User login
Official Newspaper of the American College of Surgeons
Study: Half of doctors sued by age 55
By age 55, nearly half of physicians have been sued for malpractice, with general surgeons and obstetricians-gynecologists facing the highest lawsuit risks, according to data from the America Medical Association.
Investigators with the AMA surveyed 3,500 postresidency physicians who were not employed by the federal government. Findings show that the probability of getting sued increases with age, and that male doctors are more likely to be sued than female physicians. For example, only 8% of doctors under 40 have been sued, compared to nearly half of physicians over age 54, the study found. In addition, nearly 40% of male physicians have been sued over the course of their careers, compared with 23% of female doctors.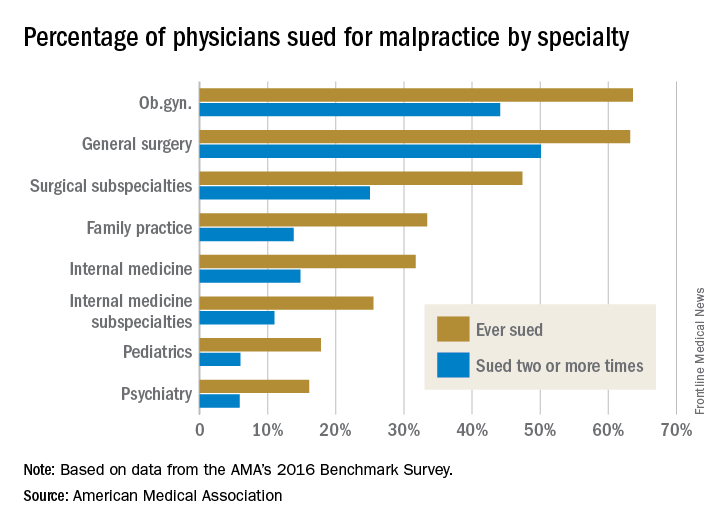
Employed physicians were no more or less likely than were physician-owners to have been sued. In addition, while solo practitioners had more claims filed against them than did doctors in single-specialty groups, the estimate was not statistically significant.
In a second report, an analysis showed the average expense incurred during a medical liability claim is $54,165 – a 65% increase since 2006. For the study, the AMA analyzed data from PIAA, a trade association for the medical professional liability insurance industry, and evaluated payments, expenses, and claim disposition within a sample of 90,473 medical liability claims that closed between 2006 and 2015.
Only 7% of claims were decided by a trial verdict with the vast majority (88%) won by the defendant health care provider. In about 25% of claims, a payment was paid to the plaintiff. The average indemnity payment to a plaintiff was $365,503 and the median payment was $200,000.
The new research paints a bleak picture of physicians’ experiences with medical liability claims and the associated cost burdens on the health system, AMA President David O. Barbe, MD, said in a statement.
“Even though the vast majority of claims are dropped, dismissed, or withdrawn, the heavy cost associated with a litigious climate takes a significant financial toll on our health care system when the nation is working to reduce unnecessary health care costs,” Dr. Barbe said.
By age 55, nearly half of physicians have been sued for malpractice, with general surgeons and obstetricians-gynecologists facing the highest lawsuit risks, according to data from the America Medical Association.
Investigators with the AMA surveyed 3,500 postresidency physicians who were not employed by the federal government. Findings show that the probability of getting sued increases with age, and that male doctors are more likely to be sued than female physicians. For example, only 8% of doctors under 40 have been sued, compared to nearly half of physicians over age 54, the study found. In addition, nearly 40% of male physicians have been sued over the course of their careers, compared with 23% of female doctors.
Employed physicians were no more or less likely than were physician-owners to have been sued. In addition, while solo practitioners had more claims filed against them than did doctors in single-specialty groups, the estimate was not statistically significant.
In a second report, an analysis showed the average expense incurred during a medical liability claim is $54,165 – a 65% increase since 2006. For the study, the AMA analyzed data from PIAA, a trade association for the medical professional liability insurance industry, and evaluated payments, expenses, and claim disposition within a sample of 90,473 medical liability claims that closed between 2006 and 2015.
Only 7% of claims were decided by a trial verdict with the vast majority (88%) won by the defendant health care provider. In about 25% of claims, a payment was paid to the plaintiff. The average indemnity payment to a plaintiff was $365,503 and the median payment was $200,000.
The new research paints a bleak picture of physicians’ experiences with medical liability claims and the associated cost burdens on the health system, AMA President David O. Barbe, MD, said in a statement.
“Even though the vast majority of claims are dropped, dismissed, or withdrawn, the heavy cost associated with a litigious climate takes a significant financial toll on our health care system when the nation is working to reduce unnecessary health care costs,” Dr. Barbe said.
By age 55, nearly half of physicians have been sued for malpractice, with general surgeons and obstetricians-gynecologists facing the highest lawsuit risks, according to data from the America Medical Association.
Investigators with the AMA surveyed 3,500 postresidency physicians who were not employed by the federal government. Findings show that the probability of getting sued increases with age, and that male doctors are more likely to be sued than female physicians. For example, only 8% of doctors under 40 have been sued, compared to nearly half of physicians over age 54, the study found. In addition, nearly 40% of male physicians have been sued over the course of their careers, compared with 23% of female doctors.
Employed physicians were no more or less likely than were physician-owners to have been sued. In addition, while solo practitioners had more claims filed against them than did doctors in single-specialty groups, the estimate was not statistically significant.
In a second report, an analysis showed the average expense incurred during a medical liability claim is $54,165 – a 65% increase since 2006. For the study, the AMA analyzed data from PIAA, a trade association for the medical professional liability insurance industry, and evaluated payments, expenses, and claim disposition within a sample of 90,473 medical liability claims that closed between 2006 and 2015.
Only 7% of claims were decided by a trial verdict with the vast majority (88%) won by the defendant health care provider. In about 25% of claims, a payment was paid to the plaintiff. The average indemnity payment to a plaintiff was $365,503 and the median payment was $200,000.
The new research paints a bleak picture of physicians’ experiences with medical liability claims and the associated cost burdens on the health system, AMA President David O. Barbe, MD, said in a statement.
“Even though the vast majority of claims are dropped, dismissed, or withdrawn, the heavy cost associated with a litigious climate takes a significant financial toll on our health care system when the nation is working to reduce unnecessary health care costs,” Dr. Barbe said.
No link found between OR skullcaps and infection
JACKSONVILLE, FLA. – Surgeons who choose to wear a skullcap in the OR can point to yet another study with evidence to bolster their preference.
Two major hospital and nursing credentialing organizations have recommended that hospitals ban skullcaps from the operating room as a practice to control surgical site infections, but a study of almost 2,000 operations at an academic medical center has found that strictly enforcing the ban had no impact on infection rates, according to results of a study presented at the Association for Academic Surgery/Society of University Surgeons Academic Surgical Congress.
The study, conducted at Thomas Jefferson University in Philadelphia, showed that rates of surgical site infections (SSIs) were almost identical in the year before and the year after the institution implemented the skullcap ban. “The overall surgical site infection rate was 5.4%, and there were no differences in surgical site infections before or after the headwear policy was adopted,” said Arturo J. Rios-Diaz, MD. The Joint Commission and the Association of periOperative Registered Nurses recommend against the use of skullcaps.
The study reviewed American College of Surgeons National Surgical Quality Improvement Program data on 1,901 patients who had 1,950 clean or clean-contaminated general surgery procedures in 2015, the year before the ban was implemented, and in 2016 (767 in 2015 and 1,183 in 2016). The most common procedures were colectomy (18.2%), pancreatectomy (13.5%), and ventral hernia repair (9.9%). The study excluded orthopedic and vascular operations and any cases with sepsis or an active infection at the time of surgery.
There were some differences between the pre- and postban patient groups. The preban group was younger (median age, 57.91 years vs. 59.75, P = .01) but had more patients who were obese, measured as body mass index above 30 kg/m2 (42.37% vs. 35.23%, P less than .01), and smokers (16.18% vs. 12.27%, P = .02). Wound classification also differed: clean, 38.55% before vs. 43.91% after; and clean-contaminated, 61.45% vs. 56.09% (P = .02). All other demographic and clinical characteristics were similar between the two groups.
“In multivariate logistic regression models controlling for these confounders, there was no association of the banning of skullcaps with decreased surgical site infection rates,” Dr. Rios-Diaz said.
“The adoption of guidelines targeted to optimize patient care should always be welcomed by surgeons,” he said. “However, if they’re going to be implemented on a national level, these policies must be based on higher levels of evidence, so further studies are warranted to assess the validity of the [Joint Commission] headwear guidelines.” According to Dr. Rios-Diaz, the recommendations from the Association of periOperative Registered Nurses are based on two case series from the 1960s and 1970s.
Thomas Jefferson University once again allows skullcaps in the OR, he said.
Dr. Rios-Diaz and his coauthors had no financial relationships to disclose.
SOURCE: Rios-Diaz AJ et al. Annual Academic Surgical Congress. Abstract 09.11.
JACKSONVILLE, FLA. – Surgeons who choose to wear a skullcap in the OR can point to yet another study with evidence to bolster their preference.
Two major hospital and nursing credentialing organizations have recommended that hospitals ban skullcaps from the operating room as a practice to control surgical site infections, but a study of almost 2,000 operations at an academic medical center has found that strictly enforcing the ban had no impact on infection rates, according to results of a study presented at the Association for Academic Surgery/Society of University Surgeons Academic Surgical Congress.
The study, conducted at Thomas Jefferson University in Philadelphia, showed that rates of surgical site infections (SSIs) were almost identical in the year before and the year after the institution implemented the skullcap ban. “The overall surgical site infection rate was 5.4%, and there were no differences in surgical site infections before or after the headwear policy was adopted,” said Arturo J. Rios-Diaz, MD. The Joint Commission and the Association of periOperative Registered Nurses recommend against the use of skullcaps.
The study reviewed American College of Surgeons National Surgical Quality Improvement Program data on 1,901 patients who had 1,950 clean or clean-contaminated general surgery procedures in 2015, the year before the ban was implemented, and in 2016 (767 in 2015 and 1,183 in 2016). The most common procedures were colectomy (18.2%), pancreatectomy (13.5%), and ventral hernia repair (9.9%). The study excluded orthopedic and vascular operations and any cases with sepsis or an active infection at the time of surgery.
There were some differences between the pre- and postban patient groups. The preban group was younger (median age, 57.91 years vs. 59.75, P = .01) but had more patients who were obese, measured as body mass index above 30 kg/m2 (42.37% vs. 35.23%, P less than .01), and smokers (16.18% vs. 12.27%, P = .02). Wound classification also differed: clean, 38.55% before vs. 43.91% after; and clean-contaminated, 61.45% vs. 56.09% (P = .02). All other demographic and clinical characteristics were similar between the two groups.
“In multivariate logistic regression models controlling for these confounders, there was no association of the banning of skullcaps with decreased surgical site infection rates,” Dr. Rios-Diaz said.
“The adoption of guidelines targeted to optimize patient care should always be welcomed by surgeons,” he said. “However, if they’re going to be implemented on a national level, these policies must be based on higher levels of evidence, so further studies are warranted to assess the validity of the [Joint Commission] headwear guidelines.” According to Dr. Rios-Diaz, the recommendations from the Association of periOperative Registered Nurses are based on two case series from the 1960s and 1970s.
Thomas Jefferson University once again allows skullcaps in the OR, he said.
Dr. Rios-Diaz and his coauthors had no financial relationships to disclose.
SOURCE: Rios-Diaz AJ et al. Annual Academic Surgical Congress. Abstract 09.11.
JACKSONVILLE, FLA. – Surgeons who choose to wear a skullcap in the OR can point to yet another study with evidence to bolster their preference.
Two major hospital and nursing credentialing organizations have recommended that hospitals ban skullcaps from the operating room as a practice to control surgical site infections, but a study of almost 2,000 operations at an academic medical center has found that strictly enforcing the ban had no impact on infection rates, according to results of a study presented at the Association for Academic Surgery/Society of University Surgeons Academic Surgical Congress.
The study, conducted at Thomas Jefferson University in Philadelphia, showed that rates of surgical site infections (SSIs) were almost identical in the year before and the year after the institution implemented the skullcap ban. “The overall surgical site infection rate was 5.4%, and there were no differences in surgical site infections before or after the headwear policy was adopted,” said Arturo J. Rios-Diaz, MD. The Joint Commission and the Association of periOperative Registered Nurses recommend against the use of skullcaps.
The study reviewed American College of Surgeons National Surgical Quality Improvement Program data on 1,901 patients who had 1,950 clean or clean-contaminated general surgery procedures in 2015, the year before the ban was implemented, and in 2016 (767 in 2015 and 1,183 in 2016). The most common procedures were colectomy (18.2%), pancreatectomy (13.5%), and ventral hernia repair (9.9%). The study excluded orthopedic and vascular operations and any cases with sepsis or an active infection at the time of surgery.
There were some differences between the pre- and postban patient groups. The preban group was younger (median age, 57.91 years vs. 59.75, P = .01) but had more patients who were obese, measured as body mass index above 30 kg/m2 (42.37% vs. 35.23%, P less than .01), and smokers (16.18% vs. 12.27%, P = .02). Wound classification also differed: clean, 38.55% before vs. 43.91% after; and clean-contaminated, 61.45% vs. 56.09% (P = .02). All other demographic and clinical characteristics were similar between the two groups.
“In multivariate logistic regression models controlling for these confounders, there was no association of the banning of skullcaps with decreased surgical site infection rates,” Dr. Rios-Diaz said.
“The adoption of guidelines targeted to optimize patient care should always be welcomed by surgeons,” he said. “However, if they’re going to be implemented on a national level, these policies must be based on higher levels of evidence, so further studies are warranted to assess the validity of the [Joint Commission] headwear guidelines.” According to Dr. Rios-Diaz, the recommendations from the Association of periOperative Registered Nurses are based on two case series from the 1960s and 1970s.
