User login
The American Journal of Orthopedics is an Index Medicus publication that is valued by orthopedic surgeons for its peer-reviewed, practice-oriented clinical information. Most articles are written by specialists at leading teaching institutions and help incorporate the latest technology into everyday practice.
Spontaneous, Chronic Expanding Posterior Thigh Hematoma Mimicking Soft-Tissue Sarcoma in a Morbidly Obese Pregnant Woman
Soft-tissue sarcomas are quite rare, with an annual incidence of 20 to 30 per 1,000,000 persons in the United States.1 Because of their heterogeneous presentation, they remain a diagnostic challenge and are often initially confused for more common, benign disorders.2 Chronic expanding hematoma, first described by Friedlander and colleagues3 in 1968, is a rare entity that is particularly difficult to distinguish from soft-tissue malignancy.3-5 Chronic expanding hematoma is defined as a hematoma that gradually expands over 1 month or longer, is absent of neoplastic change on histologic sections, and does not occur in the setting of coagulopathy.6
Typically associated with remote trauma, these lesions often present as a slowly growing mass on the anterior or lateral thigh, calf, or buttock.3-4,7-9 They have been reported to persist as long as 46 years, with sizes ranging from 3 to 55 cm in maximum diameter.7 On imaging, they have a cystic appearance with a dense fibrous capsule.7-8 Most cases resolve uneventfully after drainage or marginal excision, although some cases require repeated intervention.7 This case report describes a morbidly obese patient with a chronic expanding hematoma in the distal posterior thigh whose definitive treatment was delayed 6 months because of her pregnancy status and inability to lie prone for open biopsy. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 27-year-old morbidly obese woman, who was pregnant at 12 weeks gestation, was seen in an orthopedic oncology clinic with a 1-month history of a slowly growing, painful posterior thigh mass. She had no history of cancer or bleeding disorder, and denied a history of trauma or constitutional symptoms consistent with malignancy. Coagulation studies were normal. Magnetic resonance imaging (MRI) obtained 2 weeks prior in the emergency room showed a cystic lesion with mass-like components in the posterior compartment of the distal right thigh, measuring 17 cm longitudinally. The lesion was located adjacent to, but not involving, the sciatic nerve and femoral vasculature. On initial examination, the large soft-tissue mass was evident and moderately painful to palpation; no skin changes were noted, and the patient had a normal sensorimotor examination. Fine-needle aspiration was performed, which resulted in amorphous debris consistent with hematoma.
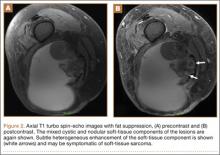
Repeat MRI 2 months later showed increased size of the lesion (9.5×10.5 cm axial, 22.0 cm craniocaudal). Although most findings of a more extensive imaging protocol, including precontrast and postcontrast sequences, were consistent with hematoma, the lesion also had several characteristics that indicated soft-tissue sarcoma. Specifically, findings suggestive of chronic hematoma included the hyperintense short tau inversion recovery (STIR) T1/T2 signal of the cystic component consistent with proteinaceous fluid and the low STIR TI/T2 signal of the periphery consistent with a rim of hemosiderin (Figure 1). Additionally, the cystic component of the lesion had multiple fine septations that are atypical for a hematoma (Figure 1), and several lymph nodes greater than 1.7 cm in short axis were noted in the anterior thigh and hemipelvis that were suspicious of metastatic lymphadenopathy. The encapsulated appearance of the lesion with a sharply defined margin and short transition zone were also reassuring findings for a benign lesion (Figures 1, 2A, 2B). However, several findings were identified that suggested soft-tissue sarcoma, including a nodular soft-tissue component on the medial wall of the lesion that had heterogeneous enhancement with contrast (Figure 2B). We, therefore, proceeded with ultrasound-guided core needle biopsy of the mass and cytologic sampling of the fluid components, which were again consistent with hematoma; no evidence of internal vascular flow was noted on Doppler ultrasound. Ultrasound-guided right inguinal lymph node biopsy was also performed and was negative for malignancy. Because of her large body habitus and pregnancy status, it was agreed that open biopsy should be delayed until after delivery to avoid placing the patient in a prone position.
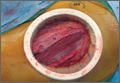
The patient visited the emergency room several times during the following months because of intermittent exacerbations of her lower extremity pain, swelling, and occasional paresthesias. About 6 months after initial presentation, repeat MRI again showed increased size of the mass (13.5×13.5 cm axial, 28 cm craniocaudal). There was also increased displacement of the adjacent neurovascular structures but no evidence of deep vein thrombosis. Because of concerns about the increased symptomatology of her thigh mass and possible sampling error of the previous biopsies, an elective cesarean section was performed at 35 weeks gestation. One week later, after clearance by her obstetrician, we proceeded with open biopsy of the mass in prone position. Initial sampling was negative for malignancy on frozen section; then, we expressed 1.75 L of brown fluid and solidified blood products, irrigated copiously, and placed a surgical drain. The permanent histologic specimens were again consistent with hematoma, and microbial cultures were negative. A week later, the patient accidentally removed her drain, and she presented with a fever (101°F) on postoperative day (POD) 15. Computed tomography showed reaccumulation of fluid; duplex ultrasound was negative. She was placed on cephalexin and underwent ultrasound-guided replacement of the drain with removal of an additional 750 mL fluid on POD 20. She drained an additional 150 to 200 mL/d for 1 month, with marked improvement in her leg swelling and knee range of motion. The drainage decreased during the next 3 weeks, and the drain was removed on POD 75.
Discussion
The presence of a hematoma in the extremities is usually a straightforward diagnosis. However, the unusual circumstances of this case highlight all the indications for investigation for possible soft-tissue sarcoma when a patient presents with what appears to be a benign condition.
Hematomas are rare in the absence of trauma or coagulopathy, with chronic expansion of hematomas rarer still.4,7,10-11 The patient had no evidence of coagulopathy because of her ability to have an uncomplicated pregnancy and elective cesarean section. She denied a history of trauma, and the location of her hematoma at the posterior distal thigh is an uncommon site of injury. In this setting, fine-needle aspiration and serial imaging to assess for progressive increase in lesion size were indicated to rule out malignancy.2
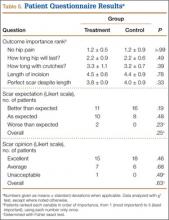
MRI is the gold-standard imaging modality for distinguishing soft-tissue masses from hematomas.5,12-14 Unlike the typical appearance of a hematoma, sarcomas of the soft-tissue extremities are often complex cystic lesions with multiple septations, internal soft-tissue components, and relatively ill-defined margins.15-17 However, as a hematoma becomes chronic, it can develop a fibrinous capsule, and the contents can manifest an atypical, heterogeneous appearance from scattered, progressive accumulation of blood products that is essentially indistinguishable from sarcomas on imaging.5
Because of the expansion of the hematoma and the atypical appearance of the mass on imaging, repeated core biopsy and, eventually, open biopsy were indicated, despite a preliminary negative diagnosis based on fine-needle aspiration. This resulted from the possibility of sampling error that is particularly relevant to cystic sarcomas, because only portions of the mass may be composed of malignant cells.2 An unusual aspect of this case is the regional lymphadenopathy noted on MRI, because regional lymphatic spread is a known mechanism of metastasis in soft-tissue sarcomas.18 However, the inguinal biopsies showed a chronic inflammatory infiltrate and were negative for malignancy, and enlarged nodes were not seen on imaging several months later. It is possible that the lymphadenopathy resulted from an unrelated process; alternatively, it may have been secondary to impaired lymphatic drainage because of mass effect from the hematoma, which also caused temporary lower extremity swelling.
The distal posterior thigh is an unreported location for a chronic expanding hematoma. Our patient developed slowly progressive lower-limb swelling and, eventually, paresthesias because of displacement of the neurovasculature, an unusual sequela that was recently reported in a similar case of an acute spontaneous hematoma in a patient on warfarin.19 Rupture of a Baker cyst is a possible inciting factor in our patient, although the proximal location of the lesion and the clearly defined tissue plane on MRI between the hematoma and the popliteal region make this unlikely. Finally, the patient’s lesion showed no evidence of vascular flow on Doppler ultrasonography, although giant hematomas secondary to popliteal aneurysm rupture have been reported.20-22
Conclusion
This case highlights the features of a chronic expanding hematoma that can suggest soft-tissue sarcoma and shows the recommended diagnostic steps to differentiate the 2 conditions. This case also describes an unreported location for a chronic expanding hematoma with resulting progressive neurovascular displacement caused by mass effect. We recommend careful monitoring of patients with similarly expansile lesions in this region for signs of neurovascular compromise.
1. O’Sullivan B, Pisters PW. Staging and prognostic factor evaluation in soft tissue sarcoma. Surg Oncol Clin N Am. 2003;12(2):333-353.
2. Rougraff B. The diagnosis and management of soft tissue sarcomas of the extremities in the adult. Curr Probl Cancer. 1999;23(1):1-50.
3. Friedlander HL, Bump RG. Chronic expanding hematoma of the calf. A case report. J Bone Joint Surg Am. 1968;50(6):1237-1241.
4. Liu CW, Kuo CL, Tsai TY, Lin LC, Wu CC. Massive gluteal mass mimicking sarcoma: chronic expanding hematoma. Formosan J Musculoskeletal Disord. 2011;2(3):106-108.
5. Taieb S, Penel N, Vanseymortier L, Ceugnart L. Soft tissue sarcomas or intramuscular haematomas? Eur J Radiol. 2009;72(1):44-49.
6. Reid JD, Kommareddi S, Lankerani M, Park MC. Chronic expanding hematomas. A clinicopathologic entity. JAMA. 1980;244(21):2441-2442.
7. Okada K, Sugiyama T, Kato H, Tani T. Chronic expanding hematoma mimicking soft tissue neoplasm. J Clin Oncol. 2001;19(11):2971-2972.
8. Negoro K, Uchida K, Yayama T, Kokubo Y, Baba H. Chronic expanding hematoma of the thigh. Joint Bone Spine. 2012;79(2):192-194.
9. Goddard MS, Vakil JJ, McCarthy EF, Khanuja HS. Chronic expanding hematoma of the lateral thigh and massive bony destruction after a failed total hip arthroplasty. J Arthroplasty. 2011;26(2):338.e13-.e15.
10. Radford DM, Schuh ME, Nambisan RN, Karakousis CP. Pseudo-tumor of the calf. Eur J Surg Oncol. 1993;19(3):300-301.
11. Mann HA, Hilton A, Goddard NJ, Smith MA, Holloway B, Lee CA. Synovial sarcoma mimicking haemophilic pseudotumour. Sarcoma. 2006;2006:27212.
12. Kransdorf MJ, Murphey MD. Radiologic evaluation of soft-tissue masses: a current perspective. AJR Am J Roentgenol. 2000;175(3):575-587.
13. Vanel D, Verstraete KL, Shapeero LG. Primary tumors of the musculoskeletal system. Radiol Clin North Am. 1997;35(1):213-237.
14. Siegel MJ. Magnetic resonance imaging of musculoskeletal soft tissue masses. Radiol Clin North Am. 2001;39(4):701-720.
15. O’Connor EE, Dixon LB, Peabody T, Stacy GS. MRI of cystic and soft-tissue masses of the shoulder joint. AJR Am J Roentgenol. 2004;183(1):39-47.
16. Bermejo A, De Bustamante TD, Martinez A, Carrera R, Zabia E, Manjon P. MR imaging in the evaluation of cystic-appearing soft-tissue masses of the extremities. Radiographics. 2013;33(3):833-855.
17. Morrison C, Wakely PE Jr, Ashman CJ, Lemley D, Theil K. Cystic synovial sarcoma. Ann Diagn Pathol. 2001;5(1):48-56.
18. Eilber FC, Rosen G, Nelson SD, et al. High-grade extremity soft tissue sarcomas: factors predictive of local recurrence and its effect on morbidity and mortality. Ann Surg. 2003;237(2):218-226.
19. Kuo CH. Peripheral neuropathy and lower limb swelling caused by a giant popliteal fossa hematoma. Neurol Sci. 2012;33(2):475-476.
20. Reijnen MM, de Rhoter W, Zeebregts CJ. Treatment of a symptomatic popliteal pseudoaneurysm using a stent-graft and ultrasound-guided evacuation of the haematoma. Emerg Radiol. 2009;16(2):167-169.
21. Rossi FH, Veith FJ, Lipsitz EC, Izukawa NM, Oliveira LA, Silva DG. Giant femoropopliteal artery aneurysm and vein rupture. Vascular. 2004;12(4):263-265.
22. Lamoca LM, Alerany MB, Hernando LL. Endovascular therapy for a ruptured popliteal aneurysm. Catheter Cardiovasc Interv. 2010;75(3):427-429.
Soft-tissue sarcomas are quite rare, with an annual incidence of 20 to 30 per 1,000,000 persons in the United States.1 Because of their heterogeneous presentation, they remain a diagnostic challenge and are often initially confused for more common, benign disorders.2 Chronic expanding hematoma, first described by Friedlander and colleagues3 in 1968, is a rare entity that is particularly difficult to distinguish from soft-tissue malignancy.3-5 Chronic expanding hematoma is defined as a hematoma that gradually expands over 1 month or longer, is absent of neoplastic change on histologic sections, and does not occur in the setting of coagulopathy.6
Typically associated with remote trauma, these lesions often present as a slowly growing mass on the anterior or lateral thigh, calf, or buttock.3-4,7-9 They have been reported to persist as long as 46 years, with sizes ranging from 3 to 55 cm in maximum diameter.7 On imaging, they have a cystic appearance with a dense fibrous capsule.7-8 Most cases resolve uneventfully after drainage or marginal excision, although some cases require repeated intervention.7 This case report describes a morbidly obese patient with a chronic expanding hematoma in the distal posterior thigh whose definitive treatment was delayed 6 months because of her pregnancy status and inability to lie prone for open biopsy. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 27-year-old morbidly obese woman, who was pregnant at 12 weeks gestation, was seen in an orthopedic oncology clinic with a 1-month history of a slowly growing, painful posterior thigh mass. She had no history of cancer or bleeding disorder, and denied a history of trauma or constitutional symptoms consistent with malignancy. Coagulation studies were normal. Magnetic resonance imaging (MRI) obtained 2 weeks prior in the emergency room showed a cystic lesion with mass-like components in the posterior compartment of the distal right thigh, measuring 17 cm longitudinally. The lesion was located adjacent to, but not involving, the sciatic nerve and femoral vasculature. On initial examination, the large soft-tissue mass was evident and moderately painful to palpation; no skin changes were noted, and the patient had a normal sensorimotor examination. Fine-needle aspiration was performed, which resulted in amorphous debris consistent with hematoma.
Repeat MRI 2 months later showed increased size of the lesion (9.5×10.5 cm axial, 22.0 cm craniocaudal). Although most findings of a more extensive imaging protocol, including precontrast and postcontrast sequences, were consistent with hematoma, the lesion also had several characteristics that indicated soft-tissue sarcoma. Specifically, findings suggestive of chronic hematoma included the hyperintense short tau inversion recovery (STIR) T1/T2 signal of the cystic component consistent with proteinaceous fluid and the low STIR TI/T2 signal of the periphery consistent with a rim of hemosiderin (Figure 1). Additionally, the cystic component of the lesion had multiple fine septations that are atypical for a hematoma (Figure 1), and several lymph nodes greater than 1.7 cm in short axis were noted in the anterior thigh and hemipelvis that were suspicious of metastatic lymphadenopathy. The encapsulated appearance of the lesion with a sharply defined margin and short transition zone were also reassuring findings for a benign lesion (Figures 1, 2A, 2B). However, several findings were identified that suggested soft-tissue sarcoma, including a nodular soft-tissue component on the medial wall of the lesion that had heterogeneous enhancement with contrast (Figure 2B). We, therefore, proceeded with ultrasound-guided core needle biopsy of the mass and cytologic sampling of the fluid components, which were again consistent with hematoma; no evidence of internal vascular flow was noted on Doppler ultrasound. Ultrasound-guided right inguinal lymph node biopsy was also performed and was negative for malignancy. Because of her large body habitus and pregnancy status, it was agreed that open biopsy should be delayed until after delivery to avoid placing the patient in a prone position.
The patient visited the emergency room several times during the following months because of intermittent exacerbations of her lower extremity pain, swelling, and occasional paresthesias. About 6 months after initial presentation, repeat MRI again showed increased size of the mass (13.5×13.5 cm axial, 28 cm craniocaudal). There was also increased displacement of the adjacent neurovascular structures but no evidence of deep vein thrombosis. Because of concerns about the increased symptomatology of her thigh mass and possible sampling error of the previous biopsies, an elective cesarean section was performed at 35 weeks gestation. One week later, after clearance by her obstetrician, we proceeded with open biopsy of the mass in prone position. Initial sampling was negative for malignancy on frozen section; then, we expressed 1.75 L of brown fluid and solidified blood products, irrigated copiously, and placed a surgical drain. The permanent histologic specimens were again consistent with hematoma, and microbial cultures were negative. A week later, the patient accidentally removed her drain, and she presented with a fever (101°F) on postoperative day (POD) 15. Computed tomography showed reaccumulation of fluid; duplex ultrasound was negative. She was placed on cephalexin and underwent ultrasound-guided replacement of the drain with removal of an additional 750 mL fluid on POD 20. She drained an additional 150 to 200 mL/d for 1 month, with marked improvement in her leg swelling and knee range of motion. The drainage decreased during the next 3 weeks, and the drain was removed on POD 75.
Discussion
The presence of a hematoma in the extremities is usually a straightforward diagnosis. However, the unusual circumstances of this case highlight all the indications for investigation for possible soft-tissue sarcoma when a patient presents with what appears to be a benign condition.
Hematomas are rare in the absence of trauma or coagulopathy, with chronic expansion of hematomas rarer still.4,7,10-11 The patient had no evidence of coagulopathy because of her ability to have an uncomplicated pregnancy and elective cesarean section. She denied a history of trauma, and the location of her hematoma at the posterior distal thigh is an uncommon site of injury. In this setting, fine-needle aspiration and serial imaging to assess for progressive increase in lesion size were indicated to rule out malignancy.2
MRI is the gold-standard imaging modality for distinguishing soft-tissue masses from hematomas.5,12-14 Unlike the typical appearance of a hematoma, sarcomas of the soft-tissue extremities are often complex cystic lesions with multiple septations, internal soft-tissue components, and relatively ill-defined margins.15-17 However, as a hematoma becomes chronic, it can develop a fibrinous capsule, and the contents can manifest an atypical, heterogeneous appearance from scattered, progressive accumulation of blood products that is essentially indistinguishable from sarcomas on imaging.5
Because of the expansion of the hematoma and the atypical appearance of the mass on imaging, repeated core biopsy and, eventually, open biopsy were indicated, despite a preliminary negative diagnosis based on fine-needle aspiration. This resulted from the possibility of sampling error that is particularly relevant to cystic sarcomas, because only portions of the mass may be composed of malignant cells.2 An unusual aspect of this case is the regional lymphadenopathy noted on MRI, because regional lymphatic spread is a known mechanism of metastasis in soft-tissue sarcomas.18 However, the inguinal biopsies showed a chronic inflammatory infiltrate and were negative for malignancy, and enlarged nodes were not seen on imaging several months later. It is possible that the lymphadenopathy resulted from an unrelated process; alternatively, it may have been secondary to impaired lymphatic drainage because of mass effect from the hematoma, which also caused temporary lower extremity swelling.
The distal posterior thigh is an unreported location for a chronic expanding hematoma. Our patient developed slowly progressive lower-limb swelling and, eventually, paresthesias because of displacement of the neurovasculature, an unusual sequela that was recently reported in a similar case of an acute spontaneous hematoma in a patient on warfarin.19 Rupture of a Baker cyst is a possible inciting factor in our patient, although the proximal location of the lesion and the clearly defined tissue plane on MRI between the hematoma and the popliteal region make this unlikely. Finally, the patient’s lesion showed no evidence of vascular flow on Doppler ultrasonography, although giant hematomas secondary to popliteal aneurysm rupture have been reported.20-22
Conclusion
This case highlights the features of a chronic expanding hematoma that can suggest soft-tissue sarcoma and shows the recommended diagnostic steps to differentiate the 2 conditions. This case also describes an unreported location for a chronic expanding hematoma with resulting progressive neurovascular displacement caused by mass effect. We recommend careful monitoring of patients with similarly expansile lesions in this region for signs of neurovascular compromise.
Soft-tissue sarcomas are quite rare, with an annual incidence of 20 to 30 per 1,000,000 persons in the United States.1 Because of their heterogeneous presentation, they remain a diagnostic challenge and are often initially confused for more common, benign disorders.2 Chronic expanding hematoma, first described by Friedlander and colleagues3 in 1968, is a rare entity that is particularly difficult to distinguish from soft-tissue malignancy.3-5 Chronic expanding hematoma is defined as a hematoma that gradually expands over 1 month or longer, is absent of neoplastic change on histologic sections, and does not occur in the setting of coagulopathy.6
Typically associated with remote trauma, these lesions often present as a slowly growing mass on the anterior or lateral thigh, calf, or buttock.3-4,7-9 They have been reported to persist as long as 46 years, with sizes ranging from 3 to 55 cm in maximum diameter.7 On imaging, they have a cystic appearance with a dense fibrous capsule.7-8 Most cases resolve uneventfully after drainage or marginal excision, although some cases require repeated intervention.7 This case report describes a morbidly obese patient with a chronic expanding hematoma in the distal posterior thigh whose definitive treatment was delayed 6 months because of her pregnancy status and inability to lie prone for open biopsy. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 27-year-old morbidly obese woman, who was pregnant at 12 weeks gestation, was seen in an orthopedic oncology clinic with a 1-month history of a slowly growing, painful posterior thigh mass. She had no history of cancer or bleeding disorder, and denied a history of trauma or constitutional symptoms consistent with malignancy. Coagulation studies were normal. Magnetic resonance imaging (MRI) obtained 2 weeks prior in the emergency room showed a cystic lesion with mass-like components in the posterior compartment of the distal right thigh, measuring 17 cm longitudinally. The lesion was located adjacent to, but not involving, the sciatic nerve and femoral vasculature. On initial examination, the large soft-tissue mass was evident and moderately painful to palpation; no skin changes were noted, and the patient had a normal sensorimotor examination. Fine-needle aspiration was performed, which resulted in amorphous debris consistent with hematoma.
Repeat MRI 2 months later showed increased size of the lesion (9.5×10.5 cm axial, 22.0 cm craniocaudal). Although most findings of a more extensive imaging protocol, including precontrast and postcontrast sequences, were consistent with hematoma, the lesion also had several characteristics that indicated soft-tissue sarcoma. Specifically, findings suggestive of chronic hematoma included the hyperintense short tau inversion recovery (STIR) T1/T2 signal of the cystic component consistent with proteinaceous fluid and the low STIR TI/T2 signal of the periphery consistent with a rim of hemosiderin (Figure 1). Additionally, the cystic component of the lesion had multiple fine septations that are atypical for a hematoma (Figure 1), and several lymph nodes greater than 1.7 cm in short axis were noted in the anterior thigh and hemipelvis that were suspicious of metastatic lymphadenopathy. The encapsulated appearance of the lesion with a sharply defined margin and short transition zone were also reassuring findings for a benign lesion (Figures 1, 2A, 2B). However, several findings were identified that suggested soft-tissue sarcoma, including a nodular soft-tissue component on the medial wall of the lesion that had heterogeneous enhancement with contrast (Figure 2B). We, therefore, proceeded with ultrasound-guided core needle biopsy of the mass and cytologic sampling of the fluid components, which were again consistent with hematoma; no evidence of internal vascular flow was noted on Doppler ultrasound. Ultrasound-guided right inguinal lymph node biopsy was also performed and was negative for malignancy. Because of her large body habitus and pregnancy status, it was agreed that open biopsy should be delayed until after delivery to avoid placing the patient in a prone position.
The patient visited the emergency room several times during the following months because of intermittent exacerbations of her lower extremity pain, swelling, and occasional paresthesias. About 6 months after initial presentation, repeat MRI again showed increased size of the mass (13.5×13.5 cm axial, 28 cm craniocaudal). There was also increased displacement of the adjacent neurovascular structures but no evidence of deep vein thrombosis. Because of concerns about the increased symptomatology of her thigh mass and possible sampling error of the previous biopsies, an elective cesarean section was performed at 35 weeks gestation. One week later, after clearance by her obstetrician, we proceeded with open biopsy of the mass in prone position. Initial sampling was negative for malignancy on frozen section; then, we expressed 1.75 L of brown fluid and solidified blood products, irrigated copiously, and placed a surgical drain. The permanent histologic specimens were again consistent with hematoma, and microbial cultures were negative. A week later, the patient accidentally removed her drain, and she presented with a fever (101°F) on postoperative day (POD) 15. Computed tomography showed reaccumulation of fluid; duplex ultrasound was negative. She was placed on cephalexin and underwent ultrasound-guided replacement of the drain with removal of an additional 750 mL fluid on POD 20. She drained an additional 150 to 200 mL/d for 1 month, with marked improvement in her leg swelling and knee range of motion. The drainage decreased during the next 3 weeks, and the drain was removed on POD 75.
Discussion
The presence of a hematoma in the extremities is usually a straightforward diagnosis. However, the unusual circumstances of this case highlight all the indications for investigation for possible soft-tissue sarcoma when a patient presents with what appears to be a benign condition.
Hematomas are rare in the absence of trauma or coagulopathy, with chronic expansion of hematomas rarer still.4,7,10-11 The patient had no evidence of coagulopathy because of her ability to have an uncomplicated pregnancy and elective cesarean section. She denied a history of trauma, and the location of her hematoma at the posterior distal thigh is an uncommon site of injury. In this setting, fine-needle aspiration and serial imaging to assess for progressive increase in lesion size were indicated to rule out malignancy.2
MRI is the gold-standard imaging modality for distinguishing soft-tissue masses from hematomas.5,12-14 Unlike the typical appearance of a hematoma, sarcomas of the soft-tissue extremities are often complex cystic lesions with multiple septations, internal soft-tissue components, and relatively ill-defined margins.15-17 However, as a hematoma becomes chronic, it can develop a fibrinous capsule, and the contents can manifest an atypical, heterogeneous appearance from scattered, progressive accumulation of blood products that is essentially indistinguishable from sarcomas on imaging.5
Because of the expansion of the hematoma and the atypical appearance of the mass on imaging, repeated core biopsy and, eventually, open biopsy were indicated, despite a preliminary negative diagnosis based on fine-needle aspiration. This resulted from the possibility of sampling error that is particularly relevant to cystic sarcomas, because only portions of the mass may be composed of malignant cells.2 An unusual aspect of this case is the regional lymphadenopathy noted on MRI, because regional lymphatic spread is a known mechanism of metastasis in soft-tissue sarcomas.18 However, the inguinal biopsies showed a chronic inflammatory infiltrate and were negative for malignancy, and enlarged nodes were not seen on imaging several months later. It is possible that the lymphadenopathy resulted from an unrelated process; alternatively, it may have been secondary to impaired lymphatic drainage because of mass effect from the hematoma, which also caused temporary lower extremity swelling.
The distal posterior thigh is an unreported location for a chronic expanding hematoma. Our patient developed slowly progressive lower-limb swelling and, eventually, paresthesias because of displacement of the neurovasculature, an unusual sequela that was recently reported in a similar case of an acute spontaneous hematoma in a patient on warfarin.19 Rupture of a Baker cyst is a possible inciting factor in our patient, although the proximal location of the lesion and the clearly defined tissue plane on MRI between the hematoma and the popliteal region make this unlikely. Finally, the patient’s lesion showed no evidence of vascular flow on Doppler ultrasonography, although giant hematomas secondary to popliteal aneurysm rupture have been reported.20-22
Conclusion
This case highlights the features of a chronic expanding hematoma that can suggest soft-tissue sarcoma and shows the recommended diagnostic steps to differentiate the 2 conditions. This case also describes an unreported location for a chronic expanding hematoma with resulting progressive neurovascular displacement caused by mass effect. We recommend careful monitoring of patients with similarly expansile lesions in this region for signs of neurovascular compromise.
1. O’Sullivan B, Pisters PW. Staging and prognostic factor evaluation in soft tissue sarcoma. Surg Oncol Clin N Am. 2003;12(2):333-353.
2. Rougraff B. The diagnosis and management of soft tissue sarcomas of the extremities in the adult. Curr Probl Cancer. 1999;23(1):1-50.
3. Friedlander HL, Bump RG. Chronic expanding hematoma of the calf. A case report. J Bone Joint Surg Am. 1968;50(6):1237-1241.
4. Liu CW, Kuo CL, Tsai TY, Lin LC, Wu CC. Massive gluteal mass mimicking sarcoma: chronic expanding hematoma. Formosan J Musculoskeletal Disord. 2011;2(3):106-108.
5. Taieb S, Penel N, Vanseymortier L, Ceugnart L. Soft tissue sarcomas or intramuscular haematomas? Eur J Radiol. 2009;72(1):44-49.
6. Reid JD, Kommareddi S, Lankerani M, Park MC. Chronic expanding hematomas. A clinicopathologic entity. JAMA. 1980;244(21):2441-2442.
7. Okada K, Sugiyama T, Kato H, Tani T. Chronic expanding hematoma mimicking soft tissue neoplasm. J Clin Oncol. 2001;19(11):2971-2972.
8. Negoro K, Uchida K, Yayama T, Kokubo Y, Baba H. Chronic expanding hematoma of the thigh. Joint Bone Spine. 2012;79(2):192-194.
9. Goddard MS, Vakil JJ, McCarthy EF, Khanuja HS. Chronic expanding hematoma of the lateral thigh and massive bony destruction after a failed total hip arthroplasty. J Arthroplasty. 2011;26(2):338.e13-.e15.
10. Radford DM, Schuh ME, Nambisan RN, Karakousis CP. Pseudo-tumor of the calf. Eur J Surg Oncol. 1993;19(3):300-301.
11. Mann HA, Hilton A, Goddard NJ, Smith MA, Holloway B, Lee CA. Synovial sarcoma mimicking haemophilic pseudotumour. Sarcoma. 2006;2006:27212.
12. Kransdorf MJ, Murphey MD. Radiologic evaluation of soft-tissue masses: a current perspective. AJR Am J Roentgenol. 2000;175(3):575-587.
13. Vanel D, Verstraete KL, Shapeero LG. Primary tumors of the musculoskeletal system. Radiol Clin North Am. 1997;35(1):213-237.
14. Siegel MJ. Magnetic resonance imaging of musculoskeletal soft tissue masses. Radiol Clin North Am. 2001;39(4):701-720.
15. O’Connor EE, Dixon LB, Peabody T, Stacy GS. MRI of cystic and soft-tissue masses of the shoulder joint. AJR Am J Roentgenol. 2004;183(1):39-47.
16. Bermejo A, De Bustamante TD, Martinez A, Carrera R, Zabia E, Manjon P. MR imaging in the evaluation of cystic-appearing soft-tissue masses of the extremities. Radiographics. 2013;33(3):833-855.
17. Morrison C, Wakely PE Jr, Ashman CJ, Lemley D, Theil K. Cystic synovial sarcoma. Ann Diagn Pathol. 2001;5(1):48-56.
18. Eilber FC, Rosen G, Nelson SD, et al. High-grade extremity soft tissue sarcomas: factors predictive of local recurrence and its effect on morbidity and mortality. Ann Surg. 2003;237(2):218-226.
19. Kuo CH. Peripheral neuropathy and lower limb swelling caused by a giant popliteal fossa hematoma. Neurol Sci. 2012;33(2):475-476.
20. Reijnen MM, de Rhoter W, Zeebregts CJ. Treatment of a symptomatic popliteal pseudoaneurysm using a stent-graft and ultrasound-guided evacuation of the haematoma. Emerg Radiol. 2009;16(2):167-169.
21. Rossi FH, Veith FJ, Lipsitz EC, Izukawa NM, Oliveira LA, Silva DG. Giant femoropopliteal artery aneurysm and vein rupture. Vascular. 2004;12(4):263-265.
22. Lamoca LM, Alerany MB, Hernando LL. Endovascular therapy for a ruptured popliteal aneurysm. Catheter Cardiovasc Interv. 2010;75(3):427-429.
1. O’Sullivan B, Pisters PW. Staging and prognostic factor evaluation in soft tissue sarcoma. Surg Oncol Clin N Am. 2003;12(2):333-353.
2. Rougraff B. The diagnosis and management of soft tissue sarcomas of the extremities in the adult. Curr Probl Cancer. 1999;23(1):1-50.
3. Friedlander HL, Bump RG. Chronic expanding hematoma of the calf. A case report. J Bone Joint Surg Am. 1968;50(6):1237-1241.
4. Liu CW, Kuo CL, Tsai TY, Lin LC, Wu CC. Massive gluteal mass mimicking sarcoma: chronic expanding hematoma. Formosan J Musculoskeletal Disord. 2011;2(3):106-108.
5. Taieb S, Penel N, Vanseymortier L, Ceugnart L. Soft tissue sarcomas or intramuscular haematomas? Eur J Radiol. 2009;72(1):44-49.
6. Reid JD, Kommareddi S, Lankerani M, Park MC. Chronic expanding hematomas. A clinicopathologic entity. JAMA. 1980;244(21):2441-2442.
7. Okada K, Sugiyama T, Kato H, Tani T. Chronic expanding hematoma mimicking soft tissue neoplasm. J Clin Oncol. 2001;19(11):2971-2972.
8. Negoro K, Uchida K, Yayama T, Kokubo Y, Baba H. Chronic expanding hematoma of the thigh. Joint Bone Spine. 2012;79(2):192-194.
9. Goddard MS, Vakil JJ, McCarthy EF, Khanuja HS. Chronic expanding hematoma of the lateral thigh and massive bony destruction after a failed total hip arthroplasty. J Arthroplasty. 2011;26(2):338.e13-.e15.
10. Radford DM, Schuh ME, Nambisan RN, Karakousis CP. Pseudo-tumor of the calf. Eur J Surg Oncol. 1993;19(3):300-301.
11. Mann HA, Hilton A, Goddard NJ, Smith MA, Holloway B, Lee CA. Synovial sarcoma mimicking haemophilic pseudotumour. Sarcoma. 2006;2006:27212.
12. Kransdorf MJ, Murphey MD. Radiologic evaluation of soft-tissue masses: a current perspective. AJR Am J Roentgenol. 2000;175(3):575-587.
13. Vanel D, Verstraete KL, Shapeero LG. Primary tumors of the musculoskeletal system. Radiol Clin North Am. 1997;35(1):213-237.
14. Siegel MJ. Magnetic resonance imaging of musculoskeletal soft tissue masses. Radiol Clin North Am. 2001;39(4):701-720.
15. O’Connor EE, Dixon LB, Peabody T, Stacy GS. MRI of cystic and soft-tissue masses of the shoulder joint. AJR Am J Roentgenol. 2004;183(1):39-47.
16. Bermejo A, De Bustamante TD, Martinez A, Carrera R, Zabia E, Manjon P. MR imaging in the evaluation of cystic-appearing soft-tissue masses of the extremities. Radiographics. 2013;33(3):833-855.
