User login
Official Newspaper of the American College of Surgeons
Predicting MDR Gram-negative infection mortality risk
Source control, defined as location and elimination of the source of the infection, was critical for patient survival in the case of multidrug resistant bacterial infection, according to the results of a case-control study of 62 critically ill surgical patients who were assessed between 2011 and 2014.
Researchers examined the characteristics of infected patients surviving to hospital discharge compared with those of nonsurvivors to look for predictive factors. Demographically, patients had an overall mean age of 62 years; 30.6% were women; 69.4% were white. The first culture obtained during a surgical ICU admission that grew a carbapenem-resistant Enterobacteriaceae (CRE), MDR Pseudomonas aeruginosa, or MDR Acinetobacter spp. was defined as the index culture.
“In this study, 33.9% [21/62] of critically ill surgical patients with a culture positive for MDR Gram-negative bacteria died prior to hospital discharge,” according to Andrew S. Jarrell, PharmD, of the Johns Hopkins Hospital, Baltimore, and his colleagues.
With multivariate logistic regression, achievement of source control was the only variable associated with decreased in-hospital mortality (odds ratio 0.04, 95% confidence interval, 0.003-0.52); P = .01).
“Source control status was predictive of in-hospital mortality after controlling for other factors. Specifically, the odds of in-hospital mortality were 97% lower when source control was achieved as compared to when source control was not achieved,” the authors stated (J Crit Care. 2018;43:321-6).
Scenarios in which source control was not applicable (pneumonia and urinary tract infection) were also similarly distributed between survivors and nonsurvivors, they reported.
Other than source control, the only significant risk factors for mortality, as seen in univariate analysis, all occurred prior to index culture. They were: vasopressor use (46.3% of survivors, vs. 76.2% of nonsurvivors, P = .03); mechanical ventilation (63.4% vs. 100%, P = .001); and median ICU length of stay (10 days vs. 18 days, P = .001).
“Achievement of source control stands out as a critical factor for patient survival. Clinicians should take this, along with prior ICU LOS, vasopressor use, and mechanical ventilation status, into consideration when evaluating patient prognosis,” Dr. Jarrell and his colleagues concluded.
The authors reported that they had no conflicts or source of funding.
Source: Jarrell, A.S., et al. J Crit Care. 2018;43:321-6.
Source control, defined as location and elimination of the source of the infection, was critical for patient survival in the case of multidrug resistant bacterial infection, according to the results of a case-control study of 62 critically ill surgical patients who were assessed between 2011 and 2014.
Researchers examined the characteristics of infected patients surviving to hospital discharge compared with those of nonsurvivors to look for predictive factors. Demographically, patients had an overall mean age of 62 years; 30.6% were women; 69.4% were white. The first culture obtained during a surgical ICU admission that grew a carbapenem-resistant Enterobacteriaceae (CRE), MDR Pseudomonas aeruginosa, or MDR Acinetobacter spp. was defined as the index culture.
“In this study, 33.9% [21/62] of critically ill surgical patients with a culture positive for MDR Gram-negative bacteria died prior to hospital discharge,” according to Andrew S. Jarrell, PharmD, of the Johns Hopkins Hospital, Baltimore, and his colleagues.
With multivariate logistic regression, achievement of source control was the only variable associated with decreased in-hospital mortality (odds ratio 0.04, 95% confidence interval, 0.003-0.52); P = .01).
“Source control status was predictive of in-hospital mortality after controlling for other factors. Specifically, the odds of in-hospital mortality were 97% lower when source control was achieved as compared to when source control was not achieved,” the authors stated (J Crit Care. 2018;43:321-6).
Scenarios in which source control was not applicable (pneumonia and urinary tract infection) were also similarly distributed between survivors and nonsurvivors, they reported.
Other than source control, the only significant risk factors for mortality, as seen in univariate analysis, all occurred prior to index culture. They were: vasopressor use (46.3% of survivors, vs. 76.2% of nonsurvivors, P = .03); mechanical ventilation (63.4% vs. 100%, P = .001); and median ICU length of stay (10 days vs. 18 days, P = .001).
“Achievement of source control stands out as a critical factor for patient survival. Clinicians should take this, along with prior ICU LOS, vasopressor use, and mechanical ventilation status, into consideration when evaluating patient prognosis,” Dr. Jarrell and his colleagues concluded.
The authors reported that they had no conflicts or source of funding.
Source: Jarrell, A.S., et al. J Crit Care. 2018;43:321-6.
Source control, defined as location and elimination of the source of the infection, was critical for patient survival in the case of multidrug resistant bacterial infection, according to the results of a case-control study of 62 critically ill surgical patients who were assessed between 2011 and 2014.
Researchers examined the characteristics of infected patients surviving to hospital discharge compared with those of nonsurvivors to look for predictive factors. Demographically, patients had an overall mean age of 62 years; 30.6% were women; 69.4% were white. The first culture obtained during a surgical ICU admission that grew a carbapenem-resistant Enterobacteriaceae (CRE), MDR Pseudomonas aeruginosa, or MDR Acinetobacter spp. was defined as the index culture.
“In this study, 33.9% [21/62] of critically ill surgical patients with a culture positive for MDR Gram-negative bacteria died prior to hospital discharge,” according to Andrew S. Jarrell, PharmD, of the Johns Hopkins Hospital, Baltimore, and his colleagues.
With multivariate logistic regression, achievement of source control was the only variable associated with decreased in-hospital mortality (odds ratio 0.04, 95% confidence interval, 0.003-0.52); P = .01).
“Source control status was predictive of in-hospital mortality after controlling for other factors. Specifically, the odds of in-hospital mortality were 97% lower when source control was achieved as compared to when source control was not achieved,” the authors stated (J Crit Care. 2018;43:321-6).
Scenarios in which source control was not applicable (pneumonia and urinary tract infection) were also similarly distributed between survivors and nonsurvivors, they reported.
Other than source control, the only significant risk factors for mortality, as seen in univariate analysis, all occurred prior to index culture. They were: vasopressor use (46.3% of survivors, vs. 76.2% of nonsurvivors, P = .03); mechanical ventilation (63.4% vs. 100%, P = .001); and median ICU length of stay (10 days vs. 18 days, P = .001).
“Achievement of source control stands out as a critical factor for patient survival. Clinicians should take this, along with prior ICU LOS, vasopressor use, and mechanical ventilation status, into consideration when evaluating patient prognosis,” Dr. Jarrell and his colleagues concluded.
The authors reported that they had no conflicts or source of funding.
Source: Jarrell, A.S., et al. J Crit Care. 2018;43:321-6.
FROM THE JOURNAL OF CRITICAL CARE
Key clinical point: Source control was the most important predictor of MDR Gram-negative infection mortality in hospitalized patients.
Major finding: The odds of in-hospital mortality were 97% lower when source control was achieved.
Study details: Case-control study of 62 critically ill surgical patients from 2011 to 2014 who had an MDR infection.
Disclosures: The authors reported that they had no conflicts or source of funding.
Source: Jarrell, A.S., et al. J Crit Care. 2018;43:321-6.
Greater gynecological but not medical risks with hysteroscopic sterilization
Hysteroscopic sterilization is associated with a significantly greater risk of gynecological complications but not medical or surgical complications, compared with laparoscopic sterilization, according to data from a French nationwide cohort study.
In a report published Jan. 23 in JAMA, researchers conducted a study of 105,357 women – 71,303 (67.7%) of whom underwent hysteroscopic sterilization, and 34,054 (32.3%) of whom underwent laparoscopic sterilization – and who were followed for at least 1 year after the procedure.
Women who had the hysteroscopic procedure had a nearly threefold higher risk of tubal disorder or surgery, a sevenfold higher risk of sterilization failure, and a 25-fold higher risk of undergoing a second sterilization procedure at 1 year compared with those who had the laparoscopic procedure (P less than .001 for each). These risk increases persisted even at 3 years after the procedure (hazard ratios of 1.79, 4.66, and 16.63, respectively; 95% confidence interval for each).
“A second sterilization procedure following hysteroscopic sterilization is a well-identified risk already described in phase 2 and 3 studies, in which the risk varied between 4.0% and 4.5%,” wrote Kim Bouillon, MD, PhD, of the French National Agency for Medicines and Health Products Safety, and her coauthors.
“In the present study, this risk was 4.1% at the 1-year follow-up, comparable with that reported in previous studies conducted in real-life conditions in patients who received care in public or private hospitals, and much higher than after laparoscopic sterilization.”
However, hysteroscopic sterilization was associated with a significantly reduced risk of surgical complications, compared with laparoscopic sterilization (adjusted odds ratio, 0.18; 95% CI, 0.14-0.23). The overall rate of in-hospital surgical complications was 0.13% with the hysteroscopic procedure and 0.78% with the laparoscopic procedure. Medical complications occurred in 0.06% of hysteroscopic procedures and 0.11% of laparoscopic procedures.
Women who underwent hysteroscopic procedures also had a significantly lower risk of uterine disorders (adjusted HR, 0.85; 95% CI, 0.74-0.98), uterine bleeding, and hysterectomies at 1 year, after adjustment for known hysterectomy risk factors.
The researchers noted that in absolute terms, the differences in the risk of procedural complications were very small, compared with the differences in the risk of gynecological complications.
The risk of pregnancy was significantly lower in the hysteroscopic group, compared with the laparoscopic group at 1 year after the procedure, but by 3 years’ follow-up, the difference was no longer significant.
There were no significant differences seen in the risk of medical complications such as autoimmune disease and thyroid disorders, attempted suicide, or death between the two procedures. Women who underwent hysteroscopic sterilization had a slightly lower use of analgesics, antidepressants, and benzodiazepines at 1 year that was more pronounced by 3 years.
There was a significantly higher risk of allergic reaction seen with hysteroscopic sterilization among women with prior allergies, but the authors suggested that a null overall effect and large number of tested interactions made the finding “hypothesis-generating” only.
The study was prompted by safety concerns about hysteroscopic sterilization, with the Food and Drug Administration in 2015 receiving a large number of reports of adverse events including bleeding, pelvic pain, fallopian tube perforation, unwanted pregnancy, hysterectomies, depression, and allergic reactions.
The FDA has since ordered the device manufacturer to undertake an open-label, nonrandomized study comparing outcomes between hysteroscopic and laparoscopic sterilization, which is expected to deliver results in 2023.
“To our knowledge, this is the first study aiming at comparing medical outcomes in addition to gynecological outcomes between hysteroscopic and laparoscopic sterilization,” the authors wrote, referring to their own work. They concluded, “these findings do not support increased medical risks associated with hysteroscopic sterilization.”
One author declared personal fees from Boston Scientific but no other conflicts of interest were declared.
SOURCE: Bouillon K et al. JAMA. 2018 Jan 23;319:375-87.
In 2016, in response to safety concerns about the hysteroscopic sterilization implant Essure, the Food and Drug Administration placed a “black box” warning on the device to highlight potential risks, and a global patient advocacy movement called for a ban on the product. In this environment, there is therefore a need for strong scientific evidence to inform objective decision making.
This study provides reassuring evidence that adverse outcomes are not significantly higher after hysteroscopic sterilization compared with laparoscopic sterilization, at least up to 3 years after the procedure. However, given the powerful and very public grassroots effort to ban the hysteroscopic implant and the possibility of class action litigation, the future of hysteroscopic sterilization is uncertain.
Eve Espey, MD, MPH, and Lisa G. Hofler, MD, MPH, are in the department of obstetrics and gynecology at the University of New Mexico, Albuquerque. The comments are taken from an editorial (JAMA. 2018 Jan 23;319[4]:347-50). Dr. Hofler declared personal fees and nonfinancial support from the American College of Obstetricians and Gynecologists.
In 2016, in response to safety concerns about the hysteroscopic sterilization implant Essure, the Food and Drug Administration placed a “black box” warning on the device to highlight potential risks, and a global patient advocacy movement called for a ban on the product. In this environment, there is therefore a need for strong scientific evidence to inform objective decision making.
This study provides reassuring evidence that adverse outcomes are not significantly higher after hysteroscopic sterilization compared with laparoscopic sterilization, at least up to 3 years after the procedure. However, given the powerful and very public grassroots effort to ban the hysteroscopic implant and the possibility of class action litigation, the future of hysteroscopic sterilization is uncertain.
Eve Espey, MD, MPH, and Lisa G. Hofler, MD, MPH, are in the department of obstetrics and gynecology at the University of New Mexico, Albuquerque. The comments are taken from an editorial (JAMA. 2018 Jan 23;319[4]:347-50). Dr. Hofler declared personal fees and nonfinancial support from the American College of Obstetricians and Gynecologists.
In 2016, in response to safety concerns about the hysteroscopic sterilization implant Essure, the Food and Drug Administration placed a “black box” warning on the device to highlight potential risks, and a global patient advocacy movement called for a ban on the product. In this environment, there is therefore a need for strong scientific evidence to inform objective decision making.
This study provides reassuring evidence that adverse outcomes are not significantly higher after hysteroscopic sterilization compared with laparoscopic sterilization, at least up to 3 years after the procedure. However, given the powerful and very public grassroots effort to ban the hysteroscopic implant and the possibility of class action litigation, the future of hysteroscopic sterilization is uncertain.
Eve Espey, MD, MPH, and Lisa G. Hofler, MD, MPH, are in the department of obstetrics and gynecology at the University of New Mexico, Albuquerque. The comments are taken from an editorial (JAMA. 2018 Jan 23;319[4]:347-50). Dr. Hofler declared personal fees and nonfinancial support from the American College of Obstetricians and Gynecologists.