Thomas Jefferson University once again allows skullcaps in the OR, he said.
Dr. Rios-Diaz and his coauthors had no financial relationships to disclose.
SOURCE: Rios-Diaz AJ et al. Annual Academic Surgical Congress. Abstract 09.11.
REPORTING FROM THE ACADEMIC SURGICAL CONGRESS
Key clinical point: Findings of this study do not support the ban on surgical skullcaps.
Major finding: No association was found between the skullcap ban and decreased surgical site infection.
Study details: Analysis of ACS NSQIP data on 1,950 surgical cases from before and after the skullcap ban.
Disclosures: The investigators had no financial relationships to disclose.
Source: Rios-Diaz AJ et al. Annual Academic Surgical Congress. Abstract 09.11.
Bladder cancer: Two chemoradiation therapy regimens on par for muscle-invasive disease
SAN FRANCISCO – Two concurrent chemoradiation induction regimens had similar safety and efficacy when used as part of a bladder-sparing strategy in patients with muscle-invasive bladder cancer, suggest primary results of the NRG/RTOG 0712 trial. But one offers better patient convenience.
The selective bladder-preservation paradigm entails maximal transurethral resection of the bladder tumor (TURBT) followed by induction radiation and concomitant chemotherapy, lead author John J. Coen, MD, a radiation oncologist with 21st Century Oncology, Providence, Rhode Island, noted at the 2018 Genitourinary Cancers Symposium. Patients then undergo cystoscopy to assess their response.
“A key component of this therapy is close urologic surveillance,” he noted. “This is trimodality therapy with close urologic surveillance, and cystectomy is prompted at the earliest time it’s indicated.”
The 70 patients enrolled in the multicenter randomized phase 2 trial had undergone TURBT and were randomized evenly to twice-daily radiation plus 5-flourouracil-cisplatin (the RTOG standard at the time of trial planning) or to daily radiation plus gemcitabine (a modification of a successful regimen developed at the University of Michigan). All were offered adjuvant chemotherapy regardless of whether they responded and whether they underwent consolidation therapy or cystectomy.
At a median follow-up of 5.1 years among the 52 evaluable patients, the 3-year rate of freedom from distant metastasis, the trial’s primary endpoint, was 78% with twice-daily radiation plus 5-flourouracil-cisplatin and 84% with daily radiation plus gemcitabine, according to results reported at the symposium, which was sponsored by the American Society of Clinical Oncology, ASTRO, and the Society of Urologic Oncology.
Both values were higher than the trial’s predefined benchmark of 75% for defining the regimen as promising. “This trial wasn’t necessarily powered to compare arms, so the conclusion would be that both arms exceeded the benchmark and it would be appropriate to evaluate both arms further in subsequent trials,” Dr. Coen commented.
In each trial arm, more than three-fourths of patients had a complete response, and about two-thirds of patients were alive and free of distant metastasis with their bladder intact at 3 years.
Toxicity, which was supposed to be the tie-breaker if efficacy was similar, was also essentially the same for the two regimens. Both were fairly well tolerated in the acute period; the primary grade 3 and 4 toxicities were hematologic ones.
“So where do we go from here? I think this trial does demonstrate concurrent gemcitabine is a reasonable alternative to cisplatin for patients undergoing selective bladder preservation,” Dr. Coen summarized. “This is especially important in patients with poor renal function or hearing loss. And bladder cancer is often a disease in the elderly, so tolerance of various regimens is a very important aspect to planning further trials.”
“This trial also demonstrates that daily radiation is a reasonable alternative to twice-a-day radiation, which had become the standard through the RTOG on more contemporary trials,” he added. “Daily radiation would allow wider adoption of selective bladder preservation by trimodality therapy.”
Clinical implications
“I would have to see more details about the toxicity data for the chemotherapy, the radiosensitizers,” session co-chair Yair Lotan, MD, a professor of urology at the UT Southwestern Medical Center, Dallas, Texas, commented in an interview. “But in general, if you have two equivalent protocols in terms of effectiveness, and one is less toxic or more convenient, that’s always preferable. From the standpoint of coming once a day rather than twice a day to get radiation, if I were a patient, I would prefer that regimen”
Current practice in this patient population likely hinges on where a patient is treated, he said. “Based on the new nonmetastatic muscle-invasive guidelines, many of them would be recommended surgery or possibly chemoradiation protocols, and then there are a lot of factors such as patient preference and outcomes that would probably impact the decision making.”
“Certainly, this study won’t prove standard of care because standard of care in the global picture for a patient with muscle-invasive disease really will depend on a randomized trial of surgery versus radiation or chemoradiation, multimodal therapies,” Dr. Lotan maintained. “Not every patient is even eligible; some patients with hydronephrosis or unresectable disease may not be the best candidates for multimodal therapy. But more information from these types of trials may help clinicians decide which multimodal therapy approach to use.”
Study details
Patients enrolled in NRG/RTOG 0712, a multicenter randomized phase 2 trial supported by the National Cancer Institute, had clinical T2 or T3-4a bladder cancer. Hydronephrosis and obvious lymph node involvement were exclusion criteria.
Like the rate of freedom from metastasis, the rate of complete response after induction therapy was similar for the two arms: 87.9% with twice-daily radiation plus 5-flourouracil-cisplatin and 75.8% with daily radiation plus gemcitabine.
“These rates exceed historical complete response rates, and this is likely a result of improved selection over time and more thorough TURBTs performed over time,” Dr. Coen proposed. “Over the course of multiple successive RTOG trials, our selection criteria have been refined. One excellent example would be that hydronephrosis has been excluded on more contemporary trials, but if you look at older trials, those patients were included.”
The 3-year rate of bladder-intact distant metastasis–free survival was 66.7% and 69%, respectively. “This is a very important endpoint. These results are excellent,” he commented. “And there is really no appreciable difference between the two arms on the actuarial analysis.”
As far as specific treatment failure events in the trial overall, three patients died, eight underwent cystectomy, and eight developed distant metastases. Although numbers were small, these events appeared fairly evenly distributed across arms.
The rate of grade 3 or 4 acute toxicity was 57.6% with twice-daily radiation plus 5-flourouracil-cisplatin and 54.6% with daily radiation plus gemcitabine. The large majority of events were blood and bone marrow toxicity. “It’s quite notable that the rates of GU and GI toxicity were very low,” commented Dr. Coen, who disclosed that he had no relevant conflicts of interest.
SOURCE: Coen, J. et al, 2018 Genitourinary Cancers Symposium, Abstract 408
SAN FRANCISCO – Two concurrent chemoradiation induction regimens had similar safety and efficacy when used as part of a bladder-sparing strategy in patients with muscle-invasive bladder cancer, suggest primary results of the NRG/RTOG 0712 trial. But one offers better patient convenience.
The selective bladder-preservation paradigm entails maximal transurethral resection of the bladder tumor (TURBT) followed by induction radiation and concomitant chemotherapy, lead author John J. Coen, MD, a radiation oncologist with 21st Century Oncology, Providence, Rhode Island, noted at the 2018 Genitourinary Cancers Symposium. Patients then undergo cystoscopy to assess their response.
“A key component of this therapy is close urologic surveillance,” he noted. “This is trimodality therapy with close urologic surveillance, and cystectomy is prompted at the earliest time it’s indicated.”
The 70 patients enrolled in the multicenter randomized phase 2 trial had undergone TURBT and were randomized evenly to twice-daily radiation plus 5-flourouracil-cisplatin (the RTOG standard at the time of trial planning) or to daily radiation plus gemcitabine (a modification of a successful regimen developed at the University of Michigan). All were offered adjuvant chemotherapy regardless of whether they responded and whether they underwent consolidation therapy or cystectomy.
At a median follow-up of 5.1 years among the 52 evaluable patients, the 3-year rate of freedom from distant metastasis, the trial’s primary endpoint, was 78% with twice-daily radiation plus 5-flourouracil-cisplatin and 84% with daily radiation plus gemcitabine, according to results reported at the symposium, which was sponsored by the American Society of Clinical Oncology, ASTRO, and the Society of Urologic Oncology.
Both values were higher than the trial’s predefined benchmark of 75% for defining the regimen as promising. “This trial wasn’t necessarily powered to compare arms, so the conclusion would be that both arms exceeded the benchmark and it would be appropriate to evaluate both arms further in subsequent trials,” Dr. Coen commented.
In each trial arm, more than three-fourths of patients had a complete response, and about two-thirds of patients were alive and free of distant metastasis with their bladder intact at 3 years.
Toxicity, which was supposed to be the tie-breaker if efficacy was similar, was also essentially the same for the two regimens. Both were fairly well tolerated in the acute period; the primary grade 3 and 4 toxicities were hematologic ones.
“So where do we go from here? I think this trial does demonstrate concurrent gemcitabine is a reasonable alternative to cisplatin for patients undergoing selective bladder preservation,” Dr. Coen summarized. “This is especially important in patients with poor renal function or hearing loss. And bladder cancer is often a disease in the elderly, so tolerance of various regimens is a very important aspect to planning further trials.”
“This trial also demonstrates that daily radiation is a reasonable alternative to twice-a-day radiation, which had become the standard through the RTOG on more contemporary trials,” he added. “Daily radiation would allow wider adoption of selective bladder preservation by trimodality therapy.”
Clinical implications
“I would have to see more details about the toxicity data for the chemotherapy, the radiosensitizers,” session co-chair Yair Lotan, MD, a professor of urology at the UT Southwestern Medical Center, Dallas, Texas, commented in an interview. “But in general, if you have two equivalent protocols in terms of effectiveness, and one is less toxic or more convenient, that’s always preferable. From the standpoint of coming once a day rather than twice a day to get radiation, if I were a patient, I would prefer that regimen”
Current practice in this patient population likely hinges on where a patient is treated, he said. “Based on the new nonmetastatic muscle-invasive guidelines, many of them would be recommended surgery or possibly chemoradiation protocols, and then there are a lot of factors such as patient preference and outcomes that would probably impact the decision making.”
“Certainly, this study won’t prove standard of care because standard of care in the global picture for a patient with muscle-invasive disease really will depend on a randomized trial of surgery versus radiation or chemoradiation, multimodal therapies,” Dr. Lotan maintained. “Not every patient is even eligible; some patients with hydronephrosis or unresectable disease may not be the best candidates for multimodal therapy. But more information from these types of trials may help clinicians decide which multimodal therapy approach to use.”
Study details
Patients enrolled in NRG/RTOG 0712, a multicenter randomized phase 2 trial supported by the National Cancer Institute, had clinical T2 or T3-4a bladder cancer. Hydronephrosis and obvious lymph node involvement were exclusion criteria.
Like the rate of freedom from metastasis, the rate of complete response after induction therapy was similar for the two arms: 87.9% with twice-daily radiation plus 5-flourouracil-cisplatin and 75.8% with daily radiation plus gemcitabine.
“These rates exceed historical complete response rates, and this is likely a result of improved selection over time and more thorough TURBTs performed over time,” Dr. Coen proposed. “Over the course of multiple successive RTOG trials, our selection criteria have been refined. One excellent example would be that hydronephrosis has been excluded on more contemporary trials, but if you look at older trials, those patients were included.”
The 3-year rate of bladder-intact distant metastasis–free survival was 66.7% and 69%, respectively. “This is a very important endpoint. These results are excellent,” he commented. “And there is really no appreciable difference between the two arms on the actuarial analysis.”
As far as specific treatment failure events in the trial overall, three patients died, eight underwent cystectomy, and eight developed distant metastases. Although numbers were small, these events appeared fairly evenly distributed across arms.
The rate of grade 3 or 4 acute toxicity was 57.6% with twice-daily radiation plus 5-flourouracil-cisplatin and 54.6% with daily radiation plus gemcitabine. The large majority of events were blood and bone marrow toxicity. “It’s quite notable that the rates of GU and GI toxicity were very low,” commented Dr. Coen, who disclosed that he had no relevant conflicts of interest.
SOURCE: Coen, J. et al, 2018 Genitourinary Cancers Symposium, Abstract 408
SAN FRANCISCO – Two concurrent chemoradiation induction regimens had similar safety and efficacy when used as part of a bladder-sparing strategy in patients with muscle-invasive bladder cancer, suggest primary results of the NRG/RTOG 0712 trial. But one offers better patient convenience.
The selective bladder-preservation paradigm entails maximal transurethral resection of the bladder tumor (TURBT) followed by induction radiation and concomitant chemotherapy, lead author John J. Coen, MD, a radiation oncologist with 21st Century Oncology, Providence, Rhode Island, noted at the 2018 Genitourinary Cancers Symposium. Patients then undergo cystoscopy to assess their response.
“A key component of this therapy is close urologic surveillance,” he noted. “This is trimodality therapy with close urologic surveillance, and cystectomy is prompted at the earliest time it’s indicated.”
The 70 patients enrolled in the multicenter randomized phase 2 trial had undergone TURBT and were randomized evenly to twice-daily radiation plus 5-flourouracil-cisplatin (the RTOG standard at the time of trial planning) or to daily radiation plus gemcitabine (a modification of a successful regimen developed at the University of Michigan). All were offered adjuvant chemotherapy regardless of whether they responded and whether they underwent consolidation therapy or cystectomy.
At a median follow-up of 5.1 years among the 52 evaluable patients, the 3-year rate of freedom from distant metastasis, the trial’s primary endpoint, was 78% with twice-daily radiation plus 5-flourouracil-cisplatin and 84% with daily radiation plus gemcitabine, according to results reported at the symposium, which was sponsored by the American Society of Clinical Oncology, ASTRO, and the Society of Urologic Oncology.
Both values were higher than the trial’s predefined benchmark of 75% for defining the regimen as promising. “This trial wasn’t necessarily powered to compare arms, so the conclusion would be that both arms exceeded the benchmark and it would be appropriate to evaluate both arms further in subsequent trials,” Dr. Coen commented.