17. Morrison C, Wakely PE Jr, Ashman CJ, Lemley D, Theil K. Cystic synovial sarcoma. Ann Diagn Pathol. 2001;5(1):48-56.
18. Eilber FC, Rosen G, Nelson SD, et al. High-grade extremity soft tissue sarcomas: factors predictive of local recurrence and its effect on morbidity and mortality. Ann Surg. 2003;237(2):218-226.
19. Kuo CH. Peripheral neuropathy and lower limb swelling caused by a giant popliteal fossa hematoma. Neurol Sci. 2012;33(2):475-476.
20. Reijnen MM, de Rhoter W, Zeebregts CJ. Treatment of a symptomatic popliteal pseudoaneurysm using a stent-graft and ultrasound-guided evacuation of the haematoma. Emerg Radiol. 2009;16(2):167-169.
21. Rossi FH, Veith FJ, Lipsitz EC, Izukawa NM, Oliveira LA, Silva DG. Giant femoropopliteal artery aneurysm and vein rupture. Vascular. 2004;12(4):263-265.
22. Lamoca LM, Alerany MB, Hernando LL. Endovascular therapy for a ruptured popliteal aneurysm. Catheter Cardiovasc Interv. 2010;75(3):427-429.
Severe Neurologic Manifestations of Fat Embolism Syndrome in a Polytrauma Patient
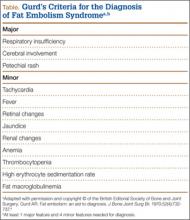
Fat embolism syndrome (FES) was first described by Von Bergmann in 1873 in a patient with a fractured femur.1 While fat within the circulation (fat embolism) is relatively common following long-bone fracture, the clinical pattern of symptoms that make up FES is less so, occurring in 1% to 3% of isolated long-bone fractures and 5% to 10% of patients with multiple skeletal trauma.1 A variety of clinical, laboratory, and imaging criteria has been described, classically by Gurd in 1970 (Table).1-6 Most commonly, however, it is a diagnosis of exclusion when the classic triad of respiratory difficulty, neurologic abnormalities, and a characteristic petechial rash are present in the appropriate clinical setting.6
The neurologic sequelae of this syndrome can range from headache, confusion, and agitation to stupor, focal neurologic signs, and, less commonly, coma.7 Onset of these symptoms usually occurs between 24 hours and 48 hours (mean, 40 hours) after trauma.1 While these neurologic manifestations occur in up to 86% of patients with FES, it is rare for them to be present without the pulmonary symptoms of dyspnea, hypoxemia, and tachypnea, which are the most common presenting symptoms of the disease.1-6 In this case report, we describe severe, rapid-onset neurologic manifestations, without the typical pulmonary involvement, as the primary clinical presentation of FES in a polytrauma patient. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
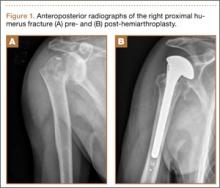
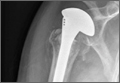
A previously healthy 50-year-old man presented to the emergency room in transfer from an outside hospital after a rollover motor vehicle collision in which he was ejected approximately 50 feet. Injuries included a right proximal humerus fracture/dislocation (Figure 1), right ulnar styloid fracture, L1 compression fracture, and multiple rib fractures. On admission, the patient had an ethanol level of 969 mg/L (.097%) and a urine drug screen positive only for opioids, presumably because of pain medication given that day. He denied a history of alcohol abuse and reported consuming 2 to 3 beers per week. The patient was awake, alert, and oriented with a Glasgow Coma Scale (GCS) of 15. He was tachycardic (heart rate, 126), tachypneic (respiratory rate, 24), and febrile (temperature, 38.6°C [101.5°F]), and his white blood cell count was elevated at 29.5×109/L. On examination, his right arm was found to be neurovascularly intact; it was placed in a sling with a forearm splint, and the patient was admitted to the intermediate special care unit on spine precautions with a plan for right shoulder hemiarthroplasty the following day.
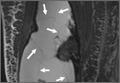
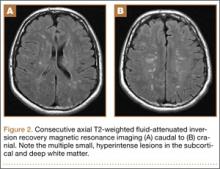
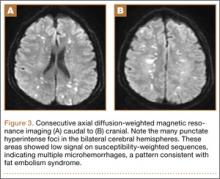
Overnight the patient’s mental status began to deteriorate, and approximately 10 hours after initial assessment, he was not answering questions but was able to respond to some commands. On hospital day 2, approximately 20 hours after initial assessment, the patient had a GCS of 8, was not responding to commands, and moved only in response to painful stimuli. The patient had been prescribed morphine by patient-controlled analgesia and had received intravenous hydromorphone on the day of admission, although the amount of medication delivered was not thought adequate to explain this deterioration. On the morning of hospital day 2, noncontrast brain computed tomography (CT) was normal with no evidence of intracranial hemorrhage or infarct. This was followed by brain magnetic resonance imaging (MRI), with the T2-weighted images showing numerous, small hyperintense lesions in subcortical and periventricular white matter, corpus callosum, basal ganglia, brain stem, and cerebellar hemispheres (Figure 2). The lesions also showed hyperintensity on diffusion-weighted MRI and were interpreted to be consistent with multiple, tiny infarcts (Figure 3). In addition, susceptibility-weighted sequences showed low signal in the same areas, suggesting multiple microhemorrhages, a pattern consistent with FES. Oxygen saturations remained 95% to 99%, and chest radiograph revealed clear lung fields without infiltrate. On hospital day 2, the patient was transferred to the intensive care unit and intubated for airway protection owing to an inability to clear secretions, although arterial blood gas levels remained normal. An echocardiogram revealed no right-to-left shunt, such as a patent foramen ovale (PFO); an electroencephalogram showed no seizure-like activity. No petechial rash was noted on skin examination. The patient was treated with supportive care. Right shoulder hemiarthroplasty was performed on hospital day 7 without complications (Figure 1). On hospital day 13, the patient was following commands and on day 14 he was extubated. His mental status continued to improve, and he was discharged to a rehabilitation facility after 36 days. On last follow-up, 6 months after initial injury, the patient was recovering well with no residual neurologic deficits and only minor limitation in range of motion of the right shoulder.
Discussion
This case presented an interesting diagnostic challenge regarding the patient’s rapid decline in mental status, with a differential diagnosis including diffuse axonal injury (DAI), anoxic brain injury, posttraumatic seizure, other intracranial pathology, such as stroke or hemorrhage, and FES. FES was diagnosed, when other possibilities were ruled out, given the characteristic findings on brain MRI described above in the context of multiple fractures.
Pathophysiology
Despite its recognition in 1873, there is no consensus on the pathophysiological mechanism that causes the clinical symptoms of FES. In the setting of trauma, there are 2 predominant theories. The mechanical theory postulates that fat globules enter the circulation through disrupted venules after the fracture of marrow-containing bones, passing to the arterial circulation through pulmonary vasculature, or paradoxically, by way of a right-to-left shunt, such as a PFO.1,3 The presence of fat in the heart, visualized as echogenic material in the right and left atria on transesophageal echocardiography, has been confirmed in multiple studies during orthopedic procedures, including total knee arthroplasty and femoral reaming.8,9 These fat particles can lodge as microembolisms in target organs such as the skin and brain. However, autopsy studies have shown a lack of correlation of the severity of symptoms and the quantity of intravascular fat.1 In addition, the typical 24- to 72-hour delay in the onset of symptoms after initial trauma would argue against a solely mechanical explanation.10
Alternatively or concomitantly, the biochemical theory proposes that embolized fat may be degraded to toxic intermediaries, such as free fatty acids and C-reactive protein, which cause end-organ damage.3 This has been shown in an animal model, in which intravascular injection of free fatty acids was associated with endothelial damage and increased capillary permeability in the lung, leading to acute respiratory distress syndrome (ARDS).11 The same mechanism could explain injury to other end organs and is consistent with the delay in onset of symptoms after acute injury. In our patient’s case, the absence of pulmonary involvement, lack of a right-to-left vascular shunt such as a PFO, and presence of a systemic inflammatory response on admission may implicate the production of toxic intermediaries from the metabolism of embolized fat as the source of this patient’s FES.
Clinical Presentation
The initial presentation of FES usually manifests as respiratory distress and hypoxia.10 Chest radiographs are often normal, as in our patient, but can show bilateral diffuse interstitial or alveolar infiltrates.2,6 CT more often has findings, including bilateral ground-glass opacities with interlobar septal thickening.12 A petechial rash can be found on the head, neck, anterior thorax, axillae, subconjunctiva, and oral mucous membranes, although it occurs in only 20% to 50% of cases.1,2,13 Neurologic sequelae are present in up to 80% of patients,7 with onset typically following pulmonary symptoms.1,10 These sequelae can range from headache, confusion, and agitation to stupor, focal neurologic signs, and, less commonly, coma.7 Onset of symptoms generally occurs between 24 and 48 hours after trauma,1 although they have been reported as early as 12 hours.10 This case is an example of an atypical course, with the initial presentation of neurologic symptoms at approximately 14 hours after trauma with rapid progression to coma without classic pulmonary symptoms.
Diagnosis
Owing to the nonspecific clinical features of FES, a variety of clinical, laboratory, and imaging criteria has been described. Of these criteria, the most frequently referenced is by Gurd in 1970,4,5 who divided the features into major and minor, with 1 major and 4 minor features required to make the diagnosis (Table). In applying these criteria to our patient, we found that he exhibited the major criteria of cerebral involvement and minor criteria of tachycardia, fever, and thrombocytopenia. Respiratory insufficiency and petechial rash, as well as jaundice, renal changes, and anemia were negative features. Retinal changes, elevated erythrocyte sedimentation rate, and fat macroglobulinemia were not tested or examined. Although in our case the clinical and laboratory criteria for the diagnosis of FES as defined by Gurd were not met, the sensitivity of Gurd’s and other criteria is debated.10
Laboratory tests specific for the disease have not been developed. Although elevated serum levels of lipase, increased blood lipid levels, and fat globules in the urine, sputum, and blood have all been proposed, they are found in trauma patients with and without FES.2,5,6
The nonspecific nature of the signs and symptoms of FES and the lack of reliable laboratory tests for diagnosis of the syndrome highlight the importance of radiographic evaluation in patients with neurologic symptoms. Brain CT scans are usually negative,14 although, in some cases, they may show diffuse edema with scattered low attenuating areas and hemorrhage.15 MRI is more sensitive, and T2-weighted images typically reveal multiple small, nonconfluent hyperintense lesions, usually in the periventricular, subcortical, and deep white matter, sometimes referred to as the “starfield” pattern.14,16 The differential diagnosis for these findings is broad and, in addition to FES, includes DAI, vasogenic edema with microinfarcts, and demyelinating disease.14 Sensitivity and specificity may be increased with the addition of diffusion-weighted MRI, which shows scattered bright spots on a dark background in a similar “starfield” pattern as on T2-weighted images.15 Susceptibility-weighted MRI has recently been introduced as having utility in the diagnosis of FES, with areas of low-signal intensity indicating diffuse microhemorrhages.17 DAI can show a similar pattern; however, the autopsy-confirmed locations of the abnormalities are distinct, with those of FES being found in cerebral and cerebellar white matter and splenium of the corpus callosum and radiographic abnormalities of DAI being found in the gray-white matter junction, dorsolateral brainstem, and splenium of corpus callosum.17
Prevention and Treatment
Of primary importance in the prevention of FES is early stabilization of fractures. Several studies have shown a decreased incidence of FES when long-bone fractures are treated with immediate operative fixation.18,19 However, in the setting of polytrauma, the desire for early definitive treatment must be balanced against the risks for the exaggerated immune response from prolonged surgery.20 The timing of fracture fixation to prevent sequelae of the inflammatory response, such as ARDS and multiple organ dysfunction syndrome, is still debated. In a review article, Pape and colleagues20 suggest classifying the multiply injured patient as stable, borderline, unstable, and in extremis based on clinical and laboratory criteria. They recommend early definitive fixation for stable patients and those patients who are borderline or unstable and responsive to resuscitation, whereas damage-control orthopedics and staged fracture fixation should be considered in the other groups.
Several pharmacologic interventions have been described, although their effects are highly variable and none have clear indications.1-3,6 The most heavily researched is corticosteroids, with the proposed mechanisms of action including blunting of the inflammatory response, stabilizing the pulmonary capillary membrane to reduce interstitial edema, preventing activation of the complement system, and retarding platelet aggregation.21 A recent meta-analysis to assess this intervention examined 6 studies with a total of 386 patients with long-bone fractures who were randomized to treatment with corticosteroids or supportive care only.22 They found a reduced risk for FES in those patients who received corticosteroids, but there was no difference in mortality between groups. Given these results, the utility of corticosteroids is still debated.
Once FES has occurred, treatment options usually focus on supportive care, with most patients having a full recovery.1,3 No specific treatments are available, and symptomatic treatment is the suggested approach, including ensuring adequate oxygenation and ventilation and providing hemodynamic support and volume and blood-product resuscitation as needed.1-3,6
Conclusion
We have presented a case of FES unique in its rapid onset, an initial presentation with neurologic manifestations without typical pulmonary involvement, and the mechanism of end-organ damage without a right-to-left shunt. This case emphasizes the importance of considering FES in the patient with deteriorating mental status in the setting of multiple fractures, particularly in the absence of other characteristic clinical findings, such as pulmonary distress and the pathognomonic petechial rash. Brain MRI can play an important role in diagnosing those patients presenting with predominantly neurological symptoms. Early recognition of this condition allows for the anticipation of complications of the disease process, such as respiratory distress, and the potential need for mechanical ventilation and hemodynamic support.
1. Johnson MJ, Lucas GL. Fat embolism syndrome. Orthopedics. 1996;19(1):41-49.
2. Levy D. The fat embolism syndrome. A review. Clin Orthop. 1990;261:281-286.
3. Mellor A, Soni N. Fat embolism. Anaesthesia. 2001;56(2):145-154.
4. Gurd AR. Fat embolism: an aid to diagnosis. J Bone Joint Surg Br. 1970:52(4):732-737.
5. Gurd AR, Wilson RI. The fat embolism syndrome. J Bone Joint Surg Br. 1974;56(3):408-416.
6. Bulger EM, Smith DG, Maier RV, Jurkovich GJ. Fat embolism syndrome. A 10-year review. Arch Surg. 1997;132(4):435-439.
7. Jacobson DM, Terrence CF, Reinmuth OM. The neurologic manifestations of fat embolism. Neurology. 1986;36(6):847-851.
8. Sulek CA, Davies LK, Enneking FK, Gearen PA, Lobato EB. Cerebral microembolism diagnosed by transcranial Doppler during total knee arthroplasty: correlation with transesophageal echocardiography. Anesthesiology. 1999;91(3):672-676.
9. Volgas DA, Burch T, Stannard JP, Ellis T, Bilotta J, Alonso JE. Fat embolus in femur fractures: a comparison of two reaming systems. Injury. 2010;41(Suppl 2):S90-S93.
10. Gupta B, D’souza N, Sawhney C, et al. Analyzing fat embolism syndrome in trauma patients at AIIMS Apex Trauma Center, New Delhi, India. J Emerg Trauma Shock. 2011;4(3):337–341.
11. King EG, Wagner WW Jr, Ashbaugh DG, Latham LP, Halsey DR. Alterations in pulmonary microanatomy after fat embolism. In vivo observations via thoracic window of the oleic acid-embolized canine lung. Chest. 1971:59(5):524-530.
12. Malagari K, Economopoulos N, Stoupis C, et al. High-resolution CT findings in mild pulmonary fat embolism. Chest. 2003:123(4):1196-1201.
13. King MB, Harmon KR. Unusual forms of pulmonary embolism. Clin Chest Med. 1994;15(3):561-580.
14. Parizel PM, Demey HE, Veeckmans G, et al. Early diagnosis of cerebral fat embolism syndrome by diffusion-weighted MRI (starfield pattern). Stroke. 2001;32(12):2942-2944.
15. Simon AD, Ulmer JL, Strottmann JM. Contrast-enhanced MR imaging of cerebral fat embolism: case report and review of the literature. AJNR Am J Neuroradiol. 2003;24(1):97-101.
16. Butteriss DJ, Mahad D, Soh C, Walls T, Weir D, Birchall D. Reversible cytotoxic cerebral edema in cerebral fat embolism. AJNR Am J Neuroradiol. 2006;27(3):620-623.
17. Zaitsu Y, Terae S, Kudo K, et al. Susceptibility-weighted imaging of cerebral fat embolism. J Comput Assist Tomogr. 2010;34(1):107-112.
18. Riska EB, Myllynen P. Fat embolism in patients with multiple injuries. J Trauma. 1982;22(11):891-894.
19. Svenningsen S, Nesse O, Finsen V, Hole A, Benum P. Prevention of fat embolism syndrome in patients with femoral fractures–immediate or delayed operative fixation? Ann Chir Gynaecol. 1987;76(3):163-166.
20. Pape HC, Tornetta P, Tarkin I, Tzioupis C, Sabeson V, Olson SA. Timing of fracture fixation in multitrauma patients: the role of early total care and damage control surgery. J Am Acad Orthop Surg. 2009;17(9):541-549.
21. Gosseling HR, Pellegrini VD Jr. Fat embolism syndrome: a review of the pathophysiology and physiological basis of treatment. Clin Orthop. 1982;165:68-82.
22. Bederman SS, Bhandari M, McKee MD, Schemitsch EH. Do corticosteroids reduce the risk of fat embolism syndrome in patients with long-bone fractures? A meta-analysis. Can J Surg. 2009:52(5):386-393.
Fat embolism syndrome (FES) was first described by Von Bergmann in 1873 in a patient with a fractured femur.1 While fat within the circulation (fat embolism) is relatively common following long-bone fracture, the clinical pattern of symptoms that make up FES is less so, occurring in 1% to 3% of isolated long-bone fractures and 5% to 10% of patients with multiple skeletal trauma.1 A variety of clinical, laboratory, and imaging criteria has been described, classically by Gurd in 1970 (Table).1-6 Most commonly, however, it is a diagnosis of exclusion when the classic triad of respiratory difficulty, neurologic abnormalities, and a characteristic petechial rash are present in the appropriate clinical setting.6
The neurologic sequelae of this syndrome can range from headache, confusion, and agitation to stupor, focal neurologic signs, and, less commonly, coma.7 Onset of these symptoms usually occurs between 24 hours and 48 hours (mean, 40 hours) after trauma.1 While these neurologic manifestations occur in up to 86% of patients with FES, it is rare for them to be present without the pulmonary symptoms of dyspnea, hypoxemia, and tachypnea, which are the most common presenting symptoms of the disease.1-6 In this case report, we describe severe, rapid-onset neurologic manifestations, without the typical pulmonary involvement, as the primary clinical presentation of FES in a polytrauma patient. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A previously healthy 50-year-old man presented to the emergency room in transfer from an outside hospital after a rollover motor vehicle collision in which he was ejected approximately 50 feet. Injuries included a right proximal humerus fracture/dislocation (Figure 1), right ulnar styloid fracture, L1 compression fracture, and multiple rib fractures. On admission, the patient had an ethanol level of 969 mg/L (.097%) and a urine drug screen positive only for opioids, presumably because of pain medication given that day. He denied a history of alcohol abuse and reported consuming 2 to 3 beers per week. The patient was awake, alert, and oriented with a Glasgow Coma Scale (GCS) of 15. He was tachycardic (heart rate, 126), tachypneic (respiratory rate, 24), and febrile (temperature, 38.6°C [101.5°F]), and his white blood cell count was elevated at 29.5×109/L. On examination, his right arm was found to be neurovascularly intact; it was placed in a sling with a forearm splint, and the patient was admitted to the intermediate special care unit on spine precautions with a plan for right shoulder hemiarthroplasty the following day.
Overnight the patient’s mental status began to deteriorate, and approximately 10 hours after initial assessment, he was not answering questions but was able to respond to some commands. On hospital day 2, approximately 20 hours after initial assessment, the patient had a GCS of 8, was not responding to commands, and moved only in response to painful stimuli. The patient had been prescribed morphine by patient-controlled analgesia and had received intravenous hydromorphone on the day of admission, although the amount of medication delivered was not thought adequate to explain this deterioration. On the morning of hospital day 2, noncontrast brain computed tomography (CT) was normal with no evidence of intracranial hemorrhage or infarct. This was followed by brain magnetic resonance imaging (MRI), with the T2-weighted images showing numerous, small hyperintense lesions in subcortical and periventricular white matter, corpus callosum, basal ganglia, brain stem, and cerebellar hemispheres (Figure 2). The lesions also showed hyperintensity on diffusion-weighted MRI and were interpreted to be consistent with multiple, tiny infarcts (Figure 3). In addition, susceptibility-weighted sequences showed low signal in the same areas, suggesting multiple microhemorrhages, a pattern consistent with FES. Oxygen saturations remained 95% to 99%, and chest radiograph revealed clear lung fields without infiltrate. On hospital day 2, the patient was transferred to the intensive care unit and intubated for airway protection owing to an inability to clear secretions, although arterial blood gas levels remained normal. An echocardiogram revealed no right-to-left shunt, such as a patent foramen ovale (PFO); an electroencephalogram showed no seizure-like activity. No petechial rash was noted on skin examination. The patient was treated with supportive care. Right shoulder hemiarthroplasty was performed on hospital day 7 without complications (Figure 1). On hospital day 13, the patient was following commands and on day 14 he was extubated. His mental status continued to improve, and he was discharged to a rehabilitation facility after 36 days. On last follow-up, 6 months after initial injury, the patient was recovering well with no residual neurologic deficits and only minor limitation in range of motion of the right shoulder.
Discussion
This case presented an interesting diagnostic challenge regarding the patient’s rapid decline in mental status, with a differential diagnosis including diffuse axonal injury (DAI), anoxic brain injury, posttraumatic seizure, other intracranial pathology, such as stroke or hemorrhage, and FES. FES was diagnosed, when other possibilities were ruled out, given the characteristic findings on brain MRI described above in the context of multiple fractures.
Pathophysiology
Despite its recognition in 1873, there is no consensus on the pathophysiological mechanism that causes the clinical symptoms of FES. In the setting of trauma, there are 2 predominant theories. The mechanical theory postulates that fat globules enter the circulation through disrupted venules after the fracture of marrow-containing bones, passing to the arterial circulation through pulmonary vasculature, or paradoxically, by way of a right-to-left shunt, such as a PFO.1,3 The presence of fat in the heart, visualized as echogenic material in the right and left atria on transesophageal echocardiography, has been confirmed in multiple studies during orthopedic procedures, including total knee arthroplasty and femoral reaming.8,9 These fat particles can lodge as microembolisms in target organs such as the skin and brain. However, autopsy studies have shown a lack of correlation of the severity of symptoms and the quantity of intravascular fat.1 In addition, the typical 24- to 72-hour delay in the onset of symptoms after initial trauma would argue against a solely mechanical explanation.10
Alternatively or concomitantly, the biochemical theory proposes that embolized fat may be degraded to toxic intermediaries, such as free fatty acids and C-reactive protein, which cause end-organ damage.3 This has been shown in an animal model, in which intravascular injection of free fatty acids was associated with endothelial damage and increased capillary permeability in the lung, leading to acute respiratory distress syndrome (ARDS).11 The same mechanism could explain injury to other end organs and is consistent with the delay in onset of symptoms after acute injury. In our patient’s case, the absence of pulmonary involvement, lack of a right-to-left vascular shunt such as a PFO, and presence of a systemic inflammatory response on admission may implicate the production of toxic intermediaries from the metabolism of embolized fat as the source of this patient’s FES.
Clinical Presentation
The initial presentation of FES usually manifests as respiratory distress and hypoxia.10 Chest radiographs are often normal, as in our patient, but can show bilateral diffuse interstitial or alveolar infiltrates.2,6 CT more often has findings, including bilateral ground-glass opacities with interlobar septal thickening.12 A petechial rash can be found on the head, neck, anterior thorax, axillae, subconjunctiva, and oral mucous membranes, although it occurs in only 20% to 50% of cases.1,2,13 Neurologic sequelae are present in up to 80% of patients,7 with onset typically following pulmonary symptoms.1,10 These sequelae can range from headache, confusion, and agitation to stupor, focal neurologic signs, and, less commonly, coma.7 Onset of symptoms generally occurs between 24 and 48 hours after trauma,1 although they have been reported as early as 12 hours.10 This case is an example of an atypical course, with the initial presentation of neurologic symptoms at approximately 14 hours after trauma with rapid progression to coma without classic pulmonary symptoms.
Diagnosis
Owing to the nonspecific clinical features of FES, a variety of clinical, laboratory, and imaging criteria has been described. Of these criteria, the most frequently referenced is by Gurd in 1970,4,5 who divided the features into major and minor, with 1 major and 4 minor features required to make the diagnosis (Table). In applying these criteria to our patient, we found that he exhibited the major criteria of cerebral involvement and minor criteria of tachycardia, fever, and thrombocytopenia. Respiratory insufficiency and petechial rash, as well as jaundice, renal changes, and anemia were negative features. Retinal changes, elevated erythrocyte sedimentation rate, and fat macroglobulinemia were not tested or examined. Although in our case the clinical and laboratory criteria for the diagnosis of FES as defined by Gurd were not met, the sensitivity of Gurd’s and other criteria is debated.10
Laboratory tests specific for the disease have not been developed. Although elevated serum levels of lipase, increased blood lipid levels, and fat globules in the urine, sputum, and blood have all been proposed, they are found in trauma patients with and without FES.2,5,6
The nonspecific nature of the signs and symptoms of FES and the lack of reliable laboratory tests for diagnosis of the syndrome highlight the importance of radiographic evaluation in patients with neurologic symptoms. Brain CT scans are usually negative,14 although, in some cases, they may show diffuse edema with scattered low attenuating areas and hemorrhage.15 MRI is more sensitive, and T2-weighted images typically reveal multiple small, nonconfluent hyperintense lesions, usually in the periventricular, subcortical, and deep white matter, sometimes referred to as the “starfield” pattern.14,16 The differential diagnosis for these findings is broad and, in addition to FES, includes DAI, vasogenic edema with microinfarcts, and demyelinating disease.14 Sensitivity and specificity may be increased with the addition of diffusion-weighted MRI, which shows scattered bright spots on a dark background in a similar “starfield” pattern as on T2-weighted images.15 Susceptibility-weighted MRI has recently been introduced as having utility in the diagnosis of FES, with areas of low-signal intensity indicating diffuse microhemorrhages.17 DAI can show a similar pattern; however, the autopsy-confirmed locations of the abnormalities are distinct, with those of FES being found in cerebral and cerebellar white matter and splenium of the corpus callosum and radiographic abnormalities of DAI being found in the gray-white matter junction, dorsolateral brainstem, and splenium of corpus callosum.17
Prevention and Treatment
Of primary importance in the prevention of FES is early stabilization of fractures. Several studies have shown a decreased incidence of FES when long-bone fractures are treated with immediate operative fixation.18,19 However, in the setting of polytrauma, the desire for early definitive treatment must be balanced against the risks for the exaggerated immune response from prolonged surgery.20 The timing of fracture fixation to prevent sequelae of the inflammatory response, such as ARDS and multiple organ dysfunction syndrome, is still debated. In a review article, Pape and colleagues20 suggest classifying the multiply injured patient as stable, borderline, unstable, and in extremis based on clinical and laboratory criteria. They recommend early definitive fixation for stable patients and those patients who are borderline or unstable and responsive to resuscitation, whereas damage-control orthopedics and staged fracture fixation should be considered in the other groups.
Several pharmacologic interventions have been described, although their effects are highly variable and none have clear indications.1-3,6 The most heavily researched is corticosteroids, with the proposed mechanisms of action including blunting of the inflammatory response, stabilizing the pulmonary capillary membrane to reduce interstitial edema, preventing activation of the complement system, and retarding platelet aggregation.21 A recent meta-analysis to assess this intervention examined 6 studies with a total of 386 patients with long-bone fractures who were randomized to treatment with corticosteroids or supportive care only.22 They found a reduced risk for FES in those patients who received corticosteroids, but there was no difference in mortality between groups. Given these results, the utility of corticosteroids is still debated.
Once FES has occurred, treatment options usually focus on supportive care, with most patients having a full recovery.1,3 No specific treatments are available, and symptomatic treatment is the suggested approach, including ensuring adequate oxygenation and ventilation and providing hemodynamic support and volume and blood-product resuscitation as needed.1-3,6
Conclusion
We have presented a case of FES unique in its rapid onset, an initial presentation with neurologic manifestations without typical pulmonary involvement, and the mechanism of end-organ damage without a right-to-left shunt. This case emphasizes the importance of considering FES in the patient with deteriorating mental status in the setting of multiple fractures, particularly in the absence of other characteristic clinical findings, such as pulmonary distress and the pathognomonic petechial rash. Brain MRI can play an important role in diagnosing those patients presenting with predominantly neurological symptoms. Early recognition of this condition allows for the anticipation of complications of the disease process, such as respiratory distress, and the potential need for mechanical ventilation and hemodynamic support.
Fat embolism syndrome (FES) was first described by Von Bergmann in 1873 in a patient with a fractured femur.1 While fat within the circulation (fat embolism) is relatively common following long-bone fracture, the clinical pattern of symptoms that make up FES is less so, occurring in 1% to 3% of isolated long-bone fractures and 5% to 10% of patients with multiple skeletal trauma.1 A variety of clinical, laboratory, and imaging criteria has been described, classically by Gurd in 1970 (Table).1-6 Most commonly, however, it is a diagnosis of exclusion when the classic triad of respiratory difficulty, neurologic abnormalities, and a characteristic petechial rash are present in the appropriate clinical setting.6
The neurologic sequelae of this syndrome can range from headache, confusion, and agitation to stupor, focal neurologic signs, and, less commonly, coma.7 Onset of these symptoms usually occurs between 24 hours and 48 hours (mean, 40 hours) after trauma.1 While these neurologic manifestations occur in up to 86% of patients with FES, it is rare for them to be present without the pulmonary symptoms of dyspnea, hypoxemia, and tachypnea, which are the most common presenting symptoms of the disease.1-6 In this case report, we describe severe, rapid-onset neurologic manifestations, without the typical pulmonary involvement, as the primary clinical presentation of FES in a polytrauma patient. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A previously healthy 50-year-old man presented to the emergency room in transfer from an outside hospital after a rollover motor vehicle collision in which he was ejected approximately 50 feet. Injuries included a right proximal humerus fracture/dislocation (Figure 1), right ulnar styloid fracture, L1 compression fracture, and multiple rib fractures. On admission, the patient had an ethanol level of 969 mg/L (.097%) and a urine drug screen positive only for opioids, presumably because of pain medication given that day. He denied a history of alcohol abuse and reported consuming 2 to 3 beers per week. The patient was awake, alert, and oriented with a Glasgow Coma Scale (GCS) of 15. He was tachycardic (heart rate, 126), tachypneic (respiratory rate, 24), and febrile (temperature, 38.6°C [101.5°F]), and his white blood cell count was elevated at 29.5×109/L. On examination, his right arm was found to be neurovascularly intact; it was placed in a sling with a forearm splint, and the patient was admitted to the intermediate special care unit on spine precautions with a plan for right shoulder hemiarthroplasty the following day.
Overnight the patient’s mental status began to deteriorate, and approximately 10 hours after initial assessment, he was not answering questions but was able to respond to some commands. On hospital day 2, approximately 20 hours after initial assessment, the patient had a GCS of 8, was not responding to commands, and moved only in response to painful stimuli. The patient had been prescribed morphine by patient-controlled analgesia and had received intravenous hydromorphone on the day of admission, although the amount of medication delivered was not thought adequate to explain this deterioration. On the morning of hospital day 2, noncontrast brain computed tomography (CT) was normal with no evidence of intracranial hemorrhage or infarct. This was followed by brain magnetic resonance imaging (MRI), with the T2-weighted images showing numerous, small hyperintense lesions in subcortical and periventricular white matter, corpus callosum, basal ganglia, brain stem, and cerebellar hemispheres (Figure 2). The lesions also showed hyperintensity on diffusion-weighted MRI and were interpreted to be consistent with multiple, tiny infarcts (Figure 3). In addition, susceptibility-weighted sequences showed low signal in the same areas, suggesting multiple microhemorrhages, a pattern consistent with FES. Oxygen saturations remained 95% to 99%, and chest radiograph revealed clear lung fields without infiltrate. On hospital day 2, the patient was transferred to the intensive care unit and intubated for airway protection owing to an inability to clear secretions, although arterial blood gas levels remained normal. An echocardiogram revealed no right-to-left shunt, such as a patent foramen ovale (PFO); an electroencephalogram showed no seizure-like activity. No petechial rash was noted on skin examination. The patient was treated with supportive care. Right shoulder hemiarthroplasty was performed on hospital day 7 without complications (Figure 1). On hospital day 13, the patient was following commands and on day 14 he was extubated. His mental status continued to improve, and he was discharged to a rehabilitation facility after 36 days. On last follow-up, 6 months after initial injury, the patient was recovering well with no residual neurologic deficits and only minor limitation in range of motion of the right shoulder.
Discussion
This case presented an interesting diagnostic challenge regarding the patient’s rapid decline in mental status, with a differential diagnosis including diffuse axonal injury (DAI), anoxic brain injury, posttraumatic seizure, other intracranial pathology, such as stroke or hemorrhage, and FES. FES was diagnosed, when other possibilities were ruled out, given the characteristic findings on brain MRI described above in the context of multiple fractures.
Pathophysiology
Despite its recognition in 1873, there is no consensus on the pathophysiological mechanism that causes the clinical symptoms of FES. In the setting of trauma, there are 2 predominant theories. The mechanical theory postulates that fat globules enter the circulation through disrupted venules after the fracture of marrow-containing bones, passing to the arterial circulation through pulmonary vasculature, or paradoxically, by way of a right-to-left shunt, such as a PFO.1,3 The presence of fat in the heart, visualized as echogenic material in the right and left atria on transesophageal echocardiography, has been confirmed in multiple studies during orthopedic procedures, including total knee arthroplasty and femoral reaming.8,9 These fat particles can lodge as microembolisms in target organs such as the skin and brain. However, autopsy studies have shown a lack of correlation of the severity of symptoms and the quantity of intravascular fat.1 In addition, the typical 24- to 72-hour delay in the onset of symptoms after initial trauma would argue against a solely mechanical explanation.10
Alternatively or concomitantly, the biochemical theory proposes that embolized fat may be degraded to toxic intermediaries, such as free fatty acids and C-reactive protein, which cause end-organ damage.3 This has been shown in an animal model, in which intravascular injection of free fatty acids was associated with endothelial damage and increased capillary permeability in the lung, leading to acute respiratory distress syndrome (ARDS).11 The same mechanism could explain injury to other end organs and is consistent with the delay in onset of symptoms after acute injury. In our patient’s case, the absence of pulmonary involvement, lack of a right-to-left vascular shunt such as a PFO, and presence of a systemic inflammatory response on admission may implicate the production of toxic intermediaries from the metabolism of embolized fat as the source of this patient’s FES.
