period.
During pregnancy
- How do you feel about your pregnancy?
- Was this pregnancy planned or unplanned, desired or not?
- Was fertility treatment needed or used?
- Did you think about stopping the pregnancy? If so, was your decision influenced by laws that restrict abortion in your state?
- Do you feel connected to the fetus?
- Do you have a room or crib at home for the baby? A car seat? Clothing? Baby supplies?
- Are you planning on breastfeeding?
- Do you have thoughts on future desired fertility and/or contraception?
- Who is your support system at home?
- How is your relationship with the baby’s father?
- How is the baby’s father’s mental well-being?
- Have you been subject to any abuse, intimate partner violence, or neglect?
- Are your other children being taken care of properly? What is the plan for them during delivery days at the hospital?
During the postpartum period
- Was your baby born prematurely?
- Did you have a vaginal or cesarean delivery?
- Did you encounter any delivery complications?
- Did you see the baby after the delivery?
- Do you feel connected to or able to bond with the baby?
- Do you have access to maternity leave from work?
- Have you had scary or upsetting thoughts about hurting your baby?
- Do you have any concerns about your treatment plan, such as medication use?
- In case of an emergency, are you aware of perinatal psychiatry resources in your area or the national maternal mental health hotline (833-852-6262)?
The American College of Obstetricians and Gynecologists clinical practice guidelines recommend that clinicians conduct depression and anxiety screening at least once during the perinatal period by using a standardized, validated tool.2 Psychiatry residents should receive adequate guidance and education about perinatal psychiatric evaluation, risk assessment, and treatment counseling. Early detection of mental health symptoms allows for early referral, close surveillance during episodes of vulnerability, and better access to mental health care during the perinatal period.
Perinatal psychiatry focuses on the evaluation, diagnosis, and treatment of mental health disorders during the preconception, pregnancy, and postpartum periods. Mood disorders, anxiety disorders, and posttraumatic stress disorder are the most common mental health conditions that arise during the perinatal period.1 Mediating factors include hormone fluctuations, sleep deprivation, trauma exposure, financial stress, having a history of psychiatric illness, and factors related to newborn care.
During the perinatal period, a comprehensive psychiatric interview is crucial. Effective screening and identification of maternal mental health conditions necessitate more than merely checking off boxes on a questionnaire. It requires compassionate, informed, and individualized conversations between physicians and their patients.
In addition to asking about pertinent positive and negative psychiatric symptoms, the following screening questions could be asked during a structured interview to identify perinatal issues during pregnancy and the postpartum period.
During pregnancy
- How do you feel about your pregnancy?
- Was this pregnancy planned or unplanned, desired or not?
- Was fertility treatment needed or used?
- Did you think about stopping the pregnancy? If so, was your decision influenced by laws that restrict abortion in your state?
- Do you feel connected to the fetus?
- Do you have a room or crib at home for the baby? A car seat? Clothing? Baby supplies?
- Are you planning on breastfeeding?
- Do you have thoughts on future desired fertility and/or contraception?
- Who is your support system at home?
- How is your relationship with the baby’s father?
- How is the baby’s father’s mental well-being?
- Have you been subject to any abuse, intimate partner violence, or neglect?
- Are your other children being taken care of properly? What is the plan for them during delivery days at the hospital?
During the postpartum period
- Was your baby born prematurely?
- Did you have a vaginal or cesarean delivery?
- Did you encounter any delivery complications?
- Did you see the baby after the delivery?
- Do you feel connected to or able to bond with the baby?
- Do you have access to maternity leave from work?
- Have you had scary or upsetting thoughts about hurting your baby?
- Do you have any concerns about your treatment plan, such as medication use?
- In case of an emergency, are you aware of perinatal psychiatry resources in your area or the national maternal mental health hotline (833-852-6262)?
The American College of Obstetricians and Gynecologists clinical practice guidelines recommend that clinicians conduct depression and anxiety screening at least once during the perinatal period by using a standardized, validated tool.2 Psychiatry residents should receive adequate guidance and education about perinatal psychiatric evaluation, risk assessment, and treatment counseling. Early detection of mental health symptoms allows for early referral, close surveillance during episodes of vulnerability, and better access to mental health care during the perinatal period.