In each trial arm, more than three-fourths of patients had a complete response, and about two-thirds of patients were alive and free of distant metastasis with their bladder intact at 3 years.
Toxicity, which was supposed to be the tie-breaker if efficacy was similar, was also essentially the same for the two regimens. Both were fairly well tolerated in the acute period; the primary grade 3 and 4 toxicities were hematologic ones.
“So where do we go from here? I think this trial does demonstrate concurrent gemcitabine is a reasonable alternative to cisplatin for patients undergoing selective bladder preservation,” Dr. Coen summarized. “This is especially important in patients with poor renal function or hearing loss. And bladder cancer is often a disease in the elderly, so tolerance of various regimens is a very important aspect to planning further trials.”
“This trial also demonstrates that daily radiation is a reasonable alternative to twice-a-day radiation, which had become the standard through the RTOG on more contemporary trials,” he added. “Daily radiation would allow wider adoption of selective bladder preservation by trimodality therapy.”
Clinical implications
“I would have to see more details about the toxicity data for the chemotherapy, the radiosensitizers,” session co-chair Yair Lotan, MD, a professor of urology at the UT Southwestern Medical Center, Dallas, Texas, commented in an interview. “But in general, if you have two equivalent protocols in terms of effectiveness, and one is less toxic or more convenient, that’s always preferable. From the standpoint of coming once a day rather than twice a day to get radiation, if I were a patient, I would prefer that regimen”
Current practice in this patient population likely hinges on where a patient is treated, he said. “Based on the new nonmetastatic muscle-invasive guidelines, many of them would be recommended surgery or possibly chemoradiation protocols, and then there are a lot of factors such as patient preference and outcomes that would probably impact the decision making.”
“Certainly, this study won’t prove standard of care because standard of care in the global picture for a patient with muscle-invasive disease really will depend on a randomized trial of surgery versus radiation or chemoradiation, multimodal therapies,” Dr. Lotan maintained. “Not every patient is even eligible; some patients with hydronephrosis or unresectable disease may not be the best candidates for multimodal therapy. But more information from these types of trials may help clinicians decide which multimodal therapy approach to use.”
Study details
Patients enrolled in NRG/RTOG 0712, a multicenter randomized phase 2 trial supported by the National Cancer Institute, had clinical T2 or T3-4a bladder cancer. Hydronephrosis and obvious lymph node involvement were exclusion criteria.
Like the rate of freedom from metastasis, the rate of complete response after induction therapy was similar for the two arms: 87.9% with twice-daily radiation plus 5-flourouracil-cisplatin and 75.8% with daily radiation plus gemcitabine.
“These rates exceed historical complete response rates, and this is likely a result of improved selection over time and more thorough TURBTs performed over time,” Dr. Coen proposed. “Over the course of multiple successive RTOG trials, our selection criteria have been refined. One excellent example would be that hydronephrosis has been excluded on more contemporary trials, but if you look at older trials, those patients were included.”
The 3-year rate of bladder-intact distant metastasis–free survival was 66.7% and 69%, respectively. “This is a very important endpoint. These results are excellent,” he commented. “And there is really no appreciable difference between the two arms on the actuarial analysis.”
As far as specific treatment failure events in the trial overall, three patients died, eight underwent cystectomy, and eight developed distant metastases. Although numbers were small, these events appeared fairly evenly distributed across arms.
The rate of grade 3 or 4 acute toxicity was 57.6% with twice-daily radiation plus 5-flourouracil-cisplatin and 54.6% with daily radiation plus gemcitabine. The large majority of events were blood and bone marrow toxicity. “It’s quite notable that the rates of GU and GI toxicity were very low,” commented Dr. Coen, who disclosed that he had no relevant conflicts of interest.
SOURCE: Coen, J. et al, 2018 Genitourinary Cancers Symposium, Abstract 408
AT THE GENITOURINARY CANCERS SYMPOSIUM
Key clinical point:
Major finding: The rate of freedom from distant metastasis at 3 years was 77.8% with twice-daily radiation plus 5-flourouracil-cisplatin and 84.0% with daily radiation plus gemcitabine.
Data source: A multicenter randomized phase 2 trial among 70 patients with muscle-invasive (cT2-4a) bladder cancer who had undergone TURBT (NRG/RTOG 0712 trial).
Disclosures: Dr. Coen disclosed that he had no relevant conflicts of interest. The trial was supported by the National Cancer Institute.
Source: Coen, J. et al, 2018 Genitourinary Cancers Symposium, Abstract 408.
Promising outcomes of thrombolysis for caval extension of iliofemoral DVT
CHICAGO – Caval extension of an acute iliofemoral deep vein thrombosis paradoxically portends better treatment outcomes than does thrombolysis of a DVT without involvement of the inferior vena cava, according to Rabih A. Chaer, MD, professor of surgery at the University of Pittsburgh.
This finding from a retrospective analysis of the University of Pittsburgh experience might seem counterintuitive. After all, caval extension clearly indicates a greater clot burden. One possible explanation: Clearing a thrombus from a large vessel, such as the inferior vena cava (IVC), provides an added protective effect. Also, since the caval segments don’t have valves – their flow is based upon negative pressure in the chest – they may not contribute as much to postthrombotic morbidity to the same extent as do thrombosed iliofemoral segments, Dr. Chaer speculated at a symposium on vascular surgery sponsored by Northwestern University.
The impetus for Dr. Chaer and coinvestigators to review the Pittsburgh experience was a lack of clarity in the literature as to the effect IVC thrombosis has on thrombolysis outcomes in patients with acute iliofemoral DVT. Even though caval thrombus extension is present in up to 22% of patients with iliofemoral DVT, current guidelines issued by the American College of Chest Physicians, the American Heart Association, and the Society for Vascular Surgery don’t address the distinction between iliofemoral DVT with and without IVC extension in regard to the occurrence of postthrombotic syndrome (PTS), the most common complication of DVT.
The incidence of PTS in patients whose iliofemoral DVT is treated by anticoagulation and compression alone is up to 50%. Mounting evidence indicates that catheter-directed thrombolysis and pharmacomechanical thrombolysis aimed at achieving early thrombus removal and symptom relief help maintain valvular competence and reduce the risk of PTS, the surgeon noted.
PTS is diagnosed using the validated Villalta scale, which incorporates clinical signs including pain on calf compression, skin edema and redness, and ulcers, as well as symptoms such as leg cramping, heaviness, itching, and paresthesia.
The Pittsburgh series included 102 consecutive patients treated with various combinations of catheter-directed or pharmacomechanical thrombolysis in 127 limbs with acute iliofemoral thrombosis. In 46 patients, the thrombus extended into the IVC, all the way up to the renal veins in most cases.
The groups with and without caval extension were similar in terms of age and prevalence of malignancy, hypercoagulable state, and clot age. However, a history of previous DVT was significantly more common in the group with IVC thrombus. Also, more than 60% of patients with caval extension got an IVC filter, a rate more than 10-fold greater than that in patients without caval extension.
In this series, caval thrombosis had no effect on the technical success of thrombolysis. The technical success rate –defined as at least 50% clot lysis – was 89% in both groups. Rates of recurrent DVT within 30 days were similar in the two groups as well: 11% in the caval thrombosis group and 14% in the noncaval group. At 2 years postintervention, 77%-78% of patients in both groups remained free of DVT recurrence. The rate of PTS – defined by a Villalta score of 5 or more – at 2 years was 34% in the noncaval group, which was significantly higher than the 11% rate in patients with IVC thrombus extension. Ultrasound-identified valve reflux was present in 51% of the noncaval group at 2 years, compared with 51% of the noncaval group.
On multivariate analysis, incomplete clot lysis was associated with nearly a 23-fold increased risk of recurrent DVT and a 5.6-fold increased risk of PTS. Caval involvement was independently associated with a 78% reduction in PTS risk.
The Society for Vascular Surgery’s guidelines recommend pharmacomechanical thrombolysis over catheter-directed thrombolysis if the expertise is available. The Pittsburgh experience speaks to the worth of that recommendation.
“Pharmacomechanical techniques can be advantageous. They can expedite the lysis process by clearing most of the clot. In our series, 20 patients were treated with pharmacomechanical techniques in a single session,” Dr. Chaer noted.
The use of IVC filters in the setting of caval extension of iliofemoral DVT is controversial, according to the surgeon: A thrombus that gets trapped in the filter is tough to remove, precluding successful recanalization.
“One-third of the patients in our series got a filter, but we’ve become more conservative nowadays. We don’t use filters anymore. But I think those patients who might benefit from an IVC filter are those who present with a PE [pulmonary embolism], because that’s telling you they might develop another PE, as well as those patients in whom pharmacomechanical thrombolysis is anticipated because we’ve seen that those patients are also more likely to develop a PE,” he said.
The University of Pittsburgh study on the effect of IVC thrombus extension has been published (J Vasc Surg Venous Lymphat Disord. 2016 Oct;4[4]:385-91).
Dr. Chaer reported serving as a paid speaker for Boston Scientific.
SOURCE: Chaer RA. Northwestern Vascular Symposium 2017.
CHICAGO – Caval extension of an acute iliofemoral deep vein thrombosis paradoxically portends better treatment outcomes than does thrombolysis of a DVT without involvement of the inferior vena cava, according to Rabih A. Chaer, MD, professor of surgery at the University of Pittsburgh.
This finding from a retrospective analysis of the University of Pittsburgh experience might seem counterintuitive. After all, caval extension clearly indicates a greater clot burden. One possible explanation: Clearing a thrombus from a large vessel, such as the inferior vena cava (IVC), provides an added protective effect. Also, since the caval segments don’t have valves – their flow is based upon negative pressure in the chest – they may not contribute as much to postthrombotic morbidity to the same extent as do thrombosed iliofemoral segments, Dr. Chaer speculated at a symposium on vascular surgery sponsored by Northwestern University.
The impetus for Dr. Chaer and coinvestigators to review the Pittsburgh experience was a lack of clarity in the literature as to the effect IVC thrombosis has on thrombolysis outcomes in patients with acute iliofemoral DVT. Even though caval thrombus extension is present in up to 22% of patients with iliofemoral DVT, current guidelines issued by the American College of Chest Physicians, the American Heart Association, and the Society for Vascular Surgery don’t address the distinction between iliofemoral DVT with and without IVC extension in regard to the occurrence of postthrombotic syndrome (PTS), the most common complication of DVT.
The incidence of PTS in patients whose iliofemoral DVT is treated by anticoagulation and compression alone is up to 50%. Mounting evidence indicates that catheter-directed thrombolysis and pharmacomechanical thrombolysis aimed at achieving early thrombus removal and symptom relief help maintain valvular competence and reduce the risk of PTS, the surgeon noted.
PTS is diagnosed using the validated Villalta scale, which incorporates clinical signs including pain on calf compression, skin edema and redness, and ulcers, as well as symptoms such as leg cramping, heaviness, itching, and paresthesia.
The Pittsburgh series included 102 consecutive patients treated with various combinations of catheter-directed or pharmacomechanical thrombolysis in 127 limbs with acute iliofemoral thrombosis. In 46 patients, the thrombus extended into the IVC, all the way up to the renal veins in most cases.
The groups with and without caval extension were similar in terms of age and prevalence of malignancy, hypercoagulable state, and clot age. However, a history of previous DVT was significantly more common in the group with IVC thrombus. Also, more than 60% of patients with caval extension got an IVC filter, a rate more than 10-fold greater than that in patients without caval extension.
In this series, caval thrombosis had no effect on the technical success of thrombolysis. The technical success rate –defined as at least 50% clot lysis – was 89% in both groups. Rates of recurrent DVT within 30 days were similar in the two groups as well: 11% in the caval thrombosis group and 14% in the noncaval group. At 2 years postintervention, 77%-78% of patients in both groups remained free of DVT recurrence. The rate of PTS – defined by a Villalta score of 5 or more – at 2 years was 34% in the noncaval group, which was significantly higher than the 11% rate in patients with IVC thrombus extension. Ultrasound-identified valve reflux was present in 51% of the noncaval group at 2 years, compared with 51% of the noncaval group.
On multivariate analysis, incomplete clot lysis was associated with nearly a 23-fold increased risk of recurrent DVT and a 5.6-fold increased risk of PTS. Caval involvement was independently associated with a 78% reduction in PTS risk.
The Society for Vascular Surgery’s guidelines recommend pharmacomechanical thrombolysis over catheter-directed thrombolysis if the expertise is available. The Pittsburgh experience speaks to the worth of that recommendation.
“Pharmacomechanical techniques can be advantageous. They can expedite the lysis process by clearing most of the clot. In our series, 20 patients were treated with pharmacomechanical techniques in a single session,” Dr. Chaer noted.
The use of IVC filters in the setting of caval extension of iliofemoral DVT is controversial, according to the surgeon: A thrombus that gets trapped in the filter is tough to remove, precluding successful recanalization.
“One-third of the patients in our series got a filter, but we’ve become more conservative nowadays. We don’t use filters anymore. But I think those patients who might benefit from an IVC filter are those who present with a PE [pulmonary embolism], because that’s telling you they might develop another PE, as well as those patients in whom pharmacomechanical thrombolysis is anticipated because we’ve seen that those patients are also more likely to develop a PE,” he said.
The University of Pittsburgh study on the effect of IVC thrombus extension has been published (J Vasc Surg Venous Lymphat Disord. 2016 Oct;4[4]:385-91).
Dr. Chaer reported serving as a paid speaker for Boston Scientific.
SOURCE: Chaer RA. Northwestern Vascular Symposium 2017.
CHICAGO – Caval extension of an acute iliofemoral deep vein thrombosis paradoxically portends better treatment outcomes than does thrombolysis of a DVT without involvement of the inferior vena cava, according to Rabih A. Chaer, MD, professor of surgery at the University of Pittsburgh.