Hysteroscopic sterilization is associated with a significantly greater risk of gynecological complications but not medical or surgical complications, compared with laparoscopic sterilization, according to data from a French nationwide cohort study.
In a report published Jan. 23 in JAMA, researchers conducted a study of 105,357 women – 71,303 (67.7%) of whom underwent hysteroscopic sterilization, and 34,054 (32.3%) of whom underwent laparoscopic sterilization – and who were followed for at least 1 year after the procedure.
Women who had the hysteroscopic procedure had a nearly threefold higher risk of tubal disorder or surgery, a sevenfold higher risk of sterilization failure, and a 25-fold higher risk of undergoing a second sterilization procedure at 1 year compared with those who had the laparoscopic procedure (P less than .001 for each). These risk increases persisted even at 3 years after the procedure (hazard ratios of 1.79, 4.66, and 16.63, respectively; 95% confidence interval for each).
“A second sterilization procedure following hysteroscopic sterilization is a well-identified risk already described in phase 2 and 3 studies, in which the risk varied between 4.0% and 4.5%,” wrote Kim Bouillon, MD, PhD, of the French National Agency for Medicines and Health Products Safety, and her coauthors.
“In the present study, this risk was 4.1% at the 1-year follow-up, comparable with that reported in previous studies conducted in real-life conditions in patients who received care in public or private hospitals, and much higher than after laparoscopic sterilization.”
However, hysteroscopic sterilization was associated with a significantly reduced risk of surgical complications, compared with laparoscopic sterilization (adjusted odds ratio, 0.18; 95% CI, 0.14-0.23). The overall rate of in-hospital surgical complications was 0.13% with the hysteroscopic procedure and 0.78% with the laparoscopic procedure. Medical complications occurred in 0.06% of hysteroscopic procedures and 0.11% of laparoscopic procedures.
Women who underwent hysteroscopic procedures also had a significantly lower risk of uterine disorders (adjusted HR, 0.85; 95% CI, 0.74-0.98), uterine bleeding, and hysterectomies at 1 year, after adjustment for known hysterectomy risk factors.
The researchers noted that in absolute terms, the differences in the risk of procedural complications were very small, compared with the differences in the risk of gynecological complications.
The risk of pregnancy was significantly lower in the hysteroscopic group, compared with the laparoscopic group at 1 year after the procedure, but by 3 years’ follow-up, the difference was no longer significant.
There were no significant differences seen in the risk of medical complications such as autoimmune disease and thyroid disorders, attempted suicide, or death between the two procedures. Women who underwent hysteroscopic sterilization had a slightly lower use of analgesics, antidepressants, and benzodiazepines at 1 year that was more pronounced by 3 years.
There was a significantly higher risk of allergic reaction seen with hysteroscopic sterilization among women with prior allergies, but the authors suggested that a null overall effect and large number of tested interactions made the finding “hypothesis-generating” only.
The study was prompted by safety concerns about hysteroscopic sterilization, with the Food and Drug Administration in 2015 receiving a large number of reports of adverse events including bleeding, pelvic pain, fallopian tube perforation, unwanted pregnancy, hysterectomies, depression, and allergic reactions.
The FDA has since ordered the device manufacturer to undertake an open-label, nonrandomized study comparing outcomes between hysteroscopic and laparoscopic sterilization, which is expected to deliver results in 2023.
“To our knowledge, this is the first study aiming at comparing medical outcomes in addition to gynecological outcomes between hysteroscopic and laparoscopic sterilization,” the authors wrote, referring to their own work. They concluded, “these findings do not support increased medical risks associated with hysteroscopic sterilization.”
One author declared personal fees from Boston Scientific but no other conflicts of interest were declared.
SOURCE: Bouillon K et al. JAMA. 2018 Jan 23;319:375-87.
Hysteroscopic sterilization is associated with a significantly greater risk of gynecological complications but not medical or surgical complications, compared with laparoscopic sterilization, according to data from a French nationwide cohort study.
In a report published Jan. 23 in JAMA, researchers conducted a study of 105,357 women – 71,303 (67.7%) of whom underwent hysteroscopic sterilization, and 34,054 (32.3%) of whom underwent laparoscopic sterilization – and who were followed for at least 1 year after the procedure.
Women who had the hysteroscopic procedure had a nearly threefold higher risk of tubal disorder or surgery, a sevenfold higher risk of sterilization failure, and a 25-fold higher risk of undergoing a second sterilization procedure at 1 year compared with those who had the laparoscopic procedure (P less than .001 for each). These risk increases persisted even at 3 years after the procedure (hazard ratios of 1.79, 4.66, and 16.63, respectively; 95% confidence interval for each).
“A second sterilization procedure following hysteroscopic sterilization is a well-identified risk already described in phase 2 and 3 studies, in which the risk varied between 4.0% and 4.5%,” wrote Kim Bouillon, MD, PhD, of the French National Agency for Medicines and Health Products Safety, and her coauthors.
“In the present study, this risk was 4.1% at the 1-year follow-up, comparable with that reported in previous studies conducted in real-life conditions in patients who received care in public or private hospitals, and much higher than after laparoscopic sterilization.”
However, hysteroscopic sterilization was associated with a significantly reduced risk of surgical complications, compared with laparoscopic sterilization (adjusted odds ratio, 0.18; 95% CI, 0.14-0.23). The overall rate of in-hospital surgical complications was 0.13% with the hysteroscopic procedure and 0.78% with the laparoscopic procedure. Medical complications occurred in 0.06% of hysteroscopic procedures and 0.11% of laparoscopic procedures.
Women who underwent hysteroscopic procedures also had a significantly lower risk of uterine disorders (adjusted HR, 0.85; 95% CI, 0.74-0.98), uterine bleeding, and hysterectomies at 1 year, after adjustment for known hysterectomy risk factors.
The researchers noted that in absolute terms, the differences in the risk of procedural complications were very small, compared with the differences in the risk of gynecological complications.
The risk of pregnancy was significantly lower in the hysteroscopic group, compared with the laparoscopic group at 1 year after the procedure, but by 3 years’ follow-up, the difference was no longer significant.
There were no significant differences seen in the risk of medical complications such as autoimmune disease and thyroid disorders, attempted suicide, or death between the two procedures. Women who underwent hysteroscopic sterilization had a slightly lower use of analgesics, antidepressants, and benzodiazepines at 1 year that was more pronounced by 3 years.
There was a significantly higher risk of allergic reaction seen with hysteroscopic sterilization among women with prior allergies, but the authors suggested that a null overall effect and large number of tested interactions made the finding “hypothesis-generating” only.
The study was prompted by safety concerns about hysteroscopic sterilization, with the Food and Drug Administration in 2015 receiving a large number of reports of adverse events including bleeding, pelvic pain, fallopian tube perforation, unwanted pregnancy, hysterectomies, depression, and allergic reactions.
The FDA has since ordered the device manufacturer to undertake an open-label, nonrandomized study comparing outcomes between hysteroscopic and laparoscopic sterilization, which is expected to deliver results in 2023.
“To our knowledge, this is the first study aiming at comparing medical outcomes in addition to gynecological outcomes between hysteroscopic and laparoscopic sterilization,” the authors wrote, referring to their own work. They concluded, “these findings do not support increased medical risks associated with hysteroscopic sterilization.”
One author declared personal fees from Boston Scientific but no other conflicts of interest were declared.
SOURCE: Bouillon K et al. JAMA. 2018 Jan 23;319:375-87.
FROM JAMA
Key clinical point: Hysteroscopic sterilization is associated with a significantly greater risk of gynecological complications – but not medical or surgical complications – compared with laparoscopic sterilization.
Major finding: The risks of repeat sterilization procedure, sterilization failure, and tubal disorder are higher with hysteroscopic sterilization than with laparoscopic sterilization, but the surgical risks are lower and there are no significant differences in other medical risks.
Data source: Nationwide cohort study of 105,357 women.
Disclosures: One author declared personal fees from Boston Scientific but no other conflicts of interest were declared.
Source: Bouillon K et al. JAMA. 2018 Jan 23;319:375-87.
Implementing enhanced recovery protocols for gynecologic surgery
“Enhanced Recovery After Surgery” (ERAS) practices and protocols have been increasingly refined and adopted for the field of gynecology, and there is hope among gynecologic surgeons – and some recent evidence – that, with the ERAS movement, we are improving patient recoveries and outcomes and minimizing the need for opioids.
This applies not only to open surgeries but also to the minimally invasive procedures that already are prized for significant reductions in morbidity and length of stay. The overarching and guiding principle of ERAS is that any surgery – whether open or minimally invasive, major or minor – places stress on the body and is associated with risks and morbidity.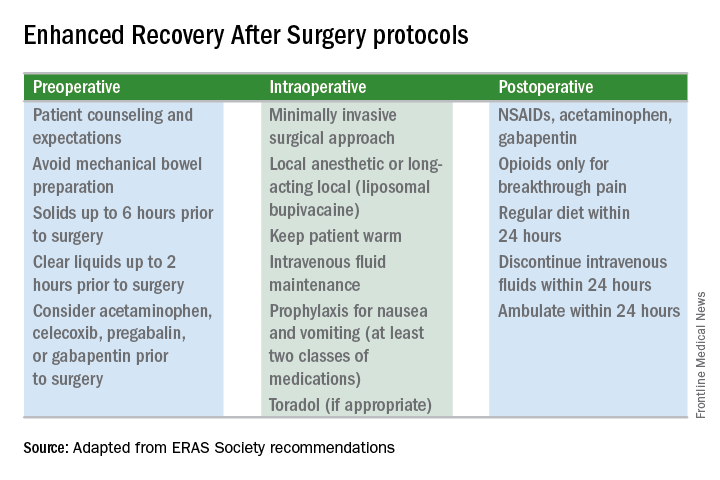
Enhanced recovery protocols are multidisciplinary, perioperative approaches designed to lessen the body’s stress response to surgery. The protocols and pathways offer us a menu of small changes that, in the aggregate, can lead to large and demonstrable benefits – especially when these small changes are chosen across the preoperative, intraoperative, and postoperative arenas and then standardized in one’s practice. Among the major components of ERAS practices and protocols are limiting preoperative fasting, employing multimodal analgesia, encouraging early ambulation and early postsurgical feeding, and creating culture shift that includes greater emphasis on patient expectations.
In our practice, we are incorporating ERAS practices not only in hopes of reducing the stress of all surgeries before, during, and after, but also with the goal of achieving a postoperative opioid-free hysterectomy, myomectomy, and extensive endometriosis surgery. (All of our advanced procedures are performed laparoscopically or robotically.)
Over the past 7 or so years, we have adopted a multimodal approach to pain control that includes a bundle of preoperative analgesics – acetaminophen, pregabalin, and celecoxib (we call it “TLC” for Tylenol, Lyrica, and Celebrex) – and the use of liposomal bupivacaine in our robotic surgeries. We are now turning toward ERAS nutritional changes, most of which run counter to traditional paradigms for surgical care. And in other areas, such as dedicated preoperative counseling, we continue to refine and improve our practices.
Improved Outcomes
The ERAS mindset notably intersected gynecology with the publication in 2016 of a two-part series of guidelines for gynecology/oncology surgery from the ERAS Society. The 8-year-old society has its roots in a study group of European surgeons and others who decided to examine surgical practices and the concept of multimodal surgical care put forth in the 1990s by Henrik Kehlet, MD, PhD, then a professor at the University of Copenhagen.
The first set of recommendations addressed pre- and intraoperative care (Gynecol Oncol. 2016 Feb;140[2]:313-22), and the second set addressed postoperative care (Gynecol Oncol. 2016 Feb;140[2]:323-32). Similar evidence-based recommendations were previously written for colonic resections, rectal and pelvic surgery, and other surgical specialties.
Most of the published outcomes of enhanced recovery protocols come from colorectal surgery. As noted in the ERAS Society gynecology/oncology guidelines, the benefits include an average reduction in length of stay of 2.5 days and a decrease in complications by as much as 50%.
There is growing evidence, however, that ERAS programs are also beneficial for patients undergoing laparoscopic surgery, and outcomes from gynecology – including minimally invasive surgery – are also being reported.
For instance, a retrospective case-control study of 55 consecutive gynecologic oncology patients treated at the University of California, San Francisco, with laparoscopic or robotic surgery and an enhanced recovery pathway – and 110 historical control patients matched on the basis of age and surgery type – found significant improvements in recovery time, decreased pain despite reduced opioid use, and overall lower hospital costs (Obstet Gynecol. 2016 Jul;128[1]:138-44).
The enhanced recovery pathway included patient education, multimodal antiemetics, multimodal analgesia, and balanced fluid administration. Early catheter removal, ambulation, and feeding were also components. Analgesia included routine preoperative gabapentin, diclofenac, and acetaminophen; routine postoperative gabapentin, NSAIDs, and acetaminophen; and transversus abdominis plane blocks in 32 of the ERAS patients.
ERAS patients were significantly more likely to be discharged on day 1 (91%, compared with 60% in the control group). Opioid use decreased by 30%, and pain scores on postoperative day 1 were significantly lower.
Another study looking at the effect of enhanced recovery implementation in gynecologic surgeries at the University of Virginia, Charlottesville, (gynecologic oncology, urogynecology, and general gynecology) similarly reported benefits for vaginal and minimally invasive procedures, as well as for open procedures (Obstet Gynecol. 2016 Sep;128[3]:457-66).