1. Howard LM, Khalifeh H. Perinatal mental health: a review of progress and challenges. World Psychiatry. 2020;19(3):313-327. doi:10.1002/wps.20769
2. American College of Obstetricians and Gynecologists. Screening and diagnosis of mental health conditions during pregnancy and postpartum. Clinical Practice Guideline Number 4. June 2023. Accessed November 3, 2023. https://www.acog.org/clinical/clinical-guidance/clinical-practice-guideline/articles/2023/06/screening-and-diagnosis-of-mental-health-conditions-during-pregnancy-and-postpartum
1. Howard LM, Khalifeh H. Perinatal mental health: a review of progress and challenges. World Psychiatry. 2020;19(3):313-327. doi:10.1002/wps.20769
2. American College of Obstetricians and Gynecologists. Screening and diagnosis of mental health conditions during pregnancy and postpartum. Clinical Practice Guideline Number 4. June 2023. Accessed November 3, 2023. https://www.acog.org/clinical/clinical-guidance/clinical-practice-guideline/articles/2023/06/screening-and-diagnosis-of-mental-health-conditions-during-pregnancy-and-postpartum
Brick and mortar: Changes in the therapeutic relationship in a postvirtual world
My colleagues and I entered the realm of outpatient psychiatry during residency at a logistically and dynamically interesting time. At the beginning of our third year in training (July 2022), almost all of the outpatients we were treating were still being seen virtually. For much of the year, they remained that way. However, with the reinstatement of the Ryan Haight Act in May 2023, I began to meet patients in person for the first time—the same patients whom I had known only virtually for the first 10 months of our therapeutic relationship. I observed vast changes in the dynamic of the room; many of these patients opened up more in their first in-person session than they had all year over Zoom.
Once in-person sessions resumed, patients who during virtual visits had assured me for almost a year that their home situation was optimized had a plethora of new things to share about their seemingly straightforward living situations. Relationships that appeared stable had more layers to reveal once the half of the relationship I was treating was now comfortably seated within the walls of my office. Problems that had previously seemed biologically based suddenly had complex sociocultural elements that were divulged for the first time. Some patients felt freer to be unrestricted in their affect, in contrast to the logistical (and metaphorical) buttoned-up virtual environment. Emotions ranged from cathartic (“It’s so great to see you in person!”) to bemused (“You’re taller/shorter, older/younger than I thought!”). The screen was gone, and the tangibility of it all breathed a different air into the room.
Virtual vs in-person: Crabs on a beach
The virtual treatment space could be envisioned as crabs in shells scattered on a beach, in which 2 crabs situated in their own shells, not necessarily adjacent to each other, could communicate. This certainly had benefits, such as the convenience of not having to move to another shell, as well as the brief but telling opportunity to gaze into their home shell environment. However, sometimes there would be disadvantages, such as interference with the connection due to static in the sand; at other times, there was the potential for other crabs to overhear and inadvertently learn of each other’s presence, thus affecting the openness of the communication. In this analogy, perhaps the equivalent of an in-person meeting would be 1 crab meandering over and the 2 crabs cohabiting a conch for the first time—it’s spacious (enough), all-enveloping, and within the harkened privacy of a shared sacred space.
A unique training experience
My co-residents and I are uniquely positioned to observe this novel phenomenon due to the timing of having entered our outpatient psychiatry training during the COVID-19 pandemic. Previous generations of residents—as well as practicing psychiatrists who had initially met their patients in person and were forced to switch to virtual sessions during the pandemic—had certain perspectives and challenges of their own, but they had a known dynamic of in-person interactions at baseline. Accordingly, residents who practiced peak- and mid-pandemic and graduated without being required to treat patients face-to-face (the classes of 2022 and 2023) might have spent entire therapeutic relationships having never met their patients in person. My class (2024) was situated in this time- and situation-bound frame in which we started virtually, and by requirements of the law, later met our patients in person. Being not only an observer but an active participant in a treatment dyad within the context of this phenomenon taught me astutely about transference, countertransference, and the holding environment. Training in psychodynamic psychotherapy has taught me about the act of listening deeply and qualities of therapeutic communication. Having the opportunity to enact these principles in such a dichotomy of treatment settings has been invaluable in my education, in getting to know different facets of my patients, and in understanding the nuances of the human experience.
My colleagues and I entered the realm of outpatient psychiatry during residency at a logistically and dynamically interesting time. At the beginning of our third year in training (July 2022), almost all of the outpatients we were treating were still being seen virtually. For much of the year, they remained that way. However, with the reinstatement of the Ryan Haight Act in May 2023, I began to meet patients in person for the first time—the same patients whom I had known only virtually for the first 10 months of our therapeutic relationship. I observed vast changes in the dynamic of the room; many of these patients opened up more in their first in-person session than they had all year over Zoom.
Once in-person sessions resumed, patients who during virtual visits had assured me for almost a year that their home situation was optimized had a plethora of new things to share about their seemingly straightforward living situations. Relationships that appeared stable had more layers to reveal once the half of the relationship I was treating was now comfortably seated within the walls of my office. Problems that had previously seemed biologically based suddenly had complex sociocultural elements that were divulged for the first time. Some patients felt freer to be unrestricted in their affect, in contrast to the logistical (and metaphorical) buttoned-up virtual environment. Emotions ranged from cathartic (“It’s so great to see you in person!”) to bemused (“You’re taller/shorter, older/younger than I thought!”). The screen was gone, and the tangibility of it all breathed a different air into the room.