This finding from a retrospective analysis of the University of Pittsburgh experience might seem counterintuitive. After all, caval extension clearly indicates a greater clot burden. One possible explanation: Clearing a thrombus from a large vessel, such as the inferior vena cava (IVC), provides an added protective effect. Also, since the caval segments don’t have valves – their flow is based upon negative pressure in the chest – they may not contribute as much to postthrombotic morbidity to the same extent as do thrombosed iliofemoral segments, Dr. Chaer speculated at a symposium on vascular surgery sponsored by Northwestern University.
The impetus for Dr. Chaer and coinvestigators to review the Pittsburgh experience was a lack of clarity in the literature as to the effect IVC thrombosis has on thrombolysis outcomes in patients with acute iliofemoral DVT. Even though caval thrombus extension is present in up to 22% of patients with iliofemoral DVT, current guidelines issued by the American College of Chest Physicians, the American Heart Association, and the Society for Vascular Surgery don’t address the distinction between iliofemoral DVT with and without IVC extension in regard to the occurrence of postthrombotic syndrome (PTS), the most common complication of DVT.
The incidence of PTS in patients whose iliofemoral DVT is treated by anticoagulation and compression alone is up to 50%. Mounting evidence indicates that catheter-directed thrombolysis and pharmacomechanical thrombolysis aimed at achieving early thrombus removal and symptom relief help maintain valvular competence and reduce the risk of PTS, the surgeon noted.
PTS is diagnosed using the validated Villalta scale, which incorporates clinical signs including pain on calf compression, skin edema and redness, and ulcers, as well as symptoms such as leg cramping, heaviness, itching, and paresthesia.
The Pittsburgh series included 102 consecutive patients treated with various combinations of catheter-directed or pharmacomechanical thrombolysis in 127 limbs with acute iliofemoral thrombosis. In 46 patients, the thrombus extended into the IVC, all the way up to the renal veins in most cases.
The groups with and without caval extension were similar in terms of age and prevalence of malignancy, hypercoagulable state, and clot age. However, a history of previous DVT was significantly more common in the group with IVC thrombus. Also, more than 60% of patients with caval extension got an IVC filter, a rate more than 10-fold greater than that in patients without caval extension.
In this series, caval thrombosis had no effect on the technical success of thrombolysis. The technical success rate –defined as at least 50% clot lysis – was 89% in both groups. Rates of recurrent DVT within 30 days were similar in the two groups as well: 11% in the caval thrombosis group and 14% in the noncaval group. At 2 years postintervention, 77%-78% of patients in both groups remained free of DVT recurrence. The rate of PTS – defined by a Villalta score of 5 or more – at 2 years was 34% in the noncaval group, which was significantly higher than the 11% rate in patients with IVC thrombus extension. Ultrasound-identified valve reflux was present in 51% of the noncaval group at 2 years, compared with 51% of the noncaval group.
On multivariate analysis, incomplete clot lysis was associated with nearly a 23-fold increased risk of recurrent DVT and a 5.6-fold increased risk of PTS. Caval involvement was independently associated with a 78% reduction in PTS risk.
The Society for Vascular Surgery’s guidelines recommend pharmacomechanical thrombolysis over catheter-directed thrombolysis if the expertise is available. The Pittsburgh experience speaks to the worth of that recommendation.
“Pharmacomechanical techniques can be advantageous. They can expedite the lysis process by clearing most of the clot. In our series, 20 patients were treated with pharmacomechanical techniques in a single session,” Dr. Chaer noted.
The use of IVC filters in the setting of caval extension of iliofemoral DVT is controversial, according to the surgeon: A thrombus that gets trapped in the filter is tough to remove, precluding successful recanalization.
“One-third of the patients in our series got a filter, but we’ve become more conservative nowadays. We don’t use filters anymore. But I think those patients who might benefit from an IVC filter are those who present with a PE [pulmonary embolism], because that’s telling you they might develop another PE, as well as those patients in whom pharmacomechanical thrombolysis is anticipated because we’ve seen that those patients are also more likely to develop a PE,” he said.
The University of Pittsburgh study on the effect of IVC thrombus extension has been published (J Vasc Surg Venous Lymphat Disord. 2016 Oct;4[4]:385-91).
Dr. Chaer reported serving as a paid speaker for Boston Scientific.
SOURCE: Chaer RA. Northwestern Vascular Symposium 2017.
REPORTING FROM THE NORTHWESTERN VASCULAR SYMPOSIUM
Splenic artery embolization increases risk of complications
LAKE BUENA VISTA, FLA. – are at higher risk of infectious complications and readmissions in the long term, according to a study presented at the annual scientific assembly of the Eastern Association for the Surgery of Trauma.
As nonoperative treatments are becoming more common for managing blunt splenic injury (BSI), it is important to understand the risks associated with splenic artery embolization (SAE) and how this treatment may be impacting a larger trend of posttrauma readmissions, according to presenter Rishi Rattan, MD, an acute care surgeon at the University of Miami.
The retrospective study included 37,986 BSI patients admitted into the National Readmissions Database from 2010 to 2014, treated with either nonoperative management (NOM), SAE, or operative management (OM).
Readmission rates for infection after 30 days were significantly higher among SAE (15.4%) and OM (21.9%) patients, compared with NOM patients (6.7%), according to Dr. Rattan. Patients who underwent SAE also had a 17.2% rate of infection after 1 year; significantly higher than the 8.1% of patients who underwent NOM, although less than the 23.2% of those who underwent OM.
For readmission due to organ surgical site infection, patients with SAE had a higher frequency at 30-day (2.9%) and 1-year (3.9%) readmission, compared with both NOM (1.3%, 1.7%) and OM (2.0%, 2.2%).
This can be particularly problematic as these organ surgical site infections, deep in the abdominal cavity around the splenic bed, are usually more complicated to manage, compared with a superficial infection, explained Dr. Rattan. Physiologically, it makes sense that having dead tissue left in the splenic bed could lead to a rise in infection, although more data are necessary to confirm that hypothesis.
SAE was a significant predictive factor for complications after BSI, increasing the odds of 30-day and 1-year readmission by 76% and 99%, respectively, from organ surgical site infection, compared with NOM (P less than .01). Other predictive factors included hospital stays longer than 4 days, not being discharged to home, and a Charlson Comorbidity index score greater than 1.
With an incidence rate of readmission among embolization patients at 30 days and 1 year double that of NOM, Dr. Rattan and fellow investigators suggest surgeons should be conscious of the risks of SAE and OM, especially as infection is a major case of morbidity after trauma in splenectomy patients.
The investigators reported no relevant financial disclosures.
LAKE BUENA VISTA, FLA. – are at higher risk of infectious complications and readmissions in the long term, according to a study presented at the annual scientific assembly of the Eastern Association for the Surgery of Trauma.
As nonoperative treatments are becoming more common for managing blunt splenic injury (BSI), it is important to understand the risks associated with splenic artery embolization (SAE) and how this treatment may be impacting a larger trend of posttrauma readmissions, according to presenter Rishi Rattan, MD, an acute care surgeon at the University of Miami.
The retrospective study included 37,986 BSI patients admitted into the National Readmissions Database from 2010 to 2014, treated with either nonoperative management (NOM), SAE, or operative management (OM).
Readmission rates for infection after 30 days were significantly higher among SAE (15.4%) and OM (21.9%) patients, compared with NOM patients (6.7%), according to Dr. Rattan. Patients who underwent SAE also had a 17.2% rate of infection after 1 year; significantly higher than the 8.1% of patients who underwent NOM, although less than the 23.2% of those who underwent OM.
For readmission due to organ surgical site infection, patients with SAE had a higher frequency at 30-day (2.9%) and 1-year (3.9%) readmission, compared with both NOM (1.3%, 1.7%) and OM (2.0%, 2.2%).
This can be particularly problematic as these organ surgical site infections, deep in the abdominal cavity around the splenic bed, are usually more complicated to manage, compared with a superficial infection, explained Dr. Rattan. Physiologically, it makes sense that having dead tissue left in the splenic bed could lead to a rise in infection, although more data are necessary to confirm that hypothesis.
SAE was a significant predictive factor for complications after BSI, increasing the odds of 30-day and 1-year readmission by 76% and 99%, respectively, from organ surgical site infection, compared with NOM (P less than .01). Other predictive factors included hospital stays longer than 4 days, not being discharged to home, and a Charlson Comorbidity index score greater than 1.
With an incidence rate of readmission among embolization patients at 30 days and 1 year double that of NOM, Dr. Rattan and fellow investigators suggest surgeons should be conscious of the risks of SAE and OM, especially as infection is a major case of morbidity after trauma in splenectomy patients.
The investigators reported no relevant financial disclosures.
LAKE BUENA VISTA, FLA. – are at higher risk of infectious complications and readmissions in the long term, according to a study presented at the annual scientific assembly of the Eastern Association for the Surgery of Trauma.
As nonoperative treatments are becoming more common for managing blunt splenic injury (BSI), it is important to understand the risks associated with splenic artery embolization (SAE) and how this treatment may be impacting a larger trend of posttrauma readmissions, according to presenter Rishi Rattan, MD, an acute care surgeon at the University of Miami.
The retrospective study included 37,986 BSI patients admitted into the National Readmissions Database from 2010 to 2014, treated with either nonoperative management (NOM), SAE, or operative management (OM).
Readmission rates for infection after 30 days were significantly higher among SAE (15.4%) and OM (21.9%) patients, compared with NOM patients (6.7%), according to Dr. Rattan. Patients who underwent SAE also had a 17.2% rate of infection after 1 year; significantly higher than the 8.1% of patients who underwent NOM, although less than the 23.2% of those who underwent OM.
For readmission due to organ surgical site infection, patients with SAE had a higher frequency at 30-day (2.9%) and 1-year (3.9%) readmission, compared with both NOM (1.3%, 1.7%) and OM (2.0%, 2.2%).
This can be particularly problematic as these organ surgical site infections, deep in the abdominal cavity around the splenic bed, are usually more complicated to manage, compared with a superficial infection, explained Dr. Rattan. Physiologically, it makes sense that having dead tissue left in the splenic bed could lead to a rise in infection, although more data are necessary to confirm that hypothesis.
SAE was a significant predictive factor for complications after BSI, increasing the odds of 30-day and 1-year readmission by 76% and 99%, respectively, from organ surgical site infection, compared with NOM (P less than .01). Other predictive factors included hospital stays longer than 4 days, not being discharged to home, and a Charlson Comorbidity index score greater than 1.
With an incidence rate of readmission among embolization patients at 30 days and 1 year double that of NOM, Dr. Rattan and fellow investigators suggest surgeons should be conscious of the risks of SAE and OM, especially as infection is a major case of morbidity after trauma in splenectomy patients.
The investigators reported no relevant financial disclosures.
REPORTING FROM EAST 2018
Key clinical point: Splenic artery embolization can increase risk of infectious complications in patients with blunt splenic injury.
Major finding: Patients who underwent splenic artery embolization had an infectious complication rate of 20% after 1 year.
Data source: Study of 37,986 blunt splenic injury patients gathered from the Nationwide Readmissions Database during 2010-2014.
Disclosures: Investigators reported no relevant financial disclosures.
Congress extends CHIP, funds opioid crisis response following temporary shutdown
Congress, despite a second shutdown in less than a month, was able to pass a number of financial extenders to fund key health care programs.
The bipartisan spending bill (H.R. 1892), passed in the early morning hours on Feb. 9 by a 71-28 vote in the Senate (16 Republicans and 12 Democrats voted against it, and Sen. John McCain [R-Ariz.] was not present) and a 240-186 vote in the House (67 Republicans and 119 Democrats voted against and 5 representatives did not vote). President Trump signed the bill later that morning.
The spending bill and continuing resolution to fund the government through March 23 includes $6 billion to fund treatment for opioid addiction and other mental health issues, $2 billion in additional funding for the National Institutes of Health, and 4 additional years of funding for the Children’s Health Insurance Program. The additional CHIP funding extends the program for a total of 10 years.
The funding bill also made a technical correction to the Merit-based Incentive Payment System (MIPS) track of the Medicare Quality Payment Program. It removes Part B drug reimbursement from the MIPS payment adjustment, so any positive or negative change to physician payments based on the MIPS score will only be applied to physician fee schedule payments.
The bill also repeals the Independent Payment Advisory Board, a panel created by the Affordable Care Act that would have the power to slash Medicare spending under certain budget circumstances. That board was never convened.
The funding legislation also accelerates closure of the Medicare Part D “donut hole,” the coverage gap in which beneficiaries must pay 100% of medication costs prior to entering catastrophic coverage.
Just over $7 billion was provided for community health centers and Medicare’s therapy caps were repealed.
While the funding bill was written in the Senate with bipartisan input and received bipartisan support, Sen. Rand Paul (R-Ky.) held up votes over objections to the more than $1 trillion it will add to the nation’s debt, as well as for the fact that there was no opportunity to introduce and vote on amendments, leading to an hours-long government shutdown.
There also were concerns about two issues that could have derailed the vote in the House. Democrats wanted to add language to address immigrants brought to this nation illegally as children, while some Republicans did not want to increase the federal debt. However, there were enough votes to pass the funding legislation.
Congress, despite a second shutdown in less than a month, was able to pass a number of financial extenders to fund key health care programs.
The bipartisan spending bill (H.R. 1892), passed in the early morning hours on Feb. 9 by a 71-28 vote in the Senate (16 Republicans and 12 Democrats voted against it, and Sen. John McCain [R-Ariz.] was not present) and a 240-186 vote in the House (67 Republicans and 119 Democrats voted against and 5 representatives did not vote). President Trump signed the bill later that morning.
The spending bill and continuing resolution to fund the government through March 23 includes $6 billion to fund treatment for opioid addiction and other mental health issues, $2 billion in additional funding for the National Institutes of Health, and 4 additional years of funding for the Children’s Health Insurance Program. The additional CHIP funding extends the program for a total of 10 years.