In the minimally invasive group, investigators compared 324 patients before ERAS implementation with 249 patients afterward and found that the median length of stay was unchanged (1 day). However, intraoperative and postoperative opioid consumption decreased significantly and – even though actual pain scores improved only slightly – patient satisfaction scores improved markedly among post-ERAS patients. Patients gave higher marks, for instance, to questions regarding pain control (“how well your pain was controlled”) and teamwork (“staff worked together to care for you”).
Reducing Opioids
New opioid use that persists after a surgical procedure is a postsurgical complication that we all should be working to prevent. It has been estimated that 6% of surgical patients – even those who’ve had relatively minor surgical procedures – will become long-term opioid users, developing a dependence on the drugs prescribed to them for postsurgical pain.
This was shown last year in a national study of insurance claims data from between 2013 and 2014; investigators identified adults without opioid use in the year prior to surgery (including hysterectomy) and found that 5.9%-6.5% were filling opioid prescriptions 90-180 days after their surgical procedure. The incidence in a nonoperative control cohort was 0.4% (JAMA Surg. 2017 Jun 21;152[6]:e170504). Notably, this prolonged use was greatest in patients with prior pain conditions, substance abuse, and mental health disorders – a finding that may have implications for the counseling we provide prior to surgery.
It’s not clear what the optimal analgesic regimen is for minimally invasive or other gynecologic surgeries. What is clearly recommended, however, is that the approach be multifaceted. In our practice, we believe that the preoperative use of acetaminophen, pregabalin, and celecoxib plays an important role in reducing postoperative pain and opioid use. But we also have striven to create a practice-wide culture shift (throughout the operating and recovery rooms), for instance, that encourages using the least amounts of narcotics possible and using the shortest-acting formulations possible.
Transversus abdominis plane (TAP) blocks are also often part of ERAS protocols; they have been shown in at least two randomized controlled trials of abdominal hysterectomy to reduce intraoperative fentanyl requirements and to reduce immediate postoperative pain scores and postoperative morphine requirements (Anesth Analg. 2008 Dec;107[6]:2056-60; J Anaesthesiol Clin Pharmacol. 2014 Jul-Sep;30[3]:391-6).
More recently, liposomal bupivacaine, which is slowly released over several days, has gained traction as a substitute for standard bupivacaine and other agents in TAP blocks. In one recent retrospective study, abdominal incision infiltration with liposomal bupivacaine was associated with less opioid use (with no change in pain scores), compared with bupivacaine hydrochloride after laparotomy for gynecologic malignancies (Obstet Gynecol. 2016 Nov;128[5]:1009-17). It’s significantly more expensive, however, making it likely that the formulation is being used more judiciously in minimally invasive gynecologic surgery than in open surgeries.
Because of costs, we currently are restricted to using liposomal bupivacaine in our robotic surgeries only. In our practice, the single 20 mL vial (266 mg of liposomal bupivacaine) is diluted with 20 mL of normal saline, but it can be further diluted without loss of efficacy. With a 16-gauge needle, the liposomal bupivacaine is distributed across the incisions (usually 20 mL in the umbilicus with a larger incision and 10 mL in each of the two lateral incisions). Patients are counseled that they may have more discomfort after 3 days, but by this point most are mobile and feeling relatively well with a combination of NSAIDs and acetaminophen.
With growing visibility of the problem of narcotic dependence in the United States, patients seem increasingly receptive and even eager to limit or avoid the use of opioids. Patients should be counseled that minimizing or avoiding opioids may also speed recovery. Narcotics cause gut motility to slow down, which may hinder mobilization. Early mobilization (within 24 hours) is among the enhanced recovery elements that the ERAS Society guidelines say is “of particular value” for minimally invasive surgery, along with maintenance of normothermia and normovolemia with maintenance of adequate cardiac output.
Selecting Steps
Our practice is also trying to reduce preoperative bowel preparation and preoperative fasting, both of which have been found to be stressful for the body without evidence of benefit. These practices can lead to insulin resistance and hyperglycemia, which are associated with increased morbidity and length of stay.
It is now recommended that clear fluids be allowed up to 2 hours before surgery and solids up to 6 hours before. Some health systems and practices also recommend presurgical carbohydrate loading (for example, 10 ounces of apple juice 2 hours before surgery) – another small change on the ERAS menu – to further reduce postoperative insulin resistance and help the body cope with its stress response to surgery.
Along with nutritional changes are also various measures aimed at optimizing the body’s functionality before surgery (“prehabilitation”), from walking 30 minutes a day to abstaining from alcohol for patients who drink heavily.
Throughout the country, enhanced recovery protocols are taking shape in gynecologic surgery. ERAS was featured in an aptly titled panel session at the 2017 annual meeting of the American Association of Gynecologic Laparoscopists: “Outpatient Hysterectomy, ERAS, and Same-Day Discharge: The Next Big Thing in Gyn Surgery.” Others are applying ERAS to scheduled cesarean sections. And in our practice, I believe that if we continue making small changes, we will reach our goal of opioid-free recoveries and a better surgical experience for our patients.
Kirsten Sasaki, MD, is an associate of the Advanced Gynecologic Surgery Institute. She reported that she has no disclosures relevant to this Master Class.
“Enhanced Recovery After Surgery” (ERAS) practices and protocols have been increasingly refined and adopted for the field of gynecology, and there is hope among gynecologic surgeons – and some recent evidence – that, with the ERAS movement, we are improving patient recoveries and outcomes and minimizing the need for opioids.
This applies not only to open surgeries but also to the minimally invasive procedures that already are prized for significant reductions in morbidity and length of stay. The overarching and guiding principle of ERAS is that any surgery – whether open or minimally invasive, major or minor – places stress on the body and is associated with risks and morbidity.
Enhanced recovery protocols are multidisciplinary, perioperative approaches designed to lessen the body’s stress response to surgery. The protocols and pathways offer us a menu of small changes that, in the aggregate, can lead to large and demonstrable benefits – especially when these small changes are chosen across the preoperative, intraoperative, and postoperative arenas and then standardized in one’s practice. Among the major components of ERAS practices and protocols are limiting preoperative fasting, employing multimodal analgesia, encouraging early ambulation and early postsurgical feeding, and creating culture shift that includes greater emphasis on patient expectations.
In our practice, we are incorporating ERAS practices not only in hopes of reducing the stress of all surgeries before, during, and after, but also with the goal of achieving a postoperative opioid-free hysterectomy, myomectomy, and extensive endometriosis surgery. (All of our advanced procedures are performed laparoscopically or robotically.)
Over the past 7 or so years, we have adopted a multimodal approach to pain control that includes a bundle of preoperative analgesics – acetaminophen, pregabalin, and celecoxib (we call it “TLC” for Tylenol, Lyrica, and Celebrex) – and the use of liposomal bupivacaine in our robotic surgeries. We are now turning toward ERAS nutritional changes, most of which run counter to traditional paradigms for surgical care. And in other areas, such as dedicated preoperative counseling, we continue to refine and improve our practices.
Improved Outcomes
The ERAS mindset notably intersected gynecology with the publication in 2016 of a two-part series of guidelines for gynecology/oncology surgery from the ERAS Society. The 8-year-old society has its roots in a study group of European surgeons and others who decided to examine surgical practices and the concept of multimodal surgical care put forth in the 1990s by Henrik Kehlet, MD, PhD, then a professor at the University of Copenhagen.
The first set of recommendations addressed pre- and intraoperative care (Gynecol Oncol. 2016 Feb;140[2]:313-22), and the second set addressed postoperative care (Gynecol Oncol. 2016 Feb;140[2]:323-32). Similar evidence-based recommendations were previously written for colonic resections, rectal and pelvic surgery, and other surgical specialties.
Most of the published outcomes of enhanced recovery protocols come from colorectal surgery. As noted in the ERAS Society gynecology/oncology guidelines, the benefits include an average reduction in length of stay of 2.5 days and a decrease in complications by as much as 50%.
There is growing evidence, however, that ERAS programs are also beneficial for patients undergoing laparoscopic surgery, and outcomes from gynecology – including minimally invasive surgery – are also being reported.
For instance, a retrospective case-control study of 55 consecutive gynecologic oncology patients treated at the University of California, San Francisco, with laparoscopic or robotic surgery and an enhanced recovery pathway – and 110 historical control patients matched on the basis of age and surgery type – found significant improvements in recovery time, decreased pain despite reduced opioid use, and overall lower hospital costs (Obstet Gynecol. 2016 Jul;128[1]:138-44).
The enhanced recovery pathway included patient education, multimodal antiemetics, multimodal analgesia, and balanced fluid administration. Early catheter removal, ambulation, and feeding were also components. Analgesia included routine preoperative gabapentin, diclofenac, and acetaminophen; routine postoperative gabapentin, NSAIDs, and acetaminophen; and transversus abdominis plane blocks in 32 of the ERAS patients.
ERAS patients were significantly more likely to be discharged on day 1 (91%, compared with 60% in the control group). Opioid use decreased by 30%, and pain scores on postoperative day 1 were significantly lower.
Another study looking at the effect of enhanced recovery implementation in gynecologic surgeries at the University of Virginia, Charlottesville, (gynecologic oncology, urogynecology, and general gynecology) similarly reported benefits for vaginal and minimally invasive procedures, as well as for open procedures (Obstet Gynecol. 2016 Sep;128[3]:457-66).
In the minimally invasive group, investigators compared 324 patients before ERAS implementation with 249 patients afterward and found that the median length of stay was unchanged (1 day). However, intraoperative and postoperative opioid consumption decreased significantly and – even though actual pain scores improved only slightly – patient satisfaction scores improved markedly among post-ERAS patients. Patients gave higher marks, for instance, to questions regarding pain control (“how well your pain was controlled”) and teamwork (“staff worked together to care for you”).
Reducing Opioids
New opioid use that persists after a surgical procedure is a postsurgical complication that we all should be working to prevent. It has been estimated that 6% of surgical patients – even those who’ve had relatively minor surgical procedures – will become long-term opioid users, developing a dependence on the drugs prescribed to them for postsurgical pain.
This was shown last year in a national study of insurance claims data from between 2013 and 2014; investigators identified adults without opioid use in the year prior to surgery (including hysterectomy) and found that 5.9%-6.5% were filling opioid prescriptions 90-180 days after their surgical procedure. The incidence in a nonoperative control cohort was 0.4% (JAMA Surg. 2017 Jun 21;152[6]:e170504). Notably, this prolonged use was greatest in patients with prior pain conditions, substance abuse, and mental health disorders – a finding that may have implications for the counseling we provide prior to surgery.
It’s not clear what the optimal analgesic regimen is for minimally invasive or other gynecologic surgeries. What is clearly recommended, however, is that the approach be multifaceted. In our practice, we believe that the preoperative use of acetaminophen, pregabalin, and celecoxib plays an important role in reducing postoperative pain and opioid use. But we also have striven to create a practice-wide culture shift (throughout the operating and recovery rooms), for instance, that encourages using the least amounts of narcotics possible and using the shortest-acting formulations possible.
Transversus abdominis plane (TAP) blocks are also often part of ERAS protocols; they have been shown in at least two randomized controlled trials of abdominal hysterectomy to reduce intraoperative fentanyl requirements and to reduce immediate postoperative pain scores and postoperative morphine requirements (Anesth Analg. 2008 Dec;107[6]:2056-60; J Anaesthesiol Clin Pharmacol. 2014 Jul-Sep;30[3]:391-6).
More recently, liposomal bupivacaine, which is slowly released over several days, has gained traction as a substitute for standard bupivacaine and other agents in TAP blocks. In one recent retrospective study, abdominal incision infiltration with liposomal bupivacaine was associated with less opioid use (with no change in pain scores), compared with bupivacaine hydrochloride after laparotomy for gynecologic malignancies (Obstet Gynecol. 2016 Nov;128[5]:1009-17). It’s significantly more expensive, however, making it likely that the formulation is being used more judiciously in minimally invasive gynecologic surgery than in open surgeries.
Because of costs, we currently are restricted to using liposomal bupivacaine in our robotic surgeries only. In our practice, the single 20 mL vial (266 mg of liposomal bupivacaine) is diluted with 20 mL of normal saline, but it can be further diluted without loss of efficacy. With a 16-gauge needle, the liposomal bupivacaine is distributed across the incisions (usually 20 mL in the umbilicus with a larger incision and 10 mL in each of the two lateral incisions). Patients are counseled that they may have more discomfort after 3 days, but by this point most are mobile and feeling relatively well with a combination of NSAIDs and acetaminophen.
With growing visibility of the problem of narcotic dependence in the United States, patients seem increasingly receptive and even eager to limit or avoid the use of opioids. Patients should be counseled that minimizing or avoiding opioids may also speed recovery. Narcotics cause gut motility to slow down, which may hinder mobilization. Early mobilization (within 24 hours) is among the enhanced recovery elements that the ERAS Society guidelines say is “of particular value” for minimally invasive surgery, along with maintenance of normothermia and normovolemia with maintenance of adequate cardiac output.
Selecting Steps
Our practice is also trying to reduce preoperative bowel preparation and preoperative fasting, both of which have been found to be stressful for the body without evidence of benefit. These practices can lead to insulin resistance and hyperglycemia, which are associated with increased morbidity and length of stay.
It is now recommended that clear fluids be allowed up to 2 hours before surgery and solids up to 6 hours before. Some health systems and practices also recommend presurgical carbohydrate loading (for example, 10 ounces of apple juice 2 hours before surgery) – another small change on the ERAS menu – to further reduce postoperative insulin resistance and help the body cope with its stress response to surgery.
Along with nutritional changes are also various measures aimed at optimizing the body’s functionality before surgery (“prehabilitation”), from walking 30 minutes a day to abstaining from alcohol for patients who drink heavily.