Virtual vs in-person: Crabs on a beach
The virtual treatment space could be envisioned as crabs in shells scattered on a beach, in which 2 crabs situated in their own shells, not necessarily adjacent to each other, could communicate. This certainly had benefits, such as the convenience of not having to move to another shell, as well as the brief but telling opportunity to gaze into their home shell environment. However, sometimes there would be disadvantages, such as interference with the connection due to static in the sand; at other times, there was the potential for other crabs to overhear and inadvertently learn of each other’s presence, thus affecting the openness of the communication. In this analogy, perhaps the equivalent of an in-person meeting would be 1 crab meandering over and the 2 crabs cohabiting a conch for the first time—it’s spacious (enough), all-enveloping, and within the harkened privacy of a shared sacred space.
A unique training experience
My co-residents and I are uniquely positioned to observe this novel phenomenon due to the timing of having entered our outpatient psychiatry training during the COVID-19 pandemic. Previous generations of residents—as well as practicing psychiatrists who had initially met their patients in person and were forced to switch to virtual sessions during the pandemic—had certain perspectives and challenges of their own, but they had a known dynamic of in-person interactions at baseline. Accordingly, residents who practiced peak- and mid-pandemic and graduated without being required to treat patients face-to-face (the classes of 2022 and 2023) might have spent entire therapeutic relationships having never met their patients in person. My class (2024) was situated in this time- and situation-bound frame in which we started virtually, and by requirements of the law, later met our patients in person. Being not only an observer but an active participant in a treatment dyad within the context of this phenomenon taught me astutely about transference, countertransference, and the holding environment. Training in psychodynamic psychotherapy has taught me about the act of listening deeply and qualities of therapeutic communication. Having the opportunity to enact these principles in such a dichotomy of treatment settings has been invaluable in my education, in getting to know different facets of my patients, and in understanding the nuances of the human experience.
My colleagues and I entered the realm of outpatient psychiatry during residency at a logistically and dynamically interesting time. At the beginning of our third year in training (July 2022), almost all of the outpatients we were treating were still being seen virtually. For much of the year, they remained that way. However, with the reinstatement of the Ryan Haight Act in May 2023, I began to meet patients in person for the first time—the same patients whom I had known only virtually for the first 10 months of our therapeutic relationship. I observed vast changes in the dynamic of the room; many of these patients opened up more in their first in-person session than they had all year over Zoom.
Once in-person sessions resumed, patients who during virtual visits had assured me for almost a year that their home situation was optimized had a plethora of new things to share about their seemingly straightforward living situations. Relationships that appeared stable had more layers to reveal once the half of the relationship I was treating was now comfortably seated within the walls of my office. Problems that had previously seemed biologically based suddenly had complex sociocultural elements that were divulged for the first time. Some patients felt freer to be unrestricted in their affect, in contrast to the logistical (and metaphorical) buttoned-up virtual environment. Emotions ranged from cathartic (“It’s so great to see you in person!”) to bemused (“You’re taller/shorter, older/younger than I thought!”). The screen was gone, and the tangibility of it all breathed a different air into the room.
Virtual vs in-person: Crabs on a beach
The virtual treatment space could be envisioned as crabs in shells scattered on a beach, in which 2 crabs situated in their own shells, not necessarily adjacent to each other, could communicate. This certainly had benefits, such as the convenience of not having to move to another shell, as well as the brief but telling opportunity to gaze into their home shell environment. However, sometimes there would be disadvantages, such as interference with the connection due to static in the sand; at other times, there was the potential for other crabs to overhear and inadvertently learn of each other’s presence, thus affecting the openness of the communication. In this analogy, perhaps the equivalent of an in-person meeting would be 1 crab meandering over and the 2 crabs cohabiting a conch for the first time—it’s spacious (enough), all-enveloping, and within the harkened privacy of a shared sacred space.
A unique training experience
My co-residents and I are uniquely positioned to observe this novel phenomenon due to the timing of having entered our outpatient psychiatry training during the COVID-19 pandemic. Previous generations of residents—as well as practicing psychiatrists who had initially met their patients in person and were forced to switch to virtual sessions during the pandemic—had certain perspectives and challenges of their own, but they had a known dynamic of in-person interactions at baseline. Accordingly, residents who practiced peak- and mid-pandemic and graduated without being required to treat patients face-to-face (the classes of 2022 and 2023) might have spent entire therapeutic relationships having never met their patients in person. My class (2024) was situated in this time- and situation-bound frame in which we started virtually, and by requirements of the law, later met our patients in person. Being not only an observer but an active participant in a treatment dyad within the context of this phenomenon taught me astutely about transference, countertransference, and the holding environment. Training in psychodynamic psychotherapy has taught me about the act of listening deeply and qualities of therapeutic communication. Having the opportunity to enact these principles in such a dichotomy of treatment settings has been invaluable in my education, in getting to know different facets of my patients, and in understanding the nuances of the human experience.