The funding bill also made a technical correction to the Merit-based Incentive Payment System (MIPS) track of the Medicare Quality Payment Program. It removes Part B drug reimbursement from the MIPS payment adjustment, so any positive or negative change to physician payments based on the MIPS score will only be applied to physician fee schedule payments.
The bill also repeals the Independent Payment Advisory Board, a panel created by the Affordable Care Act that would have the power to slash Medicare spending under certain budget circumstances. That board was never convened.
The funding legislation also accelerates closure of the Medicare Part D “donut hole,” the coverage gap in which beneficiaries must pay 100% of medication costs prior to entering catastrophic coverage.
Just over $7 billion was provided for community health centers and Medicare’s therapy caps were repealed.
While the funding bill was written in the Senate with bipartisan input and received bipartisan support, Sen. Rand Paul (R-Ky.) held up votes over objections to the more than $1 trillion it will add to the nation’s debt, as well as for the fact that there was no opportunity to introduce and vote on amendments, leading to an hours-long government shutdown.
There also were concerns about two issues that could have derailed the vote in the House. Democrats wanted to add language to address immigrants brought to this nation illegally as children, while some Republicans did not want to increase the federal debt. However, there were enough votes to pass the funding legislation.
Congress, despite a second shutdown in less than a month, was able to pass a number of financial extenders to fund key health care programs.
The bipartisan spending bill (H.R. 1892), passed in the early morning hours on Feb. 9 by a 71-28 vote in the Senate (16 Republicans and 12 Democrats voted against it, and Sen. John McCain [R-Ariz.] was not present) and a 240-186 vote in the House (67 Republicans and 119 Democrats voted against and 5 representatives did not vote). President Trump signed the bill later that morning.
The spending bill and continuing resolution to fund the government through March 23 includes $6 billion to fund treatment for opioid addiction and other mental health issues, $2 billion in additional funding for the National Institutes of Health, and 4 additional years of funding for the Children’s Health Insurance Program. The additional CHIP funding extends the program for a total of 10 years.
The funding bill also made a technical correction to the Merit-based Incentive Payment System (MIPS) track of the Medicare Quality Payment Program. It removes Part B drug reimbursement from the MIPS payment adjustment, so any positive or negative change to physician payments based on the MIPS score will only be applied to physician fee schedule payments.
The bill also repeals the Independent Payment Advisory Board, a panel created by the Affordable Care Act that would have the power to slash Medicare spending under certain budget circumstances. That board was never convened.
The funding legislation also accelerates closure of the Medicare Part D “donut hole,” the coverage gap in which beneficiaries must pay 100% of medication costs prior to entering catastrophic coverage.
Just over $7 billion was provided for community health centers and Medicare’s therapy caps were repealed.
While the funding bill was written in the Senate with bipartisan input and received bipartisan support, Sen. Rand Paul (R-Ky.) held up votes over objections to the more than $1 trillion it will add to the nation’s debt, as well as for the fact that there was no opportunity to introduce and vote on amendments, leading to an hours-long government shutdown.
There also were concerns about two issues that could have derailed the vote in the House. Democrats wanted to add language to address immigrants brought to this nation illegally as children, while some Republicans did not want to increase the federal debt. However, there were enough votes to pass the funding legislation.
Adjuvant chemo halves DFS events in upper-tract urothelial cancer
SAN FRANCISCO – Patients who have undergone nephro-ureterectomy for upper-tract urothelial cancer (UTUC) fare much better if they are given adjuvant chemotherapy, according to the first results of the POUT trial reported at the 2018 Genitourinary Cancers Symposium.
Standard treatment for this rare cancer is radical nephro-ureterectomy followed by surveillance, noted lead author Alison Jane Birtle, MD, MRCP, FRCR, a consultant clinical oncologist at the Rosemere Cancer Centre, Royal Preston Hospital, Preston, United Kingdom. Evidence has been insufficient to recommend adjuvant therapy.
“We know that UTUC shares a similar etiology with bladder cancer, where there is strong evidence for chemotherapy,” she said. “Trials of adjuvant chemotherapy in bladder cancer have been challenging because of cystectomy being a much more morbid operation. So adjuvant chemotherapy after nephro-ureterectomy should be much easier to give because of the less morbid procedure.”
The POUT (Peri-Operative Chemotherapy Versus sUrveillance in Upper Tract Urothelial Cancer, NCT0199397) investigators enrolled in the trial 261 patients from 57 UK centers who had undergone radical nephro-ureterectomy for urothelial cancer of the renal pelvis or ureter. Patients were randomized evenly to receive surveillance or adjuvant platinum-based combination chemotherapy, with specific platinum (cisplatin or carboplatin) based on glomerular filtration rate (GFR).
Trial enrollment was stopped early, after a median follow-up of 19.3 months, because of efficacy of chemotherapy, Dr. Birtle reported at the symposium, which was sponsored by the American Society of Clinical Oncology, ASTRO, and the Society of Urologic Oncology.
Main results showed that risks of both disease-free survival events and metastasis-free survival events were 51% lower for the chemotherapy group as compared with the surveillance group. Overall survival showed a trend toward benefit as well.
Not surprisingly, grade 3 or worse adverse events during treatment were about four times more common with chemotherapy, but the treatment was overall feasible and safe, even though the majority of patients were older than 60 years.
“Based on these results, adjuvant platinum-based chemotherapy should be considered a new standard of care in these patients,” Dr. Birtle maintained. “The next thing is how are we going to move this data forward? We are looking at the successor study at the moment, which is currently in development…[POUT] has been a triumph of UK urologists really getting behind it, but to do a further study, it would be great to look at international collaboration.”
Optimal timing of chemotherapy, before or after surgery, remains an open question, according session co-chair Jeanny B. Aragon-Ching, MD, a medical oncologist with the Inova Medical Group, Fairfax, Virginia.
“This trial offers high-level evidence for consideration of adjuvant chemotherapy in those who are unable to receive neoadjuvant chemotherapy in UTUC, although it is still important to evaluate what the prospective neoadjuvant chemotherapy data in UTUC would show,” she said in an interview. “Ongoing studies include the ECOG 8141 study (clinicaltrials.gov NCT02412670), but certainly, several retrospective trials showed benefit of neoadjuvant chemotherapy in UTUC.”
Other viewpoints
“It’s fantastic that we finally have data in upper-tract cancer. However, I worry about how this data is going to be interpreted, and I argue that it should not be considered the standard of care,” session attendee Matthew Campbell, MD, of the MD Anderson Cancer Center, Houston, commented during a question and answer period.
“I believe that this is showing that chemotherapy is very important in this disease, and if anything, it should be moved into the neoadjuvant setting, where more patients will be cisplatin candidates. Though there is challenge with staging upper-tract disease, at MD Anderson, we consider patients with high-grade disease or hydronephrosis to be at high enough risk to consider for neoadjuvant chemotherapy, and that’s been our approach.”
“When we looked at developing POUT, there was a big debate about whether it should be a neoadjuvant or adjuvant study,” Dr. Birtle replied. UK oncologists expressed concern that not all patients have histologic confirmation of UTUC preoperatively; therefore, chemotherapy could lead to overtreatment for some.
“We plan to go back and look at the CT urograms and the diagnostic imaging done prior to surgery just to see if we can be 100% confident in our diagnostic accuracy preoperatively, to see if we would be more confident with a neoadjuvant study,” she added. The investigators have also reviewed data from the British Association of Urological Surgeons on the postoperative dip in GFR. “It didn’t really seem from that 2015 data that there was a huge unmet need for patients postoperatively, where we would have missed them had we not treated them preoperatively,” she said.
On a related note, session attendee Surena Matin, MD, also of MD Anderson, asked “How many patients were potentially precluded because of their GFR, and do you have any data regarding response rates in the carboplatin arm?”
The main reason for exclusion was ineligible pathology and not GFR, according to Dr. Birtle. “This goes back to the previous question of can you be certain that a patient has locally advanced disease prior to nephro-ureterectomy? About 60% of patients who were thought to have muscle-invasive disease ultimately on nephro-ureterectomy didn’t.”
Confidence intervals for chemotherapy benefit in the subgroup given carboplatin were wide and overlapped unity, but still favoring benefit and falling within the overall treatment effect, she said.
Session attendee Joaquim Bellmunt, MD, Dana-Farber Cancer Institute, Boston, noted that the chemotherapy benefit was not significant in that subgroup and also in the subgroups with lymph node–positive disease and with positive margins. “I think that saying … chemotherapy is for everybody based on this trial” is incorrect, he asserted. “It may be good just to tone down the message that this is the new standard of care.” He further questioned the trial’s early stopping, noting that continuing would have provided more information in these patients.
Those subgroups were small, so analyses were underpowered to definitively rule out chemotherapy benefit, according to Dr. Birtle. The investigators had intensive discussion about the recommendation to stop early, because of a goal to determine overall survival impact. Ultimately, “when we saw the data in terms of disease-free survival and metastasis-free survival, the magnitude of the effect was so big that we felt it was uncomfortable and unethical not to offer patients treatment,” she said.
Study details
Patients in POUT’s chemotherapy arm received four cycles of chemotherapy—gemcitabine-cisplatin if their GFR was 50 mL/min or higher, or gemcitabine-carboplatin if their GFR was 30-49 mL/min—starting within 90 days of nephro-ureterectomy.
Of note, approximately 40% of all patients in the trial were aged 70 years or older, including the 5% who were aged 80 years or older. “This is very reassuring for a study of adjuvant platinum-based chemotherapy,” Dr. Birtle commented. Fully 71.2% of the chemotherapy patients received all four planned cycles.
In an intention-to-treat analysis, risk of disease-free survival events (death from any cause, metastasis, or any ureteric or renal bed recurrence) was sharply reduced with chemotherapy versus surveillance (hazard ratio, 0.49; P=.001). The proportion of patients event free at 2 years was 71% in the chemotherapy group and 54% in the surveillance group. Benefit was generally similar across subgroups, and findings were much the same after adjustment for nodal involvement, microscopic margin status, and planned chemotherapy regimen (hazard ratio, 0.47; P=.001).
Risk of metastasis-free survival events was also sharply lower with chemotherapy (hazard ratio, 0.49; P=.002), with a proportion event free at 2 years of 74% in the chemotherapy group and 60% in the surveillance group. These findings were also much the same after adjustment for the above factors (hazard ratio, 0.47; P=.002).
Overall survival tended to be better with chemotherapy than with surveillance (hazard ratio, 0.55), but data are still immature for this endpoint.
The rate of grade 3 or worse adverse events during the treatment period was 53.2% with chemotherapy and 13.5% with surveillance; events with chemotherapy were as expected, with neutropenia, thrombocytopenia, and gastrointestinal events predominating. The rate of febrile neutropenia was 5.7% with gemcitabine-cisplatin and 7.8% with gemcitabine-carboplatin, but there were no neutropenic deaths.
The rate of grade 3 or worse adverse events for the entire trial period was 62.1% with chemotherapy and 24.8% with surveillance.
Data on nephrotoxicity are still being evaluated, according to Dr. Birtle. Only seven patients who started on gemcitabine-cisplatin had to switch to gemcitabine-carboplatin because their GFR fell.
In a related translational study, the investigators are evaluating both the baseline CT urograms and the resected tumors to identify prognostic and predictive markers, she said.
Dr. Birtle disclosed that she receives honoraria from Roche, Janssen, Astellas, and Bayer, and is a consultant to Sanofi-Aventis. The trial was funded by the UK Clinical Trials Awards and Advisory Committee.
SOURCE: Birtle A et al. Genitourinary Cancers Symposium. Abstract 407
SAN FRANCISCO – Patients who have undergone nephro-ureterectomy for upper-tract urothelial cancer (UTUC) fare much better if they are given adjuvant chemotherapy, according to the first results of the POUT trial reported at the 2018 Genitourinary Cancers Symposium.
Standard treatment for this rare cancer is radical nephro-ureterectomy followed by surveillance, noted lead author Alison Jane Birtle, MD, MRCP, FRCR, a consultant clinical oncologist at the Rosemere Cancer Centre, Royal Preston Hospital, Preston, United Kingdom. Evidence has been insufficient to recommend adjuvant therapy.
“We know that UTUC shares a similar etiology with bladder cancer, where there is strong evidence for chemotherapy,” she said. “Trials of adjuvant chemotherapy in bladder cancer have been challenging because of cystectomy being a much more morbid operation. So adjuvant chemotherapy after nephro-ureterectomy should be much easier to give because of the less morbid procedure.”
The POUT (Peri-Operative Chemotherapy Versus sUrveillance in Upper Tract Urothelial Cancer, NCT0199397) investigators enrolled in the trial 261 patients from 57 UK centers who had undergone radical nephro-ureterectomy for urothelial cancer of the renal pelvis or ureter. Patients were randomized evenly to receive surveillance or adjuvant platinum-based combination chemotherapy, with specific platinum (cisplatin or carboplatin) based on glomerular filtration rate (GFR).
Trial enrollment was stopped early, after a median follow-up of 19.3 months, because of efficacy of chemotherapy, Dr. Birtle reported at the symposium, which was sponsored by the American Society of Clinical Oncology, ASTRO, and the Society of Urologic Oncology.
Main results showed that risks of both disease-free survival events and metastasis-free survival events were 51% lower for the chemotherapy group as compared with the surveillance group. Overall survival showed a trend toward benefit as well.
Not surprisingly, grade 3 or worse adverse events during treatment were about four times more common with chemotherapy, but the treatment was overall feasible and safe, even though the majority of patients were older than 60 years.
“Based on these results, adjuvant platinum-based chemotherapy should be considered a new standard of care in these patients,” Dr. Birtle maintained. “The next thing is how are we going to move this data forward? We are looking at the successor study at the moment, which is currently in development…[POUT] has been a triumph of UK urologists really getting behind it, but to do a further study, it would be great to look at international collaboration.”