Throughout the country, enhanced recovery protocols are taking shape in gynecologic surgery. ERAS was featured in an aptly titled panel session at the 2017 annual meeting of the American Association of Gynecologic Laparoscopists: “Outpatient Hysterectomy, ERAS, and Same-Day Discharge: The Next Big Thing in Gyn Surgery.” Others are applying ERAS to scheduled cesarean sections. And in our practice, I believe that if we continue making small changes, we will reach our goal of opioid-free recoveries and a better surgical experience for our patients.
Kirsten Sasaki, MD, is an associate of the Advanced Gynecologic Surgery Institute. She reported that she has no disclosures relevant to this Master Class.
“Enhanced Recovery After Surgery” (ERAS) practices and protocols have been increasingly refined and adopted for the field of gynecology, and there is hope among gynecologic surgeons – and some recent evidence – that, with the ERAS movement, we are improving patient recoveries and outcomes and minimizing the need for opioids.
This applies not only to open surgeries but also to the minimally invasive procedures that already are prized for significant reductions in morbidity and length of stay. The overarching and guiding principle of ERAS is that any surgery – whether open or minimally invasive, major or minor – places stress on the body and is associated with risks and morbidity.
Enhanced recovery protocols are multidisciplinary, perioperative approaches designed to lessen the body’s stress response to surgery. The protocols and pathways offer us a menu of small changes that, in the aggregate, can lead to large and demonstrable benefits – especially when these small changes are chosen across the preoperative, intraoperative, and postoperative arenas and then standardized in one’s practice. Among the major components of ERAS practices and protocols are limiting preoperative fasting, employing multimodal analgesia, encouraging early ambulation and early postsurgical feeding, and creating culture shift that includes greater emphasis on patient expectations.
In our practice, we are incorporating ERAS practices not only in hopes of reducing the stress of all surgeries before, during, and after, but also with the goal of achieving a postoperative opioid-free hysterectomy, myomectomy, and extensive endometriosis surgery. (All of our advanced procedures are performed laparoscopically or robotically.)
Over the past 7 or so years, we have adopted a multimodal approach to pain control that includes a bundle of preoperative analgesics – acetaminophen, pregabalin, and celecoxib (we call it “TLC” for Tylenol, Lyrica, and Celebrex) – and the use of liposomal bupivacaine in our robotic surgeries. We are now turning toward ERAS nutritional changes, most of which run counter to traditional paradigms for surgical care. And in other areas, such as dedicated preoperative counseling, we continue to refine and improve our practices.
Improved Outcomes
The ERAS mindset notably intersected gynecology with the publication in 2016 of a two-part series of guidelines for gynecology/oncology surgery from the ERAS Society. The 8-year-old society has its roots in a study group of European surgeons and others who decided to examine surgical practices and the concept of multimodal surgical care put forth in the 1990s by Henrik Kehlet, MD, PhD, then a professor at the University of Copenhagen.
The first set of recommendations addressed pre- and intraoperative care (Gynecol Oncol. 2016 Feb;140[2]:313-22), and the second set addressed postoperative care (Gynecol Oncol. 2016 Feb;140[2]:323-32). Similar evidence-based recommendations were previously written for colonic resections, rectal and pelvic surgery, and other surgical specialties.
Most of the published outcomes of enhanced recovery protocols come from colorectal surgery. As noted in the ERAS Society gynecology/oncology guidelines, the benefits include an average reduction in length of stay of 2.5 days and a decrease in complications by as much as 50%.
There is growing evidence, however, that ERAS programs are also beneficial for patients undergoing laparoscopic surgery, and outcomes from gynecology – including minimally invasive surgery – are also being reported.
For instance, a retrospective case-control study of 55 consecutive gynecologic oncology patients treated at the University of California, San Francisco, with laparoscopic or robotic surgery and an enhanced recovery pathway – and 110 historical control patients matched on the basis of age and surgery type – found significant improvements in recovery time, decreased pain despite reduced opioid use, and overall lower hospital costs (Obstet Gynecol. 2016 Jul;128[1]:138-44).
The enhanced recovery pathway included patient education, multimodal antiemetics, multimodal analgesia, and balanced fluid administration. Early catheter removal, ambulation, and feeding were also components. Analgesia included routine preoperative gabapentin, diclofenac, and acetaminophen; routine postoperative gabapentin, NSAIDs, and acetaminophen; and transversus abdominis plane blocks in 32 of the ERAS patients.
ERAS patients were significantly more likely to be discharged on day 1 (91%, compared with 60% in the control group). Opioid use decreased by 30%, and pain scores on postoperative day 1 were significantly lower.
Another study looking at the effect of enhanced recovery implementation in gynecologic surgeries at the University of Virginia, Charlottesville, (gynecologic oncology, urogynecology, and general gynecology) similarly reported benefits for vaginal and minimally invasive procedures, as well as for open procedures (Obstet Gynecol. 2016 Sep;128[3]:457-66).
In the minimally invasive group, investigators compared 324 patients before ERAS implementation with 249 patients afterward and found that the median length of stay was unchanged (1 day). However, intraoperative and postoperative opioid consumption decreased significantly and – even though actual pain scores improved only slightly – patient satisfaction scores improved markedly among post-ERAS patients. Patients gave higher marks, for instance, to questions regarding pain control (“how well your pain was controlled”) and teamwork (“staff worked together to care for you”).
Reducing Opioids
New opioid use that persists after a surgical procedure is a postsurgical complication that we all should be working to prevent. It has been estimated that 6% of surgical patients – even those who’ve had relatively minor surgical procedures – will become long-term opioid users, developing a dependence on the drugs prescribed to them for postsurgical pain.
This was shown last year in a national study of insurance claims data from between 2013 and 2014; investigators identified adults without opioid use in the year prior to surgery (including hysterectomy) and found that 5.9%-6.5% were filling opioid prescriptions 90-180 days after their surgical procedure. The incidence in a nonoperative control cohort was 0.4% (JAMA Surg. 2017 Jun 21;152[6]:e170504). Notably, this prolonged use was greatest in patients with prior pain conditions, substance abuse, and mental health disorders – a finding that may have implications for the counseling we provide prior to surgery.
It’s not clear what the optimal analgesic regimen is for minimally invasive or other gynecologic surgeries. What is clearly recommended, however, is that the approach be multifaceted. In our practice, we believe that the preoperative use of acetaminophen, pregabalin, and celecoxib plays an important role in reducing postoperative pain and opioid use. But we also have striven to create a practice-wide culture shift (throughout the operating and recovery rooms), for instance, that encourages using the least amounts of narcotics possible and using the shortest-acting formulations possible.
Transversus abdominis plane (TAP) blocks are also often part of ERAS protocols; they have been shown in at least two randomized controlled trials of abdominal hysterectomy to reduce intraoperative fentanyl requirements and to reduce immediate postoperative pain scores and postoperative morphine requirements (Anesth Analg. 2008 Dec;107[6]:2056-60; J Anaesthesiol Clin Pharmacol. 2014 Jul-Sep;30[3]:391-6).
More recently, liposomal bupivacaine, which is slowly released over several days, has gained traction as a substitute for standard bupivacaine and other agents in TAP blocks. In one recent retrospective study, abdominal incision infiltration with liposomal bupivacaine was associated with less opioid use (with no change in pain scores), compared with bupivacaine hydrochloride after laparotomy for gynecologic malignancies (Obstet Gynecol. 2016 Nov;128[5]:1009-17). It’s significantly more expensive, however, making it likely that the formulation is being used more judiciously in minimally invasive gynecologic surgery than in open surgeries.
Because of costs, we currently are restricted to using liposomal bupivacaine in our robotic surgeries only. In our practice, the single 20 mL vial (266 mg of liposomal bupivacaine) is diluted with 20 mL of normal saline, but it can be further diluted without loss of efficacy. With a 16-gauge needle, the liposomal bupivacaine is distributed across the incisions (usually 20 mL in the umbilicus with a larger incision and 10 mL in each of the two lateral incisions). Patients are counseled that they may have more discomfort after 3 days, but by this point most are mobile and feeling relatively well with a combination of NSAIDs and acetaminophen.
With growing visibility of the problem of narcotic dependence in the United States, patients seem increasingly receptive and even eager to limit or avoid the use of opioids. Patients should be counseled that minimizing or avoiding opioids may also speed recovery. Narcotics cause gut motility to slow down, which may hinder mobilization. Early mobilization (within 24 hours) is among the enhanced recovery elements that the ERAS Society guidelines say is “of particular value” for minimally invasive surgery, along with maintenance of normothermia and normovolemia with maintenance of adequate cardiac output.
Selecting Steps
Our practice is also trying to reduce preoperative bowel preparation and preoperative fasting, both of which have been found to be stressful for the body without evidence of benefit. These practices can lead to insulin resistance and hyperglycemia, which are associated with increased morbidity and length of stay.
It is now recommended that clear fluids be allowed up to 2 hours before surgery and solids up to 6 hours before. Some health systems and practices also recommend presurgical carbohydrate loading (for example, 10 ounces of apple juice 2 hours before surgery) – another small change on the ERAS menu – to further reduce postoperative insulin resistance and help the body cope with its stress response to surgery.
Along with nutritional changes are also various measures aimed at optimizing the body’s functionality before surgery (“prehabilitation”), from walking 30 minutes a day to abstaining from alcohol for patients who drink heavily.
Throughout the country, enhanced recovery protocols are taking shape in gynecologic surgery. ERAS was featured in an aptly titled panel session at the 2017 annual meeting of the American Association of Gynecologic Laparoscopists: “Outpatient Hysterectomy, ERAS, and Same-Day Discharge: The Next Big Thing in Gyn Surgery.” Others are applying ERAS to scheduled cesarean sections. And in our practice, I believe that if we continue making small changes, we will reach our goal of opioid-free recoveries and a better surgical experience for our patients.
Kirsten Sasaki, MD, is an associate of the Advanced Gynecologic Surgery Institute. She reported that she has no disclosures relevant to this Master Class.
Clinical trial: Telescopic vs. balloon dissection for hernia
A randomized clinical trial will compare two techniques for achieving extraperitoneal space during hernia surgery.
The in operative times, early postoperative pain scores, surgical complications, and rate of hernia recurrence. The Spacemaker Balloon Dissector will be used for the trial.
The techniques will be timed and measured in minutes from incision to end of procedure. Pain outcomes will be measured using the Numeric Pain Rating Scale (NRS-11) at postoperative days 1, 7, and 30. Intraoperative complications, 30-day infections, and 1-year recurrence will be reported in numbers and percent as appropriate.
The study is sponsored by The Cleveland Clinic.
For more details about the trial, go to www.clinicaltrials.gov.
SOURCE: Clinical Trial NCT03276871.
A randomized clinical trial will compare two techniques for achieving extraperitoneal space during hernia surgery.
The in operative times, early postoperative pain scores, surgical complications, and rate of hernia recurrence. The Spacemaker Balloon Dissector will be used for the trial.
The techniques will be timed and measured in minutes from incision to end of procedure. Pain outcomes will be measured using the Numeric Pain Rating Scale (NRS-11) at postoperative days 1, 7, and 30. Intraoperative complications, 30-day infections, and 1-year recurrence will be reported in numbers and percent as appropriate.
The study is sponsored by The Cleveland Clinic.
For more details about the trial, go to www.clinicaltrials.gov.
SOURCE: Clinical Trial NCT03276871.
A randomized clinical trial will compare two techniques for achieving extraperitoneal space during hernia surgery.
The in operative times, early postoperative pain scores, surgical complications, and rate of hernia recurrence. The Spacemaker Balloon Dissector will be used for the trial.
The techniques will be timed and measured in minutes from incision to end of procedure. Pain outcomes will be measured using the Numeric Pain Rating Scale (NRS-11) at postoperative days 1, 7, and 30. Intraoperative complications, 30-day infections, and 1-year recurrence will be reported in numbers and percent as appropriate.
The study is sponsored by The Cleveland Clinic.
For more details about the trial, go to www.clinicaltrials.gov.
SOURCE: Clinical Trial NCT03276871.
FROM CLINICALTRIALS.GOV
Sex disparities seen in surgical professorships
Female surgeons were less likely than were male surgeons to be full professors in 2014, according to an analysis that adjusted for a number of “factors known to influence academic rank independently of sex.”
The overall , after adjustment for such factors as age, years since residency, subspecialty, total publications, and clinical trial participation. “This methodological rigor is a central and unique strength of this analysis and bolsters the validity of the study findings,” wrote Daniel M. Blumenthal, MD, of Massachusetts General Hospital and Harvard Medical School, Boston, and his associates. The report was published in Annals of Surgery (2018 Jan 12. doi: 10.1097/SLA.0000000000002662).