Clinical Presentation
The initial presentation of FES usually manifests as respiratory distress and hypoxia.10 Chest radiographs are often normal, as in our patient, but can show bilateral diffuse interstitial or alveolar infiltrates.2,6 CT more often has findings, including bilateral ground-glass opacities with interlobar septal thickening.12 A petechial rash can be found on the head, neck, anterior thorax, axillae, subconjunctiva, and oral mucous membranes, although it occurs in only 20% to 50% of cases.1,2,13 Neurologic sequelae are present in up to 80% of patients,7 with onset typically following pulmonary symptoms.1,10 These sequelae can range from headache, confusion, and agitation to stupor, focal neurologic signs, and, less commonly, coma.7 Onset of symptoms generally occurs between 24 and 48 hours after trauma,1 although they have been reported as early as 12 hours.10 This case is an example of an atypical course, with the initial presentation of neurologic symptoms at approximately 14 hours after trauma with rapid progression to coma without classic pulmonary symptoms.
Diagnosis
Owing to the nonspecific clinical features of FES, a variety of clinical, laboratory, and imaging criteria has been described. Of these criteria, the most frequently referenced is by Gurd in 1970,4,5 who divided the features into major and minor, with 1 major and 4 minor features required to make the diagnosis (Table). In applying these criteria to our patient, we found that he exhibited the major criteria of cerebral involvement and minor criteria of tachycardia, fever, and thrombocytopenia. Respiratory insufficiency and petechial rash, as well as jaundice, renal changes, and anemia were negative features. Retinal changes, elevated erythrocyte sedimentation rate, and fat macroglobulinemia were not tested or examined. Although in our case the clinical and laboratory criteria for the diagnosis of FES as defined by Gurd were not met, the sensitivity of Gurd’s and other criteria is debated.10
Laboratory tests specific for the disease have not been developed. Although elevated serum levels of lipase, increased blood lipid levels, and fat globules in the urine, sputum, and blood have all been proposed, they are found in trauma patients with and without FES.2,5,6
The nonspecific nature of the signs and symptoms of FES and the lack of reliable laboratory tests for diagnosis of the syndrome highlight the importance of radiographic evaluation in patients with neurologic symptoms. Brain CT scans are usually negative,14 although, in some cases, they may show diffuse edema with scattered low attenuating areas and hemorrhage.15 MRI is more sensitive, and T2-weighted images typically reveal multiple small, nonconfluent hyperintense lesions, usually in the periventricular, subcortical, and deep white matter, sometimes referred to as the “starfield” pattern.14,16 The differential diagnosis for these findings is broad and, in addition to FES, includes DAI, vasogenic edema with microinfarcts, and demyelinating disease.14 Sensitivity and specificity may be increased with the addition of diffusion-weighted MRI, which shows scattered bright spots on a dark background in a similar “starfield” pattern as on T2-weighted images.15 Susceptibility-weighted MRI has recently been introduced as having utility in the diagnosis of FES, with areas of low-signal intensity indicating diffuse microhemorrhages.17 DAI can show a similar pattern; however, the autopsy-confirmed locations of the abnormalities are distinct, with those of FES being found in cerebral and cerebellar white matter and splenium of the corpus callosum and radiographic abnormalities of DAI being found in the gray-white matter junction, dorsolateral brainstem, and splenium of corpus callosum.17
Prevention and Treatment
Of primary importance in the prevention of FES is early stabilization of fractures. Several studies have shown a decreased incidence of FES when long-bone fractures are treated with immediate operative fixation.18,19 However, in the setting of polytrauma, the desire for early definitive treatment must be balanced against the risks for the exaggerated immune response from prolonged surgery.20 The timing of fracture fixation to prevent sequelae of the inflammatory response, such as ARDS and multiple organ dysfunction syndrome, is still debated. In a review article, Pape and colleagues20 suggest classifying the multiply injured patient as stable, borderline, unstable, and in extremis based on clinical and laboratory criteria. They recommend early definitive fixation for stable patients and those patients who are borderline or unstable and responsive to resuscitation, whereas damage-control orthopedics and staged fracture fixation should be considered in the other groups.
Several pharmacologic interventions have been described, although their effects are highly variable and none have clear indications.1-3,6 The most heavily researched is corticosteroids, with the proposed mechanisms of action including blunting of the inflammatory response, stabilizing the pulmonary capillary membrane to reduce interstitial edema, preventing activation of the complement system, and retarding platelet aggregation.21 A recent meta-analysis to assess this intervention examined 6 studies with a total of 386 patients with long-bone fractures who were randomized to treatment with corticosteroids or supportive care only.22 They found a reduced risk for FES in those patients who received corticosteroids, but there was no difference in mortality between groups. Given these results, the utility of corticosteroids is still debated.
Once FES has occurred, treatment options usually focus on supportive care, with most patients having a full recovery.1,3 No specific treatments are available, and symptomatic treatment is the suggested approach, including ensuring adequate oxygenation and ventilation and providing hemodynamic support and volume and blood-product resuscitation as needed.1-3,6
Conclusion
We have presented a case of FES unique in its rapid onset, an initial presentation with neurologic manifestations without typical pulmonary involvement, and the mechanism of end-organ damage without a right-to-left shunt. This case emphasizes the importance of considering FES in the patient with deteriorating mental status in the setting of multiple fractures, particularly in the absence of other characteristic clinical findings, such as pulmonary distress and the pathognomonic petechial rash. Brain MRI can play an important role in diagnosing those patients presenting with predominantly neurological symptoms. Early recognition of this condition allows for the anticipation of complications of the disease process, such as respiratory distress, and the potential need for mechanical ventilation and hemodynamic support.
1. Johnson MJ, Lucas GL. Fat embolism syndrome. Orthopedics. 1996;19(1):41-49.
2. Levy D. The fat embolism syndrome. A review. Clin Orthop. 1990;261:281-286.
3. Mellor A, Soni N. Fat embolism. Anaesthesia. 2001;56(2):145-154.
4. Gurd AR. Fat embolism: an aid to diagnosis. J Bone Joint Surg Br. 1970:52(4):732-737.
5. Gurd AR, Wilson RI. The fat embolism syndrome. J Bone Joint Surg Br. 1974;56(3):408-416.
6. Bulger EM, Smith DG, Maier RV, Jurkovich GJ. Fat embolism syndrome. A 10-year review. Arch Surg. 1997;132(4):435-439.
7. Jacobson DM, Terrence CF, Reinmuth OM. The neurologic manifestations of fat embolism. Neurology. 1986;36(6):847-851.
8. Sulek CA, Davies LK, Enneking FK, Gearen PA, Lobato EB. Cerebral microembolism diagnosed by transcranial Doppler during total knee arthroplasty: correlation with transesophageal echocardiography. Anesthesiology. 1999;91(3):672-676.
9. Volgas DA, Burch T, Stannard JP, Ellis T, Bilotta J, Alonso JE. Fat embolus in femur fractures: a comparison of two reaming systems. Injury. 2010;41(Suppl 2):S90-S93.
10. Gupta B, D’souza N, Sawhney C, et al. Analyzing fat embolism syndrome in trauma patients at AIIMS Apex Trauma Center, New Delhi, India. J Emerg Trauma Shock. 2011;4(3):337–341.
11. King EG, Wagner WW Jr, Ashbaugh DG, Latham LP, Halsey DR. Alterations in pulmonary microanatomy after fat embolism. In vivo observations via thoracic window of the oleic acid-embolized canine lung. Chest. 1971:59(5):524-530.
12. Malagari K, Economopoulos N, Stoupis C, et al. High-resolution CT findings in mild pulmonary fat embolism. Chest. 2003:123(4):1196-1201.
13. King MB, Harmon KR. Unusual forms of pulmonary embolism. Clin Chest Med. 1994;15(3):561-580.
14. Parizel PM, Demey HE, Veeckmans G, et al. Early diagnosis of cerebral fat embolism syndrome by diffusion-weighted MRI (starfield pattern). Stroke. 2001;32(12):2942-2944.
15. Simon AD, Ulmer JL, Strottmann JM. Contrast-enhanced MR imaging of cerebral fat embolism: case report and review of the literature. AJNR Am J Neuroradiol. 2003;24(1):97-101.
16. Butteriss DJ, Mahad D, Soh C, Walls T, Weir D, Birchall D. Reversible cytotoxic cerebral edema in cerebral fat embolism. AJNR Am J Neuroradiol. 2006;27(3):620-623.
17. Zaitsu Y, Terae S, Kudo K, et al. Susceptibility-weighted imaging of cerebral fat embolism. J Comput Assist Tomogr. 2010;34(1):107-112.
18. Riska EB, Myllynen P. Fat embolism in patients with multiple injuries. J Trauma. 1982;22(11):891-894.
19. Svenningsen S, Nesse O, Finsen V, Hole A, Benum P. Prevention of fat embolism syndrome in patients with femoral fractures–immediate or delayed operative fixation? Ann Chir Gynaecol. 1987;76(3):163-166.
20. Pape HC, Tornetta P, Tarkin I, Tzioupis C, Sabeson V, Olson SA. Timing of fracture fixation in multitrauma patients: the role of early total care and damage control surgery. J Am Acad Orthop Surg. 2009;17(9):541-549.
21. Gosseling HR, Pellegrini VD Jr. Fat embolism syndrome: a review of the pathophysiology and physiological basis of treatment. Clin Orthop. 1982;165:68-82.
22. Bederman SS, Bhandari M, McKee MD, Schemitsch EH. Do corticosteroids reduce the risk of fat embolism syndrome in patients with long-bone fractures? A meta-analysis. Can J Surg. 2009:52(5):386-393.
1. Johnson MJ, Lucas GL. Fat embolism syndrome. Orthopedics. 1996;19(1):41-49.
2. Levy D. The fat embolism syndrome. A review. Clin Orthop. 1990;261:281-286.
3. Mellor A, Soni N. Fat embolism. Anaesthesia. 2001;56(2):145-154.
4. Gurd AR. Fat embolism: an aid to diagnosis. J Bone Joint Surg Br. 1970:52(4):732-737.
5. Gurd AR, Wilson RI. The fat embolism syndrome. J Bone Joint Surg Br. 1974;56(3):408-416.
6. Bulger EM, Smith DG, Maier RV, Jurkovich GJ. Fat embolism syndrome. A 10-year review. Arch Surg. 1997;132(4):435-439.
7. Jacobson DM, Terrence CF, Reinmuth OM. The neurologic manifestations of fat embolism. Neurology. 1986;36(6):847-851.
8. Sulek CA, Davies LK, Enneking FK, Gearen PA, Lobato EB. Cerebral microembolism diagnosed by transcranial Doppler during total knee arthroplasty: correlation with transesophageal echocardiography. Anesthesiology. 1999;91(3):672-676.
9. Volgas DA, Burch T, Stannard JP, Ellis T, Bilotta J, Alonso JE. Fat embolus in femur fractures: a comparison of two reaming systems. Injury. 2010;41(Suppl 2):S90-S93.
10. Gupta B, D’souza N, Sawhney C, et al. Analyzing fat embolism syndrome in trauma patients at AIIMS Apex Trauma Center, New Delhi, India. J Emerg Trauma Shock. 2011;4(3):337–341.
11. King EG, Wagner WW Jr, Ashbaugh DG, Latham LP, Halsey DR. Alterations in pulmonary microanatomy after fat embolism. In vivo observations via thoracic window of the oleic acid-embolized canine lung. Chest. 1971:59(5):524-530.
12. Malagari K, Economopoulos N, Stoupis C, et al. High-resolution CT findings in mild pulmonary fat embolism. Chest. 2003:123(4):1196-1201.
13. King MB, Harmon KR. Unusual forms of pulmonary embolism. Clin Chest Med. 1994;15(3):561-580.
14. Parizel PM, Demey HE, Veeckmans G, et al. Early diagnosis of cerebral fat embolism syndrome by diffusion-weighted MRI (starfield pattern). Stroke. 2001;32(12):2942-2944.
15. Simon AD, Ulmer JL, Strottmann JM. Contrast-enhanced MR imaging of cerebral fat embolism: case report and review of the literature. AJNR Am J Neuroradiol. 2003;24(1):97-101.
16. Butteriss DJ, Mahad D, Soh C, Walls T, Weir D, Birchall D. Reversible cytotoxic cerebral edema in cerebral fat embolism. AJNR Am J Neuroradiol. 2006;27(3):620-623.
17. Zaitsu Y, Terae S, Kudo K, et al. Susceptibility-weighted imaging of cerebral fat embolism. J Comput Assist Tomogr. 2010;34(1):107-112.
18. Riska EB, Myllynen P. Fat embolism in patients with multiple injuries. J Trauma. 1982;22(11):891-894.
19. Svenningsen S, Nesse O, Finsen V, Hole A, Benum P. Prevention of fat embolism syndrome in patients with femoral fractures–immediate or delayed operative fixation? Ann Chir Gynaecol. 1987;76(3):163-166.
20. Pape HC, Tornetta P, Tarkin I, Tzioupis C, Sabeson V, Olson SA. Timing of fracture fixation in multitrauma patients: the role of early total care and damage control surgery. J Am Acad Orthop Surg. 2009;17(9):541-549.
21. Gosseling HR, Pellegrini VD Jr. Fat embolism syndrome: a review of the pathophysiology and physiological basis of treatment. Clin Orthop. 1982;165:68-82.
22. Bederman SS, Bhandari M, McKee MD, Schemitsch EH. Do corticosteroids reduce the risk of fat embolism syndrome in patients with long-bone fractures? A meta-analysis. Can J Surg. 2009:52(5):386-393.
Evaluation of Wound Healing After Direct Anterior Total Hip Arthroplasty With Use of a Novel Retraction Device
It is thought that, by placing more emphasis on soft-tissue preservation, minimally invasive surgery total hip arthroplasty (MIS-THA) results in less soft-tissue trauma, less blood loss, and earlier recovery.1-3 Despite these improvements over standard methods, there is a concern that the vigorous retraction needed for proper visualization through smaller incisions could injure soft tissues.4-7 Single-incision direct anterior THA (DA-THA) has gained in popularity because of the true intermuscular/internervous plane through which the procedure can be performed with relatively minimal muscle dissection using MIS techniques.8,9 This approach may offer quicker recovery and superior stability in comparison with nonintermuscular methods, which unavoidably cause more muscle damage.10-12
Although the evidence of these early gains is encouraging, several studies have found high complication rates with DA-THA.8,13-17 Noted disadvantages include a steep learning curve, lateral femoral cutaneous neurapraxia, need for a specialized table, and higher fracture and wound complication rates. Not surprisingly, with increased surgeon experience, the complication rate decreased substantially.14,15 However, wound-related complications remained steady, with 2 recent large studies reporting rates of 4.6% and 2.1%.14,15 The thin anterior skin, high tensional forces along the groin crease and perpendicular to the typical DA incision, and less resilient soft-tissue envelope are postulated reasons for wound-related issues, which are likely magnified in patients who are more obese.15,16
A novel device designed to lessen tissue damage is the ring retractor (Figure 1). Used initially in general surgery and obstetrics, it consists of 2 semirigid polymer rings connected by a flexible cylindrical polymer membrane.18-20 The lower ring is tucked and anchored underneath the wound edge, and then the upper ring is rolled down and cinched onto the skin. The resultant tension on the polymer sleeve—imparted by the rigidity of the ring—provides strong, evenly distributed wound-edge retraction. It also provides a physical barrier between the wound edge and the rest of the operative field. Proponents of the ring retractor claim increased wound-edge moisture, less bruising, and reduced local trauma compared with standard metal retractors alone.
Wound-edge retractor forces are doubled during MIS-THA compared with conventional THA.14-20 This may explain reports of worse scar cosmesis with MIS-THA. Given the theoretical benefits of minimized wound-edge trauma, the ring retractor may improve scar appearance compared with standard retraction alone. Any clinically relevant effect on cosmesis should be readily apparent to justify use of the retractor in this regard. Although some surgeons routinely use the device for primary THA, it has not been the subject of any recent orthopedic studies.
In the present study, we prospectively investigated wound cosmesis with and without use of the ring retractor in patients undergoing DA-THA.
Materials and Methods
This prospective, single-center, randomized study was reviewed and approved by the institutional review board at our facility. Consent was obtained from all participating patients.
We evaluated 50 surgical incisions in 48 patients. Eligible participants were over age 18 years and undergoing primary DA-THA. Exclusion criteria included previous surgery on the affected hip, a pathological hip condition requiring an extensile exposure, systemic inflammatory illness, chronic corticosteroid use, and dermatologic abnormality of the incisional area. One patient was having simultaneous bilateral THAs, and another was having staged bilateral THAs. Each hip in these patients was given its own case number and treated separately. Of the 49 patients who met all the inclusion criteria, only 1 decided not to participate (Figure 2).
Stratified randomization with permuted block size (sex, body mass index [BMI]) was used to assign patients in a 1:1 ratio to either the treatment group or the control group. In the treatment group, the Protractor Incision Protector and Retractor (Gyrus ACMI, Southborough, Massachusetts) was used with standard metal retractors. In the control group, only standard metal retractors were used. Patients were blinded to their group assignments, and surgeons were informed about each assignment only after the initial incision was made.
Clinical research investigators were blinded to the groups’ prospectively collected data. Collection time points were preoperative clinical visit, day of surgery through discharge, and 2-, 6-, and 12-week postoperative follow-ups. Day-of-surgery data included estimated intraoperative blood loss, operative side, operative time, intraoperative complications, and American Society of Anesthesiologists (ASA) physical status classification. Total length of stay, pain scores (range, 0-10), estimated drain output, and blood-transfusion data were also recorded. To evaluate whether the device had any effect on short-term functional outcome, we collected Harris Hip Scores (HHS) and Short Form–12 (SF-12, Version 2) scores at the preoperative and 6-week postoperative visits. We also documented any wound-healing-related issues or complications that occurred up until the final visit.
To account for any effect of nutrition status on wound healing, we obtained pre-albumin and albumin levels and absolute lymphocyte counts from the preoperative electronic records. We used an albumin level under 3.5 g/dL and an absolute lymphocyte count under 1500/µL for our analysis, as these cutoffs have been associated with wound complications after primary THA.21 There is no similarly established threshold for pre-albumin level, so we used values under 20 mg/L based on comparable literature.22,23
At each postoperative visit, standardized high-resolution images were obtained. At the 12-week visit, patients completed 2 Likert scales regarding their overall opinion of their scars and how their scars compared with their expectations. They also ranked 5 separate THA-related outcomes in order of importance (Appendix).
Photographs were evaluated by 2 blinded plastic surgeons (Dr. Friedman and Dr. Jack) using 2 grading systems—the Stony Brook Scar Evaluation Scale (SBSES)24 (Table 1) and a modified Manchester Scar Scale (MSS)25 (Table 2). We used these systems because they were photograph-based, psychometrically studied, and specifically designed to assess surgical incision healing with established validity and reliability.24-27 A particular advantage, strictly related to cosmetic outcome, is their validity in scoring scars from high-definition photographs in a different place or time. The SBSES, an ordinal wound evaluation scale that measures short-term cosmetic outcomes, consists of 6 items, each receiving 1 or 0 point, yielding a total score between 0 (worst) and 5 (best). The modified MSS includes a visual analog scale (VAS), which has a vertical hash marked on a 10-cm line and is scored between 0 (excellent) and 10 (poor) to 1 decimal point.26,28 This value is added to grades on color, surface appearance, contour, and distortion, resulting in a score between 4 (best) and 24 (worst). The primary outcome measures were Likert-scale responses obtained at final visit and SBSES/MSS scores for each visit; 12-week scores were the primary end point.
Operative Procedure
Experienced fellowship-trained orthopedic surgeons performed all procedures. A modified Hueter approach was used for exposure.9 Mean incision length was about 12 cm. For the treatment group, the ring retractor was inserted at the level of the tensor fascia, with the inferior ring resting between the fascia and the subcutaneous layer and the superior ring cinched over the skin (Figure 3). The device is made in 4 different sizes for incisions from 2.5 to 17 cm; our study population required only 1 size. Otherwise, the surgical protocol was based on that described by Matta and colleagues.8 Wound closure (over a drain) was performed according to a standardized protocol—running No. 1 Vicryl suture for the superficial tensor fascia, interrupted 2-0 Vicryl for the deep dermal layer, and subcutaneous 4-0 Monocryl for the skin followed by application of Dermabond (Ethicon, Somerville, New Jersey) and Tegaderm +Pad (3M, St. Paul, Minnesota) for outer dressing, which was replaced on postoperative day 2 and removed at the 2-week visit.
Statistical Methods
An a priori sample-size calculation was performed. Power performed in a base of a prior study that evaluated anterolateral and posterolateral THA scars using a VAS, a component of the MSS, suggested a sample size of 16 per group to detect the minimal clinically important difference of 1.5 cm: SD (σ) = 1.5 cm, α = 0.05, β = 0.20.29,30 In addition, a general estimate for detecting a 1-unit change on an ordinal scale (σ = 1.0, α = 0.05, β = 0.20) resulted in the same number. We conservatively decided to enroll 25 patients per arm in case of larger true variance.
The Wilcoxon rank sum test was used for comparisons of continuous data between groups. Differences between means were analyzed with 2-sided t tests. Categorical data were compared with the Pearson χ2 test or the Fisher exact test, as indicated. Ordinal ranking scores were compared with the Mantel-Haenszel test. Multivariate logistic regression was applied to identify the significant independent predictors of better scar grades for each surgeon by considering candidate variables with Ps < .20 in the univariate analysis.
Results
We found no differences in demographic or perioperative characteristics between treatment and control groups (Tables 3, 4). The groups showed similar mean improvements in their respective 6-week HHS (38.7 and 36.4 points; P = .65), SF-12 physical component summary scores (11.8 and 14.5 points; P = .37), and SF-12 mental component summary scores (5.1 and 3.7; P = .70).
Patient questionnaire outcomes are listed in Table 5. For the control group, 25/25 image sets were obtained at the 2-week visit, 25/25 at the 6-week visit, and 24/25 at the 12-week visit. For the treatment group, there were 23/25, 24/25, and 23/25 images sets, respectively.
When surgeon scoring was analyzed separately, SBSES and MSS scores were similar between treatment and control groups, with 1 exception: 2-week MSS scores were better for the treatment group according to surgeon A (P = .026). When grades were averaged, SBSES scores were again similar at all time points (Figure 4A); MSS scores were better for the treatment group at 2 weeks (P = .036) and equivalent at all other time points (Figure 4B). For the SBSES, Spearman correlation coefficient ρ with 95% confidence interval (CI) was 0.37
(95% CI, 0.08-0.66) at 2 weeks, 0.48 (95% CI, 0.20-0.76) at 6 weeks, and 0.62 (95% CI, 0.33-0.91) at 12 weeks. Following the same pattern for the MSS, ρ was 0.20 (95% CI, –0.09 to 0.49), 0.51 (95% CI, 0.23-0.79), and 0.32 (95% CI, 0.03-0.61).
Independent multivariate analysis revealed that age over 65 years was a significant predictor of worse scores. On SBSES, the odds ratio (OR) was 1.15 (95% CI, 1.07-1.24) for surgeon A and 1.11 (95% CI, 1.05-1.18) for surgeon B. On MSS, the OR was 0.89 (95% CI, 0.84-0.94) for surgeon A and 0.95 (95% CI, 0.91-0.99) for surgeon B. The likelihood of having worse SBSES scores according to surgeon A was 4.72 times higher if the pre-albumin level was under 20 mg/L (95% CI, 1.15-19.36). Albumin level under 3.5 g/dL and absolute lymphocyte count under 1500 cells/µL were not found to be independent predictors of poorer scores.
Patients’ overall opinion (P = .63) and assessment of their scars relative to expectations (P = .25) on the Likert scales were not different between groups. More scars exceeded patients’ expectations and had more excellent ratings in the control group. The 2 groups were similar with regard to relative importance of various patient-related outcomes. Factors most important to overall outcome were relief of hip pain, followed by implant longevity and length of recovery. Least important were incision-related variables.
There were only 3 minor noninfectious wound complications (6%), 2 in the treatment group and 1 in the control group. In the treatment group, a 67-year-old man with diabetes (ASA class III; BMI, 32.1 kg/m2; received transfusion) had 2 small areas (<5 mm) of superficial ulceration at 6-week follow-up—one at the proximal aspect of the incision and the other near the midpoint along the flexion crease. Both lesions resolved by 12-week follow-up. Also in the treatment group, a 77-year-old woman (ASA class II; BMI, 24.9 kg/m2; received transfusion) at 6 weeks had a spitting suture, which was removed in clinic without further issue. In the control group, a 55-year-old woman (ASA class II; BMI, 27.4 kg/m2) had a suture reaction near the proximal aspect of her incision 3 weeks after surgery. This reaction, which presented as a mild, localized erythema without pain, tenderness, or drainage, resolved by 6-week follow-up. None of these wound complications required intervention beyond observation.
Discussion
This study was designed to provide a bipartisan measure of wound-healing cosmesis after DA-THA. Scar evaluation by blinded plastic surgeons served as a standardized, clinical assessment, whereas the patient questionnaire offered a more subjective appraisal. The modified MMS25 and the SBSES24 are the only 2 wound-grading systems designed and validated for photographic assessment of postsurgical scars. Most scar evaluation schemes pertain to burn or traumatic scars.26,27,31 As a result, many earlier studies intending to compare incisional scars used poorly suited evaluation systems.
The current literature includes reports on 3 studies with scoring-based scar assessment in THA; all used grading systems designed for either burns or traumatic wounds, but 2 also used a VAS.32-34 VASs have been validated for measuring wound cosmesis but are entirely subjective and without structure and provide no feedback as to why a scar was rated good or bad.24 Mow and colleagues32 prospectively compared scars after standard posterior or MIS approaches and found no differences according to a scoring system intended for burn scars. In our study population, we found no group differences in patients’ cosmesis of their scars.
Although scars can take a year or longer to fully mature, researchers from the University of Michigan discovered that scar appearance at 1 year correlates highly with cosmesis 12 weeks after closure, though poorly with cosmesis 10 days after closure.35 Therefore, any observed differences in scar cosmesis between groups at 12-week follow-up would likely persist, whereas differences at 2-week follow-up would have little bearing on ultimate appearance. For this reason, our primary outcome measure was healing process and cosmesis at 12 weeks. High wound complication rates have been reported for MIS-DA-THA.8,14-16 Jewett and Collis15 noted a 4.6% wound complication rate (3% noninfectious ulcerative dehiscence, 1.6% superficial infection), which is comparable to the 6% rate found in this study. However, there likely is some variability across studies in what constitutes a wound complication or superficial infection. Of our 3 wound complications—stitch reaction, spitting suture, small noninfectious ulceration—only the ulceration was of a severity similar to that reported by Jewett and Collis.15 Matta and colleagues8 reported only 3 wound complications (in 494 patients), all severe enough to require operative intervention. One explanation for this low complication rate is use of a ring retractor, as it is routinely depicted in their technique paper. However, no specific reference is made to gauge how often the device was used.
Rates of superficial infection after DA-THA range from 0.6% to 1.6% in 3 large observational studies (combined deep infection rate, 0.43%).8,14,15 In 2 of these studies, all patients with superficial infection underwent formal débridement, though none developed deep infection. A prospective randomized study of 221 patients who underwent colorectal surgery—where perioperative infectious morbidity ranges from 25% to 50%—found that ring retractor use significantly reduced superficial wound infection rates (8.1% vs 0%). A significant reduction in wound infection was shown in a similarly designed study involving 48 patients who had open appendectomy (14.6% vs 1.6%). The device had no effect on deep infection in either general surgery study. The wound infection rates reported in these general surgery studies are markedly higher than those in our study population. As a result, the effect of the ring retractor on wound infection in DA-THA may be less. Regardless of the effect on deep infection, fewer superficial infections, which often require operative intervention, would be of considerable benefit.
Below-threshold albumin level and absolute lymphocyte count have been associated with wound-healing complications after hip replacement.21 In the present study, pre-albumin level under 20 mg/L was the only nutritional marker predictive of poor wound appearance, but this finding was seen only in SBSES scores from surgeon A. Subgroup analysis did not reveal any relationship between wound appearance and any of the recorded demographic or perioperative variables, but for a small predictive influence with age over 65 years.
This study had some limitations. Our findings cannot be generalized to all patients who undergo THA, as only DA incisions were studied. Results also may not be generalizable to non-fellowship-trained orthopedists. In addition, selection bias likely resulted from including patients already selected for the DA approach. Using digital images for evaluation (vs real-life evaluation) may have affected reliability as well. Last, by not incorporating texture, we omitted a potentially informative feature from scoring.
It is paramount that surgeons undergo diligent training before undertaking this approach for minimizing unwanted results; furthermore, higher early complication rates level off with increased surgeon experience.14,36,37 We recommend meticulous soft-tissue handling, cautious retraction, and careful patient selection (relative contraindication for patients with an abdominal pannus overlying the incision) as primary measures for minimizing incisional trauma and potential wound-healing complications.38 Preservation of the tensor fascia is also crucial,39 as it is the only closable layer separating deep and superficial compartments. Without good closure of the tensor fascia, there is no containment or tamponade of deep bleeding, which can facilitate hematoma formation.
In the population studied, we found no significant long-term differences in cosmetic appearance (based on clinician or patient evaluation) between wounds managed with and without the ring retractor. Our data do not support routine use of the ring retractor, during DA-THA, for improved wound cosmesis. Whether the device has any significant role in reducing the number of wound complications in THA is yet to be determined. Last, the ring retractor may have a role in other areas of orthopedic surgery, such as hip fractures in the elderly or orthopedic oncology. In situations like these, where adequate nutrition and immunocompetency may be lacking, the added protection provided by the device may translate into a more notable benefit than in elective THA.
1. Laffosse JM, Chiron P, Tricoire JL, Giordano G, Molinier F, Puget J. Prospective and comparative study of minimally invasive posterior approach versus standard posterior approach in total hip replacement [in French]. Rev Chir Orthop Reparatrice Appar Mot. 2007;93(3):228-237.
2. Smith TO, Blake V, Hing CB. Minimally invasive versus conventional exposure for total hip arthroplasty: a systematic review and meta-analysis of clinical and radiological outcomes. Int Orthop. 2011;35(2):173-184.
3. Wright JM, Crockett HC, Delgado S, Lyman S, Madsen M, Sculco TP. Mini-incision for total hip arthroplasty: a prospective, controlled investigation with 5-year follow-up evaluation. J Arthroplasty. 2004;19(5):538-545.
4. Mardones R, Pagnano MW, Nemanich JP, Trousdale RT. The Frank Stinchfield Award: muscle damage after total hip arthroplasty done with the two-incision and mini-posterior techniques. Clin Orthop. 2005;(441):63-67.
5. Müller M, Tohtz S, Dewey M, Springer I, Perka C. Age-related appearance of muscle trauma in primary total hip arthroplasty and the benefit of a minimally invasive approach for patients older than 70 years. Int Orthop. 2011;35(2):165-171.
6. Noble PC, Johnston JD, Alexander JA, et al. Making minimally invasive THR safe: conclusions from biomechanical simulation and analysis. Int Orthop. 2007;31(suppl 1):S25-S28.
7. Bremer AK, Kalberer F, Pfirrmann CW, Dora C. Soft-tissue changes in hip abductor muscles and tendons after total hip replacement: comparison between the direct anterior and the transgluteal approaches. J Bone Joint Surg Br. 2011;93(7):886-889.
8. Matta JM, Shahrdar C, Ferguson T. Single-incision anterior approach for total hip arthroplasty on an orthopaedic table. Clin Orthop. 2005;(441):115-124.
9. Rachbauer F, Kain MSH, Leunig M. The history of the anterior approach to the hip. Orthop Clin North Am. 2009;40(3):311-320.
10. Bergin PF, Doppelt JD, Kephart CJ, et al. Comparison of minimally invasive direct anterior versus posterior total hip arthroplasty based on inflammation and muscle damage markers. J Bone Joint Surg Am. 2011;93(15):1392-1398.
11. Mayr E, Nogler M, Benedetti MG, et al. A prospective randomized assessment of earlier functional recovery in THA patients treated by minimally invasive direct anterior approach: a gait analysis study. Clin Biomech. 2009;24(10):812-818.
12. Meneghini RM, Pagnano MW, Trousdale RT, Hozack WJ. Muscle damage during MIS total hip arthroplasty: Smith-Petersen versus posterior approach. Clin Orthop. 2006;(453):293-298.
13. Sculco TP. Anterior approach in THA improves outcomes: opposes. Orthopedics. 2011;34(9):e459-e461.
14. Bhandari M, Matta JM, Dodgin D, et al; Anterior Total Hip Arthroplasty Collaborative Investigators. Outcomes following the single-incision anterior approach to total hip arthroplasty: a multicenter observational study. Orthop Clin North Am. 2009;40(3):329-342.
15. Jewett BA, Collis DK. High complication rate with anterior total hip arthroplasties on a fracture table. Clin Orthop. 2011;469(2):503-507.
16. Barton C, Kim PR. Complications of the direct anterior approach for total hip arthroplasty. Orthop Clin North Am. 2009;40(3):371-375.
17. Bender B, Nogler M, Hozack WJ. Direct anterior approach for total hip arthroplasty. Orthop Clin North Am. 2009;40(3):321-328.
18. Pelosi MA 2nd, Pelosi MA 3rd. Self-retaining abdominal retractor for minilaparotomy. Obstet Gynecol. 2000;96(5, pt 1):775-778.
19. Lee P, Waxman K, Taylor B, Yim S. Use of wound-protection system and postoperative wound-infection rates in open appendectomy: a randomized prospective trial. Arch Surg. 2009;144(9):872-875.
20. Horiuchi T, Tanishima H, Tamagawa K, et al. Randomized, controlled investigation of the anti-infective properties of the Alexis retractor/protector of incision sites. J Trauma. 2007;62(1):212-215.
21. Greene KA, Wilde AH, Stulberg BN. Preoperative nutritional status of total joint patients. Relationship to postoperative wound complications. J Arthroplasty. 1991;6(4):321-325.
22. Alijanipour P, Heller S, Parvizi J. Prevention of periprosthetic joint infection: what are the effective strategies? J Knee Surg. 2014;27(4):251-258.
23. Suarez JC, Slotkin EM, Alvarez AM, Szubski CR, Barsoum WK, Patel PD. Prospective, randomized trial to evaluate efficacy of a thrombin-based hemostaticagent in total knee arthroplasty. J Arthroplasty. 2014;29(10):1950-1955.
24. Singer AJ, Arora B, Dagum A, Valentine S, Hollander JE. Development and validation of a novel scar evaluation scale. Plast Reconstr Surg. 2007;120(7):1892-1897.
25. Beausang E, Floyd H, Dunn KW, Orton CI, Ferguson MW. A new quantitative scale for clinical scar assessment. Plast Reconstr Surg. 1998;102(6):1954-1961.
26. Durani P, McGrouther DA, Ferguson MW. Current scales for assessing human scarring: a review. J Plast Reconstr Aesthet Surg. 2009;62(6):713-720.