Optimal timing of chemotherapy, before or after surgery, remains an open question, according session co-chair Jeanny B. Aragon-Ching, MD, a medical oncologist with the Inova Medical Group, Fairfax, Virginia.
“This trial offers high-level evidence for consideration of adjuvant chemotherapy in those who are unable to receive neoadjuvant chemotherapy in UTUC, although it is still important to evaluate what the prospective neoadjuvant chemotherapy data in UTUC would show,” she said in an interview. “Ongoing studies include the ECOG 8141 study (clinicaltrials.gov NCT02412670), but certainly, several retrospective trials showed benefit of neoadjuvant chemotherapy in UTUC.”
Other viewpoints
“It’s fantastic that we finally have data in upper-tract cancer. However, I worry about how this data is going to be interpreted, and I argue that it should not be considered the standard of care,” session attendee Matthew Campbell, MD, of the MD Anderson Cancer Center, Houston, commented during a question and answer period.
“I believe that this is showing that chemotherapy is very important in this disease, and if anything, it should be moved into the neoadjuvant setting, where more patients will be cisplatin candidates. Though there is challenge with staging upper-tract disease, at MD Anderson, we consider patients with high-grade disease or hydronephrosis to be at high enough risk to consider for neoadjuvant chemotherapy, and that’s been our approach.”
“When we looked at developing POUT, there was a big debate about whether it should be a neoadjuvant or adjuvant study,” Dr. Birtle replied. UK oncologists expressed concern that not all patients have histologic confirmation of UTUC preoperatively; therefore, chemotherapy could lead to overtreatment for some.
“We plan to go back and look at the CT urograms and the diagnostic imaging done prior to surgery just to see if we can be 100% confident in our diagnostic accuracy preoperatively, to see if we would be more confident with a neoadjuvant study,” she added. The investigators have also reviewed data from the British Association of Urological Surgeons on the postoperative dip in GFR. “It didn’t really seem from that 2015 data that there was a huge unmet need for patients postoperatively, where we would have missed them had we not treated them preoperatively,” she said.
On a related note, session attendee Surena Matin, MD, also of MD Anderson, asked “How many patients were potentially precluded because of their GFR, and do you have any data regarding response rates in the carboplatin arm?”
The main reason for exclusion was ineligible pathology and not GFR, according to Dr. Birtle. “This goes back to the previous question of can you be certain that a patient has locally advanced disease prior to nephro-ureterectomy? About 60% of patients who were thought to have muscle-invasive disease ultimately on nephro-ureterectomy didn’t.”
Confidence intervals for chemotherapy benefit in the subgroup given carboplatin were wide and overlapped unity, but still favoring benefit and falling within the overall treatment effect, she said.
Session attendee Joaquim Bellmunt, MD, Dana-Farber Cancer Institute, Boston, noted that the chemotherapy benefit was not significant in that subgroup and also in the subgroups with lymph node–positive disease and with positive margins. “I think that saying … chemotherapy is for everybody based on this trial” is incorrect, he asserted. “It may be good just to tone down the message that this is the new standard of care.” He further questioned the trial’s early stopping, noting that continuing would have provided more information in these patients.
Those subgroups were small, so analyses were underpowered to definitively rule out chemotherapy benefit, according to Dr. Birtle. The investigators had intensive discussion about the recommendation to stop early, because of a goal to determine overall survival impact. Ultimately, “when we saw the data in terms of disease-free survival and metastasis-free survival, the magnitude of the effect was so big that we felt it was uncomfortable and unethical not to offer patients treatment,” she said.
Study details
Patients in POUT’s chemotherapy arm received four cycles of chemotherapy—gemcitabine-cisplatin if their GFR was 50 mL/min or higher, or gemcitabine-carboplatin if their GFR was 30-49 mL/min—starting within 90 days of nephro-ureterectomy.
Of note, approximately 40% of all patients in the trial were aged 70 years or older, including the 5% who were aged 80 years or older. “This is very reassuring for a study of adjuvant platinum-based chemotherapy,” Dr. Birtle commented. Fully 71.2% of the chemotherapy patients received all four planned cycles.
In an intention-to-treat analysis, risk of disease-free survival events (death from any cause, metastasis, or any ureteric or renal bed recurrence) was sharply reduced with chemotherapy versus surveillance (hazard ratio, 0.49; P=.001). The proportion of patients event free at 2 years was 71% in the chemotherapy group and 54% in the surveillance group. Benefit was generally similar across subgroups, and findings were much the same after adjustment for nodal involvement, microscopic margin status, and planned chemotherapy regimen (hazard ratio, 0.47; P=.001).
Risk of metastasis-free survival events was also sharply lower with chemotherapy (hazard ratio, 0.49; P=.002), with a proportion event free at 2 years of 74% in the chemotherapy group and 60% in the surveillance group. These findings were also much the same after adjustment for the above factors (hazard ratio, 0.47; P=.002).
Overall survival tended to be better with chemotherapy than with surveillance (hazard ratio, 0.55), but data are still immature for this endpoint.
The rate of grade 3 or worse adverse events during the treatment period was 53.2% with chemotherapy and 13.5% with surveillance; events with chemotherapy were as expected, with neutropenia, thrombocytopenia, and gastrointestinal events predominating. The rate of febrile neutropenia was 5.7% with gemcitabine-cisplatin and 7.8% with gemcitabine-carboplatin, but there were no neutropenic deaths.
The rate of grade 3 or worse adverse events for the entire trial period was 62.1% with chemotherapy and 24.8% with surveillance.
Data on nephrotoxicity are still being evaluated, according to Dr. Birtle. Only seven patients who started on gemcitabine-cisplatin had to switch to gemcitabine-carboplatin because their GFR fell.
In a related translational study, the investigators are evaluating both the baseline CT urograms and the resected tumors to identify prognostic and predictive markers, she said.
Dr. Birtle disclosed that she receives honoraria from Roche, Janssen, Astellas, and Bayer, and is a consultant to Sanofi-Aventis. The trial was funded by the UK Clinical Trials Awards and Advisory Committee.
SOURCE: Birtle A et al. Genitourinary Cancers Symposium. Abstract 407
SAN FRANCISCO – Patients who have undergone nephro-ureterectomy for upper-tract urothelial cancer (UTUC) fare much better if they are given adjuvant chemotherapy, according to the first results of the POUT trial reported at the 2018 Genitourinary Cancers Symposium.
Standard treatment for this rare cancer is radical nephro-ureterectomy followed by surveillance, noted lead author Alison Jane Birtle, MD, MRCP, FRCR, a consultant clinical oncologist at the Rosemere Cancer Centre, Royal Preston Hospital, Preston, United Kingdom. Evidence has been insufficient to recommend adjuvant therapy.
“We know that UTUC shares a similar etiology with bladder cancer, where there is strong evidence for chemotherapy,” she said. “Trials of adjuvant chemotherapy in bladder cancer have been challenging because of cystectomy being a much more morbid operation. So adjuvant chemotherapy after nephro-ureterectomy should be much easier to give because of the less morbid procedure.”
The POUT (Peri-Operative Chemotherapy Versus sUrveillance in Upper Tract Urothelial Cancer, NCT0199397) investigators enrolled in the trial 261 patients from 57 UK centers who had undergone radical nephro-ureterectomy for urothelial cancer of the renal pelvis or ureter. Patients were randomized evenly to receive surveillance or adjuvant platinum-based combination chemotherapy, with specific platinum (cisplatin or carboplatin) based on glomerular filtration rate (GFR).
Trial enrollment was stopped early, after a median follow-up of 19.3 months, because of efficacy of chemotherapy, Dr. Birtle reported at the symposium, which was sponsored by the American Society of Clinical Oncology, ASTRO, and the Society of Urologic Oncology.
Main results showed that risks of both disease-free survival events and metastasis-free survival events were 51% lower for the chemotherapy group as compared with the surveillance group. Overall survival showed a trend toward benefit as well.
Not surprisingly, grade 3 or worse adverse events during treatment were about four times more common with chemotherapy, but the treatment was overall feasible and safe, even though the majority of patients were older than 60 years.
“Based on these results, adjuvant platinum-based chemotherapy should be considered a new standard of care in these patients,” Dr. Birtle maintained. “The next thing is how are we going to move this data forward? We are looking at the successor study at the moment, which is currently in development…[POUT] has been a triumph of UK urologists really getting behind it, but to do a further study, it would be great to look at international collaboration.”
Optimal timing of chemotherapy, before or after surgery, remains an open question, according session co-chair Jeanny B. Aragon-Ching, MD, a medical oncologist with the Inova Medical Group, Fairfax, Virginia.
“This trial offers high-level evidence for consideration of adjuvant chemotherapy in those who are unable to receive neoadjuvant chemotherapy in UTUC, although it is still important to evaluate what the prospective neoadjuvant chemotherapy data in UTUC would show,” she said in an interview. “Ongoing studies include the ECOG 8141 study (clinicaltrials.gov NCT02412670), but certainly, several retrospective trials showed benefit of neoadjuvant chemotherapy in UTUC.”
Other viewpoints
“It’s fantastic that we finally have data in upper-tract cancer. However, I worry about how this data is going to be interpreted, and I argue that it should not be considered the standard of care,” session attendee Matthew Campbell, MD, of the MD Anderson Cancer Center, Houston, commented during a question and answer period.
“I believe that this is showing that chemotherapy is very important in this disease, and if anything, it should be moved into the neoadjuvant setting, where more patients will be cisplatin candidates. Though there is challenge with staging upper-tract disease, at MD Anderson, we consider patients with high-grade disease or hydronephrosis to be at high enough risk to consider for neoadjuvant chemotherapy, and that’s been our approach.”
“When we looked at developing POUT, there was a big debate about whether it should be a neoadjuvant or adjuvant study,” Dr. Birtle replied. UK oncologists expressed concern that not all patients have histologic confirmation of UTUC preoperatively; therefore, chemotherapy could lead to overtreatment for some.
“We plan to go back and look at the CT urograms and the diagnostic imaging done prior to surgery just to see if we can be 100% confident in our diagnostic accuracy preoperatively, to see if we would be more confident with a neoadjuvant study,” she added. The investigators have also reviewed data from the British Association of Urological Surgeons on the postoperative dip in GFR. “It didn’t really seem from that 2015 data that there was a huge unmet need for patients postoperatively, where we would have missed them had we not treated them preoperatively,” she said.
On a related note, session attendee Surena Matin, MD, also of MD Anderson, asked “How many patients were potentially precluded because of their GFR, and do you have any data regarding response rates in the carboplatin arm?”
The main reason for exclusion was ineligible pathology and not GFR, according to Dr. Birtle. “This goes back to the previous question of can you be certain that a patient has locally advanced disease prior to nephro-ureterectomy? About 60% of patients who were thought to have muscle-invasive disease ultimately on nephro-ureterectomy didn’t.”
Confidence intervals for chemotherapy benefit in the subgroup given carboplatin were wide and overlapped unity, but still favoring benefit and falling within the overall treatment effect, she said.
Session attendee Joaquim Bellmunt, MD, Dana-Farber Cancer Institute, Boston, noted that the chemotherapy benefit was not significant in that subgroup and also in the subgroups with lymph node–positive disease and with positive margins. “I think that saying … chemotherapy is for everybody based on this trial” is incorrect, he asserted. “It may be good just to tone down the message that this is the new standard of care.” He further questioned the trial’s early stopping, noting that continuing would have provided more information in these patients.
Those subgroups were small, so analyses were underpowered to definitively rule out chemotherapy benefit, according to Dr. Birtle. The investigators had intensive discussion about the recommendation to stop early, because of a goal to determine overall survival impact. Ultimately, “when we saw the data in terms of disease-free survival and metastasis-free survival, the magnitude of the effect was so big that we felt it was uncomfortable and unethical not to offer patients treatment,” she said.
Study details
Patients in POUT’s chemotherapy arm received four cycles of chemotherapy—gemcitabine-cisplatin if their GFR was 50 mL/min or higher, or gemcitabine-carboplatin if their GFR was 30-49 mL/min—starting within 90 days of nephro-ureterectomy.
Of note, approximately 40% of all patients in the trial were aged 70 years or older, including the 5% who were aged 80 years or older. “This is very reassuring for a study of adjuvant platinum-based chemotherapy,” Dr. Birtle commented. Fully 71.2% of the chemotherapy patients received all four planned cycles.
In an intention-to-treat analysis, risk of disease-free survival events (death from any cause, metastasis, or any ureteric or renal bed recurrence) was sharply reduced with chemotherapy versus surveillance (hazard ratio, 0.49; P=.001). The proportion of patients event free at 2 years was 71% in the chemotherapy group and 54% in the surveillance group. Benefit was generally similar across subgroups, and findings were much the same after adjustment for nodal involvement, microscopic margin status, and planned chemotherapy regimen (hazard ratio, 0.47; P=.001).
Risk of metastasis-free survival events was also sharply lower with chemotherapy (hazard ratio, 0.49; P=.002), with a proportion event free at 2 years of 74% in the chemotherapy group and 60% in the surveillance group. These findings were also much the same after adjustment for the above factors (hazard ratio, 0.47; P=.002).
Overall survival tended to be better with chemotherapy than with surveillance (hazard ratio, 0.55), but data are still immature for this endpoint.
The rate of grade 3 or worse adverse events during the treatment period was 53.2% with chemotherapy and 13.5% with surveillance; events with chemotherapy were as expected, with neutropenia, thrombocytopenia, and gastrointestinal events predominating. The rate of febrile neutropenia was 5.7% with gemcitabine-cisplatin and 7.8% with gemcitabine-carboplatin, but there were no neutropenic deaths.
The rate of grade 3 or worse adverse events for the entire trial period was 62.1% with chemotherapy and 24.8% with surveillance.
Data on nephrotoxicity are still being evaluated, according to Dr. Birtle. Only seven patients who started on gemcitabine-cisplatin had to switch to gemcitabine-carboplatin because their GFR fell.