Differences favoring men were seen in eight of the nine subspecialties included in the study, with vascular surgery being the exception (adjusted odds ratio, 1.63). Of the other eight, however, only in general surgery (adjusted odds ratio, 0.68) was the difference significant, the investigators said.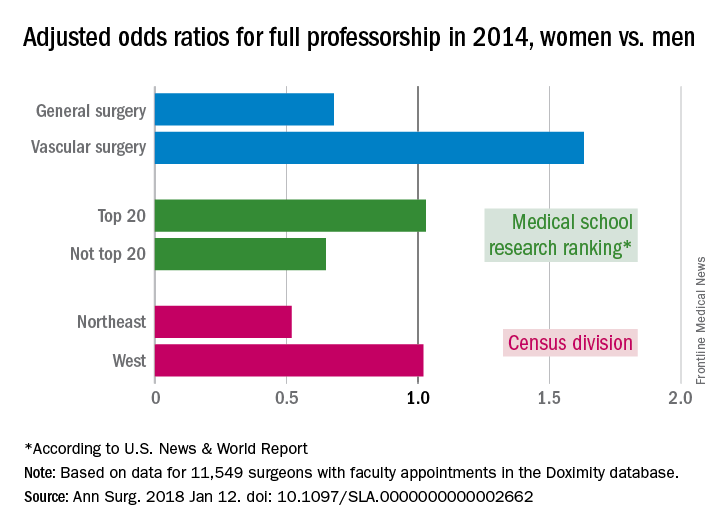
Another factor included in the analysis was the research ranking of the medical schools. There was no significant sex disparity for faculty members at medical schools ranked in the top 20 by U.S. News & World Report (adjusted odds ratio, 1.03), but schools outside the top 20 had an adjusted odds ratio (AOR) of 0.65 for women, compared with men. Geographically speaking, the largest disadvantage for female surgeons among the four main census divisions was in the Northeast (AOR, 0.52), with the West generating relative equality (AOR, 1.02) and the South (AOR, 0.83) and the Midwest (AOR, 0.87) in between, they reported.
Dr. Blumenthal and his associates used a cross-sectional database maintained by Doximity, an online networking company. The analysis included data for 11,549 surgeons with faculty appointments in 2014: 3,080 were full professors (7% were women), 2,931 were associate professors (13.8% were women), and 5,538 were assistant professors (19.4% were women).
Dr. Blumenthal reported research funding from a fellowship at Harvard. The investigators declared no conflict of interests.
SOURCE: Blumenthal DM et al. Ann Surg. 2018 Jan 12. doi: 10.1097/SLA.0000000000002662.
Female surgeons were less likely than were male surgeons to be full professors in 2014, according to an analysis that adjusted for a number of “factors known to influence academic rank independently of sex.”
The overall , after adjustment for such factors as age, years since residency, subspecialty, total publications, and clinical trial participation. “This methodological rigor is a central and unique strength of this analysis and bolsters the validity of the study findings,” wrote Daniel M. Blumenthal, MD, of Massachusetts General Hospital and Harvard Medical School, Boston, and his associates. The report was published in Annals of Surgery (2018 Jan 12. doi: 10.1097/SLA.0000000000002662).
Differences favoring men were seen in eight of the nine subspecialties included in the study, with vascular surgery being the exception (adjusted odds ratio, 1.63). Of the other eight, however, only in general surgery (adjusted odds ratio, 0.68) was the difference significant, the investigators said.
Another factor included in the analysis was the research ranking of the medical schools. There was no significant sex disparity for faculty members at medical schools ranked in the top 20 by U.S. News & World Report (adjusted odds ratio, 1.03), but schools outside the top 20 had an adjusted odds ratio (AOR) of 0.65 for women, compared with men. Geographically speaking, the largest disadvantage for female surgeons among the four main census divisions was in the Northeast (AOR, 0.52), with the West generating relative equality (AOR, 1.02) and the South (AOR, 0.83) and the Midwest (AOR, 0.87) in between, they reported.
Dr. Blumenthal and his associates used a cross-sectional database maintained by Doximity, an online networking company. The analysis included data for 11,549 surgeons with faculty appointments in 2014: 3,080 were full professors (7% were women), 2,931 were associate professors (13.8% were women), and 5,538 were assistant professors (19.4% were women).
Dr. Blumenthal reported research funding from a fellowship at Harvard. The investigators declared no conflict of interests.
SOURCE: Blumenthal DM et al. Ann Surg. 2018 Jan 12. doi: 10.1097/SLA.0000000000002662.
Female surgeons were less likely than were male surgeons to be full professors in 2014, according to an analysis that adjusted for a number of “factors known to influence academic rank independently of sex.”
The overall , after adjustment for such factors as age, years since residency, subspecialty, total publications, and clinical trial participation. “This methodological rigor is a central and unique strength of this analysis and bolsters the validity of the study findings,” wrote Daniel M. Blumenthal, MD, of Massachusetts General Hospital and Harvard Medical School, Boston, and his associates. The report was published in Annals of Surgery (2018 Jan 12. doi: 10.1097/SLA.0000000000002662).
Differences favoring men were seen in eight of the nine subspecialties included in the study, with vascular surgery being the exception (adjusted odds ratio, 1.63). Of the other eight, however, only in general surgery (adjusted odds ratio, 0.68) was the difference significant, the investigators said.
Another factor included in the analysis was the research ranking of the medical schools. There was no significant sex disparity for faculty members at medical schools ranked in the top 20 by U.S. News & World Report (adjusted odds ratio, 1.03), but schools outside the top 20 had an adjusted odds ratio (AOR) of 0.65 for women, compared with men. Geographically speaking, the largest disadvantage for female surgeons among the four main census divisions was in the Northeast (AOR, 0.52), with the West generating relative equality (AOR, 1.02) and the South (AOR, 0.83) and the Midwest (AOR, 0.87) in between, they reported.
Dr. Blumenthal and his associates used a cross-sectional database maintained by Doximity, an online networking company. The analysis included data for 11,549 surgeons with faculty appointments in 2014: 3,080 were full professors (7% were women), 2,931 were associate professors (13.8% were women), and 5,538 were assistant professors (19.4% were women).
Dr. Blumenthal reported research funding from a fellowship at Harvard. The investigators declared no conflict of interests.
SOURCE: Blumenthal DM et al. Ann Surg. 2018 Jan 12. doi: 10.1097/SLA.0000000000002662.
FROM ANNALS OF SURGERY
Major medical, insurance, health groups agree on pre-auth improvement areas
The prior authorization approval process required by health insurance companies for patients’ medical treatments, also called preapproval, eats up countless hours of time and costs, over $80,000 per year, per provider. The average provider deals with 35 of these prior authorization requests per day, and each request takes an average of 20 minutes. Physicians, pharmacists, hospitals, medical groups, and health insurance companies are working together to come up with a solution.
The American Hospital Association, America’s Health Insurance Plans, American Medical Association, American Pharmacists Association, Blue Cross Blue Shield Association, and Medical Group Management Association announced a consensus statement delineating where they agree the health care industry can improve the prior authorization process.
Most of the solutions outlined in the document are intuitive – they include reducing the number of health care professionals subject to prior authorization requirements based on their performance; adherence to evidence-based medical practices or participation in a value-based agreement with the health insurance provider; reviewing the services and medications that require prior authorization and eliminating requirements for therapies that no longer warrant them; improving communications between health insurance providers, health care professionals, and patients to provide clarity on prior authorization requirements and changes; protecting continuity of care for patients; improving formulary information and coverage restrictions at point-of-care; and adopting national electronic standards for prior authorization.
Jack Resneck Jr., MD, chair-elect of the AMA board of trustees, described the document as a “good initial step” toward reducing the difficulties imposed by prior authorizations.
Prior authorization requests are particularly burdensome for medications that are expensive, a headache that doctors working with patients who have rheumatoid arthritis or lupus know well.
As insurance and provider groups work to improve the prior authorization process “it will be vital that they consider the issue from the perspective of general practitioners as well as specialists, the latter of whom prescribe more of the specialty tier medications that are subject to more protocols before patients can access these often life-improving medications,” Stephen Marmaras, director of policy and advocacy at Global Healthy Living Foundation, said in an interview. “Ultimately, improved communication between both parties – the physician offices and the payers – will allow us to identify barriers existing in current appeals processes and work toward collectively building solutions that benefit patients, particularly those with chronic disease who rely on stable access to medications.”
Sean Fahey, MD, chair of the American College of Rheumatology’s insurance subcommittee, said that, while the consensus statement is “a step in the right direction, like a lot of things, the devil is in the details.
“There’s good concepts in the statement without a whole lot of specifics,” Dr. Fahey said. Most changes will be addressed at the state level, because the federal legislature is very hesitant to legislate decisions for nongovernment insurance.
“A lot of the ideas set forth in this consensus statement are wonderful,” said Dr. Fahey. “Unfortunately for our patients, many of their medications are ludicrously expensive. … Every time you write a prescription for one of these medications, after appropriate therapy, you have to do [a preauthorization] just to get the medicine that people want and need. It’s frustrating that the issue of drug cost is driving the whole process. For a $60,000 a year price you’re going to have to do a preauthorization every single time, as opposed to a drug that’s $100 a year.”
Still, the statement is “an important step” toward ultimately making vital medications “more accessible for patients,” Dr. Fahey said.
The prior authorization approval process required by health insurance companies for patients’ medical treatments, also called preapproval, eats up countless hours of time and costs, over $80,000 per year, per provider. The average provider deals with 35 of these prior authorization requests per day, and each request takes an average of 20 minutes. Physicians, pharmacists, hospitals, medical groups, and health insurance companies are working together to come up with a solution.
The American Hospital Association, America’s Health Insurance Plans, American Medical Association, American Pharmacists Association, Blue Cross Blue Shield Association, and Medical Group Management Association announced a consensus statement delineating where they agree the health care industry can improve the prior authorization process.
Most of the solutions outlined in the document are intuitive – they include reducing the number of health care professionals subject to prior authorization requirements based on their performance; adherence to evidence-based medical practices or participation in a value-based agreement with the health insurance provider; reviewing the services and medications that require prior authorization and eliminating requirements for therapies that no longer warrant them; improving communications between health insurance providers, health care professionals, and patients to provide clarity on prior authorization requirements and changes; protecting continuity of care for patients; improving formulary information and coverage restrictions at point-of-care; and adopting national electronic standards for prior authorization.
Jack Resneck Jr., MD, chair-elect of the AMA board of trustees, described the document as a “good initial step” toward reducing the difficulties imposed by prior authorizations.
Prior authorization requests are particularly burdensome for medications that are expensive, a headache that doctors working with patients who have rheumatoid arthritis or lupus know well.
As insurance and provider groups work to improve the prior authorization process “it will be vital that they consider the issue from the perspective of general practitioners as well as specialists, the latter of whom prescribe more of the specialty tier medications that are subject to more protocols before patients can access these often life-improving medications,” Stephen Marmaras, director of policy and advocacy at Global Healthy Living Foundation, said in an interview. “Ultimately, improved communication between both parties – the physician offices and the payers – will allow us to identify barriers existing in current appeals processes and work toward collectively building solutions that benefit patients, particularly those with chronic disease who rely on stable access to medications.”
Sean Fahey, MD, chair of the American College of Rheumatology’s insurance subcommittee, said that, while the consensus statement is “a step in the right direction, like a lot of things, the devil is in the details.
“There’s good concepts in the statement without a whole lot of specifics,” Dr. Fahey said. Most changes will be addressed at the state level, because the federal legislature is very hesitant to legislate decisions for nongovernment insurance.
“A lot of the ideas set forth in this consensus statement are wonderful,” said Dr. Fahey. “Unfortunately for our patients, many of their medications are ludicrously expensive. … Every time you write a prescription for one of these medications, after appropriate therapy, you have to do [a preauthorization] just to get the medicine that people want and need. It’s frustrating that the issue of drug cost is driving the whole process. For a $60,000 a year price you’re going to have to do a preauthorization every single time, as opposed to a drug that’s $100 a year.”
Still, the statement is “an important step” toward ultimately making vital medications “more accessible for patients,” Dr. Fahey said.
The prior authorization approval process required by health insurance companies for patients’ medical treatments, also called preapproval, eats up countless hours of time and costs, over $80,000 per year, per provider. The average provider deals with 35 of these prior authorization requests per day, and each request takes an average of 20 minutes. Physicians, pharmacists, hospitals, medical groups, and health insurance companies are working together to come up with a solution.
The American Hospital Association, America’s Health Insurance Plans, American Medical Association, American Pharmacists Association, Blue Cross Blue Shield Association, and Medical Group Management Association announced a consensus statement delineating where they agree the health care industry can improve the prior authorization process.
Most of the solutions outlined in the document are intuitive – they include reducing the number of health care professionals subject to prior authorization requirements based on their performance; adherence to evidence-based medical practices or participation in a value-based agreement with the health insurance provider; reviewing the services and medications that require prior authorization and eliminating requirements for therapies that no longer warrant them; improving communications between health insurance providers, health care professionals, and patients to provide clarity on prior authorization requirements and changes; protecting continuity of care for patients; improving formulary information and coverage restrictions at point-of-care; and adopting national electronic standards for prior authorization.
Jack Resneck Jr., MD, chair-elect of the AMA board of trustees, described the document as a “good initial step” toward reducing the difficulties imposed by prior authorizations.
Prior authorization requests are particularly burdensome for medications that are expensive, a headache that doctors working with patients who have rheumatoid arthritis or lupus know well.
As insurance and provider groups work to improve the prior authorization process “it will be vital that they consider the issue from the perspective of general practitioners as well as specialists, the latter of whom prescribe more of the specialty tier medications that are subject to more protocols before patients can access these often life-improving medications,” Stephen Marmaras, director of policy and advocacy at Global Healthy Living Foundation, said in an interview. “Ultimately, improved communication between both parties – the physician offices and the payers – will allow us to identify barriers existing in current appeals processes and work toward collectively building solutions that benefit patients, particularly those with chronic disease who rely on stable access to medications.”
Sean Fahey, MD, chair of the American College of Rheumatology’s insurance subcommittee, said that, while the consensus statement is “a step in the right direction, like a lot of things, the devil is in the details.
“There’s good concepts in the statement without a whole lot of specifics,” Dr. Fahey said. Most changes will be addressed at the state level, because the federal legislature is very hesitant to legislate decisions for nongovernment insurance.