27. Fearmonti R, Bond J, Erdmann D, Levinson H. A review of scar scales and scar measuring devices. Eplasty. 2010;10:e43.
28. Duncan JA, Bond JS, Mason T, et al. Visual analogue scale scoring and ranking: a suitable and sensitive method for assessing scar quality? Plast Reconstr Surg. 2006;118(4):909-918.
29. Quinn JV, Wells GA. An assessment of clinical wound evaluation scales. Acad Emerg Med. 1998;5(6):583-586.
30. Livesey C, Wylde V, Descamps S, et al. Skin closure after total hip replacement: a randomised controlled trial of skin adhesive versus surgical staples. J Bone Joint Surg Br. 2009;91(6):725-729.
31. Atiyeh BS. Nonsurgical management of hypertrophic scars: evidence-based therapies, standard practices, and emerging methods. Aesthetic Plast Surg. 2007;31(5):468-492.
32. Mow CS, Woolson ST, Ngarmukos SG, Park EH, Lorenz HP. Comparison of scars from total hip replacements done with a standard or a mini-incision. Clin Orthop. 2005;(441):80-85.
33. Khan RJ, Fick D, Yao F, et al. A comparison of three methods of wound closure following arthroplasty: a prospective, randomised, controlled trial. J Bone Joint Surg Br. 2006;88(2):238-242.
34. Goldstein WM, Ali R, Branson JJ, Berland KA. Comparison of patient satisfaction with incision cosmesis after standard and minimally invasive total hip arthroplasty. Orthopedics. 2008;31(4):368.
35. Quinn J, Wells G, Sutcliffe T, et al. Tissue adhesive versus suture wound repair at 1 year: randomized clinical trial correlating early, 3-month, and 1-year cosmetic outcome. Ann Emerg Med. 1998;32(6):645-649.
36. Alberti LR, Petroianu A, Zac RI, Andrade JC Jr. The effect of surgical procedures on serum albumin concentration. Chirurgia (Bucur). 2008;103(1):39-43.
37. Berend KR, Lombardi AV Jr, Seng BE, Adams JB. Enhanced early outcomes with the anterior supine intermuscular approach in primary total hip arthroplasty. J Bone Joint Surg Am. 2009;91(suppl 6):107-120.
38. Mutnal A, Patel P, Cardona L, Suarez J. Periprosthetic Propionibacterium granulosum joint infection after direct anterior total hip arthroplasty: a case report. JBJS Case Connector. 2011;1(2):e10.
39. Alvarez AM, Suarez JC, Patel P, Benton EG. Fluoroscopic imaging of acetabular cup position during THA through a direct anterior approach. Orthopedics. 2013;36(10):776-777. Erratum in: Orthopedics. 2014;37(1):16.
It is thought that, by placing more emphasis on soft-tissue preservation, minimally invasive surgery total hip arthroplasty (MIS-THA) results in less soft-tissue trauma, less blood loss, and earlier recovery.1-3 Despite these improvements over standard methods, there is a concern that the vigorous retraction needed for proper visualization through smaller incisions could injure soft tissues.4-7 Single-incision direct anterior THA (DA-THA) has gained in popularity because of the true intermuscular/internervous plane through which the procedure can be performed with relatively minimal muscle dissection using MIS techniques.8,9 This approach may offer quicker recovery and superior stability in comparison with nonintermuscular methods, which unavoidably cause more muscle damage.10-12
Although the evidence of these early gains is encouraging, several studies have found high complication rates with DA-THA.8,13-17 Noted disadvantages include a steep learning curve, lateral femoral cutaneous neurapraxia, need for a specialized table, and higher fracture and wound complication rates. Not surprisingly, with increased surgeon experience, the complication rate decreased substantially.14,15 However, wound-related complications remained steady, with 2 recent large studies reporting rates of 4.6% and 2.1%.14,15 The thin anterior skin, high tensional forces along the groin crease and perpendicular to the typical DA incision, and less resilient soft-tissue envelope are postulated reasons for wound-related issues, which are likely magnified in patients who are more obese.15,16
A novel device designed to lessen tissue damage is the ring retractor (Figure 1). Used initially in general surgery and obstetrics, it consists of 2 semirigid polymer rings connected by a flexible cylindrical polymer membrane.18-20 The lower ring is tucked and anchored underneath the wound edge, and then the upper ring is rolled down and cinched onto the skin. The resultant tension on the polymer sleeve—imparted by the rigidity of the ring—provides strong, evenly distributed wound-edge retraction. It also provides a physical barrier between the wound edge and the rest of the operative field. Proponents of the ring retractor claim increased wound-edge moisture, less bruising, and reduced local trauma compared with standard metal retractors alone.
Wound-edge retractor forces are doubled during MIS-THA compared with conventional THA.14-20 This may explain reports of worse scar cosmesis with MIS-THA. Given the theoretical benefits of minimized wound-edge trauma, the ring retractor may improve scar appearance compared with standard retraction alone. Any clinically relevant effect on cosmesis should be readily apparent to justify use of the retractor in this regard. Although some surgeons routinely use the device for primary THA, it has not been the subject of any recent orthopedic studies.
In the present study, we prospectively investigated wound cosmesis with and without use of the ring retractor in patients undergoing DA-THA.
Materials and Methods
This prospective, single-center, randomized study was reviewed and approved by the institutional review board at our facility. Consent was obtained from all participating patients.
We evaluated 50 surgical incisions in 48 patients. Eligible participants were over age 18 years and undergoing primary DA-THA. Exclusion criteria included previous surgery on the affected hip, a pathological hip condition requiring an extensile exposure, systemic inflammatory illness, chronic corticosteroid use, and dermatologic abnormality of the incisional area. One patient was having simultaneous bilateral THAs, and another was having staged bilateral THAs. Each hip in these patients was given its own case number and treated separately. Of the 49 patients who met all the inclusion criteria, only 1 decided not to participate (Figure 2).
Stratified randomization with permuted block size (sex, body mass index [BMI]) was used to assign patients in a 1:1 ratio to either the treatment group or the control group. In the treatment group, the Protractor Incision Protector and Retractor (Gyrus ACMI, Southborough, Massachusetts) was used with standard metal retractors. In the control group, only standard metal retractors were used. Patients were blinded to their group assignments, and surgeons were informed about each assignment only after the initial incision was made.
Clinical research investigators were blinded to the groups’ prospectively collected data. Collection time points were preoperative clinical visit, day of surgery through discharge, and 2-, 6-, and 12-week postoperative follow-ups. Day-of-surgery data included estimated intraoperative blood loss, operative side, operative time, intraoperative complications, and American Society of Anesthesiologists (ASA) physical status classification. Total length of stay, pain scores (range, 0-10), estimated drain output, and blood-transfusion data were also recorded. To evaluate whether the device had any effect on short-term functional outcome, we collected Harris Hip Scores (HHS) and Short Form–12 (SF-12, Version 2) scores at the preoperative and 6-week postoperative visits. We also documented any wound-healing-related issues or complications that occurred up until the final visit.
To account for any effect of nutrition status on wound healing, we obtained pre-albumin and albumin levels and absolute lymphocyte counts from the preoperative electronic records. We used an albumin level under 3.5 g/dL and an absolute lymphocyte count under 1500/µL for our analysis, as these cutoffs have been associated with wound complications after primary THA.21 There is no similarly established threshold for pre-albumin level, so we used values under 20 mg/L based on comparable literature.22,23
At each postoperative visit, standardized high-resolution images were obtained. At the 12-week visit, patients completed 2 Likert scales regarding their overall opinion of their scars and how their scars compared with their expectations. They also ranked 5 separate THA-related outcomes in order of importance (Appendix).
Photographs were evaluated by 2 blinded plastic surgeons (Dr. Friedman and Dr. Jack) using 2 grading systems—the Stony Brook Scar Evaluation Scale (SBSES)24 (Table 1) and a modified Manchester Scar Scale (MSS)25 (Table 2). We used these systems because they were photograph-based, psychometrically studied, and specifically designed to assess surgical incision healing with established validity and reliability.24-27 A particular advantage, strictly related to cosmetic outcome, is their validity in scoring scars from high-definition photographs in a different place or time. The SBSES, an ordinal wound evaluation scale that measures short-term cosmetic outcomes, consists of 6 items, each receiving 1 or 0 point, yielding a total score between 0 (worst) and 5 (best). The modified MSS includes a visual analog scale (VAS), which has a vertical hash marked on a 10-cm line and is scored between 0 (excellent) and 10 (poor) to 1 decimal point.26,28 This value is added to grades on color, surface appearance, contour, and distortion, resulting in a score between 4 (best) and 24 (worst). The primary outcome measures were Likert-scale responses obtained at final visit and SBSES/MSS scores for each visit; 12-week scores were the primary end point.
Operative Procedure
Experienced fellowship-trained orthopedic surgeons performed all procedures. A modified Hueter approach was used for exposure.9 Mean incision length was about 12 cm. For the treatment group, the ring retractor was inserted at the level of the tensor fascia, with the inferior ring resting between the fascia and the subcutaneous layer and the superior ring cinched over the skin (Figure 3). The device is made in 4 different sizes for incisions from 2.5 to 17 cm; our study population required only 1 size. Otherwise, the surgical protocol was based on that described by Matta and colleagues.8 Wound closure (over a drain) was performed according to a standardized protocol—running No. 1 Vicryl suture for the superficial tensor fascia, interrupted 2-0 Vicryl for the deep dermal layer, and subcutaneous 4-0 Monocryl for the skin followed by application of Dermabond (Ethicon, Somerville, New Jersey) and Tegaderm +Pad (3M, St. Paul, Minnesota) for outer dressing, which was replaced on postoperative day 2 and removed at the 2-week visit.
Statistical Methods
An a priori sample-size calculation was performed. Power performed in a base of a prior study that evaluated anterolateral and posterolateral THA scars using a VAS, a component of the MSS, suggested a sample size of 16 per group to detect the minimal clinically important difference of 1.5 cm: SD (σ) = 1.5 cm, α = 0.05, β = 0.20.29,30 In addition, a general estimate for detecting a 1-unit change on an ordinal scale (σ = 1.0, α = 0.05, β = 0.20) resulted in the same number. We conservatively decided to enroll 25 patients per arm in case of larger true variance.
The Wilcoxon rank sum test was used for comparisons of continuous data between groups. Differences between means were analyzed with 2-sided t tests. Categorical data were compared with the Pearson χ2 test or the Fisher exact test, as indicated. Ordinal ranking scores were compared with the Mantel-Haenszel test. Multivariate logistic regression was applied to identify the significant independent predictors of better scar grades for each surgeon by considering candidate variables with Ps < .20 in the univariate analysis.
Results
We found no differences in demographic or perioperative characteristics between treatment and control groups (Tables 3, 4). The groups showed similar mean improvements in their respective 6-week HHS (38.7 and 36.4 points; P = .65), SF-12 physical component summary scores (11.8 and 14.5 points; P = .37), and SF-12 mental component summary scores (5.1 and 3.7; P = .70).
Patient questionnaire outcomes are listed in Table 5. For the control group, 25/25 image sets were obtained at the 2-week visit, 25/25 at the 6-week visit, and 24/25 at the 12-week visit. For the treatment group, there were 23/25, 24/25, and 23/25 images sets, respectively.
When surgeon scoring was analyzed separately, SBSES and MSS scores were similar between treatment and control groups, with 1 exception: 2-week MSS scores were better for the treatment group according to surgeon A (P = .026). When grades were averaged, SBSES scores were again similar at all time points (Figure 4A); MSS scores were better for the treatment group at 2 weeks (P = .036) and equivalent at all other time points (Figure 4B). For the SBSES, Spearman correlation coefficient ρ with 95% confidence interval (CI) was 0.37
(95% CI, 0.08-0.66) at 2 weeks, 0.48 (95% CI, 0.20-0.76) at 6 weeks, and 0.62 (95% CI, 0.33-0.91) at 12 weeks. Following the same pattern for the MSS, ρ was 0.20 (95% CI, –0.09 to 0.49), 0.51 (95% CI, 0.23-0.79), and 0.32 (95% CI, 0.03-0.61).
Independent multivariate analysis revealed that age over 65 years was a significant predictor of worse scores. On SBSES, the odds ratio (OR) was 1.15 (95% CI, 1.07-1.24) for surgeon A and 1.11 (95% CI, 1.05-1.18) for surgeon B. On MSS, the OR was 0.89 (95% CI, 0.84-0.94) for surgeon A and 0.95 (95% CI, 0.91-0.99) for surgeon B. The likelihood of having worse SBSES scores according to surgeon A was 4.72 times higher if the pre-albumin level was under 20 mg/L (95% CI, 1.15-19.36). Albumin level under 3.5 g/dL and absolute lymphocyte count under 1500 cells/µL were not found to be independent predictors of poorer scores.
Patients’ overall opinion (P = .63) and assessment of their scars relative to expectations (P = .25) on the Likert scales were not different between groups. More scars exceeded patients’ expectations and had more excellent ratings in the control group. The 2 groups were similar with regard to relative importance of various patient-related outcomes. Factors most important to overall outcome were relief of hip pain, followed by implant longevity and length of recovery. Least important were incision-related variables.
There were only 3 minor noninfectious wound complications (6%), 2 in the treatment group and 1 in the control group. In the treatment group, a 67-year-old man with diabetes (ASA class III; BMI, 32.1 kg/m2; received transfusion) had 2 small areas (<5 mm) of superficial ulceration at 6-week follow-up—one at the proximal aspect of the incision and the other near the midpoint along the flexion crease. Both lesions resolved by 12-week follow-up. Also in the treatment group, a 77-year-old woman (ASA class II; BMI, 24.9 kg/m2; received transfusion) at 6 weeks had a spitting suture, which was removed in clinic without further issue. In the control group, a 55-year-old woman (ASA class II; BMI, 27.4 kg/m2) had a suture reaction near the proximal aspect of her incision 3 weeks after surgery. This reaction, which presented as a mild, localized erythema without pain, tenderness, or drainage, resolved by 6-week follow-up. None of these wound complications required intervention beyond observation.
Discussion
This study was designed to provide a bipartisan measure of wound-healing cosmesis after DA-THA. Scar evaluation by blinded plastic surgeons served as a standardized, clinical assessment, whereas the patient questionnaire offered a more subjective appraisal. The modified MMS25 and the SBSES24 are the only 2 wound-grading systems designed and validated for photographic assessment of postsurgical scars. Most scar evaluation schemes pertain to burn or traumatic scars.26,27,31 As a result, many earlier studies intending to compare incisional scars used poorly suited evaluation systems.
The current literature includes reports on 3 studies with scoring-based scar assessment in THA; all used grading systems designed for either burns or traumatic wounds, but 2 also used a VAS.32-34 VASs have been validated for measuring wound cosmesis but are entirely subjective and without structure and provide no feedback as to why a scar was rated good or bad.24 Mow and colleagues32 prospectively compared scars after standard posterior or MIS approaches and found no differences according to a scoring system intended for burn scars. In our study population, we found no group differences in patients’ cosmesis of their scars.
Although scars can take a year or longer to fully mature, researchers from the University of Michigan discovered that scar appearance at 1 year correlates highly with cosmesis 12 weeks after closure, though poorly with cosmesis 10 days after closure.35 Therefore, any observed differences in scar cosmesis between groups at 12-week follow-up would likely persist, whereas differences at 2-week follow-up would have little bearing on ultimate appearance. For this reason, our primary outcome measure was healing process and cosmesis at 12 weeks. High wound complication rates have been reported for MIS-DA-THA.8,14-16 Jewett and Collis15 noted a 4.6% wound complication rate (3% noninfectious ulcerative dehiscence, 1.6% superficial infection), which is comparable to the 6% rate found in this study. However, there likely is some variability across studies in what constitutes a wound complication or superficial infection. Of our 3 wound complications—stitch reaction, spitting suture, small noninfectious ulceration—only the ulceration was of a severity similar to that reported by Jewett and Collis.15 Matta and colleagues8 reported only 3 wound complications (in 494 patients), all severe enough to require operative intervention. One explanation for this low complication rate is use of a ring retractor, as it is routinely depicted in their technique paper. However, no specific reference is made to gauge how often the device was used.
Rates of superficial infection after DA-THA range from 0.6% to 1.6% in 3 large observational studies (combined deep infection rate, 0.43%).8,14,15 In 2 of these studies, all patients with superficial infection underwent formal débridement, though none developed deep infection. A prospective randomized study of 221 patients who underwent colorectal surgery—where perioperative infectious morbidity ranges from 25% to 50%—found that ring retractor use significantly reduced superficial wound infection rates (8.1% vs 0%). A significant reduction in wound infection was shown in a similarly designed study involving 48 patients who had open appendectomy (14.6% vs 1.6%). The device had no effect on deep infection in either general surgery study. The wound infection rates reported in these general surgery studies are markedly higher than those in our study population. As a result, the effect of the ring retractor on wound infection in DA-THA may be less. Regardless of the effect on deep infection, fewer superficial infections, which often require operative intervention, would be of considerable benefit.
Below-threshold albumin level and absolute lymphocyte count have been associated with wound-healing complications after hip replacement.21 In the present study, pre-albumin level under 20 mg/L was the only nutritional marker predictive of poor wound appearance, but this finding was seen only in SBSES scores from surgeon A. Subgroup analysis did not reveal any relationship between wound appearance and any of the recorded demographic or perioperative variables, but for a small predictive influence with age over 65 years.
This study had some limitations. Our findings cannot be generalized to all patients who undergo THA, as only DA incisions were studied. Results also may not be generalizable to non-fellowship-trained orthopedists. In addition, selection bias likely resulted from including patients already selected for the DA approach. Using digital images for evaluation (vs real-life evaluation) may have affected reliability as well. Last, by not incorporating texture, we omitted a potentially informative feature from scoring.
It is paramount that surgeons undergo diligent training before undertaking this approach for minimizing unwanted results; furthermore, higher early complication rates level off with increased surgeon experience.14,36,37 We recommend meticulous soft-tissue handling, cautious retraction, and careful patient selection (relative contraindication for patients with an abdominal pannus overlying the incision) as primary measures for minimizing incisional trauma and potential wound-healing complications.38 Preservation of the tensor fascia is also crucial,39 as it is the only closable layer separating deep and superficial compartments. Without good closure of the tensor fascia, there is no containment or tamponade of deep bleeding, which can facilitate hematoma formation.
In the population studied, we found no significant long-term differences in cosmetic appearance (based on clinician or patient evaluation) between wounds managed with and without the ring retractor. Our data do not support routine use of the ring retractor, during DA-THA, for improved wound cosmesis. Whether the device has any significant role in reducing the number of wound complications in THA is yet to be determined. Last, the ring retractor may have a role in other areas of orthopedic surgery, such as hip fractures in the elderly or orthopedic oncology. In situations like these, where adequate nutrition and immunocompetency may be lacking, the added protection provided by the device may translate into a more notable benefit than in elective THA.
It is thought that, by placing more emphasis on soft-tissue preservation, minimally invasive surgery total hip arthroplasty (MIS-THA) results in less soft-tissue trauma, less blood loss, and earlier recovery.1-3 Despite these improvements over standard methods, there is a concern that the vigorous retraction needed for proper visualization through smaller incisions could injure soft tissues.4-7 Single-incision direct anterior THA (DA-THA) has gained in popularity because of the true intermuscular/internervous plane through which the procedure can be performed with relatively minimal muscle dissection using MIS techniques.8,9 This approach may offer quicker recovery and superior stability in comparison with nonintermuscular methods, which unavoidably cause more muscle damage.10-12
Although the evidence of these early gains is encouraging, several studies have found high complication rates with DA-THA.8,13-17 Noted disadvantages include a steep learning curve, lateral femoral cutaneous neurapraxia, need for a specialized table, and higher fracture and wound complication rates. Not surprisingly, with increased surgeon experience, the complication rate decreased substantially.14,15 However, wound-related complications remained steady, with 2 recent large studies reporting rates of 4.6% and 2.1%.14,15 The thin anterior skin, high tensional forces along the groin crease and perpendicular to the typical DA incision, and less resilient soft-tissue envelope are postulated reasons for wound-related issues, which are likely magnified in patients who are more obese.15,16
A novel device designed to lessen tissue damage is the ring retractor (Figure 1). Used initially in general surgery and obstetrics, it consists of 2 semirigid polymer rings connected by a flexible cylindrical polymer membrane.18-20 The lower ring is tucked and anchored underneath the wound edge, and then the upper ring is rolled down and cinched onto the skin. The resultant tension on the polymer sleeve—imparted by the rigidity of the ring—provides strong, evenly distributed wound-edge retraction. It also provides a physical barrier between the wound edge and the rest of the operative field. Proponents of the ring retractor claim increased wound-edge moisture, less bruising, and reduced local trauma compared with standard metal retractors alone.
Wound-edge retractor forces are doubled during MIS-THA compared with conventional THA.14-20 This may explain reports of worse scar cosmesis with MIS-THA. Given the theoretical benefits of minimized wound-edge trauma, the ring retractor may improve scar appearance compared with standard retraction alone. Any clinically relevant effect on cosmesis should be readily apparent to justify use of the retractor in this regard. Although some surgeons routinely use the device for primary THA, it has not been the subject of any recent orthopedic studies.
In the present study, we prospectively investigated wound cosmesis with and without use of the ring retractor in patients undergoing DA-THA.
Materials and Methods
This prospective, single-center, randomized study was reviewed and approved by the institutional review board at our facility. Consent was obtained from all participating patients.
We evaluated 50 surgical incisions in 48 patients. Eligible participants were over age 18 years and undergoing primary DA-THA. Exclusion criteria included previous surgery on the affected hip, a pathological hip condition requiring an extensile exposure, systemic inflammatory illness, chronic corticosteroid use, and dermatologic abnormality of the incisional area. One patient was having simultaneous bilateral THAs, and another was having staged bilateral THAs. Each hip in these patients was given its own case number and treated separately. Of the 49 patients who met all the inclusion criteria, only 1 decided not to participate (Figure 2).
Stratified randomization with permuted block size (sex, body mass index [BMI]) was used to assign patients in a 1:1 ratio to either the treatment group or the control group. In the treatment group, the Protractor Incision Protector and Retractor (Gyrus ACMI, Southborough, Massachusetts) was used with standard metal retractors. In the control group, only standard metal retractors were used. Patients were blinded to their group assignments, and surgeons were informed about each assignment only after the initial incision was made.
Clinical research investigators were blinded to the groups’ prospectively collected data. Collection time points were preoperative clinical visit, day of surgery through discharge, and 2-, 6-, and 12-week postoperative follow-ups. Day-of-surgery data included estimated intraoperative blood loss, operative side, operative time, intraoperative complications, and American Society of Anesthesiologists (ASA) physical status classification. Total length of stay, pain scores (range, 0-10), estimated drain output, and blood-transfusion data were also recorded. To evaluate whether the device had any effect on short-term functional outcome, we collected Harris Hip Scores (HHS) and Short Form–12 (SF-12, Version 2) scores at the preoperative and 6-week postoperative visits. We also documented any wound-healing-related issues or complications that occurred up until the final visit.
To account for any effect of nutrition status on wound healing, we obtained pre-albumin and albumin levels and absolute lymphocyte counts from the preoperative electronic records. We used an albumin level under 3.5 g/dL and an absolute lymphocyte count under 1500/µL for our analysis, as these cutoffs have been associated with wound complications after primary THA.21 There is no similarly established threshold for pre-albumin level, so we used values under 20 mg/L based on comparable literature.22,23
At each postoperative visit, standardized high-resolution images were obtained. At the 12-week visit, patients completed 2 Likert scales regarding their overall opinion of their scars and how their scars compared with their expectations. They also ranked 5 separate THA-related outcomes in order of importance (Appendix).
Photographs were evaluated by 2 blinded plastic surgeons (Dr. Friedman and Dr. Jack) using 2 grading systems—the Stony Brook Scar Evaluation Scale (SBSES)24 (Table 1) and a modified Manchester Scar Scale (MSS)25 (Table 2). We used these systems because they were photograph-based, psychometrically studied, and specifically designed to assess surgical incision healing with established validity and reliability.24-27 A particular advantage, strictly related to cosmetic outcome, is their validity in scoring scars from high-definition photographs in a different place or time. The SBSES, an ordinal wound evaluation scale that measures short-term cosmetic outcomes, consists of 6 items, each receiving 1 or 0 point, yielding a total score between 0 (worst) and 5 (best). The modified MSS includes a visual analog scale (VAS), which has a vertical hash marked on a 10-cm line and is scored between 0 (excellent) and 10 (poor) to 1 decimal point.26,28 This value is added to grades on color, surface appearance, contour, and distortion, resulting in a score between 4 (best) and 24 (worst). The primary outcome measures were Likert-scale responses obtained at final visit and SBSES/MSS scores for each visit; 12-week scores were the primary end point.
Operative Procedure
Experienced fellowship-trained orthopedic surgeons performed all procedures. A modified Hueter approach was used for exposure.9 Mean incision length was about 12 cm. For the treatment group, the ring retractor was inserted at the level of the tensor fascia, with the inferior ring resting between the fascia and the subcutaneous layer and the superior ring cinched over the skin (Figure 3). The device is made in 4 different sizes for incisions from 2.5 to 17 cm; our study population required only 1 size. Otherwise, the surgical protocol was based on that described by Matta and colleagues.8 Wound closure (over a drain) was performed according to a standardized protocol—running No. 1 Vicryl suture for the superficial tensor fascia, interrupted 2-0 Vicryl for the deep dermal layer, and subcutaneous 4-0 Monocryl for the skin followed by application of Dermabond (Ethicon, Somerville, New Jersey) and Tegaderm +Pad (3M, St. Paul, Minnesota) for outer dressing, which was replaced on postoperative day 2 and removed at the 2-week visit.
Statistical Methods
An a priori sample-size calculation was performed. Power performed in a base of a prior study that evaluated anterolateral and posterolateral THA scars using a VAS, a component of the MSS, suggested a sample size of 16 per group to detect the minimal clinically important difference of 1.5 cm: SD (σ) = 1.5 cm, α = 0.05, β = 0.20.29,30 In addition, a general estimate for detecting a 1-unit change on an ordinal scale (σ = 1.0, α = 0.05, β = 0.20) resulted in the same number. We conservatively decided to enroll 25 patients per arm in case of larger true variance.
The Wilcoxon rank sum test was used for comparisons of continuous data between groups. Differences between means were analyzed with 2-sided t tests. Categorical data were compared with the Pearson χ2 test or the Fisher exact test, as indicated. Ordinal ranking scores were compared with the Mantel-Haenszel test. Multivariate logistic regression was applied to identify the significant independent predictors of better scar grades for each surgeon by considering candidate variables with Ps < .20 in the univariate analysis.
Results
We found no differences in demographic or perioperative characteristics between treatment and control groups (Tables 3, 4). The groups showed similar mean improvements in their respective 6-week HHS (38.7 and 36.4 points; P = .65), SF-12 physical component summary scores (11.8 and 14.5 points; P = .37), and SF-12 mental component summary scores (5.1 and 3.7; P = .70).
Patient questionnaire outcomes are listed in Table 5. For the control group, 25/25 image sets were obtained at the 2-week visit, 25/25 at the 6-week visit, and 24/25 at the 12-week visit. For the treatment group, there were 23/25, 24/25, and 23/25 images sets, respectively.
When surgeon scoring was analyzed separately, SBSES and MSS scores were similar between treatment and control groups, with 1 exception: 2-week MSS scores were better for the treatment group according to surgeon A (P = .026). When grades were averaged, SBSES scores were again similar at all time points (Figure 4A); MSS scores were better for the treatment group at 2 weeks (P = .036) and equivalent at all other time points (Figure 4B). For the SBSES, Spearman correlation coefficient ρ with 95% confidence interval (CI) was 0.37
(95% CI, 0.08-0.66) at 2 weeks, 0.48 (95% CI, 0.20-0.76) at 6 weeks, and 0.62 (95% CI, 0.33-0.91) at 12 weeks. Following the same pattern for the MSS, ρ was 0.20 (95% CI, –0.09 to 0.49), 0.51 (95% CI, 0.23-0.79), and 0.32 (95% CI, 0.03-0.61).
Independent multivariate analysis revealed that age over 65 years was a significant predictor of worse scores. On SBSES, the odds ratio (OR) was 1.15 (95% CI, 1.07-1.24) for surgeon A and 1.11 (95% CI, 1.05-1.18) for surgeon B. On MSS, the OR was 0.89 (95% CI, 0.84-0.94) for surgeon A and 0.95 (95% CI, 0.91-0.99) for surgeon B. The likelihood of having worse SBSES scores according to surgeon A was 4.72 times higher if the pre-albumin level was under 20 mg/L (95% CI, 1.15-19.36). Albumin level under 3.5 g/dL and absolute lymphocyte count under 1500 cells/µL were not found to be independent predictors of poorer scores.
Patients’ overall opinion (P = .63) and assessment of their scars relative to expectations (P = .25) on the Likert scales were not different between groups. More scars exceeded patients’ expectations and had more excellent ratings in the control group. The 2 groups were similar with regard to relative importance of various patient-related outcomes. Factors most important to overall outcome were relief of hip pain, followed by implant longevity and length of recovery. Least important were incision-related variables.
There were only 3 minor noninfectious wound complications (6%), 2 in the treatment group and 1 in the control group. In the treatment group, a 67-year-old man with diabetes (ASA class III; BMI, 32.1 kg/m2; received transfusion) had 2 small areas (<5 mm) of superficial ulceration at 6-week follow-up—one at the proximal aspect of the incision and the other near the midpoint along the flexion crease. Both lesions resolved by 12-week follow-up. Also in the treatment group, a 77-year-old woman (ASA class II; BMI, 24.9 kg/m2; received transfusion) at 6 weeks had a spitting suture, which was removed in clinic without further issue. In the control group, a 55-year-old woman (ASA class II; BMI, 27.4 kg/m2) had a suture reaction near the proximal aspect of her incision 3 weeks after surgery. This reaction, which presented as a mild, localized erythema without pain, tenderness, or drainage, resolved by 6-week follow-up. None of these wound complications required intervention beyond observation.
Discussion
This study was designed to provide a bipartisan measure of wound-healing cosmesis after DA-THA. Scar evaluation by blinded plastic surgeons served as a standardized, clinical assessment, whereas the patient questionnaire offered a more subjective appraisal. The modified MMS25 and the SBSES24 are the only 2 wound-grading systems designed and validated for photographic assessment of postsurgical scars. Most scar evaluation schemes pertain to burn or traumatic scars.26,27,31 As a result, many earlier studies intending to compare incisional scars used poorly suited evaluation systems.
The current literature includes reports on 3 studies with scoring-based scar assessment in THA; all used grading systems designed for either burns or traumatic wounds, but 2 also used a VAS.32-34 VASs have been validated for measuring wound cosmesis but are entirely subjective and without structure and provide no feedback as to why a scar was rated good or bad.24 Mow and colleagues32 prospectively compared scars after standard posterior or MIS approaches and found no differences according to a scoring system intended for burn scars. In our study population, we found no group differences in patients’ cosmesis of their scars.
Although scars can take a year or longer to fully mature, researchers from the University of Michigan discovered that scar appearance at 1 year correlates highly with cosmesis 12 weeks after closure, though poorly with cosmesis 10 days after closure.35 Therefore, any observed differences in scar cosmesis between groups at 12-week follow-up would likely persist, whereas differences at 2-week follow-up would have little bearing on ultimate appearance. For this reason, our primary outcome measure was healing process and cosmesis at 12 weeks. High wound complication rates have been reported for MIS-DA-THA.8,14-16 Jewett and Collis15 noted a 4.6% wound complication rate (3% noninfectious ulcerative dehiscence, 1.6% superficial infection), which is comparable to the 6% rate found in this study. However, there likely is some variability across studies in what constitutes a wound complication or superficial infection. Of our 3 wound complications—stitch reaction, spitting suture, small noninfectious ulceration—only the ulceration was of a severity similar to that reported by Jewett and Collis.15 Matta and colleagues8 reported only 3 wound complications (in 494 patients), all severe enough to require operative intervention. One explanation for this low complication rate is use of a ring retractor, as it is routinely depicted in their technique paper. However, no specific reference is made to gauge how often the device was used.
Rates of superficial infection after DA-THA range from 0.6% to 1.6% in 3 large observational studies (combined deep infection rate, 0.43%).8,14,15 In 2 of these studies, all patients with superficial infection underwent formal débridement, though none developed deep infection. A prospective randomized study of 221 patients who underwent colorectal surgery—where perioperative infectious morbidity ranges from 25% to 50%—found that ring retractor use significantly reduced superficial wound infection rates (8.1% vs 0%). A significant reduction in wound infection was shown in a similarly designed study involving 48 patients who had open appendectomy (14.6% vs 1.6%). The device had no effect on deep infection in either general surgery study. The wound infection rates reported in these general surgery studies are markedly higher than those in our study population. As a result, the effect of the ring retractor on wound infection in DA-THA may be less. Regardless of the effect on deep infection, fewer superficial infections, which often require operative intervention, would be of considerable benefit.
Below-threshold albumin level and absolute lymphocyte count have been associated with wound-healing complications after hip replacement.21 In the present study, pre-albumin level under 20 mg/L was the only nutritional marker predictive of poor wound appearance, but this finding was seen only in SBSES scores from surgeon A. Subgroup analysis did not reveal any relationship between wound appearance and any of the recorded demographic or perioperative variables, but for a small predictive influence with age over 65 years.
This study had some limitations. Our findings cannot be generalized to all patients who undergo THA, as only DA incisions were studied. Results also may not be generalizable to non-fellowship-trained orthopedists. In addition, selection bias likely resulted from including patients already selected for the DA approach. Using digital images for evaluation (vs real-life evaluation) may have affected reliability as well. Last, by not incorporating texture, we omitted a potentially informative feature from scoring.
It is paramount that surgeons undergo diligent training before undertaking this approach for minimizing unwanted results; furthermore, higher early complication rates level off with increased surgeon experience.14,36,37 We recommend meticulous soft-tissue handling, cautious retraction, and careful patient selection (relative contraindication for patients with an abdominal pannus overlying the incision) as primary measures for minimizing incisional trauma and potential wound-healing complications.38 Preservation of the tensor fascia is also crucial,39 as it is the only closable layer separating deep and superficial compartments. Without good closure of the tensor fascia, there is no containment or tamponade of deep bleeding, which can facilitate hematoma formation.
In the population studied, we found no significant long-term differences in cosmetic appearance (based on clinician or patient evaluation) between wounds managed with and without the ring retractor. Our data do not support routine use of the ring retractor, during DA-THA, for improved wound cosmesis. Whether the device has any significant role in reducing the number of wound complications in THA is yet to be determined. Last, the ring retractor may have a role in other areas of orthopedic surgery, such as hip fractures in the elderly or orthopedic oncology. In situations like these, where adequate nutrition and immunocompetency may be lacking, the added protection provided by the device may translate into a more notable benefit than in elective THA.
1. Laffosse JM, Chiron P, Tricoire JL, Giordano G, Molinier F, Puget J. Prospective and comparative study of minimally invasive posterior approach versus standard posterior approach in total hip replacement [in French]. Rev Chir Orthop Reparatrice Appar Mot. 2007;93(3):228-237.