In a related translational study, the investigators are evaluating both the baseline CT urograms and the resected tumors to identify prognostic and predictive markers, she said.
Dr. Birtle disclosed that she receives honoraria from Roche, Janssen, Astellas, and Bayer, and is a consultant to Sanofi-Aventis. The trial was funded by the UK Clinical Trials Awards and Advisory Committee.
SOURCE: Birtle A et al. Genitourinary Cancers Symposium. Abstract 407
AT THE GENITOURINARY CANCERS SYMPOSIUM
Key clinical point:
Major finding: Compared with surveillance, adjuvant chemotherapy halved risk of disease-free survival events (hazard ratio, 0.49; P=.001).
Data source: A phase 3 randomized trial of adjuvant platinum-based chemotherapy versus surveillance in 261 patients with upper-tract urothelial cancer (POUT trial).
Disclosures: Dr. Birtle disclosed that she receives honoraria from Roche, Janssen, Astellas, and Bayer, and is a consultant to Sanofi-Aventis. The trial was funded by the UK Clinical Trials Awards and Advisory Committee.
Source: Birtle A et al. Genitourinary Cancers Symposium. Abstract 407.
Reported penicillin allergies hike inpatient costs
Total inpatient costs for patients who report being allergic to penicillin are much higher than for those who don’t report an allergy, according to a recent systematic review and meta-analysis.
The review, which eventually included 30 articles, found that total inpatient costs ranged from an average $1,145-$4,254 higher per patient with a reported penicillin allergy compared to nonallergic patients, said T. Joseph Mattingly, PharmD, and his associates. Outpatient prescription costs were also estimated to be steeper, running $14-$93 higher per patient who reported a penicillin allergy.
Although 10%-20% of patients report a penicillin allergy, “[a] majority of patients who report PCN [penicillin] allergy are not truly allergic upon confirmatory testing,” Dr. Mattingly and his colleagues wrote.
This overreporting of penicillin allergies is a problem for the patient and the health care system because “reported antibiotic allergies have been associated with suboptimal antibiotic therapy, increased antimicrobial resistance, increased length of stay, increased antibiotic-related adverse events, increased rates of C. difficile infection, intensive care unit (ICU) admission, death, as well as increased treatment cost,” said Dr. Mattingly and his coauthors.
Health care providers often “tend to take reported allergies at face value,” said coauthor Anne Fulton, suggesting that primary care practices can help by considering skin testing for those patients who carry a label of penicillin allergy, but don’t have a documented confirmatory test. The cost for a commonly used skin test for penicillin allergy runs about $200, said Ms. Fulton, a doctoral candidate at the University of Maryland, Baltimore, in an interview.
When conducting the meta-analysis, Dr. Mattingly and his coauthors converted all figures to 2017 U.S. dollars, using Consumer Price Index figures to adjust for inflation. This yields conservative estimates for cost, as drug and health care prices have far outstripped the general rate of inflation during the period in which the studies occurred, Ms. Fulton acknowledged.
The investigators highlighted the need for ongoing study in this area. “To our knowledge, there are no evaluations of long-term outpatient outcomes related to the effects of PCN allergy and the potential impact of delabeling patients who do not have a true allergy,” they wrote.
Ms. Fulton agreed, noting that the studies covered in the meta-analysis were primarily focused on short-term outcomes, though there are many potential long-term benefits to delabeling patients who are not truly penicillin allergic.
For the patient, this includes the opportunity to receive optimal antimicrobial therapy, as well as potential savings in copays and other out-of-pocket expenses for outpatient medications, she said.
As antimicrobial resistance becomes an ever more pressing problem, there are more opportunities for targeted therapy if inappropriate allergy labeling is addressed, Ms. Fulton added.
Further study should use “cost-effectiveness analysis methods that include societal and health sector perspectives capturing immediate and future outcomes and costs to evaluate the use of skin-testing procedures in either inpatient or outpatient settings,” the investigators wrote.
The study was supported by ALK, the manufacturer of Pre-Pen, a commercially available penicillin allergy skin test.
SOURCE: Mattingly TJ et al. J Allergy Clin Immunol Pract. 2018 Jan 31. doi: 10.1016/j.jaip.2017.12.033.
Total inpatient costs for patients who report being allergic to penicillin are much higher than for those who don’t report an allergy, according to a recent systematic review and meta-analysis.
The review, which eventually included 30 articles, found that total inpatient costs ranged from an average $1,145-$4,254 higher per patient with a reported penicillin allergy compared to nonallergic patients, said T. Joseph Mattingly, PharmD, and his associates. Outpatient prescription costs were also estimated to be steeper, running $14-$93 higher per patient who reported a penicillin allergy.
Although 10%-20% of patients report a penicillin allergy, “[a] majority of patients who report PCN [penicillin] allergy are not truly allergic upon confirmatory testing,” Dr. Mattingly and his colleagues wrote.
This overreporting of penicillin allergies is a problem for the patient and the health care system because “reported antibiotic allergies have been associated with suboptimal antibiotic therapy, increased antimicrobial resistance, increased length of stay, increased antibiotic-related adverse events, increased rates of C. difficile infection, intensive care unit (ICU) admission, death, as well as increased treatment cost,” said Dr. Mattingly and his coauthors.
Health care providers often “tend to take reported allergies at face value,” said coauthor Anne Fulton, suggesting that primary care practices can help by considering skin testing for those patients who carry a label of penicillin allergy, but don’t have a documented confirmatory test. The cost for a commonly used skin test for penicillin allergy runs about $200, said Ms. Fulton, a doctoral candidate at the University of Maryland, Baltimore, in an interview.
When conducting the meta-analysis, Dr. Mattingly and his coauthors converted all figures to 2017 U.S. dollars, using Consumer Price Index figures to adjust for inflation. This yields conservative estimates for cost, as drug and health care prices have far outstripped the general rate of inflation during the period in which the studies occurred, Ms. Fulton acknowledged.
The investigators highlighted the need for ongoing study in this area. “To our knowledge, there are no evaluations of long-term outpatient outcomes related to the effects of PCN allergy and the potential impact of delabeling patients who do not have a true allergy,” they wrote.
Ms. Fulton agreed, noting that the studies covered in the meta-analysis were primarily focused on short-term outcomes, though there are many potential long-term benefits to delabeling patients who are not truly penicillin allergic.
For the patient, this includes the opportunity to receive optimal antimicrobial therapy, as well as potential savings in copays and other out-of-pocket expenses for outpatient medications, she said.
As antimicrobial resistance becomes an ever more pressing problem, there are more opportunities for targeted therapy if inappropriate allergy labeling is addressed, Ms. Fulton added.
Further study should use “cost-effectiveness analysis methods that include societal and health sector perspectives capturing immediate and future outcomes and costs to evaluate the use of skin-testing procedures in either inpatient or outpatient settings,” the investigators wrote.
The study was supported by ALK, the manufacturer of Pre-Pen, a commercially available penicillin allergy skin test.
SOURCE: Mattingly TJ et al. J Allergy Clin Immunol Pract. 2018 Jan 31. doi: 10.1016/j.jaip.2017.12.033.
Total inpatient costs for patients who report being allergic to penicillin are much higher than for those who don’t report an allergy, according to a recent systematic review and meta-analysis.
The review, which eventually included 30 articles, found that total inpatient costs ranged from an average $1,145-$4,254 higher per patient with a reported penicillin allergy compared to nonallergic patients, said T. Joseph Mattingly, PharmD, and his associates. Outpatient prescription costs were also estimated to be steeper, running $14-$93 higher per patient who reported a penicillin allergy.
Although 10%-20% of patients report a penicillin allergy, “[a] majority of patients who report PCN [penicillin] allergy are not truly allergic upon confirmatory testing,” Dr. Mattingly and his colleagues wrote.
This overreporting of penicillin allergies is a problem for the patient and the health care system because “reported antibiotic allergies have been associated with suboptimal antibiotic therapy, increased antimicrobial resistance, increased length of stay, increased antibiotic-related adverse events, increased rates of C. difficile infection, intensive care unit (ICU) admission, death, as well as increased treatment cost,” said Dr. Mattingly and his coauthors.
Health care providers often “tend to take reported allergies at face value,” said coauthor Anne Fulton, suggesting that primary care practices can help by considering skin testing for those patients who carry a label of penicillin allergy, but don’t have a documented confirmatory test. The cost for a commonly used skin test for penicillin allergy runs about $200, said Ms. Fulton, a doctoral candidate at the University of Maryland, Baltimore, in an interview.
When conducting the meta-analysis, Dr. Mattingly and his coauthors converted all figures to 2017 U.S. dollars, using Consumer Price Index figures to adjust for inflation. This yields conservative estimates for cost, as drug and health care prices have far outstripped the general rate of inflation during the period in which the studies occurred, Ms. Fulton acknowledged.
The investigators highlighted the need for ongoing study in this area. “To our knowledge, there are no evaluations of long-term outpatient outcomes related to the effects of PCN allergy and the potential impact of delabeling patients who do not have a true allergy,” they wrote.
Ms. Fulton agreed, noting that the studies covered in the meta-analysis were primarily focused on short-term outcomes, though there are many potential long-term benefits to delabeling patients who are not truly penicillin allergic.
For the patient, this includes the opportunity to receive optimal antimicrobial therapy, as well as potential savings in copays and other out-of-pocket expenses for outpatient medications, she said.
As antimicrobial resistance becomes an ever more pressing problem, there are more opportunities for targeted therapy if inappropriate allergy labeling is addressed, Ms. Fulton added.
Further study should use “cost-effectiveness analysis methods that include societal and health sector perspectives capturing immediate and future outcomes and costs to evaluate the use of skin-testing procedures in either inpatient or outpatient settings,” the investigators wrote.
The study was supported by ALK, the manufacturer of Pre-Pen, a commercially available penicillin allergy skin test.
SOURCE: Mattingly TJ et al. J Allergy Clin Immunol Pract. 2018 Jan 31. doi: 10.1016/j.jaip.2017.12.033.
FROM JOURNAL OF ALLERGY AND CLINICAL IMMUNOLOGY: IN PRACTICE
Key clinical point: Inpatient costs were $1,145 – $4,254 higher for those reporting penicillin allergy.
Major finding: Though most studies addressed inpatient admissions, outpatient costs were also significantly higher.
Study details: Systematic review and meta-analysis of 30 articles addressing reported penicillin allergy.
Disclosures: The study was sponsored by ALK.
Source: Mattingly TJ et al. J Allergy Clin Immunol Pract. 2018 Jan 31. doi: 10.1016/j.jaip.2017.12.033.
Bipartisan Senate budget deal boosts health programs
In a rare show of bipartisanship for the mostly polarized 115th Congress, Republican and Democratic Senate leaders announced a 2-year budget deal that would increase federal spending for defense as well as key domestic priorities, including many health programs.
Not in the deal, for which the path to the president’s desk remains unclear, is any bipartisan legislation aimed at shoring up the Affordable Care Act’s individual health insurance marketplaces. Senate Majority Leader Mitch McConnell (R-Ky.) promised Sen. Susan Collins (R-Maine) a vote on health legislation in exchange for her vote for the GOP tax bill in December. So far, that vote has not materialized.
The deal does appear to include almost every other health priority Democrats have been pushing the past several months, including 2 years of renewed funding for community health centers and a series of other health programs Congress failed to provide for before they technically expired last year.
“I believe we have reached a budget deal that neither side loves but both sides can be proud of,” Senate Minority Leader Chuck Schumer (D-N.Y.) said on the Senate floor. “That’s compromise. That’s governing.”
Said McConnell, “This bill represents a significant bipartisan step forward.”
Senate leaders are still negotiating last details of the accord, including the size of a cut to the ACA’s Prevention and Public Health Fund, which would help offset the costs of this legislation.
According to documents circulating on Capitol Hill, the deal includes $6 billion in funding for treatment of mental health issues and opioid addiction, $2 billion in extra funding for the National Institutes of Health, and an additional 4-year extension of the Children’s Health Insurance Program (CHIP), which builds on the 6 years approved by Congress in January.
In the Medicare program, the deal would accelerate the closing of the “doughnut hole” in Medicare drug coverage that requires seniors to pay thousands of dollars out-of-pocket before catastrophic coverage kicks in. It would also repeal the controversial Medicare Independent Payment Advisory Board (IPAB), which is charged with holding down Medicare spending for the federal government if it exceeds a certain level. Members have never been appointed to the board, however, and its use has not so far been triggered by Medicare spending. Both the closure of the doughnut hole and creation of the IPAB were part of the ACA.
The agreement would also fund a host of more limited health programs – some of which are known as “extenders” because they often ride along with other, larger health or spending bills.
Those programs include more than $7 billion in funding for the nation’s federally funded community health centers. The clinics serve 27 million low-income people and saw their funding lapse last fall – a delay advocates said had already complicated budgeting and staffing decisions for many clinics.
And in a victory for the physical therapy industry and patient advocates, the accord would permanently repeal a limit on Medicare’s coverage of physical therapy, speech-language pathology and outpatient treatment. Previously, the program capped coverage after $2,010 worth of occupational therapy and another $2,010 for speech-language therapy and physical therapy combined. But Congress had long taken action to delay those caps or provide exemptions – meaning they had never actually taken effect.
According to an analysis by the nonpartisan Congressional Budget Office, permanently repealing the caps would cost about $6.47 billion over the next decade.
Lawmakers would also forestall cuts mandated by the ACA to reduce the payments made to so-called Disproportionate Share Hospitals, which serve high rates of low-income patients. Those cuts have been delayed continuously since the law’s 2010 passage.
Limited programs are also affected. The deal would fund for 5 years the Maternal, Infant and Early Childhood Home Visiting Program, a program that helps guide low-income, at-risk mothers in parenting. It served about 160,000 families in fiscal year 2016.