“A lot of the ideas set forth in this consensus statement are wonderful,” said Dr. Fahey. “Unfortunately for our patients, many of their medications are ludicrously expensive. … Every time you write a prescription for one of these medications, after appropriate therapy, you have to do [a preauthorization] just to get the medicine that people want and need. It’s frustrating that the issue of drug cost is driving the whole process. For a $60,000 a year price you’re going to have to do a preauthorization every single time, as opposed to a drug that’s $100 a year.”
Still, the statement is “an important step” toward ultimately making vital medications “more accessible for patients,” Dr. Fahey said.
Surgical LAA occlusion tops anticoagulation for AF thromboprotection
Surgical left atrial appendage occlusion may be just as good as anticoagulation at preventing thromboembolic events in older people with atrial fibrillation, with less risk of bleeding into the brain, according to a database review of more than 10,000 patients.
Among elderly atrial fibrillation patients who underwent heart surgery with no oral follow-up oral anticoagulation, those who had the left atrial appendage surgically occluded were 74% less likely than were those who did not to be readmitted for a major thromboembolic event within 3 years, and 68% less likely to be readmitted for a hemorrhagic stroke, researchers at Duke University in Durham, N.C., found.
“The current study demonstrated that S-LAAO [surgical left atrial appendage occlusion] was associated with a significantly lower rate of thromboembolism among patients without oral anticoagulation. In the cohort of patients discharged with oral anticoagulation, S-LAAO was not associated with [reduced] thromboembolism but was associated with a lower risk for hemorrhagic stroke presumably related to eventual discontinuation of oral anticoagulation among S-LAAO patients,” reported Daniel Friedman, MD, and his coinvestigators. The study was published Jan. 23 in JAMA.
In short, the findings suggest that shutting down the left atrial appendage in older patients offers the same stroke protection as anticoagulation, but without the bleeding risk. Given the low rates of anticoagulant use, physicians have been considering that approach for a while. Even so, it’s only been a weak (IIb) recommendation so far in AF guidelines because of the lack of evidence.
That might change soon, but “additional randomized studies comparing S-LAAO without anticoagulation [versus] systemic anticoagulation alone will be needed to define the optimal use of S-LAAO,” said Dr. Friedman, a cardiothoracic surgeon at Duke, and his colleagues. Those studies are in the works.
The team found 10,524 older patients in the Society of Thoracic Surgeons Adult Cardiac Surgery Database during 2011-2012, and linked them to Medicare data so they could be followed for up to 3 years. About a third of the subjects had stand-alone coronary artery bypass grafting; the rest had mitral or aortic valve repairs with or without CABG.
The investigators compared outcomes among the 37% (3,892) who had S-LAAO with outcomes among those who did not. Participants were a median of 76 years of age, 61% were men, and they were all at high risk for AF stroke.
After a mean follow-up of 2.6 years, subjects who received S-LAAO without postoperative anticoagulation had a significantly lower risk of readmission for thromboembolism – stroke, transient ischemic attack, or systemic embolism – compared with those who received neither S-LAAO nor anticoagulation (unadjusted rate 4.2% versus 6.0%; adjusted subdistribution hazard ratio [sHR] 0.26, 95% CI, 0.17-0.40, P less than .001).
There was no extra embolic stroke protection from S-LAAO in patients who were discharged on anticoagulation (sHR 0.88, 95% CI, 0.56-1.39; P = .59), but the risk of returning with a hemorrhagic stroke was considerably less (sHR 0.32, 95% CI, 0.17-0.57, P less than .001).
The S-LAAO group more commonly had nonparoxysmal AF, a higher ejection fraction, a lower mortality risk score, and lower rates of common stroke risks, such as diabetes, hypertension, and prior stroke. The Duke team adjusted for those and a long list of other confounders, including smoking, age, preoperative warfarin, and academic hospital status.
There were important limitations. No one knows what surgeons did to close the LAA, or how well it worked, and most patients discharged on anticoagulation were sent home on warfarin, not the newer direct oral anticoagulants.
The investigators noted that “the strongest data to date for LAAO come from randomized trials comparing warfarin with percutaneous LAAO using the WATCHMAN device” from Boston Scientific.
The reduction in cardiovascular mortality in those trials appeared to be driven by a reduction in hemorrhagic stroke and occurred despite increased rates of ischemic stroke, they said.
The current study, however, showed that S-LAAO was associated with a significantly lower rate of thromboembolism in patients without oral anticoagulation, the authors said.
The work was funded, in part, by the Food and Drug Administration. Dr. Friedman reported grants from Boston Scientific and Abbott. Other authors reported financial relations with those and several other companies.
SOURCE: Friedman DJ, et. al. JAMA. 2018;319(4):365-74. doi: 10.1001/jama.2017.20125.
The implications of the study may have far-reaching consequences on the best treatment to reduce both thromboembolism and hemorrhage associated with AF treatment.
There is a strong signal that S-LAAO may be equivalent to anticoagulation prophylaxis to avoid thromboembolism in certain patients. This possibility is intriguing because it suggests that S-LAAO may be as effective as anticoagulation and could potentially avoid the bleeding risks associated with anticoagulation. A reasonable hypothesis based on the authors’ findings is that ablation procedures that occlude the left atrial appendage are adequate treatments to avoid thromboembolism and to minimize postoperative anticoagulation-related hemorrhage. This somewhat novel hypothesis, if true, could avoid a significant morbidity associated with anticoagulation while providing adequate treatment for thromboembolic complications of AF.
Importantly, the results suggest that failure to perform an S-LAAO at the time of cardiac operation in patients with nonvalvular AF is associated with significantly increased intermediate-term thromboembolic risk.
Victor M. Ferraris , MD, PhD, a cardiothoracic surgeon at the University of Kentucky, Lexington, made his comments in an accompanying editorial. He had no conflicts of interest.
The implications of the study may have far-reaching consequences on the best treatment to reduce both thromboembolism and hemorrhage associated with AF treatment.
There is a strong signal that S-LAAO may be equivalent to anticoagulation prophylaxis to avoid thromboembolism in certain patients. This possibility is intriguing because it suggests that S-LAAO may be as effective as anticoagulation and could potentially avoid the bleeding risks associated with anticoagulation. A reasonable hypothesis based on the authors’ findings is that ablation procedures that occlude the left atrial appendage are adequate treatments to avoid thromboembolism and to minimize postoperative anticoagulation-related hemorrhage. This somewhat novel hypothesis, if true, could avoid a significant morbidity associated with anticoagulation while providing adequate treatment for thromboembolic complications of AF.
Importantly, the results suggest that failure to perform an S-LAAO at the time of cardiac operation in patients with nonvalvular AF is associated with significantly increased intermediate-term thromboembolic risk.
Victor M. Ferraris , MD, PhD, a cardiothoracic surgeon at the University of Kentucky, Lexington, made his comments in an accompanying editorial. He had no conflicts of interest.
The implications of the study may have far-reaching consequences on the best treatment to reduce both thromboembolism and hemorrhage associated with AF treatment.
There is a strong signal that S-LAAO may be equivalent to anticoagulation prophylaxis to avoid thromboembolism in certain patients. This possibility is intriguing because it suggests that S-LAAO may be as effective as anticoagulation and could potentially avoid the bleeding risks associated with anticoagulation. A reasonable hypothesis based on the authors’ findings is that ablation procedures that occlude the left atrial appendage are adequate treatments to avoid thromboembolism and to minimize postoperative anticoagulation-related hemorrhage. This somewhat novel hypothesis, if true, could avoid a significant morbidity associated with anticoagulation while providing adequate treatment for thromboembolic complications of AF.
Importantly, the results suggest that failure to perform an S-LAAO at the time of cardiac operation in patients with nonvalvular AF is associated with significantly increased intermediate-term thromboembolic risk.
Victor M. Ferraris , MD, PhD, a cardiothoracic surgeon at the University of Kentucky, Lexington, made his comments in an accompanying editorial. He had no conflicts of interest.
Surgical left atrial appendage occlusion may be just as good as anticoagulation at preventing thromboembolic events in older people with atrial fibrillation, with less risk of bleeding into the brain, according to a database review of more than 10,000 patients.
Among elderly atrial fibrillation patients who underwent heart surgery with no oral follow-up oral anticoagulation, those who had the left atrial appendage surgically occluded were 74% less likely than were those who did not to be readmitted for a major thromboembolic event within 3 years, and 68% less likely to be readmitted for a hemorrhagic stroke, researchers at Duke University in Durham, N.C., found.
“The current study demonstrated that S-LAAO [surgical left atrial appendage occlusion] was associated with a significantly lower rate of thromboembolism among patients without oral anticoagulation. In the cohort of patients discharged with oral anticoagulation, S-LAAO was not associated with [reduced] thromboembolism but was associated with a lower risk for hemorrhagic stroke presumably related to eventual discontinuation of oral anticoagulation among S-LAAO patients,” reported Daniel Friedman, MD, and his coinvestigators. The study was published Jan. 23 in JAMA.
In short, the findings suggest that shutting down the left atrial appendage in older patients offers the same stroke protection as anticoagulation, but without the bleeding risk. Given the low rates of anticoagulant use, physicians have been considering that approach for a while. Even so, it’s only been a weak (IIb) recommendation so far in AF guidelines because of the lack of evidence.
That might change soon, but “additional randomized studies comparing S-LAAO without anticoagulation [versus] systemic anticoagulation alone will be needed to define the optimal use of S-LAAO,” said Dr. Friedman, a cardiothoracic surgeon at Duke, and his colleagues. Those studies are in the works.
The team found 10,524 older patients in the Society of Thoracic Surgeons Adult Cardiac Surgery Database during 2011-2012, and linked them to Medicare data so they could be followed for up to 3 years. About a third of the subjects had stand-alone coronary artery bypass grafting; the rest had mitral or aortic valve repairs with or without CABG.
The investigators compared outcomes among the 37% (3,892) who had S-LAAO with outcomes among those who did not. Participants were a median of 76 years of age, 61% were men, and they were all at high risk for AF stroke.
After a mean follow-up of 2.6 years, subjects who received S-LAAO without postoperative anticoagulation had a significantly lower risk of readmission for thromboembolism – stroke, transient ischemic attack, or systemic embolism – compared with those who received neither S-LAAO nor anticoagulation (unadjusted rate 4.2% versus 6.0%; adjusted subdistribution hazard ratio [sHR] 0.26, 95% CI, 0.17-0.40, P less than .001).
There was no extra embolic stroke protection from S-LAAO in patients who were discharged on anticoagulation (sHR 0.88, 95% CI, 0.56-1.39; P = .59), but the risk of returning with a hemorrhagic stroke was considerably less (sHR 0.32, 95% CI, 0.17-0.57, P less than .001).
The S-LAAO group more commonly had nonparoxysmal AF, a higher ejection fraction, a lower mortality risk score, and lower rates of common stroke risks, such as diabetes, hypertension, and prior stroke. The Duke team adjusted for those and a long list of other confounders, including smoking, age, preoperative warfarin, and academic hospital status.
There were important limitations. No one knows what surgeons did to close the LAA, or how well it worked, and most patients discharged on anticoagulation were sent home on warfarin, not the newer direct oral anticoagulants.
The investigators noted that “the strongest data to date for LAAO come from randomized trials comparing warfarin with percutaneous LAAO using the WATCHMAN device” from Boston Scientific.
The reduction in cardiovascular mortality in those trials appeared to be driven by a reduction in hemorrhagic stroke and occurred despite increased rates of ischemic stroke, they said.
The current study, however, showed that S-LAAO was associated with a significantly lower rate of thromboembolism in patients without oral anticoagulation, the authors said.
The work was funded, in part, by the Food and Drug Administration. Dr. Friedman reported grants from Boston Scientific and Abbott. Other authors reported financial relations with those and several other companies.
SOURCE: Friedman DJ, et. al. JAMA. 2018;319(4):365-74. doi: 10.1001/jama.2017.20125.
Surgical left atrial appendage occlusion may be just as good as anticoagulation at preventing thromboembolic events in older people with atrial fibrillation, with less risk of bleeding into the brain, according to a database review of more than 10,000 patients.
Among elderly atrial fibrillation patients who underwent heart surgery with no oral follow-up oral anticoagulation, those who had the left atrial appendage surgically occluded were 74% less likely than were those who did not to be readmitted for a major thromboembolic event within 3 years, and 68% less likely to be readmitted for a hemorrhagic stroke, researchers at Duke University in Durham, N.C., found.
“The current study demonstrated that S-LAAO [surgical left atrial appendage occlusion] was associated with a significantly lower rate of thromboembolism among patients without oral anticoagulation. In the cohort of patients discharged with oral anticoagulation, S-LAAO was not associated with [reduced] thromboembolism but was associated with a lower risk for hemorrhagic stroke presumably related to eventual discontinuation of oral anticoagulation among S-LAAO patients,” reported Daniel Friedman, MD, and his coinvestigators. The study was published Jan. 23 in JAMA.
In short, the findings suggest that shutting down the left atrial appendage in older patients offers the same stroke protection as anticoagulation, but without the bleeding risk. Given the low rates of anticoagulant use, physicians have been considering that approach for a while. Even so, it’s only been a weak (IIb) recommendation so far in AF guidelines because of the lack of evidence.
That might change soon, but “additional randomized studies comparing S-LAAO without anticoagulation [versus] systemic anticoagulation alone will be needed to define the optimal use of S-LAAO,” said Dr. Friedman, a cardiothoracic surgeon at Duke, and his colleagues. Those studies are in the works.