2. Smith TO, Blake V, Hing CB. Minimally invasive versus conventional exposure for total hip arthroplasty: a systematic review and meta-analysis of clinical and radiological outcomes. Int Orthop. 2011;35(2):173-184.
3. Wright JM, Crockett HC, Delgado S, Lyman S, Madsen M, Sculco TP. Mini-incision for total hip arthroplasty: a prospective, controlled investigation with 5-year follow-up evaluation. J Arthroplasty. 2004;19(5):538-545.
4. Mardones R, Pagnano MW, Nemanich JP, Trousdale RT. The Frank Stinchfield Award: muscle damage after total hip arthroplasty done with the two-incision and mini-posterior techniques. Clin Orthop. 2005;(441):63-67.
5. Müller M, Tohtz S, Dewey M, Springer I, Perka C. Age-related appearance of muscle trauma in primary total hip arthroplasty and the benefit of a minimally invasive approach for patients older than 70 years. Int Orthop. 2011;35(2):165-171.
6. Noble PC, Johnston JD, Alexander JA, et al. Making minimally invasive THR safe: conclusions from biomechanical simulation and analysis. Int Orthop. 2007;31(suppl 1):S25-S28.
7. Bremer AK, Kalberer F, Pfirrmann CW, Dora C. Soft-tissue changes in hip abductor muscles and tendons after total hip replacement: comparison between the direct anterior and the transgluteal approaches. J Bone Joint Surg Br. 2011;93(7):886-889.
8. Matta JM, Shahrdar C, Ferguson T. Single-incision anterior approach for total hip arthroplasty on an orthopaedic table. Clin Orthop. 2005;(441):115-124.
9. Rachbauer F, Kain MSH, Leunig M. The history of the anterior approach to the hip. Orthop Clin North Am. 2009;40(3):311-320.
10. Bergin PF, Doppelt JD, Kephart CJ, et al. Comparison of minimally invasive direct anterior versus posterior total hip arthroplasty based on inflammation and muscle damage markers. J Bone Joint Surg Am. 2011;93(15):1392-1398.
11. Mayr E, Nogler M, Benedetti MG, et al. A prospective randomized assessment of earlier functional recovery in THA patients treated by minimally invasive direct anterior approach: a gait analysis study. Clin Biomech. 2009;24(10):812-818.
12. Meneghini RM, Pagnano MW, Trousdale RT, Hozack WJ. Muscle damage during MIS total hip arthroplasty: Smith-Petersen versus posterior approach. Clin Orthop. 2006;(453):293-298.
13. Sculco TP. Anterior approach in THA improves outcomes: opposes. Orthopedics. 2011;34(9):e459-e461.
14. Bhandari M, Matta JM, Dodgin D, et al; Anterior Total Hip Arthroplasty Collaborative Investigators. Outcomes following the single-incision anterior approach to total hip arthroplasty: a multicenter observational study. Orthop Clin North Am. 2009;40(3):329-342.
15. Jewett BA, Collis DK. High complication rate with anterior total hip arthroplasties on a fracture table. Clin Orthop. 2011;469(2):503-507.
16. Barton C, Kim PR. Complications of the direct anterior approach for total hip arthroplasty. Orthop Clin North Am. 2009;40(3):371-375.
17. Bender B, Nogler M, Hozack WJ. Direct anterior approach for total hip arthroplasty. Orthop Clin North Am. 2009;40(3):321-328.
18. Pelosi MA 2nd, Pelosi MA 3rd. Self-retaining abdominal retractor for minilaparotomy. Obstet Gynecol. 2000;96(5, pt 1):775-778.
19. Lee P, Waxman K, Taylor B, Yim S. Use of wound-protection system and postoperative wound-infection rates in open appendectomy: a randomized prospective trial. Arch Surg. 2009;144(9):872-875.
20. Horiuchi T, Tanishima H, Tamagawa K, et al. Randomized, controlled investigation of the anti-infective properties of the Alexis retractor/protector of incision sites. J Trauma. 2007;62(1):212-215.
21. Greene KA, Wilde AH, Stulberg BN. Preoperative nutritional status of total joint patients. Relationship to postoperative wound complications. J Arthroplasty. 1991;6(4):321-325.
22. Alijanipour P, Heller S, Parvizi J. Prevention of periprosthetic joint infection: what are the effective strategies? J Knee Surg. 2014;27(4):251-258.
23. Suarez JC, Slotkin EM, Alvarez AM, Szubski CR, Barsoum WK, Patel PD. Prospective, randomized trial to evaluate efficacy of a thrombin-based hemostaticagent in total knee arthroplasty. J Arthroplasty. 2014;29(10):1950-1955.
24. Singer AJ, Arora B, Dagum A, Valentine S, Hollander JE. Development and validation of a novel scar evaluation scale. Plast Reconstr Surg. 2007;120(7):1892-1897.
25. Beausang E, Floyd H, Dunn KW, Orton CI, Ferguson MW. A new quantitative scale for clinical scar assessment. Plast Reconstr Surg. 1998;102(6):1954-1961.
26. Durani P, McGrouther DA, Ferguson MW. Current scales for assessing human scarring: a review. J Plast Reconstr Aesthet Surg. 2009;62(6):713-720.
27. Fearmonti R, Bond J, Erdmann D, Levinson H. A review of scar scales and scar measuring devices. Eplasty. 2010;10:e43.
28. Duncan JA, Bond JS, Mason T, et al. Visual analogue scale scoring and ranking: a suitable and sensitive method for assessing scar quality? Plast Reconstr Surg. 2006;118(4):909-918.
29. Quinn JV, Wells GA. An assessment of clinical wound evaluation scales. Acad Emerg Med. 1998;5(6):583-586.
30. Livesey C, Wylde V, Descamps S, et al. Skin closure after total hip replacement: a randomised controlled trial of skin adhesive versus surgical staples. J Bone Joint Surg Br. 2009;91(6):725-729.
31. Atiyeh BS. Nonsurgical management of hypertrophic scars: evidence-based therapies, standard practices, and emerging methods. Aesthetic Plast Surg. 2007;31(5):468-492.
32. Mow CS, Woolson ST, Ngarmukos SG, Park EH, Lorenz HP. Comparison of scars from total hip replacements done with a standard or a mini-incision. Clin Orthop. 2005;(441):80-85.
33. Khan RJ, Fick D, Yao F, et al. A comparison of three methods of wound closure following arthroplasty: a prospective, randomised, controlled trial. J Bone Joint Surg Br. 2006;88(2):238-242.
34. Goldstein WM, Ali R, Branson JJ, Berland KA. Comparison of patient satisfaction with incision cosmesis after standard and minimally invasive total hip arthroplasty. Orthopedics. 2008;31(4):368.
35. Quinn J, Wells G, Sutcliffe T, et al. Tissue adhesive versus suture wound repair at 1 year: randomized clinical trial correlating early, 3-month, and 1-year cosmetic outcome. Ann Emerg Med. 1998;32(6):645-649.
36. Alberti LR, Petroianu A, Zac RI, Andrade JC Jr. The effect of surgical procedures on serum albumin concentration. Chirurgia (Bucur). 2008;103(1):39-43.
37. Berend KR, Lombardi AV Jr, Seng BE, Adams JB. Enhanced early outcomes with the anterior supine intermuscular approach in primary total hip arthroplasty. J Bone Joint Surg Am. 2009;91(suppl 6):107-120.
38. Mutnal A, Patel P, Cardona L, Suarez J. Periprosthetic Propionibacterium granulosum joint infection after direct anterior total hip arthroplasty: a case report. JBJS Case Connector. 2011;1(2):e10.
39. Alvarez AM, Suarez JC, Patel P, Benton EG. Fluoroscopic imaging of acetabular cup position during THA through a direct anterior approach. Orthopedics. 2013;36(10):776-777. Erratum in: Orthopedics. 2014;37(1):16.
1. Laffosse JM, Chiron P, Tricoire JL, Giordano G, Molinier F, Puget J. Prospective and comparative study of minimally invasive posterior approach versus standard posterior approach in total hip replacement [in French]. Rev Chir Orthop Reparatrice Appar Mot. 2007;93(3):228-237.
2. Smith TO, Blake V, Hing CB. Minimally invasive versus conventional exposure for total hip arthroplasty: a systematic review and meta-analysis of clinical and radiological outcomes. Int Orthop. 2011;35(2):173-184.
3. Wright JM, Crockett HC, Delgado S, Lyman S, Madsen M, Sculco TP. Mini-incision for total hip arthroplasty: a prospective, controlled investigation with 5-year follow-up evaluation. J Arthroplasty. 2004;19(5):538-545.
4. Mardones R, Pagnano MW, Nemanich JP, Trousdale RT. The Frank Stinchfield Award: muscle damage after total hip arthroplasty done with the two-incision and mini-posterior techniques. Clin Orthop. 2005;(441):63-67.
5. Müller M, Tohtz S, Dewey M, Springer I, Perka C. Age-related appearance of muscle trauma in primary total hip arthroplasty and the benefit of a minimally invasive approach for patients older than 70 years. Int Orthop. 2011;35(2):165-171.
6. Noble PC, Johnston JD, Alexander JA, et al. Making minimally invasive THR safe: conclusions from biomechanical simulation and analysis. Int Orthop. 2007;31(suppl 1):S25-S28.
7. Bremer AK, Kalberer F, Pfirrmann CW, Dora C. Soft-tissue changes in hip abductor muscles and tendons after total hip replacement: comparison between the direct anterior and the transgluteal approaches. J Bone Joint Surg Br. 2011;93(7):886-889.
8. Matta JM, Shahrdar C, Ferguson T. Single-incision anterior approach for total hip arthroplasty on an orthopaedic table. Clin Orthop. 2005;(441):115-124.
9. Rachbauer F, Kain MSH, Leunig M. The history of the anterior approach to the hip. Orthop Clin North Am. 2009;40(3):311-320.
10. Bergin PF, Doppelt JD, Kephart CJ, et al. Comparison of minimally invasive direct anterior versus posterior total hip arthroplasty based on inflammation and muscle damage markers. J Bone Joint Surg Am. 2011;93(15):1392-1398.
11. Mayr E, Nogler M, Benedetti MG, et al. A prospective randomized assessment of earlier functional recovery in THA patients treated by minimally invasive direct anterior approach: a gait analysis study. Clin Biomech. 2009;24(10):812-818.
12. Meneghini RM, Pagnano MW, Trousdale RT, Hozack WJ. Muscle damage during MIS total hip arthroplasty: Smith-Petersen versus posterior approach. Clin Orthop. 2006;(453):293-298.
13. Sculco TP. Anterior approach in THA improves outcomes: opposes. Orthopedics. 2011;34(9):e459-e461.
14. Bhandari M, Matta JM, Dodgin D, et al; Anterior Total Hip Arthroplasty Collaborative Investigators. Outcomes following the single-incision anterior approach to total hip arthroplasty: a multicenter observational study. Orthop Clin North Am. 2009;40(3):329-342.
15. Jewett BA, Collis DK. High complication rate with anterior total hip arthroplasties on a fracture table. Clin Orthop. 2011;469(2):503-507.
16. Barton C, Kim PR. Complications of the direct anterior approach for total hip arthroplasty. Orthop Clin North Am. 2009;40(3):371-375.
17. Bender B, Nogler M, Hozack WJ. Direct anterior approach for total hip arthroplasty. Orthop Clin North Am. 2009;40(3):321-328.
18. Pelosi MA 2nd, Pelosi MA 3rd. Self-retaining abdominal retractor for minilaparotomy. Obstet Gynecol. 2000;96(5, pt 1):775-778.
19. Lee P, Waxman K, Taylor B, Yim S. Use of wound-protection system and postoperative wound-infection rates in open appendectomy: a randomized prospective trial. Arch Surg. 2009;144(9):872-875.
20. Horiuchi T, Tanishima H, Tamagawa K, et al. Randomized, controlled investigation of the anti-infective properties of the Alexis retractor/protector of incision sites. J Trauma. 2007;62(1):212-215.
21. Greene KA, Wilde AH, Stulberg BN. Preoperative nutritional status of total joint patients. Relationship to postoperative wound complications. J Arthroplasty. 1991;6(4):321-325.
22. Alijanipour P, Heller S, Parvizi J. Prevention of periprosthetic joint infection: what are the effective strategies? J Knee Surg. 2014;27(4):251-258.
23. Suarez JC, Slotkin EM, Alvarez AM, Szubski CR, Barsoum WK, Patel PD. Prospective, randomized trial to evaluate efficacy of a thrombin-based hemostaticagent in total knee arthroplasty. J Arthroplasty. 2014;29(10):1950-1955.
24. Singer AJ, Arora B, Dagum A, Valentine S, Hollander JE. Development and validation of a novel scar evaluation scale. Plast Reconstr Surg. 2007;120(7):1892-1897.
25. Beausang E, Floyd H, Dunn KW, Orton CI, Ferguson MW. A new quantitative scale for clinical scar assessment. Plast Reconstr Surg. 1998;102(6):1954-1961.
26. Durani P, McGrouther DA, Ferguson MW. Current scales for assessing human scarring: a review. J Plast Reconstr Aesthet Surg. 2009;62(6):713-720.
27. Fearmonti R, Bond J, Erdmann D, Levinson H. A review of scar scales and scar measuring devices. Eplasty. 2010;10:e43.
28. Duncan JA, Bond JS, Mason T, et al. Visual analogue scale scoring and ranking: a suitable and sensitive method for assessing scar quality? Plast Reconstr Surg. 2006;118(4):909-918.
29. Quinn JV, Wells GA. An assessment of clinical wound evaluation scales. Acad Emerg Med. 1998;5(6):583-586.
30. Livesey C, Wylde V, Descamps S, et al. Skin closure after total hip replacement: a randomised controlled trial of skin adhesive versus surgical staples. J Bone Joint Surg Br. 2009;91(6):725-729.
31. Atiyeh BS. Nonsurgical management of hypertrophic scars: evidence-based therapies, standard practices, and emerging methods. Aesthetic Plast Surg. 2007;31(5):468-492.
32. Mow CS, Woolson ST, Ngarmukos SG, Park EH, Lorenz HP. Comparison of scars from total hip replacements done with a standard or a mini-incision. Clin Orthop. 2005;(441):80-85.
33. Khan RJ, Fick D, Yao F, et al. A comparison of three methods of wound closure following arthroplasty: a prospective, randomised, controlled trial. J Bone Joint Surg Br. 2006;88(2):238-242.
34. Goldstein WM, Ali R, Branson JJ, Berland KA. Comparison of patient satisfaction with incision cosmesis after standard and minimally invasive total hip arthroplasty. Orthopedics. 2008;31(4):368.
35. Quinn J, Wells G, Sutcliffe T, et al. Tissue adhesive versus suture wound repair at 1 year: randomized clinical trial correlating early, 3-month, and 1-year cosmetic outcome. Ann Emerg Med. 1998;32(6):645-649.
36. Alberti LR, Petroianu A, Zac RI, Andrade JC Jr. The effect of surgical procedures on serum albumin concentration. Chirurgia (Bucur). 2008;103(1):39-43.
37. Berend KR, Lombardi AV Jr, Seng BE, Adams JB. Enhanced early outcomes with the anterior supine intermuscular approach in primary total hip arthroplasty. J Bone Joint Surg Am. 2009;91(suppl 6):107-120.
38. Mutnal A, Patel P, Cardona L, Suarez J. Periprosthetic Propionibacterium granulosum joint infection after direct anterior total hip arthroplasty: a case report. JBJS Case Connector. 2011;1(2):e10.
39. Alvarez AM, Suarez JC, Patel P, Benton EG. Fluoroscopic imaging of acetabular cup position during THA through a direct anterior approach. Orthopedics. 2013;36(10):776-777. Erratum in: Orthopedics. 2014;37(1):16.
Arm Pain in Young Baseball Players Is Common, Yet Preventable
A majority of healthy, youth baseball players report some baseline arm pain and fatigue, and many players suffer adverse psychosocial effects from this pain, according to a study published online ahead of print November 3 in the American Journal of Sports Medicine.
“Both nationally and internationally, we’re witnessing a troubling increase of elbow and shoulder injuries in young baseball players,” said study leader Christopher S. Ahmad, MD, Chief of Sports Medicine and Professor of Orthopedic Surgery at New York-Presbyterian/Columbia and head team physician for the New York Yankees.
Dr. Ahmad and his research colleagues designed a questionnaire to learn more about the frequency, severity, and psychosocial effects of arm pain among active adolescent baseball payers. The questionnaire was completed by 203 players from New York and New Jersey, who were between the ages of 8 and 18. All of the surveys were completed without input from parents or coaches.
Among the survey’s findings was that 74% of players reported having arm pain while throwing. Just 26% said they “never” had arm pain while throwing.
The study also found that:
• 54% reported that arm pain limited the number of innings they could play.
• 75% reported that arm pain limited how hard they could throw.
• 80% reported having arm pain the day after throwing.
• 82% reported arm fatigue during a game or practice.
Pitchers, compared with infielders and outfielders, were especially likely to have played with pain. One-quarter of pitchers reported that they “often” or “always” had pain the day after throwing. “These pitchers likely represent one of the higher-risk groups for incurring a future overuse injury and thus warrant particularly high monitoring,” said Dr. Ahmad.
Almost half (47%) of players reported that they had been encouraged to continue playing in a practice or game even though they were having pain. One in eight players ages 17 to 18 reported that they “always” felt encouraged to continue playing despite having arm pain. A majority of players reported that arm pain caused them to experience less enjoyment while playing and that it was responsible for holding them back from being a better player.
“It’s alarming that so many young baseball players are encouraged to play with pain,” said Dr. Ahmad. “Years ago, prior to concussion protocols, we observed something similar in football, where players who suffered a concussion were routinely sent back into the game after ‘recovering’ for a few minutes. The initial concussion lowered the threshold for another concussion, and the repeated concussions put the player at risk for permanent damage. I think we’re seeing a similar problem in baseball, where playing with arm pain is setting the stage for more serious injury.”
Dr. Ahmad suspects that this phenomenon has contributed to the recent rise in “Tommy John” surgeries among college and professional baseball players. According to Dr. Ahmad, current precautions and guidelines are inadequate for preventing injury. “It’s not enough to set pitch counts based on a player’s age,” he said. “While some 14 year olds are already quite mature, in terms of their skeletal structure, others haven’t even started their growth spurt yet. We need to come up with more individualized throwing programs and better ways to detect which players are at risk for injury.”
Dr. Ahmad is currently investigating the use of ultrasound for correlating arm pain with tissue damage.
Suggested Reading
Makhni EC, Morrow ZS, Luchetti TJ, et al. Arm pain in youth baseball players: a survey of healthy players. Am J Sports Med. 2014 Nov 3. pii: 0363546514555506. [Epub ahead of print]
A majority of healthy, youth baseball players report some baseline arm pain and fatigue, and many players suffer adverse psychosocial effects from this pain, according to a study published online ahead of print November 3 in the American Journal of Sports Medicine.
“Both nationally and internationally, we’re witnessing a troubling increase of elbow and shoulder injuries in young baseball players,” said study leader Christopher S. Ahmad, MD, Chief of Sports Medicine and Professor of Orthopedic Surgery at New York-Presbyterian/Columbia and head team physician for the New York Yankees.
Dr. Ahmad and his research colleagues designed a questionnaire to learn more about the frequency, severity, and psychosocial effects of arm pain among active adolescent baseball payers. The questionnaire was completed by 203 players from New York and New Jersey, who were between the ages of 8 and 18. All of the surveys were completed without input from parents or coaches.
Among the survey’s findings was that 74% of players reported having arm pain while throwing. Just 26% said they “never” had arm pain while throwing.
The study also found that:
• 54% reported that arm pain limited the number of innings they could play.
• 75% reported that arm pain limited how hard they could throw.
• 80% reported having arm pain the day after throwing.
• 82% reported arm fatigue during a game or practice.
Pitchers, compared with infielders and outfielders, were especially likely to have played with pain. One-quarter of pitchers reported that they “often” or “always” had pain the day after throwing. “These pitchers likely represent one of the higher-risk groups for incurring a future overuse injury and thus warrant particularly high monitoring,” said Dr. Ahmad.
Almost half (47%) of players reported that they had been encouraged to continue playing in a practice or game even though they were having pain. One in eight players ages 17 to 18 reported that they “always” felt encouraged to continue playing despite having arm pain. A majority of players reported that arm pain caused them to experience less enjoyment while playing and that it was responsible for holding them back from being a better player.
“It’s alarming that so many young baseball players are encouraged to play with pain,” said Dr. Ahmad. “Years ago, prior to concussion protocols, we observed something similar in football, where players who suffered a concussion were routinely sent back into the game after ‘recovering’ for a few minutes. The initial concussion lowered the threshold for another concussion, and the repeated concussions put the player at risk for permanent damage. I think we’re seeing a similar problem in baseball, where playing with arm pain is setting the stage for more serious injury.”
Dr. Ahmad suspects that this phenomenon has contributed to the recent rise in “Tommy John” surgeries among college and professional baseball players. According to Dr. Ahmad, current precautions and guidelines are inadequate for preventing injury. “It’s not enough to set pitch counts based on a player’s age,” he said. “While some 14 year olds are already quite mature, in terms of their skeletal structure, others haven’t even started their growth spurt yet. We need to come up with more individualized throwing programs and better ways to detect which players are at risk for injury.”
Dr. Ahmad is currently investigating the use of ultrasound for correlating arm pain with tissue damage.
A majority of healthy, youth baseball players report some baseline arm pain and fatigue, and many players suffer adverse psychosocial effects from this pain, according to a study published online ahead of print November 3 in the American Journal of Sports Medicine.
“Both nationally and internationally, we’re witnessing a troubling increase of elbow and shoulder injuries in young baseball players,” said study leader Christopher S. Ahmad, MD, Chief of Sports Medicine and Professor of Orthopedic Surgery at New York-Presbyterian/Columbia and head team physician for the New York Yankees.
Dr. Ahmad and his research colleagues designed a questionnaire to learn more about the frequency, severity, and psychosocial effects of arm pain among active adolescent baseball payers. The questionnaire was completed by 203 players from New York and New Jersey, who were between the ages of 8 and 18. All of the surveys were completed without input from parents or coaches.
Among the survey’s findings was that 74% of players reported having arm pain while throwing. Just 26% said they “never” had arm pain while throwing.
The study also found that:
• 54% reported that arm pain limited the number of innings they could play.
• 75% reported that arm pain limited how hard they could throw.
• 80% reported having arm pain the day after throwing.
• 82% reported arm fatigue during a game or practice.
Pitchers, compared with infielders and outfielders, were especially likely to have played with pain. One-quarter of pitchers reported that they “often” or “always” had pain the day after throwing. “These pitchers likely represent one of the higher-risk groups for incurring a future overuse injury and thus warrant particularly high monitoring,” said Dr. Ahmad.
Almost half (47%) of players reported that they had been encouraged to continue playing in a practice or game even though they were having pain. One in eight players ages 17 to 18 reported that they “always” felt encouraged to continue playing despite having arm pain. A majority of players reported that arm pain caused them to experience less enjoyment while playing and that it was responsible for holding them back from being a better player.
“It’s alarming that so many young baseball players are encouraged to play with pain,” said Dr. Ahmad. “Years ago, prior to concussion protocols, we observed something similar in football, where players who suffered a concussion were routinely sent back into the game after ‘recovering’ for a few minutes. The initial concussion lowered the threshold for another concussion, and the repeated concussions put the player at risk for permanent damage. I think we’re seeing a similar problem in baseball, where playing with arm pain is setting the stage for more serious injury.”
Dr. Ahmad suspects that this phenomenon has contributed to the recent rise in “Tommy John” surgeries among college and professional baseball players. According to Dr. Ahmad, current precautions and guidelines are inadequate for preventing injury. “It’s not enough to set pitch counts based on a player’s age,” he said. “While some 14 year olds are already quite mature, in terms of their skeletal structure, others haven’t even started their growth spurt yet. We need to come up with more individualized throwing programs and better ways to detect which players are at risk for injury.”
Dr. Ahmad is currently investigating the use of ultrasound for correlating arm pain with tissue damage.
Suggested Reading
Makhni EC, Morrow ZS, Luchetti TJ, et al. Arm pain in youth baseball players: a survey of healthy players. Am J Sports Med. 2014 Nov 3. pii: 0363546514555506. [Epub ahead of print]
Suggested Reading
Makhni EC, Morrow ZS, Luchetti TJ, et al. Arm pain in youth baseball players: a survey of healthy players. Am J Sports Med. 2014 Nov 3. pii: 0363546514555506. [Epub ahead of print]
Anemia Versus Transfusion: Does Blood Conservation Increase the Risk of Complications?
More than 13 million units of blood are transfused each year. Although transfusion can certainly be lifesaving, numerous studies over the past 20 years have shown significant, dose-dependent increases in morbidity, mortality, and cost with each unit of packed red blood cells (pRBCs) transfused.1 Transfusion is one of the most common interventions in the critically ill population; however the negative effects of transfusion-related infection are well documented in the recent literature.1-7 There is no question that transfusion of blood products can be lifesaving to acutely ill trauma patients, but there is little evidence regarding when transfusions are indicated in asymptomatic anemic patients who are no longer in need of acute resuscitation.
Several studies have analyzed healthy individuals with an isovolemic reduction in hemoglobin (Hgb) level to 5.0 g/dL.8,9 They have found no significant compromise in oxygen delivery to the tissues. Currently, there is a lack of clinical data to suggest adequate RBC transfusion endpoints in trauma surgery.10 Given the lack of evidence to support transfusion triggers for young, healthy, asymptomatic orthopedic trauma patients, we decided to investigate whether a more conservative transfusion strategy might be as safe as a more liberal strategy.
Materials and Methods
After obtaining approval from our institutional review board, we performed a retrospective observational cohort analysis of patients treated at a level I trauma center between September 2006 and February 2009. The trauma registry included all patients who underwent surgery performed by a single orthopedic fellowship–trained trauma surgeon. All patients who had a recorded Hgb level of 9.0 g/dL or less at any time during their admission were included; they were considered no longer volume-depleted after initial resuscitation. Exclusion criteria were age under 18 years or over 50 years; pregnancy; head injury; and preexisting heart, pulmonary, or renal disease.
Initially, 963 patients were identified as orthopedic trauma patients treated by Dr. Mullis within the defined period. After inclusion and exclusion criteria were used to limit this database, the charts of the 109 patients who met the above criteria were reviewed. By chart review or telephone follow-up, 104 patients with 1-year follow-up were identified, and their cases became the basis for our analysis. Demographic information, length of hospital stay, surgeries performed, number of pRBC units transfused, Hgb level prompting transfusion, lowest recorded Hgb level, complications, and Injury Severity Score (ISS) were recorded for each patient. Seventy-two patients (69%) were male, 32 (31%) female. Mean age of the study population was 33 years.
Patients were divided into 2 groups by lowest Hgb level before first transfusion—under 7.0 g/dL and 7.0 g/dL or higher—and then by whether they had been transfused. General guidelines for erythrocyte transfusion on the orthopedic trauma service included patients who were symptomatic at rest (headache, dizziness, or shortness of breath) and asymptomatic patients with Hgb levels under 5.0 g/dL. For patients with varying (lesser) degrees of anemia, transfusion typically depended on clinical symptoms and overall decrease in Hgb level from that recorded on admission.
Patient charts were reviewed for complications extending through a 1-year period after initial discharge from the inpatient service. Patients who had not received follow-up treatment through a known outpatient clinic were contacted by telephone to ascertain outcome. Overall, 5 of the 109 patients were lost at 1-year follow-up, leaving 104 patients with 1-year follow-up (95%). Primary outcome of the study was postoperative complications. Superficial wound infection was defined as cellulitis near the surgical site within 1 year, requiring oral antibiotics; deep wound infection was defined as any related infection within 1 year of injury, requiring intravenous antibiotics or surgical débridement in the operating room. The review for complications included superficial infection, deep infection, urinary tract infection, pneumonia, pulmonary embolism, deep venous thrombosis, acute renal failure or insufficiency, nonunion, delayed union, compartment syndrome, osteomyelitis, nerve palsy, anoxic brain injury, cardiac ischemia or infarct, pancreatitis, and death.
Statistical Methods
The primary focus of this analysis was to determine if patients’ risk of complication at 1-year follow-up was affected by anemia—lowest recorded Hgb level before first transfusion for transfused patients, or lowest Hgb level during hospital stay for nontransfused patients—or whether transfusion itself might be a risk factor for complication. Multiple logistic regression models were used to determine the likelihood each group would have a complication. The dependent variable was complication rate; the explanatory variables included whether the patient was transfused, anemia/Hgb level (under 7 g/dL vs 7 g/dL or higher), and the 2-way interaction. Other possible explanatory variables entered into the model were age, sex, ISS, and whether the patient had had multiple surgeries. As the sample size was small, these variables were entered into the regression model one at a time. Results are presented as odds ratios (ORs) with corresponding 95% confidence intervals (CIs) and P values. The analysis was performed with SAS Version 9.1 (SAS Institute, Cary, North Carolina). Tests were considered statistically significant with P < .05 and marginally significant with P < .10. OR above 1 indicated that the odds of a complication occurring were higher in the exposed group (transfused patients) than in the unexposed group (nontransfused patients).
Results
The charts of 104 patients were reviewed and included in this analysis. Sixty-two patients (60%) had received a transfusion; 42 (40%) had not. Before first transfusion, 21 (34%) of the 62 transfused patients had Hgb levels under 7.0 g/dL, and the other 41 (66%) had Hgb levels of 7.0 g/dL or higher. Of the 42 nontransfused patients, 8 (19%) had lowest Hgb levels under 7.0 g/dL, and the other 34 (81%) had Hgb levels of 7.0 g/dL or higher (Table 1).
The transfused patients, considering all levels of anemia, had a mean ISS of 16.1 (range, 1-45), a mean of 2.0 operations (range, 1-6), a mean hospital stay of 18 days (range, 1-73 days), and a mean age of 34 years (range, 18-50 years). The nontransfused patients, considering all levels of anemia, had a mean ISS of 14.1 (range, 4-43), a mean of 1.4 operations (range, 1-5), a mean hospital stay of 10 days (range, 1-42 days), and a mean age of 33 years (range, 18-50 years). In the transfusion group, the mean number of transfused pRBC units was 6.9 (range, 1-31), or 7.8 units for patients with Hgb levels under 7 g/dL and 6.4 units for patients with Hgb levels of 7 g/dL or higher. At 1-year follow-up, complications were observed in 41 (66%) of the 62 transfused patients and in 17 (40%) of the 42 nontransfused patients (Table 1). The different types of complications seen in each group are listed in Table 2.
Statistical Analysis
Patients were divided into 2 groups by Hgb level—under 7.0 g/dL and 7.0 g/dL or higher—and then by whether they received pRBC transfusion. In addition, which patients had a complication over a 1-year period were identified.
For each group, we calculated sample size, number of complications, complication rate, and 95% CI for proportions. For transfused patients with Hgb level of 7.0 g/dL or higher, the complication rate was 71% (29/41). For nontransfused patients with Hgb of 7.0 g/dL or higher, the complication rate was 41% (14/34). Similarly, for transfused patients with Hgb under 7.0 g/dL, the complication rate was 57% (12/21). Last, for nontransfused patients with Hgb under 7.0 g/dL, the complication rate was 38% (3/8) (Table 3).
Transfused patients had a significantly higher risk of complication (OR, 3.1; 95% CI, 1.4-7.1; P < .01). Severity of anemia was not found to be independently associated with increased risk of complication (OR, 0.6; 95% CI, 0.3-1.6; P = .33) (Table 4). The interaction term was removed and eliminated from further analysis, as it was not found to be significant (P = .45).
Furthermore, the possibility of confounding variables (eg, age, sex, ISS, number of surgeries performed) was considered by including them in the model one at a time. From these logistic regression models, which included whether patients were transfused and level of anemia, an increased risk of complication (OR, 1.8; 95% CI, 1.1-2.9; P = .02) was found for each additional surgery, while receiving transfusion remained statistically significant (OR, 2.5; 95% CI, 1.0-5.8; P < .04). Age, sex, and ISS were not shown to be significantly associated with an increased complication rate (Ps = .71, .32, and .13, respectively).
We performed a subanalysis of the transfused patients to determine the impact of number of units transfused on complication rate. Each additional unit of pRBCs transfused increased the risk of complication, indicating a dose-dependent response (OR, 1.3; 95% CI, 1.04-1.51; P = .02).
Discussion
Transfusion is a generally accepted and common intervention both in the intensive care unit and in the perioperative period. However, there is little evidence to support routine transfusion of asymptomatic orthopedic trauma patients who are no longer within the initial resuscitative period after trauma. Nevertheless, the practice is routinely done based on expert opinion (level 5 evidence). The anemia protocol for our orthopedic trauma service routinely allowed the Hgb levels of asymptomatic healthy patients to drop to under 7.0 g/dL without transfusion; when other services were consulted or were primary, however, these asymptomatic patients were still routinely transfused based on practitioners’ practice patterns and anecdotal experiences.
In hemodynamically unstable patients, there is no acceptable substitute for blood transfusion. Blood replacement remains essential in the case of acute hemorrhage. However, the complications associated with transfusion should lead us to avoid, or at least minimize, unnecessary transfusion in young asymptomatic patients who are not actively bleeding in the postresuscitative period. In our study, we did not seek causation of increased complications with transfusion but assessed whether the risk of anemia outweighed the risk of transfusion in young, healthy, asymptomatic trauma patients who were no longer in the initial resuscitation period.
Our study was designed to evaluate a conservative transfusion strategy used in orthopedic trauma patients. We hypothesized that the risk of anemia in these asymptomatic patients would be lower than the risk of transfusing asymptomatic patients in the perioperative period. In addition, we thought the level of anemia would play a less significant role in the postoperative complication rate relative to transfusion itself. Our results suggest that a more conservative transfusion strategy of allowing asymptomatic patients to become and remain anemic even to a Hgb level of 5 g/dL may be as safe as a more liberal transfusion strategy of keeping patients at a Hgb level higher than 7 g/dL. In general, the complication rate was 66% for transfused patients and 40% for nontransfused patients. These results remain significant after correcting for possible confounding factors, including age, sex, ISS, and number of surgeries.
The results of this study do not suggest that there may not be complications associated with anemia; a 40% complication rate even in the nontransfused group is high. One might expect that patients who had isolated injuries and never developed anemia with an Hgb level under 9 g/dL might have an even lower complication rate. In the group used for inclusion in this study, however, there was not a significant increased risk for patients who tolerated a lower anemia (Hgb, <7 g/dL), whereas transfusion to keep the Hgb level above 7 g/dL appeared to correlate with a significant risk of complication and appeared to be dose-dependent. It should be noted that the complications in both the transfusion and anemia groups are not necessarily related to transfusion or anemia, as many factors in a retrospective study cannot be controlled. These findings simply suggest that it might be as safe to use a conservative transfusion strategy as a liberal transfusion strategy in this patient population.