“We are relieved that there is a deal for a 5-year reauthorization of MIECHV,” Lori Freeman, CEO of the Association of Maternal & Child Health Programs – an advocacy group – said in an emailed statement. “States, home visitors, and families have been in limbo for the past several months, and this news will bring the stability they need to continue this successful program.”
And the budget deal funds programs that encourage doctors to practice in medically underserved areas, providing just under $500 million over the next 2 years for the National Health Service Corps and another $363 million over 2 years to the Teaching Health Center Graduate Medical Education program, which places medical residents in Community Health Centers.
Kaiser Health News correspondent Emmarie Huetteman contributed to this article. KHN’s coverage of these topics is supported by Heising-Simons Foundation and The David and Lucile Packard Foundation. Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
In a rare show of bipartisanship for the mostly polarized 115th Congress, Republican and Democratic Senate leaders announced a 2-year budget deal that would increase federal spending for defense as well as key domestic priorities, including many health programs.
Not in the deal, for which the path to the president’s desk remains unclear, is any bipartisan legislation aimed at shoring up the Affordable Care Act’s individual health insurance marketplaces. Senate Majority Leader Mitch McConnell (R-Ky.) promised Sen. Susan Collins (R-Maine) a vote on health legislation in exchange for her vote for the GOP tax bill in December. So far, that vote has not materialized.
The deal does appear to include almost every other health priority Democrats have been pushing the past several months, including 2 years of renewed funding for community health centers and a series of other health programs Congress failed to provide for before they technically expired last year.
“I believe we have reached a budget deal that neither side loves but both sides can be proud of,” Senate Minority Leader Chuck Schumer (D-N.Y.) said on the Senate floor. “That’s compromise. That’s governing.”
Said McConnell, “This bill represents a significant bipartisan step forward.”
Senate leaders are still negotiating last details of the accord, including the size of a cut to the ACA’s Prevention and Public Health Fund, which would help offset the costs of this legislation.
According to documents circulating on Capitol Hill, the deal includes $6 billion in funding for treatment of mental health issues and opioid addiction, $2 billion in extra funding for the National Institutes of Health, and an additional 4-year extension of the Children’s Health Insurance Program (CHIP), which builds on the 6 years approved by Congress in January.
In the Medicare program, the deal would accelerate the closing of the “doughnut hole” in Medicare drug coverage that requires seniors to pay thousands of dollars out-of-pocket before catastrophic coverage kicks in. It would also repeal the controversial Medicare Independent Payment Advisory Board (IPAB), which is charged with holding down Medicare spending for the federal government if it exceeds a certain level. Members have never been appointed to the board, however, and its use has not so far been triggered by Medicare spending. Both the closure of the doughnut hole and creation of the IPAB were part of the ACA.
The agreement would also fund a host of more limited health programs – some of which are known as “extenders” because they often ride along with other, larger health or spending bills.
Those programs include more than $7 billion in funding for the nation’s federally funded community health centers. The clinics serve 27 million low-income people and saw their funding lapse last fall – a delay advocates said had already complicated budgeting and staffing decisions for many clinics.
And in a victory for the physical therapy industry and patient advocates, the accord would permanently repeal a limit on Medicare’s coverage of physical therapy, speech-language pathology and outpatient treatment. Previously, the program capped coverage after $2,010 worth of occupational therapy and another $2,010 for speech-language therapy and physical therapy combined. But Congress had long taken action to delay those caps or provide exemptions – meaning they had never actually taken effect.
According to an analysis by the nonpartisan Congressional Budget Office, permanently repealing the caps would cost about $6.47 billion over the next decade.
Lawmakers would also forestall cuts mandated by the ACA to reduce the payments made to so-called Disproportionate Share Hospitals, which serve high rates of low-income patients. Those cuts have been delayed continuously since the law’s 2010 passage.
Limited programs are also affected. The deal would fund for 5 years the Maternal, Infant and Early Childhood Home Visiting Program, a program that helps guide low-income, at-risk mothers in parenting. It served about 160,000 families in fiscal year 2016.
“We are relieved that there is a deal for a 5-year reauthorization of MIECHV,” Lori Freeman, CEO of the Association of Maternal & Child Health Programs – an advocacy group – said in an emailed statement. “States, home visitors, and families have been in limbo for the past several months, and this news will bring the stability they need to continue this successful program.”
And the budget deal funds programs that encourage doctors to practice in medically underserved areas, providing just under $500 million over the next 2 years for the National Health Service Corps and another $363 million over 2 years to the Teaching Health Center Graduate Medical Education program, which places medical residents in Community Health Centers.
Kaiser Health News correspondent Emmarie Huetteman contributed to this article. KHN’s coverage of these topics is supported by Heising-Simons Foundation and The David and Lucile Packard Foundation. Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
In a rare show of bipartisanship for the mostly polarized 115th Congress, Republican and Democratic Senate leaders announced a 2-year budget deal that would increase federal spending for defense as well as key domestic priorities, including many health programs.
Not in the deal, for which the path to the president’s desk remains unclear, is any bipartisan legislation aimed at shoring up the Affordable Care Act’s individual health insurance marketplaces. Senate Majority Leader Mitch McConnell (R-Ky.) promised Sen. Susan Collins (R-Maine) a vote on health legislation in exchange for her vote for the GOP tax bill in December. So far, that vote has not materialized.
The deal does appear to include almost every other health priority Democrats have been pushing the past several months, including 2 years of renewed funding for community health centers and a series of other health programs Congress failed to provide for before they technically expired last year.
“I believe we have reached a budget deal that neither side loves but both sides can be proud of,” Senate Minority Leader Chuck Schumer (D-N.Y.) said on the Senate floor. “That’s compromise. That’s governing.”
Said McConnell, “This bill represents a significant bipartisan step forward.”
Senate leaders are still negotiating last details of the accord, including the size of a cut to the ACA’s Prevention and Public Health Fund, which would help offset the costs of this legislation.
According to documents circulating on Capitol Hill, the deal includes $6 billion in funding for treatment of mental health issues and opioid addiction, $2 billion in extra funding for the National Institutes of Health, and an additional 4-year extension of the Children’s Health Insurance Program (CHIP), which builds on the 6 years approved by Congress in January.
In the Medicare program, the deal would accelerate the closing of the “doughnut hole” in Medicare drug coverage that requires seniors to pay thousands of dollars out-of-pocket before catastrophic coverage kicks in. It would also repeal the controversial Medicare Independent Payment Advisory Board (IPAB), which is charged with holding down Medicare spending for the federal government if it exceeds a certain level. Members have never been appointed to the board, however, and its use has not so far been triggered by Medicare spending. Both the closure of the doughnut hole and creation of the IPAB were part of the ACA.
The agreement would also fund a host of more limited health programs – some of which are known as “extenders” because they often ride along with other, larger health or spending bills.
Those programs include more than $7 billion in funding for the nation’s federally funded community health centers. The clinics serve 27 million low-income people and saw their funding lapse last fall – a delay advocates said had already complicated budgeting and staffing decisions for many clinics.
And in a victory for the physical therapy industry and patient advocates, the accord would permanently repeal a limit on Medicare’s coverage of physical therapy, speech-language pathology and outpatient treatment. Previously, the program capped coverage after $2,010 worth of occupational therapy and another $2,010 for speech-language therapy and physical therapy combined. But Congress had long taken action to delay those caps or provide exemptions – meaning they had never actually taken effect.
According to an analysis by the nonpartisan Congressional Budget Office, permanently repealing the caps would cost about $6.47 billion over the next decade.
Lawmakers would also forestall cuts mandated by the ACA to reduce the payments made to so-called Disproportionate Share Hospitals, which serve high rates of low-income patients. Those cuts have been delayed continuously since the law’s 2010 passage.
Limited programs are also affected. The deal would fund for 5 years the Maternal, Infant and Early Childhood Home Visiting Program, a program that helps guide low-income, at-risk mothers in parenting. It served about 160,000 families in fiscal year 2016.
“We are relieved that there is a deal for a 5-year reauthorization of MIECHV,” Lori Freeman, CEO of the Association of Maternal & Child Health Programs – an advocacy group – said in an emailed statement. “States, home visitors, and families have been in limbo for the past several months, and this news will bring the stability they need to continue this successful program.”
And the budget deal funds programs that encourage doctors to practice in medically underserved areas, providing just under $500 million over the next 2 years for the National Health Service Corps and another $363 million over 2 years to the Teaching Health Center Graduate Medical Education program, which places medical residents in Community Health Centers.
Kaiser Health News correspondent Emmarie Huetteman contributed to this article. KHN’s coverage of these topics is supported by Heising-Simons Foundation and The David and Lucile Packard Foundation. Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
MedPAC: Medicare hospital readmissions program is working
WASHINGTON – The Medicare Hospital Readmissions Reduction Program is working, according to an original analysis of Medicare claims data presented at a meeting of the Medicare Payment Advisory Commission.
“First, readmissions declined,” MedPAC staff member Jeff Stensland, PhD, said during a congressionally mandated staff report to the commissioners. “Second, while observation stays increased, they did not fully offset the decrease in readmissions. Third, while [emergency department] visits also increased, those increases appear to largely be due to factors other than the readmission program. And fourth, in addition, all the evidence we examined suggests that the readmissions program did not result in increased mortality.”
including a reduction in readmissions and patients spending less time in the hospital with “at least equal outcomes,” Dr. Stensland said at the meeting.
Taxpayers benefited from a $2 billion reduction in spending on readmissions, which will “help extend the viability of the Medicare Trust Fund.” He noted that improvements to the program will be discussed at future MedPAC meetings.
Not all MedPAC commissioners agreed with the staff analysis.
David Nerenz, PhD, of the Henry Ford Health System, Detroit, also was not convinced the program was having an impact, noting that hospital readmissions began to decline even before the program started.
In looking at a graph presented that showed this trend, “I was impressed by the fact that the trend line started coming down all the way to the left side of the graph, and what my eye was impressed with was more just the continuation rather than a change, so I guess I feel cautious saying the program had certain effects because they certainly don’t jump off the graph visually,” Dr. Nerenz said. “I’m not disputing the numbers, but to say just as a clear unqualified conclusion the program reduced readmissions, I’m not so sure.”
It is likely premature to make any firm conclusions about how effectively this program decreases unnecessary utilization of hospitals. However, it is heartening to know that it did not increase mortality. The one variable that would best control readmissions is patient education. What constitutes an emergency requiring hospital evaluation and potential admission is often not explained to the patient by you and me.
It is likely premature to make any firm conclusions about how effectively this program decreases unnecessary utilization of hospitals. However, it is heartening to know that it did not increase mortality. The one variable that would best control readmissions is patient education. What constitutes an emergency requiring hospital evaluation and potential admission is often not explained to the patient by you and me.
It is likely premature to make any firm conclusions about how effectively this program decreases unnecessary utilization of hospitals. However, it is heartening to know that it did not increase mortality. The one variable that would best control readmissions is patient education. What constitutes an emergency requiring hospital evaluation and potential admission is often not explained to the patient by you and me.
WASHINGTON – The Medicare Hospital Readmissions Reduction Program is working, according to an original analysis of Medicare claims data presented at a meeting of the Medicare Payment Advisory Commission.
“First, readmissions declined,” MedPAC staff member Jeff Stensland, PhD, said during a congressionally mandated staff report to the commissioners. “Second, while observation stays increased, they did not fully offset the decrease in readmissions. Third, while [emergency department] visits also increased, those increases appear to largely be due to factors other than the readmission program. And fourth, in addition, all the evidence we examined suggests that the readmissions program did not result in increased mortality.”
including a reduction in readmissions and patients spending less time in the hospital with “at least equal outcomes,” Dr. Stensland said at the meeting.
Taxpayers benefited from a $2 billion reduction in spending on readmissions, which will “help extend the viability of the Medicare Trust Fund.” He noted that improvements to the program will be discussed at future MedPAC meetings.
Not all MedPAC commissioners agreed with the staff analysis.
David Nerenz, PhD, of the Henry Ford Health System, Detroit, also was not convinced the program was having an impact, noting that hospital readmissions began to decline even before the program started.
In looking at a graph presented that showed this trend, “I was impressed by the fact that the trend line started coming down all the way to the left side of the graph, and what my eye was impressed with was more just the continuation rather than a change, so I guess I feel cautious saying the program had certain effects because they certainly don’t jump off the graph visually,” Dr. Nerenz said. “I’m not disputing the numbers, but to say just as a clear unqualified conclusion the program reduced readmissions, I’m not so sure.”
WASHINGTON – The Medicare Hospital Readmissions Reduction Program is working, according to an original analysis of Medicare claims data presented at a meeting of the Medicare Payment Advisory Commission.
“First, readmissions declined,” MedPAC staff member Jeff Stensland, PhD, said during a congressionally mandated staff report to the commissioners. “Second, while observation stays increased, they did not fully offset the decrease in readmissions. Third, while [emergency department] visits also increased, those increases appear to largely be due to factors other than the readmission program. And fourth, in addition, all the evidence we examined suggests that the readmissions program did not result in increased mortality.”
including a reduction in readmissions and patients spending less time in the hospital with “at least equal outcomes,” Dr. Stensland said at the meeting.
Taxpayers benefited from a $2 billion reduction in spending on readmissions, which will “help extend the viability of the Medicare Trust Fund.” He noted that improvements to the program will be discussed at future MedPAC meetings.
Not all MedPAC commissioners agreed with the staff analysis.
David Nerenz, PhD, of the Henry Ford Health System, Detroit, also was not convinced the program was having an impact, noting that hospital readmissions began to decline even before the program started.
In looking at a graph presented that showed this trend, “I was impressed by the fact that the trend line started coming down all the way to the left side of the graph, and what my eye was impressed with was more just the continuation rather than a change, so I guess I feel cautious saying the program had certain effects because they certainly don’t jump off the graph visually,” Dr. Nerenz said. “I’m not disputing the numbers, but to say just as a clear unqualified conclusion the program reduced readmissions, I’m not so sure.”
REPORTING FROM MEDPAC