The team found 10,524 older patients in the Society of Thoracic Surgeons Adult Cardiac Surgery Database during 2011-2012, and linked them to Medicare data so they could be followed for up to 3 years. About a third of the subjects had stand-alone coronary artery bypass grafting; the rest had mitral or aortic valve repairs with or without CABG.
The investigators compared outcomes among the 37% (3,892) who had S-LAAO with outcomes among those who did not. Participants were a median of 76 years of age, 61% were men, and they were all at high risk for AF stroke.
After a mean follow-up of 2.6 years, subjects who received S-LAAO without postoperative anticoagulation had a significantly lower risk of readmission for thromboembolism – stroke, transient ischemic attack, or systemic embolism – compared with those who received neither S-LAAO nor anticoagulation (unadjusted rate 4.2% versus 6.0%; adjusted subdistribution hazard ratio [sHR] 0.26, 95% CI, 0.17-0.40, P less than .001).
There was no extra embolic stroke protection from S-LAAO in patients who were discharged on anticoagulation (sHR 0.88, 95% CI, 0.56-1.39; P = .59), but the risk of returning with a hemorrhagic stroke was considerably less (sHR 0.32, 95% CI, 0.17-0.57, P less than .001).
The S-LAAO group more commonly had nonparoxysmal AF, a higher ejection fraction, a lower mortality risk score, and lower rates of common stroke risks, such as diabetes, hypertension, and prior stroke. The Duke team adjusted for those and a long list of other confounders, including smoking, age, preoperative warfarin, and academic hospital status.
There were important limitations. No one knows what surgeons did to close the LAA, or how well it worked, and most patients discharged on anticoagulation were sent home on warfarin, not the newer direct oral anticoagulants.
The investigators noted that “the strongest data to date for LAAO come from randomized trials comparing warfarin with percutaneous LAAO using the WATCHMAN device” from Boston Scientific.
The reduction in cardiovascular mortality in those trials appeared to be driven by a reduction in hemorrhagic stroke and occurred despite increased rates of ischemic stroke, they said.
The current study, however, showed that S-LAAO was associated with a significantly lower rate of thromboembolism in patients without oral anticoagulation, the authors said.
The work was funded, in part, by the Food and Drug Administration. Dr. Friedman reported grants from Boston Scientific and Abbott. Other authors reported financial relations with those and several other companies.
SOURCE: Friedman DJ, et. al. JAMA. 2018;319(4):365-74. doi: 10.1001/jama.2017.20125.
FROM JAMA
Key clinical point: Surgical left atrial appendage occlusion (S-LAAO) is probably just as good as anticoagulation at preventing thromboembolic events in older people with atrial fibrillation, with less risk of bleeding into the brain.
Major finding: within 3 years, and 68% less likely to be readmitted for a hemorrhagic stroke.
Study details: Database review of more than 10,000 elderly AF patients followed for up to 3 years after cardiac surgery.
Disclosures: The work was funded, in part, by the Food and Drug Administration. The authors had financial ties to Boston Scientific, Abbott, and several other companies.
Source: Friedman DJ, et. al. JAMA. 2018;319(4):365-74. doi: 10.1001/jama.2017.20125
Gastrografin offers an alternative to surgery for SBO
LAKE BUENA VISTA, FLA. – Gastrografin significantly decreased the need for an operation in small bowel obstruction (SBO) patients, even among patients who had never undergone abdominal surgery, according to a study presented at the annual scientific assembly of the Eastern Association for the Surgery of Trauma.
“Small bowel obstruction is a common clinical problem in the United States, with 15 out of 100 admissions for abdominal pain related to SBO,” said Morgan Collom, DO, surgical resident at Medical City Fort Worth (Tex.). “Many studies conclude that [operative exploration] is not needed and it is feasible to perform nonoperative conservative management, yet still argue that small obstruction in a virgin abdomen patient should undergo mandatory exploration to avoid missing a diagnosis of malignancy.”
Investigators studied 601 SBO patients admitted to one of 14 institutions included in an EAST database between February 2015 and December 2016 for this prospective study.
Of those included, 500 had previous abdominal surgery and the others had never had surgery. Gastrografin (Bracco Diagnostics) was used to treat their bowel obstruction.
Those with previous abdominal surgery were more likely to be over age 65 years (48% vs. 36%), be female (50% vs. 25%), have a history of cancer (42.6% vs. 18.8%), and have a prior admission of SBO (41.2% vs 8.9%), according to Dr. Collom.
Among patients who previously had surgery, operative exploration was 50% less likely (odds ratio = .51, P = .04) than among those who had never had surgery. In a comparison of patients with and without previous surgery, introducing Gastrografin evened out the likelihood for an operation (OR = .17 and .21, respectively). Overall, those who received Gastrografin were 86% less likely to undergo bowel exploration(OR = .14, P less than .01).
Of the 36 NAS patients treated with Gastrografin, 33 underwent successful, nonoperative therapy, and 3 underwent a therapeutic laparotomy for a malignancy.
During a question-and-answer session, audience members called to attention the issue of the database used, which does not review complications or recurrences after 30 days or any missed abnormalities, indicating that some malignancies may have developed after the therapy.
Dr. Collom acknowledged the limitation and agreed that the next study would need to address this.
The investigators reported no relevant financial disclosures.
SOURCE: EAST Scientific Assembly abstract No. 24.
LAKE BUENA VISTA, FLA. – Gastrografin significantly decreased the need for an operation in small bowel obstruction (SBO) patients, even among patients who had never undergone abdominal surgery, according to a study presented at the annual scientific assembly of the Eastern Association for the Surgery of Trauma.
“Small bowel obstruction is a common clinical problem in the United States, with 15 out of 100 admissions for abdominal pain related to SBO,” said Morgan Collom, DO, surgical resident at Medical City Fort Worth (Tex.). “Many studies conclude that [operative exploration] is not needed and it is feasible to perform nonoperative conservative management, yet still argue that small obstruction in a virgin abdomen patient should undergo mandatory exploration to avoid missing a diagnosis of malignancy.”
Investigators studied 601 SBO patients admitted to one of 14 institutions included in an EAST database between February 2015 and December 2016 for this prospective study.
Of those included, 500 had previous abdominal surgery and the others had never had surgery. Gastrografin (Bracco Diagnostics) was used to treat their bowel obstruction.
Those with previous abdominal surgery were more likely to be over age 65 years (48% vs. 36%), be female (50% vs. 25%), have a history of cancer (42.6% vs. 18.8%), and have a prior admission of SBO (41.2% vs 8.9%), according to Dr. Collom.
Among patients who previously had surgery, operative exploration was 50% less likely (odds ratio = .51, P = .04) than among those who had never had surgery. In a comparison of patients with and without previous surgery, introducing Gastrografin evened out the likelihood for an operation (OR = .17 and .21, respectively). Overall, those who received Gastrografin were 86% less likely to undergo bowel exploration(OR = .14, P less than .01).
Of the 36 NAS patients treated with Gastrografin, 33 underwent successful, nonoperative therapy, and 3 underwent a therapeutic laparotomy for a malignancy.
During a question-and-answer session, audience members called to attention the issue of the database used, which does not review complications or recurrences after 30 days or any missed abnormalities, indicating that some malignancies may have developed after the therapy.
Dr. Collom acknowledged the limitation and agreed that the next study would need to address this.
The investigators reported no relevant financial disclosures.
SOURCE: EAST Scientific Assembly abstract No. 24.
LAKE BUENA VISTA, FLA. – Gastrografin significantly decreased the need for an operation in small bowel obstruction (SBO) patients, even among patients who had never undergone abdominal surgery, according to a study presented at the annual scientific assembly of the Eastern Association for the Surgery of Trauma.
“Small bowel obstruction is a common clinical problem in the United States, with 15 out of 100 admissions for abdominal pain related to SBO,” said Morgan Collom, DO, surgical resident at Medical City Fort Worth (Tex.). “Many studies conclude that [operative exploration] is not needed and it is feasible to perform nonoperative conservative management, yet still argue that small obstruction in a virgin abdomen patient should undergo mandatory exploration to avoid missing a diagnosis of malignancy.”
Investigators studied 601 SBO patients admitted to one of 14 institutions included in an EAST database between February 2015 and December 2016 for this prospective study.
Of those included, 500 had previous abdominal surgery and the others had never had surgery. Gastrografin (Bracco Diagnostics) was used to treat their bowel obstruction.
Those with previous abdominal surgery were more likely to be over age 65 years (48% vs. 36%), be female (50% vs. 25%), have a history of cancer (42.6% vs. 18.8%), and have a prior admission of SBO (41.2% vs 8.9%), according to Dr. Collom.
Among patients who previously had surgery, operative exploration was 50% less likely (odds ratio = .51, P = .04) than among those who had never had surgery. In a comparison of patients with and without previous surgery, introducing Gastrografin evened out the likelihood for an operation (OR = .17 and .21, respectively). Overall, those who received Gastrografin were 86% less likely to undergo bowel exploration(OR = .14, P less than .01).
Of the 36 NAS patients treated with Gastrografin, 33 underwent successful, nonoperative therapy, and 3 underwent a therapeutic laparotomy for a malignancy.
During a question-and-answer session, audience members called to attention the issue of the database used, which does not review complications or recurrences after 30 days or any missed abnormalities, indicating that some malignancies may have developed after the therapy.
Dr. Collom acknowledged the limitation and agreed that the next study would need to address this.
The investigators reported no relevant financial disclosures.
SOURCE: EAST Scientific Assembly abstract No. 24.
REPORTING FROM EAST 2018
Key clinical point: Gastrografin is an effective alternative to operative exploration in small bowel obstruction patients with or without a history of surgical intervention.
Major finding: Treatment with Gastrografin (Bracco Diagnostics) reduced the risk of operative exploration for patients with small bowel obstruction (OR = .14, P less than .01).
Data source: Prospective, observational study of 601 small bowel obstruction patients seen at 14 institutions between February 2015 and December 2016.
Disclosures: The investigators reported no relevant financial disclosures.
Source: Collom et al. EAST Scientific Assembly, abstract 24.
CHIP out of cash amid government shutdown
The Children’s Health Insurance Plan remains without funding as a temporary government funding bill that included a 6-year extension of the popular bipartisan program failed to garner enough support in the Senate.
On Jan. 19, to clear that hurdle.
Other Republican senators who voted against the bill were Jeff Flake of Arizona, Lindsey Graham of South Carolina, Mike Lee of Utah, and Rand Paul of Kentucky.
Democratic senators who voted for the bill were Joe Donnelly of Indiana, Heidi Heitkamp of North Dakota, Doug Jones of Alabama, Joe Manchin of West Virginia, and Claire McCaskill of Missouri.
The continuing resolution also featured a few other health care provisions, including delays to the medical device tax and the so-called Cadillac tax on high-valued health insurance plans offered by employers.
In comments made on the Senate floor prior to the vote, Finance Committee Chairman Orrin Hatch (R-Utah) called out Democrats for failing to pass the bill.
“There’s really nothing wrong with the substance of the bill, Mr. President, or at least very few of our Democratic colleagues are complaining about what’s actually in the bill” he said. “Instead, they’re complaining about what’s not in it.”
Democrats made their stand on the absence of language on Deferred Action for Childhood Arrivals (DACA), which addresses the immigration status of young people – often called Dreamers – who were brought to this country illegally as children.
Democrats also complained that funding for community health centers was not included.
Sen. Hatch, one of the original authors of the CHIP authorizing legislation, continued to argue for the program and the overall funding bill.
“This new bill before us would reauthorize CHIP for 6 six years,” he noted. “A 6-year extension would be the longest in the history of the program. In all other respects, the bill is identical to the one the Finance Committee reported [in September] with broad bipartisan support,” although no further action was taken on it after receiving near unanimous support in committee.
Finance Committee Ranking Member Ron Wyden (D-Ore.) took exception to the characterization that Democrats were not in support of the CHIP provision.
“The Chairman and I did negotiate a CHIP extension back in September, and the Senate Finance did report it out in a near unanimous, bipartisan basis,” he said. “CHIP could have passed the Congress within days, but the House Republicans had other ideas.”
Sen. Wyden noted that CHIP reauthorization was attached to other legislative actions in the House that all failed to garner enough support instead of being sent through as a stand-alone bill that likely would have received large bipartisan support.
And even though the extension would be for 6 years, Sen. Wyden also questioned why it wasn’t receiving permanent authorization.
“Congress learned that making CHIP permanent actually saves taxpayer dollars,” he said. “It’s a better deal than a 6-year extension, less of an expense for the taxpayer. True fiscal conservatives ought to be tripping over themselves to pass a permanent bill without preconditions. But at every turn in the CHIP debate, Republican leaders have found a new hostage.”
Sen. McConnell said following the failed voted that the Senate would resume activities beginning at noon on Jan. 20 – with votes expected – in hopes of finding a solution.
The Children’s Health Insurance Plan remains without funding as a temporary government funding bill that included a 6-year extension of the popular bipartisan program failed to garner enough support in the Senate.
On Jan. 19, to clear that hurdle.
Other Republican senators who voted against the bill were Jeff Flake of Arizona, Lindsey Graham of South Carolina, Mike Lee of Utah, and Rand Paul of Kentucky.
Democratic senators who voted for the bill were Joe Donnelly of Indiana, Heidi Heitkamp of North Dakota, Doug Jones of Alabama, Joe Manchin of West Virginia, and Claire McCaskill of Missouri.
The continuing resolution also featured a few other health care provisions, including delays to the medical device tax and the so-called Cadillac tax on high-valued health insurance plans offered by employers.