Although our study is retrospective, prospective randomized studies in the elderly and in the critical care population have shown conservative transfusion guidelines are at least as safe as liberal transfusion strategies.2,11 One study randomized intensive care unit patients with Hgb levels under 9.0 g/dL to 2 groups, one with liberal and the other with restrictive protocols for pRBC unit transfusion.2 The liberal group maintained Hgb levels between 10.0 and 12.0 g/dL, and the restricted group kept Hgb levels between 7.0 and 9.0 g/dL. Thirty-day mortality was significantly lower in less acutely ill patients and younger patients (<55 years old) in the restrictive group than in the liberal group. It was concluded that a restrictive strategy of RBC transfusion is at least as effective as, and possibly superior to, a liberal transfusion strategy in the critically ill when considering short- and long-term outcomes. Another prospective study randomized elderly patients (N = 2016) with hip fractures and cardiovascular risk factors to a liberal transfusion strategy (if Hgb level fell under 10 g/dL) or a restrictive transfusion strategy (if Hgb level fell under 8 g/dL). The study found no difference between the 2 groups.11
The deleterious effect of allogeneic blood transfusion on the immune system is complex and has been linked to the down-regulation of cellular immunity, including decreased function of natural killer cells, decreased function of macrophages and monocytes, and increased numbers of suppressor T cells.12,13 This minimized immune response has been associated with a multitude of infectious morbidities in various patient populations.7 A meta-analysis of 20 studies reviewing outcomes of the effects of transfusion on postoperative bacterial infection found strong evidence supporting a correlation.5 Their analysis found an OR of 5.3 (range, 5.0-5.4) for infectious complication after allogeneic transfusion in the trauma population, and an OR of 3.5 (range, 1.4-15.2) considering all patient populations.
Similar results showing increased risk of infectious morbidities associated with transfusion were found in other studies involving the critically ill, patients after hip arthroplasty, and cardiothoracic surgery and general trauma populations.1,3,4,14,15 Furthermore, these results were seen in a dose-dependent response leading to increased incidence of complication with each unit of blood transfused.
Our study did not focus only on infection but included other complications (eg, cardiac, renal, and brain ischemia) that might be associated with anemia or transfusion. It is intuitive that anemia can cause ischemic events but less intuitive that allogeneic transfusion can also cause ischemic events because of the poor deformability of the cells due to storage, which can lead to “sludging” in capillaries throughout the body.16 This has been shown to be important in animal models, but it is unclear what poses more risk in humans—anemia without transfusion or the initial insult from transfusion, before the body clears the “waste” from stored cells and the remaining viable cells gain oxygen-carrying capacity.
Our study has several limitations. The number of patients who had severe anemia (Hgb level, <7 g/dL) and were not transfused is relatively small compared with the numbers in the other groups used for comparison. Because our study was retrospective, we could only find associations and not prove causation. This is significant, as the higher complication rate seen with transfusions may only be caused by the transfusion as a predictor of a patient requiring more complex surgery with higher blood loss (and higher risk of complication) or other such risk factors that led to transfusion, but not the transfusion itself causing the complication. An attempt was made to remove this potential bias by controlling for age, sex, ISS, and whether the patient had multiple surgeries. However, there may have been other significant confounding variables not excluded. As complications were assessed by chart review, they may not include those that occurred at other institutions and that were never reported to the practitioners at our facility (though we did have the ability to search records of neighboring institutions electronically when electronic medical records were available). That no functional outcomes were included in this retrospective review might make the complication rate appear more or less sensitive than the patients’ own opinions regarding their outcomes. All these weaknesses could call into question whether the statistically significant higher risk associated with allogeneic transfusion found in this study is real, but the focus and reason for pursuing this study were to determine if permissive anemia was dangerous or would be associated with a higher risk of complications than routine allogeneic transfusion of asymptomatic patients to treat a laboratory value.
Strengths of the study include the review of a single surgeon’s practice with a written protocol in place for anemic orthopedic trauma patients. The 95% follow-up (104/109 patients) is good for this type of trauma population. Although this series is retrospective, it is reasonably large for a subgroup of young, healthy orthopedic trauma patients to study the effects of anemia or transfusion. Whether transfused or not, many of these patients tolerated Hgb levels under 7 g/dL, which gave a large enough comparison group to evaluate the independent effects of transfusion (or of using transfusion as a marker for complication risk) or anemia as a risk factor. As a result, it appears that a more conservative transfusion strategy may be as safe as a more liberal transfusion strategy. The results of this retrospective study were used to design a prospective multidisciplinary pilot study randomizing patients to either a liberal or a conservative transfusion strategy to determine which approach might carry higher risks of complications.
Conclusion
The results of this retrospective study suggest that a conservative transfusion strategy in a young, healthy, euvolemic asymptomatic patient who is not actively bleeding may be as safe as a liberal transfusion strategy and potentially may have fewer complications than does transfusion for a conventional laboratory value. Our study results do not suggest that transfusions should be held in patients who are symptomatic at rest or in patients who are being actively resuscitated, as transfusion can be lifesaving under these circumstances. A prospective randomized study has begun at our institution with enrollment expected to take 2 years with another year needed to complete 1-year follow-up of all patients.
1. Leal-Noval SR, Rincón-Ferrari MD, García-Curiel A, et al. Transfusion of blood components and postoperative infection in patients undergoing cardiac surgery. Chest. 2001;119(5):1461-1468.
2. Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340(6):409-417.
3. Carson JL, Altman DG, Duff A, et al. Risk of bacterial infection associated with allogeneic blood transfusion among patients undergoing hip fracture repair. Transfusion. 1999;39(7):694-700.
4. Edna TH, Bjerkeset T. Association between blood transfusion and infection in injured patients. J Trauma. 1992;33(5):659-661.
5. Hill GE, Frawley WH, Griffith KE, Forestner JE, Minei JP. Allogeneic blood transfusion increases the risk of postoperative bacterial infection: a meta-analysis. J Trauma. 2003;54(5):908-914.
6. Vincent JL, Baron JF, Reinhart K, et al; ABC (Anemia and Blood Transfusion in Critical Care) Investigators. Anemia and blood transfusion in critically ill patients. JAMA. 2002;288(12):1499-1507.
7. Taylor RW, Manganaro L, O’Brien J, Trottier SJ, Parkar N, Veremakis C. Impact of allogenic packed red blood cell transfusion on nosocomial infection rates in the critically ill patient. Crit Care Med. 2002;30(10):2249-2254.
8. Leung JM, Weiskopf RB, Feiner J, et al. Electrocardiographic ST-segment changes during acute, severe isovolemic hemodilution in humans. Anesthesiology. 2000;93(4):1004-1010.
9. Weiskopf RB, Viele MK, Feiner J, et al. Human cardiovascular and metabolic response to acute, severe isovolemic anemia. JAMA. 1998;279(3):217-221.
10. Johnston P, Wynn-Jones H, Chakravarty D, Boyle A, Parker MJ. Is perioperative blood transfusion a risk factor for mortality or infection after hip fracture? J Orthop Trauma. 2006;20(10):675-679.
11. Carson JL, Terrin ML, Noveck H, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365(26):2453-2462.
12. Blumberg N. Deleterious clinical effects of transfusion immunomodulation: proven beyond a reasonable doubt. Transfusion. 2005;45(2 suppl):33S-39S.
13. Triulzi DJ, Vanek K, Ryan DH, Blumberg N. A clinical and immunologic study of blood transfusion and postoperative bacterial infection in spinal surgery. Transfusion. 1992;32(6):517-524.
14. Shorr AF, Jackson WL. Transfusion practice and nosocomial infection: assessing the evidence. Curr Opin Crit Care. 2005;11(5):468-472.
15. Engoren MC, Habib RH, Zacharias A, Schwann TA, Riordan CJ, Durham SJ. Effect of blood transfusion on long-term survival after cardiac operation. Ann Thorac Surg. 2002;74(4):1180-1186.
16. Tsai AG, Cabrales P, Intaglietta M. Microvascular perfusion upon exchange transfusion with stored red blood cells in normovolemic anemic conditions. Transfusion. 2004;44(11):1626-1634.
More than 13 million units of blood are transfused each year. Although transfusion can certainly be lifesaving, numerous studies over the past 20 years have shown significant, dose-dependent increases in morbidity, mortality, and cost with each unit of packed red blood cells (pRBCs) transfused.1 Transfusion is one of the most common interventions in the critically ill population; however the negative effects of transfusion-related infection are well documented in the recent literature.1-7 There is no question that transfusion of blood products can be lifesaving to acutely ill trauma patients, but there is little evidence regarding when transfusions are indicated in asymptomatic anemic patients who are no longer in need of acute resuscitation.
Several studies have analyzed healthy individuals with an isovolemic reduction in hemoglobin (Hgb) level to 5.0 g/dL.8,9 They have found no significant compromise in oxygen delivery to the tissues. Currently, there is a lack of clinical data to suggest adequate RBC transfusion endpoints in trauma surgery.10 Given the lack of evidence to support transfusion triggers for young, healthy, asymptomatic orthopedic trauma patients, we decided to investigate whether a more conservative transfusion strategy might be as safe as a more liberal strategy.
Materials and Methods
After obtaining approval from our institutional review board, we performed a retrospective observational cohort analysis of patients treated at a level I trauma center between September 2006 and February 2009. The trauma registry included all patients who underwent surgery performed by a single orthopedic fellowship–trained trauma surgeon. All patients who had a recorded Hgb level of 9.0 g/dL or less at any time during their admission were included; they were considered no longer volume-depleted after initial resuscitation. Exclusion criteria were age under 18 years or over 50 years; pregnancy; head injury; and preexisting heart, pulmonary, or renal disease.
Initially, 963 patients were identified as orthopedic trauma patients treated by Dr. Mullis within the defined period. After inclusion and exclusion criteria were used to limit this database, the charts of the 109 patients who met the above criteria were reviewed. By chart review or telephone follow-up, 104 patients with 1-year follow-up were identified, and their cases became the basis for our analysis. Demographic information, length of hospital stay, surgeries performed, number of pRBC units transfused, Hgb level prompting transfusion, lowest recorded Hgb level, complications, and Injury Severity Score (ISS) were recorded for each patient. Seventy-two patients (69%) were male, 32 (31%) female. Mean age of the study population was 33 years.
Patients were divided into 2 groups by lowest Hgb level before first transfusion—under 7.0 g/dL and 7.0 g/dL or higher—and then by whether they had been transfused. General guidelines for erythrocyte transfusion on the orthopedic trauma service included patients who were symptomatic at rest (headache, dizziness, or shortness of breath) and asymptomatic patients with Hgb levels under 5.0 g/dL. For patients with varying (lesser) degrees of anemia, transfusion typically depended on clinical symptoms and overall decrease in Hgb level from that recorded on admission.
Patient charts were reviewed for complications extending through a 1-year period after initial discharge from the inpatient service. Patients who had not received follow-up treatment through a known outpatient clinic were contacted by telephone to ascertain outcome. Overall, 5 of the 109 patients were lost at 1-year follow-up, leaving 104 patients with 1-year follow-up (95%). Primary outcome of the study was postoperative complications. Superficial wound infection was defined as cellulitis near the surgical site within 1 year, requiring oral antibiotics; deep wound infection was defined as any related infection within 1 year of injury, requiring intravenous antibiotics or surgical débridement in the operating room. The review for complications included superficial infection, deep infection, urinary tract infection, pneumonia, pulmonary embolism, deep venous thrombosis, acute renal failure or insufficiency, nonunion, delayed union, compartment syndrome, osteomyelitis, nerve palsy, anoxic brain injury, cardiac ischemia or infarct, pancreatitis, and death.
Statistical Methods
The primary focus of this analysis was to determine if patients’ risk of complication at 1-year follow-up was affected by anemia—lowest recorded Hgb level before first transfusion for transfused patients, or lowest Hgb level during hospital stay for nontransfused patients—or whether transfusion itself might be a risk factor for complication. Multiple logistic regression models were used to determine the likelihood each group would have a complication. The dependent variable was complication rate; the explanatory variables included whether the patient was transfused, anemia/Hgb level (under 7 g/dL vs 7 g/dL or higher), and the 2-way interaction. Other possible explanatory variables entered into the model were age, sex, ISS, and whether the patient had had multiple surgeries. As the sample size was small, these variables were entered into the regression model one at a time. Results are presented as odds ratios (ORs) with corresponding 95% confidence intervals (CIs) and P values. The analysis was performed with SAS Version 9.1 (SAS Institute, Cary, North Carolina). Tests were considered statistically significant with P < .05 and marginally significant with P < .10. OR above 1 indicated that the odds of a complication occurring were higher in the exposed group (transfused patients) than in the unexposed group (nontransfused patients).
Results
The charts of 104 patients were reviewed and included in this analysis. Sixty-two patients (60%) had received a transfusion; 42 (40%) had not. Before first transfusion, 21 (34%) of the 62 transfused patients had Hgb levels under 7.0 g/dL, and the other 41 (66%) had Hgb levels of 7.0 g/dL or higher. Of the 42 nontransfused patients, 8 (19%) had lowest Hgb levels under 7.0 g/dL, and the other 34 (81%) had Hgb levels of 7.0 g/dL or higher (Table 1).
The transfused patients, considering all levels of anemia, had a mean ISS of 16.1 (range, 1-45), a mean of 2.0 operations (range, 1-6), a mean hospital stay of 18 days (range, 1-73 days), and a mean age of 34 years (range, 18-50 years). The nontransfused patients, considering all levels of anemia, had a mean ISS of 14.1 (range, 4-43), a mean of 1.4 operations (range, 1-5), a mean hospital stay of 10 days (range, 1-42 days), and a mean age of 33 years (range, 18-50 years). In the transfusion group, the mean number of transfused pRBC units was 6.9 (range, 1-31), or 7.8 units for patients with Hgb levels under 7 g/dL and 6.4 units for patients with Hgb levels of 7 g/dL or higher. At 1-year follow-up, complications were observed in 41 (66%) of the 62 transfused patients and in 17 (40%) of the 42 nontransfused patients (Table 1). The different types of complications seen in each group are listed in Table 2.
Statistical Analysis
Patients were divided into 2 groups by Hgb level—under 7.0 g/dL and 7.0 g/dL or higher—and then by whether they received pRBC transfusion. In addition, which patients had a complication over a 1-year period were identified.
For each group, we calculated sample size, number of complications, complication rate, and 95% CI for proportions. For transfused patients with Hgb level of 7.0 g/dL or higher, the complication rate was 71% (29/41). For nontransfused patients with Hgb of 7.0 g/dL or higher, the complication rate was 41% (14/34). Similarly, for transfused patients with Hgb under 7.0 g/dL, the complication rate was 57% (12/21). Last, for nontransfused patients with Hgb under 7.0 g/dL, the complication rate was 38% (3/8) (Table 3).
Transfused patients had a significantly higher risk of complication (OR, 3.1; 95% CI, 1.4-7.1; P < .01). Severity of anemia was not found to be independently associated with increased risk of complication (OR, 0.6; 95% CI, 0.3-1.6; P = .33) (Table 4). The interaction term was removed and eliminated from further analysis, as it was not found to be significant (P = .45).
Furthermore, the possibility of confounding variables (eg, age, sex, ISS, number of surgeries performed) was considered by including them in the model one at a time. From these logistic regression models, which included whether patients were transfused and level of anemia, an increased risk of complication (OR, 1.8; 95% CI, 1.1-2.9; P = .02) was found for each additional surgery, while receiving transfusion remained statistically significant (OR, 2.5; 95% CI, 1.0-5.8; P < .04). Age, sex, and ISS were not shown to be significantly associated with an increased complication rate (Ps = .71, .32, and .13, respectively).
We performed a subanalysis of the transfused patients to determine the impact of number of units transfused on complication rate. Each additional unit of pRBCs transfused increased the risk of complication, indicating a dose-dependent response (OR, 1.3; 95% CI, 1.04-1.51; P = .02).
Discussion
Transfusion is a generally accepted and common intervention both in the intensive care unit and in the perioperative period. However, there is little evidence to support routine transfusion of asymptomatic orthopedic trauma patients who are no longer within the initial resuscitative period after trauma. Nevertheless, the practice is routinely done based on expert opinion (level 5 evidence). The anemia protocol for our orthopedic trauma service routinely allowed the Hgb levels of asymptomatic healthy patients to drop to under 7.0 g/dL without transfusion; when other services were consulted or were primary, however, these asymptomatic patients were still routinely transfused based on practitioners’ practice patterns and anecdotal experiences.
In hemodynamically unstable patients, there is no acceptable substitute for blood transfusion. Blood replacement remains essential in the case of acute hemorrhage. However, the complications associated with transfusion should lead us to avoid, or at least minimize, unnecessary transfusion in young asymptomatic patients who are not actively bleeding in the postresuscitative period. In our study, we did not seek causation of increased complications with transfusion but assessed whether the risk of anemia outweighed the risk of transfusion in young, healthy, asymptomatic trauma patients who were no longer in the initial resuscitation period.
Our study was designed to evaluate a conservative transfusion strategy used in orthopedic trauma patients. We hypothesized that the risk of anemia in these asymptomatic patients would be lower than the risk of transfusing asymptomatic patients in the perioperative period. In addition, we thought the level of anemia would play a less significant role in the postoperative complication rate relative to transfusion itself. Our results suggest that a more conservative transfusion strategy of allowing asymptomatic patients to become and remain anemic even to a Hgb level of 5 g/dL may be as safe as a more liberal transfusion strategy of keeping patients at a Hgb level higher than 7 g/dL. In general, the complication rate was 66% for transfused patients and 40% for nontransfused patients. These results remain significant after correcting for possible confounding factors, including age, sex, ISS, and number of surgeries.
The results of this study do not suggest that there may not be complications associated with anemia; a 40% complication rate even in the nontransfused group is high. One might expect that patients who had isolated injuries and never developed anemia with an Hgb level under 9 g/dL might have an even lower complication rate. In the group used for inclusion in this study, however, there was not a significant increased risk for patients who tolerated a lower anemia (Hgb, <7 g/dL), whereas transfusion to keep the Hgb level above 7 g/dL appeared to correlate with a significant risk of complication and appeared to be dose-dependent. It should be noted that the complications in both the transfusion and anemia groups are not necessarily related to transfusion or anemia, as many factors in a retrospective study cannot be controlled. These findings simply suggest that it might be as safe to use a conservative transfusion strategy as a liberal transfusion strategy in this patient population.
Although our study is retrospective, prospective randomized studies in the elderly and in the critical care population have shown conservative transfusion guidelines are at least as safe as liberal transfusion strategies.2,11 One study randomized intensive care unit patients with Hgb levels under 9.0 g/dL to 2 groups, one with liberal and the other with restrictive protocols for pRBC unit transfusion.2 The liberal group maintained Hgb levels between 10.0 and 12.0 g/dL, and the restricted group kept Hgb levels between 7.0 and 9.0 g/dL. Thirty-day mortality was significantly lower in less acutely ill patients and younger patients (<55 years old) in the restrictive group than in the liberal group. It was concluded that a restrictive strategy of RBC transfusion is at least as effective as, and possibly superior to, a liberal transfusion strategy in the critically ill when considering short- and long-term outcomes. Another prospective study randomized elderly patients (N = 2016) with hip fractures and cardiovascular risk factors to a liberal transfusion strategy (if Hgb level fell under 10 g/dL) or a restrictive transfusion strategy (if Hgb level fell under 8 g/dL). The study found no difference between the 2 groups.11
The deleterious effect of allogeneic blood transfusion on the immune system is complex and has been linked to the down-regulation of cellular immunity, including decreased function of natural killer cells, decreased function of macrophages and monocytes, and increased numbers of suppressor T cells.12,13 This minimized immune response has been associated with a multitude of infectious morbidities in various patient populations.7 A meta-analysis of 20 studies reviewing outcomes of the effects of transfusion on postoperative bacterial infection found strong evidence supporting a correlation.5 Their analysis found an OR of 5.3 (range, 5.0-5.4) for infectious complication after allogeneic transfusion in the trauma population, and an OR of 3.5 (range, 1.4-15.2) considering all patient populations.
Similar results showing increased risk of infectious morbidities associated with transfusion were found in other studies involving the critically ill, patients after hip arthroplasty, and cardiothoracic surgery and general trauma populations.1,3,4,14,15 Furthermore, these results were seen in a dose-dependent response leading to increased incidence of complication with each unit of blood transfused.
Our study did not focus only on infection but included other complications (eg, cardiac, renal, and brain ischemia) that might be associated with anemia or transfusion. It is intuitive that anemia can cause ischemic events but less intuitive that allogeneic transfusion can also cause ischemic events because of the poor deformability of the cells due to storage, which can lead to “sludging” in capillaries throughout the body.16 This has been shown to be important in animal models, but it is unclear what poses more risk in humans—anemia without transfusion or the initial insult from transfusion, before the body clears the “waste” from stored cells and the remaining viable cells gain oxygen-carrying capacity.
Our study has several limitations. The number of patients who had severe anemia (Hgb level, <7 g/dL) and were not transfused is relatively small compared with the numbers in the other groups used for comparison. Because our study was retrospective, we could only find associations and not prove causation. This is significant, as the higher complication rate seen with transfusions may only be caused by the transfusion as a predictor of a patient requiring more complex surgery with higher blood loss (and higher risk of complication) or other such risk factors that led to transfusion, but not the transfusion itself causing the complication. An attempt was made to remove this potential bias by controlling for age, sex, ISS, and whether the patient had multiple surgeries. However, there may have been other significant confounding variables not excluded. As complications were assessed by chart review, they may not include those that occurred at other institutions and that were never reported to the practitioners at our facility (though we did have the ability to search records of neighboring institutions electronically when electronic medical records were available). That no functional outcomes were included in this retrospective review might make the complication rate appear more or less sensitive than the patients’ own opinions regarding their outcomes. All these weaknesses could call into question whether the statistically significant higher risk associated with allogeneic transfusion found in this study is real, but the focus and reason for pursuing this study were to determine if permissive anemia was dangerous or would be associated with a higher risk of complications than routine allogeneic transfusion of asymptomatic patients to treat a laboratory value.
Strengths of the study include the review of a single surgeon’s practice with a written protocol in place for anemic orthopedic trauma patients. The 95% follow-up (104/109 patients) is good for this type of trauma population. Although this series is retrospective, it is reasonably large for a subgroup of young, healthy orthopedic trauma patients to study the effects of anemia or transfusion. Whether transfused or not, many of these patients tolerated Hgb levels under 7 g/dL, which gave a large enough comparison group to evaluate the independent effects of transfusion (or of using transfusion as a marker for complication risk) or anemia as a risk factor. As a result, it appears that a more conservative transfusion strategy may be as safe as a more liberal transfusion strategy. The results of this retrospective study were used to design a prospective multidisciplinary pilot study randomizing patients to either a liberal or a conservative transfusion strategy to determine which approach might carry higher risks of complications.
Conclusion
The results of this retrospective study suggest that a conservative transfusion strategy in a young, healthy, euvolemic asymptomatic patient who is not actively bleeding may be as safe as a liberal transfusion strategy and potentially may have fewer complications than does transfusion for a conventional laboratory value. Our study results do not suggest that transfusions should be held in patients who are symptomatic at rest or in patients who are being actively resuscitated, as transfusion can be lifesaving under these circumstances. A prospective randomized study has begun at our institution with enrollment expected to take 2 years with another year needed to complete 1-year follow-up of all patients.
More than 13 million units of blood are transfused each year. Although transfusion can certainly be lifesaving, numerous studies over the past 20 years have shown significant, dose-dependent increases in morbidity, mortality, and cost with each unit of packed red blood cells (pRBCs) transfused.1 Transfusion is one of the most common interventions in the critically ill population; however the negative effects of transfusion-related infection are well documented in the recent literature.1-7 There is no question that transfusion of blood products can be lifesaving to acutely ill trauma patients, but there is little evidence regarding when transfusions are indicated in asymptomatic anemic patients who are no longer in need of acute resuscitation.
Several studies have analyzed healthy individuals with an isovolemic reduction in hemoglobin (Hgb) level to 5.0 g/dL.8,9 They have found no significant compromise in oxygen delivery to the tissues. Currently, there is a lack of clinical data to suggest adequate RBC transfusion endpoints in trauma surgery.10 Given the lack of evidence to support transfusion triggers for young, healthy, asymptomatic orthopedic trauma patients, we decided to investigate whether a more conservative transfusion strategy might be as safe as a more liberal strategy.
Materials and Methods
After obtaining approval from our institutional review board, we performed a retrospective observational cohort analysis of patients treated at a level I trauma center between September 2006 and February 2009. The trauma registry included all patients who underwent surgery performed by a single orthopedic fellowship–trained trauma surgeon. All patients who had a recorded Hgb level of 9.0 g/dL or less at any time during their admission were included; they were considered no longer volume-depleted after initial resuscitation. Exclusion criteria were age under 18 years or over 50 years; pregnancy; head injury; and preexisting heart, pulmonary, or renal disease.
Initially, 963 patients were identified as orthopedic trauma patients treated by Dr. Mullis within the defined period. After inclusion and exclusion criteria were used to limit this database, the charts of the 109 patients who met the above criteria were reviewed. By chart review or telephone follow-up, 104 patients with 1-year follow-up were identified, and their cases became the basis for our analysis. Demographic information, length of hospital stay, surgeries performed, number of pRBC units transfused, Hgb level prompting transfusion, lowest recorded Hgb level, complications, and Injury Severity Score (ISS) were recorded for each patient. Seventy-two patients (69%) were male, 32 (31%) female. Mean age of the study population was 33 years.
Patients were divided into 2 groups by lowest Hgb level before first transfusion—under 7.0 g/dL and 7.0 g/dL or higher—and then by whether they had been transfused. General guidelines for erythrocyte transfusion on the orthopedic trauma service included patients who were symptomatic at rest (headache, dizziness, or shortness of breath) and asymptomatic patients with Hgb levels under 5.0 g/dL. For patients with varying (lesser) degrees of anemia, transfusion typically depended on clinical symptoms and overall decrease in Hgb level from that recorded on admission.
Patient charts were reviewed for complications extending through a 1-year period after initial discharge from the inpatient service. Patients who had not received follow-up treatment through a known outpatient clinic were contacted by telephone to ascertain outcome. Overall, 5 of the 109 patients were lost at 1-year follow-up, leaving 104 patients with 1-year follow-up (95%). Primary outcome of the study was postoperative complications. Superficial wound infection was defined as cellulitis near the surgical site within 1 year, requiring oral antibiotics; deep wound infection was defined as any related infection within 1 year of injury, requiring intravenous antibiotics or surgical débridement in the operating room. The review for complications included superficial infection, deep infection, urinary tract infection, pneumonia, pulmonary embolism, deep venous thrombosis, acute renal failure or insufficiency, nonunion, delayed union, compartment syndrome, osteomyelitis, nerve palsy, anoxic brain injury, cardiac ischemia or infarct, pancreatitis, and death.
Statistical Methods
The primary focus of this analysis was to determine if patients’ risk of complication at 1-year follow-up was affected by anemia—lowest recorded Hgb level before first transfusion for transfused patients, or lowest Hgb level during hospital stay for nontransfused patients—or whether transfusion itself might be a risk factor for complication. Multiple logistic regression models were used to determine the likelihood each group would have a complication. The dependent variable was complication rate; the explanatory variables included whether the patient was transfused, anemia/Hgb level (under 7 g/dL vs 7 g/dL or higher), and the 2-way interaction. Other possible explanatory variables entered into the model were age, sex, ISS, and whether the patient had had multiple surgeries. As the sample size was small, these variables were entered into the regression model one at a time. Results are presented as odds ratios (ORs) with corresponding 95% confidence intervals (CIs) and P values. The analysis was performed with SAS Version 9.1 (SAS Institute, Cary, North Carolina). Tests were considered statistically significant with P < .05 and marginally significant with P < .10. OR above 1 indicated that the odds of a complication occurring were higher in the exposed group (transfused patients) than in the unexposed group (nontransfused patients).
Results
The charts of 104 patients were reviewed and included in this analysis. Sixty-two patients (60%) had received a transfusion; 42 (40%) had not. Before first transfusion, 21 (34%) of the 62 transfused patients had Hgb levels under 7.0 g/dL, and the other 41 (66%) had Hgb levels of 7.0 g/dL or higher. Of the 42 nontransfused patients, 8 (19%) had lowest Hgb levels under 7.0 g/dL, and the other 34 (81%) had Hgb levels of 7.0 g/dL or higher (Table 1).
The transfused patients, considering all levels of anemia, had a mean ISS of 16.1 (range, 1-45), a mean of 2.0 operations (range, 1-6), a mean hospital stay of 18 days (range, 1-73 days), and a mean age of 34 years (range, 18-50 years). The nontransfused patients, considering all levels of anemia, had a mean ISS of 14.1 (range, 4-43), a mean of 1.4 operations (range, 1-5), a mean hospital stay of 10 days (range, 1-42 days), and a mean age of 33 years (range, 18-50 years). In the transfusion group, the mean number of transfused pRBC units was 6.9 (range, 1-31), or 7.8 units for patients with Hgb levels under 7 g/dL and 6.4 units for patients with Hgb levels of 7 g/dL or higher. At 1-year follow-up, complications were observed in 41 (66%) of the 62 transfused patients and in 17 (40%) of the 42 nontransfused patients (Table 1). The different types of complications seen in each group are listed in Table 2.
Statistical Analysis
Patients were divided into 2 groups by Hgb level—under 7.0 g/dL and 7.0 g/dL or higher—and then by whether they received pRBC transfusion. In addition, which patients had a complication over a 1-year period were identified.
For each group, we calculated sample size, number of complications, complication rate, and 95% CI for proportions. For transfused patients with Hgb level of 7.0 g/dL or higher, the complication rate was 71% (29/41). For nontransfused patients with Hgb of 7.0 g/dL or higher, the complication rate was 41% (14/34). Similarly, for transfused patients with Hgb under 7.0 g/dL, the complication rate was 57% (12/21). Last, for nontransfused patients with Hgb under 7.0 g/dL, the complication rate was 38% (3/8) (Table 3).
Transfused patients had a significantly higher risk of complication (OR, 3.1; 95% CI, 1.4-7.1; P < .01). Severity of anemia was not found to be independently associated with increased risk of complication (OR, 0.6; 95% CI, 0.3-1.6; P = .33) (Table 4). The interaction term was removed and eliminated from further analysis, as it was not found to be significant (P = .45).
Furthermore, the possibility of confounding variables (eg, age, sex, ISS, number of surgeries performed) was considered by including them in the model one at a time. From these logistic regression models, which included whether patients were transfused and level of anemia, an increased risk of complication (OR, 1.8; 95% CI, 1.1-2.9; P = .02) was found for each additional surgery, while receiving transfusion remained statistically significant (OR, 2.5; 95% CI, 1.0-5.8; P < .04). Age, sex, and ISS were not shown to be significantly associated with an increased complication rate (Ps = .71, .32, and .13, respectively).
We performed a subanalysis of the transfused patients to determine the impact of number of units transfused on complication rate. Each additional unit of pRBCs transfused increased the risk of complication, indicating a dose-dependent response (OR, 1.3; 95% CI, 1.04-1.51; P = .02).
Discussion
Transfusion is a generally accepted and common intervention both in the intensive care unit and in the perioperative period. However, there is little evidence to support routine transfusion of asymptomatic orthopedic trauma patients who are no longer within the initial resuscitative period after trauma. Nevertheless, the practice is routinely done based on expert opinion (level 5 evidence). The anemia protocol for our orthopedic trauma service routinely allowed the Hgb levels of asymptomatic healthy patients to drop to under 7.0 g/dL without transfusion; when other services were consulted or were primary, however, these asymptomatic patients were still routinely transfused based on practitioners’ practice patterns and anecdotal experiences.
In hemodynamically unstable patients, there is no acceptable substitute for blood transfusion. Blood replacement remains essential in the case of acute hemorrhage. However, the complications associated with transfusion should lead us to avoid, or at least minimize, unnecessary transfusion in young asymptomatic patients who are not actively bleeding in the postresuscitative period. In our study, we did not seek causation of increased complications with transfusion but assessed whether the risk of anemia outweighed the risk of transfusion in young, healthy, asymptomatic trauma patients who were no longer in the initial resuscitation period.
Our study was designed to evaluate a conservative transfusion strategy used in orthopedic trauma patients. We hypothesized that the risk of anemia in these asymptomatic patients would be lower than the risk of transfusing asymptomatic patients in the perioperative period. In addition, we thought the level of anemia would play a less significant role in the postoperative complication rate relative to transfusion itself. Our results suggest that a more conservative transfusion strategy of allowing asymptomatic patients to become and remain anemic even to a Hgb level of 5 g/dL may be as safe as a more liberal transfusion strategy of keeping patients at a Hgb level higher than 7 g/dL. In general, the complication rate was 66% for transfused patients and 40% for nontransfused patients. These results remain significant after correcting for possible confounding factors, including age, sex, ISS, and number of surgeries.
The results of this study do not suggest that there may not be complications associated with anemia; a 40% complication rate even in the nontransfused group is high. One might expect that patients who had isolated injuries and never developed anemia with an Hgb level under 9 g/dL might have an even lower complication rate. In the group used for inclusion in this study, however, there was not a significant increased risk for patients who tolerated a lower anemia (Hgb, <7 g/dL), whereas transfusion to keep the Hgb level above 7 g/dL appeared to correlate with a significant risk of complication and appeared to be dose-dependent. It should be noted that the complications in both the transfusion and anemia groups are not necessarily related to transfusion or anemia, as many factors in a retrospective study cannot be controlled. These findings simply suggest that it might be as safe to use a conservative transfusion strategy as a liberal transfusion strategy in this patient population.
Although our study is retrospective, prospective randomized studies in the elderly and in the critical care population have shown conservative transfusion guidelines are at least as safe as liberal transfusion strategies.2,11 One study randomized intensive care unit patients with Hgb levels under 9.0 g/dL to 2 groups, one with liberal and the other with restrictive protocols for pRBC unit transfusion.2 The liberal group maintained Hgb levels between 10.0 and 12.0 g/dL, and the restricted group kept Hgb levels between 7.0 and 9.0 g/dL. Thirty-day mortality was significantly lower in less acutely ill patients and younger patients (<55 years old) in the restrictive group than in the liberal group. It was concluded that a restrictive strategy of RBC transfusion is at least as effective as, and possibly superior to, a liberal transfusion strategy in the critically ill when considering short- and long-term outcomes. Another prospective study randomized elderly patients (N = 2016) with hip fractures and cardiovascular risk factors to a liberal transfusion strategy (if Hgb level fell under 10 g/dL) or a restrictive transfusion strategy (if Hgb level fell under 8 g/dL). The study found no difference between the 2 groups.11
The deleterious effect of allogeneic blood transfusion on the immune system is complex and has been linked to the down-regulation of cellular immunity, including decreased function of natural killer cells, decreased function of macrophages and monocytes, and increased numbers of suppressor T cells.12,13 This minimized immune response has been associated with a multitude of infectious morbidities in various patient populations.7 A meta-analysis of 20 studies reviewing outcomes of the effects of transfusion on postoperative bacterial infection found strong evidence supporting a correlation.5 Their analysis found an OR of 5.3 (range, 5.0-5.4) for infectious complication after allogeneic transfusion in the trauma population, and an OR of 3.5 (range, 1.4-15.2) considering all patient populations.
Similar results showing increased risk of infectious morbidities associated with transfusion were found in other studies involving the critically ill, patients after hip arthroplasty, and cardiothoracic surgery and general trauma populations.1,3,4,14,15 Furthermore, these results were seen in a dose-dependent response leading to increased incidence of complication with each unit of blood transfused.
Our study did not focus only on infection but included other complications (eg, cardiac, renal, and brain ischemia) that might be associated with anemia or transfusion. It is intuitive that anemia can cause ischemic events but less intuitive that allogeneic transfusion can also cause ischemic events because of the poor deformability of the cells due to storage, which can lead to “sludging” in capillaries throughout the body.16 This has been shown to be important in animal models, but it is unclear what poses more risk in humans—anemia without transfusion or the initial insult from transfusion, before the body clears the “waste” from stored cells and the remaining viable cells gain oxygen-carrying capacity.
Our study has several limitations. The number of patients who had severe anemia (Hgb level, <7 g/dL) and were not transfused is relatively small compared with the numbers in the other groups used for comparison. Because our study was retrospective, we could only find associations and not prove causation. This is significant, as the higher complication rate seen with transfusions may only be caused by the transfusion as a predictor of a patient requiring more complex surgery with higher blood loss (and higher risk of complication) or other such risk factors that led to transfusion, but not the transfusion itself causing the complication. An attempt was made to remove this potential bias by controlling for age, sex, ISS, and whether the patient had multiple surgeries. However, there may have been other significant confounding variables not excluded. As complications were assessed by chart review, they may not include those that occurred at other institutions and that were never reported to the practitioners at our facility (though we did have the ability to search records of neighboring institutions electronically when electronic medical records were available). That no functional outcomes were included in this retrospective review might make the complication rate appear more or less sensitive than the patients’ own opinions regarding their outcomes. All these weaknesses could call into question whether the statistically significant higher risk associated with allogeneic transfusion found in this study is real, but the focus and reason for pursuing this study were to determine if permissive anemia was dangerous or would be associated with a higher risk of complications than routine allogeneic transfusion of asymptomatic patients to treat a laboratory value.
Strengths of the study include the review of a single surgeon’s practice with a written protocol in place for anemic orthopedic trauma patients. The 95% follow-up (104/109 patients) is good for this type of trauma population. Although this series is retrospective, it is reasonably large for a subgroup of young, healthy orthopedic trauma patients to study the effects of anemia or transfusion. Whether transfused or not, many of these patients tolerated Hgb levels under 7 g/dL, which gave a large enough comparison group to evaluate the independent effects of transfusion (or of using transfusion as a marker for complication risk) or anemia as a risk factor. As a result, it appears that a more conservative transfusion strategy may be as safe as a more liberal transfusion strategy. The results of this retrospective study were used to design a prospective multidisciplinary pilot study randomizing patients to either a liberal or a conservative transfusion strategy to determine which approach might carry higher risks of complications.
Conclusion
The results of this retrospective study suggest that a conservative transfusion strategy in a young, healthy, euvolemic asymptomatic patient who is not actively bleeding may be as safe as a liberal transfusion strategy and potentially may have fewer complications than does transfusion for a conventional laboratory value. Our study results do not suggest that transfusions should be held in patients who are symptomatic at rest or in patients who are being actively resuscitated, as transfusion can be lifesaving under these circumstances. A prospective randomized study has begun at our institution with enrollment expected to take 2 years with another year needed to complete 1-year follow-up of all patients.
1. Leal-Noval SR, Rincón-Ferrari MD, García-Curiel A, et al. Transfusion of blood components and postoperative infection in patients undergoing cardiac surgery. Chest. 2001;119(5):1461-1468.
2. Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340(6):409-417.
3. Carson JL, Altman DG, Duff A, et al. Risk of bacterial infection associated with allogeneic blood transfusion among patients undergoing hip fracture repair. Transfusion. 1999;39(7):694-700.
4. Edna TH, Bjerkeset T. Association between blood transfusion and infection in injured patients. J Trauma. 1992;33(5):659-661.
5. Hill GE, Frawley WH, Griffith KE, Forestner JE, Minei JP. Allogeneic blood transfusion increases the risk of postoperative bacterial infection: a meta-analysis. J Trauma. 2003;54(5):908-914.
6. Vincent JL, Baron JF, Reinhart K, et al; ABC (Anemia and Blood Transfusion in Critical Care) Investigators. Anemia and blood transfusion in critically ill patients. JAMA. 2002;288(12):1499-1507.
7. Taylor RW, Manganaro L, O’Brien J, Trottier SJ, Parkar N, Veremakis C. Impact of allogenic packed red blood cell transfusion on nosocomial infection rates in the critically ill patient. Crit Care Med. 2002;30(10):2249-2254.
8. Leung JM, Weiskopf RB, Feiner J, et al. Electrocardiographic ST-segment changes during acute, severe isovolemic hemodilution in humans. Anesthesiology. 2000;93(4):1004-1010.
9. Weiskopf RB, Viele MK, Feiner J, et al. Human cardiovascular and metabolic response to acute, severe isovolemic anemia. JAMA. 1998;279(3):217-221.
10. Johnston P, Wynn-Jones H, Chakravarty D, Boyle A, Parker MJ. Is perioperative blood transfusion a risk factor for mortality or infection after hip fracture? J Orthop Trauma. 2006;20(10):675-679.
11. Carson JL, Terrin ML, Noveck H, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365(26):2453-2462.
12. Blumberg N. Deleterious clinical effects of transfusion immunomodulation: proven beyond a reasonable doubt. Transfusion. 2005;45(2 suppl):33S-39S.
13. Triulzi DJ, Vanek K, Ryan DH, Blumberg N. A clinical and immunologic study of blood transfusion and postoperative bacterial infection in spinal surgery. Transfusion. 1992;32(6):517-524.
14. Shorr AF, Jackson WL. Transfusion practice and nosocomial infection: assessing the evidence. Curr Opin Crit Care. 2005;11(5):468-472.
15. Engoren MC, Habib RH, Zacharias A, Schwann TA, Riordan CJ, Durham SJ. Effect of blood transfusion on long-term survival after cardiac operation. Ann Thorac Surg. 2002;74(4):1180-1186.
16. Tsai AG, Cabrales P, Intaglietta M. Microvascular perfusion upon exchange transfusion with stored red blood cells in normovolemic anemic conditions. Transfusion. 2004;44(11):1626-1634.
1. Leal-Noval SR, Rincón-Ferrari MD, García-Curiel A, et al. Transfusion of blood components and postoperative infection in patients undergoing cardiac surgery. Chest. 2001;119(5):1461-1468.
2. Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340(6):409-417.
3. Carson JL, Altman DG, Duff A, et al. Risk of bacterial infection associated with allogeneic blood transfusion among patients undergoing hip fracture repair. Transfusion. 1999;39(7):694-700.
4. Edna TH, Bjerkeset T. Association between blood transfusion and infection in injured patients. J Trauma. 1992;33(5):659-661.
5. Hill GE, Frawley WH, Griffith KE, Forestner JE, Minei JP. Allogeneic blood transfusion increases the risk of postoperative bacterial infection: a meta-analysis. J Trauma. 2003;54(5):908-914.
6. Vincent JL, Baron JF, Reinhart K, et al; ABC (Anemia and Blood Transfusion in Critical Care) Investigators. Anemia and blood transfusion in critically ill patients. JAMA. 2002;288(12):1499-1507.
7. Taylor RW, Manganaro L, O’Brien J, Trottier SJ, Parkar N, Veremakis C. Impact of allogenic packed red blood cell transfusion on nosocomial infection rates in the critically ill patient. Crit Care Med. 2002;30(10):2249-2254.
8. Leung JM, Weiskopf RB, Feiner J, et al. Electrocardiographic ST-segment changes during acute, severe isovolemic hemodilution in humans. Anesthesiology. 2000;93(4):1004-1010.
9. Weiskopf RB, Viele MK, Feiner J, et al. Human cardiovascular and metabolic response to acute, severe isovolemic anemia. JAMA. 1998;279(3):217-221.
10. Johnston P, Wynn-Jones H, Chakravarty D, Boyle A, Parker MJ. Is perioperative blood transfusion a risk factor for mortality or infection after hip fracture? J Orthop Trauma. 2006;20(10):675-679.
11. Carson JL, Terrin ML, Noveck H, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365(26):2453-2462.
12. Blumberg N. Deleterious clinical effects of transfusion immunomodulation: proven beyond a reasonable doubt. Transfusion. 2005;45(2 suppl):33S-39S.
13. Triulzi DJ, Vanek K, Ryan DH, Blumberg N. A clinical and immunologic study of blood transfusion and postoperative bacterial infection in spinal surgery. Transfusion. 1992;32(6):517-524.
14. Shorr AF, Jackson WL. Transfusion practice and nosocomial infection: assessing the evidence. Curr Opin Crit Care. 2005;11(5):468-472.
15. Engoren MC, Habib RH, Zacharias A, Schwann TA, Riordan CJ, Durham SJ. Effect of blood transfusion on long-term survival after cardiac operation. Ann Thorac Surg. 2002;74(4):1180-1186.
16. Tsai AG, Cabrales P, Intaglietta M. Microvascular perfusion upon exchange transfusion with stored red blood cells in normovolemic anemic conditions. Transfusion. 2004;44(11):1626-1634.
Osteoporosis Can Affect Men on Large Scale, Too
Significantly fewer men received evaluation for osteoporosis following a distal radial fracture, with rates of evaluation unacceptably low according to published guidelines, according to a study published November 5 in the Journal of Bone and Joint Surgery.
“Given that the prevalence of fragility fractures among men is expected to increase threefold by the year 2050, adequately evaluating and treating men for osteoporosis is of paramount importance,” said lead author Tamara Rozental, MD, an investigator in the Department of Orthopedic Surgery at Beth Israel Deaconess Medical Center and an Associate Professor of Orthopedic Surgery at Harvard Medical School.
Dr. Rozental, who specializes in hand, wrist, and elbow injuries, examined five years of data from 2007 to 2012, from patients who suffered a distal radial fracture.
“We know that a distal radial fracture can often be an early indication of bone loss. We typically see this type of fracture 10 to 15 years before we might see a hip fracture,” said Dr. Rozental. “When we treat fractures of the wrist, it gives us the opportunity to do a bone mass density evaluation and, if necessary, get patients into treatment with the goal of preventing more serious injury, like a hip fracture down the line.”
Even though existing clinical practice guidelines recommend bone mass density evaluation after hip fracture for both men and women, studies continue to show that screening rates are unacceptably low, particularly among men. Dr. Rozental examined the data to see if the same trend would play out when examining clinical follow up to wrist fractures.
Fifty-three percent of women received dual x-ray absorptiometry, compared with only 18% of men. In addition, 21% of men versus 55% of women initiated treatment with calcium and vitamin D supplements within six months of injury, and 3% of men versus 22% of women began taking bisphosphonates.
Studies have shown that men have twice the mortality rate of women both during initial hospitalization and in the year following a hip fracture. Survival rates following a wrist fracture also are lower among men.
“Treating men for bone fractures, but not the underlying cause, places them at a greater risk for future bone breaks and related complications,” said Dr. Rozental. “The results of this study lead us to suggest that men over the age of 50 with fractures of the distal radius should undergo further clinical assessment and bone density testing to better identify those at high risk for future fracture as well as those who would benefit from further treatment.”
Suggested Reading
Harper CM, Fitzpatrick SK, Zurakowski D, Rozental TD. Distal radial fractures in older men: a missed opportunity? J Bone Joint Surg Am. 2014;96(21):1820-1827.
Significantly fewer men received evaluation for osteoporosis following a distal radial fracture, with rates of evaluation unacceptably low according to published guidelines, according to a study published November 5 in the Journal of Bone and Joint Surgery.
“Given that the prevalence of fragility fractures among men is expected to increase threefold by the year 2050, adequately evaluating and treating men for osteoporosis is of paramount importance,” said lead author Tamara Rozental, MD, an investigator in the Department of Orthopedic Surgery at Beth Israel Deaconess Medical Center and an Associate Professor of Orthopedic Surgery at Harvard Medical School.
Dr. Rozental, who specializes in hand, wrist, and elbow injuries, examined five years of data from 2007 to 2012, from patients who suffered a distal radial fracture.
“We know that a distal radial fracture can often be an early indication of bone loss. We typically see this type of fracture 10 to 15 years before we might see a hip fracture,” said Dr. Rozental. “When we treat fractures of the wrist, it gives us the opportunity to do a bone mass density evaluation and, if necessary, get patients into treatment with the goal of preventing more serious injury, like a hip fracture down the line.”
Even though existing clinical practice guidelines recommend bone mass density evaluation after hip fracture for both men and women, studies continue to show that screening rates are unacceptably low, particularly among men. Dr. Rozental examined the data to see if the same trend would play out when examining clinical follow up to wrist fractures.
Fifty-three percent of women received dual x-ray absorptiometry, compared with only 18% of men. In addition, 21% of men versus 55% of women initiated treatment with calcium and vitamin D supplements within six months of injury, and 3% of men versus 22% of women began taking bisphosphonates.
Studies have shown that men have twice the mortality rate of women both during initial hospitalization and in the year following a hip fracture. Survival rates following a wrist fracture also are lower among men.
“Treating men for bone fractures, but not the underlying cause, places them at a greater risk for future bone breaks and related complications,” said Dr. Rozental. “The results of this study lead us to suggest that men over the age of 50 with fractures of the distal radius should undergo further clinical assessment and bone density testing to better identify those at high risk for future fracture as well as those who would benefit from further treatment.”
Significantly fewer men received evaluation for osteoporosis following a distal radial fracture, with rates of evaluation unacceptably low according to published guidelines, according to a study published November 5 in the Journal of Bone and Joint Surgery.
“Given that the prevalence of fragility fractures among men is expected to increase threefold by the year 2050, adequately evaluating and treating men for osteoporosis is of paramount importance,” said lead author Tamara Rozental, MD, an investigator in the Department of Orthopedic Surgery at Beth Israel Deaconess Medical Center and an Associate Professor of Orthopedic Surgery at Harvard Medical School.
Dr. Rozental, who specializes in hand, wrist, and elbow injuries, examined five years of data from 2007 to 2012, from patients who suffered a distal radial fracture.
“We know that a distal radial fracture can often be an early indication of bone loss. We typically see this type of fracture 10 to 15 years before we might see a hip fracture,” said Dr. Rozental. “When we treat fractures of the wrist, it gives us the opportunity to do a bone mass density evaluation and, if necessary, get patients into treatment with the goal of preventing more serious injury, like a hip fracture down the line.”
Even though existing clinical practice guidelines recommend bone mass density evaluation after hip fracture for both men and women, studies continue to show that screening rates are unacceptably low, particularly among men. Dr. Rozental examined the data to see if the same trend would play out when examining clinical follow up to wrist fractures.
Fifty-three percent of women received dual x-ray absorptiometry, compared with only 18% of men. In addition, 21% of men versus 55% of women initiated treatment with calcium and vitamin D supplements within six months of injury, and 3% of men versus 22% of women began taking bisphosphonates.
Studies have shown that men have twice the mortality rate of women both during initial hospitalization and in the year following a hip fracture. Survival rates following a wrist fracture also are lower among men.
“Treating men for bone fractures, but not the underlying cause, places them at a greater risk for future bone breaks and related complications,” said Dr. Rozental. “The results of this study lead us to suggest that men over the age of 50 with fractures of the distal radius should undergo further clinical assessment and bone density testing to better identify those at high risk for future fracture as well as those who would benefit from further treatment.”
Suggested Reading
Harper CM, Fitzpatrick SK, Zurakowski D, Rozental TD. Distal radial fractures in older men: a missed opportunity? J Bone Joint Surg Am. 2014;96(21):1820-1827.
Suggested Reading
Harper CM, Fitzpatrick SK, Zurakowski D, Rozental TD. Distal radial fractures in older men: a missed opportunity? J Bone Joint Surg Am. 2014;96(21):1820-1827.
Inflammation Causes Painful Sensitization in Knee Osteoarthritis
BOSTON—Inflammation related to synovitis or effusion may drive increased sensitization in knee osteoarthritis, according to research presented at the 2014 American College of Rheumatology Annual Meeting.
“It is widely recognized that the level of pain patients experience is not always what one would expect based upon what is seen on their x-rays,” said lead author Tuhina Neogi, MD, PhD, of Boston University School of Medicine.
Using data from the Multicenter Osteoarthritis Study (MOST), researchers looked at test results obtained from 1,111 subjects with or at risk of knee osteoarthritis, including x-rays, magnetic resonance imaging scans (MRI), and standardized somatosensory evaluations of two measures that give insights into the presence of sensitization. These measures were obtained at the knee at baseline and again two years later. The mean age of the subjects in the study was 66.9. The mean body mass index was 29.7, and 62% were female.
The researchers looked at how synovitis, effusion, and bone marrow lesions (BMLs) seen at the baseline assessment might be related to the new development of temporal summation in the same knee two years later among those who did not show signs of it at the baseline visit. They also assessed changes in pressure pain thresholds levels in the same knee between baseline and the visit two years later in all the subjects.
A total of 22.6% developed incident temporal summation by the two-year study visit. Between the baseline and two-year visit, changes in the pressure pain thresholds levels ranged from -7.35 to 7.15 kg/cm2. Synovitis was associated with significant decreases in pressure pain thresholds. Effusion was significantly associated with incident temporal summation. Bone marrow lesions presence or burden was not associated with temporal summation or change in pressure pain thresholds.
The study’s authors concluded that inflammation, such as that associated with synovitis or effusion, may drive sensitization in knee osteoarthritis, while bone marrow lesions do not appear to do so. Furthermore, researchers suggested that early targeting of inflammation in knee osteoarthritis may prevent sensitization and helping to reduce pain severity in people with knee osteoarthritis.
“This is the first such study in knee osteoarthritis to obtain sensitization measures at more than one time-point in such a large number of individuals, providing insights for the first time into how sensitization may develop or change over time in this disease,” said Dr. Neogi.
BOSTON—Inflammation related to synovitis or effusion may drive increased sensitization in knee osteoarthritis, according to research presented at the 2014 American College of Rheumatology Annual Meeting.
“It is widely recognized that the level of pain patients experience is not always what one would expect based upon what is seen on their x-rays,” said lead author Tuhina Neogi, MD, PhD, of Boston University School of Medicine.
Using data from the Multicenter Osteoarthritis Study (MOST), researchers looked at test results obtained from 1,111 subjects with or at risk of knee osteoarthritis, including x-rays, magnetic resonance imaging scans (MRI), and standardized somatosensory evaluations of two measures that give insights into the presence of sensitization. These measures were obtained at the knee at baseline and again two years later. The mean age of the subjects in the study was 66.9. The mean body mass index was 29.7, and 62% were female.
The researchers looked at how synovitis, effusion, and bone marrow lesions (BMLs) seen at the baseline assessment might be related to the new development of temporal summation in the same knee two years later among those who did not show signs of it at the baseline visit. They also assessed changes in pressure pain thresholds levels in the same knee between baseline and the visit two years later in all the subjects.
A total of 22.6% developed incident temporal summation by the two-year study visit. Between the baseline and two-year visit, changes in the pressure pain thresholds levels ranged from -7.35 to 7.15 kg/cm2. Synovitis was associated with significant decreases in pressure pain thresholds. Effusion was significantly associated with incident temporal summation. Bone marrow lesions presence or burden was not associated with temporal summation or change in pressure pain thresholds.
The study’s authors concluded that inflammation, such as that associated with synovitis or effusion, may drive sensitization in knee osteoarthritis, while bone marrow lesions do not appear to do so. Furthermore, researchers suggested that early targeting of inflammation in knee osteoarthritis may prevent sensitization and helping to reduce pain severity in people with knee osteoarthritis.
“This is the first such study in knee osteoarthritis to obtain sensitization measures at more than one time-point in such a large number of individuals, providing insights for the first time into how sensitization may develop or change over time in this disease,” said Dr. Neogi.
BOSTON—Inflammation related to synovitis or effusion may drive increased sensitization in knee osteoarthritis, according to research presented at the 2014 American College of Rheumatology Annual Meeting.
“It is widely recognized that the level of pain patients experience is not always what one would expect based upon what is seen on their x-rays,” said lead author Tuhina Neogi, MD, PhD, of Boston University School of Medicine.
Using data from the Multicenter Osteoarthritis Study (MOST), researchers looked at test results obtained from 1,111 subjects with or at risk of knee osteoarthritis, including x-rays, magnetic resonance imaging scans (MRI), and standardized somatosensory evaluations of two measures that give insights into the presence of sensitization. These measures were obtained at the knee at baseline and again two years later. The mean age of the subjects in the study was 66.9. The mean body mass index was 29.7, and 62% were female.
The researchers looked at how synovitis, effusion, and bone marrow lesions (BMLs) seen at the baseline assessment might be related to the new development of temporal summation in the same knee two years later among those who did not show signs of it at the baseline visit. They also assessed changes in pressure pain thresholds levels in the same knee between baseline and the visit two years later in all the subjects.
A total of 22.6% developed incident temporal summation by the two-year study visit. Between the baseline and two-year visit, changes in the pressure pain thresholds levels ranged from -7.35 to 7.15 kg/cm2. Synovitis was associated with significant decreases in pressure pain thresholds. Effusion was significantly associated with incident temporal summation. Bone marrow lesions presence or burden was not associated with temporal summation or change in pressure pain thresholds.
The study’s authors concluded that inflammation, such as that associated with synovitis or effusion, may drive sensitization in knee osteoarthritis, while bone marrow lesions do not appear to do so. Furthermore, researchers suggested that early targeting of inflammation in knee osteoarthritis may prevent sensitization and helping to reduce pain severity in people with knee osteoarthritis.
“This is the first such study in knee osteoarthritis to obtain sensitization measures at more than one time-point in such a large number of individuals, providing insights for the first time into how sensitization may develop or change over time in this disease,” said Dr. Neogi.
Total Hip Replacement: An Excellent Option to Relieve Pain in Young Juvenile Arthritis Patients
BOSTON—A new study finds that total hip replacement (THR) is an excellent option for patients under age 35, when traditional treatments fail to provide relief. The study, presented at the 2014 American College of Rheumatology Annual Meeting, found that hip replacement lasted at least 10 years in 85% of juvenile idiopathic arthritis (JIA) patients. Twenty years later, 50% of the patients needed a revision surgery.
“Joint replacement can free patients from a life of unrelenting pain. It can enable those in a wheel chair to walk again. Patients can go back to school or work and get their lives back,” said Mark P. Figgie, MD, senior author of the study and Chief of the Surgical Arthritis Service at the Hospital for Special Surgery in New York.
This study evaluated the longevity of implants in juvenile idiopathic arthritis patients ages 35 or younger who underwent hip replacement at Hospital for Special Surgery. “This study followed one of the largest cohorts of patients with JIA to see how they fared 10 years after total hip replacement,” said coinvestigator Ishaan Swarup, MD, an orthopedic resident at the Hospital for Special Surgery. “It is also one of the few studies to look at patient-reported measures, such as pain and the ability to perform activities of daily living.”
Data were collected retrospectively for 56 patients. Forty-one patients had undergone bilateral hip replacement, while 15 individuals had only one side replaced, for a total of 97 hip replacement surgeries. The mean time for follow-up was 12 years. The 10-year and 20-year implant survival was 85% and 50%, respectively.
The researchers found that hip replacement in patients who were 25 or older lasted longer compared to total hip replacement in younger patients. There were no other significant differences in implant longevity based on gender or the use of custom versus standard implants.
Overall, male patients reported better outcomes with respect to activities of daily living. Patients who had received custom hip implants did worse in their reporting of pain and the ability to perform daily activities.
“We were not surprised that the patients who received custom implants had lower scores, since the very fact that they needed a custom implant meant they had more severe joint deformities and more severe disease,” stated Dr. Figgie.
BOSTON—A new study finds that total hip replacement (THR) is an excellent option for patients under age 35, when traditional treatments fail to provide relief. The study, presented at the 2014 American College of Rheumatology Annual Meeting, found that hip replacement lasted at least 10 years in 85% of juvenile idiopathic arthritis (JIA) patients. Twenty years later, 50% of the patients needed a revision surgery.
“Joint replacement can free patients from a life of unrelenting pain. It can enable those in a wheel chair to walk again. Patients can go back to school or work and get their lives back,” said Mark P. Figgie, MD, senior author of the study and Chief of the Surgical Arthritis Service at the Hospital for Special Surgery in New York.
This study evaluated the longevity of implants in juvenile idiopathic arthritis patients ages 35 or younger who underwent hip replacement at Hospital for Special Surgery. “This study followed one of the largest cohorts of patients with JIA to see how they fared 10 years after total hip replacement,” said coinvestigator Ishaan Swarup, MD, an orthopedic resident at the Hospital for Special Surgery. “It is also one of the few studies to look at patient-reported measures, such as pain and the ability to perform activities of daily living.”
Data were collected retrospectively for 56 patients. Forty-one patients had undergone bilateral hip replacement, while 15 individuals had only one side replaced, for a total of 97 hip replacement surgeries. The mean time for follow-up was 12 years. The 10-year and 20-year implant survival was 85% and 50%, respectively.
The researchers found that hip replacement in patients who were 25 or older lasted longer compared to total hip replacement in younger patients. There were no other significant differences in implant longevity based on gender or the use of custom versus standard implants.
Overall, male patients reported better outcomes with respect to activities of daily living. Patients who had received custom hip implants did worse in their reporting of pain and the ability to perform daily activities.
“We were not surprised that the patients who received custom implants had lower scores, since the very fact that they needed a custom implant meant they had more severe joint deformities and more severe disease,” stated Dr. Figgie.
BOSTON—A new study finds that total hip replacement (THR) is an excellent option for patients under age 35, when traditional treatments fail to provide relief. The study, presented at the 2014 American College of Rheumatology Annual Meeting, found that hip replacement lasted at least 10 years in 85% of juvenile idiopathic arthritis (JIA) patients. Twenty years later, 50% of the patients needed a revision surgery.
“Joint replacement can free patients from a life of unrelenting pain. It can enable those in a wheel chair to walk again. Patients can go back to school or work and get their lives back,” said Mark P. Figgie, MD, senior author of the study and Chief of the Surgical Arthritis Service at the Hospital for Special Surgery in New York.
This study evaluated the longevity of implants in juvenile idiopathic arthritis patients ages 35 or younger who underwent hip replacement at Hospital for Special Surgery. “This study followed one of the largest cohorts of patients with JIA to see how they fared 10 years after total hip replacement,” said coinvestigator Ishaan Swarup, MD, an orthopedic resident at the Hospital for Special Surgery. “It is also one of the few studies to look at patient-reported measures, such as pain and the ability to perform activities of daily living.”
Data were collected retrospectively for 56 patients. Forty-one patients had undergone bilateral hip replacement, while 15 individuals had only one side replaced, for a total of 97 hip replacement surgeries. The mean time for follow-up was 12 years. The 10-year and 20-year implant survival was 85% and 50%, respectively.
The researchers found that hip replacement in patients who were 25 or older lasted longer compared to total hip replacement in younger patients. There were no other significant differences in implant longevity based on gender or the use of custom versus standard implants.
Overall, male patients reported better outcomes with respect to activities of daily living. Patients who had received custom hip implants did worse in their reporting of pain and the ability to perform daily activities.
“We were not surprised that the patients who received custom implants had lower scores, since the very fact that they needed a custom implant meant they had more severe joint deformities and more severe disease,” stated Dr. Figgie.
Manual Therapy and Exercise Improve Pain and Function in Osteoarthritis
BOSTON—Patients with hip and knee osteoarthritis (OA) may improve their pain, stiffness, and physical function with sustained physical exercise, manual therapy, or both, according to research presented at the 2014 American College of Rheumatology Annual Meeting.
“The aim of this study was to establish whether providing a comprehensive program of exercise or manual therapy results in significant additional benefits, over and above usual medical care,” said lead author J. Haxby Abbott, DPT, PhD, at the University of Otago in Dunedin, New Zealand.
The participants’ progress was measured using the Western Ontario and McMaster (WOMAC) osteoarthritis index, which calculates scores on a scale of 0 to 240. Lower WOMAC scores indicate improvements in pain, stiffness, and physical function. Participants were also given several physical performance tests, Timed Up and Go, 40-meter fast-paced walk, and a 30-second sit-to-stand. At baseline, the mean age of the osteoarthritis patients in the study was 66, with a mean WOMAC score of 100.8.
After two years, all the participants who engaged in regular exercise, manual therapy, or a combination of both showed improved WOMAC scores that were superior to those who had only the usual medical care for osteoarthritis.
Participants receiving exercise therapy in addition to their usual care showed improvement of 31.7 WOMAC points compared to usual care alone. Participants receiving manual therapy in addition to their usual care showed a relative improvement of 30.1 WOMAC points.
While the difference in WOMAC improvement for participants receiving combined exercise therapy and manual therapy in addition to usual care did not meet the a priori threshold for clinical significance (28 points), there was a trend towards benefit; with this group improving 26.2 WOMAC points more than usual care only. Those participants in the exercise therapy group showed greater mean changes on most physical performance tests than anyone in the other groups.
Adding either exercise therapy or manual therapy to usual medical care is beneficial for people with hip and knee osteoarthritis, the study’s authors concluded. “This study showed that benefits imparted by a comprehensive program of exercise therapy or manual therapy, provided by physical therapists, remain significant to at least two years follow-up,” said Dr. Abbott.
BOSTON—Patients with hip and knee osteoarthritis (OA) may improve their pain, stiffness, and physical function with sustained physical exercise, manual therapy, or both, according to research presented at the 2014 American College of Rheumatology Annual Meeting.
“The aim of this study was to establish whether providing a comprehensive program of exercise or manual therapy results in significant additional benefits, over and above usual medical care,” said lead author J. Haxby Abbott, DPT, PhD, at the University of Otago in Dunedin, New Zealand.
The participants’ progress was measured using the Western Ontario and McMaster (WOMAC) osteoarthritis index, which calculates scores on a scale of 0 to 240. Lower WOMAC scores indicate improvements in pain, stiffness, and physical function. Participants were also given several physical performance tests, Timed Up and Go, 40-meter fast-paced walk, and a 30-second sit-to-stand. At baseline, the mean age of the osteoarthritis patients in the study was 66, with a mean WOMAC score of 100.8.
After two years, all the participants who engaged in regular exercise, manual therapy, or a combination of both showed improved WOMAC scores that were superior to those who had only the usual medical care for osteoarthritis.
Participants receiving exercise therapy in addition to their usual care showed improvement of 31.7 WOMAC points compared to usual care alone. Participants receiving manual therapy in addition to their usual care showed a relative improvement of 30.1 WOMAC points.
While the difference in WOMAC improvement for participants receiving combined exercise therapy and manual therapy in addition to usual care did not meet the a priori threshold for clinical significance (28 points), there was a trend towards benefit; with this group improving 26.2 WOMAC points more than usual care only. Those participants in the exercise therapy group showed greater mean changes on most physical performance tests than anyone in the other groups.
Adding either exercise therapy or manual therapy to usual medical care is beneficial for people with hip and knee osteoarthritis, the study’s authors concluded. “This study showed that benefits imparted by a comprehensive program of exercise therapy or manual therapy, provided by physical therapists, remain significant to at least two years follow-up,” said Dr. Abbott.
BOSTON—Patients with hip and knee osteoarthritis (OA) may improve their pain, stiffness, and physical function with sustained physical exercise, manual therapy, or both, according to research presented at the 2014 American College of Rheumatology Annual Meeting.
“The aim of this study was to establish whether providing a comprehensive program of exercise or manual therapy results in significant additional benefits, over and above usual medical care,” said lead author J. Haxby Abbott, DPT, PhD, at the University of Otago in Dunedin, New Zealand.
The participants’ progress was measured using the Western Ontario and McMaster (WOMAC) osteoarthritis index, which calculates scores on a scale of 0 to 240. Lower WOMAC scores indicate improvements in pain, stiffness, and physical function. Participants were also given several physical performance tests, Timed Up and Go, 40-meter fast-paced walk, and a 30-second sit-to-stand. At baseline, the mean age of the osteoarthritis patients in the study was 66, with a mean WOMAC score of 100.8.
After two years, all the participants who engaged in regular exercise, manual therapy, or a combination of both showed improved WOMAC scores that were superior to those who had only the usual medical care for osteoarthritis.
Participants receiving exercise therapy in addition to their usual care showed improvement of 31.7 WOMAC points compared to usual care alone. Participants receiving manual therapy in addition to their usual care showed a relative improvement of 30.1 WOMAC points.
While the difference in WOMAC improvement for participants receiving combined exercise therapy and manual therapy in addition to usual care did not meet the a priori threshold for clinical significance (28 points), there was a trend towards benefit; with this group improving 26.2 WOMAC points more than usual care only. Those participants in the exercise therapy group showed greater mean changes on most physical performance tests than anyone in the other groups.
Adding either exercise therapy or manual therapy to usual medical care is beneficial for people with hip and knee osteoarthritis, the study’s authors concluded. “This study showed that benefits imparted by a comprehensive program of exercise therapy or manual therapy, provided by physical therapists, remain significant to at least two years follow-up,” said Dr. Abbott.
The Patient Relations and Service Recovery Guide: A Colorful Approach to Handling Upset and Angry Patients
Tearful breakdowns and loud outbursts—they happen with orthopedic patients even in the best of practices. And if you are an orthopedic surgeon who has rarely or never experienced a patient in emotional distress, just talk with your staff—they have no doubt experienced this many times.
There is something about orthopedic conditions—they carry with them an increased likelihood of emotional adverse effects for patients and their loved ones. Inhibited movement can lead to palpable frustration and depression. Time off from work may cause financial hardship and an identity crisis for a family breadwinner. Physical pain can cause the patient to become depressed, angry, or dependent on prescription medication. Medications can cause a change in disposition or outlook. These realities make orthopedic surgery practices particularly predisposed to patient relations risks and service recovery opportunities.
As a practice management consultant and former executive director of an orthopedic practice, I have observed and participated in patient relations and service recovery efforts at many levels. Particularly proud of the way our staff and physicians prevented and handled these and having spent many years traveling by air under the color-coded TSA (Transportation Security Administration) security level indicator system, I created the Patient Relations and Service Recovery Guide (Figure) to help practices gain perspective, have a vocabulary, and develop practical methods for mitigating patient relations risks and responding to incidents and complaints.
The Patient Relations and Service Recovery Guide
The Patient Relations and Service Recovery Guide shows the relationship between the practice as a whole and the patient as an individual.
Green and Red
Green describes the elements of service orientation that the practice must consistently demonstrate and convey to each individual from the point of access, through treatment, and, finally, during account settlement. If you think you have a systemic problem with anything under the Green heading, you probably need a practice management or service orientation consultant, not this article. Red shows the other end of the spectrum—a complete degeneration, worst-case scenario. As with problems in the Green category, this article will not help you in these Red situations, for which you need experienced legal counsel immediately.