In comments made on the Senate floor prior to the vote, Finance Committee Chairman Orrin Hatch (R-Utah) called out Democrats for failing to pass the bill.
“There’s really nothing wrong with the substance of the bill, Mr. President, or at least very few of our Democratic colleagues are complaining about what’s actually in the bill” he said. “Instead, they’re complaining about what’s not in it.”
Democrats made their stand on the absence of language on Deferred Action for Childhood Arrivals (DACA), which addresses the immigration status of young people – often called Dreamers – who were brought to this country illegally as children.
Democrats also complained that funding for community health centers was not included.
Sen. Hatch, one of the original authors of the CHIP authorizing legislation, continued to argue for the program and the overall funding bill.
“This new bill before us would reauthorize CHIP for 6 six years,” he noted. “A 6-year extension would be the longest in the history of the program. In all other respects, the bill is identical to the one the Finance Committee reported [in September] with broad bipartisan support,” although no further action was taken on it after receiving near unanimous support in committee.
Finance Committee Ranking Member Ron Wyden (D-Ore.) took exception to the characterization that Democrats were not in support of the CHIP provision.
“The Chairman and I did negotiate a CHIP extension back in September, and the Senate Finance did report it out in a near unanimous, bipartisan basis,” he said. “CHIP could have passed the Congress within days, but the House Republicans had other ideas.”
Sen. Wyden noted that CHIP reauthorization was attached to other legislative actions in the House that all failed to garner enough support instead of being sent through as a stand-alone bill that likely would have received large bipartisan support.
And even though the extension would be for 6 years, Sen. Wyden also questioned why it wasn’t receiving permanent authorization.
“Congress learned that making CHIP permanent actually saves taxpayer dollars,” he said. “It’s a better deal than a 6-year extension, less of an expense for the taxpayer. True fiscal conservatives ought to be tripping over themselves to pass a permanent bill without preconditions. But at every turn in the CHIP debate, Republican leaders have found a new hostage.”
Sen. McConnell said following the failed voted that the Senate would resume activities beginning at noon on Jan. 20 – with votes expected – in hopes of finding a solution.
The Children’s Health Insurance Plan remains without funding as a temporary government funding bill that included a 6-year extension of the popular bipartisan program failed to garner enough support in the Senate.
On Jan. 19, to clear that hurdle.
Other Republican senators who voted against the bill were Jeff Flake of Arizona, Lindsey Graham of South Carolina, Mike Lee of Utah, and Rand Paul of Kentucky.
Democratic senators who voted for the bill were Joe Donnelly of Indiana, Heidi Heitkamp of North Dakota, Doug Jones of Alabama, Joe Manchin of West Virginia, and Claire McCaskill of Missouri.
The continuing resolution also featured a few other health care provisions, including delays to the medical device tax and the so-called Cadillac tax on high-valued health insurance plans offered by employers.
In comments made on the Senate floor prior to the vote, Finance Committee Chairman Orrin Hatch (R-Utah) called out Democrats for failing to pass the bill.
“There’s really nothing wrong with the substance of the bill, Mr. President, or at least very few of our Democratic colleagues are complaining about what’s actually in the bill” he said. “Instead, they’re complaining about what’s not in it.”
Democrats made their stand on the absence of language on Deferred Action for Childhood Arrivals (DACA), which addresses the immigration status of young people – often called Dreamers – who were brought to this country illegally as children.
Democrats also complained that funding for community health centers was not included.
Sen. Hatch, one of the original authors of the CHIP authorizing legislation, continued to argue for the program and the overall funding bill.
“This new bill before us would reauthorize CHIP for 6 six years,” he noted. “A 6-year extension would be the longest in the history of the program. In all other respects, the bill is identical to the one the Finance Committee reported [in September] with broad bipartisan support,” although no further action was taken on it after receiving near unanimous support in committee.
Finance Committee Ranking Member Ron Wyden (D-Ore.) took exception to the characterization that Democrats were not in support of the CHIP provision.
“The Chairman and I did negotiate a CHIP extension back in September, and the Senate Finance did report it out in a near unanimous, bipartisan basis,” he said. “CHIP could have passed the Congress within days, but the House Republicans had other ideas.”
Sen. Wyden noted that CHIP reauthorization was attached to other legislative actions in the House that all failed to garner enough support instead of being sent through as a stand-alone bill that likely would have received large bipartisan support.
And even though the extension would be for 6 years, Sen. Wyden also questioned why it wasn’t receiving permanent authorization.
“Congress learned that making CHIP permanent actually saves taxpayer dollars,” he said. “It’s a better deal than a 6-year extension, less of an expense for the taxpayer. True fiscal conservatives ought to be tripping over themselves to pass a permanent bill without preconditions. But at every turn in the CHIP debate, Republican leaders have found a new hostage.”
Sen. McConnell said following the failed voted that the Senate would resume activities beginning at noon on Jan. 20 – with votes expected – in hopes of finding a solution.
HHS creates new religious freedoms division
The Trump administration has announced a new division within the U.S. Health & Human Services Department that aims to protect health care providers who have religious or moral objections to performing medical services, such as abortion.
In a Jan. 18 announcement, HHS Office for Civil Rights Director Roger Severino said the new Conscience and Religious Freedom Division will focus on outreach, policy making, and vigorously enforcing existing federal laws that protect conscience and religious freedom rights. The new branch will enable health providers to file conscience and religious freedom complaints, which will then be investigated by the division.
Opinions on the new division from physicians and medical associations were mixed. The American College of Physicians (ACP) cautioned that the division’s creation must not lead to discrimination against any category of class of patients, as guided by medical professions’ ethical obligations.
“ACP would be particularly concerned if the new HHS division takes any actions that would result in denial of access to appropriate health care based on gender, gender identity, sexual orientation, race, ethnicity, or other personal characteristics,” ACP President Jack Ende, MD, said in a statement. “ACP will evaluate the newly formed division as it begins operating, informed by our ethics and public policy positions. Those state that physicians have a professional obligation to not discriminate against any class of patients, but also that a physician may have a conscientious objection to providing a specific medical service to a patient.”
The Catholic Medical Association (CMA) applauded the new HHS division, saying it’s about time that the religious and conscience rights of health providers were protected by the federal government. In the past, health providers could be drawn into medical activities in a hospital or health care system that they found moral objectionable with no recourse, said Kathleen Raviele, MD, a board member for the Catholic Medical Association and a Decatur, Ga.–based ob.gyn.
“The fact that individual health care providers are going to have a place to go if they feel they have valid complaint is really momentous,” Dr. Raviele said in an interview. “It’s respecting the rights of all of us and not just some special interest groups ... I also think [the new division] will make states [and] hospital systems less likely to try to infringe on people’s conscientious rights.”
The HIV Medicine Association called the formation of the new HHS division appalling, and said the office appears to protect health care providers who discriminate against vulnerable patients, such as women, LGBTQ patients, and minorities.
“The new division, designed to ‘protect’ health care providers who discriminate in the care and services they provide, defies the fundamental medical ethic to first do no harm,” HIV Medicine Association Chair Melanie Thompson, MD, said in a statement. “Using federal dollars to shield providers who choose to discriminate rather than protect vulnerable patients and provide services to improve their health is counter to the mission of HHS, wasteful of scarce federal funds, and will result in delayed or lack of care for vulnerable individuals, threatening their health and lives.”
The HHS Conscience and Religious Freedom Division is necessary to address the “sustained attack on conscience rights,” said Jane Orient, MD, executive director for the conservative Association of American Physicians and Surgeons.
“Many powerful and influential forces are attempting to impose their views on Americans, predominantly on Christians, forcing them to perform acts they believe to be immoral, [or risk] losing their job, their business, their livelihood, or even their life savings,” Dr. Orient said in an interview. “...We hope that the OCR will help to return our nation to its foundational principles of freedom and respect for people of conscience.”
The Trump administration has announced a new division within the U.S. Health & Human Services Department that aims to protect health care providers who have religious or moral objections to performing medical services, such as abortion.
In a Jan. 18 announcement, HHS Office for Civil Rights Director Roger Severino said the new Conscience and Religious Freedom Division will focus on outreach, policy making, and vigorously enforcing existing federal laws that protect conscience and religious freedom rights. The new branch will enable health providers to file conscience and religious freedom complaints, which will then be investigated by the division.
Opinions on the new division from physicians and medical associations were mixed. The American College of Physicians (ACP) cautioned that the division’s creation must not lead to discrimination against any category of class of patients, as guided by medical professions’ ethical obligations.
“ACP would be particularly concerned if the new HHS division takes any actions that would result in denial of access to appropriate health care based on gender, gender identity, sexual orientation, race, ethnicity, or other personal characteristics,” ACP President Jack Ende, MD, said in a statement. “ACP will evaluate the newly formed division as it begins operating, informed by our ethics and public policy positions. Those state that physicians have a professional obligation to not discriminate against any class of patients, but also that a physician may have a conscientious objection to providing a specific medical service to a patient.”
The Catholic Medical Association (CMA) applauded the new HHS division, saying it’s about time that the religious and conscience rights of health providers were protected by the federal government. In the past, health providers could be drawn into medical activities in a hospital or health care system that they found moral objectionable with no recourse, said Kathleen Raviele, MD, a board member for the Catholic Medical Association and a Decatur, Ga.–based ob.gyn.
“The fact that individual health care providers are going to have a place to go if they feel they have valid complaint is really momentous,” Dr. Raviele said in an interview. “It’s respecting the rights of all of us and not just some special interest groups ... I also think [the new division] will make states [and] hospital systems less likely to try to infringe on people’s conscientious rights.”
The HIV Medicine Association called the formation of the new HHS division appalling, and said the office appears to protect health care providers who discriminate against vulnerable patients, such as women, LGBTQ patients, and minorities.
“The new division, designed to ‘protect’ health care providers who discriminate in the care and services they provide, defies the fundamental medical ethic to first do no harm,” HIV Medicine Association Chair Melanie Thompson, MD, said in a statement. “Using federal dollars to shield providers who choose to discriminate rather than protect vulnerable patients and provide services to improve their health is counter to the mission of HHS, wasteful of scarce federal funds, and will result in delayed or lack of care for vulnerable individuals, threatening their health and lives.”
The HHS Conscience and Religious Freedom Division is necessary to address the “sustained attack on conscience rights,” said Jane Orient, MD, executive director for the conservative Association of American Physicians and Surgeons.
“Many powerful and influential forces are attempting to impose their views on Americans, predominantly on Christians, forcing them to perform acts they believe to be immoral, [or risk] losing their job, their business, their livelihood, or even their life savings,” Dr. Orient said in an interview. “...We hope that the OCR will help to return our nation to its foundational principles of freedom and respect for people of conscience.”
The Trump administration has announced a new division within the U.S. Health & Human Services Department that aims to protect health care providers who have religious or moral objections to performing medical services, such as abortion.
In a Jan. 18 announcement, HHS Office for Civil Rights Director Roger Severino said the new Conscience and Religious Freedom Division will focus on outreach, policy making, and vigorously enforcing existing federal laws that protect conscience and religious freedom rights. The new branch will enable health providers to file conscience and religious freedom complaints, which will then be investigated by the division.
Opinions on the new division from physicians and medical associations were mixed. The American College of Physicians (ACP) cautioned that the division’s creation must not lead to discrimination against any category of class of patients, as guided by medical professions’ ethical obligations.
“ACP would be particularly concerned if the new HHS division takes any actions that would result in denial of access to appropriate health care based on gender, gender identity, sexual orientation, race, ethnicity, or other personal characteristics,” ACP President Jack Ende, MD, said in a statement. “ACP will evaluate the newly formed division as it begins operating, informed by our ethics and public policy positions. Those state that physicians have a professional obligation to not discriminate against any class of patients, but also that a physician may have a conscientious objection to providing a specific medical service to a patient.”
The Catholic Medical Association (CMA) applauded the new HHS division, saying it’s about time that the religious and conscience rights of health providers were protected by the federal government. In the past, health providers could be drawn into medical activities in a hospital or health care system that they found moral objectionable with no recourse, said Kathleen Raviele, MD, a board member for the Catholic Medical Association and a Decatur, Ga.–based ob.gyn.
“The fact that individual health care providers are going to have a place to go if they feel they have valid complaint is really momentous,” Dr. Raviele said in an interview. “It’s respecting the rights of all of us and not just some special interest groups ... I also think [the new division] will make states [and] hospital systems less likely to try to infringe on people’s conscientious rights.”
The HIV Medicine Association called the formation of the new HHS division appalling, and said the office appears to protect health care providers who discriminate against vulnerable patients, such as women, LGBTQ patients, and minorities.
“The new division, designed to ‘protect’ health care providers who discriminate in the care and services they provide, defies the fundamental medical ethic to first do no harm,” HIV Medicine Association Chair Melanie Thompson, MD, said in a statement. “Using federal dollars to shield providers who choose to discriminate rather than protect vulnerable patients and provide services to improve their health is counter to the mission of HHS, wasteful of scarce federal funds, and will result in delayed or lack of care for vulnerable individuals, threatening their health and lives.”
The HHS Conscience and Religious Freedom Division is necessary to address the “sustained attack on conscience rights,” said Jane Orient, MD, executive director for the conservative Association of American Physicians and Surgeons.
“Many powerful and influential forces are attempting to impose their views on Americans, predominantly on Christians, forcing them to perform acts they believe to be immoral, [or risk] losing their job, their business, their livelihood, or even their life savings,” Dr. Orient said in an interview. “...We hope that the OCR will help to return our nation to its foundational principles of freedom and respect for people of conscience.”