We’ll now explore the stories, challenges, opportunities, and practical suggestions for the Blue, Yellow, and Orange categories. The Blue and Yellow categories in the Figure are shaded in grey as a depiction of the interactive, fluid nature of these situations. In addition, they are situations that have developed and can be resolved within and by the practice.
Blue
Patients are very comfortable complaining to the receptionist, x-ray technician, and medical assistant about any number of perceived shortcomings, but when you walk in the examination room, not a word. This is a reality I am sure you have heard about from your staff, and it puts them in a position to observe and determine if a patient’s frustration is escalating. Telephone and front desk receptions are first in line. Patients will say to a telephone receptionist, “I have called 3 times yesterday and twice today and the doctor/nurse still hasn’t called back.” Front desk receptionists will also observe dynamics in the seating area. Staff are your partners in patient relations and service recovery. Working together effectively will help you address issues in the Blue and Yellow areas.
Create an environment that prevents patient discontent and supports service orientation goals. A hospital-based practice that I once managed was a flagship for service excellence goals of a Fortune 150 corporation, had a large seating area, and was close to the airport in a city with multiple company properties; we frequently had executives showing up unannounced, and, because of company politics, it seemed like they were actively looking for instances of substandard service. More importantly, though, we had patients. We established “Waiting Stories” as a performance standard for the receptionists. That is, at any moment, the receptionist was able to recap the “story” of each person in the seating area. The “story” is the reason the person was there, the appointment time, and the cause of the delay, if the wait time was excessive. We all knew this was a performance standard for our practice, so if a receptionist called back to the clinic to find out the reason for a backup in throughput, everyone was respectful and responsive to the inquiry.
The receptionists quickly became effective in judging situations and mitigating or avoiding breakdowns in service and communication. We also implemented an easy and quick notification code for when they needed help handling a service recovery situation. The responses and support in those situations were unwavering, consistent, and blame-free. We would debrief after a significant situation was resolved to determine if there were systemic or response improvement opportunities.
Communication among staff is essential for preventing or mitigating patient discontent. All practices experience service and throughput errors occasionally: a quiet, uncomplaining patient inadvertently doesn’t get called back and remains in the seating area unnoticed; a call doesn’t get returned; x-ray breaks down and a spine patient has to make a painful walk; the physician has to interrupt the encounter to take an important call; etc. Stuff happens. Individually, these breaches are tolerable to most patients. Unfortunately, there can be a cumulative element—when various service mishaps happen to the same patient. This is when communication and support among the staff and with the manager become especially important. If a patient has weathered a rough or long wait or has expressed some dissatisfaction while in the reception area, it’s probably a good idea to let the back-office staff know, so they can show a little extra compassion and be cognizant of additional situations.
Clinical staff and the physicians must convey support and appreciation to front-line staff who observe and share that a patient may be prone to distress, so that they will continue to participate in active incident prevention and service recovery.
Heightening awareness on the part of your staff—especially, receptionists, technicians, medical assistants, and collectors—goes a long way toward getting patient discontent issues settled before they get out of hand. As executive director of a large orthopedic surgery practice, I was particularly proud of our staff’s sensitivity to patient discontent, their sense of when it might be helpful to bring in a manager, and the managers’ ability both to recover many situations and to know when it was most effective to get help and support from either one of the executive team or physicians.
I can remember one patient that both front-line staff and the manager determined needed some service recovery intervention. She had been visibly upset at the end of her final postoperative visit with the physician. The staff noticed and called the manager in. The patient mentioned to the manager that she had been to another orthopedic surgeon who had told her that the surgery our physician had performed was not the right one and that he would have done things differently. The patient said she just didn’t know what to do. Our manager had the keen sense to know that she should get help to recover the situation within our practice. She and all of the staff were always supported when they asked for help, and the physicians were good about expressing their gratitude to the staff for their efforts. The manager escorted the upset patient to my office where we talked—well, she mostly talked and I listened. It turned out that her injuries had prevented her from attending games during her only child’s senior soccer season. I know, it sounds more like therapy—it was a lot of listening and compassion on my part. Eventually, she got around to thanking me for listening. And while that was not the end of it (there was another conversation), she did not take any action against our physician. See the “Talking It Through” Box.
Another group of staff who can identify issues is billing and collections. Often a patient will experience a minor cumulative series of service breaches (eg, long wait, perceived physician distraction, long hold times on the phone) and then lose it when they receive a bill that is incorrect, late, or confusing. The staff members answering those calls also need to feel supported in asking for help from a manager or another associate, either during the call or by suggesting that someone call the patient back.
Empowering staff or managers with tangible service recovery courtesies is also a good idea. We gave our staff coupons from the sundries shop in the building, so that when experiencing a particularly long wait, the patient could go down and get a complimentary snack. We also had 1 or 2 occasions when a patient drove a great distance to see the physician and experienced a significant service breach. As part of our response, we gave the patient a gas card.
Blue is the category in which the staff’s keen observation and true teamwork and support come into play when a situation or developing situation is identified.
Yellow
Yellow, while still contained within the practice, is overt. There has been an incident and/or a communication (letter or call) to the manager or physician. In Yellow, we are beyond the cooperative staff observation and sensory skills—we know something has happened. A situation might be physical- and/or facility-based, eg, a patient or family member had a minor stumble on a doormat, and though luckily they had not appeared injured and the physician checked them out, it was an incident. The other sign of a Yellow situation is that a patient or family member has written a letter to the practice to express their dissatisfaction. In either case, as dreadful as it may seem or as busy as you may be, follow up promptly.
In the case of an incident in the practice, the doctor or manager can call the patient that evening to check in and make sure all is well. Upon receiving a letter, the treating physician and manager should take a minute to discuss and agree on a response plan. Sometimes the situation may call for patient discharge from the practice—only the physician can determine that. Other times, the content of the letter may cause you to consult an attorney or your malpractice insurance carrier. The letters sometimes voice service-oriented complaints and can be addressed by the manager with a phone call and conversation as described in the Blue section above.
Orange
As a consultant, I have assisted many physicians in responding to individual patient complaints to their state medical board (SMB). I have seen a 15-page, single-spaced, typewritten letter with photographs (of the patient’s 70-lb pannus, no less), a 4-sentence letter in childlike grammar and handwriting, and many in between. The spelling, grammar, punctuation, coherence, and brevity of the letter do not matter. Your feelings on the validity of the complaint (ie, “That’s total BS!”) don’t matter. The perceived mental health of the patient (ie,“Well, he’s crazy! Ask my nurse.”) does not matter. Your SMB takes each and every complaint letter very seriously and so must you. One complaint spiraling out of control can be all it takes for you to lose your license. Having said that, individual patient SMB complaints are not uncommon; even the best physicians receive them.
Here are some thoughts to keep in mind regarding individual patient SMB complaints. An individual patient SMB complaint:
◾ Typically comes to you via US mail with no receipt signature required. Lots of us do so much online these days we can go weeks, perhaps months, without looking at our mail—even if staff members have opened it.
Suggestion: Make sure the staff looks at mail and is able to judge what requires action and what should be brought to your attention. Provide appreciation and detailed feedback when staff members bring something to you and do not misdirect negative reactions regardless of the content. You would rather staff members feel comfortable bringing something to your attention that is immaterial than keep something important from you out of fear of displeasing you.
◾ Includes a SMB response deadline that may give you as little as 1 or 2 weeks.
Suggestion: Meet the deadline. If you have or are going to miss the deadline or know that you cannot meet it, have your staff call the SMB office and abjectly request an extension.
◾ Is coming from physicians as members of the SMB, even though it may have the names of physicians you know, perhaps friends, on the letterhead.
Suggestions:
1. The physicians are not your colleagues in this situation. In this capacity, each physician is a member of an oversight board that serves and protects the people of your state. Don’t try to address the situation with a phone call or comment on the golf course.
2. Reply in the format the board has requested—a letter. Open your response letter with a statement that acknowledges the work and responsibility of the SMB and your appreciation, for example:
Esteemed Board,
While I regret that a patient complaint associated with me has come to your attention, I am grateful that the physicians and the people of [your state] have an oversight body to ensure the integrity of medical care delivered and received. Thank you for your service.
◾ Is likely to make you feel angry, indignant, unappreciated, hurt, bewildered, etc.
Suggestion: Breathe, vent to someone you can trust, exercise, get a good night’s sleep, and/or other calming, self-preservation tactics. Repeat as necessary so as not to allow these emotions a place in your response.
◾ May or may not include a request for a copy of the complete medical record.
Suggestion: If the medical record is not requested, do not send it. If the medical record is requested, send it in its entirety, as is. Do not make changes, edits, or amendments to the medical record as a response to the complaint.
◾ May be brief, vague, long, articulate, well thought-out and well structured, and/or ridiculous. Regardless of education level, profession, age, and socioeconomic status, any of your patients may write a complaint letter to the SMB, who then must address it.
Suggestions:
1. Demonstrate respect for the board’s time and service by writing a response letter of respectable length and substance regardless of the brevity of the complaint. Brief responses to the SMB may be perceived as arrogant and irreverent, and this is the exact situation and group of people in the entire state in which and before whom you do not want to be thought of that way.
2. Summarize the case with detail and substance in the letter, even if the medical record will be included in the response. Identify the actual complaints and address them in an organized way, an objective voice, and a logical order. Describe the time, thought, and follow-up you have put into addressing the situation. For instance, if the complaint includes a legitimate reference to a delay in test results or an unreturned phone call, provide a broad description of having reviewed and modified the process with your staff to understand where the gap occurred and having taken measures to help keep it from happening again.
◾ Will likely require that a copy of your response be made available or sent to the complainant.
Suggestions:
1. You are writing to 2, maybe 3, recipients: the SMB, the complainant, and the complainant’s attorney. Even if it is clear the patient did not consult a lawyer to write the complaint, it is best to write the response as though it will be read by an attorney.
2. Take the time and deliberation necessary for a multiple-draft writing process. Get help from someone to assure you have addressed all the issues in an organized, objective way.
◾ May lead to a request from the SMB that you appear before them in response to the original complaint letter and/or to clarify your response to a complaint letter. This is an indication of an investigation that has escalated beyond the patient SMB complaint letters addressed in this article; consult an experienced attorney who represents you.
Sometimes other state oversight bodies will receive complaints directly from patients and follow up with you. Consult your attorney, risk management consultant, or malpractice coverage representative for guidance if you are unsure as to the jurisdiction or how to respond.
Conclusion
Most of your practice operates in the Green, no doubt. It is simply not noticeable or memorable when everything goes smoothly. When incidents occur that require service recovery, I hope this guide and commentary will offer perspective on the full range of patient relations and service recovery, provide stories and experiences that might help, and offer general tips and suggestions.
Tearful breakdowns and loud outbursts—they happen with orthopedic patients even in the best of practices. And if you are an orthopedic surgeon who has rarely or never experienced a patient in emotional distress, just talk with your staff—they have no doubt experienced this many times.
There is something about orthopedic conditions—they carry with them an increased likelihood of emotional adverse effects for patients and their loved ones. Inhibited movement can lead to palpable frustration and depression. Time off from work may cause financial hardship and an identity crisis for a family breadwinner. Physical pain can cause the patient to become depressed, angry, or dependent on prescription medication. Medications can cause a change in disposition or outlook. These realities make orthopedic surgery practices particularly predisposed to patient relations risks and service recovery opportunities.
As a practice management consultant and former executive director of an orthopedic practice, I have observed and participated in patient relations and service recovery efforts at many levels. Particularly proud of the way our staff and physicians prevented and handled these and having spent many years traveling by air under the color-coded TSA (Transportation Security Administration) security level indicator system, I created the Patient Relations and Service Recovery Guide (Figure) to help practices gain perspective, have a vocabulary, and develop practical methods for mitigating patient relations risks and responding to incidents and complaints.
The Patient Relations and Service Recovery Guide
The Patient Relations and Service Recovery Guide shows the relationship between the practice as a whole and the patient as an individual.
Green and Red
Green describes the elements of service orientation that the practice must consistently demonstrate and convey to each individual from the point of access, through treatment, and, finally, during account settlement. If you think you have a systemic problem with anything under the Green heading, you probably need a practice management or service orientation consultant, not this article. Red shows the other end of the spectrum—a complete degeneration, worst-case scenario. As with problems in the Green category, this article will not help you in these Red situations, for which you need experienced legal counsel immediately.
We’ll now explore the stories, challenges, opportunities, and practical suggestions for the Blue, Yellow, and Orange categories. The Blue and Yellow categories in the Figure are shaded in grey as a depiction of the interactive, fluid nature of these situations. In addition, they are situations that have developed and can be resolved within and by the practice.
Blue
Patients are very comfortable complaining to the receptionist, x-ray technician, and medical assistant about any number of perceived shortcomings, but when you walk in the examination room, not a word. This is a reality I am sure you have heard about from your staff, and it puts them in a position to observe and determine if a patient’s frustration is escalating. Telephone and front desk receptions are first in line. Patients will say to a telephone receptionist, “I have called 3 times yesterday and twice today and the doctor/nurse still hasn’t called back.” Front desk receptionists will also observe dynamics in the seating area. Staff are your partners in patient relations and service recovery. Working together effectively will help you address issues in the Blue and Yellow areas.
Create an environment that prevents patient discontent and supports service orientation goals. A hospital-based practice that I once managed was a flagship for service excellence goals of a Fortune 150 corporation, had a large seating area, and was close to the airport in a city with multiple company properties; we frequently had executives showing up unannounced, and, because of company politics, it seemed like they were actively looking for instances of substandard service. More importantly, though, we had patients. We established “Waiting Stories” as a performance standard for the receptionists. That is, at any moment, the receptionist was able to recap the “story” of each person in the seating area. The “story” is the reason the person was there, the appointment time, and the cause of the delay, if the wait time was excessive. We all knew this was a performance standard for our practice, so if a receptionist called back to the clinic to find out the reason for a backup in throughput, everyone was respectful and responsive to the inquiry.
The receptionists quickly became effective in judging situations and mitigating or avoiding breakdowns in service and communication. We also implemented an easy and quick notification code for when they needed help handling a service recovery situation. The responses and support in those situations were unwavering, consistent, and blame-free. We would debrief after a significant situation was resolved to determine if there were systemic or response improvement opportunities.
Communication among staff is essential for preventing or mitigating patient discontent. All practices experience service and throughput errors occasionally: a quiet, uncomplaining patient inadvertently doesn’t get called back and remains in the seating area unnoticed; a call doesn’t get returned; x-ray breaks down and a spine patient has to make a painful walk; the physician has to interrupt the encounter to take an important call; etc. Stuff happens. Individually, these breaches are tolerable to most patients. Unfortunately, there can be a cumulative element—when various service mishaps happen to the same patient. This is when communication and support among the staff and with the manager become especially important. If a patient has weathered a rough or long wait or has expressed some dissatisfaction while in the reception area, it’s probably a good idea to let the back-office staff know, so they can show a little extra compassion and be cognizant of additional situations.
Clinical staff and the physicians must convey support and appreciation to front-line staff who observe and share that a patient may be prone to distress, so that they will continue to participate in active incident prevention and service recovery.
Heightening awareness on the part of your staff—especially, receptionists, technicians, medical assistants, and collectors—goes a long way toward getting patient discontent issues settled before they get out of hand. As executive director of a large orthopedic surgery practice, I was particularly proud of our staff’s sensitivity to patient discontent, their sense of when it might be helpful to bring in a manager, and the managers’ ability both to recover many situations and to know when it was most effective to get help and support from either one of the executive team or physicians.
I can remember one patient that both front-line staff and the manager determined needed some service recovery intervention. She had been visibly upset at the end of her final postoperative visit with the physician. The staff noticed and called the manager in. The patient mentioned to the manager that she had been to another orthopedic surgeon who had told her that the surgery our physician had performed was not the right one and that he would have done things differently. The patient said she just didn’t know what to do. Our manager had the keen sense to know that she should get help to recover the situation within our practice. She and all of the staff were always supported when they asked for help, and the physicians were good about expressing their gratitude to the staff for their efforts. The manager escorted the upset patient to my office where we talked—well, she mostly talked and I listened. It turned out that her injuries had prevented her from attending games during her only child’s senior soccer season. I know, it sounds more like therapy—it was a lot of listening and compassion on my part. Eventually, she got around to thanking me for listening. And while that was not the end of it (there was another conversation), she did not take any action against our physician. See the “Talking It Through” Box.
Another group of staff who can identify issues is billing and collections. Often a patient will experience a minor cumulative series of service breaches (eg, long wait, perceived physician distraction, long hold times on the phone) and then lose it when they receive a bill that is incorrect, late, or confusing. The staff members answering those calls also need to feel supported in asking for help from a manager or another associate, either during the call or by suggesting that someone call the patient back.
Empowering staff or managers with tangible service recovery courtesies is also a good idea. We gave our staff coupons from the sundries shop in the building, so that when experiencing a particularly long wait, the patient could go down and get a complimentary snack. We also had 1 or 2 occasions when a patient drove a great distance to see the physician and experienced a significant service breach. As part of our response, we gave the patient a gas card.
Blue is the category in which the staff’s keen observation and true teamwork and support come into play when a situation or developing situation is identified.
Yellow
Yellow, while still contained within the practice, is overt. There has been an incident and/or a communication (letter or call) to the manager or physician. In Yellow, we are beyond the cooperative staff observation and sensory skills—we know something has happened. A situation might be physical- and/or facility-based, eg, a patient or family member had a minor stumble on a doormat, and though luckily they had not appeared injured and the physician checked them out, it was an incident. The other sign of a Yellow situation is that a patient or family member has written a letter to the practice to express their dissatisfaction. In either case, as dreadful as it may seem or as busy as you may be, follow up promptly.
In the case of an incident in the practice, the doctor or manager can call the patient that evening to check in and make sure all is well. Upon receiving a letter, the treating physician and manager should take a minute to discuss and agree on a response plan. Sometimes the situation may call for patient discharge from the practice—only the physician can determine that. Other times, the content of the letter may cause you to consult an attorney or your malpractice insurance carrier. The letters sometimes voice service-oriented complaints and can be addressed by the manager with a phone call and conversation as described in the Blue section above.
Orange
As a consultant, I have assisted many physicians in responding to individual patient complaints to their state medical board (SMB). I have seen a 15-page, single-spaced, typewritten letter with photographs (of the patient’s 70-lb pannus, no less), a 4-sentence letter in childlike grammar and handwriting, and many in between. The spelling, grammar, punctuation, coherence, and brevity of the letter do not matter. Your feelings on the validity of the complaint (ie, “That’s total BS!”) don’t matter. The perceived mental health of the patient (ie,“Well, he’s crazy! Ask my nurse.”) does not matter. Your SMB takes each and every complaint letter very seriously and so must you. One complaint spiraling out of control can be all it takes for you to lose your license. Having said that, individual patient SMB complaints are not uncommon; even the best physicians receive them.
Here are some thoughts to keep in mind regarding individual patient SMB complaints. An individual patient SMB complaint:
◾ Typically comes to you via US mail with no receipt signature required. Lots of us do so much online these days we can go weeks, perhaps months, without looking at our mail—even if staff members have opened it.
Suggestion: Make sure the staff looks at mail and is able to judge what requires action and what should be brought to your attention. Provide appreciation and detailed feedback when staff members bring something to you and do not misdirect negative reactions regardless of the content. You would rather staff members feel comfortable bringing something to your attention that is immaterial than keep something important from you out of fear of displeasing you.
◾ Includes a SMB response deadline that may give you as little as 1 or 2 weeks.
Suggestion: Meet the deadline. If you have or are going to miss the deadline or know that you cannot meet it, have your staff call the SMB office and abjectly request an extension.
◾ Is coming from physicians as members of the SMB, even though it may have the names of physicians you know, perhaps friends, on the letterhead.
Suggestions:
1. The physicians are not your colleagues in this situation. In this capacity, each physician is a member of an oversight board that serves and protects the people of your state. Don’t try to address the situation with a phone call or comment on the golf course.
2. Reply in the format the board has requested—a letter. Open your response letter with a statement that acknowledges the work and responsibility of the SMB and your appreciation, for example:
Esteemed Board,
While I regret that a patient complaint associated with me has come to your attention, I am grateful that the physicians and the people of [your state] have an oversight body to ensure the integrity of medical care delivered and received. Thank you for your service.
◾ Is likely to make you feel angry, indignant, unappreciated, hurt, bewildered, etc.
Suggestion: Breathe, vent to someone you can trust, exercise, get a good night’s sleep, and/or other calming, self-preservation tactics. Repeat as necessary so as not to allow these emotions a place in your response.
◾ May or may not include a request for a copy of the complete medical record.
Suggestion: If the medical record is not requested, do not send it. If the medical record is requested, send it in its entirety, as is. Do not make changes, edits, or amendments to the medical record as a response to the complaint.
◾ May be brief, vague, long, articulate, well thought-out and well structured, and/or ridiculous. Regardless of education level, profession, age, and socioeconomic status, any of your patients may write a complaint letter to the SMB, who then must address it.
Suggestions:
1. Demonstrate respect for the board’s time and service by writing a response letter of respectable length and substance regardless of the brevity of the complaint. Brief responses to the SMB may be perceived as arrogant and irreverent, and this is the exact situation and group of people in the entire state in which and before whom you do not want to be thought of that way.
2. Summarize the case with detail and substance in the letter, even if the medical record will be included in the response. Identify the actual complaints and address them in an organized way, an objective voice, and a logical order. Describe the time, thought, and follow-up you have put into addressing the situation. For instance, if the complaint includes a legitimate reference to a delay in test results or an unreturned phone call, provide a broad description of having reviewed and modified the process with your staff to understand where the gap occurred and having taken measures to help keep it from happening again.
◾ Will likely require that a copy of your response be made available or sent to the complainant.
Suggestions:
1. You are writing to 2, maybe 3, recipients: the SMB, the complainant, and the complainant’s attorney. Even if it is clear the patient did not consult a lawyer to write the complaint, it is best to write the response as though it will be read by an attorney.
2. Take the time and deliberation necessary for a multiple-draft writing process. Get help from someone to assure you have addressed all the issues in an organized, objective way.
◾ May lead to a request from the SMB that you appear before them in response to the original complaint letter and/or to clarify your response to a complaint letter. This is an indication of an investigation that has escalated beyond the patient SMB complaint letters addressed in this article; consult an experienced attorney who represents you.
Sometimes other state oversight bodies will receive complaints directly from patients and follow up with you. Consult your attorney, risk management consultant, or malpractice coverage representative for guidance if you are unsure as to the jurisdiction or how to respond.
Conclusion
Most of your practice operates in the Green, no doubt. It is simply not noticeable or memorable when everything goes smoothly. When incidents occur that require service recovery, I hope this guide and commentary will offer perspective on the full range of patient relations and service recovery, provide stories and experiences that might help, and offer general tips and suggestions.
Tearful breakdowns and loud outbursts—they happen with orthopedic patients even in the best of practices. And if you are an orthopedic surgeon who has rarely or never experienced a patient in emotional distress, just talk with your staff—they have no doubt experienced this many times.
There is something about orthopedic conditions—they carry with them an increased likelihood of emotional adverse effects for patients and their loved ones. Inhibited movement can lead to palpable frustration and depression. Time off from work may cause financial hardship and an identity crisis for a family breadwinner. Physical pain can cause the patient to become depressed, angry, or dependent on prescription medication. Medications can cause a change in disposition or outlook. These realities make orthopedic surgery practices particularly predisposed to patient relations risks and service recovery opportunities.
As a practice management consultant and former executive director of an orthopedic practice, I have observed and participated in patient relations and service recovery efforts at many levels. Particularly proud of the way our staff and physicians prevented and handled these and having spent many years traveling by air under the color-coded TSA (Transportation Security Administration) security level indicator system, I created the Patient Relations and Service Recovery Guide (Figure) to help practices gain perspective, have a vocabulary, and develop practical methods for mitigating patient relations risks and responding to incidents and complaints.
The Patient Relations and Service Recovery Guide
The Patient Relations and Service Recovery Guide shows the relationship between the practice as a whole and the patient as an individual.
Green and Red
Green describes the elements of service orientation that the practice must consistently demonstrate and convey to each individual from the point of access, through treatment, and, finally, during account settlement. If you think you have a systemic problem with anything under the Green heading, you probably need a practice management or service orientation consultant, not this article. Red shows the other end of the spectrum—a complete degeneration, worst-case scenario. As with problems in the Green category, this article will not help you in these Red situations, for which you need experienced legal counsel immediately.
We’ll now explore the stories, challenges, opportunities, and practical suggestions for the Blue, Yellow, and Orange categories. The Blue and Yellow categories in the Figure are shaded in grey as a depiction of the interactive, fluid nature of these situations. In addition, they are situations that have developed and can be resolved within and by the practice.
Blue
Patients are very comfortable complaining to the receptionist, x-ray technician, and medical assistant about any number of perceived shortcomings, but when you walk in the examination room, not a word. This is a reality I am sure you have heard about from your staff, and it puts them in a position to observe and determine if a patient’s frustration is escalating. Telephone and front desk receptions are first in line. Patients will say to a telephone receptionist, “I have called 3 times yesterday and twice today and the doctor/nurse still hasn’t called back.” Front desk receptionists will also observe dynamics in the seating area. Staff are your partners in patient relations and service recovery. Working together effectively will help you address issues in the Blue and Yellow areas.
Create an environment that prevents patient discontent and supports service orientation goals. A hospital-based practice that I once managed was a flagship for service excellence goals of a Fortune 150 corporation, had a large seating area, and was close to the airport in a city with multiple company properties; we frequently had executives showing up unannounced, and, because of company politics, it seemed like they were actively looking for instances of substandard service. More importantly, though, we had patients. We established “Waiting Stories” as a performance standard for the receptionists. That is, at any moment, the receptionist was able to recap the “story” of each person in the seating area. The “story” is the reason the person was there, the appointment time, and the cause of the delay, if the wait time was excessive. We all knew this was a performance standard for our practice, so if a receptionist called back to the clinic to find out the reason for a backup in throughput, everyone was respectful and responsive to the inquiry.
The receptionists quickly became effective in judging situations and mitigating or avoiding breakdowns in service and communication. We also implemented an easy and quick notification code for when they needed help handling a service recovery situation. The responses and support in those situations were unwavering, consistent, and blame-free. We would debrief after a significant situation was resolved to determine if there were systemic or response improvement opportunities.
Communication among staff is essential for preventing or mitigating patient discontent. All practices experience service and throughput errors occasionally: a quiet, uncomplaining patient inadvertently doesn’t get called back and remains in the seating area unnoticed; a call doesn’t get returned; x-ray breaks down and a spine patient has to make a painful walk; the physician has to interrupt the encounter to take an important call; etc. Stuff happens. Individually, these breaches are tolerable to most patients. Unfortunately, there can be a cumulative element—when various service mishaps happen to the same patient. This is when communication and support among the staff and with the manager become especially important. If a patient has weathered a rough or long wait or has expressed some dissatisfaction while in the reception area, it’s probably a good idea to let the back-office staff know, so they can show a little extra compassion and be cognizant of additional situations.
Clinical staff and the physicians must convey support and appreciation to front-line staff who observe and share that a patient may be prone to distress, so that they will continue to participate in active incident prevention and service recovery.
Heightening awareness on the part of your staff—especially, receptionists, technicians, medical assistants, and collectors—goes a long way toward getting patient discontent issues settled before they get out of hand. As executive director of a large orthopedic surgery practice, I was particularly proud of our staff’s sensitivity to patient discontent, their sense of when it might be helpful to bring in a manager, and the managers’ ability both to recover many situations and to know when it was most effective to get help and support from either one of the executive team or physicians.
I can remember one patient that both front-line staff and the manager determined needed some service recovery intervention. She had been visibly upset at the end of her final postoperative visit with the physician. The staff noticed and called the manager in. The patient mentioned to the manager that she had been to another orthopedic surgeon who had told her that the surgery our physician had performed was not the right one and that he would have done things differently. The patient said she just didn’t know what to do. Our manager had the keen sense to know that she should get help to recover the situation within our practice. She and all of the staff were always supported when they asked for help, and the physicians were good about expressing their gratitude to the staff for their efforts. The manager escorted the upset patient to my office where we talked—well, she mostly talked and I listened. It turned out that her injuries had prevented her from attending games during her only child’s senior soccer season. I know, it sounds more like therapy—it was a lot of listening and compassion on my part. Eventually, she got around to thanking me for listening. And while that was not the end of it (there was another conversation), she did not take any action against our physician. See the “Talking It Through” Box.
Another group of staff who can identify issues is billing and collections. Often a patient will experience a minor cumulative series of service breaches (eg, long wait, perceived physician distraction, long hold times on the phone) and then lose it when they receive a bill that is incorrect, late, or confusing. The staff members answering those calls also need to feel supported in asking for help from a manager or another associate, either during the call or by suggesting that someone call the patient back.
Empowering staff or managers with tangible service recovery courtesies is also a good idea. We gave our staff coupons from the sundries shop in the building, so that when experiencing a particularly long wait, the patient could go down and get a complimentary snack. We also had 1 or 2 occasions when a patient drove a great distance to see the physician and experienced a significant service breach. As part of our response, we gave the patient a gas card.
Blue is the category in which the staff’s keen observation and true teamwork and support come into play when a situation or developing situation is identified.
Yellow
Yellow, while still contained within the practice, is overt. There has been an incident and/or a communication (letter or call) to the manager or physician. In Yellow, we are beyond the cooperative staff observation and sensory skills—we know something has happened. A situation might be physical- and/or facility-based, eg, a patient or family member had a minor stumble on a doormat, and though luckily they had not appeared injured and the physician checked them out, it was an incident. The other sign of a Yellow situation is that a patient or family member has written a letter to the practice to express their dissatisfaction. In either case, as dreadful as it may seem or as busy as you may be, follow up promptly.
In the case of an incident in the practice, the doctor or manager can call the patient that evening to check in and make sure all is well. Upon receiving a letter, the treating physician and manager should take a minute to discuss and agree on a response plan. Sometimes the situation may call for patient discharge from the practice—only the physician can determine that. Other times, the content of the letter may cause you to consult an attorney or your malpractice insurance carrier. The letters sometimes voice service-oriented complaints and can be addressed by the manager with a phone call and conversation as described in the Blue section above.
Orange
As a consultant, I have assisted many physicians in responding to individual patient complaints to their state medical board (SMB). I have seen a 15-page, single-spaced, typewritten letter with photographs (of the patient’s 70-lb pannus, no less), a 4-sentence letter in childlike grammar and handwriting, and many in between. The spelling, grammar, punctuation, coherence, and brevity of the letter do not matter. Your feelings on the validity of the complaint (ie, “That’s total BS!”) don’t matter. The perceived mental health of the patient (ie,“Well, he’s crazy! Ask my nurse.”) does not matter. Your SMB takes each and every complaint letter very seriously and so must you. One complaint spiraling out of control can be all it takes for you to lose your license. Having said that, individual patient SMB complaints are not uncommon; even the best physicians receive them.
Here are some thoughts to keep in mind regarding individual patient SMB complaints. An individual patient SMB complaint:
◾ Typically comes to you via US mail with no receipt signature required. Lots of us do so much online these days we can go weeks, perhaps months, without looking at our mail—even if staff members have opened it.
Suggestion: Make sure the staff looks at mail and is able to judge what requires action and what should be brought to your attention. Provide appreciation and detailed feedback when staff members bring something to you and do not misdirect negative reactions regardless of the content. You would rather staff members feel comfortable bringing something to your attention that is immaterial than keep something important from you out of fear of displeasing you.
◾ Includes a SMB response deadline that may give you as little as 1 or 2 weeks.
Suggestion: Meet the deadline. If you have or are going to miss the deadline or know that you cannot meet it, have your staff call the SMB office and abjectly request an extension.
◾ Is coming from physicians as members of the SMB, even though it may have the names of physicians you know, perhaps friends, on the letterhead.
Suggestions:
1. The physicians are not your colleagues in this situation. In this capacity, each physician is a member of an oversight board that serves and protects the people of your state. Don’t try to address the situation with a phone call or comment on the golf course.
2. Reply in the format the board has requested—a letter. Open your response letter with a statement that acknowledges the work and responsibility of the SMB and your appreciation, for example:
Esteemed Board,
While I regret that a patient complaint associated with me has come to your attention, I am grateful that the physicians and the people of [your state] have an oversight body to ensure the integrity of medical care delivered and received. Thank you for your service.
◾ Is likely to make you feel angry, indignant, unappreciated, hurt, bewildered, etc.
Suggestion: Breathe, vent to someone you can trust, exercise, get a good night’s sleep, and/or other calming, self-preservation tactics. Repeat as necessary so as not to allow these emotions a place in your response.
◾ May or may not include a request for a copy of the complete medical record.
Suggestion: If the medical record is not requested, do not send it. If the medical record is requested, send it in its entirety, as is. Do not make changes, edits, or amendments to the medical record as a response to the complaint.
◾ May be brief, vague, long, articulate, well thought-out and well structured, and/or ridiculous. Regardless of education level, profession, age, and socioeconomic status, any of your patients may write a complaint letter to the SMB, who then must address it.
Suggestions:
1. Demonstrate respect for the board’s time and service by writing a response letter of respectable length and substance regardless of the brevity of the complaint. Brief responses to the SMB may be perceived as arrogant and irreverent, and this is the exact situation and group of people in the entire state in which and before whom you do not want to be thought of that way.
2. Summarize the case with detail and substance in the letter, even if the medical record will be included in the response. Identify the actual complaints and address them in an organized way, an objective voice, and a logical order. Describe the time, thought, and follow-up you have put into addressing the situation. For instance, if the complaint includes a legitimate reference to a delay in test results or an unreturned phone call, provide a broad description of having reviewed and modified the process with your staff to understand where the gap occurred and having taken measures to help keep it from happening again.
◾ Will likely require that a copy of your response be made available or sent to the complainant.
Suggestions:
1. You are writing to 2, maybe 3, recipients: the SMB, the complainant, and the complainant’s attorney. Even if it is clear the patient did not consult a lawyer to write the complaint, it is best to write the response as though it will be read by an attorney.
2. Take the time and deliberation necessary for a multiple-draft writing process. Get help from someone to assure you have addressed all the issues in an organized, objective way.
◾ May lead to a request from the SMB that you appear before them in response to the original complaint letter and/or to clarify your response to a complaint letter. This is an indication of an investigation that has escalated beyond the patient SMB complaint letters addressed in this article; consult an experienced attorney who represents you.
Sometimes other state oversight bodies will receive complaints directly from patients and follow up with you. Consult your attorney, risk management consultant, or malpractice coverage representative for guidance if you are unsure as to the jurisdiction or how to respond.
Conclusion
Most of your practice operates in the Green, no doubt. It is simply not noticeable or memorable when everything goes smoothly. When incidents occur that require service recovery, I hope this guide and commentary will offer perspective on the full range of patient relations and service recovery, provide stories and experiences that might help, and offer general tips and suggestions.